

Top 17 Clinical Research Organizations (CRO) in 2023
In clinical research and treatment development, clinical research organizations (CROs) are frequently a sponsor’s most important partner and ally.
Depending on the nature of the clinical trial, and your existing capabilities as a sponsor to run the trial, the CRO company of your choice will typically be responsible for facilitating most of the micro and macro processes that go into designing and running a successful clinical trial.
When contracting a CRO to help you with your trial, you are transferring over a large portion of responsibility into the hands of your clinical research partner. The CRO of your choice will have the responsibility to control a variety of factors and processes of a clinical trial, and depending on their expertise, team structures, service offerings, internal resources and many other capabilities.
Your ability to find and contract a top CRO company that is the right fit for your unique trial will be a determinant of whether or not you will be able to operate a high-quality clinical trial that meets your expected timelines, budget and delivers a top-notch patient experience.
At ClaraHealth (a patient-centric recruitment acceleration platform) , we have put together an extensive list of the top CRO companies in the US and around the world.
This is not a cro rankings list, but rather a compiled list of some of the top clinical research organizations around the world. We have highlighted their strengths and core service offerings to make it easier for you to find the right fit clinical research partner.
In addition, we’ve put together a list of 9 fundamental questions to ask the prospective clinical research organization , which will help you to save time and ensure a right fit in picking the CRO.
Formerly known as Quintiles and IMS Health, IQVIA is one of the largest CROs in the world, with a large range of service offerings to help advance clinical research.
The company was founded in North Carolina in 1982, and has since grown to over 88,000 employees in more than 100 countries.
Some clinical trial solutions offered by IQVIA include:
- Assistance with protocol design
- Design of phase 1 clinical trials
- Assessment and improvement of phase 2 and 3 clinical trials
- Site identification & selection
- Patient recruitment
- Access to global laboratories via their wholly owned subsidiary Q2 Solutions
Parexel is a global clinical research organization that was founded in 1982, and specializes in conducting clinical studies on behalf of its pharmaceutical partners in order to accelerate and ensure the drug approval process of up-and-coming potential treatments. It currently operates in more than 50 countries, and is run by more than 18,000 employees around the world.
The company has a wide range of service offerings, covering nearly every type of clinical trial service to assist sponsors in running successful clinical studies.
Some clinical trial solutions offered by Parexel include:
- Clinical trial design and development for early phase, phase 2 & 3, and late phase clinical trials
- Clinical data management
- Decentralized clinical trials
- Clinical supply chain management
- Medical writing
- Regulatory affairs consulting
- Pharmacovigilance
3. PRA Health Sciences
PRA Health Sciences is one of the largest contract research organizations in the world. Founded in 1976 under the name “Anti-Inflammatory Drug Study Group”, the company was renamed to PRA in 1982. PRA Health Sciences employees more than 17,000 people, and provides coverage to more than 90 countries.
In 2021, PRA Health Sciences was acquired by the Ireland-headquartered global CRO leader ICON, which is also reviewed in this list.
Some clinical trial solutions offered by PRA Health Sciences include:
- Decentralized Clinical Trials Platform
- Protocol Consultation & Study Design
- Onsite Support services
- Customized Solutions for Biotech (such as asset valuation, regulatory strategy, engagement and support, drug development strategy and funding solutions)
- Clinical Diagnostics
- Site Commercial Solutions
- PRA’s Laboratories for Drug Development
Headquartered in Ireland, ICON was founded in 1990 in Dublin by co-founders John Climax and Ronan Lambre. The company has since grown to be one of the largest CROs in the world. As of September 2020, the company employs more than 15,000 people in 94 locations and across 40 countries.
ICON offers clinical research services which include consulting, clinical development and commercialization across a wide range of therapeutic areas.
In 2021, ICON acquired PRA Health Sciences, which is another CRO and global leader in clinical research services.
Some clinical trial solutions offered by ICON:
- Commercial Positioning
- Early Phase
- Functional Services Provision
- Laboratories
- Language Services
- Medical Imaging
- Real World Intelligence
- Site & Patient Solutions
- COVID-19 Clinical Operations
5. Syneos Health
Formerly known as InVentiv Health Incorporated and INC Research, Syneos Health is a publicly listed and global contract research organization. The company is based in Morrisville, North Carolina, and specializes in assisting companies with late-stage clinical trials. Syneos Health currently employs more than 25,000 people, and has offices across 91 locations.
In early 2018, INC Research was acquired inVentiv Health, and the merged company was named Syneos Health.
Some clinical trial solutions offered by Syneos Health include:
- Decentralized Clinical Trials Solutions
- Bioanalytical Solutions
- Phase II-III/Phase IIIb-IIIV
- Medical Device Diagnostics
- Clinical Data Management
- Clinical Project Management
- Clinical Monitoring
- Drug Safety & Pharmacovigilance
- Site and Patient Access
6. Labcorp Drug Development (Formerly Covance)
Formerly known as Covance and renamed to Labcorp Drug Development in early 2021, this CRO is one of the largest contract research organizations in the world. The company claims to provide the world’s largest central laboratory network, and has been rated as one of the best places to work for LGBTQ+ equality by the Human Rights Campaign organization in 2018 to 2021. Currently, Labcorp employs over 70,000 people and is able to support clinical research efforts in almost 100 countries around the world.
Some clinical trial solutions offered by Labcorp Drug Development include:
- Preclinical Services
- Clinical Trials
- Clinical Trial Laboratory Services
- Post-Marketing Solutions
- Medical Devices
- Data & Technology
Also known as Pharmaceutical Product Development, PPD is a large global contract research organization headquartered in Wilmington, North Carolina. Started as a one-person consulting firm in 1985, PPD has grown to over 27,000 employees worldwide, and provides a wide range of clinical research services to pharmaceutical and biotech companies.
Some clinical trial solutions offered by PPD include:
- Clinical Development
- Early Development
- Peri- and Post-Approval
- PPD Biotech
- PPD Laboratories
- Product Development and Consulting
- Site and Patient Centric Solutions
8. Fisher Clinical Services
Part of Thermo Fisher Scientific, Fisher Clinical Services is a global clinical research organization with headquarters in Center Valley, Philadelphia.
The company has been in the business of clinical supply chain management for over 20 years, and is focused exclusively on working with the packaging and distribution requirements of clinical trials across the globe.
Some clinical trial solutions offered by Fisher Clinical Services include:
- Biologistics Management
- Cell & Gene Therapy
- Clinical Ancillary Management
- Clinical Label Services
- Clinical Trial Packaging & Storage
- Clinical Supply Optimization Services
- Cold Chain Management & Expertise
- Direct-to-Patient
- Distribution & Logistics
- Strategic Comparator Sourcing
- Public Health Research
Established in 1997 under the name Kiecana Clinical Research, KCR is a full-service contract research organization that provides a variety of services for clinical monitoring, safety & pharmacovigilance, clinical project management, quality assurance and regulatory affairs.
KCR operates globally, and has offices in North America, Western Europe, Central Europe and Eartern Europe. The company currently employs more than 700 staff.
Some clinical trial solutions offered by KCR include:
- Trial Execution
10. Medpace
Founded in 1992 and based in Cincinnati, Ohio, Medpace is a midsize clinical contract research organization. The company has operations in over 45 countries, and employs over 2,800 people. Medpace provides support services for Phase I-IV clinical trials for pharmaceutical and biotechnology companies, which include central laboratory services and regulatory services.
Some clinical trial solutions offered by Medpace include:
- Biostatistics and Data Sciences
- Clinical Trial Management
- Drug Safety and Pharmacovigilance
- Medical Writing
- Quality Assurance
- Regulatory Affairs
- Risk-Based Monitoring
- Medpace Laboratories
11. Clintec
Now in business for over 22 years, Clintec is a medium-sized global contract research organization for pharmaceutical, biotech and medical device industries, with large expertise in oncology and rare diseases.
The company provides the flexibility and agility of a smaller-sized CRO, while also having a wide global coverage that large CRO companies are known for. Clintec is based in more than 50 countries, and was acquired by the leading global CRO IQVIA in late 2018.
Some clinical trial solutions offered by Clintec include:
- Project Management
- Data Management
- Biostatistics
- Global Feasibilities
- Patient Recruitment & Retention
12. Worldwide Clinical Trials
Bringing over 30 years of experience to the clinical research market, Worldwide Clinical Trials is a leading medium-sized global contract research organization. Founded by physicians with a dedication and commitment to advancing medical research, Worldwide Clinical Trials was the first customer-centric CRO.
Currently the company has coverage in more than 60 countries, and has extensive experience in a wide range of therapeutic areas, including central nervous system, metabolic, cardiovascular, oncology, rare diseases and general medicine.
Some clinical trial solutions offered by Worldwide Clinical Trials include:
- Bioanalytical Lab
- Early Phase Development
- Clinical Phase IIB-II Clinical Trials
- Phase IIIB-IV Clinical Trials
- Trial Management Technologies
Named #1 CRO in the world for operational excellence at the 2021 CRO Leadership Awards, CTI Clinical Trial And Consulting Services is a medium-sized global contract research organization that has been serving pharmaceutical companies since 1999.
Based in Covington, Kentucky, CTI has offices around the world in more than 60 countries, with coverage in North America, Europe, Latin America, Middle-East, Africa, and Asia-Pacific regions.
Some clinical trial solutions offered by CTI include:
- Feasibility
- Regulatory Affairs Study Start-Up
- Medical Monitoring
- Safety & Pharmacovigilance
- Clinical Services
14. Wuxi AppTec
Founded in 2000 as WuXi PharmaTech in the city of Wuxi, China, Wuxi AppTec has grown from a single laboratory into a leading global contract research organization with more than 28,000 employees, including 23,000 scientists and more than 30 research & development and manufacturing sites around the world.
With offices in Asia, U.S, Europe and the Middle East, the company is able to provide coverage to more than 30 countries around the world.
Some clinical trial solutions offered by Wuxi AppTec include:
- Small Molecule Drug R&D and Manufacturing
- Cell Therapy and Gene Therapy
- Drug R&D and Medical Device Testing
- Clinical Services (Phase I-IV)
15. Advanced Clinical
Founded in 1994 and based out of Deerfield, Illinois, Advanced Clinical is a midsize and full-service CRO that helps sponsors with running clinical trials. The company employs more than 700 staff, and offers a wide variety of services across many therapeutic areas. Advanced Clinical has global representation in over 50 countries around the world.
Some clinical trial solutions offered by Advanced Clinical include:
- eTMF & Document Management
- Global Medical Services
- Quality & Validation
16. Pharm-Olam
Pharm-Olam is a leading midsize CRO with global headquarters located in Houston, Texas and its European headquarters in Bracknell, United Kingdom. The company employs more than 800 staff, and has 25 offices around the world, with a global coverage in more than 60 countries.
The company has therapeutic expertise in 5 areas, including Rare & Orphan Disease, Infectious Disease & Vaccine, Oncology-Hematology, Allergy and Autoimmune.
Some clinical trial solutions offered by Pharm-Olam include:
- Study Feasibility
- Site Activation
- Patient Recruitment
- Medical Affairs
- Compliance & Training
- Clinical Monitoring & Operations
17. Clinipace
Founded in 2003 and based out of Morrisville, North Carolina, Clinipace is a global midsize full-service CRO with a focus on solution customization for clinical trials. The company has a large global coverage in more than 50 countries, and has offices in North America, South America, Europe and Asia-Pacific regions.
Clinipace’s therapeutic focus areas include Oncology, Nephrology and Urology, Rare Disease, Gastroenterology and Women’s Health. The company also has complete therapeutic expertise in Infectious Disease & Vaccines, Cardiology, CNS, Immunology, and Respiratory.
Some clinical trial solutions offered by Clinipace include:
- Clinical Analytics
- Clinical Technology and Ecosystem
- Functional Service Partnership (FSP)
- Regulatory & Strategic Product Development
9 Fundamental Questions To Ask A Top CRO Company Before Signing The Contract
1. which services does the cro provide.
CROs offload a lot of operational tasks from trial sponsors, which can touch any component of clinical trial operations. From formulating an overall study strategy and implementing technologies to support the operational processes of the trial, to picking and identifying sites, and supporting patients during the trial, the range of clinical services offered by a CRO tends to be vast and inclusive of all the typical services and support you will require for running a successful clinical trial.
However, not all CROs are the same in their service offerings, or are able to offer the same depth of capability within a seemingly same clinical trial support process. For this reason it is important to understand exactly which kind of clinical services and support you are looking to receive from the prospective CRO when running your clinical trial.
While services such as clinical monitoring and clinical trial management are offered by the majority of CROs, the specific needs of each trial are unique, and for this reason it is important to first identify what will be the unique services your trial requires. Completing this internal analysis first will help you to understand the extent to which a potential CRO partner will be able to provide all of these services.
Some CROs specialize in specific clinical trial functions which the company may label as a “core services”, in which case this is a sign the company will have more expertise, experience, and will be set up in a way to maximize their capabilities in providing support for these services compared to other services that the CRO offers.
For example, a CRO may include patient recruitment as part of its “core services”, which implies that they are highly skilled in and have the necessary infrastructure to design and implement a high-quality patient recruitment strategy.
Clara Health CRO Support Services: At Clara Health our specialty services include technology-augmented digital and patient advocacy recruitment, as well as patient support via our signature patient recruitment platform, which we use to upgrade clinical trials and deliver results sponsors look for in their recruitment and retention campaigns.
At Clara, we work alongside CROs to supplement and support clinical trials with modern and personalized capabilities that CROs do not typically have the bandwidth, corporate structure or infrastructure to support.
If you would like to learn more about exactly how our platform can upgrade your unique trial, feel free to book a Free 30 Minute Consultation Session Here with one of our in-house experts.
2. What Related Experience Does The CRO Have?
It is helpful to ask the prospective CRO company if they have any relevant experience in running clinical trials that would be an asset in designing and running your study. Previous experience in a related therapeutic area or in running a trial with a similar design allows CROs to have a deeper understanding into potential opportunities and challenges, increasing the likelihood of your clinical study being successful.
For example, if a sponsor is planning to run a trial in oncology, for the purpose of site identification and selection it would be valuable to partner with a CRO vendor that has expertise in this area, as they likely already have a good understanding of which sites will lead to optimal results.
However, it is also important to consider all factors when selecting a CRO vendor and not to rely on therapeutic experience as the sole qualifier for whether or not a potential CRO is a fit for your trial. While previous experience is beneficial, some sponsors close themselves off from working with vendors that have not worked in their therapeutic area, which significantly limits options when choosing a CRO partner that is truly a good fit for their clinical study.
This can impact the end result of your clinical study, as sponsors that are not successful in choosing a CRO vendor that is the right overall fit may face difficulties if the needs of their clinical study aren’t being properly met.
Clara Health: We have worked to provide support for clinical trials across a wide range of therapeutic areas and trial designs. Our specialty is filling in the gaps that CROs traditionally did not have to think about, which include digital patient recruitment, patient advocacy recruitment, and technology-augmented patient support.
Additionally, we are constantly building our proprietary data and running tests in a variety of therapeutic areas. These research efforts allow us to have a detailed understanding of the expected level of difficulty when recruiting particular patient populations, as well as allow us to predict with accuracy which segments of the targeted population will be likely to qualify in a particular study.
3. What Are The Communication Workflows & Expectations For Performing And Delivering Contracted Services?
It is important that you clarify what the expectations for communication will be between your prospective CRO vendor and your internal teams, as you will most likely be working with the CRO of your choice for the entire duration of your clinical trial.
There are a vast variety of factors and success determinants for a clinical trial, which are continuously undergoing change as the study unfolds. For this reason, it is recommended that you work with a CRO that is proactive in their communication, so that you are kept up to date with information about important changes as your clinical trial progresses.
A vendor that is proactive rather than reactive in their communication and approach to dealing with arising issues is one of the most important qualities in CRO. Challenging situations will naturally arise, and the promptness with which they are taken care of will significantly impact your clinical trial’s degree of success. Therefore, seeking a vendor that is able to match the standard of communication that you as a sponsor would like to experience throughout the duration of your partnership is one of the most critical steps in determining which CRO is the right fit for your clinical trial.
We’ve included a few additional questions pertaining to the communication structure and reporting expectations that you can ask a prospective CRO vendor to determine the degree of fit in this particular category:
Communication Expectations:
- If we were to move forward with you, which of your team members will be our main point of contact?
- How available will you be outside of the scheduled meetings to address any of our concerns or additional requests?
- What will be the frequency at which update meetings will be conducted, and who will be present at those meetings?
- Which clinical study processes will be reported on, and what will be the workflow for how we will receive this information?
- What will be the cadence at which we will receive progress reports?
- Would we be able to access metrics electronically via an interactive dashboard, or will you send us formal reports?
Clara Health: At Clara Health, we directly interact and actively work with several key stakeholders involved in running a clinical trial, which includes sponsors, CROs, sites, and patients. This unique position allows us to have a centralized perspective which helps us to see all the moving parts of a clinical trial at the same time, which helps to identify issues and relay this vital information and insight back to the sponsor (or other appropriate stakeholders) in the shortest time possible.
The ability to access this perspective allows us to gather the most accurate, complete, and up-to-date information about how the clinical trial is unfolding, and quickly becomes very valuable to sponsors for their clinical trial.
As an example, we may receive feedback from patients about having an unsatisfactory experience with a particular study site. We are able to aggregate and analyze this information, and relay our findings back to the sponsor and the study site to improve the experience for other patients.
4. What Is The CRO’s Client Satisfaction Record?
It is a good practice to request information or metrics from the prospective CRO vendor that can point to the degree of satisfaction of their past clients. Prior to signing the contract, vendors will naturally do their best to uplift their image and future value to you during their sales conversations with you and your team. It can be tricky to get an objective understanding of what the partnership experience will actually entail, especially when there are multiple vendors fighting for your commitment.
We recommend that you ask the prospective vendor to provide success metrics regarding areas of clinical trial operations that are going to be important for your trial.
For example, you may be interested in learning about the vendor’s relationship to finances, in which case it will be useful to ask them about situations in which they went over the planned budget, and investigate into the reasons behind that. Alternatively you may be concerned about potential delays in timelines, in which case it would be helpful to learn about metrics regarding the CRO’s ability to meet timeline expectations.
You may also request to talk to the prospective CRO’s past clients, which will help you to gain insight into what the relationship was like and give you the opportunity to examine if the way in which the particular CRO manages its relationships and performs its services meets the expectations that you would have for your potential relationship and for your clinical trial.
Clara Health: At Clara Health, our relationships with our partners and with our patients are most important to us. In the unique position where we fit in the clinical trial process, we have the opportunity to directly co-create the clinical trial patient experience with a variety of stakeholders, including sponsors, sites, CROs, and patients.
Our company’s values and culture have been directed and developed to be such that the client and patient experience is at the top of priority for all of our internal teams, and we work to provide the best quality of care to all stakeholders.
We have many testimonials from every type of partner we’ve worked with which we can happily share with you.
5. How Do You Adapt When Encountering Challenges With Running A Clinical Trial?
It is inevitable that challenges and unforeseen changes will arise throughout the operational clinical trial process, and for this reason it is important to work with a CRO vendor that can provide you with evidence of their flexibility and ability to adapt to sudden changes.
The ideal CRO partner is one that is highly consultative throughout the entire process, and has an ability and the initiative to deal with challenges at their seed stage, prior to them turning into major obstacles for the success of your trial.
CROs naturally have a large reach, and there are a lot of different clinical trial mechanisms and processes that are under their control. They are able to monitor and respond to what is going on in every key link in the chain of the clinical trial operation.
It is reasonable to expect this level of oversight from a CRO, and additional questions that can help you gain insight into this include:
- What are some examples where the CRO was effective at monitoring the health of clinical trials they’ve helped operate in the past?
- How quickly does the CRO respond to challenges or opportunities for improving the clinical trial experience?
- How well does the CRO gather & process information from study sites, study teams, patients & the sponsor, and what are their typical data analysis workflows?
It is also recommended to speak to the prospective CROs past clients to help you gain insight into how well they respond and adapt to the naturally arising challenges in clinical trials.
Clara Health: While CROs do have a large reach within the clinical trial, no CRO has complete visibility into every clinical process. They are not typically set up to support full visibility, which can manifest as a potential threat to your clinical trial as it unfolds. This is especially true for parts of the clinical trial processes that CROs naturally do not specialize and often subcontract, such as clinical trial recruitment.
At Clara, we are in a unique position in relation to other key partners involved in operating the clinical trial. We are in direct and frequent contact with patients, CROs, study sites, study teams, and the sponsor, and have a very deep understanding of the patient pipeline. This allows us the unique ability to go very deep into specific parts of the recruitment chain and investigate what is working and what is not working.
In addition, Clara functions as a resource for all partners in the clinical trial. For example, we work directly with site teams to ensure that they have access to a 3rd party that they can relay their needs to and receive fast support in case there is anything they require that can improve the patient recruitment process.
6. Which Parts Of Operating The Clinical Trial Will You Be Outsourcing?
Since there are so many processes and mechanisms that go into operating a clinical trial, CROs will always outsource some parts of running and managing the study. While you can expect that the prospective CRO will subcontract some of the work, it is important to find out which exact parts the clinical study will be outsourced.
There are certain basic and key clinical processes (such as site selection) that CROs almost always help with, and if you find that these parts of your trial are going to be subcontracted to another company, it is recommended to find out why the CROs operations are set up this way and how this would impact the service you will receive.
Ultimately what matters to you as a partner and client is that the quality of service and care that you will receive will be up to standard, and meet what was promised and what you are expecting. While this trust is important after you have signed the contract, it is recommended that prior to entering into such a significant commitment that you have evidence and the conviction that the CRO of your choice is truly the right fit and will deliver the quality of service that was being discussed.
Since it is impossible to predict exactly what the quality of this relationship and services performed will actually be like in practice, it is recommended that you understand the details of what will be done for your trial and how. Investigating how the CRO outsources and subcontracts services for a clinical trial will help you to gain necessary insight that you would need to make the correct vendor selection decision.
Clara Health: At Clara, we maximize the effectiveness of the digital component across the entire digital & recruitment spectrum, which is added on top of the existing capabilities of the CROs and other vendors involved in operating your clinical trial. In addition, we offer services that augment the CROs efforts, which has the potential to significantly improve the patient experience, operations flows, recruitment and retention performance, which is so important in ensuring the success of a clinical trial.
For example, if a CRO wants to have a great site relationship, we are able to come in as a third party on behalf of the sponsor and CRO and act as a resource and additional support for sites.
In another example, If a sponsor wants to have great relationships with the patient community, Clara is able to come in on behalf of the sponsor and develop these relationships while being perceived more neutrally by the patient community.
7. Do You Have Experience Running International Trials?
If you are planning on operating an international clinical trial, it is recommended to work with a CRO that has extensive experience in this area. While many CROs will offer near-global coverage, the level of experience with specific geographic locations can significantly vary from one vendor to another.
It is important to work with a CRO that has experience running clinical trials in the specific countries and regions you are planning to conduct your research in. Being compliant with the local rules and regulations for clinical testing is a very complex process that requires existing understanding and familiarity in order to ensure logistical smoothness and to mitigate legal risks. In operating a clinical trial, there are a multitude of clinical services and processes, which can greatly vary across the many regions in which you can conduct clinical testing.
A CRO that is lacking experience in operating international trials or operating in particular regions where you plan on conducting research may not be able to meet your desired quality and agility expectations, and therefore may not be the right fit for your international clinical trial.
Clara Health: In the past, we have provided international patient recruitment and digitally-augmented trial support services for clinical trials in the EU, Canada, UK, Australia and South America.
Clara Health is fully compliant to operate international studies everywhere in the world, with the exception of Russia and China.
8. What Is Your Relationship With Patients?
Patient-centric approach to designing and operating a clinical trial is becoming more and more crucial in the clinical research space. The ability of a sponsor and their CRO partner to understand the needs and characteristics of their target patient community is a significant determinant of whether or not the study will be a success.
A sponsor that has close and authentic relationships with the patient community tends to have a deeper understanding of how to create the best clinical trial experience that will attract patients and keep their interest throughout the clinical trial.
In addition, strong relationships with patients allow sponsors and CROs to forecast recruitment and patient retention pipeline with much higher accuracy. This ability is critical for ensuring the success of the trial and mitigating the risk of low enrollment. After an understanding of the patient population is acquired, sponsors gain the necessary insight to design a clinical trial that is not only favorable to their research results, but is also practical and will result in the enrollment numbers they are looking for.
While many CROs have already recognized the importance of patient-centricity and evolved the ways in which they design and operate clinical trials, other CROs have not yet made such a pivot in their values. It is important to understand the degree of importance the prospective CRO places on creating a favorable patient experience, and what kind of infrastructure the company has to support it.
At Clara, we recommend choosing a CRO partner that is adapting to the patient-centric model which is becoming more and more important for running a successful clinical trial.
Clara Health: Since early stages of our development, we’ve had a dedicated patient advocacy team that has been integral in shaping our company’s vision and operations. We have built our entire platform and recruitment infrastructure around creating the best experience for patients. Our teams, corporate values, service offerings and company infrastructure all work in the service of the patient.
In addition, over the many years of being in business we have heavily invested in building authentic patient community relationships that span across a variety of therapeutic areas. This has given us a unique ability to receive feedback directly from patients that is genuine and authentic around marketing materials, strategy for patient recruitment, and other services that we build for specific trials.
This ability to build partnerships with the patient community in an authentic way gives us a very unique ability to engage with the patient community on behalf of a pharmaceutical company, allowing our sponsor & CRO partners the opportunity to start conversations with patients through our in-house patient advocacy team.
If you would like to learn how Clara can help you to build a strong & authentic relationship with your target patient community, get in touch with us and we’d be happy to share our capabilities and previous results with you as they relate to your current or upcoming clinical trial.
9. How Is The CRO Going To Utilize Patient Input For Developing The Trial?
In the initial stages of clinical trial design, sponsors often determine the ideal patient profiles that would help them to drive the most favorable research outcomes for their study. While it is important for the success of your trial to determine who your ideal patients are, very often these projections do not match up with what is viable in practice.
At Clara, we often encounter study protocols that are not set up realistically for successful recruitment to be possible.
Common mistakes that are made when determining trial eligibility criteria and trial design include:
- Overestimating the interest in the clinical trial from the target patient population
- A lack of patient focus in the trial design
- A lack of convenience for patients in their participation
- Complicated and/or inefficient study experience flows
- Crafting the eligibility criteria around the patient population that is most likely to lead to favorable study outcomes, without conducting sufficient research to more accurately estimate the recruitment and retention difficulty of the group for a particular study
It is natural for there to be a “push & pull” between the research ideal and the real world practicality. It is important to determine the correct balance between these two sides for your trial, as going too far in either direction will decrease the chance of your clinical study’s success.
The nature of the industry as it is right now is such that there is excess research idealization and not enough emphasis on patient centricity. This distorted orientation has resulted in many clinical trials being unsuccessful, negatively impacting sponsors, patients and the entire clinical trials industry.
The ideal CRO partner should help you make sure that your protocol design sets your study up for success. The CRO should be able to help you determine the proper balance between the research ideal and the real world practicality, and back up their findings with sufficient research and patient data that can project your trial being a success.
Clara Health: When formulating a recruitment and retention plan for our clients, we begin with conducting thorough research into the target trial patient population. This allows us to get a clear understanding of which recruitment channels will yield the best results and what kind of marketing materials will resonate with the prospective study participants.
To ensure accuracy and real-world applicability of our research, we consult and collaborate with our internal patient advocacy and patient support teams, as well as with our clients and patients representing the target trial patient profiles. We then tie our findings back with any existing proprietary data that we have in connection with the therapeutic area or the prospective target patient group.
Our unique position within the clinical recruitment chain gives us the presence and deep-rooted access needed to effectively tap into any of the three patient traffic sources: digital recruitment, offline recruitment, or patient advocacy recruitment.
Once a recruitment campaign has gone live, we constantly monitor, analyze and optimize our performance to make sure that the processes we have in place are as efficient as possible and drive the greatest results. In addition, we have the capability to layer in any traditional advertising (such as billboard ads) if requested by the study sponsor.

Supporting clinical trials across border
Facilitating european clinical research.
ECRIN, the European Clinical Research Infrastructure Network, facilitates multinational clinical research, through the provision of advice and services for the set-up and management of investigator or SME led clinical studies in Europe. ECRIN unites national networks of clinical trial units across Europe, through its scientific partners, to fulfil its vision of generating scientific evidence to optimise medical practice.

Supporting clinical research
Clinical operations
Support for the management of multinational clinical trials, from project conception through to operational management
Data centre certification
Ensures compliant, effective, and efficient data management services for European, non-commercial data centres
Infrastructure Development
Develop and strengthen our capacity, tools, and services for the benefit of the clinical research community
Share expertise with the clinical research community.
Access & cost of services
Learn more about how to access our clinical operation services.
We provide our 13 Member and Observer countries with diverse trial support services and contribute to infrastructure development projects with European and international partners.
ECRIN Tools

Adaptive Platform Trial Toolbox
The Adaptive Platform Trial Toolbox aims to collect the accumulated knowledge, experience, and resources (collectively termed as ‘tools’) from multiple projects and trials into a practical and guided toolbox to facilitate planning and conduct of future adaptive platform trials in any therapeutic area.

Clinical Research Metadata Repository
The Clinical Research Metadata Repository is the free online tool to help scientific researchers find documents and data linked to a clinical research study, and to obtain information on the accessibility of those results.

Rare Diseases Clinical Trials Toolbox
The toolbox aims to collect the accumulated knowledge, experience, and resources generated by previous projects and/or research infrastructures and other organisations into a practical and guided toolbox to help understand the regulations and requirements for conducting trials, with special focus on investigator-initiated trials for rare diseases.

Paediatric Tools
A series of tools and procedures to support the setup and management of multinational neonatal and paediatric clinical trials in Europe were developed through the PedCRIN project.

Risk Based Monitoring Toolbox
The Risk-Based Monitoring Toolbox provides information on tools available for risk assessment, monitoring and study conduct, the institutions where they are used, and other relevant details such as links and user feedback.

Regulatory and Ethical Tools (CAMPUS)
ECRIN CAMPUS is a central resource for information about clinical trial regulatory and ethical requirements covering 22 European countries and multiple study types such as clinical drug trials, clinical investigations of medical devices, combination drug-device studies and nutritional studies.

CZECRIN celebrates its 10th anniversary

EOSC Future project comes to a close

Tools for EU clinical studies: paediatric and neonatal tools

EOSC-ENTRUST kick-off meeting

EU-X-CT Public Stakeholders’ Forum

12th European Conference on Rare Diseases & Orphan Products (ECRD 2024)

2nd EU-Africa PerMed Summer School

International Clinical Trials Day (ICTD) 2024: Data Centric Clinical Research

the EJP RD Final Conference
List of Clinical Research Organizations (CRO) for Clinical Trials in Europe (Part 1)
Patricio ledesma, contract research organization, 24 january, 2024.

Contact us at [email protected] if you need CRO services for clinical trials in Europe.
Here you can find a comprehensive list of small and medium-sized European contract research organizations (CROs) specialized in clinical trial management.
These are full service CROs focused on managing phase I-IV clinical trials, including services such as regulatory affairs, site management, monitoring, data management, pharmacovigilance, biostatistics, and medical writing, among others.
The CROs listed are located in EU member states or associated countries, which have been alphabetically ordered.
This list is structured in two parts. The first part of the list (presented in this article) contains the following countries: Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, and Greece.
The second part of the list can be seen in this link , and includes the following countries: Hungary, Ireland, Italy, Latvia, Lithuania, Netherlands, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, and Ukraine.
Clinical Investigation Support (CIS)
CIS is a completely independent, internationally operating contract research organisation. CIS offers consulting services in clinical research and development, in particular in the areas of quality assurance and regulatory affairs. CIS has a documented broad international experience in West and East Europe, but also in non-European countries.
J&P Medical Research
J&P Medical Research Ltd. offers the entire range of clinical research services for phases II-IV. Our interdisciplinary principal investigators and research staff have long-standing expertise in conducting clinical phase II and phase I-IV clinical studies. Our outstanding reputation stems from diligently focusing on planning and conducting scientific studies, pharmacokinetic and statistical analyses, clinical trial monitoring and the preparation of standardized medical documents.
pan Clinical Research Consulting
pan Clinical Research Consulting provides the following services: Clinical trial services and consulting (Phase I-IV), site identification / investigator recruitment, study setup (including investigator / hospital contract management), regulatory authority -and Ethics Committee- submissions, monitoring, project management and resourcing of Clinical Research Associates (CRAs).
Archer Research
Archer Research is a contract research organization dedicated to helping companies and healthcare professionals with their clinical investigations. Our expertise is strongly focused on the medical device industry. Archer Research is located in the heart of Europe with a fast connection between Belgium, France, The Netherlands and Germany.
DICE is a CRO specialized in clinical study design, biostatistics, clinical data management, medical monitoring, medical writing, and central imaging review. Our mission is to enhance access to medical treatment by delivering excellence in the design and execution of clinical trials.
Sillar Clinical
Sillar Clinical is specialized in assisting companies from clinical development strategy to the full setup and management of clinical trials. In parallel we offer GCP auditing services and vendor assessments.
4Clinics is a Contract Research Organization (CRO) providing data management, biostatistics, scientific writing, regulatory affairs and clinical operations services for clinical, observational and epidemiological studies with a particular expertise in vaccines, immunotherapeutics, cell and gene therapies with a dedicated branch for medical devices.
August Research
August Research is a CRO providing the following clinical trial services: study feasibilities and strategy consultation/country selection, regulatory and ethics submissions, site and investigator contract preparations/negotiations, all monitoring and study oversight (during start-up, initiation, maintenance, close-out), local drug and equipment management, project management, clinical study supplies provisioning, site/investigator payments, investigator meeting organization, document archiving, insourcing/ FSP projects, and legal representative services.
Comac Medical
Comac Medical offers a broad range of early to late phase drug development services managed by professionals fully devoted to the clients’ programs and needs. The services can be contracted as part of a full-service integrated program or as stand-alone solutions. We work in partnership with our clients to maximize opportunities, remaining flexible to individual requirements and meeting key milestones on time and on budget.
Convex Clinical Research
Convex is a clinical research services provider in Bulgaria with extensive experience in the management of clinical trials. As a local CRO we deliver high quality clinical trial services in Bulgaria and in other countries in Eastern Europe through our partnering companies. Convex offers a full range of services in clinical study management for: Phase I–IV studies on drugs, bioequivalence and bioavailability studies, clinical studies on food supplements, clinical studies on medical devices, and clinical studies on vaccines.
Ramus Medical
Ramus Medical is a full service CRO established in 2009 by Assoc. Prof. Dr. Rossen Mihaylov, Managing Director and Founder, having its headquarters in Sofia, Bulgaria. The company provides services from protocol development to final report through RA consultancy, clinical monitoring, site management, GxP auditing, bioanalysis (HPLC and LC-MS/MS), statistics and data management.
Marti Farm Ltd, company based in Zagreb, Croatia, is a Contract Research Organization supporting the needs of the pharmaceutical, biotechnology, medical devices, food supplements, cosmetics and healthcare industries across a variety of core services areas. We are internationally recognized as a reliable partner and provider of consultancy and regulatory services across pharmacovigilance, registration and market access, clinical trials, patient support programs and medical writing.
Optimapharm
Established in 2006, Optimapharm is a full-service Contract Research Organization that provides tailored solutions for our clients, and has a high-level of expertise in managing trials across Europe. With unrivalled access to patients in the emerging markets of Eastern and South Eastern Europe, and with strong presence in Western Europe, our operational excellence and customer-focused approach has resulted in our achieving a 95% repeat business level.
We are an independent, private company providing high quality clinical trial monitoring services in the South-Eastern Europe region. We provide a full range of activities required for the regular and legal conduct of a clinical trial, including: site selection, feasibility and evaluation; negotiation of contracts with institutions and investigators; regulatory submissions and obtaining of approvals; obtaining import permits and organizing the import of study medication and all study materials; all monitoring activities including Site Initiation Visit, Interim Monitoring Visits and Close-Out Visits; SUSAR reporting; drug safety reporting; 24 hour expert support; communication between trial sponsor and trial site; monitoring (phases I-IV); pharmacovigilance; translation of clinical trial-related documentation; and specialized medical and certified translations.
BECRO is an independent contract services organization (CRO) founded by professionals with research and clinical experience in academia, regulatory authorities and pharmaceutical industry in order to promote excellence in clinical research. Our services include clinical trial monitoring, data management and biometrics, pharmaceutical market research, pharmaceutical advisory boards, and regulatory affairs.
ClinBAY’s services involve statistical consulting and programming for clinical trials. We manage approximately 40 clinical trials per year, spanning from phase I to phase IV. Our consultants are also available for functional sourcing support in Biometry. Last, we develop custom data analysis packages in R and SAS.
Ilikos Drug Development Solutions
ILIKOS offers comprehensive site evaluation and monitoring services from feasibility through site close-out across North America, Europe and the META (Middle-East, Turkey and Africa) regions. Our monitors have 10+ years of experience on average along with excellent understanding of regulatory guidelines in their region.
Czech Republic
A-Pharma is a privately owned Czech CRO (Contract research organization) founded in 1996. The company can offer complete services in the field of clinical trials, medical devices, drug registration, data management, biostatistics, quality assurance including GCP auditing, translations of medical text and GCP trainings.
NEOX is a CRO providing drug development, medical device development, pharmacovigilance and regulatory, real world evidence/ data and HEOR, data management and biostatistics, and professional life sciences, medical and regulatory translation services.
Pharmservice
Founded in 2003, Pharmservice is a Clinical Research Organization (CRO) and Pharmaceutical Consulting Company delivering high quality services in the Czech and Slovak Republics. Our clinical research services include start-up and regulatory, clinical trial monitoring, data management and analysis, project management, site contract negotiation, post marketing studies (PMS), regulatory affairs and pharmacovigilance services.
Prague Clinical Services
Prague Clinical Services is a full-service, independent contract research organisation. Our services include clinical operations, regulatory services, medical writing, data management, biostatistics, quality assurance, pharmacovigilance, bridging studies, and medical monitoring.
Larix is a family founded, privately owned CRO offering full service (statistics, data management, clinical operations, medical writing, pharmacovigilance) solutions. Our offices are located near Copenhagen, in the middle of the Medicon Valley region and we have strong ties to the thriving pharmaceutical and biotech activities in this region. In 2015 Larix expanded to Sweden, Norway and Finland and now ranks as one of the largest local Nordic CROs.
NBCD is dedicated to clinical drug development in osteoarthritis. We specialize in design and execution of clinical trials within both pain and DMOAD compounds for osteoarthritis. We are experts in placebo response mitigation and selecting the right patients. Our background makes us experts in precision medicine using unique biomarker technologies. We support our biotech and pharmaceutical clients advance their osteoarthritis compounds through all clinical development phases.
Qmed Consulting
Qmed Consulting is a global full-service Contract Research Organization (CRO) based in Copenhagen, Denmark. We provide strategic consulting services in connection with medical device approval as well as best-in-class expertise in clinical affairs, regulatory affairs, quality management and commercial healthcare.
Clinical Accelerator
Clinical Accelerator is a clinical CRO and a patient enrollment organization operating since 2006. We also manage a network of investigators and patient referring physicians who are our valuable partners in implementing clinical studies. We place our studies in Central and Eastern Europe, the region with many well documented advantages for clinical trials. As a clinical CRO, Clinical Accelerator offers a full range of clinical trial services from protocol development and regulatory affairs through clinical conduct to data management, biostatistics and study reporting. We have performed numerous projects in all main therapeutic areas, including oncology, CNS, cardiology, pulmonology, GI, dermatology and ophthalmology.
Gaea was established in 1994 as a clinical consultancy to assist in clinical development plans, and to provide project management within Sponsors. Gaea expanded to become a trial enrollment acceleration company working at problematic sites that are common in many trials. In 2010 Gaea became a full-service CRO in Europe, from the UK. In 2018 Gaea exited the UK and moved European trial management to Estonia and Ukraine. Gaea works with difficult to enrol trials and complex IMP in oncology, acute care, intensive care, and neurology.
AKL Clinical Trials & Consulting
AKL clinical trial services include feasibility studies, site selection and pre-study activities, regulatory and ethics submissions (Finland), budget and contract negotiation support, site initiation and site staff training, site monitoring, site close out activities, and trial management.
Established in 2005, Crown CRO is a privately owned CRO with Finnish roots and a global network. We specialize in customer-oriented, competitive, and flexible services for clinical trials and regulatory affairs in Europe and the United States, serving the pharmaceutical, biotech, functional food, and medical device industries as well as contract research organizations. Our headquarters are located in Finland, and we have a strong presence in Sweden, Denmark, Norway, Estonia, Latvia, and Lithuania.
4Pharma is a contract research organization (CRO) providing medical research services to pharmaceutical, biotechnology, medical device and food companies. Focused on biostatistics, data management and medical writing, 4Pharma employees offer a unique blend of industry experience, service provider flexibility and the academic excellence of truly data driven mindsets.
Biomedical and Global Clinical Solutions
Biomedical and Global Clinical Solutions (BG ClinicalS Ltd) is an independent, privately held Contract Research Organization (CRO) located in southwest France. BG ClinicalS expertise applies to the full process of phase I to III biomedical research studies, from concept to clinical report. BG ClinicalS excellence in trial management addresses most expectations of our customers in pharmaceutical, biotech companies and agro-food/nutraceutical industries.
ClinSearch is a French Clinical Research Organization which provides clinical development and commercialization services for medical device and drug development on a European level. We are committed to offering individualized, cost-effective solutions to managing pre- or post-marketing studies in Europe, as well as obtaining reimbursement in key markets. From developing study methodology, to writing and reviewing study documents, conducting regulatory and site feasibility, adapted electronic data management solutions, monitoring in Europe, producing top-notch study and statistical reports: we have it all under our roof in Malakoff, South-West Paris.
Excelya stands apart from all other clinical development companies working for healthcare businesses. We are the only independent European CRO providing R&D players with full-service offers within a cooperative framework. Our clients can depend on the highest degree of involvement to guarantee the excellence they need. Our customized solutions perfectly match the scope and specificity of our clients’ research projects. We galvanise the finest scientific and human skills to be found in the clinical, biometric, regulatory, medical writing, data science and pharmacovigilance fields.
ExperTrials
ExperTrials is a full-service Clinical Contract Research Organisation (CRO) dedicated to biotech and medtech startups. Our service portfolio includes clinical operations, quality assurance, regulatory affairs, pharmacovigilance and safety, medical monitoring, and vendors management.
Pharmaspecific
Founded in 2010, Pharmaspecific is an ISO9001 certified company located in the Paris region. We are both a CRO and also an SMO (site management organization) in France. Specialists in clinical research, we provide a complete service for the conduct and track of clinical and observational studies. We collaborate with pharmaceutical companies (human and veterinary drugs), CROs, biotechs, clinical sites and medical device companies.
PopsiCube-Fovea
Popsicube-Fovea is not only a CRO. We act in several collaborative projects in the area of the future of healthcare, like personalized and predictive medicine, and artificial intelligence, where our combined expertise in clinical trials and new technologies have great applications. We provide clinical services, data management, biostatistics, innovative data collection tools, secured gateway, biosensors, and tailor made applications.
CONET GmbH is a privately owned, well established full-service CRO (Contract Research Organization) headquartered in Mannheim, Germany, with a representative office in Moscow, Russia. For more than 14 years, CONET offers clinical trial management service from small pilot studies to large multicenter international clinical trials for all phases (1-4), including pediatric clinical trials as well as IITs and medical device trials in accordance with EU Directives, internationally accepted guidelines and standards, like ICH-GCP or ISO EN 14155 and national laws.
FGK Clinical Research
FGK Clinical Research is a Europe-based CRO of an ideal size for cooperation with smaller and middle-size biotech, pharmaceutical or medical device companies. We currently have 140 employees in-house at our offices – all very experienced in their own fields and trained according to the latest standards in clinical research. Our services include consulting, regulatory affairs, project management / monitoring, data management / biostatistics, pharmacovigilance, medical writing, quality assurance, medical safety, and eSolutions.
Sacura is a full-service contract research organization granting a high level of quality (FDA approved), flexible structures and qualified staff. We are dedicated to the success of our clients by providing extensive support through a network of veterans previously experienced in leading positions in the big pharmaceutical industry to cover the entire life cycle process of your product. We are experts in the set-up, managing and performance of clinical trials. We are experienced in more than 70 indications and more than 130 different studies.
SCIRENT was founded in 2012 with the vision to help clients develop innovative medical drugs and devices for patients with cardiovascular diseases. Our dedicated team of experienced clinical and regulatory professionals shares your enthusiasm for developing effective therapies for heart patients. What makes us different from our competitors is our profound expertise in cardiovascular therapeutics and diagnostics. Our CRO offers access to an excellent network of dedicated trial sites in Europe and North America with extensive experience in cardiovascular trials.
SSS International Clinical Research
As an internationally operating Clinical Research Organisation (CRO), we have managed clinical trials in almost 20 countries. Starting with quality assurance services back in 1993 we have successfully run more than 250 clinical trials and non-interventional studies to date. Our clients are ‘big pharma’ (4 out of the top ten), biotech, diagnostics and medtech companies. We are a full-service provider covering every aspect of clinical research. SSS International Clinical Research is DIN EN ISO 9001:2015 certified.
ANTAEA is one of the largest regional Contract Research Organizations (CROs), providing high quality services in South-East Europe, Middle East and Africa with coverage in Greece, Cyprus, Egypt, Lebanon, Jordan, Tunisia, Morocco, Gulf states, Saudi Arabia, Sudan and Ghana. Since 1998, we provide professional flexible solutions supporting our clients to optimize allocation of their research resources achieving timely results in a cost effective manner. ANTAEA is committed to deliver services of scientific quality, management expertise, and precision based on reliable technologies. To date, we have performed over 520 clinical trials and over 1900 Registration & Regulatory submissions
Operating for more than two decades in Greece and Cyprus, CORONIS has been delivering a full spectrum of services to national and international pharmaceutical, biotechnology and medical device industries and other healthcare-related organisations. The configuration of our services has followed our customers’ demands and has been built according to the following main categories: clinical development, real world data, commercialization, regulatory affairs, vigilance, training and learning.
Health Data Specialists (HeaDS) is a full-service European Contract Research Organization (CRO) dedicated to the conduct of clinical studies, with particular expertise in hemato-oncology studies. We provide the complete range of clinical trial services, from start-up to completion, from Phase I to IV. In addition, we provide strategic oncology drug development consulting and expert advice as we understand the challenges of developing anti-cancer drugs in the complex, highly competitive and rapidly developing oncology market.
NEXT CRO is a highly added value, Contract Research Organisation (CRO), running Clinical Trials in Greece, Turkey and Cyprus, and with its alliances, globally, offering “plug and play” study teams. NEXT CRO provides added value clinical trial services in all therapeutic areas, including rare diseases.
ZEINCRO is a contract research organization (CRO) that provides its services to more than 21 countries in Europe, with special focus on the region of Central and South-Eastern Europe. ZEINCRO provides clinical trial services to the global drug development industry and offers additional services in the areas of post-marketing safety and efficacy studies, regulatory affairs and pharmacovigilance (IRISG), Phase I studies, including risk management plans.
Click here to see the second part of this CRO list.

Patricio Ledesma ( B.Eng Concordia University, Montreal, Canada and Master’s Degree in Clinical Trials, University of Seville, Spain) is the Head of Clinical Operations and Founder at Sofpromed CRO. Patricio is a professional consultant providing comprehensive, one-stop expert advice and guidance to biotechnology and pharmaceutical companies worldwide in the field of clinical trials and drug development. He is personally and enthusiastically devoted to helping biotech Chief Executive, Operations, Scientific, Medical, and Regulatory Officers in the planning and execution of phase I-IV clinical trials across North America, Europe, Asia-Pacific, Latin America, and Middle East regions. You can contact Patricio at: +34 607 939 266 [email protected]
To our articles and updates
- Follow Follow
You may also be interested…

What Are Community-Based Clinical Trials?
Community-Based Clinical Trials
If you are a Sponsor seeking to run a community-based clinical trial in underserved populations, please contact us at [email protected] Clinical trials are fundamental to advance medical knowledge and improve patient care. However, one significant challenge in...


Clinical Research Site Networks: Accelerating Clinical Trials
Site Networks
If you are a Sponsor seeking to run a clinical trial through a clinical research site network, please contact us at [email protected] Clinical research plays a central role in advancing medical treatments and improving healthcare outcomes. To ensure the smooth...

How Community-Based Clinical Trials Are Advancing Healthcare in Underserved Populations
If you are a Sponsor interested in running a community-based clinical trial in underserved populations, please contact us at [email protected] Clinical trials are instrumental in advancing healthcare by evaluating the safety and effectiveness of new treatments and...

Advantages of Clinical Research Site Networks in North Carolina
If you are a Sponsor interested in running a clinical trial through a clinical research site network in North Carolina, please contact us at [email protected] Clinical research plays a pivotal role in advancing medical knowledge, improving patient care, and driving...

Benefits of Clinical Research Site Networks in Illinois
If you are a Sponsor interested in running a clinical trial through a clinical research site network in Illinois, please contact us at [email protected] Clinical site networks play a central role in advancing medical research and improving patient care. In this...

What is a Contract Research Organization (CRO)?

How to Select a Clinical Research Organization (CRO)

Top Clinical Research Organizations (CRO) in the United States
Patricio Ledesma ( B.Eng Concordia University, Montreal, Canada and Master’s Degree in Clinical Trials, University of Seville, Spain) is the Head of Clinical Operations and Founder at Sofpromed CRO. Patricio is a professional consultant providing comprehensive, one-stop expert advice and guidance to biotechnology and pharmaceutical companies worldwide in the field of clinical trials and drug development. He is personally and enthusiastically devoted to helping biotech Chief Executive, Operations, Scientific, Medical, and Regulatory Officers in the planning and execution of phase I-IV clinical trials across North America, Europe, Asia-Pacific, Latin America, and Middle East regions. You can contact Patricio at: +34 607 939 266 [email protected]
Working Groups
- Become a member
- File Sharing
- COVID-19 Information & Guidance
- Webinars & Trainings
- About the GDPR Code
- How to Adhere
Latest News
- Newsletters
- Publications
Join our Working Groups
Our activities, thanks to the expertise of our members, we can provide :.
Get involved in one of EUCROF’s many working groups to help shape the thinking of regulatory and industry stakeholders
Join our webinars and take advantage of our experts to keep fully trained on key areas of interest in clinical research
Subscribe to our quarterly newsletter to stay in touch with all forthcoming events, our numerous achievements and initiatives involving EUCROF
EUCROF eNews
- Biotechnology
- Medical Device
07/02/24 - Draft European Code of Conduct - PRESS RELEASE
25-26/01/24 - act eu clinical trials analytics multi-stakeholder workshop., 31/10/23 – meet the eucrof talent engagement task force.
We use cookies to improve your experience on our website.

A unique CRO
The CERC is a unique Contract Research Organization based in Massy, France, and presided by five medical directors, Marie-Claude Morice, Philip Urban, Philippe Garot, Davide Capodanno and Antoinette Neylon.
The CERC was conceived and founded by physicians with wide experience in clinical research. Their renown and expertise in this field have enabled them to generate optimal interaction with their industrial partners and other medical research teams focusing on the development of innovative techniques, concepts and treatments.
Prestigious members
The prestigious members of the CERC’s Medical Advisory Council provide unparalleled guidance and expert support in a broad range of clinical trials dedicated to the assessment of interventional coronary and peripheral revascularization, structural and valvular heart disease treatments and adjunctive pharmacology.
The CERC has an excellent track record in regulatory guidance, trial design, global study management and monitoring, CEC/DSMB coordination, core-lab activities for pre- and post- market drug and device trials.
About this website
This website supports the running of clinical trials for human medicines in the European Union (EU) and European Economic Area (EEA).
Use of Clinical Trials Information System becomes mandatory for new clinical trial applications in the EU
From 31 January 2023, all initial clinical trial applications in the European Union (EU) must be submitted via the Clinical Trials Information System (CTIS). CTIS is now the single-entry point for sponsors and regulators of clinical trials for the submission and assessment of clinical trial data. This follows a one-year transition, during which sponsors could choose whether to apply for a new clinical trial in the EU/EEA in line with the Clinical Trials Directive or under the new Clinical Trials Regulation (CTR), which entered into application on 31 January 2022.
In the past, sponsors had to submit clinical trial applications separately to national competent authorities (NCAs) and ethics committees in each country to gain regulatory approval to run a clinical trial. Registration and the posting of results were also separate processes. With CTIS, sponsors can now apply for authorisations in up to 30 EU/EEA countries at the same time and with the same documentation. The system includes a public, searchable database for healthcare professionals, patients, and other interested parties.
The CTR foresees a three-year transition period, from 2022 to 2025. The first milestone has been reached today; in the next two years, by 31 January 2025, all ongoing trials that were approved under the Clinical Trials Directive will be governed by the new Regulation and will have to be transitioned to CTIS.
The application of the CTR strengthens Europe as an attractive location for clinical research. The new regulation streamlines the processes for the application and supervision of clinical trials, and their public registration: all clinical trial sponsors will now use the same system and follow the same procedures to apply for the authorisation of a clinical trial, no matter where they are located and which National Competent Authority (NCA) or national ethics committee they are dealing with.
The authorisation and oversight of clinical trials is the responsibility of EU/EEA Member States while the European Medicines Agency (EMA) is responsible for maintaining CTIS. The European Commission (EC) oversees the implementation of the Clinical Trials Regulation.
- This press release, together with all related documents, is available on the Agency's website.
- More information on the work of the European Medicines Agency can be found on its website: European Medicines Agency
Introduction
A clinical trial is a study performed to investigate the safety or efficacy of a medicine. For medicines intended for human use, these studies are carried out in people who volunteer.
Clinical trials in the EU and EEA are governed by the Clinical Trials Regulation (Regulation (EU) No 536/2014) which came into application on 31 January 2022. It is part of a broad initiative to transform the EU/EEA clinical trials environment in support of large clinical trials in multiple European countries, to the benefit of medical innovation and patients.
The regulation of clinical trials aims to ensure that the rights, safety and well-being of clinical trial participants are protected and the results of clinical trials are reliable and informative.
- Clinical trial sponsors (usually researchers or companies that oversee a clinical trial and collect and analyse the data) can use this website to apply for permission to run a clinical trial anywhere in the EU/EEA including in multiple countries, provide updates to national regulators about a trial, and submit trial results.
- EU/EEA national regulators can use it to process clinical trial applications collaboratively, request further information, authorise or refuse a trial and oversee an authorised trial.
- Anybody can use it to view information on clinical trials in the EU and EEA from 31 January 2022.
Clinical trial sponsors and EU/EEA national regulators work in the Clinical Trials Information System, the system underpinning this website.
The EU Member States and EEA countries, the European Commission and EMA launched the Clinical Trials Information System on 31 January 2022. On the same day, EMA launched this website. EMA maintains this website in collaboration with the EU Member States and EEA countries, and the European Commission.
CTIS is a registered data provider for the World Health Organization (WHO). Data from authorised trials published on this website, excluding those with category 1 deferrals of the main characteristics, is included in the search portal of WHO's International Clinical Trials Registry Platform (ICTRP) . This applies to relevant clinical trial data, as required by WHO , which has been published on CTIS since the launch of the system on 31 January 2022.
Publicly accessible registries that are data providers to WHO's ICTRP are accepted by the International Committee of Medical Journal Editors (ICMJE), according to its recommendations .
For more information on the regulation of clinical trials in human medicines, see:
- Clinical trials in human medicines
Secure workspaces
This website supports the business processes of clinical trial sponsors and national regulators throughout the lifecycle of a clinical trial, via the secure Sponsor workspace and Authority workspace Users need to log into these secure workspaces.
Clinical trial sponsors can apply for authorisation to run a clinical trial in up to 30 EEA countries via a single application in this website. They can also carry out tasks including liaising with national regulators while a trial is ongoing and recording the results of the trial.
National regulators can work together using this website on the assessment and authorisation of a clinical trial in several countries.
They can also use this website together with other systems to work together on clinical trial oversight, including monitoring and assessing safety-related data in the context of a clinical trial.
CTIS for sponsors
Clinical trial sponsors and other organisations involved in running clinical trials can apply to run a trial and can manage an ongoing trial in up to 30 countries in the European Union and European Economic Area via the Clinical Trials Information System (CTIS).
CTIS for authorities
Regulatory authorities, such as national competent authorities and ethics committees of EU Member States and European Economic Area countries can participate in the assessment, authorisation or oversight of a trial.
Searching for clinical trials
This website contains limited information on individual clinical trials entered since its launch on 31 January 2022.
It will gradually contain more information as clinical trial sponsors and EU/EEA regulators use it to initiate and oversee clinical trials in the EU and EEA.
For information on individual clinical trials initiated before 31 January 2022 see:
To find detailed information on clinical trials granted or refused permission in the EU and EEA via the Clinical Trial Information System, this website has a search function.
You can view information on individual clinical trials as soon as it becomes available, such as the EU clinical trial number, name and address of the trial sponsor, start and end dates of participant recruitment and of the trial itself.
Further information, including the identity of the investigational medicine and details of the trial design, is also made available via this website, but its publication may be deferred to protect legitimate economic interests.
- Find out more and search for clinical trials
Training and support
Training and supporting materials are available from EMA on how to use the Clinical Trials Information System, and a dedicated user support service is available:
Legal framework
The Clinical Trials Information System, the system underpinning this website, serves to implement EU pharmaceutical law in the Clinical Trials Regulation (Regulation (EU) No 536/2014) . The goal of the Regulation is to create a favourable environment for conducting clinical trials in the EU, to ensure the EU remains an attractive region for clinical research, with the highest standards of safety for participants and transparency of information.
It does this by harmonising the submission, assessment and supervision processes for clinical trials supported by this website. Before the launch of this website, sponsors had to submit clinical trial applications separately to national competent authorities and ethics committees in each country to gain regulatory approval to run a clinical trial.
The assessment, authorisation and supervision of clinical trials are the responsibilities of EU Member States and EEA countries.
Transition period
Under the Clinical Trials Regulation, EU Member States and EEA countries will carry out their legal responsibilities to assess and oversee clinical trials using the Clinical Trials Information System from its launch on 31 January 2022.
- Clinical trial sponsors can choose whether to apply to start a clinical trial via the Clinical Trials Information System or under the Clinical Trials Directive until 30 January 2023.
- From 31 January 2023 onwards, clinical trial sponsors will need to apply to start a clinical trial via the Clinical Trials Information System.
- By 31 January 2025 , any ongoing trials approved under the Clinical Trials Directive will fall under the Regulation and information about them will need to be transferred to the Clinical Trials Information System.
For more information:
- EMA: Clinical trials in human medicines
- EMA: Clinical Trials Regulation
- European Commission, EudraLex - Volume 10: Clinical trials guidelines
EMA news and events
Find EMA news and events related to clinical trials in the EU and the EEA.
This site uses cookies to offer you a better browsing experience. Find out more on how we use cookies and how you can change your settings .
Your cookie preferences have been saved. For more information and to change your preferences at any time, see our Cookies page .
- Medical Devices
- Advanced Therapy Medicinal Product
- Neurology / Psychiatry
- Cardiovascular
- Ophthalmology
- Gastroenterology
- Gynecology / Urology
- Endocrine / Metabolic Disorders
- Dermatology
- Rare Diseases
Representative Services
- Regulatory Affairs
- Medical Writing
- Project Management / Monitoring
- Quality Assurance
- Data Management / Biostatistics
- Medical Safety
Pharmacovigilance
- Memberships / Accreditations
- Corporate Development
- Locations / Contact
FLEXIBLE EXPERTS & RELIABLE SOLUTIONS
In clinical research, smallest details matter. Our commitment is to highest standards in quality, service and client confidentiality.

Minimizing Risks for maximum Benefits.
Keeping a watchful eye on the safety of your products, we collect and report your safety data. Because safety comes first.

Your Premium European Legal Representative.
Operating in all of the European Union and Switzerland, we are your link to successful business throughout Europe.

We are a full service contract research organization offering a complete range of clinical development and consulting services to pharmaceutical, biotechnology and medical device companies.
Deutsche Biotechnologietage
FGK participates with Edgar Fenzl, Ursula Türcke and Martin Krauss at DBT in Berlin, Germany.
FGK Clinical Research GmbH Acquires Clinicology Ltd., Expanding Territorial Footprint in Europe
Press Release
Our experience spans multiple fields of medical indications.
Clinical studies with medical devices require a contract research organization that is familiar with the specific regulatory requirements.
▷FGK has broad experience in conducting clinical trials with all forms of biologics including cell therapy, ATMP and GMO.
As full service contract research organization, we have completed numerous clinical trials spanning a broad variety of indications in oncology.
CRO experienced in neurology − Our clinical research experts have extensive experience with novel therapeutic approaches
New challenges in dental clinical trials require profound support for study centers. ▷FGK can support sponsors with this
▷FGK has broad experience in cardiology and in vascular indications, including clinical research with e.g. stents, balloons and ablation systems.
▷FGK is a CRO with broad experience in infectious diseases research. Your clinical study can benefit from expert knowledge gained in various indications
▷FGK supports sponsors of clinical studies in ophthalmic indications. ✓ A contract research organization with experience in opthalmology
Clinical study support for pulmonology research. ▷FGK has an established network of investigational sites specialized in different methodologies
Based on many years of comprehensive experience in the design and conduct of clinical trials in various gastroenterological indications we can provide sound advice and service
▷FGK Clinical Research is experienced in conducting studies in classical indications of gynecology and urology.
Endocrine and metabolic diseases span a highly diversified range of conditions. ▷FGK has performed international Phase I studies up to post market studies
Clinical studies in dermatology use key imaging tools in evaluating efficacy endpoints. ▷FGK can advise on the best approach and support trial conduct
Clinical trials in rare diseases present challenges that need to be addressed early in the study design. ▷FGK knows how to overcome common obstacles
FGK Clinical Research GmbH
Heimeranstrasse 35 80339 Munich · Germany
T: +49 89 893 119 - 0 F: +49 89 893 119 - 20
info @ fgk-cro . com
- News & Events
- Privacy Policy
2nd Floor 53-55 Totleben Blvd. Sofia 1606, Bulgaria
Ulica grada Vukovara 284 Almeria Tower Zagreb 1000, Croatia
Czech Republic
Jachymova 26/2, 110 00, Prague 1, Czech Republic
Maison La Défense, 12 place de La Défense, 92974 Paris la Défense cedex France
5, Chavchavadze Avenue Tbilisi, Georgia
Tbilisi (Depot)
Unter den Linden 21 10117 Berlin, Germany
Bazis Office Center Kft., 1027 Budapest, Horvat u. 14-24, Hungary
Eurosky Tower Ufficio B/4 2˚Piano Via Giorgio Ribotta n.21 00144 Rome, Italy
Prosta Tower 13th floor ul. Prosta 32 00-838 Warsaw, Poland
B-dul Iuliu Maniu nr 7 Cladirea A, Intrarea 3, Etaj 1, Sector 6 Bucharest, 061072, Romania
3rd Floor Airport City, Omladinskih brigada 90b, Belgrade, 11070, Serbia
Levent Mahallesi Cömert Sokak No:1, Yapı Kredi Plaza C Blok Kat:17 No:40-41, Ofis DO-1, Beşiktaş/ İstanbul
1, Acad.Proskury str Kharkov, 61070 Ukraine
12 Kurenivsky lane, office B-101 Kyiv, Ukraine
Kyiv (Depot)
22, Sem'i Prahoyvykh str., Kyiv, Ukraine
United Kingdom
1st Floor Waterfront House Beeston Business Park Beeston, Nottingham NG9 1LA, UK
Cookies on the Worldwide Clinical Trials website
We use cookies to improve your browsing experience and help us improve our websites. We have carefully selected third parties that use cookies to achieve purposes illustrated in the cookie policy. For more information. Read more about online privacy statement . By closing this banner or continuing to browse our website, you agree to our use of such cookies.

CONTRACT RESEARCH ORGANIZATION
Dedicated to help companies and
health care professionals with their clinical research in Europe.
Your personal partner to future proof your clinical evidence
MEDICAL DEVICE REGULATION
We support you with the extensive and complex MDR clinical evidence requirements and challenges
FULL-SERVICE CRO
Supporting every step of your clinical research. We offer high-quality services for consultancy, regulatory and clinical trial management, as well as data management, biostatistics and medical writing.
SPECIALIZED IN MEDICAL DEVICES
We deliver valuable clinical data for medical devices to meet the medical needs for the patient and to continuously monitor the quality and safety of the device during the device lifecycle.
WITH A PERSONAL APPROACH
Our dedicated monitors are a supportive extension of the study team. We are actively involved, enforcing our relationships and thereby reaching your project goals and timelines.

We match our services, expertise, budget & timelines to your specific clinical needs

OUR PARTNERS

Archer Research is a Contract Research Organization based in the heart of Europe.
Introducing: Our dynamic team of experienced research professionals eager to advise you and drive your project with the highest quality.
Let us do what we do best. Let us run your project. We are crazy about clinical research!
Nice to meet you!

Karen Gabriels
PhD, Clinical Research Manager
MDR expert hel ping you find your way through the regulatory web.

Mieke Tempels
MSc, Clinical Operations & Quality Manager
Clinical operations expert, supporting the team in delivering all projects with the highest quality.

Dorien Haesen
PhD, Clinical Research Professional
Project management expert with the highest dedication to support the site staff as an extension of the study team.

Stephanie De Munter
Project management expert with the highest dedication to supporting the site staff as an extension of the study team.

Clinical Research Associat e
Data expert with the highest dedication to supporting the site staff as an extension of the study team.

Clotilde Thumser-Henner
PhD, Clinical Research Associate

Clinical Research Associate

MSc, Clinical Trial Assistant
Administrative wonder woman providing the best support for your project.

Eline Bruyninx
Management Assistant
Dynamic organisation talent, making our days super efficient and bright.
WHAT PEOPLE SAY
"Very pleasant cooperation, professional, high quality, always available, good service."
— Prof. Dr. Erik Debing
Head of the Department of Vascular Surgery,
NEWS & EVENTS

* Happy holidays from team Archer! *

First cadaver testing of a very innovative device

Ask Archer | Tips towards clinical data that meet all regulatory requirements

NEED SUPPORT WITH YOUR CLINICAL RESEARCH?
Let our research professionals assist you.
Agoralaan A bis (BioVille) - 3590 Diepenbeek - Belgium
- Biospecimen Solutions Precision Value & Health Careers

- Clinical Trial Services Overview
- Global Clinical Trial Support
Cost-effective European CRO Services
Precision provides clinical research organization services throughout Europe, with clinical trials focused in Central and South-Eastern Europe (CEE) to leverage the operational efficiencies available in these regions. Efficiencies include faster patient recruitment due to the centralized healthcare systems in CEE countries and highly-engaged clinical trial investigators who produce high-quality work. We generally recommend our clients include a significant number of sites in CEE to ensure that their studies finish on schedule.
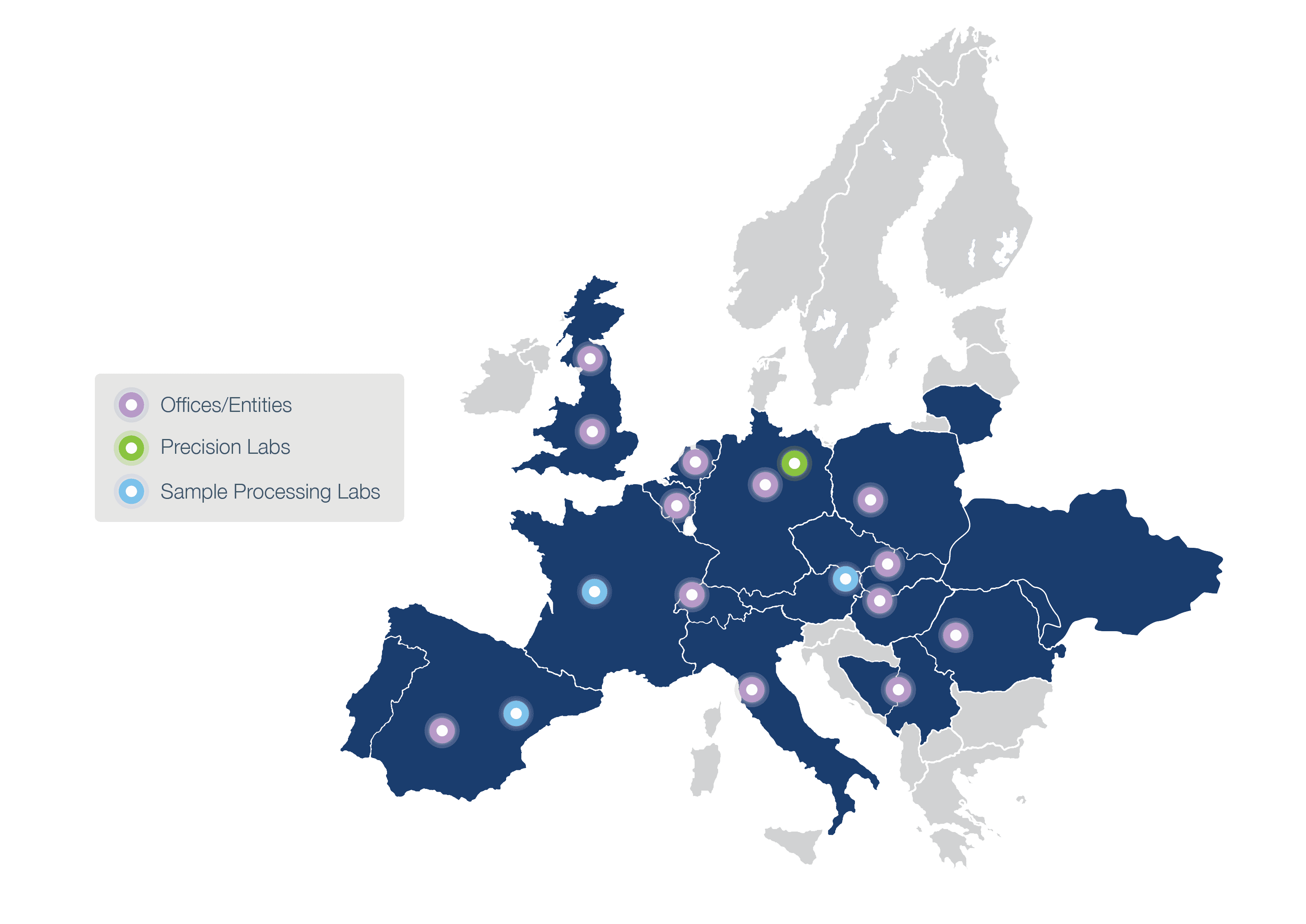
Delivering integrated Clinical Trial Services, Data Science, and Specialty Lab Services worldwide
Our european locations provide a strategic footprint for recruitment and execution.
- Amsterdam, NL
- Belgrade, SER
- Berlin, GER
- Bratislava, SK
- Bucharest, ROU
- Budapest, HU
- Edinburgh, UK
- Geneva, SUI
- Lausanne, SUI
- Royston, UK
- Windsor, UK
Highlighting our extensive Central and South-Eastern European operations
Precision’s place as the leading European CRO was solidified in 2018 with the acquisition of Argint International, the premiere CRO of Central and South-Eastern Europe.
Argint’s strong presence in Hungary, Poland, Romania, Slovakia, and Serbia gives researchers across the globe increased access to investigators and patients throughout the CEE.
Our people are our most precious resource. We have an exceptional team of clinical, in-country project managers and regulatory experts, all of whom are committed to helping our clients run successful studies.
All members of our European CRO team are fluent in English and multiple other European languages.
Learn how Precision’s European CRO services can advance your development program.
Related clinical trial services, clinical trial design.
We harness advanced trial-design approaches-including basket, umbrella, and adaptive trials-to realize the full impact of biomarker-driven clinical research. Deep experience in these highly complex trial designs maximizes both insights and efficiency.
Biostatistics
Our seasoned biostatisticians and statistical programmers deliver insight into every phase of your trial, from study design to regulatory submissions, all backed by meticulous documentation and data monitoring.
Clinical Sample Management
We manage sample inventories across a global network of labs to supply real-time processing in 55 countries, then consolidate data from central labs, screening labs, and specialty labs with clinical data to create actionable reports.
Explore our other areas of expertise
Precision for Medicine is part of the Precision Medicine Group, an integrated team of experts that extends Precision for Medicine’s therapeutic development capabilities beyond approval and into launch strategies, marketing communication, and payer insights. As one company, the Precision Medicine Group helps pharmaceutical and life-sciences clients conquer product development and commercialization challenges in a rapidly evolving environment.

Europe Clinical Research Career Guide

Clinical research remains a field with significant career potential worldwide. Clinical research associates (CRAs) continue to be highly sought after, overseeing lab and research processes. This role offers financial security and the possibility of frequent work travel. This guide provides an updated overview of becoming a CRA in the EU.
EU CRA salaries still vary considerably. While Payscale may cite older data for the Netherlands (€42,874), consult recent job postings and salary comparison websites for the most accurate figures. Glassdoor ( https://www.glassdoor.com/index.htm ) or Indeed ( https://www.indeed.jobs/ ) can be helpful resources. Germany, for instance, might show a higher average closer to Salaryexpert's €59,969 estimate (though this could also fluctuate). Researching your specific country's CRA salary range is crucial.
Requirements to Work:
Successful CRAs come from diverse backgrounds, but the minimum education requirement in most EU postings remains a Bachelor's degree with a strong science focus. Many companies still seek candidates with 1-2 years of relevant experience. Be aware that some companies may prioritize experience within the country they operate in. Bilingualism remains important – fluency in English and the country's official language is generally expected.
Additional Considerations:
Clinical Trial Regulations : The EU has its own set of clinical trial regulations (GCP). Consider familiarizing yourself with these for a competitive edge. You can find information on the EU GCP guidelines on the European Commission website ( https://www.ema.europa.eu/en/human-regulatory-overview/research-and-development/compliance-research-and-development/good-clinical-practice ).
Continuing Education : Staying updated on the latest advancements in clinical research through conferences or online courses can enhance your skillset.
CROs vs. Pharma : Research opportunities exist with both Contract Research Organizations (CROs) and pharmaceutical companies. Explore which environment best suits your career goals.
Conclusion:
While clinical research is a global field, job requirements and salaries can differ across the EU. This guide aims to provide a starting point. Consider further research tailored to your specific country and desired career path within clinical research.
Montenegro Clinical Research Career Guide
Remote monitoring is the future of clinical research.
Therapeutic Expertise
Participate

Explore end-to-end solutions throughout development — from portfolio optimization and regulatory strategy, to Phase I-IV clinical trials, market access planning, and more.
- Portfolio management and asset valuation
- Early development and innovation
- Integrated clinical development
- Approval and access
- Value substantiation lifecycle management
HOW WE DO IT
- Operational excellence
- Delivery models
- Building patient insights into assets, profile and claims
- Portfolio Optimization
- Asset Valuation and Indication Prioritization
- Early Evidence Review
- Model-Based Drug Development
- Integrated Development Strategy and Planning
- Phase I Clinical Trials
- Proof of Concept Studies: Phase IB-IIA
- Patient Engagement Strategy and Enrollment Solutions
- Patient Inclusion
- Site Alliance Network and KOL Engagement
- Protocol Optimization
- Regulatory Strategy
- Market Access Strategy and Delivery
- Biomarker and Genomic Medicine Strategy
- Clinical Trial Supply & Logistics
- Medical Communications
- Phase IIB-IV Clinical Trials
- Real World Evidence
- Protocol-Driven, Customized Site Solution Strategy
- Regulatory Strategy, Submissions, Compliance, and Outsourcing
- Clinical Development Technology Optimization
- Global Regulatory Submissions and Outsourcing
- Compliance and Risk Management
- Real-World Evidence, Market Access Strategy and Planning
- Regulatory Compliance, Drug Safety and Pharmacovigilance
- Lifecycle Optimization
- Leveraging AI and digital in clinical development
- Biotech Clinical Trial Solutions
- Gain an advantage through FSP

Utilize our expertise across therapeutic areas, combining innovative trial designs, leading clinical and regulatory expertise, global reach, and a passion for changing patient lives.
- Neuroscience
- General medicine
- Infectious disease & vaccines
- Inflammation & immunology
Cross-Therapeutic Expertise
- Cell & gene therapies
- Rare diseases

Our experts help you stay at the forefront of the industry - and ahead of change.
New Medicines, Novel Insights
- Advancing rare disease drug development
- Accelerating development of cell and gene therapies
- Achieving patient-guided drug development
Discussions on Diversity
- Chapter 1 Bridging the Gap
- Chapter 2 Beyond the Binary
The Regulatory Navigator
- Exploring ICH Q5A revision 2
- Potency assurance for CGT products: FDA's new draft guidance
- The FDA’s final guidance: CAR-T product development
Latest Report

New Medicines, Novel Insights: Achieving patient-guided drug development

Thinking about joining a clinical trial? Learn the drug development process, what it’s like to participate, how to find a trial, and answers to frequently asked questions.
INTERESTED IN PARTICIPATING?
- Healthy Volunteers
- Patient Volunteers
- Patient Advocacy Groups
HEAR FROM REAL PATIENTS
- Patient Stories
TRIAL SITES

Want to collaborate with us to offer clinical trials at your site? We would welcome the opportunity to discuss.
We are one of the largest CROs in the world, speeding life-changing medicine to market by engaging patients With Heart ™. Learn about who we are, what we do, and what we believe.
- Management team
- Global reach
- Our DE&I strategy
- Compliance, Tax & Privacy
- Meet us at an event
- Jamie Macdonald
- Peyton Howell
- Michael Crowley
- Jonathan Shough
- Sheri McCoy
- Michael Bruun
- John Groetelaars
- Susan Salka
- Global Reach
- Our DE&I Strategy
- Our ESG Strategy
- Compliance & Ethics
- Tax Strategy
- Privacy Policy
- Supplier Data Privacy Requirements
- Terms of Use
- French Gender Pay Data
- UK Gender Pay Data
- UK Modern Slavery Act Statement
- EU-U.S. Data Privacy Framework (EU-U.S. DPF)
- Supplier Diversity Policy
- World Vaccine Congress
- Patients as Partners U.S.
- MAPS Americas
- ASCPT 2024 Annual Meeting
- Managing Complexity in Cell and Gene Trials: Establishing an RBQM Strategy that Drives Better Performance
- EDI in clinical research: The case for adaptive strategies at every step
- 17th ISCR ANNUAL CONFERENCE 2024
- IncluDE Site Solutions Summit
- AAN Annual Meeting
- ASGCT Annual Meeting
- Dermatology Drug Development Summit EU
- World Orphan Drug Congress US
- AACR Annual Meeting
- BIO International Convention
- DIA Global Annual Meeting
- International Society for Cell & Gene Therapy
- Final countdown: How to transition your trials under EU-CTR
- SCRS: Oncology Site Solutions Summit
- Accelerating Vaccine Development Through Operational Excellence
- Alzheimer’s Disease vs Multiple Sclerosis Drug Development: Similarities, Differences & New Perspectives
What can we help you find today?
The perspectives and opinions expressed in this material represent those of the patient advocate only and should not be considered a solicitation, promotion or advertisement for any services of Parexel, or any drugs or therapies, including those under development. Participating in clinical trials for investigational medicines offers patients potential benefits, such as access to cutting-edge treatments and expert medical care, while contributing to medical research. However, risks may include side effects, unpredictable outcomes, and time commitment. Careful assessment of these factors helps patients make informed decisions. The content of this material, including graphics, images and text, is provided for informational purposes only and does not constitute medical advice, diagnosis or treatment. Please consult your healthcare professional for medical advice. The patient advocate has provided their consent for the use and distribution of this content.
Patient Story
- Cell & Gene Therapy
- Rare Diseases
- Inflammation & Immunology
- Neurosciences
One night, while watching TV with her husband, Robyn felt a lump in her neck.
She had large B-cell lymphoma — an aggressive cancer diagnosed in 150,000 patients each year globally.
As a doctor, she knew the risk. She got a CAT scan, looked at the images, and her world stopped.
Four years later the lymphoma returned. Robyn’s care team prescribed a further six cycles of a brutal chemotherapy, followed by an autologous stem cell transplant (ASCT) and external beam radiation. Robyn’s ASCT treatment was further complicated by septic shock requiring a stay in intensive care.
Robyn entered remission again, but it was short-lived, with the lymphoma returning nine months later. Treatment options for Robyn were now bleak and limited.
Robyn immediately sought treatment. With her husband by her side, she underwent many rounds of chemotherapy and went into remission.
Feeling defeated, she found a clinical trial for car t-cell therapy, a new treatment that reengineers white blood cells to target and eradicate cancer., she thought this was her last chance., a week after receiving treatment, her lymph nodes shrunk. within three months, there was no evidence of the disease., today, she’s cancer free — and sharing her experiences to help us better meet the needs of patients like her in our cell and gene therapy trials., lives can change when you design cell and gene therapy trials with speed and precision..
- Utilize the right experts, focused on the right indications
- Access even hard-to-find patient populations
- Pinpoint the perfect sites for your trial
- Satisfy global regulations, to get your treatment to market safely and quickly
What we do, we do
Our Experts
Our cell and gene therapy specialists collaborate to help get your innovative treatments to patients like Robyn faster.
MORE EXPERTS
Steve Winitsky
Vice President, Tech...
Stefan Zietze
Executive Director, ...
Hicham El Bariqi
Director, Cell & Gen...
Jingke Yang, M.D., Ph.D.
Global TA Section He...
Chris Learn, Ph.D., PMP

Vice President, Cell and Gene Therapy, Center of Excellence
With 20+ years of trial execution and team management experience, Chris leads development for our cell and gene therapeutic area. He reinforces our patient-first focus to help ensure your trials are designed to meet patient needs.
"Being able to capture and understand the patient’s perspective — not just as a patient, but as a person — and use their insight to guide my decisions is what it means to work With Heart™."
Our diverse experiences ensure you get the expertise you need, no matter the indication.
Our experience with CAGT clinical trial sites around the world allows us to accelerate study start-ups.
- North America
- South America
- Middle East & Africa
Access to global EMR data enables us to pinpoint the perfect sites for your trial.

Our cross-functional team overcomes any technical, logistical, and strategic challenges.
Our highly tailored recruitment and retention strategies drive access to even hard-to-find patient populations.
What can we do to help you change patient lives?
See CAGT capabilities Visit all therapeutic areas Explore CAGT careers
Want our latest insights for cell and gene therapy?
Ready to speak to someone on our team?
Are you a patient interested in a clinical trial?
We focus on patients, because they inspire us to deliver better trials, faster than ever . So we can make a difference for more patients like Robyn.
Who we are,, parexel is proudly among the world’s largest clinical research organizations.
A dedicated CRO providing the full range of Phase I to IV clinical development services and leveraging the breadth of our clinical, regulatory and therapeutic expertise , our team of more than 21,000 global professionals works in partnership with biopharmaceutical leaders and sites to design and deliver clinical trials with patients in mind , to make clinical research a care option for anyone, anywhere.

Europe has a consistent reputation for quality research.

George Clinical In Europe
Europe enjoys a consistent reputation for quality research and has some of the best healthcare systems in the world.
Consisting of >50 countries, Europe has a very large and diverse patient population with access to private, academic and national hospitals.
In comparison with the healthcare systems of other developed nations, the UK’s National Health Service (NHS) is rated as one of the best. The Department of Health created the National Institute for Health Research to establish a high-a health system within the NHS. The Clinical Research Network is part of this organization.
The Clinical Research Network provides a good infrastructure to support clinical research in the UK and the European Union, and provides support including identifying and recruiting patients, providing training so that researchers have access to experienced people.
What are the benefits of conducting clinical trials in the UK?
- Large patient population across Eastern and Western Europe
- Centralized submission process for clinical trials
- In comparison with the healthcare systems of other developed nations, the UK’s National Health Service (NHS) is rated as one of the best
- Clinical Research Networks (CRN) provide a good infrastructure to support clinical research
- The CRN provides support including identifying and recruiting patients, providing training so that researchers have access to experienced people
What are the prevalent diseases in the UK?
- Heart disease
- Respiratory disease
- Liver disease

[email protected]

- Corporate News
- Case Studies
Add George Clinical to your network
© George Clinical 2024 All Rights Reserved | Privacy Policy | Modern Slavery & Human Trafficking Statement | GDPR Compliance Statement
- Conference Coverage
- CRO/Sponsor
- 2023 Salary Survey

- Publications
- Conferences
The Current Status of European Research Related to COVID-19: The EUCROF Perspective
- Marta Lettieri
- Donato Bonifazi
- Stefano Marini
- Martine Dehlinger-Kremer, PhD
Successful collaborations between Pharma/Biotech/Device industries and CROs will play a key role in an effective fight against the global COVID-19 pandemic.
The COVID-19 pandemic has affected different countries in different ways, but across the world it has had three common, defining characteristics: speed and scale (i.e., the disease spread quickly with potential to overwhelm the country's health systems); severity (an estimated 20% of cases are severe or critical, with increased risk in older age groups or those with underlying conditions); societal and economic disruption (particularly regarding shocks to healthcare systems and measures taken to control transmission.) 1 Clinical research plays a crucial role in developing diagnostics and medicines to fight the ongoing COVID-19 pandemic, through rapid and accurate clinical trials, and collect robust and reliable outcomes, in a variety of different settings, on a large scale. Intensive global research collaborations among all stakeholders and industry involving all countries are necessary to enable identification and testing of effective treatments, vaccines and diagnostics.
The European CRO Federation, EUCROF, is a non-profit entity founded in 2005 and representing the interests of Contract-Clinical Research Organizations (CROs) in Europe towards regulatory bodies, the bio-pharmaceutical and medical device industry, healthcare professionals and patient’s associations. The Federation consists of Members (National CRO associations) from 12 countries, including Belgium with BeCRO, Czech Republic with ACRO-CZ, France with AFCROs, Germany with BVMA, Greece with HACRO, Italy with AICRO, the Netherlands with ACRON, Romania with ACCSCR, Slovakia with SACROP, Spain with AECIC, Turkey with SAKDER, UK with CCRA, and 15 Associate Members based in Albania, Austria, Bulgaria, Croatia, Denmark, Georgia, Serbia, Spain, Sweden, Switzerland (2), Ukraine (2), United Kingdom (2) as well as three Partner Members based outside Europe, in Algeria, Egypt and Israel. EUCROF is intensively engaged with its members and other involved stakeholders in the conduct of COVID-19 related clinical research. Through its large Network, represented by over 385 affiliated member-CROs and 25 countries, EUCROF contributes to have a wide perspective on the impact COVID-19 pandemic has having on ongoing and upcoming Clinical Trials.
The article provides the results of a brief survey conducted by the European CRO Federation (EUCROF) with the goal of collating data focused on COVID-19 research activities of its members. The survey was sent to all affiliated member-CROs to obtain an overview of the extent of contribution of EUCROF members to COVID-19 relatedresearch, and to collect information on pandemic impact on CTs, measuring the active participation to research activities planned to face coronavirus outbreak.
The online quick survey was developed through Google Forms and was distributed by email to all affiliated members-CROs, in March 2020. The survey included the following questions regarding the CROs' research activities:
- Has your company been approached to participate in any research activities associated with COVID-19?
- If yes, is your company actively participating in research activities now and scheduled to do so in the next 3 to 6 months?
- a potential therapeutic?
- and/or a vaccine?
- and/or a diagnostic?
- Are the services provided on a national or international level?
- Is your CRO national or multinational?
- If yes to either, please specify what activities you will be contributing to the research? Services provided by the CRO (tick all that apply)
Questions 1-2 of the survey were aimed to understand whether the pandemic had an impact on the clinical studies management activities, and if so, question 3 investigated to what extent the EUCROF affiliated member-CROs were to be involved in the next future (3-6 months). Questions 4 and 5 were aimed to qualify whether and how many respondents provided services at national or international level, and if respondent CROs were national or multinational. Finally, question 6 investigated which services were more frequently provided.
Results were collected from March 2020 to May 2020, analyzed electronically through Google Forms statistics and exported into graphs using Excel.
In total, 68 responses to the survey were received from affiliated member-CROs and analyzed electronically. The analysis of the results shows that 53 out of 68 of the respondent CROs (78%, as shown in Figure 1) were involved in COVID-19-related research activities, of which 48 (91%) expect to maintain and continue with this research for at least further 3-6 months.
Figure 1 . Answers from question 1. Percentage of respondent CROs who positively declared to be involved/participate in COVID-19 research.
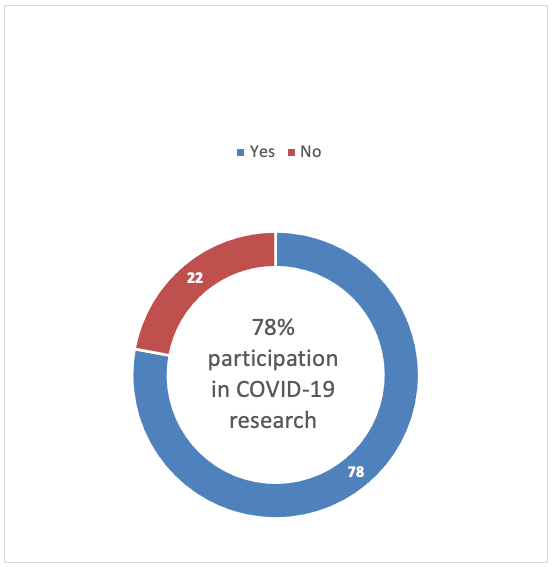
Two Year Trial Data Show Long-Term Efficacy of Izervay for Geographic Atrophy Secondary to Age-Related Macular Degeneration
Phase III GATHER2 trial data show Izervay reduced the rate of geographic atrophy lesion growth across every month and every-other-month dosing through two years of treatment.
Potential First in Class Treatment for Cirrhosis Shows Positive Results in Phase II Trial
LPCN 1148 is an oral treatment that has shown promise preventing recurrence of overt hepatic encephalopathy and treating sarcopenia.

FDA Fast Tracks Johnson & Johnson’s Nipocalimab for Fetal Neonatal Alloimmune Thrombocytopenia
Johnson & Johnson is moving forward with a pair of Phase III trials of nipocalimab to reduce the risk of fetal neonatal alloimmune thrombocytopenia in alloimmunized pregnant patients.

Designing Clinical Studies in Pain Management
In part 2 of this video interview, Greg Sturmer, co-founder and CEO of Elysium Therapeutics discusses what industry should be keeping top of mind when it comes to designing and executing pain clinical studies.
Phase III NextCOVE Trial Data Show Efficacy of Moderna's Next-Generation COVID-19 Vaccine
mRNA-1283 was found to elicit a higher immune response against SARS-CoV-2 than Moderna's licensed COVID-19 vaccine, mRNA-1273.

The State of the Opioid Epidemic
In part 1 of this video interview, Greg Sturmer, co-founder and CEO of Elysium Therapeutics discusses the current state of opioid usage including how pain treatment is often mismanaged and how the industry can adopt greater safety standards in developing pain therapies.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Skip to main content
- Skip to main menu
- Skip to user menu

Filter News
- All (806,545)
- Topic (764,546)
- Hotbed/Location (734,852)
- Career Advice (3,891)
- Insights (176)
- Webinars (8)
- Podcasts (35)
Veeda Clinical Research Acquires European CRO - Heads - Expanding Global Reach & Capabilities
Published: Mar 26, 2024
DUBLIN and AHMEDABAD, India, March 26, 2024 /PRNewswire/ -- Veeda Clinical Research Limited , a full-service contract research organization (CRO) with a proven record of drug development success, announced that it has acquired Heads , a privately held European CRO, which specializes in conducting clinical trials in oncology. Established in 2010, Heads has an operational presence in 25 multiple strategically important locations across Europe, North America and the Asia Pacific region. Through this acquisition, Veeda enters the league of global CROs with integrated capabilities to extend contract research services from discovery to clinical development extending to post commercial launch.

Sharing details related to common synergies between Veeda and Heads , Dr. Mahesh Bhalgat, Group – CEO of Veeda Clinical Research , said, "With the growing emphasis on global clinical trials, this acquisition now positions Veeda to offer access to a very diverse population for conducting large scale multi-geography trials efficiently. Both organizations are focused towards driving equitable access to trials and fostering the development of innovative treatments worldwide. The acquisition brings together a unique team of scientists and researchers, having deep therapeutic area expertise in the Oncology research. This equips Veeda to build long and enduring site relationships across geographies."
Elaborating on the prospects of this partnership, Mr. Binoy Gardi, Executive Vice Chairperson of Veeda Clinical Research, said, "As we integrate the operations of both the companies, this is an exciting opportunity for Veeda and Heads to expand markets, leverage complimentary capabilities and add value to our clients. We believe that our combined strengths will enable us to drive greater efficiency, innovation, and excellence in delivering research outcomes for drug development programs of our clients worldwide."
"There is a very strong cultural fit between Heads and Veeda," said Dr. George Kouvatseas, Partner, Heads . "Both companies share a dedication to scientific leadership, expansion of highly specialized services to meet the emerging needs of our clients, and an uncompromising focus on quality. During the integration phase, Heads will continue to offer uninterrupted support to client programs. The Veeda and Heads organization together are committed to nimble operations through a structured integration process without impact to ongoing client programs."
About Veeda Clinical Research Limited:
Veeda Clinical Research Limited ("Veeda") is one of the largest independent, full-service clinical research organizations, headquartered in Ahmedabad, India. Veeda offers a range of bioequivalence studies and early and late phase clinical trials. Veeda has successfully completed several regulatory inspections and is approved by USFDA, UK MHRA, ANVISA (Brazil), and WHO. Veeda has experience in conducting complex clinical studies. Visit https://www.veedacr.com/ .
About Heads:
Heads is a full-service Global Contract Research Organization (CRO) dedicated to the conduct of clinical studies, with expertise in haemato-oncology studies. It provides complete range of clinical trial services, from start-up to completion, from Phase I to IV. In addition, it offers strategic oncology drug development consulting and expert advice, as the team understands the challenges of developing anti-cancer drugs in the complex, highly competitive and rapidly developing oncology market. Visit https://heads-research.com/ .
Media contact:
Siddharth Baad Corporate Communications Veeda Clinical Research [email protected]
Logo: https://mma.prnewswire.com/media/2368181/Veeda_Heads_Logo.jpg
SOURCE Veeda Clinical Research Limited
Back to news
This site is part of the Informa Connect Division of Informa PLC
This site is operated by a business or businesses owned by Informa PLC and all copyright resides with them. Informa PLC's registered office is 5 Howick Place, London SW1P 1WG. Registered in England and Wales. Number 3099067.
- Informa Connect
Veeda buys Heads just weeks after appointing new CEO

Share this article
Indian clinical research organization (CRO) Veeda Clinical Research Limited has acquired European headquartered counterpart Heads, citing its global research and oncology experience as motivations.
The deal – financial terms of which were not disclosed – is part of an effort to grow Veeda’s global presence in line with customer demand for clinical trial capacity in multiple markets according to CEO, Mahesh Bhalgat.
"With the growing emphasis on global clinical trials, this acquisition now positions Veeda to offer access to a very diverse population for conducting large scale multi-geography trials efficiently. Both organizations are focused towards driving equitable access to trials and fostering the development of innovative treatments worldwide.
“The acquisition brings together a unique team of scientists and researchers, having deep therapeutic area expertise in oncology research. This equips Veeda to build long and enduring site relationships across geographies,” he said.
Heads has offices or an operational presence in 25 locations across Europe, North America, and Asia according to its website. Going forward, details of how the firms will work together were not provided.
However, Heads partner, George Kouvatseas said the CRO would “continue to offer uninterrupted support to client programs.”
Kouvatseas added that “The Veeda and Heads organization together are committed to nimble operations through a structured integration process without impact to ongoing client programs," he added.
The takeover is Veeda’s first deal since industry veteran Bhalgat was appointed CEO in early March . The move fits with comments company director, Vivek Chhachhi, made at the time.
“Mahesh brings global experience from organizations such as Amgen, Sanofi, Syngene and others to drive international standards into Veeda.
“He will focus on business growth, including mergers and acquisitions, leveraging technology and client centricity to bring the best of Veeda's service portfolios to the global clients.”
Unsplash/kellysikkema
Related Articles
Fsd hires ingenu for phase i ms drug study, frontage and eps team to take on japan’s “drug lag”, sign up for clinical insider email updates.

IMAGES
COMMENTS
Contract Research Organizations in Europe in alphabetical order. 54gene's Clinical Programs Group (CPG) Website: www.54gene.com Email: [email protected] Phone: +971 58 594 9926; +1 310-266-9926. 54gene's Clinical Programs Group (CPG) is a contract research organization that provides innovative, customizable, and cost-effective clinical trials ...
Baláti CRO is a small family owned contract research organization (CRO) offering a comprehensive range of services for the pharmaceutical, biotechnological and medical device industry with a special focus on phase 2-4 clinical trials in Hungary. Our services include clinical trial management, quality assurance, and medical writing activities.
9. KCR. Established in 1997 under the name Kiecana Clinical Research, KCR is a full-service contract research organization that provides a variety of services for clinical monitoring, safety & pharmacovigilance, clinical project management, quality assurance and regulatory affairs.
ECRIN, the European Clinical Research Infrastructure Network, facilitates multinational clinical research, through the provision of advice and services for the set-up and management of investigator or SME led clinical studies in Europe. ECRIN unites national networks of clinical trial units across Europe, through its scientific partners, to ...
EUCROF has 13 Working Groups: Clinical Trial Centres: this WG was created at the beginning of 2020. The primary objective of the Clinical Trial Centres Working Group is to look for synergies between CROs and Research Sites / Clinical Trial Centres to enforce and guarantee the clinical research in Europe. It is indispensable to ensure that sites ...
ClinSearch is a French Clinical Research Organization which provides clinical development and commercialization services for medical device and drug development on a European level. We are committed to offering individualized, cost-effective solutions to managing pre- or post-marketing studies in Europe, as well as obtaining reimbursement in ...
Join our webinars and take advantage of our experts to keep fully trained on key areas of interest in clinical research. Read more. ... Draft European Code of Conduct - PRESS RELEASE. 7 February 2024 Latest News Leave a comment. Leave review. Read More. 25-26/01/24 - ACT EU Clinical Trials Analytics multi-stakeholder workshop. 30 January 2024 ...
A unique CRO. The CERC is a unique Contract Research Organization based in Massy, France, and presided by five medical directors, Marie-Claude Morice, Philip Urban, Philippe Garot, Davide Capodanno and Antoinette Neylon. The CERC was conceived and founded by physicians with wide experience in clinical research. Their renown and expertise in ...
The EU Member States and EEA countries, the European Commission and EMA launched the Clinical Trials Information System on 31 January 2022. On the same day, EMA launched this website. EMA maintains this website in collaboration with the EU Member States and EEA countries, and the European Commission. The assessment, authorisation and ...
Full Service Clinical Research Organization in Europe . Expertise. Overview; Medical Devices; Advanced Therapy Medicinal Product ... We are a full service contract research organization offering a complete range of clinical development and consulting ... CRO experienced in neurology − Our clinical research experts have extensive experience ...
United Kingdom. Nottingham. 1st Floor Waterfront House Beeston Business Park Beeston, Nottingham NG9 1LA, UK. Our global CRO spans 60+ countries, all throughout Europe, focusing on clinical research that makes scientific & medical advancement personal.
Who we are. Parexel is among the world's largest clinical research organizations (CROs), providing the full range of Phase I to IV clinical development services to help lifesaving treatments reach patients faster. Leveraging the breadth of our clinical, regulatory, and therapeutic expertise, our team of more than 21,000 global professionals ...
Archer Research is a Contract Research Organization based in the heart of Europe. Introducing: Our dynamic team of experienced research professionals eager to advise you and drive your project with the highest quality. Let us do what we do best. Let us run your project. We are crazy about clinical research! Nice to meet you!
Cost-effective European CRO Services. Precision provides clinical research organization services throughout Europe, with clinical trials focused in Central and South-Eastern Europe (CEE) to leverage the operational efficiencies available in these regions. Efficiencies include faster patient recruitment due to the centralized healthcare systems ...
Munich-based FGK Clinical Research has acquired Clinicology Ltd., the contract research organizations announced Feb. 21 in a press release. The terms of the deal were not disclosed. "The ...
Clinical research remains a field with significant career potential worldwide. Clinical research associates (CRAs) continue to be highly sought after, overseeing lab and research processes. This role offers financial security and the possibility of frequent work travel. This guide provides an updated overview of becoming a CRA in the EU.
ProRelix Research is a Clinical Research Organization (CRO) with offices in the USA and India along with site networks in Australia, Thailand, Africa, and Europe supporting the clinical development of innovative new healthcare inventions and innovations to improve the health of patients. Every project is equally important to us. We first listen to you and strategize second, aiming to a long ...
Parexel is proudly among the world's largestclinical research organizations. A dedicated CRO providing the and leveraging the breadth of our , our team of more than 21,000 global professionals works in partnership with to design and deliver clinical trials , to make clinical research a care option for anyone, anywhere.
The Clinical Research Network is part of this organization. The Clinical Research Network provides a good infrastructure to support clinical research in the UK and the European Union, and provides support including identifying and recruiting patients, providing training so that researchers have access to experienced people.
Ad Hoc Clinical. Ad Hoc Clinical is a small CRO, located in the heart of Europe. In beautiful, peaceful Ypres/ Belgium. We offer 'ad hoc' clinical research services and support. You can rely on us for your European clinical research activities and suppo... 💻 Website ↗ 📞 +32 57 40 05 30 View all details.
Dublin (Ireland)/Ahmedabad (India), March 27, 2024: Veeda Clinical Research Limited, a full-service contract research organization (CRO) with a proven record of drug development success, announced that it has acquired Heads, a privately held European CRO, which specializes in conducting clinical trials in oncology.
The European CRO Federation, EUCROF, is a non-profit entity founded in 2005 and representing the interests of Contract-Clinical Research Organizations (CROs) in Europe towards regulatory bodies, the bio-pharmaceutical and medical device industry, healthcare professionals and patient's associations.
DUBLIN and AHMEDABAD, India, March 26, 2024 /PRNewswire/ -- Veeda Clinical Research Limited, a full-service contract research organization (CRO) with a proven record of drug development success, announced that it has acquired Heads, a privately held European CRO, which specializes in conducting clinical trials in oncology.Established in 2010, Heads has an operational presence in 25 multiple ...
The takeover is Veeda's first deal since industry veteran Bhalgat was appointed CEO in early March. The move fits with comments company director, Vivek Chhachhi, made at the time. "Mahesh brings global experience from organizations such as Amgen, Sanofi, Syngene and others to drive international standards into Veeda. "He will focus on ...
In addition, more clinical research is needed to provide effectiveness data for the use of rFVIIa in sPPH, specifically regarding the combination with current standard of care (tranexamic acid and fibrinogen); at the time of ongoing severe bleeding after balloon tamponade; evaluation of the optimal timing of administration of rFVIIa in sPPH ...