July 26, 2011

The Science Behind Dreaming
New research sheds light on how and why we remember dreams--and what purpose they are likely to serve
By Sander van der Linden

Getty Images
For centuries people have pondered the meaning of dreams. Early civilizations thought of dreams as a medium between our earthly world and that of the gods. In fact, the Greeks and Romans were convinced that dreams had certain prophetic powers. While there has always been a great interest in the interpretation of human dreams, it wasn’t until the end of the nineteenth century that Sigmund Freud and Carl Jung put forth some of the most widely-known modern theories of dreaming. Freud’s theory centred around the notion of repressed longing -- the idea that dreaming allows us to sort through unresolved, repressed wishes. Carl Jung (who studied under Freud) also believed that dreams had psychological importance, but proposed different theories about their meaning.
Since then, technological advancements have allowed for the development of other theories. One prominent neurobiological theory of dreaming is the “activation-synthesis hypothesis,” which states that dreams don’t actually mean anything: they are merely electrical brain impulses that pull random thoughts and imagery from our memories. Humans, the theory goes, construct dream stories after they wake up, in a natural attempt to make sense of it all. Yet, given the vast documentation of realistic aspects to human dreaming as well as indirect experimental evidence that other mammals such as cats also dream, evolutionary psychologists have theorized that dreaming really does serve a purpose. In particular, the “threat simulation theory” suggests that dreaming should be seen as an ancient biological defence mechanism that provided an evolutionary advantage because of its capacity to repeatedly simulate potential threatening events – enhancing the neuro-cognitive mechanisms required for efficient threat perception and avoidance.
So, over the years, numerous theories have been put forth in an attempt to illuminate the mystery behind human dreams, but, until recently, strong tangible evidence has remained largely elusive.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Yet, new research published in the Journal of Neuroscience provides compelling insights into the mechanisms that underlie dreaming and the strong relationship our dreams have with our memories. Cristina Marzano and her colleagues at the University of Rome have succeeded, for the first time, in explaining how humans remember their dreams. The scientists predicted the likelihood of successful dream recall based on a signature pattern of brain waves. In order to do this, the Italian research team invited 65 students to spend two consecutive nights in their research laboratory.
During the first night, the students were left to sleep, allowing them to get used to the sound-proofed and temperature-controlled rooms. During the second night the researchers measured the student’s brain waves while they slept. Our brain experiences four types of electrical brain waves: “delta,” “theta,” “alpha,” and “beta.” Each represents a different speed of oscillating electrical voltages and together they form the electroencephalography (EEG). The Italian research team used this technology to measure the participant’s brain waves during various sleep-stages. (There are five stages of sleep; most dreaming and our most intense dreams occur during the REM stage.) The students were woken at various times and asked to fill out a diary detailing whether or not they dreamt, how often they dreamt and whether they could remember the content of their dreams.
While previous studies have already indicated that people are more likely to remember their dreams when woken directly after REM sleep, the current study explains why. Those participants who exhibited more low frequency theta waves in the frontal lobes were also more likely to remember their dreams.
This finding is interesting because the increased frontal theta activity the researchers observed looks just like the successful encoding and retrieval of autobiographical memories seen while we are awake. That is, it is the same electrical oscillations in the frontal cortex that make the recollection of episodic memories (e.g., things that happened to you) possible. Thus, these findings suggest that the neurophysiological mechanisms that we employ while dreaming (and recalling dreams) are the same as when we construct and retrieve memories while we are awake.
In another recent study conducted by the same research team, the authors used the latest MRI techniques to investigate the relation between dreaming and the role of deep-brain structures. In their study, the researchers found that vivid, bizarre and emotionally intense dreams (the dreams that people usually remember) are linked to parts of the amygdala and hippocampus. While the amygdala plays a primary role in the processing and memory of emotional reactions, the hippocampus has been implicated in important memory functions, such as the consolidation of information from short-term to long-term memory.
The proposed link between our dreams and emotions is also highlighted in another recent study published by Matthew Walker and colleagues at the Sleep and Neuroimaging Lab at UC Berkeley, who found that a reduction in REM sleep (or less “dreaming”) influences our ability to understand complex emotions in daily life – an essential feature of human social functioning. Scientists have also recently identified where dreaming is likely to occur in the brain. A very rare clinical condition known as “Charcot-Wilbrand Syndrome” has been known to cause (among other neurological symptoms) loss of the ability to dream. However, it was not until a few years ago that a patient reported to have lost her ability to dream while having virtually no other permanent neurological symptoms. The patient suffered a lesion in a part of the brain known as the right inferior lingual gyrus (located in the visual cortex). Thus, we know that dreams are generated in, or transmitted through this particular area of the brain, which is associated with visual processing, emotion and visual memories.
Taken together, these recent findings tell an important story about the underlying mechanism and possible purpose of dreaming.
Dreams seem to help us process emotions by encoding and constructing memories of them. What we see and experience in our dreams might not necessarily be real, but the emotions attached to these experiences certainly are. Our dream stories essentially try to strip the emotion out of a certain experience by creating a memory of it. This way, the emotion itself is no longer active. This mechanism fulfils an important role because when we don’t process our emotions, especially negative ones, this increases personal worry and anxiety. In fact, severe REM sleep-deprivation is increasingly correlated to the development of mental disorders. In short, dreams help regulate traffic on that fragile bridge which connects our experiences with our emotions and memories.
Are you a scientist who specializes in neuroscience, cognitive science, or psychology? And have you read a recent peer-reviewed paper that you would like to write about? Please send suggestions to Mind Matters editor Gareth Cook, a Pulitzer prize-winning journalist at the Boston Globe. He can be reached at garethideas AT gmail.com or Twitter @garethideas .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 14 October 2019
Predicting the affective tone of everyday dreams: A prospective study of state and trait variables
- Eugénie Samson-Daoust 1 ,
- Sarah-Hélène Julien 1 ,
- Dominic Beaulieu-Prévost ORCID: orcid.org/0000-0001-7926-5295 2 &
- Antonio Zadra ORCID: orcid.org/0000-0003-3671-7081 1 , 3
Scientific Reports volume 9 , Article number: 14780 ( 2019 ) Cite this article
3924 Accesses
10 Citations
5 Altmetric
Metrics details
- Human behaviour
Although emotions are reported in a large majority of dreams, little is known about the factors that account for night-to-night and person-to-person variations in people’s experience of dream affect. We investigated the relationship between waking trait and state variables and dream affect by testing multilevel models intended to predict the affective valence of people’s everyday dreams. Participants from the general population completed measures of personality and trauma history followed by a three-week daily journal in which they noted dream recall, valence of dreamed emotions and level of perceived stress for the day as well as prior to sleep onset. Within-subject effects accounted for most of the explained variance in the reported valence of dream affect. Trait anxiety was the only variable that significantly predicted dream emotional valence at the between-subjects level. In addition to highlighting the need for more fine-grained measures in this area of research, our results point to methodological limitations and biases associated with retrospective estimates of general dream affect and bring into focus state variables that may best explain observed within-subject variance in emotions experienced in everyday dreams.
Similar content being viewed by others
Evidence of an active role of dreaming in emotional memory processing shows that we dream to forget
Dreams share phenomenological similarities with task-unrelated thoughts and relate to variation in trait rumination and COVID-19 concern

Emotion dysregulation mediates the relationship between nightmares and psychotic experiences: results from a student population
Introduction.
Despite decades of advances in dream research, relatively little is known about how dreams are formed and what factors predict their content and emotional tone. One of the most widely studied models of dream content is the continuity hypothesis of dreaming 1 , 2 which posits that dreams are generally continuous with the dreamer’s current thoughts, concerns and salient experiences. In line with this conceptualization of dreams, a large proportion of dream research 1 , 3 , 4 , 5 , 6 , 7 has been dedicated to quantifying various dimensions of people’s dream reports and investigating their relationship to different aspects of people’s waking life. While much of this work has helped refine our understanding of which aspects of waking life (e.g., day-to-day actions, ongoing concerns, learning tasks, stressful experiences, psychological well-being) are most likely to be reflected or embodied in various facets of people’s dreams (e.g., settings, interpersonal interactions, activities, thematic contents), attempts to identify factors accounting for night-to-night or person-to-person variations in the intensity and valence of dream affect have yielded mixed results 7 , 8 , 9 , 10 , 11 , 12 , 13 .
Given that emotions are present in a vast majority of home and laboratory dream reports 7 , 14 , 15 , 16 , 17 and that some theorists 18 , 19 , 20 believe that affect plays a key role in structuring dream content, elucidating why people experience negatively toned dreams on some nights and positively toned dreams on others is of prime importance. Among the most studied factors hypothesised to influence dream valence are stress 21 , 22 , 23 , 24 , trait or personality characteristics 25 , 26 , 27 , history of traumatic experiences 28 , 29 , 30 , 31 , and psychological well-being 7 , 18 , 32 , 33 . Relatedly, one neurocognitive model 34 , 35 of dysphoric and everyday dream production suggests that variations in the frequency and intensity of negative dream emotions are partially determined by affect load , or day-to-day variations in emotional stress, and that the relation between dream content and stress varies as a function of affect distress , or the disposition to experience events with distressing, reactive emotions.
Many of the factors believed to predict the experience of negative dreams, including trauma history and psychopathology, have been associated with disturbed dreaming 28 , 36 , 37 , 38 and likely contribute to the development and heightening of affect distress 34 , 39 . Similarly, other dispositional traits related to the concept of affect distress, such as boundary thinness 40 (used to describe particularly sensitive and vulnerable individuals prone to mixing thoughts, images and feelings) and trait anxiety 41 (stable individual differences in the tendency to experience anxiety across situations) are also correlated with indices of negative dream content, including frequency of bad dreams and nightmares 27 , 33 , 42 , 43 , 44 , 45 . Thus, affect distress may be viewed as encompassing a range of factors known to impact dream affect, including trauma history, psychopathology, trait anxiety, and boundary thinness.
While several studies have investigated the differential impact of state and trait factors on dream content 7 , 11 , 12 , 32 , 42 , 46 , 47 , 48 , 49 , 50 , most have focused solely on nightmares, have been purely retrospective in nature, or did not weigh state-related findings against trait factors such as personality or psychopathology. Only two studies 42 , 48 have ever used a prospective design to assess the effect of trait and daily state measures on everyday dreams. The first one 42 assessed state anxiety and depression (what the authors termed “mood”) in relation to trait measures believed to underlie nightmare occurrence. They found statistically significant correlations between their state and trait variables and nightmare frequency, but only in individuals with thin psychological boundaries. The second study 48 obtained similar results in that daily stress was found to statistically predict general sleep-related experiences—a concept elaborated by Watson 51 to describe nocturnal phenomena such as nightmares, falling dreams, flying dreams and sleep paralysis—but only in young adults scoring high on a measure of trait dissociation (the tendency to experience psychological detachment from reality).
In sum, in addition to giving rise to inconsistent results, research on the determinants of dream affect has been limited by the often retrospective nature of the study design, single measurement points, focus on nightmare incidence or broad sleep-related experiences, and a failure to evaluate the interactive role of state and trait factors within a larger conceptual framework. We therefore used a prospective, multilevel design to investigate the interplay between daily fluctuations in perceived levels of stress and trait indices of affect distress as determinants of dream affect. Individuals from the general population first completed questionnaire measures of sleep and dream experiences, trait anxiety, boundary thinness, trauma history, and PTSD symptoms, followed by at least three consecutive weeks of daily assessments of perceived stress as well as dream recall, including the emotional valence associated with each remembered dream. Since daily measures ( N = 2538) were nested within individuals ( N = 128), multilevel hierarchical linear modelling (HLM) analyses were performed in order to examine the distinctive effect of state and trait variables.
Descriptive statistics and intercorrelations of tested variables
Table 1 presents the means, standard deviations and zero-order Pearson correlations between study variables. Daily measures were averaged per participant over the study’s duration to investigate their association to trait variables. All observed correlations were in the expected direction. The highest obtained correlation ( r = 0.752) was between the mean daily level of maximum stress and the mean level of stress prior to bedtime. The fact that daily maximum stress was more strongly correlated with daily dream valence ( r = 0.300) than was daily stress prior to bedtime ( r = 0.185) suggests that the two variables tapped into different facets of perceived stress. As can be seen in the table, trait anxiety was statistically correlated with a majority of other studied variables, while sex did not show statistically significant correlations with any of the other measures.
Multilevel models predicting dream valence as outcome
A total of 1700 nights led to a dream recall in participants over the study’s three-week duration, of which 1653 (97.2%) contained ratings on the dream’s emotional valence. Of the 1700 nights, 773 (45.5%) yielded more than one recalled dream and participants reported an average of 6.9 dreams per week. Figure 1 presents the distribution of dream valence ratings for the 1653 dream reports. The mean dream valence score was 5.08 ( SD = 2.27), or at the midpoint of the positive to negative rating scale. As can also be seen in the figure, highly positive dreams (scores of 1 or 2) were approximately twice as frequent as highly negative ones (scores of 9 or 10).
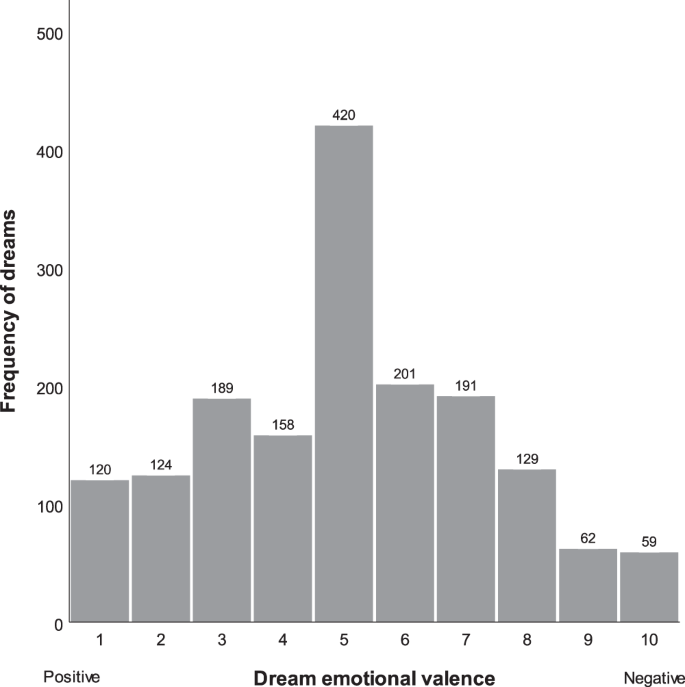
Distribution of dream emotional valence for 1653 dream reports.
Table 2 presents the intercepts-only model (i.e., unconditional model) for daily measures of dream valence. The intraclass correlation was 0.161, indicating that 16.1% of the variance in dream valence occurred between subjects, while 83.9% of the variance occurred within subjects (i.e., across days).
Table 3 presents the multilevel model predicting dream valence using trait (Level-2) and state (Level-1) predictors. At Level-2, when all predictors were entered in the model as fixed terms, trait anxiety (STAI-T) was the only variable to statistically predict dream valence. At Level-1, neither of the two daily measures of perceived stress statistically predicted the dream valence experienced on the subsequent night. Dream recall frequency per night was the only statistically significant Level-1 predictor. This measure was used as a control variable since dream valence was only provided for the best remembered dream on a given night when more than one dream was recalled (45.5% had multiple recalls) and thus the two variables were not entirely independent.
When standardized scores for trait anxiety (ZSTAI-T) were entered as a single predictor of dream valence in a separate model, it was found to be an even better predictor ( p < 0.001) than when it was considered alongside other predictor variables, with each increase in standard deviation STAI-T scores explaining a 0.33 unit increase in dream valence ratings. This model reduced the unexplained between-subject variance by 11.6%, thus explaining a total of 1.9% of the variance in dream valence ratings obtained over the study’s 3-week duration.
Post Hoc multilevel models predicting dream valence as an outcome variable
Since interactions between predictors could potentially explain why neither of our perceived stress variables predicted dream valence 42 , 48 , we tested for possible interactions, particularly between trait variables (Level-2) and daily perceived stress (Level-1), but did not find a statistically significant interaction that could predict dream valence. The only statistically significant interaction predicting dream valence was between trait anxiety (STAI-T) scores and dream recall frequency ( p = 0.007), which was positive and expected since the dream valence rating of the most vivid or best-remembered dream on a given night can increase when a greater number of dreams is recalled on that night.
Since daily perceived stress did not predict the dream valence experienced on the subsequent night, models testing for potential a dream-lag effect (i.e., increased incorporation in dreams of events having occurred 5–7 days prior to the dream) 52 , 53 were also computed post hoc. Separate datasets pairing daily perceived stress levels from previous days (i.e., two to seven days prior to recalled dreams) with reported valence of subsequently recalled dream were generated. No statistically significant effect of perceived stress from the past 2 to 7 days on dream valence was found in any of the datasets tested, thus refuting a possible delayed effect of perceived stress on subsequently experienced dream affect.
Additional multilevel models predicting perceived stress as outcome
Using a reversed model, we aimed to predict daily stress scores (both maximum and prior to bedtime) using dream valence and DRF from the preceding night, along with the other predictor variables. The models only yielded a statistically significant effect of trait anxiety as a predictor of both maximum ( p = 0.031) and bedtime stress levels ( p = 0.007) (see Supplementary Tables S1 and S2 for more details).
We investigated the relationship between waking trait and state variables and dream affect by testing multilevel models aiming to predict the affective valence of people’s everyday dreams. Moreover, this was the first time a prospective day-by-day design was used to test predictors of dream valence at the between-subject as well as within-subject levels of variance. The results showed that daily measures of perceived stress collected from a non-clinical sample of adults do not, as suggested by some theorists, predict the emotional valence of dreams experienced later that night, nor on immediately subsequent nights. This study is also the first to identify trait anxiety as a key dispositional variable in predicting dream valence, even when trait measures are weighed against state variables.
Taken as a whole, these results run counter to previous findings indicating that state variables are better predictors of dysphoric dream frequency than are dispositional traits 46 , 47 , and that daily stress or mood interacts with trait variables to predict nightmares 42 , 48 . Previous positive results could be due to methodological considerations as these studies either lacked a multilevel, prospective design, focused on nightmare occurrence 42 , 46 , 47 or general sleep-related experiences 48 instead of everyday dreams, or focused on undergraduate (often psychology) students instead of recruiting participants from the general adult population 46 , 47 , 48 .
Our results are reminiscent of Cellucci and Lawrence’s study 49 of nightmare sufferers showing that daily ratings of general and maximum anxiety were statistically correlated with nightmare frequency and intensity in only a small minority of participants. Since trait variables were not assessed in their study, why nightmare occurrence was related to daily anxiety in some participants but not others remains to be determined. In line with this question, Soffer-Dudek and Shahar 48 found that daily stress predicted “general sleep-related experiences” only in individuals scoring high in trait dissociation (a trait strongly correlated with boundary thinness), while Blagrove and Fisher 42 found that correlations between state anxiety and nightly incidence of nightmares were only statistically significant in participants scoring high on boundary thinness. While the interplay between dispositional and state factors underlying nightmare occurrence may play a role in the emotional tone of everyday dreams, the current study showed no statistical interactions between various trait variables and daily levels of perceived stress in predicting dream valence.
With respect to the other dispositional traits investigated, it is noteworthy that although traumatic experiences, including aversive events during one’s childhood, are well-documented correlates of disturbed dreaming 21 , 34 , 54 , 55 , 56 , we found no statistically significant effect of trauma history on everyday dream affect. Most findings linking trauma and dream content, however, have come from work focused on trauma-related nightmares, typically in patients diagnosed with PTSD. By contrast, only 23 (18%) of our participants had a cut-off score of 3 or greater on the PC-PTSD (indicative of ongoing trauma-related difficulties) and only 16% reported more than one dream with an affect score of 9 or 10 (indicative of a nightmare) during the three weeks of the study. In fact, as shown in Fig. 1 , dreams with highly intense negative affect represented less than 8% of the over 1600 dream reports collected in the current study.
Similarly, while boundary thinness has been linked to dream content variables such as high dream recall, frequent nightmares and negatively-toned dreams 26 , 43 , 57 , 58 , 59 , it had no predictive value in our models of everyday dream valence. This trait variable may be better suited to the study of nightmare sufferers, a population specifically investigated by Hartmann et al . 59 when developing this personality construct, or to individuals prone to particularly vivid or bizarre dreams 26 .
Turning to the construct of affect load, the current study did not find evidence to support the idea that daily variations in perceived stress are temporally related night-to-night variations in dream affect. It should be noted that studies having reported an effect of affect load on the emotional content of dreams did so by measuring affect load retrospectively (e.g., for the past month) at a single point in time 7 , 46 , 47 rather than on a day-to-day basis. This underscores the importance of how state factors are assessed since correlates of retrospectively estimated state variables can be biased by dispositional factors (e.g., personality) and are not necessary correlates of prospective, day-to-day measurements of these constructs. In fact, this is not the first time in dream research that prospective study designs have yielded findings contradicting results obtained with retrospective measurements of dream-related variables, including correlates of dream recall and dream content 60 , 61 , 62 .
The concept of affect load may also need to be better defined to allow for more directly comparable study results. For example, in exploring the effects of stress on dreams, researchers have investigated acute stressors 63 , 64 , experimental stressors 22 , 65 , emotional stressors 66 , as well as cumulative stressors 21 . Additionally, in light of the recently proposed social simulation theory of dream function 67 in which dreaming is conceptualized as simulating social skills and bonds to strengthen waking social relationships, the study of social or interpersonal stressors 68 in relation to dream content may be particularly valuable, especially since a vast majority of dream reports feature social interactions 5 , 15 , 69 and that concerns of an interpersonal nature are frequent in everyday dreams 1 , 3 . Moreover, as suggested by some researchers 50 , dream content may be more reactive to the emotional nature of stressors than to the stressors per se . Finally, it is important to note that our participants were not particularly stressed—or at least did not perceive that they were—during the 3-week study as reflected by their mean score of 3.6 (out of 9) on our measure of daily maximum stress and 1.7 (out of 9) for daily bedtime stress. It is possible that direct or interaction effects of state and trait variables on dream affect become heightened, and thus more readily observable, during periods of acute or chronic stress.
When stress or affect load are studied in relation to dream content, they are usually assessed with self-report questionnaires. However, subjective levels of perceived stress can differ from variations or patterns in the biological markers of cortisol 70 , 71 . It is thus possible that physiological modulation of stress response, as opposed to subjective stress perception, plays a role in people’s nightly experience of dream affect. Of note, Nagy et al . 72 found a blunted cortisol awakening response in women reporting frequent nightmares, which was independent of lifestyle, psychiatric symptoms and demographic variables. This led the authors to hypothesize that low cortisol reactivity could be a trait-like feature of nightmare sufferers. Similarly, some researchers 73 have suggested that the gradual rise in people’s cortisol level from the middle of the night until its peak in the morning could account for observed increases in dream emotionality, bizarreness, vividness and length across the night 74 , independently of sleep stage. The use of biomarkers such as cortisol, which can be sampled in saliva 72 , could therefore be of particular interest in investigating the range and intensity of dream emotions reported both within and across nights.
Furthermore, since dream emotional valence was measured for the best-recalled dream upon awakening in the morning, the current study is limited to a narrow portion of participants’ sleep mentation. In addition, given the recency of morning dreams 75 and the aforementioned increase in dreamlike qualities of sleep mentation across the night, dream emotional valence was likely based on dreams occurring moments before morning awakenings. Affect load could thus have been processed through the emotional valence of dreams that were not collected in the present study (i.e., dreams from earlier periods of the night or other forms of unrecalled sleep mentation). Such a hypothesis could be tested with serial laboratory-based awakenings for dream collection across the sleep period, although the proportion of dreams containing emotions as well as their valence tend to differ when they are self-reported in the laboratory 13 , 14 , 17 , 76 as opposed to participants’ natural home enviornment 16 , 77 , 78 , 79 .
Finally, our sample of over 1600 dream reports revealed a roughly equal distribution of positive and negative emotions, as well as a higher proportion of intense positive emotions as opposed to negative emotions. This finding adds to the growing evidence showing that when the presence and valence of dreamed emptions are scored by the participants themselves as opposed to by external judges, as done in early studies of dream content 15 , a considerably higher proportion (70% to 100%) of dream reports are found to contain emotions 16 , 77 , 78 , 79 and that positive dream affect is particularly more frequent than when dream reports are assessed by external raters 17 , 79 . These findings also highlight the interest of investigating positive dimensions of waking states, such as mindfulness 27 and positive emotions 7 in relation to dream affect. In a related vein, the study of how self-regulation techniques such as relaxation and meditation may modulate the impact of state and trait factors on dream content also merits investigation.
In sum, results of the present study showed that trait anxiety, but not day-to-day levels of perceived stress, predicted the affective tone of home dream reports and revealed a potential bias in previous studies associated with the use of one-time retrospective assessments of state variables in predicting night-to-night variations in dream affect. The present results also underscore the need for additional research on factors underlying the valence of emotions experienced in everyday dreams as opposed to focusing solely on nightmares or trauma-related dreams. In particular, the study of different categories of stressors and the use of stress biomarkers could be particularly useful in elucidating the differential impact of state and trait factors on dream content.
Data were collected as part of a larger online study conducted on the Qualtrics Research Suite platform. After providing informed consent, participants were emailed a link giving them access to the study materials. Participants first completed a series of questionnaires on sleep, personality, trait anxiety and trauma history. They then received, over a maximum of four consecutive weeks, daily scheduled notifications to complete a questionnaire on dream recall in the morning as well as an evening questionnaire on the stress and emotions experienced that day. The project was approved by the Arts and Science Research Ethics Committee of the Université de Montréal, Canada (Project no. CERAS-2017-18-013-P) and all research was performed in accordance with their guidelines and regulations.
Participants
One hundred and twenty-eight non-paid participants (98 women, 30 men, M age = 42.55, SD age = 14.63, range = 19–76 years) were recruited from the general adult population between February and July 2018 via ads in free local newspapers (74.9% of sample), social networks (9.4%), email lists (8.6%) and community posters (7.1%). Study materials were available in both French and English to reflect the bilingual nature of Montreal, Canada. One hundred and twelve of the 128 volunteers (87.5%) completed the study in French. Eighty-eight participants (68.8% of sample) were working at the time of study, 20 (15.6%) were students, 12 (9.4%) were retired, 5 (3.9%) were unemployed, and 3 (2.3%) did not specify their occupation. Of the 285 people who initially expressed interest in the study, 151 provided written informed consent and completed the first set of questionnaires. Of these 151 participants, 23 (18 women, 5 men) were excluded for providing fewer than three consecutive days of matching stress and dream valence data. Participants’ morning dream data were paired with their stress ratings completed prior to bedtime the night before. Sixty-six of 128 participants (51.6%) completed one or more days of data collection beyond the 21 consecutive days required. These data were included in the analyses as they contained validly paired evening stress and morning dream valence scores.
Retrospective measures
Participants first completed a general Sleep and Dream Questionnaire 33 used to assess basic sleep, dream and demographic variables.
Boundary thinness
The short form of the Boundary Questionnaire (BQ18) 80 , which contains 18 items derived from the original Boundary Questionnaire 40 , was used to measure boundary thinness or thickness, a personality trait associated with various aspects of dreaming 57 , including high dream recall 43 and nightmare prevalence 58 . People with thin psychological boundaries are typically described as being creative, sensitive, vulnerable and easily mixing thoughts, images and feelings. The total score of the BQ18 consists of a sum of the ratings (ranging from 0 to 4) on the 18 items after inverting the ratings on 4 items. Scores on the BQ18 are positively correlated ( r = 0.87, N = 856) with total scores on the original Boundary Questionnaire 80 . Cronbach’s alpha (α) for the BQ18 in the present study was 0.70.
Trait anxiety
The Trait scale of the State-Trait Anxiety Inventory – Form Y (STAI-T) 81 measures anxiety as an enduring personality trait and consists of 20 statements that pertain to how participants “generally feel.” Each item is rated on a 4-point Likert scale. The total score is calculated as a sum of all the ratings (ranging from 0 to 80), with a higher score indicating higher trait anxiety. The STAI-T is widely used and has been translated in multiple languages, including in French Canadian 82 . The latter shows a correlation of r = 0.82 with the original English version and a test-retest correlation of r = 0.94. The original French-Canadian translation shows strong internal consistency (α = 0.91) and an identical reliability (α = 0.91) obtained in the present study.
Youth trauma
A shortened French version 83 of the Early Trauma Inventory Self Report (ETISR-SF) 84 was used to assess a range of physical, emotional, and sexual abuse experiences that may have occurred before the age of 18. The seven items, presented in “Yes-No” format, yield a total score ranging between 0 and 7. Cronbach’s alpha (α) for the ETISR-SF in the present study was 0.73.
Posttraumatic stress disorder
The Primary Care PTSD Screen (PC-PTSD) 85 measures four factors specific to posttraumatic stress disorder (PTSD): reexperiencing, avoidance, hyperarousal and numbing. A positive response to any of the yes/no items indicates that the responder may have PTSD or trauma-related problems, and a cut-off score of 3 is recommended to detect positive cases. Cronbach’s alpha (α) for the PC-PTSD in the present study was 0.75.
Prospective measures
Dream recall and content were assessed each morning via URL links emailed to each participant at 3:00 AM. To ensure that reported dream recall data was for the targeted day, daily links expired at 6:00 PM. This time range was sufficiently broad to accommodate participants’ occupations and schedules. Reminders were automatically sent out at 3:00 PM if the morning questionnaire had not been completed by that time. Waking perceived stress for the day was measured prior to bedtime with links sent out at 6:00 PM and expiring at 3:00 AM. A reminder was sent at 12:00 AM (i.e. midnight) if participants had not completed the evening questionnaire by that time.
Dream affect and content
Dream recall was assessed with a single item, “Did you dream last night?” and a “Yes-No” answer format. If “No” was selected, participants had the option of returning to the questionnaire if ever they remembered a dream later in the day. If participants answered “Yes,” they were required to indicate if they remembered one, two, or three or more dreams from that night. These values were used to calculate participants’ dream recall frequency. Participants then had to indicate (for the most vivid or best-remembered dream from the night if more than one dream was recalled), the dream’s emotional valence by answering the question, “What was the general emotion of your dream?” using a 10-point Likert scale ranging from positive (1) to negative (10).
Perceived stress
Two daily measures of perceived stress were completed prior to bedtime using a 10-point Likert scale ranging from not stressed at all (0) to extremely stressed (9). The first measure required participants to rate the maximum level of stress experienced that day while the second required participants to rate their stress level at the time of questionnaire completion (i.e., prior to bedtime). These scales, reviewed by Dr. Sonia J. Lupien, director of the Centre for Studies on Human Stress ( https://humanstress.ca/ ), were used instead of more exhaustive instruments such as the Daily Stress Inventory 86 due to the multi-week nature of the study and our desire to limit volunteers’ workload.
Statistical analyses
Data were analyzed using hierarchical linear modeling (HLM) with IBM SPSS Statistics (version 25), where affect load (level 1: affective dream content [outcome], perceived stress [predictor]) was underpinned by the participants’ dispositional measures (level 2 predictors: trait anxiety, boundary thinness, trauma history, PTSD, sex, age). The level of statistical significance for every analysis was set at p = 0.05. This type of multilevel analysis is ideally suited to such a dataset as it a) allows for the analysis of multiple relationships while considering shared variance at both levels, b) takes into account dependency across measurement time points, c) doesn’t require balanced designs in which different individuals have a fixed number of prospective data points without any missing data, and d) has fewer assumptions and is less likely to underestimate error than other statistical methods 87 .
Although dream valence was the main outcome variable of interest, models predicting daily perceived stress were also tested to investigate possible effects of dreamed emotions on daytime stress. Dream valence had a normal distribution and enough anchor points (10) to approximate continuity. It was thus tested using linear mixed-effects modeling (MIXED command). Since both measures of daily perceived stress were positively skewed, they were tested under a Poisson distribution using a generalized estimating equation (GENLIN command) which, in both cases, presented a better model fit than with a normal distribution under a linear mixed-effects model.
When dream valence was the outcome variable, measures of daily stress from the preceding day were used as Level-1 predictors while trait, trauma and demographic variables were used as Level-2 predictors. Since dream recall frequency was measured daily, it was also used as a Level-1 predictor to assess its possible mediating effect on dream valence and other predictor variables, with values from 1 (one dream remembered on that night) to 3 (three or more dreams remembered). When daily stress was the outcome of interest, the dataset was shifted in order for a given night’s dream valence to be paired with levels of perceived stress of the following day. Considering that participants’ first daily measurement was for perceived stress, there was a smaller total of 2410 observations, not 2538, because the first stress values and last dream valence values were unpaired and thus excluded.
We first computed an intercepts-only model where time was not specified as a repeated measures variable and no predictors entered. This procedure is recommended to determine the amount of between-subject variance in the outcome variable, also known as the intraclass correlation 88 . The intraclass correlation was thus calculated by dividing the value of the intercept (between-group) variance by the sum of the residual (within-group) variance and intercept.
We then progressively added predictors to the unconditional model, beginning with individual Level-2 predictors. All Level-2 variables were grand mean centered. Level-1 stress predictor variables were centered to each participants’ mean for the duration of the study to account for dispositional biases in reported self-ratings.
Finally, post hoc analyses were performed to test alternate hypotheses. Interactions were tested between predictors to assess whether the model generalized to the whole sample or if some effects were moderated by other variables. We individually tested and reported the potential moderating effects of every level 2 predictor and of dream recall and valence (level 1) on each of the two level 1 stress predictors. The effect on dream valence of the stress variables from 2 to 7 days ago was also tested using lagged independent variables.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Domhoff, G. W. Finding meaning in dreams: a quantitative approach ., https://doi.org/10.1007/978-1-4899-0298-6 (Plenum Press, 1996).
Book Google Scholar
Hall, C. S. & Nordby, V. J. The individual and his dreams . (Signet, 1972).
Domhoff, G. W. The emergence of dreaming: mind-wandering, embodied simulation, and the default network . (Oxford University Press, 2018).
Han, H. J., Schweickert, R., Xi, Z. & Viau-Quesnel, C. The cognitive social network in dreams: transitivity, assortativity, and giant component proportion are monotonic. Cogn. Sci. 40 , 671–696 (2016).
Article PubMed Google Scholar
Pesant, N. & Zadra, A. Dream content and psychological well-being: a longitudinal study of the continuity hypothesis. J. Clin. Psychol. 62 , 111–121 (2006).
Schredl, M. & Erlacher, D. Relation between waking sport activities, reading, and dream content in sport students and psychology students. J. Psychol. 142 , 267–275 (2008).
Sikka, P., Pesonen, H. & Revonsuo, A. Peace of mind and anxiety in the waking state are related to the affective content of dreams. Sci. Rep. 8 , 1–13 (2018).
Article CAS Google Scholar
Davidson, J., Lee-Archer, S. & Sanders, G. Dream imagery and emotion. Dreaming 15 , 33–47 (2005).
Article Google Scholar
Gilchrist, S., Davidson, J. & Shakespeare-Finch, J. Dream emotions, waking emotions, personality characteristics and well-being — a positive psychology approach. Dreaming 17 , 172–185 (2007).
Nixon, A., Robidoux, R., Dale, A. L. & De Koninck, J. Pre-sleep and post-sleep mood as a complementary evaluation of emotionally impactful dreams. Int. J. Dream Res. 10 , 141–150 (2017).
Google Scholar
Schredl, M. Factors affecting the continuity between waking and dreaming: emotional intensity and emotional tone of the waking-life event. Sleep Hypn. 8 , 1–5 (2006).
Schredl, M. & Reinhard, I. The continuity between waking mood and dream emotions: direct and second-order effects. Imagin. Cogn. Pers. 29 , 271–282 (2010).
St-Onge, M., Lortie-Lussier, M., Mercier, P., Grenier, J. & De Koninck, J. Emotions in the diary and REM dreams of young and late adulthood women and their relation to life satisfaction. Dreaming 15 , 116–128 (2005).
Fosse, R., Stickgold, R. & Hobson, J. A. The mind in REM sleep: reports of emotional experience. Sleep 24 , 1–9 (2001).
Hall, C. S. & Van de Castle, R. L. The content analysis of dreams. Central Psychology Series (Appleton-Century-Crofts, 1966).
Merritt, J. M., Stickgold, R., Pace-Schott, E. F., Williams, J. & Hobson, J. A. Emotion profiles in the dreams of men and women. Conscious. Cogn. 3 , 46–60 (1994).
Sikka, P., Valli, K., Virta, T. & Revonsuo, A. I know how you felt last night, or do I? Self- and external ratings of emotions in REM sleep dreams. Conscious. Cogn. 25 , 51–66 (2014).
Cartwright, R. D. The twenty-four hour mind: the role of sleep and dreaming in our emotional lives . (Oxford University Press, 2010).
Hartmann, E. The nature and functions of dreaming . (Oxford University Press, 2010).
Kramer, M. The selective mood regulatory function of dreaming: an update and revision. In The functions of dreaming . (eds Moffitt, A., Kramer, M. & Hoffmann, R.) 139–195 (State University of New York Press, 1993).
Cook, C. A. L., Caplan, R. D. & Wolowitz, H. Nonwaking responses to waking stressors: dreams and nightmares. J. Appl. Soc. Psychol. 20 , 199–226 (1990).
De Koninck, J. & Koulack, D. Dream content and adaptation to a stressful situation. J. Abnorm. Psychol. 84 , 250–260 (1975).
Delorme, M. A., Lortie-Lussier, M. & De Koninck, J. Stress and coping in the waking and dreaming states during an examination period. Dreaming 12 , 171–183 (2002).
Roberts, J., Lennings, C. J. & Heard, R. Nightmares, life stress, and anxiety: an examination of tension reduction. Dreaming 19 , 17–29 (2009).
Blagrove, M. & Pace-Schott, E. F. Trait and neurobiological correlates of individual differences in dream recall and dream content. In International Review of Neurobiology: Dreams and Dreaming . (eds Clow, A. & McNamara, P.) 92 , 155–180 (Elsevier, 2010).
Hartmann, E., Rosen, R. & Rand, W. Personality and dreaming: boundary structure and dream content. Dreaming 8 , 31–39 (1998).
Simor, P., Köteles, F., Sándor, P., Petke, Z. & Bódizs, R. Mindfulness and dream quality: the inverse relationship between mindfulness and negative dream affect. Scand. J. Psychol. 52 , 369–375 (2011).
Duval, M., McDuff, P. & Zadra, A. Nightmare frequency, nightmare distress, and psychopathology in female victims of childhood maltreatment. J. Nerv. Ment. Dis. 201 , 767–772 (2013).
Esposito, K., Benitez, A., Barza, L. & Mellman, T. A. Evaluation of dream content in combat-related PTSD. J. Trauma. Stress 12 , 681–687 (1999).
Article CAS PubMed Google Scholar
Helminen, E. & Punamäki, R.-L. Contextualized emotional images in children’s dreams: psychological adjustment in conditions of military trauma. Int. J. Behav. Dev. 32 , 177–187 (2008).
Valli, K. et al . The threat simulation theory of the evolutionary function of dreaming: evidence from dreams of traumatized children. Conscious. Cogn. 14 , 188–218 (2005).
Blagrove, M., Farmer, L. & Williams, E. The relationship of nightmare frequency and nightmare distress to well-being. J. Sleep Res. 13 , 129–136 (2004).
Zadra, A. & Donderi, D. C. Nightmares and bad dreams: their prevalence and relationship to well-being. J. Abnorm. Psychol. 109 , 273–281 (2000).
Levin, R. & Nielsen, T. A. Disturbed dreaming, posttraumatic stress disorder, and affect distress: a review and neurocognitive model. Psychol. Bull. 133 , 482–528 (2007).
Levin, R. & Nielsen, T. A. Nightmares, bad dreams and emotion dysregulation: a review and new neurocognitive model of dreaming. Curr. Dir. Psychol. Sci. 18 , 84–88 (2009).
Sandman, N. et al . Nightmares as predictors of suicide: an extension study including war veterans. Sci. Rep. 7 , 1–7 (2017).
Schredl, M. & Engelhardt, H. Dreaming and psychopathology: dream recall and dream content of psychiatric inpatients. Sleep Hypn. 3 , 44–54 (2001).
Soffer-Dudek, N. Arousal in nocturnal consciousness: how dream- and sleep-experiences may inform us of poor sleep quality, stress, and psychopathology. Front. Psychol. 8 , 1–10 (2017).
Nielsen, T. A. The stress acceleration hypothesis of nightmares. Front. Neurol. 8 , 1–23 (2017).
Hartmann, E. Boundaries in the mind: a new psychology of personality . (Basic Books, 1991).
Sylvers, P., Lilienfeld, S. O. & LaPrairie, J. L. Differences between trait fear and trait anxiety: implications for psychopathology. Clin. Psychol. Rev. 31 , 122–137 (2011).
Blagrove, M. & Fisher, S. Trait-state interactions in the etiology of nightmares. Dreaming 19 , 65–74 (2009).
Schredl, M., Schäfer, G., Hofmann, F. & Jacob, S. Dream content and personality: thick vs. thin boundaries. Dreaming 9 , 257–263 (1999).
Sándor, P., Horváth, K., Bódizs, R. & Konkolÿ Thege, B. Attachment and dream emotions: The mediating role of trait anxiety and depression. Curr. Psychol . 1–10, https://doi.org/10.1007/s12144-018-9890-y (2018).
Nielsen, T. A. et al . Development of disturbing dreams during adolescence and their relation to anxiety symptoms. Sleep 23 , 1–10 (2000).
Levin, R., Fireman, G., Spendlove, S. & Pope, A. The relative contribution of affect load and affect distress as predictors of disturbed dreaming. Behav. Sleep Med. 9 , 173–183 (2011).
Schredl, M. Effects of state and trait factors on nightmare frequency. Eur. Arch. Psychiatry Clin. Neurosci. 253 , 241–247 (2003).
Soffer-Dudek, N. & Shahar, G. Daily stress interacts with trait dissociation to predict sleep-related experiences in young adults. J. Abnorm. Psychol. 120 , 719–729 (2011).
Cellucci, A. J. & Lawrence, P. S. Individual differences in self-reported sleep variable correlations among nightmare sufferers. J. Clin. Psychol. 34 , 721–725 (1978).
Malinowski, J. E. & Horton, C. L. Evidence for the preferential incorporation of emotional waking-life experiences into dreams. Dreaming 24 , 18–31 (2014).
Watson, D. Dissociations of the night: individual differences in sleep-related experiences and their relation to dissociation and schizotypy. J. Abnorm. Psychol. 110 , 526–535 (2001).
Nielsen, T. A., Kuiken, D., Alain, G., Stenstrom, P. & Powell, R. A. Immediate and delayed incorporations of events into dreams: Further replication and implications for dream function. J. Sleep Res. 13 , 327–336 (2004).
Eichenlaub, J.-B. et al . The nature of delayed dream incorporation (‘dream-lag effect’): personally significant events persist, but not major daily activities or concerns. J. Sleep Res. 28 , 1–8 (2019).
Agargun, M. Y. et al . Nightmares and dissociative experiences: the key role of childhood traumatic events. Psychiatry Clin. Neurosci. 57 , 139–145 (2003).
Wittmann, L., Schredl, M. & Kramer, M. Dreaming in posttraumatic stress disorder: a critical review of phenomenology, psychophysiology and treatment. Psychother. Psychosom. 76 , 25–39 (2007).
Duval, M. & Zadra, A. Frequency and content of dreams associated with trauma. Sleep Med. Clin. 5 , 249–260 (2010).
Aumann, C., Lahl, O. & Pietrowsky, R. Relationship between dream structure, boundary structure and the big five personality dimensions. Dreaming 22 , 124–135 (2012).
Zborowski, M., McNamara, P., Hartmann, E., Murphy, M. & Mattle, L. Boundary structure related to sleep measures and to dream content. Sleep 21S , 284 (1998).
Hartmann, E., Russ, D., Oldfield, M., Sivan, I. & Cooper, S. Who has nightmares? Arch. Gen. Psychiatry 44 , 49–56 (1987).
Wood, J. M. & Bootzin, R. R. The prevalence of nightmares and their independence from anxiety. J. Abnorm. Psychol. 99 , 64–68 (1990).
Robert, G. & Zadra, A. Measuring nightmare and bad dream frequency: impact of retrospective and prospective instruments. J. Sleep Res. 17 , 132–139 (2008).
Beaulieu-Prévost, D. & Zadra, A. Absorption, psychological boundaries and attitude towards dreams as correlates of dream recall: two decades of research seen through a meta-analysis. J. Sleep Res. 16 , 51–59 (2007).
Wood, J. M., Bootzin, R. R., Rosenhan, D., Nolen-Hoeksema, S. & Jourden, F. Effects of the 1989 San Francisco earthquake on frequency and content of nightmares. J. Abnorm. Psychol. 101 , 219–224 (1992).
Soffer-Dudek, N. & Shahar, G. Effect of exposure to terrorism on sleep-related experiences in Israeli young adults. Psychiatry Interpers. Biol. Process. 73 , 264–276 (2010).
Koulack, D., Prevost, F. & De Koninck, J. Sleep, dreaming, and adaptation to a stressful intellectual activity. Sleep 8 , 244–253 (1985).
Cartwright, R. D., Lloyd, S., Knight, S. & Trenholme, I. Broken dreams: a study of the effects of divorce and depression on dream content. Psychiatry 47 , 251–9 (1984).
Revonsuo, A., Tuominen, J. & Valli, K. Avatars in the machine: dreaming as a simulation of social reality. In Open Mind: Philosophy and the Mind Sciences in the 21st Century (eds Metzinger, T. & Windt, J. M.) 2 , 1295–1322 (MIND Group, 2016).
Takahashi, T. et al . Anxiety, reactivity, and social stress-induced cortisol elevation in humans. Neuroendocrinol. Lett. 26 , 351–354 (2005).
CAS PubMed Google Scholar
Tuominen, J., Stenberg, T., Revonsuo, A. & Valli, K. Social contents in dreams: an empirical test of the Social Simulation Theory. Conscious. Cogn. 69 , 133–145 (2019).
van Eck, M. M., Berkhof, H., Nicolson, N. A. & Sulon, J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosom. Med. 58 , 447–458 (1996).
Hirvikoski, T., Lindholm, T., Nordenström, A., Nordström, A. L. & Lajic, S. High self-perceived stress and many stressors, but normal diurnal cortisol rhythm, in adults with ADHD (attention-deficit/hyperactivity disorder). Horm. Behav. 55 , 418–424 (2009).
Nagy, T. et al . Frequent nightmares are associated with blunted cortisol awakening response in women. Physiol. Behav. 147 , 233–237 (2015).
Payne, J. D. Memory consolidation, the diurnal rhythm of cortisol, and the nature of dreams: a new hypothesis. In International Review of Neurobiology: Dreams and Dreaming (eds Clow, A. & McNamara, P.) 92, 101–134 (Elsevier, 2010).
Carr, M. & Solomonova, E. Dream recall and content in different stages of sleep and time-of-night effect. In Dreams: Understanding Biology, Psychology, and Culture (eds Valli, K., Hoss, R. J. & Gongloff, R. P.) 188–194 (Greenwood Publishing Group, 2019).
Trinder, J. & Kramer, M. Dream recall. Am. J. Psychiatry 128 , 296–301 (1971).
Foulkes, D., Sullivan, B., Kerr, N. H. & Brown, L. Appropriateness of dream feelings to dreamed situations. Cogn. Emot. 2 , 29–39 (1988).
Nielsen, T. A., Deslauriers, D. & Baylor, G. W. Emotions in dream and waking event reports. Dreaming 1 , 287–300 (1991).
Schredl, M. & Doll, E. Emotions in diary dreams. Conscious. Cogn. 7 , 634–646 (1998).
Sikka, P., Feilhauer, D., Valli, K. & Revonsuo, A. How you measure is what you get: differences in self- and external ratings of emotional experiences in home dreams. Am. J. Psychol. 130 , 367–384 (2017).
Kunzendorf, R. G., Hartmann, E., Cohen, R. & Cutler, J. Bizarreness of the dreams and daydreams reported by individuals with thin and thick boundaries. Dreaming 7 , 265–271 (1997).
Spielberger, C. D., Gorsuch, R. L., Lushene, R. E., Vagg, P. R. & Jacobs, G. A. Manual for the State-Trait Anxiety Inventory (Form Y): Self-Evaluation Questionnaire. Consulting Psychologists Press , https://doi.org/10.5370/JEET.2014.9.2.478 (Consulting Psychologists Press, 1983).
Gauthier, J. & Bouchard, S. Adaptation canadienne-française de la forme revisée du State-Trait Anxiety Inventory de Spielberger. Rev. Can. des Sci. du Comport. 25 , 559–578 (1993).
Hébert, M., Cyr, M. & Zuk, S. Traduction et adaptation française du Early Trauma Inventory Self-Report - Short Form (ETISR-SF, 2007) de Bremner, Bolus et Mayer (2008).
Bremner, J. D., Bolus, R. & Mayer, E. A. Psychometric properties of the Early Trauma Inventory-Self Report. J. Nerv. Ment. Dis. 195 , 211–218 (2007).
Article PubMed PubMed Central Google Scholar
Prins, A. et al . The primary care PTSD screen (PC–PTSD): development and operating characteristics. Prim. Care Psychiatry 9 , 9–14 (2004).
Brantley, P. J., Waggoner, C. D., Jones, G. N. & Rappaport, N. B. A daily stress inventory: development, reliability, and validity. J. Behav. Med. 10 , 61–73 (1987).
Woltman, H., Feldstain, A., MacKay, C. & Rocchi, M. An introduction to hierarchical linear modeling. Tutor. Quant. Methods Psychol. 8 , 52–69 (2012).
Tabachnick, B. G. & Fidell, L. S. Using multivariate statistics , https://doi.org/10.1037/022267 (Pearson Education Inc., 2019).
Download references
Acknowledgements
This research was funded by a grant from the Social Sciences and Humanities Research Council of Canada (SSHRC #435-2015-1181) and from the Canadian Institutes of Health Research (CIHR # MOP 97865) to A.Z. The authors would like to thank Pierre McDuff for his help with statistical analyses, the Interdisciplinary Research Centre on Intimate Relationship Problems and Sexual Abuse (CRIPCAS) and the Centre for Studies on Human Stress (CSHS) for their assistance in the early phases of the study.
Author information
Authors and affiliations.
Department of Psychology, Université de Montréal, Montreal, QC, Canada
Eugénie Samson-Daoust, Sarah-Hélène Julien & Antonio Zadra
Department of Sexology, Université du Québec à Montréal (UQAM), Montreal, QC, Canada
Dominic Beaulieu-Prévost
Center for Advanced Research in Sleep Medicine (CARSM), Hôpital du Sacré-Coeur de Montréal, Montreal, QC, Canada
Antonio Zadra
You can also search for this author in PubMed Google Scholar
Contributions
E.S.D. contributed to study design, conducted the study, analysed the results and wrote the manuscript draft. S.H.J. contributed to the study design and data collection. D.B.P. contributed to the statistical analyses and reviewed the manuscript. A.Z. obtained the grants, designed and supervised the study and reviewed the manuscript.
Corresponding author
Correspondence to Antonio Zadra .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary tables, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Samson-Daoust, E., Julien, SH., Beaulieu-Prévost, D. et al. Predicting the affective tone of everyday dreams: A prospective study of state and trait variables. Sci Rep 9 , 14780 (2019). https://doi.org/10.1038/s41598-019-50859-w
Download citation
Received : 28 May 2019
Accepted : 15 September 2019
Published : 14 October 2019
DOI : https://doi.org/10.1038/s41598-019-50859-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

- Table of Contents
- Random Entry
- Chronological
- Editorial Information
- About the SEP
- Editorial Board
- How to Cite the SEP
- Special Characters
- Advanced Tools
- Support the SEP
- PDFs for SEP Friends
- Make a Donation
- SEPIA for Libraries
- Entry Contents
Bibliography
Academic tools.
- Friends PDF Preview
- Author and Citation Info
- Back to Top
Dreams and Dreaming
Dreams and dreaming have been discussed in diverse areas of philosophy ranging from epistemology to ethics, ontology, and more recently philosophy of mind and cognitive science. This entry provides an overview of major themes in the philosophy of sleep and dreaming, with a focus on Western analytic philosophy, and discusses relevant scientific findings.
1.1 Cartesian dream skepticism
1.2 earlier discussions of dream skepticism and why descartes’ version is special, 1.3 dreaming and other skeptical scenarios, 1.4 descartes’ solution to the dream problem and real-world dreams, 2.1 are dreams experiences, 2.2 dreams as instantaneous memory insertions, 2.3 empirical evidence on the question of dream experience, 2.4 dreams and hallucinations, 2.5 dreams and illusions, 2.6 dreams as imaginative experiences, 2.7 dreaming and waking mind wandering, 2.8 the problem of dream belief, 3.1 dreaming as a model system and test case for consciousness research, 3.2 dreams, psychosis, and delusions, 3.3 beyond dreams: dreamless sleep experience and the concepts of sleep, waking, and consciousness, 4. dreaming and the self, 5. immorality and moral responsibility in dreams, 6.1 the meaning of dreams, 6.2 the functions of dreaming, 7. conclusions, other internet resources, related entries, 1. dreams and epistemology.
Dream skepticism has traditionally been the most famous and widely discussed philosophical problem raised by dreaming (see Williams 1978; Stroud 1984). In the Meditations , Descartes uses dreams to motivate skepticism about sensory-based beliefs about the external world and his own bodily existence. He notes that sensory experience can also lead us astray in commonplace sensory illusions such as seeing things as too big or small. But he does not think such cases justify general doubts about the reliability of sensory perception: by taking a closer look at an object seen under suboptimal conditions, we can easily avoid deception. By contrast, dreams suggest that even in a seemingly best-case scenario of sensory perception (Stroud 1984), deception is possible. Even the realistic experience of sitting dressed by the fire and looking at a piece of paper in one’s hands (Descartes 1641: I.5) is something that can, and according to Descartes often does, occur in a dream.
There are different ways of construing the dream argument. A strong reading is that Descartes is trapped in a lifelong dream and none of his experiences have ever been caused by external objects (the Always Dreaming Doubt ; see Newman 2019). A weaker reading is that he is just sometimes dreaming but cannot rule out at any given moment that he is dreaming right now (the Now Dreaming Doubt ; see Newman 2019). This is still epistemologically worrisome: even though some of his sensory-based beliefs might be true, he cannot determine which these are unless he can rule out that he is dreaming. Doubt is thus cast on all of his beliefs, making sensory-based knowledge slip out of reach.
Cartesian-style skeptical arguments have the following form (quoted from Klein 2015):
- If I know that p , then there are no genuine grounds for doubting that p .
- U is a genuine ground for doubting that p .
- Therefore, I do not know that p .
If we apply this to the case of dreaming, we get:
- If I know that I am sitting dressed by the fire, then there are no genuine grounds for doubting that I am really sitting dressed by the fire.
- If I were now dreaming, this would be a genuine ground for doubting that I am sitting dressed by the fire: in dreams, I have often had the realistic experience of sitting dressed by the fire when I was actually lying undressed in bed!
- Therefore, I do not know that I am now sitting dressed by the fire.
Importantly, both strong and weak versions of the dream argument cast doubt only on sensory-based beliefs, but leave other beliefs unscathed. According to Descartes, that 2+3=5 or that a square has no more than 4 sides is knowable even if he is now dreaming:
although, in truth, I should be dreaming, the rule still holds that all which is clearly presented to my intellect is indisputably true. (Descartes 1641: V.15)
By Descartes’ lights, dreams do not undermine our ability to engage in the project of pure, rational enquiry (Frankfurt 1970; but see Broughton 2002).
Dream arguments have been a staple of philosophical skepticism since antiquity and were so well known that in his objections to the Meditations , Hobbes (1641) criticized Descartes for not having come up with a more original argument. Yet, Descartes’ version of the problem, more than any other, has left its mark on the philosophical discussion.
Earlier versions tended to touch upon dreams just briefly and discuss them alongside other examples of sensory deception. For example, in the Theaetetus (157e), Plato has Socrates discuss a defect in perception that is common to
dreams and diseases, including insanity, and everything else that is said to cause illusions of sight and hearing and the other senses.
This leads to the conclusion that knowledge cannot be defined through perception.
Dreams also appear in the canon of standard skeptical arguments used by the Pyrrhonists. Again, dreams and sleep are just one of several conditions (including illness, joy, and sorrow) that cast doubt on the trusthworthiness of sensory perception (Diogenes Laertius, Lives of Eminent Philosophers; Sextus Empiricus, Outlines of Pyrrhonism) .
Augustine ( Against the Academics ; Confessions) thought the dream problem could be contained, arguing that in retrospect, we can distinguish both dreams and illusions from actual perception (Matthew 2005: chapter 8). And Montaigne ( The Apology for Raymond Sebond ) noted that wakefulness itself teems with reveries and illusions, which he thought were even more epistemologically worrisome than nocturnal dreams.
Descartes devoted much more space to the discussion of dreaming and cast it as a unique epistemological threat distinct from both waking illusions and evil genius or brain-in-a-vat-style arguments. His claim that he has often been deceived by his dreams implies he also saw dreaming as a real-world (rather than merely hypothetical) threat.
This is further highlighted by the intimate, first-person style of the Meditations . Their narrator is supposed to exemplify everyone’s epistemic situation, illustrating the typical defects of the human mind. Readers are further drawn in by Descartes’ strategy of moving from commonsense examples towards more sophisticated philosophical claims (Frankfurt 1970). For example, Descartes builds up towards dream skepticism by first considering familiar cases of sensory illusions and then deceptively realistic dreams.
Finally, much attention has been devoted to several dreams Descartes reportedly had as a young man. Some believe these dreams embodied theoretical doubts he developed in the Discourse and Meditations (Baillet 1691; Leibniz 1880: IV; Cole 1992; Keefer 1996). Hacking (2001:252) suggests that for Descartes, dream skepticism was not just a philosophical conundrum but a source of genuine doubt. There is also some discussion about the dream reports’ authenticity (Freud 1940; Cole 1992; Clarke 2006; Browne 1977).
In the Meditations , after discussing the dream argument, Descartes raises the possibility of an omnipotent evil genius determined to deceive us even in our most basic beliefs. Contrary to dream deception, Descartes emphasizes that the evil genius hypothesis is a mere fiction. Still, it radicalizes the dream doubt in two respects. One, where the dream argument left the knowability of certain general truths intact, these are cast in doubt by the evil genius hypothesis . Two, where the dream argument, at least on the weaker reading, involves just temporary deception, the evil genius has us permanently deceived.
One modernized version, the brain-in-a-vat thought experiment, says that if evil scientists placed your brain in a vat and stimulated it just right, your conscious experience would be exactly the same as if you were still an ordinary, embodied human being (Putnam 1981). In the Matrix -trilogy (Chalmers 2005), Matrixers live unbeknownst to themselves in a computer simulation. Unlike the brain-in-a-vat , they have bodies that are kept alive in pods, and flaws in the simulation allow some of them to bend its rules to their advantage.
Unlike dream deception, which is often cast as a regularly recurring actuality (cf. Windt 2011), brain-in-a-vat-style arguments are often thought to be merely logically or nomologically possible. However, there might be good reasons for thinking that we actually live in a computer simulation (Bostrom 2003), and if we lend some credence to radical skeptical scenarios, this may have consequences for how we act (Schwitzgebel 2017).
Even purely hypothetical skeptical scenarios may enhance their psychological force by capitalizing on the analogy with dreams. Clark (2005) argues that the Matrix contains elements of “industrial-strength deception” in which both sensory experience and intellectual functioning are exactly the same as in standard wake-states, whereas other aspects are more similar to the compromised reasoning and bizarre shifts that are the hallmark of dreams.
At the end of the Sixth Meditation , Descartes suggests a solution to the dream problem that is tied to a reassessment of what it is like to dream. Contrary to his remarks in the First Meditation , he notes that dreams are only rarely connected to waking memories and are often discontinuous, as when dream characters suddenly appear or disappear. He then introduces the coherence test:
But when I perceive objects with regard to which I can distinctly determine both the place whence they come, and that in which they are, and the time at which they appear to me, and when, without interruption, I can connect the perception I have of them with the whole of the other parts of my life, I am perfectly sure that what I thus perceive occurs while I am awake and not during sleep. (Meditation VI. 24)
For all practical purposes, he has now found a mark by which dreaming and waking can be distinguished (cf. Meditation I.7), and even if the coherence test is not fail-safe, the threat of dream deception has been averted.
Descartes’ remarks about the discontinuous and ad hoc nature of many dreams are backed up by empirical work on dream bizarreness (see Hobson 1988; Revonsuo & Salmivalli 1995). Still, many of his critics were not convinced this helped his case against the skeptic. Even if Descartes’ revised phenomenological description characterizes most dreams, one might occasionally merely dream of successfully performing the test (Hobbes 1641), and in some dreams, one might seem to have a clear and distinct idea but this impression is false (Bourdin 1641). Both the coherence test and the criterion of clarity and distinctness would then be unreliable.
How considerations of empirical plausibility impact the dream argument continues to be a matter of debate. Grundmann (2002) appeals to scientific dream research to introduce an introspective criterion: when we introspectively notice that we are able to engage in critical reflection, we have good reason to think that we are awake and not dreaming. However, this assumes critical reasoning to be uniformly absent in dreams. If attempts at critical reasoning do occur in dreams and if they generally tend to be corrupted, the introspective criterion might again be problematic (Windt 2011, 2015a). There are also cases in which even after awakening, people mistake what was in fact a dream for reality (Wamsley et al. 2014). At least in certain situations and for some people, dream deception might be a genuine cause of concern (Windt 2015a).
2. The ontology of dreams
In what follows, the term “conscious experience” is used as an umbrella term for the occurrence of sensations, thoughts, impressions, emotions etc. in dreams (cf. Dennett 1976). These are all phenomenal states: there is something it is like to be in these states for the subject of experience (cf. Nagel 1974). To ask about dream experience is to ask whether it is like something to dream while dreaming, and whether what it is like is similar to (or relevantly different from) corresponding waking experiences.
Cartesian dream skepticism depends on a seemingly innocent background assumption: that dreams are conscious experiences. If this is false, then dreams are not deceptive experiences during sleep and we cannot be deceived, while dreaming, about anything at all. Whether dreams are experiences is a major question for the ontology of dreams and closely bound up with dream skepticism.
The most famous argument denying that dreams are experiences was formulated by Norman Malcolm (1956, 1959). Today, his position is commonly rejected as implausible. Still, it set the tone for the analysis of dreaming as a target phenomenon for philosophy of mind.
For Malcolm, the denial of dream experience followed from the conceptual analysis of sleep: “if a person is in any state of consciousness it logically follows that he is not sound asleep” (Malcolm 1956: 21). Following some remarks of Wittgenstein’s (1953: 184; see Chihara 1965 for discussion), Malcolm claimed
the concept of dreaming is derived, not from dreaming, but from descriptions of dreams, i.e., from the familiar phenomenon that we call “telling a dream”. (Malcolm 1959:55)
Malcolm argued that retrospective dream reports are the sole criterion for determining whether a dream occurred and there is no independent way of verifying dream reports. While first-person, past-tense psychological statements (such as “I felt afraid”) can at least in principle be verified by independent observations (but see Canfield 1961; Siegler 1967; Schröder 1997), he argued dream reports (such as “in my dream, I felt afraid”) are governed by different grammars and merely superficially resemble waking reports. In particular, he denied dream reports imply the occurrence of experiences (such as thoughts, feelings, or judgements) in sleep:
If a man had certain thoughts and feelings in a dream it no more follows that he had those thoughts and feelings while asleep, than it follows from his having climbed a mountain in a dream that he climbed a mountain while asleep. (Malcolm 1959/1962: 51–52)
What exactly Malcolm means by “conscious experience” is unclear. Sometimes he seems to be saying that conscious experience is conceptually tied to wakefulness (Malcolm 1956); other times he claims that terms such as mental activity or conscious experience are vague and it is senseless to apply them to sleep and dreams (Malcolm 1959: 52).
Malcolm’s analysis of dreaming has been criticized as assuming an overly strict form of verificationism and a naïve view of language and conceptual change. A particularly counterintuitive consequence of his view is that there can be no observational evidence for the occurrence of dreams in sleep aside from dream reports. This includes behavioral evidence such as sleepwalking or sleeptalking, which he thought showed the person was partially awake; as he also thought dreams occur in sound sleep, such sleep behaviors were largely irrelevant to the investigation of dreaming proper. He also claimed adopting a physiological criterion of dreaming (such as EEG measures of brain activity during sleep) would change the concept of dreaming, which he argued was tied exclusively to dream reporting. This claim was particularly radical as it explicitly targeted the discovery of REM sleep and its association with dreaming (Dement & Kleitman 1957), which is commonly regarded as the beginning of the science of sleep and dreaming. Malcolm’s position was that the very project of a science of dreaming was misguided.
Contra Malcolm, most assume that justification does not depend on strict criteria with the help of which the truth of a statement can be determined with absolute certainty, but “on appeals to the simplicity, plausibility, and predictive adequacy of an explanatory system as a whole” (Chihara & Fodor 1965: 197). In this view, behavioral and/or physiological evidence can be used to verify dream reports (Ayer 1960) and the alleged principled difference between dream reports and other first-person, past-tense psychological sentences (Siegler 1967; Schröder 1997) disappears.
Putnam noted that Malcolm’s analysis of the concept of dreaming relies on the dubious idea that philosophers have access to deep conceptual truths that are hidden to laypeople:
the lexicographer would undoubtedly perceive the logical (or semantical) connection between being a pediatrician and being a doctor, but he would miss the allegedly “logical” character of the connection between dreams and waking impressions. […] this “depth grammar” kind of analyticity (or “logical dependence”) does not exist. (Putnam 1962 [1986]: 306)
Nagel argued that even if one accepts Malcolm’s analysis of the concept of dreaming,
it is a mistake to invest the demonstration that it is impossible to have experiences while asleep with more import than it has. It is an observation about our use of the word “experience”, and no more. It does not imply that nothing goes on in our minds while we dream. (Nagel 1959: 114)
Whether dream thoughts, feelings or beliefs should count as real instances of their kind now becomes an open question, and in any case there is no conceptual contradiction involved in saying one has experiences while asleep and dreaming.
To ask about dream experience is also to ask whether there is something it is like to dream during sleep as opposed to there just being something it is like to remember dreaming after awakening. Dennett’s (1976, 1979) cassette theory says dreams are the product of instantaneous memory insertion at the moment of the awakening, as if a cassette with pre-scripted dreams had been inserted into memory, ready for replay. Dennett claims the cassette theory and the view that dreams are experiences can deal equally well with empirical evidence for instance on the relationship between dreaming and REM sleep. The cassette theory is preferable because it is more parsimonious, positing only an unconscious dream composition process rather than an additional conscious presentation process in sleep. For Dennett, the important point is that it is impossible to distinguish between the two rival theories based on dream recall; the question of dream experience should be settled by independent empirical evidence.
While Dennett shares Malcolm’s skepticism about dream experience, this latter claim is diametrically opposed to Malcolm’s rejection of a science of dreaming. For Dennett, the unreliability of dream recall also is not unique, but exemplifies a broader problem with memory reports: we generally cannot use retrospective recall to distinguish conscious experience from memory insertion (Dennett 1991; see also Emmett 1978).
An earlier and much discussed (Binz 1878; Goblot 1896; Freud 1899; Hall 1981; Kramer 2007:22–24) version of Dennett’s cassette theory goes back to Maury’s (1861) description of a long and complex dream about the French revolution that culminated in his execution at the guillotine, at which point Maury suddenly awoke to find that the headboard had fallen on his neck. Because the dream seemed to systematically build up to this dramatic conclusion, which in turn coincided with a sudden external event, he suggested that such cases were best explained as instantaneous memory insertions experienced at the moment of awakening. Similarly, Gregory (1916) described dreams are psychical explosions occurring at the moment of awakening.
The trustworthiness of dream reports continues to be contentious. Rosen (2013) argues that dream reports are often fabricated and fail to accurately describe experiences occurring during sleep. By contrast, Windt (2013, 2015a) argues that dream reports can at least under certain conditions (such as in laboratory studies, when dreams are reported immediately after awakening by trained participants) be regarded as trustworthy sources of evidence with respect to previous experience during sleep.
Unlike Malcolm, many believe that whether dreams are experiences is an empirical question; and unlike Dennett, the predominant view is that the empirical evidence does indeed support this claim (Flanagan 2000; Metzinger 2003; Revonsuo 2006; Rosen 2013; Windt 2013, 2015a).
A first reason for thinking that dreams are experiences during sleep is the relationship between dreaming and REM (rapid eye movement) sleep. Researchers in the 1950s discovered that sleep is not a uniform state of rest and passivity, but there is a sleep architecture involving different stages of sleep that is relatively stable both within and across individuals (Aserinsky & Kleitman 1953, 1955; Dement & Kleitman 1957). Following sleep onset, periods of non-REM (or NREM) sleep including slow wave sleep (so called because of the presence of characteristic slow-wave, high-voltage EEG activity) are followed by periods of high-frequency, low-voltage activity during REM sleep. EEG measures from REM sleep strongly resemble waking EEG. REM sleep is additionally characterized by rapid eye movements and a near-complete loss of muscle tone (Dement 1999: 27–50; Jouvet 1999).
The alignment between conscious experience on the one hand and wake-like brain activity and muscular paralysis on the other hand would seem to support the experiential status of dreams as well as explain the outward passivity that typically accompanies them. Reports of dreaming are in fact much more frequent following REM (81.9%) than NREM sleep awakenings (43%; Nielsen 2000). REM reports tend to be more elaborate, vivid, and emotionally intense, whereas NREM reports tend to be more thought-like, confused, non-progressive, and repetitive (Hobson et al. 2000). These differences led to the idea that REM sleep is an objective marker of dreaming (Dement & Kleitman 1957; Hobson 1988: 154).
Attempts to identify dreaming with mental activity during REM sleep have not, however, been successful, and many now hold that dreams can occur in all stages of sleep (e.g., Antrobus 1990; Foulkes 1993b; Solms 1997, 2000; Domhoff 2003; Nemeth & Fazekas 2018). In recent years there has been renewed interest in NREM sleep for the study of dreaming (Noreika et al. 2009; Siclari et al. 2013, 2017). This suggests the inference from the physiology of REM sleep to the phenomenology of dreaming is not straightforward.
A second line of evidence comes from lucid dreams, or dreams in which one knows one is dreaming and often has some level of dream control (Voss et al. 2013; Voss & Hobson 2015; Baird et al. 2019). The term lucid dreaming was coined by van Eeden (1913), but Aristotle ( On Dreams ) already noted that one can sometimes be aware while dreaming that one is dreaming.
Scientific evidence that lucid dreaming is real and a genuine sleep phenomenon comes from laboratory studies (Hearne 1978; LaBerge et al. 1981) showing lucid dreamers can use specific, pre-arranged patterns of eye movements (e.g., right-left-right-left) to signal in real-time that they are now lucid and engaging in dream experiments. These signals are clearly identifiable on the EOG and suggest a correspondence between dream-eye movements and real-eye movements (as predicted by the so-called scanning hypothesis ; see Dement & Kleitman 1957; Leclair-Visonneau et al. 2010). Retrospective reports confirm that the dreamer really was lucid and signalled lucidity (Dresler et al. 2012; Stumbrys et al. 2014).
Signal-verified lucid dreams have been used to study muscular activity accompanying body movements in dreams (Erlacher et al. 2003; Dresler et al. 2011), for advanced EEG analysis of brain activity during lucid dreaming (Voss et al. 2009), and imaging studies (Dresler et al. 2011, 2012). Eye signals can also be used to measure the duration of different activities performed in lucid dreams; contrary to the cassette theory, lucid dreams have temporal extension and certain dream actions even seem to take slightly longer than in waking (Erlacher et al. 2014). There have also been attempts to induce lucidity through non-invasive electrical stimulation during sleep (Stumbrys et al. 2013; Voss et al. 2014). The combination of signal-verified lucid dreaming with volitional control over dream content, retrospective report, and objective sleep measures has been proposed to provide controlled conditions for the study of conscious experience in sleep and a new methodology for investigating the relationship between conscious experience and neurophysiological processes (Baird et al.2019).
A third line of evidence (Revonsuo 2006: 77) comes from dream-enactment behavior (Nielsen et al. 2009), most prominently in patients with REM-sleep behavior disorder (RBD; Schenck & Mahowald 1996; Schenck 2005; Leclair-Visonneau et al. 2010). Due to a loss of the muscular atonia that accompanies REM sleep in healthy subjects, these patients show complex, seemingly goal-directed outward behaviors such as running or fighting off an attacker during REM sleep. Retrospective dream reports often match these behaviors, suggesting that patients literally act out their dreams during sleep.
While persuasive, these lines of evidence might not satisfy skeptics about dream experience. They might worry that results from lucid dreaming and dream enactment do not generalize to ordinary, non-lucid dreams; they might also construe alternative explanations that do not require conscious experience in sleep. There are also methodological concerns, for instance about how closely sleep-behaviors actually match dream experience. A key issue is that to support the experiential status of dreams, evidence from sleep polysomnography, signal verified lucid dreams, or sleep behavior requires convergence with retrospective dream reports. This means trusting dream reports is built into any attempt to empirically resolve the question of dream experience – which then invites the familiar skeptical concerns. Again, an anti-skeptical strategy may be to appeal to explanatory considerations. In this view, the convergence of dream reports and objective polysomnographic or behavioral observations is best explained by the assumption that dreams are experiences in sleep, and this assumption is strengthened by further incoming findings. This strategy places dream reports at the center of scientific dream research while avoiding the contentious claim that their trustworthiness, and with it the experiential status of dreams, can be demonstrated conclusively by independent empirical means (Windt 2013, 2015a).
Even where philosophers agree dreams are experiences, they often disagree on how exactly to characterize dreaming relative to wake-state psychological terms. Often, questions about the ontology of dreaming intersect with epistemological issues. Increasingly, they also incorporate empirical findings.
The standard view is that dreams have the same phenomenal character as waking perception in that they seemingly put us in contact with mind-independent objects, yet no such object is actually being perceived. This means dreams count as hallucinations in the philosophical sense (Crane & French 2017; Macpherson 2013). Even if, in a particularly realistic dream, my visual experience was exactly as it would be if I were awake (I could see my bedroom, my hands on the bed sheets, etc.), as long as my eyes were closed during the episode, I would not, literally, be seeing anything.
There is some controversy in the psychological literature about whether dreams should be regarded as hallucinations. Some believe the term hallucination should be reserved for clinical contexts and wake-state pathologies (Aleman & Larøi 2008: 17; but see ffytche 2007; ffytche et al. 2010).
The view that dreams involve hallucinations is implicit in Descartes’ assumption that even when dreaming,
it is certain that I seem to see light, hear a noise, and feel heat; this cannot be false, and this is what in me is properly called perceiving ( sentire ). (Descartes 1641: II.9)
It also lies at the heart of Aristotle’s ( On Dreams ) assumption that dreams result from the movements of the sensory organs that continue even after the original stimulus has ceased. He believed that in the silence of sleep, these residual movements result in vivid sensory imagery that is subjectively indistinguishable from genuine perception (see also Dreisbach 2000; Barbera 2008).
The assumption of phenomenological equivalence between dream and waking experience can also be found in Berkeley’s (1710: I.18) idealist claim that the existence of external bodies is not necessary for the production of vivid, wake-like perceptual experience. Similarly, Russell defended sense-data theory by noting that in dreams,
I have all the experiences that I seem to have; it is only things outside my mind that are not as I believe them to be while I am dreaming. (Russell 1948: 149–150)
Elsewhere, he argued dreams and waking life
must be treated with equal respect; it is only by some reality not merely sensible that dreams can be condemned. (Russell 1914: 69)
Hume was less clear on this matter, proposing that dreams occupy an intermediate position between vivid and largely non-voluntary sensory impressions and ideas, or “the faint images of previous impressions in thinking and reasoning” (Hume 1739: 1.1.1.1). On the one hand, as mere creatures of the mind, Hume wanted to categorize dreams as ideas. On the other hand, he acknowledged that in sleep, “our ideas can approach the vivacity of sensory impressions” (Hume 1739: 1.1.1.1). Dreams do not fit comfortably into Hume’s attempt to draw a dichotomous distinction between impressions, including perception, and ideas, including sensory imagination (Ryle 1949; Waxman 1994; Broughton 2006).
Phenomenologists often focus not so much on the quality of dream imagery as on the overall character of experience, noting that dreams are experienced as reality; as in waking perception, we simply feel present in a world. This also sets dreams apart from waking fantasy and daydreams (Husserl 1904/1905; Uslar 1964; Conrad 1968; Globus 1987: 89.
At its strongest, the hallucination view claims that dreaming and waking experience are identical in both the quality of sensory imagery and their overall, self-in-a-world structure (Revonsuo 2006: 84). This claim is central to the virtual reality metaphor , according to which consciousness itself is dreamlike and waking perception a kind of online hallucination modulated by the senses (Llinás & Ribary 1994; Llinás & Paré 1991; Revonsuo 2006; Metzinger 2003, 2009).
This seems to be empirically supported. Neuroimaging studies (Dang-Vu et al. 2007; Nir & Tononi 2010; Desseilles et al. 2011) show that the predominance of visual and motor imagery as well as strong emotions in dreams is paralleled by high activation of the corresponding brain areas in REM sleep, which may exceed waking; at the same time, the cognitive deficits often thought to characterize dreams such as the loss of self-awareness, the absence of critical thinking, delusional reasoning, and mnemonic deficits fit in well with the comparative deactivation of frontal areas (Hobson et al. 2000). Hobson (1988, Hobson et al. 2000) has argued that the vivid, hallucinatory character of dreaming results from the fact that in REM sleep, the visual and motor areas are activated in the same way as in waking perception, the sole difference being dreams’ dependence on internal signal generation. Horikawa and colleagues (2013) used neuroimaging data from sleep onset to predict the types of objects described in mentation reports, which they took to support the perceptual equivalence between dreaming and waking.
Generally, versions of the hallucination view that suggest dreams replicate all aspects of waking perception are too vague to be informative. Especially for subtle perceptual activities (such as visual search), we might not know enough about dream phenomenology to make any strong claims (Nielsen 2010). Specifying points of similarity leads to a more informative and precise, but likely also more nuanced view. Dreams are heterogeneous, and some might be more perception-like while others resemble imagination (Windt 2015a). There might also be differences between or even within specific types of imagery. For example, visual imagery might be quite different from touch sensations, which tend to be rare in dreams (Hobson 1988). Visual dream imagery might overall resemble waking perception but lack color saturation, background detail and focus (Rechtschaffen & Buchignani, 1992). Classifying dreams as either hallucinatory or imaginative is further complicated by the fact that there is strong overlap in cortical activity associated with both visual imagery and perception (Zeidman & Maguire, 2016). This means even a strong overlap in cortical activity between, say, visual dream imagery and visual perception does not necessarily set dreaming apart from waking imagination.
This is also true for evidence on eye movements in dreams. LaBerge and colleagues (2018) recently showed that eye tracking of objects is smooth in lucid dreaming and perceiving, but not in imagining. Drawing from this evidence, Rosen (forthcoming) suggests many dreams mimic the phenomenology of interacting with a stable world, including eye movements and visual search. Others argue we should not analogize dream imagery to mind-independent, scannable objects and that eye movements might instead be implicated in the generation of dream imagery (Windt 2018).
Another way to make sense of the claim that dreaming has the same phenomenal character as waking perception is to say some kinds of dream imagery are illusory: they involve misperception of an external object as having different properties than it actually has (cf. Smith 2002; Crane & French 2017). The illusion view disagrees with the hallucination view on whether dreams have a contemporaneous external stimulus source.
The illusion view has fallen out of favor but has a long history. The Ancients believed dreams have bodily sources. This idea underlies the practice of using dreams to diagnose illness, as practiced in the shrines at Epidaurus (Galen On Diagnosis in Dreams ; van de Castle 1994). Aristotle ( On Dreams ) thought some dreams are caused by indigestion, and Hobbes adopted this view, claiming different kinds of dreams could be traced to different bodily sensations. For instance, “lying cold breedeth Dreams of Feare, and raiseth the thought and Image of some fearfull object” (Hobbes 1651: 91).
Appeals to the bodily sources of dreaming became especially popular in the 19 th and early 20 th centuries. Many believed specific dream themes such as flying were linked to sleeping position (Macnish 1838; Scherner 1861; Vold 1910/1912; Ellis 1911) and realizing, in sleep, that one’s feet are not touching the ground (Bergson 1914).
There were also attempts to explain the phenomenology of dreaming by appealing to the absence of outward movement. The lack of appropriate feedback and of movement and touch sensations was thought to cause dreams of being unable to move (Bradley 1894) or of trying but failing to do something (Gregory 1918).
Some proponents of the “ Leibreiztheorie ” (or somatic-stimulus theory) of dreaming attempted to go beyond anecdotal observations to conduct controlled experiments. Weygandt (1893) investigated the influence of various factors including breathing, blood circulation, temperature changes, urge to urinate, sleeping position, and visual or auditory stimulation during sleep on dream content (see Schredl 2010 for details). Singer (1924) proposed experiments on stimulus incorporation in dreams can inform claims on the ontology of dreaming: If dreams are sensations, a particular auditory stimulus should increase the frequency of dreams in nearby sleepers as well as the frequency of sound in their dreams, and it should decrease the range of quality and intensity of these dreams, making them overall more similar and predictable.
Newer studies provide evidence for the incorporation of external stimuli in dreams, including light flashes, sounds, sprays of water applied to the skin (Dement & Wolpert 1958), thermal (Baldridge 1966), electrical (Koulack 1969), and verbal stimuli (Berger 1963; Breger et al. 1971; Hoelscher et al., 1981), as well as blood pressure cuff stimulation on the leg (Nielsen et al. 1995; Sauvageau et al. 1998).
Muscular activity also often leaves its mark on dreams. It occurs throughout sleep but is especially frequent in REM sleep, mostly in the form of twitching but occasionally also in the form of larger, seemingly goal-directed movements (Blumberg 2010; Blumberg & Plumeau 2016). The relation between outward and dream movements is complex: in some cases, outward movements might mirror dream movements, while in others, sensory feedback might prompt dream imagery (Windt 2018).
Generally, it seems external and bodily stimuli can be related to varying degrees to dream and sleep onset imagery (Nielsen 2017; Windt 2018; Windt et al. 2016). Some of these cases appear to fit the concept of illusion, as in when the sound of the alarm clock is experienced, in a dream, as a siren, or when blood pressure cuff inflation on the leg leads to dreams of wearing strange shoes (Windt 2018; for these and other examples, see Nielsen et al. 1995). In other cases, such as when blood pressure cuff stimulation on the leg prompts a dream of seeing someone else’s leg being run over, describing this as illusory misperception might be less straightforward.
Saying that dreams can be prompted by external stimuli and that in some cases these are best described as illusions is different from the stronger claim, sometimes advanced by historical proponents of somatic-stimulus theory, that dreams generally are caused by external or bodily stimuli. As an example of the stronger claim, consider Wundt’s proposal that the
ideas which arise in dreams come, at least to a great extent, from sensations, especially from those of the general sense, and are therefore mostly illusions of fancy, probably only seldom pure memory ideas which hence become hallucinations. (Wundt 1896: 179)
This claim is likely too strong. It is also likely that appeals to external or bodily stimuli on their own cannot fully explain dream imagery, including when and how external stimuli are incorporated in dreams. Sensory incorporation in dreams is often hard to predict and indirect; associated imagery seems related not just to stimulus intensity, but also to short- and long term memories. A full explanation of dream content additionally has to take the cognitive and memory sources of dreaming into account (Windt 2018; Nielsen 2017; cf. Silberer 1919).
The most important rival to the hallucination view is that dreams are imaginative experiences (Liao & Gendler 2019; Thomas 2014). This can mean dream imagery involves imaginings rather than percepts (including hallucinations or illusions; McGinn 2004), that dream beliefs are imaginative and not real beliefs (Sosa 2007), or both (Ichikawa 2008, 2009). An important advantage is that by assimilating dreams to commonplace mental states such as waking fantasy and daydreaming, rather than a rare and often pathological occurrence such as hallucinations, it provides a more unified account of mental life (Stone 1984). However, the reasons for adopting the imagination view are diverse, and dreams have been proposed to resemble imaginings and differ from perception along a number of dimensions (e.g. McGinn 2004, 2005a,b; Thomas 2014). This issue is complicated by the fact that there is little agreement on the definition of imagination and its relation to perception (Kind 2013).
One way is to deny dreams involve presence or the feeling of being in a world, which many believe is central to waking perception. Imagination theorists compare the sense in which we feel present in our dreams to cognitive absorption, as when we are lost in a novel, film, or vivid daydream (Sartre 1940; McGinn 2004; but see Hering 1947; Globus 1987). Some argue that reflexive consciousness or meta-awareness (as in lucid dreams) interrupts cognitive absorption and terminates the ongoing dream (Sartre 1940), essentially denying lucid dreams are possible.
Another issue is whether dreams are subject to the will (Ichikawa 2009). Imagination is often characterized as active and under our control (Wittgenstein 1967: 621, 633), involving “a special effort of the mind” (Descartes 1641: VI, 2), whereas perception is passive. Because dreams just seem to happen to us without being under voluntary control, they present an important challenge for the imagination view. Ichikawa (2009) argues lucid control dreams show dreams are generally subject to the will even where they are not under deliberate control.
Dreams are widely described as more indeterminate than waking perception (James 1890: 47; Stone 1984). In scientific dream research, vagueness is regarded as one of three main subtypes of bizarreness (Hobson 1988; Revonsuo & Salmivalli 1995). An example are dream characters who are identified not by their behavior or looks, but by just knowing (Kahn et al. 2000, 2002; Revonsuo & Tarkko 2002). Dreams are also attention-dependent and lack foreground-background structure (Thompson 2014); while it is tempting to construe the dream world as rich in detail, there is no more to dreams than meets the eye, and many think dream experience is exhausted by what is the focus of selective attention (Hunter 1983; Thompson 2014).
Indeterminacy is also related to the question of whether we dream in color or in black and white. Based on a review of historical and recent studies, Schwitzgebel (2002, 2011) argues there has been a shift in theories on dream color that coincides with the rise first of black-and-white and then color television. He argues it is unlikely that dreams themselves changed from colored to black and white and back to colored, proposing that a change in opinion is a more plausible explanation. Maybe dreams were either black and white or colored all along; or maybe they are indeterminate with respect to color, as may be the case for imagined or fictional objects; were this the case, it would strengthen the imagination view (Ichikawa 2009). Schwitzgebel’s main point is that reports of colored dreaming are unreliable and our opinions about dreams can be mistaken (but see Windt 2013, 2015a). This relates to Schwitzgebel’s (2011; Hurlburt & Schwitzgebel 2007) general skepticism about the reliability of introspection.
The issue of dream color has led to a number of follow-up studies (Schwitzgebel 2003; Schwitzgebel et al. 2006; Murzyn 2008; Schredl et al. 2008; Hoss 2010). They suggest most people dream in color and a small percentage describe grayscale or even mixed dreams (Murzyn 2008) or dreams involving moderate color saturation (Rechtschaffen and Buchignani 1992). Indeterminacy is rarely reported.
The imagination view has consequences for Cartesian dream skepticism. If dream pain does not feel like real pain, there is a fail-safe way to determine whether one is now dreaming: one need only pinch oneself (Nelson 1966; Stone 1984; but see Hodges & Carter 1969; Kantor 1970). As Locke put it,
if our dreamer pleases to try, whether the glowing heat of a glass furnace, be barely a wandering imagination in a drowsy man’s fancy, by putting his hand into it, he may perhaps be wakened into a certainty greater than he could wish, that it is something more than bare imagination. (Locke 1689: IV.XI.8)
If dreaming feels different from waking, this raises the question why we tend to describe dreams in the same terms as waking perception. Maybe this is because most people haven’t thought about these matters and they would find the imagination view plausible if they considered it (Ichikawa 2009). Or maybe
it is just because we all know that dreams are throughout un like waking experiences that we can safely use ordinary expressions in the narration of them. (Austin 1962: 42)
Some authors classify dreams as imaginings while acknowledging they feel like perceiving. For example, Hobbes describes dreams as “the imaginations of them that sleep” (Hobbes 1651: 90), and imagination as a “ decaying sense ” (Hobbes 1651: 88). Yet he also uses the concepts of imagination and fancy to describe perception and argues “their appearance to us is Fancy, the same waking, that dreaming” (Hobbes 1651: 86).
In the scientific literature, the imagination view is complemented by cognitive theories. Foulkes (1978: 5) describes dreaming as a form of thinking with its own grammar and syntax, but allows that dream imagery is sufficently perception-like to deceive us. Domhoff’s neurocognitive model of dreaming (2001, 2003) emphasizes the dependence of dreaming on visuospatial skills and on a network including the association areas of the forebrain. The theory draws from findings on the partial or global cessation of dreaming following brain lesions (cf. Solms 1997, 2000), evidence that dreaming develops gradually and in tandem with visuospatial skills in children (Foulkes 1993a, 1999; but see Resnick et al. 1994), and results from dream content analysis supporting the continuity of dreaming with waking concerns and memories (the so-called continuity hypothesis ; see Domhoff 2001, 2003; Schredl & Hofmann 2003; Schredl 2006; see also Nir & Tononi 2010).
A number of researchers have begun to consider dreaming in the context of theories of mind wandering. Mind wandering is frequent in waking and involves spontaneous thoughts that unfold dynamically and are only weakly constrained by ongoing tasks and environmental demands (Schooler et al. 2011; Smallwood & Schooler 2015; Christoff et al. 2016). Based on phenomenological and neurophysiological similarities, dreams have been proposed to be an intensified form of waking mind wandering (Pace-Schott 2007, 2013; Domhoff 2011; Wamsley 2013; Fox et al. 2013). This basic idea seems to have been anticipated by Leibniz, who noted that the spontaneous formation of visions in dreams surpasses the capacity of our waking imagination (Leibniz, Philosophical Papers and Letters , Vol. I, 177–178).
The analogy between dreams and waking mind wandering has been discussed in the context of cognitive agency. Metzinger (2013a,b, 2015) describes dreams and waking mind wandering as involving a cyclically recurring loss of mental autonomy, or the ability to deliberately control one’s conscious thought processes. Dreams and waking mind wandering are not mental actions but unintentional mental behaviors, comparable to subpersonal processes such as breathing or heartbeat. Because dreaming and waking mind wandering make up a the majority of our conscious mental lives, he argues that cognitive agency and mental autonomy are the exception, not the rule.
This raises the question of how to make sense of lucid control dreams, which involve both meta-awareness and agency. Windt and Voss (2018) argue that in such cases, spontaneous processes including imagery formation co-exist alongside more deliberate, top-down control; they also argue metacognitive insight and control themselves can have spontaneous elements. This suggests spontaneity and control are not opposites, but a more complex account is needed. Possibly, certain dreams and instances of waking mind wandering can be both spontaneous and agentive.
The analogy with mind wandering might help move forward the debate on the ontology of dreaming. In this debate, a common assumption is that dreams can be categorized as either hallucinatory or imaginative. Yet the application of these terms to dreams quickly runs into counterexamples and it is unclear they are mutually exclusive. One option is pluralism (Rosen 2018b), in which some aspects of dreaming are hallucinatory, others imaginative, and yet again others illusory. Another is that dreams are sui generis, combining aspects associated with wake states such as hallucinating, imagining, or perceiving in a novel manner without mimicking them completely. Windt (2015a) proposes that mind wandering, which describes a range of mental states loosely characterized by their spontaneous and dynamic character, might be particularly suitable for the characterization of dreaming precisely because that term leaves open more specific questions on the phenomenology of dreaming, allowing for variation in control, determinacy, and so on. This might be a good starting point for describing what is unique about dreaming while also acknowledging continuities across sleep-wake states and capitalizing on the strengths of the hallucination, illusion, imagination, and cognitive views.
The second strand of the imagination view argues that dream beliefs are not real beliefs, but propositional imaginings. This may or may not be combined with the claim that dream imagery is imaginative rather than perceptual (Sosa 2007; Ichikawa 2009).
Denying that dream beliefs have the status of real beliefs only makes sense before the background of a specific account of what beliefs are and how they are distinguished from other mental states such as delusions or propositional imaginings. For instance, Ichikawa (2009) argues that if we follow interpretationist or dispositionalist accounts of belief, dream beliefs fall short of real beliefs. He claims dream beliefs lack connection with perceptual experience and fail to motivate actions; consequently, they do not have the same functional role as real beliefs. Moreover, we cannot ascribe dream beliefs to a person by observing them lying asleep in bed. Dream beliefs are often inconsistent with longstanding waking beliefs and acquired and discarded without any process of belief revision (Ichikawa 2009).
This analysis of dream beliefs has consequences for skepticism. If dream beliefs are propositional imaginings, then we do not falsely believe while dreaming that we are now awake, but only imagine that we do (Sosa 2007). It is not clear though that this protects us from deception. If dream beliefs fall short of real beliefs, this might even make the specter of dream deception more worrisome: in mistaking dream beliefs for the real thing, we would now be deceived about the status of our own mental states (Ichikawa 2008).
It is also not clear whether the same type of argument extends to mental states other than beliefs. As Lewis points out, a person might
in fact believe or realize in the course of a dream that he was dreaming, and even if we said that, in such case, he only dreamt that he was dreaming, this still leaves it possible for someone who is asleep to entertain at the time the thought that he is asleep. (Lewis 1969: 133)
Mental states other than believing such as entertaining, thinking, or minimally appraisive instances of taking for granted might be sufficient for deception (Reed 1979).
The debate about dream beliefs is paralleled by a debate about whether delusions are beliefs or imaginings (see Currie 2000; Currie & Ravenscroft 2002; McGinn 2004; Bayne & Pacherie 2005; Bortolotti 2009; Gendler 2013). Both debates might plausibly inform each other, especially as dreams are sometimes proposed to be delusional (Hobson 1999).
3. Dreaming and theories of consciousness
Dreams are a global state of consciousness in which experience arises under altered behavioral and neurophysiological conditions as compared to standard wakefulness; unlike other altered states of consciousness (such as drug-induced or deep meditative states) and pathological wake states (such as psychosis or neurological syndromes), dreams occur spontaneously and regularly in healthy subjects. For both reasons, many regard dreams as a test case for theories of consciousness or even an ideal model system for consciousness research (Churchland 1988; Revonsuo 2006).
Existing proposals differ on the phenomenology of dreaming: referring to dream bizarreness, Churchland describes dream experience as robustly different from waking, whereas Revonsuo argues dreaming is similar to waking and the purest form of experience:
the dreaming brain brings out the phenomenal level of organization in a clear and distinct form. Dreaming is phenomenality pure and simple, untouched by external physical stimulation or behavioural activity. (Revonsuo 2006: 75)
Revonsuo argues dreaming reveals the basic, state-independent structure of consciousness to be immersive: “dreaming depicts consciousness first and foremost as a subjective world- for-me ” (Revonsuo 2006: 75). This leads him to introduce the “world-simulation metaphor of consciousness”, according to which consciousness itself is essentially simulational and dreamlike. This is taken to support internalism about conscious experience.
This latter claim is also contentious. Noë (2004: 213) argues that phenomenological differences between dreaming and waking (such as greater instability of visual dream imagery) result from the lack of dynamic interaction with the environment in dreams. He proposes this shows that neural states are sufficient for dreaming but denies they are also sufficient for perceptual experience.
A possible problem for both views is their reliance on background assumptions about the phenomenology of dreaming and its disconnection from environmental stimuli and bodily sensations. Windt (2015a, 2018) argues both internalism and externalism mistakenly assume dreams to be isolated from external sensory input and own-body perception; she believes both the phenomenology of dreaming and its correlation with external stimuli are complex and variable. She argues the analysis of dreaming does not clearly support either side in the debate on internalism vs externalism (but see Rosen 2018a). Generally, in the absence of a well worked out theory of dreaming and its sleep-stage and neural correlates, proposals for using dreaming as a model system or test case run the risk of relying on an oversimplified description of the target phenomenon (Windt & Noreika 2011).
Recent accounts appealing to generative models and predictive processing (Clark 2013b; Hohwy 2013) suggest a new, unified account of perception, imagination, and dreaming. In these accounts, different mental states, including perception and action, embody different strategies of hypothesis testing and prediction error minimization. Perception is the attempt to model the hidden external causes of sensory stimuli; action involves keeping the internal model stable while changing the sensory input. Clark argues that on such a model,
systems that know how to perceive an object as a cat are thus systems that, ipso facto , are able to use a top-down cascade to bring about the kinds of activity pattern that would be characteristic of the presence of a cat. […] Perceivers like us, if this is correct, are inevitably potential dreamers and imaginers too. Moreover, they are beings who, in dreaming and imagining, are deploying many of the very same strategies and resources used in ordinary perception. (Clark 2013a: 764)
Predictive processing accounts have also been used to explain specific features of dreaming. Bizarreness has been associated with the comparative lack of external stimulus processing, implying dream imagery is relatively unconstrained by prediction errors (cf. Hobson & Friston 2012; Fletcher & Frith 2008; Bucci & Grasso 2017). Windt (2018) suggests a predictive processing account of dream imagery generation that links bodily self-experience to own-body perception and subtle motor behaviors such as twitching in REM sleep (Blumberg 2010; Blumberg & Plumeau 2016). She argues that movement sensations in dreams, in relation to REM-sleep related muscle twitching, involve a form of bodily self-sampling in which coordinated muscular activity contributes to the generation and maintenance of a body model. This is important because in predictive processing accounts neither the bodily nor the external causes of sensory inputs are known; at the same time, having an accurate body model is a prerequisite for action, requiring the system to disambiguate between self- and other generated changes to sensory inputs. Especially in early development, sleep might provide the ideal conditions for exploring one’s own body via subtle but coordinated muscular activity while processing of visual and auditory stimuli is reduced.
Dreams have also been suggested as a test case for whether phenomenal consciousness can be divorced from cognitive access (e.g., Block 2007; but see Cohen & Dennett 2011). Sebastián (2014a) argues that dreams provide empirical evidence that conscious experience can occur independently of cognitive access. This is because during (non-lucid) REM-sleep dreams, the dorsolateral prefrontal cortex (dlPFC) as the most plausible mechanism underlying cognitive access is selectively deactivated (see also Pantani et al. 2018). This would challenge theories linking conscious experience to access, such as higher-order-thought theory (Sebastián 2014b). However, both the hypoactivation of the dlPCF in REM sleep and its association with cognitive access have been debated. Fazekas and Nemeth (2018) suggest that certain kinds of cognitive access may be independent of dlPFC activation, necessitating a more complex account.
Dreaming has been suggested as a model system not just of waking consciousness in general, but also of psychotic wake states in particular. The analogy between dreaming and madness has a long philosophical history (Plato, Phaedrus ; Kant 1766; Schopenhauer 1847) and finds particularly stark expression in Hobson’s claim that “dreaming is not a model of a psychosis. It is a psychosis. It’s just a healthy one” (Hobson 1999: 44). Gottesmann (2006) proposes dreaming as a neurophysiological model of schizophrenia. There is a rich discussion on the theoretical and methodological implications of dream research for psychiatry (see Scarone et al. 2007; d’Agostino et al. 2013; see Windt & Noreika 2011 as well as the other papers in this special issue) and a number of studies have investigated differences in dream reports from schizophrenic and healthy subjects (Limosani et al. 2011a,b).
Rather than likening dreaming to waking in general or specific wake states such as psychosis, there have also been attempts to compare specific dream phenomena to wake-state delusions. Gerrans (2012, 2013, 2014) focuses on character misidentification in dreams and delusions of hyperfamiliarity (such as the Frégoli delusion, in which strangers are mistakenly identified as family members, and déjà vu ) to argue that anomalous experience and faulty reality testing both play a role in delusion formation. Rosen (2015) analyzes instances of thought insertion and of auditory hallucinations, which are key symptoms of schizophrenia, to raise broader questions about the altered sense of agency in dreams as compared to waking.
Philosophers have focused almost exclusively on dreaming, largely leaving to the side questions about dreamless sleep including whether it is uniformly unconscious. In recent years there has been a surge of interest in the possibility of dreamless sleep experience and foundational issues about the definition of sleep and waking. This has been paralleled by growing interest in dreaming in NREM sleep.
Conceptually, interest in dreamless sleep experience has been facilitated by the precise definition of dreaming offered by simulation views (Revonsuo et al. 2015). If dreams are immersive sleep experiences characterized by their here -and- now structure, it makes sense to ask whether this is true for all or just a subset of sleep-related experiences and whether non-immersive sleep experiences exist. By contrast, if dreaming is broadly identified with any conscious mentation in sleep (Pagel et al. 2001), there is no conceptual space for dreamless sleep experience.
Following Thompson's (2014, 2015) discussion of dreamless sleep in Indian and Buddhist philosophy, Windt and colleagues (2016; see also Windt 2015b) introduce a framework for different kinds of dreamless sleep experience ranging from thinking and isolated imagery, perception, or bodily sensations, where these lack integration into a scene, to minimal kinds of experience lacking imagery or specific thought contents. A possible example of minimal phenomenal experience in sleep are white dreams, where people report having had experiences during sleep but cannot remember any details. Taken at face value, some white dream reports might describe experiences that lack reportable content (Windt 2015b); others might describe forgotten dreams or dreams with degraded content (Fazekas et al. 2018). Another example are reports of witnessing dreamless sleep, as described in certain meditation practices. This state is said to involve non-conceptual awareness of sleep, again in the absence of imagery or specific thought contents, and loss of sense of self (Thompson 2014, 2015). Some schools in Buddhist philosophy explain claims of deep and dreamless sleep by saying we never fully lose consciousness in sleep (Prasad 2000, 66; and Thompson 2014, 2015).
Empirically, interest in dreamless sleep experience is paralleled by increasing interest in experiences in NREM sleep (Fazekas et al. 2018). Most researchers now accept that dreaming is not confined to REM sleep, but also occurs at sleep onset and in NREM sleep. The deeper stages of NREM sleep are particularly interesting as they involve roughly similar proportions of dreaming, unconscious sleep, and white dreams (Noreika et al. 2009: Siclari et al. 2013, 2017). In the search for the neural correlates of dreaming vs unconscious dreamless sleep, this makes comparisons within the same sleep stage possible and avoids confounds involved in comparing presumably dreamful REM sleep with presumably dreamless NREM sleep. Findings suggest that activity in the same parietal hot zone underlies dreaming in both NREM and REM sleep (Siclari et al. 2017).
Where sleep and dream research have traditionally tried to identify the sleep stage correlates of dreaming, newer research suggests local changes occurring independently of sleep stages might in fact be more relevant. Traditionally regarded as global, whole-brain phenomena, there is now increasing evidence that sleep itself is locally driven, and local changes in sleep depth might be associated with changes in sleep-related experience (Siclari & Tononi 2017; Andrillon et al. 2019). While sleep and dream research are often considered as separate fields, changes in how sleep in general and sleep stages in particular are defined appear closely associated with changes in the theoretical conception of dreaming and its empirical investigation.
Historically, discoveries about dreaming have precipitated changing conceptions of sleep (for an excellent history of the study of sleep and dreaming, see Kroker 2007). Following Aristotle ( On Sleeping and Waking ), sleep was traditionally defined in negative terms as the absence of wakefulness and perception. This is still reflected in Malcolm’s assumption that “to a person who is sound asleep, ‘dead to the world’, things cannot even seem” (Malcolm 1956: 26). With the discovery of REM sleep, sleep came to be regarded as a heterogeneous phenomenon characterized by the cyclic alteration of different sleep stages. REM sleep was now considered as “neither sleeping nor waking. It was obviously a third state of the brain, as different from sleep as sleep is from wakefulness” (Jouvet 1999: 5). The folk-psychological dichotomy between sleep and wakefulness now seemed oversimplified and empirically implausible. At the same time dreaming, which had previously been considered as an intermediate state of half-sleeping and half-waking, came to be regarded as a genuine sleep phenomenon, but narrowed to REM sleep. Today, the framework for describing dreams and other sleep-related experiences is more precise, but dreaming has also been cast adrift from REM sleep.
A closely associated issue is how to define waking. Crowther’s (2018) capacitation thesis casts waking consciousness as a state in which the individual is fully switched on to their environment, but also to their own epistemic (cf. O’Shaugnessy 2002) and agentive potential; the waking individual is empowered to act and think in certain ways, though this potential need not be actualized. By contrast, dreaming is an “imagining-of consciousness” (O’Shaughnessy 2002: 430) and consciousness is conceptually tied to wakefulness. Because in lucid dreams, the epistemic and agentive profile of waking is at least partly realized, they might, according to Crowther, be regarded as closer to waking than nonlucid dreams.
This account of waking and sleep may also have consequences for the imagination model of dreaming and dream skepticism (Soteriou 2017). As in the imagination model, dreaming would be passive and action, including cognitive agency, would be tied to waking. If dreaming nonetheless involved passive episodes of imagining oneself to be active, one would be unable to tell that one were dreaming and imagining, as this insight would require the exercise of real agency. The sceptical consequence would be that when dreaming, one would lose agency as well as the capacity to gain insight into one’s current state. Yet our ability to know we are waking when waking would be unscathed; according to Soteriou, waking would thus have an epistemic function connected to the capacity to exercise agency over our mental lives.
Finally, definitions of consciousness themselves are bound up with conceptions of sleep and dreaming. As dreaming went from a state whose experiential status was doubted to being widely recognized as a second global state of consciousness, consciousness sometimes came to be defined contrastively as that which disappears in deep, dreamless sleep and reappears in waking and dreaming (Searle 2000; Tononi 2008). In light of dreamless sleep experience, such definitions are problematic (Thompson 2014, 2015; Windt 2015b; Windt et al. 2016). Dreamless sleep experience has been proposed to be particularly relevant for understanding minimal phenomenal experience, or the conditions under which the simplest kind of conscious experience arises (Windt 2015b). The investigation of dreamless sleep might thus shed light on the transition from unconscious sleep to sleep-related experience.
We almost always have a self in dreams, though this self can sometimes be a slightly different (e.g. older or younger) version of our waking self or even a different person entirely. Dreams therefore raise interesting questions about the identity between the dream and waking self. Locke (1689) invites us to imagine two men alternating in turns between sleep and wakefulness and sharing one continuously thinking soul (Locke 1689: II.I.12). He argues that if one man retained no memory of the soul’s thoughts and perceptions while it was linked to the other man’s body, they would be distinct persons. His position is that personal identity depends on psychological continuity, including recall: in the absence of recall, as illustrated by the toy example of two people sharing one soul, continuous conscious thinking does not suffice for identity. Locke also rejects the possibility of unrecalled dreams and the idea that we dream throughout sleep, remembering only a small proportion of our dreams (Locke 1689: II.I.19).
Valberg distinguishes between the subject of the dream (i.e., the dream self) and the sleeping person who is the dreamer of the dream and recalls it upon awakening (Valberg 2007). He argues that awakening from a dream involves crossing a chasm between discrete worlds with discrete spaces and times; it does not make sense to say that “the ‘I’ at these times [is] a single individual who crosses from one world to the other” (Valberg 2007: 69). According to Valberg, this is relevant to dream skepticism because there is no simple way to make sense of the claims that it is I who emerge from a dream or that I was the victim of dream deception.
Vicarious dreams, or dreams in which the protagonist of the dream seems to be a different person from the dreamer, are particularly puzzling with respect to identity. They may even raise the question of whether the dream self has an independent existence (Rosen & Sutton 2013: 1047). Such dreams are superficially similar to cases in which we imagine being another person, but according to Rosen and Sutton require a different explanation: in the case of dreaming, the imagined person’s thoughts are not framed as diverging from one’s own and one does not retain one’s own perspective in addition to the imagined one; in nonlucid dreams, only the perspective of the dream’s protagonist is retained.
The dream self is also at the center of simulation views of dreaming, which define dreaming via its immersive, here and now character as the experience of a self in a world. This leads to further questions about the phenomenology of self-experience in dreams and how it is different from waking self-experience. Different versions of the simulation view focus on different aspects of self- and world experience in dreams, ranging from social simulation (Revonsuo et al. 2015) to the typical features of selfhood in dreams (Revonsuo 2005, 2006, Metzinger 2003, 2009) to the minimal conditions for experiencing oneself as a self in dreams and what this tells us about minimal phenomenal selfhood in general (Windt 2015a, 2018). Yet these different versions of the simulation view are largely complementary and together have forged unity in a field that was previously hampered by lack of agreement about the definition of dreaming. They also integrate the philosophy of dreaming and scientific dream research.
As so often in debates about dreaming, there is disagreement about basic phenomenological questions. Revonsuo (2005) describes self-experience including bodily experience in dreams as identical to waking, whereas Metzinger (2003, 2009; see also Windt & Metzinger 2007) argues that important layers of waking self-experience (such as autobiographical memory, agency, a stable first-person perspective, metacognitive insight, and self-knowledge) are missing in nonlucid dreams. He argues this is due to the cognitive and mnemonic deficit that characterizes nonlucid dreams (cf. Hobson et al. 2000). Windt (2015a) analyzes the range of cognitive and bodily self-experience in dreams, both of which she describes as variable. She argues that in a majority of cases, dreams are weakly phenomenally embodied states in which bodily experience is largely related to movement sensations but a detailed and integrated body representation is lacking; instead, bodily experience in dreams is largely indeterminate (for an attempt to test this empirically, see Koppehele-Gossel et al. 2016). She proposes this is because dreams are also weakly functionally embodied states, in which the specific pattern of bodily experience reflects altered processing of bodily sensations (as in the illusion view). She also analyzes instances of bodiless dreams, in which dreamers say they experienced themselves as disembodied entities, to argue that self-experience can be reduced to pure spatiotemporal-self-location (Windt 2010); she proposes these cases can help identify the conditions for the emergence of minimal phenomenal selfhood (Blanke & Metzinger 2009; see also Metzinger 2013b).
How the phenomenology of dreaming compares to waking and what to say about how the dream self relates to the waking self bears on questions about the moral status of dreams. For Augustine ( Confessions ) dreams were a cause of moral concern because of their indistinguishability from waking life. What particularly worried him about dreams of sexual acts was their vividness, as well as the feeling of pleasure and seeming acquiescence or consent on the part of the dreamer. He concluded, however, that the transition from sleep to wakefulness involves a radical chasm, enabling the dreamer to awaken with a clear conscience and absolving them from taking responsibility for their dream actions.
What exactly Augustine thought the chasm between dreaming and waking consists in allows for different interpretations (Matthews 1981). Firstly, if the dream and waking self are not identical, then waking Augustine is not morally responsible for dream-Augustine’s actions. Secondly, actions performed in dreams might be morally irrelevant because they did not really happen. And thirdly, assuming that moral responsibility requires the ability to act otherwise, dreams provide no grounds for moral concern because we cannot refrain from having certain types of dreams.
The issue of dream immorality may also present a choice point between different accounts of moral evaluation. Where internalists assume the moral status of a person’s actions is entirely determined by internal factors such as intentions and motives, externalists look beyond these to the effects of actions. Driver (2007) argues that the absurdity of dream immorality itself should count against purely internalist accounts; yet she also acknowledges this absurdity is not a necessary feature of dreams.
Central to the question of dream immorality is the status of dreams as actions rather than mere behaviors. Mullane (1965) argues that while we don’t have full control over our dreams, they are not completely involuntary either; as is the case for blushing, considerable effort is required to attain control over our dreams and in some cases they can even be considered as actions. That lucid dream control is, to some extent, a learnable skill (Stumbrys et al. 2014) lends some support to this claim.
6. The meaning of dreams and the functions of dreaming
Philosophical discussions of dreaming tend to focus on (a) dream deception and (b) questions about the ontology of dreaming, its moral status, etc., that tend to intersect with dream skepticism. By contrast, the main source of interest in dreams outside of philosophy traditionally has been dream interpretation and whether dreams are a source of knowledge and insight. Historically, the epistemic status of dreams and the use of prophetic and diagnostic dreams was not just a theoretical, but a practical problem (Barbera 2008). Different types of dreams were distinguished by their putative epistemic value. Artemidorus, for instance, used the term enhypnion to refer to dreams that merely reflect the sleeper’s current bodily or psychological state and hence do not merit further interpretation, whereas he reserved the term oneiron for meaningful and symbolic dreams of divine origin.
The practice of dream interpretation was famously attacked by Aristotle in On Prophecy in Sleep . He denied that dreams are of divine origin, but allowed that occasionally, small affections of the sensory organs as might stem from distant events that cannot be perceived in waking are perceptible in the quiet of sleep. He also believed such dreams were mostly likely to occur in dullards whose minds resemble an empty desert – an assessment that was not apt to encourage interest in dreams (Kroker 2007: 37). A similarly negative view was held by early modern philosophers who believed dreams were often the source of superstitious beliefs (Hobbes 1651; Kant 1766; Schopenhauer 1847).
In Freudian dream theory, dream interpretation once more assumed a prominent role as the royal road to knowledge of the unconscious. This was associated with claims about the psychic sources of dreaming. Freud (1899) also rejected the influence of external or bodily sources, as championed by contemporary proponents of somatic-stimulus theory.
In the neuroscience of dreaming, Hobson famously argued that dreams are the product of the random, brain-stem driven activation of the brain during sleep (Hobson 1988) and at best enable personal insights in the same way as a Rorschach test (Hobson et al. 2000). Dennett (1991) illustrates the lack of design underlying the production of dream narratives through the “party game of psychoanalysis”, which involves an aimless game of question-and-answer. In the game, players follow simple rules to jointly produce narratives that can seem symbolic and meaningful, even though no intelligent and deliberate process of narration was involved.
Even if we grant that dreams are not messages from a hidden entity in need of decoding, this does not imply that dream interpretation cannot be a personally meaningful source of insight and creativity (Hobson & Wohl 2005). Whether and under which conditions, and following which methods, dream interpretation can lead to personally significant insights is an empirical question that is only beginning to be investigated systematically (see Edwards et al. 2013).
Finally, throughout history, views on the epistemic status of dreams and the type of knowledge to be gained from dream interpretation (e.g., knowledge about the future, diagnosis of physical illness, or insights about one’s current concerns) often changed in tandem with views on the origin and sources of dreaming, which gradually moved from divine origins and external sources, via the body, to the unconscious, and finally to the brain.
Different theories on the functions of dreaming have been proposed and the debate is ongoing. An important distinction is between the functions of sleep stages and the functions of dreaming. Well-documented functions of REM sleep include thermoregulation and the development of cortical structures in birds and mammals, as well as neurotransmitter repletion, the reconstruction and maintenance of little-used brain circuits, the structural development of the brain in early developmental phases, as well as the preparation of a repertoire of reflexive or instinctive behaviors (Hobson 2009). Yet none of these functions are obviously linked to dreaming. An exception is protoconsciousness theory, in which REM sleep plays an important role in foetal development by providing a virtual world model even before the emergence of full-blown consciousness (Hobson 2009: 808) .
Numerous studies have investigated the contribution of sleep to memory consolidation, with different sleep stages promoting different types of memories (Diekelmann et al. 2009; Walker 2009). However, only a few studies have investigated the relationship between dream content and memory consolidation in sleep (for a review, see Nielsen & Stenstrom 2005). Dreams rarely involve episodic replay of waking memories (Fosse et al. 2003). The incorporation of memory sources seems to follow a specific temporal pattern in which recent memories are integrated with older but semantically related memories (Blagrove et al. 2011). Nielsen (2017) presents a model of how external and bodily stimuli on one hand and short- and long-term memories on the other hand form seemingly novel, complex, and dreamlike images at sleep onset; he proposes these microdreams shed light on the formation and sources of more complex dreams. There is also some evidence that dream imagery might be associated with memory consolidation and task performance after sleep, though this is preliminary (Wamsley & Stickgold 2009, 2010; Wamsley et al. 2010).
Prominent theories on the function of dreaming focus on bad dreams and nightmares. It has long been thought that dreaming contributes to emotional processing and that this is particularly obvious in the dreams of nightmare sufferers or in dreams following traumatic experiences (e.g., Hartmann 1998; Nielsen & Lara-Carrasco 2007; Levin & Nielsen 2009; Cartwright 2010; Perogamvros et al. 2013). Based on the high prevalence of negative emotions and threatening dream content, threat simulation theory suggests that the evolutionary function of dreaming lies in the simulation of ancestral threats and the rehearsal of threatening events and avoidance skills in dreams has an adaptive value by enhancing the individual’s chances of survival (see Revonsuo 2000; Valli 2008). A more recent proposal is social simulation theory, in which social imagery in dreams supports social cognition, bonds, and social skills. (Revonsuo et al. 2015).
An evolutionary perspective can also be fruitfully applied to specific aspects of dream phenomenology. According to the vigilance hypothesis , natural selection disfavored the occurrence of those types of sensations during sleep that would compromise vigilance (Symons 1993). Dream sounds, but also smells or pains might distract attention from the potentially dangerous surroundings of the sleeping subject, and the vigilance hypothesis predicts that they only rarely occur in dreams without causing awakening. By contrast, because most mammals sleep with their eyes closed and in an immobile position, vivid visual and movement hallucinations during sleep would not comprise vigilance and thus can occur in dreams without endangering the sleeping subject. Focusing on the stuff dreams are not made of might then be at least as important for understanding the function of dreaming as developing a positive account.
Finally, even if dreaming in general and specific types of dream content in particular were found to be strongly associated with specific cognitive functions, it would still be possible that dreams are mere epiphenomena of brain activity during sleep (Flanagan 1995, 2000). It is also possible that the function of dreams is not knowable (Springett 2019).
A particular problem for any theory on the function of dreaming is to explain why a majority of dreams are forgotten and how dreams can fulfill their putative function independently of recall. Crick and Mitchinson (1983) famously proposed that REM sleep “erases” or deletes surplus information and unnecessary memories, which would suggest that enhanced dream recall is counterproductive. Another problem is that dreaming can be lost selectively and independently of other cognitive deficits (Solms 1997, 2000).
Some of the problems that arise for theories on the functions of dreaming can be avoided if we do not assume that dreaming has a specific function, separate from the function(s) of conscious wakeful states. This depends on the broader taxonomy of dreaming in relation to wakeful states. For example, if dreaming is continuous with waking mind wandering, imagination, and/or own-body perception, we should not expect it to have a unique function, but rather to express a similar function as these wakeful states, perhaps to varying degrees. Nor should we expect dreams to have a single function; the functions of dreaming might be as varied and complex as those of consciousness, and given the complexity of the target phenomenon, the failure to pin down a single function should not be surprising (Windt 2015a).
Questions about dreaming in different areas of philosophy such as epistemology, ontology, philosophy of mind and cognitive science, and ethics are closely intertwined. Scientific evidence from sleep and dream research can meaningfully inform the philosophical discussion and has often done so in the past. The discussion of dreaming has also often functioned as a lens on broader questions about knowledge, morality, consciousness, and self. Long a marginalized area, the philosophy of dreaming and of sleep is central to important philosophical questions and increasingly plays an important role in interdisciplinary consciousness research, for example in the search for the neural correlates of conscious states, in conscious state taxonomies, and in research on the minimal conditions for phenomenal selfhood and conscious experience.
- Aleman, A. and F. Larøi, 2008, Hallucinations: The Science of Idiosyncratic Perception , Washington, DC: American Psychological Association.
- Andrillon, T., J. Windt, T. Silk, S. Drummond, M. Bellgrove, and N. Tsuchiya, 2019, “Does the mind wander when the brain takes a break? Local sleep in wakefulness, attentional lapses and mind-wandering”, Frontiers in Neuroscience , 13(949), doi:10.3389/fnins.2019.00949
- Antrobus, J.S., 1990, “The Neurocognition of Sleep Mentation: Rapid Eye Movements, Visual Imagery, and Dreaming”, in Sleep and Cognition , R. Bootzin, J. Kihlstrom, and D. Schacter (eds.), Washington: American Psychological Association, pp. 3–24.
- Aristotle, On Dreams , in Aristotle: On the Soul. Parva Naturalia. On Breath , (Loeb Classical Library No. 288), W.S. Hett (ed.), Cambridge, MA & London: Harvard University Press, 1986.
- –––, On Prophecy in Sleep , in Aristotle: On the Soul. Parva Naturalia. On Breath , (Loeb Classical Library No. 288), W.S. Hett (ed.), Cambridge, MA & London: Harvard University Press, 1986.
- –––, On Sleeping and Waking , in Aristotle: On the Soul. Parva Naturalia. On Breath , (Loeb Classical Library No. 288), W.S. Hett (ed.), Cambridge, MA & London: Harvard University Press, 1986.
- Artemidorus, The Interpretation of Dreams , R.J. White (ed.), New Jersey: Noyes Press, 1975.
- Aserinsky, E. and N. Kleitman, 1953, “Regularly Occurring Periods of Eye Motility and Concurrent Phenomena during Sleep”, Sleep Medicine Review , 118: 273–274.
- –––, 1955, “Two Types of Ocular Motility Occurring in Sleep”, Journal of Applied Physiology , 8: 1–10.
- Augustine, Against the Academics , J.J. O’Meara (ed.), Westminster, MD: Newman Press, 1950.
- –––, Confessions , H. Chadwick (ed.), Oxford: Oxford University Press, 1991.
- Austin, J.L., 1962, Sense and Sensibilia , G.J. Warnock (ed.), Oxford & New York: Oxford University Press.
- Ayer, A.J., 1960, “Professor Malcolm on Dreams”, Journal of Philosophy , 57: 517–535.
- Baillet, A., 1691, La Vie de Monsieur Descartes , Paris: La Table Ronde.
- Baird, B., S. Mota-Rolim, and M. Dresler, 2019, “The cognitive neuroscience of lucid dreaming”, Neuroscience & Biobehavioral Reviews , 100: 305–323.
- Baldridge, B.J., 1966, “Physical Concomittants of Dreaming and the Effect of Stimulation on Dreams”, Ohio State Medical Journal , 62: 1272–1275.
- Barbera, J., 2008, “Sleep and Dreaming in Greek and Roman Philosophy”, Sleep Medicine , 9: 906–910.
- Barrett, D. and P. McNamara (eds.), 2007a, The New Science of Dreaming, Volume 1: Biological Aspects , Westport: Praeger Perspectives.
- ––– (eds.), 2007b, The New Science of Dreaming, Volume 2: Content, Recall, Personality Correlates , Westport: Praeger Perspectives.
- ––– (eds.), 2007c, The New Science of Dreaming, Volume 3: Cultural and Theoretical Perspectives , Westport: Praeger Perspectives.
- Bayne, T.J. and E. Pacherie, 2005, “In Defence of the Doxastic Conception of Delusions”, Mind and Language , 20(2): 163–188.
- Berger, R.J., 1963, “Experimental Modification of Dream Content by Meaningful Verbal Stimuli”, British Journal of Psychiatry , 109: 722–740.
- Bergson, H., 1914, Dreams , E.E. Slosson (ed. and trans.), New York: B.W. Huebsch. [ Bergson 1914 available online ]
- Berkeley, G., 1710, “A Treatise Concerning the Principles of Human Knowledge”, in Principles of Human Knowledge and Three Dialogues , H. Robinson (ed.), Oxford: Oxford University Press, pp. 1–96.
- Binz, C., 1878, Über den Traum , Bonn: Adolph Marcus.
- Blanke, O. and T. Metzinger, 2009, “Full-Body Illusions and Minimal Phenomenal Selfhood”, Trends in Cognitive Sciences , 13(1): 7–13.
- Block, N., 2007, “Consciousness, Accessibility, and the Mesh between Psychology and Neuroscience”, Behavioral and Brain Sciences , 30: 499–548.
- Blumberg, M., 2010, “Beyond dreams: do sleep-related movements contribute to brain development?”, Frontiers in Neurology , 1(140), doi:10.3389/fneur.2010.00140
- Blumberg, M., and A. Plumeau, 2016, “A new view of ‘dream enactment’ in REM sleep behavior disorder”, Sleep Medicine Reviews , 30: 34–42.
- Bortolotti, L., 2009, Delusions and Other Irrational Beliefs , Oxford: Oxford University Press.
- Bostrom, N., 2003, “Are We Living in a Computer Simulation?”, The Philosophical Quarterly , 53(211): 243–255.
- Bourdin, P., 1641, “Seventh Set of Objections”, reprinted in Moriarty 2008: 214–231.
- Bradley, F.H., 1894, “The Failure of Movement in Dream”, Mind , 3(11): 373–377.
- Breger, L., I. Hunter, and R.W. Lane, 1971, The Effect of Stress on Dreams , New York: International Universities Press.
- Broughton, J., 2002, Descartes’s Method of Doubt , Princeton & Oxford: Princeton University Press.
- –––, 2006, “Impressions and Ideas”, in The Blackwell Guide to Hume’s Treatise , S. Traiger (ed.), Malden, MA & Oxford: Blackwell, pp. 43–57.
- Browne, A., 1977, “Descartes’s Dreams”, Journal of the Warburg and Courtauld Institutes , 40: 256–273.
- Bucci, A., and M. Grasso, 2017, “Sleep and dreaming in the predictive processing framework”, in T. Metzinger and W. Wiese (eds.), Philosophy and Predictive Processing (Volume 6), Frankfurt am Main: MIND Group.
- Canfield, J.V., 1961, “Judgments in Sleep”, The Philosophical Review , 70: 224–230.
- Cartwright, R.D., 2010, The Twenty-four Hour Mind: The Role of Sleep and Dreaming in our Emotional Lives , Oxford: Oxford University Press.
- Chalmers, D.J., 2005, “The Matrix as Metaphysics”, in Grau 2005: 132–176.
- Chihara, C.S., 1965, “What Dreams Are Made On”, Theoria , 31: 145–158.
- Chihara, C.S. and J.A. Fodor, 1965, “Operationalism and Ordinary Language: A Critique of Wittgenstein”, American Philosophical Quarterly 2: 281–295; page reference is to the reprint in Dunlop 1977.
- Christoff, K., Z. Irving, K. Fox, R. Spreng, and J. Andrews-Hanna, 2016, “Mind-wandering as spontaneous thought: a dynamic framework”, Nature Reviews Neuroscience , 17(11): 718–731.
- Churchland, P.S., 1988, “Reduction and the Neurobiological Basis of Consciousness”, in Consciousness in Contemporary Science , A. Marcel and E. Bisiach (eds.), Oxford: Oxford University Press, pp. 273–304.
- Clark, A., 2005, “The Twisted Matrix: Dream, Simulation, or Hybrid?”, in Grau 2005: 177–197.
- –––, 2013a, “Dreaming the Whole Cat: Generative Models, Predictive Processing, and the Enactivist Conception of Perceptual Experience”, Mind , 121(483), 753–771.
- –––, 2013b, “Whatever Next? Predictive Brains, Situated Agents, and the Future of Cognitive Science”, Behavioral and Brain Sciences , 36(03), 181–204.
- Clarke, D.M., 2006, Descartes: A Biography , Cambridge & New York: Cambridge University Press.
- Cohen, M.A. and D.C. Dennett, 2011, “Consciousness Cannot Be Separated from Function”, Trends in Cognitive Sciences , 15(8), 358–364.
- Cole, J.R., 1992, The Olympian Dreams and Youthful Rebellion of René Descartes , Urbana & Chicago: University of Illinois Press.
- Conrad, T., 1968, Zur Wesenslehre des psychischen Lebens und Erlebens , (Phaenomenologica: Vol. 27), DenHaag: Nijhoff.
- Crane, T. and C. French, 2017, “The Problem of Perception”, The Stanford Encyclopedia of Philosophy (Spring 2017 Edition), Edward N. Zalta (ed.), URL = < https://plato.stanford.edu/archives/spr2017/entries/perception-problem/ >.
- Crick, F. and G. Mitchinson, 1983, “The Function of Dream Sleep”, Nature , 304, 111–114.
- Crowther, T., 2018, “Experience, dreaming, and the phenomenology of wakeful consciousness”, in F. Dorsch, F. MacPherson, & M. Nida-Rumelin (eds.), Phenomenal Presence , Oxford: Oxford University Press.
- Currie, G., 2000, “Imagination, Delusion and Hallucination”, Mind & Language , 15(1), 168–183.
- Currie, G. and I. Ravenscroft, 2002, Recreative Minds: Imagination in Philosophy and Psychology , Oxford: Clarendon Press.
- D’Agostino, A., A. Castelnovo, and S. Scarone, 2013, “Dreaming and the Neurobiology of Self: Recent Advances and Implications for Psychiatry”, Frontiers in Psychology , 4, doi:10.3389/fpsyg.2013.00680 [ available online ]
- Dang-Vu, T.T., M. Schabus, M. Desseilles, S. Schwartz, P. Maquet, 2007, “Neuroimaging of Sleep and Dreaming”, in Barrett and McNamara 2007a: 95–114.
- Dement, W.C., 1999, The Promise of Sleep. The Scientific Connection between Health, Happiness and a Good Night’s Sleep , London: Pan Books.
- Dement, W.C. and N. Kleitman, 1957, “The Relation of Eye Movements during Sleep to Dream Activity: An Objective Method for the Study of Dreaming”, Journal of Experimental Psychology , 53: 89–97.
- Dement, W.C. and E. Wolpert, 1958, “The Relation of Eye Movements, Body Motility, and External Stimuli to Dream Content”, Journal of Experimental Psychology , 55: 543–553.
- Dennett, D.C., 1976, “Are dreams experiences?”, The Philosophical Review , 85(2): 151–171.
- –––, 1979, “The Onus Re Experiences: A Reply to Emmett”, Philosophical Studies , 35(April): 315–318.
- –––, 1991, Consciousness Explained . Boston, New York & London: Little, Brown and Company.
- Descartes, R., 1637, Discourse on the Method of Rightly Conducting the Reason, and Seeking Truth in the Sciences ., G.B. Rawlings (ed.). [ Descartes 1637 available online ]
- –––, 1641, Meditations on First Philosophy , reprinted in Moriarty 2008: 1–64.
- Desseilles, M., T.T. Dang-Vu, V. Sterpenich, and S. Schwartz, 2011, “Cognitive and Emotional Processes during Dreaming: A Neuroimaging View”, Consciousness and Cognition , 20(4): 998–1008.
- Diekelmann, S., I. Wilhelm, and J. Born, 2009, “The Whats and Whens of Sleep-Dependent Memory Consolidation”, Sleep Medicine Reviews , 13(5): 309–321.
- Diogenes Laertius, Lives of Eminent Philosophers , R.D. Hicks (ed.), Cambridge MA: Harvard University Press, 1943.
- Domhoff, G.W., 2001, “A New Neurocognitive Theory of Dreams”, Dreaming , 11: 13–33.
- –––, 2003, The Scientific Study of Dreams: Neural Networks, Cognitive Development, and Content Analysis , Washington, DC: American Psychological Association.
- –––, 2011, “The Neural Substrate for Dreaming: Is It a Subsystem of the Default Network?”, Consciousness and Cognition , 20(4): 1163–1174.
- Dreisbach, C., 2000, “Dreams in the History of Philosophy”, Dreaming , 10(1): 31–41.
- Dresler, M., S.P. Koch, R. Wehrle, V.I. Spoormaker, F. Holsboer, A. Steiger, P. Sämann, H. Obrig, and M. Czisch, 2011, “Dreamed Movement Elicits Activation in the Sensorimotor Cortex”, Current Biology , 21(21): 1833–1837.
- Dresler, M., R. Wehrle, V.I. Spoormaker, S.P. Koch, F. Holsboer, A. Steiger, H. Obrig, P. Sämann, and M. Czisch, 2012, “Neural Correlates of Dream Lucidity Obtained from Contrasting Lucid versus Non-lucid REM Sleep: A Combined EEG/fMRI Case Study”, Sleep , 35(7): 1017–1020.
- Driver, J., 2007, “Dream immorality”, Philosophy , 82(1): 5–22.
- Dunlop, C.E.M. (ed.), 1977, Philosophical Essays on Dreaming . Ithaca & London: Cornell University Press.
- Edwards, C.L., P.M. Ruby, J.E. Malinowski, P.D. Bennett, and M.T. Blagrove, 2013, “Dreaming and Insight”, Frontiers in Psychology , 4, doi:10.3389/fpsyg.2013.00979 [ available online ]
- Ellis, H., 1911, The World of Dreams , London: Constable & Company LTD.
- Emmett, K., 1978, “Oneiric Experiences”, Philosophical Studies , 34(November): 445–450.
- Erlacher, D., M. Schredl, and S. LaBerge, S., 2003, “Motor Area Activation during Dreamed Hand Clenching: A Pilot Study on EEG Alpha Band”, Sleep and Hypnosis , 5: 182–187.
- Erlacher, D., M. Schädlich, T. Stumbrys, and M. Schredl, 2014, “Time for Actions in Lucid Dreams: Effects of Task Modality, Length, and Complexity”, Frontiers in Psychology , 4, doi:10.3389/fpsyg.2013.01013 [ available online ]
- Fazekas, P., and G. Nemeth, 2018, “Dream experiences and the neural correlates of perceptual consciousness and cognitive access”, Philosophical Transactions of the Royal Society B (Biological Sciences), 373(1755): 20170356.
- Fazekas, P., G. Nemeth, and M. Overgaard, 2019, “White dreams are made of colours: What studying contentless dreams can teach about the neural basis of dreaming and conscious experiences”, Sleep Medicine Reviews , 43: 84–91.
- ffytche, D.H., 2007, “Visual Hallucinatory Syndromes: Past, Present, and Future”, Dialogues in Clinical Neuroscience , 9(2): 173–189.
- ffytche, D.H., J.D. Blom, and M. Catani, 2010, “Disorders of Visual Perception”, Journal of Neurology, Neurosurgery & Psychiatry , 81(11): 1280–1287.
- Flanagan, O., 1995, “Deconstructing Dreams: The Spandrels of Sleep”, The Journal of Philosophy , 92: 5–27.
- –––, 2000, Dreaming Souls: Sleep, Dreams, and the Evolution of the Conscious Mind , Oxford & New York: Oxford University Press.
- Fletcher, P.C. and C.D. Frith, 2008, “Perceiving is Believing: A Bayesian Approach to Explaining the Positive Symptoms of Schizophrenia”, Nature Reviews Neuroscience , 10(1): 48–58.
- Fosse, M.J., R. Fosse, J.A. Hobson, and R.J. Stickgold, 2003, “Dreaming and Episodic Memory: A Functional Dissociation?”, Journal of Cognitive Neuroscience , 15: 1–9.
- Foulkes, D., 1978, A Grammar of Dreams , New York: Basic Books.
- –––, 1993a, “Children’s Dreaming”, in Dreaming as Cognition , C. Cavallero and D. Foulkes (eds.), New York: Harvester Wheatsheaf, pp. 114–132.
- –––, 1993b, “Dreaming and REM Sleep”, Journal of Sleep Research , 2:199–202.
- –––, 1999, Children’s Dreaming and the Development of Consciousness , Cambridge, MA and London: Harvard University Press.
- Fox, K.C.R., S. Nijeboer, E. Solomonova, G.W. Domhoff, and K. Christoff, 2013, “Dreaming as Mind Wandering: Evidence from Functional Neuroimaging and First-Person Content Reports”, Frontiers in Human Neuroscience , 7, doi:10.3389/fnhum.2013.00412 [ available online ]
- Frankfurt, H.G., 1970, Demons, Dreamers, and Madmen: The Defense of Reason in Descartes’s Meditations , Princeton & Oxford: Princeton University Press.
- Freud, S., 1899, Die Traumdeutung , Frankfurt am Main: Fischer Taschenbuch Verlag (2003).
- –––, 1940, Schriften über Träume und Traumdeutungen , Frankfurt am Main: Fischer Taschenbuch Verlag (2003).
- Galen, On Diagnosis in Dreams , L. Pearcy (ed.), < available online >.
- Gerrans, P., 2012, “Dream Experience and a Revisionist Account of Delusions of Misidentification”, Consciousness and Cognition , 21(1): 217–227.
- –––, 2013, “Delusional Attitudes and Default Thinking”, Mind & Language , 28(1): 83–102.
- –––, 2014, “Pathologies of Hyperfamiliarity in Dreams, Delusions and Déjà Vu”, Frontiers in Psychology , 5, doi:10.3389/fpsyg.2014.00097 [ available online ]
- Globus, G.G., 1987, Dream Life, Wake Life: The Human Condition Through Dreams , New York: State University of New York Press.
- Goblot, 1896, “Sur le souvenir des rêves”, Revue philosophique , XLII .
- Gottesmann, C., 2006, “The Dreaming Sleep Stage: A New Neurobiological Model of Schizophrenia?”, Neuroscience , 140: 1105–1115.
- Grau, C. (ed.), 2005, Philosophers Explore the Matrix , C. Grau (ed.), Oxford: Oxford University Press.
- Gregory, J.C., 1916, “Dreams as Psychical Explosions”, Mind , 25(98): 193–205.
- –––, 1918, “The Dream of ‘Frustrated Effort’: A Suggested Explanation”, Mind , 27(105): 125–128.
- Grundmann, T., 2002, “Die Struktur des skeptischen Traumarguments”, Grazer Philosophische Studien , 64: 57–81.
- Hacking, I., 2001, “Dreams in Place”, Journal of Aesthetics and Art Criticism , 59(3):244–260.
- Hall, C.S., 1981, “Do We Dream during Sleep? Evidence for the Globot Hypothesis”, Perceptual and Motor Skills , 53(1): 239–246.
- Hartmann, E., 1998, Dreams and Nightmares: The New Theory on the Origin and Meaning of Dreams , Cambridge: Perseus Books.
- Hearne, K., 1978, Lucid Dreams: An Electro-Physiological and Psychological Study , (Ph.D. thesis), University of Liverpool, England. [ Hearne 1978 available online ]
- Hering, J., 1947, “Concerning Image, Idea, and Dream”, Philosophy and Phenomenological Research , 8(2): 188–205.
- Hobbes, T., 1651, Leviathan , C.B. Macpherson (ed.), London: Penguin (1985).
- –––, 1641, “Third Set of Objections”, reprinted in Moriarty 2008: 107–124.
- Hobson, J.A., 1988, The Dreaming Brain , New York: Basic Books.
- –––, 1999, Dreaming as Delirium. How the Brain Goes Out of Its Mind , Cambridge MA: MIT Press.
- –––, 2009, “REM Sleep and Dreaming: Towards a Theory of Protoconsciousness”, Nature Reviews Neuroscience , 10(November): 803–813.
- Hobson, J. and K. Friston, 2012, “Waking and Dreaming Consciousness: Neurobiological and Functional Considerations”, Progress in Neurobiology , 98(1): 82–98.
- Hobson, J., E.F. Pace-Schott, and R. Stickgold, 2000, “Dreaming and the Brain. Toward a Cognitive Neuroscience of Conscious States”, Behavioral and Brain Sciences , 23: 793–842.
- Hobson, J. and H. Wohl, 2005, From Angels to Neurones. Art and the New Science of Dreaming , Parma: Mattioli 1885.
- Hodges, M. and W.R. Carter, 1969, “Nelson on Dreaming a Pain”, Philosophical Studies , 20(3): 43–46.
- Hoelscher, T.J., E. Klinger, and S.G. Barta, 1981, “Incorporation of Concern- and Nonconcern-related Verbal Stimuli into Dream Content”, Journal of Abnormal Psychology , 90: 88–91.
- Hohwy, J., 2013, The Predictive Mind , Oxford: Oxford University Press.
- Horikawa, T., M. Tamaki, Y. Miyawaki, and Y. Kamitani, 2013, “Neural Decoding of Visual Imagery during Sleep”, Science 340 (6132): 639–642.
- Hoss, R.J., 2010, “Content Analysis on the Potential Significance of Color in Dreams: A Preliminary Investigation”, International Journal of Dream Research , 3(1): 80–90.
- Hume, D., 1739, A Treatise of Human Nature , D.F. Norton, M.J. Norton (eds.), Oxford & New York: Oxford University Press (2000).
- Hunter, J. F. M., 1983, “The difference between dreaming and being awake”, Mind , 92(365): 80–93.
- Hurlburt, R.T. and E. Schwitzgebel, 2007, Describing Inner Experience?: Proponent Meets Skeptic , Cambridge, MA & London: MIT Press.
- Husserl, E., 1904/1905, Phantasie und Bildbewußtsein , E. Marbach (ed.), Hamburg: Felix Meiner (2006).
- Ichikawa, J., 2008, “Scepticism and the Imagination Model of Dreaming”, The Philosophical Quarterly , 58(232): 519–527.
- –––, 2009, “Dreaming and Imagination”, Mind & Language , 24(1): 103–121.
- James, W., 1890, The Principles of Psychology , Volume Two, New York: Dover Publications (1950).
- Jouvet, M., 1999, The Paradox of Sleep: The Story of Dreaming , L. Garey (ed.), Cambridge & London: MIT Press.
- Kahn, D., E.F. Pace-Schott, and J.A. Hobson, 2002, “Emotion and Cognition: Feeling and Character Identification in Dreaming”, Consciousness and Cognition , 11: 34–50.
- Kahn, D., R. Stickgold, E.F. Pace-Schott, and J.A. Hobson, 2000, “Dreaming and Waking Consciousness: A Character Recognition Study”, Journal of Sleep Research , 9: 317–325.
- Kant, I., 1766 [2002], Träume eines Geistersehers, erläutert durch Träume der Metaphysik , Stuttgart: Reclam.
- Kantor, J., 1970, “Pinching and Dreaming”, Philosophical Studies , 21(1–2): 28–32.
- Keefer, M.H., 1996, “The Dreamer’s Path: Descartes and the Sixteenth Century”, Renaissance Quarterly , 49(1): 30–76.
- Kind, A., 2013, “The heterogeneity of the imagination”, Erkenntnis , 78(1): 141–159.
- Klein, P., 2015, “Skepticism”, The Stanford Encyclopedia of Philosophy (Summer 2015 Edition), Edward N. Zalta (ed.), URL = < https://plato.stanford.edu/archives/sum2015/entries/skepticism/ >.
- Koppehele-Gossel, J., A. Klimke, K. Schermelleh-Engel, and U. Voss, 2016, “A template model of embodiment while dreaming: Proposal of a mini-me.” Consciousness and Cognition , 46: 148–162.
- Koulack, D., 1969, “Effects of Somatosensory Stimulation on Dream Content”, Archives of General Psychiatry , (20): 718–725.
- Kramer, M., 2007, The Dream Experience: A Systematic Exploration , New York & London: Routledge.
- Kroker, K., 2007, The Sleep of Others and the Transformations of Sleep Research , Toronto: University of Toronto Press.
- LaBerge, S., 2007, “Lucid Dreaming”, in Barrett and McNamara 2007a: 307–328.
- LaBerge, S., L.E. Nagel, W.C. Dement, and V.P. Zarcone, 1981, “Lucid Dreaming Verified by Volitional Communication during REM Sleep”, Perceptual and Motor Skills , 52: 727–732.
- LaBerge, S., B. Baird, and P. Zimbardo, 2018, “Smooth tracking of visual targets distinguishes lucid REM sleep dreaming and waking perception from imagination”, Nature Communications , 9(1), doi:10.1038/s41467-018-05547-0
- Leclair-Visonneau, L., D. Oudiette, B. Gaymard, S. Leu-Semenescu, and I. Arnulf, 2010, “Do the Eyes Scan Dream Images during Rapid Eye Movement Sleep? Evidence from the Rapid Eye Movement Sleep Behaviour Disorder Model”, Brain , 133(6): 1737–1746.
- Leibniz, G.W., 1880, Die Philosophischen Schriften , Berlin: Weidmannsche Buchhandlung.
- –––, Philosophical Papers and Letters , L.E. Loemker (ed.), Chicago: University of Chicago Press, 1956.
- Levin, R. and T.A. Nielsen, 2009, “Nightmares, Bad Dreams, and Emotions Dysregulation: A Review and New Neurocognitive Model of Dreaming”, Current Directions in Psychological Science , 18(2): 84–88.
- Lewis, H.D., 1969, The Elusive Mind , London: George Allan & Unwin LTD.
- Liao, Shen-yi and Gendler, Tamar, “Imagination”, The Stanford Encyclopedia of Philosophy (Spring 2019 Edition), Edward N. Zalta (ed.), URL = < https://plato.stanford.edu/archives/spr2019/entries/imagination/ >.
- Limosani, I., A. D’Agostino, M.L. Manzone, and S. Scarone, 2011a, “The Dreaming Brain/Mind, Consciousness and Psychosis”, Consciousness & Cognition , 20(4): 987–992.
- –––, 2011b, “Bizarreness in Dream Reports and Waking Fantasies of Psychotic, Schizophrenic and Manic Patients: Empirical Evidences and Theoretical Consequences”, Psychiatry Research , 189(2): 195–199.
- Llinás, R. and D. Paré, 1991, “Of Dreaming and Wakefulness”, Neuroscience , 44: 521–535.
- Llinás, R. and U. Ribary, 1994, “Perception as an Oneiric-like State Modulated by the Senses”, in Large-Scale Neuronal Theories of the Brain , C. Koch and J. Davis (eds.), Cambridge, MA: MIT Press, pp. 111–124.
- Locke, J., 1689, An Essay Concerning Human Understanding , R. Woolhouse (ed.), London: Penguin (1997).
- Macnish, R., 1838, The Philosophy of Sleep , Glasgow: W.R. M’Phun.
- Macpherson, F., 2013, “The Philosophy and Psychology of Hallucination: An Introduction”, in Hallucination. Philosophy and Psychology , F. Macpherson and D. Platchias (eds.), Cambridge MA: MIT Press, pp. 1–38.
- Malcolm, N., 1956, “Dreaming and Skepticism”, The Philosophical Review , 65(1): 14–37.
- –––, 1959, Dreaming , London: Routledge & Kegan Paul.
- Matthews, G.B., 1981, “On Being Immoral in a Dream”, Philosophy , 56(January): 47–64.
- –––, 2005, Augustine , Malden, MA: Blackwell Pub.
- Maury, L.-F. A., 1861, Le sommeil et les rêves: études psychologiques sur ces phénomènes et les divers états qui s’y rattachent , Paris: Didier et Cie Libraires-Éditeurs.
- McGinn, C., 2004, Mindsight: Image, Dream, Meaning , Cambridge, MA & London: Harvard University Press.
- –––, 2005a, “The Matrix of Dreams”, in Grau 2005: 62–70.
- –––, 2005b, The Power of Movies: How Screen and Mind Interact , New York: Vintage Books.
- Metzinger, T., 2003, Being No One: The Self-Model Theory of Subjectivity , Cambridge, MA & London: MIT Press.
- –––, 2009, The Ego Tunnel: The Science of the Mind and the Myth of the Self , New York: Basic Books.
- –––, 2013a, “The Myth of Cognitive Agency: Subpersonal Thinking as a Cyclically Recurring Loss of Mental Autonomy”, Frontiers in Psychology , 4, doi:10.3389/fpsyg.2013.00931 [ available online ]
- –––, 2013b, “Why Are Dreams Interesting for Philosophers? The Example of Minimal Phenomenal Selfhood, Plus an Agenda for Future Research”, Frontiers in Psychology , 4, doi:10.3389/fpsyg.2013.00746 [ available online ]
- –––, 2015, “M-autonomy”, Journal of Consciousness Studies , 22(11–12): 270–302.
- Montaigne, M. de, “The Apology for Raymond Sebond”, in Readings in the History of Philosophy , R.M. Popkin (ed.), The Philosophy of the 16th and 17th Centuries , Toronto: Collier-Macmillan (1966): 70–81.
- Moriarty, M. (ed.), 2008, Meditations, Objections, and Replies , Oxford: Oxford University Press.
- Mullane, H., 1965, “Moral Responsibility for Dreams”, Dialogue , 4(02): 224–229.
- –––, 1966, “Dreaming as an Action”, Dialogue , 5(02): 239–242.
- Murzyn, E., 2008, “Do We Only Dream in Colour? A Comparison of Reported Dream Colour in Younger and Older Adults with Different Experiences of Black and White Media”, Consciousness and Cognition , 17: 1228–1237.
- Nagel, T., 1959, “Dreaming”, Analysis , 19(5): 112–116.
- –––, 1974, “What Is It Like to Be a Bat?”, Philosophical Review , 83: 435–450.
- Nelson, J.O., 1966, “Can One Tell that He is Awake by Pinching Himself?”, Philosophical Studies , 17(6): 81–84.
- Nemeth, G., and P. Fazekas, 2018, “Beyond the REM--NREM Dichotomy: A Multidimensional Approach to Understanding Dreaming”, Journal of Consciousness Studies , 25(11–12): 13–33.
- Newman, L., 2019, “Descartes’ Epistemology”, The Stanford Encyclopedia of Philosophy (Spring 2019 Edition), Edward N. Zalta (ed.), URL = < https://plato.stanford.edu/archives/spr2019/entries/descartes-epistemology/ >.
- Nielsen, T.A., 2000, “A Review of Mentation in REM and NREM Sleep: ‘Covert’ REM Sleep as a Possible Reconciliation of Two Opposing Models”, Behavioral and Brain Sciences , 23(6): 851–866.
- –––, 2010, “Dream Analysis and Classification: The Reality Simulation Perspective”, in Principles and Practice of Sleep Medicine , M. Kryger, T. Roth, and W.C. Dement (eds.), New York: Elsevier, pp. 595–603.
- –––, 2017, “Microdream neurophenomenology”, Neuroscience of Consciousness , 1: nix001; doi:10.1093/nc/nix001
- Nielsen, T.A. and J. Lara-Carrasco, 2007, “Nightmares, Dreaming, and Emotion Regulation: A Review”, in Barrett and McNamara 2007b: 253–284.
- Nielsen, T.A. and P. Stenstrom, 2005, “What Are the Memory Sources of Dreaming?”, Nature , 437(7063): 1286–1289.
- Nielsen, T.A., L. Ouellet, and A.L. Zadra, 1995, “Pressure Stimulation during REM Sleep Alters Dream Limb Activity and Body Bizarreness”, Sleep Research , 24: 134.
- Nielsen, T.A., C. Svob, and D. Kuiken, 2009, “Dream-Enacting Behaviors in a Normal Population”, Sleep , 32(12): 1629–1636.
- Nir, Y. and G. Tononi, 2010, “Dreaming and the Brain: From Phenomenology to Neurophysiology”, Trends in Cognitive Sciences , 14(2): 88–100.
- Noë, A., 2004, Action in Perception , Cambridge, MA & London: MIT Press.
- Noreika, V., K. Valli, H. Lahtela, and A. Revonsuo. “Early-night serial awakenings as a new paradigm for studies on NREM dreaming”, International Journal of Psychophysiology , 74(1): 14–18.
- O’Shaughnessy, B., 2002, “Dreaming”, Inquiry , 45: 399–432.
- Pace-Schott, E.F., 2007, “The Frontal Lobes and Dreaming”, in Barrett and McNamara 2007b: 115–154.
- –––, 2013, “Dreaming as a Story-Telling Instinct”, Frontiers in Psychology , 4, doi:10.3389/fpsyg.2013.00159 [ available online ]
- Pagel, J.F., M. Blagrove, R. Levin, B. States, B. Stickgold, and S. White, 2001, “Definitions of Dream: A Paradigm for Comparing Field Descriptive Specific Studies of Dream”, Dreaming , 11: 195–202.
- Pantani, M., A. Tagini, and A. Raffone, 2018, “Phenomenal consciousness, access consciousness and self across waking and dreaming: bridging phenomenology and neuroscience”, Phenomenology and the Cognitive Sciences 17(1): 175–197.
- Perogamvros, L., T.T. Dang-Vu, M. Desseilles, S. Schwartz, 2013, “Sleep and Dreaming Are for Important Matters”, Frontiers in Psychology , 4, doi:10.3389/fpsyg.2013.00474 [ available online ]
- Plato, Phaedrus , B. Jowett (ed.), in The Internet Classics Archive , available online .
- –––, Theaetetus , B. Jowett (ed.), in The Internet Classics Archive , available online .
- Prasad, H.S., 2000, “Dreamless Sleep and Soul: a controversy between Vedānta and Buddhism”, Asian Philosophy , 10(1): 61–73. doi:10.1080/09552360050001770
- Putnam, H., 1981, “Brains in a Vat”, in Reason, Truth and History , H. Putnam (ed.), Cambridge & New York: Cambridge University Press, pp. 1–21.
- –––, 1962 [1986], “Dreaming and ‘Depth Grammar’”, in Analytical Philosophy First Series , R. Butler (ed.), Oxford: Basil Blackwell & Mott; page reference is to the reprint in H. Putnam (ed.), 1986, Philosophical Papers, Vol. 2.: Mind, Language and Reality , Cambridge: University Press, pp. 304–324.
- Rechtschaffen, A. and C. Buchignani, 1992, “The Visual Appearance of Dreams”, in The Neuropsychology of Sleep and Dreaming , J.S. Antrobus and M. Bertini (eds.), Hillsdale, N.J.: Lawrence Erlbaum, pp. 143–156.
- Reed, T.M., 1979, “Dreams, Scepticism and Waking Life”, in Body, Mind, and Method. Essays in Honor of Virgil C. Aldrich , D.F. Gustafson and B.L. Tapscott (eds.), Dodrecht & Boston: D. Reidel Publishing Company, pp. 37–64.
- Resnick, J., R. Stickgold, C.D. Rittenhouse, and J.A. Hobson, 1994, “Self-Representation and Bizarreness in Children’s Dream Reports Collected in the Home Setting”, Consciousness and Cognition , 3: 30–45.
- Revonsuo, A., 2000, “The Reinterpretation of Dreams: An Evolutionary Hypothesis of the Function of Dreaming”, Behavioral and Brain Sciences , 23: 877–901.
- –––, 2005, “The Self in Dreams”, in The Lost Self. Pathologies of the Brain and Identity , T.E. Feinberg and J.P. Keenan (eds.), Oxford: Oxford University Press, pp. 206–219.
- –––, 2006, Inner Presence: Consciousness as a Biological Phenomenon , Cambridge, MA & London: MIT Press.
- Revonsuo, A. and C. Salmivalli, 1995, “A Content Analysis of Bizarre Elements in Dreams”, Dreaming , 5:169–187.
- Revonsuo, A. and K. Tarkko, 2002, “Binding in Dreams. The Bizarreness of Dream Images and the Unity of Consciousness”, Journal of Consciousness Studies , 9: 3–24.
- Revonsuo, A., J. Tuominen, and K. Valli, 2015, “The Avatars in the Machine – Dreaming as a Simulation of Social Reality”, in T. Metzinger and J. M. Windt (eds.), Open MIND , Frankfurt am Main: MIND Group.
- Rosen, M.G., 2013, “What I Make up when I Wake up: Anti-Experience Views and Narrative Fabrication of Dreams”, Frontiers in Psychology , 4, doi:10.3389/fpsyg.2013.00514 [ available online ]
- –––, 2015, “I’m thinking your thoughts while I sleep: Sense of agency and ownership over dream thought”, Psychology of Consciousness: Theory, Research, and Practice , 2(3): 326–339.
- –––, 2018a, “Enactive or inactive? Cranially envatted dream experience and the extended conscious mind”, Philosophical Explorations , 21(2): 295–318.
- –––, 2018b, “How bizarre? A pluralist approach to dream content”, Consciousness and Cognition , 62: 148–162.
- –––, forthcoming, “Dreaming of a stable world: vision and action in sleep”, first online 22 February 2019 Synthese , doi:10.1007/s11229-019-02149-1
- Rosen, M.G. and J. Sutton, 2013, “Self-Representation and Perspectives in Dreams”, Philosophy Compass , 8(11): 1041–1053.
- Russell, B., 1914, Our Knowledge of the External World as a Field for Scientific Method in Philosophy , London & New York: Routledge (2009).
- –––, 1948, Human Knowledge: It’s Scope and Limits , London & New York: Routledge (2009).
- Ryle, G., 1949, The Concept of Mind , Chicago: University of Chicago Press, 2000.
- Sartre, J.-P., 1940, L’imaginaire: Psychologie phénoménologique de l’imagination , Paris: Gallimard, 2005.
- Sauvageau, A., T.A. Nielsen, and J. Montplaisir, 1998, “Effects of Somatosensory Stimulation on Dream Content in Gymnasts and Control Participants: Evidence of Vestibulomotor Adaptation in REM Sleep”, Dreaming , 8: 125–134.
- Scarone, S., M.L. Manzone, O. Gambini, I. Kantzas, I. Limosani, A. D’Agostino, and J.A. Hobson, 2007, “The Dream as a Model for Psychosis: An Experimental Approach Using Bizarreness as a Cognitive Marker”, Schizophrenia Bulletin , 34(3): 515–522.
- Schenck, C.H. 2005, Paradox Lost: Midnight in the Battleground of Sleep and Dreams , Minneapolis: Extreme Nights, LLC.
- Schenck, C.H. and M.W. Mahowald, 1996, “REM Sleep Parasomnias”, Neurological Clinics , 14: 697–720.
- Scherner, K.A., 1861, Das Leben des Traumes , Berlin: Verlag von Heinrich Schindler.
- Schooler, J.W., J. Smallwood, K. Christoff, T.C. Handy, E.D. Reichle, and M.A. Sayette, 2011, “Meta-awareness, Perceptual Decoupling and the Wandering Mind”, Trends in Cognitive Sciences , 15(7): 319–326.
- Schopenhauer, A., 1847, Kleinere Schriften , Zürich: Haffmans Verlag (1988).
- Schredl, M., 2006, “Factors Affecting the Continuity between Waking and Dreaming: Emotional Intensity and Emotional Tone of the Waking-Life Event”, Sleep and Hypnosis , 8: 1–5.
- –––, 2010, “History of Dream Research: The Dissertation ‘Entstehung der Träume (Origin of Dreams)’ of Wilhelm Weygandt Published in 1893”, International Journal of Dream Research , 3(1): 95–97.
- Schredl, M. and F. Hofmann, 2003, “Continuity between Waking Activities and Dream Activities”, Consciousness and Cognition , 12: 298–308.
- Schredl, M., A. Fuchedzhieva, H. Hämig, and V. Schindele, 2008, “Do We Think Dreams Are in Black and White Due to Memory Problems?”, Dreaming , 18(3): 175–180.
- Schröder, S., 1997, “The Concept of Dreaming: On Three Theses by Malcolm”, Philosophical Investigations , 20(1): 15–38.
- Schwitzgebel, E., 2002, “Why Did We Think We Dreamed in Black and White?”, Studies in History and Philosophy of Science , 33: 649–660.
- –––, 2003, “Do People Still Report Dreaming in Black and White? An Attempt to Replicate a Questionnaire from 1942”, Perceptual and Motor Skills , 96: 25–29.
- –––, 2011, Perplexities of Consciousness , Cambridge & London: MIT Press.
- –––, 2017, “1% Skepticism”, Noûs , 51(2): 271–290.
- Schwitzgebel, E., C. Huang, and Y. Zhou, 2006, “Do We Dream in Color? Cultural Variations and Skepticism”, Dreaming , 16(1): 36–42.
- Searle, J., 2000, “Consciousness”, Annual Review of Neuroscience , 23: 557–578.
- Sebastián, M., 2014a, “Dreams: An Empirical Way to Settle the Discussion between Cognitive and Non-Cognitive Theories of Consciousness”, Synthese , 191(2): 263–285.
- –––, 2014b, “Not a HOT dream”, In Consciousness inside and out: Phenomenology, neuroscience, and the nature of experience, Springer, Dordrecht, pp. 415–432.
- Sextus Empiricus, Outlines of Pyrrhonism , R.G. Bury (ed.), Cambridge, MA: Harvard University Press (1987).
- Siclari, F., and G. Tononi, 2017, “Local aspects of sleep and wakefulness”, Current Opinion in Neurobiology , 44: 222–227.
- Siclari, F., J. LaRocque, B. Postle, and G. Tononi, 2013, “Assessing sleep consciousness within subjects using a serial awakening paradigm”, Frontiers in psychology , 4, doi:10.3389/fpsyg.2013.00542
- Siclari, F., B. Baird, L. Perogamvros, G. Bernardi, J. LaRocque, B. Riedner, M. Boly, B. Postle, and G. Tononi, 2017, “The neural correlates of dreaming”, Nature Neuroscience , 20(6): 872.
- Siegler, F.A., 1967, “Remembering Dreams”, The Philosophical Quarterly , 17: 14–24.
- Silberer, H., 1919, Der Traum: Einführung in die Traumpsychologie , Stuttgart: Ferdinand Enke.
- Singer, E.A., 1924, “On Pain in Dreams”, The Journal of Philosophy , 21(22): 589–601.
- Smallwood, J. and J.W. Schooler, 2015, “The science of mind wandering: empirically navigating the stream of consciousness”, Annual review of psychology , 66: 487–518.
- Smith, A.D., 2002, The Problem of Perception , Cambridge, Mass.: Harvard University Press.
- Solms, M., 1997, The Neuropsychology of Dreams: A Clinico-Anatomical Study , Mahwah, NJ: Lawrence Erlbaum.
- –––, 2000, “Dreaming and REM Sleep Are Controlled by Different Brain Mechanisms”, Behavioral and Brain Sciences , 23: 843–850.
- Sosa, E., 2007, A Virtue Epistemology: Apt Belief and Reflective Knowledge , Volume I, Oxford: Clarendon Press.
- Soteriou, M., forthcoming, “Dreams, agency, and judgement”, Synthese , first online 20 July 2017, doi: 10.1007/s11229-017-1496-7
- Springett, B., 2019, “Can we know about the evolution of dreaming?”, Dreaming , 29(3): 220.
- Stickgold, R. and M.P. Walker (eds.), 2009, The Neuroscience of Sleep , London, Burlington MA, San Diego: Elsevier: Academic Press.
- Stone, J., 1984, “Dreaming and Certainty”, Philosophical Studies , 45(May): 353–368.
- Stroud, B., 1984, The Significance of Philosophical Scepticism , Oxford: Clarendon Press.
- Stumbrys, T., D. Erlacher, and M. Schredl, 2013, “Testing the involvement of the prefrontal cortex in lucid dreaming: a tDCS study”, Consciousness and Cognition , 22(4): 1214–1222.
- Stumbrys, T., D. Erlacher, M. Johnson, and M. Schredl, M., 2014, “The Phenomenology of Lucid Dreaming: An Online Survey”, American Journal of Psychology , 127(2): 191–204.
- Symons, D., 1993, “The Stuff that Dreams Aren’t Made of: Why Wake-State and Dream-State Sensory Experiences Differ”, Cognition , 47(3): 181–217.
- Thomas, N., 2014, “The multidimensional spectrum of imagination: Images, dreams, hallucinations, and active, imaginative perception”, Humanities 3(2): 132–184.
- Thompson, E., 2014, Waking, dreaming, being: Self and consciousness in neuroscience, meditation, and philosophy , New York: Columbia University Press.
- –––, 2015, “Dreamless Sleep, the Embodied Mind, and Consciousness – The Relevance of a Classical Indian Debate to Cognitive Science”, in T. Metzinger and J. M. Windt (eds.), Open MIND , Frankfurt am Main: MIND Group.
- Tononi, G., 2008, “Consciousness as integrated information: a provisional manifesto”, Biological Bulletin , 215: 216–242.
- Uslar, D. von, 1964, Der Traum als Welt: Untersuchungen zur Ontologie und Phänomenologie des Traums , Pfullingen: Neske.
- Valberg, J.J., 2007, Dream, Death, and the Self , Princeton & Oxford: Princeton University Press.
- Valli, K., 2008, Threat Simulation: The Function of Dreaming? , doctoral thesis, Turku: Annales Universitatis Turkuensis.
- van de Castle, R.L., 1994, Our Dreaming Mind , New York: Ballantine Books.
- van Eeden, F., 1913, “A Study of Dreams”, Proceedings of the Society for Psychical Research , 26: 431–461.
- Vold, J.M., 1910/1912, Über den Traum , Vol. I and II, edited by O. Klemm, Leipzig: Johann Ambrosius Barth.
- Voss, U., R. Holzmann, I. Tuin, and J.A. Hobson, 2009, “Lucid Dreaming: A State of Consciousness with Features of Both Waking and Non-Lucid Dreaming”, Sleep , 32(9): 1191–1200.
- Voss, U., K. Schermelleh-Engel, J. Windt, C. Frenzel, and A. Hobson, 2013, “Measuring consciousness in dreams: the lucidity and consciousness in dreams scale”, Consciousness and Cognition , 22(1): 8–21.
- Voss, U. and A. Hobson, 2015, “What is the state-of-the-art on lucid dreaming? – Recent advances and questions for future research”, in T. Metzinger & J. M. Windt (eds.), Open MIND , Frankfurt a. MIND Group.
- Voss, U., R. Holzmann, A. Hobson, W. Paulus, J. Koppehele-Gossel, A. Klimke, and M. Nitsche, 2014, “Induction of self awareness in dreams through frontal low current stimulation of gamma activity”, Nature Neuroscience , 17(6): 810.
- Walker, M.P., 2009, “Sleep-Dependent Memory Processing”, in Stickgold and Walker 2009: 230–240.
- Wamsley, E.J., 2013, “Dreaming, Waking Conscious Experience, and the Resting Brain: Report of Subjective Experience as a Tool in the Cognitive Neurosciences”, Frontiers in Psychology , 4, doi:10.3389/fpsyg.2013.00637 [ available online ]
- Wamsley, E.J. and R. Stickgold, 2009, “Incorporation of Waking Events into Dreams”, in Stickgold and Walker 2009: 330–336.
- –––, 2010, “Dreaming and Offline Memory Processing”, Current Biology , 20(23): R1010–1013.
- Wamsley, E.J., M. Tucker, J.D. Payne, J.A. Benavides, and R. Stickgold, 2010, “Dreaming of a Learning Task Is Associated with Enhanced Sleep-Dependent Memory Consolidation”, Current Biology , 20(9): 850–855.
- Wamsley, E., C. Donjacour, T. Scammell, G. Lammers, and R. Stickgold, 2014, “Delusional confusion of dreaming and reality in narcolepsy”, Sleep , 37(2): 419–422.
- Waxman, W., 1994, Hume’s Theory of Consciousness , Cambridge & New York: Cambridge University Press.
- Weygandt, W., 1893, Entstehung der Träume , Leipzig: Grübel & Sommerlatte.
- Williams, B., 1978, Descartes: The Project of Pure Enquiry , Middlesex: Pelican Books.
- Windt, J.M., 2010, “The Immersive Spatiotemporal Hallucination Model of Dreaming”, Phenomenology and the Cognitive Sciences , 9: 295–316.
- –––, 2011, “Altered Consciousness in Philosophy”, in Altering Consciousness. Multidisciplinary Perspectives, Volume 1: History, Culture, and the Humanities , E. Cardeña and M. Winkelman (eds.), Santa Barbera, Denver & Oxford: Praeger, pp. 229–254.
- –––, 2013, “Reporting Dream Experience: Why (Not) to Be Skeptical about Dream Reports”, Frontiers in Human Neuroscience , 7, doi:10.3389/fnhum.2013.00708 [ available online ]
- –––, 2015a, Dreaming: A Conceptual Framework for Philosophy of Mind and Empirical Research. Cambridge, MA: MIT Press.
- –––, 2015b, “Just in time – dreamless sleep experience as pure subjective temporality”, in T. Metzinger & J.M. Windt (eds.), Open MIND , Frankfurt am Main: MIND Group.
- –––, 2018, “Predictive brains, dreaming selves, sleeping bodies: how the analysis of dream movement can inform a theory of self-and world-simulation in dreams”, Synthese , 195(6): 2577–2625.
- Windt, J.M. and T. Metzinger, 2007, “The Philosophy of Dreaming and Self-Consciousness: What Happens to the Experiential Subject during the Dream State?”, in Barrett and McNamara 2007c: 193–248.
- Windt, J.M. and V. Noreika, 2011, “How to Integrate Dreaming into a General Theory of Consciousness—A Critical Review of Existing Positions and Suggestions for Future Research”, Consciousness and Cognition , 20(4): 1091–1107.
- Windt, J.M., and U. Voss, 2018, “Spontaneous thought, insight, and control in lucid dreams”, in The Oxford Handbook of Spontaneous Thought: Mind-Wandering, Creativity, and Dreaming , Oxford: Oxford University Press, pp. 385–410.
- Windt, J.M., T. Nielsen, and E. Thompson, 2016, “Does consciousness disappear in dreamless sleep?”, Trends in cognitive sciences , 20(12): 871–882.
- Wittgenstein, L., 1953, Philosophical Investigations , G.E.M. Anscombe (ed.), Oxford: Blackwell.
- –––, 1967, Zettel , G.E.M. Anscombe and G.H. von Wright (eds.), Berkeley: University of California Press.
- Wundt, W.M., 1896, Grundriss der Psychologie , Leipzig: Engelman; page reference is to the excerpt translated by C.H. Judd, reprinted in R.L. Woods and H.B. Greenhouse (eds.), The New World of Dreams , New York: Macmilllan (1974), pp. 179–180.
- Zeidman, P., and E. Maguire, 2016, “Anterior hippocampus: the anatomy of perception, imagination and episodic memory”, Nature Reviews Neuroscience , 17(3): 173.
How to cite this entry . Preview the PDF version of this entry at the Friends of the SEP Society . Look up topics and thinkers related to this entry at the Internet Philosophy Ontology Project (InPhO). Enhanced bibliography for this entry at PhilPapers , with links to its database.
- Philosophy of Dreaming , entry in the Internet Encyclopedia of Philosophy .
- Dreams , PhilPapers collection.
- Dreams and Skepticism , PhilPapers collection.
belief | Berkeley, George | delusion | Descartes, René: epistemology | imagination | Locke, John | perception: the problem of | personal identity | personal identity: and ethics | Plato: on knowledge in the Theaetetus | sense data | skepticism | skepticism: and content externalism
Acknowledgments
I want to thank Regina Fabry and two anonymous reviewers for helpful comments and constructive criticism on an earlier version of this manuscript. And as always, I am greatly indebted to Stefan Pitz for his support.
Copyright © 2019 by Jennifer M. Windt < jennifer . windt @ monash . edu >
- Accessibility
Support SEP
Mirror sites.
View this site from another server:
- Info about mirror sites
The Stanford Encyclopedia of Philosophy is copyright © 2023 by The Metaphysics Research Lab , Department of Philosophy, Stanford University
Library of Congress Catalog Data: ISSN 1095-5054
Frontiers for Young Minds

- Download PDF
The Science of Dreams

Dreams are a common experience. Some are scary, some are funny. Recent research into how the brain works helps us understand why we dream. Strange combinations of ideas in our dreams may make us more creative and give us ideas that help us to solve problems. Or, when memories from the day are repeated in the brain during sleep, memories may get stronger. Dreams may also improve our moods. Together, these studies show that dreams and sleep are important for performing well when we are awake.
When she was 8, my daughter told me about one of her dreams. She was in a spaceship with some animals. Although she knew she was in a spaceship in her dream, when telling me about the dream, she realized the spaceship was actually a washing machine. At times, she and the animals would be out in space, but they also came back to earth. She told me the dream with a laugh and then moved on with her day, ignoring the crazy animals and spaceships that entertained her in her sleep.
Since we remember our dreams and then often forget them, what is their purpose? Why do we dream about the things we do? New research tools, particularly those that can be used to investigate the brain, are being used to answer these questions.
What Are Dreams?
Although it is hard to define what a dream is, for the purpose of this article, we will define dreams as our thoughts during sleep that we recall when we wake up. So, sleeping dreams are not the same as “daydreaming.” Dreams are mostly visual (made up of scenes and faces; sound, taste, and smell are rare in dreams [ 1 ]). Dreams can range from truly strange to rather boring, snapshots from a recent event.
To study dreams, scientists need a measure of dreaming. Most studies use dream reports (a person writes out her dreams when she wakes up) or questionnaires (a person answers questions like “How many dreams have you recalled in the past month?” [ 2 ]). Dreams are more likely to be recalled when a person is woken up from REM sleep. REM sleep is a type of sleep that is named for the rapid eye movements that can be measured during this stage of sleep. We do not dream as much in non-REM sleep, the sleep stages that make up the rest of the night, and dream reports from non-REM sleep are often less strange.
Dream frequency (how often dreams happen) and content (what dreams are about) is very different for everyone, and there are many reasons why this may be true. For example, you will remember dreams more if you are woken up by someone or by an alarm clock. This might be because you can still recall that dream memory while it is fresh but, if you wake up on your own, you will transition through a few sleep stages and possibly lose that dream memory. Dream recall changes with age, too. Older people are less likely to report dreaming. This could also be related to memory: since older people have weaker memories, it could be that they dream but cannot remember their dreams by the time they wake up. A brain area called the medial prefrontal cortex is also related to dream recall. If this brain area is damaged, the person recalls few dreams, which may mean the person dreams less (or not at all). Also, how tightly packed the brain cells are in the medial prefrontal cortex can vary from person to person, which may cause some healthy people to dream more or less than other healthy people. There are also genes that affect how much REM sleep people get. People with less REM sleep may not have the strange dreams that tend to come in REM. So, how long you sleep, your age, and your genetics may all explain why you dream more or less than someone else.
Do dreams actually happen while we sleep, or are they ideas that come to us when we wake up and we just “feel” like it happened during sleep? A recent study using a type of brain imaging called magnetic resonance imaging or (MRI: Read more in the Young Minds article “How Is Magnetic Resonance Imaging Used to Learn About the Brain?” [ 3 ]) helped answer this question ( Figure 1A ). The scientists made maps of the brain activity that occurred when people looked at pictures of things—keys, beds, airplanes. Later, the people in the study slept in the MRI machine. The scientists matched the pattern of brain activity from the people as they slept to brain activity patterns for the pictures they viewed earlier, and then chose the best match ( Figures 1B,C ). This match predicted what the person said they dreamed about 60% of the time. Although 60% is not perfect, it is better than guessing! [ 4 ]. This means that dreams are created in the brain during sleep.
- Figure 1 - (A) Magnetic resonance imaging (MRI) is a way to investigate the brain.
- The person lies on a bed inside a giant magnet. (B) MRI can measure the structure of the brain and the areas of the brain that are active. (C) MRI was used to measure dreaming. First, while the participant was awake, they viewed thousands of pictures in the MRI. This told scientists the specific brain responses to specific pictures. Later, when the participant slept in the MRI, scientists measured the brain activity patterns and matched this to the brain responses to the pictures the participant saw when they were awake. Scientists guessed that the best match would tell them what the participant was dreaming about. By asking the participant about their dreams in the MRI, scientists found that the dreams did tend to match the pictures predicted by the brain activity.
Dreams Support Memories
What is the purpose of our dreams? Researchers have found that sleep is important for memory (see this Frontiers for Young Minds article ; “Thanks for the Memories…” [ 5 ]). Memories move from temporary storage in the hippocampus , a brain structure that is very important for short-term memory, to permanent storage in other parts of the brain. This makes the memories easier to remember later. Memories improve with sleep because the memories are replayed during sleep [ 6 ]. If you want to learn all the words to your favorite scene in a movie, you might re-watch that scene over and over again. The brain works the same way: neurons (brain cells) that are active with learning are active again and replay the learned material during sleep. This helps store the memory more permanently.
Memory replay may show up in our dreams. Dreams in non-REM sleep, when most memory replay happens, often contain normal people and objects from recent events. However, sleep switches between non-REM and REM sleep (see Figure 2 ). So, bizarre dreams in REM sleep may come from a combination of many different recent memories, which were replayed in non-REM sleep, and get jumbled up during REM sleep. If dreams help with memory processing, does that mean your memories are not being processed if you do not dream? No. Memories are moving to storage even if we do not dream.
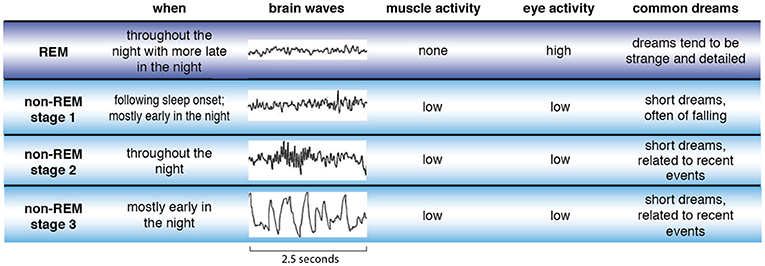
- Figure 2 - There are four types of sleep—REM sleep (purple) and three stages of non-REM sleep (blue).
- REM stands for rapid eye movements, which happen during this stage of sleep. During REM sleep, muscle and brain activity also differ from other sleep stages. Characteristics of dreams tend to be different for each of these sleep stages.
Dreams Improve Creativity and Problem Solving
My daughter’s dream of a spaceship made a great story that she recited to me, and later, to her classmates. The images were intense and interesting, inspiring her to draw scenes in a notebook and write about the dream for school. This is an example of how dreams can help make us more creative. Mary Shelley, the author of the book Frankenstein, got the idea for her book from a dream. Even scientists get ideas from dreams [ 7 ].
To measure creative problem solving, scientists used a remote associates task, in which three unrelated words are shown, and the person is to come up with a word they have in common. For instance, HEART, SIXTEEN, and COOKIES seem unrelated until you realize they all are related to SWEET (sweetheart, sweet sixteen, and cookies are sweet) ( Figure 3 ). The scientists wanted to see whether sleep helped people do better on this task. They found that people were better at thinking of the remote solution if they had a nap, particularly a nap with REM sleep. Given that REM is when most bizarre dreaming occurs, this supports the idea that these dreams might help us find creative solutions to problems [ 8 ].
- Figure 3 - REM sleep helps people find creative solutions.
- In the morning, participants did two tasks to test creativity and problem solving (A) . They did one task again in the afternoon. In between, they either stayed awake (“wake” group) or took a nap. Those that took naps either did not have REM sleep in their nap (“nREM” group) or had both nREM and REM sleep (“nREM + REM” group). (B) If subjects stayed awake between the morning and afternoon tests (yellow bar), they did not improve on the task. They also did not improve if they had a nap that was only nREM sleep (light blue bar). But, if they had a nap with both nREM and REM sleep, they did better in the afternoon compared with when they did the task in the morning (dark blue bar). So, REM sleep must help us find creative solutions (from Cai et al. [ 8 ]).
This study and research like it gives us reason to believe that REM dreams may help us be more creative and solve problems. Many different memories may be activated at the same time and when these memories are mixed together, the result when we wake up may be both the memory of a strange dream and a unique perspective on problems.
Dreams Regulate Our Moods and Emotions
Dreams are usually emotional. One study found that most dreams are scary, angry, or sad.
Dreams might seem to be emotional simply because we tend to remember emotional things better than non-emotional things. For example, in waking life, the day you got a puppy is more memorable than a normal school day. So, dreams about emotional events might be remembered more easily than boring, non-emotional dreams. It is also possible that dreams are emotional because one job of dreams is to help us process emotions from our day [ 9 ]. This may be why the amygdala , an area of the brain that responds to emotions when we are awake, is active during REM sleep. If you had a sad day, you are more likely to have sad dreams. But, sleep also improves mood–sleep after a disagreement or sad event will make you happier.
Dreams could also help prepare us for emotional events, through something called threat simulation theory [ 10 ]. For example, when I dreamt that my young daughter, who could not swim, fell into a swimming pool, recall of that dream convinced me to sign her up for swim lessons. By simulating this fearful situation, I could prevent it by being prepared.
These studies show us that sleep and dreams are important for our emotions. By processing emotions in sleep, we may be better prepared and in a better mood the next day.
Conclusions
There are different ways scientists measure dreams—from asking questions to using MRI. These studies show us that activity in the brain while we sleep gives us the interesting dreams we recall when we wake up. These dreams help us remember things, be more creative, and process our emotions.
We know most kids do not get enough sleep. Some diseases (like Alzheimer’s disease) also make people sleep less, while others (like REM sleep behavior disorder and mood disorders) affect dreams directly. It is important to study sleep and dreams to understand what happens when we do not get enough sleep and how we can treat people with these diseases.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rapid Eye Movement (REM) : ↑ A stage of sleep in which the eyes move rapidly and there is no muscle activity.
Medial Prefrontal Cortex : ↑ A specific area in the front of the brain that is associated with dream recall but also has a role in memory and decision-making.
Magnetic Resonance Imaging (MRI) : ↑ A tool used to take pictures of internal body parts (including the brain). MRI can also be used to measure the activity in the brain.
Hippocampus : ↑ An area in the brain that is thought to be important for short-term memory.
Neuron : ↑ A cell in the nervous system (brain and spinal cord) that can transmit information to other cells.
Amygdala : ↑ An area of the brain involved in the experience of emotions.
Threat Simulation Theory : ↑ A theory of dreaming that says that threats (things that could be bad) are simulated or practiced in your dreams to prepare you for those situations when you are awake.
1. ↑ Zandra, A. L., Nielsen, T. A., and Donderi, D. C. 1998. Prevalence of auditory, olfactory, and gustatory experiences in home dreams. Percept. Mot. Skills 87:819–26.
2. ↑ Schredl, M. 2002. Questionnaires and diaries as research instruments in dream research: methodological issues. Dreaming 12:17–26. doi: 10.1023/A:1013890421674
3. ↑ Hoyos, P., Kim, N., and Kastner, S. 2019. How Is Magnetic Resonance Imaging Used to Learn About the Brain? Front. Young Minds . 7:86. doi: 10.3389/frym.2019.00086
4. ↑ Horikawa, T., Tamaki, M., Miyawaki, Y., and Kamitani, T. 2013. Neural decoding of visual imagery during sleep. Science 340:639–42. doi: 10.1126/science.1234330
5. ↑ Davachi, L., and Shohamy, D. 2014. Thanks for the Memories.… Front. Young Minds. 2:23. doi: 10.3389/frym.2014.00023
6. ↑ O’Neill, J., Senior, T. J., Allen, K., Huxter, J. R., and Csicsvari, J. 2008. Reactivation of experience-dependent cell assembly patterns in the hippocampus. Nat. Neurosci . 11:209–15. doi: 10.1038/nn2037
7. ↑ Barrett, D. 2001. The Committee of Sleep: How artists, scientists, and athletes use dreams for creative problem-solving–and How You Can Too . New York, NY: Crown.
8. ↑ Cai, D. J., Mednick, S. A., Harrison, E. M., Kanady, J. C., and Mednick, S. C. 2009. REM, not incubation, improves creativity by priming associative networks. Proc. Natl. Acad. Sci. U.S.A . 106:10130–4. doi: 10.1073/pnas.0900271106
9. ↑ Cremone, A., Kurdziel, L. B. F., Fraticelli, A., McDermott, J., and Spencer, R. M. C. 2017. Napping reduces emotional attention bias during early childhood. Dev. Sci . 20:e12411. doi: 10.1111/desc.12411
10. ↑ Revonsuo, A. 2000. The reinterpretation of dreams: an evolutionary hypothesis of the function of dreaming. Behav. Brain Sci . 23:877–901. doi: 10.1017/s0140525x00004015
Study finds novel evidence that dreams reflect multiple memories, anticipate future events
Dreams focused on future events utilize memories of multiple past experiences.
Dreams result from a process that often combines fragments of multiple life experiences and anticipates future events, according to novel evidence from a new study.
Results show that 53.5% of dreams were traced to a memory, and nearly 50% of reports with a memory source were connected to multiple past experiences. The study also found that 25.7% of dreams were related to specific impending events, and 37.4% of dreams with a future event source were additionally related to one or more specific memories of past experiences. Future-oriented dreams became proportionally more common later in the night.
"Humans have struggled to understand the meaning of dreams for millennia," said principal investigator Erin Wamsley, who has a doctorate in cognitive neuroscience and is an associate professor in the department of psychology and program in neuroscience at Furman University in Greenville, South Carolina. "We present new evidence that dreams reflect a memory-processing function. Although it has long been known that dreams incorporate fragments of past experience, our data suggest that dreams also anticipate probable future events."
The study involved 48 students who spent the night in the laboratory for overnight sleep evaluation using polysomnography. During the night, participants were awakened up to 13 times to report on their experiences during sleep onset, REM sleep, and non-REM sleep. The following morning, participants identified and described waking life sources for each dream reported the previous evening. A total of 481 reports were analyzed.
"This is a new description of how dreams draw simultaneously from multiple waking-life sources, utilizing fragments of past experience to construct novel scenarios anticipating future events," said Wamsley.
According to Wamsley, the proportional increase of future-oriented dreams later in the night may be driven by temporal proximity to the upcoming events. While these dreams rarely depict future events realistically, the activation and recombination of future-relevant memory fragments may nonetheless serve an adaptive function.
The research abstract was published recently in an online supplement of the journal Sleep and will be presented as a poster beginning June 9 during Virtual SLEEP 2021. SLEEP is the annual meeting of the Associated Professional Sleep Societies, a joint venture of the American Academy of Sleep Medicine and the Sleep Research Society.
- Sleep Disorders
- Spirituality
- Obstructive Sleep Apnea
- Disorders and Syndromes
- Multiple Sclerosis
- K-12 Education
- Post-traumatic stress disorder
- Confirmation bias
- Synesthesia
- Psychopathology
- Philosophy of mind
- Psychological trauma
Story Source:
Materials provided by American Academy of Sleep Medicine . Note: Content may be edited for style and length.
Journal Reference :
- Erin Wamsley. 034 Dreaming as Constructive Episodic Future Simulation . Sleep , 2021; 44 (Supplement_2): A15 DOI: 10.1093/sleep/zsab072.033
Cite This Page :
Explore More
- Fossil Frogs Share Their Skincare Secrets
- Fussy Eater? Most Parents Play Short Order Cook
- Precise Time Measurement: Superradiant Atoms
- Artificial Cells That Act Like Living Cells
- Affordable and Targeted Anticancer Agent
- This Alloy Is Kinky
- Giant Galactic Explosion: Galaxy Pollution
- Flare Erupting Around a Black Hole
- Two Species Interbreeding Created New Butterfly
- Warming Antarctic Deep-Sea and Sea Level Rise
Trending Topics
Strange & offbeat.
Suggestions or feedback?

MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
A new way to control experimentation with dreams
Press contact :.
Previous image Next image
The study of dreams has entered the modern era in exciting ways, and researchers from MIT and other institutions have created a community dedicated to advancing the field, lending it legitimacy and expanding further research opportunities.
In a new paper , researchers from the Media Lab’s Fluid Interfaces group introduce a novel method called “ Targeted Dream Incubation ” (TDI). This protocol, implemented through an app in conjunction with a wearable sleep-tracking sensor device, not only helps record dream reports, but also guides dreams toward particular themes by repeating targeted information at sleep onset, thereby enabling incorporation of this information into dream content. The TDI method and accompanying technology serve as tools for controlled experimentation in dream study, widening avenues for research into how dreams impact emotion, creativity, memory, and beyond.
The paper, “Dormio: A Targeted Dream Incubation Device,” is co-authored by lead researcher Adam Haar Horowitz and professor of media arts and sciences Pattie Maes, who is also head of the Fluid Interfaces group. Additional authors on the paper are Tony J. Cunningham, postdoc at Beth Israel Deaconess Medical Center and Harvard Medical School, and Robert Stickgold, director of the Center for Sleep and Cognition at Beth Israel Deaconess Medical Center and professor of psychiatry at Harvard Medical School.
Previous neuroscience studies from researchers such as sleep and cognitive sciences expert Stickgold show that hypnagogia (the earliest sleep stage) is similar to the REM stage in terms of brainwaves and experience; however, unlike REM, individuals can still hear audio during hypnagogia while they dream.
“This state of mind is trippy, loose, flexible, and divergent,” explains Haar Horowitz. “It’s like turning the notch up high on mind-wandering and making it immersive — being pushed and pulled with new sensations like your body floating and falling, with your thoughts quickly snapping in and out of control.”
To facilitate the TDI protocol, an interdisciplinary team at the Media Lab designed and developed Dormio , a sleep-tracking device that can alter dreams by tracking hypnagogia and then delivering audio cues based on incoming physiological data, at precise times in the sleep cycle, to make dream direction possible. Upon awakening, a person’s guided dream content can be used to complete tasks such as creative story writing, and compared experimentally to waking thought content.
“Dormio takes dream research to a new level, interacting directly with an individual’s dreaming brain and manipulating the actual content of their dreams,” says Stickgold. “The potential value of Dormio for enhancing learning and creativity are literally mind-blowing.”
The Media Lab team’s first pilot study using Dormio demonstrated dream incubation and creativity augmentation in six people, and was presented alt.CHI in 2018. Multiple scientists began reaching out to the team expressing interest in replicating the dream-control research. These requests led to the first Dream Engineering workshop, which was held at the Media Lab in January 2019, organized by Maes, Haar Horowitz, and Judith Amores from the Fluid Interfaces group, and Michelle Carr, visiting researcher from the University of Rochester Sleep and Neurophysiology Laboratory. The workshop brought together many of the world’s leading dream researchers, including pioneers such as Deirdre Barrett, Bjorn Rasch, Ken Paller, and Stephen LaBerge, to brainstorm new technologies for studying, recording, and influencing dreams.
The talks and technologies presented at the workshop further led to a Special Issue on Dream Engineering for the journal Consciousness and Cognition , with Maes, Haar Horowitz, Amores, and Carr serving as guest editors.
“Most sleep and dream studies have so far been limited to university sleep labs and have been very expensive, as well as cumbersome, for both researchers and participants,” says Maes. “Our research group is excited to be pioneering new, compact, and cheap technologies for studying sleep and interfacing with dreams, thereby opening up opportunities for more studies to happen and for these experiments to take place in natural settings. Apart from benefiting scientists, this work has the potential to lead to new commercial technologies that go beyond sleep tracking to issue interventions that affect sleep onset, sleep quality, sleep-based memory consolidation, and learning.”
The research itself is central to Haar Horowitz’s thesis work in the Program of Media Arts and Sciences. This past year, he ran a larger dream study with 50 subjects, which replicated and extended the results of the previous study.
“We showed that dream incubation is tied to performance benefits on three tests of creativity, by both objective and subjective metrics,” Haar Horowitz states. “Dreaming about a specific theme seems to offer benefits post-sleep, such as on creativity tasks related to this theme. This is unsurprising in light of historical figures like Mary Shelley or Salvador Dalí, who were inspired creatively by their dreams. The difference here is that we induce these creatively beneficial dreams on purpose, in a targeted manner.”
An enhanced Dormio device has now also been built, as well as an analysis platform, streaming platform, an iOS app for audio capture and streaming, and a web app for audio capture, storage, and streaming. These mobile and online platforms allow the TDI method to be shared through a variety of open source technologies.
A number of other universities have likewise begun related Dormio studies; these include Duke University, Boston College, Harvard University, the University of Rochester, and the University of Chicago.
The Media Lab research team is also leading collaborations with artists, using dreams to create new artwork and augment artistic creativity. This work, which mixes sleep science and media art, has been shown at the Beijing Biennale and Ars Electronica festival, and a new collaboration with installation artist Carsten Holler looks to create an overnight experimental art piece.
The Dormio development team includes researchers Haar Horowitz, Tomás Vega, Ishaan Grover, Pedro Reynolds-Cuéllar, Oscar Rosello, Abhinandan Jain, and Eyal Perry, along with students in the MIT Undergraduate Research Opportunities Program Matthew Ha, Christina Chen, and Kathleen Esfahany.
Share this news article on:
Press mentions.
MIT researchers have developed a new wearable device, called Dormio, that can be used to record and even guide a person’s dreams, reports Mashable . Dormio is aimed at providing “insights into how dreams work and their effect on various things like memory, emotion, creativity.”
Previous item Next item
Related Links
- Paper: "Dormio: A targeted dream incubation device"
- Targeted Dream Incubation
- Fluid Interfaces research group
- MIT Media Lab
Related Topics
- Undergraduate Research Opportunities Program (UROP)
- School of Architecture and Planning
- Neuroscience
- Brain and cognitive sciences
Related Articles

Study: Better sleep habits lead to better college grades

Decoding hidden dreams
How brain waves guide memory formation
More mit news.
3 Questions: A shared vocabulary for how infectious diseases spread
Read full story →

Seven from MIT elected to American Academy of Arts and Sciences for 2024
Two MIT teams selected for NSF sustainable materials grants

Study demonstrates efficacy of MIT-led Brave Behind Bars program

Bringing an investigator’s eye to complex social challenges

MIT announces 2024 Bose Grants
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
Find anything you save across the site in your account
The Haiti That Still Dreams
By Edwidge Danticat

I often receive condolence-type calls, e-mails, and texts about Haiti. Many of these messages are in response to the increasingly dire news in the press, some of which echoes what many of us in the global Haitian diaspora hear from our family and friends. More than fifteen hundred Haitians were killed during the first three months of this year, according to a recent United Nations High Commissioner for Human Rights report, which described the country’s situation as “ cataclysmic .” Women and girls are routinely subjected to sexual violence. Access to food, water, education, and health care is becoming more limited, with more than four million Haitians, around a third of the population, living with food insecurity, and 1.4 million near starvation. Armed criminal groups have taken over entire neighborhoods in Port-au-Prince and the surrounding areas, carrying out mass prison breaks and attacks on the city’s airport, seaport, government buildings, police stations, schools, churches, hospitals, pharmacies, and banks, turning the capital into an “ open air prison .”
Even those who know the country’s long and complex history will ask, “Why can’t Haiti catch a break?” We then revisit some abridged version of that history. In 1804, after a twelve-year revolution against French colonial rule, Haiti won its independence, which the United States and several European powers failed to recognize for decades. The world’s first Black republic was then forced to spend sixty years paying a hundred-and-fifty-million francs (now worth close to thirty billion dollars) indemnity to France . Americans invaded and then occupied Haiti for nineteen years at the beginning of the twentieth century. The country endured twenty-nine years of murderous dictatorship under François Duvalier and his son, Jean-Claude, until 1986. In 1991, a few months after Haiti’s first democratically elected President, Jean-Bertrand Aristide, took office, he was overthrown in a coup staged by a military whose members had been trained in the U.S. Aristide was elected again, then overthrown again, in 2004, in part owing to an armed rebellion led by Guy Philippe, who was later arrested by the U.S. government for money laundering related to drug trafficking. Last November, six years into his nine-year prison sentence, Philippe was deported by the U.S. to Haiti. He immediately aligned himself with armed groups and has now put himself forward as a Presidential candidate.
In 2010, the country was devastated by a 7.0-magnitude earthquake, which killed more than two hundred thousand people. Soon after, United Nations “peacekeepers” dumped feces in Haiti’s longest river, causing a cholera epidemic that killed more than ten thousand people and infected close to a million. For the past thirteen years, Haiti has been decimated by its ruling party, Parti Haïtien Tèt Kale (P.H.T.K.), which rose to power after a highly contested election in 2011. In that election, the U.S.—then represented by Secretary of State Hillary Clinton—and the Organization of American States helped the candidate who finished in third place, Michel Martelly, claim the top spot. Bankrolled by kidnapping, drug trafficking, business élites, and politicians, armed groups have multiplied under P.H.T.K, committing massacres that have been labelled crimes against humanity. In 2021, a marginally elected President, Jovenel Moïse, was assassinated in his bedroom , a crime for which many of those closest to him, including his wife, have been named as either accomplices or suspects.

The unasked question remains, as W. E. B. Du Bois wrote in “ The Souls of Black Folk ,” “How does it feel to be a problem?”
I deeply honor Haiti’s spirit of resistance and long history of struggle, but I must admit that sometimes the answer to that question is that it hurts. Sometimes it hurts a lot, even when one is aware of the causes, including the fact that the weapons that have allowed gangs to take over the capital continue to flow freely from Miami and the Dominican Republic, despite a U.N. embargo. Internally, the poorest Haitians have been constantly thwarted by an unequal and stratified society, which labels rural people moun andeyò (outside people), and which is suffused with greedy and corrupt politicians and oligarchs who scorn the masses from whose tribulations they extract their wealth.
Recently, at a loved one’s funeral, in Michigan, the spectre of other Haitian deaths was once again on the minds of my extended family members. Everywhere we gather, Haiti is with us, as WhatsApp messages continuously stream in from those who chose to stay in Haiti and can’t leave because the main airport is closed, and others who have no other home. In Michigan, during chats between wake, funeral, and repast, elders brought up those who can’t get basic health care, much less a proper burial or any of the rituals that are among our most sacred obligations. “Not even a white sheet over those bodies on the street,” my mother-in-law, who is eighty-nine, said, after receiving yet another image of incinerated corpses in Port-au-Prince. At least after the 2010 earthquake, sheets were respectfully placed on the bodies pulled from the rubble. Back then, she said, the armed young men seemed to have some reverence for life and some fear of death.
Lately, some of our family gatherings are incantations of grief. But they can also turn into storytelling sessions of a different kind. They are opportunities for our elders to share something about Haiti beyond what our young ones, like everyone else, see on the news. The headlines bleed into their lives, too, as do the recycled tropes that paint us as ungovernable, failures, thugs, and even cannibals. As with the prayers that we recite over the dead, words still have power, the elders whisper. We must not keep repeating the worst, they say, and in their voices I hear an extra layer of distress. They fear that they may never see Haiti again. They fear that those in the next generation, some of whom have never been to Haiti, will let Haiti slip away, as though the country they see in the media—the trash-strewn streets and the barricades made from the shells of burnt cars, the young men brandishing weapons of war and the regular citizens using machetes to defend themselves—were part of some horror film that they can easily turn off. The elders remind us that we have been removed, at least physically, from all of this by only a single generation, if not less.
We are still human beings, the elders insist—“ Se moun nou ye .” We are still wozo , like that irrepressible reed that grows all over Haiti. For a brief moment, I think someone might break into the Haitian national anthem or sing a few bars of the folk song “ Ayiti Cheri .” (“Beloved Haiti, I had to leave you to understand.”) Instead, they hum the music that the wozo has inspired : “ Nou se wozo / Menm si nou pliye, nou pap kase. ” Even if we bend, we will not break.

Except we are breaking. “It pains me to see people living in constant fear,” the Port-au-Prince-based novelist and poet Évelyne Trouillot recently wrote to me in an e-mail. “I dream of a country where children are not afraid to dream.” Internationally, U.S. deportations continue , Navy ships are ready to be deployed to intercept migrant boats, and Haitian asylum seekers could once again end up imprisoned on Guantánamo, as they did in the early nineteen-nineties. In conversations, whether with strangers or with younger family members, someone inevitably asks, “Is there any hope?”
I have hope, I say, because I grew up with elders, both in Haiti and here in the U.S., who often told us, “ Depi gen souf gen espwa ”—as long as there’s breath, there’s hope. I have hope, too, because the majority of Haitians are under twenty-five years old, as are many members of our family. Besides, how can we give in to despair with eleven million people’s lives in the balance? Better yet, how can we reignite that communal grit and resolve that inspired us to defeat the world’s greatest armies and then pin to our flag the motto “ L’union fait la force ”? Unity is strength.
The elders also remind us that Haiti is not just Port-au-Prince. As more and more of the capital’s residents are forced to return to homesteads and ancestral villages, the moun andeyò have much to teach other Haitians. “Historically, the moun andeyò have always been the preserver of Haiti’s cultural and traditional ethos,” Vivaldi Jean-Marie, a professor of African American and African-diaspora studies at Columbia University, told me. Rural Haitians, who have lived for generations without the support of the state, have had no choice but to rely on one another in close and extended family structures called lakou . “This shared awareness—I am because we are—will prevail beyond this difficult chapter in Haitian history,” Jean-Marie said.
Finally, I have hope because in Haiti, as the American writer and art collector Selden Rodman has written, “ art is joy .” This remains true even as some of the country’s most treasured cultural institutions, including the National School of the Arts and the National Library, have been ransacked. In the summer of 2023, Carrefour Feuilles, a district in Port-au-Prince that many writers, visual artists, and musicians call home, was attacked by armed criminal groups. The onslaught led to a petition that collected close to five thousand signatures. It read in part, “How many more hundreds of our women and children must be raped, executed, burned before the public authorities do everything possible to put an end to the plague of gangs and their sponsors?”
A few days later, the homes of two of the signatories, the multimedia artist Lionel St. Eloi and the writer Gary Victor, were taken over by a gang. The last time I saw St. Eloi was in 2019, in the courtyard of Port-au-Prince’s Centre d’Art, where he had a series of metal birds on display, their bejewelled bodies and beaks pointing toward the sky. Allenby Augustin, the Centre d’Art’s executive director, recently described how some artists, afraid of having to suddenly flee their homes and leave their work behind, bring their pieces to the center or keep them in friends’ homes in different parts of the city. Others add the stray bullets that land inside their studios— bal pèdi or bal mawon —to their canvasses.
St. Eloi, the patriarch of a family of artists, had lived in Carrefour Feuilles since the seventies, working with young people there. “The youth who were neglected or who could not afford to go to school were taken in by our family,” one of St. Eloi’s sons, the musician Duckyns (Zikiki) St. Eloi, told me. “We taught them to paint, to play guitar, and to play the drums. Now they are hired to run errands for gangsters who put guns in their hands.” In spite of what has happened, he still believes that art can turn some things around. He recently sent me a picture of a work by his younger brother Anthony—an image depicting gang members wearing brightly colored balaclavas and holding pencils, a book, a paint palette, a camera, and a musical instrument. “If there are gangs, we’d be better off with art gangs,” Zikiki said. “Gangs that paint, make music, recite poetry. Art is how we bring our best face to the world. Art is how we dream.” ♦
New Yorker Favorites
A Harvard undergrad took her roommate’s life, then her own. She left behind her diary.
Ricky Jay’s magical secrets .
A thirty-one-year-old who still goes on spring break .
How the greatest American actor lost his way .
What should happen when patients reject their diagnosis ?
The reason an Addams Family painting wound up hidden in a university library .
Fiction by Kristen Roupenian: “Cat Person”
Sign up for our daily newsletter to receive the best stories from The New Yorker .
By signing up, you agree to our User Agreement and Privacy Policy & Cookie Statement . This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

By Rebecca Mead

By Rivka Galchen
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
Discrimination Experiences Shape Most Asian Americans’ Lives
4. asian americans and discrimination during the covid-19 pandemic, table of contents.
- Key findings from the survey
- Most Asian Americans have been treated as foreigners in some way, no matter where they were born
- Most Asian Americans have been subjected to ‘model minority’ stereotypes, but many haven’t heard of the term
- Experiences with other daily and race-based discrimination incidents
- In their own words: Key findings from qualitative research on Asian Americans and discrimination experiences
- Discrimination in interpersonal encounters with strangers
- Racial discrimination at security checkpoints
- Encounters with police because of race or ethnicity
- Racial discrimination in the workplace
- Quality of service in restaurants and stores
- Discrimination in neighborhoods
- Experiences with name mispronunciation
- Discrimination experiences of being treated as foreigners
- In their own words: How Asian Americans would react if their friend was told to ‘go back to their home country’
- Awareness of the term ‘model minority’
- Views of the term ‘model minority’
- How knowledge of Asian American history impacts awareness and views of the ‘model minority’ label
- Most Asian Americans have experienced ‘model minority’ stereotypes
- In their own words: Asian Americans’ experiences with the ‘model minority’ stereotype
- Asian adults who personally know an Asian person who has been threatened or attacked since COVID-19
- In their own words: Asian Americans’ experiences with discrimination during the COVID-19 pandemic
- Experiences with talking about racial discrimination while growing up
- Is enough attention being paid to anti-Asian racism in the U.S.?
- Acknowledgments
- Sample design
- Data collection
- Weighting and variance estimation
- Methodology: 2021 focus groups of Asian Americans
- Appendix: Supplemental tables
Following the coronavirus outbreak, reports of discrimination and violence toward Asian Americans increased. A previous Pew Research Center survey of English-speaking Asian adults showed that as of 2021, one-third said they feared someone might threaten or physically attack them. English-speaking Asian adults in 2022 were also more likely than other racial or ethnic groups to say they had changed their daily routines due to concerns they might be threatened or attacked. 19
In this new 2022-23 survey, Asian adults were asked if they personally know another Asian person in the U.S. who had been attacked since the pandemic began.
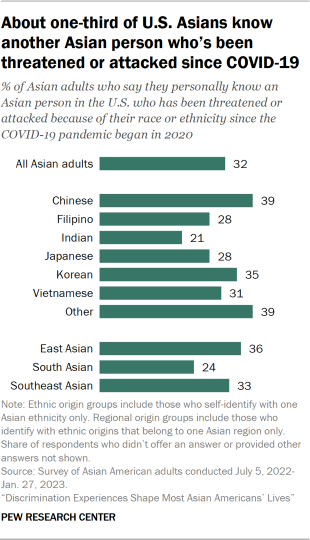
About one-third of Asian adults (32%) say they personally know an Asian person in the U.S. who has been threatened or attacked because of their race or ethnicity since the COVID-19 pandemic began in 2020.
Whether Asian adults know someone with this experience varies across Asian ethnic origin groups:
- About four-in-ten Chinese adults (39%) say they personally know another Asian person who has been threatened or attacked since the coronavirus outbreak. Similar shares of Korean adults (35%) and those who belong to less populous Asian origin groups (39%) – those categorized as “other” in this report – say the same.
- About three-in-ten Vietnamese (31%), Japanese (28%) and Filipino (28%) Americans and about two-in-ten Indian adults (21%) say they know another Asian person in the U.S. who has been the victim of a racially motivated threat or attack.
Additionally, there are some differences by regional origin groups:
- Overall, similar shares of East and Southeast Asian adults say they know another Asian person who’s been threatened or attacked because of their race or ethnicity (36% and 33%, respectively).
- A somewhat smaller share of South Asian adults say the same (24%).
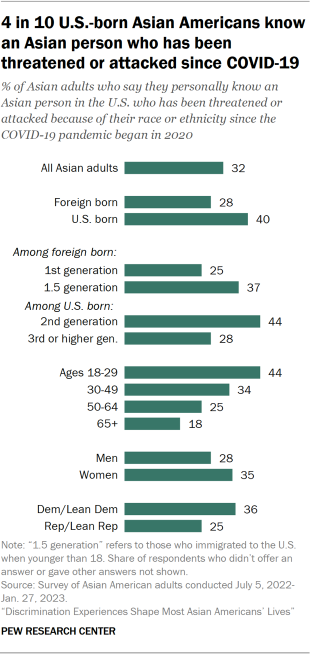
There are also differences across nativity and immigrant generations:
- U.S.-born Asian adults are more likely than Asian immigrants to say they know another Asian person who has been threatened or attacked during the COVID-19 pandemic (40% vs. 28%, respectively).
- Among immigrants, those who are 1.5 generation – those who came to the U.S. as children – are more likely than the first generation – those who immigrated as adults – to say they know someone with this experience (37% vs. 25%).
- And among U.S.-born Asian Americans, 44% of second-generation adults say this, compared with 28% of third- or higher-generation Asian adults.
Whether Asian Americans personally know another Asian person who was threatened or attacked because of their race or ethnicity since the beginning of the pandemic also varies across other demographic groups:
- Age: 44% of Asian adults under 30 years old say they know someone who has been threatened or attacked during the pandemic, compared with 18% of those 65 and older.
- Gender: Asian women are somewhat more likely than men to say they know an Asian person in the U.S. who has been threatened or attacked during the COVID-19 pandemic (35% vs. 28%, respectively).
- Party: 36% of Asian Democrats and Democratic leaners say they know another Asian person who has been threatened or attacked because of their race or ethnicity, higher than the share among Republicans and Republican leaners (25%).
Heightened anti-Asian discrimination during the COVID-19 pandemic
These survey findings follow a spike in reports of discrimination against Asian Americans during the COVID-19 pandemic. The number of federally recognized hate crime incidents of anti-Asian bias increased from 158 in 2019 to 279 in 2020 and 746 in 2021, according to hate crime statistics published by the FBI . In 2022, the number of anti-Asian hate crimes decreased for the first time since the coronavirus outbreak, to 499 incidents. Between March 2020 and May 2023, the organization Stop AAPI Hate received more than 11,000 self-reported incidents of anti-Asian bias, the vast majority of which involved harassment, bullying, shunning and other discrimination incidents.
Additionally, previous research found that calling COVID-19 the “Chinese Virus,” “Asian Virus” or other names that attach location or ethnicity to the disease was associated with anti-Asian sentiment in online discourse. Use of these phrases by politicians or other prominent public officials, such as by former President Donald Trump , coincided with greater use among the general public and more frequent instances of bias against Asian Americans.
In the 2021 Pew Research Center focus groups of Asian Americans, participants discussed their experiences of being discriminated against because of their race or ethnicity during the COVID-19 pandemic.
Participants talked about being shamed in both public and private spaces. Some Asian immigrant participants talked about being afraid to speak out because of how it might impact their immigration status:
“I was walking in [the city where I live], and a White old woman was poking me in the face saying, ‘You are disgusting,’ and she was trying to hit me. I ran away crying. … At the time, I was with my boyfriend, but he also just came to the U.S., so we ran away together thinking that if we cause trouble, we could be deported.”
–Immigrant woman of Korean origin in late 20s (translated from Korean)
“[A very close friend of mine] lived at [a] school dormitory, and when the pandemic just happened … his room was directly pasted with the adhesive tape saying things like ‘Chinese virus quarantine.’”
–Immigrant man of Chinese origin in early 30s (translated from Mandarin)
Many participants talked about being targeted because others perceive them as Chinese , regardless of their ethnicity:
“I think the crimes [that happened] against other Asian people can happen to me while going through COVID-19. When I see a White person, I don’t know if their ancestors are Scottish or German, so they will look at me and think the same. It seems that they can’t distinguish between Korean and Chinese and think that we are from Asia and the onset of COVID-19 is our fault. This is something that can happen to all of us. So I think Asian Americans should come together and let people know that we are also human and we have rights. I came to think about Asian Americans that they shouldn’t stay still even if they’re trampled on.”
–Immigrant woman of Korean origin in early 50s (translated from Korean)
“Even when I was just getting on the bus, [people acted] as if I was carrying the virus. People would not sit with me, they would sit a bit far. It was because I look Chinese.”
–Immigrant woman of Bhutanese origin in early 30s (translated from Dzongkha)
Amid these incidents, some participants talked about feeling in community and kinship with other Asian people:
“[When I hear stories about Asian people in the news,] I feel like automatically you just have a sense of connection to someone that’s Asian. … [I]t makes me and my family and everyone else that I know that is Asian super mad and upset that this is happening. [For example,] the subway attacks where there was a mother who got dragged down the stairs for absolutely no reason. It just kind of makes you scared because you are Asian, and I would tell my mom, ‘You’re not going anywhere without me.’ We got pepper spray and all of that. But there is definitely a difference because you just feel a connection with them no matter if you don’t know them.”
–U.S.-born woman of Taiwanese origin in early 20s
“[A]s a result of the pandemic, I think we saw an increase in Asian hate in the media. I think that was one time where I realized as an Asian person, I felt a lot of pain. I felt a lot of fear, I felt a lot of anger and frustration for my community. … I think it was just at that specific moment when I saw the Asian hate, Asian hate crimes, and I realized, ‘Oh, they’re targeting my people.’ I don’t know how to explain it exactly. I never really referred to myself just plainly as an Asian American, but when I saw it in that media and I saw people who looked like me or people who I related with getting hurt and mistreated, I felt anger for that community, for my community.””
–U.S.-born woman of Korean origin in late teens
Some connected discrimination during the pandemic to other times of heightened anti-Asian discrimination . For example, one woman connected anti-Asian discrimination during COVID-19 to the period after Sept. 11:
“[T]he hate crimes I’m reading about now are towards Chinese [people] because of COVID, but I remember after 9/11, that was – I remember the looks that people would give me on the subway but also reading the violent acts committed towards Indians of all types, just the confusion – I mean, I say confusion but I mean really they wanted to attack Muslims, but they didn’t care, they were just looking for a brown person to attack. So there’s always something that happens that then suddenly falls on one community or another.”
–U.S.-born man of Indian origin in late 40s
- Pew Research Center’s American Trends Panel surveys of Asian adults were conducted only in English and are representative of the English-speaking Asian adult population. In 2021, 70% of Asian adults spoke only English or said they speak English “very well,” according to a Pew Research Center analysis of the 2021 American Community Survey. By contrast, the Center’s 2022-23 survey of Asian Americans was conducted in six languages, including Chinese (Simplified and Traditional), English, Hindi, Korean, Tagalog and Vietnamese. ↩
Sign up for our weekly newsletter
Fresh data delivery Saturday mornings
Sign up for The Briefing
Weekly updates on the world of news & information
- Asian Americans
- Discrimination & Prejudice
- Immigration Issues
- Race Relations
- Racial Bias & Discrimination
Key facts about Asian Americans living in poverty
Methodology: 2023 focus groups of asian americans, 1 in 10: redefining the asian american dream (short film), the hardships and dreams of asian americans living in poverty, key facts about asian american eligible voters in 2024, most popular, report materials.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Age & Generations
- Coronavirus (COVID-19)
- Economy & Work
- Family & Relationships
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- Methodological Research
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Politics & Policy
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
Terms & Conditions
Privacy Policy
Cookie Settings
Reprints, Permissions & Use Policy
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Environ Res Public Health

The Effects of Sleep Quality on Dream and Waking Emotions
Francesca conte.
1 Department of Psychology, University of Campania L. Vanvitelli, Viale Ellittico 31, 81100 Caserta, Italy; [email protected] (O.D.R.); [email protected] (M.L.R.); [email protected] (G.F.)
Nicola Cellini
2 Department of General Psychology, University of Padova, Via Venezia 8, 35131 Padova, Italy; [email protected]
3 Department of Biomedical Sciences, University of Padova, Via Ugo Bassi 58/B, 35131 Padova, Italy
4 Padova Neuroscience Center, University of Padova, Via Giuseppe Orus 2, 35131 Padova, Italy
5 Human Inspired Technology Center, University of Padova, Via Luzzatti 4, 35121 Padova, Italy
Oreste De Rosa
Marissa lynn rescott, serena malloggi.
6 Department NEUROFARBA, University of Firenze, Via di San Salvi 12, 50135 Firenze, Italy; [email protected] (S.M.); [email protected] (F.G.)
Fiorenza Giganti
Gianluca ficca, associated data.
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
Despite the increasing interest in sleep and dream-related processes of emotion regulation, their reflection into waking and dream emotional experience remains unclear. We have previously described a discontinuity between wakefulness and dreaming, with a prevalence of positive emotions in wakefulness and negative emotions during sleep. Here we aim to investigate whether this profile may be affected by poor sleep quality. Twenty-three ‘Good Sleepers’ (GS) and 27 ‘Poor Sleepers’ (PS), identified through the Pittsburgh Sleep Quality Index (PSQI) cut-off score, completed three forms of the modified Differential Emotions Scale, assessing, respectively, the frequency of 22 emotions over the past 2 weeks, their intensity during dreaming and during the previous day. The ANOVA revealed a different pattern of emotionality between groups: GS showed high positive emotionality in wakefulness (both past 2 weeks and 24 h) with a significant shift to negative emotionality in dreams, while PS showed evenly distributed emotional valence across all three conditions. No significant regression model emerged between waking and dream affect. In the frame of recent hypotheses on the role of dreaming in emotion regulation, our findings suggest that the different day/night expression of emotions between groups depends on a relative impairment of sleep-related processes of affect regulation in poor sleepers. Moreover, these results highlight the importance of including sleep quality assessments in future dream studies.
1. Introduction
The interaction between sleep and affective brain function has received attention only in the last couple of decades. As pointed out by Walker and van der Helm [ 1 ], this delay appears surprising in light of two observations. On one hand, there is significant overlap between sleep physiology and the brain networks and neurochemical processes involved in affective modulation; in addition, sleep dysfunctions co-occur with remarkable frequency in most affective psychiatric disorders [ 1 ].
Despite the dearth of past research on the topic, recent work has begun to point out the importance of sleep for the regulation of emotions (see, e.g., [ 2 ] for a recent review). The role of sleep in affective processing is generally explained in light of the peculiar neurophysiology of sleep, and REM sleep in particular (see, e.g., [ 1 , 3 ]). In fact, this sleep state is associated with a relative deactivation of several areas of the neocortex [ 4 , 5 ], paralleling an increased activity in subcortical regions [ 4 , 6 ]. This pattern of activation, accompanied by the distinctive neurochemical balance occurring during REM sleep [ 7 , 8 ], is believed to provide optimal conditions for offline processing of emotional information.
In line with the prominent involvement hypothesized for REM sleep in emotional processing, the most recent theoretical approaches propose an important role of mental activity occurring during sleep (i.e., dreaming, according to Schredl and Wittman’s definition [ 9 ]) in these complex regulatory processes. At the biological level, it is supported by the existence of largely overlapping neural networks sustaining both (REM) dreaming and emotional processing (extensively reviewed in [ 10 ]). Indeed, several models propose that dreaming actively participates in the regulation of prior daytime emotions by facilitating the resolution of emotional conflicts [ 11 , 12 ], enhancing fear-extinction processes [ 3 ], and depotentiating the affective tone initially associated with waking events [ 1 ]. Another set of hypotheses focuses instead on the role of dreaming in optimizing affective reactions to future waking events: dreaming would allow an offline simulation of threatening or social episodes and a rehearsal of the corresponding threat- or social coping skills (respectively the “threat simulation theory” [ 13 ] and the “social simulation theory” [ 14 ]). Ultimately, both types of models converge in suggesting that waking and dream emotions are closely connected and that emotional processing occurring in dreams promotes adaptive behavioral responses to the challenges of waking life.
However, a clear understanding of the relationship between waking and dream emotions and their expression in subjective daytime consciousness and sleep mentation is still lacking. A recent study by our group [ 15 ] has addressed this issue in a sample of healthy adults: emotions of the last recalled dream, as well as those of the previous day and previous two weeks, were collected (through the modified Differential Emotions Scale, mDES [ 16 , 17 ]) and compared. Our findings mainly highlighted a discontinuity between waking and dream affect, with positive emotionality prevailing during the past two weeks as well as the day before the dream and reduced in the dream, while negative emotionality of the dream was similar to that of the preceding two weeks but significantly increased relative to the previous day. This interesting pattern of results opened the way to several hypotheses, such as the possibility that positive and negative emotions experienced in wakefulness may undertake different but parallel sleep-related regulation pathways.
As also suggested in the discussion of those findings [ 15 ], another intriguing hypothesis is that the relationships between waking and dream emotions (plausibly reflecting affective regulation processes) may be modulated by sleep quality. In fact, in the last couple of decades, a vast amount of research has focused on the effects of sleep disruption on several aspects of affective processing.
One night of sleep deprivation is sufficient to increase subjective reports of stress, anxiety, and anger in response to low-stress situations [ 18 ] and to increase impulsivity toward negative stimuli [ 19 ]. Moreover, after one night of sleep deprivation, subjects evaluated neutral pictures more negatively than control participants [ 20 , 21 ], independently of negative mood [ 20 ]. Impairments of emotion recognition [ 22 ] and expression [ 23 ] have been observed as well after single-night sleep deprivation.
Other studies provide evidence of emotional dysregulation following sleep deprivation using neural and physiological measures of emotionality. Enhanced amygdala reactivity in response to emotionally negative pictures, paralleled by a reduction of functional connectivity with medial prefrontal regions (believed to exert top-down regulatory control on the amygdala), has been detected after one night of sleep deprivation [ 24 ] as well as after five nights of sleep restriction [ 25 ]. Also, sleep loss has been shown to amplify pupil diameter responses during passive viewing of negative emotional pictures [ 26 ] and to increase sympathetic dominance of the autonomic nervous system, indexed by changes in heart rate variability [ 27 ].
An impact of sleep loss on affective processing has also been described in more ecologically relevant paradigms, i.e., based on cumulative sleep restriction protocols or on samples with impaired sleep quality. For instance, negative emotional changes have been reported in both adults [ 28 ] and adolescents [ 29 ] after several days of sleep restriction. Furthermore, poor subjective sleep quality has been associated with higher negative [ 30 , 31 ] and lower positive emotionality [ 30 , 31 , 32 ] and with decreased ability in cognitive reappraisal [ 33 ]. Habitual self-reported sleep quality has also been found to moderate the relationship between threat-related amygdala reactivity, negative affect, and perceived stress [ 34 ]. Furthermore, Tempesta et al. [ 21 ] showed that poor sleepers (classified through the Pittsburgh Sleep Quality Index, PSQI [ 35 ]) evaluated neutral pictures more negatively than good sleepers.
In sum, this brief review of data provides strong support to the idea that sleep disruption impairs affective regulation. In light of the aforementioned hypotheses on dreams as a reflection of ongoing emotional processing, dream emotions of individuals with disturbed sleep may represent an interesting object of study. The very few studies addressing this issue show that dreams of insomniacs [ 36 , 37 , 38 ] and narcolecptic subjects [ 39 ] are more negatively toned than those of good sleepers; also, nightmare frequency appears to be more elevated in individuals with poor sleep quality [ 40 , 41 , 42 , 43 ]. However, focusing exclusively on dream emotions, these studies do not allow the authors to make hypotheses on the possible differences between good and poor sleepers in emotion regulatory processes, which are probably better expressed in the relationships between waking and dream emotions rather than in dream emotions alone.
Indeed, several hypotheses on the presentation of waking and dream emotions in good and poor sleepers may be put forward. For instance, the profile of differences between daytime and dream emotionality observed in our previous study [ 15 ] could emerge in poor sleepers as well, indicating the presence of a similar pathway of affective processing notwithstanding the possible dysfunctionality of emotion regulation processes in poor sleepers observed in previous literature (e.g., [ 21 , 33 ]). Alternatively, poor sleepers could display an inverse pattern of emotionality in wakefulness and dreaming relative to good sleepers, with negative tone predominant in wakefulness and a positive rebound in sleep. Also, at variance with good sleepers, poor sleepers could manifest a more evenly distributed emotional tone (similar in both states of consciousness), and so on. The possibilities are multiplied when considering the time span over which these mechanisms unfold: for instance, each dream may process emotions experienced the day before, a few days before (in analogy with literature on the “dream lag” and “day-residue” effect [ 44 , 45 ]), or during wider daytime spans (e.g., the last few weeks, the general “time period”), etc.
Therefore, here we conduct an exploratory study to investigate the relationships between waking emotions and those of the subsequent night’s dreams in a sample of good and poor sleepers identified through the PSQI [ 35 ]. Specific aims of our study are:
- to compare, between good and poor sleepers, the prevalent emotional valence of the dream with that of the previous day and previous weeks;
- to assess the possibility that waking emotionality predicts dream emotionality in good and poor sleepers;
- to confirm findings from previous literature on dream emotional valence in good and poor sleepers using an instrument, the mDES [ 16 , 17 ], which addresses a repertoire of emotions broader than the ones commonly used in dream literature.
2. Materials and Methods
2.1. participants and procedure.
Figure 1 displays the recruitment and selection process. Four hundred volunteers from the cities of Naples and Caserta (Italy) were screened through a brief ad-hoc interview to collect general demographic data and information on medical conditions and life habits. The interview was conducted via telephone by a psychologist from the Sleep Lab of the University of Campania. Two hundred and twelve healthy participants (163 F, 49 M; mean age: 25 ± 8 years) were thus selected for the study, according to the following inclusion criteria: age between 18 and 65 years; absence of any relevant somatic or psychiatric disorder; absence of any sleep apnea or respiratory disorder symptoms; having a regular sleep–wake pattern; absence of sleep disorders; no history of drug or alcohol abuse; limited caffeine (no more than 150 mg caffeine per day, corresponding to about three cups of espresso or one cup of American coffee) and alcohol (no more than 250 mL per day) consumption.

Flowchart of the participant recruitment and selection process.
The whole selected sample ( N = 212) participated in a larger study [ 15 ], which included a validation of the Italian version of the mDES [ 16 , 17 ]. Thus, two forms of the questionnaire (WAKE-24 h and WAKE-2 weeks, assessing the frequency of specific emotions over the past 2 weeks and their intensity in the past 24 h, respectively) were administered to participants along with the Mannheim Dream Questionnaire (MADRE [ 46 ]) to collect data on dream recall frequency and several variables related to dreams, and the PSQI [ 35 ], in its Italian version [ 47 ], to assess habitual subjective sleep quality.
Of the 212 participants included in the validation study, 50 (38 F, 12 M; mean age: 24.6 ± 6.4 years) volunteered to take part in a second phase of the study, i.e., the assessment of relationships between waking and dream emotions. Participants received 10 copies of the WAKE-24 h mDES, with the instruction to complete one each night at bedtime, referring to the emotions experienced during that particular day. This had to be done until the day they recalled a dream. On the morning they recalled a dream, they had to fill in the DREAM mDES, specifically referring to the emotions experienced during the dream. Data collection was thus ended as soon as the mDES ratings of one dream were provided by each participant.
While our previous study [ 15 ] focused on differences between waking and dream emotions in the general sample, this study analyses the same dataset with regard to sleep quality, i.e., by dividing the final sample ( N = 50) into a group of ‘Good Sleepers’ and a group of ‘Poor Sleepers’ (GS and PS, respectively) based on the PSQI cut-off score (scores ≥ 5 indicate poor sleep quality [ 35 ]).
2.2. Instruments
- Italian version of the mDES: The original mDES [ 16 , 17 ] consists of 20 items corresponding to 20 different emotions (10 positive and 10 negative) whose intensity over the past 24 h is rated on a five-point Likert scale (from 0 = Not at all, to 4 = Extremely). Each category is described by three adjectives (e.g., “Grateful, appreciative, or thankful”): for clarity purposes, throughout the manuscript the noun referring to the first of the three adjectives will be used to identify specific emotion categories (e.g., “Gratefulness”). The Italian version [ 15 ] includes two additional positive emotions (“sexual/desiring/flirtatious” and “sympathy/concern/compassion”), which were included in the earlier version of the instrument [ 16 ]. In addition to this standard version (labeled WAKE-24 h mDES [ 15 ]), two other forms of the scale were developed in our previous study [ 15 ], assessing, respectively, the frequency of each emotion over the past two weeks (WAKE-2 weeks mDES) and the intensity of emotions experienced during the last recalled dream (DREAM mDES). The specific instructions provided in the DREAM and the WAKE-24 h mDES versions are: “Please think back to how you have felt during your last recalled dream/last 24 h. Using the 0–4 scale below, indicate the greatest amount that you’ve experienced each of the following feelings.” As for the WAKE-2 weeks form, the instructions are: “Please think back to how you have felt during the past two weeks. Using the 0–4 scale below, indicate the frequency with which you’ve experienced each of the following feelings.” (from 0 = Never, to 4 = Very frequently). The mDES also allows the use of aggregate measures of positive and negative emotionality (the Positive Affect (PA) and Negative Affect (NA) subscales, i.e., average scores of the positive and negative emotion items, respectively), which have shown to have high internal reliability, ranging from 0.82 to 0.94 [ 48 , 49 ]. The scale has been validated on the Greek [ 50 ] and Italian [ 15 ] populations and has shown to have good psychometric properties in its various translations [ 15 , 50 , 51 , 52 , 53 ].
- PSQI [ 35 ]: This questionnaire assesses sleep quality and disturbances over a 1-month time interval. It consists of 19 individual items which generate seven component scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication and daytime dysfunction. The sum of scores for these seven components yields one global score, ranging from 0 to 21, with 5 as a cut-off score which allows to differentiate good from poor sleepers [ 35 ] (higher scores indicate worse sleep quality). Here we use the Italian version of the PSQI [ 47 ], which has been validated on the Italian population [ 47 ].
- MADRE questionnaire [ 46 ]: This questionnaire measures several variables related to dreams such as frequency of dream recall, nightmares and lucid dreaming, attitude towards dreams and the effects of dreams on waking life. We report frequency of dreams, lucid dreams, and nightmares, as well as intensity of the dream experience, attitude towards dreams and correlates of dreams (the sum of items 13-14-15-16-17), all referring to how the contents of dreams are used in terms of problem solving and creativity (see [ 54 ]).
2.3. Data Analysis
Differences between GS and PS in age, gender distribution and MADRE scores were assessed using independent t -test, χ 2 (for categorical data) and Mann–Whitney test (for ordinal data). To assess the differences between groups in emotional valence of dreams and previous wakefulness, we conducted a 2 (Group: GS, PS) × 3 (Condition: WAKE-2 weeks, WAKE-24 h, DREAM) mixed ANOVA, with Δ mDES score (PA minus NA, i.e., an aggregate measure of valence, with positive values indicating positive valence and negative values indicating negative valence) as dependent variable. We used η 2 p as a measure of effect size and the Holm test for post-hoc analysis.
Also, in order to explore the potential predictors of dream emotions, we conducted, separately for GS and PS, a linear regression with DREAM Δ scores as dependent variables and WAKE-2 weeks and WAKE-24 h Δ scores as predictors. For each significant predictor, we reported the unstandardized (b) and the standardized (β) coefficient. All analyses were conducted using JAMOVI 1.2.27 and a p < 0.05 was considered statistically significant.
3.1. Descriptives
The sample was made up of 38 females (76%) and 12 males (24%), with an age range of 19 to 52 years.
A total of 50 DREAM mDES and 50 WAKE-2 weeks mDES (one per participant) were collected. As for the WAKE-24 h version, 84 scales were collected in total (50 referring to the day immediately preceding the dream and the remaining referred to the previous days); in fact, 30 participants (60%) recalled a dream after 1 night, 6 participants (12%) after 2 nights, and the remaining 14 (28%) after 3 nights. Only the 50 WAKE-24 h mDES scales (one per participant) referring to the day before the recalled dream were included in data analyses.
Twenty-seven participants (54% of the sample) reported a PSQI > 5 and were thus classified as PS [ 35 ], while the remaining 23 subjects (46%) made up the GS group. GS and PS were similar in terms of age (GS: 25.26 ± 7.39 vs. PS: 23.96 ± 5.04, t = 0.734, p = 0.466, Cohen’s d = 0.21) and gender distribution (GS: 5 M, 18 F vs. PS: 7 M, 20 F, χ 2 1 = 0.119, p = 0.730), while they significantly differed in PSQI scores (GS: 3.70 ± 0.92 vs. PS: 7.93 ± 1.83, t = −10.496, p < 0.001, Cohen’s d = −2.837).
3.2. MADRE Scores in Good and Poor Sleepers
The two groups showed similar dream frequency (median = 4, W = 301, p = 0.858), intensity of the dream experience (median = 2.5, W = 307.5, p = 0.959), attitude towards dreams (median = 2.4, W = 703.5, p = 0.770) and correlates of the dream experience (mean = 11, W = 286.5, p = 0.647). A significant difference was observed for the frequency of lucid dreams (W = 194.5, p = 0.023), with higher frequency in PS (median = 4) compared to GS (median = 3). The frequency of nightmares was nominally higher (W = 224.5, p = 0.091) in PS (median = 3) relative to GS (median = 4).
3.3. Characterisctics of Dream Emotions
3.3.1. good sleepers.
In GS, scores at the PA and NA subscales of the DREAM mDES showed a higher intensity of negative emotionality in the dream (PA: 0.80 ± 0.58 vs. NA: 1.40 ± 1.30; t22 = −2.29, p = 0.032, Cohens’ d = −0.48).
Looking at the specific emotions, all dreams contain at least 8 emotions and all of the 22 emotions are reported at least once. On average, GS reported 12.08 ± 4.79 dream emotions. As displayed in Figure 2 , the most frequent emotion is Sadness (reported by 91.3% of the participants), followed by Fear (82.6%) and Anger (78.3%), while the least frequent are Sensuality (30.4%) and Inspiredness (30.43%).

Proportion of Good Sleepers reporting each of the 22 emotions during the dream.
The most intensely experienced emotions during the dream were mostly negative ( Figure 3 ): Sadness (2.00 ± 0.28) was followed by Fear (1.87 ± 1.32), Stress (1.878 ± 1.28), Anger (1.70 ± 1.40), and Awe (1.61 ± 1.12).

Scores of each emotion in the WAKE-2 weeks, WAKE-24 h, and DREAM mDES in Good Sleepers. Panels ( a , b ) display positive and negative emotions, respectively. Error bars represent standard error of the means.
3.3.2. Poor Sleepers
Scores at the PA and NA subscales of the DREAM mDES did not differ (PA: 1.10 ± 0.76 vs. NA: 1.13 ± 0.81; t26 = −0.10, p = 0.925, Cohens’ d = −0.02), indicating an equal intensity of positive and negative emotionality in the dreams of PS.
As for specific emotions, all dreams contain at least 5 emotions and all of the 22 emotions are reported at least once. On average, PS reported 12.63 ± 4.39 dream emotions. As displayed in Figure 4 , the most frequent emotion is Awe (reported by 81.5% of the participants), followed by Pride (77.8%) and Solidarity (74.1%), while the least frequent are Gratefulness (37.04%) and Sensuality (29.6%).

Proportion of Poor Sleepers reporting each of the 22 emotions during the dream.
Although PA and NA scores did not differ, the most intensely experienced emotions during the dream were mostly negative ( Figure 5 ): Awe (1.74 ± 0.22) was followed by Anger (1.55 ± 0.25), Sadness (1.52 ± 0.26), Fear (1.48 ± 0.27), and Stress (1.41 ± 0.27).

Scores of each emotion in the WAKE-2 weeks, WAKE-24 h, and DREAM mDES in Poor Sleepers. Panels ( a , b ) display positive and negative emotions, respectively. Error bars represent standard error of the means.
3.4. Differences between Waking and Dream Emotions in Good and Poor Sleepers
The ANOVA on Δ mDES scores yielded a significant main effect of condition (F 2,96 = 15.41, p < 0.001, η p 2 = 0.24), with a decrease of delta scores (i.e., more negative emotionality) in the DREAM compared to WAKE-2 weeks and WAKE-24 h (all p holm ’s < 0.001), and no difference between WAKE-2 weeks and WAKE-24 h (p holm = 0.923). Although we did not find a main effect of Group (F 1,48 = 0.40, p = 0.528, η p 2 < 0.01), we observed a significant Group × Condition interaction (F 2,96 = 4.72, p = 0.011, η p 2 = 0.09, Figure 6 ): only GS displayed a reduction of delta scores from wakefulness to dream (WAKE-2 weeks vs. DREAM: p holm < 0.001; WAKE-24 h vs. DREAM: p holm < 0.001), while PS did not show any significant change (all p holm ’s > 0.644). No between-groups differences were observed in any of the three conditions (all p holm ’s > 0.643, Table 1 ).

Change in Δ mDES scores (PA minus NA) as a function of condition (WAKE-2 weeks, WAKE-24 h, and DREAM) in Good and Poor Sleepers. The orange area indicates positive affect and the blue area indicates negative affect. Error bars represent standard error of the means.
Mean and standard error of Δ scores in the two groups across conditions.
3.5. Predictors of Dream Emotional Valence (Δ mDES Scores) in Good and Poor Sleepers
In GS, linear regression analysis showed that neither WAKE-2 weeks nor WAKE-24 h Δ scores were predictive of DREAM Δ scores (F 2,20 = 0.04, p = 0.952, Adj. R2 < 0.01). The same result was observed in PS (F 2,24 = 0.99, p = 0.387, Adj. R2 < 0.01).
4. Discussion
This study investigated the relationships between dream emotions and those experienced during the previous days (both the day before the recalled dream and over the two weeks preceding it) in good and poor sleepers. In the frame of theoretical models on the role of dreaming in emotion regulation, postulating a close link between waking and dream emotionality, we aimed to assess the influence of poor sleep quality on this relationship. In fact, though previous literature has already shown the prevalence of negatively toned dreams in populations with disturbed sleep, we believe that affect regulation processes are plausibly better expressed in the interplay between waking and dream emotions rather than in dream emotions alone.
4.1. Proportion of Good and Poor Sleepers
Before discussing our main results, it is worth commenting on the high proportion of poor sleepers that emerged in our sample (54%). Considering that most of our participants were university students (mean age: 24.6 ± 6.4 years), this result is in line with those of several wide survey studies assessing the prevalence of poor sleep quality through the PSQI on similar populations and age groups. Indeed, the proportion of poor sleepers was over 40% in Mah et al. [ 55 ] and exceeded 60% in Lund et al. [ 56 ] and Becker et al. [ 57 ].
4.2. Results from the MADRE Questionnaire in Good and Poor Sleepers
Data from the MADRE questionnaire show that GS and PS are similar in most dream related variables, including dream frequency, intensity of dreams, attitude towards dreams, and perceived effects of dreams on waking life problem solving and creativity skills. However, PS show a higher frequency both of nightmares and lucid dreams. As for nightmares, this finding is consistent with previous studies showing increased nightmare frequency in poor sleepers [ 40 , 41 , 42 , 43 ]. Also, lucid dreaming has sometimes been associated with disrupted sleep [ 58 , 59 ]. Interestingly, nightmares and lucid dreaming have been conceptualized as belonging to a common domain involving unusual cognitions and perceptions in wakefulness and sleep [ 60 ], which would be linked to arousal and hypervigilance intruding in the sleep state [ 58 , 61 ] and thus could be viewed as indicators of poor sleep quality [ 58 ]. In other words, these hypotheses point to the existence of a close link between the quality of physiological sleep features and that of subjective sleep mentation.
4.3. Frequency and Valence of Dream Emotions in Good and Poor Sleepers
GS and PS reported on average a similar number of dream emotions (slightly more than 12), suggesting that the average amount of emotions (12.38) found in our previous study on the whole sample [ 15 ] was not affected by sleep quality. The number of emotions in our two samples is slightly higher than that reported in previous literature using the same self-report scale [ 62 ], probably because of methodological differences (see [ 15 ]).
As for emotional valence, GS displayed higher negative than positive emotionality (scores at the NA subscale) in the dream, whereas, in PS’ dreams, positive and negative emotionality appeared with equal intensity (no difference between PA and NA scores of the DREAM mDES). This finding well accounts for the one emerged in our previous study [ 15 ], in which NA scores were slightly higher than those at the PA subscale, but the difference failed to reach significance. The higher negative affect observed in GS is coherent with the finding that specific negative emotions were the most frequent as well as the most intense in this sample; also, it is in line with several previous studies showing a prevalence of negative emotions in dreams (e.g., [ 63 , 64 , 65 , 66 ], but see also [ 67 ] for a discussion on the differences between self and external ratings of dream emotions). Instead, the evenly distributed emotional tone observed in PS’ dreams apparently contradicts existing literature on populations with sleep impairments [ 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 ], which points to more negatively toned dreams in these individuals. However, our analysis of specific emotions showed that, although positive emotions were the most frequently reported by PS, their negative emotions were the most intense. In addition, it must be considered that: (a) the instrument we used is quite different from those commonly used in dream research, since it includes a much broader repertoire of emotions and a more balanced number of positive and negative items, thus reducing the risk of underestimating the presence of positive emotionality; (b) results obtained on sleep disordered populations [ 36 , 37 , 38 , 39 ] are not fully comparable to those observed in healthy samples reporting poor sleep quality; (c) the higher frequency of nightmares observed in individuals with poor sleep quality (both in our present study and in previous research [ 40 , 41 , 42 , 43 ]) does not necessarily imply that their dreams are generally more negatively valenced (in fact, emotionality may be viewed as a “tonic” feature of sleep mentation, while nightmares, or lucid dreams, may be better conceptualized as “phasic” events, although the notion of a continuum between bad dreams and nightmares is sustained by several authors [ 68 , 69 ]).
4.4. Relationships between Waking and Dream Emotions in Good and Poor Sleepers
The main finding of our study is the difference observed between GS and PS in the profile of waking and dream emotionality. While GS display a striking inflection of emotional tone from wakefulness to the dream (i.e., affective tone is prevalently positive both during the previous weeks and the previous day and becomes extremely negative in the dream), PS’ emotionality remains stable across conditions. Specifically, in PS, differences between positive and negative emotionality (i.e., delta values) are very close to zero in all three scales.
First of all, this pattern of data suggests that habitual sleep quality significantly affects the interplay of emotional expression across wakefulness and dreaming. This observation is particularly important in light of the numerous discrepancies existing in data on dream features and especially dream emotionality (see [ 62 , 67 , 70 ]). Controversial results in this field are usually explained through methodological biases as well as biases linked to the retrospective nature of dream descriptions [ 62 , 67 , 70 ]. Our data prompt us to consider sleep quality as an additional factor affecting dream emotional experience, and thus able to confound results when not controlled for. Therefore, we believe that future dream investigations should include assessments of sleep quality even when addressing nonclinical samples.
At the theoretical level, the differences observed between GS and PS appear to reflect a different functionality of sleep–wake emotion regulation processes, in line with the recent models on dream-related affect regulation [ 1 , 3 , 11 , 12 , 13 , 14 ]. In other words, the lack of oscillations in prevalent emotional valence of PS (expressed by their flattened curve of Δ mDES scores across daytime and sleep) may depend on a relative impairment of their emotion regulation processes, whose effectiveness would instead be expressed by the opposite emotional tone in wakefulness and dreams of GS. Specifically, GS display a prevalence of positive affect during daytime and negative affect during the dream. As suggested in Conte et al. [ 15 ], the negative emotions experienced more frequently or intensely in the general period in which the dream occurs would be those in need of regulation during sleep, whereas positive emotions, requiring less modulation, would be underrepresented in the dream. Also, the predominant positive affect observed during wakefulness in GS would at least partly depend on effective sleep-related modulation that occurred in previous dreams. As for PS, they showed lower positive emotionality than GS both during the two weeks and the day preceding the dream (although the differences between groups did not reach significance). This observation is in line with past literature showing lower well-being and positive affect in poor sleepers [ 30 , 31 , 32 , 71 ], which also is plausibly linked to less effective sleep-related affect modulation. Moreover, as suggested above, the fact that negative affect does not prevail in PS’ dreams could indicate poor functioning of sleep-related emotion regulation.
A complementary explanation may also be proposed, referring to the recent hypothesis of a dream rebound of thoughts suppressed during wakefulness [ 72 , 73 , 74 ], which, in turn, can be traced back to Freud’s idea [ 75 ] that dreams reflect the return of mental contents inhibited during the waking hours. This kind of mechanism was plausibly active in GS, whose negative emotions, excluded from waking consciousness in favor of positive ones, may have rebounded in the dream. The process of negative affect suppression could instead have been ineffective in PS, possibly due to the fact that disrupted sleep is linked to deficits in higher cognitive functions including inhibition (e.g., [ 19 , 76 , 77 ]). In line with Malinowski et al. [ 78 ], who showed that successful suppression of thoughts and their rebound in the dream benefit the emotional response to pleasant and unpleasant thoughts, it may be hypothesized that, in good sleepers, the dream rebound of negative emotions reflects their effective processing in sleep, irrespective of the specific episodic memories (thoughts, events, etc.) that generated them.
In sum, our findings are probably the result of two parallel mechanisms: a general day-night emotion regulation process (with prevalent negative emotions of daytime being processed in sleep and thus reappearing in dreaming) and the specific suppression (either deliberate or automatic) of certain negative emotions during wakefulness with consequent rebound in the dream for regulation purposes.
It must be acknowledged here that our regression analysis on waking and dream delta scores did not yield significant results. In fact, emotional tone of the previous day and previous two weeks did not predict that of the dream in either group of participants. This result is consistent with three other studies which found few [ 79 ], small [ 80 ], or no correlations [ 81 ] between corresponding dream and previous daytime emotions. The absence, to date, of data on direct relationships between waking and dream affect does not lend support to our main hypothesis, i.e., the interpretation of our data in the frame of theories on dream-related emotion regulation [ 1 , 3 , 11 , 12 , 13 , 14 ]. However, clearer associations between waking and dream affect could exist across different time spans and in different directions than those investigated here and in the abovementioned studies [ 79 , 80 , 81 ]. In fact, as pointed out in the introduction, each dream could process emotions experienced the day before, a few days before (in analogy with literature on the “dream lag” and “day-residue” effect [ 44 , 45 ]), or during wider daytime spans (e.g., the last few weeks, the general “time period”, etc.). Also, as predicted by the “simulation models” [ 13 , 14 ], dream emotionality could reveal stronger associations with future rather than past waking affect, a possibility to be investigated in forthcoming studies.
Furthermore, it may be speculated that poor sleepers rate their dreams as less negatively toned compared to good sleepers also because of a different general perception of the dreaming experience. In other words, they could retrospectively evaluate their dream experience as more positive than it actually was since the simple fact of having dreamed, per se, represents for them a sign of having slept well (good sleepers would obviously have no such bias). This interesting possibility could be usefully investigated in future research.
Finally, our findings allow us to extend the discussion of our previous work on the same sample [ 15 ], by underlining the influence exerted by poor habitual sleep quality on waking and dream emotional expression. In fact, here we observed that the opposite prevalent emotional tone of wakefulness and dreams, emerged in the previous study, well describes GS’ profile, while PS display an equal amount of positive and negative affect in both states. The hypotheses made on these findings are coherent with the main interpretations discussed in our previous work. However, the current data allow us to exclude a couple of alternative explanations advanced on those data [ 15 ]. Specifically, we proposed that participants may have undergone some sort of social desirability effect in compiling the scales (see, e.g., [ 82 ]); in other words, they would have more easily identified positive emotions (coherent with a positive image of the self) in wakefulness and negative emotions in the dream (which is experienced as “involuntary”). Similarly, we acknowledged a possible recall bias linked to the time frame of events to which the emotions refer. In the DREAM mDES, the participant is focusing on a much shorter time frame compared to those of the daytime scales (2 weeks and 24 h). Among this limited pool of memories, the negative ones could appear more salient and thus be more easily recognized (according to the widely held tenet in psychology that “bad is stronger than good” [ 83 ]). While these two hypotheses may have applied to our GS group, we see no reason why PS would not have equally undergone these types of biases: thus, the different emotional profile emerged in the latter group induces us to rule out these possibilities.
4.5. Limitations
Our results should be considered in light of some limitations to be overcome in future research. The main limitation is the use of a self-report measure of sleep quality rather than standard polysomnography for the identification of good and poor sleepers. However, it must be noted that groups of good and poor sleepers classified through the PSQI have been shown to significantly differ in polysomnographic sleep measures in several previous studies [ 35 , 84 , 85 ].
Furthermore, according to some authors [ 67 , 86 ], self-ratings of dream emotions based on emotion rating scales may be biased by demand characteristics of the rating task (i.e., individuals may be primed by answer options) or phenomena such as the positivity offset (i.e., the tendency to experience mildly positive mood most of the time); still, several authors argue that self-ratings more validly represent dream emotional experiences [ 65 , 87 ].
5. Conclusions
In conclusion, to the best of our knowledge, this is the first study to investigate differences between good and poor sleepers in the profile of emotionality across wakefulness and dreaming. Overall, our findings show that good sleepers experience a notable change in emotionality between wakefulness and dreaming, with a prevalence of positive affect during daytime and predominant negative affect during dreaming, whereas poor sleepers are characterized by equal intensity of positive and negative emotionality in both states. In the frame of recent theoretical models postulating a role of dreaming in affect regulation, the lack of changes in prevalent emotional valence across states observed in the latter group may be interpreted as reflecting ineffective sleep-related emotional processing. Furthermore, regardless of the theoretical framework, our results highlight that sleep quality is associated with notable differences in the expression of waking and dream emotions which should not be neglected in future dream research. Therefore, our findings definitely encourage researchers to include sleep quality assessments in dream studies (both on clinical and nonclinical samples) and prompt future investigations on sleep-impaired populations as a privileged object of study in the field of research on dreaming and emotion regulation processes.
Author Contributions
All authors contributed in a meaningful way to this manuscript. Conceptualization, F.C., F.G. and G.F.; Methodology, F.C. and G.F.; Formal analysis, N.C. and O.D.R.; Investigation, O.D.R. and S.M.; Writing—original draft preparation, F.C. and N.C.; Writing—review and editing, F.C., M.L.R. and G.F.; Visualization, F.C. and N.C.; Supervision, F.G. and G.F.; Project administration, F.C., F.G. and G.F. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Institutional Review Board Statement
The study design was submitted to the Ethical Committee of the Department of Psychology, University of Campania “L. Vanvitelli”, which approved the research (code 1/2017) and certified that the involvement of human participants was performed according to acceptable standards.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
Dream content. Dreaming was first investigated on an experimental level in the nineteenth century. Calkins published the first statistical results about dreaming and argued that some aspects of dream content could be quantified.Later, questionnaires and automatic analysis of the lexical content of dream reports allowed psychologists to show that dream content has some precise phenomenological ...
dreams is related to wish fulfillment. Freud believed that the manifest content of a dream, or. the actual imagery and eve nts of the dream, serve d to disguise the latent content or the ...
The Science Behind Dreaming. New research sheds light on how and why we remember dreams--and what purpose they are likely to serve. For centuries people have pondered the meaning of dreams. Early ...
Contemporary dream research. Although dreams have fascinated us since the dawn of time, their rigorous, scientific study is a recent development[1-4] (Supplementary Fig. 1).In The interpretation of dreams [] Freud predicted that "Deeper research will one day trace the path further and discover an organic basis for the mental event."Recent work, which we review in this article, begins to ...
Abstract. The function of dreams is a longstanding scientific research question. Simulation theories of dream function, which are based on the premise that dreams represent evolutionary past ...
1.1. The REM‐NREM sleep dichotomy. A classical view of the neurobiological basis of the oneiric activity postulates the existence of a close relationship between dream experience and REM sleep (Hobson et al., 2000; Nielsen, 2000).This hypothesis was based on early electroencephalographic (EEG) observations showing that >70% of individuals awakened during REM sleep reported dreams, while ...
The paper confronts psychoanalytic dream theories with the findings of empirical dream research. It summarizes the discussion in psychoanalysis around the function of dreams (e.g. as the guardian of sleep), wish-fullfilment or compensation, whether there is a difference between latent and manifest content, etc.
In line with this conceptualization of dreams, a large proportion of dream research 1,3,4,5,6,7 has been dedicated to quantifying various dimensions of people's ... Calls for Papers ...
Dreaming is a multidisciplinary journal, the only professional journal devoted specifically to dreaming. The journal publishes scholarly articles related to dreaming from any discipline and viewpoint. This includes: biological aspects of dreaming and sleep/dream laboratory research; psychological articles of any kind related to dreaming;
Abstract. This paper presents an evolutionary argument for the role of dreams in the development of human cognitive processes. While a theory by Revonsuo (2000) proposes that dreams allow for threat rehearsal and therefore provide an evolutionary advantage, the goal of this paper is to extend this argument by commenting on other fitness ...
A set of 1000 dream reports hand-coded by Hall and Van de Castle themselves in the late 1940s and early 1950s [ 16 ]. Two hundred American university students (100 male and 100 female) in Cleveland (Ohio) were asked to write down five dreams each. Normative values in dream studies are generally based on this set.
There is a rich discussion on the theoretical and methodological implications of dream research for psychiatry (see Scarone et al. 2007; d'Agostino et al. 2013; see Windt & Noreika 2011 as well as the other papers in this special issue) and a number of studies have investigated differences in dream reports from schizophrenic and healthy ...
Dreams are mental, emotional, or sensory experiences that take place during sleep. Sleep experts continue to study what happens in the brain during sleep, but no one knows for sure why we dream. Dreams are the most common and intense during REM sleep when brain activity increases. Dreaming is normal and healthy, but frequent nightmares can ...
Introduction. Whilst lucid dreaming (LD) is defined as being aware of dreaming whilst dreaming, a misconception exists in the public domain as a referral to controlling dream content and plot (Neuhäusler et al., 2018).This misconception reflects a number of widely-held beliefs about the nature of dreaming, which in part this commentary will seek to explain and rectify.
2 The research project 'Structural Dream Analysis' There is also a long tradition of clinical research on dreams in psychoanalysis (Fonagy et al. 2012). A problem with these approaches is that they often include assumptions taken from psychoanalytic theories, e.g. that the dream serves as a guardian of sleep.
Also, dream experience is preserved after pharmacological suppression of REM sleep (Landolt et al., 2001; Oudiette et al., 2012). Finally, dream recall has been recently associated with a similar electrophysiological response after REM and NREM sleep (D'Atri et al., 2019; Siclari et al., 2017). These results suggest that (a) dream and REM sleep ...
Dreams are a common experience. Some are scary, some are funny. Recent research into how the brain works helps us understand why we dream. Strange combinations of ideas in our dreams may make us more creative and give us ideas that help us to solve problems. Or, when memories from the day are repeated in the brain during sleep, memories may get stronger. Dreams may also improve our moods ...
The study also found that 25.7% of dreams were related to specific impending events, and 37.4% of dreams with a future event source were additionally related to one or more specific memories of ...
Upon awakening, a person's guided dream content can be used to complete tasks such as creative story writing, and compared experimentally to waking thought content. "Dormio takes dream research to a new level, interacting directly with an individual's dreaming brain and manipulating the actual content of their dreams," says Stickgold.
So we can add an extra premise to the dreaming argument to represent its dependence on this assumption: (1) Sometimes when you're dreaming, you can't tell whether or not you're dreaming. (1a) Any course of experience you can have while awake can also be had while having one of those sorts of dreams. (2) So, even when you're awake, you ...
Last November, six years into his nine-year prison sentence, Philippe was deported by the U.S. to Haiti. He immediately aligned himself with armed groups and has now put himself forward as a ...
In 2021, Asian households had a median net worth of $320,900, compared with $250,400 for White households. The median net worth of Hispanic households ($48,700) and Black households ($27,100) was much less. In dollar amounts, the wealth gap between White households and Black and Hispanic households increased from 2019 to 2021.
NREM dreams tend to be less emotional and visually vivid, as well as more thought-like (Cavallero, Cicogna, Natale, Occhionero and Zito, 1992; Hobson, Pace-Schott and Stickgold, 2000). Research suggests that lucid dreams, on the other hand, are predominantly a REM sleep phenomenon (LaBerge et al., 1986; LaBerge et al., 1981c). However, this ...
Girls are more likely than boys to say it would be difficult for them to give up social media (58% vs. 49%). Older teens are also more likely than younger teens to say this: 58% of those ages 15 to 17 say it would be very or somewhat hard to give up social media, compared with 48% of those ages 13 to 14. Teens are more likely to say social ...
How Pew Research Center will report on generations moving forward. Journalists, researchers and the public often look at society through the lens of generation, using terms like Millennial or Gen Z to describe groups of similarly aged people. This approach can help readers see themselves in the data and assess where we are and where we're ...
Introduction. Dreams may help to process and integrate people's daily experiences (e.g., De Monchaux 1993; Wamsley and Stickgold 2011), and it is thought that meaningful experiences, particularly those that are threatening (Revonsuo 2000), are at the forefront of dream material (Erikson 1954; Jung 1948/1974).Research based in self-determination theory (SDT; Ryan and Deci 2000, 2017) has ...
Capture land is a Jamaican colloquialism for land that is (or is presumed to be) occupied without the authorization of the landowner. Working through two quotes, "just to make life for a time," and, "we've always been here," this article examines how the practice of capture indexes abolitionist spatial practices of varied temporalities: rooted in belonging to place, but also alighting ...
Following the coronavirus outbreak, reports of discrimination and violence toward Asian Americans increased. A previous Pew Research Center survey of English-speaking Asian adults showed that as of 2021, one-third said they feared someone might threaten or physically attack them. English-speaking Asian adults in 2022 were also more likely than other racial or ethnic groups to say they had ...
1. Introduction. The interaction between sleep and affective brain function has received attention only in the last couple of decades. As pointed out by Walker and van der Helm [], this delay appears surprising in light of two observations.On one hand, there is significant overlap between sleep physiology and the brain networks and neurochemical processes involved in affective modulation; in ...
Gene editing has the potential to solve fundamental challenges in agriculture, biotechnology, and human health. CRISPR-based gene editors derived from microbes, while powerful, often show significant functional tradeoffs when ported into non-native environments, such as human cells. Artificial intelligence (AI) enabled design provides a powerful alternative with potential to bypass ...