Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 06 June 2022

The burden and risks of emerging complications of diabetes mellitus
- Dunya Tomic ORCID: orcid.org/0000-0003-2471-2523 1 , 2 ,
- Jonathan E. Shaw ORCID: orcid.org/0000-0002-6187-2203 1 , 2 na1 &
- Dianna J. Magliano ORCID: orcid.org/0000-0002-9507-6096 1 , 2 na1
Nature Reviews Endocrinology volume 18 , pages 525–539 ( 2022 ) Cite this article
47k Accesses
234 Citations
56 Altmetric
Metrics details
- Diabetes complications
- Type 1 diabetes
- Type 2 diabetes
The traditional complications of diabetes mellitus are well known and continue to pose a considerable burden on millions of people living with diabetes mellitus. However, advances in the management of diabetes mellitus and, consequently, longer life expectancies, have resulted in the emergence of evidence of the existence of a different set of lesser-acknowledged diabetes mellitus complications. With declining mortality from vascular disease, which once accounted for more than 50% of deaths amongst people with diabetes mellitus, cancer and dementia now comprise the leading causes of death in people with diabetes mellitus in some countries or regions. Additionally, studies have demonstrated notable links between diabetes mellitus and a broad range of comorbidities, including cognitive decline, functional disability, affective disorders, obstructive sleep apnoea and liver disease, and have refined our understanding of the association between diabetes mellitus and infection. However, no published review currently synthesizes this evidence to provide an in-depth discussion of the burden and risks of these emerging complications. This Review summarizes information from systematic reviews and major cohort studies regarding emerging complications of type 1 and type 2 diabetes mellitus to identify and quantify associations, highlight gaps and discrepancies in the evidence, and consider implications for the future management of diabetes mellitus.
With advances in the management of diabetes mellitus, evidence is emerging of an increased risk and burden of a different set of lesser-known complications of diabetes mellitus.
As mortality from vascular diseases has declined, cancer and dementia have become leading causes of death amongst people with diabetes mellitus.
Diabetes mellitus is associated with an increased risk of various cancers, especially gastrointestinal cancers and female-specific cancers.
Hospitalization and mortality from various infections, including COVID-19, pneumonia, foot and kidney infections, are increased in people with diabetes mellitus.
Cognitive and functional disability, nonalcoholic fatty liver disease, obstructive sleep apnoea and depression are also common in people with diabetes mellitus.
As new complications of diabetes mellitus continue to emerge, the management of this disorder should be viewed holistically, and screening guidelines should consider conditions such as cancer, liver disease and depression.
Similar content being viewed by others
Type 2 diabetes mellitus in older adults: clinical considerations and management
The cross-sectional and longitudinal relationship of diabetic retinopathy to cognitive impairment: a systematic review and meta-analysis

Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025
Introduction.
Diabetes mellitus is a common, albeit potentially devastating, medical condition that has increased in prevalence over the past few decades to constitute a major public health challenge of the twenty-first century 1 . Complications that have traditionally been associated with diabetes mellitus include macrovascular conditions, such as coronary heart disease, stroke and peripheral arterial disease, and microvascular conditions, including diabetic kidney disease, retinopathy and peripheral neuropathy 2 (Fig. 1 ). Heart failure is also a common initial manifestation of cardiovascular disease in patients with type 2 diabetes mellitus (T2DM) 3 and confers a high risk of mortality in those with T1DM or T2DM 4 . Although a great burden of disease associated with these traditional complications of diabetes mellitus still exists, rates of these conditions are declining with improvements in the management of diabetes mellitus 5 . Instead, as people with diabetes mellitus are living longer, they are becoming susceptible to a different set of complications 6 . Population-based studies 7 , 8 , 9 show that vascular disease no longer accounts for most deaths among people with diabetes mellitus, as was previously the case 10 . Cancer is now the leading cause of death in people with diabetes mellitus in some countries or regions (hereafter ‘countries/regions’) 9 , and the proportion of deaths due to dementia has risen since the turn of the century 11 . In England, traditional complications accounted for more than 50% of hospitalizations in people with diabetes mellitus in 2003, but for only 30% in 2018, highlighting the shift in the nature of complications of this disorder over this corresponding period 12 .
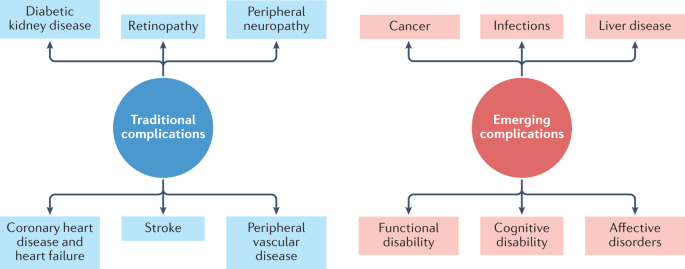
The traditional complications of diabetes mellitus include stroke, coronary heart disease and heart failure, peripheral neuropathy, retinopathy, diabetic kidney disease and peripheral vascular disease, as represented on the left-hand side of the diagram. With advances in the management of diabetes mellitus, associations between diabetes mellitus and cancer, infections, functional and cognitive disability, liver disease and affective disorders are instead emerging, as depicted in the right-hand side of the diagram. This is not an exhaustive list of complications associated with diabetes mellitus.
Cohort studies have reported associations of diabetes mellitus with various cancers, functional and cognitive disability, liver disease, affective disorders and sleep disturbance, and have provided new insights into infection-related complications of diabetes mellitus 13 , 14 , 15 , 16 , 17 . Although emerging complications have been briefly acknowledged in reviews of diabetes mellitus morbidity and mortality 11 , 17 , no comprehensive review currently specifically provides an analysis of the evidence for the association of these complications with diabetes mellitus. In this Review, we synthesize information published since the year 2000 on the risks and burden of emerging complications associated with T1DM and T2DM.
Diabetes mellitus and cancer
The burden of cancer mortality.
With the rates of cardiovascular mortality declining amongst people with diabetes mellitus, cancer deaths now constitute a larger proportion of deaths among this population in some countries/regions 8 , 9 . Although the proportion of deaths due to cancer appears to be stable, at around 16–20%, in the population with diabetes mellitus in the USA 7 , in England it increased from 22% to 28% between 2001 and 2018 (ref. 9 ), with a similar increase reported in Australia 8 . Notably, in England, cancer has overtaken vascular disease as the leading cause of death in people with diabetes mellitus and it is the leading contributor to excess mortality in those with diabetes mellitus compared with those without 9 . These findings are likely to be due to a substantial decline in the proportion of deaths from vascular diseases, from 44% to 24% between 2001 and 2018, which is thought to reflect the targeting of prevention measures in people with diabetes mellitus 18 . Over the same time period, cancer mortality rates fell by much less in the population with diabetes mellitus than in that without diabetes 9 , suggesting that clinical approaches for diabetes mellitus might focus too narrowly on vascular complications and might require revision 19 . In addition, several studies have reported that female patients with diabetes mellitus receive less-aggressive treatment for breast cancer compared with patients without diabetes mellitus, particularly with regard to chemotherapy 20 , 21 , 22 , suggesting that this treatment approach might result in increased cancer mortality rates in women with diabetes mellitus compared with those without diabetes mellitus. Although substantial investigation of cancer mortality in people with diabetes mellitus has been undertaken in high-income countries/regions, there is a paucity of evidence from low-income and middle-income countries/regions. It is important to understand the potential effect of diabetes mellitus on cancer mortality in these countries/regions owing to the reduced capacity of health-care systems in these countries/regions to cope with the combination of a rising prevalence of diabetes mellitus and rising cancer mortality rates in those with diabetes mellitus. One study in Mauritius showed a significantly increased risk of all-cause cancer mortality in patients with T2DM 23 , but this study has yet to be replicated in other low-income and middle-income countries/regions.
Gastrointestinal cancers
Of the reported associations between diabetes mellitus and cancer (Table 1 ), some of the strongest have been demonstrated for gastrointestinal cancers.
Hepatocellular carcinoma
In the case of hepatocellular carcinoma, the most rigorous systematic review on the topic — comprising 18 cohort studies with a combined total of more than 3.5 million individuals — reported a summary relative risk (SRR) of 2.01 (95% confidence interval (CI) 1.61–2.51) for an association with diabetes mellitus 24 . This increased risk of hepatocellular carcinoma with diabetes mellitus is supported by the results of another systematic review that included case–control studies 25 . Another review also found that diabetes mellitus independently increased the risk of hepatocellular carcinoma in the setting of hepatitis C virus infection 26 .
Pancreatic cancer
The risk of pancreatic cancer appears to be approximately doubled in patients with T2DM compared with patients without T2DM. A meta-analysis of 36 studies found an adjusted odds ratio (OR) of 1.82 (95% CI 1.66–1.89) for pancreatic cancer among people with T2DM compared with patients without T2DM 27 (Table 1 ). However, it is possible that these findings are influenced by reverse causality — in this scenario, diabetes mellitus is triggered by undiagnosed pancreatic cancer 28 , with pancreatic cancer subsequently being clinically diagnosed only after the diagnosis of diabetes mellitus. Nevertheless, although the greatest risk (OR 2.05, 95% CI 1.87–2.25) of pancreatic cancer was seen in people diagnosed with T2DM 1–4 years previously compared with people without T2DM, those with a diagnosis of T2DM of more than 10 years remained at increased risk of pancreatic cancer (OR 1.51, 95% CI 1.16–1.96) 27 , suggesting that reverse causality can explain only part of the association between T2DM and pancreatic cancer. Although T2DM accounts for ~90% of all cases of diabetes mellitus 29 , a study incorporating data from five nationwide diabetes registries also reported an increased risk of pancreatic cancer amongst both male patients (HR 1.53, 95% CI 1.30–1.79) and female patients (HR 1.25, 95% CI 1.02–1.53) with T1DM 30 .
Colorectal cancer
For colorectal cancer, three systematic reviews have shown a consistent 20–30% increased risk associated with diabetes mellitus 31 , 32 , 33 . One systematic review, which included more than eight million people across 30 cohort studies, reported an incidence SRR of 1.27 (95% CI 1.21–1.34) of colorectal cancer 31 , independent of sex and family history (Table 1 ). Similar increases in colorectal cancer incidence in patients with diabetes mellitus were reported in a meta-analysis of randomized controlled trials (RCTs) and cohort studies 32 and in a systematic review that included cross-sectional studies 33 .
Female-specific cancers
Endometrial, breast and ovarian cancers all occur more frequently in women with diabetes mellitus than in women without diabetes mellitus.
Endometrial cancer
For endometrial cancer, one systematic review of 29 cohort studies and a combined total of 5,302,259 women reported a SRR of 1.89 (95% CI 1.46–2.45) and summary incidence rate ratio (IRR) of 1.61 (95% CI 1.51–1.71) 34 (Table 1 ). Similar increased risks were found in two systematic reviews incorporating cross-sectional studies 35 , 36 , one of which found a particularly strong association of T1DM (relative risk (RR) 3.15, 95% CI 1.07–9.29) with endometrial cancer.
Breast cancer
The best evidence for a link between diabetes mellitus and breast cancer comes from a systematic review of six prospective cohort studies and more than 150,000 women, in which the hazard ratio (HR) for the incidence of breast cancer in women with diabetes mellitus compared with women without diabetes mellitus was 1.23 (95% CI 1.12–1.34) 32 (Table 1 ). Two further systematic reviews have also shown this increased association 37 , 38 .
The association of diabetes mellitus with breast cancer appears to vary according to menopausal status. In a meta-analysis of studies of premenopausal women with diabetes mellitus, no significant association with breast cancer was found 39 , whereas in 11 studies that included only postmenopausal women, the SRR was 1.15 (95% CI 1.07–1.24). The difference in breast cancer risk between premenopausal and postmenopausal women with diabetes mellitus was statistically significant. The increased risk of breast cancer after menopause in women with diabetes mellitus compared with women without diabetes mellitus might result from the elevated concentrations and increased bioavailability of oestrogen that are associated with adiposity 40 , which is a common comorbidity in those with T2DM; oestrogen synthesis occurs in adipose tissue in postmenopausal women, while it is primarily gonadal in premenopausal women 41 . Notably, however, there is evidence that hormone-receptor-negative breast cancers, which typically carry a poor prognosis, occur more frequently in women with breast cancer and diabetes mellitus than in women with breast cancer and no diabetes mellitus 42 , indicating that non-hormonal mechanisms also occur.
Ovarian cancer
Diabetes mellitus also appears to increase the risk of ovarian cancer, with consistent results from across four systematic reviews. A pooled RR of 1.32 (95% CI 1.14–1.52) was reported across 15 cohort studies and a total of more than 2.3 million women 43 (Table 1 ). A SRR of 1.19 (95% CI 1.06–1.34) was found across 14 cohort studies and 3,708,313 women 44 . Similar risks were reported in meta-analyses that included cross-sectional studies 45 , 46 .
Male-specific cancers: prostate cancer
An inverse association between diabetes mellitus and prostate cancer has been observed in a systematic review (RR 0.91, 95% CI 0.86–0.96) 47 , and is probably due to reduced testosterone levels that occur secondary to the low levels of sex hormone-binding globulin that are commonly seen in men with T2DM and obesity 48 . Notably, however, the systematic review that showed the inverse association involved mostly white men (Table 1 ), whereas a systematic review of more than 1.7 million men from Taiwan, Japan, South Korea and India found that diabetes mellitus increased prostate cancer risk 49 , suggesting that ethnicity might be an effect modifier of the diabetes mellitus–prostate cancer relationship. The mechanisms behind this increased risk in men in regions of Asia such as Taiwan and Japan, where most study participants came from, remain unclear. Perhaps, as Asian men develop diabetes mellitus at lower levels of total adiposity than do white men 50 , the adiposity associated with diabetes mellitus in Asian men might have a lesser impact on sex hormone-binding globulin and testosterone than it does in white men. Despite the reported inverse association between diabetes mellitus and prostate cancer in white men, however, evidence suggests that prostate cancers that do develop in men with T2DM are typically more aggressive, conferring higher rates of disease-specific mortality than prostate cancers in men without diabetes mellitus 51 .
An assessment of cancer associations
As outlined above, a wealth of data has shown that diabetes mellitus is associated with an increased risk of various cancers. It has been argued, however, that some of these associations could be due to detection bias resulting from increased surveillance of people with diabetes mellitus in the immediate period after diagnosis 52 , or reverse causality, particularly in the case of pancreatic cancer 53 . However, neither phenomenon can account for the excess risks seen in the longer term. An Australian study exploring detection bias and reverse causality found that standardized mortality ratios (SMRs) for several cancer types in people with diabetes mellitus compared with the general population fell over time, but remained elevated beyond 2 years for pancreatic and liver cancers 54 , suggesting that diabetes mellitus is a genuine risk factor for these cancer types.
A limitation of the evidence that surrounds diabetes mellitus and cancer risk is high clinical and methodological heterogeneity across several of the large systematic reviews, which makes it difficult to be certain of the effect size in different demographic groups. Additionally, many of the studies exploring a potential association between diabetes mellitus and cancer were unable to adjust for BMI, which is a major confounder. However, a modelling study that accounted for BMI found that although 2.1% of cancers worldwide in 2012 were attributable to diabetes mellitus as an independent risk factor, twice as many cancers were attributable to high BMI 55 , so it is likely that effect sizes for cancer risk associated with diabetes mellitus would be attenuated after adjustment for BMI. Notably, however, low-income and middle-income countries/regions had the largest increase in the numbers of cases of cancer attributable to diabetes mellitus both alone and in combination with BMI 55 , highlighting the need for public health intervention, given that these countries/regions are less equipped than high-income countries/regions to manage a growing burden of cancer.
As well as the cancer types outlined above, diabetes mellitus has also been linked to various other types of cancer, including kidney cancer 56 , bladder cancer 57 and haematological malignancies; however, the evidence for these associations is not as strong as for the cancers discussed above 58 . Diabetes mellitus might also be associated with other cancer types such as small intestine cancer, but the rarity of some of these types makes it difficult to obtain sufficient statistical power in analyses of any potential association.
Potential aetiological mechanisms
Several aetiological mechanisms that might be involved in linking diabetes mellitus to cancer have been proposed, including hyperinsulinaemia, hyperglycaemia, inflammation and cellular signalling mechanisms.
Hyperinsulinaemia
Most cancer cells express insulin receptors, through which hyperinsulinaemia is thought to stimulate cancer cell proliferation and metastasis 59 . Hyperinsulinaemia might also promote carcinogenesis through increased local levels of insulin-like growth factor 1 (IGF1), which has potent mitogenic and anti-apoptotic activities 60 , owing to decreased levels of insulin-like growth factor binding proteins. As outlined above, people with diabetes mellitus show a strong risk of pancreatic and liver cancers; this increased risk might occur because insulin is produced by pancreatic β-cells and transported to the liver via the portal vein 61 , thereby exposing the liver and pancreas to high levels of endogenous insulin 59 .
Hyperglycaemia and inflammation
Hyperglycaemia can induce DNA damage 62 , increase the generation of reactive oxygen species 63 and downregulate antioxidant expression 64 , all of which are associated with cancer development. Inflammatory markers, including cytokines such as IL-6, appear to have an important role in the association between diabetes and cancer 65 .
Cellular signalling mechanisms
Several cellular signalling components are common to the pathogenesis of T2DM and cancer. These include the mechanistic target of rapamycin (mTOR), a central controller of cell growth and proliferation; AMP-activated protein kinase, a cellular energy sensor and signal transducer 66 ; and the phosphatidylinositol 3-kinase (PI3K)–AKT pathway, which transduces growth factor signals during organismal growth, glucose homeostasis and cell proliferation 67 . Dysregulation of any of these cellular signalling components or pathways could contribute to the development of cancer and metabolic disorders, including T2DM, and glucose-lowering drugs such as metformin have been associated with a reduction in cancer cell proliferation through effective inhibition of some of these components 68 .
Diabetes mellitus and infections
Infection-related complications.
Although infection has long been recognized as a complication of diabetes mellitus, an association between diabetes mellitus and infection has not been well documented in epidemiological studies 69 . Only in the past decade have major studies quantified the burden of infection-related complications in people with diabetes mellitus and explored the specific infections accounting for this burden. In a US cohort of 12,379 participants, diabetes mellitus conferred a significant risk of infection-related hospitalization, with an adjusted HR of 1.67 (95% CI 1.52–1.83) compared with people without diabetes mellitus 70 (Table 2 ). The association was most pronounced for foot infections (HR 5.99, 95% CI 4.38–8.19), with significant associations also observed for respiratory infection, urinary tract infection, sepsis and post-operative infection, but not for gastrointestinal infection, a category that included appendicitis and gastrointestinal abscesses but not viral or bacterial gastroenteritis. Interestingly, a report from Taiwan demonstrated an association between the use of metformin and a lower risk of appendicitis 71 .
In an analysis of the entire Hong Kong population over the period 2001–2016, rates of hospitalization for all types of infection remained consistently higher in people with diabetes mellitus than in those without diabetes mellitus 72 . The strongest association was seen for hospitalization due to kidney infections, for which the adjusted RR was 4.9 (95% CI 3.9–6.2) in men and 3.2 (95% CI 2.8–3.7) in women with diabetes mellitus compared with those without diabetes mellitus in 2016 (Table 2 ). Diabetes mellitus roughly doubled the risk of hospitalization from tuberculosis or sepsis. The most common cause of infection-related hospitalization was pneumonia, which accounted for 39% of infections across the study period, while no other single cause accounted for more than 25% of infections across the same period. Pneumonia-related hospitalization rates increased substantially from 2001 to 2005, probably as a result of the 2003 severe acute respiratory syndrome (SARS) epidemic and the decreased threshold for pneumonia hospitalization in the immediate post-epidemic period. Rates for hospitalization for influenza increased from 2002 to 2016, possibly because of changes in the virus and increased testing for influenza. Declining rates of hospitalization for tuberculosis, urinary tract infections, foot infections and sepsis could be due to improvements in the management of diabetes mellitus.
Infection-related mortality rates were found to be significantly elevated among 1,108,982 Australians with diabetes mellitus studied over the period 2000–2010 compared with rates in people without diabetes mellitus 73 . For overall infection-related mortality, SMRs were 4.42 (95% CI 3.68–5.34) for T1DM and 1.47 (95% CI 1.42–1.53) for people with T2DM compared with those without diabetes mellitus (Table 2 ). Substantially higher infection-related mortality rates were seen in people with T1DM compared with those with T2DM for all infection types, even after accounting for age. Hyperglycaemia is thought to be a driver of infection amongst people with diabetes mellitus (see below) 73 , which might explain the higher SMRs amongst people with T1DM, in whom hyperglycaemia is typically more severe, than in those with T2DM. The highest SMRs were seen for osteomyelitis, and SMRs for septicaemia and pneumonia were also greater than 1.0 for both types of diabetes mellitus compared with those without diabetes mellitus.
Post-operative infection
Post-operative infection is also an important complication of diabetes mellitus. In a meta-analysis, diabetes mellitus was found to be associated with an OR of 1.77 (95% CI 1.13–2.78) for surgical site infection across studies that adjusted for confounding factors 74 (Table 2 ). The effect size appears to be greatest after cardiac procedures, and one US study of patients undergoing coronary artery bypass grafting found diabetes mellitus to be an independent predictor of surgical site infection, with an OR of 4.71 (95% CI 2.39–9.28) compared with those without diabetes mellitus 75 . Risks of infection of more than threefold were reported in some studies of gynaecological 76 and spinal surgery 77 in people with diabetes mellitus compared with those without diabetes mellitus. Increased risks of infection among people with diabetes mellitus were also observed in studies of colorectal and breast surgery and arthroplasty, suggesting that the association between diabetes mellitus and post-operative infection is present across a wide range of types of surgery 74 .
Respiratory infections
The incidence of hospitalizations due to respiratory infections among people with diabetes mellitus was increasing substantially even before the onset of the coronavirus disease 2019 (COVID-19) pandemic, probably owing to increased life expectancy in these patients as well as an increased likelihood of them being hospitalized for conditions such as respiratory infections, which occur mostly in older age 12 . This rising burden of respiratory infection, in combination with the rising prevalence of diabetes mellitus, highlights the importance of addressing the emerging complications of diabetes mellitus to minimize impacts on health-care systems in current and future global epidemics.
Although diabetes mellitus does not appear to increase the risk of becoming infected with COVID-19 (ref. 78 ), various population-based studies have reported increased risks of COVID-19 complications among people with diabetes mellitus. In a study of the total Scottish population, people with diabetes mellitus were found to have an increased risk of fatal or critical care unit-treated COVID-19, with an adjusted OR of 1.40 (95% CI 1.30–1.50) compared with those without diabetes mellitus 79 (Table 2 ). The risk was particularly high for those with T1DM (OR 2.40, 95% CI 1.82–3.16) 79 . Both T1DM and T2DM have been linked to a more than twofold increased risk of hospitalization with COVID-19 in a large Swedish cohort study 80 . In South Korean studies, T2DM was linked to intensive care unit admission among patients with COVID-19 infection 81 , and diabetes mellitus (either T1DM or T2DM) was linked to a requirement for ventilation and oxygen therapy 82 in patients with COVID-19. Diabetes mellitus appears to be the primary predisposing factor for opportunistic infection with mucormycosis in individuals with COVID-19 (ref. 83 ). The evidence for diabetes mellitus as a risk factor for post-COVID-19 syndrome is inconclusive 84 , 85 . Interestingly, an increase in the incidence of T1DM during the COVID-19 pandemic has been reported in several countries/regions 86 , and some data suggest an increased risk of T1DM after COVID-19 infection 87 , but the evidence regarding a causal effect is inconclusive.
Pneumonia, MERS, SARS and H1N1 influenza
The data regarding diabetes mellitus and COVID-19 are consistent with the published literature regarding other respiratory infections, such as pneumonia, for which diabetes mellitus has been shown to increase the risk of hospitalization 88 and mortality 88 , with similar effect sizes to those seen for COVID-19, compared with no diabetes mellitus. Diabetes mellitus has also been also linked to adverse outcomes in people with Middle East respiratory syndrome (MERS), SARS and H1N1 influenza 89 , 90 , 91 , 92 , suggesting that mechanisms specific to COVID-19 are unlikely to be responsible for the relationship between diabetes mellitus and COVID-19. Unlike the case for COVID-19, there is evidence that people with diabetes mellitus are at increased risk of developing certain other respiratory infections, namely pneumonia 93 and possibly also MERS 94 .
The mechanisms that might link diabetes mellitus and infection include a reduced T cell response, reduced neutrophil function and disorders of humoral immunity.
Mononuclear cells and monocytes of individuals with diabetes mellitus secrete less IL-1 and IL-6 than the same cells from people without diabetes mellitus 95 . The release of IL-1 and IL-6 by T cells and other cell types in response to infection has been implicated in the response to several viral infections 96 . Thus, the reduced secretion of these cytokines in patients with diabetes mellitus might be associated with the poorer responses to infection observed among these patients compared with people without diabetes mellitus.
In the context of neutrophil function, hyperglycaemic states might give rise to reductions in the mobilization of polymorphonuclear leukocytes, phagocytic activity and chemotaxis 97 , resulting in a decreased immune response to infection. Additionally, increased levels of glucose in monocytes isolated from patients with obesity and/or diabetes mellitus have been found to promote viral replication in these cells, as well as to enhance the expression of several cytokines, including pro-inflammatory cytokines that are associated with the COVID-19 ‘cytokine storm’; furthermore, glycolysis was found to sustain the SARS coronavirus 2 (SARS-CoV-2)-induced monocyte response and viral replication 98 .
Elevated glucose levels in people with diabetes mellitus are also associated with an increase in glycation, which, by promoting a change in the structure and/or function of several proteins and lipids, is responsible for many of the complications of diabetes mellitus 99 . In people with diabetes mellitus, antibodies can become glycated, a process that is thought to impair their biological function 100 . Although the clinical relevance of this impairment is not clear, it could potentially explain the results of an Israeli study that reported reduced COVID-19 vaccine effectiveness among people with T2DM compared with those without T2DM 101 .
Diabetes mellitus and liver disease
Nonalcoholic fatty liver disease.
The consequences of nonalcoholic fatty liver disease (NAFLD) make it important to recognize the burden of this disease among people with diabetes mellitus. NAFLD and nonalcoholic steatohepatitis (NASH; an advanced form of NAFLD) are major causes of liver transplantation in the general population. In the USA, NASH accounted for 19% of liver transplantations in 2016 — second only to alcoholic liver disease, which was the cause of 24% of transplantations 102 . In Australia and New Zealand, NAFLD was the primary diagnosis in 9% of liver transplant recipients in 2019, only slightly below the figure for alcoholic cirrhosis of 13% 103 . In Europe, NASH increased as the reason for transplantations from 1% in 2002 to more than 8% in 2016, in parallel with the rising prevalence of diabetes mellitus 104 .
NAFLD is highly prevalent among people with T2DM. In a systematic review of 80 studies across 20 countries/regions, the prevalence of NAFLD among 49,419 people with T2DM was 56% 105 , while the global prevalence of NAFLD in the general population is estimated to be 25% 106 . In a Chinese cohort study of 512,891 adults, diabetes mellitus was associated with an adjusted HR of 1.76 (95% CI 1.47–2.16) for NAFLD compared with no diabetes mellitus 107 (Table 3 ). Another smaller longitudinal Chinese study also reported an increased risk of developing NAFLD among those with T2DM compared with those without T2DM 108 . However, most evidence regarding the association between NAFLD and diabetes mellitus is from cross-sectional studies 109 , 110 , 111 .
NASH and fibrosis
Diabetes mellitus appears to enhance the risk of NAFLD complications, including NASH and fibrosis. An analysis of 892 people with NAFLD and T2DM across 10 studies showed that the prevalence of NASH was 37% (ref. 105 ); figures for the prevalence of NASH in the general population with NAFLD vary greatly across different study populations, ranging from 16% to 68% 112 . Amongst 439 people with T2DM and NAFLD in seven studies, 17% had advanced fibrosis 105 . An analysis of 1,069 people with NAFLD in a US study found that diabetes mellitus was an independent predictor for NASH (OR 1.93, 95% CI 1.37–2.73) and fibrosis (3.31, 95% CI 2.26–4.85) 113 .
Bidirectional relationship between diabetes mellitus and liver disease
The relationship between diabetes mellitus and NAFLD is bidirectional, as NAFLD is associated with an increased risk of developing T2DM 114 . There is also a notable bidirectional relationship between diabetes mellitus and liver cirrhosis. The prevalence of diabetes mellitus in people with liver cirrhosis has been reported as 20–63%, depending on the severity of liver damage, aetiology and diagnostic criteria 115 . In an Italian study of 401 participants with cirrhosis, 63% of those with decompensated liver disease had diabetes mellitus compared with 10% of those with well-compensated liver disease 116 , suggesting that diabetes mellitus is more common in severe cases of liver damage. The association between diabetes mellitus and cirrhosis also varies according to the cause of liver disease. In a US study of 204 people with cirrhosis, the prevalence of diabetes mellitus was 25% among those with cirrhosis caused by hepatitis C virus, 19% among those with cirrhosis from alcoholic liver disease and only 1% among those with cirrhosis due to cholestatic liver disease 117 . Among the causes of cirrhosis, haemochromatosis has the strongest association with diabetes mellitus, with diabetes mellitus mainly resulting from the iron deposition that is characteristic of haemochromatosis 118 .
Several factors have been implicated in the aetiology of liver disease in people with diabetes mellitus, with insulin resistance being the most notable 119 .
Insulin resistance
Insulin resistance causes lipolysis, thereby increasing the circulating levels of free fatty acids, which are then taken up by the liver as an energy source 120 . These fatty acids overload the mitochondrial β-oxidation system in the liver, resulting in the accumulation of fatty acids and, consequently, NAFLD 121 . Of those individuals with NAFLD, 2–3% develop hepatic inflammation, necrosis and fibrosis, which are the hallmarks of NASH 122 . The exact mechanisms leading to steatohepatitis are unclear, although dysregulated peripheral lipid metabolism appears to be important 14 .
Ectopic adipose deposition
Excessive or ectopic deposition of adipose tissue around the viscera and in the liver might be an important mechanism underlying both T2DM and liver disease, particularly NAFLD 123 . Dysfunction of long-term adipose storage in white adipose tissue is known to lead to ectopic adipose deposition in the liver. In this state, increased levels of fatty acyl-coenzyme As, the activated form of fatty acids, might lead to organ dysfunction, including NAFLD 124 . Ectopic adipose deposition leading to organ-specific insulin resistance has emerged as a major hypothesis for the pathophysiological basis of T2DM, and ectopic adipose in the pancreas could contribute to β-cell dysfunction and, thus, the development of T2DM 125 .
Diabetes mellitus and affective disorders
The prevalence of depression appears to be high among people with diabetes mellitus. The strongest evidence for an association comes from a systematic review of 147 studies among people with T2DM, which revealed a mean prevalence of depression of 28% 126 , while the global prevalence of depression in the general population is estimated at around 13% 127 . For T1DM, a systematic review reported a pooled prevalence of depression of 12% compared with only 3% in those without T1DM 128 . The risk of depression among people with diabetes mellitus appears to be roughly 25% greater than the risk in the general population, with consistent findings across several meta-analyses (Table 4 ). A 2013 study found an adjusted RR of 1.25 (95% CI 1.10–1.44) for incident depression among people with diabetes mellitus compared with those without diabetes mellitus 129 . Another systematic review of people with T2DM reported a near identical effect size 130 .
Anxiety and eating disorders
Evidence exists for an association of diabetes mellitus with anxiety, and of T1DM with eating disorders. In a systematic review involving 2,584 individuals with diabetes mellitus, a prevalence of 14% was found for generalized anxiety disorder and 40% for anxiety symptoms, whereas the prevalence of generalized anxiety disorder in the general population is estimated as only 3–4% 131 . People with diabetes mellitus had an increased risk of anxiety disorders (OR 1.20, 95% CI 1.10–1.31) and anxiety symptoms (OR 1.48, 95% CI 1.02–1.93) compared with those without diabetes mellitus in a meta-analysis 132 (Table 4 ), although these findings were based on cross-sectional data. Across 13 studies, 7% of adolescents with T1DM were found to have eating disorders, compared with 3% of peers without diabetes mellitus 133 .
Broader psychological impacts
There is a substantial literature on a broad range of psychological impacts of diabetes mellitus. Social stigma 134 can have profound impacts on the quality of life of not only people with diabetes mellitus, but their families and carers, too 135 . In a systematic review, diabetes mellitus distress was found to affect around one-third of adolescents with T1DM, which was consistent with the results of studies of adults with diabetes mellitus 136 . Diabetes mellitus burnout appears to be a distinct concept, and is characterized by exhaustion and detachment, accompanied by the experience of a loss of control over diabetes mellitus 137 .
Diabetes mellitus and depression appear to have common biological origins. Activation of the innate immune system and acute-phase inflammation contribute to the pathogenesis of T2DM — increased levels of inflammatory cytokines predict the onset of T2DM 138 — and there is growing evidence implicating cytokine-mediated inflammation in people with depression in the absence of diabetes mellitus 139 . Dysregulation of the hypothalamic–pituitary–adrenal axis is another potential biological mechanism linking depression and diabetes mellitus 140 . There have been numerous reports of hippocampal atrophy, which might contribute to chronic activation of the hypothalamic–pituitary–adrenal axis, in individuals with T2DM as well as those with depression 141 , 142 . A meta-analysis found that, although hypertension modified global cerebral atrophy in those with T2DM, it had no effect on hippocampal atrophy 143 . This suggests that, although global cerebral atrophy in individuals with T2DM might be driven by atherosclerotic disease, hippocampal atrophy is an independent effect that provides a common neuropathological aetiology for the comorbidity of T2DM with depression. There is a lack of relevant information regarding the potential aetiological mechanisms that link diabetes to other affective disorders.
Diabetes mellitus and sleep disturbance
Obstructive sleep apnoea.
Obstructive sleep apnoea (OSA) is highly prevalent among people with diabetes mellitus. In a systematic review of 41 studies of adults with diabetes mellitus, the prevalence of OSA was found to be 60% 144 , whereas reports for OSA prevalence in the general population range from 9% to 38% 145 . In a UK study of 1,656,739 participants, T2DM was associated with an IRR for OSA of 1.48 (95% CI 1.42–1.55) compared with no T2DM 146 . A population-based US study reported a HR of 1.53 (95% CI 1.32–1.77) for OSA in people with T2DM compared with those without diabetes mellitus 147 . However, the association in this latter report was attenuated after adjustment for BMI and waist circumference (1.08, 95% CI 1.00–1.16), suggesting that the excess risk of OSA among people with diabetes mellitus might be mainly explained by the comorbidity of obesity. Although most studies on OSA have focused on T2DM, a meta-analysis of people with T1DM revealed a similar prevalence of 52% 148 ; however, this meta-analysis was limited to small studies. The association between T2DM and OSA is bidirectional: the severity of OSA was shown to be positively associated with the incidence of T2DM, independent of adiposity, in a large US cohort study 149 .
The mechanism by which T2DM might increase the risk of developing OSA is thought to involve dysregulation of the autonomic nervous system leading to sleep-disordered breathing 150 . Conversely, the specific mechanism behind OSA as a causative factor for T2DM remains poorly understood. It has been suggested that OSA is able to induce insulin resistance 151 , 152 and is a risk factor for the development of glucose intolerance 152 . However, once T2DM has developed, there is no clear evidence that OSA worsens glycaemic control, as an RCT of people with T2DM found that treating OSA had no effect on glycaemic control 153 .
Diabetes mellitus and cognitive disability
Dementia and cognitive impairment.
Dementia is emerging as a major cause of mortality in both individuals with diabetes mellitus and the general population, and is now the leading cause of death in some countries/regions 9 . However, compared with the general population, diabetes mellitus increases the risk of dementia, particularly vascular dementia. The association is supported by several systematic reviews, including one of eight population-based studies with more than 23,000 people, which found SRRs of 2.38 (95% CI 1.79–3.18) for vascular dementia and 1.39 (95% CI 1.16–1.66) for Alzheimer disease comparing people with diabetes mellitus with those without diabetes mellitus 154 (Table 4 ). Similar results, as well as a RR of 1.21 (95% CI 1.02–1.45) for mild cognitive impairment (MCI), were reported across 19 population-based studies of 44,714 people, 6,184 of whom had diabetes mellitus 155 . Two meta-analyses of prospective cohort studies have shown increased risks of all-cause dementia in people with diabetes mellitus compared with those without diabetes mellitus 156 , 157 , and T2DM has been shown to increase progression to dementia in people with MCI 158 .
The boundaries between Alzheimer disease and vascular dementia remain controversial, and these conditions are often difficult to differentiate clinically 159 . Consequently, vascular dementia might have been misdiagnosed as Alzheimer disease in some studies investigating diabetes mellitus and dementia, resulting in an overestimation of the effect size of the association between diabetes mellitus and Alzheimer disease. Although a cohort study found a significant association between diabetes mellitus and Alzheimer disease using imaging 160 , autopsy studies have failed to uncover an association between diabetes mellitus and Alzheimer disease pathology 161 , 162 , suggesting that vascular mechanisms are the key driver of cognitive decline in people with diabetes mellitus.
Another important finding is a 45% prevalence of MCI among people with T2DM in a meta-analysis, compared with a prevalence of 3–22% reported for the general population 163 . Notably, however, the prevalence of MCI in individuals with T2DM was similar in people younger than 60 years (46%) and those older than 60 years (44%), which is at odds with previous research suggesting that MCI is most common in older people, particularly those aged more than 65 years 164 However, another meta-analysis found cognitive decline in people with T2DM who are younger than 65 years 165 , suggesting that a burden of cognitive disease exists among younger people with diabetes mellitus.
Although there is solid evidence that links diabetes mellitus to cognitive disability, our understanding of the underlying mechanisms is incomplete. Mouse models suggest a strong association between hyperglycaemia, the advanced glycation end products glyoxal and methylglyoxal, enhanced blood–brain barrier (BBB) permeability and cognitive dysfunction in both T1DM and T2DM 166 . The BBB reduces the access of neurotoxic compounds and pathogens to the brain and sustains brain homeostasis, so disruption to the BBB can result in cognitive dysfunction through dysregulation of transport of molecules between the peripheral circulation and the brain 167 . There appears to be a continuous relationship between glycaemia and cognition, with associations found between even high-normal blood levels of glucose and cognitive decline 168 . Another hypothetical mechanism involves a key role for impaired insulin signalling in the pathogenesis of Alzheimer disease. Brain tissue obtained post mortem from individuals with Alzheimer disease showed extensive abnormalities in insulin and insulin-like growth factor signalling mechanisms compared with control brain tissue 169 . Although the synthesis of insulin-like growth factors occurred normally in people with Alzheimer disease, their expression levels were markedly reduced, which led to the subsequent proposal of the term ‘type 3 diabetes’ to characterize Alzheimer disease.
Diabetes mellitus and disability
Functional disability.
Disability (defined as a difficulty in functioning in one or more life domains as experienced by an individual with a health condition in interaction with contextual factors) 170 is highly prevalent in people with diabetes mellitus. In a systematic review, lower-body functional limitation was found to be the most prevalent disability (47–84%) among people with diabetes mellitus 171 The prevalence of difficulties with activities of daily living among people with diabetes mellitus ranged from 12% to 55%, although most studies were conducted exclusively in individuals aged 60 years and above, so the results are not generalizable to younger age groups. A systematic review showed a significant association between diabetes mellitus and falls in adults aged 60 years and above 172 . A 2013 meta-analysis 173 showed an increased risk of mobility disability, activities of daily living disability and independent activities of daily living disability among people with diabetes mellitus compared with those without diabetes mellitus (Table 4 ). Although this analysis included cross-sectional data, results were consistent across longitudinal and cross-sectional studies, suggesting little effect of reverse causality. However, people with functional disabilities that limit mobility (for example, people with osteoarthritis or who have had a stroke) might be more prone to developing diabetes mellitus owing to physical inactivity 174 .
Workplace productivity
Decreased productivity while at work, increased time off work and early dropout from the workforce 175 are all associated with diabetes mellitus, probably partly due to functional disability, and possibly also to comorbidities such as obesity and physical inactivity 176 . Given that young-onset diabetes is becoming more common, and most people with diabetes mellitus in middle-income countries/regions are less than 65 years old 177 , a pandemic of diabetes mellitus-related work disability among a middle-aged population does not bode well for the economies of these regions.
The mechanisms by which diabetes mellitus leads to functional disability remain unclear. One suggestion is that hyperglycaemia leads to systemic inflammation, which is one component of a multifactorial process that results in disability 154 . The rapid loss of skeletal muscle strength and quality seen among people with diabetes mellitus might be another cause of functional disability 178 (Box 1 ). In addition, complications of diabetes mellitus, including stroke, peripheral neuropathy and cardiac dysfunction, can obviously directly cause disability 179 .
Box 1 Diabetes mellitus and skeletal muscle atrophy
Individuals with diabetes mellitus exhibit skeletal muscle atrophy that is typically mild in middle age and becomes more substantial with increasing age.
This muscle loss leads to reduced strength and functional capacity and, ultimately, increased mortality.
Skeletal muscle atrophy results from a negative balance between the rate of synthesis and degradation of contractile proteins, which occurs in response to disuse, ageing and chronic diseases such as diabetes mellitus.
Degradation of muscle proteins is more rapid in diabetes mellitus, and muscle protein synthesis has also been reported to be decreased.
Proposed mechanisms underlying skeletal muscle atrophy include systemic inflammation (affecting both protein synthesis and degradation), dysregulation of muscle protein anabolism and lipotoxicity.
Mouse models have also revealed a key role for the WWP1/KLF15 pathway, mediated by hyperglycaemia, in the pathogenesis of muscle atrophy.
See refs 195 , 196 , 197 , 198 .
Diabetes management and control
Although a detailed discussion of the impacts of anti-diabetes mellitus medications and glucose control on emerging complications is beyond the scope of this Review, their potential effect on these complications must be acknowledged.
Medications
Anti-diabetes mellitus medications and cancer.
In the case of cancer as an emerging complication, the use of medications for diabetes mellitus was not controlled for in most studies of diabetes mellitus and cancer and might therefore be a confounding factor. People taking metformin have a lower cancer risk than those not taking metformin 180 . However, this association is mainly accounted for by other factors. For example, metformin is less likely to be administered to people with diabetes mellitus who have kidney disease 181 , who typically have longer duration diabetes mellitus, which increases cancer risk. A review of observational studies into the association between metformin and cancer found that many studies reporting significant reductions in cancer incidence or mortality associated with metformin were affected by immortal time bias and other time-related biases, casting doubt on the ability of metformin to reduce cancer mortality 182 . Notably, the use of insulin was associated with an increased risk of several cancers in a meta-analysis 183 . However, in an RCT of more than 12,000 people with dysglycaemia, randomization to insulin glargine (compared with standard care) did not increase cancer incidence 184 . Furthermore, cancer rates in people with T1DM and T2DM do not appear to vary greatly, despite substantial differences in insulin use between people with these types of diabetes mellitus.
Anti-diabetes mellitus medications and other emerging complications
Anti-diabetes medications appear to affect the onset and development of some other emerging complications of diabetes mellitus. Results from RCTs suggest that metformin might confer therapeutic effects against depression 185 , and its use was associated with reduced dementia incidence in a systematic review 186 . In an RCT investigating a potential association between metformin and NAFLD, no improvement in NAFLD histology was found among people using metformin compared with those given placebo 187 . An RCT reported benefits of treatment with the glucagon-like peptide 1 receptor agonist dulaglutide on cognitive function in a post hoc analysis 188 , suggesting that trials designed specifically to test the effects of dulaglutide on cognitive function should be undertaken.
Glucose control
Another important consideration is glycaemic control, which appears to have variable effects on emerging complications. A meta-analysis found no association of glycaemic control with cancer risk among those with diabetes mellitus 189 , and an RCT found no effect of intensive glucose lowering on cognitive function in people with T2DM 190 . However, glycaemic control has been associated with improved physical function 191 , decreased COVID-19 mortality 192 and a decreased risk of NAFLD 193 in observational studies of patients with diabetes mellitus; notably, no RCTs have yet confirmed these associations.
Conclusions
With advances in the management of diabetes mellitus and associated increased life expectancy, the face of diabetes mellitus complications is changing. As the management of glycaemia and traditional complications of diabetes mellitus is optimized, we are beginning instead to see deleterious effects of diabetes mellitus on the liver, brain and other organs. Given the substantial burden and risk of these emerging complications, future clinical and public health strategies should be updated accordingly. There is a need to increase the awareness of emerging complications among primary care physicians at the frontline of diabetes mellitus care, and a place for screening for conditions such as depression, liver disease and cancers in diabetes mellitus guidelines should be considered. Clinical care for older people with diabetes mellitus should target physical activity, particularly strength-based activity, to reduce the risk of functional disability in ageing populations. Ongoing high-quality surveillance of diabetes mellitus outcomes is imperative to ensure we know where the main burdens lie. Given the growing burden of these emerging complications, the traditional management of diabetes mellitus might need to broaden its horizons.
Zimmet, P., Alberti, K. G. M. M. & Shaw, J. Global and societal implications of the diabetes epidemic. Nature 414 , 782–787 (2001).
Article CAS PubMed Google Scholar
Fowler, M. J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 26 , 77–82 (2008).
Article Google Scholar
Shah, A. D. et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol. 3 , 105–113 (2015).
Article PubMed PubMed Central Google Scholar
Bertoni, A. G. et al. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 27 , 699–703 (2004).
Article PubMed Google Scholar
Gregg, E. W. et al. Changes in diabetes-related complications in the United States, 1990–2010. N. Engl. J. Med. 370 , 1514–1523 (2014).
Gregg, E. W., Sattar, N. & Ali, M. K. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 4 , 537–547 (2016).
Gregg, E. W. et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet 391 , 2430–2440 (2018).
Harding, J. L., Shaw, J. E., Peeters, A., Davidson, S. & Magliano, D. J. Age-specific trends from 2000–2011 in all-cause and cause-specific mortality in type 1 and type 2 diabetes: a cohort study of more than one million people. Diabetes Care 39 , 1018–1026 (2016).
Pearson-Stuttard, J. et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 9 , 165–173 (2021).
Einarson, T. R., Acs, A., Ludwig, C. & Panton, U. H. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 17 , 83 (2018).
Pearson-Stuttard, J., Buckley, J., Cicek, M. & Gregg, E. W. The changing nature of mortality and morbidity in patients with diabetes. Endocrinol. Metab. Clin. North Am. 50 , 357–368 (2021).
Pearson-Stuttard, J. et al. Trends in leading causes of hospitalisation of adults with diabetes in England from 2003 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 10 , 46–57 (2022).
Pearson-Stuttard, J., Blundell, S., Harris, T., Cook, D. G. & Critchley, J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 4 , 148–158 (2016).
Tolman, K. G., Fonseca, V., Dalpiaz, A. & Tan, M. H. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care 30 , 734–743 (2007).
Chatterjee, S. et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 39 , 300–307 (2016).
Tsilidis, K. K., Kasimis, J. C., Lopez, D. S., Ntzani, E. E. & Ioannidis, J. P. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 350 , g7607 (2015).
Harding, J. L., Pavkov, M. E., Magliano, D. J., Shaw, J. E. & Gregg, E. W. Global trends in diabetes complications: a review of current evidence. Diabetologia 62 , 3–16 (2019).
Unal, B., Critchley, J. A. & Capewell, S. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation 109 , 1101–1107 (2004).
Pearson-Stuttard, J., Ezzati, M. & Gregg, E. W. Multimorbidity — a defining challenge for health systems. Lancet Public. Health 4 , e599–e600 (2019).
Lee, L., Cheung, W. Y., Atkinson, E. & Krzyzanowska, M. K. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J. Clin. Oncol. 29 , 106–117 (2011).
Srokowski, T. P., Fang, S., Hortobagyi, G. N. & Giordano, S. H. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J. Clin. Oncol. 27 , 2170–2176 (2009).
Gross, C. P., McAvay, G. J., Guo, Z. & Tinetti, M. E. The impact of chronic illnesses on the use and effectiveness of adjuvant chemotherapy for colon cancer. Cancer 109 , 2410–2419 (2007).
Harding, J. L. et al. All-cause cancer mortality over 15 years in multi-ethnic Mauritius: the impact of diabetes and intermediate forms of glucose tolerance. Int. J. Cancer 131 , 2385–2393 (2012).
Wang, C. et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int. J. Cancer 130 , 1639–1648 (2012).
El-Serag, H. B., Hampel, H. & Javadi, F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin. Gastroenterol. Hepatol. 4 , 369–380 (2006).
Desbois, A. C. & Cacoub, P. Diabetes mellitus, insulin resistance and hepatitis C virus infection: a contemporary review. World J. Gastroenterol. 23 , 1697–1711 (2017).
Article CAS PubMed PubMed Central Google Scholar
Huxley, R., Ansary-Moghaddam, A., Berrington De González, A., Barzi, F. & Woodward, M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br. J. Cancer 92 , 2076–2083 (2005).
Gullo, L. et al. Diabetes and the risk of pancreatic cancer. N. Engl. J. Med. 331 , 81–84 (1994).
Gershell, L. Type 2 diabetes market. Nat. Rev. Drug Discov. 4 , 367–368 (2005).
Carstensen, B. et al. Cancer incidence in persons with type 1 diabetes: a five-country study of 9,000 cancers in type 1 diabetic individuals. Diabetologia 59 , 980–988 (2016).
Jiang, Y. et al. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur. J. Epidemiol. 26 , 863–876 (2011).
De Bruijn, K. M. J. et al. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br. J. Surg. 100 , 1421–1429 (2013).
Deng, L., Gui, Z., Zhao, L., Wang, J. & Shen, L. Diabetes mellitus and the incidence of colorectal cancer: an updated systematic review and meta-analysis. Dig. Dis. Sci. 57 , 1576–1585 (2012).
Liao, C., Zhang, D., Mungo, C., Andrew Tompkins, D. & Zeidan, A. M. Is diabetes mellitus associated with increased incidence and disease-specific mortality in endometrial cancer? A systematic review and meta-analysis of cohort studies. Gynecol. Oncol. 135 , 163–171 (2014).
Saed, L. et al. The effect of diabetes on the risk of endometrial cancer: an updated a systematic review and meta-analysis. BMC Cancer 19 , 527 (2019).
Friberg, E., Orsini, N., Mantzoros, C. S. & Wolk, A. Diabetes mellitus and risk of endometrial cancer: A meta-analysis. Diabetologia 50 , 1365–1374 (2007).
Anothaisintawee, T. et al. Risk factors of breast cancer: a systematic review and meta-analysis. Asia-Pac. J. Public Health 25 , 368–387 (2013).
Larsson, S. C., Mantzoros, C. S. & Wolk, A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int. J. Cancer 121 , 856–862 (2007).
Boyle, P. et al. Diabetes and breast cancer risk: a meta-analysis. Br. J. Cancer 107 , 1608–1617 (2012).
Rinaldi, S. et al. Anthropometric measures, endogenous sex steroids and breast cancer risk in postmenopausal women: a study within the EPIC cohort. Int. J. Cancer 118 , 2832–2839 (2006).
Michels, K. B. et al. Type 2 diabetes and subsequent incidence of breast cancer in the nurses’ health study. Diabetes Care 26 , 1752–1758 (2003).
Bronsveld, H. K. et al. Diabetes and breast cancer subtypes. PLoS ONE 12 , e0170084 (2017).
Article PubMed PubMed Central CAS Google Scholar
Zhang, D., Li, N., Xi, Y., Zhao, Y. & Wang, T. Diabetes mellitus and risk of ovarian cancer. A systematic review and meta-analysis of 15 cohort studies. Diabetes Res. Clin. Pract. 130 , 43–52 (2017).
Weng, L., Wang, L., Zhang, J., Wang, B. & Liu, H. Association between diabetes mellitus and subsequent ovarian cancer in women: a systematic review and meta-analysis of cohort studies. Medicine 96 , e6396 (2017).
Wang, L., Zhong, L., Xu, B., Chen, M. & Huang, H. Diabetes mellitus and the risk of ovarian cancer: a systematic review and meta-analysis of cohort and case-control studies. BMJ Open 10 , e040137 (2020).
Lee, J. Y. et al. Diabetes mellitus and ovarian cancer risk: a systematic review and meta-analysis of observational studies. Int. J. Gynecol. Cancer 23 , 402–412 (2013).
Bonovas, S., Filioussi, K. & Tsantes, A. Diabetes mellitus and risk of prostate cancer: a meta-analysis. Diabetologia 47 , 1071–1078 (2004).
Shikata, K., Ninomiya, T. & Kiyohara, Y. Diabetes mellitus and cancer risk: review of the epidemiological evidence. Cancer Sci. 104 , 9–14 (2013).
Long, X. J., Lin, S., Sun, Y. N. & Zheng, Z. F. Diabetes mellitus and prostate cancer risk in Asian countries: a meta-analysis. Asian Pac. J. Cancer Preven. 13 , 4097–4100 (2012).
Rhee, E. J. Diabetes in Asians. Endocrinol. Metab. 30 , 263–269 (2015).
Article CAS Google Scholar
Bensimon, L., Yin, H., Suissa, S., Pollak, M. N. & Azoulay, L. Type 2 diabetes and the risk of mortality among patients with prostate cancer. Cancer Causes Control. 25 , 329–338 (2014).
Johnson, J. A., Bowker, S. L., Richardson, K. & Marra, C. A. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia 54 , 2263–2271 (2011).
Johnson, J. A. et al. Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia 55 , 1607–1618 (2012).
Harding, J. L., Shaw, J. E., Peeters, A., Cartensen, B. & Magliano, D. J. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care 38 , 264–270 (2015).
Pearson-Stuttard, J. et al. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol. 6 , e6–e15 (2018).
Larsson, S. C. & Wolk, A. Diabetes mellitus and incidence of kidney cancer: a meta-analysis of cohort studies. Diabetologia 54 , 1013–1018 (2011).
Xu, X. et al. Diabetes mellitus and risk of bladder cancer: a meta-analysis of cohort studies. PLoS ONE 8 , e58079 (2013).
Gong, I. Y. et al. Association between diabetes and haematological malignancies: a population-based study. Diabetologia 64 , 540–551 (2021).
Giovannucci, E. et al. Diabetes and cancer: a consensus report. Diabetes Care 33 , 1674–1685 (2010).
Weinstein, D., Simon, M., Yehezkel, E., Laron, Z. & Werner, H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab. Res. Rev. 25 , 41–49 (2009).
Najjar, S. M. & Perdomo, G. Hepatic insulin clearance: mechanism and physiology. Physiology 34 , 198–215 (2019).
Lorenzi, M., Montisano, D. F., Toledo, S. & Barrieux, A. High glucose induces DNA damage in cultured human endothelial cells. J. Clin. Invest. 77 , 322–325 (1986).
Robertson, R., Zhou, H., Zhang, T. & Harmon, J. S. Chronic oxidative stress as a mechanism for glucose toxicity of the beta cell in type 2 diabetes. Cell Biochem. Biophys. 48 , 139–146 (2007).
Turturro, F., Friday, E. & Welbourne, T. Hyperglycemia regulates thioredoxin-ROS activity through induction of thioredoxin-interacting protein (TXNIP) in metastatic breast cancer-derived cells MDA-MB-231. BMC Cancer 7 , 96 (2007).
Wu, Y., Liu, Y., Dong, Y. & Vadgama, J. Diabetes-associated dysregulated cytokines and cancer. Integr. Cancer Sci. Ther. 3 , 370–378 (2016).
PubMed PubMed Central Google Scholar
Inoki, K., Kim, J. & Guan, K. L. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu. Rev. Pharmacol. Toxicol. 52 , 381–400 (2012).
Huang, X., Liu, G., Guo, J. & Su, Z. Q. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 14 , 1483–1496 (2018).
Zhao, Y. et al. Metformin is associated with reduced cell proliferation in human endometrial cancer by inbibiting PI3K/AKT/mTOR signaling. Gynecol. Endocrinol. 34 , 428–432 (2018).
Knapp, S. Diabetes and infection: is there a link?-A mini-review. Gerontology 59 , 99–104 (2013).
Fang, M. et al. Diabetes and the risk of hospitalisation for infection: the atherosclerosis risk in communities (ARIC) study. Diabetologia 64 , 2458–2465 (2021).
Tseng, C.-H. Metformin use is associated with a reduced risk of acute appendicitis in Taiwanese patients with type 2 diabetes mellitus. Sci. Rep. 11 , 12400 (2021).
Luk, A. O. Y. et al. Temporal trends in rates of infection-related hospitalisations in Hong Kong people with and without diabetes, 2001–2016: a retrospective study. Diabetologia 64 , 109–118 (2021).
Magliano, D. J. et al. Excess risk of dying from infectious causes in those with type 1 and type 2 diabetes. Diabetes Care 38 , 1274–1280 (2015).
Martin, E. T. et al. Diabetes and risk of surgical site infection: a systematic review and meta-analysis. Infect. Control. Hosp. Epidemiol. 37 , 88–99 (2016).
Trussell, J. et al. Impact of a patient care pathway protocol on surgical site infection rates in cardiothoracic surgery patients. Am. J. Surg. 196 , 883–889 (2008).
Coleman, J. S. et al. Surgical site infections after hysterectomy among HIV-infected women in the HAART era: a single institution’s experience from 1999–2012. Am. J. Obstet. Gynecol. 210 , 117.e111–117.e117 (2014).
Friedman, N. D., Sexton, D. J., Connelly, S. M. & Kaye, K. S. Risk factors for surgical site infection complicating laminectomy. Infect. Control. Hosp. Epidemiol. 28 , 1060–1065 (2007).
Apicella, M. et al. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 8 , 782–792 (2020).
McGurnaghan, S. J. et al. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 9 , 82–93 (2021).
Rawshani, A. et al. Severe COVID-19 in people with type 1 and type 2 diabetes in Sweden: a nationwide retrospective cohort study. Lancet Reg. Health Eur. 4 , 100105 (2021).
You, J. H. et al. Clinical outcomes of COVID-19 patients with type 2 diabetes: a population-based study in Korea. Endocrinol. Metab. 35 , 901–908 (2020).
Moon, S. J. et al. Independent impact of diabetes on the severity of coronavirus disease 2019 in 5,307 patients in South Korea: a nationwide cohort study. Diabetes Metab. J. 44 , 737–746 (2020).
Aranjani, J. M., Manuel, A., Razack, H. I. A. & Mathew, S. T. Covid-19–associated mucormycosis: evidence-based critical review of an emerging infection burden during the pandemic’s second wave in India. PLoS. Negl. Trop. Dis. 15 , e0009921 (2021).
Crankson, S., Pokhrel, S. & Anokye, N. K. Determinants of COVID-19-related length of hospital stays and long COVID in Ghana: a cross-sectional analysis. Int. J. Environ. Res. Public Health 19 , 527 (2022).
Bellan, M. et al. Respiratory and psychophysical sequelae among patients with covid-19 four months after hospital discharge. JAMA Netw. Open 4 , e2036142 (2021).
Gottesman, B. L., Yu, J., Tanaka, C., Longhurst, C. A. & Kim, J. J. Incidence of new-onset type 1 diabetes among US children during the COVID-19 global pandemic. JAMA Pediatr. 176 , 414–415 (2022).
Barrett, C. E. et al. Risk for newly diagnosed diabetes <30 days after SARS-CoV-2 infection among persons aged >18 years-United States, March 1, 2020-June 28, 2021. Morb. Mortal. Wkly. Rep. 71 , 59–65 (2022).
Kornum, J. B. et al. Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case-control study. Diabetes Care 31 , 1541–1545 (2008).
Matsuyama, R., Nishiura, H., Kutsuna, S., Hayakawa, K. & Ohmagari, N. Clinical determinants of the severity of Middle East respiratory syndrome (MERS): a systematic review and meta-analysis. BMC Public Health 16 , 1203 (2016).
Badawi, A. & Ryoo, S. G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int. J. Infect. Dis. 49 , 129–133 (2016).
Badawi, A. & Ryoo, S. G. Prevalence of diabetes in the 2009 influenza A (H1N1) and the middle east respiratory syndrome coronavirus: a systematic review and meta-analysis. J. Public. Health Res. 5 , 130–138 (2016).
Yang, J. K. et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 23 , 623–628 (2006).
Ehrlich, S. F., Quesenberry, C. P. Jr, Van Den Eeden, S. K., Shan, J. & Ferrara, A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care 33 , 55–60 (2010).
Alraddadi, B. M. et al. Risk factors for primary middle east respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg. Infect. Dis. 22 , 49–55 (2016).
Geerlings, S. E. & Hoepelman, A. I. M. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 26 , 259–265 (1999).
Velazquez-Salinas, L., Verdugo-Rodriguez, A., Rodriguez, L. L. & Borca, M. V. The role of interleukin 6 during viral infections. Front. Microbiol. 10 , 1057 (2019).
Joshi, N., Caputo, G. M., Weitekamp, M. R. & Karchmer, A. W. Infections in patients with diabetes mellitus. N. Engl. J. Med. 341 , 1906–1912 (1999).
Codo, A. C. et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/Glycolysis-dependent axis. Cell Metab. 32 , 437–446.e435 (2020).
Miyazawa, T., Nakagawa, K., Shimasaki, S. & Nagai, R. Lipid glycation and protein glycation in diabetes and atherosclerosis. Amino Acids 42 , 1163–1170 (2012).
Peleg, A. Y., Weerarathna, T., McCarthy, J. S. & Davis, T. M. E. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab. Res. Rev. 23 , 3–13 (2007).
Barda, N., Dagan, N. & Balicer, R. D. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. reply. N. Engl. J. Med. 384 , 1970 (2021).
PubMed Google Scholar
Cholankeril, G. & Ahmed, A. Alcoholic liver disease replaces hepatitis C virus infection as the leading indication for liver transplantation in the United States. Clin. Gastroenterol. Hepatol. 16 , 1356–1358 (2018).
Fink, M. & Byrne, M. Australia and New Zealand Liver and Intestinal Transplant Registry Annual Report 2019 (Melbourne, Victoria, Australia, 2019).
Haldar, D. et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: a European liver transplant registry study. J. Hepatol. 71 , 313–322 (2019).
Younossi, Z. M. et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J. Hepatol. 71 , 793–801 (2019).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64 , 73–84 (2016).
Pang, Y. et al. Diabetes, plasma glucose, and incidence of fatty liver, cirrhosis, and liver cancer: a prospective study of 0.5 million people. Hepatology 68 , 1308–1318 (2018).
Li, Y. et al. Bidirectional association between nonalcoholic fatty liver disease and type 2 diabetes in Chinese population: evidence from the Dongfeng-Tongji cohort study. PLoS ONE 12 , e0174291 (2017).
Mansour-Ghanaei, F. et al. Prevalence of non-alcoholic fatty liver disease in patients with diabetes mellitus, hyperlipidemia, obesity and polycystic ovary syndrome: a cross-sectional study in north of Iran. Diabetes Metab. Syndr. 13 , 1591–1596 (2019).
Leite, N. C., Salles, G. F., Araujo, A. L., Villela-Nogueira, C. A. & Cardoso, C. R. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 29 , 113–119 (2009).
Singh, S. P. et al. Risk factors associated with non-alcoholic fatty liver disease in Indians: a case-control study. J. Clin. Exp. Hepatol. 5 , 295–302 (2015).
Dufour, J.-F. et al. The global epidemiology of nonalcoholic steatohepatitis (NASH) and associated risk factors–a targeted literature review. Endocr. Metab. Sci. 3 , 100089 (2021).
Loomba, R. et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 56 , 943–951 (2012).
Anstee, Q. M., Targher, G. & Day, C. P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 10 , 330–344 (2013).
Holstein, A., Hinze, S., Thießen, E., Plaschke, A. & Egberts, E. H. Clinical implications of hepatogenous diabetes in liver cirrhosis. J. Gastroenterol. Hepatol. 17 , 677–681 (2002).
Del Vecchio Blanco, C., Gentile, S., Marmo, R., Carbone, L. & Coltorti, M. Alterations of glucose metabolism in chronic liver disease. Diabetes Res. Clin. Pract. 8 , 29–36 (1990).
Zein, N. N., Abdulkarim, A. S., Wiesner, R. H., Egan, K. S. & Persing, D. H. Prevalence of diabetes mellitus in patients with end-stage liver cirrhosis due to hepatitis C, alcohol, or cholestatic disease. J. Hepatol. 32 , 209–217 (2000).
Niederau, C. et al. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N. Engl. J. Med. 313 , 1256–1262 (1985).
Larter, C. Z. & Farrell, G. C. Insulin resistance, adiponectin, cytokines in NASH: which is the best target to treat? J. Hepatol. 44 , 253–261 (2006).
Marchesini, G. et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am. J. Med. 107 , 450–455 (1999).
Angulo, P. Medical progress: nonalcoholic fatty liver disease. N. Engl. J. Med. 346 , 1221–1231 (2002).
Porepa, L., Ray, J. G., Sanchez-Romeu, P. & Booth, G. L. Newly diagnosed diabetes mellitus as a risk factor for serious liver disease. CMAJ 182 , E526–E531 (2010).
Stefan, N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 8 , 616–627 (2020).
Stefan, N., Häring, H. U. & Cusi, K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 7 , 313–324 (2019).
Sattar, N. & Gill, J. M. R. Type 2 diabetes as a disease of ectopic fat? BMC Med. 12 , 123 (2014).
Harding, K. A. et al. Depression prevalence in type 2 diabetes is not related to diabetes–depression symptom overlap but is related to symptom dimensions within patient self-report measures: a meta-analysis. Diabet. Med. 36 , 1600–1611 (2019).
Lim, G. Y. et al. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci. Rep. 8 , 2861 (2018).
Roy, T. & Lloyd, C. E. Epidemiology of depression and diabetes: a systematic review. J. Affect. Disord. 142 , S8–S21 (2012).
Rotella, F. & Mannucci, E. Diabetes mellitus as a risk factor for depression. A meta-analysis of longitudinal studies. Diabetes Res. Clin. Pract. 99 , 98–104 (2013).
Nouwen, A. et al. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia 53 , 2480–2486 (2010).
Grigsby, A. B., Anderson, R. J., Freedland, K. E., Clouse, R. E. & Lustman, P. J. Prevalence of anxiety in adults with diabetes a systematic review. J. Psychosom. Res. 53 , 1053–1060 (2002).
Smith, K. J. et al. Association of diabetes with anxiety: a systematic review and meta-analysis. J. Psychosom. Res. 74 , 89–99 (2013).
Young, V. et al. Eating problems in adolescents with type1 diabetes: a systematic review with meta-analysis. Diabet. Med. 30 , 189–198 (2013).
Schabert, J., Browne, J. L., Mosely, K. & Speight, J. Social stigma in diabetes: a framework to understand a growing problem for an increasing epidemic. Patient 6 , 1–10 (2013).
Barnard, K. D., Speight, J. & Skinner, T. C. Quality of life and impact of continuous subcutaneous insulin infusion for children and their parents. Pract. Diabetes Int. 25 , 278–283 (2008).
Hagger, V., Hendrieckx, C., Sturt, J., Skinner, T. C. & Speight, J. Diabetes distress among adolescents with type 1 diabetes: a systematic review. Curr. Diabetes Rep. 16 , 1–14 (2016).
Abdoli, S. et al. New insights into diabetes burnout and its distinction from diabetes distress and depressive symptoms: a qualitative study. Diabetes Res. Clin. Pract. 169 , 108446 (2020).
Pickup, J. C. & Crook, M. A. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia 41 , 1241–1248 (1998).
Dantzer, R., O’Connor, J. C., Freund, G. G., Johnson, R. W. & Kelley, K. W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9 , 46–56 (2008).
Prestele, S., Aldenhoff, J. & Reiff, J. [The HPA-axis as a possible link between depression, diabetes mellitus and cognitive dysfunction]. Fortschr. Neurol. Psychiatr. 71 , 24–36 (2003).
Cole, J., Costafreda, S. G., McGuffin, P. & Fu, C. H. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. J. Affect. Disord. 134 , 483–487 (2011).
Gold, S. M. et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 50 , 711–719 (2007).
Moulton, C. D., Costafreda, S. G., Horton, P., Ismail, K. & Fu, C. H. Y. Meta-analyses of structural regional cerebral effects in type 1 and type 2 diabetes. Brain Imaging Behav. 9 , 651–662 (2015).
Khalil, M., Power, N., Graham, E., Deschênes, S. S. & Schmitz, N. The association between sleep and diabetes outcomes – systematic review. Diabetes Res. Clin. Pract. 161 , 108035 (2020).
Senaratna, C. V. et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep. Med. Rev. 34 , 70–81 (2017).
Subramanian, A. et al. Risk of incident obstructive sleep apnea among patients with type 2 diabetes. Diabetes Care 42 , 954–963 (2019).
Huang, T. et al. A population-based study of the bidirectional association between obstructive sleep apnea and type 2 diabetes in three prospective U.S. Cohorts. Diabetes Care 41 , 2111–2119 (2018).
Reutrakul, S. et al. Sleep characteristics in type 1 diabetes and associations with glycemic control: systematic review and meta-analysis. Sleep. Med. 23 , 26–45 (2016).
Nagayoshi, M. et al. Obstructive sleep apnea and incident type 2 diabetes. Sleep. Med. 25 , 156–161 (2016).
Ficker, J. H. et al. Obstructive sleep apnoea and diabetes mellitus: the role of cardiovascular autonomic neuropathy. Eur. Respir. J. 11 , 14–19 (1998).
Young, T., Peppard, P. E. & Taheri, S. Excess weight and sleep-disordered breathing. J. Appl. Physiol. 99 , 1592–1599 (2005).
Ip, M. S. M. et al. Obstructive sleep apnea is independently associated with insulin resistance. Am. J. Respir. Crit. Care Med. 165 , 670–676 (2002).
Shaw, J. E. et al. The effect of treatment of obstructive sleep apnea on glycemic control in type 2 diabetes. Am. J. Respir. Crit. Care Med. 194 , 486–492 (2016).
Lu, F. P., Lin, K. P. & Kuo, H. K. Diabetes and the risk of multi-system aging phenotypes: A systematic review and meta-analysis. PLoS ONE 4 , e4144 (2009).
Cheng, G., Huang, C., Deng, H. & Wang, H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern. Med. J. 42 , 484–491 (2012).
Li, X. Y. et al. Midlife modifiable risk factors for dementia: a systematic review and meta-analysis of 34 prospective cohort studies. Curr. Alzheimer Res. 16 , 1254–1268 (2019).
Article PubMed CAS Google Scholar
Xue, M. et al. Diabetes mellitus and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 144 prospective studies. Ageing Res. Rev. 55 , 100944 (2019).
Pal, K., Mukadam, N., Petersen, I. & Cooper, C. Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: a systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 53 , 1149–1160 (2018).
Biessels, G. J., Staekenborg, S., Brunner, E., Brayne, C. & Scheltens, P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 5 , 64–74 (2006).
Peila, R., Rodriguez, B. L. & Launer, L. J. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia aging study. Diabetes 51 , 1256–1262 (2002).
Abner, E. L. et al. Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimer’s Dement. 12 , 882–889 (2016).
Matioli, M. N. P. S. et al. Association between diabetes and causes of dementia: evidence from a clinicopathological study. Dement. Neuropsychol. 11 , 406–412 (2017).
You, Y. et al. The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Acta Diabetol. 58 , 671–685 (2021).
Langa, K. M. & Levine, D. A. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA 312 , 2551–2561 (2014).
Pelimanni, E. & Jehkonen, M. Type 2 diabetes and cognitive functions in middle age: a meta-analysis. J. Int. Neuropsychol. Soc. 25 , 215–229 (2019).
Rom, S. et al. Hyperglycemia and advanced glycation end products disrupt BBB and promote occludin and claudin-5 protein secretion on extracellular microvesicles. Sci. Rep. 10 , 7274 (2020).
Hussain, B., Fang, C. & Chang, J. Blood–brain barrier breakdown: an emerging biomarker of cognitive impairment in normal aging and dementia. Front. Neurosci. 15 , 688090 (2021).
Anstey, K. J., Sargent-Cox, K., Eramudugolla, R., Magliano, D. J. & Shaw, J. E. Association of cognitive function with glucose tolerance and trajectories of glucose tolerance over 12 years in the AusDiab study. Alzheimers Res. Ther. 7 , 48 (2015).
Steen, E. et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease - Is this type 3 diabetes? J. Alzheimer’s Dis. 7 , 63–80 (2005).
Leonardi, M., Bickenbach, J., Ustun, T. B., Kostanjsek, N. & Chatterji, S. The definition of disability: what is in a name? Lancet 368 , 1219–1221 (2006).
Lisy, K., Campbell, J. M., Tufanaru, C., Moola, S. & Lockwood, C. The prevalence of disability among people with cancer, cardiovascular disease, chronic respiratory disease and/or diabetes: a systematic review. Int. J. Evid. Based Healthc. 16 , 154–166 (2018).
Yang, Y., Hu, X., Zhang, Q. & Zou, R. Diabetes mellitus and risk of falls in older adults: a systematic review and meta-analysis. Age Ageing 45 , 761–767 (2016).
Wong, E. et al. Diabetes and risk of physical disability in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 1 , 106–114 (2013).
Havercamp, S. M., Scandlin, D. & Roth, M. Health disparities among adults with developmental disabilities, adults with other disabilities, and adults not reporting disability in North Carolina. Public. Health Rep. 119 , 418–426 (2004).
Herquelot, E., Guéguen, A., Bonenfant, S. & Dray-Spira, R. Impact of diabetes on work cessation: data from the GAZEL cohort study. Diabetes Care 34 , 1344–1349 (2011).
Virtanen, M. et al. Work disability among employees with diabetes: latent class analysis of risk factors in three prospective cohort studies. PLoS ONE 10 , e0143184 (2015).
Cho, N. H. et al. IDF Diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 138 , 271–281 (2018).
Seok, W. P. et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care 30 , 1507–1512 (2007).
Stratton, I. M. et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br. Med. J. 321 , 405–412 (2000).
DeCensi, A. et al. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prev. Res. 3 , 1451–1461 (2010).
Inzucchi, S. E., Lipska, K. J., Mayo, H., Bailey, C. J. & McGuire, D. K. Metformin in patientswith type 2 diabetes and kidney disease a systematic review. JAMA 312 , 2668–2675 (2014).
Suissa, S. & Azoulay, L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 35 , 2665–2673 (2012).
Karlstad, Ø. et al. Use of insulin and insulin analogs and risk of cancer-systematic review and meta-analysis of observational studies. Curr. Drug. Saf. 8 , 333–348 (2013).
Bordeleau, L. et al. The association of basal insulin glargine and/or n-3 fatty acids with incident cancers in patients with dysglycemia. Diabetes Care 37 , 1360–1366 (2014).
Guo, M. et al. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin. Exp. Pharmacol. Physiol. 41 , 650–656 (2014).
CAS PubMed Google Scholar
Campbell, J. M. et al. Metformin use associated with reduced risk of dementia in patients with diabetes: a systematic review and meta-analysis. J. Alzheimer’s Dis. 65 , 1225–1236 (2018).
Haukeland, J. W. et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand. J. Gastroenterol. 44 , 853–860 (2009).
Cukierman-Yaffe, T. et al. Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial. Lancet Neurol. 19 , 582–590 (2020).
Johnson, J. A. & Bowker, S. L. Intensive glycaemic control and cancer risk in type 2 diabetes: a meta-analysis of major trials. Diabetologia 54 , 25–31 (2011).
Launer, L. J. et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 10 , 969–977 (2011).
Jia, Y. et al. Associations of the glycaemic control of diabetes with dementia and physical function in rural-dwelling older Chinese adults: a population-based study. Clin. Interv. Aging 16 , 1503–1513 (2021).
Lesniak, C. et al. Inpatient glycemic control and outcome of COVID-19 patients: a retrospective cohort. SAGE Open. Med. 9 , 20503121211039105 (2021).
Afolabi, B. I. et al. The relationship between glycaemic control and non-alcoholic fatty liver disease in Nigerian type 2 diabetic patients. J. Natl Med. Assoc. 110 , 256–264 (2018).
Nouwen, A. et al. Longitudinal associations between depression and diabetes complications: a systematic review and meta-analysis. Diabet. Med. 36 , 1562–1572 (2019).
Perry, B. D. et al. Muscle atrophy in patients with Type 2 diabetes mellitus: roles of inflammatory pathways, physical activity and exercise. Exerc. Immunol. Rev. 22 , 94–109 (2016).
Hirata, Y. et al. Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 axis. JCI Insight 4 , e124952 (2019).
Article PubMed Central Google Scholar
Bassil, M. S. & Gougeon, R. Muscle protein anabolism in type 2 diabetes. Curr. Opin. Clin. Nutr. Metab. Care 16 , 83–88 (2013).
Meex, R. C. R., Blaak, E. E. & van Loon, L. J. C. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes. Rev. 20 , 1205–1217 (2019).
Download references
Acknowledgements
D.T. is supported by an Australian Government Research Training Program (RTP) Scholarship and Monash Graduate Excellence Scholarship. J.E.S. is supported by a National Health and Medical Research Council Investigator Grant. D.J.M. is supported by a National Health and Medical Research Council Senior Research Fellowship.
Author information
These authors jointly supervised this work: Jonathan E. Shaw and Dianna J. Magliano.
Authors and Affiliations
Baker Heart and Diabetes Institute, Melbourne, Victoria, Australia
Dunya Tomic, Jonathan E. Shaw & Dianna J. Magliano
School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia
You can also search for this author in PubMed Google Scholar

Contributions
D.T. researched data for the article and wrote the article. J.E.S and D.J.M. contributed substantially to discussion of the content. D.T., J.E.S. and D.J.M reviewed and/or edited the manuscript before submission.
Corresponding author
Correspondence to Dianna J. Magliano .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Endocrinology thanks Emily Gallagher, Norbert Stefan and Assaad Eid for their contribution to the peer review of this work.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fundamental skills required to independently care for oneself such as eating, bathing and mobility.
Activities that allow an individual to live independently in a community.
The error in estimating the association between an exposure and an outcome that results from misclassification or exclusion of time intervals.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Tomic, D., Shaw, J.E. & Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol 18 , 525–539 (2022). https://doi.org/10.1038/s41574-022-00690-7
Download citation
Accepted : 06 May 2022
Published : 06 June 2022
Issue Date : September 2022
DOI : https://doi.org/10.1038/s41574-022-00690-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Spontaneous akt2 deficiency in a colony of nod mice exhibiting early diabetes.
- Julie Hervé
- Karine Haurogné
- Blandine Lieubeau
Scientific Reports (2024)
Insights and implications of sexual dimorphism in osteoporosis
- Yuan-Yuan Zhang
- Zhisen Shen
Bone Research (2024)
Fingerprinting hyperglycemia using predictive modelling approach based on low-cost routine CBC and CRP diagnostics
- Kashif Asghar
- Safee Ullah Chaudhary
Exploring the mechanism of Jinlida granules against type 2 diabetes mellitus by an integrative pharmacology strategy
- Liang Zhong
- Zanchao Liu
Exosomes derived from mesenchymal stem cells in diabetes and diabetic complications
- Yu-Rui Jiao
- Kai-Xuan Chen
- Chang-Jun Li
Cell Death & Disease (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Reference Manager
- Simple TEXT file
People also looked at
Hypothesis and theory article, type 2 diabetes mellitus: a pathophysiologic perspective.

- Department of Medicine, Duke University, Durham, NC, United States
Type 2 Diabetes Mellitus (T2DM) is characterized by chronically elevated blood glucose (hyperglycemia) and elevated blood insulin (hyperinsulinemia). When the blood glucose concentration is 100 milligrams/deciliter the bloodstream of an average adult contains about 5–10 grams of glucose. Carbohydrate-restricted diets have been used effectively to treat obesity and T2DM for over 100 years, and their effectiveness may simply be due to lowering the dietary contribution to glucose and insulin levels, which then leads to improvements in hyperglycemia and hyperinsulinemia. Treatments for T2DM that lead to improvements in glycemic control and reductions in blood insulin levels are sensible based on this pathophysiologic perspective. In this article, a pathophysiological argument for using carbohydrate restriction to treat T2DM will be made.
Introduction
Type 2 Diabetes Mellitus (T2DM) is characterized by a persistently elevated blood glucose, or an elevation of blood glucose after a meal containing carbohydrate ( 1 ) ( Table 1 ). Unlike Type 1 Diabetes which is characterized by a deficiency of insulin, most individuals affected by T2DM have elevated insulin levels (fasting and/or post glucose ingestion), unless there has been beta cell failure ( 2 , 3 ). The term “insulin resistance” (IR) has been used to explain why the glucose levels remain elevated even though there is no deficiency of insulin ( 3 , 4 ). Attempts to determine the etiology of IR have involved detailed examinations of molecular and intracellular pathways, with attribution of cause to fatty acid flux, but the root cause has been elusive to experts ( 5 – 7 ).
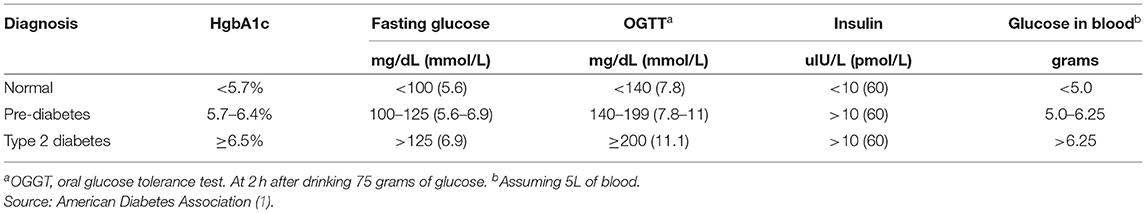
Table 1 . Definition of type 2 diabetes mellitus.
How Much Glucose Is in the Blood?
Keeping in mind that T2DM involves an elevation of blood glucose, it is important to understand how much glucose is in the blood stream to begin with, and then the factors that influence the blood glucose—both exogenous and endogenous factors. The amount of glucose in the bloodstream is carefully controlled—approximately 5–10 grams in the bloodstream at any given moment, depending upon the size of the person. To calculate this, multiply 100 milligrams/deciliter × 1 gram/1,000 milligrams × 10 deciliters/1 liter × 5 liters of blood. The “zeros cancel” and you are left with 5 grams of glucose if the individual has 5 liters of blood. Since red blood cells represent about 40% of the blood volume, and the glucose is in equilibrium, there may be an extra 40% glucose because of the red blood cell reserve ( 8 ). Adding the glucose from the serum and red blood cells totals about 5–10 grams of glucose in the entire bloodstream.
Major Exogenous Factors That Raise the Blood Glucose
Dietary carbohydrate is the major exogenous factor that raises the blood glucose. When one considers that it is common for an American in 2021 to consume 200–300 grams of carbohydrate daily, and most of this carbohydrate is digested and absorbed as glucose, the body absorbs and delivers this glucose via the bloodstream to the cells while attempting to maintain a normal blood glucose level. Thinking of it in this way, if 200–300 grams of carbohydrates is consumed in a day, the bloodstream that holds 5–10 grams of glucose and has a concentration of 100 milligrams/deciliter, is the conduit through which 200,000–300,000 milligrams (200 grams = 200,000 milligrams) passes over the course of a day.
Major Endogenous Factors That Raise the Blood Glucose
There are many endogenous contributors that raise the blood glucose. There are at least 3 different hormones that increase glucose levels: glucagon, epinephrine, and cortisol. These hormones increase glucose levels by increasing glycogenolysis and gluconeogenesis ( 9 ). Without any dietary carbohydrate, the normal human body can generate sufficient glucose though the mechanism of glucagon secretion, gluconeogenesis, glycogen storage and glycogenolysis ( 10 ).
Major Exogenous Factors That Lower the Blood Glucose
A reduction in dietary carbohydrate intake can lower the blood glucose. An increase in activity or exercise usually lowers the blood glucose ( 11 ). There are many different medications, employing many mechanisms to lower the blood glucose. Medications can delay sucrose and starch absorption (alpha-glucosidase inhibitors), slow gastric emptying (GLP-1 agonists, DPP-4 inhibitors) enhance insulin secretion (sulfonylureas, meglitinides, GLP-1 agonists, DPP-4 inhibitors), reduce gluconeogenesis (biguanides), reduce insulin resistance (biguanides, thiazolidinediones), and increase urinary glucose excretion (SGLT-2 inhibitors). The use of medications will also have possible side effects.
Major Endogenous Factors That Lower the Blood Glucose
The major endogenous mechanism to lower the blood glucose is to deliver glucose into the cells (all cells can use glucose). If the blood glucose exceeds about 180 milligrams/deciliter, then loss of glucose into the urine can occur. The blood glucose is reduced by cellular uptake using glut transporters ( 12 ). Some cells have transporters that are responsive to the presence of insulin to activate (glut4), others have transporters that do not require insulin for activation. Insulin-responsive glucose transporters in muscle cells and adipose cells lead to a reduction in glucose levels—especially after carbohydrate-containing meals ( 13 ). Exercise can increase the glucose utilization in muscle, which then increases glucose cellular uptake and reduce the blood glucose levels. During exercise, when the metabolic demands of skeletal muscle can increase more than 100-fold, and during the absorptive period (after a meal), the insulin-responsive glut4 transporters facilitate the rapid entry of glucose into muscle and adipose tissue, thereby preventing large fluctuations in blood glucose levels ( 13 ).
Which Cells Use Glucose?
Glucose can used by all cells. A limited number of cells can only use glucose, and are “glucose-dependent.” It is generally accepted that the glucose-dependent cells include red blood cells, white blood cells, and cells of the renal papilla. Red blood cells have no mitochondria for beta-oxidation, so they are dependent upon glucose and glycolysis. White blood cells require glucose for the respiratory burst when fighting infections. The cells of the inner renal medulla (papilla) are under very low oxygen tension, so therefore must predominantly use glucose and glycolysis. The low oxygen tension is a result of the countercurrent mechanism of urinary concentration ( 14 ). These glucose-dependent cells have glut transporters that do not require insulin for activation—i.e., they do not need insulin to get glucose into the cells. Some cells can use glucose and ketones, but not fatty acids. The central nervous system is believed to be able to use glucose and ketones for fuel ( 15 ). Other cells can use glucose, ketones, and fatty acids for fuel. Muscle, even cardiac muscle, functions well on fatty acids and ketones ( 16 ). Muscle cells have both non-insulin-responsive and insulin-responsive (glut4) transporters ( 12 ).
Possible Dual Role of an Insulin-Dependent Glucose-Transporter (glut4)
A common metaphor is to think of the insulin/glut transporter system as a key/lock mechanism. Common wisdom states that the purpose of insulin-responsive glut4 transporters is to facilitate glucose uptake when blood insulin levels are elevated. But, a lock serves two purposes: to let someone in and/or to keep someone out . So, one of the consequences of the insulin-responsive glut4 transporter is to keep glucose out of the muscle and adipose cells, too, when insulin levels are low. The cells that require glucose (“glucose-dependent”) do not need insulin to facilitate glucose entry into the cell (non-insulin-responsive transporters). In a teleological way, it would “make no sense” for cells that require glucose to have insulin-responsive glut4 transporters. Cells that require glucose have glut1, glut2, glut3, glut5 transporters—none of which are insulin-responsive (Back to the key/lock metaphor, it makes no sense to have a lock on a door that you want people to go through). At basal (low insulin) conditions, most glucose is used by the brain and transported by non-insulin-responsive glut1 and glut3. So, perhaps one of the functions of the insulin-responsive glucose uptake in muscle and adipose to keep glucose OUT of the these cells at basal (low insulin) conditions, so that the glucose supply can be reserved for the tissue that is glucose-dependent (blood cells, renal medulla).
What Causes IR and T2DM?
The current commonly espoused view is that “Type 2 diabetes develops when beta-cells fail to secrete sufficient insulin to keep up with demand, usually in the context of increased insulin resistance.” ( 17 ). Somehow, the beta cells have failed in the face of insulin resistance. But what causes insulin resistance? When including the possibility that the environment may be part of the problem, is it possible that IR is an adaptive (protective) response to excess glucose availability? From the perspective that carbohydrate is not an essential nutrient and the change in foods in recent years has increased the consumption of refined sugar and flour, maybe hyperinsulinemia is the cause of IR and T2DM, as cells protect themselves from excessive glucose and insulin levels.
Insulin Is Already Elevated in IR and T2DM
Clinical experience of most physicians using insulin to treat T2DM over time informs us that an escalation of insulin dose is commonly needed to achieve glycemic control (when carbohydrate is consumed). When more insulin is given to someone with IR, the IR seems to get worse and higher levels of insulin are needed. I have the clinical experience of treating many individuals affected by T2DM and de-prescribing insulin as it is no longer needed after consuming a diet without carbohydrate ( 18 ).
Diets Without Carbohydrate Reverse IR and T2DM
When dietary manipulation was the only therapy for T2DM, before medications were available, a carbohydrate-restricted diet was used to treat T2DM ( 19 – 21 ). Clinical experience of obesity medicine physicians and a growing number of recent studies have demonstrated that carbohydrate-restricted diets reverse IR and T2DM ( 18 , 22 , 23 ). Other methods to achieve caloric restriction also have these effects, like calorie-restricted diets and bariatric surgery ( 24 , 25 ). There may be many mechanisms by which these approaches may work: a reduction in glucose, a reduction in insulin, nutritional ketosis, a reduction in metabolic syndrome, or a reduction in inflammation ( 26 ). Though there may be many possible mechanisms, let's focus on an obvious one: a reduction in blood glucose. Let's assume for a moment that the excessive glucose and insulin leads to hyperinsulinemia and this is the cause of IR. On a carbohydrate-restricted diet, the reduction in blood glucose leads to a reduction in insulin. The reduction in insulin leads to a reduction in insulin resistance. The reduction in insulin leads to lipolysis. The resulting lowering of blood glucose, insulin and body weight reverses IR, T2DM, AND obesity. These clinical observations strongly suggest that hyperinsulinemia is a cause of IR and T2DM—not the other way around.
What Causes Atherosclerosis?
For many years, the metabolic syndrome has been described as a possible cause of atherosclerosis, but there are no RCTs directly targeting metabolic syndrome, and the current drug treatment focuses on LDL reduction, so its importance remains controversial. A recent paper compared the relative importance of many risk factors in the prediction of the first cardiac event in women, and the most powerful predictors were diabetes, metabolic syndrome, smoking, hypertension and BMI ( 27 ). The connection between dietary carbohydrate and fatty liver is well-described ( 28 ). The connection between fatty liver and atherosclerosis is well-described ( 29 ). It is very possible that the transport of excess glucose to the adipose tissue via lipoproteins creates the particles that cause the atherosclerotic damage (small LDL) ( Figure 1 ) ( 30 – 32 ). This entire process of dietary carbohydrate leading to fatty liver, leading to small LDL, is reversed by a diet without carbohydrate ( 26 , 33 , 34 ).
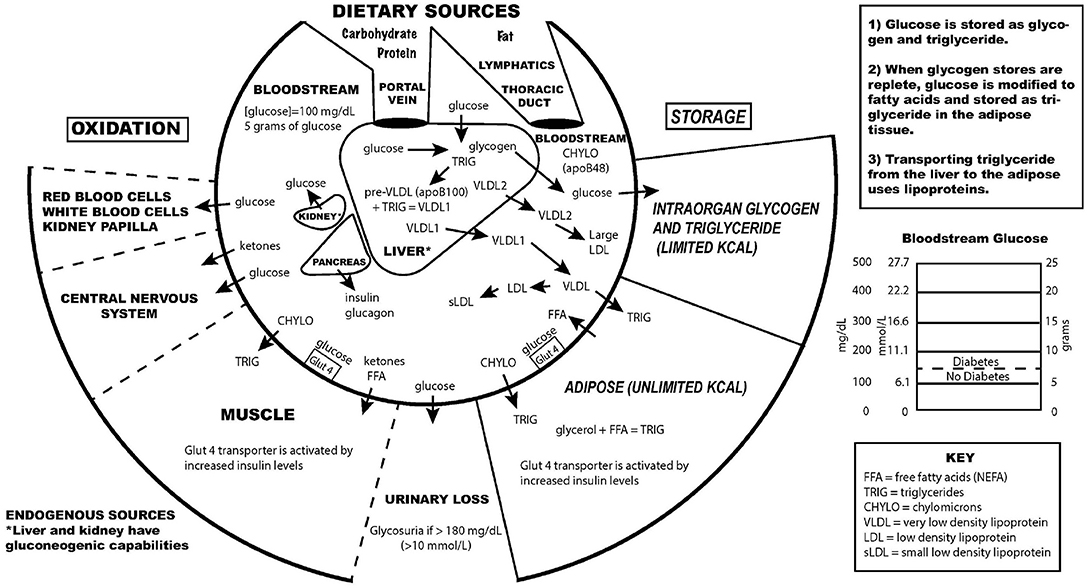
Figure 1 . Key aspects of the interconnection between glucose and lipoprotein metabolism.
Reducing dietary carbohydrate in the context of a low carbohydrate, ketogenic diet reduces hyperglycemia and hyperinsulinemia, IR and T2DM. In the evaluation of an individual for a glucose abnormality, measure the blood glucose and insulin levels. If the insulin level (fasting or after a glucose-containing meal) is high, do not give MORE insulin—instead, use an intervention to lower the insulin levels. Effective ways to reduce insulin resistance include lifestyle, medication, and surgical therapies ( 23 , 35 ).
The search for a single cause of a complex problem is fraught with difficulty and controversy. I am not hypothesizing that excessive dietary carbohydrate is the only cause of IR and T2DM, but that it is a cause, and quite possibly the major cause. How did such a simple explanation get overlooked? I believe it is very possible that the reductionistic search for intracellular molecular mechanisms of IR and T2DM, the emphasis on finding pharmaceutical (rather than lifestyle) treatments, the emphasis on the treatment of high total and LDL cholesterol, and the fear of eating saturated fat may have misguided a generation of researchers and clinicians from the simple answer that dietary carbohydrate, when consumed chronically in amounts that exceeds an individual's ability to metabolize them, is the most common cause of IR, T2DM and perhaps even atherosclerosis.
While there has historically been a concern about the role of saturated fat in the diet as a cause of heart disease, most nutritional experts now cite the lack of evidence implicating dietary saturated fat as the reason for lack of concern of it in the diet ( 36 ).
The concept of comparing medications that treat IR by insulin-sensitizers or by providing insulin itself was tested in the Bari-2D study ( 37 ). Presumably in the context of consuming a standard American diet, this study found no significant difference in death rates or major cardiovascular events between strategies of insulin sensitization or insulin provision.
While lifestyle modification may be ideal to prevent or cure IR and T2DM, for many people these changes are difficult to learn and/or maintain. Future research should be directed toward improving adherence to all effective lifestyle or medication treatments. Future research is also needed to assess the effect of carbohydrate restriction on primary or secondary prevention of outcomes of cardiovascular disease.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
EW receives royalties from popular diet books and is founder of a company based on low-carbohydrate diet principles (Adapt Your Life, Inc.).
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care . (2016) 39 (Suppl. 1):S13–22. doi: 10.2337/dc16-S005
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Bogardus C, Lillioja S, Howard BV, Reaven G, Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. (1984) 74:1238–46. doi: 10.1172/JCI111533
3. Reaven GM. Compensatory hyperinsulinemia and the development of an atherogenic lipoprotein profile: the price paid to maintain glucose homeostasis in insulin-resistant individuals. Endocrinol Metab Clin North Am. (2005) 34:49–62. doi: 10.1016/j.ecl.2004.12.001
4. DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. (1991) 14:173–94. doi: 10.2337/diacare.14.3.173
5. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. (2005) 365:1415–28. doi: 10.1016/S0140-6736(05)66378-7
6. Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A. Insulin resistance: review of the underlying molecular mechanisms. J Cell Physiol. (2019) 234:8152–61. doi: 10.1002/jcp.27603
7. Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. (2000) 106:171–6. doi: 10.1172/JCI10583
8. Guizouarn H, Allegrini B. Erythroid glucose transport in health and disease. Pflugers Arch. (2020) 472:1371–83. doi: 10.1007/s00424-020-02406-0
9. Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. (2017) 13:572–87. doi: 10.1038/nrendo.2017.80
10. Tondt J, Yancy WS, Westman EC. Application of nutrient essentiality criteria to dietary carbohydrates. Nutr Res Rev. (2020) 33:260–70. doi: 10.1017/S0954422420000050
11. Colberg SR, Hernandez MJ, Shahzad F. Blood glucose responses to type, intensity, duration, and timing of exercise. Diabetes Care. (2013) 36:e177. doi: 10.2337/dc13-0965
12. Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. (2013) 34:121–38. doi: 10.1016/j.mam.2012.07.001
13. Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. (2002) 3:267–77. doi: 10.1038/nrm782
14. Epstein FH. Oxygen and renal metabolism. Kidney Int. (1997) 51:381–5. doi: 10.1038/ki.1997.50
15. Cahill GF. Fuel metabolism in starvation. Annu Rev Nutr. (2006) 26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258
16. Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. (2020) 370:364–8. doi: 10.1126/science.abc8861
17. Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. (2017) 66:241–55. doi: 10.2337/db16-0806
18. Westman EC, Yancy WS, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab. (2008) 5:36. doi: 10.1186/1743-7075-5-36
CrossRef Full Text | Google Scholar
19. Allen F. The treatment of diabetes. Boston Med Surg J. (1915) 172:241–7. doi: 10.1056/NEJM191502181720702
20. Osler W, McCrae T. The Principles and Practice of Medicine . 9th ed. New York and London: Appleton & Company (1923).
21. Lennerz BS, Koutnik AP, Azova S, Wolfsdorf JI, Ludwig DS. Carbohydrate restriction for diabetes: rediscovering centuries-old wisdom. J Clin Invest. (2021) 131:e142246. doi: 10.1172/JCI142246
22. Steelman GM, Westman EC. Obesity: Evaluation and Treatment Essentials . 2nd ed. Boca Raton: CRC Press, Taylor & Francis Group (2016). 340 p.
23. Athinarayanan SJ, Adams RN, Hallberg SJ, McKenzie AL, Bhanpuri NH, Campbell WW, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol. (2019) 10:348. doi: 10.3389/fendo.2019.00348
24. Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. (2011) 54:2506–14. doi: 10.1007/s00125-011-2204-7
25. Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. (2010) 33:1438–42. doi: 10.2337/dc09-2107
26. Bhanpuri NH, Hallberg SJ, Williams PT, McKenzie AL, Ballard KD, Campbell WW, et al. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: an open label, non-randomized, controlled study. Cardiovasc Diabetol. (2018) 17:56. doi: 10.1186/s12933-018-0698-8
27. Dugani SB, Moorthy MV, Li C, Demler OV, Alsheikh-Ali AA, Ridker PM, et al. Association of lipid, inflammatory, and metabolic biomarkers with age at onset for incident coronary heart disease in women. JAMA Cardiol. (2021) 6:437–47. doi: 10.1001/jamacardio.2020.7073
28. Duwaerts CC, Maher JJ. Macronutrients and the adipose-liver axis in obesity and fatty liver. Cell Mol Gastroenterol Hepatol. (2019) 7:749–61. doi: 10.1016/j.jcmgh.2019.02.001
29. Zhang L, She Z-G, Li H, Zhang X-J. Non-alcoholic fatty liver disease: a metabolic burden promoting atherosclerosis. Clin Sci Lond Engl. (1979) 134:1775–99. doi: 10.1042/CS20200446
30. Horton TJ, Drougas H, Brachey A, Reed GW, Peters JC, Hill JO. Fat and carbohydrate overfeeding in humans: different effects on energy storage. Am J Clin Nutr. (1995) 62:19–29. doi: 10.1093/ajcn/62.1.19
31. Packard C, Caslake M, Shepherd J. The role of small, dense low density lipoprotein (LDL): a new look. Int J Cardiol. (2000) 74 (Suppl. 1):S17–22. doi: 10.1016/S0167-5273(99)00107-2
32. Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. (2020) 41:2313–30. doi: 10.1093/eurheartj/ehz962
33. Yancy WS, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. (2004) 140:769. doi: 10.7326/0003-4819-140-10-200405180-00006
34. Tendler D, Lin S, Yancy WS, Mavropoulos J, Sylvestre P, Rockey DC, et al. The effect of a low-carbohydrate, ketogenic diet on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. (2007) 52:589–93. doi: 10.1007/s10620-006-9433-5
35. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. (1995) 222:339–50. doi: 10.1097/00000658-199509000-00011
36. Astrup A, Magkos F, Bier DM, Brenna JT, de Oliveira Otto MC, Hill JO, et al. Saturated fats and health: a reassessment and proposal for food-based recommendations: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 76:844–57. doi: 10.1016/j.jacc.2020.05.077
37. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med . (2009) 360:2503–15. doi: 10.1056/NEJMoa0805796
Keywords: type 2 diabetes, insulin resistance, pre-diabetes, carbohydrate-restricted diets, hyperinsulinemia, hyperglycemia
Citation: Westman EC (2021) Type 2 Diabetes Mellitus: A Pathophysiologic Perspective. Front. Nutr. 8:707371. doi: 10.3389/fnut.2021.707371
Received: 09 May 2021; Accepted: 20 July 2021; Published: 10 August 2021.
Reviewed by:
Copyright © 2021 Westman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eric C. Westman, ewestman@duke.edu
This article is part of the Research Topic
Carbohydrate-restricted Nutrition and Diabetes Mellitus
- Cancer Nursing Practice
- Emergency Nurse
- Evidence-Based Nursing
- Learning Disability Practice
- Mental Health Practice
- Nurse Researcher
- Nursing Children and Young People
- Nursing Management
- Nursing Older People
- Nursing Standard
- Primary Health Care
- RCN Nursing Awards
- Nursing Live
- Nursing Careers and Job Fairs
- CPD webinars on-demand
- --> Advanced -->

- Clinical articles
- CPD articles
- CPD Quizzes
- Expert advice
- Clinical placements
- Study skills
- Clinical skills
- University life
- Person-centred care
- Career advice
- Revalidation
Evidence and practice
Diabetes mellitus: an overview of the types, symptoms, complications and management, linda cloete lecturer, school of nursing, avondale university, wahroonga, sydney nsw, australia.
• To learn about the types of diabetes mellitus and associated presentation
• To refresh your knowledge of the complications that are associated with diabetes
• To understand the nurse’s role in managing patients with diabetes
The incidence of diabetes mellitus is rapidly increasing, and this condition often results in significant metabolic disease and severe complications. Nurses have a crucial role in monitoring, educating and supporting people with diabetes, as well as their families and significant others. This article provides an overview of the main types and common symptoms of diabetes, its acute and long-term complications and its management. It also outlines the nurse’s role in diabetes care, which frequently includes assessing and empowering patients.
Nursing Standard . 37, 1, 61-66. doi: 10.7748/ns.2021.e11709
This article has been subject to external double-blind peer review and checked for plagiarism using automated software
@Cloetelinda
None declared
Cloete L (2021) Diabetes mellitus: an overview of the types, symptoms, complications and management. Nursing Standard. doi: 10.7748/ns.2021.e11709
Published online: 28 October 2021
blood glucose - clinical - diabetes - diabetic foot ulcers - diabetic ketoacidosis - glycaemic control - hyperglycaemia - hypoglycaemia - insulin - type 1 diabetes - type 2 diabetes
User not found
Want to read more?
Already have access log in, 3-month trial offer for £5.25/month.
- Unlimited access to all 10 RCNi Journals
- RCNi Learning featuring over 175 modules to easily earn CPD time
- NMC-compliant RCNi Revalidation Portfolio to stay on track with your progress
- Personalised newsletters tailored to your interests
- A customisable dashboard with over 200 topics
Alternatively, you can purchase access to this article for the next seven days. Buy now
Are you a student? Our student subscription has content especially for you. Find out more

05 January 2022 / Vol 37 issue 1
TABLE OF CONTENTS
DIGITAL EDITION
- LATEST ISSUE
- SIGN UP FOR E-ALERT
- WRITE FOR US
- PERMISSIONS
Share article: Diabetes mellitus: an overview of the types, symptoms, complications and management
We use cookies on this site to enhance your user experience.
By clicking any link on this page you are giving your consent for us to set cookies.
A distributed system of wearable analyzers for the diagnosis of peripheral blood flow disorders in type 2 diabetes mellitus
- Published: 08 May 2024
Cite this article

- E. V. Zharkikh 1 &
- A. V. Dunaev 1
This paper discusses a method for the diagnosis of peripheral blood flow disorders in type 2 diabetes mellitus (DM) using a distributed system of wearable blood microcirculation analyzers based on laser Doppler flowmetry. This method was tested in a clinical setting. The research resulted in the development of a classification model based on linear discriminant analysis and allowing the presence of peripheral blood flow disorders in patients with type 2 diabetes to be identified with sensitivity and specificity indicators of 0.88 and 0.90 respectively.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Dunaev AV (2022) Multimodal optical diagnostics of the microcirculatory tissue systems of the human body. TNT, Staryi Oskol
Google Scholar
Krupatkin AI, Sidorov VV (2013) Functional diagnostics of the state of microcirculatory tissue systems: fluctuations, information, nonlinearity. Guidelines for doctors. Librokom, Moscow
Caballero AE, Arora S, Saouaf R, Lim SC, Smakowski P, Park JY, King GL, LoGerfo FW, Horton ES, Veves A (1999) Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes 48(9):1856–1862
Article CAS PubMed Google Scholar
Martini R, Bagno A (2018) The wavelet analysis for the assessment of microvascular function with the laser doppler fluxmetry over the last 20 years. Looking for hidden informations. Clin Hemorheol Microcirc 70(2):213–229
Article PubMed Google Scholar
Kralj L, Lenasi H (2023) Wavelet analysis of laser doppler microcirculatory signals: current applications and limitations. Front Physiol 13(1):1076445
Article PubMed PubMed Central Google Scholar
Kulikov DA, Glazkov AA, Kovaleva YA, Balashova NV, Kulikov AV (2017) Prospects of Laser Doppler flowmetry application in assessment of skin microcirculation in diabetes. Diabetes mellitus 20(4):279–285
Article Google Scholar
Fuchs D, Dupon PP, Schaap LA, Draijer R (2017) The association between diabetes and dermal microvascular dysfunction non-invasively assessed by laser doppler with local thermal hyperemia: a systematic review with meta-analysis. Cardiovasc Diabetol 16(1):11
Sidorov VV, Rybakov YL, Gusakov VM, Evtushenko GS (2021) A system of local analyzers for non-invasive diagnostics of the general state of the compartments of the microcirculatory tissue system of human skin. Meditsinsk Tekhn 330(6):4–6
Zharkikh EV (2023) Modeling of the diagnostic volume for a portable laser Doppler flowmetry device. Fundam Priklad Prob Tekhn Tekhnol 357(1):140–147
Jörneskog G, Brismar K, Fagrell B (1995) Skin capillary circulation severely impaired in toes of patients with IDDM, with and without late diabetic complications. Diabetologia 38(4):474–480
Urbancic-Rovan V, Stefanovska A, Bernjak A, Ažman-Juvan K, Kocijančič A (2004) Skin blood flow in the upper and lower extremities of diabetic patients with and without autonomic neuropathy. J Vasc Res 41(6):535–545
Jonasson H, Bergstrand S, Nystrom FH, Länne T, Östgren CJ, Bjarnegård N, Fredriksson I, Larsson M, Strömberg T (2017) Skin microvascular endothelial dysfunction is associated with type 2 diabetes independently of microalbuminuria and arterial stiffness. Diab Vasc Dis Res 14(4):363–371
Download references
Acknowledgements
The authors would like to thank the CEO of the Scientific and Production Enterprise “LAZMA”, V.V. Sidorov Ph.D., for comprehensive assistance at all stages of this research.
This study was carried out with financial support from the Russian Science Foundation within the framework of project No. 23-25-00522.
Author information
Authors and affiliations.
Research and Development Center of Biomedical Photonics, Orel State University named after I. S. Turgenev, Orel, Russian Federation
E. V. Zharkikh & A. V. Dunaev
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to E. V. Zharkikh .
Additional information
Translated from Meditsinskaya Tekhnika , Vol. 58, No. 1, pp. 1–4, January-February, 2024.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Original article submitted July 20, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Zharkikh, E., Dunaev, A.V. A distributed system of wearable analyzers for the diagnosis of peripheral blood flow disorders in type 2 diabetes mellitus. Biomed Eng (2024). https://doi.org/10.1007/s10527-024-10354-7
Download citation
Published : 08 May 2024
DOI : https://doi.org/10.1007/s10527-024-10354-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
This high blood sugar often causes nerve damage, kidney damage, eye damage, heart disease, skin hearing impairment, etc. Loss of limb, blindness is also some of the effects [3]. ... An Approach ...
Type 1 diabetes cannot be prevented with current knowledge. Effective approaches are available to prevent type2 diabetes and to prevent the complications and premature death that can result from all types of diabetes. These include policies and practices across whole populations and within specific settings (school,
I. Introduction. Diabetes mellitus, a metabolic disease defined by elevated fasting blood glucose levels due to insufficient insulin production, has reached epidemic proportions worldwide (World Health Organization, 2020).Type 1 and type 2 diabetes (T1D and T2D, respectively) make up the majority of diabetes cases with T1D characterized by autoimmune destruction of the insulin-producing ...
Table 7 Assessment of effectiveness of diabetes services 27 Table 8 Stratification of level of risk of developing diabetic foot problems or the need for an amputation 33 Figures Fig. 1 Protocol for control of blood glucose in type 2 diabetes* 16 Fig. 2 Type 2 diabetes management protocol derived from WHO-PEN 25 Fig. 3 Examples of foot ulcers 30
CLASSIFICATION OF DIABETES MELLITUS. Although classification of diabetes is important and has implications for the treatment strategies, this is not an easy task and many patients do not easily fit into a single class especially younger adults[1,4-6] and 10% of those initially classified may require revision[].The classical classification of diabetes as proposed by the American Diabetes ...
The best evidence for a link between diabetes mellitus and breast cancer comes from a systematic review of six prospective cohort studies and more than 150,000 women, in which the hazard ratio (HR ...
Type 2 diabetes accounts for nearly 90% of the approximately 537 million cases of diabetes worldwide. The number afected is increasing rapidly with alarming trends in children and young adults (up to age 40 years). Early detection and proactive management are crucial for prevention and mitigation of microvascular and macrovascular complications ...
Diabetes mellitus (DM) underscores a rising epidemic orchestrating critical socio-economic burden on countries globally. Different treatment options for the management of DM are evolving rapidly because the usual methods of treatment have not completely tackled the primary causes of the disease and are laden with critical adverse effects. Thus, this narrative review explores different ...
Type 2 Diabetes Mellitus (T2DM) is characterized by chronically elevated blood glucose (hyperglycemia) and elevated blood insulin (hyperinsulinemia). When the blood glucose concentration is 100 milligrams/deciliter the bloodstream of an average adult contains about 5-10 grams of glucose. Carbohydrate-restricted diets have been used effectively to treat obesity and T2DM for over 100 years ...
Diabetes remains a substantial public health issue. Type 2 diabetes, which makes up the bulk of diabetes cases, is largely preventable and, in some cases, potentially reversible if identified and managed early in the disease course. However, all evidence indicates that diabetes prevalence is increasing worldwide, primarily due to a rise in obesity caused by multiple factors. Preventing and ...
This article provides an overview of the main types and common symptoms of diabetes, its acute and long-term complications and its management. It also outlines the nurse's role in diabetes care, which frequently includes assessing and empowering patients. Nursing Standard . 37, 1, 61-66. doi: 10.7748/ns.2021.e11709. Peer review.
within the paper and its Supporting Information files. Funding: This study was supported by National Natural Science Foundation of China (81670741). The funder had no role in study design, data ... Publication trends of research on diabetes mellitus and T cells (1997-2016): A 20-year bibliometric study ...
The chronic complications of type 2 diabetes are a major cause of mortality and disability worldwide [ 1, 2 ]. Clinical research is the main way to gain knowledge about long-term diabetic complications and reduce the burden of diabetes. This allows for designing effective programs for screening and follow-up and fine-targeted therapeutic ...
2 National Institutes of Health (NIH) urging patients to "Take Charge of Your Diabetes"5 and "Conquer Diabetes".6 One of the main goals of USDHHS's report, Healthy People 2010, is to improve the quality of life for persons with diabetes.7 Taking control of diabetes to improve quality of life has put the spotlight on
Diabetes mellitus is a common endocrine disorder, and affects more than 100 million people worldwide (World Health Organization, 1994). It is recognized as being a syndrome, a collection of disorders that have hyperglycaemia and glucose intolerance as a hallmark, due either to insulin deficiency or to impaired effectiveness of insulin's ...
Introduction. Diabetes mellitus (DM) is probably one of the oldest diseases known to man. It was first reported in Egyptian manuscript about 3000 years ago. 1 In 1936, the distinction between type 1 and type 2 DM was clearly made. 2 Type 2 DM was first described as a component of metabolic syndrome in 1988. 3 Type 2 DM (formerly known as non-insulin dependent DM) is the most common form of DM ...
The present study utilized a correlational. design to examine the relationships among diabetes distress, social support, self-efficacy, and. performance of diabetes self-care activities. A total of 33 adults with T2DM participated in the. study by completing a battery of surveys regarding performance of diabetes self-care activities.
This paper discusses a method for the diagnosis of peripheral blood flow disorders in type 2 diabetes mellitus (DM) using a distributed system of wearable blood microcirculation analyzers based on laser Doppler flowmetry. This method was tested in a clinical setting. The research resulted in the development of a classification model based on linear discriminant analysis and allowing the ...
Abstract. In recent years, numerous studies have explored the quality of life (QoL) in those with diabetes mellitus. The aim of this scoping review was to explore the current state of knowledge on QoL and its various associated factors among people with diabetes in India. Three databases were searched (PubMed, Scopus, and Medline) and the ...