If energy cannot be created or destroyed, where does it come from?
9 March 2022
Shutterstock/Relight Motion
Herman D’Hondt
Sydney, New South Wales, Australia
It may sound incredible, but many scientists believe that the total energy of the universe is zero. Hence, no energy needed to be “created” when the universe came into existence.
While it seems obvious that there is a huge amount of energy in the particles and radiation that pervade the universe, this energy may be balanced by negative energy caused by the gravitational attraction between the particles. As Stephen Hawking explained, when you pull two objects apart, you need to expend energy to overcome the gravity that pulls them together. As it takes positive energy to separate them, gravity must be negative energy.
If that theory is correct, then there was never any need to create energy or matter – they cancel each other out. That implies that the big bang could have started as a simple statistical fluctuation.
It also means that it may be possible to create our own big bang: just squeeze some vacuum in the right direction and bingo, a new universe, no energy required.
Mike Follows
Sutton Coldfield, West Midlands, UK
Energy can be transferred between different stores. Energy and matter are equivalent, so nuclear energy, for example, starts off as matter. When an inventory is taken of all the matter and energy in the universe, it adds up to zero, which seems counter-intuitive to say the least.
The zero-energy universe hypothesis is the simplest explanation of this, as outlined in 1973 by Edward Tryon, who was the first to suggest that our universe is the result of a quantum fluctuation.
According to Albert Einstein, mass and energy are equivalent and, according to Tryon, the positive energy associated with mass is counterbalanced by gravitational potential energy, which is negative. Also, according to quantum field theory, particles routinely pop into and out of existence. Tryon suggested that the same could happen for a universe.
Villembits, France
The amount of energy/matter in the universe is constant and derives from the mysterious big bang. Over time, this becomes more and more spread out and less and less useful, even though the actual amount doesn’t change.
Take the sun as an example. Its nuclear fusion reactions turn matter (think of it as concentrated energy) into visible sunlight and other forms of energy. The sunlight hits a green leaf on Earth and the solar energy is now transferred into a chemical energy store as oxygen is separated from carbon dioxide and water, leaving carbohydrate in the leaf.
We eat the leaf and breathe in the oxygen. The respiration reaction in our muscle cells allows the energy to be used to move our arm as we hammer in a nail. The arm, nail, hammer and the air absorb the sound, get hot and radiate infrared heat to outer space.
So the energy concentrated in the original hydrogen atoms in the sun is now scattered into the universe. Low-grade and almost useless, but still the same amount we started with.
To answer this question – or ask a new one – email [email protected] .
Questions should be scientific enquiries about everyday phenomena, and both questions and answers should be concise. We reserve the right to edit items for clarity and style. Please include a postal address, daytime telephone number and email address.
New Scientist Ltd retains total editorial control over the published content and reserves all rights to reuse question and answer material that has been submitted by readers in any medium or in any format.
Terms and conditions apply .

Sign up to our weekly newsletter
Receive a weekly dose of discovery in your inbox! We'll also keep you up to date with New Scientist events and special offers.
More from New Scientist
Explore the latest news, articles and features
Sleeping bumblebees can survive underwater for a week
Starfish have hundreds of feet but no brain – here's how they move.
Subscriber-only
The man reinventing economics with chaos theory and complexity science
Environment, our plans to tackle climate change with carbon storage don't add up, popular articles.
Trending New Scientist articles
Can Solar Energy Replace Fossil Fuel?
Tony chen november 7, 2020, submitted as coursework for ph240 , stanford university, fall 2020, introduction.
Solar energy is becoming more and more popular in the United States. With more people becoming more conscious about the effects of global warming, the interest in solar energy to replace fossil fuels has also greatly increased. In order for solar energy to achieve this feat, large solar farms, order of magnitude larger than the typical solar farm shown in Fig. 1 would need to be constructed. In this thought experiment, I want to explore the hypothesis that solar energy can replace fossil fuel as the main source of energy production in the United States.
Solar Panel Efficiency
Solar panels efficiency has been the fundamental limiting factor of solar energy harvesting. From ShockleyQueisser limit calculating the maximum efficiency of solar energy conversion to be 33.7% with a single p-n junction to the maximum theoretical efficiency cap of 68.7% with an infinite stacking of cells from skylight. [1] In this calculation, I will be using the current state-of-the-art of 29%. [2]
Generating Power Is Not the Whole Story
To be able to generate the power is only part of the equation. The next immediate need is to be able to store the energy, as a buffer, for when solar panels are not active such as at night or during rainy and cloudy days. This gives incentives to construct solar farms in areas of dry weather, long day time, and cheap land. The next part of this solar power system is the transmission of power from one or multiple central locations in the United States to the rest of the country. Some regions of the country is not suitable for solar panels, which then must rely on other states to produce and transmit power. This not only creates additional infrastructure needs but also potentially geopolitical issues between states. The last consideration is the maintenance cost of the proposed solar farm, although this could create a lot of local jobs for the locations of the solar farms. This also could be a hotly contested political issue as states which are currently big on fossil fuel electricity generation would lose a large number of jobs to states which are ideal for solar farms.
What Would It Take to Replace
First, let's calculate roughly how much solar energy are there per square meters:
Given the previously quoted current best solar panel conversion rate:
And the current energy consumption of the United States, and given the hypothesis that solar energy will be the dominant source of energy generation, we will assume that 51% of this energy will need to be produced by solar: [3]
So this can lead us to how many square meters of land needs to be covered by solar panel:
To more easily put this number into perspective in the United States, we can convert this number into 25560 square miles. To put this number into perspective, it is would be about covering about 21% of the land area of the state of New Mexico.
Now that we have calculated the land area that would need to be covered with solar panels to make this hypothesis work, let's talk about the other part of this thought experience, definitely the harder part, which is the cost. What makes this calculation hard is the uncertainty of price, especially given the scale of this project. Ignoring the cost of the infrastructure of transmitting the power to the rest of the nation, the main costs of this giant solar farm is the cost of the solar panels and the cost of the battery needed to store the energy. With current solar farms, at a large scale, usually cost about $1 per Watt it generates. [4]
This calculation does not take into consideration of the battery needed to store the power for day/night cycles and potential bad weathers, land cost and the maintenance cost of the solar farm, which would be significant as well.
Let's talk about the area first. Building one giant solar farm that takes up 21% of the land area of New Mexico definitely sounds not feasible. However, this definitely would not be the way of possible implementation. Most likely multiple mega-large scale solar farms would be constructed at multiple states with a lot of sunlight hours and clear days, such as Arizona, California, Nevada, New Mexico, Georgia, Colorado, Idaho, Kansas, Oklahoma, Utah, Wyoming and Texas. If spread out in these 11 states, we are only looking at about 2% of the total land area of these states.
The above calculation shows a lower-bound starting cost of more than $1700 billion for this project. The approved 2019 U.S. military budget of $686.1 billion. [5] So this project would require multiple years of budget and spending similar to the scale of U.S. military budget. However, the additional cost of upkeeping of the system, the infrastructure required to transmit and deliver the power, and the geopolitical issue between states require much further discussion.
© Tony Chen. The author warrants that the work is the author's own and that Stanford University provided no input other than typesetting and referencing guidelines. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] A. De Vos and H. Pauwels, "On The Thermodynamic Limit of Photovoltaic Energy Conversion," Appl. Phys. 25 , 119 (1981).
[2] J. Temple, " These Flexible Solar Cells Bring Us Closer to Kicking the Fossil-Fuel Habbit ," MIT Technology Review, 16 May 19.
[3] " October 2020 Monthly Energy Review ," U.S. Energy Information Administration, 27 Oct 20.
[4] A. Sharma, "A Comprehensive Study of Solar Power in India and World," Renew. Sust. Energ. Rev. 15 , 1767 (2011).
[5] " 2019 Defense Budget Overview ," Office of the Under Secretary of Defense (Comptroller), Feburary 18.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Comput Neurosci
The Energy Homeostasis Principle: Neuronal Energy Regulation Drives Local Network Dynamics Generating Behavior
Rodrigo c. vergara.
1 Neurosystems Laboratory, Faculty of Medicine, Biomedical Neuroscience Institute, Universidad de Chile, Santiago, Chile
Sebastián Jaramillo-Riveri
2 School of Biological Sciences, Institute of Cell Biology, University of Edinburgh, Edinburgh, United Kingdom
Alejandro Luarte
3 Cellular and Molecular Neurobiology Laboratory, Faculty of Medicine, Biomedical Neuroscience Institute, Universidad de Chile, Santiago, Chile
Cristóbal Moënne-Loccoz
4 Motor Control Laboratory, Faculty of Medicine, Biomedical Neuroscience Institute, Universidad de Chile, Santiago, Chile
5 Department of Health Sciences, Faculty of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile
Rómulo Fuentes
Andrés couve, pedro e. maldonado.
A major goal of neuroscience is understanding how neurons arrange themselves into neural networks that result in behavior. Most theoretical and experimental efforts have focused on a top-down approach which seeks to identify neuronal correlates of behaviors. This has been accomplished by effectively mapping specific behaviors to distinct neural patterns, or by creating computational models that produce a desired behavioral outcome. Nonetheless, these approaches have only implicitly considered the fact that neural tissue, like any other physical system, is subjected to several restrictions and boundaries of operations. Here, we proposed a new, bottom-up conceptual paradigm: The Energy Homeostasis Principle, where the balance between energy income, expenditure, and availability are the key parameters in determining the dynamics of neuronal phenomena found from molecular to behavioral levels. Neurons display high energy consumption relative to other cells, with metabolic consumption of the brain representing 20% of the whole-body oxygen uptake, contrasting with this organ representing only 2% of the body weight. Also, neurons have specialized surrounding tissue providing the necessary energy which, in the case of the brain, is provided by astrocytes. Moreover, and unlike other cell types with high energy demands such as muscle cells, neurons have strict aerobic metabolism. These facts indicate that neurons are highly sensitive to energy limitations, with Gibb's free energy dictating the direction of all cellular metabolic processes. From this activity, the largest energy, by far, is expended by action potentials and post-synaptic potentials; therefore, plasticity can be reinterpreted in terms of their energy context. Consequently, neurons, through their synapses, impose energy demands over post-synaptic neurons in a close loop-manner, modulating the dynamics of local circuits. Subsequently, the energy dynamics end up impacting the homeostatic mechanisms of neuronal networks. Furthermore, local energy management also emerges as a neural population property, where most of the energy expenses are triggered by sensory or other modulatory inputs. Local energy management in neurons may be sufficient to explain the emergence of behavior, enabling the assessment of which properties arise in neural circuits and how. Essentially, the proposal of the Energy Homeostasis Principle is also readily testable for simple neuronal networks.
Introduction
Throughout evolution, the development of the nervous system has enabled animals with the capacity to manifest ever-growing complex behavior, which has helped them survive in a changing environment. Understanding how neurons arrange themselves into neural networks that work at producing different behaviors has always been a major goal of neuroscience. Various conceptual frameworks have aimed to explain how behavior emerges from neuronal activity. Arguably, the most relevant is the Neuron Doctrine, proposed by Santiago Ramón y Cajal and further developed by Heinrich Waldeyer-Hartz and Horace Barlow (Barlow, 1972 ; Bock, 2013 ). Since then, the same logic has spread into coding paradigms (Lettvin et al., 1959 ; Fairhall, 2014 ; Yuste, 2015 ), especially in information processing frameworks (Fodor, 1983 ; Friston, 2002 ; Robbins, 2010 ; Lorenz et al., 2011 ), and has been scaled from neurons up to neural networks (Yuste, 2015 ). A common and key element of these conceptual approaches has been to find neuronal correlates of behaviors, effectively associating specific behaviors with distinct neural patterns. This top-down approach (using behavior as a reference to be mapped into neuronal circuits) has been very successful in providing single-unit or network models that can implement the observed behaviors, yet simultaneously, may make difficult the capture of the emergence of behavior, which is by-large a bottom-up phenomenon. This methodological approach also limits our capacity of predicting the boundaries of the capabilities or the spectrum of behaviors of a given system, because we map or associate only those behaviors that have been well-characterized. More importantly, all theoretical approaches, to our knowledge, have only implicitly addressed the fact that neural tissue, like any other physical system, is subjected to several restrictions and boundaries of operations.
Cells use energy to stay alive and at the same time, maintain some reserves to respond and adapt to dynamic situations, maintaining their homeostasis . For neurons, energy availability would be further important, as their energy expenses are high, as compared to other somatic cells (Attwell and Laughlin, 2001 ; Shulman et al., 2004 ). Indeed, the metabolic consumption of the brain, which represents 20% of whole-body oxygen consumption, contrasts with the neural tissue representing only 2% of whole body weight (Shulman et al., 2004 ). Interestingly, the total brain energy consumption increases proportionally with the number of neurons among different species, including humans (Herculano-Houzel, 2011 ), and the total energy expenditure associated to a neuron during the signaling and resting states is constant in different mammalian species (Hyder et al., 2013 ). Thus, neurons seem to present a highly specialized system for managing their energy demands.
Several evidences demonstrate that it is reasonable to assume a constant value for energy availability for neurons over the long term (energetic homeostasis). For instance, cultured neurons exhibit a steady value for free adenosine triphosphate (ATP) in basal conditions, which transiently decrease during the induction of glutamatergic synaptic activity through various energy challenges (Marcaida et al., 1995 , 1997 ; Rangaraju et al., 2014 ; Lange et al., 2015 ). This tight energy management suggests a relevant role for neuronal energy homeostasis on neuronal and network functional properties.
Here, we propose a new bottom-up conceptual paradigm for neuronal networks: The Energy Homeostasis Principle. Under this principle, the condition of maintaining neuronal homeostasis triggers synaptic changes in the individual but connected neurons, resulting in the local energy balance scaling up to a network property. This conceptual framework supposes that energy management might be critical in determining plasticity, network functional connectivity, and ultimately behavior.
Cellular Homeostasis and Gibbs Free Energy
In this article, we propose that behavior may raise as an emergent property rooted in energy requirement of neurons, thus, we would like to start from the level of biochemistry and metabolism. As such, we will begin with the fact that cells are dynamic molecular machines that require the nutrient intake to stay alive. Many biological processes are thermodynamically unfavorable, and through metabolism, cells draw energy from nutrients, and generate metabolic resources necessary to drive their cellular activities (Hofmeyr and Cornish-Bowden, 2000 ) (for a schematic, see Figure 1A ). Cellular homeostasis can be defined as a state where the production and consumption of metabolic resources balance each-other, and thus their concentration is constant in time. For our specific context, balancing the intake and consumption of metabolic resources will unavoidably have a global impact on the cellular processes. The network of metabolic processes is large and complex, limiting, to some extent, our capacity to predict cellular behavior using basic principles. Nonetheless, biochemical reactions must be consistent with the laws of thermodynamics.

Homeostasis requires a balance between ATP production (metabolism) and ATP consumption (synaptic activity): (A) Production of energy molecules by metabolism supports neuron activity, in addition to cell maintenance processes, most notably by ATP [A(t)]. Neuronal homeostasis depends on a balance between production and consumption of high-energy molecules. (B) Synaptic activity has been estimated to amount for half of total ATP consumption [diagram redrawn from (Harris et al., 2012 )]. For cellular homeostasis to be achieved, neurons must regulate their activity and metabolism in response to changing external perturbations. We propose that regulatory mechanisms, responsible for changes synaptic plasticity, reflect the requirement for maintaining a constant level of energy resources available for neurons to function.
Thermodynamics can help us understand how a system evolves in time through the comparison of the thermodynamic potential between an initial and final state. For processes at a constant temperature and pressure, the thermodynamic potential is given by Gibbs Free Energy (G). This thermodynamic potential will dictate a directional bias of chemical reactions. The Gibbs Free Energy—supporting cellular processes—is provided with a finite amount of metabolic resources. Thus, there is a trade-off between the potential for metabolic work and metabolic expenses, which, we propose, may explain some well-established phenomenology of how cells respond to external perturbations. Additionally, the change in the Gibbs Free Energy (ΔG) and the rate associated with chemical transformations are related (Crooks, 1999 ). To illustrate the relation between thermodynamics and kinetics, for a reversible reaction X⇔Y, the following relation constrains kinetic rates:
where R is Gas constant, and T the absolute temperature (Cannon and Baker, 2017 ). This equation describes the relation between the mean rates of any pair of reversible processes (from X to Y, and from Y to X) and the difference in Gibbs Free Energy between the states. Note that by definition the Gibbs Free Energy assumes Temperature to be constant. In simple terms, the difference ΔG{X⇒Y} can be thought of as a “directional bias,” indicating how favorable one direction is over the other. In more detail, the Gibbs Free Energy is divided into two components, Enthalpy (H) and Entropy (S):
Where, T is the absolute temperature (Silbey et al., 2004 ). In the context of chemical transformations, Enthalpy is a measure of the energy required to form a given substance, disregarding interactions with other molecules; whereas Entropy can be interpreted as a correction accounting for all possible combinations by which molecules can react (Danos and Oury, 2013 ). Given the combinatorial nature of entropy, it can also be interpreted as a measure of disorder or information, but certain care must be taken for this interpretation to have physical meaning (Jaynes, 1965 ). We wish to recognize that the direct application of thermodynamics to biology has many challenges, particularly in describing macro-molecular processes (Cannon, 2014 ; Cannon and Baker, 2017 ; Ouldridge, 2018 ), combining large systems of reactions (e.g., kinetic parameters may be required), and accounting for fluctuations from average behavior (Marsland and England, 2018 ).
A long-standing observation in biology, rooted in thermodynamic laws is that for cells to function, they must couple unfavorable reactions (ΔG > 0) with more favorable ones (ΔG < 0). Common examples of unfavorable processes are the synthesis of macromolecules, and the maintenance of membrane potential; which are coupled with the hydrolysis of ATP, and GTP providing more favorable ΔG (Nicholls, 2013 ). In turn, ATP, GTP, and monomers for macro-molecules are synthesized from nutrients through metabolism (Nicholls, 2013 ). For instance, the maximum free energy provided by ATP hydrolysis is related to the concentration of ATP, ADP, and phosphate.
Where, Δ G ° is the standard free energy, and log, the natural logarithm. For generality, we will call hereafter “energy resources” the set of reactants that allow cells to maintain unfavorable reactions in the direction conducive to cellular functioning and survival. We wish to emphasize that balancing the internal production and consumption of metabolic resources by different reactions is critical, given that metabolic resources are finite and shared by many cellular processes. Thus, cells must manage their internal production and consumption of metabolic resources to stay alive and remain functional, which may be of special consequence to cellular activities with high energy demands, such as synaptic activity in neurons. Given that neurons are active most of the time, it is reasonable to expect that current and future disposal of energy resources is privileged, which may be reflected in the regulatory mechanisms responsible for synaptic plastic changes. In the following section, we will explain how current evidence regarding neuron plasticity appears to support a relatively simple rule: maintain the levels of energy disposal constant, by reducing the consumption of energy resources (e.g., reducing discharge rate, post-synaptic potential), or by increasing high-energy molecule production (e.g., mitochondria and interactions with glia).
Energy Management of Brain Neurons
Neurons are the paramount example of energy expenditure for their function and survival. This situation is reflected in their large metabolic rates and by the comparatively higher sensibility of brain tissues to oxygen and glucose deprivation (Ames, 2000 ). Reactions controlling the conversion of nutrients into available cytosolic levels of ATP are important to generate the potential metabolic work that is available to a neuron at any given time. During normal conditions, the primary energy substrate in the brain for neurons is blood-derived glucose; however, when at elevated levels in the blood, ketone bodies and lactate can be used as energy sources as well (Magistretti and Allaman, 2018 ). The glycolytic pathway is the first step to glucose processing, where two pyruvates and two ATPs are generated from one molecule of glucose. In addition, the pyruvate could either be reduced to lactate or enter the Krebs cycle to produce metabolic reducing intermediates that will generate nearly 29 additional ATP molecules per glucose (through oxidative phosphorylation in the mitochondria). Although neurons and astrocytes are capable of glucose uptake and performing both glycolysis and the Krebs cycle, accumulated evidence supports the hypothesis that neurons may “outsource” glycolytic activity to astrocytes under activity conditions (Weber and Barros, 2015 ). In addition, the central nervous system is provided with small glycogen reserves, which are predominantly present in astrocytes (Brown and Ransom, 2007 ), but also found in neurons (Saez et al., 2014 ). In any case, the lactate derived from glycogen break-down may also provide ATP to the neurons under ischemic or sustained electric activity conditions (Brown and Ransom, 2007 ).
ATP sources change dynamically with neuronal activity and several mechanisms account for this fine-tuning response. First, neuronal mitochondria are capable of raising ATP synthesis in response to increased synaptic stimuli (Jekabsons and Nicholls, 2004 ; Connolly et al., 2014 ; Rangaraju et al., 2014 ; Toloe et al., 2014 ; Lange et al., 2015 ). Although the molecular meditators for this activation are not completely elucidated, the increase of the respiratory rate of an isolated mitochondria correlates well with the ADP concentration (Brown, 1992 ), and neuronal mitochondrial function has been satisfactorily modeled considering the changes in ATP and ADP levels (Berndt et al., 2015 ). As an alternative mechanism, it has been reported that operating on milder stimulation conditions, the activity of Na-pump rapidly induces ATP synthesis of the mitochondria, in response to neuronal activity independent from changes in the adenosine nucleotides (Baeza-Lehnert et al., 2018 ). Second, neuronal activity is known to elicit local increases in blood flow (neurovascular coupling), glucose uptake, and oxygen consumption (Sokoloff, 2008 ). Coherently, glucose uptake and glycolytic rate of astrocytes are further increased in response to the activity of excitatory neurons, potentially as a consequence of the local rise of glutamate, ammonium (NH 4 ), nitric oxide (NO), and importantly, K + (Magistretti and Allaman, 2018 ). As such, an increased glycolytic rate on astrocytes leads to lactate accumulation that is shuttled into neurons which generate ATP through oxidative phosphorylation. Thus, in CNS neurons, different neuronal and non-neuronal ATP sources work “on demand,” depending on the local levels of synaptic activity.
What Is ATP Used for in Neurons?
Neurons are perhaps the largest eukaryotic cell in nature, their surface may be up to 10,000 times larger than an average cell (Horton and Ehlers, 2003 ). The large size of neurons supposes that structural processes, such as protein and lipid synthesis or the traffic of subcellular organelles, should be sustained by high levels of ATP synthesis. In addition to this fact, energy consumption during signaling is far more important. Indeed, it has been estimated that nearly 75% of the gray-matter energy budget is used during signaling; a number that is coherent with the decrease of energy consumption, observed under anesthesia, and is estimated to be around 20% of the total energy budget (Attwell and Laughlin, 2001 ; Harris et al., 2012 ).
Most of the neuron's energy budget during signaling is used to restore ion gradients across the plasma membrane, mediated by the action of different ATP-dependent pumps. For example, assuming an average firing rate of 4 Hz, a presynaptic neuron's ATP is mostly used for restoring the Na+ gradient due to action potentials, and to sustain the resting potential (22% and 20% of energy consumption, respectively). Meanwhile, at the post-synaptic neuron, ATP is primarily used to extrude ions participating in post-synaptic currents—about 50% of the energy consumption (Harris et al., 2012 ). More detailed descriptions of the neuron energy budget is provided in Figure 1B .
Neuron's ATP Availability Is Tightly Regulated
All cellular organizations require a minimum amount of ATP for survival. It is well-known that when ATP levels decrease below a certain threshold for different eukaryotic cells, apoptosis or necrosis is induced (Eguchi et al., 1997 ). Nevertheless, determining the maximum and minimum thresholds of a cell's ATP requirement for not only to survive but to realize a specialized function, is less apparent. In any case, this feature must be necessarily shaped by evolutionary adaptations of cells to their specific tissue environment. It is not completely clear how a neuron's ATP levels, during rest and upon activity, may impact its structure and function. Interestingly, by computational and mathematical modeling, it has been proposed that a compromise among energy consumption and information processing capacity has shaped the fundamental features of neuronal structure and physiology, including neuronal body size, ion channel density, and the size and frequency of synaptic inputs (Sengupta et al., 2013 ). For example, a larger neuronal body has a better capacity to discriminate and respond to different synaptic inputs (coding capacity), but at the cost of higher energy consumption. On the other hand, with a fixed size for the soma, the ion channel density required to obtain maximum energy efficiency is at a lower value than the density needed to maximize the coding capacity. Similarly, although small synaptic inputs at low frequencies are energetically more efficient, better coding capacity arises with larger inputs and rates. These energy constraints may have introduced important consequences during cellular evolution, such that neurons with similar shape and function may harbor similar metabolic features, even across different species.
Remarkably, it has been found that energy consumption of neurons, across the brains of varying species, is constant (Herculano-Houzel, 2011 ). This result supposes a critical restriction for the function of neuronal networks and their coding properties. For example, sparse coding i.e., brain computations that emerge from the increased firing rate of a few neurons during a task, has been proposed as a mechanistic solution to the limited energy availability for brain neurons (Attwell and Laughlin, 2001 ; Laughlin, 2001 ; Lennie, 2003 ; Weber and Barros, 2015 ). Thus, it is also possible that variables such as the ATP cytosolic concentration may have been finely tuned during evolution to allow for the emergence of fundamental properties, including some forms of synaptic plasticity.
Accumulating evidence supports that neurons, in time, harbor a narrow window of ATP cytosolic concentration availability [A(t)]. Despite not having dynamic measurements with absolute values of A(t), different experimental approaches on cultured neurons show that this variable tends to remain constant at resting conditions and after momentary synaptic challenges. Accordingly, 60 min of different sorts of glutamatergic stimulation leads to a nearly 5-fold decrease of A(t) (Marcaida et al., 1995 , 1997 ), but when a brief glutamatergic or electric stimulation is applied, only a transient and reversible decrease on ATP levels occurs and the A(t) is subsequently restored to basal levels (Rangaraju et al., 2014 ; Lange et al., 2015 ).
Tight management of A(t) also operates on axonal compartments with important functional consequences. For instance, isolated axons from the optical nerve, under low glucose conditions, demonstrate a pronounced decay of ATP levels during high-frequency stimulation (50–100 Hz) (Trevisiol et al., 2017 ). Interestingly, compound action potentials (CAPs), generated by those stimulated axons, are reduced to the same extent and in high coincidence as the A(t), suggesting that electric activity depends on A(t) (Trevisiol et al., 2017 ). In addition, isolated axons exhibit a constant value for A(t), which immediately and steeply decays after the inhibition of glycolysis and oxidative phosphorylation, in concomitance with CAPs. However, when inhibitors are washed out, both A(t) and CAPs return to basal levels, further supporting that the system tends to reach a constant value for A(t). The tendency of the system to set a constant value for A(t) is also manifest in conditions where expenditures are highly reduced. For example, A(t) remains constant on pre-synaptic terminals of cultured hippocampal neurons, despite the inhibition of action potential firing due to incubation with the Na + channel blocker Tetrodotoxin (TTX) (Rangaraju et al., 2014 ). Conversely, the same study showed that electrical stimulation of 10 Hz by 1 min, concomitantly evokes ATP synthesis on pre-synaptic terminals, restoring A(t) to basal levels (Rangaraju et al., 2014 ). From now on, we will call the basal value of A(t) as the homeostatic availability of ATP (A H ).
Mechanisms accounting for the intrinsic control of A H in neurons are less explored than in other cells. In the short term, there is a direct and fast effect of ATP molecules and their hydrolysis products, such as AMP/ADP, over the activity of different metabolic enzymes and ion channels. Indeed, neurons are largely known for being extremely, even disproportionately, sensitive to decreases in ATP sources, leading to a fast and significant inhibition of electrical activity (Ames, 2000 ). For example, ATP-sensitive K + channels open during decreased ATP levels, hyperpolarizing the neuron to reduce endocytosis and the opening of voltage-sensitive Na + channels, thus preventing the ATP expenditure associated to both processes (Ben-Ari et al., 1990 ). On the other hand, it has been elegantly shown that action potential firing on pre-synaptic terminals' gate activity-driven ATP production is also required to allow proper synaptic transmission (Rangaraju et al., 2014 ). This close dependency of ATP levels to synaptic functioning has suggested that the affinity constant for ATP (e.g., K m ) of different pre-synaptic enzymes, might be close to certain resting ATP levels (Rangaraju et al., 2014 ). It is tempting to speculate that the fine-tuning of the affinity constant from key enzymes might be a broader phenomenon in neurons. In addition, it is known that calcium entry, which is transiently modified by electrical activity, is capable of orchestrating changes in ATP production. For example, synaptic stimulation with brief NMDA pulses, not only lead to pronounced increases of cytosolic calcium levels, but also of the mitochondrial matrix, whose ATP producing enzymes are known to be stimulated by calcium increases (Tarasov et al., 2012 ; Lange et al., 2015 ). Indeed, transient increases of calcium levels are thought to be a sort of metabolic alarm which prepares cells to confront high energy demands by increasing ATP production by the mitochondria (Bhosale et al., 2015 ).
As a complementary mechanism, changes in the ATP and AMP ratio gate the activity of other metabolic sensors which, in turn, induce a specific signaling cascade for short and long-term adaptations of neuronal functions. For example, all known eukaryotic cells, including neurons, harbor energy sensors, such as AMP-activated protein kinase (AMPK), which tend to restore ATP concentration by decreasing anabolic and/or energy consuming processes, while increasing energy production through catabolism post-energy challenges (Potter et al., 2010 ; Hardie, 2011 ; Hardie et al., 2012 ). AMPK is a highly evolutionary-conserved serine/threonine kinase enzyme that is activated either by diminished cellular energy (high AMP/ATP ratio) and/or through increased calcium (Hardie et al., 2012 ). Recent evidence shows that in dorsal root ganglion neurons—which express the transient receptor potential ankyrin 1 (TRPA1) channel for thermal and pain transduction—the AMPK activation results in a fast, down-regulation of membrane-associated TRPA1 and its channel activity within minutes, which is consistent with lowering energy expenditure by diminishing post-synaptic currents (Wang et al., 2018 ). Furthermore, it has been demonstrated that calcium overload, induced by an excitotoxic NMDA stimulus on cultured cortical neurons, can be reduced by the activation of AMPK, which would save the energy involved in the reversal of a Ca ++ potential (Anilkumar et al., 2013 ). Interestingly, the actions of the catalytic subunit of neuronal AMPK also includes the inhibition of axon outgrowth and dendritic arborization during neuronal development, for adapting to metabolic stress mediated by the suppression of Akt and mTOR signaling pathways (Ramamurthy et al., 2014 ). This result suggests that AMPK may also operate in mediating structural synaptic changes during the activity of mature neurons, contributing to control energy expenditures in the long-term. Furthermore, it has been shown that the maintenance of long-term potentiation (LTP), which is energetically demanding, is dampened when AMPK activity is pharmacologically activated (mimicking a low ATP/AMP ratio), or conversely, LTP could be rescued when an ATP mimetic, ara-A, was added during an energy challenge. Thus, under low energy conditions, neuronal AMPK tends to inhibit changes on ionic gradients and reduce changes on cytoarchitecture, which can upregulate the value of A(t), impacting plastic capacity as well.
Summarizing, each neuron has a certain amount of ATP available to them, which is constantly consumed by their different functions which can mostly be explained using ion gradient changes on axons and dendrites. At the same time, ATP production will compensate the ATP expenditure reaching an A H that should remain constant until another specific synaptic challenge arrives ( Figure 2 ). In the next section, we will discuss the potential functional consequences of these adaptations in special cases of neuronal plasticity.

Neuron activity induces changes in metabolism and synaptic activity to maintain homeostatic energy levels: Schematic illustration of neuronal response to an increase in activity. Above, in scale of colors, ATP concentration indicating from low to high A, with A H at the center. Below, step 1 depicts a resting state with A(t 0 ) = A H . In time step 2, the increase in activity would result in additional ATP consumption, and therefore reduce ATP concentration. We propose that neurons would respond to this perturbation by decreasing ATP consumption [C(t)] and increasing its ATP production [P(t)], as represented in time step 3. Finally, neurons would return to homeostatic levels of ATP (A H ), which is illustrated in time step 4. During all these steps, A(t) is colored following the above ATP concentration color scale. Some factors that contribute to ATP consumption are as follows: synaptic post-excitatory currents (excitatory inputs), firing rate (excitatory outputs), ion channel density, size of soma, dendrite arborization and axonal length, neurotransmitter recycling and release, and cytoarchitecture adaptations. Whereas, factors contributing to ATP production are: glycolysis supported by glucose or glycogen breakdown, oxidative phosphorylation supported by neuronal pyruvate, astrocyte-derived lactate, and ketone bodies. Neurons possess regulatory mechanisms that sense current energy levels (here represented by a rotating wheel with a floating balloon), and control production and consumption, to maintain homeostasis. Examples of these control mechanisms would include ATP-Sensitive K+ channels, and AMPK signaling.
Revisiting Neuronal Plasticity Under the Perspective of Energy Constraints
A narrow window of ATP cytosolic concentration across time supports a bottom-up view of neuronal energy constraints, which may explain some well-described plastic adaptations from the literature. Measurements of glucose and oxygen consumption (reflecting energy consumption) have not distinguished between the contribution from glial and neuronal metabolism and the total energy expenditure attributed to one neuron (Hyder et al., 2013 ). Nonetheless, neurons would keep energy availability during the increment of energy demands, which include action potentials, potential propagation or dendritic depolarization, by dynamically sharing expenses with astrocytes glial cells (Hyder et al., 2006 ; Barros, 2013 ). It is worth mentioning that energy management is partly performed by these latter cells (Magistretti, 2006 ; Magistretti and Allaman, 2015 ). Indeed, we must consider that ATP neural production is provided by the local pyruvate and glial lactate. Where a theoretical model aimed to explain brain energy availability from rat and human brains, it indirectly suggested that glial and neuron lactate sources may dynamically vary across different species and activity levels, with the condition of maintaining a rather constant energy production (Hyder et al., 2013 ).
We will follow a very simplified view of ATP metabolism characterized by two collections of processes: Those that produce ATP (e.g., from local pyruvate and glial lactate), and those that consume ATP (e.g., recovery of ion gradients, structural and functional synapse maintenance). We can formalize the effect of these processes on ATP concentration (A) by a simply differential equation:
Where, P ( t, A , …) is a function that represents the sum of all reaction rates that produce ATP (e.g., anaerobic and aerobic metabolism), whereas C ( t, A , …) is the sum of all reaction rates that consume ATP (e.g., membrane repolarization, structural and functional synapse maintenance). Both production ( P ) and consumption ( C ) rates are dynamic (they depend on time), but, more importantly, they depend on the levels of ATP available ( A ). Homeostasis will be achieved when production and consumption rates are equal, and the concentration of ATP is constant in time. We will represent the homeostatic concentration of ATP by A H .
We can interpret the observations of relatively constant ATP concentrations in neurons, as reflecting the action of feedbacks that adjust ATP production ( P ) and consumption ( C ) rates, compensating deviations of ATP ( A ), such that neurons return to homeostatic ATP levels ( A H ). We can expect that in case cells have an excess of ATP, they would respond by decreasing production or/and increasing consumption; and analogously, in case ATP levels are reduced, cells would respond by increasing production or/and decreasing consumption. We will call this regulation the neuron “energy management,” and summarize it mathematically using these equations:
Meaning that the differences between A with A H determines whether ATP production ( P ) and consumption ( C ) processes increase, decrease, or maintain their rates over time. Note that we also consider the possibility that neurons may respond to energy challenges by adjusting production and consumption, but it must be at least one of those variables.
It is critical to notice that this formalization makes some important simplifications. First, we understand that in addition to ATP, the concentrations of ADP, AMP, and other energy resources do determine homeostasis and influence neuronal changes. ATP is a reasonable departure point, given its prevalence in metabolism and the evidence supporting its role in synaptic plasticity, and therefore, will be the main example of energy resource exploit in our argument. An additional reasonable assumption is that the magnitude of the change in reaction rates should correlate with the magnitude of the distance to homeostasis, which we have omitted from the equations but will become relevant later in our argument for proposing experiments. We expect to expand toward a more detailed formalism in future work. Despite its simplicity, we think that our model can help to understand several previous studies and propose some experiments aimed at empirically evaluating the relation between energy resource availability and neural plasticity. We expect that simple phenomenological models, such as ours, will encourage both theoretical and experimental efforts, provided they can be readily falsified empirically, and be compared to theoretical derivations from biochemical first principles.
The tendency to set ATP at A H might be compatible with homeostatic plastic changes that return a neuronal network to a basal firing rate, after prolonged periods of increased or decreased synaptic activity (homeostatic synaptic plasticity). Accordingly, it has been theoretically proposed that the excitability threshold of neurons might be a direct function of ATP (Huang et al., 2007 ) . For example, the K ATP channel-opener diazoxide decreases bursting and regular firing activity of the immature entorhinal cortex neurons (Lemak et al., 2014 ), which is coherent with a tight association of firing rates with contingent ATP concentration. Also, theoretically, neuronal circuits governed by purely Hebbian-plasticity rules are predicted to converge on instability, or to the opposite—total inactivity (Miller and MacKay, 1994 ). One possible solution to enable neuronal circuits to remain responsive is to limit the amount of synaptic strength per neuron. At least on excitatory synapses, this problem has shown to be solved by another form of synaptic plasticity termed “homeostatic synaptic plasticity,” and more specifically, “synaptic scaling” (Turrigiano et al., 1998 ; Turrigiano, 2012 ). Synaptic scaling emerges to counteract the effects of long periods of increased or decreased synaptic activity in a multiplicative manner, thus allowing neurons to continuously reset the weight of their synaptic inputs to remain responsive to new environmental and cellular contexts. In the long term, the consequence of this regulation is that the firing rate of cortical cells in culture is sustained to an average set point (Turrigiano, 2012 ). As far as we know, no attempt has been made to relate or prove the influence of neuronal energy load or A(t) on this phenomenon.
Simple experiments on synaptic scaling could be performed to examine whether the tendency to reach A H has a predictive value on the synaptic activity of neuronal networks. As shown in the seminal experiments of Turrigiano's group, when a GABAergic inhibitor bicuculline (Bic) is acutely added to cultured neurons, it produces a significant increase in average firing rate. However, during 48 h of stimulation, firing rates return to control values. On the other hand, neuronal firing rates can be completely abolished soon after adding either tetrodotoxin (TTX) or 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). Nevertheless, during the 48 h of incubation, activity levels also return to a basal value (Turrigiano et al., 1998 ). The observed adaptive changes that operate in the long-term makes this experiment ideal for manipulating energy parameters.
Similar to the experiment of synaptic scaling performed by Turrigiano's group, in our theoretical experiment, cultured neurons would be submitted to 48 h of synaptic activity stimulation with Bic. During the stimulus, A(t) will transiently decrease, inducing plastic changes on the network that will return ATP concentration to A H , in a given time period ( t 1 ) ( Figure 3A ). In all conditions, P(t) and C(t) change accordingly with A(t), following Equations 4 and 5. However, if, during the stimulus, the neurons were pharmacologically modified to partially decrease ATP production [e.g., by blocking oxidative phosphorylation with sodium azide], expenditures C(t) are expected to be rapidly lowered and the time window required to return to the A H value will be shortened ( Figure 3B ). Conversely, one could “enlarge” the theoretical value of A H on cultured neurons by adding an ATP mimetic, such as ara-A. Here we assume that ara-A would cause inhibition of AMPK signaling, and that concentrations employed are low enough not to disturb the ATP synthesis. Thus, we propose that the neurons will take more time to return to A H ( Figure 3C ) . Under these three conditions, the firing rate of neurons should also be adapted to the same level as in the initial state, before stimulation, as well as ATP concentrations should return to the homeostatic value A H .

Hypothetical experiment to evaluate the influence of energy availability on synaptic scaling: The influence of energy availability in synaptic plasticity could be evaluated empirically. Here we propose a simple hypothetical scenario and explain what outcomes we predict under each condition. (A) Cultured neurons are stimulated with bicuculine (Bic) for 48 h (denoted by a black bar), which presumably induces transient changes in ATP concentration [A(t)]. Neurons respond by increasing ATP production [P(t)] and reducing ATP consumption [C(t)] by reducing its firing rate, which leads to the reestablishment of homeostatic ATP concentration within the time window enclosed by dotted lines. (B) During stimulation, cultured neurons can be pharmacologically treated to partially inhibit oxidative phosphorylation (i.e., reducing ATP synthesis) denoted by a black arrow. Following the Energy Homeostasis Principle, we propose this will result in a further reduction of ATP concentration, which will induce an accelerated reduction in ATP consumption through the reduction of synapse firing rates. Thus, we propose that in this scenario the time window required to return to homeostasis is shortened. (C) Almost an identical protocol to (B) is applied to neurons; however, using an ATP mimetic molecule (denoted by the black arrow). We assume that ATP mimetic molecules would delay the reduction of synapsis firing rate by allosterically inhibiting AMPK, resulting in an enlarged period of energy consumption. Thus, we propose a wider time window before reaching A H . All graphics follow Equations 4 and 5, with the additional assumption that the magnitude of the adjustment of P(t) and C(t) are proportional to the distance of ATP levels A(t) to homeostatic levels A H . Results from these kinds of experiments could advance the understanding (and potentially manipulate) of the mechanisms responsible for neural adaptations, uncovering the relevant role of metabolic elements, such as metabolic sensors and/or nutrient availability.
From Molecules to Behavioral Homeostasis
In the previous sections, we have discussed how the energy homeostasis can affect synaptic plasticity in one neuron. Subsequently, this plasticity can impact other neurons that will trigger the same control systems to keep their A H . Since energy demands are transferred through synapses, and synapses appear or disappear according to energy demands, a network homeostasis comes into play. In this section, we argue that energy constraints scale up a level of organization and how homeostasis in one level is affected by homeostasis in the others.
From Neurons to a Neural Network
The first level is single neuron homeostasis, which is the balance between C(t) and P(t) in single neurons. Importantly, as far as an action potential producing a post-synaptic potential goes, it necessarily imposes an increment in C(t) for the post-synaptic neuron. As such, neurons manage their energy needs which also present an external demand from pre-synaptic neurons, and also imposes an energy demand over the post-synaptic neurons. The fact that a local increase in the C(t) can produce a change in post-synaptic neuron's C(t) supports that energy management is also a neural population property, which we will name network homeostasis. The single neuron homeostasis is closely related to the network homeostasis through a two-way directional interaction, where the network structure imposes constraints on the range of possible homeostatic states that a neuron can achieve, which will, in turn, pose stress on the network through interactions with neighboring neurons. In the same way, these neurons will respond by modifying their synaptic weights (also known as network connectivity), the number and the location of their synapses, thus changing the functionality of the neural network structure (maybe even micro-anatomically). In any condition that causes an imbalance between C(t) and P(t), the neurons will tend to change. Since neurons activate each other through synapses, this means that the activity of the pre-synaptic neurons will induce metabolic work in the post-synaptic ones. In turn, a post-synaptic neuron will modulate its synaptic weight to couple the input from the pre-synaptic neuron to its own metabolic needs. This process will continue recursively until the neurons balance their C(t) and P(t), in which case the network would have reached homeostasis. Essentially, network homeostasis is driven by the needs of each neuron, as each of them will change in an attempt to reach their own A H . Note that it is not necessary that every neuron should reach its own A H , as the connectivity of activity within the network may not allow them to improve further. However, every single neuron must have enough P(t) to devote toward maintenance processes required to stay alive. As such, network homeostasis becomes a neural population property.
Network homeostasis is tightly related to single neuron homeostasis; therefore, neural network homeostasis will be only achieved when several of the neurons that compose it individually can maintain themselves within homeostatic ranges (e.g., achieving A H ). It is known that synaptic and dendrite pruning are a part of healthy development (Huttenlocher, 1990 ; Riccomagno and Kolodkin, 2015 ), which we could interpret as adjustments required to couple with the trade-off between maintaining the structure vs. the energy spent in action and post-synaptic potentials. In worse cases where suboptimal conditions are imposed on a single neuron by the neural network homeostasis, we expect to find neuron death. This phenomenon is documented as a part of normal brain development in some species (Huttenlocher, 1990 ), and also in pathological conditions (Perry et al., 1991 ; Kostrzewa and Segura-Aguilar, 2003 ; Pino et al., 2014 ).
From Neural Networks to Behavior
Behavior can be broadly described as the set of actions performed by an organism, or anything that an organism does that involves movement and response to stimulation. These actions are adaptive when they increase the survival and reproduction probability. In a top-down interpretation of behavior, these actions are the result of the activation of the neuronal circuit that developed evolutionary to fulfill a need. Nonetheless, according to the Energy Homeostasis Principle, at the neural circuitry level, the actions performed by an organism are out of spatial and temporal context, since all the cells experiences are perturbations of the network activity. For a given neuron, the activity dynamics is dependent on the cumulative synaptic currents, regardless of the type of pre-synaptic cells that evoked them, or in the case of sensory receptors, the type of energy that is transduced. Similarly, it makes no difference for a given neuron to have neuron-neuron or neuro-muscular/endocrine synapses. Conversely, we can reinterpret behavior as the observed consequence of the homeostatic activity of an extended neural network (brain) which interacts with the environment. Sensory input and motor outputs can thus be viewed as “environmental synapsis.” Under this framework, what we call behavior may be not necessarily be different from the range of actions neurons engage in any circuit.
However, the interaction with the environment has an important difference that will impact the energy balance in the neuronal network. We can operationalize behavior in a neural system as a set of inputs and outputs that occur in a closed-loop manner. For instance, when we move our eyes, the brain is generating output activity, which in turn modifies the subsequent input to the retina. These dynamics occur for all sensory systems, where motor acts modify sensory inputs (Ahissar and Assa, 2016 ). In this process, for each brain action, we should expect changes to occur in some sensory inputs. In other words, behavior can be seen as one of the ways in which the brain stimulates itself.
In principle, this closed-loop scheme would enable the brain to completely predict the sensory consequence of the motor actions. This processes of active inference is in line with previous proposals such as Friston's free energy principle and predictive coding (Friston, 2010 ; Schroeder et al., 2010 ). It is crucial to note that Friston's Free Energy Principle used an informational approach where aspects such as temperature do not refer to the absolute temperature measured in an experimental setting. As such, the Energy Homeostasis Principle does not conceptually overlap with Friston's proposal; they can be considered as complementary. From a bottom-up view, Friston's proposal answers the epiphenomena, which can be related to information processing, rather omitting the underlying physiological constraints. However, in any of these proposals, there is an agreement that the brain is capable of predicting sensory input, and that it seems to reduce uncertainty as far as possible. In the case of Friston's proposal, it refers to the reduction of informational uncertainty, while in the Energy Homeostasis Principle, it refers to the reduction of energy sensorial input uncertainty.
Parsimoniously, the brain cannot fully predict the sensory inputs that occur after every motor act, because changes that are independent of the action of the organism also occur in the environment, and these changes may be critical to its survival. According to the Energy Homeostasis Principle, we should expect that neural networks will operate in a way that will favor the behavioral input activities within homeostatic energy ranges. If a given input is energetically too demanding, we should expect a change in behavior. If a given set of motor activity consistently produces an energy stressor input, it will cause synaptic changes in the brain, as the energy balance processes are spread over the neuronal network.
Sensory input represents the major energy challenge in the brain, while the motor output is the only way a neural network can modify this input. This way, the neuron in the network has the chance to regulate its C(t), given the pressure representing sensory input. Neural networks will restrict the palette of behaviors that can be observed, while behavior will impose energy demands that the neural network will couple with by modifying behavior. For these reasons, behavior can also be considered as a phenomenon which affects the energy homeostasis in a two-way direction. Thus, an at least three-level nested system can be depicted, where each level will have a two-way interaction with each other (see Figure 4 ).

Energy homeostasis: An integrated view of neurons, networks and behavior schematic of the three nested levels of the energy homeostasis system. Each level represents one unit and its proximal operations. Neuron refers to one neuron which must manage its energy consumption, which will trigger neuron-plastic changes. Many of these neurons will build a network, which has connectivity properties and population energy demands. Many networks working together will deploy behavior through motor actions, while also receive the sensory input. All levels present a two-way energy interaction between behavior, neural networks, and neurons. The figure intends to present how sensory input can be considered an energy demand at network and neuron levels, while motor output through behavior gives room to control part of the sensory input.
According to the Energy Homeostasis Principle, a key aspect to explaining adaptive behavior must reside within the brain's macro-structure and evolutionary mechanisms. Given a certain palette of sensory specializations, set of effectors, and brain structures, it will impose the range of possible energy disposal attractors that can emerge. For instance, the number of sensory cells, and their sensitivity to stimuli will determine the energy input imposed on neural tissue. The effectors given will determine the space in which a neural network must control that input. The macrostructure of the brain will then impose general restrictions on the neural network homeostasis. For instance, the visual cortex mainly receives visual input, and the communication through brain regions is mainly achieved using major tracts. As such, the series of C(t), imposed by one neuron on another, must follow the paths allowed by the macrostructure. This means, that neural network homeostasis will not only be a function of energy input provided by the sensory cells, and the chances to control it using effectors, but also of macro anatomical restrictions produced by genetic mechanisms (Gilbert, 2000a , b ).
Evolutive pressures must act over all traits—genetic, physiological, and behavioral—of the organism (Darwin, 2003 ). As such, evolutive pressures have selected a sensory and effectors pallet, as well as a brain macrostructure. We conjecture that from the set of behaviors that satisfy the energy constrictions, behaviors that statistically improve the chances of surviving will be selected. We propose that the macro-anatomical structures impose a certain system of dynamic energy management among neural networks, which force the emergence of a certain set of energy attractors producing, in turn, a specific set of behaviors. It is important to consider that in animals that display a large set of behaviors, probably what is selected is the ability to learn. This concretely would mean that the selected is not a specific behavior, rather the flexibility with which an organism must adapt behaviorally during its own life.
Given this bottom-up view, we conclude the existence of behaviors strictly required for survival, and others which might present adaptive advantages given the specific context of the organism. In human primates, for example, there is a vast set of behaviors that are not strictly for the survival of the single individual in any context, yet they exist, such as leisure activities, those related to the production of art in its multiple forms, and even pathological behaviors which might directly impact the individual's health or survival. As far as these non-strictly adaptive behaviors do not impact the organism's life, they might be highly adaptive in certain contexts.
In any case, evolutionary mechanisms will shape the nervous system's macrostructure and behavior so that both are aligned in a way where solving the energy constraints of a single neuron and the neural network will lead to survival. If not, that macrostructure is expected to be lost, as those organisms will die. In fact, there is no need that all these levels work in alignment per se, rather they must only be aligned to survive. Evolutive pressures will remove the organisms where the three level systems present goals that don't benefit each other. Since we can only see the animals that present all three level goals aligned, we have historically thought that neurons, neural networks, and organisms share the same goal. We proposed here that evolution shaped organisms, so when a neuron solves their needs, the behavior emerges as an epiphenomenon, which enables the organism to solve its needs, hence surviving.
Perspectives of Reinterpretation
In this section, we aim to contrast our proposal with evidence and highlight the corollary aspects which can open new avenues of research. Concretely, we will evaluate if we can reassess evidence, considering the Energy Homeostasis Principle. We think that this proposal is parsimonious as in spirit the rule is simple, what makes it complex is the wide range of interactions and properties that can emerge from the neural interaction constraint imposed by this rule. We believe that our proposal captures the essence of the concept of Braitenberg's vehicles (Braitenberg, 1986 ) 1 and provides a plausible solution to the dynamics elaborated there. Naturally, Energy Homeostasis Principle still has some limitations. It is unclear how we can scale up this principle in networks as large as the brain. The metabolic mechanisms in neurons are quite complex, and still we need more empirical information to tune the mathematical modeling. We decided to use ATP as an energetic proxy, but many other molecules are used by neurons as energetic resources, and may present a dual signaling-resource condition activating control systems. We have mention abundant literature that shows an association between energy related variables and neural activity. However, we have not presented direct evidence of how energy constraints shape plasticity and neural network properties. Despite these limitations, the Energy Homeostasis Principle can be tested empirically by associating plasticity markers with energy availability, production, and consumption as mentioned in section “Revisiting neuronal plasticity under the perspective of energy constraints.” More importantly, this proposal serves new empiric avenues to study the working of the brain. For instance, plasticity has always been thought to be the changes required to fix a given behavior. However, according to our proposal, plasticity is a process that takes place not only during learning but continuously, as a core component of the constant deployment of behavioral changes. As such, plasticity might not only be a determinant of behavior acquisition but a key aspect of ongoing behavior. In the following subsections, we will briefly discuss different strategies which can be used to extend further from Equation (5), an example of evidence interpreted considering the Energy Homeostasis Principle, and then discuss other theoretical and empirical avenues which can be reinterpreted based on this paradigm.
Modeling Strategies to Implement Energy Homeostasis Principle
We did not extend our mathematical definitions beyond (Equations 4 and 5) as we aim to set a theoretical ground fertile for different modeling strategies. Equations (4) and (5) describe a quite simple idea that neurons take resources to couple with their energetic demands, and that these two must balance each other in order for the neuron to survive. However, the specific strategies used to operationalize the terms within (Equations 4 and 5) was purposefully left open to avoid constrains into specific modeling paradigms. Equations (1–3) were included to better formalize the problem at a metabolic level. These equations are relevant to build the theoretical argument, however, we would not consider them necessary for modeling, at least in a first approach.
In general terms, Energy Homeostasis Principle requires a dynamic modeling, and a topographic or structural component ideally framed from bottom-up. There is already an example that fits with these requirements (Yuan et al., 2018 ). In this work, they used the ratio between the energy consumed in synaptic transmission and the total metabolic energy consumed in synaptic transmission and dendritic integration over time. This ratio is used as a third component of Hebbian synaptic plasticity, allowing it to change synaptic weighs according to this energetic ratio and pre-synaptic activity. This is a nice example of how to include energetics constraints in neural activity modeling. Under the Energy Homeostasis principle view, the ratio does not make sense in terms of metabolism and neuron needs, because it only address energy consumption, without considering the impacts in productions and availability. Therefore, ignoring the restrictions in energy consumption derived from production and availability. This consumption ratio make sense under a top-down view supported in an information codification logic. Therefore, we suggest to define that ratio according to Equation (4), including consumption, availability, and production following the control mechanisms here presented.
Besides this particular model, graph theory could represent a starting point to define the structure of a dynamic network, in which nodes properties can be updated in a temporal fashion. Graph theory is already used to recall the structural properties of brain networks (Feng et al., 2007 ; De Vico Fallani et al., 2014 ), therefore, without a doubt it will be suitable representation which can be extended to consider the energetic management. Moreover, graph theory contains a vast amount of metrics to characterize networks (Costa et al., 2007 ), and more importantly, could allow to contrast those metrics against real data (Demirtaş and Deco, 2018 ; Klinger, 2018 ).
Strategies such as Free Energy Principle (Friston, 2010 ), or those that profit of predictive coding conceptions (Spratling, 2008 ; Schroeder et al., 2010 ; Huang and Rao, 2011 ), can also serve as a basis for energy homeostatic modeling. However, we suggest to use energy consumption instead of neural activity as predictor. In this case reducing surprise would be analogous to reduce the chances of a neuron to be driven out of energy homeostasis. Energy availability and production ideas are more complex to include. In general terms, and based on the concepts exposed in the previous sections, energy consumption is constrained to energy availability and production. As such, to adapt a predictive coding paradigm requires to include energetic restrictions, which must take into account the rate and amount of energy or activity equivalents that can be managed by the neurons within physiological ranges.
Many other strategies can be used. The above mentioned are often used in neuroscience, however, any modeling strategy that suits the temporal dynamic of energy management, and its topographical bottom-up properties, should be able to capture the essence of the Energy Homeostasis Principle.
Hybrots: An Analysis Using the Energy Homeostasis Principle
Let us discuss the energy principle proposed here in the context of a simple, in vivo network model. Empirically, one critical aspect of relating energy management with behavior is the major challenge of controlling sensory inputs. Most of experimental, in vivo animal models are not only sensible to acoustic, visual, physical, and chemical stimuli of the environment, but also to proprioceptive inputs, such as muscle contraction, tendon tension, blood acidification, hormone levels, among others. Strictly speaking, there is no way to properly control the sensory input of an animal in vivo , and the behavioral in vitro protocol seems to be unreal. Nonetheless, there are some protocols that can be considered as initial efforts of trying to build in vitro behavioral protocols. Specifically, some reports demonstrate that if we connect a neuronal culture of dissociated cortical or hippocampal neurons to an external device, coherent behavior can be obtained (Novellino et al., 2007 ; Tessadori et al., 2013 ).
Concretely, a system decodes the firing rate of the neurons in the culture and generates an output which is used to control two wheels of a vehicle. The vehicle has distance sensors. The sensor activity is coded to electrical pulses delivered back to the culture. The stimulation frequency is a function of the distance to an obstacle in front of the sensors. If the vehicle gets closer to an obstacle, then the stimulation frequency increases. If the vehicle crashes into an obstacle, a stimulation (20 Hz for 2 s) is delivered, which is previously known to trigger plasticity (Jimbo et al., 1999 ; Tateno and Jimbo, 1999 ; Madhavan et al., 2007 ; Chiappalone et al., 2008 ; le Feber et al., 2010 ). Leaving the vehicle in a circular maze with several obstacles under the operation of this protocol will cause it to “learn” to navigate, while avoiding impacts with obstacles (Tessadori et al., 2012 ). This model constitutes a protocol that enables studying the molecular, electrophysiological, and behavioral properties of neural processing simultaneously; above all, it allows the full control of the sensory input that this network will have.
Is this learning-like phenomenon compatible with the Energy Homeostasis Principle? When a single neuron is submitted to constant stimulation, we expect to have a 1–1 stimulation-action potential response. However, at a frequency stimulation as low as 10 Hz, the neurons will decay over time until they are unresponsive, or their response is importantly delayed (Gal et al., 2010 ). If interpreted through the Energy Homeostasis Principle we can hypothesize the following mechanism. First, we can postulate that at a frequency of 10 Hz or higher, stimulations become energetically stressful. As a response, neurons will respond with modifications in their synaptic weights in the short term, and with changes on their cytoarchitecture in the long term. Both processes will result in changes to the network structure. Each time the vehicle crashes, a stressful 20 Hz pulse will be delivered inducing plasticity. Functional restructuration is expected at each impact; leading to a random walk through different neural functional configurations, where each neuron will jump from state to state to minimize energy stress (see Figure 5 ). It is expected that those network configurations that decrease the effects of the sensory input will reduce energy stress due to impacting obstacles. But the best network configuration to the energy stress is indeed to avoid it. Eventually, a network configuration will arise which will prevent the vehicle from crashing. Since no energy stress will be delivered as a sensory input with this configuration, this structure will seemingly stabilize on a configuration of homeostatic energy expenditure ( Figure 5 ). We are aware that the above interpretation may oversimplify the actual mechanisms followed by the neurons. Neuronal changes are most likely not completely random and more complex regulations may be taking place. However, we want to point out that they can be sufficient to explain the phenomenology of the observations. As such, energy management, as a local rule, will impact the neural network structure as an emergent property, where, in turn, it will impact behavior. Critically, in this example, we have focused on sensorial input as an increment of neural activity. This might not always be the case (such as under sensorial isolation). Despite that, under this specific scenario, we propose that networks will minimize energy consumption; the goal is to arrive to A H , not to the minimum possible energy expenditure. Therefore, if the sensorial input would move A(t) below A H , we would expect network modifications to increase expenses. In any case, the obtained behaviors must be at least compatible with the dynamic constraints imposed by C(t), despite it being too high or low. In this example, behavior emerged to satisfy the energy needs of the neuron by means of C(t). Finally, from all the vehicle movements, only a few, like avoiding the obstacle, might be interpreted as purposeful from an observer's point of view, the remaining ones may be considered a random trajectory. Importantly, this attribute is provided by the observer, as the neurons would only be managing their energetic demands. More research is required to evaluate what is happening with behavior , when the obstacles are out of the sensor's range along with the learning curve of the vehicle. Nonetheless, the Energy Homeostasis Principle allowed us to propose this hypothesis ( Figure 5C ), and it can be empirically addressed. Naturally, using the same experimental approach, we can evaluate how plasticity is affected by energetic demands induced electrically or by altering neurotransmitter concentrations. We can use the vehicle's behavior, or we can use the Graph Theory index already used to characterize networks (Costa et al., 2007 ) to associate neural network properties with energetic demands and metabolic activity.

Depiction of Braitenberg's vehicle behavior as a controlled platform to study learning and network energy adaptation: In this figure we hypothesize, based on the Energy Homeostasis Principle, how a hybrot would learn. (A) Different behaviors observed at different learning levels of the Braitenberg's vehicle (top panel), and the corresponding sensory input (bottom panel) from an obstacle (circle centered to x symbol) is detected by a sensor (dashed area), while the vehicle explores the environment. (B) Learning curve of the vehicle when learning to avoid the obstacles, passing from frequent collisions to full avoidance behavior. (C) Network energy adaptation triggered by the sensory input while minimizing the energy stress.
The Neuron Doctrine and the Energy Homeostasis Principle
Historically, the primary efforts to connect neuron activity with neural network dynamics and behavior was first proposed in 1888 (Barlow, 1972 ; Bock, 2013 ), which is referred to as “The Neuron Doctrine,” maintained and developed to this day (Dehaene, 2003 ; Moser and Moser, 2015 ; Sheppard, 2016 ). In general terms, this theoretical proposal tries, in dual form, to solve the information coding and processing problem and has been supported by intracranial recordings, where abundant examples can be found (Lettvin et al., 1959 ; Fairhall, 2014 ; Moser and Moser, 2015 ). Specifically, neurons are expected to code for specific properties of the environment, where its activity is associated with the detection of specific stimuli. For instance, neurons in the primary visual cortex of mammals are selectively sensible to oriented bars (Hubel and Wiesel, 1962 ; Taylor, 1978 ), while in the Lateral Geniculate there is evidence supporting the existence of circular receptive fields representing portions of visual space (Reid and Shapley, 1992 ). In these cases, neurons have receptive fields, which can be interpreted as a specific topologic relation of V1 with a certain retinal region; and therefore, with the image. Receptive fields with the same selectivity feature can also be found in the tactile, and auditory primary cortex, evidence of which is often interpreted as environmental stimuli being coded as a map in the brain (Penfield and Boldrey, 1937 ; Penfield, 1965 ; Ruth Clemo and Stein, 1982 ). This classic evidence is also theoretically line up with the recent hippocampus where neurons (Moser and Moser, 2015 ).
Critically, most of the evidence supporting the neuron doctrine is associated to the neuron discharge rate. Since this discharge rate is a part of the C(t), it necessarily means that most of the evidence supporting the neuron doctrine supports the Energy Homeostasis Principle as well. For this reason, it is plausible to consider that most of the neuron doctrine evidence is also evidence indicating how energy expenses of one neuron can be directly associated with behavior. Furthermore, high discharge rates, as mentioned above, are expected to trigger plasticity mechanisms. Also, only a low percentage of neurons present high discharge rates (Olshausen and Field, 2005 ), which should be expected under the Energy Homeostasis Principle scope. Moreover, due to the fact that high discharge rates might trigger changes in functional connectivity (synaptic weights), it should not be surprising that when presenting more complex visual scenes, classic receptive fields are no longer detectable (Fairhall, 2014 ). We may consider that classic stimulation visual protocols impose an energy input, reflected in the high discharge rate, which needs to be managed. In contrast, visual scenes are regularly experienced, therefore already managed energy, and the firing pattern are considerably lower. As such, we think that the neuron doctrine is not necessarily wrong, rather it has not focused on how the discharge rate is a proxy of energy demands imposed on neurons, which in turn affects their homeostasis. Also, that plasticity might have a functional role in ongoing behavior rather than only stabilizing learned behaviors.
The neural doctrine paradigm has been closely related to information coding paradigms. The coding paradigms follow the same logic as the genetic code; the idea that information is universally coded using the same dictionary or codebook. In the case of genetics, what we call the genetic code, is an arrangement of how the sequence of nucleic acids informs specific amino acid sequences when assembling proteins. In the case of a neural code, the assumption is that environmental stimuli are translated into brain activity, which is then translated into motor output. More specifically, it is possible to map specific neuron activities to specific properties of the environment. For instance, the intensity of the stimulation can be mapped to the discharge rate of the sensory neurons (Gardner and Johnson, 2013 ). The transduction of the stimuli is usually non-linear and sensitive to differences with previous stimulation rather than the raw value of the stimuli—Weber's law (Gardner and Johnson, 2013 ). This adaptation law has a direct interpretation in the context of energy expenditure by neurons, as neurons coding raw stimuli would demand a greater energy supply. Weber's law has also been extended to complex cognitive functions, such as quantity estimations (Dehaene, 2003 )—where discharge rates are used as the code of quantities for specific neurons—suggesting that energy saving may be a strategy widely used by neurons.
Of course, the discharge rate is far from being the only neural code proposed. Coupled with the complexity of sensory activity, temporal coding was proposed, where the exact temporal relationship between each neuron spike would be the key to understanding how environmental information is translated into brain activity (Connor and Johnson, 1992 ; Friston, 1997 ). Temporal coding is implicitly related to energy demands, as the time between action potentials trigger plasticity mechanisms, associated with one of the most expensive items of the neuron physiology—post-synaptic potential and plastic mechanisms (Attwell and Laughlin, 2001 ). Another strategy was population coding (Georgopoulos et al., 1986 ; Nicolelis, 2003 ; Moxon and Foffani, 2015 ). Population coding uses the activity of a high number of neurons, where the discharge rate, timing, and as many properties can be extracted make it possible for a human or non-human primate to move a robotic arm or similar, with the brain. As more neurons are included, more information is obtained, and we should expect that we will better predict the arm movement. This approximation is good when the aim is to predict behavior but is not useful to understand how behavior emerges from neural activity. If reassessed using Energy Homeostasis Principle, we interpret that population coding works as it is a good assessment of neural network homeostasis, implicitly providing information about plastic changes and neural energy management. Up to some extent, all approaches have to do with when, how much, and which neurons are discharging, which in turn can be interpreted as when and how much energy is expended by individual neurons and the network.
When evaluating evidence related to a whole-brain approach, the neuron doctrine is mostly applied by associating the bold signals of brain regions to specific behaviors. Critically, the fMRI signal is derived, to some extent, by the changes triggered through the glia to couple with the energy demands (Otsu et al., 2015 ). Therefore, we can interpret that energy management associated to glial function, is already associated directly with behavior. Moreover, it suggests that energy management can be mapped into networks associated to specific behaviors. Naturally, the specifics in which Energy Homeostasis Principle would impact large networks like brains is still elusive, and it probably would require to incorporate formally the functional properties of the glia.
In general, the fMRI approach strongly resembles the serial symbolic programming paradigms, where a module can be homologized to a programming function, and the network would be the general architecture of the software. The loss of a programming function leads to the loss of a specific functionality of the software. This metaphor was addressed in classic literature (Hovland, 1960 ; Searle, 1980 ), suggesting that the brain processes information using a symbolic serial paradigm. As such, most of the neural correlates within the neurocognitive domain are interpreted as information processing, ranging from a strictly symbolic to a correlative information approach. However, using a bottom-up approach and the Energy Homeostasis Principle, those attributions are an observer's bias, as the one described in Braitenberg's vehicles (Braitenberg, 1986 ). Behavioral functions of a neuron or the neural network would be the epiphenomena of neurons regulating their own homeostasis. In fact, as explained in the previous section, we can describe how the vehicle learns to avoid obstacles without using any informational, symbolic, or teleological explanations. Using this bottom-up approach, it is expected that an informational approach will be useful, as far as the neurons' and the neural network's needs are aligned with the organism's. However, it should be interpreted as an epiphenomenon of neural networks solving their own needs.
Reinterpreting Evidence Toward New Research Avenues
As we have discussed above, energy management, though implicitly considered, is a key feature of the nervous system. This necessarily means that most of our current evidence can be reinterpreted in the light of the Energy Homeostasis Principle. We expect that this reinterpretation will trigger new ideas and strategies to understand the neural phenomena. As an example, we may try to explain the neuronal changes associated with learning processes, based on iconic paradigms such as the long-term potentiation (LTP) and depression (LTD) (Nabavi et al., 2014 ; Jia and Collingridge, 2017 ). Both phenomena involve a large amount of energy expense where the ATP could be followed to understand the phenomena of plasticity as one of energy management. This is key, considering that even the Hebbian rules (Kempter et al., 1999 ), operates differently, according to the neuron type (Abbott and Nelson, 2000 ), highlighting the difficulties in predicting plasticity according to neural activity. At the same time, the calcium ion plays a critical signaling role within neural physiology, where we should ask if it might be a signal of energy expenditure. It is known that metabolic processes sense the ATP-AMP ratio (Ames, 2000 ), however, they have not been studied in association to the plasticity phenomena.
Consequentially, we can assess energy management and not solely from a molecular or electrophysiological perspective. For instance, can we consider inhibitory neurons as an adaptive feature to control brain energy expenditure? This is most intriguing if we consider that inhibitory neurons are key to increasing the neural circuits' controlling properties (e.g. negative feedback structures).
Simultaneously, the central nervous system is the only structure of the body which is actively isolated from the vascular system. It has its own system to maintain stable the neuron proximal environment. Moreover, astrocytes coordinate themselves through calcium waves, producing local changes in blood flow and hyperemia (increase on blood irrigation) (Otsu et al., 2015 ). The brain-blood barrier is not only a filter, but it works functionally to support the energy demands of the neural networks. In fact, synapses are currently suggested as tripartite structures (neuron-neuron and astrocyte) (Wang and Bordey, 2008 ), where the glutamate-release excitatory synapses are proposed to control neurovascular coupling, and thus, brain energy during conditioning and behavior (Robinson and Jackson, 2016 ). This would be a clear example of a neural activity involving external support for energy management.
Moreover, there is a vast number of shapes for neural cells. It is currently unknown why some neurons display large, dendritic arborizations and short axons, while others present long axons and rather small dendritic arborizations. Similarly, there are varying basal discharge rates of activity. We think it is worth exploring whether the likelihood of particular morphologies and rate of activities are associated with energy constraints. For instance, can a neuron manage to maintain a long axon and at the same time a huge dendritic arborization where it must maintain a large number of dendritic spines? If we explore the evidence we already have, we are confident that new insights into neuron morphology will appear. Even more, if an unlikely neuron shape or size which is energetically more expensive presents itself; we should expect that those neurons would be more sensible to energy demands and may be more susceptible to neural death (Le Masson et al., 2014 ). In fact, Paul Bolam proposed that the reason behind Parkinson's is due to the dying out of dopaminergic neurons because of their huge size, which is very expensive in energy terms (Bolam and Pissadaki, 2012 ; Pissadaki and Bolam, 2013 ). It is most likely that many of these traits are genetically determined, however, energy constraints might limit the possible morphological variety. Furthermore, that genetic determinants of neuron specializations may be triggered in response to the C(t).
Finally, the Energy Homeostasis Principle paradigm, combined with a bottom-up view, allows us to reinterpret behavior in a much more flexible way. Animals display many behaviors that are not intrinsically adaptive. Leisure activities are an evident example. Why the dog likes to go for the ball or follow a car? Why would we like to learn how to play the piano or to paint? Using a top-down approach would force us to interpret that evolution endorses us with a leisure activity brain module and that all behaviors are somehow beneficial. It seems more parsimonious to think that evolution restricted the system through macrostructure, so that survival-related brain functions will be selected and inherited. Above all, a wide set of diverse, seemingly useless behaviors can appear, without compromising organism survival or neural needs. Therefore, the only constraint for behavior is that the organisms must stay alive and that the sensory input can be successfully managed, in terms of its energy demand, by the neural networks and the neurons within them. As we already explained before, we think that in the cases of the vehicles controlled by neural cultures, the rules of the stimulation given is critical in understanding how they learn to avoid obstacles. From all the works that reported learning-like properties of in vitro dissociated cultures of neurons (Novellino et al., 2007 ; Mulas and Massobrio, 2010 ; Tessadori et al., 2012 ), two main conclusions can be obtained: (1) learning-like properties are not dependent on a priori, highly intricate and sophisticated neural structures, and (2) there is at least one property which does not require a brain evolution argument to explain the emergence of behavior (but probably requires a neural tissue evolution argument). This would be particularly important in relation to behaviors that are not directly tied to survival.
Because of the space limitations, many of these latter considerations are laid out in a basic form. Nonetheless we stress that some of these speculations can be assessed by reviewing the current literature under the Energy Homeostasis Principle rationale. However, the proposal may encourage the development of falsifiable hypotheses, allowing for the testing of these intuitions through empiric work. Therefore, we propose the principle as a novel paradigm from which we can reinterpret neuroscience experimental data, as well inspire the design of experiments which may connect biochemical knowledge to cognitive neuroscience.
Author Contributions
RV, SJ-R, and AL developed the initial general argument. SJ-R was the main contributor to the metabolic and biochemistry sections. AL was the main contributor to the single neuron's physiological sections. RV was the main contributor to the neural networks and behavior sections. PM contributed by articulating the different sections to build the manuscript. RV wrote the first draft. CM-L designed and built all the figures, based on all the authors' recommendations. CM-L, AC, and RF contributed with key observations that refined the general argument. All authors revised and edited the manuscript. RV and PM edited the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Dr. Felipe Barros for his valuable comments in an earlier version of the manuscript.
1 Braitenberg vehicle is a concept proposed by Braitenberg ( 1986 ), referring to a simple vehicle that has two sensors, each connected to an effector, i.e., a wheel, that provides the vehicle the ability to navigate in a given environment. The activation of the sensors can increase or decrease the speed of the respective wheel. In addition to the sensor-effector relationship, the physical configuration of the sensors and the wheels will determine the navigation “behavior” of the vehicle when the stimuli are present in the environment. One major conclusion of this concept is that complex behaviors arise from relatively simple properties (sensors, circuitry, and effectors) of the system in interaction with the environment.
Funding. This work was supported by the post-doctoral FONDECYT grant 3160403, given to RV, the Ph.D. scholarship by The Darwin Trust of Edinburgh, given to SJ-R, the regular FONDECYT grant 1151478, given to RF, the BNI Puente post-doctoral fellowship, given to CM-L, and the BNI Millennium Institute No. P09-015-F, given to AC.
- Abbott L. F., Nelson S. B. (2000). Synaptic plasticity: taming the beast . Nat. Neurosci. 3 , 1178–1183. 10.1038/81453 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ahissar E., Assa E. (2016). Perception as a closed-loop convergence process . Elife 5 , 1–26. 10.7554/eLife.12830 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ames A. (2000). CNS energy metabolism as related to function . Brain Res. Brain Res. Rev. 34 , 42–68. 10.1016/S0165-0173(00)00038-2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anilkumar U., Weisová P., Düssmann H., Concannon C. G., König H. G., Prehn J. H. M. (2013). AMP-activated protein kinase (AMPK)-induced preconditioning in primary cortical neurons involves activation of MCL-1 . J. Neurochem. 124 , 721–734. 10.1111/jnc.12108 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Attwell D., Laughlin S. B. (2001). An energy budget for signalling in the grey matter of the brain . J. Cereb. Blood Flow Metab. 21 , 1133–1145. 10.1097/00004647-200110000-00001 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baeza-Lehnert F., Saab A. S., Gutiérrez R., Larenas V., Díaz E., Horn M. (2018). Non-Canonical control of neuronal energy status by the Na + pump . Cell Metab. 29 , 668–680.e4. 10.1016/j.cmet.2018.11.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barlow H. B. (1972). Single units and sensation: a neuron doctrine for perceptual psychology? Perception 1 , 371–394. 10.1068/p010371 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barros L. F. (2013). Metabolic signaling by lactate in the brain . Trends Neurosci. 36 , 396–404. 10.1016/j.tins.2013.04.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ben-Ari Y., Krnjević K., Crépel V. (1990). Activators of ATP-sensitive K+ channels reduce anoxic depolarization in CA3 hippocampal neurons . Neuroscience 37 , 55–60. 10.1016/0306-4522(90)90191-6 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Berndt N., Kann O., Holzhütter H. G. (2015). Physiology-based kinetic modeling of neuronal energy metabolism unravels the molecular basis of NAD(P)H fluorescence transients . J. Cereb. Blood Flow Metab . 35 , 1494–1506. 10.1038/jcbfm.2015.70 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhosale G., Sharpe J. A., Sundier S. Y., Duchen M. R. (2015). Calcium signaling as a mediator of cell energy demand and a trigger to cell death . Ann. N. Y. Acad. Sci. 1350 , 107–116. 10.1111/nyas.12885 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bock O. (2013). Cajal, Golgi, Nansen, Schäfer and the neuron doctrine . Endeavour 37 , 228–234. 10.1016/j.endeavour.2013.06.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bolam J. P., Pissadaki E. K. (2012). Living on the edge with too many mouths to feed: why dopamine neurons die . Mov. Disord. 27 , 1478–1483. 10.1002/mds.25135 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Braitenberg V. (1986). Vehicles: Experiments in Synthetic Psychology, 2nd Edn. Cambridge: MIT Press, 168. [ Google Scholar ]
- Brown A. M., Ransom B. R. (2007). Astrocyte glycogen and brain energy metabolism . Glia 55 , 1263–1271. 10.1002/glia.20557 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brown G. C. (1992). Control of respiration and ATP synthesis in mammalian mitochondria and cells . Biochem. J. 284 , 1–13. 10.1042/bj2840001 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cannon W. R. (2014). Concepts, challenges, and successes in modeling thermodynamics of metabolism . Front. Bioeng. Biotechnol. 2 :53. 10.3389/fbioe.2014.00053 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cannon W. R., Baker S. E. (2017). Non-steady state mass action dynamics without rate constants: dynamics of coupled reactions using chemical potentials . Phys. Biol. 14 :055003. 10.1088/1478-3975/aa7d80 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chiappalone M., Massobrio P., Martinoia S. (2008). Network plasticity in cortical assemblies . Eur. J. Neurosci. 28 , 221–237. 10.1111/j.1460-9568.2008.06259.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Connolly N. M. C., Dussmann H., Anilkumar U., Huber H. J., Prehn J. H. M. (2014). Single-cell imaging of bioenergetic responses to neuronal excitotoxicity and oxygen and glucose deprivation . J. Neurosci. 34 , 10192–10205. 10.1523/JNEUROSCI.3127-13.2014 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Connor C. E., Johnson K. O. (1992). Neural coding of tactile texture: comparison of spatial and temporal mechanisms for roughness perception . J. Neurosci. 12 , 3414–3426. 10.1523/JNEUROSCI.12-09-03414.1992 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Costa L. D. F., Rodrigues F. A., Travieso G., Villas Boas P. R. (2007). Characterization of complex networks: a survey of measurements . Adv. Phys. 56 , 167–242. 10.1080/00018730601170527 [ CrossRef ] [ Google Scholar ]
- Crooks G. E. (1999). Entropy production fluctuation theorem and the nonequilibrium work relation for free energy differences . Phys. Rev. E 60 , 2721–2726. 10.1103/PhysRevE.60.2721 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Danos V., Oury N. (2013). Equilibrium and termination II: the case of petri nets . Math. Struct. Comput. Sci. 23 , 290–307. 10.1017/S0960129512000126 [ CrossRef ] [ Google Scholar ]
- Darwin C. (2003). On the Origin of Species by Means of Natural Selection . ed Carroll J. (Peterborough, ON: Broadview Press; ), 672. [ Google Scholar ]
- De Vico Fallani F., Richiardi J., Chavez M., Achard S. (2014). Graph analysis of functional brain networks: practical issues in translational neuroscience . Philos. Trans. R. Soc. B Biol. Sci. 369 :20130521. 10.1098/rstb.2013.0521 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dehaene S. (2003). The neural basis of the Weber-Fechner law: a logarithmic mental number line . Trends Cogn. Sci. 7 , 145–147. 10.1016/S1364-6613(03)00055-X [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Demirtaş M., Deco G. (2018). Computational models of dysconnectivity in large-scale resting-state networks , in Computational Psychiatry: Mathematical Modeling of Mental Illness , eds Anticevic A., Murray J. D. (San Diego, CA: Elsevier Academic Press; ), 87–116. 10.1016/B978-0-12-809825-7.00004-3 [ CrossRef ] [ Google Scholar ]
- Eguchi Y., Shimizu S., Tsujimoto Y. (1997). Intracellular ATP levels determine cell death fate by apoptosis or necrosis . Cancer Res. 57 , 1835–1840. [ PubMed ] [ Google Scholar ]
- Fairhall A. (2014). The receptive field is dead . Long live the receptive field? Curr. Opin. Neurobiol. 25 , 9–12. 10.1016/j.conb.2014.02.001 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Feng J., Jost J., Qian M. (2007). Networks: From Biology to Theory , eds Feng J., Jost J., Qian M. (London: Springer; ). 10.1007/978-1-84628-780-0 [ CrossRef ] [ Google Scholar ]
- Fodor J. A. (1983). The Modularity of Mind: An Essay on Faculty Psychology. Cambridge, MA: MIT Press, 145. 10.7551/mitpress/4737.001.0001 [ CrossRef ] [ Google Scholar ]
- Friston K. (1997). Another neural code? Neuroimage 5 , 213–220. 10.1006/nimg.1997.0260 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Friston K. (2002). Beyond phrenology: what can neuroimaging tell us about distributed circuitry? Annu. Rev. Neurosci. 25 , 221–250. 10.1146/annurev.neuro.25.112701.142846 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Friston K. (2010). The free-energy principle: a unified brain theory? Nat. Rev. Neurosci. 11 , 127–138. 10.1038/nrn2787 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gal A., Eytan D., Wallach A., Sandler M., Schiller J., Marom S. (2010). Dynamics of excitability over extended timescales in cultured cortical neurons . J. Neurosci. 30 , 16332–16342. 10.1523/JNEUROSCI.4859-10.2010 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gardner E. P., Johnson K. O. (2013). Sensory coding , in Principles of Neural Science , eds Kandel E. R., Schwartz J. H., Jessell T. (New York, NY: McGraw-Hill; ), 449–474. 10.1126/science.158.3799.399 [ CrossRef ] [ Google Scholar ]
- Georgopoulos A. P., Schwartz A. B., Kettner R. E. (1986). Neuronal population coding of movement direction . Science 233 , 1416–1419. 10.1126/science.3749885 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gilbert S. F. (2000a). Neural crest cells and axonal specificity , in Developmental Biology , ed Gilbert S. F. (Sunderland, MA: Sinauer Associates; ), 407–441. [ Google Scholar ]
- Gilbert S. F. (2000b). The central nervous system and the epidermis , in Developmental Biology , ed Gilbert S. F. (Sunderland, MA: Sinauer Associates; ), 379–410. [ Google Scholar ]
- Hardie D. G. (2011). Sensing of energy and nutrients by AMP-activated protein kinase . Am. J. Clin. Nutr. 93 , 891S−896S. 10.3945/ajcn.110.001925 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hardie D. G., Ross F. A., Hawley S. A. (2012). AMPK: a nutrient and energy sensor that maintains energy homeostasis . Nat. Rev. Mol. Cell Biol. 13 , 251–262. 10.1038/nrm3311 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harris J. J., Jolivet R., Attwell D. (2012). Synaptic energy use and supply . Neuron 75 , 762–777. 10.1016/j.neuron.2012.08.019 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Herculano-Houzel S. (2011). Scaling of brain metabolism with a fixed energy budget per neuron: Implications for neuronal activity, plasticity and evolution . PLoS ONE . 6 :e17514. 10.1371/journal.pone.0017514 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hofmeyr J.-H. S., Cornish-Bowden A. (2000). Regulating the cellular economy of supply and demand . FEBS Lett. 476 , 47–51. 10.1016/S0014-5793(00)01668-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Horton A. C., Ehlers M. D. (2003). Neuronal polarity and trafficking . Neuron . 40 , 277–295. 10.1016/S0896-6273(03)00629-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hovland C. I. (1960). Computer simulation of thinking . Am. Psychol. 15 , 687–693. 10.1037/h0044165 [ CrossRef ] [ Google Scholar ]
- Huang C.-W., Huang C.-C., Cheng J.-T., Tsai J.-J., Wu S.-N. (2007). Glucose and hippocampal neuronal excitability: Role of ATP-sensitive potassium channels . J. Neurosci. Res. 85 , 1468–1477. 10.1002/jnr.21284 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Huang Y., Rao R. P. N. (2011). Predictive coding . Wiley Interdiscip. Rev. Cogn. Sci. 2 , 580–593. 10.1002/wcs.142 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hubel D. H., Wiesel T. N. (1962). Receptive fields, binocular interaction and functional architecture in the cat's visual cortex . J. Physiol. 160 , 106–54. 10.1113/jphysiol.1962.sp006837 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Huttenlocher P. R. (1990). Morphometric study of human cerebral cortex development . Neuropsychologia 28 , 517–527. 10.1016/0028-3932(90)90031-I [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hyder F., Patel A. B., Gjedde A., Rothman D. L., Behar K. L., Shulman R. G. (2006). Neuronal–glial glucose oxidation and glutamatergic–GABAergic function . J. Cereb. Blood Flow Metab. 26 , 865–877. 10.1038/sj.jcbfm.9600263 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hyder F., Rothman D. L., Bennett M. R. (2013). Cortical energy demands of signaling and nonsignaling components in brain are conserved across mammalian species and activity levels . Proc. Natl. Acad. Sci. U.S.A. 110 , 3549–3554. 10.1073/pnas.1214912110 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jaynes E. T. (1965). Gibbs vs Boltzmann entropies . Am. J. Phys. 33 , 391–398. 10.1119/1.1971557 [ CrossRef ] [ Google Scholar ]
- Jekabsons M. B., Nicholls D. G. (2004). In situ respiration and bioenergetic status of mitochondria in primary cerebellar granule neuronal cultures exposed continuously to glutamate . J. Biol. Chem. 279 , 32989–3000. 10.1074/jbc.M401540200 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jia Z., Collingridge G. L. (2017). Learning about synaptic GluA3 . Neuron 93 , 254–256. 10.1016/j.neuron.2017.01.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jimbo Y., Tateno T., Robinson H. P. C. (1999). Simultaneous induction of pathway-specific potentiation and depression in networks of cortical neurons . Biophys. J. 76 , 670–678. 10.1016/S0006-3495(99)77234-6 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kempter R., Gerstner W., Van Hemmen J. L. (1999). Hebbian learning and spiking neurons . Phys. Rev. E 59 , 4498–4514. 10.1103/PhysRevE.59.4498 [ CrossRef ] [ Google Scholar ]
- Klinger E. G. (2018). Approximate Bayesian Model Selection for Local Cortical Networks at Cellular Resolution . Available online at: https://mediatum.ub.tum.de/doc/1426522/file.pdf (accessed July 6, 2019).
- Kostrzewa R. M., Segura-Aguilar J. (2003). Novel mechanisms and approaches in the study of neurodegeneration and neuroprotection. A review . Neurotox. Res. 5 , 375–383. 10.1007/BF03033166 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lange S. C., Winkler U., Andresen L., Byhr,ø M., Waagepetersen H. S., Hirrlinger J., et al.. (2015). Dynamic changes in cytosolic ATP levels in cultured glutamatergic neurons during NMDA-induced synaptic activity supported by glucose or lactate . Neurochem. Res. 40 , 2517–2526. 10.1007/s11064-015-1651-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Laughlin S. B. (2001). Energy as a constraint on the coding and processing of sensory information . Curr. Opin. Neurobiol. 11 , 475–480. 10.1016/S0959-4388(00)00237-3 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- le Feber J., Stegenga J., Rutten W. L. C. (2010). The effect of slow electrical stimuli to achieve learning in cultured networks of rat cortical neurons . PLoS ONE 5 :e8871. 10.1371/journal.pone.0008871 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Le Masson G., Przedborski P., Abbott L. F. (2014). A computational model of motor neuron degeneration . Neuron 83 , 975–988. 10.1016/j.neuron.2014.07.001 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lemak M. S., Voloshanenko O., Draguhn A., Egorov A. V. (2014). KATP channels modulate intrinsic firing activity of immature entorhinal cortex layer III neurons . Front. Cell. Neurosci. 8 :255. 10.3389/fncel.2014.00255 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lennie P. (2003). The cost of cortical computation . Curr. Biol. 13 , 493–497. 10.1016/S0960-9822(03)00135-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lettvin J., Maturana H., McCulloch W. S., Pitts W. H. (1959). What the frog's eye tells the frog's brain , in Proceedings of the IRE. Available online at: http://ieeexplore.ieee.org/xpls/abs_all.jsp?arnumber=4065609 (accessed May 2, 2012).
- Lorenz D. M., Jeng A., Deem M. W. (2011). The emergence of modularity in biological systems . Phys. Life Rev. 8 , 129–160. 10.1016/j.plrev.2011.02.003 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Madhavan R., Chao Z. C., Potter S. M. (2007). Plasticity of recurring spatiotemporal activity patterns in cortical networks . Phys. Biol. 4 , 181–193. 10.1088/1478-3975/4/3/005 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Magistretti P. J. (2006). Neuron – glia metabolic coupling and plasticity . J. Exp. Biol . 209 , 2304–2311. 10.1242/jeb.02208 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Magistretti P. J., Allaman I. (2015). A cellular perspective on brain energy metabolism and functional imaging . Neuron 86 , 883–901. 10.1016/j.neuron.2015.03.035 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Magistretti P. J., Allaman I. (2018). Lactate in the brain: from metabolic end-product to signalling molecule . Nat. Rev. Neurosci. 19 , 235–249. 10.1038/nrn.2018.19 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Marcaida G., Min ana M. D., Grisolía S., Felipo V. (1995). Lack of correlation between glutamate-induced depletion of ATP and neuronal death in primary cultures of cerebellum . Brain Res. 695 , 146–150. 10.1016/0006-8993(95)00703-S [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Marcaida G., Miñana M.-D., Grisolia S., Felipo V. (1997). Determination of intracellular ATP in primary cultures of neurons . Brain Res. Protoc. 1 , 75–78. 10.1016/S1385-299X(96)00009-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Marsland R., England J. (2018). Limits of predictions in thermodynamic systems: a review . Reports Prog. Phys. 81 :016601. 10.1088/1361-6633/aa9101 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Miller K. D., MacKay D. J. C. (1994). The role of constraints in hebbian learning . Neural Comput. 6 , 100–126. 10.1162/neco.1994.6.1.100 [ CrossRef ] [ Google Scholar ]
- Moser M.-B., Moser E. I. (2015). Where am I? Where am i going? Sci. Am. 314 , 26–33. 10.1038/scientificamerican0116-26 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moxon K. A., Foffani G. (2015). Brain-machine interfaces beyond neuroprosthetics . Neuron 86 , 55–67. 10.1016/j.neuron.2015.03.036 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mulas M., Massobrio P. (2010). A simulated neuro-robotic environment for bi-directional closed-loop experiments . Communication 1 , 179–186. 10.2478/s13230-011-0004-x [ CrossRef ] [ Google Scholar ]
- Nabavi S., Fox R., Proulx C. D., Lin J. Y., Tsien R. Y., Malinow R. (2014). Engineering a memory with LTD and LTP . Nature 511 , 348–352. 10.1038/nature13294 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nicholls D. (2013). Bioenergetics , 4th Edn. London: Academic Press. [ Google Scholar ]
- Nicolelis M. A. L. (2003). Brain-machine interfaces to restore motor function and probe neural circuits . Nat. Rev. Neurosci. 4 , 417–422. 10.1038/nrn1105 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Novellino A., D'Angelo P., Cozzi L., Chiappalone M., Sanguineti V., Martinoia S. (2007). Connecting Neurons to a mobile robot: an in vitro bidirectional neural interface . Comput. Intell. Neurosci. 2007 , 1–13. 10.1155/2007/12725 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Olshausen B. A., Field D. J. (2005). How close are we to understanding v1? Neural Comput. 17 , 1665–1699. 10.1162/0899766054026639 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Otsu Y., Couchman K., Lyons D. G., Collot M., Agarwal A., Mallet J.-M., et al.. (2015). Calcium dynamics in astrocyte processes during neurovascular coupling . Nat. Neurosci. 18 , 210–218. 10.1038/nn.3906 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ouldridge T. E. (2018). The importance of thermodynamics for molecular systems, and the importance of molecular systems for thermodynamics . Nat. Comput. 17 , 3–29. 10.1007/s11047-017-9646-x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Penfield W. (1965). Speech, perception and the uncommitted cortex , in Brain and Conscious Experience , ed Eccless J. C. (Berlin; Heidelberg: Springer; ), 217–237. [ Google Scholar ]
- Penfield W., Boldrey E. (1937). Somatic motor and sensory representation in man . Brain , 389–443. 10.1093/brain/60.4.389 [ CrossRef ] [ Google Scholar ]
- Perry E. K., McKeith I., Thompson P., Marshall E., Kerwin J., Jabeen S., et al.. (1991). Topography, extent, and clinical relevance of neurochemical deficits in dementia of Lewy body type, Parkinson's disease, and Alzheimer's disease . Ann. N. Y. Acad. Sci. 640 , 197–202. 10.1111/j.1749-6632.1991.tb00217.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pino O., Guílera G., Gómez-Benito J., Najas-García A., Rufián S., Rojo E. (2014). Neurodevelopment or neurodegeneration: Review of theories of schizophrenia . Actas Esp. Psiquiatr. 42 , 185–195. Available online at: https://www.actaspsiquiatria.es/repositorio/16/90/ENG/16-90-ENG-185-195-865491.pdf (accessed July 6, 2019). [ PubMed ] [ Google Scholar ]
- Pissadaki E. K., Bolam J. P. (2013). The energy cost of action potential propagation in dopamine neurons: clues to susceptibility in Parkinson's disease . Front. Comput. Neurosci. 7 :13. 10.3389/fncom.2013.00013 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Potter W. B., O'Riordan K. J., Barnett D., Osting S. M. K., Wagoner M., Burger C., et al.. (2010). Metabolic regulation of neuronal plasticity by the energy sensor AMPK . PLoS ONE . 5 :e8996. 10.1371/journal.pone.0008996 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ramamurthy S., Chang E., Cao Y., Zhu J., Ronnett G. V. (2014). AMPK activation regulates neuronal structure in developing hippocampal neurons . Neuroscience 259 , 13–24. 10.1016/j.neuroscience.2013.11.048 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rangaraju V., Calloway N., Ryan T. A. (2014). Activity-driven local ATP synthesis is required for synaptic function . Cell 156 , 825–835. 10.1016/j.cell.2013.12.042 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reid R. C., Shapley R. M. (1992). Spatial structure of cone inputs to receptive fields in primate lateral geniculate nucleus . Nature 356 , 716–718. 10.1038/356716a0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Riccomagno M. M., Kolodkin A. L. (2015). Sculpting neural circuits by axon and dendrite pruning . Annu. Rev. Cell Dev. Biol. 31 , 779–805. 10.1146/annurev-cellbio-100913-013038 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Robbins P. (2010). Modularity of Mind. Standford Encycl. Phylosophy . Available online at: http://plato.stanford.edu/entries/modularity-mind/ (accessed October 19, 2011).
- Robinson M. B., Jackson J. G. (2016). Astroglial glutamate transporters coordinate excitatory signaling and brain energetics . Neurochem. Int. 98 , 56–71. 10.1016/j.neuint.2016.03.014 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ruth Clemo H., Stein B. E. (1982). Somatosensory cortex: a new somatotopic representation . Brain Res. 235 , 162–168. 10.1016/0006-8993(82)90207-4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Saez I., Duran J., Sinadinos C., Beltran A., Yanes O., Tevy M. F., et al.. (2014). Neurons have an active glycogen metabolism that contributes to tolerance to hypoxia . J. Cereb. Blood Flow Metab. 34 , 945–955. 10.1038/jcbfm.2014.33 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schroeder C. E., Wilson D. A., Radman T., Scharfman H., Lakatos P. (2010). Dynamics of Active Sensing and perceptual selection . Curr. Opin. Neurobiol. 20 , 172–176. 10.1016/j.conb.2010.02.010 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Searle J. R. (1980). Minds, brains, and programs . Behav. Brain Sci. 3 , 417–424. 10.1017/S0140525X00005756 [ CrossRef ] [ Google Scholar ]
- Sengupta B., Faisal A. A., Laughlin S. B., Niven J. E. (2013). The effect of cell size and channel density on neuronal information encoding and energy efficiency . J. Cereb. Blood Flow Metab. 33 , 1465–1473. 10.1038/jcbfm.2013.103 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sheppard G. M. (2016). Foundations of the Neuron Doctrine: 25th Anniversary Edition . Oxford: Oxford University Press. 10.1093/med/9780190259389.001.0001 [ CrossRef ] [ Google Scholar ]
- Shulman R. G., Rothman D. L., Behar K. L., Hyder F. (2004). Energetic basis of brain activity: Implications for neuroimaging . Trends Neurosci. 27 , 489–495. 10.1016/j.tins.2004.06.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Silbey R. J., Alberty R. A., Moungi B. G. (2004). Physical Chemistry, 4th Edn . Hoboken, NJ: Wiley. [ Google Scholar ]
- Sokoloff L. (2008). The physiological and biochemical bases of functional brain imaging . Cogn. Neurodyn . 2 , 1–5. 10.1007/978-1-4020-8387-7_58 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Spratling M. W. (2008). Reconciling predictive coding and biased competition models of cortical function . Front. Comput. Neurosci. 2 :4. 10.3389/neuro.10.004.2008 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tarasov A. I., Griffiths E. J., Rutter G. A. (2012). Regulation of ATP production by mitochondrial Ca2+ . Cell Calcium . 52 , 28–35. 10.1016/j.ceca.2012.03.003 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tateno T., Jimbo Y. (1999). Activity-dependent enhancement in the reliability of correlated spike timings in cultured cortical neurons . Biol. Cybern. 80 , 45–55. 10.1007/s004220050503 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Taylor C. (1978). Theory of edge detection . Proc. R. Soc. Lond. B Biol. Sci. 207 , 187–217. 10.1098/rspb.1980.0020 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tessadori J., Bisio M., Martinoia S., Chiappalone M. (2012). Modular neuronal assemblies embodied in a closed-loop environment: toward future integration of brains and machines . Front. Neural Circuits 6 :99. 10.3389/fncir.2012.00099 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tessadori J., Venuta D., Kumar S. S., Bisio M., Pasquale V., Chiappalone M. (2013). Embodied neuronal assemblies: a closed-loop environment for coding and decoding studies , in 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER) (San Diego, CA: ), 899–902. 10.1109/NER.2013.6696080 [ CrossRef ] [ Google Scholar ]
- Toloe J., Mollajew R., Kügler S., Mironov S. L. (2014). Metabolic differences in hippocampal Rett neurons revealed by ATP imaging . Mol. Cell. Neurosci . 59 , 47–56. 10.1016/j.mcn.2013.12.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Trevisiol A., Saab A. S., Winkler U., Marx G., Imamura H., Möbius W., et al.. (2017). Monitoring ATP dynamics in electrically active white matter tracts . Elife 6 :e24241. 10.7554/eLife.24241 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Turrigiano G. (2012). Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function . Cold Spring Harb. Perspect. Biol. 4 :a005736–a005736. 10.1101/cshperspect.a005736 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Turrigiano G., Leslie K. R., Desai N. S., Rutherford L. C., Nelson S. B. (1998). Activity-dependent scaling of quantal amplitude in neocortical neurons . Nature . 391 , 892–896. 10.1038/36103 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang D. D., Bordey A. (2008). The astrocyte odyssey . Prog. Neurobiol. 86 , 342–367. 10.1016/j.pneurobio.2008.09.015 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang S., Kobayashi K., Kogure Y., Yamanaka H., Yamamoto S., Yagi H., et al.. (2018). Negative regulation of TRPA1 by AMPK in primary sensory neurons as a potential mechanism of painful diabetic neuropathy . Diabetes 67 , 98–109. 10.2337/db17-0503 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Weber B., Barros L. F. (2015). The astrocyte: powerhouse and recycling center . Cold Spring Harb. Perspect. Biol. 7 :a020396. 10.1101/cshperspect.a020396 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yuan Y., Huo H., Zhao P., Liu J., Liu J., Xing F., et al.. (2018). Constraints of metabolic energy on the number of synaptic connections of neurons and the density of neuronal networks . Front. Comput. Neurosci. 12 :91. 10.3389/fncom.2018.00091 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yuste R. (2015). From the neuron doctrine to neural networks . Nat. Rev. Neurosci. 16 , 487–497. 10.1038/nrn3962 [ PubMed ] [ CrossRef ] [ Google Scholar ]
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- How to Write a Strong Hypothesis | Steps & Examples
How to Write a Strong Hypothesis | Steps & Examples
Published on May 6, 2022 by Shona McCombes . Revised on November 20, 2023.
A hypothesis is a statement that can be tested by scientific research. If you want to test a relationship between two or more variables, you need to write hypotheses before you start your experiment or data collection .
Example: Hypothesis
Daily apple consumption leads to fewer doctor’s visits.
Table of contents
What is a hypothesis, developing a hypothesis (with example), hypothesis examples, other interesting articles, frequently asked questions about writing hypotheses.
A hypothesis states your predictions about what your research will find. It is a tentative answer to your research question that has not yet been tested. For some research projects, you might have to write several hypotheses that address different aspects of your research question.
A hypothesis is not just a guess – it should be based on existing theories and knowledge. It also has to be testable, which means you can support or refute it through scientific research methods (such as experiments, observations and statistical analysis of data).
Variables in hypotheses
Hypotheses propose a relationship between two or more types of variables .
- An independent variable is something the researcher changes or controls.
- A dependent variable is something the researcher observes and measures.
If there are any control variables , extraneous variables , or confounding variables , be sure to jot those down as you go to minimize the chances that research bias will affect your results.
In this example, the independent variable is exposure to the sun – the assumed cause . The dependent variable is the level of happiness – the assumed effect .
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example
Step 1. Ask a question
Writing a hypothesis begins with a research question that you want to answer. The question should be focused, specific, and researchable within the constraints of your project.
Step 2. Do some preliminary research
Your initial answer to the question should be based on what is already known about the topic. Look for theories and previous studies to help you form educated assumptions about what your research will find.
At this stage, you might construct a conceptual framework to ensure that you’re embarking on a relevant topic . This can also help you identify which variables you will study and what you think the relationships are between them. Sometimes, you’ll have to operationalize more complex constructs.
Step 3. Formulate your hypothesis
Now you should have some idea of what you expect to find. Write your initial answer to the question in a clear, concise sentence.
4. Refine your hypothesis
You need to make sure your hypothesis is specific and testable. There are various ways of phrasing a hypothesis, but all the terms you use should have clear definitions, and the hypothesis should contain:
- The relevant variables
- The specific group being studied
- The predicted outcome of the experiment or analysis
5. Phrase your hypothesis in three ways
To identify the variables, you can write a simple prediction in if…then form. The first part of the sentence states the independent variable and the second part states the dependent variable.
In academic research, hypotheses are more commonly phrased in terms of correlations or effects, where you directly state the predicted relationship between variables.
If you are comparing two groups, the hypothesis can state what difference you expect to find between them.
6. Write a null hypothesis
If your research involves statistical hypothesis testing , you will also have to write a null hypothesis . The null hypothesis is the default position that there is no association between the variables. The null hypothesis is written as H 0 , while the alternative hypothesis is H 1 or H a .
- H 0 : The number of lectures attended by first-year students has no effect on their final exam scores.
- H 1 : The number of lectures attended by first-year students has a positive effect on their final exam scores.
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
A hypothesis is not just a guess — it should be based on existing theories and knowledge. It also has to be testable, which means you can support or refute it through scientific research methods (such as experiments, observations and statistical analysis of data).
Null and alternative hypotheses are used in statistical hypothesis testing . The null hypothesis of a test always predicts no effect or no relationship between variables, while the alternative hypothesis states your research prediction of an effect or relationship.
Hypothesis testing is a formal procedure for investigating our ideas about the world using statistics. It is used by scientists to test specific predictions, called hypotheses , by calculating how likely it is that a pattern or relationship between variables could have arisen by chance.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, November 20). How to Write a Strong Hypothesis | Steps & Examples. Scribbr. Retrieved April 16, 2024, from https://www.scribbr.com/methodology/hypothesis/
Is this article helpful?
Shona McCombes
Other students also liked, construct validity | definition, types, & examples, what is a conceptual framework | tips & examples, operationalization | a guide with examples, pros & cons, what is your plagiarism score.
Geography Department Penn State
- Course Development
- Syllabus (Online)
- Syllabus (Resident)
- Instructor Information
- TA Information
- Adobe Connect
- Box at Penn State
- Google Apps
- PASS Explorer
- Personal Web Space
- Sites at Penn State (Blogs)
- VoiceThread
- Citation Styles
- Writing Style Guides
- Creating a Presentation
- Using the Penn State Library
- Class Communication Guidelines
- Getting Help!
- Teaching Your Class
- To Do: Proofreading and Accessibility
Formulating and Writing Hypotheses
After exploring and visualizing your data, the first step to hypothesis testing is writing your hypotheses to test. Data visualization and summarizing your data (e.g., with the ) help in formulating these hypotheses, in particular with defining the appropriate inequality (whether to use < or > or ≠) in the alternative hypothesis. We can break down the formulation of hypotheses into the following steps below.
Three Steps to Writing a Hypothesis
Write your question in plain language. Based upon the data at hand, and the prior exploratory data analysis (e.g., visualization, summarizing), what question are you seeking to answer? An example might be: “Are PM2.5 concentrations unsafe in certain areas?” It is important to frame the question such that it can answered by the data you have. For instance, you wouldn’t want to ask a question about all pollution when you only have data on particulate matter concentration.
Write your hypothesis in plain language. The goal in this step is to take a stance on the answer to the question posed in Step 1. Following the example given above, a viable hypothesis is: “Yes, PM2.5 concentrations are at unsafe levels.” This statement doesn’t need to be correct, since the rest of the hypothesis testing procedure will test this statement. In particular, it will tell you whether or not the data support this statement .
Write your statistical hypotheses. The task here is really to convert your plain-language hypothesis from Step 2 into statistical notation. Specifically, it needs to be written in terms of the appropriate population parameter for your question. For example, in order to say that PM2.5 concentrations are safe or unsafe, we can look at the mean ( μ ), since this would capture the general, average PM2.5 conditions. Furthermore, it needs to be written in terms of two different hypotheses:
The null hypothesis (or just “null” for short). Here you are stating that the population parameter for your variable of interest is equal to a specific value (single-sample tests) or another population parameter (two-sample tests). Again, this is a hypothesis so it does not need to be correct; this is what will be tested. The null hypothesis should capture the contradiction to your hypothesis from Step 2. So, for example, with the EPA defining the threshold between safe and unsafe PM2.5 levels as 12 micrograms per cubic meter ( 40 C.F.R. § 50.18 (2013) ), our null hypothesis would be μ = 12 μ g / m 3 . (You might be wondering why ≤ is not used instead of =, since safe levels would be anything at or below 12 μ g / m 3 . Indeed, many statistics texts introduce the null hypothesis this way, and really either way is allowable. However, when using ≤ (or ≥ in other situations), you end up only testing the = portion of this statement anyway.)
The alternative hypothesis (“alternative” for short). Here you are replacing the equality in the null hypothesis with an inequality (>, <, or ≠). The correct choice is that which aligns with your hypothesis in Step 2. So, following the example above, our alternative hypothesis is μ > 12 μ g / m 3 , because any average PM2.5 exceeding 12 μ g / m 3 is unsafe.
The subsequent hypothesis testing procedure will give us a quantitative metric (the p-value) for saying whether or not the data support the alternative hypothesis. This is framed as either:
We reject the null hypothesis in favor of the alternative hypothesis, or
We fail to reject the null hypothesis.
Note that the focus here is on rejecting or not rejecting the null hypothesis. This is because the null contains the equality statement, and thus something definitive to test. However, as we will see later, this null is tested against the alternative, which is why in the first option we can say “in favor of the alternative”.
Because our statistical hypotheses need to written in terms of the population parameters, let's review some common parameters in the table below:
This lesson will only cover tests for some of these parameters, and correlation and slope will be covered in Linear Regression.
Assess It: Check Your Knowledge
Knowledge check.
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
AP®︎/College Biology
Course: ap®︎/college biology > unit 7.
- Earth formation
- Beginnings of life
- Origins of life
Hypotheses about the origins of life
- The RNA origin of life
- Origins of life on Earth
Key points:
- The Earth formed roughly 4.5 billion years ago, and life probably began between 3.5 and 3.9 billion years ago.
- The Oparin-Haldane hypothesis suggests that life arose gradually from inorganic molecules, with “building blocks” like amino acids forming first and then combining to make complex polymers.
- The Miller-Urey experiment provided the first evidence that organic molecules needed for life could be formed from inorganic components.
- Some scientists support the RNA world hypothesis , which suggests that the first life was self-replicating RNA. Others favor the metabolism-first hypothesis , placing metabolic networks before DNA or RNA.
- Simple organic compounds might have come to early Earth on meteorites.
Introduction
When did life appear on earth, the earliest fossil evidence of life, how might life have arisen.
- Simple inorganic molecules could have reacted (with energy from lightning or the sun) to form building blocks like amino acids and nucleotides, which could have accumulated in the oceans, making a "primordial soup." 3
- The building blocks could have combined in further reactions, forming larger, more complex molecules (polymers) like proteins and nucleic acids, perhaps in pools at the water's edge.
- The polymers could have assembled into units or structures that were capable of sustaining and replicating themselves. Oparin thought these might have been “colonies” of proteins clustered together to carry out metabolism, while Haldane suggested that macromolecules became enclosed in membranes to make cell-like structures 4 , 5 .
From inorganic compounds to building blocks
Were miller and urey's results meaningful, from building blocks to polymers, what was the nature of the earliest life, the "genes-first" hypothesis, the "metabolism-first" hypothesis, what might early cells have looked like, another possibility: organic molecules from outer space.
- Miller, Urey, and others showed that simple inorganic molecules could combine to form the organic building blocks required for life as we know it.
- Once formed, these building blocks could have come together to form polymers such as proteins or RNA.
- Many scientists favor the RNA world hypothesis, in which RNA, not DNA, was the first genetic molecule of life on Earth. Other ideas include the pre-RNA world hypothesis and the metabolism-first hypothesis.
- Organic compounds could have been delivered to early Earth by meteorites and other celestial objects.
Works cited:
- Harwood, R. (2012). Patterns in palaeontology: The first 3 billion years of evolution. Palaeontology , 2(11), 1-22. Retrieved from http://www.palaeontologyonline.com/articles/2012/patterns-in-palaeontology-the-first-3-billion-years-of-evolution/ .
- Wacey, D., Kilburn, M. R., Saunders, M., Cliff, J., and Brasier, M. D. (2011). Microfossils of sulphur-metabolizing cells in 3.4-billion-year-old rocks of Western Australia. Nature Geoscience , 4 , 698-702. http://dx.doi.org/10.1038/ngeo1238 .
- Primordial soup. (2016, January 20). Retrieved May 22, 2016 from Wikipedia: https://en.wikipedia.org/wiki/Primordial_soup .
- Gordon-Smith, C. (2003). The Oparin-Haldane hypothesis. In Origin of life: Twentieth century landmarks . Retrieved from http://www.simsoup.info/Origin_Landmarks_Oparin_Haldane.html .
- The Oparin-Haldane hypothesis. (2015, June 14). In Structural biochemistry . Retrieved May 22, 2016 from Wikibooks: https://en.wikibooks.org/wiki/Structural_Biochemistry/The_Oparin-Haldane_Hypothesis .
- Kimball, J. W. (2015, May 17). Miller's experiment. In Kimball's biology pages . Retrieved from http://www.biology-pages.info/A/AbioticSynthesis.html#Miller's_Experiment .
- Earth’s early atmosphere. (Dec 2, 2011). In Astrobiology Magazine . Retrieved from http://www.astrobio.net/topic/solar-system/earth/geology/earths-early-atmosphere/ .
- McCollom, T. M. (2013). Miller-Urey and beyond: What have learned about prebiotic organic synthesis reactions in the past 60 years? Annual Review of Earth and Planetary Sciences , 41_, 207-229. http://dx.doi.org/10.1146/annurev-earth-040610-133457 .
- Powner, M. W., Gerland, B., and Sutherland, J. D. (2009). Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature , 459 , 239-242. http://dx.doi.org/10.1038/nature08013 .
- Lurquin, P. F. (June 5, 2003). Proteins and metabolism first: The iron-sulfur world. In The origins of life and the universe (pp. 110-111). New York, NY: Columbia University Press.
- Ferris, J. P. (2006). Montmorillonite-catalysed formation of RNA oligomers: The possible role of catalysis in the origins of life. Philos. Trans. R. Soc. Lond. B. Bio.l Sci ., 361 (1474), 1777–1786. http://dx.doi.org/10.1098/rstb.2006.1903 .
- Kimball, J. W. (2015, May 17). Assembling polymers. In Kimball's biology pages . Retrieved from
- Montmorillonite. (2016, 28 March). Retrieved May 22, 2016 from Wikipedia: https://en.wikipedia.org/wiki/Montmorillonite .
- Wilkin, D. and Akre, B. (2016, March 23). First organic molecules - Advanced. In CK-12 biology advanced concepts . Retrieved from http://www.ck12.org/book/CK-12-Biology-Advanced-Concepts/section/10.8/ .
- Hollenstein, M. (2015). DNA catalysis: The chemical repertoire of DNAzymes. Molecules , 20 (11), 20777–20804. http://dx.doi.org/10.3390/molecules201119730 .
- Breaker, R. R. and Joyce, G. F. (2014). The expanding view of RNA and DNA function. Chemistry & biology , 21 (9), 1059–1065. http://dx.doi.org/10.1016/j.chembiol.2014.07.008 .
- Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., and Walter, P. (2002). A pre-RNA world probably predates the RNA world. In Molecular biology of the cell (4th ed.). New York, NY: Garland Science. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK26876/#_A1124_ .
- Moran, L. A. (2009, May 15). Metabolism first and the origin of life. In Sandwalk: Strolling with a skeptical biochemist . Retrieved from http://www.simsoup.info/Origin_Landmarks_Oparin_Haldane.html .
- Kimball, J. W. (2015, May 17). The first cell? In Kimball's biology pages . Retrieved from http://www.biology-pages.info/A/AbioticSynthesis.html#TheFirstCell?
- Kimball, J. W. (2015, May 17). Molecules from outer space? In Kimball's biology pages . Retrieved from http://www.biology-pages.info/A/AbioticSynthesis.html#Molecules_from_outer_space? .
- Jeffs, W. (2006, November 30). NASA scientists find primordial organic matter in meteorite. In NASA news . Retrieved from http://www.nasa.gov/centers/johnson/news/releases/2006/J06-103.html .
Additional references:
Want to join the conversation.
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Post-lockdown teaching support
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More from navigation items
Energy content in foods
In association with Nuffield Foundation
- Four out of five
- No comments
Try this class experiment to investigate how much energy different foods contain
In this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food.
This is a class experiment in which different groups can investigate different foodstuffs. If each group investigates two foodstuffs – one in common with the rest of class to provide a common baseline, and the other a different foodstuff from the rest – a comparative table of energy in different foods can be drawn up from the class results. This should be possible to achieve in 45–60 minutes.
- Eye protection
- Thermometer (–10 to 110 °C), short, stirring type
- Boiling tube, or metal calorimeter (or similar metal container) (see note 4 below)
- Measuring cylinder, 25 cm 3
- Bunsen burner
- Heat resistant mat
- Mounted needle
- Stand and clamp
- Balance, weighing to 0.1 g
Use of a variety of dry foodstuffs, such as:
- Mini-marshmallows
- Popcorn (already popped)
- Broad beans (dried)
See notes 5, 6 and 7 below for additional guidance.
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Students must be instructed NOT to taste or eat any of the foods used in the experiment.
- While boiling tubes are easy to use for this experiment, the poor thermal conductivity of glass may be a major cause of error. Metal containers, such as copper calorimeters or tin cans of similar dimensions that can be held in a clamp, provide more effective heat transfer to the water. Note that this benefit is lost if the can is stood on a tripod, as the latter will also be heated.
- Check in advance for common allergy problems, eg peanuts.
- Some foodstuffs can be burned safely and easily using a mounted needle. Others may melt and drop off the needle, so burning on an old metal teaspoon is an alternative method – this can also be used for liquid foodstuffs, such as olive oil. High protein foodstuffs may produce pungent fumes, and should be burned in a fume cupboard. Each foodstuff provided should be tested beforehand to check that it is capable of sustained combustion without having to be relit repeatedly.
- One foodstuff needs to be selected as suitable for the standard experiment used by each group. Enough samples of approximately the same mass of this foodstuff need to be provided for the class. A trial run before the lesson should be carried out to establish that it will burn readily, sustain combustion and leave little unburnt residue, and that the mass of this foodstuff that will cause a temperature rise in the water used of around 20–30 °C.
Using a test tube
- Measure 10 cm 3 of water into the test tube.
- Clamp the test tube in the retort stand at an angle as shown in the diagram, and over a heat resistant mat.
Source: Royal Society of Chemistry
The equipment required to investigate the energy content of different foodstuffs
- Weigh a small piece of food and record the mass.
- Take the temperature of the water and record it in the table.
- Fix the food on the end of the mounted needle. If the food is likely to melt when heated put it on a teaspoon instead of on the needle.
- Ignite the food using a Bunsen burner, and immediately hold it about 1 cm below the test tube and above a heat resistant mat. If the flame goes out, quickly relight it.
- When the food stops burning, stir the water with the thermometer and record the temperature.
- If there is a significant amount of unburnt food left on the needle, reweigh this and record the mass remaining.
- Empty the test tube and refill it with another 10 cm 3 of cold water. Repeat the experiment using a different food.
Using a metal container
Instructions as for a test tube, except:
- Use a larger volume of water, eg 25 or 50 cm 3 , and a larger food sample.
- Clamp the container in a level position above a heat resistant mat.
Teaching notes
This experiment provides an opportunity to use a temperature sensor linked to a data logger instead of a thermometer.
One foodstuff should be preselected to be the one used by all groups to standardise their experiments with each other (see Health, safety and technical notes ), while each group needs to be allocated a different foodstuff for the second run.
As the same amount of water is heated each time, the temperature rise can be used to compare the amount of heat energy given off per gram of each foodstuff by dividing the rise by the mass of foodstuff burnt.
For classes familar with the equation q = m × C × Δ T , where q is the heat energy, m the mass of water heated, C the specific heat of water (4.2 J g –1 deg – 1 ) and Δ T the temperature rise, this can be used to calculate the energy (in J) absorbed by the water each time. Dividing this by the mass of foodstuff burnt gives the heat energy absorbed by the water in J per g, as shown in the table below.
Each group needs to prepare a results table along the lines of the example below:
Class results for the heat absorbed by water per gram of food may then be collected and compared on a class spreadsheet prepared by the teacher. After this has been done, a class discussion of ‘fair test’ problems will be appropriate, identifying sources of error and ideas for improving the technique used.
Additional information
This is a resource from the Practical Chemistry project , developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology .
© Nuffield Foundation and the Royal Society of Chemistry
- 11-14 years
- 14-16 years
- 16-18 years
- Practical experiments
- Thermodynamics
- Quantitative chemistry and stoichiometry
- Analytical chemistry
Specification
- The quantity of heat energy released can be determined experimentally and calculated using Eₕ=cmΔT.
- Fats and oils are: a concentrated source of energy; essential for the transport and storage of fat-soluble vitamins in the body.
- 2.8.7 calculate enthalpy changes from experimental data using the equation q = mcΔT;
- determine the enthalpy changes for combustion and neutralisation using simple apparatus; and
- 2. Develop and use models to describe the nature of matter; demonstrate how they provide a simple way to to account for the conservation of mass, changes of state, physical change, chemical change, mixtures, and their separation.
- 9. Consider chemical reactions in terms of energy, using the terms exothermic, endothermic and activation energy, and use simple energy profile diagrams to illustrate energy changes.
Related articles

Why fermented foods are good for your gut – and your teaching
2024-04-15T05:30:00Z By Emma Davies
From kimchi to kefir, tuck into the complex chemistry of fermentation and its health potential

How to teach extraction of metals at 14–16
2024-04-09T07:20:00Z By Niall Begley
Solidify learners’ understanding of extraction processes with these tips, misconception busters and teaching ideas

Sniffing out the science of smells
2024-03-25T04:00:00Z By Hayley Bennett
What makes a bad smell smell bad? Sniff out the chemical culprits behind obnoxious odours
No comments yet
Only registered users can comment on this article., more from experiments.

‘Gold’ coins on a microscale | 14–16 years
By Dorothy Warren and Sandrine Bouchelkia
Practical experiment where learners produce ‘gold’ coins by electroplating a copper coin with zinc, includes follow-up worksheet

Practical potions microscale | 11–14 years
By Kirsty Patterson
Observe chemical changes in this microscale experiment with a spooky twist.

Antibacterial properties of the halogens | 14–18 years
By Kristy Turner
Use this practical to investigate how solutions of the halogens inhibit the growth of bacteria and which is most effective
- Contributors
- Email alerts
Site powered by Webvision Cloud
- Skip to main content
- Keyboard shortcuts for audio player

- LISTEN & FOLLOW
- Apple Podcasts
- Google Podcasts
- Amazon Music
- Amazon Alexa
Your support helps make our show possible and unlocks access to our sponsor-free feed.
When Life Gives You Lemons...Make A Battery

Emily Kwong

Rebecca Ramirez
Madeline K. Sofia

Electrical circuit with lemons. A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb. Science Photo Libra/Getty Images hide caption
Electrical circuit with lemons. A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb.
We're going "Back To School" today, revisiting a classic at-home experiment that turns lemons into batteries — powerful enough to turn on a clock or a small lightbulb. But how does the science driving the "lemon battery" show up in those household batteries we use daily?
Short Wave host Maddie Sofia and reporter Emily Kwong speak with environmental engineer Jenelle Fortunato about the fundamentals of electric currents and the inner workings of batteries.
Fortunato is a postdoctoral researcher at North Carolina State University studying materials for electrodes that can be used in solid-state batteries.
A few years ago, she brought the "lemon battery" to classrooms through Penn State's Science U program. Middle schoolers got particularly invested in the experimental possibilities.
"They hooked up like 20 lemons, three cups of lemon juice, an apple, three different light bulbs and a buzzer buzzing. And it was... it was chaos...I was in awe. Leave it to kids to come up with something like that," Fortunato said.
You can build your very own lemon battery using Science U's design here , written by Fortunato and Christopher Gorski of Penn State College of Engineering.
A reminder: Do NOT play with household batteries. Be safe out there, scientists!
You can read more about Fortunato's research here .
This episode was produced by Rebecca Ramirez, edited by Viet Le and fact-checked by Rasha Aridi. J. Czys and Josh Newell were the audio engineers. Special thanks to Short Wave listener Violet Thomas for inviting us to dig deeper into battery science.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

Photosynthesis
- Last updated
- Save as PDF
- Page ID 2855
Introduction
Before beginning this exercise, it is necessary to understand that photosynthesis uses light energy to synthesize carbohydrate from carbon dioxide. The equation is below.
\[\mathrm{6CO_2 + 6H_2O + Energy \rightarrow C_6H_{12}O_6 + 6O_2}\]
This process requires light for some of the reactions.
It is also necessary to understand that the plant is constantly undergoing cellular respiration according to the equation below.
\[\mathrm{C_6H_{12}O_6 + 6O_2 \rightarrow 6CO_2 + 6H_2O + Energy}\]
Notice that these two equations appear to be opposites.
When plants are exposed to light, photosynthesis and cellular respiration both occur. In the dark, only cellular respiration occurs.
We will study photosynthesis in an aquatic plant ( Elodea ) We can measure the rate of photosynthesis and cellular respiration by measuring the amount of CO 2 given off or taken up by the plant.
Carbon dioxide combines with water to form carbonic acid (H 2 CO 3 ) which dissociates into hydrogen ions (H + ) and bicarbonate ions (HCO 3 - ). The pH drops due to the presence of hydrogen ions.
\[\mathrm{CO_2 + H_2O \leftrightarrow H_2CO_3 \leftrightarrow H^+ + \sideset{ }{_{3}^{-}}{HCO}}\]
Respiring plants release CO 2 into the water, causing the pH to decline. During photosynthesis, plants take up CO 2 and the pH increases.
Using the pH Probe
Set up the pH probe by following the instructions given in the link below.
Instructions for setting up and using the pH probe.
Procedure for Measuring Photosynthesis
Create a hypothesis for this experiment.
Be sure to use tap water for the experiments below. Distilled water should not be used.
After the probe is set up (see the step above), obtain two large test tubes for this experiment. A ring stand can be used to hold the tubes as shown in the photograph below.
Rinse both tubes and stoppers thoroughly with tap water to remove any traces of contaminants that might affect pH.
Fill each tube with tap water to approximately 2 cm from the top.
Cut a sprig of Elodea that is long enough to fill the entire length of one tube but not protrude from the water. The length of the stem can be adjusted, if necessary, by cutting a piece from the base of the stem. Put the plant in one of the tubes. The other tube will serve as a control.
Clamp the two tubes on a ring stand to hold them.
Insert one pH probe into each test tube.
When the pH stabilizes, record the pH in each tube and then turn on the lamp and begin timing the experiment. The pH probes can be kept in the tubes until the experiment is finished.
Record the pH of the water in each tube every 10 minutes for one hour. Your data table will have 7 pH readings for each tube.
You should begin the chromatography procedure (below) while waiting to take pH readings.
Chromatography
During photosynthesis, light energy is absorbed by photosynthetic pigments. Chlorophyll A is the main photosynthetic pigment but chlorophyll B, carotenes, and xanthophylls also absorb light. Each pigment absorbs a specific range of colors but all of them together enable the plant to use a larger amount of light. These pigments absorb red and blue light best and absorb green the least. Plants look green; because the green light is not absorbed by the plant; it is reflected.
Chromatography is a technique used to separate the components of a mixture. In this investigation, you will use chromatography to separate and identify several photosynthetic pigments in a solution prepared from spinach leaves.
Paper chromatography can be used to separate the components of a mixture based on their polarity. Some of the mixture is placed on one end of a piece of paper and that end of the paper is immersed in a nonpolar liquid (see the diagram below).
As the liquid moves up the paper, the molecules of the sample mixture will also move. Polar molecules within the sample will spend most of their time bound to the polar surface of the paper and will therefore not move very much. Nonpolar molecules, however, will spend most of their time dissolved in the liquid as it moves up the paper. When the liquid reaches the top of the paper, these molecules will also have traveled most of the way to the top. The two types of molecules (polar and nonpolar) are now separated.
It is useful to determine the relative distance moved by a particular kind of molecule using chromatography. This value is known as R f . For example, if the molecules move half as far as the solvent traveled, the R f = 0.5. If the molecules moved 1/4 the distance, the R f = 0.25. The maximum value for R f is therefore 1.0.
Using a pencil, put a small dot in the center of a strip of chromatography paper 2 cm. from one end.
Put a hole in the other end of the paper so that it can be suspended on a wire clip inserted in a cork stopper as shown in the photograph above. The paper should be able to reach to within 1 cm of the bottom of the tube but not touch the bottom.
When the apparatus is adjusted properly, remove the paper so that you can add pigment extract.
Caution - The remainder of this procedure should be conducted under the hood because the vapors from the chemicals used are toxic and carcinogenic (cause cancer).
Use a micropipette to place a 5 ul of pigment extract on the dot that you marked on the paper. The extract should be placed directly on top of the dot. It will spread, producing a small circular spot. Allow the spot to dry for 1 minute and repeat this procedure 4 more times for a total of 5 applications of pigment extract. Allow the spot to dry between each application.
While waiting for the paper to dry after the fifth application, add chromatography solvent to the bottom of the chromatography tube. Add enough solvent so that the end of the chromatography paper will be immersed in the solvent but the spot with pigment extract will remain above the solvent. It is important not to immerse the pigment spot. This tube should be kept under the hood at all times.
When the spot has dried, suspend the paper vertically in a chromatography tube. If possible, keep the paper from touching the sides of the tube.
Check the movement of the solvent after 20 minutes. The paper should be removed before the solvent reaches the top of the paper.
Before the solvent dries, draw a line on the paper that shows how far the solvent moved. One end of the paper should have a dot that indicates where the pigments were applied and the other end should have a line that indicates how far the solvent traveled.
Allow the paper to dry under the hood. After the paper is dried, you may bring it out from under the hood.
Do not discard the chromatography solution down the drain, discard it in the beaker provided under the hood.
The photosynthetic pigments will be separated in the following order beginning with the highest R f : beta-carotenes, xanthophylls, chlorophyll a, chlorophyll b. Beta-carotenes are orange or orange-yellow, xanthophylls are yellow, chlorophyll A is blue-green, and chlorophyll B is olive-green.
The proportion of the total distance moved by the spot is often calculated in chromatography procedures. To calculate this value, you must first measure the distance moved by the solvent. This can be done by measuring the distance from the spot to the top of the paper. Next, measure the distance moved by each of the pigments.
R f = distance moved by the pigment/distance moved by the solvent
For each of the pigments (beta-carotenes, xanthophylls, chlorophyll a, chlorophyll b) record the distance moved and the Rf.
One member of your group should paste the chromatogram in their notebook. The names of the members of your group should be listed next to the chromatogram.
Create a graph of your results from the photosynthesis experiment. Put time on the X-axis and pH on the Y-axis. If you have a notebook with quad-ruled pages, you can draw the graph directly in your notebook. If not, use graph paper or use a computer graphing program such as Excel or LibreOffice. Adjust the graph so that the minimum value of the Y-axis is a number that is slightly less than the minimum value observed in the table above. The maximum value should be equal to or slightly greater than the maximum value of the data. For example, suppose that the minimum pH that you measured was 5.9 and the maximum was 7.3. You could make the minimum on the graph 5.5 and the maximum 7.5. Be sure that you clearly identify the two lines on the graph. For example, you could use circles for points on one line and squares for points on the other line.
- What will happen to the pH of water when CO 2 is dissolved in the water?
- Write the equation that describes what happens when CO 2 is dissolved in water.
- What will happen to the pH of water when CO 2 is removed from the water?
- Two kinds of metabolic reactions caused the pH to change in the tube with the plant. One of these processes caused the pH to increase, the other caused it to decrease. Name these processes and tell which causes the pH to increase and which causes the pH to decline.
- The two processes listed above had opposite effects on the pH. Why did the pH increase in the light tube if both of these process were occurring at the same time?
- Explain why the values produced from the tube with the plant do not reflect the total photosynthesis (total CO 2 consumption) of the plant. [Hint - Think about your answer to questions 4 and 5.]
- Explain why the pH of the tube with the plant might stop increasing toward the end of the 60 minute time period. The diagram above may be helpful for answering this question.
Contributors
Michael J. Gregory, Ph.D. ( Clinton Community College)
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 05 March 1998

The hydrogen hypothesis for the first eukaryote
- William Martin 1 &
- Miklós Müller 2
Nature volume 392 , pages 37–41 ( 1998 ) Cite this article
13k Accesses
868 Citations
64 Altmetric
Metrics details
A new hypothesis for the origin of eukaryotic cells is proposed, based on the comparative biochemistry of energy metabolism. Eukaryotes are suggested to have arisen through symbiotic association of an anaerobic, strictly hydrogen-dependent, strictly autotrophic archaebacterium (the host) with a eubacterium (the symbiont) that was able to respire, but generated molecular hydrogen as a waste product of anaerobic heterotrophic metabolism. The host's dependence upon molecular hydrogen produced by the symbiont is put forward as the selective principle that forged the common ancestor of eukaryotic cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
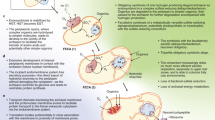
The Syntrophy hypothesis for the origin of eukaryotes revisited
Purificación López-García & David Moreira

Eukaryogenesis and oxygen in Earth history
Daniel B. Mills, Richard A. Boyle, … Timothy M. Lenton
The role of mitochondrial energetics in the origin and diversification of eukaryotes
Paul E. Schavemaker & Sergio A. Muñoz-Gómez
Müller, M. The hydrogenosome. J. Gen. Microbiol. 139 , 2879–2889 (1993).
Article Google Scholar
Cavalier-Smith, T. Eukaryotes with no mitochondria. Nature 326 , 332–333 (1987).
Article ADS CAS Google Scholar
Sogin, M. L., Silberman, J. D., Hinkle, G. & Morrison, H. G. Problems with molecular diversity in the Eukarya. Symp. Soc. Gen. Microbiol. 54 , 167–184 (1996).
Google Scholar
Whatley, J. M., John, P. & Whatley, F. R. From extracellular to intracellular: the establishment of mitochondria and chloroplasts. Proc. R. Soc. Lond. B 204 , 165–187 (1979).
Gray, M. W. & Doolittle, W. F. Has the endosymbiont hypothesis been proven? Microbiol. Rev. 46 , 1–42 (1982).
CAS PubMed PubMed Central Google Scholar
Cavalier-Smith, T. The origin of eukaryote and archaebacterial cells. Ann. NY Acad. Sci. 503 , 7–54 (1987).
ADS Google Scholar
Cavalier-Smith, T. & Chao, E. E. Molecular phylogeny of the free-living archaezoan Trepomonas agilis and the nature of the first eukaryote. J. Mol. Evol. 43 , 551–562 (1996).
Zillig, W. et al . Did eukaryotes originate by a fusion event? Endocytobiosis Cell Res. 6 , 1–25 (1989).
Gupta, R. S. & Golding, G. B. The origin of the eukaryotic cell. Trends Biochem. Sci. 21 , 166–171 (1996).
Article CAS Google Scholar
Lake, J. A. & Rivera, M. C. Was the nucleus the first endosymbiont? Proc. Natl Acad. Sci. USA 91 , 2880–2881 (1994).
Clark, C. G. & Roger, A. J. Direct evidence for secondary loss of mitochondria in Entamoeba histolytica . Proc. Natl Acad. Sci. USA 92 , 6518–6521 (1995).
Henze, K. et al . Anuclear gene of eubacterial origin in Euglena reflects cryptic endosymbioses during protist evolution. Proc. Natl Acad. Sci. USA 92 , 9122–9126 (1995).
Keeling, P. W. & Doolittle, W. F. Evidence that eukaryotic triosephosphate isomerase is of alpha-proteobacterial origin. Proc. Natl Acad. Sci. USA 94 , 1270–1275 (1997).
Doolittle, W. F. Some aspects of the biology of cells and their possible evolutionary significance. Symp. Soc. Gen. Microbiol. 54 , 1–21 (1996).
Rosenthal, B. et al . Evidence for the bacterial origin of genes encoding fermentation enzymes of the amitochondriate protozoan parasite Entamoeba histolytica . J. Bacteriol. 179 , 3736–3745 (1997).
Searcy, D. G. in The Origin and Evolution of the Cell (eds Hartman, H. & Matsuno, K.) 47–78 (World Scientific, Singapore, (1992)).
de Duve, C. Blueprint for a Cell: the Nature and Origin of Life (Patterson, Burlington, NC, (1991)).
Coombs, G. H. & Müller, M. in Biochemistry and Molecular Biology of Parasites (eds Marr, J. J. & Müller, M.) 33–47 (Academic, London, (1995)).
Book Google Scholar
Müller, M. in Evolutionary Relationships Among Protozoa (eds Coombs, G. H., Vickermann, K., Sleigh, M. A. & Warren, A.) 109–132 (Chapman Hall, London, (1998)).
Müller, M. Energy metabolism of protozoa without mitochondria. Annu. Rev. Microbiol. 42 , 465–488 (1988).
Müller, M. in Christian Gottfried Ehrenberg-Festschrift anla¨ßlich der 14. Wissenschaftlichen Jahrestagung der Deutschen Gesellschaft für Protozoologie, 9.-11. Marz 1995 in Delitzsch (Sachsen) (eds Schlegel, M. & Hausmann, K.) 63–76 (Leipziger Universitätsverlag, Leipzig, (1996)).
Iwabe, N., Kuma, K.-I., Hasegawa, M., Osawa, S. & Miyata, T. Evolutionary relationship of archaebacteria, eubacteria and eukaryotes inferred from phylogenetic trees of duplicated genes. Proc. Natl Acad. Sci. USA 86 , 9355–9359 (1989).
Woese, C., Kandler, O. & Wheelis, M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria and Eukarya. Proc. Natl Acad. Sci. USA 87 , 4576–4579 (1990).
Langer, D., Hain, J., Thuriaux, P. & Zillig, W. Transcription in Archaea: similarity to that in Eukarya. Proc. Natl Acad. Sci. USA 92 , 5768–5772 (1995).
Yamamoto, A., Hashimoto, T., Asaga, E., Hasegawa, M. & Goto, N. Phylogenetic position of the mitochondrion-lacking protozoan Trichomonas tenax , based on amino acid sequences of elongation factors 1-α and 2. J. Mol. Evol. 44 , 98–105 (1997).
Horner, D. S., Hirt, R. P., Kilvington, S., Lloyd, D. & Embley, T. M. Molecular data suggest an early acquisition of the mitochondrion endosymbiont. Proc. R. Soc. Lond. B 263 , 1053–1059 (1996).
Bui, E. T. N., Bradley, P. J. & Johnson, P. J. Acommon evolutionary origin for mitochondria and hydrogenosomes. Proc. Natl Acad. Sci. USA 93 , 9651–9656 (1996).
Germot, A., Philippe, H. & Le Guyader, H. Presence of a mitochondrial-type 70-kDa heat shock protein in Trichomonas vaginalis suggests a very early mitochondrial endosymbiosis in eukaryotes. Proc. Natl Acad. Sci. USA 93 , 14614–14617 (1996).
Roger, A. J., Clark, C. G. & Doolittle, W. F. Apossible mitochondrial gene in the early-branching amitochondriate protist Trichomonas vaginalis . Proc. Natl Acad. Sci. USA 93 , 14618–14622 (1996).
Hrdý, I. & Müller, M. Primary structure and eubacterial relationships of the pyruvate : ferredoxin oxidoreductase of the amitochondriate eukaryote, Trichomonas vaginalis . J. Mol. Evol. 41 , 388–396 (1995).
Article ADS Google Scholar
Blattner, F. R. et al . The complete genome sequence of Escherichia coli K-12. Science 277 , 1453–1474 (1997).
Kaneko, T. et al . Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3 , 109–136 (1996).
Sánchez, L. B. & Müller, M. Purification and characterization of the acetate forming enzyme, acetyl-CoA synthetase (ADP-forming) from the amitochondriate protist, Giardia lamblia . FEBS Lett. 378 , 240–244 (1996).
Schönheit, P. & Schäfer, T. Metabolism of hyperthermophiles. World. J. Microbiol. Biotechnol. 11 , 26–57 (1995).
Markoŝ, A., Miretsky, A. & Müller, M. Aglyceraldehyde-3-phosphate dehydrogenase with eubacterial features in the amitochondriate eukaryote Trichomonas vaginalis . J. Mol. Evol. 37 , 631–643 (1993).
Martin, W. & Schnarrenberger, C. The evolution of the Calvin cycle from prokaryotic to eukaryotic chromosomes: a case study of functional redundancy in ancient pathways through endosymbiosis. Curr. Genet. 32 , 1–18 (1997).
Fenchel, T. & Finlay, B. J. Ecology and Evolution in Anoxic Worlds (Oxford Univ. Press, Oxford, (1995)).
Gibson, J. L. & Tabita, F. R. The molecular regulation of the reductive pentose phosphate pathway in proteobacteria and cyanobacteria. Arch. Microbiol. 166 , 141–150 (1996).
Murrel, J. C. Genetics and molecular biology of methanotrophs. FEMS Microbiol. Lett. 88 , 233–248 (1992).
Thauer, R. K., Hedderich, R. & Fischer, R. in Methanogenesis: Ecology, Physiology, Biochemistry and Genetics (ed. Ferry, J. G.) 209–252 (Chapman & Hall, New York, (1993)).
Conrad, R. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2, and NO). Microbiol. Rev. 60 , 609–640 (1996).
Bryant, M. P., Wolin, E. A., Wolin, M. J. & Wolfe, R. S. Methanobacillus omelianskii , a symbiotic association of two species of bacteria. Arch. Microbiol. 59 , 20–31 (1967).
CAS Google Scholar
Broers, C. A. M., Stumm, C. K., Vogels, G. D. & Brugerolle, G. Psalteriomonas lanterna gen. nov., sp. nov., a free living amoboflagellate isolated from freshwater anaerobic sediments. Eur. J. Protistol. 25 , 369–380 (1990).
Embley, T. M. et al . Multiple origins of anaerobic ciliates with hydrogenosomes within the radiation of aerobic ciliates. Proc. R. Soc. Lond. B 262 , 87–93 (1995).
Finlay, kB. J., Embley, T. M. & Fenchel, T. Anew polymorphic methanogen, closely related to Methanocorpusculum parvum , living in stable symbiosis within the anaerobic ciliate Trimyema sp. J. Gen. Microbiol. 139 , 371–378 (1993).
Stevens, T. O. & McKinley, J. P. Lithoautotrophic microbial ecosystems in deep basalt aquifers. Science 270 , 450–454 (1995).
Brinkmann, H. & Martin, W. Higher plant chloroplast and cytosolic 3-phosphoglycerate kinases: a case of endosymbiotic gene replacement. Plant. Mol. Biol. 30 , 65–75 (1996).
Kasting, J. F. Earth's early atmosphere. Science 259 , 920–926 (1993).
Poole, A. M., Jeffares, D. C. & Penny, D. The path from the RNA world. J. Mol. Evol. 46 , 1–17 (1998).
Rospert, S. et al . Methyl-coenzyme M reductase and other enzymes involved in methanogenesis from CO2and H2in the extreme thermophile Methanopyrus kandleri . Arch. Microbiol. 156 , 49–55 (1991).
Download references
Acknowledgements
We thank H. Brinkmann, M. Embley, K. Henze, R. Herrmann, R. Hensel, D.Oesterheld and L. Sánchez for critical comments on the manuscript and gratefully acknowledge financial support from the Deutsche Forschungsgemeinschaft (W.M.) and the National Institutes of Health (M.M.).
Author information
Authors and affiliations.
Institut für Genetik, Technische Universität Braunschweig, Spielmannstrasse 7, D-38023, Braunschweig, Germany
William Martin
The Rockefeller University, 1230 York Avenue, New York, 10021, New York, USA
Miklós Müller
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to William Martin or Miklós Müller .
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Martin, W., Müller, M. The hydrogen hypothesis for the first eukaryote. Nature 392 , 37–41 (1998). https://doi.org/10.1038/32096
Download citation
Issue Date : 05 March 1998
DOI : https://doi.org/10.1038/32096
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Human genetic associations of the airway microbiome in chronic obstructive pulmonary disease.
- Jingyuan Gao
- Yuqiong Yang
Respiratory Research (2024)
Horizontal gene transfer in eukaryotes: aligning theory with data
- Patrick J. Keeling
Nature Reviews Genetics (2024)
A break in mitochondrial endosymbiosis as a basis for inflammatory diseases
- Michael P. Murphy
- Luke A. J. O’Neill
Nature (2024)
Actin cytoskeleton and complex cell architecture in an Asgard archaeon
- Thiago Rodrigues-Oliveira
- Florian Wollweber
- Christa Schleper
Nature (2023)
Eco-evolutionary modelling of microbial syntrophy indicates the robustness of cross-feeding over cross-facilitation
Scientific Reports (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Addressing the Environmental Kuznets Curve in the West African Countries: Exploring the Roles of FDI, Corruption, and Renewable Energy
- Published: 15 April 2024
Cite this article
- Lobna Abid 1 , 3 ,
- Sana Kacem 1 , 4 &
- Haifa Saadaoui 2
Environmental degradation and economic growth are two intricately related issues whose impact is in constant increase within a global context marked by climate risks and corruption, notably in certain African countries. This research work examines the impacts of economic growth, corruption, renewable energy, and foreign direct investment on carbon dioxide emissions for a set of West African economies between 1990 and 2020. The current paper uses the PMG-ARDL panel method in order to assess the relationships between the various variables invested. The results are indicative of the long-term effects of variables. These findings demonstrate that GDP per capita has a positive and significant effect on CO2 emissions, and that the Kuznet curve is not validated in this case. Moreover, FDI confirms the pollution heaven hypothesis as it reduces environmental quality in the long run. In contrast, renewable energy consumption and control corruption in West African countries constitute significant factors in the fight for environmental quality. The causality outcomes reveal that there exist one way of unidirectional link between CO2 to both income and corruption, and a one direction causality from FDI to CO2 emissions. Meanwhile, the link between renewable energy and CO2 emissions is neutral. In this respect, this research offers outstanding findings to help maintain influential procedures for environmental sustainability within the West African framework.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Abdouli, M., & Hammami, S. (2017). Investigating the causality links between environmental quality, foreign direct investment and economic growth in MENA countries. International Business Review, 26 (2), 264–278.
Article Google Scholar
Acheampong, A. O., Adams, S., & Boateng, E. (2019). Do globalization and renewable energy contribute to carbon emissions mitigation in Sub-Saharan Africa? Science of the Total Environment, 677 , 436–446.
Akhbari, R., & Nejati, M. (2019). The effect of corruption on carbon emissions in developed and developing countries: Empirical investigation of a claim. Heliyon, 5 (9), e02516. https://doi.org/10.1016/j.heliyon.2019.e02516
Alaganthiran, J. R., & Anaba, M. I. (2022). The effects of economic growth on carbon dioxide emissions in selected Sub-Saharan African (SSA) countries. Heliyon, 8 (11).
Alam, M. S. (2022). Is trade, energy consumption and economic growth threat to environmental quality in Bahrain–Evidence from VECM and ARDL bound test approach. International Journal of Emergency Services, 11 (3), 396–408. https://doi.org/10.1108/IJES-12-2021-0084
Amri, F., Zaied, Y. B., & Lahouel, B. B. (2019). ICT, total factor productivity, and carbon dioxide emissions in Tunisia. Technological Forecasting and Social Change, 146 (C), 212–217. https://doi.org/10.1016/j.techfore.2019.05.028
Asongu, S. A., El Montasser, G., & Toumi, H. (2016). Testing the relationships between energy consumption, CO 2 emissions, and economic growth in 24 African countries: A panel ARDL approach. Environmental Science and Pollution Research, 23 (7), 6563–6573. https://doi.org/10.1007/s11356-015-5883-7
Awan, A. M., & Azam, M. (2022). Evaluating the impact of GDP per capita on environmental degradation for G-20 economies: Does N-shaped environmental Kuznets curve exist? Environment, Development and Sustainability, 24 , 11103–11126. https://doi.org/10.1007/s10668-021-01899-8
Azam, M., Khan, A. Q., Abdullah, H. B., & Qureshi, M. E. (2016). The impact of CO 2 emissions on economic growth: Evidence from selected higher CO 2 emissions economies. Environmental Science and Pollution Research, 23 (7), 6376–6389. https://doi.org/10.1007/s11356-015-5817-4
Aziz, N., Sharif, A., Raza, A., & Rong, K. (2020). Revisiting the role of forestry, agriculture, and renewable energy in testing environment Kuznets curve in Pakistan: Evidence from quantile ARDL approach. Environmental Science and Pollution Research, 27 (9), 10115–10128. https://doi.org/10.1007/s11356-020-07798-1
Bakare, I. A., & Ozegbe, A. E. (2022). The dynamics of corruption, human capital development and economic performance in Nigeria. Journal of Management Scholarship, 1 (1), 41–52. https://doi.org/10.38198/JMS/1.1.2022.7
Balsalobre-Lorente, D., Ibáñez-Luzón, L., Usman, M., & Shahbaz, M. (2022). The environmental Kuznets curve, based on the economic complexity, and the pollution haven hypothesis in PIIGS countries. Renewable Energy, 185 , 1441–1455. https://doi.org/10.1016/j.renene.2021.10.059
Belaïd, F., & Zrelli, M. H. (2019). Renewable and non-renewable electricity consumption, environmental degradation and economic development: Evidence from Mediterranean countries. Energy Policy, 133 , 110929.
Ben Jebli, M., et al. (2016). Testing environmental Kuznets curve hypothesis: The role of renewable and non-renewable energy consumption and trade in OECD countries. Ecological Indicators, 60 , 824–831. https://doi.org/10.1016/j.ecolind.2015.08.031
Ben Jebli, M., & Ben Youssef, S. (2017). The role of renewable energy and agriculture in reducing CO 2 emissions: Evidence for North Africa countries. Ecological Indicators, 74 , 295–301. https://doi.org/10.1016/j.ecolind.2016.11.032
Bergougui, B. (2024). Moving toward environmental mitigation in Algeria: Asymmetric impact of fossil fuel energy, renewable energy and technological innovation on CO2 emissions. Energy Strategy Reviews, 51 , 101281.
Boufateh, T. (2019). The environmental Kuznets curve by considering asymmetric oil price shocks: Evidence from the top two. Environmental Science and Pollution Research, 26 , 706–720.
Boukhelkhal, A. (2022). Energy use, economic growth and CO 2 emissions in Africa: Does the environmental Kuznets curve hypothesis exist? New evidence from heterogeneous panel under cross-sectional dependence. Environment, Development and Sustainability, 24 (11), 13083–13110.
Bouyghrissi, S., Murshed, M., Jindal, A., Berjaoui, A., Mahmood, H., & Khanniba, M. (2022). The importance of facilitating renewable energy transition for abating CO2 emissions in Morocco. Environmental Science and Pollution Research, 29 (14), 20752–20767.
Breitung, J. (2000). The local power of some unit root tests for panel data. In B. Baltagi (Ed.), Advances in Econometrics 15. Nonstationary panels, panel cointegration, and dynamic panels (pp. 161–178). JAI Press.
Chapter Google Scholar
Breusch, T. S., & Pagan, A. R. (1980). The Lagrange multiplier test and its application to model specifications in econometrics. Review of Economic Studies, 47 (1), 239–253. https://doi.org/10.2307/2297111
Çakmak, E. E., & Acar, S. (2022). The nexus between economic growth, renewable energy and ecological footprint: An empirical evidence from most oil-producing countries. Journal of Cleaner Production, 352 , 131548.
Danish, B. M. A. (2017). Dynamic linkages between road transport energy consumption, economic growth, and environmental quality: evidence from Pakistan. Environmental Science and Pollution Research, 25 , 7541–7552. https://doi.org/10.1007/s11356-017-1072-1
Danish, & Ulucak, R. (2022). Analyzing energy innovation-emissions nexus in China: A novel dynamic simulation method. Energy, 244 , 123010.
Danmaraya, I. A., & Danlami, A. H. (2022). Impact of hydropower consumption, foreign direct investment and manufacturing performance on CO2 emissions in the ASEAN-4 countries. International Journal of Energy Sector Management, 16 (5), 856–875.
Dickey, D. A., & Fuller, W. A. (1979). Distribution of the estimators for autoregressive time series with a unit root. Journal of the American Statistical Association, 74 (366a), 427–431.
Damania, R., Fredriksson, P. G., & List, J. A. (2003). Trade liberalization, corruption, and environmental policy formation: Theory and evidence. Journal of Environmental Economics and Management, 46 (3), 490–512.
Danmaraya, I. A., & Danlami, A. H. (2021). Impact of hydropower consumption, foreign direct investment and manufacturing performance on CO 2 emissions in the ASEAN-4 countries. International Journal of Energy Sector Management, 16 (5), 856–875. https://doi.org/10.1108/IJESM-06-2021-0019
Demena, B. A., & Afesorgbor, S. K. (2020). The effect of FDI on environmental emissions: Evidence from a meta-analysis. Energy Policy, 138 (c), 111192.
Dinda, S. (2004). Environmental Kuznets curve hypothesis: A survey. Ecological Indicators, 49 (4), 431–455. https://doi.org/10.1016/j.ecolecon.2004.02.011
Dogan, E., & Seker, F. (2016). Determinants of CO 2 emissions in the European Union: The role of renewable and non-renewable energy. Renewable Energy, 94 , 429–439.
Dumitrescu, E. I., & Hurlin, C. (2012). Testing for Granger non-causality in heterogeneous panels. Economic Modelling, 29 (4), 1450–1460.
Farhani, S., & Shahbaz, M. (2014). What role of renewable and non-renewable electricity consumption and output is needed to initially mitigate CO2 emissions in MENA region? Renewable and Sustainable Energy Reviews, 40 , 80–90.
Fredriksson, P. G., & Neumayer, E. (2016). Corruption and climate change policies: do the bad old days matter? Environmental and resource economics, 63 , 451–469.
Fakher, H. A., Ahmed, Z., Acheampong, A. O., & Nathaniel, S. P. (2023). Renewable energy, nonrenewable energy, and environmental quality nexus: An investigation of the N-shaped environmental Kuznets curve based on six environmental indicators. Energy, 263 (A), 125660. https://doi.org/10.1016/j.energy.2022.125660
Fang, X., Nie, L., & Mu, H. (2020). Research progress on logistics network optimization under low carbon constraints. IOP Conference Series: Earth and Environmental Science, 615 . https://doi.org/10.1088/1755-1315/615/1/012060
Ganda, F. (2020). The influence of corruption on environmental sustainability in the developing economies of Southern Africa. Heliyon, 6 (7), e4416. https://doi.org/10.1016/j.heliyon.2020.e04387
Ghazouani, T. (2021). Impact of FDI inflow, crude oil prices, and economic growth on CO2 emission in Tunisia: Symmetric and asymmetric analysis through ARDL and NARDL approach. Environmental Economics, 12 (1), 1.
Goodness, C. A., & Prosper, E. E. (2017). Effect of economic growth on CO2 emission in developing countries Evidence from a dynamic panel threshold model. Cogent Economics & Finance, 5 (1), 1379239. https://doi.org/10.1080/23322039.2017.1379239
Grossman, G. M., & Kreuger, A.B. (1991). Environmental impacts of a North American free trade agreement. NBER Working Paper 3914 ,.
Google Scholar
Grossman, G. M., & Krueger, A. B. (1995). Economic growth and the environment. The Quarterly Journal of Economics, 110 (2), 353–377. https://doi.org/10.2307/2118443
Habib, S., Abdelmonen, S., & Khaled, M. (2020). The effect of corruption on the environmental quality in African countries: A panel quantile regression analysis. Journal of the Knowledge Economy, Springer; Portland International Center for Management of Engineering and Technology (PICMET), 11 (2), 788–804.
Haug, A. A., & Ucal, M. (2019). The role of trade and FDI for CO2 emissions in Turkey: Nonlinear relationships. Energy Economics, 81 , 297–307.
Hossain, M. R., Rej, S., Awan, A., Bandyopadhyay, A., Islam, M. S., Das, N., & Hossain, M. E. (2023). Natural resource dependency and environmental sustainability under N-shaped EKC: The curious case of India. Resources Policy, 80 , 103150.
Hou, H., Feng, X., Zhang, Y., Bai, H., Ji, Y., & Xu, H. (2021). Energy-related carbon emissions mitigation potential for the construction sector in China. Environmental Impact Assessment Review, 89 , 106599.
Hwang, Y., Kim, C.-B., & Yu, C. (2023). The effect of corruption on environmental quality: Evidence from a panel of CIS countries. Journal of the Knowledge Economy . https://doi.org/10.1007/s13132-023-01236-6
Ibrahiem, D. M. (2020). Do technological innovations and financial development improve environmental quality in Egypt? Environmental Science and Pollution Research, 27 (10), 10869–10881.
IEA. (2021). Available at https://www.iea.org/data-and-statistics
Im, K. S., Pesaran, M. H., & Shin, Y. (2003). Testing for unit roots in heterogenous panel. Journal of Econometrics, 115 , 53–74.
Inglesi-Lotz, R., & Dogan, E. (2018). The role of renewable versus non-renewable energy to the level of CO 2 emissions a panel analysis of sub-Saharan Africa’s Βig 10 electricity generators. Renewable Energy, 123 (C), 36–43.
Jahanger, A., Awan, A., Anwar, A., & Adebayo, T. S. (2023, August). Greening the Brazil, Russia, India, China and South Africa (BRICS) economies: Assessing the impact of electricity consumption, natural resources, and renewable energy on environmental footprint. In Natural resources forum (Vol. 47, No. 3, pp. 484–503). Blackwell Publishing Ltd.
Jebabli, I., Lahiani, A., & Mefteh-Wali, S. (2023). Quantile connectedness between CO2 emissions and economic growth in G7 countries. Resources Policy, 81 , 103348.
Khalid, W., Özdeşer, H., & Jalil, A. (2021). An empirical analysis of inter-factor and inter-fuel substitution in the energy sector of Pakistan. Renewable Energy, 177 (c), 953–966.
Khan, Z., Ali, M., Jinyu, L., et al. (2020). Consumption-based carbon emissions and trade nexus: Evidence from nine oil exporting countries. Energy Economics, 89 . https://doi.org/10.1016/j.eneco.2020.104806
Kirikkaleli, D. (2020). New insights into an old issue: Exploring the nexus between economic growth and CO2 emissions in China. Environmental Science and Pollution Research, 27 (32), 40777–40786.
Leitão, N. C. (2021). The effects of corruption, renewable energy, trade and CO 2 emissions. Economies, 9 (2), 62. https://doi.org/10.3390/economies9020062
Levin, A., & Lin, C. F. (1992). Unit root test in panel data: Asymptotic and finite-sample properties. Discussion Paper (pp. 92–93). Department of Economics, University of California at San Diego.
Levin, A., & Lin, C. F. (1993). Unit root test in panel data: New results. Discussion Paper (pp. 93–56). Department of Economics, University of California at San Diego.
Li, B., & Haneklaus, N. (2021). The role of renewable energy, fossil fuel consumption, urbanization and economic growth on CO2 emissions in China. Energy Report, 7 , 783–793.
Li, B., & Haneklaus, N. (2022). The potential of India’s net-zero carbon emissions: Analyzing the effect of clean energy, coal, urbanization, and trade openness. Energy Reports, 8 , 724–733.
Liang, W., & Yang, M. (2019). Urbanization, economic growth and environmental pollution: Evidence from China. Sustainable Computing: Informatics and Systems, 21 , 1–9. https://doi.org/10.1016/j.suscom.2018.11.007
Liao, J., Liu, X., Zhou, X., & Tursunova, N. R. (2023). Analyzing the role of renewable energy transition and industrialization on ecological sustainability: Can green innovation matter in OECD countries. Renewable Energy, 204 , 141–151.
List, J., & Gallet, C. (1999). The environmental Kuznets curve: Does one size fit all? Ecological Economics, 31 (3), 409–423. https://doi.org/10.1016/S0921-8009(99)00064-6
Lopez, R., & Mitra, S. (2000). Corruption, pollution, and the Kuznets environment curve. Journal of Environmental Economics and Management, 40 , 137–150. https://doi.org/10.1006/jeem.1999.1107
Lv, Z., & Gao, Z. (2021). The effect of corruption on environmental performance: Does spatial dependence play a role? Economic Systems, 45 (2), 100773. https://doi.org/10.1016/j.ecosys.2020.100773
Maddala, G., & Wu, S. (1999). A comparative study of unit root tests and a new simple test. Oxford Bulletin of Economics and Statistics, 61 , 631–652.
Massagony, A., & Budiono. (2023). Is the Environmental Kuznets Curve (EKC) hypothesis valid on CO2 emissions in Indonesia? International Journal of Environmental Studies, 80 (1), 20–31.
Mongo, M., Belaïd, F., & Ramdani, B. (2021). The effects of environmental innovations on CO2 emissions: Empirical evidence from Europe. Environmental Science & Policy, 118 , 1–9.
Mukhtarov, S., Aliyev, F., Aliyev, F., & Ajayi, R. (2022). Renewable energy consumption and carbon emissions: Evidence from an oil-rich economy. Sustainability, 15 (1), 1–12.
Musah, M., Gyamfi, B. A., Kwakwa, P. A., & Agozie, D. Q. (2023). Realizing the 2050 Paris climate agreement in West Africa: The role of financial inclusion and green investments. Journal of Environmental Management, 340 , 117911.
Naeem, M. A., Appiah, M., Taden, J., Amoasi, R., & Gyamfi, B. A. (2023). Transitioning to clean energy assessing the impact of renewable energy, bio-capacity and access to clean fuel on carbon emissions in OECD economies. Energy Economics, 127 , 107091.
Namahoro, J. P., Wu, Q., Zhou, N., & Xue, S. (2021). Impact of energy intensity, renewable energy, and economic growth on CO 2 emissions: Evidence from Africa across regions and income levels. Renewable and Sustainable Energy Reviews, 147 , 111233.
Nathaniel, S. P., & Adeleye, N. (2021). Environmental preservation amidst carbon emissions, energy consumption, and urbanization in selected African countries: Implication for sustainability. Journal of Cleaner Production, 285 , 125409.
Nathaniel, S. P., & Iheonu, C. I. (2019). Carbon dioxide abatement in Africa: The role of renewable and non-renewable energy consumption. Science of the Total Environment, 679 , 337–345. https://doi.org/10.1016/j.scitotenv.2019.05.011
Njoh, A. J. (2021). Renewable energy as a determinant of inter-country differentials in CO 2 emissions in Africa. Renewable Energy, 172 , 1225–1232.
Nkemdilim, I., Ike, O. C., & Ozegbe, A. E. (2023). Corruption, environmental sustainability and economic performance in emerging economies: Evidence from Nigeria. International Journal of Management, Economics and Social Sciences, 12 (1), 52–78. https://doi.org/10.32327/IJMESS/12.1.2023.3
Nourry, M. (2007). La croissance économique est-elle un moyen de lutte contre la pollution ? Revue Française D’économie, 21 (3), 137–176. https://www.persee.fr/doc/rfeco_0769-0479_2007_num_21_3_1605
Ohajionu, U. C., Gyamfi, B. A., Haseki, M. I., & Bekun, F. V. (2022). Assessing the linkage between energy consumption, financial development, tourism and environment: Evidence from method of moments quantile regression. Environmental Science and Pollution Research, 29 , 30004–30018. https://doi.org/10.1007/s11356-021-17920-6
Onofrei, M., Vatamanu, A. F., & Cigu, E. (2022). The relationship between economic growth and CO2 emissions in EU countries: A cointegration analysis. Frontiers in Environmental Science, 10 , 934885.
Omri, E., & Saadaoui, H. (2022). An empirical investigation of the relationships between nuclear energy, economic growth, trade openness, fossil fuels, and carbon emissions in France: Fresh evidence using asymmetric cointegration. Environmental Science and Pollution Research , 30 (5).
Omri, E., & Saadaoui, H. (2023). An empirical investigation of the relationships between nuclear energy, economic growth, trade openness, fossil fuels, and carbon emissions in France: Fresh evidence using asymmetric cointegration. Environmental Science and Pollution Research, 30 (5), 13224–13245.
Panait, M., Janjua, L. R., Apostu, S. A., & Mihăescu, C. (2023). Impact factors to reduce carbon emissions. Evidences from Latin America. Kybernetes, 52 (11), 5669–5686. https://doi.org/10.1108/K-05-2022-0712
Pata, U. K., & Caglar, A. E. (2021). Investigating the EKC hypothesis with renewable energy consumption, human capital, globalization and trade openness for China: evidence from augmented ARDL approach with a structural break. Energy, 216 , 119220.
Pata, U. K., & Samour, A. (2022). Do renewable and nuclear energy enhance environmental quality in France? A new EKC approach with the load capacity factor. Progress in Nuclear Energy, 149 , 104249.
Pata, U. K., Yilanci, V., Hussain, B., & Naqvi, S. A. A. (2022). Analyzing the role of income inequality and political stability in environmental degradation: evidence from South Asia. Gondwana Research, 107 (1), 13–29. https://doi.org/10.1016/j.gr.2022.02.009
Pedroni, P. (1999). Critical values for cointegration tests in heterogeneous panels with multiple regressors. Oxford Bulletin of Economics and statistics, 61 (S1), 653–670.
Pedroni, P. (2004). Panel cointegration: Asymptotic and finite sample properties of pooled time series tests with an application to the PPP hypothesis. Econometric Theory, 20 (2), 597–625. https://doi.org/10.1017/S0266466604203073
Perone, G. (2024). The relationship between renewable energy production and CO 2 emissions in 27 OECD countries: A panel cointegration and Granger non-causality approach. Journal of Cleaner Production, 434 , 139655. https://doi.org/10.1016/j.jclepro.2023.139655
Perrings, C., & Pearce, D. W. (1994). Threshold effects and incentives for the conservation of biodiversity. Environmental and Resource Economics, 4 , 13–28. https://doi.org/10.1007/BF00691930
Pesaran, M. H. (1997). The role of economic theory in modelling the long run. The Economic Journal, 107 (440), 178–191.
Pesaran, M. H. (2007). A simple panel unit root test in the presence of cross-section dependence. Journal of Applied Econometrics, 22 (2), 265–312. https://doi.org/10.1002/jae.951
Pesaran, M. H. (2004a). General diagnostic tests for cross section dependence in panels. University of Cambridge, Faculty of Economics, Cambridge Working Papers in Economics No. 0435. http://www.econ.cam.ac.uk/research-files/repec/cam/pdf/cwpe0435.pdf .
Pesaran, M. H. (2004b). General diagnostic tests for cross section dependence in panels. Cambridge Working Papers in Economics 0435 . Faculty of Economics, University of Cambridge.
Pesaran, M. H., Shin, Y., & Smith, R. P. (1999). Pooled mean group estimation of dynamic heterogeneous panels. Journal of the American Statistical Association, 94 (446), 621–634.
Qamruzzaman, M. (2021). Nexus between environmental quality, institutional quality and trade openness through the channel of FDI: An application of common correlated effects estimation (CCEE), NARDL, and asymmetry causality. Environmental Science and Pollution Research, 28 , 52475–52498. https://doi.org/10.1007/s11356-021-14269-8
Rahman, M. M., & Alam, K. (2022). Effects of corruption, technological innovation, globalisation, and renewable energy on carbon emissions in Asian countries. Utilities Policy, 79 (c), 101448. https://doi.org/10.1016/j.jup.2022.101448
Rahman, M. M., Saidi, K., & Mbarek, M. B. (2020). Economic growth in South Asia: the role of CO2 emissions population density and trade openness. Heliyon, 6 (5).
Raihan, A., & Tuspekova, A. (2023). The role of renewable energy and technological innovations toward achieving Iceland’s goal of carbon neutrality by 2040. Journal of Technology Innovations and Energy, 2 (1), 22–37. https://doi.org/10.56556/jtie.v2i1.421
Saadaoui, H. (2022). The impact of financial development on renewable energy development in the MENA region: The role of institutional and political factors. Environmental Science and Pollution Research, 29 , 39461–39472. https://doi.org/10.1007/s11356-022-18976-8
Saadaoui, H., & Chtourou, N. (2023). Do institutional quality, financial development, and economic growth improve renewable energy transition? Some evidence from Tunisia. Journal of the Knowledge Economy, 14 , 2927–2958. https://doi.org/10.1007/s13132-022-00999-8
Sadiq, M., Hassan, S. T., Khan, I., & Rahman, M. M. (2023). Policy uncertainty, renewable energy, corruption and CO 2 emissions nexus in BRICS-1 countries: A panel CS-ARDL approach. Environment, Development and Sustainability , 1–27. https://doi.org/10.1007/s10668-023-03546-w
Sahoo, M., & Sethi, N. (2021). The intermittent effects of renewable energy on ecological footprint: Evidence from developing countries. Environmental Science and Pollution Research, 28 , 56401–56417.
Saidi, K., & Ben Mbarek, M. (2017). Dynamic relationship between CO 2 emissions, energy consumption and economic growth in three North African countries. International Journal of Sustainable Energy, 36 (9), 840–854. https://doi.org/10.1080/14786451.2015.1102910
Saidi, K., & Hammami, S. (2015). The impact of CO 2 emissions and economic growth on energy consumption in 58 countries. Energy Reports, 1 , 62–70. https://doi.org/10.1016/j.egyr.2015.01.003
Saidi, K., & Omri, A. (2020). The impact of renewable energy on carbon emissions and economic growth in 15 major renewable energy-consuming countries. Environmental Research, 186 , 109567. https://doi.org/10.1016/j.envres.2020.109567
Salari, M., Javid, R. J., & Noghanibehambari, H. (2021). The nexus between CO2 emissions, energy consumption, and economic growth in the US. Economic Analysis and Policy, 69 , 182–194.
Seker, F., Ertugrul, H. M., & Cetin, M. (2015). The impact of foreign direct investment on environmental quality: A bounds testing and causality analysis for Turkey. Renewable and Sustainable Energy Reviews, 52 (C), 347–356. https://doi.org/10.1016/j.rser.2015.07.118
Sekrafi, H., & Sghaier, A. (2018). The effect of corruption on carbon dioxide emissions and energy consumption in Tunisia. PSU Research Review, 2 (1), 81–95. https://doi.org/10.1108/PRR-11-2016-0008
Shafik, N. (1994). Economic development and environmental quality: An econometric analysis. Oxford Economic Papers, 46 (1), 757–773. https://doi.org/10.1093/oep/46.Supplement_1.757
Shafik, N., & Bandyopadhyay, S. (1992). Economic growth and environmental quality: Time series and cross-country evidence. World development working paper WPS 904 . World Bank.
Shahbaz, M., Balsalobre-Lorente, D., & Sinha, A. (2019). Foreign direct investment–CO 2 emissions nexus in Middle East and North African countries: Importance of biomass energy consumption. Journal of Cleaner Production, 217 , 603–614. https://doi.org/10.1016/j.jclepro.2019.01.282
Shahbaz, M., Khan, S., Ali, A., & Bhattacharya, M. (2017). The impact of globalization on CO2 emissions in China. The Singapore Economic Review, 62 . https://doi.org/10.1142/S021759081740033
Sharma, R., Shahbaz, M., Sinha, A., & Vo, X. V. (2021). Examining the temporal impact of stock market development on carbon intensity: Evidence from South Asian countries. Journal of Environmental Management, 297 , 113248.
Sharma, S. S. (2011). Determinants of carbon dioxide emissions: Empirical evidence from 69 countries. Applied Energy, 88 (1), 376–382. https://doi.org/10.1016/j.apenergy.2010.07.022
Shinwari, R., Wang, Y., Maghyereh, A., & Awartani, B. (2022). Does Chinese foreign direct investment harm CO2 emissions in the belt and road economies. Environmental Science and Pollution Research, 29 (26), 39528–39544.
Shoaib, H. M., Rafique, M. Z., Nadeem, A. M., & Huang, S. (2020). Impact of financial development on CO2 emissions: A comparative analysis of developing countries (D8) and developed countries (G8). Environmental Science and Pollution Research, 27 , 12461–12475.
Sulaiman, J., Azlinda, A., & Saboori, B. (2013). The potential of renewable energy: Using the environmental Kuznets curve model. American Journal of Environmental Science, 9 (2), 103–112. https://doi.org/10.3844/ajessp.2013.103.112
Sultana, N., Rahman, M., Khanan, R., & Kkabir, Z. (2022). Environmental quality and its nexus with informal economy, corruption control, energy use, and socioeconomic aspects: The perspective of emerging economies. Heliyon, 8 (6), 1–11.
Tahir, T., Luni, T., Majeed, M. T., & Zafar, A. (2021). The impact of financial development and globalization on environmental quality: Evidence from South Asian economies. Environmental Science and Pollution Research, 28 , 8088–8101.
Tang, C. F., & Tan, B. W. (2015). The impact of energy consumption, income and foreign direct investment on carbon dioxide emissions in Vietnam. Energy, 79 , 447–454. https://doi.org/10.1016/j.energy.2014.11.033
To, A. H., Ha, D. T. T., Nguyen, H. M., & Vo, D. H. (2019). The impact of foreign direct investment on environment degradation: Evidence from emerging markets in Asia. International Journal of Environmental Research and Public Health, 16 (9), 1636.
Usman, M., Jahanger, A., Radulescu, M., & Lorente, D. B. (2022). Do nuclear energy, renewable energy, and environmentalrelated technologies asymmetrically reduce ecological footprint? Evidence from Pakistan. Energies, 15 (9), 3448. https://doi.org/10.3390/en15093448
Usman, O. (2022). Modelling the economic and scocial issues related to environmental quality in Nigeria: The role of economic growth and internal conflict. Environmental Pollution and Pollution Research, 29 , 39209–39227. https://doi.org/10.1007/s11356-021-18157-z
Usman, O., Rafndadi, A. A., & Sarkodie, S. A. (2021). Conflicts and ecological footprint in MENA countries: Implications for sustainable terrestrial ecosystem. Environment Science Pollution Research, 28 (42), 59988–59999. https://doi.org/10.1007/s11356-021-14931-1
Uzar, U. (2020). Political economy of renewable energy: Does institutional quality make a difference in renewable energy consumption? Renewable Energy, 155 , 591–603.
Wang, Q., Zhang, F., & Li, R. (2023). Free trade and carbon emissions revisited: the asymmetric impacts of trade diversification and trade openness. Sustainable Development.
Wang, Z., Zhang, B., & Wang, B. (2018). The moderating role of corruption between economic growth and CO2 emissions: evidence from BRICS economies. Energy, 148 , 506–513.
Wang, S., Zhao, D., & Chen, H. (2020). Government corruption, resource misallocation, and ecological efficiency. Energy Economics, 85 (C). https://doi.org/10.1016/j.eneco.2019.104573
WDI. (2021). Available at https://databank.worldbank.org/source/world-development-indicators
Wei, C., Ren, S., Yang, P., Wang, Y., He, X., Xu, Z., et al. (2021). Effects of irrigation methods and salinity on CO2 emissions from farmland soil during growth and fallow periods. Science of the Total Environment, 752 , 141639.
Wen, J., Mughal, N., Zhao, J., Shabbir, M. S., Niedbała, G., Jain, V., & Anwar, A. (2021). Does globalization matter for environmental degradation? Nexus among energy consumption, economic growth, and carbon dioxide emission. Energy policy, 153 , 112230.
Wilson, J. K., & Damania, R. (2005). Corruption, political competition and environmental policy. Journal of Environmental Economics and Management, 49 (3), 516–535. https://doi.org/10.1016/j.jeem.2004.06.004
Xie, P., Yang, F., Mu, Z., & Gao, S. (2020). Influencing factors of the decoupling relationship between CO2 emission and economic development in China’s power industry. Energy, 209 , 118341.
Xu, F., Huang, Q., Yue, H., et al. (2020). Reexamining the relationship between urbanization and pollutant emissions in China based on the STIRPAT model. Journal of Environmental Management, 273 , 111134. https://doi.org/10.1016/j.jenvman.2020.111134
Xue, C., Shahbaz, M., Ahmed, Z., Ahmad, M., & Sinha, A. (2022). Clean energy consumption, economic growth, and environmental sustainability: what is the role of economic policy uncertainty? Renewable Energy, 184 , 899–907.
Yahaya, N. S., Mohd-Jali, M. R., & Raji, J. O. (2020). The role of financial development and corruption in environmental degradation of Sub-Saharan African countries. Management of Environmental Quality, 31 (4), 895–913. https://doi.org/10.1108/MEQ-09-2019-0190
Yang, F., Yuan, H., & Yi, N. (2022). Natural resources, environment and the sustainable development. Urban Climate, 42 . https://doi.org/10.1016/j.uclim.2022.101111
Yousefi-Sahzabi, A., Sasaki, K., Yousefi, H., Pirasteh, S., & Sugai, Y. (2011). GIS aided prediction of CO2 emission dispersion from geothermal electricity production. Journal of Cleaner Production, 19 (17-18), 1982–1993.
Yilanci, V., & Pata, U. K. (2020). Convergence of per capita ecological footprint among the ASEAN-5 countries: Evidence from a non-linear panel unit root test. Ecological Indicators, 113 , 106178. https://doi.org/10.1016/j.ecolind.2020.106178
Zhang, M., Yang, Z., Liu, L., & Zhou, D. (2021). Impact of renewable energy investment on carbon emissions in China - An empirical study using a nonparametric additive regression model. Science of the Total Environment, 785 , 147109. https://doi.org/10.1016/j.scitotenv.2021.147109
Zhang, Y., & Zhang, S. (2018). The impacts of GDP, trade structure, exchange rate and FDI inflows on China’s carbon emissions. Energy Policy, 120 (C), 347–353. https://doi.org/10.1016/j.enpol.2018.05.056
Zhou, A., & Li, J. (2019). Heterogeneous role of renewable energy consumption in economic growth and emissions reduction: Evidence from a panel quantile regression. Environmental Science and Pollution Research, 26 (22), 22575–22595. https://doi.org/10.1007/s11356-019-05447-w
Zoundi, Z. (2017). CO2 emissions, renewable energy and the environmental Kuznets curve, a panel cointegration approach. Renewable and Sustainable Energy Reviews, 72 , 1067–1075.
Download references
Availability of Data and Material
All data is provided in the results section of this manuscript.
Author information
Authors and affiliations.
University of Sfax, Tunisia, Higher Institute of Business Administration of Sfax, Laboratory of Probability and Statistics - PB 1171, Sfax, Tunisia
Lobna Abid & Sana Kacem
University of Sfax, Tunisia, Higher Institute of Business Administration of Sfax, Laboratory of Governance, Finance and Accounting-LR 13ES19, Sfax, Tunisia
Haifa Saadaoui
University of Sfax, Tunisia, Faculty of Economics and Management of Sfax, LED, Tunisia, Sfax
Sfax, Tunisia
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to this work.
Corresponding author
Correspondence to Lobna Abid .
Ethics declarations
Ethics approval.
Not applicable
Consent to Participate
Consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Abid, L., Kacem, S. & Saadaoui, H. Addressing the Environmental Kuznets Curve in the West African Countries: Exploring the Roles of FDI, Corruption, and Renewable Energy. J Knowl Econ (2024). https://doi.org/10.1007/s13132-024-01858-4
Download citation
Received : 08 April 2022
Accepted : 13 February 2024
Published : 15 April 2024
DOI : https://doi.org/10.1007/s13132-024-01858-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Economic growth
- CO2 emissions
- Renewable energy
- West African economies
JEL Classification
- Find a journal
- Publish with us
- Track your research
Hemodynamic Flow Hypothesis for Energy Dissipation in the Equine
Paperzz.com, your paperzz.
© Copyright 2024 Paperzz
Help | Advanced Search
Astrophysics > High Energy Astrophysical Phenomena
Title: first search for high-energy neutrino emission from galaxy mergers.
Abstract: The exact sources of high-energy neutrinos detected by the IceCube neutrino observatory still remain a mystery. For the first time, this work explores the hypothesis that galaxy mergers may serve as sources for these high-energy neutrinos. Galaxy mergers can host very high-energy hadronic and photohadronic processes, which may produce very high-energy neutrinos. We perform an unbinned maximum-likelihood-ratio analysis utilizing the galaxy merger data from six catalogs and 10 years of public IceCube muon-track data to quantify any correlation between these mergers and neutrino events. First, we perform the single source search analysis, which reveals that none of the considered galaxy mergers exhibit a statistically significant correlation with high-energy neutrino events detected by IceCube. Furthermore, we conduct a stacking analysis with three different weighting schemes to understand if these galaxy mergers can contribute significantly to the diffuse flux of high-energy astrophysical neutrinos detected by IceCube. We find that upper limits (at $95\%$ c.l.) of the all flavour high-energy neutrino flux, associated with galaxy mergers considered in this study, at $100$ TeV with spectral index $\Gamma=-2$ are $2.57\times 10^{-18}$, $8.51 \times 10^{-19}$ and $2.36 \times 10^{-18}$ $\rm GeV^{-1}\,cm^2\,s^{-1}\,sr^{-1}$ for the three weighting schemes. This work shows that these selected galaxy mergers do not contribute significantly to the IceCube detected high energy neutrino flux. We hope that in the near future with more data, the search for neutrinos from galaxy mergers can either discover their neutrino production or impose more stringent constraints on the production mechanism of high-energy neutrinos within galaxy mergers.
Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- INSPIRE HEP
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .

- WiSER to Spotlight Role of Women Entrepreneurs and Mentors at World Future Energy Summit …
WiSER to Spotlight Role of Women Entrepreneurs and Mentors at World Future Energy Summit 2024

Women in Sustainability, Environment and Renewable Energy (WiSER) - Masdar’s global initiative that champions women as drivers of sustainable change and innovation – will be placing female entrepreneurs and mentors center stage at the World Future Energy Summit (WFES).
In an afternoon of dedicated programming at the Masdar Pavilion on April 18, three panels will explore innovative solutions and opportunities in climate finance for female entrepreneurs, highlight the transformative role of female mentorship in fostering leadership and promoting diversity and inclusion, and examine the nexus between SDG5 and environmental action to ensure women's perspectives from the Global South are integrated into policy and practice.
Bringing together female entrepreneurs, thought leaders, business executives and academics, WiSER’s program at WFES aims to enlighten, challenge and inform audiences as part of WiSER’s commitment to addressing the under-representation of women in the renewable energy sector and highlighting the barriers they face in accessing climate finance and education.

Dr Lamya Fawwaz, Executive Director for Brand & Strategic Initiatives, Masdar, and Program Director of WiSER, said: “WiSER is dedicated to inspiring and championing the next generation of women sustainability leaders. Empowering female entrepreneurs and delivering impactful mentorship programs are two important mechanisms to achieving gender equity. Our panel discussions at WFES will draw on the expertise of a range of distinguished speakers to identify solutions that will unlock climate finance and foster leadership.”
WiSER, a Masdar initiative, was established as a result of the UAE leadership’s longstanding commitment to the role of women; a commitment traced back to the vision of the late founding father Sheikh Zayed bin Sultan Al Nahyan. Aligned with the UAE’s net-zero goals, WiSER is rooted in a recognition that more needs to be done to support women in the global mission to tackle climate change.
Hosted by Masdar, WFES is the leading business event for future energy, clean technology and sustainability and takes place from April 16-18 at Abu Dhabi National Exhibition Centre (ADNEC).
The Masdar Pavilion will be situated in Hall 5 at ADNEC. Full details of WiSER’s program at WFES can be found here .
About WiSER
Women in Sustainability, Environment and Renewable Energy (WiSER) is a global platform that champions women as leaders of sustainable change. Since 2015, WiSER has focused on the core pillars of education, engagement, and empowerment in its workshops, mentoring and programs. Established by the UAE’s two leading sustainability champions – Masdar and the Zayed Sustainability Prize – WiSER embodies the country’s vision for empowering women and building a more sustainable future for all.
About Masdar
Masdar (Abu Dhabi Future Energy Company) is one of the world’s fastest-growing renewable energy companies. As a global clean energy pioneer, Masdar is advancing the development and deployment of solar, wind, geothermal, battery storage and green hydrogen technologies to accelerate the energy transition and help the world meet its net-zero ambitions. Established in 2006, Masdar has developed and invested in projects in over 40 countries with a combined capacity of over 20 gigawatts (GW), providing affordable clean energy access to those who need it most and helping to power a more sustainable future.
Masdar is jointly owned by TAQA, ADNOC, and Mubadala, and is targeting a renewable energy portfolio capacity of 100GW by 2030 while aiming to be a leading producer of green hydrogen by the same year.
© 2000 - 2024 Al Bawaba (www.albawaba.com)
Background Information
Masdar is a global leader in renewable energy and sustainable urban development. Over the last decade, we have pioneered commercially viable solutions in clean energy, sustainable real estate and clean technology in the UAE and around the world.
Headquartered in Abu Dhabi, we develop renewable energy projects, realise low-carbon urban development, while advancing clean-tech innovation. We also deliver world-class knowledge and industry platforms.
At Masdar, we are supporting the UAE’s transition towards a knowledge-based economy. We aim to be a model for the commercial adoption of clean technologies, and a showcase of the UAE’s wider commitment to sustainability.
Company Profile
Check out our pr service.

'Czechoslovakia supports Ukraine!' Biden in trouble AGAIN

Video: Massive landslide due to heavy rains and floods in the UAE

Is Dr. Food abusing his wife Shrouq Sunshine?

Iranian journalist, newspaper under probe for criticizing Tehran's attack on Israel

Video: Alligator spotted in Jordan putting citizens and sheep in danger
- Open access
- Published: 12 April 2024
Different profiles of soil phosphorous compounds depending on tree species and availability of soil phosphorus in a tropical rainforest in French Guiana
- Albert Gargallo-Garriga 1 , 2 ,
- Jordi Sardans 3 , 4 ,
- Joan Llusià 3 , 4 ,
- Guille Peguero 3 , 4 ,
- Marta Ayala-Roque 2 ,
- Elodie A. Courtois 5 , 6 ,
- Clément Stahl 7 ,
- Otmar Urban 3 ,
- Karel Klem 3 ,
- Pau Nolis 8 ,
- Miriam Pérez-Trujillo 8 ,
- Teodor Parella 8 ,
- Andreas Richter 9 ,
- Ivan A. Janssens 5 &
- Josep Peñuelas 3 , 4
BMC Plant Biology volume 24 , Article number: 278 ( 2024 ) Cite this article
79 Accesses
6 Altmetric
Metrics details
The availability of soil phosphorus (P) often limits the productivities of wet tropical lowland forests. Little is known, however, about the metabolomic profile of different chemical P compounds with potentially different uses and about the cycling of P and their variability across space under different tree species in highly diverse tropical rainforests.
We hypothesised that the different strategies of the competing tree species to retranslocate, mineralise, mobilise, and take up P from the soil would promote distinct soil 31 P profiles. We tested this hypothesis by performing a metabolomic analysis of the soils in two rainforests in French Guiana using 31 P nuclear magnetic resonance (NMR). We analysed 31 P NMR chemical shifts in soil solutions of model P compounds, including inorganic phosphates, orthophosphate mono- and diesters, phosphonates, and organic polyphosphates. The identity of the tree species (growing above the soil samples) explained > 53% of the total variance of the 31 P NMR metabolomic profiles of the soils, suggesting species-specific ecological niches and/or species-specific interactions with the soil microbiome and soil trophic web structure and functionality determining the use and production of P compounds. Differences at regional and topographic levels also explained some part of the the total variance of the 31 P NMR profiles, although less than the influence of the tree species. Multivariate analyses of soil 31 P NMR metabolomics data indicated higher soil concentrations of P biomolecules involved in the active use of P (nucleic acids and molecules involved with energy and anabolism) in soils with lower concentrations of total soil P and higher concentrations of P-storing biomolecules in soils with higher concentrations of total P.
Conclusions
The results strongly suggest “niches” of soil P profiles associated with physical gradients, mostly topographic position, and with the specific distribution of species along this gradient, which is associated with species-specific strategies of soil P mineralisation, mobilisation, use, and uptake.
Peer Review reports
Tropical forests are characterised by high biodiversity and biomass despite growing in strongly weathered soils. All tropical rainforests tend to have high productivity, rapid nutrient turnover, highly weathered soil, and low soil pH [ 1 ]. Tropical regions, such as those in Africa, Asia, and South America, have distinct geological histories that underlie the high biodiversity [ 2 ]. The distribution of plant species and soils are highly variable at local scales within tropical regions [ 3 ]. Several mechanisms have been proposed to explain the coexistence of many plant species in small areas in tropical forest i.e. the high biodiversity observed in tropical forests [ 4 , 5 ]. Many of these mechanisms include heterogeneous disturbances and systems of regeneration [ 6 ]. A diverse topography [ 7 ], species-specific defenses against herbivores [ 8 ], soil traits heterogeneity [ 9 , 10 , 11 ], and differences in nutrient availability [ 10 , 12 , 13 , 14 ] are the other most frequently discussed mechanisms.
The amount of P and its availability limits the productivity of many terrestrial and aquatic ecosystems [ 15 ]. Stocks of total soil P, the chemical forms of P, and P availability in the soil change as ecosystems age and develop, and these transformations can strongly influence ecosystem properties [ 16 , 17 , 18 ]. In particular, biological productivity in wet tropical forests is frequently limited by the availability of soil P [ 19 , 20 ]. Soil taxonomic studies (Soil Survey Staff 2006) classify most tropical forest soils as Oxisols, Ultisols, Alfisols, Inceptisols, or Entisols [ 21 ]. P in these soils usually occurs as organic P and as occluded inorganic forms as part of pedogenic minerals. Occluded forms of P are especially common in older soils (Oxisols, Ultisols, and Alfisols), which frequently have very low or negligible amounts of primary minerals in their profiles and low concentrations of orthophosphate, which is the chemical form of P directly available to plants [ 17 , 22 ]. In contrast, organic P can account for a considerable proportion of total P in tropical mineral soils, accounting for an average of 29 ± 3% of the total P in equatorial forests and in Panama (soil organic P in lowland tropical forests) [ 23 , 24 ].
The turnover of organic P is the primary source of P for microbes and plants (soluble inorganic phosphate ions) in tropical soils [ 25 , 26 ], even though several plant-soil processes can make organic and inorganic/occluded P available to plants, such as mobilisation by roots [ 27 ] or the chemical reduction of iron-phosphate complexes under anaerobic conditions [ 28 ]. Organic P in forest soils is derived from fresh organic matter (leaf litter), microbial biomass, and non-microbial biomass. In general there is evidence suggesting rapid decomposition of leaf litter in the humid tropics (< 1 year; [ 29 ], despite other studies have observed slower rates of leaf litter decomposition (> 1 year) [ 30 ]. Litter nonetheless provides most of the P that supports growth in strongly weathered tropical soils. This phenomenon of “ direct nutrient cycling ” in tropical forests is characterised by the fast release of biologically available phosphate to roots and mycorrhizae by the decomposition of leaf litter and the consequent fast absorption of P by microbes and plants, which is so rapid that the plant-soil P cycle closes, nearly preventing P from being lost via leaching or sorption to iron and aluminium oxides [ 31 ].
Knowledge of the various chemical types of soil organic P and of how plants and microbes use these forms of P is essential to better understand the global cycling and use of P in the plant-soil systems of tropical rainforests. 31 P nuclear magnetic resonance (NMR) is an excellent tool for studying soil organic P because it provides quantitative data and allows the comparison of the various chemical forms of P [ 32 , 33 , 34 , 35 ]. In fact, 31 P NMR allows to discern different molecules where P is present, molecules with different metabolic function such as phosphate mono- and di-esters such as nucleic acids, ATP or acilglyrates involved directly in metabolism pathways, metabolism control and energy transfer from molecules such as orthophosphate, pyrophosphates and polyphosphates, which are involved in P storage. For example, 31 P NMR in soil studies provides data on the proportion of diester P associated with changes in microbial P compounds [ 36 , 37 ] and the ratio of monoester P to diester P, information needed to characterise the lability and rate of turnover of soil organic P.
In these conditions of high species diversity under strong P limitation, we hypothesised, as suggested in previous reports [ 38 ], a long-term adaptation of sympatric species to maximise the capacity of taking up P while avoiding competition. We tested this hypothesis using 31 P NMR spectroscopy in a P metabolomics study to determine the allocation of P to different biological functions in the soil. Our objective was to investigate the variation in soil P profiles along topographic gradients in two tropical forests with highly diverse compositions of tree species for determining whether changes in the profiles of soil organic P were correlated with changes in the composition of tree species and/or with distinct topographic environments and sites. We hypothesised that tree species would use P in specific ways, because the species occupy different functional [ 39 ] and biogeochemical [ 40 ] niches. Biogeochemical niche hypothesis captures niche parameters through species-specific elemental composition and stoichiometry [ 40 , 41 , 42 , 43 ]. The assumptions underlying it are based on the idea that each species is a unique genetic pool of individuals, a product of long-term evolutionary processes, so each species should have a specific structure and functionality (from gene expression to physiological processes). Since the fundamental biological processes (e.g. growth, secondary metabolism, reproduction and storage) have distinct rates in different species, depending on what selection has shaped, the different species have to allocate elements to various traits of tissues and organs differentially. The different use of bio-elements has proved to be even more different in sympatric species as a trait that could avoid direct competition [ 40 ]. Thus, as phosphorus is frequently limiting in tropical rainforests, we should expect that each species tends to have its own P-use strategy (P-metabolome niche) and that this should be underlying the higher species diversity of this high diverse ecosystem. We hypothesized that the tree species would use P in specific ways because each species occupies different functional [ 39 ] and biogeochemical [ 40 ] niches, thus providing species-specific litter that would accordingly modify the 31 P-NMR profile of the underlying soil. Our specific expectations were: (1) species composition would influence the proportions of different P compounds in the underlying soil, and (2) the 31 P NMR profiles would differ amongst and within sites (due to factors such as topography), indicative of differences in P use.
1D 31 P NMR
The spectra of acid-insoluble compounds indicated three main P resonances: monoesters, phospholipids (orthophosphate diester), and DNA. With NMR we were able to determine the family of P-compounds (Fig. 1 ), but we could not determine the exact compounds. Signals from nucleic acids (DNA: −0.37 ppm) and phospholipids were differentiated in the orthophosphate diester region and were readily identified in the soil samples. Inorganic and organic polyphosphates were differentiated by the presence of a signal at − 9 ppm from the α phosphate of organic polyphosphates. Some orthophosphate monoesters, such as mononucleotides derived from RNA and phosphatidyl choline, degraded rapidly to orthophosphate diesters in NaOH-EDTA, although DNA and phospholipids were more stable. The 31 P NMR spectra of NaOH-EDTA extracts from all soil samples generally presented monoesters ( ∼ 25%) at 3.4 to 5.4 ppm, unhydrolysed diesters ( ∼ 20%) at − 1 to 2.3 ppm, and DNA ( ∼ 20%) at − 0.3 ppm. The less abundant forms were polyphosphate ( ∼ 10%) at − 5.6 to − 3.8 ppm, inorganic orthophosphate (hereafter ‘phosphate’; ∼ 10%) at 5.7 to 6.5 ppm, and the pyrophosphate inorganic form of P ( ∼ 5%) at 0.5 to 0.6 ppm. Glucose-6 phosphates had low resonance intensities, at 5.3 to 5.4 ppm.
31 P NMR chromatogram with the different molecules shown in the chromatogram
Soil P compounds and nutrients
In the PCA of organic P compounds; nutrients N, P, and K; enzymatic activity, and ecophysiological variables PC1 axis correlated best with site whereas PC2 correlates with samples of different topographic positions within each site (PC2) (Fig. 2 ). We examined the PCA results to identify the components that correlated best with site for better understanding the relationships of site with organic P compounds and the concentrations of C, N, P, and K. The PC2 axes correlated with topographic position and separated samples from the top, slope, and bottom positions, in Nourague and those of top and slope with respect to those of bottom in Paracou site.
Principal component analyses of soil organic P compounds (green), nutrients (red), d 13 C, d 15 N, enzymatic activities, and ecophysiological variables (black) indicating significant effects of topographic position and site, with corresponding standard deviations Polyphos (polyphosphate); pyrophos (pyrophosphate) polyphosphos (polyphosphonate); orthophos (orthophosphate), orthophosmono (orthophosphate monosester), orthophosphate-diester (orthophosdiester); glucose6phos (glucose-6phosphates); SPAD—chlorophyll content; C—carbon; N—nitrogen; K—potassium; P—phosphorus. The abbreviations in the legend refer to the site (Paracou and Nouragues) and topographic position: T, top; S, slope; and B, bottom
The PCA results also indicated that soil with higher total P concentrations had higher concentrations of organic P compounds involved in storage (polyphosphates and polyphophos) and of C-free forms of P (orthophosphates and pyrophosphates). In contrast, soils with the lowest total P concentrations had higher concentrations of P compounds involved with genetic information, energy transfer, and protein anabolism such as orthophosphate monosester and orthophosphate-diester. Soils at the upper topographic positions had more total P (Table 1 ), which strongly correlated with the activities of acid and alkaline phosphatase, suggesting a greater “investment” of microbes and plants to allocation in P acquisition from soil when the level of total P is high. Tree characteristics also differed amongst the plots at the three topographic positions with wood density tending to be higher in the top plots whereas growth rate, and tree height tending to be higher in the bottom plots (Margalef et al. 2018). Each species occupied a different position in the 2D-plot of the PCA analysis as reinforced by the results of the PERMANOVAS (Table S3 ). The profiles of organic P compounds in the 31 P NMR did not significantly vary between sites (Paracou vs. Nouragues; pseudo-F = 1.93; R 2 = 0.015, P = 0.08), nor in function of topographic positions (top, slope, and bottom; pseudo-F = 1.73; R 2 = 0.027, P = 0.568)) (Table S2 ). The interaction between topographic position and site was neither significant (pseudo-F = 5.68; R 2 = 0.089, P = 0.195) (Table S2 ). Species had a significant effect on 31 P NMR profile (pseudo-F = 3.09; R 2 = 0.53, P < 0.001) (Table S3 ).
The concentrations of the most abundant fractions of P (residual P, total P extractable by NaOH-EDTA, organic P, and inorganic P) varied amongst the soil samples, without any clear trends (Fig. 2 ). The total P concentration was 58–299 mg kg − 1 ; 56–88% was extracted by NaOH-EDTA, and the nonextracted fraction represented ‘residual P’. For the P extracted with NaOH-EDTA, 59–74% was organic and 26–41% was inorganic. Thus, most P was present in organic compounds than in inorganic forms suggesting a high biological use of P. Moreover, among these organic forms the highest proportion of P was found in molecules involved in active functions, such as growth, cellular control, or energy transfer. This is the case of the di-ester forms present in nucleic acid chains or storing energy molecules such as ATP.
The ratio of monoester to diester P was near 1, and diester-2 P was more abundant than diester-1 P, except for soils at the upper topographic position (in which the ratios of monoesters to diesters and diester-1 [phospholipids] to diester-2 [DNA and acid-unstable compounds] in the NaOH-EDTA extracts were similar).
Species-specific utilization of phosphorus
Our findings support the hypothesis that soil phosphorus (P) profiles differ among samples collected beneath different tree species. The soil samples associated with each tree species occupy distinct positions in a 2D plot representing the main functional groups of metabolic P molecules and nutrient concentrations in the soils (Fig. 3 ; Table 2 , and table S4 ). Species accounted for over half of the total variance in 31P NMR data. These consistent results indicate a species-specific pattern of P uptake and utilization, as well as species-specific interactions between trees and microbes (including mycorrhiza) and P transformations specific to the microbes associated with each tree species. Additionally, we observed a clear gradient in P compounds from high anabolic energy to storage, with the highest concentrations of P compounds associated with anabolic energy in soils with the lowest total P concentrations.
Principal component analyses of soil organic P compounds (green), nutrients (red), d 13 C, d 15 N, enzymatic activities, and ecophysiological variables (black) with species scores mean position, with corresponding confidence intervals (95%). Polyphos (polyphosphate); pyrophos (pyrophosphate) polyphosphos (polyphosphonate); orthophos (orthophosphate), orthophosmono (orthophosphate monosester), orthophosphate-diester (orthophosdiester); glucose6phos (glucose-6phosphates); SPAD—chlorophyll content; C—carbon; N—nitrogen; K—potassium; P—phosphorus. The abbreviations in the legend refer to the tree species: Aro, Aniba rosaeodora ; Bpr, Bocoa prouacensis ; Car, Chrysophyllum argenteum ; Cde, Capiro decorticans ; Cfr, Catostemma fragrans ; Cgl, Caryocar glabrum ; Csa, Chrysophyllum sanguinolentum ; Csu, Carapa surimensis ; Ctu, Chimarrhis turbita ; Dgu, Dicorynia guianensis ; Dod, Dipteryx odorata ; Dva, Drypetes variabilis ; Eco, Eschweilera coriacea ; Ede, Eschweilera decolorans ; Efa, Eperua falcata ; Egr, Eperua grandiflora ; Emy, Eugenia ; Hbi, Hirtella bicornis ; hco, Hymanea courbaril ; Lal, Licania alba ; Mco, Moronobea coccinea ; Mve, Micropholis venulosa ; Oas, Oxandra asbeckii ; Peu, Pouteria eugeniifolia ; Pgu, Paloue guianensis ; Pop, Protium opacum ; Ppt, Pradosia ptychandra ; Sel, Sloanea ; Sgr, Sapotaceae grandiflora ; Spr, Sterculia pruriens ; Ssp, Sterculia speciose ; TCl, Tovomita clusiaceae ; Tmy, Tetragastris ; Tpr, Talisia praealta ; Vam Vouacapoua america ; and Vsa, Vochysia sabatieri
Polyphosphates and polyphosphonates are chains of orthophosphates that serve as stored phosphate compounds. Our results revealed higher concentrations of orthophosphate, pyrophosphates, polyphosphonates, and polyphosphates in soils with higher total P concentrations and lower P availability at our study sites. Proportionally, more P was allocated to active metabolic molecules (e.g., DNA) and energy transfer (poly P, pyro P) in soils with lower total P concentrations and higher P availability. Higher concentrations of P storage compounds in the soil can be associated with low P availability, ensuring a controlled source of P for microbes when accessing soil P is challenging [ 44 ]. Moreover, the investment of microbes in building phosphatases was significantly lower in conditions of low carbon (C) and high poly P concentrations. This suggests that high polyphosphate concentrations may be associated with C conservation, particularly in sites where the soil has a high capacity to retain P. In these cases, higher polyphosphate reserves can serve as a P conservation trait, preserving C and providing a biological mechanism for accessing P from P reserves in microbial cells when necessary. Similarly, under conditions of very low C availability, higher PolyP levels may act as a compound for storing energy to conserve C [ 42 ].
Another study in the same ecosystem reported clear species-specific signatures in general foliar metabolomics profiles, indicating functional niches for dominant tree species in the region [ 43 , 44 ]. Our results align with our initial hypothesis that the quantities of different P compounds would differ among soil samples due to interspecific differences in P utilization strategies and cycling. This finding is consistent with previous observations [ 45 , 46 ]. For example, in a Panama rainforest, Condit et al. (2013) found that dry-season intensity and soil phosphorus were the strongest predictors, affecting the distribution of over half of a set of 550 tree species.
Our findings are also in line with previous studies that have identified associations between sets of species and specific habitats in tropical rainforests. The distribution of tree species is influenced by spatial variability in soil properties, such as nutrient availability and topography [ 47 , 48 , 49 , 50 ]. An extension of classical ecological niche theory, known as the “biogeochemical niche hypothesis“ [ 45 ], suggests that each species tends to reach an optimal chemical composition linked to a specific function that allows it to survive in its niche [ 39 , 51 , 52 ]. Our study provides evidence that organic P measurements can be used to characterize soil niches. The measurement and identification of different organic P molecules can serve as a powerful tool for characterizing most P-limited rainforests [ 23 , 53 ]. In these cases, “P-metabolome niches” arise due to soil niche differentiation along natural spatial gradients of biotic and abiotic factors that influence P availability, total concentrations, and chemical forms of P, which, in turn, are associated with P utilization and cycling. Tropical trees may exhibit finely subdivided niches [ 54 ], although direct evidence for measurable variables of overall functional differences among sympatric species and geological processes is currently limited. However, our results clearly indicate that soil beneath each tree species possesses a specific soil P-metabolome. The distinct strategies of individual tree species for P uptake, litter quality, retranslocation, allocation, soil exudates, and biotic interactions with soil microorganisms and other tree species contribute to a micro-scale soil space with a unique P-metabolome profile.
Topography and regional factors
In addition to the differences in P-metabolome profiles in soils associated with different tree species, we also observed relationships between P-metabolome profiles and other factors such as site and topographic position (Fig. 2 ). These relationships suggest that microsite variations in P-metabolome profiles are influenced by biotic conditions and associated with distinct soil traits, including texture, that vary across different topographic situations. Topography creates diverse soil P conditions, with higher total P concentrations and lower P availability at higher elevations, and vice versa at lower elevations. This finding is consistent with more conservative resource-use traits observed at higher elevations, such as the higher wood density of trees, and the opposite pattern at lower elevations. Furthermore, the spread of species along the 2D space generated by the two principal component axes was also associated with these findings. Thus, each species has developed a specific niche for highly efficient P utilization along the gradient, resulting in a distinctive distribution profile of P compounds that maximizes their use efficiency. These results also suggest that a “niche” of P profiles is associated with a physical gradient, primarily determined by topographic positions, and reflects species-specific strategies for mineralizing, mobilizing, utilizing, and acquiring soil P.
The abundance of organic P compounds, such as diesters and phosphonates, varied according to site and topographic position. Specifically, DNA and polyphosphate concentrations increased from the bottom to the top. DNA, polyphosphate, and phosphonates are abundant in soils with high microbial activity [ 31 ]. Site and topographic position explained some of the variance in the P profiles, likely due to clay content, which can form organomineral complexes and better retain organic material, including organic P. Variations in P profiles may also be influenced by metal oxides, as they can strongly occlude P, including organic P molecules. The occasional flooding of the bottom plots may have influenced the redox states of metal oxides (specifically iron and manganese oxides; aluminum oxides are less affected), which, in turn, could have affected the adsorption/desorption dynamics of P fractions in the soil.
Furthermore, the higher presence of mono- and diesters observed in bottom soils with higher sand content aligns with previous findings. A greater presence of monoester phosphorous compounds has been linked to the decomposition of organic matter rich in P metabolites, such as phosphatidylcholine and phospholipids [ 31 , 55 ]. Similarly, higher soil concentrations of diester phosphates have been positively associated with higher concentrations of sand-sized fractions compared to silt and clay fractions [ 56 , 57 ].
Significant variations were observed in the ratios of monoesters to diesters and the ratios of different diesters (diester-1 and diester-2; Table 1 ) across different parts of the 31P NMR spectra. Gressel et al. (1996) suggested that the correlation between monoester P and alkyl C in the organic horizons of a tropical forest soil indicates that the mineralization of monoester P fractions is linked to the decomposition of plant structural components, and that a significant portion of the monoester P in NaOH extracts may originate from hydrolyzed phospholipids derived from plants. The alkaline hydrolysis of phosphatidylcholine to monoesters is well-documented [ 31 , 55 , 58 ]. Soils in semi-arid northern Tanzania [ 59 ], enriched with diesters in sand-sized fractions relative to silt and clay fractions, likely derive these diesters from plants. Labile organic P compounds (e.g., phosphonates and diesters) and phosphate diesters (e.g., DNA and other diesters) often exhibit inverse relationships. For instance, diester P may increase while phosphonates decrease when comparing soils from a native savanna to Oxisol soil of an improved pasture in Colombia [ 36 , 53 ]. Similarly, in a Spodosol soil of a spruce-fir forest, diester P (0 ppm) increased while unidentified diester compounds (1.5 to 2.5 ppm) decreased with increasing decomposition levels [ 46 ].
The top plots had higher concentrations of total P but lower levels of available P due to adsorption onto fine-grained particles, particularly highly reactive P oxides. This occlusion mechanism of P requires greater investments in microbial and root activities to acquire P, which is consistent with the higher activities of acid and alkaline phosphatases observed. However, the higher presence of molecules associated with P storage (orthophosphate, pyrophosphates, and polyphosphate inorganic chains) in these top soils is consistent with a more conservative strategy [ 47 , 48 , 49 , 50 , 51 ], where more P is stored within cells to support biological functions that require P, independent of P uptake from the soil. Furthermore, in top soils, the limited availability of soil P was associated with tree species characterized by higher wood density, typically exhibiting more conservative traits. These findings provide further evidence of the links between soil P status, species distribution, and their life strategies in this tropical rainforest [ 52 , 53 , 54 ].
In conclusion, our study reveals that variations in P-metabolome profiles in soils are not only influenced by different tree species but also by other factors such as site and topographic position. Topography creates distinct soil P conditions, which, combined with the spread of species along the gradient, leads to species-specific strategies for P utilization and uptake. Additionally, the abundance of organic P compounds varies with site and topographic position, influenced by factors such as clay content and metal oxides. The presence of mono- and diesters in soils correlates with organic matter decomposition and sand content. The ratios of different P compounds further highlight the complex dynamics of P cycling in the soil. Our findings contribute to a better understanding of the interactions between vegetation, soil, and P dynamics in tropical rainforest ecosystems.
The P metabolomic profiles of soil samples collected at different sites under trees in French Guianese rainforests differed greatly, with very significant differences amongst soils collected beneath different tree species. This result is consistent with the ecological niche theory and the biogeochemical niche hypothesis (a correspondence of species with specific environmental conditions) in this highly diverse tropical ecosystem. Using 31 P-metabolomic profiles to analyse the functions of different plant species in a community thus allowed us to identify different soil 31 P NMR profiles associated with each tree species.
Our multivariate analyses of the soil 31 P NMR metabolomics data notably indicated a trend to find higher soil concentrations of P biomolecules associated with low P use and high P storage under higher total P concentrations at the top sites, coinciding with tree species with more conservative strategies associated with denser wood and with a soil texture and mineral composition providing high P immobilization capacity. The bottom sites tended, instead, to have higher soil concentrations of P biomolecules associated with biological activity under lower total P concentrations, but higher P-availability, coinciding with tree species with less dense wood and a soil coarser texture.
Our combined analysis of soil elemental composition and P metabolomics provides an improved understanding of environmentally linked shifts in soil P concentrations and availability with different allocations of P for growth and other functions, such as storage, defense, reproduction, or resistance that stress the key role of this element in tropical rainforest functioning. As more P is available, more P is invested in active metabolism and functional activity and less in storage.
French Guiana is on the northeastern coast of South America between 2°10′ and 5°45′N and 51°40′ and 54°30′W. 97% of the region is covered by lowland wet tropical forest [ 55 ]. A pronounced dry season, characterised by < 100 mm precipitation per month, extends from September to November and is associated with the displacement of the intertropical convergence zone. Mean daily temperature is 25.8 °C and varies by only 2 °C throughout the year; daily temperatures vary by 7 °C during the rainy season and by 10 °C in the dry season [ 56 , 57 ].
Field work was conducted at two sites of mature lowland tropical rainforest: the Paracou Research Station (5°18′N, 52°53′W) and the Nouragues Research Station (4°05′N, 52°40′W). Mean annual rainfall is 2990 mm at Nouragues and 3160 mm at Paracou [ 58 ], although the dry season is more severe at Paracou [ 58 ]. Three topographical locations were selected at each site: top of hills (top), middle of slopes at an intermediate elevation (slope), and bottom of slopes at a low elevation, immediately above a creek [ 59 ].
Study plots
We established 12 plots of 0.25 ha at each site stratified by three topographic positions to account for the heterogeneous soil texture: top of the hills, slope and bottom of the valleys, thus with 4 plots in each topographic position. In each plot, we delimited a central 20 × 20 m quadrat where we marked five evenly spaced sampling points around which we focused all our measurements. This design thus contained a total of 120 sampling points (2 sites × 3 topographic positions × 4 replicate plots per topographic position × 5 sampling points in each plot). In each sampling point we annotated the nearest tree in function of the linear distance to the trunk base. The bottom plots at both sites had higher sand contents and lower clay contents than the top and slope plots [ 57 ]. All trees (diameter at breast height ≥ 10 cm) within the 0.25-ha plots were mapped, tagged, and identified to species or genus using herbarium vouchers for determining the richness of the tree species for each plot.
Sample collection
Thus, we collected five soil samples per plot to a depth of 15 cm (Topsoil) in June (wet season) 2015. Each sample was collected using an auger/corer and was a composite of three borings near (< 2 m apart) each other. All voucher specimens were deposited in the Herbarium of International Center for Tropical Botany in Miami, FL 33,199 USA. A total of 120 samples were collected around the trees. We aliquoted 5 g of each sample for 31 P NMR analyses that were immediately placed into a paper bag and frozen in liquid nitrogen before transporting the samples to the laboratory. The rest of soil sample was transported to the lab and stored in plastic zip bags at 4ºC until all the other analyses (within 4 weeks). Soil storage at 4ºC has been shown to keep enzymatic activity of tropical soils better than frozen samples. Fresh soil was sieved to 2 mm; for each sample one part was used for enzymatic activity analyses and the other part was dried 24 h at 105ºC for gravimetric water content. The other soil variables were analyzed from the same soil samples as those used in the 31 P NMR analyses to determine the effect of the tree species.
Environmental biotic and abiotic data
We compiled data for 28 variables describing the pools of soil nutrients, activities of extracellular enzymes, and aboveground tree-community data for each site to characterise the potential micro-environmental and biotic drivers. Nutrient concentrations and ratios, d 13 C, d 15 N, enzymatic activities, and ecophysiological variables are abbreviated as: C, N, P, C:N, C:P, N:P, K, d 13 C, d 15 N, leu (enzymes leucine), gly (enzymes glycine aminopeptidases), alkp (enzymes alkaline phosphatases), acidp (enzymes acid phosphatases), bgluc (enzymes β- glucosidase), p_olsen (amount of soil phosphorus available by Olsen test), p_bray (amount of soil phosphorus available by Bray test), scw_lab (laboratory surface water content), swc_field (field surface water content), surf_temp (soil surface temperature), EC (soil electric conductivity, mS/cm), MBP (microbial biomass P, µg P / g soil DW), MBP_Ke-P (microbial biomass P, µg P / g soil DW with factor KeP = 0.40), MBP_P recov (microbial biomass P, µg P / g soil DW with P-recovery factor), MBP_Ke-P + P recov (microbial biomass P, ug P / g soil DW with P-recovery factor and KeP), TEP (Total extractable P, µg P / g soil DW), TEP_recov P (Total extractable P, µg P / g soil DW with recovery factor P applied ), OEP (Organic extractable P, µg P / g soil DW), and PmgkgLOQ (P concentration in soil, mg/kg Limit of Quantification). All the acronyms used throughout the text are described in Table S1 .
Nutrient pools
We collected soil cores from the topsoil of each sampling point using a soil auger (4 cm in diameter and 15 cm in length; Van Walt, Haslemere, UK) to analyse the nutrient status. The samples were sieved to 2 mm and then freeze-dried (Alpha 1–2 LDplus, Martin Chirst Freeze Dryers, Osterode, Germany). Subsamples were pulverised in a ball mill (MM400, Retsch, Haan, Germany) for the analysis of elemental composition. We weighed 0.15–0.2 g of soil using an MX5 microbalance (Mettler Toledo, Columbus, USA) for determining the concentrations of total carbon (C) and nitrogen (N) by combustion coupled to an isotopic ratio mass spectrometer at the Stable Isotopes Facility (UC Davis, USA). Concentrations of total P and potassium (K) were determined by diluting 0.25 g of soil with an acid mixture of HNO 3 (60%) and H 2 O 2 (30% w/v) and digested in a MARS Xpress microwave oven (CEM Corporation, Matthews, USA). The digested solutions were then diluted to final volumes of 50 mL with ultrapure water and 1% HNO 3 . Blank solutions (5 mL of HNO 3 with 2 mL of H 2 O 2 but no sample biomass) were regularly analysed. The content of each element was determined using inductively coupled plasma/optical emission spectrometry (ICP-OES Optima 4300DV, PerkinElmer, Wellesley, USA). We used the standard certified biomass NIST 1573a to assess the accuracy of the biomass digestion and analytical procedures.
Determination of activities of extracellular enzymes
We determined the activities of the extracellular enzymes β-glucosidase, leucine and glycine aminopeptidases, and acid and alkaline phosphatases (βgluc, leu, gly, acidP, and alkP, respectively) in all the 120 topsoil samples. The activities of these enzymes can serve as proximal variables of microbial nutritional metabolism and depend mostly on the interaction of the relationship between supply and demand with environmental kinetics [ 60 ]. Fresh subsamples of 2-mm sieved soil were stored in ziplock plastic bags and stored at 4 °C until analysis. We quantified the maximum potential activities of each enzyme by colorimetric assays using p -nitrophenylphosphate and p -nitroaniline derivative chromogenic substances. These enzymes are involved in the mineralization of C, N and P (Sinsabaugh and Shah, 2012). β -glucosidase participates in the decomposition of plant tissues, catalyzing the hydrolysis of 1–4 glucosidic bonds of labile cellulose (cellobiose and cellodextrins) to yield glucose. Leucine and glycine amino-peptidases cleave N-terminal residues from proteins and peptides. Acid and alkaline phosphatases release orthophosphate from organic P compounds like labile nucleic acids, phospholipids and inositol phosphates by the hydrolyzation of oxygen-P bonds.
Soil microbial biomass P and soil extractable P
Phosphorus in the microbial biomass (MBP) was measured using the chloroform fumigation extraction method according to Brookes et al. (1982) and Brookes et al. (1985). Two subsamples (10 g fresh weight) of sieved soil were taken for each topsoil sample. One subsample was fumigated for 24 h with chloroform and extracted with 0.5 M of NaHCO 3 (10:1 v:w) after 30 min shaking. The other subsample was directly extracted following the same protocol. The extracts were then filtered with Whatman 42 equivalent paper. Total P content in the extracts was determined after digestion of 2.5 g of aliquot with HNO 3 in a microwave oven (MARS Xpress, CEM Corporation, Matthews, USA). The digested solutions were then diluted to a final volume of 50 mL with ultrapure water and 1% HNO 3 . Blank solutions were regularly analysed in parallel. P concentration in the digested samples and blanks was determined using inductively coupled plasma/optical emission spectrometry (ICP-OES Optima 4300DV, Perkin-Elmer, Wellesley, USA). The microbial biomass P content was calculated from the difference between fumigated and non-fumigated samples and expressed per unit of soil dry mass. Given that inorganic P molecules can absorb onto soil surfaces (organic matter and minerals) it is necessary to account for this effect during the extraction of P from soil, this is done through the application of an empirically determined “P-recovery factor”. Thus, a known amount of inorganic P (12.5 µg of inorganic P added as KH 2 PO 4 ) was added to some of the controls to calculate the P-recovery factor in our soils. Microbial biomass was then calculated using the fumigated and non-fumigated samples corrected with the P-recovery coefficient (MBP_P.recov). Microbial biomass P was also corrected to take into account the efficiency of the fumigation (MBP_Ke.P), using the conversion factor KeP = 0.40 (Jenkinson et al. 2004). The factor KeP is an empirical coefficient used to relate the quantity of material solubilized by chloroform to the size of the original biomass, in this case it indicates that with fumigation 40% of the biomass-P is extracted as inorganic P. We have used all the microbial biomass P variables with and without corrections (MBP, MBP_P.recov, MBP_Ke.P and MBP_Ke.P…P.recov) in the analyses to give an overview of the methodological issues related to the quantification of extractable P in soils and microbes and to provide additional information for those readers with a background in soil biogeochemistry.
The total P concentration measured in non-fumigated bicarbonate-extracts was referred as the total extractable P (TEP) fraction. One aliquot of the non-fumigated extracts was used to determine the inorganic extractable P (P_Olsen) by Olsen’s method (Watanabe and Olsen, 1965). The organic extractable P (OEP) in the non-fumigated extracts was calculated as the difference between TEP and P_Olsen. All bicarbonate-extractable P fractions were expressed as µg of P per gram dry soil. Soil extractable P was also determined with Bray-P (P_Bray) acid fluoride extraction (Bray and Kurtz 1945) in oven-dried soil subsamples.
Sample processing for 31 P NMR analysis
The soils were frozen in liquid nitrogen, lyophilised, and stored in paper bags at -80 °C. The samples were ground with a ball mill at 1500 rpm for 3 min, and the fine powder was stored at -80 °C until extraction of the metabolites.
One-dimensional 31 P NMR
Conventional one-dimensional (1D) 31 P NMR (Box 1) was used to quantify the main organic and inorganic forms of extractable P using NaOH-EDTA, including DNA, total diesters and monoesters, phosphonates, pyrophosphate, and polyphosphate. All the identification of the target P classes was based on chemical shifts and previous reported data (Turner et al. 2003; Vestergren et al. 2012). P was extracted by shaking 1.5 g of dry ground soil from each composite sample for 4 h in 30 mL of a solution containing 250 mM NaOH and 50 mM Na 2 EDTA (Cade-Menun and Preston 1996). The extracts were centrifuged at 14 000 g for 30 min, and the supernatant (23 mL) was frozen at − 80 °C overnight and then lyophilised. Lyophilisation yielded 750 ± 50 mg of material, 80 mg of which was redissolved in 640 µL (1:8 w/v) of a solution containing 530 µL of D 2 O, 10 µL of 14.2 M NaOD, and 50 µL of 16 mM methylene diphosphonic acid trisodium salt (MDPA, Sigma-Aldrich product number M1886). The MDPA served as a P reference for quantifying individual compounds, and each 50-µL spike contained 50 µg of P. The redissolved solution was vortexed for 2 min and centrifuged at 10 000 g for 5 min, and 560 µL was then transferred to a 5-mm NMR tube for 1D 31 P NMR.
NMR spectra for 31 P were obtained using an Avance III 600 MHz spectrometer (Bruker, Ettlingen, Germany) operating at 161.76 MHz. NaOH-EDTA extracts were analysed using a 3.9-µs pulse (90°), a relaxation delay time of 2.0 s, an acquisition time of 0.9 s, and broadband proton decoupling. We recorded 15 000 scans per sample, and the experimental time was 12.5 h. Spectra were processed with a line broadening of 2 Hz, and chemical shifts of signals were determined in parts per million (ppm) relative to an external standard (85% orthophosphoric acid, H 3 PO 4 ). The main chemical types of P compounds were identified based on previously reported chemical shifts [ 32 , 61 ]. Peaks were first identified using an automatic procedure for fitting peaks; peaks that were clearly visible were manually selected using TopSpin 2.0 NMR software (Bruker, Germany). Signal areas were calculated by the deconvolution and integration of individual peaks. Concentrations of P compounds (mg P kg –1 soil) were calculated using the known P concentration of MDPA spiked in the sample, and concentrations per weight of air-dried soil were given. All NMR spectra were processed using TopSpin 2.0.
Signal intensity in the 31 P spectra was assigned to the different types of P by first integrating across the following regions of broad chemical shifts: -21.5 to -18.5 ppm for nonterminal polyphosphate (poly P), -5.3 to -4.8 ppm for pyrophosphate (pyro P), -4.8 to -4.0 for terminal poly P, -1.5 to 2.5 for diester-P, and 2.5 to 7 ppm for orthophosphate (ortho-P and monoester-P). Deconvolution was then used to determine the intensity of up to 16 resonances in the ortho-P and monoester-P regions. Deconvolution analysis began by manually identifying chemical shifts of peaks and shoulders. Peak chemical shifts varied only slightly amongst the samples. The orthophosphate signal shifted the most (range: 5.56–5.74 ppm), which shifted amongst soil samples, sites, species, and topographic positions. This variation was most likely due to slight differences in pH amongst the samples, because the orthophosphate peak is highly sensitive to variation in pH [ 62 ]. Thus, 31 P NMR allows the determination of the family of P-compounds (Fig. 3 ), but not of the exact compounds.
31 P nuclear magnetic resonance ( 31 P-NMR) is an analytical NMR technique that allows the detection of both the concentration and the chemical form of the most abundant phosphorus isotope, 31 P, in the analytical sample. In the concrete case of 31 P this analytical tool allows to separately detect the intensity of the signal (proportional to concentration in the analyzed sample) of each molecular structure where the 31 P is located. In this way, we can detect the most abundant types of compounds containing 31 P in biological samples. We explored different aqueous/organic solvent methods to extract as many groups of compounds as possible and the preliminary results showed that using an aqueous solution allowed extracting most of groups compounds. Standard 1D 31 P NMR was used to quantify concentrations of the main organic and inorganic P classes, including DNA, total orthophosphate diesters and monoesters, phosphonates, pyrophosphate and polyphosphate. Phosphorus was extracted by shaking 1.5 g of dry and ground soil from each composite soil sample in 30 mL of a solution containing 250 mM NaOH and 50 mM Na2EDTA (ethylenediaminetetraacetate) for 4 h (Cade-Menun and Preston 1996). Identification of the target P classes was based on chemical shifts and previous reported data [ 32 , 63 ].
Statistical analyses
The relationships of the P compounds with species, site and topographic position were identified by a PERMANOVA [ 64 ] of the NMR data for each soil sample. Euclidean distance, species identity, site, and topographic position were the fixed factors, and plot was a random effect, with 2000 permutations. Differences amongst species, site, and topographic position and amongst the P compounds most responsible for these differences were determined by comparing the areas of the different metabolite peaks normalized relatively to internal standards. Principal component analyses (PCAs) were used for processing the “omic” data sets together with the other potential influencing soil parameters, to detect the part of the variance explained by species [ 65 ]. Pearson’s correlation was used to identify the relationships of the 1D NMR measurements and the relationships between the concentrations of nutrients and organic P compounds. Error estimates are standard errors of the mean unless otherwise stated.
All statistical procedures were performed using R v 3.5 ( www.r-project.org ) with the SEQKNN, VEGAN, FACTOEXTRA, FACTOMINER, DPLYR, RANDOMFOREST, and MIXOMICS packages.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Baraloto C, Rabaud S, Molto Q, Blanc L, Fortunel C, Harault B, et al. Disentangling stand and environmental correlates of aboveground biomass in amazonian forests. Glob Chang Biol. 2011;17:2677–88.
Article Google Scholar
Corlett RT, Primack RB. Tropical rainforests and the need for cross-continental comparisons. Trends Ecol Evol. 2006;21:105–7.
Vitousek PM, Sanford RL Jr. Nutrient cycling in moist tropical forest. Ann Rev Ecol Syst. 1986;17:137–67.
Rothstein DE, Vitousek PM, Simmons BL. An exotic Tree alters Decomposition and Nutrient Cycling in a hawaiian montane forest. Ecosystems. 2004;7:805–14.
Article CAS Google Scholar
Allison SD, Vitousek PM. Rapid nutrient cycling in leaf litter from invasive plants in Hawai. Oecologia. 2004;141:612–9.
Article PubMed Google Scholar
Connell JH. Diversity in tropical rain forests and coral reefs. Science (1979). 1978;199:1302–10.
Mandl NA, Kessler M, Robbert Gradstein S. Effects of environmental heterogeneity on species diversity and composition of terrestrial bryophyte assemblages in tropical montane forests of southern Ecuador. Plant Ecol Divers. 2009;2:313–21.
Becerra JX. On the factors that promote the diversity of herbivorous insects and plants in tropical forests. Proc Natl Acad Sci. 2015;112:6098–103.
Article CAS PubMed PubMed Central Google Scholar
Aiba SI, Sawada Y, Takyu M, Eino T, Kitayama K, Repin R. Structure, floristics and diversity of tropical montane rain forests over ultramafic soils on Mount Kinabalu (Borneo) compared with those on non-ultramafic soils. Aust J Bot. 2015;63:191–203.
John R, Dalling JW, Harms KE, Yavitt JB, Stallard RF, Mirabello M, et al. Soil nutrients influence spatial distributions of tropical tree species. Proc Natl Acad Sci U S A. 2007;104:864–9.
Martins KG, Marques MCM, dos Santos E, Marques R. Effects of soil conditions on the diversity of tropical forests across a successional gradient. Ecol Manage. 2015;349:4–11.
LeBauer DS, Treseder KK. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology. 2008;89:371–9.
Fujii K, Shibata M, Kitajima K, Ichie T, Kitayama K, Turner BL. Plant–soil interactions maintain biodiversity and functions of tropical forest ecosystems. Ecol Res. 2017;33:1–12.
Google Scholar
Xu W, Ci X, Song C, He T, Zhang W, Li Q, et al. Soil phosphorus heterogeneity promotes tree species diversity and phylogenetic clustering in a tropical seasonal rainforest. Ecol Evol. 2016;6:8719–26.
Article PubMed PubMed Central Google Scholar
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, et al. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett. 2007;10:1135–42.
Wardle DA, Walker LR, Bardgett RD. Ecosystem Properties and Forest decline in contrasting long-term. Sci (1979). 2004;305:509–14.
Walker TW, Syers JK. The fate of phosphorus during pedogenesis. Geoderma. 1976;15:1–19.
Kichenin E, Wardle DA, Peltzer DA, Morse CW, Freschet GT. Contrasting effects of plant inter- and intraspecific variation on community-level trait measures along an environmental gradient. Funct Ecol. 2013;27:1254–61.
Tanner EVJ, Vitousek PM, Cuevas E. Experimental investigation of nutrient limitation of Forest Growth on Wet Tropical mountains. Ecology. 1998;79:10–22.
Vitousek PM. Nutrient cycling and limitation: Hawai ‘i as a model system. Princeton. Princeton, New Jersey, USA.; 2004.
Sanchez PA, Logan TJ. Myths and science about the Chemistry and Fertility of soils in the tropics. SSSA Special Publication. 1992;29:35–35.
CAS Google Scholar
Tiessen H, Stewart JWB, Cole CV. Pathways of Phosphorus transformations in Soils of Differing Pedogenesis 1. Soil Sci Soc Am J. 1984;48:853–8.
Harrison AF. Soil organic phosphorus: a review of world literature. Commonweal. 1987.
Turner BL, Engelbrecht BMJ. Soil organic phosphorus in lowland tropical rain forests. Biogeochemistry. 2011;103:297–315.
Tiessen H, Salcedo IH, Sampaio EVSB. Nutrient and soil organic matter dynamics under shifting cultivation in semi-arid northeastern. Agric Ecosyst Environ. 1992;38:139–51.
Johnson AH, Frizano J, Vann DR. Biogeochemical implications of labile phosphorus in forest soils determined by the Hedley fractionation procedure. Oecologia. 2003;135:487–99.
Cade-Menun BJ. Characterizing phosphorus in environmental and agricultural samples by 31P nuclear magnetic resonance spectroscopy. Talanta. 2005;66:359–71.
Article CAS PubMed Google Scholar
Chacon N, Silver WL, Dubinsky EA, Cusack DF. Iron reduction and soil phosphorus solubilization in humid tropical forests soils: the roles of labile carbon pools and an electron shuttle compound. Biogeochemistry. 2006;78:67–84.
Sampaio EVDSB, Dall’Olio A, Nunes KS, de Lemos EEP. A model of litterfall, litter layer losses and mass transfer in a humid tropical forest at Pernambuco, Brazil. J Trop Ecol. 1993;9:291–301.
HaTtenschwiler S, Coq S, Barantal S, Handa IT. Leaf traits and decomposition in tropical rainforests: revisiting some commonly held views and towards a new hypothesis. New Phytol. 2011;189:950–65.
Stark NM, Jordan CF. Nutrient Retention by the Root Mat of an amazonian rain forest author (s): Nellie M. Stark and Carl F. Jordan published by : Ecological Society of America NUTRIENT RETENTION BY THE ROOT MAT OF AN. Ecology. 1978;59:434–7.
Turner BL, Mahieu N, Condron LM. Phosphorus-31 nuclear magnetic resonance spectral assignments of Phosphorus compounds in Soil NaOH–EDTA extracts. Soil Sci Soc Am J. 2003;67:497–510.
Cade-Menun BJ, Liu CW, Nunlist R, McColl JG. Soil and litter Phosphorus-31 nuclear magnetic resonance spectroscopy. J Environ Qual. 2002;31:457–65.
CAS PubMed Google Scholar
Vincent AG, Turner BL, Tanner EVJ. Soil organic phosphorus dynamics following perturbation of litter cycling in a tropical moist forest. Eur J Soil Sci. 2010;61:48–57.
Vestergren J, Vincent AG, Jansson M, Persson P, Ilstedt U, Giesler R. High-resolution characterization of Organic Phosphorus in Soil extracts using 2D 1 H – 31 P NMR correlation spectroscopy. Environ Sci Technol. 2012;46:3950–6.
Sumann M, Amelung W, Haumaier L, Zech W. Climatic effects on Soil Organic Phosphorus in the North American Great Plains identified by Phosphorus-31 nuclear magnetic resonance. Soil Sci Soc Am J. 1998;62:1580–6.
Guggenberger G, Christensen BT, Rubaek G, Zech W. Land-use and fertilization effects on P forms in two EuroPean soils: resin extraction and 31P-NMR analysis. Eur J Soil Sci. 1996;47:605–14.
Turner BL. Soil organic phosphorus in tropical forests: an assessment of the NaOH-EDTA extraction procedure for quantitative analysis by solution 31P NMR spectroscopy. Eur J Soil Sci. 2008;59:453–66.
Díaz S, Kattge J, Cornelissen JHC, Wright IJ, Lavorel S, Dray S, et al. The global spectrum of plant form and function. Nature. 2016;529:167–71.
Peñuelas J, Fernández-Martinez M, Ciais P, Jou D, Piao S, Obersteiner M, et al. The bioelements, the elementome, and the biogeochemical niche. Ecology. 2019;100:e02652.
Peñuelas J, Sardans J, Ogaya R, Estiarte M. Nutrient stoichiometric relations and biogeochemical niche in coexisting plant species: Effect of simulated climate change. Pol J Ecol. 2008;56:613–22.
Penuelas J, Sardans J, Llusià J, Owen SM, Carnicer J, Giambelluca TW, et al. Faster returns on ‘leaf economics’ and different biogeochemical niche in invasive compared with native plant species. Glob Chang Biol. 2010;16:2171–85.
Sardans J, Peñuelas J. Climate and taxonomy underlie different elemental concentrations and stoichiometries of forest species: the optimum biogeochemical niche. Plant Ecol. 2014;215:441–55.
Turner BL, Haygarth PM. Phosphatase activity in temperate pasture soils: potential regulation of labile organic phosphorus turnover by phosphodiesterase activity. Sci Total Environ. 2005;344:27–36.
Ecological Niches: Linking Classical and Contemporary Approaches - Jonathan M. Chase,Mathew A. Leibold - Google Libros. University of Chica….
Turner BL, Brenes-Arguedas T, Condit R. Pervasive phosphorus limitation of tree species but not communities in tropical forests. Nature. 2018;555:367–70.
Sabatier D, Grimaldi M, Prévost MF, Guillaume J, Godron M, Dosso M, et al. The influence of soil cover organization on the floristic and structural heterogeneity of a guianan rain forest. Plant Ecol. 1997;131:81–108.
Pélissier R, Dray S, Sabatier D. Within-plot relationships between tree species occurrences and hydrological soil constraints: an example in French Guiana investigated through canonical correlation analysis. Plant Ecol. 2002;162:143–56.
Moulatlet GM, Rennó CD, Figueiredo FOG, Ruokolainen K, Banon L, Emilio T, et al. The role of topographic-derived hydrological variables in explaining plant species distributions in Amazonia. Acta Amazon. 2022;52:218–28.
Lopez OR, Kursar TA. Does flood tolerance explain tree species distribution in tropical seasonally flooded habitats? Oecologia. 2003;136:193–204.
Lescure J-P, Boulet R. Relationships between Soil and Vegetation in a tropical rain forest in French Guiana. Biotropica. 1985;17:155.
Fritsch E, Herbillon AJ, Do Nascimento NR, Grimaldi M, Melfi AJ. From Plinthic Acrisols to Plinthosols and Gleysols: iron and groundwater dynamics in the tertiary sediments of the upper Amazon basin. Eur J Soil Sci. 2007;58:989–1006.
Chacon N, Flores S, Gonzalez A. Implications of iron solubilization on soil phosphorus release in seasonally flooded forests of the lower Orinoco River, Venezuela. Soil Biol Biochem. 2006;38:1494–9.
Allié E, Pélissier R, Engel J, Petronelli P, Freycon V, Deblauwe V, et al. Pervasive Local-Scale Tree-Soil Habitat Association in a Tropical Forest Community. PLoS ONE. 2015;10:e0141488.
Me Chave JR, Ra BR, Dubois M-A. Estimation of biomass in a neotropical forest of French Guiana: spatial and temporal variability. J Trop Ecol. 2001;17:79–96.
Gourlet-Fleury S, Guehl J-M, Laroussinie O. Ecology and management of a neotropical rainforest: lessons drawn from Paracou, a long-term experimental research site in French Guiana. Elseiver. 2004.
Courtois EA, Stahl C, Van den Berge J, Bréchet L, Van Langenhove L, Richter A, et al. Spatial variation of Soil CO2, CH4 and N2O Fluxes across Topographical Positions in Tropical Forests of the Guiana Shield. Ecosystems. 2018. https://doi.org/10.1007/s10021-018-0232-6 .
Aguilos M, Hérault B, Burban B, Wagner F, Bonal D. Agricultural and Forest Meteorology what drives long-term variations in carbon fl ux and balance in a tropical rainforest in French Guiana ? Agric Meteorol. 2018;253–4 February:114–23.
Soong JL, Janssens IA, Grau O, Margalef O, Stahl C, Van Langenhove L, et al. Soil properties explain tree growth and mortality, but not biomass, across phosphorus-depleted tropical forests. Sci Rep 2020. 2020;10:1.
Sinsabaugh RL, Follstad Shah JJ. Ecoenzymatic Stoichiometry and Ecological Theory. Annu Rev Ecol Evol Syst. 2012;43:313–43.
Vestergren J, Vincent AG, Jansson M, Persson P, Ilstedt U, Gröbner G, et al. High-resolution characterization of Organic Phosphorus in Soil extracts using 2D 1 H– 31 P NMR correlation spectroscopy. Environ Sci Technol. 2012;46:3950–6.
Smernik RJ, Doolette AL, Marschner P, Bu EK, Stonor R, Wakelin SA et al. Soil Biology & Biochemistry Forms of phosphorus in bacteria and fungi isolated from two Australian soils. 2008;40:1908–15.
Vestergren J, Vincent AG, Jansson M, Persson P, Ilstedt U, Gröbner G, et al. High-resolution characterization of organic phosphorus in soil extracts using 2D 1H-31P NMR correlation spectroscopy. Environ Sci Technol. 2012;46:3950–6.
Anderson MJ, Gorley RN, Clarke KR, PERMANOVA + for. PRI-MER: Guide to Software and Statistical Methods. Primer-E. Plymouth,UK.; 2008.
Smith CA, Want EJ, ’maille O, Abagyan G, Siuzdak R. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling using nonlinear peak alignment, matching, and identification. Anal Chem. 2006;78:779–87.
Download references
Acknowledgements
We appreciate the cooperation of all the people that help in anything related with this research.
This research was supported by the European Research Council Synergy grant ERC-2013-SyG-610028 IMBALANCE-P, the European FP7 S-Clima project PIEF-GA-2013-626234, the Spanish Government grant PID2019-110521GB-I00, the Fundacion Ramon Areces grant ELEMENTAL-CLIMATE, and the Catalan Government grant SGR 2017 − 1005. We thank the staff of the Nouragues station managed by USR mixte LEEISA (CNRS; Cayenne) and the Paracou station managed by UMR Ecofog (CIRAD, INRA; Kourou). Both research stations benefit from “Investissement d’Avenir” grants managed by Agence Nationale de la Recherche (CEBA: ANR-10-LABX-25-01; ANAEE-France: ANR-11-INBS-0001). A.G.G. was supported by the Ministry of Education, Youth and Sports of CR within the project Mobility CzechGlobe, CZ.02.2.69/0.0/0.0/16_027/0008137. A.G.G. and O.U. were also supported by the project SustES (CZ.02.1.01/0.0/0.0/16_019/0000797).
Author information
Authors and affiliations.
Global Change Research Institute, The Czech Academy of Sciences, Belidla 986/4a, Brno, CZ-60300, Czech Republic
Albert Gargallo-Garriga
Universitat Autònoma de Barcelona, Bellaterra, 08193, Spain
Albert Gargallo-Garriga & Marta Ayala-Roque
Global Ecology Unit, CSIC, CREAF-CSIC-UAB, Bellaterra, 08193, Catalonia, Spain
Jordi Sardans, Joan Llusià, Guille Peguero, Otmar Urban, Karel Klem & Josep Peñuelas
CREAF, Cerdanyola del vallès, Barcelona, Catalonia, 08193, Spain
Jordi Sardans, Joan Llusià, Guille Peguero & Josep Peñuelas
Centre of Excellence PLECO (Plants and Ecosystems), Department of Biology, University of Antwerp, Wilrijk, Belgium
Elodie A. Courtois & Ivan A. Janssens
Laboratoire écologie, évolution, Interactions des Systèmes Amazoniens (LEEISA), Université de Guyane, CNRS, IFREMER, Cayenne, France
Elodie A. Courtois
UMR ECOFOG - Ecologie des forêts de Guyane, Kourou cedex, 97379, France
Clément Stahl
Servei de Ressonància Magnètica Nuclear, Universitat Autònoma de Barcelona, Bellaterra, 08193, Spain
Pau Nolis, Miriam Pérez-Trujillo & Teodor Parella
Centre for Microbiology and Environmental Systems Science, University of Vienna, Althanstr. 14, Vienna, 1090, Austria
Andreas Richter
You can also search for this author in PubMed Google Scholar
Contributions
A.G.G., J.S. and J.P. designed the study with the help of all co-authors. A.G.G., J.L., G.P., M.A.R, E.C., C.S., O.U., K.K., P.N., M.P., T.P., A.R., I.J., J.S. and J.P. authors participated in the field measurements or chemical and statistical analyses. All authors contributed to the writing of the manuscript and the drafting of the figures. All authors read and approved the final version of the manuscript.
Corresponding author
Correspondence to Albert Gargallo-Garriga .
Ethics declarations
Ethical approval and consent to participate.
The owner gave permission for sampling.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Gargallo-Garriga, A., Sardans, J., Llusià, J. et al. Different profiles of soil phosphorous compounds depending on tree species and availability of soil phosphorus in a tropical rainforest in French Guiana. BMC Plant Biol 24 , 278 (2024). https://doi.org/10.1186/s12870-024-04907-x
Download citation
Received : 20 January 2023
Accepted : 13 March 2024
Published : 12 April 2024
DOI : https://doi.org/10.1186/s12870-024-04907-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- P metabolomic niche
- Metabolomics
BMC Plant Biology
ISSN: 1471-2229
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
The role of the hypothesis of an energy system in the human body. First, the hypothesis that there is an energy system in the human body would lead to a more comprehensive and in-depth understanding of TCM theory and methodology. Further, such a hypothesis could help to explain the phenomena of life, the universe, and nature.
A hypothesis is a tentative, testable answer to a scientific question. Once a scientist has a scientific question she is interested in, the scientist reads up to find out what is already known on the topic. Then she uses that information to form a tentative answer to her scientific question. Sometimes people refer to the tentative answer as "an ...
The zero-energy universe hypothesis is the simplest explanation of this, as outlined in 1973 by Edward Tryon, who was the first to suggest that our universe is the result of a quantum fluctuation.
2.1. What is renewable energy led economic growth hypothesis? In agreement with Bercu et al. (Citation 2019) energy and its consumption has a significant impact on the development of a sustainable economy.Several amount studies for different countries confirmed the energy-led growth hypothesis i.e., renewable energy is the driver of economic growth (Singh et al., Citation 2019).
And the current energy consumption of the United States, and given the hypothesis that solar energy will be the dominant source of energy generation, we will assume that 51% of this energy will need to be produced by solar: [3] 1.055 × 10 20 Joules × 0.51 = 5.38 × 10 19 Joules So this can lead us to how many square meters of land needs to be ...
In this work, we reflect upon the energy balance hypothesis of obesity. International organizations, the general population and many scientists hold the belief that obesity is indisputably caused ...
Although this hypothesis is widely accepted, empirical support so far has been equivocal. ... Isler, K. & van Schaik, C. P. Costs of encephalisation: the energy trade-off hypothesis tested on ...
Nonetheless, the Energy Homeostasis Principle allowed us to propose this hypothesis (Figure 5C), and it can be empirically addressed. Naturally, using the same experimental approach, we can evaluate how plasticity is affected by energetic demands induced electrically or by altering neurotransmitter concentrations.
5. Phrase your hypothesis in three ways. To identify the variables, you can write a simple prediction in if…then form. The first part of the sentence states the independent variable and the second part states the dependent variable. If a first-year student starts attending more lectures, then their exam scores will improve.
This study examines the effects of clean energy consumption, green innovation, and technological diffusion on environmental sustainability from 1990 to 2018 in selected European Union countries. This relationship has been frequently examined in the literature within the framework of the environmental Kuznets curve (EKC) hypothesis.
Write your hypothesis in plain language. The goal in this step is to take a stance on the answer to the question posed in Step 1. Following the example given above, a viable hypothesis is: "Yes, PM2.5 concentrations are at unsafe levels.". This statement doesn't need to be correct, since the rest of the hypothesis testing procedure will ...
The Earth formed roughly 4.5. . billion years ago, and life probably began between 3.5. . and 3.9. . billion years ago. The Oparin-Haldane hypothesis suggests that life arose gradually from inorganic molecules, with "building blocks" like amino acids forming first and then combining to make complex polymers.
The PL hypothesis (PLH) suggests that the dilution of protein in energy-dense foods, particularly those rich in carbohydrates and fats, combines with protein leverage to contribute to the global ...
For classes familar with the equation q = m × C × Δ T, where q is the heat energy, m the mass of water heated, C the specific heat of water (4.2 J g -1 deg -1) and Δ T the temperature rise, this can be used to calculate the energy (in J) absorbed by the water each time. Dividing this by the mass of foodstuff burnt gives the heat energy ...
The environmental Kuznets curve (EKC) has been analyzed in many studies, but none of them has focused on countries with high energy imports. COP27 reiterated that fossil fuels are the main cause of carbon dioxide (CO2) emissions and highlighted the importance of net-zero targets for CO2 reduction by 2050. Analyzing the determinants of CO2 emissions in countries that import a lot of fossil ...
Electrical circuit with lemons. A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb. We're going "Back To School ...
The causal relationship between economic growth and renewable energy development is also explained in the literature in the form of four testable hypotheses: growth hypothesis, conservation ...
Chromatography. Create a hypothesis for this experiment. During photosynthesis, light energy is absorbed by photosynthetic pigments. Chlorophyll A is the main photosynthetic pigment but chlorophyll B, carotenes, and xanthophylls also absorb light.
A hypothesis for obtaining energy from gravity in conjunction with time [closed] Ask Question Asked 4 years, 2 months ago. Modified 4 years, 2 months ago. ... The ball has more energy than it did before, because you put energy into the ball at this step. Specifically, you put in $10000-1960=8040$ J of energy ...
Full-text available. Nov 2010. Nikhil Kaza. Residential energy consumption accounts for 22% of the total energy consumption in the US. However, the impacts of local planning policies, such as ...
2013. TLDR. The purpose of the work described in this thesis is to investigate how the performance of the PC is achieved, with reference to structure, mechanical properties and possible transduction mechanisms, and to provide novel insights into the three-dimensional structure of the intact corpuscle. Expand. 1.
A new hypothesis for the origin of eukaryotic cells is proposed, based on the comparative biochemistry of energy metabolism. Eukaryotes are suggested to have arisen through symbiotic association ...
Environmental degradation and economic growth are two intricately related issues whose impact is in constant increase within a global context marked by climate risks and corruption, notably in certain African countries. This research work examines the impacts of economic growth, corruption, renewable energy, and foreign direct investment on carbon dioxide emissions for a set of West African ...
energy is dissipated within the equine foot at ground impact. Our hypothesis proposes that the high transient energy forces. produced within the horse's foot are dissipated via the rapid flow. and movement of blood through an extensive and tortuous. vascular network of small caliber veno-venous anastomoses.
The exact sources of high-energy neutrinos detected by the IceCube neutrino observatory still remain a mystery. For the first time, this work explores the hypothesis that galaxy mergers may serve as sources for these high-energy neutrinos. Galaxy mergers can host very high-energy hadronic and photohadronic processes, which may produce very high-energy neutrinos. We perform an unbinned maximum ...
Masdar is a global leader in renewable energy and sustainable urban development. Over the last decade, we have pioneered commercially viable solutions in clean energy, sustainable real estate and ...
Soil P compounds and nutrients. In the PCA of organic P compounds; nutrients N, P, and K; enzymatic activity, and ecophysiological variables PC1 axis correlated best with site whereas PC2 correlates with samples of different topographic positions within each site (PC2) (Fig. 2).We examined the PCA results to identify the components that correlated best with site for better understanding the ...