
- About Grants
- How to Apply - Application Guide
- Write Application

Develop Your Budget
Cost considerations, budgets: getting started.
- Allowable direct vs. allowable F&A costs
- Modular vs. Detailed Budgets
Modular Budgets
- Detailed Budget: Personnel (Sec A & B)
- Detailed Budget: Equipment, Travel, and Trainee Costs (Sec C, D, and E)
- Detailed Budget: Other Direct Costs (Sec F)
Consortiums/Subawards
Understanding the out years.
- Other resources
As you begin to develop a budget for your research grant application and put all of the relevant costs down on paper, many questions may arise. Your best resources for answering these questions are the grants or sponsored programs office within your own institution, your departmental administrative officials, and your peers. They can answer questions such as:
- What should be considered a direct cost or indirect cost?
- What is the fringe benefit rate?
- What is the graduate student stipend rate?
- What Facilities and Administrative (F&A) costs rate should I use?
Below are some additional tips and reminders we have found to be helpful for preparing a research grant application, mainly geared towards the SF424 (R&R) application. (Note: these tips do not supersede the budget instructions found in the relevant application instruction guide found on the How to Apply - Application Guide page.
An applicant's budget request is reviewed for compliance with the governing cost principles and other requirements and policies applicable to the type of recipient and the type of award. Any resulting award will include a budget that is consistent with these requirements. Information on the applicable cost principles and on allowable and unallowable costs under NIH grants is provided in the NIH Grants Policy Statement, Section 7.2 The Cost Principles Statement under Cost Considerations /grants/policy/nihgps/HTML5/section_7/7_cost_consideration.htm . In general, NIH grant awards provide for reimbursement of actual, allowable costs incurred and are subject to Federal cost principles /grants/policy/nihgps/HTML5/section_7/7.2_the_cost_principles.htm .
The cost principles address four tests that NIH follows in determining the allowability of costs. Costs charged to awards must be allowable, allocable, reasonable, necessary, and consistently applied regardless of the source of funds. NIH may disallow the costs if it determines, through audit or otherwise, that the costs do not meet the tests of allowability, allocability, reasonableness, necessity, and consistency.
- II.1 (Mechanism of Support),
- II.2 (Funds Available),
- III.2 (Cost Sharing or Matching), and
- IV.5 (Funding Restrictions).
- Identify all the costs that are necessary and reasonable to complete the work described in your proposal.
- Throughout the budgeting process, round to whole dollars and use only U.S. dollars.
- Reviewers look for reasonable costs and will judge whether your request is justified by your aims and methods.
- Reviewers will consider the person months you've listed for each of the senior/key personnel and will judge whether the figures are in sync with reviewer expectations, based on the research proposed.
- Significant over- or under-estimating suggests you may not understand the scope of the work. Despite popular myth, proposing a cost-sharing (matching) arrangement where you only request that NIH support some of the funding while your organization funds the remainder does not normally impact the evaluation of your proposal. Only a few select programs require cost-sharing, and these programs will address cost-sharing in the funding opportunity.
Direct Costs: Costs that can be identified specifically with a particular sponsored project, an instructional activity, or any other institutional activity, or that can be directly assigned to such activities relatively easily with a high degree of accuracy.
F&A Costs: Necessary costs incurred by a recipient for a common or joint purpose benefitting more than one cost objective, and not readily assignable to the cost objectives specifically benefitted, without effort disproportionate to the results achieved. To facilitate equitable distribution of indirect expenses to the cost objectives served, it may be necessary to establish a number of pools of F&A (indirect) costs. F&A (indirect) cost pools must be distributed to benefitted cost objectives on bases that will produce an equitable result in consideration of relative benefits derived.
- The total costs requested in your budget will include allowable direct costs (related to the performance of the grant) plus allowable F&A costs. If awarded, each budget period of the Notice of Award will reflect direct costs, applicable F&A, and in the case of SBIR or STTR awards, a "profit" or fee .
- For most institutions the negotiated F&A rate will use a modified total direct cost base, which excludes items such as: equipment, student tuition, research patient care costs, rent, and sub-recipient charges (after the first $25,000). Check with your sponsored programs office to find out your negotiated direct cost base.
- When calculating whether your direct cost per year is $500,000 or greater, do not include any sub-recipient F&A in the base but do include all other direct costs as well as any equipment costs. NOTE: Direct cost requests equal to or greater than $500,000 require prior approval from the NIH Institute/Center before application submission. For more information, see NIH Guide Notice NOT-OD-02-004 .
- For many SBIR/STTR recipients, 40% of modified total direct costs is a common F&A rate, although rates at organizations may vary.
Modular versus Detailed Budgets
The NIH uses 2 different formats for budget submission depending on the total direct costs requested and the activity code used.
The application forms package associated with most NIH funding opportunities includes two optional budget forms—(1) R&R Budget Form; and, (2) PHS 398 Modular Budget Form. NIH applications will include either the R&R Budget Form or the PHS 398 Modular Budget Form, but not both. To determine whether to use a detailed versus modular budget for your NIH application, see the flowchart below.
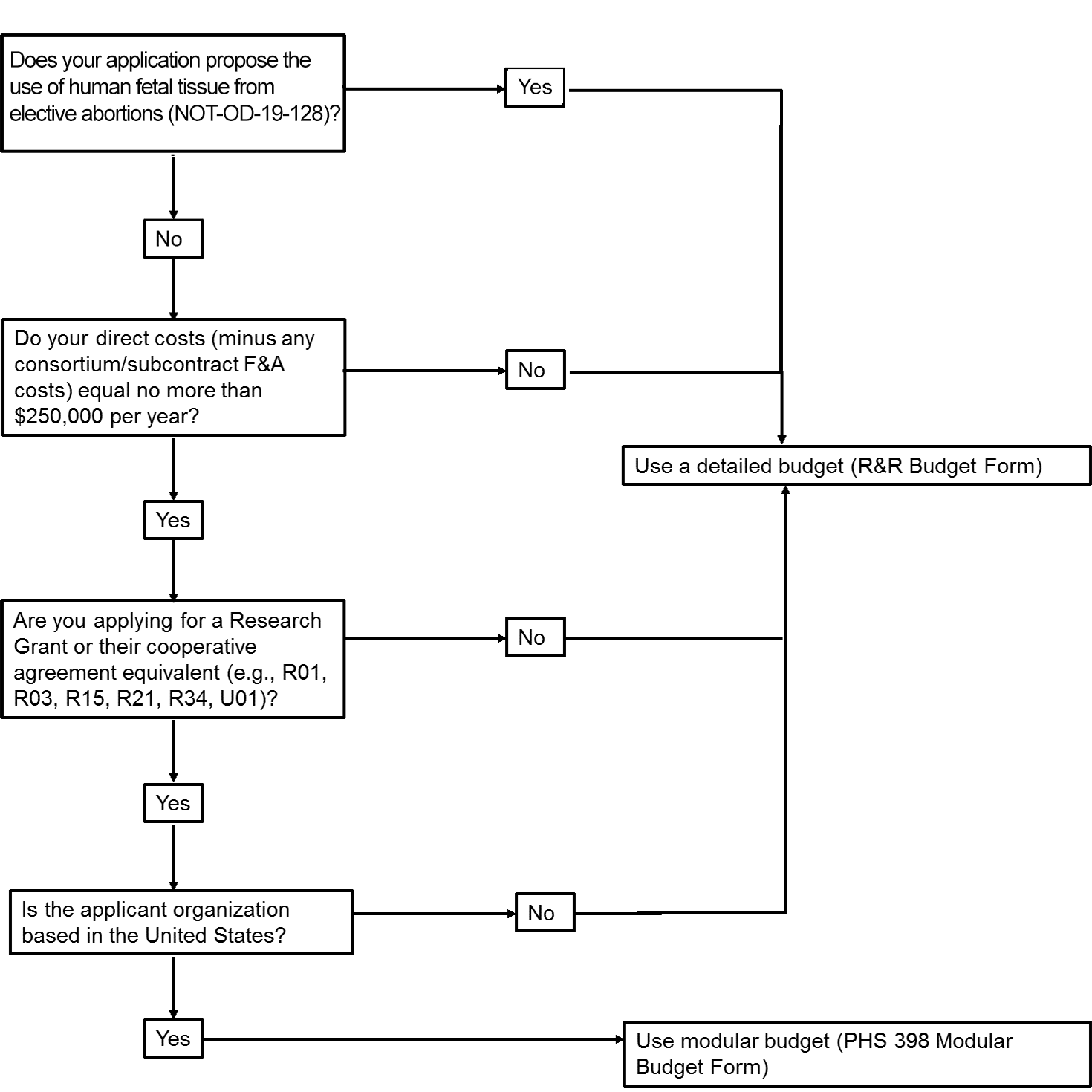
NIH uses a modular budget format to request up to a total of $250,000 of direct costs per year (in modules of $25,000, excluding consortium F&A costs) for some applications, rather than requiring a full detailed budget. The modular budget format is NOT accepted for
- SBIR and STTR grant applications,
- applications from foreign (non-U.S.) institutions (must use detailed budget even when modular option is available), or
- applications that propose the use of human fetal tissue (HFT) obtained from elective abortions (as defined in NOT-OD-19-128 for HFT) whether or not costs are incurred.
Creating a modular budget
- Select the PHS398 Modular Budget form for your submission package, and use the appropriate set of instructions from the electronic application user's guide. You do not need to submit the SF424 (R&R) Budget form if you submit the PHS398 Modular Budget form.
- Consider creating a detailed budget for your own institution's use including salaries, equipment, supplies, graduate student tuition, etc. for every year of funds requested. While the NIH will not ask for these details, they are important for you to have on hand when calculating your F&A costs base and writing your justification, and for audit purposes.
- In order to determine how many modules you should request, subtract any consortium F&A from the total direct costs, and then round to the nearest $25,000 increment.
A modular budget justification should include:
- Personnel Justification: The Personnel Justification should include the name, role, and number of person-months devoted to this project for every person on the project. Do not include salary and fringe benefit rate in the justification, but keep in mind the legislatively mandated salary cap when calculating your budget. [When preparing a modular budget, you are instructed to use the current cap when determining the appropriate number of modules.]
- Consortium Justification: If you have a consortium/subcontract, include the total costs (direct costs plus F&A costs), rounded to the nearest $1,000, for each consortium/subcontract. Additionally, any personnel should include their roles and person months; if the consortium is foreign, that should be stated as well.
- Additional Narrative Justification: Additional justification should include explanations for any variations in the number of modules requested annually. Also, this section should describe any direct costs that were excluded from the total direct costs (such as equipment, tuition remission) and any work being conducted off-site, especially if it involves a foreign study site or an off-site F&A rate.
See the NIH Modular Research Grant Applications page and the NIH Grants Policy Statement for more information.
Detailed Budget: Personnel (Sections A & B)
Personnel make up sections A and B of the SF424 (R&R) Budget form. All personnel from the applicant organization dedicating effort to the project should be listed on the personnel budget with their base salary and effort, even if they are not requesting salary support.
- Effort : Effort must be reported in person months. For help converting percent effort to person months, see Usage of Person Months FAQs .
- Salary Caps: NIH will not pay requested salary above the annual salary cap, which can be found at Salary Cap Summary . If salary is requested above the salary cap, NIH will reduce that line item to the salary cap, resulting in a reduced total award amount. In future years, if the salary cap increases, recipients may rebudget to pay investigator salaries up to the new salary cap, but NIH will not increase the total award amount. If you are preparing a detailed budget, you are instructed to base your request on actual institutional base salaries (not the cap) so that NIH staff has the most current information in hand at the time of award and can apply the appropriate salary cap at that time.
- Fringe Benefits: The fringe benefits rate is based on your institution's policy; the NIH does not have a pre-set limit on fringe benefits. More information on what is included as fringe benefits can be found in the Grants Policy Statement at /grants/policy/nihgps/HTML5/section_12/12.8.1_salaries_and_fringe_benefits.htm . If you have questions about what rate to use, consult your institution's sponsored programs office.
- Senior/Key Personnel: The Senior/Key Personnel section should include any senior or key personnel from the applicant organization who are dedicating effort to this project. "Other Significant Contributors" who dedicate negligible effort should not be included. Some common significant contributors include: 1) CEOs of companies who provide overall leadership, but no direct contribution to the research; and 2) mentors for K awardees, who provide advice and guidance to the candidate but do not work on the project. Likewise, any consultants or collaborators who are not employed by the applicant organization should not be included in section A, but rather should be included in section F.3 of the budget (for consultants) or in section A of the consortium/subaward budget page (for collaborators).
- Postdoctoral Associates: Postdocs can be listed in either section A or B depending on their level of involvement in project design and execution. If listed in section B, include the individuals' names and level of effort in the budget justification section.
- Other Personnel: Other personnel can be listed by project role. If multiple people share the same role such as "lab technician", indicate the number of personnel to the left of the role description, add their person months together, and add their requested salaries together. The salaries of secretarial/clerical staff should normally be treated as F&A costs. Direct charging of these costs may be appropriate where a major project or activity explicitly budgets for administrative or clerical services and individuals involved can be specifically identified with the project or activity [see Exhibit C of OMB Circular A-21 (relocated to 2 CFR, Part 220)]. Be specific in your budget justifications when describing other personnel's roles and responsibilities.
Detailed Budget: Equipment, Travel, and Trainee Costs (Sections C, D, and E)
- Generally equipment is excluded from the F&A base, so if you have something with a short service life (< 1 year), even if it costs more than $5,000, you are better off including it under "supplies".
- If you request equipment that is already available (listed in the Facilities & Other Resources section, for example), the narrative justification must explain why the current equipment is insufficient to accomplish the proposed research and how the new equipment's use will be allocated specifically to the proposed research. Otherwise, NIH may disallow this cost.
- General purpose equipment, such as desktop computers and laptops, that will be used on multiple projects or for personal use should not be listed as a direct cost but should come out of the F&A costs, unless primarily or exclusively used in the actual conduct of the proposed scientific research.
- While the application does not require you to have a price quote for new equipment, including price quotes in your budget justification can aid in the evaluation of the equipment cost to support the project.
- Trainee Costs: Leave this section blank unless otherwise stated in the funding opportunity. Graduate student tuition remission can be entered in section F.8.
Detailed Budget: Other Direct Costs (Section F)
- Materials and Supplies: In the budget justification, indicate general categories such as glassware, chemicals, animal costs, including an amount for each category. Categories that include costs less than $1,000 do not have to be itemized.
- Animal Costs: While included under "materials and supplies", it is often helpful to include more specific details about how you developed your estimate for animal costs. Include the number of animals you expect to use, the purchase price for the animals (if you need to purchase any), and your animal facility's per diem care rate, if available. Details are especially helpful if your animal care costs are unusually large or small. For example, if you plan to follow your animals for an abnormally long time period and do not include per diem rates, the reviewers may think you have budgeted too much for animal costs and may recommend a budget cut.
- Publication Costs: You may include the costs associated with helping you disseminate your research findings from the proposed research. If this is a new application, you may want to delay publication costs until the later budget periods, once you have actually obtained data to share.
- Consultant Services: Consultants differ from Consortiums in that they may provide advice, but should not be making decisions for the direction of the research. Typically, consultants will charge a fixed rate for their services that includes both their direct and F&A costs. You do not need to report separate direct and F&A costs for consultants; however, you should report how much of the total estimated costs will be spent on travel. Consultants are not subject to the salary cap restriction; however, any consultant charges should meet your institution's definition of "reasonableness".
- ADP/Computer Services: The services you include here should be research specific computer services- such as reserving computing time on supercomputers or getting specialized software to help run your statistics. This section should not include your standard desktop office computer, laptop, or the standard tech support provided by your institution. Those types of charges should come out of the F&A costs.
- Justify basis for costs, itemize by category.
- Enter the total funds requested for alterations and renovations. Where applicable, provide the square footage and costs.
- If A&R costs are in excess of $300,000 further limitations apply and additional documentation will be required.
- The names of any hospitals and/or clinics and the amounts requested for each.
- If both inpatient and outpatient costs are requested, provide information for each separately.
- Provide cost breakdown, number of days, number of patients, costs of tests/treatments.
- Justify the costs associated with standard care or research care. (Note: If these costs are associated with patient accrual, restrictions may be justified in the Notice of Award.) (See NIH Grants Policy Statement NIH Grants Policy Statement, Research Patient Care Costs )
- Tuition: In your budget justification, for any graduate students on your project, include what your school's tuition rates are. You may have to report both an in-state and out-of-state tuition rate. Depending on your school stipend and tuition levels, you may have to budget less than your school's full tuition rate in order to meet the graduate student compensation limit (equivalent to the NRSA zero-level postdoctorate stipend level).
- Human Fetal Tissue (HFT) from elective abortions: If your application proposes the use of human fetal tissue obtained from elective abortions (as defined in NOT-OD-19-128 ), you must include a line item titled “Human Fetal Tissue Costs” on the budget form and an explanation of those costs in the budget justification.
- Other: Some types of costs, such as entertainment costs, are not allowed under federal grants. NIH has included a list of the most common questionable items in the NIH Grants Policy Statement ( /grants/policy/nihgps/HTML5/section_7/7_cost_consideration.htm ). If NIH discovers an unallowable cost in your budget, generally we will discount that cost from your total award amount, so it is in your best interest to avoid requesting unallowable costs. If you have any question over whether a cost is allowable, contact your sponsored programs office or the grants management specialist listed on the funding opportunity.
If you are using the detailed budget format, each consortium you include must have an independent budget form filled out.
- In the rare case of third tier subawards, section F.5 "subawards/consortium/contractual" costs should include the total cost of the subaward, and the entire third tier award is considered part of the direct costs of the consortium for the purposes of calculating the primary applicant's direct costs.
- Cost Principles. Regardless of what cost principles apply to the parent recipient, the consortium is held to the standards of their respective set of cost principles.
- Consortium F&A costs are NOT included as part of the direct cost base when determining whether the application can use the modular format (direct costs < $250,000 per year), or determining whether prior approval is needed to submit an application (direct costs $500,000 or more for any year). NOTE: The $500K prior approval policy does not apply to applications submitted in response to RFAs or in response to other funding opportunities including specific budgetary limits above $500K.
- F&A costs for the first $25,000 of each consortium may be included in the modified total direct cost base, when calculating the overall F&A rate, as long as your institution's negotiated F&A rate agreement does not express prohibit it.
- If the consortium is a foreign institution or international organization, F&A for the consortium is limited to 8%.
- Consortiums should each provide a budget justification following their detailed budget. The justification should be separate from the primary recipient's justification and address just those items that pertain to the consortium.
- We do not expect your budget to predict perfectly how you will spend your money five years down the road. However, we do expect a reasonable approximation of what you intend to spend. Be thorough enough to convince the reviewers that you have a good sense of the overall costs.
- In general, NIH does not have policy on salary escalation submitted in an application. We advise applicants to request in the application the actual costs needed for the budget period and to request cost escalations only if the escalation is consistent with institutional policy. See Salary Cap Summary and https://grants.nih.gov/faqs#/fy2012_salary_cap_faqs.htm .
- Any large year-to-year variation should be described in your budget justification. For example, if you have money set aside for consultants only in the final year of your budget, be sure to explain why in your justification (e.g. the consultants are intended to help you with the statistical interpretation of the data and therefore are not needed before the final year).
- In general, NIH recipients are allowed a certain degree of latitude to rebudget within and between budget categories to meet unanticipated needs and to make other types of post-award changes. Some changes may be made at the recipient's discretion as long as they are within the limits established by NIH. In other cases, NIH prior written approval may be required before a recipient makes certain budget modifications or undertakes particular activities (such as change in scope). See NIH Grants Policy Statement - Changes in Project and Budget .
Other resources to help you create your budget
This page last updated on: September 11, 2019
- Bookmark & Share
- E-mail Updates
- Help Downloading Files
- Privacy Notice
- Accessibility
- National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
- NIH... Turning Discovery Into Health

The Ultimate Guide to Clinical Trial Costs
- by Kunal Sampat
- January 29, 2022
- in Clinical Project Management

Have you been tasked to develop a clinical trial budget? Well, you’re in luck because I’m going to share everything you need to know about clinical trial costs.
Clinical trial budgets are often put together in haste. The focus is on getting the product to market as quickly as possible. Or revenues and profits.
Developing a clinical trial budget can be a confusing exercise for sponsors and CROs. There are too many cost variables to account for.
This post covers the key cost drivers for medical device clinical trials. If you are a researcher or financial analyst working in clinical research space or simply curious about clinical trial costs, this post will serve you well.
So let’s get started.
1. Patient Grant Costs
Patient grant costs are broken down into screening, baseline and follow-up visits and medical imaging costs.
a. Screen Failures
Clinical trial protocols have inclusion and exclusion criteria to qualify patients. Strict inclusions and exclusion criteria reduce the available patient pool for trial enrollment. Clinical sites spend physician and site coordinator time to screen for potential patients.
During the budgeting process, map out the complete patient screening workflow. Speak with a few clinical sites to understand how many patients they would have to see in order to find one qualified patient.
For example, a site may need to screen four patients to find one qualified patient. Understand how many hours the site is spending on screening activities and reimburse accordingly. Therefore it’s not unusual to reimburse sites anywhere between $50 to $250+ per screen failure.
b. Baseline/index Procedure and Follow-up Visits
Depending on the clinical trial design, data is collected at baseline or index procedures and follow-up visits. The site coordinator is generally responsible for entering the data in the case report form. Sites are reimbursed for the time spent to collect clinical trial data.
Based on number and type data fields you are collecting, you’ll want to estimate the site coordinator time needed to collect and input trial data. Then multiply the estimated coordinator time by the hourly bill rate to obtain the fair market value for each patient visit.
In some cases, sponsors may choose to reimburse patients. Reimbursement for patients can include paying for their participation, reimbursement for travel, meals or overnight hotel stays.
c. Non-standard of Care Tests
A clinical trial may require non-standard of care tests such as medical imaging scans. Insurance companies or medical care agencies generally do not reimburse non-standard of care costs. Therefore you should include them in your clinical trial budget.
d. Procedure Costs
Medical payor such as Medicare or private insurance may reimburse clinical trial procedure costs. If procedure reimbursement is available, you don’t need to budget for the procedure cost. In case a brand new procedure where no reimbursement available, budget for the procedure costs.
2. Site costs
A. site start-up fees.
Clinical sites spend significant time to initiate a new clinical trial. Sites are responsible for site-specific informed consent development, Ethics Committee (EC)/ Investigational Review Board (IRB) submissions, staff training including participation in investigator/ site coordinator meetings and site initiation visits and execute a clinical trial contract. It is typical for a sponsor to pay anywhere between $3500 – $7500+ in site start-up fees.
b. Ethics Committee (EC)/ Institutional Review Board (IRB) Fees
EC/IRB fees are in addition to site start-up fees. These fees cover the time spent by EC/IRB to plan and conduct a review of the clinical trial protocol and other associated materials. Many EC/IRBs update and publish their rates annually.
c. Close-out fees
Close-out fees include time spent by site staff to reconcile clinical trial data, finances, and regulatory documents during study closure. Not all sites require this payment but, in recent years, this cost has become a more common line item in the study budget.
d. Storage Fees
Government regulations require that clinical trial data be stored after study close-out. The duration for storage can range from 2-years to permanent storage. Thus it’s not uncommon for sites to have boxes of regulatory paperwork that need to be stored once a clinical trial ends. The storage fees vary by country and site.
Some sponsors make arrangements for the site to send trial documents to an offsite storage location. Due to country-specific regulations, a site might be unable to move documents outside their country.
e. Administrative Overhead
Clinical sites may require as much as 30% administrative overhead in addition to per patient grant amount. This cost covers management and legal resources needed to provide clinical research oversight and legal review of clinical contracts respectively.
f. Site Management Organization (SMO)
In certain countries such as Japan, data entry and collection tasks are outsourced to SMOs. For post-approval studies, sites do not research coordinator support. Thus sponsors are expected to hire SMOs to support the site or pay the sites to hire their preferred SMOs.
3. Non-patient costs
A. clinical evaluation committee (cec).
Adverse event and endpoint data is adjudicated by a non-biased, independent CEC. CEC is generally composed of 3 or more physicians. CEC members review adverse events and trial endpoints in a team setting or independently.
A sponsor can hire physicians to serve as the CEC and reimburse them at fair market value rates. It is more cost effective for the sponsor to contract with physicians directly. But the sponsor has to assign its own resources to manage the CEC.
The other option is for the sponsor to outsource management and conduct of CEC activities. However, this option is more expensive because you are hiring professionals to manage the CEC.
CEC is a very important component of medical device clinical trial. Adjudicated adverse event data is highly regarded by regulatory agencies and the physician community. In many cases, it is a requirement to have adjudicated adverse event data in order to get the product on the market.
b. Data Safety Monitoring Board (DSMB)
DSMB is sometimes known as the Data Monitoring Committee (DMC). According to IMARC research , the purpose of the DMC is to advise the sponsor on continuing safety of the trial subjects and those yet to be recruited and provide continuing validity and scientific merit of the study.
For budgeting purposes, it is important to know that DSMB is required during the trial enrollment phase. In some cases, DSMB meetings occur until all patients have reached their primary endpoint. The decision of whether or not to conduct DSMB meetings after the primary endpoint is reached is up to the sponsor.
c. Physician consulting
Physicians are consulted during all phases of a clinical trial. Physician guidance is needed to develop clinical trial strategy, enrollment plan, final data analysis, and publication plans.
Physician consulting costs can be anywhere between $150 – $600+ per hour. The billable rate varies based on the physician’s medical expertise and geographical location. If a clinical trial is interesting to the physician, he or she may be willing to provide consulting services at little or no cost.
d. Independent core lab analysis
Many medical device trials collect imaging data such as angiograms, CT scans, and X-Rays. Since this data comes from multiple sites, variability is expected. An independent core lab standardizes the collection and analysis of imaging data.
Corelab costs can add up quickly. Costs depend upon the number of images analyzed per patient, the time it takes for the core lab to analyze the data, and the duration of the trial.
Corelabs usually hire analysts to collect and calibrate data from different sites. The final analysis is usually done by a physician. Given the complexity of imaging data collection and analysis combined with the importance of core lab data to regulatory agencies, it is important that adequate and accurate budget is allocated for independent core lab analysis.
e. Medical product cost
Once you are ready to enroll patients in the clinical trial, you’ll need to ship the medical product to the sites. Most sites will expect to receive the medical product for free. The only exception is when conducting post-approval trials for commercially available medical product.
Medical device and biologics manufacturers may conduct a trial for clinical indication expansion. For example, a stent company may conduct a trial to get their heart stent approved for use in different anatomy. For such expansion trials, sponsors may need to provide commercially available medical product to sites at no cost.
Whether or not you want to provide the medical product at no cost is a business decision. When investigational medical product is provided at no cost, sites can enroll faster and have a much stronger, collaborative relationship with the Sponsor.
4. Labor Costs
In order to conduct a clinical trial, you need to hire people that have expertise in clinical research and clinical trial management. Depending on the size of the trial and the number of trials conducted, resource allocations vary. Therefore the amount of labor needed to run a study also varies.
a. Clinical Research Assistants or Associates (CRAs)
CRAs are primarily responsible for monitoring clinical trial data that is collected during the course of the study. They visit clinical research sites to ensure data is collected in a compliant manner.
b. Project Manager (also known as Clinical Trial Manager or Study Manager)
A project manager’s responsibilities can vary from one organization to another. Project managers are like “general contractors.” A project manager is responsible for managing the clinical trial budget, resources, and timelines. The core function of a project manager is to resolve or escalate issues that come up during the course of a clinical study.
c. Clinical Data Manager
A data manager’s job is to address data discrepancy issues by generating queries to sites. Data managers may also be responsible for implementing an electronic data capture system or paper case report forms needed to collect trial data.
d. Clinical Research Scientist
The scientist is primarily responsible for developing the clinical strategy for a trial. Individuals with Ph.D. or M.D. degrees are usually the right fit for this role. In some organizations, the project manager also plays the role of the scientist.
e. Biostatistician
A biostatistician is responsible for developing a statistical analysis plan (SAP). The SAP documents on the data will be analyzed during the course of the study. A statistician or statistical programmer is also responsible for programming data tables that are incorporated in the final clinical study reports.
Clinical research is a regulated industry. Quality plays an important role in ensuring sponsors, CROs, and clinical sites are conducting the trial in a compliant manner. Thus a quality associate or manager helps an organization create and implement standard operating procedures (SOPs).
Salaries for these roles can vary by geography and experience. The above list is not comprehensive. However, it should give you an idea of the core resources needed to conduct a medical device clinical trial.
5. Site Management
A. pre-study visits.
Prior to inviting any site to participate in a clinical trial, you want to conduct a pre-study visit, also known as the site assessment visit. This visit becomes even more important if you don’t have any prior experience working with the site in a clinical or commercial setting.
Although sites don’t charge for this visit, the sponsor will need to pay for travel and CRA labor costs.
b. Site Initiation Visits (SIV)
Once the site has received Institutional Review Board (IRB) or Ethics Committee (EC) approval and the trial contract has been signed, it’s time to activate the site for patient enrollment.
A SIV is conducted when you are ready to activate the site. SIV involves training the site on the clinical protocol and any other study-specific requirements.
Similar to the pre-study visit, the sponsor will need to pay for travel and CRA labor costs.
c. Monitoring – Remote, Virtual, In-person
Once patients are enrolled in the study, it is critical to collect data in compliance with regulations and the clinical study protocol. This is when monitoring comes into play.
A CRA, sometimes known as the site monitor, visits clinical sites at regular intervals to ensure compliance.
In recent years, due to the push for a reduction in clinical trial costs, several sponsors have started to monitor remotely rather than conducting an in-person monitoring trip.
d. Close-out
Once all patients at a site have completed their follow-up visits, it’s time to conduct a close-out visit. Any open items related to study conduct are addressed during the close-out visit.
Although it’s always nice to have in-person close-out visits, it’s acceptable to close trials via remote close-out calls.
6. Miscellaneous
A. investigator meetings.
Investigator Meetings serve to kick-off a new clinical trial. Site investigators and research coordinators are invited to participate in a 1-2 day meeting. These meetings serve to educate site personnel on the clinical trial protocol and any other trial specific requirements.
These meetings can be quite expensive and the sponsor pays for attendee airfare, hotel, and meals.
Plan and budget for ad hoc travel. Clinical research is highly regulated. You’ll need to visit a site to address a compliance issue or help them prepare for an audit. In other cases, you want to visit a site to motivate them to enroll patients. Whatever the case may be, it’s always good to have a bit of money set aside for travel.
c. Document Translations
Document translations cost increase significantly depending on the countries in which the clinical trial is conducted. Sites where English is not the primary language, you may receive a request for translation of key documents such as the protocol and site-specific informed consent in the local language.
Also if the adverse event source documents from non-English speaking sites are in their native language, additional costs will incur to translate documents into English for event adjudication purposes.
d. Technology solutions
To conduct clinical trials, you need systems such as Clinical Trial Management System (CTMS), Electronic Data Capture (EDC), Electronic Trial Master File (eTMF), Interactive Voice/Web Response System (IxRS). These systems manage site contact information, collect clinical data and maintain clinical trial records. Budget monthly or annual license fees associated with these systems. Additionally, you need staff to manage and maintain these systems.
e. Regulatory filing fees
Don’t overlook regulatory filing fees. These fees can run into thousands of dollars. Depending on the class of medical device, different applications are filed with regulatory agencies, competent authorities and notified bodies.
7. Other Clinical Trial Cost Factors
A. protocol amendments.
Due to unforeseen circumstances, a clinical protocol amendment may be necessary. A protocol amendment has many downstream effects that can increase the cost of a clinical trial.
A protocol amendment usually leads to additional IRB/EC fees, site costs, regulatory re-submissions and more.
b. Inflation, Value Added Tax (VAT) and Foreign Exchange
Don’t forget to factor inflation for multi-year clinical trials. Generally speaking, plan for a minimum of 3% inflation rate.
For sites in countries such as Australia and Europe, add VAT for the research services. The VAT can be upwards of 12% on all research services.
For trials conducted in multiple countries, pay attention to foreign exchange rates. At a minimum, an annual review of exchange rates is advised. Adjust clinical trial cost projections based on exchange rates.
c. Trial enrollment delays
Enrolling in trials is a tricky business. It takes longer to complete enrollment and initial projections are overly optimistic. Therefore account for these delays when you develop your clinical trial budget.

Conclusion:
We’ve covered a lot of ground in this Ultimate Guide to Clinical Trial Costs. To summarize, you should now have a solid understanding of these factors that impact clinical trial costs:
- Patient grant amounts such as screen failure costs, data entry costs, and travel reimbursement
- Site costs such as site start-up fees, EC/IRB fees, close-out and storage fees
- Non-patient costs such as core laboratory fees, clinical events committee and data safety monitoring board
- Labor costs such as clinical research employee salaries or contractor payments
- Site management costs such as pre-study, site initiation, monitoring, and close-out visits
- Miscellaneous costs such as travel, technology solutions, and regulatory filing costs
- Other factors such as value-added tax, inflation, protocol amendment and delays in enrollment
What’s your best tip to planning a clinical trial budget? Leave in the comment section below.
Clinical Research Billing for Small to Medium Sites with Kristi Etchberger
Integrating ehr and edc systems with hugh levaux, 29 thoughts on "the ultimate guide to clinical trial costs", overview of clinical research | clinical trial podcast, role of a clinical project manager | clinical trial podcast, as the cost of clinical trials climbs, here are 3 ways to save - archemedx, best practices and assumptions for clinical trial budgeting - cereblis.
Tidor Morgan
Kunal, thanks for summarising this so concisely…. a really useful read and reference point for future discussion.
Hi Tidor, I’m so glad to hear you found this post useful. This should severe as a useful guide to the clinical community when it comes to planning trial budgets. Thanks again for taking the time to read and comment.
Nice summary Kunal. The overview is very concise and you touched on all the important aspects to consider when budgeting for a clinical trial.
Hey Chris, Thanks for the positive feedback 🙂 It’s good to hear from industry professionals such as yourself who developed hundreds of budget models and scenarios. If you have any other insights or suggestions, please do let me know.
Thank you for writing the article and sharing the excel file. These are great!
Kunal Sampat
Thanks Ehsan! Let me know if you have any follow-up questions. Happy to help!
Thank you, Ehsan!
Hi Kunal, what a tremendous resource you’ve provided here, thank you! I’m looking for someone to consult on a budget for a medical device trial. Do you know anyone offering these services?
Excellent, thank you.
Thank you, Laura. Glad to hear your feedback.
Thank you, Laura
Vladimir Shnaydman
Hi Kunal, Several questions. 1. How budgeting is coordinated with site selection? 2. How did you included risk in budgeting process? 3. What are major cost drivers for a clinical trial budget? Thank you, Vladimir
Hi Vladimir,
1. budgeting in most cases would not be coordinated with site selection 2. many of the clinical trial costs are tried to patient recruitment. patient recruitment is a dynamic process. you will likely need to re-forecast your budget on a regular basis depending on how fast or slow you enroll 3. Major cost drives are generally patient grant costs, labor costs, and monitoring. Every study is different and there may be other high ticket items.
This post should be renamed Clinical Trial Budgeting for dummies, as it gives an informative yet easy to understand breakdown of the whole process. Thank you Kunal.
Hi Ivy, Thank you 🙂 Kunal
Thanks Kunal , this is helpful. I may also add that it might also be required to include the product’s manufacturing costs in a study budget, especially in budgets of small Biotech companies.
Hi Uri, Yes, I agree. Product manufacturing costs should be part of the study budget. I’ve mentioned “device costs” in this article. Will update it to include “biologics costs” as well. Thanks for the input.
Francis Akenami
Hi Kunal – thank you for the comprehensive presentation. Where do you include costs for Medical Writing Services such content development for Protocols, Clinical Study Reports, Clinical Evaluation Reports, New Drug Applications and publications in peer-reviewed journals? Can they be added to the overall cost of Clinical Trials? I should think so since a Clinical Trial cannot be considered complete if those are missing.
Gerard Abate
What is the average cost per patient for a CRO for an interventional trial?
It depends on the study design.
Really got a good understanding of the basics especially when I am in a project involving a major player in the clinical trials domain. Thanks a lot!
Kenneth Quintana
What is the average time a cro spends on final data analysis? Thank You
This average time a CRO spends time on data analysis can vary based on study design (ex: how complex are your statistics), quality of the data collected (ex: lot of missing data = more time needed), and resources (ex: do you have a team to do the work). I would say plan for 3-months but it can take more or less time based on the above factors
raveena aher
thank you for sharing your blogs
Thankyou for sharing Clinical Trial Costs with us.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Budget Development
Budget development is an important piece of clinical trial management. There are different considerations if the budget will be federally funded, funded by industry or if the trial is investigator initiated.
Federal Budgets
When requesting funding for a study, all study-related costs should be noted in the budget, including personnel, consultants, equipment, supplies, travel, and other expenses. NIH applications have special budget and justification forms that contain detailed instructions. Penn investigators must have their grant budgets approved by Office of Research Support Services (ORSS) .
Industry Sponsored Budgets
When considering an industry sponsored-study, all study related expenses should be determined and compared to the overall reimbursement offered by the sponsor to ensure the study is financially feasible.
- The sponsor of a clinical trial generally sends a protocol and budget overview/template for the trial.
- After reviewing the protocol and events schedule, create an internal budget to reflect all costs, including time and effort of all personnel, in order to conduct the trial.
- Note : You may not bill insurance for a drug, test, device, or service paid for by the sponsor.
- Determine if the labs and testing procedures will be done in-house or at the sponsor’s site.
- Determine if there will be professional charges required for the technical tests performed.
- A study start-up fee must be included in order to cover the following costs: protocol review, staff training, budget preparation, regulatory documents, and administrative fees.
- There are one-time costs that should be included in the budget, if applicable: IRB fees, IRB continuing review fees, IRB amendment fees, investigational drug set-up fees, MCA preparation fee, archive fees, and advertising costs.
- PSOM has instituted a clinical trial indirect cost rate of 39%, which is an additional charge that must be applied to the total direct study costs. Only IRB fees are exempt. Indirect costs cover a small part of PSOM’s infrastructure costs for research.
- For multi-year clinical trials, consider adding an inflation rate of 5% to the per-completed-subject cost.
- Holdback on a trial should not exceed 10%.
Investigator Initiated Studies Budgets
Penn sponsor-investigators should develop a budget based upon the expected expenses at each site. Billing rates for the same procedure will vary from place to place.
To develop a budget work with your department BA and the Office of Clinical Research Finance group can also assist.
Budget Preparation and Management FAQs
Q: who should i contact to help me prepare a budget .
A: You should work with your department BA when developing a clinical research budget. The Office of Clinical Research (OCR) Finance Services can assist when needed.
Q: What resources are available to help create a clinical research budget?
A: The Office of Clinical Research (OCR) has developed a template to help create clinical research budgets. In addition to the template, Costfinder can be used to look up hospital services by searching on CPT codes to find given research rates.
In addition to the template, use the Cost Finder application (navigate to the Forms, Tools and Templates library) and search in categories
Q: What is a prospective reimbursement analysis (PRA)?
A: The PRA is a questionnaire designed to make a determination if a clinical trial is a qualifying clinical trial. A qualifying clinical trial means standard of care services can be charged to insurance if needed.
Q: What is a Medicare coverage analysis (MCA)?
A: A Medicare Coverage Analysis (MCA) is a document that determines the appropriate payer (sponsor, Medicare, or third-party) for each item and service required by a clinical research trial. A MCA is required for all clinical trials in which tests, procedures, and interventions associated with a clinical trial are invoiced to third-party payers, and/or when research procedures are paid for by sponsors.
Q: What are typical items that should be included in a budget?
A: When requesting funding for a study, all study-related costs should be noted in the budget, including personnel, consultants, equipment, supplies, travel, and other expenses. Some common expenses are:
- Start-up costs
- Site Visit & Site Initiation Costs
- Institutional Review Board (IRB) fees
- Investigational Drug Service (IDS) fees
- Blood collection tubes, chemicals, dry ice
- Centrifuges, mass spectrometers, liquid simulation counters
- Technology (e.g. telephone, computer)
- Shipping/Packaging Supplies (e.g. dry ice)
- Advertising/Recruitment
- Archival fees
- Clinical Research Computing Unit (CRCU) fees
- Biostatistics and Epidemiology Consulting Center (BECC) fees
- External Institution or Contract Research Organization (CRO)
- Service Contracts (e.g. instrument maintenance)
- Training/Seminar/Conference (directly related to the research project) travel expenses
- Screen failures
- Travel Expenses
- Document Preparation
- Document Submission
- Medical Director
- Medical Record Retrieval
- Data Management
- Data Safety Monitoring Board
- Closeout Fees
Q: What is our current overhead rate?
A: The current overhead rate can be found on the Office of Research Services (ORS) .
Q: How do I pay for hospital services?
A: The university uses the research billing application (RBA) to generate research billing numbers (RBN). A research billing number (RBN) is a protocol-specific number used by the University of Pennsylvania Health System (UPHS) to bill research-only UPHS services/procedures to a School of Medicine fund set up for the protocol (e.g., research fund, departmental fund, etc.). There can only be one RBN per IRB Protocol Number. All studies, regardless of payor, need to be registered in the Research Billing Application before they are loaded into Penn Chart (Epic) and enrollment can begin. A User Guide for the RBA is available.
Hospital fees are made up of either technical fees or professional fees. The definition for each is below:
Technical Fees - A technical fee is the cost incurred for use of the mechanical equipment and processing. Tests/procedures that are study related and are not "standard of care" must be charged to the research budget. All costs should be based on the currently approved technical "research rate".
Professional Fees - The professional fee is the physician's charge for interpretation of diagnostic procedures/tests. It is important to note, if there is a professional fee associated with a test/procedure, you must include that charge in your expenses. A limited number of laboratory tests have professional fees associated with them. All radiology and cardiology procedures have an associated professional fee, as do various other procedures. All costs should be based on the currently approved professional "research rate".
Q: Is it allowable to supplement patient insurance costs with research funds?
A: Only for hardship purposes can research funds be used to offset unpaid insurance claims, deductibles or co-pays. Medicare’s policy is referenced below:
A research patient must, like all other patients, be responsible for deductibles and co-payments. Investigators may not induce patients to participate in clinical trials and fore go standard therapy by promising to waive these payments. Nor may the investigator offer as an enrollment incentive any free items or services to patients unless these items or services are customarily provided without charge to patients not enrolled in clinical research. (This does not prohibit, however, hardship discounts when applicable.)
Medicare has no obligation to pay for items and services if a provider treats Medicare beneficiaries differently from non-Medicare patients or if other situations trigger Medicare exclusions. The provision sets out limited situations (such as patient indigency) when waiving charges for non-Medicare patients will not disturb Medicare coverage.
The “No Legal Obligation to Pay” provision addresses scenarios such as billing Medicare for a service while not billing non-Medicare patients for the same service. This provision of the manual operates to prohibit billing Medicare for the same service that is provided free to non-Medicare beneficiaries. In such a case, Medicare has no legal obligation to pay for the service and the provider also cannot charge the Medicare beneficiary.
Into this provision CMS inserted clinical research situations. The Special Edition Article advances the idea that if a provider does not charge a non-Medicare enrollee for a research study service, then the Medicare enrollee must also receive that same study service free. If the provider does not pursue collections against the research subject after the patient’s insurance denies coverage, CMS argues that the provider’s actions disallow billing for the same service for Medicare patients enrolled in the study.
Q: What financial management requirements are needed?
A: Each sponsor has their own regulations on all financial reporting and retention of documents. Review the contracts associated with each study to ensure financial requirements are being met. To ensure proper financial management, study expenditures should be reviewed on a regularly basis and should comply to the University’s policy 2106 which can be found here: https://www.finance.upenn.edu/policy/2106-financial-responsibility/ .
Q: I received an effort report, what do I need to do?
A: The Effort Reporting System (ERS) is used to certify effort applied to research. Effort reporting is mandated by the federal government. If you receive an effort report, you should log into ERS, review the effort report, suggest changes if needed and certify the report. Effort Reporting System FAQs
Q: How do I hire new staff?
A: Penn uses Workday@Penn for all its human resource needs. All new positions must be submitted in Workday for approval. Please consult with your Business Administrator (BA) and Human Resource Manager prior to posting a position.
For more information regarding hiring a staff member
Q: How do I reimburse patient stipends?
A: Penn’s preferred method for patient reimbursement is Greenphire. Greenphire is a reloadable prepaid clincard. More information about Greenphire click here: https://www.finance.upenn.edu/payments-disbursing-funds/paying-program-participants-via-clincard .
Q: What payment mechanisms does Penn accept from external funding sponsors?
A: Funding sponsors can pay via check or wire transfers. Click here for additional information https://researchservices.upenn.edu/areas-of-service/research-operations-and-cash-management/
Your session is about to expire
Clinical trial budget template, introduction: how to prepare a clinical trial budget.
Preparing a comprehensive and practical clinical trial budget requires thorough planning and foresight. Budgeting is one of the first steps involved in planning a clinical trial, and is a central component of a feasibility study, which is done to ensure that the study is well positioned to successfully answer a relevant research hypothesis, within given time and monetary constraints.
Clinical trial budgeting requires extreme detail; it is not just about accounting for the obvious costs, but also includes consideration of less apparent expenses that could arise during the course of research study. It's crucial for sponsors to develop an accurate budget estimate in order to ensure a smooth-running trial, effective agreements with sites and providers, and to avoid unexpected financial stress.
As for the best way to go about preparing a clinical trial budget, there is no single answer. Depending on the sponsoring organization’s past experience and partnerships, the therapeutic area and type of study, and many other factors, the budget will reflect different expenses. Nonetheless, there are still primary cost categories that are common to most clinical studies. Thus, one option is to begin with an established clinical trial budget template, which will provide an excellent starting point for eventual customization to the specific study at hand. This option may be particularly attractive for those conducting their first trials; experienced sponsors may also have an internal budget template that they adjust for each new trial.
Of course, the budget can be constructed anew from the ground up, but it becomes even more important to carefully review it to ensure that all costs – including potential/unexpected costs (which can be significant) – are taken into consideration. The main types of expenses that should always be included in a clinical trial budget are explored in further detail below.
What should be included in a clinical trial budget?
A detailed clinical trial budget should account for various cost categories, which can be organized in different ways depending on the structure of agreements and the design of the trial. [1] , [2]
Start-up (up-front) costs:
- Preparation of regulatory documentation
- Investigator meetings
- IRB submission and review
- Site selection and site initiation visits
- Storage and archiving dees
- Recruitment advertising and outreach efforts
- FDA audit fees
- Pharmacy fees and costs of the investigational product (supplies of the study drug)
Invoiceable costs (event-based) refer to aspects that may be billed on a temporal basis, either for patient care/interventions or for periodic reviews and regulatory submissions, including concepts like:
- Periodic safety reporting, i.e., the ICH E2F DSUR
- Medical procedures
- General patient care
- Study-related training
- Auditing fees
Patient-related expenditures / per-patient costs are another significant component of clinical trial expenses. Some of the patient care costs categorized under invoiceable costs (above) could also be included as per-patient costs. These costs, which are directly dependent on the number of patients enrolled (or screened), may relate to aspects such as:
- Participant recruitment and screening costs (including screen failures)
- Compensation/reimbursements made to patients for their participation or for travel expenses, accommodation, etc. (as specified in the trial protocol)
- Procedures and tests (could also be categorized as invoiceable costs)
Staffing / personnel costs include salaries for principal investigator(s), nurses, study coordinators, data managers, and other personnel directly involved in conducting, directing, and overseeing the research study.
Indirect costs, or “Facilities & Administration (F&A)” fees encompass administrative overheads, as public research institutions must recover all costs of for-profit research conducted using their facilities and administrative procedures. [3] The infrastructure is usually shared between various users (both public and private entities) simultaneously, and thus they tend to be complex to calculate or predict. Indirect or F&A fees include things such as:
- data management services
- insurance coverage related to the study
- institutional overhead charges
Hidden costs : In addition to the routine operational expenses, it's vital to allocate funds towards potential contingencies which could emerge unexpectedly. These could include things such as additional monitoring visits in the case of excessive queries to be resolved or severe adverse events, re-submissions and re-consenting for interim protocol amendments, unexpected delays, or costs related to early termination (amongst many other possibilities).
What is the average budget for a clinical trial?
The cost of conducting a clinical trial varies widely according to a number of factors, making it difficult to state an 'average' budget. The cost of conducting a clinical trial is directly dependent on:
- The type of intervention being studied (investigational drug, medical device, behavioral intervention)
- The complexity of the study design
- The phase of the trial
- The duration of the study, including the follow-up phase
- The therapeutic area of the disease and/or intervention
To give a rough guideline, small-scale observational studies will typically be one of the least expensive types of trial to conduct. Based on a 2016 study analyzing data for the period of 2004–2012 in the U.S.A., approximate/average costs for interventional clinical trials according to the phase are as follows [4] :

Top cost drivers of clinical trial expenses
The same study offered further insights into clinical trial expenditure, such as indicating which therapeutic areas tended to involve more costly studies and which types of expenses were the main contributors to overall trial costs.
It was found that the top 3 drivers of clinical trial costs were the following categories of expenses [4] :
- Clinical procedure costs, representing 15%-22% of total costs
- Administrative staff costs, representing 11%-29% of total costs
- Site monitoring costs, representing 9%-14% of total costs
In terms of therapeutic area, the ranges of overall trial costs across disciplines were narrower for phase I clinical trials and phase II clinical trials, while the overall cost of phase III trials varied more significantly, with trials in the field of pain and anesthesia being the most costly. [4]
The fast route: Accelerate study start up by using a clinical trial budget template
Preparation of the clinical trial budget can be expedited significantly by utilizing a well-structured template that has been used for prior studies. A good budget template will allow sponsors to organize projected expenses in predetermined categories, helping ensure that no costs are overlooked during the planning stage.
As mentioned, each study budget is unique, so the basic idea would be to check a few of the templates available through reputed academic institutions (linked below), find one that reflects your expense structure more or less, and then customize it until it captures everything you need it to, in a way that makes the information legible and accessible for any stakeholders who may need to review it.
For organizations that conduct multiple trials, employing a standardized template also supports straightforward inter-project comparisons and facilitates auditing procedures when necessary. If you’re conducting your first trials, pay attention to what works and what doesn’t work in the budget template, and refine it for subsequent studies.
See the following section for direct links to a few clinical trial budget template excel spreadsheets, which can be used directly, customized, or simply used to gather ideas for generating your own budget from scratch.
Clinical trial budget example templates
Some great examples of clinical trial budgets can be found at the following resources. Note that most of these are direct download links to clinical trial budget template excel sheets (clicking them will open the dialogue for downloading the budget template directly to your computer).
1. University of Tennessee Health Science Center (UTHSC): Sponsor study budget template
https://uthsc.edu/research/sponsored-programs/agreements/documents/budget-template-010909.xls
2. Oregon Health & Science University (OHSU): Study budget template https://www.ohsu.edu/sites/default/files/2019-05/budget_template.xls
3. University of Arizona: Industry-sponsored clinical trial budget worksheet
https://medicine.arizona.edu/sites/default/files/study_budget_spreadsheet_templates_and_sample.xls
4. Virginia Commonwealth University: Industry clinical trial budget template
https://research.vcu.edu/media/office-of-research-and-innovation/documents/industry_clinical_trial_budget_template.xlsx
5. Smartsheet’s Clinical research budget template
https://www.smartsheet.com/sites/default/files/2020-03/IC-Clinical-Research-Budget-10628.xlsx
Other Trials to Consider
NNC6019-0001
Topical lidocaine patch, difamilast ointment, vivacit-e liner, popular categories.
Hidradenitis Suppurativa Clinical Trials 2024
Follicular Lymphoma Clinical Trials 2024
Cataract Clinical Trials 2024
Prostate Clinical Trials 2024
Endometrial Cancer Clinical Trials 2024
Lenalidomide Clinical Trials
Tymlos Clinical Trials
Paid Clinical Trials in Milwaukee, WI
Forteo Clinical Trials
Aplastic Anemia Clinical Trials 2023
Popular guides.
- Utility Menu
FAS Research Administration Services
- Budget Templates
The budget templates below reflect FY24 Fringe Benefits and Indirect Cost rates .
Internal Budget - Federal This Excel budget template contains commonly used expense types and automatically calculates fringe and overhead rates. It is formulated for Modified Total Direct Costs (MTDC).
Internal Budget - Federal - NSF This budget template is formulated for Modified Total Direct Costs (MTDC) on NSF budgets and is formatted for easy entry into the NSF budget form pages.
Internal Budget - Federal - NIH This budget template is formulated for Modified Total Direct Costs (MTDC) on NIH "R&R" detailed budgets and is formatted for easy entry into the R&R budget pages. Internal Budget - Non-Federal The Excel budget template contains commonly used expense types and automatically calculates fringe and overhead rates. Non-federal sponsor rates should be calculated on Total Direct Costs. See also: FAS and SEAS Policy on Assessments
- Getting Started
- Proposal Development Resources
- Fringe Benefits
- Other Direct Costs
- Indirect Costs
- Cost Sharing
- Review & Submission
- Award Setup
Jimmy Matejek-Morris
... Read more about Jimmy Matejek-Morris

Katherine Zuccala
... Read more about Katherine Zuccala

Clinical Trial Budget Costing Tool
The costing tool is a spreadsheet to assist in the process of study costing for research being conducted by NSW Public Health Organisations.
In 2017, the Office for Health and Medical Research (OHMR) initiated a project to develop a standard Budget Costing Tool (‘the Tool’) for clinical trials. In partnership with Sydney Local Health District (SLHD), the Tool was built and validated, using as a model the successful UK National Institute of Health Research budget costing tool. This work was undertaken as part of the wider initiative with Advanced Health Research and Translation Committees (AHRTCs) and Centres for Innovation in Regional Health to streamline the governance process for clinical trials through the development of clinical trial support platforms within the AHRTCs (the Clinical Trial Support Unit Project). At the Clinical Trial Support Unit presentations, we confirmed that SLHD and OHMR worked together to develop and validate a Budget Costing Tool which is available for use.
Clinical trials budget costing tool template
XLSM - 331 KB
User Manual for Budget Costing Tool
PDF - 769 KB
The Tool was developed to:
- pro vide a consistent framework and methodology for cost calculations
- support swift local site budget negotiation and study set-up
- support full cost reimbursement of industry studies
- ensure that departments conducting studies have an accurate picture of the financial implications when conducting a clinical trial so that decisions can be made based on sound information
- support a proposed policy change that will require all clinical trials to make use of a budget costing tool as part of the set-up process.
Staff consulted
Consultation was undertaken across the health system for preparation of the Tool. The following staff were consulted:
- Merela Ghazal, Research Business Manager, Clinical Research Centre, Sydney Local Health District
- Louise Ford, Clinical Trials Business Manager, Clinical Research Centre, Sydney Local Health District
- Virginia Turner, Manager, Concord Research Office, Sydney Local Health District
- Maree Larkin, Research Governance Officer, RPAH Research Ethics and Governance Office, Sydney Local Health District
- Dr Janet Macpherson, Development Manager Cell & Molecular Therapies, RPAH, Sydney Local Health District
- Kylie Becker, Research Governance Officer, Northern Sydney Local Health District
- Maria Mury, Research Governance and Compliance Manager, Northern Sydney Local Health District
- Amy Boland, Clinical Research Manager, Clinical Research Centre, The Sydney Children’s Hospitals Network
- Nisha Berthon-Jones, Clinical Research Manager, Sydney Children’s Hospital, Randwick, The Sydney Children’s Hospitals Network
- Sandra Lowe, Clinical Research Manager, Sydney Children’s Hospital, Randwick, The Sydney Children’s Hospitals Network
- Kelsey Dobell-Brown, Manager – Clinical Trials Support Unit, South Western Sydney Local health District
- A/Prof Meera Agar, Director of Palliative Care, Staff Specialist Palliative Medicine South Western Sydney Local Health District, and Staff Specialist – Research South Western Sydney Local Health District Palliative Care Service Clinical Trials Director, Ingham Institute of Applied Medical Research
- Mr. Mark Smith, Research Development Manager, Western Sydney Local Health District
- Dalia Younan, Finance Manager, Research & Education Network, Westmead Hospital, Western Sydney Local Health District
- Kellie Hansen, Research Office Manager, Western Sydney Local Health District
- Margaret Piper – Research Governance Officer, Western Sydney Local Health District
- Sharon Lee, Clinical Trials Manager, Research & Education Network, Western Sydney Local Health District
- Helene Abouyanni, Director of Operations, Research & Education Network, Western Sydney Local Health District
- Vicky Wegner, Clinical Trials Manager of the Crown Princess Mary Cancer Centre
- Penny Mahairas, Executive Officer, Research Office (Ethics), Nepean Blue Mountains Local Health District
- Gina Oliver, Research Governance Officer, Research Office, Nepean Blue Mountains Local Health District
- Deborah Adrian, Executive Officer (Ethics), Research Support Office, South Eastern Sydney Local Health District
- Rosemary Carney, Research Governance Officer/Project Coordinator, NSW Ambulance
- Nicole Gerrand, Manager, Research Ethics and Governance, Hunter New England Local Health District
Updated 4 months ago
Popular Searches:
- clinical trial expertise
- clinical trial support
- clinical trials
- collaboration opportunities
- commercialisation
- early phase clinical trials
- establish a clinical trial
- ethics and governance
- facilitate resolution
- good clinical practice
- human research ethics committee
- key contacts
- motor neurone disease
- news articles
- problem solving
- publications
- solution service
- standard operating procedures
- start a clinical trial
- translational research
Clinical Trial Templates to Start Your Clinical Research
By Kate Eby | May 13, 2019
- Share on Facebook
- Share on LinkedIn
Link copied
In this article, you will find everything you need to start your clinical research trials, with easy-to-understand guidance and terminology, 26 adaptable templates, and project plans in Microsoft Word, Excel, Project, and SharePoint formats.
Included on this page, you'll find details on what a research protocol is, project management for clinical trials , research compliance templates , and post-clinical study research documentation and templates
What Is the Research Protocol?
All clinical research starts with the research protocol , a document that details all aspects of the trial: its background, rationale, objectives, design, methodology, statistical analysis plan, and organization. With the protocol, you can make sure you protect the participants and collect the data. Using protocol templates, you can start thinking through what you need to meet compliance standards with the Food and Drug Administration (FDA) and clinical study best practices.

Download Research Protocol Template - Word
The full research protocol includes the following sections and topics:
- Title Pages: These pages provide general information about the protocol, including name, number, version number and date, trial phase, investigational product name, investigational new drug (IND) number, sponsor (or principal investigator in academia), funding organization, medical monitor, and coordinating center. The pages include the principal investigator’s signature (or sponsor), as well as site-specific information, such as the agreement, and protocol details. They also detail the study team and site, particularly in the case of multiple teams and sites.
- Objectives: List the study’s primary and secondary objectives.
- Background Information: Describe the problem under study and priority. Include the medical and scientific rationale that justifies researching the problem. Include data from other studies relevant to this proposed research. Include the name and description of the proposed intervention, including the dosage, route of administration, period, and frequency of intervention.
- Study Design: Describe the methodology and how it will answer the study question. This should include the type of study, primary and secondary outcome(s), population, sample size, study location, period of enrollment and follow-up, intervention and route of administration, randomization (as necessary), and any other relevant protocol information.
- Selection and Exclusion of Subjects: Provide statements describing how the participants must meet all the inclusion and exclusion criteria, and list the criteria. Clearly define the study population. For example, list the demographic criteria, required laboratory data, any prior therapies allowed or disallowed, ability to understand and meet all study requirements, if contraception is necessary, exclusion criteria such as specific health status, use of excluded drugs, cancer status, and chemical dependency status.
- Study Enrollment Procedures: Describe the methods and procedures for identifying and enrolling subjects, how they are documented, how consent is obtained, and any randomization procedures.
- Study Intervention, Duration, and Route of Administration: This section should describe each intervention and duration, as well as how each is administered. List expected adverse effects and dose escalation, if applicable. Discuss how the intervention is acquired, stored, and disposed of, as well as documentation for intervention accountability. In addition, note the medications restricted, allowed, and required, along with the extent to which these medications are tracked and documented.
- Study Procedures: This section includes a study evaluation schedule (presented as a chart) and explanations of the required assessments, what each period is, and any special considerations or instructions necessary. These should match what is available in the column headers of the chart above, and they should include information on the screening or baseline assessments, randomization, blinding, follow-up visits, and final assessments.
- Safety Assessment: List any expected adverse events, and how these could be managed. Mention any toxicities seen in earlier IND studies here. Also, include safety measures as identified in laboratory findings, methods and timing for safety parameters based on the risk profile, definitions for adverse events (AE) and serious adverse events (SAE) and laboratory values used to identify their possibility, timeframes for reporting and collecting information on AEs and SAEs, the reporting system, how you will follow up on AEs, and the specific guidelines for independent monitoring.
- Intervention Discontinuation: List criteria for intervention discontinuation and how you could meet them. Also list possible reasons for discontinuation, any modifications to the schedule should it be discontinued, duration of follow-up, any temporary discontinuation criteria, or any evaluations should participants be temporarily or permanently discontinued from the study.
- Statistical and Analytical Considerations: Include primary and secondary statistical hypotheses, why you chose the study design, the primary and secondary outcome measures, and the validity and reliability of these measures. Also discuss sample size and randomization, treatment assignment procedures, how you define the population, any interim analyses, primary and secondary outcome analyses, the statistical methods you use to consider any necessary intervention effect between groups, and if necessary, the expected positive within group correlations among different study arms.
- Data Collection: Detail how you will gather the data, the required forms, how to keep these forms confidential, and what source data to expect. Note site responsibility for data collection and management, and (if necessary) the responsibilities of the coordinating center.
- Quality Assurance: Describe training for study staff, whether there is a control committee and their required practices, any quality control metrics, how you will identify and document protocol deviations, how you will assure protocol compliance, and the schedule for reviews. If you have a manual of procedures (MOP), reference it here.
- Participants Rights: Include references to the Institutional Review Board (IRB) requirements, informed consent documents, procedures for participant confidentiality, and study discontinuation requirements.
- Committees: List any committees associated with the study, along with their roles.
- Publication: Outline the requirements and procedures for publication.
- References: List any citations referenced in this protocol.
- Supplements/Appendices: Include any additional documentation.
To track every aspect of the proposed research for each participant, create a case report form (CRF) that you can use in both paper and electronic formats. With CRFs, you can collect and analyze data for analysis, and then generate a conclusion for your study. For more information on the distinct phases of clinical trials, see “ Understanding the Phases of Clinical Trials .”
Concept Protocol Template
Before you start your full protocol, consider putting together a concept protocol. A concept protocol helps you introduce an abstract project to stakeholders and encourage discussion around the proposed project.
Download Concept Protocol Template for Clinical Research
Phase 1 Clinical Trial Protocol Template

For nonclinical research or clinical trials that are Phase 0 or Phase 1, use this free template. Phase 1 or nonclinical trials do not require the same amount of detail as a full study protocol.
Download Phase 1 Clinical Trial Protocol Template - Word
Research Compliance Templates
By training staff members on the research protocol, you’ll help them meet compliance standards and understand the purpose and details of the study. Use a training log to record all training that the site study staff completes, signing the log entry for verification.
Download Protocol Training Log Template
Excel | Word | PDF | Smartsheet
Protocol Deviation Template
Protocol deviations are inadvertent or unplanned changes or noncompliance with the research protocol. These events do not increase risk or decrease benefit, nor do they impinge on participants’ safety or rights. They do not compromise study data, but you should capture the deviation for reference.
Download Protocol Deviation Log Template
Excel | Word | PDF
Delegation of Authority Log Template
Once you’ve trained your staff and figured out their roles and responsibilities, the principal investigator must delegate authority. The delegation of authority log should be filled out and signed prior to the study’s start.
Download Delegation of Authority Log Template
Site Selection Visit Form Template
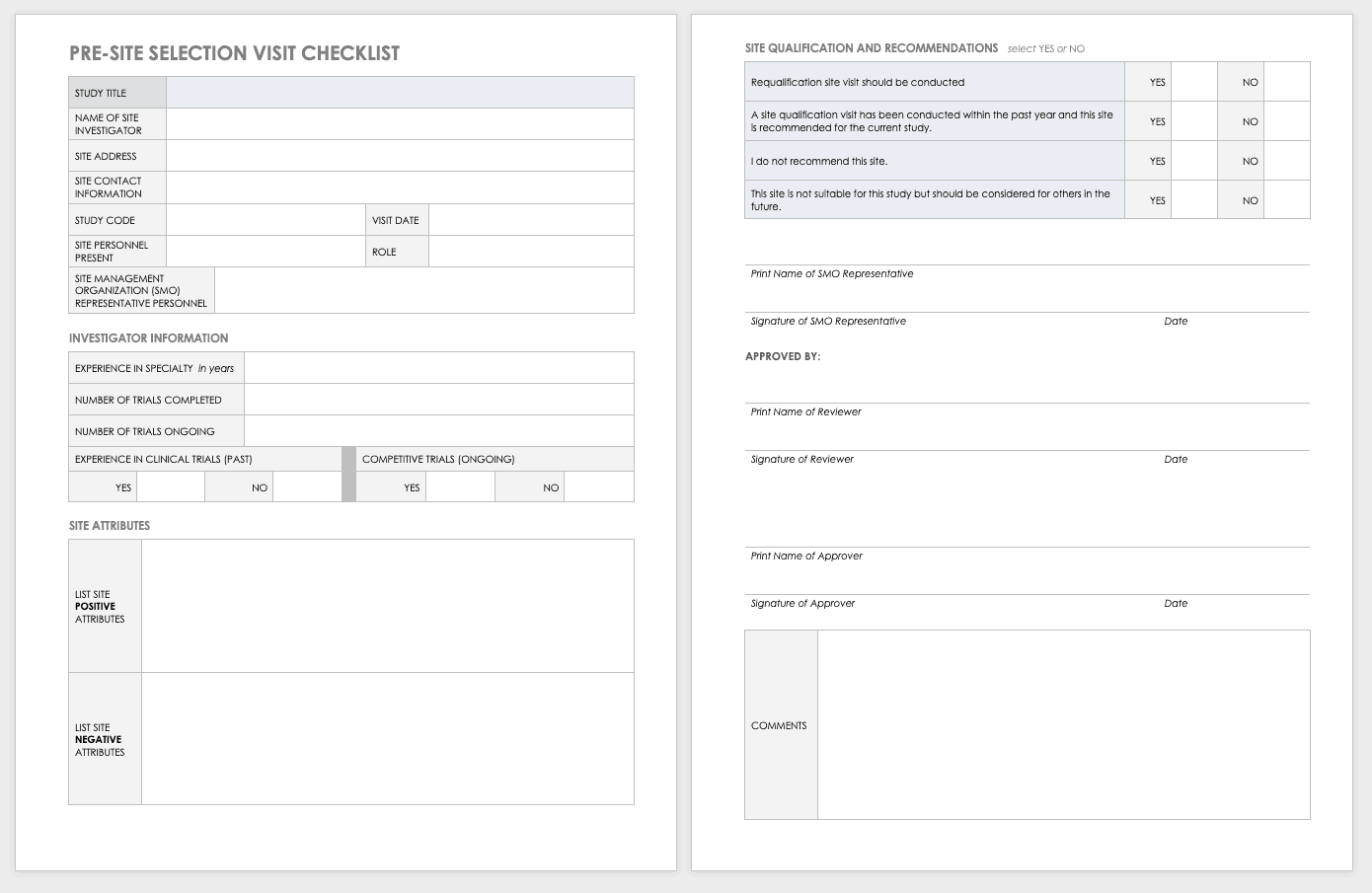
The sponsor must perform a site visit to determine its suitability as part of a multisite study. This means taking a tour to determine whether the site has the capabilities to meet the sponsor’s goals.
Download Site Selection Visit Form Template
Word | PDF | Smartsheet
Study Site Initiation Checklist
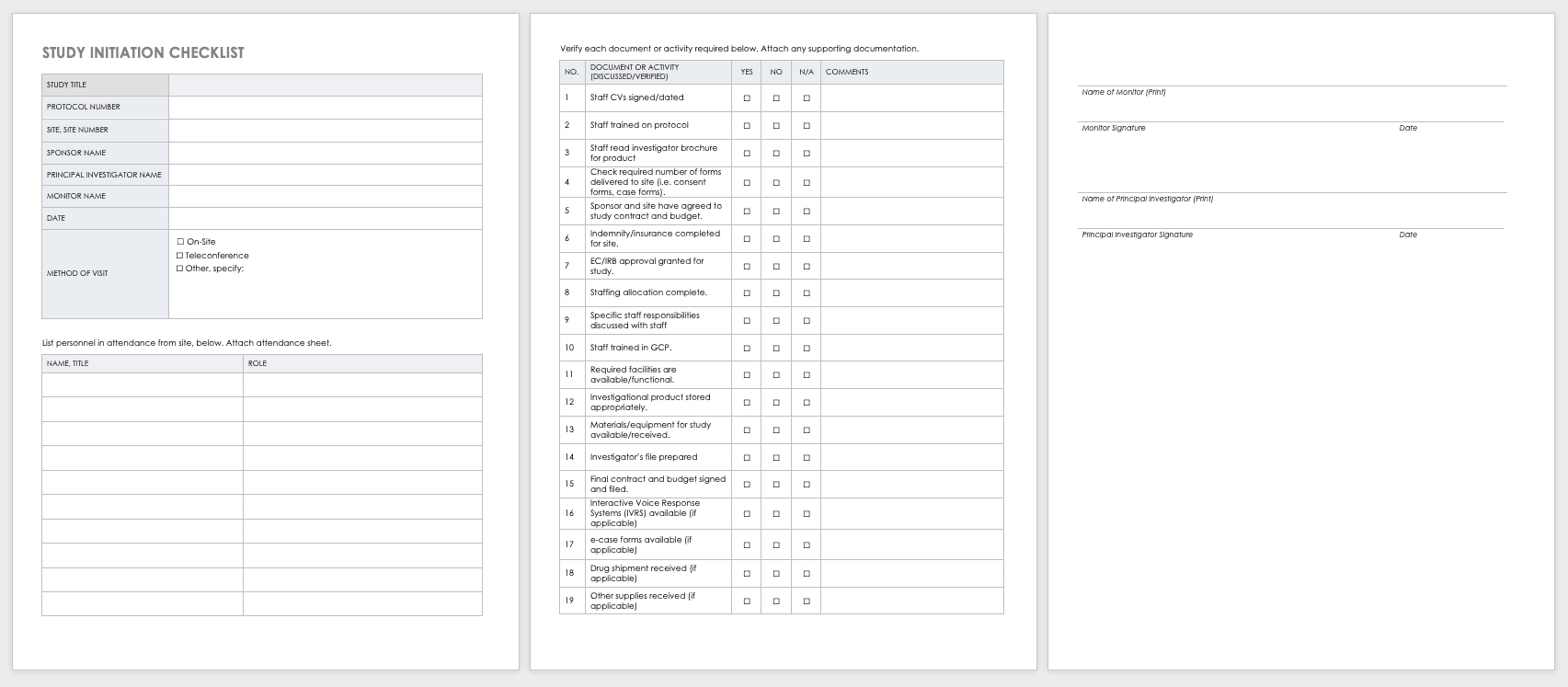
Teams must also perform an inspection to determine if a site has the appropriate staff, training, equipment, and supplies to be part of a multisite trial.
Download Study Site Initiation Checklist
Project Management for Clinical Trials, Practices, Templates, and Documents
Clinical trials are big projects. If the organization is not used to planning and wants to conduct clinical research, it must hire a project manager and work with senior leadership to introduce planning into the organization.
Together, they should develop the main goals and define their limits and the terms of success. They should set out a strategy for which tasks and sets of tasks to perform and in what manner. Test any planning tools or software before the trials start. When possible, use templates to ensure consistency and best practices.
Once the trial starts, evaluate your systems with standardized metrics. The project manager can track study deviations and apply corrective actions. Use the lessons learned from past and current projects to help guide future projects. Employing consistent tools gives you the opportunity to draw from a reservoir of data.
Clinical research can cost billions of dollars and years of time, resources, and effort. As
such, project management best practices and methodologies are critical to the success of a clinical trial, according to experts .
Many software systems are available to manage clinical trials. When very specialized, these are referred to as clinical trial management systems (CTMSs). However, other platforms can also manage clinical trials and may already be embedded with your information technology. Regardless of the platform you use, you should have full project management functionality, such as planning and reporting modules, as well as the ability to track participant contact information, deadlines, and milestones.
You may want to consider the following project management documents for your clinical research.
Project Management Plan (PMP) for Clinical Trials
A PMP delineates and acts as an agreed-upon document of scope, responsibilities, and guidance. You can use it throughout the project to help stay on track. Every clinical trial has difficult milestones, but a good project management plan can help you sidestep some of the regular issues.
You have many PMP software platforms to choose from, but regardless of your ultimate decision, your PMP must focus on protocol adherence, subject care, and service quality, along with how to achieve each standard. Here are the sections you should include in your PMP for a clinical trial:
- Project Objectives: This is an outline of the research objectives for the study, your quantifying standards, and your goals.
- Background and Strategic Context: By documenting background and context, you establish a foundation for decisions and discussion to follow.
- Study Governance: The governance covers the roles and responsibilities in the project, encouraging open communication, sharing, and accountability.
- Stakeholder Management Plan: This plan details how the staff and investigators will collaborate and effectively communication with stakeholders. This could include (as per the roles and responsibilities) regular emails, newsletters, consultation, oversight, training, and documentation.
- Scope: This document delineates assumptions, constraints, and deliverables (and their expected dates).
- Project Risk Assessment: This document helps you prepare for risks and decide on the risk profile.
Clinical Research Project Activity List
A project activity list is an itemized documentation of all the activities scheduled as part of the project. This list should be very detailed, including the status and priority of the task, when it is due, and to whom it is assigned.
Download Clinical Research Project Activity List Template
Excel | Smartsheet
Clinical Trial Timeline Template
A timeline enables you and your staff to track each major portion or milestone of your clinical trial. Your timeline should include these steps:
- Choose Research Questions and Study Design: Research always begins with questions. Your research question will determine how you design your study.
- Choose Outcomes: The outcomes for any trial are dependent on many factors, including scope, health conditions under study, target population, type of intervention. One resource to help develop outcomes is Core Outcome Measures in Effectiveness Trials (COMET) . This database details core outcome sets for comparison in clinical trials.
- Prospectively Register the Trial: Whether you are working through the FDA, World Health Organization (WHO), or another national agency, study transparency is critical. Prospective registration of trials is recommended. One resource for registration is the ISRCTN registry .
- Obtain Ethics Approval: Any trial involving human participants must go through an ethics review to safeguard the subjects’ rights, safety, well-being, and dignity. There are many options for institutional review, including through a university or a private or governmental organization. Without this step, research cannot commence.
- Prospectively Publish Protocol and Analysis Plan: Before a clinical trial, you must complete some pilot research. When you publish the research leading up to a clinical trial, along with the protocol and analysis for the trial itself, you increase transparency and accountability of the research.
- Planning for the Trial and Data Management: Many clinical research professionals recommend including patients in the planning phase of clinical trials, at least as stakeholders to review the plan. By completing the plan early and allowing potential participants to review it, you help improve recruitment and retention during the trial.
- Recruitment and Retention: Recruitment is getting the right people to take part in your trial, and retention is about keeping their interest and trust. A source of unending frustration for researchers, recruitment and retention can make or break a trial.
- Identify and Manage Trial Sites and Staff: This process is not as straightforward as it is often thought to be. Study coordinators must use feasibility checklists to choose sites and figure out how to get bring on staff who have the bandwidth to recruit for the study.
- Data Collection: The methods for collecting data are critical to any study. Advance planning and structure help you stay organized, comprehensive, and transparent so that your study can have a seamless analysis and solid conclusions.
- Data analysis: Flaws in analysis can generate poor, biased, or erroneous outcomes. In advance, researchers should consider patient blinding, randomization procedures, and sequence generation.
- Findings dissemination: Some researchers recommend threading all research on a trial topic. One resource for this is CrossRef , a database that links similar research. Regardless, the point of research is to capitalize on scientific progress and move it along. By having a plan to disseminate your results, you ensure that others capitalize on your research and move the knowledge forward.
Use this free template to develop your own clinical trial timeline. Add your own steps, milestones, and dates for a comprehensive, expansive view.

Download Clinical Trial Timeline Template
For a different perspective, add your project details to this free template so you can view your timeline visually.
Download Trial Timeline and Graph Template
Microsoft Project Management for Clinical Trials
First released in 1985, Project is a well-respected Microsoft product for project management. Microsoft Project was not traditionally available as a part of Office Suites, a package of programs for professionals and professional organizations. However, Microsoft recently included it as a part of the Windows 2016 suite.
Microsoft Project Management has the following features:
- Built-in templates
- Project portfolio management
- IT management
- Presentations
- Out-of-the-box reports
- Multiple timelines
- Real-time reporting
- Dependency management
- Priority assignment
- Lean management
- Gantt charts/project mapping
- Calendar views
- Setting baselines/KPIs
- Project budgeting
- Issue tracking
- Task creation
- Resource management
- Cloud access
Microsoft Project has built-in templates that you can apply to clinical trial management.
Microsoft SharePoint for Clinical Trials
SharePoint is a collaboration platform that is integrated with Microsoft Office. SharePoint manages and stores documents , and it enables multiple users to access the documents via their own site or a standardized Microsoft site. A subscription to Microsoft Office 365’s SharePoint does not require a server, but customization options are limited; the flexible authentication and authorization systems are built in.
SharePoint Server, available in Standard or Enterprise versions, can be developed as either
virtual or hosted services in a business’s IT department. SharePoint Server enables the organization to control the SharePoint features available to staff, and you can scale it to meet different numbers of users.
Windows SharePoint Services 3.0 is a Microsoft-hosted version that comes with Microsoft Office. Microsoft provides a template in SharePoint for Clinical Trials: Clinical Trial Initiation and Management application template for Windows SharePoint Services 3.0 . You can download and add this template to your SharePoint Services, which enables you to create the following:
- Clinical Trial Protocols: This includes the objectives, study design, project plan, subject selection, and budget.
- Protocol Documents: This includes additional documents relative to your study.
- Calendar: Track milestones in the project.
- Threaded Document Discussions: Team members can start and track discussions within documents.
- Task Creation and Assignment: You can create and assign tasks to users, who receive email notifications.
- Archiving: You can move documents or groups of documents to archive status, keeping them but not making them visible.
The clinical trial template has site lists of libraries for clinical trial protocols, protocol documents, announcements, calendars, issues, tasks, and document discussions. These can be further customized with different versions of SharePoint. To download this template, you will need access to SharePoint Server 3.0.
Clinical Research Budget Plan Template
In many instances, you set the clinical trial budget after much negotiation with a sponsor. Other times, you need to build a budget before the sponsor is even on board, as a way to convince them of the project’s feasibility. The key cost drivers for any clinical research project are the following:
- Patient Grants: These include the costs for screening failures, baseline patient measurements, and procedural costs.
- Site Costs: This covers any expenses associated with the site, such as start-up fees, IRB fees, storage fees, and site management costs.
- Non-Patient Costs: This includes consultation fees, monitoring board fees, and any medical device costs.
- Labor Costs: You must account for all the staff required for the project and their full-time equivalency (FTE).
- Site Management: These costs include pre-study visits, initiation fees, monitoring, and close-out fees.
- Miscellaneous: These include investigator meetings, any technology needs, and ad hoc travel.
- Unexpected Costs: These are costs resulting from protocol amendments, value added tax (VAT), delays, and inflation.
Before you start putting together your research budget, you must gather the following:
- Schedule of assessments from the protocol
- Standard institutional fees from your institution, if applicable
- Evaluation and procedural costs
- Staff allocation and their hourly rates
- Indirect cost rate
- Subject compensation costs
- Data storage fee estimate
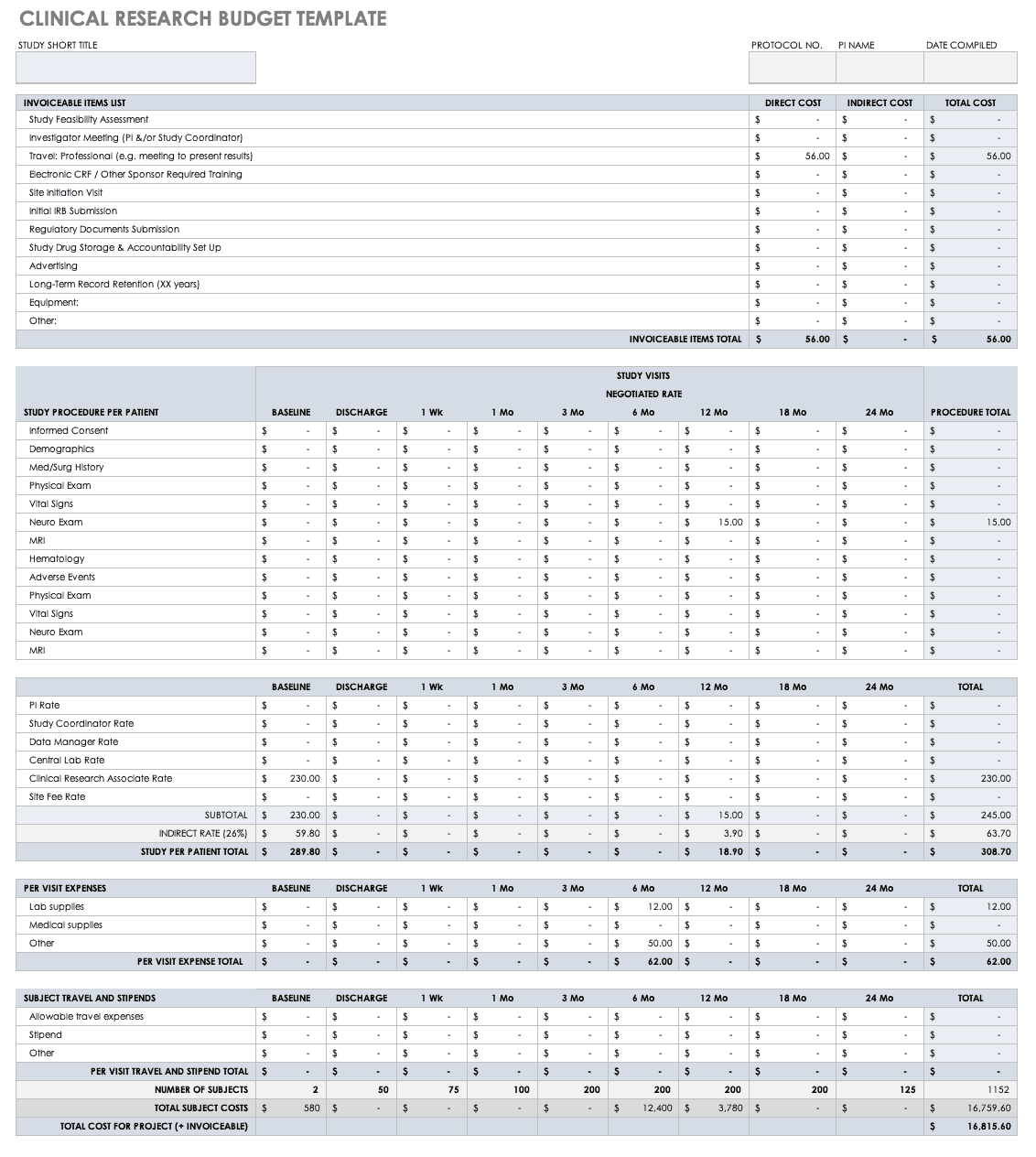
Put together your own clinical trial budget with this free clinical research budget template.
Download Clinical Research Budget Template - Excel
Clinical Research Tracking Log Templates
Clinical research requires scrupulous planning, a well-developed team, regulatory adherence, and above all, excellent documentation. It is therefore critical for clinical trial project managers to have a completed scope of work and to develop all the forms and templates before the trial begins. Some of these documents are for planning, and some, like those included below, are for operational purposes.
Regulatory Binder Checklist

Strong clinical practice thrives with a regulatory binder checklist. This checklist keeps track of all paper versions of essential regulatory study documents. Each document should also include any electronic locations. This document should be regularly updated, customized for unique studies, and stored in reverse chronological order.
Download Regulatory Binder Checklist
Clinical Study Document Tracking Log
It is important to not only track all paperwork related to a clinical trial, but also be able to locate it easily between various staff and sites. A clinical trial document tracking log can help you keep a written trail of the documents and when they were submitted and approved. You should also keep copies of the documents with the log. Use this free template to develop your own clinical study document tracking log. You can also adapt the log for specific correspondence, such as documents relating to FDA or IRB submissions, but it should not be mixed with regulatory documentation.
Download Clinical Study Document Tracking Log
Data and Safety Monitoring Plan (DSMP) Template
Before you can undertake a study, you must develop a DSMP for how to keep participants safe and how to secure data and ensure accuracy. The DSMP has several sections:
- The study purpose
- An adherence statement
- Any protocol amendments
- Multisite agreements
- A plan for subject privacy
- Confidentiality during adverse event reporting
- Expected risks
- Adverse events, unanticipated problems, and serious adverse events: how they are defined, their relation to the study, expectations, severity grading, and reporting procedures in single-site and multisite trials, and whether they are IND or non-IND studies
- Events of special interest
- Pregnancy reporting
- Rules to halt the study for participants
- Quality control and quality assurance
- Subject accrual and compliance
- Sample size justification
- Stoppage rules
- Monitoring committee designation
- Safety review plan
- Study report plan for independent monitors
- Plan to submit reports from onsite monitoring and audits
- Data handling and record keeping
- Informed consent
- Reporting changes in study status

Create your own data and safety monitoring plan using this free template. It lays out each section so you can specify them for your research. The principal investigator should sign and date this document once it is complete so that it may be filed.
Download Data and Safety Monitoring Plan Template - Word
Research Communication Plan Template
A communication plan should describe how you will converse with internal and external stakeholders during your project. Your communication plan should include a brief overview of your project and a breakdown of the messages you need to get out. You should adapt the messages for different audiences and define who will deliver these messages. The messages should include the following:
- The purpose and benefits of the research
- The known effectiveness of the intervention, or (if the intervention is under study) the disclosure that the effectiveness is unknown
- How participants will be protected
- The risks and benefits of participating

Develop your own communication plan using this free clinical trial communication plan template. This template also includes a section for situation analysis and risk analysis that asks for inputs on strengths, weaknesses, opportunities, and threats.
Download Clinical Trial Communication Plan Template - Word
Participant Management in Clinical Trials Using Templates
A few main documents help ensure that your participants are tracked and well-cared for before and during your research study.
Enrollment Log for Clinical Trials Template
This log keeps track of everyone that has been enrolled for participation in your study. This does not mean that they have met the eligibility requirements or have been otherwise screened, but it is a record that they have signed up to be admitted.
Download Enrollment Log for Clinical Trials Template
Informed Consent Form Templates
Informed consent is the central tenet of ethical research with human subjects. The consent process typically involves a researcher delineating what is involved in the study, its risks and benefits, what a participant’s duties entail, and answering any questions they have. Before you perform any research, make sure the informed consent document is signed and the participant receives a copy, unless the informed consent document has been waived by an institutional review board (IRB). Federal regulations 45 CFR 46.116 govern what you must provide in the informed consent process in the United States.
To prepare informed consent documentation, researchers must do the following:
- Use plain, easily understandable language no higher than an 8th-grade reading level.
- Tailor documents to the potential population.
- Avoid technical jargon.
- Use the second or third person (you/he/she) to present study details.
- Include a statement of agreement.
- Ensure that the consent document is consistent with information in the IRB application.
These templates assist the principal investigator in the design of their informed consent forms (ICFs). You can adapt them to accommodate the details of any study and include both the information sheet and the consent form. Modify each section with the appropriate description described in italics. Use the general template for any type of research.
Download General Informed Consent Template - Word
Use the clinical trial template for medical research.

Download Informed Consent for Clinical Trials Template - Word
Eligibility Criteria (Inclusion/Exclusion) Checklist
Eligibility criteria are an essential part of clinical trials. They define the population under investigation.
Inclusion criteria are the standards that participants must meet to enroll in the study. For example, in a study on a new diabetes medication, you would likely want participants who have already been diagnosed with diabetes.
Exclusion criteria specify the characteristics that disqualify participants from taking part in the research. For example, in the diabetes study above, the proposed diabetes drug may target a specific age demographic. One exclusion criterion could be a participant whose age falls outside of the range.
Download Eligibility Checklist Inclusion-Exclusion Template
Concomitant Medication Log Template
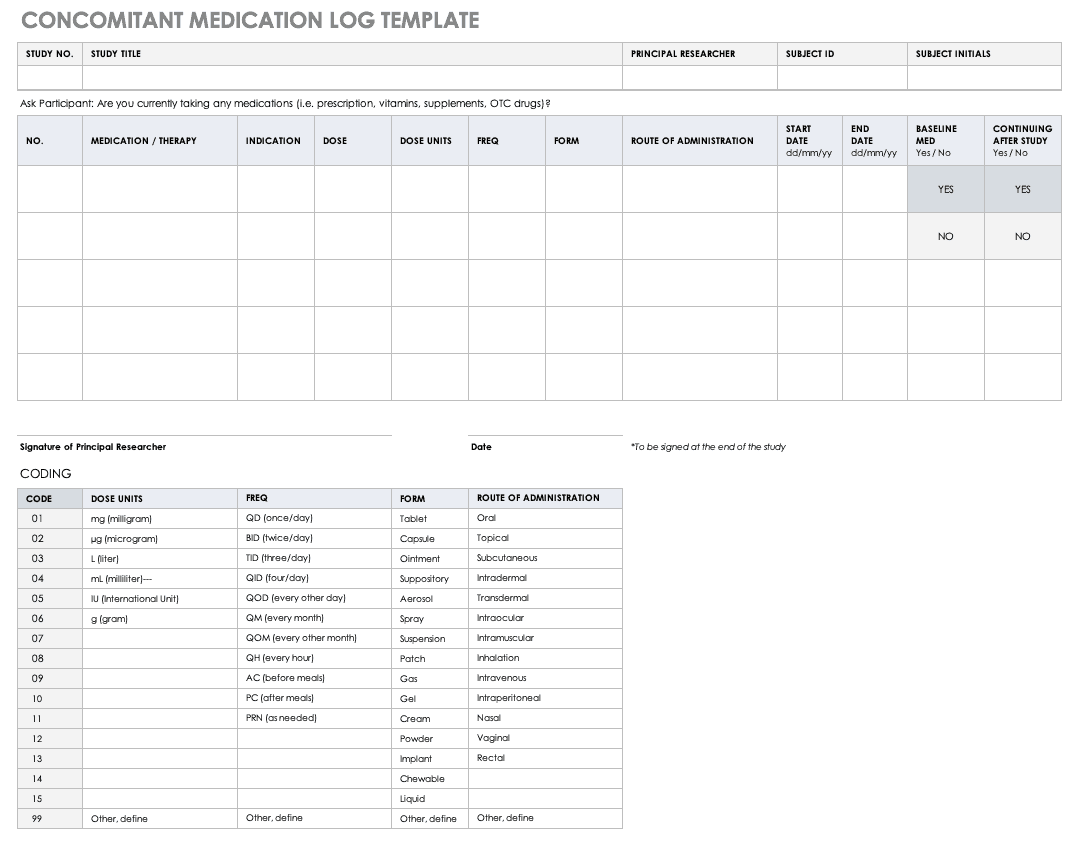
Properly documenting any medications that participants are taking is imperative to understanding the reactions occurring in their bodies, as well as what could spur adverse and severe adverse events during the study. Fill out a concomitant medication log for every participant and account for everything participants take, even seemingly innocuous items like multivitamins.
Download Concomitant Medication Log Template
Excel | Word | PDF
Adverse Event Form
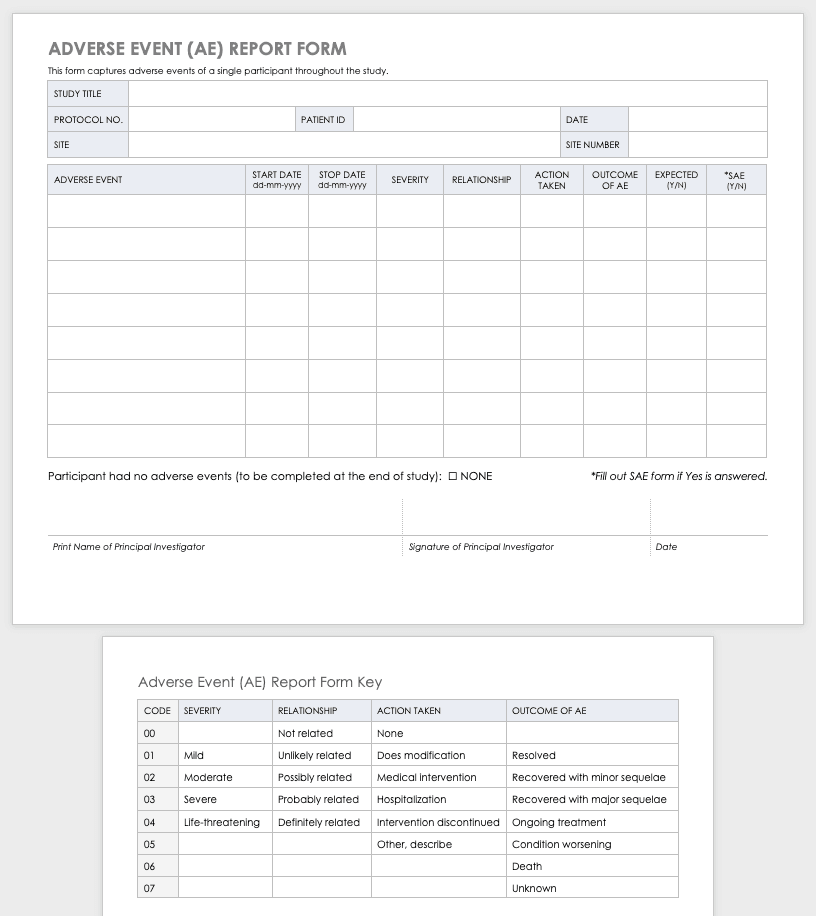
Clinical research can result in complications for the participants and trigger an adverse or severe adverse event. An adverse or severe adverse event is when participants in a clinical trial have negative medical symptoms that can be shown in laboratory or physical testing. Each participant in a clinical trial should have an adverse event log that tracks any adverse events through the duration of the study.
Download Adverse Event Form Template
Severe Adverse Event Form

A severe adverse event (SAE) is a special case of an adverse event in which the outcomes are acute. Examples of SAEs include death, life-threatening complications, or anything leading to immediate hospitalization, physical disability, or congenital abnormalities. Log SAEs in the AE form, but fill out an additional SAE form.
Download Severe Adverse Event Form Template
Word | PDF | Smartsheet
Post-Clinical Study Research Documentation and Templates
After you complete or terminate a clinical trial, you should prepare several additional documents. Here are some examples of this documentation:
- Investigational Product Accountability Log: You generally provide an accountability log to the authorities that tracks drug products to show product disposition and accountability per participant. It also helps you track the drug product stock and any imbalance at the end of the study.
- Investigational Product Destruction: Due to regulations governing the proper disposition of investigational products in clinical research, you must properly dispose of products left at the end of a study (as evidenced by the product accountability log). This form describes and ensures that you have properly handled any leftover products.
- Close-out Checklist/Report: A study close-out checklist and report helps ensure that you complete all closing procedures, archive the paperwork, and resolve electronic data.
Clinical Study Summary Report Template

Assemble the summary report at the end of a study to get results into the sponsor’s or public’s hands while you complete the full report. A summary report is typically about 2-3 page-long document that encompasses the highlights from the trial.
Download Study Summary Report Template - Word
Clinical Study Report (Full) Template

The full clinical study report (CSR) encompasses all aspects and details of the research you’ve conducted. It is not a sales or marketing tool; instead, it is a scientific report details the methodology and shows scientific rigor.
Download Clinical Study Report Template - Word
Public Links and Resources for Clinical Trials
The following are publicly available resources, tools, and links for clinical trial practitioners and principal investigators:
- PROMIS : Patient-Reported Outcomes Measurement Information System (PROMIS) software gives clinicians health status patient measures that are physical, mental, and social patient-reported metrics. Funded by the National Institutes of Health (NIH), PROMIS can be used in clinical trials as measures of conditions and disease and as a comparison to the general population. The measures in PROMIS are free to administer on paper, by computer (computer adaptive tests), or with an app. The computer adaptive tests may be conducted on REDCap , Assessment Center , or Epic .
- REDCap: REDCap (Research Electronic Data Capture) is an electronic data capture system that works on browsers to develop research databases. It was developed at Vanderbilt University to support clinical research data collection and is a free resource to nonprofit organizations. It is limited to organizations joining the REDCap consortium and is not open-source or available for commercial use.
- Good Clinical Practice (GCP) Training: GCP is an international quality standard designed for use by staff involved in clinical trials. The guidelines for this are from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). These regulate the ethical guidelines, documentation, record keeping, training, facilities, technology, and inspections. The purpose of these guidelines is to keep clinical trials scientifically rigorous and to delineate the roles and responsibilities of research staff. The National Institutes of Health administers training for GCP.
- Quality Management Study-wide Review Tool: Developed by the NIH, this review tool is for PIs and study teams to manage their quality reviews, and may be customized for unique studies.
- Quality Management Subject Review Tool: Also developed by the NIH, this review tool provides study teams the structure for review of participant data, and may be customized for the unique study. This should be developed in concert with the DSMP.
- AccrualNet: AccrualNet is sponsored by the National Cancer Institute (NCI), and offers advice and training to staff on how to recruit study participants.
- Regulatory Education for Industry (REdI): The FDA offers a Clinical Investigator Training Course for researchers conducting investigational new drug (IND) or device exemption (IDE) studies.
- ResearchMatch: Available to volunteers and researchers affiliated with the NIH Clinical and Translational Science Award (CTSA) program, this site helps match prospective participants with specific studies.
- Grant Policies and Guidance: The NIH and National Center for Complementary and Integrative Health (NCCIH) offer links to many resources that are policy- and grant-specific to the NIH and NCCIH, updated regularly.
- Protocol Amendments: The NIH and NCCIH offer regularly updated guidance for NIH policy and protocol changes.
- Clinical Terms of Award for Human Subjects Research: The NIH and NCCIH offer guidance for clinical trial grant awardees for compliance.
- NIH Single IRB (sIRB) Policy for Multisite Research: The NIH offers a FAQ page for multisite research that includes policy, contract and application information, responsibilities, exceptions, and costs.
- Dictionary of Cancer Terms: The National Cancer Institute (NCI) offers a dictionary of cancer terms for researchers and laypersons. You can add this dictionary to your website as a widget.
- Informed Consent FAQs: The U.S. Department of Health and Human Services (HHS) and the Office for Human Research Protections (OHRP) offer a FAQ page about informed consent for researchers and lay persons.\Informed Consent Language (ICL) Database: The National Comprehensive Cancer Network (NCCN) offers a database to help write informed consents. This database is specific to medical conditions and different risk language.
Improve Clinical Trial Research with Smartsheet for Healthcare
Empower your people to go above and beyond with a flexible platform designed to match the needs of your team — and adapt as those needs change.
The Smartsheet platform makes it easy to plan, capture, manage, and report on work from anywhere, helping your team be more effective and get more done. Report on key metrics and get real-time visibility into work as it happens with roll-up reports, dashboards, and automated workflows built to keep your team connected and informed.
When teams have clarity into the work getting done, there’s no telling how much more they can accomplish in the same amount of time. Try Smartsheet for free, today.
Discover why over 90% of Fortune 100 companies trust Smartsheet to get work done.
- About Research & Innovation
- Advanced Cardiovascular Care
- Health Equity
- Inflammation
- Maternal and Fetal Medicine
- Musculoskeletal Care
- Neuroscience
- Opioid & Pain
- Research Centers
- Departments
- About the Office of Research
- Funding & Proposal Development
- Regulatory Review & Compliance
- Research Project Management
- Cores & Resources
- Education & Training
- Peter Arvan Lab
- Antiphospholipid Syndrome Research Labs
- J. Michelle Kahlenberg Lab
- John Varga Lab (ScleroLab)
- Mulholland Lab
- Pasca di Magliano Lab
- Raghavendran Lab
- ALS Center of Excellence
- Institute for Heart & Brain Health
- Center for Basic & Translational Science
- Center for Bioethics and Social Sciences in Medicine
- Biomedical Research Core Facilities
- IT Route Map
- Clinical Research Route Map
- Commercialization Route Map
- Great Minds, Greater Discoveries
- Research Scouts
- Meet the ROMS Team
- ROMS Fellowship Application Information
- Working with a ROMS Fellow
- Pandemic Research Recovery
- Research Climate Council
- Support for Outstanding Research
- Research News Trivia
- Frequently Asked Questions (FAQ)
- Billing Calendar & Study Applications
- CRAO Frequently Asked Questions (FAQ)
- Pre-Onboarding Considerations
- Access Biospecimen
- Biospecimen Processing
- Biospecimen Inventory
- Regulatory & Governance
- Equipment & Instrumentation
- Central Biorepository Pricelist
- CBR Frequently Asked Questions (FAQ)
- Clinical Trials Support Units
- Clinical Research Coordinator Career Ladder
- Clinical Trials Management Systems: Accounts & Support
- Research Pharmacy
- Off-Campus Clinical Research
- Statistical Support for Large-Scale Clinical Trials
- MCRU Services
- MCRU Lab Study Team Guidelines
- MCRU Frequently Asked Questions (FAQ)
- Michigan Medicine Site Profile
- CTSO Frequently Asked Questions (FAQ)
- Self-Serve Data Tools
- Custom Data Request
- Data & Biospecimen Sharing
- Student Access to Patient Data
- FFMI fastPACE
- Frankel Cardiovascular Center Innovation Program
- Rogel Cancer Center Innovation Program
- Global Health Commercialization Competition
- Biomedical Innovation 101
- Certificate in Biomedical Innovation & Entrepreneurship
- Fast Forward Medical Innovation Fellowship Program
- Biotech Career Development Program
- Drug Discovery & Development Course
- Innovation Studio
- Oncology Drug Discovery & Development (3D) Workshop
- FFMI fastPACE Train-the-Trainer
- Intellectual Property in the Academic Setting
- Academic Drug Discovery
- Medical Device Regulation
- Idea to Impact Webinar Series
- 6 Tips for Customer Discovery
- Interacting with Industry
- Ask the Experts: A Biomedical Innovation Forum
- Business Development
- Michigan Biomedical Venture Fund
- R01 Boot Camp
- Facility & Resource Profile Templates
- BMRC Bridging Support Program for Biomedical Research
- Medical School Limited Submissions
- Budgeting & Costs
- NIH Specifics, Tips & Tricks
- Grant Routing Deadlines
- Proposal Review Checklists
- Department Contacts
- Authorized Signers
- Research Data Analytics
- Grant Processing Advisory Committee (GPAC)
- Post-Award Advisory Committee (PAAC)
- Grants Frequently Asked Questions (FAQ)
- Board Nominations Sought
- Informed Consent & Assent Templates
- IRBMED Seminar Series
- Information for IRB Members
- Information for Study Subjects
- SignNow Electronic Signature Service
- IRBMED Frequently Asked Questions (FAQ)
- IRBMED Contacts & Roster

The Grant Services & Analysis Office curated the following information on budgeting various types of proposals.
Institutional Cost Sharing Policy - This policy describes the process and conditions for obtaining a research cost share commitment from the Medical School Dean's Office, HHC, or EVPMA. Further, this also addresses the process for Medical School units seeking a cost share from the Office of the Vice President for Research (OVPR). Indirect Cost Recovery Policy - This policy describes the Medical School's expectations regarding recovery of indirect costs. Indirect Cost Waiver Policy - This policy describes the process that should be followed by Medical School units seeking an indirect cost waiver. Indirect Cost Waivers on Federal Patient Pass-Through Costs - This policy describes the process and conditions for Medical School units seeking a reduction of indirect costs on pass-through expenses related to clinical trial patient payments. Recovering Full Indirects: Industry-Funded Research - This policy describes the required process that should be used to negotiate the indirect cost rate with industry sponsors. Data & Biospecimen Sharing - University of Michigan policy is that data and biospecimens should be collected under transparent, informed consent that permits and promotes the maximal use and value of the data and biospecimens consistent with the permission of the donors.
We have been asked to provide tools to facilitate budgeting and translation of the budget into sponsor forms. These formats may help ease the transition. If you have any comments, improvements, or suggested new formats, please contact [email protected] .
All templates have formulas embedded. They will "break" if formulas are overwritten. We recommend always doing a "Save As" to preserve the original formulas. Please read all instructions included!
SF424 Modular (Excel) This excel form will help you take cost estimates, average over time, and determine how to populate a modular SF424 budget. It includes both a worksheet for drafting a budget, as well as carrying the totals into replica 424 forms. Also provides calculations for face page and PAF routing form.
SF424 Categorical (Excel) Like the modular, this includes a worksheet for details in the non-personnel categories that will roll up into the main form and this will give you the placement of costs on the 424 pages. Also provides numbers for face page and PAF form.
PHS 398 Single Budget with a Straight Increase (Excel) This form is all but obsolete for use with NIH and the form has not been updated, so the subcontract costs are totaled a bit differently on the current 398 form and the checklist is no longer a combined multi-year total. HOWEVER, this is a good format for checking a budget, playing with internal budgets, or mocking up numbers for another sponsor.
PHS 398 Budgets Change Year to Year (Excel) This form is also old and does not conform to current 398 placement of subcontract costs or checklist information. However, it is a reasonable template for mocking up internal budgets, especially complex ones that have constantly changing costs. It will increase costs by a standard %, but requires re-entry of all effort and other costs with space to enter detailed information. It has similar functionality to the SF424 Traditional, but is for those that are more comfortable in the 398 world.
PHS 398 Program Projects with a Straight Increase (Excel) This form allows you to add up to 8 budgets and calculate your cumulative totals. You may also track indirect cost exclusions by project and have those accumulate for the final checklist calculation. Like the two previous, it has not been updated for the change in subcontract position or the indirect cost calculation by year instead of total, but it does offer a very close look and feel with final numbers.
Quick Calculations (Excel) This is a non-form driven template that allows you to quickly calculate the Modular and K Award costs, including numbers for face page and PAF form.
MoUs are required to be filled out for all faculty with a dual VA/UM appointment. The attached Excel form contains all needed MoU, cost share, and HR distribution information – including the application of PHS Salary Cap I & II. Please see below for Salary Cap guidance.
- 2024 VA MoU - 1/1/2024 and After (Excel)
- 2023 VA MoU - 1/1/2023 and After (Excel)
- 2022 VA MoU - 1/2/2022 and After (Excel)
- 2021 VA MoU - 1/3/2021 and After (Excel)
- 2020 VA MoU - 1/5/2020 and After (Excel)
- 2019 VA MoU - 1/6/2019 and After (Excel)
- 2018 VA MoU - 1/7/2018 through 1/5/2019 (Excel)
- 2017 VA MoU - 1/8/2017 through 1/6/2018 (Excel)
- 2016 VA MoU -1/10/2016 through 1/7/2017 (Excel)
- 2015 VA MoU - 1/11/2015 through 1/9/2016 (Excel)
- 2014 VA MoU - 1/12/2014 through 1/10/15 (Excel)
- Oldest VA MoU - Prior to 1/12/2014 (Excel)
See Handling Salary Over the Cap .
As you are building budgets, or looking at rebudgeting in post-award, it is important to understand the different methods of calculating effort and the impact of different employment arrangements for those at the UM and expectations of particular sponsors on what to implement.
In addition to instructions on handling effort in budgets, we also have a few YouTube videos to explain effort appointments and calculating effort spent on a project. Please consider watching the videos in our Video Library for more context.
Internal budgets and some external sponsors tend to calculate in terms of a percentage. Whether the individual has a full 1.0 FTE or a partial appointment, for example 0.8FTE, all employees of UM have 100% appointments to allocate. Budgets should be built on the institutional base and the % of time (out of 100%) is applied to calculate the reasonable amount to charge the sponsor. See additional information further below in the UM/VA Appointments section
Example 1: Full 1.0 FTE:
The staff member will work half their time on the project, it is 50% requested.
Example 2: Partial 0.8 FTE:
The staff member will work half their time on the project, 50% of their university appointment requested.
Note: The institutional base for the two individuals will be different, but the effort % is the same.
Many sponsors require that effort be requested in person months rather than by %. Person months can be broken down into calendar, academic and summer months. Medical School faculty typically have calendar year appointments, therefore effort is reflected in calendar months. We highly recommend working with a participating academic unit closely if you have participants that are on an academic or summer month appointment. Some high-level notes are below.
To calculate calendar months take the % effort the person will spend on the project and multiply by the FTE appointment at the U and multiply by 12 (for the 12 months in the year).
Example 1: Full 1.0 FTE:
The PI will be working on the project at 25% effort. 25% (or .25) x (1.0 FTE x 12 months) = 3.0 calendar months
Example 2: Partial 0.8 FTE:
The PI will be working on the project at 25% effort. 25% (or .25) x (0.8 FTE x 12 months) = 2.4 calendar months
Note: To calculate academic or summer months, it is a similar method of applying effort against the FTE and base calendar months; however, the calendar months become the number months of the appointment (9 months for academic/summer months depend on the home unit and the individual’s employment arrangement. This is why it is important to stay in close contact.)
Occasionally sponsors (including federal contracts) ask for budgets based on a per labor hour calculation. It is important to know that the University’s effort tracking system does not allow exempt employees to track effort on an hourly basis. Instead, we recognize a work week that is “normalized” to 40 hours and a total work year of 2080 hours. The Medical School requires that a statement to this effect be listed on the budget shared with the sponsor so that the institutional practices are clear. A statement could be phrased: Effort represented is based on a standard 40 hour work week. Effort tracking systems will be collected in % effort and converted for the recovery. It is easiest to think about the effort needed in terms of a percentage and convert it to the needed hours requested.
A technician will be working on the project at 25% effort. 25% (or .25) x (2080 hours) = 520 hours
A technician will be working on the project at 25% of their partial appointment. 25% (or .25) x (0.8 FTE x 2080 hours) = 416 hours
The National Institutes of Health (NIH) and other sponsors under the DHHS (CDC, FDA, AHRQ, HRSA, SAHMSA, etc) will not pay requested salary above their congressionally mandated salary cap regardless of which type of effort representation (%, months, or hours). If salary is requested above the imposed salary cap, the sponsor will adjust the total amount of the award accordingly. Please visit our page on Handling Salary Over the Cap for additional information on how this should be handled in both pre- and post-award.
The VA MoU & VA Cost share template file contains all needed MoU, cost share and HR distribution information. Please see above for guidance.
When working with faculty that have a dual appointment with the VA, it is important to remember that you must always calculate % of University Effort starting with hours (not $$). For each of the cases below, please consult their VA MoU for hours at the UM and calculate:
% of University effort = avg # hrs/wk on the project divided by # hrs/avg wk at the UM
With the % of University Effort calculated, proceed with the type of sponsor:
- Indicate effort proposed (in budget and justification) out of 100% of the University appointment (University effort = avg # hrs/wk on project divided by avg # hrs/wk at UM)
- Indicate that all effort on the proposal comes from the University appointment
- Institutional Salary Base to use: University Comp Rate
- Calculation of Salary Requested: University Comp Rate * % of University Effort
- Language to use in Justification: Dr. X will have 50% of his University appointment devoted to this project.
- # of months at the UM as reflected on the MoU worksheet * % of University Effort
- Example: 3 months at the UM * 50% UM effort = 1.5 calendar months
- Note: In calendar months, you do not have to say “of University appointment”
- Name – UM / Months devoted to project in the Calendar Months column / Univ Comp Rate / salary requested
Note: You are not required under NIH guidelines to list the Institutional Base (Comp Rate) on the SF424 form at time of application. If you do list it, because it is calendar months, the math may look “funny” and you will need to include an explanation in the justification (see below). You also may choose to leave the Institutional Base blank and provide that information to NIH if/when it is requested. Departments may institute rules on how all applications will be treated. Please contact your department Grant Administrator for any local practice.
In the justification :
- Example: 3 mnths at the UM * 50% UM effort = 1.5 calendar months
- Indicate that there is a dual appointment with the VA, but all effort charged to the proposal comes from the University of Michigan (you may have additional time from the VA appointment reflected and no dollars recovered)
- Example Language to use: Dr. X will have 1.5 months devoted to this project. He also has a VA appointment, however all effort on this project will be from the University appointment. A MoU is on file describing the relationship.
- Additionally, if you are listing the Institutional Base on the budget grid, it is appropriate to say: Dr. X has a 0.00 FTE at the university and the institutional base reflects the reduced appointment.
If it is a NIH K-Award proposal
- Examples: If the announcement requires 75% effort or 9 calendar months, then 75% of 40 hours is 30 recorded hours reflected on the University side of the MoU to apply OR 75% of 12 calendar months is 9 calendar months documented.
- % University Effort is avg # hrs/wk on the project divided by avg # hrs/wk at UM
- # Calendar Months is # of months at the UM as reflected on the MoU worksheet * % of University Effort
- Example Language to use: Dr. X will have Y calendar months from his University appointment devoted to this project. He also has a VA appointment and a copy of the Memorandum of Understanding between the VA and University has been included in the proposal.
- Institutional Salary Base to use: University Comp Rate
Below are some common components of proposal budgets. You are encouraged to also discuss expected approaches with your department as the financial risk for cost overruns and short fall for budget lies within. Therefore, the sub-units may have preferences in budget approaches. A list of your department contacts are available here .
Project Teams assembling budgets are encouraged to think about the full cost of the project. Any work proposed under the aims that does not recover funds from the sponsor (whether because we didn’t budget in the proposal or accept the aims without full funding) is a form of institutional “investment” in the project.
Below are the categories that are traditionally requested to be addressed and justified. While always recommended, some sponsors do not require the detail in the budget or even provision of the justification. In those cases, it is the Principal Investigator’s decision on how to address with the best grantsmanship outcome. See our Budget Justification page for more information on the justification component, particularly when working with the NIH.
Personnel Salary
Salary of personnel who will be spending time on the project are included in the budget. Provide the name, title, amount of time on the project, institutional base salary (not necessarily full-time rate) and the amount of support requested. In addition, include a narrative on what each person will do on the project and tie to the specific aims presented.
For information on calculating calendar months, dealing with the NIH cap or Split UM/VA appointments view Budgeting Personnel above.
Personnel Fringe Benefits
Fringe Benefits are calculated on all salaries. Many departments have a standard rate they use when preparing budgets. You may choose to use an average rate over the project or actual. Actual fringe rates are available from the campus website .
Consultant Costs
Consultant services may be deemed necessary as a part of a grant proposal. The consultant’s expertise, primary organizational affiliation, and amount of proposed compensation are normally detailed in the budget justification. You will want to compare to the work statement provided by the consultant.
Equipment is defined by the federal government as an item of property that has an acquisition cost of $5,000 or more and an expected service life of two or more years. Proposed equipment should be itemized by descriptive name and estimated cost. Shipping and/or installation charges associated with equipment acquisitions should be included in the cost estimates but generally are not itemized.
Items costing less than $5,000 or with a life expectancy of less than two years normally should be included under "Materials and Supplies." Also, non-federal sponsors have a different threshold and individual instructions from the sponsor need to be consulted.
Detail on each trip, the destination, purpose, and expense, should be incorporated, including transportation, lodging, and per diem. Some sponsors request explicit explanation if there is foreign travel. Occasionally, sponsors also want extreme detail (e.g., miles driven * rate per mile). Be aware, scrutiny of the mathematical validity will be applied in the review process.
Materials and Supplies
Consumable supplies such as office supplies, glassware, chemicals, and computer supplies would normally be included in this category.
- Publications
The costs of preparing and publishing the results of the research project may be included in the budget request for most sponsors. Sponsors have a vested interest in making sure results are distributed and normally welcome this budget line. Costs may include printing, illustrations, and other journal costs.
Subcontract Costs
Subcontracts are typically established when funds awarded to the University will be paid to an organization outside of the University for work performed on the project. For more information view Consortium/Contractual Arrangements on our NIH Specifics, Tips & Tricks page.
Facility & Administrative Costs (F&A or Indirect Costs)
Overhead must be requested at the institutionally established rate based on the type of activity being proposed. This may only be waived if a sponsor has a published policy restricting the payment of indirect costs that applies to all applicants. See the University’s indirect cost rates and the Medical School’s position on recovery of overhead .
Further, for federally sponsored projects and non-federally sponsored projects that recover the federal rate, the University uses a Modified Total Direct Cost (MTDC) base for calculating indirect costs. The following expenses are excluded from the direct costs base under MTDC:
- Equipment purchased for $5,000 or more with a life expectancy of less than two years
- Alterations and renovations of facilities
- Patient care costs (as charged through the Clinical Pricing Tool)
- Subcontracts – the first $25,000 of each subcontract is included in the base for calculating indirect costs, any amount over $25,000 gets excluded from the indirect costs base
Industry sponsored studies may be for activity related to investigator-initiated research, acting as one of multiple sites on a clinical trial, or performing service agreements for standard activities. Regardless of activity or contract method, there are some approaches and costs to keep in mind.
When preparing a budget for an industry sponsor, consider using a full cost budget. A full cost budget shows only the total costs of conducting the study, as opposed to the standard budget where direct and indirect (F&A) costs are shown separately in addition to the total cost. For example, instead of requesting $1,000 for travel and $500 for overhead, ask for $1,500 for travel. Representing costs as a full cost may remove some of the discussion about the institutional overhead recovery.
Industry Sponsored Agreements are not limited to Clinical Trials. Below are some tips that will help smooth the negotiation with the sponsor, as well as project execution on all types of industry agreements.
Read the Protocol or Research Plan
- Whether it is industry or investigator-initiated, the protocol or work plan will help determine many budget issues and planning concerns as you go forward
- Ask the PI to consciously determine if the level of scientific endeavor is worth exploration of the relationship
Determine the Timeline
- With project plan in mind, determine the amount of time the project will take, keeping in mind the patient population, milestones (including enrollment targets, adverse events), and final publications that might influence the timeline
*Decision Point: Is it reasonable to pursue the project?
- If it is, and this is a clinical trial with a draft agreement provided, complete a CTRF in the eRPM system in order to provide ORSP with the draft agreement to begin negotiation of non-financial terms.
- If it is not, please cancel any related activity created in the eResearch systems.
Determine the Costs
Independent of any financial indications from the sponsor, internally determine the cost of doing the project. Cost out all real expenses over the life of the project.
Considerations:
- Faculty time
- Technical staff support time (i.e. research assistants, nurses)
- Administrative/financial accounting time (non-federal sponsors allow recovery of these costs as a direct item)
- GSRA? If yes, must include tuition as well as time
- IRB fee (if applicable)
- Administrative unit start-up fees (i.e. Cancer Center or MICHR CTO, MCRU initiation)
- Drug set up and dispensing
- Advertising
- Screen Fail Compensation
- Patient Visits
- Patient Tests
- Patient Reimbursements (subject fees, travel reimbursement, volunteer parking, focus group food)
- Adverse Event Reporting
- Animal Expenses (purchase, housing, care, maintenance)
- Computer for data collection
- Filing Cabinets for data storage
- Off-site Space
- Note: This may not be in the form of an incentive per patient! That would be against IRB requirements and University compensation. Instead, compensation for recruiting time must be done as a component of institutional effort.
- Indirect Cost (F&A) Recovery - Need help deciding which rate applies? Call the Grants Office 734-763-4272 or email [email protected]
After all potential costs have been determined separate the costs into two categories:
- Requested from the industry partner
- Institutionally supported
For the expenses being requested from the industry partner, separate budget items by type of cost
- IRB, Advertising, Screen Fails, Drug Dispensing Set-Up, Cancer Center Clinical Trials Office administrative fee, Investigators’ meetings during the trial
- Variable costs associated with ongoing effort or supplies needed to complete the project
- Office Visits, Tests, Blood Draws, Screen Fails, Subject Fees
- Publications, Data Storage, IDS Pharmacy Maintenance and Closeout
Negotiating the Budget
List all costs as a Total Cost (a combination of direct and indirect costs)
- Industry sponsors are likely to be looking for a bottom line to fund, so provide only a total cost for the project or by line item.
- $500 for travel to a meeting would show up as $775 with an indirect cost rate of 55% ($500 direct + $275 indirect = $775)
- In Post-Award, how expenses are applied in the General Ledger will assign the costs appropriately
Consider negotiating flat up-front or close-out costs as separate reimbursements, rather than rolled into a per patient cost.
- A contract that garners $1800 for an IRB Fee and $1562 for advertising regardless of patient enrollment is much more viable if it enrolls no patients in competitive enrollment than one that has those costs built into the per patient amount.
*Decision Point: Revisit the protocol and budget again:
- Is it still worth doing the project based on the funds you can recover from the sponsor v. costs of doing project? Is the patient population still viable and attainable at this point?
- If it is not, please cancel any related activity in the eResearch systems.
Routing the Industry Project on a PAF
Once you have a draft agreement, expectation of doing the project, and a reasonable budget agreement with the sponsor (even if it is not in the final contract terms, as long as you have email confirmation of the representation) route the PAF through eRPM with the following attachments:
- Statement of Work (can be the opening sections of the protocol)
- Draft of agreement (unless investigator-initiated)
- If it is cost-reimbursable and a budget with built in overhead was used, there should also be an internal budget attached showing the split of DC and IDC.
- If a per-patient reimbursement, include a copy of the payment terms from the draft agreement
Routing Hints:
- If a payment schedule is included in the contract, the PAF should equal the contract “worth”
- Estimate a probable maximum number of patients to budget against – any increase in dollars over the original PAF means routing an incremental PAF for the project down the road
- Include on the PAF any budget comments that would be helpful in determining the contract worth (i.e. number of patients anticipated, basic breakdown of costs, etc.)
The Clinical Trials Support Office (CTSO) is the management team for the seven trans-departmental Clinical Trials Support Units (CTSUs) . The CTSO offers enterprise-wide standards, policies, and a common infrastructure. All clinical trials at U-M are required to be supported by a CTSU.
Budgeting Fixed Price Agreements
As part of the Contracting Demonstration Project to streamline the contracting of industry-sponsored clinical trials, the Medical School Office of Research has engaged ORSP and Sponsored Programs in conversations about the inclusion of internal budgets as part of the official University file – specifically for sponsored fixed price or capitated agreements.
We have agreed it is the fiscal responsibility of the department to ensure that the use of capitated/fixed payments are accurately budgeted and expended to the benefit of the project. In order to recognize where that responsibility lies and to reduce the administrative burden of responding to other campus units, ORSP, Sponsored Programs, and the Medical School Office of Research will not require review of an internal budget.
Effective immediately, these agreements will be reviewed based on the draft agreement and protocol attached to the Proposal Approval Form (PAF) in the eResearch Proposal Management (eRPM) system. However, departments are strongly advised to create a culture where a protocol budget is formulated by the project team and then evaluated for feasibility at the chair level before authorizing the protocol and draft agreement to go forward in the eRPM system.
Further information provided through the departments and administrative structure advising how dollars should be described on the eRPM PAF, as well as best practices in reviewing the financial obligations, are included below.
Specific Instructions and Principles Regarding Fixed Price/Capitated Payments
Departments are advised to evaluate a full cost budget of the protocol prior to PAF approval. Reviewing for:
- Accuracy of costs based on the protocol requirements and institutional cost structure
- Reasonableness of effort budgeted for the time period
- Margin of needed finances for institutional costs v. the amount the sponsor is willing to pay under the contract
Complete the PAF using the following bullet points as a guide:
- Attach to the PAF only the draft version of the contract and the protocol. No internal expense budget should be attached.
- The correct IDC rate should be listed on the PAF.
- Brief notes may be added to the PAF budget notes section (or an attachment may be uploaded under the internal documents section) that explains the estimated contract recovery – based on number of enrollees, clinical services provided, or start-up costs included. This will be similar to what has been historically referred to as the “Revenue” representation on the internal budget.
- The DC / IDC split of the revenue amount may be added to the PAF project dollars as well as Initial Budget period. For help with the calculation, feel free to contact the Grants Office. These figures are captured for school data purposes. IF the unit chooses not to fill out for fixed price agreements, we will complete on the unit’s behalf at the Medical School.
- If the department chooses, you may also capture effort related to the Key Personnel for data use on the PAF in the personnel section, but these numbers will not be validated by any unit in the review process past the Department level.
As we continue reviewing the clinical trials process and introducing measures to streamline the contracting process, further instructions on completion of the use of the system, PAF, and routing may be issued.
What is an IRBMED Fee?
There are three types of IRBMED fees:
- A full IRBMED Fee is assessed on industry projects that are industry sponsored and the MEDIRB serves as the local site IRB.
- A ceded IRBMED fee is assessed on industry projects where U-M will cede oversight to an external IRB (typically commercial).
- sIRB IRB fees are assessed when MEDIRB will serve as the single IRB of record for the project.
The fees help underwrite a portion of the office and the administrative support needed to handle the IRB review and approval process. Not all sponsors allow this type of a fee (for instance, it is prohibited on federal agreements).
When Is an IRBMED Fee Charged?
A full IRBMED fee will be charged if the following conditions are met:
- new project/contract
- is using our IRBMED for IRB review of a project
- is sponsored by industry/for-profit
- and is awarded.
A ceded IRBMED fee is charged if the following conditions are met:
- is using an external IRB for review
Single IRB (sIRB) fees are charges when
- IRBMED is the IRB of Record for the study.
- More information about the fee structure for qualifying studies and budgeting for these fees in grant applications and contracts is located at IRBMED sIRB Review Fees in Section II: IRBMED Accepts Oversight (sIRB).
It is key to note that currently the industry full and ceded IRBMED fees are charged on each new sponsor contract and not multiple charges throughout the life of the contract/protocol. Amendments are not subject to an IRBMED recovery; however, stand-alone contracts, even if affiliated with an existing IRB approval, are assessed the fee.
For more information about the fees and local budgeting, please refer to our internally facing IRB website . Language to share with sponsors during the negotiation process is available in Section V: Fees and Budgeting for Industry-Sponsored Research on our externally facing IRB website .
The IRB Fee is assessed after the award notice is issued in the eRPM system and will be charged in two components (SUB and additional funds). If you have questions about a fee charged to your P/G or the applicability when formulating a budget, please contact Grant Services & Analysis at [email protected] .
Whether you’re working on an industry sponsored study and/or a clinical trial the following tips, listed by class code, will help you get the PAF through the routing process.
On-Campus Organized Research (22000):
- If a payment schedule is included in the contract, the PAF and internal budget should equal the contract “worth”
- If no effort is in the budget for the investigators, indicate in the budget notes whether the time needed will be provided from departmental (or other) funds.
- Estimate a probable maximum number of subjects, procedures, and invoiceable items to budget against – keeping in mind that any increase in dollars over the original PAF means routing an incremental PAF for the project later on
- Remember to include the IRBMED Fee in the budget for all industry sponsored studies (see above)
Clinical Trial Site Activity (31200):
- An internal budget is not required for fixed price reimbursements
- Estimate a probable maximum number of subjects, procedures, and invoiceable items to estimate the amounts for the PAF – keeping in mind that any increase in dollars over the original PAF means routing an incremental PAF for the project later on
- Remember a Clinical Trial Routing Form (CTRF) is required
After an institutional review of patient care related charges on federal grants that fall under our negotiated Research Patient Care Agreement * and related agency policy statements, we have affirmed that these expenses are exempt from indirect cost recovery.
If you are working on a federal project (or any sponsored project recovering the full federal rate) and have expenses that fall under the Research Patient Care Agreement, these costs should be reviewed back to 7/1/2011 to make sure that they are not recovering IDC in post-award. Additionally, as you prepare proposals for external sponsors, please be sure to exclude costs under the agreement from the Modified Total Direct Cost (MTDC) base. Proposals have been submitted to federal agencies with these costs excluded from IDC recovery since FY12.
If you have any questions about the review of expenses on an active project, please contact your Sponsored Programs Coordinator. If you are preparing a proposal or have questions about the impact to awarded budgets, you may contact Heather Offhaus of the Medical School Grant Review & Analysis Office ( [email protected] ).
*The rates in the Research Patient Care Agreement have been loaded into the clinical pricing tool and MBECT systems.
NOTE: On non-federal projects that are recovering less than our negotiated research rate (currently 55.5%), the MTDC and exclusions do not apply. For instance, non-federal projects termed clinical trials would have no Research Patient Care exclusions, but are already able to use the substantially discounted recovery rates.
This content was last reviewed 6/2013.
Both Principal Investigators and core recharge units themselves, sometimes have difficulty explaining the roles of recharge units in the budgets and justifications of grant proposals. There is a grantsmanship struggle representing specific personnel with effort on the proposal budget and justification in the ”Personnel” section, versus including the recharge activity as an ”Other” expense. Investigators often feel that proposing use of a recharge unit may not carry as much weight under peer review as a named individual might.
To further complicate the issue, there may be times when faculty (or staff) - particularly Core or Unit Directors, serve multiple roles on the same project. A portion of the Director’s effort could be expended on the recharge activity and accurately reflected as a component of the recharge rate, and s/he is also asked to serve in a capacity that requires unique scientific contributions on the same proposal.
On the other hand, the University is required by federal law to treat costs consistently, therefore all recharge service units must have their rates approved prior to charging customers for the services they provide. And because they are approved recharge rates, they must be demonstrated on budgets as standard service fees and not personnel or other separate costs.
The scenarios below walk through the steps of preparing a budget with core activities included. Further, we have prepared a comprehensive document that contains additional information on these struggles as well as full examples that can be used when preparing grant proposals.
Scenario 1: Recharge activities only
Project team should:
- Budget the anticipated core expense as a single charge under “Other Expenses”
- Justify the anticipated core expense as the overall core service, not the individual activities that make up the charge’s components, under “Other Expenses”
If grantsmanship calls for a detailed description of the service providers, you may use one or all of these optional justification references:
- Include A-21 language to explain treatment of the budget
- In the “Other Expenses” section describe specific individuals that MAY work on the project as a part of the recharge unit
- Describe Core Director or staff in “Personnel” section of justification with no mention of effort or amount, noting that the expense is included as a part of the line item for Core Charges under “Other Expenses” AND is budgeted under “Other Expenses”
Any of the last three options must include very specific language. You may contact the Grant Review & Analysis Office ( [email protected] ) for assistance in crafting this type of justification.
Scenario 2: Effort includes recharge along with other project specific activities of individuals
- Budget/Justify the anticipated core recharge expense under “Other Expenses”
- Budget/Justify the additional activity under “Personnel” and “Materials/Supplies,” depending on items.
If the PI doesn’t think that is detailed enough description of the services, they may use an optional justification reference (see optional justification references in Scenario 1) .
The purpose of this page is to give a working definition of budget terms that turn up in pre- and post-award administration. These are not the "legal" definition. If there are terms you would like to suggest adding or if a link to the "legal" definition would be helpful, please send an email to Heather Offhaus .
Sponsored Research v. Gifts
Although alphabetically this isn't first, this is one of the first distinctions to be made. Sponsored Research is auditable. In exchange for the money sent to you to do research, the sponsor expects it to be used for a particular project with certain restrictions on spending, a financial report due, services in return, or some auditable promises were made from the university. A gift is a sum of money with no strings attached. They aren't specific on what research you have to use it, they aren't interested in more than a blurb for their financial offerings, and there is no commitment of how funds might be spent. Sponsored research can be broken down into several categories such as grants, contracts, clinical trials, material transfers, other sponsored activity, etc.
This is a peculiar term for certain budget items. The Office of Management and Budget (Federal Government) publishes rules and conditions for use of federal moneys. One of their circulars was numbered A-21 and it directly impacts institutions of higher education that conduct research. In it, they spell out that certain costs cannot be charged to a grant unless they are specific to the project. Most of the components are costs that would normally be covered under the Administrative portion of the F&A rate (see below.) For instance, if you want to charge a sum of money to a grant for copying charges, it has to be justified in the application as project specific (i.e. why do you need it?) for it to be allowable (Copy charges are requested to cover disseminating data results to the 3 subcontract sites). If it is not justified and approved, the charges cannot hit a federal account. This applies mainly to federal proposals. See Cost Accounting Standards for the implications on non-federal sponsors.
Conflict of Interest (COI)
Many institutions now ask you to declare long before award if you will or will not have a conflict of interest. At UM it is the following statement (Yes/No): Do the Project Investigator, Participating Investigators, or other key investigators has significant financial or management interest in the proposed project that may constitute the basis for a conflict of interest?
Cost Accounting Standards
Although it has many issues involved, the basic ideas are that all sponsors should 1) be treated equally and 2) be able to audit for university support "promised" in a proposal. The first of the two basically means that the government wants us to be consistent in how we account for items. If someone non-federal gets a "break" for something, in the same way the government should get that break. Whether it be an indirect cost rate, how we charge out salaries, or allowable costs on grants. Conversely, if the government guidelines limit us, we should also be limiting what we charge other sponsors. So, A-21 items, although a government requirement, should be a non-federal guideline. This isn't to say that you cannot charge certain A-21 costs to a proposal, but should try to be consistent on the allowable charges. As far as the 2nd principle, see Cost Sharing. The term most synonymous with Cost Accounting Standards might be consistency.
Cost Sharing
This easily could be also termed Resource Sharing. Any time we commit effort, supplies, space or other item to a sponsor as indication of support for the project within the university, we have to account for how that commitment will be matched. If you represent that a faculty member will be on a grant with 10% effort, it is assumed that the agency awarded the project taking that into consideration. At any time they can come and ask us if the resource (in this case salary) is being provided and audit us for this cost. At UM it is extremely important to account for where that support will come from before submission of a sponsored research project. There are two types of cost sharing. The definitions vary from institution to institution, but UM has a fairly mainstream understanding.
Mandatory Cost Sharing
Any time the resource is quantifiable, it is considered mandatory (and auditable.) This would be an effort level (which is translatable into dollars), a specific amount for a piece of equipment or supply, or anything else you can put in terms of dollars.
Voluntary Cost Sharing
This consists of things that are non-quantifiable. This may be a statement in the justification that says: "The PI will provide other necessary supplies to this project." or "The tuition will be paid from departmental funds." Another instance is when the sponsor imposes a limit. The NIH salary cap is congressionally set. Any time a person's academic base exceeds that amount, the department has to voluntarily cost share the amount over the imposed cap. (Current cap is available here .)
Direct Costs
These are the dollars directly associated with research investigations. It could be professional salary, travel, consulting fees, or equipment (among others). The cost must have a direct benefit to the project.
Indirect Costs (or Overhead or Facilities and Administrative Costs (F&A))
These are what in the business world would be considered overhead. Facilities charges include lights, water, electricity, network hook-ups for the computer, telephone lines. A good rule of thumb is anything behind the wall. Administrative costs are secretarial support, general office supplies, and the cost of people that administer sponsored projects. Basically anything "common" that does not directly benefit a specific project.
These come under a variety of names. They are costs that have to be borne in certain circumstances under a university's accounting/charging system. They all have separate rules and will vary by institution. We bring them up so that you know the types of fees to look for and examples of what they might do. See the Institutional Review Board Fee (IRBMED Fee) webpage for more information.
Patient Care
Many times, institutions that have clinical care as part of research charge less for certain procedures on a research proposal. In these cases, you need to find out if the prices include the F&A/Indirect charges already. If they do, you can't charge a sponsor for the F&A associated since it is built in and charging would constitute "double-dipping."
Research Participation
There are several different titles of those who participate on a project. In many cases they are different shades of gray.
Project Director - The person who meets the eligibility requirements of the university and is responsible for the academic and budgetary performance.
Principal Investigator - The person who meets the eligibility requirements of the sponsor and is responsible for the academic and budgetary performance (usually the same person).
Co-Investigator - The NIH defines this as people who contribute significantly to the scientific progress of the proposal.
Collaborators and Consultants - These terms are fairly interchangeable? The only distinction that has been explained is that Collaborators tend to be those who work in the field of the research and are willing to offer advice and consultation on the direction of the project. Consultants then would be outside the PI's realm of expertise that are willing to advise from a different professional perspective. Then again, the definitions have also been flipped.
Subcontracts
This is an agreement between another site (corporation, university, hospital) and the parent institution submitting the proposal to perform a component of the research. It is a subcontract if the site’s work involves specific people who impact the course of research. They would be subject to their own internal cost structures. Sometimes the term is confused with contracted services. If you have questions on the differences, please contact your department research administrator.
We transform lives through bold discovery, compassionate care and innovative education.
- Diversity, Equity & Inclusion
- News & Stories
- Find a Doctor
- Conditions & Treatments
- Patient & Visitor Guide
- Patient Portal
- Clinical Trials
- Research Labs
- Cores and Resources
- Programs & Admissions
- Our Community
- Departments, Centers & Offices
- About the Medical School
Global Footer Secondary Navigation
- Skip to navigation
- Skip to content
- UMB Shuttle
University of Maryland, Baltimore
About UMB History, highlights, administration, news, fast facts
- Accountability and Compliance
- Administration and Finance
- Center for Information Technology Services
- Communications and Public Affairs
- Community Engagement
- Equity, Diversity, and Inclusion
- External Relations
- Government Affairs
- Philanthropy
- Office of the President
- Office of the Provost
Research and Development
- University Counsel
- Administrative Officers
- Boards of Visitors
- Faculty Senate
- Staff Senate
- Center for Health and Homeland Security
- Council for the Arts & Culture
- Interprofessional Education
- Leaders in Education: Academy of Presidential Scholars
- Middle States Self-Study
- President's Council for Women
- President's Symposium and White Paper Project
- For the Media
- Steering Committee Roster
- Logistics Committee Roster
- UMB Police and Public Safety
- Graduation Celebration 2024
- Founders Week
- UMB Holiday Craft Fair
Academics Schools, policies, registration, educational technology
- School of Dentistry
- Graduate School
- School of Medicine
- School of Nursing
- School of Pharmacy
- School of Social Work
- Carey School of Law
- Health Sciences and Human Services Library
- Thurgood Marshall Law Library
Admissions Admissions at UMB are managed by individual schools.
- Carey School of Law Admissions
- Graduate School Admissions
- School of Dentistry Admissions
- School of Medicine Admissions
- School of Nursing Admissions
- School of Pharmacy Admissions
- School of Social Work Admissions
- Tuition and Fees
- Student Insurance
- Academic Calendar
- Financial Assistance for Prospective Students
- Financial Assistance for Current Students
- Financial Assistance for Graduating Students
Research Offices, contracts, investigators, UMB research profile
- Organized Research Centers and Institutes
- UMB Institute for Clinical & Translational Research
- Sponsored Programs Administration
- Sponsored Projects Accounting and Compliance (SPAC)
- Kuali Research
- Clinical Trials and Corporate Contracts
- CICERO Log-in
- Conflict of Interest
- Human Research Protections
- Environmental Health and Safety
- Export Compliance
- Effort Reporting
- Research Policies and Procedures
- Center for Innovative Biomedical Resources
- Baltimore Life Science Discovery Accelerator (UM-BILD)
- Find Funding
- File an Invention Disclosure
- Global Learning for Health Equity Network
- Manage Your Grant
- Research Computing
- UM Research HARBOR
- Center for Violence Prevention
- Office of Research and Development
- Center for Clinical Trials and Corporate Contracts
- Technology Transfer/UM Ventures
- Contact Research and Development
Services For students, faculty, and staff, international and on-campus
- Student Health Resources
- Educational Support and Disability Services
- Writing Center
- URecFit and Wellness
- Intercultural Leadership and Engagement
- Educational Technology
- Student Counseling Center
- UMB Scholars for Recovery
- UMB Student Affairs
- Human Resource Services
- Travel Services
- Strategic Sourcing and Acquisition Services
- Office of the Controller
- Office of the Ombuds
- Employee Assistance Program (EAP)
- Workplace Mediation Service
- Faculty Center for Teaching and Learning
- UMB Travel: Start Here
- International Students, Scholars, and Employees
- Center for Global Engagement
- International Travel SOS
- International Operations
- Parking and Transportation Services
- UMB shuttle
- SMC Campus Center Event Services
- Donaldson Brown Riverfront Event Center
- All-Gender Bathrooms
- Environmental Services
- Interprofessional Program for Academic Community Engagement
University Life Alerts, housing, dining, calendar, libraries, and recreation
- Emergency Reference Guide
- Campus Life Weekly with USGA
- Starting a New Universitywide Organization
- University Student Government Association
- Planned Closures
- Intramural Sports
- Safety Education
- About URecFit and Wellness
- How to Get Your One Card
- One Card Uses
- Lost One Card
- One Card Policies
- Photo Services
- One Card Forms
- One Card FAQs
- Office Hours and Directions
Give to UMB Sustain excellence and meet UMB's educational needs for today and tomorrow.

Thank You for Your Gift to UMB
The University of Maryland, Baltimore (UMB) is excited to share its new online giving page.
With enhanced searchability, a streamlined checkout process, and new ways to give such as Venmo, PayPal, Apple Pay, and Google Pay in addition to credit card, donors can support UMB quickly and securely.
- Ways to Give
- Where to Give
- Staying Connected: You and UMB
- The UMB Foundation
- Office of Philanthropy
- Maryland Charity Campaign

- Budgets and Expenses
Clinical Trial Budgets
620 W. Lexington St. Fourth Floor Baltimore, MD 21201
P 410-706-6723
Whether you are developing your own budget or the sponsor presents you with one, your clinical study budget should cover all study-related costs, including fees and indirect costs.
Most of your costs will be calculated per patient and/or per procedure, but some may be one-time costs or fees. Examples of costs that may not be included in the per patient cost include:
- Advertisement costs.
- Screen failures.
- Prorated payment for subjects who are terminated, drop out, or are lost to follow-up.
- Startup costs: Upfront, nonrefundable payment to defray the cost of startup work such as preparing regulatory documents, attending investigator meetings, site initiation training, enrollment efforts, etc.
- Payment for close-down costs in the case of early termination by the sponsor.
For clinical trials proposed under a federal grant or contract or similar mechanism, be sure that personnel costs are appropriately allocated to the personnel budget or to the per-patient study costs.
The protocol may state that certain items or services are "free” to all research subjects. The budget and/or agreement should include these services. Even if these services normally would be considered standard of care, if the sponsor has agreed to pay for them, they may not be billed to the patient or the patient's insurer .
Procedures for Research Study Participant Payments
If a budget offered by a sponsor is inadequate to cover costs, you will need sufficient evidence to support your request for additional funds. The more ammunition you have in support of reasonable and customary costs in your area, the better your chance of obtaining increased funding.
Budget review
Numerous rules and regulations govern the conditions under which a clinical service, item, or test may be billed to the patient, to Medicare, or to a third-party insurer. It is essential to understand whether a trial involving a test drug or device is a “ Qualifying Clinical Trial ” as defined by the regulations of the Centers for Medicare and Medicaid Services (CMS). Further, the study budget must be analyzed to ensure that study costs are correctly distinguished from costs related to the standard care for a patient.
The University of Maryland Medical System (UMMS) Research Billing Compliance Office will work with the principal investigator and study staff to determine whether the trial is a Qualifying Clinical Trial and to help develop the financial aspects of the budget. Ultimately, the clinical study funding agreement or award terms must harmonize with the study budget, the study protocol, the research subject informed consent document, and any other study-related documents. The investigator and department remain responsible for development of the grant or contract proposal and budget. Contact the UMMS Department of Finance at 410-328-5991 for assistance or more information.
The University of Maryland, Baltimore is the founding campus of the University System of Maryland. 620 W. Lexington St., Baltimore, MD 21201 | 410-706-3100 © 2023-2024 University of Maryland, Baltimore. All rights reserved.
Customize Your Path
Filters Applied
Customize Your Experience.
Utilize the "Customize Your Path" feature to refine the information displayed in myRESEARCHpath based on your role, project inclusions, sponsor or funding, and management center.
Commercial or industry sponsored clinical trial budgets
Need assistance with industry sponsored budgets?
Get help with industry sponsored budgets.
Contact the DOCR OneDuke Budget Leads:
- Email: [email protected]
The task of developing budgets that support industry-sponsored research is an activity involving the Duke Office of Clinical Research (DOCR), Office of Research Contracts (ORC), School of Medicine Finance office, and the Clinical Research Unit (CRU). The CRU should be contacted first w hen industry-sponsored research projects (clinical trial or clinical research) become available.
CRU submits protocol shell in iRIS; syncs with Oncore
CRU attaches final protocol and draft budget/contract in OnCore; requests study initiation meeting
DOCR determines need for Protocol Calendar; attaches Task List
DOCR creates Protocol Calendar, links to OnCore Charge Master
DOCR/Budgeter completes appropriate tabs; creates preliminary budget workbook
CRU reviews budget and preps for study initiation meeting
Study initiation meeting is conducted; Protocol Calendar updated as needed
DOCR sends Protocol Calendar to team for review
CRU approves Protocol Calendar
CRU negotiates budget and payment terms (study initiation meeting must happen prior to this step)
* Access the Understand pre-award activities and spending page for guidance on obtaining pre-award spending approval, if needed.
CRU provides budget approval to ORC; copies DOCR and SOMF
DOCR/Budgeter enters negotiated amounts; attaches executed contract in OnCore
FPM reviews PC and Financial Consoles before CRU sign-off
DOCR completes sign-offs; releases Protocol Calendar/Budget
- CRU attaches the preliminary contract in OnCore.
- CRU sends the preliminary contract to ORC so that they may begin negotiations of the legal language.
- CRU reviews payment terms and contacts CRO/Sponsor to negotiate before study initiation meeting, if needed.
- After budget and contract are finalized, CRU obtains required signatures.
- Attach fully executed contract in OnCore.
- CRU attaches the preliminary budget in OnCore.
- CRU negotiates budget with CRO/Sponsor.
- DOCR Budgeter creates budget and enters FPM-approved negotiated amounts in OnCore
- CRU enters and routes project in SPS, if have not already completed
- CRU Finance Manager completes the CRU Finance Sign-off in Oncore.
*NOTES for pricing: Research and Retail rates are in the OnCore Charge Master. For the CPT codes not in the Charge Master, submit a completed PRMO Grant Pricing Request, via a Service Now ticket, with a comment (Procedure/event is not in the OnCore Charge Master). For supplies not in fee schedule, send SAP number (material number), vendor and description to Revenue Manager. For “bundled” procedures, request a bill of known patients who underwent the same or similar procedure from Revenue Manager. For supplies not in fee schedule, send SAP number (material number), vendor and description to Revenue Manager
- Conference Coverage
- CRO/Sponsor
- 2023 Salary Survey

- Publications
- Conferences
Key Cost Drivers in Clinical Research: Guide to Successful Budgeting
It's almost impossible to map out study budgets with absolute precision. Planning for the unexpected costs, such as those associated with slow enrollment, protocol amendments, and other contingencies—is vital.
Proper study budget is vital for a successful trial for both parties, contract research organizations (CRO) and pharma companies. Therefore, it is essential to take the time and prepare your financial plan by thoroughly thinking of all expenses.
So, what does a typical clinical trial budget consist of? First of all, the study budget can be broken into two main big parts: the cost of CRO services, and pass-through costs (PTC).
PTC, for their part, are all the expenses that are passed directly to the Sponsor at actual cost:
- Site and PI grant fees —grant fees paid to principal investigators and sites for the recruitment of patients; these costs often comprise the most significant part of the study budget;
- Vendors’ costs —Central Labs, IDMC, depots, ePRO, and any other vendors, if they are subcontracted by the CRO;
- Comparator and concomitant medication —depending on the study design, purchase, and logistics of these might add up to quite a significant sum that should be planned for in advance.
- Study supplies, equipment —complexity of study procedures may require specific equipment not usually used at site in routine practice, so there might be a need to purchase it for the study. Sometimes it can be expensive to procure, deliver, and then at the end of the study, collect it from the site.
- Travel costs, investigator meeting costs —keep in mind that travel costs may also form a substantial part of the budget, depending on the study design and needs (for example, frequency of on-site monitoring visits and site geography). Another important factor to be taken into consideration, especially when planning a multinational clinical study, are currency fluctuations that may influence the cost of travel, which is very hard to predict or plan for.
- Logistics and courier costs, depot services —these costs do not only depend on the study geography, but on the number of subjects (and, as a consequence—sites) as well. The larger the sample size is, the more medication and biosamples shipments, thus the more expensive the study is. And again, do not forget about currency fluctuations. So, it is imperative that logistics managers help plan the budget as precisely as possible.
- Regulatory authorities’ fees and patients’ insurance —make sure to include all local RA (as well as LECs—where applicable) fees and patients’ insurance that directly depends on the sample size and on the study phase and local country requirements.
- Questionnaire license fees —don’t forget about the licensing fees that can be both paid and free of charge. In certain cases, they can be accompanied by considerable costs.
- Taxes and bank commissions —local specific requirements for taxes and bank commissions vary among countries. So, your partner CRO should be responsible for double-checking that.
Taking a deeper dive into the structure of the CRO service costs we will see the following:
- Site management and monitoring —one of the highest in the overall structure of service fees, and it may account for the largest part of the clinical study budget. Amount of monitoring (including site initiation visits, interim monitoring visits, and closeout visits) will depend on both the sample size and Sponsor’s requirements towards SDV (Source Data Verification) percentage.
- EDC, data management and biostatistics —in general, data management costs strongly depend on the number of patients and sites. Another powerful cost driver for the cost of these services, is the complexity of CRF (which, in turn, depends on the amount of study procedures) as well as the duration of the study.
- Project management —the cost of project management (PM) depends on study duration and complexity (including number of sites, countries and specific vendors involved). And even though there is no direct connection between the number of subjects and cost of PM, if the increased number of subjects requires involvement of additional sites or even countries—this of course will add to the project management cost.
- RA support/CTA submission —geography definitely has an impact on the cost of regulatory support services, as the duration of clinical trial approval and the CTA process itself varies (sometimes significantly) from country to country. Different countries and regions may have their own requirements for submission packages and sometimes it can be a challenge to put everything together. For instance, in Russia, the package is relatively easy to collect as it is similar to what EMA and FDA require, but all documents should be translated into Russian language, which takes time and some additional costs. In Ukraine, the package is also simple, as they EU CTA for initial submission and EU requirements (for example, submission of complete IMPD for IMP), but the Ministry of Health requires a lot of documents from investigators, not only principal investigators. Accreditation and certification of each medical institution involved in the trial have to be collected and reviewed for validity. Of course, collection of such packages takes more time and costs are higher with respect to the services. Meanwhile, there is no need in full translation of study core documents into Ukrainian.
- Medical writing —even though the cost of services connected with medical writing, and CTA package preparation are normally lower than other project costs, it is hard to overestimate the importance of biostatisticians’ and medical writers’ work - both experts’ decisions regarding study approval and the efficiency of the clinical trial depend directly on properly and expertly prepared study design.
- Logistics —when we say “logistics” here, we are talking about CRO support in coordinating the import and shipments of investigational drugs, study supplies, concomitant or comparator medications, biosamples (while the cost of those shipments are pass-through costs, described above).
- Pharmacovigilance and quality assurance —quality assurance costs spent on maintenance of QA and QC systems, training and audit expenses, as well as PVG, even though highly important, usually comprise a smaller part of the overall study budget.
What if out-of-scope happens?
However, contingencies are hard to avoid in real life, therefore it is necessary to be ready to face the out-of-scope. So, how to find ways to effectively manage it? Indeed, with large-scale international studies, especially in the long term, it is almost impossible to map out study budgets with 100% precision. The majority of companies have all the responsibilities of project managers or those of other project-related specialists listed in their SOPs and project management plans. What is more, take a tip from insiders—if a project manager not only presents an issue of out-of-scope to Sponsor, but also comes up with a possible solution within the current study budget, it will be clear evidence of his professional competence and individual approach to clients. There might be some internal monetary resources if you look into the budget carefully. For example, depending on how the enrollment goes at each site, you can adjust monitoring frequency and extend the break between visits but make them longer. This is an opportunity to cut back on travel costs which, in case of big countries like Russia, might be quite significant. Besides, the CRO service cost for a two-day monitoring visit will be lower than two one-day visits, as the CRA will not have to spend additional time travelling to site. One more thing which can help avoid out-of-scope situations is an accurate and detailed budget grid. For example, the budget template of OCT Clinical CRO consists of over 400 items and covers all the tasks that will be done for a particular project, which provides maximum transparency and reduces the risks of hidden costs. Planning for the unexpected, such as costs associated with slow enrollment, protocol amendments, additional IMP imports and other contingencies—is, indeed, of paramount importance.
Irina Petrova , MD, is the Director of Clinical Operations, OCT Clinical

Tips to Rescue a Clinical Trial Before It’s Too Late
Why constant communication and transparency are paramount to successful partnerships between pharmaceutical companies and CROs

Focus on Fundamentals for Better Collaboration Across Research Sites and Sponsors
Improving the site-sponsor relationship can get trials off on the right foot and on a path for success.

When FMVs Collide: Coming to Terms with Fair Market Value
With variation in costs between different stakeholders in clinical trials, there is often disagreement on fair market value.

Top 3 Most Read CRO/Sponsor Feature Articles of 2023
Authors highlighted outsourcing and the impact of the COVID-19 pandemic in these CRO/sponsor articles from 2023.

Anticipating Near-Term Structural Change in the Outsourcing Landscape
Can full-service outsourcing to CROs by large biopharma companies sustainably prosecute a clinical development portfolio?

Harmonizing Outsourcing to Keep Clinical Trials on Track
A shared partnership culture is critical in navigating today’s complex terrain.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Ayurveda Integr Med
- v.10(2); Apr-Jun 2019
How to plan and write a budget for research grant proposal?
Medical research can have an enormous positive impact on human health. Health research improves the quality of human lives and society which plays a vital role in social and economic development of the nation. Financial support is crucial for research. However, winning a research grant is a difficult task. A successful grant-winning application requires two key elements: one is an innovative research problem with best probable idea/plan for tackling it and appropriate planning of budget. The aim of the present paper is to give an insight on funding agencies providing funding for health research including traditional Indian medicine (from an Indian perspective) and key points for planning and writing budget section of a grant application.
1. Introduction
Why health science research is important and why should it to be funded? Science and technology innovations and health research can have an enormous impact on human health. They improve public health, quality of human lives, longevity and have made society better [1] , [2] . Healthy humans with better quality of life are crucial for the social and economical development of the nation [3] . Medical research led to the expansion of knowledge about health problems/conditions and their mechanism, risk factors, outcomes of treatments or interventions, preventive measures and proper management. Clinical studies or trials provide important information about the safety and efficacy of a drug/intervention. Innovative basic science research had led to the discovery of new technology, efficient diagnostic and therapeutic devices. So, currently, an effort with multidisciplinary approach is a demand for better understanding of clinical conditions and providing safest health care to the community [2] , [4] .
Whether it is basic or applied, clinical or non-clinical, all research needs financial support. Considering the importance of research in economic growth of a nation, many countries are increasing their budget for research and development in science. A study on impact of GDP (Gross Domestic Product) on research and development in science among Asian countries has found that one who spends more on research has more research outcomes in the form of total number of research documents, citations per documents and h-index [5] . About 95% of the NIH (National Institutes of Health, USA), budget goes directly to research awards, programs, and centers; training programs; and research and development contracts [6] . Total expenditure carried out for research in India is too less than USA and China. Percentage of GDP for research and development in India is 0.88%, while South Korea, USA and China have 4.292%, 2.742 and 2.1% respectively [7] .
Owing to the increasing competition among the researchers, especially the young ones, for their academic growth, preparing and planning a winning research proposal becomes very essential. A successful grant-winning application requires two key elements: (1) innovative research problem with best probable idea/plan for tackling it and (2) appropriate planning of budget. The aim of the present paper is to give an insight on funding agencies (from an Indian perspective) and key points for planning and writing budget section of a grant application.
2. What is the purpose of the budget plan in a grant application?
A budget is the quantitative expression of a financial plan for future expenses on the project in a given period of time [8] . Budget plan is a key element of a grant application. It demonstrates the required cost for the proposed project. It is a prediction of expenses and serves a plan for funders on how the organization will operate the project, spend the money in a given set of period and where their money will go. It shows the funders exactly what they can support and also helps the institution and investigating team in management of the project. Moreover, budget plan requires for accountability [9] .
3. Which are the funding agencies that sponsor health research in India?
Various national and international sponsoring agencies have identified health problems of priority for funding a research. Some of the leading funding agencies providing grant for health research including alternative systems of medicine in India are given in Table 1 . State Universities/deemed Universities also have a provision of funding for medical research.
Table 1
List of funding agencies those promote health research.
4. What constitutes a research project budget?
Proforma of the research grant applications and presentation of budget section may vary among the sponsoring agencies. However, major parts of budget plan in the applications of the above mentioned funding agencies are quite similar. The budget section is broadly divided into two categories: direct and indirect costs.
4.1. Direct costs:
These are the costs incurred specifically to carry out a project [10] . Direct costs include expenses towards personnel, materials, equipments, consumables and travel. These particulars are further categorized into recurring and non-recurring expenses on the basis of their occurrence during the study period. A brief description of the sub-sections under direct cost is given below:
4.1.1. Personnel:
Budget for personnel can be mentioned in this section in case human resources are required for the study and as per funding agency guidelines. Salaries with allowances can be budgeted for human resources such as site manager, research assistant, junior research fellow (JRF), senior research fellow (SRF), research associate, technician, data entry operator and attender. Most of the Indian funding agencies do not have a provision for salaries for the principal investigator (PI) and co-investigators (Co-PI). Ministry of AYUSH [11] and Rajiv Gandhi University of Health Science (RGUHS), Karnataka [12] provide one-time minimal fees for investigators and supporting staff respectively. There is a provision for salaries of investigators in Wellcome trust-DBT India alliance grants [13] .
4.1.2. Recurring expenses:
Recurring expenses are those which are variable and which keep on occurring throughout the entire project duration. Particulars categorized in this category are consumables, chemicals, glasswares, laboratory test charges, diagnostic kits, stationery, prints, photocopies, communication, postage, telephone charges, survey tools, questionnaires, publication charges, reprints, binding etc. Other expenses could be allowances for patients/participants, food charges and physician fees.
4.1.3. Non-recurring expenses:
Non-recurring expenses are those which are one-time in nature or which do not recur at regular intervals. Particulars included in this category are equipments or instruments with its accessories, software's, computer, printer, electrical and electronic items and accessories of the existing instrument in your lab. Percentage of budget allocated for equipment varies among the funding agencies from 25% to 90% of the entire budget. Some of the agencies do not have provision for equipment in budget. Vision Group on Science and Technology allocated their maximum grant (up to 90%) for development of infrastructure of laboratories [14] .
4.1.4. Traveling expenses:
Budget allocated for traveling can be used for attending meetings, conferences, workshops and training programs. Foreign travel is not allowed by any Indian funding agency. Traveling expenses for collection of data, survey and visit to other centers in multicentric study can be budgeted in this sub-section.
4.2. Indirect costs:
These are the costs which cannot be directly attributed to specific expenses of a project, but are required to run a project. It is also termed as overhead charges. Laboratory, electricity, water, library and other facilities are provided by the institution to run a proposed research project. Therefore, a fixed cost (usually) of about 5–15% of the total budget is provisioned as institutional overhead charges which goes to the institution directly. The range may, however, be flexible on the basis of the type of funding agency.
5. Budget justification
Most of the funding agencies require submission of a budget justification with all the items described above. Sometimes it is also called as budget narrative. Explanation of need for each line item in the budget with item-wise and year-wise breakdown has to be provided. Quantification of total costs of each line-item and document cost calculation should be done. When writing a budget justification, it is important to follow the same order as that in an itemized budget. For example, if equipment such as color doppler is required, then justify the need of a device with respect to the proposed methodology of the study. Similarly, for non-recurring expenses, breakdown the consumables item-wise and year-wise with its cost and calculation according to the protocol of the study and justify accordingly.
6. Budget summary
An item-wise and year wise summary of the total budget is usually required in most of the applications. Budget summary outlines the proposed grant and often (most of the format) appears at the beginning of the proposal. It should always be prepared at the end, after the grant proposal has been completely developed. A sample budget summary (as an example) for a proposed study for the duration of three years is shown in Table 2 . In the personnel section, a research fellow salary with allowances is budgeted year-wise. The salary of the research fellow for the first and second year is Rs. 2,30,000 per year (JRF) with an enhancement to Rs 2, 59,000 for the third year (SRF) as per the guidelines of the funding agency. As non-recurring expenses are one time in nature, a budget for equipment was budgeted only for the first year. Under the section of recurring expenses, more budgets are allocated in the second year for consumables because recruitment of subjects in large number will be done during the second year of the proposed study. Similarly, expenses toward travel, investigator fee and other miscellaneous costs year-wise have been budgeted. The emoluments and guidelines on service conditions for research personnel employed in research project by ICMR has been given in reference section [15] , [16] .
Table 2
Sample budget summary (year wise).
7. How to plan a simple research budget?
Planning of the research budget begins with an innovative research question, objectives and design of the study. Before starting to write a budget plan, it is essential to understand the expectations of funding agencies, University/Institute and the team of researchers. It is imperative to keep in mind that the research proposal will be reviewed by both scientific and financial (non-scientific) experts. Hence, the proposal should be prepared in such a way that it can be easily understood by even non-scientific experts.
Firstly, a list of what is essential and would add value for research such as focus of research, primary and secondary outcomes of the study, the source of the sample, study setting, sample design and sample size, techniques used to collect data, method of data analysis and available resources should be made [17] .
Secondly, the instructions, format of the application and rules of the funding agency should be read thoroughly. Budget specifications, limitations of recurring and non-recurring costs, and necessity of budget justification with cost breakdown should be checked. Note that one should not deviate or modify the proforma of the funding agency.
Thirdly, a list of items should be made and categorized into recurring and non-recurring expenses. Breakdown of the budget into item-wise and year-wise with cost calculation should be done. It should be ensured that costs are reasonable, allowable and related to the research proposal, so that the budget appears realistic. Travel expenses should be calculated as per the rules of the funding agency.
Fourthly, item-wise and year-wise justification of the requirement in a same sequence of format should be provided. A well-justified budget can enhance the evaluation of the research proposal by reviewers and funding body.
The last most important part is to review the budget and verify the costs and calculation. It is better, if other research team members can review the budget plan and re-calculate the costs thoroughly. Remember, too high budget and too low budget with respect to the research proposal are suspicious and chances of receiving a grant are less.
Sources of funding
Conflict of interest.
Peer review under responsibility of Transdisciplinary University, Bangalore.
- Sponsored Projects Administration
- Tools & Resources
Forms & Templates
Forms & templates.
SPA offers a variety of its forms, templates, and worksheets, in PDF and Excel format. In order to read, print, search, and fill-in PDF forms, you will need a copy of Adobe Acrobat or Adobe Reader .
Forms must be downloaded in order to save them. To do so, right-click the link, click "Save Target As...", then choose the destination on your computer.
New! How to open pdf forms in Chrome
- eRA Commons Account Registration Obtain eRA Commons account & affiliate with UTHealth
- Individual Research Conflict of Interest Form
- Multiple PI Leadership Plan
- Other Support Example Template - Must be utilized when responding to any JIT request and when submitting revised other support with annual NIH progress reports.
- Transfer In Information Sheet Complete for incoming faculty and send to [email protected]
- Unobligated Balance Calculation
- LOI Template - UTHealth as Subrecipient – To be utilized for subaward in proposals. Must be completed and uploaded to Uploads and Reviews when a Subaward In proposal is routed to the Grants and Award Management Team for review.
- LOI Template - Foreign Subrecipient – To be used when a foreign subaward is included on a NIH grant proposal that will be submitted by UTHealth. This will be uploaded in the Research Plan tab in the Consortium/Contractual Arrangements section once completed.
- Cost Transfer Request Used by departments to transfer expenses onto or off of a sponsored project. (Guidance is provided on the form for backup support.)
- Contract Residual Closeout Form Used to request the closure of grants and contracts when funds vest with UT. (Rename and save the PDF form to your hard drive, complete and forward within your department for approval. Return to PAF when completed.) Clinical Research Accounts (with fund code 57001) should not use this form. For accounts with fund code 57001 the “Clinical Research Account Close Out Form” listed under CLINICAL RESEARCH FINANCE below must be used.
- Gift Card Reimbursement Form Include with the non-po voucher documentation when requesting reimbursement of gift cards purchased.
- Modular Grant Information Use when preparing modular grant budgets.
- Person Months Conversion Chart Use to convert % to person months
- Program Income Setup Request Form
- Relinquishing of Grants Procedures for departments and PAF to follow when a PI relinquishes a project.
- Salary Cap Worksheet (For both NIH & CPRIT) Accompanies PA's with federal grants that are subject to the federal Salary Cap.
- Split Account Request Template Used to add an additional sub-project to a main project.
- There are at least two schools, or
- It is multi-PI between two departments, or
- The funding for the second account includes: > 20% salary/effort of the investigator, additional personnel expenses, or non-personnel expenses in addition to the salary.
- Stipend Payments Guidance for departments to use when submitting stipends on a project.
- Subcontractor Tracking Form Used to monitor subcontractor invoices receipt and payment. Departments can optionally use the form.
CLINICAL RESEARCH FINANCE
- Clinical Research Account Close Out Form Used to request the closure of Clinical Research accounts/transfer of residual funds. Rename and save the PDF form to your hard drive, complete and forward within your department for approval. Return to CRF mailbox when completed.) Only use this form if the fund code of the research account is 57001
- Checklist to Expedite Device Study Review A form requested by Memorial Hermann Healthcare System to expedite the review of Device Studies to be conducted at MHHS. This form should be completed and submitted to MHHS as soon as possible.
- CAIB Tool Guidance Document This document provides guidance on how to complete the Coverage Analysis/Internal Budget tool. (CAIB tool)
- Clinical Research Charge Document Log
- Clinical Trial Budget Template with Coverage Analysis Grid (CAIB tool) Development of a clinical trial coverage analysis grid and budget. It contains internal price lists and formulas for effort calculations. FOR INTERNAL USE ONLY.
- Contract/ Coverage Analysis/ Proposal Record Workflow Document This workflow document provides an overview of the start -up process in SPA for industry initiated clinical studies.
- Coverage Analysis Fees - Industry Sponsored and Departmentally Funded Studies This sheet provides a breakdown of fees with and without IDC applied.
- Coverage Analysis Fee Memo for Industry Sponsors This memo provides information to provide to Industry Sponsors regarding coverage analysis fees.
- EPIC Tip Sheet- Study Coordinator Guide This tip sheet discusses how to link patients to studies, how to link visit encounters to studies, and how to review charges for research visits in Epic
- EPIC Tip Sheet- Manage Study Record This tip sheet discusses how to manage the study record in Epic
- Link/ Associate Upcoming Research Visits in Epic - This is a short video (78 seconds) showing how to create a report in Epic showing the upcoming visits scheduled for all patients linked to your study(ies) in Epic. You will need to cut and paste: Upcoming/Current Admissions for Patients Associated with <Insert Research Study> into the “Report Library” in Epic.
- Harris Health Patient Registration Form
- MHH Patient Registration Form
- Link/Associate Patient to Study in Epic- GIF - This is a short video recording showing a researcher linking a patient to a study in Epic (60 seconds no sound). For detailed guidance on how to link/associate a patient to a study in Epic please refer to “EPIC Tip Sheet- Study Coordinator Guide
- Submit CTA to SPA Checklist This checklist can be used as a guide to help ensure you have completed all of the steps for submitting agreements for new clinical studies to SPA.
- Clinical Study START Proposal Record Budget Checklist This checklist is used by the CRFA team to review the proposal record budget for Clinical studies. This includes the review of the attached internal budget (on the CAIB tool) and the budget data entered into the START budget tab. This can be used as a guide to help ensure you have completed all of the needed data for submitting START Proposal records to SPA for new applicable clinical studies.
BUDGET TEMPLATES
- Clinical Trial Budget Template with Coverage Analysis Grid Development of a clinical trial coverage analysis grid and budget. It contains internal price lists and formulas for effort calculations. FOR INTERNAL USE ONLY.
- Complex Detailed Budget and Directions Assist in the development of internal budgets for proposals. Use this template for large proposals with numerous personnel and expense lines, such as program projects.
- Set-Up Budget Worksheet for Subcontracts Required for all non-cash basis award set-ups, including cost reimbursable, fee-for-service, and milestone payment accounts with subcontracts.
- Set-up Budget Worksheet and Directions Required for all non-cash basis award set-ups, including cost reimbursable, fee-for-service, and milestone payment accounts.
- Simple Detailed Budget and Directions Assist in the development of internal budgets for proposals. Use this template for standard proposals.
- ECC Access Request Form Gain access to ECC, effort reporting system
- Funding History Access Request Form (email [email protected] for access)
- Effort Minimum Waiver Request a waiver to minimum effort requirements/policy (DocuSign)
- Effort Maximum Waiver Request a waiver to maximum effort requirements/policy (DocuSign)
- Effort Commitment Template Effort Commitment Excel form
SYSTEM ACCESS FORMS
- ECC Access Request Form - Gain access to ECC effort reporting system
- eRA Commons Account Registration - Obtain eRA Commons account & affiliate with UTHealth
- NSF FastLane Account Registration - Instructions to obtain NSF Fastlane account
- Sponsor/Subcontractor Request Form - New! Request a new UTHealth START sponsor or subcontractor
- Subcontractor Personnel Request Form - Request access for Subcontractor Personnel
- UTHealth START Delegation Form - Users can delegate tasks/To Do Items to other UTHealth employees
- UTHealth START Access Request Form - New! Gain access to UTHealth STARRT system
How to Develop a Clinical Trial Budget: Download Excel Worksheet
Patricio ledesma, clinical trial costs, 23 september, 2020.

This article explains how to develop a per patient clinical trial budget (test, procedure, and staff costs to be paid to the hospital for each recruited subject). If you want to download a free (per patient) Excel clinical trial budget worksheet, please visit this page . If you wish to learn about all the costs of a clinical trial, check this article . You can also download a CRO trial quote here .
Clinical trials generate several costs at the hospitals or clinical centers in which they are conducted.
Per-patient trial costs linked to a clinical site are those expenses derived from procedures and tests required per protocol and performed in the study visits.
A typical way of structuring hospital-related study costs is to divide the costs in the following two categories: (1) Per-patient costs (including procedures/tests, staff costs, and overheads), and (2) Clinical site fees.
Per Patient Costs
Per patient costs comprise clinical procedure/test expenses, staff costs, and overheads, and they are distributed in study visits (according to the calendar of assessments of the protocol).
As an example, we can consider a cancer clinical trial with the following visits:
- Screening visit / Baseline
- Treatment visits: Cycle 1 Day 1, Cycle 2 Day 1, Cycle 3 Day 1, Cycle 4 Day 1, and Cycle 5 Day 1 (21-day cycles)
- End of Treatment visit
- Follow-up visits to evaluate survival: One every 3 months during 1 year
Every clinical protocol is different and every drug has its own administration scheme, but the calendar of assessments described in this article provides a good basic idea of a typical visit calendar in oncology.
Screening Visit (Baseline)
The screening visit (baseline) is the first visit of the patient in the trial, and it is used to evaluate the eligibility of the patient (inclusion / exclusion criteria are verified).
In oncology, this initial stage normally includes the informed consent signature, tumor block collection (biopsy) for central diagnosis confirmation and/or translational studies, medical history review, collection of demographics, height, weight, vital signs, ECOG performance status, physical exam, blood count, serum biochemistry, coagulation test, pregnancy test, quality of life questionnaires, adverse events/concomitant medications, PK blood draws, electrocardiogram, and CT scan (to measure the tumor size).
In addition to the procedure/test costs for each visit, staff costs must be considered. This is essentially the time dedicated by hospital personnel: the study coordinator, the physician (investigator), and nurses.
Finally, you can add a 20-25% of overhead on the sum of procedure/test costs plus staff costs.
Screening visit / Baseline total = Procedures/tests ($2,350.00) + Staff costs ($320.00) + Overheads ($667.50) = $3,337.50
Treatment Visits
The treatment visits are those hospital visits in which the patient receives the study drug (e.g. chemotherapy infusion).
The first treatment visit will be Cycle 1 Day 1, and in the example we are describing in this article the patient will have a total of 5 treatment visits.
A typical oncology treatment visit will include the evaluation of basic elements such as weight, vital signs, ECOG, physical exam, complete blood count, full serum chemistry, adverse events, and concomitant medications.
The treatment visits (not all of them) will also include additional tests such as pregnancy tests, quality of life questionnaires, PK blood draws, and radiological assessments (CT scans).
In our example of 5 treatment visits, these are the approximate costs (including procedures/tests, staff, and overhead costs):
- Cycle 1 Day 1 = $718.75
- Cycle 2 Day 1 = $787.50
- Cycle 3 Day 1 = $2,431.25 (includes CT scan)
- Cycle 4 Day 1 = $787.50
- Cycle 5 Day 1 = $2,343.75 (includes CT scan)
- Treatment visits total = $7,068.75
End of Treatment Visit
The end of treatment visit is usually conducted 2-4 weeks after the last dose received by the patient. This visit includes the assessment of weight, vital signs, ECOG, physical exam, blood count, biochemistry, pregnancy test, quality of life form, adverse events, concomitant medications, PK blood extraction, and ECG.
End of treatment visit total = $1,125.00
Follow up Visits
Overall survival is an important endpoint in oncology trials. For this reason, once the patient has completed the study therapy, long-term follow-up visits are done to assess vital status.
In our example, follow-up visits are proposed every 3 months during one year of follow-up (4 follow-up visits in total).
During follow-up visits at least the following assessments are performed: weight, vital signs, and physical exam. Radiology tests may also be done to monitor the evolution of the tumor.
Follow-up visits total (4 visits) = $1,900.00
Therefore, the per patient costs adding up all the concepts mentioned above are: $13,431.25.
Clinical Sites Fees
Finally, clinical sites may ask for a number of additional fees, which we summarize here:
- Trial start-up fee = $1,500.00
- Site contract fee = $2,000.00
- Trial close-out fee = $1,500.00
- Pharmacy set-up fee = $2,000.00
- Total clinical site fees = $7,000.00
Other Potential Costs
There are more hospital costs that could go into the trial budget (not included in the example described in this article). These other costs may be optional and will depend on the trial sponsor’s particular strategy:
- Screen failures: Some patients start the screening process but they end up being ineligible for trial participation because they fail to meet some inclusion criteria or they finally refuse to be enrolled. Sponsors may decide to pay for screen failures, covering the costs of the screening activities actually completed.
- Patient transportation: Some sponsors may want to pay for patient transportation costs (e.g. taxi expenses to visit the hospital).
- File storage: At the end of the trial, physical documents / files will have to be stored for a number of years. Some hospitals will charge the sponsor for such storage while other sponsors can decide to keep the folders at its own offices.
Let’s say we have a phase 1 clinical trial in oncology recruiting a total of 18 patients. Then, the total budget of the study (related to hospital costs) will be:
18 patients x $13,431.25 + $7,000 = $248,762.50
Very important: Per patient costs can vary substantially depending on the country. For instance, a clinical trial in the United States will usually have higher costs than a study in Europe.
Please also note these are hospital-related costs only. A clinical trial involves many other expenses.
You can obtain a free quote describing all clinical trial costs here:
Download a Full CRO Clinical Trial Quotation Example
If you want to download an editable Excel worksheet with the detailed per-patient budget described in this article, please click here.
Patricio Ledesma ([email protected]) helps biotech companies navigate the complexities of clinical trial planning and development in the US and Europe; from drug manufacturing to the publication of trial results. He provides comprehensive assistance for the entire trial process, a one-stop approach to avoid looking for multiple vendors. Check this article to learn how he can help.

Patricio Ledesma ( B.Eng Concordia University, Montreal, Canada and Master’s Degree in Clinical Trials, University of Seville, Spain) is the Head of Clinical Operations and Founder at Sofpromed CRO. Patricio is a professional consultant providing comprehensive, one-stop expert advice and guidance to biotechnology and pharmaceutical companies worldwide in the field of clinical trials and drug development. He is personally and enthusiastically devoted to helping biotech Chief Executive, Operations, Scientific, Medical, and Regulatory Officers in the planning and execution of phase I-IV clinical trials across North America, Europe, Asia-Pacific, Latin America, and Middle East regions. You can contact Patricio at: +34 607 939 266 [email protected]
To our articles and updates
- Follow Follow
You may also be interested…

What Are Community-Based Clinical Trials?
Community-Based Clinical Trials
If you are a Sponsor seeking to run a community-based clinical trial in underserved populations, please contact us at [email protected] Clinical trials are fundamental to advance medical knowledge and improve patient care. However, one significant challenge in...

Clinical Research Site Networks: Accelerating Clinical Trials
Site Networks
If you are a Sponsor seeking to run a clinical trial through a clinical research site network, please contact us at [email protected] Clinical research plays a central role in advancing medical treatments and improving healthcare outcomes. To ensure the smooth...

How Community-Based Clinical Trials Are Advancing Healthcare in Underserved Populations
If you are a Sponsor interested in running a community-based clinical trial in underserved populations, please contact us at [email protected] Clinical trials are instrumental in advancing healthcare by evaluating the safety and effectiveness of new treatments and...

Advantages of Clinical Research Site Networks in North Carolina
If you are a Sponsor interested in running a clinical trial through a clinical research site network in North Carolina, please contact us at [email protected] Clinical research plays a pivotal role in advancing medical knowledge, improving patient care, and driving...

Benefits of Clinical Research Site Networks in Illinois
If you are a Sponsor interested in running a clinical trial through a clinical research site network in Illinois, please contact us at [email protected] Clinical site networks play a central role in advancing medical research and improving patient care. In this...

Advantages of Clinical Research Site Networks in Pennsylvania
If you are a Sponsor interested in running a clinical trial through a clinical research site network, please contact us at [email protected] Pennsylvania is a hub for clinical research, with numerous reputable clinical site networks offering a wide range of trials to...

Benefits of Clinical Research Site Networks in New York
If you are a Sponsor interested in running a clinical trial through a clinical research site network in New York, please contact us at [email protected] New York, with its vibrant healthcare landscape, is home to several prominent clinical research site networks.In...

Enhancing Clinical Trial Diversity Through Community-Based Site Networks
If you are a Sponsor interested in running a clinical trial through a community-based clinical research site network, please contact us at [email protected] One significant challenge in clinical trials is the lack of diversity among participants, particularly from...
Patricio Ledesma ( B.Eng Concordia University, Montreal, Canada and Master’s Degree in Clinical Trials, University of Seville, Spain) is the Head of Clinical Operations and Founder at Sofpromed CRO. Patricio is a professional consultant providing comprehensive, one-stop expert advice and guidance to biotechnology and pharmaceutical companies worldwide in the field of clinical trials and drug development. He is personally and enthusiastically devoted to helping biotech Chief Executive, Operations, Scientific, Medical, and Regulatory Officers in the planning and execution of phase I-IV clinical trials across North America, Europe, Asia-Pacific, Latin America, and Middle East regions. You can contact Patricio at: +34 607 939 266 [email protected]

This content is onyen-protected and requires you to log in to view it. Please click here to log in.
Budget Development Tools

IMAGES
VIDEO
COMMENTS
Develop Your Budget. As you begin to develop a budget for your research grant application and put all of the relevant costs down on paper, many questions may arise. Your best resources for answering these questions are the grants or sponsored programs office within your own institution, your departmental administrative officials, and your peers.
For multi‐center stroke trials, you will need to prepare a concept proposal and preliminary budget. Once concept proposal is deemed feasible and approved for submission, need to have final budget. It needs to be as accurate as possible since if you do need to resubmit, you can only have up to 10% budget increase.
Policy Statement, con't. F&A costs are charged to individual awards as direct costs are incurred. F&A is applied to the direct costs base, Modified Total Direct Costs (MTDC) for most federal awards. Industry-sponsored clinical trials must be charged a rate of 30.00% of TDC, including all fees and invoiceable expenses.
Download Clinical Trial Budget Template 4. Labor Costs. In order to conduct a clinical trial, you need to hire people that have expertise in clinical research and clinical trial management. Depending on the size of the trial and the number of trials conducted, resource allocations vary. Therefore the amount of labor needed to run a study also ...
Clinical Research Resource Feasibility Assessment Budget/ Finance ; Clinical Trial Protocol Template This protocol template is designed to help research teams develop a clinical trial protocol that includes an investigational intervention (drug, biologic, vaccine or device). Protocol Templates
The sponsor of a clinical trial generally sends a protocol and budget overview/template for the trial. After reviewing the protocol and events schedule, create an internal budget to reflect all costs, including time and effort of all personnel, in order to conduct the trial. ... The Office of Clinical Research (OCR) has developed a template to ...
It was found that the top 3 drivers of clinical trial costs were the following categories of expenses [4]: Clinical procedure costs, representing 15%-22% of total costs. Administrative staff costs, representing 11%-29% of total costs. Site monitoring costs, representing 9%-14% of total costs.
The best practice for developing a budget is to create a comprehensive study or trial budget and then break down costs by year. The presentation from the NIH also divides budgets into two types: an overall and a site-specific budget. The overall budget refers to the budget for the entire multicenter trial or a single-center trial.
University of Florida requires all clinical research be conducted in compliance with applicable State and Federal regulations, and UF policies. It is critical that a budget be developed that details all costs associated with conducting the study in order for the Principal Investigator and University of Florida to be in compliance with regulatory requirements and…
Internal Budget - Federal - NIH. This budget template is formulated for Modified Total Direct Costs (MTDC) on NIH "R&R" detailed budgets and is formatted for easy entry into the R&R budget pages. Internal Budget - Non-Federal. The Excel budget template contains commonly used expense types and automatically calculates fringe and overhead rates.
Clinical Trial Budget Costing Tool. The costing tool is a spreadsheet to assist in the process of study costing for research being conducted by NSW Public Health Organisations. In 2017, the Office for Health and Medical Research (OHMR) initiated a project to develop a standard Budget Costing Tool ('the Tool') for clinical trials.
All clinical research starts with the research protocol, a document that details all aspects of the trial: its background, rationale, objectives, design, methodology, statistical analysis plan, and organization.With the protocol, you can make sure you protect the participants and collect the data. Using protocol templates, you can start thinking through what you need to meet compliance ...
Your clinical trial costs will also vary depending on how long you're running it. There is a reason why some trials can cost upwards of $100 million to run! The average cost of a Phase I study is $30 million and the average cost of a Phase II study is $50 million. These costs include staffing and site fees paid to multiple CROs for full ...
Budget Terminology. Grant Services & Analysis. Phone: 734-763-4272. Email: [email protected]. Grant Services & Analysis is a unit of the Medical School Office of Research, where our mission is to foster an environment of innovation and efficiency that serves the Michigan Medicine research community and supports biomedical science from insight ...
Clinical Trial Budgets. Whether you are developing your own budget or the sponsor presents you with one, your clinical study budget should cover all study-related costs, including fees and indirect costs. Most of your costs will be calculated per patient and/or per procedure, but some may be one-time costs or fees.
The CRU should be contacted first when industry-sponsored research projects (clinical trial or clinical research) become available. The following is a general overview of the industry sponsored budget process; however, each CRU may have its own specific process and forms. CRU led items are yellow, DOCR led items are green, misc items are grey.
TCCCR has designed an Excel template that reflects the true cost of conducting research by including these so-called hidden costs in the budget request (Table 1). The template takes into consideration fees for research personnel at all levels, including the principal investigator, site manager, clinical research coordinators, and regulatory ...
Proper study budget is vital for a successful trial for both parties, contract research organizations (CRO) and pharma companies. Therefore, it is essential to take the time and prepare your financial plan by thoroughly thinking of all expenses. ... For example, the budget template of OCT Clinical CRO consists of over 400 items and covers all ...
Budget plan is a key element of a grant application. It demonstrates the required cost for the proposed project. It is a prediction of expenses and serves a plan for funders on how the organization will operate the project, spend the money in a given set of period and where their money will go.
Clinical Research Accounts (with fund code 57001) should not use this form. For accounts with fund code 57001 the "Clinical Research Account Close Out Form" listed under CLINICAL RESEARCH FINANCE below must be used. ... Clinical Trial Budget Template with Coverage Analysis Grid Development of a clinical trial coverage analysis grid and ...
Let's say we have a phase 1 clinical trial in oncology recruiting a total of 18 patients. Then, the total budget of the study (related to hospital costs) will be: 18 patients x $13,431.25 + $7,000 = $248,762.50. Very important: Per patient costs can vary substantially depending on the country. For instance, a clinical trial in the United ...
Research Budget Development Template, Master Revenue and Expense Tracker, and instructions. Skip to main content. Submit Search. ... Office of Clinical Trials CB 1651 720 Martin Luther King, Jr. Blvd. Chapel Hill, NC 27599-1651. Ph: 919-843-2698 Fax: 919-843-2399. General Inbox: [email protected]
Medical Research Budget Template. researchamerica.org. Download. A research proposal budget sample is in many ways similar to the research budget sample, only that at this stage, it is still a proposal. This sample is often written in a linear or tabular format and it details all the expenses that are associated with the proposal project.