
Artificial Intelligence in Medicine pp 1469–1477 Cite as

Artificial Intelligence in Critical Care
The Path from Promise to Practice
- Alfredo Vellido 3 , 4 &
- Vicent Ribas 5
- Reference work entry
- First Online: 18 February 2022
318 Accesses
The hectic domain of critical care is, arguably, one of the most demanding instances of multidisciplinary medical decision-making. It is also a domain infused with monitoring and life-sustaining technologies that leave behind the type of digital data trail that is ideal for the deployment of artificial intelligence and, particularly, machine learning methods and analytical pipelines. These data analysis methods should provide medical decision support to intensivists, but there is yet no clear framework or standard set of guidelines on how to do so. In this chapter, we aim to provide readers with information on the current landscape of applications of machine learning in critical care and also with a clear outline of some of the many challenges yet to overcome to guarantee the success of such applications, including the problem of model interpretability and explainability, the technology compliance with current legislation, or the education of medical practitioners in the area of data analytics using open-source software.
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Cosgriff CV, Celi LA, Stone DJ. Critical care, critical data. Biomed Eng Comput Biol. 2019;10:1179597219856564.
Article Google Scholar
Ravì D, Wong C, Deligianni F, Berthelot M, Andreu-Pérez J, Lo B, Yang GZ. Deep learning for health informatics. IEEE J Biomed Health. 2017;21(1):4–21.
Cabitza F, Rasoini R, Gensini GF. Unintended consequences of machine learning in medicine. JAMA. 2017;318(6):517–8.
Tu JV. Advantages and disadvantages of using artificial neural networks versus logistic regression for predicting medical outcomes. J Clin Epidemiol. 1996;49(11):1225–31.
Article CAS Google Scholar
Doshi-Velez F, Kim B. Towards a rigorous science of interpretable machine learning. 2017. arXiv preprint. arXiv:1702.08608.
Google Scholar
Bacciu D, Lisboa PJ, Martín JD, Stoean R, Vellido A. Bioinformatics and medicine in the era of deep learning. In: Proceedings of the 26th European symposium on artificial neural networks, computational intelligence and machine learning (ESANN 2018), Bruges, 2018. p. 345–54.
Vellido A, Martín JD, Rossi F, Lisboa PJG. Seeing is believing: the importance of visualization in real-world machine learning applications. In: Proceedings of the 19th European symposium on artificial neural networks (ESANN), 2011. p. 219–26.
Bhanot G, Biehl M, Villmann T, Zühlke D. Biomedical data analysis in translational research: integration of expert knowledge and interpretable models. In: Proceedings of the 25th European symposium on artificial neural networks, computational intelligence and machine learning (ESANN), 2017. p. 177–86.
Vellido A. The importance of interpretability and visualization in Machine Learning for applications in medicine and health care. Neural Comput Appl. ePub ahead of press. https://doi.org/10.1007/s00521-019-04051-w .
Waring J, Lindvall C, Umeton R. Automated machine learning: review of the state-of-the-art and opportunities for healthcare. Artif Intell Med. 2020;104:101822.
Safdar S, Zafar S, Zafar N, Khan NF. Machine learning based decision support systems (DSS) for heart disease diagnosis: a review. Artif Intell Rev. 2017;50(4):597–623.
Vellido A, Ribas V, Morales C, Ruiz-Sanmartín A, Ruiz-Rodríguez JC. Machine learning for critical care: state-of-the-art and a sepsis case study. Biomed Eng Online. 2018;17(S1):135.
Dreiseitl S, Binder M. Do physicians value decision support? A look at the effect of decision support systems on physician opinion. Artif Intell Med. 2005;33(1):25–30.
Rhee C, Jones TM, Hamad Y, Pande A, Varon J, O’Brien C, Anderson DJ, Warren DK, Dantes RB, Epstein L, Klompas M. Prevalence, underlying causes, and preventability of sepsis-associated mortality in US acute care hospitals. JAMA Netw Open. 2019;2(2):e187571.
https://www.global-sepsis-alliance.org/sepsis
Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med. 2014;20(4):195–203.
Nguyen D, Ngo B, van Sonnenberg E. AI in the intensive care unit: up-to-date review. J Intensive Care Med. 2020. ePub ahead of publication. https://doi.org/10.1177/0885066620956620 .
Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM, Shankar-Hari M, Singer M, Deutschman CS. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):762–74.
Seymour CW, Kennedy JN, Wang S, Chang CC, Elliott CF, Xu Z, Berry S, Clermont G, Cooper G, Gomez H, Huang DT. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019;321(20):2003–17.
Ribas VJ, Vellido A, Ruiz-Rodríguez JC, Rello J. Severe sepsis mortality prediction with logistic regression over latent factors. Expert Syst Appl. 2012;39(2):1937–43.
Raghu A, Komorowski M, Celi LA, Szolovits P, Ghassemi M. Continuous state-space models for optimal sepsis treatment-a deep reinforcement learning approach. arXiv preprint arXiv:1705.08422. 2017 May 23.
Aushev A, Ribas Ripoll V, Vellido A, Aletti F, Bollen Pinto B, Bendjelid K, Herpain A, Hendrik Post E, Romay Medina E, Ferrer R, Baselli G. Feature selection for the accurate prediction of septic and cardiogenic shock ICU mortality in the acute phase. PLoS One. 2018;13(11):e0199089.
Ripoll VJ, Vellido A, Romero E, Ruiz-Rodríguez JC. Sepsis mortality prediction with the quotient basis kernel. Artif Intell Med. 2014;61(1):45–52.
Goodman B, Flaxman S. European Union regulations on algorithmic decision making and a “right to explanation”. AI Mag. 2017;38(3):50–57.
Reiz AN, de la Hoz MA, García MS. Big data analysis and machine learning in intensive care units. Med Intensiva (English Edition). 2019;43(7):416–26.
Yoon J, Drumright LN, Van Der Schaar M. Anonymization through data synthesis using generative adversarial networks (ADS-GAN). IEEE J Biomed Health Inform. 2020;24(8):2378–88.
McLennan S, Shaw D, Celi LA. The challenge of local consent requirements for global critical care databases. Intensive Care Med. 2019;45(2):246–8.
Tanner A. Our bodies, our data: how companies make billions selling our medical records. Beacon Press; 2017.
Hueso M, de Haro L, Calabia J, Dal-Ré R, Tebé C, Gibert K, Cruzado JM, Vellido A. Leveraging data science for a personalized haemodialysis. Kidney Dis. 2020. ePub ahead of print. https://doi.org/10.1159/000507291 .
Caban JJ, Joshi A, Nagy P. Rapid development of medical imaging tools with open-source libraries. J Digit Imaging. 2007;20(Suppl 1):83–93.
Karopka T, Schmuhl H, Demski H. Free/libre open source software in health care: a review. Healthc Inform Res. 2014;20(1):11–22.
Aminpour F, Sadoughi F, Ahamdi M. Utilization of open source electronic health record around the world: a systematic review. J Res Med Sci. 2014;19(1):57–64.
PubMed PubMed Central Google Scholar
Alaa AM, van der Schaar M. Autoprognosis: automated clinical prognostic modeling via Bayesian optimization with structured kernel learning. arXiv preprint arXiv:1802.07207. 2018.
Download references
Author information
Authors and affiliations.
Universitat Politècnica de Catalunya, Barcelona, Spain
Alfredo Vellido
Intelligent Data Science and Artificial Intelligence (IDEAI-UPC) Research Center, Barcelona, Spain
Eurecat, Barcelona, Spain
Vicent Ribas
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Alfredo Vellido .
Editor information
Editors and affiliations.
Department of Women’s and Children’s Health, Karolinska Institutet, Stockholm, Sweden
Niklas Lidströmer
Imperial College London, London, UK
Hutan Ashrafian
Electronic Supplementary Materials
(MP4 56481 kb)
Rights and permissions
Reprints and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this entry
Cite this entry.
Vellido, A., Ribas, V. (2022). Artificial Intelligence in Critical Care. In: Lidströmer, N., Ashrafian, H. (eds) Artificial Intelligence in Medicine. Springer, Cham. https://doi.org/10.1007/978-3-030-64573-1_174
Download citation
DOI : https://doi.org/10.1007/978-3-030-64573-1_174
Published : 18 February 2022
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-64572-4
Online ISBN : 978-3-030-64573-1
eBook Packages : Medicine Reference Module Medicine
Share this entry
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Artificial intelligence systems for complex decision-making in acute care medicine: a review
Affiliation.
- 1 The Sleep and Breathing Research Institute, 1251 Dublin Rd, Columbus, OH 43215 USA.
- PMID: 30733829
- PMCID: PMC6357484
- DOI: 10.1186/s13037-019-0188-2
The integration of artificial intelligence (AI) into acute care brings a new source of intellectual thought to the bedside. This offers great potential for synergy between AI systems and the human intellect already delivering care. This much needed help should be embraced, if proven effective. However, there is a risk that the present role of physicians and nurses as the primary arbiters of acute care in hospitals may be overtaken by computers. While many argue that this transition is inevitable, the process of developing a formal plan to prevent the need to pass control of patient care to computers should not be further delayed. The first step in the interdiction process is to recognize; the limitations of existing hospital protocols, why we need AI in acute care, and finally how the focus of medical decision making will change with the integration of AI based analysis. The second step is to develop a strategy for changing the focus of medical education to empower physicians to maintain oversight of AI. Physicians, nurses, and experts in the field of safe hospital communication must control the transition to AI integrated care because there is significant risk during the transition period and much of this risk is subtle, unique to the hospital environment, and outside the expertise of AI designers. AI is needed in acute care because AI detects complex relational time-series patterns within datasets and this level of analysis transcends conventional threshold based analysis applied in hospital protocols in use today. For this reason medical education will have to change to provide healthcare workers with the ability to understand and over-read relational time pattern centered communications from AI. Medical education will need to place less emphasis on threshold decision making and a greater focus on detection, analysis, and the pathophysiologic basis of relational time patterns. This should be an early part of a medical student's education because this is what their hospital companion (the AI) will be doing. Effective communication between human and artificial intelligence requires a common pattern centered knowledge base. Experts in safety focused human to human communication in hospitals should lead during this transition process.
Publication types
- Reference Manager
- Simple TEXT file
People also looked at
Review article, artificial intelligence in critical illness and its impact on patient care: a comprehensive review.

- 1 Khyber Medical College, Peshawar, Khyber Pakhtunkhwa, Pakistan
- 2 Ghulam Muhammad Mahar Medical College, Sukkur, Sindh, Pakistan
- 3 Jinnah Sindh Medical University, Karachi, Sindh, Pakistan
- 4 Health Services Academy, Islamabad, Pakistan
Artificial intelligence (AI) has great potential to improve the field of critical care and enhance patient outcomes. This paper provides an overview of current and future applications of AI in critical illness and its impact on patient care, including its use in perceiving disease, predicting changes in pathological processes, and assisting in clinical decision-making. To achieve this, it is important to ensure that the reasoning behind AI-generated recommendations is comprehensible and transparent and that AI systems are designed to be reliable and robust in the care of critically ill patients. These challenges must be addressed through research and the development of quality control measures to ensure that AI is used in a safe and effective manner. In conclusion, this paper highlights the numerous opportunities and potential applications of AI in critical care and provides guidance for future research and development in this field. By enabling the perception of disease, predicting changes in pathological processes, and assisting in the resolution of clinical decisions, AI has the potential to revolutionize patient care for critically ill patients and improve the efficiency of health systems.
1. Introduction
The word Artificial Intelligence (AI) describes the methods by which a system may imitate human cognitive functions, such as reasoning capacity, decision-making, generalization, or learning from past experiences, to achieve goals without being expressly programmed for specific activities. AI is characterized as intelligent machines, as opposed to the intelligence of individuals or other living things ( 1 ). The areas of learning algorithms, processing natural languages, and robotics may thus fall under the umbrella of artificial intelligence (AI), which has the potential to advance biomedical research, primary care, and health systems. These fields can be adapted to almost any area of medicine.
One of the most hotly contested uses of artificial intelligence (AI) in the healthcare industry has been the development of technology. The use of software, algorithms for machine learning, or artificial intelligence (AI) to simulate mental abilities in the interpretation, evaluation, and comprehension of healthcare data is referred to as AI in healthcare. For instance, AI-based medical algorithms used in mammograms help radiologists by providing a second opinion while aiding in the diagnosis of breast cancer ( 1 ).
AI was used in the healthcare industry to produce well-performing medicine. For instance, Insilico Medical has created AI algorithms that can halt viral infection. By providing nutritional guidance to expectant mothers based on their health state and algorithm estimates, another proposal seeks to safeguard them. Epileptic seizure detection, another excellent use of AI, assisted in lessening the severity of epileptic convulsions. With AI and the creation of a cutting-edge movement-detecting device, early stroke might also be accurately predicted.
Although using AI in medical healthcare seems to have the potential to drastically increase the effectiveness of clinical diagnosis and biomedicine in general, it has also raised some ethical questions. One of the main obstacles for medical AI is safety. IBM Watson for oncology is a very good example. It uses AI algorithms to analyse data from patient records and assist physicians in exploring cancer options for treatment for their patient populations. However, it has since come under fire for allegedly making risky and unreliable cancer therapy recommendations.
The quality of medical treatment for critically ill patients has greatly improved due to advancements in care standards ( 1 ). Despite this progress, traditional critical care has limitations in fully understanding and addressing the complexities of patients’ health, predicting deterioration, and providing timely treatment. The advent of advanced monitoring systems and non-invasive and invasive treatments has improved bedside care, but it is yet to be determined if these advancements represent the next step in critical care medicine. Artificial intelligence (AI) aims to help computers identify patterns in complex and diverse data, which was once only possible in limited fields like physics or astronomy due to limited computing resources. However, with the recent growth in computing power, AI can now be applied to other fields, including critical care medicine, where there is an abundance of complex data ( 2 ). According to a recent study ( 3 ), the number of articles about AI in the field of critical care medicine (CCM) has been increasing rapidly, particularly from 2018 to 2020. The majority of these articles are of high quality and come from top-ranked journals. Research into artificial intelligence (AI) has shown promise in terms of predicting disease outcomes and improving patient care ( 3 ).
While there are increasing numbers of studies using AI-powered models in the intensive care unit (ICU), our understanding of AI’s potential in critical care is still limited. Additionally, there are challenges that AI must overcome before becoming a routine part of clinical practice. Using the most recent literature, this review aims to improve understanding of the applications of AI in critical illness and its impact on patient care, and it makes recommendations for the future.
A comprehensive search was carried out in PubMed, Google Scholar, PLOS One, and Scopus for all relevant literature using the following terms: “critical care,” “intensive care medicine,” “ICU medicine,” “artificial intelligence,” “AI,” “machine learning,” and “critical illness” from January 2018 through February 2023 in the English language. Similar articles were also reviewed using the suggested articles for each paper, and gray literature was also searched using relevant terms. All papers were imported into reference management software, and duplicates were removed. Older versions of the same papers were not included if newer versions were available. All relevant papers were read, and corresponding authors were contacted using email if the full text of a paper was not available. No unpublished papers were included in our review.
3. Applications of AI in critical care patient management
AI has a multitude of diverse applications for the care of critically ill patients. The Figure 1 includes the recognition of disease, the prediction of disease progression, and the recognition of unique patterns in complex patient data. AI can also significantly aid caregivers in complex decision-making, as shown in Figure 1 .
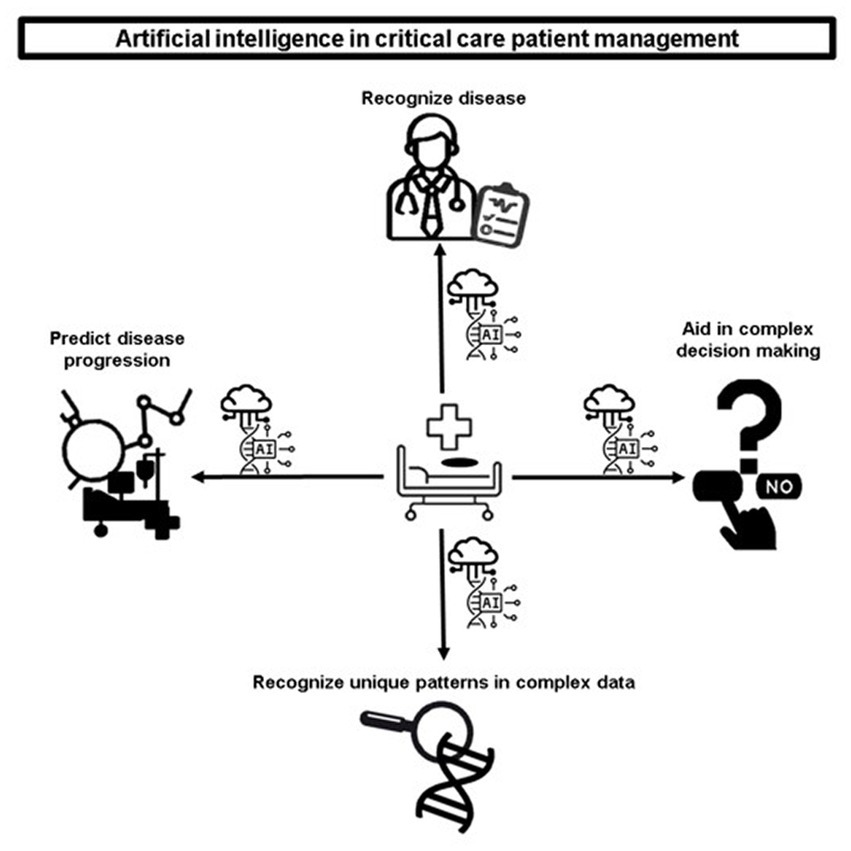
Figure 1 . Artificial intelligence in critical care patient management.
3.1. Recognition of disease
Diagnosing the source of a critically ill patient’s clinical decline can be a complex task due to the subtle onset of the disease or the presence of other conditions that obscure the main issue. Properly understanding the underlying context can be a challenging feat. For example, the presence of pulmonary infiltrates does not always indicate an excessive accumulation of fluid in the air sacs; it could be a sign of cardiac-related pulmonary edema, fluid in the pleural cavity, inflammation- or infection-related fluid buildup, or blood collections from trauma. Without proper clinical context and additional testing, appropriate and prompt treatment may be hindered. Artificial intelligence (AI) can aid in the medical diagnosis of critically ill patients by utilizing its advanced text and image processing abilities ( 4 ). A machine learning model, for instance, can differentiate congestive heart failure from other lung diseases and quantify pulmonary edema using a technique that provides a probabilistic manner for describing an observation ( 5 ). Furthermore, recent advancements in image analysis using convolutional neural networks have enabled the evaluation of traumatic brain injury with more accuracy than manual methods when viewed on head computed tomography scans ( 6 ). In a retrospective analysis by Prasad et al. ( 7 ), a reinforcement learning (RL) approach was used to develop a treatment protocol for electrolyte replacements in an ICU setting. This system provides recommendations for patient care that can be continuously updated based on the patient’s specific needs. The RL algorithm used available data from electronic health records, including vital signs, lab test results, and information about administered drugs and procedures, to estimate a patient-specific protocol for electrolyte repletion at six-hour intervals. The recommendations were presented by the AI algorithm in an interpretable and hierarchical manner, with the system first suggesting whether electrolyte replacement is needed and the best route for it, followed by the most appropriate dosage if the clinician chose to administer it. The RL system provided a more controlled and data-driven approach to electrolyte repletion as compared to traditional provider- or protocol-driven methods, which are often prone to error and deviation. This system also allows for greater flexibility and adaptability, considering patient context and clinical priorities. Optimal RL policy is reported to be able to recommend electrolyte replacements in a more targeted manner, potentially reducing the number of repletion events and the cost and time associated with unnecessary or repeat orders. Additionally, the system uses a reward and punishment system, reducing the costs and risks associated with intravenous delivery ( 7 ). This is not to underscore the value and significance of care-givers in the critical care setting; instead, it is a remarkable example of how new technologies such as AI can have a significant impact on the care of critically ill patients.
3.2. Prediction of disease progression using random forest models
Predicting disease progression is crucial for critically ill patients, as a delay in detecting clinical instability can result in harm or death ( 4 ). A dynamic random forest model is a type of machine learning algorithm that can be used to predict outcomes in the critical care setting. It works by using an ensemble of decision trees that can adapt and update in real-time as new data becomes available. A study by Yoon et al. ( 2 ) found that a dynamic model using random forest classification could predict cardiorespiratory instability, defined as a combination of hypotension, tachycardia, respiratory distress, or decreased oxygen saturation 90 min before it occurred in reality ( 2 , 4 ). The use of AI and machine learning has expanded across various fields such as public health, disease prediction, and drug development, including the ability to predict viral mutations before they arise ( 4 ). The power of AI approaches continues to be utilized in a wide range of disease prediction and drug development applications ( 8 ). In a study by Davoudi et al. ( 9 ), tachycardia, which frequently precedes shock, was predicted 75 min before its onset using a random forest model ( 9 ). Although not in the critical care setting, hypotension was also predicted prior to its occurrence in the operating room and confirmed by a randomized controlled trial, reducing the rate of intraoperative hypotension to 1.2% ( 10 , 11 ). In the critical care space, the prediction of hypotension events in the ICU has already been achieved using a random forest model that analyzed electronic health records and vital signs data, with 92.7% sensitivity, 15 min before the event even occurred ( 12 ). Another area where machine learning, a subset technology of artificial intelligence is in the assessment of pain in critically ill patients. In a study by Kobayashi et al. ( 13 ) which focused on using machine learning to assess the pain experienced by ICU patients, reported that vital signs, which are measured continuously in the ICU, can be used to predict pain with an accuracy upwards of 85% using a random forest (RF) model. This shows that machine learning can be used to continuously evaluate pain, which is important for pain management and the use of pain medication in ICUs. Their study also suggests that the use of an automated and continuous pain assessment algorithm may help relieve pain in patients who cannot communicate which could improve their life expectancy ( 13 ). All these examples show how the utilization of such models can prove significantly useful for management of critically ill patients.
3.3. Recognition of unique patterns in complex data
Critical illness is a complex condition that presents itself in various and unpredictable ways, leading to organ dysfunction and complicating the disease and recovery processes. To effectively manage these critical states, a careful consideration of underlying etiologies and clinical conditions is necessary. AI can help by recognizing unique patterns within complex data and identifying specific phenotypes or endotypes that reflect the individual’s critical state, leading to more personalized treatment plans ( 14 ). This relies heavily on access to large amounts of training data and phenotypic information. The complexity of medical care is highlighted by the fact that the same symptoms can be caused by different underlying conditions, making it difficult to provide personalized treatment. Diseases such as brain disorders, cardiovascular issues, and digestive problems are examples of this complexity. Innovative techniques and tools have been used to achieve personalized phenotyping in patients, combining practical experiences and scientific knowledge to realize the potential for using AI in a systems medicine approach to personalize medical care ( 15 ). The advancement of AI techniques has enabled researchers to uncover the underlying causes of various phenotypes, including genetic variations and cancer diseases, and by utilizing these tools and combining them with other methods, the biomedical field will be able to advance their knowledge and understanding of the relationship between genomics and expressions in diseases, promising faster and more accurate discoveries ( 16 ). This exemplifies how AI can serve as an aid to personalized patient care for critically ill patients.
3.4. Aid to complex decision-making in critical care
AI has the potential to assist doctors in the complex process of assessing patient risk levels for treatments, determining those who are most likely to experience a sudden deterioration, and analyzing multiple small outcomes to enhance overall patient outcomes. However, the complexity of AI techniques can affect physician comprehension and interpretation of results ( 17 ). To overcome this challenge, it is important for medical education to involve physicians in model creation and educate them in this field. AI platforms have the potential to be more efficient in some aspects compared to caregivers. For example, when compared to senior consultants, an AI platform such as Childhood Cataract Cruiser has proven to be more efficient and time-efficient for diagnoses, with high patient satisfaction rates ( 18 ). Such platforms can also be tested in the critical care setting. If they prove successful, it could significantly increase the efficiency of care delivery in the ICU. One-size-fits-all solutions are not effective in dealing with complex problems, as evidenced by the lack of improvement in septic shock outcomes in recent years despite various treatment guidelines ( 19 , 20 ). Utilizing the concept of reinforcement learning has the potential to offer individualized solutions to the diverse nature of septic shock and varying host responses. A study by Komorowski et al. ( 21 ) used reinforcement learning on time series data with 44 features collected from mechanically ventilated patients, which resulted in improved outcomes compared to standard clinical care, reducing 90 day and ICU mortality rates ( 21 ). AI can also perform real-time electrocardiogram analysis to detect myocardial infarctions. A study by Chen et al. ( 22 ) reported using AI-assisted real-time analysis of electrocardiograms in the prehospital setting and found that it was feasible and had the potential to reduce delays in treatment times for patients requiring percutaneous coronary interventions ( 22 ). These examples demonstrate the use of AI for therapeutic guidance in medical decision-making for critically ill patients with good efficacy.
3.5. Intelligent decision making intervention in critical illness
By assisting in decision-making and enabling healthcare professionals to concentrate their efforts on investing more time with patients, artificial intelligence can help to promote shared decision-making (SDM) ( 22 ). AI technologies offer a wide range of information and have the capacity to evaluate enormous amounts of data and find correlations that scholars and healthcare professionals would have overlooked ( 23 ). The bioethics of employing AI for health decision-making, the challenges involved, patients’ and healthcare practitioners’ perspectives of AI-based decision aids, and how it should be included to provide patient-centered healthcare are all topics of developing study. Nevertheless, little is known about the actual application of AI in SDM or how it may help with the decision-making phase of SDM.
4. Challenges and obstacles to AI in critical care patient management
Despite the potential benefits of AI in healthcare, particularly in the critical care setting, it is important to be aware of the potential challenges and obstacles that may arise when implementing AI models for critically ill patients. These roadblocks should not be ignored or overlooked, as they can have significant consequences for patient care and outcomes. The Figure 2 includes interpretability, data privacy and sharing, decreased clinical readiness and sub-optimal adherence to standard. A figure depicting the challenges and ethical concerns of AI in critical care patient management is shown in Figure 2 .
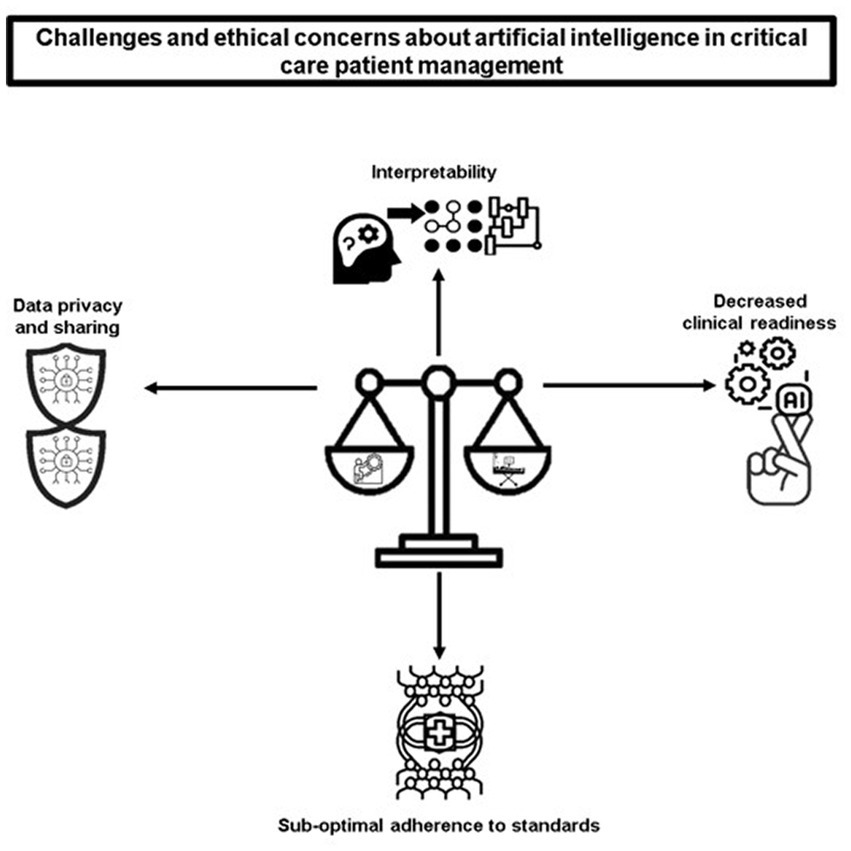
Figure 2 . Challenges and ethical concerns of AI in critical care patient management.
4.1. Interpretability of AI in the intensive care unit
The deployment of AI in a healthcare setting, specifically at the bedside, requires careful planning and consideration of key factors such as usability and trustworthiness. The involvement of all relevant stakeholders, including patients, clinicians, researchers, and hospital administrators, is crucial for the success of the deployment. To ensure that the AI systems are effective and well-received, the implementation strategy should focus on creating models with a manageable amount of information that is presented in an understandable and visually appealing manner. This can be achieved through the use of interpretable logic and a user-friendly graphic interface. One of the key challenges in deploying AI systems at the bedside is ensuring that the AI-generated alerts are accurate and not overwhelming, so as to prevent alarm fatigue ( 23 ). In recent research on predicting hypotension in the ICU, the use of a stacked random forest model was found to reduce the number of alerts tenfold while still maintaining accuracy ( 12 ). To build trust and acceptance of AI systems among end users, it is important to understand the AI-generated predictions and recommendations. Despite the complex nature of many AI models, researchers are working to enhance their interpretability. The creation of a graphic user interface is essential for the effective deployment of AI systems at the bedside, as it helps to improve hospital workflow and reduce the burden on healthcare workers. Additionally, the use of deep learning in the analysis of patient behavior and environmental stimuli can provide useful information for detecting delirium in ICU patients ( 9 ). Care-takers are keen to understand how machines arrive at predictions that involve patient care. There are different software technologies that can help caregivers to understand how machines arrive at these predictions. One such example is the Shapley additive explanation (SHAP), a method that explains how machines arrive at individual predictions. In a study by Alderden et al. ( 24 ), the risk of developing hospital-acquired pressure-related injury (HAPrI) was analyzed in COVID-19 patients who were hospitalized in the ICU. The study aimed to utilize machine learning algorithms to create a predictive model for HAPrI risk and ensure that the model was transparent and understandable for medical professionals. The best-performing model was an ensemble SuperLearner, which showed good discrimination in HAPrI risk assessment. The use of explainable AI methods such as SHAP plots was a novel approach in this study and provided a way to visualize the relationships between the patient’s characteristics and the predictions made by the model. This study found that COVID-19 positive critical care patients have a higher risk of HAPrI compared to non-COVID patients. The use of machine learning algorithms to evaluate HAPrI risk in COVID-19 patients in the ICU is reported to be a feasible approach, and explainable AI methods such as SHAP plots provide a means of ensuring that the model is understandable and trustworthy for medical professionals. Medical professionals need to understand how the model reached its decisions for each individual patient to decide whether the model is trustworthy for that patient ( 24 ). Care-takers generally have a positive attitude towards the adoption of AI. Mlodzinski et al. ( 25 ) set out to examine the perspectives of both healthcare providers and non-providers regarding the use of machine learning (ML) in critical care. The study found that both groups generally have positive attitudes towards the use of ML in healthcare; however, non-providers with more knowledge about ML and AI are more likely to feel favorable towards its use. The study also found that there were no major differences in the level of comfort or knowledge among providers, regardless of their level of experience. Furthermore, the study identified common concerns such as systemic bias in data, patient safety, negative effects on the doctor-patient relationship, and data privacy an security. Among providers, workflow interruptions were also identified as a major concern, while limited knowledge of ML and AI was a concern among non-providers. It provided important insights into provider and non-provider perspectives on ML-based tools and will play a crucial role in optimizing their clinical utility ( 25 ). In the future, it will be important to design ICU systems that embrace the capabilities of AI and address caregiver concerns in order to enable early detection of patient deterioration and improve the accuracy and trustworthiness of AI-generated predictions. The complex nature of many AI models often makes it difficult to understand the rationale behind the computation and output, leading to resistance among healthcare professionals to adopting these models in daily practice. The fear of performing unnecessary interventions or changing treatment strategies without scientific evidence can have serious consequences, especially in critical care where patient outcomes are directly linked to such decisions ( 26 , 27 ). However, there are efforts underway to address the issue of complex AI models. ML techniques are being used to determine what kinds of strategies caregivers use to make their decisions. For example, using game theory to measure the importance of features in predicting near-term hypoxic events during surgery has helped explain the contribution of various features to the AI model’s output. This approach has been shown to provide consistent results with prior knowledge and literature, leading to improved clinical decision-making and preventing hypoxia during surgery ( 28 ). This can also be extrapolated to the critical care setting to explain the contribution of different features in the output of AI models. Additionally, providing detailed methodologies for model validation, robustness of analysis, and expert knowledge can help alleviate concerns and increase the reliability and trust in AI models ( 4 ).
In this study, in contrast to SHAP, we will concentrate on two more example post-hoc model accuracy techniques that have gained minimal attention in the physical scientific world, namely breakDown (BD) research and Ceteris-Paribus (CP) analyses. The BD technique, like the SHAP method, is founded on the variety attribution principle, which divides each observation’s estimate into its individual variable components ( 29 , 30 ). The BD values offer action descriptions of the impacts of variables in a clever way, in contrast to the SHAP values. The independence and non-interaction of the input characteristics (factors or descriptors) constitutes a component of the BD method’s presumptions ( 31 ). For BD evaluation, there are two algorithms: step-up and step-down. The step-down approach begins with a complete collection of input characteristics.
Finally, in order to minimize the proximity to the prediction models, each selected feature contribution is determined by successively eliminating one characteristic from a set accompanied by variable relaxation. In contrast to the step-down approach, the step-up method begins with a null set and proceeds in the other manner. In feature contributions, both techniques have been proved to deliver consistent results.
On the contrary hand, the CP profiles, also known as individual conditional expectancies (ICE) plots, assess the impact of a variable from a learned ML model while assuming that the levels of all other variables remain constant (akin to what-if analysis).
Using CP profiles, one can quickly see how the source and responses are connected and how the projected response depends on a characteristic (e.g., in a non-linear, linear or complex). In this approach, the CP analysis aids in quantifying the influence of a particular variable on the conclusions drawn from a black version and offers a brief, visual description of the functional form linking an input with an output. From either the SHAP or BD analysis, it is difficult to draw conclusions regarding this type of functional reliance. Hence, adding CP profile plots to SHAP and BD studies is of great utility.
4.2. Reproducibility issues of AI systems during application
Frequently, determining the causative factors of deteriorating patients from the complete list of differential diagnoses is tough, because of the subtle feature of early illness progression or the existence of co-existing disorders disguising the underlying problem. Above all, it is important to accurately interpret the underlying context, which is sometimes difficult to do. For instance, it is not enough to infer that pulmonary infiltrates are caused by an excessive amount of alveolar fluid. These may signify pleural effusion, pulmonary embolism fluid from an infection or inflammation, pulmonary edema with a cardiac origin, collections of blood due to trauma, or any of these conditions. Lacking clinical context and additional testing, proper and prompt care might be delayed. AI might aid in such circumstances by obtaining a more exact diagnosis, given enhanced text and picture processing power. Using a machine learning algorithm, congestive heart failure (CHF) could be distinguished from other cause of lung illness ( 32 ), and the quantity of pulmonary edema brought on by the CHF could be measured using semi-supervised machine learning and a finite difference autoencoder. An AI model was used to evaluate imaging data from hospitalized patients in during acute pulmonary syndrome coronavirus 2 (SARS-CoV-2) epidemic in order to identify coronavirus disease 2019 (COVID-19) ( 33 ).
The application of AI in the clinical setting is hindered by a lack of sufficient clinical trials and experiments, leading to a low rate of reproducibility and future analysis. A review of 172 AI solutions created from chart data revealed that the clinical readiness level of AI was low, with 93% of the analyzed solutions falling below stage 4 for real-world application and only 2% undergoing prospective validation ( 29 ). The reproducibility of AI solutions is uncertain due to limitations in data openness and algorithmic complexity, and there are no clear protocols in place to examine this thoroughly. A study showed that attempting to reproduce mortality prediction projects resulted in large sample size differences in half of the experiments, highlighting the importance of accurate labeling, clinical context, and precise reporting methods ( 30 ). Adherence to reporting standards and the risk of bias are also sub-optimal, as a study of 81 non-randomized and 10 randomized trials using deep learning showed that only 6 of the 81 non-randomized studies had been tested in a real-world clinical setting, and 72% of the studies had high risks of bias ( 31 ). Even more complex AI models, such as reinforcement learning, face challenges as they require significant computational resources and are difficult to test on patients in a clinical environment. However, new approaches such as inverse reinforcement learning may offer a solution by inferring information about rewards, potentially making decision-assisting engines more robust and reliable with varying input data, which is crucial in critical care data science where data is vast and extremely diverse ( 34 ).
5. Ethical concerns
The use of AI in critical care is a new and developing field, and the ethical issues that may arise from its use are not fully understood. However, there are a few aspects that can be discussed to anticipate potential ethical dilemmas. One issue is data privacy and sharing. The process of collecting and manipulating data to find patterns could lead to the leakage of confidential information, particularly during the pre-processing stage and external validation. De-identification and novel models such as federated learning might help to minimize data leakage and increase the speed of the validation process ( 4 ). Another ethical concern is the safety of AI models in patient care. The maturity metric used for self-driving cars has been used to describe the safety of AI models, with 6 levels ranging from no automation to full automation ( 35 ). Based on this scale, most AI-driven solutions would currently fall into the categories of partial or no automation, meaning that human oversight and decision-making are still required. This also raises questions about patient autonomy and informed consent, as AI recommendations may not always align with a patient’s preferences. In order to address these ethical issues and overcome the limitations of AI, researchers and clinicians need to be aware of the potential problems and develop solutions to mitigate them. This also includes understanding patient perspectives and incorporating them into the development of practical and ethical AI solutions ( 4 ).

6. Guidance for future
The Figure 3 includes efficient data transfer, data de-identification, rapid processing, quality control, and decentralized federated learning. The field of AI has the potential to greatly impact critical care, but there are several steps that must be taken in order to make this happen, as highlighted in Figure 3 . Many of the recommendations have either already been implemented or are in the process of being implemented.
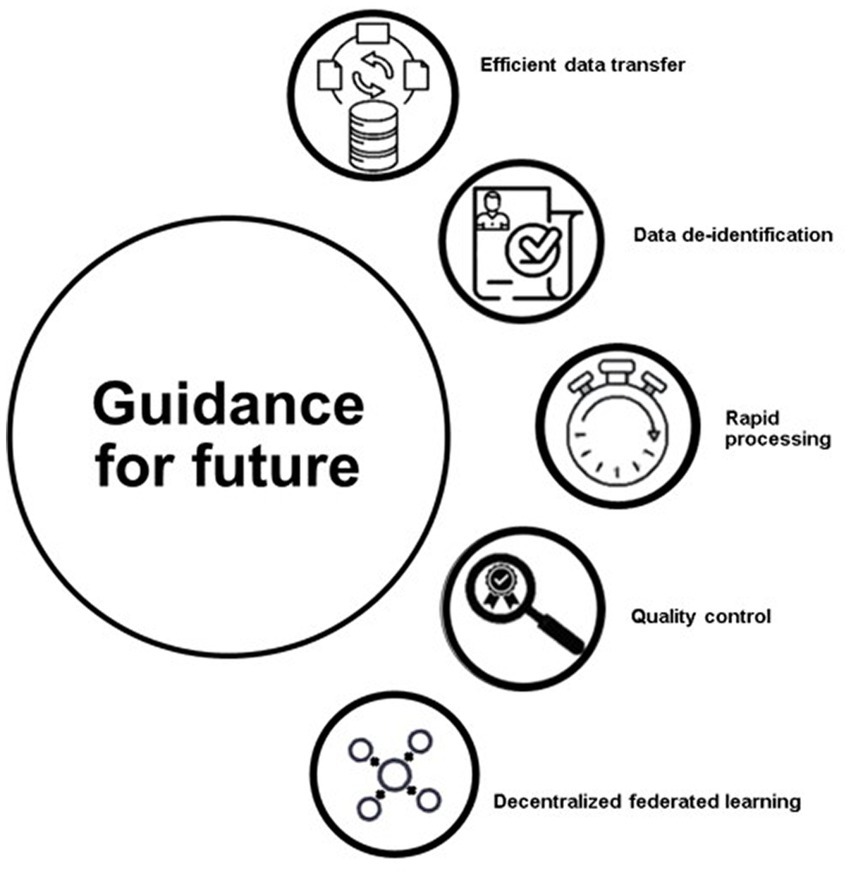
Figure 3 . Guidance for future.
One of the most important is ensuring that the data used for training AI models is properly de-identified and standardized. This is important for both privacy and data quality, as data from different hospitals may be structured differently and contain different amounts of personal information. The Society of Critical Care Medicine and the European Society of Intensive Care Medicine have developed a process for de-identifying data, that involves separating personal data from anonymous data, conducting a risk-based process to de-identify the personal data, and conducting an external review to ensure that all privacy and legal considerations are met ( 36 ). Another important step is standardizing the data in order to facilitate efficient exchange between different hospitals. This requires developing a standard format for storing and exchanging clinical and physiological data. One such format, the Hierarchical Data Format, Version 5 (HDF5), allows for the storage, compression, and real-time streaming of multiparameter data. This would allow for the integration of other types of large-scale datasets, such as those in imaging or genomics ( 37 ). Another solution is the use of federated learning, where models can be trained locally at different hospitals rather than having the data sent to a central location for training. This helps to preserve privacy and can be particularly useful when the data distribution is imbalanced or skewed. A successful example of this approach was seen during the COVID-19 pandemic, where 20 academic centers collaborated to predict clinical outcomes from COVID-19 using a federated learning approach. The AI model was trained on chest X-ray data, and achieved an average area under the curve (AUC), of 0.92 for predicting 24–72 h outcomes ( 4 ). The task of labeling events for AI models can be labor-intensive and resource-intensive, but novel AI models, such as weakly supervised learning, are being developed to make the process more efficient. This type of learning can build desired labels with only partial participation of domain experts, which preserves resources. Additionally, clinical trials can also be designed with AI models to maximize benefits and minimize risks to participants, as well as to make the best use of limited resources. One example of this is the Randomized Embedded Multifactorial Adaptive Platform for Community-Acquired Pneumonia (REMAP-CAP) trial, which uses a Bayesian inference model, to identify the optimal treatment for community-acquired pneumonia and has contributed to improved survival among critically ill COVID-19 patients ( 38 ). The labeling process for AI models can be a difficult and resource-intensive task. To make this process more efficient, new AI models such as weakly supervised learning have been developed. This method of learning allows for the partial involvement of domain experts and can reduce resource usage. For example, in the case of COVID-19 patients visiting the emergency department, weakly supervised learning was used in conjunction with medical ontologies and expert-driven rules to classify patients with related symptoms. This combination of weakly supervised learning and pretrained language models improved performance compared to a majority vote classifier, reducing the cost of creating classifiers in a short period of time, especially during a pandemic when experts may not be available for labeling. Innovative trial designs can also be developed with AI models to make the best use of resources and minimize risks to participants ( 39 ). This platform, initially developed for community-acquired pneumonia, has continued to enroll patients during the COVID-19 pandemic and has contributed to improved survival among critically ill patients ( 4 , 40 – 42 ). For an AI model to be useful in real-life settings, it needs to provide important information in a timely manner, especially for critically ill patients who require quick feedback. The AI model should have a fast data pre-processing platform, parsimoniously feature input data, and deliver output rapidly. To date, no such model has been developed that can successfully do the above-mentioned tasks in such a quick manner. Although true real-time prediction is a challenging task, the application of a real-time AI model in the critical care environment could offer significant benefits without delay. Once the AI model is deemed useful in a clinical setting, quality assessment efforts should follow to ensure its maturity and integration with healthcare. The National Academy of Medicine of the United States has published a white paper on AI use in healthcare, emphasizing the development of guidelines and legal terms for safer, more effective, and personalized medicine ( 43 ).
7. Conclusion
The utilization of artificial intelligence (AI) in critical care presents numerous opportunities for enhancing outcomes in critically ill patients by enabling the perception of disease, predicting changes in pathological processes, recognizing unique patterns in disease presentations, and assisting in the process of clinical decision-making in a symbiotic fashion with care-givers. Moreover, AI can facilitate the understanding of medical processes by presenting recommendations for patient care in an interpretable and hierarchical manner through techniques such as reinforcement learning. The technology has the potential to improve understanding of the diverse clinical needs of critically ill patients, risk assessment for treatments, and the analysis of patient outcomes.
Author contributions
MS and MI: data curation, formal analysis, investigation, conceptualization, supervision, visualization, writing – original draft, and writing – review and editing. HM: data curation, formal analysis, investigation, methodology, writing – original draft, writing – review and editing, and supervision. All authors reviewed the final version of the paper and approved it for submission and publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AI, Artificial intelligence; ML, Machine learning; RL, Reinforcement learning; ICU, Intensive care unit; CCM, Critical care medicine; RF, Random forest; SHAP, Shapley additive explanations; HAPrI, Hospital-acquired pressure-related injury; COVID-19, Coronavirus disease – 2019; HDF5, Hierarchical data format-version 5; AUC, Area under the curve; REMAP-CAP, Randomized embedded multifactorial adaptive platform for community-acquired pneumonia.
1. Zimmerman, JE, Kramer, AA, and Knaus, WA. Changes in hospital mortality for United States intensive care unit admissions from 1988 to 2012. Crit Care . (2013) 17:R81. doi: 10.1186/cc12695
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Yoon, JH, and Pinsky, MR. Predicting adverse hemodynamic events in critically ill patients. Curr Opin Crit Care . (2018) 24:196–203. doi: 10.1097/MCC.0000000000000496
3. Cui, X, Chang, Y, Yang, C, Cong, Z, Wang, B, and Leng, Y. Development and trends in artificial intelligence in critical care medicine: a bibliometric analysis of related research over the period of 2010–2021. J Pers Med . (2023) 13:50. doi: 10.3390/jpm13010050
CrossRef Full Text | Google Scholar
4. Yoon, JH, Pinsky, MR, and Clermont, G. Artificial intelligence in critical care medicine. Crit Care . (2022) 26:75. doi: 10.1186/s13054-022-03915-3
5. Seah, JCY, Tang, JSN, Kitchen, A, Gaillard, F, and Dixon, AF. Chest radiographs in congestive heart failure: visualizing neural network learning. Radiology . (2019) 290:514–22. doi: 10.1148/radiol.2018180887
6. Monteiro, M, Newcombe, VFJ, Mathieu, F, Adatia, K, Kamnitsas, K, Ferrante, E, et al. Multiclass semantic segmentation and quantification of traumatic brain injury lesions on head CT using deep learning: an algorithm development and multicentre validation study. Lancet Digital Health . (2020) 2:e314–22. doi: 10.1016/S2589-7500(20)30085-6
7. Prasad, N, Mandyam, A, Chivers, C, Draugelis, M, Hanson, CW 3rd, Engelhardt, BE, et al. Guiding efficient, effective, and patient-oriented electrolyte replacement in critical care: an artificial intelligence reinforcement learning approach. J Personalized Med . (2022) 12:661. doi: 10.3390/jpm12050661
8. Zhang, Q, Xie, Q, and GJCToIT, W. A survey on rough set theory and its applications. CAAI Trans Intel Technol . (2016) 1:323–33. doi: 10.1016/j.trit.2016.11.001
9. Davoudi, A, Malhotra, KR, Shickel, B, Siegel, S, Williams, S, Ruppert, M, et al. Intelligent ICU for autonomous patient monitoring using pervasive sensing and deep learning. Sci Rep . (2019) 9:8020. doi: 10.1038/s41598-019-44004-w
10. Wijnberge, M, Geerts, BF, Hol, L, Lemmers, N, Mulder, MP, Berge, P, et al. Effect of a machine learning-derived early warning system for intraoperative hypotension vs standard care on depth and duration of intraoperative hypotension during elective noncardiac surgery: the HYPE randomized clinical trial. JAMA . (2020) 323:1052–60. doi: 10.1001/jama.2020.0592
11. Joosten, A, Rinehart, J, van der Linden, P, Alexander, B, Penna, C, de Montblanc, J, et al. Computer-assisted individualized hemodynamic management reduces intraoperative hypotension in intermediate- and high-risk surgery: a randomized controlled trial. Anesthesiology . (2021) 135:258–72. doi: 10.1097/ALN.0000000000003807
12. Yoon, JH, Jeanselme, V, Dubrawski, A, Hravnak, M, Pinsky, MR, and Clermont, G. Prediction of hypotension events with physiologic vital sign signatures in the intensive care unit. Crit Care . (2020) 24:661. doi: 10.1186/s13054-020-03379-3
13. Kobayashi, N, Shiga, T, Ikumi, S, Watanabe, K, Murakami, H, and Yamauchi, M. Semi-automated tracking of pain in critical care patients using artificial intelligence: a retrospective observational study. Sci Rep . (2021) 11:5229. doi: 10.1038/s41598-021-84714-8
14. Yoon, JH, Pinsky, MR, and Clermont, G. Artificial Intelligence in critical care medicine. Crit Care . (2022) 26:75. doi: 10.1186/s13054-022-03915-3
15. Cesario, A, D’Oria, M, Bove, F, Privitera, G, Boškoski, I, Pedicino, D, et al. Personalized clinical phenotyping through systems medicine and artificial intelligence. J Pers Med . (2021) 11:265. doi: 10.3390/jpm11040265
16. Frey, LJ. Artificial intelligence and integrated genotype–phenotype identification. Genes . (2019) 10:18. doi: 10.3390/genes10010018
17. Azar, AT, and El-Metwally, SM. Decision tree classifiers for automated medical diagnosis. Neural Comput Appl . (2013) 23:2387–403. doi: 10.1007/s00521-012-1196-7
18. Lin, H, Li, R, Liu, Z, Chen, J, Yang, Y, Chen, H, et al. Diagnostic efficacy and therapeutic decision-making capacity of an artificial intelligence platform for childhood cataracts in eye clinics: a multicentre randomized controlled trial. eClinicalMedicine . (2019) 9:52–9. doi: 10.1016/j.eclinm.2019.03.001
19. Rivers, E, Nguyen, B, Havstad, S, Ressler, J, Muzzin, A, Knoblich, B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. New England J Med . (2001) 345:1368–77. doi: 10.1056/NEJMoa010307
20. PRISM InvestigatorsRowan, KM, Angus, DC, Bailey, M, Barnato, AE, Bellomo, R, et al. Early, goal-directed therapy for septic shock—a patient-level meta-analysis. New England J Med . (2017) 376:2223–34. doi: 10.1056/NEJMoa1701380
21. Komorowski, M, Celi, LA, Badawi, O, Gordon, AC, and Faisal, AA. The artificial intelligence clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med . (2018) 24:1716–20. doi: 10.1038/s41591-018-0213-5
22. Chen, K-W, Wang, Y-C, Liu, M-H, Tsai, B-Y, Wu, M-Y, Hsieh, P-H, et al. Artificial intelligence-assisted remote detection of ST-elevation myocardial infarction using a mini-12-lead electrocardiogram device in prehospital ambulance care. Front Cardiovasc Med . (2022) 9:1001982. doi: 10.3389/fcvm.2022.1001982
23. Hravnak, M, Pellathy, T, Chen, L, Dubrawski, A, Wertz, A, Clermont, G, et al. A call to alarms: current state and future directions in the battle against alarm fatigue. J Electrocardiol . (2018) 51:S44–8. doi: 10.1016/j.jelectrocard.2018.07.024
24. Alderden, J, Kennerly, SM, Wilson, A, Dimas, J, McFarland, C, Yap, DY, et al. Explainable artificial intelligence for predicting hospital-acquired pressure injuries in COVID-19-positive critical care patients. Comput Inf Nurs . (2022) 40:659–65. doi: 10.1097/CIN.0000000000000943
25. Mlodzinski, E, Wardi, G, Viglione, C, Nemati, S, Crotty Alexander, L, and Malhotra, A. Assessing barriers to implementation of machine learning and artificial intelligence-based tools in critical care: web-based survey study. JMIR Perioperative Med . (2023) 6:e41056. doi: 10.2196/41056
26. Rudin, C. Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat Mach Intell . (2019) 1:206–15. doi: 10.1038/s42256-019-0048-x
27. Durán, JM, and Jongsma, KR. Who is afraid of black box algorithms? On the epistemological and ethical basis of trust in medical AI. J Med Ethics . (2021):medethics-2020-106820. doi: 10.1136/medethics-2020-106820
28. Lundberg, SM, Nair, B, Vavilala, MS, Horibe, M, Eisses, MJ, Adams, T, et al. Explainable machine-learning predictions for the prevention of hypoxaemia during surgery. Nat Biomed Eng . (2018) 2:749–60. doi: 10.1038/s41551-018-0304-0
29. Fleuren, LM, Thoral, P, Shillan, D, Ercole, A, and Elbers, PWG. Machine learning in intensive care medicine: ready for take-off? Intensive Care Med . (2020) 46:1486–8. doi: 10.1007/s00134-020-06045-y
30. Johnson, AE, Pollard, TJ, and Mark, RG, Reproducibility in critical care: a mortality prediction case study. Machine learning for healthcare conference PMLR; (2017).
Google Scholar
31. Nagendran, M, Chen, Y, Lovejoy, CA, Gordon, AC, Komorowski, M, Harvey, H, et al. Artificial intelligence versus clinicians: systematic review of design, reporting standards, and claims of deep learning studies. BMJ . (2020) 368:m689. doi: 10.1136/bmj.m689
32. Seah, JCY, Tang, JSN, Kitchen, A, Gaillard, F, and Dixon, AF. Chest radiographs in congestive heart failure: visualizing neural network learning. Radiology . (2019) 290:514–22. doi: 10.1148/radiol.2018180887
33. Horng, S, Liao, R, Wang, X, Dalal, S, Golland, P, and Berkowitz, SJ. Deep learning to quantify pulmonary edema in chest radiographs. Radiol Artif Intell . (2021) 3:e190228. doi: 10.1148/ryai.2021190228
34. Fu, J, Luo, K, and S, Levine. Learning robust rewards with adversarial inverse reinforcement learning (2017).
35. Topol, EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med . (2019) 25:44–56. doi: 10.1038/s41591-018-0300-7
36. Thoral, PJ, Peppink, JM, Driessen, RH, Sijbrands, EJG, Kompanje, EJO, Kaplan, L, et al. Sharing ICU patient data responsibly under the Society of Critical Care Medicine/European Society of Intensive Care Medicine Joint Data Science Collaboration: the Amsterdam University Medical Centers database (AmsterdamUMCdb) example. Crit Care Med . (2021) 49:e563–77. doi: 10.1097/CCM.0000000000004916
37. Laird, P, Wertz, A, Welter, G, Maslove, D, Hamilton, A, Heung Yoon, J, et al. The critical care data exchange format: a proposed flexible data standard for combining clinical and high-frequency physiologic data in critical care. Physiol Meas . (2021) 42:065002. doi: 10.1088/1361-6579/abfc9b
38. Angus, DC, Berry, S, Lewis, RJ, Al-Beidh, F, Arabi, Y, van Bentum-Puijk, W, et al. The REMAP-CAP (randomized embedded multifactorial adaptive platform for community-acquired pneumonia) study. Rationale and design. Ann Am Thorac Soc . (2020) 17:879–91. doi: 10.1513/AnnalsATS.202003-192SD
39. Fries, JA, Steinberg, E, Khattar, S, Fleming, SL, Posada, J, Callahan, A, et al. Ontology-driven weak supervision for clinical entity classification in electronic health records. Nat Commun . (2021) 12:2017. doi: 10.1038/s41467-021-22328-4
40. Angus, DC, Derde, L, Al-Beidh, F, Annane, D, Arabi, Y, Beane, A, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA . (2020) 324:1317–29. doi: 10.1001/jama.2020.17022
41. Gordon, AC, Mouncey, PR, Al-Beidh, F, Rowan, KM, Nichol, AD, Arabi, YM, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med . (2021) 384:1491–502. doi: 10.1056/NEJMoa2100433
42. Patel, BN, Rosenberg, L, Willcox, G, Baltaxe, D, Lyons, M, Irvin, J, et al. Human-machine partnership with artificial intelligence for chest radiograph diagnosis. NPJ Digital Medicine . (2019) 2:111. doi: 10.1038/s41746-019-0189-7
43. Matheny, ME, Whicher, D, and Thadaney Israni, S. Artificial intelligence in health care: a report from the National Academy of medicine. JAMA . (2020) 323:509–10. doi: 10.1001/jama.2019.21579
Keywords: artificial intelligence, intensive care units, critical illness, risk assessment, decision making
Citation: Saqib M, Iftikhar M, Neha F, Karishma F and Mumtaz H (2023) Artificial intelligence in critical illness and its impact on patient care: a comprehensive review. Front. Med . 10:1176192. doi: 10.3389/fmed.2023.1176192
Received: 28 February 2023; Accepted: 04 April 2023; Published: 20 April 2023.
Reviewed by:
Copyright © 2023 Saqib, Iftikhar, Neha, Karishma and Mumtaz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hassan Mumtaz, [email protected]
This article is part of the Research Topic
Clinical Application of Artificial Intelligence in Emergency and Critical Care Medicine, Volume IV
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Artificial Intelligence in Critical Care Medicine
Joo heung yoon.
1 Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh, Pittsburgh, PA USA
Michael R. Pinsky
2 Department of Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA USA
Gilles Clermont
Associated data.
Not applicable.
This article is one of ten reviews selected from the Annual Update in Intensive Care and Emergency Medicine 2022. Other selected articles can be found online at https://www.biomedcentral.com/collections/annualupdate2022 . Further information about the Annual Update in Intensive Care and Emergency Medicine is available from https://link.springer.com/bookseries/8901 .
Introduction
The clinical outcome of critically ill patients has improved significantly and to an unprecedented level as standards of care have improved [ 1 ]. However, conventional critical care practice still has limitations in understanding the complexity of acuity, handling extreme individual heterogeneity, anticipating deterioration, and providing early treatment strategies before decompensation. Critical care medicine has seen the arrival of advanced monitoring systems and various non-invasive and invasive treatment strategies to provide timely intervention for critically-ill patients. Whether the mergence of such systems represents the next step in improving bedside care is an existing, yet unproven possibility.
The simplified concept of artificial intelligence (AI) is to allow computers to find patterns in a complex environment of multidomain and multidimensional data, with the prerequisite that such patterns would not be recognized otherwise. Previously, applying the concept in real life required a tremendous amount of computing time and resources. This could only be done in limited fields, including physics or astronomy. However, with recent exponential growth in computing power and portability, the power of AI became available to many fields, including critical care medicine where data are vast, abundant, and complex [ 2 ]. More and more clinical investigations are being performed using AI-driven models to leverage the data in the intensive care unit (ICU), but our understanding of the power and utility of AI in critical care medicine is still quite rudimentary. In addition, there are many obstacles and pitfalls for AI to overcome before becoming a core component of our daily clinical practice.
In this chapter, we seek to introduce the roles of AI with the potential to change the landscape of our conventional practice patterns in the ICU, describe its current strengths and pitfalls, and consider future promise for critical care medicine.
Applications of AI in Critical Care
Disease identification.
Oftentimes, finding the root cause of clinical deterioration from the exhaustive list of differential diagnoses is challenging, because of the insidious characteristic of early disease progression or the presence of co-existing conditions masking the main problem (Fig. 1 ). More than anything, the underlying context should be deciphered correctly, often a challenging task. For example, pulmonary infiltrates cannot simply be assumed to represent excessive alveolar fluid. They could indicate pulmonary edema from a cardiac cause, pleural effusion, parapneumonic fluid from inflammation or infection, or in some cases collections of blood as a result of trauma. Without clinical context and further testing, adequate and timely management could be delayed. AI could assist in such cases by obtaining a more precise diagnosis, given advanced text and image processing capability. The presence of congestive heart failure (CHF) could be differentiated from other causes of lung disease using a machine learning model [ 3 ], and amounts of pulmonary edema secondary to the CHF could be quantified with semi-supervised machine learning using a variational autoencoder [ 4 ]. During the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, imaging data from patients admitted to hospital were processed to detect coronavirus disease 2019 (COVID-19) using an AI model [ 5 ]. With recent efforts in image segmentation and quantification of lesions by convolutional neural networks, a type of algorithm particularly apt at interpreting images, the presence of traumatic brain injury (TBI) on head computed tomography (CT) could be evaluated with higher accuracy than manual reading [ 6 ]. Similarly, traumatic hemoperitoneum was quantitatively visualized and measured using a multiscale residual neural network [ 7 ].

Conceptual role of artificial intelligence (AI)-driven predictive analytics on disease progression. The AI model enables timely detection or prediction of disease enabling clinicians to manage critically ill patients earlier (green line) than conventional strategy (yellow dotted line)
Disease Evolution Prediction
Disease detection and prediction of disease evolution is one of the holy grails for critically ill patients. Given that the disease process is a continuum, instability of clinical condition can take various paths, even prior to ICU admission [ 8 ]. In a series of step-down unit patients who experienced cardiorespiratory instability (defined as hypotension, tachycardia, respiratory distress, or a desaturation event using numerical thresholds), a dynamic model using a random forest classification showed that a personalized risk trajectory predicted deterioration 90 min ahead of the crisis [ 9 ].
In the ICU, rapid clinical deterioration is common, and the result can be irreversible and even lead to mortality if detected late. Thus, efforts are being made to predict such hemodynamic decompensation. Tachycardia, one of the most commonly observed deviations from normality prior to shock, was predicted 75 min prior to development using a normalized dynamic risk score trajectory with a random forest model [ 10 ]. Hypotension, a manifestation of shock, was also predicted in the operating room [ 11 ]. The utility of a machine learning model in reducing intraop-erative hypotension was further confirmed in a randomized controlled trial in patients having intermediate and high-risk surgery, with hypotension occurring in 1.2% of patients managed with an AI-driven intervention, versus 21.5% using conventional methods [ 12 ]. Hypotension events have also been predicted in the ICU where vital sign granularities are lower and datasets contain more noise. Using electronic health record (EHR) as well as physiologic numeric vital sign data, clinically relevant hypotension events were predicted with a random forest model, achieving a sensitivity of 92.7%, with the average area under the curve (AUC) of 0.93 at 15 min before the actual event [ 13 ].
Hypoxia and respiratory distress have also been major targets for prediction, the roles of which have expanded during the recent coronavirus pandemic. In the first few months of the pandemic, AI-driven models were used to predict the progression of COVID-19, using imaging, biological, and clinical variables [ 14 ]. Cardiac arrest has also been predicted using an electronic Cardiac Arrest Risk Triage (eCART) score from EHR data, showing non-inferior scores compared to conventional early warning scoring systems [ 15 ]. Sepsis has also been predicted, with an AUC of 0.85 using Weibull-Cox proportional hazards model on high-resolution vital sign time series data and clinical data [ 16 ]. Other clinical outcomes may be predicted using AI models, including mortality after TBI [ 17 ] or mortality of COVID-19 patients with different risk profiles [ 18 ].
Disease Phenotyping
Critical illness is complex and its manifestations can rarely be reduced to typical presentations. Rather, critical illness manifests in a lot of different ways (inherent heterogeneity), and carries significant risks for organ dysfunction that can subsequently complicate the underlying disease process or recovery processes. Such syndromes should not be treated blindly without careful consideration of underlying etiologies or clinical conditions for a given individual. Moreover, the complex critical states change over time, such that clinicians cannot rely on assessments from even a few hours earlier. Yet, evidence-based guidelines should be followed whenever these exist. With its strong capability of pattern recognition from complex data, AI could delineate distinctive phenotypes or endotypes that could reflect influences from the critical state and hence open up avenues to personalize management, integrated into existing guidelines.
Sepsis, one of the most common ICU conditions, is a highly heterogenous syndrome, and has been a favorite target of AI algorithms. Recently, using different clinical trial cohorts, sepsis was clustered into four phenotypes (α, 3, γ, and 6) by consensus K-means clustering, a type of unsupervised machine learning model. The phenotypes had distinctive demographic characteristics, different biochemical presentations, correlated to host-response patterns, and were eventually associated with different clinical outcomes [ 19 ]. Such phenotypes are of a descriptive nature, are useful in describing case-mix, and could represent targets for predictive enrichment of clinical trials. However, they are not at this juncture based in mechanism and thus are not therapeutically actionable. Nevertheless, further explorations using richer data might allow a greater degree of actionability.
In the acute respiratory distress syndrome (ARDS), latent class analysis (LCA) revealed two subtypes (hypo- and hyper-inflammatory subtypes) linked with different clinical characteristics, treatment responses, and clinical outcomes [ 20 ]. A parsimonious model was developed and achieved similar performance to the initial LCA using a smaller set of classifier variables (interleukin [IL]-6, -8), protein C, soluble tumor necrosis factor (TNF) receptor 1, bicarbonate, and vasopressors). This result was validated in a secondary analysis of three different randomized clinical trials [ 21 ]. This machine learning-driven ARDS phenotyping has expanded our knowledge in assessing and treating complex disease, and become one of the criteria for predictive enrichment of future clinical trials.
Dynamic phenotyping for prediction of clinical deterioration can be performed on time series data. Using analysis of 1/20 Hz granular physiologic vital sign data, several unique phenotypes, including persistently high, early onset, and late onset deterioration, were identified prior to overt cardiorespiratory deterioration, using K-means clustering (Fig. 2 ) [ 9 ]. Time-series of images can be clustered for dynamic phenotyping, as performed using transesophageal echocardiographic monitoring images in patients with septic shock: using a hierarchical clustering method, septic shock was clustered into three cardiac deterioration patterns and two responses to interventions, which were linked with clinical outcome with different day 7 and ICU mortality rates [ 22 ].

Dynamic, personal risk trajectory prior to cardiorespiratory instability (CRI). Black line represents control subjects. Orange line (5) indicates ‘persistent high’, purple line (4) indicates ‘early rise’, and green (3), blue (2), and red (1) lines indicate ‘late rise’ to CRI. Adapted from [ 9 ] with permission of the American Thoracic Society.
Copyright © 2021 American Thoracic Society
Guiding Clinical Decisions
For a complex problem, one-size-fits-all solutions do not work well. Over the last decade, research has failed to improve the outcome of septic shock with different treatment guidelines [ 23 , 24 ]. The extreme heterogeneity of septic shock, various underlying conditions, and different host-responses could be at least partially addressed by AI to provide individualized solutions using reinforcement learning. The algorithm in reinforcement learning is designed to detect numerous variables in a given state to build an action model, which then learns from the reward or penalty from the results of the action. Applying this to the sepsis population, reinforcement learning could provide optimal sequential decision-making solutions for sepsis treatment, showcasing the potential impact of AI to generate personalized solutions [ 25 ]. In patients receiving mechanical ventilation, time series data with 44 features were extracted and reinforcement learning (Markov Decision Process) resulted in better results compared to physicians’ standard clinical care, with target outcomes of 90-day and ICU mortality [ 26 ]. These examples demonstrate the role AI may have in guiding important decision-making for critically ill patients. The notion of AI’s therapeutic utility could be more pronounced and provocative in different clinical environments, such as critical case scenarios in remote areas where clinicians are not available and patient transfer is not possible, or resource-limited settings where treatment options are limited. Because the optimality of such treatment recommendations is computed from retrospective and observational datasets, it is imperative that recommendation sequences or policies originating from such AI systems be fully analyzed and then tested prospectively before clinical implementation.
Implementation
An important consideration in successful deployment of AI at the bedside is system usability and trustworthiness. Deployment of such systems should involve all stakeholders, including clinicians and patients (end users), researchers (producers), and hospital administrators (logistics and management). In a specific research project, an implementation strategy entails creating models with adequate amounts of information (not a ‘flood’ of information with alarms), delivered with understandable (interpretable) logics, and placed on a visually appealing vehicle or dashboard—a graphic user interface. These systems, when deployed as alerting tools, must be accurate enough and parsimonious enough to prevent alarm fatigue, which leads to delays in detecting, and intervening for, developing crises [ 27 ]. In recent work on prediction of hypotension in the ICU, researchers found that AI-generated alerts could be reduced tenfold while maintaining sensitivity, when they used a stacked random forest model, or a model checking on another model before generating alerts [ 13 ].
Understanding AI-derived predictions and recommendations is arguably an important component of AI acceptance at the bedside. Although complex models can be thought of as ‘black-boxes’, an enormous effort is underway to enhance model interpretability and explainability. For example, in a recent report on hypoxia detection, researchers adopted concepts from game theory to differentially weight predictive physiological readouts during surgery, as an attempt to interpret the clinical drivers of hypoxic alarming from an AI system [ 28 ]. Creating the graphic user interface is necessary not only for the AI output to be delivered to the bedside, but also to improve hospital workflow and alleviate nursing burden. As shown in recent work, deep learning could be used to analyze fiducial points from the face, postures, and action of patients, and from environmental stimuli to discriminate delirious and non- delirious ICU patients [ 29 ]. Future ICU design should embrace the functionalities of AI solutions to enable clinicians to react earlier to any potential deterioration, and researchers to build models that perform better using more comprehensive data, and presented in such a way that it will be readily available, highly accurate, and trusted by bedside clinicians.
Pitfalls of AI in Critical Care
As much as the power of the AI model changes the current landscape of data analysis and plays an important role in assisting early diagnosis and management, there are many road blocks that should not be overlooked when introducing AI models for critically ill patients.
Explainability and Interpretability
Many AI models have complex layers of nodes that enable the characteristics of input data to be more meaningful in revealing hidden patterns. While the model may produce seemingly accurate output through that process, oftentimes the rationale of the computation cannot be provided to the end users. In the clinical environment, this can create strong resistance to accepting AI models into daily practice, as clinicians fear that performing unnecessary interventions or changing a treatment strategy without supporting scientific evidence could easily violate the first rule of patient care, primum non nocere . In critical care medicine, such a move could be directly and rapidly associated with mortality. On the other hand, many novel treatments did not have enough evidence when first introduced in the history of medicine, and ‘black-box’ models do not need to be completely deciphered, advocating the use of inherently interpretable AI [ 30 ]. Another recent approach argues that providing detailed methodologies for the model validation, robustness of analysis, history of successful/unsuccessful implementations, and expert knowledge could alleviate epistemological and methodological concerns and gain reliability and trust [ 31 ].
Multiple efforts have been introduced to overcome the complexity of deep learning models. Explaining feature contribution on a dynamic time series dataset became possible by leveraging game theory into measuring feature importance, when predicting near-term hypoxic events during surgery [ 32 ]. In that report, contributing features explained by SHapley Additive exPlanation (SHAP) showed consistency with the literature and prior knowledge from anesthesiologists for upcoming hypoxia risks. Moreover, anesthesiologists were able to make better clinical decisions to prevent intraoperative hypoxia when assisted by the explainable AI model.
Lack of Robustness
The readiness of AI for the real-life clinical environment is limited by the lack of adequate clinical experiments and trials, with a disappointingly low rate of reproducibility and prospective analyses. In a recent review of 172 AI-driven solutions created from routinely collected chart data, the clinical readiness level for AI was low. In that study, the maturity of the AI was classified into nine stages corresponding to real world application [ 33 ]. Strikingly, around 93% of all analyzed articles remained below stage 4, with no external validation process, and only 2% of published studies had performed prospective validation. Thus, current AI models in critical care medicine have largely been generated using retrospective data, without external validation or prospective evaluation.
Reproducibility of AI solutions is not guaranteed and no clear protocols exist to examine this thoroughly. As mentioned above, AI solutions already have limitations in terms of data openness and almost inexplicable algorithmic complexity, so the lack of reproducibility on top of these factors could significantly impact the fidelity of the AI model. A recent study attempted to reproduce 38 experiments for 28mortality prediction projects using the Medical Information Mart for Intensive Care (MIMIC-III) database, and reported large sample size differences in about a half the experiments [ 34 ]. This problem highlights the importance of accurate labeling, understanding the clinical context to create the study population, as well as precise reporting methods including data pre-processing and featurization.
Adherence to reporting standards and risks of bias is also sub-optimal, as a study that analyzed 81 non-randomized and 10 randomized trials using deep learning showed only 6 of 81 non-randomized studies had been tested in a real-world clinical setting and 72% of studies showed high risks of bias [ 35 ]. Hence, considering the scientific rigor of conventional randomized controlled trials needed to prove scientific hypothesis, the maturity and robustness of AI-driven models would be even less convincing for everyday practice.
More complex and sophisticated AI models, like reinforcement learning are also not free from challenges, as such intricate models require a lot of computational resources and are difficult to test on patients in order to train or test the models in a clinical environment. Inverse reinforcement learning, which infers information about rewards, could be a new model-agnostic reinforcement learning approach to constructing decision-making trajectories, because this approach alleviates the stress of manually designing a reward function [ 36 ]. With those algorithmic advances, decision-assisting engines can be more robust and reliable when input data varies, which may be a great asset to critical care data science where work is conducted with enormous quantity and extreme heterogeneity of data.
Ethical Concerns
Use of AI in critical care is still a new field to most researchers and clinicians. We will not really appreciate what ethical issues we will encounter until AI becomes more widely used and apparent in the development pipeline and bedside applications. However, given the nature of AI characteristics and current AI-driven solutions, a few aspects can be discussed to look around the corner into likely ethical dilemmas of AI models in critical care. The first issue is in data privacy and sharing. Innovation in data science allows us to collect and manipulate data to find hidden patterns, during which course collateral data leakage could pose threats, especially in its pre-processing and in external validation steps towards generalization. It is very hard to remove individual data points from the dataset once they are already being used by the AI model. De-identification and parallel/distributive computing could provide some solutions to data management, and novel models, including federated learning, might minimize data leakage and potentially speed up the multicenter validation process.
A second issue in ethics is safety of the AI model at the bedside. To semi-quantitatively describe the safety of the model, the analogous maturity metric used by self-driving cars was used for clinical adaptability of AI-driven solutions, with 6 levels [ 37 ]: 0 (no automation) to 2 (partial automation) represent situations where the human driver monitors the environment; 3 (conditional automation) to 5 (full automation) represent situations where the system is monitoring the environment rather than any human involvement. According to this scale, if used in real life, most of the AI-driven solutions developed would fall into categories 1 or 2. This concept signifies that the safety and accountability of the AI model cannot be blindly guaranteed, and decision making by clinicians remains an integral part of patient care. Also, the autonomy of individual patients has never been more important, including generating informed consent or expressing desire to be treated in life-threatening situations—here the AI recommendations might not be aligned with those of the patient. Recognition of such ethical issues and preparing for potential solutions to overcome limitations of AI, as well as understanding more about patient perspectives could allow researchers and clinicians to develop more practical and ethical AI solutions.
Future Tasks of AI in Critical Care
Data de-identification/standardization/sharing strategy.
Like any other clinical research, AI solutions need validation from many different angles. External validation, which uses input data from other environments, is one of the most common ways to generalize a model. Although external validation and prospective study designs certainly require collaborative data pools and concerted efforts, creating such a healthy ecosystem for AI research in critical care demands considerable groundwork.
De-identification of the healthcare data is probably the first step to ascertain data privacy and usability. The Society of Critical Care Medicine (SCCM)/European Society of Intensive Care Medicine (ESICM) Joint Data Science Task Force team has published the process to create a large-scale database from different source databases, including the following steps: (1) using an anonymization threshold, personal data are separated from anonymous data; (2) iterative, risk-based process to de-identify personal data; (3) external review process to ensure privacy and legal considerations to abide within the European General Data Protection Regulation (GDPR) [ 38 ]. Such a de-identification process would ascertain safe data transfer and could further facilitate high-quality AI model training.
Other important groundwork for the multi-center collaboration is data standardization. Individual hospital systems have developed numerous different data labeling strategies in different EHR layers. Even within the same hospital system, small discrepancies, including the number of decimals, commonly used abbreviations, and data order within the chart, could be stumbling blocks for systematic data standardization. In addition, data with higher granularity, including physiologic waveform data, are even harder to standardize, as there are no distinctive labels to express the values in a structured way. To address this, international researchers have developed a standardized format to facilitate efficient exchange of clinical and physiologic data [ 39 ]. In this Hierarchical Data Format-version 5 (HDF5)-based critical care data exchange format, multiparameter data could be stored, compressed, and streamed real time. This type of data exchange format would allow integration of other types of large-scale datasets as well, including imaging or genomics.
While one cannot completely remove the data privacy and governance concerns, rapid collaboration can be facilitated when those are less of an issue. An example is federated learning, where models can be designed to be dispatched to local centers for training, instead of data from participating centers collected to one central location for model training. While the data are not directly exposed to the outside environment, the model could still be trained by outside datasets with comparable efficacy and performance [ 40 ]. Federated learning could be even more useful when the data distribution among different centers is imbalanced or skewed, demonstrating the real-world collaboration environment [ 41 ]. A comprehensive federated learning project was performed during the COVID-19 pandemic. Across the globe, 20 academic centers collaborated to predict clinical outcomes from COVID-19 by constructing federated learning within a strong cloud computing system [ 42 ]. During the study phase, researchers developed an AI model to predict the future oxygen requirements of patients with symptomatic COVID-19 using chest X-ray data, which was then dispatched to participating hospitals. The trained model was calibrated with shared partial-model weights, then the averaged global model was generated, while privacy was preserved in each hospital system. In that way, the AI model achieved an average AUC > 0.92 for predicting 24- to 72-h outcomes. In addition, about a 16% improvement in average AUC, with a 38% increase in generalizability was observed when the model was tested with federated learning compared to the prediction model applied to individual centers. This report exemplifies the potential power of a federated learning-based collaborative approach, albeit the source data (chest X-ray and other clinical data) are relatively easy to standardize for the federated learning system to work on.
Novel AI Models and Trial Designs
Labeling target events for AI models is a daunting, labor-intensive task, and requires a lot of resources. To make the task more efficient, novel AI models, such as weakly supervised learning, have been introduced. Weakly supervised learning can build desired labels with only partial participation of domain experts, and may potentially preserve resource use. One example was provided by performing weakly supervised classification tasks using medical ontologies and expert-driven rules on patients visiting the emergency department with COVID-19 related symptoms [ 43 ]. When ontology-based weak supervision was coupled with pretrained language models, the engineering cost of creating classifiers was reduced more than for simple weakly supervised learning, showing an improved performance compared to a majority vote classifier. The results showed that this AI model could make unstructured chart data available for machine learning input, in a short period of time, without an expert labeling process in the midst of a pandemic. Future clinical trials could also be designed with AI models, especially to maximize benefits and minimize risks to participants, as well as to make the best use of limited resources. One example of such an innovative design is the REMAP-CAP (Randomized Embedded Multifactorial Additive Platform for Community-Acquired Pneumonia), which adopted a Bayesian inference model. In detail, this multicenter clinical trial allows randomization with robust causal inference, creates multiple intervention arms across multiple patient subgroups, provides response-adaptive randomization with preferential assignment, and provides a novel platform with perpetual enrollment beyond the evaluation of the initial treatments [ 44 ]. The platform, initially developed to identify optimal treatment for community-acquired pneumonia, continued to enroll throughout the COVID-19 pandemic, and has contributed to improved survival among critically ill COVID-19 patients [ 45 , 46 ].
Real-Time Application
To establish a valuable AI system in the real-life setting, the model should be able to deliver important information in a timely manner. In critically ill patients, the feedback time should be extremely short, sometimes less than a few minutes. Prediction made too early would have enough time to formulate the model, but have less predictive power, and prediction made very close to target events would have higher performance, with no time to curate the input data and run the model for output.
To be used in the real-life environment, a real time AI model should be equipped with a very fast data pre-processing platform, and able to parsimoniously featurize to update the model with new input data simultaneously. The output should also be delivered to the bedside rapidly. In that strict sense of real time, almost no clinical studies have accomplished real-time prediction. A few publications claim real-time prediction, but most of them used retrospective data, and failed to demonstrate continuous real-time data pre-processing without time delay. Using a gradient boosting tree model, one study showed dynamic ‘real-time’ risks of the onset of sepsis from a large retrospective dataset. The duration of ICU stay was divided into three periods (0–9 h, 10–49 h, and more than 50 h of ICU stay), partitioned to reflect different sepsis onset events, and resulted in different utility scores in each phase [ 47 ]. While this provides valuable information on different performances of the AI model for different durations of ICU stay, use of continuous real-time pre-processing without a time delay was not demonstrated. Another study using a recurrent neural network on postoperative physiologic vital sign data produced a high positive predictive value of 0.90 with sensitivity of 0.85 in predicting mortality, and was superior to the conventional metric to predict mortality and other complications [ 48 ]. The study also showed that the predictive difference between the AI solution and conventional methods was evident from the beginning of the ICU stay. However, prediction from the earliest part of the ICU stay also does not qualify as true real-time prediction. Although this is a challenging task for current technology, application of the real- time AI model to the critical care environment could yield significant benefit in downstream diagnostic or therapeutic options without time delay.
Quality Control After Model Deployment
Once the AI model achieves high performance and is deemed to be useful in a real-life clinical setting, implementation strategies as well as quality assessment efforts should follow. Anticipating such changes in the clinical/administrative landscape, the National Academy of Medicine of the United States has published a white paper on AI use in healthcare, in which the authors urge the development of guidelines and legal terms for safer, more efficacious, and personalized medicine [ 49 ]. In particular, for the maturity of AI solutions and their integration with healthcare, the authors suggested addressing implicit and explicit bias, contextualizing a dialogue of transparency and trust, developing and deploying appropriate training and educational tools, and avoiding over-regulation or over-legislation of AI solutions.
Rapid development and realization of AI models secondary to an unprecedented increase in computing power has invigorated research in many fields, including medicine. Many research topics in critical care medicine have employed the concept of AI to recognize hidden disease patterns among the extremely heterogeneous and noise-prone clinical datasets. AI models provide useful solutions in disease detection, phenotyping, and prediction that might alter the course of critical diseases. They may also lead to optimal, individualized treatment strategies when multiple treatment options exist. However, at the current stage, development and implementation of AI solutions face many challenges. First, data generalization is difficult without proper groundwork, including de-identification and standardization. Second, AI models are not robust, with sub-optimal adherence to reporting standards, a high risk of bias, lack of reproducibility, and without proper external validation with open data and transparent model architecture. Third, with the nature of the obscurity and probabilistic approach, AI models could lead to unforeseen ethical dilemmas. For the successful implementation of AI into clinical practice in the future, collaborative research efforts with plans for data standardization and sharing, advanced model development to ascertain data security, real-time application, and quality control are required.
Acknowledgements
Authors' contributions.
JHY, MRP, and GC have conceptualized and written the manuscript. All authors have agreed to be personally accountable for their contributions. All authors read and approved the final manuscript before submission.
National Institute of Health (NIH)—K23GM138984 (Yoon JH), R01NR013912 (Pinsky MR, Clermont G), R01GM117622 (Pinsky MR, Clermont G). Publication costs were funded by NIH K23GM138984 (Yoon JH).
Availability of data and materials
Declarations.
Authors have no competing financial or non-financial interests for current work.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
April 1, 2021. This blog post is the first of two that excerpt a HealthStream article, "The Validity of the Jane® Competency System AI Critical Thinking Assessments," by Randy L. Carden, Statistical Consultant, HealthStream. The development of critical thinking/judgment skills by nurses is of paramount importance in the healthcare industry ...
Acute Care Ai Critical Thinking Assessment acute-care-ai-critical-thinking-assessment 4 Downloaded from archive.nafc.org on 2020-12-05 by guest We'll explore the benefits of Acute Care Ai Critical Thinking Assessment, incorporating practical exercises and self-assessment tools into your study Acute Care Ai Critical Thinking Assessment.
AI and simulation enable application at a much higher level while eliminating the biggest risk of developing critical thinking skills in the healthcare environment—the fact that it's a matter of life and death.". Freedom to Make and Learn from Wrong Decisions. AI and simulation remove the effects of bad decisions, of life or death as the ...
Critical Thinking in Respiratory Care Shelley C. Mishoe 2001-06 This basic text teaches respiratory therapy content in a framework that promotes students' decision-making skills. The problem-based learning approach relies on clinical cases and a systematic method in which students may develop a deep and broad understanding of respiratory care ...
Harnessing AI. Augmenting nursing skills and knowledge with the assistive technology of AI can optimize acute care discharge processes and postacute care events and actions. The possibilities for nurses to further enhance patient care transitions in our digitally driven world by harnessing AI are immense. Predictive AI and conversational AI ...
AI is being developed to detect critical, highly complex and time dependent conditions such as adverse drug reactions and sepsis [6, 7] in acute care environments where timely nuanced communication is pivotal. It is likely that within 5 years, AI based protocols will replace many of the threshold based protocols which are presently providing ...
Acute Care Ai Critical Thinking Assessment Cumulated Index Medicus 1997 Critical Care Transport American Academy of Orthopaedic Surgeons (AAOS), 2009-11-09 Welcome ... state-of-the-art information on management of critical care patients. Crisp Assessment Logical Operations LLC 2002-02-12 By learning the critical thinking strategies
Critical thinking is a short-term process that stops when a nurse has acquired extensive clinical experience. JoAnne is a newly licensed nurse who is working on her critical thinking skills. She is contemplating her plan of care of for one her pts. The pt's name is Mr. Hernandez.
Today, critical care nurses in some hospitals use AI to enhance patient monitoring, develop nursing care plans, handle administrative tasks, and improve clinical decision-making. 1 In the future, AI will likely provide real-time insights for early detection and diagnosis, facilitate efficient allocation of health care resources, and streamline ...
The First Clinical Competency System with AI. Jane harnesses the power of artificial intelligence (AI) to create a system that personalizes competency development at scale, quickly identifies risk and opportunity, and improves quality outcomes by focusing on both knowledge retention and critical thinking. Leveraging decades of research and with ...
ARTIFICIAL INTELLIGENCE | Determining the Role of AI in a Dynamic Health Care Environment 6 6 EXECUTIVE DIALOGUE | Sponsored by Cerner | 2020 own instincts and assessment as to when someone is declining. It is an art and a science. We need both. BRADTMILLER: We definitely feel that it will help nurses. I love the acuity idea that Ginger just ...
HealthStream has introduced the Jane™ system, the first-ever AI-based competency assessment solution using a conversational format. Powered by IBM Watson, Jane is a highly reliable, effective, and evidence-based method for the assessment and validation of clinical competency. What's more, Jane is a comprehensive system and virtual nurse ...
-Secure airway -Perform a focused neuro assessment a long with cardiopulmonary; VS (HR, RR, Spo2, BP and temp) and a GSC-HOB 30-45 degrees-Maintain adequate oxygenation/elevation (supplemental O2)-Temperature should be kept within certain limits.-Contact MD and anticipate orders for CT scan or MRI, CSF drainage if needed, osmotic diuretics, medications for blood pressure control.
The hectic domain of critical care is, arguably, one of the most demanding instances of multidisciplinary medical decision-making. It is also a domain infused with monitoring and life-sustaining technologies that leave behind the type of digital data trail that is ideal for the deployment of artificial intelligence and, particularly, machine learning methods and analytical pipelines.
The integration of artificial intelligence into acute care AI and the acute care environment The future of medical AI extends far beyond the interpret-ation of medical images, pathology slides and radiographs. AI is being developed to detect critical, highly complex and time dependent conditions such as adverse drug reac-tions and sepsis [6, 7 ...
The current pandemic is accelerating the need to take advantage of AI to make real time clinical and operational decisions, wisely integrating the vast amount of heterogeneous data and emerging knowledge generated in the critical care environment . Safe, effective, efficient, and ethical clinical management of COVID-19 patients in ICUs urgently ...
AI, together with critical thinking and human judgment, can guide all phases of the Nursing Process by heightening the speed and accuracy of evaluation, anticipation, synthesis and knowledge ...
The integration of artificial intelligence (AI) into acute care brings a new source of intellectual thought to the bedside. This offers great potential for synergy between AI systems and the human intellect already delivering care. This much needed help should be embraced, if proven effective. However, there is a risk that the present role of ...
Artificial intelligence (AI) has great potential to improve the field of critical care and enhance patient outcomes. This paper provides an overview of current and future applications of AI in critical illness and its impact on patient care, including its use in perceiving disease, predicting changes in pathological processes, and assisting in clinical decision-making. To achieve this, it is ...
April 1, 2021. Assessing clinical competency is essential for knowing whether healthcare staff members are able to provide care that leads to positive outcomes. In our article excerpted here, "Using Artificial Intelligence to Assess Critical Thinking for Healthcare," HealthStream's Joe Caracci, MBA, BSN, RN; AVP, Clinical Assessments ...
As our flagship clinical development solution, HealthStream's Jane® is the World's First Digital Mentor for Nurses. Jane® harnesses the power of artificial intelligence (AI) to create a system that personalizes competency development at scale, quickly identifies risk and opportunity, and improves quality outcomes by focusing on critical ...
Artificial intelligence (AI) has great potential to improve the field of critical care and enhance patient outcomes. This paper provides an overview of current and future applications of AI in critical illness and its impact on patient care, including its use in perceiving disease, predicting changes in pathological processes, and assisting in clinical decision-making.
Many research topics in critical care medicine have employed the concept of AI to recognize hidden disease patterns among the extremely heterogeneous and noise-prone clinical datasets. AI models provide useful solutions in disease detection, phenotyping, and prediction that might alter the course of critical diseases.