- Information for: Future Student Current Student Graduate Student Faculty/Staff Alumni and Donors
- Life at WOU
Home » Student Resources » Online Chemistry Textbooks » CH104: Chemistry and the Environment » CH104: Chapter 4 – Covalent Bonds and Molecular Compounds
- CH104: Chemistry and the Environment

Chapter 4 – Covalent Bonds and Molecular Compounds
4.1 introduction to covalent molecules and compounds, how to recognize covalent bonds, 4.2 electron sharing, single covalent bonds between the same atoms, single covalent bonds between different atoms, multiple covalent bonds, coordinate covalent bonds, 4.3 electronegativity and bond polarity, 4.4 properties of molecular compounds, 4.5 naming binary molecular compounds, 4.6 focus on the environment – the love canal, 4.7 chapter summary, 4.8 references.
Chemical bonds are generally divided into two fundamentally different types: ionic and covalent. In reality, however, the bonds in most substances are neither purely ionic nor purely covalent, but lie on a spectrum between these extremes. Although purely ionic and purely covalent bonds represent extreme cases that are seldom encountered in any but very simple substances, a brief discussion of these two extremes helps explain why substances with different kinds of chemical bonds have very different properties. Ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally consist of molecules, which are groups of atoms in which one or more pairs of electrons are shared between bonded atoms. In a covalent bond, atoms are held together by the electrostatic attraction between the positively charged nuclei of the bonded atoms and the negatively charged electrons they share. This chapter will focus on the properties of covalent compounds.
Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has the fundamental chemical properties of a covalent compound. Thus, the term molecular compound is used to describe elements that are covalently bonded and to distinguish the compounds from ionic compounds. Some pure elements exist as covalent molecules. Hydrogen, nitrogen, oxygen, and the halogens occur naturally as the diatomic (“two atoms”) molecules H 2 , N 2 , O 2 , F 2 , Cl 2 , Br 2 , and I 2 (part (a) in Figure 4.1 ). Similarly, a few pure elements exist as polyatomic (“many atoms”) molecules, such as elemental phosphorus and sulfur, which occur as P 4 and S 8 (part (b) in Figure 4.1 ).
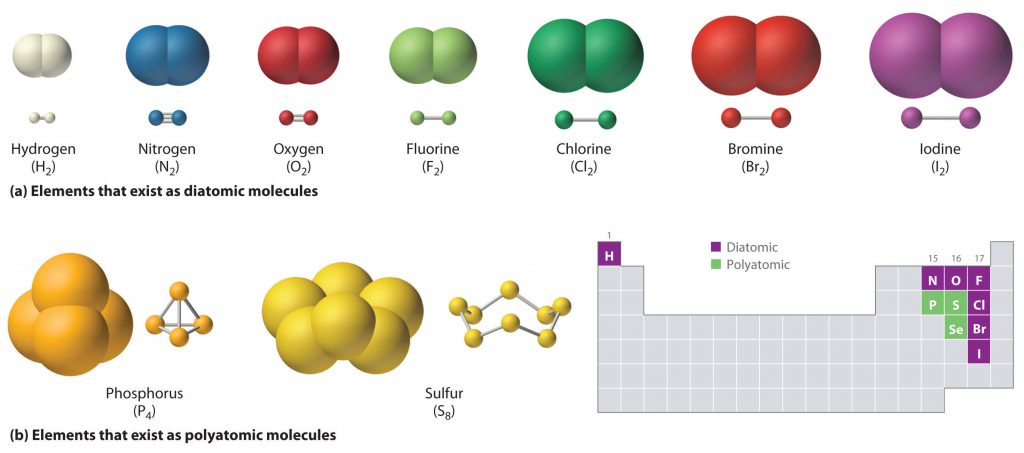
Figure 4.1 Elements That Exist as Covalent Molecules. (a) Several elements naturally exist as diatomic molecules, in which two atoms (E) are joined by one or more covalent bonds to form a molecule with the general formula E2. (b) A few elements naturally exist as polyatomic molecules, which contain more than two atoms. For example, phosphorus exists as P4 tetrahedra—regular polyhedra with four triangular sides—with a phosphorus atom at each vertex. Elemental sulfur consists of a puckered ring of eight sulfur atoms connected by single bonds. Selenium is not shown due to the complexity of its structure.
Each covalent compound is represented by a molecular formula, which gives the atomic symbol for each component element, in a prescribed order, accompanied by a subscript indicating the number of atoms of that element in the molecule. The subscript is written only if the number of atoms is greater than 1. For example, water, with two hydrogen atoms and one oxygen atom per molecule, is written as H 2 O. Similarly, carbon dioxide, which contains one carbon atom and two oxygen atoms in each molecule, is written as C O 2 .

Covalent compounds that predominantly contain carbon and hydrogen are called organic compounds . The convention for representing the formulas of organic compounds is to write carbon first, followed by hydrogen and then any other elements in alphabetical order (e.g., CH 4 O is methyl alcohol, a fuel). Compounds that consist primarily of elements other than carbon and hydrogen are called inorganic compounds ; they include both covalent and ionic compounds. The convention for writing inorganic compounds, involves listing the component elements beginning with the one farthest to the left in the periodic table, as in CO 2 or SF 6 . Those in the same group are listed beginning with the lower element and working up, as in ClF. By convention, however, when an inorganic compound contains both hydrogen and an element from groups 13–15, hydrogen is usually listed last in the formula. Examples are ammonia (NH 3 ) and silane (SiH 4 ). Compounds such as water, whose compositions were established long before this convention was adopted, are always written with hydrogen first: Water is always written as H 2 O, not OH 2 . Typically this distinguishes when hydrogen is participating in a covalent bond rather than an ionic interaction, as seen in many of the inorganic acids, such as hydrochloric acid (HCl) and sulfuric acid (H 2 SO 4 ), as described in chapter 3 .
In Chapter 3, we saw that ionic compounds are composed predominantly of a metal + a nonmetal. Covalent molecules, on the otherhand, are typically composed of two nonmetals or a nonmetal and a metalloid. This is an initial screening method that you can use to categorize compounds into the ionic or the covalent cagetogy.
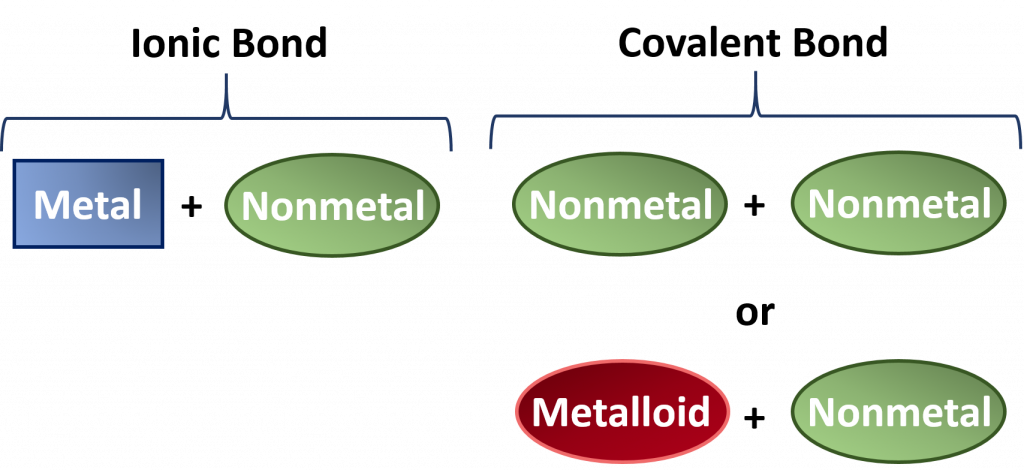
Figure 4.2 Recognizing Ionic vs Covalent Compounds. Typically compounds that are formed from a combination of a metal with a nonmetal have more ionic bond character whereas compounds formed from two nonmetals or a metalloid and a nonmetal show more covalent character. Although compounds usually lie on a spectrum somewhere between fully ionic and fully covalent character, for naming purposes, this guideline works well.
Chapter 3 described how electrons can be transferred from one atom to another so that both atoms have an energy-stable outer electron shell following the octet rule . However, there is another way an atom can achieve a full valence shell: atoms can share electrons to reach the octet state (or the duet state in the case of hydrogen).
This concept can be illustrated by using two hydrogen atoms, each of which has a single electron in its valence shell. (For small atoms such as hydrogen atoms, the valence shell will be the first shell, which holds only two electrons.) We can represent the two individual hydrogen atoms as follows:
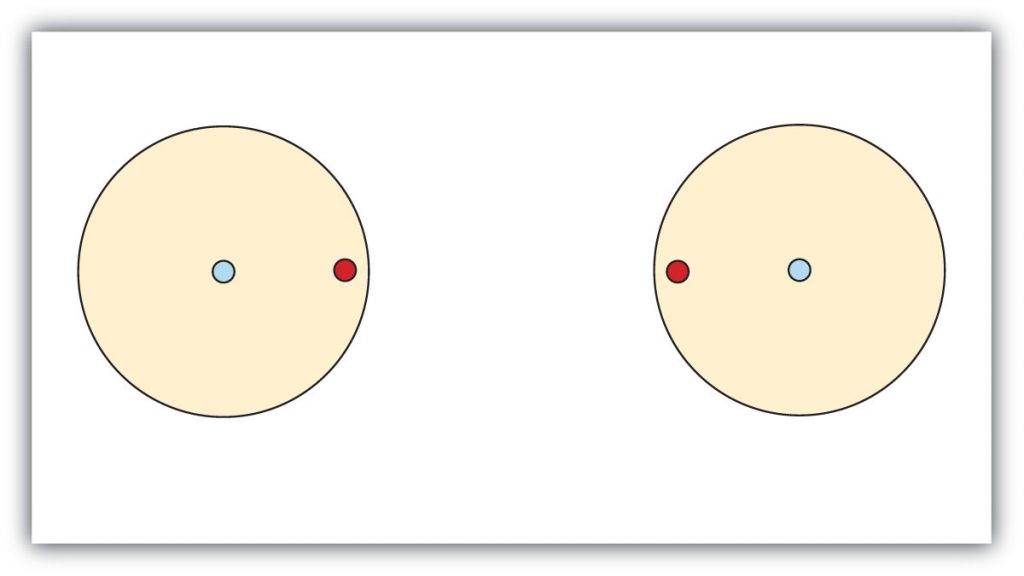
In this situation neither hydrogen can reach the preferred duet state. In contrast, when two hydrogen atoms get close enough together to share their electrons, they can be represented as follows:
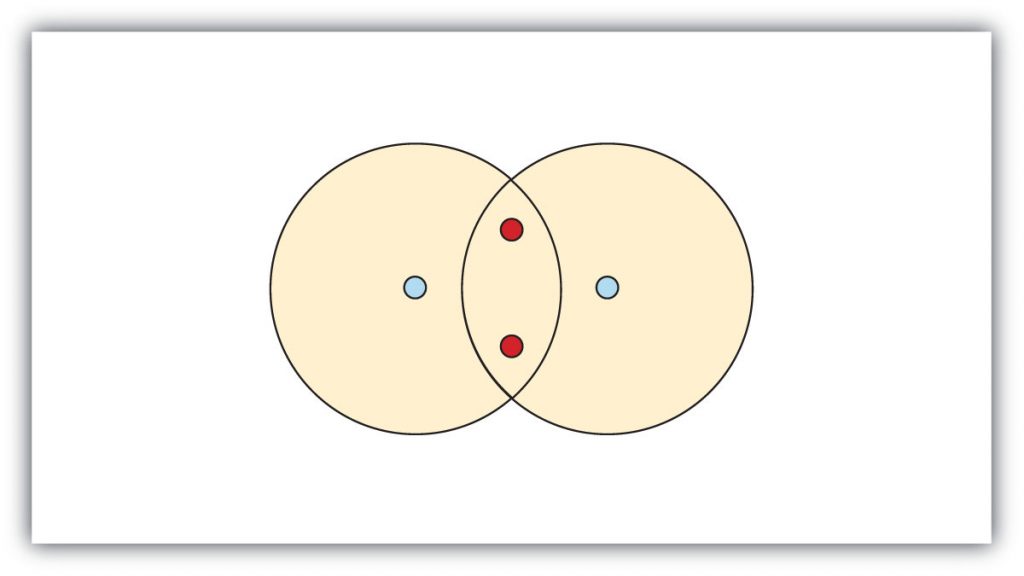
By sharing their valence electrons, both hydrogen atoms now have two electrons in their respective valence shells. Because each valence shell is now filled, this arrangement is more stable than when the two atoms are separate. In this configuration, each hydrogen has an electron configuration equivalent to that of the noble gas, helium. The sharing of electrons between atoms is called a covalent bond , and the two electrons that join atoms in a covalent bond are called a bonding pair of electrons . A discrete group of atoms connected by covalent bonds is called a molecule —the smallest part of a compound that retains the chemical identity of that compound. For example, one molecule of water would contain two hydrogen atoms and one oxygen atom (H 2 O).
Chemists frequently use Lewis electron dot diagrams to represent covalent bonding in molecular substances. For example, the Lewis diagrams of two separate hydrogen atoms are as follows:
The Lewis diagram of two hydrogen atoms sharing electrons looks like this:
This depiction of molecules is simplified further by using a dash to represent a covalent bond. The hydrogen molecule is then represented as follows:
Remember that the dash, also referred to as a single bond, represents a pair of bonding electrons.
The bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10 −11 m, or 74 picometers (pm; 1 pm = 1 × 10 −12 m). This particular bond length represents a balance between several forces: (1) the attractions between oppositely charged electrons and nuclei, (2) the repulsion between two negatively charged electrons, and (3) the repulsion between two positively charged nuclei. If the nuclei were closer together, they would repel each other more strongly; if the nuclei were farther apart, there would be less attraction between the positive and negative particles.
Fluorine is another element whose atoms bond together in pairs to form diatomic (two-atom) molecules. Two separate fluorine atoms have the following electron dot diagrams:

Each fluorine atom contributes one valence electron, making a single bond and giving each atom a complete valence shell, which fulfills the octet rule:

The circles show that each fluorine atom has eight electrons around it. As with hydrogen, we can represent the fluorine molecule with a dash in place of the bonding electrons:
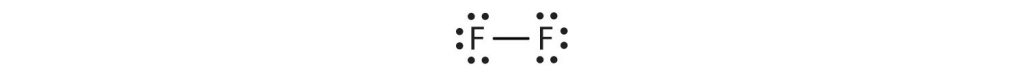
Each fluorine atom has six electrons, or three pairs of electrons, that are not participating in the covalent bond. Rather than being shared, they are considered to belong to a single atom. These are called nonbonding pairs (or lone pairs) of electrons.
Now that we have looked at electron sharing between atoms of the same element, let us look at covalent bond formation between atoms of different elements. Consider a molecule composed of one hydrogen atom and one fluorine atom:

Each atom needs one additional electron to complete its valence shell. By each contributing one electron, they make the following molecule:
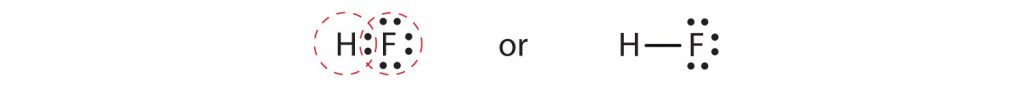
In this molecule, the hydrogen atom does not have nonbonding electrons, while the fluorine atom has six nonbonding electrons (three lone electron pairs). The circles show how the valence electron shells are filled for both atoms (recall that hydrogen is filled with two electrons).
Larger molecules are constructed in a similar fashion, with some atoms participating in more than one covalent bond. For example, water, with two hydrogen atoms and one oxygen atom, and methane (CH 4 ), with one carbon atom and four hydrogen atoms, can be represented as follows:

Atoms typically form a characteristic number of covalent bonds in compounds. Figure 4.3 shows valence electron configurations of each element family (or column).
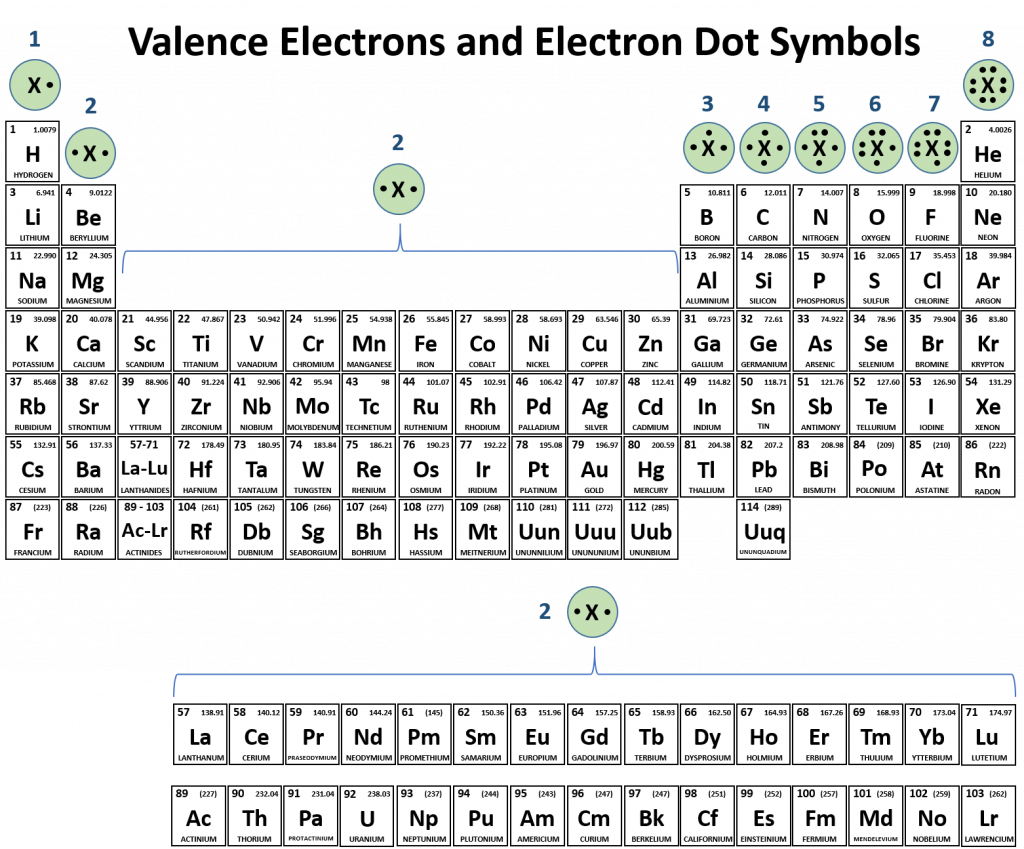
Fig 4.3 Periodic Table with Lewis Structures. Each family shows a representative lewis structure for that group of elements. For the nonmetals (Families 4A, 5A, 6A, and 7A) they can accept a complementary number of shared bonds to reach the octet state. Family 4A can share 4 covalent bonds (4 + 4 = 8), whereas Families 5A, 6A, and 7A can share 3, 2, and 1 covalent bond(s), respectively, to achieve the octet state. Exceptions to the octet rule do exist. For example, hydrogen can be considered to be in Group 1 or Group 7A because it has properties similar to both groups. Hydrogen can participate in either ionic or covalent bonding. When participating in covalent bonding, hydrogen only needs two electrons to have a full valence shell. As it has one electron to start with, it can only make one covalent bond. Similarly, boron has 3 electrons in its outer shell. This nonmetal typically forms 3 covalent bonds, having a maximum of 6 electrons in its outer shell. Thus, boron can never reach the octet state. Other atoms can have expanded orbitals and accept additional covalent bonds. Two of these that are important for living systems are sulfur and phosphorus. By the octet rule, sulfur can make 2 covalent bonds and phosphorus 3 covalent bonds. Sulfur can also have expanded orbitals to accept 4 or 6 covalent bonds, and phosphorus can expand to 5 covalent bonds.

In many molecules, the octet rule would not be satisfied if each pair of bonded atoms shares only two electrons. Consider carbon dioxide (CO 2 ). If each oxygen atom shares one electron with the carbon atom, we get the following:
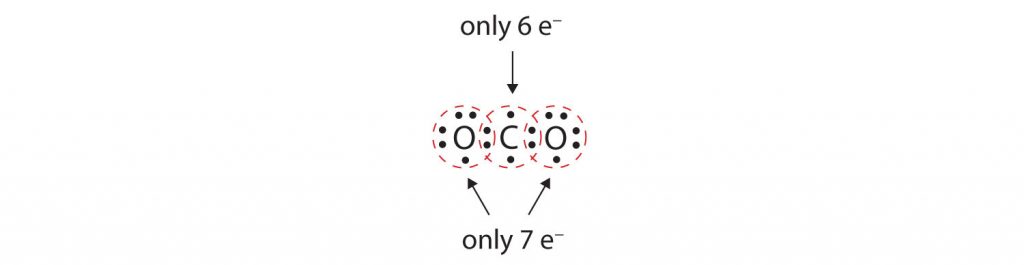
This does not give either the carbon or oxygen atoms a complete octet; The carbon atom only has six electrons in its valence shell and each oxygen atom only has seven electrons in its valence shell. Thus, none of the atoms can reach the octet state in the current configuration. As written, this would be an unstable molecular conformation.
Sometimes more than one pair of electrons must be shared between two atoms for both atoms to have an octet. In carbon dioxide, a second electron from each oxygen atom is also shared with the central carbon atom, and the carbon atom shares one more electron with each oxygen atom:
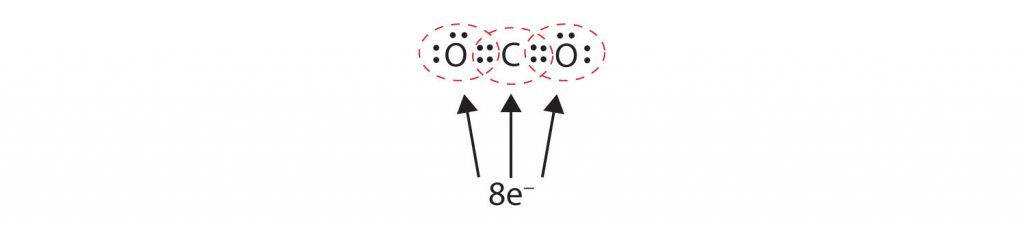
In this arrangement, the carbon atom shares four electrons (two pairs) with the oxygen atom on the left and four electrons with the oxygen atom on the right. There are now eight electrons around each atom. Two pairs of electrons shared between two atoms make a double bond between the atoms, which is represented by a double dash:
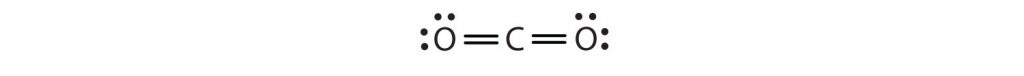
Some molecules contain triple bonds, covalent bonds in which three pairs of electrons are shared by two atoms. A simple compound that has a triple bond is acetylene (C 2 H 2 ), whose Lewis diagram is as follows:

A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from the same atom. A covalent bond is formed by two atoms sharing a pair of electrons. The atoms are held together because the electron pair is attracted by both of the nuclei. In the formation of a simple or ordinary covalent bond, each atom supplies one electron to the bond – but that does not have to be the case. In the case of a coordinate covalent bond, one atom supplies both of the electrons and the other atom does not supply any of the electrons. The following reaction between ammonia and hydrochloric acid demonstrates the formation of a coordinate covalent bond between ammonia and a hydrogren ion (proton).
The reaction between ammonia and hydrochloric acid
If these colorless gases are allowed to mix, a thick white smoke of solid ammonium chloride is formed.
Video provided by North Carolina School of Science and Mathematics
The overall reaction is
Ammonium ions, NH 4 + , are formed by the transfer of a hydrogen ion (a proton) from the hydrochloric acid molecule to the lone pair of electrons on the ammonia molecule. To visualize this reaction, we can use electron dot configurations to observe the electron movement during the reaction. First recall the valence electron states for all of the atoms involved in the reaction:
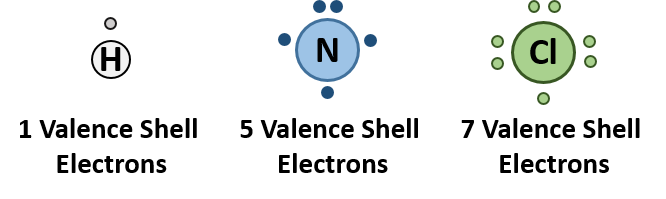
On the left side of the equation (to the left of the arrow) are the reactants of the reaction (ammonia and hydrochloric acid). On the right side of the reaction (to the right of the arrow) is the product of the reaction, the ionic compound – ammonium chloride. The diagram below shows the electron and proton movement during the reaction.
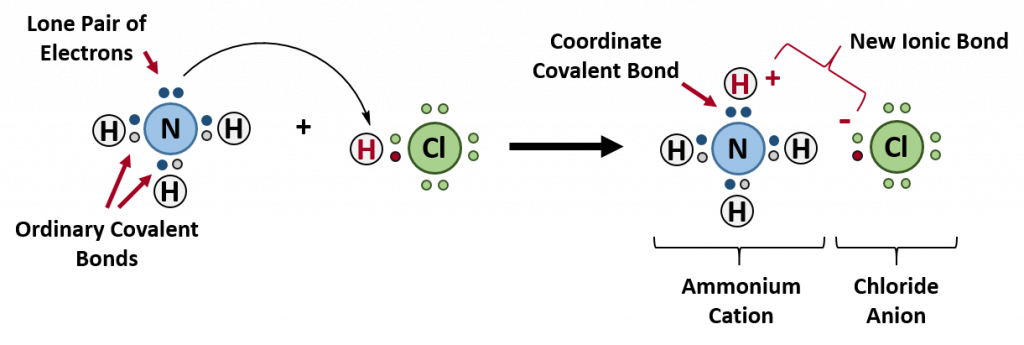
Figure 4.4 Formation of Ammonium Chloride. When the ammonium ion, NH 4 + , is formed, the fourth hydrogen (shown in red) is attached by a coordinate covalent bond, because only the hydrogen’s nucleus is transferred from the chlorine to the nitrogen. The hydrogen’s electron is left behind on the chlorine to form a negative chloride ion. Once the ammonium ion has been formed it is impossible to tell any difference between the coordinate covalent and the ordinary covalent bonds, all of the hydrogens are equivalent in the molecule and the extra positive charge is distributed throughout the molecule. Although the electrons are shown differently in the diagram, there is no difference between them in reality. In simple diagrams, a coordinate bond is shown by a curved arrow. The arrow points from the atom donating the lone pair to the atom accepting it.
Although we defined covalent bonding as electron sharing, the electrons in a covalent bond are not always shared equally by the two bonded atoms. Unless the bond connects two atoms of the same element, there will always be one atom that attracts the electrons in the bond more strongly than the other atom does, as shown in Figure 4.5. When such an imbalance occurs, there is a resulting buildup of some negative charge (called a partial negative charge and designated δ−) on one side of the bond and some positive charge (designated δ+) on the other side of the bond. A covalent bond that has an unequal sharing of electrons, as in part (b) of Figure 4.5, is called a polar covalent bond . A covalent bond that has an equal sharing of electrons (part (a) of Figure 4.5) is called a nonpolar covalent bond .
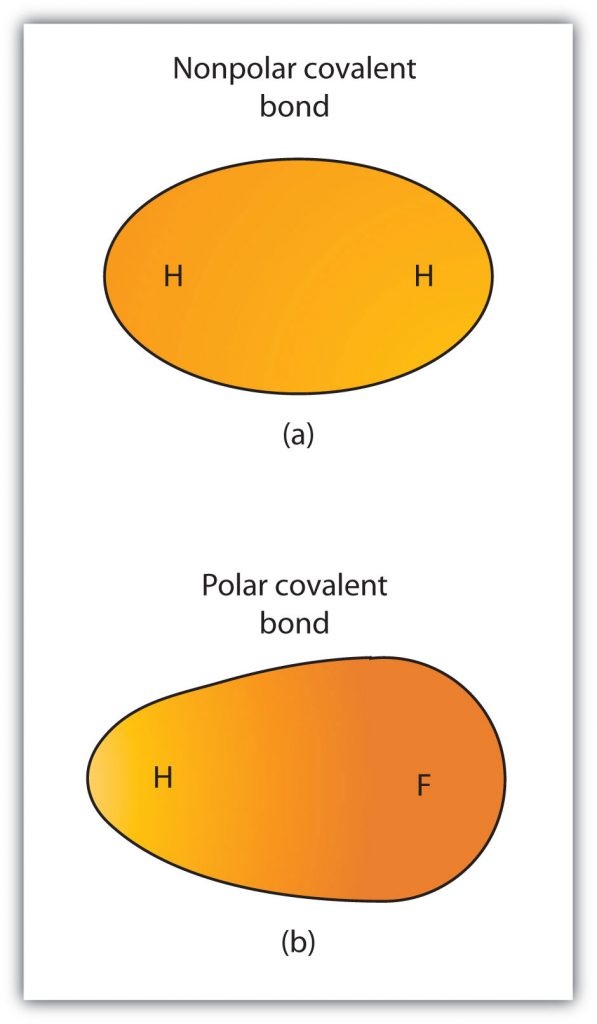
Figure 4.5 Polar versus Nonpolar Covalent Bonds. (a) The electrons in the covalent bond are equally shared by both hydrogen atoms. This is a nonpolar covalent bond. (b) The fluorine atom attracts the electrons in the bond more than the hydrogen atom does, leading to an imbalance in the electron distribution. This is a polar covalent bond.
Any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. Some bonds between different elements are only minimally polar, while others are strongly polar. Ionic bonds can be considered the ultimate in polarity, with electrons being transferred completely rather than shared. To judge the relative polarity of a covalent bond, chemists use electronegativity , which is a relative measure of how strongly an atom attracts electrons when it forms a covalent bond.
There are various numerical scales for rating electronegativity. Figure 4.6 shows one of the most popular— the Pauling scale . The polarity of a covalent bond can be judged by determining the difference in the electronegativities between the two atoms making the bond. The greater the difference in electronegativities, the greater the imbalance of electron sharing in the bond.
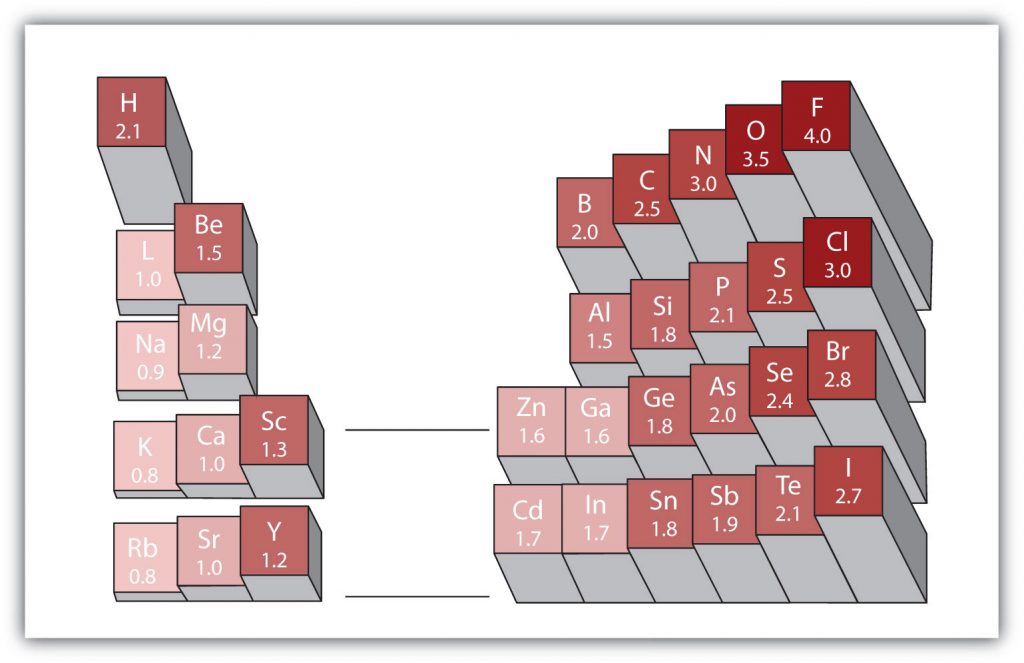
Figure 4.6 Electronegativities of Various Elements. The Pauling Scale for electronegativities has the value for fluorine atoms set at 4.0, the highest value.

Although there are no hard and fast rules, the general rule is that a difference in electronegativity less than 0.4 indicates the bond is nonpolar; when the difference is greater than 0.4, the bond is considered polar. When the difference in electronegativities is large enough (generally greater than about 1.8), the resulting compound is considered ionic rather than covalent. An electronegativity difference of zero, of course, indicates a nonpolar covalent bond. Examples of electronegativity difference are shown in Figure 4.7.
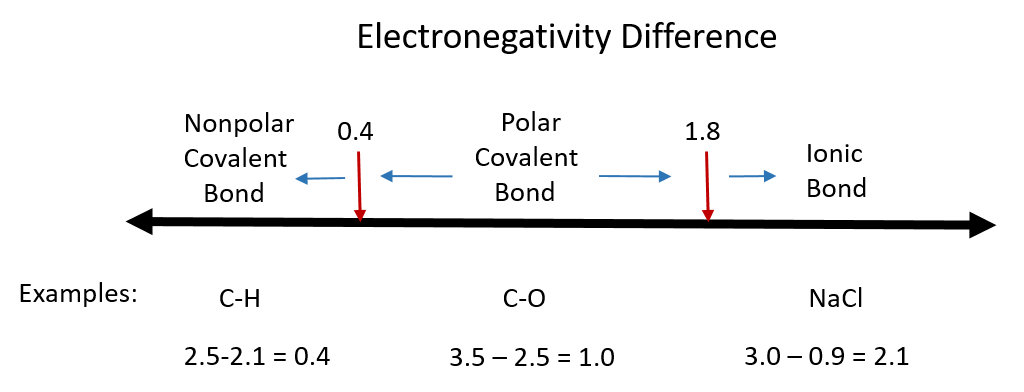
Figure 4.7 Electronegativity Difference Diagram. The diagram above is a guide for discerning what type of bond forms between two different atoms. By taking the difference between the electronegativity values for each of the atoms involved in the bond, the bond type and polarity can be predicted. Note that full ionic character is rarely reached, however when metals and nonmetals form bonds, they are named using the rules for ionic bonding.
When a molecule’s bonds are polar, the molecule as a whole can display an uneven distribution of charge, depending on how the individual bonds are oriented. For example, the orientation of the two O–H bonds in a water molecule (Figure 4.8) is bent: one end of the molecule has a partial positive charge, and the other end has a partial negative charge. In short, the molecule itself is polar. The polarity of water has an enormous impact on its physical and chemical properties. (For example, the boiling point of water [100°C] is high for such a small molecule and is due to the fact that polar molecules attract each other strongly.) In contrast, while the two C=O bonds in carbon dioxide are polar, they lie directly opposite each other in the molecule and so cancel each other’s effects. Thus, carbon dioxide molecules are nonpolar overall. This lack of polarity influences some of carbon dioxide’s properties. (For example, carbon dioxide becomes a gas at −77°C, almost 200° lower than the temperature at which water boils.)
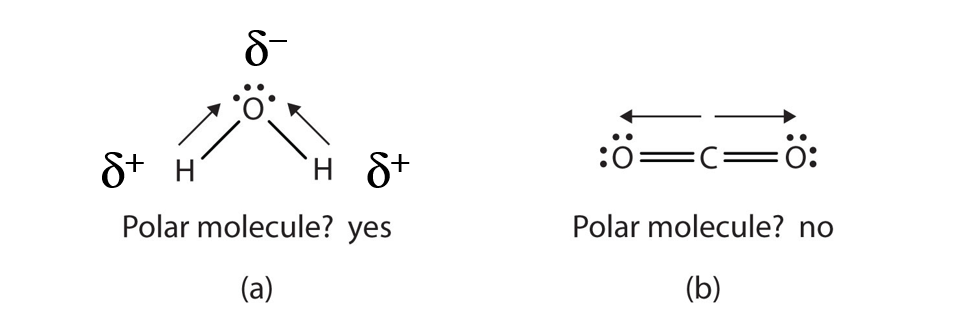
Figure 4.8 Physical Properties and Polarity. The physical properties of water (a) and carbon dioxide (b) are affected by their molecular polarities. Note that the arrows in the diagram always point in the direction where the electrons are more strongly attracted. In this diagram, the delta symbol (δ) is used with a (+) or (-) symbol to represent partial positive and partial negative charge distribution in polar covalent bonds. Note that the electrons shared in polar covalent bonds will be attracted to and spend more time around the atom with the higher electronegativity value. When the polarity is equal and directly opposing, as in the case of carbon dioxide (b), the overall molecule will have no overall charge.

(BACK TO THE TOP)
Molecular compounds have many properties that differ from ionic compounds. Some of the generalizations for this group include much lower melting and boiling points when compared with their ionic counterpoints. For example, water (H 2 O) has a melting point of 4 o C and a boiling point of 100 o C compared with NaCl that has a melting point of 801 o C and a boiling point of 1,413 o C. This is because the full charges created in ionic bonds have much stronger attractive force than the comparatively weak partial charges created in covalent molecules. thus, ionic compounds tend to form very strong crystalline lattice structures due to the repeating charges of the cation and anion components. Covalent compounds, on the otherhand, do not typically have such well-structured 3-dimensional shapes. Thus they tend to be more brittle and break more easily when in solid form, and many are found in liquid and gas phases. In addition, due to their lack of charges, they tend to be poor electrical and thermal conductors. Many are also insoluble in water due to their nonpolar nature (ie oil and water don’t mix).
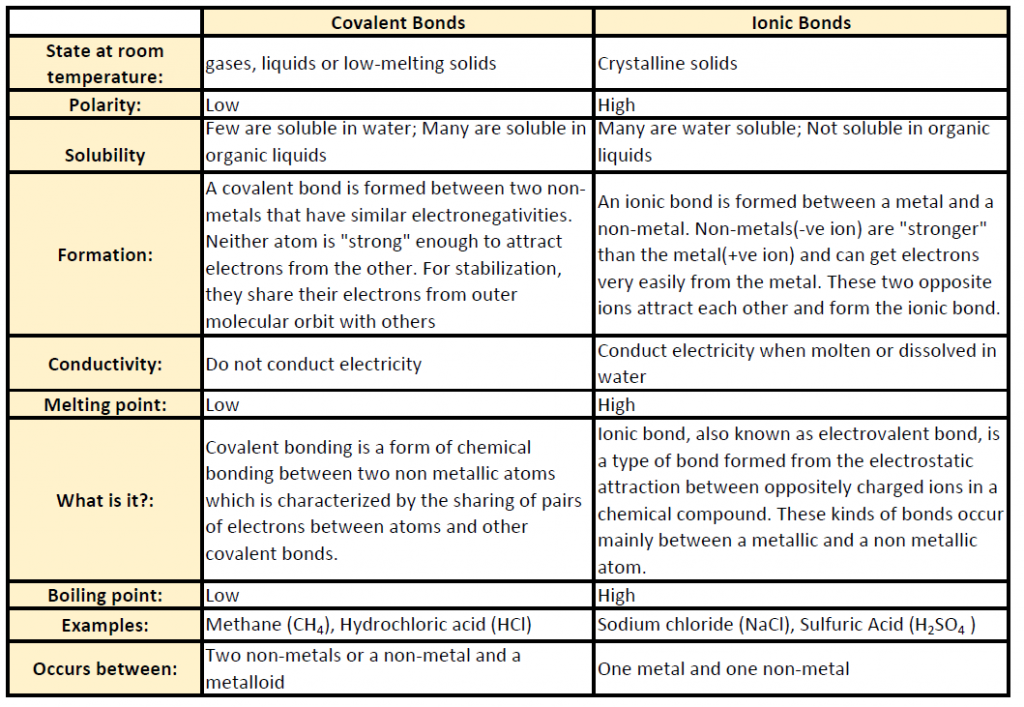
Table 4.1 shows common differences between covalent and ionic compounds.
Table 4.1 Comparison of Ionic and Covalent Compounds
Recall that a molecular formula shows the number of atoms of each element that a molecule contains. A molecule of water contains two hydrogen atoms and one oxygen atom, so its formula is H 2 O . A molecule of octane, which is a component of gasoline, contains 8 atoms of carbon and 18 atoms of hydrogen. The molecular formula of octane is C 8 H 18 . When writing the chemical formula the element that is the least electronegative (the element that is farther left or further down within the same family group) is written first while the more electronegative element is written second. You will be required to know how to name simple binary covalent compounds (compounds composed of two different elements)

Figure 4.9 Nitrogen dioxide ( NO 2 ) is a reddish-brown toxic gas that is a prominent air pollutant produced by internal combustion engines.
The elements that combine to form binary molecular compounds are both nonmetal atoms or they are a combination of a nonmetal and a metalloid. This contrasts with ionic compounds, which were formed from a metal ion and a nonmetal ion. Therefore, binary molecular compounds are different because ionic charges cannot be used to name them or to write their formulas. Another difference is that two nonmetal atoms will frequently combine with one another in a variety of ratios. Consider the elements nitrogen and oxygen. They combine to make several compounds including
NO , NO 2 , and N 2 O
They all can’t be called nitrogen oxide. How would someone know which one you were talking about? Each of the three compounds has very different properties and reactivity. A system to distinguish between compounds such as these is necessary.
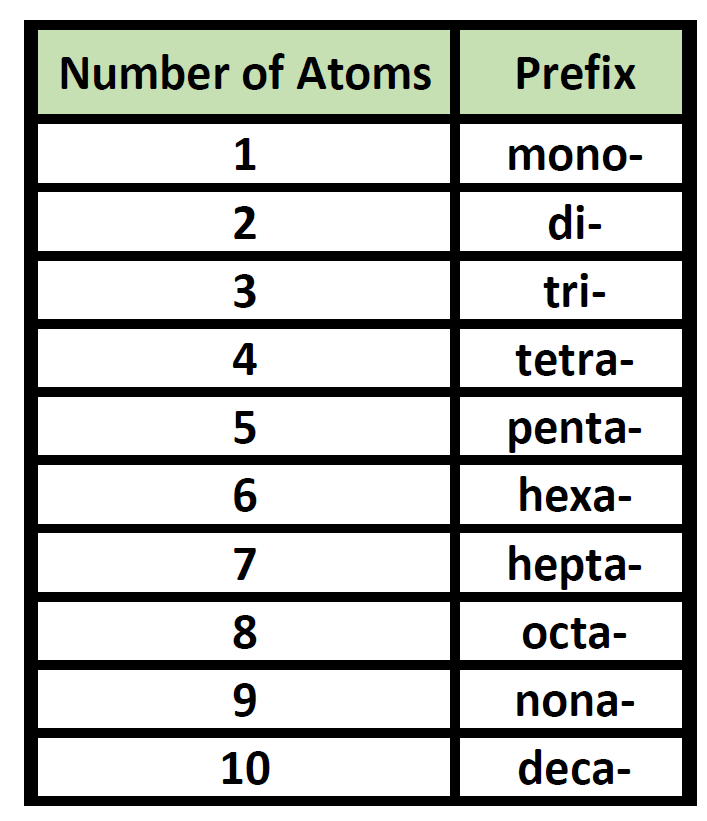
Prefixes are used in the names of binary molecular compounds to identify the number of atoms of each element. The table below shows the prefixes up to ten.
Table 4.2 Prefixes used for Nomenclature of Binary Covalent Molecules
The rules for using the prefix system of nomenclature of binary compounds can be summarized as follows.
- Generally, the less-electronegative element is written first in the formula, though there are a few exceptions. Exception 1 : Carbon is always first in a formula. Exception 2: When hydrogen is participating in a covalent bond, it is typically written in the second postion (For example: hydrogen is after nitrogen in a formula such as NH 3 ) Overall, t he order of common nonmetals in binary molecular compounds is C , P , N , H , S , I , Br , Cl , O ,
- When naming the first element, use the full name of the element and the appropriate prefix if there are more than one atom of that element in the formula. If there is only one atom for the first element, the term mono- is NOT used, but is implied. For example, CO is carbon monoxide, not monocarbon monoxide.
- For the second element the ending of the element’s name is typically changed to ‘ -ide’ and the appropriate prefix is always used for the second element.
Note: the a or o at the end of a prefix is usually dropped from the name when the name of the element begins with a vowel. As an example, four oxygen atoms, is tetroxide instead of tetraoxide. Some examples of molecular compounds are listed in Table 4 .3 .
Table 4.3 Examples of Naming Covalent Molecules
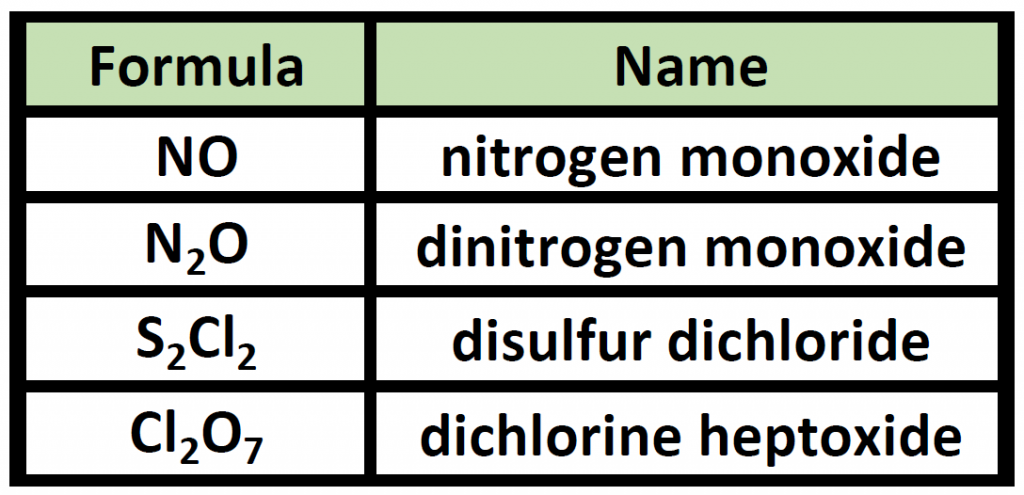
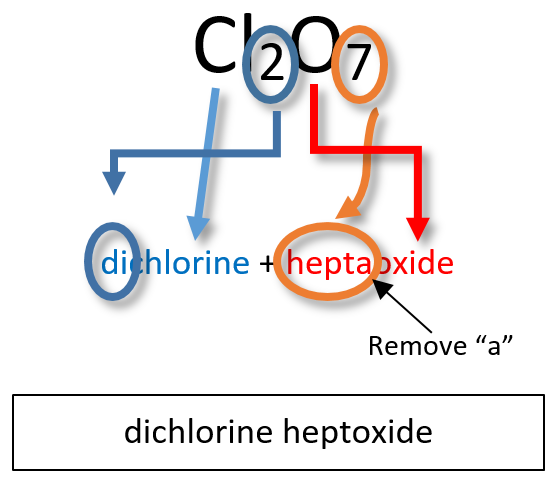
Notice that the mono- prefix is not used with the nitrogen in the first compound, but is used with the oxygen in both of the first two examples. The S 2 Cl 2 emphasizes that the formulas for molecular compounds are not reduced to their lowest ratios. The o of the mono- and the a of hepta- are dropped from the name when paired with oxide. For example:
The Environmental Protection Agency
On December 3rd of 1970 the United States Environmental Protection Agency (EPA) was proposed by President Richard Nixon to be an agency of the federal government charged with protecting human health and the environment. The agency was charged with writing and enforcing regulations based on laws passed by Congress.
The main focus of the EPA as an agency is to conduct research, make environmental assessments, and provide educational materials for use by Congress and the American people regarding environmental and health concerns. The EPA also enforces national standards under a variety of environmental laws, such as the clean air act, and can levy fines, sanctions, and other measures on violators. Within this section, we will learn about creation of the Comprehensive Environmental Response, Compensation, and Liability Act, that is also known as ‘Superfund ‘. The Superfund program was instituted in 1980 and designed to identify and clean up the worst of the hazardous chemical waste sites in the U.S. The first of these sites was the Love Canal described in the next section.
The Love Canal
One of the most famous and important examples of groundwater pollution in the U.S. is the Love Canal tragedy in Niagara Falls, New York (Figure 4.10). It is important because the pollution disaster at Love Canal, along with similar pollution calamities at that time (Times Beach, Missouri and Valley of Drums, Kentucky), helped to create Superfund .

Figure 4.10. Love Canal. Source: US Environmental Protection Agency
Love Canal is a neighborhood in Niagara Falls named after a large ditch (approximately 15 m wide, 3–12 m deep, and 1600 m long) that was dug in the 1890s for hydroelectric power. The ditch was abandoned before it actually generated any power and went mostly unused for decades, except for swimming by local residents. In the 1920s Niagara Falls began dumping urban waste into Love Canal, and in the 1940s the U.S. Army dumped waste from World War II there, including waste from the frantic effort to build a nuclear bomb. Hooker Chemical Company purchased the land in 1942 and lined it with clay. Then, the company put into Love Canal an estimated 21,000 tons of hazardous chemical waste, including the carcinogens benzene, dioxin, and polychlorobisphenols (PCBs) in large metal barrels and covered them with more clay (Figure 4.11).
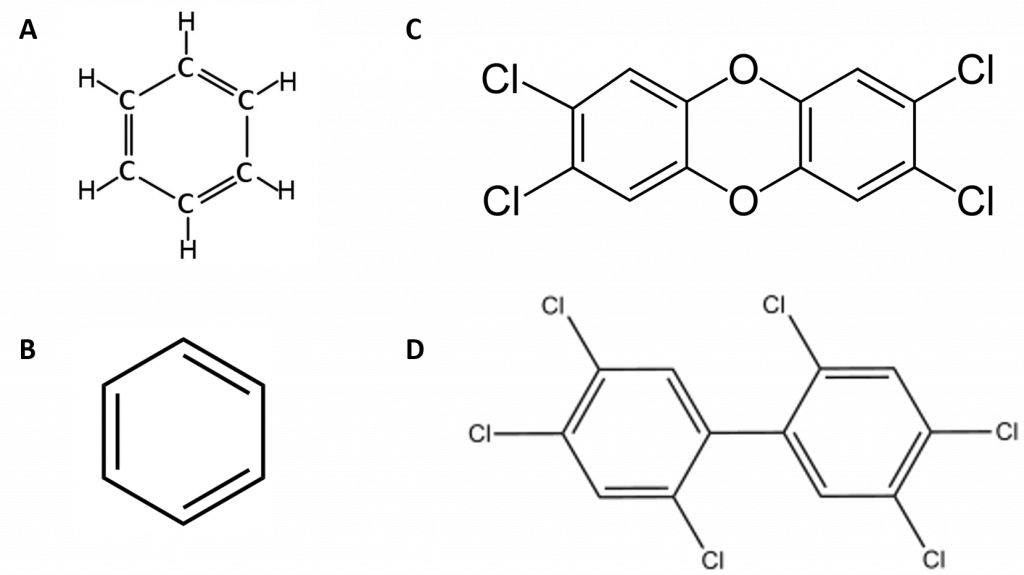
Figure 4.11 Examples of Carcinogens disposed of in the Love Canal. (A) and (B) are representations of Benzene. In (A) all of the atoms in benzene are shown. (B) is a representative line structure of benzene where carbon atoms are indicated at every bend in every line and hydrogen atoms are implied. (C) line structure of dioxin, and (D) is a line diagram of a representative PCB.
In 1953, Hooker sold the land to the Niagara Falls school board for $1, and included a clause in the sales contract that both described the land use (filled with chemical waste) and absolved them from any future damage claims from the buried waste. The school board promptly built a public school on the site and sold the surrounding land for a housing project that built 200 or so homes along the canal banks and another 1,000 in the neighborhood (Figure 4.10). During construction, the canal’s clay cap and walls were breached, damaging some of the metal barrels.
Eventually, the chemical waste seeped into people’s basements, and the metal barrels worked their way to the surface. Trees and gardens began to die; bicycle tires and the rubber soles of children’s shoes disintegrated in noxious puddles. From the 1950s to the late 1970s, residents repeatedly complained of strange odors and substances that surfaced in their yards. Eckardt Beck, the EPA administrator for the region from 1977-1979 recalled in the EPA Journal, “I visited the canal area at that time. Corroding waste-disposal drums could be seen breaking up trough the grounds of backyards. Trees and gardens were turning black and dying. One entire swimming pool had been popped up from its foundation, afloat now on a small sea of chemicals. Puddles of noxious substances were pointed out to be by the residents. Some of these puddles were in their yards, some were in their basements, others yet on the school grounds. Everywhere the air had a faint, choking smell. Children returned from play with burns on their hands and faces. And then there were the birth defects. The New York State Health Department is continuing an investigation into a disturbingly high rate of miscarriages, along with five birth-defect cases detected thus far in the area.” 10
In 1978 President Carter declared a state of emergency at Love Canal, making it the first human-caused environmental problem to be designated that way. The Love Canal incident became a symbol of improperly stored chemical waste. Clean up of Love Canal, which was funded by Superfund and completely finished in 2004, involved removing contaminated soil, installing drainage pipes to capture contaminated groundwater for treatment, and covering it with clay and plastic. In 1995, Occidental Chemical (the modern name for Hooker Chemical) paid $102 million to Superfund for cleanup and $27 million to Federal Emergency Management Association for the relocation of more than 1,000 families. New York State paid $98 million to EPA and the US government paid $8 million for pollution by the Army. The total clean up cost was estimated to be $275 million.
The Love Canal tragedy helped to create Superfund, which has analyzed tens of thousands of hazardous waste sites in the U.S. and cleaned up hundreds of the worst ones. Nevertheless, over 1,000 major hazardous waste sites with a significant risk to human health or the environment are still in the process of being cleaned.
Suggested Assignment:
1. Explore the EPA Superfund Website.
2. Scroll down to the bottom of the page and select ‘Sites Where You Live’
3. Select ‘Your State’ and Show ‘All Sites’.
4. Select a Superfund site within your state and write a 500 word summary describing your chosen site. In the first paragraph, describe the type of toxic waste that is found at the site and what the health and environmental impacts are due to the pollution. In a second paragraphs, describe who is responsible for generating the pollution and what has or is currently being done to restore and clean up the site. Be sure to include the monetary cost of the clean up in your response if it is disclosed and what parties are paying for the clean up.
Atoms can share pairs of valence electrons to obtain a valence shell octet. This sharing of electrons is a covalent bond . A species formed from covalently bonded atoms is a molecule and is represented by a molecular formula , which gives the number of atoms of each type in the molecule. The two electrons shared in a covalent bond are called a bonding pair of electrons . The electrons that do not participate in covalent bonds are called nonbonding pairs (or lone pairs ) of electrons . A covalent bond consisting of one pair of shared electrons is called a single bond .
Covalent bonds occur between nonmetal atoms. Naming simple covalent compounds follows simple rules similar to those for ionic compounds. However, for covalent compounds, numerical prefixes are used as necessary to specify the number of atoms of each element in the compound.
In some cases, more than one pair of electrons is shared to satisfy the octet rule. Two pairs of electrons are shared by two atoms to make a double bond . Three pairs of atoms are shared to make a triple bond . Single, double, and triple covalent bonds may be represented by one, two, or three dashes, respectively, between the symbols of the atoms. In the case of a coordinate covalent bond , one atom supplies both of the electrons and the other atom does not supply any of the electrons.
To judge the relative polarity of a covalent bond, chemists use electronegativity , which is a relative measure of how strongly an atom attracts electrons when it forms a covalent bond. The greater the electronegativity difference between the atoms involved in the covalent bond, the more polarity the bond displays.
In comparison to ionic compounds, covalent molecules tend to have lower melting and boiling points, are less soluble in water, and are poor conductors of electricity. These major differences are largely due to increased polarity of ionic bonds when compared with covalent bonds.
Chapter 4 materials have been adapted from the following creative commons resources unless otherwise noted:
1. Organic Chemistry Portal. WikiUniversity. Available at: https://en.wikiversity.org/wiki/Portal:Organic_chemistry
2. Anonymous. (2012) Introduction to Chemistry: General, Organic, and Biological (V1.0). Published under Creative Commons by-nc-sa 3.0. Available at: http://2012books.lardbucket.org/books/introduction-to-chemistry-general-organic-and-biological/index.html
3. Poulsen, T. (2010) Introduction to Chemistry. Published under Creative Commons by-nc-sa 3.0. Available at: http://openedgroup.org/books/Chemistry.pdf
4. Molecules and Molecular Compounds. (2017) Libretexts. Available at: https://chem.libretexts.org/Textbook_Maps/General_Chemistry_Textbook_Maps/Map%3A_Chemistry%3A_The_Central_Science_(Brown_et_al.)/02._Atoms,_Molecules,_and_Ions/2.6%3A_Molecules_and_Molecular_Compounds
5. Clark, J. (2017) ‘General Principles of Chemical Bonding’ Published by Libretexts. Available at: https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Coordinate_(Dative_Covalent)_Bonding
6. OpenStax (2015) Atoms, Isotopes, Ions, and Molecules: The Building Blocks. OpenStax CNX.Available at: http://cnx.org/contents/be8818d0-2dba-4bf3-859a-737c25fb2c99@12 .
7. Wikipedia, Ionic Compound. Available at: https://en.wikipedia.org/wiki/Ionic_compound
8. Physical and Theoretical Chemistry (2017) Libretexts. Available at: https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Covalent_Bonds_vs_Ionic_Bonds .
9. Lois Gibbs. (1998) Love Canal the story continues. Published by the Center for Health, Environment and Justice. Available at: http://chej.org/wp-content/uploads/Love-Canal-PDF-v1.pdf
10. Beck, E.C. (1979) The Love Canal Tragedy. EPA Journal. Available at: https://archive.epa.gov/epa/aboutepa/love-canal-tragedy.html
- CH104 – Chapter 1: Measurements in Chemistry
- CH104 – Chapter 2: Atoms and The Periodic Table
- CH104: Chapter 3 – Ions and Ionic Compounds
- CH104: Chapter 4 – Covalent Bonds and Molecular Compounds
- CH104: Chapter 5 – Chemical Reactions
- CH104: Chapter 6 – Quantities in Chemical Reactions
- CH104: Chapter 7 – Solutions

WESTERN OREGON UNIVERSITY 345 Monmouth Ave. N. Monmouth OR 97361
503-838-8000 | 1-877-877-1593
Campus Maps Canvas Find People Portal Virtual Tour WOU Email Technical Support
A-Z Index Accessibility Academic Calendar Class Schedule Jobs at WOU Partnerships Student Services
Western Oregon University’s Land Acknowledgement Western Oregon University in Monmouth, OR is located within the traditional homelands of the Luckiamute Band of Kalapuya. Following the Willamette Valley Treaty of 1855 (Kalapuya etc. Treaty), Kalapuya people were forcibly removed to reservations in Western Oregon. Today, living descendants of these people are a part of the Confederated Tribes of Grand Ronde Community of Oregon and the Confederated Tribes of the Siletz Indians .
Accessibility Public Records Privacy Student Consumer Information
WOU prohibits discrimination on the basis of race, color, sex, national or ethnic origin, age, religion, marital status, disability, veteran status, sexual orientation, gender identity, and gender expression in all programs, activities and employment practices as required by Title IX, other applicable laws, and policies. Retaliation is prohibited by WOU.

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Covalent Bond Definition and Examples

A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Usually, sharing electrons gives each atom a full valence shell and makes the resulting compound more stable than its constituent atoms are on their own. Covalent bonds usually form between nonmetals . Examples of covalent compounds include hydrogen (H 2 ), oxygen (O 2 ), carbon monoxide (CO), ammonia (NH 3 ), water (H 2 O), and all organic compounds . There are compounds that contain both covalent and ionic bonds , such as potassium cyanide (KCN) and ammonium chloride (NH 4 Cl).
What Is a Covalent Bond?
Covalent bonding is one of the main types of chemical bonds , along with ionic and metallic bonds. Unlike these other bonds, covalent bonding involves the sharing of electron pairs between atoms. These shared electrons exist in the outer shell of the atom, the so-called valence shell .
The water molecule (H 2 O) is an example of compound with covalent bonds. The oxygen atom shares one electron with each of the two hydrogen atoms, forming two covalent bonds.
Octet Rule and Covalent Bonding
The concept of covalent bonding ties in with the octet rule . This rule states that atoms combine in such a way that each atom has eight electrons in its valence shell, resembling the electronic configuration of a noble gas . By sharing electrons through covalent bonding, atoms effectively fill their outer shells and satisfy the octet rule.
Covalent Bond vs Ionic and Metallic Bonds
Covalent bonds differ significantly from ionic and metallic bonds . Ionic bonds form when one atom gives up one or more electrons to another atom, forming ions that attract each other due to their opposite charges. Sodium chloride (NaCl) is an example of a compound with ionic bonds.
Metallic bonds, on the other hand, form between metal atoms. In these bonds, electrons are not shared or transferred between atoms but instead move freely in what is sometimes referred to as an “electron sea”. This fluidity of electrons gives metals their unique properties, such as electrical conductivity and malleability.
Types of Covalent Bonds
Covalent bonds are either polar covalent bonds or nonpolar covalent bonds.
A nonpolar covalent bond forms when two atoms with the same electronegativity share electrons equally, as in a molecule of hydrogen gas (H 2 ).
A polar covalent bond, on the other hand, forms when the atoms involved in the bond have different electronegativities, resulting in unequal sharing of electrons. The atom with the higher electronegativity pulls the shared electrons closer, creating a region of slight negative charge, while the other atom becomes slightly positive. An example is water (H 2 O), where the oxygen atom is more electronegative than the hydrogen atoms.
Electronegativity and the Type of Bonding
Electronegativity is a measure of an atom’s tendency for attracting a bonding pair of electrons. The electronegativity values, proposed by Linus Pauling, range from around 0.7 to 4.0. The higher the electronegativity, the greater an atom’s attraction for bonding electrons.
When considering whether a bond is ionic or covalent, the difference in electronegativity between the two atoms a helpful guideline.
- If the electronegativity difference is greater than 1.7, the bond is ionic. This is because the more electronegative atom attracts the electron(s) so strongly that it effectively “steals” them from the other atom.
- If the electronegativity difference is less than 1.7 but greater than 0.5, the bond is polar covalent. The atoms do not share electrons equally. The more electronegative atom attracts the electron pair. This leads to a separation of charge, with the more electronegative atom carrying a slight negative charge and the other atom a slight positive charge.
- If the electronegativity difference is less than 0.5, the bond is nonpolar covalent. The atoms share the electron pair more or less equally.
However, these are just guidelines and there is no absolute cut-off value that cleanly separates ionic and covalent bonds. In reality, many bonds falling somewhere in between. Also, electronegativity is not the only factor that determines the type of bond formed. Other factors also play a role, including the size of the atoms, the lattice energy, and the overall structure of the molecule.
Single, Double, and Triple Bonds
Covalent bonds exist as single, double, or triple bonds. In a single covalent bond, two atoms share one pair of electrons. Hydrogen gas (H 2 or H-H) has a single covalent bond, where each hydrogen atom shares its single electron with the other.
In a double bond, atoms share two pairs of electrons. A typical example is oxygen gas (O 2 or O=O), where each oxygen atom shares two electrons with the other. A double bond is stronger than a single bond, but less stable.
Triple bonds involve the sharing of three pairs of electrons, as seen in nitrogen gas (N 2 or N≡N). The triple bond is strongest, yet least stable.
Properties of Covalent Compounds
Compounds that have covalent bonds often share several common properties .
- Low Melting and Boiling Points : Covalent compounds generally have lower melting and boiling points than ionic bonds due to the weaker forces of attraction between molecules.
- Poor Conductivity : Most covalent compounds do not conduct electricity because they lack free-moving charges (such as ions or delocalized electrons) that are necessary for the flow of electrical current. There are exceptions, such as graphite, which conducts electricity due to the delocalization of its electrons. Thermal conductivity varies widely among covalent compounds. For example, diamond, a form of carbon with each carbon atom covalently bonded to four other carbon atoms, is one of the best known thermal conductors. In contrast, many other covalently bonded substances, like water or polymers, are relatively poor thermal conductors.
- Insolubility in Water : Many covalent compounds are nonpolar and are not soluble in water. Water and ethanol are examples of polar covalent compounds that do dissolve ionic compounds and other polar compounds.
- Solubility in Organic Solvents : While nonpolar covalent compounds don’t dissolve well in water, they often dissolve well in organic solvents like benzene or in nonpolar solvents such as carbon tetrachloride. This is due to the ‘like dissolves like’ principle, where polar substances dissolve polar substances, and nonpolar substances dissolve nonpolar substances.
- Lower Density : Covalent compounds generally have lower densities than ionic compounds. This is because the atoms in covalently bonded substances are not packed as closely together as in ionic substances. As a result, they are lighter for their size.
- Brittle Solids : When covalent compounds do form solids, they are generally brittle. They are not ductile or malleable. This is due to the nature of their bonds. If a layer of atoms gets shifted, it disrupts the network of covalent bonds and the substance breaks.
- Atkins, Peter; Loretta Jones (1997). Chemistry: Molecules, Matter and Change . New York: W.H. Freeman & Co. ISBN 978-0-7167-3107-8.
- Langmuir, Irving (1919). “The Arrangement of Electrons in Atoms and Molecules”. Journal of the American Chemical Society . 41 (6): 868–934. doi: 10.1021/ja02227a002
- Lewis, Gilbert N. (1916). “The Atom and the Molecule”. Journal of the American Chemical Society . 38 (4): 772. doi: 10.1021/ja02261a002
- Pauling, Linus (1960). The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry . ISBN 0-801-40333-2. doi: 10.1021/ja01355a027
- Weinhold, F.; Landis, C. (2005). Valency and Bonding . Cambridge University Press. ISBN 0521831288.
Related Posts

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

Chemical Bonding (Worksheet)
- Last updated
- Save as PDF
- Page ID 3142

- Richard Banks
- Boise State
Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Work in groups on these problems. You should try to answer the questions without referring to your textbook. If you get stuck, try asking another group for help.
Learning Objectives
- Be able to define covalent bonds, polar covalent bonds, ionic bonds, electronegativity, dipoles, formal charge, molecular formula, structural formula and electron-dot formula.
- Be able to recognize whether the type of bond between two atoms is covalent, polar covalent or ionic.
- Be able to draw electron-dot formulas for simple molecules.
- Be able to calculate the charge on an atom given the electron-dot formula.
Bond Classifications
Chemical bonds are the attractive forces that hold atoms together in the form of compounds. A chemical bond is formed when electrons are shared between two atoms. There are three types of bonds: covalent bonds , polar covalent bonds and ionic bonds . The simplest example of bonding is demonstrated by the H 2 molecule. We can see that each hydrogen atom has a single electron from the periodic table. If two hydrogen atoms come together to form a bond, then each hydrogen atom effectively has a share in both electrons and thus each resembles an inert gas structure. The two electrons that are shared are considered to be a chemical bond and can be represented with either two dots or a single dash between the two bonded atoms.
Covalent Bond: A covalent bond is formed when there is an equal sharing of electrons between two atoms. The atoms forming a covalent bond must have relatively equal attraction for the electrons. The bonds between the carbon atom and the hydrogen atoms in the compound methane CH 4 are examples of covalent bonds between two different elements.
Polar covalent bond: If the atoms differ in their attraction for electrons, a polar-covalent bond will be formed. Water is an example of a molecule with this type of bond.
The attraction of electrons to the nucleus of an atom is a very important property of atoms and is called electronegativity . The difference in electronegativity between bonded atoms determines the bond polarity , which is determined by the relative difference in electronegativity between two atoms that are joined together by a chemical bond. A dipole results from an unequal distribution of the electron pair in the bond between the atoms.
Ionic bonds: An ionic bond is formed when an electron is essentially transferred from one atom of a pair to the other creating ions. Sodium chloride is an example of a compound with this type of bond. These compounds exist as a crystal lattice with all the ions stacked in definite patterns. The following picture is a representation of a cubic crystal of sodium chloride. The chloride ions are represented by the purple spheres.
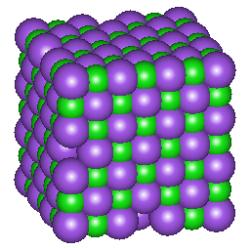
Remember that a cation is a positively charged atom or molecule (one that has lost one or more electrons) and that an anion is a negatively charged atom or molecule (one that has gained one or more electrons). The ions are held together by electrostatic attractions. Ionic compounds have high melting points and many are soluble in water.
Most compounds that contain metals are ionic. For the purpose of this course, you may assume that all compounds that contain metals are ionic. If a compound contains the ammonium ion, it will be ionic. That is why the compound ammonium nitrate is written as NH 4 NO 3 rather than N 2 H 4 O 3 . This alerts people to the fact that the compound contains the ammonium group and the nitrate group.
Methods of Showing Formulas of Compounds
- Molecular formulas show only the type and number of atoms in a molecule.
- Structural formulas show the atoms in their correct placement in the molecule.
- Electron-dot formulas are similar to structural formulas but also include all of the non-bonding outer electrons. Knowledge of electron placement aids in understanding how molecules and elements react with one another.
Drawing Electron-Dot Formulas
Step 1: Sum up the total number of valence electrons for the elements in the molecule. If the molecule is charged, subtract one electron for each positive charge or add one electron for each negative charge.
Step 2: Write the structure for the molecule with a pair of electrons (or a dash) between each atom. Groups of atoms will usually have the less electronegative atom surrounded by atoms having greater electronegativity. Never place a hydrogen atom in the center since it can only form one bond. Think about the valence of each atom and make sure that these valences are not exceeded except in the case of ions. Ions always have more or less bonds than the normal uncharged atom. The common valence or number of bonds formed for some common atoms are:
- O and S = 2
- F, Cl, Br and I = 1
Step 3: Place electrons around the outer atoms to fill their outer shells. Most atoms require eight electrons (the octet rule) so they will resemble an inert gas. Elements in Group I to Group 13 often do not follow the octet rule and have less than eight electrons in the final formula.
Step 4: Subtract the number of electrons used so far from the total calculated in Step 1 and place these remaining electrons on the central atom or atoms.
Step 5: If the central atom ends up with less than 8 electrons, then it probably forms a multiple bond with an adjacent outer atom.
Step 6: Finally you should calculate the formal charge on the atoms.
Example 1: Methane CH 4
The first electron-dot formula we shall draw will be methane, CH 4 .
Step 1 : The first thing that you must do is to determine the number of electrons available for the formula.
- Hydrogen is a Group 1 element and thus has 1 valance electron. Since there are 4 hydrogen atoms, the total for hydrogen will be 4 electrons.
- Carbon is in Group 14 element thus has 4 outer electrons (remember that we don't use the atomic number for electron-dot formulas).
The total number of electrons for all atoms is 8.
Step 2: The next step is to determine which atom will be in the center of the molecule. Hydrogen atoms are only able to form one bond to other atoms so we need to put the carbon atom in the center.
Step 3: The next step requires drawing a "C" and "H" and place 2 electrons between these atoms.
Step 4: All you have to do now is draw all of the other hydrogen atoms around the carbon atom and place 2 electrons between these hydrogen atoms and the carbon atom. This gives you a total of 8 electrons and you have drawn the electron-dot formula for methane.
Step 5: A full octet. No further effort needed.
Step 6 : Calculating the Formal Charges for elements in methane.
The formal charge for each hydrogen atom is calculated by the following calculation...
\[f_c = 1 - \dfrac{1}{2}(2) -0 =0 \nonumber \]
The first number represents the Roman Numeral over the periodic table column. The number "2" in the parentheses represents the shared electrons and there are no non-bonding electrons so the last number is zero.
The formal charge for the carbon atom is calculated by the following calculation...
\[f_c = 4- \dfrac{1}{2}(8) -6 =1 \nonumber \]
Example 2: Water H 2 O
Then let's try a compound that has non-bonding electron pairs: water.
- Hydrogen is a Group 1 element and thus has 1 valance electron. Since there are 2 hydrogen atoms, the total for hydrogen will be 2 electrons.
- Oxygen is a Group 16 element thus has 6 outer electrons (remember that we don't use the atomic number for electron-dot formulas).
Step 2: The next step is to determine which atom will be in the center of the molecule. Hydrogen atoms are only able to form one bond to other atoms so we need to put the oxygen atom in the center.
Step 3: The next thing that you must do is to draw an "O" and "H" and place 2 electrons between these atoms.
Step 4: Now repeat this with the second "H". Don't worry about where these atoms are positioned around the oxygen atom, it does not matter for this representation.
Step 5: Now all you have to do is place the remaining 4 electrons on the oxygen atom. The hydrogen atoms can only share in 2 electrons so don't put more electrons around them. This gives you a total of 8 electrons and you have drawn the electron-dot formula for water.
Step 6 : Calculating the Formal Charge for atoms in water.
The formal charge for the hydrogen atom is calculated by the following calculation...
The formal charge for the oxygen atom is calculated by the following calculation...
\[f_c = 6- \dfrac{1}{2}(2) -4 =1 \nonumber \]
Example 3: Hydroxide Ion
The next example will be an ion with a negative charge, the hydroxide ion, \(\ce{HO^{-}}\).
Step 1: The first thing that you must do is to determine the number of electrons available for the formula?
- Hydrogen is is a Group 1 element and thus has 1 electron.
- Oxygen is a Group 16 element and thus has 6 outer electrons.
- We must add one electron for the negative charge.
The total number of electrons for both atoms including the extra electron for the negative charge is 8.
Step 2: Draw the hydrogen atom next to the oxygen atom and place a pair of electrons between the atoms.
Step 3: The last thing needed to complete the formula is to place the remaining 6 electrons around the oxygen atom.
Step 6 : Calculating the Formal Charge for the Hydroxide Ion, \(\ce{HO^{-}}\).
\[f_c = 6- \dfrac{1}{2}(2) -6 =1 \nonumber \]

Example 4: Ammonium Ion
Now let's see what happens when we have a positive charge and draw the ammonium ion, NH 4 + .
Step 1 : The first thing that you must do is to determine the number of electrons available for the formula?
- Hydrogen is a Group 1 element and thus has 1 electron. Since there are 4 hydrogen atoms, we have 4 electrons.
- Nitrogen is is a Group 15 element and thus has 5 outer electrons.
- We must subtract one electron for the positive charge.
The total number of electrons for all atoms including the loss of an electron for the positive charge is 8.
Steps 2-5: Draw the nitrogen atom in the center and place the 4 hydrogen atoms around it. Draw a pair of electrons between each pair. This actually finishes the formula. Notice that this structure looks exactly like the structure for methane except that the center atom is a nitrogen atom. When two structures have identical electron-dot formulas, they are considered to be isoelectronic .
The formal charge for each of the atoms is calculated by the following calculation:
The formal charge for the nitrogen atom is calculated by the following calculation:
\[f_c = 5- \dfrac{1}{2}(8) -6 = +1 \nonumber \]
Example 5: Molecular Oxygen
Finally, let's draw a compound that requires multiple bonds and draw the formula for oxygen, O2.
- Oxygen is a Group 16 element and thus has 6 outer electrons...since there are 2 oxygen atoms, there will be a total of 12 electrons.
Step 2: Draw the 2 oxygen atoms with a pair of electrons between them.
Step 3: The next thing that you must do is to draw the remaining electrons in pairs around each of the oxygen atoms giving neither atom more than 8 total electrons.
Step 4: The oxygen atom on the right has 8 electrons but the oxygen atom on the left has only 6 electrons. The only way to correct this problem is to create a multiple bond between the oxygen atoms. Take a pair of electrons from the oxygen atom on the right and place this pair between the 2 atoms.
The formal charge for each oxygen atom is calculated by the following calculation...
\[f_c = 6 - \dfrac{1}{2}(2) - 4 = 0 \nonumber \]
- Define a covalent bond.
- Define a polar-covalent bond.
- Define an ionic bond.
- Define electronegativity. Why does electronegativity increase going from left to right in a row in the periodic table?
- Why does electronegativity increase going from bottom to top in a column in the periodic table?
Describe the type of bond represented by the dash for each of the following compounds using the definitions in Q1. Consider only the atoms to which the dash is directly connected.
- H–CH 3
- H–NH 2
- Na–ONa
- Na–Br
- F–CH 3
Draw the electron-dot formulas for the following compounds or ions and calculate the formal charges for every atom.
Chemical Bonds in Compounds Quiz
Self-Test for Bonds, Electron Transfer, and Compounds
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
- shared equally
- shared unequally
An example would be the bond between two hydrogen atoms to form hydrogen gas.
The alkaline earths are the second group (column) in the periodic table. Atoms of these elements form ions with a +2 charge. Knowing the usual valence of elements in a group helps when it comes to predicting the compounds that can be formed.
- iron chloride
- iron (I) chloride
- iron (II) chloride
- iron (III) chloride
Transition metals can have different valences, so it's important to list the valence in a compound name.
- covalent, dinitrogen tetroxide
- covalent, nitrogen tetroxide
- ionic, nitrogen oxide
- ionic, dinitrogen oxide
Nonmetals form covalent bonds with each other. Rather than call any combination of nitrogen and oxygen "nitrogen oxide", you should specify how many atoms of each type are present.
- polar covalent
- nonpolar covalen
Nonmetals form covalent bonds. Since the electronegativity values are not identical, you know it's a polar bond.
Answer this by looking up the atomic number (number of protons) on the periodic table. The number of protons and electrons is not the same, so you're dealing with an ion. If there are more protons than electrons, there's a net positive charge. If there are more electrons than protons, it's an ion with a negative charge.
- MgNO₃
- Mg₂NO₃
- Mg(NO₃)₂
- Mg₂(NO₃)₃
Polyatomic ions are groups of atoms bound together that act as a cation or anion to form bonds and make compounds. To answer this question, you need to know the formula of a nitrate .
The tri- prefix in the name means "three".
- loses 2 electrons to form a magnesium ion with a 2- charge
- gains 2 electrons to form a magnesium ion with a 2- charge
- loses 2 electrons to form a magnesium ion with a 2+ charge
- gains 2 electrons to form a magnesium ion with a 2+ charge
Magnesium and other alkaline earth atoms form cations with a 2+ charge. To get that positive charge, you need 2 more protons than electrons.
The electron dot diagram shows how many lone electron pairs are present, which is similar to the valence on the atom.
:max_bytes(150000):strip_icc()/scientist-holding-molecular-model-in-laboratory-660493183-57e01d945f9b586516829442.jpg)
You're on track to learn more about chemical bonds and how they work. Your biggest friend when it comes to understanding chemical bonding is the periodic table because it's organized to group elements with similar charges together (for example, all of the alkali metals carry a +1 charge). Electronegativity is a periodic table trend . Atoms with the same electronegativity form nonpolar covalent bonds. Atoms with similar but not identical electronegativity (two different nonmetals) form polar covalent bonds. When the electronegativity difference is large (think metals with nonmetals), you get ionic bonds.
When you balance chemical formulas, remember the electrical charges cancel out. So, if you have two positive charges, you form a neutral compound if it bonds with two negative charges.
From here, you might want to review the types of chemical bonds and how chemical formulas work . If you're ready for another quiz, see if you understand the basics about atoms and their parts .
:max_bytes(150000):strip_icc()/molecular-structure-547999397-57e01bff5f9b58651682623b.jpg)
Bravo! You understand how chemical bonds form and how electrons are transferred or shared to form ions and compounds. If you're ever in doubt about the type of bonds formed between atoms, look at their position on the periodic table. Atoms with the same electronegativity (like two oxygen atoms) form nonpolar covalent bonds. Atoms with close electronegativity values (like two nonidentical nonmetals) form polar covalent bonds. If the electronegativity difference is large (between a metal and a nonmetal) then ionic bonds form.
From here, you can test yourself to see if you know the trends in the periodic table or you might wish to review the types of chemical bonds .
If you're ready for another quiz, find out which type of mad scientist you are or you can practice naming ionic compounds .
- Why Do Atoms Create Chemical Bonds?
- What Are Some Examples of Covalent Compounds?
- What Is a Covalent Bond in Chemistry?
- The Main Types of Chemical Bonds
- Examples of Ionic Bonds and Compounds
- Compounds With Both Ionic and Covalent Bonds
- Polar Bond Definition and Examples
- Predicting Formulas of Compounds with Polyatomic Ions
- Ionic vs Covalent Bonds - Understand the Difference
- What Type of Bonds Does Carbon Form?
- Atoms and Atomic Theory - Study Guide
- Formulas of Ionic Compounds
- Types of Chemical Bonds in Proteins
- Predicting Formulas of Ionic Compounds
- Bonds Definition in Chemistry
- Electronegativity and Chemical Bonding
Structural Formulas for C 4 H 10 O Isomers Kekulé Formula Condensed Formula Shorthand Formula
Constitutional isomers have the same molecular formula, but their physical and chemical properties may be very different. For an example Click Here .
Our ability to draw structural formulas for molecules is remarkable. To see how this is done Click Here .
The manner in which atomic orbitals overlap to form molecular orbitals is actually more complex than the localized examples given above. These are useful models for explaining the structure and reactivity of many organic compounds, but modern molecular orbital theory involves the creation of an orbital correlation diagram . Two examples of such diagrams for the simple diatomic elements F 2 and N 2 will be drawn above when the appropriate button is clicked. The 1s and 2s atomic orbitals do not provide any overall bonding, since orbital overlap is minimal, and the resulting sigma bonding and antibonding components would cancel. In both these cases three 2p atomic orbitals combine to form a sigma and two pi-molecular orbitals, each as a bonding and antibonding pair. The overall bonding order depends on the number of antibonding orbitals that are occupied. The subtle change in the energy of the σ 2p bonding orbital, relative to the two degenerate π-bonding orbitals, is due to s-p hybridization that is unimportant to the present discussion.
One example of the advantage offered by the molecular orbital approach to bonding is the oxygen molecule . Here, the correlation diagram correctly accounts for the paramagnetic character of this simple diatomic compound. Likewise, the orbital correlation diagram for methane provides another example of the difference in electron density predicted by molecular orbital calculations from that of the localized bond model. Click on the compound names for these displays.
This page is the property of William Reusch. Comments, questions and errors should be sent to [email protected] . These pages are provided to the IOCD to assist in capacity building in chemical education. 05/05/2013
Have you ever wondered how atoms are held together? In this simulation, you will learn the basics about atomic bonding in ionic and covalent compounds and how to distinguish those compounds experimentally. With all this knowledge, you will help your friend analyze two mysterious substances he received from an alchemist. By testing their specific physical properties, you’ll be able to identify the nature of the substances. Finally, you will learn how ions form lattice structures and how this influences the property of a compound.
Test solubility and conductivity
Atoms can interact in many different ways, giving compound-specific properties. In the first mission of the Ionic and Covalent Bonds simulation, your task is to choose appropriate laboratory equipment to test the solubility and conductivity of the two substances. You will explore how these properties differ in ionic and covalent compounds.
Draw Lewis dot structures
In the second part of the Ionic and Covalent Bonds simulation, you will learn about the octet rule and how to apply this to building Lewis dot structures in a virtual drawing activity. You will see that there are many ways that covalent bonds can be formed, depending on the compound and electron configuration. You will also learn how to identify lone electron pairs in a covalent bond.
Determine melting points
In the last mission, your task is to determine the melting point of the two substances by using a melting point apparatus. You will explore how your results are connected to the ability of ions to form a lattice structure.
Will you be able to identify the two mysterious substances and help your friend cure his migraine?
Ionic and Covalent Bonds | Virtual Lab

Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
Unordered list
Superscript
About This Simulation
Join your friend on a quest to analyze two mysterious substances he got from an alchemist to cure his migraine and learn how atoms connect.
Learning Objectives
- Describe the formation of ionic and covalent bonds
- Identify anions and cations
- Apply the octet rule
- Describe ionic lattice structure
- Explain the formation of single, double, and triple bonds
- Distinguish between ionic compounds and covalent compounds

Lab Techniques
- Conductivity measurement
- Melting point determination
Related Standards
- 2.3. Structure of Ionic Solids
- 2.5 Lewis Diagrams
- 2.1 Types of Chemical Bonds
- 4.1 Ionic bonding and structure
- 4.3 Covalent structures
- 4.2 Covalent bonding
Learn More About This Simulation
Experience labster for yourself.
75% of students show high engagement and improved grades with Labster
Easily bolster your learning objectives with relevant, interactive content
Practice a lab procedure or visualize theory through narrative-driven scenarios

Related Course Packages
Organic chemistry, intro chemistry (100), general, organic, and biological chemistry i, general chemistry (101 and 102), +35 other courses, related simulations.

Ionic and Covalent Bonds

Homeostatic Control: How does the human body keep itself in balance?
Ever wondered how your body constantly regulates itself to stay healthy? Visit the Homeostatic Control lab to learn all about the concept of homeostasis and how it can be applied to a wide range of systems, from blood pressure to body temperature.

Thin Layer Chromatography: Separate a mixture and monitor a reaction's progress
Discover the intermolecular interactions involved in Thin Layer Chromatography. Utilize this newfound knowledge to assemble, run, and analyze a TLC experiment to monitor the progress of a reaction.
For Science Programs Providing a Learning Advantage

“They did the simulation at home, then completed the in-person lab within 30 minutes, no questions asked, and passed the quiz with flying colors.”

"I saw some of the students who clearly didn’t necessarily like sitting there reading a book discover they could turn on Labster and keep up with the rest of the class because it spoke to them.

"Having something that's engaging for the students gives teachers that opportunity to breathe and get excited again. Because they're seeing the kids light up, they're seeing the kids engage with content."

"The question always is, ‘Can we demonstrate that the students are meeting course outcomes?’ Check! We can do that.”

"We surveyed over 400 students. More than 90% thought Labster was easy to navigate, and that it was fun, but more importantly, most of them felt confident that they could execute the labs in person. And that confidence is a big deal."

“The Labster simulations get students to do things, and they're not just sitting there consuming a webinar where their mind can drift. They become an active participant in that learning experience.”

Find answers to frequently asked questions.
Labster is hosted online, which means that students only have to login from their internet browsers once an account is created.
Labster is only available for purchase by faculty and administration at academic institutions. To procure Labster, simply reach out to us on our website. Schedule a demo, book a meeting to discuss pricing, start a free trial, or simply fill out our contact form.
Labster simulations are created by real scientists and designed with unparalleled interactivity. Unlike point and click competitors, Labster simulations immerse students and encourage mastery through active learning.
Labster supports a wide range of courses at the high school and university level across fields in biology, chemistry and physics. Some simulations mimic lab procedures with high fidelity to train foundational skills, while others are meant to bring theory to life through interactive scenarios.
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
High school chemistry
Course: high school chemistry > unit 5.
- London dispersion forces introduction
- Dipole–dipole forces
Hydrogen bonding
- Understand: intermolecular forces
- Apply: intermolecular forces
Want to join the conversation?
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

Video transcript
KEY - Introduction to Ionic & Covalent Bonding
Related documents

Study collections
- noah durbin
Add this document to collection(s)
You can add this document to your study collection(s)
Add this document to saved
You can add this document to your saved list
Suggest us how to improve StudyLib
(For complaints, use another form )
Input it if you want to receive answer

COMMENTS
Covalent compounds do not have strong attractions between molecules and so have very low melting points, unlike ionic compounds. Does your explanation contain the following? Check all of them. Study with Quizlet and memorize flashcards containing terms like Identify arrows pointing to bonding electrons., Identify arrows pointing to nonbonding ...
Chemical reactions result in the gain, loss, or rearrangement of valence electrons. For main group elements, the number of valence electrons equals eight minus the element's group number. Core electrons are not involved in bonding or in chemical reactions. a. 1 only b. 2 only c. 3 only d. 1 and 3 e. 1, 2, and 3. Answer.
Covalent bonding is an important and extensive concept in chemistry, and it will be treated in considerable detail in a later chapter of this text. We can often identify molecular compounds on the basis of their physical properties. Under normal conditions, molecular compounds often exist as gases, low-boiling liquids, and low-melting solids ...
Chapter 4 - Covalent Bonds and Molecular Compounds. Chemical bonds are generally divided into two fundamentally different types: ionic and covalent. In reality, however, the bonds in most substances are neither purely ionic nor purely covalent, but lie on a spectrum between these extremes. Although purely ionic and purely covalent bonds ...
Note: We have just defined a molecule to be any distinct group of atoms bound together with covalent bonds. However, a few teachers and textbooks define a molecule more narrowly. According to this less common definition, a molecule is a group of covalently bound atoms of the same element only.In the rest of this article, we will stick to the broader definition of molecule because it is more ...
Which scenario would cause a covalent bond to form? Choose 1 answer: Choose 1 answer: (Choice A) A hydrogen atom with a slight positive charge is attracted to a negative charge of another molecule or atom. A. ... The constant motion of electrons and the creation of charge imbalances bonds two molecules together. C.
34) A _____ covalent bond between the same two atoms is the longest. A) single B) double C) triple D) they are all the same length. E) strong 35) As the number of covalent bonds between two atoms increases, the distance between the atoms _____ and the strength of the bond between them _____. A) increases, increases
A covalent bond is a type of chemical bond characterized by two atoms sharing valence electrons. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Usually, sharing electrons gives each atom a full valence shell and makes the resulting compound more stable than its constituent atoms are on their own.
So the difference in electronegativity is somewhere between 1.5 and 2.1, between a polar covalent bond and an ionic bond. So most textbooks we'll see approximately somewhere around 1.7. So if you're higher than 1.7, it's generally considered to be mostly an ionic bond. Lower than 1.7, in the polar covalent range.
Chemical bonds are the attractive forces that hold atoms together in the form of compounds. A chemical bond is formed when electrons are shared between two atoms. There are three types of bonds: covalent bonds, polar covalent bonds and ionic bonds. The simplest example of bonding is demonstrated by the H 2 molecule. We can see that each ...
Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. The binding arises from the electrostatic attraction of their nuclei for the same electrons. A bond forms when the bonded atoms have a lower total energy than that of widely separated atoms.
PART 3: Use Lewis dot structures to show the covalent bonding in the following pairs of elements. Once you have determined the structure for the molecule, write its structural formula in the space provided; use a dash to represent a shared pair of electrons, and dots to show unshared electrons.
Student Exploration: Covalent Bonds. Vocabulary: covalent bond, diatomic molecule, Lewis diagram, molecule, noble gases, nonmetal, octet rule, shell, valence, valence electron. Prior Knowledge Questions (Do these BEFORE using the Gizmo.) There are eight markers in a full set, but Flora and Frank each only have seven markers.
7. Ionic compounds may contain polyatomic ions. For example, the formula of magnesium nitrate is: MgNO₃. Mg₂NO₃. Mg (NO₃)₂. Mg₂ (NO₃)₃. Polyatomic ions are groups of atoms bound together that act as a cation or anion to form bonds and make compounds. To answer this question, you need to know the formula of a nitrate.
A compound containing covalent bonds. Covalent compounds have these properties. This is equal to the number of electrons gained, lost or shared by an atom during bonding. This family of elements never exists as ions or in molecules. Two or more atoms bonded together ...
Consider a hydrocarbon with a molecular structure consisting of a simple chain of four carbon atoms, CH 3 CH 2 CH 2 CH 3. The molecular formula is C 4 H 10 (the maximum number of bonded hydrogens by the 2n + 2 rule). If the four carbon atoms form a ring, two hydrogens must be lost.
Ionic bond: when two atoms (metal and non-metal) have opposite charges then transfers electrons. A chemical compound with an ionic bond will be held together with an electrostatic force. b. Covalent bond: when two atoms (both non-metal) of the same charges will share electrons to equal 8 electrons (octate rule).
You will see that there are many ways that covalent bonds can be formed, depending on the compound and electron configuration. You will also learn how to identify lone electron pairs in a covalent bond. Determine melting points. In the last mission, your task is to determine the melting point of the two substances by using a melting point ...
11. Contrast sigma bonds and pi bonds. A sigma bond is a single covalent bond formed from the direct overlap of orbitals. A pi bond is the parallel overlap of p orbitals. 12. Apply Create a graph using the bond- dissociation energy data in Table 8.2 and the bond-length data in Table 8.1.
Hydrogen bonding. Hydrogen bonding is a special type of dipole-dipole interaction that occurs between the lone pair of a highly electronegative atom (typically N, O, or F) and the hydrogen atom in a N-H, O-H, or F-H bond. Hydrogen bonds can form between different molecules (intermolecular hydrogen bonding) or between different parts of ...
Silicon crystal lattice with one broken bond. It requires energy to break a covalent bond. The required energy is called the "bandgap" (bandgap of Silicon is ~1.12 eV) A broken bond results in one negatively charged "free electron" and one positively charged "hole". The "free electron" can freely move around in the crystal.
KEY - Introduction to Ionic & Covalent Bonding. 1. Define ionic bond: Chemical bond where electron(s) are transferred from a cation (usually a metal) to an anion (a nonmetal or polyatomic). The resulting opposite charges attract and the bond gives the atoms involved a full octet. 2.
FlexBook Platform®, FlexBook®, FlexLet® and FlexCard™ are registered trademarks of CK-12 Foundation.