BioNTech doses first patient in herpes vaccine candidate clinical trial
- Medium Text

Sign up here.
Reporting by Tristan Chabba in Gdansk Editing by Miranda Murray
Our Standards: The Thomson Reuters Trust Principles. New Tab , opens new tab


Business Chevron

Computer parts maker Logitech's fourth-quarter sales rise
Logitech International increased its sales during its fourth quarter, the computer peripherals maker said on Tuesday, snapping two-and-a-half years of sales downturns after a pandemic-driven boom.

With a herpes vaccine on the horizon, will the stigma persist?

When I was 9 years old, I came down with a terrifying bout of pneumonia and ended up in the hospital for a week. I remember having a panic attack when I couldn’t catch my breath and my mother, scared, called a doctor for help. He arrived quickly and calmed me down with his gentle bedside manner. He helped me take deep, slow breaths through an oxygen mask, to the tune of his voice as he counted down from 10. I egged myself on, knowing I had to relax or I’d get transferred to the local children’s hospital.
A few days later, I arrived back at school. I was in fourth grade. I had recovered but still had trouble breathing. Whenever my immune system runs low, I’m at greater risk for a herpes outbreak , and that’s exactly what happened. My face had erupted in giant, oozing cold sores. During recess, I sat on a bench alone listening to Coldplay’s “Yellow” on my Walkman. A group of older, prettier and more popular girls approached me. I pulled one of my earbuds out to hear what they were saying, only to find they were taunting me. “AIDS Face.” “Pimple Mouth.” “Zit Lips.”
I’ve had herpes for as long as I can remember, likely contracting the virus as a grabbing toddler reaching for my mother’s face.
As these cruel names were hurled at me, I trembled, cried and hugged my legs to my chest. When treating cold sores, time is of the essence. The second you feel a tingle, you need to treat the afflicted area. This helps mitigate the severity of the breakout . However, for a period of my childhood, I chose inaction, too traumatized by the stigma to do anything about it anymore. Instead, I leaned into being the weird kid and a social pariah, allowing my face to be riddled with herpes. While being infected with the virus is common and technically not a big deal, I was astronomically ashamed and isolated. In pop culture, the word herpes is near synonymous with dirty and that’s how I felt — dirty.
I’ve had herpes for as long as I can remember, likely contracting the virus as a grabbing toddler reaching for my mother’s face. Over the decades, I have spent a considerable amount of time agonizing over how to skip work, school and social events. When hiding from the world, I have tried every home remedy, topical cream and ointment and antiviral drug available. Sadly, there is no cure for herpes, only options to limit or prevent outbreaks . But a new vaccine on the horizon could prove to be a game changer.
Moderna is developing a vaccine using mRNA technology to treat the herpes simplex virus (HSV). There are two HSV virus types — HSV-1, the one I have, that affects the mouth, face and genitals, and HSV-2, which predominantly affects the genitals. However, both viruses can spread to other parts of the body. In the United States, of people aged 14 to 49, 47.8 percent have HSV-1 and 11.9 percent have HSV-2 , according to the Centers for Disease Control and Prevention. Many people living with herpes don’t know they have it, which means these figures may be far greater. HSV remains latent in the body , staying alive through the lifelong infection of a given person. When reactivated, HSV results in visible outbreaks. The vaccine will protect against HSV-2 and provide cross-protection for HSV-1 as a suppressive antiviral treatment.
The CDC recommends against widespread testing for herpes as, alongside the risk of false positives, “the risk of shaming and stigmatizing people outweighs the potential benefits.” Throughout my life, the social stigma surrounding herpes has proven more disastrous for my mental health than the virus itself. For so long, I assumed I wasn’t likable, let alone loveable. I believed I would be consigned to a life without sex and intimacy, having internalized harmful myths about a generally harmless infection. When I’ve had an outbreak, I’ve often chosen abstinence over disclosure, too fearful of rejection to open up. Interestingly, many people don’t even realize that having had chickenpox or shingles means they’ve been infected by a member of the herpes family . (Moderna is also developing a vaccine that would reduce the rate of the varicella-zoster virus that causes shingles.) But it’s the sexual component of HSV-1 and 2 that remains socially lethal.
The CDC recommends against widespread testing for herpes as, alongside the risk of false positives, “the risk of shaming and stigmatizing people outweighs the potential benefits.”
Much of the hysteria affecting the social status of herpes has been generated by the media and pharmaceutical companies. A 1982 TIME magazine cover labeled genital herpes “Today’s Scarlet Letter.” Authors of the cover story , John Leo and Maureen Dowd, posited that it could cause the sexual revolution to grind to a shrieking halt. Even more dramatic, the story argued that herpes was “altering sexual rites in America, changing courtship patterns, sending thousands of sufferers spinning into months of depression and self-exile and delivering a numbing blow to the one-night stand.”
Given the stigma around HIV at the time, perhaps the increased awareness about herpes did make some people change their sexual behaviors, but we also know that any activity that was deemed sexually deviant was used as a scapegoat to make sex seem shameful. A 2016 Vice exposé found that, starting in the 1970s, there is evidence that “big pharma” likely conjured up and perpetuated stigma to increase sales of a new drug, one that couldn’t be used to treat all members of the herpes family. To advertise the drug, herpes had to be pushed as a disease worthy of attention, the answer to which was a sex panic.
In the age of medical misinformation, vaccines themselves are misunderstood. For example, in general, they have been said to cause autism, despite no scientific evidence. The misinformation seems to increase when it comes to newly available ones; look no further than conspiracy theories swirling around Covid-19 vaccines , which were rumored to contain infertility agents or spread HIV — another notoriously stigmatized STI. The mRNA technology used to create these life-saving Covid-19 vaccines opened up the door for those Moderna is currently developing to treat herpes. In the near future, it’s possible that people will be prevented from ever getting herpes and that those with it won’t have to suffer through outbreaks anymore. I’ve wondered if the social stigma will persist and if kids like myself will be spared the pain I have experienced since childhood.
Deidre Olsen is an award-nominated writer based in Berlin. She is writing a memoir about self-destruction, healing and resilience.
Vaccine shows promise against herpes virus
New study demonstrates candidate's potential to generate antibodies, limit viral shedding.
A genetically edited form of a herpes simplex virus -- rewired to keep it from taking refuge in the nervous system and eluding an immune response -- has outperformed a leading vaccine candidate in a new study from the University of Cincinnati, Northwestern University and the University of Nebraska-Lincoln.
Published Nov. 6 in the journal Nature Vaccines , the study found that vaccinating guinea pigs with the modified live virus significantly increased the production of virus-combating antibodies. When challenged with a virulent strain of herpes simplex virus, the vaccinated animals displayed fewer genital lesions, less viral replication and less of the viral shedding that most readily spreads infection to others.
The modified virus is actually a form of herpes simplex virus type 1, best known for causing cold sores around the lip. The fact that it demonstrated cross-protection against HSV type 2 -- the sexually transmitted type usually responsible for genital herpes -- suggests that an HSV-2-specific edition of the vaccine could prove even more effective, the researchers said.
The World Health Organization estimates that more than 500 million people have HSV-2, which persists for a lifetime and often flares up in response to stress. In addition to causing blisters, HSV-2 increases the risk for HIV infection and may contribute to Alzheimer's disease or other forms of dementia.
Despite the prevalence of the viruses, more than four decades of research have yet to yield an approved vaccine for HSV-1 or HSV-2. Part of the difficulty: The alphaherpesviruses, which include HSV, have evolved an especially sophisticated way of evading the immune responses aimed at destroying them.
After infecting mucosal tissues of the mouth or genitourinary tract, HSV works its way to the tips of sensory nerves that transmit signals responsible for the sensations of pain, touch and the like. With the help of a specialized molecular switch, the virus then breaks into the nerve cell, hitching a ride on the molecular equivalent of a trolley car that transports it along a nerve fiber and into the nucleus of a sensory neuron. Whereas the mucosal infection is soon cleared by the immune response, the infected neurons become a sanctuary from the body's immune system, with HSV leaving only when stirred by rises in steroids or other stress-elevated hormones in the host.
Nebraska's Gary Pickard and Patricia Sollars, alongside Northwestern's Gregory Smith and Tufts University's Ekaterina Heldwein, have spent years studying how to prevent HSV from reaching the safety of the nervous system. Heldwein advanced those efforts when she characterized the architecture of a certain alphaherpesvirus protein, pUL37, that the team suspected was integral to the virus moving along nerve fibers. Computer analyses based on that architecture suggested that three regions of the protein might prove important to the process.
Smith then carefully plucked out and replaced five codons, the fundamental coding information in the DNA, from the viral genome of each region. The researchers hoped that those mutations might help impede the virus from invading the nervous system.
Their hopes were rewarded when Pickard and Sollars injected mice with a virus modified in region 2, or R2, of the protein. Rather than advancing deeper into the nervous system, the virus was stuck at the nerve terminal. But the team also knew that modifying HSV could have unintended consequences.
"You can keep the virus from getting into the nervous system," said Pickard, professor of veterinary medicine and biomedical sciences at Nebraska. "That's not that hard to do by making broadly debilitating mutations. But when you knock down the virus so much that it doesn't replicate well, you are not rewarded with a robust immune response that can protect you from future exposures."
So the researchers were heartened when further studies showed that the R2-mutated virus performed well as a vaccine in mice. Moreover, it circumvented certain stubborn issues that have cropped up with other vaccine approaches. Some approaches have involved challenging the immune system with only a subset of HSV components, or antigens, priming the body to recognize them but potentially miss others. Some have modified the virus so that it can replicate just once, preventing long-term persistence in the nervous system but also reducing spread in mucosal tissues and, by extension, a stout immune response.
"So it's the same story over and over again: Either your subunit vaccine doesn't present enough antigens, or you make the live virus essentially so sick that it doesn't work really well to generate an immune response," Pickard said. "That's why we're so optimistic about our R2 platform, because it avoids all those problems."
David Bernstein, a researcher at Cincinnati Children's Hospital Medical Center who evaluates herpesvirus vaccine candidates through a program supported by the National Institutes of Health, took note of the team's success and reached out to Northwestern's Smith in 2018. Armed with an R2-modified form of HSV-1, Bernstein decided to test its effectiveness against HSV-2 infection in guinea pigs. As promising as their prior results had been, Pickard conceded that he wasn't sure an HSV-1 vaccine would be up to the task of generating immunity against HSV-2.
But just one of the dozen R2-inoculated guinea pigs developed acute lesions after being injected with HSV-2, compared with five of 12 animals receiving another promising vaccine candidate that recently failed a human clinical trial. Whereas that latter vaccine candidate had no discernible effect on the number of days that guinea pigs shed the virus, the team's R2 vaccine cut the shedding period from 29 days to about 13. And unlike the guinea pigs receiving no vaccine or the other candidate, those receiving the R2 vaccine showed no sign of HSV-2 in the cluster of brain cells that normally house it. Neutralizing antibodies, meanwhile, registered about three times higher in the R2-inoculated guinea pigs than in those inoculated with the other vaccine candidate.
"The fact that the viral shedding was knocked down so much with the R2 vaccine is really important, because it's the viral shedding -- even if it doesn't cause lesions -- that can then pass on the virus," Pickard said. "If you have genital herpes, you can pass that on to your significant other, not knowing that you're doing it. It's very problematic. So the fact that the shedding was knocked down so much is a really good sign."
With an HSV-1 version of the R2 vaccine showing such promising cross-protection against its sexually transmitted counterpart, the researchers' to-do list now includes making and testing an HSV-2 vaccine against the HSV-2 virus.
"If you're making antibodies against the proteins of that particular virus, it stands to reason (that) it would work better than if you're making an antibody against something that's slightly different," he said. "So that's our expectation."
'IT'S GOING TO HAVE A BIG IMPACT'
Around the time that Bernstein and his NIH program were expressing interest in the R2 vaccine design, Pickard and Smith were launching a startup, Thyreos LLC, aimed at further developing and eventually licensing their R2 vaccine design.
Fittingly for a couple of researchers based in Nebraska and Illinois, the duo is working on vaccines for livestock -- cattle and hogs, specifically -- that contend with alphaherpesviruses of their own. In cattle, the bovine herpesvirus can cause respiratory disease, curb appetite and even contribute to aborted calves, all of which add up to billions of dollars in lost revenue annually. Though a modified live-virus vaccine for cattle does exist, it also gets into the bovine nervous system. And that, Pickard said, can spell trouble in cattle just as easily as in people.
"What happens, then, is that when those cows are loaded on a truck and shipped to a feedlot, it's a stressful environment," he said. "The virus hiding in the immune system reactivates. They start shedding the virus from excretions in their nose, and they can then pass that virus on to other animals in that feedlot, and the cattle can get respiratory disease.
"So the fact that our R2-modified viruses don't enter the nervous system is not just an academic thing. Actually, it has a real, practical application for the cattle industry."
As they prepare to embark on a new series of studies that they hope will show the R2 design's superiority to the current industrywide vaccine, Pickard and Smith are also kicking off an initial round of seed funding for the enterprise.
Given that the team initially developed its R2 design in the alphaherpesvirus that infects pigs -- the so-called pseudorabies virus -- Pickard also expressed confidence in the design's promise for protecting hogs. In the late 1990s and early 2000s, the United States waged a successful campaign to eradicate pseudorabies from the country, in large part through vaccination. As with cattle, though, the vaccine can enter the nervous system of hogs and has proven less successful in countries that are less vigilant about outbreaks.
"Again, we are pretty confident that our pseudorabies virus R2 vaccine is going to be more effective than what's been out there," Pickard said. "In terms of protecting pigs, it's going to have a big impact at some point.
"These pathogens can survive trans-Pacific transport in feed ingredients or feed products. When you talk to people who are concerned about biosecurity, they say that whatever is going on elsewhere in the world in terms of these viruses, eventually, they may show up here. It's just a matter of time."
- Cold and Flu
- Infectious Diseases
- HIV and AIDS
- HPV vaccine
- Sexually transmitted disease
- Epstein-Barr virus
- Encephalitis
Story Source:
Materials provided by University of Nebraska-Lincoln . Original written by Scott Schrage. Note: Content may be edited for style and length.
Journal Reference :
- David I. Bernstein, Rhonda D. Cardin, Gregory A. Smith, Gary E. Pickard, Patricia J. Sollars, David A. Dixon, Rajamouli Pasula, Fernando J. Bravo. The R2 non-neuroinvasive HSV-1 vaccine affords protection from genital HSV-2 infections in a guinea pig model . npj Vaccines , 2020; 5 (1) DOI: 10.1038/s41541-020-00254-8
Cite This Page :
Explore More
- Mice Given Mouse-Rat Brains Can Smell Again
- New Circuit Boards Can Be Repeatedly Recycled
- Collisions of Neutron Stars and Black Holes
- Advance in Heart Regenerative Therapy
- Bioluminescence in Animals 540 Million Years Ago
- Profound Link Between Diet and Brain Health
- Loneliness Runs Deep Among Parents
- Food in Sight? The Liver Is Ready!
- Acid Reflux Drugs and Risk of Migraine
- Do Cells Have a Hidden Communication System?
Trending Topics
Strange & offbeat.
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- COVID-19 Vaccines
- Occupational Therapy
- Healthy Aging
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Progress in Developing a Herpes Vaccine
- Where Research Stands
- Existing Vaccines
- Development Priorities
Barriers and Successes
Frequently asked questions.
The search for a vaccine to protect against oral and genital herpes has been a long one. Researchers have been experimenting with possible vaccines since at least the early 1930s. To date, they've seen little success. While herpes vaccines have been successful in mice, they've been largely unsuccessful in human trials.
This article explains the steps that have been taken thus far to develop vaccines for oral and genital herpes, the reasons why these vaccines are important, and the roadblocks keeping researchers from better preventing or controlling herpes infections.
Where Herpes Vaccine Research Stands
Although some vaccines for these herpes types have initially appeared to have promise, stringent testing has shown them to be no better than a sham vaccine, or placebo .
With that said, newer approaches to vaccine development—including genetic editing—have begun to show promise in early-stage animal research, offering a glimpse of hope of a possible breakthrough.
Existing Herpes Vaccines
Technically speaking, there are already herpes vaccines on the market. They just don't protect you from herpes simplex virus (HSV) type 1 (the type most commonly associated with oral herpes) or HSV type 2 (the type most commonly associated with genital herpes).
Rather, the two vaccines currently available protect against a type of herpes virus known as varicella-zoster virus (VZV), more commonly called the chickenpox virus.
Once a chickenpox infection resolves, the virus remains in a dormant (latent) state. It does so in a cluster of nerve cells called the dorsal root ganglion , where it could reactivate later in life. If it does, it causes shingles (herpes zoster).
The shingles vaccine and chickenpox vaccine both guard against the virus, but in different ways:
- The chickenpox vaccine is typically given in early childhood to protect you from becoming infected with VZV.
- The shingles vaccine is given from the age of 50 to prevents the reactivation of VZV.
These are similar to the two types of vaccines that have been proposed to protect against oral and genital herpes. One type aims to prevent the virus from infecting people who've never had it, while the other aims to protect against outbreaks in people who already have herpes.
Protecting people who have had herpes from future outbreaks is important because once you're infected with a herpes virus, it stays in your body forever. It goes dormant, but can suddenly reactivate at any point, bringing on symptoms again.
An adult in their 60s, for example, might develop shingles due to a reactivation of VZV that they acquired when they had chickenpox as a child.
Oral and genital herpes outbreaks can recur in the same way.
The chickenpox vaccine protects you from a type of herpes. The shingles vaccine protects you from reactivation of that same virus. However, this is not the type of herpes associated with oral or genital cases.
Herpes Vaccine Priorities
Theoretically, it makes sense that a vaccine could work to prevent oral and genital herpes outbreaks. After all, in many people, the immune system controls herpes infections so that they never have symptoms.
This makes herpes a good target for a therapeutic vaccine —that is, one that treats rather than prevents disease. However, the herpes simplex viruses have proven to be difficult to control with vaccines.
In 2017, the World Health Organization (WHO) defined a series of priorities for developing a herpes vaccine:
- Reduce the number of people who become infected with human immunodeficiency virus ( HIV ) due to a herpes genital infection. (Having genital sores increases your risk of getting HIV.)
- Reduce the number of people negatively affected by HSV by reducing physical symptoms, psychological symptoms, and serious consequences such as infection in newborns (neonatal herpes).
- Reduce the impact of herpes infection on reproductive health.
The WHO suggests that two types of vaccines could be useful for herpes simplex infections:
- Prophylactic vaccines, like the chickenpox vaccine, would help prevent people from ever getting herpes.
- Therapeutic vaccines, like the shingles vaccine, would reduce the number of outbreaks.
Developing vaccines that can prevent oral or genital herpes infection and reactivation are worldwide goals. This is not just because of a desire to reduce complications of HSV itself, but to address the increased risk of HIV infection that comes with genital herpes.
Some promising trials of herpes vaccines have been performed. However, to date, no human trials have shown high enough efficacy to bring a herpes vaccine to market.
Scientists have several hurdles to face when developing a vaccine to protect against oral or genital herpes.
No animal model perfectly replicates HSV infection in humans. Several vaccine candidates have shown promise in animal studies but have, thus far, not been effective in clinical trials in humans.
Aside from mice, rabbits and guinea pigs are also being used to develop therapeutic herpes vaccines (for eye and genital herpes, respectively). Early results have been promising, but current animal models still don't do a great job of showing how the disease progresses in humans.
Herpes vaccines are also difficult to study for several other practical reasons:
- Limited study population: Researchers need to test a lot of people to see if a vaccine works. Those people can be hard to find.
- Asymptomatic infection: Because many infected people never have herpes symptoms, assessing the effectiveness of a preventive vaccine means having to actively test to see whether they've been infected with the virus since getting the shot.
- Viral shedding: Scientists have to test the possibility that the virus will be shed, or release particles that can infected others. Low viral shedding translates to a lower risk of infections.
Addressing any of these factors can make vaccine trials slow-going, burdensome, impractical, and expensive.
A 2020 study from researchers from the University of Cincinnati, Northwestern University, and the University of Nebraska-Lincoln offers hope of a possible breakthrough.
According to the research, a genetically modified form of herpes simplex virus type 1 was able to prevent symptoms of herpes simplex virus type 2 in guinea pigs. The response was far more robust than seen with any herpes vaccine study to date. It significantly slowed the virus's replication and showed less viral shedding.
Another research team at the University of California, Irvine, School of Medicine proposed the use of lasers as part of the vaccination procedure. Their goal was to stimulate the development of immune cells in the layers of the skin where herpes reactivation occurs.
The procedure involved mice. It, too, showed promise in preventing genital herpes, improving the effect of an experimental vaccine.
Although it's far too soon to tell whether the studies will lead to a successful vaccine, these advances are considered significant.
The lack of a vaccine for oral or genital herpes is not for a lack of effort. Several issues, including the poor translation of results in animals to results in humans, have made development challenging.
Putting an end to oral and genital herpes would have a far-reaching impact on the health of people around the world. The virus increases the risk of HIV, affects fertility, and places significant psychological and physical stress on those infected.
Unfortunately, there are a number of barriers to developing a vaccine. First, it’s hard to find people who are able to participate in the studies needed to test possible vaccines. Also, people who are infected may not have symptoms, which makes it more complicated to tell whether a vaccine is effective.
A Word From Verywell
Fortunately, you have other options for reducing the risk of transmission as research on herpes vaccines continues. Both suppressive therapy and reliably practicing safe sex can help protect the sexual partners of people with HSV infections.
No. The herpes zoster vaccine protects you against shingles (herpes zoster), a viral infection that is a reactivation of the chickenpox virus. There is currently no vaccine to protect against genital or oral herpes.
No. However, antiviral medications may prevent or reduce the severity of an oral or genital herpes outbreak.
There is some evidence that certain types of oils can ease a herpes outbreak. For instance, oregano oil has been shown to have antiviral properties that act on HSV. But more research is needed to know if these will actually shorten an outbreak.
Ghiasi H. Highly efficacious novel vaccine, humoral immunity, and ocular herpes simplex virus 1: reality or myth ? J Virol . 2017;91(23). doi:10.1128/JVI.01421-17
Crimi S, Florillo L, Bianchi A. et al. Herpes virus, oral clinical signs and QoL: systematic review of recent data . Viruses . 2019 May;11(5):463. doi:10.3390/v11050463
Wang L, Zhu L, Zhu H. Efficacy of varicella (VZV) vaccination: an update for the clinician . Ther Adv Vaccines . 2016;4(1-2):20-31. doi:10.1177/2051013616655980
Gabutti G, Bolognesi N, Sandri F, Flourescu C, Stefanati A. Varicella zoster virus vaccines: an update . Immunotargets Ther. 2019;8:15-28. doi:10.2147/ITT.S176383
National Institutes of Health. Herpes can happen to anyone .
Centers for Disease Control. Genital herpes - CDC fact sheet .
Gottlieb SL, Giersing BK, Hickling J, et al. Meeting report: Initial World Health Organization consultation on herpes simplex virus (HSV) vaccine preferred product characteristics, March 2017 . Vaccine . 2019;37(50):7408-7418. doi:10.1016/j.vaccine.2017.10.084
Spicknall IH, Looker KJ, Gottlieb SL, et al. Review of mathematical models of HSV-2 vaccination: Implications for vaccine development . Vaccine . 2019;37(50):7396-7407. doi:10.1016/j.vaccine.2018.02.067
Kuo T, Wang C, Badakshan T, Chilikuri S, BenMohamed L. The challenges and opportunities for development of a T-Cell epitope-based herpes simplex vaccine . Vaccine. 2014 Nov 28;32(50):6733-45. doi:10.1016/j.vaccine.2014.10.002
Gottlieb SL, Johnston C. Future prospects for new vaccines against sexually transmitted infections . Curr Opin Infect Dis . 2017;30(1):77-86. doi:10.1097/QCO.0000000000000343
Bernstein DI, Cardin RD, Smith GA, et al. The R2 non-neuroinvasive HSV-1 vaccine affords protection from genital HSV-2 infections in a guinea pig model . npj Vaccines , 2020:5(1):104. doi:10.1038/s41541-020-00254-8
Lopes PP, Todorov G, Pham TT, Nesburn AB, Bahraoui E, Benmohamed L. Laser adjuvant-assisted peptide vaccine promotes skin mobilization of dendritic cells and enhances protective CD8 T and T cell responses against herpesvirus infection and disease . J Virol . 2018;92(8). doi:10.1128/JVI.02156-17
MedlinePlus. Genital herpes .
FDA. Zostavax (Herpes Zoster Vaccine) Questions and Answers .
Center for Disease Control and Prevention. Is there a Cure or Treatment for Herpes?
Wang L, Wang D, Wu X, Xu R, Li Y. Antiviral mechanism of carvacrol on HSV-2 infectivity through inhibition of RIP3-mediated programmed cell necrosis pathway and ubiquitin-proteasome system in BSC-1 cells . BMC Infect Dis . 2020;20(1):832. doi:10.1186/s12879-020-05556-9
Awasthi S, Friedman HM. Status of prophylactic and therapeutic genital herpes vaccines. Curr Opin Virol . 2014 Jun;6:6-12. doi:10.1016/j.coviro.2014.02.006
Rajčáni J, Bánáti F, Szenthe K, Szathmary S. The potential of currently unavailable herpes virus vaccines. Expert Rev Vaccines . 2018 Mar;17(3):239-248. doi: 10.1080/14760584.2018.1425620
World Health Organization. WHO preferred product characteristics for herpes simplex virus vaccines .
By Elizabeth Boskey, PhD Boskey has a doctorate in biophysics and master's degrees in public health and social work, with expertise in transgender and sexual health.
- History, Facts & Figures
- YSM Dean & Deputy Deans
- YSM Administration
- Department Chairs
- YSM Executive Group
- YSM Board of Permanent Officers
- FAC Documents
- Current FAC Members
- Appointments & Promotions Committees
- Ad Hoc Committees and Working Groups
- Chair Searches
- Leadership Searches
- Organization Charts
- Faculty Demographic Data
- Professionalism Reporting Data
- 2022 Diversity Engagement Survey
- State of the School Archive
- Faculty Climate Survey: YSM Results
- Strategic Planning
- Mission Statement & Process
- Beyond Sterling Hall
- COVID-19 Series Workshops
- Previous Workshops
- Departments & Centers
- Find People
- Biomedical Data Science
- Health Equity
- Inflammation
- Neuroscience
- Global Health
- Diabetes and Metabolism
- Policies & Procedures
- Media Relations
- A to Z YSM Lab Websites
- A-Z Faculty List
- A-Z Staff List
- A to Z Abbreviations
- Dept. Diversity Vice Chairs & Champions
- Dean’s Advisory Council on Lesbian, Gay, Bisexual, Transgender, Queer and Intersex Affairs Website
- Minority Organization for Retention and Expansion Website
- Office for Women in Medicine and Science
- Committee on the Status of Women in Medicine Website
- Director of Scientist Diversity and Inclusion
- Diversity Supplements
- Frequently Asked Questions
- Recruitment
- By Department & Program
- News & Events
- Executive Committee
- Aperture: Women in Medicine
- Self-Reflection
- Portraits of Strength
- Mindful: Mental Health Through Art
- Event Photo Galleries
- Additional Support
- MD-PhD Program
- PA Online Program
- Joint MD Programs
- How to Apply
- Advanced Health Sciences Research
- Clinical Informatics & Data Science
- Clinical Investigation
- Medical Education
- Visiting Student Programs
- Special Programs & Student Opportunities
- Residency & Fellowship Programs
- Center for Med Ed
- Organizational Chart
- Leadership & Staff
- Committee Procedural Info (Login Required)
- Faculty Affairs Department Teams
- Recent Appointments & Promotions
- Academic Clinician Track
- Clinician Educator-Scholar Track
- Clinican-Scientist Track
- Investigator Track
- Traditional Track
- Research Ranks
- Instructor/Lecturer
- Social Work Ranks
- Voluntary Ranks
- Adjunct Ranks
- Other Appt Types
- Appointments
- Reappointments
- Transfer of Track
- Term Extensions
- Timeline for A&P Processes
- Interfolio Faculty Search
- Interfolio A&P Processes
- Yale CV Part 1 (CV1)
- Yale CV Part 2 (CV2)
- Samples of Scholarship
- Teaching Evaluations
- Letters of Evaluation
- Dept A&P Narrative
- A&P Voting
- Faculty Affairs Staff Pages
- OAPD Faculty Workshops
- Leadership & Development Seminars
- List of Faculty Mentors
- Incoming Faculty Orientation
- Faculty Onboarding
- Past YSM Award Recipients
- Past PA Award Recipients
- Past YM Award Recipients
- International Award Recipients
- Nominations Calendar
- OAPD Newsletter
- Fostering a Shared Vision of Professionalism
- Academic Integrity
- Addressing Professionalism Concerns
- Consultation Support for Chairs & Section Chiefs
- Policies & Codes of Conduct
- Health & Well-being
- First Fridays
- Fund for Physician-Scientist Mentorship
- Grant Library
- Grant Writing Course
- Mock Study Section
- Research Paper Writing
- Funding Opportunities
- Join Our Voluntary Faculty
- Child Mental Health: Fostering Wellness in Children
- Faculty Resources
- Research by Keyword
- Research by Department
- Research by Global Location
- Translational Research
- Research Cores & Services
- Program for the Promotion of Interdisciplinary Team Science (POINTS)
- CEnR Steering Committee
- Experiential Learning Subcommittee
- Goals & Objectives
- Issues List
- Print Magazine PDFs
- Print Newsletter PDFs
- YSM Events Newsletter
- Social Media
- Patient Care
INFORMATION FOR
- Residents & Fellows
- Researchers
How the Stigma of Herpes Harms Patients and Stymies Research for a Cure
“Well, my life is over,” Christopher Pickering remembers thinking.
It was the height of the COVID-19 pandemic when he first noticed the painful urination, swollen lymph nodes, over 20 blisters in his pubic area, and a dull, aching, burning pain. “This isn’t anything, it’s just a friction burn,” he assured himself, trying to suppress his gut feeling on what the blisters actually meant. “I can’t be the kind of person who gets [herpes].” Because COVID had shut down all the sexual health clinics in Toronto, he found himself wandering into a busy walk-in clinic. The doctor confirmed his symptoms were likely herpes , swabbed one of the blisters, and quickly sent him away with a prescription to help him get through his first outbreak.
On the bus ride home, antivirals in hand, Pickering processed his new reality. Now a patient advocate on TikTok , he speaks openly to his over 100,000 followers about his diagnosis and journey to acceptance. But in that moment, he thought, “no one is going to accept me.”
Herpes simplex virus 1 and 2 [HSV-1 and HSV-2] infections are exceedingly common. As many as half to 60 percent of adults in United States have oral herpes, caused by HSV-1, and one of six individuals lives with genital herpes, caused by either HSV-1 or HSV-2. Despite this, the effects of the stigmatization of herpes are pervasive. Upon diagnosis, many patients have found that their providers aren’t well-informed about the condition, which got wide public attention in the 1970s and 80s that receded when deadly HIV/AIDS took hold.
The attention faded, but the virus didn’t. Patients who are infected often go on to experience severe mental health impacts in addition to the physical symptoms, but fear of stigmatization keeps many from advocating for themselves. Furthermore, the lack of funding can stymie research that could be life-changing for the millions living with the condition. As the COVID-19 pandemic and prevalence of long COVID highlight the urgent need to better understand the long-lasting impacts of viral infections, researchers and patients alike hope it will also generate interest in other chronic viral illnesses like herpes.
“Herpes can be a devastating disease. But because it’s sexually transmitted, people don’t want to talk about it,” says Akiko Iwasaki, PhD , Sterling Professor of Immunobiology and professor of dermatology; of molecular, cellular & developmental biology; and of epidemiology (microbial diseases). In her lab at Yale School of Medicine, Iwasaki has developed a therapeutic vaccine that can successfully shut down the reactivation of genital herpes in guinea pigs, but lack of investment has hindered it from progressing to human trials. “People used to email me all the time asking when a vaccine would be available, saying their lives had been destroyed by the virus. But without interest from pharmaceutical companies, we can’t go any farther.”
The Psychosocial Impact of Genital Herpes
Sexually transmitted infections are surging nationwide. Between 2017 and 2021, the Centers for Disease Control and Prevention (CDC) reported that cases of gonorrhea increased by 28 percent, and syphilis by 74 percent. Less testing and education during the height of the COVID-19 pandemic, says Dana Dunne, MD, MHS , associate professor of medicine (infectious diseases), contributed largely to this surge. “Our ability to test and treat sexually transmitted infections really shut down between 2020 and 2021,” she says. “So now that we’re able to test more frequently again, we’re seeing a lot more cases because of the ongoing, unchecked transmission when we were unable to intervene.”
However, the 2021 report has a notable omission—herpes. Due to its difficulty to track and a lack of accurate testing, the CDC doesn’t recommend that the infection be included in standard sexually transmitted infection panels. But chronic viral infection, especially HSV-2, can significantly impact a patient’s quality of life.
People used to email me all the time asking when a vaccine would be available, saying their lives had been destroyed by the virus. But without interest from pharmaceutical companies, we can’t go any farther. Akiko Iwasaki, PhD
When people acquire herpes, the virus stays mostly asleep in the body for the rest of their lives, emerging just from time to time. Most people living with herpes—as many as 90 percent of them—either are asymptomatic or have symptoms so subtle they don’t realize they are infected. In symptomatic cases, HSV-1 is associated with cold sores, and while it can be transmitted through kissing, it also can be passed through more informal kinds of contact such as sharing cups. HSV-2, on the other hand, causes genital sores and is typically acquired through sexual contact. However, both HSV-1 and HSV-2 can cross anatomic sites—individuals can get HSV-1 on their genitals, and more rarely, HSV-2 in their mouths.
Despite their similarities, HSV-1 and HSV-2 have vastly different psychosocial implications, says Dunne. While there isn’t much perceived stigma around getting a cold sore, she explains, people with HSV-2 often face fear and uncertainty about how to disclose their status to their partners. “They feel like they’re branded with a letter H on their forehead,” she says. This can make patients reluctant to get tested for it and physicians conflicted on when to test.
“The most significant challenge of living with herpes by far—and I don’t think this is covered anywhere close to what it should be in the media and scientific community—is the psychological impact,” says Brandon [name changed for privacy], a 42-year-old attorney, who has put off re-entering the dating pool after his recent divorce. “I can’t for the life of me put any energy into dating anymore because I just don’t know how to tell people about this diagnosis.”
“Living with herpes feels like a prison sentence,” says Natalie [name also changed], a nurse practitioner who sat on an ice pack during her interview to cope with the pain of one of her outbreaks, which are frequent despite taking antivirals. “You’ll have these wonderful periods of time where you have no outbreaks, but you still have this dread knowing it will come back. It’s devastating.”
Lack of Education and Effective Herpes Testing Are Barriers to Patients
Furthermore, many patients say that a lack of accurate testing and ignorance about the condition among their providers pose further challenges. There are two ways to test for the virus. For a patient with an active outbreak, clinicians can directly swab the sore and provide a highly accurate result. Asymptomatic patients can seek out a blood test, but there is significant risk of receiving a false positive. “Current testing measures are absolutely not adequate. We’ve had the same tests on the market for decades,” says Jeffrey Klausner, MD , professor of clinical population and public health sciences at Keck School of Medicine of the University of Southern California. “There just hasn’t been a push or demand for expansion of confirmatory testing.”
“I believe a lot of doctors avoid using the blood test because they don’t know how to counsel patients if it’s positive,” Dunne adds. “Physicians need to know how to counsel patients, as well as be familiar with how to recognize a true positive and false positive and how to troubleshoot the tests that are inconclusive.”
Fourteen years ago, Natalie went to two different clinics, where her symptoms were dismissed as a urinary tract infection or yeast infection. Although she was not yet a nurse practitioner, she regrets not demanding further testing, a decision that haunts her to this day. She ended up transmitting the virus to her now-husband, who was diagnosed immediately after his symptoms appeared. “I live with the shame that I am a victim and also that I became a spreader,” she says. “My husband says I am his dream woman, but that I am also his nightmare because of what I did.”
When Brandon was diagnosed after noticing a pimple-like sore on his groin ten days after protected intercourse with a new partner, he asked an internal medicine physician about how to disclose it. The doctor told him that if he didn’t have active outbreaks, he had nothing to worry about. “Conflicting advice by doctors has put a huge psychological burden on me,” he says.
The root of the widespread ignorance among providers, says Klausner, begins in part during medical school. For about 10 years, he taught a course on sexually transmitted infections at the University of California, Los Angeles, School of Medicine, where he had about 55 minutes to cover over a dozen different infections. “We don’t provide enough opportunity for education and training around sexual health in medical school,” he says. “Our society has always had a difficult time talking about sexual health, and as a result so many medical professionals are poorly trained in how to listen to and counsel patients.”
Lack of Funding Hinders Herpes Vaccine Research
For decades, scientists have tried and failed to develop vaccines for herpes. The evasive nature of the virus makes it notoriously difficult to treat. But in her lab at Yale, Iwasaki has discovered promising clues for an effective vaccination.
Conventional vaccines boost immunity by triggering antibody development. But after vaccinating mice for HSV-2 with conventional vaccines, Iwasaki’s team learned that the animals failed to develop T cells or antibodies in the genital tract. In addition, the virus displays molecules that inactivate antibodies from attacking the virus. The limited access of T cells and antibodies to the viral entry site, and the antibody evasion strategies, likely explain why previous herpes vaccines have failed.
Based on that insight, roughly a decade ago the team developed a vaccine strategy called “prime-and-pull.” Through this mechanism, the researchers used a vaccine to generate T cell immunity in guinea pigs [prime]. Then, they used a cream that can induce chemokines—signaling molecules that can direct immune cells toward an infected site—to attract the T cells into the vaginal tissue [pull].
The strategy was a success —the team found that their vaccine could shut down the reactivation of herpes in infected guinea pigs. But the lack of funding has brought their work to a halt. “We’re looking for partners to be able to take this to humans, and that’s been the bottleneck ever since,” Iwasaki says.
She believes the stigma around sexually transmitted diseases is likely one of the obstacles to getting her vaccine funded. Research shows that the human papillomavirus (HPV) vaccine Gardasil, for example, is dramatically effective in preventing cervical cancer—reducing rates in women by nearly 90 percent. But despite its benefits, convincing parents to vaccinate their children is challenging, experts say.
“We have this fabulous anti-cancer vaccine designed to wipe out cervical cancer. But parents will see that it is ‘an STD vaccine’ and think we’re accusing their son or daughter of being high-risk sexually,” says Sten Vermund, MD, PhD , Anna M.R. Lauder Professor of Public Health. “I tell them that HPV is so ubiquitous and easy to contract that most women will acquire it at some point in their life. It’s not a marker of unsafe sex, but many parents still refuse.”
A herpes vaccine may face similar resistance, making it economically unattractive for the drug industry. “When pharmaceutical companies need to pour millions of dollars into developing a vaccine, if the uptake is going to be low, the profit doesn’t justify the effort,” says Iwasaki. “This makes STD vaccines difficult, even for something so obviously beneficial, safe, and effective as Gardasil.”
As interest in mRNA vaccines grows post-COVID, Moderna and Pfizer have begun working on such a vaccine for herpes. But in a recent talk to the World Health Organization (WHO), Iwasaki warned of the limitations of mRNA vaccines in treating herpes. “If you just make a vaccine without thinking about the mucosal tissue, it’s not going to work,” she said. “Just making an intra-muscular vaccine alone—I think it will be very difficult for such a vaccine to be effective.”
Advocates for Herpes Patients Rise Above the Stigma
The COVID-19 pandemic has shed light on the highly pervasive nature of viruses. As hundreds of millions of individuals recover from a SARS-CoV-2 infection, as many as one in eight experience lingering physical, cognitive, and neurological symptoms known as long COVID. While post-viral syndromes are not new, they have long been ignored by researchers. However, the magnitude of “long haulers” became too large for the medical community to sweep under the rug.
Many viral infections have been linked to autoimmune and neurological disease. For instance, research suggests that the Epstein-Barr virus (EBV), a member of the herpes virus family that is known for causing mononucleosis, may play a role in triggering multiple sclerosis. Individuals infected with EBV are 32 times more likely to develop the disease. Research has also uncovered potential links between HSV-1 and Alzheimer’s disease—the virus has been linked to amyloid plaque-like formations in human brain-like tissue. More research will be needed to see if herpes is causing the neurodegenerative disease, says Iwasaki. But this will require more investment in studying the infection.
As patients with long COVID struggle to find answers, many have rallied to advocate awareness about the need for more research. But patients with herpes face a much greater stigma that leaves many fearful to speak up about the need for new treatments. Now, as the medical community shows a growing interest in poorly understood viruses, patients are beginning to take a stand. In 2020, Herpes Cure Advocacy , the first grassroots international advocacy organization for HSV-1 and HSV-2, was born. The organization is advocating for the National Institutes of Health (NIH) to invest more in therapeutic cure research and for the CDC to establish an HSV taskforce. “Our job is to raise awareness, try to share medically accurate information, and hold public health agencies accountable to try to change the field the best we can,” says Kimberley, a patient advocate who founded the organization. For privacy, she has asked that her last name not be included.
For Pickering, who continues to make videos about herpes on TikTok and other social media platforms, learning to question the stigma about herpes helped him become less afraid of it. “People are not judging you if you don’t judge yourself,” he says in his recent YouTube video . “The stigma might never go away in the heads of everyone else and in society, but you can realize what the stigma is about, that you don’t have to hold onto it, and that you don’t have to be impacted by someone’s harsh words because those harsh words don’t make any sense.”
And advocacy efforts are beginning to pay off. For instance, prodded by Herpes Cure Advocacy, the Office of the Assistant Secretary for Health is working on the first national strategy for the cure, treatment, and prevention of herpes.
“Public health officials have a lot of competing priorities, so if we want change, we need more voices,” says Klausner. “If we want a cure and vaccines for herpes, we need to scream and yell for them.”
Featured in this article
- Akiko Iwasaki, PhD Sterling Professor of Immunobiology and Professor of Dermatology and of Molecular, Cellular, and Developmental Biology and of Epidemiology (Microbial Diseases); Investigator, Howard Hughes Medical Institute
- Dana Dunne, MD, MHS Associate Professor of Medicine (Infectious Diseases); Associate DIO; GME Director, Educator Development, Department of Medicine; Associate, Teaching and Learning Center; Associate Chair for Education and Academic Affairs, Internal Medicine; Director of YMS Coaching Program , Office of Curriculum
- Sten H. Vermund, MD, PhD Anna M.R. Lauder Professor of Public Health
- Share full article
Advertisement
Supported by
To Patients, Herpes Can Be Devastating. To Many Doctors, It’s Not a Priority.
Billions of people live with the infection, but there has been scant progress for treatments and tests.

By Dani Blum
When Lauren went to her doctors with stinging clusters of sores on her genitals, she assumed the pain was from a urinary tract infection. But at the OB-GYN, her doctor swabbed the bumps and told her that the rash was herpes. “No,” she remembered responding. “It’s not.”
At the time, Lauren, who asked that her last name be withheld in order to talk about personal health issues, was a 19-year-old college student. She was in a two-year monogamous relationship with her second-ever sexual partner — a guy who occasionally dealt with an errant blister on his lip.
They hadn’t known that oral herpes could induce cold sores, and that HSV-1, the virus that causes oral herpes, could be transferred to the genitals. Lauren’s boyfriend was convinced that she had cheated on him, and he broke up with her, she said.
Lauren became withdrawn and almost failed out of college. “You think, Why does anything even matter anymore?” she said. “I’m never going to date. I’m never going to have a boyfriend.”
That was in 2013. Over the last decade, Lauren has had only a few additional outbreaks, none as painful as her first. The mental strain — the depression she fell into after the diagnosis, the fear that future partners wouldn’t accept her — has been, by far, the hardest part of managing the disease. “It attacks your self-worth,” she said.
Herpes is extremely common: The World Health Organization estimates that 3.7 billion people live with HSV-1, some oral and some genital. And cases like Lauren’s, where HSV-1 spreads to the genitals during oral sex, have sharply increased over the past two decades, said Dr. Jonathan Zenilman, a professor of medicine at Johns Hopkins University School of Medicine who specializes in sexually transmitted infections.
But herpes isn’t a top priority for researchers, said Dr. Larry Corey, a professor and virologist at Fred Hutch Cancer Center in Seattle who has studied the virus. It isn’t even the top priority among those who study sexually transmitted infections, he added. “The disease has been sort of ignored by both the pharmaceutical industry as well as the medical research establishment,” he said.
There are several potential reasons for this, experts theorize, including the relatively mild physical symptoms for most patients, clinicians’ reluctance to discuss sexual health and how hard it is to develop a vaccine for herpes.
“The fact that a lot of the toll is psychological makes physicians not that interested in it,” said Dr. Anna Wald, a clinical virologist and a professor of medicine at the University of Washington School of Medicine.
There has been little progress on more accurate tests, vaccines or additional treatments over the last few decades, Dr. Wald said. Part of the challenge is that the herpes virus can hide inside neurons that are shielded from the immune system, making the body’s immune response insufficient at eradicating the virus, she said — that’s why herpes remains in a person’s body for life. Vaccine attempts, so far, have not stimulated an immune response that can control the virus or prevent infection, she said.
If a patient does not have symptoms, doctors typically diagnose herpes with an antibody test that is frequently inaccurate. Up to half of positive commercial test results could be false, according to past research . There is another antibody test, called the herpes Western blot, that scientists consider the gold standard in diagnosing herpes — but the test is only available through the University of Washington, which can be cumbersome and expensive for patients to obtain. Testing is typically reliable when a patient has symptoms; doctors can swab a lesion and run a highly sensitive molecular test.
The U.S. Preventive Services Task Force doesn’t recommend routine genital herpes screenings for people without symptoms, in part because false positive rates are so high. On Tuesday, the task force reaffirmed its recommendation . In a related paper , a group of doctors wrote that the recommendation was, in part, based on “psychosocial harms” associated with false positives on herpes tests.
And so the virus continues to spread essentially unchecked — exacerbated by just how ineffective the most widely available tests for herpes are, said Terri Warren, a nurse practitioner who has researched herpes.
As cases circulate, patients are left grappling with a diagnosis that can be psychologically devastating, Dr. Zenilman said.
“You can control the symptoms,” he added. “But lots of people feel stigmatized, dirty.”
How herpes got sidelined
Herpes can be severe in certain cases: Babies can contract neonatal herpes from their mothers, putting them at risk for severe complications and even death . For people who are immunocompromised , outbreaks can be more prolonged and painful. In the vast majority of cases, though, people will have very mild symptoms, and many will have none. That’s part of the reason the infection is so pervasive: People pass it onto partners without knowing they have herpes.
Those who contract HSV-1 may develop blisters on or around their mouths or, in some cases, on their genitals. HSV-2, the other predominant strain, is usually characterized by one or more lesions around the genitals or the rectum. In the United States, around one in six people between the ages of 14 and 49 has genital herpes, and over half of adults have oral herpes.
Antiviral medications help reduce the amount of the virus a person sheds, lowering the chance that someone with herpes will pass it on to a sexual partner. Some patients take antivirals daily; others only take medication when they have an outbreak. But the risk of spreading herpes is never zero. The disease lingers in the body, putting the onus on patients to disclose their diagnosis to anyone with whom they have intimate contact.
When Lauren started dating after her diagnosis, she found herself staying in relationships for longer than she might otherwise, scared nobody else would want to be with her. “I thought I was going to die alone,” she said.
Brittany, 29, who asked that her last name be withheld in order to discuss her personal health, only thinks about her HSV-2 when she scrolls through a dating app. In the two years since she was diagnosed, she’s only had one outbreak. Still, when she looks at each profile, she wonders how the man would respond to learning about her diagnosis. “I just worry so much that people are going to judge me,” she said. “That no matter how I present it to them, I’ll still face rejection. That weighs heavily on me.”
Some men have told her, flat-out, that they would never date someone with herpes, but what bothers her, too, are the ones who say, “I’m so sorry this happened to you.”
“I don’t want people to feel sorry for me,” she said. “I wake up every day and I’m fine.”
Scientists have worked on herpes vaccines in fits and starts since the 1970s, said Dr. Harvey Friedman, a professor of medicine at the University of Pennsylvania Perelman School of Medicine who has studied the disease for over 40 years. But past attempts have failed, for reasons researchers are still trying to uncover.
Because herpes has been around for so long, the viruses have evolved alongside us, making them more difficult to eradicate, said Christine Johnston, an associate professor at the University of Washington School of Medicine who has studied herpes.
There are new vaccines under development . Dr. Friedman is working with BioNTech on an HSV-2 vaccine candidate that was given to the first human subject in December . But none are in late-stage clinical trials, said Dr. Ina Park, a professor of family and community medicine at the University of California, San Francisco, and author of “Strange Bedfellows: Adventures in the Science, History, and Surprising Secrets of S.T.D.s.” “There’s nothing anywhere close to prime time,” she said.
‘One of the biggest secret societies’
When Ella Dawson, 30, contracted genital HSV-1 in college, she started to post openly about her diagnosis on social media. To her surprise, people came out of the woodwork to share their stories — friends, relatives, even a cashier who worked at the grocery store on campus. Many told her that they had never disclosed their diagnosis to anyone other than a sexual partner.
“It’s one of the biggest secret societies in the world,” said Ms. Dawson, a novelist and writer who often speaks publicly about her experience with herpes.
Courtney Brame, 34, started the herpes education advocacy organization and podcast Something Positive for Positive People after his own HSV-2 diagnosis. He’s seen how the disease “completely shatters a person’s identity,” he said, partly because of how central sexuality can be to someone’s self-worth. “They don’t feel like they have anything to contribute to a relationship now, just because they have herpes,” he said. “It’s like, ‘Who’s going to want me now that I have this?’”
Mr. Brame has seen this in his own life. He was once messaging a woman on Tinder who brought up her struggle with chronic asthma; when he disclosed his own chronic condition, she stopped responding. But more often than facing rejection, when he shares his diagnosis, he said, he gets a different response: Women share that they, too, have herpes.
Herpes stigma stems in part from the idea that people with the infection have done something “wrong,” Dr. Park said. But you can exercise every precaution and still get it, she added — condoms do not entirely prevent transmission, and you don’t even need to have penetrative sex to contract the virus.
Though condoms can reduce the risk of transmission, not everyone with herpes will use a barrier method in long-term, monogamous relationships. In 2021, Something Positive for Positive People conducted a survey of over 1,000 people diagnosed with herpes; around 66 percent said a partner had consented to sex without a condom or other barrier method. And there is little research on how the virus spreads between women who have sex with women, Dr. Park said.
Medical providers, in general, often don’t receive extensive education on talking to patients about sexual health, Dr. Johnston said. When it comes to herpes in particular, “health care providers can be really insensitive about it and minimize it,” she said. “This is thought of more as a nuisance than a serious infection.”
“Clinicians don’t want to deal with this,” Ms. Warren said. “It involves people talking about sex. They’re crying, they’re going to have to talk about various specifics like is oral sex OK, is anal sex OK — I don’t think they want to go there,” she said.
Without support from doctors, or medical innovations to cure the infection, people with herpes are left “dealing with two viruses at the same time,” as Ms. Dawson put it. “You’re dealing with the physical symptoms of the virus,” she said, “and you’re dealing with the mental strain.”
Dani Blum is a reporter for Well. More about Dani Blum
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
News releases.
Media Advisory
Wednesday, April 12, 2017
NIH scientists advance understanding of herpesvirus infection
Protein complexes identified that control infection and reactivation.

Herpes simplex virus (HSV) infections last a lifetime. Once a person has been infected, the virus can remain dormant (latent) for years before periodically reactivating to cause recurrent disease. This poorly understood cycle has frustrated scientists for years. Now, National Institutes of Health scientists have identified a set of protein complexes that are recruited to viral genes and stimulate both initial infection and reactivation from latency. Environmental stresses known to regulate these proteins also induce reactivation.
Globally, the World Health Organization estimates that 500 million people are infected with HSV-2 while two-thirds of the population are infected with HSV-1. These viruses cause human diseases ranging from oral cold sores to genital lesions to serious eye conditions that can lead to blindness. In infants, HSV can cause neurological and developmental problems. People infected with HSV also have an enhanced risk of acquiring or transmitting human immunodeficiency virus (HIV).
Scientists at NIH’s National Institute of Allergy and Infectious Diseases previously made progress toward understanding the role of cellular protein HCF-1 in initiating HSV infection and reactivation. HCF-1 and associated proteins are recruited to the viral genome to enable the virus to replicate and spread. This previous work identified targets for the development of therapeutics to suppress infection and reactivation.
Their latest work, with collaborators from Princeton University, Princeton, New Jersey, identifies new HCF-1 protein complexes that play additional roles in initiating viral infection and reactivation. The scientists found they could reactivate latent HSV in a mouse model using compounds that turn on components of these HCF-1 protein complexes. Interestingly, some of these HCF-1-associated proteins also are involved in HIV reactivation from latency.
The researchers are continuing to investigate the protein complexes involved in promoting HSV gene expression, infection, and reactivation from latency. Identifying these complexes and understanding the mechanisms by which they function can potentially reveal additional targets for the development of new therapeutics.
R. Alfonso-Dunn et al . Transcriptional elongation of HSV Immediate Early genes by the Super Elongation Complex drives lytic infection and reactivation from latency. Cell Host & Microbe . DOI: 10.1016/j.chom.2017.03.007 (2017).
Related Work
Y. Liang et al. Inhibition of the histone demethylase LSD1 blocks alpha-herpesvirus lytic replication and reactivation from latency. Nature Medicine DOI:10.1038/nm.2051 (2009).
Y. Liang et al. Targeting the JMJD2 histone demethylases to epigenetically control herpesvirus infection and reactivation from latency. Science Translational Medicine DOI: 10.1126/scitranslmed.3005145 (2013).
J. Hill et al. Inhibition of LSD1 reduces herpesvirus infection, shedding, and recurrence by promoting epigenetic suppression of viral genomes. Science Translational Medicine DOI: 10.1126/scitranslmed.3010643 (2014).
Thomas M. Kristie, Ph.D., Chief of the Molecular Genetics Section of NIAID’s Laboratory of Viral Diseases, is available to comment on this study.
To schedule interviews, please contact Ken Pekoc, (301) 402-1663, [email protected] .
NIAID conducts and supports research — at NIH, throughout the United States, and worldwide — to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website .
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
Connect with Us
- More Social Media from NIH
Genital Herpes – CDC Basic Fact Sheet

The Search for a Herpes Vaccine
The need for a herpes vaccine is clear: about half a billion people worldwide between the ages of 15-49 have genital herpes infection caused by either HSV-1 or HSV-2 , according to the World Health Organization (WHO). In the United States alone, an estimated 1 in 6 adults have genital herpes, with around 300,000 new infections diagnosed each year.
While HSV is typically a mild infection, there are potential health risks associated with it, including neonatal herpes , a serious and sometimes fatal condition that occurs when HSV is passed to an infant during delivery. Another concern is the increased risk of HIV infection. The risk of getting HIV (if exposed) is increased 2-3 fold for someone with genital HSV-2 infection.
Researchers have been working on developing herpes vaccines for decades. There have been a number of clinical trials aimed at testing both therapeutic (intended to reduce recurrences and viral shedding in people who are already infected with HSV) and preventive (designed to prevent infection) vaccine candidates.
How do clinical trials work?
Before a treatment regimen or vaccine can become standard, it must go through a clinical trial . Clinical trials test if a potential treatment or vaccine is safe and effective in humans. Clinical trials go through a series of phases, starting with a smaller group of patients and expanding to a much larger group.
Herpes Vaccine Clinical Trials
There are currently both preventive and therapeutic vaccines under development. While the primary focus is on HSV-2, the primary cause of genital infection, HSV-2 vaccines may also have benefits in preventing or treating HSV-1 infection. In addition to work being done in the preclinical stage, there are several vaccines in clinical trials.
- ClinicalTrials.gov
- CenterWatch.com
- Herpes Cure Pipeline 2.0
For more on herpes vaccine development, see this article.
More to Explore

Five Things You Should Know about Herpes
ASHA answers many questions from the public about STIs, including about genital herpes. Here we offer five things to know about how to manage this common infection.

Genital Herpes and Pregnancy
If you are pregnant and you have genital herpes, you may be concerned about the risk of spreading the infection to your baby. Be reassured that the risk is extremely small.

Herpes and Relationships
The best way for couples to deal with herpes is to talk about it openly and make decisions together. So what’s the best way to start the conversation?

Herpes Testing
Is that sore or rash actually genital herpes? Can you tell by just looking? Can a healthcare provider? No! When it comes to diagnosing genital herpes, it takes more than a look.
New Research Highlights the Need for Improved Herpes Diagnostics
A paper published ahead of print in Sexually Transmitted Diseases finds that commercial blood tests commonly used to diagnose herpes simplex virus (HSV) are frequently not reliable, especially in those with “low positive” results.

Signs and Symptoms of Herpes—What You Should Know
Most people with herpes won’t experience symptoms, but knowing what to look for can make you more aware.
American Sexual Health Association
ASHA believes that all people have the right to the information and services that will help them to have optimum sexual health. We envision a time when stigma is no longer associated with sexual health and our nation is united in its belief that sexuality is a normal, healthy, and positive aspect of human life.
GET INVOLVED
ASHA WEBSITES
© 2024 American Sexual Health Association
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 29 April 2024
Ligand-based drug design against Herpes Simplex Virus-1 capsid protein by modification of limonene through in silico approaches
- Md. Rezaul Islam 1 , 11 ,
- Md. Shafiqul Islam Sovon 11 , 2 ,
- Ummy Amena 11 , 3 ,
- Miadur Rahman 11 , 4 ,
- Md. Eram Hosen 11 , 5 ,
- Ajoy Kumer 11 , 6 , 7 ,
- Mohammed Bourhia 8 ,
- Yousef A. Bin Jardan 9 ,
- Samir Ibenmoussa 10 &
- Gezahign Fentahun Wondmie 11
Scientific Reports volume 14 , Article number: 9828 ( 2024 ) Cite this article
Metrics details
- Computational biology and bioinformatics
- Drug discovery
The pharmacological effects of limonene, especially their derivatives, are currently at the forefront of research for drug development and discovery as well and structure-based drug design using huge chemical libraries are already widespread in the early stages of therapeutic and drug development. Here, various limonene derivatives are studied computationally for their potential utilization against the capsid protein of Herpes Simplex Virus-1. Firstly, limonene derivatives were designed by structural modification followed by conducting a molecular docking experiment against the capsid protein of Herpes Simplex Virus-1. In this research, the obtained molecular docking score exhibited better efficiency against the capsid protein of Herpes Simplex Virus-1 and hence we conducted further in silico investigation including molecular dynamic simulation, quantum calculation, and ADMET analysis. Molecular docking experiment has documented that Ligands 02 and 03 had much better binding affinities (− 7.4 kcal/mol and − 7.1 kcal/mol) to capsid protein of Herpes Simplex Virus-1 than Standard Acyclovir (− 6.5 kcal/mol). Upon further investigation, the binding affinities of primary limonene were observed to be slightly poor. But including the various functional groups also increases the affinities and capacity to prevent viral infection of the capsid protein of Herpes Simplex Virus-1. Then, the molecular dynamic simulation confirmed that the mentioned ligands might be stable during the formation of drug-protein complexes. Finally, the analysis of ADMET was essential in establishing them as safe and human-useable prospective chemicals. According to the present findings, limonene derivatives might be a promising candidate against the capsid protein of Herpes Simplex Virus-1 which ultimately inhibits Herpes Simplex Virus-induced encephalitis that causes interventions in brain inflammation. Our findings suggested further experimental screening to determine their practical value and utility.
Introduction
Infectious diseases affecting the central nervous system (CNS) has developed a significant challenge to human health, often resulting in severe neurological complications and long-term cognitive impairments 1 . Among these diseases, herpes simplex virus-induced encephalitis (HSVE) is one of the most life thretening problem due to its capacity to induce substantial brain inflammation and damage. HSVE is primarily caused by herpes simplex virus type 1 (HSV-1) and herpes simplex virus type 2 (HSV-2) 2 , 3 . The most common cause of sporadic viral encephalitis is herpes simplex virus encephalitis (HSE) Despite of targeted antiviral therapy, it is still difficult to fight against HSV-1. There is evidence suggesting that chronic neuroinflammation plays a role in the development of HSV-1, even though the innate immune system is crucial for controlling HSV-1 within the brain 4 . Herpetic stromal keratitis (HSK), an immunoinflammatory condition resulting from herpes simplex virus (HSV) eye infection, can cause visual impairment. T cells are responsible for regulating this condition, and they can be reduced in size through the use of anti-inflammatory medications and treatments that modify the ratio of affected cells 5 . HSV-1 and HSV-2 infections continue to be a significant global health concern despite the availability of antiviral treatments. The increasing prevalence of drug resistance in immunocompromised patients raises serious concerns and emphasizes the urgent need for the rapid development of new, effective treatment options 6 .
Over 60% of people in the United States and 90% of people worldwide are infected with the neurotropic HSV-1. HSV-1, the leading infectious cause of encephalitis and corneal blindness, causes various biochemical and physical symptoms including cold sores and encephalitis 7 , 8 . While HSV-1 infection is widespread, there is currently limited vaccination or treatment available for it. Nucleoside analogs, which inhibit viral genome replication, constitute more than 75% of HSV-1 antiviral medications. Antivirals are effective in shortening the duration and frequency of outbreaks but only work against active infections; they have no impact on latent infections, which can be reactivated. The main limitations of current therapeutic approaches include the requirement for an active infection, high dosages, and the emergence of antiviral resistance. The emergence of antiviral resistance, a challenge in managing HSV-1 and other viral diseases, is significantly facilitated by targeting a shared mechanism of action. Most HSV-1 antivirals exhibit limited absorption, necessitating a high dose frequency of up to three to five times daily to maintain therapeutic levels. An exception is Valacyclovir, a pro-drug of Acyclovir, which has a better absorption profile than its parent medication and allows for a reduced dose frequency. Acyclovir, a synthetic nucleoside analog, is the cornerstone of HSV-1 infection treatment. It targets viral DNA polymerase and prevents viral replication due to its affinity for viral thymidine kinase 9 , 10 . The blood–brain barrier (BBB), consisting of endothelium tightly connected with glial limitans lining the CNS parenchyma, prevents pathogens from entering the CNS during infection 11 . In contrast to rabies, the breakdown of the BBB is a significant pathogenic mechanism in the development of HSE 12 . The lysis infection of neurons and glial cells, along with neuroinflammatory processes, leads to brain injury 13 . The initial release of cytokines such as type I IFN, IL-6, IL-1β, IFN-γ, and TNF results in BBB rupture when neurons and glia recognize HSV-1 through the action of toll-like receptors (TLRs) 14 . The HSV-1 infection triggers an increased inflammatory response by attracting immune cells to the brain parenchyma, resulting in more neuronal damage. In the mouse brainstem, perivascular infiltrates near HSV-infected cells contain CD4 + or CD8 + T cells and macrophages 15 . HSV-related diseases have proven challenging to prevent and treat over the years, necessitating the development of new vaccines, or drugs. The high infection rate of HSV makes it incurable and difficult to prevent, resulting in millions of cases worldwide 16 . The research community and scientists face a challenging task in searching for potential drug candidates. However, new drug development takes five to ten years, outpacing a single molecule, and costs an estimated $800 million to bring to market 17 . Thus, more innovative strategies are needed to find effective antiviral drugs and lower the probability of clinical trial failure. To decrease this cost burden, resource, personnel, time, and reagent waste, pharmaceutical companies have focused on computer-aided drug design (CADD) in recent years 18 , 19 , 20 . Using computer-aided methods makes it possible to screen molecules virtually for various pharmacological activities, saving time and money compared to more conventional methodological techniques. Therefore, this novel technology has been used in this study to identify an effective drug targeting HSVE through Computational Drug Design Approach.
The main objective of this study to combat HSV-1 by targeting the capsid protein, the protective surface that encapsulates the virus's genetic material 21 , 22 . Through meticulous molecular design, our modifications are strategically crafted to infiltrate the intricate structure of the capsid protein. This approach offers enhanced selectivity and efficacy, promising hope in the ongoing HSV-1 battle and potentially paving the way for targeted antiviral therapeutics. The capsid protein of HSV-1 is crucial in encapsulating the viral genome, protecting it during transmission and entry into host cells, making it a key target for virus replication and spread. Genetic engineering allows for modifications to prevent key interactions, improving treatment selectivity and efficacy while minimizing protein structure and function disruption. This comprehensive approach underscores the significance of targeting the capsid protein and elucidates the therapeutic strategy's promise.
Material and methods
Geometry optimization and ligand preparation.
Density Functional Theory (DFT) was utilized in DMol3's Material Studio 08 to optimize molecules. The B3LYP functional and DND basis with semi-core pseudo-potentials and DNP + basis set were utilized. These adjustments were made with precision in mind, especially when managing electronegative atoms such as oxygen. After geometric optimization, vibrational frequencies from DMol3 were used to identify important molecular frontier orbitals (HOMO and LUMO) 23 , 24 . These orbitals were represented by diagrams, which are essential for comprehending chemical reactivity and electron dispersion. The study sought to obtain profound understanding of molecular behavior by computational tools, supporting additional analysis and interpretation 25 , 26 . Our study focused on molecular frontier orbitals after geometric optimization, identifying the HOMO–LUMO. Using vibrational frequencies taken from the DMol3 code in Material Studio 08, we carefully developed charts that displayed these important orbitals and their corresponding sizes. This detailed investigation not only revealed the complex electron inside the molecule, but it also give important new information on its electrical structure and reactivity.
Lipinski rule and pharmacokinetics
The SwissADME online website was used to get the pharmacokinetics, drug-likeness score, and characteristics ( http://www.swissadme.ch/index.php ) 27 . This online repository contains all the information needed for developing new medicines. To describe the drug-likeness metrics of ligands, the following data were measured: topological polar surface area (TPSA) 2, molecular weight, hydrogen bond donors (HBD), hydrogen bond acceptors (HBA), bond rotation numbers (NRB), and lipophilicity.
ADMET profile prediction
Absorption, distribution, metabolism, excretion, and toxicity are all referred to as ADMET. This parameter has been regarded as the most important and potentially useful for drug discovery and development. The Swiss ADME and pkCSM online tools at “ http://biosig.unimelb.edu.au/pkcsm/predictionsingle/adme1643650057.59 ” have provided the ADMET measurements 28 . Although this web tool reports on between 60 and 70 characteristics. The BBB, water solubility, total clearance, maximum tolerance rate, hepatotoxicity, oral rat acute toxicity (LD50), oral rat chronic toxicity, skin sensitization, etc., are just a few of the potential factors that we have concentrated on.
Target structure preparation and molecular docking analysis
The capsid protein of HSV-1 (PDB ID: 1NO7) was downloaded from data bank (PDB) ( http://www.rcsb.org ) 29 . Following that, the water molecules found in the protein, heteroatoms, and other materials were effectively removed, and the prepared protein using the discovery studio application. Finally, this prepared protein was saved in PDB format. Next, the PyRx application was utilized to conduct molecular docking analysis using the AutoDock vina functionalities, allowing for the determination of the binding affinity to each ligand. When conducting molecular docking experiments, the protein was entered as a macromolecule, while the ligand was entered as a ligand. After reloading the ligand, the optimization process focused on maximizing energy and grid surface area. This involved adjusting the center and dimensions of the grid 30 , 31 . Once the docking experiment was done, the dock molecules were picked up and uploaded into the Pymol software version PyMolV2.3 ( https://pymol.org/2/ ) and generated a complex for molecular dynamic simulation 32 , 33 . Then, the ligand–protein docking complex’s active side and non-covalent contacts were identified using Discovery Studio visualization and ChimeraX at the endpoint.

Molecular dynamic simulation
The molecular dynamics simulations were conducted using the Assisted Model Building with Energy Refinement (AMBER) 14 force field and Yet Another Scientific Artificial Reality Application (YASARA) dynamics program 34 . The hydrogen bond network was cleaned up and optimized along with the docked complexes. The General AMBER Force Field (GAFF) was used to create the ligands' topology files, and AM1BCC charges were assigned. The TIP3P solvation model with periodic boundary conditions was used to generate a cubic simulation cell 35 . The simulation parameters were set to 0.9% NaCl, 310 K, and pH 7.4 to produce a neutral system 36 . With an 8.0 cutoff radius, the particle mesh Ewald (PME) approach was used to calculate the long-range electrostatic interactions. The simulation was run with a 2.0 fs time step. Using the steepest gradient algorithm and simulated annealing techniques (5000 cycles), the primary energy minimization was carried out. The simulated trajectories were used to analyze a number of characteristics, including RMSD, Rg, SASA, hydrogen bonds, RMSF, and MMPBSA binding free energy. Every 100 ps, during the simulation's 100 ns runtime, a snapshot of the trajectory was taken 37 .
Result analysis
Structural activity relationship (sar) studies.
The structure–activity relationship (SAR) enables desired effects during the development of the new drug, such as identifying new drug candidates and enhancing their desirable functionalities. This method is gaining popularity day by day to design specific drug molecules against certain diseases. Limonene has already been reported as broad-spectrum antimicrobial properties. So, the natural limonene is modified by adding different functional groups such as benzene, COOH, NO 2 , CH 2 –OH, and CH 2 –O–CH 3 to get better efficiency. After modification of the structure of limonene, it is observed that the efficiencies and pharmacokinetics are improved against HSV-1 targeted protein. Besides, the mentioned functional group strongly impacts aqueous solubility, ADMET, and binding affinities. So, it is clearly understood that the structural activity relationship plays a vital in finding new bioactive molecules. The main objective is to determine how different functional groups impact the drug properties of limonene. The modified structures are given in Fig. 1 .
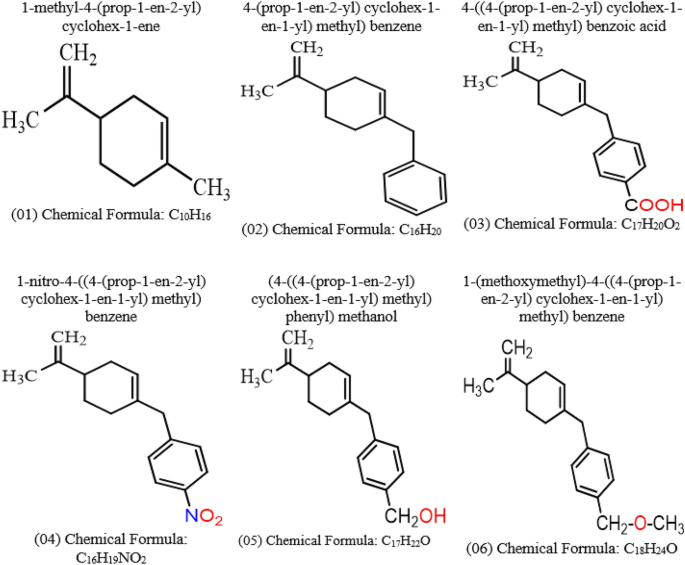
Chemical structure of limonene and its derivatives.
Pharmacokinetics and drug likeness
The evaluation of the possibility of a specific biochemical to be utilized as an oral medication relating to bioavailability and physicochemical properties is known as pharmacological drug-likeness, determined by the SwissADME web tool 25 , 27 . This is a crucial investigation since it is believed that nine out of ten targeted drugs do not reach the final stages due to their adverse effects, which results in vast amounts of wasted expenses, time, and human resources 38 . This issue arises because there is a lack of proper identification of the suitable physiochemical features of medication. The Lipinski rule is the fundamental and novel strategy to reduce the chance of failing, also called drug likeness 39 . This investigation mentioned that all the ligands have a potential bioavailability score (0.55–0.85), whereas only ligand 04 has a maximum bioavailability score of 0.85 due to carboxylic group attachment. Besides, the number of rotatable bonds , hydrogen bond acceptor, and hydrogen bond donor ranges are 01–04 for all ligands where the molecular weight is about 136.23 Dalton–257.33 Dalton. Finally, all the drugs accepted druglikeness parameters such as Lipinski rule for all ligands. Therefore, it is reasonable to conclude that the ligands might be used without risk. The conclusive findings of the pharmacokinetics and drug-likeness analyses are shown in Table 1 .
Molecular docking against capsid protein of Herpes Simplex Virus-1
The molecular docking has been conducted to determine the binding affinity when drug protein make complex each other. The generated pharmacological data of binding affinity was determined using molecular docking computations. In these simulations, bioactive components of the ligands interact with a peptide from capsid protein of HSV-1. The protein–ligand interaction is essential in development, especially in in silico and virtually screening drug development 40 , 41 .
In our investigation, the initial docking score was − 5.6 kcal/mol against capsid protein of HSV-1. But, with the addition of a functional group or side chain, the docking score has gradually increased and crossed the FDA-approved standard Acyclovir score.
The predicted score has displayed in Table 2 for better understanding. In details description, the molecular docking score ranges for capsid protein of HSV-1, and they all are showing the better binding impact than the standard Acyclovir. So, they could be regarded as better compounds than the standards. It is noted that when we have modified the primary structures of limonene, the binding affinity was obtained better effectiveness in comparison with the primary structures of limonene, and it is said that the functional groups play significant roles to be effectiveness, and potentiality of binding affinity. Hence, overall, the finding reported that ligands 02 and 03 are the most active molecules, and due to the addition of Benzene ring and –COOH functional group.
Protein–ligand interaction, molecular docking poses, and active site analysis
The most significant point to note when developing a novel drug is how a weak or covalent bonds between a ligand and a protein is configured. This investigation approximates the binding affinity or energy of various substances with the proteins of the pathogens. Measurements of the bond distance, active side, and protein ligand interaction aided researchers better understand how the chemical and the protein interact with each other. According to the graphical representation, the protein–ligand complex was actively generated by the ligand and protein, which appeared to form pockets and display various active amino acid residues. With the help of Biovia Discovery Studio version 2021 42 , this entire interaction was formed based on the highest docking score, and given in Fig. 2 . The docking pocket represents how a drug binds with ligands and in which position of protein they are engaged.
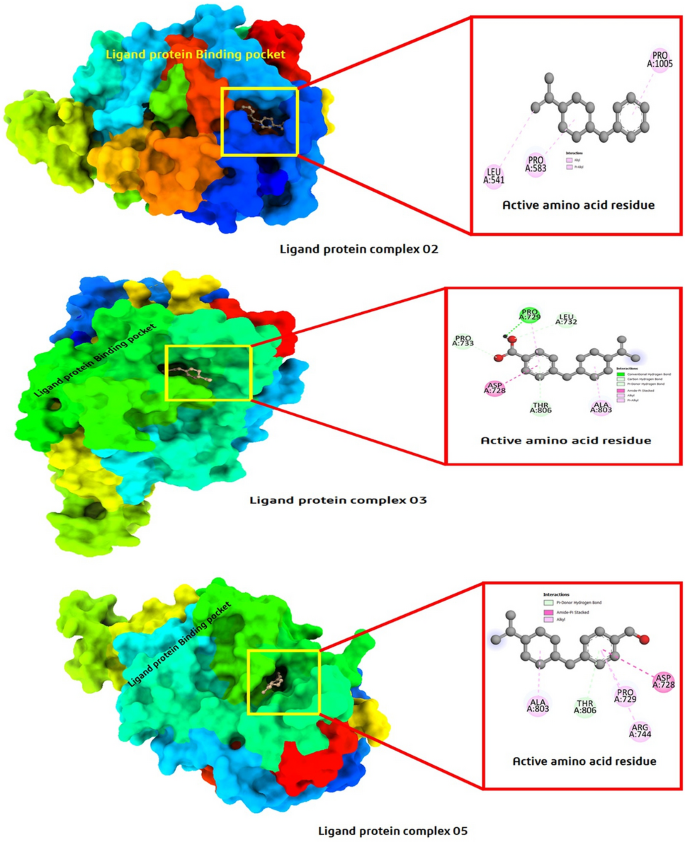
Protein–ligand interaction diagram and active site analysis. The Pink colour represent Pi-alkyl bonds, and the deep green colour hydrongen bonds.
Frontier molecular orbitals and quantum calculation
In broad terms, chemical descriptors are essential to any organic or physiologically active drug-like compound. The Highest Occupied Molecular Orbital (HOMO) orbital is the one that might function as an electron donor due to being the highest energy orbital that encompasses electrons. On the other hand, the Lowest Unoccupied Molecular Orbital (LUMO) orbital is the one that might function as an electrophile because it is the lowest energy orbital that has space to receive electron 42 , 43 . The magnitudes of εLUMO, εHOMO, and the energy gap differentiation (Δε), hardness (η), and softness (S) of the six molecules 01–06 are displayed in Table 3 . These data estimates were computed using the B3LYP functional.
Comparing the molecular frontier orbital properties of six drugs (01 to 06) against accepted standard ranges provides insights into their reactivity and pharmacological potential. The negative values of both LUMO and HOMO across all drugs indicate their ability to accept and donate electrons, respectively, for drug interaction. The relatively small energy gaps suggest moderate to high reactivity. Furthermore, hardness values ranging from 3.566 to 3.8605 imply moderate stability, while softness values between 0.2590 and 0.2804 indicate moderate reactivity. Overall, these findings contribute to understanding the pharmacological characteristics and reactivity profiles of the drugs, facilitating their development and optimization.
We find consistent trends that reflect the electrical characteristics of the six medications when we compare their HOMO and LUMO values. The range of values for the HOMO is − 7.402 to − 8.397, and the range for the LUMO is − 0.090 to − 0.704. These negative values imply that all medications have electron-donating (HOMO) and electron-accepting (LUMO) properties, which are advantageous for molecular interactions. Furthermore, there is moderate to high reactivity indicated by the relatively modest energy gaps between HOMO and LUMO, which range from 7.132 to 7.721 (Table 3 ). All medications have constant negative values for HOMO and LUMO, indicating their ability to donate and take electrons, which is essential for pharmacological interactions and reactivity 44 , 45 .
Therefore, these compounds may have potential as drug candidates. Additionally, the softness values range from 0.2590 to 0.2804; generally, drugs with lower softness values decompose more quickly.
On the other hand, one of the essential characteristics of a material is its hardness, which reflects its resilience. A higher hardness value indicates greater strength and stability of the compound. The information in Table 3 reveals that hardness is often correlated to the energy gap. Ligand 05 shows the lowest hardness value, suggesting that this material has slightly inferior stability and softness, which means it may decompose more rapidly than others. Supplementary Fig. S1 has shown the Frontier Molecular Orbitals (HOMO and LUMO) diagram.
Molecular of electrostatic potential (MEP) charge distribution map
Molecular Electrostatic Potential (MEP) is a valuable tool for integrating various physicochemical features of drug molecules, such as dipole moment, electronegativity, and partial charges. It provides insights into the electrophilic and nucleophilic regions of compounds. By analyzing the distribution of potential energy formed by the molecule's electrons and nuclei, MEP serves as a static dispersion of potential and aids in defining and predicting the functional behavior of a compound. Electrostatic potentials have played a significant role in investigating diverse phenomena in biological, physical, and related fields.
Figure 6 illustrates the dynamically calculated MEP values for compounds (01–06) of limonene derivatives, which were predicted using the DFT method. In this representation, the red color indicates the lowest electrostatic potential energy intensity, representing the most significant negative area and the location most susceptible to electrophilic attacks. Conversely, the blue zone in the MEP signifies the maximum value of the potential electrical charge, indicating both the maximal positive region and a place favorable for nucleophilic substitution. The white color represents no potential or zero potential, as demonstrated in Supplementary Fig. S2 .
Overall, the red and blue zones on all the molecules are identical, suggesting that these chemicals are almost equally prone to electrophilic reactions and nucleophilic attacks. However, a crucial finding indicates that their red regions are considerably more significant than their blue regions, highlighting their higher propensity for electrophilic reactions".
ADMET data investigation
When it comes to drug development, using techniques such as in silico pharmacokinetics and ADMET analysis is essential. These approaches facilitate determine the chemical and physical characteristics of compounds, ensuring the process more efficient and cost-effective 46 , 47 . Using computational tools like SwissADME and pkCSM, we executed ADMET analysis on limonene derivatives (Ligand 01–06) 48 . This investigation is essential to evaluate pharmacokinetics properties such as absorption, distribution, metabolism, excretion, and toxicity. These factors have significance to maintaining the efficacy and effectiveness of potential oral medications. The investigation addressed a wide range of factors related to the behavior of these substances. This included their solubility in water, how easily they can pass through Caco-2 cells, their absorption rates in the gastrointestinal tract, and any potential interactions with metabolic enzymes and transporters.
When it comes to absorption, understanding the water solubility (Log S) values may provide us useful information about how well compounds may disintegrate in water. This is essential because it affects how well they are absorbed in the gastrointestinal tract 49 . Based on the results of this study, it can be observed that Ligands 02 and 06 have Log S values indicating moderately solubility. On the other hand, the other compounds show better soluble characteristics which implies a promising potential for absorption. The solubility is ranges are considered by the Log S scale: insoluble < − 10 poorly < − 6, moderately < − 4 soluble < − 2 very < 0 < highly 50 , 51 , 52 .
It is important to note that positive Caco-2 permeability values indicate efficient absorption across intestinal epithelial cells. This further supports the compounds' suitability for oral administration through assuring their beneficial passage through the intestinal barrier 53 , 54 .
In addition, the results indicate positive findings regarding distribution, metabolism, and excretion (Table 4 ). It is worth mentioning that the compounds exhibit remarkable rates of GI absorption, with Ligands 03, 04, and 05 achieving full absorption (100%). Furthermore, the molecules' potential to cross the blood–brain barrier highlights their possible therapeutic benefits, with the exception of Ligand 02. When it comes to metabolic considerations, there are interesting interactions with CYP450 enzymes. It's fascinating to observe how different compounds can exhibit inhibition of CYP450 1A2 while not affecting 2C9. This data is essential for predicting possible drug-drug interactions and providing guidance for dosage adjustments.
In addition, analyzing the overall clearance rates offers valuable information about how quickly the compounds are eliminated from the body. The clearance ranges are consistently favorable, suggesting efficient elimination. It is important to mention that Ligand 04 might be excreted through the kidneys using the OCT2 substrate pathway. This indicates the importance of monitoring patients with impaired renal function. In general, conducting thorough ADMET evaluations is essential for understanding the safety and pharmacokinetic properties of limonene derivatives. These theoretical ADMET finding might be helpful to the researcher for the subsequent stages of preclinical and clinical development, with a particular emphasis on improving efficacy and safety.
Aquatic and non-aquatic toxicity
Table 5 lists the aquatic and non-aquatic toxicities, which are essential for determining whether drugs or other substances are tolerable in the environment before and after use 25 . When directly indicated, none of the chemicals exhibit hepatotoxicity or toxicity to AMES., indicating that they will not be carcinogenic to humans or laboratory animals or induce liver toxicity. The vast majority of drugs, except for compounds 04 and 05, caused by AMES. The finding has been reported that the max. tolerated dose ranges from 0.309 mg/kg/day to 0. 770 mg/kg/day, oral rat acute toxicity (LD50) range from 1.747 mol/kg to 2.482 mol/kg. Finally, the oral rat chronic toxicity range is about 1.037 mg/kg/day to 4.689 mg/kg/day for all compounds. Therefore, it might be concluded that they do not threaten humans or the environment, and further experimental studies need to be established as safe medication.
Molecular dynamic simulation result analysis
Root mean square deviation (rmsd), and radius of gyration (rg) analysis.
MD simulation was conducted to ensure the stability of drug-protein complex at 100 ns 55 , 56 . In this work, we quantitatively evaluated structural alterations, stability, and dynamics in biomolecular systems using RMSD analysis, a critical tool in molecular dynamics simulations. In particular, we performed an RMSD study on complexes (called complex_03 and complex_05) that were generated between the HSV-1 capsid protein and two limonene derivatives. As shown in Fig. 3 A, these profiles were compared to those of the regular Acyclovir. The mean RMSD values that were acquired were 1.92 Å for complex_03 and 2.04 Å for complex_05. These results were in close agreement with the conventional Acyclovir's RMSD value of 1.99 Å. We saw an early increase in RMSD values for all complexes during the first few nanoseconds of the experiment. After this first spike, though, the complexes showed a tendency to hold onto stability, while little fluctuations were noticed here and there over the experiment. Like Fig. 3 A illustrates, these variations often fell between 1.50 and 2.50 Å. Despite the small variations between complexes, they did not significantly impact overall stability or cause significant structural changes.
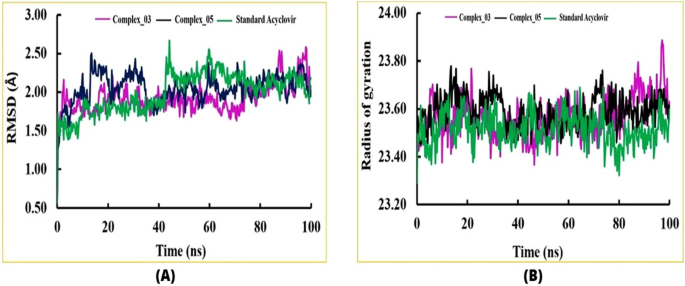
Two key metrics: ( A ) Root Mean Square Deviation (RMSD), and ( B ) Radius of Gyration (Rg). These analyses were carried out on complexes formed by limonene derivatives, specifically complex_03 and complex_05, in comparison to the standard compound Acyclovir. In our visual representations, complex_03 is denoted by a pink color, complex_05 by black, and Acyclovir by green. By investigating the RMSD and Rg values, we aimed to discern any significant differences or similarities between the behavior of the limonene derivatives complexes and the standard Acyclovir throughout the duration of the molecular dynamics.
Secondly, Rg analysis is a versatile tool in MD simulations that provides valuable information about the structural dynamics and compactness of biomolecules. It aids in understanding conformational changes, stability, interactions, and folding processes, making it an integral part of drug discovery focused on molecular dynamics studies 9 . Initially, the radius of gyration (Rg) profiles for all three complexes exhibited an upward trend up to 20 ns (ns). Subsequently, these profiles maintained a stable configuration from 20 to 65 ns. However, after a slight downward movement between approximately 75 ns and 83 ns, an increase in Rg values was observed. Of particular interest is the fact that all of the complexes displayed a similar pattern in their Rg profiles when compared to the standard. The observed pattern in the Rg profiles suggests a common behavior across all complexes and the standard. The initial upward movement in Rg indicates a phase of structural expansion or rearrangement within the first 20 ns. The subsequent stable profile from 20 to 65 ns implies that the complexes reached a relatively equilibrium-like state, characterized by minimal changes in their overall size and conformation. The subsequent increase in Rg values after the slight downward movement between 75 and 83 ns could indicate a phase of structural changes or unfolding. The consistent pattern among the complexes and the standard suggests that there might be shared underlying factors influencing their dynamics (Fig. 3 B).
Solvent accessible surface area (SASA) analysis
The SASA analysis serves to quantify the fraction of a molecule's external surface that remains exposed and open for attachment with surrounding solvent molecules. This investigation is particularly significant in understanding protein stability and folding dynamics 10 .
During our study, the SASA profiles of both complex_03 and complex_05 exhibited a consistent level of stability, marked by minor fluctuations. Interestingly, this stability closely resembled the SASA profile of the standard Acyclovir (Fig. 4 ). This observation indicates that the surface accessibility of solvent molecules to complexes complex_03 and complex_05 remained relatively unchanged, with only slight variations throughout the simulation. Remarkably, this pattern of stability mirrored the behaviour seen in the SASA profile of the standard Acyclovir. In essence, the SASA profiles suggest that the external surfaces of all three entities complex_03, complex_05, and Acyclovir interacted with the surrounding solvent molecules in a comparable manner. This consistent trend may imply a shared mode of surface interaction, possibly linked to similar structural characteristics or dynamic behaviours among the molecules. This aims to assess the level of surface accessibility to solvent molecules for each compound. We compared the SASA values of limonene derivatives complexes and Acyclovir to identify any significant differences in their behavior during the simulated period.
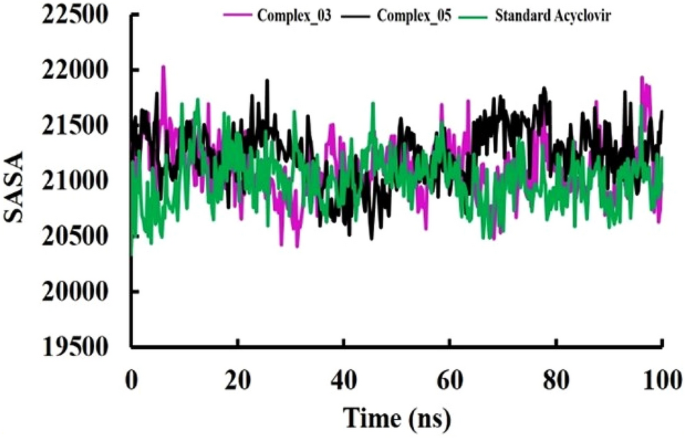
Solvent accessible surface area (SASA).
Hydrogen bonds analysis
Hydrogen bond analysis is an essential component of molecular dynamics (MD) simulation studies due to the significant role that hydrogen bonds play in shaping the structure, dynamics, and interactions of biomolecules. Based on our discoveries, we observed that the hydrogen bond patterns for all three complexes initially showed an upward trend up to 15 ns. This finding implies that during the initial phase of the simulation, hydrogen bonds were actively forming, potentially indicating structural adjustments or interactions among the components. Subsequently, complex_03 and complex_05 maintained stable hydrogen bond profiles that were notably similar throughout the entire 100 ns simulation period. This stability closely resembled the hydrogen bond profile of the standard Acyclovir. However, it's worth noting that the standard Acyclovir exhibited slightly higher hydrogen bond values compared to the other two complexes throughout the simulation (Fig. 5 ). The subsequent stability in hydrogen bond profiles for complex_03 and complex_05 suggests that these complexes stable into configurations where their hydrogen bonding patterns remained relatively constant over time. Furthermore, the similarity in hydrogen bond profiles between complex_03, complex_05, and Acyclovir underscores a potential commonality in their structural behaviors or interactions.
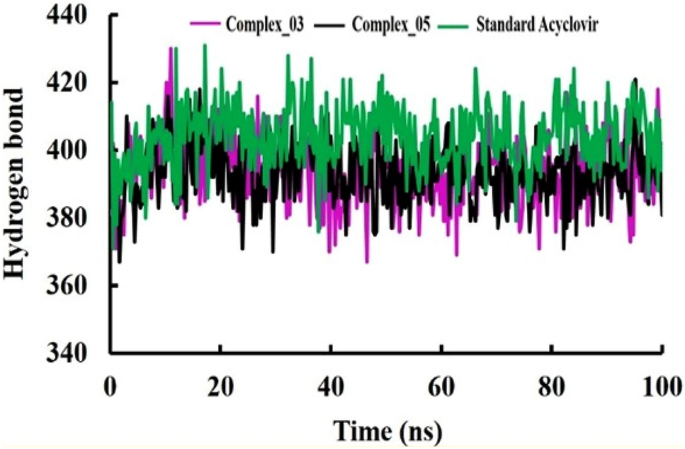
The hydrogen bonds within complexes formed by limonene derivatives complex_03 and complex_05, comparing them to the standard Acyclovir. This analysis aimed to elucidate the hydrogen bonding patterns and dynamics within each complex.
Root-mean-square fluctuation (RMSF) analysis
In this study we used RMSF to investigate the flexibility and dynamic behavior of each of the atoms or residues within a biomolecular system, it is also utilized to identify specific residues that contribute to these fluctuations. The two-limonene derivatives complex_03 and complex_05 showed an excellent level of similar pattern RMSF profile with standard Acyclovir. However, some residue fluctuated more than 4 Å such as Arg34, Leu41, Leu58, Asp62, Pro63, Gly215, Pro349, Ala418, Asn419, Thr420, Ala421, and His542 (Fig. 6 ). The combined similarity in RMSF profiles along with the notable flexible behavior of specific residues provides valuable insights into the dynamic behavior of the complexes. Despite the overall similarity in RMSF profiles, certain residues within these complexes demonstrated a higher degree of flexibility or mobility during the simulation. The residues mentioned, such as Arg34, Leu41, and others, could potentially be part of flexible loops, active site regions, or points of interaction within the complexes. The increased flexibility in these residues might have functional implications, such as facilitating binding events, accommodating conformational changes, or participating in molecular recognition processes.
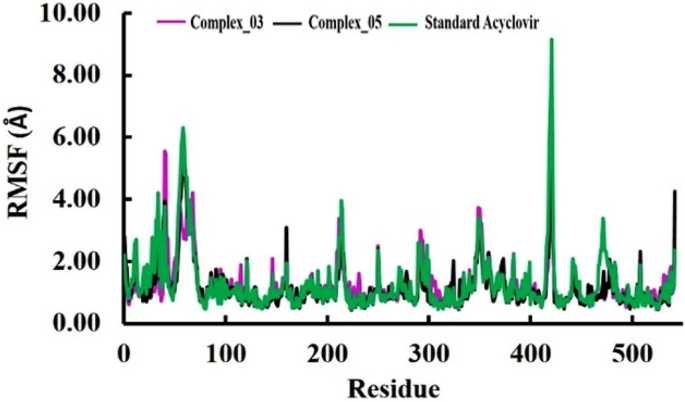
The analysis of Root-mean-square fluctuation (RMSF). The RMSF analysis enabled us to investigate the flexibility and dynamics of the protein–ligand complexes. We analyzed the differences in binding stability and dynamics between the limonene derivatives complexes and Acyclovir by comparing their RMSF profiles.
MMPBSA binding energy
The MMPBSA binding energy is a quantitative measure of the net energy change that occurs when a protein–ligand complex is formed compared to the individual unbound components. In our investigation, we utilized Molecular Mechanics/Poisson-Boltzmann Surface Area (MMPBSA) calculations to investigate the binding free energy associated with protein–ligand interactions. These MMPBSA binding free energies were computed for all complexes over a 100 ns simulation period, as depicted in Fig. 7 . Our study revealed that the average MMPBSA binding energies for the three complexes complex_03, complex_05, and standard Acyclovir were calculated as − 22.94, 91.35, and − 178.81 kJ/mol, respectively. Throughout the study of the 100 ns simulation period, the MMPBSA binding energy profiles for the three complexes exhibited distinct patterns of stability. Notably, despite this variation in profiles, the complexes' binding energies remained relatively constant over time. These findings imply that complex_05 exhibited a relatively weaker binding interaction compared to complex_03 and standard Acyclovir. Acyclovir, the standard reference, exhibited the strongest binding interaction, indicated by a significant negative MMPBSA binding energy.
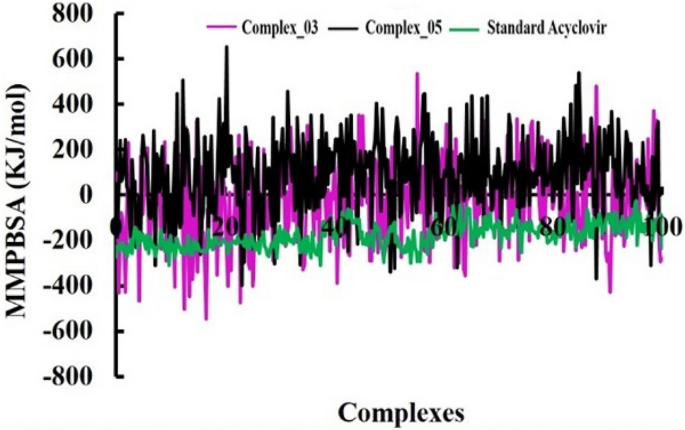
The analysis of MMPBSA analysis binding energy at 100 ns molecular dynamics simulations periods.
The four layers that make up the HSV-1 particle include an inner core that families the viral DNA, a protein shell known as the capsid, a multiprotein layer known as the tegument, and an envelope made of viral glycoproteins that are generated from cellular membranes. The most widely recognized theory explaining HSV-1 postulates that capsids are formed in the nucleus, integrate viral DNA, fuse with the outer nuclear membrane to lose the first envelope, and then emerge into the cytoplasm nude 57 , 58 , 59 . The Herpesviridae family is associated with various diseases such as infectious mononucleosis, nasopharyngeal cancer, Kaposi's sarcoma, and herpes lesions of the genitalia and lips 21 . All three herpesvirus subfamilies—α-, β-, and γ-herpesviruses—have nine known human herpesviruses. These viruses are categorized based on biological characteristics, such as variations in tissue tropism. The α-herpesvirus subfamily of viruses includes varicella-zoster virus (which causes chickenpox and shingles), herpes simplex virus type 1 (HSV-1, causes cold sores), and type 2 (HSV-2, causes genital herpes). These viruses can cause lifelong latent infections in the peripheral nervous systems of their hosts.
The study of HSV-1 structure-activity correlations is an essential for developing advanced antiviral drugs that specifically target the virus. Through comprehension of the ways in which specific structural characteristics influence the effectiveness and specificity of existing antiviral medications, scientists can develop novel molecules that possess enhanced potency and reduced off-target effects. Multi-targeted medicines can be developed as a result of investigating the connections between molecular structures and antiviral activity, which may uncover synergy between various therapeutic targets or pathways involved in the virus's replication cycle. By utilizing structure–activity insights to optimize pharmacokinetic features, these molecules can further boost their potential for therapeutic use, and possible to optimize pharmacokinetic features, the molecules in question can further improve their potential for therapeutic use in vivo 60 , 61 . These discoveries also provide new molecular targets and pathways for preventing HSV-1 proliferation, opening the door for new antiviral treatments. All things considered, utilizing the structure–activity knowledge gained from HSV-1 research should transform the development and refinement of next-generation antiviral drugs, providing a more effective means of combating HSV-1 infections.
One well-known cyclic monoterpene is limonene. This hydrocarbon is an olefin (C 10 H 16 ), and it exists in two optical forms. (++)-One of the most significant and widely used terpenes in the flavor and fragrance industry is limonene. Limonene (both optical forms) is present in over 300 plant essential oils derived from a wide range of species, such as fir, orange, lemon, and mint 62 . One of the most common smells used in cosmetic formulations, limonene is a monoterpene that is mostly found in cleaning and food products. Limonene has the therapeutic effects such as anti-inflammatory, antioxidant, antinociceptive, anticancer, antidiabetic, antiviral, and gastroprotective properties 63 . Essential oils are complex combinations of constituents from multiple functional group groups. The system can differentiate between monoterpenes, phenylpropanes, and other constituents based on their structural composition. The study tested the antiviral properties of monoterpene compounds, limonene and β-pinene, in essential oils against HSV-1 in vitro. Both limonene and beta-pinenene completely eliminated viral infectivity. Monoterpenes have been identified as having an antiviral action mechanism, but they only show moderate activity when administered to host cells before or after HSV entry. Both monoterpenes demonstrated strong anti-HSV-1 efficacy upon direct attachment with free virus particles. The viral infection was reduced inactive by the dose-dependent interactions between the two studied medications and HSV-1 64 . The main objective of our study was to identify how structural modification, and different function group can affect the binding affinity, and pharmacological effectiveness against capsid protein of HSV-1. Our study found that addition of different functional group can improve the binding affinity compare to the primary compounds. Now, according to the literature finding, and our in silico study both can be compared, and concluded that Limonene can actively inhibit the capsid protein of HSV-1, and fulfil the main objective the investigation.
This research has amplified and integrated limonene derivatives as an innovative antiviral drugs using computer-aided methodologies and performed different computational approaches against capsid protein of HSV-1 . After a comprehensive investigation, it is reported that the maximum binding affinity score for capsid protein of HSV-1 has been displayed − 7.4 kcal/mol and − 7.1 kcal/mol. Besides, the drug-likeness of all compounds is entirely accepted. Therefore, the drug development and implementation process may be guided by anticipating the in silico modeling of ADMET features for safe medicine.
Additionally, it obtained an improved ADMET profile for all drugs with low toxicity and greater to moderate solubility; most of the medication can cross the BBB and may be metabolized by CYP450 1A2 inhibitor. The chemical descriptors HOMO–LUMO reported that the energy gap for ligands 01–06 is around 7.174–7.693, while the softness value is between 0.2590 and 0.2804. Finally, molecular dynamic modeling has demonstrated that they are stable drugs in many conditions and ought to demonstrate improved stability once they enter the biological system. Therefore, it may be inferred that researcher searching for antiviral medications against for capsid protein of HSV-1 are capable of developing innovative drug.
Data availability
All data are available in this manuscript.
Swanson, P. A. II. & McGavern, D. B. Viral diseases of the central nervous system. Curr. Opin. Virol. 11 , 44–54 (2015).
Article PubMed PubMed Central Google Scholar
Arduino, P. G. & Porter, S. R. Herpes Simplex Virus Type 1 infection: Overview on relevant clinico-pathological features. J. Oral Pathol. Med. 37 , 107–121 (2008).
Article PubMed Google Scholar
Rozenberg, F., Deback, C. & Agut, H. Herpes simplex encephalitis: From virus to therapy. Infect. Disord. Drug Targets 11 , 235–250 (2011).
Article CAS PubMed Google Scholar
Hayes, C. K. et al. ASC-dependent inflammasomes contribute to immunopathology and mortality in herpes simplex encephalitis. PLoS Pathog. 17 , e1009285 (2021).
Article CAS PubMed PubMed Central Google Scholar
Berber, E. & Rouse, B. T. Controlling herpes simplex virus-induced immunoinflammatory lesions using metabolic therapy: A comparison of 2-deoxy-d-glucose with metformin. J. Virol. 96 , e00688-e722 (2022).
Krawczyk, A. et al. Overcoming drug-resistant herpes simplex virus (HSV) infection by a humanized antibody. Proc. Natl. Acad. Sci. 110 , 6760–6765 (2013).
Article ADS CAS PubMed PubMed Central Google Scholar
Looker, K. J. et al. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE 10 , e0140765 (2015).
Griffiths, S. J. et al. A systematic analysis of host factors reveals a Med23-interferon-λ regulatory axis against herpes simplex virus type 1 replication. PLoS Pathog. 9 , e1003514 (2013).
Greco, A., Diaz, J., Thouvenot, D. & Morfin, F. Novel targets for the development of anti-herpes compounds. Infect. Disord. Drug Targets 7 , 11–18 (2007).
Luganini, A. et al. Inhibition of herpes simplex virus type 1 and type 2 infections by peptide-derivatized dendrimers. Antimicrob. Agents Chemother. 55 , 3231–3239 (2011).
Daneman, R. & Prat, A. The blood–brain barrier. Cold Spring Harb. Perspect. Biol. 7 , a020412 (2015).
Liu, H., Qiu, K., He, Q., Lei, Q. & Lu, W. Mechanisms of blood-brain barrier disruption in herpes simplex encephalitis. J. Neuroimmune Pharmacol. 14 , 157–172 (2019).
Lundberg, P. et al. The immune response to herpes simplex virus type 1 infection in susceptible mice is a major cause of central nervous system pathology resulting in fatal encephalitis. J. Virol. 82 , 7078–7088 (2008).
Mancini, M. & Vidal, S. M. Insights into the pathogenesis of herpes simplex encephalitis from mouse models. Mamm. Genome 29 , 425–445 (2018).
Chan, W., Javanovic, T. & Lukic, M. Infiltration of immune T cells in the brain of mice with herpes simplex virus-induced encephalitis. J. Neuroimmunol. 23 , 195–201 (1989).
Wijesinghe, V. N. et al. Current vaccine approaches and emerging strategies against herpes simplex virus (HSV). Expert Rev. Vaccines 20 , 1077–1096 (2021).
Rahman, M. M. et al. Use of computer in drug design and drug discovery: A review. Int. J. Pharma Life Sci. 1 , 12955 (2012).
Article Google Scholar
Nascimento, I. J. D. S., de Aquino, T. M. & da Silva-Júnior, E. F. The new era of drug discovery: The power of computer-aided drug design (CADD). Lett. Drug Des. Discov. 19 , 951–955 (2022).
Article CAS Google Scholar
Kumar, A., Rajendran, V., Sethumadhavan, R. & Purohit, R. Evidence of colorectal cancer-associated mutation in MCAK: A computational report. Cell Biochem. Biophys. 67 , 837–851 (2013).
Kumar, A. et al. Computational SNP analysis: Current approaches and future prospects. Cell Biochem. Biophys. 68 , 233–239 (2014).
Dai, X. & Zhou, Z. H. Structure of the herpes simplex virus 1 capsid with associated tegument protein complexes. Science 360 , 7298 (2018).
Baines, J. D. Herpes simplex virus capsid assembly and DNA packaging: A present and future antiviral drug target. Trends Microbiol. 19 , 606–613 (2011).
Rahman, M. A., Matin, M. M., Kumer, A., Chakma, U. & Rahman, M. J. Modified D-glucofuranoses as new black fungus protease inhibitors: Computational screening, docking, dynamics, and QSAR study. Trends Microbiol. 10 , 195–209 (2022).
Google Scholar
Delley, B. Ground-state enthalpies: Evaluation of electronic structure approaches with emphasis on the density functional method. J. Phys. Chem. A 110 , 13632–13639 (2006).
Kumer, A. et al. Investigation of the new inhibitors by sulfadiazine and modified derivatives of α-D-glucopyranoside for white spot syndrome virus disease of shrimp by in silico: Quantum calculations, molecular docking, ADMET and molecular dynamics study. Molecules 27 , 3694 (2022).
Bleken, F. et al. Thermochemistry of organic reactions in microporous oxides by atomistic simulations: Benchmarking against periodic B3LYP. J. Phys. Chem. A 114 , 7391–7397 (2010).
Daina, A., Michielin, O. & Zoete, V. J. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 7 , 1–13 (2017).
Pires, D. E., Blundell, T. L. & Ascher, D. B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 58 , 4066–4072 (2015).
Bowman, B. R., Baker, M. L., Rixon, F. J., Chiu, W. & Quiocho, F. A. Structure of the herpesvirus major capsid protein. EMBO J. 22 , 757–765 (2003).
Kondapuram, S. K., Sarvagalla, S. & Coumar, M. S. Docking-based virtual screening using PyRx tool: Autophagy target Vps34 as a case study. in Molecular Docking for Computer-Aided Drug Design , 463–477 (Elsevier, 2021).
Chigurupati, S. et al. Molecular docking of phenolic compounds and screening of antioxidant and antidiabetic potential of Moringa oleifera ethanolic leaves extract from Qassim region, Saudi Arabia. Saudi J. Biol. Sci. 29 , 854–859 (2022).
Yuan, S., Chan, H. S. & Hu, Z. Using PyMOL as a platform for computational drug design. Wiley Interdiscipl. Rev. Comput. Mol. Sci. 7 , e1298 (2017).
Sharma, J., Bhardwaj, V. K., Das, P. & Purohit, R. Identification of naturally originated molecules as γ-aminobutyric acid receptor antagonist. J. Biomol. Struct. Dyn. 39 , 911–922 (2021).
Hu, X. et al. Molecular dynamics simulation of the interaction of food proteins with small molecules. Food Chem. 1 , 134824 (2022).
Grover, A., Shandilya, A., Punetha, A., Bisaria, V. S. & Sundar, D. Inhibition of the NEMO/IKKβ association complex formation, a novel mechanism associated with the NF-κB activation suppression by Withania somnifera’s key metabolite withaferin A. BMC Genom. 1 , 1–11 (2010).
Jagusiak, A., Chlopas, K., Zemanek, G., Wolski, P. & Panczyk, T. Controlled release of doxorubicin from the drug delivery formulation composed of single-walled carbon nanotubes and congo red: A molecular dynamics study and dynamic light scattering analysis. Pharmaceutics 12 , 622 (2020).
Dasmahapatra, U. et al. In-silico molecular modelling, MM/GBSA binding free energy and molecular dynamics simulation study of novel pyrido fused imidazo [4, 5-c] quinolines as potential anti-tumor agents. Front. Chem. 10 , 991369 (2022).
Athar, M., Sona, A. N., Bekono, B. D. & Ntie-Kang, F. J. Fundamental physical and chemical concepts behind “drug-likeness” and “natural product-likeness. Phys. Sci. Rev. 4 , 101 (2019).
Protti, Í. F. et al. Do drug-likeness rules apply to oral prodrugs?. ChemMedChem 16 , 1446–1456 (2021).
Caldwell, G. W. In silico tools used for compound selection during target-based drug discovery and development. Expert Opinion on Drug Discovery 10 (8), 901–923 (2015).
Reddy, A. S., Pati, S. P., Kumar, P. P., Pradeep, H. N., & Sastry, G. N. Virtual screening in drug discovery-a computational perspective. Current Protein and Peptide Science 8 (4), 329–351 (2007).
Ts, X. & Joe, I. H. FT-IR, Raman and DFT study of 2-amino-5-fluorobenzoic acid and its biological activity with other halogen (Cl, Br) substitution. Spectrochim. Acta A 79 , 332–337 (2011).
Almutairi, M. S., Soumya, S., Al-Wabli, R. I., Joe, I. H. & Attia, M. I. Density functional theory calculations, vibration spectral analysis and molecular docking of the antimicrobial agent 6-(1, 3-benzodioxol-5-ylmethyl)-5-ethyl-2-{[2-(morpholin-4-yl) ethyl] sulfanyl} pyrimidin-4 (3H)-one. Open Chem. 16 , 653–666 (2018).
Mumit, M. A. et al. DFT studies on vibrational and electronic spectra, HOMO–LUMO, MEP, HOMA, NBO and molecular docking analysis of benzyl-3-N-(2, 4, 5-trimethoxyphenylmethylene) hydrazinecarbodithioate. J. Mol. Struct. 1220 , 128715 (2020).
Ayalew, M. E. DFT studies on molecular structure, thermodynamics parameters, HOMO-LUMO and spectral analysis of pharmaceuticals compound quinoline (Benzo [b] Pyridine). J. Biophys. Chem. 13 , 29–42 (2022).
Lombardo, F. et al. In Silico absorption, distribution, metabolism, excretion, and pharmacokinetics (ADME-PK): Utility and best practices. an industry perspective from the international consortium for innovation through quality in pharmaceutical development: Miniperspective. J. Med. Chem. 60 , 9097–9113 (2017).
Chandrasekaran, B., Abed, S. N., Al-Attraqchi, O., Kuche, K. & Tekade, R. K. Computer-aided prediction of pharmacokinetic (ADMET) properties. in Dosage Form Design Parameters , 731–755 (Elsevier, 2018).
Azzam, K. A. SwissADME and pkCSM webservers predictors: An integrated online platform for accurate and comprehensive predictions for in silico ADME/T properties of artemisinin and its derivatives. Compl. Use Miner. Resourc. 325 , 14–21 (2023).
Avdeef, A. Absorption and Drug Development: Solubility, Permeability, and Charge State (Wiley, 2012).
Book Google Scholar
Debnath, B. & Ganguly, S. J. Synthesis, biological evaluation, in silico docking, and virtual ADME studies of 2-[2-Oxo-3-(arylimino) indolin-1-yl]-N-arylacetamides as potent anti-breast cancer agents. Monatsh. Chem. 147 , 565–574 (2016).
Lipinski, C. A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 44 , 235–249 (2000).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 23 , 3–25 (1997).
Jarc, T. et al. Demonstrating suitability of the Caco-2 cell model for BCS-based biowaiver according to the recent FDA and ICH harmonised guidelines. J. Pharm. Pharmacol. 71 , 1231–1242 (2019).
Cabrera-Pérez, M. Á. et al. In silico assessment of ADME properties: Advances in Caco-2 cell monolayer permeability modeling. Curr. Top. Med. Chem. 18 , 2209–2229 (2018).
PubMed Google Scholar
Kumar, A., Rajendran, V., Sethumadhavan, R. & Purohit, R. Molecular dynamic simulation reveals damaging impact of RAC1 F28L mutation in the switch I region. PLoS ONE 8 , e77453 (2013).
Bera, K. et al. An in silico molecular dynamics simulation study on the inhibitors of SARS-CoV-2 proteases (3CLpro and PLpro) to combat COVID-19. Mol. Simul. 47 , 1168–1184 (2021).
Enquist, L. W., Husak, P. J., Banfield, B. W. & Smith, G. A. Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus Res. 51 , 237–347 (1998).
Mettenleiter, T. C., Klupp, B. G. & Granzow, H. Herpesvirus assembly: an update. Virus Res. 143 , 222–234 (2009).
Johnson, D. C. & Baines, J. D. Herpesviruses remodel host membranes for virus egress. Nat. Rev. Microbiol. 9 , 382–394 (2011).
Gianti, E. & Zauhar, R. J. Structure–activity relationships and drug design. in Remington , 129–153 (Elsevier, 2021).
Guha, R. On exploring structure–activity relationships. In Silico Models Drug Discov. 1 , 81–94 (2013).
Jongedijk, E. et al. Biotechnological production of limonene in microorganisms. Appl. Microbiol. Biotechnol. 100 , 2927–2938 (2016).
Vieira, A. J., Beserra, F. P., Souza, M., Totti, B. & Rozza, A. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 283 , 97–106 (2018).
Astani, A. & Schnitzler, P. Antiviral activity of monoterpenes beta-pinene and limonene against herpes simplex virus in vitro. Iran. J. Microbiol. 6 , 149 (2014).
PubMed PubMed Central Google Scholar
Download references
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R457), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and affiliations.
Department of Pharmacy, Faculty of Allied Health Sciences, Daffodil International University, Dhaka, Bangladesh, 1207
Md. Rezaul Islam
Department of Pharmacy, Faculty of Pharmacy, University of Dhaka, Dhaka, 1000, Bangladesh
Md. Shafiqul Islam Sovon
Department of Pharmacy, Faculty of Life & Earth Sciences, Jagannath University, Dhaka, Bangladesh
Department of Pharmaceutical Sciences, North South University, Dhaka, 1219, Bangladesh
Miadur Rahman
Department of Genetic Engineering and Biotechnology, University of Rajshahi, Rajshahi, 6205, Bangladesh
Md. Eram Hosen
Department of Chemistry, College of Arts and Sciences, International University of Business Agriculture and Technology (IUBAT), Dhaka, 1216, Bangladesh
Center for Global Health Research, Saveetha Institute of Medical and Technical Sciences in Saveetha Medical College and Hospital, Chennai, India
Laboratory of Biotechnology and Natural Resources Valorization, Faculty of Sciences, Ibn Zohr University, 80060, Agadir, Morocco
Mohammed Bourhia
Department of Pharmaceutics, College of Pharmacy, King Saud University, P.O. Box 11451, Riyadh, Saudi Arabia
Yousef A. Bin Jardan
Laboratory of Therapeutic and Organic Chemistry, Faculty of Pharmacy, University of Montpellier, 34000, Montpellier, France
Samir Ibenmoussa
Department of Biology, Bahir Dar University, P.O. Box 79, Bahir Dar, Ethiopia
Md. Rezaul Islam, Md. Shafiqul Islam Sovon, Ummy Amena, Miadur Rahman, Md. Eram Hosen, Ajoy Kumer & Gezahign Fentahun Wondmie
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization, original draft writing, reviewing, and editing: Md. Rezaul Islam, Md. Shafiqul Islam Sovon, Ummy Amena, Miadur Rahman. Formal analysis, investigations, funding acquisition, reviewing, and editing: Md. Eram Hosen, Ajoy Kumer, Mohammed Bourhia. Resources, data validation, data curation, and supervision: Yousef A. Bin Jardan, Samir Ibenmoussa, Gezahign Fentahun Wondmie. All authors have agreed with the content and all have given explicit consent to publish.
Corresponding authors
Correspondence to Mohammed Bourhia or Gezahign Fentahun Wondmie .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary figures., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Islam, M.R., Islam Sovon, M.S., Amena, U. et al. Ligand-based drug design against Herpes Simplex Virus-1 capsid protein by modification of limonene through in silico approaches. Sci Rep 14 , 9828 (2024). https://doi.org/10.1038/s41598-024-59577-4
Download citation
Received : 24 January 2024
Accepted : 12 April 2024
Published : 29 April 2024
DOI : https://doi.org/10.1038/s41598-024-59577-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Herpes Simplex Virus-1
- Molecular docking
- Molecular dynamics simulation
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Integr Med (Encinitas)
- v.16(3); 2017 Jun
Lysine for Herpes Simplex Prophylaxis: A Review of the Evidence
Herpes simplex virus (HSV) has been implicated in the etiology of recurrent aphthous ulcers, cold sores, and genital sores.
Primary Study Objective
To summarize the research evidence for use of L-lysine to prevent HSV disease recurrence, a use not widely recognized by doctors.
Methods/Design
Two scientists conducted a literature search of EMBASE, Medline, AMED, and CINAHL for the expanded terms lysine and herpes simplex or HSV in the title field and then independently screened the abstracts for clinically relevant articles. Disagreements on article inclusion were discussed before the literature was reviewed to see whether lysine is effective for preventing herpes simplex relapse.
Intervention
Oral L-lysine supplements were taken daily.
Primary Outcome Measures
Described individually for each trial reviewed.
L-lysine supplementation appears to be ineffective for prophylaxis or treatment of herpes simplex lesions with doses of less than 1 g/d without low-arginine diets. Doses in excess of 3 g/d appear to improve patients’ subjective experience of the disease.
Longer duration controlled studies of daily lysine doses exceeding 1.2 g/d are required to definitively test its role in herpes simplex prophylaxis. Patients with cardiovascular or gallbladder disease should be cautioned and warned of the theoretical risks of lysine supplementation.
Cold sores are a common recurrent problem and genital sores less so, caused by variants of the herpes simplex virus (HSV) sometimes treated with aciclovir, famciclovir, or valaciclovir. 1 It has been postulated that HSV may also contribute to RAUs 2 and estimated that up to 90% of the adult population is infected with HSV, which lies dormant in nervous tissue until relapse is triggered by stress or immune dysfunction. Based on tissue culture studies, it is thought that lysine-arginine balance may affect HSV expression. 3 Arginine-rich proteins are required for reproduction of HSV, whereas lysine competitively inhibits their synthesis. 2 L-lysine (the biologically active isomer of lysine) has been used by the general public and alternative health practitioners. A daily dose of 50 mg/kg of body weight has previously been recommended for cold sores 4 but this is not often advised by doctors due to conflicting evidence. 5 Patients requiring preventative or frequent oral acyclovir may prefer to try dietary changes or lysine supplements. Doses of up to 3 g/day are thought to be harmless 5 as oral lysine toxicity has not occurred in humans. 6 Two scientists conducted a literature search of EMBASE, Medline, AMED, and CINAHL for the expanded terms lysine and herpes simplex or HSV in the title field and then independently screened the abstracts for clinically relevant articles. Any disagreement on article inclusion was discussed and then the literature was reviewed to see whether lysine affects herpes simplex relapse. The evidence base is summarized in Table 1 .
Summary of Previously Reviewed Literature
a As cited in studies denoted by reference numbers.
Of the articles in Table 1 only, Thein and Hurt 16 was selected for inclusion in a Cochrane Skin Group review of randomized controlled trials, and this was deemed to be “very low” quality evidence due to imprecision and bias.
Summary of 2 Trials Not Included in Previous Literature Reviews
Wright 2 conducted a study of 28 patients with RAUs and 14 patients with herpes labialis using a 500 mg/day prophylactic dose and 4-g treatment dose, followed up by telephone interview after 6 months. All but 1 patient with herpes labialis and 1 with RAUs reported that lysine was effective. Patients were nonconcordant with prophylactic lysine, in some cases due to forgetfulness and in other cases due to believing they had been cured. Starting the treatment dose at onset of herpes labialis, prodrome was effective for only reducing the duration of lesions in 25% of patients. Two RAU patients required 1 g of lysine daily for prevention. One patient found no benefit from lysine but the other 25 patients found 500 mg/day to be 100% effective for prophylaxis.
Ozden et al 17 tested the relationship between HSV status and RAUs and the effects of L-lysine supplementation on the latter. Saliva and serum samples were collected from 30 RAU subjects and 17 healthy subjects without histories of oral mucosal disease before and after treatment. Saliva was tested for HSV-1 DNA by real-time polymerase chain reaction. Serum was tested for HSV-1 immunoglobulin G (IgG) by enzyme-linked immunosorbent assay. The RAU patients were randomly divided into placebo (n = 14) and lysine (n = 16) groups. Experimental subjects took 1260 mg of lysine daily for 2 months, increased to 2520 mg/day if prodromal symptoms occurred. Number of episodes, ulcer duration, number of ulcers per attack, severity of symptoms, and subjects’ subjective perception of degree of success of therapy on a scale from 1 to 10 were recorded. A total of 100% of RAU and 76.5% of healthy subjects tested positive for HSV-1 IgG antibody. HSV-1 DNA was present in 1 of 17 healthy controls (5.9%) and in 4 of 30 RAU patients. Two months of lysine supplementation did not significantly affect these biological markers. Ulcers did not occur in 18.7% of the lysine group and 28.5% of the placebo group during the trial. Ulcer recurrence was decreased in 62.5% of the lysine group and 14.2% of the placebo group (χ² = 7.97, P < .05). One placebo patient experienced increased ulcer recurrence. Decreased numbers of lesions per attack were reported by 56.2% of the lysine group (χ² = 8.37, P < .05) and 1 placebo patient (7.1%). Average perceived success was 7.5 ± 1.4 in the lysine group and 3.8 ± 2.0 in the placebo group ( t = 5.85, P < .001). There were no significant between-group differences for lesion duration or severity. This study was limited by its short duration.
Potential Risks and Adverse Effects or Long-term Lysine Use
Arginine deficiency may occur with kidney or small bowel pathology, sepsis, sickle cell disease, burns, trauma, or surgery. It is essential for growth, wound healing, endothelial function, and nitric oxide production. Lysine should, therefore, be used with caution in patients with cardiovascular disease, impotence, gallstones, asthma, or immune dysfunction. 18
Of the 12 studies analyzed in this paper, only 8 were placebo controlled, 7 of which were double-blind, 7 randomized, and 3 of which used cross-over methodology to minimize time effects. Ten trials tested prophylactic lysine to prevent cold-sores or mouth ulcers. Two studies 2 , 12 reported 500 mg/day to be effective for reducing lesion frequency, but neither of these were controlled or statistically tested. In contrast, 2 double-blind controlled trials showed no significant prophylaxis with 624 mg/day 3 , 7 , 9 , 17 or 750 mg/day. 14 Two double-blind placebo controlled trials showed clinically significant reductions in recurrence rates with 1 g/day 14 and 1248 mg/day, 3 , 7 , 9 , 17 in contrast to 2 that showed no significant difference with 1.2 g/day 3 , 7 , 14 ,19 and 1260 mg/day. 17 One randomized trial with 34 experimental patients taking 3 g of lysine daily and 25 control patients taking placebo demonstrated a statistically significant difference in reduction of recurrence rate. 3 , 7 , 9 , 15 , 17 , 18
Two randomized controlled trials showed no significant therapeutic effect from lysine supplements to treat active herpes simplex sores. One of these was with 1 g/day for 5 days, 3 , 7 , 10 , 13 , 14 whereas the other was with 2520 mg/day. 17 Only 25% of patients in an uncontrolled trial of a 4 g lysine daily treatment dose reported reduced duration of lesions. 2 This literature review revealed no convincing evidence for the use of lysine to treat herpes simplex sores.
Meta-analyses and randomized controlled trials are considered the best standard of evidence on which to base health care interventions. Only randomized controlled trials were considered for the Cochrane Skin Group review of treatments for herpes simplex. 16 Randomized controlled trials are expensive and, therefore, likely to be funded by only state health systems if significant cost-savings or improvements in quality of life are likely to result, or by the private sector if profit is likely to result. Lysine supplementation is not expected to significantly improve public health or generate sufficient profit to attract the funding required for randomized controlled trials. There is therefore a bias in research toward pharmaceutical as opposed to nutritional management of herpes simplex. This bias may extend to other diseases that are affected by nutritional imbalance, and it transfers power from patients to pharmaceutical companies.
Conclusions
L-lysine supplementation appears to be ineffective for prophylaxis or treatment of herpes simplex lesions with doses of less than 1 g/day without low-arginine diets. Findings from 1 small double-blind, randomized, placebo-controlled trial suggest that doses in excess of 3 g/day may reduce recurrence rates and improve patients’ self-reported symptoms. Longer-duration, randomized controlled studies of lysine doses exceeding 1.2 g/day are required to definitively test its role in herpes simplex prophylaxis and determine the minimum reliably effective daily dose. This literature review uncovered no convincing evidence that lysine can be used to treat active herpes simplex lesions. In the absence of such research, on the balance of possible risk to benefit, clinicians could consider advising patients that there is a theoretical role of lysine supplementation in the prevention of herpes simplex sores but the research evidence is insufficient to back this. Patients with cardiovascular or gallbladder disease should be cautioned and warned of the theoretical risks.
Biographies
Venthan J. Mailoo, BSc(Hons), MBBS, is anaesthetics senior house officer at Northampton General Hospital in Northhampton, United Kingdom.
Sanketh Rampes is a medical student at Kings College London in London, United Kingdom.
Appendix 1. Key Points
- Cold sores are a common problem caused by herpes simplex virus (HSV) variants.
- Lysine-arginine balance is thought to affect HSV expression.
- Oral lysine has been investigated in the prevention of HSV recurrence. Trials have yielded mixed results.
- Doses less than 1 g appear ineffective in prophylaxis or treatment of HSV lesions, whereas doses in excess of 3 g appear to improve patient’s subjective experience of the disease.
- Longer trials using daily L-lysine doses exceeding 1.2 g are required to definitively determine its role in HSV prophylaxis.
A Guide To Herpes
Home / Clinical Trials / A Guide to Herpes – Cures & Clinical Trials
Herpes Clinical Trial Recruiting Now
What is herpes.
Herpes is a sexually transmitted disease (STD) caused by the herpes simplex virus (HSV). It is one of the most common STDs in the world, and it is estimated that approximately one in eight people have been infected by the virus. Around 80% of those infected don’t even know they have the virus. It is impossible to know exactly how many people have the virus, since many cases are asymptomatic or never diagnosed.
What Causes Herpes?
Herpes is caused by two different viruses: HSV1 and HSV2. HSV1 usually causes sores around the mouth, while HSV2 causes genital herpes.
The infection is spread by skin-to-skin contact, and it can be transmitted through oral, vaginal, and anal sex, and kissing. It can also be spread through contact with lesions from other areas of the body. The virus goes into the body through small lesions in the skin, or through the mucosae in the mouth, penis, vagina, cervix, or anus.
After the infection, the patient might develop unspecific symptoms such as fever, fatigue, nausea, myalgia, adenopathy, along with the characteristic sores. Sores are small blisters which can sting or burn. They are usually grouped in clusters and they become crusted before healing. They don’t leave scars and resolve spontaneously. In some cases, patients won’t develop sores, instead displaying only irritated skin. Women can also present with vaginal discharge. Some people can be infected and remain asymptomatic; however, they can still spread the virus to other people.
After the first episode, the virus can become latent and remain in sensory nerves. This is known as the latent stage; afterwards, the virus can affect the skin again, usually along the pathway of the nerve where it has remained. These are the recurring episodes of the virus.
Fortunately, outbreaks tend to become less frequent and painful over time.
Risk factors for acquiring the infection include:
- Multiple sex partners
- Previous STDs
- Female gender
- Having unprotected sex
- Early age during first sexual intercourse
Match to Herpes Clinical Trials
How is herpes diagnosed.
A sample will be taken from a sore and tested to determine whether HSV is present in the lesion, thus confirming the diagnosis. However, a negative result does not rule out herpes. Samples should be taken from new ulcers, where it is more likely to find the virus.
Blood tests are also carried out to determine the presence of antibodies against HSV. This test can determine if the infection is new or a repeat outbreak. It is usually very difficult, if not impossible, to point to the exact moment a person was infected with the virus. If herpes is diagnosed, tests should be carried out to discard other STDs, since they can exist as comorbidities.
What Are Some Herpes Clinical Trial Recruiting Now?
Treating herpes.
Herpes Cure Information
>> Match With A Herpes Trial Here
There isn’t a cure for herpes, and although the sores heal in days or weeks, the virus never leaves the body. However, some medications can help make the outbreak pass faster.
NSAIDs such as paracetamol can be taken to reduce the discomfort during an outbreak. Ice packs, salt baths, and local anesthetic creams can also be applied.
Over-the-counter medications:
- Abreva Docosanol
Antiviral medications:
- Valacyclovir
- Famciclovir
- Penciclovir: only available for topical application
The dosage and length of treatment with these medications will depend on the location and chronicity of the lesions. Immunocompromised patients can develop life-threatening infections due to HSV (such as encephalitis or pneumonitis), and in these cases, acyclovir is often used in high doses. If acyclovir-resistant HSV is encountered, it is usually treated with cidofovir and foscarnet; however, these drugs can cause kidney toxicity. Prophylactic treatment can also be administered with antiviral drugs to prevent or shorten future outbreaks.
Lifestyle Changes
If you have been infected with the herpes simplex virus, make sure to adopt habits that minimize the risk of infecting other people, such as using condoms to prevent infection during intercourse, avoiding having multiple sex partners, avoiding intercourse during an active outbreak. Be honest with your partners about having the virus, and consider contacting previous partners if you aren’t sure where you became infected.
Make sure to discuss all your therapeutic options with your doctor, since the proper treatment can make outbreaks shorter and less painful. Seek professional help if you are having difficulty coping with the diagnosis. It is normal to feel shock, anger and embarrassment after diagnosis; however, it is important to know that although there is a stigma associated with herpes, it is a manageable condition that hardly ever leads to serious complications and you can lead a normal life after being infected.
Tips for Friends and Family
- Be kind to your partner if they have HSV and don’t make them feel ashamed or stigmatize the disease. Practice safe sex, and ask your doctor about prophylactic treatment to minimize the risk of infection.
- Herpes can’t be spread through contact with personal objects, so there is no danger in sharing objects like towels, sheets, toilets, or going swimming together.
- Avoid intimate contact with your loved one when they have active sores.
- Consider couples counseling to deal with the diagnosis.
- If you are interested, look for clinical trials near me
- Knott, L. (Oct 16th, 2017) Genital Herpes. Recovered from https://patient.info/health/sexually-transmitted-infections-leaflet/genital-herpes
- Genital herpes. 2015. Recovered from https://www.betterhealth.vic.gov.au/health/conditionsandtreatments/genital-herpes
Other Conditions
- Acute Aortic Dissection
- Ankylosing Spondylitis
- Alzheimer’s
- Auditory Loss and Deafness
- Autoimmune Hepatitis
- Bronchiectasis
- Breast Cancer
- Esophageal Cancer
- Lung Cancer
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer
- Compensation
- COPD Treatments
- Coronavirus
- Crohn’s Disease
- Diet And Nutrition
- Endometriosis
- Fibromyalgia
- Hepatitis B
- Hepatitis C
- Mindfulness
- Multiple Sclerosis
- Myasthenia Gravis
- Ophthalmology
- Osteoarthritis
- Parkinson’s Disease
- PBC and PSC
- Plant Based Diet
- Plastic Surgery
- Pulmonary Fibrosis
- Ulcerative Colitis
- Urinary Tract Infections
- Weight Loss
Paid Vaccine Trial For Women 16-17 Years Old
CMVictory: a vaccine trial for women between 16-17 years old.
Paid Clinical Trials Nationwide
Nationwide clinical trials offering up to $3,000 for participation and treatment.
Paid Vaccine Study For Teens
Vaccine study that aims to protect teens ages 12–17 against Epstein-Barr virus (EBV) and infectious mononucleosis (mono).
Paid Clinical Studies Nationwide
Sign up to receive email notifications on clinical trials for individual conditions in your local area.
Articles from Emerging Infectious Diseases

Volume 30, Number 5—May 2024
[PDF - 14.66 MB - 228 pages]
Research Letters
About the cover.
Crimean-Congo hemorrhagic fever (CCHF), caused by CCHF virus, is a tickborne disease that can cause a range of illness outcomes, from asymptomatic infection to fatal viral hemorrhagic fever; the disease has been described in >30 countries. We conducted a literature review to provide an overview of the virology, pathogenesis, and pathology of CCHF for clinicians. The virus life cycle and molecular interactions are complex and not fully described. Although pathogenesis and immunobiology are not yet fully understood, it is clear that multiple processes contribute to viral entry, replication, and pathological damage. Limited autopsy reports describe multiorgan involvement with extravasation and hemorrhages. Advanced understanding of CCHF virus pathogenesis and immunology will improve patient care and accelerate the development of medical countermeasures for CCHF.
Crimean-Congo hemorrhagic fever (CCHF) is a tickborne infection that can range from asymptomatic to fatal and has been described in >30 countries. Early identification and isolation of patients with suspected or confirmed CCHF and the use of appropriate prevention and control measures are essential for preventing human-to-human transmission. Here, we provide an overview of the epidemiology, clinical features, and prevention and control of CCHF. CCHF poses a continued public health threat given its wide geographic distribution, potential to spread to new regions, propensity for genetic variability, and potential for severe and fatal illness, in addition to the limited medical countermeasures for prophylaxis and treatment. A high index of suspicion, comprehensive travel and epidemiologic history, and clinical evaluation are essential for prompt diagnosis. Infection control measures can be effective in reducing the risk for transmission but require correct and consistent application.
Crimean-Congo hemorrhagic fever virus (CCHFV) is the most geographically widespread tickborne viral infection worldwide and has a fatality rate of up to 62%. Despite its widespread range and high fatality rate, no vaccines or treatments are currently approved by regulatory agencies in the United States or Europe. Supportive treatment remains the standard of care, but the use of antiviral medications developed for other viral infections have been considered. We reviewed published literature to summarize the main aspects of CCHFV infection in humans. We provide an overview of diagnostic testing and management and medical countermeasures, including investigational vaccines and limited therapeutics. CCHFV continues to pose a public health threat because of its wide geographic distribution, potential to spread to new regions, propensity for genetic variability, potential for severe and fatal illness, and limited medical countermeasures for prophylaxis and treatment. Clinicians should become familiar with available diagnostic and management tools for CCHFV infections in humans.
Jamestown Canyon virus (JCV) is a mosquitoborne orthobunyavirus in the California serogroup that circulates throughout Canada and the United States. Most JCV exposures result in asymptomatic infection or a mild febrile illness, but JCV can also cause neurologic diseases, such as meningitis and encephalitis. We describe a case series of confirmed JCV-mediated neuroinvasive disease among persons from the provinces of British Columbia, Alberta, Quebec, and Nova Scotia, Canada, during 2011–2016. We highlight the case definitions, epidemiology, unique features and clinical manifestations, disease seasonality, and outcomes for those cases. Two of the patients (from Quebec and Nova Scotia) might have acquired JCV infections during travel to the northeastern region of the United States. This case series collectively demonstrates JCV’s wide distribution and indicates the need for increased awareness of JCV as the underlying cause of meningitis/meningoencephalitis during mosquito season.
We analyzed hospital discharge records of patients with coccidioidomycosis-related codes from the International Classification of Diseases, 10th revision, Clinical Modification, to estimate the prevalence of hospital visits associated with the disease in Texas, USA. Using Texas Health Care Information Collection data for 2016–2021, we investigated the demographic characteristics and geographic distribution of the affected population, assessed prevalence of hospital visits for coccidioidomycosis, and examined how prevalence varied by demographic and geographic factors. In Texas, 709 coccidioidomycosis-related inpatient and outpatient hospital visits occurred in 2021; prevalence was 3.17 cases per 100,000 total hospital visits in 2020. Geographic location, patient sex, and race/ethnicity were associated with increases in coccidioidomycosis-related hospital visits; male, non-Hispanic Black, and Hispanic patients had the highest prevalence of coccidioidomycosis compared with other groups. Increased surveillance and healthcare provider education and outreach are needed to ensure timely and accurate diagnosis and treatment of coccidioidomycosis in Texas and elsewhere.
High incidences of congenital syphilis have been reported in areas along the Pacific coast of Colombia. In this retrospective study, conducted during 2018–2022 at a public hospital in Buenaventura, Colombia, we analyzed data from 3,378 pregnant women. The opportunity to prevent congenital syphilis was missed in 53.1% of mothers because of the lack of syphilis screening. Characteristics of higher maternal social vulnerability and late access to prenatal care decreased the probability of having > 1 syphilis screening test, thereby increasing the probability of having newborns with congenital syphilis. In addition, the opportunity to prevent congenital syphilis was missed in 41.5% of patients with syphilis because of the lack of treatment, which also increased the probability of having newborns with congenital syphilis. We demonstrate the urgent need to improve screening and treatment capabilities for maternal syphilis, particularly among pregnant women who are more socially vulnerable.
Understanding SARS-CoV-2 infection in populations at increased risk for poor health is critical to reducing disease. We describe the epidemiology of SARS-CoV-2 infection in Kakuma Refugee Camp Complex, Kenya. We performed descriptive analyses of SARS-CoV-2 infection in the camp and surrounding community during March 16, 2020‒December 31, 2021. We identified cases in accordance with national guidelines.We estimated fatality ratios and attack rates over time using locally weighted scatterplot smoothing for refugees, host community members, and national population. Of the 18,864 SARS-CoV-2 tests performed, 1,024 were positive, collected from 664 refugees and 360 host community members. Attack rates were 325.0/100,000 population (CFR 2.9%) for refugees,150.2/100,000 population (CFR 1.11%) for community, and 628.8/100,000 population (CFR 1.83%) nationwide. During 2020–2021, refugees experienced a lower attack rate but higher CFR than the national population, underscoring the need to prioritize SARS-CoV-2 mitigation measures, including vaccination.
Considering patient room shortages and prevalence of other communicable diseases, reassessing the isolation of patients with Clostridioides difficile infection (CDI) is imperative. We conducted a retrospective study to investigate the secondary CDI transmission rate in a hospital in South Korea, where patients with CDI were not isolated. Using data from a real-time locating system and electronic medical records, we investigated patients who had both direct and indirect contact with CDI index patients. The primary outcome was secondary CDI transmission, identified by whole-genome sequencing. Among 909 direct and 2,711 indirect contact cases, 2 instances of secondary transmission were observed (2 [0.05%] of 3,620 cases), 1 transmission via direct contact and 1 via environmental sources. A low level of direct contact (113 minutes) was required for secondary CDI transmission. Our findings support the adoption of exhaustive standard preventive measures, including environmental decontamination, rather than contact isolation of CDI patients in nonoutbreak settings.
During the 2022 multicountry mpox outbreak, the United Kingdom identified cases beginning in May. UK cases increased in June, peaked in July, then rapidly declined after September 2022. Public health responses included community-supported messaging and targeted mpox vaccination among eligible gay, bisexual, and other men who have sex with men (GBMSM). Using data from an online survey of GBMSM during November–December 2022, we examined self-reported mpox diagnoses, behavioral risk modification, and mpox vaccination offer and uptake. Among 1,333 participants, only 35 (2.6%) ever tested mpox-positive, but 707 (53%) reported behavior modification to avoid mpox. Among vaccine-eligible GBMSM, uptake was 69% (95% CI 65%–72%; 601/875) and was 92% (95% CI 89%–94%; 601/655) among those offered vaccine. GBMSM self-identifying as bisexual, reporting lower educational qualifications, or identifying as unemployed were less likely to be vaccinated. Equitable offer and provision of mpox vaccine are needed to minimize the risk for future outbreaks and mpox-related health inequalities.
We investigated clinically suspected measles cases that had discrepant real-time reverse transcription PCR (rRT-PCR) and measles-specific IgM test results to determine diagnoses. We performed rRT-PCR and measles-specific IgM testing on samples from 541 suspected measles cases. Of the 24 IgM-positive and rRT-PCR–negative cases, 20 were among children who received a measles-containing vaccine within the previous 6 months; most had low IgG relative avidity indexes (RAIs). The other 4 cases were among adults who had an unknown previous measles history, unknown vaccination status, and high RAIs. We detected viral nucleic acid for viruses other than measles in 15 (62.5%) of the 24 cases with discrepant rRT-PCR and IgM test results. Measles vaccination, measles history, and contact history should be considered in suspected measles cases with discrepant rRT-PCR and IgM test results. If in doubt, measles IgG avidity and PCR testing for other febrile exanthematous viruses can help confirm or refute the diagnosis.
To determine the kinetics of hepatitis E virus (HEV) in asymptomatic persons and to evaluate viral load doubling time and half-life, we retrospectively tested samples retained from 32 HEV RNA-positive asymptomatic blood donors in Germany. Close-meshed monitoring of viral load and seroconversion in intervals of ≈4 days provided more information about the kinetics of asymptomatic HEV infections. We determined that a typical median infection began with PCR-detectable viremia at 36 days and a maximum viral load of 2.0 × 10 4 IU/mL. Viremia doubled in 2.4 days and had a half-life of 1.6 days. HEV IgM started to rise on about day 33 and peaked on day 36; IgG started to rise on about day 32 and peaked on day 53 . Although HEV IgG titers remained stable, IgM titers became undetectable in 40% of donors. Knowledge of the dynamics of HEV viremia is useful for assessing the risk for transfusion-transmitted hepatitis E.
We evaluated Q fever prevalence in blood donors and assessed the epidemiologic features of the disease in Israel in 2021. We tested serum samples for Coxeilla burnetii phase I and II IgG using immunofluorescent assay, defining a result of > 200 as seropositive. We compared geographic and demographic data. We included 1,473 participants; 188 (12.7%) were seropositive. The calculated sex- and age-adjusted national seroprevalence was 13.9% (95% CI 12.2%–15.7%). Male sex and age were independently associated with seropositivity (odds ratio [OR] 1.6, 95% CI 1.1–2.2; p = 0.005 for male sex; OR 1.2, 95% CI 1.01–1.03; p<0.001 for age). Residence in the coastal plain was independently associated with seropositivity for Q fever (OR 1.6, 95% CI 1.2–2.3; p<0.001); residence in rural and farming regions was not. Q fever is highly prevalent in Israel. The unexpected spatial distribution in the nonrural coastal plain suggests an unrecognized mode of transmission.
During December 11, 2020–March 29, 2022, the US government delivered ≈700 million doses of COVID-19 vaccine to vaccination sites, resulting in vaccination of ≈75% of US adults during that period. We evaluated accessibility of vaccination sites. Sites were accessible by walking within 15 minutes by 46.6% of persons, 30 minutes by 74.8%, 45 minutes by 82.8%, and 60 minutes by 86.7%. When limited to populations in counties with high social vulnerability, accessibility by walking was 55.3%, 81.1%, 86.7%, and 89.4%, respectively. By driving, lowest accessibility was 96.5% at 15 minutes. For urban/rural categories, the 15-minute walking accessibility between noncore and large central metropolitan areas ranged from 27.2% to 65.1%; driving accessibility was 79.9% to 99.5%. By 30 minutes driving accessibility for all urban/rural categories was >95.9%. Walking time variations across jurisdictions and between urban/rural areas indicate that potential gains could have been made by improving walkability or making transportation more readily available.
We estimated COVID-19 transmission potential and case burden by variant type in Alberta, British Columbia, and Ontario, Canada, during January 23, 2020–January 27, 2022; we also estimated the effectiveness of public health interventions to reduce transmission. We estimated time-varying reproduction number (R t ) over 7-day sliding windows and nonoverlapping time-windows determined by timing of policy changes. We calculated incidence rate ratios (IRRs) for each variant and compared rates to determine differences in burden among provinces. R t corresponding with emergence of the Delta variant increased in all 3 provinces; British Columbia had the largest increase, 43.85% (95% credible interval [CrI] 40.71%–46.84%). Across the study period, IRR was highest for Omicron (8.74 [95% CrI 8.71–8.77]) and burden highest in Alberta (IRR 1.80 [95% CrI 1.79–1.81]). Initiating public health interventions was associated with lower R t and relaxing restrictions and emergence of new variants associated with increases in R t .
We conducted a large surveillance study among members of an integrated healthcare delivery system in Pacific Northwest of the United States to estimate medical costs attributable to medically attended acute gastroenteritis (MAAGE) on the day care was sought and during 30-day follow-up. We used multivariable regression to compare costs of MAAGE and non-MAAGE cases matched on age, gender, and index time. Differences accounted for confounders, including race, ethnicity, and history of chronic underlying conditions. Analyses included 73,140 MAAGE episodes from adults and 18,617 from children who were Kaiser Permanente Northwest members during 2014–2016. Total costs were higher for MAAGE cases relative to non-MAAGE comparators as were costs on the day care was sought and costs during follow-up. Costs of MAAGE are substantial relative to the cost of usual-care medical services, and much of the burden accrues during short-term follow-up.
We investigated links between antimicrobial resistance in community-onset bacteremia and 1-year bacteremia recurrence by using the clinical data warehouse of Europe’s largest university hospital group in France. We included adult patients hospitalized with an incident community-onset Staphylococcus aureus , Escherichia coli , or Klebsiella spp. bacteremia during 2017–2019. We assessed risk factors of 1-year recurrence using Fine–Gray regression models. Of the 3,617 patients included, 291 (8.0%) had > 1 recurrence episode. Third-generation cephalosporin (3GC)-resistance was significantly associated with increased recurrence risk after incident Klebsiella spp. (hazard ratio 3.91 [95% CI 2.32–6.59]) or E. coli (hazard ratio 2.35 [95% CI 1.50–3.68]) bacteremia. Methicillin resistance in S. aureus bacteremia had no effect on recurrence risk. Although several underlying conditions and infection sources increased recurrence risk, 3GC-resistant Klebsiella spp. was associated with the greatest increase. These results demonstrate a new facet to illness induced by 3GC-resistant Klebsiella spp. and E. coli in the community setting.
We conducted a cross-sectional study in wild boar and extensively managed Iberian pig populations in a hotspot area of Crimean-Congo hemorrhagic fever virus (CCHFV) in Spain. We tested for antibodies against CCHFV by using 2 ELISAs in parallel. We assessed the presence of CCHFV RNA by means of reverse transcription quantitative PCR protocol, which detects all genotypes. A total of 113 (21.8%) of 518 suids sampled showed antibodies against CCHFV by ELISA. By species, 106 (39.7%) of 267 wild boars and 7 (2.8%) of 251 Iberian pigs analyzed were seropositive. Of the 231 Iberian pigs and 231 wild boars analyzed, none tested positive for CCHFV RNA. These findings indicate high CCHFV exposure in wild boar populations in endemic areas and confirm the susceptibility of extensively reared pigs to CCHFV, even though they may only play a limited role in the enzootic cycle.
African swine fever virus (ASFV) genotype II is endemic to Vietnam. We detected recombinant ASFV genotypes I and II (rASFV I/II) strains in domestic pigs from 6 northern provinces in Vietnam. The introduction of rASFV I/II strains could complicate ongoing ASFV control measures in the region.
In a representative sample of female children and adolescents in Germany, Toxoplasma gondii seroprevalence was 6.3% (95% CI 4.7%–8.0%). With each year of life, the chance of being seropositive increased by 1.2, indicating a strong force of infection. Social status and municipality size were found to be associated with seropositivity.
We describe the detection of Paranannizziopsis sp. fungus in a wild population of vipers in Europe. Fungal infections were severe, and 1 animal likely died from infection. Surveillance efforts are needed to better understand the threat of this pathogen to snake conservation.
We evaluated the in vitro effects of lyophilization for 2 vesicular stomatitis virus–based vaccines by using 3 stabilizing formulations and demonstrated protective immunity of lyophilized/reconstituted vaccine in guinea pigs. Lyophilization increased stability of the vaccines, but specific vesicular stomatitis virus–based vaccines will each require extensive analysis to optimize stabilizing formulations.
We report a cluster of serogroup B invasive meningococcal disease identified via genomic surveillance in older adults in England and describe the public health responses. Genomic surveillance is critical for supporting public health investigations and detecting the growing threat of serogroup B Neisseria meningitidis infections in older adults.
We detected Mayaro virus (MAYV) in 3.4% (28/822) of febrile patients tested during 2018–2021 from Roraima State, Brazil. We also isolated MAYV strains and confirmed that these cases were caused by genotype D. Improved surveillance is needed to better determine the burden of MAYV in the Amazon Region.
Across 133 confirmed mpox zoonotic index cases reported during 1970–2021 in Africa, cases occurred year-round near the equator, where climate is consistent. However, in tropical regions of the northern hemisphere under a dry/wet season cycle, cases occurred seasonally. Our findings further support the seasonality of mpox zoonotic transmission risk.
We investigated molecular evolution and spatiotemporal dynamics of atypical Legionella pneumophila serogroup 1 sequence type 1905 and determined its long-term persistence and linkage to human disease in dispersed locations, far beyond the large 2014 outbreak epicenter in Portugal. Our finding highlights the need for public health interventions to prevent further disease spread.
Norovirus is a major cause of acute gastroenteritis; GII.4 is the predominant strain in humans. Recently, 2 new GII.4 variants, Hong Kong 2019 and San Francisco 2017, were reported. Characterization using GII.4 monoclonal antibodies and serum demonstrated different antigenic profiles for the new variants compared with historical variants.
Cruise ships carrying COVID-19–vaccinated populations applied near-identical nonpharmaceutical measures during July–November 2021; passenger masking was not applied on 2 ships. Infection risk for masked passengers was 14.58 times lower than for unmasked passengers and 19.61 times lower than in the community. Unmasked passengers’ risk was slightly lower than community risk.
During a 2023 outbreak of Mycoplasma pneumoniae –associated community-acquired pneumonia among children in northern Vietnam, we analyzed M. pneumoniae isolated from nasopharyngeal samples. In almost half (6 of 13) of samples tested, we found known A2063G mutations (macrolide resistance) and a novel C2353T variant on the 23S rRNA gene.
We report the detection of Crimean-Congo hemorrhagic fever virus (CCHFV) in Corsica, France. We identified CCHFV African genotype I in ticks collected from cattle at 2 different sites in southeastern and central-western Corsica, indicating an established CCHFV circulation. Healthcare professionals and at-risk groups should be alerted to CCHFV circulation in Corsica.
In Latin America, rabies virus has persisted in a cycle between Desmodus rotundus vampire bats and cattle, potentially enhanced by deforestation. We modeled bovine rabies virus outbreaks in Costa Rica relative to land-use indicators and found spatial-temporal relationships among rabies virus outbreaks with deforestation as a predictor.
With the use of metagenomic next-generation sequencing, patients diagnosed with Whipple pneumonia are being increasingly correctly diagnosed. We report a series of 3 cases in China that showed a novel pattern of movable infiltrates and upper lung micronodules. After treatment, the 3 patients recovered, and lung infiltrates resolved.
Dogs are known to be susceptible to influenza A viruses, although information on influenza D virus (IDV) is limited. We investigated the seroprevalence of IDV in 426 dogs in the Apulia region of Italy during 2016 and 2023. A total of 14 samples were positive for IDV antibodies, suggesting exposure to IDV in dogs.
We report the detection of OXA-181 carbapenemase in an azithromycin-resistant Shigella spp. bacteria in an immunocompromised patient. The emergence of OXA-181 in Shigella spp. bacteria raises concerns about the global dissemination of carbapenem resistance in Enterobacterales and its implications for the treatment of infections caused by Shigella bacteria.
Although a vaccine against SARS-CoV-2 Omicron-XBB.1.5 variant is available worldwide and recent infection is protective, the lack of recorded infection data highlights the need to assess variant-specific antibody neutralization levels. We analyzed IgG levels against receptor-binding domain–specific SARS-CoV-2 ancestral strain as a correlate for high neutralizing titers against XBB variants.
We describe a feline sporotrichosis cluster and zoonotic transmission between one of the affected cats and a technician at a veterinary clinic in Kansas, USA. Increased awareness of sporotrichosis and the potential for zoonotic transmission could help veterinary professionals manage feline cases and take precautions to prevent human acquisition.
We report a clinical isolate of Burkholderia thailandensis 2022DZh obtained from a patient with an infected wound in southwest China. Genomic analysis indicates that this isolate clusters with B. thailandensis BPM, a human isolate from Chongqing, China. We recommend enhancing monitoring and surveillance for B. thailandensis infection in both humans and livestock.
To determine changes in Bordetella pertussis and B. parapertussis detection rates, we analyzed 1.43 million respiratory multiplex PCR test results from US facilities from 2019 through mid-2023. From mid-2022 through mid-2023, Bordetella spp. detection increased 8.5-fold; 95% of detections were B. parapertussis. While B. parapertussis rates increased, B. pertussis rates decreased.
We report a case of Sphingobium yanoikuyae bacteremia in an 89-year-old patient in Japan. No standard antimicrobial regimen has been established for S. yanoikuyae infections. However, ceftriaxone and ceftazidime treatments were effective in this case. Increased antimicrobial susceptibility data are needed to establish appropriate treatments for S. yanoikuyae .
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 30, Number 6—June 2024
Perspective.
- Decolonization and Pathogen Reduction to Prevent Antimicrobial Resistance and Healthcare-Associated Infections M. R. Mangalea et al.
- Deciphering Unexpected Vascular Locations of Scedosporium spp. and Lomentospora prolificans Fungal Infections, France C. Vignals et al.
- An Electronic Health Record–Based Algorithm for Respiratory Virus–like Illness N. M. Cocoros et al.
- Severe Human Parainfluenza Virus Community- and Healthcare-Acquired Pneumonia in Adults at Tertiary Hospital in Seoul, South Korea, 2010–2019 J. H. Park et al.
- SARS-CoV-2 Disease Severity in Children during Pre-Delta, Delta, and Omicron Periods, Colorado L. Bankers et al.
- Effectiveness of 23-Valent Pneumococcal Polysaccharide Vaccine Against Invasive Pneumococcal Disease in Follow-Up Study, Denmark K. Nielsen et al.
- Chest Radiograph Screening for Detection of Subclinical Tuberculosis in Asymptomatic Household Contacts, Peru Q. Tan et al.
- Outbreak of Highly Pathogenic Avian Influenza Virus H5N1 in Seals in the St. Lawrence Estuary, Quebec, Canada S. Lair et al.
- Carbapenem-Resistant and Extended-Spectrum β-Lactamase–Producing Enterobacterales Cases among Children, United States, 2016–2020 H. N. Grome et al.
- Antibodies to H5N1 Influenza A Virus in Retrieving Hunting Dogs, Washington State, USA J. D. Brown et al.
We characterized the evolution and molecular characteristics of avian influenza A(H7N9) viruses isolated in China during 2021–2023. We systematically analyzed the 10-year evolution of the hemagglutinin gene to determine the evolutionary branch. Our results showed recent antigenic drift, providing crucial clues for updating the H7N9 vaccine and disease prevention and control.
- Burkholderia semiarida as Cause of Recurrent Pulmonary Infection in Immunocompetent Patient, China D. Kuang et al.
- SARS-CoV-2 in Captive Nonhuman Primates, Spain, 2020–2023 D. Cano-Terriza et al.
- Infection- and Vaccine-Induced SARS-CoV-2 Seroprevalence in Persons 0–101 Years of Age, Japan, 2023 R. Kinoshita et al.
- Zoonotic Ancylostoma ceylanicum Infection in Coyotes from the Guanacaste Conservation Area, Costa Rica, 2021 P. A. Zendejas-Heredia et al.
- Detection of Encephalitozoon cuniculi in Cerebrospinal Fluid from Immunocompetent Patients, Czech Republic B. Sak et al.
- Emergence of Group B Streptococcus Disease in Pigs and Porcupines, Italy C. Garbarino et al.
- Molecular Identification of Fonsecaea monophora , Novel Agent of Fungal Brain Abscess S. Gourav et al.
During May–July 2023, a cluster of 7 patients at local hospitals in Florida, USA, received a diagnosis of Plasmodium vivax malaria. Whole-genome sequencing of the organism from 4 patients and phylogenetic analysis with worldwide representative P. vivax genomes indicated probable single parasite introduction from Central/South America.
Because novel SARS-CoV-2 variants continue to emerge, immunogenicity of XBB.1.5 monovalent vaccines against live clinical isolates needs to be evaluated. We report boosting of IgG (2.1×), IgA (1.5×), and total IgG/A/M (1.7×) targeting the spike receptor-binding domain and neutralizing titers against WA1 (2.2×), XBB.1.5 (7.4×), EG.5.1 (10.5×), and JN.1 (4.7×) variants.
Using the GISAID EpiCoV database, we identified 256 COVID-19 patients in Japan during March 31–December 31, 2023, who had mutations in the SARS-CoV-2 nonstructural protein 5 conferring ensitrelvir resistance. Ongoing genomic surveillance is required to monitor emergence of SARS-CoV-2 mutations that are resistant to anticoronaviral drugs.
- Novel Avian Influenza A(H5N6) in Wild Birds, South Korea, 2023 A. Cho et al.
Volume 30, Number 7—July 2024
We report highly pathogenic avian influenza A(H5N1) virus in dairy cattle and cats in Kansas and Texas, United States, which reflects the continued spread of clade 2.3.4.4b viruses that entered the country in late 2021. Infected cattle experienced nonspecific illness, reduced feed intake and rumination, and an abrupt drop in milk production, but fatal systemic influenza infection developed in domestic cats fed raw (unpasteurized) colostrum and milk from affected cows. Cow-to-cow transmission appears to have occurred because infections were observed in cattle on Michigan, Idaho, and Ohio farms where avian influenza virus–infected cows were transported. Although the US Food and Drug Administration has indicated the commercial milk supply remains safe, the detection of influenza virus in unpasteurized bovine milk is a concern because of potential cross-species transmission. Continued surveillance of highly pathogenic avian influenza viruses in domestic production animals is needed to prevent cross-species and mammal-to-mammal transmission.
- Borrelia miyamotoi -associated Acute Meningoencephalitis, Minnesota, United States J. M. Kubiak et al.
Research Letter
- Pasteurella bettyae Infections in Men Who Have Sex With Men, France A. Li et al.
Medscape, LLC is pleased to provide online continuing medical education (CME) for selected journal articles, allowing clinicians the opportunity to earn CME credit. In support of improving patient care, these activities have been planned and implemented by Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. CME credit is available for one year after publication.
Active CME Articles
During October 2021–June 2023, a total of 392 cases of acute hepatitis of unknown etiology in children in the United States were reported to Centers for Disease Control and Prevention as part of national surveillance. We describe demographic and clinical characteristics, including potential involvement of adenovirus in development of acute hepatitis, of 8 fatally ill children who met reporting criteria. The children had diverse courses of illness. Two children were immunocompromised when initially brought for care. Four children tested positive for adenovirus in multiple specimen types, including 2 for whom typing was completed. One adenovirus-positive child had no known underlying conditions, supporting a potential relationship between adenovirus and acute hepatitis in previously healthy children. Our findings emphasize the importance of continued investigation to determine the mechanism of liver injury and appropriate treatment. Testing for adenovirus in similar cases could elucidate the role of the virus.
In 2022, concurrent outbreaks of hepatitis A, invasive meningococcal disease (IMD), and mpox were identified in Florida, USA, primarily among men who have sex with men. The hepatitis A outbreak (153 cases) was associated with hepatitis A virus genotype IA. The IMD outbreak (44 cases) was associated with Neisseria meningitidis serogroup C, sequence type 11, clonal complex 11. The mpox outbreak in Florida (2,845 cases) was part of a global epidemic. The hepatitis A and IMD outbreaks were concentrated in Central Florida and peaked during March–June, whereas mpox cases were more heavily concentrated in South Florida and had peak incidence in August. HIV infection was more common (52%) among mpox cases than among hepatitis A (21%) or IMD (34%) cases. Where feasible, vaccination against hepatitis A, meningococcal disease, and mpox should be encouraged among at-risk groups and offered along with program services that target those groups.
Disseminated leishmaniasis (DL) is an emergent severe disease manifesting with multiple lesions. To determine the relationship between immune response and clinical and therapeutic outcomes, we studied 101 DL and 101 cutaneous leishmaniasis (CL) cases and determined cytokines and chemokines in supernatants of mononuclear cells stimulated with leishmania antigen. Patients were treated with meglumine antimoniate (20 mg/kg) for 20 days (CL) or 30 days (DL); 19 DL patients were instead treated with amphotericin B, miltefosine, or miltefosine and meglumine antimoniate. High levels of chemokine ligand 9 were associated with more severe DL. The cure rate for meglumine antimoniate was low for both DL (44%) and CL (60%), but healing time was longer in DL (p = 0.003). The lowest cure rate (22%) was found in DL patients with >100 lesions. However, meglumine antimoniate/miltefosine treatment cured all DL patients who received it; therefore, that combination should be considered as first choice therapy.
Streptococcus suis , a zoonotic bacterial pathogen circulated through swine, can cause severe infections in humans. Because human S. suis infections are not notifiable in most countries, incidence is underestimated. We aimed to increase insight into the molecular epidemiology of human S. suis infections in Europe. To procure data, we surveyed 7 reference laboratories and performed a systematic review of the scientific literature. We identified 236 cases of human S. suis infection from those sources and an additional 87 by scanning gray literature. We performed whole-genome sequencing to type 46 zoonotic S. suis isolates and combined them with 28 publicly available genomes in a core-genome phylogeny. Clonal complex (CC) 1 isolates accounted for 87% of typed human infections; CC20, CC25, CC87, and CC94 also caused infections. Emergence of diverse zoonotic clades and notable severity of illness in humans support classifying S. suis infection as a notifiable condition.
During January–August 2021, the Community Prevalence of SARS-CoV-2 Study used time/location sampling to recruit a cross-sectional, population-based cohort to estimate SARS-CoV-2 seroprevalence and nasal swab sample PCR positivity across 15 US communities. Survey-weighted estimates of SARS-CoV-2 infection and vaccine willingness among participants at each site were compared within demographic groups by using linear regression models with inverse variance weighting. Among 22,284 persons > 2 months of age and older, median prevalence of infection (prior, active, or both) was 12.9% across sites and similar across age groups. Within each site, average prevalence of infection was 3 percentage points higher for Black than White persons and average vaccine willingness was 10 percentage points lower for Black than White persons and 7 percentage points lower for Black persons than for persons in other racial groups. The higher prevalence of SARS-CoV-2 infection among groups with lower vaccine willingness highlights the disparate effect of COVID-19 and its complications.
Invasive fusariosis can be life-threatening, especially in immunocompromised patients who require intensive care unit (ICU) admission. We conducted a multicenter retrospective study to describe clinical and biologic characteristics, patient outcomes, and factors associated with death and response to antifungal therapy. We identified 55 patients with invasive fusariosis from 16 ICUs in France during 2002–2020. The mortality rate was high (56%). Fusariosis-related pneumonia occurred in 76% of patients, often leading to acute respiratory failure. Factors associated with death included elevated sequential organ failure assessment score at ICU admission or history of allogeneic hematopoietic stem cell transplantation or hematologic malignancies. Neither voriconazole treatment nor disseminated fusariosis were strongly associated with response to therapy. Invasive fusariosis can lead to multiorgan failure and is associated with high mortality rates in ICUs. Clinicians should closely monitor ICU patients with a history of hematologic malignancies or stem cell transplantation because of higher risk for death.
Using whole-genome sequencing, we characterized Escherichia coli strains causing early-onset sepsis (EOS) in 32 neonatal cases from a 2019–2021 prospective multicenter study in France and compared them to E. coli strains collected from vaginal swab specimens from women in third-trimester gestation. We observed no major differences in phylogenetic groups or virulence profiles between the 2 collections. However, sequence type (ST) analysis showed the presence of 6/32 (19%) ST1193 strains causing EOS, the same frequency as in the highly virulent clonal group ST95. Three ST1193 strains caused meningitis, and 3 harbored extended-spectrum β-lactamase. No ST1193 strains were isolated from vaginal swab specimens. Emerging ST1193 appears to be highly prevalent, virulent, and antimicrobial resistant in neonates. However, the physiopathology of EOS caused by ST1193 has not yet been elucidated. Clinicians should be aware of the possible presence of E. coli ST1193 in prenatal and neonatal contexts and provide appropriate monitoring and treatment.
We describe detection of the previously rarely reported gram-positive bacterium Auritidibacter ignavus in 3 cases of chronic ear infections in Germany. In all 3 cases, the patients had refractory otorrhea. Although their additional symptoms varied, all patients had an ear canal stenosis and A. ignavus detected in microbiologic swab specimens. A correct identification of A. ignavus in the clinical microbiology laboratory is hampered by the inability to identify it by using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Also, the bacterium might easily be overlooked because of its morphologic similarity to bacterial species of the resident skin flora. We conclude that a high index of suspicion is warranted to identify A. ignavus and that it should be particularly considered in patients with chronic external otitis who do not respond clinically to quinolone ear drop therapy.
We reviewed invasive Nocardia infections in 3 noncontiguous geographic areas in the United States during 2011–2018. Among 268 patients with invasive nocardiosis, 48.2% were from Minnesota, 32.4% from Arizona, and 19.4% from Florida. Predominant species were N. nova complex in Minnesota (33.4%), N. cyriacigeorgica in Arizona (41.4%), and N. brasiliensis in Florida (17.3%). Transplant recipients accounted for 82/268 (30.6%) patients overall: 14 (10.9%) in Minnesota, 35 (40.2%) in Arizona, and 33 (63.5%) in Florida. Manifestations included isolated pulmonary nocardiosis among 73.2% of transplant and 84.4% of non–transplant patients and central nervous system involvement among 12.2% of transplant and 3.2% of non–transplant patients. N. farcinica (20.7%) and N. cyriacigeorgica (19.5%) were the most common isolates among transplant recipients and N. cyriacigeorgica (38.0%), N. nova complex (23.7%), and N. farcinica (16.1%) among non–transplant patients. Overall antimicrobial susceptibilities were similar across the 3 study sites.
We collected stool from school-age children from 352 households living in the Black Belt region of Alabama, USA, where sanitation infrastructure is lacking. We used quantitative reverse transcription PCR to measure key pathogens in stool that may be associated with water and sanitation, as an indicator of exposure. We detected genes associated with > 1 targets in 26% of specimens, most frequently Clostridioides difficile (6.6%), atypical enteropathogenic Escherichia coli (6.1%), and enteroaggregative E. coli (3.9%). We used generalized estimating equations to assess reported risk factors for detecting > 1 pathogen in stool. We found no association between lack of sanitation and pathogen detection (adjusted risk ratio 0.95 [95% CI 0.55–1.7]) compared with specimens from children served by sewerage. However, we did observe an increased risk for pathogen detection among children living in homes with well water (adjusted risk ratio 1.7 [95% CI 1.1–2.5]) over those reporting water utility service.
Campylobacter fetus accounts for 1% of Campylobacter spp. infections, but prevalence of bacteremia and risk for death are high. To determine clinical features of C. fetus infections and risks for death, we conducted a retrospective observational study of all adult inpatients with a confirmed C. fetus infection in Nord Franche-Comté Hospital, Trevenans, France, during January 2000–December 2021. Among 991 patients with isolated Campylobacter spp. strains, we identified 39 (4%) with culture-positive C. fetus infections, of which 33 had complete records and underwent further analysis; 21 had documented bacteremia and 12 did not. Secondary localizations were reported for 7 (33%) patients with C. fetus bacteremia, of which 5 exhibited a predilection for vascular infections (including 3 with mycotic aneurysm). Another 7 (33%) patients with C. fetus bacteremia died within 30 days. Significant risk factors associated with death within 30 days were dyspnea, quick sequential organ failure assessment score > 2 at admission, and septic shock.
Group A Streptococcus (GAS) primary peritonitis is a rare cause of pediatric acute abdomen (sudden onset of severe abdominal pain); only 26 pediatric cases have been reported in the English language literature since 1980. We discuss 20 additional cases of pediatric primary peritonitis caused by GAS among patients at Starship Children’s Hospital, Auckland, New Zealand, during 2010–2022. We compare identified cases of GAS primary peritonitis to cases described in the existing pediatric literature. As rates of rates of invasive GAS increase globally, clinicians should be aware of this cause of unexplained pediatric acute abdomen.
In Mississippi, USA, infant hospitalization with congenital syphilis (CS) spiked by 1,000%, from 10 in 2016 to 110 in 2022. To determine the causes of this alarming development, we analyzed Mississippi hospital discharge data to evaluate trends, demographics, outcomes, and risk factors for infants diagnosed with CS hospitalized during 2016–2022. Of the 367 infants hospitalized with a CS diagnosis, 97.6% were newborn, 92.6% were covered by Medicaid, 71.1% were African American, and 58.0% were nonurban residents. Newborns with CS had higher odds of being affected by maternal illicit drug use, being born prematurely (<37 weeks), and having very low birthweight (<1,500 g) than those without CS. Mean length of hospital stay (14.5 days vs. 3.8 days) and mean charges ($56,802 vs. $13,945) were also higher for infants with CS than for those without. To address escalation of CS, Mississippi should invest in comprehensive prenatal care and early treatment of vulnerable populations.
Ongoing surveillance after pneumococcal conjugate vaccination (PCV) deployment is essential to inform policy decisions and monitor serotype replacement. We report serotype and disease severity trends in 3,719 adults hospitalized for pneumococcal disease in Bristol and Bath, United Kingdom, during 2006–2022. Of those cases, 1,686 were invasive pneumococcal disease (IPD); 1,501 (89.0%) had a known serotype. IPD decreased during the early COVID-19 pandemic but during 2022 gradually returned to prepandemic levels. Disease severity changed throughout this period: CURB65 severity scores and inpatient deaths decreased and ICU admissions increased. PCV7 and PCV13 serotype IPD decreased from 2006–2009 to 2021–2022. However, residual PCV13 serotype IPD remained, representing 21.7% of 2021–2022 cases, indicating that major adult PCV serotype disease still occurs despite 17 years of pediatric PCV use. Percentages of serotype 3 and 8 IPD increased, and 19F and 19A reemerged. In 2020–2022, a total of 68.2% IPD cases were potentially covered by PCV20.
Borrelia miyamotoi , transmitted by Ixodes spp. ticks, was recognized as an agent of hard tick relapsing fever in the United States in 2013. Nine state health departments in the Northeast and Midwest have conducted public health surveillance for this emerging condition by using a shared, working surveillance case definition. During 2013–2019, a total of 300 cases were identified through surveillance; 166 (55%) were classified as confirmed and 134 (45%) as possible. Median age of case-patients was 52 years (range 1–86 years); 52% were male. Most cases (70%) occurred during June–September, with a peak in August. Fever and headache were common symptoms; 28% of case-patients reported recurring fevers, 55% had arthralgia, and 16% had a rash. Thirteen percent of patients were hospitalized, and no deaths were reported. Ongoing surveillance will improve understanding of the incidence and clinical severity of this emerging disease.
During 2006–2021, Canada had 55 laboratory-confirmed outbreaks of foodborne botulism, involving 67 cases. The mean annual incidence was 0.01 case/100,000 population. Foodborne botulism in Indigenous communities accounted for 46% of all cases, which is down from 85% of all cases during 1990–2005. Among all cases, 52% were caused by botulinum neurotoxin type E, but types A (24%), B (16%), F (3%), and AB (1%) also occurred; 3% were caused by undetermined serotypes. Four outbreaks resulted from commercial products, including a 2006 international outbreak caused by carrot juice. Hospital data indicated that 78% of patients were transferred to special care units and 70% required mechanical ventilation; 7 deaths were reported. Botulinum neurotoxin type A was associated with much longer hospital stays and more time spent in special care than types B or E. Foodborne botulism often is misdiagnosed. Increased clinician awareness can improve diagnosis, which can aid epidemiologic investigations and patient treatment.
Corynebacterium ulcerans is a closely related bacterium to the diphtheria bacterium C. diphtheriae , and some C. ulcerans strains produce toxins that are similar to diphtheria toxin. C. ulcerans is widely distributed in the environment and is considered one of the most harmful pathogens to livestock and wildlife. Infection with C. ulcerans can cause respiratory or nonrespiratory symptoms in patients. Recently, the microorganism has been increasingly recognized as an emerging zoonotic agent of diphtheria-like illness in Japan. To clarify the overall clinical characteristics, treatment-related factors, and outcomes of C. ulcerans infection, we analyzed 34 cases of C. ulcerans that occurred in Japan during 2001–2020. During 2010–2020, the incidence rate of C. ulcerans infection increased markedly, and the overall mortality rate was 5.9%. It is recommended that adults be vaccinated with diphtheria toxoid vaccine to prevent the spread of this infection.
Mycolicibacterium neoaurum is a rapidly growing mycobacterium and an emerging cause of human infections. M. neoaurum infections are uncommon but likely underreported, and our understanding of the disease spectrum and optimum management is incomplete. We summarize demographic and clinical characteristics of a case of catheter-related M. neoaurum bacteremia in a child with leukemia and those of 36 previously reported episodes of M. neoaurum infection. Most infections occurred in young to middle-aged adults with serious underlying medical conditions and commonly involved medical devices. Overall, infections were not associated with severe illness or death. In contrast to other mycobacteria species, M. neoaurum was generally susceptible to multiple antimicrobial drugs and responded promptly to treatment, and infections were associated with good outcomes after relatively short therapy duration and device removal. Delays in identification and susceptibility testing were common. We recommend using combination antimicrobial drug therapy and removal of infected devices to eradicate infection.
We retrospectively reviewed consecutive cases of mucormycosis reported from a tertiary-care center in India to determine the clinical and mycologic characteristics of emerging Rhizopus homothallicus fungus. The objectives were ascertaining the proportion of R. homothallicus infection and the 30-day mortality rate in rhino-orbital mucormycosis attributable to R. homothallicus compared with R. arrhizus. R. homothallicus accounted for 43 (6.8%) of the 631 cases of mucormycosis. R. homothallicus infection was independently associated with better survival (odds ratio [OR] 0.08 [95% CI 0.02–0.36]; p = 0.001) than for R. arrhizus infection (4/41 [9.8%] vs. 104/266 [39.1%]) after adjusting for age, intracranial involvement, and surgery. We also performed antifungal-susceptibility testing, which indicated a low range of MICs for R. homothallicus against the commonly used antifungals (amphotericin B [0.03–16], itraconazole [0.03–16], posaconazole [0.03–8], and isavuconazole [0.03–16]). 18S gene sequencing and amplified length polymorphism analysis revealed distinct clustering of R. homothallicus .
Zoonotic outbreaks of sporotrichosis are increasing in Brazil. We examined and described the emergence of cat-transmitted sporotrichosis (CTS) caused by the fungal pathogen Sporothrix brasiliensis . We calculated incidence and mapped geographic distribution of cases in Curitiba, Brazil, by reviewing medical records from 216 sporotrichosis cases diagnosed during 2011–May 2022. Proven sporotrichosis was established in 84 (39%) patients and probable sporotrichosis in 132 (61%). Incidence increased from 0.3 cases/100,000 outpatient visit-years in 2011 to 21.4 cases/100,000 outpatient visit-years in 2021; of the 216 cases, 58% (n = 126) were diagnosed during 2019–2021. The main clinical form of sporotrichosis was lymphocutaneous (63%), followed by localized cutaneous (24%), ocular (10%), multisite infections (3%), and cutaneous disseminated (<0.5%). Since the first report of CTS in Curitiba in 2011, sporotrichosis has increased substantially, indicating continuous disease transmission. Clinician and public awareness of CTS and efforts to prevent transmission are needed.
Babesiosis is a globally distributed parasitic infection caused by intraerythrocytic protozoa. The full spectrum of neurologic symptoms, the underlying neuropathophysiology, and neurologic risk factors are poorly understood. Our study sought to describe the type and frequency of neurologic complications of babesiosis in a group of hospitalized patients and assess risk factors that might predispose patients to neurologic complications. We reviewed medical records of adult patients who were admitted to Yale-New Haven Hospital, New Haven, Connecticut, USA, during January 2011–October 2021 with laboratory-confirmed babesiosis. More than half of the 163 patients experienced > 1 neurologic symptoms during their hospital admissions. The most frequent symptoms were headache, confusion/delirium, and impaired consciousness. Neurologic symptoms were associated with high-grade parasitemia, renal failure, and history of diabetes mellitus. Clinicians working in endemic areas should recognize the range of symptoms associated with babesiosis, including neurologic.
Tularemia is a zoonotic infection caused by Francisella tularensis . Its most typical manifestations in humans are ulceroglandular and glandular; infections in prosthetic joints are rare. We report 3 cases of F. tularensis subspecies holarctica –related prosthetic joint infection that occurred in France during 2016–2019. We also reviewed relevant literature and found only 5 other cases of Francisella -related prosthetic joint infections worldwide, which we summarized. Among those 8 patients, clinical symptoms appeared 7 days to 19 years after the joint placement and were nonspecific to tularemia. Although positive cultures are typically obtained in only 10% of tularemia cases, strains grew in all 8 of the patients. F. tularensis was initially identified in 2 patients by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; molecular methods were used for 6 patients. Surgical treatment in conjunction with long-term antimicrobial treatment resulted in favorable outcomes; no relapses were seen after 6 months of follow-up.

Search for articles with a simple interface. Powered by PubMed.

Search for articles by multiple keywords or authors. Results are searchable and sortable. Powered by PubMed.
Find articles by topic country with the article map. Results are searchable and sortable.

Find articles on specific topics such as Ebola, Zika, and Influenza.

Browse articles by type, such as research, letters, or dispatches.

You can find all of EID’s contents on PubMed Central.
What's New

Highly Pathogenic Avian Influenza A(H5N1) Virus Clade 2.3.4.4b Infections in Wild Terrestrial Mammals, United States, 2022 — (Length: 30:05)
Length: 30:05
To receive email updates about this page, enter your email address:
- audio icon EID Podcasts
- twitter icon EID on Twitter
- linkedin icon EID on LinkedIn
- insta icon EID on Instagram
- fb icon EID on Facebook

- rss icon Subscribe to RSS Feeds
- book icon PubMed Central external icon
- Morbidity and Mortality Weekly Report (MMWR)
- Preventing Chronic Disease Journal
- Public Health Image Library (PHIL)
- Science Briefs
- Vital Signs
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Article Quick Search
By continuing to browse the site you are agreeing to our use of cookies and similar tracking technologies described in our privacy policy .
Resource Library
The AAEP develops numerous resources to assist veterinarians and the broader equine industry with issues affecting the horse and connection to those with common goals.
Search & Filter
Resource Type
Audience Type
Snake Bite Vaccination Guidelines
Tetanus Vaccination Guidelines
Tetanus toxoid is a core equine vaccine and should be included in equine immunization programs for every horse annually.
Venezuelan Equine Encephalomyelitis Vaccination Guidelines
Equine Viral Arteritis (EVA) Vaccination Guidelines
Equine Influenza Vaccination Guidelines
West Nile Virus Vaccination Guidelines
- Next »
Join Us Today!
Our community of horse doctors connects you to more than 9,000 veterinarians and veterinary students who make a difference every day in horse health, just like you!


IMAGES
VIDEO
COMMENTS
Fred Hutch researchers have developed a gene therapy approach that can destroy up to 95% of herpes virus in infected nerve cells of mice. The method involves using multiple gene-cutting enzymes and carrier viruses to target and disable the virus genes.
Learn about new drugs and microbicides that may prevent or treat genital herpes, a common sexually transmitted infection. Find out how to join clinical trials and access experimental products.
Learn about the latest developments and challenges of herpes vaccine candidates based on different technologies and clinical trials. Find out the latest news, updates, and resources from the NIH, WHO, and other global partners.
In response to the persistent health challenges of herpes simplex virus 1 (HSV-1) and HSV-2, today the National Institutes of Health released the Strategic Plan for Herpes Simplex Virus Research. An NIH-wide HSV Working Group developed the plan, informed by feedback from more than 100 representatives of the research and advocacy communities and interested public stakeholders.
Researchers at Fred Hutchinson Cancer Center report that an experimental gene therapy can reduce or eliminate viral shedding in mice with herpes simplex virus infections. The therapy uses enzymes to cut up viral genes in nerve clusters, but also raises concerns about potential toxicity.
BioNtech has dosed the first patient with its BNT163 herpes vaccine candidate designed to prevent genital lesions as part of a first-in-human Phase 1 clinical research study, the German vaccine ...
Moderna is developing a vaccine using mRNA technology to treat the herpes simplex virus (HSV). There are two HSV virus types — HSV-1, the one I have, that affects the mouth, face and genitals ...
Herpes virus articles from across Nature Portfolio. Herpesvirus is an infectious agent belonging to the virus family Herpesviridae that causes latent and lytic infections in a wide range of ...
Herpes simplex virus (HSV), known as herpes, is a common infection that can cause painful blisters or ulcers. It primarily spreads by skin-to-skin contact. ... WHO and partners are also supporting research to develop new strategies for prevention and control of HSV infections, such as vaccines and topical microbicides. Related.
Herpes news. Read the latest research on the herpes virus, including new treatment options. ... A research team has now introduced a completely new approach for treating herpes. Their method is ...
A genetically edited form of a herpes simplex virus has outperformed a leading vaccine candidate in a new study. When challenged with a virulent strain of the sexually transmitted HSV-2 ...
According to the research, a genetically modified form of herpes simplex virus type 1 was able to prevent symptoms of herpes simplex virus type 2 in guinea pigs. The response was far more robust than seen with any herpes vaccine study to date. It significantly slowed the virus's replication and showed less viral shedding.
Research has also uncovered potential links between HSV-1 and Alzheimer's disease—the virus has been linked to amyloid plaque-like formations in human brain-like tissue. More research will be needed to see if herpes is causing the neurodegenerative disease, says Iwasaki. But this will require more investment in studying the infection.
In 2021, Something Positive for Positive People conducted a survey of over 1,000 people diagnosed with herpes; around 66 percent said a partner had consented to sex without a condom or other ...
Herpes simplex virus (HSV) infections last a lifetime. Once a person has been infected, the virus can remain dormant (latent) for years before periodically reactivating to cause recurrent disease. ... the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services ...
Herpes is a lifelong infection that can cause oral or genital sores. Learn why there is no cure, how to prevent it, and what clinical trials are investigating potential vaccines and drugs.
Herpes simplex research includes all medical research that attempts to prevent, treat, or cure herpes, as well as fundamental research about the nature of herpes.Examples of particular herpes research include drug development, vaccines and genome editing.HSV-1 and HSV-2 are commonly thought of as oral and genital herpes respectively, but other members in the herpes family include chickenpox ...
One point I want to make is that the research is very much focused on herpes simplex viruses 1 and 2 right now. Our paper studied herpes simplex virus 1 because of the experimental model we used.
Herpes sores usually appear as one or more blisters on or around the genitals, rectum or mouth. This is known as having an "outbreak". The blisters break and leave painful sores that may take a week or more to heal. Flu-like symptoms (e.g., fever, body aches, or swollen glands) also may occur during the first outbreak.
The Search for a Herpes Vaccine. The need for a herpes vaccine is clear: about half a billion people worldwide between the ages of 15-49 have genital herpes infection caused by either HSV-1 or HSV-2, according to the World Health Organization (WHO). In the United States alone, an estimated 1 in 6 adults have genital herpes, with around 300,000 ...
Genital herpes is a common sexually transmitted infection (STI). The herpes simplex virus (HSV) causes genital herpes. Genital herpes can often be spread by skin-to-skin contact during sexual activity. Some people infected with the virus may have very mild symptoms or no symptoms. They can still able to spread the virus.
In this research, the obtained molecular docking score exhibited better efficiency against the capsid protein of Herpes Simplex Virus-1 and hence we conducted further in silico investigation ...
Herpes simplex virus (HSV) has been implicated in the etiology of recurrent aphthous ulcers, cold sores, and genital sores.To summarize the research evidence for use of L-lysine to prevent HSV disease recurrence, a use not widely recognized by doctors.Two ...
To inform surveillance, prevention, and management strategies for the varicella zoster virus (VZV) during the COVID-19 pandemic, this study aimed to evaluate the risk of herpes zoster (HZ) occurrence/recurrence following COVID-19 infection and vaccination.
HSV1 usually causes sores around the mouth, while HSV2 causes genital herpes. The infection is spread by skin-to-skin contact, and it can be transmitted through oral, vaginal, and anal sex, and kissing. It can also be spread through contact with lesions from other areas of the body.
Emerging Infectious Diseases is a peer-reviewed, monthly journal published by the Centers for Disease Control and Prevention (CDC). It offers global health professionals the latest scientific information on emerging infectious diseases and trends. Articles provide the most up-to-date information on infectious diseases and their effects on global health.
The AAEP develops numerous resources to assist veterinarians and the broader equine industry with issues affecting the horse and connection to those with common goals. Home. Guidelines & Resources. Resource Library.
Ribonuclease P (RNase P) complexed with an external guide sequence (EGS) represents a promising nucleic acid-based gene targeting approach for gene expression knock-down and modulation. The RNase P-EGS strategy is unique as an EGS can be designed to basepair any mRNA sequence and recruit intracellular RNase P for hydrolysis of the target mRNA. In this study, we provide the first direct ...