The elusive ethics of animal ethics committees
Honorary Associate, Australian Institute for Primary Care & Ageing, La Trobe University
The University of Melbourne

Disclosure statement
Monika Merkes is a financial member of Humane Research Australia and on the HRA Advisory Panel.
Rob Buttrose does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
University of Melbourne provides funding as a founding partner of The Conversation AU.
La Trobe University provides funding as a member of The Conversation AU.
View all partners
More than six million animals are used in experiments in Australia each year. Many endure pain and distress, and most are killed after their use.
The research community claims that our regulatory framework ensures animals are only used for scientific purposes when their use is essential and justified. We challenge this “clean image” and believe much animal experimentation today is unreasonable and continues the abuses of the past .
We also believe that the Australian public is being kept in the dark about the research use of animals. As in the cases of live exports and factory farming, public disclosure will be the key agent for change.
Animal advocacy groups argue unethical research that causes harm to animals and has insufficient benefits is still routinely approved in Australia. There are many examples – shaking lambs’ heads until they die to test hypotheses about “shaken baby syndrome”, breast implants in pigs, feeding junk food to rats and brain surgery on marmosets.
That such studies have proceeded after ethics approval suggests to critics of current practice that something is seriously amiss.
Animal experimentation in Australia is governed by the National Health and Medical Research Council’s (NHMRC) Code of Practice for the Care and Use of Animals for Scientific Purposes and state laws. According to the NHMRC, the purpose of the Code is to “ensure the ethical and humane care and use of animals used for scientific purposes as defined in the Code.”
This self-regulatory system is currently under review . Although compliance is strongly impressed on institutions and researchers, there are no penalties for not complying.
In many respects, the purpose of the Code is to legitimise the interests of the “industry”. The Code allows acts against animals which, if committed by an ordinary person outside a research institution, would be regarded as offences under the animal cruelty legislation.
Animal Ethics Committees
Under the Code, ethical review is conducted on every research proposal by an Animal Ethics Committee (AEC). These committees must have at least four members, with one having a commitment to animal welfare (the Category C member) and another who “should be viewed by the wider community as bringing a completely independent view to the AEC”, normally a layperson (the Category D member).
Approval decisions are made on the basis of consensus and any proposal must satisfy the committee that the requirements of replacement (not using animals where possible), reduction (reducing the number of animals used) and refinement (minimising impact) have been considered.
The contentious projects we mentioned above suggest that self-regulation of the animal research community is either too weak or not being properly complied with, or both. We’re puzzled that the AEC member with a commitment to animal welfare and the layperson assented to these experiments.
We suspect that in these cases the independent members were not independent or, if they were, perhaps they were restricted in expressing their point of view. Several critics (for example, here and here ) have claimed these are common problems with the operation of AECs.
Independently of the experience of AEC members, the principles and overall approach of the Code itself show how difficult it can be to reject projects as unethical. Despite some seemingly strong provisions to protect animals, we believe the Code biases AECs toward approval.
Under the Code, animal lives have no intrinsic value. As long as their suffering can be minimised “where possible”, they can be used and then disposed of in scientific projects of “merit”. The Code uses a lot of words, such as “necessary”, “essential” and “justified”, but is short on criteria for how these critical terms should be interpreted.
In the crude harm-to-benefit calculation that the Code sees as constituting ethical assessment, benefit (mostly to humans) will nearly always outweigh harm to animals even before the particulars of a project are examined. Because if there’s little harm caused when pain and discomfort are minimised as much as possible (relative to a project’s objectives), and killing an animal after use is doing it no harm, then minor benefits, and even minor potential benefits, will be sufficient to justify using animals.
Lack of disclosure and transparency
Like many before us, we discovered that it’s very difficult to find publicly available documents detailing the deliberations and decisions of AECs. This is despite the fact that the NHMRC and universities (big users of research animals and the focus of this article) are funded by taxpayers.
We could easily retrieve the AEC terms of reference for most institutions, as well as meeting dates and, in some cases, even the names of AEC members. But we found very few instances where an associated animal welfare organisation of a Category C member was named.
We couldn’t find the applications for the contentious studies listed above, nor the records of the AECs that approved them. In fact, we couldn’t find the AEC applications records for any research project that uses animals – that information is just not in the public domain.
When we contacted the major universities, we either received no reply, were directed to irrelevant information on a website, or were told that these details were the subject of confidentiality agreements to protect intellectual property. But how can intellectual property be an issue after research is published, as in the cases we cite?
Surely the veil of secrecy that hangs over AECs is unreasonable. The public should know the details of ethical assessments of animal use projects and be assured that animal welfare and community points of view are adequately represented.
Animal experiments with dubious benefits coupled with the lack of transparency have led us to believe that research involving animals approved under the Code is not necessarily essential and justified. If our assessment is correct, we doubt that animal ethics processes are as rigorous as the research community often claims.
A vital and urgent first step to change this situation and start seeing a reduction in the number of the animals used for experimental purposes is greater transparency. In particular, universities need to allow greater public scrutiny of their Animal Ethics Committees.
- Animal welfare
- Animal testing
- Animal ethics
- Animal ethics committee

Deputy Social Media Producer

Research Fellow /Senior Research Fellow – Implementation Science

Associate Professor, Occupational Therapy

GRAINS RESEARCH AND DEVELOPMENT CORPORATION CHAIRPERSON

Faculty of Law - Academic Appointment Opportunities
Ethical Guidelines for the Use of Animals in Research
Given by the National Committee for Research Ethics in Science and Technology (NENT), 2018.

Download as pdf
About the guidelines
These guidelines have been prepared by the National Committee for Research Ethics in Science and Technology (NENT). Their purpose is to provide ethical guidelines for researchers and other people who are considering experiments on animals. The guidelines will be useful when planning projects, assessing them, and when reporting and publishing findings and results. They are also intended to contribute to reflection on research ethics and the use of animals in research in both research communities and in the public debate.
The overarching framework for these guidelines is provided by the Guidelines for Research Ethics in Science and Technology (2016), particularly guidelines 12 and 13. A consultation process and a subsequent workshop organised by NENT in the autumn of 2016 found that relevant players see a need for a set of guidelines that can systematise and elaborate on the ethical responsibility inherent in the use of animals in research. This is the background for the current guidelines.
In Norway, the use of laboratory animals is governed by the Regulations Relating to the Use of Animals in Research, which follow from the Animal Welfare Act. The EEA Agreement obliges Norway to implement EU Directive 2010/63/EU on the Protection of Animals used for Scientific Purposes. These rules provide a zero vision for research using animals. In Norway, the Gene Technology Act provides the legal framework for research on such organisms.
Many of the ethical obligations stipulated in these guidelines are also laid down in applicable legislation. Researchers who violate the guidelines can face legal sanctions. In that case, it is because they have broken the law, not primarily because they have violated the guidelines for research ethics. NENT does not have access to any sanctions of its own. NENT's role in following up the guidelines is to provide advice and recommendations, help increase awareness of animal welfare, and to stimulate continued discussion about research that involves animals.
Ethics and Experiments on Animals
The ethical assessments related to the use of animals in research are wide-ranging. It is generally thought that it may be necessary to use laboratory animals in some cases in order to create improvements for people, animals or the environment. At the same time, the general opinion is that animals have a moral status, and that our treatment of them should be subject to ethical considerations. Such views are reflected in the following positions:
(i) Animals have an intrinsic value which must be respected.
(ii) Animals are sentient creatures with the capacity to feel pain, and the interests of animals must therefore be taken into consideration.
(iii) Our treatment of animals, including the use of animals in research, is an expression of our attitudes and influences us as moral actors.
The guidelines reflect all these positions, and stipulate principles and considerations that can be used as tools when balancing between harm and benefit. The three Rs (Replace, Reduce, Refine) are established principles that are also enshrined in legislation. These principles can establish absolute limits for experiments on animals, even when there are great benefits. These principles also state what can reasonably be considered harm and benefit, and the principles thus facilitate good assessments. Assessments of harm and benefit associated with experiments on animals are particularly demanding, because experiments may result in researchers intentionally causing actual harm to animals, while the future benefits are often uncertain.
The guidelines are dynamic and must be reviewed in line with technological developments and the appearance of new ethical issues. New gene technology methods create new opportunities for the use of genetically modified animals in research, which is a growing trend. Genetically modifying laboratory animals, i.e. changing the genetic material of laboratory animals using gene technology, gives rise to a special responsibility in that this method entails a double intervention: first, intervention in the animal's genetic material and second, use of the animal as a research object. This practice has the potential to change our view of humans and our attitudes towards generating or eliminating genetic characteristics in ourselves.
These guidelines provide a framework that also covers ethical questions associated with the use of genetically modified animals in research.
Definitions
In these guidelines, the term «research» must be understood broadly, and include planning, execution and dissemination. The guidelines primarily address the «researcher» but apply to any person involved when animals are used for research, including funding and approval bodies, which are also responsible for making ethical assessments of projects involving experiments on animals.
The guidelines cover «laboratory animals», as defined in the Regulations Relating to the Use of Animals in Research, but also cover all animals that are otherwise impacted by research activities.
1. Respect for animals' dignity
Researchers must have respect for animals' worth, regardless of their utility value, and for animals' interests as living, sentient creatures. Researchers must be respectful when choosing their topic and methods, and when disseminating their research. Researchers must provide care that is adapted to the needs of each laboratory animal.
2. Responsibility for considering options ( Replace )
Researchers are responsible for studying whether there are alternatives to experiments on animals. Alternative options must be prioritised if the same knowledge can be acquired without using laboratory animals. If no good options are available, researchers should consider whether the research can be postponed until alternative methods have been developed. When justifying experiments on animals, researchers therefore must be able to account for the absence of options and the need to acquire knowledge immediately.
3. The principle of proportionality: responsibility for considering and balancing suffering and benefit
Researchers must consider the risk that laboratory animals experience pain and other suffering (see guideline 5) and assess them in relation to the value of the research for animals, people or the environment. Researchers are responsible for considering whether the experiment may result in improvements for animals, people or the environment. The possible benefits of the study must be considered, substantiated and specified in both the short and the long term. The responsibility also entails an obligation to consider the scientific quality of the experiments and whether the experiments will have relevant scientific benefits.
Suffering can only be caused to animals if this is counterbalanced by a substantial and probable benefit for animals, people or the environment.
There are many different methods for analysing harm and benefit. Research institutions should provide training on suitable models, and researchers are responsible for using such methods of analysis when planning experiments on animals.
4. Responsibility for considering reducing the number of animals ( Reduce )
Researchers are responsible for considering whether it is possible to reduce the number of animals the experiment plans to use and must only include the number necessary to maintain the scientific quality of the experiments and the relevance of the results. This means, among other things, that researchers must conduct literature studies, consider alternative experiment designs and perform design calculations before beginning experiments.
5. Responsibility for minimising the risk of suffering and improving animal welfare ( Refine )
Researchers are responsible for assessing the expected effect on laboratory animals. Researchers must minimise the risk of suffering and provide good animal welfare. Suffering includes pain, hunger, thirst, malnutrition, abnormal cold or heat, fear, stress, injury, illness and restrictions on the ability to behave normally/naturally.
A researcher's assessment of what is considered acceptable suffering should be based on the animals that suffer the most. If there are any doubts regarding perceived suffering, consideration of the animals must be the deciding factor.
Researchers must not only consider the direct suffering that may be endured during the experiment itself, but also the risk of suffering before and after the experiment, including trapping, labelling, anaesthetising, breeding, transportation, stabling and euthanising. This means that researchers must also take account of the need for periods of adaptation before and after the experiment.
6. Responsibility for maintaining biological diversity
Researchers are responsible for ensuring that the use of laboratory animals does not endanger biological diversity. This means that researchers must consider the consequences to the stock and to the ecosystem as a whole. The use of endangered and vulnerable species must be reduced to an absolute minimum. When there is credible, but uncertain, knowledge that the inclusion of animals in research or the use of certain methods may have ethically unacceptable consequences for the stock and the ecosystem as a whole, researchers must observe the precautionary principle.[ 1 ]
7. Responsibility when intervening in a habitat
Researchers are responsible for reducing disruption and any impact on the natural behaviour of individual animals, including those that are not direct subjects of research, as well as of populations and their surroundings. Certain research and technology-related projects, like those regarding environmental technology and environmental surveillance, may impact on animals and their living conditions, for example as a result of installing radar masts, antennas or other measurement instruments. In such cases, researchers must seek to observe the principle of proportionality (see guideline 3) and minimise the possible negative impact.
8. Responsibility for openness and sharing of data and material
Researchers are responsible for ensuring that there is transparency about research findings and facilitating the sharing of data and material from experiments on animals. Such transparency and sharing are important in order to avoid unnecessary repetition of experiments. Transparency is also important in order to ensure that the public are informed and is part of researchers' responsibility for dissemination.
In general, the negative results of experiments on animals should be public knowledge. Disclosing negative results may give other researchers information about which experiments are not worth pursuing, shine a light on unfortunate research design, and help reduce the use of animals in research.
9. Requirement of expertise on animals
Researchers and other parties who handle live animals must have adequately updated and documented expertise on animals. This includes specific knowledge about the biology of the animal species in question, and a willingness and ability to take care of animals properly.
10. Requirement of due care
There are national laws and rules and international conventions and agreements regarding the use of laboratory animals, and both researchers and research managers must comply with these. Any person who plans to use animals in experiments must familiarise themselves with the current rules.
References and useful resources
[ 1 ] The Norwegian National Committee for Research Ethics in Science and Technology (NENT). Guidelines for research ethics in science and technology (2007) 2016. Oslo.
Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32010L0063
Regulation on the capture and collection of wild animals for scientific or other special purposes (Forskrift om innfanging og innsamling av vilt for vitenskapelige eller andre særlige formål). 2003. https://lovdata.no/dokument/LTI/forskrift/2003-03-14-349
Regulation on Animal Experimentation (Forskrift om bruk av dyr i forsøk). 2015. https://lovdata.no/dokument/SF/forskrift/2015-06-18-761
Act of 2 April 1993 No. 38 Relating to the Production and Use of Genetically Modified Organisms, etc. (Gene Technology Act) (Lov om framstilling og bruk av genmodifiserte organismer m.m. 1993). https://www.regjeringen.no/en/ dokumenter/gene-technology-act/id173031/
The Animal Welfare Act. 2009. https://www.regjeringen.no/en/dokumenter/animal-welfare-act/id571188/
The ARRIVE Guidelines (Animal Research: Reporting of In Vivo Experiments). 2010. https://www.nc3rs.org.uk/sites/default/files/documents/Guidelines/NC3Rs%20ARRIVE%20Guidelines%202013.pdf
The Norwegian Food Safety Authority's instructions on the management of the Regulation on Animal Experimentation (Mattilsynets instruks om forvaltningen av Forsøksdyrforskriften): https://www.mattilsynet.no/dyr_og_dyrehold/dyrevelferd/forsoksdyr/ instruks_om_mattilsynets_forvaltning_av_forsoksdyrforskriften.21015/binary/Instruks%20om%20Mattilsynets%20forvaltning%20av%20forsøksdyrforskriften
PREPARE (Planning Research and Experimental Procedures on Animals: Recommendations for Excellence) guidelines. 2017. https://norecopa.no/prepare
Research & Innovation Office
Institutional Animal Care and Use Committee
- Regulatory Charge
- Governing Principles
- Regulatory Documents
- Institutional Official
- Committee Membership
- Member Sign In
- Submit & Maintain Protocols
- Criteria of Review
- Help Submitting Protocols
- Finding Models & Alternatives
- Research with Client-Owned Animals
- Research Involving Surgery
- Responding to Committee Stipulations
- Requesting Changes
- Inspections
- Animal Use Guidelines & Exceptions
- Mandatory Training
- Optional Training
- Recordkeeping
- Ethics of Animal Use in Research
Laws & Regulations
Reporting concerns, other guidelines.
- Pain & Distress
The use of animals in research, teaching and testing is a controversial ethical and political issue. Much of the discussion about this issue revolves around the relative value, often referred to as 'moral value', of humans and animals. When the needs of animals and humans come into conflict, which takes precedence? Today there exists a wide spectrum of views on this subject, ranging from those concerned with animal 'rights' to those who view animals only as a resource to be exploited. All of these viewpoints have contributed to the development of ethical principles of animal use. These in turn have shaped animal use regulations promulgated by the USDA and the Public Health Service, and reinforced by organizations such as AAALAC , AALAS and the AVMA .
Current legislation also recognizes that there are diverse viewpoints about the moral value of animals. Thus, all live animal use in research, teaching or testing must be reviewed by a committee (the IACUC) with diverse membership. This evaluation includes an emphasis on minimizing the overall use of animals.
Proposals for animal use are reviewed based on the potential for learning new information, or for teaching skills or concepts that cannot be obtained using an alternative. There are provisions for ensuring that animal use is performed in as humane a manner as possible, minimizing pain, distress or discomfort. These provisions include a requirement for a veterinarian to be employed at each institution, so that the needs of the animals are looked after by someone trained in, and sympathetic toward animals' needs. It is also required that all personnel with animal contact be trained in appropriate handling techniques and that they be skilled in any experimental procedures that will be performed. Finally, basic husbandry requirements are specified, ensuring that an animal's food, water and shelter will be provided for in an optimal manner. Deviations from the numerous requirements are granted by the IACUC only if adequate scientific justification is given that the proposed experiment is scientifically and socially important, and that any methods to alleviate pain or distress would frustrate the experimental objectives.
Animals have been used throughout history for anatomical and physiological research as well as for testing new medications and toxic substances. Many medical advances, including vaccines for polio and rabies, the development of certain antibiotics, cancer treating agents and transplant medicines, have been developed thanks to the use of animals in research.
The use of animals in research is a privilege and not a right. A research institution that receives money and support from the public is responsible for conducting research humanely and responsibly according to the limits set by society and regulatory bodies.
Animal Welfare Act
The Animal Welfare Act (AWA) was passed in 1966. This act licenses dealers, exhibitors and breeders of animals, regulates research facilities that use animals, sets standards for the humane care and treatment of animals, and regulates the transportation of animals. The Act has been amended multiple times adding further protections for animals covered by the Act. The AWA specifically exempts birds, mice, rats, amphibians and reptiles used in research as well as agricultural animals that are used for agricultural production.
The United States Department of Agriculture is the government agency that is responsible for the enforcement of this act. Facilities must submit an annual report to the USDA. The USDA conducts unannounced inspections of research facilities at least once a year. If violations of the Act are found, fines can be imposed or research activities can be stopped.
Public Health Service Policy
The Public Health Service (PHS) Policy on the Humane Care and Use of Laboratory Animals is based on the United States Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training. This policy covers all research that is funded by the National Institutes of Health (NIH) using vertebrate species of animals including birds, mice, and rats.
Institutions covered by this policy must follow the Guide for the Care and Use of Laboratory Animals (see below) and annually submit a written document called an Animal Welfare Assurance to NIH, which documents how the institution is complying with all the regulations covering animals used in research. The Office of Laboratory Animal Welfare (OLAW) at NIH is the agency that is responsible for enforcement of the PHS policy.
Guide for the Care and Use of Laboratory Animals
The Guide for the Care and Use of Laboratory Animals ("The Guide"), first developed in 1963, is a manual for research facilities receiving public funding for research using animals. The latest (2011) version of the Guide , sets specific standards for the care and use of laboratory animals. It addresses institutional responsibilities, husbandry and housing standards, veterinary care and physical plant specifications. It is written by experts in laboratory animal care and is published by the National Research Council.
AAALAC stands for the Association for the Assessment and Accreditation of Laboratory Animal Care. This is an independent (non-government) and voluntary accreditation organization. AAALAC accredits laboratory animal facilities through a process of intensive inspections (every 3 years) and reports (yearly). AAALAC follows the high standards put forth in the Guide. Accreditation, while voluntary, represents commitment to excellence in animal care and is an important factor to many funding agencies.
University of Minnesota Policy
The Regents Policy on Animal Care and Use addresses the use of all animals in research, teaching or display at the University of Minnesota. This policy follows from the federal and other laws and regulations. It addresses the roles and responsibilities of the Institutional Official, the Institutional Animal Care and Use Committee (IACUC), Research Animal Resources, and the University Community.
The Institutional Official (IO) is appointed by the University President and reports directly to him/her as well as to the federal authorities regarding compliance with all laws and regulations governing the use of laboratory animals in research and teaching. The President has formally delegated responsibility to appoint IACUC members to the Institutional Official.
The IACUC, which is a committee mandated by the AWA and the PHS policy, reviews and approves all activities involving animals at the University of Minnesota. The AWA and PHS policy have specific membership requirements for the committee. There must be at least:
- one veterinarian (with laboratory animal background and programmatic responsibility at the institution),
- one member of the community (non-affiliated member to represent the public interest),
- one scientist who uses animals in research, and
- one non-scientist member.
University policy states that the committee should have at least 5 members, but the committee has many members, including several student members and ex-officio representatives from Occupational Health & Safety and the Department of Environmental Health and Safety.
The committee reviews all animal care and use protocols to ensure:
- that the use of animals is necessary to achieve the stated objectives,
- that pain and distress is minimized, and
- that all the laws and policies for the use of animals are followed.
The committee also ensures the humane care of animals through the inspection of animal housing and use facilities twice a year and by investigating any complaints made regarding animal use. The committee is also responsible for reporting any instances of non-compliance and recommending corrective action.
Research Animal Resources (RAR) is designated by University policy as the program that provides the housing and husbandry as well as the veterinary care for the laboratory animals on the Twin Cities campus. They are also designated to serve as a consultation resource for the care and use of animals in research and teaching.
University policy also lists the roles and responsibilities of the University community. The University researchers and staff are to be appropriately trained and qualified to conduct activities with animals and are to abide by the decisions of the University and the IACUC.
For serious questions or concerns about animal welfare, the process of review, or about committee decisions, contact:
Shashank Priya Institutional Official (612) 624-5054 [email protected]
Joanne Billings Deputy Institutional Official (612) 624-0999 [email protected]
You may also report animal welfare concerns or policy violations via the University of Minnesota's reporting system. The UReport provides a way for University community members to report violations of rules, regulations and policies. The report can be made anonymously.
Note that, by federal law, no facility employee, Committee member, or laboratory personnel shall be discriminated against or be subject to any reprisal for reporting violations of any regulation or standards.
Agricultural
The Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching is a text published by the Federation of Animal Sciences Societies. This Guide addresses standards for agricultural animal husbandry, housing and veterinary care. It does not apply to agricultural animals used for biomedical type research or teaching.
The standards are slightly different than those listed in the Guide for the Care and Use of Laboratory Animals. For example, cage space requirements may differ slightly between the two texts. Although this text is not regulatory, the University uses its provisions and principles as the basis for its care and use programs involving animals used for production or agricultural research.
There are several references available for the use of fish , amphibians and mammals in wildlife research . Again, these documents are not regulatory documents but are excellent references for the care and handling of these animals.
Pain & Distress
The AWA defines a painful procedure in an animal as: "any procedure that would reasonably be expected to cause more than slight or momentary pain or distress in a human being to which that procedure was applied, that is, pain in excess of that caused by injections or other minor procedures." Pain can be acute, short lived - or chronic, lasting a long time. The signs manifesting acute or chronic pain may differ and may be different in different species. Prey species of animals can be adept at hiding signs of pain or illness and may be more difficult to assess discomfort.
Signs of Acute Pain in Animals
- vocalization
- attempts to escape the stimulus
- aggressive responses
- increased heart and respiratory rates
- anorexia or shaking
Signs of Chronic Pain
- weight loss
- poor or unkempt hair coats
- depression or lethargy
- general debilitation
Distress is currently defined as "a state in which an animal cannot escape from or adapt to the external or internal stressors or conditions it experiences resulting in negative effects upon its well being…" Distress differs from stress, which is a physiological reaction that can lead to an adaptive response.
Principle IV of the US Government Principles states that unless the contrary is established, the assumption must be made that a procedure that causes pain or distress in a human being will cause pain and distress in an animal.
Alternatives
Current regulations stress the need to search for and utilize alternatives to procedures on animals that cause more than momentary pain or distress. The concept of the three "R"s has been used when thinking about alternatives to animal use. This concept was developed in 1959 by Russell and Burch in their book: The Principles of Humane Animal Experimental Techniques.
The three "R"s are Replacement, Reduction, and Refinement. Investigators at the University of Minnesota, who use animals that may undergo more than momentary pain or distress, should consider the three “R”s when conducting procedures which may be painful or distressful.
Replacement of animals with other systems may be an option. Computer modeling or in vitro testing may be a substitute for animal models. "Lower" or non-vertebrate animals, such as the fruit fly may be used in some situations rather than a higher order animal.
Reduction of the number of animals used for research is also an important concept. This is done mostly through experimental design and the use of statistics. The use of too few animals could result in statistically invalid results, which could necessitate the use of even more animals in subsequent experiments. Pilot studies to help determine statistical parameters can sometimes assist in determination of group sizes. Reduction of pain and distress may also actually require the use of more animals so that repeated procedures are not conducted on the same animal.
Refinement refers to methods that decrease the amount of pain and distress experienced by the animals that are actually needed to perform an experiment. This is not only done through the use of pain relieving measures such as anesthetics and analgesics whenever possible, but also through environmental enrichment.
The use of early endpoints can also be a form of refinement. For instance if an animal were to suffer from an early indicator of disease or a tumor reaches a certain measurable size, this could be used as the endpoint. The animals should be humanely euthanized at this point rather than waiting until the death of the animal.
For more examples of replacement/reduction/refinement and searches for alternatives, see IACUC's web page, “Finding Models and Alternatives”.
Ethical Review of Animal Research and the Standards of Procedural Justice: A European Perspective
Affiliation.
- 1 Research Centre for Public Policy and Regulatory Governance, University of Silesia in Katowice, 11b Bankowa str, 40-007, Katowice, Poland. [email protected].
- PMID: 34283345
- PMCID: PMC8566386
- DOI: 10.1007/s11673-021-10111-5
Committees established for the ethical review of research involving animals have become a widespread legal standard around the world. Despite many differences in their composition, powers, and institutional settings, they share many common problems related to the well-established standards of procedural justice in administrative practice. The paper adapts the general theory of procedural justice to the specific context of ethical review committees. From this perspective, the main concerns over the procedural aspects of the ethical evaluation of research projects are identified and examined. They include in particular the standards of the committees' composition, impartiality, fair hearing, appeal, transparency, and benevolence. Their proper reflection in the regulatory regimes of animal ethics committees is necessary to secure the standards of fairness of the ethical review itself. This, in turn, is a condition of the moral and social legitimacy of all administrative and quasi-administrative procedures, including the committees' operations (irrespective of whether they are legally entrusted with the task of authorizing or only evaluating research projects).
Keywords: Animal experimentation; Animal welfare; Ethical committees; Ethics; Law; Procedural justice; Research ethics.
© 2021. The Author(s).
- Animal Experimentation*
- Ethical Review
- Ethics Committees, Research
- Social Justice
Ethical Issues in Animal Research
- First Online: 16 November 2022
Cite this chapter

- Gerard Marshall Raj 4 &
- Rekha Priyadarshini 4
1018 Accesses
1 Citations
Contribution of animals in biomedical research—though in varied proportions—is indispensable. Both the cases for and against the use of laboratory animals are equally debatable. Apart from fundamental biological research, animals were extensively utilized in drug toxicity testings including non-pharmaceutical chemical safety assessments and also in biomedical teaching and training. However, with the growing understanding of animal experimentations and animal ethics—particularly with greater application of the 3R principles—nowadays, the experimental procedures involving animals warrant even more judicious perusal. Whenever feasible, the principle of replacement (absolute or relative) is given prime importance and engagement of appropriate alternatives to animal experiments (non-animal testing methods) is highly recommended. Reduction and refinement (and rehabilitation , the 4th R) are secondary principles of humane animal experimentations. This Chapter includes discussion on the principles of animal ethics, the evolution of ethical issues in animal experiments, the 3R approach including the alternatives to animal experiments, the present status of animal experimentations, and the various guidelines related to animal research.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Bibliography
Akbarsha MA, Pereira S. Mahatma gandhi-doerenkamp center for alternatives to use of animals in life science education. J Pharmacol Pharmacother. 2010;1(2):108–10. https://doi.org/10.4103/0976-500X.72353 .
Article Google Scholar
Akbarsha MA, Zeeshan M, Pereira S. Alternatives discussed at Indian science congress. ALTEX. 2012;29(2):216–8. https://doi.org/10.14573/altex.2012.2.216 .
Badyal DK, Desai C. Animal use in pharmacology education and research: the changing scenario. Indian J Pharmacol. 2014;46(3):257–65. https://doi.org/10.4103/0253-7613.132153 .
Caloni F, De Angelis I, Hartung T. Replacement of animal testing by integrated approaches to testing and assessment (IATA): a call for in vivitrosi. Arch Toxicol. 2022. Epub ahead of print. doi: https://doi.org/10.1007/s00204-022-03299-x .
Cheluvappa R, Scowen P, Eri R. Ethics of animal research in human disease remediation, its institutional teaching; and alternatives to animal experimentation. Pharmacol Res Perspect. 2017 Aug;5(4):e00332. https://doi.org/10.1002/prp2.332 .
CPCSE. Compendium of CPCSEA, 2018. Available from: http://cpcsea.nic.in
Google Scholar
CPCSEA. Standard Operating Procedures (SOP) for IAEC, 2010 [Internet]. 2010 [cited 2022 May 6]. Available from: http://cpcsea.nic.in/WriteReadData/userfiles/file/SOP_CPCSEA_inner_page.pdf.
Doke SK, Dhawale SC. Alternatives to animal testing: a review. Saudi Pharm J. 2015 Jul;23(3):223–9. https://doi.org/10.1016/j.jsps.2013.11.002 .
Festing S, Wilkinson R. The ethics of animal research. Talking Point on the use of animals in scientific research. EMBO Rep. 2007 Jun;8(6):526–30. doi: 10.1038/sj.embor.7400993. PMID: 17545991; PMCID: PMC2002542.
Hubrecht RC, Carter E. The 3Rs and humane experimental technique: implementing change. Animals (Basel). 2019 Sep 30;9(10):754. https://doi.org/10.3390/ani9100754 .
ICCVAM (Interagency Coordinating Committee on the Validation of Alternative Methods). A Strategic Roadmap for Establishing New Approaches to Evaluate the Safety of Chemicals and Medical Products in the United States [Internet]. 2018 [cited 2022 April 17]. Available from: https://dx.doi.org/10.22427/NTP-ICCVAM-ROADMAP2018.
INSA. Man, Animal and Science, 2011. [Internet]. 2011 [cited 2022 May 6]. Available from: https://www.insaindia.res.in/pdf/FINAL_DOCUMENT_ON_MAN_ANIMAL_SCIENCE.pdf.
Knight A. 127 million non-human vertebrates used worldwide for scientific purposes in 2005. Altern Lab Anim. 2008;36(5):494–6.
Article CAS Google Scholar
Knight J, Rovida C, Kreiling R, Zhu C, Knudsen M, Hartung T. Continuing animal tests on cosmetic ingredients for REACH in the EU. ALTEX. 2021;38(4):653–68. https://doi.org/10.14573/altex.2104221 .
Kojima H, Seidle T, Spielmann H. Alternatives to animal testing. Proceedings of Asian Congress. 2016. Available from: https://link.springer.com/book/10.1007/978-981-13-2447-5.
Liebsch M, Grune B, Seiler A, Butzke D, Oelgeschläger M, Pirow R, Adler S, Riebeling C, Luch A. Alternatives to animal testing: current status and future perspectives. Arch Toxicol. 2011 Aug;85(8):841–58. https://doi.org/10.1007/s00204-011-0718-x .
National Research Council. Guide for the care and use of laboratory animals: Eighth Edition. Washington, DC: The National Academies Press [Internet]. 2011 [cited 2022 May 11]. Available from: https://doi.org/10.17226/12910.
NMC. Minimum Requirements for Annual M.B.B.S. Admissions Regulations, 2020 [Internet]. 2020 [cited 2022 March 21]. Available from: https://www.nmc.org.in/ActivitiWebClient/open/getDocument?path=/Documents/Public/Portal/NmcGazette/Medical%20College%20MSR%20regulations%202020.pdf.
Pereira S, Tettamanti M. Ahimsa and alternatives: the concept of the 4th R. The CPCSEA in India. ALTEX. 2005;22(1):3–6.
Pereira S, Veeraraghavan P, Ghosh S, Gandhi M. Animal experimentation and ethics in India: the CPCSEA makes a difference. Altern Lab Anim. 2004 Jun;32(Suppl 1B):411–5. https://doi.org/10.1177/026119290403201s67 .
Sakuratani Y, Horie M, Leinala E. Integrated approaches to testing and assessment: OECD activities on the development and use of adverse outcome pathways and case studies. Basic Clin Pharmacol Toxicol. 2018 Sep;123(Suppl 5):20–8. https://doi.org/10.1111/bcpt.12955 .
Smith AJ, Hawkins P. Good science, good sense and good sensibilities: the three Ss of Carol Newton. Animals (Basel). 2016;6(11):70. https://doi.org/10.3390/ani6110070 .
Stephens ML. Personal reflections on Russell and Burch, FRAME, and the HSUS. Altern Lab Anim. 2009 Dec;37(Suppl 2):29–33. https://doi.org/10.1177/026119290903702S21 .
Tannenbaum J, Bennett BT. Russell and Burch's 3Rs then and now: the need for clarity in definition and purpose. J Am Assoc Lab Anim Sci. 2015 Mar;54(2):120–32.
The Norwegian National Research Ethics Committees. Ethical Guidelines for the Use of Animals in Research, 2018 [Internet]. 2019 [cited 2022 Feb 24]. Available from: https://www.forskningsetikk.no/en/guidelines/science-and-technology/ethical-guidelines-for-the-use-of-animals-in-research/.
UGC. Guidelines for discontinuation of dissection and animal· experimentation in Zoology/ Life Sciences in a phased manner [Internet]. 2011 [cited 2022 March 20]. Available from: https://www.ugc.ac.in/pdfnews/6686154_guideline.pdf.
UGC. Dissection and animal experimentation in zoology /life sciences and allied disciplines in undergraduate, postgraduate and research programmes [Internet]. 2014 [cited 2022 March 20]. Available from: https://www.ugc.ac.in/pdfnews/6819407_ugcletterzoology.pdf.
Download references
Author information
Authors and affiliations.
Department of Pharmacology, All India Institute of Medical Sciences (AIIMS) Bibinagar, Hyderabad, Telangana, India
Gerard Marshall Raj & Rekha Priyadarshini
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Gerard Marshall Raj .
Editor information
Editors and affiliations.
Department of Pharmacology, Thanjavur Medical College, Thanjavur, Tamil Nadu, India
Mageshwaran Lakshmanan
Department of Pharmacology, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, Pondicherry, India
Deepak Gopal Shewade
Gerard Marshall Raj
Rights and permissions
Reprints and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this chapter
Raj, G.M., Priyadarshini, R. (2022). Ethical Issues in Animal Research. In: Lakshmanan, M., Shewade, D.G., Raj, G.M. (eds) Introduction to Basics of Pharmacology and Toxicology. Springer, Singapore. https://doi.org/10.1007/978-981-19-5343-9_49
Download citation
DOI : https://doi.org/10.1007/978-981-19-5343-9_49
Published : 16 November 2022
Publisher Name : Springer, Singapore
Print ISBN : 978-981-19-5342-2
Online ISBN : 978-981-19-5343-9
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- www.mandela.ac.za

Change the world
Office of Research Development
- Message from the Director
- RD Workshops
- FindFunding@RD
- Postgraduate Research Scholarships
- Internal Research Bursaries (IRB's)
- Student Account Refunds
- Postdoctoral and Research Fellowships
- Research Ethics Committee: Human (REC-H)
Research Ethics Committee: Animal (REC-A)
- Unit for Statistical Consultation
- RD Facebook Page
The Nelson Mandela University Research Ethics Committee (Animal) (REC-A) is charged with monitoring the treatment of animals used in both research and teaching at the institution. It has the responsibility of reviewing all protocols involving animal use in order to ensure that they are in accordance with acceptable ethical and scientific standards as described in the South African National Standard for the Care and Use of Animals for Scientific Purposes 10386:2021. The REC-A assists the institution in complying with legislation and creating an awareness of ethical conduct in researchers and teachers.
Respect for animals must underpin all decisions and actions of the people involved in the care and use of animals for scientific purposes. This respect is demonstrated by
- using animals only when it is justified,
- supporting the well-being of the animals involved,
- avoiding or minimizing harm, including pain, suffering, distress and lasting harm, to those animals,
- applying high standards of scientific integrity,
- the Replacement of animals with alternatives;
- the Reductio n in the number of animals used;
- the Refinement of techniques used to minimize the adverse impact on animals; and
- the knowledge and acceptance of one’s Responsibilities .
SANS 10386:2021_Ed 2: The care and use of animals for scientific purposes (Please note: This document is subject to copyright and is only available to staff and students at Nelson Mandela University. It may reside on a LAN, WAN, intranet, internet or ECM server and is available in accordance with copyright exploitation agreement no. 014/009/21-006, valid until 2023-03-31. Only students, lecturers and staff members of the Nelson Mandela University may make paper copies of the standard. No paper copy may be photocopied or reproduced in any way.’)
Applying for Ethics Approval from REC-A:
All applications for approval by Mandela staff and/or registered students must be submitted online on MEOS (Mandela Ethics Online System). This includes the following:
- Research studies: Any activity involving the acquisition, keeping or use of live animals for a research study
- Practicals: Any activity involving the acquisition, keeping or use of live animals for practical work
- Use of an External Animal Sample Collection: Any activity that requires the acquisition of already existing animal samples
- Use of Data from an Animal Sample Collection: Any activity where there there is a need for previously collected/secondary data to be used in a study
- Extension Applications
- Amendments to an Approved Protocol
- Reporting of an Unexpected Mortality/Adverse Event
- Reporting the Closure of a Research Study
Reporting on protocols not approved on MEOS:
Progress reports, amendments, extensions, etc. for protocols that were not approved on MEOS cannot be submitted on MEOS. The processes to be followed can be found on the previous REC-A webpage .
Reporting Research Misconduct:
Any member of the public or Nelson Mandela fraternity who is concerned that Mandela staff or student research might be contravening ethical research principles is asked to report their concerns. The reporting process as well as further information on what constitutes research misconduct is available on the Report Research Misconduct page.

Meeting Dates
Reference documentation, mandela university animal ethics reference documentation:.
- REC-A Standard Operating Procedures (2023): The Nelson Mandela University Research Ethics Committee - Animal is charged with monitoring the treatment of animals used in research and teaching at the University. This work-in-progress document outlines the standard operating procedures of REC-A.
- REC-A Terms of Reference : This can be found on the REC-A Terms of Reference and Standard Operating Procedures document of 2016.
- Guidelines for Ethical Conduct in the Care and Handling of Animals used for Research and Education at the Nelson Mandela University
- Nelson Mandela University Code of Conduct for Researchers: This policy gives expression to the values that apply at the Nelson Mandela University and to which all researchers (students, academic and support staff) commit themselves in their research activities.
- Nelson Mandela University Research Ethics Policy : This policy aims to promote awareness of fundamental ethical standards, principles and practices when conducting research with human participants.
Recommended Reading:
- South African Medical Research Council Research Ethics Policy
- South African Medical Research Council Guidelines on the Responsible Conduct of Research
- Department of Health Research Ethics Guidelines (2015) : This document provides the minimum national benchmark of norms and standards for conducting responsible and ethical research.
- World Organisation for Animal Health: Terrestrial Animal Health Code (2019)
How-To Users Guides / Videos
Meos applicant/supervisor user guides:.
- Applicant User Guide (ver 1.5, updated 19 Jun '23)
- Update Project (ver 1.0, published Aug '23)
- Responding to a Signature Request (ver 1.0, published 2 Jun '23)
MEOS How-to Video:
- Starting an ethics application (4.30 min)

For Enquiries about the REC-A Ethics Application Process:
Imtiaz Khan ( [email protected] ) Terry Adams ( [email protected] )
For Enquiries about Animal Ethics Principles:
Shaun Welman ( [email protected] ) Pierre Pistorius ( [email protected] )
For Technical Enquiries about the Online System:
Work@Mandela
FindIt@Mandela

Commercial Services@Mandela
Tel: +27 (0) 41 504 1111
Fax: +27 (0) 41 504 2574 / 2731
Email: [email protected]
PO Box 77000, Nelson Mandela University
Gqeberha, 6031, South Africa
Connect@Mandela
- A - Z Index
- Privacy statement

Privacy statement Mail & Portals --> BEE & Tax Certificate PAIA ISPA FAQ Sitemap A - Z Index --> WCMS

- info@unizik.edu.ng
- Nnamdi Azikiwe University PMB 5025, Awka, Anambra State
Animal Research Ethics Committee
Animal research ethics committee application form.
It is the responsibility of the Principal Investigator to ensure that all facets of animal care and use meet the requirements of the Code of Practice for the Care of Use of Animal for Scientific purposes in Nnamdi Azikiwe University. This includes a responsibility to protect and promote the welfare of animals used.
The Code of Practice embodies the principles of:
- Replacement of animal use
- Reduction of animal use
- Refinement of animal use
It is important to consider these principles when designing and carrying out projects. Under the Animal Research Act (1985), approval by an Animal Research Ethics Committee (AREC) is required for the use of any experimental animal for research and teaching. Approval can be given for up to 3 years. Annual renewal of approval is conditional on submission of all required paperwork and compliance with the Code. The AREC must assess the impact of all procedures and the project as a whole on animals.
- Any activity involving the acquisition of animals for research, education or practical work hereafter called a study requires completion of this form and submission for approval to the Animal Research Ethics Committee.
- Each researcher or investigator has the primary responsibility for ensuring that animal carcasses or parts are kept and used where it is protected from other students, staff and private individuals.
- Complete all fields in the form below and enter NONE for items not relevant to your study.
- Ethics approval remains valid for three years, provided that the approval protocols and conditions remain unchanged.
- How to Proceed
a. Fill the application form by clicking Fill Application Form
b. Complete the form and click on the Submit button to ensure confirmation of submission.
- Open access
- Published: 31 January 2023
Final analysis of the phase 3 randomized clinical trial comparing HD201 vs. referent trastuzumab in patients with ERBB2-positive breast cancer treated in the neoadjuvant setting
- Xavier Pivot 1 ,
- Alexey Georgievitch Manikhas 2 ,
- Volodymyr Shamrai 3 ,
- Giorgi Dzagnidze 4 ,
- Hwoei Fen Soo Hoo 5 ,
- Viriya Kaewkangsadan 6 ,
- Fausto Petrelli 7 ,
- Cristian Villanueva 8 ,
- Jamie Kim 9 ,
- Sumita Pradhan 9 ,
- Litha Jaison 9 ,
- Peggy Feyaerts 9 ,
- Leonard Kaufman 10 ,
- Marie-Paule Derde 10 ,
- Filip Deforce 10 &
- David G. Cox 1
BMC Cancer volume 23 , Article number: 112 ( 2023 ) Cite this article
1774 Accesses
17 Altmetric
Metrics details
The TROIKA trial established that HD201 and trastuzumab were equivalent in terms of primary endpoints (total pathological complete response) following neoadjuvant treatment. The objective of the present analysis was to compare survival outcomes and final safety.
In the TROIKA trial, patients with ERBB2-positive early breast cancer were randomized and treated with either HD201 or the referent trastuzumab. Eligible patients received 8 cycles of either HD201 or referent trastuzumab (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) every 3 weeks in combination with 8 cycles of chemotherapy (4 cycles of docetaxel, 75 mg/m 2 , followed by 4 cycles of epirubicin, 75 mg/m 2 , and cyclophosphamide, 500 mg/m 2 ) in the neoadjuvant setting. The patients then underwent surgery followed by 10 cycles of adjuvant HD201 or referent trastuzumab according to their initial randomization to complete one year of trastuzumab-directed therapy. Event-free and overall survival rates were calculated using Kaplan–Meier analysis. The hazard ratio for event-free survival was estimated by Cox proportional hazards regression.
The final analysis was performed after all patients completed the study at a median follow-up of 37.7 months (Q1-Q3, 37.3–38.1 months). A total of 502 randomized patients received either HD201 or the referent trastuzumab, and 474 (94.2%) were eligible for inclusion in the per-protocol set. In this population, the 3-year event-free survival rates were 85.6% (95% CI: 80.28–89.52) and 84.9% (95% CI: 79.54–88.88) in the HD201 and referent trastuzumab groups, respectively (log rank p = 0.938) (HR 1.02, 95% CI: 0.63–1.63; p = 0.945). The 3-year overall survival rates were comparable between the HD201 (95.6%; 95% CI: 91.90–97.59) and referent trastuzumab treatment groups (96.0%, 95% CI: 92.45–97.90) (log rank p = 0.606). During the posttreatment follow-up period, adverse events were reported for 64 (27.4%) and 72 (29.8%) patients in the HD201 and the reference trastuzumab groups, respectively. Serious adverse events were rare and none of which were related to the study treatment.
Conclusions
This final analysis of the TROIKA trial further confirms the comparable efficacy and safety of HD201 and trastuzumab.
Trial registration
ClinicalTrials.gov identifier: NCT03013504.
Peer Review reports
Introduction
In the primary analysis of the prospective, randomized, multicenter phase 3 TROIKA study, HD201, a trastuzumab biosimilar, was shown to be equivalent to the referent trastuzumab in patients with ERBB2-positive early breast cancer (EBC) based on the primary endpoints of locally assessed total pathologic complete response (tpCR) [ 1 ].
The relationship between tpCR status and survival has been extensively debated following a meta-analysis indicating that tpCR status predicts survival outcome in patients with ERBB2-positive EBC [ 2 ]. Regulatory agencies have acknowledged this relationship by authorizing several compounds on this early criterion for activity [ 3 , 4 , 5 , 6 , 7 ]. The neoadjuvant setting can be definitively considered the new era for development in ERBB2-positive breast cancer [ 8 ]. It remains reassuring that in most cases, the conclusion derived from the early criteria of pathologic complete response (pCR) has been confirmed by survival outcome analysis [ 9 , 10 , 11 ]. In this final analysis of the TROIKA study, we report the long-term efficacy and safety outcomes at 3 years of follow-up.
Study design and patients
TROIKA (NCT03013504) was a multicenter, randomized, phase 3 trial previously detailed in the publication reporting the primary analysis [ 1 ]. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Approval of the study protocol and all accompanying documents provided to the patients was obtained from independent ethics committees at participating institutions, and all patients provided voluntary written informed consent. Key eligibility criteria were age ≥ 18 years; ERBB2-positivity; new diagnosis; unilateral, operable breast cancer; and a baseline left ventricular ejection fraction ≥ 55%.
Patients were enrolled and randomized using a block of 8 in a ratio of 1:1 to receive either HD201 or referent trastuzumab (loading dose: 8 mg/kg; maintenance dose: 6 mg/kg) every 3 weeks, administered concurrently with 8 cycles of chemotherapy (4 cycles of docetaxel [75 mg/m 2 ], followed by 4 cycles of epirubicin [75 mg/m 2 ]/cyclophosphamide [500 mg/m 2 ]) in the neoadjuvant setting. After surgery, patients received an additional 10 cycles of HD201 or referent trastuzumab in the adjuvant setting according to the previous allocation.
Secondary objectives included evaluation of event-free survival (EFS) (defined as the time from randomization to the first observation of disease progression, including local and distant recurrence, second primary cancer, or death due to any cause), overall survival (OS) (defined as the time from randomization to death), safety, and immunogenicity. Exploratory analyses were conducted for EFS including locally assessed tpCR and bpCR as covariates.
Statistical analysis
Target sample sizes and statistical power calculations for the primary analysis have been reported previously [ 1 ]. Statistical analyses were performed with SAS (version 9.4; SAS Institute Inc., NC, USA). Kaplan–Meier analysis was used to estimate EFS and OS rates. Cox proportional hazards regression analyses providing hazard ratios (HRs) and 95% confidence intervals (95% CIs) for EFS adjusted for region, stage, and tumor hormonal receptor status are presented. Survival analyses were conducted in the per-protocol set (PPS), including all patients who received the study treatment (without a major protocol deviation affecting the primary efficacy assessment) and who underwent surgery after the completion of neoadjuvant treatment or did not undergo surgery because of lack of efficacy, and analysis was also performed in the modified full analysis set (mFAS), including all patients who received at least 1 dose of study medication (Fig. 1 ). Safety analyses were descriptive and conducted in all patients who received at least one dose of treatment. Adverse events (AEs) and serious AEs (SAEs) were recorded and graded per standard common technology criteria for adverse events (CTCAE).
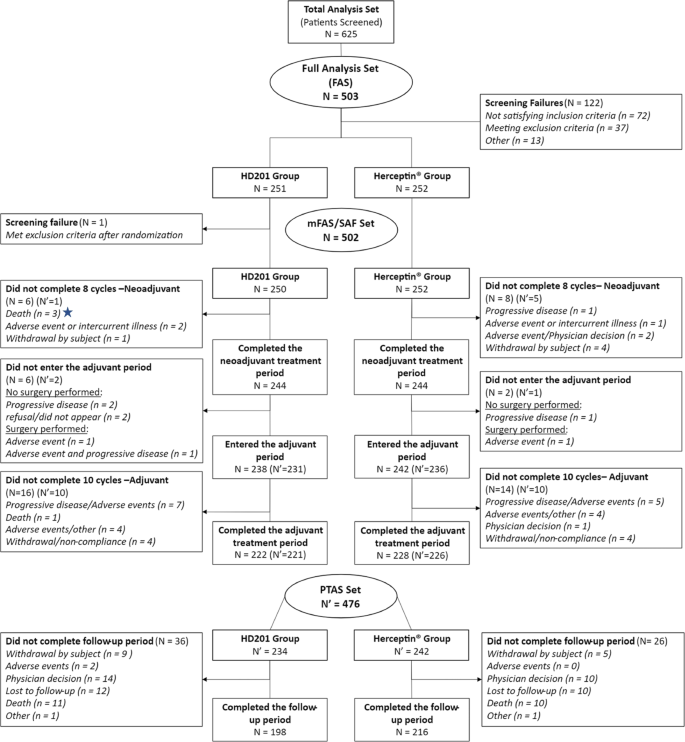
Patient distribution: CONSORT diagram
Patient population
This analysis was performed after all patients completed the study at a median follow-up of 37.7 months (Q1-Q3, 37.3–38.1 months). The mFAS comprised 502 randomized and treated patients, among whom 250 (49.8%) were in the HD201 group and 252 (50.2%) were in the referent trastuzumab group and were included between February 19 and September 21, 2018, across 70 centers in 12 countries. A total of 28 patients with mFAS were excluded from the PPS (12 patients in the HD201 treatment group and 16 patients in the referent trastuzumab group). The PPS thus comprised 238 patients in the HD201 treatment group and 236 patients in the referent trastuzumab treatment group. Baseline demographics and disease characteristics were well balanced between the study arms as reported previously [ 1 ].
In the PPS, the 3-year EFS rates were 85.6% (95% CI: 80.28–89.52) and 84.9% (95% CI: 79.54–88.88) in the HD201 and referent trastuzumab groups, respectively (log rank p = 0.938) (Fig. 2 A). The Cox proportional HR adjusted for region, stage, and tumor hormonal receptor status was 1.02 (95% CI: 0.63–1.63; p = 0.945) (Fig. 2 A). The 3-year OS rates were comparable for the HD201 (95.5%; 95% CI: 91.90–97.59) and referent trastuzumab treatment groups (96.0%, 95% CI: 92.45–97.90) (log rank p = 0.606) (Fig. 2 B). These results for EFS and OS were similar to those in the mFAS population (Figs. 2 E and F). The sensitivity analysis searching heterogeneity of treatment effect according to the disease characteristics did not observed any discordances between the two arms in terms of survival outcomes.
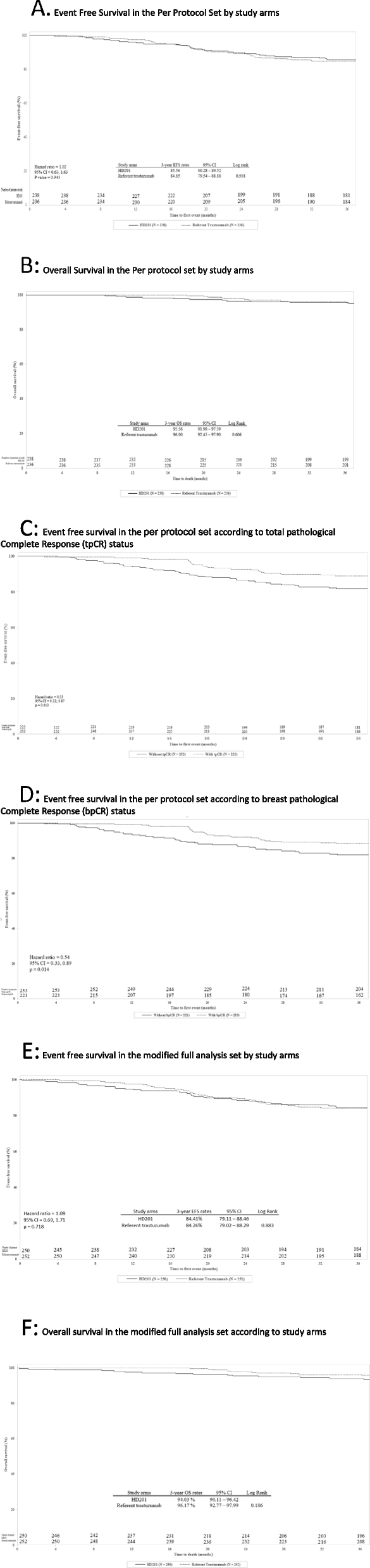
Event-Free Survival and Overall Survival in the Per Protocol set (PPS) and in the modified Full Analysis set (mFAS). A EFS by study arm in the PPS. B OS by study arm in the PPS. C EFS by tpCR status in the PPS. D EFS by bpCR status in the PPS. E Event free survival in the mFAS. F Overall survival in the mFAS. bpCR, breast pathologic complete response; CI, confidence interval; EFS, event-free survival; HR, hazard ratio; PPS, per protocol set; OS, overall survival; pCR, pathologic complete response; tpCR, total pathologic complete response
Locally assessed pCR and long-term efficacy
In the PPS, in both treatment arms, 3-year EFS was more better for patients achieving a tpCR (locally assessed) than for those with residual disease, with 10.8% (24/222) versus 17.9% (45/252) of patients with events counting for EFS, respectively (HR 0.53, 95% CI 0.32–0.87; p = 0.013) (Fig. 2 C). Similarly, 3-year EFS was more favorable for patients achieving a bpCR (locally assessed) than for those without (HR 0.54, 95% CI 0.33–0.89; p = 0.014) (Fig. 2 D).
Long-term safety
During the posttreatment follow-up period, PTAEs were reported for 64 (27.4%) and 72 (29.8%) patients in the HD201 and the referent trastuzumab groups, respectively (Table 1 ). PTAEs with severity grade 3 or higher were reported for 7 (3.0%) patients and 13 (5.4%) patients, and serious PTAEs were reported for 4 (1.7%) patients and 5 (2.1%) patients, respectively. No serious PTAEs related to study treatment were reported during the posttreatment follow-up period. Overall, no noteworthy differences were found between the two groups.
The phase 3 TROIKA study in patients with ERBB2-positive EBC is the conclusive step in the investigation of HD201 and the referent trastuzumab in the extensive comparison of the two supporting the development of the biosimilar candidate [ 1 ]. Analysis of the secondary long-term efficacy endpoints, EFS and OS, after 3 years of follow-up continues to support the equivalence of HD201 to referent trastuzumab established by the primary analysis based on the tpCR criterion. Most recurrent events in ERBB2-positive breast cancer have been reported to occur within 3 years, and this duration appears sufficient to provide adequate evidence to support efficacy and safety conclusions [ 12 , 13 , 14 ]. Achieving tpCR was associated with longer EFS in both treatment arms, and these results were consistent with those observed in other studies assessing neoadjuvant trastuzumab [ 9 , 10 , 11 , 14 ].
The overall safety profile of HD201 and trastuzumab at the 3-year follow-up remains consistent with the safety profiles observed in previous studies, post-treatment adverse events are unrelated or unlikely to the study drug, and rarely, events related to the study drug occurred in the post-treatment follow-up period.
Limitations of the study include the use of newer anti-HER2 agents, which could impact survival in patients with relapse and were not assessed in this study. In addition, subgroup analyses are limited by their small and unbalanced sample sizes.
This final analysis of TROIKA further supports the comparability of the efficacy and safety of HD201 and the referent trastuzumab.
Availability of data and materials
Data types: Deidentified participant data.
How to access data: The application providing the project details should be submitted to Prestige Bio Pharma,(2 Science Park Dr, #04–13/14 Ascent Tower B, Singapore Science Park, Singapore 118,222) or by email at [email protected] or [email protected] . Then the request need to be approved by the steering committee of the study before release the data.
Restriction: The steering committee of the trial approval based on the scientific assessment of the application is requested to release the data.
Pivot X, Georgievich MA, Shamrai V, Dzagnidze G, Soo Hoo HF, Kaewkangsadan V, Petrelli F, Villanueva C, Nikolaevich LO, Hii J, et al. Efficacy of HD201 vs referent trastuzumab in patients with erbb2-positive breast cancer treated in the neoadjuvant setting: a multicenter phase 3 randomized clinical trial. JAMA Oncol. 2022;8(5):698–705.
Article Google Scholar
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (London, England). 2014;384(9938):164–72.
Stebbing J, Baranau Y, Baryash V, Manikhas A, Moiseyenko V, Dzagnidze G, Zhavrid E, Boliukh D, Stroyakovskii D, Pikiel J, et al. CT-P6 compared with reference trastuzumab for HER2-positive breast cancer: a randomised, double-blind, active-controlled, phase 3 equivalence trial. Lancet Oncol. 2017;18(7):917–28.
Article CAS Google Scholar
von Minckwitz G, Colleoni M, Kolberg HC, Morales S, Santi P, Tomasevic Z, Zhang N, Hanes V. Efficacy and safety of ABP 980 compared with reference trastuzumab in women with HER2-positive early breast cancer (LILAC study): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2018;19(7):987–98.
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32.
Ismael G, Hegg R, Muehlbauer S, Heinzmann D, Lum B, Kim SB, Pienkowski T, Lichinitser M, Semiglazov V, Melichar B, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13(9):869–78.
Pivot X, Bondarenko I, Nowecki Z, Dvorkin M, Trishkina E, Ahn JH, Vinnyk Y, Im SA, Sarosiek T, Chatterjee S, et al. Phase iii, Randomized, double-blind study comparing the efficacy, safety, and immunogenicity of sb3 (trastuzumab biosimilar) and reference trastuzumab in patients treated with neoadjuvant therapy for human epidermal growth factor receptor 2-positive early breast cancer. J Clin Oncol. 2018;36(10):968–74.
Pivot X, Cox DG. A new era for treatment development in HER2-positive breast cancer. Lancet Oncol. 2018;19(2):160–2.
Pivot X, Bondarenko I, Nowecki Z, Dvorkin M, Trishkina E, Ahn JH, Im SA, Sarosiek T, Chatterjee S, Wojtukiewicz MZ, et al. A phase III study comparing SB3 (a proposed trastuzumab biosimilar) and trastuzumab reference product in HER2-positive early breast cancer treated with neoadjuvant-adjuvant treatment: Final safety, immunogenicity and survival results. Eur J Cancer. 2018;93:19–27.
Jackisch C, Stroyakovskiy D, Pivot X, Ahn JS, Melichar B, Chen SC, Meyenberg C, Al-Sakaff N, Heinzmann D, Hegg R. Subcutaneous vs Intravenous Trastuzumab for Patients With ERBB2-Positive Early Breast Cancer: Final Analysis of the HannaH Phase 3 Randomized Clinical Trial. JAMA Oncol. 2019;5(5):1–5. https://doi.org/10.1001/jamaoncol.2019.0339 .
Stebbing J, Baranau YV, Baryash V, Manikhas A, Moiseyenko V, Dzagnidze G, Zhavrid E, Boliukh D, Pikiel J, Eniu AE, et al. Long-term efficacy and safety of CT-P6 versus trastuzumab in patients with HER2-positive early breast cancer: final results from a randomized phase III trial. Breast Cancer Res Treat. 2021;188(3):631–40.
Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, Lortholary A, Espie M, Fumoleau P, Serin D, et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet (London, England). 2019;393(10191):2591–8.
von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, Suter T, Arahmani A, Rouchet N, Clark E, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med. 2017;377(2):122–31.
Gianni L, Eiermann W, Semiglazov V, Lluch A, Tjulandin S, Zambetti M, Moliterni A, Vazquez F, Byakhov MJ, Lichinitser M, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15(6):640–7.
Download references
Acknowledgements
Funding/Support: This study was sponsored by Prestige Biopharma Ltd.
Role of the Funder/Sponsor: The funding source validated the study as designed by the trial’s steering committee, as well as subsequent amendments. The sponsor organized the management and the conduction of the study, including the collection of data. The data were analyzed by DICE with Drs Derde and Kaufman. The data were interpreted by the trial’s steering committee, including Drs. Pivot, Cox, Deforce, Feyaerts, and Derde, independently from the sponsor. The preparation of the manuscript was performed by Drs. Pivot and Derde and reviewed and approved by all enclosed authors. The sponsor approved the manuscript and agreed to submit the manuscript for publication.
Author information
Authors and affiliations.
Institute of Cancer Strasbourg, 17 Rue Albert Calmette, 67033, Strasbourg, France
Xavier Pivot & David G. Cox
St Petersburg GBUZ City Clinical Oncology Dispensary, St Petersburg, Russia
Alexey Georgievitch Manikhas
Vinnytsia Regional Clinical Oncological Dispensary, Vinnytsia, Ukraine
Volodymyr Shamrai
S. Khechinashvili University Hospital, Tbilisi, Georgia
Giorgi Dzagnidze
Penang General Hospital, Penang Island, Malaysia
Hwoei Fen Soo Hoo
Department of Surgery, Phramongkutklao Hospital, Bangkok, Thailand
Viriya Kaewkangsadan
Oncology Unit, ASST Bergamo Ovest, Bergamo, Trevigilio, Italy
Fausto Petrelli
Clinique Clementville, Montpellier, Montpellier, France
Cristian Villanueva
Prestige BioPharma Ltd, Singapore, Singapore
Jamie Kim, Sumita Pradhan, Litha Jaison & Peggy Feyaerts
DICE, Naamloze Vennootschap, Dilbeek, Belgium
Leonard Kaufman, Marie-Paule Derde & Filip Deforce
You can also search for this author in PubMed Google Scholar
Contributions
Dr. Pivot had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Pivot, Kaufman. Acquisition, analysis, or interpretation of data: Pivot, Dzagnidze, Georgeivich, Shamrai, Fen, Kaewkangsadan, Petrelli, Villanueva, Kim, Pradhan, Jaison, Feyaerts, Kaufman, Derde, Deforce, Cox. Drafting of the manuscript: Pivot, Derde. Critical revision of the manuscript for important intellectual content: Pivot, Georgeivich, Fen, Kaewkangsadan, Petrelli, Villanueva, Kim, Pradhan, Jaison, Feyaerts, Kaufman, Derde, Deforce, Cox. Statistical analysis: Pivot, Kaufman, Derde, Deforce, Cox. Administrative, technical, or material support: Kim, Pradhan, Jaison, Feyaerts, Supervision: Pivot, Feyaerts. All author read and accept the final manuscript.
Corresponding author
Correspondence to Xavier Pivot .
Ethics declarations
Ethics approval and consent to participate.
TROIKA trial (NCT03013504) that was reported according to the Enhancing the Quality and Transparency Of Health Research guidelines. The TROIKA trial was conducted according to the ethical principles of good clinical practice and was approved by ethics committees in each involved country. All patients signed an informed consent to participate in the trial which are available at request submitted to Prestige Bio Pharma, (2 Science Park Dr, #04–13/14 Ascent Tower B, Singapore Science Park, Singapore 118222). An independent monitoring committee monitored the study.
List of the ethics committees / institutional review board that approved the study.
Consent for publication
Not applicable.
Competing interests
Conflict of Interest Disclosures: Dr. Pivot reported being an unpaid adviser for Prestige Biopharma. Dr Dzagnidze reported personal fees from Khechinashvili University Hospital during the conduct of the study. Dr Kaewkangsadan reported grants from Prestige BioPharma during the conduct of the study. Drs Derde, Kaufman, and Deforce are/were employees of DICE Ltd. and had a memorandum of understanding with Prestige BioPharma Ltd. No other disclosures were reported.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Pivot, X., Manikhas, A.G., Shamrai, V. et al. Final analysis of the phase 3 randomized clinical trial comparing HD201 vs. referent trastuzumab in patients with ERBB2-positive breast cancer treated in the neoadjuvant setting. BMC Cancer 23 , 112 (2023). https://doi.org/10.1186/s12885-023-10574-2
Download citation
Received : 30 September 2022
Accepted : 23 January 2023
Published : 31 January 2023
DOI : https://doi.org/10.1186/s12885-023-10574-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Trastuzumab
- Breast cancer
- Neoadjuvant
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Reset your password
21. July 2021
Developing an ethics and compliance programme for Moscow City Transport organisations and beyond

A new sector-wide integrity programme seeks to transform and harmonise standards of ethics and compliance across all Moscow City Transport organisations.
Alexandr Rusetskiy, a Deputy General Director in the Moscow Directorate of Transport Services, together with Ilsur Akhmetshin of the Compliance-Elements partnership, explain the motivation, approach and challenges in this effort to bring state-of-the-art anti-corruption practices to the sector and beyond.
Briefly, what is the Sectoral Ethics & Compliance Program for Moscow City Transport Organisations?
It is a new compliance programme designed to help Moscow City Transport organisations and their business partners improve and harmonise anti-corruption compliance and business ethics.
It contains 11 focus areas in line with Russian and international guidance and laws on anti-corruption compliance. These include crucial topics such as leadership, risk assessments, codes of conduct, third-party due diligence and communications.
See the programme and its English translation , which also lists all the international and national texts on which the programme is based.
Who is it for?
The programme is designed to be applicable to all private and state-owned companies in the Moscow Transport industry, which employs around 35,000 people.
The companies range from very large organisations like the Moscow Metro to smaller companies like the Moscow Directorate of Transport Services, which is the first organisation to be piloting the programme before it is rolled out across the sector.
However, the principles are universal and the programme could be adapted for use in any industry sector in Russia, as well as in countries with similar legal frameworks and business cultures.
What is special about the programme?
It is the first time that a sector-wide integrity programme has been developed in Russia. Generally, companies are left to develop their own anti-corruption compliance programmes separately, if they develop one at all.
When companies work on ethical issues separately, it takes many more resources – financial and human. Company leaders and compliance officers can’t learn from one another. It is also more difficult to measure the effectiveness of anti-corruption measures.
This means that many companies have a compliance programme on paper, but it is not up to international standards and is poorly implemented in practice. This is a problem not only in Russia but also in many other countries.
By adopting the universal rules and principles contained in this sectoral programme, companies will find it more straightforward to develop strong anti-corruption compliance measures and make sure they are implemented in practice. Regulators will also be better able to evaluate implementation. Greater harmony and transparency will benefit the reputation of the sector and minimise risks of corruption scandals.
The programme is also unique in responding specifically to the situation of state-owned enterprises, which make up a large proportion of Moscow Transport companies.
How do you start on such an ambitious programme to enhance ethical standards in this challenging context?
We started with a pilot project in the Moscow Directorate of Transport Services. We are part way through an action plan to implement the various aspects, including a reporting hotline and a training curriculum for new and existing compliance officers and the company leadership.
After Alexander reported on the initial achievements to Moscow Government colleagues, it was decided to roll the programme out to other companies in the sector. It is important to demonstrate solid results and to get buy-in. Many company leaders who were initially surprised at these new “rules” are now keen to implement the programme themselves.
It also takes a lot of hard work and talking to people to explain what the ethics and compliance programme is about and what benefits it brings.
Talking to whom?
With the companies themselves, with government partners and with members of civil society and academia.
Both of us are deeply involved in Russian compliance networks and business associations, which offer a rich forum for sharing experiences and widening perspectives. Among these forums, we would like to mention the Russian Business Ethics Network , which is part of the European Business Ethics Network, and the Moscow Chamber of Commerce and Industry , in which we lead the Compliance and Ethics Committee and a Working Group.
It is also important to exchange with academics and students engaged in compliance, as these are influential in shaping the future of ethical business in our country. So we have been holding events at Russia’s leading business and finance academies.
In this way, we both benefit from and contribute to collective discussions around anti-corruption compliance in Russia. Personal relationships and networks are crucial to communicating and demonstrating the benefits of a sectoral programme on ethics and compliance.
We hope that in time, as the programme rolls out, compliance officers and company leaders will be able to exchange knowledge and experience on ethical issues in the framework of a more formal Collective Action initiative. That way, as the programme extends across the sector and beyond, everyone will be helping to raise standards of business integrity and spread a culture of ethics and compliance in their domains.
For more information, download the Compliance Programme or contact Ilsur or Alexandr using the details below.

Sticks and carrots: New UN Resolution calls on governments to provide incentives for companies to implement anti-corruption measures

Culture and corruption: a complex relationship

Fighting corruption to promote peace and security: Basel Peace Forum 2024
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Final analysis of the phase 3 randomized clinical trial comparing HD201 vs. referent trastuzumab in patients with ERBB2-positive breast cancer treated in the neoadjuvant setting
Xavier pivot.
1 Institute of Cancer Strasbourg, 17 Rue Albert Calmette, 67033 Strasbourg, France
Alexey Georgievitch Manikhas
2 St Petersburg GBUZ City Clinical Oncology Dispensary, St Petersburg, Russia
Volodymyr Shamrai
3 Vinnytsia Regional Clinical Oncological Dispensary, Vinnytsia, Ukraine
Giorgi Dzagnidze
4 S. Khechinashvili University Hospital, Tbilisi, Georgia
Hwoei Fen Soo Hoo
5 Penang General Hospital, Penang Island, Malaysia
Viriya Kaewkangsadan
6 Department of Surgery, Phramongkutklao Hospital, Bangkok, Thailand
Fausto Petrelli
7 Oncology Unit, ASST Bergamo Ovest, Bergamo, Trevigilio Italy
Cristian Villanueva
8 Clinique Clementville, Montpellier, Montpellier, France
9 Prestige BioPharma Ltd, Singapore, Singapore
Sumita Pradhan
Litha jaison, peggy feyaerts, leonard kaufman.
10 DICE, Naamloze Vennootschap, Dilbeek, Belgium
Marie-Paule Derde
Filip deforce, david g. cox, associated data.
Data types: Deidentified participant data.
How to access data: The application providing the project details should be submitted to Prestige Bio Pharma,(2 Science Park Dr, #04–13/14 Ascent Tower B, Singapore Science Park, Singapore 118,222) or by email at [email protected] or [email protected]. Then the request need to be approved by the steering committee of the study before release the data.
Restriction: The steering committee of the trial approval based on the scientific assessment of the application is requested to release the data.
The TROIKA trial established that HD201 and trastuzumab were equivalent in terms of primary endpoints (total pathological complete response) following neoadjuvant treatment. The objective of the present analysis was to compare survival outcomes and final safety.
In the TROIKA trial, patients with ERBB2-positive early breast cancer were randomized and treated with either HD201 or the referent trastuzumab. Eligible patients received 8 cycles of either HD201 or referent trastuzumab (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) every 3 weeks in combination with 8 cycles of chemotherapy (4 cycles of docetaxel, 75 mg/m 2 , followed by 4 cycles of epirubicin, 75 mg/m 2 , and cyclophosphamide, 500 mg/m 2 ) in the neoadjuvant setting. The patients then underwent surgery followed by 10 cycles of adjuvant HD201 or referent trastuzumab according to their initial randomization to complete one year of trastuzumab-directed therapy. Event-free and overall survival rates were calculated using Kaplan–Meier analysis. The hazard ratio for event-free survival was estimated by Cox proportional hazards regression.
The final analysis was performed after all patients completed the study at a median follow-up of 37.7 months (Q1-Q3, 37.3–38.1 months). A total of 502 randomized patients received either HD201 or the referent trastuzumab, and 474 (94.2%) were eligible for inclusion in the per-protocol set. In this population, the 3-year event-free survival rates were 85.6% (95% CI: 80.28–89.52) and 84.9% (95% CI: 79.54–88.88) in the HD201 and referent trastuzumab groups, respectively (log rank p = 0.938) (HR 1.02, 95% CI: 0.63–1.63; p = 0.945). The 3-year overall survival rates were comparable between the HD201 (95.6%; 95% CI: 91.90–97.59) and referent trastuzumab treatment groups (96.0%, 95% CI: 92.45–97.90) (log rank p = 0.606). During the posttreatment follow-up period, adverse events were reported for 64 (27.4%) and 72 (29.8%) patients in the HD201 and the reference trastuzumab groups, respectively. Serious adverse events were rare and none of which were related to the study treatment.
Conclusions
This final analysis of the TROIKA trial further confirms the comparable efficacy and safety of HD201 and trastuzumab.
Trial registration
ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03013504","term_id":"NCT03013504"}} NCT03013504 .
Introduction
In the primary analysis of the prospective, randomized, multicenter phase 3 TROIKA study, HD201, a trastuzumab biosimilar, was shown to be equivalent to the referent trastuzumab in patients with ERBB2-positive early breast cancer (EBC) based on the primary endpoints of locally assessed total pathologic complete response (tpCR) [ 1 ].
The relationship between tpCR status and survival has been extensively debated following a meta-analysis indicating that tpCR status predicts survival outcome in patients with ERBB2-positive EBC [ 2 ]. Regulatory agencies have acknowledged this relationship by authorizing several compounds on this early criterion for activity [ 3 – 7 ]. The neoadjuvant setting can be definitively considered the new era for development in ERBB2-positive breast cancer [ 8 ]. It remains reassuring that in most cases, the conclusion derived from the early criteria of pathologic complete response (pCR) has been confirmed by survival outcome analysis [ 9 – 11 ]. In this final analysis of the TROIKA study, we report the long-term efficacy and safety outcomes at 3 years of follow-up.
Study design and patients
TROIKA ( {"type":"clinical-trial","attrs":{"text":"NCT03013504","term_id":"NCT03013504"}} NCT03013504 ) was a multicenter, randomized, phase 3 trial previously detailed in the publication reporting the primary analysis [ 1 ]. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Approval of the study protocol and all accompanying documents provided to the patients was obtained from independent ethics committees at participating institutions, and all patients provided voluntary written informed consent. Key eligibility criteria were age ≥ 18 years; ERBB2-positivity; new diagnosis; unilateral, operable breast cancer; and a baseline left ventricular ejection fraction ≥ 55%.
Patients were enrolled and randomized using a block of 8 in a ratio of 1:1 to receive either HD201 or referent trastuzumab (loading dose: 8 mg/kg; maintenance dose: 6 mg/kg) every 3 weeks, administered concurrently with 8 cycles of chemotherapy (4 cycles of docetaxel [75 mg/m 2 ], followed by 4 cycles of epirubicin [75 mg/m 2 ]/cyclophosphamide [500 mg/m 2 ]) in the neoadjuvant setting. After surgery, patients received an additional 10 cycles of HD201 or referent trastuzumab in the adjuvant setting according to the previous allocation.
Secondary objectives included evaluation of event-free survival (EFS) (defined as the time from randomization to the first observation of disease progression, including local and distant recurrence, second primary cancer, or death due to any cause), overall survival (OS) (defined as the time from randomization to death), safety, and immunogenicity. Exploratory analyses were conducted for EFS including locally assessed tpCR and bpCR as covariates.
Statistical analysis
Target sample sizes and statistical power calculations for the primary analysis have been reported previously [ 1 ]. Statistical analyses were performed with SAS (version 9.4; SAS Institute Inc., NC, USA). Kaplan–Meier analysis was used to estimate EFS and OS rates. Cox proportional hazards regression analyses providing hazard ratios (HRs) and 95% confidence intervals (95% CIs) for EFS adjusted for region, stage, and tumor hormonal receptor status are presented. Survival analyses were conducted in the per-protocol set (PPS), including all patients who received the study treatment (without a major protocol deviation affecting the primary efficacy assessment) and who underwent surgery after the completion of neoadjuvant treatment or did not undergo surgery because of lack of efficacy, and analysis was also performed in the modified full analysis set (mFAS), including all patients who received at least 1 dose of study medication (Fig. 1 ). Safety analyses were descriptive and conducted in all patients who received at least one dose of treatment. Adverse events (AEs) and serious AEs (SAEs) were recorded and graded per standard common technology criteria for adverse events (CTCAE).

Patient distribution: CONSORT diagram
Patient population
This analysis was performed after all patients completed the study at a median follow-up of 37.7 months (Q1-Q3, 37.3–38.1 months). The mFAS comprised 502 randomized and treated patients, among whom 250 (49.8%) were in the HD201 group and 252 (50.2%) were in the referent trastuzumab group and were included between February 19 and September 21, 2018, across 70 centers in 12 countries. A total of 28 patients with mFAS were excluded from the PPS (12 patients in the HD201 treatment group and 16 patients in the referent trastuzumab group). The PPS thus comprised 238 patients in the HD201 treatment group and 236 patients in the referent trastuzumab treatment group. Baseline demographics and disease characteristics were well balanced between the study arms as reported previously [ 1 ].
In the PPS, the 3-year EFS rates were 85.6% (95% CI: 80.28–89.52) and 84.9% (95% CI: 79.54–88.88) in the HD201 and referent trastuzumab groups, respectively (log rank p = 0.938) (Fig. 2 A). The Cox proportional HR adjusted for region, stage, and tumor hormonal receptor status was 1.02 (95% CI: 0.63–1.63; p = 0.945) (Fig. 2 A). The 3-year OS rates were comparable for the HD201 (95.5%; 95% CI: 91.90–97.59) and referent trastuzumab treatment groups (96.0%, 95% CI: 92.45–97.90) (log rank p = 0.606) (Fig. 2 B). These results for EFS and OS were similar to those in the mFAS population (Figs. 2 E and F). The sensitivity analysis searching heterogeneity of treatment effect according to the disease characteristics did not observed any discordances between the two arms in terms of survival outcomes.

Event-Free Survival and Overall Survival in the Per Protocol set (PPS) and in the modified Full Analysis set (mFAS). A EFS by study arm in the PPS. B OS by study arm in the PPS. C EFS by tpCR status in the PPS. D EFS by bpCR status in the PPS. E Event free survival in the mFAS. F Overall survival in the mFAS. bpCR, breast pathologic complete response; CI, confidence interval; EFS, event-free survival; HR, hazard ratio; PPS, per protocol set; OS, overall survival; pCR, pathologic complete response; tpCR, total pathologic complete response
Locally assessed pCR and long-term efficacy
In the PPS, in both treatment arms, 3-year EFS was more better for patients achieving a tpCR (locally assessed) than for those with residual disease, with 10.8% (24/222) versus 17.9% (45/252) of patients with events counting for EFS, respectively (HR 0.53, 95% CI 0.32–0.87; p = 0.013) (Fig. 2 C). Similarly, 3-year EFS was more favorable for patients achieving a bpCR (locally assessed) than for those without (HR 0.54, 95% CI 0.33–0.89; p = 0.014) (Fig. 2 D).
Long-term safety
During the posttreatment follow-up period, PTAEs were reported for 64 (27.4%) and 72 (29.8%) patients in the HD201 and the referent trastuzumab groups, respectively (Table (Table1). 1 ). PTAEs with severity grade 3 or higher were reported for 7 (3.0%) patients and 13 (5.4%) patients, and serious PTAEs were reported for 4 (1.7%) patients and 5 (2.1%) patients, respectively. No serious PTAEs related to study treatment were reported during the posttreatment follow-up period. Overall, no noteworthy differences were found between the two groups.
Safety results for the post treatment period
PTAE Post treatment Adverse Event
The phase 3 TROIKA study in patients with ERBB2-positive EBC is the conclusive step in the investigation of HD201 and the referent trastuzumab in the extensive comparison of the two supporting the development of the biosimilar candidate [ 1 ]. Analysis of the secondary long-term efficacy endpoints, EFS and OS, after 3 years of follow-up continues to support the equivalence of HD201 to referent trastuzumab established by the primary analysis based on the tpCR criterion. Most recurrent events in ERBB2-positive breast cancer have been reported to occur within 3 years, and this duration appears sufficient to provide adequate evidence to support efficacy and safety conclusions [ 12 – 14 ]. Achieving tpCR was associated with longer EFS in both treatment arms, and these results were consistent with those observed in other studies assessing neoadjuvant trastuzumab [ 9 – 11 , 14 ].
The overall safety profile of HD201 and trastuzumab at the 3-year follow-up remains consistent with the safety profiles observed in previous studies, post-treatment adverse events are unrelated or unlikely to the study drug, and rarely, events related to the study drug occurred in the post-treatment follow-up period.
Limitations of the study include the use of newer anti-HER2 agents, which could impact survival in patients with relapse and were not assessed in this study. In addition, subgroup analyses are limited by their small and unbalanced sample sizes.
This final analysis of TROIKA further supports the comparability of the efficacy and safety of HD201 and the referent trastuzumab.
Acknowledgements
Authors’ contributions.
Dr. Pivot had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Pivot, Kaufman. Acquisition, analysis, or interpretation of data: Pivot, Dzagnidze, Georgeivich, Shamrai, Fen, Kaewkangsadan, Petrelli, Villanueva, Kim, Pradhan, Jaison, Feyaerts, Kaufman, Derde, Deforce, Cox. Drafting of the manuscript: Pivot, Derde. Critical revision of the manuscript for important intellectual content: Pivot, Georgeivich, Fen, Kaewkangsadan, Petrelli, Villanueva, Kim, Pradhan, Jaison, Feyaerts, Kaufman, Derde, Deforce, Cox. Statistical analysis: Pivot, Kaufman, Derde, Deforce, Cox. Administrative, technical, or material support: Kim, Pradhan, Jaison, Feyaerts, Supervision: Pivot, Feyaerts. All author read and accept the final manuscript.
Funding/Support: This study was sponsored by Prestige Biopharma Ltd.
Role of the Funder/Sponsor: The funding source validated the study as designed by the trial’s steering committee, as well as subsequent amendments. The sponsor organized the management and the conduction of the study, including the collection of data. The data were analyzed by DICE with Drs Derde and Kaufman. The data were interpreted by the trial’s steering committee, including Drs. Pivot, Cox, Deforce, Feyaerts, and Derde, independently from the sponsor. The preparation of the manuscript was performed by Drs. Pivot and Derde and reviewed and approved by all enclosed authors. The sponsor approved the manuscript and agreed to submit the manuscript for publication.
Availability of data and materials
Declarations.
TROIKA trial ( {"type":"clinical-trial","attrs":{"text":"NCT03013504","term_id":"NCT03013504"}} NCT03013504 ) that was reported according to the Enhancing the Quality and Transparency Of Health Research guidelines. The TROIKA trial was conducted according to the ethical principles of good clinical practice and was approved by ethics committees in each involved country. All patients signed an informed consent to participate in the trial which are available at request submitted to Prestige Bio Pharma, (2 Science Park Dr, #04–13/14 Ascent Tower B, Singapore Science Park, Singapore 118222). An independent monitoring committee monitored the study.
List of the ethics committees / institutional review board that approved the study.
Not applicable.
Conflict of Interest Disclosures: Dr. Pivot reported being an unpaid adviser for Prestige Biopharma. Dr Dzagnidze reported personal fees from Khechinashvili University Hospital during the conduct of the study. Dr Kaewkangsadan reported grants from Prestige BioPharma during the conduct of the study. Drs Derde, Kaufman, and Deforce are/were employees of DICE Ltd. and had a memorandum of understanding with Prestige BioPharma Ltd. No other disclosures were reported.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
Questions about these guidelines should be referred to the APA Committee on Animal Research and Ethics (CARE) via email at [email protected], by phone at 202-336-6000, or in writing to the American Psychological Association, Science Directorate, Office of Research Ethics, 750 First St., NE, Washington, DC 20002-4242
Thus, animal ethics committees commonly review research projects with reference to the 4 Rs principles . The first "R", Reduction means that the experimental design is examined to ensure that researchers have reduced the number of experimental animals in a research project to the minimum required for reliable data .
Hereinafter, by "animal ethics committees" or "AECs," I am referring to the bodies entrusted with the task of the ethical evaluation of research involving living animals. The need for such evaluation is usually restricted to vertebrate animals only, which, according to scientific evidence, are believed to be sentient.
1. Introduction. In response to growing public concerns about the welfare of animals in laboratories stemming from exposés of abuse at several high-profile federally-funded research facilities, in 1985 U.S. Congress and the Public Health Service (PHS) mandated the institutional animal care and use committee (IACUC) system to oversee vertebrate animal use and ensure compliance with federal ...
Animal Ethics Committees. Under the Code, ethical review is conducted on every research proposal by an Animal Ethics Committee (AEC). These committees must have at least four members, with one ...
The ethics of using nonhuman animals in biomedical research is usually seen as a subfield of animal ethics. In recent years, however, the ethics of animal research has increasingly become a subfield within research ethics under the term "animal research ethics". Consequently, ethical issues have become prominent that are familiar in the context of human research ethics, such as autonomy or ...
research in animal ethics and food ethics. She is also a member of the committee for ethics and education in animal research at the Swedish Board of Agriculture since its establishment in 2008. Mickey Gjerris is a bioethicist at The University of Copenhagen where he for more than 15 years has been doing research in (among other subjects) ani-
From left to right: Donna Korol, PhD; Brett Frye, PhD; Erica Glasper, PhD; Melvin Rouse, PhD; Alex Ohpir, PhD; and Jeff Grimm, PhD. APA Services held a Hill Day for APA's Committee on Animal Research and Ethics (care) on March 22, 2024, to advocate for animal research and address the misinformation surrounding it.Accompanied by APA Advocacy staff, the six CARE members met with staff from ...
The Norwegian National Committee for Research Ethics in Science and Technology (NENT). Guidelines for research ethics in science and technology (2007) 2016. Oslo. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes.
The Regents Policy on Animal Care and Use addresses the use of all animals in research, teaching or display at the University of Minnesota. This policy follows from the federal and other laws and regulations. It addresses the roles and responsibilities of the Institutional Official, the Institutional Animal Care and Use Committee (IACUC ...
The Emergence of Animal Research Ethics. In his contribution to The Routledge Companion to Bioethics, Tom L. Beauchamp (2014, p. 262) calls animal research ethics "a recently coined term".It is, indeed, only in the last decade, that animal research has been discussed extensively within the framework of philosophical research ethics, but the term "animal research ethics" goes back at ...
Committees established for the ethical review of research involving animals have become a widespread legal standard around the world. Despite many differences in their composition, powers, and institutional settings, they share many common problems related to the well-established standards of procedural justice in administrative practice.
The Committee is competent to approve or refuse, from a point of view of ethical acceptability, the research projects involving animals that are carried out abroad. In addition to the AREC review, projects needs authorization from the relevant country's competent authority on animal research.
This Chapter includes discussion on the principles of animal ethics, the evolution of ethical issues in animal experiments, the 3R approach including the alternatives to animal experiments, the present status of animal experimentations, and the various guidelines related to animal research. Download chapter PDF.
The Nelson Mandela University Research Ethics Committee (Animal) (REC-A) is charged with monitoring the treatment of animals used in both research and teaching at the institution. It has the responsibility of reviewing all protocols involving animal use in order to ensure that they are in accordance with acceptable ethical and scientific ...
The Code of Practice embodies the principles of: It is important to consider these principles when designing and carrying out projects. Under the Animal Research Act (1985), approval by an Animal Research Ethics Committee (AREC) is required for the use of any experimental animal for research and teaching. Approval can be given for up to 3 years.
Statement of ethics. The study was approved by the Moscow City Ethics Committee (protocol № 50/69_13.10.2020) of the Research Institute of the Organization of Health and Healthcare Management. All procedures were performed in accordance with the ethical standards of the local research committee and with the declaration of Helsinki.
Abstract. An Ethics Committee (EC) is an independent body composed of members with expertise in both scientific and nonscientific arenas which functions to ensure the protection of human rights and the well-being of research subjects based on six basic principles of autonomy, justice, beneficence, nonmaleficence, confidentiality, and honesty.
Human Research Ethics Committee USM, Division of Research & Innovation (R&I), USM Health Campus, 16150, Kubang Kerian, Kelantan. 458-006. Medical Research and Ethics Committee, National Institutes of Health, Ministry of Health Malaysia, Block A, Level 2, No 1, Jalan Setia Murni U13/52, Seksyen U13, Setia Alam, 40170, Shah Alam, Selangor.
A new sector-wide integrity programme seeks to transform and harmonise standards of ethics and compliance across all Moscow City Transport organisations. Alexandr Rusetskiy, a Deputy General Director in the Moscow Directorate of Transport Services, together with Ilsur Akhmetshin of the Compliance-Elements partnership, explain the motivation, approach and challenges in this effort to bring ...
Human Research Ethics Committee USM, Division of Research & Innovation (R&I), USM Health Campus, 16150, Kubang Kerian, Kelantan: 458-006: Medical Research and Ethics Committee, National Institutes of Health, Ministry of Health Malaysia, Block A, Level 2, No 1, Jalan Setia Murni U13/52, Seksyen U13, Setia Alam, 40170, Shah Alam, Selangor: