- Research article
- Open access
- Published: 04 June 2021

Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews
- Israel Júnior Borges do Nascimento 1 , 2 ,
- Dónal P. O’Mathúna 3 , 4 ,
- Thilo Caspar von Groote 5 ,
- Hebatullah Mohamed Abdulazeem 6 ,
- Ishanka Weerasekara 7 , 8 ,
- Ana Marusic 9 ,
- Livia Puljak ORCID: orcid.org/0000-0002-8467-6061 10 ,
- Vinicius Tassoni Civile 11 ,
- Irena Zakarija-Grkovic 9 ,
- Tina Poklepovic Pericic 9 ,
- Alvaro Nagib Atallah 11 ,
- Santino Filoso 12 ,
- Nicola Luigi Bragazzi 13 &
- Milena Soriano Marcolino 1
On behalf of the International Network of Coronavirus Disease 2019 (InterNetCOVID-19)
BMC Infectious Diseases volume 21 , Article number: 525 ( 2021 ) Cite this article
16k Accesses
28 Citations
13 Altmetric
Metrics details
Navigating the rapidly growing body of scientific literature on the SARS-CoV-2 pandemic is challenging, and ongoing critical appraisal of this output is essential. We aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Nine databases (Medline, EMBASE, Cochrane Library, CINAHL, Web of Sciences, PDQ-Evidence, WHO’s Global Research, LILACS, and Epistemonikos) were searched from December 1, 2019, to March 24, 2020. Systematic reviews analyzing primary studies of COVID-19 were included. Two authors independently undertook screening, selection, extraction (data on clinical symptoms, prevalence, pharmacological and non-pharmacological interventions, diagnostic test assessment, laboratory, and radiological findings), and quality assessment (AMSTAR 2). A meta-analysis was performed of the prevalence of clinical outcomes.
Eighteen systematic reviews were included; one was empty (did not identify any relevant study). Using AMSTAR 2, confidence in the results of all 18 reviews was rated as “critically low”. Identified symptoms of COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%) and gastrointestinal complaints (5–9%). Severe symptoms were more common in men. Elevated C-reactive protein and lactate dehydrogenase, and slightly elevated aspartate and alanine aminotransferase, were commonly described. Thrombocytopenia and elevated levels of procalcitonin and cardiac troponin I were associated with severe disease. A frequent finding on chest imaging was uni- or bilateral multilobar ground-glass opacity. A single review investigated the impact of medication (chloroquine) but found no verifiable clinical data. All-cause mortality ranged from 0.3 to 13.9%.
Conclusions
In this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic were of questionable usefulness. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards.
Peer Review reports
The spread of the “Severe Acute Respiratory Coronavirus 2” (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [ 1 ]. The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [ 2 ], causing massive economic strain, and escalating healthcare and public health expenses [ 3 , 4 ].
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 [ 1 ], and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data [ 5 ]. The living map of COVID-19 evidence, curated by the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre), contained more than 40,000 records by February 2021 [ 6 ]. More than 100,000 records on PubMed were labeled as “SARS-CoV-2 literature, sequence, and clinical content” by February 2021 [ 7 ].
Due to publication speed, the research community has voiced concerns regarding the quality and reproducibility of evidence produced during the COVID-19 pandemic, warning of the potential damaging approach of “publish first, retract later” [ 8 ]. It appears that these concerns are not unfounded, as it has been reported that COVID-19 articles were overrepresented in the pool of retracted articles in 2020 [ 9 ]. These concerns about inadequate evidence are of major importance because they can lead to poor clinical practice and inappropriate policies [ 10 ].
Systematic reviews are a cornerstone of today’s evidence-informed decision-making. By synthesizing all relevant evidence regarding a particular topic, systematic reviews reflect the current scientific knowledge. Systematic reviews are considered to be at the highest level in the hierarchy of evidence and should be used to make informed decisions. However, with high numbers of systematic reviews of different scope and methodological quality being published, overviews of multiple systematic reviews that assess their methodological quality are essential [ 11 , 12 , 13 ]. An overview of systematic reviews helps identify and organize the literature and highlights areas of priority in decision-making.
In this overview of systematic reviews, we aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Methodology
Research question.
This overview’s primary objective was to summarize and critically appraise systematic reviews that assessed any type of primary clinical data from patients infected with SARS-CoV-2. Our research question was purposefully broad because we wanted to analyze as many systematic reviews as possible that were available early following the COVID-19 outbreak.
Study design
We conducted an overview of systematic reviews. The idea for this overview originated in a protocol for a systematic review submitted to PROSPERO (CRD42020170623), which indicated a plan to conduct an overview.
Overviews of systematic reviews use explicit and systematic methods for searching and identifying multiple systematic reviews addressing related research questions in the same field to extract and analyze evidence across important outcomes. Overviews of systematic reviews are in principle similar to systematic reviews of interventions, but the unit of analysis is a systematic review [ 14 , 15 , 16 ].
We used the overview methodology instead of other evidence synthesis methods to allow us to collate and appraise multiple systematic reviews on this topic, and to extract and analyze their results across relevant topics [ 17 ]. The overview and meta-analysis of systematic reviews allowed us to investigate the methodological quality of included studies, summarize results, and identify specific areas of available or limited evidence, thereby strengthening the current understanding of this novel disease and guiding future research [ 13 ].
A reporting guideline for overviews of reviews is currently under development, i.e., Preferred Reporting Items for Overviews of Reviews (PRIOR) [ 18 ]. As the PRIOR checklist is still not published, this study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 statement [ 19 ]. The methodology used in this review was adapted from the Cochrane Handbook for Systematic Reviews of Interventions and also followed established methodological considerations for analyzing existing systematic reviews [ 14 ].
Approval of a research ethics committee was not necessary as the study analyzed only publicly available articles.
Eligibility criteria
Systematic reviews were included if they analyzed primary data from patients infected with SARS-CoV-2 as confirmed by RT-PCR or another pre-specified diagnostic technique. Eligible reviews covered all topics related to COVID-19 including, but not limited to, those that reported clinical symptoms, diagnostic methods, therapeutic interventions, laboratory findings, or radiological results. Both full manuscripts and abbreviated versions, such as letters, were eligible.
No restrictions were imposed on the design of the primary studies included within the systematic reviews, the last search date, whether the review included meta-analyses or language. Reviews related to SARS-CoV-2 and other coronaviruses were eligible, but from those reviews, we analyzed only data related to SARS-CoV-2.
No consensus definition exists for a systematic review [ 20 ], and debates continue about the defining characteristics of a systematic review [ 21 ]. Cochrane’s guidance for overviews of reviews recommends setting pre-established criteria for making decisions around inclusion [ 14 ]. That is supported by a recent scoping review about guidance for overviews of systematic reviews [ 22 ].
Thus, for this study, we defined a systematic review as a research report which searched for primary research studies on a specific topic using an explicit search strategy, had a detailed description of the methods with explicit inclusion criteria provided, and provided a summary of the included studies either in narrative or quantitative format (such as a meta-analysis). Cochrane and non-Cochrane systematic reviews were considered eligible for inclusion, with or without meta-analysis, and regardless of the study design, language restriction and methodology of the included primary studies. To be eligible for inclusion, reviews had to be clearly analyzing data related to SARS-CoV-2 (associated or not with other viruses). We excluded narrative reviews without those characteristics as these are less likely to be replicable and are more prone to bias.
Scoping reviews and rapid reviews were eligible for inclusion in this overview if they met our pre-defined inclusion criteria noted above. We included reviews that addressed SARS-CoV-2 and other coronaviruses if they reported separate data regarding SARS-CoV-2.
Information sources
Nine databases were searched for eligible records published between December 1, 2019, and March 24, 2020: Cochrane Database of Systematic Reviews via Cochrane Library, PubMed, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Web of Sciences, LILACS (Latin American and Caribbean Health Sciences Literature), PDQ-Evidence, WHO’s Global Research on Coronavirus Disease (COVID-19), and Epistemonikos.
The comprehensive search strategy for each database is provided in Additional file 1 and was designed and conducted in collaboration with an information specialist. All retrieved records were primarily processed in EndNote, where duplicates were removed, and records were then imported into the Covidence platform [ 23 ]. In addition to database searches, we screened reference lists of reviews included after screening records retrieved via databases.
Study selection
All searches, screening of titles and abstracts, and record selection, were performed independently by two investigators using the Covidence platform [ 23 ]. Articles deemed potentially eligible were retrieved for full-text screening carried out independently by two investigators. Discrepancies at all stages were resolved by consensus. During the screening, records published in languages other than English were translated by a native/fluent speaker.
Data collection process
We custom designed a data extraction table for this study, which was piloted by two authors independently. Data extraction was performed independently by two authors. Conflicts were resolved by consensus or by consulting a third researcher.
We extracted the following data: article identification data (authors’ name and journal of publication), search period, number of databases searched, population or settings considered, main results and outcomes observed, and number of participants. From Web of Science (Clarivate Analytics, Philadelphia, PA, USA), we extracted journal rank (quartile) and Journal Impact Factor (JIF).
We categorized the following as primary outcomes: all-cause mortality, need for and length of mechanical ventilation, length of hospitalization (in days), admission to intensive care unit (yes/no), and length of stay in the intensive care unit.
The following outcomes were categorized as exploratory: diagnostic methods used for detection of the virus, male to female ratio, clinical symptoms, pharmacological and non-pharmacological interventions, laboratory findings (full blood count, liver enzymes, C-reactive protein, d-dimer, albumin, lipid profile, serum electrolytes, blood vitamin levels, glucose levels, and any other important biomarkers), and radiological findings (using radiography, computed tomography, magnetic resonance imaging or ultrasound).
We also collected data on reporting guidelines and requirements for the publication of systematic reviews and meta-analyses from journal websites where included reviews were published.
Quality assessment in individual reviews
Two researchers independently assessed the reviews’ quality using the “A MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2)”. We acknowledge that the AMSTAR 2 was created as “a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both” [ 24 ]. However, since AMSTAR 2 was designed for systematic reviews of intervention trials, and we included additional types of systematic reviews, we adjusted some AMSTAR 2 ratings and reported these in Additional file 2 .
Adherence to each item was rated as follows: yes, partial yes, no, or not applicable (such as when a meta-analysis was not conducted). The overall confidence in the results of the review is rated as “critically low”, “low”, “moderate” or “high”, according to the AMSTAR 2 guidance based on seven critical domains, which are items 2, 4, 7, 9, 11, 13, 15 as defined by AMSTAR 2 authors [ 24 ]. We reported our adherence ratings for transparency of our decision with accompanying explanations, for each item, in each included review.
One of the included systematic reviews was conducted by some members of this author team [ 25 ]. This review was initially assessed independently by two authors who were not co-authors of that review to prevent the risk of bias in assessing this study.
Synthesis of results
For data synthesis, we prepared a table summarizing each systematic review. Graphs illustrating the mortality rate and clinical symptoms were created. We then prepared a narrative summary of the methods, findings, study strengths, and limitations.
For analysis of the prevalence of clinical outcomes, we extracted data on the number of events and the total number of patients to perform proportional meta-analysis using RStudio© software, with the “meta” package (version 4.9–6), using the “metaprop” function for reviews that did not perform a meta-analysis, excluding case studies because of the absence of variance. For reviews that did not perform a meta-analysis, we presented pooled results of proportions with their respective confidence intervals (95%) by the inverse variance method with a random-effects model, using the DerSimonian-Laird estimator for τ 2 . We adjusted data using Freeman-Tukey double arcosen transformation. Confidence intervals were calculated using the Clopper-Pearson method for individual studies. We created forest plots using the RStudio© software, with the “metafor” package (version 2.1–0) and “forest” function.
Managing overlapping systematic reviews
Some of the included systematic reviews that address the same or similar research questions may include the same primary studies in overviews. Including such overlapping reviews may introduce bias when outcome data from the same primary study are included in the analyses of an overview multiple times. Thus, in summaries of evidence, multiple-counting of the same outcome data will give data from some primary studies too much influence [ 14 ]. In this overview, we did not exclude overlapping systematic reviews because, according to Cochrane’s guidance, it may be appropriate to include all relevant reviews’ results if the purpose of the overview is to present and describe the current body of evidence on a topic [ 14 ]. To avoid any bias in summary estimates associated with overlapping reviews, we generated forest plots showing data from individual systematic reviews, but the results were not pooled because some primary studies were included in multiple reviews.
Our search retrieved 1063 publications, of which 175 were duplicates. Most publications were excluded after the title and abstract analysis ( n = 860). Among the 28 studies selected for full-text screening, 10 were excluded for the reasons described in Additional file 3 , and 18 were included in the final analysis (Fig. 1 ) [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ]. Reference list screening did not retrieve any additional systematic reviews.
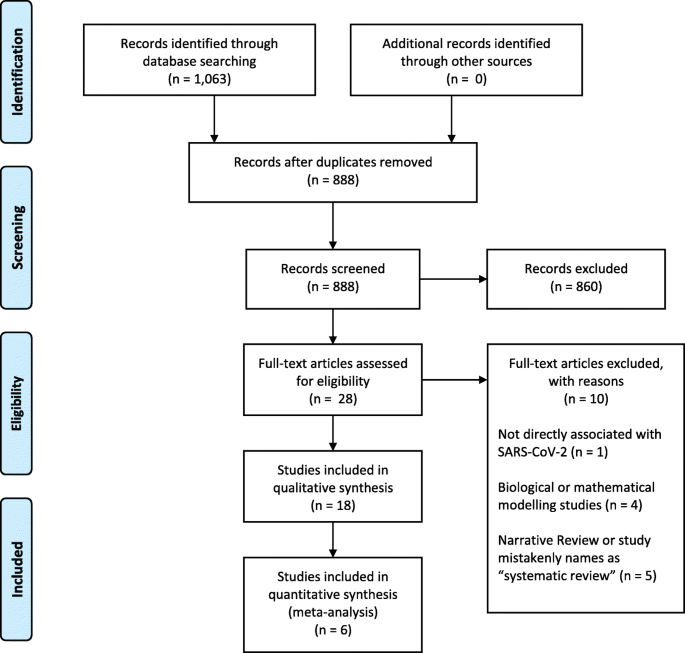
PRISMA flow diagram
Characteristics of included reviews
Summary features of 18 systematic reviews are presented in Table 1 . They were published in 14 different journals. Only four of these journals had specific requirements for systematic reviews (with or without meta-analysis): European Journal of Internal Medicine, Journal of Clinical Medicine, Ultrasound in Obstetrics and Gynecology, and Clinical Research in Cardiology . Two journals reported that they published only invited reviews ( Journal of Medical Virology and Clinica Chimica Acta ). Three systematic reviews in our study were published as letters; one was labeled as a scoping review and another as a rapid review (Table 2 ).
All reviews were published in English, in first quartile (Q1) journals, with JIF ranging from 1.692 to 6.062. One review was empty, meaning that its search did not identify any relevant studies; i.e., no primary studies were included [ 36 ]. The remaining 17 reviews included 269 unique studies; the majority ( N = 211; 78%) were included in only a single review included in our study (range: 1 to 12). Primary studies included in the reviews were published between December 2019 and March 18, 2020, and comprised case reports, case series, cohorts, and other observational studies. We found only one review that included randomized clinical trials [ 38 ]. In the included reviews, systematic literature searches were performed from 2019 (entire year) up to March 9, 2020. Ten systematic reviews included meta-analyses. The list of primary studies found in the included systematic reviews is shown in Additional file 4 , as well as the number of reviews in which each primary study was included.
Population and study designs
Most of the reviews analyzed data from patients with COVID-19 who developed pneumonia, acute respiratory distress syndrome (ARDS), or any other correlated complication. One review aimed to evaluate the effectiveness of using surgical masks on preventing transmission of the virus [ 36 ], one review was focused on pediatric patients [ 34 ], and one review investigated COVID-19 in pregnant women [ 37 ]. Most reviews assessed clinical symptoms, laboratory findings, or radiological results.
Systematic review findings
The summary of findings from individual reviews is shown in Table 2 . Overall, all-cause mortality ranged from 0.3 to 13.9% (Fig. 2 ).
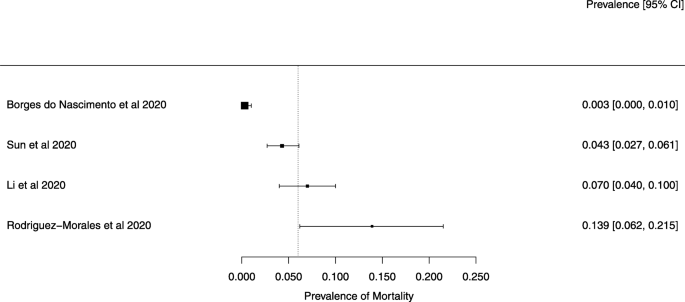
A meta-analysis of the prevalence of mortality
Clinical symptoms
Seven reviews described the main clinical manifestations of COVID-19 [ 26 , 28 , 29 , 34 , 35 , 39 , 41 ]. Three of them provided only a narrative discussion of symptoms [ 26 , 34 , 35 ]. In the reviews that performed a statistical analysis of the incidence of different clinical symptoms, symptoms in patients with COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%), gastrointestinal disorders, such as diarrhea, nausea or vomiting (5.0–9.0%), and others (including, in one study only: dizziness 12.1%) (Figs. 3 , 4 , 5 , 6 , 7 , 8 and 9 ). Three reviews assessed cough with and without sputum together; only one review assessed sputum production itself (28.5%).
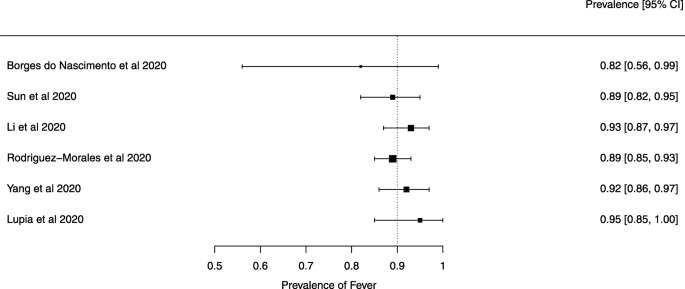
A meta-analysis of the prevalence of fever
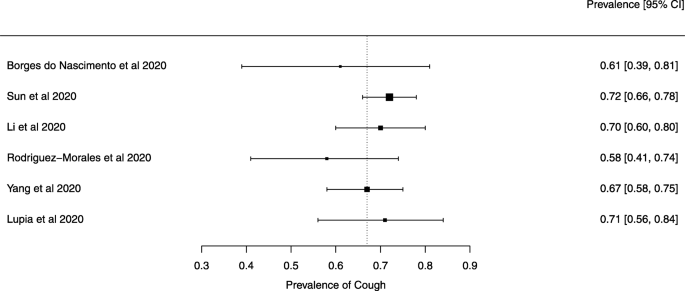
A meta-analysis of the prevalence of cough
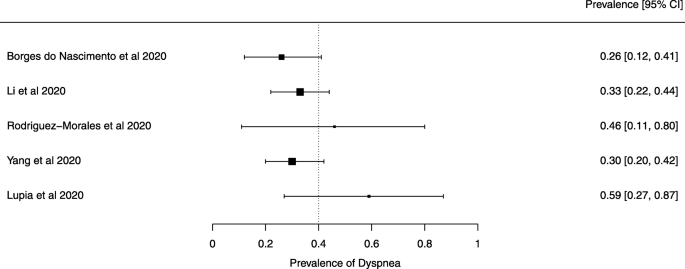
A meta-analysis of the prevalence of dyspnea
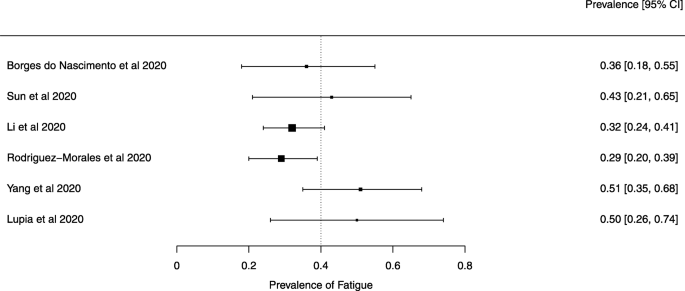
A meta-analysis of the prevalence of fatigue or myalgia
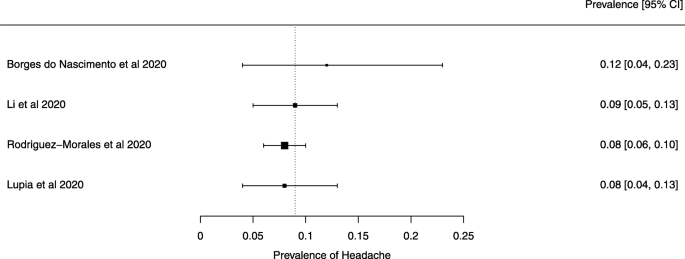
A meta-analysis of the prevalence of headache
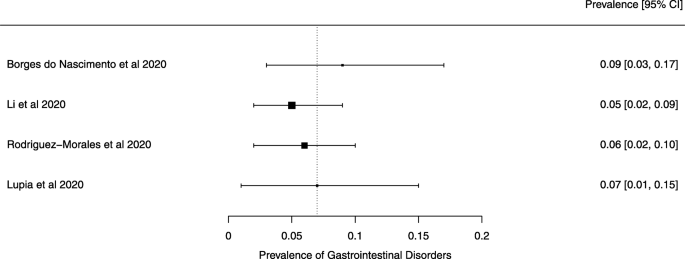
A meta-analysis of the prevalence of gastrointestinal disorders
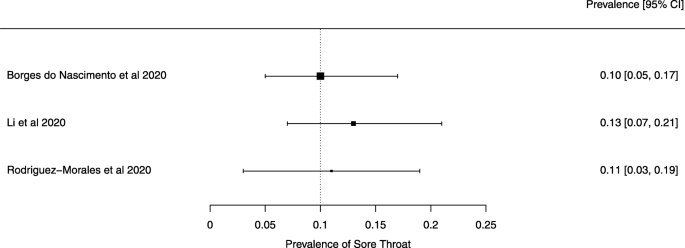
A meta-analysis of the prevalence of sore throat
Diagnostic aspects
Three reviews described methodologies, protocols, and tools used for establishing the diagnosis of COVID-19 [ 26 , 34 , 38 ]. The use of respiratory swabs (nasal or pharyngeal) or blood specimens to assess the presence of SARS-CoV-2 nucleic acid using RT-PCR assays was the most commonly used diagnostic method mentioned in the included studies. These diagnostic tests have been widely used, but their precise sensitivity and specificity remain unknown. One review included a Chinese study with clinical diagnosis with no confirmation of SARS-CoV-2 infection (patients were diagnosed with COVID-19 if they presented with at least two symptoms suggestive of COVID-19, together with laboratory and chest radiography abnormalities) [ 34 ].
Therapeutic possibilities
Pharmacological and non-pharmacological interventions (supportive therapies) used in treating patients with COVID-19 were reported in five reviews [ 25 , 27 , 34 , 35 , 38 ]. Antivirals used empirically for COVID-19 treatment were reported in seven reviews [ 25 , 27 , 34 , 35 , 37 , 38 , 41 ]; most commonly used were protease inhibitors (lopinavir, ritonavir, darunavir), nucleoside reverse transcriptase inhibitor (tenofovir), nucleotide analogs (remdesivir, galidesivir, ganciclovir), and neuraminidase inhibitors (oseltamivir). Umifenovir, a membrane fusion inhibitor, was investigated in two studies [ 25 , 35 ]. Possible supportive interventions analyzed were different types of oxygen supplementation and breathing support (invasive or non-invasive ventilation) [ 25 ]. The use of antibiotics, both empirically and to treat secondary pneumonia, was reported in six studies [ 25 , 26 , 27 , 34 , 35 , 38 ]. One review specifically assessed evidence on the efficacy and safety of the anti-malaria drug chloroquine [ 27 ]. It identified 23 ongoing trials investigating the potential of chloroquine as a therapeutic option for COVID-19, but no verifiable clinical outcomes data. The use of mesenchymal stem cells, antifungals, and glucocorticoids were described in four reviews [ 25 , 34 , 35 , 38 ].
Laboratory and radiological findings
Of the 18 reviews included in this overview, eight analyzed laboratory parameters in patients with COVID-19 [ 25 , 29 , 30 , 32 , 33 , 34 , 35 , 39 ]; elevated C-reactive protein levels, associated with lymphocytopenia, elevated lactate dehydrogenase, as well as slightly elevated aspartate and alanine aminotransferase (AST, ALT) were commonly described in those eight reviews. Lippi et al. assessed cardiac troponin I (cTnI) [ 25 ], procalcitonin [ 32 ], and platelet count [ 33 ] in COVID-19 patients. Elevated levels of procalcitonin [ 32 ] and cTnI [ 30 ] were more likely to be associated with a severe disease course (requiring intensive care unit admission and intubation). Furthermore, thrombocytopenia was frequently observed in patients with complicated COVID-19 infections [ 33 ].
Chest imaging (chest radiography and/or computed tomography) features were assessed in six reviews, all of which described a frequent pattern of local or bilateral multilobar ground-glass opacity [ 25 , 34 , 35 , 39 , 40 , 41 ]. Those six reviews showed that septal thickening, bronchiectasis, pleural and cardiac effusions, halo signs, and pneumothorax were observed in patients suffering from COVID-19.
Quality of evidence in individual systematic reviews
Table 3 shows the detailed results of the quality assessment of 18 systematic reviews, including the assessment of individual items and summary assessment. A detailed explanation for each decision in each review is available in Additional file 5 .
Using AMSTAR 2 criteria, confidence in the results of all 18 reviews was rated as “critically low” (Table 3 ). Common methodological drawbacks were: omission of prospective protocol submission or publication; use of inappropriate search strategy: lack of independent and dual literature screening and data-extraction (or methodology unclear); absence of an explanation for heterogeneity among the studies included; lack of reasons for study exclusion (or rationale unclear).
Risk of bias assessment, based on a reported methodological tool, and quality of evidence appraisal, in line with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method, were reported only in one review [ 25 ]. Five reviews presented a table summarizing bias, using various risk of bias tools [ 25 , 29 , 39 , 40 , 41 ]. One review analyzed “study quality” [ 37 ]. One review mentioned the risk of bias assessment in the methodology but did not provide any related analysis [ 28 ].
This overview of systematic reviews analyzed the first 18 systematic reviews published after the onset of the COVID-19 pandemic, up to March 24, 2020, with primary studies involving more than 60,000 patients. Using AMSTAR-2, we judged that our confidence in all those reviews was “critically low”. Ten reviews included meta-analyses. The reviews presented data on clinical manifestations, laboratory and radiological findings, and interventions. We found no systematic reviews on the utility of diagnostic tests.
Symptoms were reported in seven reviews; most of the patients had a fever, cough, dyspnea, myalgia or muscle fatigue, and gastrointestinal disorders such as diarrhea, nausea, or vomiting. Olfactory dysfunction (anosmia or dysosmia) has been described in patients infected with COVID-19 [ 43 ]; however, this was not reported in any of the reviews included in this overview. During the SARS outbreak in 2002, there were reports of impairment of the sense of smell associated with the disease [ 44 , 45 ].
The reported mortality rates ranged from 0.3 to 14% in the included reviews. Mortality estimates are influenced by the transmissibility rate (basic reproduction number), availability of diagnostic tools, notification policies, asymptomatic presentations of the disease, resources for disease prevention and control, and treatment facilities; variability in the mortality rate fits the pattern of emerging infectious diseases [ 46 ]. Furthermore, the reported cases did not consider asymptomatic cases, mild cases where individuals have not sought medical treatment, and the fact that many countries had limited access to diagnostic tests or have implemented testing policies later than the others. Considering the lack of reviews assessing diagnostic testing (sensitivity, specificity, and predictive values of RT-PCT or immunoglobulin tests), and the preponderance of studies that assessed only symptomatic individuals, considerable imprecision around the calculated mortality rates existed in the early stage of the COVID-19 pandemic.
Few reviews included treatment data. Those reviews described studies considered to be at a very low level of evidence: usually small, retrospective studies with very heterogeneous populations. Seven reviews analyzed laboratory parameters; those reviews could have been useful for clinicians who attend patients suspected of COVID-19 in emergency services worldwide, such as assessing which patients need to be reassessed more frequently.
All systematic reviews scored poorly on the AMSTAR 2 critical appraisal tool for systematic reviews. Most of the original studies included in the reviews were case series and case reports, impacting the quality of evidence. Such evidence has major implications for clinical practice and the use of these reviews in evidence-based practice and policy. Clinicians, patients, and policymakers can only have the highest confidence in systematic review findings if high-quality systematic review methodologies are employed. The urgent need for information during a pandemic does not justify poor quality reporting.
We acknowledge that there are numerous challenges associated with analyzing COVID-19 data during a pandemic [ 47 ]. High-quality evidence syntheses are needed for decision-making, but each type of evidence syntheses is associated with its inherent challenges.
The creation of classic systematic reviews requires considerable time and effort; with massive research output, they quickly become outdated, and preparing updated versions also requires considerable time. A recent study showed that updates of non-Cochrane systematic reviews are published a median of 5 years after the publication of the previous version [ 48 ].
Authors may register a review and then abandon it [ 49 ], but the existence of a public record that is not updated may lead other authors to believe that the review is still ongoing. A quarter of Cochrane review protocols remains unpublished as completed systematic reviews 8 years after protocol publication [ 50 ].
Rapid reviews can be used to summarize the evidence, but they involve methodological sacrifices and simplifications to produce information promptly, with inconsistent methodological approaches [ 51 ]. However, rapid reviews are justified in times of public health emergencies, and even Cochrane has resorted to publishing rapid reviews in response to the COVID-19 crisis [ 52 ]. Rapid reviews were eligible for inclusion in this overview, but only one of the 18 reviews included in this study was labeled as a rapid review.
Ideally, COVID-19 evidence would be continually summarized in a series of high-quality living systematic reviews, types of evidence synthesis defined as “ a systematic review which is continually updated, incorporating relevant new evidence as it becomes available ” [ 53 ]. However, conducting living systematic reviews requires considerable resources, calling into question the sustainability of such evidence synthesis over long periods [ 54 ].
Research reports about COVID-19 will contribute to research waste if they are poorly designed, poorly reported, or simply not necessary. In principle, systematic reviews should help reduce research waste as they usually provide recommendations for further research that is needed or may advise that sufficient evidence exists on a particular topic [ 55 ]. However, systematic reviews can also contribute to growing research waste when they are not needed, or poorly conducted and reported. Our present study clearly shows that most of the systematic reviews that were published early on in the COVID-19 pandemic could be categorized as research waste, as our confidence in their results is critically low.
Our study has some limitations. One is that for AMSTAR 2 assessment we relied on information available in publications; we did not attempt to contact study authors for clarifications or additional data. In three reviews, the methodological quality appraisal was challenging because they were published as letters, or labeled as rapid communications. As a result, various details about their review process were not included, leading to AMSTAR 2 questions being answered as “not reported”, resulting in low confidence scores. Full manuscripts might have provided additional information that could have led to higher confidence in the results. In other words, low scores could reflect incomplete reporting, not necessarily low-quality review methods. To make their review available more rapidly and more concisely, the authors may have omitted methodological details. A general issue during a crisis is that speed and completeness must be balanced. However, maintaining high standards requires proper resourcing and commitment to ensure that the users of systematic reviews can have high confidence in the results.
Furthermore, we used adjusted AMSTAR 2 scoring, as the tool was designed for critical appraisal of reviews of interventions. Some reviews may have received lower scores than actually warranted in spite of these adjustments.
Another limitation of our study may be the inclusion of multiple overlapping reviews, as some included reviews included the same primary studies. According to the Cochrane Handbook, including overlapping reviews may be appropriate when the review’s aim is “ to present and describe the current body of systematic review evidence on a topic ” [ 12 ], which was our aim. To avoid bias with summarizing evidence from overlapping reviews, we presented the forest plots without summary estimates. The forest plots serve to inform readers about the effect sizes for outcomes that were reported in each review.
Several authors from this study have contributed to one of the reviews identified [ 25 ]. To reduce the risk of any bias, two authors who did not co-author the review in question initially assessed its quality and limitations.
Finally, we note that the systematic reviews included in our overview may have had issues that our analysis did not identify because we did not analyze their primary studies to verify the accuracy of the data and information they presented. We give two examples to substantiate this possibility. Lovato et al. wrote a commentary on the review of Sun et al. [ 41 ], in which they criticized the authors’ conclusion that sore throat is rare in COVID-19 patients [ 56 ]. Lovato et al. highlighted that multiple studies included in Sun et al. did not accurately describe participants’ clinical presentations, warning that only three studies clearly reported data on sore throat [ 56 ].
In another example, Leung [ 57 ] warned about the review of Li, L.Q. et al. [ 29 ]: “ it is possible that this statistic was computed using overlapped samples, therefore some patients were double counted ”. Li et al. responded to Leung that it is uncertain whether the data overlapped, as they used data from published articles and did not have access to the original data; they also reported that they requested original data and that they plan to re-do their analyses once they receive them; they also urged readers to treat the data with caution [ 58 ]. This points to the evolving nature of evidence during a crisis.
Our study’s strength is that this overview adds to the current knowledge by providing a comprehensive summary of all the evidence synthesis about COVID-19 available early after the onset of the pandemic. This overview followed strict methodological criteria, including a comprehensive and sensitive search strategy and a standard tool for methodological appraisal of systematic reviews.
In conclusion, in this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all the reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic could be categorized as research waste. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards to provide patients, clinicians, and decision-makers trustworthy evidence.
Availability of data and materials
All data collected and analyzed within this study are available from the corresponding author on reasonable request.
World Health Organization. Timeline - COVID-19: Available at: https://www.who.int/news/item/29-06-2020-covidtimeline . Accessed 1 June 2021.
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html . Accessed 1 June 2021.
Anzai A, Kobayashi T, Linton NM, Kinoshita R, Hayashi K, Suzuki A, et al. Assessing the Impact of Reduced Travel on Exportation Dynamics of Novel Coronavirus Infection (COVID-19). J Clin Med. 2020;9(2):601.
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. https://doi.org/10.1126/science.aba9757 .
Article CAS PubMed PubMed Central Google Scholar
Fidahic M, Nujic D, Runjic R, Civljak M, Markotic F, Lovric Makaric Z, et al. Research methodology and characteristics of journal articles with original data, preprint articles and registered clinical trial protocols about COVID-19. BMC Med Res Methodol. 2020;20(1):161. https://doi.org/10.1186/s12874-020-01047-2 .
EPPI Centre . COVID-19: a living systematic map of the evidence. Available at: http://eppi.ioe.ac.uk/cms/Projects/DepartmentofHealthandSocialCare/Publishedreviews/COVID-19Livingsystematicmapoftheevidence/tabid/3765/Default.aspx . Accessed 1 June 2021.
NCBI SARS-CoV-2 Resources. Available at: https://www.ncbi.nlm.nih.gov/sars-cov-2/ . Accessed 1 June 2021.
Gustot T. Quality and reproducibility during the COVID-19 pandemic. JHEP Rep. 2020;2(4):100141. https://doi.org/10.1016/j.jhepr.2020.100141 .
Article PubMed PubMed Central Google Scholar
Kodvanj, I., et al., Publishing of COVID-19 Preprints in Peer-reviewed Journals, Preprinting Trends, Public Discussion and Quality Issues. Preprint article. bioRxiv 2020.11.23.394577; doi: https://doi.org/10.1101/2020.11.23.394577 .
Dobler CC. Poor quality research and clinical practice during COVID-19. Breathe (Sheff). 2020;16(2):200112. https://doi.org/10.1183/20734735.0112-2020 .
Article Google Scholar
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. https://doi.org/10.1371/journal.pmed.1000326 .
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 1-purpose, eligibility, search and data extraction. Syst Rev. 2017;6(1):231. https://doi.org/10.1186/s13643-017-0617-1 .
Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. 2020. Available from www.training.cochrane.org/handbook .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane. 2020; Available from www.training.cochrane.org/handbook .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18. https://doi.org/10.1186/s13643-018-0914-3 .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):29. https://doi.org/10.1186/s13643-018-0768-8 .
Hunt H, Pollock A, Campbell P, Estcourt L, Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39. https://doi.org/10.1186/s13643-018-0695-8 .
Pollock M, Fernandes RM, Pieper D, Tricco AC, Gates M, Gates A, et al. Preferred reporting items for overviews of reviews (PRIOR): a protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):335. https://doi.org/10.1186/s13643-019-1252-9 .
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–30.
Krnic Martinic M, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med Res Methodol. 2019;19(1):203. https://doi.org/10.1186/s12874-019-0855-0 .
Puljak L. If there is only one author or only one database was searched, a study should not be called a systematic review. J Clin Epidemiol. 2017;91:4–5. https://doi.org/10.1016/j.jclinepi.2017.08.002 .
Article PubMed Google Scholar
Gates M, Gates A, Guitard S, Pollock M, Hartling L. Guidance for overviews of reviews continues to accumulate, but important challenges remain: a scoping review. Syst Rev. 2020;9(1):254. https://doi.org/10.1186/s13643-020-01509-0 .
Covidence - systematic review software. Available at: https://www.covidence.org/ . Accessed 1 June 2021.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Borges do Nascimento IJ, et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9(4):941.
Article PubMed Central Google Scholar
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. https://doi.org/10.1186/s40249-020-00646-x .
Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–83. https://doi.org/10.1016/j.jcrc.2020.03.005 .
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8. https://doi.org/10.1007/s00392-020-01626-9 .
Article CAS PubMed Google Scholar
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–83. https://doi.org/10.1002/jmv.25757 .
Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–1. https://doi.org/10.1016/j.pcad.2020.03.001 .
Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–8. https://doi.org/10.1016/j.ejim.2020.03.014 .
Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–1. https://doi.org/10.1016/j.cca.2020.03.004 .
Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. https://doi.org/10.1016/j.cca.2020.03.022 .
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–95. https://doi.org/10.1111/apa.15270 .
Lupia T, Scabini S, Mornese Pinna S, di Perri G, de Rosa FG, Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020;21:22–7. https://doi.org/10.1016/j.jgar.2020.02.021 .
Marasinghe, K.M., A systematic review investigating the effectiveness of face mask use in limiting the spread of COVID-19 among medically not diagnosed individuals: shedding light on current recommendations provided to individuals not medically diagnosed with COVID-19. Research Square. Preprint article. doi : https://doi.org/10.21203/rs.3.rs-16701/v1 . 2020 .
Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586–92. https://doi.org/10.1002/uog.22014 .
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JIP, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9(3):623.
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. https://doi.org/10.1016/j.tmaid.2020.101623 .
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. https://doi.org/10.2214/AJR.20.23034 .
Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–7. https://doi.org/10.1002/jmv.25735 .
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. https://doi.org/10.1016/j.ijid.2020.03.017 .
Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Investig. 2020;50(3):e13209. https://doi.org/10.1111/eci.13209 .
Article CAS Google Scholar
Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwanica. 2006;15(1):26–8.
Google Scholar
Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–7. https://doi.org/10.1097/01.mlg.0000249922.37381.1e .
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–7. https://doi.org/10.1016/S1473-3099(20)30244-9 .
Wolkewitz M, Puljak L. Methodological challenges of analysing COVID-19 data during the pandemic. BMC Med Res Methodol. 2020;20(1):81. https://doi.org/10.1186/s12874-020-00972-6 .
Rombey T, Lochner V, Puljak L, Könsgen N, Mathes T, Pieper D. Epidemiology and reporting characteristics of non-Cochrane updates of systematic reviews: a cross-sectional study. Res Synth Methods. 2020;11(3):471–83. https://doi.org/10.1002/jrsm.1409 .
Runjic E, Rombey T, Pieper D, Puljak L. Half of systematic reviews about pain registered in PROSPERO were not published and the majority had inaccurate status. J Clin Epidemiol. 2019;116:114–21. https://doi.org/10.1016/j.jclinepi.2019.08.010 .
Runjic E, Behmen D, Pieper D, Mathes T, Tricco AC, Moher D, et al. Following Cochrane review protocols to completion 10 years later: a retrospective cohort study and author survey. J Clin Epidemiol. 2019;111:41–8. https://doi.org/10.1016/j.jclinepi.2019.03.006 .
Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Med. 2015;13(1):224. https://doi.org/10.1186/s12916-015-0465-6 .
COVID-19 Rapid Reviews: Cochrane’s response so far. Available at: https://training.cochrane.org/resource/covid-19-rapid-reviews-cochrane-response-so-far . Accessed 1 June 2021.
Cochrane. Living systematic reviews. Available at: https://community.cochrane.org/review-production/production-resources/living-systematic-reviews . Accessed 1 June 2021.
Millard T, Synnot A, Elliott J, Green S, McDonald S, Turner T. Feasibility and acceptability of living systematic reviews: results from a mixed-methods evaluation. Syst Rev. 2019;8(1):325. https://doi.org/10.1186/s13643-019-1248-5 .
Babic A, Poklepovic Pericic T, Pieper D, Puljak L. How to decide whether a systematic review is stable and not in need of updating: analysis of Cochrane reviews. Res Synth Methods. 2020;11(6):884–90. https://doi.org/10.1002/jrsm.1451 .
Lovato A, Rossettini G, de Filippis C. Sore throat in COVID-19: comment on “clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis”. J Med Virol. 2020;92(7):714–5. https://doi.org/10.1002/jmv.25815 .
Leung C. Comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1431–2. https://doi.org/10.1002/jmv.25912 .
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. Response to Char’s comment: comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1433. https://doi.org/10.1002/jmv.25924 .
Download references
Acknowledgments
We thank Catherine Henderson DPhil from Swanscoe Communications for pro bono medical writing and editing support. We acknowledge support from the Covidence Team, specifically Anneliese Arno. We thank the whole International Network of Coronavirus Disease 2019 (InterNetCOVID-19) for their commitment and involvement. Members of the InterNetCOVID-19 are listed in Additional file 6 . We thank Pavel Cerny and Roger Crosthwaite for guiding the team supervisor (IJBN) on human resources management.
This research received no external funding.
Author information
Authors and affiliations.
University Hospital and School of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Israel Júnior Borges do Nascimento & Milena Soriano Marcolino
Medical College of Wisconsin, Milwaukee, WI, USA
Israel Júnior Borges do Nascimento
Helene Fuld Health Trust National Institute for Evidence-based Practice in Nursing and Healthcare, College of Nursing, The Ohio State University, Columbus, OH, USA
Dónal P. O’Mathúna
School of Nursing, Psychotherapy and Community Health, Dublin City University, Dublin, Ireland
Department of Anesthesiology, Intensive Care and Pain Medicine, University of Münster, Münster, Germany
Thilo Caspar von Groote
Department of Sport and Health Science, Technische Universität München, Munich, Germany
Hebatullah Mohamed Abdulazeem
School of Health Sciences, Faculty of Health and Medicine, The University of Newcastle, Callaghan, Australia
Ishanka Weerasekara
Department of Physiotherapy, Faculty of Allied Health Sciences, University of Peradeniya, Peradeniya, Sri Lanka
Cochrane Croatia, University of Split, School of Medicine, Split, Croatia
Ana Marusic, Irena Zakarija-Grkovic & Tina Poklepovic Pericic
Center for Evidence-Based Medicine and Health Care, Catholic University of Croatia, Ilica 242, 10000, Zagreb, Croatia
Livia Puljak
Cochrane Brazil, Evidence-Based Health Program, Universidade Federal de São Paulo, São Paulo, Brazil
Vinicius Tassoni Civile & Alvaro Nagib Atallah
Yorkville University, Fredericton, New Brunswick, Canada
Santino Filoso
Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, Ontario, Canada
Nicola Luigi Bragazzi
You can also search for this author in PubMed Google Scholar
Contributions
IJBN conceived the research idea and worked as a project coordinator. DPOM, TCVG, HMA, IW, AM, LP, VTC, IZG, TPP, ANA, SF, NLB and MSM were involved in data curation, formal analysis, investigation, methodology, and initial draft writing. All authors revised the manuscript critically for the content. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Livia Puljak .
Ethics declarations
Ethics approval and consent to participate.
Not required as data was based on published studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Search strategies used in the study.
Additional file 2: Appendix 2.
Adjusted scoring of AMSTAR 2 used in this study for systematic reviews of studies that did not analyze interventions.
Additional file 3: Appendix 3.
List of excluded studies, with reasons.
Additional file 4: Appendix 4.
Table of overlapping studies, containing the list of primary studies included, their visual overlap in individual systematic reviews, and the number in how many reviews each primary study was included.
Additional file 5: Appendix 5.
A detailed explanation of AMSTAR scoring for each item in each review.
Additional file 6: Appendix 6.
List of members and affiliates of International Network of Coronavirus Disease 2019 (InterNetCOVID-19).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Borges do Nascimento, I.J., O’Mathúna, D.P., von Groote, T.C. et al. Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews. BMC Infect Dis 21 , 525 (2021). https://doi.org/10.1186/s12879-021-06214-4
Download citation
Received : 12 April 2020
Accepted : 19 May 2021
Published : 04 June 2021
DOI : https://doi.org/10.1186/s12879-021-06214-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Evidence-based medicine
- Infectious diseases
BMC Infectious Diseases
ISSN: 1471-2334
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Contact Tracing
- Pandemic Data Initiative
- Webcasts & Videos
- 30-Minute COVID-19 Briefing
Research Papers
Jhu has stopped collecting data as of.
After three years of around-the-clock tracking of COVID-19 data from...
The Johns Hopkins Coronavirus Resource Center has collected, verified, and published local, regional, national, and international pandemic data since it launched in March 2020. From the beginning, the information has been freely available to all — researchers, institutions, the media, the public, and policymakers. As a result, the CRC and its data have been cited in many published research papers and reports. Here we have gathered publications authored by CRC team members that focus on the CRC or its data.
July 14, 2022
Misaligned Federal and State Covid data limits demographic insights
CDC underreports cases and deaths among African American and Hispanic or Latino individuals.
February 17, 2022
Experts Call for Open Public Health Data
Johns Hopkins team highlighted the urgent need for better COVID data collection.
Unifying Epidemiologists and Economists
Researchers from disparate fields join to chart a new path for formulating policies in response to future pandemics.
Mobility Data Supported Social Distancing
Study found that physical distancing was an effective COVID mitigation strategy.
Johns Hopkins Engineers Build COVID Dashboard
Lancet Infectious Diseases published first paper detailing how the global map was built.

Researchers Identify Disparities in COVID Testing
Johns Hopkins team conducted an analysis of state-published demographic data

COVID-19 Research Articles Downloadable Database
March 19, 2020
Updated January 12, 2024
COVID-19 Research Guide Home
- Research Articles Downloadable Database
- COVID-19 Science Updates
- Databases and Journals
- Secondary Data and Statistics
Important announcement:
The CDC Database of COVID-19 Research Articles became a collaboration with the WHO to create the WHO COVID-19 database during the pandemic to make it easier for results to be searched, downloaded, and used by researchers worldwide.
The last version of the CDC COVID-19 database was archived and remain available on this website. Please note that it has stopped updating as of October 9, 2020 and all new articles were integrated into the WHO COVID-19 database . The WHO Covid-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). Its content remains searchable and spans the time period March 2020 to June 2023. Since June 2023, manual updates to the database have been discontinued.
If you have any questions, concerns, or problems accessing the WHO COVID-19 Database please email the CDC Library for assistance.
Materials listed in these guides are selected to provide awareness of quality public health literature and resources. A material’s inclusion does not necessarily represent the views of the U.S. Department of Health and Human Services (HHS), the Public Health Service (PHS), or the Centers for Disease Control and Prevention (CDC), nor does it imply endorsement of the material’s methods or findings.
Below are options to download the archive of COVID-19 research articles. You can search the database of citations by author, keyword (in title, author, abstract, subject headings fields), journal, or abstract when available. DOI, PMID, and URL links are included when available.
This database was last updated on October 9, 2020 .
- The CDC Database of COVID-19 Research Articles is now a part of the WHO COVID-19 database . Our new search results are now being sent to the WHO COVID-19 Database to make it easier for them to be searched, downloaded, and used by researchers worldwide. The WHO Covid-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). Its content remains searchable and spans the time period March 2020 to June 2023. Since June 2023, manual updates to the database have been discontinued.
- To help inform CDC’s COVID-19 Response, as well as to help CDC staff stay up to date on the latest COVID-19 research, the Response’s Office of the Chief Medical Officer has collaborated with the CDC Office of Library Science to create a series called COVID-19 Science Update . This series, the first of its kind for a CDC emergency response, provides brief summaries of new COVID-19-related studies on many topics, including epidemiology, clinical treatment and management, laboratory science, and modeling. As of December 18, 2021, CDC has stopped production of the weekly COVID-19 Science Update.
Excel download:
- Articles from August until October 9 2020 [XLS – 29 MB]
- Articles from December 2019 through July 2020 [XLS – 45 MB]
- The CDC Database of COVID-19 Research Articles is now a part of the WHO COVID-19 database . Our new search results are now being sent to the WHO COVID-19 Database to make it easier for them to be searched, downloaded, and used by researchers worldwide.
- October 8 in Excel [XLS – 1 MB]
- October 7 in Excel [XLS – 1 MB]
- October 6 in Excel [XLS – 1 MB]
- Note the main Excel file can also be sorted by date added.
Citation Management Software (EndNote, Mendeley, Zotero, Refman, etc.) download:
- Part 1 [ZIP – 38 MB]
- Part 2 [ZIP – 43 MB]
- October 8 in citation management software format [RIS – 2 MB]
- October 7 in citation management software format [RIS – 2 MB]
- October 6 in citation management software format [RIS – 2 MB]
- Note the main RIS file can also be sorted by date added.
The COVID-19 pandemic is a rapidly changing situation. Some of the research included above is preliminary. Materials listed in this database are selected to provide awareness of quality public health literature and resources. A material’s inclusion does not necessarily represent the views of the U.S. Department of Health and Human Services (HHS), the Public Health Service (PHS), or the Centers for Disease Control and Prevention (CDC), nor does it imply endorsement of the material’s methods or findings.
To access the full text, click on the DOI, PMID, or URL links. While most publishers are making their COVID-19 content Open Access, some articles are accessible only to those with a CDC user id and password. Find a library near you that may be able to help you get access to articles by clicking the following links: https://www.worldcat.org/libraries OR https://www.usa.gov/libraries .
CDC users can use EndNote’s Find Full Text feature to attach the full text PDFs within their EndNote Library. CDC users, please email Martha Knuth for an EndNote file of all citations. Once you have your EndNote file downloaded, to get the full-text of journal articles listed in the search results you can do the following steps:
- First, try using EndNote’s “Find Full-Text” feature to attach full-text articles to your EndNote Library.
- Next, check for full-text availability, via the E-Journals list, at: http://sfxhosted.exlibrisgroup.com/cdc/az .
- If you can’t find full-text online, you can request articles via DocExpress, at: https://docexpress.cdc.gov/illiad/
The following databases were searched from Dec. 2019-Oct. 9 2020 for articles related to COVID-19: Medline (Ovid and PubMed), PubMed Central, Embase, CAB Abstracts, Global Health, PsycInfo, Cochrane Library, Scopus, Academic Search Complete, Africa Wide Information, CINAHL, ProQuest Central, SciFinder, the Virtual Health Library, and LitCovid. Selected grey literature sources were searched as well, including the WHO COVID-19 website, CDC COVID-19 website, Eurosurveillance, China CDC Weekly, Homeland Security Digital Library, ClinicalTrials.gov, bioRxiv (preprints), medRxiv (preprints), chemRxiv (preprints), and SSRN (preprints).
Detailed search strings with synonyms used for COVID-19 are below.
Detailed search strategy for gathering COVID-19 articles, updated October 9, 2020 [PDF – 135 KB]
Note on preprints: Preprints have not been peer-reviewed. They should not be regarded as conclusive, guide clinical practice/health-related behavior, or be reported in news media as established information.
Materials listed in these guides are selected to provide awareness of quality public health literature and resources. A material’s inclusion does not necessarily represent the views of the U.S. Department of Health and Human Services (HHS), the Public Health Service (PHS), or the Centers for Disease Control and Prevention (CDC), nor does it imply endorsement of the material’s methods or findings. HHS, PHS, and CDC assume no responsibility for the factual accuracy of the items presented. The selection, omission, or content of items does not imply any endorsement or other position taken by HHS, PHS, and CDC. Opinion, findings, and conclusions expressed by the original authors of items included in these materials, or persons quoted therein, are strictly their own and are in no way meant to represent the opinion or views of HHS, PHS, or CDC. References to publications, news sources, and non-CDC Websites are provided solely for informational purposes and do not imply endorsement by HHS, PHS, or CDC.
To receive the COVID-19 Science Update, please enter your email address to subscribe today.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Coronavirus disease 2019 (COVID-19): A literature review
Affiliations.
- 1 Medical Research Unit, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; Tropical Disease Centre, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; Department of Microbiology, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia. Electronic address: [email protected].
- 2 Division of Infectious Diseases, AichiCancer Center Hospital, Chikusa-ku Nagoya, Japan. Electronic address: [email protected].
- 3 Department of Family Medicine, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia. Electronic address: [email protected].
- 4 Department of Pulmonology and Respiratory Medicine, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia. Electronic address: [email protected].
- 5 School of Medicine, The University of Western Australia, Perth, Australia. Electronic address: [email protected].
- 6 Siem Reap Provincial Health Department, Ministry of Health, Siem Reap, Cambodia. Electronic address: [email protected].
- 7 Department of Microbiology and Parasitology, Faculty of Medicine and Health Sciences, Warmadewa University, Denpasar, Indonesia; Department of Medical Microbiology and Immunology, University of California, Davis, CA, USA. Electronic address: [email protected].
- 8 Medical Research Unit, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; Tropical Disease Centre, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; Department of Microbiology, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; Department of Clinical Microbiology, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia. Electronic address: [email protected].
- 9 Department of Epidemiology, University of Michigan, Ann Arbor, Michigan, MI 48109, USA. Electronic address: [email protected].
- 10 Medical Research Unit, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; Tropical Disease Centre, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; Department of Microbiology, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia. Electronic address: [email protected].
- PMID: 32340833
- PMCID: PMC7142680
- DOI: 10.1016/j.jiph.2020.03.019
In early December 2019, an outbreak of coronavirus disease 2019 (COVID-19), caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), occurred in Wuhan City, Hubei Province, China. On January 30, 2020 the World Health Organization declared the outbreak as a Public Health Emergency of International Concern. As of February 14, 2020, 49,053 laboratory-confirmed and 1,381 deaths have been reported globally. Perceived risk of acquiring disease has led many governments to institute a variety of control measures. We conducted a literature review of publicly available information to summarize knowledge about the pathogen and the current epidemic. In this literature review, the causative agent, pathogenesis and immune responses, epidemiology, diagnosis, treatment and management of the disease, control and preventions strategies are all reviewed.
Keywords: 2019-nCoV; COVID-19; Novel coronavirus; Outbreak; SARS-CoV-2.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Publication types
- Betacoronavirus
- Clinical Trials as Topic
- Coronavirus Infections* / epidemiology
- Coronavirus Infections* / immunology
- Coronavirus Infections* / therapy
- Coronavirus Infections* / virology
- Disease Outbreaks* / prevention & control
- Pneumonia, Viral* / epidemiology
- Pneumonia, Viral* / immunology
- Pneumonia, Viral* / therapy
- Pneumonia, Viral* / virology
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Browse by collection
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 14, Issue 5
- Prevalence and health effects of post-COVID-19 condition in Africa: a scoping review protocol
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Edward Wilson Ansah ,
- http://orcid.org/0009-0007-2287-0102 Promise Kwame Salu ,
- Martin Sumani Daanko ,
- David N Banaaleh ,
- http://orcid.org/0000-0002-9582-8884 Mustapha Amoadu
- Department of Health, Physical Education and Recreation , University of Cape Coast , Cape Coast , Ghana
- Correspondence to Mr Promise Kwame Salu; promise.salu{at}stu.ucc.edu.gh
Introduction SARS-CoV-2 pandemic has caused global devastations in the social, economic and health systems of every nation, but disproportionately the nations in Africa. Apart from its grave effects on the global systems, is the persistence of post-COVID-19 condition in individuals infected with the virus. Therefore, the aim of this scoping review is to collate and summarise the existing research evidence about the prevalence and health effects of post-COVID-19 infection conditions in Africa.
Methods and analysis Five main databases will be thoroughly searched from 1 September 2023 to 30 April 2024, for eligible articles based on the inclusion and exclusion criteria. These databases include PubMed, Central, Scopus, Dimensions AI and JSTOR. Meanwhile, Arksey and O’Malley guidelines will guide this scoping review using article published between 1 January 2020 and 30 April 2024. This review will provide a useful insight into the prevalence of the post-COVID-19 symptoms and their health effects within the population in Africa. The results and findings of the review will be valuable for health system interventions, including restructuring and reorientation of health systems in the continent.
Ethics and dissemination This scoping review will involve analysis of secondary data, therefore, no ethical approval is needed. Dissemination of the results will be done through international journals and available research conferences.
- Post-Acute COVID-19 Syndrome
- Epidemiology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjopen-2023-082519
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
STRENGTHS AND LIMITATIONS OF THIS STUDY
This scoping review will provide a comprehensive synthesis of existing evidence on post-COVID-19 condition in Africa, which may inform healthcare strategies and interventions and public health planning in the continent.
The consultation with diverse stakeholders, including medical professionals and individuals experiencing post-COVID-19 complications, will enhance the validity and relevance of the findings.
By including both peer-reviewed articles and grey literature, the study will offer a broad overview of the prevalence and health effects of post-COVID-19 condition in the region.
Quality appraisal of included studies will not be conducted, potentially limiting the robustness of the findings.
The review will only include studies published in English, potentially excluding relevant literature published in other languages.
Introduction
SARS-CoV-2 caused the COVID-19 pandemic and has changed the global dynamics, including the well-being of individuals and the society since the outbreak in the late 2019 in Wuhan, China. 1 COVID-19 poses significant challenges and continues to profoundly affect the global healthcare system. 2 Unfortunately, the disease has killed approximately 7 million persons globally from an estimated 800 million confirmed cases as of September 2023. 3 It is estimated that 10 million confirmed cases of COVID-19 infections occurred in Africa, of which about 175 496 died as of 16 February 2024. 3
Recent global evidence suggests that many COVID-19-infected persons continue to experience symptoms or conditions post the acute phase of the infection. 1 Post-COVID-19 condition is the continuation or development of new symptoms 3 months after the initial infection, where symptoms would have to last for 2 months or longer, with no other explanation other than COVID-19 infection. 4 Post-COVID-19 condition is characterised by several symptoms, such as fatigue, malaise, altered smell and taste, breathlessness, and cognitive impairments. 5–7 Organ damage has also been reported. 8
Globally, post-COVID-19 condition is estimated at 0.43%. 2 In the United States of America (USA), it was estimated that as of 2022, 6.9% of adults have had post-COVID-19 conditions with 3.4% living with the conditions. 9 In the United Kngdom (UK), as of February 2023, 2 million (3.1%) of the population have reported post-COVID-19 condition. 10 In Africa, about 41% of the populations are experiencing post-COVID-19 conditions. 11 Unfortunately, the estimates stated above do not provide a comprehensive analysis of post-COVID-19 conditions in Africa. This is because there could be under-reporting of the conditions due to difficulties in testing and diagnosis of these symptoms in many low-resource settings. Therefore, it is difficult to comprehensively appreciate the health effects of COVID-19 and its implications on health systems in Africa. This would make it difficult for the various health systems in the continent to plan for effective and efficient management of the COVID-19 conditions and associated challenges to the healthcare system. Unfortunately, the persistence of post-COVID-19 condition is likely to put more pressure on the already burdened African health systems and further retrogress its socioeconomic and health development agenda.
There are a few existing reviews from Africa 11–14 on post-COVID-19 condition. The challenge is that these reviews failed to address how post-COVIID-19 conditions can complicate the ever-burdened health systems at the various countries in Africa. Moreover, though these reviews provide up-to-date evidence of the prevalence of COVID-19 conditions, their comprehensibility may be limited. This is because such methods of synthesis like systematic reviews and meta-analysis carry out a quality appraisal of selected records which has a high propensity to screen-out many relevant articles. Meanwhile, it is very important that we understand the common COVID-19 symptoms, the critical implications of the conditions on patients and how they are affecting health systems in low-resourced countries.
Post-COVID-19 conditions do not only affect the healthcare systems in these least developed countries, but also the citizens who were infected with the virus. This could raise the dependency ratio not necessarily because of the increased in the aged population, but the health implications of post-COVID-19 conditions, including the various forms of disabilities. Therefore, there will be the need for evidence-based policy, health system restructuring and reorientation to meet such increasing health demands. Thus, evidence from comprehensive reviews may be essential to inform these interventions. Therefore, a more comprehensive review like a scoping review will map available evidence on the prevalence, common symptoms, health effects of post-COVID-19 conditions and provide how they are affecting the health system in Africa. This scoping review aims at providing critical insights into the prevalence of post-COVID-19 condition, health effects on patients and the implications for the health system in Africa. This will be beneficial for healthcare strategies, interventions and public health planning in Africa. This review will inform tailored interventions, guide research priorities and contribute to global understanding while supporting better care for individuals affected by post-COVID-19 conditions in Africa. Ultimately, the evidence will improve patients’ well-being and healthcare outcomes, and form the basis for future studies, including systematic reviews.
Method and analysis
This review will follow the guideline by Arksey and O’Malley 15 for a scoping review. The six-stage guideline includes identifying and stating the research questions, identifying relevant studies, study selection, data collection (data extraction), data summary and synthesis of results and consultation. The guideline by Arksey and O’Malley is prefer, over other similar ones because it gives room for consultation, which is critical to scoping review. In addition, we will report the findings from this scoping review based on the protocol by Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR). 16
Research question(s)
Two research questions will guide the review: what is the prevalence of post-COVID-19 condition in Africa? and what are the health effects of post-COVID-19 condition in Africa?
Identification of relevant literature
Search for literature started from 1 September 2023 and will continue until 30 April 2024, in five main databases, including PubMed, Central, Scopus, Dimensions AI and JSTOR. Additional search will be conducted in Google Scholar, ProQuest, Google and institutional repositories such as WHO Library and university online repositories. While WHO Library is an open access library, our university library (Sam Jonah Library, University of Cape Coast) also has a collection of databases and has access to many repositories where we can access relevant articles.
Medical Subject Headings (MeSH) terms together with controlled vocabularies will be used to conduct an initial search in PubMed. These search terms will be modified to suit searches in the other databases (see online supplemental file 1 for details). Moreover, all identified records will be saved into the Mendeley Software where they will be merged (to remove duplicates) for further screening. See table 1 for the search strategy conducted in PubMed. A librarian at the Sam Jonah Library, University of Cape Coast, will be consulted throughout the literature search process. Table 2 presents the eligibility criteria for the review.
Supplemental material
- View inline
Search strategy for search in PubMed
Eligibility criteria for screening search results and full-text records
Selection of relevant studies
The selection of articles will go through series of screening. The screening of titles and abstracts will be carried out after the duplicate records are identified and merged using the Mendeley software. Titles and abstracts will be screened by 15 graduate students under the guidance of PKS, MSD and DNB. Five students will form a group (three groups), and these three groups of students will independently screen the retrieved records. Moreover, there will be cross-over screening, where a group will cross-check the screening done by another group. Any unresolved discrepancy will be dealt with by authors EWA and MA. This will be to ensure that all relevant full-text records are selected into the review. The screening of the titles and abstracts will be done to select eligible full-text records. The reference lists of full-text eligible records will be further searched for additional relevant records. Full-text records would be finally screened against the eligibility criteria to select studies to be included in the scoping review. Figure 1 is a model PRISMA flow diagram for the search and screening processes.
- Download figure
- Open in new tab
- Download powerpoint
The Preferred Reporting Items for Systematic Reviews and Meta-analyses Extension for Scoping Reviews flow diagram will guide the scoping review process. The diagram outlines the identification, screening, eligibility and inclusion of studies in the review. Initially, the total number of papers identified through database search will be recorded in the box labelled ‘records identified through database search’ and papers identified through other sources will be recorded in the ‘additional records identified through other sources’ box. After duplicates are removed, the remaining number of papers will be recorded in the box labelled ‘records after duplicates removed’. On completion of title and abstract screening, the number of papers selected will be recorded in the box labelled ‘records screened’ and the number of papers excluded will be recorded in the box labelled ‘records excluded’. Papers identified through expert consultation and reference check will be recorded in the boxes labelled ‘records identified through consultation’ and ‘records identified through reference check’, respectively. The total number of papers that will be subjected to full-text screening which will be the sum of papers selected after title and abstract screening, papers identified through consultation and reference check will be recorded in the box labelled ‘full-text articles assessed for eligibility’. When full-text screening is completed, the number of excluded papers (with reasons) will be recorded in the box labelled ‘records excluded with reasons’ and the number of papers finally selected will be recorded in the box labelled ‘studies included in scoping review’.
Data extraction
Data extraction form will be designed, tested and used for the charting of the data, according to author and date, purpose of the study, country of the study, design and sample size used, study population, sampling procedure used, data collection method, and findings on the prevalence of post-COVID-19 conditions, common symptoms of post-COVID-19 conditions and health effects, including limitations and disabling effects on the victims. Moreover, we will critically search the included studies and extract data on the stated implications of post-COVID-19 conditions and their health effects on the health systems in Africa.
Two authors (MSD and DNB) will carry out the data extraction independently. Then, authors PKS and MA will review the data extracted by the MSD and DNB. The data extraction process will ensure that we capture relevant information that are needed to answer the stated research questions. Author EWA will help to resolve any challenges that may ensue during the data extraction phase.
Data summary and synthesis of results
Thematic analysis will be employed in reportng the results of this scoping review. This involves systematically coding the extracted data to identify recurring patterns or themes which will then be organised and interpreted according to the stated research questions. The process will begin with authors familiarising themselves with the data through thorough reading, followed by generating initial codes to identify specific concepts or patterns. Subsequently, codes will be grouped into themes based on similarities and relationships, which are then reviewed and refined iteratively to ensure accuracy and coherence. Each theme will be defined and named descriptively to reflect its content and relevance to the research questions. Narrative reports will be generated to present the synthesised themes in a coherent manner, which will be accompanied by illustrative quotes or examples from the included studies. This approach allows for a comprehensive exploration of the prevalence, health effects and implications of post-COVID-19 conditions on health systems in Africa, which provide valuable insights into the topic under investigation.
Consultation
Consultation in the scoping review process is essential for enhancing the validity and relevance of findings. Beyond review and subject experts, consulting medical doctors, individuals experiencing post-COVID-19 complications and other care providers offers valuable perspectives to this review. Medical doctors provide insights into clinical aspects and treatment modalities while individual patents may share their lived experiences of post-COVID-19 conditions. Meanwhile, other care providers will offer perspectives on how they deliver care and support patients with post-COVID-19 conditions. By engaging these diverse stakeholders, the review will provide a comprehensive evidence to make meaningful recommendations for policy-makers, healthcare providers and patients, which ultimately will contribute to addressing the challenges of post-COVID-19 complications in the continent.
Patient and public involvement
The first part of presenting the findings of this review will cover the search results, and the screening processes which will be presented in PRISMA flow diagram (see a model PRISMA flow diagram in figure 1 ). Then, the rest of the findings will be presented using qualitative thematic content analysis based on the stated research questions. Thus, we will present our findings based on the characteristics of the included studies, the prevalence of the post-COVID-19 conditions (general prevalence and common assumptions), common symptoms, health complications of COVID-19 on patients and how post-COVID-19 conditions are affecting the health system in Africa, among others. Moreover, quality appraisal of included studies will not be conducted in this review. This is because such quality appraisals are not mandatory for scoping reviews. 15 In addition, such quality appraisal of evidence may lead to the removal of some potentially relevant articles, which may rather limit the robustness and comprehensiveness of the findings. Furthermore, we will provide a narrative summary of our findings and do an in-depth discussion that will delve into the implications of post-COVID-19 conditions on the healthcare systems in Africa.
This scoping review will contribute to a deeper understanding of the prevalence and health effects of post-COVID-19 conditions in Africa, thereby addressing a significant gap in the existing literature. By synthesising available evidence and highlighting the broader health implications of post-COVID-19 conditions, this study will underscore the urgent need for targeted interventions to address the long-term health consequences of the pandemic on the continent’s health system. Furthermore, our findings will underscore the importance of strengthening health systems in Africa to effectively manage the impacts of post-COVID-19 conditions on individuals and communities. Moving forward, it will be imperative that policy-makers, healthcare providers and researchers collaborate to develop evidence-based strategies aimed at improving healthcare delivery and addressing the multifaceted challenges posed by post-COVID-19 conditions. By leveraging the insights gleaned from this review, stakeholders can work towards building more resilient and responsive health systems capable of meeting the evolving healthcare needs of the populations in Africa in the aftermath of the pandemic or any other wide spread disaster.
Ethics and dissemination
This study will be based on the analysis of secondary data or existing published articles and will not directly involve human participants. Consequently, there is no requirement for ethical approval for this scoping review. The outcome of this scoping review will be published in international peer-review journals and or presented at research conferences.
Ethics statements
Patient consent for publication.
Not applicable.
- Nowell J , et al
- Haupert SR ,
- Zimmermann L , et al
- World Health Organization
- Aiyegbusi OL ,
- Hughes SE ,
- Turner G , et al
- Ssentongo AE , et al
- Michelen M ,
- Manoharan L ,
- Elkheir N , et al
- Alberts J , et al
- Adjaye-Gbewonyo D ,
- Vahratian A ,
- Perrine CG , et al
- Office for National Statistics
- Nyasulu PS ,
- Tamuzi JL ,
- Chimene M ,
- Moyo P , et al
- Müller SA ,
- Mumm R , et al
- Frallonardo L ,
- Segala FV ,
- Chhaganlal KD , et al
- Tricco AC ,
- Zarin W , et al
Contributors Conceptualisation: EWA. Methodology: EWA, PKS, MSD, DNB and MA. Supervision: EWA and MA. Writing–original draft: PKS, MSD and DNB. Writing–review and editing: EWA, MA, PKS and MSD.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Latest science news, discoveries and analysis

'Orangutan, heal thyself': First wild animal seen using medicinal plant

UTIs make life miserable — scientists are finding new ways to tackle them
Found: the dial in the brain that controls the immune system

Controversial virus-hunting scientist skewered at US COVID-origins hearing
Chinese virologist who was first to share covid genome sleeps on street after lab shuts, why is exercise good for you scientists are finding answers in our cells, scientists tried to give people covid — and failed, why it’s essential to study sex and gender, even as tensions rise, how to meet africa’s grand challenges with african know-how alfred r. bizoza.

Sex and gender discussions don't need to be toxic

Plagiarism in peer-review reports could be the ‘tip of the iceberg’
Dad’s microbiome can affect offspring’s health — in mice
‘Shut up and calculate’: how Einstein lost the battle to explain quantum reality
The science of 3 body problem: what’s fact and what’s fiction, do cutting-edge car-t-cell therapies cause cancer what the data say, this social sciences hub galvanized india’s dynamic growth. can it survive, first fetus-to-fetus transplant demonstrated in rats.
Male–female comparisons are powerful in biomedical research — don’t abandon them

We need more-nuanced approaches to exploring sex and gender in research
Allen j. bard obituary: electrochemist whose techniques underpin clinical diagnostics, materials discovery and more, support communities that will lose out in the energy transition, current issue.

Marsupial genomes reveal how a skin membrane for gliding evolved
A recently quenched galaxy 700 million years after the big bang, a magnetar giant flare in the nearby starburst galaxy m82, research analysis.
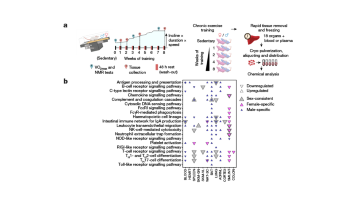
Endurance exercise causes a multi-organ full-body molecular reaction
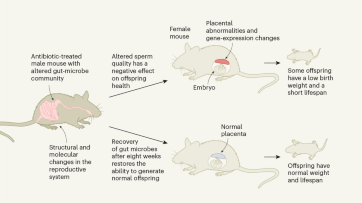
Dad’s gut microbes matter for pregnancy health and baby’s growth
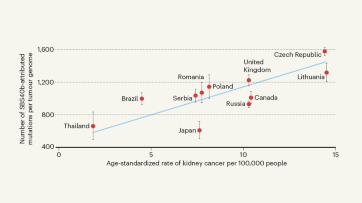
Genomics reveal unknown mutation-promoting agents at global sites
Cells destroy donated mitochondria to build blood vessels
Intel brings quantum-computing microchips a step closer, resilience lessons from ancient societies are still relevant today, how to stop students cramming for exams send them to sea, robust optical clocks promise stable timing in a portable package.

I strive to make the Great Barrier Reef more resilient to heat stress

Scientists urged to collect royalties from the ‘magic money tree’

Breaking ice, and helicopter drops: winning photos of working scientists
My pi yelled at me and i’m devastated. what do i do, us national academies report outlines barriers and solutions for scientist carers, books & culture.

How volcanoes shaped our planet — and why we need to be ready for the next big eruption

Dogwhistles, drilling and the roots of Western civilization: Books in brief

The AI tuner
Las borinqueñas remembers the forgotten puerto rican women who tested the first pill, cosmic rentals, nature podcast.

Latest videos
Nature briefing.
An essential round-up of science news, opinion and analysis, delivered to your inbox every weekday.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

COMMENTS
of COVID-19 can explain 40% of the delayed graduation gap (as well as a substantial part of the gap for other outcomes) between lower- and higher-income students. To our knowledge, this is the rst paper to shed light on the e ects of COVID-19 on college students' experiences. The treatment e ects that we nd are large in economic terms.
in the wake of COVID-19, including unemployment, mental illness, and addiction; and, (3) the importance of moderating factors (e.g., age, race and ethnicity, gender, personality, family status, and culture) for which there are likely to be disparate COVID-19 impacts.
The spread of the "Severe Acute Respiratory Coronavirus 2" (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [].The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [], causing massive economic strain ...
The 25 most downloaded Nature Communications articles* on COVID-19 published in 2021 illustrate the collaborative efforts of the international community to combat the ongoing pandemic.These papers ...
COVID-19 can involve persistence, sequelae, and other medical complications that last weeks to months after initial recovery. This systematic review and meta-analysis aims to identify studies ...
Abstract. In early December 2019, an outbreak of coronavirus disease 2019 (COVID-19), caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), occurred in Wuhan City, Hubei Province, China. On January 30, 2020 the World Health Organization declared the outbreak as a Public Health Emergency of International Concern.
Introduction. The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over 192 million cases and 4.1 million deaths as of July 22, 2021. 1 This pandemic has brought along a massive burden in morbidity and mortality in the healthcare systems. Despite the implementation of stringent public health measures, there ...
Coronavirus disease 2019 (COVID-19) is a highly contagious viral illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 has had a catastrophic effect on the world, resulting in more than 6 million deaths worldwide. After the first cases of this predominantly respiratory viral illness were reported in Wuhan, Hubei Province, China, in late December 2019, SARS ...
The Johns Hopkins Coronavirus Resource Center has collected, verified, and published local, regional, national and international pandemic data since it launched in March 2020. From the beginning, the information has been freely available to all — researchers, institutions, the media, the public, and policymakers. As a result, the CRC and its data have been cited in many published research ...
As reported by the CDC, from February 12 to April 2, 2020, of 149,760 cases of confirmed COVID-19 in the United States, 2572 (1.7%) were children aged <18 years, similar to published rates in ...
Detailed search strategy for gathering COVID-19 articles, updated October 9, 2020 [PDF - 135 KB] The CDC Database of COVID-19 Research Articles is now a part of the WHO COVID-19 database. Our new search results are now being sent to the WHO COVID-19 Database to make it easier for them to be searched, downloaded, and used by researchers ...
Abstract. In early December 2019, an outbreak of coronavirus disease 2019 (COVID-19), caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), occurred in Wuhan City, Hubei Province, China. On January 30, 2020 the World Health Organization declared the outbreak as a Public Health Emergency of International Concern.
According to a study published in 2019, An-. Corona virus causes respiratory infection including pneumonia, cold, sneezing and coughing while in animal it causes. diarrhea and upper respiratory ...
DRAFT Prepared by the SAGE Working Group on COVID-19 Vaccines 22 December 2020 5 . even death. Several occupational risks for health workers emerged or were amplified by the COVID-19 response, including (1) occupational infections with COVID-19, (2) skin disorders and heat stress
Download PDF. Article; ... A total of 14787 COVID-19 papers were identified as of May 14, 2020 and 4892 duplicate articles were removed. ... the accelerated publication of COVID-19 research was ...
A study shows that this disease has an impact on the mental, physical and social well-being of health care professionals and three groups of people are prone to the complication of covid-19. Introduction The world health organization defines the Corona virus disease (covid-19) is an infectious disease which is caused by a newly discovered corona virus [1]. It was formerly known as severe acute ...
There have been around 96,000 reported cases of coronavirus disease 2019 (COVID-2019) and 3300 reported deaths to date (05/03/2020). The disease is transmitted by inhalation or contact with infected droplets and the incubation period ranges from 2 to 14 d. The symptoms are usually fever, cough, sore throat, breathlessness, fatigue, malaise ...
Introduction SARS-CoV-2 pandemic has caused global devastations in the social, economic and health systems of every nation, but disproportionately the nations in Africa. Apart from its grave effects on the global systems, is the persistence of post-COVID-19 condition in individuals infected with the virus. Therefore, the aim of this scoping review is to collate and summarise the existing ...
COVID-19 has spread more widely and rapidly than SARS, affecting a larger number of people (Wu et al., Citation 2020). The COVID-19 pandemic began its rapid spread before the Spring Festival (Sparkes et al., Citation 2021), whereas the SARS epidemic started spreading after the festival had concluded. This suggests that the COVID-19 spread was ...
The COVID-19 pandemic has mobilized the world scientific community in 2020, especially in the life sciences [ 1, 2 ]. In the first three months after the pandemic, the number of scientific papers about COVID-19 was fivefold the number of articles on H1N1 swine influenza [ 3 ]. Similarly, the number of clinical trials related to COVID-19 ...
PDF | In this paper, we discussed basic information of Plagiarism, meaning, types and form of plagiarism. ... Methods Consecutively occurring original research or reviews relating to the COVID-19 ...
Download MP3. In the final Coronapod of 2020, we dive into the scientific literature to reflect on the COVID-19 pandemic. Researchers have discovered so much about SARS-CoV-2 - information that ...
The COVID-19 outbreak has posed an unprecedented challenge to humanity and science. On the one side, public and private incentives have been put in place to promptly allocate resources toward research areas strictly related to the COVID-19 emergency. However, research in many fields not directly related to the pandemic has been displaced. In this paper, we assess the impact of COVID-19 on ...
research conducted at the WIV was funded by NIAID and may have started the COVID-19 pandemic—the NIH began compliance actions regarding the grant. These actions centered around both administrative and scientific failures on the part of EcoHealth and resulted in the eventual suspension of EcoHealth's grant and the debarment of the WIV.
listed in Appendix I regarding or referencing the origins of COVID-19. 17. All documents and communications between or among EcoHealth Alliance, Inc, including any of its subsidiaries or affiliated individuals and any employee or contractor of the Defense Advanced Research Project agency regarding the DEFUSE proposal for the project entitled ...
Download PDF. The COVID-19 ... More than 30,000 of the COVID-19 articles published in 2020 were preprints — between 17% and 30% of total COVID-19 research papers (depending on database searched ...
Find breaking science news and analysis from the world's leading research journal.