Vitamin Assignment
Visit this website: http://www.healthaliciousness.com/
Peruse it to see all of the foods with their mineral contents. Select 3 veggies, 3 fruits, and 3 fish to investigate. Then make columns or a spreadsheet with these 9 foods and their Daily Values. Find the following information for each food, and compare side by side on the worksheet.
Daily Value of:
- caloric content
- fat content
- sodium content
- fiber content
- top five minerals
In a brief paragraph, be sure to write why these particular foods were selected and what was discovered about the foods and their nutrients.
Peruse it to see all of the foods with their vitamin contents. Select 3 veggies, 3 fruits, and 3 fish to investigate. Then make columns or a spreadsheet with these 9 foods and their Daily Values. Find the following information for each food and compare side by side on the worksheet.
- list the top five vitamins
In a brief paragraph, be sure to write why these particular foods were chosen and what was discovered about the foods and their nutrients.

Search code, repositories, users, issues, pull requests...
Provide feedback.
We read every piece of feedback, and take your input very seriously.
Saved searches
Use saved searches to filter your results more quickly.
To see all available qualifiers, see our documentation .
- Notifications
VIT Assignments for Matlab and stuff. I store my usual bullshit here.
gaganmalvi/matlab_examples
Folders and files, contributors 2.
- MATLAB 94.7%
Vitamins and Minerals

Vitamins and minerals are micronutrients required by the body to carry out a range of normal functions. However, these micronutrients are not produced in our bodies and must be derived from the food we eat.
Vitamins are organic substances that are generally classified as either fat soluble or water soluble. Fat-soluble vitamins ( vitamin A , vitamin D , vitamin E , and vitamin K ) dissolve in fat and tend to accumulate in the body. Water-soluble vitamins ( vitamin C and the B-complex vitamins , such as vitamin B6 , vitamin B12 , and folate ) must dissolve in water before they can be absorbed by the body, and therefore cannot be stored. Any water-soluble vitamins unused by the body is primarily lost through urine.
Minerals are inorganic elements present in soil and water, which are absorbed by plants or consumed by animals. While you’re likely familiar with calcium , sodium , and potassium , there is a range of other minerals, including trace minerals (e.g. copper , iodine , and zinc ) needed in very small amounts.
In the U.S., the National Academy of Medicine (formerly the Institute of Medicine) develops nutrient reference values called the Dietary Reference Intakes (DRIs) for vitamins and minerals. [1] These are intended as a guide for good nutrition and as a scientific basis for the development of food guidelines in both the U.S. and Canada. The DRIs are specific to age, gender, and life stages, and cover more than 40 nutrient substances. The guidelines are based on available reports of deficiency and toxicity of each nutrient. Learn more about vitamins and minerals and their recommended intakes in the table below.
What about multivitamins?
A diet that includes plenty of fruits, vegetables , whole grains , good protein packages , and healthful fats should provide most of the nutrients needed for good health. But not everyone manages to eat a healthful diet. Multivitamins can play an important role when nutritional requirements are not met through diet alone. Learn more about vitamin supplementation .
Did you know?
Vitamins and their precise requirements have been controversial since their discovery in the late 1800s and early 1900s. It was the combined efforts of epidemiologists, physicians, chemists, and physiologists that led to our modern day understanding of vitamins and minerals. After years of observation, experiments, and trial and error, they were able to distinguish that some diseases were not caused by infections or toxins—a common belief at the time—but by vitamin deficiencies. [2] Chemists worked to identify a vitamin’s chemical structure so it could be replicated. Soon after, researchers determined specific amounts of vitamins needed to avoid diseases of deficiency.
In 1912, biochemist Casimir Funk was the first to coin the term “vitamin” in a research publication that was accepted by the medical community, derived from “vita” meaning life, and “amine” referring to a nitrogenous substance essential for life. [3] Funk is considered the father of vitamin therapy, as he identified nutritional components that were missing in diseases of deficiency like scurvy (too little vitamin C ), beri-beri (too little vitamin B1 ), pellagra (too little vitamin B3 ), and rickets (too little vitamin D ). The discovery of all vitamins occurred by 1948.
Vitamins were obtained only from food until the 1930s when commercially made supplements of certain vitamins became available. The U.S government also began fortifying foods with specific nutrients to prevent deficiencies common at the time, such as adding iodine to salt to prevent goiter, and adding folic acid to grain products to reduce birth defects during pregnancy. In the 1950s, most vitamins and multivitamins were available for sale to the general public to prevent deficiencies, some receiving a good amount of marketing in popular magazines such as promoting cod liver oil containing vitamin D as bottled sunshine.
- Dietary Reference Intakes for Calcium, Phosphorous, Magnesium, Vitamin D, and Fluoride (1997); Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline (1998); Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids (2000); Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (2001); and Dietary Reference Intakes for Calcium and Vitamin D (2011) . These reports may be accessed via www.nap.edu .
- Semba RD. The discovery of the vitamins. Int J Vitam Nutr Res . 2012 Oct 1;82(5):310-5.
- Piro A, Tagarelli G, Lagonia P, Tagarelli A, Quattrone A. Casimir Funk: his discovery of the vitamins and their deficiency disorders. Ann Nutr Metab . 2010;57(2):85-8.
Last reviewed March 2023
Terms of Use
The contents of this website are for educational purposes and are not intended to offer personal medical advice. You should seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The Nutrition Source does not recommend or endorse any products.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

10: Vitamin C Analysis (Experiment)
- Last updated
- Save as PDF
- Page ID 95879

- Santa Monica College
- To standardize a \(\ce{KIO3}\) solution using a redox titration.
- To analyze an unknown and commercial product for vitamin C content via titration.
- To compare your results for the commercial product with those published on the label.
Note: You will need to bring a powdered or liquid drink, health product, fruit samples, or other commercial sample to lab for vitamin C analysis. You will need enough to make 500 mL of sample for use in 3-5 titrations. Be sure the product you select actually contains vitamin C (as listed on the label or in a text or website) and be sure to save the label or reference for comparison to your final results. Be careful to only select products where the actual vitamin C content in mg or percent of RDA (recommended daily allowance) is listed. The best samples are lightly colored and/or easily pulverized.
The two reactions we will use in this experiment are:
\[\ce{KIO3(aq) + 6 H+(aq) +5 I- (aq)→ 3 I2(aq) + 3 H2O(l) + K+(aq) } \quad \quad \text{generation of }\ce{I2} \label{1}\]
\[\underbrace{\ce{C6H8O6(aq)}}_{\text{vitamin C(ascorbic acid)}}\ce{ + I2(aq) →C6H6O6(aq) +2 I- (aq) + 2 H+(aq) } \quad \quad \text{oxidation of vitamin C}\label{2}\]
Reaction \ref{1} generates aqueous iodine, \(\ce{I2}\) ( aq ). This is then used to oxidize vitamin C (ascorbic acid, \(\ce{C6H8O6}\)) in reaction \ref{2}. Both of these reactions require acidic conditions and so dilute hydrochloric acid, \(\ce{HCl}\) ( aq ), will be added to the reaction mixture. Reaction one also requires a source of dissolved iodide ions, \(\ce{I^-}\) ( aq ). This will be provided by adding solid potassium iodide, \(\ce{KI}\) ( s ), to the reaction mixture.
This is a redox titration. The two relevant half reactions for reaction \ref{2} above are:
Reduction half reaction for Iodine at pH 5:
\[\ce{I2 +2e^{⎯} → 2I^{⎯}}\]
Oxidation half reaction for vitamin C (\(\ce{C6H8O6}\)) at pH 5:
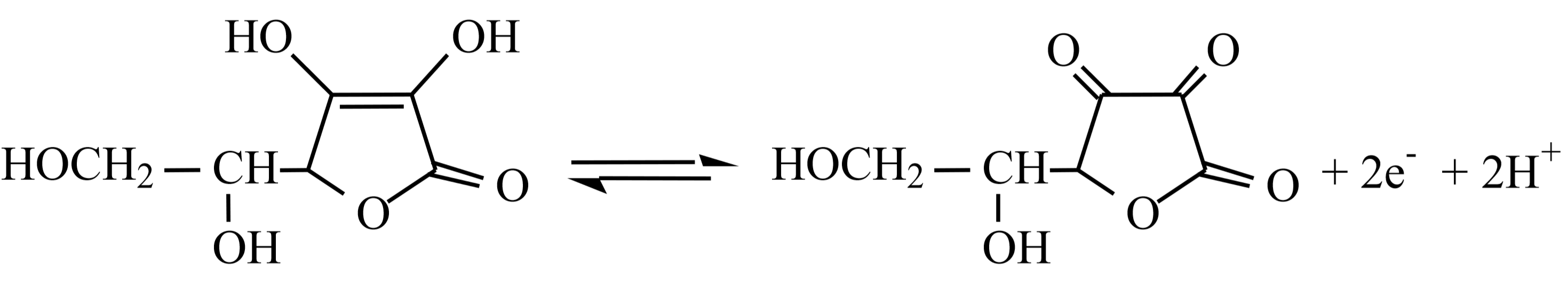
A few drops of starch solution will be added to help determine the titration endpoint. When the vitamin C (ascorbic acid) is completely oxidized, the iodine, \(\ce{I2}\) ( aq ), will begin to build up and will react with the iodide ions, \(\ce{I^-}\) ( aq ), already present to form a highly colored blue \(\ce{I3^-}\)-starch complex, indicating the endpoint of our titration.
Vitamin C: An Important Chemical Substance
Vitamin C , known chemically as ascorbic acid , is an important component of a healthy diet. The history of Vitamin C revolves around the history of the human disease scurvy, probably the first human illness to be recognized as a deficiency disease. Its symptoms include exhaustion, massive hemorrhaging of flesh and gums, general weakness and diarrhea. Resultant death was common. Scurvy is a disease unique to guinea pigs, various primates, and humans. All other animal species have an enzyme which catalyzes the oxidation of L- gluconactone to L-ascorbic acid, allowing them to synthesize Vitamin C in amounts adequate for metabolic needs.
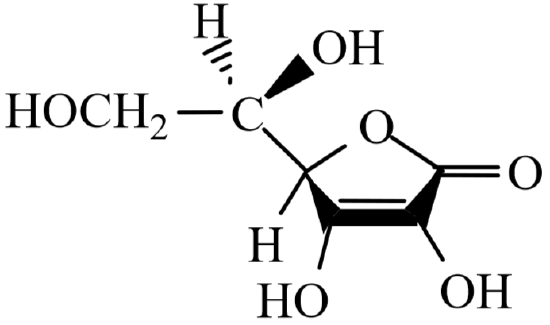
L-Ascorbic Acid -- Vitamin C
As early as 1536, Jacques Cartier, a French explorer, reported the miraculous curative effects of infusions of pine bark and needles used by Native Americans. These items are now known to be good sources of ascorbic acid. However, some 400 years were to pass before Vitamin C was isolated, characterized, and synthesized. In the late 1700's, the British Navy ordered the use of limes on ships to prevent scurvy. This practice was for many years considered to be quackery by the merchant marines, and the Navy sailors became known as “Limeys”. At that time scurvy aboard sailing vessels was a serious problem with often up to 50% of the crew dying from scurvy on long voyages.
The RDA ( Recommended Daily Allowance ) for Vitamin C put forward by the Food and Nutrition Board of the National Research Counsel is 60 mg/day for adults. It is recommended that pregnant women consume an additional 20 mg/day. Lactating women are encouraged to take an additional 40 mg/day in order to assure an adequate supply of Vitamin C in breast milk. Medical research shows that 10 mg/day of Vitamin C will prevent scurvy in adults. There has been much controversy over speculation that Vitamin C intake should be much higher than the RDA for the prevention of colds and flu. Linus Pauling, winner of both a Nobel Prize in Chemistry and the Nobel Peace Prize, has argued in his book, Vitamin C and the Common Cold , that humans should be consuming around 500 mg of Vitamin C a day (considered by many doctors to be an excessive amount) to help ward off the common cold and prevent cancer.
Vitamin C is a six carbon chain, closely related chemically to glucose. It was first isolated in 1928 by the Hungarian-born scientist Szent-Gyorgi and structurally characterized by Haworth in 1933. In 1934, Rechstein worked out a simple, inexpensive, four-step process for synthesizing ascorbic acid from glucose. This method has been used for commercial synthesis of Vitamin C. Vitamin C occurs naturally primarily in fresh fruits and vegetables.
Table 1: Vitamin C content of some foodstuffs
From Roberts, Hollenberg, and Postman, General Chemistry in the Laboratory .
Work in groups of three, dividing the work into three parts (standardization, unknown analysis, and food products) among your group members and then compare data if you are to finish in one period. Work carefully: your grade for this experiment depends on the accuracy and precision of each of your final results.
Materials and Equipment
You will need the following additional equipment for this experiment: 3 Burets, 1 Mortar and pestle, 1 Buret stand
Avoid contact with iodine solutions, as they will stain your skin. Wear safety glasses at all times during the experiment.
WASTE DISPOSAL : You may pour the blue colored titrated solutions into the sink. However, all unused \(\ce{KIO3}\) (after finishing parts A-C) must go in a waste container for disposal. This applies to all three parts of the experiment.
Proper Titration Techniques
Using a Buret
Proper use of a buret is critical to performing accurate titrations. Your instructor will demonstrate the techniques described here.
- Rinsing: Always rinse a buret (including the tip) before filling it with a new solution. You should rinse the buret first with deionized water, and then twice with approximately 10-mL aliquots of the solution you will be using in the buret. Be sure to swirl the solution to rinse all surfaces. If you are using an acid or base solution be careful to avoid spilling the solution on hands or clothing.
- Filling: Mount the buret on a buret stand. Be sure that the tip fits snuggly into the buret and is pressed all the way in. If the tip is excessively loose, exchange it for a tighter fitting one. Using a funnel rinsed in the same manner as the buret, fill the buret with the titrant to just below the 0.00 mL mark. There is no need to fill the buret to exactly 0.00 mL since you will use the difference between the ending and starting volumes to determine the amount delivered. When the buret is full, remove the funnel as drops remaining in or around the funnel can creep down and alter your measured volume. If you overfill the buret, drain a small amount into an empty beaker. Do not re-use this "extra" solution as it may have been contaminated by the beaker or diluted slightly by any water present in the beaker. Always pour fresh solution into the buret.
- Removing Air Bubbles: Often air bubbles will be trapped in the tip of a newly filled buret. These can be difficult to see and troublesome as they alter the measured volume when they escape. To remove air bubbles hold the buret over an open beaker and open the stopcock fully to allow solution to flow out of the buret. Your instructor will demonstrate this technique. Refill the buret as necessary.
- Reading the Buret: You should always read the volume in a buret from the bottom of the meniscus viewed at eye level (see Figure 1). A black or white card held up behind the buret helps with making this reading. Burets are accurate to ±0.02 mL and all readings should be recorded to two decimal places. Be sure to record both the starting and ending volumes when performing a titration. The difference is the volume delivered.
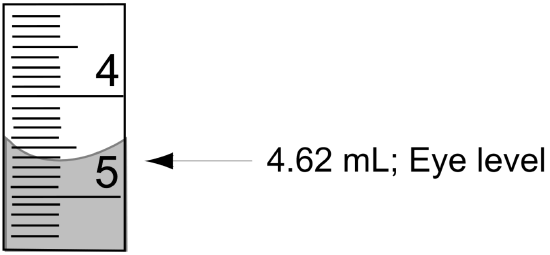
Figure 1: Reading a Buret
Good Titration Techniques
Throughout your scientific careers you will probably be expected to perform titrations; it is important that you learn proper technique. In performing a titration generally an indicator that changes color is added to a solution to be titrated (although modern instruments can now perform titrations automatically by spectroscopically monitoring the absorbance). Add titrant from the buret dropwise , swirling between drops to determine if a color change has occurred. Only if you know the approximate end-point of a titration should you add titrant faster, but when you come within a few milliliters of the endpoint you should begin to slow down and add titrant dropwise.
As you become proficient in performing titrations you will get a "feeling" for how much to open the stopcock to deliver just one drop of titrant. Some people become so proficient that they can titrate virtually "automatically" by allowing the titrant to drip out of the buret dropwise while keeping a hand on the stopcock, and swirling the solution with the other hand. If you do this, be sure that the rate at which drops are dispensed is slow enough that you can stop the flow before the next drop forms! Overshooting an end-point by even one drop is often cause for having to repeat an entire titration. Generally, this will cost you more time than you will gain from a slightly faster droping rate.
Refill the buret between titrations so you won’t go below the last mark. If a titration requires more than the full volume of the buret, you should either use a larger buret or a more concentrated titrant. Refilling the buret in the middle of a trial introduces more error than is generally acceptable for analytical work.
Set-up and Preparation of Equipment
- Clean and rinse a large 600-mL beaker using deionized water. Label this beaker “standard \(\ce{KIO3}\) solution.”
- From the large stock bottles of ~0.01 M \(\ce{KIO3}\) obtain about 600 mL of \(\ce{KIO3}\) solution. This should be enough \(\ce{KIO3}\) for your group for all three parts of the experiment including rinsings. The reason for collecting one beaker of stock is there is no guarantee that different batches of \(\ce{KIO3}\) from the stockroom will have the same exact molarity. By having one beaker of stock you ensure that all your trials come from the same solution. (If you run out of stock or spill this solution accidentally you will need to repeat part A on the new solution).
- Clean and rinse three burets once with deionized water and then twice with small (5-10 ml) aliquots of standard \(\ce{KIO3}\) from your large beaker. Pour the rinsings into a waste beaker.
- Fill each of the burets (one for each part of the experiment) with \(\ce{KIO3}\) from your beaker. Remove any air bubbles from the tips. The starting volumes in each of the burets should be between 0.00 mL and 2.00 mL. If you use a funnel to fill the burets be sure it is cleaned and rinsed in the same way as the burets and removed from the buret before you make any readings to avoid dripping from the funnel into the buret.
Each of the following parts should be performed simultaneously by different members of your group. You do not have enough time to do these sequentially and finish in one lab period.
Part A: Standardization of your \(\ce{KIO3}\) solution
The \(\ce{KIO3}\) solution has an approximate concentration of about ~0.01 M. You will need to determine exactly what the molarity is to three significant figures. Your final calculated results for each trial of this experiment should differ by less than ± 0.0005 M. Any trials outside this range should be repeated. You will need to calculate in advance how many grams of pure Vitamin C powder (ascorbic acid, \(\ce{C6H8O6}\)) you will need to do this standardization (this is part of your prelaboratory exercise). Remember that your buret holds a maximum of 50.00 mL of solution and ideally you would like to use between 25-35 mL of solution for each titration (enough to get an accurate measurement, but not more than the buret holds).
- Calculate the approximate mass of ascorbic acid you will need and have your instructor initial your calculations on the data sheet.
- Weigh out approximately this amount of ascorbic acid directly into a 250-mL Erlenmeyer flask. Do not use another container to transfer the ascorbic acid as any loss would result in a serious systematic error. Record the mass added in each trial to three decimal places in your data table. It is not necessary that you weigh out the exact mass you calculated, so long as you record the actual mass of ascorbic acid added in each trial for your final calculations.
- Dissolve the solid ascorbic acid in 50-100 mL of deionized water in an Erlenmeyer flask.
- Add approximately 0.5-0.6 g of \(\ce{KI}\), 5-6 mL of 1 M \(\ce{HCl}\), and 3-4 drops of 0.5% starch solution to the flask. Swirl to thoroughly mix reagents.
- Begin your titration. As the \(\ce{KIO3}\) solution is added, you will see a dark blue (or sometimes yellow) color start to form as the endpoint is approached. While adding the \(\ce{KIO3}\) swirl the flask to remove the color. The endpoint occurs when the dark blue color does not fade after 20 seconds of swirling.
- Calculate the molarity of this sample. Repeat the procedure until you have three trials where your final calculated molarities differ by less than ± 0.0005 M.
Part B: Vitamin C Unknown (internal control standard)
- Obtain two Vitamin C tablets containing an unknown quantity of Vitamin C from your instructor.
- Weigh each tablet and determine the average mass of a single tablet.
- Grind the tablets into a fine powder using a mortar and pestle.
- Weigh out approximately 0.20-0.25 grams of the powdered unknown directly into a 250-mL Erlenmeyer flask. Do not use another container to transfer the sample as any loss would result in a serious systematic error. Record the mass added in each trial to three decimal places in your data table.
- Dissolve the sample in about 100 mL of deionized water and swirl well. Note that not all of the tablet may dissolve as commercial vitamin pills often use calcium carbonate (which is insoluble in water) as a solid binder.
- Add approximately 0.5-0.6 g of \(\ce{KI}\), 5-6 mL of 1 M \(\ce{HCl}\), and 2-3 drops of 0.5% starch solution to the flask before beginning your titration. Swirl to mix.
- Perform two more trials. If the first titration requires less than 20 mL of \(\ce{KIO3}\), increase the mass of unknown slightly in subsequent trials.
- Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of Vitamin C contained in each tablet. Be sure to use the average molarity for \(\ce{KIO3}\) determined in Part A for these calculations. Your results should be accurate to at least three significant figures. Repeat any trials that seem to differ significantly from your average.
Part C: Fruit juices, foods, health-products, and powdered drink mixes
Solids samples
- Pulverize solid samples (such as vitamin pills, cereals, etc.) with a mortar and pestle. Powdered samples (such as drink mixes) may be used directly.
- Weigh out enough powdered sample, so that there will be about 100 mg of ascorbic acid (according to the percentage of the RDA or mg/serving listed by the manufacturer) in each trial.
- Add the sample to a 250-mL Erlenmeyer flask containing 50-100 mL of water. (Note: If your sample is highly colored, you might want to dissolve the KI in the water before adding the mix, so that you can be sure it dissolves).
- Begin your titration. As the \(\ce{KIO3}\) solution is added, you will see a dark blue (or sometimes yellow or black depending on the color of your sample) color start to form as the endpoint is approached. While adding the \(\ce{KIO3}\) swirl the flask to remove the color. The endpoint occurs when the dark color does not fade after 20 seconds of swirling.
- Calculate the milligrams of ascorbic acid per gram of sample. Be sure to use the average molarity determined for the \(\ce{KIO3}\) in Part A for these calculations. Your results should be accurate to at least three significant figures. Repeat any trials that seem to differ significantly from your average.
Liquid samples
- If you are using a pulpy juice, strain out the majority of the pulp using a cloth or filter.
- Using a graduated cylinder, measure out at least 100 mL of your liquid sample. Record the volume to three significant figures (you will calculate the mass of ascorbic acid per milliliter of juice).
- Add this liquid to an Erlenmeyer flask.
- Begin your titration. As the \(\ce{KIO3}\) solution is added, you will see a dark blue (or sometimes yellow or black depending on the color of your sample) color start to form as the endpoint is approached. While adding the \(\ce{KIO3}\) swirl the flask to remove the color. The endpoint occurs when the dark color does not fade after 20 seconds of swirling. With juices it sometimes takes a little longer for the blue color to fade, in which case the endpoint is where the color is permanent.
- Perform two more trials. If the first titration requires less than 20 mL of \(\ce{KIO3}\), increase the volume of unknown slightly in subsequent trials.
- Calculate the milligrams of ascorbic acid per milliliter of juice. Be sure to use the average molarity determined for the \(\ce{KIO3}\) in Part A for these calculations. Your results should be accurate to at least three significant figures. Repeat any trials that seem to differ significantly from your average.
Pre-laboratory Assignment: Vitamin C Analysis
- If an average lemon yields 40 mL of juice, and the juice contains 50 mg of Vitamin C per 100 mL of juice, how many lemons would one need to eat to consume the daily dose of Vitamin C recomended by Linus Pauling? Show all work.
- Why are \(\ce{HCl}\), \(\ce{KI}\), and starch solution added to each of our flasks before titrating in this experiment? What is the function of each?
- \(\ce{HCl}\):
- \(\ce{KI}\):
- A label states that a certain cold remedy contains 200% of the US Recommended Daily Allowance (RDA) of Vitamin C per serving, and that a single serving is one teaspoon (about 5 mL). Calculate the number of mg of Vitamin C per serving and per mL for this product. Show all work.
- Based on the balanced reactions \ref{1} and \ref{2} for the titration of Vitamin C, what is the mole ratio of \(\ce{KIO3}\) to Vitamin C from the combined equations?
_______ moles \(\ce{KIO3}\) : _______ moles Vitamin C (ascorbic acid)
- Assuming that you want to use about 35 mL of \(\ce{KIO3}\) for your standardization titration in part A, about how many grams of ascorbic acid should you use? (you will need this calculation to start the lab). Show all work.
Hint: you will need to use the approximate \(\ce{KIO3}\) molarity given in the lab instructions and the mole ratio you determined in the prior problem.
Lab Report: Vitamin C Analysis
Mass of ascorbic acid to be used for standardization of ~0.01 M \(\ce{KIO3}\): __________ g ______Instructor’s initials
Supporting calculations:
Standardization Titration Data:
*All values should be with in ±0.0005 M of the average; trials outside this range should be crossed out and a fourth trial done as a replacement. Express your values to the correct number of significant figures. Show all your calculations on the back of this sheet.
- Average Molarity of \(\ce{KIO3}\):
"Internal Control Sample" (unknown) code:
Mass of Tablet 1:
Mass of Tablet 2:
Average mass:
Control Standard (Unknown) Titration Data:
* Express your values to the correct number of significant figures. Show all your calculations on the back of this sheet.
- ____________mg/g
- ____________mg/tablet
Name of Sample Used: ________________________________________________________
- Briefly describe the sample you chose to examine and how you prepared it for analysis. You may continue on the back if necessary:
Part C Titration Data:
*Express your values to the correct number of significant figures. Show all your calculations on the back of this sheet.
Average ascorbic acid :
- What is the concentration of Vitamin C listed on the packaging by the manufacturer or given in the reference source? This can be given in units of %RDA, mg/g, mg/mL, mg/serving, or %RDA per serving. Be sure to include the exact units cited.
- Manufacturer’s claim: ____________________________ (value and units)
- Serving Size (if applicable): ________________________ (value and units)
____________ mg / g or mL
- If your reference comes from a text book or the internet give the citation below. If it comes from a product label please remove the label and attach it to this report.
- Using your average milligrams of Vitamin C per gram or milliliter of product from part C as the "correct" value, determine the percent error in the manufacturer or text’s claim (show calculations)?
- What can you conclude about the labeling of this product or reference value? How do you account for any discrepancies? Does the manufacturer or reference overstate or understate the amount of Vitamin C in the product? If so, why might they do this? Explain below. Use the back of this sheet if necessary.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- NIST Author Manuscripts

Value Assignment of Vitamin D Metabolites in Vitamin D Standardization Program (VDSP) Serum Samples
Karen w. phinney.
Biomolecular Measurement Division and Chemical Sciences Division, National Institute of Standards and Technology, Gaithersburg, MD 20899 USA
Johanna E. Camara
Susan s.-c. tai, lane c. sander, stephen a. wise, linde a.c. de grande.
Laboratory for Analytical Chemistry, Faculty of Pharmaceutical Sciences, Ghent University, Ghent, Belgium
Linda M. Thienpont
Antonio m. possolo.
Statistical Engineering Division, National Institute of Standards and Technology, Gaithersburg, MD 20899 USA
Blaza Toman
Christopher t. sempos.
Office of Dietary Supplements, National Institutes of Health, Bethesda, MD 20892 USA
Joseph M. Betz
Paul m. coates, associated data.
Assay variability has been cited as an obstacle to establishing optimal vitamin D exposure. As part of the Vitamin D Standardization Program (VDSP) effort to standardize the measurement of total 25(OH)D, value assignment of total 25(OH)D in 50 single donor serum samples was performed using two isotope-dilution LC-MS/MS methods. Both methods are recognized as reference measurement procedures (RMPs) by the Joint Committee for Traceability in Laboratory Medicine. These samples and their assigned values serve as the foundation for several aspects of the VDSP. To our knowledge, this is the first time that two RMPs have been used to assign 25(OH)D values to such a large number of serum samples.
Introduction
Measurement of circulating 25-hydroxyvitamin D [25(OH)D] is the accepted indicator of vitamin D exposure ( 1 , 2 ). Several factors, including media attention, have led to considerable growth in the number of 25(OH)D tests performed each year. As a result, there has also been an increase in the number of assay platforms, both commercial and laboratory-developed, for 25(OH)D. Numerous publications have described comparisons among immunoassays for 25(OH)D or have compared immunoassay approaches to those based upon liquid chromatography coupled to mass spectrometry (LC-MS or LC-MS/MS) ( 3 – 6 ). Although such studies are informative from the standpoint of characterizing assay performance, they have also highlighted the lack of comparability among different assays. Assay variability has been identified as a significant obstacle to the accurate diagnosis of vitamin D deficiency ( 7 – 9 ).
A primary objective of the Vitamin D Standardization Program (VDSP) is the standardization of total 25(OH)D measurements over time, location, and laboratory procedure ( 10 , 11 ). Total 25(OH)D is defined in the context of the VDSP as the sum of the 25(OH)D 2 and 25(OH)D 3 concentrations. Standardization of total 25(OH)D measurements is necessary for comparing data across different populations, pooling and interpreting the results of research studies, and for ensuring appropriate decision making by medical professionals ( 12 , 13 ). To achieve this objective, the VDSP incorporates several components designed to link the results obtained by the end user of a routine assay to a true value as determined by a reference measurement procedure (RMP) ( 11 ). These components comprise a reference measurement system and include reference methods, reference materials, accuracy-based performance testing (PT)/external quality assessment (EQA) schemes, and an assay standardization-certification program. The VDSP has also undertaken a research program directed toward understanding and improving the laboratory measurement of total 25(OH)D.
As part of this VDSP effort, concentrations of total 25(OH)D were determined in 50 single donor serum samples selected to span a range from approximately 10 nmol/L to 150 nmol/L and which included samples with endogenous 25(OH)D 2 . These samples and their assigned values will serve as the foundation for several aspects of the VDSP, including assessment of the performance of assays for 25(OH)D, evaluating the commutability of reference materials and quality assurance testing materials, and in developing study designs for standardizing data from completed national health surveys. Concentrations of total 25(OH)D were determined by two independent isotope-dilution (ID) liquid chromatography/tandem mass spectrometry (LC-MS/MS) methods developed by the National Institute of Standards and Technology (NIST)( 14 ) and by Ghent University ( 15 ). Both methods are recognized as RMPs by the Joint Committee for Traceability in Laboratory Medicine (JCTLM) ( 16 ).
Each of the 50 samples was analyzed in triplicate by Ghent University and in duplicate by NIST. Standard Reference Material (SRM) 972 Vitamin D in Human Serum ( 17 ) was employed as a measurement quality assurance material by both laboratories. Each laboratory provided results for 25(OH)D 2 , 25(OH)D 3 , and 3-epi-25(OH)D 3 . Although the biological function of the 3-epimer of 25(OH)D 3 has not yet been elucidated, it is detectable in many adult serum samples and can interfere with the determination of 25(OH)D 3 by mass spectrometry-based methods ( 18 , 19 ). The protocols used for assigning values to each sample and for evaluating uncertainties associated with the assigned values are described in this manuscript along with observations about the performance of each of the RMPs.
Experimental
Single donor serum samples were collected by Solomon Park Research Laboratories (Kirkland, WA) in accordance with specifications provided by the Centers for Disease Control and Prevention (CDC) and using the procedures described in the Clinical and Laboratory Standards Institute (CLSI) C37-A protocol but without filtration ( 20 ). Serum samples were initially screened by LC-MS/MS at CDC to identify those suitable for inclusion in the study. These orientation values were provided to the two reference laboratories, NIST and Ghent University, to assist in determining appropriate calibration solution and internal standard concentrations for the RMPs.
Measurements by ID LC-MS/MS at NIST
The NIST RMPs for 25(OH)D 2 and 25(OH)D 3 have been described previously ( 14 ), and details specific to this study are included in the supplemental material . Briefly, serum samples were spiked with internal standard solution, the pH was adjusted, and the samples were subjected to liquid-liquid extraction with hexane:ethyl acetate. Each sample was analyzed on two different days, and isotopically labeled internal standards [25(OH)D 2 - d 3 , 25(OH)D 3 - d 6 , and 3-epi-25(OH)D 3 - d 3 ] were utilized for the quantification of 25(OH)D 2 , 25(OH)D 3 , and 3-epi-25(OH)D 3 , respectively. Two analysts performed the measurements at NIST, with each analyst responsible for 25 of the single donor samples. In addition, two different tandem mass spectrometry (MS/MS) instruments, an API 4000 and an API 5000 (both from AB Sciex), were employed for the measurement of the desired analytes. Limits of quantitation (LOQs) for 25(OH)D 2 , 25(OH)D 3 , and 3-epi-25(OH)D 3 were 0.5 ng/g for one analyst and 1.0 ng/g for the other. Results were reported in mass fractions (ng/g) and were converted to ng/mL using the measured density of each serum sample. Density measurements were performed by Ghent University.
Measurements by ID LC-MS/MS at Ghent
The Ghent University RMPs for 25(OH)D 2 and 25(OH)D 3 have been outlined previously ( 15 ), and details specific to this study are provided in the supplemental information . Samples were spiked with internal standard, alkalinized, extracted with hexane, and fractionated chromatographically. Each sample was analyzed on three different days, and isotopically labeled internal standards [25(OH)D 2 - d 6 and 25(OH)D 3 - d 6 ] were utilized for quantification of 25(OH)D 2 and 25(OH)D 3 , respectively. Concentrations of 3-epi-25(OH)D 3 were estimated by assuming equivalent response factors for 25(OH)D 3 and 3-epi-25(OH)D 3 . All results were converted from ng/g to ng/mL using the measured density of each serum sample. The LOQs for 25(OH)D 2 , 25(OH)D 3 , and 3-epi-25(OH)D 3 were 0.6 ng/mL.
Results and Discussion
Routine assays for 25(OH)D differ in the way that values for total 25(OH)D are obtained and reported. Immunoassays are generally developed to detect both 25(OH)D 3 and 25(OH)D 2 , although there has been debate about the extent of cross-reactivity with 25(OH)D 2 for certain assays ( 21 – 23 ). Cross-reactivity with other hydroxylated metabolites can also occur and contribute to the observed total 25(OH)D concentration ( 24 ). On the other hand, cross-reactivity with 3-epimers of 25(OH)D is generally thought to be low for most immunoassays and is not likely to contribute to the value obtained for total 25(OH)D ( 21 ).
In mass spectrometry-based platforms, the individual metabolites 25(OH)D 2 and 25(OH)D 3 can be detected and quantified. Values for total 25(OH)D are then obtained by summing the concentrations of 25(OH)D 2 and 25(OH)D 3 . As noted previously, the 3-epimer of 25(OH)D 3 can interfere with the determination of 25(OH)D 3 in mass spectrometry assays because it has the same molecular mass and generates similar fragmentation patterns. Failure to resolve this isomer chromatographically can result in overestimation of the concentration of 25(OH)D 3 ( 25 ). Routine mass spectrometry methods may employ chromatographic approaches that do not resolve the epimers in the interest of higher sample throughput.
Previous assessments of assay platforms for 25(OH)D have illustrated both within-method and between-method variability ( 13 , 26 , 27 ). Lack of method comparability and changes in assay performance over time have proven to be obstacles in establishing recommendations for optimal vitamin D exposure ( 8 , 28 , 29 ). The majority of assay comparisons reported to date have been limited in their ability to assess performance of 25(OH)D assays because they have not employed reference methods or reference materials as an anchor point for the comparisons. A study by Moon et al. used Standard Reference Material (SRM) 972 Vitamin D in Human Serum to evaluate the accuracy of several 25(OH)D assays, but some of the method bias observed may have been linked to the fact that certain levels of SRM 972 were prepared using non-human serum or exogenous 25(OH)D 2 ( 5 ). Nevertheless, the authors concluded that the use of uniform decision points for identifying vitamin D deficiency was problematic without assay standardization.
As a foundation for standardization of 25(OH)D measurements, the VDSP elected to assign values for total 25(OH)D to a set of 50 single-donor human serum samples having endogenous vitamin D metabolite concentrations. These samples could be utilized in a variety of ways including an evaluation of routine assay accuracy (bias) and precision. RMPs are the preferred approach to value assignment of reference materials and other samples used in standardization efforts. Hence, the RMPs developed by NIST and by Ghent University were used to assign values for vitamin D metabolites in these VDSP samples. The serum samples were selected to span a wide range of 25(OH)D concentrations and included samples with endogenous 25(OH)D 2 .
This study represents the first time, to our knowledge, that two RMPs have been used to assign 25(OH)D values to such a large number of samples. Although the methods are both based upon ID-LC-MS/MS, they differ in the specific procedures used for sample preparation and analysis. Therefore, one aspect of this study involved assessing the concordance of results from the two methods. The use of two or more independent analytical methods is a common approach to value assignment of reference materials at NIST because it reduces the likelihood of undetected measurement errors or bias ( 30 , 31 ).
A few differences between the NIST and Ghent methods are worth noting prior to discussion of specific results for 25(OH)D 2 , 25(OH)D 3 , 3-epi-25(OH)D 3 , and total 25(OH)D. Both methods employ liquid-liquid extraction for isolation of the vitamin D metabolites. The Ghent method includes an additional fractionation step by Sephadex LH20 chromatography. The Ghent method also employs a two-dimensional chromatographic approach, with a C 4 column in the first dimension and either a C 18 or cyanopropyl (CN) column in the second dimension. The C 18 column was used for quantification of 25(OH)D 2 , and the CN column provided chromatographic separation between 25(OH)D 3 and 3-epi-25(OH)D 3 . In the Ghent method, the concentration of 3-epi-25(OH)D 3 in each sample was estimated by assuming equal response factors for 25(OH)D3 and its 3-epimer. In the NIST method, a pentafluorophenyl (PFP) chromatographic stationary phase was used for the determination of 25(OH)D 2 , and a CN column was used for 25(OH)D 3 and 3-epi-25(OH)D 3 . Isotopically labeled internal standards were employed for all three analytes in the NIST measurements.
Only 17 of the 50 samples had 25(OH)D 2 concentrations that were above the LOQ for both laboratories; results for those samples with 25(OH)D 2 concentrations below the LOQ are not included in Table 1 . The fact that 25(OH)D 2 concentrations were below the LOQ in more than half the samples was not surprising because, in general, 25(OH)D 2 is only detected in serum if supplementation with vitamin D 2 has occurred. The concentrations ranged from approximately 1.4 nmol/L to 19 nmol/L. Good agreement between the two methods (NIST and Ghent) was consistently observed, even when concentrations of 25(OH)D 2 were < 5 nmol/L, and relative standard deviations (%RSD) for the two methods were nearly always less than 3.5%.
Summary of NIST and Ghent results for 25(OH)D 2 in 50 single donor samples. The mean values (nmol/L) for each laboratory are listed with relative standard deviations (% RSD) in parentheses. Samples with 25(OH)D 2 concentrations below the LOQ are not included in the table.
In most individuals, 25(OH)D 3 is the major contributor to the observed total 25(OH)D concentration, and 25(OH)D 3 was detected at concentrations above the LOQ in all 50 samples ( Table 2 ). Both the LC-MS/MS methods incorporated chromatographic resolution of 25(OH)D 3 from its 3-epimer to ensure that the presence of 3-epi-25(OH)D 3 in the samples did not introduce measurement bias. Concentrations of 25(OH)D 3 in the 50 samples ranged from approximately 12 nmol/L to 140 nmol/L, as shown in Table 2 . Excellent measurement precision was observed for both methods across this concentration range, with %RSD values frequently below 2%.
Summary of NIST and Ghent results for 25(OH)D 3 in 50 single donor samples. The mean values (nmol/L) for each laboratory are presented with relative standard deviations (% RSD) in parentheses.
3-Epi-25(OH)D 3
The majority of the samples (40 of 50) were found to have concentrations of 3-epi-25(OH)D 3 above the LOQ ( Table 3 ). The observed results are in agreement with previous studies reporting that 3-epi-25(OH)D 3 is present in most adult sera ( 18 , 19 ). Because the concentrations of 3-epi-25(OH)D 3 determined by the Ghent laboratory were estimated by assuming equivalent response factors for 25(OH)D 3 and 3-epi-25(OH)D 3 , they were not used in the final value assignment. Nevertheless, the Ghent results provide supporting evidence for the assigned values and generally were in good agreement with the NIST data. Concentrations of 3-epi-25(OH)D 3 in the 40 samples ranged from approximately 1.4 nmol/L to 15 nmol/L.
Summary of NIST and Ghent results for 3-epi-25(OH)D 3 in 50 single donor samples. The mean values (nmol/L) for each laboratory are presented with relative standard deviations (% RSD) in parentheses. Samples with 3-epi-25(OH)D 3 concentrations below the LOQ are not included in the table.
Comparison of Method Results/Value Assignment
Figure 1 shows a Bland-Altman plot ( 32 ) comparing the NIST and Ghent results for total 25(OH)D, which as noted previously, corresponded to the sum of the 25(OH)D 2 and 25(OH)D 3 concentrations. The total 25(OH)D concentrations included values for 25(OH)D 2 that were below the LOQ. Results for each sample (blue dots) are shown with standard uncertainties (u) for both the average of method results and their difference ( 33 ). The results suggest a tendency for the NIST RMP to measure slightly lower concentrations of total 25(OH)D than the Ghent RMP, with an average difference of −0.52 ng/mL (u = 0.04 ng/mL). This corresponds to a relative average difference between methods less than 1.8% based on the median concentration (30.17 ng/mL) of total 25(OH)D in these samples. The Bland-Altman plot also suggests that the differences between methods may increase with higher total 25(OH)D concentrations, but the slope of a Deming regression line fitted to the data ( 34 ), taking into account the uncertainties in both variables, did not differ significantly from zero (results not shown). Although the method comparison does reveal a small but statistically significant difference between the averages, it is impossible to attribute any bias to one laboratory or the other, or both, without further study. Results from each laboratory for the measurement quality assurance material SRM 972 were also used to evaluate bias, and none of the biases differed significantly from zero.

Bland-Altman plot comparing total 25(OH)D results from the NIST and Ghent methods. Each sample (blue dot) is shown with the associated standard uncertainties (u) for the method average (horizontal lines) and the method difference (vertical lines). The solid red line represents an outlier-resistant summary of the method differences, and the dashed red lines are the limits of agreement.
Values for 25(OH)D 2 and 25(OH)D 3 were assigned to each of the 50 samples based upon the average of the averages of determinations made by NIST and Ghent. Results for 3-epi-25(OH)D 3 were based solely on the average of results from NIST. The assigned values for all 50 samples are summarized in Table 4 , and the distribution of metabolites for each of the 50 samples is shown in Figure 2 . An uncertainty evaluation was also performed for each of the assigned values, including 25(OH)D 2 , 25(OH)D 3 , 3-epi-25(OH)D 3 , and total 25(OH)D and included uncertainty components arising from within-laboratory and between-laboratory measurement dispersion as well as from calibration of the methods ( 33 ). As can be seen in the table, most of the values have small uncertainties, and the median coefficient of variation for total 25(OH)D was 1.8%.

Distribution of vitamin D metabolites in the 50 VDSP single donor samples (1–50). All results in nmol/L.
Assigned values (nmol/L) for the 50 VDSP samples. Relative expanded uncertainties are given in parentheses.
Conclusions
Standardization of 25(OH)D measurements is important for accurate and consistent identification of inadequate and/or deficient vitamin D levels and related health consequences in individuals and populations. Value assignment of the 50 single donor samples described here represents a fundamental component of the VDSP and supports its goal to improve clinical and public health practice worldwide. In addition, this study provided evidence of the comparability of two RMPs for vitamin D metabolites, providing further confidence in measurements based on these methods.
Supplementary Material
Certain commercial equipment, instruments, or materials are identified in this paper in order to specify the experimental procedure adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the materials or equipment identified are necessarily the best available for the purpose.
Contributor Information
Karen W. Phinney, Biomolecular Measurement Division and Chemical Sciences Division, National Institute of Standards and Technology, Gaithersburg, MD 20899 USA.
Johanna E. Camara, Biomolecular Measurement Division and Chemical Sciences Division, National Institute of Standards and Technology, Gaithersburg, MD 20899 USA.
Susan S.-C. Tai, Biomolecular Measurement Division and Chemical Sciences Division, National Institute of Standards and Technology, Gaithersburg, MD 20899 USA.
Lane C. Sander, Biomolecular Measurement Division and Chemical Sciences Division, National Institute of Standards and Technology, Gaithersburg, MD 20899 USA.
Stephen A. Wise, Biomolecular Measurement Division and Chemical Sciences Division, National Institute of Standards and Technology, Gaithersburg, MD 20899 USA.
Linde A.C. De Grande, Laboratory for Analytical Chemistry, Faculty of Pharmaceutical Sciences, Ghent University, Ghent, Belgium.
Linda M. Thienpont, Laboratory for Analytical Chemistry, Faculty of Pharmaceutical Sciences, Ghent University, Ghent, Belgium.
Antonio M. Possolo, Statistical Engineering Division, National Institute of Standards and Technology, Gaithersburg, MD 20899 USA.
Blaza Toman, Statistical Engineering Division, National Institute of Standards and Technology, Gaithersburg, MD 20899 USA.
Christopher T. Sempos, Office of Dietary Supplements, National Institutes of Health, Bethesda, MD 20892 USA.
Joseph M. Betz, Office of Dietary Supplements, National Institutes of Health, Bethesda, MD 20892 USA.
Paul M. Coates, Office of Dietary Supplements, National Institutes of Health, Bethesda, MD 20892 USA.

- TILT Higher Ed Examples and Resources
The following resources from the Transparency in Learning and Teaching project (TILT Higher Ed) can help faculty, educational developers and administrators to apply the Transparency Framework (of purpose/task/criteria) in contexts including assignments, curricula, assessment and strategic initiatives, all toward the goal of enhancing student success equitably. If you have developed TILT-focused tools or publications you would like to share, please contact [email protected]
Introduction to Transparency in Learning and Teaching
- Transparency and Problem-centered Learning (7-minute overview)
- Transparent Instruction and Its Impact on Learning, University of Tokyo TV (45 minutes)
- Transparency Framework for academic work
- Unwritten Rules for College Success, 39 second video
- Transparency Framework 1) Purpose, 44 second video
- Transparency Framework 2) Task, 25 second video
- Transparency Framework 3) Criteria, 24 second video
- Nave, Lillian. " Transparent Design with Mary-Ann Winkelmes. " Think UDL, Episode 76, 9 December 2021
- Christopher, K. (2018). "What are we doing and why? Transparent assignment design benefits students and faculty alike." The Flourishing academic: A Blog for teacher-scholars. Duquesne University Center for Teaching Excellence, April 16, 2018.
- Willingham-McLain, L. (2017). Just a TAD: Transparent assignment design. The Flourishing academic: A Blog for teacher-scholars. Duquesne University Center for Teaching Excellence. December 8, 2017.
- Cepek, R. (2017). Parallelograms and poetry: Helping first generation students connect. The Flourishing academic: A Blog for teacher-scholars. Duquesne University Center for Teaching Excellence. October 5, 2017.
- Yong, Darryl. "How Transparency Improves Learning." Teaching Tidbits (Mathematical Association of America blog), October 24, 2017.
- Nichols, Karen. "Remember 'Transparency' in Your Instructional Continuity Preparations." >CAT FooD, August 18, 2017.
- Mulnix, Amy B. "The Power of Transparency in Your Teaching." Faculty Focus: Higher Ed Teaching Strategies, November 6, 2016.
- Gambill, Sandy. "Transparent Assignments." Inclusive Teaching, November 30, 2016.
- Volk, Steven. Revealing the Secret Handshakes: The Rules of Clear Assignment Design." Article of the Week: Teaching and Learning at Oberlin College, September 27, 2015.
- Mary-Ann Winkelmes: "Transparency in Teaching and Learning interview,Smart Talks", Project Information Literacy
- “Small Teaching Changes, Big Learning Benefits” video interview with Mary-Ann Winkelmes, ACUE Community ‘Q’ Blog, Expert Series, December 2016.
- Great Conversations: Mary-Ann Winkelmes video interview at Indiana University (6 min:47 sec - 10 min: 25 sec)
- Faculty at 7 institutions reflect on their use of transparent assignment design (as part of an AAC&U project funded by TG Philanthropy) in the "Transparency and Problem-Centered Learning" issue of Peer Review, (Winter/Spring 2016) vol.18, no. 1/2.
- Faculty at University of Nevada, Las Vegas reflect on their use of transparently designed assignments in “Benefits (some unexpected) of Transparently Designed Assignments.” National Teaching and Learning Forum 24, 4 (May 2015), pages 4-6.
- Faculty at Texas Tech University discuss the design process and impacts of transparent assignments in their courses: Transparent Assignment Design at Texas Tech: A Panel Discussion, 13 th Annual Advancing Teaching and Learning Conference, Texas Tech University, March 3, 2017.
- Fukuda, D. 2018. Promote active learning in group projects through the use of the transparent assignment framework. In Chen, B., deNoyelles, A., & Thompson, K. (Eds.), Teaching Online Pedagogical Repository . Orlando, FL: University of Central Florida Center for Distributed Learning. Retrieved March 4, 2020.
- Turlington, Anita; Shimkus, Jim. (2017). "TILTing the Writing Across the Curriculum Program at UNG."
- Ou, J. (2018, June), Board 75 : Work in Progress: A Study of Transparent Assignments and Their Impact on Students in an Introductory Circuit Course Paper presented at 2018 ASEE Annual Conference & Exposition , Salt Lake City, Utah.
- Kane, J. & Mushtare, R. (Hosts). (2023 May) , Transparency in Learning and Teaching (episode 290). [Audio podcast episode]. In Tea for Teaching. https://teaforteaching.com/290-transparency-in-learning-and-teaching/
- Bruff, D. (Host). (January 2023), Transparent Teaching with Mary-Ann Winkelmes (Episode 5). [Audio podcast episode]. In Intentional Teaching
Example A: Sociology
Example B: Science 101
Example C: Psychology
Example D: Communications
Authors of Examples A-D describe the outcomes of their assignment revisions
Example E: Biology
Discussion Questions (about Examples A-E)
Example F: Library research Assignment
Example G: Criminal Justice In-Class activity
Example H: Criminal Justice Assignment
Example I: Political Science Assignment
Example J: Criteria for Math Writing
Example K - Environmental History
Example L - Calculus
Example M - Algebra
Example N - Finance
- Transparent Assignment Template for instructors
- Checklist for Designing Transparent Assignments
- Measuring Transparency: A Learning-focused Assignment Rubric (Palmer, M., Gravett, E., LaFleur, J.)
- Assignment Cues to use when designing an assignment (adapted from Bloom’s Taxonomy) for faculty
- Transparent Equitable Learning Readiness Assessment for Teachers
- Transparent Assignment Template for students (to help students learn to parse assignments; also to frame a conversation to gather feedback from your students about how to make assignments’ more transparent and relevant for them)
- Transparent Assignment Template for students (to help students learn to parse assignments; also to frame a conversation to gather feedback from your students about how to make assignments more transparent and relevant for them)
- Transparent Equitable Learning Framework for Students (to frame a conversation with students about how to make the purposes, tasks and criteria for class activities transparent and relevant for them)
- TILT and Align Your Assessment
- TILT Strategic Planning Worksheet
- Transparent Equitable Collaboration Framework for Staff
- Unwritten Rules: Transparent Assignment Framework for Students
- Transparent Equitable Learning Framework for Students
Workshop Videos and Slides
For faculty.
- Transparent Assignments Promote Equitable Opportunities for Students’ Success videorecording (University of Nevada, Las Vegas, April 29, 2016).
- Transparent Assignment Design faculty workshop videorecording (“Using Transparent Assignments to Increase Students' Success,” Mary-Ann Winkelmes, keynote workshop, 13th Annual Advancing Teaching and Learning Conference, Texas Tech University, March 3, 2017).
- Part 1) Research findings
- Part 2) Example Assignments
- Part 3) Peer feedback on your own assignments
FOR FACULTY DEVELOPERS
- Faculty workshop slides, Indianapolis Assessment Conference
- TILT Workshop Slides and Notes for Facilitators
- Train the Trainers webinar recording
- NILOA Charrette and Feedback with TILT
- TILT Course Sequencing Worksheet
FOR INSTITUTIONAL LEADERS
- Transparency and Equity webinar recording (hosted by AAC&U, NILOA, TILT)
For institutions, results can include increased retention and completion rates. For participating instructors, individualized reports identify small teaching adjustments best suited to improving students’ learning for the specific population of students in their courses. Ongoing analysis explores teaching/learning adjustments that improve learning outcomes, specific to discipline, class size, level of expertise, and student demographics.
A national study by the Association of American Colleges and Universities, funded by TG Philanthropy, demonstrated that transparency around academic work enhances students’ success at statistically significant levels, with even greater benefits for historically underserved students (with a medium-to-large sized magnitude of effect) [Winkelmes et al., Peer Review 2016]. Students who receive transparent instruction about the purposes, tasks and criteria for their academic work report gains in three areas that are important predictors of students’ success:
- academic confidence,
- sense of belonging, and
- mastery of the skills that employers value most when hiring.
Important studies have already connected academic confidence and sense of belonging with students’ greater persistence and higher grades [Walton and Cohen, Science 2011; Aronson, Fried, Good, 2002, Brady, Cohen, et al., Science Advances 2020. ]
- To bring a Transparency Project workshop to your institution, please contact Mary-Ann Winkelmes at [email protected]
- Frequently asked questions
- Transparent Methods: Examples
- Winkelmes, M. (2023). Introduction to Transparency in Learning and Teaching. Perspectives In Learning, 20 (1). Retrieved from https://csuepress.columbusstate.edu/pil/vol20/iss1/2
- Brown, J., et al. (2023). Perspectives in Learning: TILT Special Issue, 20 (1). Retrieved from https://csuepress.columbusstate.edu/pil/vol20/iss1/
- Winkelmes, M. (2022). “Assessment in Class Meetings: Transparency Reduces Systemic Inequities.” In Henning, G. W., Jankowski, N. A., Montenegro, E., Baker, G. R., & Lundquist, A. E. (Eds.). (2022). Reframing Assessment to Center Equity: Theories, Models, and Practices. Stylus Publishing, LLC.
- Howard, Tiffiany, Mary-Ann Winkelmes, and Marya Shegog. “ Transparency Teaching in the Virtual Classroom: Assessing the Opportunities and Challenges of Integrating Transparency Teaching Methods with Online Learning.” Journal of Political Science Education, June 2019.
- Palmer, M. S., Gravett, E. O., & LaFleur, J. (2018). Measuring transparency: A learning‐focused assignment rubric . To Improve the Academy, 37(2), 173-187. doi:10.1002/tia2.20083
- Winkelmes, M., Allison Boye and Suzanne Tapp, ed.s. (2019). Transparent Design in Higher Education Teaching and Leadership. Stylus Publishing.
- Humphreys, K., Winkelmes, M.A., Gianoutsos, D., Mendenhall, A., Fields, L.A., Farrar, E., Bowles-Terry, M., Juneau-Butler, G., Sully, G., Gittens, S. Cheek, D. (forthcoming 2018). Campus-wide Collaboration on Transparency in Faculty Development at a Minority-Serving Research University. In Winkelmes, Boye, Tapp, (Eds.), Transparent Design in Higher Education Teaching and Leadership.
- Copeland, D.E., Winkelmes, M., & Gunawan, K. (2018). Helping students by using transparent writing assignments. In T.L. Kuther (Ed.), Integrating Writing into the College Classroom: Strategies for Promoting Student Skills, 26-37. Retrieved from the Society for the Teaching of Psychology website.
- Winkelmes, Mary-Ann, Matthew Bernacki, Jeffrey Butler, Michelle Zochowski, Jennifer Golanics, and Kathryn Harriss Weavil. "A Teaching Intervention that Increases Underserved College Students’ Success."Peer Review (Winter/Spring 2016).
- Transparency and Problem-Centered Learning. (Winter/Spring 2016) Peer Review vol.18, no. 1/2.b
- Winkelmes, Mary-Ann. Small Teaching Changes, Big Learning Benefits.” ACUE Community ‘Q’ Blog, December, 2016.
- Winkelmes, Mary-Ann. “Helping Faculty Use Assessment Data to Provide More Equitable Learning Experiences.” NILOA Guest Viewpoints. Urbana, IL: University of Illinois and Indiana University, National Institute for Learning Outcomes Assessment, March 17, 2016.
- Gianoutsos, Daniel, and Mary-Ann Winkelmes.“Navigating with Transparency: Enhancing Underserved Student Success through Transparent Learning and Teaching in the Classroom and Beyond.” Proceedings of the Pennsylvania Association of Developmental Educators (Spring 2016).
- Sodoma, Brian.“The End of Busy Work.” UNLV Magazine 24,1 (Spring 2016): 16-19.
- Cook, Lisa and Daniel Fusch. One Easy Way Faculty Can Improve Student Success." Academic Impressions (March 10, 2016).
- Head, Alison and Kirsten Hosteller. "Mary-Ann Winkelmes: Transparency in Teaching and Learning," Project Information Literacy, Smart Talk Interview, no. 25. Creative Commons License 3.0 : 2 September 2015.
- Winkelmes, Mary-Ann, et al. David E. Copeland, Ed Jorgensen, Alison Sloat, Anna Smedley, Peter Pizor, Katharine Johnson, and Sharon Jalene. “Benefits (some unexpected) of Transparent Assignment Design.” National Teaching and Learning Forum, 24, 4 (May 2015), 4-6.
- Winkelmes, Mary-Ann. “Equity of Access and Equity of Experience in Higher Education.” National Teaching and Learning Forum, 24, 2 (February 2015), 1-4.
- Cohen, Dov, Emily Kim, Jacinth Tan, Mary-Ann Winkelmes, “A Note-Restructuring Intervention Increases Students’ Exam Scores.” College Teaching vol. 61, no. 3 (2013): 95-99.
- Winkelmes, Mary-Ann."Transparency in Teaching: Faculty Share Data and Improve Students' Learning.” Liberal Education Association of American Colleges and Universities (Spring 2013).
- Winkelmes, Mary-Ann. “Transparency in Learning and Teaching: Faculty and students benefit directly from a shared focus on learning and teaching processes.” NEA Higher Education Advocate (January 2013): 6 - 9.
- Bhavsar, Victoria Mundy. (2020). A Transparent Assignment to Encourage Reading for a Flipped Course, College Teaching, 68:1, 33-44, DOI: 10.1080/87567555.2019.1696740
- Bowles-Terry, Melissa, John C. Watts, Pat Hawthorne, and Patricia Iannuzzi. “ Collaborating with Teaching Faculty on Transparent Assignment Design .” In Creative Instructional Design: Practical Applications for Librarians, edited by Brandon K. West, Kimberly D. Hoffman, and Michelle Costello, 291–311. Atlanta: American Library Association, 2017.
- Leuzinger, Ryne and Grallo, Jacqui, “ Reaching First- Generation and Underrepresented Students through Transparent Assignment Design .” (2019). Library Faculty Publications and Presentations. 11. https://digitalcommons.csumb.edu/lib_fac/11
- Fuchs, Beth, “ Pointing a Telescope Toward the Night Sky: Transparency and Intentionality as Teaching Techniques ” (2018). Library Presentations. 188. https://uknowledge.uky.edu/libraries_present/188
- Ferarri, Franca; Salis, Andreas; Stroumbakis, Kostas; Traver, Amy; and Zhelecheva, Tanya, “ Transparent Problem-Based Learning Across the Disciplines in the Community College Context: Issues and Impacts ” (2015).NERA Conference Proceedings 2015. 9. https://opencommons.uconn.edu/nera-2015/9
- Milman, Natalie B. Tips for Success: The Online Instructor's (Short) Guide to Making Assignment Descriptions More Transparent . Distance Learning. Greenwich Vol. 15, Iss. 4, (2018): 65-67. 3
Offer research-based explanations about concepts or tasks that students often struggle to master in your discipline [See examples below including Bloom, Bransford, Gregorc, Light, Perry.]
- Ryjova, Yana. What is the Transparency in Learning and Teaching in Higher Education Project (TILT Higher Ed)? Interview with Dr. Mary-Ann Winkelmes." Hixson-Lied Success Scholar Newsletter. Las Vegas: University of Nevada, Las Vegas, Academic Success Center, March 2016.
- Sodoma, Brian. Forget the What: It's the How and Why That Matters." UNLV News Center, January 21, 2016.
- Summers, Keyonna. “Newsmakers 2015: People.” UNLV News Center, January 7, 2016.
- Berrett, Dan. “The Unwritten Rules of College.” Chronicle of Higher Education, September 21, 2015.
- Adolfo Guzman-Lopez, “Researchers say as college demographics change, so must teaching.” 89.3 KPCC Southern California Public Radio. March 13, 2015.
- “Transparency and Problem-Centered Learning.” Association of American Colleges and Universities website, retrieved November 5, 2014.
- “Mary-Ann Winkelmes and UNLV's Transparency in Teaching and Learning in Higher Education Project.” Accomplishments, UNLV News Center, September 2014.
- “New Project Will Engage Minority-Serving Institutions to Research Effect of Faculty Intentionality in Problem-Centered Educational Practices on the Success of Students Who Have Historically Been Underserved in Higher Education.” Association of American Colleges & Universities press release, August 4, 2014.
- “UNLV Partners with AAC&U to Lead National Project to Improve Under-Represented Students’ Success.” UNLV Research and Economic Development press release. August 7, 2014.
- Mellon grant in partnership with Berea College (2017-2021)
- Robert J. Menges Award for Outstanding Research in Educational Development, 2012, from Professional Organizational Development Network in Higher Education
- TG Philanthropy grant in partnership with Association of American Colleges and Universities(2014-2016)
University of Illinois
- Application to Institutional Review Board, University of Illinois at Urbana-Champaign
- Exempt Research Application
- University of Illinois Institutional Review Board Certification of principal investigator
- Collaborative Institutional Training Initiative (CITI) Certification of principal investigator
- Approvals from Institutional Review Board, University of Illinois at Urbana-Champaign:
- November 18, 2009
- May 5, 2010
- November 8, 2010
- December 9, 2011
- November 19, 2012
- February 12, 2013

University of Nevada, Las Vegas
- Approvals from Institutional Review Board, University of Nevada, Las Vegas:
- August 23, 2013 Application for Exempt Status and Approval
- November 2014 Modification request, Updated exempt application, Approval
- September 2015 Modification Request and October 13, 2015 Approval
- December 2015 Modification Request and Approval
- July 12 2016 Modification Request and Approval
- July 28, 2016 Modification Request and Approval
- July 28, 2016 Continuing Review Approval
- August 2016 Modification Request and September 1, 2016 Approval
- October 27, 2016 Modification Request and Approval
- March 20, 2017 Modification Request and Approval
- September 2017 Modification Request and Approval
- October 2017 Modification Request and Approval
- February 2018 Modification Request and Approval
- October 2018 Modification Request and Approval
- Principal Investigator's 2013-2018 Collaborative Institutional Training Initiative (CITI) Certification
- Principal Investigator's 2018-2023 Collaborative Institutional Training Initiative (CITI) Certification
Brandeis University
- March 28, 2019 Application for Exempt Status and Approval
- April 18, 2019 Modification Request and Approval
- May 6 2022 Mod and Approval
- Exempt determination through Jun 30 2030
Additional Materials to Support Assignment Design
Organizing Assignment-Design Work on Your Campus: A Tool Kit of Resources and Materials.
A Library of DQP Assignments: Building Capacity for a New Model of Assessment
AAC&U VALUE Rubrics (Valid Assessment of Learning in Undergraduate Education)
Decoding Assignments
Please send to [email protected] any additional materials and resources that you develop and would like to share.
Mary-Ann Winkelmes, Ph.D.
Principal Investigator and Founder, TILT Higher Ed

Copyright © 2009-2023 M.A. Winkelmes. TILT Higher Ed © 2009-2023 by Mary-Ann Winkelmes and materials on this website are licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License (CC BY-NC-SA 4.0) except where otherwise noted. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
2009-2023 M.A. Winkelmes
TILT Higher Ed © 2009-2023 by Mary-Ann Winkelmes and materials on this website are licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License (CC BY-NC-SA 4.0) except where otherwise noted. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
- Biology Article
- Vitamins Types Sources
Vitamins - Types, Sources and its Significance

What are Vitamins?
The vitamins are natural and essential nutrients, required in small quantities and play a major role in growth and development, repair and healing wounds, maintaining healthy bones and tissues, for the proper functioning of an immune system, and other biological functions. These essential organic compounds have diverse biochemical functions.
There are thirteen different types of vitamins and all are required for the metabolic processes. The discovery of the vitamins was begun in the year 1912 by a Polish American biochemist Casimir Funk. Based on his research and discoveries on vitamins, their sources, functions and deficiency disorders, he is considered as the father of vitamins and vitamin therapy.
Similar to minerals, vitamins cannot be synthesized by our body. Therefore, we need to get them from the food we consume or in extreme cases supplements to keep ourselves healthy.
Also refer: Vitamins and Minerals
Types of Vitamins
Based on the solubility, Vitamins have been classified into two different groups:
- Fat-Soluble Vitamins.
- Water-Soluble Vitamins.
Fat-soluble vitamin
Fat-soluble vitamins are stored in the fat cells and as the name suggests, these vitamins require fat in order to be absorbed. Vitamin A, D, E and K are fat-soluble vitamins.
Water-soluble vitamin
Water-soluble vitamins are not stored in our body as its excess gets excrete through the urine. Therefore, these vitamins need to be replenished constantly. Vitamin B and C are water-soluble vitamins.
Also Read: Difference between Vitamins and Minerals
Sources of Vitamins
The human body is so designed that it takes what it needs from the food we eat and then it passes out waste as excreta.
These organic substances are abundantly found in both plants and animals source and play a vital role in both growth and development and optimal health.
Listed below are the different types of vitamins along with their sources.
The best sources of fat-soluble vitamins include:
- Vitamin A : Found in potato, carrots, pumpkins, spinach, beef and eggs.
- Vitamin D : Found in fortified milk and other dairy products.
- Vitamin E : Found in fortified cereals, leafy green vegetables, seeds, and nuts.
- Vitamin K : Found in dark green leafy vegetables and in turnip or beet green.
Also Read: Vitamin D Deficiency Symptoms
vitamin C are abundantly found in all citrus fruits. Other sources of Vitamin B and C include:
- Vitamin B1 or Thiamin : Found in pork chops, ham, enriched grains and seeds.
- Vitamin B2 or Riboflavin : Found in whole grains, enriched grains and dairy products.
- Vitamin B3 or Niacin : Found in mushrooms, fish, poultry, and whole grains.
- Vitamin B5 or Pantothenic Acid: Found in chicken, broccoli, legumes and whole grains.
- Vitamin B6 or Pyridoxine : Found in fortified cereals and soy products.
- Vitamin B7 or Biotin : Found in many fruits like fruits and meats.
- Vitamin B9 or Folic Acid : Found in leafy vegetables.
- Vitamin B12 : Found in fish, poultry, meat and dairy products.
- Vitamin C: Found in citrus fruits and juices, such as oranges and grapefruits.
Also read: Vitamin B-12 Deficiency
Learn more in detail about the Vitamins, different types of Vitamins, its sources, deficiency and other related topics at BYJU’S Biology.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Biology related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Thanks for solving my problem
thank you, sir
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

116 Vitamins Essay Topic Ideas & Examples
🏆 best vitamins topic ideas & essay examples, ⭐ simple & easy vitamins essay titles, 📌 most interesting vitamins topics to write about, 👍 good research topics about vitamins, ❓ questions about vitamins.
- Importance of Vitamin C for the Human Body The nutrient is soluble in water and is not stored in the body of human beings. Ascorbic acid is the only vitamin that interacts with both the vitamins and minerals for the benefit of our […]
- Vitamin A: Description and Usage Vitamin A found in fruits and vegetable is referred to as Provitamin A carotenoids, these vitamins A are converted into retinol in the body where one molecule of beta carotene produces two molecule of Vitamin […] We will write a custom essay specifically for you by our professional experts 808 writers online Learn More
- Promotional Strategy of Swisse Vitamins Pty Ltd This study aims at critically analyzing the impact of the use of the ambassadors as a promotional strategy for this firm in creating awareness of the products of this company.
- Comparison of Vitamin C Content in Different Fruits Over 80% of vitamin C in the human diet is obtained from fruits and vegetables. To compare vitamin C content in fresh, frozen and canned pineapples and strawberries.
- Comparison of Vitamin C Levels in Different Vegetables To determine the effect of processing on the vitamin C content of peas and carrots. To establish the difference in vitamin C levels in the different types of peas and carrot samples.
- Vitamins and Dietary Supplement But before this can be done there is a need to understand what the difference between vitamins and dietary supplements. The capsules taken in as dietary supplements are similar looking to the vitamins in capsule […]
- Comparison of Vitamin C Levels in Pineapples and Strawberry This experiment will be comparing the content of vitamin C in fresh, frozen and canned fruits using the direct colorimetric technique.
- An Intervention to Address Vitamin A Deficiency in Ethiopia Irrespective of the definition adopted for vitamin A deficiency, vitamin A plays an important role in the functioning of the body.
- Xerophthalmia: Providing Vitamin A Supplements The lack of vitamin A is a serious concern, especially for kids in underdeveloped nations. The incidence of vitamin A insufficiency generally in India is 17.
- Vitamin and Supplement Treatments: Good or Bad? The notion that vitamin therapy can play a role in the treatment of diseases of the musculoskeletal system is being popularized.
- Vitamin D: Functions and Effects As in the case of LDL, the exact nature of the relationship between vitamin D and HDL remains not fully understood.
- Low Vitamin D and Risk of Premature Death Categories of clear communication index, including the Main Message and Call to Action, Language, Information Design, State of the Science, Behavioral Recommendations, Numbers, and Risks, will evaluate the general consumer publication.
- Vitamin D and Dyslipidemia in Metabolic Syndrome The deficiency of vitamin D indicates a key link to the development of cardiovascular disease and different cardiovascular risk factors like dyslipidemia.
- Complementary Vitamins: Supplements vs. Dietary Sources Some situations compel people to consider the use of additional medicines to increase the supply of vitamins and folic acid in the body.
- The Effect of Vitamin E on Cardiovascular Diseases In conclusion, the apparent difference is linked with the bias during the selection of participants for each study, as observational studies tend to be less objective.
- Vitamin E for Prevention of Heart Diseases As experiments on the benefits of vitamin E show, ‘swimming’ is not always the key to a completely healthy life, in which the risk of a heart attack is reduced to a minimum.
- Vitamin C Serums: Myths Debunked Vitamin C is indeed an important component for healthy skin since it aids in the creation of the skin barrier and collagen in the dermis, as well as the capacity to combat skin oxidation and […]
- Is Vitamin D the New Super Nutrient? The other significance of vitamin D in the body is that it can be manufactured when exposed to sunlight. What sets vitamin D apart from the other nutrients and arguably makes it a super nutrient […]
- Iodine and Vitamin C and Their Function in Body The paper describes the symptoms of toxicity and deficiency of iodine and vitamin C as well as their impact on people’s organisms.
- Vitamin A Supplementation for Infants The meeting was conducted to ensure endless supplementation of Vitamin A to children within the age bracket of 6-59 months of age.
- Evaluating the Effect of Vitamin B12 on Athletic Performance and Strength However, it is crucial to choose the right type of water, as in some cases, it can lead to a deficiency of vitamins, including B12.
- Overview of The Necessity of Vitamin B12 Therefore low serum levels of vitamin B12 should be the indicator of vitamin B12 deficiency and could e confirmed by high MMA in the blood.
- Vitamin C in the Manufactured Products Only the ascorbic acid or vitamin C in the drink will react with the dye. The reduction of vitamin C will be negligible.
- The International Vitamin Corporation in the U.S. Steven Dai’s primary reason for building a vitamin and nutrition supplement factory in the U.S.is to build a long-term market for Chinese customers.
- The Relationship Between Vitamin D Deficiency and Asthma Disease in Children The reaction of the host on the respiratory infections is closely correlated with the deficiency of the vitamin D [1]. This is because of the suggestion that providing vitamin D supplements to patients with low […]
- Vitamin D: Impact on the Immune System The assertions by some researchers are that vitamin D is immunosuppressive while others argue that the vitamin activates the immune system.
- Vitamin D Deficiency: Causes and Consequences The subject is also important since it highlights the predisposing factors of Vitamin D and ways of eradicating it to stop diseases like rickets from affecting infants and children in the world. What is the […]
- Vitamin C in Nutrition: An Informative Leaflet What you have to understand is that Vitamin C plays an important role in the body since it is required in the production of collagen which is a type of protein used in the connective […]
- Prognostic Role of Vitamin D, Vitamin D Levels in Children For instance, the sun is believed to be beneficial for the skin because it helps in the synthesis of vitamin D.however, the sun rays are widely believed to be one of the major causes of […]
- Vitamin B6: Biochemical Overview In order to maintain the proper percentage of Vitamin B6 in the patient’s body, it is imperative that the dietary allowances of the vitamin should be in direct proportion to the patient’s age; more to […]
- Vitamin A Intake and Elevated Serum Retinol Levels The study, therefore, arrived at a conclusion that intake of vitamin A and serum level was high in participants with CF and PI.
- Vitamin D, Round up Glyphosate, Nicotine and Sodium Nitrate Toxicity The curve takes a sigmoid shape The NOAEL is the upper limit which courses no adverse effects and for vitamin D it is 100 mg/L.
- The Drug Interaction With Herbal Products: Vitamins On the other hand, Vitamin C, biotin, pantothenic acid and riboflavin have a very low likelihood of causing toxicity even when taken in large quantities.
- Vitamin C. Recommended Dietary Allowance It also discusses the complications associated with inadequate RDA for vitamin C as well as the effects of excessive intake of the vitamin on the body.
- Vitamin D and Calcium Supplementation Intending to seek facts and evidence surrounding the health benefits of Ca and vitamin D, the research established research gaps like the high prevalence of vitamin D and Ca deficiencies among the study group regardless […]
- The Vitamin Myth: Do We Need Supplements This revelation was a clear indication that the intake of vitamins was dangerous and capable of triggering the occurrence of cancer. The second interesting issue is the argument that vitamin supplements are dangerous and capable […]
- Dietary Supplements: Vitamins Nowadays, there exists a great variety of medications aimed at treating a particular health issue. Due to their number, some of them have quite similar names that can potentially confuse both the patient and pharmacist (Levinthal, 2014). Sometimes, these drugs can be mistaken due to their common active substance, so they do not do serious […]
- Nutritional Science: Vitamins C, E and D This post attempts to discuss the benefits, as well as the detriments, of using E and D vitamin supplements. First of all, it is important to note the positive effects vitamin D has on human […]
- Vitamin C Test: Medical Analysis Thus, the concentration of vitamin C in each solution influences the number of iodine drops that are needed in order to change the color of the homogenate solution to black.
- Vegetarian Diet and Proper Amount of Vitamins Issue This difference was accounted for by 14% lower zinc levels in the vegetarian diet and 21% less efficient absorption of zinc while eating it.
- B12 Vitamin: Risks and Benefits Vitamin B12, also known as cobalamin, is a water-soluble vitamin that contributes to blood cell formation, the work of the nervous system, and metabolism.
- Vitamin C Reducing the Incidence of Common Cold The purpose of this paper is to understand the effects of vitamin C supplements have on the frequency and incidence of the common cold.
- Vitamin C Against Common Cold Incidence Based on the above issues, the findings of the EBP question will help in providing clarity regarding the efficacy of Vitamin C in reducing the symptoms of the common cold.
- The Impact of Health Supplements and Vitamins For instance, vitamin D and calcium can be included in the health supplements to keep the bones of the user stronger.
- Equine Nutrition: Calcium, Phosphorus and Vitamin D Importance Horses require calcium, phosphorus, and vitamin D as macronutrients that are important in the formation and the growth of bones. Hence, horses require calcium to speed the blood clotting process and promote healing of wounds […]
- “Vitamin-Enriched” Bottled Water: PEST Analysis The new “vitamin-enriched” bottled water to be launched in the market requires a critical environmental analysis to guarantee its success in the realms of sales and market penetration. Recognition and ratification of technology is a […]
- Competition in Energy Drinks, Sports Drinks & Vitamin Enhanced Beverage The major strategy of the beverage companies has been to diversify and make enormous extension of their brands in the market.
- The Role of Vitamin D for Tuberculosis Treatment This study investigates the use of vitamin D for the deterrence and cure of tuberculosis and other contagious infections. The unearthing of vitamin D as a therapeutic agent begins with the detection of rickets as […]
- Fat- and Water-Soluble Vitamins In turn, its shortage of these chemical compounds can impair the development of a fetus. For example, the shortage of B vitamins can impair the functioning of the brain.
- The Relationship Between Loss of Bone Mass and Deficiency in Vitamin D After Hematopoietic SCT Bone mineral density was reduced before SCT in 49% of the patients. Pathologic bone mineral density values before SCT were mainly seen in patients with acute leukemia, 55% of the patients with acute leukemia had […]
- The Difference Between Natural and Synthetic Vitamins
- The Role and Importance of Vitamins, Nutrients and Minerals to Our Body
- The Importance of Vitamins and Its Effects on Children
- What Are Vitamins and Why You Need Them
- What Else Are You Getting With Vitamins Supplements
- Vitamins How to Get the Most From Your Diet
- Food Tests to Determine the Presence of Sugar, Starch, Vitamins, and Other Nutrients
- What Circumstances Can Water Soluble Vitamins Be Toxic
- An Analysis of the Advertisement of Vitafusion’s Men’s Vitamins
- An Analysis of Essential Vitamins and Nutrients in Having a Healthy Lifestyle
- Vitamins and Minerals in Modern Society
- What’s the Importance of Vitamins in Our Life
- The Need for Americans to Supplement Their Vitamins
- The Benefits of Natural Vitamins and Supplements
- The Effect of Vitamins on Health and Health
- Water and Fat Soluble Vitamins
- What Are the B Complex Vitamins
- Get a Better Health by Using Vitamins Supplements
- Using Vitamins Supplements as a Treatment
- The Ideas That Strict Diets and Vitamins Can Cure Your Childs Autism
- Vitamins and Herbal Dietary Supplements Market
- Vitamins: Essential for Growth and Development
- Vitamins and Supplements Sector 2015: Market Numbers and Growth Projections
- The Use of Vitamins to Battle Acne
- We All Need These Vitamins Vitamin B12 and Folacin
- Vitamins vs. Chemotherapy and Radiation for Cancer Therapy
- The Essentials of Good Health and the Need for Supplementing Vitamins
- Vitamins: Vitamin and Red Blood Cells
- The Problem With Taking Too Many Vitamins
- Vitamins for Stress Beat Stress With Nutrition
- The Effect of Steaming Process on Fat Soluble Vitamins Content and Fatty Acid Profile in Bluefish and Rainbow Trout Fillets
- The Important Vitamins in the Prevention of the Heart Disease
- The Debate Over the Use of Natural vs. Synthetic Vitamins
- The Importance of Gluten Free Vitamins to Health Care
- Vitamins and Coenzymes in Homocysteine Metabolism
- The Importance of Vitamins and Supplements in Maintaining a Healthy Body
- The Description of Vitamins and Their Importance in the Body
- When and How to Take Vitamins Supplements
- The Use of Herbal Supplements, Vitamins, and Treatment
- Vitamins for Female Hair Loss Prevention
- Vitamins & Nutrient Supplements: History, Use, Effectiveness
- What Is the Difference Between Natural and Synthetic Vitamins?
- What Is the Role and Importance of Vitamins, Nutrients, and Minerals for Our Body?
- How Important Are Vitamins in Life?
- How Do Vitamins Affect Children?
- What Are the Most Important Vitamins?
- What Else Do You Get From Vitamin Supplements?
- Are Vitamins Really Good for Human Health?
- Which Vitamin Is Most Powerful?
- What Can We Know Useful About Vitamins?
- How to Get the Most Out of Your Diet?
- What Tests Are Available to Determine the Presence of Sugar, Starch, Vitamins, and Other Nutrients?
- How to Improve Your Love Life With Vitamins?
- How to Make Vitamins Simple and Understandable?
- Under What Circumstances Can Water-Soluble Vitamins Be Toxic?
- What Do We Borrow About Vitamins and Minerals in Today’s Society?
- What Is the Need for Americans to Supplement Their Vitamins?
- What Are the Benefits of Natural Vitamins and Supplements?
- How Do Vitamins Affect Health?
- What Is the Main Biochemical Function of B Vitamins?
- What Are the B Complex Vitamins?
- How to Use Vitamin Supplements as a Treatment?
- What Are the Problems With Taking Too Many Vitamins?
- What Is the Relationship Between Vitamins and Smoking Cessation?
- Why Can’t Vitamins Cure a Cold?
- How Vitamins Can Help Eliminate Fatigue?
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, March 2). 116 Vitamins Essay Topic Ideas & Examples. https://ivypanda.com/essays/topic/vitamins-essay-topics/
"116 Vitamins Essay Topic Ideas & Examples." IvyPanda , 2 Mar. 2024, ivypanda.com/essays/topic/vitamins-essay-topics/.
IvyPanda . (2024) '116 Vitamins Essay Topic Ideas & Examples'. 2 March.
IvyPanda . 2024. "116 Vitamins Essay Topic Ideas & Examples." March 2, 2024. https://ivypanda.com/essays/topic/vitamins-essay-topics/.
1. IvyPanda . "116 Vitamins Essay Topic Ideas & Examples." March 2, 2024. https://ivypanda.com/essays/topic/vitamins-essay-topics/.
Bibliography
IvyPanda . "116 Vitamins Essay Topic Ideas & Examples." March 2, 2024. https://ivypanda.com/essays/topic/vitamins-essay-topics/.
- Dietary Supplements Questions
- Vegetarianism Essay Ideas
- Malnutrition Titles
- Veganism Essay Ideas
- Therapy Paper Topics
- Stress Titles
- Allergy Research Ideas
- Cooking Questions
- Food Essay Ideas
- Health Promotion Research Topics
- Sugar Paper Topics
- Weight Loss Essay Titles
- Meat Research Ideas
- Wellness Essay Topics
- Anorexia Essay Ideas
ED10FA2 – From graduate to mentor: completing the Victorian Institute of Teaching (VIT) inquiry
Oct 7, 2019 | Teacher wellbeing and resillience | 0 comments

The Victorian Institute of Teaching (VIT) is an independent statutory authority for the teaching profession, whose primary function is to regulate members of the teaching profession.
Most searchable topics
Renewal of registration
Technical support
Pre-service teachers
For employers

IMAGES
COMMENTS
Documenting Evidence VIT. Published on Nov 10, 2015. Kids education. Leah Cardenas. Leah Cardenas. Issuu converts static files into: digital portfolios, online yearbooks, online catalogs, digital ...
stephjs81. • 3 yr. ago. just fyi, camps are cancelled for the term. I considered doing my inquiry in term 4 but I teach secondary and most of my kids will be done with academic work in week 6, unless exams are cancelled. Also, report writing will need to be started in week 7 and there was no way I could do both.
Peruse it to see all of the foods with their vitamin contents. Select 3 veggies, 3 fruits, and 3 fish to investigate. Then make columns or a spreadsheet with these 9 foods and their Daily Values. Find the following information for each food and compare side by side on the worksheet. Daily Value of: caloric content; fat content; sodium content ...
vitamin B 1. component of a coenzyme in carbohydrate metabolism; supports normal nerve function. impairment of the nerves and heart muscle wasting. riboflavin. vitamin B 2. component of coenzymes required for energy production and lipid, vitamin, mineral, and drug metabolism; antioxidant. inflammation of the skin, tongue, and lips; ocular ...
The architecture of the ViT with specific details on the transformer encoder and the MSA block. Keep this picture in mind. Picture from Bazi et. al.. By the picture, we see that the input image (a ...
Remember, it is about being authentic, this is not a graded assignment based on the outcomes of student growth. To support your reflection, consider the following questions: ... including videos and worked examples of the portfolio. ... Victorian Institute of Teaching 2019a, Australian Professional Standards for Teachers, viewed 6 August 2019 ...
VIT Assignments for Matlab and stuff. I store my usual bullshit here. - GitHub - gaganmalvi/matlab_examples: VIT Assignments for Matlab and stuff. I store my usual bullshit here.
What are the 13 types of vitamins? The list of essential vitamins includes the following 13 vitamins: Vitamin A, Vitamin B1, Vitamin B2, Vitamin B3, Vitamin B5, Vitamin B6, Vitamin B7, Vitamin B9 ...
Recommended Daily Intake of Vitamins and Minerals for Adults. Vitamin (Common Names) Recommended Dietary Allowance (RDA) or Daily Adequate Intake (AI)*. Upper Limit. Women. Men. Vitamin A (preformed = retinol; beta-carotene can be converted to Vitamin A) 700 micrograms (2,333 IU) 900 micrograms (3,000 IU)
PO Box 531, Collins Street West VIC 8007 | e. [email protected] | t. 1300 888 067 | f. (03) 8601 6101 | www.vit.vic.edu.au Documenting evidence This is a sample of one early childhood teacher's evidence. It is important to note that evidence may vary significantly from this particular sample due to the context of each early
VIT. University; Vellore Institute of Technology; Courses (345) ... The objective of this assignment is to explore and analyze the distinct consumer buying behaviors associated with utilitarian products of low value and hedonic products of high value. ... Choose a product that fulfills a basic need or serves a practical purpose. Examples: Basic ...
The VIT will assess the PRT's application along with the panel recommendation. When all requirements have been met, the PRT will be granted full registration. ... this is not a university assignment - you should present enough information to understand the Inquiry question and evidence used to demonstrate your proficiency ... examples of ...
PURPOSE: The purpose of this assignment is to take an in-depth look at your personal vitamin and mineral intake. You'll. also discuss a case study about vitamin and mineral supplements and the DASH Diet. Then, you'll evaluate your overall vitamin and mineral intake and discuss specific ways to improve. INSTRUCTIONS
Vitamin C is a six carbon chain, closely related chemically to glucose. It was first isolated in 1928 by the Hungarian-born scientist Szent-Gyorgi and structurally characterized by Haworth in 1933. In 1934, Rechstein worked out a simple, inexpensive, four-step process for synthesizing ascorbic acid from glucose.
Check Pages 1-40 of VIT Project in the flip PDF version. VIT Project was published by jedwards on 2020-05-20. ... Flipped learning caters perfectly to these learners since all theory and examples are presented as videos that can be watched, paused, watched again, allowing these learners extra time to absorb the English used within the videos ...
Higher Chemistry - assignment. Assignment 2023 (All links open to PDF files) Full project: Vitamin C Concentration in Orange Juice. Full Project Candidate Evidence. Full Project Commentary. Sections: Summary, analysis, and evaluation sections. Sections Candidate Evidence. Sections Commentary. Assignment 2022 (All links open to PDF files)
Value assignment of the 50 single donor samples described here represents a fundamental component of the VDSP and supports its goal to improve clinical and public health practice worldwide. In addition, this study provided evidence of the comparability of two RMPs for vitamin D metabolites, providing further confidence in measurements based on ...
The following resources from the Transparency in Learning and Teaching project (TILT Higher Ed) can help faculty, educational developers and administrators to apply the Transparency Framework (of purpose/task/criteria) in contexts including assignments, curricula, assessment and strategic initiatives, all toward the goal of enhancing student ...
The best sources of fat-soluble vitamins include: Vitamin A: Found in potato, carrots, pumpkins, spinach, beef and eggs. Vitamin D: Found in fortified milk and other dairy products. Vitamin E: Found in fortified cereals, leafy green vegetables, seeds, and nuts. Vitamin K: Found in dark green leafy vegetables and in turnip or beet green.
For instance, vitamin D and calcium can be included in the health supplements to keep the bones of the user stronger. Equine Nutrition: Calcium, Phosphorus and Vitamin D Importance. Horses require calcium, phosphorus, and vitamin D as macronutrients that are important in the formation and the growth of bones.
Vitamin C is the compound ascorbic acid with the molecular formula C6H8O6. The body uses ascorbic acid when making collagen, a protein which helps skin, bone, hair and blood vessels stick together. Ascorbic acid also helps the body absorb iron. We need to take in about 90 mg each day.
This might include collecting work samples, recording conversations with colleagues, photos, and videos of your practice. ... Remember, it is about being authentic, this is not a graded assignment based on the outcomes of student growth. To support your reflection, consider the following questions: ... Victorian Institute of Teaching 2019b ...
The Victorian Institute of Teaching (VIT) is an independent statutory authority for the teaching profession, whose primary function is to regulate members of the teaching profession. Read more. How can we help? Most searchable topics View all . Renewal of registration. Technical support. Pre-service teachers. For employers.