

50+ Chemistry IA Ideas with Research Question Examples
One of the biggest challenges facing students taking IB chemistry is coming up with a good Internal Assessment (IA) idea .
It’s got to be something suitably demanding for diploma-level study, it’s got to be something relevant to the chemistry syllabus, it’s got to be something you can’t just look up the answer to in a textbook, and crucially, it’s got to be a topic the student is personally engaged with.
Many students are unsure how to relate the IB chemistry topics to a real-world situation or problem that they can investigate. To help with this, I’ve produced a list of chemistry IA ideas, together with some example Research Questions (RQ).
I’ve grouped them together according to the main experimental technique or measurement method.
A big fat disclaimer
This is a list of ideas only , intended as a source of inspiration for students who are stuck for an idea.
It is a starting point for further research, not a list of off-the-shelf projects you can select from and take to your teacher.
Please do not just copy directly from this list!
There are several reasons why you shouldn’t do this:
Firstly, one of the internal assessment criteria is Personal Engagement. This is about how well you engage with the project and make it your own .
To achieve the maximum score “ The evidence of personal engagement with the exploration is clear with significant independent thinking, initiative or creativity. The justification given for choosing the research question and/or the topic under investigation demonstrates personal significance, interest or curiosity. There is evidence of personal input and initiative in the designing, implementation or presentation of the investigation. ”
It should be clear from these guidelines that you need to choose your own project and completely own it .
Secondly, some of the projects below are just plain bad for reasons I’ve outlined in my post about research questions .
These reasons include:
- The RQ is just a title
- The RQ is unfocused and unclear – impossible to understand what the aims are
- The RQ doesn’t include any variables
- Project is unimaginative
- Project has obvious outcomes
- Project is just a series of measurements or a synthesis, rather than an investigation
I’ve given a weaker and a stronger example research question for each category, but be aware that the stronger research question is not necessarily a good research question!
With that out of the way, here’s the list:
Share This Post
Related posts.

AQA A-Level Chemistry Paper 3 Hard Questions

Hard Exam Questions from AQA A-Level Chemistry Paper 1
2023 IB Chemistry Syllabus Update: What’s Changed?

Hard A-Level Chemistry Questions: AQA AS Paper 1 and 2
Limiting and excess reactants in chemistry
12 comments.
Hello Sir, One of my students wants to conduct research about the amount of calcium in eggshells of different colors. However, He can’t explain to himself the relationship between water and calcium ion concentration. Could you please elaborate so I can explain him?
Hi, I’m not sure I understand the question – eggshell colour is due to the presence of pigments rather than Ca2+ ion concentration?
Egg shell is a finish – Like – Matt finish, Low sheen, Egg shell, Semi gloss, Gloss etc. Within the finish, there are many colours produced – depending on the paint company – from 100 to 10000. Most of them gives the colours through tinting platform – Base + tinter combination. There is a variation of CaCo3 between the bases. Hope it helps
Any ideas how I could incorporate something to do with seaweed?
Found so much value from your post! Thank you!!
Really a very helpful article thanks for sharing and keep on sharing!
My student wanted to do how peroxides can be a good preservation agent for milk
how better can he frame his RQ
Hello, I’ve been struggling for a while to come up with a type of question to be able to do an IA with. I’m interested in a topic having to do with pesticides but the thing is that I am not really sure how to convert it into an actual strong question. Can you please help? help/tips would be greatly helpful! Please and Thank you!
Hi, I wanted to determine protein content of something over different cooking time but I did some research on the google and I found that proteins are not affected by heating. So if I do this experiment, I will not see any difference in the protein content. Shoul I do this experiment? If I do this experiment and I write it as a IA, Will I get higher points? Will conclusion affect my point. Thank you
Hi, thanks for this nice article! I was thinking of investigating fragrance esters,specifically whether amount of unsaturation affects smell characteristics and volatility of the molecule? I am not sure how I can develop this up into an RQ. Any help you can give is appreciated. TIA
Hello Astridde, glad you find the article useful. The issue I can see to begin with is you have two dependent variables, volatility and smell characteristics. Volatility can be quantified and measured (e.g. vapour pressure) but ‘smell characteristics’ you would have to think how you can quantify and measure. Linking volatility and your independent variable (number of double bonds?) also sounds too simplistic to me as you can quite easily predict that relationship.
The earlier post I wrote on research questions will hopefully help.
[…] Don’t just copy an existing IA you found online, such as one from my list here! […]
Leave A Comment Cancel reply

20 IB Chemistry IA ideas

IB Chemistry IA - Insider Tips & Tricks To Guarantee A 7
Unlock the secrets to acing your IB Chemistry IA with our insider tips & tricks. Boost your grade to a 7 guaranteed! Don't miss out.
Table of content
The essence of ib chemistry, ib chemistry ia upcoming amendments, writing the ia - is it a daunting task, grading rubrics (chemistry ia criteria breakdown), personal engagement (criterion a), exploration (criterion b), analysis (criterion c), evaluation (criterion d), communication (criterion e), structuring your ib chemistry ia the right way, background/introduction, equipment and materials, safety, environmental and ethical considerations, methodology/procedure, data collection, data processing, conclusion and analysis, works cited, 7 pro tips to write a top-notch ib chemistry ia, 23 ib chemistry ia ideas to get you started, let's wrap it up.
Welcome aboard to the world of IB chemistry, which is more than just the study of chemicals!
This guide is here to help you score the 7 you’ve been aiming for by breaking down the Chemistry IA Rubric, the marking criteria, and 30+ Chemistry, IA Topic Ideas, to make it a smooth ride.
If you are reading this blog, you are earnestly committed to boosting your IB Chemistry IA score.
The good news is that this is your one-stop destination for everything you need to know. Let’s start with the basics.
IB Chemistry is undoubtedly an essential subject that elaborates on the chemical concepts underlying our physical environment and the biological processes that lie under it.
The emphasis of IB Chemistry lies in the practical approach. Students doing the IB Chemistry IA are taught to design investigations and data collection to arrive at the results and familiarize themselves with how the more significant scientific community functions. For this purpose, every student of the DP is expected to write an IA.
Don't worry. It's not as tough as you might think it is!
Now that you’ve gone through the curriculum and have a broad understanding of this course, you should also know that the IB Chemistry syllabus is under revision and subject to change post-2023.
Regarding the same, the following is stated by the IB:
- The ‘scientific investigation’ (internal assessment) will also see a change, with the opportunity for students to collaborate with each other within small groups. Where appropriate, the students will also be able to share similar methodologies, provided that the independent or dependent variables differ and the data collected is unique to each student.
- Students will continue to submit individual reports with a maximum word count of 3000 words.
- The revised criteria will emphasize higher-order thinking skills, with 50% of the marks allocated for Conclusion and Evaluation.
To convey the aforementioned in simple words, you will be pleased to work on your IA with your friends while submitting individual reports.
Additionally, the Conclusion and Evaluation segments of the IA must be taken seriously to score well.
Please keep checking the IB’s official website or register with us to stay in the loop for all the updates!
People will say it’s tough. We say it’s tough only if you want it to be.
To most of us, the IB Chemistry IA is a never-ending maze of dead ends.
Like all other IAs, it carries a huge weightage of 20% of your final score, making it all the more strenuous to be taken lightly.
One may cram their way through the External examinations. Still, the IB Chemistry IA, which emphasizes independent research, personal engagement and thorough analysis, seems like an insuperable obstacle.
All IB Chemistry students will readily admit how they grapple with scoring well in their IAs. It is one of the most challenging subjects owing mainly to the need for more clarity of information, the relevance of the research question to the Chemistry syllabus prescribed by IB, and the uniqueness of the topic, to name a few.
However, it isn’t all bad news. We at Nail IB are devoted to providing the best guide to cracking your IB Chemistry IA like a pro!
However, before we start, we have one tip that can completely change your IB Chemistry game.
Go through as many sample IAs as possible.
Ideally, go through the good and bad ones and reverse engineer the format, language and tone to form the base. Reading the previous submissions also boosts your confidence and gives you a better thought process. Nail IB’s experts bring you the best IB Chemistry HL and SL Notes to make it super easy.
These notes cover quizzes, past papers , samples IAs and much more.
Name it!
And Nail IB will have it!
Now, going back to the IA.
The Internal Assessment is worth 20% of the final assessment , which amounts to 24 marks . The performance in internal assessment at both HL and SL is marked on the standard criteria mentioned below.
The scientific investigation has to be completed within 10 hours, and submission of a report of 6-12 pages long is sufficient.
A longer write-up is generally penalized for lack of brevity.
To achieve a Super 7, you must meet all the criteria mentioned below.
To successfully nail the IB Chemistry IA, it is inevitable to familiarize ourselves with the Assessment Criteria and their respective markings.
Figure 1 - Table On IB Chemistry IA Criterion
This means the extent to which the student engages with the exploration and makes it their own.
This would include addressing personal interests or expressing independent thinking and creativity in the investigation.
Students must establish a scientific context for their work, state a straightforward research question, and use concepts and techniques appropriate to the Diploma Programme level.
The safety, environmental and ethical considerations are also evaluated alongside this.
The background information must be appropriate and enhance the understanding of the context of the investigation.
Assess the extent to which the student’s report provides evidence that the student has selected, recorded, processed and interpreted the data relevant to the research question and supports the conclusion.
A detailed conclusion has to be described and justified with strengths and weaknesses of the investigation, such as data limitations, sources of error and a clear understanding of the methodological issues involved in establishing the conclusion.
The presentation of the investigation needs to be clear and well-structured.
The focus, process and outcomes must be stated coherently.
Subject-specific terminologies should be employed with the appropriate use of diagrammatic presentation of the data.
References and citations should also be stated clearly.
Typically speaking, your IA should be divided into 8 sections.
The following paragraphs will elaborate on how to nail each so you, as an aspiring IB Chemistry student, can get closer to that sweet 7!
This is the opening part of the IA, and it sets the document's momentum by mentioning its aim and context.
Begin with how the Research Question resonates with you personally.
Next, explain the importance of the Research Question on real-world issues. Make sure that this is mentioned specifically and explicitly.
Lastly, elaborate on how it could be beneficial to the scientific community at large.
Next comes the hypothesis.
A hypothesis is the statement of an educated guess in the experiment based on observations. This segment should be short. You may justify the choice by using the Chemistry concepts learnt in class.
Selecting variables is central to any investigation.
3 types of variables should be kept in mind:
- Dependent variables,
- Independent variables, and
- Confounding variables
List all of these and evaluate why these variables were chosen and how they could skew the data and affect the experiment.
Remember to mention how you plan on monitoring and controlling them.
Give a complete list of each equipment and material you've employed in your IA.
Carefully choose the glassware and instruments that could maximize your accuracy and, at the same, minimize the inaccuracies.
You may also like to justify your preferred apparatus and preferred material.
Be especially careful of the details. For example, write 100 ml graduated cylinder instead of the graduated cylinder.
Please list all the safety concerns and why they are essential to keep an eye on.
Be careful of how they affect your experiment.
For instance, products can pollute the water system and must be stored in a waste container.
Plan your experiment well in advance to minimize the possibility of errors.
Record and report all measured values. Report all significant figures. For example, 5.0 or 5.1 instead of 5.
Multiple trials must be conducted to ensure sufficient relevant data for complete analysis.
This is where you show how you have processed the data to draw conclusions from your hypothesis.
It can be represented in the following formats:
- Data tables: suitable titles must be provided for each of them. All columns and rows should be labelled.
- Data Presentation: Ample use of diagrammatic representation of data through tables, graphs, pie charts etc., should be employed.
- Qualitative data: Positively mention qualitative data. For instance, the bubbling of gases or change of colour.
The conclusion is a rephrasing of the research question, along with the mention of the data processed. Ascertain the correlation between the hypothesis and the findings of your experiment. Explain the results as clearly as possible.
Correlate the arguments to concepts of Chemistry covered in the course taught at school. You may even go beyond the course to substantiate your thesis with a more complex concept.
Finally, your conclusion must also refer to the graph presented.
Every lab has a minimum of 4 sources of errors that could skew the data collected and cause ambiguity. So each of them must be mentioned and explained how they were controlled. Suggestions for improvements may also be presented.
Explain the limitations of the variables used, the reliability of the data presented and the certainty of the conclusion derived in clear words.
Also, maintain the balance between the strengths and weaknesses of the experiment. Attempt to name an equal number of strengths and weaknesses to create a balanced opinion.
Here you're supposed to mention any novel factor or variable that may be referred to for further investigation.
Academic honesty is a virtue that IB values in high regard. An exhaustive bibliography list, citations and references must be recorded. Remember to use MLA format and keep the bibliography in alphabetic order.
Please read this thoroughly before you sit down to write your Chemistry IA:
- There are 2 points for personal engagement. The research question must resonate with you personally.
- However, it is essential to note that the research question can be a more complex idea. Still, the approach to the research should be fresh and original with a personal perspective.
- Choose a research topic that allows for a ‘hypothesis’ where the outcome is not 100 % obvious, leaving some room for analysis and interpretation.
- Although it sounds obvious, it is surprising how many students overlook this. The variables must be clearly thought through along with the research question. Ideally, the variables must be enlisted with the research question itself. Refer to the previous segment of this guide, where we discuss variables in depth.
- One must be as straightforward as possible in their method and the material section. For instance, do more than just mention 5 beakers. Instead, write 5 glass beakers of 5 ml each, eliminating the uncertainty of the equipment used.
- Different alternatives can also be noted when developing the methodology.
- It forms the backbone of the experiment. A well-done investigation is NEVER complete without sources of systematic errors.
- Mention each source of error with a suggestion for improvements.
- Linking the research question to the wider world is imperative. This could either be discussed in the introduction or the conclusion.
- What should not be neglected is the application of the research topic in real life or industries, providing a ‘bigger picture perspective which the IB appreciates.
- Don’t be disheartened if you DO NOT have much to say in your evaluation. This is not a bad thing. You don’t get marked down in your IA if your experiment doesn’t work. You are marked down if you do not analyze properly.
Having familiarized ourselves with the knowledge required to write an excellent Chemistry IA, let us now look at a few fascinating topics for an IA.
- How does the heating of everyday acidic food items such as lemon juice affect the concentration of ascorbic acid in it?
- Measurement of the pH differences between two acidic samples.
- Catalase test to differentiate between Clostridium strains and Bacillus species.
- Use Complexometric titration with Ethylenediamine tetraacetic acid (EDTA) to compare the hardness of various water samples, possibly regular tap water, purified water or water from a nearby pond.
- Creating an electrolytic cell to measure the impact on the steel corrosion rate due to temperature changes.
- Using a water calorimeter experiment to check the impact on Gasoline's combustion enthalpy after adding ethanol.
- Measuring the impact of heating on dissolved oxygen concentration in water. Suitability of various lipid samples for making soaps
- Measurement of the Biological Oxygen Demand of water samples.
- Ascertaining the molecular formula of salt samples using thermal decomposition
- Using acid-base titration to ascertain the efficacy of indigestion tablets
- Synthesizing everyday medicines like paracetamol or aspirin
- Analyses of different soil samples to determine nutrients like phosphorous, nitrogen etc.
- Investigating the change in the reaction rate with a difference in the surface area.
- Ascertaining the iron content in Iron supplements using redox titration.
- Determining the enthalpy of neutralization of various acids and alkalis using calorimetry.
- Effect on the enthalpy of different salts using Hess' Law
- Understanding the importance of concentration of metals on electrode potentials
- Measuring the percentage of unsaturated fats in different oil samples using iodine numbers
- Comparison of lab-created esters from acids and alcohol with commercial perfumes
- Investigating different factors affecting electroplating
- Measurement of the concentration of zinc in dietary supplements
- Studying the vitamin content in different food items
- Measuring the changes of select nutrients (such as iron, Vitamin C, etc.) during fruit ripening stages.
Make sure that you begin writing your Chemistry IA well in advance to avoid any last-minute jitters or a hastily compiled report that would surely break your chances of excellent grades!
Found this guide helpful?
There’s more on our blog space , so bookmark us and don’t miss out on some great IB tips and tricks!
Nail IB is the most enormous IB resources repository on the internet.
We promise that writing your IB Externals and IAs will be a breeze with us. Register here now!
IB Resources you will love!
55234 + free ib flashcards, 136 + free ia samples, 3962 + ib videos by experts, 20099 + ib sample practice questions, ib resources for 30 + subjects.

IB Chemistry IA: 60 Examples and Guidance
Charles Whitehouse
The IB Diploma programme offers a variety of assessments for students, including Internal Assessments (IAs), which are pieces of coursework marked by students’ teachers. The Chemistry IA is an assessment designed to test students' understanding of the material they have learned in their chemistry course and their ability to conduct independent research. The investigation should be a self-directed study that demonstrates the student's ability to design, execute, and evaluate a scientific investigation.
What is the IA?
The IA consists of a laboratory report that students must complete during their IB chemistry course. For assessments before May 2025, the report should be 6 to 12 pages in length and should include a research question, a methodology section, data analysis, and a conclusion. From May 2025 , the report should be a maximum of 3,000 words.
What should the IA be about?
When choosing a topic for their IA, expert IB tutors recommend that the students should keep in mind that the investigation should be related to the content of the IB Chemistry course. It should also be practical, feasible, and of sufficient complexity to demonstrate their understanding of the subject matter. Some examples of topics that have been used in the past include the determination of the concentration of a substance in a solution, the effect of temperature on a chemical reaction, and the rate of a chemical reaction.
What should the IA contain?
Once a topic has been chosen, students should write a research proposal outlining their investigation. The proposal should include a clear research question, a brief literature review, a detailed methodology, and a list of the equipment and materials that will be needed. The proposal should also include a risk assessment, outlining any hazards associated with the investigation and the measures that will be taken to minimize them.
After the proposal has been approved, students can begin their investigation. They should keep a detailed laboratory notebook, including all the data they collect, any observations they make, and any calculations they perform. They should also take photographs or videos of their experiment to document the process.
Once the investigation is complete, students should analyze their data and draw conclusions. They should process their data using appropriate techniques, such as statistical analysis or graphing, and present it in a clear and concise manner. They should also evaluate their methodology and results, highlighting any limitations or uncertainties.
Finally, students should write a report, summarizing their investigation. The report should include an introduction, a method section, a results section, a discussion section, and a conclusion. The report should also include a list of references, citing any sources that were used in the research proposal or during the investigation.
Have a look at our comprehensive set resources for IB Chemistry developed by expert IB teachers and examiners!
- IB Chemistry 2024 Study Notes
- IB Chemistry 2025 Study Notes
- IB Chemistry 2024 Questions
- IB Chemistry 2025 Questions
How can I do well in the IA?
To prepare for the IA, students should ensure that they understand the material covered in their chemistry course and should practice writing lab reports. They should also seek feedback from their teachers on their writing skills and their understanding of the research process, and can also enlist the help of an IB Chemistry tutor .
Before starting the IA, students should also familiarize themselves with the assessment criteria and the guidelines provided by the IB. This will allow them to show their full potential and achieve the highest mark possible. Students should also make sure that their report is well-written and properly formatted, and that it includes all the required sections.
The assessment criteria include the following:
Personal engagement : Students should engage with the exploration, which can be demonstrated through independent thinking and creativity. The research question or topic should be linked to something of personal significance or interest, and the student should show initiative in implementing the investigation. (2 marks)
Exploration : Students should identify a relevant and fully-focused research question, which is explored with appropriate background information and investigated with an appropriate methodology. The student should consider the safety, ethical, or environmental issues that are relevant to the methodology. (6 marks)
Analysis : Students should demonstrate the ability to analyze data and draw conclusions. They should show that they have used appropriate techniques to process and present data, and that they have identified patterns and trends in the data. The report should include quantitative and qualitative data, which supports a detailed and valid conclusion, following appropriate data processing. (6 marks)
Evaluation : Students should demonstrate an understanding of the limitations and uncertainties of their investigation. They should critically evaluate their methodology and results, and suggest ways in which the investigation could be improved or extended. (6 marks)
Communication : The investigation should be clearly presented, with an effective structure, concise writing, and appropriate use of subject-specific terminology. (4 marks)
What are some example research questions?
Here are a few examples of potential research questions compiled by expert Chemistry tutors that could inspire your Chemistry IA:
1 - How does the concentration of a solution affect the rate of reaction between hydrochloric acid and magnesium?
Conduct a series of experiments in which hydrochloric acid is added to different concentrations of magnesium in solution. The rate of reaction could be measured by tracking the production of hydrogen gas over time. The concentration of the solution could be varied by diluting the hydrochloric acid with water. The results could be plotted on a graph to show the relationship between concentration and rate of reaction. Control variables such as temperature and stirring rate would need to be kept constant throughout the experiments.
2 - Can the purity of a sample of aspirin be determined using thin-layer chromatography?
A sample of the aspirin would be dissolved in a suitable solvent and spotted onto a thin-layer chromatography plate. The plate would then be placed in a developing chamber containing a suitable solvent. As the solvent moves up the plate, it will separate the different components of the sample based on their polarity. The purity of the aspirin can be determined by comparing the distance traveled by the aspirin spot to the distance traveled by any impurities or other components present in the sample. This can be done by measuring the Rf value (the ratio of the distance traveled by the spot to the distance traveled by the solvent) for each component. A pure sample of aspirin would have an Rf value of 1, while impurities or other components would have lower Rf values.
3 - Investigating the effect of temperature on the solubility of a salt in water.
Prepare a saturated solution of the salt at room temperature. Then, heat the solution to a higher temperature and add more of the salt until it reaches saturation again. The amount of salt that can dissolve in the water at each temperature can be measured by weighing the solution before and after adding the salt. This process can be repeated at different temperatures to create a solubility curve. The curve can then be used to determine the effect of temperature on the solubility of the salt in water.
4 - How does the concentration of hydrochloric acid affect the reaction rate with sodium thiosulfate?
Conduct a series of experiments in which different concentrations of hydrochloric acid are mixed with a fixed amount of sodium thiosulfate. The reaction rate can be measured by observing the time it takes for the solution to turn cloudy, indicating that the reaction has occurred. The concentration of hydrochloric acid that produces the fastest reaction rate can be determined, and a graph can be created to show the relationship between concentration and reaction rate. Control variables such as temperature and stirring should be kept constant throughout the experiments.
5 - Can the enthalpy change of a chemical reaction be determined using Hess's law and calorimetry?
Use calorimetry to measure the enthalpy change of the individual reactions involved in the chemical reaction of interest. Then, use Hess's law to calculate the enthalpy change of the overall reaction. This would involve setting up a calorimeter, measuring the initial and final temperatures of the reactants and products, and calculating the heat absorbed or released during the reaction. The enthalpy change of the individual reactions could be determined by conducting them separately and measuring the heat change.
6 - Investigating the effect of different types of catalysts on the rate of decomposition of hydrogen peroxide.
Set up an experiment in which hydrogen peroxide is mixed with different types of catalysts, such as manganese dioxide, copper oxide, or iron oxide. The rate of decomposition of the hydrogen peroxide can be measured by monitoring the release of oxygen gas using a gas syringe or pressure sensor. The experiment would need to be repeated with each type of catalyst, and the results can be compared to determine which catalyst is most effective at increasing the rate of decomposition. Control variables such as temperature, concentration of hydrogen peroxide, and volume of catalyst would need to be kept constant.
7 - How does the pH of a solution affect the solubility of a sparingly soluble salt?
Prepare a solution of the sparingly soluble salt in water at a known concentration. Vary the pH of the solution using acidic or basic solutions. The solubility of the salt can be determined by measuring the concentration of the salt in the solution using techniques such as spectrophotometry or gravimetry. The solubility of the salt can then be plotted against the pH of the solution to determine the effect of pH on solubility. This process would need to be repeated for different concentrations of the salt to determine the impact of concentration on solubility.
8 - Can the concentration of a solution be determined using acid-base titration?
To determine the concentration of a solution using acid-base titration, a known volume of the solution would be added to a flask and an indicator would be added. A standardized solution of a strong acid or base would then be slowly added to the flask until the endpoint is reached, indicating that all the acid or base has reacted with the solution. The concentration of the solution can then be calculated based on the volume and concentration of the standardized solution used in the titration. This process would need to be repeated for each solution being tested.
9 - Investigating the effect of different types of surfactants on the surface tension of water.
Prepare solutions of different concentrations of the surfactants being tested. A drop of each solution would be placed on the surface of water and the surface tension of the water would be measured using a tensiometer. The process would be repeated for each concentration of surfactant being tested. The results would be plotted on a graph to determine the relationship between the concentration of surfactant and the surface tension of water.
10 - How does the concentration of a solution affect the rate of reaction between sodium thiosulfate and hydrochloric acid?
Conduct a series of experiments in which different concentrations of sodium thiosulfate and hydrochloric acid are mixed together in a controlled environment. The rate of reaction can be measured by observing the time it takes for the solution to turn cloudy due to the formation of sulfur. The concentration of the solution can be varied by diluting or concentrating the solutions before mixing them together. By comparing the rate of reaction at different concentrations, the relationship between concentration and rate of reaction can be determined.
11 - Can the concentration of copper in a brass alloy be determined using atomic absorption spectroscopy?
Prepare a series of standard solutions of known copper concentrations using a pure copper sample. The brass alloy would then be dissolved in acid and the resulting solution would be analyzed using atomic absorption spectroscopy. The absorption of light by the copper atoms in the solution would be measured and compared to the absorption of the standard solutions to determine the concentration of copper in the brass alloy. This process would need to be repeated for each brass alloy being tested.
12 - Investigating the effect of the length of an alkane chain on its boiling point.
Prepare a series of alkane samples with varying chain lengths. Each sample would be heated and the temperature at which it boils would be recorded. The boiling point of each alkane sample would be plotted against its chain length to determine the relationship between the two variables. This experiment would need to be repeated multiple times to ensure accuracy and consistency of results.
13 - How does the pH of a solution affect the color of an indicator?
Select an appropriate indicator that changes color within the pH range being tested. Prepare solutions with different pH values by adding acids or bases to a neutral solution. Add a small amount of the indicator to each solution and observe the color change. Record the pH value at which the color change occurs for each indicator. This experiment can be repeated with different indicators to compare their sensitivity to changes in pH.
14 - Can the concentration of iron in a solution be determined using spectrophotometry?
Prepare a series of standard solutions with known concentrations of iron. The absorbance of each standard solution would be measured using a spectrophotometer, which would create a calibration curve. A sample of the unknown solution containing iron would then be measured for its absorbance, and the concentration of iron in the solution can be determined by comparing its absorbance to the calibration curve. This process would need to be repeated for each solution being tested.
15 - Investigating the effect of the concentration of a solution on the rate of reaction between potassium permanganate and oxalic acid .
Set up a series of experiments in which different concentrations of the potassium permanganate solution are mixed with a fixed concentration of oxalic acid. The rate of reaction could be measured by monitoring the color change of the solution over time, as the potassium permanganate is reduced by the oxalic acid. The concentration of the potassium permanganate solution that produces the fastest rate of reaction could be determined, and the effect of varying concentrations of oxalic acid could also be investigated. Control variables such as temperature and stirring rate would need to be kept constant throughout the experiments.
16 - How does the presence of a common ion affect the solubility of a salt?
Prepare solutions of the salt at different concentrations and add a known amount of the common ion to each solution. The solubility of the salt in each solution can then be determined by measuring the amount of salt that remains undissolved after stirring the solution for a set period of time. Comparing the solubility of the salt in solutions with and without the common ion would determine the effect of the common ion on the salt's solubility. This process would need to be repeated for different concentrations of the common ion to determine the concentration at which it has the greatest effect on the salt's solubility.
17 - Can the rate constant of a chemical reaction be determined using kinetics experiments?
Conduct a series of experiments in which the concentration of reactants is varied while keeping all other variables constant. The rate of the reaction can be measured by monitoring the change in concentration of the reactants or products over time. The rate constant can then be calculated using the rate equation for the reaction. This process would need to be repeated for different temperatures and concentrations to determine the effect of these variables on the rate constant.
18 - Investigating the effect of different types of acids and bases on the pH of a buffer solution.
Prepare a buffer solution with a known pH and add different types of acids and bases to it. The pH of the buffer solution would be measured using a pH meter or indicator paper before and after the addition of each acid or base. The change in pH would indicate the effect of the acid or base on the buffer solution. This process would need to be repeated for each type of acid and base being tested. The results could be compared to determine which types of acids and bases have the greatest impact on the pH of the buffer solution.
19 - How does the concentration of a solution affect the absorbance of light by a colored compound?
Prepare a series of solutions with varying concentrations of the colored compound. Use a spectrophotometer to measure the absorbance of light by each solution at a specific wavelength. Plot the absorbance values against the concentration of the colored compound to create a calibration curve. Use this curve to determine the concentration of the colored compound in an unknown solution by measuring its absorbance and comparing it to the calibration curve. This process would need to be repeated for each colored compound being tested.
20 - Can the concentration of ammonia in a solution be determined using acid-base titration?
Prepare a standardized solution of a known concentration of acid or base. A sample of the ammonia solution would be titrated with the acid or base solution until the endpoint is reached, indicating that all the ammonia has reacted with the acid or base. The concentration of ammonia in the solution can then be calculated based on the volume and concentration of the acid or base solution used in the titration. This process would need to be repeated for each ammonia solution being tested.
21 - Investigating the effect of different types of buffers on the pH of a solution.
Prepare solutions of different buffers and measure their pH using a pH meter. Then, add a small amount of acid or base to each solution and measure the change in pH. The buffer that maintains the pH closest to its original value would be the most effective. This process would need to be repeated for each type of buffer being tested. The results could be graphed to visually compare the effectiveness of each buffer.
22 - How does the concentration of a solution affect the rate of reaction between iodine and sodium thiosulfate?
Prepare solutions of different concentrations of sodium thiosulfate and iodine. The reaction between the two solutions can be timed and the rate of reaction calculated for each concentration. The results can be graphed to show the relationship between concentration and reaction rate. This experiment would need to be repeated multiple times to ensure accuracy and to account for any experimental error.
23 - Can the concentration of a metal ion in a solution be determined using complexometric titration?
Prepare a standardized solution of a chelating agent, such as EDTA, of a known concentration. A sample of the metal ion solution would be titrated with the chelating agent until the endpoint is reached, indicating that all the metal ions have reacted with the chelating agent. The concentration of the metal ion in the solution can then be calculated based on the volume and concentration of the chelating agent used in the titration. This process would need to be repeated for each metal ion being tested.
24 - Investigating the effect of the length of a chain on the rate of esterification.
Set up an experiment in which different lengths of chains are used in the esterification reaction. The reaction could be monitored by measuring the amount of product formed over time using a spectrophotometer or by analyzing the product using gas chromatography. The rate of esterification could be calculated by determining the slope of the reaction curve. Comparing the rates of esterification for the different chain lengths would determine the effect of chain length on the reaction rate.
25 - How does the pH of a solution affect the rate of reaction between sodium thiosulfate and hydrochloric acid?
Set up a series of solutions with varying pH levels using hydrochloric acid and sodium thiosulfate. The reaction between the two chemicals would be timed and the time taken for the solution to turn cloudy would be recorded. The experiment would need to be repeated multiple times for each pH level to ensure accuracy. The data collected would then be used to plot a graph of the reaction rate against pH level, allowing for the relationship between pH and reaction rate to be determined.
26 - Can the concentration of a solution be determined using gravimetric analysis?
In gravimetric analysis, a known mass of the substance being analyzed is dissolved in a solvent and then reacted with a reagent that forms a precipitate with the substance of interest. The precipitate is then filtered, dried, and weighed to determine its mass. From this, the mass of the original substance can be calculated using stoichiometry. Therefore, to determine the concentration of a solution using gravimetric analysis, a known volume of the solution would need to be evaporated to dryness, and the resulting solid would be weighed. The mass of the solid would then be used to calculate the concentration of the original solution.
27 - Investigating the effect of different types of surfactants on the emulsification of oil and water.
Create a series of oil and water emulsions using different types and concentrations of surfactants. The emulsions could be visually inspected for stability and the time it takes for the oil and water to separate could be recorded. The effectiveness of each surfactant in emulsifying the oil and water could be compared by analyzing the data collected. Additionally, the size and distribution of the droplets in the emulsion could be measured using microscopy to gain a more detailed understanding of the emulsification process.
28 - How does the concentration of a solution affect the rate of reaction between potassium permanganate and hydrogen peroxide?
Set up a series of experiments in which different concentrations of potassium permanganate and hydrogen peroxide are mixed together. The reaction rate could be measured by tracking the change in color of the solution over time, as the potassium permanganate is reduced. The concentration of the reactants could be varied by diluting them with water, and the reaction rate could be measured for each concentration. The results could then be plotted on a graph to show the relationship between concentration and reaction rate.
29 - Can the concentration of sulfate ions in a solution be determined using gravimetric analysis?
To determine the concentration of sulfate ions in a solution using gravimetric analysis, a known volume of the solution would be evaporated to dryness to obtain the sulfate ions in solid form. The mass of the solid sulfate would be measured and compared to the mass of the original sample to determine the percentage of sulfate ions present. This process would need to be repeated for multiple samples of the solution to ensure accuracy and precision in the results.
30 - Investigating the effect of different types of acids and bases on the rate of reaction between hydrochloric acid and sodium thiosulfate.
Set up an experiment in which hydrochloric acid and sodium thiosulfate are mixed with different types and concentrations of acids and bases. The reaction between the two chemicals would produce a yellow precipitate of sulfur, which would gradually become less visible as the reaction progresses. The time taken for the precipitate to disappear could be measured and used to calculate the rate of reaction. Comparing the rates of reaction for the different groups would determine the effect of the acids and bases on the reaction between hydrochloric acid and sodium thiosulfate.
31 - Investigating the effects of different types of catalysts on the rate of a chemical reaction.
Set up an experiment in which a chemical reaction is carried out with different catalysts. The reaction should be monitored using a suitable method such as spectrophotometry or gas chromatography to determine the rate of the reaction. The same reaction should be carried out with each catalyst, and the results should be compared to determine the effect of the catalyst on the rate of the reaction. Control variables such as temperature, pressure, and concentration of reactants should be kept constant to ensure accurate results.
32 - How does the concentration of a reactant affect the rate of a chemical reaction?
Conduct a series of experiments in which the concentration of the reactant is varied while keeping all other variables constant. The rate of the chemical reaction can be measured by monitoring the change in concentration of the reactant or product over time. A graph can be plotted to show the relationship between the concentration of the reactant and the rate of the reaction. This can be used to determine the rate law for the reaction, which can then be used to predict the rate of the reaction under different conditions.
33 - Investigating the properties of different types of acids and bases and their behavior in different solutions.
Conduct experiments in which different types of acids and bases are added to different solutions, such as water or other acids/bases. The behavior of the acids and bases can be observed, such as whether they dissolve or react with the solution, and the pH of the solution can be measured. The properties of the acids and bases, such as their strength and reactivity, can be compared based on their behavior in the different solutions. This could also involve testing the effect of different concentrations of the acids and bases on the pH of the solution.
34 - How does the temperature affect the solubility of a solute in a solvent?
Prepare a solution of the solute in the solvent at a known concentration and temperature. The solution would then be cooled or heated to different temperatures and the solubility of the solute in the solvent would be measured at each temperature. This could be done by adding a known amount of the solute to the solvent at each temperature and measuring the amount of solute that dissolves. The results could be plotted on a solubility curve to show the relationship between temperature and solubility.
35 - Investigating the properties of different types of polymers and their behavior in different environments.
Conduct experiments in which different types of polymers are exposed to different environmental conditions, such as temperature, humidity, and UV radiation. The behavior of the polymers could be observed and measured using techniques such as tensile testing, thermal analysis, and microscopy. Comparing the properties and behavior of the different polymers in different environments would provide insights into their suitability for various applications.
Get expert help with your IB Chemistry
The world's leading online IB Chemistry tutoring provider trusted by students, parents, and schools globally.
4.92 /5 based on 480 reviews
36 - How does the concentration of a solute affect the osmotic pressure of a solution?
Set up a series of solutions with varying concentrations of the solute and measure the osmotic pressure of each solution using an osmometer. The osmotic pressure can be calculated by measuring the change in pressure as the solution is introduced to a semi-permeable membrane. The results can then be plotted on a graph to determine the relationship between solute concentration and osmotic pressure. This experiment could be repeated with different solutes to compare their effects on osmotic pressure.
37 - Investigating the properties of different types of surfactants and their behavior in different solutions.
Conduct experiments in which different types of surfactants are added to different solutions, such as water or oil. The behavior of the surfactants can be observed, including their ability to reduce surface tension and form micelles. The properties of the surfactants can also be tested, such as their solubility in different solvents and their stability under different conditions. The results of these experiments can be used to compare the effectiveness of different surfactants in different applications, such as in cleaning products or in the production of emulsions.
38 - How does the temperature affect the conductivity of an electrolyte solution?
Conductivity measurements of an electrolyte solution would need to be taken at different temperatures using a conductivity meter. The temperature of the solution can be controlled using a water bath or other temperature control device. The conductivity readings can be plotted against temperature to determine the effect of temperature on conductivity. The experiment would need to be repeated multiple times to ensure accuracy and consistency of results.
39 - Investigating the properties of different types of metal alloys and their behavior under different conditions.
Conduct experiments on different types of metal alloys under varying conditions such as temperature, pressure, and exposure to different chemicals. The properties of the alloys such as strength, ductility, and corrosion resistance could be measured and compared to determine their behavior under different conditions. This would require specialized equipment such as a tensile testing machine and a corrosion testing apparatus. The results of these experiments could be used to optimize the use of different alloys in various applications.
40 - How does the concentration of a solution affect the boiling and freezing points of the solvent?
Conduct an experiment in which different concentrations of a solution are prepared and their boiling and freezing points are measured using a thermometer. The data collected can be used to create a graph showing the relationship between concentration and boiling/freezing point. This graph can be used to determine the effect of concentration on the boiling and freezing points of the solvent. Control variables such as pressure and volume of the solution should be kept constant throughout the experiment.
41 - Investigating the properties of different types of gas laws and their behavior under different conditions.
Conduct experiments using different gases, such as helium, nitrogen, and oxygen, under varying conditions of temperature and pressure. The behavior of the gases could be observed using equipment such as pressure gauges and thermometers. The data collected could then be analyzed to determine the properties of each gas and how they behave under different conditions. This could include measuring the volume of gas at different pressures, or the pressure of gas at different temperatures. The results could then be used to develop mathematical models of gas behavior, such as the ideal gas law.
42 - How does the concentration of a solution affect the rate of diffusion and effusion?
Set up a series of experiments in which solutions of varying concentrations are placed in separate compartments of a diffusion or effusion apparatus. The rate of diffusion or effusion could be measured by tracking the movement of a dye or gas through a semi-permeable membrane separating the compartments. The rate of diffusion or effusion could then be compared across the different concentrations to determine the effect of concentration on the rate of diffusion or effusion. Control variables such as temperature and pressure would need to be kept constant throughout the experiments.
43 - Investigating the properties of different types of nuclear reactions and their behavior under different conditions.
Conduct experiments with different types of nuclear reactions, such as fission and fusion, under varying conditions such as temperature, pressure, and reactant concentration. The behavior of the reactions can be observed and recorded, and data can be analyzed to determine the properties of each type of reaction. This could include factors such as energy release, reaction rate, and byproducts produced. The results of these experiments can be used to better understand the behavior of nuclear reactions and their potential applications.
44 - How does the temperature affect the viscosity of a liquid?
Measure the viscosity of a liquid at different temperatures using a viscometer. The temperature of the liquid can be controlled using a water bath or other heating/cooling apparatus. The viscosity can be measured by timing how long it takes for the liquid to flow through the viscometer at each temperature. The results can be plotted on a graph to show how the viscosity changes with temperature. This can help determine the optimal temperature for the liquid's intended use or provide insight into the physical properties of the liquid.
45 - Investigating the properties of different types of organic compounds and their behavior under different conditions.
Conduct a series of experiments to investigate the properties of different types of organic compounds. This could involve testing their solubility in different solvents, their reactivity with other compounds, their melting and boiling points, and their behavior under different conditions such as heat or pressure. The results of these experiments could be used to develop a better understanding of the behavior and properties of organic compounds, which could have applications in fields such as medicine, agriculture, and materials science.
46 - How does the concentration of a solution affect the pH of the solution?
Prepare solutions of varying concentrations of an acidic or basic substance, such as hydrochloric acid or sodium hydroxide. The pH of each solution would be measured using a pH meter or indicator paper. The results would be recorded and analyzed to determine the relationship between the concentration of the solution and its pH. A graph could be created to visualize this relationship.
47 - Investigating the properties of different types of electrochemical cells and their behavior under different conditions.
Set up different electrochemical cells using different electrodes and electrolytes. Measure the voltage and current produced by each cell under different conditions such as temperature, concentration of electrolyte, and electrode surface area. Analyze the data to determine the behavior of each cell and compare their properties. This could include calculating the cell potential, determining the rate of reaction, and identifying any limitations or advantages of each type of cell.
48 - How does the concentration of a solution affect the color and absorption spectrum of a chromophore?
Prepare a series of solutions with varying concentrations of the chromophore. The absorption spectra of each solution could be measured using a spectrophotometer, and the color of each solution could be observed visually. By comparing the absorption spectra and colors of the different solutions, the relationship between concentration, color, and absorption spectrum of the chromophore could be determined. This could be further analyzed using mathematical models to predict the absorption spectrum and color of solutions with different concentrations of the chromophore.
49 - Investigating the properties of different types of covalent compounds and their behavior under different conditions.
Conduct experiments on different covalent compounds under varying conditions such as temperature, pressure, and pH levels. Observe and record their behavior, including changes in state, solubility, and reactivity. Analyze the data to determine the properties of each compound and how they respond to different conditions. This could involve using techniques such as spectroscopy, chromatography, and mass spectrometry to identify and characterize the compounds. The results could be used to develop a better understanding of the behavior of covalent compounds and their potential applications in various fields.
50 - How does the temperature affect the rate of diffusion and effusion?
Set up an experiment in which a gas is released in a container at a constant rate and the time it takes for the gas to diffuse or effuse through a small opening is measured at different temperatures. The temperature can be varied by immersing the container in a water bath of different temperatures. The rate of diffusion or effusion can be calculated based on the time taken for the gas to pass through the opening, and the temperature can be varied to determine its effect on the rate of diffusion or effusion. The results can be plotted on a graph to visualize the relationship between temperature and the rate of diffusion or effusion.
51 - Investigating the properties of different types of intermolecular forces and their behavior under different conditions.
Conduct experiments using different substances with different types of intermolecular forces, such as hydrogen bonding, dipole-dipole interactions, and London dispersion forces. The substances could be tested under different conditions, such as temperature and pressure, to observe how the intermolecular forces affect their behavior. The results could be analyzed to determine the properties of each type of intermolecular force and how they interact with each other. This could lead to a better understanding of the behavior of substances in various environments.
52 - How does the concentration of a solution affect the rate of an acid-base titration?
Prepare a standardized solution of a strong acid or base of known concentration. A sample of the solution being tested would be titrated with the acid or base solution until the endpoint is reached, indicating that all the acid or base has reacted with the solution. The concentration of the solution being tested can then be calculated based on the volume and concentration of the acid or base solution used in the titration. This process would need to be repeated for solutions of varying concentrations to determine the effect of concentration on the rate of the acid-base titration.
53 - Investigating the properties of different types of coordination compounds and their behavior under different conditions.
Conduct experiments to observe the behavior of different coordination compounds under varying conditions such as temperature, pH, and concentration. The properties of the compounds such as color, solubility, and stability could be measured and compared. The results could be analyzed to determine the effect of the different conditions on the behavior of the coordination compounds. This could provide insight into the potential applications of these compounds in various fields such as medicine or materials science.
54 - How does the concentration of a solution affect the equilibrium constant of a chemical reaction?
Conduct a series of experiments in which the concentration of a reactant or product is varied while keeping other variables constant. The equilibrium constant of the chemical reaction can then be calculated using the concentrations of the reactants and products at equilibrium. This process would need to be repeated for different initial concentrations of the reactants to determine the effect of concentration on the equilibrium constant. Graphing the data would help visualize the relationship between concentration and equilibrium constant.
55 - Investigating the properties of different types of chromatography and their behavior in different separation techniques.
Conduct a series of experiments using different types of chromatography, such as paper chromatography, thin-layer chromatography, and gas chromatography. Each experiment would involve separating a mixture of substances using the chosen chromatography technique and analyzing the results to determine the effectiveness of the technique in separating the substances. The behavior of the chromatography technique could be studied by varying the solvent used, the type of stationary phase, and other experimental conditions. The results of the experiments could be compared to determine the most effective chromatography technique for different types of separations.
56 - How does the temperature affect the activation energy of a chemical reaction?
Conduct a series of experiments in which the same chemical reaction is carried out at different temperatures. The activation energy of the reaction can be calculated by measuring the rate of the reaction at each temperature and using the Arrhenius equation to determine the activation energy. The results can be plotted on a graph to show the relationship between temperature and activation energy. This would help to determine how temperature affects the rate of chemical reactions.
57 - Investigating the properties of different types of solid-state materials and their behavior under different conditions.
Conduct experiments on different types of solid-state materials, such as metals, ceramics, and polymers, under different conditions such as temperature, pressure, and humidity. The properties that could be investigated include strength, elasticity, conductivity, and thermal expansion. The results of these experiments could be used to compare the behavior of different materials and to identify the most suitable material for a particular application. The data collected could also be used to develop models and simulations to predict the behavior of materials under different conditions.
58 - How does the concentration of a solution affect the rate of a redox reaction?
Conduct a series of experiments in which the concentration of a solution is varied while keeping all other variables constant. The redox reaction could be monitored using a colorimetric assay or by measuring the change in pH of the solution. The rate of the reaction could then be calculated based on the change in absorbance or pH over time. By comparing the rates of the reaction at different concentrations, the effect of concentration on the rate of the redox reaction could be determined.
59 - Investigating the properties of different types of nanomaterials and their behavior under different conditions.
Conduct experiments with different types of nanomaterials, varying their size, shape, and composition, and observe their behavior under different conditions such as temperature, pressure, and exposure to different chemicals. The properties of the nanomaterials, such as their conductivity, reactivity, and strength, could be measured using various techniques such as microscopy, spectroscopy, and mechanical testing. The results could be analyzed to determine the optimal conditions for each type of nanomaterial and to compare their properties to identify the most suitable material for specific applications.
60 - How does the concentration of a solution affect the rate of a precipitation reaction?
Set up multiple solutions of the same reactants with varying concentrations. The rate of precipitation can be measured by tracking the time it takes for the precipitate to form or by measuring the amount of precipitate formed over a set period of time. By comparing the rates of precipitation in the different solutions, the effect of concentration on the rate of the reaction can be determined. Control variables such as temperature and stirring rate would need to be kept constant.
Remember to come up with your own original IA topic and check it with your teacher. It should be practical to conduct and relevant to the syllabus. This is a great opportunity to develop your personal interests, while advancing your knowledge of the chemistry curriculum. Online tutors agree that this list is quite extensive and can help IB students a lot with their IB Chemistry IA.
TutorChase's IB Chemistry Study Notes , IB Past Papers and IB Chemistry Questions are the perfect resource for students who want to get a 7 in their IB Chemistry exams and also prepare for the internal assessment. They are completely free, cover all topics in depth, and are structured by topic so you can easily keep track of your progress.
How is the IA graded?
The IA is worth 20% of the final grade for the IB chemistry course, whether you are studying at Higher or at Standard Level. This applies for assessments both before and after May 2025. It is graded by the student’s teacher, who is trained and certified by the International Baccalaureate organization. The report is then sent to a moderator, who will check that the report adheres to the IB guidelines and that the grade awarded is appropriate.
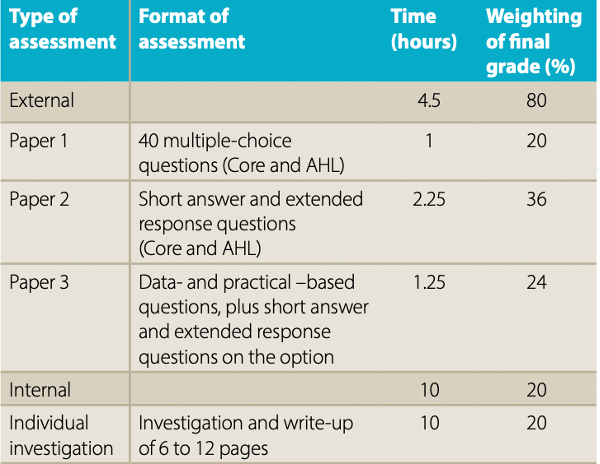
Source: IB Chemistry Subject Brief, pre-May 2025
In summary, the IA in the IB is an opportunity for students to demonstrate their understanding of the chemistry curriculum, as well as their ability to conduct independent research. It consists of a laboratory report and a reflective statement, and is worth 20% of the final grade for the course. To prepare for the assessment, students should ensure that they understand the material covered in their IB chemistry course , practice writing lab reports, and seek feedback from their teachers or tutors.
Need help from an expert?
The world’s top online tutoring provider trusted by students, parents, and schools globally.
Study and Practice for Free
Trusted by 100,000+ Students Worldwide
Achieve Top Grades in Your Exams with our Free Resources:
STUDY NOTES
Expert-crafted notes designed to make learning the material engaging and clear.
PRACTICE QUESTIONS
Comprehensive questions to boost your revision and exam preparedness.
PAST EXAM PAPERS
Extensive collection of previous exam papers for effective revision.
Need Expert Help?
If you’re looking for assistance with IB Chemistry, get in touch with the TutorChase team and we’ll be able to provide you with an expert IB Chemistry tutor . We’ll be there every step of the way!

Professional tutor and Cambridge University researcher

Written by: Charles Whitehouse
Charles scored 45/45 on the International Baccalaureate and has six years' experience tutoring IB and IGCSE students and advising them with their university applications. He studied a double integrated Masters at Magdalen College Oxford and has worked as a research scientist and strategy consultant.
Related Posts

IB Biology IA: 60 Examples and Guidance

IB Maths IA: 60 Examples and Guidance

IB Chemistry: A Complete Guide (2024)

Hire a tutor
Please fill out the form and we'll find a tutor for you
- Select your country
- Afghanistan
- Åland Islands
- American Samoa
- Antigua and Barbuda
- Bosnia and Herzegovina
- Bouvet Island
- British Indian Ocean Territory
- Brunei Darussalam
- Burkina Faso
- Cayman Islands
- Central African Republic
- Christmas Island
- Cocos (Keeling) Islands
- Congo, The Democratic Republic of the
- Cook Islands
- Cote D'Ivoire
- Czech Republic
- Dominican Republic
- El Salvador
- Equatorial Guinea
- Falkland Islands (Malvinas)
- Faroe Islands
- French Guiana
- French Polynesia
- French Southern Territories
- Guinea-Bissau
- Heard Island and Mcdonald Islands
- Holy See (Vatican City State)
- Iran, Islamic Republic Of
- Isle of Man
- Korea, Democratic People'S Republic of
- Korea, Republic of
- Lao People'S Democratic Republic
- Libyan Arab Jamahiriya
- Liechtenstein
- Macedonia, The Former Yugoslav Republic of
- Marshall Islands
- Micronesia, Federated States of
- Moldova, Republic of
- Netherlands
- Netherlands Antilles
- New Caledonia
- New Zealand
- Norfolk Island
- Northern Mariana Islands
- Palestinian Territory, Occupied
- Papua New Guinea
- Philippines
- Puerto Rico
- Russian Federation
- Saint Helena
- Saint Kitts and Nevis
- Saint Lucia
- Saint Pierre and Miquelon
- Saint Vincent and the Grenadines
- Sao Tome and Principe
- Saudi Arabia
- Serbia and Montenegro
- Sierra Leone
- Solomon Islands
- South Africa
- South Georgia and the South Sandwich Islands
- Svalbard and Jan Mayen
- Switzerland
- Syrian Arab Republic
- Taiwan, Province of China
- Tanzania, United Republic of
- Timor-Leste
- Trinidad and Tobago
- Turkmenistan
- Turks and Caicos Islands
- United Arab Emirates
- United Kingdom
- United States
- United States Minor Outlying Islands
- Virgin Islands, British
- Virgin Islands, U.S.
- Wallis and Futuna
- Western Sahara

Still have questions? Let’s get in touch.
25 IB Chemistry IA Topic Ideas

We all know that scoring superbly on internal assessments is a great way to boost our IB grades. But how do we get started on a lab report and, crucially, what should we write about? In general, keep in mind that for your Chemist ry IA we want to measuring how changing one variable has an effect on another variable.
If you’re struggling with a topic, consider some easily altered variables (temperature, surface area, concentration) and explore what effect changing these may have on your dependent variable, which should ideally be easy to measure (pH, gas produced, temperature, etc.). However, if you’re still feeling stuck then this week we’re going to take a look at 25 Chemistry IA topic ideas that we hope will spark some ideas for you!
NOTE: These topics are purely meant as inspiration and are not to be chosen blindly. Even though many of these topics led to high scores for some of our graduates in the past, it is important that you listen to the advice of your subject teacher before choosing any topic!
Get Support from A Top Tutor Today
At Lanterna we have hundreds of tutors who smashed Chemistry. They know exactly how to get an 7 in the exam and IA, and can give you tips and tricks on how you can do the same. What are you waiting for? Get your own tutor today !
1, Calculating absolute zero using gas volume
2, exploring the vitamin content of various healthy foodstuffs, 3, studying the dissolved oxygen content of a water body, 4, investigating the concentration of drugs within tablets (this could even be explored across different brands), 5, using calorimetry to determine enthalpy changes/the enthalpy of neutralisation, 6, determining the activation energy of a reaction, 7, exploring conditions under which lipase can be denatured, 8, exploring the speed of various chemical reactions using a spectrometer, 9, what is the activation energy needed to decompose a compound such as hydrogen peroxide, 10, find the calcium content of different milk brands, 11, explore the optimal conditions to electroplate metals by considering a variety of external factors, 12, using thermal decomposition try to identify the type of salt present in some compound, grab free chem resources , 13, explore and distinguish between methanol and ethanol using iodine and sodium hydroxide solutions, 14, use paper chromatography to separate pigments present in a tree leaf, 15, explore the effect of temperature on the strength of a ferromagnet, 16, describe the effect of varying temperature on the formation of rust on steel, 17, measure the amount of free caffeine in different coffee, tea, or other drink brands, 18, can different fruits be used in order to chelate heavy metals from polluted water sources, 19, an analysis into the different edta contents of a variety of shower cleaners, 20, speed of denaturation in various animal proteins using uv light, 21, synthesizing the sweetener dulcin from paracetamol, 22, considering and exploring the effectiveness of various brands of salts for snow removal, 23, using paper chromatography to analyse the various dyes present in different brands of jelly candy. , 24, measuring the change in iron levels of avocados as they go through different ripening stages, 25, measuring the energy content of a packet of cheetos.
For getting the ball rolling after you’ve grabbed a great Chemistry IA topic, there are numerous great websites with tips . But non-chemists scientists out there please don’t feel left out! Check out our previous blog about generating some inspiration for your reports! And finally, if you do require any additional assistance with tackling your IA’s then our online private tuition services might be perfect for you…
Check Out Our 30 Bio IA Topic Ideas !
Share article links
Related Articles

- Most Popular
30 IB Biology IA Topic Ideas!
Are you struggling with choosing your topic for your IB Biology IA? Don’t worry, we’ve all been there. Finding a topic is one of the – if not THE – most important part of writing your IA, so we want to make sure that you get it right! Luckily, there are so many great topics […]
20 IB Physics IA Topic Ideas!
Choosing where to start with an IA can be the hardest part, and this is definitely true for the Physics IA. We know that our topic has to be somewhat related to the syllabus, but where should we focus? Thankfully, we’ve asked some of our favourite IB graduates for some of the ideas they pursued! […]
25 History IA Topic Ideas!
Are you about to start your History internal assessment? We know the struggle. One of the most difficult parts about the task is finding a good History IA topic because it feels like you can just write about anything. The IB breaks it down into 7 main different types of topics that you can choose, […]

- Saesha Grover
IB Chemistry Internal Assessment Solved: A Guide to Acing Your Chemistry IA
The IB Chemistry IA is often dreaded by students, as they frequently envision completing notoriously difficult experiments and spending hours at a desk trying to formulate topic ideas. Given the complexity of the Chemistry subject, with numerous topics and subtopics that demand intricate knowledge and application, securing a high grade on your Internal Assessment can be the make-or-break factor for achieving your desired score in final exams and finding solace as you enter the exam room. In this guide, I will not only break down how to secure a strong Grade 7 on your Chemistry IA, but I will also share how this can be achieved in both an experimental and data-based IA . The guiding principle: keep it simple, keep it clear.
1.0 Picking your research question:
1.1: Keeping it Simple
Selecting an appropriate research question is an important step in ensuring success in your IB Chemistry IA. I can assure you that there is no need to opt for an overly complicated research question – a principle I adhered to, resulting in a 22/24 on my Chemistry HL Internal Assessment ( keep it simple, keep it clear! ). The rationale behind this, is that your goal is to showcase a thorough understanding of your chosen topic throughout your IA, rather than inadvertently revealing gaps in your knowledge.
1.2: Check out the Academic Literature
Furthermore, I strongly advocate for choosing a topic with plenty of existing academic literature. This will provide you with several sources and reports you can draw upon in your conclusion and analysis within your Internal Assessment . This allows you to make effective comparisons between the outcomes of your experiment and established findings .
1.3: Don’t Neglect a Data-based IA
In addition, I want to emphasise the potential of doing a data-based IA. In a data-based IA, you may opt for running a virtual experiment, or analysing data on a topic to answer a selected research question. If you are doing the latter, note that there are various online sources for data for Chemistry Internal Assessments, such as ChemSpider, the CRC Handbook, and PubChem. These platforms offer a lot of varied information that can be harnessed for data-based investigations.
2.0 Personal Engagement:
In your introduction, ensure that you demonstrate your personal engagement with the topic you have selected. Your personal engagement is essentially what sparked your interest in the topic you explore in your Internal Assessment. Reveal what genuinely ignited your interest, avoiding elaborate stories . Whether it's a concept learned in class or a discovered exception (e.g., trends in melting points, electronegativity, boiling points or solubility properties of elements), keep it simple, keep it clear.
3.0 Risk Assessment and Variables:
For the risk assessment and variables section of your Internal Assessment, make sure you address safety, ethical AND environmental concerns if you are doing an experimental IA. However, if you are doing a data-based IA , you can say that these were not of concern in your investigation.
When discussing the different variables in your investigation, do not just state what they are. Discuss how these variables will be manipulated and provide clear and convincing justification for why you are manipulating the variable in this way. Ensure you state which unit you are using for your experiment, and if you are not using conventional SI units, justify why .
For instance, in a database IA, do your database sources mainly cite temperature values in degrees Celsius, and is that the reason for why you are using that unit?
4.0 Methodology:
4.1: Creating a Methodology for a Databased IA
When conducting a database-based Internal Assessment (IA), a common misconception is that this type of investigation does not require a detailed methodology section. However, this is arguably one of the most important parts of your IA . In your methodology for this type of IA, clearly outline the values you plan to extract from the databases that you find, and explain how you will ensure accuracy in your experiment while presenting your data. And of course, remember to keep it simple, keep it clear .
To further enhance your databased IA , create a section beneath your methodology that serves the purpose of justifying the selection of databases for your investigation . Here, delve into discussions about the reliability of authors of the databases, and try to ascertain whether the sourced data has been cross-checked .
4.2: An Extra Tip I have for Databased IAs
An additional recommendation I have for your database IA is to actively compare multiple sources for a specific data point value that you are researching. For instance, different sources may state different values for the melting point of an element. By doing so, you can identify discrepancies between source values. This comparative analysis will ultimately contribute significantly to the overall clarity of your IA ( keep it simple, keep it clear! ).
5.0 Data Collection:
5.1: Clarity
In your data collection, I highly recommend being wary of your unit representation and notation. Your units should be in your table headings , not inside the rest of the table as well. Small things like these can go a long way. Also, make it clear when you are referring to raw versus processed data, and include sample calculations in the processed data section of your IA .
5.2: Data Based IA
In some of the data you find for an IB Chemistry IA, you may be given a range of values in tabulated form, especially instances where you require a property of a chemical unit.

In instances like these, you might make the decision to take the midpoint of your data point values. If you do this, again, be upfront, and explain why you made this decision. If something does not go your way, you can insert phrases such as “in view of this observation” or “due to this' and this helps your examiner follow your line of thinking .
6.0 Conclusion:
I recommend reminding the examiner of the purpose of your investigation at the start of your conclusion. Refer to your figures! What did Figure 1 tell you? Is this consistent with literature? Why or why not? Was there an exception in the results that you found? Use this information to discuss some of the weaknesses or limitations of your investigation. Additionally, in your figures within your IA, try and include error bars if relevant, as this allows you to discuss things like the lack or presence of error bar overlap, as well as the size of error bars and what this means in the context of the reliability of the trends that you observe in your investigation .
7.0 Extensions:
Something that I have noticed a lot of students miss in their investigations or often do not go into adequate detail over, is the extensions in their investigation. I would not brush past this in a couple of sentences, but rather would recommend writing in extensions to your IA that can tie easily onto your research question. I would also recommend explaining why or how the extensions would develop your understanding of the investigation.
In essence, “keep it simple, keep it clear” , is a mantra you should keep in mind throughout writing up your IB Chemistry Internal Assessment. This approach ensures not only a smoother IA writing process, but also a greater likelihood of achieving success in this assessment, whether it be experimental or databased!
And that's it! Best of luck for the Chemistry Internal Assessment. You’ve got this!!!
- Internal Assessment Guides
Recent Posts
IB Sports Science Internal Assessment Solved: A Guide to Acing Your SEHS IA
The Complete IB Physics Internal Assessment Guide
IB Biology Internal Assessment Solved: A Guide to Acing Your Biology IA

IB Chemistry IA examples
Type a search phrase to find the most relevant Chemistry IA examples for you
Not sure what to search for? You can always look through our example Internal Assessments below for inspiration.
All Chemistry IA Examples
Filter exemplars.
Starting from the May 2025 session, the Chemistry IA requirements have changed. We created a couple of exemplars to show you how the new IA should look like. It's OK to refer to the old Chemistry IA exemplars (since the new IA is quite similar) for inspiration/ideas, but make sure to follow the new requirements.
What is the effect of the temperature (30, 40, 50, 60, 70 °C) on the buffering capacity (mol/dm3) of phosphate buffer, as measured using titration with 0.1 mol/dm3 HCl?
How does the pka value of certain weak acids present in food (2.85, 2.99, 3.13, 4.10, 4.76) affect the loss in mass of calcium carbonate (in grams, measured using an electronic balance, ±0.01 g), and how does the addition of sodium fluoride solution affect this reaction, keeping the concentration and temperature of the acid solutions and sodium fluoride solutions, the time taken for the reaction and the surface area of the calcium carbonate chips controlled, want to get full marks for your ia allow us to review it for you 🎯, what is the effect of boiling in 100°c and filtering using the carbon filter on the hardness of tap water (mol/dm3) as measured using edta complexometric titration, what is the effect of changing temperatures (20, 40, 60, 80, 100 °c) on the amount of iodine remaining in iodized salt (in grams) after exposure to heat during cooking, as measured by an iodometric titration, how does the source of caffeine (lipton earl grey, lipton yellow label, remsey earl grey, milton earl grey classic) affect its content (in g), determined using the dcm-facilitated separation funnel technique, fast track your coursework with mark schemes moderated by ib examiners. upgrade now 🚀, how does changing the current [3.0, 3.5, 4.0, 4.5, 5.0 a] in electrolysis affect the mass of products formed at the cathode in the electrolysis of copper and aluminium respectively, how does the distance from the city centre (1.3 km, 3.1 km, 4.1 km, 6 km, 7.1 km) affect the number of millimoles of the dissolved oxygen concentration in city ponds as measured by the winkler titration method, what is the effect of cooking temperature (10, 25, 40, 55, 70 °c) of ipomoea aquatica on its concentration of iron (ii) ions, as measured by the titration volume of 0.002m potassium permanganate solution required to completely oxidize iron (ii) ions in a solution of ipomoea aquatica after cooking it for 10 minutes, how effective are natural antacids such as cumin and fennel seeds in comparison to synthetic antacids containing calcium carbonate and simethicone in neutralizing excess hydrochloric acid using back titration, what effect does the temperature of the solution (k) have on the rate of decomposition (cm3 s -1) of carbonic acid (h2co3) to carbon dioxide (co2) and water (h2o) and therefore what is the activation energy (kjmol-1) of this reaction, to what extent does the activation energy of an iodine clock reaction change when catalyzed by copper (ii) sulfate (cuso4), investigating the caffeine content (mg) in different brands of black tea (tetley black tea, american breakfast black tea, filiz black tea, & red label tea) using dichloromethane in a separating funnel, what is the correlation between electric current and the amount of mass that is electroplated, and, hence, is there an optimum current, how does the increase in temperature (30.0oc, 35.0oc, 40.0oc, 45.0oc, 50.0oc) of a solution of tetraborate (b4o5(oh)4) 2- (aq) effect the volume (cm3 ) of 0.100m hcl(aq) required to titrate it, and on the solubility equilibrium constant (ksp), using titration, how does temperature – 283, 293, 303, 313, 323, 333 (k ± 0.1) – affect the equilibrium constant (kc) of the iron (iii) thiocyanate solution equilibrium, determined by measuring the absorbance (± 0.01) of a 470 nm wavelength using a vernier colorimeter., how does temperature (at 25 oc – 65 oc with 10oc intervals) affect the equilibrium constant for the reversible reaction between tetrachlorocobalt(ii) [co(cl)4]2- and hexaaquacobalt(ii) [co(h2o)]2+, how does the varying concentrations of hydrogen peroxide affect the initial and average redox reaction rate between sodium thiosulfate and hydrogen peroxide in an alkaline condition by measuring the change in ph using a ph meter, the effect of temperature on buffering capacity of a phosphate buffer, the effect of halogen electronegativity, number of halogen atoms, and distance from the acidic hydrogen on the pka of carboxylic acids., which is the optimal incubation temperature in between 2-35 ∞c for the oxidation of ethanol in order to figure out the concentration of ethanol in albani odense classic beers by performing a redox back titration with sodium thiosulfate and how does that value compare to the theoretical one, which appears in the beer can, how does temperature affect the critical micelle concentration (cmc) of the common ionic surface active agent (surfactant) sodium dodecyl sulphate measured by using the change in the rate of conductivity as concentration is increased, what is the difference in activation energy (kjmol") between the uncatalyzed and iron (ii) sulfate (feso#) catalyzed oxidation of iodide ions by potassium iodate (kio$) in the iodate variation of the iodine clock reaction, determined by analyzing the rate of reaction at different temperatures (10.0°c, 20.0°c, 30.0°c, 40.0°c, 50.0°c)n, how does the temperature of high-calcium milk affect its calcium concentration (mg/cm3) as measured through a complexometric titration utilising eriochrome black t (ebt) indicator, how do different shelf lives (t = 6 months, 12 months, 24 months, 36 months, 48 months) of common polish cooking oils: [flaxseed oil (lenvitol), sesame oil (diamond), canola oil (kujawski), olive oil (antico frantoio), coconut oil (enerbio)] affect their iodine value (𝐼𝑉 [𝑔] ∈ [12.39 ∪ 194.72] ± 1.3), measured by back-to-back titration of the oil sample dissolved in acetone (c3h6o, 99.5%) reacted by iodine solution (ki3, 0.050 𝑚𝑜𝑙 ∙ 𝑑𝑚−3) with sodium thiosulfate (na2s2o3, 0.100 𝑚𝑜𝑙 ∙ 𝑑𝑚−3) in the presence of a starch indicator under constant pressure 1013.00 hpa ± 0.13 hpa and in air temperature 20.00°c ± 0.05°c, how does the initial temperature of water (50.0°c, 60.0°c, 70.0°c, 80.0°c, 90.0°c, 100.0°c) in which two kombu varieties (s. japonica var. japonica and s. japonica var. ochotensis) are steeped affect its calcium content (in mg) in the prepared kombu-dashi, determined by complexometric titration with disodium edetate dihydrate 0.125 mol l-1 solution and eriochrome black t indicator, determining the optimal temperature for the fishes in my aquarium based on their oxygen need by using the winkler’s method., how does the ph of a water sample affect its biological oxygen demand over a course of 5 days, using the winkler method, how does increasing the temperature of a 0.01m naoh solution affect the rate of hydrolysis of 0.01m acetylsalicylic acid as measured by the spectrophotometry of iron (iii) salicylate, how does the temperature of a 1.5m copper sulfate solution electrolyte (40oc, 50oc, 60oc, 70oc, 80oc) impact the mass of copper deposited after a set timeframe of 30 minutes of electrolysis with zinc electrodes, with reference to the theoretical values given by faraday’s laws of electrolysis, which of the 5 processed orange juice brands in the market has the least concentration of ascorbic acid measured in moldm-3 using acid-base titration with a solution of 0.05 moldm-3 sodium hydroxide, how does changing the temperature (10°c, 25°c, 40°c, 55°c, 70°c) at which kangkung is cooked at affect its concentration of iron (ii) ions, as measured by the volume of 0.002m potassium permanganate needed to completely oxidise iron (ii) ions in a solution of water spinach, while keeping cooking time constant at 10 minutes, how does an increase in ionic radii (10-12 m) of central metal ions that are period 4 transition metals (co2+, cu2+, ni2+, mn2+, fe2+) affect the energy difference in the d-orbital splitting (kj/mol) of color complexes calculated through highest absorbance on a spectrophotometer, what is the effect of changing the cooking temperature (25.0 °c, 55.0 °c, 70.0 °c, 85.0 °c, and 100.0 °c) on the amount of iron (fe2+) that leaches from spinacia oleracea, using a redox titration, determine whether the dissociation constant (pka) of 3 weak acids, ethanoic acid, propanoic acid, and butanoic acid, changes at 7 different temperatures (303 k,308 k,313 k, 318k,323k, 328k, 333k), what is the effect of temperature on the light output of glow sticks over time, how do varying concentrations (%10, %30, %50, %70, %96) of aqueous solutions of ethanol (c2h5oh) have an effect on the rate (kpa min-1 ) of reaction in the dehydration of ethanol, recording the amount of pressure (kpa) released by a gas pressure sensor (± 0.01 kpa) under the same time (min) periods, with catalyst titanium dioxide (tio2), how does the temperature (20℃, 40℃, 60℃, 80℃, 100℃) of heating dairy milk affect the amount (mg) of ionic calcium (ca2+) present in the milk determined using a complexometric titration, what is the antioxidant content [μmol/dm3 of filtrate] in superfoods: fucus, chlorella, spirulina, matcha, barley, wheatgrass and kale, how significant is the variation in calcium content (mg) of milk depending on the stage of processing it has undergone (raw, pasteurized, or uht), as determined by the complexometric titration against edta, which d-block metal catalyst from zinc(ii) sulfate(znso4) and manganese(ii) sulfate(mnso4) will lower the activation energy (kjmol-1) the most in the reaction between sodium thiosulfate and iron(iii) nitrate, by calculating the time taken for the completion of the reaction at varying temperatures of 25°c, 30°c, 35°c, 40°c, 45°c.

20 IB Chemistry IA Topics
An introduction to ib chemistry ia’s.
IB Chemistry IA’s (Internal Assessments) are essays that students in the International Baccalaureate program must write in order to be successful in the program. Each essay examines a different concept in chemistry, and requires comprehensive research and investigation into the subject. While completing the essays is often daunting, they are an invaluable asset to those seeking to excel in the program, as each essay carries a weight of 25% of their overall grade.
The topics for IB Chemistry IA’s vary greatly, ranging from analyzing stoichiometric processes, to studying the effects of light on photosynthesis. Furthermore, each essay must meet the criteria set forth by the International Baccalaureate program, and must follow a specific structure and style. In the following guide, we will present 20 IB Chemistry IA topics, and provide insight and guidance for each one.
The 20 IB Chemistry IA topics we will explore in this guide are:
🎓✍️ Acing Your Internal Assessment Has Never Been Easier! ✍️🎓
Are you struggling with your Internal Assessment ? Let our experts take care of it! We’ve successfully completed hundreds of IA projects across different IB courses, and we know the IB criterium inside out.
🌟 Our writers are all human and do not use CHAT-GPT, ensuring a unique and personalized touch to your project. Plus, our service is 100% confidential and risk-free, so you can trust us with your academic success.
Don’t miss out on this opportunity to secure the grade you deserve! Get started with our IB IA Writing Service today! 💡📚🔝

- Thermochemistry
- Redox Reactions
- Equilibrium
- Acid-Base Chemistry
- Periodic Trends
- Atomic Structure
- Molecular Structure
- Energy and Bonding
- Nuclear Chemistry
- Chemical Equations
- Voltaic Cells
- Descriptive Chemistry
- Quantitative Chemistry
- Bioinorganic Chemistry
- Environmental Chemistry
- Organometallic Chemistry
- Surface Chemistry
- Analytical Chemistry
We hope that this guide will provide an informative and comprehensive look into the topics of IB Chemistry IA’s. With our help, students can gain a greater understanding of the topics and make their essays stand out amongst the rest.
What is an IB Chemistry IA?
IB Chemistry IA’s, also known as Internal Assessments, are a critical part of the International Baccalaureate Chemistry course. It is a project that requires you to undertake a practical experiment and write up your work. This will account for 20-40% of your overall grade.
The experiment is designed to be student-led and it allows you to demonstrate all the key skills you have learnt throughout the year. These include your knowledge of the subject matter, data-gathering, lab safety and technical reporting.
The aim of the assessment is to show the examiner that you can ask a scientific question, plan the apparatus and method, carry out the experiment with precision, analyze and interpret the data, and write up your conclusions.
The report form needs to be completed correctly, so it is important that you familiarize yourself with its structure. This includes a title, hypothesis, materials and methods, results, discussion/conclusion, references and bibliography.
You will be marked on a variety of criteria such as accuracy of data, depth of discussion and style of writing. If you follow the assessment criteria and take the time to plan and write your report carefully, you are sure to do well.
General Tips for Achieving Success With IB Chemistry IA Reports
Essay structure and style, carry out your own research, edit and proofread your work, seek feedback from others, topic 1: preparation of salts.
The preparation of salts is a key part of IB Chemistry IAs. Salts are compounds that are made up of cations and anions, which are oppositely charged particles. When these two particles form a bond, a salt is created. In this topic, you’ll learn about the different types of salts, how to create them in your own lab, and how to approach the topic in your IA.
Types of Salts
There are many different types of salts, some of which include aluminum, ammonium, calcium, magnesium, sodium, and potassium salts. Each salt is unique in its own way and requires different amounts of ions to form them. Understanding the different types of salts will help you accurately depict the preparation of salts in your lab.
Creating Salts in the Lab
In order to successfully create salts, you must first have the cations and anions, acid, and base. To combine all these elements and create a salt, you must use the neutralization reaction. This reaction occurs when an acid reacts with a base and creates water and the salt. To achieve this in the lab, you must measure out the correct amounts of acid and base and mix them together. Once they are mixed, the salt will form and can then be collected.
Approaching the Topic in Your IA
When writing your IA on the topic of salts, there are a few things to keep in mind. First, be sure to include the background and methods used throughout the experiment. You must also be sure to discuss any findings or observations you may have noticed during the experiment. Finally, any conclusion from the experiment must be discussed, as well as any sources of error or uncertainties. Writing the IA following these steps will ensure that your IA is successful.
The second topic is all about acid-base titrations. Titration is a method that is used to determine the concentration of an unknown acid or base solution by adding small amounts of a known standard solution until the reaction is complete. This process is often done using a burette and indicator solution.
When studying titrations in IB Chemistry, you need to make sure you understand the concept of equivalence point. This is the point at which the amount of base added to the acid is exactly equal to the amount of acid originally present. Once you understand this, you can learn to calculate the concentrations of solutions before and after the reaction. It’s also important to understand the concept of end point. This is the point at which the acid-base reaction is complete and no further addition of either solution is required to reach the equivalence point.
In order to approach this topic successfully, it’s important to brush up on your basic knowledge about acids, bases, and pH. It’s also essential to understand different types of indicators and how they are used to determine the end point of the titration. Additionally, it is important to be able to compare titrations to other experiments such as oxidation-reduction titrations. Finally, practice calculating concentrations and finding the amount of solutions needed for an accurate titration.
Topic 3: Kinetics
Kinetics is the study of the rate of chemical reactions. It is a very important topic in IB Chemistry IA’s as it requires students to understand how different variables such as temperature, pressure, reactants and catalysts, affect the speed at which a reaction occurs.
When writing your IA report on kinetics, there are certain things you should focus on. Firstly, it is important to understand the general equation for the rate of a reaction, which is: Rate of reaction = k [reactants]^n. From this equation, you can infer that the rate of the reaction is dependent on the reactant concentrations and catalysts involved.
It is also important to pay close attention to the order of the reaction. The order determines how the rate of the reaction changes depending on the concentration of the reactants. For example, if the order of the reaction is 0, then the rate of the reaction will not be affected by the concentration of reactants; however, if the order is 1, then the rate will increase as the concentration of reactants increases.
Finally, when writing an IA on kinetics, make sure to include a discussion of catalyst mechanisms and their effect on the rate of the reaction. You may also want to include experiments that demonstrate the effects of temperature and other variables on the rate of the reaction. Make sure that you explain all of your results clearly and thoroughly.
Topic 4: Investigating the Entropy Change of a Substance
The entropy change of a substance is a measure of the disorder or randomness of molecules in the reaction. Entropy can be calculated by measuring the heat exchange when the reaction occurs and calculating the pressure, volume, temperature, and total number of molecules.
When investigating this topic, it is important to pay attention to the conditions that the substances are in before and after the reaction. This will help determine the entropy change of the reaction. A change in entropy can tell us whether the reaction is an exothermic or endothermic reaction, as well as other useful information.
To approach this topic, begin by researching the basics of entropy and familiarizing yourself with the equation that must be used, S = k lnW. Then, decide what experiment you would like to do, and how you are going to measure entropy. It is also important to choose a suitable reaction to calculate the entropy change for. Consider the effects of the different compounds in the reaction on the entropy.
Once you have chosen your experiment and set it up, begin to collect data. Depending on the experiment, this can involve measuring the temperature, pressure, and volume, amongst other parameters. Record all of your data meticulously in order to understand the exact changes taking place in the reaction.
Finally, calculate the entropy change of the reaction using the equation S=klnW. This will tell you the entropy change of the reaction, as well as giving you information about whether the reaction is endothermic or exothermic.
Topic 5: Electrochemistry
Electrochemistry is a branch of chemistry which studies the chemical changes caused by electric current, or the interchange of chemical and electrical energy. It includes topics such as galvanic cells, electrolysis, redox reactions and acid-base titrations.
To write a successful IB Chemistry IA regarding electrochemistry, it can be helpful to focus on the different variables that affect the rate of a reaction. These variables generally include the concentration of the solution, temperature, surface area of the reactants, pressure, and the presence of a catalyst. By studying how these variables impact the electrochemical reaction you can gain insight into the rate of the reaction, the amount of product formed, and the energy produced.
It can also be beneficial to discuss the structure of different electrochemical cells, such as galvanic, electrolytic, and fuel cells. Additionally, an IB Chemistry IA on electrochemistry should also explore topics within electroanalytical chemistry such as voltammetry, potentiometry, and amperometry.
When approaching this topic, it is important to research all the necessary information and become familiar with the different processes involved in electrochemistry. It will also be useful to create diagrams and sketches to help explain the different concepts covered in your IA.
Topic 6: Investigating the Effect of Temperature on the Rate of Reaction
In this topic, you will be investigating how temperature affects the rate of a chemical reaction. It is important to understand that when a reaction takes place, it takes some energy for it to occur and the reactants must overcome an energy barrier called an “activation energy” before they can form products.
The higher the activation energy, the slower the reaction; therefore, the rate at which products are formed is determined by the amount of energy the reactants have available. Temperature has a direct impact on the rate at which a reaction can take place. As temperature increases, so too does the amount of energy available to the reactants, thus allowing them to overcome the activation energy and speed up the rate at which the reaction takes place.
When conducting your investigation, it is important to ensure you have a safe environment for the experiment to take place in and that you understand the necessary precautions required. You should also calculate the theoretical rate of reaction at different temperatures, which can provide useful background information for comparison against actual results.
When analyzing the data obtained from the experiment, it is important to consider factors such as validity (whether the results were influenced by any outside factors) and accuracy (how close the results were to predictions made prior to the experiment). Additionally, it can be beneficial to create a graph in order to visualize any trends or patterns observed in your data.
Overall, this is an engaging and interesting topic that allows one to explore the relationship between temperature and reaction rate. With the correct planning and analysis of results, this can be a practical and informative experience.
We have made it to the seventh topic of this guide—Topic 7! This topic is all about titration, which is a fancy word for measuring how much of a substance you have in a solution.
When you are doing titration, you need to start by making a solution of a known concentration (this is called a standard solution). Then you will use that solution to find out the concentration of the unknown solution. This is done by adding the standard solution to the unknown one, drop by drop, until an endpoint is reached—this is when a color change happens and shows how much of the unknown solution has been added.
To succeed at titration, you will need to know how to make a standard solution, measure accurately, and identify when the endpoint has been reached. All of these skills take practice and experience, so be sure to get lots of practice before tackling this section of the IA.
Here are some tips for approaching titration:
- Always use an accurate measuring device (preferably a burette) when adding the standard solution to the unknown solution.
- Make sure you pay attention to the color change that indicates the endpoint has been reached.
- Be sure to calculate and record your results, as they are the main part of the IA.
Titration can be tricky, but with practice and patience, you can do it! So take the time to practice, and you will soon become an expert in titration.
The eighth topic of your IB Chemistry IA might be about acids and bases. The goal of this topic is for you to understand how acids and bases react in different environments. You’ll need to be able to recognize indicators such as pH paper or universal indicator, as well as other measurements used to assess the acidity or basicity of a solution.
You’ll also explore the different types of salts and the effect they have on the acidic or basic nature of a solution. It’s important to conduct various tests to determine the concentration of anions and cations in a solution and to understand their properties.
When approaching this topic, it’s important to keep track of the observed data and the process that led to the observation. Good record-keeping will aid in replicating experiments and effectively demonstrating the concept or principles being studied in the lab. It’s also important to consider what factors could be contributing to any anomalies seen in the data.
Finally, it’s essential to think critically about the results and implications of your experiments. This will help to further develop your research questions and objectives and guide subsequent experiments.
You have now learned about the 20 IB Chemistry IA topics in detail. These topics can range from exploring the properties of different types of molecules, to researching biological processes or chemical reactions.
We have also looked at some tips for successfully completing your IB Chemistry IA report. This includes taking into account essay structure, style, and research techniques.
By now you should have a good idea about what is expected from an IB Chemistry IA. Remember, it’s important to stay focused, and keep track of what you have accomplished so far. Doing this will help you stay organized, and ensure that your report meets all assessment requirements.
Good luck! And don’t forget to take the time to enjoy the process, as this is an opportunity to apply your knowledge in a meaningful way.

Nick Radlinsky
Nick Radlinsky is a devoted educator, marketing specialist, and management expert with more than 15 years of experience in the education sector. After obtaining his business degree in 2016, Nick embarked on a quest to achieve his PhD, driven by his commitment to enhancing education for students worldwide. His vast experience, starting in 2008, has established him as a reputable authority in the field.
Nick's article, featured in Routledge's " Entrepreneurship in Central and Eastern Europe: Development through Internationalization ," highlights his sharp insights and unwavering dedication to advancing the educational landscape. Inspired by his personal motto, "Make education better," Nick's mission is to streamline students' lives and foster efficient learning. His inventive ideas and leadership have contributed to the transformation of numerous educational experiences, distinguishing him as a true innovator in his field.
You Might Be Interested
- Psychology Internal Assessment Topics
- Business IA topics. Guide with examples
- How to Choose Math IA Topic
- IB Biology IA Topics That Don’t Require Experiment
- Biology IA topics
- A Guide to Choosing and Creating Compelling Math IA SL Topics
- How to write physics IA. Comprehensive Guide
- How to write Biology Internal Assessment. Comprehensive Guide
- Economics IA topic ideas
- Math SL Internal Assessment Ideas
- Geography IA ideas
- IB Internal Assessment Rubric: Grading Criteria and How to Excel
- How to write an IB Internal Assessment
Looking for more help with your Internal Assessment? Check out our IB IA Writing Service or buy Internal Assessment .
2024 November TOK Essay Prompts | How to Write Them?
In this comprehensive guide, an experienced IB writer shares essential insights and strategies specifically tailored to mastering TOK essay prompts. From analyzing the nuances of knowledge acquisition in different areas of knowledge to considering the dynamic interplay between artistic creativity and scientific methodology, this article offers a deep immersion into each prompt.
How Long Is IB EE? Minimum and Maximum Word Count
Balancing word count limits requires careful planning and consideration of every word you write. In this guide, I’ll share strategies and insights from years of mentoring IB students to help you master the art of word count management in your extended essay.
TOK Essay Word Count. Min & Max
In this guide, we discuss the crucial parameters set by the International Baccalaureate for minimum and maximum word counts. Through the insights of an experienced IB writer, this article offers practical strategies for staying within these limits while improving the quality and depth of your essay.
How Long Is IB IA? Average IA Word Count
From my experience as IB tutor, a frequent question among students is, “How Long Is IB IA?” This question is crucial as the IA represents a significant component of the IB diploma, reflecting a student’s ability to apply classroom knowledge in a real-world context.
IB Extended Essay Rubric. Grading Criteria
Understanding the IB extended essay rubric is essential for success. The rubric provides a framework that grades students on several key criteria including the sharpness of their research question, the rigor of their methodology, the breadth and depth of their knowledge, the fluidity and clarity of their argumentation, and their personal engagement with the research topic.
IB TOK Essay Rubric. Grading Criteria
This article provides essential insights and strategies for understanding the assessment process and helping you write essays that meet and exceed the rigorous standards of the IB curriculum. Whether you’re striving for clarity of argument, effective integration of knowledge, or personal engagement, our tips will help you achieve a higher score.
© 2023 I Bstudenthelp.com. This website is owned and operated by Udeepi OU Harju maakond, Tallinn, Lasnamäe linnaosa, Sepapaja tn 6, 15551. Disclaimer : Services we provide are only to assist the buyer like a guideline to complete any kind of writing assignment. Privacy Policy Terms and Conditions Cookie Policy Revision Policy Refund Policy

- Customer Reviews
- Extended Essays
- IB Internal Assessment
- Theory of Knowledge
- Literature Review
- Dissertations
- Essay Writing
- Research Writing
- Assignment Help
- Capstone Projects
- College Application
- Online Class
IB Chemistry IA Guide: Format, Topics, Rubric, And Assessment
by Antony W
October 20, 2022
For most students, the IB Chemistry IA (Internal Assessment) assignment lurks on the path to academic success like a sphinx with an impossible riddle. The analogy is quite right too, given that the assignments take up a hefty 20% of your total marks in the coursework.
If you are reading this, you are likely confused by the guesswork that surrounds the entire exam. Luckily for you, we at Help for Assessments are old hands at chemistry assignments including IB internal assessments. We will guide you through the process as we debunk the 'nightmare' that is a chemistry IA to make sure you ace it.
If you would love a little more practical help than this detailed guide, our team of experts is at your disposal to carry out your chemistry IA with polished skill. As a multi-faceted team, we are experts in every form of academic writing assignments and projects. Don't be shy, check out our range of services and order your very own writing expert today.
If you still want to proceed, its time you put your apprehension away as we take a practical look into how you can pass the chemistry IA.
This guide will explore the sections and format of a chemistry IA, a look into the marking criteria used, and suggestions at every point to help show you what is expected of you.
What is a Chemistry IA
The IB chemistry IA is an investigative essay that seeks to explain the chemistry behind common real-world phenomena. It seeks to assess your grasp of chemistry up to the level achieved in your coursework through practical application of the skills gained.
Unlike regular test which many students cram their way through, the chemistry IA needs a high level of independent skill, research work, and personal effort to complete.
Coupled with all the other stressful assignments in other subjects, the chemistry IA sometimes feels like an insurmountable problem partly because of the sheer amount of guesswork.
The assignment seeks to develop independent thinking and creative application of concepts learned to the real world. If you can do this successfully, you will earn top marks in the assignment. The first task you have with the chemistry internal assignment is choosing an appropriate topic.
Choosing a Topic for Your Chemistry IA
The first consideration you should take into account when choosing a topic for the IB chemistry IA is that it should be personal. That means it should be one that interests you personally, adapted to your particular cause, and related with a personal touch throughout the text.
To achieve full marks, your topic and essay must meet the conditions set out by the descriptors on IB’s chem page .
However, in the search for authenticity and originality, many students get themselves in a rut trying to invent procedures and experiments. Notice that the paper is looking for independent thinking, creativeness, and initiative.
The best way to achieve these criteria without knocking your brains out is to adapt existing experiments, procedures, or other ideas to your investigation. What you need to showcase is authentic inspiration and highly personalized methods, which you can easily do by adapting existing ones.
Let's say, for example, that you want to investigate the calories in the food you eat. Of course, that is a washed-out idea. How about you give it a new twist and investigate how cooking Ideal Protein packs affects their calorie content? This is something you can do easily with a calorimeter in the lab.
For those who don’t know, Ideal Protein is a popular, albeit shady, weight loss program when dieters substitute daily food for prepackaged ultra-refined protein packets. If you have ever tried it, you can tie it to the investigation to show innovation and inspiration.
The same goes for other common chemistry experiments you will find online, in books, journals, magazines, and other common sources.
With a topic in hand, it's time to plan the investigation and structure it accordingly.
Chemistry IA Format
You will be carrying out the IA assignment in stages, but very little of it will be actual lab work. Most of the work is in the research involved and crafting the actual write up.
So, while we are confident you can handle the actual experimentation (your instructor will be more than glad to help), let us show you how to structure the write up.
There will be seven important parts of the write up, which for the sake of simplicity we will keep calling an essay. The image below will give you a rough idea of how each section appears, but we shall also take a more in-depth look into some of the parts.
Parts of a Chemistry IA
The following image shows a sample structure of a chemistry IA. Please note that it is strictly for guidance only; in no way is it to be taken as a must-use format. Your instructor will usually provide exhaustive instructions in this regard.

Introduction
Your introduction is meant to tell your reader why they should keep reading. Here, give in very brief detail what your experiment is about, why you chose it, and stress your personal connection to the subject matter. You first person ‘I’ or ‘We’ pronouns to achieve this.
a) Background
As part of the Introduction, the background is meant to enhance the reader’s understanding of the context of your investigation. Include a general review of the science behind the concept, backed by facts and evidence.
You can also include the equations and mathematical formulas that you will be using in the assignment.
b) Hypothesis
The hypothesis is meant to be a justification for the choice of science concepts you use to explain the claim under investigation.
More technically, it should be a specific prediction about the outcome of your experiment or how the dependent variable will change with the independent one.
For example, with our example, our hypothesis might be:
“Using calorimeters to find out whether Ideal Protein packs change in calorie content value upon cooking in various ways.”
In any other discipline, this section would probably be called ‘methodology’ or ‘procedure.’ This is the part where you tell, in complete detail, how you carry out your experiment.
a) Research Question
The design part starts with a research question in the form ‘how is x dependent on Y?’ It should be a highly specific question outlining the variance you are investigating, stated without any ambiguity.
b) Variables
Outline fully what your variables are, outlining the three types:
- Independent variables - the factor you manipulate and set parameters for.
- Dependent variable - the variable you want to determine by measuring or calculating.
- Controlled variable - other variables or factors that influence the outcome and which should either be controlled or taken into consideration in the outcome.
As part of the design, the method or procedure is a detailed explanation of how you carried out the experiment or tests required to measure the variables. No matter how strong the temptation is, do not take this section off a chemistry book or lap report.
Most students trip on this section because they forget the procedure is supposed to be specially adapted and personalized for this assessment.
If you were choosing a reagent, state why you chose it. If you are choosing a flask, show the reasoning behind your choice. For example, 'use a glass stirring rod instead of a metallic one to prevent reaction with the reagent …'
It is also a good idea to include a diagram of your setup and describe any modifications you had to improvise. A picture will also do.
a) Raw Data
Record your data as soon as you collect it. This is usually done in tables where you can compare the variables easily and fill in the gaps quickly. You need to collect both quantitative (measured, e.g. 10g of residue) and qualitative data (observed, e.g. color change, bubbles).
Report every measured value and include every field on the same table where possible. If any of the data values don't match, you can enter it in different fields.
b) Analysis
Use your measured data to calculate totals, averages, correlations, among others. Draw graphs and charts as needed. Be sure to show the entirety of your working including the formulas you used. Note any uncertainties and margins of error expected.
If you have an accepted value for that kind of experiment, compare the two and see how close they come to each other. If you have graphs, make them appealing, give them names, and name them accordingly.
The conclusion should be a rewording of your research question to give an answer based on the trend observed and proved by your results. The conclusion is a three-part section that also includes:
a) Evaluation
The evaluation is a critical review of your methods and how well the procedure you chose worked to your benefit (or not). If there are any discordant data/outlier points, discuss them here.
b) Improvements
Suggest any improvements or changes you would make if you were to repeat the experiment. What would you advise anyone else using the same procedure for the experiment? Do you have any suggestions to make it better?
Marking Criteria for Your Chemistry IA
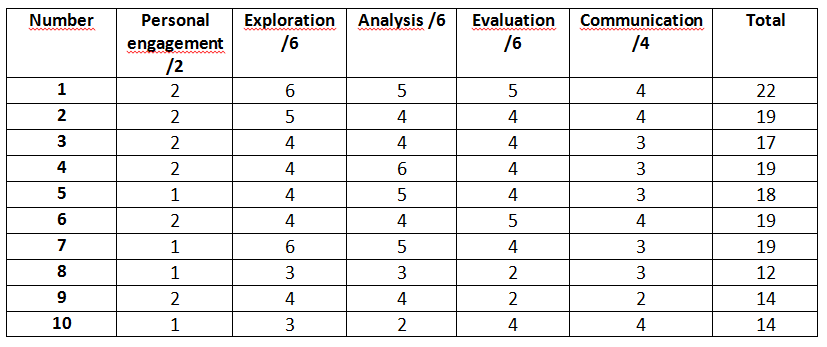
By now, you can tell that personal engagement is a pretty big deal in your chemistry IA, but it is not the only one.
Markers will rely on several other criteria to grade your work, including:
1. Exploration
Exploration is a measure of how well you establish a scientific context for the project. It measures how well focused and grounded your research question is, as well as your awareness and knowledge of safety, environmental, and ethical concerns involved, if any.
2. Methodology
This criterion measures how clearly and thoroughly you set out your procedure. You should have at least 5 variations or manipulations of the independent variable and 3 trials for each.
You should collect as many significant data points as possible, set out any safety procedures taken, and qualify each choice of equipment and supplies.
3. Analysis
This metric measures your data collection, organizational, and presentation skills. All calculations must be shown clearly, formulas displayed, and all qualitative and quantitative data recorded.
4. Evaluation
This metric contributes 6 marks to the total 20 and assesses how well the results obtained serve to prove/disprove your research question in the accepted context.
You also get points for suggesting useful improvements, noting and owning up to any weakness of the methodology, and describing the strength of the correlation between the results and the hypothesis.
5. Communication
This criterion is a measure of how clearly and effectively you presented yourself in the essay.
6. Personal Engagement
As discussed, you will need to completely own the entire project from start to finish. Choose every word with care, especially if you use textbook sources along the way. Cite each of these appropriately because, needless to say, plagiarism is a capital crime in the assessment.
Need Help With Your Chemistry IA Assignment?
Many students are tempted to rush through with the assignment to be ‘over and done with’ it. However, such IB assessments are tricky because they test not only the outcomes, but also the process, the student’s state of mind, and the overall circumstances surrounding the assessment.
If you truly want to be 'over and done with' the assignment, the only way to do it is to be diligent and do it as meticulously as you can. However, you can also hand over the entire problem to chemistry internal assessment experts at Help for Assessments to write it for you.
With years of experience and skill in the market, we will take the burden off your hands and do the entire assessment for you in record time and at the lowest prices. If you want to experience total peace of mind, order our stellar services here. We are here for you, always.
About the author
Antony W is a professional writer and coach at Help for Assessment. He spends countless hours every day researching and writing great content filled with expert advice on how to write engaging essays, research papers, and assignments.
Leave a Reply
Your email address will not be published. Required fields are marked *

25+ IB Chemistry IA Topics and How to Complete Them ⚗️

Internal Assessment is an integral part of the International Baccalaureate (IB) Diploma Programme in chemistry, comprising 20% of the final assessment. The IA requires students to conduct an independent laboratory investigation and write a report on their findings.So this where the next question comes – why is it important to select a right Chemistry IA topic.
Why choosing a good topic for your IB Chemistry IA is important?
Choosing a good IA topic is crucial as it forms the foundation of the investigation and can make or break the project. So if you do not want to risk the final mark, consider selecting a topic you will be able to cover.
First, let’s take a closer look at how you should choose a topic for your Chemistry Internal Assessment.
When choosing a topic for your IB Chemistry IA, it’s important to consider the following factors:
- Relevance to the course: The topic should align with the objectives and content of the IB Chemistry course. It should demonstrate your understanding of key concepts and theories covered in the syllabus.
- Interest and curiosity: Select a topic that genuinely interests you and arouses your curiosity. This will motivate you to conduct thorough research and engage with the project more deeply.
- Feasibility and resources: Ensure that the topic is feasible and you have access to the resources needed to conduct the investigation. This includes equipment, chemicals, and data sources.
- Originality and innovation: While it’s unnecessary to come up with a completely new topic, selecting an original and innovative angle can make your IA stand out and demonstrate your creativity and critical thinking skills.
- Clarity and specificity: Choose a specific research question that can be clearly defined and answered within the project’s scope. This will help you stay focused and avoid getting overwhelmed by the vastness of the subject.
A well-chosen topic can help you score higher grades in your IA as it provides a clear and organized framework for the investigation. A good topic also allows you to apply your knowledge and skills to a real-world problem or question, which is the ultimate goal of the IA. Therefore, take your time and carefully consider your options before finalizing your IA topic.
Here are some tips for selecting the best chemistry IA topics:
- Choose a topic that interests you: The IA is a long-term project and requires a lot of time and effort. It is important to choose a topic that you are genuinely interested in, as it will keep you motivated throughout the process.
- Make sure the topic is feasible: The IA should be realistic and achievable because you should complete them within the given time frame. Consider factors such as the availability of equipment, materials, and resources.
- Keep it simple: While it is tempting to choose a complex and ambitious topic, it is important to keep it simple and focused. A narrow and well-defined topic is easier to investigate and allows you to delve deeper into the subject.
- Consider the relevance of the topic: Choose a topic that is relevant and timely, and has real-world applications. This will make the IA more meaningful and engaging.
Here are some examples of good chemistry IA topics:
- The effect of pH on the rate of enzyme-catalyzed reactions
- The synthesis and characterization of nanoparticles
- The determination of the equilibrium constant of a chemical reaction
- The analysis of food additives using spectroscopy techniques
- The determination of the molar mass of a volatile liquid using the Ideal Gas Law
Want some complex Chemistry IA ideas?

Here are some more detailed chemistry IA topics that explore the speed of different chemical reactions using a spectrometer:
- Investigating the effect of temperature on the rate of a chemical reaction
In this IA, you could use a spectrometer to measure the absorbance of a reactant or product as a function of time at different temperatures. This will allow you to determine the rate of the reaction at each temperature and plot a rate versus temperature graph.
You could also use this data to determine the activation energy of the reaction.
- Determining the rate law of a chemical reaction
In this IA, you could use a spectrometer to measure the absorbance of a reactant or product as a function of time at different concentrations. This will allow you to determine the rate of the reaction at each concentration and plot a rate versus concentration graph.
You could then use this data to determine the rate law of the reaction and calculate the rate constant.
- Analyzing the kinetics of a catalyzed reaction
In this IA, you could use a spectrometer to measure the absorbance of a reactant or product as a function of time in the presence and absence of a catalyst. This will allow you to compare the rate of the reaction with and without the catalyst and determine the effect of the catalyst on the rate.
You could also use this data to calculate the rate constant and activation energy for the catalyzed and uncatalyzed reactions.
- Evaluating the effect of a solvent on the rate of a chemical reaction
In this IA, you could use a spectrometer to measure the absorbance of a reactant or product as a function of time in different solvents. This will allow you to determine the reaction rate in each solvent and compare the rates.
You can use this data to determine the effect of the solvent on the rate constant and activation energy of the reaction.
If you want, you can buy IB Chemistry IA at our writing service.
These topics will surely give you some ideas for your chemistry IA! But I know that you want more, so let’s continue with other ideas.
And some more Chemistry IA topics for you:
Here are five more chemistry IA topics related to the effect of temperature on activation energy:
- Investigating the effect of pH on the activation energy of an enzyme-catalyzed reaction
In this IA, you could measure the rate of an enzyme-catalyzed reaction at different pH values and use this data to calculate the activation energy.
You can use a spectrometer to measure the absorbance of a reactant or product as a function of time at each pH value to determine the rate of the reaction.
- Analyzing the effect of a solvent on the activation energy of a chemical reaction
In this IA, you could measure the rate of a chemical reaction in different solvents and use this data to calculate the activation energy.
You could also use a spectrometer to measure the absorbance of a reactant or product as a function of time in each solvent to determine the rate of the reaction.
- Evaluating the effect of a catalyst on the activation energy of a chemical reaction
In this IA, you could measure the rate of a chemical reaction in the presence and absence of a catalyst and use this data to calculate the activation energy for both cases.
Use a spectrometer to measure the absorbance of a reactant or product as a function of time with and without the catalyst to determine the rate of the reaction.
- Determining the effect of temperature on the stability of a compound
In this IA, you could measure a compound’s decomposition rate at different temperatures and use this data to calculate the activation energy.
You could also use a spectrometer to measure the absorbance of the decomposition products as a function of time at each temperature to determine the reaction rate.
- Analyzing the effect of a reactant concentration on the activation energy of a chemical reaction
In this IA, you could measure the rate of a chemical reaction at different reactant concentrations and use this data to calculate the activation energy.
And now use a spectrometer to measure the absorbance of a reactant or product as a function of time at each concentration to determine the rate of the reaction.
And five more Chemistry IA ideas you can do at home:

Here are five more chemistry IA topics related to investigating energy contents in packaged chips:
- Analyzing the nutritional value of packaged chips using proximate analysis
In this IA, you could perform proximate analysis on a variety of packaged chips to determine their protein, carbohydrate, fat, and fiber content. You could also calculate the caloric value of each chip based on the proximate analysis data.
- Evaluating the effect of frying temperature on the oil content of packaged chips
In this IA, you could fry chips at different temperatures and use a moisture analyzer to determine the oil content of each batch.
Then compare the oil content of the chips fried at different temperatures to see if the frying temperature affects the oil content.
- Determining the preservative content of packaged chips using spectrophotometry
In this IA, you could use spectrophotometry to analyze the preservative content of different brands of packaged chips.
You could compare the preservative content of each brand to see if there are significant differences.
- Investigating the effect of storage temperature on the shelf life of packaged chips
In this IA, you could store chips at different temperatures and use a moisture analyzer to determine the moisture content at regular intervals.
Then you can plot the moisture content versus time to see how the storage temperature affects the shelf life of the chips.
- Analyzing the effect of packaging material on the oxygen content of packaged chips
In this IA, you could compare the oxygen content of chips packaged in different materials using a gas chromatograph.
You could then determine the effect of the packaging material on the oxygen content and shelf life of the chips.
Want some more Chem IA topics?
Here are five more chemistry IA topics related to the effect of sodium fluoride on tooth decay in the presence of carbonated drinks:
- Investigating the effect of different pH levels on the effectiveness of sodium fluoride in preventing tooth decay
In this IA, you could expose teeth to different concentrations of sodium fluoride at different pH levels and measure the amount of tooth decay using a tooth decay model.
You can compare the effect of the pH on the effectiveness of the sodium fluoride in preventing tooth decay.
- Evaluating the effect of different sweeteners on the tooth decay prevention properties of sodium fluoride
In this IA, using a tooth decay model, you could expose teeth to different concentrations of sodium fluoride in the presence of different sweeteners and measure the amount of tooth decay.
You could then compare the effect of the sweeteners on the effectiveness of the sodium fluoride in preventing tooth decay.
- Analyzing the effect of different types of carbonated drinks on the tooth decay prevention properties of sodium fluoride
In this IA, you could expose teeth to different concentrations of sodium fluoride in the presence of different types of carbonated drinks and measure the amount of tooth decay using a tooth decay model.
Then – compare the effect of the carbonated drinks on the effectiveness of the sodium fluoride in preventing tooth decay.
- Determining the effect of different storage temperatures on the stability of sodium fluoride in a toothpaste formulation
In this IA, you could prepare a toothpaste formulation containing sodium fluoride and store it at different temperatures. You could then measure the stability of the sodium fluoride at regular intervals using a spectrophotometer.
Also, you could compare the stability of the sodium fluoride at different storage temperatures to see if the temperature affects its stability.
- Investigating the effect of different brushing agents on the tooth decay prevention properties of sodium fluoride
In this IA, you could expose teeth to different concentrations of sodium fluoride in the presence of different types of toothpaste formulations containing various types of abrasives and measure the amount of tooth decay using a tooth decay model.
Afterwards, compare the abrasives’ effect on sodium fluoride’s effectiveness in preventing tooth decay.
Don’t let the stress of choosing an IA topic
Hold you back..
Are you struggling to come up with topic suggestions for your IB Internal Assessment?
Our experienced writers can help you choose the perfect topic for your IA
Tailored to your specific subject and requirements.
Simply click:

And last but not least – topics related to the current situation worldwide:
Here are five chemistry IA topics related to modern times:
- Investigating the environmental impact of plastic pollution
In this IA, you could analyze the chemical composition of plastic waste and determine its environmental impact.
Also, compare the environmental impact of different types of plastics to see if some are more harmful than others.
- Analyzing the effect of air pollution on plant growth
In this IA, you could expose plants to different levels of air pollution and measure the effect on their growth using various parameters such as height, weight, and leaf area.
You could also use spectrophotometry to measure the concentration of pollutants in the air and compare it to the effect on plant growth.
- Evaluating the effectiveness of natural antimicrobial agents against pathogenic bacteria
In this IA, you could test the antimicrobial activity of natural compounds such as essential oils against pathogenic bacteria and compare their effectiveness to synthetic antimicrobial agents.
Use spectrophotometry to measure the concentration of the antimicrobial agents and determine the minimum inhibitory concentration (MIC).
- Determining the effect of electronic waste on soil and water quality
In this IA, you could expose soil and water samples to electronic waste and measure the effect on their quality using various parameters such as pH, conductivity, and nutrient content.
You could also use spectrophotometry to measure the concentration of heavy metals and other contaminants in the soil and water.
- Analyzing the chemical composition of alternative energy sources
In this IA, you could analyze the chemical composition of different alternative energy sources such as biofuels, solar cells, and batteries and compare their properties.
Once again, you can also use spectroscopy techniques such as infrared spectroscopy and X-ray fluorescence to determine the chemical structure and elemental composition of the energy sources.
If you are also searching for other IA topics, make sure to read other related articles:
- Biology IA topics without experiment
- Experiment based Bio Internal Assessment topics
- History IA topic ideas
- Math IA topics for HL and SL students
- IB Business IA ideas
- Topics for Physics IA
- Environmental Science IA topics
Select topic for Chemistry IA that suits YOU!
Remember, the IA is an opportunity for you to demonstrate your understanding and skills in chemistry.
So please choose a topic that challenges and engages you, and have fun with it!
If you need assistance with choosing a topic or writing your Chemistry IA, IB writing service is here to help you. Simply contact us.
Get hot offers and discounts for your IB Assignments
Our writing solutions cater to all disciplines within the IB program, and we specialize in crafting academic papers for students of all levels. We follow the IB criteria.
Adhering strictly to the rigorous standards set by the IB, we deploy a methodical approach to our writing process. This ensures that every piece of content we generate not only meets but exceeds the expectations set within the program.
Contact us:
Latest Articles:

November 2024 TOK Essay Titles. Short Description for Each Topic and How to Write It

Interdisciplinary Topics in Extended Essays

How to Create a Research Question for Your IB Extended Essay?
Our services:.
- Buy Internal Assessment
- Buy Math IA
- Buy Extended Essay
- Buy TOK Essay
- Buy TOK Exhibition
IBWritingService.com is an independent academic writing aid with no official ties to the International Baccalaureate Organization (IBO). Our use of “IB” in the domain and title is purely for identification, and we neither claim nor imply any endorsement or partnership with the IBO. Our services aim to support students’ educational needs without violating IBO policies. Trademarks mentioned are property of their owners and do not suggest affiliations. By using our services, you acknowledge our non-affiliation with the IBO and that we’re not a substitute for IBO requirements. We deny any liability for use of our services in relation to the IBO.
ALL PAPERS WRITTEN BY OUR EXPERTS AS PART OF THIS WRITING SERVICE ARE FOR REFERENCE PURPOSES ONLY. WHEN USING CONTENT PURCHASED FROM THIS WEBSITE, IT MUST BE PROPERLY REFERENCED.
- Terms & Conditions
- Revision Policy
- Privacy Policy
- Refund Policy
- Cookie Policy
© 2023. All Rights Reserved.
Practical Science
Table of Contents


How to write the perfect Chemistry Internal Assessment (IA)
The Internal Assessment (IA) for Chemistry in the International Baccalaureate Diploma Programme (IBDP) is an experimental investigation that requires students to demonstrate their ability to design and conduct experiments, analyze data, and draw conclusions. Here is a plan for a Chemistry IA: Topic Selection: Choose a topic that interests you and aligns with your learning objectives. Examples of topics could be, but not limited to:
- Determination of the concentration of an acid using titration
- Investigating the rate of reaction of magnesium and hydrochloric acid
- Investigating the effect of concentration on reaction rate
- Investigating the effect of temperature on the reaction rate
- Investigating the effect of pH on the reaction rate
Research Question: Formulate a research question that clearly identifies the variables you will be investigating. Ensure that the research question is focused and specific, and the variables are measurable.
Experimental Design: Design an experiment that will allow you to collect quantitative data that will help you to answer your research question. Ensure that your experimental design is detailed, safe, and reliable. Include an appropriate number of trials, controls, and independent variables. Make sure that your methodology aligns with best practices in experimental design.
Data Collection: Collect data using your experimental design. Ensure that you record all relevant data accurately, and take steps to reduce sources of error. Collect sufficient data to draw reliable conclusions.
Data Analysis: Analyze the data you have collected using appropriate statistical methods. Use your data to answer your research question, and draw conclusions based on your results. Consider any sources of error and their potential impact on your results.
Conclusion and Evaluation: Summarize your findings and draw a conclusion that answers your research question. Evaluate your experiment, including strengths and limitations, and consider how you might improve it in the future.
Communication: Write a report that clearly and concisely communicates your research question, experimental design, data, analysis, and conclusion. Use appropriate scientific language and structure. Include any necessary calculations, graphs, and diagrams to support your conclusions. Ensure that you cite all sources of information used in your report.
Reflection: Reflect on the process of conducting your IA. Consider what you learned, how you might improve your experimental design, and what you might do differently in future experiments.
Overall, ensure that you follow all guidelines and instructions provided by your teacher and the IB. Make sure that your IA demonstrates your ability to apply scientific knowledge, conduct research, and communicate your findings effectively.
Share this:
- Click to share on Twitter (Opens in new window)
- Click to share on Facebook (Opens in new window)
- Click to print (Opens in new window)
- Click to email a link to a friend (Opens in new window)
Type your email…
- Already have a WordPress.com account? Log in now.
- Subscribe Subscribed
- Copy shortlink
- Report this content
- View post in Reader
- Manage subscriptions
- Collapse this bar
- How It Works
- Prices & Discounts
How to Select the Perfect Chemistry IA Topic for IB Standard Level
Table of contents
Choosing a topic for your chemistry internal assessment can feel like being a kid in a candy store - so many options, each more exciting than the last. It's your chance to dive deep into the vast and varied world of chemistry, exploring the areas that fascinate you the most.
The IA is an integral part of the IB SL chemistry course. It provides a unique opportunity to carry out an in-depth investigation on a chosen chemistry topic, applying the knowledge and skills you've learned throughout your studies.
In this blog post, we aim to guide you through this selection process, providing you with 30 chemistry IA topic ideas and tips on how to choose the right one for you. Whether you're passionate about organic chemistry or fascinated by the behavior of molecules, there's something here for everyone.
30 IB Chemistry IA Topic Ideas
Choosing a chemistry IA topic isn't just about picking something off a list; it's about finding a subject that piques your curiosity, matches your interests, and offers a viable path for investigation. To help spark your inspiration, we've curated a list of 30 good chemistry IA topics suitable for IB Standard Level:
1. Analysis of vitamin C content in different types of fruit
2. Investigating the effect of temperature on enzyme activity
3. The chemistry of natural water pollution cleaners
4. Analysis of caffeine concentration in different types of coffee
5. Investigation of acidity levels in various types of soda
6. The impact of sunlight on dye degradation
7. Extraction and analysis of essential oils from plants
8. Studying the rate of fermentation under different conditions
9. Investigating the effect of pH on the corrosion of metals
10. The chemistry behind non-stick pans
11. Analyzing the presence of heavy metals in local soils
12. Investigating the effect of salinity on water freezing point
13. Understanding the chemistry of batteries
14. Study on the rate of reaction between magnesium and hydrochloric acid
15. Analysis of sugar content in various types of beverages
16. The chemistry of climate change: Carbon sequestration techniques
17. Investigating the properties of homemade plastic
18. Analysis of different antacid effectiveness in neutralizing stomach acid
19. Understanding the chemical processes in brewing beer
20. Investigating the extraction of DNA from fruits
21. Studying the process of rust and ways to prevent it
22. The chemistry behind fireworks: Why do they show different colors?
23. Investigating the synthesis and properties of biodiesel
23. Analysis of fluoride concentration in different brands of toothpaste
25. Studying the effect of light exposure on the color of soft drinks
26. Understanding the chemistry of soaps and detergents
27. Investigating the rate of photosynthesis under different light colors
28. Analysis of the chemical composition of different types of honey
29. Studying the chemical changes in ripening fruits
30. The chemistry behind airbags: How do they work so fast?
These topics represent a broad range of areas in chemistry. They are intended to inspire your thought process and guide you toward a subject that you find intriguing.
Tips for Selecting the Right Topic for Chemistry IA
Choosing a chemistry IA topic can be exciting, but it can also be daunting. After all, the topic you select will guide your research and learning for the duration of the project. To ensure that you make a choice that will keep you engaged and motivated, here are a few tips:
Align with Your Interests : You're more likely to stay motivated if you're genuinely curious about your topic. Think about the areas of chemistry that have intrigued you during your studies. Are you drawn to organic chemistry? Or maybe you're fascinated by biochemistry? Start there.
Consider Your Resources : Keep in mind the resources you have available to you. This includes both tangible resources like lab equipment and materials, as well as intangible ones like time and guidance from your teacher.
Think About the Scope : Be wary of choosing a topic that's too broad or too complex. Remember, this is a Standard Level IA, so aim for depth over breadth. It's better to thoroughly explore one aspect of a topic than to skim over many.
Relevance to the Syllabus : Although the IA is an independent project, it should still relate to the material you've covered in your IB Chemistry course. Make sure your chosen topic aligns with the syllabus.
Real-World Connection : Topics with real-world connections can be more engaging and satisfying to explore. Consider choosing a topic that relates to everyday life, environmental issues, or current scientific research.
Remember, there's no "perfect" chemistry IA topic. The best topic for your Chemistry IB IA is the one that sparks your curiosity and aligns with your resources and objectives.
From Chemistry Topic to Research Question
Once you've chosen a broad topic that interests you, the next step is to narrow it down into a focused research question. This process can often feel as challenging as choosing the topic itself, but don't worry - we're here to guide you through it.
Be Specific : The more specific your research question, the easier it will be to stay focused during your investigation. For example, instead of a broad topic like "The Chemistry of Coffee", you might narrow it down to "Analysis of caffeine concentration in different types of coffee".
Consider Feasibility : Your research question should be something you can feasibly investigate within the constraints of your resources. Think about what materials you have access to, the amount of time you have, and the guidance available to you.
Align with the IB Criteria : Remember that the IA is not just about conducting an experiment. It also involves evaluating your method, analyzing your data, and drawing conclusions. Ensure that your research question allows for this type of analysis.
Seek Feedback: Don't hesitate to discuss your ideas with your teachers or classmates. They can offer valuable perspectives and help you refine your research question.
Turning your topic into a research question is a crucial step in your Chemistry IA journey. It's the question that will guide your exploration and determine the direction of your investigation.
Utilizing Available Resources When Writing Chemistry IA
There are numerous resources available to you that can provide guidance and support when you need help with your chemistry IA.
Your Teacher : Your Chemistry teacher is a valuable resource. They understand the requirements of the IA and can provide guidance on your chosen topic, research question, and experimental design.
Textbooks and Class Materials : Your textbooks and class materials can help provide the foundational knowledge you need for your IA.
Online Resources : There are numerous online resources available that can provide additional support. Websites like Khan Academy can help reinforce concepts, while research databases can provide scientific literature related to your chosen topic.
Writing Services : If you're feeling overwhelmed with your IA, there are professional IA services where you can buy chemistry IA online . These assessment help services can help you through the process and ensure that your IA meets the highest academic standards or you can request them to write it from scratch for you.
Remember, your IA is an opportunity for you to showcase your understanding of chemistry. Utilize the resources available to you to ensure that your work is the best it can be.
With the right topic, a well-crafted research question, and effective use of resources, you're on the right track toward a successful project.
Remember that the key to a successful IA is not just about selecting a good chemistry IA topic but also about maintaining your curiosity and enthusiasm throughout the process. This journey will require effort, creativity, and critical thinking, but the result will be incredibly rewarding - a deep understanding of a chemistry IA topic you're passionate about and a high-quality piece of scientific research to be proud of.
Share this article
Achieve Academic Success with Expert Assistance!
Crafted from Scratch for You.
Ensuring Your Work’s Originality.
Transform Your Draft into Excellence.
Perfecting Your Paper’s Grammar, Style, and Format (APA, MLA, etc.).
Calculate the cost of your paper
Get ideas for your essay
Tiber Tutor
Explored ib chemistry .
- Mathematics: AA SL
- Mathematics: AA HL
- Mathematics: AI SL
- Mathematics: AI HL
- Biology SL (FE2025)
- Biology HL (FE2025)
- Biology SL (FE2016)
- Biology HL (FE2016)
- Chemistry SL (FE2025)
- Chemistry HL (FE2025)
- Chemistry SL (FE2016)
- Chemistry HL (FE2016)
- Physics SL (FE2025)
- Physics HL (FE2025)
- Physics SL (FE2016)
- Physics HL (FE2016)
- Business SL (FE2024)
- Business HL (FE2024)
- Business SL (FE2016)
- Business HL (FE2016)
- Economics SL
- Economics HL
- Psychology SL
- Psychology HL
- Computer Science SL
- Computer Science HL
- English A LAL SL
- English A LAL HL
20 Amazing Chemistry IA Ideas to nail your IB Score
Stuck on how to choose your IB Chemistry internal assessment topic. We get it picking a relevant topic particularly when you are just confined to school laboratories with a tight schedule is not less than resolving any mystery. The fear of selecting a perfect topic that is solid and high scoring inevitably raises stress among the students.
Well, that’s a norm in the IB program. But practicing a simple approach and unique prospect can be helpful to some extent in reducing the bigger complexities.
In this article, you will find some amazing topics ideas that you can consider for your IB Chemistry internal assessment with maximum possibilities of scoring high.
Stoichiometry Relations
- Determine whether the oxidation of a coffee or tea affects its pH level.
- Find the content of calcium in milk by using Ethylenediaminetetraacetic acid (EDTA) titration.
- Calculate iron percentage in iron compounds by using redox titration.
- Determine the water of crystallization by using acid-base titration.
- Find the zinc concentration in a dietary supplement.
- Find the drug content in some tablets.
Chemical Bonding
- Explore how the solubility of salts is affected by the hardness of the water.
- Find the molecular formula of an unknown salt.
- Find the effect of boiling time on the protein concentration of milk by the bicinchoninic acid assay and colorimetry method.
Energetics
- Separate the tree leaves pigments by using the paper Chromatography method.
- Calculate the energy and calorie content of a Cheetos packet.
Chemical Kinetics
- Find the activation energy of a reaction.
- Determine how the rate of reaction increases by the increase in temperature.
- Examine the unsaturation level in various oil brands.
- Analyze the denaturation of lipase under various conditions.
Electrochemical Reactions
- Examine the factors affecting electroplating.
- Find how the strength of ferromagnets varies with temperature.
Organic Chemistry
- By using Sodium Hydroxide solutions separate the ethanol and methanol compounds.
- Try synthesizing a compound such as Paracetamol and aspirin.
- Design and test the buffer solutions.
Disclaimer:
- The topics suggested in this article are only for the motivational purpose and to give students some ideas of how you can link some real-world investigations with your IB chemistry internal assessment to score a good grade.
- Choose a topic that meets the IB syllabus criteria.
- Do not just copy-paste from the list mentioned.
- Always consult your instructor before finalizing any topic.
- Try coming up with your creative idea of investigating a particular topic.
Start your Free Trial today
Unrestricted access to IBDP questions, key concepts, solutions, performance reports and much more…
*Invite 2 students to subscribe to Blen and unlock FREE Access to IBDP content, assessments, assignments, students’ performance reports.
Navigating the Environmental Systems and Societies (ESS) IA
Mastering the ib english oral commentary, an ultimate handbook for ib math internal assessment (ia), a comprehensive guide to choosing history ia topics, unveiling the perfect economics ia: exploring real-world applications, a comprehensive manual for crafting ib psychology internal assessment (ia), stuck for ideas for your chemistry ia, the power of mathematics ia: unlocking creativity and success.

Blen is a powerful learning and teaching platform, thoughtfully designed for the IBDP community. With 100% IBDP Curriculum aligned resources such as interactive questions, key concepts, adaptive mock tests, assignments and detailed reports, unlock your true IBDP potential.
Be the first to know about our news and special offers direct to your inbox.
I consent to the processing of my personal data, in order to receive news and updates of Blen, according to the " Privacy Statement "

Bavesh Blogs
A step-by-step guide to writing a good ib chemistry internal assessment + my own.
Written by:
It can be a bit daunting to write up your experiment once you’ve done it, especially with the weirdly worded marking rubric and the challenge of explaining why you may have done things in a peculiar way. But fear not, for this guide should help you write up a killer IA in no time, with examples from my project along the way!
It should help you tick off everything in the mark scheme – just follow it step-by-step and most of the hard work will be done for you automatically. Remember to not copy my IA idea or some of the words I use… word-for-word because the IB dishonesty ban-hammer is omnipresent.
Of course, this isn’t the only way to write an IA but I think it’s a pretty fool-proof way to make sure you hit as many of those 24 points you can.
Introduction and Background Theory
1) Explain why you chose this specific investigation and why it’s personal to you, but you don’t need a “When I was five…”

2) I would just make the title of your IA your research question, structured with a clear “How does the IV affect the DV”. Then you can justify your methodology in the introduction (usually this involves saying why you chose this method over another to measure your dependent variable or alter the independent variable).

3) Next it’s time to hit them with some background theory! It does not have to be crazy. Most importantly it must be über-specific to your research question and not just be a paragraph from your IB textbook.
Imagine you’re trying to explain to your classmate i) Why your experiment should work (why does acid break starch, in my case or e.g. why does temperature increase the rate of your reaction) and ii) How you are planning to measure your variables to support that hypothesis.

But the key thing in this section is to pretend you’re an academic and find some similar experiments online (try Google Scholar) and reference what they did. Again, it has to actually be relevant. But the point is it makes it seem as if you are contributing to a field, and that’s pretty dope! I got to include some fancy diagrams to show a proposed mechanism to the reaction I was investigating, for example.

Methodology (Exploration)
1) Hopefully you get a chance to do a trial run of your experiment or something vaguely similar before you were let loose on all the chemicals. It’s fine if it goes a bit wrong – it’s probably actually a good thing because you can write a lot of stuff the IB will reward you lovingly for. It’s actually this part where you show personal engagement (not childhood stories).
Explain how you changed up the quantities you chose, how many repeats you did, how you set up the equipment in an innovative way, the ranges of your variables. Extra brownie points if you can tell them how any of these may reduce the systematic errors (especially if you had to change your whole methodology).
I essentially got to write a whole page about my faults because my experiment trial run straight up did not go so well.

2) Next up is to introduce what chemicals (including their concentrations/moles/volumes) and apparatus you’re gonna use for the final experiment. Include the uncertainties associated with the apparatuses here, lest you forget the numbers later on. The best way to do this is in a table.

3) Then go ahead and write up the method you actually used in your final experiment, using numbered step-by-step instructions. You probably will have to use some numbers in this section and sometimes this can be complicated – for traffic light/equilibrium reactions or similar ones you may be changing multiple concentrations to yield the same volume of solutions and the best way to present this would be in a table.
Make it seem as if you’re doing enough experiments and repeats (usually 3-5) that this makes up 4-6 hours of lab work, as the IB expects and, even if it didn’t work out, emphasise that you did as much as you could in that time frame to collect a sufficient data set to support your hypothesis.

4) You won’t get brownie points for spamming your assignment with selfies you took in the lab. It’s more professional to sketch out your equipment setup if it needs a bit of explaining in the typical pre-GCSE way (like drawing a three-line tripod for a bunsen burner) otherwise ditch the pictures/figures entirely.

5) If you’re doing a bit of a complicated experiment, chances are you are going to have to do some fiddling around with the uncertainties of your independent, dependent and possibly even control variables. This is a good place to introduce them, as well as re-state your independent and dependent variables clearly. If you had to dilute a solution or had to use pipettes a different number of times for different solutions, uncertainties for the same variable could be quite different and things can get pretty yucky. In this case, you don’t need to be exhaustive, but show the method you used to calculate the uncertainty for an exemplar concentration.

6) To get the highest band of exploration, you need to take into consideration “all or nearly all” the significant factors that can affect your data and present error. The fool-proof way to tick off this box is to do this next section really well – control variables. If you can’t actually control a variable for whatever reason, you must monitor it (e.g. so you can say ambient temperature stayed the same). You can take an entire page if you need to and the best way to do it is to come up with at least five and put them in a table like this:

7) Great! You’re almost done with this bit. There’s a pesky bit in the Exploration section you need to hit to score well here, but it’s easy to do in a few sentences – “The report shows evidence of some awareness of the significant safety, ethical or environmental issues that are relevant to the methodology of the investigation”
- Safety. For this bit, state basically every chemical you are using and its maximal concentration or mass in your experiment. Then look up the hazards using CLEAPSS and reference them (just search the relevant chemicals in the search bar), no matter how small (for harmless powders this can simply be something like respiratory hazard). You can also mention that you need to be careful about glassware and hot liquids. Then state what safety protocol you are going to take to minimise the hazard. 3 sentences each – done.

- You can tick off the environmental and ethical concerns in one sentence each. Mention how the chemicals are disposed so they minimise harm to aquatic life (usually via a chemical sink or thorough dilution) and you can say there are no major ethical implications apart from the usage of your schools resources/chemicals etc.
Results and Data Processing (Analysis)
WOO!! Now onto the bit where everyone changes their data but doesn’t know what the data was supposed to show in the first place and inevitably messes up by trying to show they didn’t mess up in the lab. Save yourself the trouble. If you think your results show the exact opposite of whatever you wanted, it’s ok . It makes for a more interesting second half of your report anyway, usually. Besides, the IB demands academic honesty! 🙂
1) Qualitative Results. For some reason lots of people forget this, but you absolutely need it to get more than 2/6 marks in the Analysis section of the mark scheme! Just say what you saw (a color change or effervescense – a fancy word for bubbling). If nothing much happened you can always say the powder disappeared (or diluted if you’re a non-believer) or settled on the bottom. Again, you don’t need pictures unless they’re absolutely vital (you can always make it the feature image in your blog on how to write an IA in the future, anyway).
2) Quantitative Results. Present it as a raw data table. Invariably some of you will have pages upon pages of data and obviously you shouldn’t present all of this here – just take a snippet (nobody really knows if you should include all of the rest in an appendix at the end, but I would advise against having an appendix at all).

3) You can choose to do this after your graph but you need to discuss your anomalies and the major limitation of your experiment (maybe it was just that you didn’t end up doing enough repeats to generate reliable data). If you have enough data (which you almost 90% will NOT) you may introduce standard deviations on your data.
4) Look at this graph! Hopefully, you can generate a line graph with your hopefully non-categorical data. Remember the general pointers: the data should take up most of the space of the axes, a title, error margins/uncertainties on both of the axes and best fit lines or curves, as relevant. You can also pop in a R^2 value (Excel will do this automatically for you if you ask). Again, if you have enough data you can pop in error bars, but you don’t need them (much to biologists’ upset). You can circle your anomalies if it makes them clearer.

5) Describe your graph simply e.g. “negative linear correlation” and comment on the R^2 values (these show how well the trendlines fit the data you gave). Comment on how many of your points were anomalous and what you would have expected from the literature data value/literature explanation/previous experiments/hypothesis (in a descending order of preference). If there is a literature value, you can work out a percentage error.
6) Conclusion! My graph shows that …… this makes sense/doesn’t align with my hypothesis because ….. One exception to this was …..
K.I.S.S – Keep it simple, silly!

7) Data processing. This will REALLY depend on the experiment you performed, but you need to do some sort of rigorous calculation or technique that is relevant to helping you answer your research question. The IB care a lot about the accuracy of your calculations and the maintenance of an appropriate degree of significant figures.
If you had a graph, a good way to do this may be to use Min-Max error processing to see how big your final uncertainties actually were (this is different to the uncertainties you had already measured and stated previously). You can just show an example of how you did this if you had multiple lines and then present the results in a table.

A few extra tick marks to make sure you fulfil in this section are:
- Comparing the size of your trend to the magnitude of your uncertainties and errors
- Whether there was actually enough data to make a solid conclusion
- Whether your control variables tampered with your final results
- How closely your repeats were to each other (i.e. precision) – this affects how valid your conclusion may be
- Being honest and critical! More on this in the next bit….
Judgin’ and Extendin’ (Evaluation)
You’re probably on the last page or so of your write-up now and it can be tempting to say that you can just improve next time by completely changing your methodology to use fancy, expensive apparatus or just do a billion more repeats but this is a section that is actually really important. In just a few paragraphs you can score up to a third of your total IA mark!
1) Evaluate your strengths. Maybe you kept the room the same temperature, that’s worth something! You can say even these can be improved farther e.g. using draft excluders.
2) Evaluate your weaknesses. There probably were inevitably some human and random errors and these are worth a mention. However, the main point of this section is highlighting the unavoidable systematic errors that arose from your methodology and how improvements could be made to minimise them if you were to do it all over again. If you’re struck for ideas mention more precise apparatus, a bigger sample size or a larger range for your independent variable. Also ensure to compare how big systematic vs random errors were in impacting how strong of a conclusion you could have made. Again, I would use a table:

3) To finish up, write a sentence suggesting a similar but realistic experiment that could be useful, usually to explore a similar topic e.g. if you investigated glucose, you could try looking at galactose next time.
Communication
YOUR IA SHOULD BE NO LONGER THAN 12 PAGES.
If it is, the first step is to cut out the fluff. Please be careful with changing table margins (though having things tabulated in the first place can save some valuable space, hence why I used so many) and font sizes/spacing. The IB don’t like this.
You can also save space by not being silly. You don’t need a page long bibliography at the end: it is perfectly acceptable to use a footnote and numbers-in-text approach to referencing. Just keep it consistent and have the bare minimum like access dates for websites. Ask your teacher, but I really don’t think you need an appendix either – nobody knows if you will be penalised for it, but it almost certainly won’t be read if your IA gets externally moderated, anyway.

Make sure everything is anonymous, you have page numbers, all the graphs are labelled and it looks nice, large and pretty. Tables must have their relevant uncertainties (once in the column heading, unless they’re all different) and make sure your numbers have units. You can even number your equations if you used quite a few, otherwise treat everything else (even tables) as figures and number them accordingly. You can use a text box to do this.

The last check is finding anywhere in your IA you used the words accuracy, precision, reliability and error. Make sure you used all of them correctly and ask someone if in doubt. This really annoys teachers and moderators alike if you mess it up.
Congratulations! That’s one fine IA if I say so myself 🙂
Share this:
Leave a comment cancel reply.
Latest Articles

How to do well in Stage 2 Exams (KCL Medicine) + Quesmed Review

Chronicles of Spending 2,600 Hours on a School Bus

My Journey to the Himalayas!

- Already have a WordPress.com account? Log in now.
- Subscribe Subscribed
- Copy shortlink
- Report this content
- View post in Reader
- Manage subscriptions
- Collapse this bar
IB CRASH COURSE FOR MAY SESSION 2024
For more details related to IBDP 1 Crash Course, Please Download IBDP 1 Brochure. For more details related to IBDP 2 Crash Course, Please Download IBDP 2 Brochure. For more details related to IBMYP Crash Course, Please Download IBMYP Brochure.
For Any Queries related to crash course, Please call at +918825012255

IB Chemistry IA Guide: Everything you need to know
- April 24, 2023

Table of Contents
- 1.1 Introduction:
- 1.2 Materials and Methods:
- 1.3 Data Collection and Analysis:
- 1.4 Results and Discussion:
- 1.5 Conclusion:
- 1.6 Evaluation:
Chemistry is a fascinating subject that involves the study of matter and its properties, composition, and reactions. The Internal Assessment (IA) is an essential part of the International Baccalaureate (IB) program. It is an opportunity for students to showcase their understanding of chemistry and apply it to a real-world problem or investigation. The IA is graded on a scale of 1 to 7, with 7 being the highest possible score. For IB students, the Internal Assessment (IA) is an important component of the chemistry course. It allows students to demonstrate their understanding of the subject and apply their knowledge to a real-world problem or investigation.
Structure of the Chemistry IA:
Introduction:.
This section should provide an overview of the research question or problem that the IA is investigating. It should include a clear hypothesis and any relevant background information.
Bad IA Introduction:
In this IA, we conducted an experiment to see the reaction between hydrogen peroxide and potassium iodide. We wanted to see how fast the reaction would occur and if it would change with different concentrations of hydrogen peroxide. We think that this experiment is important because hydrogen peroxide is used in many different applications, and it is important to understand its chemical properties.
Explanation: This introduction is too brief and lacks detail. It doesn’t provide a clear research question or hypothesis, which makes it difficult for the reader to understand the purpose of the experiment. It also doesn’t explain why the experiment is significant and how it relates to previous research or real-world applications.
Good IA Introduction:
The experiment conducted in this IA aims to investigate the effect of different concentrations of hydrogen peroxide on the rate of reaction with potassium iodide. This experiment is significant because it provides insight into the reaction kinetics of hydrogen peroxide and potassium iodide and how their reaction rates change as the concentration of hydrogen peroxide varies. Additionally, this experiment is relevant in understanding the properties of hydrogen peroxide and its potential applications in various industries. The hypothesis for this experiment is that as the concentration of hydrogen peroxide increases, the reaction rate will also increase.
Explanation: This introduction provides a clear research question and states the significance of the experiment. The hypothesis is also clearly stated and relates to the research question. The introduction provides enough context for the reader to understand the importance of the experiment.
Materials and Methods:
In this section, you should describe the materials and methods used to carry out the experiment. You should also describe any safety precautions that were taken.
Bad Materials and Methods Section:
- Incomplete list of materials and equipment used, with no details about their specifications or sources.
- Vague or confusing description of the experimental procedure, with missing steps or unclear instructions.
- No mention of any safety precautions taken during the experiment.
- Lack of detail about how the data was collected and recorded, including measurements or observations.
- No discussion of potential sources of error or limitations of the experimental design.
Explanation: A bad materials and methods section lacks sufficient detail to allow others to replicate the experiment. It may be confusing or incomplete, making it difficult to understand the experimental procedures. The section also fails to include any information about safety precautions or potential sources of error, which can impact the reliability and validity of the experiment.
Good Materials and Methods Section:
- Detailed list of materials and equipment used in the experiment, including their specifications, manufacturers, and sources.
- Clear and concise description of the experimental procedure, including step-by-step instructions that can be easily followed by others.
- Explanation of any safety precautions taken during the experiment, such as the use of protective equipment and proper handling of hazardous chemicals.
- Clear and concise explanation of how the data was collected and recorded, including any relevant measurements or observations.
- Discussion of any potential sources of error or limitations of the experimental design.
Explanation: A good materials and methods section provides a clear and detailed description of the experimental procedures used in the study. It should be written in a way that allows others to replicate the experiment and obtain similar results. The section should also include any relevant safety precautions and potential sources of error to ensure the reliability and validity of the experiment.
Data Collection and Analysis:
This section should describe the data that was collected during the experiment and how it was analyzed. This can include tables, graphs, and other visual representations of the data.
Bad Data Collection and Analysis Section:
- Incomplete or unclear presentation of the data, with missing or inaccurate information.
- Lack of detail about the methods used to analyze the data, or inadequate explanation of the statistical tests or other techniques used.
- Inaccurate reporting of the results, with incorrect units or significant figures.
- No discussion of trends or patterns observed in the data, or no reference to the research question or hypothesis.
- No comparison of the results to previous research or theories in the field.
Explanation: A bad data collection and analysis section may be incomplete or unclear, making it difficult for the reader to understand the results. It may lack detail about the methods used to analyze the data, or inaccurately report the results. The section may also fail to identify any trends or patterns in the data or compare the results to previous research or theories, making it difficult to assess the significance of the experiment.
Good Data Collection and Analysis Section:
- Clear and concise presentation of the data, including tables, graphs, or charts.
- Thorough explanation of the methods used to analyze the data, including statistical tests or other techniques.
- Accurate and precise reporting of the results, with appropriate units and significant figures.
- Discussion of any trends or patterns observed in the data, with references to the research question or hypothesis.
- Comparison of the results to previous research or theories in the field.
Explanation: A good data collection and analysis section provides a clear and thorough presentation of the data, with a detailed explanation of the methods used to analyze it. It accurately reports the results and identifies any trends or patterns observed in the data. The section also includes a comparison of the results to previous research or theories, demonstrating the significance of the experiment.
Results and Discussion:
In this section, you should present your findings and discuss their significance. This can include a comparison of your results to previous research or theories, as well as an explanation of any unexpected or anomalous results.
Bad Results and Discussion Section:
- Unclear or confusing presentation of the results, with no discussion of any trends or patterns observed in the data.
- Superficial or incomplete discussion of the implications of the results, with no clear relation to the research question or hypothesis.
- No discussion of potential sources of error or limitations of the experimental design that may have impacted the results.
- Lack of detail about the significance of the results, with no discussion of their contributions to the understanding of the field or real-world applications.
- No reference to previous research or theories in the field, with no comparison of the results to existing knowledge.
Explanation: A bad results and discussion section may be unclear or confusing, making it difficult for the reader to understand the implications of the results. The section may also lack detail about potential sources of error or limitations of the experimental design that may have impacted the results. The significance of the results may be superficial or incomplete, with no clear relation to the research question or hypothesis. The section may also fail to reference previous research or theories in the field, making it difficult to assess the significance of the results.
Good Results and Discussion Section:
- Clear and concise presentation of the results, including any trends or patterns observed in the data.
- Thorough discussion of the implications of the results, including how they relate to the research question or hypothesis.
- Explanation of any potential sources of error or limitations of the experimental design that may have impacted the results.
- Discussion of the significance of the results, including how they contribute to the understanding of the field or have real-world applications.
- Reference to previous research or theories in the field, with a comparison of the results to existing knowledge.
Explanation: A good results and discussion section presents the results in a clear and concise manner, with a thorough discussion of their implications. The section includes an explanation of any potential sources of error or limitations of the experimental design that may have impacted the results. The significance of the results is discussed, including how they contribute to the understanding of the field or have real-world applications. The section also references previous research or theories in the field, with a comparison of the results to existing knowledge.

Conclusion:
This section should summarize the main findings of the experiment and any conclusions that can be drawn from them. You can also suggest areas for future research.
Bad Conclusion:
- Merely restates the results without providing any new insights or analysis.
- Fails to reflect on the significance of the results or relate them to the research question or hypothesis.
- Does not identify any limitations of the experimental design or suggest future areas of research.
- Is too brief or lacks any substantive content.
- Does not leave a lasting impression on the reader or emphasize the importance of the research.
Explanation: A bad conclusion merely restates the results without providing any new insights or analysis. It may fail to reflect on the significance of the results or relate them to the research question or hypothesis. The conclusion may also not identify any limitations of the experimental design or suggest future areas of research. It may be too brief or lack any substantive content, and does not leave a lasting impression on the reader or emphasize the importance of the research.
Good Conclusion:
- Summarizes the key findings of the experiment, including any trends or patterns observed in the data.
- Restates the research question or hypothesis, and discusses how the results relate to them.
- Reflects on the significance of the results, including how they contribute to the understanding of the field or have real-world applications.
- Identifies any limitations of the experimental design and suggests future areas of research.
- Leaves a lasting impression on the reader and emphasizes the importance of the research.
Explanation: A good conclusion summarizes the key findings of the experiment, restating the research question or hypothesis and discussing how the results relate to them. The significance of the results is reflected upon, including their contributions to the understanding of the field or real-world applications. Any limitations of the experimental design are identified, and future areas of research are suggested. The conclusion leaves a lasting impression on the reader and emphasizes the importance of the research.
Evaluation:
In this final section, you should reflect on the strengths and weaknesses of the experiment, including any limitations or sources of error. You can also discuss any modifications that could be made to improve the experiment in the future.
Bad Evaluation Section:
- Fails to provide a critical evaluation of the experimental design or methodology used.
- Does not reflect on any limitations or sources of error in the experimental design or suggest improvements for future research.
- Does not discuss the reliability and validity of the results, including the precision and accuracy of the measurements.
- Does not compare the results to previous research or theories in the field, or explain any discrepancies or inconsistencies.
- Does not reflect on the overall success of the experiment or its potential impact on the field.
Explanation: A bad evaluation section fails to provide a critical evaluation of the experimental design or methodology used. It may not reflect on any limitations or sources of error in the experimental design or suggest improvements for future research. The section may not discuss the reliability and validity of the results, including the precision and accuracy of the measurements. It may not compare the results to previous research or theories in the field, or explain any discrepancies or inconsistencies. The section may not reflect on the overall success of the experiment or its potential impact on the field.
Good Evaluation Section:
- Provides a critical evaluation of the experimental design, including the methodology, procedures, and instruments used.
- Reflects on any limitations or sources of error in the experimental design and suggests improvements for future research.
- Discusses the reliability and validity of the results, including the precision and accuracy of the measurements.
- Compares the results to previous research or theories in the field, and explains any discrepancies or inconsistencies.
- Reflects on the overall success of the experiment and its potential impact on the field.
Explanation: A good evaluation section provides a critical evaluation of the experimental design, including the methodology, procedures, and instruments used. It reflects on any limitations or sources of error in the experimental design and suggests improvements for future research. The section discusses the reliability and validity of the results, including the precision and accuracy of the measurements. It compares the results to previous research or theories in the field, and explains any discrepancies or inconsistencies. The section reflects on the overall success of the experiment and its potential impact on the field.
Here are a few pointers on how to format your Chemistry IA well:
- Cover Page: Include a cover page with the title of the IA, your name, date of submission, and any other relevant information, such as your school and the subject in which you conducted the experiment.
- Table of Contents: Include a table of contents that lists the different sections of the IA, including the introduction, materials and methods, data collection and analysis, results and discussion, evaluation, and conclusion.
- Page Numbers: Number the pages of your IA and include them in the table of contents.
- Font and Spacing: Use a clear and legible font, such as Times New Roman or Arial, and set the font size to 12 points. Use double-spacing throughout the IA, except for the table of contents, figures, and tables, which may require single-spacing.
- Headings and Subheadings: Use headings and subheadings to clearly separate and organize each section of the IA. Use a consistent formatting style, such as bold or italicized text, for all headings and subheadings.
- Figures and Tables: Include any figures or tables in the appropriate sections of the IA and label them clearly. Refer to the figures and tables in the text and explain their significance in the results and discussion section.
- Citations and References: Use appropriate citation style, such as APA or MLA , to cite any sources used in your IA. Include a reference list at the end of the IA that lists all sources cited in the text.
- Appendices: Include any additional materials, such as raw data or calculations, in the appendices section of the IA.
- Proofread: Before submitting your IA, proofread it carefully to ensure that it is free of grammatical errors and typos. Ask someone else to review it as well to catch any mistakes you may have missed.
In conclusion, the structure and format of a Chemistry IA play an essential role in ensuring that the research is well-organised, informative, and understandable. A well-structured IA consists of several key components, including a clear introduction, detailed methodology, accurate data analysis, insightful results, and thoughtful evaluation. By following a consistent formatting style, including clear headings, page numbers, and references, you can present your research in a professional manner and showcase your scientific knowledge and skills.
Ultimately, a well-written Chemistry IA not only demonstrates your understanding of the subject matter but also serves as a valuable resource for others in the scientific community. By paying attention to the structure and format of your IA, you can produce a high-quality research document that contributes to the field of chemistry and beyond.
You May Also Like!

Inside GWU: George Washington University Admissions
Table of Contents1 Introduction to GWU2 Admission Requirements3 Application Process4...

Inside Brown University: Demystifying Admission Requirements
Table of Contents1 Introduction to Brown University2 Admission Requirements for...

Emerson College Admission Requirements: A Closer Look
Table of Contents1 Introduction to Emerson College2 Admission Requirements for...

Bowdoin Admission Requirements: Your Path to Excellence
Table of Contents1 Introduction to Bowdoin College2 Admission Process and...

Boston University’s Admission Requirements Unraveled
Table of Contents1 Introduction to Boston University2 Requirements for Undergraduate...

BYU Admission Requirements: A Closer Look
Table of Contents1 Introduction to BYU2 Overview of BYU’s Admission...
Leave a Reply Cancel reply
You must be logged in to post a comment.
We Are Here To Help You To Excel in Your Exams!
Book your free demo session now, head office.
- HD-213, WeWork DLF Forum, Cyber City, DLF Phase 3, DLF, Gurugram, Haryana-122002
- +919540653900
- [email protected]
Tychr Online Tutors
IB Online Tutor
Cambridge Online Tutor
Edexcel Online Tutors
AQA Online Tutors
OCR Online Tutors
AP Online Tutors
SAT Online Tuition Classes
ACT Online Tuition Classes
IB Tutor in Bangalore
IB Tutors In Mumbai
IB Tutors In Pune
IB Tutors In Delhi
IB Tutors In Gurgaon
IB Tutors In Noida
IB Tutors In Chennai
Quick Links
Who We Are?
Meet Our Team
Our Results
Our Testimonials
Let’s Connect!
Terms & Conditions
Privacy Policy
Refund Policy
Recent Articles
International ib tutors.
IB Tutor in Singapore
IB Tutor in Toronto
IB Tutor in Seattle
IB Tutor in San Diego
IB Tutor in Vancouver
IB Tutor in London
IB Tutor in Zurich
IB Tutor in Basel
IB Tutor in Lausanne
IB Tutor in Geneva
IB Tutor in Ontario
IB Tutor in Boston
IB Tutor in Kowloon
IB Tutor in Hong Kong
IB Tutor in San Francisco
IB Tutor in Dallas
IB Tutor in Houston
IB Tutor in Chicago
IB Tutor in New York City
IB Tutor in Brooklyn
IB Tutor in Washington
IB Tutor in Berkshire
IB Tutor in Sussex
IB Tutor in Melbourne
IB Tutor in Western Australia
Ⓒ 2023 TYCHR ACADEMY | All Rights Reserved
Dive in, explore, and experience firsthand what sets us apart.

ANNOUNCEMENT
Download our Successful College Application Guide developed by counselors from the University of Cambridge for institutions like Oxbridge alongside other Ivy Leagues . To join our college counseling program, call at +918825012255
We are hiring a Business Development Associate and Content Writer and Social Media Strategist at our organisation TYCHR to take over the responsibility of conducting workshops and excelling in new sales territory. View More
IA Criteria Checklist
Use the following checklist to guide you through the planning, carrying out and write up of your Internal Assessment
Click the link for audio of this page
Below are some frequently asked questions regarding the Chemistry Internal Assessment
Jump to a question:
How do I decide on a topic?
Try to choose a topic that you find personally interesting or relevant to your studies, it will be easier to demonstrate personal engagement and interest
How do I decide on a my Research Question?
Consider the variables you wish to measure, and the data you are collecting to formulate a detailed RQ
How do I demonstrate personal engagement?
It is important that you justify the reasons behind choosing your research area and your RQ. Making the experiement your own and novel design of practical work / carrying out preliminary investigations all demonstrate personal engagement
How do I get good marks for the communication criteria?
Reports should be written in the 3rd person and care taken with spelling, grammar and punctuation. The report needs to follow a logical order and all chemical terms used appropriately
Do I have to do an experiment?
No, you can choose to do a database IA
Do I have to order my own chemicals / equipment ?
No, we will provide as long as the requisition form is handed in on time
How will I be assessed?
Your teacher will mark each IA and then it will be moderated by the other chemistry teachers. A sample is submitted to IB for final marks
How long does the IA take ?
2 weeks of lessons for the action phase and the write up. Around 10 hours total
Do I have to cite my research ?
Yes, all sources must be cited
Do I have to include every piece of data?
Clear samples of raw data must be included and all data collected can be contained in the appendix
How long should it be?
No more than 12 pages
2024 Chemistry Appreciation Night
A Chemistry Celebration The University of Iowa 2024
Event Schedule 5:00 PM - Reception & Poster session*
6:00 PM - Ceremony
- Welcome – Prof. Renée Cole (DEO)
- Undergraduate Awards – Prof. Mona Maalouf
- Chemistry & Sustainability Study Abroad- Prof. Adam Brummett
- Graduate Awards – Prof. Scott Shaw
- CGSA Awards – Samantha Kruse & Darby Duffy
- Closing remarks – Prof. Renée Cole
Undergraduate Awards & Recognition
Department Awards Donald J. Burton & Margaret A. Burton Scholarship
- Asya Lengel
E. David Cater Scholarship
- Kailey Krigas
Russell K. Simms Scholarship
- Ferris Bissen
- Parker Kintigh
Chemistry Alumni Awards
Senior Recipient
- Casey Huettman
Junior Recipient
- Jenna Ringwald
Sophomore Recipient
- Nadia Arsenijevic
CRC Freshman Chemistry Award
- Lily Nelson
Merck Index Award
American Institute of Chemists Award
- Ruben Delatorre
ACS Division of Analytical Chemistry Award
ACS Division of Inorganic Chemistry Award
ACS Division of Organic Chemistry Award
- Karah Putnam
ACS Division of Physical Chemistry Award
- Griffin Hancock
Summer Undergraduate Research Award
- Cade Hoambrecker
Viksnins, Harris, & Padys Poster Award
Andrew Burgess
Carson Lovig
Chemistry & Sustainability Student Abroad Scholarship
- Jessica Miller
- Michael Lauer
- Carsen Mellinger
- Ella Schlueter
B.S. and B.A. Graduates
Fall 2023: Rachael Bertolino (BS), Steven Palacios (BS), Aasthika Das (BA), Richard Whiteside (BS), Trevor Larkin (BS), Jackson Tupper (BS)
Spring 2024: BS : Sophie Alessio, Emma Barker, Leighton Barnes, Tommy Branstad Phillips, Aidan Craven, Sam Dolde, Griffin Hancock, Carter Swanson, Ferris Bissen, Julianne Green, Victor Calderon
BA : Ben Alexander, Anna Cacini, Brady Dunn, Aidan Freshly, Emry Hahn, Peyton Hedger, Cassidy Kamman, Kierra McKernan, Abril Mendez, Emily Miller, Lily Poci, Karah Putnam, Matthew Salas, Samantha Schwarte, Hajar Smith, Claire Stevens, Mackenzee Albert, Bergen Ensminger, Mackenzie Green, Cole Hillier, Kieran McLaughlin, Casey Huettman
Graduate Student Awards & Recognition
Department Awards
A. Lynn Anderson Award for Research Excellence
- To be announced
Kathryn K. & James D. Steele Scholarship
- Andrew Lazicki
- Darby Duffy
University Recognition
Ballard and Seashore Fellowship
- Piyumi B. Wijesirigunawardana, Fall 2024
- Dilini M. Kirindigoda Gamage, Fall 2024
- Colleen Lasar, Spring 2024
- Andrea Van Wyk, Spring 2024
- Chamika Madawala, Spring 2024
Graduate College Post-Comprehensive Research Fellowship
- Grace Akporere, Fall 2024
- Emmanuel Y Bonsu, Fall 2024
- Andrew Lazicki, Spring 2024
- Josie Welker, Spring 2024
Graduate College Summer (2024) Fellowship
- Uchechukwu Akporere
- Emmanuel Bonsu
- Thomas Chiarella
- Akalanka Ekanayake
- Janeshta Fernando
- Dilini Kirindigoda Gamage
- Heather Koska
- Jeewani Meepage
- Janadhi Nakath Durage
- Dunya Sembukuttiarachchige
- Mai Yer Yang
OVPR Dare to Discover Banner
- Samantha Kruse
External Recognition NSF Graduate Research Fellowship
- Abby Carlin
NSF Graduate Research Fellowship Honorable Mention
- Nicole Shapiro
2023-2024 M.S. Graduates & Degrees Received
Summer 2023: Jalen Dickson, Andrew Horvath, Grant Shivers
Fall 2023: Collin Hill, Janadi Nakath Durage, Bishnu Neupane, Raphael Ogbodo, Jacob Schuely
Spring 2024: Leslie Bolda, Bichitra Borah
2023-2024 Ph.D. Graduates
Summer 2023: Jacob Hackbarth, Whitney Harmon, Hong Bok Lee, Leah Scharlott, Joshua Zgrabik
Fall 2023: Joshua Coduto, Kasun Dadallagei, Hoang Dang, Christopher Hartwick, Christopher Knutson, Ishanka Liyanage, Nicholas Luedtke, Chamari Mampage, Arjun Paudel, Nimesh Ranasinghe Arachchige
Spring 2024: Matthew Emerson, Tiron Jahinge, Chamika Madawala, Hannah Nennig, Celymar Ortiz de Leon, Andrea Van Wyk
CGSA Peer Awards
The Graduating class of 2024 recognizes these UI faculty and staff members for making a positive difference in their lives during their time at the University of Iowa. President Barbara Wilson, Executive Vice President and Provost Kevin Kregel, and Vice President for Student Life Sarah Hansen join in thanking all of the valuable faculty and staff members who work every day to make a difference for our students. And congratulations to the class of 2024!
Aditi Bhattacherjee
Adam Brummett
David Cwiertny
Caleb DeWitt
Tori Forbes
Dominic Frisbee
Maxwell Geng
Rebecca Laird
Johna Leddy
Mouna Maalouf
David Martin
Louis Messerle
Chris Pigge
Binaya Shrestha
Michael Sinnwell
Betsy Stone
Amy Strathman
Alexei Tivanski
Florence Williams
Names were collected through the UI Senior Exit Survey, which all undergraduates are invited to complete at the time they file to graduate. Names in bold were mentioned more than 5 times.
Chemistry Appreciation & Awards Night Poster Session Participants
Iowa Reading Research Center

Dialogic Reading: Having a Conversation about Books
(View and download versions of this post in languages besides English at the bottom of this page)
Parents and teachers alike nearly universally accept the importance of reading to children. Even pediatricians and hospitals encourage it . However, reading aloud can have greater educational value than serving as a filler activity before bedtime or lunch.
It is easy to simply take a book of a shelf, read it, and maybe ask a few questions at the end of the story without giving much thought to the selection of the book or the purpose of reading the story. Maybe it is the child’s favorite, or it is just the right length for the time allotted. Maybe it fits with the class theme of the week. However, these are all adult-directed examples. The adult selects the book, reads the story, and asks the questions or leads the discussion.
A more engaging and productive alternative is interactive reading or dialogic reading. Dialogic reading involves an adult and child having a dialogue around the text they are reading. Their conversation includes defining new vocabulary, improving verbal fluency, introducing story components, and developing narrative skills.
When parents and teachers properly plan and execute dialogic reading, research suggests it can be particularly effective at improving skills such as print awareness, oral language, and comprehension. Most importantly, it helps model how good readers think about the text as they are reading it. The benefits of dialogic reading are not just for young children. Although the concept originated with picture book reading for preschoolers (Whitehurst et al., 1988), a wide body of research has extended its use to other ages and populations including students with disabilities (What Works Clearinghouse, 2010), struggling readers (Swanson et al., 2011), and English language learners (Brannon & Dauksas, 2014).
Different Levels of Questions in Dialogic Reading
A traditional implementation of dialogic reading involves repeatedly reading the same book and interacting around three levels of questions (Flynn, 2011).
- Level 1 questions are basic “wh” type questions focused on what can immediately be seen (or read) in the text. Level 1 also includes introducing new vocabulary. For example, while pointing to a picture, the adult reader may ask, “What type of feet does this animal have?” Here the adult is looking for a specific, correct response to expand the child’s oral vocabulary.
- Level 2 questions are open-ended and are used to solicit the child’s feedback. For example, the adult reader might ask, “What is happening in this part of the story?” Here the adult is trying to encourage the child to share what he or she is thinking about and make meaning from the text.
- Level 3 questions are more advanced and introduce concepts like text features and story components. For example, the adult reader might ask, “Who was the main character and how did he feel?” This also may include questions that distance the student from the text and connect the story to their own life. For example, “How would you feel if that happened to you?”
Differences Between Correct Ways and Incorrect Ways to Utilize Dialogic Reading
Dialogic reading....
- Is not "teacher reads, students listen"
- Is not teacher led
- Is not limited to narrative text with questions and discussions at the end
- Is not only for very young children
- Is interactive
- Is student centered
- Is conducted with narrative and expository text with questions and discussion throughout
- Is an activity requiring careful planning
- Is for students at all levels
Although several strategies exist to facilitate the three tiers of questions in dialogic reading, the two most popular are known by the acronyms CROWD and PEER . Both have been covered in previous IRRC blog posts, and this post will expand on the two strategies and provide another resource to help with planning a dialogic reading activity.
Covering the Various Question Tiers with CROWD
CROWD is used to remember the types of questions to ask: c ompletion, r ecall, o pen-ended, “ w h” questions, and d istancing. An extension of CROWD is CROWD-HS, which is used to encourage distancing prompts related to h ome and s chool. Here is an example of CROWD-HS questions for the well-known story of The Three Little Pigs .
C ompletion question: “I’ll huff, and I’ll puff, and I’ll __________ ___________ _________ ___________.”
Answer: Blow your house down.
R ecall question: Which house couldn’t the Big Bad Wolf blow down?
Answer: The one made of bricks.
O pen-ended question: Why do you think the first pig built his house out of straw?
Answer: It was the easiest to build. He was lazy.
“ W h” question: What kind of animal was after the pigs?
Answer: Wolf.
D istancing: How do you think the pigs felt when the wolf tried to get them?
Answer: (Answers will vary.) Scared, angry, sad.
H ome question: If you had to build a playhouse at home, what kind would you build?
Answer: (Answers will vary.) Tree house, tent, fort.
S chool question: The wolf was a bully. He was mean to the three little pigs. What should you do if someone is bullying you at school?
Answer: (Answers will vary.) Tell a teacher. Tell them to stop. Ignore them.
Prompting More Dialogue with PEER+PA
CROWD-HS is helpful for remembering the types of questions to ask, but remember that the point is to foster a dialogue about the text, not prompt one-word answers. The PEER ( p rompt, e valuate, e xpand, and r epeat) strategy can be used to help the adult encourage deeper responses. An extension of PEER is PEER+PA, which is used to remind the adult to p raise the child for engaging in conversation and help the child a pply the response so it is meaningful. These components are applied throughout the dialogue with the child. Here is an example of using PEER+PA with one of the above CROWD-HS questions:
P rompt the child to say something about the book.
Adult: What kind of animal was after the three little pigs?
Child: Wolf.
E valuate the child’s response.
Adult thinks to self, “Yes, it was a wolf, but we can add more to that response.”
E xpands the child’s response.
Adult: Yes, it was a big, bad wolf!
R epeat the prompt.
Child: A big, bad wolf!
P raise and A pply the child’s response.
Adult: That’s right! The big bad wolf was after the three little pigs. Good job remembering the story! How would you feel if you saw a big bad wolf?
Child: (Answers will vary.) I’d be scared and run away.
Teaching Vocabulary Through Dialogic Reading
Recall that an important goal of dialogic reading is to expand the oral language skills of students, particularly vocabulary. One way to do this is to engage in pre-reading activities to teach key vocabulary. Just as there are levels of questions to facilitate dialogue about the text, it is easy to think about vocabulary words in three tiers:
- Tier 1 words are basic vocabulary words that should be in the child’s receptive and expressive vocabularies. Tier 1 words in the story of The Three Little Pigs would include: house, pig, wolf.
- Tier 2 words occur with high frequency in texts for mature language users. These words should be taught to help expand the receptive and expressive vocabularies of the child. A Tier 2 word in the story of The Three Little Pigs might be eldest (some versions of the story characterize the first pig as the eldest of the three).
- Tier 3 words are domain specific and best learned when needed in a content area or in a specific context. These words are less likely to occur in a story like The Three Little Pigs. However, a more sophisticated version of the story might include the Tier 3 word for the bricklaying tool the third pig used: trowel.
Although some words can be introduced during dialogic reading, it is important to carefully identify and pre-teach any words that might otherwise interfere with a student’s comprehension of the book. The adult reader can identify Tier 2 words that will be helpful for understanding the current text and that the child is likely to use or read in other settings.
Now that we are swimming in levels, tiers, and alphabet soup, how do we keep our heads above water, bring it all together, and actually engage in dialogic reading? The most important thing is to adequately prepare! This includes selecting appropriate text that encourages dialogue, identifying vocabulary to pre-teach, thinking of CROWD-HS questions, and anticipating child responses with which you can use PEER+PA to expand the dialogue. The Interactive Reading Guide below can be put in every book to help you plan the vocabulary and questions you will include. The questions should be asked throughout the text, not just at the end, so do not forget to record a page number for each! This will make it easier for you or someone else to locate the targets for the conversation starters while implementing dialogic reading.
Bookmarks/Book Inserts to Aid in Dialogic Reading
Set of Three Interactive Reading Guide Book Inserts for Download
Set of Four CROWD Bookmarks for Download
Set of Four PEER Bookmarks for Download
Brannon, D., & Dauksas, L. (2014). The effectiveness of dialogic reading in increasing English language learning preschool children’s expressive language. International Research in Early Childhood Education , 5 , 1-10.
Flynn, K. S. (2011). Developing children’s oral language skills through dialogic reading: Guidelines for implementation. Teaching Exceptional Children , 44 , 8-16. doi: 10.1177/004005991104400201
Stephenson, J. (2010). Book reading as an intervention context for children beginning to use graphic symbols for communication. Journal of Developmental and Physical Disabilities , 22 , 257-271. doi: 10.1007/s10882-009-9164-6
Swanson, E., Vaughn, S., Wanzek, J., Petscher, Y., Heckert, J., Cavanaugh, C., . . . Tackett, K. (2011). A synthesis of read-aloud interventions on early reading outcomes among preschool through third graders at risk for reading difficulties. Journal of Learning Disabilities , 44 , 258-275. doi: 10.1177/0022219410378444 | Full article
What Works Clearinghouse (2010). What Works Clearinghouse intervention report early childhood education interventions for children with disabilities: Dialogic reading. Washington, DC: Institute for Education Sciences. Retrieved from http://ies.ed.gov/ncee/wwc/InterventionReport/136
Whitehurst, G. J., Falco, F. L., Lonigan, C. J., Fischel, J. E., DeBaryshe, B. D., Valdez-Menchaca, M. C, & Caulfield, M. (1988). Accelerating language development through picture book reading. Fischel, J. E., DeBaryshe, B. D., Valdez-Menchaca, M. C, & Caulfield, M. (1988). Accelerating language development through picture book reading. Development Psychology , 24 , 552-559. doi: 10.1037/0012-1649.24.4.552
Translations
In order to provide this post and information about dialogic reading in the home to more families in Iowa, we have provided the following versions of the post and associated bookmarks and inserts, translated from English.
Dialogic Reading: Having a Conversation about Books (Lees in Dialoog: 'N Gesprek Oor Boeke)
Interactive reading guide book inserts (Interaktiewe Leesgids)
CROWD bookmarks (VOOWD Boekmerk)
PEER bookmarks (VEBH Boekmerk)
Dialogic Reading: Having a Conversation about Books (القراءة الحوارية: التحدث حول الكتب)
Interactive reading guide book inserts (مجموعة من ثلاثة فواصل تفاعلية للقراءة لتنزيلها)
CROWD bookmarks (مجموعة من علامات مميزة من CROWD لتنزيلها)
PEER bookmarks (مجموعة من علامات مميزة من PEER لتنزيلها.)
Dialogic Reading: Having a Conversation about Books (Dijaloško čitanje: razgovaranje o knjigama)
Interactive reading guide book inserts (Komplet tri knjižna umetka za Vodič za interaktivno čitanje koji možete preuzeti)
CROWD bookmarks (Komplet četiri CROWD knjižne oznake koje možete preuzeti)
PEER bookmarks (Komplet četiri PEER knjižne oznake koje možete preuzeti)
Dialogic Reading: Having a Conversation about Books (ဒိုုင္ယာေလာဂ်စ္ ရီးဒင္း - စာအုုပ္မ်ားအေၾကာင္း အျပန္အလွန္ေျပာဆိုုေဆြးေႏြးျခင္း )
Interactive reading guide book inserts (ေဒါင္းလုုတ္ လုုပ္ရန္ ျပန္လွန္ဆက္သြယ္စာဖတ္ပံုုလမ္းညႊန္ စာအုုပ္တြင္းထည့္သြင္းသည့္အရာသံုုးခုုပါ ဆက္)
CROWD bookmarks (ေဒါင္းလုုတ္လုုပ္ရန္ CROWD စာအုုပ္မွတ္ ေလးခုုပါ ဆက္)
PEER bookmarks (ေဒါင္းလုုတ္လုုပ္ရန္ PEER စာအုုပ္မွတ္ ေလးခုုပါ ဆက္)
Dialogic Reading: Having a Conversation about Books (Lecture dialogique : Conversation à propos de livres)
Interactive reading guide book inserts (Inserts de livre du guide de lecture interactive à télécharger)
CROWD bookmarks (marques-pages CMOGD à télécharger)
PEER bookmarks (marques-pages IEER à télécharger)
Dialogic Reading: Having a Conversation about Books (Dialogisches Lesen: Ein Gespräch Über Bücher)
Interactive reading guide book inserts (Satz von drei interaktiven Leseführer Buchzeichen zum Herunterladen)
CROWD bookmarks (Satz von vier CROWD Lesezeichen zum Herunterladen)
PEER bookmarks (Satz von vier PEER Lesezeichen zum Herunterladen)
Interactive reading guide book inserts
CROWD bookmarks
PEER bookmarks
Dialogic Reading: Having a Conversation about Books (대화식 독서: 책에 관해 대화하기)
Interactive reading guide book inserts (다운로드를 위한 세 가지 대화식 독서 가이드 책 표식 세트)
CROWD bookmarks (다운로드를 위한 네 가지 CROWD 북마크 세트)
PEER bookmarks (다운로드를 위한 네 가지 PEER 북마크 세트)
Dialogic Reading: Having a Conversation about Books (ການອ່ານແບບໂຕ້ຕອບ: ດຳເນີນການສົນທະນາກ່ຽວກັບປຶ້ມ)
Interactive reading guide book inserts (ຊຸດແນວສອດຂັ້ນໜ້າປຶ້ມແນະນຳການອ່ານແບບສື່ສານຕອບກັບສາມຢ່າງສຳລັບການດາວໂຫຼດ)
CROWD bookmarks (ຊຸດເຄື່ອງໝາຍຂັ້ນໜ້າປຶ້ມ CROWD ສີ່ຢ່າງສຳລັບການດາວໂຫຼດ)
PEER bookmarks (ຊຸດເຄື່ອງໝາຍຂັ້ນໜ້າປຶ້ມ PEER ສີ່ຢ່າງສຳລັບການດາວໂຫຼດ)
Chinese (Mandarin)
Dialogic Reading: Having a Conversation about Books (对话式阅读:开启一场与书籍的对话)
Interactive reading guide book inserts (提供下载的一套三个互动式阅读指南插件)
CROWD bookmarks (提供下载的一套四个CROWD书签)
PEER bookmarks (提供下载的一套四个PEER书签)
Marshallese
Dialogic Reading: Having a Conversation about Books (Riit im Bwebwenato: Kōnono kōn Bok ko)
Interactive reading guide book inserts (Guide n̄an Riit Ippān Doon)
CROWD bookmarks (CROWD Kakōļļe in Bok)
PEER bookmarks (PEER Kakōļļe in Bok)
Dialogic Reading: Having a Conversation about Books (वार्तालापपूर्ण पढाइ (Dialogic Reading): पुस्तकहरूका विषयमा कुराकानी गर्नु)
Interactive reading guide book inserts (तीन वटा सहभागी मुलक पढाइ गाइड पुस्तक डाउनलोड)
CROWD bookmarks (चार वटा क्राउड (CROWD) बुकमार्क्सहरू डाउनलोड)
PEER bookmarks (चार वटा पियर (PEER) बुकमार्क्सहरू डाउनलोड)
Serbo-Croatian
Dialogic Reading: Having a Conversation about Books (Aqrinta Wadahadalka: Lahaanshaha Wada sheekeysiga Ku saabsan Buuggaagta)
Interactive reading guide book inserts (Daji Sadex jaheynada Aqrinta Isdhaxgalka ee Gelinada Buugga ee Dajisashada)
CROWD Boookmarks (Daji Afar Aastaamaha buugga CROWD ee Dajisashada)
PEER bookmarks (Daji Afar Aastaamaha buugga PEER ee Dajisashada)
Dialogic Reading: Having a Conversation about Books (Lectura dialógica: Conversemos sobre los libros)
Interactive reading guide book inserts (Conjunto de tres inserciones en la Guía de lectura interactiva para descargar)
CROWD bookmarks (Conjunto de cuatro marcadores CROWD para descargar)
PEER bookmarks (Conjunto de cuatro marcadores PEER para descargar)
Dialogic Reading: Having a Conversation about Books (Usomaji wa Majibizano: Kuwa na Mazungumzo Kuhusu Vitabu)
Interactive reading guide book inserts (Seti ya Vitabu Vitatu vya Mwongozo wa Usamaji wa Kuingiliana kwaajili ya Kupakua)
CROWD bookmarks (Seti ya Alama za CROWD nne kwaajili ya Kupakua)
PEER bookmarks (Seti ya Alama za PEER nne kwaajili ya Kupakua)
Dialogic reading: Having a Conversation about Books (Đọc sách Đối thoại: Thực hiện Đối thoại về Sách)
Interactive reading guide book inserts (Bộ Ba Chèn sách Hướng dẫn Đọc Tương tác để Tải xuống)
CROWD bookmarks (Bộ Bốn Đánh dấu sách CROWD để Tải xuống)
PEER bookmarks (Bộ Bốn Đánh dấu sách PEER để Tải xuống)
- dialogic reading
- translated posts
Hide and seek between atoms: Find the dopant
Professor Si-Young Choi, and So-Yeon Kim and Yu-Jeong Yang, PhD candidates, from the Department of Materials Science and Engineering at Pohang University of Science and Technology (POSTECH) along with Dr. Sungho Choi from the Korea Research Institute of Chemical Technology (KRICT) and Dr. Sora Lee and Chiho Jo from LG Energy Solution have made a breakthrough in understanding the stabilization mechanism of surface structures in high-capacity, high-nickel Cathode materials through single-element doping in their collaborative research through quantitative analysis. Their work was published in Chemical Engineering Journal , an international journal in the field of chemical engineering.
In the quest to extend the driving range of electric vehicles, there's a growing need for cathode materials with a higher capacity to store more power. Nickel (Ni) is widely used in electric vehicle batteries due to its high energy density. High-nickel compounds like LiNi 0.8 Co 0.1 Mn 0.1 O 2 are common cathode materials, boasting substantial nickel content. However, as the concentration of nickel rises, a concerning phenomenon emerges: nickel ions infiltrate the lithium layer by exchanging positions with similarly sized nickel and lithium ions along certain surfaces. This excessive cation mixing has been linked to diminished battery performance.
To address this issue, recent research has focused on incorporating metal ions as dopants. These metal cations are placed within the transition metal or lithium layers of high-nickel cathode materials. Precise the doping sites is crucial to understand their effect on the structural stability of the cathode materials. However, the small quantity of metal cations added to enhance cathode performance poses challenges in pinpointing their exact locations and studying the stabilization mechanism.
In this research, the team developed a deep learning AI technique to quantitatively analyze cation mixing using atomic structure images. They combined this approach with atomic-scale electron microscopy (HAADF-STEM), allowing them to visualize, for the first time, the location of aluminum (Al), titanium (Ti), and zirconium (Zr) metal dopants at sub-molar concentrations (mol %) in high-nickel cathode materials. Through this method, they were able to examine how these dopants affect the surface structure and electrochemical properties of the cathode material.
The examination revealed that the introduction of three metal cations into the transition metal layer fortified the bonds between nickel and oxygen atoms, thereby curbing cation mixing and enhancing structural stability. Among aluminum, titanium, and zirconium, all contributed to increased discharge capacity and retention in the high-capacity nickel cathode material with titanium exhibiting the most pronounced effect. This marks the first quantitative assessment and analysis of cation mixing defects, a domain previously restricted to qualitative examination.
POSTECH Professor Si-Young Choi who led the research stated, "We developed a deep learning technology for the quantitative analysis of cation mixing in high-nickel cathode materials, enhancing the effectiveness of atomic-scale structural analysis." He expressed expectation by saying, "Our aim is to lay the groundwork for technologies that analyze highly sensitive materials, thereby advancing the understanding of performance enhancement mechanisms for next-generation cathode materials."
The research was conducted with support from the Nanomaterial Technology Development Program of the Ministry of Science and ICT, the Dual-Use Technology Projects of the Ministry of Trade, Industry and the Energy and Defense Acquisition Program Administration, the National research Facilities & Equipment Center of the Korea Basic Science Institute under the Ministry of Education, and LG Energy Solution.
1. Doping The deliberate introduction of impurities during crystal fabrication to alter the electrical, optical, and structural properties of a material
2. Dopant A purposely added impurity during the doping process
3. HAADF-STEM (High-Angle Annular Dark-Field Scanning Transmission Electron Microscopy) A STEM analysis technique utilizing an annular detector with an internal angle of 70 mrad or more. In HAADF-STEM images, signal intensity correlates with the square of the atomic number, making it particularly useful for analyzing heavy elements. This form of Scanning Transmission Electron Microscopy (STEM) employs a scanning transmission electron microscope with remarkable resolution in the tens of picometers range, facilitating detailed analysis of atomic structures
- Materials Science
- Engineering and Construction
- Civil Engineering
- Engineering
- Energy and Resources
- Battery electric vehicle
- Alternative fuel vehicle
- Hybrid vehicle
- Electricity
- Electrical conduction
- Electric power
- Flexible-fuel vehicle
- Antikythera mechanism
Story Source:
Materials provided by Pohang University of Science & Technology (POSTECH) . Note: Content may be edited for style and length.
Journal Reference :
- So-Yeon Kim, Yu-Jeong Yang, Eun Gyu Lee, Min-Su Kim, Kyoung-June Go, Minseuk Kim, Gi-Yeop Kim, Sora Lee, Chiho Jo, Sungho Choi, Si-Young Choi. Site selectivity of single dopant in high-nickel cathodes for lithium-ion batteries . Chemical Engineering Journal , 2024; 482: 148869 DOI: 10.1016/j.cej.2024.148869
Cite This Page :
Explore More
- Fastest Rate of CO2 Rise Over Last 50,000 Years
- Like Dad and Like Mum...all in One Plant
- What Makes a Memory? Did Your Brain Work Hard?
- Plant Virus Treatment for Metastatic Cancers
- Controlling Shape-Shifting Soft Robots
- Brain Flexibility for a Complex World
- ONe Nova to Rule Them All
- AI Systems Are Skilled at Manipulating Humans
- Planet Glows With Molten Lava
- A Fragment of Human Brain, Mapped
Trending Topics
Strange & offbeat.
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation
- hot-topics Extras
- Newsletters
- Reading room
Tell us what you think. Take part in our reader survey
Celebrating twenty years
- Back to parent navigation item
- Collections
- Water and the environment
- Chemical bonding
- Antimicrobial resistance
- Energy storage and batteries
- AI and automation
- Sustainability
- Research culture
- Nobel prize
- Food science and cookery
- Plastics and polymers
- Periodic table
- Coronavirus

- More from navigation items
Electrochemical acid–base reactions can be fine tuned to control reactivity

- No comments
Two new ways to control and optimise electrochemical reactions at electrified interfaces have been discovered. These insights could help to design new catalysts for battery technology and hydrogen production, crucial for the green transition.
Electrolysis involves passing an electric current through a substance to induce chemical reactions, a particularly important process for green hydrogen production and battery technology. However, there is limited understanding of acid–base chemistry at the metal/water interfaces where key steps of electrocatalysis take place. Although it is theorised that acid–base chemistry at the electrochemical interface differs from that in bulk, the relationship between these effects, as well as assessing a key metric – the acid dissociation constant (p K a ) values – quantitatively has been difficult.
Scientists from Ruhr University Bochum have now discovered two factors that govern the complex behaviour at electrified gold/water interfaces. A new model to rationalise shifts in the acid dissociation constant values at metal aqueous interfaces has been proposed based on two mechanisms: local hydrophobicity and local electric fields.
The team used surface enhanced Raman spectroscopy (SERS) in combination with modelling to study local and site-specific acid–base chemistry properties of the amino acid glycine. ‘Vibrational spectroscopy is a very good choice because [SERS] is very sensitive and allows you to reliably get the information about the change in protonation state of the two polar groups of the molecule,’ comments lead study author Simone Pezzotti .
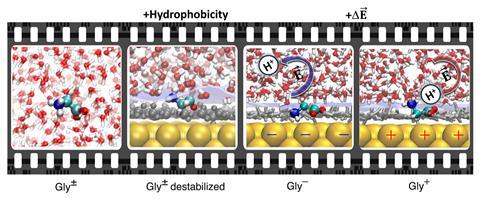
Source: © 2024 Steffen Murke et al
Proposed mechanism tuning acid–base chemistry at the electrified metal–water interface. From left to right, the sketches illustrate that local hydrophobicity at the interface destabilises the zwitterionic form of glycine
‘[Glycine] has two groups that can take or give a proton and when you play with it, you can see that it’s not even a question of local pH,’ says Pezzotti. Compared with the known p K a values of glycine in bulk, the p K a value of both carboxyl and amino groups at the gold/water interface is shifted towards neutrality – thus, the pH window for the existence of the glycine zwitterion is reduced. Simulations confirmed that the two charged groups are partially desolvated at both gold/water and air/water interfaces.
‘From these simulations, we could understand why this zwitterion shrinks and the reason is that at the interface, even though gold interacts very well with water, we do have hydrophobicity.’ A hydrophobic water interface very close to the metal destabilises the zwitterion – it becomes like mixing water and oil. The local pH value at a hydrophobic interface provides a very different solvation environment to the bulk one. ‘This provides a direct quantitative measure of how much less good as a solvent water is when it’s close to a surface than when it is far away,’ explains Ian McCrum , an electrochemistry researcher at Clarkson University in the US, who wasn’t involved with the work.
This was not the only outcome observed – the reduced stability of the zwitterion becomes amplified with increasing applied potential. The local electric field (the sum of the fields from the electrified gold surface, as well as the interfacial water) can either stabilise or destabilise a bond. Electric fields play a significant role as they can catalyse chemical reactions in liquid water – breaking and forming bonds. ‘If we fix the pH and we just play with the voltage, we can induce chemistry,’ says Pezzotti, adding that additional local electric fields induce proton transfer on the glycine. The local electric fields drive the protonation of amino groups not only in hydrophobic solutes, but also in hydrophilic ones.
‘I think that [the research] is going to move us in the direction of understanding how solvent and electric field work together to affect reactions occurring at surfaces,’ says McCrum. The results show that acid–base chemistry at electrified interfaces can be tuned by not only variations in hydrophobicity but also by a variation in applied voltage. ‘There are really a lot of opportunities to tune these two contributions and … tune the chemistry,’ adds Pezzotti.
S Murke et al , J. Am. Chem. Soc. , 2024, DOI: 10.1021/jacs.3c13633

More from Zahra Khan

First regular molecular fractal in nature

Intelligent ionotronic wood device can keep an eye on people’s health

New ‘supermolecule’ demonstrated for the first time at record-breaking ultracold temperature
- electrochemistry
- Physical chemistry
Related articles
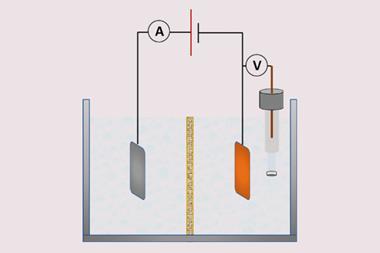
Reference electrode misuse sparks alarm among electrochemists
2023-08-21T08:06:00Z
By George Barsted
Rapid alternating polarity brings new life to 189-year-old electrochemical reaction
2023-04-11T14:00:00Z
By Andy Extance

Electrochemistry offers new way to tackle rising carbon dioxide – extract it from seawater
2023-03-10T09:31:00Z
By Rebecca Trager

Photochemistry enables safer method for reprocessing plutonium and uranium mixtures
2022-10-06T13:56:00Z
By Aphra Murray

Electrochemical ‘game-changer’ could make aniline production greener
2022-08-05T08:30:00Z
By James Urquhart

Electrocatalytic hydrogen atom transfer could give industrial organic synthesis a boost
2022-06-09T08:30:00Z
By Kira Welter
No comments yet
Only registered users can comment on this article., more from news.

Rodents’ striking orange teeth not down to iron-rich enamel as thought
2024-05-13T13:30:00Z
By Ada McVean

Biomass, plastic waste and carbon dioxide feedstocks key to cutting chemical industry’s emissions
2024-05-13T09:30:00Z
By Jamie Durrani

Lead found in Beethoven’s hair reveals news insight into his ailing health
2024-05-13T08:34:00Z
By Julia Robinson

Precious metal-free catalyst conjured using sugar can turn CO 2 into chemical feedstock
2024-05-10T14:56:00Z
By Tim Wogan

Explainer: what is tear gas?
2024-05-09T14:33:00Z
By Rachel Brazil

Claims of tear gas use against Ukrainian troops ‘insufficiently substantiated’
2024-05-09T13:56:00Z
- Contributors
- Terms of use
- Accessibility
- Permissions
- This website collects cookies to deliver a better user experience. See how this site uses cookies .
- This website collects cookies to deliver a better user experience. Do not sell my personal data .
- Este site coleta cookies para oferecer uma melhor experiência ao usuário. Veja como este site usa cookies .
Site powered by Webvision Cloud

COMMENTS
50+ Chemistry IA Ideas with Research Question Examples One of the biggest challenges facing students taking IB chemistry is coming up with a good Internal Assessment (IA) idea. It's got to be something suitably demanding for diploma-level study, it's got to be something relevant to the chemistry syllabus, it's got ...
Your IB Chemistry IA is your mini-research project that accounts for 20% of your total grade. Your research question sets the base for your overall performance as it states the aim and context of your IA. Your IB Chemistry IA research question, in turn, can only be effectively framed once the topic you pick for your IA is inspired by past ...
The Internal Assessment (IA) is a written piece of work that students need to complete for all their IB classes. In chemistry, the IA consists of an individual research project which allows the student to independently design and carry out an experiment whilst making both quantitative and qualitative observations (with the guidance of their ...
Coming up with an idea for your IB Chemistry IA is sometimes the hardest part. Get your Chemistry brain working with this helpful list of 50 suggestions for your IB Chemistry Internal Assessment. Written by an experienced IB teacher, these are practical ideas to help you find a project that can work with the resources and time available to you.
IB Chemistry IA ideas. VSEPR theory predicts the shape and bond angles of molecules based on the number of bonding and non-bonding pairs of electrons around the central atom. It does not consider the identity of the atoms or groups attached to the central atom however, and this does have an effect.
Please read this thoroughly before you sit down to write your Chemistry IA: Unique Research question There are 2 points for personal engagement. The research question must resonate with you personally. However, it is essential to note that the research question can be a more complex idea. Still, the approach to the research should be fresh and ...
The IA consists of a laboratory report that students must complete during their IB chemistry course. For assessments before May 2025, the report should be 6 to 12 pages in length and should include a research question, a methodology section, data analysis, and a conclusion. From May 2025, the report should be a maximum of 3,000 words.
25 IB Chemistry IA Topic Ideas. We all know that scoring superbly on internal assessments is a great way to boost our IB grades. But how do we get started on a lab report and, crucially, what should we write about? In general, keep in mind that for your Chemistry IA we want to measuring how changing one variable has an effect on another variable.
1.1: Keeping it Simple. Selecting an appropriate research question is an important step in ensuring success in your IB Chemistry IA. I can assure you that there is no need to opt for an overly complicated research question - a principle I adhered to, resulting in a 22/24 on my Chemistry HL Internal Assessment (keep it simple, keep it clear!).
We created a couple of exemplars to show you how the new IA should look like. It's OK to refer to the old Chemistry IA exemplars (since the new IA is quite similar) for inspiration/ideas, but make sure to follow the new requirements. What is the effect of the temperature (30, 40, 50, 60, 70 °C) on the buffering capacity (mol/dm3) of phosphate ...
The topics for IB Chemistry IA's vary greatly, ranging from analyzing stoichiometric processes, to studying the effects of light on photosynthesis. Furthermore, each essay must meet the criteria set forth by the International Baccalaureate program, and must follow a specific structure and style.
30+ Chemistry IA Topic Ideas to Get You Started. The following are just but ideas to give you a clear picture of what a suitable Chemistry IA topic should look like. It's important to keep in mind that these are not by any means research questions. They're either general questions or statement, which you can modify and make as specific as ...
October 20, 2022. For most students, the IB Chemistry IA (Internal Assessment) assignment lurks on the path to academic success like a sphinx with an impossible riddle. The analogy is quite right too, given that the assignments take up a hefty 20% of your total marks in the coursework. If you are reading this, you are likely confused by the ...
Here are some examples of good chemistry IA topics: The effect of pH on the rate of enzyme-catalyzed reactions. The synthesis and characterization of nanoparticles. The determination of the equilibrium constant of a chemical reaction. The analysis of food additives using spectroscopy techniques.
Here is a plan for a Chemistry IA: Topic Selection: Choose a topic that interests you and aligns with your learning objectives. Examples of topics could be, but not limited to: Research Question: Formulate a research question that clearly identifies the variables you will be investigating. Ensure that the research question is focused and ...
To help spark your inspiration, we've curated a list of 30 good chemistry IA topics suitable for IB Standard Level: 1. Analysis of vitamin C content in different types of fruit. 2. Investigating the effect of temperature on enzyme activity. 3.
A student's Research Question, or RQ, is the starting point for their IA. Equally so, it is the first point of criticism for a teacher grading the work. Consequently, a well formed Research Question is essential to ensure a high quality IA. There are certain essential criteria that an RQ must meet to guarantee a well-performing IA.
View all Tiber Tutor topic 1 resources for free (2016) Join 120,000 students, across 100 countries, in 460 IB schools. That's half of the IB science graduates worldwide. View IB chemistry IA examples, download Internal assessments for IB chemistry IA ideas, and browse chem IA topics and example IA courseworks.
In this article, you will find some amazing topics ideas that you can consider for your IB Chemistry internal assessment with maximum possibilities of scoring high. Stoichiometry Relations. Determine whether the oxidation of a coffee or tea affects its pH level. Find the content of calcium in milk by using Ethylenediaminetetraacetic acid (EDTA ...
Introduction and Background Theory. 1) Explain why you chose this specific investigation and why it's personal to you, but you don't need a "When I was five…". 2) I would just make the title of your IA your research question, structured with a clear "How does the IV affect the DV".
The IA is graded on a scale of 1 to 7, with 7 being the highest possible score. For IB students, the Internal Assessment (IA) is an important component of the chemistry course. It allows students to demonstrate their understanding of the subject and apply their knowledge to a real-world problem or investigation. Structure of the Chemistry IA:
Below are some frequently asked questions regarding the Chemistry Internal Assessment. Jump to a question: How do I decide on a topic? How do I decide on a my Research Question? ... How do I decide on a my Research Question? Consider the variables you wish to measure, and the data you are collecting to formulate a detailed RQ ...
A Chemistry Celebration The University of Iowa 2024 Event Schedule5:00 PM - Reception & Poster session*6:00 PM - CeremonyWelcome - Prof. Renée Cole (DEO)Undergraduate Awards - Prof. Mona MaaloufChemistry & Sustainability Study Abroad- Prof. Adam BrummettGraduate Awards - Prof. Scott ShawCGSA Awards
Dialogic reading... Is interactive; Is student centered; Is conducted with narrative and expository text with questions and discussion throughout; Is an activity requiring careful planning; Is for students at all levels; Although several strategies exist to facilitate the three tiers of questions in dialogic reading, the two most popular are known by the acronyms CROWD and PEER.
Ames, IA Audra worked in REG's chemistry lab where she assisted with quality control tests on biodiesel samples from refineries. She also tested samples generated by the lab engineers who are always trying to find better feedstocks or improve the processes at the plants to make a better product. Lastly, she got to spend some time working with
Pohang University of Science & Technology (POSTECH). "Hide and seek between atoms: Find the dopant." ScienceDaily. ScienceDaily, 13 May 2024. <www.sciencedaily.com / releases / 2024 / 05 ...
High concentrations of lead in two locks of hair from Ludwig van Beethoven suggest that exposure to the element - through plumbed wine (added as a sweetener and preservative), diet and medical ...
2024 AP Exam Dates. The 2024 AP Exams will be administered in schools over two weeks in May: May 6-10 and May 13-17. AP coordinators are responsible for notifying students when and where to report for the exams. Early testing or testing at times other than those published by College Board is not permitted under any circumstances.
'I think that [the research] is going to move us in the direction of understanding how solvent and electric field work together to affect reactions occurring at surfaces,' says McCrum. The results ...