An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Respir Med Case Rep

Case report: Three adult brothers with cystic fibrosis (delF508-delF508) maintain unusually preserved clinical profile in the absence of standard CF care
We present three cases in this report. Three adult brothers, homozygous for the delF508 cystic fibrosis mutation, have maintained an unusually preserved clinical condition even though they did not attend a CF Clinic during their childhood, do not attend a CF Clinic now, and do not follow standard CF care guidelines. The brothers use an alternative CF treatment regimen on which they have maintained normal lung function, height/weight, and bloodwork, and they utilize less than half the recommended dosage of pancreatic enzymes. The brothers culture only methicillin-sensitive Staphylococcus aureus, and have never cultured any other bacteria. Highly effective modulator therapies, such as elexacaftor/tezacaftor/ivacaftor, do not substantially reduce infection and inflammation in vivo in CF patients, and thus these three case reports are of special note in terms of suggesting adjunct therapeutic approaches. Finally, these three cases also raise important questions about standard CF care guidelines.
- • Three adult brothers, delF508 cystic fibrosis (CF) homozygotes, maintain unusually preserved clinical condition absent standard CF care.
- • An alternative CF treatment regimen has kept their lung function, weight/height, and lab parameters normal, with low pancreatic enzyme dose.
- • The brothers culture only methicillin-sensitive Staphylococcus aureus, and have never cultured any other bacteria.
- • Highly effective modulator therapies (HEMT) for CF do not substantially reduce infection and inflammation in vivo; these cases are thus of note.
- • These cases also raise important questions about standard CF care guidelines.
1. Introduction
Cystic fibrosis (CF) is a serious and life-shortening genetic disorder affecting approximately 70,000 persons worldwide [ 1 ]. Respiratory failure is the foremost cause of death in CF patients, and lung transplantation is often considered in end-stage CF disease. For those born with CF in the last five years, median predicted survival age is now 44, which is decades longer than survival rates in the recent past [ 2 ]. Indeed, new advances in CF modulator therapy and CF gene therapy may eventually provide a normal life expectancy for these individuals.
A key approach in fighting the ravages of CF while waiting for more advanced treatments to be developed has been to slow the inexorable decline in lung function. Typical rate of lung function decline in CF is approximately −1.2 to −1.6 FEV1% per year [ 3 ]. Rate of decline is strongly associated with type of CF mutation. The three most severe classes of CFTR, Classes I, II, and III, represent defects in protein production, protein processing, and protein regulation, respectively [ 4 ]. The most common CF-causing mutation is delF508, occurring in 70% of cases, which is a Class II mutation [ 5 ]. Being homozygous for the delF508 mutation confers a severe phenotype, including pancreatic insufficiency and a steeper rate of decline in lung function over time [ 6 ]. In the United States, it is estimated that approximately 50% of those with cystic fibrosis are homozygous for delF508 [ 7 ]. Standard clinical care for severe mutation cases is often aggressive, including but not limited to daily airway clearance, use of pancreatic enzymes at the level of 500-2,500 lipase units/kg/meal (and enteric feeding if adequate weight percentile cannot be maintained), common and repeated use of oral, inhaled and intravenous antibiotics, daily intake of water-miscible versions of fat-soluble vitamins, and quarterly CF Clinic visits where lung function parameters and cultures of lung bacteria and fungi are assessed [ 8 , 9 ]. Pulmonary exacerbations often result in hospitalization, which may occur one or more times per year, typically lasting 14–21 days and including intensive antibiotic treatment and chest physical therapy. Everyday treatment burden is high, with estimates of 2–3 hours per day, with adherence at an estimated 50% or less [ 10 ]. The mean annual cost of standard supportive CF care in the US in 2016 (in 2019 dollars), before CFTR modulator therapies, was estimated to average $77,143, with severe non-transplant cases experiencing multiple pulmonary exacerbations costing on average triple or quadruple that amount [ 11 ]. With the average cost of elexacaftor/tezacaftor/ivacaftor (Trikafta) treatment currently over $311,000 per year, average standard supportive CF care costs were expected to double in 2019 [ 12 ] and increase further over time, perhaps quadrupling, with wider adoption of that treatment by all eligible patients.
Here we report on three adult brothers who are delF508 homozygotes, and yet who have maintained an unusually preserved clinical profile in the absence of standard CF clinical care. At the time of this writing, Brother A is 23 years old, Brother B is 21 years old, and Brother C is 18 years old. They are full-blooded siblings.
2. Case reports
2.1. brother a.
Brother A, now aged 23, was born full-term weighing 10 lbs. 2 oz. to a carrier mother experiencing gestational diabetes who subsequently breastfed him. His weight percentile decreased significantly over time, and at 6 months, after a course of oral antibiotics for a suspected ear infection, he developed a severe Vitamin K deficiency manifesting in quarter-sized black bruises on his body, as well as Pseudo-Bartter Syndrome. He was hospitalized until IV fluids stabilized his condition and normalized his electrolytes. Vitamin K shots were also administered. At 9 months of age, he was diagnosed with cystic fibrosis, and the genetic mutation analysis identified him as a delF508 homozygote. Between the time of his hospitalization and his diagnosis, he suffered from malnutrition with accompanying protein edema and his weight percentile, which had been over 97th percentile when born, was under the 5th percentile adjusted for age and sex. Once started on pancreatic enzymes (CREON 5) after diagnosis, his weight percentile increased to approximately the 30th percentile.
Approximately one year after diagnosis, the parents of Brother A elected to depart from standard CF care, including an election to stop attending the CF Clinic, while continuing to be under the care of their family pediatrician. The treatment plan for the brothers is described in detail in a later section. The only prescription medicine taken during his childhood and continuing to this day remains CREON 5/6, with Brother A utilizing 4 CREON 5/6 per meal, less than half the lowest recommended dose for his weight. In the teen years, Brother A experienced three episodes of heat exhaustion requiring IV fluid stabilization in an emergency room, has had one endoscopic sinus cleaning for sinus pain at age 20, and also underwent an appendectomy for appendicitis at age 23, but otherwise has had no major clinical issues, though exhibiting digital clubbing. Brother A played ice hockey throughout his childhood and teen years. His height, weight, lung function, and lab results at age 23 are provided in Table 1 .
Clinical parameters, Brother A.
2.2. Brother B
Brother B, now aged 21, was born full-term, weighing 8 lbs. 8 oz., the mother supplementing with oral glutathione (GSH) during the pregnancy and subsequently breastfeeding him. Brother B has never attended a CF Clinic, was diagnosed at 2 weeks of age, and was under the care of the family's pediatrician only. Brother B's only prescription medication during his childhood was CREON 5/6, just as with Brother A, utilizing 4 capsules per meal. Brother B has never needed to be hospitalized or have surgery or antibiotics. While Brother B does not exhibit digital clubbing; when recovering from respiratory viruses, he does manifest a cough that lingers longer than it lingers for his brothers, though the cough ultimately resolves. Brother B played ice hockey in childhood and teen years, as well as participated in gymnastics, cross-country running, track and field, and weight-lifting. His height, weight, lung function, and lab results at age 21 are provided in Table 2 .
Clinical parameters, Brother B.
2.3. Brother C
Brother C, now aged 18, was born full-term weighing 9 lbs. 2 oz., the mother supplementing with oral glutathione (GSH) during the pregnancy and subsequently breastfeeding him. Brother C has never attended a CF Clinic, was diagnosed at 2 weeks of age, and was under the care of the family's pediatrician only. Brother C's only prescription medication during his childhood was CREON 5/6, just as with Brothers A and B, utilizing 4 capsules per meal. Brother C has never needed to be hospitalized, or have surgery or antibiotics. Brother C does not exhibit digital clubbing. Brother C played ice hockey in childhood and teen years, as well as participated in gymnastics. His height, weight, lung function, and lab results at age 18 are provided in Table 3 .
Clinical parameters, Brother C.
3. Description of treatment
Given the severity of the genotype involved and the almost complete non-adherence to standard CF guidelines (with the exception of a significantly lower-than-average dose of prescription pancreatic enzymes and a standard dose of water-miscible fat soluble vitamins), the preserved clinical profile of these three brothers is noteworthy. However, the family developed a regimen that went well beyond pancreatic enzymes and water-miscible vitamins. The treatment regimen is provided in Table 4 .
Description of Daily Regimen.

4. Discussion
There are several possibilities for the preserved clinical status of these three brothers in the absence of standard CF care:
- a) They avoided the CF Clinic setting. Recent research [ 13 ] has shown that Pseudomonas infections are more prevalent and lung function lower among CF patients in standard care versus CF patients in a telemedicine setting. It is possible these three brothers benefitted from not attending a standard CF Clinic, especially since during their childhood years at the turn of the century, Clinic infection control was not emphasized. For example, during Brother A's first few CF Clinic visits as an infant, families were expected to wait together in a communal area with communal toys, and health care professionals at the Clinic wore neither masks nor gloves as they moved from exam room to exam room.
- b) With the exception of Brother A, Brothers B and C have used no antibiotics at all. Brother A has only used antibiotics three times in his life; the first use in infancy precipitated Pseudo-Bartter Syndrome, leading to his diagnosis with cystic fibrosis. The other two uses were incident to endoscopic sinus scraping and an appendectomy. Recent research has shown the importance of the gut microbiome in maintenance of health (including respiratory function), digestion and immune signaling, and this is true in the case of cystic fibrosis as well [ [14] , [15] , [16] ]. As David Pride, Associate Director of Microbiology at UC San Diego, notes in an address to the 2019 North American Cystic Fibrosis Conference [ 17 ], “It is important to preserve our microbiomes because they play important roles in preventing pathogens from establishing infections, in the development of our immune systems to recognize and kill pathogens, and in metabolic processes such as the digestion of foods. Indiscriminate uses of antibiotics can have profound and long-lasting effects upon our microbiomes by killing many of the bacteria that make up our microbiome; thus, limiting their use may aid in keeping us healthy.”
- Prevalent, sometimes chronic, antibiotic use among CF patients results in a significant gut dysbiosis [ 18 ]. In addition, it has been noted that aggressive antibiotic use in CF, usually incident to the first manifestation of Staphylococcus aureus (SA), may allow Pseudomonas aeruginosa a greater foothold [ 19 ], and that aggressive treatment of Pseudomonas may, in turn, promote drug resistance and may allow additional bacteria, such as Stenotrophomonas maltophilia, an opportunity to proliferate [ 20 ]. Perhaps a preserved gut microbiome due to non-use of antibiotics may have played a role in the brothers' preserved clinical condition; this may also help account for the brothers’ significantly lower level of need for pancreatic enzymes. Perhaps also the decision not to aggressively treat their light to moderate growth of methicillin-sensitive SA may have precluded additional bacteria, including drug-resistant bacteria, from emerging.
- c) Other standard daily CF treatments were not employed, either, which might help account for their preserved clinical condition. For example, the brothers do not use bronchodilators; and beta-2 agonist bronchodilators, such as albuterol, have recently been shown to significantly reduce delF508 CFTR activation [ 21 ]. This reduction is even evident when CFTR modulators are used, with the finding of a more than 60% reduction of modulator-corrected CFTR activation in vitro, “sufficient to abrogate VX809/VX770 modulation of F508-del CFTR” [ 21 ]. In addition, the brothers do not use DNase, which has been associated with increased levels of neutrophil elastase in past research [ 22 ]. Last, after Brother A transitioned to his new treatment regimen at approximately 23 months of age, chest percussive therapy (CPT) was discontinued, and neither Brother B nor C underwent CPT at all. A Cochrane meta-review found that while CPT constituted the lion's share of treatment time burden in CF, the evidence that outcomes of CPT differed from no CPT was “very low quality” [ 23 ].
- d) Glutathione (GSH) is heavily emphasized in the brothers' daily regimen. Levels of GSH are strongly decreased in the extracellular milieu of CF patients, as its efflux from epithelial cells is compromised by CFTR mutation [ 24 ]. In the non-CF research literature, GSH in its ratio of reduced to oxidized forms (GSH:GSSG) has been shown to be the foundation of redox signaling in the body; GSH is also the body's primary water-soluble antioxidant and a potent mucolytic, and conserves NO through formation of GSNO. Given its pivotal roles, it is not surprising to find that GSH deficiency is noted in several other severe respiratory illnesses besides CF, including ARDS, COPD, IIP, IPF, IRDS, and DFA, and GSH deficiency is a key catalyst for (and GSH dosing a key treatment of) cachexia [ 24 ]. The use of GSH in the treatment of CF may reduce systemic inflammation, lessen the viscosity of mucus, and catalyze the efficacy of the immune system, including through GSNO. Indeed, a clinical study by Visca et al. found significantly increased BMI [ 25 ], significantly increased lung function [ 26 ], and even improved bacteriological results [ 27 ] from the daily use of oral glutathione in children with CF at a dose of 30 mg/lb body weight/day, spread out over 3–4 doses, over a time period of 6 months. In addition, the parents of these brothers noted a sudden increase in both saliva and appetite in Brother A after glutathione (GSH) was introduced when he was two years of age. Brothers B and C, on GSH from two weeks of birth (and with the mother supplementing with oral glutathione throughout pregnancy with these two brothers), never displayed low saliva or low appetite. The preserved clinical status of these three brothers may perhaps be related to this glutathione-heavy regimen.
- e) Other aspects of the brothers' regimen may offset their disease condition. The use of probiotics [ 28 ], the heavy emphasis on antioxidants in addition to glutathione (such as C, CoQ10, Alpha-lipoic acid, D, E, etc. [ 29 ]), amino acids (such as cysteine [ 30 ], carnitine [ 31 ], choline [ 32 , 33 ], taurine [ 34 ], and glycine [ 35 ]), curcumin [ 36 ], and additional digestive support beyond enzymes (lecithin, bile acid). It is possible that some or all of these supplementation efforts also helped to preserve the clinical status of the three brothers. In addition, exclusive breastfeeding of CF infants has been linked to significantly higher FEV1 at age 5 (difference significant at p ≤ 0.001 between breastfed and formula fed CF infants), perhaps contributing to the preservation of lung function beyond that time frame [ 37 ].
- f) Modifier alleles may be present. While no in-depth analysis of the brothers' genetic profile has been performed beyond the identification of their CF mutations, there are known modifier alleles that serve to lessen (or exacerbate) the severity of CF (see, for example [ 38 ]). It is possible all three brothers inherited some propitious set of modifier alleles.
5. Conclusion
In conclusion, while it is encouraging and heartening that new CF therapies, such as elexacaftor/tezacaftor/ivacaftor (Trikafta) and other HEMT (highly effective modulator therapies), now exist, it is instructive to consider how this family was able to preserve the clinical condition of three brothers, all delF508 homozygotes, in the absence of those therapies, and even in the absence of standard CF care. While HEMT certainly increase CFTR activity, there is substantially less effect on infection and inflammation in vivo [ 39 ]. As recently noted by Singh et al., “[I]f infection and inflammation become uncoupled from CFTR activity in established disease [due to HEMT use], drugs targeting CFTR may need to be initiated very early in life, or used in combination with agents that suppress infection and inflammation ” [ 39 ; emphasis ours]. These case reports may speak to that proposition.
Furthermore, each possible explanation for that preservation is an occasion for reflection on the current standard of CF care. We may feel to ask questions such as, “From the point of view of the patient's health, is the entire concept of the CF Clinic inherently flawed? Is the frequent, sometimes chronic, use of antibiotics and certain other medications in CF care a real double-edged sword for CF patients, with disadvantages possibly outweighing advantages in many cases? Are there measures we can take now, relatively inexpensive measures such as the use of glutathione (GSH) and other antioxidants and amino acids, that will help preserve the clinical status of CF patients, and that might synergize with cutting-edge treatments such as CFTR modulators to improve and safeguard health to an even greater degree, and which should be initiated as early in life as possible, possibly while the fetus is still in utero ?” The experience of these three brothers, so removed from standard CF care and yet so well preserved in their clinical status, highlights the need to consider such questions more urgently than we perhaps have heretofore considered them.
Funding sources
This work was supported by the Utah Valley Institute of Cystic Fibrosis, for publication costs only.
Acknowledgements
The author wishes to acknowledge Valerie M. Hudson, who assisted with the writing of this article.
We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs

Chapter 19: Case Study: Cystic Fibrosis
Julie M. Skrzat; Carole A. Tucker
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Introduction.
- Examination: Age 2 Months
- Evaluation, Diagnosis, and Prognosis
- Intervention
- Conclusion of Care
- Examination: Age 8 Years
- Examination: Age 16 Years
- Recommended Readings
- Full Chapter
- Supplementary Content
C ystic fibrosis (CF) is an autosomal recessive condition affecting approximately 30,000 Americans and 70,000 people worldwide. According to the Cystic Fibrosis Foundation ( Cystic Fibrosis Foundation, 2019a ), approximately 1,000 new cases are diagnosed yearly in the United States, with a known incidence of 1 per 3,900 live births. The disease prevalence varies greatly by ethnicity, with the highest prevalence occurring in Western European descendants and within the Ashkenazi Jewish population.
The CF gene, located on chromosome 7, was first identified in 1989. The disease process is caused by a mutation to the gene that encodes for the CF transmembrane conductance regulator (CFTR) protein. This mutation alters the production, structure, and function of cyclic adenosine monophosphate (cAMP), a dependent transmembrane chloride channel carrier protein found in the exocrine mucus glands throughout the body. The mutated carrier protein is unable to transport chloride across the cell membrane, resulting in an electrolyte and charge imbalance. Diffusion of water across the cell membrane is thus impaired, resulting in the development of a viscous layer of mucus. The thick mucus obstructs the cell membranes, traps nearby bacteria, and incites a local inflammatory response. Subsequent bacterial colonization occurs at an early age and ultimately this repetitive infectious process leads to progressive inflammatory damage to the organs involved in individuals with CF.
Pop-up div Successfully Displayed
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Global health
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 14, Issue 11
- Cystic fibrosis: a diagnosis in an adolescent
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0001-9674-0879 Monica Bennett 1 ,
- Andreia Filipa Nogueira 1 ,
- Maria Manuel Flores 2 and
- Teresa Reis Silva 1
- 1 Pediatric , Centro Hospitalar e Universitario de Coimbra EPE , Coimbra , Portugal
- 2 Pediatric , Centro Hospitalar do Baixo Vouga EPE , Aveiro , Aveiro , Portugal
- Correspondence to Dr Monica Bennett; acinomaicila{at}gmail.com
Most patients with cystic fibrosis (CF) develop multisystemic clinical manifestations, the minority having mild or atypical symptoms. We describe an adolescent with chronic cough and purulent rhinorrhoea since the first year of life, with diagnoses of asthma, allergic rhinitis and chronic rhinosinusitis. Under therapy with long-acting bronchodilators, antihistamines, inhaled corticosteroids, antileukotrienes and several courses of empirical oral antibiotic therapy, there was no clinical improvement. There was no reference to gastrointestinal symptoms. Due to clinical worsening, extended investigations were initiated, which revealed Pseudomonas aeruginosa in sputum culture, sweat test with a positive result and heterozygosity for F508del and R334W mutations in genetic study which allowed to confirm the diagnosis of CF. In this case, heterozygosity with a class IV mutation can explain the atypical clinical presentation. It is very important to consider this diagnosis when chronic symptoms persist, despite optimised therapy for other respiratory pathologies and in case of isolation of atypical bacterial agents.
- cystic fibrosis
- pneumonia (respiratory medicine)
https://doi.org/10.1136/bcr-2021-245971
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
A high degree of diagnostic suspicion is of fundamental importance when chronic symptoms persist, despite optimised therapy for previous diagnoses and in case of isolation of atypical bacterial agents in microbiological studies.
This case describes an adolescent with a chronic cough since the first year of life, adequate weight gain and normal pubertal development, without improvement with optimised therapy for other respiratory pathologies. There was no reference to gastrointestinal symptoms. There was clinical worsening at 13 years of age and isolation of Pseudomonas aeruginosa in sputum culture. After extensive investigation, including sweat test and genetic study, it was possible to confirm the diagnosis of cystic fibrosis (CF).
Case presentation
A 13-year-old female teenager presented with chronic cough and purulent rhinorrhoea with periods of intermittent clinical worsening with associated fever since the first year of life. This was accompanied by various medical specialties, with diagnoses of asthma, allergic rhinitis and chronic rhinosinusitis. She was under therapy with long-acting bronchodilators, antihistamines, inhaled corticosteroids, and antileukotrienes and submitted to several courses of empirical oral antibiotic therapy, without sustained and effective clinical improvement. She presented an adequate height–weight evolution, with a body mass index (BMI) at 50th−85th percentile and normal pubertal development, no reference to gastrointestinal symptoms or previous hospitalisations. Her family background was irrelevant. Due to clinical worsening, with emetising cough associated with intermittent fever and night sweats, a pulmonary CT scan was performed, which revealed parenchymal densification, air bronchogram, thickened bronchi, mucoid impaction and mediastinal adenopathies. Observed in the emergency department, the objective examination highlighted bibasal crackles on pulmonary auscultation, without other alterations. She was treated with clarithromycin, later associated with co-amoxiclav. An extended investigation was initiated, which revealed erythrocyte sedimentation rate of 52 mm/hour, C reactive protein test of 4.10 mg/dL, negative BK and interferon gamma release assay test, and isolation of P. aeruginosa in sputum culture. The antibiotic therapy was changed to ciprofloxacin and sweat tests were performed with positive results on two occasions (102 and 110 mmol/L). Later, a genetic study revealed heterozygosity for the F508del and R334W mutations, which confirmed the diagnosis of CF. Faecal elastase was performed, and the result was normal (>500 µg/g).
After antimicrobial therapy with ciprofloxacin, she maintained P. aeruginosa, and methicillin-sensitive Staphylococcus aureus (MSSA) was now discovered in the sputum. For this reason, she was hospitalised for intravenous eradication. After 2 weeks of antibiotic therapy with meropenem, gentamicin and teicoplanin, P. aeruginosa was eradicated but not MSSA. Linezulide was prescribed for 2 weeks, with a good response, and the microbiological study was negative.
Outcome and follow-up
During the follow-up period (2 years), she continued having frequent respiratory infections, with isolation of P. aeruginosa and MSSA in respiratory secretions intermittently, requiring the need for several courses of antibiotic therapy. The antibiogram of P. aeruginosa has remained sensible. Currently, she continues follow-up in a specialised fibrosis cystic centre, under inhaled therapy with colistin/tobramycin, hypertonic saline, salbutamol, dornase alfa, budesonide/formoterol, chest physiotherapy and oral azithromycin prophylaxis. Her pulmonary function is normal with a currently forced expiratory volume in 1 s of 87% and she shows adequate height−weight evolution, with BMI maintained at P50–85. The sweat chloride test was not repeated after confirmed diagnosis.
CF is one of the most commonly diagnosed genetic disorders 1 and the most common life-shortening autosomal recessive disease among Caucasian populations, with a frequency of 1 in 2000–3000 live births. 2 CF is caused by mutations in a single large gene on chromosome 7 that encodes the cystic fibrosis transmembrane conductance regulator ( CFTR ) protein.
There are more than 2000 mutations/variations of the CFTR gene reported and listed in the CFTR mutation database. A small subset are CF disease-causing mutations, of which the majority are associated with pancreatic insufficiency and a smaller subset are associated with pancreatic sufficiency. Most of the known mutations/variations related to CF are described in the CFTR2 database (Clinical and Functional Translation of CFTR). This website provides information about what is currently known about specific genetic variants or variant combination and is a useful resource to correlate clinical measures to the large number of variants identified to date. 3 4
Clinical disease requires disease-causing mutations in both copies of the CFTR gene. Mutations of the CFTR gene have been divided into five different classes. The most common mutation is F508del which is included in category class II mutations—defective protein processing. Approximately 50% of patients with CF are homozygous for this mutation, and 90% will carry at least one copy of this gene. In general, mutations in classes I−III cause more severe disease than those in classes IV and V. Class IV and V mutations are associated with moderate phenotypes and pancreatic sufficiency. 5 The R334W is a rare mutation included in class IV—defective conduction and associated with pancreatic sufficiency. 5 6 Those with less severe mutations present with pancreatic sufficiency and single organ manifestations of CF. Some of these patients would fulfil the diagnostic criteria for CF and some would be classified as having a CFTR-related disorder if the diagnosis of CF cannot be fulfilled. 7
The phenotypic expression of disease varies widely, based on CFTR-related (genotype-related) and non-CFTR-related factors (environmental and other genetic modifiers). Genotype–phenotype correlations are weak for pulmonary disease in CF and somewhat stronger for the pancreatic insufficiency phenotype. 5
Many studies in different individuals heterozygous for CFTR gene mutation have been performed to find out the association of CFTR gene mutation with asthma. The results are inconclusive, as some of the studies have shown positive association, whereas other could find either protective or no association. 8 Also, at this time, there is no evidence for a specific association between CFTR gene mutation and other allergic manifestations.
Clinical manifestations are multisystemic and heterogeneous. 9 The first symptoms of the disease usually appear in the first years of life, and most patients develop a multisystem disease, with predominantly respiratory and digestive symptoms. 2 5 10 The usual presenting symptoms and signs include persistent pulmonary infection, pancreatic insufficiency and elevated sweat chloride levels. However, many patients demonstrate mild or atypical symptoms, and clinicians should remain alert to the possibility of CF even when only a few of the usual features are present. 2 Progressive pulmonary involvement is the main cause of morbidity and mortality. Clinically significant pancreatic insufficiency eventually develops in approximately 85% of individuals with CF. The remaining 10%–15% of patients with CF remain pancreatic sufficient throughout childhood and early adulthood, but these individuals are at risk of pancreatitis. Pancreatic exocrine function may be evaluated indirectly by measurement of faecal elastase, which is clinically practical but has limited accuracy. Low levels of faecal elastase suggest pancreatic insufficiency and support a diagnosis of CF. 2 5 11–13
The diagnosis of CF is based on compatible clinical findings with biochemical or genetic confirmation. The sweat chloride test is the mainstay of laboratory confirmation, although tests for specific mutations, nasal potential difference (NPD), immunoreactive trypsinogen, stool faecal fat or pancreatic enzyme secretion may also be useful in some cases.
Both of the following criteria must be met to diagnose CF: (1) clinical symptoms consistent with CF in at least one organ system, or positive newborn screen or having a sibling with CF; and (2) evidence of cystic CFTR dysfunction (any of the following): elevated sweat chloride ≥60 mmol/L; presence of two disease-causing mutations in the CFTR gene, one from each parental allele; abnormal NPD.
Sweat chloride test ≥60 mmol/L is considered abnormal. If confirmed on a second occasion, this is sufficient to confirm the diagnosis of CF in patients with clinical symptoms of CF. Positive results of sweat testing should be further evaluated by CFTR sequencing. Determining the CFTR genotype is important because the results may affect treatment choices as well as confirm the diagnosis. For patients with inconclusive results of sweat chloride and DNA testing, measurement of NPD can be used to further evaluate for CFTR dysfunction. 5 14
Newborn screening programmes for CF are now performed routinely in several countries, which contributed to a dramatic increase in the number of CF cases identified before presenting with symptoms. The rationale for this screening is that early detection of CF may lead to earlier intervention and improved outcomes because the affected individuals are diagnosed, referred and treated earlier in life compared with individuals who are diagnosed after presenting with symptomatic CF. In Portugal and some other European countries, this programme was implemented less than 10 years ago, contributing to a late diagnosis in older children.
There are different neonatal screening programmes that include biochemical screening and/or DNA assays with panels to test for the most common CFTR mutations in the local population. Most programmes test for between 23 and 40 mutations, and some programmes even perform adjunctive full gene sequencing. Screening for a greater number of mutations increases the likelihood of identifying infants with CF and also increases the identification of rare or unique sequence mutations, making interpretation of the result more complicated. As only a limited number of mutations are evaluated on the genetic screens, it is possible to miss the diagnosis. Thus, it is important to follow such children closely, with particular attention to weight gain and recurrent respiratory infections. Clinicians should consider CF in individuals with suggestive symptoms, even when results of the newborn screen are negative or equivocal. 5 14
In the case described here, heterozygosity with a class IV mutation, usually associated with an intermediate phenotype and pancreatic sufficiency, may explain the atypical clinical presentation and consequent diagnosis only in adolescents. We also hypothesise that this child’s allergic manifestations may have delayed the diagnosis.
As the spectrum of clinical presentation is very variable, it is very important for clinicians from multiple specialties to be vigilant and suspect this diagnosis in conditions such as recurrent pulmonary infection, male infertility, pancreatitis, nasal polyposis and malabsorption even in patients with negative newborn screening. 2 10 13
Learning points
There is a wide spectrum of manifestations of cystic fibrosis (CF). These variations and wide spectrum are based on cystic fibrosis transmembrane conductance regulator (CFTR)-related (genotype-related) and non-CFTR-related factors (environmental and other genetic modifiers).
Most patients with CF develop multisystemic and heterogeneous clinical manifestations, with predominantly respiratory and digestive symptoms.
A minority have mild or atypical symptoms.
Heterozygosity with a class IV mutation usually is associated with an intermediate phenotype and pancreatic sufficiency and can explain the atypical clinical presentation.
It is very important to consider this diagnosis when chronic symptoms persist, despite optimised therapy for other respiratory pathologies and in case of isolation of atypical bacterial agents in microbiological studies.
Ethics statements
Patient consent for publication.
Consent obtained from parent(s)/guardian(s)
- Dickinson KM ,
- ↵ Cystic fibrosis mutation database . Available: http://www.genet.sickkids.on.ca/Home.html
- ↵ Clinical and functional translation of CFTR . Available: https://cftr2.org/
- Ellis L , et al
- Awasthi S ,
- Gartner S ,
- Salcedo Posadas A ,
- García Hernández G
- Castellani C ,
- Linnane B ,
- Pranke I , et al
- Farrell PM ,
- Ren CL , et al
- Kharrazi M ,
- Bishop T , et al
Contributors MB cared for study patient, planned and wrote the article. AFN collected data. MMF provided and cared for study patient, served as scientific advisors and critically reviewed the study proposal. TRS cared for study patient, served as scientific advisors and critically reviewed the study proposal.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

Case Study: Cystic Fibrosis - CER
- Last updated
- Save as PDF
- Page ID 26446
This page is a draft and is under active development.
Part I: A Case of Cystic Fibrosis
Dr. Weyland examined a six month old infant that had been admitted to University Hospital earlier in the day. The baby's parents had brought young Zoey to the emergency room because she had been suffering from a chronic cough. In addition, they said that Zoey sometimes would "wheeze" a lot more than they thought was normal for a child with a cold. Upon arriving at the emergency room, the attending pediatrician noted that salt crystals were present on Zoey's skin and called Dr. Weyland, a pediatric pulmonologist. Dr. Weyland suspects that baby Zoey may be suffering from cystic fibrosis.
CF affects more than 30,000 kids and young adults in the United States. It disrupts the normal function of epithelial cells — cells that make up the sweat glands in the skin and that also line passageways inside the lungs, pancreas, and digestive and reproductive systems.
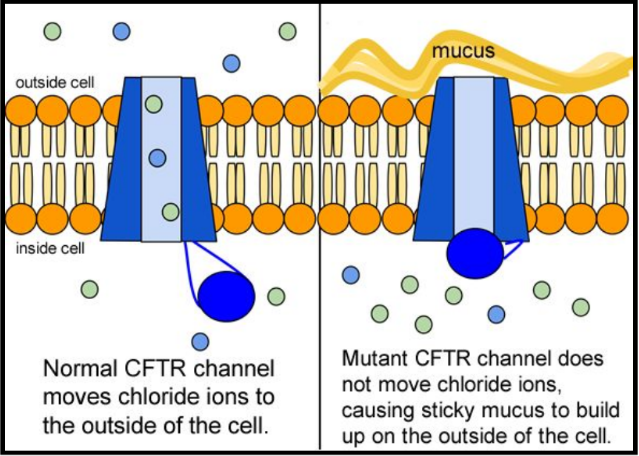
The inherited CF gene directs the body's epithelial cells to produce a defective form of a protein called CFTR (or cystic fibrosis transmembrane conductance regulator) found in cells that line the lungs, digestive tract, sweat glands, and genitourinary system.
When the CFTR protein is defective, epithelial cells can't regulate the way that chloride ions pass across cell membranes. This disrupts the balance of salt and water needed to maintain a normal thin coating of mucus inside the lungs and other passageways. The mucus becomes thick, sticky, and hard to move, and can result in infections from bacterial colonization.
- "Woe to that child which when kissed on the forehead tastes salty. He is bewitched and soon will die" This is an old saying from the eighteenth century and describes one of the symptoms of CF (salty skin). Why do you think babies in the modern age have a better chance of survival than babies in the 18th century?
- What symptoms lead Dr. Weyland to his initial diagnosis?
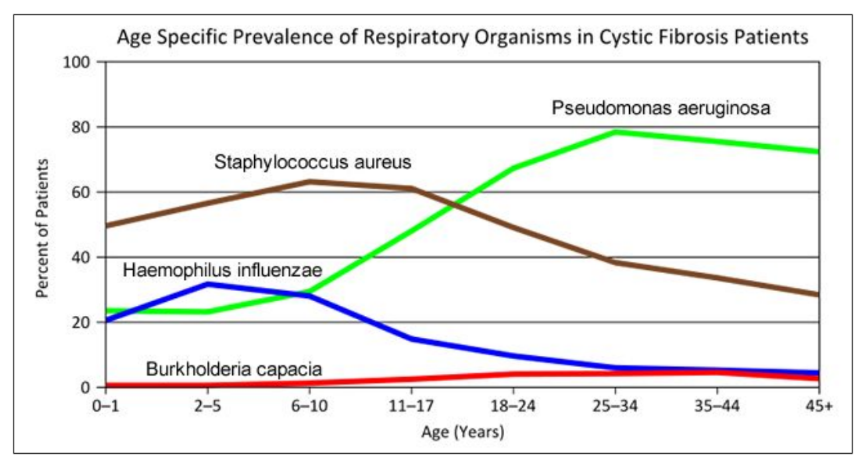
- Consider the graph of infections, which organism stays relatively constant in numbers over a lifetime. What organism is most likely affecting baby Zoey?
- What do you think is the most dangerous time period for a patient with CF? Justify your answer.
Part II: CF is a disorder of the cell membrane.
Imagine a door with key and combination locks on both sides, back and front. Now imagine trying to unlock that door blind-folded. This is the challenge faced by David Gadsby, Ph.D., who for years struggled to understand the highly intricate and unusual cystic fibrosis chloride channel – a cellular doorway for salt ions that is defective in people with cystic fibrosis.
His findings, reported in a series of three recent papers in the Journal of General Physiology, detail the type and order of molecular events required to open and close the gates of the cystic fibrosis chloride channel, or as scientists call it, the cystic fibrosis transmembrane conductance regulator (CFTR).
Ultimately, the research may have medical applications, though ironically not likely for most cystic fibrosis patients. Because two-thirds of cystic fibrosis patients fail to produce the cystic fibrosis channel altogether, a cure for most is expected to result from research focused on replacing the lost channel.
5. Suggest a molecular fix for a mutated CFTR channel. How would you correct it if you had the ability to tinker with it on a molecular level?
6. Why would treatment that targets the CFTR channel not be effective for 2⁄3 of those with cystic fibrosis?
7. Sweat glands cool the body by releasing perspiration (sweat) from the lower layers of the skin onto the surface. Sodium and chloride (salt) help carry water to the skin's surface and are then reabsorbed into the body. Why does a person with cystic fibrosis have salty tasting skin?
Part III: No cell is an island
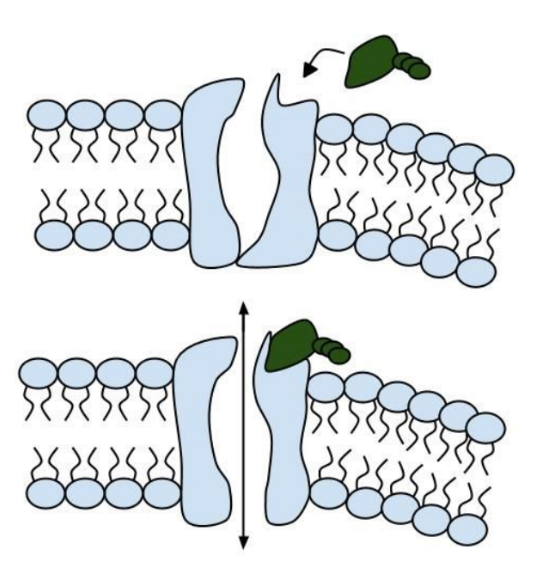
Like people, cells need to communicate and interact with their environment to survive. One way they go about this is through pores in their outer membranes, called ion channels, which provide charged ions, such as chloride or potassium, with their own personalized cellular doorways. But, ion channels are not like open doors; instead, they are more like gateways with high-security locks that are opened and closed to carefully control the passage of their respective ions.
In the case of CFTR, chloride ions travel in and out of the cell through the channel’s guarded pore as a means to control the flow of water in and out of cells. In cystic fibrosis patients, this delicate salt/water balance is disturbed, most prominently in the lungs, resulting in thick coats of mucus that eventually spur life-threatening infections. Shown below are several mutations linked to CFTR:
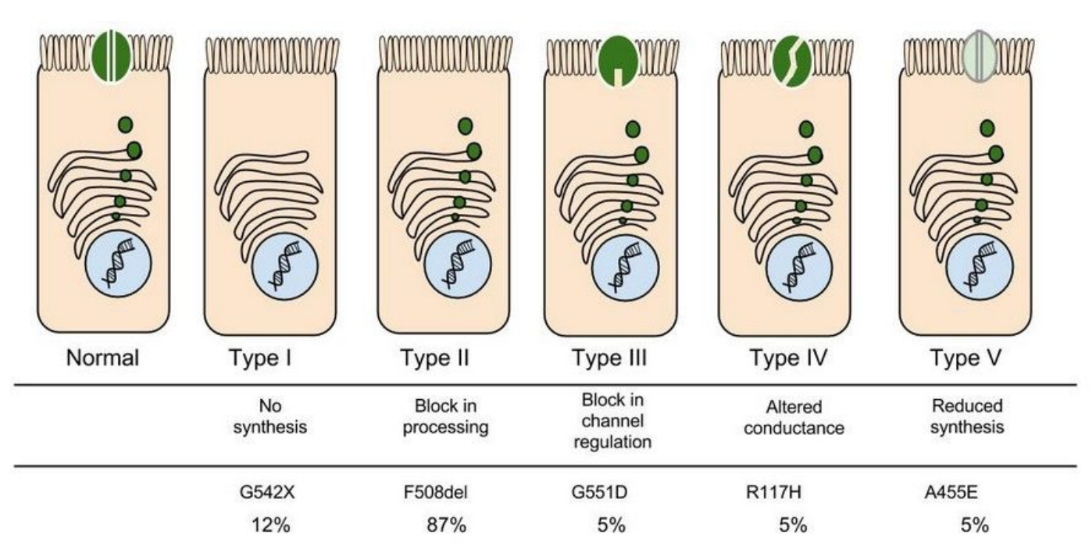
8. Which mutation do you think would be easiest to correct. Justify your answer. 9. Consider what you know about proteins, why does the “folding” of the protein matter?
Part IV: Open sesame
Among the numerous ion channels in cell membranes, there are two principal types: voltage-gated and ligand-gated. Voltage-gated channels are triggered to open and shut their doors by changes in the electric potential difference across the membrane. Ligand-gated channels, in contrast, require a special “key” to unlock their doors, which usually comes in the form of a small molecule.
CFTR is a ligand-gated channel, but it’s an unusual one. Its “key” is ATP, a small molecule that plays a critical role in the storage and release of energy within cells in the body. In addition to binding the ATP, the CFTR channel must snip a phosphate group – one of three “P’s” – off the ATP molecule to function. But when, where and how often this crucial event takes place has remains obscure.
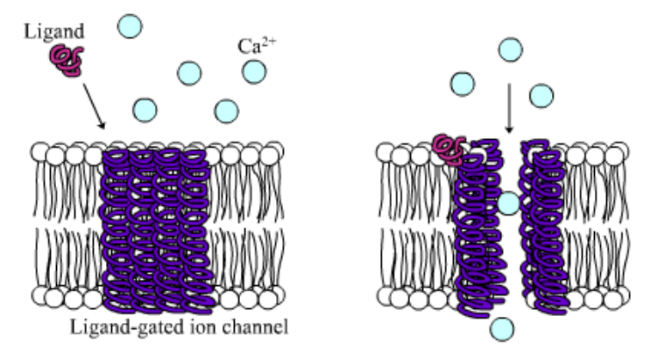
10. Compare the action of the ligand-gated channel to how an enzyme works.
11. Consider the model of the membrane channel, What could go wrong to prevent the channel from opening?
12. Where is ATP generated in the cell? How might ATP production affect the symptoms of cystic fibrosis?
13. Label the image below to show how the ligand-gated channel for CFTR works. Include a summary.
Part V: Can a Drug Treat Zoey’s Condition?
Dr. Weyland confirmed that Zoey does have cystic fibrosis and called the parents in to talk about potential treatments. “Good news, there are two experimental drugs that have shown promise in CF patients. These drugs can help Zoey clear the mucus from his lungs. Unfortunately, the drugs do not work in all cases.” The doctor gave the parents literature about the drugs and asked them to consider signing Zoey up for trials.
The Experimental Drugs
Ivacaftor TM is a potentiator that increases CFTR channel opening time. We know from the cell culture studies that this increases chloride transport by as much as 50% from baseline and restores it closer to what we would expect to observe in wild type CFTR. Basically, the drug increases CFTR activity by unlocking the gate that allows for the normal flow of salt and fluids.
In early trials, 144 patients all of whom were age over the age of 12 were treated with 150 mg of Ivacaftor twice daily. The total length of treatment was 48 weeks. Graph A shows changes in FEV (forced expiratory volume) with individuals using the drug versus a placebo. Graph B shows concentrations of chloride in patient’s sweat.
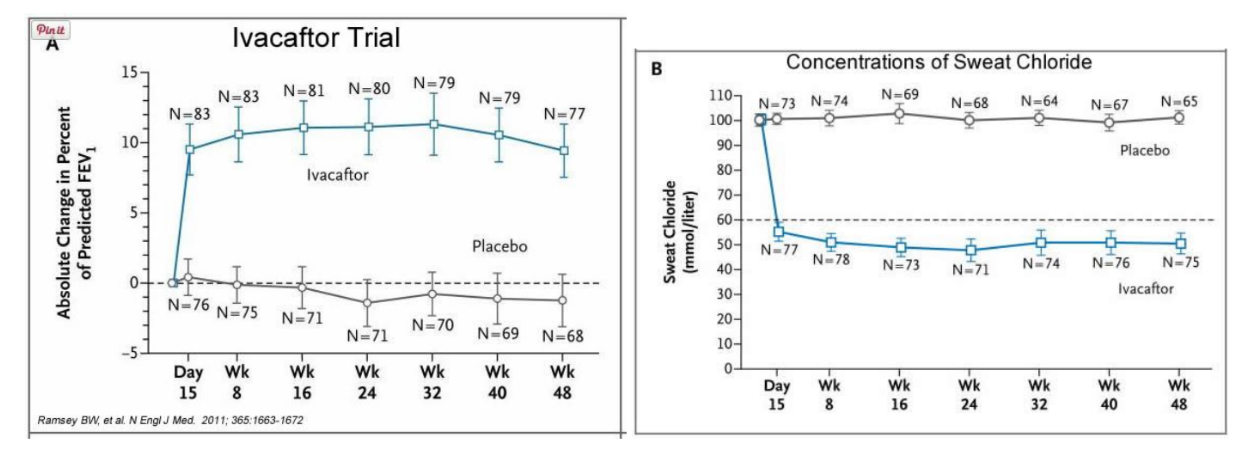
14. What is FEV? Describe a way that a doctor could take a measurement of FEV.
15. Why do you think it was important to have placebos in both of these studies?
16. Which graph do you think provides the most compelling evidence for the effectiveness of Ivacafor? Defend your choice.
17. Take a look at the mutations that can occur in the cell membrane proteins from Part III. For which mutation do you think Ivacaftor will be most effective? Justify your answer.
18. Would you sign Zoey up for clinical trials based on the evidence? What concerns would a parent have before considering an experimental drug?
Part VI: Zoey’s Mutation
Dr. Weyland calls a week later to inform the parents that genetic tests show that Zoey chromosomes show that she has two copies of the F508del mutation. This mutation, while the most common type of CF mutation, is also one that is difficult to treat with just Ivacaftor. There are still some options for treatment.
In people with the most common CF mutation, F508del, a series of problems prevents the CFTR protein from taking its correct shape and reaching its proper place on the cell surface. The cell recognizes the protein as not normal and targets it for degradation before it makes it to the cell surface. In order to treat this problem, we need to do two things: first, an agent to get the protein to the surface, and then ivacaftor (VX-770) to open up the channel and increase chloride transport. VX-809 has been identified as a way to help with the trafficking of the protein to the cell surface. When added VX-809 is added to ivacaftor (now called Lumacaftor,) the protein gets to the surface and also increases in chloride transport by increasing channel opening time.
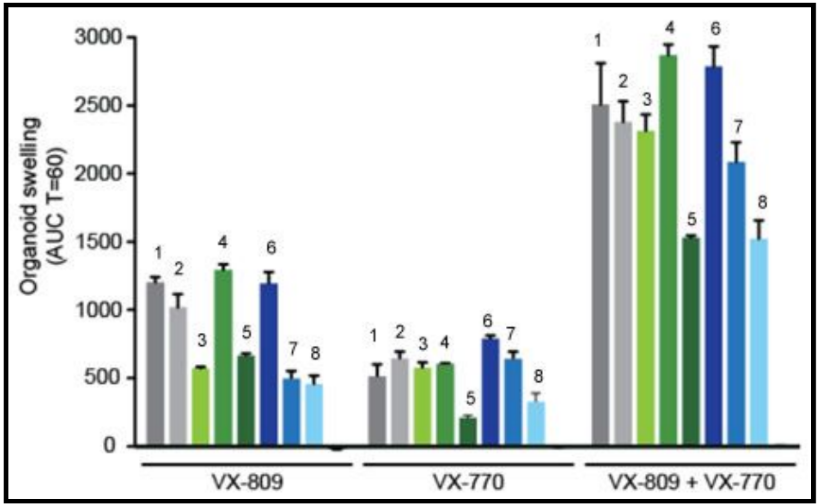
In early trials, experiments were done in-vitro, where studies were done on cell cultures to see if the drugs would affect the proteins made by the cell. General observations can be made from the cells, but drugs may not work on an individual’s phenotype. A new type of research uses ex-vivo experiments, where rectal organoids (mini-guts) were grown from rectal biopsies of the patient that would be treated with the drug. Ex-vivo experiments are personalized medicine, each person may have different correctors and potentiators evaluated using their own rectal organoids. The graph below shows how each drug works for 8 different patients (#1-#8)
19. Compare ex-vivo trials to in-vitro trials.
20. One the graph, label the group that represents Ivacaftor and Lumacaftor. What is the difference between these two drugs?
21. Complete a CER Chart. If the profile labeled #7 is Zoey, rank the possible drug treatments in order of their effectiveness for her mutation. This is your CLAIM. Provide EVIDENCE to support your claim. Provide REASONING that explains why this treatment would be more effective than other treatments and why what works for Zoey may not work for other patients. This is where you tie the graph above to everything you have learned in this case. Attach a page.
- Gene Therapy
Gene Therapy Case Study: Cystic Fibrosis
- Publications
- Conferences & Events
- Professional Learning
- Science Standards
- Awards & Competitions
- Daily Do Lesson Plans
- Free Resources
- American Rescue Plan
- For Preservice Teachers
- NCCSTS Case Collection
- Partner Jobs in Education
- Interactive eBooks+
- Digital Catalog
- Regional Product Representatives
- e-Newsletters
- Bestselling Books
- Latest Books
- Popular Book Series
- Prospective Authors
- Web Seminars
- Exhibits & Sponsorship
- Conference Reviewers
- National Conference • Denver 24
- Leaders Institute 2024
- National Conference • New Orleans 24
- Submit a Proposal
- Latest Resources
- Professional Learning Units & Courses
- For Districts
- Online Course Providers
- Schools & Districts
- College Professors & Students
- The Standards
- Teachers and Admin
- eCYBERMISSION
- Toshiba/NSTA ExploraVision
- Junior Science & Humanities Symposium
- Teaching Awards
- Climate Change
- Earth & Space Science
- New Science Teachers
- Early Childhood
- Middle School
- High School
- Postsecondary
- Informal Education
- Journal Articles
- Lesson Plans
- e-newsletters
- Science & Children
- Science Scope
- The Science Teacher
- Journal of College Sci. Teaching
- Connected Science Learning
- NSTA Reports
- Next-Gen Navigator
- Science Update
- Teacher Tip Tuesday
- Trans. Sci. Learning
MyNSTA Community
- My Collections
Maggie’s Illness
Protein Structure and Function in Cystic Fibrosis
By Michaela Gazdik Stofer
Share Start a Discussion

This directed case study examines the molecular basis of cystic fibrosis to emphasize the relationship between the genetic code stored in a DNA sequence and the encoded protein’s structure and function. Cystic fibrosis is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) protein that functions to help maintain salt and water balance along the surface of the lung and gastrointestinal tract. This case introduces students to “Maggie,” who has just been diagnosed with cystic fibrosis. The students must identify the mutation causing Maggie’s disease by transcribing and translating a portion of the wildtype and mutated CFTR gene. Students then compare the three-dimensional structures of the resulting proteins to better understand the effect a single amino acid mutation can have on the overall shape of a protein. Students also review the concepts of tonicity and osmosis to examine how the defective CFTR protein leads to an increase in the viscosity of mucus in cystic fibrosis patients. This case was developed for use in an introductory college-level biology course but could also be adapted for use in an upper-level cell or molecular biology course.
Download Case
Date Posted
- Generate a protein sequence through transcription and translation of a given DNA gene sequence.
- Explain the chemistry of amino acid side chains and their importance in protein folding.
- Describe how a mutation in a protein sequence leads to changes in the overall tertiary structure of the protein.
- Examine various levels of protein structure using Cn3D to view three-dimensional protein structures from NCBI’s Entrez Structure database.
- Relate the loss of function of the CFTR protein to the physiological causes of cystic fibrosis.
Protein structure; transcription; translation; DNA mutation; cystic fibrosis; genetic disease; protein function; protein folding; protein; CFTR; Cn3D
Subject Headings
EDUCATIONAL LEVEL
Undergraduate lower division, Undergraduate upper division
TOPICAL AREAS
TYPE/METHODS
Teaching Notes & Answer Key
Teaching notes.
Case teaching notes are protected and access to them is limited to paid subscribed instructors. To become a paid subscriber, purchase a subscription here .
Teaching notes are intended to help teachers select and adopt a case. They typically include a summary of the case, teaching objectives, information about the intended audience, details about how the case may be taught, and a list of references and resources.
Download Notes
Answer Keys are protected and access to them is limited to paid subscribed instructors. To become a paid subscriber, purchase a subscription here .
Download Answer Key
Materials & Media
Supplemental materials.
The following two files should be viewed with the Cn3D software to view a single domain of the CFTR and ∆F508 CFTR proteins.
You may also like
Web Seminar
Join us on Thursday, June 13, 2024, from 7:00 PM to 8:00 PM ET, to learn about the science and technology of firefighting. Wildfires have become an e...
Join us on Thursday, October 10, 2024, from 7:00 to 8:00 PM ET, for a Science Update web seminar presented by NOAA about climate science and marine sa...
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 20 April 2024
The changing epidemiology of pulmonary infection in children and adolescents with cystic fibrosis: an 18-year experience
- Jagdev Singh 1 , 2 ,
- Sharon Hunt 1 ,
- Sharon Simonds 1 ,
- Christie Boyton 1 ,
- Anna Middleton 1 ,
- Matthew Elias 2 ,
- Susan Towns 1 , 3 ,
- Chetan Pandit 1 , 3 ,
- Paul Robinson 1 , 3 ,
- Dominic A. Fitzgerald 1 , 3 &
- Hiran Selvadurai 1 , 3
Scientific Reports volume 14 , Article number: 9056 ( 2024 ) Cite this article
Metrics details
- Bacterial infection
- Cystic fibrosis
The impact of evolving treatment regimens, airway clearance strategies, and antibiotic combinations on the incidence and prevalence of respiratory infection in cystic fibrosis (CF) in children and adolescents remains unclear. The incidence, prevalence, and prescription trends from 2002 to 2019 with 18,339 airway samples were analysed. Staphylococcus aureus [− 3.86% (95% CI − 5.28–2.43)] showed the largest annual decline in incidence, followed by Haemophilus influenzae [− 3.46% (95% CI − 4.95–1.96)] and Pseudomonas aeruginosa [− 2.80%95% CI (− 4.26–1.34)]. Non-tuberculous mycobacteria and Burkholderia cepacia showed a non-significant increase in incidence. A similar pattern of change in prevalence was observed. No change in trend was observed in infants < 2 years of age. The mean age of the first isolation of S. aureus ( p < 0.001), P. aeruginosa ( p < 0.001), H. influenza ( p < 0.001), Serratia marcescens ( p = 0.006) and Aspergillus fumigatus ( p = 0.02) have increased. Nebulised amikacin (+ 3.09 ± 2.24 prescription/year, p = 0.003) and colistin (+ 1.95 ± 0.3 prescriptions/year, p = 0.032) were increasingly prescribed, while tobramycin (− 8.46 ± 4.7 prescriptions/year, p < 0.001) showed a decrease in prescription. Dornase alfa and hypertonic saline nebulisation prescription increased by 16.74 ± 4.1 prescriptions/year and 24 ± 4.6 prescriptions/year ( p < 0.001). There is a shift in CF among respiratory pathogens and prescriptions which reflects the evolution of cystic fibrosis treatment strategies over time.
Introduction
The management of pulmonary infections is critical in the care of individuals with cystic fibrosis (CF). Despite an increase in the median survival age over recent years, chronic pulmonary infection and concomitant airway inflammation leading to respiratory failure still account for 80–95% of deaths in individuals with CF 1 , 2 . This vicious cycle of infection and inflammation begins early in life, resulting in a decline in lung function, poorer nutrition, and structural lung abnormalities 3 .
Assessing long-term epidemiological trends in CF among children poses significant challenges, with studies often limited to registry reports, of a limited timeframe 4 , involve a small number of children and adolescents 5 , focus on specific organisms of interest 6 , 7 , or are derived from results obtained from bronchioalveolar sampling alone 8 , 9 . Furthermore, larger studies conducted before the year 2000 may not reflect recent advancements in CF treatment 10 , 11 , 12 , 13 , 14 , highlighting the need to evaluate any changes in the incidence and prevalence of CF bacterial pathogens to establish a reference point for future therapeutic interventions.
To this end, we conducted a study to investigate the trends in the incidence and prevalence of respiratory pathogens among children and adolescents with CF since the turn of the new millennium. By evaluating long-term longitudinal data within a clinical setting in the modern era of eradication therapy 15 , we would like to determine the changes that may have occurred in different age groups over time.
Methodology
Study population.
Children and adolescents with CF between birth to 18 years of age who were managed within a large CF centre in Australia between January 2002 and December 2019 were included in this study. Universal newborn screening of cystic fibrosis had been well-established before the study period 16 . Data collected from their existing electronic medical record included; the microbiological culture result (method of collection, date during which sample was collected with the corresponding age of the child or adolescent), and hospital pharmacy-based medication prescription data. This study was approved by the Ethics Committee of the Sydney Children’s Hospital Network (2020/ETH00815) and was conducted based on local guidelines and regulations. Exemption from consent was obtained from, and approved by the same committee.

Clinical routine during the study period
In our centre which encompasses a large region in New South Wales, outpatient (CF clinic) reviews occur four times a year, with infants or those who are clinically unwell reviewed on a more frequent basis. During these visits, airway samples are routinely collected regardless of the presence or absence of symptoms either through spontaneous expectoration (typically in older children), oropharyngeal suctioning performed by a trained CF nurse (typically in younger children), or via bronchoalveolar lavage (BAL). Airway samples microbiological cultures are ordered based on either BAL culture order label (samples obtained via BAL) or sputum CF culture order label (samples obtained through either spontaneously expectorated sputum or airway sample obtained from oropharyngeal suctioning).
All infants less than one year of age have been prescribed oral flucloxacillin or occasionally amoxicillin and clavulanic acid from diagnosis as part of our CF clinics’ routine Staphylococcus aureus prophylaxis approach for over 20 years.
In terms of the microbiological practices which has remained consistent during this study period, sputum specimens have been set up on (1) MacConkey agar for gram-negative bacteria e.g., coliforms, Pseudomonas aeruginosa, and Inquilinus limosus , (2) Anaerobically incubated chocolate agar with Bacitracin for Haemophilus influenzae . (3) Mannitol salt agar for S. aureus (4) Horse blood agar for e.g., Streptococcus pneumoniae and Moraxella catarrhalis . (5) Cepacia agar for Burkholderia cepacia and incubated for 7 days. (6) Non-tuberculous mycobacteria (NTM) testing is performed in an external Mycobacterium Reference Laboratory (MRL) using the automated blood culture system (BD BACTEC™) and testing occurs annually. Matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry (MS) has been used since 2015 for the rapid identification of organisms.
The microbiologist's report on the results of the collected airway samples is routinely reviewed by the CF team within 5–7 days after the samples are obtained. Treatment, where applicable following discussion with the primary CF physician is then prescribed. The treatment strategy includes; admission for parenteral antibiotics, a course of oral antibiotics, and/or nebulised antibiotic treatment.
Case definitions and stratification
Incidence was defined as the first time a respiratory pathogen of interest is isolated from the sputum of the child or adolescent with CF. Once the child or adolescent is an incident case for that particular pathogen, they were excluded from the denominator for the subsequent years.
Prevalence was defined as a child or adolescent with a respiratory pathogen isolated from their sputum in a specific year. Once the child or adolescent is a prevalent case for that particular pathogen, any further positive culture of the same pathogen isolated from the same child or adolescent was excluded for the remainder of that year.
Nine organisms of clinical interest in CF were selected for analysis. This includes; S. aureus, P. aeruginosa, H. influenza, Aspergillus fumigatus, Serratia marcescens, NTM, B. cepacia, Achromobacter xylosoxidans , and Stenotrophomonas maltophilia 17 .
The cohort was divided into four age groups: < 2 years, 2–5 years, 6–11 years, and > 12 years. The rationale behind this age group includes (1) biological variability in terms of differences in microbiome composition, immune system development and environmental exposure e.g. home or pre-school (2) management approaches such as methods of physiotherapy, lung function testing or the availability of medications such as dornase alfa (3) to align with existing clinical trials in CF transmembrane conductance regulator (CFTR) and CF registry reports.
In terms of medications prescribed and obtained from the hospital pharmacy, prescription of oral antimicrobials (including amoxicillin and clavulanic acid, ciprofloxacin, trimethoprim/sulfamethoxazole, flucloxacillin, and itraconazole), nebulised antimicrobials (including amikacin, colistin, and tobramycin), and other medications (including dornase-alfa, hypertonic saline nebules, and CFTR modulators and correctors) were reviewed.
Statistical analysis
We used descriptive statistics to summarise the data, reporting organism incidence and prevalence as n (%). To assess changes over time, we calculated the annual incidence and prevalence of each organism based on individual airway samples, and used regression analysis to evaluate these measures. Based on the coefficients obtained from the regression model, the average change in incidence and prevalence was presented. Prescription trends were also analysed on an individual basis. Results are reported as % change (with 95% confidence intervals) for incidence and prevalence, and as number of prescriptions/year ± standard deviation for medications prescribed. Changes in the mean age of first organism isolation were assessed using analysis of variance. All statistical calculations were performed using the SPSS Statistic Data Editor (IBM Version 28, New York, USA, 2021). Statistical significance was defined as p < 0.05.
Study population and bacterial samples
During the study period, 419 children and adolescents with CF were followed up with 206 (49.2%) born on, or after 1st January 2002. A total of 18,339 airway samples were collected during the study period with 401 (2.2%) collected via bronchioalveolar lavage, with the remaining samples obtained from expectorated sputum or oropharyngeal suction.
Out of the total airway samples that were collected, 724 (3.9%) samples met the criteria for incidence and 15,332 (83.6%) samples met the criteria for prevalence as defined in the methodology of this study were included in the analysis.
Incidence and prevalence of respiratory pathogens
Throughout the entire study period, S. aureus (25.1%), P. aeruginosa (26.2%), and H. influenzae (17.9%) exhibited the highest incidence among respiratory pathogens. Together, these pathogens accounted for 70% of the overall incidence over 18 years. In contrast, B. cepacia (0.69%), A. xylosoxidans (2.1%), and NTM (3.7%) had the lowest incidence across the study period, collectively representing 6.5% of the overall incidence over 18 years (Table 1 ).
Throughout the entire study period, S. aureus (47.8%), P. aeruginosa (34.5%), and A. fumigatus (8.4%) exhibited the highest prevalence among respiratory pathogens. Together, these organisms constituted almost 95% of the overall prevalence over 18 years. In contrast, the least prevalent respiratory pathogens were NTM (0.72%), B. cepacia (0.69%), and A. xylosoxidans (0.48%) throughout the study period. Collectively, these organisms represented less than two percent of the overall prevalence over 18 years (Table 2 ).
Changes in age of first isolation of respiratory pathogens
The ages at which these pathogens were first isolated are as follows: S. aureus (3.35 ± 2.1 years), H. influenza (4.28 ± 2.7 years), S. marcescens (5.24 ± 4.09 years), P. aeruginosa (5.27 ± 2.9 years), A. fumigatus (7.31 ± 2.85 years). This is followed by S. maltophilia (8.95 ± 2.95 years), B. cepacia (9.055 ± 2.3 years), NTM (11.38 ± 2.06 years), A. xylosoxidans (11.71 ± 2.86 years).
Over time, respiratory pathogens have shown an increase in the mean age of the first isolation: S. aureus ( p < 0.001), P. aeruginosa ( p < 0.001), H. influenza ( p < 0.001), S. marcescens ( p = 0.006), A. Fumigatus ( p = 0.02), B. cepacia ( p = 0.58), NTM ( p = 0.052), S. marcescens ( p = 0.308), S. maltophilia ( p = 0.47), A. xylosoxidans ( p = 0.80). The changes over years of these respiratory pathogens are illustrated in Fig. 1 .
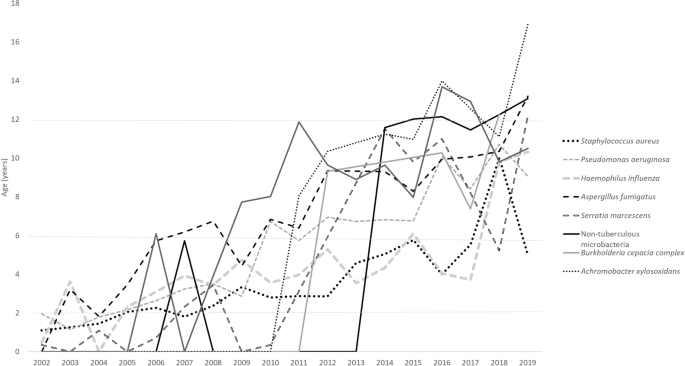
Mean age group of the first culture of CF organisms.
Changes of overall and age-specific incidence and prevalence of CF organisms from 2002 to 2019
Amongst the organisms with the highest incidence, S. aureus showed the largest decline in incidence over time, followed by H. influenza and P. aeruginosa . Meanwhile, NTM and B. cepacia showed a non-significant increase in incidence. A similar pattern of change in prevalence was observed (Tables 1 , 2 ).
With respect to age groups, incidence of S. aureus, P. aeruginosa, H. influenza and A. fumigatus in children < 2 years of age have remained unchanged. A similar pattern of change in prevalence was observed. Meanwhile, NTM showed a significant increase in both incidence and prevalence in children 6–11 years of age.
Throughout this study, a total of 29,203 medications (oral antimicrobials n = 18,367, 62.9%) were prescribed. The antibiotics that were increasingly prescribed include amikacin (3.09 ± 2.24 prescription/year, p = 0.003), amoxicillin/clavulanic acid (8.98 ± 2.17 prescriptions/year, p < 0.001), colistin (1.95 ± 0.3 prescriptions/year, p = 0.032), trimethoprim/sulfamethoxazole (18.1 ± 8.7, p < 0.001). Flucloxacillin (− 4.48 ± 1.073, p < 0.001), tobramycin (− 8.46 ± 4.7, p < 0.001) showed a decrease in prescription. Ciprofloxacin (− 6.049 ± 5.1 prescriptions/year, p = 0.068) and itraconazole (− 4.53 ± 1 prescriptions/year, p = 0.07) did not show any significant change over time.
Dornase alfa prescription increased by 16.74 ± 4.1 prescriptions/year ( p < 0.001). The prescription of hypertonic saline nebulisation increased by 24 ± 4.6 prescriptions/year ( p < 0.001). There were 7 children or adolescents on CFTR corrector or modulator therapy.
This paediatric-focused study evaluates annual changes in the incidence and prevalence rates of respiratory pathogens across different age groups, while also comparing medication prescription trends over an 18-year period. This study provides valuable data from a real-world clinical setting where infants under the age of one receive universal antimicrobial prophylaxis and, standardised respiratory pathogen surveillance is conducted by qualified personals using consistent sampling and microbiological testing protocols. In particular, obtaining samples through sputum and oropharyngeal suctioning is considered to have the highest concordance with BAL samples, rendering them more representative of lower airway infections compared to other sampling methods like throat or cough swabs 18 . The findings contribute to our understanding of the long-term trends in respiratory pathogens and associated clinical management in the paediatric population, particularly in the modern era of eradication therapy 15 .
Our study showed that together, S. aureus and P. aeruginosa make up the majority of respiratory pathogens both in terms of incidence (51.3%) and prevalence (82.3%). Data preceding 2000, report prevalence of these two respiratory pathogens to be higher at 95% 14 .
Registry data taken from 2018 to 2020 showed a prevalence of P. aeruginosa of 20.9% 17 and S. aureus of 55.26% in children and adolescents under the age of 18. In comparison, our data shows a recent prevalence of P. aeruginosa of 17.6% and S. aureus of 45.3%. Of the less frequent respiratory pathogens, NTM prevalence was 4.3% from registry data vs 3.7% from our cohort and B .cepacia was 3.2% vs. 1.3% respectively.
In a recent publication by VanDevanter et al., a trend of decline in P. aeruginosa prevalence was observed, as evidenced by the examination and presentation of registry data within a comparable time frame 19 . Following this, Fischer et al. raised a crucial question regarding whether the observed changes in P. aeruginosa over time were also apparent in other respiratory pathogens of interest in CF 20 . We have demonstrated that over the past 18 years, the incidence and prevalence of the most common respiratory pathogens in CF such as S. aureus , P. aeruginosa , H. influenzae and A. fumigatus have decreased steadily. This significant decline of between 2 and 4% of individual respiratory pathogens are observed both in the incidence and prevalence. Meanwhile, less common organisms such as NTM , B. cepacia and A. xylosoxidans, S. maltophilia showed no significant change in terms of incidence and prevalence.
We also found that the incidence and prevalence of respiratory pathogens remain unchanged for infants up to 2 years of age across all respiratory pathogens. Additionally, we have found that our cohort of children and adolescents with CF are found to have a positive airway sample culture for these respiratory pathogens significantly later that the earlier years of this study.
Our centre has adopted the universal use of S. aureus prophylactic antibiotics in infants diagnosed with CF preceding this study period. In a systematic analysis performed which reviewed four studies, there was a weak indication that P.aeruginosa was isolated less frequently in children under three years and more frequently in children between three to six years in the prophylactic group 21 . In contrast, despite our universal use of prophylactic antibiotics in infants, our study shows (1) a decline in the incidence and prevalence of P. aeruginosa , (2) no significant increase in the incidence and prevalence of organisms such as NTM and B. cepacia (3) an increase in the mean age of first isolation of respiratory pathogens of interest, (4) no change of incidence and prevalence of respiratory pathogen < 2 years of age. A contributing factor in terms of improvements in infection control practices may have helped keep our incidence and prevalence lower than the national average. While being potentially circumstantial, these findings suggest that the use of prophylactic anti-staphylococcal antibiotics is not associated with an increase in P. aeruginosa or increase in prevalence of other less common respiratory pathogen. Prospective studies such as the CF-START study in evaluating outcomes of prophylactic treatments will hopefully provide conclusive proof of its benefits and safety 21 .
By examining prescription trends, we have found that there is a rise in the use of anti-pseudomonal nebulised antibiotics such as amikacin and colistin. This suggests that P.aeruginosa is being more aggressively treated over time as both this antibiotics are considered as second line after tobramycin 22 . However, the increase in use of amikacin could also be attributed to an increase in NTM incidence and prevalence. Encouragingly, we have found that the emphasis on respiratory clearance has increased over time with the significant increase in the prescription of dornase alpha and hypertonic saline in our cohort.
Our study comes with certain limitations that warrant consideration. Firstly, the sputum and prescription data lack representation from external laboratories or pharmacies, potentially limiting the comprehensiveness of our findings. Additionally, we did not culture anaerobic bacteria and did not routinely test for co-infection with respiratory viruses, leading to an omission in addressing potential co-infections among these organisms in our study. Moreover, the annual frequency of NTM testing, as opposed to routine CF airway sample cultures, may result in an underrepresentation of NTM within our study cohort.
Thirdly, our data originated from a single CF centre in Australia, raising concerns about the generalisability of our findings to a broader population. Fourthly, our incidence calculation may involve a small number of children or adolescents intermittently found to have these respiratory pathogens in their airway samples. Finally, the relatively limited sample size of children and adolescents on CFTR modulators or correctors is noteworthy, as our study predates the widespread adoption that followed the approval and government funding of these medications in Australia. Current evidence suggests that while it may more difficult to obtain sputum samples in children on CFTR therapy, its’ impact on the growth of specific bacterial pathogens needs to be closely examined 23 . The low number of children or adolescents on CFTR modulators or correctors is an important aspect of this study as it will enable future comparison in a post-modulator era in the management of CF.
Our study has several strengths. First, we analysed a large number of sputum samples, both overall and in different age groups, providing a longitudinal comparison of changes in CF treatment over the past 18 years. This is the first study of such magnitude in children and adolescents with CF, providing age-specific incidence and prevalence, as well as prescription trends. In particular, our review of incidences of these organisms and the age of first positive culture provides additional information towards our understanding of CF respiratory pathogens over the past two decades.
Second, our study includes a large cohort of children born on or after January 1st, 2002, when newborn screening has already been well-established, allowing us to assess the acquisition of respiratory pathogens from shortly after birth over the past 18 years. Third, the practice of using prophylactic anti-staphylococcus antibiotics universally has given us the opportunity to assess the outcomes of its’ use over a significantly long period of time. While strong conclusions cannot be made without a non-prophylactic control arm, it does provide insight into the long-term impact of its’ implementation on respiratory pathogens in our cohort.
In summary, our study shows a change in the epidemiology of CF pathogens in a single large paediatric clinic that practices universal prophylaxis in children. First, we observed a decline in the incidence and prevalence of the most commonly found CF pathogens such as S. aureus, P. aeruginosa, H. influenzae, and A. fumigatus , as well as a delay in the first acquisition of these pathogens. However, less common pathogens such as S. marcescens , NTM, B. cepacia, A. xylosoxidans , and S. maltophilia did not show significant changes. Second, we found no change in the incidence or prevalence of respiratory pathogens in infants under 2 years of age over time.
Data availability
Data is available from the corresponding author, upon reasonable request.
Flume, P. A. et al. Cystic fibrosis pulmonary guidelines: Chronic medications for maintenance of lung health. Am. J. Respir. Crit. Care Med. 176 , 957–969 (2007).
Article CAS PubMed Google Scholar
Lyczak, J. B., Cannon, C. L. & Pier, G. B. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15 , 194–222. https://doi.org/10.1128/CMR.15.2.194-222.2002 (2002).
Article CAS PubMed PubMed Central Google Scholar
Pillarisetti, N. et al. Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am. J. Resp. Crit. Care Med. 184 , 75–81. https://doi.org/10.1164/rccm.201011-1892OC (2011).
Article PubMed Google Scholar
Spicuzza, L. et al. Emerging pathogens in cystic fibrosis: Ten years of follow-up in a cohort of patients. Eur. J. Clin. Microbiol. Infect. Dis. 28 , 191–195 (2009).
Coburn, B. et al. Lung microbiota across age and disease stage in cystic fibrosis. Sci. Rep. 5 , 10241. https://doi.org/10.1038/srep10241 (2015).
Article ADS CAS PubMed PubMed Central Google Scholar
Abidin, N. Z. et al. Trends in nontuberculous mycobacteria infection in children and young people with cystic fibrosis. J. Cystic Fibrosis 20 , 737–741 (2021).
Article Google Scholar
Miall, L. S., McGinley, N. T., Brownlee, K. G. & Conway, S. P. Methicillin resistant Staphylococcus aureus (MRSA) infection in cystic fibrosis. Arch. Dis. Childhood 84 , 160–162. https://doi.org/10.1136/adc.84.2.160 (2001).
Article CAS Google Scholar
Breuer, O. et al. Changing prevalence of lower airway infections in young children with cystic fibrosis. Am. J. Resp. Crit. Care Med. 200 , 590–599. https://doi.org/10.1164/rccm.201810-1919OC (2019).
Hilliard, T. N. et al. Bronchoscopy following diagnosis with cystic fibrosis. Arch. Dis. Childhood 92 , 898–899. https://doi.org/10.1136/adc.2006.105825 (2007).
Gilligan, P. H. Microbiology of airway disease in patients with cystic fibrosis. Clin. Microbiol. Rev. 4 , 35–51 (1991).
Burns, J. L. et al. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin. Infect. Dis. 27 , 158–163 (1998).
Govan, J. & Nelson, J. Microbiology of lung infection in cystic fibrosis. Br. Med. Bull. 48 , 912–930 (1992).
Millar, F., Simmonds, N. & Hodson, M. Trends in pathogens colonising the respiratory tract of adult patients with cystic fibrosis, 1985–2005. J. Cystic Fibrosis 8 , 386–391 (2009).
Razvi, S. et al. Respiratory microbiology of patients with cystic fibrosis in the United States, 1995 to 2005. Chest 136 , 1554–1560. https://doi.org/10.1378/chest.09-0132 (2009).
Ramsay, K. A. et al. The changing prevalence of pulmonary infection in adults with cystic fibrosis: A longitudinal analysis. J. Cystic Fibrosis Off. J. Eur. Cystic Fibrosis Soc. 16 , 70–77. https://doi.org/10.1016/j.jcf.2016.07.010 (2017).
McKay, K. O., Waters, D. L. & Gaskin, K. J. The influence of newborn screening for cystic fibrosis on pulmonary outcomes in New South Wales. J. Pediatrics 147 , S47–S50. https://doi.org/10.1016/j.jpeds.2005.08.013 (2005).
Ahern, S. et al. The Australian cystic fibrosis data registry: Annual report 2019 (2021).
Schultz, A. & Caudri, D. Cough swabs less useful but induced sputum very useful in symptomatic older children with cystic fibrosis. Lancet Resp. Med. 6 , 410–411 (2018).
VanDevanter, D. R., LiPuma, J. J. & Konstan, M. W. Longitudinal bacterial prevalence in cystic fibrosis airways: Fact and artifact. J. Cystic Fibrosis Off. J. Eur. Cystic Fibrosis Soc. https://doi.org/10.1016/j.jcf.2023.09.011 (2023).
Fischer, A. J. & Planet, P. J. A birth cohort approach to understanding cystic fibrosis lung infections. J. Cystic Fibrosis Off. J. Eur. Cystic Fibrosis Soc. https://doi.org/10.1016/j.jcf.2023.10.014 (2023).
Rosenfeld, M., Rayner, O. & Smyth, A. R. Prophylactic anti-staphylococcal antibiotics for cystic fibrosis. Cochrane Database System. Rev. https://doi.org/10.1002/14651858.CD001912.pub5 (2020).
Elborn, J., Hodson, M. & Bertram, C. Implementation of European standards of care for cystic fibrosis—control and treatment of infection. J. Cystic fibrosis 8 , 211–217 (2009).
Nichols, D. P. et al. PROMISE: Working with the CF community to understand emerging clinical and research needs for those treated with highly effective CFTR modulator therapy. J. Cystic Fibrosis 20 , 205–212. https://doi.org/10.1016/j.jcf.2021.02.003 (2021).
Download references
Acknowledgements
We extend our gratitude to The Cure4CF Foundation and The Team Simon Foundation for Cystic Fibrosis for their generous financial support towards this study.
Author information
Authors and affiliations.
Department of Respiratory Medicine, The Children’s Hospital at Westmead, Sydney, NSW, Australia
Jagdev Singh, Sharon Hunt, Sharon Simonds, Christie Boyton, Anna Middleton, Susan Towns, Chetan Pandit, Paul Robinson, Dominic A. Fitzgerald & Hiran Selvadurai
Department of Pharmacy, The Children’s Hospital at Westmead, Sydney, NSW, Australia
Jagdev Singh & Matthew Elias
Discipline of Child and Adolescent Health, Sydney Medical School, University of Sydney, Sydney, NSW, Australia
Susan Towns, Chetan Pandit, Paul Robinson, Dominic A. Fitzgerald & Hiran Selvadurai
You can also search for this author in PubMed Google Scholar
Contributions
H.S. and J.S. conceived the research question. J.S., H.S. and D.F. designed the study and analysis plan. J.S., S.H., S.S., C.B., A.M. and M.E. collected the data. J.S. performed the statistical analysis. H.S., D.F., P.R., S.T. and C.P. reviewed the data. J.S. drafted the initial and final versions of the manuscript. All authors critically reviewed early and final versions of the manuscript.
Corresponding author
Correspondence to Jagdev Singh .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Singh, J., Hunt, S., Simonds, S. et al. The changing epidemiology of pulmonary infection in children and adolescents with cystic fibrosis: an 18-year experience. Sci Rep 14 , 9056 (2024). https://doi.org/10.1038/s41598-024-59658-4
Download citation
Received : 31 August 2023
Accepted : 12 April 2024
Published : 20 April 2024
DOI : https://doi.org/10.1038/s41598-024-59658-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
REVIEW article
Occurrence of covid-19 in cystic fibrosis patients: a review.

- 1 Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
- 2 Department of Bacteriology and Virology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
- 3 Department of Bacteriology, Pasteur Institute of Iran, Tehran, Iran
- 4 Department of Microbiology, Islamic Azad University, Arak, Iran
- 5 Nervous System Stem Cells Research Center, Semnan University of Medical Sciences, Semnan, Iran
- 6 Department of Medical Microbiology, School of Medicine, Shahed University, Tehran, Iran
- 7 Pediatric Pulmonary Disease and Sleep Medicine Research Center, Pediatric Center of Excellence, Children's Medical Center, Tehran, Iran
- 8 Cystic Fibrosis Research Center, Iran CF Foundation (ICFF), Tehran, Iran
Cystic fibrosis (CF) is a genetic ailment caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. This autosomal recessive disorder is characterized by diverse pathobiological abnormalities, such as the disorder of CFTR channels in mucosal surfaces, caused by inadequate clearance of mucus and sputum, in addition to the malfunctioning of mucous organs. However, the primary motive of mortality in CF patients is pulmonary failure, which is attributed to the colonization of opportunistic microorganisms, formation of resistant biofilms, and a subsequent decline in lung characteristics. In December 2019, the World Health Organization (WHO) declared the outbreak of the radical coronavirus disease 2019 (COVID-19) as a worldwide public health crisis, which unexpectedly spread not only within China but also globally. Given that the respiration system is the primary target of the COVID-19 virus, it is crucial to investigate the impact of COVID-19 on the pathogenesis and mortality of CF patients, mainly in the context of acute respiratory distress syndrome (ARDS). Therefore, the goal of this review is to comprehensively review the present literature on the relationship between cystic fibrosis, COVID-19 contamination, and development of ARDS. Several investigations performed during the early stages of the virus outbreak have discovered unexpected findings regarding the occurrence and effectiveness of COVID-19 in individuals with CF. Contrary to initial expectancies, the rate of infection and the effectiveness of the virus in CF patients are lower than those in the overall population. This finding may be attributed to different factors, including the presence of thick mucus, social avoidance, using remedies that include azithromycin, the fairly younger age of CF patients, decreased presence of ACE-2 receptors, and the effect of CFTR channel disorder on the replication cycle and infectivity of the virus. However, it is important to notice that certain situations, which include undergoing a transplant, can also doubtlessly boost the susceptibility of CF patients to COVID-19. Furthermore, with an increase in age in CF patients, it is vital to take into account the prevalence of the SARS-CoV-2 virus in this population. Therefore, ordinary surveillance of CF patients is vital to evaluate and save the population from the capability of transmission of the virus given the various factors that contribute to the spread of the SARS-CoV-2 outbreak in this precise organization.
Introduction
Cystic fibrosis (CF) is a genetic ailment resulting from an autosomal recessive trait. In the United States of America, the estimated prevalence of CF disease is 0.797 per 10,000 individuals; at the same time, among Caucasians who are born alive, the superiority is 1 in 2,500 ( Stern, 1997 ). An association related to CF has expressed concerns about the potential impact of COVID-19 on CF patients, as previous viral infections have been associated with severe outcomes and consequences. Furthermore, a significant number of CF patients already have impaired lung function ( Lambrecht et al., 2001 ). In the United States of America, the estimated prevalence of CF disease is 0.797 per 10,000 individuals, while in Caucasians who are born alive, the prevalence is 1 in 2,500 ( Stern, 1997 ). The CF association is expressing significant concerns about the potential impact of the coronavirus disease on individuals with CF, as previous viral infections have been linked to more severe outcomes. Additionally, a significant proportion of CF patients already have impaired lung function ( Lambrecht et al., 2001 ).
Etiopathology of cystic fibrosis (CF)
The prevalence of CF has increased due to a genetic mutation within a specific gene located on chromosome 7. This gene encodes a protein referred to as the cystic fibrosis transmembrane conductance regulator (CFTR), which consists of 1,480 amino acids. The CFTR protein has a vital function in regulating the movement of electrolytes at some point of epithelial-cell membranes, and it is also believed to have an effect on intracellular membranes ( Stern, 1997 ; Terlizzi et al., 2022a , b ).
One of the foremost complications related to CF is continuous pulmonary infections, which might be the leading cause of respiratory failure in individuals with CF ( Meng et al., 2020 ). A study carried out with the aid of the Cystic Fibrosis Foundation Patient Registry (CFFPR) from 2017 to 2021 centered on newborn infants and found that CF patients have a median lifespan of ~53 years ( Taylor-Cousar et al., 2023 ). In individuals with CF, the presence of two faulty CF genes and malfunctioning elements of the genetic machinery can result in multiplied stages of mucus in diverse organs, which can cause similar complications and impair the regular functioning of these organs.
This situation could have detrimental effects on the lungs, pancreas, liver, intestines, and salivary glands. On the other hand, although the CFTR channel and chloride ions are not functioning properly due to insufficient shipping, it causes the production of thick mucus. Mucous production obstructs the airways, making it hard to breathe and increasing the probability of bacterial lung infections in affected individuals ( Blevings et al., 2022 ).
Immune responses in cystic fibrosis
CF patients affected with COVID-19 show an exaggerated inflammatory reaction characterized by the release of positive chemical compounds and cells such as IL-8, TNF, mucin, polymorphonuclear leukocytes (PMNs), and serine proteases. These robust inflammatory reactions contribute to the further development of CF symptoms. The abnormal innate immune responses found in CF patients may be attributed to the CFTR mutation, which negatively affects the function of the epithelial innate immune system.
Under these circumstances, the NF-κB pathway, which induces activation of the positive genes, becomes active, resulting in a multiplied secretion of IL-8. The law of TLR4 and IFN-γ expression and accumulation is compromised in a weakened immune system. Additionally, the activation of pulmonary dendritic cells (DCs), which play a crucial role in T-cell-established immunity and immune surveillance, is decreased ( Lambrecht et al., 2001 ).
Epidemiology and clinical outcomes of COVID-19
Among the extremely good human coronaviruses (HCoVs), the novel coronavirus (SARS-CoV-2) is significant. HCoVs, which are RNA-enveloped viruses, can be transmitted from animals, such as rodent and bat families, to human beings. In December 2019, the World Health Organization (WHO) identified SARS-CoV-2 in Wuhan, China, ultimately leading to the prevalence of acute respiration distress syndrome (ARDS). On 11 March 2020, the novel coronavirus, known as COVID-19, was declared an international pandemic. It is crucial to highlight that SARS-CoV-2 is a newly diagnosed strain that has not been previously detected in humans. The outbreak ended with over 381,000 individuals being infected across 195 countries, with the number of deaths exceeding 16,000. In patients having inflammation with severe acute respiration syndrome coronavirus 2 (SARS-CoV-2), common signs and symptoms include low-grade fever, fatigue, dry cough, sore throat, diarrhea, anosmia, and loss of flavor or scent. While many people with this contamination might also be asymptomatic or experience slightly higher breathing tract symptoms, there are times when they will develop intense and acute respiratory distress syndrome (ARDS; Chams et al., 2020 ; Chen et al., 2020 ). Contracting COVID-19 can bring about numerous organ-related troubles, such as acute kidney injury, vascular blood clots, endothelial cell damage, and shock. These issues and headaches can substantially increase pressure on society and healthcare systems ( Bradley et al., 2020 ; Chams et al., 2020 ; Yohannes, 2021 ).
Microbiome changes linked to COVID-19
The microbiome consists of a wide variety of microorganisms, including bacteria, fungi, viruses, and protozoans, that live in distinct organs at some point in the human body. These microorganisms have a substantial function in regulating cell metabolisms and biological signaling pathways ( Gilbert et al., 2018 ). Evidence supports the critical involvement of the microbiome community, especially intestinal microbiota, in maintaining a properly functioning bodily system, immune responses, and metabolic functions. In cystic fibrosis patients, there is regular dysbiosis of the intestinal microbiota over time, characterized by a reduction in the abundance of useful bacteria and an increase in pathogens. This imbalance can bring about inflammation and compromise immune responses. However, the impact of this dysbiosis on the susceptibility to or transmission of COVID-19 in these patients remains inadequately explored. Subsequent medical evaluations have found the prevalence of intestinal dysbiosis and changes in the respiration microbiome in people affected by COVID-19 ( Segal et al., 2020 ). An observation in 2022 potentiated that the depletion of Faecalibacterium prausnitzii ought to contribute to respiratory and lung disorders along with allergies and cystic fibrosis ( Vernocchi et al., 2018 ; Demirci et al., 2019 ). F. prausnitzii is believed to possess anti-inflammatory properties, protecting against various gastrointestinal illnesses, including Crohn's disease ( Parada Venegas et al., 2019 ; Leylabadlo et al., 2020 ). Furthermore, more recent research on COVID-19-recovered patients with persistent symptoms discovered that post-exercising chest tightness becomes inversely associated with the relative abundance of F. prausnitzii ( Zhou et al., 2021 ). Throughout the duration of hospitalization in Hong Kong, the stool samples of the 15 COVID-19 patients displayed the growth of opportunistic bacteria such as Rothia, Streptococcus, Actinomyces , and Veillonella , as indicated by similar examinations. Additionally, a lower bacterial range was found in these samples ( Gu et al., 2020 ). Moreover, alterations in the gut and lung microbiomes may facilitate the infiltration of the SARS-CoV-2 virus into lung tissue. The lung microbiomes play critical roles in the onset, development, and efficacy of therapeutic interventions ( Ye et al., 2020 ).
Entry of SARS-CoV-2 of SARSCoV-2; outcome in CF patients
The SARS-CoV-2 virus enters cells by using the spike (S) protein, which is located on its surface. This protein is made up of two parts, S1 and S2. The S1 subunit attaches to the angiotensin-converting enzyme 2 (ACE2), which is the main receptor on the surface of certain cells in the airway epithelium. These cells include airway epithelial cells, goblet secretory cells, and type II cells during pneumocystis infection. When the virus enters a host cell, the S1 domain of the S protein binds to ACE2, causing the S1 subunit to be cut by cellular proteases. Then, the S2 subunit helps the viral membrane fuse with the host cell membrane, allowing viral components to be released into the host cell's cytoplasm ( Shirato et al., 2017 ; Chams et al., 2020 ; Touret et al., 2020 ; Zhao et al., 2022 ).
As shown in Figure 1 , When the spike protein attaches to the ACE2 cell membrane protein, an association is formed between the virus and the cell. The enzymes TMPRSS2 and furin help in the entry of the virus into the cell, and individuals with CF may have altered versions of these enzymes ( Chams et al., 2020 ; Peckham et al., 2020 ). There is a z report of a decreased level of TMPRSS2 among CF patients compared to the control group, although it was not significant, and there is also an increase in the TMPRSS2 enzyme in CF patients by flagellin of Pseudomonas aeruginosa ( Bitossi et al., 2021 ; Ruffin et al., 2021 ).
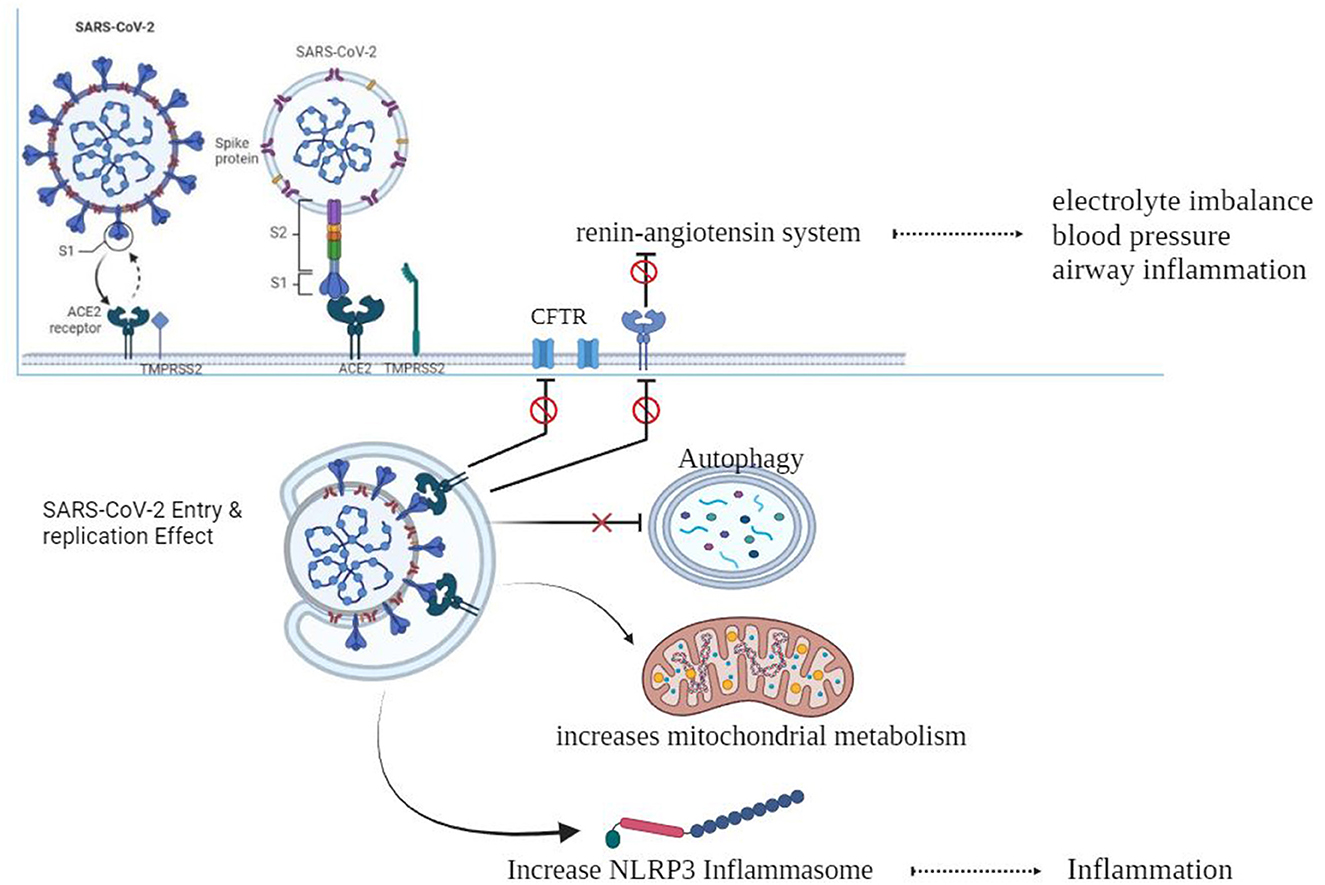
Figure 1 . SARS-CoV-2 and cystic fibrosis.
After entering the cell, the virus can be affected by excessive inflammation and CF-related cellular processes like autophagy, mitophagy, endosomal function, and cellular metabolism. SARS-CoV-2 may take advantage of these cellular processes to replicate itself ( Peckham et al., 2020 ).
CF can result in electrolyte abnormalities, while the SARS-CoV-2 infection hampers the functioning of pulmonary ACE2, resulting in the disruption of the renin–angiotensin system (RAS). This disruption has detrimental outcomes on fluid and electrolyte balance, blood pressure, and airway irritation. Furthermore, it additionally complements vascular permeability in the airways, as indicated in previous studies ( Scurati-Manzoni et al., 2014 ; Bekassy et al., 2021 ).
Replication of SARSCoV-2; outcome in CF patients
The three-chymotrypsin-like cysteine protease (3CLpro) of SARSCoV-2 is a vital component of the virus that plays a key function in virus replication and is conserved. This protease cleaves the polyproteins to generate 16 useful non-structural proteins (NSPs). The cleaved NSPs have essential functions in assembly and replication ( Mody et al., 2021 ). Figure 2 illustrates the precise interaction between viral S-glycoproteins and the cell ACE2 receptor, which allows the entry of SARS-CoV-2 into cells. The spike glycoprotein is cleaved through TMPRSS2, which helps fusion among the host cellular membrane and the virus envelope ( Hoffmann et al., 2020 ). Viral RNA is translated into non-structural proteins (NSPs). (iii) Furthermore, the virus utilizes the host cellular machinery to translate viral proteins. The RNA genome is replicated and translated into double-membrane vesicles (DMVs), which resemble bubble-like endoplasmic structures. (v) Finally, the mature virion is transported to the Golgi bodies, where it is launched through exocytosis ( Yuan et al., 2020 ; Roingeard et al., 2022 ).
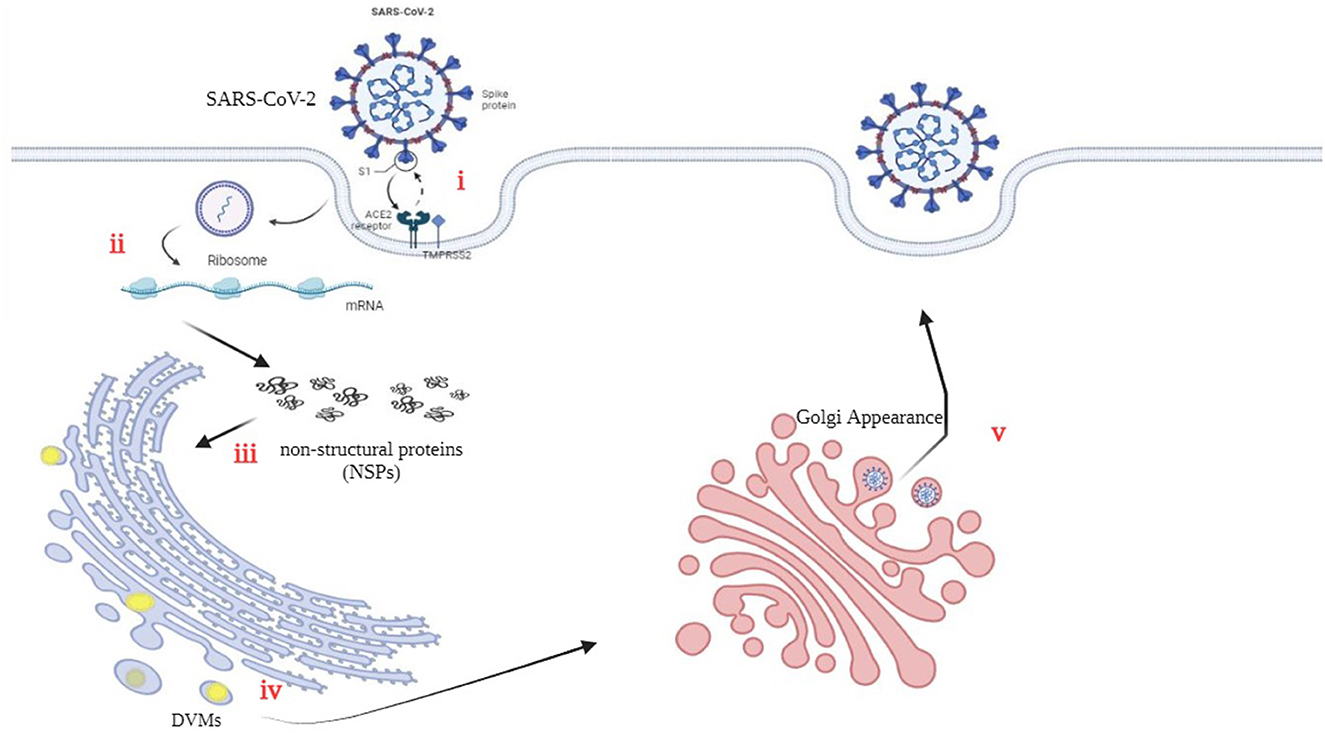
Figure 2 . An illustrative representation of the replication cycle in SARS-CoV-2.
The availability of the CFTR protein for the SARS-CoV 3CL protease may result in its cleavage ( Peckham et al., 2020 ). Additionally, evidence suggests that the airway tract microbiota, thick secretions, and autophagy induction in CF patients may play a protective role against viral infections. Autophagy, as an immune mechanism in CF disease, could be heightened and actively contributes to the antiviral response ( van Ewijk et al., 2005 ; Junkins et al., 2014 ; Mingione et al., 2020 ; Pehote and Vij, 2020 ).
Immune system status in patients affected with COVID-19 simultaneously with CF
At the onset of contamination, SARS-CoV-2 penetrates host cells, permitting its genome to enter the cytoplasm and initiate a pro-inflammatory reaction via diverse signaling pathways. During the preliminary levels of viral infection in airway epithelial cells, regulated and programmed cell death responses known as pyroptosis take place, releasing seasoned-inflammatory cytokines and chemokines such as IL-6, IFNγ, MCP1, and IP-10. In the presence of inflammatory cytokines, immune cells are recruited to eliminate host epithelial cells, thereby diminishing inflammatory immune responses in the lungs. Nevertheless, in SARS-COV-2 positive patients, induction of a cytokine storm, a deadly immune system reaction that may damage multiple organs, including the coronary heart, liver, and kidneys, in the long run may result in organ failure ( Tay et al., 2020 ; Yapasert et al., 2021 ).
The innate immune system plays an essential role in the preliminary defense in opposition to COVID-19 ( Diamond and Kanneganti, 2022 ). Among the various cytokines being considered for the improvement of COVID-19, interleukin-6 (IL-6) often emerges as the most significant. Elevated levels of IL-6 are directly associated with the development and mortality rates of this viral infection, which may be triggered by the initiation of SARS-CoV-2. Importantly, this research shows that lowering IL-6 levels ought to serve as a protective measure against SARS-CoV-2 infection, specifically in the context of cytokine storms ( Chams et al., 2020 ; Coomes and Haghbayan, 2020 ; McGonagle et al., 2020 ). In a comprehensive study involving 39 CF patients with persistent pulmonary infections, an evaluation of accumulated sputum found a decrease in IL-6 and interleukin-10 (IL-10) levels, followed by an increase in interleukin-8 (IL-8) levels. The production of IL-6 within localized sputum was found to decrease, even as systemic IL-6 levels remained unaffected ( Majka et al., 2021 ).
The airway tract of people with cystic fibrosis suffering lung damage indicates an increased concentration of neutrophil elastase, a dangerous pathogen, leading to a decrease in pulmonary function and activities. The incidence of pulmonary infections, together with acute respiratory distress syndrome (ARDS), related to COVID-19, has been linked to the increased secretion of excess neutrophil elastase. According to available evidence, the capacity treatment (therapy, cure, medication, repair, solution, medicine, fix, correct, solutions, solve, prevent, remedies, recourse) for lung damage and ARDS includes the use of neutrophil elastase inhibitors ( Mohamed et al., 2020 ; Sahebnasagh et al., 2020 ; Yang and Montgomery, 2021 ; Terlizzi et al., 2022a , b ). Nebulized dornase alfa, which breaks down DNA and promotes the discharge of extracellular traps of neutrophils, has been identified as having a useful function in the development of lung disease. Its use has shown promise in improving lung characteristics in cystic fibrosis patients and can be taken into consideration for trials for COVID-19 treatment. Although the current research proposes against the persistent use of nebulized dornase alfa, the administration of azithromycin has been indicated as an effective remedy for COVID-19 in some research ( Kournoutou and Dinos, 2022 ).
Epidemiological and clinical outcomes of SARS-CoV-2 in CF patients
Recent findings propose that the majority of children infected with the quite contagious SARS-CoV-2 virus exhibit mild signs of inflammation. However, it remains uncertain whether children with continual respiratory symptoms experience exacerbated symptoms due to SARS-CoV-2. Nevertheless, initial studies suggest that most children infected with SARS-CoV-2 exhibit mild signs and symptoms ( Borch et al., 2022 ). The recent findings revealed that children recognized with bronchial asthma and cystic fibrosis, who have reduced weight and contracted SARS-CoV-2, did not display a considerable decrease in lung characteristics. This finding is especially important as many children with bronchopulmonary dysplasia (BPD) and other breathing disorders require ventilator assistance, making them more prone to infections inside this group ( Ward, 2016 ; Kaore and Kaore, 2021 ). A study carried out across more than one country, together with the United States, verified that the duration of infection in people with CF corresponds to that of the general population. However, due to the small sample size of the studies, definitive and particular conclusions were hard to draw. Therefore, it is widely suggested that people with CF adhere strictly to public health policies to safeguard themselves from infections ( Jin et al., 2020 ). In cases in which people are recognized as having cystic fibrosis (CF) and sooner or later are identified as having SARS-CoV-2, the severity of infection can be further intensified, potentially leading to an escalation in signs and symptoms ( Hong et al., 2020 ). Several investigations were conducted in this specific context. In February 2020, a total of 13 individuals recognized with cystic fibrosis (CF) were found to be suffering from COVID-19 ( Fainardi et al., 2020 ).
The COVID-19 outbreak in Northern Italy had a substantial effect on CF patients, especially those living in areas with an excessive occurrence of the ailment. It is known that every CF patient who contracted the virus had been infected through their circle of relatives, such as close people or related people. Among those patients, 61.5% experienced slight breathing difficulties; at the same time, the remaining 38.5% required more intensive clinical interventions. Furthermore, out of the 13 patients, COVID-19 was detected in others as well. However, in May 2020, a significant number of patients, especially 85%, had recovered ( Fainardi et al., 2020 ).
In contrast to European nations with similar populations, the areas in northern Italy, namely, Lombardia, Emilia-Romagna, Veneto, and Piemont, witnessed a substantially higher mortality rate due to the referred infection.
COVID-19 has been observed to have an excessive effect on the elderly population, with men being more vulnerable to infection than women. Consequently, a considerable number of elderly individuals in Italy have a higher mortality rate ( Salzberger et al., 2021 ). Moreover, people with underlying health conditions, which include diabetes, cardiovascular diseases, or most cancers, are at a higher risk of death than those without these health conditions. Recent studies have additionally suggested cases of COVID-19 infections in kids ( Mehrabani, 2020 ). Notably, the general population tested for an additional occurrence of SARS-CoV-2 (0.15%) in comparison to CF patients (0.07%; Cosgriff et al., 2020 ). This unexpected discovery could potentially be ascribed to the relatively younger age of patients affected with CF. The utilization of antibiotic treatment among CF patients confers protection against SARS-CoV-2.
CF patients also take precautions to prevent the spread of infection, including self-isolation, which probably contributes to the low occurrence of SARS-CoV-2 infection in this population ( Bezzerri et al., 2020 ; Biondo et al., 2022 ). The Cystic Fibrosis Center in Parma endorsed self-isolation measures, including hand hygiene, sporting face masks, and remote verbal exchange, to reveal and avoid group activities throughout the pandemic period in Italy. Based on the results, it could be concluded that individuals suffering from extreme respiration infection as a result of the coronavirus may be correctly managed and guarded ( Fainardi et al., 2020 ).
In an examination conducted in France, the findings imply that patients with excessive lung ailments resulting from COVID-19 may be affected. A study conducted in France determined that, out of 31 CF patients who have mild COVID-19, only 0.41% of them had inflammation, which resulted in a significant decrease (93% less) compared to the total number of members ( Corvol et al., 2020 ). The study also found that CF patients with COVID-19 had a higher average age than the general population ( Mathew et al., 2021 ), suggesting a correlation between age and coinfection with the virus. Symptoms such as fever, fatigue, and aggravated cough were mentioned in 28 patients, while the other three patients did not display any signs ( Corvol et al., 2020 ).
Another study in Spain, at some stage in the peak of the preliminary wave of the pandemic, used RT-qPCR to confirm the presence of COVID-19 in CF patients ( Mondejar-Lopez et al., 2020 ). CF patients with COVID-19 had a higher likelihood of being hospitalized as compared to the general population. Moreover, people with CF are more likely to experience worsening of their persistent lung ailment, which may lead to respiratory tract viral infections. Research has shown that CF patients have a lower prevalence of COVID-19 infection than the overall population ( Mondejar-Lopez et al., 2020 ).
Cystic fibrosis treatment: outcome in SARS-CoV-2 infection
Prolonged usage of antibiotics, such as azithromycin, acknowledged for its anti-inflammatory properties, can suppress viral infections in CF patients. Moreover, this antibiotic reveals antiviral consequences in a model of continuous obstructive pulmonary disorder (COPD; Khezri et al., 2021 ; Suarez-Reyes and Villegas-Valverde, 2021 ). Azithromycin, an often prescribed antibiotic for cystic fibrosis (CF), holds promise for decreasing the severity of COVID-19 because of its potential to modulate the immune response and show slight antiviral properties ( Echeverria-Esnal et al., 2020 ; Ghazy et al., 2020 ; Touret et al., 2020 ). Furthermore, mutations in the CFTR gene may impact the performance of ACE2 and TMPRSS2 proteins, resulting in reduced susceptibility to SARS-CoV-2 infection ( Stanton et al., 2020 ).
The significance of microorganisms, mainly Pseudomonas aeruginosa and Staphylococcus aureus , in the treatment of continual and acute infections is noteworthy. When infection arises inside the lungs, it leads to pulmonary exacerbation (PEx), inflicting a decline in lung characteristics and negatively affecting normal, excellent lifestyles ( Lam et al., 2015 ).
Previous research has indicated that bacterial infections can affect immune responses to viral infections ( Nilashi et al., 2020 ). A previous study found that viral infections currently do not have a sizeable impact on lung features in patients with CF ( Smith et al., 1980 ). In fact, it can be asserted that the suppression of bacterial colonization, in particular Pseudomonas aeruginosa as formerly stated, is essential in dealing with CF infection. The presence of viral microorganisms played a considerable position in the exacerbation of CF ( Wark et al., 2012 ).
Medications and specialized treatments administered to people with CF can relieve the severity of COVID-19. It is plausible that pills used in the treatment of CF sufferers may be connected to a reduction in COVID-19 symptoms, playing an important function in mitigating the severity of the disorder ( Gaudio, 2020 ; Porter et al., 2023 ).
Effect of host factors of CF patients on SARS-CoV-2 infection
The variation in COVID-19 and mortality rates among distinct countries, in conjunction with the various clinical manifestations of the viral infection in patients, has been drastically documented ( Sorci et al., 2020 ). Host genetic elements were recognized as vital elements influencing the pathogenicity of COVID-19 ( Tharappel et al., 2020 ; Jafarpour et al., 2021 ). Specifically, individuals carrying single pathogenic versions of the CFTR gene (CF vendors) are more susceptible to respiratory tract infections and severe COVID-19 ( Baldassarri et al., 2021 ). ACE polymorphisms have additionally been related to COVID-19, as studies have indicated that patients with the ACE D/D polymorphism showcase advanced medical signs and symptoms and a higher danger of lung damage in comparison to people with I/I or D/I polymorphisms ( Karakaş Çelik et al., 2021 ). Furthermore, studies have validated that ACE polymorphisms can affect ACE2 expression, leading to the CF phenotype and pulmonary inflammation associated with the development of COVID-19 ( Vitiello et al., 2023 ). It is critical to highlight that the angiotensin-converting enzyme 2 (ACE2) serves as the crucial host receptor for SARS-CoV-2 entry via the spike (S) protein on the virus surface ( Zhang et al., 2020 ).
Impact of COVID-19 on individuals with cystic fibrosis
To gain a complete knowledge of the effect of COVID-19 on individuals with cystic fibrosis, it is essential to accumulate additional proof and records. The European Cystic Fibrosis Society (ECFS) performs an important role in this regard by gathering proof statistics and disseminating well-timed and vital documents from various areas across Europe ( Colombo et al., 2020 ). Respiratory infections affecting the respiratory system are more intense in cystic fibrosis patients than the overall population ( Yu and Kotsimbos, 2023 ). Furthermore, complications and destructive effects on lung characteristics were determined to be increasing among individuals with cystic fibrosis ( Flume et al., 2019 ). While a few individuals with cystic fibrosis may additionally experience respiratory fitness troubles, others may also be afflicted by a chronic airway ailment characterized by intense signs and symptoms ( Hisert et al., 2017 ). It is crucial to note that the signs of cystic fibrosis differ significantly from the clinical manifestations of COVID-19 ( Al Lawati et al., 2023 ).
Investigations have confirmed that organ transplantation is a determinant of COVID-19 prevalence. Consequently, it has been found that lung transplant recipients with COVID-19 infection have a higher mortality rate than those note affected with COVID-19 ( Pereira et al., 2020 ; Hall et al., 2022 ). In patients with cystic fibrosis (CF), mainly those experiencing extreme respiratory infections, it is important that we no longer miss the possibility of COVID-19 contamination because of reduced immunity. Despite the incredibly low prevalence of SARS-CoV-2 infections in CF people, it is more important to impress upon this population the importance of using numerous measures together with effective somatic and public distancing, as well as using structures and practices aimed toward infection management. These measures should be incorporated into ordinary CF care. Additionally, different elements can also make a contribution to the management of COVID-19 in CF patients ( Mathew et al., 2021 ).
Hence, it is more feasible for people with mild COVID-19 to be mistakenly diagnosed as having CF, while those with slight signs and symptoms can be perceived as healthy individuals. To tackle this trouble, a low-threshold trial is proposed to be implemented, which will facilitate early detection. Given the cutting-edge instances, a wide variety of households have expressed issues concerning the right of entry to medicinal drugs and food supplies ( Burgel and Goss, 2021 ; Lim et al., 2022 ). To maintain excellent health, patients with cystic fibrosis and their families must adhere to certain principles. It has been observed that CF patients have been successful in averting infection with SARS-CoV-19 ( Colombo et al., 2020 ). Researchers are presently accumulating various statistics to determine the elements that affect the severity of COVID-19 within the cystic fibrosis patients. With the spread of the SARS-CoV-2 pandemic, it is essential for us to accumulate diverse records to understand how this viral infection influences specific patient groups with unique illnesses, which includes cystic fibrosis ( Carr et al., 2022 ). One study found that 181 cystic fibrosis patients (32 post-transplant) from 19 countries were infected with SARS-CoV, and infection with SARS-CoV-2 had similar effects as determined within the general population ( McClenaghan et al., 2020 ). One study demonstrated that only a small number of individuals from the Cystic Fibrosis Registry had been diagnosed with or examined for COVID-19 on a month-to-month basis as of January 2020 ( Berardis et al., 2020 ; Colombo et al., 2020 ; Corvol et al., 2020 ; Cosgriff et al., 2020 ; McClenaghan et al., 2020 ; Scagnolari et al., 2020 ; Bain et al., 2021 ; Naehrlich et al., 2021 ).
The association between scientific cycles and certain elements, which include older age, CF-associated diabetes, decreased lung features within the 12 months before contamination, and having gone through an organ transplant, has been observed ( Jardel et al., 2018 ; Mainbourg et al., 2019 ). Despite the higher-than-anticipated effects in a large cohort, probably because of the noticeably younger CF population as compared to other continual situations, it is critical to say that SARS-CoV-2 is not a benign ailment for all individuals in this group of affected persons ( Hadi et al., 2021 ). The COVID-19 global pandemic as a result of SARS-CoV-2 has had a huge impact and continues to unfold globally, with CF being identified as a potential factor for negative effects ( Taylor-Cousar et al., 2023 ). Multiple national CF registries from diverse countries have documented a lower prevalence of SARS-CoV-2 contamination in people with cystic fibrosis (PwCF) in comparison to the general population ( Cosgriff et al., 2020 ; Flume et al., 2022 ). Furthermore, it has been discovered that more young PwCF tend to have milder signs and symptoms and inflammation with SARS-CoV-2. However, individuals with compromised immune systems and people with impaired lung function are more likely to experience intense results ( Burgel and Goss, 2021 ). Another study applied the multicenter research community TriNETX method to research the medical outcomes of COVID-19 contamination in a large cohort of PwCF in an evaluation of the general populace. The findings found that, out of the 507,810 individuals aged 6 years or older, women constituted the majority ( n = 225, 53.32%), and the common age at COVID-19 analysis among CF patients was ~46.6 years. The majority of the participants were of Caucasian ethnicity ( n = 309, 73.22%). Higher rates of hospitalization, acute renal damage, and critical care needs were found in PwCF following robust propensity matching.
Hospitalization became vital for 10% of patients with COVID-19 ( Hadi et al., 2021 ). Despite the reality that CF patients have a decreased prevalence of obesity and a lesser median age, these elements no longer offer protection against intense disease. A prospective multicenter cohort study, which involved 32 CF centers and 6,597 patients, was conducted to study the symptoms and scientific path of SARS-CoV-2 infection in PwCF. It was found that dysfunctional kidneys, respiration systems, dying facts, and unrivaled reviews were also improved in PwCF at 30 days ( Colombo et al., 2021 ). To ensure proper follow-up, facilities reached out to individuals showing symptoms indicative of COVID-19. According to a recent publication ( Tedbury et al., 2023 ), CFTR may additionally affect the severity of SARS-CoV-2 infection and COVID-19 ailments in CF patients ( Vitiello et al., 2023 ). Meanwhile, a literature evaluation ( Marques et al., 2023 ) emphasizes the restricted understanding regarding the effects of COVID-19 on CF patients. The evaluation indicates that CF patients may be at a greater risk of intense infection from COVID-19 due to their underlying lung disease and other comorbidities ( Marques et al., 2023 ). Additionally, an examination carried out in Brazil indicates using observational studies to assess the results of COVID-19 on individuals diagnosed with cystic fibrosis ( Table 1 ; Sorci et al., 2020 ).
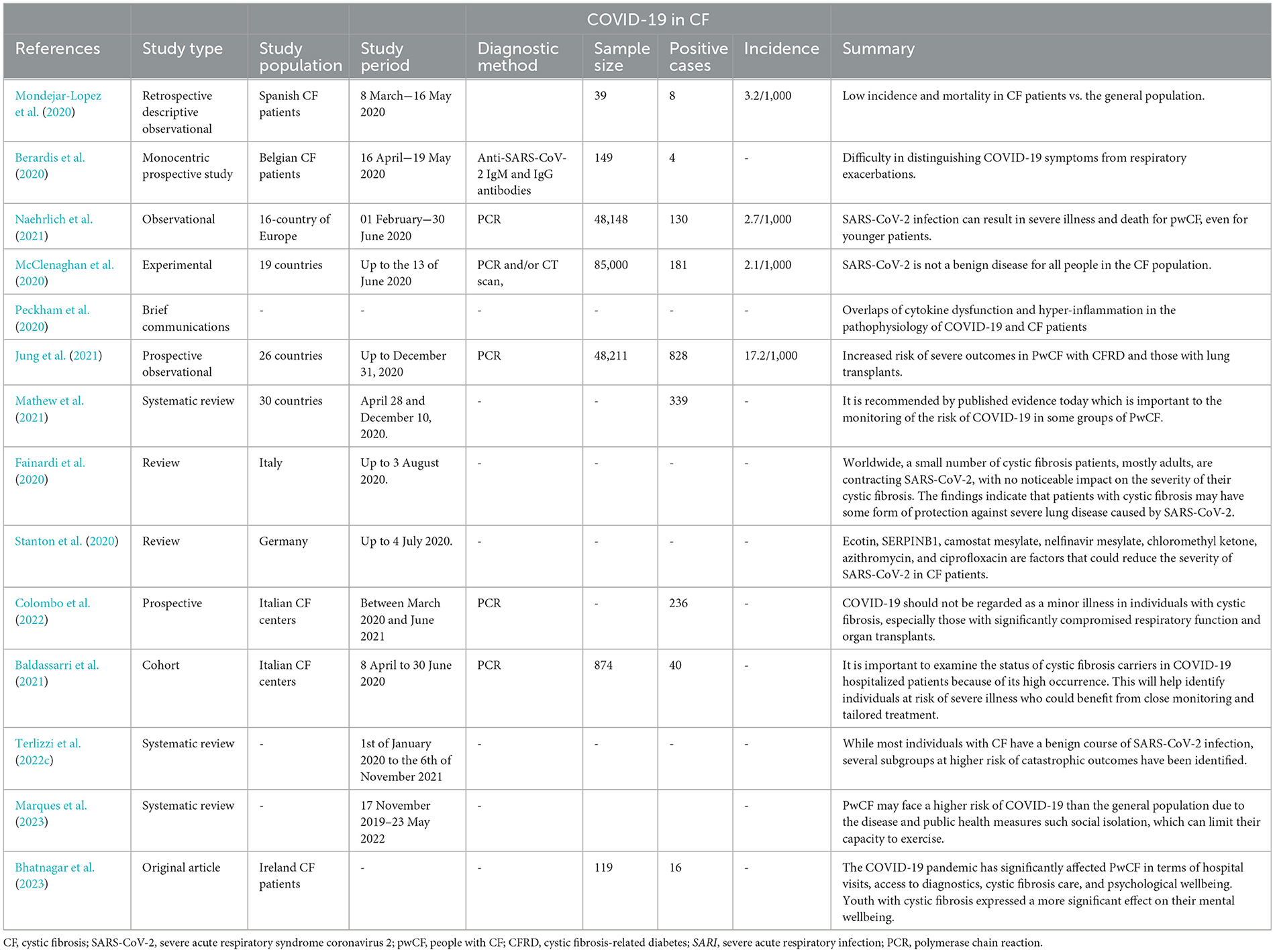
Table 1 . Studies that have been done in the field of COVID-19 infection in CF patients.
Various breathing infections have posed a hazard to individuals with CF, regularly leading to excessive consequences. However, the contribution of viral infections to CF pulmonary decline remains a subject of research. Research suggests that CF patients are prone to acute breathing viral infections, yet it appears that most people have not been critically laid low with SARS-CoV-2. Conversely, reports advise that CF patients who have undergone transplantation and finally got mild infections have been laid low with the severe SARS-CoV-2 strain. Another cause of the particularly low occurrence of this virus among CF patients is the commonly lower age of this populace. Given that many CF patients are young people and have constrained receptors for the SARS-CoV-2 virus, the occurrence and severity of the ailment are generally decreasing in this group. Recent research has proposed numerous protective mechanisms for SARS-CoV-2 infection in CF patients. These investigations have highlighted the complete function of CFTR in SARS-CoV-2 replication, as CFTR deficiency results in reduced viral replication. Panou (2018) and Lotti et al. (2022) have said that inhibiting CFTR in primary kidney cells inflamed with BK polyomavirus (BKPyV) drastically reduces the transportation of virions to the ER. Recent studies have indicated that impaired CFTR features may also have a huge effect on viral replication. Specifically, the activation of the SARS-CoV-2 S protein depends on endolysosomal proteases in acidification, and deacidification of this organelle has been shown to prevent viral infection. Mutations in the CFTR gene can result in an increase in organelle pH, resulting in altered glycosylation patterns of ACE-2 and/or TMPRSS-2, which could affect the results of SARS-CoV-2 infection. Furthermore, modifications in ionic balance can modify intracellular pH, leading to large modifications in viral protein assembly and structure ( Stanton et al., 2020 ).
From a pathogenic perspective, increased stages of neutrophil elastase are associated with extended lung damage and reduced pulmonary characteristics in CF ( Dittrich et al., 2018 ; Barth et al., 2020 ). Consequently, neutrophil elastase inhibitors are being actively examined in trials for CF treatments ( Barth et al., 2020 ). It is worth noting that an imbalance of seasoned-inflammatory neutrophil elastase is also implicated in the improvement of acute respiratory distress syndrome (ARDS) associated with COVID-19 ( Polverino et al., 2017 ). Therefore, neutrophil elastase inhibitors were proposed as capacity-repurposed treatments for ARDS and the associated lung damage ( Sahebnasagh et al., 2020 ).
Additionally, nebulized dornase alfa, a typically used CF treatment, is presently undergoing trials for COVID-19 treatment ( Southern et al., 2019 ; Earhart et al., 2020 ). Its proposed protective impact is attributed to its clearance of neutrophil extracellular traps, which play a pathogenic function in SARS-CoV-2 infection ( Okur et al., 2020 ). Interestingly, preliminary studies show that dornase alfa is effective in proscribing the in vitro contamination of green monkey and bovine kidney cell strains with the aid of SARS-CoV-2 ( Okur et al., 2020 ).
In conclusion, azithromycin, another usually prescribed antimicrobial agent, has been proven to have antiviral effects against SARS-CoV-2. In the preliminary research, the use and effectiveness of nebulized dornase alfa have not yet been truly defined, but the use of azithromycin was referred to in four research studies ( Corvol et al., 2020 ; Moeller et al., 2020 ; Mondejar-Lopez et al., 2020 ; Bain et al., 2021 ), both as a pre-present treatment or as a capability treatment for COVID-19. It is possible that CF patients exposed to SARS-CoV-2 can be on medications that could alleviate the severity of COVID-19.
Overall, the studies referred to in this evaluation advise that different factors might also help lessen the severity of SARS-CoV-2 in CF sufferers and identify potential goals for further studies to mitigate the severity of SARS-CoV-2 in both CF patients and the general population. These factors encompass ecotin, SERPINB1, camostat mesylate, nelfinavir mesylate, chloromethyl ketone, azithromycin, and ciprofloxacin, some of which are presently being tested in scientific trials ( Stanton et al., 2020 ). Additionally, the composition of the lung microbiome in CF patients, which includes opportunistic pathogens, non-tuberculosis mycobacteria (NTM), and fungi, may play a role in the functioning of COVID-19. Further research in those areas ought to offer valuable insights and future prospects for investigation.
While the findings advocate that CF patients can be truly covered in opposition to extreme lung disorders resulting from SARS-CoV-2, the medium and long-term effects of SARS-CoV-2 on infected CF patients remain unknown. Further research on a larger population of CF patients is needed to determine the proper impact of SARS-CoV-2 on CF lung ailment. Additionally, given the different factors contributing to the spread of SARS-CoV-2, it is encouraged that CF patients be closely monitored to shield them from the risk of COVID-19 contamination.
Author contributions
FA: Data curation, Software, Writing – original draft. NR: Data curation, Writing – original draft. HA: Conceptualization, Supervision, Writing – original draft. PS: Writing – review & editing. MS: Project administration, Supervision, Validation, Writing – review & editing. MMod: Writing – review & editing, Visualization. MMoe: Data curation, Conceptualization, investigation, Software, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Al Lawati, A., Al Balushi, A., and Al Salimi, S. (2023). COVID-19 in a cystic fibrosis patient. Oman Med. J . 38:e490. doi: 10.5001/omj.2023.10
PubMed Abstract | Crossref Full Text | Google Scholar
Bain, R., Cosgriff, R., Zampoli, M., Elbert, A., Burgel, P. R., Carr, S. B., et al. (2021). Clinical characteristics of SARS-CoV-2 infection in children with cystic fibrosis: an international observational study. J. Cyst. Fibr . 20, 25–30. doi: 10.1016/j.jcf.2020.11.021
Baldassarri, M., Fava, F., Fallerini, C., Daga, S., Benetti, E., Zguro, K., et al. (2021). Severe COVID-19 in hospitalized carriers of single CFTR pathogenic variants. J. Personal. Med . 11:558. doi: 10.3390/jpm11060558
Barth, P., Bruijnzeel, P., Wach, A., Kessler, O. S., Hooftman, L., Zimmermann, J., et al. (2020). Single dose escalation studies with inhaled POL6014, a potent novel selective reversible inhibitor of human neutrophil elastase, in healthy volunteers and subjects with cystic fibrosis. J. Cyst. Fibr . 19, 299–304. doi: 10.1016/j.jcf.2019.08.020
Bekassy, Z., Lopatko Fagerström, I., Bader, M., and Karpman, D. (2021). Crosstalk between the renin-angiotensin, complement and kallikrein-kinin systems in inflammation. Nat. Rev. Immunol . 2021, 1–18. doi: 10.1038/s41577-021-00634-8
Berardis, S., Verroken, A., Vetillart, A., Struyf, C., Gilbert, M., Gruson, D., et al. (2020). SARS-CoV-2 seroprevalence in a Belgian cohort of patients with cystic fibrosis. J. Cyst. Fibr . 19, 872–874. doi: 10.1016/j.jcf.2020.08.005
Bezzerri, V., Lucca, F., Volpi, S., and Cipolli, M. (2020). Does cystic fibrosis constitute an advantage in COVID-19 infection? Ital. J. Pediatr . 46, 1–3. doi: 10.1186/s13052-020-00909-1
Bhatnagar, R., Tecklenborg, S., Segurado, R., Watt, P., McAuley, N., Fitzpatrick, P., et al. (2023). Impact of COVID-19 pandemic on health care system, work, and mental well-being of people with cystic fibrosis. Irish J. Med. Sci. 23, 1–8. doi: 10.1007/s11845-023-03391-w
Biondo, C., Midiri, A., Gerace, E., Zummo, S., and Mancuso, G. (2022). SARS-CoV-2 infection in patients with cystic fibrosis: what we know so far. Life 12:2087. doi: 10.3390/life12122087
Bitossi, C., Frasca, F., Viscido, A., Oliveto, G., Scordio, M., Belloni, L., et al. (2021). SARS-CoV-2 entry genes expression in relation with interferon response in cystic fibrosis patients. Microorganisms 9:93. doi: 10.3390/microorganisms9010093
Blevings, P. J., Moore, J. E., and Millar, B. C. (2022). Cystic fibrosis: mutations, modulators and microbiology. J. Prev. Diagnost. Treat. Strat. Med . 1:30. doi: 10.4103/jpdtsm.jpdtsm_10_22
Crossref Full Text | Google Scholar
Borch, L., Holm, M., Knudsen, M., Ellermann-Eriksen, S., and Hagstroem, S. (2022). Long COVID symptoms and duration in SARS-CoV-2 positive children-a nationwide cohort study. Eur. J. Pediatr . 181, 1597–1607. doi: 10.1007/s00431-021-04345-z
Bradley, B. T., Maioli, H., Johnston, R., Chaudhry, I., Fink, S. L., Xu, H., et al. (2020). Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet 396, 320–332. doi: 10.1016/S0140-6736(20)31305-2
Burgel, P. R., and Goss, C. (2021). COVID-19 outcomes in people with cystic fibrosis. Curr. Opin. Pulmon. Med . 27, 538–543. doi: 10.1097/MCP.0000000000000823
Carr, S. B., McClenaghan, E., Elbert, A., Faro, A., Cosgriff, R., Abdrakhmanov, O., et al. (2022). Factors associated with clinical progression to severe COVID-19 in people with cystic fibrosis: a global observational study. J. Cyst. Fibr . 21, e221–e231. doi: 10.1016/j.jcf.2022.06.006
Chams, N., Chams, S., Badran, R., Shams, A., Araji, A., Raad, M., et al. (2020). COVID-19: a multidisciplinary review. Front. Publ. Health 8:383. doi: 10.3389/fpubh.2020.00383
Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., Han, Y., et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395, 507–513. 10.1016/S0140-6736(Zhaoet al., 2022)30211-7
PubMed Abstract | Google Scholar
Colombo, C., Alicandro, G., Daccó, V., Gagliano, V., Morlacchi, L. C., Casciaro, R., et al. (2021). SARS-CoV-2 infection in cystic fibrosis: a multicentre prospective study with a control group, Italy, February-July 2020. PLoS ONE 16:e0251527. doi: 10.1371/journal.pone.0251527
Colombo, C., Burgel, P. R., Gartner, S., van Koningsbruggen-Rietschel, S., Naehrlich, L., Sermet-Gaudelus, I., et al. (2020). Impact of COVID-19 on people with cystic fibrosis. Lancet Respirat. Med . 8, e35–e36. doi: 10.1016/S2213-2600(20)30177-6
Colombo, C., Cipolli, M., Daccò, V., Medino, P., Alghisi, F., Ambroni, M., et al. (2022). Clinical course and risk factors for severe COVID-19 among Italian patients with cystic fibrosis: a study within the Italian Cystic Fibrosis Society. Infection 50, 671–679. doi: 10.1007/s15010-021-01737-z
Coomes, E. A., and Haghbayan, H. (2020). Interleukin-6 in COVID-19: a systematic review and meta-analysis. Rev. Med. Virol . 30, 1–9. doi: 10.1002/rmv.2141
Corvol, H., de Miranda, S., Lemonnier, L., Kemgang, A., Reynaud Gaubert, M., Chiron, R., et al. (2020). First wave of COVID-19 in French patients with cystic fibrosis. J. Clin. Med . 9:3624. doi: 10.3390/jcm9113624
Cosgriff, R., Ahern, S., Bell, S. C., Brownlee, K., Burgel, P. R., Byrnes, C., et al. (2020). A multinational report to characterise SARS-CoV-2 infection in people with cystic fibrosis. J. Cyst. Fibr . 19, 355–358. doi: 10.1016/j.jcf.2020.04.012
Demirci, M., Tokman, H., Uysal, H., Demiryas, S., Karakullukcu, A., Saribas, S., et al. (2019). Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergologia et Immunopathologia 47, 365–371. doi: 10.1016/j.aller.2018.12.009
Diamond, M. S., and Kanneganti, T. D. (2022). Innate immunity: the first line of defense against SARS-CoV-2. Nat. Immunol . 23, 165–176. doi: 10.1038/s41590-021-01091-0
Dittrich, A. S., Kühbandner, I., Gehrig, S., Rickert-Zacharias, V., Twigg, M., Wege, S., et al. (2018). Elastase activity on sputum neutrophils correlates with severity of lung disease in cystic fibrosis. Eur. Respirat. J . 51:2017. doi: 10.1183/13993003.01910-2017
Earhart, A., Holliday, Z., Hofmann, H., and Schrum, A. (2020). Consideration of dornase alfa for the treatment of severe COVID-19 acute respiratory distress syndrome. N. Microbes N. Infect . 35:100689. doi: 10.1016/j.nmni.2020.100689
Echeverria-Esnal, D., Martin-Ontiyuelo, C., Navarrete-Rouco, M. E., De-Antonio, M., Ferrandez, O., Horcajada, J. P., et al. (2020). Could azithromycin play a role in the treatment of COVID-19? A review. Authorea 2020:16292378. doi: 10.22541/au.159170711.16292378
Fainardi, V., Longo, F., Chetta, A., Esposito, S., and Pisi, G. (2020). SARS-CoV-2 infection in patients with cystic fibrosis. An overwiew. Acta Bio Medica 91:e2020035. doi: 10.23750/abm.v91i3.10391
Flume, P., Rowe, S., Miller, S., Sorscher, E., Elkins, M., Robinson, M., et al. (2019). “Pulmonary complications of cystic fibrosis,” in Seminars in Respiratory and Critical Care Medicine . New York, NY: Thieme Medical Publishers.
Google Scholar
Flume, P. A., Saiman, L., and Marshall, B. (2022). The impact of COVID-19 in cystic fibrosis. Archivos de Bronconeumologia 58:466. doi: 10.1016/j.arbres.2021.12.003
Gaudio, D. (2020). SARS-CoV-2 and COVID-19: between pathophysiology complexity and therapeutic uncertainty. Physiol. Rev . 2020, 1−10.
Ghazy, R. M., Almaghraby, A., Shaaban, R., Kamal, A., Beshir, H., Moursi, A., et al. (2020). A systematic review and meta-analysis on chloroquine and hydroxychloroquine as monotherapy or combined with azithromycin in COVID-19 treatment. Sci. Rep . 10:22139. doi: 10.1038/s41598-020-77748-x
Gilbert, J. A., Blaser, M. J., Caporaso, J. G., Jansson, J. K., Lynch, S. V., Knight, R., et al. (2018). Current understanding of the human microbiome. Nat. Med . 24, 392–400. doi: 10.1038/nm.4517
Gu, S., Chen, Y., Wu, Z., Chen, Y., Gao, H., Lv, L., et al. (2020). Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin. Infect. Dis . 71, 2669–2678. doi: 10.1093/cid/ciaa709
Hadi, Y. B., Lakhani, D. A., Naqvi, S. F., Fatima, N. U., and Sarwari, A. R. (2021). Outcomes of SARS-CoV-2 infection in patients with cystic fibrosis: a multicenter retrospective research network study. Respirat. Med . 188:106606. doi: 10.1016/j.rmed.2021.106606
Hall, V. G., Solera, J. T., Al-Alahmadi, G., Marinelli, T., Cardinal, H., Poirier, C., et al. (2022). Severity of COVID-19 among solid organ transplant recipients in Canada, 2020–2021: a prospective, multicentre cohort study. CMAJ 194, E1155–E1163. doi: 10.1503/cmaj.220620
Hisert, K. B., Heltshe, S. L., Pope, C., Jorth, P., Wu, X., Edwards, R. M., et al. (2017). Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am. J. Respirat. Crit. Care Med . 195, 1617–1628. doi: 10.1164/rccm.201609-1954OC
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., et al. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280.e8. doi: 10.1016/j.cell.2020.02.052
Hong, N., Yu, W., Xia, J., Shen, Y., Yap, M., Han, W., et al. (2020). Evaluation of ocular symptoms and tropism of SARS-CoV-2 in patients confirmed with COVID-19. Acta Ophthalmol . 98, e649–e655. doi: 10.1111/aos.14445
Jafarpour, R., Pashangzadeh, S., and Dowran, R. (2021). Host factors: implications in immunopathogenesis of COVID-19. Pathol. Res. Pract . 228:153647. doi: 10.1016/j.prp.2021.153647
Jardel, S., Touzet, S., Poupon-Bourdy, S., Nove-Josserand, R., Durieu, I., Reynaud, Q., et al. (2018). Cystic fibrosis related diabetes before lung transplantation impacts survival but not long-term renal function. Revue d'Épidémiologie et de Santé Publique 66, S324–S325. doi: 10.1016/j.respe.2018.05.235
Jin, Y. H., Cai, L., Cheng, Z. S., Cheng, H., Deng, T., Fan, Y. P., et al. (2020). A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Milit. Med. Res . 7, 1–23. doi: 10.1186/s40779-020-0233-6
Jung, A., Orenti, A., Dunlevy, F., Aleksejeva, E., Bakkeheim, E., Bobrovnichy, V., et al. (2021). Factors for severe outcomes following SARS-CoV-2 infection in people with cystic fibrosis in Europe. ERJ Open Res . 7:411. doi: 10.1183/23120541.00411-2021
Junkins, R. D., McCormick, C., and Lin, T. J. (2014). The emerging potential of autophagy-based therapies in the treatment of cystic fibrosis lung infections. Autophagy 10, 538–547. doi: 10.4161/auto.27750
Kaore, S. N., and Kaore, N. M. (2021). Arginine and citrulline as nutraceuticals: efficacy and safety in diseases. Nutraceuticals 55, 925–944. doi: 10.1016/B978-0-12-821038-3.00055-0
Karakaş Çelik, S., Çakmak Genç, G., Pişkin, N., Açikgöz, B., Altinsoy, B., Kurucu Işsiz, B., et al. (2021). Polymorphisms of ACE (I/D) and ACE2 receptor gene (Rs2106809, Rs2285666) are not related to the clinical course of COVID-19: a case study. J. Med. Virol . 93, 5947–5952. doi: 10.1002/jmv.27160
Khezri, M. R., Zolbanin, N. M., Ghasemnejad-Berenji, M., and Jafari, R. (2021). Azithromycin: immunomodulatory and antiviral properties for SARS-CoV-2 infection. Eur. J. Pharmacol . 905:174191. doi: 10.1016/j.ejphar.2021.174191
Kournoutou, G. G., and Dinos, G. (2022). Azithromycin through the Lens of the COVID-19 treatment. Antibiotics 11:1063. doi: 10.3390/antibiotics11081063
Lam, J. C., Somayaji, R., Surette, M. G., Rabin, H. R., and Parkins, M. D. (2015). Reduction in Pseudomonas aeruginosa sputum density during a cystic fibrosis pulmonary exacerbation does not predict clinical response. BMC Infect. Dis . 15, 1–12. doi: 10.1186/s12879-015-0856-5
Lambrecht, B., Prins, B., and Hoogsteden, H. (2001). Lung dendritic cells and host immunity to infection. Eur. Respirat. J . 18, 692–704. doi: 10.1183/09031936.01.18040692
Leylabadlo, H. E., Ghotaslou, R., Feizabadi, M. M., Farajnia, S., Moaddab, S. Y., Ganbarov, K., et al. (2020). The critical role of Faecalibacterium prausnitzii in human health: an overview. Microb. Pathog . 149:104344. doi: 10.1016/j.micpath.2020.104344
Lim, J. T., Ly, N. P., Willen, S. M., Iwanaga, K., Gibb, E. R., Chan, M., et al. (2022). Food insecurity and mental health during the COVID-19 pandemic in cystic fibrosis households. Pediatr. Pulmonol . 57, 1238–1244. doi: 10.1002/ppul.25850
Lotti, V., Merigo, F., Lagni, A., Di Clemente, A., Ligozzi, M., Bernardi, P., et al. (2022). CFTR modulation reduces SARS-CoV-2 infection in human bronchial epithelial cells. Cells 11, 1347. doi: 10.3390/cells11081347
Mainbourg, S., Philit, F., Touzet, S., Nove-Josserand, R., Durupt, S., Sénéchal, A., et al. (2019). Cystic fibrosis-related diabetes before lung transplantation is associated with lower survival but does not affect long-term renal function. Pediatr. Pulmonol . 54, 977–983. doi: 10.1002/ppul.24307
Majka, G., Mazurek, H., Strus, M., Ciszek-Lenda, M., Szatanek, R., Pac, A., et al. (2021). Chronic bacterial pulmonary infections in advanced cystic fibrosis differently affect the level of sputum neutrophil elastase, IL-8 and IL-6. Clin. Exp. Immunol . 205, 391–405. doi: 10.1111/cei.13624
Marques, L. S., Boschiero, M. N., Sansone, N. M. S., Brienze, L. R., and Marson, F. A. L. (2023). Epidemiological profile of hospitalized patients with cystic fibrosis in Brazil due to severe acute respiratory infection during the COVID-19 pandemic and a systematic review of worldwide COVID-19 in those with cystic fibrosis. Healthcare 2023:31936. doi: 10.3390/healthcare11131936
Mathew, H. R., Choi, M. Y., Parkins, M. D., and Fritzler, M. J. (2021). Systematic review: cystic fibrosis in the SARS-CoV-2/COVID-19 pandemic. BMC Pulmon. Med . 21, 1–11. doi: 10.1186/s12890-021-01528-0
McClenaghan, E., Cosgriff, R., Brownlee, K., Ahern, S., Burgel, P. R., Byrnes, C. A., et al. (2020). The global impact of SARS-CoV-2 in 181 people with cystic fibrosis. J. Cyst. Fibr . 19, 868–871. doi: 10.1016/j.jcf.2020.10.003
McGonagle, D., Sharif, K., O'Regan, A., and Bridgewood, C. (2020). The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev . 19:102537. doi: 10.1016/j.autrev.2020.102537
Mehrabani, S. (2020). COVID-19 infection and children: a comprehensive review. Int. J. Prev. Med . 11:20. doi: 10.4103/ijpvm.IJPVM_277_20
Meng, L., Hua, F., and Bian, Z. (2020). Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J. Dental Res . 99, 481–487. doi: 10.1177/0022034520914246
Mingione, A., Ottaviano, E., Barcella, M., Merelli, I., Rosso, L., Armeni, T., et al. (2020). Cystic fibrosis defective response to infection involves autophagy and lipid metabolism. Cells 9:1845. doi: 10.3390/cells9081845
Mody, V., Ho, J., Wills, S., Mawri, A., Lawson, L., Ebert, M. C., et al. (2021). Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents. Commun. Biol . 4:93. doi: 10.1038/s42003-020-01577-x
Moeller, A., Thanikkel, L., Duijts, L., Gaillard, E. A., Garcia-Marcos, L., Kantar, A., et al. (2020). COVID-19 in children with underlying chronic respiratory diseases: survey results from 174 centres. ERJ Open Res . 6:409. doi: 10.1183/23120541.00409-2020
Mohamed, M. M., El-Shimy, I. A., and Hadi, M. A. (2020). Neutrophil Elastase Inhibitors: A Potential Prophylactic Treatment Option for SARS-CoV-2-Induced Respiratory Complications? (Berlin: Springer), 1–2.
Mondejar-Lopez, P., Quintana-Gallego, E., Giron-Moreno, R. M., Cortell-Aznar, I., de Valbuena-Maiz, M. R., Diab-Caceres, L., et al. (2020). Impact of SARS-CoV-2 infection in patients with cystic fibrosis in Spain: incidence and results of the national CF-COVID-19-Spain survey. Respirat. Med . 170:106062. doi: 10.1016/j.rmed.2020.106062
Naehrlich, L., Orenti, A., Dunlevy, F., Kasmi, I., Harutyunyan, S., Pfleger, A., et al. (2021). Incidence of SARS-CoV-2 in people with cystic fibrosis in Europe between February and June 2020. J. Cyst. Fibr . 20, 566–577. doi: 10.1016/j.jcf.2021.03.017
Nilashi, M., Samad, S., Yusuf, S. Y. M., and Akbari, E. (2020). Can complementary and alternative medicines be beneficial in the treatment of COVID-19 through improving immune system function? J. Infect. Publ. Health 13:893. doi: 10.1016/j.jiph.2020.05.009
Okur, H. K., Yalcin, K., Tastan, C., Demir, S., Yurtsever, B., Karakus, G., et al. (2020). Preliminary report of in vitro and in vivo effectiveness of dornase alfa on SARS-CoV-2 infection. N. Microb. N. Infect . 37:100756. doi: 10.1016/j.nmni.2020.100756
Panou, M.-M. (2018). Role of cellular ion channels in the BK polyomavirus life cycle (PhD thesis). University of Leeds.
Parada Venegas, D., De la Fuente, M. K., Landskron, G., González, M. J., Quera, R., Dijkstra, G., et al. (2019). Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol . 277:1486. doi: 10.3389/fimmu.2019.01486
Peckham, D., McDermott, M. F., Savic, S., and Mehta, A. (2020). COVID-19 meets cystic fibrosis: for better or worse? Genes Immun . 21, 260–262. doi: 10.1038/s41435-020-0103-y
Pehote, G., and Vij, N. (2020). Autophagy augmentation to alleviate immune response dysfunction, and resolve respiratory and COVID-19 exacerbations. Cells 9:1952. doi: 10.3390/cells9091952
Pereira, M. R., Mohan, S., Cohen, D. J., Husain, S. A., Dube, G. K., Ratner, L. E., et al. (2020). COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am. J. Transpl . 20, 1800–1808. doi: 10.1111/ajt.15941
Polverino, E., Rosales-Mayor, E., Dale, G. E., Dembowsky, K., and Torres, A. (2017). The role of neutrophil elastase inhibitors in lung diseases. Chest 152, 249–262. doi: 10.1016/j.chest.2017.03.056
Porter, J. C., Inshaw, J., Solis, V. J., Denneny, E., Evans, R., Temkin, M. I., et al. (2023). Nebulised dornase alfa reduces inflammation and improves clinical outcomes in severe COVID-19: a randomised clinical trial. eLife 12:2. doi: 10.7554/eLife.87030.2
Roingeard, P., Eymieux, S., Burlaud-Gaillard, J., Hourioux, C., Patient, R., Blanchard, E., et al. (2022). The double-membrane vesicle (DMV): a virus-induced organelle dedicated to the replication of SARS-CoV-2 and other positive-sense single-stranded RNA viruses. Cell. Mol. Life Sci . 79:425. doi: 10.1007/s00018-022-04469-x
Ruffin, M., Bigot, J., Calmel, C., Mercier, J., Givelet, M., Oliva, J., et al. (2021). Flagellin from Pseudomonas aeruginosa modulates SARS-CoV-2 infectivity in cystic fibrosis airway epithelial cells by increasing TMPRSS2 expression. Front. Immunol . 12:714027. doi: 10.3389/fimmu.2021.714027
Sahebnasagh, A., Saghafi, F., Safdari, M., Khataminia, M., Sadremomtaz, A., Ghaleno, H. R., et al. (2020). Neutrophil Elastase inhibitor (Sivelestat), may be a promising therapeutic option for Management of Acute Lung Injury/acute respiratory distress syndrome or disseminated intravascular coagulation in COVID-19. Authorea 2020:13251. doi: 10.1111/jcpt.13251
Salzberger, B., Buder, F., Lampl, B., Ehrenstein, B., Hitzenbichler, F., Holzmann, T., et al. (2021). Epidemiology of SARS-CoV-2. Infection 49, 233–239. doi: 10.1007/s15010-020-01531-3
Scagnolari, C., Bitossi, C., Frasca, F., Viscido, A., Oliveto, G., Scordio, M., et al. (2020). No detection of SARS-CoV-2 in cystic fibrosis patients at the Regional (Lazio) Reference Center for CF in Italy. J. Cyst. Fibr . 19, 837–838. doi: 10.1016/j.jcf.2020.06.018
Scurati-Manzoni, E., Fossali, E. F., Agostoni, C., Riva, E., Simonetti, G. D., Zanolari-Calderari, M., et al. (2014). Electrolyte abnormalities in cystic fibrosis: systematic review of the literature. Pediatr. Nephrol . 29, 1015–1023. doi: 10.1007/s00467-013-2712-4
Segal, J. P., Mak, J. W., Mullish, B. H., Alexander, J. L., Ng, S. C., Marchesi, J. R., et al. (2020). The gut microbiome: an under-recognised contributor to the COVID-19 pandemic? Therapeut. Adv. Gastroenterol . 13:1756284820974914. doi: 10.1177/1756284820974914
Shirato, K., Kanou, K., Kawase, M., and Matsuyama, S. (2017). Clinical isolates of human coronavirus 229E bypass the endosome for cell entry. J. Virol . 91:16. doi: 10.1128/JVI.01387-16
Smith, C. B., Kanner, R. E., Golden, C. A., Klauber, M. R., and Renzetti Jr, A. D. (1980). Effect of viral infections on pulmonary function in patients with chronic obstructive pulmonary diseases. J. Infect. Dis . 141, 271–280. doi: 10.1093/infdis/141.3.271
Sorci, G., Faivre, B., and Morand, S. (2020). Explaining among-country variation in COVID-19 case fatality rate. Sci. Rep . 10:18909. doi: 10.1038/s41598-020-75848-2
Southern, K. W., Clancy, J. P., and Ranganathan, S. (2019). Aerosolized agents for airway clearance in cystic fibrosis. Pediatr. Pulmonol . 54, 858–864. doi: 10.1002/ppul.24306
Stanton, B. A., Hampton, T. H., and Ashare, A. (2020). SARS-CoV-2 (COVID-19) and cystic fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol . 319, L408–L415. doi: 10.1152/ajplung.00225.2020
Stern, R. C. (1997). The diagnosis of cystic fibrosis. N. Engl. J. Med . 336, 487–491. doi: 10.1056/NEJM199702133360707
Suarez-Reyes, A., and Villegas-Valverde, C. A. (2021). Implications of low-grade inflammation in SARS-CoV-2 immunopathology. Medicc Rev . 23, 42–54. doi: 10.37757/MR2021.V23.N2.4
Tay, M. Z., Poh, C. M., Rénia, L., MacAry, P. A., and Ng, L. F. (2020). The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol . 20, 363–374. doi: 10.1038/s41577-020-0311-8
Taylor-Cousar, J. L., Shteinberg, M., Cohen-Cymberknoh, M., and Jain, R. (2023). The impact of highly effective cystic fibrosis transmembrane conductance regulator modulators on the health of female subjects with cystic fibrosis. Clin. Therapeut . 45, 278–289. doi: 10.1016/j.clinthera.2023.01.016
Tedbury, P. R., Manfredi, C., Degenhardt, F., Conway, J., Horwath, M. C., McCracken, C., et al. (2023). Mechanisms by which the cystic fibrosis transmembrane conductance regulator may influence SARS-CoV-2 infection and COVID-19 disease severity. FASEB J . 37:e23220. doi: 10.1096/fj.202300077R
Terlizzi, V., Castellani, C., Taccetti, G., and Ferrari, B. (2022a). Dornase alfa in cystic fibrosis: indications, comparative studies and effects on lung clearance index. Ital. J. Pediatr . 48:141. doi: 10.1186/s13052-022-01331-5
Terlizzi, V., Motisi, M. A., Pellegrino, R., Padoan, R., and Chiappini, E. (2022c). Risk factors for severe COVID-19 in people with cystic fibrosis: a systematic review. Front. Pediatr . 10:958658. doi: 10.3389/fped.2022.958658
Terlizzi, V., Parisi, G. F., Ferrari, B., Castellani, C., Manti, S., Leonardi, S., et al. (2022b). Effect of dornase alfa on the lung clearance index in children with cystic fibrosis: a lesson from a case series. Children 9:1625. doi: 10.3390/children9111625
Tharappel, A. M., Samrat, S. K., Li, Z., and Li, H. (2020). Targeting crucial host factors of SARS-CoV-2. ACS Infect. Dis . 6, 2844–2865. doi: 10.1021/acsinfecdis.0c00456
Touret, F., Gilles, M., Barral, K., Nougairède, A., Van Helden, J., Decroly, E., et al. (2020). In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci. Rep . 10:13093. doi: 10.1038/s41598-020-70143-6
van Ewijk, B. E., van der Zalm, M. M., Wolfs, T. F., and van der Ent, C. K. (2005). Viral respiratory infections in cystic fibrosis. J. Cyst. Fibr . 4, 31–36. doi: 10.1016/j.jcf.2005.05.011
Vernocchi, P., Del Chierico, F., Russo, A., Majo, F., Rossitto, M., Valerio, M., et al. (2018). Gut microbiota signatures in cystic fibrosis: loss of host CFTR function drives the microbiota enterophenotype. PLoS ONE 13:e0208171. doi: 10.1371/journal.pone.0208171
Vitiello, A., Sabbatucci, M., Silenzi, A., Capuano, A., Rossi, F., Zovi, A., et al. (2023). The impact of SARS-CoV-2 infection in patients with cystic fibrosis undergoing CFTR channel modulators treatment: a literature review. Respirat. Res . 24:278. doi: 10.1186/s12931-023-02593-1
Ward, J. (2016). The Coalition Chronicle .
Wark, P. A., Tooze, M., Cheese, L., Whitehead, B., Gibson, P. G., Wark, K. F., et al. (2012). Viral infections trigger exacerbations of cystic fibrosis in adults and children. Eur. Respirat. J . 40, 510–512. doi: 10.1183/09031936.00202311
Yang, C., and Montgomery, M. (2021). Dornase alfa for cystic fibrosis. Cochr. Datab. Syst. Rev . 2021:CD001127. doi: 10.1002/14651858.CD001127.pub5
Yapasert, R., Khaw-On, P., and Banjerdpongchai, R. (2021). Coronavirus infection-associated cell death signaling and potential therapeutic targets. Molecules 26:7459. doi: 10.3390/molecules26247459
Ye, Z. W., Yuan, S., Yuen, K. S., Fung, S. Y., Chan, C. P., Jin, D. Y., et al. (2020). Zoonotic origins of human coronaviruses. Int. J. Biol. Sci . 16:1686. doi: 10.7150/ijbs.45472
Yohannes, A. M. (2021). COPD patients in a COVID-19 society: depression and anxiety. Exp. Rev. Respirat. Med . 15, 5–7. doi: 10.1080/17476348.2020.1787835
Yu, C., and Kotsimbos, T. (2023). Respiratory infection and inflammation in cystic fibrosis: a dynamic interplay among the host, microbes, and environment for the ages. Int. J. Mol. Sci . 24:4052. doi: 10.3390/ijms24044052
Yuan, S., Peng, L., Park, J. J., Hu, Y., Devarkar, S. C., Dong, M. B., et al. (2020). Nonstructural protein 1 of SARS-CoV-2 is a potent pathogenicity factor redirecting host protein synthesis machinery toward viral RNA. Mol. Cell 80, 1055–1066.e6. doi: 10.1016/j.molcel.2020.10.034
Zhang, H., Penninger, J. M., Li, Y., Zhong, N., and Slutsky, A. S. (2020). Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intens. Care Med . 46, 586–590. doi: 10.1007/s00134-020-05985-9
Zhao, M. M., Zhu, Y., Zhang, L., Zhong, G., Tai, L., Liu, S., et al. (2022). Novel cleavage sites identified in SARS-CoV-2 spike protein reveal mechanism for cathepsin L-facilitated viral infection and treatment strategies. Cell Disc . 8:53. doi: 10.1038/s41421-022-00419-w
Zhou, Y., Zhang, J., Zhang, D., Ma, W. L., and Wang, X. (2021). Linking the gut microbiota to persistent symptoms in survivors of COVID-19 after discharge. J. Microbiol . 59, 941–948. doi: 10.1007/s12275-021-1206-5
Keywords: cystic fibrosis (CF), SARS-CoV-2, Pseudomonas aeruginosa , immune system, respiratory virus, pathogenesis
Citation: Abolhasani FS, Moein M, Rezaie N, Sheikhimehrabadi P, Shafiei M, Afkhami H and Modaresi M (2024) Occurrence of COVID-19 in cystic fibrosis patients: a review. Front. Microbiol. 15:1356926. doi: 10.3389/fmicb.2024.1356926
Received: 16 December 2023; Accepted: 11 March 2024; Published: 17 April 2024.
Reviewed by:
Copyright © 2024 Abolhasani, Moein, Rezaie, Sheikhimehrabadi, Shafiei, Afkhami and Modaresi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Morvarid Shafiei, dr.m.shafiei@pasteur.ac.ir ; Hamed Afkhami, hamedafkhami70@gmail.com
† These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Case Study - What is the Relationship Between the Cell Membrane and Cystic Fibrosis?
CF affects more than 30,000 kids and young adults in the United States. It disrupts the normal function of epithelial cells — cells that make up the sweat glands in the skin and that also line passageways inside the lungs, pancreas, and digestive and reproductive systems.
The inherited CF gene directs the body's epithelial cells to produce a defective form of a protein called CFTR (or cystic fibrosis transmembrane conductance regulator) found in cells that line the lungs, digestive tract, sweat glands, and genitourinary system.
When the CFTR protein is defective, epithelial cells can't regulate the way that chloride ions pass across cell membranes. This disrupts the balance of salt and water needed to maintain a normal thin coating of mucus inside the lungs and other passageways. The mucus becomes thick, sticky, and hard to move, and can result in infections from bacterial colonization.
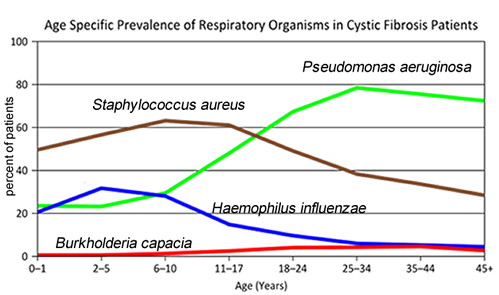
1. "Woe to that child which when kissed on the forehead tastes salty. He is bewitched and soon will die"
This is an old saying from the eighteenth century and describes one of the symptoms of CF (salty skin). Why do you think babies in the modern age have a better chance of survival than babies in the 18th century?
2. What symptoms lead Dr. Weyland to his initial diagnosis?
3. Consider the graph of infections, which organism stays relatively constant in numbers over a lifetime?
What organism is most likely affecting baby Zoey?
4. Explain how the CF gene affects the cell membrane.
5. Consider what you know about TONICITY and the cell membrane. Why is it important to regulate salt in cells?
Part II: CF is a disorder of the cell membrane.
Imagine a door with key and combination locks on both sides, back and front. Now imagine trying to unlock that door blind-folded. This is the challenge faced by David Gadsby, Ph.D., who for years struggled to understand the highly intricate and unusual cystic fibrosis chloride channel – a cellular doorway for salt ions that is defective in people with cystic fibrosis.
His findings, reported in a series of three recent papers in the Journal of General Physiology, detail the type and order of molecular events required to open and close the gates of the cystic fibrosis chloride channel, or as scientists call it, the cystic fibrosis transmembrane conductance regulator (CFTR).
Ultimately, the research may have medical applications, though ironically not likely for most cystic fibrosis patients. Because two-thirds of cystic fibrosis patients fail to produce the cystic fibrosis channel altogether, a cure for most is expected to result from research focused on replacing the lost channel.
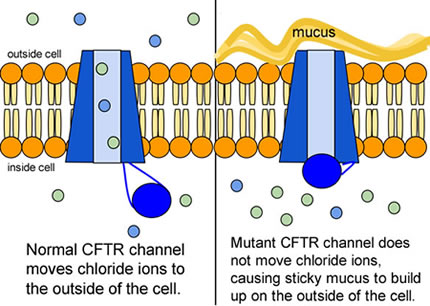
6. Compare the normal and the mutant CFTR protein. How would you correct the mutant protein if you had the ability to tinker with it on a molecular level?
7. Why would treatment that targets the CFTR channel not be effective for ⅔ of those with cystic fibrosis? 8. Sweat glands cool the body by releasing perspiration (sweat) from the lower layers of the skin onto the surface. Sodium and chloride (salt) help carry water to the skin's surface and are then reabsorbed into the body. Why does a person with cystic fibrosis have salty tasting skin?
Part III: No cell is an island
Like people, cells need to communicate and interact with their environment to survive. One way they go about this is through pores in their outer membranes, called ion channels, which provide charged ions, such as chloride or potassium, with their own personalized cellular doorways. But, ion channels are not like open doors; instead, they are more like gateways with high-security locks that are opened and closed to carefully control the passage of their respective ions.
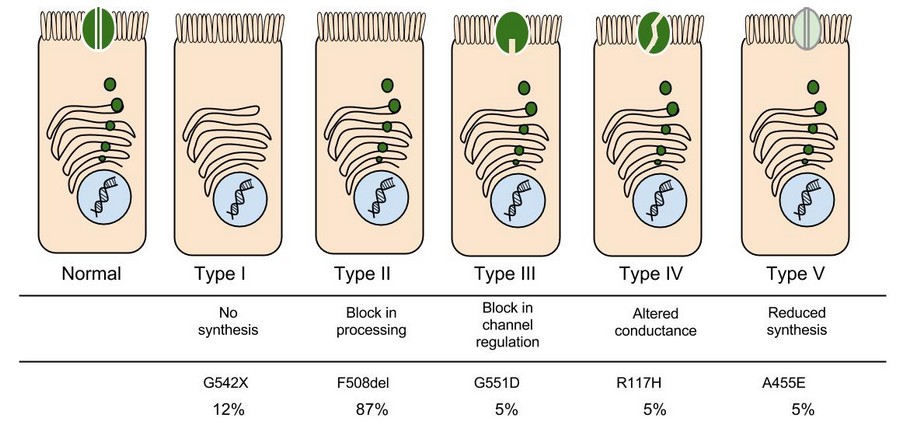
9. Which mutation do you think would be easiest to correct? Justify your answer.
10. Consider what you know about proteins, why does the "folding" of the protein matter?
Part IV: Open Sesame
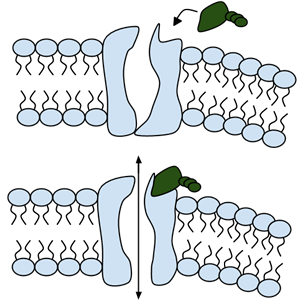
Among the numerous ion channels in cell membranes, there are two principal types: voltage-gated and ligand-gated. Voltage-gated channels are triggered to open and shut their doors by changes in the electric potential difference across the membrane. Ligand-gated channels, in contrast, require a special “key” to unlock their doors, which usually comes in the form of a small molecule.
CFTR is a ligand-gated channel, but it’s an unusual one. Its “key” is ATP, a small molecule that plays a critical role in the storage and release of energy within cells in the body. In addition to binding the ATP, the CFTR channel must snip a phosphate group – one of three “P’s” – off the ATP molecule to function. But when, where and how often this crucial event takes place has remained obscure.
11. Label the image to the right to show how the ligand-gated channel for CFTR works. (Structures: Ligand-gated channel protein, ATP, phospholipids). Summarize how this channels works.
12. Where is ATP generated in the cell? How might ATP production affect the symptoms of cystic fibrosis?
Part V: Can a Drug Treat Zoey's Condition?
Dr. Weyland confirmed that Zoey does have cystic fibrosis and called the parents in to talk about potential treatments. “Good news, there are two experimental drugs that have shown promise in CF patients. These drugs can help Zoey clear the mucus from her lungs. Unfortunately, the drugs do not work in all cases.” The doctor gave the parents literature about the drugs and asked them to consider signing Zoey up for trials.
The Experimental Drugs
Ivacaftor ™ is a potentiator that increases CFTR channel opening time. We know from the cell culture studies that this increases chloride transport by as much as 50% from baseline and restores it closer to what we would expect to observe in wild type CFTR. Basically, the drug increases CFTR activity by unlocking the gate that allows for the normal flow of salt and fluids.
In early trials, 144 patients all of whom were over the age of 12 were treated with 150 mg of Ivacaftor twice daily. The total length of treatment was 48 weeks. Graph A shows changes in FEV (forced expiratory volume) with individuals using the drug versus a placebo. Graph B shows concentrations of chloride in patient’s sweat.
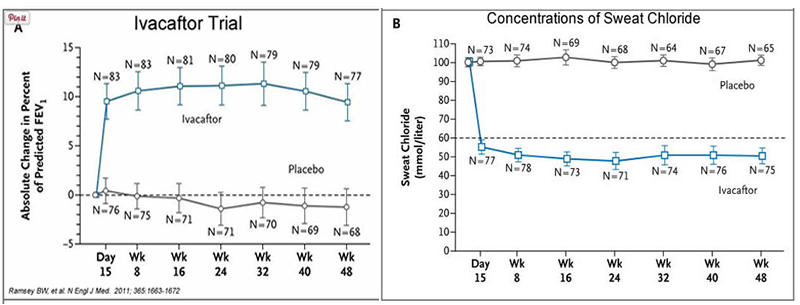
13. What is FEV (if you're not sure, look this one up)? Describe a way that a doctor could take a measurement of FEV.
14. Why do you think it was important to have placebos in both of these studies?
15. Which graph do you think provides the most compelling evidence for the effectiveness of Ivacaftor. Defend your choice.
16. Take a look at the mutations that can occur in the cell membrane protein from Part III. For which mutation do you think Ivacaftor will be most effective. Justify your answer.
17. Would you sign Zoey up for clinical trials based on the evidence? What concerns would a parent have before considering an experimental drug?
Part VI: Zoey's Mutation
Dr. Weyland calls a week later to inform the parents that genetic tests show that Zoey chromosomes show that she has two copies of the F508del mutation. This mutation, while the most common type of CF mutation, is also one that is difficult to treat with just Ivacaftor. There are still some options for treatment.
In people with the most common CF mutation, F508del, a series of problems prevents the CFTR protein from taking its correct shape and reaching its proper place on the cell surface. The cell recognizes the protein as not normal and targets it for degradation before it makes it to the cell surface. In order to treat this problem, we need to do two things: first, an agent to get the protein to the surface, and then ivacaftor (VX-770) to open up the channel and increase chloride transport. VX-809 has been identified as a way to help with the trafficking of the protein to the cell surface. When added VX-809 is added to ivacaftor (now called Lumacaftor,) the protein gets to the surface and also increases in chloride transport by increasing channel opening time.
In early trials, experiments were done in-vitro, where studies were done on cell cultures to see if the drugs would affect the proteins made by the cell. General observations can be made from the cells, but drugs may not work on an individual’s phenotype. A new type of research uses ex-vivo experiments, where rectal organoids (mini-guts) were grown from rectal biopsies of the patient that would be treated with the drug. Ex-vivo experiments are personalized medicine, each person may have different correctors and potentiators evaluated using their own rectal organoids.
The graph below shows how each drug works for 8 different patients (#1-#8). Swelling in the organoid indicates the the channels within the cell membrane are allowing material to pass.
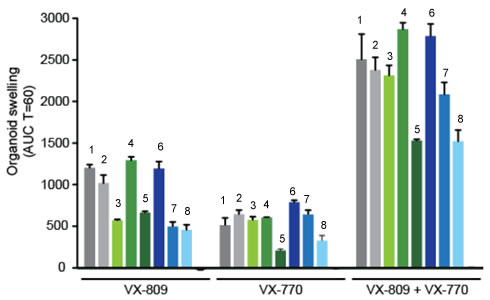
19. . Compare ex-vivo trials to in-vitro trials.
20. One the graph, label the group that represents Ivacaftor and Lumacaftor. What is the difference between these two drugs?
21. Complete a CER Chart.
If the profile labeled #7 is Zoey, rank the possible drug treatments in order of their effectiveness for her mutation. This is your CLAIM. Provide EVIDENCE to support your claim Provide REASONING that explains why this treatment would be more effective than other treatments and why what works for Zoey may not work for other patients. This is where you tie the graph above to everything you have learned in this case. Attach a page.

Source & Credits
- "CFTR Protein Panels" by Lbudd14 - Own work. Licensed under Creative Commons Attribution-Share Alike 3.0 via Wikimedia Commons
- http://newswire.rockefeller.edu/2003/12/19/scientists-finally-pry-stubborn-cellular-door-ajar/
- http://en.wikipedia.org/wiki/Cystic_fibrosis
- http://www.medscape.org/viewarticle/806649_transcript
- http://www.cff.org/research/clinicalresearch/faqs/combinedkalydeco-vx-809/#Expanded-Access
- Ifacaftor Trial Graph: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3230303/
- Organoid swelling graph: http://www.potentiate.info/?q=trio-clinical-trial-ivacaftor-genistein
- Life expectancy graph: http://www.nationaljewish.org/healthinfo/conditions/cysticfibrosis/life-expectancy/
Follow-up Article: What it's like to have two kids with cystic fibrosis More information at John Hopkins Cystic Fibrosis Center
Other Resources on Cystic Fibrosis
Cystic Fibrosis Mutations Cell Membrane and Transport (Slides)

IMAGES
VIDEO
COMMENTS
Cystic fibrosis (CF) is a serious and life-shortening genetic disorder affecting approximately 70,000 persons worldwide . Respiratory failure is the foremost cause of death in CF patients, and lung transplantation is often considered in end-stage CF disease.
The clinical course of patients with cystic fibrosis (CF) is variable and probably determined by many interacting factors. We aimed to examine the influence of early social and clinical factors on long-term survival. A case-control study of adult CF patients was used to compare long-term survivors (aged ≥40 yrs) with patients who died before reaching 30 yrs of age. Each case (n = 78) was ...
To the Editor: The Case Record by Mojica et al. (Dec. 27 issue) 1 highlights the importance of considering the diagnosis of cystic fibrosis in adults. We reviewed 842 cases of cystic fibrosis in ...
In my experience, non-adherence is often not a case of people choosing not to take their medication. It has much more to do with finding the mental resilience needed to cope with the burden of cystic fibrosis, as well as a desire to fit in with peers, and the demands of work and school. Helping people and their families to better manage anxiety ...
Cystic fibrosis (CF) is an autosomal recessive condition affecting approximately 30,000 Americans and 70,000 people worldwide. According to the Cystic Fibrosis Foundation (Cystic Fibrosis Foundation, 2019a), approximately 1,000 new cases are diagnosed yearly in the United States, with a known incidence of 1 per 3,900 live births. The disease ...
Most patients with cystic fibrosis (CF) develop multisystemic clinical manifestations, the minority having mild or atypical symptoms. We describe an adolescent with chronic cough and purulent rhinorrhoea since the first year of life, with diagnoses of asthma, allergic rhinitis and chronic rhinosinusitis. Under therapy with long-acting bronchodilators, antihistamines, inhaled corticosteroids ...
Case report: A 25-year-old man diagnosed with CF at 15 by sweat test and genetic study demonstrating F508del mutation. He presents exacerbation of bronchiectasis due to an infectious cause, reporting increase of cough, with greenish sputum production and no improvement factors. In addition to cough, the patient reports ventilatory-dependent ...
The inherited CF gene directs the body's epithelial cells to produce a defective form of a protein called CFTR (or cystic fibrosis transmembrane conductance regulator) found in cells that line the lungs, digestive tract, sweat glands, and genitourinary system. When the CFTR protein is defective, epithelial cells can't regulate the way that ...
In addition, many states have introduced newborn screening for CF, resulting in the detection of asymptomatic infants with CF. Case 12. Failure to Thrive: Workup Results in Diagnosis of Cystic Fibrosis. Mr. and Mrs. M, a white couple, have two children, a four-year-old son and a three-month-old daughter. The three-month-old has had considerable ...
Genetic Science Learning Center. (2012, December 1) Gene Therapy Case Study: Cystic Fibrosis. Retrieved April 15, 2024, from https://learn.genetics.utah.edu/content ...
This directed case study examines the molecular basis of cystic fibrosis to emphasize the relationship between the genetic code stored in a DNA sequence and the encoded protein's structure and function. Cystic fibrosis is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) protein that functions to help ...
Universal newborn screening of cystic fibrosis had been well-established before the study period 16. Data collected from their existing electronic medical record included; the microbiological ...
This case study is a follow-up to the Cystic Fibrosis Case Study where students explore how changes in transport proteins affects the movement of ions, resulting in a build-up of chloride ions and the symptoms of the disease. Students were introduced to the idea that different mutations can cause differences in the transport proteins, but in ...
Introduction. Cystic fibrosis (CF) is a genetic ailment resulting from an autosomal recessive trait. In the United States of America, the estimated prevalence of CF disease is 0.797 per 10,000 individuals; at the same time, among Caucasians who are born alive, the superiority is 1 in 2,500 (Stern, 1997).An association related to CF has expressed concerns about the potential impact of COVID-19 ...
unfolding case study history of present problem: justin ewing is boy with history of cystic fibrosis (cf) who for the past two days has had fever of 102.1 (38.9. ... Cystic Fibrosis case study. Course: Family Health Nursing (NUR 338) 10 Documents. Students shared 10 documents in this course. University: University of Massachusetts Dartmouth.
This case study asks students to examine a case of cystic fibrosis. As students read the symptoms and gather evidence about membrane proteins, they learn that CF is really a disorder of membrane permeability. ... Weyland suspects that baby Zoey may be suffering from cystic fibrosis. CF affects more than 30,000 kids and young adults in the ...
suggest that they add extra salt to debbie's diet and watch her for dehydation. Study with Quizlet and memorize flashcards containing terms like Which statement by the mother supports the diagnosis of CF, which documentation further supports the diagnosis of CF, what information will the nurse include when teaching about the sweat test and more.
ABSTRACTToday, more than 90% of people with cystic fibrosis (pwCF) are eligible for the highly effective cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapy called elexacaf...