An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager

Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Rice: Importance for Global Nutrition
Affiliations.
- 1 USDA, ARS, Beltsville Human Nutrition Research Center.
- 2 USDA, ARS, Adaptive Cropping Systems Laboratory.
- PMID: 31619630
- DOI: 10.3177/jnsv.65.S2
Rice, a staple food for more than half of the world's population, is grown in >100 countries with 90% of the total global production from Asia. Although there are more than 110,000 cultivated varieties of rice that vary in quality and nutritional content, after post-harvest processing, rice can be categorized as either white or brown. Regional and cultural preferences as well as need for stability during storage and transport are the final determinants of market availability and final consumption. In addition to calories, rice is a good source of magnesium, phosphorus, manganese, selenium, iron, folic acid, thiamin and niacin; but it is low in fiber and fat. Although brown rice is promoted as being "healthier" because of bioactive compounds, including minerals and vitamins not present in white rice after polishing, white rice is more widely consumed than brown. This is for several reasons, including cooking ease, palatability, and shelf life. Polished rice has a higher glycemic load and may impact glucose homeostasis but when combined with other foods, it can be considered part of a "healthy" plate. With the projected increase in the global population, rice will remain a staple. However, it will be important to encourage intake of the whole grain (brown rice) and to identify ways to harness the phytonutrients that are lost during milling. Furthermore, as the world faces environmental challenges, changing demographics and consumer demands, farmers, healthcare providers, food manufacturers and nutritionists must work collaboratively to assure adequate supply, nutritional integrity and sustainability of rice production systems globally.
Keywords: global nutrition; health; rice.
PubMed Disclaimer
Similar articles
- Improving Rice Grain Quality Through Ecotype Breeding for Enhancing Food and Nutritional Security in Asia-Pacific Region. Alam M, Lou G, Abbas W, Osti R, Ahmad A, Bista S, Ahiakpa JK, He Y. Alam M, et al. Rice (N Y). 2024 Aug 5;17(1):47. doi: 10.1186/s12284-024-00725-9. Rice (N Y). 2024. PMID: 39102064 Free PMC article. Review.
- Brown Rice, a Diet Rich in Health Promoting Properties. Lee JS, Sreenivasulu N, Hamilton RS, Kohli A. Lee JS, et al. J Nutr Sci Vitaminol (Tokyo). 2019;65(Supplement):S26-S28. doi: 10.3177/jnsv.65.S26. J Nutr Sci Vitaminol (Tokyo). 2019. PMID: 31619639
- Rice bran: a novel functional ingredient. Sharif MK, Butt MS, Anjum FM, Khan SH. Sharif MK, et al. Crit Rev Food Sci Nutr. 2014;54(6):807-16. doi: 10.1080/10408398.2011.608586. Crit Rev Food Sci Nutr. 2014. PMID: 24345050 Review.
- An overview of global rice production, supply, trade, and consumption. Muthayya S, Sugimoto JD, Montgomery S, Maberly GF. Muthayya S, et al. Ann N Y Acad Sci. 2014 Sep;1324:7-14. doi: 10.1111/nyas.12540. Epub 2014 Sep 15. Ann N Y Acad Sci. 2014. PMID: 25224455 Review.
- Cooking rice in excess water reduces both arsenic and enriched vitamins in the cooked grain. Gray PJ, Conklin SD, Todorov TI, Kasko SM. Gray PJ, et al. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2016;33(1):78-85. doi: 10.1080/19440049.2015.1103906. Epub 2015 Nov 2. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2016. PMID: 26515534
- Plant growth-promoting rhizobacterium Bacillus megaterium modulates the expression of antioxidant-related and drought-responsive genes to protect rice ( Oryza sativa L.) from drought. Lee S, Kim JA, Song J, Choe S, Jang G, Kim Y. Lee S, et al. Front Microbiol. 2024 Aug 21;15:1430546. doi: 10.3389/fmicb.2024.1430546. eCollection 2024. Front Microbiol. 2024. PMID: 39234545 Free PMC article.
- Estimation of Proximate Composition in Rice Using ATR-FTIR Spectroscopy and Chemometrics. Siregar S, Nurhikmat A, Amdani RZ, Hatmi RU, Kobarsih M, Kusumaningrum A, Karim MA, Dameswari AH, Siswanto N, Siswoprayogi S, Yuliyanto P. Siregar S, et al. ACS Omega. 2024 Jul 16;9(30):32760-32768. doi: 10.1021/acsomega.4c02816. eCollection 2024 Jul 30. ACS Omega. 2024. PMID: 39100304 Free PMC article.
- Enhancing aromatic rice production through agronomic and nutritional management for improved yield and quality. Patra PS, Saha R, Ahmed AS, Kanjilal B, Debnath MK, Paramanik B, Hoque A, Kundu A, Adhikary P, Biswas A, Dey P, Biswas A. Patra PS, et al. Sci Rep. 2024 Jul 5;14(1):15555. doi: 10.1038/s41598-024-65476-5. Sci Rep. 2024. PMID: 38969735 Free PMC article.
- Application of Silicon Influencing Grain Yield and Some Grain Quality Features in Thai Fragrant Rice. Chan-In P, Jamjod S, Prom-U-Thai C, Rerkasem B, Russell J, Pusadee T. Chan-In P, et al. Plants (Basel). 2024 May 12;13(10):1336. doi: 10.3390/plants13101336. Plants (Basel). 2024. PMID: 38794407 Free PMC article.
- New Insights into the Genetic Basis of Lysine Accumulation in Rice Revealed by Multi-Model GWAS. He L, Sui Y, Che Y, Liu L, Liu S, Wang X, Cao G. He L, et al. Int J Mol Sci. 2024 Apr 25;25(9):4667. doi: 10.3390/ijms25094667. Int J Mol Sci. 2024. PMID: 38731885 Free PMC article.
- Search in MeSH
Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- J-STAGE, Japan Science and Technology Information Aggregator, Electronic
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cochrane Database Syst Rev
Fortification of rice with vitamins and minerals for addressing micronutrient malnutrition
Rice fortification with vitamins and minerals has the potential to increase the nutrition in rice‐consuming countries where micronutrient deficiencies exist. Globally, 490 million metric tonnes of rice are consumed annually. It is the dominant staple food crop of around three billion people.
To determine the benefits and harms of rice fortification with vitamins and minerals (iron, vitamin A, zinc or folic acid) on micronutrient status and health‐related outcomes in the general population.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, and 16 other databases all up to 10 December 2018. We searched ClinicalTrials.gov, and World Health Organization International Clinical Trials Registry Platform (ICTRP) on 10 December 2018.
Selection criteria
We included randomised and quasi‐randomised trials (with either individual or cluster randomisation) and controlled before‐and‐after studies. Participants were populations older than two years of age (including pregnant women) from any country. The intervention was rice fortified with at least one micronutrient or a combination of several micronutrients (iron, folic acid, zinc, vitamin A or other vitamins and minerals) compared with unfortified rice or no intervention.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Two review authors independently screened studies and extracted data.
Main results
We included 17 studies (10,483 participants) and identified two ongoing studies. Twelve included studies were randomised‐controlled trials (RCTs), with 2238 participants after adjusting for clustering in two cluster‐RCTs, and five were non‐randomised studies (NRS) with four controlled before‐and‐after studies and one cross‐sectional study with a control (8245 participants). Four studies were conducted in India, three in Thailand, two in the Philippines, two in Brazil, one each in Bangladesh, Burundi, Cambodia, Indonesia, Mexico and the USA. Two studies involved non‐pregnant, non‐lactating women and 10 involved pre‐school or school‐age children.
All 17 studies reported fortification with iron. Of these, six studies fortified rice with iron only; 11 studies had other micronutrients added (iron, zinc and vitamin A, and folic acid). One study had one arm each with vitamin A alone and carotenoid alone. Elemental iron content ranged from 0.2 to 112.8 mg/100 g uncooked rice given for a period varying from two weeks to 48 months.
Thirteen studies did not clearly describe either sequence generation or allocation concealment. Eleven studies had a low attrition rate. There was no indication of selective reporting in the studies. We considered two RCTs at low overall risk of bias and 10 at high overall risk of bias. One RCT was at high or unclear risk of bias for most of the domains. All controlled before‐and‐after studies had a high risk or unclear risk of bias in most domains. The included studies were funded by Government, private and non‐governmental organisations, along with other academic institutions. The source of funding does not appear to have altered the results. We used the NRS in the qualitative synthesis but we excluded them from the quantitative analysis and review conclusions since they provided mostly contextual information and limited quantitative information.
Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)
Fortification of rice with iron (alone or in combination with other micronutrients) may make little or no difference in the risk of having anaemia (risk ratio (RR) 0.72, 95% confidence interval (CI) 0.54 to 0.97; I 2 = 74%; 7 studies, 1634 participants; low‐certainty evidence) and may reduce the risk of iron deficiency (RR 0.66, 95% CI 0.51 to 0.84; 8 studies, 1733 participants; low‐certainty evidence). Rice fortification may increase mean haemoglobin (mean difference (MD) 1.83, 95% CI 0.66 to 3.00; I 2 = 54%; 11 studies, 2163 participants; low‐certainty evidence) and it may make little or no difference to vitamin A deficiency (with vitamin A as one of the micronutrients in the fortification arm) (RR 0.68, 95% CI 0.36 to 1.29; I 2 = 37%; 4 studies, 927 participants; low‐certainty evidence). One study reported that fortification of rice (with folic acid as one of the micronutrients) may improve serum or plasma folate (nmol/L) (MD 4.30, 95% CI 2.00 to 6.60; 215 participants; low‐certainty evidence). One study reported that fortification of rice with iron alone or with other micronutrients may slightly increase hookworm infection (RR 1.78, 95% CI 1.18 to 2.70; 785 participants; low‐certainty evidence). We are uncertain about the effect of fortified rice on diarrhoea (RR 3.52, 95% CI 0.18 to 67.39; 1 study, 258 participants; very low‐certainty evidence).
Rice fortified with vitamin A alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)
One study had one arm providing fortified rice with vitamin A only versus unfortified rice. Fortification of rice with vitamin A (in combination with other micronutrients) may increase mean haemoglobin (MD 10.00, 95% CI 8.79 to 11.21; 1 study, 74 participants; low‐certainty evidence). Rice fortified with vitamin A may slightly improve serum retinol concentration (MD 0.17, 95% CI 0.13 to 0.21; 1 study, 74 participants; low‐certainty evidence).
No studies contributed data to the comparisons of rice fortification versus no intervention. The studies involving folic acid and zinc also involved iron in the fortification arms and hence we reported them as part of the first comparison.
Authors' conclusions
Fortification of rice with iron alone or in combination with other micronutrients may make little or no difference in the risk of having anaemia or presenting iron deficiency and we are uncertain about an increase in mean haemoglobin concentrations in the general population older than 2 years of age. Fortification of rice with iron and other micronutrients such as vitamin A or folic acid may make little or no difference in the risk of having vitamin A deficiency or on the serum folate concentration. There is limited evidence on any adverse effects of rice fortification.
Plain language summary
What is the aim of this review?
The aim of this Cochrane Review was to assess whether rice fortification with one or more vitamins and minerals in the general population aged two years or older improves nutritional status.
Key messages
Fortification of rice with iron alone or in combination with other micronutrients may make little or no difference in the risk of having anaemia but probably reduces the risk of iron deficiency and increases mean haemoglobin concentrations in population aged two years or above. If vitamin A is added, it may reduce the risk of having vitamin A deficiency and when folic acid is added, fortified rice may slightly increase serum folate concentrations.
What was studied in this review?
Micronutrient malnutrition compromises the health and well‐being of populations in many low‐ and middle‐income countries. Fortification is the addition of nutrients to foods to improve their nutritional quality. Rice is widely consumed as a staple food and is suitable for adopting as a food vehicle for fortification. This review addresses the benefits and harms of rice fortification with vitamins and minerals on micronutrient status along with health‐related outcomes, among participants aged two years and above, in addition to outcomes relevant to deficiencies in iron, vitamin A, zinc and folate.
What are the main results of the review?
We identified 17 studies (involving 10,483 participants) from Bangladesh, Brazil, Burundi, Cambodia, India, Indonesia, Mexico, Philippines, Thailand and USA. Twelve were randomised studies (2238 participants); 10 involved children, and two studies involved non‐pregnant non‐lactating women. In addition to iron, some studies had vitamin A, zinc or folic acid as fortifying agents, alone or in combination. Five non‐randomised studies (8245 participants), were assessed to augment the information on implementation and impact of fortification. The included studies were funded by government, private and non‐governmental organisations, along with other academic institutions. The source of funding does not appear to have altered the results.
We are uncertain about whether rice fortification with iron and other micronutrients reduces the risk of having anaemia although this intervention may increase mean haemoglobin concentrations (a biomarker of anaemia). We are uncertain if fortification of rice with iron alone or in combination with other micronutrients as compared to without any fortification, reduces the risk of iron deficiency.
Furthermore, consumption of vitamin A in the fortified rice, may make little difference on haemoglobin and serum retinol concentrations (a biomarker of vitamin A nutrition). We do not know whether fortification of rice has any adverse effects, in the mid‐ or long‐term, as the evidence was very limited. We found that the overall certainty of the evidence ranged from very low to low. Also, all studies used iron to fortify rice, so the effect of isolated nutrients may be hidden. There was no significant publication bias across the studies.
How up‐to‐date is this review?
The review authors searched for studies that had been published up to 10 December 2018.
Summary of findings
Summary of findings for the main comparison.
| general population older than 2 years of age (including pregnant women) from any country Burundi, Cambodia, India, Indonesia, Mexico, Philippines, Thailand and USA rice fortified with iron alone or in combination with other micronutrients unfortified rice (no micronutrients added) | ||||||
| (95% CI) | ||||||
| (defined as haemoglobin below the WHO cut‐off, adjusted for altitude as appropriate) | Study population | RR 0.72 (0.54 to 0.97) | 1634 (7 RCTs) | ⊕⊕⊝⊝ | Included studies: ; ; ; ; ; ; | |
| 388 per 1000 | 279 per 1000 (209 to 376) | |||||
| (as defined by study authors, based on a biomarker of iron status) | Study population | RR 0.66 (0.51 to 0.84) | 1733 (8 RCTs) | ⊕⊕⊝⊝ | Included studies: ; ; ; ; ; ; ; | |
| 228 per 1000 | 150 per 1000 (116 to 191) | |||||
| (in g/L) | The mean haemoglobin concentration (g/L) in the intervention groups was 1.83 higher (0.66 to 3.00 higher) | ‐ | 2163 (11 RCTs) | ⊕⊕⊝⊝ | Included studies: ; ; ; ; ; ; ; ; ; ; | |
| (as defined by the study authors) | Study population | RR 0.68 (0.36 to 1.29) | 927 (4 RCTs) | ⊕⊕⊝⊝ | Included studies: ; ; ; | |
| 105 per 1000 | 71 per 1000 (38 to 135) | |||||
| (nmol/L) | The mean serum or plasma folate (nmol/L) in the intervention group was 4.30 higher (2.00 to 6.60 higher) | ‐ | 215 (1 RCT) | ⊕⊕⊝⊝ | Included study: | |
| (hookworm infection risk) | Study population | RR 1.78 (1.18 to 2.70) | 785 (1 RCT) | ⊕⊕⊝⊝ | Included study: | |
| 119 per 1000 | 211 per 1000 (140 to 320) | |||||
| (as defined by study authors) | Study population | RR 3.52 (0.18 to 67.39) | 258 (1 RCT) | ⊕⊝⊝⊝ | Included study: | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| * (and its 95% confidence interval) is based on the assumed risk in the comparison group and the of the intervention (and its 95% CI). confidence interval; randomised controlled trial; risk ratio; World Health Organization | ||||||
| we are very confident that the true effect lies close to that of the estimate of the effect. we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded 2 levels: one for serious limitations in study design or execution (risk of bias) and one for indirectness. The baseline characteristics were not similar in all groups and the method of randomisation was unclear in half of the studies. Also studies used different cut‐off levels of haemoglobin to define anaemia. Hardinsyah 2016 ; Parker 2015 (C) ; Perignon 2016 (C) ; Radhika 2011 used WHO cut‐off levels, Hotz 2008 used CDC criteria and Angeles‐Agdeppa 2008 and Thankachan 2012 did not name the criteria they used. 2 Downgraded 2 levels: one for serious limitations in study design or execution (risk of bias) and one for indirectness as most of the studies, except one ( Hotz 2008 ), were conducted in children. There was negligible inconsistency among the studies. 3 Downgraded 2 levels: one for serious limitations in study design or execution (risk of bias) and one for indirectness. Most of the included studies (except Hotz 2008 ; Losso 2017 ) were carried out among children. Losso 2017 was carried out in USA, which is a different study setting as compared to all other studies included. 4 Downgraded 2 levels: one for serious risk of bias and one for inconsistency. Findings from the studies crossed line of no effect except one study ( Thankachan 2012 ), which showed clear benefit due to fortification. 5 Downgraded 2 levels for risk of bias being serious in the included study ( Hardinsyah 2016 ), having selection bias, reporting bias and presence of other bias. 6 Downgraded 2 levels: one for inconsistency and one for indirectness. Only one study in children assessed this adverse effect of hookworm infection in an endemic setting to soil‐transmitted helminth infections among participating children ( Perignon 2016 (C) ). 7 Downgraded 3 levels: one for inconsistency, one for indirectness and one for imprecision. Only one study in children reported on this outcome and assessed it through asking participating children about symptoms and signs during the previous week ( Thankachan 2012 ). Wide confidence intervals.
Summary of findings 2
| general population older than 2 years of age (including pregnant women) from any country India rice fortified with vitamin A alone or in combination with other micronutrients unfortified rice (no micronutrients added) | |||||
| (95% CI) | |||||
| (g/L) | MD 10 higher (8.79 higher to 11.21 higher) | ‐ | 74 (1 RCT) | ⊕⊕⊕⊝ | Included study: |
| (µmol/L) | MD 0.17 higher (0.13 higher to 0.21 higher) | ‐ | 74 (1 RCT) | ⊕⊕⊕⊝ | Included study: |
| * (and its 95% confidence interval) is based on the assumed risk in the comparison group and the of the intervention (and its 95% CI). confidence interval; mean difference; randomised controlled trial; risk ratio | |||||
| we are very confident that the true effect lies close to that of the estimate of the effect. we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1 Downgraded by 2 levels: one level for risk of bias and one level for indirectness. The only study was carried out in India with a small sample size (250 children aged 5‐8 years) attending a school with a subsidised lunch feeding programme ( Hussain 2014 ).
Description of the condition
Adequate vitamin and mineral nutrition is required for optimal growth and development of children and for the maintenance of adequate health and nutrition of adult populations. Vitamin and mineral deficiencies may result in conditions such as anaemia, blindness, birth defects, retarded growth, diminished mental development and other poor health outcomes ( Howson 1998 ; Oakley 2004 ; Darnton‐Hill 2005 ; A2Z Project 2008 ). Micronutrient deficiencies have also long been demonstrated to increase the risk of morbidity and, in some cases, mortality, especially from infection ( Bhaskaram 2002 ; Singhal 2002 ; Black 2003 ). They also significantly and negatively impact on socioeconomic development at the individual, community and national levels ( Darnton‐Hill 2005 ). Iron, vitamin A, iodine and zinc deficiencies constitute the world’s most common micronutrient deficiencies ( WHO 2009b ).
The World Health Organization (WHO) estimates that approximately 1.6 billion people are anaemic worldwide with half being women and children under five years of age ( WHO 2015a ). It is estimated that in 2016, 41.7% of children, 40% of pregnant women and 32.5% of non‐pregnant women had anaemia ( Stevens 2013 ; WHO 2015a ; WHO 2019 ). Although anaemia can be caused by multiple factors, iron deficiency is estimated to account for up to 50% at least of the anaemia burden, making it the single most widespread nutritional deficiency in the world ( Graham 2001 ; Rastogi 2002 ; Stoltzfus 2011 ). Other conditions such as parasitic infections, inherited haemoglobin disorders, or nutritional deficiencies such as of folate or vitamin B 12 can also cause anaemia ( WHO 2017 ). Thus, low haemoglobin concentrations are indicators of both poor nutrition and poor health ( WHO 2011a ). Before birth and during the first years of life, iron deficiency affects growth, neurodevelopment and cognitive performance ( Lozoff 2006 ; Carter 2010 ), and may increase susceptibility to infections ( Scrimshaw 2010 ). In adults, iron deficiency and anaemia cause the loss of healthy and productive lives due to their effects on work and physical capacity ( Haas 1996 ). Pregnant women with iron deficiency are at higher risk of suboptimal pregnancy outcomes, including complications at delivery, low birthweight infants and preterm births ( Peña‐Rosas 2015 ).
Vitamin A deficiency causes xerophthalmia, which leads to night blindness and weakens the immune system, thereby increasing the risk of childhood morbidity and mortality ( Sommer 1996 ). Vitamin A deficiency may increase the risk of morbidity and mortality during infancy, pregnancy and in the postpartum period ( Sommer 1996 ; West 1999 ). It is estimated that vitamin A deficiency results in 18 million disability‐adjusted life years (DALYs) lost, a measure of overall disease burden that is expressed as the number of years lost due to ill‐health, disability or early death ( WHO 2002 ). Vitamin A deficiency occurs mostly after prolonged deprivation of this vitamin ( WHO/FAO 2004 ), and is a significant public health problem in many low‐ and middle‐income countries, most seriously affecting young children, women of reproductive age and pregnant women. According to recent estimates, 190 million preschool‐age children (under five years of age) and 19.1 million pregnant women have inadequate concentrations of retinol. Roughly 45% of all preschool‐age children and pregnant women with vitamin A deficiency live in the WHO regions of South‐East Asia, while Africa accounts for another 30% of cases ( WHO 2009b ). An analysis of trends of vitamin A deficiency showed a decline in the overall prevalence from 39% to 29% from 1991 to 2013, but Africa and South Asia had the least decline ( Stevens 2015 ). Vitamin A deficiency alone is responsible for almost 6% of child deaths under the age of five years in Africa and 8% in South‐East Asia ( WHO 2009a ). It was estimated that in 2013, 1.7% of all deaths in children younger than five years were attributable to vitamin A deficiency ( Stevens 2015 ).
Zinc deficiency is considered to be associated with morbidity and mortality in low‐ and middle‐income countries. Severe zinc deficiency in children may cause short stature, impaired immune function and other disorders, and is a significant cause of respiratory infections, malaria and diarrhoeal disease ( WHO 2002 ). Adequate zinc nutrition is essential for human health because of zinc’s critical structural and functional roles in multiple enzyme systems that are involved in gene expression, cell division and growth, and immunologic and reproductive functions ( Hess 2009 ). Although there is very limited national‐ or first administrative‐level survey data on the prevalence of zinc deficiency, it has been estimated that zinc deficiency is responsible for approximately 4% of child mortality and DALYs ( Black 2008 ). An estimated 17.3% of the global population is at risk of inadequate zinc intake. The regional estimated prevalence of inadequate zinc intake ranged from 7.5% in high‐income regions to 30% in South Asia. These country‐specific prevalences of inadequate zinc intake were calculated based on the estimated absorbable zinc content of the national food supply ( Wessells 2012 ).
Inadequate intake is a leading cause of folate deficiency and insufficiency in the population although increased requirements from pregnancy or neoplastic diseases, malabsorptive conditions, use of antifolate drugs or other metabolic inhibitors can also cause folate deficiency ( Bailey 2015 ). Inadequate periconceptional folate status and folic acid intake are associated with congenital malformations including neural tube defects ( IOM 2003 ). Folic acid is a synthetic form of folate used in supplements and fortified foods (like wheat and maize flour) to reduce the occurrence of neural tube defects (NTDs). These defects include spina bifida (or cleft spine), where there is an opening in one or more of the bones (vertebrae) of the spinal column and anencephaly where the head (cephalic) end of the neural tube fails to close. It has been demonstrated through controlled studies that the risk of neural tube defects can be substantially reduced (risk ratio (RR) 0.31, 95% confidence interval (CI) 0.17 to 0.58; 5 studies, 6708 births; high‐certainty evidence) with daily folic acid supplementation, alone or in combination with other vitamins and minerals ( De‐Regil 2015 ). The effectiveness of mandatory folic acid fortification in wheat flour programmes has also been documented by a decline in the prevalence of neural tube defects, in the USA, Canada, Costa Rica, Chile and South Africa ( Berry 2010 ). In general, populations from lower socioeconomic status do not consume sufficient high‐folate‐content foods, and although their diets may be adequate in folate intake to preventing clinical deficiency (i.e. megaloblastic anaemia), they may be insufficient to reach red blood cell folate concentrations associated with optimal health and fetal development (i.e. greatest NTD risk reduction) in women of reproductive age, that is concentrations above 400 ng/mL (906 nmol/L) ( WHO 2015b ).
Other vitamins and minerals
In addition to iron, vitamin A, zinc and folate deficiencies, those of iodine, calcium, vitamin B 12 and vitamin D impair health and development. For example, iodine deficiency is a major threat to the health and development of populations worldwide, particularly in preschool children and pregnant women, resulting in goitre, stillbirth and miscarriage, hypothyroidism, and impaired growth ( Andersson 2012 ). Vitamin D deficiency (defined as low concentrations of serum 25‐hydroxyvitamin D) may be a common health problem worldwide ( Bandeira 2006 ; Palacios 2014 ). A recent review found an important proportion of infants, children, adolescents, adults and older persons living in different countries with low serum vitamin D concentrations ( Palacios 2014 ). These low concentrations were seen in all age groups, but in particular in girls and women from the Middle East. Vitamin D deficiency and/or perturbations of vitamin D metabolism; very low chronic calcium intake or a combination of both vitamin D deficiency and low chronic calcium intake, can cause nutritional rickets. Rickets is mostly associated with very low calcium intake in older children while in adolescents, the studies suggest that nutritional rickets is more associated with vitamin D deficiency ( Munns 2016 ).
Intervention strategies for micronutrient malnutrition
Current recommended intervention strategies for the prevention and treatment of micronutrient deficiencies include either one or a combination of supplementation, food‐based approaches such as dietary diversification, mass food fortification or point‐of‐use food fortification; other public health control measures include deworming, health and nutrition education ( Howson 1998 ; Zimmermann 2007 ; WHO 2011c ). These strategies can be delivered through at least four platforms, the health systems, agriculture, market‐based, and social protection programmes ( Olney 2012 ). Supplementation is still the most widely practiced intervention to control iron ( WHO 2011b ; WHO 2011d ; WHO 2016 ) and vitamin A deficiencies in high‐risk populations ( WHO 2011e ).
Some adverse effects observed with high‐dose supplements, as well as active participation from users, may affect compliance and the long‐term sustainability of such programmes. Supplementation programmes ( Baltussen 2004 ; Alderman 2007 ), usually face logistical and human‐resource constraints, such as bad road networks and generally fragile institutions, which may hinder their effectiveness, especially in low‐ and middle‐income countries where the intervention is needed most ( Zimmermann 2007 ). In such cases, mass fortification of staple foods becomes an important option to combat vitamin and mineral deficiencies. There are fewer concerns related to mass food fortification and it can be a complementary intervention to supplementation for efforts to reduce vitamin and mineral deficiencies.
Meeting the recommended dietary intakes ( WHO/FAO 2004 ), through the daily diet is desirable but not always possible for many populations. Poor dietary diversity and dependence on cereal‐based diets, which are common in low‐ and middle‐income countries, are major contributing factors to the high prevalence of micronutrient deficiencies ( Welch 1999 ). Cereals, in addition to being poor sources of vitamins and minerals, also contain high quantities of other dietary compounds, such as phytates, which decrease the absorption of certain micronutrients, often called 'anti‐nutrients' ( Graham 2001 ). For instance, iron and zinc absorption is significantly inhibited by phytic acid, present in cereals and other grains; polyphenols, contained in red wine and chocolate; or calcium, abundant in dairy products ( Gibson 1998 ; Hurrell 2010 ; Kim 2011 ). On this basis, dietary bioavailability of iron has been estimated to be in the range of 14% to 18% for mixed diets and 5% to 12% for vegetarian diets.
Cereals, however, are overwhelmingly the major source of food supplies for direct human consumption. In 2014, around 2.5 billion tonnes of cereals were produced with roughly 1.1 billion tonnes (43%) used as food; around 900 million tonnes (35%) used as animal feed and the remaining 500 million tonnes were diverted to industrial usage or seed, or were wasted ( FAO 2016 ). While rice is produced in vast areas of the world, the physical requirements for growing this crop are limited to certain zones. Rice is the primary staple for more than half the world’s population. Production and consumption is greatest in Asia ( Muthayya 2014 ), and in recent years, it has also become an important staple in Africa ( FAO 2012 ). About 741 million tonnes of rice (paddy) were harvested in 2014 ( FAOSTAT 2016 ). The milled equivalent of rice produced is 490 million tonnes ( FAO 2016 ).
Description of the intervention
Fortification is “the addition of one or more essential nutrients to a food, whether or not it is normally contained in the food, for the purpose of preventing or correcting a demonstrated deficiency of one or more nutrients in the general population or specific population groups" ( Codex Alimentarius 1994 ). This process usually takes place during food processing by the food industry at a central level so that it reaches the intended population en masse and does not require the active participation of end users. While there are some different definitions for enrichment, for the purposes of this review, we used enrichment and fortification interchangeably.
Results of a study in Vietnamese school children showed that iron‐fortified rice noodles are efficacious in reducing anaemia and improving haemoglobin and iron status indicators ( Huong 2006 ). In places where rice is a staple food, iron fortification has been shown to reduce the prevalence of iron‐deficiency anaemia from 100% to 33% among preschool age children ( Angeles‐Agdeppa 2008 ), particularly when there is strong political support and intensive social marketing activities as well as efforts to keep the cost affordable (Angeles‐Agdeppa 2011). Zinc fortification of cereals can boost total zinc consumed daily and absorbed zinc in infants, young children and adults ( Brown 2007 ). Although less frequent, fortification of wheat and maize flours with vitamin A has the technological and biological potential to palliate this deficiency ( Klemm 2010 ). Perhaps the most well known area of micronutrient fortification is that of folic acid, in both wheat and maize flours, and its effect on the prevention of birth defects ( WHA 2010 ). Well conducted studies from several countries have documented decreases of 26% to 42% in the occurrence of neural tube defect (NTD)‐affected births after implementation of national regulations mandating wheat flour fortification with folic acid ( WHO 2009b ). Food fortification brings together the benefit of energy, fat and protein, and the complementary roles of vitamins and minerals to enhance the stability and bioavailability of vitamins and minerals used to fortify foods ( Best 2011 ). In addition, this strategy has a dual advantage of reaching a wider and larger proportion of the population than supplementation without requiring radical changes in food consumption patterns ( Howson 1998 ).
Food fortification practices vary nationally. The choice of nutrients (in this context also known as fortificants) varies according to their bioavailability. In the case of iron, for instance, many compounds such as ferrous sulphate, ferrous fumarate, ferric pyrophosphate and electrolytic iron powder can be used in food fortification ( WHO/FAO 2006 ). However, many cereal foods are fortified with low‐cost iron powders with absorption of iron lower than 2% ( Hurrell 2010 ). For vitamin A fortification, retinyl palmitate and acetate are frequently used while the synthetic form of folic acid is used to improve folate status.
A concern expressed by a few people about food fortification is related to the possible toxicity of excessive vitamins and minerals among all groups, particularly those that are not at risk of deficiencies ( Garcia‐Casal 2019 ). This is especially so with iron excess ( Gordeuk 1987 ), which may affect the risk of colonic adenomas and cancer ( Muthunayagam 2009 ), and a potentially more pathogenic gut microbiota that is associated with higher gut inflammation ( Zimmermann 2010 ). Excess and chronic vitamin A intake during pregnancy has been shown to increase the risk of teratogenicity ( Rothman 1995 ), and hip fracture ( Penniston 2003 ). A hypothetical association between the prolonged consumption of folic acid‐enriched cereals and the increase in the incidence of colorectal cancer in the USA and Canada ( Mason 2007 ), has been challenged with other studies where such an association has not been demonstrated ( EFSA 2009 ). Another concern may relate to the possibility of over‐consumption of rice given the potential benefits of additional vitamins and minerals. As a public health intervention, the use of a vehicle would imply not encouraging the population to consume greater amounts of the 'fortified' rice. Higher consumption of white rice is associated with a significantly increased risk of type 2 diabetes, especially in Asian (Chinese and Japanese) populations ( Hu 2012 ).
Micronutrient deficiencies of public health significance are all widespread in most high rice‐consuming countries ( Juliano 1993 ; MIcronutrient Initiative/UNICEF 2004 ), and rice fortification has the potential to fill an obvious gap in current nutrition programmes and help aid vulnerable populations that are currently out of reach. A fundamental requirement in the adoption of food fortification as a public health intervention is the selection of the most appropriate and suitable food that will serve as a vehicle for the extra nutrients. It needs to be eaten in large amounts by the target population and be affordable and available all year round ( Dexter 1998 ; WHO/FAO 2006 ). Although almost all foods can be fortified, cereals are widely grown, produced and consumed in low‐ and middle‐income countries ( Welch 1999 ), making them important vehicles for fortification. Improving the micronutrient content of cereals or their subproducts could provide a sustainable solution to the worldwide problem of micronutrient deficiencies, particularly in populations where there is a marked social characterisation of eating habits ( Prättälä 2012 ), and where the fortified foods will be reaching those in need of the vitamins and minerals. Poor children and their mothers systematically lag behind the better‐off in terms of mortality, morbidity and undernutrition. Evaluations of the equity impact of health programmes and nutrition interventions are scarce. There are, however, some results suggesting that innovative approaches can effectively promote equity through, for example, employing appropriate delivery channels; removing financial barriers; and monitoring implementation, coverage and impact with an equity lens. Mandatory fortification of staple foods being consumed by the most vulnerable segments of the populations would potentially provide vitamins and minerals to those in a vulnerable situation ( WHO 2010 ), although it is clear that tackling inequities requires the involvement of various programmes and stakeholders, both within and outside the health sector, that can help address social determinants ( WHO 2010 ).
How the intervention might work
Rice is a globally produced, milled and traded staple food with an annual production and consumption worldwide of about 490 million tonnes. It is the dominant staple food crop of around three billion people worldwide, providing up to 50% to 60% of their daily energy and protein intake ( IRRI 2010 ). Rice is cultivated in almost all parts of the world as it can grow in a wide range of soil and environmental conditions ( Juliano 1993 ). It is estimated that 90% of the world's rice is produced in Asia ( Juliano 1993 ; Muthayya 2014 ). China and India consume 50% of the world's rice and per capita consumption is highest in Asia ( Muthayya 2014 ). High consumption has been reported in Latin America and Caribbean countries as well as in sub‐Saharan Africa ( Muthayya 2014 ). With its popularity, reach and quantum of consumption, rice far exceeds the requirements for adoption as a vehicle for food fortification for the purposes of a population‐level intervention.
Globally, the main rice processing method is milling. The process is aimed at producing a maximum yield of unbroken milled rice compared to flour or meal in other cereals ( Dexter 1998 ). The process involves cleaning the paddy or rough rice (un‐hulled rice grain) and de‐hulling (removing hull, germ and bran layers) to produce brown rice ( Dexter 1998 ). Brown rice consists of an average weight of 6% to 7% bran, 90% endosperm and 2% to 3% embryo ( Saunders 1979 ). Further milling to remove the bran layer yields white rice. On average, paddy rice produces 25% hulls, 10% bran, and 65% white rice ( Chen 1998 ). In some countries the milled white rice is coated with talc and glucose to improve its appearance ( Dexter 1998 ). The various forms of rice are presented in Table 3 . Milled white rice is low in vitamins and minerals as these vitamins (B vitamins) and minerals (iron) are found predominately in the germ and bran layers ( Dexter 1998 ). Parboiling is one of the ways by which nutrients in the rice grain can be partially preserved. The parboiling process of soaking the rough rice, applying heat, drying and milling results in the transfer of nutrients to the inner endosperm layer from the bran before milling ( Dexter 1998 ). Parboiling is expensive and the end product, referred to as ‘golden colour rice’, may not be readily acceptable to consumers ( Dexter 1998 ). The different types rice are depicted in Table 3 .
| (paddy rice) | Rice kernels still enclosed in an inedible, protective hull |
| Rice with only the hull removed. Bran layers and rice germ remain, giving the rice a brown colour | |
| Rice pressurised to gelatinise the starch within the rice kernel, resulting in a firmer, more separate grain that is more stable and less susceptible to overcooking than regular‐milled white rice | |
| (milled rice) | Polished whole rice, or polished rice. Hull, bran layer and germ have all been removed |
| Regular milled white rice, parboiled milled white rice, and brown rice can be precooked and dehydrated before packaging. Examples of precooked rice are quick‐cooking rice, instant rice, and boil‐in‐the‐bag rice | |
| Cooked grains are individually frozen before packaging | |
| Kernels can be processed in a number of different ways and shapes to meet particular manufacturing need |
Adapted from Dexter 1998 .
Previous attempts to fortify rice by simply adding a micronutrient powder to the rice that adheres to the grains by electrostatic forces (dusting) have proven unsuccessful ( Leon Guerrero 2009 ), due to the typical washing and cooking methods employed in most developing countries, which results in the rinsing away of the enrichment. Three more sophisticated methods have been developed to overcome this problem ( A2Z Project 2008 ). Coating involves spraying of the surface of ordinary rice grains in several layers with a vitamin and mineral mix to form a protective coating that will not easily rinse off the surface when washed ( Kyritsia 2011 ). The grains (fortified premix) contain high concentrations of vitamin and mineral fortificants and must be blended with natural rice (that is commonly 1 part fortified premix to 199 parts untreated milled rice) to produce fortified rice. The extrusion technology is a totally different concept in rice fortification. In hot extrusion, a dough made of rice flour, vitamin and mineral mix and water is passed through a single or twin screw extruder and shaped into partially precooked grain‐like structures resembling rice grains; that is then blended with natural polished rice at a ratio of about 1:200 to produce fortified rice. This process involves relatively high temperatures (70 to 110 °C) obtained by preconditioning or heat transfer through steam‐heated barrel jackets, or both. The cold extrusion follows a similar process at low temperature (below 70 °C) that does not primarily utilise any additional heat and produces uncooked, opaque fortified premix grains with a slightly softer consistency. This is then blended with natural polished rice at a ratio of about 1:200 to produce fortified rice.
Rice is a highly culturally‐sensitive commodity ( Hariyadi 2011 ). Growing, selecting and cooking of rice grains are subject to regional, national and even local preferences. It is estimated that a large proportion of key vitamins and minerals are lost during milling ( DSM/Buhler 2010 ). Additionally, rinsing and washing are common cooking methods which can potentially dissolve added or restored nutrients. There are many different ways of cooking rice. These are 1) soaking, and boiling with excess water; 2) boiling in excess water; 3) boiling without excess water; 4) rinsing and boiling without excess water; and 5) frying and boiling without excess water. The use of these cooking preparations could have different retentions of micronutrients in fortified rice kernels as some vitamins are sensitive to heat and others are water‐soluble ( WHO/FAO 2006 ). Cultural preferences for specific types of rice characteristics may represent a barrier to mass fortification in some settings. A technical challenge is to produce fortified rice that resembles natural rice and resists normal meal preparation and cooking processes.
A study conducted as far back as 1948 in the Philippines demonstrated the effects of rice fortification in the prevention of beriberi ( Salcedo 1950 ). In Brazil, a bioavailability study with vitamin A‐fortified rice showed an improvement in children's retinol levels ( Flores 1994 ). Another study among young children from 6 to 24 months of age in Brazil found that rice fortified with micronized iron pyrophosphate was more effective than iron drops in decreasing anaemia from 100% to 62%, and iron deficiency from 69% to 25%, and improving iron status ( Beinner 2010 ). In a study in India, fortified rice in school‐age children attending school showed a reduction of iron‐deficiency anaemia from 78% at baseline to 25% in the iron group ( Moretti 2006a ). In another setting, the feeding of rice fortified with microencapsulated, micronized iron pyrophosphate to improve the iron status of women in Mexico showed significant increases in plasma ferritin concentrations and estimated body iron stores as well as a significant decrease in plasma transferrin receptor concentrations. Fortified rice reduced the prevalence of anaemia by 80% and iron deficiency by 29% in Mexican women working in a factory ( Hotz 2008 ).
This review attempts to evaluate, based on existing research, the effectiveness of rice fortification as a public health intervention. The World Health Organization and Centers for Disease Control and Prevention (WHO/CDC) logic model for micronutrient interventions in public health depicts the programme theory and plausible relationships between inputs and expected improvements in Sustainable Development Goals and can be adapted to different contexts ( WHO/CDC 2016 ). The effectiveness of rice fortification in public health depends on several factors related to policies and legislation regulations; production and supply of the fortified rice; the development of delivery systems for the fortified rice; the development and implementation of external and internal food quality control systems; and the development and implementation of strategies for information, education and communication for behaviour change among consumers. A generic logic model for micronutrient interventions that depicts these processes and outcomes is presented in Figure 1 .

WHO/CDC logic model for micronutrients interventions in public health (with permission from WHO)
The high consumption of polished rice as a staple food in many settings has been associated with an increased risk of diabetes and other chronic diseases although the results of the studies have been conflicting. One systematic review and meta‐analysis has been published with respect to polished rice and diabetes studies ( Hu 2012 ), and another has been published with respect to rice and the incidence of chronic diseases including diabetes ( Saneei 2017 ). The earlier meta‐analysis included four prospective cohort studies and found that higher white rice consumption was associated with increased risk of developing type‐2 diabetes in comparison with lower intake levels (relative risk 1.27 (95% CI 1.04 to 1.54; Hu 2012 ). This association was stronger for Asian (Chinese and Japanese) populations, although the dose‐response relations indicated that even for Western populations with typically low intake levels, white rice consumption may still modestly increase risk of diabetes. A more recent meta‐analysis ( Saneei 2017 ), did not show an increased risk of diabetes with higher rice consumption due to an additional study from Spain ( Soriguer 2013 ), which showed that a negative association was found between white rice intake and the six‐year incidence of diabetes. No disaggregation of the estimates was done in Saneei 2017 for origin of population but both Hu 2012 and Saneei 2017 showed an increased risk of diabetes or chronic diseases among women who consumed more rice. Rice fortification policies may have to take into account the possible increased risk of diabetes and other diseases with rice consumption and identify fortification levels targeting existing rice consumption levels as has been done for salt iodisation and salt reduction policies ( WHO 2014 ). Additionally, the fortification of this staple food may affect acceptability of the fortified rice and thus potentially change dietary patterns ( Khanh 2014 ).
Why it is important to do this review
Vitamin and mineral deficiencies are important public health concerns worldwide. Among the options to address these deficiencies, mass fortification represents an appealing intervention as it takes advantage of the existing market and delivery systems, does not require the active participation of vulnerable populations to increase food intake or diversify the diet, and has few safety concerns. Rice represents a suitable vehicle for fortification as it is considered a staple food in most of the world, especially in regions where micronutrient deficiencies are most evident.
Wheat and maize flour fortification with iron alone, or in combination with folic acid and other micronutrients, has been implemented in more than 50 countries ( CDC 2008 ; WHO 2009b ) and is showing promising results in reducing anaemia and neural tube defects ( Centeno Tablante 2019 ; Garcia‐Casal 2018 ). Based on this experience, an increasing number of countries across the world are rapidly adopting fortification of rice as a means to fight malnutrition. Mandatory fortification of rice has been adopted in some countries, such as the Philippines, Costa Rica, Papua New Guinea and Nicaragua ( GAIN 2010 ). Fortified rice is sold in China using a multi‐micronutrient formula and in Japan enriched rice has been on the market since 1981. The USA has a mandatory food standard for 'enriched rice', prescribing levels of thiamin, niacin, riboflavin, folic acid and iron to be added to rice for enrichment. Although this requirement only applies in order for rice to be labelled as 'enriched' ( FDA 2001 ), 70% of the rice eaten in the USA is enriched or fortified ( American Rice Inc 2004 ; A2Z Project 2008 ). In India, Brazil and Colombia, fortified rice is currently being distributed through public safety net programmes ( Tsang 2016 ).
Despite this interest, to date there has been no systematic assessment of the benefits and harms of this intervention to inform policymaking and assist countries in the design and implementation of appropriate food‐fortification programmes, except for one systematic review carried out on interventions among children between 6 to 59 months ( Hijar 2015 ). Rice fortification was concluded to be effective for correcting and improving iron deficiency in children aged under five years of age.
Criteria for considering studies for this review
Types of studies.
We included randomised controlled trials (RCTs). Such studies provide information on whether fortified rice is effective and can actually achieve changes in health and vitamin and mineral status for those receiving the intervention.
Food fortification is, however, an intervention that aims at reaching the entire population of a country or large sections of the population and is frequently delivered through the food system. Therefore we have also included data from other study designs.
In summary, we aimed to include the following study designs.
- RCTs, with randomisation at either the individual or cluster level
- Quasi‐RCTs (where allocation of treatment has been made, for example, by alternate allocation, date of birth, or alphabetical order)
- Non‐randomised controlled trials
- cohort studies (prospective and retrospective);
- controlled before‐and‐after studies with at least two control and two intervention sites;
- interrupted time series with at least three measure points both before and after the intervention.
We analysed results from controlled non‐randomised and observational study designs separately from randomised and quasi‐randomised study designs.
We did not consider before‐and‐after studies without a control group for inclusion in this review. Results from these studies are presented in a table but are not included in a meta‐analysis and do not directly inform the conclusions of the review. Such studies provide information on the implementation, feasibility and other contextual factors relating to the interventions under review. We did not include cross‐over trials.
Types of participants
General population older than two years of age (including pregnant women) from any country. We excluded studies of interventions targeted toward participants with a critical illness or severe co‐morbidities.
Types of interventions
Interventions in the review were those in which rice had been fortified with at least one micronutrient or a combination of several micronutrients (iron, folic acid, zinc, vitamin A or other vitamins and minerals) irrespective of the method of fortification technology used. Fortified rice, for the purposes of this review, refers to the addition of a micronutrient premix to ordinary rice using any rice fortification technologies, such as hot extrusion, cold extrusion, coating or dusting ( A2Z Project 2008 ). We included studies with co‐interventions, that is, fortified rice with education, if the comparison group also received the education component in addition to the unfortified rice.
Comparisons include the following.
Rice fortified with iron alone or in combination with other micronutrients versus no intervention
Rice fortified with vitamin a alone or in combination with other micronutrients versus no intervention, rice fortified with zinc alone or in combination with other micronutrients versus unfortified rice (no micronutrients added), rice fortified with zinc alone or in combination with other micronutrients versus no intervention, rice fortified with folic acid alone or in combination with other micronutrients versus unfortified rice (no micronutrients added), rice fortified with folic acid alone or in combination with other micronutrients versus no intervention.
If studies examined the effects of two or more nutrients along with iron, we included them in the first comparison only to avoid duplication. If the studies had micronutrients in their fortification arms without iron, we included them in the further comparisons.
We excluded studies comparing rice fortification with other forms of micronutrient interventions (i.e. supplementation or dietary diversification) or the fortification of other food vehicles. We also excluded in‐vitro studies and those examining the effect of bio‐fortified rice (nutrient‐dense staple crops of rice using conventional breeding practices and modern biotechnology).
Types of outcome measures
Primary outcomes.
The primary outcomes across all populations in this review were the presence of anaemia, iron deficiency, haemoglobin concentrations and adverse effects.
- Anaemia (defined as haemoglobin (Hb) below the WHO cut‐off, adjusted for altitude as appropriate ( WHO 2011a ), or as defined by the study authors)
- Iron deficiency (as defined by study authors, based on a biomarker of iron status)
Haemoglobin concentration (g/L)
- Vitamin A deficiency (as defined by study authors, by using a biomarker; only for vitamin A‐fortified rice as intervention)
- Serum or plasma folate (nmol/L) (only for folic acid‐fortified rice as intervention)
- Any adverse effects (as defined by study authors)
Additional primary outcomes of interest differed by participant group, as listed below.
Children (2 to 11.9 years of age)
- Diarrhoea (as defined by study authors)
- Respiratory infections (as defined by study authors)
- All‐cause death
Pregnant women
- Congenital anomalies (neural tube defect, cleft lip, cleft palate, congenital cardiovascular defects and others as defined by study authors; only for folic acid‐fortified rice as intervention)
- Miscarriage
Secondary outcomes
Secondary outcomes included the following.
- Serum or plasma retinol (µmol/L) (only for vitamin A‐fortified rice as intervention)
Serum or plasma zinc (µmol/L)
- Anthropometric measures (height‐for‐age Z‐score and weight‐for‐height Z‐score for children, body mass index (BMI) for adults)
Risk of iron overload (defined by serum ferritin higher than 150 µg/L in women and higher than 200 µg/L in men)
- Clinical malaria (as defined by study authors)
- Severe malaria (as defined by study authors)
- Night blindness (defined as the reported inability to see after dusk by people who typically report having normal vision during the day; only for vitamin A‐fortified rice as intervention)
For those studies that delivered the intervention at the first administrative level or higher (i.e. non‐randomised studies) we examined the same variables at an ecological level (for example prevalence of anaemia or congenital anomalies rates).
Search methods for identification of studies
Electronic searches.
We searched the following international and regional sources.
International databases
- Cochrane Central Register of Controlled Trials (CENTRAL; Issue 7 2012 to Issue 12 2018) via Cochrane Register of Studies Online (CRSO)
- MEDLINE (OVID; 1948 to 10 December 2018)
- Embase (OVID; 1980 to 10 December 2018)
- CINAHL EBSCOhost (1937 to 10 December 2018)
- Web of Science (ISI) SCI, SSCI, CPCI‐exp & CPCI‐SSH (until 10 December 2018)
- POPLINE (www.popline.org/; 10 December 2018)
- AGRICOLA (Ebsco; 10 December 2018)
- ClinicalTrials.gov (searched 10 December 2018)
- WHO International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch; searched 10 December 2018)
- BIOSIS (ISI; 2012 to 10 December 2018)
Regional databases
- IBECS (ibecs.isciii.es; searched 10 December 2018)
- SciELO (Scientific Electronic Library Online; www.scielo.br; searched 10 December 2018)
- African Index Medicus (AIM; www.globalhealthlibrary.net/php/index.php?lang=en; searched 10 December 2018)
- Index Medicus for the Eastern Mediterranean Region (IMEMR; www.globalhealthlibrary.net/php/index.php?lang=en; searched 10 December 2018)
- LILACS (Latin American and Caribbean Health Sciences Literature; lilacs.bvsalud.org/en; searched 10 December 2018)
- PAHO (Pan American Health Library; www1.paho.org/english/DD/IKM/LI/library.htm; searched 10 December 2018)
- WHOLIS (WHO Library; dosei.who.int/; searched 10 December 2018)
- WPRIM (Western Pacific Region Index Medicus; www.wprim.org/; searched 10 December 2018)
- Index Medicus for the South‐East Asia Region (IMSEAR; imsear.hellis.org; searched 10 December 2018)
- IndMED, Indian medical journals; medind.nic.in/imvw/; searched to 10 December 2018)
- Native Health Research Database; hslic‐nhd.health.unm.edu; searched to 10 December 2018)
For these sources, we searched WorldCat, Networked Digital Library of Theses and Dissertations, DART‐Europe E‐theses Portal, Australasian Digital Theses Program, Theses Canada Portal and ProQuest‐Desertations and Theses.
We handsearched the five journals with the highest number of included studies in the last 12 months to capture any article that may not have been indexed in the databases at the time of the search. As rice fortification technologies are relatively novel we limited the search, from 1960 to present, for all databases, although some had no time restrictions.
We contacted Cochrane Public Health's Information Specialist to search the Cochrane Public Health Group Specialised Register. The search used keyword and controlled vocabulary (when available), using the search terms set out in the Appendices and adapting them as appropriate for each database (see Appendix 1 ).
We did not apply any language restrictions. If we identified articles written in a language other than English, we commissioned their translation into English. If this was not possible, we sought advice from Cochrane Public Health. We stored these articles in the 'Awaiting assessment' section of the review until a translation is available.
Searching other resources
For assistance in identifying ongoing or unpublished studies, we contacted the Department of Nutrition for Health and Development and WHO regional offices, the nutrition section of the United Nations Children's Fund (UNICEF), the World Food Programme (WFP), the US Centers for Disease Control and Prevention (CDC), US Agency for International Development (USAID) micronutrient programme, Nutrition International, the Global Alliance for Improved Nutrition (GAIN), Hellen Keller International (HKI), Sight and Life Foundation, PATH, the Wright Group, premix producers DSM and BASF, Food Fortification Initiative (FFI) and the Rice Fortification Resource Group (March 2019).
Selection of studies
Two review authors (JPP, PM) independently screened the titles and abstracts of articles retrieved by each search to assess initial eligibility. After the initial screening, we then retrieved full copies of all eligible papers and screened them for eligibility as determined by the inclusion and exclusion criteria listed above. When we were unable to reject a title or abstract with certainty, we obtained the full text of the article for further evaluation. If we could not obtain full articles, we attempted to contact the study authors to obtain further details of the study. Failing this, we classified studies as 'awaiting assessment' until further information is published or made available to us. We resolved disagreements at any stage of the eligibility assessment process through discussion and consultation with two other review authors (SN, LMR), where necessary.
Data extraction and management
Two review authors independently extracted data in duplicate using customised data extraction forms based on those from Cochrane Handbook ( Higgins 2019 ), Cochrane Public Health ( Cochrane PHG 2010 ), and Cochrane Effective Practice and Organisation of Care ( EPOC 2017 ).
All review authors were involved in piloting the form using a subset of articles in order to enhance consistency amongst review authors; based on this, we modified the form as necessary. We collected information on study design, study setting, participants (number and characteristics) and provided a full description of the interventions examined. We extracted details of outcomes measured (including a description of how and when outcomes were measured) and results.
Two review authors (JPP, LMR) designed the form, so that we were able to record results for our prespecified outcomes as well as for other non‐specified outcomes, although we did not use such outcomes to underpin any of our conclusions. We also extracted additional items relating to study recruitment and the implementation of the intervention, including number of sites for an intervention, whether recruitment was similar at different sites, levels of compliance and use of rice in different sites within studies, resources required for implementation, and whether studies had conducted a process evaluation. We also recorded whether or not studies included specific strategies to address diversity or disadvantage. We used the PROGRESS (place of residence, race/ethnicity, occupation, gender, religion, education, socioeconomic status, capital) checklist to collect information on whether or not studies had reported data by sociodemographic characteristics known to be important from an equity perspective ( Ueffing 2011 ).
Two review authors (JPP, JAS) entered data into Review Manager 5 software and checked for accuracy ( Review Manager 2014 ).
Assessment of risk of bias in included studies
We used the EPOC 'RIsk of bias' tool for studies with a separate control group to assess the risk of bias of all studies at study level for primary outcomes ( EPOC 2017 ). This tool includes five domains of bias: selection, performance, attrition, detection and reporting; as well as an 'other bias' category to capture other potential threats to validity.
Two review authors independently assessed risk of bias in duplicate (JPP, PM) for each study and resolved any disagreement by discussion or by involving an additional review author (SN).
Assessing risk of bias in randomised trials and quasi‐randomised trials
1. sequence generation (checking for possible selection bias).
We assessed studies as:
- low risk of bias if there is a random component in the sequence generation process (e.g. random number table; computer random number generator);
- high risk of bias if a non‐random approach has been used (e.g. odd or even date of birth; hospital or clinic record number). Non‐randomised studies should be scored 'high';
- unclear risk of bias if not specified in the paper.
2. Allocation concealment (checking for possible selection bias)
- low risk of bias if participants and investigators enrolling participants could not foresee assignment because an appropriate method was used to conceal allocation (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes). We gave this rating to studies where the unit of allocation was by institution and allocation was performed on all units at the start of the study;
- high risk of bias if participants of investigators enrolling participants could possibly foresee assignments and potentially introduce selection bias (e.g. open random allocation; unsealed or non‐opaque envelopes);
- unclear.
3. Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias)
- low risk of bias if outcomes were measured prior to the intervention, and no important differences were present across intervention groups;
- high risk of bias if important differences in outcomes between groups were present prior to intervention and were not adjusted for in the analysis;
- unclear risk of bias if there was no baseline measure of outcome (note: if 'high' or 'unclear' but there is sufficient information to do an adjusted analysis, the assessment should be 'low').
4. Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias)
- low risk of bias if baseline characteristics are reported and similar across intervention groups;
- high risk of bias if baseline characteristics are not reported or if there are differences across groups;
- unclear risk of bias if it is not clear (e.g. characteristics mentioned in text but no data presented).
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts and protocol deviations)
We assessed outcomes in each included study as:
- low risk of bias due to incomplete outcome data, which could be either that there were no missing outcome data or the missing outcome data were unlikely to bias the results based on the following considerations: study authors provided transparent documentation of participant flow throughout the study, the proportion of missing data was similar in the intervention and control groups, the reasons for missing data were provided and balanced across the intervention and control groups, the reasons for missing data were not likely to bias the results (e.g. moving house).
- high risk of bias if missing outcome data was likely to bias the results. Studies will also receive this rating if an 'as‐treated' (per protocol) analysis is performed with substantial differences between the intervention received and that assigned at randomisation, or if potentially inappropriate methods for imputation have been used;
- unclear risk of bias.
6. Blinding (checking for possible performance and detection bias)
We assessed the risk of performance bias associated with blinding as:
- low, high or unclear risk of bias for participants;
- low, high or unclear risk of bias for personnel.
We assessed the risk of detection bias associated with blinding as:
- low, high or unclear risk of bias for outcome assessors.
Whilst assessed separately, we combined the results in a single evaluation of risk of bias associated with blinding as follows:
- low risk of bias if there was blinding of participants and key study personnel and it was unlikely to have been broken, or the outcomes are objective. This rating will also be given to studies where either participants and key study personnel were not blinded but outcome assessment was blinded and the non‐blinding of others was unlikely to introduce bias;
- high risk of bias if there was no blinding or incomplete blinding or if there was blinding that was likely to have been broken and the outcome or outcome assessment was likely to be influenced by a lack of blinding;
7. Contamination (checking for possible performance bias)
- low risk of bias if allocation was by community, institution or practice and it is unlikely that the control group received the intervention;
- high risk of bias if it is likely that the control group received the intervention;
- unclear risk of bias if it is possible that contamination occurred but the risk of this happening is not clear.
8. Selective reporting bias
- low risk of bias if it is clear, either by availability of the study protocol or otherwise, that all prespecified outcomes that are of interest in the review have been reported;
- high risk of bias if it is clear that not all of the study's prespecified outcomes have been reported, or reported outcomes were not prespecified (unless justification for reporting is provided), or outcomes of interest are reported incompletely and cannot be used, or where one or more of the primary outcomes is reported using measurements or analysis methods that were not prespecified, or finally if the study report fails to include an important outcome that would be expected to have been reported;
9. Other sources of bias
Other possible sources of bias were described for each included study and a rating of low, high or unclear risk of bias was given for this item.
In addition to the above criteria, we also assessed cluster‐RCTs with the following criteria:
1. Recruitment bias
- low risk of bias if individuals were recruited to the study before the clusters were randomised;
- high risk of bias if individuals were recruited to the study after the clusters were randomised;
2. Baseline imbalance
- low risk of bias if baseline characteristics were reported and were similar across clusters or if study authors used stratified or pair‐matched randomisation of clusters;
- high risk of bias if baseline characteristics were not reported or if there were differences across clusters;
- Unclear risk of bias.
3. Loss of clusters
- low risk of bias if no complete clusters were lost or omitted from the analysis;
- high risk of bias if complete clusters were lost or omitted from the analysis;
4. Incorrect analysis
- low risk of bias if study authors appropriately accounted for clusters in the analysis or provided enough information for review authors to account for clusters in the meta‐analysis;
- High risk of bias if study authors did not appropriately account for clusters in the analysis or did not provide enough information for review authors to account for clusters in the meta‐analysis;
5. Compatibility with individual RCTs
- low risk of bias if effects of the intervention were likely not altered by the unit of randomisation;
- high risk of bias if effects of the intervention were likely altered by the unit of randomisation;
Overall risk of bias
For all included studies, we summarised the overall risk of bias by primary outcome within each study at the study level. Studies at high risk of bias were those with high or unclear risk of bias in the following domains: allocation concealment, similarity of baseline outcome measurements, and incomplete outcome data. We judged the overall risk of bias of each study as 'low' if we had assessed all three domains at low risk; and 'high' when we had assessed one or more of the domains at either high or unclear risk. Judgements took into account the likely magnitude and direction of bias and whether it was likely to impact on the findings of the study.
Measures of treatment effect
For dichotomous outcomes we have presented proportions, and for two‐group comparisons we have presented results as average risk ratio (RR) with 95% confidence interval (CI). We have reported results for continuous outcomes as the mean difference (MD) with 95% CI if studies measured outcomes in the same way. Where some studies reported endpoint data and others reported changes from baseline data (with errors), we combined these in the meta‐analysis if the outcomes had been reported using the same scale. We used standardised mean difference (SMD) with 95% CI to combine studies that measured the same outcome (for example haemoglobin) but used different methods.
For studies with multiple arms reporting a continuous variable as an outcome, we calculated the weighted average for single pair‐wise results in the meta analysis.
Unit of analysis issues
Cluster‐randomised trials.
We combined results from both cluster‐randomised and individually randomised studies if there was little heterogeneity between the studies. We labelled cluster‐randomised trials with a 'C'. If the authors of cluster‐randomised trials had conducted their analyses at a different level to that of allocation, and they had not appropriately accounted for the cluster design in their analyses, we calculated studies' effective sample sizes to account for the effect of clustering in the data. We utilised the intra‐cluster correlation coefficient (ICC) derived from the study (if available) or from another source (for example using the ICCs derived from other, similar studies) ( Adams 2004 ; Gulliford 1999 ), and then calculated the design effect with the formula provided in the Cochrane Handbook for Systematic Reviews of Interventions ( Higgins 2011 ). We reported this and undertook sensitivity analysis to investigate the effect of variations in ICC.
We made an adjustment in the number of participants for design effect for both the continuous outcome of haemoglobin concentrations and dichotomous outcomes of anaemia, iron deficiency and vitamin A deficiency in two studies ( Parker 2015 (C) ; Perignon 2016 (C) . We used the design effect calculated for anaemia for calculating the total number of participants in iron deficiency, vitamin A deficiency and haemoglobin concentration. We used the mean and standard deviations of haemoglobin concentration in the analysis without making any changes. The details of adjustments for design effect in each of the studies are provided in Characteristics of included studies .
Studies with more than two treatment groups
If we identified studies with more than two intervention groups (multi‐arm studies), where possible we combined groups to create a single pair‐wise comparison or used the methods set out in the Cochrane Handbook for Systematic Reviews of Interventions to avoid double counting study participants ( Higgins 2011 ). If two or more study arms shared the control group, we divided the control group over the number of relevant subgroup categories to avoid double counting the participants (for dichotomous data, we divided the events and the total population while for continuous data we assumed the same mean and standard deviation but divided the total population). The details are described in the Characteristics of included studies tables.
Dealing with missing data
We noted missing outcome data and levels of attrition for included studies on the data extraction form. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. The denominator for each outcome in each study was the number randomised minus any participants whose outcomes we knew to be missing. For missing summary data, we contacted lead study authors for clarification or, if possible, we estimated missing summary data using other statistical information (for example confidence intervals, standard errors) provided in the primary paper and imputed the standard deviation either from other studies in the same systematic review or from studies in another systematic review.
Assessment of heterogeneity
We examined forest plots from a meta‐analysis to visually determine the level of heterogeneity (in terms of the size or direction of treatment effect) between studies. We used Tau², I² statistic ( Higgins 2003 ) and Chi² statistic to quantify the level of heterogeneity among the studies in each analysis ( Deeks 2017 ). We regarded substantial or considerable heterogeneity as Tau² greater than 0 and either I² statistic greater than 30% or a low P value (< 0.10) in the Chi² test. We noted this in the text and explored it using prespecified subgroup analyses mentioned below. Caution was taken in the interpretation of those results with high levels of unexplained heterogeneity.
Assessment of reporting biases
Where we suspected reporting bias (see 'Selective reporting bias' above) we attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and we thought that the missing data would introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
We did not anticipate that there would be sufficient studies contributing data for any particular outcome for us to examine possible publication bias; if more than 10 studies reporting the same outcome of interest were available, we planned to generate funnel plots in Review Manager 2014 and visually examine them for asymmetry. Where we pooled studies in a meta‐analysis we ordered studies in terms of weight so that a visual examination of forest plots might allow us to assess whether the results from smaller and larger studies were similar or if there were any apparent differences according to study size.
Data synthesis
We carried out a meta‐analysis to provide an overall estimate of treatment effect when more than one study examined the same intervention, provided that studies used similar methods and measured the same outcome in similar ways in similar populations. We used a random‐effects model meta‐analysis for combining data as we anticipated that there might be natural heterogeneity between studies attributable to the difference. We used narrative synthesis, guided by the data extraction form in terms of the ways in which studies were grouped and summarised, to describe the outcomes, explore intervention processes, and describe the impact of interventions by sociodemographic characteristics known to be important from an equity perspective based on the PROGRESS framework, where this information was available.
We did not combine results from randomised and non‐randomised trials together in a meta‐analysis, and we have not presented pooled estimates for non‐randomised studies with different types of study design. We have reported the results of the controlled before‐and‐after studies in narrative form.
Assessing the certainty of evidence
For the assessment across studies, we used MECIR (Methodological Expectations of Cochrane Intervention Reviews) conduct standards ( MECIR 2018 ), and we employed the GRADE approach to interpret findings ( Langendam 2013 ). The GRADE profiler ( GRADEpro GDT 2015 ) allowed us to import data from Review Manager 2014 to create 'Summary of findings' tables. For each of the outcomes, two review authors (JPP, MNGC) assessed the certainty of evidence of included studies independently, using the GRADE approach ( Balshem 2011 ). We have listed the primary outcomes for each comparison with estimates of relative effects along with the number of participants and studies contributing data for those outcomes. These tables provide outcome‐specific information concerning the overall certainty of evidence from studies included in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes we considered. We included only primary outcomes in the 'Summary of findings' tables. We prepared 'Summary of findings' tables for the comparisons including rice fortified with iron alone versus unfortified rice, vitamin A alone or in combination with other micronutrients versus unfortified rice, zinc alone or in combination with other micronutrients versus unfortified rice, and folic acid alone or in combination with other micronutrients versus unfortified rice. The outcomes included in these were anaemia, iron deficiency, haemoglobin concentration, vitamin A deficiency, diarrhoea, respiratory infections, all‐cause death, and any adverse effects (see Table 1 ; Table 2 ).
For assessments of the overall certainty of evidence for each outcome that included pooled data from included studies from RCTs only, we downgraded the evidence from 'high certainty' by one level for serious (or by two for very serious) study limitations (risk of bias), indirectness of evidence (due to the use of different cut‐offs, for example), serious inconsistency, imprecision of effect estimates or potential publication bias. Data from observational studies started at low certainty. This assessment was limited only to the studies included in this review and as we did not consider there was a serious risk of publication bias, we did not downgrade in this domain.
Subgroup analysis and investigation of heterogeneity
Where possible we conducted subgroup analysis to explore heterogeneity according to the following subgroups.
- Micronutrient content: single nutrient versus two or more nutrients
- Rice fortification method: hot extrusion versus cold extrusion versus coating versus dusting
- Cooking method most commonly used in study setting (as reported): soaking, and boiling with excess water versus boiling in excess water versus boiling without excess water versus rinsing and boiling without excess water versus frying and boiling without excess water versus unknown/unreported
- Public health significance of anaemia at baseline in the target group: not a problem (lower than 5%) versus mild and moderate (5% to 39.9%) versus severe (40% and more) versus mixed/unknown/unreported
- Malaria endemicity at the time that the study was conducted: some malaria risk setting versus malaria‐free area versus unknown/unreported malaria setting.
We examined differences between subgroups by visual inspection of the CIs; non‐overlapping CIs suggesting a statistically significant difference in treatment effect between the subgroups. We also used the approach of Borenstein 2008 to formally investigate differences between two or more subgroups. We conducted analyses in RevMan 5 ( Review Manager 2014 ). We limited this analysis to those outcomes for which three or more studies contributed data.
Sensitivity analysis
We carried out sensitivity analysis to examine the effects of removing studies at high risk of bias (those with high or unclear risk of bias for allocation concealment, similarity of baseline outcome measurements, incomplete outcome data) from the meta‐analysis. For cluster‐randomised trials, we carried out sensitivity analysis using a range of ICC on overall effect estimate and have reported these effects.
Description of studies
Results of the search.
Our search strategy identified 28,730 references (22,147 references after removing duplicates) for possible inclusion. We screened a total of 58 full‐text articles for potential inclusion for the analyses. We included 17 studies (28 records). We excluded 22 studies (28 records) with reasons and identified two ongoing or unpublished studies ( NCT02714075 ; NCT03056625 ). All 17 included studies were reported in English. We have summarised the study selection process in Figure 2 . Of the 17 studies, 12 RCTs contributed to the meta‐analysis.

PRISMA study flow diagram
Included studies
We have presented the details of included studies, including participants, interventions, outcomes, source of funding, and results of contact with the study authors, in the Characteristics of included studies . We have given a summary of the general characteristics of the included studies in Table 4 . Twelve studies were reported from Asian countries ( Angeles‐Agdeppa 2008 ; Ara 2019 ; Gershoff 1977 ; Hardinsyah 2016 ; Hussain 2014 ; Moretti 2006b ; Perignon 2016 (C) ; Pinkaew 2013 ; Pinkaew 2014 ; Radhika 2011 ; Salcedo 1950 ; Thankachan 2012 ), two from Brazil ( Della Lucia 2016 ; Nogueira Arcanjo 2013 ), one from Mexico ( Hotz 2008 ), one from the USA (Louisiana; Losso 2017 ) and one study from Burundi ( Parker 2015 (C) ). The PROGRESS‐Plus framework characteristics are given in Table 5 . Each of the 17 included studies had different levels of micronutrient concentrations per 100 grams of uncooked rice, and we have given details of the micronutrient fortification profile in Table 6 .
| | ||||
| (Philippines) | 180 anaemic children aged 6‐9 years excluding severe anaemia (Hb < 70 g/L), history of blood disorders and other haemoglobinopathies | ), micronized ferric pyrophosphate (ExFeP80); and cooked unfortified rice | 6 months | High |
| (Indonesia) | 200 post‐menarchal adolescent girls 14‐18 years of age attending boarding school | ); group 2 (n = 100) received meals prepared with unfortified rice. , or tofu. | 4 months | High |
| (Mexico) | 180 non‐pregnant, non‐lactating women 18‐49 years of age with moderate to low Hb concentrations from 6 factories | 6 months | High | |
| (India) | 222 iron‐ and vitamin A‐depleted children 5‐8 years of age attending a subsidised lunch feeding programme | 6 months | High | |
| (USA) | 17 menstruating women with iron‐deficiency anaemia | 2 weeks | High | |
| (India) | 184 iron‐depleted children aged 6‐13 years from a primary school serving the Rock‐Colony neighbourhood | 7 months | Low | |
| (Thailand) | The study was conducted in 8 primary schools with children aged 4‐12 years and they were mainly from low‐income families. | 5 months | High | |
| (Thailand) | One primary school in the Muang district, of Thailand with children aged 8‐12 years, were the study participants | 2 months | High | |
| (India) | 140 children aged between 5 and 11 years (with haemoglobin > 70 g/L) | 8 months | Low | |
| (India) | Total of 258 anaemic (Hb concentrations 115 g/L for 6–11 years and 120 g/L for 12 years) children attending 4 primary schools aged 6‐12 years | 6 months | High | |
| (Burundi) | The study included 1071 children from 12 schools in Burundi aged between 7 and 11 years | 7 months | High | |
| (Cambodia) | The study was a double‐blind cluster‐randomised, placebo‐controlled trial conducted among a total of 2440 school‐going children aged 6‐16 years. | 6 months | High | |
| (Bangladesh) | 870 women aged 15‐49 years excluding severe anaemia (435/group) at baseline and 800 (400/group) at end line | 12 months | High | |
| (Brazil) | 131 non‐anaemic children between 2 and 6 years old, of both genders, participated in the study. | 4 months | High | |
| (Thailand) | 2250 children aged 1.5‐9 years from 29 villages | 4 years | High | |
| (Brazil) | 303 children 2‐5 years of age attending 2 public schools in City of Sobral‐Ceará, in the northeast of Brazil, between August and December 2010 | 18 weeks | High | |
| (Philippines) | 574 children aged between 3 and 18 years 2188 Government employees with their families 1416 military personnel (clinical assessment limited to 350 in the experimental group and 116 in the control group) | 8 months | High | |
| controlled before‐and‐after study; haemoglobin; randomised controlled trial | ||||
| | | ||||||||
| Metro Manila, Division Pasig; Philippines | No specific mention, apart from the locality of the school in the capital city | School children | Male 99 + female 81 | No religion mentioned; children going to San Joaquin Elementary School (public) | Not mentioned | Not mentioned | Anaemic children; sexual orientation not mentioned | This study was carried out among 180 anaemic children going to a government elementary school. | |
| Vulnerable Group Development (VDG) beneficiaries in 5 districts of Bangladesh | Not mentioned specifically, however, they were the local resident women. | It included professional workers, unskilled workers, agricultural labourers, home servants and housewives. Most of the study population were housewives | Non‐pregnant women aged 15‐49 years | No religion mentioned; nearly 25% without any education | No direct estimate provided; however, most of the study participants were from lower socioeconomic strata | Not mentioned | Women with severe anaemia were excluded. Sexual orientation is not mentioned | The study was carried out among 870 women of reproductive age and local residents of Bangladesh | |
| Brazil | Not specified | School‐going children | No religion mentioned, attending philanthropic schools | Not mentioned | Not mentioned | Children, 2‐6 years old | This study was carried out in 2 public schools among non‐anaemic children 2‐6 years of age during 4 consecutive months. | ||
| Chiang Mai villages in tile valley of the Ping River, Thailand | Thai children | Children in the community | Male 1121 + female 1109 | No religion mentioned. Children in the study villages | Not mentioned | Low/middle | Normal children; sexual orientation not mentioned | The study included 2230 children attending pre‐school and school from the low/middle social background | |
| Medan of North Sumatra Province, Indonesia | The majority of participants' ethnicity was Javanese and Bataknese | Teenage girls attending boarding school | Female | There is mention of the Ramadan fasting month during the second week of June | The family income ranges from 4.9 million to 5.5 million Rupiahs (Approximately 340 to 390 US Dollars) | Not mentioned | Age 14‐18 years of age | This study was carried out among post‐menarchal adolescent girls attending boarding school in Indonesia. The study lasted 4 months. | |
| Morelos State, Mexico | Mexican women | Factory workers | Women only | No religion mentioned; 18‐49 years | Low/middle school | Low/middle | Anaemic women; sexual orientation not mentioned | This study included women with altitude‐adjusted Hb concentrations between 105 and 135 g/L from low/middle social background, non‐pregnant and non‐lactating. | |
| India | Iron and vitamin A‐depleted 5‐8‐year‐old children attending a subsidised lunch feeding programme | Children attending a school‐based feeding programme | Not specified | Not reported | Not reported | Not mentioned, although programme is subsidised | 5‐8 years of age | This study included 222 children aged 5‐8 years attending a school where there was a subsidised lunch feeding programme in India receiving a 200‐250 g meal of cooked rice daily. | |
| Baton Rouge, USA | In the iron‐fortified group: 4 white, 3 black or African‐American, 1 Asian, 1 other; in the unfortified rice group: 3 white, 2 black or African American, 1 Asian | Women only | Not reported | Not mentioned | Not mentioned | 18‐50 years of age | This study included women with iron‐deficiency anaemia recruited through web and phone interviews and then in a clinic. | ||
| Franciscan primary school serving the population of Rock‐Colony neighbourhood, in crowded urban slum of Bangaoore; India | Indian | School‐going children | Not mentioned | 6‐13 years | Low | Low | Children with iron deficiency; sexual orientation not mentioned | Study included children having iron deficiency from an urban slum neighbourhood in India, belonging to low socioeconomic status and low social class | |
| Public schools in City of Sobral‐Ceará, in the northeast of Brazil | Not reported | School‐going children | Fortified rice group: 65 male: 73 female; unfortified rice group: 79 male: 86 female | 2‐5 years of age | Not reported. Family income 300 USD or less (it is unclear if this is weekly or monthly income ‐ not reported). 126/138 participants from iron‐fortified group versus 154/165 participants from unfortified group. | Not mentioned | Children 2‐5 years of age. Other information not reported | This before‐and‐after study included children 2‐5 years of age from 2 public schools in northeast Brazil receiving the school lunch programme and the fortified/unfortified intervention once a week. | |
| The study was carried out in Muyinga Province in Burundi catering to mainly agrarian population | Burundians | School‐going children | Female: 51.1% in intervention arm, 55.3% in control arm | Religion was not mentioned. 7‐11 years | Mean socioeconomic status score quintile = 3.03 (1.45) for intervention arm and 2.97 (1.37) for control arm | Not mentioned | Children with Hb level 70‐110 g/L and those who had not taken any nutritional supplements during the past 1 month since commencement of the study were included. Sexual orientation is not mentioned. | This cluster‐RCT included 904 children who were mild to moderately anaemic from the selected schools of Burundi and mainly with an agricultural background. | |
| The study was carried out in Kampung Speu Province of Cambodia | Cambodians | School‐going children | Male and female participants had equal representation (50% each) | 6‐16 years | Not mentioned | Not mentioned | Excluding severely anaemic children. All in the eligible age group were included in the study. Sexual orientation not mentioned | The cluster‐RCT included children from selected schools of Cambodia in KamPong Speu province with rice farming as a predominant occupation and income source. | |
| Satun province, west coast of southern Thailand | Thai Muslims | School children | Male, 98 + female 105 | Majority Muslim, age group of 7‐12 years | Low | Low/middle | Children with zinc deficiency; sexual orientation not mentioned | This study included school‐going children from low socioeconomic status and having zinc deficiency in Thailand. | |
| Muang District, Satun Province of southern Thailand | Thai Muslims | School Children | Males, 24 and females, 26 | Majority Muslims in the age group 8‐12 years | Low | Low/middle | Children who had consumed the triple‐fortified rice before or showed clinical symptoms of VAD (Bitots spot or ocular signs of xerophthalmia) or serum retinol values of < 0.7m mol/L were excluded | This study included school‐going children from low socioeconomic status and having zinc deficiency in Thailand. | |
| Village of Keesara; Andhra Pradesh State in India | Indian | School children | Male 56 + female 90 | No mention of religion; age group of 5‐11 years | Low/middle | Low/middle | Anaemic children; sexual orientation not mentioned | The study included anaemic children from low‐middle socioeconomic background belonging to a rural area in India. | |
| Bataan, Philippines | Filipinos | Children and military personnel | Male and female, but proportions not reported | No mention of religion or education | Children lived in a welfare institution; military personnel were fully employed | Not mentioned | No exclusions were reported; sexual orientation was not mentioned | The study was conducted among children living in a welfare institution and among military personnel in the Philippines. | |
| Primary schools in Bangalore Urban District of Karnataka State; South India | Indians | School children | Male 47%, female 53% | Hindu > Christians > Muslim; 6‐12 years | Low/middle school | Low | Anaemic children; sexual orientation not mentioned | This study included anaemic school going children from low socioeconomic background from an urban area India. | |
| haemoglobin; randomised controlled trial | |||||||||
| | | | | (thiamin) | (riboflavin) | (niacin) | (pyridoxine) | (cobalamin) | |
| 6.25 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| (CBA) | 6 | 0.15 | 4.00 | 130 | 0.40 | 1.0 | |||
| (CBA) | 8.4 | ‐ | 4.20 | 144 | 0.72 | ‐ | ‐ | ‐ | ‐ |
| (CBA) | 0.2 | 0.81 | 0.087 | 0.04 | |||||
| 0.2 | 0.81 | 0.087 | 0.04 | ||||||
| 0.2 | 0.81 | 0.087 | 0.04 | ||||||
| 10.8 | 0.28 | 5.20 | 145 | ‐ | 3.2 | ||||
| 26.6 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 4 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| ‐ | 1.20 (as beta‐carotene) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| ‐ | 0.18 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 4 | 0.18 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 4 | 1.20 (as beta‐carotene) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 18 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 20 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| (CBA) | 112.8 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| 11.9 | ‐ | 5.70 | 400 | 1.80 | ‐ | ‐ | ‐ | ‐ | |
| 10.67 | ‐ | 3.04 | 170 | 1.06 | ‐ | ‐ | ‐ | ‐ | |
| 7.55 | 0.64 | 2.02 | 280 | 1.43 | ‐ | 12.57 | ‐ | 3.8 | |
| 7.46 | 0.29 | 3.68 | 140 | 0.69 | ‐ | 7.98 | 0.92 | 1.26 | |
| 20 | 2.10 | 18 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 20 | 2.10 | 18 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 15 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| (CBA) | 2.86 | ‐ | ‐ | ‐ | 0.44 | ‐ | 0.33 | ‐ | ‐ |
| 12.5 | 0.50 | 3 | 75 | 0.38 | ‐ | 5 | 0.38 | 0.75 | |
| 6.25 | 0.50 | 3 | 75 | 0.38 | ‐ | 5 | 0.38 | 0.75 | |
| cluster randomised; controlled before‐and‐after study | |||||||||
a One international unit (IU) vitamin A is equivalent to 0.0003 mg of retinol, 0.0006 mg of beta‐carotene and 0.0012 mg of other pro‐vitamin A carotenoids.
Study designs
Out of the 17 included studies (28 records), twelve were RCTs ( Angeles‐Agdeppa 2008 ; Hardinsyah 2016 ; Hotz 2008 ; Hussain 2014 ; Losso 2017 ; Moretti 2006b ; Parker 2015 (C) ; Perignon 2016 (C) ; Pinkaew 2013 ; Pinkaew 2014 ; Radhika 2011 ; Thankachan 2012 ), two of which were cluster‐randomised trials ( Parker 2015 (C) ; Perignon 2016 (C) . For distinguishing them from individual randomised trials, their names are denoted with '(C)'. Five studies were controlled before‐and‐after studies ( Ara 2019 ; Della Lucia 2016 ; Gershoff 1977 ; Nogueira Arcanjo 2013 ; Salcedo 1950 ).
Overall, eight RCTs had 2‐arms ( Hardinsyah 2016 ; Hotz 2008 ; Losso 2017 ; Moretti 2006b ; Parker 2015 (C) ; Pinkaew 2013 ; Pinkaew 2014 ; Radhika 2011 ), two studies had three arms ( Angeles‐Agdeppa 2008 ; Thankachan 2012 ), and one study had five arms (including a placebo and traditional feeding intervention) as a part of the FORISCA‐UltraRice+NutriRice study ( Perignon 2016 (C) ). One RCT had six arms with five fortification groups in addition to control group ( Hussain 2014 ). The studies had various types of randomisation procedures, sequence generation, allocation concealment, blinding as described in Characteristics of included studies .
One before‐and‐after study had five arms ( Gershoff 1977 ) and four had two arms ( Ara 2019 ; Della Lucia 2016 ; Nogueira Arcanjo 2013 ; Salcedo 1950 ).
Twelve studies were carried out in Asia, four in the Americas and one in Africa. The studies in Asia were carried out in Bangladesh ( Ara 2019 ), Cambodia ( Perignon 2016 (C) ), India ( Hussain 2014 ; Moretti 2006b ; Radhika 2011 ; Thankachan 2012 ), Indonesia ( Hardinsyah 2016 ), Philippines ( Angeles‐Agdeppa 2008 ; Salcedo 1950 ) and Thailand ( Gershoff 1977 ; Pinkaew 2013 ; Pinkaew 2014 ). The four studies in the Americas were conducted in Brazil ( Della Lucia 2016 ; Nogueira Arcanjo 2013 ), Mexico ( Hotz 2008 ) and in the USA ( Losso 2017 ). The study in Africa was conducted in Burundi ( Parker 2015 (C) ). The Indian studies had school children in an urban area ( Thankachan 2012 ), urban slum ( Moretti 2006b ), and rural school setting ( Radhika 2011 ). The Mexican study ( Hotz 2008 ), recruited women from six factories without any specific mention of ethnicity or race. The studies from the Philippines had urban school children in Manila ( Angeles‐Agdeppa 2008 ), and children from a welfare institution and military personnel ( Salcedo 1950 ). The study in Burundi was conducted among rural school age children from Muyinga Province ( Parker 2015 (C) ). The study in Cambodia was among rural school children in Kampung Speu ( Perignon 2016 (C) ), while the Indonesian study included teenage girls from a boarding school in the area of Medan ( Hardinsyah 2016 ).
Malaria endemicity
One study reported that it was conducted in a malaria‐endemic area ( Parker 2015 (C) ). Four studies reported to be from non‐endemic areas for malaria ( Angeles‐Agdeppa 2008 ; Moretti 2006b ; Perignon 2016 (C) ; Thankachan 2012 ). Other studies did not report on endemicity for malaria ( Ara 2019 ; Della Lucia 2016 ; Gershoff 1977 ; Hardinsyah 2016 ; Hotz 2008 ; Hussain 2014 ; Losso 2017 ; Nogueira Arcanjo 2013 ; Pinkaew 2013 ; Pinkaew 2014 ; Radhika 2011 ; Salcedo 1950 ).
Participants
The 17 included studies had a total of 10,483 participants. The controlled before‐and‐after studies were conducted in children aged two to six years of age ( Della Lucia 2016 ; Nogueira Arcanjo 2013 ), 18 months to nine years of age ( Gershoff 1977 ), and one was among women aged between 15 and 49 years ( Ara 2019 ). One controlled before‐and‐after study was on infants to adults as it was prompted by clinical beriberi which cut across ages ( Salcedo 1950 ). Among the RCTs, two were conducted among non‐pregnant, non‐lactating women 18 to 49 years of age ( Hotz 2008 ; Losso 2017 ). All other included studies were carried out among preschool and school age children. RCTs involved children aged between 5 to 18 years of age ( Angeles‐Agdeppa 2008 ; Hardinsyah 2016 ; Hussain 2014 ; Moretti 2006b ; Parker 2015 (C) ; Perignon 2016 (C) ; Pinkaew 2013 ; Pinkaew 2014 ; Radhika 2011 ; Thankachan 2012 ). There were no studies reporting data exclusively on adolescents beyond the age of 12 years, adult men or pregnant women.
Anaemia prevalence
The baseline prevalence of anaemia ranged from 5% to 62% in the studies in children ( Angeles‐Agdeppa 2008 ; Hussain 2014 ; Moretti 2006b ; Nogueira Arcanjo 2013 ; Parker 2015 (C) ; Perignon 2016 (C) ; Pinkaew 2014 ; Radhika 2011 ; Thankachan 2012 ), and around 21% (intervention: 21.4 %; control: 20.4%) in one study among women ( Hotz 2008 ), while the other study on women included women with iron deficiency as determined by serum ferritin or serum iron value, or both ( Losso 2017 ). The study done on teenagers ( Hardinsyah 2016 ), had a baseline anaemia level of 34%. Two studies from India had an anaemia prevalence of around 40% and above ( Radhika 2011 ; Thankachan 2012 ). A third study from India reported iron‐deficiency anaemia of around 30% suggesting a much higher anaemia rate ( Moretti 2006b ). The two studies that included only anaemic children reported 38% ( Parker 2015 (C) ), and 45% ( Angeles‐Agdeppa 2008 ), during screening. Three studies had an anaemia prevalence between 10% to 18% ( Perignon 2016 (C) ; Pinkaew 2013 ; Pinkaew 2014 ). The controlled before‐and‐after study Ara 2019 had a baseline level of anaemia of 42%.
In total, five studies reported on stunting at baseline ranging from 12% to 40% ( Angeles‐Agdeppa 2008 ; Perignon 2016 (C) ; Pinkaew 2014 ; Radhika 2011 ; Thankachan 2012 ), one study reported less than 10% stunting in both intervention and control arms ( Hussain 2014 ). Two studies reported the height‐for‐age Z‐score ( Moretti 2006b ; Pinkaew 2013 ) as −1.3 and −0.8 respectively. One study ( Perignon 2016 (C) ), reported both: 40% stunting; −1.75 Z‐score). None of the studies restricted inclusion of participants or analysed their data based on stunting status.
Thirteen studies had preschool or school‐going children as participants ( Angeles‐Agdeppa 2008 ; Della Lucia 2016 ; Gershoff 1977 ; Hardinsyah 2016 ; Hussain 2014 ; Moretti 2006b ; Nogueira Arcanjo 2013 ; Parker 2015 (C) ; Perignon 2016 (C) ; Pinkaew 2013 ; Pinkaew 2014 ; Radhika 2011 ; Thankachan 2012 ). One study had women factory workers ( Hotz 2008 ), and another did not mention the occupation of the participants ( Losso 2017 ). The Ara 2019 study had mostly housewives (> 70% in each of the groups) followed by unskilled workers (˜7.82% to 11.26%).
Gender allocation was reported in all but three studies ( Hussain 2014 ; Moretti 2006b ; Salcedo 1950 ). All the three studies among adults included all women ( Ara 2019 ; Hotz 2008 ; Losso 2017 ). There were no differences in gender between treatment groups at baseline in any study. Six studies had more female participants ( Della Lucia 2016 ; Parker 2015 (C) ; Pinkaew 2013 ; Pinkaew 2014 ; Radhika 2011 ; Thankachan 2012 ). One study was carried out among teenage girls ( Hardinsyah 2016 ). Two studies had more male participants ( Angeles‐Agdeppa 2008 ; Perignon 2016 (C) ). One before‐and‐after study had roughly equal numbers ( Gershoff 1977 ), while one had more women ( Nogueira Arcanjo 2013 ).
Religion/culture
No specific mention was made about the religion of the study population in 12 studies ( Angeles‐Agdeppa 2008 ; Ara 2019 ; Della Lucia 2016 ; Gershoff 1977 ; Hotz 2008 ; Hussain 2014 ; Losso 2017 ; Moretti 2006b ; Nogueira Arcanjo 2013 ; Perignon 2016 (C) ; Radhika 2011 ; Salcedo 1950 ). However one of these studies ( Losso 2017 ), had mixed ethnic background participants. One study from India ( Thankachan 2012 ), reported that the majority of the study participants were Hindus (74% in fortification groups and 65% in control groups). Two studies from Thailand had a predominantly Muslim population ( Pinkaew 2013 ; Pinkaew 2014 ). The study from Indonesia had Javanese and Bataknese ethnic participants predominantly ( Hardinsyah 2016 ).
Socioeconomic status
The studies were conducted mostly among those with low‐socioeconomic status. Three studies from India ( Moretti 2006b ; Radhika 2011 ; Thankachan 2012 ), and two studies from Thailand ( Pinkaew 2013 ; Pinkaew 2014 ), were carried out among children from low socioeconomic backgrounds. One study from India was in an urban area ( Thankachan 2012 ), urban slum ( Moretti 2006b ), and rural area ( Radhika 2011 ). Other studies did not specify the socioeconomic status of their participants.
Twelve studies ( Angeles‐Agdeppa 2008 ; Della Lucia 2016 ; Hardinsyah 2016 ; Hussain 2014 ; Moretti 2006b ; Nogueira Arcanjo 2013 ; Parker 2015 (C) ; Perignon 2016 (C) ; Pinkaew 2013 ; Pinkaew 2014 ; Radhika 2011 ; Thankachan 2012 ), reported studies on pre‐school and school‐going children. One community‐based study ( Salcedo 1950 ), and two other studies ( Hotz 2008 ; Losso 2017 ), were on women whose educational status was not reported. One study included pre‐school children attending a day‐care centre ( Gershoff 1977 ). In Ara 2019 , nearly 25% of the study population did not have any education, the remaining participants all had primary school education and above.
Social capital
The studies did not report issues related to inequity, access to food or any particular instances of preferences of certain social classes. However, the included studies were carried out in predominantly lower socioeconomic settings.
Interventions
Micronutrient content.
All studies fortified rice with iron and some studies added various additional combinations of micronutrients. Seven studies ( Hardinsyah 2016 ; Hussain 2014 ; Parker 2015 (C) ; Perignon 2016 (C) ; Pinkaew 2013 ; Pinkaew 2014 ; Thankachan 2012 ), and four controlled before‐and‐after studies fortified with multiple micronutrients ( Ara 2019 ; Della Lucia 2016 ; Gershoff 1977 ; Salcedo 1950 ). Among them, six studies ( Hardinsyah 2016 ; Parker 2015 (C) ; Perignon 2016 (C) ; Pinkaew 2013 ; Pinkaew 2014 ; Thankachan 2012 ), compared the effectiveness of multiple‐micronutrient‐fortified rice with that of unfortified rice. One RCT reported different micronutrients and their effects on each arm, compared with the control arm, which received unfortified rice ( Hussain 2014 ), in which out of five intervention arms, one arm each received iron alone, beta carotene, retinyl palmitate, iron plus retinyl palmitate and iron plus beta carotene. Five studies ( Angeles‐Agdeppa 2008 ; Hotz 2008 ; Losso 2017 ; Moretti 2006b ; Radhika 2011 ), and one controlled before‐and‐after study ( Nogueira Arcanjo 2013 ), included iron alone for rice fortification (with various quantities of iron and its compounds).
For the RCTs with multiple micronutrients, two studies ( Pinkaew 2013 ; Pinkaew 2014 ), reported rice fortified with three micronutrients (iron, zinc and vitamin A); one study ( Parker 2015 (C) ), with four micronutrients (iron, zinc, thiamine and folic acid) and two ( Hardinsyah 2016 ; Perignon 2016 (C) ), with four or more micronutrients. All the multiple micronutrient studies had an arm with at least iron and zinc. One study ( Gershoff 1977 ), reported the field effect of consumption of rice fortified with lysine, threonine, thiamin, riboflavin, vitamin A and iron. One study ( Thankachan 2012 ), compared the effectiveness of high iron (12.5 mg/100 g natural rice) with that of low iron (6.25 mg/100 g of natural rice) fortification, along with vitamin A, thiamine, niacin, vitamin B 6 , vitamin B 12 , folate, iron and zinc and no fortification of rice. Another study ( Angeles‐Agdeppa 2008 ) compared the effectiveness of ferrous sulphate‐fortified rice and micronized dispersible ferric pyrophosphate‐fortified rice with that of unfortified rice, wherein each of the three arms received in 160 g (1 cup) of cooked rice, ferrous sulphate (ExFeSO4), micronized ferric pyrophosphate (ExFeP80) or no added fortificant (control).
In all the included studies, the amount of elemental iron per 100 g of rice ranged from 0.2 mg to 112.8 mg. Vitamin A was a fortificant in three studies. The amount of vitamin A per 100 g of rice ranged from 0.15 mg ( Ara 2019 ) to 2.1 mg ( Pinkaew 2013 ; Pinkaew 2014 ). The amount of zinc per 100 g of rice ranged from 2 mg ( Perignon 2016 (C) ) to 18 mg ( Pinkaew 2013 ; Pinkaew 2014 ). One study carried out among women had ferrous sulphate 18 mg/100 g of fortified rice for the intervention arm and unfortified rice for the control arm ( Losso 2017 ). Fortification details per 100 g of uncooked rice are given in Table 6 .
Rice fortification method
One study reported using extrusion as a fortification method without indicating the temperature ( Angeles‐Agdeppa 2008 ). Seven studies used hot‐extrusion process only ( Ara 2019 ; Hardinsyah 2016 ; Moretti 2006b ; Parker 2015 (C) ; Pinkaew 2013 ; Pinkaew 2014 ; Thankachan 2012 ), and three studies reported a cold extrusion process only ( Della Lucia 2016 ; Hotz 2008 ; Radhika 2011 ). One study included 2 arms with hot extrusion and one arm with cold extrusion ( Perignon 2016 (C) ). Three studies reported using coating in the fortification ( Gershoff 1977 ; Losso 2017 ; Salcedo 1950 ), and two studies did not report the method ( Hussain 2014 ; Nogueira Arcanjo 2013 ).
Iron compounds
The included studies used three types of iron compounds. The before‐and‐after study ( Gershoff 1977 ) used ferric phosphate tetrahydrate. One study ( Angeles‐Agdeppa 2008 ), used ferrous sulphate as one of two iron fortificants. Nine studies that were part of the meta‐analysis used ferric pyrophosphate. Micronized iron reduces the particle size to promote absorption. Two studies used a micronized ferric pyrophosphate, which had a particle size of 0.3 μm and was encapsulated ( Angeles‐Agdeppa 2008 ; Hotz 2008 ). One study, which used cold extrusion, reported a particle size of 3.1 μm ( Radhika 2011 ). Five studies described the iron used as micronized ground ferric pyrophosphate ( Moretti 2006b ; Perignon 2016 (C) ; Pinkaew 2013 ; Pinkaew 2014 ; Thankachan 2012 ), and three of these specified the particle size as 2.5 μm ( Moretti 2006b ; Pinkaew 2013 ; Pinkaew 2014 ). One study described the iron compound as ferric pyrophosphate without any reference to micronization or particle size ( Parker 2015 (C) ). Other studies had micronized ground ferric pyrophosphate with 4 mg iron ( Hussain 2014 ), 10 mg iron ( Pinkaew 2014 ), 11.8 mg per 100 g ( Parker 2015 (C) ); 10.6 mg per 11 g rice for cold‐extruded and 7.5 mg per 100 g rice for hot‐extruded ( Perignon 2016 (C) ), 20 mg iron ( Moretti 2006b ; Hotz 2008 ), 0.8% by weight ( Gershoff 1977 ). One study on women reported ferrous sulphate as the iron compound used ( Losso 2017 ). Two studies did not specify the iron compound ( Ara 2019 ; Hardinsyah 2016 ).
Cooking methods
In three studies, rice was washed prior to cooking ( Angeles‐Agdeppa 2008 ; Moretti 2006b ; Radhika 2011 ). However, the manner of cooking was not described in sufficient detail to categorise based on the protocol for this review. In ( Moretti 2006b ), in addition to rice being washed in preparation for cooking, rice portions were cooked with seasoning ingredients in household pressure cookers for 8 min after reaching peak pressure, after which pressure was released. Test servings contained 35 g cooked rice.
One study used the absorption method of cooking rice ( Perignon 2016 (C) ; author correspondence). Lunch menus were prepared in rotating order and usually consisted of rice together with chicken or fish and occasionally with vegetables. The schools also provided free milk (200 mL) daily to all children. Weekly iron supplementation, which had been given to the children by health officers or village health volunteers before the intervention, was not provided during the intervention. This was to improve the chance of showing an improvement in iron status even though it was not the primary outcome measure ( Pinkaew 2014 ). In Burundi, parent committees performed all the cooking ( Parker 2015 (C) ). The other studies did not describe the manner of cooking rice.
All but one study ( Parker 2015 (C) ), described the accompanying dishes or menu. The study on female teenagers ( Hardinsyah 2016 ), reported the cooking methods as pouring one sack of fortified rice into a plastic bucket to wash the rice, and steaming for about one hour until the rice was cooked well. One study ( Losso 2017 ), was done as a pilot among women from the Baton Rouge area (Louisiana (LA), USA), wherein they tested the iron retention of fortified rice by several methods of cooking; however there was no description of the type cooking used for the clinical study. Similarly, Ara 2019 did not specify the method of cooking.
Comparisons
Twelve studies (2201 participants) made this comparison.
No studies contributed data to this comparison.
One study (74 participants) made this comparison, having a vitamin A‐only arm versus a control arm.
Of the 12 RCTs included in the meta‐analysis, seven reported on anaemia ( Angeles‐Agdeppa 2008 ; Hardinsyah 2016 ; Hotz 2008 ; Parker 2015 (C) ; Perignon 2016 (C) ; Radhika 2011 ; Thankachan 2012 ). Five out of the twelve RCTs used WHO thresholds to define anaemia based on serum haemoglobin concentrations (Hb) ( Hardinsyah 2016 ; Moretti 2006b ; Parker 2015 (C) ; Perignon 2016 (C) ; Radhika 2011 ), three used a cut‐off level but did not mention the cut‐off criteria ( Angeles‐Agdeppa 2008 , Pinkaew 2013 Thankachan 2012 ), one RCT ( Hotz 2008 ), used CDC 1989 criteria and three RCTs did not report the cut‐off used to define anaemia ( Hussain 2014 ; Losso 2017 ; Pinkaew 2014 ). Among the included non‐randomised studies, three mentioned Hb cut‐off to define anaemia, but did not specify the criteria ( Ara 2019 ; Della Lucia 2016 ; Gershoff 1977 ), one used WHO criteria ( Nogueira Arcanjo 2013 ), and one study did not report a Hb cut‐off ( Salcedo 1950 ). The details of the cut‐off used in each included study are given in Table 7 . We used data from these studies in the meta‐analysis irrespective of the criteria used by them to define anaemia. Three studies reported iron‐deficiency anaemia ( Moretti 2006b ; Radhika 2011 ; Thankachan 2012 ); however we did not use these data in quantitative synthesis.
| Anaemia was defined as haemoglobin concentration in blood < 120 g/L | Not mentioned | |
| < 120 g/L in non‐pregnant and non‐lactating women | Not mentioned | |
| ≥ 110 g/L was used as a cut off for including children in the study. Anaemia was not defined | Not reported | |
| Haemoglobin levels were categorised as deficient < 100, low 100‐90 (g/L) | Not mentioned | |
| Severe anaemia: < 80 g/L; moderate anaemia: 80‐109 g/L; mild anaemia: 110‐119 g/L; non anaemia: ≥ 120 g/L | WHO ( ) | |
| < 122 g/L, adjusted for average altitude of the study sites (1100 m) with the use of an equation | CDC ( ) | |
| < 110 g/L and severely anaemic (Hb < 75 g/L) were excluded | Not mentioned | |
| Not reported (iron‐deficiency anaemia was defined based on iron and ferritin levels in serum) | Not reported | |
| < 115 g/L in children aged 5–11 years | WHO ( ) | |
| < 110 g/L in children < 5 years of age | WHO ( ) | |
| For school‐aged children at 1500 m above sea level, mild anaemia was defined as Hb 115‐119 g/L, moderate anaemia Hb 85‐114 g/L, and severe anaemia Hb < 85 g/L | WHO ( ) | |
| < 115 g/L for children aged 6‐11 years, < 120 g/L for children aged 12‐14 years and girls aged ≥ 15 years and < 130 g/L for boys aged ≥ 15 years | WHO ( ) | |
| < 120 g/L | Not mentioned | |
| Not reported | Not reported | |
| In children aged 5–11 years, anaemia (mild to moderate) was defined as Hb 70‐115 g/L. | WHO ( ) | |
| Not reported | Not reported | |
| < 115 g/L in children aged 6–11 years and < 120 g/L in participants aged ≥ 12 years | Not mentioned | |
| Centers for Disease Control and Prevention; haemoglobin; World Health Organization | ||
Nine studies reported iron deficiency ( Angeles‐Agdeppa 2008 ; Hardinsyah 2016 ; Hotz 2008 ; Hussain 2014 ; Moretti 2006b ; Perignon 2016 (C) ; Pinkaew 2013 ; Radhika 2011 ; Thankachan 2012 ); we included eight in the meta‐analysis. We did not include data from Hussain 2014 because the subgroup‐specific details were not available for further analysis.
To evaluate iron deficiency, seven studies used plasma ferritin concentrations (Angeles‐Agdeppa 2008; Hotz 2008; Hussain 2014 ; Losso 2017; Moretti 2006a; Radhika 2011; Thankachan 2012), four studies used serum transferrin receptor concentrations ( Hotz 2008 ; Losso 2017 ; Moretti 2006b Thankachan 2012 ), wherein two studies (Moretti 2006a; Thankachan 2012 ), used and reported both these parameters.
Eleven studies reported mean haemoglobin concentrations ( Angeles‐Agdeppa 2008 ; Hardinsyah 2016 ; Hotz 2008 ; Hussain 2014 ; Losso 2017 ; Moretti 2006b ; Parker 2015 (C) ; Perignon 2016 (C) ; Pinkaew 2013 ; Radhika 2011 ; Thankachan 2012 ), four studies evaluated vitamin A deficiency ( Hardinsyah 2016 ; Perignon 2016 (C) ; Pinkaew 2014 ; Thankachan 2012 ), and one study reported plasma folate concentration ( Hardinsyah 2016 ). One study reported the level of diarrhoea ( Thankachan 2012 ). No studies compared the groups for respiratory infections (as measured by study authors) or all‐cause death in their outcomes. One study reported on hookworm infection ( Perignon 2016 (C) ). One study reported abdominal pain ( Thankachan 2012 ). No studies reported congenital anomalies or miscarriage (no studies were carried out among pregnant women). Five studies reported serum retinol ( Angeles‐Agdeppa 2008 ; Hardinsyah 2016 ; Pinkaew 2013 ; Pinkaew 2014 ; Thankachan 2012 ), three studies reported plasma zinc concentration ( Hardinsyah 2016 ; Pinkaew 2014 ; Thankachan 2012 ).
One study ( Moretti 2006b ), reported anthropometric measurements (weight‐for‐age, height‐for‐age, weight‐for‐height in terms of Z‐score) whereas Angeles‐Agdeppa 2008 reported prevalence of wasting and stunting. No studies compared the risk of iron overload (defined by serum ferritin higher than 150 µg/L in women and higher than 200 µg/L in men), clinical and severe malaria, night blindness (defined as the inability to see after dusk by people who typically report having normal vision during the day; only for vitamin A fortified rice as an intervention), across the groups
The controlled before‐and‐after studies reported biochemical outcomes relevant to iron, vitamin A, zinc and folate in addition to clinical outcomes such as beriberi ( Della Lucia 2016 ; Gershoff 1977 ; Nogueira Arcanjo 2013 ; Salcedo 1950 ). In Ara 2019 , they included anaemia, mean haemoglobin (g/L), zinc deficiency (< 10.1 mmol/L), mean serum zinc (mmol/L), morbidity (last two weeks), diarrhoea, fever and inflammation (CRP >10.0 mg/L) as the outcomes. Gershoff 1977 study reported the effect of consumption of rice fortified with multiple vitamins, however they did not report the total number of outcomes in the entire study population, hence their report did not reach the stage of meta‐analysis.
Sources of funding
Most included studies were funded by one or more agencies from the government sector, private sector, academic organisations, or non‐government organisations. One study did not report the source of financial support ( Losso 2017 ).
Angeles‐Agdeppa 2008 stated its source of funding was The International Life Sciences Institute Center for Health Promotion of Japan (ILSI CHP, Japan), and the ILSI CHP, Atlanta, USA. Taiyo Kagaku, Japan donated the necessary fortificant used in this study.
DSM Nutritional Products (India) provided fortified rice for Thankachan 2012 . Another study, Gershoff 1977 , reported that they were supported in part by the United States Agency for International Development and the Fund for Research and Teaching, Department of Nutrition, Harvard School of Public Health. Also, that Ajinomoto Company, Tokyo, Japan supplied the rice fortification grains.
Two RCTs ( Pinkaew 2013 ; Pinkaew 2014 ), were supported by Medicor Foundation (Triesen, Liechtenstein), the Royal Thai Government Scholarship. Also, Pinkaew 2014 study was supported by the International Atomic Energy Agency (Vienna, Austria). The Micronutrient Initiative, Ottawa, Canada, along with the Swiss Federal Institute of Technology, Zurich, Switzerland, and St John’s Academy of Health Sciences, Bangalore, India supported Moretti 2006b and Dr. Paul Lohmann (GmbH, Emmerthal, Germany) provided the iron and zinc compounds, and DSM Nutritional Products Ltd. (Basel, Switzerland) provided the vitamin A compound for Moretti 2006b ; Pinkaew 2013 ; and Pinkaew 2014 . Radhika 2011 was funded by the Department of Biotechnology, New Delhi, India. The Program for Appropriate Technology in Health (PATH), Seattle, USA provided the Ultra Rice premix for them. United States Department of Agriculture (USDA) and the Open Road Alliance funded one study ( Parker 2015 (C) ). USDA, the World Food Programme‐DSM Consortium, and the Institut de Recherce pour le Development (IRD) supported Perignon 2016 (C) .
A subcontract grant from PATH through an original grant from Bill & Melinda Gates Foundation provided the funding for Hotz 2008 . The Food and Nutrition Society of Indonesia supported Hardinsyah 2016 . Williams‐Waterman Fund Committee of the Research Corporation, New York City funded Salcedo 1950 , and Hoffmann‐LaRoche, Inc., Nutley, New Jersey, USA donated the premix that they used. Della Lucia 2016 had financial support from O Programa Institucional de Bolsas de Iniciação Científica e Tecnológica da (PROBIC/FAPEMIG), PIBIC/CNPq and FAPEMIG and PATH donated the fortified rice. The United Nations World Food Programme (Grant # 1209) funded the study conducted in Bangladesh by Ara 2019
Excluded studies
We excluded 28 articles from 22 studies after assessing the full‐text articles. The details of excluded studies along with the reasons for exclusion are given in Characteristics of excluded studies .
See Figure 2 for reasons for excluding the articles. Nine studies were related to a different type of intervention or rice was not the medium of intervention ( Arsenault 2010 ; Bagni 2009 ; Barboza 2011 ; Castro 2017 ; Finkelstein 2013 ; Graham 2007 ; Haskell 2005 ; Sridevi 2013 ; Vitolol 1998 ), six studies with participants outside the age range of interest ( Beinner 2010 ; Ma 2016 ; Nogueira Arcanjo 2012 ; Pham 2012 ; Skau 2015 ; Walter 1993 ); five studies with different type of study design ( Ando 2012 ; Angeles‐Agdeppa 2011 ; Florentino 1998 ; Huo 2014 ; Kagawa 2017 ), and two studies had insufficient information ( Hyun 2015 ; Pham Van 2013 ).
We have provided details of two ongoing studies in Characteristics of ongoing studies table.
Risk of bias in included studies
We used standardised domains to assess the risk of bias of included studies (including individually and cluster‐randomised trials) ( Higgins 2019 ). We assessed the primary outcomes for risk of bias at the study level. We considered additional domains for risk of bias among the cluster‐RCTs. We presented them in the 'Risk of bias' table in the Characteristics of included studies section, and give a summary of the ’Risk of bias’ analyses in Figure 3 and Figure 4 . Among the 12 RCTs contributing to the meta‐analysis, two studies had a low overall risk of bias ( Moretti 2006b ; Radhika 2011 ), having low risk in allocation concealment, differences in baseline outcome measures, and incompleteness of outcome data. We assessed all other RCTS to be at high overall risk of bias. The five controlled before‐and‐after studies had high or unclear risk of bias for most domains ( Ara 2019 ; Della Lucia 2016 ; Gershoff 1977 ; Nogueira Arcanjo 2013 ; Salcedo 1950 ). Two of these studies had a documented difference in baseline outcome measures ( Della Lucia 2016 ; Nogueira Arcanjo 2013 ). Also, Ara 2019 had different baseline and end‐line populations.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Four studies were controlled before‐and‐after studies ( Ara 2019 ; Della Lucia 2016 ; Gershoff 1977 ; Nogueira Arcanjo 2013 ), and one was a controlled cross‐sectional study ( Salcedo 1950 )

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
Sequence generation
We noted three studies adequately describing the sequence generation for the recruitment of study participants in their respective studies ( Hotz 2008 ; Perignon 2016 (C) ; Thankachan 2012 ), and we graded them to be at low risk of bias. We assessed four studies at high risk of bias for not having random sequence generation ( Ara 2019 ; Della Lucia 2016 ; Gershoff 1977 ; Salcedo 1950 ), and 10 studies to be at unclear risk ( Angeles‐Agdeppa 2008 ; Hardinsyah 2016 ; Hussain 2014 ; Losso 2017 ; Moretti 2006b ; Nogueira Arcanjo 2013 ; Parker 2015 (C) ; Pinkaew 2013 ; Pinkaew 2014 ; Radhika 2011 ) ; all four studies at high risk of bias for random sequence generation were before‐and‐after comparison studies.
Allocation concealment
Five studies reported allocation concealment completely in their methods ( Della Lucia 2016 ; Hardinsyah 2016 ; Moretti 2006b ; Perignon 2016 (C) ; Radhika 2011 ), and we assessed them to be at low risk of bias. Six studies did not effectively conceal allocation ( Angeles‐Agdeppa 2008 ; Ara 2019 ; Gershoff 1977 ; Pinkaew 2013 ; Pinkaew 2014 ; Salcedo 1950 ), and we assessed them at high risk of bias, and six studies were at unclear risk ( Hotz 2008 ; Hussain 2014 ; Losso 2017 ; Nogueira Arcanjo 2013 ; Parker 2015 (C) ; Thankachan 2012 ).
Similarity of baseline characteristics
We assessed 13 studies to be at low risk of bias for similarity of baseline characteristics ( Angeles‐Agdeppa 2008 ; Ara 2019 ; Hardinsyah 2016 ; Hotz 2008 ; Hussain 2014 ; Losso 2017 ; Moretti 2006b ; Nogueira Arcanjo 2013 ; Pinkaew 2013 ; Pinkaew 2014 ; Radhika 2011 ; Salcedo 1950 ; Thankachan 2012 ). Two studies were at high risk of bias ( Gershoff 1977 ; Perignon 2016 (C) ), and two studies were at unclear risk ( Della Lucia 2016 ; Parker 2015 (C) ).
Overall, seven studies were at high risk of selection bias ( Angeles‐Agdeppa 2008 ; Ara 2019 ; Gershoff 1977 ; Perignon 2016 (C) ; Pinkaew 2013 ; Pinkaew 2014 ; Salcedo 1950 ) with high risk of bias in any one of the domains of selection bias.
For similarity of baseline outcome measurements, nine studies were at low risk of bias ( Angeles‐Agdeppa 2008 ; Hotz 2008 ; Hussain 2014 ; Losso 2017 ; Moretti 2006b ; Pinkaew 2013 ; Pinkaew 2014 ; Radhika 2011 ; Thankachan 2012 ), seven studies were at high risk of bias ( Ara 2019 ; Della Lucia 2016 ; Gershoff 1977 ; Hardinsyah 2016 ; Nogueira Arcanjo 2013 ; Parker 2015 (C) ; Perignon 2016 (C) ), and we assessed one study as unclear risk of bias ( Salcedo 1950 ).
Eight studies described the blinding process for the participants adequately and we graded them to be at low of bias in blinding the participants ( Angeles‐Agdeppa 2008 ; Hardinsyah 2016 ; Losso 2017 ; Moretti 2006b ; Nogueira Arcanjo 2013 ; Perignon 2016 (C) ; Radhika 2011 ; Thankachan 2012 ), five studies were at low risk of bias in blinding the outcome assessment ( Angeles‐Agdeppa 2008 ; Moretti 2006b ; Nogueira Arcanjo 2013 ; Radhika 2011 ; Thankachan 2012 ), and seven studies were at low risk in terms of contamination ( Ara 2019 ; Hardinsyah 2016 ; Hussain 2014 ; Losso 2017 ; Nogueira Arcanjo 2013 ; Parker 2015 (C) ; Perignon 2016 (C) ). Overall, four studies ( Hardinsyah 2016 ; Losso 2017 ; Nogueira Arcanjo 2013 ; Perignon 2016 (C) ), were at low risk of performance bias and five studies gave an account of blinding the outcome assessors which we assessed to be at low risk of detection bias ( Angeles‐Agdeppa 2008 ; Moretti 2006b ; Nogueira Arcanjo 2013 ; Radhika 2011 ; Thankachan 2012 ).
We assessed 13 studies at unclear risk of blinding. Two studies were at unclear risk of blinding the participants and personnel ( Della Lucia 2016 ; Parker 2015 (C) ), six studies for blinding of outcome assessment ( Della Lucia 2016 ; Hardinsyah 2016 ; Hussain 2014 ; Perignon 2016 (C) ; Pinkaew 2013 ; Pinkaew 2014 ), and nine studies for contamination ( Della Lucia 2016 ; Gershoff 1977 ; Hotz 2008 ; Moretti 2006b ; Pinkaew 2013 ; Pinkaew 2014 ; Radhika 2011 ; Salcedo 1950 ; Thankachan 2012 ).
Ten studies were at high risk of blinding. Seven studies were at high risk of blinding of the participants ( Ara 2019 ; Gershoff 1977 ; Hotz 2008 ; Hussain 2014 ; Pinkaew 2013 ; Pinkaew 2014 ; Salcedo 1950 ), six studies for blinding of outcome assessment ( Ara 2019 ; Gershoff 1977 ; Hotz 2008 ; Losso 2017 ; Parker 2015 (C) ; Salcedo 1950 ), and one study was at high risk of contamination ( Angeles‐Agdeppa 2008 ).
Incomplete outcome data
We assessed 11 studies to be at low risk of bias for completeness of outcome data ( Angeles‐Agdeppa 2008 ; Hardinsyah 2016 ; Hussain 2014 ; Losso 2017 ; Moretti 2006b ; Nogueira Arcanjo 2013 ; Perignon 2016 (C) ; Pinkaew 2013 ; Pinkaew 2014 ; Radhika 2011 ; Thankachan 2012 ), and they reported minimum loss to follow‐up rates among the study participants. Two studies were at high risk of bias ( Hotz 2008 ; Salcedo 1950 ), because of high rates of dropout from the study, and we assessed four studies at unclear risk of bias due to inadequate description of attrition ( Ara 2019 ; Della Lucia 2016 ; Gershoff 1977 ; Parker 2015 (C) ).
Selective reporting
There was no indication of selective reporting by any of the studies from published records, however we did not have access to the study protocols. Ten studies reported all of their pre‐specified outcomes, including the insignificant ones, and we assessed them to be at low risk of bias ( Angeles‐Agdeppa 2008 ; Ara 2019 ; Losso 2017 ; Moretti 2006b ; Parker 2015 (C) ; Perignon 2016 (C) ; Pinkaew 2013 ; Pinkaew 2014 ; Radhika 2011 ; Thankachan 2012 ); one of these studies provided a study registration number for the protocol ( Pinkaew 2014 ). We assessed one study at high risk of reporting bias because the study participants were given rewards for maintaining highest attendance in the schools in which the attendance rate was not steady ( Hardinsyah 2016 ). We assessed six studies to be at unclear risk of bias ( Della Lucia 2016 ; Gershoff 1977 ; Hotz 2008 ; Hussain 2014 ; Nogueira Arcanjo 2013 ; Salcedo 1950 )
Other potential sources of bias
We could not identify other potential sources of bias in the included studies. For some studies, industry provided the fortificants or the rice fortification grains. We assessed three studies to be at low risk of bias ( Angeles‐Agdeppa 2008 ; Parker 2015 (C) ; Perignon 2016 (C) ), one study at high risk of bias ( Hardinsyah 2016 ), and 13 studies at unclear risk of bias ( Ara 2019 ; Della Lucia 2016 ; Gershoff 1977 ; Hotz 2008 ; Hussain 2014 ; Losso 2017 ; Moretti 2006b ; Nogueira Arcanjo 2013 ; Pinkaew 2013 ; Pinkaew 2014 ; Radhika 2011 ; Salcedo 1950 ; Thankachan 2012 ).
We evaluated and determined additional criteria for risk of bias in cluster‐randomised studies (i.e. recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, compatibility with individual RCTs) for two studies ( Parker 2015 (C) ; Perignon 2016 (C) ). Among these criteria, Perignon 2016 (C) was at unclear risk for baseline imbalance because they did not describe clusters and did not report any process of statistical adjustment for clustering. For all remaining criteria, we judged both the studies to be at low risk of bias.
Effects of interventions
See: Table 1 ; Table 2
A summary of the effects of interventions is given in Table 1 and Table 2 .
We included 12 RCTs in the meta‐analysis. All 12 RCTs contained at least one arm with iron and compared with unfortified rice. No RCT had a 'no intervention' arm in their study. Of the 12 RCTs, six RCTs fortified with iron only, five RCTs fortified with iron and other micronutrients and one RCT had several fortified groups including iron only, vitamin A only and multiple micronutrients. Few of the pre‐specified outcome measures in this review were not reported by any of the included studies. We analysed the results using a random‐effects model, since all the included studies had significant heterogeneity. See Data and analyses for a detailed description of pre‐specified outcomes and their results.
We carried out sensitivity analyses for two cluster‐randomised trials ( Parker 2015 (C) ; Perignon 2016 (C) ), with different values of ICC and examined their effect on the effect estimates (RR) for two outcomes: anaemia and mean haemoglobin concentration. We observed that ICC did not change the direction of effects of interventions significantly for any outcome. We have presented the details of these sensitivity analyses in Table 8 . We also carried out sensitivity analysis by excluding the single RCT ( Parker 2015 (C) ), with a high/unclear risk of bias in eight out of 15 domains (including the additional domains for cluster‐RCTs) for two outcomes, anaemia and mean haemoglobin.
| ( ; ; ; ; ; ; ) | (0) | 0.83 (0.64 to 1.08) | 0.06 | 16.06 | 0.01 | 63% |
| (0.001) | 0.83 (0.64 to 1.08) | 0.06 | 15.72 | 0.02 | 62% | |
| (0.002) | 0.83 (0.64 to 1.08) | 0.06 | 15.71 | 0.02 | 62% | |
| (0.005) | 0.83 (0.64 to 1.07) | 0.06 | 15.12 | 0.02 | 60% | |
| (0.01) | 0.83 (0.64 to 1.08) | 0.06 | 14.80 | 0.02 | 59% | |
| (0.02723) | 0.83 (0.64 to 1.07) | 0.05 | 13.08 | 0.04 | 54% | |
| (0.1) | 0.81 (0.64 to 1.03) | 0.04 | 10.03 | 0.12 | 40% | |
| (0) | 0.83 (0.67 to 1.03) | 0.04 | 13.17 | 0.04 | 54% | |
| (0.001) | 0.83 (0.67 to 1.04) | 0.04 | 13.15 | 0.04 | 54% | |
| (0.002) | 0.83 (0.66 to 1.04) | 0.04 | 13.16 | 0.04 | 54% | |
| (0.005) | 0.83 (0.66 to 1.05) | 0.04 | 13.12 | 0.04 | 54% | |
| (0.01) | 0.83 (0.65 to 1.05) | 0.05 | 13.12 | 0.04 | 54% | |
| (0.02723) | 0.83 (0.64 to 1.07) | 0.05 | 13.08 | 0.04 | 54% | |
| ( 0.1) | 0.83 (0.63 to 1.09) | 0.06 | 13.08 | 0.04 | 54% | |
| ( ; ; ; ; ; ; ; ; ; ; ) | (0) | 1.69 (0.48 to 2.91) | 1.82 | 24.15 | 0.007 | 59% |
| (0.001) | 1.70 (0.48 to 2.92) | 1.81 | 23.90 | 0.008 | 58% | |
| (0.002) | 1.71 (0.49 to 2.93) | 1.81 | 23.69 | 0.008 | 58% | |
| (0.005) | 1.73 (0.51 to 2.96) | 1.80 | 23.18 | 0.01 | 57% | |
| (0.01) | 1.77 (0.54 to 3.00) | 1.79 | 22.62 | 0.01 | 56% | |
| ) (0.02723) | 1.85 (0.61 to 3.10) | 1.77 | 21.73 | 0.02 | 54% | |
| ) (0.1) | 1.98 (0.71 to 3.25) | 1.76 | 20.96 | 0.02 | 52% | |
| ) (0) | 1.85 (0.61 to 3.09) | 1.77 | 21.98 | 0.02 | 55% | |
| ) (0.001) | 1.85 (0.61 to 3.09) | 1.77 | 21.97 | 0.02 | 54% | |
| ) (0.002) | 1.85 (0.61 to 3.09) | 1.77 | 21.96 | 0.02 | 54% | |
| ) (0.005) | 1.85 (0.61 to 3.10) | 1.77 | 21.93 | 0.02 | 54% | |
| ) (0.01) | 1.85 (0.61 to 3.10) | 1.77 | 21.89 | 0.02 | 54% | |
| ) (0.02723) | 1.85 (0.61 to 3.10) | 1.77 | 21.73 | 0.02 | 54% | |
| ) (0.1) | 1.86 (0.61 to 3.11) | 1.78 | 21.15 | 0.02 | 53% | |
| cluster‐randomised trial; confidence interval; intra‐cluster correlation coefficient; mean difference; risk ratio | ||||||
No studies looked at fortified rice versus no intervention, and we could not examine comparisons 2 and 4 to 8 because there were no studies looking at vitamin A, folic acid and zinc with other micronutrients that did not also include iron, and there were no studies with a 'no intervention' arm. However we undertook meta‐analysis for vitamin A versus unfortified rice since one study ( Hussain 2014 ), reported an intervention arm with vitamin A only compared with unfortified rice. We included this in comparison 3.
Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice
There were 12 studies (2201 participants) included in this comparison ( Angeles‐Agdeppa 2008 ; Hardinsyah 2016 ; Hotz 2008 ; Hussain 2014 ; Losso 2017 ; Moretti 2006b ; Parker 2015 (C) ; Perignon 2016 (C) ; Pinkaew 2013 ; Pinkaew 2014 ; Radhika 2011 ; Thankachan 2012 ). These studies comprise all the data included in the synthesis of this Review. We included five non‐randomised studies in this comparison ( Ara 2019 ; Della Lucia 2016 ; Gershoff 1977 ; Nogueira Arcanjo 2013 ; Salcedo 1950 ) for qualitative assessment.
Anaemia (defined as haemoglobin (Hb) below the WHO cut‐off, adjusted for altitude as appropriate or as defined by the study authors)
We included seven studies in the analysis ( Angeles‐Agdeppa 2008 ; Hardinsyah 2016 ; Hotz 2008 ; Parker 2015 (C) ; Perignon 2016 (C) ; Radhika 2011 ; Thankachan 2012 ). The studies had a duration of four months ( Hardinsyah 2016 ), six months ( Angeles‐Agdeppa 2008 ; Hotz 2008 ; Parker 2015 (C) ; Perignon 2016 (C) ; Thankachan 2012 ), and eight months ( Radhika 2011 ). The detailed results are presented in Analysis 1.1 . Overall, the children who consumed iron‐fortified rice had similar levels of anaemia to controls at the end of the follow‐up period (RR 0.72, 95% CI 0.54 to 0.97; 7 studies; 1634 participants; low‐certainty evidence). Heterogeneity was high (Tau² = 0.10; Chi² = 23.27, df = 6; P = 0.0007; I 2 = 74%) and the results have to be interpreted with caution. One study ( Angeles‐Agdeppa 2008 ), reported direction of benefit favouring fortification, whereas Hotz 2008 ; Parker 2015 (C) ; Perignon 2016 (C) ; Radhika 2011 and Thankachan 2012 showed ambiguous direction. Exclusion of two studies, which clearly favoured fortification ( Angeles‐Agdeppa 2008 ; Hardinsyah 2016 ), changed the effect such that there was no effect on anaemia (RR 0.92, 95% CI 0.76 to 1.12; 1248 participants) and reduced heterogeneity among studies (Tau² = 0.01; Chi² = 5.08, df = 4; P = 0.28; I 2 = 21%). Exclusion of Parker 2015 (C) revealed a slight reduction in anaemia with fortification of rice (RR 0.66, 95% CI 0.49 to 0.89; I 2 = 61%; 1336 participants) and it reduced the heterogeneity slightly.

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 1 Anaemia (defined as haemoglobin (Hb) below the WHO cut‐off, adjusted for altitude as appropriate).
There was no clear evidence of differences between subgroups in terms of reduction of anaemia in any of the following subgroup analyses.

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 2 Anaemia (subgroup: by micronutrient content).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 3 Anaemia (subgroup: by rice fortification method).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 4 Anaemia (subgroup: by cooking method most commonly used in trial setting).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 5 Anaemia (subgroup: by public health significance of anaemia at baseline ).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 6 Anaemia (subgroup: by malaria endemicity).
Iron deficiency
We included eight studies in the analysis ( Angeles‐Agdeppa 2008 ; Hardinsyah 2016 ; Hotz 2008 ; Moretti 2006b ; Perignon 2016 (C) ; Pinkaew 2013 ; Radhika 2011 ; Thankachan 2012 ). We did not include data from Hussain 2014 in the meta‐analysis because the subgroup details (for each arm) related to iron deficiency were not available, since the study authors reported data only for overall fortification versus unfortified rice. The intervention in the included studies lasted four months ( Hardinsyah 2016 ), five months ( Pinkaew 2013 ), six months ( Angeles‐Agdeppa 2008 ; Hotz 2008 ; Perignon 2016 (C) ; Thankachan 2012 ), seven months ( Moretti 2006b ) and eight months ( Radhika 2011 ). Details are presented in Analysis 1.7 . Participants consuming rice fortified with iron or in combination with other micronutrients may have slightly lower levels of iron deficiency compared to those consuming unfortified rice (RR 0.66, 95% CI 0.51 to 0.84; 8 studies, 1733 participants; low‐certainty evidence). Heterogeneity was low (Tau² = 0.02; Chi² = 8.60, df = 7; P = 0.28; I 2 = 19%). The pooled estimate favours fortification, in which direction of benefit was positive for fortification in Moretti 2006b and Radhika 2011 . Also Hussain 2014 showed favourable effects towards fortification (48 out of 185 in the fortification arms together and 19 out of 37 in the control arm).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 7 Iron deficiency (as defined by study authors, based on a biomarker of iron status).
There were no significant differences in iron deficiency due to consumption of iron‐fortified rice across the subgroups.

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 8 Iron deficiency (subgroup: by micronutrient content).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 9 Iron deficiency (subgroup: by rice fortification method).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 10 Iron deficiency (subgroup: by cooking method most commonly used in trial setting).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 11 Iron deficiency (subgroup: by public health significance of anaemia at baseline ).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 12 Iron deficiency (subgroup: by malaria endemicity).
We included 11 studies in the analysis ( Angeles‐Agdeppa 2008 ; Hardinsyah 2016 ; Hotz 2008 ; Hussain 2014 ; Losso 2017 ; Moretti 2006b ; ; Parker 2015 (C) ; Perignon 2016 (C) ; Pinkaew 2013 ; Radhika 2011 ; Thankachan 2012 ). The duration of the intervention varied between two weeks ( Losso 2017 ), and eight months ( Radhika 2011 ). The other included studies provided the intervention for four months ( Hardinsyah 2016 ), five months ( Pinkaew 2013 ), six months ( Angeles‐Agdeppa 2008 ; Hotz 2008 ; Hussain 2014 ; Perignon 2016 (C) ; Thankachan 2012 ) and seven months ( Moretti 2006b ; Parker 2015 (C) ). Details are presented in Analysis 1.13 . Consuming rice fortified with iron or in combination with other micronutrients may increase haemoglobin concentrations (g/L) in comparison to consuming unfortified rice (MD 1.83, 95% CI 0.66 to 3.00; 11 studies, 2163 participants; low‐certainty evidence). Heterogeneity was substantial (Tau² = 1.58; Chi² = 21.86, df = 10; P = 0.02; I² = 54%) and results should be interpreted with caution. The direction of benefit was towards fortification in three studies ( Angeles‐Agdeppa 2008 ; Hardinsyah 2016 ; Perignon 2016 (C) ). Exclusion of Parker 2015 (C) did not change the direction of the effect or the heterogeneity.

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 13 Haemoglobin concentration (g/L).
There were no clear differences between other subgroups, or any obvious asymmetry in the funnel plot ( Figure 5 ).

Funnel plot of comparison 1. Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added), outcome 1.13, haemoglobin concentration (g/L)

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 14 Haemoglobin concentration (subgroup: by micronutrient content).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 15 Haemoglobin concentration (subgroup: by rice fortification method).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 16 Haemoglobin concentration (subgroup: by cooking method most commonly used in trial setting).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 17 Haemoglobin concentration (subgroup: by public health significance of anaemia at baseline).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 18 Haemoglobin concentration (subgroup: by malaria endemicity).
Vitamin A deficiency (as defined by the study authors)
Four studies contributed data for vitamin A deficiency ( Hardinsyah 2016 ; Perignon 2016 (C) ; Pinkaew 2014 ; Thankachan 2012 ). Details are provided in Analysis 1.19 . Consumption of fortified rice may make little or no difference to vitamin A deficiency (RR 0.68, 95% CI 0.36 to 1.29; 927 participants; 4 studies, low‐certainty evidence). Heterogeneity was marginally high (Tau² = 0.16; Chi² = 4.77, df = 3; P = 0.19; I² = 37%) and results have to be interpreted with caution. We did not include data from Hussain 2014 in the meta‐analysis, since the study authors reported vitamin A deficiency for the fortified rice group overall versus unfortified rice (37 out of 185 in the fortified arm and 22 out of 37 in the control arm). However their overall estimates had significant decrease in vitamin A deficiency in the fortified arm compared to control arm at the end of the six‐month intervention period.

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 19 Vitamin A deficiency (as defined by study authors, by using a biomarker of vitamin A).
There are no significant differences in the risk ratio across the subgroups. The details of subgroup analyses are presented below.

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 20 Vitamin A deficiency (subgroup: by micronutrient content).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 21 Vitamin A deficiency (subgroup: by rice fortification method).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 22 Vitamin A deficiency (subgroup: by cooking method most commonly used in trial setting).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 23 Vitamin A deficiency (subgroup: by public health significance of anaemia at baseline ).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 24 Vitamin A deficiency (subgroup: by malaria endemicity).
Serum or plasma folate (nmol/L)
One study reported the level or comparison of serum folate ( Hardinsyah 2016 ). The direction of benefit in this study was slightly towards fortified rice (MD 4.30, 95% CI 2.00 to 6.60; 1 study, 215 participants; low‐certainty evidence). The details are given in Analysis 1.25 .

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 25 Serum or plasma folate (nmol/L).
Any adverse effects (hookworm infection risk)
One study ( Perignon 2016 (C) ), reported that children given fortified rice were more likely to have hookworm infection compared to those given unfortified rice (RR 1.78, 95% CI 1.18 to 2.70, 1 study, 785 participants; low‐certainty evidence; Analysis 1.26 ).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 26 Any adverse effects.
Any adverse effects (abdominal pain more than three days)
One study ( Thankachan 2012 ), reported that children given fortified rice were just as likely to have abdominal pain compared to those given unfortified rice (average RR 0.77, 95% CI 0.42 to 1.42; Analysis 1.26 ).
Diarrhoea (as defined by study authors; for studies among children aged 2 to 11.9 years of age)
One study reported the comparison of diarrhoeal episodes across the fortified and unfortified groups ( Thankachan 2012 ). There was no difference in the risk of diarrhoea (average RR 3.52, 95% CI 0.18 to 67.39; 1 study, 258 participants, very low‐certainty evidence; Analysis 1.27 ).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 27 Diarrhoea (as defined by study authors).
Respiratory infections (for studies among children aged 2 to 11.9 years of age)
The included studies mentioned the episodes of respiratory infections in intervention and control groups during the study period, however, none reported the differences across the groups following rice fortification.
All‐cause death (for studies among children aged 2 to 11.9 years of age)
None of the included studies reported deaths.
Congenital anomalies (for studies among pregnant women with folic acid‐fortified rice as intervention)
No studies involved pregnant women.
Miscarriage (for studies among pregnant women)
Serum or plasma retinol (µmol/L)
Five studies reported the outcome of serum retinol (µmol/L) across the iron‐fortified and unfortified groups ( Angeles‐Agdeppa 2008 ; Hardinsyah 2016 ; Hussain 2014 ; Pinkaew 2014 ; Thankachan 2012 ). Details are presented in Analysis 1.28 . Participants consuming rice fortified with iron alone or other micronutrients had a marginally higher plasma retinol compared to those consuming unfortified rice (MD 0.04, 95% CI −0.13 to 0.21; 5 studies; 727 participants). Heterogeneity was high (Tau² = 0.03; Chi² = 60.39, df = 4 P < 0.00001; I 2 = 93%). Two studies favoured fortification ( Angeles‐Agdeppa 2008 ; Hussain 2014 ), and three had ambiguous results ( Hardinsyah 2016 ; Pinkaew 2014 ; Thankachan 2012 ). Heterogeneity was explained by one study ( Hardinsyah 2016 ), and its removal showed a higher mean difference favouring fortification (MD 0.20, 9% CI 0.18 to 0.22). This study had seven micronutrients.

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 28 Serum or plasma retinol (µmol/L).
Three studies reported the effectiveness of multiple micronutrient‐fortified rice on the level of serum or plasma zinc among children ( Hardinsyah 2016 ; Pinkaew 2014 ; Thankachan 2012 ). See Analysis 1.29 for details (MD 0.38, 95% CI −0.08 to 0.83; I 2 = 28%; 3 studies, 618 participants).

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 29 Serum or plasma zinc (µmol/L).
Anthropometric measures
One study ( Moretti 2006b ), contributed data to the outcome height‐for‐age Z‐score and weight‐for‐height Z‐score. The mean difference for height‐for‐age Z‐score was 0.02 (95% CI −0.32 to 0.36; 1 study, 184 participants; Analysis 1.30 ) and for weight‐for‐height Z‐score mean difference was 0.13 (95% CI −0.19 to 0.45; 1 study, 184 participants; Analysis 1.31 ). For both these outcomes, fortification may or may not make a difference.

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 30 Height‐for‐age Z‐score.

Comparison 1 Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice (no micronutrients added)., Outcome 31 Weight‐for‐height Z‐score.
No studies reported the aspects of iron overload.
Clinical and severe malaria
None of the included studies had details on malaria across any of the study groups. Few studies had declared they were conducted in malaria non‐endemic areas.
Night blindness (defined as the inability to see after dusk by people who typically report having normal vision during the day; only for vitamin A‐fortified rice as an intervention)
No studies with vitamin A reported night blindness.
No studies contributed data for this outcome.
Rice fortified with vitamin A alone or in combination with other micronutrients versus unfortified rice
We included one study in this comparison ( Hussain 2014 ).
Anaemia (defined as haemoglobin (Hb) below the WHO cut‐off, adjusted for altitude as appropriate)
One study contributed data for this analysis ( Hussain 2014 ). The study had five intervention arms; we included the retinyl palmitate arm with the fortification arm and the arm without fortification as the control arm for this comparison. The study authors reported a significant increase in the haemoglobin concentration in the vitamin A‐fortified arm compared to the unfortified control arm (MD 10.00, 95% CI 8.79 to 11.21; 1 study, 74 participants; low‐certainty evidence; Analysis 2.1 ).

Comparison 2 Rice fortified with vitamin A alone or in combination with other micronutrients versus unfortified rice (no micronutrients added), Outcome 1 Haemoglobin concentration (g/L).
Vitamin A deficiency (as defined by study authors, by using a biomarker)
We did not include any studies in this meta‐analysis. One study ( Hussain 2014 ), reported vitamin A deficiency; however there were no data on vitamin A deficiency status in the arm fortified with vitamin A only. Their estimates are reported for the fortification arms in total. Details of their findings are in comparison 1.
One study contributed data to this analysis ( Hussain 2014 ). Fortification of rice with vitamin A probably increases the serum retinol concentration compared to unfortified rice (MD 0.17, 95% CI 0.13 to 0.21; 1 study, 74 participants; low‐certainty evidence; Analysis 2.2 ).

Comparison 2 Rice fortified with vitamin A alone or in combination with other micronutrients versus unfortified rice (no micronutrients added), Outcome 2 Serum or plasma retinol (µmol/L).
Any adverse effects (abdominal pain more than 3 days)
Summary of non‐randomised studies.
The value of the controlled before‐and‐after studies was mixed. Salcedo 1950 documented a reduction in clinical beriberi. Gershoff 1977 reported on the changes in amino acid intake and weight and length but did not make any formal estimates of change. One of the more recent studies, Nogueira Arcanjo 2013 , comparing iron‐fortified rice versus unfortified rice showed no change in mean haemoglobin but with an increase in anaemia in the unfortified group. The study comparing multiple micronutrient‐fortified rice with unfortified rice compared outcomes relative to baseline values within the fortified group and the unfortified group ( Della Lucia 2016 ). In the fortified group, there was an improvement in folic acid, thiamine and serum zinc, but not for haemoglobin or ferritin. In the unfortified group, there was an improvement in thiamine and ferritin concentrations. The groups were unbalanced with respect to ferritin levels, being significantly higher in the fortified group at baseline.
In Nogueira Arcanjo 2013 , the groups were different in the prevalence of anaemia at baseline: 8.9% (11/120 were anaemic) in the group receiving iron‐fortified rice, and 20.8% (30/144 were anaemic) in the group receiving unfortified rice (P = 0.009). At the end of the study, the groups remained different, 10.5% (13/120) among the participants receiving iron‐fortified rice, and 37.5% (54/144) among those receiving unfortified rice (P < 0.001). There was a statistically significant increase in anaemia prevalence in the control group receiving standard unfortified rice; however, the iron‐fortified rice group did not present a statistically significant change in the number of anaemic children after the intervention. Also, the groups were similar for haemoglobin concentration: 120.6 ± 10.1 g/L in the fortified rice group versus 124.0 ± 41.4 g/L in the unfortified rice group, P = 0.38; but after intervention, there was no significant difference between the groups, P = 0.56. The after intervention period haemoglobin concentrations were 121.4 ± 10.6 g/L in the fortified rice group and 122.9 ± 24.8 g/L. Among only anaemic participants, in the group receiving iron‐fortified rice before intervention, mean haemoglobin value was 101.2 ± 8.5 g/L (n = 11) and 115.6 ± 8.6 after intervention, P = 0.0003; in the control group receiving unfortified rice (n = 30), mean haemoglobin concentrations changed from 108.3 ± 11.1 g/L at baseline to 109.4 ± 11.8 g/L after intervention, P = 0.18. For the standard rice school: baseline mean haemoglobin was 124.0 ± 41.4 g/L, and after intervention 122.9 ± 24.8, P = 0.78. Considering only anaemic participants, there was a significant increase in haemoglobin means before and after intervention, P = 0.003 in the fortified rice school.
In the other controlled before‐and‐after study ( Gershoff 1977 ), the average haemoglobin concentration among those children consuming less than 10% or no fortified rice for all ages (5 to 9 years of age) was 114.6 g/L ± 6.6 (n = 135) in 1971 and changed to 119.8 g/L ± 12.6 (n = 135) in 1975. On the other hand, the average haemoglobin concentration among those children consuming more than 66% of fortified rice for all ages (5 to 9 years) was 117.7 g/L ± 8.8 (n = 61) in 1971 and 122.5 g/L ± 11.8 (n = 61) in 1975. These changes were not significant.
Summary of main results
We have summarised the findings here and in Table 1 and Table 2 . We included 17 studies in this review, 12 of which were RCTs. Two of the RCTs were cluster‐randomised trials. Five studies had a before‐and‐after study design that compared micronutrient‐fortified rice with unfortified rice. All the included studies had unfortified rice in the control arm. There were no studies comparing fortified rice with no intervention arm.
We considered 12 RCTs for the meta‐analysis. Ten RCTs were conducted in children and two were among non‐pregnant and non‐lactating women. All studies included in this review had unfortified rice as the control. All the studies included iron in their intervention arms (one study by Hussain 2014 with multiple arms had one fortification group with vitamin A only). Five studies included rice fortified with iron alone and one study had one arm with iron alone as the intervention. Seven studies used multiple micronutrients (six in children; one in women). Vitamin A, zinc, folate and vitamin B were the additional micronutrients. Four studies fortified with vitamin A, five studies fortified with zinc and three studies fortified with folic acid. The fortification profile of the included studies is shown in Table 6 .
Participants consuming rice fortified with iron alone or in combination with other micronutrients were just as likely to be anaemic as those taking unfortified rice (7 RCTs, low‐certainty evidence); however, fortification of rice may reduce the risk of iron deficiency (8 RCTs, low‐certainty evidence). Considering the low level of certainty, rice fortification may make an improvement in the mean haemoglobin levels (g/L; 11 RCTs). Consumption of fortified rice may make little or no difference to vitamin A deficiency (4 RCTs, low‐certainty evidence), it may improve serum or plasma folate (1 RCT). Two studies reported on three adverse effects of diarrhoea, hookworm infection risk and abdominal pain of more than three days' duration. We are uncertain about the risk of diarrhoea (1 RCT, very low‐certainty evidence). Children given fortified rice may have a higher risk of hookworm infection compared to those given unfortified rice (1 RCT, low‐certainty evidence) and there may not be any difference in terms of abdominal pain of more than three days.
We noted a slight improvement in serum or plasma retinol (µmol/L) concentration among the participants consuming fortified rice (5 RCTs). There is no difference in serum zinc concentration (µmol/L; 3 RCTs), height‐for‐age Z‐score (1 RCT) and weight‐for‐height Z‐score (1 RCT) with fortification.
We included one RCT under the comparison of vitamin A alone or in combination with other micronutrients versus unfortified rice, since all other RCTs that included vitamin A in their fortification arm, also had iron. So they were only included in the first comparison, to avoid duplication.
Participants consuming rice fortified with vitamin A alone tend to have higher mean haemoglobin levels (1 RCT) and serum retinol levels (1 RCT).
The controlled before‐and‐after studies were significant in showing the first evidence of a clinical outcome attributable most likely to rice fortification ( Salcedo 1950 ), and the extent by which dietary content is improved ( Gershoff 1977 ). The limitations of unbalanced groups, most likely due to the absence of random allocation into treatment groups, limits our interpretation of the two studies in Brazil ( Della Lucia 2016 ; Nogueira Arcanjo 2013 ), however both provide a foundation for planning studies on zinc and folate, both of which have very few studies.
Overall completeness and applicability of evidence
The review includes studies on preschool and school‐age children, and non‐pregnant, non‐lactating women. We found no studies for adolescents only (except one study that included adolescent girls), pregnant or lactating women or adult males. All but one study were from low‐ and middle‐income countries, where anaemia prevalence among children aged 6 to 59 months of age ranged between 26% to 59% ( WHO 2015a ). The studies with serum retinol as outcomes were conducted in countries where the prevalence of vitamin A deficiency among preschoolers ranged from 16% to 62% ( WHO 2009a ).
Almost all randomised clinical trials used micronized ferric pyrophosphate and the hot extrusion method, with three studies including either a cold or warm extrusion arm. Of the four vitamins and minerals identified for review, studies were found to have investigated four (iron, vitamin A, folate and zinc). All but one study had haemoglobin data but not all studies reported the mean haemoglobin or anaemia rates. Although three studies included folic acid as one of the fortificants, folate status was reported in only one. Also, most of the included RCTs were conducted in school settings. The certainty of evidence for anaemia and iron deficiency was low. For haemoglobin concentration, it was very low‐certainty evidence. This being a closed and controlled set up, generalising the findings of such studies becomes a challenge for the present systematic review. There could also be an interplay of co‐interventions like other added micronutrients to the same fortified rice, dosage and absorption of iron and consumption of other nutritive items, which would alter the overall estimate of effect.
Another potential aspect of the rice fortification and its effect on malnutrition is the duration of intervention. The included studies had a duration of follow‐up from two weeks to four years. However in the included RCTs, one study had a follow‐up of two weeks, other studies ranged from four months to eight months. Thus there could be a role played by the duration of intervention and follow‐up.
Quality of the evidence
Among the RCTs that contributed to the meta‐analysis, we assessed two studies as having overall low risk of bias and one study as having a high or unclear risk of bias in most of the domains. Excluding this study favoured fortification and altered the conclusion for anaemia (from no effect to a reduction of anaemia) and the conclusion for the multiple micronutrient subgroup in comparison 1 for mean haemoglobin (from no effect to an increased mean difference). Most of these studies inadequately described their randomisation sequence generation. Close to half had an unclear description of allocation concealment and blinding of outcomes. Most of the studies had low attrition rate.
The GRADE assessment of the certainty of evidence was low for anaemia (no effect), iron deficiency (favours fortification), vitamin A deficiency (no effect), serum or plasma folate (one included study favouring fortification), and adverse events (one study reported hookworm infection risk higher with fortification) in the comparison of iron alone or in combination with other micronutrients versus no fortification. The certainty of the evidence was also low for mean haemoglobin (may favour). We rated the outcome diarrhoea (no effect) as very low‐certainty evidence. We mainly downgraded studies due to inconsistency and imprecision in the estimates. In the comparison of vitamin A alone versus no fortification, one RCT contributed data and we graded the certainty of evidence for haemoglobin concentration and serum retinol concentration as low.
Further research is very likely to have an important impact on our confidence in the estimates of effect and is likely to change the results.
Potential biases in the review process
Two review authors independently carried out the review process, with the same data extraction sheet and tools to assess risk of bias in the included studies. Many studies had minimum information regarding the randomisation procedure, allocation concealment and blinding. In the absence of precise details, we considered mutual discussion among review authors as final in this review, since these included subjective components. We also extensively searched grey literature and trials registries, along with contacting agencies involved in carrying out RCTs and subject experts; thus minimising publication bias in this review. Also there was no language limit set in this systematic review for searches as well as obtaining abstracts/full‐text articles. We sought the help of translators to convert non‐English‐language articles to English. This would minimise the language bias in this review.
Agreements and disagreements with other studies or reviews
Our review is the first systematic review and meta‐analysis specific to rice as a vehicle to fortification as a public health intervention. There are systematic reviews and meta‐analyses of micronutrient fortification of staples, condiments and processed foods including all age groups, but these do not include a subgroup analysis specifically for rice ( Das 2013 ; Gera 2012 ). Only two of the 12 studies included in our review were included in Das 2013 and our review did not include infants and young children under two years of age. In Das 2013 , staple foods fortified with iron were found to improve mean haemoglobin and serum ferritin concentrations, and reduce the risk of anaemia. Staple foods fortified with vitamin A improved mean haemoglobin and serum retinol concentrations. Cereals fortified with zinc improved zinc serum levels. Staple foods fortified with iron had no impact on iron indicators among women. The two reviews agreed that fortification with iron improved mean haemoglobin concentrations. Our review showed a reduction of iron deficiency, while Das 2013 showed an improvement in serum ferritin concentrations. There was no agreement between Das 2013 and this review on the impact on anaemia, vitamin A and zinc concentrations or deficiency. The systematic review by Gera 2012 included only apparently healthy individuals and examined the effect of fortified food items on haematological outcomes. They concluded that food items fortified with iron led to improvement in haemoglobin, serum ferritin and iron status and there by reduced the risk of anaemia.
Another systematic review and meta‐analysis evaluated the effect of fortification of staples with zinc on serum zinc levels and zinc deficiency ( Shah 2016 ). Zinc‐fortified staples were compared with food without zinc. None of the studies included in our review were included in Shah 2016 . Shah 2016 showed that fortification of foods with zinc alone, but not in combination with other micronutrients, improved serum zinc levels. Our review did not have a comparison of rice fortified with zinc alone with unfortified rice, but rice fortified with zinc and other micronutrients did not improve zinc levels. In both reviews, the certainty of evidence was low for zinc.
A systematic review of rice fortification included seven studies among children aged 6 to 59 months ( Hijar 2015 ). They did not carry out a meta‐analysis and the search strategy was more limited than that used in our review. The population also included children less than two years, who we excluded from our review. None of the studies in our review were included in the review by Hijar 2015 . They reported that rice fortification was effective in correcting iron deficiency among children aged less than five years. The improvement was not significant for vitamin A‐fortified rice.
Another systematic review of rice fortification with no age restrictions included 12 studies that fortified rice with iron, four studies that fortified with vitamin A and two that included other micronutrients ( De Pee 2017 ). They gave a description of the direction of the effects, but did not perform a meta‐analysis. The review by De Pee 2017 concluded that with the available evidence of efficacy, stability and needs, rice should be fortified with multiple micronutrients including iron, zinc, and vitamins A, B 1 (thiamin), B 3 (niacin) B 6 (pyridoxine), B 9 (folic acid) and B 12 (cobalamin). The meta‐analysis in our review shows evidence of an effect of iron on mean haemoglobin and iron deficiency; of vitamin A fortification on vitamin A deficiency; and of folate fortification on folate deficiency. Another review on issues related to rice fortification in correcting micronutrient deficiency compiled the evidence from available primary studies to conclude that rice fortification is an effective strategy ( Piccoli 2012 ).
Implications for practice
Fortification of rice has been recommended as an effective strategy for reducing micronutrient deficiencies. This review shows that fortification of rice may make no difference on anaemia and there are probable effects on blood haemoglobin concentration (an indicator used in diagnosis of anaemia). However, the review suggests that fortification of rice with iron may reduce iron deficiency. There is some evidence that these effects may be greater in single nutrient fortification than multiple micronutrient fortification. There is also evidence that fortification of rice with iron, vitamin A and other micronutrients may improve serum retinol concentrations, may not reduce the risk of vitamin A deficiency, and it may improve plasma folate. There is no evidence that fortification improves zinc levels.
This review may provide enough evidence supporting the effectiveness of fortification for anaemia and iron status. However, more evidence is needed for other micronutrients. Moreover, more thought may have to be given to the number of micronutrients added as this appears to have some bearing on the effect of fortification of rice.
Implications for research
The certainty of evidence for the outcome of anaemia and iron deficiency was low. The certainty of outcome change in mean haemoglobin was also low. There were considerable differences in the effects of single and multiple nutrient fortification. Single nutrient fortification with iron compound was found to be superior to multiple micronutrient interventions in terms of improvement in iron deficiency and haemoglobin concentration. A similar observation could be made when exploring heterogeneity in serum retinol results, with heterogeneity mainly due to one study with multiple micronutrients. Very few studies reported adverse effects, and a substantial number did not report the manner of cooking or malaria endemicity. There were only a handful of studies that used cold extrusion. We identify the following areas of research.
- More, high‐quality randomised controlled trials (RCTs) with anaemia as a reported outcome. Given that anaemia, rather than iron deficiency or mean haemoglobin is a more common indicator of national nutrition programmes, it is important to have better evidence on this outcome.
- RCTs that directly compare the efficacy or effectiveness of iron‐fortified rice, and iron and other micronutrient‐fortified rice with unfortified rice would inform policymakers on whether the reduced effect of the multiple micronutrient‐fortified rice described in this review happens in a controlled setting with the same population.
- RCTs directly comparing cold extrusion with unfortified rice would increase the evidence base for evaluating the effect of the cold extrusion method.
- RCTs should be encouraged to include the recording of solicited and unsolicited adverse effects and data relevant to rice fortification (e.g. manner of cooking) and iron metabolism (e.g. malaria and other parasitic infections like hookworm infection).
- RCTs with zinc and folate as outcomes would increase the evidence base as very few studies reported these.
Acknowledgements
We would like to thank the editorial staff of Cochrane Public Health, in particular Solange Durao, Anke Rohwer, Reza Yousefi‐Nooraie, Irma Klerings, Hilary Thomson and Newton Opiyo (Cochrane Public Health and Health Systems Network)” for their support throughout the preparation of this review. The review team also acknowledges the external referees, Mark Lawrence and Annhild Mosdøl for their useful input to this work.
We would to thank Joseph Ashong, Sumithra Muthayya, Arnaud Laillou, Christophe Guyondet, Regina Moench‐Pfanner, and Belinda J Burford for their work in the protocol and in the early stages of the review. Joseph Ashong, Sumithra Muthayya, Arnaud Laillou and Christophe Guyondet initially screened independently the records for eligibility in an early search in 2012. The whole process was repeated in 2016 to 2017 for this version of the review with a new review team.
We are grateful for technical support from Dr Lucero Lopez and Dr Ricardo X Martinez for the interpretation of the results from two non‐randomised studies.
Special thanks are due to Joanne Abbott, for her support in designing and running various updates to the search strategy. We would like to thank Mr Thomas Allen and Jose Luis Garnica from the World Health Organization Library Services for their support with the search in Agricola. We also thank all the authors who contributed additional details of their studies.
The World Health Organization retains copyright and all other rights in the manuscript of this review as submitted for publication, including any revisions or updates to the manuscript that they may make from time to time.
Appendix 1. Search strategy for electronic databases
Cochrane Central Register of Controlled Trials (CENTRAL) (CRSO)
#1 MESH DESCRIPTOR Micronutrients EXPLODE ALL TREES
#2 MESH DESCRIPTOR iron compounds
#3 MESH DESCRIPTOR zinc compounds
#4 MESH DESCRIPTOR iron, dietary EXPLODE ALL TREES
#5 ((micronutrient* or micro‐nutrient*)):TI,AB,KY
#6 ((multinutrient* or multi‐nutrient*)):TI,AB,KY
#7 ((multinutrient* or multi‐nutrient* or multi* nutrient* or multimicronutrient*)):TI,AB,KY
#8 ((multivitamin* or multi‐vitamin* or multi* vitamin*)):TI,AB,KY
#9 ( (multimineral* or multi‐mineral* or multi* mineral*)):TI,AB,KY
#10 ((trace adj (element* or mineral* or nutrient*))):TI,AB,KY
#11 ((iron or Fe or ferric* or ferrous* or zinc or Zn or vitamin* or retinol* or folate* or folic* or folacin*)):TI,AB,KY
#12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11
#13 MESH DESCRIPTOR Food, Fortified EXPLODE ALL TREES
#14 (((fortifi* or enrich* or enhanc*) adj2 (food* or rice))):TI,AB,KY
#15 MESH DESCRIPTOR Oryza sativa EXPLODE ALL TREES
#16 #13 OR #14 OR #15
#17 #12 AND #16
#18 11/07/2012 TO 08/11/16
#19 #17 AND #18
MEDLINE and Medline in Process (OVID)
1 exp Micronutrients/ or iron compounds/ or zinc compounds/ or exp iron, dietary/
2 (micronutrient$ or micro‐nutrient$).ti,ab.
3 (multinutrient$ or multi‐nutrient$).ti,ab.
4 (multinutrient$ or multi‐nutrient$ or multi$ nutrient$ or multimicronutrient$).ti,ab.
5 (multivitamin$ or multi‐vitamin$ or multi$ vitamin$).ti,ab.
6 (multimineral$ or multi‐mineral$ or multi$ mineral$).ti,ab.
7 (trace adj (element$ or mineral$ or nutrient$)).ti,ab.
8 (iron or Fe or ferric$ or ferrous$ or zinc or Zn or vitamin$ or retinol$ or folate$ or folic$ or folacin$).ti,ab.
9 or/1‐8
10 exp Food, Fortified/
11 ((fortifi$ or enrich$ or enhanc$) adj2 (food* or rice)).ti,ab.
13 Oryza sativa/
14 or/10‐13
15 9 and 14
16 exp animals/ not humans.sh.
17 15 not 16
18 (201206* or 201207* or 201208* or 201209* or 201210* or 201211* or 201212* or 2013* or 2014* or 2015* or 2016*).ed.
19 17 and 18
Embase (OVID)
16 (animal/ or nonhuman/) not human/
18 (201206* or 201207* or 201208* or 201209* or 201210* or 201211* or 201212* or 2013* or 2014* or 2015* or 2016*).dd.
CINAHL (EBSCO)
June 2012 to Nov 2016 (n=152)
S20 S18 AND S19
S19 EM 20120601‐20151130
S18 S11 AND S16 Limiters ‐ Human
S17 S11 AND S16
S16 S12 OR S13 OR S14 OR S15
S15 Oryza sativa
S14 (MH "Rice")
S13 ((fortifi* or enrich* or enhanc*) N2
(food* or rice))
S12 (MH "Food, Fortified")
S11 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR
S7 OR S8 OR S9 OR S10
S10 (iron or Fe or ferric* or ferrous* or
zinc or Zn or vitamin* or retinol* or
folate* or folic* or folacin*)
S9 (trace N1 (element* or mineral* or
nutrient*))
S8 (multimineral* or multi‐mineral* or
multi* mineral*)
S7 (multivitamin* or multi‐vitamin* or
multi* vitamin*)
S6 (multinutrient* or multi‐nutrient* or
multi* nutrient* or multimicronutrient*)
S5 (multinutrient* or multi‐nutrient*)
S4 (micronutrient* or micro‐nutrient*)
S3 (MH "Zinc Compounds")
S2 (MH "Iron Compounds+")
S1 (MH "Micronutrients")
BIOSIS & Web of Science (ISI) SCI, SSCI, CPCI‐exp & CPCI‐SSH
2012 to 7/11/16 (n=1)
#10 #9 AND #8
#9 TOPIC: (((fortifi* or enrich* or enhanc*) N2 (food* or rice)))
#8 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
#7 TOPIC: ((iron or Fe or ferric* or
ferrous* or zinc or Zn or vitamin* or
retinol* or folate* or folic* or
#6 TOPIC: ((trace N (element* or mineral*
or nutrient*)))
#5 TOPIC: ((multimineral* or multi‐
mineral* or "multi* mineral*"))
#4 TOPIC: ((multivitamin* or multi‐
vitamin* or "multi* vitamin*"))
#3 TOPIC: ((multinutrient* or multi‐
nutrient* or "multi* nutrient*" or
multimicronutrient*))
#2 TOPIC: ((multinutrient* or multi‐
#1 TOPIC: ((micronutrient* or micro‐
Popline & SCIELO
(micronutrient* OR micronutrient* OR multinutrient* OR multinutrient* OR multinutrient* OR multinutrient* OR multi* nutrient* OR multimicronutrient* OR multivitamin* OR multivitamin* OR multi* vitamin* OR multimineral* OR multimineral* OR multi* mineral* OR trace element* OR trace mineral* OR trace nutrient* OR iron OR Fe OR ferric* OR ferrous* OR zinc OR Zn OR vitamin* OR retinol* OR folate* OR folic* OR folacin*)
((fortifi* OR enrich* OR enhanc*) AND (food* OR rice))
2012‐2015
IBECS, PAHO, WHOLIS, AFRO and LILACS (BIRME)
(micronutrient$ or micro‐nutrient$) or (multinutrient$ or multi‐nutrient$) or (multinutrient$ or multi‐nutrient$ or multi$ nutrient$ or multimicronutrient$) or (multivitamin$ or multi‐vitamin$ or multi$ vitamin$) or (multimineral$ or multi‐mineral$ or multi$ mineral$) or trace element$ or trace mineral$ or trace nutrient$ or (iron or Fe or ferric$ or ferrous$ or zinc or Zn or vitamin$ or retinol$ or folate$ or folic$ or folacin$) [Words] and ((fortifi$ or enrich$ or enhanc$) and (food$ or rice)) [Words] and 2012 or 2013 or 2014 or 2015 [Country, year publication]
IMSEAR and EMRO (GLOBAL INDEX MEDICUS)
fortification
INMED & Native Health Research database
Rice AND fortify OR fortified OR enrich OR enriched
AGRICOLA (Ebsco)
#1 (ZU "micronutrients") OR (ZU "micronutrients concentration") OR (ZU "micronutrients uptake") OR (ZU "micronutrition") OR (ZU "micronycteris minuta")) OR ((ZU "iron compounds") OR (ZU "iron compounds‐congresses") OR (ZU "iron compounds‐structure") OR (ZU "iron concentration") OR (ZU "iron concretions") OR (ZU "iron consumption") OR (ZU "iron content") OR (ZU "iron contents"))) OR ((ZU "zinc compounds") OR (ZU "zinc contamination") OR (ZU "zinc content") OR (ZU "zinc coordination") OR (ZU "zinc deficiencies") OR (ZU "zinc deficiency") OR (ZU "zinc deficiency diseases") OR (ZU "zinc deficiency diseases‐congresses") OR (ZU "zinc deficiency diseases‐epidemiology") OR (ZU "zinc deficiency diseases‐united states") OR (ZU "zinc deficiency states‐congresses"))) OR ((ZU "dietary iron")) OR TI (multinutrient* OR multi‐nutrient* OR multinutrient * OR “multi‐nutrient*” OR “multi* nutrient*” OR multimicronutrient* OR multivitamin* OR multi‐vitamin* OR multi* vitamin* OR multimineral* or multi‐mineral* or multi* mineral* OR iron or Fe or ferric* or ferrous* or zinc or Zn or vitamin* or retinol* or folate* or folic* or folacin*) OR AB (multinutrient* OR multi‐nutrient* OR multinutrient* OR multi‐nutrient* OR multi* nutrient* OR multimicronutrient* OR multivitamin* OR multi‐vitamin* OR multi* vitamin* OR multimineral* OR multi‐mineral* OR multi* mineral* OR iron OR Fe OR ferric* OR ferrous* OR zinc OR Zn OR vitamin* OR retinol* OR folate* OR folic* OR folacin*)
#2 (fortified food) or (ZU "fortified food") or (ZU "fortified foods") or (ZU "fortified fruit beverages") or (ZU "fortified instant asian noodles") or (ZU "fortified milk") or (ZU "fortified musts") or (ZU "fortified seaweed extract") or (ZU "fortified soy protein powder") or (ZU "fortified vegetables") or (ZU "fortified vitamin juices") or (ZU "fortified water") or (ZU "fortifying agents") OR (ZU "rice") or (ZU "rice ( <i>oryza sativa</i> l.)") or (ZU "rice (<i>oryza sativa</i> l.)") or (ZU "rice (oriza sativa l.)") or (ZU "rice (oryza sativa cv tainung 67) suspension cells") or (ZU "rice (oryza sativa l)") or (ZU "rice (oryza sativa l. var. nipponbare)") or (ZU "rice (oryza sativa l.)") or (ZU "rice (oryza sativa l.) crop") or (ZU "rice (oryza sativa ssp. japonica)") or (ZU "rice (oryza sativa)") or (ZU "rice (oryza sativa) cells") or (ZU "rice (oryzae sativa l.)") or (ZU "rice (oryzae sativa)") or (ZU "rice (orzya sativa)") or (ZU "rice (qryza sativa l.)") or (ZU "rice act1 genes") or (ZU "rice aging") or (ZU "rice agriculture") or (ZU "rice agro‐residues") or (ZU "rice and rice products") or (ZU "rice as feeding stuff") or (ZU "rice as food") or (ZU "rice bibliography") or (ZU "rice blends") or (ZU "rice bran") or (ZU "rice bran protein") or (ZU "rice byproducts") or (ZU "rice cake") or (ZU "rice cake machines") or (ZU "rice cakes") or (ZU "rice cereals") or (ZU "rice consumption") or (ZU "rice cookie") or (ZU "rice cooking") or (ZU "rice council for market development") or (ZU "rice cracker") or (ZU "rice crackers") or (ZU "rice development") or (ZU "rice drink") or (ZU "rice economy") or (ZU "rice enrichment") or (ZU "rice exploitation") OR ((TI (fortifi* or enrich* or enhanc*) OR AB (fortifi* or enrich* or enhanc*)) N2 (TI (food* or rice) OR AB (food* or rice))) OR ((TI (fortifi* or enrich* or enhanc*) OR AB (fortifi* or enrich* or enhanc*) ) AND ((TI (food* or rice) OR AB (food* or rice)))
#3 ZU (rabbit* OR bird* OR chicken* OR soil* OR insect* OR Mice OR rat OR goat* OR sheep OR animal* OR livestock* OR cow*) OR ((ZE "animals") or (ZK "animal breeding and genetics") or (ZK "animal diseases (bacterial)") or (ZK "animal diseases (fungal)") or (ZK "animal diseases (general)") or (ZK "animal diseases (physiological)") or (ZK "animal diseases (viral)") or (ZK "animal disorders and injuries") or (ZK "animal ecology and behavior") or (ZK "animal nutrition") or (ZK "animal physiology and biochemistry") or (ZK "animal production") or (ZK "animal reproduction") or (ZK "animal science") or (ZK "animal structure") or (ZK "animal taxonomy and geography") or (ZK "pests of animals (general)") or (ZK "pests of animals (helminths)") or (ZK "pests of animals (insects and other arthropods)"))
#4 (#1 AND #2) NOT #3
#5 Limited to up to January 2017
Clinicaltrials.gov
"rice AND fortification", "rice AND enrichment","fortified rice" OR "enriched rice".
WHO International Clinical Trials Registry Platform (ICTRP)
"rice AND fortification", "rice AND enrichment","fortified rice" and "enriched rice". Duplicates were removed.
Data and analyses
Comparison 1.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7 | 1634 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.54, 0.97] | |
| 7 | 1634 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.66, 1.04] | |
| 2.1 Iron alone | 3 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.36, 1.09] |
| 2.2 Iron + other micronutrients | 4 | 1190 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.82, 1.11] |
| 7 | 1634 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.55, 0.97] | |
| 3.1 Hot extrusion | 5 | 1197 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.52, 1.01] |
| 3.2 Cold extrusion | 3 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.41, 1.38] |
| 3.3 Coating | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Dusting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Mixed/unknown/unreported | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 | 1634 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.54, 0.97] | |
| 4.1 Soaking, and boiling with excess water | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Boiling without excess water | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Rinsing and boiling without excess water | 1 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.26, 0.63] |
| 4.4 Frying and boiling without excess water | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Unknown/unreported | 6 | 1419 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.63, 1.05] |
| 7 | 1634 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.54, 0.97] | |
| 5.1 Not a problem (lower than 5%) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Mild and moderate (5% to 39.9%) | 4 | 1129 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.44, 1.06] |
| 5.3 Severe (40% and more) | 2 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.67, 1.12] |
| 5.4 Mixed/unknown/unreported | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.09, 1.10] |
| 7 | 1634 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.54, 0.97] | |
| 6.1 Some malaria risk setting | 1 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.55, 1.32] |
| 6.2 Malaria‐free area | 2 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.48, 1.03] |
| 6.3 Unknown/unreported | 4 | 786 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.34, 1.31] |
| 8 | 1733 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.51, 0.84] | |
| 8 | 1733 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.51, 0.84] | |
| 8.1 Iron alone | 4 | 628 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.40, 0.80] |
| 8.2 Iron + other micronutrients | 4 | 1105 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.57, 1.06] |
| 8 | 1733 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.52, 0.83] | |
| 9.1 Hot extrusion | 6 | 1283 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.51, 0.87] |
| 9.2 Cold extrusion | 3 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.38, 1.09] |
| 9.3 Coating | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Dusting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.5 Mixed/unknown/unreported | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 | 1733 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.51, 0.84] | |
| 10.1 Soaking, and boiling with excess water | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Boiling without excess water | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Rinsing and boiling without excess water | 1 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.51, 1.21] |
| 10.4 Frying and boiling without excess water | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Unknown/unreported | 7 | 1518 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.46, 0.84] |
| 8 | 1733 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.51, 0.84] | |
| 11.1 Not a problem (lower than 5%) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Mild and moderate (5% to 39.9%) | 4 | 1046 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.55, 1.07] |
| 11.3 Severe (40% and more) | 2 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.26, 1.27] |
| 11.4 Mixed/unknown/unreported | 2 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.39, 1.01] |
| 8 | 1733 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.51, 0.84] | |
| 12.1 Some malaria risk setting | 1 | 485 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.48, 1.53] |
| 12.2 Malaria‐free area | 3 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.41, 0.84] |
| 12.3 Mixed/unknown/unreported | 4 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.39, 0.96] |
| 11 | 2163 | Mean Difference (IV, Random, 95% CI) | 1.83 [0.66, 3.00] | |
| 11 | 2163 | Mean Difference (IV, Random, 95% CI) | 2.09 [0.75, 3.44] | |
| 14.1 Iron alone | 6 | 698 | Mean Difference (IV, Random, 95% CI) | 3.93 [1.24, 6.62] |
| 14.2 Iron + other micronutrients | 6 | 1465 | Mean Difference (IV, Random, 95% CI) | 1.06 [0.15, 1.98] |
| 11 | 2163 | Mean Difference (IV, Random, 95% CI) | 1.60 [0.81, 2.38] | |
| 15.1 Hot extrusion | 7 | 1563 | Mean Difference (IV, Random, 95% CI) | 1.93 [0.53, 3.32] |
| 15.2 Cold extrusion | 3 | 437 | Mean Difference (IV, Random, 95% CI) | 1.54 [0.58, 2.51] |
| 15.3 Coating | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 8.20 [‐12.14, 28.54] |
| 15.4 Dusting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.5 Mixed/unknown/unreported | 1 | 148 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐11.72, 3.72] |
| 11 | 2163 | Mean Difference (IV, Random, 95% CI) | 1.83 [0.66, 3.00] | |
| 16.1 Soaking, and boiling with excess water | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Boiling without excess water | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Rinsing and boiling without excess water | 1 | 215 | Mean Difference (IV, Random, 95% CI) | 3.80 [0.86, 6.74] |
| 16.4 Unknown/unreported | 10 | 1948 | Mean Difference (IV, Random, 95% CI) | 1.62 [0.43, 2.81] |
| 11 | 2163 | Mean Difference (IV, Random, 95% CI) | 1.83 [0.66, 3.00] | |
| 17.1 Not a problem (lower than 5%) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Mild and moderate (5% to 39.9%) | 6 | 1459 | Mean Difference (IV, Random, 95% CI) | 1.67 [‐0.10, 3.44] |
| 17.3 Severe (40% and more) | 2 | 360 | Mean Difference (IV, Random, 95% CI) | 1.07 [‐0.84, 2.98] |
| 17.4 Mixed/unknown/unreported | 3 | 344 | Mean Difference (IV, Random, 95% CI) | 3.42 [1.10, 5.73] |
| 11 | 2163 | Mean Difference (IV, Random, 95% CI) | 1.83 [0.66, 3.00] | |
| 18.1 Some malaria risk setting | 1 | 445 | Mean Difference (IV, Random, 95% CI) | 0.90 [0.65, 1.15] |
| 18.2 Malaria‐free area | 3 | 587 | Mean Difference (IV, Random, 95% CI) | 3.15 [0.98, 5.31] |
| 18.3 Mixed/unknown/unreported | 7 | 1131 | Mean Difference (IV, Random, 95% CI) | 1.33 [‐0.48, 3.14] |
| 4 | 927 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.36, 1.29] | |
| 4 | 927 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.36, 1.29] | |
| 20.1 Iron alone | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Iron + other micronutrients | 4 | 927 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.36, 1.29] |
| 4 | 927 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.41, 1.14] | |
| 21.1 Hot extrusion | 4 | 765 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.35, 1.39] |
| 21.2 Cold extrusion | 1 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.24, 1.54] |
| 21.3 Coating | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.4 Dusting | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.5 Mixed/unknown/unreported | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 | 927 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.36, 1.29] | |
| 22.1 Soaking, and boiling with excess water | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Boiling without excess water | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Rinsing and boiling without excess water | 1 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.47, 2.60] |
| 22.4 Frying and boiling without excess water | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.5 Unknown/unreported | 3 | 712 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.25, 1.22] |
| 4 | 927 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.36, 1.29] | |
| 23.1 Not a problem (lower than 5%) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Mild and moderate (5% to 39.9%) | 3 | 695 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.29, 1.24] |
| 23.3 Severe (40% and more) | 1 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.30, 7.07] |
| 23.4 Mixed/unknown/unreported | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 | 927 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.36, 1.29] | |
| 24.1 Some malaria risk setting | 1 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.30, 1.08] |
| 24.2 Malaria‐free area | 1 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.30, 7.07] |
| 24.3 Unknown/unreported | 2 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.12, 2.59] |
| 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | ||
| 2 | 1019 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.53, 2.76] | |
| 26.1 Hookworm infection risk | 1 | 785 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.18, 2.70] |
| 26.2 Abdominal pain more than 3 days | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.42, 1.42] |
| 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | ||
| 5 | 727 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.13, 0.21] | |
| 3 | 618 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.08, 0.83] | |
| 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | ||
| 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | ||
| 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study id].
| Methods | Double‐blinded, RCT with 3 arms | |
| Participants | A total of 180 anaemic (Hb < 120 g/L) children aged 6‐9 years of age attending San Joaquin Elementary School in Metro Manila, in the Division of Pasig City, Philippines with no severe anaemia (Hb < 70 g/L), nor history of blood disorders and other haemoglobinopathies. | |
| Interventions | Participants were randomly allocated to 1 of 3 groups: It is estimated that 160 mg cooked rice is equivalent to 66.72 g of uncooked rice. Rice was served during a 2‐week cycle with standard dishes during lunch, daily for 5 days a week for 120 days (6 months) under a supervised regimen. The separate mixtures for groups 1 and 2 were subjected to extrusion to form iron/rice‐like grains premix. This premix was blended with ordinary rice at a ratio of 2.5:100, aiming to provide 10 mg elemental iron per day. All participants received a 400 mg chewable albendazole tablet at baseline and a second dose was given at 12th week of the feeding. | |
| Outcomes | Hb, plasma ferritin, plasma retinol, and C‐reactive protein, anaemia at baseline, at 3 months and at 6 months. Weight, height, weight‐for‐age Z‐score, height‐for‐age Z‐score, weight‐for‐height Z‐score at 6 months of the intervention | |
| Notes | Source of funding: International Life Sciences Institute Center for Health Promotion of Japan (ILSI CHP, Japan), the ILSI CHP, Atlanta, Georgia, USA. Taiyo Kagaku, Japan donated the fortificant used in this study. | |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised but method unclear |
| Allocation concealment (selection bias) | High risk | Colour‐coded packages of uncooked rice were placed in respective colour‐coded kettles by a staff at the catering service. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | Low risk | The 3 groups showed similar mean values of Hb, weight and height |
| Similarity of baseline characteristics (checking for confounding,a potential consequence of selection bias) | Low risk | Participants were similar at baseline for all the variables measured. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate was 5%. It was similar across all the 3 arms of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The school officials and the researchers were blinded as to the type of rice the children were eating. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The school officials and the researchers were blinded as to the type of rice the children were eating. |
| Contamination (performance bias) | High risk | The packing of allocation of cooked rice into the lunch boxes was done by colour codes, hence, there was no point of comparison with the other cooked rice. However, after the study and data analysis, a thorough scrutiny was conducted by the researchers and noticed that brown‐coloured rice grains were visible in the uncooked rice fortified with ferrous sulphate. |
| Selective reporting (reporting bias) | Low risk | Study authors reported both significant and insignificant outcomes |
| Other bias | Low risk | There are insufficient problems with the study design to put it at high risk of bias. |
| Recruitment Bias | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Baseline imbalance | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Loss of clusters | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Incorrect analysis | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Compatibility with individual RCTs | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Methods | This was a community‐based CBA conducted in Bangladesh, done to evaluate the intervention provided by the WFP on the VGD beneficiaries. The 2 groups included 870 (435 in each group) women aged 15‐49 years; VGD beneficiaries receiving the FFR in 5 sub district‐level units called and a control group of the same age group receiving non‐FFR. These for both the groups were selected by the WFP from 5 districts across the country by using systematic random sampling method. The study was carried out between January and December 2013. A total of 400 women in each group were available for the end‐line survey. Quote "Participants for FFR group were drawn from the total list of approximately 15,000 VGD beneficiaries from 40 unions under the 5 . Similarly, participants for non‐FFR group were selected from enlisted approximately 15,000 VGD women from 53 unions of the 5 . During the end line evaluation, similar sampling approach was employed, and participants were allocated to FFR and non‐FFR group from the same sampling frame." | |
| Participants | Women aged 15–49 years‐old with possession of the VGD programme card. Those with either known/suspected chronic or congenital disease, pregnant and those with severe anaemia were excluded from the study. Advice was given to severe cases of anaemia to visit the government health facility. | |
| Interventions | The intervention group received 30 kg FFR; the control group received 30 kg non‐FFR for every month during the study period. | |
| Outcomes | Anaemia (defined as Hb level < 120 g/L in non‐pregnant and non‐lactating women), zinc deficiency (serum zinc level of < 10.10 mmol/L) and elevated CRP (> 10.0 mg/L) were measured | |
| Notes | namely Kaligonj, Sarankhola, Tungipara, Dacope and Shyamnagar in the FFR group and non‐FFR rations in the non‐FFR group. The 5 FFR were selected by the WFP from 5 districts in different geographic locations across the country. Source of funding: this research was supported by the UN WFP Grant # 1209, www1.wfp.org/countries/bangladesh | |
| Random sequence generation (selection bias) | High risk | There was no random sequence generation, since this was CBA |
| Allocation concealment (selection bias) | High risk | There was no mechanism for allocation concealment. The study participants were selected using systematic random sampling |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | High risk | The difference in the baseline prevalence of anaemia differed across the FFR and non‐FFR groups by 4.8% (38.8% in FFR versus 34% in non‐FFR). |
| Similarity of baseline characteristics (checking for confounding,a potential consequence of selection bias) | Low risk | The demographic characteristics were similar among the FFR and non‐FFR groups. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The baseline and end‐line study population was different. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding of study participants, data collectors or any person involved in the research |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of the outcome assessment done in the study |
| Contamination (performance bias) | Low risk | Since the allocation was in community and also the baseline and end‐line populations were different. |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes were reported. |
| Other bias | Unclear risk | Unclear |
| Recruitment Bias | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Baseline imbalance | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Loss of clusters | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Incorrect analysis | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Compatibility with individual RCTs | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Methods | CBA with 2 arms | |
| Participants | 143 male and female children 2‐6 years of age, with Hb concentrations ≥ 110 g/L attending 2 philanthropic preschools in Brazil were considered and 131 were eligible. Of these only 112 received parental consent to participate and underwent biochemical assessment. A total of 99 children completed all stages of the study. Children diagnosed with iron‐deficiency anaemia, who received ferrous sulphate supplementation or other nutritional supplements after evaluation by a qualified doctor of the city Health Department, were excluded from the study. | |
| Interventions | Participants from the selected preschools received, as part of school meals: Rice grains extruded from rice flour (Ultra Rice® (UR® )), produced and provided by a pasta manufacturer after authorisation by the Program for Appropriate Technology in Health (PATH). | |
| Outcomes | Erythrocytes, Hb, hematocrit, mean corpuscular volume, mean corpuscular Hb, mean corpuscular Hb concentration, folate, thiamine, ferritin, serum zinc, C‐reactive protein concentrations | |
| Notes | Source of funding: Programa de Bolsas de Iniciação Científica e Tecnológica Institucional da Fundação de Amparo à Pesquisa do Estado de Minas Gerais, Programa Institucional de Bolsas de Iniciação Científica/CNPq and Fundação de Amparo à Pesquisa do Estado de Minas Geraisprovided financial support and PATH donated the FFR. | |
| Random sequence generation (selection bias) | High risk | "Quote; The preschools were randomly selected as “control” or “test”. |
| Allocation concealment (selection bias) | Low risk | Quote: "The preschools were randomly selected as “control” or “test”." |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | High risk | Quote: "At the beginning of the study, children in the test group showed higher concentrations of erythrocyte thiamin (p = 0.012) and ferritin (p <0.001) Table 1." |
| Similarity of baseline characteristics (checking for confounding,a potential consequence of selection bias) | Unclear risk | There was no comparison of the baseline characteristics between groups. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Figure 1 shows that 13 out of 112 children at baseline "did not attend the dietetic and anthropometric assessment". The distribution between groups is not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding is not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding is not reported. |
| Contamination (performance bias) | Unclear risk | This study was conducted in 2 schools only and either FFR or non‐FFR was used in that school. Quote: "The preschools were randomly selected as "control" or "test"." |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found on clinical trials.gov |
| Other bias | Unclear risk | Unclear |
| Recruitment Bias | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Baseline imbalance | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Loss of clusters | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Incorrect analysis | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Compatibility with individual RCTs | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Methods | CBA with 2 arms | |
| Participants | 2250 children aged 1.5‐9 years from 29 villages in the province of Chiang Mai, Thailand from January 1971‐July 1975 | |
| Interventions | The villages were divided into 5 groups: Before being mixed with the other ingredients the rice has been cooked by putting it in water, bringing the water to boil and then cooking under low heat for 15 mins, by which time the rice becomes soft and the water absorbed. The fortification occurred at the mills. In some villages the rice was not fortified. | |
| Outcomes | Length, weight, hand‐wrist X‐rays, head, arm and chest circumferences, triceps and subscapular skinfold measurements, Hb and hematocrit concentrations and morbidity evaluations | |
| Notes | Source of funding: USAID | |
| Random sequence generation (selection bias) | High risk | Observational study with no random allocation |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | High risk | Groups were different. |
| Similarity of baseline characteristics (checking for confounding,a potential consequence of selection bias) | High risk | Groups were different. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was not purposely done. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open. |
| Contamination (performance bias) | Unclear risk | it is possible that contamination occurred but the risk of this happening is not clear. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Recruitment Bias | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Baseline imbalance | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Loss of clusters | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Incorrect analysis | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Compatibility with individual RCTs | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Methods | RCT with 2 arms | |
| Participants | 215 post‐menarchal anaemic and non‐anaemic adolescent girls 14‐18 years of age, with symptoms of anaemia (i.e. lethargy, weakness) assessed through self‐administered questionnaire from January‐September 2016, attending boarding school in Medan of North Sumatra Province, Indonesia. Participants with history of chronic diseases, obese, or having donated blood during the last 3 months, and practice regular fasting (intermittent fasting, and fasting on Monday and Thursday) were excluded. | |
| Interventions | Participants were randomly assigned to 1 of 2 groups: , or tofu for 4 months; The FFR contained iron, zinc, thiamin, folic acid, vitamin B , niacin, and vitamin A. The duration of intervention was about 4 months from March‐June 2016. FFR was produced by mixing rice kernel and rice with the ratio 2:100 using mixer machine. The menu cycle was changed every 2 weeks. The school meal was cooked and prepared centrally by the school kitchen. | |
| Outcomes | Hb, ferritin, serum folate, serum retinol, serum zinc, C‐reactive protein, weight, height, food intake, absenteeism, morbidity and cognitive score | |
| Notes | Source of funding: Food and Nutrition Society of Indonesia, Better Rice Initiative Asia (BRIA), a larger Public Private Partnership and Deutsche Gesellschaft fur Internationale Zusammenarbeit (GIZ) | |
| Random sequence generation (selection bias) | Unclear risk | Study reported as individually randomised but method unclear. |
| Allocation concealment (selection bias) | Low risk | Quote: "A total of 18 sacks of rice fortification was coded to show the intervention week and the serial number of sacks. For example the sack code is M1‐H3‐W2, it means the fortified rice sack is for the first week intervention (M1), on the third day of the week that is Thursday (H3) and for lunch time (W2)." |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | High risk | A total of 81.3 % of participants in the control group were not anaemic at baseline; while in the intervention group 50% were non‐anaemic. In terms of rice consumption the groups were similar. Quote: "There is no difference in the mean consumption of rice for both groups, which ranges from 147 grams to 150 grams served". |
| Similarity of baseline characteristics (checking for confounding,a potential consequence of selection bias) | Low risk | Quote: "During the baseline survey, the mean age of subjects were around 16.1 years old. Both groups have an average of normal nutrition status with mean weight 49,4 kg and height 151,5 cm. Most of the subject’s parents are high school graduates and above." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both fortified and non‐fortified rice with the dishes and vegetables in a sealed container was taken by car to the dining room (canteen) as usual. The meaning of colours of the basket only known by kitchen staff and servants." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Contamination (performance bias) | Low risk | Quote: "Quality checks of the nutrients content of the rice kernel and fortified rice were performed by an accredited laboratory analysis, namely in Laboratory Saraswanti in Bogor." |
| Selective reporting (reporting bias) | High risk | Researchers provided rewards for participants with the highest attendance to increase the compliance of participants in the second week, even though they report that the attendance was not consistent throughout the study. |
| Other bias | High risk | Quote: "Every week during the four months, the intervention subjects was given fortified cooked rice from Tuesday to Sunday (six days). Monday is a fasting day for students as suggested by the principal of the school." "Friday is a free day for the students of Ar Raudhatul Hasanah, hence parents are allowed to visit their children. They often bring in food from outside of the school, causing the subjects to be absent during meal times in the canteen." |
| Recruitment Bias | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Baseline imbalance | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Loss of clusters | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Incorrect analysis | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Compatibility with individual RCTs | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Methods | RCT with 2 arms | |
| Participants | A total of 201 nonpregnant, non lactating women 18‐49 years of age with moderate‐low altitude‐adjusted Hb concentrations (between 105 and 135 g/L) working in 6 factories in Morelos State, Mexico. Women who were currently taking, or were planning to take, iron supplements in the next 6 months or were planning to discontinue employment at their workplace in the next 6 months were excluded. | |
| Interventions | Participants were randomly assigned to 1 of 2 groups: The iron fortificant used in this study was microencapsulated, micronized ferric pyrophosphate, which is marketed as SunActive® iron. The FFR was formulated to provide 20 mg of elemental iron per daily portion during 6 months. The fortified grains were mixed directly with dry, locally obtained rice. For the first 3 months of the study, the fortified grains were added at a proportion of 2:100 (weight/weight), and each daily portion of rice consisted of 75 g of dry rice. Rice was prepared daily in a central kitchen with a standard recipe that was varied from day to day by using different combinations of 1‐3 cooked vegetables (broccoli, carrot, cauliflower, garlic, green bean, onion, parsley, pea, pepper, potato, sweet corn, and tomato) and seasonings. Portions were weighed by project personnel and packed in individual plastic containers labelled with the participant’s name and study identification number. | |
| Outcomes | Blood Hb concentration, plasma ferritin, soluble transferrin receptors, C‐reactive protein, anaemia, iron deficiency and iron body stores | |
| Notes | Source of funding: PATH through an original grant by the Bill & Melinda Gates Foundation | |
| Random sequence generation (selection bias) | Low risk | Upon enrolment, each woman was assigned a unique, consecutive identification number. These numbers were then randomly assigned by computer to 1 of 2 groups. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. There was no mention as to how the allocation was concealed. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | Low risk | Baseline characteristics are reported and similar across intervention groups. |
| Similarity of baseline characteristics (checking for confounding,a potential consequence of selection bias) | Low risk | There were no differences in characteristics of the enrolled women between the treatment and control groups at baseline. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 33 out of 103 (32%) lost to follow‐up from the non‐FFR, and 23 out of 98 (23%) from the iron‐FFR group. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The women did not know to which group they were assigned. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Only participants were blinded to the intervention groups. |
| Contamination (performance bias) | Unclear risk | Because of the large number of different lunch shifts, it was not feasible for project staff to monitor consumption of the rice. It is possible that contamination occurred but the risk of this happening is not clear. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Recruitment Bias | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Baseline imbalance | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Loss of clusters | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Incorrect analysis | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Compatibility with individual RCTs | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Methods | RCT with 6 arms | |
| Participants | 250 children aged 5‐8 years attending a school with a subsidised lunch feeding programme in India receiving a 200‐250 g meal of cooked rice daily | |
| Interventions | Participants were randomly assigned to 1 of 6 groups. The group receiving FFR (n = 185 in total) were randomly assigned to 5 subgroups based on their iron deficiency, iron‐deficiency anaemia and vitamin A deficient status at baseline. They received a lunch meal containing FFR for 6 months. The subgroups that were divided from the FFR were: The meals were consumed under direct supervision, and the daily leftovers were weighed. All the children were dewormed at baseline. Thus some children received iron‐FFR, others vitamin A‐FFR or beta‐carotene FFR and others a combination. | |
| Outcomes | Hb, serum ferritin, serum retinol, total iron binding capacity at baseline and after 6 months | |
| Notes | The data are disaggregated in this study to isolate the effect of iron alone, iron+retinyl palmitate, iron + beta‐carotene, beta‐carotene alone, retinyl palmitate alone in comparison with non‐FFR meals. For the vitamin A‐FFR comparisons we used only the group fortified with vitamin A and not the one with beta‐carotene, if the data were disaggregated. Micronutrient content: variable. There was a group using FFR in comparison with non‐FFR meal. However the fortified group was divided in several subgroups that included 1 or more micronutrients. Source of funding: the study is reported to have been made under the auspices of the public health agencies, but there is no mention of the specific agencies. The Directorate of Rice Research, Rajendranagar, Hyderabad is mentioned as provider of a sensory scale used in the study. | |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised but method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | Low risk | Quote: "The intervention groups did not differ significantly in baseline characteristics for Hb, serum ferritin, total; iron binding capacity, iron deficiency, iron‐deficiency anaemia, and vitamin A deficiency. At baseline 6.4 and 9.7 percent children were mildly stunted (HAZ < ‐1.0), 3.3 and 6.8 percent were mildly under weight (WAZ < ‐1.0) and 2.8 and 1.4 percent were mildly wasted (WHZ < ‐1.0) in fortified and control group respectively". |
| Similarity of baseline characteristics (checking for confounding,a potential consequence of selection bias) | Low risk | Quote: "The baseline characteristics of the two groups did not differ significantly after the randomisation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Out of 250 subjects enrolled, 222 completed the study. Twenty four of 28 subjects, who discontinued the study, were in micronutrient fortification group and 4 were in control group". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The distinguished colour of beta‐carotene fortified premix could not match the white colour of natural rice which also affected the taste and elongation score of such samples". Also less than a third of the panellists doing the sensory assessment part of the study could detect any difference between fortified and natural rice during sensory evaluations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study is reported as double‐blind, but it is unclear if outcome assessors were aware of the group assignments. |
| Contamination (performance bias) | Low risk | Quote: "The meals were consumed under the direct supervision, and the daily left overs were weighed". |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Recruitment Bias | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Baseline imbalance | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Loss of clusters | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Incorrect analysis | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Compatibility with individual RCTs | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Methods | RCT with 2 arms | |
| Participants | A total of 17 menstruating women 18‐50 years of age with iron‐deficiency anaemia (low iron and/or low ferritin) and otherwise healthy living in Baton Rouge, USA. Women who were pregnant, nursing, taking an iron supplement or taking a chronic medication that had not been stable for ≥ 1 month were excluded. The study was halted due to slow recruitment, the blind was broken and analysis performed after randomisation of 17 participants and completion of 15. | |
| Interventions | Participants were randomly assigned to 1 of 2 groups: The intervention lasted 2 weeks. The rice dishes were prepared in the Pennington metabolic kitchen and dispensed frozen in an insulated chest to the participants weekly in lots of 14 with instructions to heat and consume 1 rice dish twice a day. The participants were asked to return the dishes with any remaining food with the insulated chest each week. The coated iron FFR kernels were added to the regular rice at a 1:200 ratio (1 g of coated iron FFR kernels was added to 199 g of regular rice). The iron content in the finished iron‐FFR is 18 mg/100 g. | |
| Outcomes | Hb, hematocrit, serum ferritin, compliance, any adverse events (i.e. mild fatigue, mild insomnia, severe migraine, mild sinus congestion) | |
| Notes | Source of funding: rice samples including control (non‐FFR) and iron‐fortified samples were provided by The Wright Group (Crowley, USA). | |
| Random sequence generation (selection bias) | Unclear risk | Study did not report the method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | Low risk | Recruitment was based on serum hematocrit values and only those women who were anaemic were included. Quote: "Originally 20 women with iron deficiency determined by a serum ferritin (Siemens Immulite 2000) and/or a serum iron value (Beckman Coulter DXC600) below the lower limits of normal for the local laboratory (5 ng/mL and 40μg/mL, respectively) but otherwise healthy between the ages of 18 and 50 years of age were to be recruited". |
| Similarity of baseline characteristics (checking for confounding,a potential consequence of selection bias) | Low risk | Quote: "All subjects were menstruating women and the two groups were well‐matched." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Seventeen subjects were randomised to the study and 15 completed. One subject changed her mind about participating in the trial. Another subject did not return and could not be contacted." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study reported a double‐blinding to both investigators and the participants. The process of rinsing did not alter the colour or taste of rice to be consumed. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study reports a break in the blind at the time of slow down in recruitment and performance of the analysis of those participants who completed the interventions at that time. |
| Contamination (performance bias) | Low risk | Since the study was double‐blinded and both intervention arms had the same appearance of rice and container, chances of contamination were minimal. |
| Selective reporting (reporting bias) | Low risk | The women who completed the intervention were included in the analysis and all the parameters for all the 9 in intervention arm and 6 in control arm were reported. |
| Other bias | Unclear risk | Not clear. Quote: "Women who were pregnant, nursing, taking an iron supplement or taking a chronic medication that had not been stable for 1 month or longer were excluded. Although subjects were initially recruited from a study of women with irregular menses, recruitment eventually slowed." |
| Recruitment Bias | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Baseline imbalance | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Loss of clusters | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Incorrect analysis | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Compatibility with individual RCTs | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Methods | RCT with 2 arms | |
| Participants | The study included 184 iron‐depleted children aged 6‐13 years in a primary school serving the population of the Rock‐Colony neighbourhood, an urban slum in Bangalore, India. A subsidised lunch feeding programme is in place that provides the students with a 200–300‐g meal of cooked rice daily. | |
| Interventions | Participants were randomly assigned to 1 of 2 groups: The intervention lasted 7 months. Lunch was served 6 days/week (except for school holidays). 3 local recipes of rice cooked with different seasoning ingredients were presented in repeating sequence to maintain interest. The main seasoning ingredients of the 3 recipes were as follows: for tomato rice, onions and tomatoes; for lemon rice, groundnuts, roasted lentils, and lemon juice; and for vegetable , french beans, beetroot, cauliflower, carrots, and onions. At baseline and at the midpoint of the study, all participants were dewormed with 400 mg albendazole (Low‐Cost Pharmaceuticals®, Bangalore, India). As part of the current Indian national supplementation campaign, children in the study were treated with vitamin A supplements (200000 IU) 4 months before the start of the study and near the study midpoint. Quote: "Rice was washed in preparation for cooking. Rice portions were cooked with seasoning ingredients in household pressure cookers for 8 min after reaching peak pressure, after which pressure was released. Test servings contained 35 g cooked rice." | |
| Outcomes | Serum transferrin receptor, serum ferritin, Hb, C‐reactive protein at 3.5 and 7 months post‐intervention, height, weight, weekly morbidity | |
| Notes | Source of funding: Micronutrient Initiative, Ottawa, Canada; the Swiss Federal Institute of Technology, Zurich, Switzerland; and St John’s Academy of Health Sciences, Bangalore, India. Paul Lohmann GmbH (Emmerthal, Germany) provided the iron fortification compound. | |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised but method unclear. |
| Allocation concealment (selection bias) | Low risk | The iron‐FFR and non‐FFR were packaged in colour‐coded 10‐kg polyethylene bags. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | Low risk | The baseline characteristics of the 2 groups are reported and do not differ. |
| Similarity of baseline characteristics (checking for confounding,a potential consequence of selection bias) | Low risk | The baseline characteristics of the 2 groups did not differ significantly after the randomisation step. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 184 participants enrolled, 170 completed the study. 12 of the 14 participants who discontinued the study were in the iron‐fortification group and 2 were in control group. The main reasons for dropping out were leaving school (n = 9) and loss of interest in the study (n = 5). 3 participants who dropped out because of lack of interest were in the iron group and 2 were in the control group. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participants were blinded and were informed about the procedures of the test only after completion of the entire study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study was double‐blind. |
| Contamination (performance bias) | Unclear risk | It is possible that contamination occurred but the risk of this happening is not clear. |
| Selective reporting (reporting bias) | Low risk | The study protocol was approved by the institutions, that all prespecified outcomes that are of interest in the review have been reported. |
| Other bias | Unclear risk | Unclear |
| Recruitment Bias | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Baseline imbalance | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Loss of clusters | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Incorrect analysis | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Compatibility with individual RCTs | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Methods | CBA | |
| Participants | 303 children aged 2‐5 years in Brazil from 2 public schools in City of Sobral‐Ceará, in the northeast of Brazil, between August and December 2010. The mean Hb values for the groups were 120.6 ± 10.1 g/L for group receiving FFR and 124.0 ± 41.4 g/L for those receiving non‐FFR; P = 0.38; anaemia prevalence was 8.9% and 20.8%, in these groups, respectively, P = 0.009. | |
| Interventions | Participants from the 2 selected schools received: The menus at the 2 schools were equal in content; the study rice was consumed with poultry, which was the customarily consumed meal for Tuesdays at the schools. "This proportioned a weekly ingestion of 56.4 mg of elemental iron as ferric pyrophosphate per 50 g uncooked individual portion." | |
| Outcomes | Hb and anaemia | |
| Notes | Source of funding: Secretariat of Education and Secretariat of Health at the Municipal City Hall‐Sobral‐CE; Program for Appropriate Technology in Health (PATH) donated the rice to the Secretariat of Education at the Municipal City Hall‐Sobral‐CE | |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Schools were chosen and participants later invited to participate. "Prior to the study, each school was identified to receive one type of rice to avoid contamination between the different groups". |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | High risk | Anaemia was higher in the school receiving non‐FFR. |
| Similarity of baseline characteristics (checking for confounding,a potential consequence of selection bias) | Low risk | The baseline characteristics of the 2 groups are reported and do not differ. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13% attrition, but balanced between groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Staff at the schools were not aware of the rice that was being served (fortified or standard) as the rice was provided by the study team in non‐identifying packages. Data collection team was also unaware of intervention and control groups." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Data collection team was also unaware of intervention and control groups." |
| Contamination (performance bias) | Low risk | Schools were chosen and only 1 intervention given. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | Unclear |
| Recruitment Bias | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Baseline imbalance | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Loss of clusters | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Incorrect analysis | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Compatibility with individual RCTs | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Methods | Single‐blind, cluster randomised, placebo‐controlled trial | |
| Participants | 904 anaemic (Hb concentration 70‐120 g/L) school children aged 7‐11 years from 12 primary schools in the Muyinga Province, Burundi | |
| Interventions | The study team randomised 12 schools to receive either FFR or milled non‐FFR, wherein 904 participants were enrolled and received 150 g dry ration of rice: (thiamine), and 0.6 mg (600 µg) folic acid 5 days/week for 7 months; All children received beans prepared with vitamin A‐fortified oil and iodised salt as co‐intervention in both groups. All children were dewormed 2 weeks prior to the start of the study. Children did not receive the intervention in the 2 study holiday periods (December and April) consisting of 2 weeks per period and during a 2‐week period in March due to procurement problems. Milled non‐FFR or FFR was delivered every 2 weeks to the schools and parents prepared the meals under supervision of study staff. | |
| Outcomes | Change in Hb concentration expressed as change in Hb between baseline and follow‐up. Anemia improvement based on a change from mild to no anaemia or moderate anaemia to mild or no anaemia | |
| Notes | Anaemia classification was based on WHO criteria for children at 1500 m above sea level; moderate and severe anaemia was combined as 1 group in the analysis because of the few participants who had Hb < 8.5 g/L (severe). Sample size estimated not reported. The study did not report on malaria smear positivity nor presence of soil‐transmitted helminths, but malaria and hookworm infections are endemic in the area. It is indicated in the article that "Almost 80 % of the population of Burundi lives in malaria‐endemic areas, and one‐quarter lives in areas of particularly high incidence." In this cluster‐randomised trial, we made adjustment towards design effect while presenting the outcomes anaemia and Hb concentration by estimating the effective sample size. This included 6 clusters each receiving FFR and non‐FFR. The total number of participants who provided complete outcome data was 904 (461 + 443), and therefore mean cluster size was 75.33. With ICC as 0.02723, from ICC for postal code reported in other studies for outcome Hb ( ; ), the computed design effect was 3.024. For anaemia, in the intervention arm receiving iron‐FFR, sample size adjustment was made to 84 events (from n = 254) out of 152 participants (from n = 461) and in control arm with non‐FFR, numbers were adjusted to 77 events (from n = 234) out of 146 participants (from n = 443). However, for Hb concentration, only the total numbers in both intervention and control groups were adjusted as above without changing the mean and SD, thus making total number of participants in FFR group as 152 and control group as 146. Source of funding: USDA and Open Road Alliance | |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised but random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. The study team transported non‐FFR or FFR to the schools. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | High risk | Participants from intervention schools had a significantly lower Hb level and a higher proportion of children with moderate or severe anaemia. |
| Similarity of baseline characteristics (checking for confounding,a potential consequence of selection bias) | Unclear risk | The baseline characteristics (age, dietary diversity score) are reported in the text as different between groups, but the table shows only the frequency or mean, but not the P values. Calculation for the P values confirm that these are < 0.05. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of the 1071 participants, 904 (15% attrition) completed the study. From the intervention group, 81 were lost to follow‐up and from the control group, 86 were lost to follow‐up. There was no description of the reasons for attrition. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This is reported as a "single‐blind" study with participants receiving the food through the school canteen and parent volunteers using the rice allocated. However, study team food monitors on site supervised the programme and it is unclear whether these monitors were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This is reported as a "single‐blind" study and the study team measured Hb on site. |
| Contamination (performance bias) | Unclear risk | It is possible that contamination occurred but the risk of this happening is not clear. Each school received either FFR or non‐FFR coming from a central location. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were reported. This study was approved by the Comité National d’Ethique du Burundi and the PATH Research Ethics Committee. |
| Other bias | Low risk | There are no other clear signs of bias. Source of funding was declared as "Funding for this study was provided by the United States Department of Agriculture (USDA) grant #2010‐38418‐21635 with supplemental funding from Open Road Alliance." |
| Recruitment Bias | Low risk | Reported randomisation of the schools (clusters) into intervention and control arms was done after the screening of children and their recruitment into the study. |
| Baseline imbalance | Low risk | Clusters were reported to be similar in terms of demographic, social and dietary characteristics. However there was significant difference across the arms with respect to baseline outcome measurements (Hb concentration and level of anaemia). Study authors also report "The primary models were not adjusted for any additional covariates, although we did conduct a sensitivity analyses to assess whether the results differed after adjustment for characteristics that were found to be imbalanced between the intervention groups at baseline. " |
| Loss of clusters | Low risk | No clusters were lost at the follow‐up. |
| Incorrect analysis | Low risk | "Linear mixed model" was used in the analysis to account for clustering by school. |
| Compatibility with individual RCTs | Low risk | It is unlikely that cluster design would effect the outcomes in this study. |
| Methods | Cluster‐randomised, double‐blind trial | |
| Participants | 2440 school‐age children from 20 schools with Hb ≥ 70 g/L. 16 schools in Cambodia were randomly allocated into 4 groups: 3 with FFR and 1 placebo. From another 16 schools 4 schools were randomly selected to be a no‐intervention group. From each of the 20 schools, 132 children were selected after stratification by age and grade. A total 528 participants were selected per group, including the no intervention group. Of the 2640 children, 200 were not recruited for various reasons (age, severe anaemia, absence or refusal). Finally, placebo group had 427 participants, UltraRice old (URO) = 446, UltraRice new (URN) = 464 and Nutririce = 460, adding to a total of 2248, who were available for assessment at the end of follow up period. The mean age of children was 9.6 (SD 2.3) years and 49.9% of them were girls. | |
| Interventions | 4 groups received rice through a school meal. At randomisation: (thiamine), 270 µg (0.27 mg) folic acid; (thiamine), 2140 IU vitamin A, 12.57 mg vitamin B (niacin) and 3.8 µg vitamin B ; (thiamine), 140 µg (0.14 mg) folic acid, 960 IU vitamin A, 7.98 mg vitamin B (niacin), 1.26 µg vitamin B , and 0.92 mg vitamin B (pyridoxine); and A fifth group consisted of those participating in a take‐home ration with no school meals. Blending of the FFR was done at the plant with a ratio of 1:100. Packages were coded A‐H with 2 letters per intervention group to improve blinding. | |
| Outcomes | The primary outcomes of the study were the prevalence of anaemia and other micronutrient deficiencies, anthropometry, general health and well‐being and cognitive function. The secondary outcomes were prevalence of helminth infection, gut flora and immune function. This paper is limited to the impact on Hb concentration, iron and vitamin A deficiencies. | |
| Notes | Sample size estimates were calculated to detect a difference of 4 g/L. The study did not report on malaria smear positivity but reported on the presence of intestinal parasites (17.9% overall at baseline ranging from 9.4% to 23.9%) Source of funding: USDA WFP‐DSM Consortium, IRD. The study was approved by the National Ethics Committee for Health Research (NECHR) of the Ministry of Health, Phnom Penh, Cambodia, the Ministry of Education, Youth and Sports, Phnom Penh, Cambodia, and the Research Ethical Committee (REC) of PATH, Seattle, WA, USA. The study was registered at ClinicalTrials.gov (Identifier: NCT01706419). For presenting the outcomes in analyses we combined the 3 intervention groups (URO, URN and Nutririce) as intervention arm and placebo group as control arm. Only for the subgroup analysis related to cold and hot extrusion process involved in fortification, URO was considered along with half of placebo group as a separate study than the combination of URN and Nutririce compared with half of placebo group. While presenting the outcomes anaemia, Hb concentration and iron deficiency, we adjusted for the design effect by calculating the effective sample size. We estimated the design effect as 4.016 for the outcomes anaemia, Hb concentration, iron deficiency and vitamin A deficiency by considering ICC = 0.02723, ICC for postal code reported towards outcome Hb in references from other studies ( ; ). This study had mean cluster size of 111.75 (1788 participants from 16 clusters (schools), providing data for the outcome Hb). The adjusted sample size for UltraRice Old was 111 from 445, UltraRice New 116 from 464, NutriRice 112 from 454 and placebo was 106 from 425. Overall, when URO, URN and Nutririce were merged as single intervention arm and adjusted for design effect, there were 60 events of anaemia (from n = 239) out of a total of 339 participants (from n = 1363) and in the control arm, there were 22 events (from n = 89) out of 106 participants (from n = 425). Similarly, when we presented the outcome anaemia under the subgroup of type of extrusion, for URO, which was the cold extrusion arm, there were 18 anaemia events out of 111 participants and hot extrusion arm including URN and Nutririce had 42 events of anaemia out of 228 participants. For Hb concentration, the adjusted number was taken for total number and original weighted average and SD were retained in the meta‐analysis. For presenting the results of iron deficiency, we adjusted the sample size for the design effect and using the design effect for anaemia (4.016). The adjusted sample size was 37 events of iron deficiency (from n = 149) in the intervention arm having 366 (from n = 1471) and 14 events of iron deficiency (from n = 56) out of 119 participants in control arm (from n = 479). To present the outcome of vitamin A deficiency, the total sample size was adjusted to 338 (from n = 1356) in the intervention arm (URO, URN and Nutririce added together) and 104 (from n = 421) in control arm. The numbers of vitamin A deficiency (VAD) were adjusted to 24 (from n = 97) in the intervention arm and 13 (from n = 52) in the control arm. While presenting the subgroup of type of extrusion, for URO the adjusted events were 9 out of 110 participants and for hot extrusion arm (URN and Nutririce together), 15 out of 228 participants. | |
| Random sequence generation (selection bias) | Low risk | Reported as cluster‐randomised. Allocation of schools was done via a computer‐generated list. |
| Allocation concealment (selection bias) | Low risk | Random allocation was performed by a researcher not involved in the field work and the codes were kept from the field staff and other researchers. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | High risk | Baseline measures of outcome measures were reported. Authors report "There were no significant differences in age and gender between the study groups. " and "However, despite the randomisation, the prevalence of marginal vitamin A status, ferritin and soluble transferrin receptors levels, inflammation, parasite infection, and haemoglobinopathy significantly differed between groups. Furthermore, the control group was significantly different from the placebo and intervention groups for most indicators." |
| Similarity of baseline characteristics (checking for confounding,a potential consequence of selection bias) | High risk | Baseline characteristics were reported and there were no differences in age or sex, but there were significant differences in inflammation and parasite infection and haemoglobinopathy. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Around 8% of participants were not followed up at end line due to dropping out from school, transferring schools, absence, refusal to participate and severe anaemia at midline. The final sample size was below the targeted sample size of 528 per group. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was reported as double‐blind and the blinding of allocation into groups was described. Blinding of the intervention was described as being blended centrally and coded with a letter A to H. Each intervention group would receive packages with 2 letters. There was no report on whether the participants could tell whether they received FFR. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Hb was measured on site, all other assays were performed in the laboratory in Germany. Therefore the concealment of the codes were not described. |
| Contamination (performance bias) | Low risk | The study was cluster‐randomised with each cluster receiving an intervention that was labelled centrally at the rice blending plant. |
| Selective reporting (reporting bias) | Low risk | Of the primary outcomes noted above, the paper is limited to micronutrient deficiencies and these were all reported in this paper. The prevalence of anaemia was a stated primary outcome. The absence of a change in prevalence was reported in the discussion but not shown in the results. |
| Other bias | Low risk | There were no other clear signs of bias. Study authors have declared the source of funding: "USDA/FAS, WFP‐DSM consortium, and IRD" |
| Recruitment Bias | Low risk | Quote "Prior to the study, all parents of children from the 20 schools were invited to attend a meeting at which the study procedures were explained". Randomisation took place after the recruitment of the participants into the study. |
| Baseline imbalance | Unclear risk | However there is no evidence of differences across the clusters. |
| Loss of clusters | Low risk | No clusters were lost to follow‐up. |
| Incorrect analysis | Low risk | Quote: "Generalized mixed models (linear or binary logistic regression) were used to evaluate the effects of time, group, and time*group interaction on Hb, ferritin, soluble transferrin receptors, and the prevalence of vitamin A deficiency, while taking into account the random effects of individuals and school clusters." |
| Compatibility with individual RCTs | Low risk | The cluster design did not seem to effect the findings of the study. |
| Methods | RCT with 2 arms | |
| Participants | 203 school‐going children with low serum zinc concentration aged between 7‐12 years from low‐income Muslim families attending 8 primary schools in peri‐urban area of the Muang district, Satun province, southern Thailand. There were children aged between 4‐12 years in the schools (kindergarten to grade 6), who were provided with a school lunch programme for 5 days/week, with partial government subsidy | |
| Interventions | Participants were randomised to 1 of 2 groups: The rice was provided as a component of school lunch meals for 5 months. Lunch menus were changed frequently with rotation manner so that rice was given with chicken or fish and occasionally with vegetables. The schools also provided 200 mL of free milk daily to all children to improve the chance of showing an improvement in iron status even though it was not the primary outcome measure. | |
| Outcomes | Hb level, serum ferritin, transferrin, anaemia, iron‐deficiency anaemia, vitamin A deficiency, zinc deficiency | |
| Notes | Source of funding: Medicor Foundation (Triesen, Liechtenstein) and The Royal Thai Government Scholarship. Dr. Paul Lohmann GmbH (Emmerthal, Germany) provided iron and zinc compounds and DSM Nutritional Products Ltd. (Basel, Switzerland) provided the vitamin A compound. | |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported. |
| Allocation concealment (selection bias) | High risk | The individually packed rice portions were transported by research assistants from the central kitchen to the school each day. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | Low risk | The dietary intake of macro‐ and micronutrients was comparable among the study groups except for vitamin A. |
| Similarity of baseline characteristics (checking for confounding,a potential consequence of selection bias) | Low risk | There was no significant difference in any of the characteristics between the 2 groups except for BMI‐for‐age Z‐scores, which were found to be lower in the control group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate was 3%, intervention group ‐ 2%, control group ‐ 4% and it was due to loss of interest. Authors also quote "fourteen children (5 in the triple‐fortified group, 9 in the control group) were excluded from data analysis because of low school attendance (<80% during the feeding period)". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The individually packed rice portions were transported by research assistants from the central kitchen to the school each day. "The cooked rice was weighed into individual portions of 140 g into a colour‐coded container that was labelled with the child's name. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was reported a double‐blind study wherein individual rice packs in individual containers with child's name were transported from the central kitchen to the school. |
| Contamination (performance bias) | Unclear risk | Not clear. |
| Selective reporting (reporting bias) | Low risk | The study reported all the parameters concerned to the objectives. |
| Other bias | Unclear risk | Not clear. |
| Recruitment Bias | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Baseline imbalance | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Loss of clusters | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Incorrect analysis | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Compatibility with individual RCTs | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Methods | RCT with 2 arms | |
| Participants | 203 school‐going children aged between 7‐12 years from 8 primary schools in periurban area of the Muang district, Satun province, southern Thailand. The schools included children mainly from low‐income Muslim families. There were children aged between 4‐12 years in the schools (kindergarten to grade 6). They were provided with a school lunch programme for 5 days/week, with a partial government subsidy. Children who had consumed the triple‐FFR in a previous study ( ), or showed clinical symptoms of vitamin A deficiency were excluded. | |
| Interventions | Participants with low serum zinc (n = 203) were randomised to 1 of 2 groups: The rice was provided as a component of school lunch meals for 5 months. They used paired stable isotope dilution technique with labelled C ‐retinyl acetate ( C‐RID) to quantify vitamin A pool size. Lunch menus were changed frequently with rotation manner so that rice was given with chicken or fish and occasionally with vegetables. The schools also provided 200 mL of free milk daily to all children to improve the chance of showing an improvement in iron status even though it was not the primary outcome measure. Triple FFR once a day for 5 times a week. | |
| Outcomes | Hb level, serum ferritin, transferrin, anaemia, iron‐deficiency anaemia, vitamin A deficiency, zinc deficiency | |
| Notes | Source of funding: Medicor Foundation (Triesen, Liechtenstein), the International Atomic Energy Agency (Vienna, Austria), and the Royal Thai Government Scholarship. Dr. Paul Lohmann (GmbH, Emmerthal, Germany) provided the iron and zinc compounds, and DSM Nutritional Products Ltd. (Basel, Switzerland) provided the vitamin A compound. | |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported. |
| Allocation concealment (selection bias) | High risk | The individually packed rice portions were transported by research assistants from the central kitchen to the school each day. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | Low risk | The dietary intake of macro‐ and micronutrients was comparable among the study groups except for vitamin A. |
| Similarity of baseline characteristics (checking for confounding,a potential consequence of selection bias) | Low risk | There was no significant difference in any of the characteristics between the 2 groups except for BMI‐for‐age Z‐scores, which were found to be lower in the control group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Among the total of 50 children who were enrolled, 45 children completed it. 2 children dropped out because they shifted out of the study area (1 child in each group), and 3 children (1 in the intervention group and 2 in the control group) were excluded due to low compliance. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The individually packed rice portions were transported by research assistants from the central kitchen to the school each day. "The cooked rice was weighed into individual portions of 140 g into a colour coded container that was labelled with the child's name. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was reported a double‐blind study wherein individual rice packs in individual containers with child's name were transported from the central kitchen to the school. |
| Contamination (performance bias) | Unclear risk | Not clear. |
| Selective reporting (reporting bias) | Low risk | The study reported all the parameters concerned to the objectives including the findings of the pilot study. |
| Other bias | Unclear risk | Not clear. |
| Recruitment Bias | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Baseline imbalance | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Loss of clusters | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Incorrect analysis | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Compatibility with individual RCTs | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Methods | Double‐blind RCT with 2 arms | |
| Participants | 140 school children aged 5–11 years (54 boys and 86 girls) with Hb ≥ 70 g/L attending school through the public distribution system of the government of Andhra Pradesh, India | |
| Interventions | Participants were randomly allocated to 1 of 2 groups: The extruded kernels were produced by a cold‐extrusion process. Rice served in combination with a typical liquid preparation of pulse, locally called (prepared by combining dehusked split pigeon peas, tamarind pulp, and vegetables, e.g. bottle gourd, cucumber, ), or a semi‐liquid preparation of the same pulse with green leafy vegetables (e.g. spinach, amaranth, sorrel), locally called . Another variation in the menu included rice seasoned with tamarind pulp, locally called . The main ingredients used on a daily basis were tomatoes, onions, peanuts, red chilies, salt, and peanut oil. The intervention lasted 8 months. The process of blending the rice with the premix was done through manual mixing in 50‐kg batches outside rice mills or normal packaging facilities, for the sole purpose of use in the school study. All of the participating children were dewormed with 100 mg mebendazole (Mebex®, Cipla Limited) twice daily for 3 days, 1 week before initiating the feeding study. | |
| Outcomes | Average consumption amounts of the midday meal, height, weight, Hb, ferritin, and C‐reactive protein at baseline and at 8 months | |
| Notes | Source of funding: Department of Biotechnology, Government of India. PATH USA provided UltraRice premix for the use in the study. | |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. |
| Allocation concealment (selection bias) | Low risk | The codes were kept secure, and the decoding was completed after the analysis of data. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | Low risk | The prevalence of anaemia, iron deficiency, and iron‐deficiency anaemia was similar between the 2 groups at baseline. |
| Similarity of baseline characteristics (checking for confounding,a potential consequence of selection bias) | Low risk | The prevalence of anaemia, iron deficiency, and iron‐deficiency anaemia was similar between the 2 groups at baseline. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 participants were lost to follow‐up. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. The study group randomisation and the process of fortification and coding of rice were completed by a scientist not involved in the study, to ensure the double‐blinding of the study. The codes were kept secure, and the decoding was completed after the analysis of data. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The codes were kept secure, and the decoding was completed after the analysis of data. |
| Contamination (performance bias) | Unclear risk | It is possible that contamination occurred but the risk of this happening is not clear. |
| Selective reporting (reporting bias) | Low risk | The protocol was approved by the Institutional Ethical Committee of the National Institute of Nutrition and the Human Subject’s Protection Committee of PATH. |
| Other bias | Unclear risk | Unclear. |
| Recruitment Bias | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Baseline imbalance | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Loss of clusters | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Incorrect analysis | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Compatibility with individual RCTs | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Methods | Non randomised‐controlled study: cross‐sectional study with 2 arms | |
| Participants | Children from welfare institutions of selected municipalities of Bataan Province, Philippines (aged 3‐18 years). | |
| Interventions | The 7 municipalities of the east coast, with a population of 63,508 as of 1 October 1948, made up the experimental area (FFR); the remainder of the province, comprising a larger, more sparsely settled area in 5 municipalities, with a population of 29,393, became the control zone (non‐FFR). In the control zone, only the 2 municipalities of Bagac and Moron were considered. A sample was selected in each area. 3 sets of observations were made: There were 3 studies reported: Only studies 2 and 3 (n = 1703) are pertinent for this review. However, the records available of these studies do not allow for data extraction and thus do not contribute data for this review. | |
| Outcomes | For children: average increase in height (cm) and weight (kg), % gaining height and weight, red cell count (counts), increase in Hb (%) | |
| Notes | Source of funding: Williams‐Wateran Fund for the Combat of Dietary Diseases, New York, USA; Government of Bataan Province; Hoffmann‐La Roche Inc, Nutley, USA and the National Rice and Corn Corporation of Philippines | |
| Random sequence generation (selection bias) | High risk | Not used. |
| Allocation concealment (selection bias) | High risk | Quote: "Accordingly, it was early decided to set up within Bataan a control area as well as an experimental one, which might then be compared with one another with respect to morbidity, mortality and other features before and after a reasonable trial period." |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | Unclear risk | Not reported. |
| Similarity of baseline characteristics (checking for confounding,a potential consequence of selection bias) | Low risk | Quote: "Both groups were in fairly good health and showed no symptoms of thiamine deficiency at the start of or during the experiment." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only small samples were analysed from each of the universe of the population receiving the FFR or non‐FFR. Quote: "Twenty‐seven children from each group had to be dropped due to transfers and discharges from the institutions." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It was an open assessment. |
| Contamination (performance bias) | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | Unclear. |
| Recruitment Bias | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Baseline imbalance | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Loss of clusters | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Incorrect analysis | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Compatibility with individual RCTs | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Methods | Randomized, double masked, placebo controlled design. A block randomisation with a computer‐generated list in blocks of 20 was used to assign children to 1 of the 3 intervention groups. | |
| Participants | 258 children aged 6–12 years attending 4 primary schools in the Bangalore Urban District of Karnataka State in South India. This region is not endemic for malaria and the incidence of intestinal parasitic infestation is estimated to be low. | |
| Interventions | Participants were randomly assigned to 1 of 3 groups: All the FFR also contained per 100 g 0.5 mg vitamin A (as vitamin A palmitate), 0.38 mg thiamine (as thiamin mononitrate), 5 mg niacin (as niacinamide), 0.38 mg vitamin B6 (as pyridoxine hydrochloride), 0.00075 mg vitamin B‐12 (as vitamin B12 0.1%), 0.075 mg (75 µg) folic acid, and 3 mg zinc (as zinc oxide). A rotating menu of 5 traditional recipes was used: lemon rice, tamarind rice, vegetable rice, curd rice, and tomato rice. Dedicated technicians were responsible for preparing the daily meals according to the standard recipes, supervising the cooking, weighing and packing the individual rice meals in colour‐coded plastic containers, and transporting it to the schools. All study children were dewormed under the supervision of the research staff with 400 mg albendazole (Low‐Cost Pharmaceuticals) before the study and near the study midpoint. The schools were instructed not to provide iron and folic acid supplements, vitamin A supplements, and deworming medication to the children who were included in the study. | |
| Outcomes | Hb, C‐reactive protein, zinc protoporphyrin, serum ferritin, serum transferrin receptor, serum retinol, serum zinc, plasma homocysteine, serum vitamin B | |
| Notes | Source of funding: DSM, Mumbai, India | |
| Random sequence generation (selection bias) | Low risk | A block randomisation with a computer‐generated list in blocks of 20 was used to assign children to 1 of the 3 intervention groups. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | Low risk | There were no differences in baseline characteristics among the study groups except for the prevalence of low serum retinol, which was greater in the low‐iron group compared with the high‐iron group. |
| Similarity of baseline characteristics (checking for confounding,a potential consequence of selection bias) | Low risk | Children who did not complete the study were comparable to those who remained in the study by baseline characteristics (data not shown) except for the prevalence of low serum retinol, which was greater in the low‐iron group compared with the high‐iron group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was balanced among the groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Each group was randomly assigned a distinct colour code, which remained unknown to both the study staff and the children until the completion of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Each group was randomly assigned a distinct colour code, which remained unknown to both the study staff and the children until the completion of the study. |
| Contamination (performance bias) | Unclear risk | It is possible that contamination occurred but the risk of this happening is not clear. |
| Selective reporting (reporting bias) | Low risk | The study protocol was approved by the Institutional Ethical Review Board of St. John’s Medical College, Bangalore. This study was registered at the Clinical Trials Registry of India as CTRI/20/09/091/000941. |
| Other bias | Unclear risk | Unclear. |
| Recruitment Bias | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Baseline imbalance | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Loss of clusters | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Incorrect analysis | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
| Compatibility with individual RCTs | Unclear risk | Not applicable. We only evaluated this domain in cluster‐randomised studies. |
BMI: body‐mass index; CBA: controlled before‐and‐after study; FFR: fortified rice; Hb: haemoglobin; ICC: intra‐cluster correlation; RCT: randomised controlled trial; SD: standard deviation; UN: United Nations; USAID: United States Agency for International Development; USDA: United States Department of Agriculture; VGD: Vulnerable Group Development; WFP: World Food Programme
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| The type of study design is outside the scope of this review. In this study, 144 residents (100 women and 44 men) of a long‐term care health facility, Soka Royal Care Center (Soka city, Saitama prefecture of Japan) aged ≥ 65 years received folic acid‐FFR from March 2010 and were examined for blood cell parameters (n = 144) and vitamin B and/or serum folate concentrations (n = 68) after 1 year. 3 meals a day were prepared to contain 240 μg of folate derived from food stuff on average. Additional folic acid was made by adding calculated amounts of synthetic mono glutamyl folic acid‐FFR (House Wellness Foods Co) to washed rice before cooking. There was no control group. Participants who stayed more than 2 years (n = 49) were classified as high or low basal folate status. | |
| It did not involve a control group and hence was excluded.The study was carried out among 766 mothers and their children aged 6‐9 years in Orion, Bataan. This involved seeking political support, networking with local organisations, market surveys, and social marketing activities. In addition to these social marketing, the haemoglobin concentrations and levels of anaemia among mothers and children were measured before and after the introduction of the iron FFR into the market commercially. | |
| The study design and the type of intervention (micronutrient biofortification) are not within the scope of this review. A total of 480 children, aged 24‐48 months and their female caregivers residing in 2 rural districts of northern Bangladesh participated in a study that quantified their usual rice and zinc intakes and serum zinc concentrations in order to simulate the potential impact of zinc‐biofortified rice on their zinc intakes. | |
| The type of intervention is point‐of‐use fortification with iron drops and is therefore out of the scope of this review. Here, 360 children aged between 12‐60 months attending 4 public day‐care centres in Rio de Janeiro, Brasil, were randomly assigned to 1 of 2 groups: group 1 (n = 180) received a daily meal prepared with iron‐fortified rice (with iron bisglycinate); group 2 (n = 174) received non‐fortified placebo rice. The rice was fortified once a week for 16 weeks at a dose of 4.2 mg of iron for every 100g of food ready in the supplemented group. In the days of fortification, the solution was added as drops to the rice by the researcher during assembly of the dishes from lunch. If the child repeats the meal, the solution was administered proportional to the quantity of rice offered repetition. The number of drops of solution offered to every child, every day of intervention, was recorded in order to know, the end of the study, the total amount of iron offered the intervention. | |
| This was a retrospective study. Food that was supplemented was not limited to rice and since the study design was not in the scope of this review, the study was excluded. National Data Center Congenital Diseases Registry record national population base and Central American Population Center, were used and prevalence of neural tube defects before the implementation of food supplementation of folic acid and after the same were compared. | |
| The study was excluded because the participants were outside the age range and fortification is compared with another form of micronutrient intervention (supplementation). Randomised trial including 175 mildly anaemic infants and young children, 6‐24 months of age from the metropolitan region of Belo Horizonte in southeastern Brazil, who were randomly assigned to 1 of 2 groups for 5 months: group 1 received 100 g of cooked rice fortified with micronized ferric pyrophosphate and a placebo solution; group 2 received an identical, unfortified rice and iron drops. | |
| This study did not include rice as a medium of intervention and the included intervention was not fortification. Hence this study was excluded. Children aged between 2‐5 years were provided with the supplement daily as part of the afternoon snack, diluted in water. The dietary, biochemical, haematological and anthropometric parameters were measured before and after the interventions. | |
| Study was excluded since the iron fortification intervention employed was bio fortification. This study discusses the collective results of iron fortification interventions on iron status, from 3 RCTs from India and Philippines. A total of 686 participants were enrolled in the study. The primary outcome was measured in terms of serum haemoglobin and ferritin levels, Incidence of anaemia and iron‐deficiency anaemia and its resolution. There was no significant impact on the haematological outcomes during the follow‐up period, but likelihood of resolution of existing iron deficiency increased by 50%. | |
| This is a review paper on the history, technological aspects and challenges from rice fortification efforts in Phillipines. This is not the type of study considered for this review. | |
| The study combined fortification and supplementation and therefore did not meet the inclusion criteria. RCT of 116 night‐blind, pregnant women in Nepal who were assigned either a supplement providing 30 mg iron and 6 mg riboflavin or a placebo while receiving vitamin A–FFR providing 850 g retinal activity equivalents for 6 days/week under supervision for 6 weeks. Iron and riboflavin supplements significantly reduced the prevalences of riboflavin deficiency, iron‐deficiency anaemia, and abnormal papillary threshold from baseline. The study concluded that iron deficiency may limit the efficacy of vitamin A to normalise dark adaptation in pregnant Nepali women. | |
| The type of interventions and comparisons are outside the scope of this review. The study was conducted in seven 6‐week cycles between July 2000 and May 2002 in a total of 60 clusters, with each cluster forming the Nepali structure called a Village Development Committee, in the Saptari district in the eastern Terai region of Nepal; 405 pregnant women aged 18–45 years were identified and were randomly assigned to 1 of 6 treatment groups to receive a midday meal and capsule supplements 6 days/week for a period of 6 weeks: group 1 (n = 57) received 200 g cooked rice, ≈150 g vegetables with low vitamin A content (curried cauliflower or white squash or white potatoes), and a high‐dose capsule containing 2.0 mg vitamin A as retinyl palmitate in ≈200 μL corn oil); group 2 (n = 55) received 200 g cooked rice, ≈150 g curried vegetables with low vitamin A content, and a low‐dose capsule containing 850 μg vitamin A as retinyl palmitate in ≈200 μL corn oil; group 3 (n = 69) received 200 g cooked vitamin A–fortified rice containing 850 μg vitamin A, ≈150 g curried vegetables with low vitamin A content, and a capsule containing ≈200 μL corn oil; group 4 (n = 56) received 200 g cooked rice, ≈150 g curried vegetables with low vitamin A content, 8 g goat liver containing 850 μg vitamin A, and a capsule containing ≈200 μL corn oil; group 5 (n = 54) received 200 g cooked rice, ≈150 g amaranth greens containing 850 μg RE (425 μg retinol activity equivalents (RAE)) as β‐carotene, and a capsule containing 200 μL corn oil; and group 6 (n = 57) 200 g cooked rice, ≈128 g carrots containing 850 μg RE (425 μg RAE) as β‐carotene, and a capsule containing 200 μL corn oil. | |
| The study design is outside the scope of this review. In this descriptive cross‐sectional study from China, effectiveness of iron‐fortified food items among school‐age children aged between 11‐16 years of age was assessed. Iron‐fortified soy sauce, multinutrient fortified rice and vitamin A‐fortified cooking oil provided to a school cafeteria for a period of 10 months. Baseline Hb, zinc, vitamin A, serum ferritin and transferrin levels were measured at baseline and at the end of 10 months. There was a significant increase in the serum zinc, vitamin A and Hb levels and a decrease in anaemia prevalence. | |
| This is an abstract with no data available to further evaluate this study. In this study in Korea, 78 women between 19 and 35 years at risk of folate deficiency were randomly assigned to groups: fortified‐food group, non‐fortified food group, and fortified‐food group and nutrition education group and non‐fortified with nutrition education group. Instant rice fortified with 400 μg of folic acid per serving (1 bowl) was developed and used in this study. Fortified or non‐fortified food was consumed once a day for 4 weeks and nutrition education was given once a week. | |
| The study design is outside the scope of this review. Here, 1101 participants (male: n = 345, female: n = 756) aged 61 ± 10 years completed a dietary intake through a food frequency questionnaire of 56 foods and beverages commonly consumed by the Japanese population. Blood chemistry analysis included serum folate and total homocysteine concentration as well as genotype assessment. Participants then received “folate fortified rice” containing 26.7 mg folic acid, 187 mg thiamin, 66.7 mg vitamin B and 320 μg vitamin B per 100 g rice (House‐wellness Foods Company) and a folic acid‐fortified bread called Sakado Folate Bread, containing215 ± 14.7 μg folic acid per slice of 64.0 g bread. Commercially available Sakado Folate Bread contains 160 μg folic acid/slice. All participants received lecture, genotyping, blood analysis, nutrition surveys, and guidance according to the collected data. All participants were exposed to the folic acid from the fortified foods: rice and bread, as well as other foods. The study authors report that in the population of Sakado City (approximately 101,000 inhabitants in 2015) there were 693 births in 2015; consequently, a decrease in neural tube defects could not be confirmed. There is no control group and the interventions include availability of the fortified foods, the blood testing and the education component. | |
| Since the study population was < 2 years of age, it was excluded. The study included infants in the age group of 6 months being randomised to receive meat with equi‐caloric fortified cereal supplement or local cereal supplement. | |
| The participants were outside the age for inclusion in this review. Cluster‐RCT with infants, 10‐23 months of age from 2 public day‐care centres in northeast of Brazil who received either 50 g of iron fortified rice or a control rice weekly for 18 weeks. Though the effect was small, iron‐FFR was effective in increasing Hb levels and reducing anaemia in infants. | |
| The study was excluded since it was conducted among infants. In this intervention study from Vietnam, effect of micronutrient fortified complementary foods among normal infants was assessed. 29 villages were randomly divided into 3 groups and received 3 different interventions instant flour fortified with micronutrients, Traditional flour gruel fortified with micronutrients and no fortification. After 6 months of intervention, anthropometric indices were greater in the 2 intervention groups compared to the non‐intervention group. | |
| This is a poster presentation in an international congress reporting a randomised, double‐masked study of 244 reproductive‐age women, in 2 groups. No data available to further evaluate this study. A meal based on iron‐fortified rice (SunActive® FeP80) was served 6 days/week with 15 mg premix per 150g rice‐iron fortified group or 0.75mg iron/day and no added iron‐control group. Concentrations of haemoglobin, serum ferritin, transferrin receptor were measured at baseline 3 and 6 months. After intervention, in iron‐FFR group, concentrations of Hb, serum ferritin were significant higher, but no further data are available. | |
| The study was excluded since the study participants were infants. This was a randomised, single‐blind study conducted among infants to assess the impact of animal source food and micronutrient fortification on body fat composition and iron status. The infants were randomised to receive 2 different types of rice‐based complementary foods. WinFood (WF) with small fish and edible spiders and WinFood‐Lite (WF‐L) fortified with small fish, against 2 existing fortified corn‐soy blend products, CSB+ (purely plant based) and CSB++ (8% dried skimmed milk). Outcomes were assessed in terms of increments in fat‐free mass, serum transferrin and ferritin levels and anthropometric indices. | |
| The study was excluded as the vehicle of fortification was not rice. This observational study was conducted among women working in ginning industries (i.e. industry using machines separating the fibres of cotton from the seeds). 20 moderately anaemic women from a sample of 150 ginning workers were selected and given intervention in the form of micronutrient fortified soy biscuit. The haematological parameters and anthropometric indices were assessed after a period of 4 months. There was drastic improvement in the Hb levels and serum iron levels in the intervention group. | |
| The type of intervention is outside the scope of this review. This observational study was carried out to assess the impact of iron‐fortified cereal on anthropometric parameters and Hb levels among children aged 1‐4 years. 54 children, 24 in 1 room and 30 in another room in a nursery were provided with iron‐fortified cereal given in the form of cereal that provided 2‐3 g of iron for a period of 2 months. 1 group children were assisted by teachers during feeding and the other group fed on themselves. The commercial product used was a cereal porridge which was composed of 43.0% corn starch, 40.0% corn flour, 10.0% oatmeal, 4.0% rice flour, and 2.0% rye, sweetened at 7.5%. | |
| The participants were outside the age range for inclusion and type of interventions are outside the scope of this review. This study included 515 infants who weighed > 3000 g at birth and who were started on a cereal diet at 4 months of age, from a low‐ and low‐middle‐income group living in urban Santiago in Chile. The study compared iron‐FFR cereal to non‐FFR cereal in infants who were exclusively breast‐fed for > 4 months and to iron‐fortified formula in infants who were weaned to formula before 4 months of age. The design was double‐blind in respect to the presence or absence of fortification iron in the cereal or formula. Among infants weaned to formula before 4 months, the cumulative percentages of infants excluded for anaemia by 15 months were 8%, 24%, and 4%, respectively, in the fortified cereal, unfortified cereal and formula, and fortified formula groups. In infants breast‐fed for more than 4 months, the corresponding values were 13% and 27%, respectively, in the fortified and unfortified cereal groups. |
FFR: fortified rice; Hb: haemoglobin; RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Comparison of iron absorption from extruded FePP‐fortified rice containing zinc, citric acid/trisodium citrate and EDTA (MM_Rice) |
| Methods | Bioavailability study intervention model |
| Participants | Children 5‐9 years of age (both male and female) with these criteria for inclusion: iron deficient or anaemic as per definition; read and signed informed consent form by the caregiver (or has been read out to the caregiver in case of illiteracy) and absence of any metabolic, gastrointestinal or chronic diseases |
| Interventions | Administration of iron‐FFR to all participants; participants will act as their own controls and each participant will receive all foreseen treatments/interventions |
| Outcomes | Fractional iron absorption, Hb, plasma ferritin, C‐reactive protein concentrations, zinc protoporphyrin measurement as zinc to heme ratio, soluble Transferrin receptor |
| Starting date | April 2016 |
| Contact information | Prof. Dr. Michael B Zimmermann Zurich, Switzerland, Tel +41 44 632 86 57 |
| Notes | Sponsors: Swiss Federal Institute of Technology, Switzerland and University for Development Studies, Tamale, Ghana. |
| Trial name or title | Efficacy of vitamin A fortified rice in lactating Thai women (EVAL) |
| Methods | 2‐arm interventions, vitamin A‐FFR and non‐FFR with 35 participants (lactating women) per group |
| Participants | 70 healthy lactating Thai women 18‐38 years of age, with gestational age between 37 to 42 weeks, willing to breast feed for a period of 4‐6 months. Women with Hb concentrations < 70 gt/L, postpartum haemorrhage, twin birth, or low birth weight infant, or receiving vitamin A supplements during the study period will be excluded. |
| Interventions | Participants will receive 1 meal/day on weekdays for a period of 14 weeks and will be randomly assigned to receive "normal" rice 1 meal/day on weekdays for a period of 14 weeks or vitamin A‐FFR 1 meal/day. The fortified dose will be measured in the final cooked product, but we will aim to administer approximately 500 µg retinol in the rice meal. Vitamin A‐FFR rice is produced by extrusion technology by adding retinol into an artificial rice to be mixed with the normal rice. |
| Outcomes | Vitamin A liver stores, breast milk retinol, serum retinol, Hb concentration, gut microbiota |
| Starting date | 15 March 2017 |
| Contact information | Dr Siwaporn Pinkaew, Prince of Songkla University Tel +66 89 9772266 Email: [email protected] |
| Notes | Sponsor: Prince of Songkla University |
FFR: fortified rice; Hb: haemoglobin
Differences between protocol and review
We aimed at searching two databases in the protocol that were not available to us at the review stage: BIOSIS and Food Science and Technology Abstracts (FSTA). We also made the following changes from the published protocol ( Ashong 2012 ).
- We removed the subgroup analysis by prevalence of stunting in children under five years in the study site: moderate (number of children whose height‐for‐age Z‐score is between −2.0 and 2.99 standard deviations (SD) below the mean) versus severe stunting (number of children whose height‐for‐age Z‐score is less than −3.0 SD below the mean) versus mixed/unknown/unreported. This information was not available in most studies and not all studies were conducted in children. The most commonly used form of reporting was by mean Z‐score in each group rather than the prevalence.
- We removed the subgroup by iron compound as most studies used micronized pyrophosphate and ferrous sulphate, which have similar bioavailability with differences varying depending on encapsulation, particle size and other factors not captured in these studies.
- Given the different ways that diarrhoea was reported, we changed our protocol definition of three liquid stools in a single day, to any as defined by study authors in order to be able to capture this adverse effect, if present.
- We have included any adverse effects to primary outcomes. They are reported separately.
- We did not consider before‐and‐after studies without a control group for inclusion in this review as planned in protocol.
- We did not include data from non‐randomised studies in the quantitative synthesis and the review conclusions, since these studies provided little information on the comparative effectiveness of rice fortification in improving nutrition. However, we did include them in the qualitative synthesis because of their details on contextual aspects and concepts of fortification. We mentioned their findings under the subheading 'Summary of non‐randomised studies' at the end of the main synthesis.
- We have made the change from the proposed type of participants in the protocol being general population of all age groups (including pregnant women) from any country to general population older than two years of age (including pregnant women) from any country.
- We planned to examine the effects of two or more nutrients (in the same study arm) in multiple comparisons. However, owing to the duplication of findings because most of the studies had iron as one of the micronutrients in the fortification arm, we included multi nutrient studies in comparison 1 only and since one study had vitamin A only in one of the arms, we included it in comparison 2.
Contributions of authors
Protocol development: Joseph Ashong drafted an initial protocol with technical input from Sumithra Muthayya, Arnaud Laillou, Luz Maria De‐Regil and Juan Pablo Pena‐Rosas. Luz Maria De‐Regil, Belinda Burford and Juan Pablo Pena‐Rosas developed the methods of the protocol. All authors provided input and contributed to drafting the final version of the protocol.
Review development: all authors contributed to screening, extraction and assessment of data, as described in the Methods . MNGC, JPP and LMD prepared the GRADE 'Summary of findings' tables. All the review authors wrote and approved the final manuscript.
Juan Pablo Peña‐Rosas is the guarantor for the review.
Disclaimer: Juan Pablo Peña‐Rosas and Maria Nieves Garcia‐Casal are full‐time staff members of the World Health Organization. The review authors alone are responsible for the views expressed in this publication and they do not necessarily represent the official position, decisions, policy or views of the World Health Organization.
Sources of support
Internal sources.
- Centre for Health Innovation and Partnership, NSW Health, North Parramatta, Australia.
- Department of Nutrition for Health and Development, World Health Organization, Switzerland.
External sources
WHO provided partial financial support from the Department of Nutrition for Health and Development for this work for some of the authors of this review.
WHO thanks GAIN for their financial support for conducting systematic reviews on micronutrient interventions. SM received partial financial support from GAIN during the preparation of the protocol.
The National Center for Birth Defects and Developmental Disabilities provided financial support for conducting this systematic review.
WHO thanks Nutrition International for their financial support for conducting systematic reviews on micronutrient interventions.
WHO acknowledges the financial support from the Bill & Melinda Gates Foundation for the development of systematic reviews of the evidence on the effects of nutrition interventions.
Declarations of interest
Juan Pablo Peña‐Rosas (JPP) co‐ordinates the Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, which received financial resources from the Bill & Melinda Gates Foundation (2013‐2019); US Centers for Disease Control and Prevention (CDC) (2014‐2019); Nutrition International (2014‐2019) and USAID (2014‐2019). Donors do not fund specific guidelines and do not participate in any decision related to the guideline development process including the composition of policy questions, membership of the guideline groups, the conduct and interpretation of systematic reviews, or the formulation of recommendations.
Prasanna Mithra (PM) received partial financial support from the Department of Nutrition for Health and Development, World Health Organization for this work.
B Unnikrishnan (BUK) received partial financial support from the Department of Nutrition for Health and Development, World Health Organization for this work.
Nithin Kumar (NK) received partial financial support from the Department of Nutrition for Health and Development, World Health Organization for this work.
Luz Maria De‐Regil (LMD) was a full‐time staff member of Nutrition International (formerly Micronutrient Initiative), an international not‐for‐profit organisation that delivers nutrition interventions to children, women of reproductive age and pregnant women. Nutrition International supports the implementation of large‐scale research projects on food fortification. None of them met the inclusion criteria of this review. Nutrition International is a partner of the Food Fortification Initiative and receives funds from the Canadian Department of Foreign Affairs. LMD was an Editor for the Cochrane Developmental, Psychosocial and Learning Problems Group.
Sreekumar Nair (SN) received partial financial support from the Department of Nutrition for Health and Development, World Health Organization for this work.
Maria Nieves Garcia‐Casal (MNG) is a scientist in the Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, which received financial resources from the Bill & Melinda Gates Foundation (2013‐2019); US Centers for Disease Control and Prevention (CDC) (2014‐2019); Nutrition International (2014‐2019) and USAID (2014‐2019). Donors do not fund specific guidelines and do not participate in any decision related to the guideline development process including the composition of policy questions, membership of the guideline groups, the conduct and interpretation of systematic reviews, or the formulation of recommendations.
Juan Antonio Solon (JAS) is president of the Nutrition Center of the Philippines, a non‐profit organisation working towards nutrition security (www.ncp.org.ph). He received partial financial support for this work. His involvement with the review was at the final stage where he helped with the interpretation of the results.
References to studies included in this review
Angeles‐agdeppa 2008 {published data only}.
- Angeles‐Agdeppa I, Capanzana MV, Barba CV, Florentino RF, Takanashi K. Efficacy of iron‐fortified rice in reducing anemia among schoolchildren in the Philippines . International Journal for Vitamin and Nutrition Research 2008; 78 ( 2 ):74‐86. [ PubMed ] [ Google Scholar ]
Ara 2019 {published data only}
- Ara G, Khanam M, Rahman AS, Islam Z, Farhad S, Sanin KI, et al. Effectiveness of micronutrient‐fortified rice consumption on anaemia and zinc status among vulnerable women in Bangladesh . PLoS ONE 2019; 14 ( 1 ):e0210501. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Della Lucia 2016 {published data only}
- Della Lucia CM, Rodrigues KC, Rodrigues VC, Santos LL, Cardoso LM, Martino HS, et al. Diet quality and adequacy of nutrients in preschool children: should rice fortified with micronutrients be Included in school meals? . Nutrients 2016; 8 ( 5 ):1‐16. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Della Lucia CM, Santos LL, Silva BP, Anuncuiacao PC, Goncalvez Alfenas R, Castro Franceschini S, et al. Impact of rice fortified with iron, zinc, thiamine and folic acid on laboratory measurements of nutritional status of preschool children [Impacto do arroz fortificado com ferro, zinco, tiamina e ácido fólico sobre as medidas laboratoriais do estado nutricional de crianças pré‐escolares]. Ciencia & Saude Coletiva 2016; 22 ( 2 ):583‐92. [ Google Scholar ]
Gershoff 1977 {published data only}
- Gershoff SN, McGandy RB, Suttapreyasri D, Promkutkao C, Nondasuta A, Pisolyabutra U, et al. Nutrition studies in Thailand. II. Effects of fortification of rice with lysine, threonine, thiamin, riboflavin, vitamin A, and iron on preschool children . American Journal of Clinical Nutrition 1977; 30 ( 7 ):1185‐95. [ PubMed ] [ Google Scholar ]
- Gershoff SN, McGandy RB, SuttaPreyasri D, Nondasuta A, Pisolyabutra U, Tantiwongse P. Amino acid fortification of rice studies in Thailand. 1. background and baseline data . American Journal of Clinical Nutrition 1975; 28 ( 2 ):170‐82. [ PubMed ] [ Google Scholar ]
Hardinsyah 2016 {published data only}
- Hardinsyah I, Briawan D, Budianto S, Hustina P, Ghifari N, Suhandono S. Production and clinical impact study of micronutrients fortified rice for teen girls in Islamic boarding school in Medan, Indonesia. Final report . https://snrd‐asia.org/download/better_rice_initiative_asia_bria/CLINICAL‐IMPACT‐STUDY‐OF‐MICRONUTRIENTS‐FORTIFIED‐RICE‐IN‐INDONESIA.pdf (accessed 9 October 2019) . West Java: Food and Nutrition Society of Indonesia (Perhizi Pangan Indonesia), 2016.
Hotz 2008 {published data only}
- Hotz C, Porcayo M, Onofre G, Garcia‐Guerra A, Elliott T, Jankowski S, et al. Efficacy of iron‐fortified Ultra Rice in improving the iron status of women in Mexico . Food and Nutrition Bulletin 2008; 29 ( 2 ):140‐9. [ PubMed ] [ Google Scholar ]
Hussain 2014 {published data only}
- Hussain SZ, Singh B, Rather AH. Efficacy of micronutrient fortified extruded rice in improving the iron and vitamin A status in Indian schoolchildren . International Journal of Agriculture and Food Science Technology 2014; 5 ( 3 ):227‐38. [ Google Scholar ]
Losso 2017 {published data only}
- Greenway F. Pilot study of the effect of iron fortified rice in iron deficient anemic women . clinicaltrials.gov 2012; Vol. (accessed 19 July 2017).
- Losso JN, Karki N, Muyonga J, Wu Y, Fusilier K, Jacob G, et al. Iron retention in iron‐fortified rice and use of iron‐fortified rice to treat women with iron deficiency: a pilot study . BBA Clinical 2017; 8 :78‐83. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Moretti 2006b {published data only}
- Moretti D, Lee TC, Zimmermann MB, Nuessli J, Hurrell RF. Development and evaluation of iron‐fortified extruded rice grains . Journal of Food Science 2005; 70 ( 5 ):S330–S336. [ Google Scholar ]
- Moretti D, Zimmermann MB, Muthayya S, Thankachan P, Lee TC, Kurpad AV, et al. Extruded rice fortified with micronized ground ferric pyrophosphate reduces iron deficiency in Indian schoolchildren: a double‐blind randomized controlled trial . American Journal of Clinical Nutrition 2006; 84 ( 4 ):822‐9. [ PubMed ] [ Google Scholar ]
- Zimmermann MB, Muthayya S, Moretti D, Kurpad A, Hurrell RF. Iron fortification reduces blood lead levels in children in Bangalore, India . Pediatrics 2006; 117 ( 6 ):2014‐21. [ PubMed ] [ Google Scholar ]
Nogueira Arcanjo 2013 {published data only}
- Nogueira Arcanjo FP, Santos PR, Segall S. Ferric pyrophosphate fortified rice given once weekly does not increase hemoglobin levels in preschoolers . Journal of Rice Research 2013; 1 ( 2 ):1‐6. [ Google Scholar ]
Parker 2015 (C) {published data only}
- Parker ME, Mosites E, Reider K, Ndayishimiye N, Waring M, et al. A blinded, cluster‐randomized, placebo‐controlled school feeding trial in Burundi using rice fortified with iron, zinc, thiamine, and folic acid . Food and Nutrition Bulletin 2015; 36 ( 4 ):481‐92. [ PubMed ] [ Google Scholar ]
Perignon 2016 (C) {published data only}
- Gier B, Campos Ponce M, Perignon M, Fiorentino M, Khov K, Chamnan C, et al. Micronutrient‐fortified rice can increase hookworm infection risk: a cluster randomized trial . PLoS One 2016; 11 ( 1 ):e0145351. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fiorentino M, Perignon M, Kuong K, Groot R, Parker M, Burja K, et al. Effect of multi‐micronutrient‐fortified rice on cognitive performance depends on premix composition and cognitive function tested: results of an effectiveness study in Cambodian schoolchildren . Public Health Nutrition 2018; 21 ( 4 ):816‐27. [DOI: 10.1017/S1368980017002774] [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kuong K, Fiorentino M, Perignon M, Chamnan C, Parker M, Burja K, et al. What is the right indicator to evaluate impact of interventions? Examples from a rice fortification trial in Cambodia . European Journal of Nutrition & Food Safety 2015; Vol. 5, issue 5:760‐61.
- Perignon M, Fiorentino M, Kuong K, Burja K, Parker M, Sisokhom S, et al. Stunting, poor iron status and parasite infection are significant risk factors for lower cognitive performance in Cambodian school‐aged children . PLoS One 2014; 9 ( 11 ):e112605. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Perignon M, Fiorentino M, Kuong K, Dijkhuizen MA, Burja K, Parker M, et al. Impact of multi‐micronutrient fortified rice on hemoglobin, iron and vitamin A status of Cambodian schoolchildren: a double‐blind cluster‐randomized controlled trial . Nutrients 2016; 8 ( 1 ):E29. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Pinkaew 2013 {published data only}
- Pinkaew S, Winichagoon P, Hurrell RF, Wegmuller R. Extruded rice grains fortified with zinc, iron, and vitamin A increase zinc status of Thai school children when incorporated into a school lunch program . Journal of Nutrition 2013; 143 ( 3 ):362‐8. [ PubMed ] [ Google Scholar ]
Pinkaew 2014 {published data only}
- Pinkaew S, Wegmuller R, Wasantwisut E, Winichagoon P, Hurrell RF, Tanumihardjo SA. Triple‐fortified rice containing vitamin A reduced marginal vitamin A deficiency and increased vitamin A liver stores in school‐aged Thai children . Journal of Nutrition 2014; 144 ( 4 ):519‐24. [ PubMed ] [ Google Scholar ]
Radhika 2011 {published data only}
- Radhika MS, Nair KM, Kumar RH, Rao MV, Ravinder P, Reddy CG, et al. Micronized ferric pyrophosphate supplied through extruded rice kernels improves body iron stores in children: a double‐blind, randomized, placebo‐controlled midday meal feeding trial in Indian schoolchildren . American Journal of Clinical Nutrition 2011; 94 ( 5 ):1202‐10. [ PubMed ] [ Google Scholar ]
Salcedo 1950 {published data only}
- Salcedo J Jr, Bamba MD, Carrasco EO, Chan GS, Concepcion I, Jose FR, et al. Artificial enrichment of white rice as a solution to endemic beriberi: report of field trials in Bataan, Philippines . Journal of Nutrition 1950; 42 ( 4 ):501‐23. [ PubMed ] [ Google Scholar ]
- Salcedo J Jr, Concepcion I. Studies on the thiamine content of rice, vegetables, and other foods in Bataan Province . Acta Medica Philippina 1949; 5 ( 4 ):1‐19. [ PubMed ] [ Google Scholar ]
- Salcedo J Jr, Pedroche A, Panganiban EC, DeLeon JF. Artificial enrichment of white rice as a solution to endemic beriberi; preliminary report of field trials . Journal of Nutrition 1949; 38 ( 4 ):443‐51. [ PubMed ] [ Google Scholar ]
Thankachan 2012 {published data only}
- Thankachan P, Rah JH, Thomas T, Selvam S, Amalrajan V, Srinivasan K, et al. Multiple micronutrient‐fortified rice affects physical performance and plasma vitamin B‐12 and homocysteine concentrations of Indian school children . Journal of Nutrition 2012; Vol. 142, issue 5:846‐52. [ PubMed ]
References to studies excluded from this review
Ando 2012 {published data only}.
- Ando S, Sekine S, Murosaki S. Folate deficiency among aged patients and its amelioration through consumption of folic acid‐fortified rice . 文京学院大学人間学部研究紀 2012; 13 ( 3 ):123‐37. [ Google Scholar ]
Angeles‐Agdeppa 2011 {published data only}
- Angeles‐Agdeppa I, Saises M, Capanzana M, Juneja LR, Sakaguchi N. Pilot‐scale commercialization of iron‐fortified rice: effects on anemia status . Food and Nutrition Bulletin 2011; 32 ( 1 ):3‐12. [ PubMed ] [ Google Scholar ]
- Capanzana MV, Angeles‐Agdeppa IT. Social marketing efforts for the promotion of iron‐fortified rice: an ongoing study in Bataan . Annals of Nutrition and Metabolism . Basel (Switzerland): Karger International, 2009; Vol. 55:167‐8. [https://doi.org/10.1159/000248282]
Arsenault 2010 {published data only}
- Arsenault JE, Yakes EA, Hossain MB, Islam MM, Ahmed T, Hotz C, et al. The current high prevalence of dietary zinc inadequacy among children and women in rural Bangladesh could be substantially ameliorated by zinc biofortification of rice . Journal of Nutrition 2010; 140 ( 9 ):1683‐90. [ PubMed ] [ Google Scholar ]
- Arsenault JE, Yakes EA, Lewis B, Islam MM, Hossain MB, Ahmed T, et al. Potential impact of zinc‐biofortified rice on zinc intakes of young Bangladeshi children . FASEB Journal (Meeting Abstract Supplement) 334.4 2010; Vol. 24.
Bagni 2009 {published data only}
- Bagni UV, Baião MR, Santos MM, Luiz RR, Veiga GV. Effect of weekly rice fortification with iron on anemia prevalence and hemoglobin concentration among children attending public daycare centers in Rio de Janeiro, Brazil [Efeito da fortificaçao semanal do arroz com ferro quelato sobre a frequencia de anemia e concentraçao de hemoglobina em crianças de creches municipais do Rio de Janeiro, Brasil]. Cadernos De Saude Publica 2009; 25 ( 2 ):291‐302. [ PubMed ] [ Google Scholar ]
Barboza 2011 {published data only}
- Barboza Argüello MP, Umaña Solís LM. Impact of the fortification of food with folic acid on neural tube defects in Costa Rica [Impacto de la fortificación de alimentos con ácido fólico en los defectos del tubo neural en Costa Rica]. Revista Panamericana de Salud Publica 2011; 30 ( 1 ):1‐6. [ PubMed ] [ Google Scholar ]
Beinner 2010 {published data only}
- Beinner MA, Soares AD, Barros AL, Monteiro MA. Sensory evaluation of rice fortified with iron . Ciencia e Tecnologia De Alimentos 2010; 30 ( 2 ):516‐9. [ Google Scholar ]
- Beinner MA, Velasquez‐Meléndez G, Pessoa MC, Greiner T. Iron‐fortified rice is as efficacious as supplemental iron drops in infants and young children . Journal of Nutrition 2010; 140 ( 1 ):49‐53. [ PubMed ] [ Google Scholar ]
Castro 2017 {published data only}
- Castro LC, Brunoro CN, Pinheiro SH, Fortes LC, Castro FS. Improvement the nutritional status of pre‐school children following intervention with a supplement containing iron, zinc, copper, vitamin A, vitamin C and prebiotic . Ciência & Saúde Coletiva 2017; 22 ( 2 ):359‐68. [ Google Scholar ]
Finkelstein 2013 {published data only}
- Filkenstein J, Mehta S, Haas JD. Iron fortification interventions: synthesis of results from three trials in Asia . Annals of Nutrition and Metabolism 2013; 63 ( Suppl 1 ):1‐1960. [ Google Scholar ]
Florentino 1998 {published data only}
- Florentino RF, Pedro MR. Update on rice fortification in the Philippines . Food and Nutrition Bulletin 1998; 19 ( 2 ):149‐53. [ Google Scholar ]
Graham 2007 {published data only}
- Graham JM, Haskell MJ, Pandey P, Shrestha RK, Brown KH. Supplementation with iron and riboflavin enhances dark adaptation response to vitamin A ‐‐ fortified rice in iron‐deficient, pregnant, nightblind Nepali women . American Journal of Clinical Nutrition 2007; 85 ( 5 ):1375‐84. [ PubMed ] [ Google Scholar ]
- Graham JM, Pandey P, Haskell MJ, Shrestha RK, Peerson JM, Brown KH, et al. Riboflavin plus iron supplementation did not enhance improvement of dark adaptation or retinol in night blind pregnant Nepali women receiving vitamin A‐fortified Ultra‐Rice (TM) . FASEB Journal 2003; 17 ( 5 ):A1101‐2. [ Google Scholar ]
- Haskell MJ, Pandey P, Graham JM, Peerson JM, Shresthra RK, Brown KH. Response of plasma retinol and carotenoid concentrations of nightblind, pregnant Nepalese women to daily supplementation with locally available vitamin A‐rich foods or vitamin A‐fortified Ultra‐Rice (TM) . FASEB Journal 2002; 16 ( 4 ):A626. [ Google Scholar ]
Haskell 2005 {published data only}
- Haskell MJ, Pandey P, Graham JM, Peerson JM, Shrestha RK, Brown KH. Recovery from impaired dark adaptation in nightblind pregnant Nepali women who receive small daily doses of vitamin A as amaranth leaves, carrots, goat liver, vitamin A‐fortified rice, or retinyl palmitate . American Journal of Clinical Nutrition 2005; 81 ( 2 ):461‐71. [ PubMed ] [ Google Scholar ]
Huo 2014 {published data only}
- Huo J, Sun J, Huang J, Wang J, Li W, Wang B. School food fortification improves nutrition status of students from poor migrant . Journal of Food and Nutritional Disorders 2014; 3 :2. [ Google Scholar ]
Hyun 2015 {published data only}
- Hyun T, Kim NJ, Kim JH, Jang YA, Kim K, Lee Y. The effects of folic acidfortified food and nutrition education on folate nutritional status and behavioral capability in Korean women of childbearing age . FASEB Journal 2015; 29 ( 1 Supplement ):LB331. [ Google Scholar ]
Kagawa 2017 {published data only}
- Kagawa Y, Hiraoka M, Kageyama M, Kontai Y, Yurimoto M, Nishijima C, et al. Medical cost savings in Sakado City and worldwide achieved by preventing disease by folic acid fortification . Congenital Anomalies 2017; Vol. 57, issue 5:157‐65. [ PMC free article ] [ PubMed ]

Ma 2016 {published data only}
- Ma J, Sun Q, Liu J, Hu Y, Liu S, Zhang J, et al. The effect of iron fortification on iron (Fe) status and inflammation: a randomized controlled trial . Plos One 2016; 11 ( 12 ):e0167458. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Nogueira Arcanjo 2012 {published and unpublished data}
- Nogueira Arcanjo FP, Santos PR, Costa Arcanjo CP, Silverio Amancio OM, Pellegrini Braga JA. Use of iron‐fortified rice reduces anemia in infants . Journal of Tropical Pediatrics 2012; 58 ( 6 ):475‐80. [ PubMed ] [ Google Scholar ]
- Nogueira Arcanjo FP, Santos PR, Madeiro Leite AJ, Bastos Mota FS, Duarte Segall S. Rice fortified with iron given weekly increases hemoglobin levels and reduces anemia in infants: a community intervention trial . International Journal for Vitamin and Nutrition Research. Internationale Zeitschrift fur Vitamin‐ und Ernahrungsforschung. Journal International de Vitaminologie et de Nutrition 2013; 83 ( 1 ):59‐66. [ PubMed ] [ Google Scholar ]
Pham 2012 {published data only}
- Pham VP, Nguyen VH, Salvignol B, Treche S, Wieringa FT, Dijkhuizen MA, et al. A six‐month intervention with two different types of micronutrient‐fortified complementary foods had distinct short‐ and long‐term effects on linear and ponderal growth of Vietnamese infants . Journal of Nutrition 2012; 142 ( 9 ):1735‐40. [ PubMed ] [ Google Scholar ]
Pham Van 2013 {published data only}
- Pham Van T, Thi H, Nakanishi Y. Efficacy of iron fortified rice in Vietnam . Annals of Nutrition and Metabolism 2013; 63 ( suppl 1 ):1040. [ Google Scholar ]
Skau 2015 {published data only}
- Skau JK, Touch B, Chhoun C, Chea M, Unni US, Makurat J, et al. Effects of animal source food and micronutrient fortification in complementary food products on body composition, iron status, and linear growth: a randomized trial in Cambodia . American Journal of Clinical Nutrition 2015; 101 ( 4 ):742‐51. [ PubMed ] [ Google Scholar ]
Sridevi 2013 {published data only}
- Sridevi D, Radhaisri S. Impact of micronutrient fortified food supplement on nutritional profile among ginning women workers . International Journal of Research in Ayurveda & Pharmacy 2013; 4 ( 4 ):526‐9. [ Google Scholar ]
Vitolol 1998 {published data only}
- VitoloI MR, Nogueira de Campo A, KondoIII MR, Giuliano Y, Ferreira N, Lopez FA. Effect of the use of iron‐enriched cereal on the serum hemoglobin levels and anthropometric values of preschool children [Impacto do uso de cereal adicionado de ferro sobre os níveis de hemoglobina e a antropometria de pré‐escolares]. Revista de Nutrição, Campinas 1998; 11 ( 2 ):163‐71. [ Google Scholar ]
Walter 1993 {published data only}
- Walter T, Dallman PR, Pizarro F, Velozo L, Pena G, Bartholmey SJ, et al. Effectiveness of iron‐fortified infant cereal in prevention of iron‐deficiency anemia . Pediatrics 1993; 91 ( 5 ):976‐82. [ PubMed ] [ Google Scholar ]
References to ongoing studies
Nct02714075 {published data only}.
- NCT02714075. Comparison of iron absorption from extruded FePP‐fortified rice containing zinc, citric acid/trisodium citrate and EDTA (MM_Rice) . clinicaltrials.gov 2016 (accessed 09 October 2019).
NCT03056625 {published data only}
- NCT03056625. Efficacy of vitamin A fortified rice in lactating Thai women (EVAL) . clinicaltrials.gov September 2017 (accessed 09 October 2019).
Additional references
A2z project 2008.
- A2Z Project. Rice fortification in developing countries: a critical review of the technical and economic feasibility . https://www.spring‐nutrition.org/sites/default/files/a2z_materials/508‐food‐rice‐fortification‐report‐with‐annexes‐final.pdf (accessed 10 October 2019) . Washington DC: Academy for Educational Development, 2008.
- Adams G, Gulliford MC, Ukoumunne OC, Eldridge S, Chinn S, Campbell MJ. Patterns of intra‐cluster correlation from primary care research to inform study design and analysis . Journal of Clinical Epidemiology 2004; 57 :785‐94. [ PubMed ] [ Google Scholar ]
Alderman 2007
- Alderman H, Horton S. The economics of addressing nutritional anemia . In: Kraemer K, Zimmermann MB editor(s). Nutritional Anemia . Basel: Sight and Life Press, 2007:19‐35. [ Google Scholar ]
American Rice Inc 2004
- American Rice Inc. Nutritional Information . www.amrice.com/6‐4.cfm 2004.
Andersson 2012
- Andersson M, Karumbunathan V, Zimmermann MB. Global iodine status in 2011 and trends over the past decade . Journal of Nutrition 2012; 142 ( 4 ):744‐50. [ PubMed ] [ Google Scholar ]
Bailey 2015
- Bailey LB, Stover PJ, McNulty H, Fenech MF, Gregory JF 3rd, Mills JL, et al. Biomarkers of nutrition for development‐ folate review . Journal of Nutrition 2015; 145 ( 7 ):1636S‐1680S. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: Rating the quality of evidence . Journal of Clinical Epidemiology 2011; 64 ( 4 ):401‐6. [ PubMed ] [ Google Scholar ]
Baltussen 2004
- Baltussen R, Knai C, Sharan M. Iron fortification and iron supplementation are cost‐effective interventions to reduce iron deficiency in four subregions of the world . Journal of Nutrition 2004; 134 ( 10 ):2678‐84. [ PubMed ] [ Google Scholar ]
Bandeira 2006
- Bandeira F, Griz L, Dreyer P, Eufrazino C, Bandeira C, Freese E. Vitamin D deficiency: a global perspective [Deficiência de vitamina D: uma perspectiva global]. Arquivos Brasileiros de Endocrinologia e Metabologia 2006; 50 ( 4 ):640‐6. [ PubMed ] [ Google Scholar ]
- Berry RJ, Bailey L, Mulinare J, Bower C. Folic acid working group. Fortification of flour with folic acid . Food and Nutrition Bulletin 2010; 31 ( Suppl 1 ):22‐35. [ PubMed ] [ Google Scholar ]
- Best C, Neufingerl N, Rosso JM, Transler C, Briel T, Osendarp S. Can multi‐micronutrient food fortification improve the micronutrient status, growth, health, and cognition of schoolchildren? A systematic review . Nutrition Reviews 2011; 69 ( 4 ):186–204. [ PubMed ] [ Google Scholar ]
Bhaskaram 2002
- Bhaskaram P. Micronutrient malnutrition, infection, and immunity: an overview . Nutrition Reviews 2002; 60 ( 5 Pt 2 ):4‐45. [ PubMed ] [ Google Scholar ]
- Black RE. Micronutrient deficiency‐‐an underlying cause of morbidity and mortality . Bulletin of the World Health Organization 2003; 81 ( 2 ):79. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, Onis M, Ezzati M, et al. Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: global and regional exposures and health consequences . Lancet 2008; 371 ( 9608 ):243‐60. [ PubMed ] [ Google Scholar ]
Borenstein 2008
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to Meta‐Analysis (Statistics in Practice) . Chichester (UK): John Wiley & Sons, 2008. [ Google Scholar ]
- Brown KH, Wessells KR, Hess SY. Zinc bioavailability from zinc‐fortified foods . International Journal for Vitamin and Nutrition Research 2007; 77 :174‐81. [ PubMed ] [ Google Scholar ]
Carter 2010
- Carter RC, Jacobson JL, Burden MJ, Armony‐Sivan R, Dodge NC, Angelilli ML, et al. Iron deficiency anemia and cognitive function in infancy . Pediatrics 2010; 126 ( 2 ):e427‐34. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Centers for Disease Control and Prevention. Criteria for anemia in children and childbearing‐age women. . Morbidity and Mortality Weekly Report 1989; Vol. 38, issue ‐:400‐04. [ PubMed ]
- Centers for Disease Control and Prevention . Trends in wheat‐flour fortification with folic acid and iron . Morbidity and Mortality Weekly Report 2008; 57 ( 01 ):8‐10. [ PubMed ] [ Google Scholar ]
Centeno Tablante 2019
- Centeno Tablante E, Pachón H, Guetterman HM, Finkelstein JL. Fortification of wheat and maize flour with folic acid for population health outcomes . Cochrane Database of Systematic Reviews 2019, Issue 7 . [DOI: 10.1002/14651858.CD012150.pub2] [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chen H, Siebenmorgen T, Griffin K. Quality characteristics of long‐grain rice milled in two commercial systems . Cereal Chemistry 1998; 75 ( 4 ):560‐5. [ Google Scholar ]
Cochrane PHG 2010
- Cochrane Public Health Group. Guide for developing a Cochrane Protocol, Version 2 . The Cochrane Collaboration. Public Health Group, 2010. [ Google Scholar ]
Codex Alimentarius 1994
- Codex Alimentarius. In: FAO/WHO editor(s). Codex general principles for the addition of essential nutrients to foods. CAC/GL 09‐1987 (as amended 1989, 1991) . Second Edition. Vol. 4 , Rome: Joint FAO/WHO Food Stardards Programme, Codex Alimentarius Commission, 1994. [ Google Scholar ]
Darnton‐Hill 2005
- Darnton‐Hill I, Webb P, Harvey PW, Hunt JM, Dalmiya N, Chopra M, et al. Micronutrient deficiencies and gender: social and economic costs . American Journal of Clinical Nutrition 2005; 81 ( 5 ):1198S‐1205S. [ PubMed ] [ Google Scholar ]
- Das JK, Salam RA, Kumar R, Bhutta ZA. Micronutrient fortification of food and its impact on woman and child health: a systematic review . Systematic Reviews 2013; 2 :67. [ PMC free article ] [ PubMed ] [ Google Scholar ]
De Pee 2017
- Pee S, Fabrizio C, Rosenzweig J. Overview of evidence and recommendations for effective large‐scale rice fortification . In: Laura Irizarry, Marc‐André Prost, Diana Murillo editor(s). Scaling up rice fortification in Latin America and the Caribbean (sightandlife.org/wp‐content/uploads/2017/04/Scaling‐Up‐Rice‐Fortification‐WFP‐Rice‐Fortification‐ENG.pdf, accessed 10 October 2019) . Basel: Sight and Life and World Food Programme, 2017:143‐49. [ Google Scholar ]
De‐Regil 2015
- De‐Regil LM, Peña‐Rosas JP, Fernández‐Gaxiola AC, Rayco‐Solon P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects . Cochrane Database of Systematic Reviews 2015, Issue 12 . [DOI: 10.1002/14651858.CD007950.pub3] [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017 . Available from www.training.cochrane.org/handbook .
Dexter 1998
- Dexter PB. Rice fortification for developing countries . www.mostproject.org/PDF/rice4.pdf . Fayetteville, AR: OMNI/USAID, (accessed 7 May 2011). [ Google Scholar ]
DSM/Buhler 2010
- DSM Nutritional Products Ltd/Buhler AG. Nutririce process: a breakthrough in rice fortification . www.buhlergroup.com/global/downloads/NutriRice_Process_EN.pdf 2010:1‐8.
- European Food Safety Authority. Folic acid: an update on scientific developments . www.efsa.europa.eu/en/home/publication/efsafolicacid.pdf . Parma: European Food Safety Authority, 2009.
- Cochrane Effective Practice, Organisation of Care (EPOC). EPOC Resources for review authors, 2017 . Available from epoc.cochrane.org/epoc‐resources‐review‐authors .
- Food, Agriculture Organization of the United Nations. FAO Statistical Yearbook. World Food and Agriculture . www.fao.org/docrep/015/i2490e/i2490e00.htm . Rome: Food and Agriculture Organization of the United Nations, 2012.
- FAO. Food Outlook June 2016. Biannual Report on Global Food Markets . Food Outlook (accessed October 2016).
FAOSTAT 2016
- FAO Statistics Division. FAOSTAT Database . www.fao.org (accessed October 2016).
- Code of Federal Regulations. 137.350 Enriched rice . Title 21‐‐Food and Drugs. Chapter 1‐‐Food and Drug Administration, Department of Health and Human Services. Part 137 cereals flours and related products . Vol. (4‐1‐01 Edition) , Washington DC: United States Government, 2001. [ Google Scholar ]
Flores 1994
- Flores H, Guerra NB, Cavalcanti A, Campos F, Azevedo M, Silva M. Bioavailability of vitamin A in a synthetic rice premix . Journal of Food Science 1994; 59 ( 2 ):371‐2. [ Google Scholar ]
- Global Alliance for Improved Nutrition. RiFoRG. Rice Fortification Frequently Asked Questions . www.gainhealth.org/riforg/sites/default/files/RiFoRG_FAQs_Jan%202011.pdf 2010.
Garcia‐Casal 2018
- Garcia‐Casal MN, Peña‐Rosas JP, De‐Regil LM, Gwirtz JA, Pasricha SR. Fortification of maize flour with iron for controlling anaemia and iron deficiency in populations . Cochrane Database of Systematic Reviews 2018, Issue 12 . [DOI: 10.1002/14651858.CD010187.pub2] [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Garcia‐Casal 2019
- Garcia‐Casal MN, Mowson R, Rogers L, Grajeda R, Consultation working groups. Risk of excessive intake of vitamins and minerals delivered through public health interventions: objectives, results, conclusions of the meeting, and the way forward . Annals of the New York Academy of Sciences 2019; 1446 ( 1 ):5‐20. [ PubMed ] [ Google Scholar ]
- Gera T, Sachdev HS, Boy E. Effect of iron‐fortified foods on hematologic and biological outcomes: systematic review of randomized controlled trials . American Journal of Clinical Nutrition 2012; 96 ( 2 ):309‐24. [ PubMed ] [ Google Scholar ]
Gibson 1998
- Gibson RS, Ferguson EL. Nutrition intervention strategies to combat zinc deficiency in developing countries . Nutrition Research Reviews 1998; 11 ( 1 ):115‐31. [ PubMed ] [ Google Scholar ]
Gordeuk 1987
- Gordeuk VR, Bacon BR, Brittenham GM. Iron overload: causes and consequences . Annual Review of Nutrition 1987; 7 ( 1 ):485‐508. [ PubMed ] [ Google Scholar ]
GRADEpro GDT 2015 [Computer program]
- McMaster University. GRADEpro GDT:GRADEpro Guideline Development Tool . McMaster University, 2015 (developed by Evidence Prime, Inc).
Graham 2001
- Graham RD, Welch RM, Bouis HE. Addressing micronutrient malnutrition through enhancing the nutritional quality of staple foods: principles, perspectives and knowledge gaps . Advances in Agronomy 2001; 70 :77‐142. [ Google Scholar ]
Gulliford 1999
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intra class correlations for the design of community‐based surveys and intervention studies: data from the health survey for England . American Journal of Epidemiology 1999; 149 ( 9 ):876‐83. [ PubMed ] [ Google Scholar ]
- Haas JD, Murdoch S, Rivera J, Martorell R. Early nutrition and later physical work capacity . Nutrition Reviews 1996; 54 ( 2 Pt 2 ):S41‐8. [ PubMed ] [ Google Scholar ]
Hariyadi 2011
- Hariyadi P. Beyond Food Security . www.worldfoodscience.org/cms/?pid=1004751 2011.
- Hess SY, Lönnerdal B, Hotz C, Rivera JA, Brown KH. Recent advances in knowledge of zinc nutrition and human health . Food and Nutrition Bulletin 2009; 30 Suppl ( 1 ):5‐11. [ PubMed ] [ Google Scholar ]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses . BMJ 2003; 327 :557‐60. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Higgins 2011
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011 . Available from www.handbook.cochrane.org .
Higgins 2019
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. . In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Available from www.training.cochrane.org/handbook. . Cochrane, 2019. [ Google Scholar ]
- Hijar G, Aramburu A, Hurtado Y, Suarez V. Fortification of rice to correct micronutrient deficiencies in children 6 to 59 months old [Fortificación del arroz para corregir la deficiencia de micronutrientes en niños de 6 a 59 meses de edad]. Revista Panamericana de Salud Publica 2015; 37 ( 1 ):52‐8. [ PubMed ] [ Google Scholar ]
Howson 1998
- Howson CP, Kennedy ET, Horwitz A, editors. Prevention of micronutrient deficiencies: tools for policy makers and public health workers . Washington, DC: National Academy Press, 1998. [ PubMed ] [ Google Scholar ]
- Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: meta‐analysis and systematic review . BMJ 2012; 344 ( e1454 ):1‐9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Huong TL, Brouwer ID, Burema J, Nguyen KC, Kok FJ. Efficacy of iron fortification compared to iron supplementation among Vietnamese schoolchildren . Nutrition Journal 2006; 5 ( 32 ):1‐8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Hurrell 2010
- Hurrell RF, Egli I. Iron bioavailability and dietary reference values . American Journal of Clinical Nutrition 2010; 91 Suppl :1461–7. [ PubMed ] [ Google Scholar ]
- Bale JR, Stoll BJ, Lucas AO, editors. Reducing Birth Defects: Meeting the Challenge in the Developing World . Washington: National Academies Press, 2003. [ PubMed ] [ Google Scholar ]
- International Rice Research Institute. IRRI World Rice Statistics . beta.irri.org/solutions/index.php?option=com_content&task=view&id=250) (accessed 29 June 2011).
Juliano 1993
- Juliano BO. Rice in Human Nutrition . Rome: Food and Agriculture Organization of the United Nations (FAO), 1993. [ Google Scholar ]
- Khanh Van T, Burja K, Thuy Nga T, Kong K, Berger J, Gardner M, et al. Organoleptic qualities and acceptability of fortified rice in two Southeast Asian countries . Annals of the New York Academy of Sciences 2014; 1324 :48‐54. [ PubMed ] [ Google Scholar ]
- Kim EY, Pai TK, Han O. Effect of bioactive dietary polyphenols on zinc transport across the intestinal Caco‐2 cell monolayers . Journal of Agricultural Food Chemistry 2011; 59 ( 8 ):3606‐12. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Klemm RD, West KP Jr, Palmer AC, Johnson Q, Randall P, Ranum P, et al. Vitamin A fortification of wheat flour: considerations and current recommendations . Food and Nutrition Bulletin 2010; 31 Suppl ( 1 ):47‐61. [ PubMed ] [ Google Scholar ]
Kyritsia 2011
- Kyritsia A, Tziaa C, Karathanos VT. Vitamin fortified rice grain using spraying and soaking methods . Food Science and Technology 2011; 44 ( 1 ):312‐20. [ Google Scholar ]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews . Systematic Reviews 2013; 2 :81. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Leon Guerrero 2009
- Leon Guerrero RT, Gebhardt, SE, Holden J, Kretsch MJ, Todd K, Novotny R, et al. White rice sold in Hawaii, Guam, and Saipan often lacks nutrient enrichment . Journal of the American Dietetic Association 2009; 109 ( 10 ):1738–43. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Lozoff 2006
- Lozoff B, Georgieff MK. Iron deficiency and brain development . Seminars in Pediatric Neurology 2006; 13 ( 3 ):158‐65. [ PubMed ] [ Google Scholar ]
- Mason JB, Dickstein A, Jacque PF, Haggarty P, Selhub J, Dallal G, et al. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis . Cancer Epidemiology, Biomarkers & Prevention 2007; 16 :1325‐9. [ PubMed ] [ Google Scholar ]
- Methodological Expectations of Cochrane Intervention Reviews (MECIR) . methods.cochrane.org/sites/default/files/public/uploads/mecir_printed_booklet_final_v1.02.pdf January 2018.
MIcronutrient Initiative/UNICEF 2004
- Micronutrient Initiative, UNICEF. Vitamin and mineral deficiency: a global progress report . https://www.unicef.org/media/files/vmd.pdf, accessed 10 October 2019 . Ottawa: Micronutrient Intiative, 2004; Vol. ‐, issue ‐:‐. [https://www.unicef.org/media/files/vmd.pdf]
Moretti 2006a
- Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, et al. Global consensus recommendations on prevention and management of nutritional rickets [Global Consensus Recommendations on Prevention and Management of Nutritional Rickets.]. Hormone Research in Paediatrics 2016; 85 ( 2 ):83–106. [ PubMed ] [ Google Scholar ]
Muthayya 2014
- Muthayya S, Sugimoto JD, Montgomery S, Maberly GF. An overview of global rice production, supply, trade, and consumption . Annals of New York Academy of Sciences 2014; 1324 :7‐14. [ PubMed ] [ Google Scholar ]
Muthunayagam 2009
- Muthunayagam NP, Rohrer JE, Wright SE. Correlation of iron and colon adenomas . Gastroenterologie Clinique et Biologique 2009; 33 ( 4 ):435‐40. [ PubMed ] [ Google Scholar ]
Oakley 2004
- Oakley Jr GP, Bell KN, Weber MB. Recommendations for accelerating global action to prevent folic acid–preventable birth defects and other folate deficiency diseases: meeting of experts on preventing folic acid–preventable neural tube defects . Birth Defects Research Part A: Clinical and Molecular Teratology 2004; 70 ( 11 ):835‐7. [ PubMed ] [ Google Scholar ]
- Olney DK, Rawat R, Ruel MT. Identifying potential programs and platforms to deliver multiple micronutrient interventions . Journal of Nutrition 2012; 142 ( 1 ):178S‐85S. [ PubMed ] [ Google Scholar ]
Palacios 2014
- Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? . Journal of Steroid Biochemistry and Molecular Biology 2014; 144 ( Pt A ):138‐45. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Penniston 2003
- Penniston KL, Tanumihardjo SA. Vitamin A in dietary supplements and fortified foods: too much of a good thing? . Journal of the American Dietetic Association 2003; 103 ( 9 ):1185‐7. [ PubMed ] [ Google Scholar ]
Peña‐Rosas 2015
- Peña‐Rosas JP, De‐Regil LM, Garcia‐Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy . Cochrane Database of Systematic Reviews 2015, Issue 7 . [DOI: 10.1002/14651858.CD004736.pub5] [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Piccoli 2012
- Piccoli NB, Grede N, Pee S, Singhkumarwong A, Roks E, Moench‐Pfanner R, et al. Rice fortification: its potential for improving micronutrient intake and steps required for implementation at scale . Food and Nutrition Bulletin 2012; 33 ( 4 Suppl ):S360‐72. [ PubMed ] [ Google Scholar ]
Prättälä 2012
- Prättälä RS, Puska P. Social determinants of health behaviours and social change . European Journal of Public Health 2012; 22 ( 2 ):166. [ PubMed ] [ Google Scholar ]
Rastogi 2002
- Rastogi R, Mathers CD. Global burden of iron deficiency anaemia in the year 2000 . Global Burden of Diseases 2000 Working Paper, World Health Organization, Geneva 2002.
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5) . Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rothman 1995
- Rothman KJ, Moore LL, Singer MR, Nguyen US, Mannino S, Milunsky A. Teratogenicity of high vitamin A intake . New England Journal of Medicine 1995; 333 ( 21 ):1369‐73. [ PubMed ] [ Google Scholar ]
Saneei 2017
- Saneei P, Larijani B, Esmaillzadeh A. Rice consumption, incidence of chronic diseases and risk of mortality: meta‐analysis of cohort studies . Public Health Nutrition 2017; 20 ( 2 ):233‐44. [PUBMED: 27577106] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Saunders 1979
- Saunders R, Betschart A. In: Inglett GE, Charalambous G editor(s). Tropical foods: chemistry and nutrition . New York: Academic Press, 1979:191‐216. [ Google Scholar ]
Scrimshaw 2010
- Scrimshaw NS. INCAP studies of nutrition and infection . Food and Nutrition Bulletin 2010; 31 ( 1 ):54‐67. [ PubMed ] [ Google Scholar ]
- Shah D, Sachdev HS, Gera T, De‐Regil LM, Peña‐Rosas JP. Fortification of staple foods with zinc for improving zinc status and other health outcomes in the general population . Cochrane Database of Systematic Reviews 2016, Issue 6 . [DOI: 10.1002/14651858.CD010697] [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Singhal 2002
- Singhal N, Austin J. A clinical review of micronutrients in HIV infection . Journal of the International Association of Physicians in AIDS Care 2002; 1 ( 2 ):63‐75. [ PubMed ] [ Google Scholar ]
Sommer 1996
- Sommer A, West KP, editors. Vitamin A deficiency. Health, survival and vision . Oxford: Oxford University Press, 1996. [ Google Scholar ]
Soriguer 2013
- Soriguer F, Colomo N, Oliveira G, García‐Fuentes E, Esteva I, Ruiz de Adana M, et al. White rice consumption and risk of type 2 diabetes . Clinical Nutrition 2013; 32 :481‐4. [ PubMed ] [ Google Scholar ]
Stevens 2013
- Stevens GA, Finucane MM, De‐Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non‐pregnant women for 1995‐2011: a systematic analysis of population‐representative data . Lancet Global Health 2013; Vol. 1, issue 1:e16‐25. [ PMC free article ] [ PubMed ]
Stevens 2015
- Stevens GA, Bennett JE, Hennocq Q, Lu Y, De‐Regil LM, et al. Trends and mortality effects of vitamin A deficiency in children in 138 low‐income and middle‐income countries between 1991 and 2013: a pooled analysis of population‐based surveys . Lancet Global Health 2015; Vol. 3, issue 5:e528‐e536. [ PubMed ]
Stoltzfus 2011
- Stoltzfus RJ. Iron interventions for women and children in low‐income countries . Journal of Nutrition 2011; 141 ( 4 ):756S‐62S. [ PubMed ] [ Google Scholar ]
- Tsang BL, Moreno R, Dabestani N, Pachón H, Spohrer R, Milani P. Public and private sector dynamics in scaling up rice fortification: the Colombian experience and its lessons . Food and Nutrition Bulletin 2016; 37 ( 3 ):317‐328. [ PubMed ] [ Google Scholar ]
Ueffing 2011
- Ueffing E, Tugwell P, Welch V, Petticrew M, Kristjansson E, for the Campbell and Cochrane Equity Methods Group. Equity Checklist for Systematic Review Authors. Version 2011‐11‐08 . www.equity.cochrane.org/files/equitychecklist2011.pdf (accessed 13 September 2012).
- Welch RM, Graham RD. A new paradigm for world agriculture: meeting human needs: productive, sustainable, nutritious . Field Crop Research 1999; 60 ( 1‐2 ):1‐10. [ Google Scholar ]
Wessells 2012
- Wessells KR, Brown KH. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting . PloS One 2012; 7 ( 11 ):e50568. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- West Jr KP, Katz J, Khatry SK, LeClerq SC, Pradhan EK, Shrestha SR, et al. Double blind, cluster randomised trial of low dose supplementation with vitamin A or carotene on mortality related to pregnancy in Nepal . BMJ 1999; 318 ( 7183 ):570‐5. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- World Health Assembly. Resolution WHA63.17. Birth defects . Sixty‐third World Health Assembly, Geneva 17‐21 May 2010. Resolutions and decisions . Geneva: World Health Organization, 2010. [ Google Scholar ]
- World Health Organization. Iron deficiency anemia: assessment prevention and control—a guide for program managers . NHD 01, 3rd edition 2001.
- World Health Organization. The World Health Report 2002: Reducing Risks, Promoting Healthy Life . Geneva: World Health Organization, 2002. [ Google Scholar ]
- World Health Organization (WHO). Global prevalence of vitamin A deficiency in populations at risk 1993‐2005. Global Database on Vitamin A Deficiency . Geneva: World Health Organization, 2009. [ Google Scholar ]
- World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks . Geneva: World Health Organization, 2009. [ Google Scholar ]
- Barros FC, Victora CG, Scherpbier RW, Gwatkin D. Health and nutrition of children: equity and social determinants . In: Blas E, Sivasankara Kurup A editor(s). Equity, social determinants and public health programmes . Geneva: World Health Organization, 2010:32‐49. [http://whqlibdoc.who.int/publications/2010/9789241563970_eng.pdf] [ Google Scholar ]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity . WHO/NMH/NHD/MNM/11.1. Geneva: World Health Organization, 2011. [ Google Scholar ]
- World Health Organization. Intermittent iron supplementation for menstruating women . www.who.int/nutrition/publications/micronutrients/weekly_iron_folicacid.pdf (accessed 7 April 2012).
- World Health Organization. Guideline: Use of Multiple Micronutrient Powders for Home Fortification of Foods Consumed by Infants and Children 6–23 Months of Age . Geneva: World Health Organization, 2011. [ PubMed ] [ Google Scholar ]
- World Health Organization. Guideline: Intermittent Iron Supplementation in Preschool and School Age Children . Geneva: World Health Organization, 2011. [ PubMed ] [ Google Scholar ]
- World Health Organization. Guideline: Vitamin A Supplementation in Infants and Children 6–59 Months of Age . Geneva: World Health Organization, 2011. [ Google Scholar ]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System . Geneva: WHO, 2011. [ Google Scholar ]
- World Health Organization. Guideline: Fortification of Food‐Grade Salt with Iodine for the Prevention and Control of Iodine Deficiency Disorders . Geneva: World Health Organization, 2014. [ PubMed ] [ Google Scholar ]
- World Health Organization. The Global Prevalence of Anaemia in 2011 . Geneva: World Health Organization, 2015. [ Google Scholar ]
- World Health Organization. Guideline: Optimal Serum and Red Blood Cell Folate Concentrations in Women of Reproductive Age for Prevention of Neural Tube Defects . Geneva: World Health Organization, 2015. [ PubMed ] [ Google Scholar ]
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience . Geneva: World Health Organization, 2016. [ PubMed ] [ Google Scholar ]
- World Health Organization. Nutritional Anaemias: Tools for Effective Prevention and Control . Geneva: World Health Organization, 2017. [ Google Scholar ]
- World Health Organization. Global Health Observatory . www.who.int/gho/en/ (accessed 3 July 2019).
WHO/CDC 2016
- World Health Organization, Centers for Disease Control and Prevention. Logic model for micronutrient interventions in public health . Vitamin and Mineral Nutrition Information System. (WHO/NMH/NHD/MNM/16.1) . Geneva: World Health Organization, 2016.
WHO/FAO 2004
- World Health Organization, Food, Nutrition Organization of the United Nations. Vitamin and Mineral Requirements in Human Nutrition . 2nd Edition. Geneva: World Health Organization, 2004. [ Google Scholar ]
WHO/FAO 2006
- Allen L, Benoist B, Dary O, Hurrell R, editors. WHO/FAO Guidelines on Food Fortification with Micronutrients . Geneva: World Health Organization, 2006. [ Google Scholar ]
Zimmermann 2007
- Zimmermann MB, Hurrell RF. Nutritional iron deficiency . Lancet 2007; 370 ( 9586 ):511‐20. [ PubMed ] [ Google Scholar ]
Zimmermann 2010
- Zimmermann MB, Chassard C, Rohner F, N'Goran EK, Nindjin C, Dostal A, et al. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Côte d'Ivoire . American Journal of Clinical Nutrition 2010; 92 ( 6 ):1406‐15. [ PubMed ] [ Google Scholar ]
References to other published versions of this review
Ashong 2012.
- Ashong J, Muthayya S, De‐Regil LM, Laillou A, Guyondet C, Moench‐Pfanner R, et al. Fortification of rice with vitamins and minerals for addressing micronutrient malnutrition . Cochrane Database of Systematic Reviews 2012, Issue 6 . [DOI: 10.1002/14651858.CD009902; CD009902] [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Review article
- Open access
- Published: 21 October 2019
Nutritional and functional properties of coloured rice varieties of South India: a review
- Rathna Priya T. S. 1 ,
- Ann Raeboline Lincy Eliazer Nelson 1 ,
- Kavitha Ravichandran 1 &
- Usha Antony 1
Journal of Ethnic Foods volume 6 , Article number: 11 ( 2019 ) Cite this article
72k Accesses
118 Citations
139 Altmetric
Metrics details
Rice is a major cereal food crop and staple food in most of the developing countries. India stands second in the production of rice next to China. Though almost 40,000 varieties of rice are said to exist, at present, only a few varieties are cultivated extensively, milled and polished. Even if white rice is consumed by most people around the world, some specialty rice cultivars are also grown. These include the coloured and aromatic rice varieties. The nutritional profile of the specialty rice is high when compared to the white rice varieties. The coloured rice, which usually gets its colour due to the deposition of anthocyanin pigments in the bran layer of the grain, is rich in phytochemicals and antioxidants. Rice bran, a by-product of the rice milling industry is under-utilised, is rich in dietary fibre which finds application in the development of functional foods and various other value-added products. Thus, more focus on specialty rice and its by-products will not only save it from becoming extinct but also lead a step forward towards nutrition security of the country as they are abundant in vitamins, minerals and polyphenols.
Introduction
Rice is a major cereal crop consumed as a staple food by over half of the world’s population. Consumption of rice is very high in developing countries and nations in Asia. Almost 95% of the rice production is done in Asian countries and about half of the world’s population consumes it. The cultivation of rice ranks third in the production of agricultural commodity next to sugarcane and maize. It is the predominant dietary energy source of 17 countries in Asia and the Pacific, 9 countries in North and South America and 8 countries in Africa. India is one of the major centres for rice production. The area for rice cultivation in India comprises about 43,388,000 hectares of land [ 1 ] and rice contributes to 780 and 689 kcal/capita/day of the food supply in Asia and India, respectively. Furthermore, India is one of the largest countries in terms of energy consumption from agriculture and rice comprises a major part of it [ 2 ].
Rice is rich in genetic diversity, with thousands of varieties grown throughout the world and India is home to 6000 varieties, at present. Originally, India had more than 110,000 varieties of rice until 1970, which were lost during the Green Revolution with its emphasis on monoculture and hybrid crops [ 3 ]. Paddy comes in many different colours, including brown, red, purple and even black. The colourful varieties of rice are considered valuable for their health benefits. The unpolished rice with its bran has high nutrient content than milled or polished white rice. However, rice consumers prefer to consume polished white rice, despite the fact that brown rice contains valuable nutrient content [ 4 ]. A detailed analysis on the nutrient content of rice suggests that the nutrition value varies depending upon several factors such as the strain or variety (i.e. white , brown , red and black /purple), nutrient quality of the soil in which rice is cultivated, the degree of milling and the method of preparation before consumption.
Origin and spread of rice
Oryza sativa , the dominant rice species, is a member of the Poaceae family. Historically, rice was cultivated widely in the river valleys of South and Southeast Asia 10,000 years ago [ 5 ] and it is believed to have originated probably in India. Domestication of rice in India is mainly attributed to the Indus valley civilization c. 3000–1500 BC [ 6 ]; however, the evidence of rice cultivation in India has been pushed to 4000 years ahead with the discovery of rice grains and early pottery found in the site of Lahuradewa, Uttar Pradesh, situated in the middle Ganges plains dating to c. 6409 BC [ 7 , 8 ].
Rice is highly adaptable to its environment of growth and this is evident from the fact that it is grown in north-eastern parts of China at latitude 53°N, on the equator in central Sumatra, and at 35°S in New South Wales, Australia. In India, it is grown below sea level in Kerala; most rice-growing areas are present at or near sea level and also, at elevations above 2000 m in Kashmir. Today, rice is cultivated in all parts of the world except Antarctica [ 9 ].
Importance of rice in India
India ranks second in the production of rice in the world next to China, accounting for 22.5% of overall world rice production. Rice is India’s pre-eminent crop and is the staple food of the people of the eastern and southern parts of the country. Apart from being nutritionally rich, rice has greater significance in India and holds great spiritual and ritual importance. As per Indian tradition, rice is revered as a potent symbol of auspiciousness, prosperity and fertility because of its life-sustaining qualities. Several rituals involving rice are performed during different occasions and festivals. In Tamil Nadu, kolam , a kind of geometric pattern, is drawn using rice flour at the threshold of the houses by women before sunrise. Rice also plays a vital role in wedding ceremonies in India. Dhanpan is a ritual wherein the family of bridegroom sends paddy, betel and/or turmeric to the house of the bride [ 10 ]. Rice mixed with turmeric is thrown on the couples during the wedding ceremony as a symbol of prosperity, eternity, continuity and fertility. The father of the bride organises a feast called Bhat (means, boiled rice) for the family and relations of the bridegroom [ 10 ]. The brides throw five handfuls of rice before leaving their parents’ home after the wedding to wish prosperity and wealth and remain with the family members. The bride enters her new home by pushing a glass or a jar full of rice while, rice is the first food offered to the bridegroom by the bride after marriage. In Tamil Nadu, the groom is offered a special variety of rice named Maappillai Samba to improve fertility [ 11 ]. Rice also plays a vital role during the baby shower function, named godh bharai in North India, valaikaapu in Tamil Nadu and seemandham in Kerala; on the event of birth; at the time of giving first solid food to the baby that is 6 or 7 months old; and during puberty in Kerala and Tamil Nadu. Flattened rice made from a variety called Thavala Kannan is given as offering in Kerala.
Rice also plays a prominent role in cultural celebrations of India, such as the festivals are based on sowing of seeds in the paddy field, transplanting the saplings in the fields, removal of weeds from the fields, harvesting of paddy, thrashing of paddy and storage of paddy [ 10 ]. The harvest festivals include Thai Pongal celebrated in the Tamizhian calendar month of Thai (falls in the month of January) in Tamil Nadu; Onam celebrated in the Malayalam month of Chingam (falls in the month of August or September) and Sankranti in Andhra Pradesh and Telangana, Makar Sankranti in Karnataka, Na-Khuwa Bhooj in rural Assam, Nabanna in West Bengal, and Nua khia or Navanna in Odisha; and Bihu in Assam celebrates the harvest of paddy. Thus, rice has not only shaped the history, culture, diet and economy of people but also the growth stage of the rice crop marks the passage of time and season. In India, rice is considered the root of civilization [ 12 ].
Production and market demand for rice varieties
Rice is a fundamental food in many cultural cuisines around the world. According to Ricepedia, more than 90% of production and consumption of rice in the world occur in Asia and the current share in global rice consumption is around 87%. In African countries, per capita consumption continues to increase than production [ 13 ]. The volume of international rice trade has increased almost sixfold, from 7.5 million tonnes annually in the 1960s to an average of 44.2 million tonnes during 2015–2016.
Based on the global market scenario with respect to rice, the production has increased slightly with years. The use of rice as food remains predominant compared to feed and other uses. The supply and utilisation of rice have also increased slightly (Table 1 ).
Similarly, rice is a major cereal crop and is consumed as a staple food by the majority of the population in India. India is one of the major centres for the production of rice. Both the Himalayan red rice and the Assam red rice find their place in international trade. The production of rice, wheat and maize has grown steadily over this period and that of rice is the highest followed by wheat (Table 2 ). In contrast, the production of other grains such as sorghum, pearl millet, finger millet, little millet and coarse cereals have either remained steady or have declined.
Rice is consumed by the rich and poor as well as rural and urban households. The per capita net availability of food grains increased after the Green Revolution, and rice is a part of the balanced diet along with vegetables, pulses, eggs, meat and fruits. The per capita net availability of rice increased to 69.3 kg/year in 2017 from 58.0 kg/year in 1951 [ 15 , 16 ]. Although rice is widely consumed, with years, the expenditure on cereals decreased from 26.3% in 1987–1988 to 12.0% in 2011–2012 and from 15% in 1987–1988 to 7.3% in 2011–2012 in rural and urban households, respectively. This overall dip in the expenditure may be due to the fact that more money is spent on non-food items in both rural and urban households [ 16 ].
Rice varieties
Among the 40,000 varieties of rice cultivated worldwide, only two major species are cultivated widely— Oryza sativa or the Asian rice and Oryza glaberrima or the African rice. The cultivation of Oryza sativa is practised worldwide; however, the cultivation of the Oryza glaberrima is confined to Africa [ 17 ].
Oryza sativa has two major subspecies: the Indica , long-grain rice and the Japonica , round-grain rice. Japonica rice is mainly cultivated and consumed in Australia, China, Taiwan, Korea, the European Union, Japan, Russia, Turkey and the USA. Indica rice varieties are grown widely in Asia [ 17 ]. These varieties also comprise of the fragrant ones which are priced as premium. The principal fragrant varieties are Hom Mali from Thailand and the various types of Basmati exclusively grown on the Himalayan foothills of India (in the states of Haryana and Punjab) and Pakistan (in the state of Punjab) [ 18 ].
The Indian rice varieties cultivated widely are Basmati , Joha , Jyothi , Navara , Ponni , Pusa , Sona Masuri , Jaya , Kalajiri (aromatic), Boli , Palakkad Matta , etc. The coloured variety includes Himalayan red rice; Matta rice, Kattamodon , Kairali , Jyothy , Bhadra , Asha , Rakthashali of Kerala; Red Kavuni , Kaivara Samba , Mappillai Samba , Kuruvi Kar , Poongar of Tamil Nadu, etc.
The shelf life of rice
In general, it is recommended to store rice in the form of paddy rather than as milled rice, since the husk provides protection against insects and helps prevent quality deterioration. Rice can be stored for long periods only if the following three conditions are met and maintained: (1) the moisture levels of grains, 14% or less and that of seeds must be 12% or less; (2) grains must be well protected from insects, birds and rodents; and (3) grains must be protected from rains or imbibing moisture from the atmosphere. In addition to its nutritive and medicinal properties, red rice and black rice possess several other special features and the most common one is their resistance to insects and pests during storage than brown rice. From the cultivation point of view, red rice possesses resistance to drought, flood, submergence, alkalinity, salinity, and resistance to pests and diseases [ 19 ].
Structure of rice grain
The paddy (also, rough rice or rice grain) consists of the hull, an outer protective covering, and the fruit or rice caryopsis (brown or dehusked rice) [ 20 ]. Rice primarily consists of carbohydrates, proteins and small quantities of fat, ash, fibre and moisture. Vitamins and minerals are largely confined to the bran and germ [ 21 ].
The polished white rice, usually consumed, is the highly refined version of raw rice. The processing and milling of raw rice take away significant parts of the grain, namely the bran and the germ. Both bran and germ are rich in dietary fibre as well as nutrients that are beneficial for human health. Further, if white rice undergoes additional polishing, its aleurone layer getsremoved leading to loss of more nutrients, as this layer is rich in vitamin B, proteins, minerals and essential fats.
In this aspect, the coloured rice finds an advantage as a healthier alternative to white rice. Coloured rice varieties and brown rice varieties have the same harvesting process apart from possessing similar nutritional profiles. These varieties are usually either dehulled or partially hulled with the bran and germ intact. Brown rice is found worldwide, while red rice is confined to the Himalayas, Southern Tibet, and Bhutan, as well as parts of North East and South India. After the removal of husk, brown rice still consists of few outer layers—the pericarp, seed-coat and nucellus; the germ or embryo; and the endosperm. The endosperm consists of the aleurone layer, the sub-aleurone layer and the starchy or inner endosperm (Fig. 1 ). The aleurone layer encloses the embryo. Pigments are confined to the pericarp layer [ 20 ].
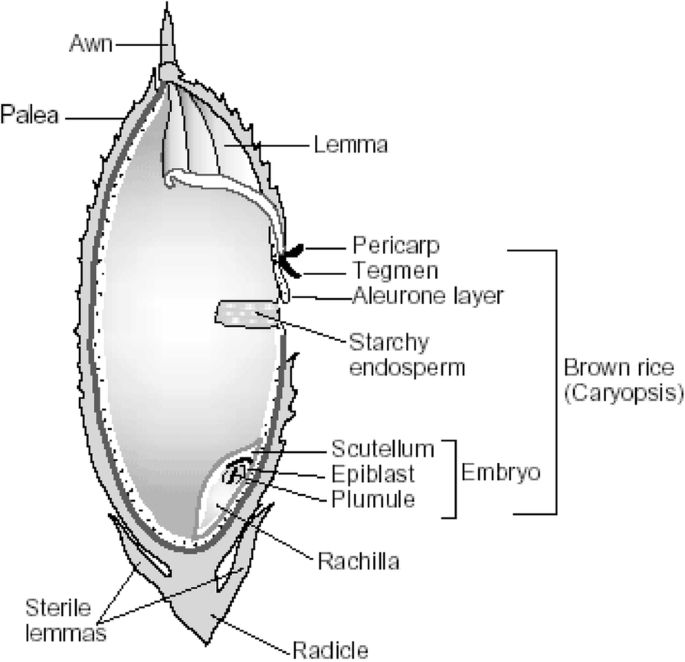
Structure of rice grain (Copyright: FAO) [ 22 ]. Paddy consists of the husk, bran, aleurone layer, starchy endosperm and embryo. Brown rice is semi-polished, so it retains embryo while white rice is more polished than brown rice, lacking bran, aleurone and embryo. The removal of bran, aleurone and embryo provides aesthetic appeal to rice and improves shelf life; however, it also removes nutrients and minerals found in the grain
The hull (also, husk) constitutes about 20% of the rough rice weight, but values range from 16 to 28%. The aleurone layer varies from one to five cell layers; it is thicker at the dorsal than at the ventral side and thicker in short-grain than in long-grain rice [ 23 ]. The aleurone and embryo cells are rich in protein and lipid bodies [ 24 ].
The different layers of rice contain different quantities of nutrients. The bran layer is rich in dietary fibre, minerals and vitamin B complex while the aleurone layer contains the least. The endosperm of rice is rich in carbohydrate and also contains a reasonable amount of digestible protein, with favourable amino acid profile than other grains [ 25 ].
Rice processing
Processing of rice mainly involves milling of rice which converts paddy into rice by removing the hull and all or part of the bran layer. Milling of rice is a crucial stage and the objective of milling is to remove the husk and bran so as to produce an edible white rice kernel that is free from impurities.
Rabbani and Ali [ 26 ] report that as a result of processing, some essential nutrients like thiamine and vitamin B are lost. The milling process followed by polishing destroys 67% of the vitamin B 3 , 80% of vitamin B 1 , 90% of vitamin B 6 , 50% of manganese and phosphorus, 60% of the iron, and all of the dietary fibre, as well as the essential fatty acids present in the raw unmilled variety.
The rough rice (also, paddy) on milling produces brown rice, milled rice, germ, bran, broken and husk. Each of these has unique properties and can be used in numerous ways. The extent of value addition in rice and rice products depends upon the utilisation pattern of these components directly or as derivatives. For coloured rice varieties, only the first three steps of milling, namely, pre-cleaning, dehusking and separation, are applied and bran and germ are left intact.
Nutritional information
Raw, long-grain white rice is a good source of carbohydrates, calcium, iron, thiamine, pantothenic acid, folate and vitamin E when compared with maize, wheat and potatoes. It does not contain vitamin C, vitamin A, beta-carotene, lutein and zeaxanthin. It is also notably low in dietary fibre.
- Coloured rice
Brown rice retains its bran layer (containing vitamins, minerals and fibre), as this has not been polished more to produce white rice. The coloured rice varieties are either semi-polished or unpolished (Fig. 2 ). Red-coloured rice varieties are known to be rich in iron and zinc, while black rice varieties are especially high in protein, fat and crude fibre. Red and black rice get their colour from anthocyanin pigments, which are known to have free radical scavenging and antioxidant capacities, as well as other health benefits.

Some traditional South Indian rice varieties. a Red Kavuni . b Kaivara Samba . c Kuruvi Kar . d Poongar . e Kattu Yanam . f Koliyal . g Maappillai Samba. h Black Kavuni . Kavuni possesses anti-microbial activity. Kaivara Samba lowers blood sugar levels. Kuruvi Kar is resistant to drought and consumed by the locals for its health benefits. Poongar is consumed by women after puberty and is believed to avert ailments associated with the reproductive system. Kattu Yanam lowers glucose level in blood and also imparts strength. Koliyal is widely consumed as puttu , a specialty dish. Maapillai Samba has a hypocholesterolemic effect and anti-cancer activity and also improves fertility in men. Black Kavuni is resistant to drought and is popular among locals for its health benefits
Brown rice is highly nutritious. It has low calorie and has a high amount of fibre. Furthermore, it is a good source of magnesium, phosphorus, selenium, thiamine, niacin, vitamin B 6 and an excellent source of manganese. Brown rice and rough rice are rich in vitamins and minerals; this is due to the fact that the vitamins are confined to the bran and husk of the paddy. Rice bran and husk contain a higher amount of calcium, zinc and iron (Table 3 ).
Rice is rich in glutamic and aspartic acids but has a lower amount of lysine. The antinutritional factors that are concentrated mainly in the bran are phytate, trypsin inhibitors, oryzacystatin and haemagglutinin-lectin [ 25 ].
The moisture content plays a significant role in determining the shelf life of foods [ 29 ]. Xheng and Lan [ 30 ] report that moisture influences the milling characteristics and the taste of cooked rice. The differences in genetic makeup and the climatic conditions in which they are cultivated determine the moisture content in rice varieties. As seen from Table 4 , the moisture content of the red rice varieties is variable from 9.3 to 12.94%, the moisture content of brown rice and milled rice is lower than other rice varieties.
Protein is the second major component next to starch; it influences the eating quality and the nutritional quality of rice. In India, the dietary supply of rice per person per day is 207.9 g, this provides about 24.1% of the required dietary protein [ 2 ]. Rice has a well-balanced amino acid profile due to the presence of lysine, in superior content to wheat, corn, millet and sorghum and thus makes the rice protein superior to other cereal grains [ 36 ]. The lysine content of rice protein is between 3.5 and 4.0%, making it the highest among cereal proteins. The endosperm protein comprises of 15% albumin (water soluble), globulin (salt soluble), 5–8% prolamin (alcohol soluble), and the rest glutelin (alkali soluble) [ 27 ].
The coloured rice has high protein content than polished white rice due to the presence of bran. The Srilankan and Chinese varieties have higher protein content than the Indian varieties (Table 4 ). Rice bran proteins are rich in albumin than endosperm proteins. The aleurone protein bodies contain 66% albumin, 7% globulin and 27% prolamin and glutelin [ 37 ].
The fat present in rice is a good source of linoleic acid and other essential fatty acids. The rice does not contain cholesterol [ 36 ]. The lipids or fats in rice are mainly confined to the rice bran (20%, dry basis). It is present as lipid bodies in the aleurone layer and bran. The core of the lipid bodies is rich in lipids and the major fatty acids are linoleic, oleic and palmitic acids [ 38 , 39 ]. Starch lipids present in rice is composed of monoacyl lipids (fatty acids and lysophosphatides) complexed with amylose [ 40 ]. The amount of fat present in various fractions of rice and red rice indicate that red rice varieties from Sri Lanka and India have about 1% fat, while the China red rice has almost doubled this value (Table 4 ).
The presence of fibre in the diet increases the bulk of faeces, which has a laxative effect in the gut. The fibre content is 0.5–1.0% for well-milled rice [ 41 ]. Arabinoxylans, along with β-d-glucan, are the major component of soluble dietary fibre in rice. In addition, rhamnose, xylose, mannose, galactose and glucose are also present in soluble dietary fibre. Insoluble dietary fibre is made up of cellulose, hemicellulose, insoluble β-glucan and arabinoxylans. However, the quantity and amount of non-starch polysaccharide depend upon the rice cultivar, the degree of milling and water solubility [ 42 ]. Among the red rice varieties, Chak-hao amubi (Manipur black rice) has a significantly lower content of crude fibre (Table 4 ).
The variation in ash content of different cultivars of rice may be due to genetic factors or the mineral content of the soil [ 43 ]. The zinc and iron content of red rice is two to three times higher than that of white rice [ 44 ]. The most common minerals found in rice include calcium, magnesium, iron and zinc (Table 3 ).
The proximate composition of rice and its fractions are influenced by the kind of rice and degree of milling, as milling completely or partially removes the bran layer, aleurone layer and embryo. Thus, variation occurs in the nutrition content between the rice fractions of the same rice variety. The variations can be found in the amount of fats, fibre and minerals present in the grain.
Phytochemical composition
The non-nutritive plant chemicals that have a protective or disease-preventing property are known as phytochemicals. The phytochemical compounds are mainly accumulated in the pericarp and bran of the rice kernel. They prevent oxidative damage in foods and also have a wide spectrum of beneficial biological activities.
Phytochemicals present in rice can be divided into the following sub-groups namely carotenoids, phenolics, alkaloids, nitrogen and organo-sulphur containing compounds. Phenolic compounds are further sub-grouped as phenolic acids, flavonoids, coumarins and tannins. Anthocyanins, the major pigment responsible for the colour of red and black rice, are a kind of flavonoids. Maapillai Samba , a kind of red rice from Tamil Nadu, has the highest amount of total polyphenolic compounds and anthocyanin content than the varieties from Sri Lanka, China red rice and Manipur black rice (Table 5 ).
The pigmented cereal grains, such as red and purple/black rice, have phytochemical compounds in higher amounts than non-pigmented varieties. The phytochemicals such as cell wall-bound phenolics and flavonoids are gaining more interest as these compounds can be broken down by digestive enzymes and gut microflora, and as a result, they can be easily absorbed into the body [ 45 ].
The coloured rice bran contains anthocyanins that possess inhibition of reductase enzyme and anti-diabetic activities [ 46 ]. The reductase inhibitors possess anti-androgen effects and are used in the treatment of benign prostatic hyperplasia and to lower urinary tract symptoms. β-sitosterol present in Maappillai Samba (Fig. 2 g) has a hypocholesterolemic effect, improves fertility and also heals colon cancer. Furthermore, stigmasterol found in this variety is a precursor in the production of semi-synthetic progesterone [ 11 ].
Garudan Samba contains 9,12-octadecadienoic acid ( Z , Z ) which has the potential to act as hypocholesterolemic, anti-arthritic, hepatoprotective, 5-alpha-reductase inhibitor, anti-histaminic, anti-coronary and anti-androgenic effects. In addition to these compounds, it also contains several other bioactive compounds [ 47 ].
3-Cyclohexene-1-methanol and α, α,4-trimethyl- present in red Kavuni (Fig. 2 a) possess the anti-microbial activity, and also, 3-hydroxy-4 methoxy benzoic acid is used as a precursor for the synthesis of morphine. In addition to these compounds, fatty acid esters and fatty acids such as dodecanoic acid, ethyl ester (lauric acid ester) and octadecanoic acid are present. Among these bioactive compounds, octadecanoic acid and ethyl esters increase low-density lipoprotein (LDL) cholesterol in the human body [ 48 ].
Health benefits
Depending upon the flavours, culinary needs, availability and its potential health benefits, people choose different varieties of rice. Rice has the ability to provide fast and instant energy. Brown rice and red rice are great sources of fibre, B vitamins, calcium , zinc and iron, manganese, selenium, magnesium and other nutrients. The red and black rice variety gets its rich colour from a group of phytochemicals called anthocyanins, which are also found in deep purple or reddish fruits and vegetables.
Diabetes mellitus
Unlike white polished rice, brown rice releases sugars slowly thus helping to stabilise blood sugar in a sustained manner. This trait makes it a better option for people who are suffering from diabetes mellitus. Further, studies in Asia have shown a relationship between the consumption of white rice and risk of type 2 diabetes. Dietary fibres reduce the absorption of carbohydrates by providing an enclosure to the food, hindering the action of hydrolytic enzymes in the small intestine on food, and increasing the viscosity of food in the intestine [ 49 ]. This plays a vital role in reducing the GI of food thereby preventing the risk of diabetes type 2 [ 50 ]. Proanthocyanidins present in red rice provide protection against type 2 diabetes [ 51 ]. Similarly, anthocyanins present in black rice is said to have a hypoglycemic effect [ 52 ].
Brown rice is rich in manganese and selenium, which play a vital role against free radicals, which acts as a major cancer-causing agent. Due to the presence of these elements and high dietary fibre, brown rice is associated with a lowered risk of cancer. Studies have also correlated the use of whole grains like brown rice with lowered levels of colon cancer. This may be related to its high fibre content, as fibre gets attached to carcinogenic substances and toxins helps to eliminate them from the body, and also keep them away from attaching to the cells in the colon. Proanthocyanins, present in red rice, modulate the inflammatory response and protect against some cancers [ 51 ]. Similarly, anthocyanins which are found abundantly in black rice have anti-carcinogenic properties based on epidemiological and in vivo animal and human-based studies [ 53 ].
Cardiovascular disease
Brown rice may help in lowering the risk of metabolic syndrome, while metabolic syndrome itself is a strong predictor of cardiovascular disease. Red rice contains magnesium that prevents the risk of heart attacks [ 54 ]. Various high-fat diet-induced risk factors for cardiovascular disease were ameliorated by anthocyanin-rich extracts from black rice in rat models [ 55 ].
Cholesterol
Brown rice contains naturally occurring bran oil, which helps in reducing LDL forms of cholesterol. Intake of black rice has found to eliminate reactive oxygen species (ROS) such as lipid peroxide and superoxide anion radicals and lower cholesterol levels due to the presence of compounds such as anthocyanins, polyphenolic compounds, flavonoids, phytic acid, vitamin E and γ-oryzanol [ 56 , 57 ]. Modulation of inflammatory responses by proanthocyanidins in red rice provided protection from cardiovascular disease [ 51 ]. Based on these studies, it is evident that whole grains can lower the chances of arterial plaque buildup, thus reducing the chances of developing heart disease.
Hypertension
Both brown and red rice have high magnesium content than white rice. Magnesium is an important mineral that plays a vital role in the regulation of blood pressure and sodium balance in the body [ 54 ].
Rice varieties such as brown, red and black rice are rich in fibre and have the ability to keep healthy bowel function and metabolic function. Anthocyanins present in red rice have properties that can help in weight management [ 54 ].
Rice protein is hypoallergenic; products from other plant sources such as soy and peanut and animal sources like eggs and milk are a good source of proteins, yet they may cause allergy when consumed. Rice protein provides a solution to this problem because it is hypoallergenic. Furthermore, the anthocyanins present in red rice also have the property to reduce allergy [ 54 ].
Medicinal uses of coloured rice
Among several types of rice, few varieties are used to treat ailments. Every variety of rice is unique in its properties, so the treatment of diseases using rice is not limited to a single variety alone. Many different varieties of rice are employed in treating ailments because of their different properties and characteristics. According to practitioners of Ayurveda, rice creates balance to the humours of the body. Rice enriches elements of the body; strengthens, revitalises and energises the body by removing toxic metabolites; regulates blood pressure; and prevents skin diseases and premature ageing. Rakthasali (a kind of red rice) is efficient in subduing disturbed humours of the body and good for fevers and ulcers; improves eyesight, health, voice and skin health; and increases fertility [ 58 , 59 , 60 , 61 ]. In Ayurveda, Sali , Sashtika and Nivara rice are used to treat bleeding from haemorrhoids (piles); Sali rice is used to treat burns and fractures; Nivara rice is used to treat cervical spondylitis, paralysis, rheumatoid arthritis, neuromuscular disorders, psoriasis, skin lesions, reduce backache, stomach ulcers and snakebite; and Nivara rice is also used in the preparation of weaning food for underweight babies [ 58 , 62 ].
Rice water prepared by soaking rice in water or boiling rice in excess water is used to control diseases. In Ayurvedic preparations, rice varieties such as Mahagandhak ras , Kamdudha ras , Sutsekhar ras , Amritanav ras , Swarnmalti ras , Pradraripu ras , Laghumai ras , Dughdavati , Pradaknasak churna , Pushpnag churna , Sangrahat bhasm and Mukta sukti are used to control ailments such as vaginal and seminal discharges, diarrhoea, constipation and dysentery [ 58 ]. Red rice varieties are known to be used in the treatment of ailments such as diarrhoea, vomiting, fever, haemorrhage, chest pain, wounds and burns [ 63 ]. Matali and Lal Dhan are used for curing blood pressure and fever in Himachal Pradesh. Another red rice variety called Kafalya from the hills of Himachal Pradesh and Uttar Pradesh is used in treating leucorrhoea and complications from abortion [ 64 ]. Kari Kagga and Atikaya from Karnataka are used for coolness and also as a tonic, whereas Neelam Samba of Tamil Nadu is used for lactating mothers [ 65 ]. Kuruvi Kar is resistant to drought and consumed by the locals for its health benefits [ 66 ]. Raktasali is efficient in subduing deranged humours [ 60 , 61 ]. It was also regarded as a good treatment for ailments such as fevers and ulcers. It is also believed that it improves eyesight and voice; acts as diuretic, spermatophytic, cosmetic and tonic; and was also antitoxic [ 59 ].
Traditional food and its importance
Ayurvedic treatises mention red rice as a nutritive food and medicine, so the red rice is eaten as a whole grain. Red rice varieties such as Bhama , Danigora , Karhani , Kalmdani , Ramdi , Muru , Hindmauri and Punaigora of Jharkhand and Chattisgarh are rich in nutrition and provide energy and satiety for a whole day [ 67 , 68 ]. Traditionally, various foods such as pongal , puttu , adai , appam , idli , dosai , idiyappam , adirasam , kozhukattai , modakam , payasam , semiya , uppuma , flaked rice, puffed rice, etc. are prepared and consumed. In Tamil Nadu and Kerala, paddy is parboiled prior to milling. This hydrothermal process facilitates the migration of nutrients such as vitamins and minerals from the bran and the aleurone layer to the endosperm [ 69 ]. Rice takes the place of major cereal consumed in the South Indian diet while it is wheat that holds the position in North Indian diet. Dosai , idli , pongal , appam , semiya , uppuma , kichadi and idiyappam are prepared and consumed for breakfast along with wide varieties of chutney. The specialty dish called puttu made from rice is also prepared and consumed for breakfast. The lunch of South India is a combination of cooked parboiled rice, poriyal , eggs, meat, sambar , dal curry, rasam , pappad , moore (buttermilk) or curd and/or dessert, payasam . The dinner usually consists of idli , dosai , idiappam , cooked rice and curries. Various other dishes are also prepared from rice and include biryani, pulao, fried rice, curd rice, tamarind rice, sambar rice, jeera rice, lemon rice, coconut rice, etc. In Tamil Nadu, appams and idlis are also made using the red rice. Koliyal and Garudan Samba ( Kaadai Kazhuththaan ) of Tamil Nadu are used in the preparation of a specialty dish called puttu [ 47 ]. Flatbread and chapatti are made from red Gunja and glutinous rice is used in making puttu , a South Indian meal [ 70 ]. Several products such as cookies, murruku (a type of South Indian snack), are also made using the various coloured rice varieties.
Rice also plays a major role in festivals celebrated in India. The harvest festivals are celebrated with several delicacies made from freshly harvested paddy. In Tamil Nadu, sarkarai pongal is made from raw rice, green gram, milk and jaggery; in Assam, fried rice balls named ghila pitha are prepared and consumed; in West Bengal, traditional Bengali delicacies are made from freshly harvested rice and jaggery, the most famous one is home-made sweets from rice pitha and karpursal or banapuli , and Basmati rice is also used to make Bengali paish .
Parboiled red rice widely consumed in Kerala includes Thondi , Matta , Paal Thondi , Kuruva , Chitteni and Chettadi . Seeraga Samba is an aromatic rice variety consumed widely in Tamil Nadu and Kerala; it is known as ‘Basmati of South India’ and used in the preparation of biryani. Similarly, Jatu of Kulu valley, Ambemohar of Maharastra, Dubraj of Madhya Pradesh, Joha of Assam, Kamod of Gujarat, Badshah bhog of West Bengal and Odisha, Radhunipagla of West Bengal, Katrini and Kalanamak of Uttar Pradesh and Bihar, Gandha samba of Kerala, Kalajira of Odisha and Chakhao varieties of Manipur are prized for its aroma [ 64 , 67 ].
Today, the spotlight is on the increased production of these traditional varieties, promoting the consumption among the younger generation and production of nutritious and novel value-added products from coloured rice.
Although India is home to traditional red rice varieties and their use has been common among the practitioners of traditional medicine and communities as part of their cultural heritage, their functional effects and health benefits in terms of modern scientific methodology are far and few. Due to the insufficient availability of data, the beneficial properties of these varieties still remain unknown to a majority of the population. So, to leverage their health benefits, extensive research on these native coloured varieties by the stakeholders needs to be promoted so that they are available to consumers as a part of the daily diet or specialty functional foods.
Availability of data and materials
Not applicable
Agricultural Statistics Division, Third advance estimates of production of food grains for 2016-17, Department of Agriculture, Cooperation and Farmers Welfare, India. https://eands.dacnet.nic.in/Advance_Estimate/3rd_Adv_Estimates2016-17_Eng.pdf . Accessed 20 Nov 2018.
Kennedy G, Burlingame B, Nguyen VN. Nutritional contribution of rice and impact of biotechnology and biodiversity in rice-consuming countries, Food and Agriculture Organization of the United Nations. http://www.fao.org/3/Y4751E/y4751e05.htm . Accessed 26 June 2019.
The Hindu, The Hindu Group (2012), ‘From 1,10,000 varieties of rice to only 6,000 now.’ http://www.thehindu.com/news/national/karnataka/from-110000-varities-of-rice-to-only-6000-now/article3284453.ece . Accessed 5 Jan 2019.
Devi GN, Padmavathi G, Babu VR, Waghray K. Proximate nutritional evaluation of rice ( Oryza sativa L.). J Rice Res. 2015;8(1):23–32.
Google Scholar
Gnanamanickam SS. Rice and its importance to human life. In: Biological Control of Rice Diseases. Progress in Biological Control 2009;(8):1-11.
Possehl GL. The Indus Civilization: a contemporary perspective. AltaMira Press. CA; Oxford: Walnut Creek; 2002.
Fuller DQ, Qin L, Harvey E. Evidence for a late onset of agriculture in the lower Yangtze region and challenges for an archaeobotany rice. In: Sanchez-Mazas A, Blench R, Ross MD, Peiros I, Lin M, editors. Past Human Migrations in East Asia, Matching Archaeology, Linguistics and Genetics. London: Routledge; 2007. p. 40–83.
Tewari R, Srivastava RK, Saraswat KS, Singh IB, Singh KK. Early farming at Lahuradewa. Pragdhara. 2008;18:347–73.
Yosida S. Climatic environment and its influence. In: Fundamentals of rice crop science. Manila: International Rice Research Institute; 1981. p. 65.
Ahuja SC, Ahuja U. Rice in religion and tradition. 2 nd International Rice Congress. 2006. https://www.researchgate.net/publication/321334487 . Accessed 23 May 2019.
Sulochana S, Singaravadivel K. A study on phytochemical evaluation of traditional rice variety of Tamil Nadu -'Maappillai Samba' by GC-MS. International Journal of Pharma and Biosciences. 2015;6(3):606–11.
Gomez KA. Rice, the Grain of culture. Siam Society Lecture Series, The Siam Society, Thailand. 2001. https://www.thairice.org/html/article/pdf_files/Rice_thegrain_of_Culture.pdf . Accessed 10 June 2018.
Ricepedia, CGIAR, IRRI, Philippines. Rice as a Crop. www.ricepedia.org/rice-as-a-crop . Accessed 10 Jan 2018.
Food and Agricultural Organisation of the United Nations. Rice Market Monitor FAO. 2017. http://www.fao.org/3/I8317EN/I8317EN.pdf . Accessed 20 Oct 2018.
Department of Agriculture, Cooperation and Farmers Welfare. Ministry of Agriculture, India. 2017. http://agricoop.nic.in/sites/default/files/pocketbook_0.pdf . Accessed 2 Mar 2019.
Directorate of Economics and Statistics (DES), Ministry of Agriculture, India. 2014. https://eands.dacnet.nic.in/PDF/Glance-2016.pdf . Accessed 20 Nov 2018.
Ricepedia, CGIAR, IRRI, Philippines. Cultivated rice species. http://ricepedia.org/rice-as-a-plant/rice-species/cultivated-rice-species . Accessed 10 Jan 2018.
Calpe C. Rice International Commodity Profile. Food and Agricultural Organisation of the United States. 2006. http://www.fao.org/fileadmin/templates/est/COMM_MARKETS_MONITORING/Rice/Documents/Rice_Profile_Dec-06.pdf . Accessed 25 Sept 2018.
Kitano H, Tamura Y, Satoh H, Nagato Y. Hierarchial regulation of organ differentiation during embryogenesis in rice. Plant J. 1993;3:607–10.
Juliano BO, Bechtel DB 1985. The rice grain and its gross composition. In: Juliano BO, editor. Rice Chemistry and Technology, American Association of Cereal Chemists: Eagan, MN, USA. 1985. p. 17-57.
Tangpinijkul N. Rice Milling System: paper prepared for a training course on Grain Post-harvest Technology Manhattan Klongluang Hotel, Pathumthani, Thailand. 2010. https://pdfs.semanticscholar.org/ce4e/874284982e5b7603b0373a554c786c763c8e.pdf . Accessed 25 June 2019.
Kennedy G, Burlingame B, Nguyen N. Nutrient impact assessment of rice in major rice-consuming countries. Food and Agriculture Organisation of the United Nations. http://www.fao.org/3/Y6159T/y6159t04.htm#Note1 . Accessed on 17 July 2019.
Del Rosario AR, Briones VP, Vidal AJ, Juliano BO. Composition and endosperm structure of developing and mature rice kernel. Cereal Chem. 1968;45:225–35.
Tanaka K, Ogawa M, Kasai Z. The Rice Scutellum. II. A comparison of scutellar and aleurone electrodense particles by transmission electron microscopy including energy-dispersive X-ray analysis. Cereal Chem. 1977;54:684–9.
Juliano BO. Rice in human nutrition. FAO Food and Nutrition Series No. 21, Rome, Italy. 1993. 162.
Rabbani GH, Ali M. New ideas and concepts, rice bran: a nutrient dense mill-waste for human nutrition. The ORION Med. J. 2009;32(3):458–62.
Juliano BO. Rice: Chemistry and Technology. 2nd ed. St. Paul, MN: Am. Assoc. Cereal Chem; 1985b. p. 774.
Pedersen B, Eggum BO. The influence of milling on the nutritive value of flour from cereal grains. Plant foods Human Nutrition. 1983;33:267–78.
Ebuehi OAT, Oyewole AC. Effect of cooking and soaking on physical characteristics, nutrient composition and sensory evaluation of indigenous and foreign rice varieties in Nigeria. African Journal of Biotechnology. 2007;6(8):1016–20.
Xheng X, Lan Y. Effects of drying temperature and moisture content on rice taste quality. Agricultural Engineering International: The CIGRE Journal 2007:9. Manuscript FP07 023.
Eggum BO, Juliano BO, Maniñgat CC. Protein and energy utilization of rice milling fractions by rats. Qual. Plant. Plant Foods Hum. Nutr. 1982;31:371–6.
Umadevi M, Pushpa R, Sampathkumar KP, Bhowmik D. Rice- Traditional medicinal plant in India. Journal of Pharmacognosy and Phytochemistry. 2012;1(1):6–12.
Longvah T, Ananthan R, Bhaskarachary K, Venkaiah K. Proximate principles and dietary fibre. Hyderabad, India: Indian Food Composition Tables, National Institute of Nutrition, Department of Health Research, Ministry of Health and Family Welfare, Government of India; 2017. p. 3.
Sompong R, Siebenhandl-Ehn S, Linsberger-Martin G, Berghofer E. Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chemistry. 2011;124:132–40.
Saikia S, Dutta H, Saikia D, Mahanta CL. Quality Characterisation and estimation of phytochemicals content and antioxidant capacity of aromatic pigmented and non-pigmented rice varieties. Food Research International. 2012;46(1):334–40.
Eggum BO. The nutritional value of rice in comparison with other cereals. In: Proceedings, Workshop on Chemical Aspects of Rice Grain Quality, IRRI. Los Banos, Laguna, The Philippines; 1979. p. 91–111.
Tanaka N, Fujita N, Nishi A, Satoh H, Hosaka Y, Ugaki M. The structure of starch can be manipulated by changing the expression levels of starch branching enzyme IIb in rice endosperm. Plant Biotechnol. J. 2004;2:207–516.
Juliano BO, Goddard MS. Cause of varietal difference in insulin and glucose responses to ingested rice. Qual. Plant. Plant Foods Hum. Nutr. 1986;36:35–41.
Tanaka Y, Resurreccion AP, Juliano BO, Bechtel DB. Properties of whole and undigested fraction of protein bodies of milled rice. Agric. Biol. Chem. 1978;42:2015–23.
Choudhury NH, Juliano BO. Effect of amylose content on the lipids of mature rice grain. Phytochemistry. 1980;19:1385–9.
Oko AO, Onyekwere SC. Studies on the proximate chemical composition and mineral element contents of five new lowland rice varieties in Ebonyi State. Int J Biotechnology Biochemistry. 2010;6(6):949–55.
Lai VMF, Lu S, He WH, Chen HH. Non-starch polysaccharide compositions of rice grains with respect to rice variety and degree of milling. Food Chemistry. 2007;101(3):1205–10.
AO O, BE U, AA E, N D. Comparative analysis of the chemical nutrient composition of selected local and newly introduced rice varieties grown in Ebonyi State of Nigeria. Int J Agriculture Forestry. 2012;2(2):16–23.
Ramaiah K, Rao MVBN. Rice breeding and genetics ICAR science monograph 19. New Delhi, India: Indian Council of Agricultural Research; 1953.
Chen C-H, Yang J-C, Uang Y-S, Lin C-J. Improved dissolution rate and oral bioavailability of lovastatin in red yeast rice products. Int J Pharm. 2013;444(1-2):18–24.
Yawadio R, Tanimori S, Morita N. Identification of phenolic compounds isolated from pigmented rices and their aldose reductase inhibitory activities. Food Chemistry. 2007;101(4):1616–25.
Sulochana S, Meyyappan RM, Singaravadivel K. Phytochemical screening and GC-MS analysis of Garudan Samba traditional rice variety. Int J Environ Agri Res. 2016;2(4):44–7.
Sulochana S, Meyyappan RM, Singaravadivel K. Mass spectrometry analysis of indian traditional variety “Red Kavuni” in comparison with high yielding popular variety of Tamil Nadu ADT 43 under raw and hydrothermally processed condition. Indo Am J Pharm Res. 2016;6(5):5358–63.
Jenkins DJA, Leeds AR, Gassell MA, Cocket B, Alberti KGM. Decrease in post-prandial insulin and glucose concentrations by gaur and pectin. Ann Intern Med. 1977;86:20–3.
Babu DP, Bhakyaraj R, Vildhyalakshmi R. A low cost nutritious food “tempeh”- a review. World J. Dairy Food Sci. 2009;4(1):222–7.
Chen M-H, McClung AM, Bergman CJ. Concentrations of ligomers and polymers of proanthocyanidins in red and purple rice bran and their relationships to total phenolics, flavonoids, antioxidant capacity and whole grain color. Food Chemistry. 2016;208:279–87.
Tsuda T, Horio F, Uchids K, Aoki H, Osawa T. Dietary cyanidin 3-O-beta-D-glucoside-rice purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003;133(7):2125–30.
Pojer E, Mattivi F, Johnson D, Stockley CS. The case for anthocyanin consumption to promote human health: a review. Compr. Rev. Food Sci, Food Saf. 2013;12(5):483–508.
Institute of Medicine (US). Standing Committee on the Scientific Evaluation of Dietary References Intakes. Dietary reference intakes for calcium, phosphorous, magnesium, vitamin D, and fluoride. Magnesium. Washington, DC: National Academies Press; 1997.
Shao Y, Xu F, Sun X, Bao J, Beta T. Identification and quantification of phenolic acids and anthocyanins as antioxidants in bran, embryo and endosperm of white, red and black rice kernels ( Oryza sativa L.). J Cereal Sci. 2014;59:211–8.
Ichikawa H, Ichiyanagi T, Xu B, Yoshii Y, Nakajima M, Konishi T. Antioxidant activity of anthocyanin extract from purple black rice. J. Med. Food. 2001;4(4):211–8.
Nam YJ, Nam SH, Kang MY. Cholesterol - lowering efficacy of unrefined bran oil from the pigmented black rice ( Oryza sativa L cv. Suwon 415) in hypercholesterolemic rats. Food Sci. Biotechnol. 2008;17:457–63.
Ahuja U, Ahuja SC, Thakrar R, Singh RK. Rice- a nutraceutical. Asian Agri-History. 2008;12(2):93–108.
Bhat FM, Riar CS. Health benefits of traditional rice varieties of temperate regions. Med. Aromat. Plants. 2015;4:198. https://doi.org/10.4172/2167-0412.1000198 .
Krishnamurthy KS. The Wealth of Susruta. Tamil Nadu, India: International Institute of Ayurveda, Coimbatore; 1991.
Kumar TT. History of rice in India. Delhi, India: Gian Publishers; 1988.
Sharma PV. Classical uses of medicinal plants. Chaukhamba Vishwabharati, Varanasi. Uttar Pradesh, India. 1996:848.
Hedge S, Yenagi NB, Kasturiba B. Indigenous knowledge of the traditional and qualified Ayurveda practitioners on the nutritional significance and use of red rice in medications. Indian journal of traditional knowledge. 2013;12:506–11.
Ahuja U, Ahuja SC, Chaudhary N, Thakrar R. Red rices-past, present, and future. Asian Agri-History. 2007;11(4):291–304.
Arumugasamy S, Jayashankar N, Subramanian K, Sridhar S, Vijayalakshmi K. Indigenous rice varieties. Centre for Indian Knowledge System (CIKS), Chennai: Tamil Nadu India; 2001.
The Hindu. The Hindu Group. India: India. Indigenous rice varieties make a comeback The Hindu Group; 2018. http://www.thehindu.com/life-and-style/food/thanals-save-our-rice-is-reviving-indigenous-rice-varieties/article22420554.ece .
Ahuja U, Ahuja SC, Thakrar R, Shobha Rani N. Scented rices of India. Asian Agri-History. 2008;12(4):267–83.
Rahman S, Sharma MP, Sahai S. Nutritional and medicinal value of some indigenous rice varieties. Indian J Traditional Knowledge. 2006;5(4):454–8.
Bhattacharya KR. Parboiling of rice. In: Champagne NET, editor. Rice chemistry and technology. American Association of Cereal Chemists Inc. St. Paul, Minnesota; 2004. p. 329–404.
Rani S, Krishnaiah K. Current status and future prospects of improving traditional aromatic rice. In: Chaudhary RC and Tran DV, editors, Specialty Rices of the World: Breeding, Production, and Marketing, FAO, Rome, Italy and Oxford IBH Publishers, India. 2001. p. 49-79.
Download references
Acknowledgements
The authors wish to thank the anonymous reviewer(s) for their suggestions.
Author information
Authors and affiliations.
Centre for Food Technology, Department of Biotechnology, Alagappa College of Technology, Anna University, Guindy, Chennai-25, India
Rathna Priya T. S., Ann Raeboline Lincy Eliazer Nelson, Kavitha Ravichandran & Usha Antony
You can also search for this author in PubMed Google Scholar
Contributions
RP initiated the idea of the article and authored all sections of the article except sections on medicinal uses of coloured rice, traditional food products and value-added products and new products. ARLEN authored sections on medicinal uses of coloured rice, traditional food products and value-added products and new products; co-authored other sections of the article KR co-authored the sections on the importance of rice in India, rice processing, production and demand of rice varieties, origin and spread of rice and value-added products and new products; and provided critical inputs to revise the manuscript. UA co-authored the sections on structure of grain, nutrition, health benefits and traditional food products; and provided critical inputs to revise the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Correspondence to Kavitha Ravichandran .
Ethics declarations
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Rathna Priya, T., Eliazer Nelson, A.R.L., Ravichandran, K. et al. Nutritional and functional properties of coloured rice varieties of South India: a review. J. Ethn. Food 6 , 11 (2019). https://doi.org/10.1186/s42779-019-0017-3
Download citation
Received : 21 January 2019
Accepted : 12 September 2019
Published : 21 October 2019
DOI : https://doi.org/10.1186/s42779-019-0017-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Polyphenols
- Phytochemicals
Journal of Ethnic Foods
ISSN: 2352-619X
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Rice research for food security and sustainable agricultural development in Asia: Achievements and future challenges
- Published: March 1995
- Volume 35 , pages 286–298, ( 1995 )
Cite this article

- Hossain M. 1 &
- Fischer K. S. 1
Rice is the major staple food of Asia, and an important source of employment and income in rural areas, particularly in low-income countries. Research has contributed significantly in achieving food security by increasing the yield potential of rice in irrigated systems, reducing the crop maturity period and achieving yield stability by developing resistance against major insects and diseases in the modern high-yielding varieties. Poverty is, however, still extensive in fragile rainfed rice ecosystems where rice yield has remained low, as scientists have yet to develop high-yielding varieties resistant to abiotic stresses and problem soils. Rice production needs to be increased by another 70% over the next 30 years to meet growing food needs. This has to be achieved with less land, less water, and less labor to accommodate the demand for these inputs from the expanding nonagricultural sectors. The challenge to the rice research community is to make further shifts in yield potential of rice for the irrigated systems, to close the yield gaps in the rainfed systems through developing resistance of high yielding varieties to abiotic stresses, and greater understanding of the interactions between genotypes and environment, developing durable resistance against pests and diseases to reduce farmers' dependence on harmful agrochemicals, and to increase efficiency in the use of water, labor and fertilizers. As further intensification of rice cultivation is inevitable, scientists must understand the negative environmental side-effects of increasing rice productivity, to develop appropriate mitigation options.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Rice Production in Africa
Rice production in australia.
Systems-based rice improvement approaches for sustainable food and nutritional security
Anderson, K.; Hayami, Y. with Associates: The Political Economy of Agricultural Protection: East Asia in International Perspective. Allen and Unwin, London 1986.
Google Scholar
Asian Development Bank: Asian Development Outlook, 1992. Oxford University Press, Hong Kong 1992.
Barker, R; Herdt, R. W.: The Rice Economy of Asia. Resources for the Future, Washington, DC 1985.
Bennett, J.: Biotechnology and the Future of Rice Production. GeoJournal 35.3, 335–337 (1995)
Cassmann, K. G.; Pingali, P. L.: Intensification of Irrigated Rice Systems: Learning from the Past to Meet Future Challenges. GeoJournal 35.3, 299–305 (1995)
Childs, Nathan W.: The world rice market: government intervention and multilateral policy reform. Economic Research Service, USDA, Washington DC, USA 1990.
Chou, C. H.; Chang, F.J.; Oka, H.I.: Allelopathic potentials of a wild rice, Oryza perennis . Taiwania 35(3):201–210 (1991)
David, C. C.; Otsuka, K.: Modern Rice Technology and Income Distribution in Asia. Lynne Reinner Publishers, Boulder and London, and International Rice Research Institute, Los Baños, Laguna, Philippines 1994.
Dingkhun, M.; Penning de Vries, F. W. T.; De Datta, S. K.; van Laar, H. H.: Concepts for a new plant type for direct seeded flooded tropical rice. In: Direct Seeded Flooded Rice in the Tropics, pp. 17–38, International Rice Research Institute, Los Baños, Philippines 1991.
Evans, L. T.: Raising the ceiling to yield: The key role of synergisms between agronomy and plant breeding. In: Muralidharan, K.; Siddiq, E. A., (eds.), New Frontiers in Rice Research, pp. 103–107. Directorate of Rice Research, Hyderabad, India 1990.
Gleick, P.H., (ed.): Water in Crisis: A Guide to the World's Fresh Water Resources. Oxford University Press, New York 1993.
Hayami, Y.; Ruhan, V. W.: Agricultural Development: An International Perspective. Johns Hopkins University Press, Baltimore 1985.
Hazell, P.B.; Ramasamy, C.: Green Revolution Reconsidered: The Impact of High Yielding Rice Varieties in South India. John Hopkins University Press, Baltimore and London 1991.
Heong, K. L.; Teng, P. S.; Moody, K.: Managing Rice Pests with Less Chemicals. GeoJournal 35.3, 339–351 (1995)
Hossain, M.: Nature and Impact of Green Revolution in Bangladesh. Research Report No. 67, International Food Policy Research Institute, Washington DC 1988.
Hossain, M.; Yifu Lin, J.: Rice production constraints in Asia. Paper presented in the Rockefeller Foundation Rice Biotechnology Meeting, held in Bali, Indonesia 1994.
Huang, J; David, C. C.: Demand for Cereal Grains in Asia: Effect of Urbanization. Social Sciences Division, International Rice Research Institute, PO Box 933, Manila, Philippines 1992.
IRRI: IRRI 2000 and Beyond. International Rice Research Institute, P.O. Box 933, 1099 Manila, Philippines 1989.
IRRI: 1993–1995: IRRI Rice Almanac. International Rice Research Institute, PO Box 933, 1099 Manila, Philippines 1993.
IRRI: IRRI Rice Facts. International Rice Research Institute, PO Box 933, 1099 Manila, Philippines 1994.
Ishii, S.: Varietal differences of photosynthesis and grain yield in rice (Oryza sativa L.). Reprinted from the Korean Journal of Crop Science 33(3) (1988)
Ito, S.; Peterson, W. W. F.; Grant, W. R.: Rice in Asia: Is it becoming an inferior good? American Journal of Agricultural Economics 71:32–42 (1989)
Jackson, M.T.: Protecting the Heritage of Rice Biodiversity. GeoJournal 35.3, 267–274 (1995)
Janoria, M. P.: A basic plant ideotype for rice. Intl. Rice Res. Newsl. 14:(3)12–13 1989.
Khush, G. S.: Varietal needs for different environments and breeding strategies. In: Muralidharan, K.; Siddiq, E.A. (eds.), New Frontiers in Rice Research, pp. 68–75. Directorate of Rice Research, Hyderabad, India 1990.
Khush, G. S.: Modern Varieties — Their Real Contribution to Food Supplies and Euqity. GeoJournal 35.3, 275–284 (1995)
Khush, G. S.: Breaking the Yield Frontier of Rice. GeoJournal 35.3, 331–334 (1995)
Khush, G. S.; Brar, D. S.: Overcoming barriers in hybridization. Distant hybrid of crop plants. Theor. Appl. Genet. (monog.) 16:47–61 (1992)
Kropff, M. J.; Cassman, K. G.; Van Laar, H. H., Peng, S.: Nitrogen and yield potential of irrigated rice. Plant and Soil 155/156: 391–394 (1993)
Kropff, M. J.; Cassman, K. G.; Peng, S.; Matthews, R. B.; Setter, T. L.: Quantitative understanding of rice yield potential. In: Cassman, K. G. (ed.), Breaking the Yield Barrier: Proceedings of a Workshop on Rice Yield Potential in Favorable Environments, Ch. 3. International Rice Research Institute, Los Baños, Philippines 1994a.
Kropff, M. J.; Williams, R. L.; Horie, T.; Angus, J. F.; Singh, U.; Centeno, H. G.; Cassman, K. G.: Predicting yield potential of rice in different environments, In: Temperate Rice — Achievements and Potential. Yanco Agricultural Research Institute, NSW, Australia 1994 b.
Lin, J.; Smith, R. J., Jr.; Dilday, R. H.: Allelopathic activity of rice germplasm onweeds. In: Proceedings of the 45th Annual Meeting of the Southern Weed Science Society, p. 99. Little Rock, Arkansas, USA 1992.
Makino, A.; Mae, T.; Ohira, K.: Photosynthesis and ribulose-1,5-biphosphate carboxylase in rice leaves from emergence through senescence. Quantitative analysis by carboxylation/oxygenation and regeneration of ribulose-1.5-biphosphate carboxylase. Planta 166:414–420 (1985)
Paddock, W.; Paddock, P.: Time of Famines. Little, Brown and Company, Canada 1967.
Peng, S.; Garcia, F.; Cassman, K.G.: Adjustment of specific leaf weight improves chlorophyll meter's estimate of rice leaf N concentration. Agron. Journal 85:987–990 1993.
Peng, S.; Khush, G.S.; Cassman, K.G.: Evolution of the new plant ideotype for increased yield potential. In: Cassman, K.G. (ed.), Breaking the Yield Barrier: Proceedings of a Workshop on rice Yield Potential in Favorable Environments, Ch 2. International Rice Research Institute, Los Baños, Philippines 1994.
Penning de Vries, F. W. T.: Improving yields: designing and testing VHYVs. In: Penning de Vries, F. W. T. et al. (ed.), Systems Simulation at IRRI, pp. 13–19. IRRI Res. paper 151. International Rice Research Institute, Los Baños, Philippines 1991.
Rola, A. C.; Pingali, P. L.: Pesticides and Rice Productivity: An Economic Assessment for the Philippines. International Rice Research Institute, PO Box 933, Manila, Philippines 1993.
Scobie, G.; McDonald, D.; Willey, R.; Gryseels, G.: Investment in rice research in the CGIAR: A global perspective. Report of the Inter-Centre Review of Rice. AGR/TAC:IAR/93/4. TAC Secretariat. FAO, Rome, Italy 1993.
Setter, T. L.; Kropff, M. J.; Cassman, K. G.; Khush, G. S.: Yield Potential of Rice: Past, Present and Future Perspectives. International Rice Research Institute, Los Baños, Philippines 1994.
Teng, P. S.: Sustainability in rice growing — Recent progress in rice research and challenges towards the next century. Paper presented at the Japan-IRRI Day Symposium, Tsukuba, Japan. International Rice Research Institute, P.O. Box 933, 1099 Manila, Philippines.
Vergara, B. S.: Raising the yield potential of rice. Philipp. Tech. J. 13:3–9 (1988)
Virmani, S. S.; Prasad, M. N.; Kumar, I.: Breaking yield barrier of rice through exploitation of heterosis. In: Muralidharan, K.; Siddiq, E. A. (eds.), New Frontiers in Rice Research, pp. 76–85. Directorate of Rice Research, Hyderabad, India 1993.
Wirjahardja, S.; Nurfilmarasa, E.: Some autoecological aspects of wild rice. In: Proceedings of the 3rd Indonesian Weed Science Conference, pp. 18–32. Bandung, Indonesia 1975.
Wopereis, M. C. S.: Quantifying the impact of soil and climate variability on rainfed rice production. Ph.D. Thesis, p. 188, Wageningen Agricultural University, The Netherlands 1993.
World Bank: Poverty: World Development Report 1990. Oxford University Press, New York 1990
World Bank: Development and the Environment: World Development Report 1992. Oxford University Press, Oxford, New York 1992.
WCED: Our Common Future. Oxford University Press, World Commission on Environment and Development. New York 1987.
Yap, C. L.: A comparison of the cost of producing rice in selected countries. FAO Economic and Social Development Paper No. 101, Rome, Food and Agriculture Organization 1992.
Zeigler, R. S.; Puckridge, D.W.: Improving Sustainable Productivity in Rice-based Rainfeld Lowland Systems of South and Southeast Asia. GeoJournal 35.3, 307–324 (1995)
Download references
Author information
Authors and affiliations.
International Rice Research Institute, PO Box 933, 1099, Manila, Philippines
Hossain M. ( Dr. ) & Fischer K. S. ( Dr. )
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
About this article
Hossain, M., Fischer, K.S. Rice research for food security and sustainable agricultural development in Asia: Achievements and future challenges. GeoJournal 35 , 286–298 (1995). https://doi.org/10.1007/BF00989136
Download citation
Issue Date : March 1995
DOI : https://doi.org/10.1007/BF00989136
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Food Security
- Irrigate System
- Durable Resistance
- Mitigation Option
- Find a journal
- Publish with us
- Track your research
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Research progress on mirnas and artificial mirnas in insect and disease resistance and breeding in plants.

1. Introduction
2. progress in mirna research on insect resistance and breeding in plants, 2.1. plant endogenous mirnas, 2.2. insect endogenous mirnas, 3. progress in mirna research on disease resistance and breeding in plants, 4. application of artificial mirnas (amirnas) in research on plant resistance, 4.1. application of amirnas for insecticidal effects in plants, 4.2. application of amirnas for disease resistance in plants, 5. prospects and conclusions, 5.1. applications of rnai technology in crop production, 5.2. challenges in the application of mirna technology in crop breeding, 5.3. conclusions, author contributions, conflicts of interest.
- Correia de Sousa, M.; Gjorgjieva, M.; Dolicka, D.; Sobolewski, C.; Foti, M. Deciphering miRNAs’ action through miRNA editing. Int. J. Mol. Sci. 2019 , 20 , 6249. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015 , 16 , 421–433. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lukasik, A.; Zielenkiewicz, P. Plant microRNAs—Novel players in natural medicine? Int. J. Mol. Sci. 2016 , 18 , 9. [ Google Scholar ] [ CrossRef ]
- Ameres, S.; Zamore, P. Diversifying microRNA sequence and function. Nat. Rev. Mol. Cell Biol. 2013 , 14 , 475–488. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gebert, L.; Macrae, I. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019 , 20 , 21–37. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yu, S.; Wang, J. The Crosstalk between MicroRNAs and Gibberellin Signaling in Plants. Plant Cell Physiol. 2020 , 61 , 1880–1890. [ Google Scholar ] [ CrossRef ]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRbase: From microRNA sequences to function. Nucleic Acids Res. 2019 , 47 , 155–162. [ Google Scholar ] [ CrossRef ]
- Mao, Y.; Liu, Y.; Chen, D.; Chen, F.; Fang, X.; Hong, G.; Wang, L.; Wang, J.; Chen, X. Jasmonate response decay and defense metabolite accumulation contributes to age-regulated dynamics of plant insect resistance. Nat. Commun. 2017 , 8 , 13925. [ Google Scholar ] [ CrossRef ]
- Wamiq, G.; Khan, J. Overexpression of ghr-miR166b generates resistance against Bemisia tabaci infestation in Gossypium hirsutum plants. Planta 2018 , 247 , 1175–1189. [ Google Scholar ] [ CrossRef ]
- Agrawal, A.; Rajamani, V.; Reddy, V.; Mukherjee, S.; Bhatnagar, R. Transgenic plants over-expressing insect-specific microRNA acquire insecticidal activity against Helicoverpa armigera : An alternative to Bt-toxin technology. Transgenic Res. 2015 , 24 , 791–801. [ Google Scholar ] [ CrossRef ]
- Ge, Y.; Han, J.; Zhou, G.; Xu, Y.; Ding, Y.; Shi, M.; Guo, C.; Wu, G. Silencing of miR156 confers enhanced resistance to brown planthopper in rice. Planta 2018 , 248 , 813–826. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shen, Y.; Yang, G.; Miao, X.; Shi, Z. OsmiR159 Modulate BPH Resistance Through Regulating G-Protein γ Subunit GS3 Gene in Rice. Rice 2023 , 16 , 30. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dai, Z.; Tan, J.; Zhou, C.; Yang, X.; Yang, F.; Zhang, S.; Sun, S.; Miao, X.; Shi, Z. The OsmiR396-OsGRF8-OsF3H-flavonoid pathway mediates resistance to the brown planthopper in rice ( Oryza sativa ). Plant Biotechnol. J. 2019 , 17 , 1657–1669. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chen, J.; Liu, Q.; Yuan, L.; Shen, W.; Shi, Q.; Qi, G.; Zhang, Z. Osa-miR162a Enhances the Resistance to the Brown Planthopper via α-Linolenic Acid Metabolism in Rice ( Oryza sativa ). J. Agric. Food Chem. 2023 , 71 , 11847–11859. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fan, D.; Ran, L.; Hu, J.; Ye, X.; Xu, D.; Li, J.; Su, H.; Wang, X.; Ren, S.; Luo, K. miR319a/TCP module and DELLA protein regulate trichome initiation synergistically and improve insect defenses in Populus tomentosa. New Phytol. 2020 , 227 , 867–883. [ Google Scholar ] [ CrossRef ]
- Zhao, W.; Li, Z.; Fan, J.; Hu, C.; Yang, R.; Qi, X.; Chen, H.; Zhao, F.; Wang, S. Identification of jasmonic acid-associated microRNAs and characterization of the regulatory roles of the miR319/TCP4 module under root-knot nematode stress in tomato. J. Exp. Bot. 2015 , 66 , 4653–4667. [ Google Scholar ] [ CrossRef ]
- Xia, X.; Shao, Y.; Jiang, J.; Du, X.; Sheng, L.; Chen, F.; Fang, W.; Guan, Z.; Chen, S. MicroRNA expression pro file during aphid feeding in chrysanthemum ( Chrysanthemum morifolium ). PLoS ONE 2015 , 10 , e0143720. [ Google Scholar ] [ CrossRef ]
- Zhang, Q.; Dou, W.; Taning, C.; Smagghe, G.; Wang, J. Regulatory roles of microRNAs in insect pests: Prospective targets for insect pest control. Curr. Opin. Biotechnol. 2021 , 70 , 158–166. [ Google Scholar ] [ CrossRef ]
- Muthu Lakshmi Bavithra, C.; Murugan, M.; Pavithran, S.; Naveena, K. Enthralling genetic regulatory mechanisms meddling insecticide resistance development in insects: Role of transcriptional and post-transcriptional events. Front. Mol. Biosci. 2023 , 10 , 1257859. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bordoloi, K.; Agarwala, N. MicroRNAs in plant insect interaction and insect pest control. Plant Gene 2021 , 26 , 100271. [ Google Scholar ] [ CrossRef ]
- Sempere, L.; Dubrovsky, E.; Dubrovskaya, V.; Berger, E. The expression of the let-7 small regulatory RNA is controlled by ecdysone duriniz metamorphosis in Drosophila melanogaster. Dev. Biol. 2002 , 244 , 170–179. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sempere, L.; Sokol, N.; Dubrovsky, E.; Berger, E.; Ambrosa, V. Temporal regulation of microRNA expression in Drosophila melanogaster mediated by hormonal signals and Broad-Complex gene activity. Dev. Biol. 2003 , 259 , 9–18. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Varghese, J.; Cohen, S. microRNA miR-14 acts to modulate a positive autoregulatory loop controlling steroid hormone signaling in Drosophila. Genes Dev. 2007 , 21 , 2277–2282. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- He, K.; Xiao, H.; Sun, Y.; Ding, S.; Situ, G.; Li, F. Transgenic microRNA-14 rice shows high resistance to rice stemborer. Plant Biotechnol. J. 2019 , 17 , 461–471. [ Google Scholar ] [ CrossRef ]
- He, K.; Xiao, H.; Sun, Y.; Situ, G.; Xi, Y.; Li, F. microRNA-14 as an efficient suppressor to switch off ecdysone production after ecdysis in insects. RNA Biol. 2019 , 16 , 1313–1325. [ Google Scholar ] [ CrossRef ]
- Epstein, Y.; Perry, N.; Volin, M.; Zohar-Fux, M.; Braun, R.; Porat-Kuperstein, L.; Toledano, H. miR-9a modulates maintenance and ageing of Drosophila germline stem cells by limiting N-cadherin expression. Nat. Commun. 2017 , 8 , 600. [ Google Scholar ] [ CrossRef ]
- Li, X.; Zhao, M.; Tian, M.; Zhao, J.; Cai, W.; Hua, H. An InR/mir-9a/NlUbx regulatory cascade regulates wing diphenism in brown planthoppers. Insect Sci. 2021 , 28 , 1300–1313. [ Google Scholar ] [ CrossRef ]
- Guo, H.; Song, X.; Wang, G.; Yang, K.; Wang, Y.; Niu, L.; Chen, X.; Fang, R. Plant-generated artificial small RNAs mediated aphid resistance. PLoS ONE 2014 , 9 , e97410. [ Google Scholar ] [ CrossRef ]
- Pruss, G.J.; Nester, E.W.; Vance, V. Infiltration with Agrobacterium tumefaciens induces host defense and development-dependent responses in the infiltrated zone. Mol. Plant Microbe Interact. 2008 , 21 , 1528–1538. [ Google Scholar ] [ CrossRef ]
- Djami-Tchatchou, A.; Dubery, I. miR393 regulation of lectin receptor-like kinases associated with LPS perception in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2019 , 513 , 88–92. [ Google Scholar ] [ CrossRef ]
- Kasschau, K.; Xie, Z.; Allen, E.; Llave, C.; Chapman, E.; Krizan, K.; Carrington, J. P1/HC-Pro, a viral suppressor of RNA silencing, interferes with Arabidopsis development and miRNA function. Dev. Cell 2003 , 4 , 205–217. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chapman, E.; Prokhnevsky, A.; Gopinath, K.; Dolja, V.; Carrington, J. Viral RNA silencing suppressors inhibit the microRNA pathway at an intermediate step. Genes Dev. 2004 , 18 , 1179–1186. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Windels, D.; Vazquez, F. miR393: Integrator of environmental cues in auxin signaling? Plant Signal. Behav. 2011 , 6 , 1672–1675. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, Y.; Cao, X.; Zhu, Y.; Yang, X.; Zhang, K.; Xiao, Z.; Wang, H.; Zhao, J.; Zhang, L.; Li, G.; et al. Osa-miR398b boosts H2O2 production and rice blast disease-resistance via multiple superoxide dismutases. New Phytol. 2019 , 222 , 1507–1522. [ Google Scholar ] [ CrossRef ]
- Gill, S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010 , 48 , 909–930. [ Google Scholar ] [ CrossRef ]
- Feng, H.; Wang, X.; Zhang, Q.; Fu, Y.; Feng, C.; Wang, B.; Huang, L.; Kang, Z. Monodehydroascorbate reductase gene, regulated by the wheat PN-2013 miRNA, contributes to adult wheat plant resistance to stripe rust through ROS metabolism. Biochim. Biophys. Acta 2014 , 1839 , 1–12. [ Google Scholar ] [ CrossRef ]
- Wu, J.; Yang, R.; Yang, Z.; Yao, S.; Zhao, S.; Wang, Y.; Li, P.; Song, X.; Jin, L.; Zhou, T.; et al. ROS accumulation and antiviral defence control by microRNA528 in rice. Nat. Plants 2017 , 3 , 485–510. [ Google Scholar ] [ CrossRef ]
- Navarro, L.; Dunoyer, P.; Jay, F.; Arnold, B.; Dharmasiri, N.; Estelle, M.; Voinnet, O.; Jones, J.D. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 2006 , 312 , 436–439. [ Google Scholar ] [ CrossRef ]
- Yin, Z.; Li, Y.; Han, X.; Shen, F. Genome-wide profiling of miRNAs and other small non-coding RNAs in the Verticillium dahliae-inoculated cotton roots. PLoS ONE 2012 , 7 , e35765. [ Google Scholar ] [ CrossRef ]
- Zhang, T.; Zhao, Y.; Zhao, J.; Wang, S.; Jin, Y.; Chen, Z.; Fang, Y.; Hua, C.; Ding, S.; Guo, H. Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nat. Plants 2016 , 2 , 16153. [ Google Scholar ] [ CrossRef ]
- Hu, G.; Lei, Y.; Liu, J.; Hao, M.; Zhang, Z.; Tang, Y.; Chen, A.; Wu, J. The ghr-miR164 and GhNAC100 modulate cotton plant resistance against Verticillium dahlia. Plant Sci. 2020 , 293 , 110438. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jiang, N.; Cui, J.; Shi, Y.; Yang, G.; Zhou, X.; Hou, X.; Meng, J.; Luan, Y. Tomato lncRNA23468 functions as a competing endogenous RNA to modulate NBSLRR genes by decoying miR482b in the tomato- Phytophthora infestans interaction. Hortic. Res. 2019 , 6 , 28. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wang, H.; Li, Y.; Chern, M.; Zhu, Y.; Zhang, L.; Lu, J.; Li, X.; Dang, W.; Ma, X.; Yang, Z.; et al. Suppression of rice miR168 improves yield, flowering time and immunity. Nat. Plants 2021 , 7 , 129–136. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yang, Z.; Hui, S.; Lv, Y.; Zhang, M.; Chen, D.; Tian, J.; Zhang, H.; Liu, H.; Cao, J.; Xie, W.; et al. miR395-regulated sulfate metabolism exploits pathogen sensitivity to sulfate to boost immunity in rice. Mol. Plant 2022 , 15 , 671–688. [ Google Scholar ] [ CrossRef ]
- Li, F.; Pignatta, D.; Bendix, C.; Brunkard, J.O.; Cohn, M.M.; Tung, J.; Sun, H.; Kumar, P.; Baker, B. MicroRNA regulation of plant innate immune receptors. Proc. Natl. Acad. Sci. USA 2012 , 109 , 1790–1795. [ Google Scholar ] [ CrossRef ]
- Luo, M.; Peng, H.; Gao, J.; Pan, G.; Zhang, Z. Identification and functional analysis of miRNAs in response to banded leaf and sheath blight in Zea mays . Chin. J. Biochem. Mol. Biol. 2012 , 28 , 1122–1132. [ Google Scholar ]
- Loreti, E.; Perata, P. Mobile plant microRNAs allow communication within and between organisms. New Phytol. 2022 , 235 , 2176–2182. [ Google Scholar ] [ CrossRef ]
- Niu, D.; Lii, Y.; Chellappan, P.; Lei, L.; Peralta, K.; Jiang, C.; Guo, J.; Coaker, G.; Jin, H. miRNA863-3p sequentially targets negative immune regulator ARLPKs and positive regulator SERRATE upon bacterial infection. Nat. Commun. 2016 , 7 , 11324. [ Google Scholar ] [ CrossRef ]
- Zhang, X.; Yuan, Y.R.; Pei, Y.; Lin, S.; Tuschl, T.; Patel, D.; Chua, N. Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev. 2006 , 20 , 3255–3268. [ Google Scholar ] [ CrossRef ]
- Schwab, R.; Ossowski, S.; Riester, M.; Warthmann, N.; Weigel, D. Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 2006 , 18 , 1121–1133. [ Google Scholar ] [ CrossRef ]
- Kryovrysanaki, N.; James, A.; Tselika, M.; Bardani, E.; Kalantidis, K. RNA silencing pathways in plant development and defense. Int. J. Dev. Biol. 2022 , 66 , 163–175. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ding, S. RNA silencing. Curr. Opin. Biotechnol. 2000 , 11 , 152–156. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, Y.; Zhang, Q.; Zhang, J.; Wu, L.; Qi, Y.; Zhou, J. Identification of microRNAs involved in pathogen-associated molecular pattern-triggered plant innate immunity. Plant Physiol. 2010 , 152 , 2222–2231. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Baldrich, P.; Kakar, K.; Sire, C.; Moreno, A.; Berger, A.; GarcíaChapa, M.; López-Moya, J.; Riechmann, J.; San Segundo, B. Small RNA profiling reveals regulation of Arabidopsis miR168 and heterochromatic siRNA415 in response to fungal elicitors. BMC Genom. 2014 , 15 , 1083. [ Google Scholar ] [ CrossRef ]
- Zhang, J.; Zhang, H.; Srivastava, A.; Pan, Y.; Bai, J.; Fang, J.; Shi, H.; Zhu, J. Knockdown of rice microRNA166 confers drought resistance by causing leaf rolling and altering stem xylem development. Plant Physiol. 2018 , 176 , 2082–2094. [ Google Scholar ] [ CrossRef ]
- Mengistu, A.; Tenkegna, T. The role of miRNA in plant-virus interaction: A review. Mol. Biol. Rep. 2021 , 48 , 2853–2861. [ Google Scholar ] [ CrossRef ]
- Jagadeeswaran, G.; Saini, A.; Sunkar, R. Biotic and abiotic stress down-regulate miR398 expression in Arabidopsis. Planta 2009 , 229 , 1009–1014. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rawat, N.; Kiran, S.; Du, D.; Gmitter, F.; Deng, Z. Comprehensive meta-analysis, co-expression, and miRNA nested network analysis identifies gene candidates in citrus against Huanglongbing disease. BMC Plant Biol. 2015 , 15 , 184. [ Google Scholar ] [ CrossRef ]
- Chisholm, S.; Coaker, G.; Day, B.; Staskawicz, B. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 2006 , 124 , 810–814. [ Google Scholar ] [ CrossRef ]
- Ngou, B.; Ding, P.; Jones, J. Thirty years of resistance: Zig-zag through the plant immune system. Plant Cell 2022 , 34 , 1447–1478. [ Google Scholar ] [ CrossRef ]
- Shivaprasad, P.; Chen, H.; Patel, K.; Bond, D.; Santos, B.; Baulcombe, D. A microRNA superfamily regulates nucleotide binding site-leucine-rich repeats and other mRNAs. Plant Cell 2012 , 24 , 859–874. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lu, S.; Sun, Y.; Chiang, V. Stress-responsive microRNAs in populus. Plant J. 2008 , 55 , 131–151. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Campo, S.; Peris-Peris, C.; Siré, C.; Moreno, A.; Donaire, L.; Zytnicki, M.; Notredame, C.; Llave, C.; San Segundo, B. Identification of a novel microRNA (miRNA) from rice that targets an alternatively spliced transcript of the Nramp6 (Natural resistance-associated macrophage protein 6) gene involved in pathogen resistance. New Phytol. 2013 , 199 , 212–227. [ Google Scholar ] [ CrossRef ]
- Li, Y.; Lu, Y.; Shi, Y.; Wu, L.; Xu, Y.; Huang, F. Multiple rice micrornas are involved in immunity against the blast fungus Magnaporthe oryzae. Plant Physiol. 2014 , 164 , 1077–1092. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Xin, M.; Wang, Y.; Yao, Y.; Xie, C.; Peng, H.; Ni, Z.; Sun, Q. Diverse set of microRNAs are responsive tompowdery mildew infection and heat stress in wheat ( Triticum aestivum L.). BMC Plant Biol. 2010 , 10 , 123. [ Google Scholar ] [ CrossRef ]
- Hua, C.; Zhao, J.; Guo, H. Trans-Kingdom RNA Silencing in Plant-Fungal Pathogen Interactions. Mol. Plant 2018 , 11 , 235–244. [ Google Scholar ] [ CrossRef ]
- Tang, J.; Gu, X.; Liu, J.; He, Z. Roles of small RNAs in crop disease resistance. Stress Biol. 2021 , 1 , 6. [ Google Scholar ] [ CrossRef ]
- Ding, T.; Li, W.; Li, F.; Ren, M.; Wang, W. microRNAs: Key Regulators in Plant Responses to Abiotic and Biotic Stresses via Endogenous and Cross-Kingdom Mechanisms. Int. J. Mol. Sci. 2024 , 25 , 1154. [ Google Scholar ] [ CrossRef ]
- Sablok, G.; Pérez-Quintero, Á.; Hassan, M.; Tatarinova, T.; López, C. Artificial microRNAs (amiRNAs) engineering—On how microRNAbased silencing methods have affected current plant silencing research. Biochem. Biophys. Res. Commun. 2011 , 406 , 315–319. [ Google Scholar ] [ CrossRef ]
- Ai, T.; Zhang, L.; Gao, Z.; Zhu, C.; Guo, X. Highly efficient virus resistance mediated by artificial microRNAs that target the suppressor of PVX and PVY in plants. Plant Biol. 2011 , 13 , 304–316. [ Google Scholar ] [ CrossRef ]
- Park, W.; Zhai, J.; Lee, J. Highly efficient gene silencing using perfect complementary artificial miRNA targeting AP1 or heteromeric artificial miRNA targeting AP1 and CAL genes. Plant Cell Rep. 2009 , 28 , 469–480. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schwab, R.; Palatnik, J.; Riester, M.; Schommer, C.; Schmidet, M.; Weigel, D. Specific effects of microRNAs on the plant transcriptome. Dev. Cell 2005 , 8 , 517–527. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Niu, Q.; Lin, S.; Reyes, J.; Chen, K.; Wu, H.; Yeh, S.; Chua, N. Expression of artificial microRNAs in transgenic Arabidopsis thaliana confers virus resistance. Nat. Biotechnol. 2006 , 24 , 1420–1428. [ Google Scholar ] [ CrossRef ]
- Zhang, X.; Li, H.; Zhang, J.; Zhang, C.; Gong, P.; Ziaf, K.; Xiao, F.; Ye, Z. Expression of artificial microRNAs in tomato confers efficient and stable virus resistance in a cellautonomous manner. Transgenic Res. 2011 , 20 , 569–581. [ Google Scholar ] [ CrossRef ]
- Saini, R.; Raman, V.; Dhandapani, G.; Malhotra, E.; Sreevathsa, R.; Kumar, P.; Sharma, T.; Pattanayak, D. Silencing of HaAce1 gene by host-delivered artificial microRNA disrupts growth and development of Helicoverpa armigera . PLoS ONE 2018 , 13 , e0194150. [ Google Scholar ] [ CrossRef ]
- Qu, J.; Ye, J.; Fang, R. Artificial microRNA-mediated virus resistance in plants. J. Virol. 2007 , 81 , 6690–6699. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Song, Y.; Han, Q.; Jiang, F.; Sun, R.; Fan, Z.; Zhu, C.; Wen, F. Effects of the sequence characteristics of miRNAs on multi-viral resistance mediated by single amiRNAs in transgenic tobacco. Plant Physiol. Biochem. 2014 , 77 , 90–98. [ Google Scholar ] [ CrossRef ]
- Zheng, X.; Weng, Z.; Li, H.; Kong, Z.; Zhou, Z.; Li, F.; Ma, W.; Lin, Y.; Chen, H. Transgenic rice overexpressing insect endogenous microRNA csu-novel-260 is resistant to striped stem borer under field conditions. Plant Biotechnol. J. 2021 , 19 , 421–423. [ Google Scholar ] [ CrossRef ]
- Jiang, S.; Wu, H.; Liu, H.; Zheng, J.; Lin, Y.; Chen, H. The overexpression of insect endogenous small RNAs in transgenic rice inhibits growth and delays pupation of striped stem borer ( Chilo suppressalis ). Pest Manag. Sci. 2017 , 73 , 1453–1461. [ Google Scholar ] [ CrossRef ]
- Liu, H.; Shen, E.; Wu, H.; Ma, W.; Chen, H.; Lin, Y. Trans-kingdom expression of an insect endogenous microRNA in rice enhances resistance to striped stem borer Chilo suppressalis . Pest Manag. Sci. 2022 , 78 , 770–777. [ Google Scholar ] [ CrossRef ]
- Li, C.; Wei, J.; Lin, Y.; Chen, H. Gene silencing using the recessive rice bacterial blight resistance gene xa13 as a new paradigm in plant breeding. Plant Cell Rep. 2012 , 31 , 851–862. [ Google Scholar ] [ CrossRef ]
- Shen, W.; Cao, S.; Liu, J.; Zhang, W.; Chen, J.; Li, J. Overexpression of an Osa-miR162a Derivative in Rice Confers Cross-Kingdom RNA Interference-Mediated Brown Planthopper Resistance without Perturbing Host Development. Int. J. Mol. Sci. 2021 , 22 , 12652. [ Google Scholar ] [ CrossRef ]
- Daborn, P.; Lumb, C.; Harrop, T.; Blasetti, A.; Pasricha, S.; Morin, S.; Mitchell, S.; Donnelly, M.; Müller, P.; Batterham, P. Using Drosophila melanogaster to validate metabolism-based insecticide resistance from insect pests. Insect Biochem. Mol. Biol. 2012 , 42 , 918–924. [ Google Scholar ] [ CrossRef ]
- Kung, Y.; Lin, S.; Huang, Y.; Chen, T.; Harish, S.; Chua, N.; Yeh, S. Multiple artificial microRNAs targeting conserved motifs of the replicase gene confer robust transgenic resistance to negative-sense single-stranded RNA plant virus. Mol. Plant Pathol. 2012 , 13 , 303–317. [ Google Scholar ] [ CrossRef ]
- Zeng, Y.; Wagner, E.; Cullen, B. Both natural and designed micro RNAs can inhibit the expression of cognate mRNAs when expressed in human cells. Mol. Cell 2002 , 9 , 1327–1333. [ Google Scholar ] [ CrossRef ]
- Parizotto, E.; Dunoyer, P.; Rahm, N.; Himber, C.; Voinnet, O. In vivo investigation of the transcription, processing, endonucleolytic activity, and functional relevance of the spatial distribution of a plant miRNA. Genes Dev. 2004 , 18 , 2237–2242. [ Google Scholar ] [ CrossRef ]
- Toppino, L.; Kooiker, M.; Lindner, M.; Dreni, L.; Rotino, G.; Kater, M. Reversible male sterility in eggplant ( Solanum melongena L.) by artificial microRNA-mediated silencing of general transcription factor genes. Plant Biotechnol. J. 2011 , 9 , 684–692. [ Google Scholar ] [ CrossRef ]
- Warthmann, N.; Chen, H.; Ossowski, S.; Weigel, D.; Hervé, P. Highly specific gene silencing by artificial miRNAs in rice. PLoS ONE 2008 , 3 , e1829. [ Google Scholar ] [ CrossRef ]
- Wagaba, H.; Patil, B.; Mukasa, S.; Alicai, T.; Fauquet, C.; Taylor, N. Artificial microRNA-derived resistance to Cassava brown streak disease. J. Virol Methods 2016 , 231 , 231–243. [ Google Scholar ] [ CrossRef ]
- Peng, J.; Chen, T.; Raja, J.; Yang, C.; Chien, W.; Lin, C.; Liu, F.; Wu, H.; Yeh, S. Broad-spectrum transgenic resistance against distinct tospovirus species at the genus level. PLoS ONE 2014 , 9 , e96073. [ Google Scholar ] [ CrossRef ]
- Chandra, T.; Jaiswal, S.; Iquebal, M.; Singh, R.; Gautam, R.; Rai, A.; Kumar, D. Revitalizing miRNAs mediated agronomical advantageous traits improvement in rice. Plant Physiol. Biochem. 2023 , 202 , 107933. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lin, S.; Wu, H.; Elena, S.; Chen, K.; Niu, Q.; Yeh, S.; Chen, C.; Chua, N. Molecular evolution of a viral noncoding sequence under the selective pressure of amiRNA-mediated silencing. PLoS Pathog. 2009 , 5 , e1000312. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Simón-Mateo, C.; García, J.A. MicroRNA-guided processing impairs Plum pox virus replication, but the virus readily evolves to escape this silencing mechanism. J. Virol. 2006 , 80 , 2429–2436. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Duan, C.G.; Wang, C.H.; Fang, R.X.; Guo, H.S. Artificial MicroRNAs highly accessible to targets confer efficient virus resistance in plants. J. Virol. 2008 , 82 , 11084–11095. [ Google Scholar ] [ CrossRef ]
- Dhandapani, R.; Gurusamy, D.; Howell, J.; Palli, S. Development of CS-TPP-dsRNA nanoparticles to enhance RNAi efficiency in the yellow fever mosquito. Aedes Aegypti. Sci. Rep. 2019 , 9 , 8775. [ Google Scholar ] [ CrossRef ]
- Niehl, A.; Heinlein, M. Perception of double-stranded RNA in plant antiviral immunity. Mol. Plant Pathol. 2019 , 20 , 1203–1210. [ Google Scholar ] [ CrossRef ]
- Wang, M.; Jin, H. Spray-induced gene silencing:A powerful innovative strategy for crop protection. Trends Microbiol. 2017 , 25 , 4–6. [ Google Scholar ] [ CrossRef ]
- Zotti, M.; Dos Santos, E.; Cagliari, D.; Christiaens, O.; Taning, C.; Smagghe, G. RNA interference technology in crop protection against arthropod pests, pathogens and nematodes. Pest Manag. Sci. 2018 , 74 , 1239–1250. [ Google Scholar ] [ CrossRef ]
- Camacho, A.; Van Deynze, A.; Chi-Ham, C.; Bennett, A.B. Genetically engineered crops that fly under the US regulatory radar. Nat. Biotechnol. 2014 , 32 , 1087–1091. [ Google Scholar ] [ CrossRef ]
- Demirer, G.S.; Zhang, H.; Goh, N.S.; González-Grandío, E.; Landry, M.P. Carbon nanotube-mediated DNA delivery without transgene integration in intact plants. Nat. Protoc. 2019 , 14 , 2954–2971. [ Google Scholar ] [ CrossRef ]
- Demirer, G.S.; Zhang, H.; Matos, J.L.; Goh, N.S.; Cunningham, F.J.; Sung, Y.; Chang, R.; Aditham, A.J.; Chio, L.; Cho, M.J.; et al. High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat. Nanotechnol. 2019 , 14 , 456–464. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tripathi, S.; Sarkar, S. Carbon dots in agricultural system. In Carbon Dots in Agricultural Systems ; Khan, R., Murali, S., Gogoi, S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 175–197. [ Google Scholar ] [ CrossRef ]
- Schwartz, S.H.; Hendrix, B.; Hoffer, P.; Sanders, R.A.; Zheng, W. Carbon Dots for Efficient Small Interfering RNA Delivery and Gene Silencing in Plants. Plant Physiol. 2020 , 184 , 647–657. [ Google Scholar ] [ CrossRef ] [ PubMed ]
Click here to enlarge figure
| miRNA | Target(s) | Plant Species | Insect Species | Function | Reference |
|---|---|---|---|---|---|
| AtmiR156 | SPL9 | A. thaliana | Helicoverpa armigera Plutella xylostella | Decrease weight and prolong larval stage | [ ] |
| Ghr-miR166b | ATP synthase gene | Gossypium hirsutum | Bemisia tabaci | Death | [ ] |
| miR-24 | Chitinase | N. tabacum | H. armigera | Death | [ ] |
| OsmiR156 | SPL14 | O. sativa | Nilaparvata lugens | Decrease fertility | [ ] |
| OsmiR159 | OsGAMYBL2 | O. sativa | N. lugens | Decrease honeydew excretion | [ ] |
| OsmiR396 | OsGRF8 | O. sativa | N. lugens | Decrease honeydew excretion | [ ] |
| OsmiR162a | OsDCL1 | O. sativa | N. lugens | Decrease fertility | [ ] |
| PtmiR319a | TCP | Populus tomentosa | Spodoptera frugiperda | Decrease feeding | [ ] |
| SlmiR319 | TCP4 | Solanum lycopersicum | Meloidogyne incognita | Decrease fertility | [ ] |
| miRNA | Target(s) | Plant Species | Pathogen Species | Function | Reference |
|---|---|---|---|---|---|
| AtmiR393 | TIR1 | A. thaliana | Pseudomonas syringae | Increase resistance | [ ] |
| GhmiR2118 | TIR-NBS-LRR genes | G. hirsutum | Verticillium dahliae | Promote target protein accumulation, enhance root defense | [ ] |
| GhmiR166, GhmiR159 | Clp-1, HiC-15 | G. hirsutum | V. dahliae | Identification and degradation | [ ] |
| GhmiR164 | GhNAC100 | G. hirsutum | V. dahliae | Increase resistance | [ ] |
| NtmiR482b | Solyc02g036270.2 | N. tabacum | Phytophthora parasitica | Increase NBS-LRR expression and resistance | [ ] |
| OsmiR168 | Ago1 | O. sativa | Magnaporthe oryzae | Enhance resistance | [ ] |
| OsmiR395 | ATP sulfurylase gene OsAPS1 | O. sativa | Xanthomonas oryzae pv. oryzae (Xoo); X. oryzae pv. oryzicola (Xoc) | Enhance broad-spectrum resistance. | [ ] |
| OsmiR398b | Superoxide dismutase | O. sativa | M. oryzae | Upregulate of H O level, increase resistance | [ ] |
| OsmiR528 | L-Ascorbate oxidase | O. sativa | Rice stripe tenuivirus | Reduce cell death and damage | [ ] |
| StmiR482, SlmiR482 | Ry, NL25, N, RB, R2, and R3a | Solanum tuberosum, S. lycopersicum | Phytophthora infestans | Genes cluster to confer resistance | [ ] |
| PN-2013 | Monodehydroascorbate reductase gene | Triticum aestivum | Puccinia striiformis | Upregulate PR gene expression, increase resistance | [ ] |
| ZmmiR168 | Ago1 | Z. mays | Rhizoctonia solani | Promote resistance gene accumulations, increase resistance | [ ] |
| Target(s) | Plant Species | Insect/Pathogen Species | Function | Reference |
|---|---|---|---|---|
| HC-Pro, p69 gene | A. thaliana | Turnip mosaic virus, Turnip yellow mosaic virus | Mediate viral RNA cleavage, Increase resistance | [ ] |
| ATP synthase gene | G. hirsutum | Bemisia Tabaci | Death | [ ] |
| 2b gene | Lycopersicum esculentummill | Cucumber mosaic virus | Target viral RNA, Increase resistance | [ ] |
| HaAce1 gene | N. tabacum | H. armigera | Induce larval death and deformity in adult | [ ] |
| 2b gene | N. tabacum | Cucumber mosaic virus | Target viral RNA, Increase resistance | [ ] |
| Nuclear inclusion b protein, coat protein | N. tabacum | Potato virus Y | Target viral RNA, Increase resistance | [ ] |
| MpAChE2 | N. tabacum | M. persicae | Decrease fecundity | [ ] |
| Ecdysone receptor, Spook gene | O. sativa | Chilo suppressalis | Death | [ ] |
| Disembodied protein | O. sativa | C. suppressalis | Death | [ ] |
| DN70206_c1_g10 | O. sativa | C. suppressalis | Decrease weight, prolong larval stage | [ ] |
| DN90065_c0_g12 | O. sativa | C. suppressalis | Decrease weight, decrease eclosion rate, prolong larval stage | [ ] |
| Xa13 | O. sativa | X. oryzae | Silence genes, increase resistance | [ ] |
| HC-Pro, p25 gene | S. tuberosum | Potato virus Y | Reduce pathogenicity | [ ] |
| HC-Pro, p25 gene | S. tuberosum | Potato virus X | Reduce pathogenicity | [ ] |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Ma, Z.; Wang, J.; Li, C. Research Progress on miRNAs and Artificial miRNAs in Insect and Disease Resistance and Breeding in Plants. Genes 2024 , 15 , 1200. https://doi.org/10.3390/genes15091200
Ma Z, Wang J, Li C. Research Progress on miRNAs and Artificial miRNAs in Insect and Disease Resistance and Breeding in Plants. Genes . 2024; 15(9):1200. https://doi.org/10.3390/genes15091200
Ma, Zengfeng, Jianyu Wang, and Changyan Li. 2024. "Research Progress on miRNAs and Artificial miRNAs in Insect and Disease Resistance and Breeding in Plants" Genes 15, no. 9: 1200. https://doi.org/10.3390/genes15091200
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
- Open access
- Published: 14 September 2024
OsWRKY70 Plays Opposite Roles in Blast Resistance and Cold Stress Tolerance in Rice
- Jiangdi Li 1 ,
- Yating Chen 1 ,
- Rui Zhang 1 ,
- Rujie Wang 1 ,
- Haiwen Zhang 2 &
- Guiqing Xiao 1
Rice volume 17 , Article number: 61 ( 2024 ) Cite this article
Metrics details
The transcription factor WRKYs play pivotal roles in the adapting to adverse environments in plants. Prior research has demonstrated the involvement of OsWRKY70 in resistance against herbivores and its response to abiotic stress. Here, we reported the functional analysis of OsWRKY70 in immunity against fungal diseases and cold tolerance. The results revealed that OsWRKY70 was induced by various Magnaporthe oryzae strains. Knock out mutants of OsWRKY70 , which were generated by the CRISPR/Cas9 system, exhibited enhanced resistance to M. oryzae . This was consistent with fortifying the reactive oxygen species (ROS) burst after inoculation in the mutants, elevated transcript levels of defense-responsive genes ( OsPR1b , OsPBZ1 , OsPOX8.1 and OsPOX22.3 ) and the observation of the sluggish growth of invasive hyphae under fluorescence microscope. RNA sequencing (RNA-seq) and quantitative real-time PCR (qRT-PCR) validations demonstrated that differentially expressed genes were related to plant-pathogen interactions, hormone transduction and MAPK cascades. Notably, OsbHLH6 , a key component of the JA signaling pathway, was down-regulated in the mutants compared to wild type plants. Further investigation confirmed that OsWRKY70 bound to the promoter of OsbHLH6 by semi-in vivo chromatin immunoprecipitation (ChIP). Additionally, the loss-function of OsWRKY70 impaired cold tolerance in rice. The enhanced susceptibility in the mutants characterized by excessive ROS production, elevated ion leakage rate and increased malondialdehyde content, as well as decreased activity of catalase (CAT) and peroxidase (POD) under low temperature stress was, which might be attributed to down-regulation of cold-responsive genes ( OsLti6b and OsICE1 ). In conclusion, our findings indicate that OsWRKY70 negatively contributes to blast resistance but positively regulates cold tolerance in rice, providing a strategy for crop breeding with tolerance to stress.
Introduction
Plant growth and development are greatly affected by biotic and abiotic stresses, including pathogen attacks, insect herbivory, extreme temperatures, high salinity and various other factors. To adapt to adverse environments, plants have evolved intricate regulatory mechanisms at the molecular, physiological, biochemical and metabolic levels (Nejat et al. 2017 ). For example, after perceiving the stimulation, plants promptly and effectively initiate extensive transcriptional reprogramming of gene expression, generating a variety of signaling molecules, including phytohormones, reactive oxygen species (ROS), calcium ions (Ca 2+ ) (Buscaill et al. 2014 ; Ng et al. 2018 ; Chen et al. 2020 ). This stress-responsive reprograming requires the coordinated and precise timing of the involvement of different types of transcription factors (TFs) in both temporal and spatial dimensions (Khan et al. 2018 ). Genetic and molecular studies have elucidated the functional attributes of TF families such as WRKY, AP2/ERF, NAC, MYB and bHLH in plants (Ng et al. 2018 ; Kajla et al. 2023 ).
WRKY TFs are among the largest transcriptional regulatory families in plants. These proteins were divided into three subgroups, namely groups I, II and III, according to the number of WRKY domains and the type of zinc finger structure (Eulgem et al. 2000 ; Rushton et al. 2010 ). Up to now, WRKY TFs have been identified in different plant species, including Arabidopsis thaliana , Glycine max , Gossypium hirsutum , Oryza sativa , Solanum tuberosum , Triticum aestivum , Zea mays (Khoso et al. 2022 ; Song et al. 2023 ; Javed et al. 2023 ). For instance, there are 90 and 128 WRKYs in Arabidopsis thaliana and Oryza sativa , respectively (Tian et al. 2020 ). Some WRKY TFs have been evaluated for their pivotal roles in plant growth and development (Wang et al. 2023a ). For example, AtWRKY10 and AtWRKY41 are involved in seed development and dormancy (Ding et al. 2014 ; Xi et al. 2021 ), while AtWRKY23 promotes lateral root growth in Arabidopsis (Grunewald et al. 2012 ). The functions of OsWRKY11 , OsWRKY36 and OsWRKY53 have been separately demonstrated in the flowering process, plant height and grain size in rice, respectively (Cai et al. 2014 ; Lan et al. 2020 ; Tian et al. 2017 ).
WRKY TFs serve as critical regulators in plant immune response, specifically binding to W-box cis-element (T)(T)TGAC(C/T) in the promoter region of target genes to modulate transcription (Bakshi et al. 2014 ; Viana et al. 2018 ; Saha et al. 2023 ). In Arabidopsis , at least 20 WRKY genes have been identified as playing significant roles in diseases or insect resistance, including AtWRKY28 / 33 / 55 / 70 / 75 (Li et al. 2006 ; Chen et al. 2013 ; Wang et al. 2019 , 2020 ; Zhou et al. 2020 ; Saha et al. 2023 ). In rice, overexpression of OsWRKY67 up-regulates defense-related genes ( PR1a , PR1b , PR4 , PR10a and PR10b ), as well as leads to rapid induction of ROS upon stimulation with chitin and flg22 (Liu et al. 2018 ). The adaptation results from the interplay between WRKYs and a variety of plant hormones. For example, OsWRKY72 directly binds to the promoter of AOS1 , which is the jasmonic acid (JA) biosynthesis enzyme gene, negatively regulates JA synthesis and resistance to Xanthomonas oryzae pv oryzae ( Xoo ) infection (Hou et al. 2019 ). OsWRKY42 is a negative regulator to M. oryzae via repressing JA signaling and OsWRKY45-2 directly activates OsWRKY13 , whose encoding protein in turn transcriptionally suppresses OsWRKY42 / OsWRKY45-2 to regulate blast resistance (Cheng et al. 2015 ). OsNPR1 , a key regulator of salicylic acid (SA)-mediated resistance against fungal, bacterial disease and herbivores (Yuan et al. 2007 ; Feng et al. 2011 ), is downstream of OsWRKY03 (Liu et al. 2005 ). Previous research has documented that WRKY TFs play critical roles in plant immune response as one of the downstream substrates of the mitogen-activated protein kinase (MAPK) cascades. For instance, OsMKK10-2-OsMPK3/OsMPK6-OsWRKY31 module participates in the biosynthesis of secondary metabolite camalexin to regulate defense for rice blast pathogen (Wang et al. 2023c ). OsWRKY53 is downstream of MAPK cascades, meanwhile, it functions as a negative feedback modulator of OsMPK3/OsMPK6 in response to striped stem borer (SSB) (Hu et al. 2015 ). Moreover, OsWRKY53 acts as an early suppressor of induced defenses to mediate the MAPK-regulated OsWRKY24 / 33 / 70 expression, as well as SSB-induced JA, JA-isoleucine (JA-Ile) and ethylene (ET) biosynthesis (Hu et al. 2015 ). Collectively, these studies confirm that WRKY genes might contribute to multiple biotic stresses through complex signaling cascades such as ROS, plant hormones and MAPKs.
In the past decades, WRKYs have gained extensive attention due to their functions in involving tolerance against abiotic stress (Khoso et al. 2022 ; Goyal et al. 2023 ). For example, AtWRKY25/26/33 positively mediates tolerance to heat stress in Arabidopsis (Li et al. 2011 ). OsWRKY10 negatively regulates thermotolerance in rice by modulating ROS homeostasis and hypersensitive response (Chen et al. 2022 ). Overexpression of OsWRKY76 up-regulates peroxidase gene OsPrx71 and lipid metabolism gene OsBURP13/OsRAFTIN1 , thus alleviating the damage of low temperatures in rice (Yokotani et al. 2013 ). Some studies have highlighted the relationship of abiotic stress with WRKY genes by regulating the dehydrate-responsive element binding proteins (DREBs) or C-repeat binding factors (CBFs). In particular, the OsWRKY63-OsWRKY76-OsDREB1B signaling cascade module is involved in the regulation of chilling tolerance (Zhang et al. 2022b ). OsWRKY28 confers salinity tolerance by directly activating OsDREB1B in rice (Zhang et al. 2023 ). Additionally, overexpression of OsWRKY55 reduced drought tolerance, is consistent with accelerated water loss and massive accumulation of ROS (Huang et al. 2021 ).
It has been confirmed that several OsWRKY TFs play multiple roles in the adaptation to both biotic and abiotic stress in rice. For instance, OsWRKY10 is involved in resistance to fungus and thermotolerance (Wang et al. 2023b ; Chen et al. 2022 ). OsWRKY24 positively regulates fungal disease resistance, which has been identified as a potential candidate gene affecting cold sensitivity (Yokotani et al. 2018 ; Wu et al. 2023 ). OsWRKY53 acts as a negative modulator in resistance of bacterial blight, cold and salt tolerance (Xie et al. 2021 ; Tang et al. 2022b ; Yu et al. 2023 ), as well as a positive regulator in blast resistance (Chujo et al. 2007 ). OsWRKY76 plays opposite roles in blast resistance and cold stress tolerance (Yokotani et al. 2013 ). Empirical evidence suggests that the adaption of a variety of stress is associated with the crosstalk between WRKYs and phytohormones. For example, overexpression of OsWRKY13 enhanced rice resistance to M. oryzae and Xoo , which was accompanied by the activation of SA signaling pathways and the suppression of JA signaling pathways (Qiu et al. 2007 ). OsWRKY45-1 and OsWRKY45-2 are involved in JA and SA signaling, whereas they play opposite roles in bacterial disease resistance (Tao et al. 2009 ). In addition, OsWRKY45-1 and OsWRKY45-2 act as negative and positive regulators in abscisic acid (ABA) signaling, respectively, while only the latter negatively regulates the tolerance of salt stress (Tao et al. 2011 ). Furthermore, knock out of OsWRKY53 mutants confer rice cold tolerance at the booting stage by repressing the anther gibberellin content (Tang et al. 2022b ). Therefore, WRKY proteins are potentially important components in plant biotic and abiotic stress responses and are associated with an array of signaling crosstalk.
OsWRKY70 encodes a rice WRKY TF belonging to group I, characterized by the presence of two WRKY domains (Zhang et al. 2015 ; Li et al. 2023 ). The resistance to herbivores of OsWRKY70 in rice has been investigated (Li et al. 2015 ; Ye et al. 2019 ). However, whether and how OsWRKY70 affects rice immunity to fungal disease and its association with abiotic stress have not been reported. Our previous study has demonstrated that OsWRKY70 is induced by cold stress (Li et al. 2023 ). Here, we observed that the transcript level of OsWRKY70 was up-regulated by different strains of M. oryzae . The biological functions of OsWRKY70 were also investigated using knock out mutants of OsWRKY70 . Our findings revealed that the loss-function of OsWRKY70 drastically enhanced resistance against M. oryzae , while attenuating cold tolerance in rice, demonstrating that OsWRKY70 plays opposite roles in immunity and cold stress response.
OsWRKY70 is a Fungal Pathogen-Induced Gene
The previous finding that OsWRKY70 acts as an early regulator of plant response to herbivores (Li et al. 2015 ) inspired us to investigate its potential role in immunity against fungal diseases. We first assessed the expression level of OsWRKY70 by quantitative PCR (qPCR) after spray inoculation with M. oryzae strain 318-2. The results showed that the transcript level of OsWRKY70 was rapidly up-regulated upon infection, achieving a peak of 11.4-fold at 24 h post-inoculation (hpi) (Fig. 1 A). To confirm whether the specific fungal pathogens, strains R01-1 and 110-2 were examined. Obviously, the expression of OsWRKY70 was significantly induced by these strains, resulting in an increase of 9.5-fold and 15.6-fold at 48 hpi, respectively (Fig. 1 B, C). Overall, these results indicate that OsWRKY70 exhibits distinct responses to diverse fungal pathogens.
M. oryzae strains infection induced the expression of OsWRKY70 . ( A-C ) 2-week-old seedlings of NIP plants were inoculated with rice blast by spraying method. The qPCR analysis of OsWRKY70 expression levels at 0, 24 and 48 h post-inoculation with M. oryzae strains 318-2 ( A ), R01-1 ( B ) and 110-2 ( C ). OsActin was used as an internal control gene. The expression level of OsWRKY70 in plants under normal condition was set as 1. Data are the means ± SD of three biological replicates. Asterisks indicate significant differences by the Student’s t-test (* P < 0.05, ** P < 0.01)
Generation and Characterization of OsWRKY70 Knock Out Mutants
To elucidate the function of OsWRKY70, two homozygous mutants, designated oswrky70-7 and oswrky70-10 , were generated by CRISPR/Cas9-mediated genome editing. They exhibited a single base A and T insertion in the target sequence of OsWRKY70 , respectively (Fig. 2 A), resulting in the early termination of translation and thus loss of the conserved WRKY domain (Fig. 2 B). Under field condition, our observations revealed that there was no significant difference between the mutants and wild type in terms of plant height, the tiller count, flag leaf and panicle length (Fig. 2 C, D; Supplemental Table 2 ). Interestingly, the grain length of the mutants was notably greater than that of wild type, while the grain width exhibited a slight increase in oswrky70-10 , resulting in a promotion in 1000-grain weight of mutants as compared to wild type (Fig. 2 E-I). These results suggest that the loss-function of OsWRKY70 affects rice grain shape rather than its growth and development.
Generation of mutants and investigations of agronomic traits. ( A ) Knock out of OsWRKY70 gene by CRISPR/Cas9 technology. Target sequences of single-guide RNA (sgRNA) were listed. The mutation sites were indicated in red. Sequencing results of the OsWRKY70 in the mutants and wild type. ( B ) Schematic diagrams of OsWRKY70 in knock out mutants. ( C, D ) The plant height at seeding stage ( C ) and heading stage ( D ). Scale bar = 2 cm and 1 dm, respectively. ( E , F ) The grain length ( E ) and grain width ( F ). Scale bar = 1 cm, n = 20. ( G-I ) The Statistical analysis of grain length ( G ), grain width ( H ) and 1000-grain weight ( I ). Data are the means ± SD of three biological replicates. Asterisks indicate significant differences by the Student’s t-test (* P < 0.05, ** P < 0.01)
Loss-Function of OsWRKY70 Enhances Resistance Against M. Oryzae
To ascertain the involvement of OsWRKY70 in rice blast resistance, we first performed spray inoculation on 2-week-old seedlings with M. oryzae 318-2. At 5 days post-inoculation (dpi), the mutants exhibited a reduced overall severity blast compared to wild type (Fig. 3 A), as determined by a decrease in the lesion numbers by approximately 47.2% (Fig. 3 B). Next, measurement of fungal growth in planta, as revealed by analyzing the genomic DNA level of the MoPOT2 gene of M. oryzae , indicated that oswrky70-7 and oswrky70-10 supported less fungal growth, resulting in a reduction of 73.3% and 97.0%, respectively, as compared with that in wild type (Fig. 3 C). Additionally, we conducted inoculation to detached leaves and punch-inoculated at 4-week-old seedings with strain 318-2. Consistent with previous findings, the mutants were less susceptible to rice blast than wild type, manifesting as significantly reduced disease lesions and lower fungal biomass (Fig. 3 D-F; Figure S1 ). To further investigate the growth characteristics of M. oryzae spores, we infected rice leaf sheath with GFP-tagged strain RB22. At 24 hpi, we observed that almost no appressoria were formed in the leaf sheath cells of mutants, whereas maturing appressoria were found in those of wild type (Fig. 3 G). At 48 hpi, only a limited number of invasive hyphae with no branch were present in the mutant cells, while numerous invasive hyphae freely spread to adjacent cells in wild type (Fig. 3 H). Collectively, these results demonstrate that OsWRKY70 negatively regulates rice immune response against M. oryzae .
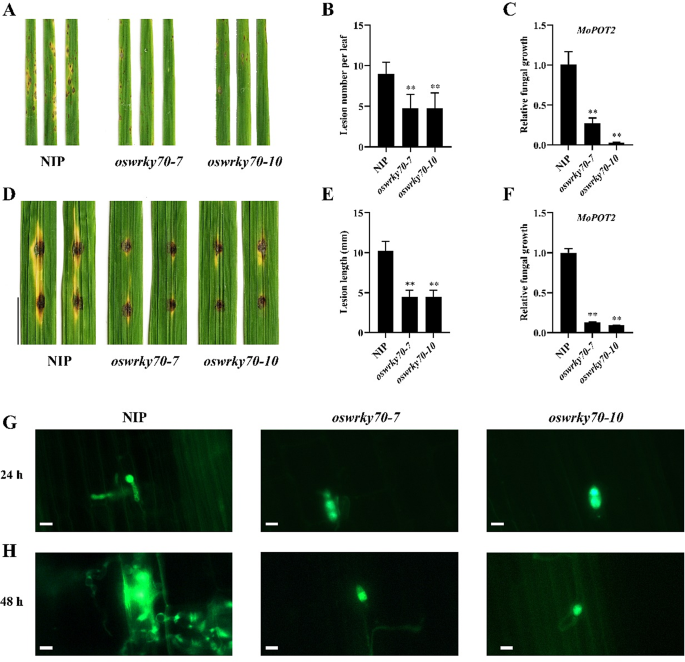
Loss-function of OsWRKY70 enhance resistance to M. oryzae. ( A ) Spray inoculation with M. oryzae spores on seedlings of mutants and wild type. ( B ) Lesion numbers on inoculated leaves at 5 days post-inoculation, n = 5. ( C ) Blast fungus biomass was determined by qPCR analysis using the ratio of M. oryzae DNA ( MoPOT2 ) to rice DNA ( OsAcitn ) in infected leaves. Data are the means ± SD of three biological replicates. Asterisks indicate significant differences by the Student’s t-test (* P < 0.05, ** P < 0.01). ( D ) The detached leaves of mutants and wild type were inoculated with the M. oryzae . Scale bar = 1 cm. ( E ) Lesion length was determined on leaves at 5 days after inoculation, n = 5. ( F ) Relative fungal biomass in the necrotic regions of the detached leaves was assessed by qPCR of the fungal MoPOT2 and normalized to rice OsActin . Data are the means ± SD of three biological replicates. Asterisks indicate significant differences by the Student’s t-test (* P < 0.05, ** P < 0.01). ( G , H ) Fluorescence microscopic observation of M. oryzae strain RB22-GFP infection on leaf sheath at 24 hpi ( G ) and 48 hpi ( H ). Scale bar = 20 μm
ROS Accumulation and Up-Regulated Defense Responsive Genes Enhance Resistance Against M. Oryzae in the Mutants
ROS bursts typically trigger plant defense responses (Li et al. 2021 ). Firstly, we conducted the analysis of ROS in the mutants and wild type after inoculation with M. oryzae by histochemical staining. The results showed that the presence of numerous reddish-brown spots on the leaves of mutants upon staining with 3, 3’-diaminobenzidine (DAB) and a significant increase in the number of blue dots were observed on the mutant leaves by nitro-blue tetrazolium (NBT) staining compared with those of wild type (Fig. 4 A, B). The findings indicate that enhanced accumulation of H 2 O 2 and superoxide anion probably contribute to resistance against rice blast disease. Subsequently, we investigated the expression patterns of defense-related genes. The results revealed that OsPBZ1 , OsPOX8.1 and OsPOX22.3 were dramatically up-regulated in the mutants compared to wild type before inoculation, except for OsPR1b (Fig. 4 C-F). Upon exposure M. oryzae strain 318-2, all four genes were a particularly notable up-regulation in the mutants compared with wild type (Fig. 4 C-F). It suggests that loss-function of OsWRKY70 activate defense response, which might play a pivotal role in response to biotic stress in rice.
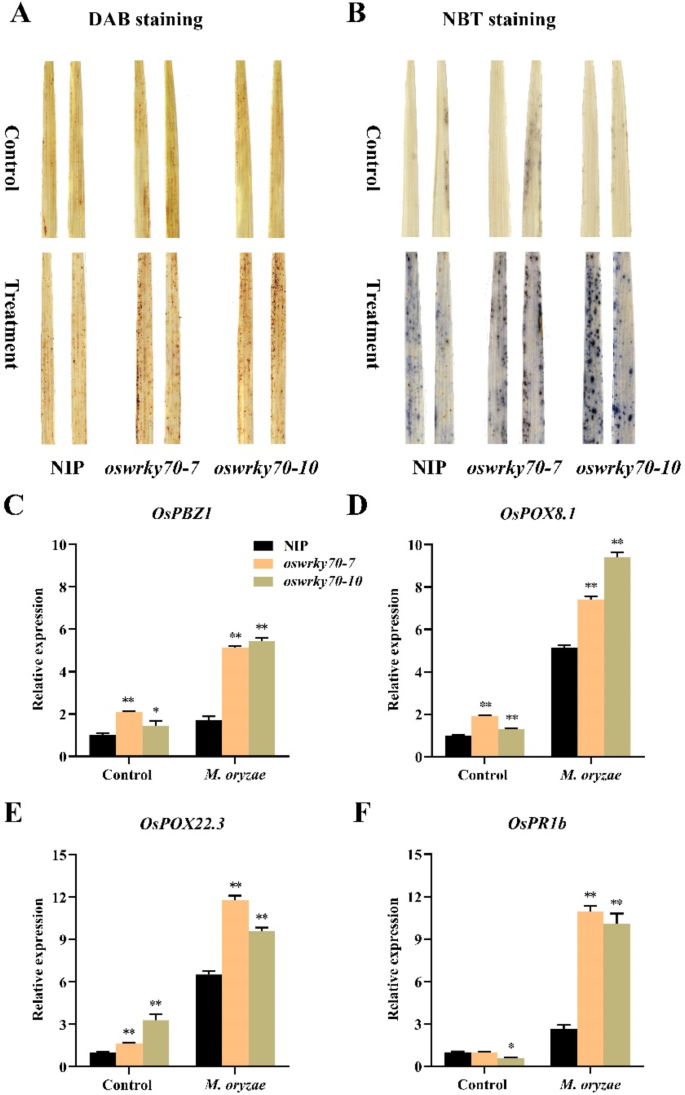
ROS accumulation and up-regulated defense responsive genes enhance M. oryzae resistance in rice. ( A , B ) DAB staining ( A ) and NBT staining showed H 2 O 2 and superoxide anion accumulation in leaves from 2-week-old seedings cultivated in normal culture and after inoculation with M. oryzae . ( C-F ) Expression levels of defense-responsive genes OsPBZ1 ( C ), OsPOX8.1 ( D ), OsPOX22.3 ( E ) and OsPR1b ( F ) in the leaves at seeding stage without inoculation and post-inoculation with M. oryzae . OsActin was used as an internal control. The expression level of the tested genes in wild type plants under normal condition was set as 1. Data are the means ± SD of three biological replicates. Asterisks indicate significant differences by the Student’s t-test (* P < 0.05, ** P < 0.01)
Transcriptome Analysis of OsWRKY70 Knock Out Mutant and qPCR Analysis
To further elucidate mechanisms underlying OsWRKY7 0-mediated immunity response in rice, we examined gene expression differences between oswrky70-7 (W7) and NIP (N) under normal condition and 48 h post-inoculation with M. oryzae using RNA sequencing (RNA-seq). Sample correlation and principal components analysis (PCA) of all genes showed that the three replicates of each treatment clustered together, suggesting good biological replicability (Supplemental Fig. 2 ). Rice genes whose transcript abundance showed a fold change (FC) ≥ 2 and false discovery rate (FDR) ≤ 0.01 were defined as differentially expressed genes (DEGs). We observed significant clustering differences between W7 and N under normal condition, which identified 2210 DEGs, including 1205 up-regulated and 1005 down-regulated genes. The data revealed that knock out of OsWRKY70 led to different transcript profiles. Additionally, a comparison of W7 and N at 48 hpi revealed 968 DEGs, containing 482 up-regulated genes, as well as 486 down-regulated genes in the mutant (Supplemental Fig. 2 ). However, the differences were not significantly enlarged by biotic stress.
KEGG classification illuminated the functional roles of these DEGs were mainly enriched into such as plant-pathogen interaction, plant hormone signal transduction, MAPK signal cascade and other metabolic processes (Fig. 5 A, B). Among them, several encoding putative NBS-LRR disease resistance protein genes, LOC_Os11g44960 , LOC_Os11g45050 and LOC_Os11g45180 , as well as the gene LOC_Os07g44130 in phenylpropanoids metabolism were significantly up-regulated in W7 (Fig. 5 C). In addition, OsbHLH6 , OsUGT74H4 and OsWRKY76 were negative regulators of disease resistance involved in JA, SA and MAPK cascade signaling, respectively (Yokotani et al. 2013 ; Meng et al. 2020 ; Wu et al. 2022 ), were also dramatically down-regulated in the mutant after inoculation (Fig. 5 C). The findings indicate that complex regulatory networks are activated in timely manners, which is crucial for rice blast resistance. To further screen candidate downstream target genes associated with the immune response of OsWRKY70, the leaves of 2-week-old mutants and wild type were individually collected for qPCR analysis after inoculation. The results revealed that the expression level of hormone, MAPK signaling and metabolic process genes was consistent with the RNA-seq analysis (Fig. 5 D-I).
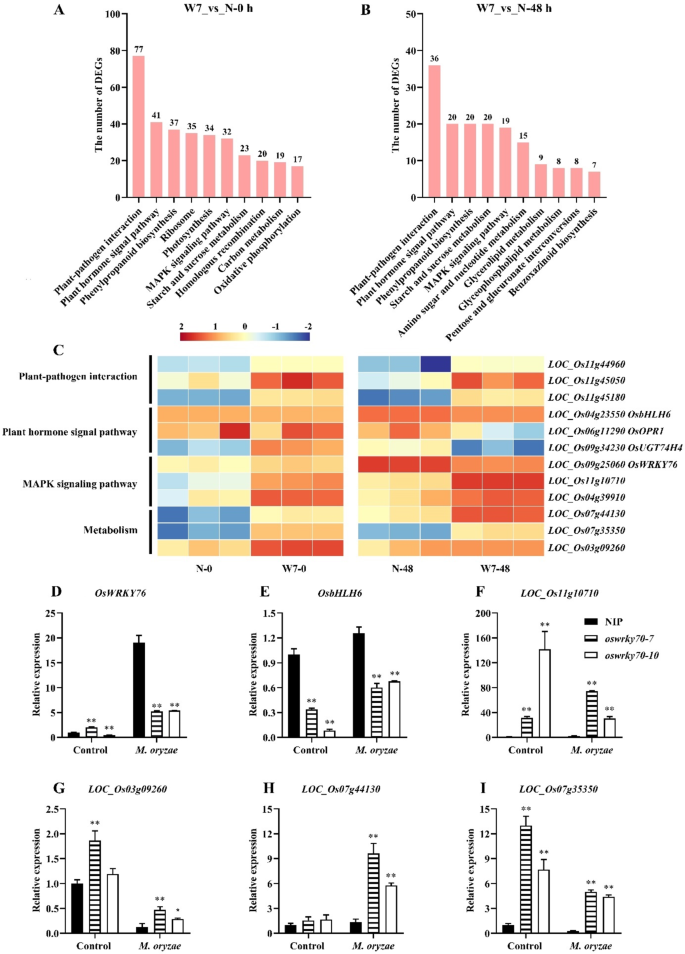
Transcriptomic analysis of oswrky70-7 before and after blast inoculation, as well as qPCR analysis to verify some DEGs. ( A , B ) KEGG pathway analysis of the DEGs between the mutant and wild type without inoculation ( A ) and 48 h post-inoculation ( B ) with M. oryzae 318-2. ( C ) Heatmaps showing the part of DEGs related to plant-pathogen interaction, plant hormone signal pathway, MAPK signaling pathway and metabolism. ( D-I) Quantitative PCR analysis of OsWRKY76 ( D ), OsbHLH6 ( E ), LOC_Os11g101710 ( F ), LOC_Os03g09260 ( G ), LOC_Os07g44130 ( H ) and LOC_Os07g35350 ( I ). 2-week-old wild-type mutants and wild type plants were grown in soil under 16 h light/8 h dark conditions and were treated under inoculation for 0 and 48 h. The leaves were collected for RNA extraction. Three biological replicates were performed. Relative expression levels were normalized by the transcript level of the OsActin gene as an internal control and the expression level of each gene of interest in wild type plants under normal condition was set as 1. Data are the means ± SD of three biological replicates. Asterisks indicate significant differences by the Student’s t-test (* P < 0.05, ** P < 0.01)
OsWRKY70 Interacts with Promoter of OsbHLH6 in Vitro
Our finding revealed that the expression level of OsbHLH6 was down-regulated in the mutants (Fig. 5 E). To further explore whether OsbHLH6 is the candidate target gene of OsWRKY70 in immune response, we initially analyzed the promoter region of OsbHLH6 and found ten W-box elements (Supplemental Table 3 ; Fig. 6 A). Notably, the candidate fragments encompassed one or two core sequences (TGAC) characteristic of W-box. Then, we conducted a semi-in vivo chromosome immunoprecipitation qPCR (ChIP-qPCR) assay to verify the interaction, which employed the purified recombinant His-OsWRKY70 protein (Fig. 6 B; Supplemental Fig. 3 ) and DNA fragments of rice genomic. The ChIP-qPCR results confirmed a significant approximately 4.0-fold enrichment of the P1 fragment in His-OsWRKY70 compared to the His control, while no enrichment was observed for the P2 fragment (Fig. 6 C). These findings suggest that OsbHLH6 might serve as a downstream target gene for OsWRKY70.
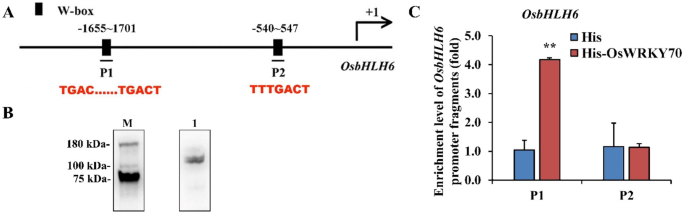
OsWRKY70 interacts the promoter of OsbHLH6 in vitro. ( A ) Schematic of the OsbHLH6 promoter. Black rectangles indicate W-box (TGAC core sequences) cis-elements in the promoter of OsbHLH6 . ( B ) The Western blot with purification of His-OsWRKY70 obtained from recombination of E. coli BL21. (Line 1, eluted His-OsWRKY70; M, protein marker). ( C ) The semi-in vivo ChIP-qPCR assay showed that His-OsWRKY70 enriched the P1 fragment of OsbHLH6 promoter. DNA fragments co-incubated with His was used as a negative control. Relative enrichment is represented as the normalized ratio of the ChIP DNA to the input genomic DNA at the site. P1 and P2 are the fragments of the promoter. Data are the means ± SD of three biological replicates. Asterisks indicate significant differences by the Student’s t-test (* P < 0.05, ** P < 0.01)
Loss-Function of OsWRKY70 Impairs Cold Tolerance in Rice
Our previous study that OsWRKY70 was induced by cold stress (Li et al. 2023 ) prompted us to investigate whether OsWRKY70 is involved in cold tolerance. Consequently, 2-week-old seedlings were subjected to 4 °C for 3 days and returned to normal growth conditions for a recovery period of 7 days. The mutants exhibited pronounced leaf curling symptoms after cold stress and a significant proportion of them could not be recovered compared with wild type (Fig. 7 A), demonstrating that OsWRKY70 is a positive regulator of cold tolerance in rice. This observation is consistent with the survival rate of oswrky70-7 and oswrky70-10 , which were 25.3% and 3.3%, compared to 42.7% of wild type (Fig. 7 B). Moreover, higher levels of electrolyte leakage and MDA content were in the mutants than that in wild type (Fig. 7 C, D). We further performed histochemical staining to detect ROS bursts in rice plants. After cold treatment, deeper reddish brown and darker bluish-purple spots in the mutant leaves than that in wild type by DAB and NBT staining (Fig. 8 A, B), reflecting excessive H 2 O 2 and superoxide anion in the mutants, respectively. Subsequently, the activity of two antioxidant enzymes crucial for scavenging ROS was examined. A significant decrease in CAT activity was observed in the oswrky70-7 compared to wild type at 24 h after cold treatment (Fig. 8 C). Especially, oswrky70-10 exhibited approximately 30% decline after 12 h of cold treatment compared to wild type (Fig. 8 C). Similarly, POD activity significantly reduced after exposure to cold for 12 h in the mutants. These results indicate that an imbalance of ROS homeostasis contributes to cellular oxidative membrane damage in OsWRKY70-regulated cold tolerance in rice. To assess the potential downstream genes of OsWRKY70 in response to cold, we detected the expression level of OsLti6b , OsICE1 and OsCOLD1 , which are cold-related genes (Kim et al. 2007 ; Ma et al. 2015 ; Zhang et al. 2017 ). The results revealed that the mutants showed down-regulation of OsLti6b and OsICE1 , while no significant difference in the expression of OsCOLD1 was observed (Fig. 8 E-G), implying that knock out of OsWRKY70 attenuates cold tolerance in rice presumably due to the suppressed expression of OsLti6b and OsICE1 .
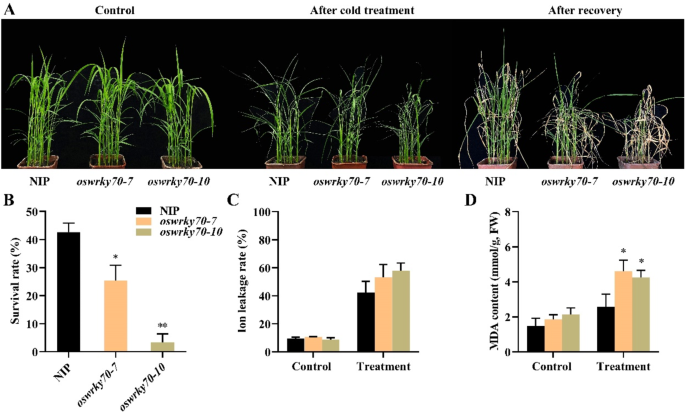
Knock out of OsWRKY70 reduced cold tolerance in rice. ( A ) 2-week-old seedlings of the mutants and wild type were subjected to 4 °C for 3 days in a growth chamber and then cold stressed recovered under 25–28 °C for 7 days. ( B ) The survival rate of seedlings after recovery were calculated. ( C ) Ion leakage rate in leaves of the cold-stressed mutants and wild type. ( D ) Content of MDA between mutants and wild type under normal condition and cold treatment. Data are the means ± SD of three biological replicates and each replicate includes at least 15 independent seedlings. Asterisks indicate significant differences by the Student’s t-test (* P < 0.05, ** P < 0.01)
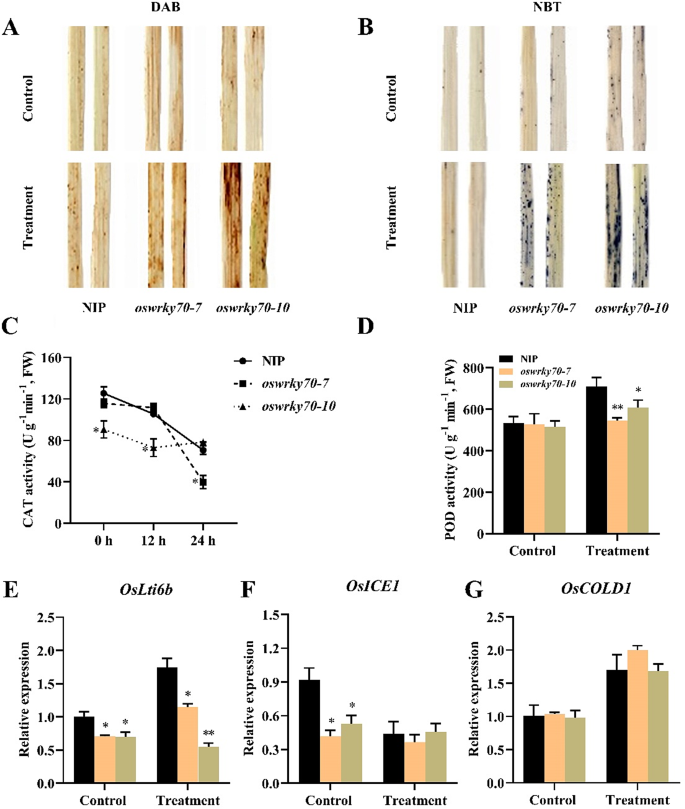
ROS burst and the expression levels of cold-related genes in oswrky70 mutants in response to cold. ( A ) H 2 O 2 accumulation checked by DAB staining. ( B ) The accumulation of superoxide anion in leaves detected by NBT staining. ( C , D ) Enzyme Activity of CAT ( C ) and POD ( D ) from seedings of 2-week-old wild type and oswrky70 mutants before and after cold stress treatment. Data are the means ± SD of three biological replicates. Asterisks indicate significant differences by the Student’s t-test (* P < 0.05, ** P < 0.01). ( E-G ) The expression levels of cold-related genes OsLti6b ( E ), OsICE1 ( F ) and OsCOLD1 ( G ) from the seedings of 2-week-old wild type mutants and mutants without and with cold treatment. Relative expression levels were normalized by the transcript level of the OsActin gene as an internal control and the expression level of each gene of interest in wild type plants under normal condition was set as 1. Data are the means ± SD of three biological replicates. Asterisks indicate significant differences by the Student’s t-test (* P < 0.05, ** P < 0.01)
Transcription factor OsWRKY70, a member of WRKY group I, has been established to function as a transcriptional activator (Li et al. 2015 ; Zhang et al. 2015 ). Phylogenetic analysis revealed that OsWRKY70 shares up to 52.43% and 62.87% amino acids identities with OsWRKY24 and OsWRKY53, respectively (Li et al. 2023 ). Repression of OsWRKY24 (Yokotani et al. 2018 ) and overexpression of OsWRKY53 (Chujo et al. 2007 ) confers blast resistance in rice. Our findings demonstrated that knock out of OsWRKY70 mutants enhanced resistance against M. oryzae (Fig. 3 ; Figure S1 ), implying that OsWRKY70 negatively regulates the fungal immunity in rice. However, it seems paradoxical that the increased transcriptional expression level of OsWRKY70 upon infection (Fig. 1 ). Similarly, this feedback-like has been reported in some rice TFs. For instance, OsbHLH6 and ONAC083 , which are induced by inoculation with M. oryzae , act as negative regulators of disease resistance (Bi et al. 2023 ; Meng et al. 2020 ). Additionally, OsWRKY24, OsWRKY53 and OsWRKY70 may be functionally redundant in grain regulation (Tang et al. 2022a ). Considering that predicted interactions of these proteins (data have no shown), we presume that up-regulated OsWRKY70 might be involved in adjusting the intensity of defense response by cooperating with OsWRKY24 or OsWRKY53 to protect the plant from biotic stress. Therefore, further generation of double and triple mutant plants for these genes will contribute to elucidating the molecular mechanism of blast resistance. Our KEGG analysis revealed that the altered genes are involved in plant-pathogen interactions, hormone signaling transduction, MAPK cascades and so on, indicating that OsWRKY70 is a key component of immunity response (Fig. 5 ). Plant NBS-LRR genes, a class of the resistance (R) genes, encode immune receptors that help defend against pathogens infection (Wang et al. 2023 d ). For instance, rice NBS-LRR protein Pit interacts with OsRac1 and induces the generation of ROS and hypersensitive response to resist the invasion of M. oryzae (Kawano et al. 2014 ). Pi63 encodes a typical NBS-LRR protein, whose expression level is closely related to disease resistance (Xu et al. 2014 ). In our study, LOC_Os11g44960 , LOC_Os11g45050 and LOC_Os11g45180 , which are assumed to encode NBS-LRR proteins, were up-regulated expression in the mutant (Fig. 5 C). It is suggested that these genes might be conferred to the resistance to blast. The activation of the JA signaling pathway improved resistance against disease in rice (Okada et al. 2015 ; Wang et al. 2021 ; Qiu et al. 2022 ). OsbHLH6 , a transcription activator, negatively regulates rice blast resistance, which has been shown to play a pivotal role in modulating the JA and SA signaling pathways (Kiribuchi et al. 2004 ; Meng et al. 2020 ). Knock out of the OsbHLH6 mutant downregulates the expression of OsJAZ family genes, which exhibits severe damage upon exposure to herbivores (Valea et al. 2022 ). In our study, the OsbHLH6 gene transcript significantly decreased in oswrky70-7 and oswrky70-10 mutants before and after inoculation compared to the wild type (Fig. 5 C, E). Given the finding that OsWRKY70 enhances rice herbivore resistance to SSB mediated by JA signaling (Li et al. 2015 ), we hypothesized that OsbHLH6 might be a potential downstream target of OsWRKY70. Then, we analyzed the promoter of OsbHLH6 and further confirmed the binding of OsWRKY70 using a semi-in vivo ChIP assay (Fig. 6 ). Therefore, it would be interesting in our future work to verify the interactions in vivo by ChIP and to generate their double mutant plants for exploring the regulatory relationships. Advanced thinking is that JA and SA signaling crosstalk commonly manifests as a reciprocal antagonism or adaptation (Thaler et al. 2012 ). In our work, the transcript level of OsUGT74H4 was decreased in mutant after infection by RNA-seq analysis (Fig. 5 C). OsUGT74H4 may inactivate SA through glycosylation modification, negatively regulating the resistance of rice to bacterial diseases (Wu et al. 2022 ). Both positive and negative transcriptional regulations of SA biosynthesis are required to fine-tune the SA levels for optimal defense without causing unnecessary fitness costs (Ding et al. 2020 ). Therefore, we suspect that knock out of OsWRKY70 might affect SA content in response to fungal pathogen infection. Phenylpropanoids are considered to be secondary metabolites involved in plant defense responses (Kishi-Kaboshi et al. 2010 ). Previous research has revealed that CYP72A1 , the cytochrome P450 gene, positively regulates the production of ROS and the accumulation of defense-related secondary metabolites in basic immune response (Zhang et al. 2022a ). The Cinnamate-4-hydroxylase (C4H) belongs to the cytochrome monooxygenase, which is the second key enzyme in the phenylpropane metabolic pathway (Yang et al. 2005 ). According to our transcriptome analysis, there were more than 20 DEGs in Phenylpropanoids biosynthesis without and after inoculation (Fig. 5 A). Among them, LOC_Os07g44130 , which encodes putative cytochrome P450, was up-regulated more than 5.5-fold in mutants after infection (Fig. 5 H). Thus, it is also worthy of further study and exploration of issues that OsWRKY70 participates in phenylpropanoids biosynthesis to adapt to biotic stress.
The loss-function of OsWRKY70 mutants reduced tolerance to cold stress (Fig. 7 ), suggesting that OsWRKY70 is a positive regulator of cold tolerance. It has been demonstrated that chilling usually causes excessive ROS accumulation in rice (Marchi et al. 2012 ; Zhang et al. 2022b ; Zhai et al. 2024 ). OsWRKY63 and OsWRKY76 might affect ROS homeostasis in the regulation of cold tolerance (Yokotani et al. 2013 ; Zhang et al. 2022b ). We observed that knock out of OsWRKY70 mutants accumulated a large amount of H 2 O 2 and superoxide anion under cold stress condition (Fig. 8 A, B). This is consistent with the measurement of reduced activities of CAT and POD, as well as increased electrolyte leakage levels and MDA content (Figs. 7 C and D and 8 C and D). Cold tolerance is primarily regulated by cold-responsive (COR) regulon (Wani et al. 2021 ). Our findings revealed that the expression level of OsLti6b and OsICE1 was significantly down-regulated in mutants (Fig. 8 E, F), which has been documented to positively regulate cold tolerance in rice (Kim et al. 2007 ; Nakamura et al. 2011 ; Xia et al. 2021 ). What is more, COR regulon also mediates the expression of AtWRKY 6/ 22 / 30 / 40 / 32 / 187 (Banerjee et al. 2015 ; Wani et al. 2021 ), hinting that there may be feedback or cooperation between OsWRKY70 and COR regulon in chilling stress. Unlike cold-responsive genes, OsCOLD1 is involved in sensing cold to trigger Ca 2+ signaling for chilling tolerance (Ma et al. 2015 ). There were no differences in the transcript level of OsCOLD1 between the mutants and wild type before and after cold treatment (Fig. 8 G), suggesting that OsCOLD1 may be the upstream of OsWRKY70 -mediated signaling. Additionally, the changes in OsCOLD1 protein structure and membrane fluidity in response to low temperature might initiate signaling (Ma et al. 2015 ). Thus, another possibility is that the mutation of OsWRKY70 affects the structure and dynamics of OsCOLD1 to regulate cold tolerance.
We demonstrated that OsWRKY70 functions as a negative regulator of rice against M. oryzae while a positive regulator of cold tolerance in rice (Figs. 3 and 7 ). Previous findings showed that temperature is an important factor affecting the occurrence of rice blast. For instance, cold summers increase the frequency and severity of fungal pathogen disease and bring heavy yield losses in the northern regions of Japan (Hironori et al. 2004 ). The blast lesion area and fungal growth in the inoculated seedlings at a warm temperature, 22 °C, is greater than those at 28 °C (Qiu et al. 2022 ). Additionally, 22 °C compromises basal resistance in rice by reducing JA biosynthesis and signaling (Qiu et al. 2022 ), suggesting that JA are involved in temperature-modulated plant resistance. We found that knock out of OsWRKY70 mutants down-regulated OsbHLH6 expression in response to rice blast (Fig. 5 E). However, the regulatory relationship between OsWRKY70 and OsbHLH6 whether involving temperature-mediated fungal pathogen resistance is yet to be clarified. In addition, more and more evidences imply that WRKY TFs are involved in the MAPK signaling pathway to adapt to biotic stress. For example, OsMKK10-20-OsMPK6 pathway is required for OsWRKY45 -mediated resistance against M. oryzae (Ueno et al. 2013 ). OsWRKY31, which is phosphorylated by OsMPKs, elevates DNA-binding activity and confers enhanced blast resistance in rice (Wang et al. 2023c ). Previously, OsMPK3 and OsMPK6 regulates OsWRKY70 in herbivore resistance (Li et al. 2015 ). OsMPK3, phosphorylated and activated by the calcium-dependent protein kinase CPK18, negatively regulates rice blast resistance (Xie et al. 2014 ). In addition to biotic stress, MAPK cascades have also been confirmed to confer abiotic stress responses, including chilling, salt and drought. For instance, OsICE1 is phosphorylated by OsMPK3, resulting in inhibition of OsICE1 ubiquitination and enhanced resistance to chilling damage (Zhang et al. 2017 ). Furthermore, OsMPK3 has positively modulated salt tolerance by attenuating the accumulation of ROS (Zhang et al. 2018 ). Consequently, further validation of the interaction with OsMPK3 and OsWRKY70 will contribute to aid in elucidating the molecular mechanism in response to fungal pathogen infection and cold stress.
Materials and methods
Plants and pathogens.
The wild type rice Oryza sativa L. japonica ‘Nipponbare’ (NIP) was used to generate knock out of OsWRKY70 mutants in this study. All rice seeds were soaked in culture dishes with water at 37℃ for one day. On the second day, the seeds were rinse for 2–3 times and added a small amount water to promote germination at 37 °C. Then, the seeds with same germination state were planted to the substrate soil. The plants were grown under normal conditions (25–28℃, 14 h light/10 h dark photoperiod, 50–75% relative humidity). For agronomic trait analysis, the tiller count, flag leaf, panicle length, grain length, grain width and 1000-grain weight of these plants were grown in the paddy fields of Hunan Agricultural University, Changsha, China.
Magnaporthe oryzae strains 318-2 from College of Agronomy in Hunan Agricultural University, as well as M. oryzae strains 110-2, R01-1 and GFP-tagged RB22 from Institute of Plant Protection in Chinese Academy of Agricultural Sciences, were cultured on oatmeal agar for 2 weeks at 28 °C and the spores were collected with sterile water containing 0.02% Tween-20. For M. oryzae strains treatment, 2-week-old seedlings of NIP were inoculated using M. strains 318-2, 110-2 and R01-1 for gene expression analysis of OsWRKY70 . The plants were sprayed with spores of M. oryzae strains (2 × 10 5 spores/mL), covered with a plastic box in the dark for 24 h (25–28 °C, approximately 100% relative humidity) and transferred to normal alternation of light and dark (25–28 °C, approximately 100% relative humidity). Leaf samples were collected at 0, 24 and 48 h post-inoculation, frozen in liquid nitrogen and stored at -80 °C until use. Each treatment was performed with three biological replicates. The control plants (0 h) were left blast-free.
Generation of OsWRKY70 Knock Out Mutants
For the construction of knock out of OsWRKY70 vector, the sequence (5’-GGACGAGCAGCAACAGTACT-3’) of OsWRKY70 genomic locus was conducted as the guide RNA through CRISPR Primer Designer v1.1.2. We designed two primers sequences (LP: 5’-TGGCGGGACGAGCAGCAACAGTACT-3’; RP: 5’-AAACAGTACTGTTGCTGCTCGTCCC-3’) to synthesized the sgRNA expression cassette. This sequence was inserted into the pHUN4c12 plasmid, which was linearized using Bsa I enzyme (NEB, USA). The vectors were introduced into Agrobacterium tumefaciens strain EHA105 through electroporation, which were further used to transform the rice callus. After screening with hygromycin and sequence confirmation, two homozygous knockouts oswrky70-7 and oswrky70-10 were obtained and the T3 generation seedlings were used for further analysis in this work. The primers used in the plant vector construction and identification are listed in Table S1 .
Fungal Pathogen Resistance Experiment
For spraying with M. oryzae spores (2 × 10 5 spores/mL), 2-week-old transgenic seedlings were used for pathogen infection. Leaves were collected at 0 and 24 h post-inoculation for expression analysis of defense-related genes. After inoculation for 5 d, the lesions were scanned and leaves were collected for DNA extraction to evaluate the relative fungal growth. The qRT-PCR was measured to analyze and compare the genomic level of M. oryzae POT2 in wild type and the mutant leaves with that of the rice OsActin as an internal control. All of primers were listed in Table S1 .
For detached leaf inoculation, leaves of 4-week-old rice seedlings were cut into pieces (about 5 cm × 1 cm) and float on the distilled water in culture dishes. Apply a drop of the spore suspension (10 µL of 4 × 10 5 spores/mL) to the wound of each leaf and keep the dishes at 25–28℃ for 5 days. (Darkness is not required). The lesions were photographed and measured at 5 d post-inoculation and leaves were collected for detecting relative biomass.
For punch inoculation, 4-week-old seedlings grown in the field were made a wound using a hole punch. 10 µL spore suspension (4 × 10 5 spores/mL) was dropped onto wound. The inoculated region was wrapped with tape to maintain humidity. At 5 d post-inoculation, the lesions were pictured and surveyed. Leaf samples were conducted for measuring relative growth of M. oryzae . Blast inoculation was performed as previously described (Gu et al. 2023 ). M. oryzae strain 318-2 was used for rice blast inoculation. All experiments were independently repeated at least three times.
For leaf sheaths infection, detached sheaths of 4-week-old rice plants were inoculated with GFP-tagged M. oryzae strain RB22 spores (4 × 10 5 spores/mL) and kept on dished with approximately 100% humidity for 24 h and 48 h in the dark. Images of conidial germination, appressorium development and invasive hyphal growth were recorded using a fluorescence microscope (Mshot, China).
Evaluation of Cold Tolerance
For cold stress tolerance assay, 2-week-old knock out of OsWRKY70 mutants and wild type plants were grown under normal condition and then transferred into a growth chamber with the temperature set at 4 °C with a cycle of 14 h light/10 h dark for 3 days, followed by transferring to the growth room with the normal condition for recovery. Plants with green leaves and healthy young leaves after transferring to the normal growth condition were considered as survivals and surviving plants were evaluated at 7 days after recovery from cold treatment. Survival rate was calculated as the ratio of the number of survived plants over the total number of treated plants. Leaf samples at 0 and 24 h were used for determination of malondialdehyde (MDA) content and electric conductivity using the colorimetric method and electrical conductivity meter, respectively (Guan et al. 2012 ). Leaves at 0, 12 and 24 h were measured the enzyme activity catalase (CAT) and Leaves at 0 and 12 h were detected the peroxidase (POD) activity, as previously described (Wang et al. 2022 ). Leaves were collected at 0 and 24 h for expression analysis of cold-related genes. The specific primers were exhibited in Table S1 . Cold treatment in each of the experiments included three biological replicates with at least 15 plants and the experiments were independently repeated three times.
Histochemical Staining Analysis
For fungal pathogen infection, 2-week-old seedlings of wild type and oswrky70 mutants were sprayed with M. oryzae 318-2 spores (2 × 10 5 spores/mL) for detection of ROS burst. Leaf samples were collected at 0 and 24 h post-inoculation to carry out Histochemical Staining. For cold stress treatment, 2-week-old transgenic plants were transferred to a growth chamber with temperature set at 4℃. Leaf samples were collected at 0 and 6 h after treatment. 3, 3’-diaminobenzidine (DAB) and nitro-blue tetrazolium (NBT) staining for H 2 O 2 and superoxide anion accumulation in plants, respectively, were conducted as previously described (Jambunathan 2010 ).
DNA Extraction
Rice leaf genomic DNA was extracted, according to the CTAB method (Semagn et al. 2014 ).
RNA Extraction and Quantitative Real‑Time PCR
Total RNA was extracted using Ultrapure RNA Kit (Cwbio, China). RNA was reverse transcribed to cDNA using HiScript III 1st Strand cDNA Synthesis Kit (Vazyme, China). Quantitative Real‑Time PCR (qRT-PCR) was performed using ChamQ Universal SYBR qPCR Master Mix (Vazyme, China). The reaction was carried out in the CFX Connect Real-Time PCR Detection System (Bio-Rad, USA). Rice OsActin gene was used as an internal standard to normalize. All of primers used for mRNA detection of target genes are shown in Table S1 . Three replicate experiments were performed for each sample. The relative quantitation method (2 −ΔΔCT ) was used to evaluate quantitative variation among replicates.
Expression and Purification of Recombinant Protein
The full-length cDNA of OsWRKY70 was PCR-amplified and cloned into the pCold-TF vector (Takara, Japan). Specific primers used for PCR amplification for this gene are listed in Table S1 . The constructs were transformed into Escherichia coli BL21 (DE3) (Vazyme, China). OsWRKY70 recombinant protein was induced by adding 0.4 mM isopropyl-β-thiogalactopyranoside (IPTG) at 15˚C for 24 h. Cells were collected and the recombinant protein was purified using ProteinIso ® Ni-NTA Resin for His (TransGen, China) according to the manufacturer’s instructions.
Transcriptome Analysis
Libraries for RNA-seq were constructed and sequenced by Biomarker Technologies Co., Ltd (Beijing, China). Briefly, RNAs were quantified by NanoDrop 2000c UV-Vis Spectrophotometer (Thermo Fisher Scientific), agarose gel electrophoresis and Agient2100/LabChip GX. mRNAs were isolated from total RNAs by poly (A) selection, fragmented into short fragments and converted to cDNAs. cDNAs were ligated to adapters and the suitable fragments were selected for PCR amplification as templates. All the RNA-seq libraries were pair-end sequenced on an Illumina NovaSeq6000 platform. mRNA sequencing data analysis were performed as reported (Love et al. 2014 ). Low-quality reads were removed and adapters were trimmed to obtain clean reads, which were mapped to the reference genome (Oryza_sativa.MSU_v7.0.genome.fa). Transcriptome analysis was performed using BMKCloud ( www.biocloud.net ). The expression level of each gene was calculated as the FPKM value (fragments per kilobase of transcript per million mapped reads). For differential gene expression analysis, fold change (FC) ≥ 2 and false discovery rate (FDR) ≤ 0.01 as screening criteria. Fold Change represents the ratio of expression between two samples (groups). False Discovery Rate (FDR) is obtained by correcting for the difference significance p-value (p-value), indicating the significance of the difference.
Semi-Vivo Chromatin Immunoprecipitation Assay
The analysis of ChIP was conducted as previously described (Li et al. 2017 ). Total DNA of Nipponbare and purified His-OsWRKY70 were used for a semi-vivo chromatin immunoprecipitation (semi-vivo ChIP) assay. 2-week-old seedlings were used for total DNA extraction. The total DNA was sheared into 200–800 bp fragments using ultrasonic crusher. The His fusion protein was affinity-purified on Ni-NTA Resin. His-OsWRKY70 and DNA fragments were co-incubated for 2 h. The incubation buffer includes: 50 mM Tris, 1mM EDTA, 100 mM KCl, adjust pH to 7.0 by HCl, 5% Glycerol, 0.1% Triton X-100; add freshly-made 100 mM DTT to reaction solution to make final concentration of DTT at 1 mM. After co-incubation, Ni-NTA Resin was washed three times using incubation buffer. Then 4 µL 5 M NaCl was added into the sample for each 100 µL volume and was incubated for 4 h to break down cross-linked His-OsWRKY70 and DNA fragments. The prepared DNA in ChIP was applied for qRT-PCR using respective primer pairs (Table S1 ) in a ChamQ Universal SYBR qPCR Master Mix with a CFX Connect Real-Time PCR Detection System. PCR reactions were performed in triplicate for each sample and the expression levels were normalized to the input sample for enrichment detection. The fold enrichment was calculated against OsActin No addition of antibodies (NoAbs) served as a negative control.
Data Analysis
For qRT-PCR analyzes, disease assays, agronomic traits assessment and cold tolerance assays significant differences between samples/lines and the corresponding controls were analyzed using two-tailed Student’s t test for pairwise comparisons.
Data Availability
No datasets were generated or analysed during the current study.
Abbreviations
Jasmonic acid
Salicylic acid
Abscisic acid
Reactive oxygen species
Mitogen-activated protein kinase
3, 3’-diaminobenzidine
Nitro-blue tetrazolium
Malondialdehyde
Differentially expressed gene
Kyoto encyclopedia of genes and genomes
Principal component analysis
Polymerase chain reaction
Quantitative real‑time PCR
Chromatin immunoprecipitation
Bakshi M, Oelmüller R (2014) WRKY transcription factors: Jack of many trades in plants. Plant Signal Behav 9:e27700. https://doi.org/10.4161/psb.27700
Article CAS PubMed PubMed Central Google Scholar
Banerjee A, Roychoudhury A (2015) WRKY proteins: signaling and regulation of expression during abiotic stress responses. ScientificWorldJournal 2015:807560. https://doi.org/10.1155/2015/807560
Bi Y, Wang H, Yuan X, Yan Y, Li D, Song F (2023) The NAC transcription factor ONAC083 negatively regulates rice immunity against Magnaporthe oryzae by directly activating transcription of the RING-H2 gene OsRFPH2-6. J Integr Plant Biol 65:854–875. https://doi.org/10.1111/jipb.13399
Article CAS PubMed Google Scholar
Buscaill P, Rivas S (2014) Transcriptional control of plant defence responses. Curr Opin Plant Biol 20:35–46. https://doi.org/10.1016/j.pbi.2014.04.004
Cai Y, Chen X, Xie K, Xing Q, Wu Y, Li J, Du C, Sun Z, Guo Z (2014) Dlf1, a WRKY transcription factor, is involved in the control of flowering time and plant height in rice. PLoS ONE 9:e102529. https://doi.org/10.1371/journal.pone.0102529
Chen X, Liu J, Lin G, Wang A, Wang Z, Lu G (2013) Overexpression of AtWRKY28 and AtWRKY75 in Arabidopsis enhances resistance to oxalic acid and sclerotinia sclerotiorum. Plant Cell Rep 32:1589–1599. https://doi.org/10.1007/s00299-013-1469-3
Chen J, Clinton M, Qi G, Wang D, Liu F, Fu ZQ (2020) Reprogramming and remodeling: transcriptional and epigenetic regulation of salicylic acid-mediated plant defense. J Exp Bot 71:5256–5268. https://doi.org/10.1093/jxb/eraa072
Chen S, Cao H, Huang B, Zheng X, Liang K, Wang G-L, Sun X (2022) The WRKY10-VQ8 module safely and effectively regulates rice thermotolerance. Plant Cell Environ 45:2126–2144. https://doi.org/10.1111/pce.14329
Cheng H, Liu H, Deng Y, Xiao J, Li X, Wang S (2015) The WRKY45-2 WRKY13 WRKY42 transcriptional regulatory cascade is required for rice resistance to fungal pathogen. Plant Physiol 167:1087–1099. https://doi.org/10.1104/pp.114.256016
Chujo T, Takai R, Akimoto-Tomiyama C, Ando S, Minami E, Nagamura Y, Kaku H, Shibuya N, Yasuda M, Nakashita H, Umemura K, Okada A, Okada K, Nojiri H, Yamane H (2007) Involvement of the elicitor-induced gene OsWRKY53 in the expression of defense-related genes in rice. Biochim Biophys Acta 1769:497–505. https://doi.org/10.1016/j.bbaexp.2007.04.006
Ding P, Ding Y (2020) Stories of salicylic acid: a Plant Defense hormone. Trends Plant Sci 25:549–565. https://doi.org/10.1016/j.tplants.2020.01.004
Ding ZJ, Yan JY, Li GX, Wu ZC, Zhang SQ, Zheng SJ (2014) WRKY41 controls Arabidopsis seed dormancy via direct regulation of ABI3 transcript levels not downstream of ABA. Plant J 79:810–823. https://doi.org/10.1111/tpj.12597
Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5:199–206. https://doi.org/10.1016/s1360-1385(00)01600-9
Feng J-X, Cao L, Li J, Duan C-J, Luo X-M, Le N, Wei H, Liang S, Chu C, Pan Q, Tang J-L (2011) Involvement of OsNPR1/NH1 in rice basal resistance to blast fungus Magnaporthe oryzae. Eur J Plant Pathol 131:221–235. https://doi.org/10.1007/s10658-011-9801-7
Article CAS Google Scholar
Goyal P, Devi R, Verma B, Hussain S, Arora P, Tabassum R, Gupta S (2023) WRKY transcription factors: evolution, regulation, and functional diversity in plants. Protoplasma 260:331–348. https://doi.org/10.1007/s00709-022-01794-7
Grunewald W, De Smet I, Lewis DR, Löfke C, Jansen L, Goeminne G, Vanden Bossche R, Karimi M, De Rybel B, Vanholme B, Teichmann T, Boerjan W, Van Montagu MCE, Gheysen G, Muday GK, Friml J, Beeckman T (2012) Transcription factor WRKY23 assists auxin distribution patterns during Arabidopsis root development through local control on flavonol biosynthesis. Proc Natl Acad Sci U S A 109:1554–1559. https://doi.org/10.1073/pnas.1121134109
Article PubMed PubMed Central Google Scholar
Gu X, Si F, Feng Z, Li S, Liang D, Yang P, Yang C, Yan B, Tang J, Yang Y, Li T, Li L, Zhou J, Li J, Feng L, Liu J-Y, Yang Y, Deng Y, Wu XN, Zhao Z, Wan J, Cao X, Song X, He Z, Liu J (2023) The OsSGS3-tasiRNA-OsARF3 module orchestrates abiotic-biotic stress response trade-off in rice. Nat Commun 14:4441. https://doi.org/10.1038/s41467-023-40176-2
Guan Q, Takano T, Liu S (2012) Genetic transformation and analysis of rice OsAPx2 gene in Medicago sativa. PLoS ONE 7:e41233. https://doi.org/10.1371/journal.pone.0041233
Hironori K, Koji D, Masashi M (2004) Abscisic acid and low temperatures suppress the whole plant-specific resistance reaction of rice plants to the infection of Magnaporthe Grisea. Physiol Mol Plant P 65:3–9. https://doi.org/10.1016/j.pmpp.2004.11.002
Hou Y, Wang Y, Tang L, Tong X, Wang L, Liu L, Huang S, Zhang J (2019) SAPK10-Mediated phosphorylation on WRKY72 releases its suppression on Jasmonic Acid Biosynthesis and bacterial blight resistance. iScience 16:499–510. https://doi.org/10.1016/j.isci.2019.06.009
Hu L, Ye M, Li R, Zhang T, Zhou G, Wang Q, Lu J, Lou Y (2015) The Rice transcription factor WRKY53 suppresses Herbivore-Induced defenses by acting as a negative feedback modulator of Mitogen-activated protein kinase activity. Plant Physiol 169:2907–2921. https://doi.org/10.1104/pp.15.01090
Huang K, Wu T, Ma Z, Li Z, Chen H, Zhang M, Bian M, Bai H, Jiang W, Du X (2021) Rice transcription factor OsWRKY55 is involved in the Drought Response and Regulation of Plant Growth. Int J Mol Sci 22:4337. https://doi.org/10.3390/ijms22094337
Jambunathan N (2010) Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. Methods Mol Biol 639:292–298. https://doi.org/10.1007/978-1-60761-702-0_18
Javed T, Gao S-J (2023) WRKY transcription factors in plant defense. Trends Genet 39:787–801. https://doi.org/10.1016/j.tig.2023.07.001
Kajla M, Roy A, Singh IK, Singh A (2023) Regulation of the regulators: transcription factors controlling biosynthesis of plant secondary metabolites during biotic stresses and their regulation by miRNAs. Front Plant Sci 14:1126567. https://doi.org/10.3389/fpls.2023.1126567
Kawano Y, Kaneko-Kawano T, Shimamoto K (2014) Rho family GTPase-dependent immunity in plants and animals. Front Plant Sci 5:522. https://doi.org/10.3389/fpls.2014.00522
Khan S-A, Li M-Z, Wang S-M, Yin H-J (2018) Revisiting the role of plant transcription factors in the battle against Abiotic stress. Int J Mol Sci 19:1634. https://doi.org/10.3390/ijms19061634
Khoso MA, Hussain A, Ritonga FN, Ali Q, Channa MM, Alshegaihi RM, Meng Q, Ali M, Zaman W, Brohi RD, Liu F, Manghwar H (2022) WRKY transcription factors (TFs): molecular switches to regulate drought, temperature, and salinity stresses in plants. Front Plant Sci 13:1039329. https://doi.org/10.3389/fpls.2022.1039329
Kim S-H, Kim J-Y, Kim S-J, An K-S, An G, Kim S-R (2007) Isolation of cold stress-responsive genes in the reproductive organs, and characterization of the OsLti6b gene from rice (Oryza sativa L). Plant Cell Rep 26:1097–1110. https://doi.org/10.1007/s00299-006-0297-0
Kiribuchi K, Sugimori M, Takeda M, Otani T, Okada K, Onodera H, Ugaki M, Tanaka Y, Tomiyama-Akimoto C, Yamaguchi T, Minami E, Shibuya N, Omori T, Nishiyama M, Nojiri H, Yamane H (2004) RERJ1, a jasmonic acid-responsive gene from rice, encodes a basic helix-loop-helix protein. Biochem Biophys Res Commun 325:857–863. https://doi.org/10.1016/j.bbrc.2004.10.126
Kishi-Kaboshi M, Okada K, Kurimoto L, Murakami S, Umezawa T, Shibuya N, Yamane H, Miyao A, Takatsuji H, Takahashi A, Hirochika H (2010) A rice fungal MAMP-responsive MAPK cascade regulates metabolic flow to antimicrobial metabolite synthesis. Plant J 63:599–612. https://doi.org/10.1111/j.1365-313X.2010.04264.x
Lan J, Lin Q, Zhou C, Ren Y, Liu X, Miao R, Jing R, Mou C, Nguyen T, Zhu X, Wang Q, Zhang X, Guo X, Liu S, Jiang L, Wan J (2020) Small grain and semi-dwarf 3, a WRKY transcription factor, negatively regulates plant height and grain size by stabilizing SLR1 expression in rice. Plant Mol Biol 104:429–450. https://doi.org/10.1007/s11103-020-01049-0
Li J, Brader G, Kariola T, Palva ET (2006) WRKY70 modulates the selection of signaling pathways in plant defense. Plant J 46:477–491. https://doi.org/10.1111/j.1365-313X.2006.02712.x
Li S, Fu Q, Chen L, Huang W, Yu D (2011) Arabidopsis thaliana WRKY25, WRKY26, and WRKY33 coordinate induction of plant thermotolerance. Planta 233:1237–1252. https://doi.org/10.1007/s00425-011-1375-2
Li R, Zhang J, Li J, Zhou G, Wang Q, Bian W, Erb M, Lou Y (2015) Prioritizing plant defence over growth through WRKY regulation facilitates infestation by non-target herbivores. Elife 4:e04805. https://doi.org/10.7554/eLife.04805
Li W, Zhu Z, Chern M, Yin J, Yang C, Ran L, Cheng M, He M, Wang K, Wang J, Zhou X, Zhu X, Chen Z, Wang J, Zhao W, Ma B, Qin P, Chen W, Wang Y, Liu J, Wang W, Wu X, Li P, Wang J, Zhu L, Li S, Chen X (2017) A natural allele of a transcription factor in Rice confers broad-spectrum Blast Resistance. Cell 170:114–126e15. https://doi.org/10.1016/j.cell.2017.06.008
Li Y, Wang L-F, Bhutto SH, He X-R, Yang X-M, Zhou X-H, Lin X-Y, Rajput AA, Li G-B, Zhao J-H, Zhou S-X, Ji Y-P, Pu M, Wang H, Zhao Z-X, Huang Y-Y, Zhang J-W, Qin P, Fan J, Wang W-M (2021) Blocking miR530 improves Rice Resistance, Yield, and Maturity. Front Plant Sci 12:729560. https://doi.org/10.3389/fpls.2021.729560
Li J, Chen Y, Zhang R, Wu B, Xiao G (2023) Expression identification of three OsWRKY genes in response to abiotic stress and hormone treatments in rice. Plant Signal Behav 18:2292844. https://doi.org/10.1080/15592324.2023.2292844
Liu XQ, Bai XQ, Qian Q, Wang XJ, Chen MS, Chu CC (2005) OsWRKY03, a rice transcriptional activator that functions in defense signaling pathway upstream of OsNPR1. Cell Res 15:593–603. https://doi.org/10.1038/sj.cr.7290329
Liu Q, Li X, Yan S, Yu T, Yang J, Dong J, Zhang S, Zhao J, Yang T, Mao X, Zhu X, Liu B (2018) OsWRKY67 positively regulates blast and bacteria blight resistance by direct activation of PR genes in rice. BMC Plant Biol 18:257. https://doi.org/10.1186/s12870-018-1479-y
Love MI, Huber W, Anders S (2014) Moderated estimation of Fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. https://doi.org/10.1186/s13059-014-0550-8
Ma Y, Dai X, Xu Y, Luo W, Zheng X, Zeng D, Pan Y, Lin X, Liu H, Zhang D, Xiao J, Guo X, Xu S, Niu Y, Jin J, Zhang H, Xu X, Li L, Wang W, Qian Q, Ge S, Chong K (2015) COLD1 confers chilling tolerance in rice. Cell 160:1209–1221. https://doi.org/10.1016/j.cell.2015.01.046
Marchi S, Giorgi C, Suski JM, Agnoletto C, Bononi A, Bonora M, De Marchi E, Missiroli S, Patergnani S, Poletti F, Rimessi A, Duszynski J, Wieckowski MR, Pinton P (2012) Mitochondria-Ros crosstalk in the control of cell death and aging. J Signal Transduct 2012:329635. https://doi.org/10.1155/2012/329635
Meng F, Yang C, Cao J, Chen H, Pang J, Zhao Q, Wang Z, Qing Fu Z, Liu J (2020) A bHLH transcription activator regulates defense signaling by nucleo-cytosolic trafficking in rice. J Integr Plant Biol 62:1552–1573. https://doi.org/10.1111/jipb.12922
Nakamura J, Yuasa T, Huong TT, Harano K, Tanaka S, Iwata T, Phan T, Iwaya M (2011) Rice homologs of inducer of CBF expression (OsICE) are involved in cold acclimation. Plant Biotechnol 28:303–309. https://doi.org/10.5511/plantbiotechnology.11.0421a
Nejat N, Mantri N (2017) Plant Immune System: crosstalk between responses to biotic and abiotic stresses the missing link in understanding Plant Defence. Curr Issues Mol Biol 23:1–16. https://doi.org/10.21775/cimb.023.001
Article PubMed Google Scholar
Ng DW-K, Abeysinghe JK, Kamali M (2018) Regulating the regulators: the Control of Transcription Factors in Plant Defense Signaling. Int J Mol Sci 19:3737. https://doi.org/10.3390/ijms19123737
Okada K, Abe H, Arimura G (2015) Jasmonates induce both defense responses and communication in monocotyledonous and dicotyledonous plants. Plant Cell Physiol 56:16–27. https://doi.org/10.1093/pcp/pcu158
Qiu D, Xiao J, Ding X, Xiong M, Cai M, Cao Y, Li X, Xu C, Wang S (2007) OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol Plant Microbe Interact 20:492–499. https://doi.org/10.1094/MPMI-20-5-0492
Qiu J, Xie J, Chen Y, Shen Z, Shi H, Naqvi NI, Qian Q, Liang Y, Kou Y (2022) Warm temperature compromises JA-regulated basal resistance to enhance Magnaporthe oryzae infection in rice. Mol Plant 15:723–739. https://doi.org/10.1016/j.molp.2022.02.014
Rushton PJ, Somssich IE, Ringler P, Shen QJ (2010) WRKY transcription factors. Trends Plant Sci 15:247–258. https://doi.org/10.1016/j.tplants.2010.02.006
Saha B, Nayak J, Srivastava R, Samal S, Kumar D, Chanwala J, Dey N, Giri MK (2023) Unraveling the involvement of WRKY TFs in regulating plant disease defense signaling. Planta 259:7. https://doi.org/10.1007/s00425-023-04269-y
Semagn K (2014) Leaf tissue sampling and DNA extraction protocols. Methods Mol Biol 1115:53–67. https://doi.org/10.1007/978-1-62703-767-9_3
Song H, Cao Y, Zhao L, Zhang J, Li S (2023) Review: WRKY transcription factors: understanding the functional divergence. Plant Sci 334:111770. https://doi.org/10.1016/j.plantsci.2023.111770
Tang J, Mei E, He M, Bu Q, Tian X (2022a) Functions of OsWRKY24, OsWRKY70 and OsWRKY53 in regulating grain size in rice. Planta 255:92. https://doi.org/10.1007/s00425-022-03871-w
Tang J, Tian X, Mei E, He M, Gao J, Yu J, Xu M, Liu J, Song L, Li X, Wang Z, Guan Q, Zhao Z, Wang C, Bu Q (2022b) WRKY53 negatively regulates rice cold tolerance at the booting stage by fine-tuning anther gibberellin levels. Plant Cell 34:4495–4515. https://doi.org/10.1093/plcell/koac253
Tao Z, Liu H, Qiu D, Zhou Y, Li X, Xu C, Wang S (2009) A pair of allelic WRKY genes play opposite roles in rice-bacteria interactions. Plant Physiol 151:936–948. https://doi.org/10.1104/pp.109.145623
Tao Z, Kou Y, Liu H, Li X, Xiao J, Wang S (2011) OsWRKY45 alleles play different roles in abscisic acid signalling and salt stress tolerance but similar roles in drought and cold tolerance in rice. J Exp Bot 62:4863–4874. https://doi.org/10.1093/jxb/err144
Thaler JS, Humphrey PT, Whiteman NK (2012) Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci 17:260–270. https://doi.org/10.1016/j.tplants.2012.02.010
Tian X, Li X, Zhou W, Ren Y, Wang Z, Liu Z, Tang J, Tong H, Fang J, Bu Q (2017) Transcription factor OsWRKY53 positively regulates Brassinosteroid Signaling and Plant Architecture. Plant Physiol 175:1337–1349. https://doi.org/10.1104/pp.17.00946
Tian F, Yang D-C, Meng Y-Q, Jin J, Gao G (2020) PlantRegMap: charting functional regulatory maps in plants. Nucleic Acids Res 48:D1104–D1113. https://doi.org/10.1093/nar/gkz1020
Ueno Y, Yoshida R, Kishi-Kaboshi M, Matsushita A, Jiang C-J, Goto S, Takahashi A, Hirochika H, Takatsuji H (2013) MAP kinases phosphorylate rice WRKY45. Plant Signal Behav 8:e24510. https://doi.org/10.4161/psb.24510
Valea I, Motegi A, Kawamura N, Kawamoto K, Miyao A, Ozawa R, Takabayashi J, Gomi K, Nemoto K, Nozawa A, Sawasaki T, Shinya T, Galis I, Miyamoto K, Nojiri H, Okada K (2022) The rice wound-inducible transcription factor RERJ1 sharing same signal transduction pathway with OsMYC2 is necessary for defense response to herbivory and bacterial blight. Plant Mol Biol 109:651–666. https://doi.org/10.1007/s11103-021-01186-0
Viana VE, Busanello C, da Maia LC, Pegoraro C, Costa de Oliveira A (2018) Activation of rice WRKY transcription factors: an army of stress fighting soldiers? Curr Opin Plant Biol 45:268–275. https://doi.org/10.1016/j.pbi.2018.07.007
Wang N, Zhao P, Ma Y, Yao X, Sun Y, Huang X, Jin J, Zhang Y, Zhu C, Fang R, Ye J (2019) A whitefly effector Bsp9 targets host immunity regulator WRKY33 to promote performance. Philos Trans R Soc Lond B Biol Sci 374:20180313. https://doi.org/10.1098/rstb.2018.0313
Wang Y, Cui X, Yang B, Xu S, Wei X, Zhao P, Niu F, Sun M, Wang C, Cheng H, Jiang Y-Q (2020) WRKY55 transcription factor positively regulates leaf senescence and the defense response by modulating the transcription of genes implicated in the biosynthesis of reactive oxygen species and salicylic acid in Arabidopsis. Development 147:dev189647. https://doi.org/10.1242/dev.189647
Wang Y, Mostafa S, Zeng W, Jin B (2021) Function and mechanism of Jasmonic Acid in Plant responses to Abiotic and Biotic stresses. Int J Mol Sci 22:8568. https://doi.org/10.3390/ijms22168568
Wang L, Zhang X, She Y, Hu C, Wang Q, Wu L, You C, Ke J, He H (2022) Physiological Adaptation Mechanisms to Drought and Rewatering in Water-Saving and Drought-Resistant Rice. Int J Mol Sci 23:14043. https://doi.org/10.3390/ijms232214043
Wang H, Chen W, Xu Z, Chen M, Yu D (2023a) Functions of WRKYs in plant growth and development. Trends Plant Sci 28:630–645. https://doi.org/10.1016/j.tplants.2022.12.012
Wang L, Fu J, Shen Q, Wang Q (2023b) OsWRKY10 extensively activates multiple rice diterpenoid phytoalexin biosynthesis genes to enhance rice blast resistance. Plant J 115:758–771. https://doi.org/10.1111/tpj.16259
Wang S, Han S, Zhou X, Zhao C, Guo L, Zhang J, Liu F, Huo Q, Zhao W, Guo Z, Chen X (2023c) Phosphorylation and ubiquitination of OsWRKY31 are integral to OsMKK10-2-mediated defense responses in rice. Plant Cell 35:2391–2412. https://doi.org/10.1093/plcell/koad064
Wang X, Xu Y, Fan H, Cui N, Meng X, He J, Ran N, Yu Y (2023d) Research progress of plant nucleotide-binding leucine-rich repeat protein. Horticulturae 9:122. https://doi.org/10.3390/horticulturae9010122
Article Google Scholar
Wani SH, Anand S, Singh B, Bohra A, Joshi R (2021) WRKY transcription factors and plant defense responses: latest discoveries and future prospects. Plant Cell Rep 40:1071–1085. https://doi.org/10.1007/s00299-021-02691-8
Wu T, Zhang H, Yuan B, Liu H, Kong L, Chu Z, Ding X (2022) Tal2b targets and activates the expression of OsF3H03g to hijack OsUGT74H4 and synergistically interfere with rice immunity. New Phytol 233:1864–1880. https://doi.org/10.1111/nph.17877
Wu B, Chen S, Cheng S, Li C, Li S, Chen J, Zha W, Liu K, Xu H, Li P, Shi S, Yang G, Chen Z, Liu K, You A, Zhou L (2023) Transcriptome analysis revealed the dynamic and Rapid Transcriptional Reprogramming Involved in cold stress and related core genes in the Rice Seedling Stage. Int J Mol Sci 24(1914). https://doi.org/10.3390/ijms24031914
Xi X, Hu Z, Nie X, Meng M, Xu H, Li J (2021) Cross Inhibition of MPK10 and WRKY10 participating in the growth of Endosperm in Arabidopsis thaliana. Front Plant Sci 12:640346. https://doi.org/10.3389/fpls.2021.640346
Xia C, Gong Y, Chong K, Xu Y (2021) Phosphatase OsPP2C27 directly dephosphorylates OsMAPK3 and OsbHLH002 to negatively regulate cold tolerance in rice. Plant Cell Environ 44:491–505. https://doi.org/10.1111/pce.13938
Xie K, Chen J, Wang Q, Yang Y (2014) Direct phosphorylation and activation of a mitogen-activated protein kinase by a calcium-dependent protein kinase in rice. Plant Cell 26:3077–3089. https://doi.org/10.1105/tpc.114.126441
Xie W, Ke Y, Cao J, Wang S, Yuan M (2021) Knock out of transcription factor WRKY53 thickens sclerenchyma cell walls, confers bacterial blight resistance. Plant Physiol 187:1746–1761. https://doi.org/10.1093/plphys/kiab400
Xu X, Hayashi N, Wang C-T, Fukuoka S, Kawasaki S, Takatsuji H, Jiang C-J (2014) Rice blast resistance gene Pikahei-1(t), a member of a resistance gene cluster on chromosome 4, encodes a nucleotide-binding site and leucine-rich repeat protein. Mol Breed 34:691–700. https://doi.org/10.1007/s11032-014-0067-6
Yang DH, Chung BY, Kim JS, Kim JH, Yun PY, Lee YK, Lim YP, Lee MC (2005) cDNA cloning and sequence analysis of Rice Cinnamate-4-Hydroxylase Gene, a cytochrome P450-Dependent monooxygenase, involving in the General Phenylpropanoid Pathway. J Plant Biol 48:311–318. https://doi.org/10.1007/bf03030528
Ye M, Glauser G, Lou Y, Erb M, Hu L (2019) Molecular Dissection of Early Defense Signaling underlying volatile-mediated defense regulation and Herbivore Resistance in Rice. Plant Cell 31:687–698. https://doi.org/10.1105/tpc.18.00569
Yokotani N, Sato Y, Tanabe S, Chujo T, Shimizu T, Okada K, Yamane H, Shimono M, Sugano S, Takatsuji H, Kaku H, Minami E, Nishizawa Y (2013) WRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance. J Exp Bot 64:5085–5097. https://doi.org/10.1093/jxb/ert298
Yokotani N, Shikata M, Ichikawa H, Mitsuda N, Ohme-Takagi M, Minami E, Nishizawa Y (2018) OsWRKY24, a blast-disease responsive transcription factor, positively regulates rice disease resistance. J Gen Plant Pathol 84:85–91. https://doi.org/10.1007/s10327-018-0768-5
Yu J, Zhu C, Xuan W, An H, Tian Y, Wang B, Chi W, Chen G, Ge Y, Li J, Dai Z, Liu Y, Sun Z, Xu D, Wang C, Wan J (2023) Genome-wide association studies identify OsWRKY53 as a key regulator of salt tolerance in rice. Nat Commun 14:3550. https://doi.org/10.1038/s41467-023-39167-0
Yuan Y, Zhong S, Li Q, Zhu Z, Lou Y, Wang L, Wang J, Wang M, Li Q, Yang D, He Z (2007) Functional analysis of rice NPR1-like genes reveals that OsNPR1/NH1 is the rice orthologue conferring disease resistance with enhanced herbivore susceptibility. Plant Biotechnol J 5:313–324. https://doi.org/10.1111/j.1467-7652.2007.00243.x
Zhai M, Chen Y, Pan X, Chen Y, Zhou J, Jiang X, Zhang Z, Xiao G, Zhang H (2024) OsEIN2-OsEIL1/2 pathway negatively regulates chilling tolerance by attenuating OsICE1 function in rice. Plant Cell Environ 47:2561–2577. https://doi.org/10.1111/pce.14900
Zhang L, Gu L, Ringler P, Smith S, Rushton PJ, Shen QJ (2015) Three WRKY transcription factors additively repress abscisic acid and gibberellin signaling in aleurone cells. Plant Sci 236:214–222. https://doi.org/10.1016/j.plantsci.2015.04.014
Zhang Z, Li J, Li F, Liu H, Yang W, Chong K, Xu Y (2017) OsMAPK3 phosphorylates OsbHLH002/OsICE1 and inhibits its Ubiquitination to activate OsTPP1 and enhances Rice Chilling Tolerance. Dev Cell 43:731–743e5. https://doi.org/10.1016/j.devcel.2017.11.016
Zhang Z, Liu H, Sun C, Ma Q, Bu H, Chong K, Xu Y (2018) A C2H2 zinc-finger protein OsZFP213 interacts with OsMAPK3 to enhance salt tolerance in rice. J Plant Physiol 229:100–110. https://doi.org/10.1016/j.jplph.2018.07.003
Zhang F, Fang H, Wang M, He F, Tao H, Wang R, Long J, Wang J, Wang G-L, Ning Y (2022a) APIP5 functions as a transcription factor and an RNA-binding protein to modulate cell death and immunity in rice. Nucleic Acids Res 50:5064–5079. https://doi.org/10.1093/nar/gkac316
Zhang M, Zhao R, Huang K, Huang S, Wang H, Wei Z, Li Z, Bian M, Jiang W, Wu T, Du X (2022b) The OsWRKY63-OsWRKY76-OsDREB1B module regulates chilling tolerance in rice. Plant J 112:383–398. https://doi.org/10.1111/tpj.15950
Zhang M, Zhao R, Wang H, Ren S, Shi L, Huang S, Wei Z, Guo B, Jin J, Zhong Y, Chen M, Jiang W, Wu T, Du X (2023) OsWRKY28 positively regulates salinity tolerance by directly activating OsDREB1B expression in rice. Plant Cell Rep 42:223–234. https://doi.org/10.1007/s00299-022-02950-2
Zhou J, Wang X, He Y, Sang T, Wang P, Dai S, Zhang S, Meng X (2020) Differential Phosphorylation of the transcription factor WRKY33 by the protein kinases CPK5/CPK6 and MPK3/MPK6 cooperatively regulates Camalexin Biosynthesis in Arabidopsis. Plant Cell 32:2621–2638. https://doi.org/10.1105/tpc.19.00971
Download references
Acknowledgements
First of all, the authors would like to appreciate to all those who have contributed to this study. The authors are grateful to Pro Yuese Ning and Pro Ruyi Wang (Institute of Plant Protection, Chinese Academy of Agricultural Sciences), Pro Wei Li (College of Plant Protection, Hunan Agricultural University), as well as College of Agronomy in Hunan Agricultural University for providing fungal pathogens and valuable suggestions.
This work was supported by grants from the Natural Science Foundation of China (31701781 and 32171950), the Scientific Research Project of Hunan Provincial Department of Education (22A0163), the Hebei Technology Innovation Center for Green Management of Soil-borne Diseases (Baoding University, Grant No. 2022K04) and the Postgraduate Scientific Research Innovation Project of Hunan Province (CX20230687).
Author information
Authors and affiliations.
College of Bioscience and Biotechnology, Hunan Agricultural University, Changsha, 410128, China
Jiangdi Li, Yating Chen, Rui Zhang, Rujie Wang, Bin Wu & Guiqing Xiao
Biotechnology Research Institute, Chinese Academy of Agricultural Sciences, Beijing, 100081, China
Haiwen Zhang
You can also search for this author in PubMed Google Scholar
Contributions
JL, GX, and HZ considered the project and designed the study. JL experimented and measured the data. YC, RZ, RW and BW reviewed and edited the manuscript.
Corresponding authors
Correspondence to Haiwen Zhang or Guiqing Xiao .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for Publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, supplementary material 3, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Li, J., Chen, Y., Zhang, R. et al. OsWRKY70 Plays Opposite Roles in Blast Resistance and Cold Stress Tolerance in Rice. Rice 17 , 61 (2024). https://doi.org/10.1186/s12284-024-00741-9
Download citation
Received : 21 July 2024
Accepted : 06 September 2024
Published : 14 September 2024
DOI : https://doi.org/10.1186/s12284-024-00741-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Blast resistance
- Cold tolerance
- Transcriptional regulation

IMAGES
VIDEO
COMMENTS
Therefore, in the review article, we have analysed the trends in rice research in the past three decades, particularly on the mega-projects that attempted to revolutionize rice yield, sustainability and quality of both Asian (Oryza sativa) and African (O. glaberrima) rice together with their impact on rice cultivation. We have also analysed the ...
SPL50 Regulates Cell Death and Resistance to Magnaporthe Oryzae in Rice. The identification of spotted leaf 50 (spl50), a novel lesion mimic mutant (LMM) in rice, provides critical insights into the mechanisms underlying programmed cell death (PCD) and innate immunity in plants. Authors: Banpu Ruan, Hui Wu, Yaohuang Jiang, Jiehua Qiu, Fei Chen ...
The completion of the rice genome in the mid-2000s marked a momentous milestone in rice research, opening seemingly endless doors for gene discovery not only in rice but also in other crops [59,60]. Rice, together with thale cress ( Arabidopsis thaliana ) that had its genome completed in 2001 [ 61 ], are the best-characterized model species in ...
The top-cited article on rice research in Indonesia and the Philippines, based on their overall global citations, is a study on water-efficient and water-saving irrigation ...
Peer review under responsibility of China National Rice Research Institute. Rice Science is an international research journal sponsored by China National Rice Research Institute. It publishes original research papers, review articles, as well as short communications on all aspects of rice sciences in English language. Some of the topics that ...
Juliano, B. Physicochemical Properties of Starch and Protein in Relation to Grain Quality and Nutritional Value of Rice 389-405 (International Rice Research Institute. Rice Breeding, Los Baños ...
At a global scale, yield potential averaged 9.5 Mg ha −1 crop −1, ranging from 5.9 to 14.8 Mg ha −1 crop −1 across the 32 rice cropping systems included in our analysis (Supplementary Fig ...
jected (based on medium fertility) to grow from 7.7 billion. in 2019 to 8.5 billion in 2030 (10% increase), and further to. 9.7 billion in 2050 (26%) and to 10.9 billion in 2100 (42%). The world's ...
In the Special Issue "Molecular Research in Rice: Agronomically Important Traits", we collected several recent studies that identified genetic factors and revealed their molecular contributions to rice agronomic traits under various cultivation conditions. Increasing grain yield is a major objective in breeding programs, because of the need ...
The aim behind the development of this rice is to improve the crop yield, nutritional value, and food safety of rice grains. This review article provides a summary of the research data on genetically modified rice and its potential role in improving the double burden of malnutrition, primarily through increasing nutritional quality as well as ...
Introduction. Oryza sativa L., the most widely grown rice, is the staple food of an estimated 3.5 billion people worldwide. 1 About 870 million people are estimated to suffer from chronic undernourishment globally, the vast majority of whom live in developing countries where rice is closely associated with food security and political stability. 2 Rice production and consumption are among the ...
The Next Generation Rice Disease Research Special Issue highlights features work from Roman-Reyna and colleagues (2020) at the International Rice Research Institute that describes the rice microbiome from the wealth of data from the 3000 Rice Genomes Project (The 3 2014). This work advances our perspectives on how we can consider associations ...
Research into rice—the world's most important food crop—is crucial for the development of technologies that will increase productivity for farmers who rely on rice for their livelihood. This is particularly the case throughout the developing countries of Asia and is also true for much of Latin America and, increasingly, Africa. The benefits of such increased productivity will flow ...
Its rice research is also responsible for developing rice varieties, including semi-dwarf and high-yielding IR8, and promoting the use of good agronomic practices (Mackill and Khush, 2018), which paved the way for the Green Revolution in Asia. By 2010, an estimated 480 rice varieties directly linked to the institute had been released in the ...
Rice, a staple food for more than half of the world's population, is grown in >100 countries with 90% of the total global production from Asia. Although there are more than 110,000 cultivated varieties of rice that vary in quality and nutritional content, after post-harvest processing, rice can b …
Rice (Oryza sativa L.) is the most widely consumed staple food for a large part of the world's human population, especially in Asia (Samal et al., 2018).Asia is on the top in terms of production and consumption of rice. According to FAO report (2016-2017), average production of rice is estimated as 5.0 × 10 8 t, and due to rise in population, the requirement is expected to increase up to 2. ...
A map of rice genome variation reveals the origin of cultivated rice. Nature 490 , 497-501 (2012). Article CAS PubMed PubMed Central ADS Google Scholar
Abstract Rice-wheat cropping system, intensively followed in Indo-Gangetic plains (IGP), played a prominent role in fulfilling the food grains demand of the increasing population of South Asia. In northern Indian plains, some practices such as intensive rice cultivation with traditional method for long-term have been associated with severe deterioration of natural resources, declining factor ...
In the past century, there have been great achievements in identifying resistance (R) genes and quantitative trait loci (QTLs) as well as revealing the corresponding molecular mechanisms for resistance in rice to major diseases and insect pests. The introgression of R genes to develop resistant rice cultivars has become the most effective and eco-friendly method to control pathogens/insects at ...
This review attempts to evaluate, based on existing research, the effectiveness of rice fortification as a public health intervention. ... We handsearched the five journals with the highest number of included studies in the last 12 months to capture any article that may not have been indexed in the databases at the time of the search. As rice ...
Rice is a major cereal food crop and staple food in most of the developing countries. India stands second in the production of rice next to China. Though almost 40,000 varieties of rice are said to exist, at present, only a few varieties are cultivated extensively, milled and polished. Even if white rice is consumed by most people around the world, some specialty rice cultivars are also grown ...
Rice 14, Article number: 101 (2021) Cite this article. On May 22, 2021, Mr. Yuan Longping, academician of Chinese Academy of Engineering and winner of the Medal of the Republic, passed away. Professor Yuan has been known as a renowned rice breeder, world hunger fighter and the father of hybrid rice. We knew Professor Yuan when we were young.
Rice is the major staple food of Asia, and an important source of employment and income in rural areas, particularly in low-income countries. Research has contributed significantly in achieving food security by increasing the yield potential of rice in irrigated systems, reducing the crop maturity period and achieving yield stability by developing resistance against major insects and diseases ...
REVIEW ARTICLE Two decades of rice research in Indonesia and the Philippines: A systematic review and research agenda for the social sciences Ginbert P. Cuaton 1 & Laurence L. Delina 1 While rice ...
MicroRNAs (miRNAs) are small, non-coding RNAs that are expressed in a tissue- and temporal-specific manner during development. They have been found to be highly conserved during the evolution of different species. miRNAs regulate the expression of several genes in various organisms, with some regulating the expression of multiple genes with similar or completely unrelated functions.
M. oryzae strains infection induced the expression of OsWRKY70.(A-C) 2-week-old seedlings of NIP plants were inoculated with rice blast by spraying method.The qPCR analysis of OsWRKY70 expression levels at 0, 24 and 48 h post-inoculation with M. oryzae strains 318-2 (A), R01-1 (B) and 110-2 (C).OsActin was used as an internal control gene. The expression level of OsWRKY70 in plants under ...