

Blood cells and its types with functions
Blood cells are the cells which are produced during hematopoiesis and found mainly in the blood. Blood is composed of the blood cells which accounts for 45% of the blood tissue by volume, with the remaining 55% of the volume composed of plasma, the liquid portion of the blood.
There are three types of blood cells. They are:
- Red blood cells (Erythrocytes)
- White blood cells (Leukocytes)
- Platelets (Thrombocytes)
1. Red Blood Cells (Erythrocytes)
- Account for approximately 40 to 45 percent of the blood.
- Biconcave disc which is round and flat, sort of like a shallow bowl.
- Disk diameter of approximately 6.2-8.2 µm.
- They have a thick rim and a thin sunken center.
- Nucleus Absent.
- Can change shape without breaking.
- Production of RBCs is controlled by erythropoietin.
- RBC contains hemoglobin (33%).
- The iron found in hemoglobin gives the blood its red color.
- RBCs cannot repair themselves.
- Life span of 120 days.
- 4 million new erythrocytes are produced per second in human adults.
- 20–30 trillion red blood cells at any given time.
- Male: 4.3-5.9 million/mm 3 and Female: 3.5-5.5 million/mm 3
- Transport oxygen from the lungs to the cells of the body.
- Pick up carbon dioxide from other tissues and unload it in the lungs.
2. White Blood Cells (Leukocytes)
- Account for only about 1% of the blood.
- 4500-11,000/mm 3
- They are the cells that make up the majority of the immune system.
- It is the part of the body that protects itself against foreign substances and various types of infections.
- They are made in the bone marrow from multi-potent cells called hematopoietic stem cells.
- They exist in all parts of the body, including the connective tissue, lymph system, and the bloodstream.
- Leukopenia is a low white blood cell count that can be caused by damage to the bone marrow from things like medications, radiation, or chemotherapy.
- Leukocytosis is a high white blood cell count that can be caused by a number of conditions, including various types of infections, inflammatory disease in the body.
- They are divided into Granulocytes (having visible granules or grains inside the cells) and Agranulocytes (free of visible grains under the microscope).
- There are five main types of WBCs.: Neutrophils (granulocytes), Eosinophils (granulocytes), Basophils (granulocytes), Lymphocytes (non-granulocytes) and Monocytes (non-granulocytes).
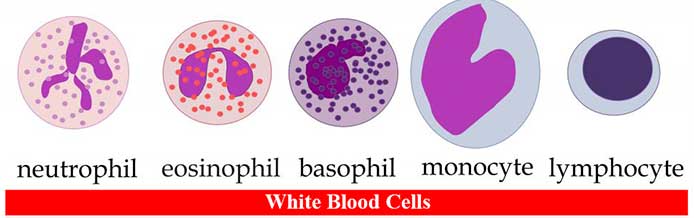
A. Neutrophils (granulocytes)
- Most common type of white blood cell.
- Accounts for 62% of Leukocytes
- Multi-lobed Nucleus present.
- Contain very fine cytoplasmic granules.
- 2000 to 7500 cells per mm 3
- Medium-sized white blood cells.
- Also called polymorphonuclear (PMN) because they have a variety of nuclear shapes.
- Diameter of 10–12 μm.
- Life span of 6 hours to few days.
- Kills bacteria through the process of phagocytosis.
- They also release a burst of super oxides that have the ability to kill many bacteria at the same time.
B. Eosinophils (granulocytes)
- 40-400 cells per mm 3
- Have large granules
- Nucleus is divided into two lobes (bi-lobed nucleus)
- Accounts for 2.3%
- Life span of 8–12 days
- Kills parasites and have a role in allergic reactions.
- Releases toxins from their granules to kill pathogens.
C. Basophils (granulocytes)
- 0-100 cells per mm 3
- Colorful when stained and looked at under the microscope
- They have a pale nucleus that is usually hidden by granules.
- Bi-lobed or Tri-lobed nucleus present.
- Diameter of 12–15 μm.
- Accounts for 0.4%
- Life span of few hours to few days.
- Functions in allergic reactions.
- Secrete anticoagulants and antibodies that have function against hypersensitivity reactions in the bloodstream.
- Basophils contain histamine, which dilates the vessels to bring more immune cells to the area of injury.
- Secrete heparin which is an anticoagulant that promotes mobility of other WBCs by preventing clotting.
D. Lymphocytes (Agranulocytes)
- Small rounded cells
- Nucleus Present
- 1300 to 4000 per mm 3
- Diameter of 7-8 μm (Small) and 12-15 μm (Large)
- Accounts for 30%
- Life span of years for memory cells and weeks for all else.
- T lymphocytes (T cells) are responsible for cell-mediated immunity.
- B lymphocytes are responsible for humoral immunity or antibody production.
- They can recognize and have a memory of invading bacteria and viruses.
- Function in destroying cancer cells.
- They present antigens to activate other cells of the immune system.
E. Monocytes (Agranulocytes)
- Largest of the types of white blood cells
- Kidney shaped nucleus present.
- 200 to 800 monocytes per mm3
- Turn into macrophages when they exit the bloodstream.
- Diameter of 15-30 μm.
- Accounts for 5.3%
- Enters the tissue, where they become larger and turn into macrophages.
- Destroy old, damaged and dead cells in the body.
3. Platelets (Thrombocytes)
- Do not reproduce.
- Small fragments of bone marrow cells.
- 150,000–400,000 platelets in each microliter of human blood.
- Platelets are the parts of cells that the body uses for clotting.
- Helps to promote other blood clotting mechanisms. Example: Secrete procoagulants (clotting factors) to promote blood clotting.
- They secrete vasoconstrictors which constrict blood vessels, causing vascular spasms in broken blood vessels.
- They secrete chemicals that attract neutrophils and monocytes to sites of inflammation.
- Dissolve blood clots when they are no longer needed.
- Digest and destroy bacteria.
- They secrete growth factors to maintain the linings of blood vessels.
- https://www.ncbi.nlm.nih.gov/books/NBK2263/
- https://www.hematology.org/Patients/Basics/
- https://www.healthline.com/health/blood-cell-disorders
- https://www.healthline.com/health/wbc-count?m=0
- https://www.urmc.rochester.edu/encyclopedia/content.aspx?ContentTypeID=160&ContentID=34
- https://en.wikipedia.org/wiki/Red_blood_cell
- https://en.wikipedia.org/wiki/White_blood_cell
- https://en.wikipedia.org/wiki/Blood_cell
- https://www.urmc.rochester.edu/encyclopedia/content.aspx?ContentTypeID=160&ContentID=35
- https://www.medicalnewstoday.com/articles/315133.php
- https://www.webmd.com/heart/anatomy-picture-of-blood#1
- https://www.mayoclinic.org/symptoms/low-white-blood-cell-count/basics/causes/sym-20050615
- https://www.mayoclinic.org/symptoms/high-white-blood-cell-count/basics/causes/sym-20050611
- https://www.fi.edu/heart/red-blood-cells
- https://web.mit.edu/scicom/www/blood.html
- https://www.boundless.com/physiology/textbooks/boundless-anatomy-and-physiology-textbook/cardiovascular-system-blood-17/white-blood-cells-166/types-of-wbcs-831-7902/
- https://www.myvmc.com/anatomy/blood-function-and-composition/
Similar Posts:
- Normal Laboratory Values of Blood, Plasma, Serum, Urine, CSF and Stool
- Differences Between B-Cells and T-Cells
- Difference between Serum and Plasma
- Histoplasma Capsulatum – Habitat, Morphology, Epidemiology, Virulence Factors, Treatment + More
6 thoughts on “Blood cells and its types with functions”
Very educative, simplified and concise
so are they bacically the same just different functions
Which part of human digestive system helps to keep human system active in process of digestion?
What part of blood type acts to stir up the reactions usually given by anxiety, how does it work?
B-lymphocytes
is there not also the types, structure, function, and origin of the list of the component of blood the origin of one cell per mm cubic.
Leave a Comment Cancel reply
Save my name and email in this browser for the next time I comment.
18.1 An Overview of Blood
Learning objectives.
By the end of this section, you will be able to:
- Identify the primary functions of blood in transportation, defense, and maintenance of homeostasis
- Name the fluid component of blood and the three major types of formed elements, and identify their relative proportions in a blood sample
- Discuss the unique physical characteristics of blood
- Identify the composition of blood plasma, including its most important solutes and plasma proteins
Recall that blood is a connective tissue. Like all connective tissues, it is made up of cellular elements and an extracellular matrix. The cellular elements—referred to as the formed elements —include red blood cells (RBCs) , white blood cells (WBCs) , and cell fragments called platelets . The extracellular matrix, called plasma , makes blood unique among connective tissues because it is fluid. This fluid, which is mostly water, perpetually suspends the formed elements and enables them to circulate throughout the body within the cardiovascular system.
Functions of Blood
The primary function of blood is to deliver oxygen and nutrients to and remove wastes from body cells, but that is only the beginning of the story. The specific functions of blood also include defense, distribution of heat, and maintenance of homeostasis.
Transportation
Nutrients from the foods you eat are absorbed in the digestive tract. Most of these travel in the bloodstream directly to the liver, where they are processed and released back into the bloodstream for delivery to body cells. Oxygen from the air you breathe diffuses into the blood, which moves from the lungs to the heart, which then pumps it out to the rest of the body. Moreover, endocrine glands scattered throughout the body release their products, called hormones, into the bloodstream, which carries them to distant target cells. Blood also picks up cellular wastes and byproducts, and transports them to various organs for removal. For instance, blood moves carbon dioxide to the lungs for exhalation from the body, and various waste products are transported to the kidneys and liver for excretion from the body in the form of urine or bile.
Many types of WBCs protect the body from external threats, such as disease-causing bacteria that have entered the bloodstream in a wound. Other WBCs seek out and destroy internal threats, such as cells with mutated DNA that could multiply to become cancerous, or body cells infected with viruses.
When damage to the vessels results in bleeding, blood platelets and certain proteins dissolved in the plasma, the fluid portion of the blood, interact to block the ruptured areas of the blood vessels involved. This protects the body from further blood loss.
Maintenance of Homeostasis
Recall that body temperature is regulated via a classic negative-feedback loop. If you were exercising on a warm day, your rising core body temperature would trigger several homeostatic mechanisms, including increased transport of blood from your core to your body periphery, which is typically cooler. As blood passes through the vessels of the skin, heat would be dissipated to the environment, and the blood returning to your body core would be cooler. In contrast, on a cold day, blood is diverted away from the skin to maintain a warmer body core. In extreme cases, this may result in frostbite.
Blood also helps to maintain the chemical balance of the body. Proteins and other compounds in blood act as buffers, which thereby help to regulate the pH of body tissues. Blood also helps to regulate the water content of body cells.
Composition of Blood
You have probably had blood drawn from a superficial vein in your arm, which was then sent to a lab for analysis. Some of the most common blood tests—for instance, those measuring lipid or glucose levels in plasma—determine which substances are present within blood and in what quantities. Other blood tests check for the composition of the blood itself, including the quantities and types of formed elements.
One such test, called a hematocrit , measures the percentage of RBCs, clinically known as erythrocytes, in a blood sample. It is performed by spinning the blood sample in a specialized centrifuge, a process that causes the heavier elements suspended within the blood sample to separate from the lightweight, liquid plasma ( Figure 18.2 ). Because the heaviest elements in blood are the erythrocytes, these settle at the very bottom of the hematocrit tube. Located above the erythrocytes is a pale, thin layer composed of the remaining formed elements of blood. These are the WBCs, clinically known as leukocytes, and the platelets, cell fragments also called thrombocytes. This layer is referred to as the buffy coat because of its color; it normally constitutes less than 1 percent of a blood sample. Above the buffy coat is the blood plasma, normally a pale, straw-colored fluid, which constitutes the remainder of the sample.
The volume of erythrocytes after centrifugation is also commonly referred to as packed cell volume (PCV) . In normal blood, about 45 percent of a sample is erythrocytes. The hematocrit of any one sample can vary significantly, however, about 36–50 percent, according to gender and other factors. Normal hematocrit values for females range from 37 to 47, with a mean value of 41; for males, hematocrit ranges from 42 to 52, with a mean of 47. The percentage of other formed elements, the WBCs and platelets, is extremely small so it is not normally considered with the hematocrit. So the mean plasma percentage is the percent of blood that is not erythrocytes: for females, it is approximately 59 (or 100 minus 41), and for males, it is approximately 53 (or 100 minus 47).
Characteristics of Blood
When you think about blood, the first characteristic that probably comes to mind is its color. Blood that has just taken up oxygen in the lungs is bright red, and blood that has released oxygen in the tissues is a more dusky red. This is because hemoglobin is a pigment that changes color, depending upon the degree of oxygen saturation.
Blood is viscous and somewhat sticky to the touch. It has a viscosity approximately five times greater than water. Viscosity is a measure of a fluid’s thickness or resistance to flow, and is influenced by the presence of the plasma proteins and formed elements within the blood. The viscosity of blood has a dramatic impact on blood pressure and flow. Consider the difference in flow between water and honey. The more viscous honey would demonstrate a greater resistance to flow than the less viscous water. The same principle applies to blood.
The normal temperature of blood is slightly higher than normal body temperature—about 38 °C (or 100.4 °F), compared to 37 °C (or 98.6 °F) for an internal body temperature reading, although daily variations of 0.5 °C are normal. Although the surface of blood vessels is relatively smooth, as blood flows through them, it experiences some friction and resistance, especially as vessels age and lose their elasticity, thereby producing heat. This accounts for its slightly higher temperature.
The pH of blood averages about 7.4; however, it can range from 7.35 to 7.45 in a healthy person. Blood is therefore somewhat more basic (alkaline) on a chemical scale than pure water, which has a pH of 7.0. Blood contains numerous buffers that actually help to regulate pH.
Blood constitutes approximately 8 percent of adult body weight. Adult males typically average about 5 to 6 liters of blood. Females average 4–5 liters.
Blood Plasma
Like other fluids in the body, plasma is composed primarily of water: In fact, it is about 92 percent water. Dissolved or suspended within this water is a mixture of substances, most of which are proteins. There are literally hundreds of substances dissolved or suspended in the plasma, although many of them are found only in very small quantities.
Interactive Link
Visit this site for a list of normal levels established for many of the substances found in a sample of blood. Serum, one of the specimen types included, refers to a sample of plasma after clotting factors have been removed. What types of measurements are given for levels of glucose in the blood?
Plasma Proteins
About 7 percent of the volume of plasma—nearly all that is not water—is made of proteins. These include several plasma proteins (proteins that are unique to the plasma), plus a much smaller number of regulatory proteins, including enzymes and some hormones. The major components of plasma are summarized in Figure 18.3 .
The three major groups of plasma proteins are as follows:
- Albumin is the most abundant of the plasma proteins. Manufactured by the liver, albumin molecules serve as binding proteins—transport vehicles for fatty acids and steroid hormones. Recall that lipids are hydrophobic; however, their binding to albumin enables their transport in the watery plasma. Albumin is also the most significant contributor to the osmotic pressure of blood; that is, its presence holds water inside the blood vessels and draws water from the tissues, across blood vessel walls, and into the bloodstream. This in turn helps to maintain both blood volume and blood pressure. Albumin normally accounts for approximately 54 percent of the total plasma protein content, in clinical levels of 3.5–5.0 g/dL blood.
- The second most common plasma proteins are the globulins . A heterogeneous group, there are three main subgroups known as alpha, beta, and gamma globulins. The alpha and beta globulins transport iron, lipids, and the fat-soluble vitamins A, D, E, and K to the cells; like albumin, they also contribute to osmotic pressure. The gamma globulins are proteins involved in immunity and are better known as antibodies or immunoglobulins . Although other plasma proteins are produced by the liver, immunoglobulins are produced by specialized leukocytes known as plasma cells. (Seek additional content for more information about immunoglobulins.) Globulins make up approximately 38 percent of the total plasma protein volume, in clinical levels of 1.0–1.5 g/dL blood.
- Fibrinogen is the third of the three major groups of plasma proteins. Like albumin and the alpha and beta globulins, fibrinogen is produced by the liver. It is essential for blood clotting, a process described later in this chapter. Fibrinogen accounts for about 7 percent of the total plasma protein volume, in clinical levels of 0.2–0.45 g/dL blood.
Other Plasma Solutes
In addition to proteins, plasma contains a wide variety of other substances. These include various electrolytes, such as sodium, potassium, and calcium ions; dissolved gases, such as oxygen, carbon dioxide, and nitrogen; various organic nutrients, such as vitamins, lipids, glucose, and amino acids; and metabolic wastes. All of these nonprotein solutes combined contribute approximately 1 percent to the total volume of plasma.
Career Connection
Phlebotomy and medical lab technology.
Phlebotomists are professionals trained to draw blood (phleb- = “a blood vessel”; -tomy = “to cut”). When more than a few drops of blood are required, phlebotomists perform a venipuncture, typically of a surface vein in the arm. They perform a capillary stick on a finger, an earlobe, or the heel of an infant when only a small quantity of blood is required. An arterial stick is collected from an artery and used to analyze blood gases. After collection, the blood may be analyzed by medical laboratories or perhaps used for transfusions, donations, or research. While many allied health professionals practice phlebotomy, the American Society of Phlebotomy Technicians issues certificates to individuals passing a national examination, and some large labs and hospitals hire individuals expressly for their skill in phlebotomy.
Medical or clinical laboratories employ a variety of individuals in technical positions:
- Medical technologists (MT), also known as clinical laboratory technologists (CLT), typically hold a bachelor’s degree and certification from an accredited training program. They perform a wide variety of tests on various body fluids, including blood. The information they provide is essential to the primary care providers in determining a diagnosis and in monitoring the course of a disease and response to treatment.
- Medical laboratory technicians (MLT) typically have an associate’s degree but may perform duties similar to those of an MT.
- Medical laboratory assistants (MLA) spend the majority of their time processing samples and carrying out routine assignments within the lab. Clinical training is required, but a degree may not be essential to obtaining a position.
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/anatomy-and-physiology-2e/pages/1-introduction
- Authors: J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble, Peter DeSaix
- Publisher/website: OpenStax
- Book title: Anatomy and Physiology 2e
- Publication date: Apr 20, 2022
- Location: Houston, Texas
- Book URL: https://openstax.org/books/anatomy-and-physiology-2e/pages/1-introduction
- Section URL: https://openstax.org/books/anatomy-and-physiology-2e/pages/18-1-an-overview-of-blood
© Dec 19, 2023 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
Module 18: The Circulatory System
Structure and function of blood, describe the structure and function of blood in the body.
Blood is important for regulation of the body’s pH, temperature, osmotic pressure, the circulation of nutrients and removal of waste, the distribution of hormones from endocrine glands, and the elimination of excess heat; it also contains components for blood clotting. Blood is made of of several components, including red blood cells, white blood cells, platelets, and the plasma, which contains coagulation factors and serum.
Learning Objectives
- Identify the role of blood in the body
- Compare red and white blood cells
- Describe the basic components of the blood
The Role of Blood in the Body
Blood, like the human blood illustrated in Figure 1 is important for regulation of the body’s systems and homeostasis. Blood helps maintain homeostasis by stabilizing pH, temperature, osmotic pressure, and by eliminating excess heat. Blood supports growth by distributing nutrients and hormones, and by removing waste. Red blood cells contain hemoglobin, which binds oxygen. These cells deliver oxygen to the cells and remove carbon dioxide.
Blood plays a protective role by transporting clotting factors and platelets to prevent blood loss after injury. Blood also transports the disease-fighting agents white blood cells to sites of infection. These cells—including neutrophils, monocytes, lymphocytes, eosinophils, and basophils—are involved in the immune response.
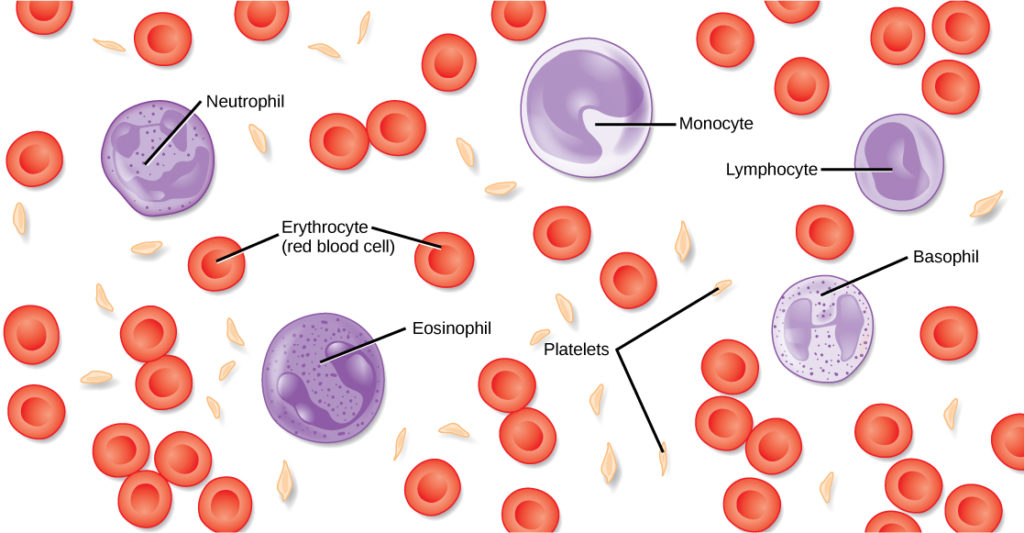
Figure 1. The cells and cellular components of human blood are shown.
Red Blood Cells
Red blood cells , or erythrocytes ( erythro – = “red”; – cyte = “cell”), are specialized cells that circulate through the body delivering oxygen to cells; they are formed from stem cells in the bone marrow. In mammals, red blood cells are small biconcave cells that at maturity do not contain a nucleus or mitochondria and are only 7–8 µm in size. In birds and non-avian reptiles, a nucleus is still maintained in red blood cells.
The red coloring of blood comes from the iron-containing protein hemoglobin, illustrated in Figure 2a. The principal job of this protein is to carry oxygen, but it also transports carbon dioxide as well. Hemoglobin is packed into red blood cells at a rate of about 250 million molecules of hemoglobin per cell. Each hemoglobin molecule binds four oxygen molecules so that each red blood cell carries one billion molecules of oxygen. There are approximately 25 trillion red blood cells in the five liters of blood in the human body, which could carry up to 25 sextillion (25 × 10 21 ) molecules of oxygen in the body at any time. In mammals, the lack of organelles in erythrocytes leaves more room for the hemoglobin molecules, and the lack of mitochondria also prevents use of the oxygen for metabolic respiration. Only mammals have anucleated red blood cells, and some mammals (camels, for instance) even have nucleated red blood cells. The advantage of nucleated red blood cells is that these cells can undergo mitosis. Anucleated red blood cells metabolize anaerobically (without oxygen), making use of a primitive metabolic pathway to produce ATP and increase the efficiency of oxygen transport.
Not all organisms use hemoglobin as the method of oxygen transport. Invertebrates that utilize hemolymph rather than blood use different pigments to bind to the oxygen. These pigments use copper or iron to the oxygen. Invertebrates have a variety of other respiratory pigments. Hemocyanin, a blue-green, copper-containing protein, illustrated in Figure 2b is found in mollusks, crustaceans, and some of the arthropods. Chlorocruorin, a green-colored, iron-containing pigment is found in four families of polychaete tubeworms. Hemerythrin, a red, iron-containing protein is found in some polychaete worms and annelids and is illustrated in Figure 2c. Despite the name, hemerythrin does not contain a heme group and its oxygen-carrying capacity is poor compared to hemoglobin.

Figure 2. In most vertebrates, (a) hemoglobin delivers oxygen to the body and removes some carbon dioxide. Hemoglobin is composed of four protein subunits, two alpha chains and two beta chains, and a heme group that has iron associated with it. The iron reversibly associates with oxygen, and in so doing is oxidized from Fe 2+ to Fe 3+ . In most mollusks and some arthropods, (b) hemocyanin delivers oxygen. Unlike hemoglobin, hemolymph is not carried in blood cells, but floats free in the hemolymph. Copper instead of iron binds the oxygen, giving the hemolymph a blue-green color. In annelids, such as the earthworm, and some other invertebrates, (c) hemerythrin carries oxygen. Like hemoglobin, hemerythrin is carried in blood cells and has iron associated with it, but despite its name, hemerythrin does not contain heme.
The small size and large surface area of red blood cells allows for rapid diffusion of oxygen and carbon dioxide across the plasma membrane. In the lungs, carbon dioxide is released and oxygen is taken in by the blood. In the tissues, oxygen is released from the blood and carbon dioxide is bound for transport back to the lungs. Studies have found that hemoglobin also binds nitrous oxide (NO). NO is a vasodilator that relaxes the blood vessels and capillaries and may help with gas exchange and the passage of red blood cells through narrow vessels. Nitroglycerin, a heart medication for angina and heart attacks, is converted to NO to help relax the blood vessels and increase oxygen flow through the body.
A characteristic of red blood cells is their glycolipid and glycoprotein coating; these are lipids and proteins that have carbohydrate molecules attached. In humans, the surface glycoproteins and glycolipids on red blood cells vary between individuals, producing the different blood types, such as A, B, and O. Red blood cells have an average life span of 120 days, at which time they are broken down and recycled in the liver and spleen by phagocytic macrophages, a type of white blood cell.
White Blood Cells
White blood cells, also called leukocytes (leuko = white), make up approximately one percent by volume of the cells in blood. The role of white blood cells is very different than that of red blood cells: they are primarily involved in the immune response to identify and target pathogens, such as invading bacteria, viruses, and other foreign organisms. White blood cells are formed continually; some only live for hours or days, but some live for years.
The morphology of white blood cells differs significantly from red blood cells. They have nuclei and do not contain hemoglobin. The different types of white blood cells are identified by their microscopic appearance after histologic staining, and each has a different specialized function. The two main groups, both illustrated in Figure 3 are the granulocytes, which include the neutrophils, eosinophils, and basophils, and the agranulocytes, which include the monocytes and lymphocytes.

Figure 3. (a) Granulocytes—including neutrophils, eosinophils and basophils—are characterized by a lobed nucleus and granular inclusions in the cytoplasm. Granulocytes are typically first-responders during injury or infection. (b) Agranulocytes include lymphocytes and monocytes. Lymphocytes, including B and T cells, are responsible for adaptive immune response. Monocytes differentiate into macrophages and dendritic cells, which in turn respond to infection or injury.
Granulocytes contain granules in their cytoplasm; the agranulocytes are so named because of the lack of granules in their cytoplasm. Some leukocytes become macrophages that either stay at the same site or move through the blood stream and gather at sites of infection or inflammation where they are attracted by chemical signals from foreign particles and damaged cells. Lymphocytes are the primary cells of the immune system and include B cells, T cells, and natural killer cells. B cells destroy bacteria and inactivate their toxins. They also produce antibodies. T cells attack viruses, fungi, some bacteria, transplanted cells, and cancer cells. T cells attack viruses by releasing toxins that kill the viruses. Natural killer cells attack a variety of infectious microbes and certain tumor cells.
One reason that HIV poses significant management challenges is because the virus directly targets T cells by gaining entry through a receptor. Once inside the cell, HIV then multiplies using the T cell’s own genetic machinery. After the HIV virus replicates, it is transmitted directly from the infected T cell to macrophages. The presence of HIV can remain unrecognized for an extensive period of time before full disease symptoms develop
Components of Blood
Hemoglobin is responsible for distributing oxygen, and to a lesser extent, carbon dioxide, throughout the circulatory systems of humans, vertebrates, and many invertebrates. The blood is more than the proteins, though. Blood is actually a term used to describe the liquid that moves through the vessels and includes plasma (the liquid portion, which contains water, proteins, salts, lipids, and glucose) and the cells (red and white cells) and cell fragments called platelets . Blood plasma is actually the dominant component of blood and contains the water, proteins, electrolytes, lipids, and glucose. The cells are responsible for carrying the gases (red cells) and immune the response (white). The platelets are responsible for blood clotting. Interstitial fluid that surrounds cells is separate from the blood, but in hemolymph, they are combined. In humans, cellular components make up approximately 45 percent of the blood and the liquid plasma 55 percent. Blood is 20 percent of a person’s extracellular fluid and eight percent of weight.
Platelets and Coagulation Factors
Blood must clot to heal wounds and prevent excess blood loss. Small cell fragments called platelets (thrombocytes) are attracted to the wound site where they adhere by extending many projections and releasing their contents. These contents activate other platelets and also interact with other coagulation factors, which convert fibrinogen, a water-soluble protein present in blood serum into fibrin (a non-water soluble protein), causing the blood to clot. Many of the clotting factors require vitamin K to work, and vitamin K deficiency can lead to problems with blood clotting. Many platelets converge and stick together at the wound site forming a platelet plug (also called a fibrin clot), as illustrated in Figure 4b. The plug or clot lasts for a number of days and stops the loss of blood. Platelets are formed from the disintegration of larger cells called megakaryocytes, like that shown in Figure 4a. For each megakaryocyte, 2000–3000 platelets are formed with 150,000 to 400,000 platelets present in each cubic millimeter of blood. Each platelet is disc shaped and 2–4 μm in diameter. They contain many small vesicles but do not contain a nucleus.

Figure 4. (a) Platelets are formed from large cells called megakaryocytes. The megakaryocyte breaks up into thousands of fragments that become platelets. (b) Platelets are required for clotting of the blood. The platelets collect at a wound site in conjunction with other clotting factors, such as fibrinogen, to form a fibrin clot that prevents blood loss and allows the wound to heal.
Plasma and Serum
The liquid component of blood is called plasma, and it is separated by spinning or centrifuging the blood at high rotations (3000 rpm or higher). The blood cells and platelets are separated by centrifugal forces to the bottom of a specimen tube. The upper liquid layer, the plasma, consists of 90 percent water along with various substances required for maintaining the body’s pH, osmotic load, and for protecting the body. The plasma also contains the coagulation factors and antibodies.
The plasma component of blood without the coagulation factors is called the serum . Serum is similar to interstitial fluid in which the correct composition of key ions acting as electrolytes is essential for normal functioning of muscles and nerves. Other components in the serum include proteins that assist with maintaining pH and osmotic balance while giving viscosity to the blood. The serum also contains antibodies, specialized proteins that are important for defense against viruses and bacteria. Lipids, including cholesterol, are also transported in the serum, along with various other substances including nutrients, hormones, metabolic waste, plus external substances, such as, drugs, viruses, and bacteria.
Human serum albumin is the most abundant protein in human blood plasma and is synthesized in the liver. Albumin, which constitutes about half of the blood serum protein, transports hormones and fatty acids, buffers pH, and maintains osmotic pressures. Immunoglobin is a protein antibody produced in the mucosal lining and plays an important role in antibody mediated immunity.
Blood Types Related to Proteins on the Surface of the Red Blood Cells
Red blood cells are coated in antigens made of glycolipids and glycoproteins. The composition of these molecules is determined by genetics, which have evolved over time. In humans, the different surface antigens are grouped into 24 different blood groups with more than 100 different antigens on each red blood cell. The two most well known blood groups are the ABO, shown in Figure 5, and Rh systems. The surface antigens in the ABO blood group are glycolipids, called antigen A and antigen B. People with blood type A have antigen A, those with blood type B have antigen B, those with blood type AB have both antigens, and people with blood type O have neither antigen. Antibodies called agglutinougens are found in the blood plasma and react with the A or B antigens, if the two are mixed. When type A and type B blood are combined, agglutination (clumping) of the blood occurs because of antibodies in the plasma that bind with the opposing antigen; this causes clots that coagulate in the kidney causing kidney failure. Type O blood has neither A or B antigens, and therefore, type O blood can be given to all blood types. Type O negative blood is the universal donor. Type AB positive blood is the universal acceptor because it has both A and B antigen. The ABO blood groups were discovered in 1900 and 1901 by Karl Landsteiner at the University of Vienna.
The Rh blood group was first discovered in Rhesus monkeys. Most people have the Rh antigen (Rh+) and do not have anti-Rh antibodies in their blood. The few people who do not have the Rh antigen and are Rh– can develop anti-Rh antibodies if exposed to Rh+ blood. This can happen after a blood transfusion or after an Rh– woman has an Rh+ baby. The first exposure does not usually cause a reaction; however, at the second exposure, enough antibodies have built up in the blood to produce a reaction that causes agglutination and breakdown of red blood cells. An injection can prevent this reaction.

Figure 5. Human red blood cells may have either type A or B glycoproteins on their surface, both glycoproteins combined (AB), or neither (O). The glycoproteins serve as antigens and can elicit an immune response in a person who receives a transfusion containing unfamiliar antigens. Type O blood, which has no A or B antigens, does not elicit an immune response when injected into a person of any blood type. Thus, O is considered the universal donor. Persons with type AB blood can accept blood from any blood type, and type AB is considered the universal acceptor.
In Summary: Structure and Function of Blood
Red blood cells are specialized cells that contain hemoglobin and circulate through the body delivering oxygen to cells. White blood cells are involved in the immune response to identify and target invading bacteria, viruses, and other foreign organisms; they also recycle waste components, such as old red blood cells.
Platelets and blood clotting factors cause the change of the soluble protein fibrinogen to the insoluble protein fibrin at a wound site forming a plug. Plasma consists of 90 percent water along with various substances, such as coagulation factors and antibodies. The serum is the plasma component of the blood without the coagulation factors.
Check Your Understanding
Answer the question(s) below to see how well you understand the topics covered in the previous section. This short quiz does not count toward your grade in the class, and you can retake it an unlimited number of times.
Use this quiz to check your understanding and decide whether to (1) study the previous section further or (2) move on to the next section.
- Introduction to Structure and Function of Blood. Provided by : Lumen Learning. License : CC BY: Attribution
- Biology. Provided by : OpenStax CNX. Located at : http://cnx.org/contents/[email protected] . License : CC BY: Attribution . License Terms : Download for free at http://cnx.org/contents/[email protected]
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-.

InformedHealth.org [Internet].
In brief: what does blood do.
Last Update: March 16, 2023 ; Next update: 2026.
Blood is a vitally important fluid for the body. It is thicker than water, and feels a bit sticky. The temperature of blood in the body is 38°C (100.4°F), which is about one degree higher than body temperature. How much blood you have depends mostly on your size and weight. A man who weighs about 70 kg (about 154 pounds) has about 5 to 6 liters of blood in his body.
- What jobs does blood do?
Transportation
The blood transports oxygen from the lungs to the cells of the body, where it is needed for metabolism. The carbon dioxide produced during metabolism is carried back to the lungs by the blood, where it is then exhaled (breathed out). Blood also provides the cells with nutrients, transports hormones and removes waste products, which organs such as the liver, the kidneys or the intestine then get rid of.
The blood helps to keep certain things in the body in balance. For instance, it makes sure that the right body temperature is maintained. This is done both through the liquid part of the blood (plasma), which can absorb or give off heat, as well as through the speed at which the blood is flowing: When the blood vessels expand, the blood flows more slowly and this causes heat to be lost. When the temperature outside the body is low, the blood vessels can contract to reduce the amount of heat lost. Even the pH value of the blood is kept at a level ideal for the body. The pH value tells us how acidic or alkaline a liquid is. A constant pH value is very important for things in the body to function properly.
This involves solid parts of the blood such as blood platelets and various substances that are dissolved in the blood plasma. If a blood vessel is damaged, these parts of the blood stick together (clot) very quickly and make sure that a scrape, for instance, stops bleeding. This prevents large amounts of blood loss. White blood cells and certain chemical messengers also play an important role in the immune system.
- What is blood made up of?
Blood is made up of about 55% blood plasma and about 45% different types of blood cells. Blood plasma is a light yellow, slightly cloudy liquid. Over 90% of blood plasma is water, while less than 10% consists of dissolved substances, mostly proteins. Blood plasma also contains electrolytes, vitamins and nutrients such as glucose and amino acids. Over 99% of the solid particles in blood are cells known as red blood cells (erythrocytes) due to their red color. The rest are pale or colorless white blood cells (leukocytes) and platelets (thrombocytes).
Blood is made up of plasma and blood cells
Red blood cells look like discs that are thinner in the middle. They can easily change shape to “squeeze through” narrow blood vessels. Unlike many other cells, red blood cells have no nucleus ("information center"). All red blood cells contain a red pigment known as hemoglobin. Oxygen binds to hemoglobin, and is transported around the body in that way. In tiny blood vessels in the lung, the red blood cells pick up oxygen from inhaled (breathed in) air and carry it through the bloodstream to all parts of the body. When they reach their goal, they release it again. The cells need oxygen for metabolism, which creates carbon dioxide as a waste product. The carbon dioxide is absorbed from the cells by the blood plasma (some of it binds to hemoglobin too) and is transported back to the lungs in the bloodstream. There it leaves the body when we breathe out.
Red blood cells can also pick up or release hydrogen and nitrogen. By picking up or releasing hydrogen they help to keep the pH of the blood stable; when they release nitrogen the blood vessels expand, and blood pressure falls. Red blood cells live for about 120 days. When they're too old or damaged, they're broken down in the bone marrow, spleen or liver.
White blood cells (leukocytes) have a cell nucleus and don't contain hemoglobin. There are different types of white blood cells. They are classified according to how their nucleus is shaped and what the inside of the cell looks like under a microscope. Granulocytes have small granules inside them. Monocytes and lymphocytes also contain granules, but their granules are extremely small and can't be seen under a microscope. White blood cells can also leave the bloodstream and move into tissues in the body.
White blood cells play an important role in the immune system . Here the different blood cells have different functions: Some fight intruders such as bacteria, viruses, parasites or fungi themselves and render them harmless. Others make antibodies, which specifically target foreign objects or germs like viruses. Leukocytes also play a part in allergic reactions : For instance, they are the reason why people with a dust mite allergy get a runny nose when they come into contact with dust. Certain lymphocytes can also kill cancerous cells. Most white blood cells have a lifespan of only a few hours to several days. Some lymphocytes can stay in the body for many years, though.
Blood platelets (thrombocytes) also look like little discs, and they also have no cell nucleus. But they are much smaller than red blood cells. They play an important role in blood clotting : If a blood vessel is damaged – for instance, if you accidentally cut yourself with a knife – the healing process begins with blood platelets gathering and clumping together on the inside of the damaged wall of the blood vessel. This quickly causes a plug to form and close the wound temporarily. At the same time, strong protein threads are made and they hold the clump in place, attached to the wound. Thrombocytes usually live only 5 to 9 days. Old thrombocytes are mainly broken down in the spleen.
- How are blood cells produced?
All solid parts of the blood come from common parent cells known as stem cells. In adults, blood cells are mainly produced in the bone marrow. The various blood cells develop in several stages from stem cells to blood cells or blood platelets. White blood cells such as lymphocytes don't only mature in the bone marrow, but also in the lymph nodes. When the cells are ready, they are released into the bloodstream. In addition to these mature cells, the blood still contains a small number of precursor cells.
Certain chemical messengers regulate the production of blood cells. For instance, the hormone erythropoietin, which is produced in the kidneys, promotes the production of red blood cells. And cytokines stimulate the production of white blood cells.
- Brandes R, Lang F, Schmidt R. Physiologie des Menschen: mit Pathophysiologie. Berlin: Springer; 2019.
- Menche N. Biologie Anatomie Physiologie. München: Urban und Fischer; 2020.
- Pschyrembel Online . 2023.
IQWiG health information is written with the aim of helping people understand the advantages and disadvantages of the main treatment options and health care services.
Because IQWiG is a German institute, some of the information provided here is specific to the German health care system. The suitability of any of the described options in an individual case can be determined by talking to a doctor. informedhealth.org can provide support for talks with doctors and other medical professionals, but cannot replace them. We do not offer individual consultations.
Our information is based on the results of good-quality studies. It is written by a team of health care professionals, scientists and editors, and reviewed by external experts. You can find a detailed description of how our health information is produced and updated in our methods.
- Cite this Page InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-. In brief: What does blood do? [Updated 2023 Mar 16].
- Disable Glossary Links
In this Page
Informed health links, recent activity.
- In brief: What does blood do? - InformedHealth.org In brief: What does blood do? - InformedHealth.org
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

Essays on blood: why do we actually have it?
Adjunct Professor, University of Technology Sydney
Disclosure statement
David Irving is employed by the Australian Red Cross Blood Service and has research collaborations with others receiving NHMRC and ARC research grants. Australian governments fund the Australian Red Cross Blood Service for the provision of blood, blood products and services to the Australian community.
University of Technology Sydney provides funding as a founding partner of The Conversation AU.
View all partners
This week we’re running a series in collaboration with the Australian Red Cross Blood Service looking at blood: what it actually does, why we need it, and what happens when something goes wrong with the fluid that gives us life. Read other articles in the series here .
Just as a village can’t grow into a city without some form of transport (road, rail or river) that provides necessary interconnections for it to flourish, living things are limited in the size they can reach unless they have some form of circulatory system to transport nutrients and remove waste.
Single celled organisms such as bacteria and fungi, and some multicellular creatures such as sponges, corals and flatworms, simply absorb the nutrients they need and get rid of their waste using a passive process known as diffusion (which is much like soaking in and draining out).
More complex animals have developed some kind of circulatory system. A variety of different systems and pumps (hearts) have developed, but they all have a few things in common. These include something to carry oxygen around their bodies, a fluid of some sort, and some “plumbing” – in humans (and a number of other species) the fluid is called blood and the plumbing is our arteries, veins and capillaries. The oxygen carrier is haemoglobin.
Depending on the organism and where it has adapted to live, its oxygen carrier can come in different forms, often giving its “blood” different colours. Spiders, crustaceans, octopuses and squid use haemocyanin, which is based on copper and gives them blue blood. This carrier works well in low oxygen environments and in the cold.
Segmented worms and some leeches use an iron based carrier called chlorocruorin, which can appear either green or red, depending on its chemical environment. Vertebrates, including humans, use haemoglobin, which makes their blood red.
A truly special case is the Antarctic icefish , which lost its haemoglobin long ago as a result of a presumably random mutation. It has adapted though, and now survives by transporting oxygen that is simply dissolved in its blood. This is possible thanks to the cold conditions it lives in.

What is our blood made of?
Human blood, and that of all creatures with backbones (Antarctic ice fish excepted), is red. The colour comes from a chemical known as haem, which contains iron. It’s the iron that is the crucial ingredient for carrying oxygen. Oxygen is needed for our cells to burn sugars, fats and proteins in a controlled way. This provides us with the energy we need to live.
Outside our bodies, we know that when iron is exposed to oxygen, it rusts. And it doesn’t easily “unrust”. But to work as an oxygen carrier in our bodies, iron needs to “rust” and “unrust” on demand - picking up oxygen where it is in plentiful supply (our lungs), and releasing it where it is required (the cells in our organs).
This on/off oxygen switch is made possible with help from complex larger molecules. The first is haem, a flat ring structure that holds an iron atom at its centre. Haem is held closely by proteins known as globin, and this combination forms haemoglobin, which is itself packaged up in red blood cells to be transported around the body.
Infographic - From animal experiments to saving lives: a history of blood transfusions
The molecular structure of haemoglobin is delicately tuned to allow it to bind oxygen in the lungs and drop it off in areas where there is less oxygen available.
Red cells are specialised parcels, lacking DNA, that are able to squeeze through the tiniest capillaries, down to four millionths of a meter (equivalent to roughly half their diameter). Their donut shape maximises their surface area to make sure they can efficiently deliver oxygen, while keeping them small enough to fit through the smallest blood vessels.

More than just the red stuff
As well as red cells, our blood contains other cells and chemicals that repair and maintain the transport system and send signals around the body.
White blood cells, also known as leukocytes, repel or destroy invaders. Some white blood cells (lymphocytes) manufacture molecules known as antibodies that tag viruses and bacteria for destruction, while others called neutrophils and macrophages (literally “big eaters”) engulf bacteria, fungi and parasites to keep our circulation clean. When neutrophils have done their job you sometimes might see them as the main component of pus.
Platelets are very small fragments of larger cells called megakaryocytes. They react to any breaches to the walls of blood vessels, gathering together and triggering reactions that form a plug (or a clot) for the damaged section. If a person doesn’t have enough platelets, they can suffer from uncontrollable bleeding.

Where does it come from?
All blood cells (red cells, white cells and platelets) develop from haematopoietic (literally meaning “blood-making”) stem cells, located in the bone marrow. It has recently been found that many platelets are made in the lungs , from megakaryocytes that have migrated there from the bone marrow.
As stem cells develop, they progressively specialise into the many different types of blood cells, making developmental choices along the way. The specialisation of cells during development is tightly controlled by a symphony of growth factors. In some types of blood cancers and serious diseases, stem cell or bone marrow transplants can be used to “reboot” the blood making system.
As our knowledge of the control of blood cell development grows, we’re making progress towards being able to reproduce this process in cells grown in the laboratory . This is still some time away from being a broadly available process, but an exciting area to watch as it develops.
Update: the sentence outlining the shape of red blood cells was incorrect and has been reworded.
- White blood cells
- Blood series
- Essays on blood
- Australian Red Cross Blood Service
- Red blood cells

Content Coordinator

Lecturer / Senior Lecturer - Marketing

Assistant Editor - 1 year cadetship

Executive Dean, Faculty of Health

Lecturer/Senior Lecturer, Earth System Science (School of Science)
Blood Cells and Their Functions Essay
Blood is the fluid that transports oxygen nutrients through the whole body and carries away the waste products of the organism. An average human adult has about five liters of blood, which constitutes 8% of the entire body weight (Shier et al., 2019). Due to its complex nature and transport function, a single drop of blood can contain a countless number of viruses. Therefore, the extraction of blood requires the strictest precautions to avoid infection.
It is essential to analyze its structure to have a better understanding of blood’s functions. Blood consists of white and red blood cells, and platelets, which are cellular fragments. Shier et al. (2019) explain the origin of blood cells: “Blood cells originate in red bone marrow from hematopoietic stem cells, also known as hemocytoblasts” (p. 531). The function of red blood cells lies in carrying oxygen from the lung to the rest of the body. They are shaped into a biconcave disc, with a thinner layer in the middle and a thicker layer around the rims. Such a shape allows them to increase the area of the surface, which, in turn, creates space for the diffusion of gases into and out of the cell (Shier et al., 2019). Moreover, this shape shortens the distance for diffusion, where the cell membrane is set closer to hemoglobin molecules. Therefore, red blood cells assist the transference of oxygen across the organism, which supplements the body with needed nutrients.
Another significant part of blood is white blood cells, or leukocytes, that serve as protectors of the organism. They fight bacteria, viruses, and other damaging bodies that threaten human health. They are responsible for preventing illnesses, and a person’s health directly depends on the quality of their work. There are five main types of white blood cells in circulating blood. They differ in the shape of their nucleus, size, and the nature of the cytoplasm. These are neutrophils, eosinophils, basophils, monocytes, and lymphocytes. The first three belong to the granulocytes group, while the last two to the agranulocytes group. They differ in the composition of their cytoplasmic granules, where granulocytes have a more prominent granular cytoplasm. Shier et al. (2019) describe the functions of each type of white blood cell. Neutrophils are the blood cells that come first at the site of the infection. Their task is to phagocytize bacteria, some viruses, and fungi. Eosinophils manage allergic reactions and protect the rest of the body from the infestation of parasitic worms. Basophils respond to neutrophils by migrating “to damaged tissues where they release histamine, which promotes inflammation and heparin, which inhibits blood clotting, actions that increase blood flow to injured tissues” (Shier et al., 2019, p. 537-39). Therefore, the function of the granulocytes group is to take the first act in fighting the infection.
Agranulocytes are responsible for the composition of the organism’s adaptive immunity to prevent potential reoccurring infections. For example, monocytes are similar to neutrophils because they also phagocytize bacteria and other debris in the tissues, only outside the bloodstream. Lymphocytes divide into two groups: T cells and B cells which are essential for the immune system. While “T cells directly attack microorganisms, tumor cells, and transplanted cells, B cells produce antibodies, which are proteins that attack foreign molecules” (Shier et al., 2019, p. 537-39). Thus, granulocytes and agranulocytes represent the protectors of the body from infections and help to develop a stronger immune system.
Shier, D., Butler, J., & Lewis, R. (2019). Hole’s human anatomy and physiology (15th ed.). McGraw-Hill Education.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2022, October 19). Blood Cells and Their Functions. https://ivypanda.com/essays/blood-cells-and-their-functions/
"Blood Cells and Their Functions." IvyPanda , 19 Oct. 2022, ivypanda.com/essays/blood-cells-and-their-functions/.
IvyPanda . (2022) 'Blood Cells and Their Functions'. 19 October.
IvyPanda . 2022. "Blood Cells and Their Functions." October 19, 2022. https://ivypanda.com/essays/blood-cells-and-their-functions/.
1. IvyPanda . "Blood Cells and Their Functions." October 19, 2022. https://ivypanda.com/essays/blood-cells-and-their-functions/.
Bibliography
IvyPanda . "Blood Cells and Their Functions." October 19, 2022. https://ivypanda.com/essays/blood-cells-and-their-functions/.
- Metabolism, Virulence Factors, Strepto Pyogenes
- Case Study on Hypersensitivity Reaction
- Streptococcus Pyogenes Overview
- Proterozoic Eon: The Eyespot and the Photoreceptors
- Homeostasis and Regulation in the Human Body
- Continuous Cell Lines and Primary Cell Cultures
- Yeast and the Fermentation Process
- Ubiquity of Bacteria: Laboratory Activity
Red Blood Cells (Erythrocytes)
Structure, Function, and Related Disorders
- Cell Biology
- Weather & Climate
- B.A., Biology, Emory University
- A.S., Nursing, Chattahoochee Technical College
Red blood cells, also called erythrocytes, are the most abundant cell type in the blood. Other major blood components include plasma, white blood cells, and platelets. The primary function of red blood cells is to transport oxygen to body cells and deliver carbon dioxide to the lungs.
A red blood cell has what is known as a biconcave shape. Both sides of the cell's surface curve inward like the interior of a sphere. This shape aids in a red blood cell's ability to maneuver through tiny blood vessels to deliver oxygen to organs and tissues.
Red blood cells are also important in determining human blood type. Blood type is determined by the presence or absence of certain identifiers on the surface of red blood cells. These identifiers, also called antigens, help the body's immune system to recognize its own red blood cell type.
Red Blood Cell Structure
DAVID MCCARTHY / Getty Images
Red blood cells have a unique structure. Their flexible disc shape helps to increase the surface area-to-volume ratio of these extremely small cells. This enables oxygen and carbon dioxide to diffuse across the red blood cell's plasma membrane more readily. Red blood cells contain enormous amounts of a protein called hemoglobin. This iron-containing molecule binds oxygen as oxygen molecules enter blood vessels in the lungs. Hemoglobin is also responsible for the characteristic red color of blood.
Unlike other cells of the body, mature red blood cells do not contain a nucleus, mitochondria, or ribosomes. The absence of these cell structures leaves room for the hundreds of millions of hemoglobin molecules found in red blood cells. A mutation in the hemoglobin gene can result in the development of sickle-shaped cells and lead to sickle cell disorder.
Red Blood Cell Production
STEVE GSCHMEISSNER / Getty Images
Red blood cells are derived from stem cells in red bone marrow. New red blood cell production, also called erythropoiesis, is triggered by low levels of oxygen in the blood. Low oxygen levels can occur for various reasons including blood loss, presence in high altitude, exercise, bone marrow damage, and low hemoglobin levels.
When the kidneys detect low oxygen levels, they produce and release a hormone called erythropoietin. Erythropoietin stimulates the production of red blood cells by red bone marrow. As more red blood cells enter blood circulation, oxygen levels in the blood and tissues increase. When the kidneys sense the increase in oxygen levels in the blood, they slow the release of erythropoietin. As a result, red blood cell production decreases.
Red blood cells circulate on average for about four months. Adults have around 25 trillion red blood cells in circulation at any given time. Due to their lack of a nucleus and other organelles, adult red blood cells can not undergo mitosis to divide or generate new cell structures. When they become old or damaged, the vast majority of red blood cells are removed from circulation by the spleen, liver, and lymph nodes . These organs and tissues contain white blood cells called macrophages that engulf and digest damaged or dying blood cells. Red blood cell degradation and erythropoiesis typically occur at the same rate to ensure homeostasis in red blood cell circulation.
Red Blood Cells and Gas Exchange
John Bavosi / Getty Images
Gas exchange is the primary function of red blood cells. The process by which organisms exchange gases between their body cells and the environment is called respiration. Oxygen and carbon dioxide are transported through the body via the cardiovascular system . As the heart circulates blood, oxygen-depleted blood returning to the heart is pumped to the lungs. Oxygen is obtained as a result of respiratory system activity.
In the lungs, pulmonary arteries form smaller blood vessels called arterioles. Arterioles direct blood flow to the capillaries surrounding lung alveoli. Alveoli are the respiratory surfaces of the lungs. Oxygen diffuses across the thin endothelium of the alveoli sacs into the blood within the surrounding capillaries. Hemoglobin molecules in red blood cells release the carbon dioxide picked up from body tissues and become saturated with oxygen. Carbon dioxide diffuses from the blood to the alveoli, where it is expelled through exhalation.
The now oxygen-rich blood is returned to the heart and pumped to the rest of the body. As the blood reaches systemic tissues, oxygen diffuses from the blood to surrounding cells. Carbon dioxide produced as a result of cellular respiration diffuses from the interstitial fluid surrounding body cells into the blood. Once in the blood, carbon dioxide is bound by hemoglobin and returned to the heart via the cardiac cycle.
Red Blood Cell Disorders
SCIEPRO / Getty Images
Diseased bone marrow can produce abnormal red blood cells. These cells may be irregular in size (too large or too small) or shape (sickle-shaped). Anemia is a condition characterized by the lack of production of new or healthy red blood cells. This means that there are not enough functioning red blood cells to carry oxygen to body cells. As a result, individuals with anemia may experience fatigue, dizziness, shortness of breath, or heart palpitations. Causes of anemia include sudden or chronic blood loss, not enough red blood cell production, and the destruction of red blood cells. Types of anemia include:
- Aplastic anemia: A rare condition in which insufficient new blood cells are produced by bone marrow due to stem cell damage. Development of this condition is associated with a number of different factors including pregnancy, exposure to toxic chemicals, the side effect of certain medications, and certain viral infections, such as HIV, hepatitis, or Epstein-Barr virus.
- Iron-deficiency anemia: A lack of iron in the body leads to insufficient red blood cell production. Causes include sudden blood loss, menstruation, and insufficient iron intake or absorption from food.
- Sickle cell anemia: This inherited disorder is caused by a mutation in the hemoglobin gene that causes red blood cells to take on a sickle shape. These abnormally shaped cells get stuck in blood vessels, blocking normal blood flow.
- Normocytic anemia: This condition results from a lack of red blood cell production. The cells that are produced, however, are of normal size and shape. This condition may result from kidney disease, bone marrow dysfunction, or other chronic diseases.
- Hemolytic anemia: Red blood cells are prematurely destroyed, typically as a result of an infection, autoimmune disorder, or blood cancer .
Treatments for anemia vary based on severity and include iron or vitamin supplements, medication, blood transfusion, or bone marrow transplantation.
- Blood Composition and Function
- Bone Marrow and Blood Cell Development
- Spleen Anatomy and Function
- Respiratory System and How We Breathe
- Cardiovascular System
- What Is Genetic Dominance and How Does It Work?
- 12 Interesting Facts About Blood
- Platelets: Cells That Clot Blood
- The Lungs and Respiration
- Biology Prefixes and Suffixes: hem- or hemo- or hemato-
- What Is Pleiotropy? Definition and Examples
- Circulatory System: Pulmonary and Systemic Circuits
- White Blood Cells—Granulocytes and Agranulocytes
- Epithelial Tissue: Function and Cell Types
- An Introduction to Types of Respiration
- The Types of Blood Vessels in Your Body
- Biology Article
Blood is one of the most important components of life. Almost any animal that possesses a circulatory system has blood. From an evolutionary perspective, blood was speculated to have risen from a type of cell that was responsible for phagocytosis and nutrition. Billions of years later, blood and the circulatory system have drastically helped the evolution of more complex lifeforms.
Types of Blood Cells
We have seen blood consist of cells known as formed elements of blood. These cells have their own functions and roles to play in the body. The blood cells which circulate all around the body are as follows:
Red blood cells (Erythrocytes)
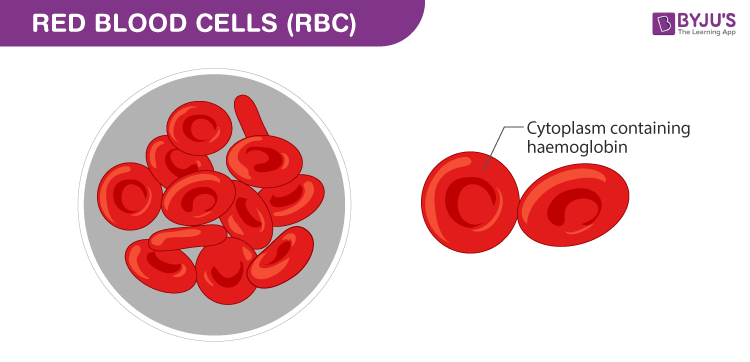
RBCs are biconcave cells without nucleus in humans; also known as erythrocytes. RBCs contain the iron-rich protein called haemoglobin; give blood its red colour. RBCs are the most copious blood cells produced in bone marrows. Their main function is to transport oxygen from and to various tissues and organs.
White blood cells (Leucocytes)
Leucocytes are colourless blood cells. They are colourless because it is devoid of haemoglobin. They are further classified as granulocytes and agranulocytes. WBCs mainly contribute to immunity and defence mechanism.
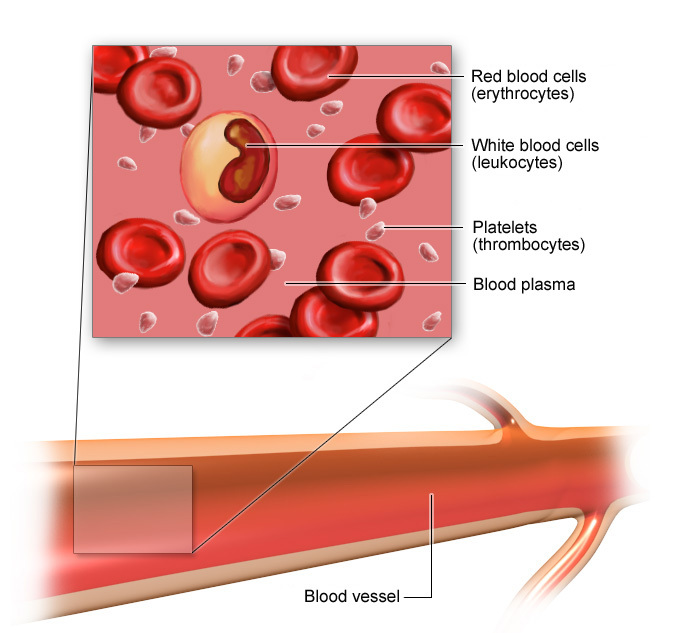
Red Blood Cells are red due to Hemoglobin , which is a transport molecule and also a pigment. As a result, blood is red.
Types of White Blood Cells
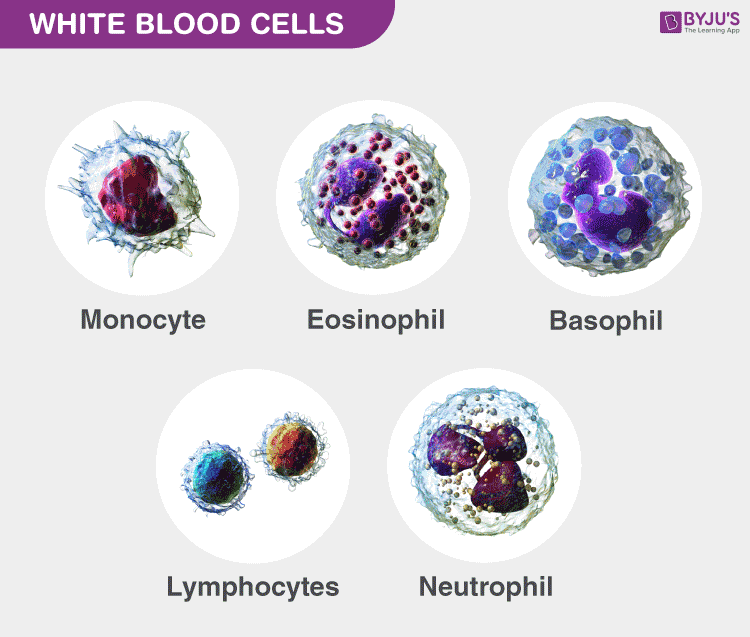
There are five different types of White blood cells and are classified mainly based on the presence and absence of granules.
Granulocytes
Agranulocytes.
There are five types of white blood cells present in the blood
They are leukocytes, with the presence of granules in their cytoplasm. The granulated cells include- eosinophil, basophil, and neutrophil.
Eosinophils
They are the cells of leukocytes, which are present in the immune system.
These cells are responsible for combating infections in parasites of vertebrates and for controlling mechanisms associated with allergy and asthma .
Eosinophil cells are small granulocyte, which are produced in the bone marrow and makes 2 to 3 per cent of whole WBCs. These cells are present in high concentrations in the digestive tract.
They are the least common of the granulocytes, ranging from 0.5 to 1 per cent of WBCs.
They contain large cytoplasmic granules, which play a vital role in mounting a non-specific immune response to pathogens, and allergic reactions by releasing histamine and dilating the blood vessels.
These white blood cells have the ability to be stained when exposed to basic dyes, hence referred to as basophil.
These cells are best known for their role in asthma and their result in inflammation and bronchoconstriction in the airways.
- They secrete serotonin, histamine and heparin.
Neutrophils
They are normally found in the bloodstream.
They are predominant cells, which are present in pus.
Around 60 to 65 per cent of WBCs are neutrophils with a diameter of 10 to 12 micrometres.
The nucleus is 2 to 5 lobed and the cytoplasm has very fine granules.
Neutrophil helps in the destruction of bacteria with lysosomes, and it acts as a strong oxidant.
Neutrophils are stained only using neutral dyes. Hence, they are called so.
Neutrophils are also the first cells of the immune system to respond to an invader such as a bacteria or a virus.
The lifespan of these WBCs extends for up to eight hours and is produced every day in the bone marrow.
They are leukocytes, with the absence of granules in their cytoplasm. Agranulocytes are further classified into monocytes and lymphocytes.
These cells usually have a large bilobed nucleus, with a diameter of 12 to 20 micrometres.
The nucleus is generally half-moon shaped or kidney-shaped and it occupies 6 to 8 per cent of WBCs.
They are the garbage trucks of the immune system.
The most important functions of monocytes are to migrate into tissues and clean up dead cells, protect against bloodborne pathogens and move very quickly to the sites of infections in the tissues .
These white blood cells have a single bean-shaped nucleus, hence referred to as Monocytes.
Lymphocytes
They play a vital role in producing antibodies.
Their size ranges from 8 to 10 micrometres.
They are commonly known as natural killer cells.
They play an important role in body defence.
These white blood cells are colourless cells formed in lymphoid tissue, hence referred to as lymphocytes.
There are two main types of lymphocytes – B lymphocytes and T lymphocytes.
These cells are very important in the immune systems and are responsible for humoral and cell-mediated immunity.
Platelets (Thrombocytes)
Thrombocytes are specialized blood cells produced from bone marrow.
Platelets come into play when there is bleeding or haemorrhage.
They help in clotting and coagulation of blood. Platelets help in coagulation during a cut or wound.
Composition of Blood: Plasma, RBCs, WBCs and platelets
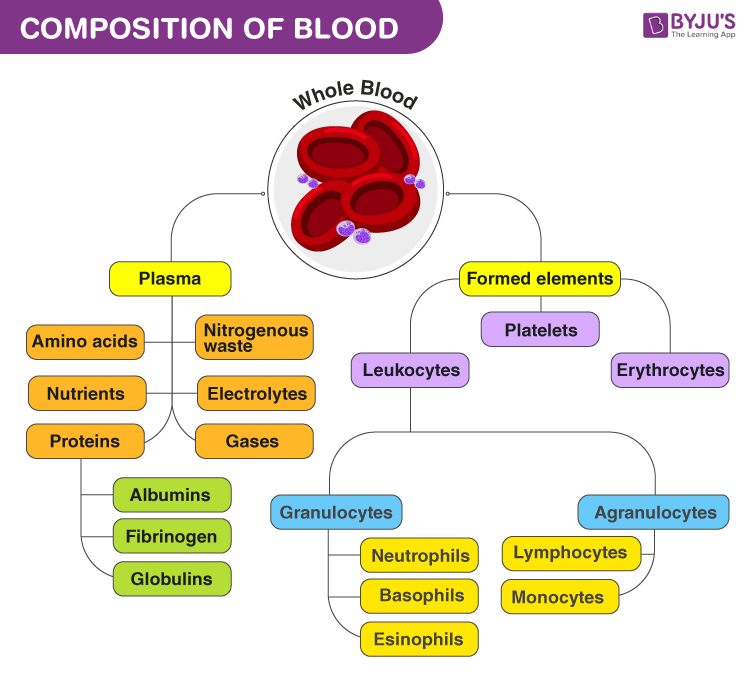
Components Of Blood
There are many cellular structures in the composition of blood. When a sample of blood is spun in a centrifuge machine, they separate into the following constituents: Plasma, buffy coat and erythrocytes. Thus blood contains RBC, WBC, platelets and plasma.
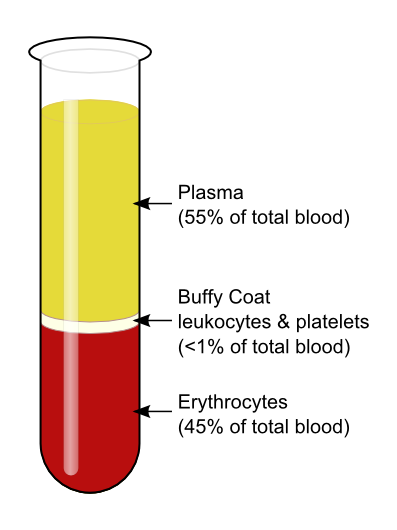
The liquid state of blood can be contributed to plasma as it makes up ~55% of blood. It is pale yellow in colour and when separated. Blood plasma consists of salts, nutrients, water and enzymes. Blood plasma also contains important proteins and other components necessary for overall health. Hence, blood plasma transfusions are given to patients with liver failure and life-threatening injuries.
Components of Blood Plasma
Blood plasma has several protein components. Proteins in blood plasma are:
- Serum globulin
- Serum albumin
The serum contains only globulin and albumin. Fibrinogen is absent in serum because it is converted into fibrin during blood clotting.
Red Blood Cells (RBC)
Red blood cells consist of Haemoglobin, a protein. They are produced by the bone marrow to primarily carry oxygen to the body and carbon dioxide away from it.
White Blood Cells (WBC)
White blood cells are responsible for fighting foreign pathogens (such as bacteria, viruses, and fungi) that enter our body. They circulate throughout our body and originate from the bone marrow.
Tiny disc-shaped cells that help regulate blood flow when any part of the body is damaged, thereby aiding in fast recovery through clotting of blood.
The above-stated elements form the composition of blood in humans. The only vertebrate without haemoglobin is the crocodile icefish. It derives its oxygen requirement directly from the cold, oxygen-rich water where it lives.
Also Read: Difference between Plasma and Serum
Blood Vessels
There are different types of blood vessels in our body each carrying out specialized functions.

Blood vessels are categorized into arteries, veins and capillaries
Recommended Video:
Types of Blood Vessels
Three types of blood vessels are:
- Capillaries
Arteries are strong tubes and muscular in nature. These blood vessels carry oxygen-rich blood from the heart to all the tissues of the body. Aorta is one of the main arteries that arise from the heart and branches further.
Veins are elastic blood vessels which carry deoxygenated blood from all parts of the body to the heart. An exception is the umbilical and pulmonary veins. The Pulmonary vein carries oxygenated blood to the heart from the lungs and the umbilical vein carries oxygenated blood from the placenta to the foetus.
On reaching tissues, arteries branch further into extremely thin tubes called capillaries. Capillaries bring about the exchange of substances between blood and tissues.
Sinusoids are a special type of wider capillaries present in bone marrow, liver, lymph nodes, spleen and some endocrine glands. They may be continuous, discontinuous or fenestrated.
Layers of Blood Vessels
Both arteries and veins consist of three layers.
Tunica Intima : It is one of the innermost and thinnest layers of arteries and veins. It comprises endothelial cells. They are in direct contact with the flow of blood.
Tunica Media : It is the middle layer of an artery or vein. Tunica media is made up of smooth muscle cells.
Tunica Externa: It surrounds tunica media. It is made up of collagen and is also supported by the elastic lamina in arteries.
Functions of Blood
Blood is responsible for the following body functions:
Fluid Connective Tissue
Blood is a fluid connective tissue composed of 55% plasma and 45% formed elements including WBCs, RBCs, and platelets. Since these living cells are suspended in plasma, blood is known as a fluid connective tissue and not just fluid.
- Provides oxygen to the cells
Blood absorbs oxygen from the lungs and transports it to different cells of the body. The waste carbon dioxide moves from the blood to the lungs and is exhaled.
Transports Hormones and Nutrients
The digested nutrients such as glucose, vitamins, minerals, and proteins are absorbed into the blood through the capillaries in the villi lining the small intestine.
The hormones secreted by the endocrine glands are also transported by the blood to different organs and tissues.
Homeostasis
Blood helps to maintain the internal body temperature by absorbing or releasing heat.
Blood Clotting at Site of Injury
The platelets help in the clotting of blood at the site of injury. Platelets along with the fibrin form clot at the wound site
Transport of waste to the Kidney and Liver
Blood enters the kidney where it is filtered to remove nitrogenous waste out of the blood plasma. The toxins from the blood are also removed by the liver.
Protection of the body against pathogens
The White Blood Cells fight against infections. They multiply rapidly during infections.
To know more about blood, its types, blood vessels, and composition of blood, please register at BYJU’S or download the BYJU’S app for further reference.
More to Explore:
- Difference Between Blood and Lymph
- Blood Groups
Frequently Asked Questions
1. what is blood, 2. state the types of blood cells found in human blood..
Blood cells are classified into the following types:
- Erythrocytes or red blood cells
- Leucocytes or white blood cells
3. State the different types of white blood cells found in the blood.
White blood cells can be classified as follows:
- lymphocytes
- neutrophils
- eosinophils

4. What are granulocytes?
Granulocytes are leukocytes with granule-like structures, that contain enzymes capable of digesting microorganisms. Granulocytes are further classified into eosinophils, basophils, and neutrophils.
5. What are agranulocytes?
Agranulocytes are a type of white blood cell that has no distinct granules in their cytoplasm. However, they form an important part of the body’s immune system. They are further classified into monocytes and lymphocytes.
6. Name the various components of blood.
Blood is primarily broken down into the following components:
7. What are the various types of blood vessels present in our body?
Blood vessels are classified as follows:
8. What are sinusoids?
Sinusoids are very small vessels predominantly located inside the bone marrow, liver and spleen. Sinusoids are usually a little larger than capillaries.
9. Name the various layers of blood vessels.
- Tunica Intima
- Tunica Media
- Tunica Adventitia or Externa
10. Name the major functions of blood.
- Helps in homeostasis
- Transports hormones and nutrients
- Help in the clotting process
11. What gives blood its red colour?
12. does plasma contain haemoglobin.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Discover BYJU’S for more concepts on Biology
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Nice explanation
It is probably the best platform for any sort of questions
THANKS HELPED A LOT!!
Thank you for giving a good explanation I’m lucky to reach this side Thank you
Very useful
Thank you Byju’s for providing me a very important notes
Content was to the point and very clear with fine points
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.


Essay on Blood
Students are often asked to write an essay on Blood in their schools and colleges. And if you’re also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic.
Let’s take a look…
100 Words Essay on Blood
What is blood.
Blood is a body fluid in humans and animals that delivers necessary substances to cells and carries waste products away. It is made up of two main components, namely, plasma and blood cells. Plasma, the liquid part, makes up about 55% of the blood’s volume.
Blood Cells
Blood cells are of three types: red blood cells, white blood cells, and platelets. Red blood cells carry oxygen from our lungs to the rest of our body. White blood cells defend our body against infections. Platelets help in blood clotting when we have a wound.
Importance of Blood
Blood plays a vital role in our body. It transports nutrients and oxygen to our body cells. It also helps in removing waste materials like carbon dioxide. Blood carries hormones and signals from one part of the body to another. It also helps in fighting against diseases.
Blood Groups
There are four main blood groups: A, B, AB, and O. Each group is either Rh positive or Rh negative. Blood group is important during blood transfusion. A person can only receive blood from a compatible blood group.
Blood Donation
Blood donation is a lifesaving act. Donated blood is used in various medical treatments and emergencies. It’s a safe process and a healthy person can donate blood every three months. It’s important to donate blood as it can save someone’s life.
250 Words Essay on Blood
Blood is a body fluid in humans and other animals that delivers necessary substances to the body’s cells. It’s like a delivery service inside our bodies. It carries food, water, and oxygen to our body parts. It also takes away waste, like carbon dioxide, from our cells.
Parts of Blood
Blood has four main parts. These are red blood cells, white blood cells, platelets, and plasma. Red blood cells carry oxygen. They are like trucks that move oxygen from our lungs to all parts of our body. White blood cells are our body’s defense team. They fight germs and help us stay healthy. Platelets are like band-aids. They help our body heal when we get a cut by making clots to stop bleeding. Plasma is a yellowish liquid that carries all these parts and more.
Blood is very important for our body. It helps keep us alive by carrying oxygen and nutrients to our cells, fighting infections, and healing wounds. Without blood, our body would not be able to function properly.
Blood Types
There are four main blood types: A, B, AB, and O. Each type can be either positive or negative. These types are important when it comes to blood transfusions. This is when blood is given from one person to another. For example, if a person with type A blood is given type B blood, it can make them very sick.
In conclusion, blood is a vital part of our body that performs many important functions. It’s like a transportation system and a defense team all in one. Understanding blood can help us appreciate how our bodies work and stay healthy.
500 Words Essay on Blood
Blood is a body fluid that delivers necessary substances like nutrients and oxygen to the cells and carries waste products away from those same cells. In simple words, blood is like a transport system inside our bodies. It is red in color and is slightly thicker than water.
Components of Blood
Blood is made up of four main components. These are red blood cells, white blood cells, platelets, and plasma.
Red blood cells, also known as RBCs, carry oxygen from our lungs to the rest of our body. They also bring back carbon dioxide from the body to the lungs, which we breathe out. This makes RBCs a very important part of our blood.
White blood cells, or WBCs, are the body’s defense system. They fight off germs, bacteria, and viruses that enter the body and help to keep us healthy.
Platelets are tiny blood cells that help the body form clots to stop bleeding. If one of your blood vessels gets damaged, it sends out signals that are picked up by platelets. The platelets then rush to the site of damage and form a clot to repair the vessel.
Plasma is the liquid part of the blood. It carries the blood cells and platelets around the body. It also carries other important things like hormones, which control many things in the body, and nutrients.
Types of Blood
There are four main types of blood: A, B, AB, and O. Each type is determined by the presence or absence of certain substances on the surface of the red blood cells. People with type A blood have A antigens, those with type B have B antigens, those with type AB have both, and those with type O have neither.
Blood is very important because it keeps us alive by carrying out many important jobs. It delivers oxygen and nutrients to our cells, takes away waste products, fights infections, and helps to heal wounds. Without blood, our bodies would not be able to function.
Blood donation is a simple, safe process where a person voluntarily agrees to have blood drawn from them to be used in medical treatments. Donated blood can be life-saving for people who have lost large amounts of blood due to accidents or surgery, and for people with certain diseases. It’s a great way to help others and make a big difference in someone’s life.
In conclusion, blood is a vital part of our body that performs many important functions. It’s a fascinating substance that’s much more than just a red liquid flowing in our veins.
That’s it! I hope the essay helped you.
If you’re looking for more, here are essays on other interesting topics:
- Essay on Blockchain Technology
- Essay on Blindness
- Essay on Black Power Movement
Apart from these, you can look at all the essays by clicking here .
Happy studying!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

The Red Blood Cells and Types of Blood
The red blood cells (RBCs) are oxygen and carbon dioxide carrying cells found in the blood serum of vertebrate organisms (Starr, 143). They transport oxygen from the lungs to the tissues, and carbon dioxide from the tissues to organs that get rid of them, such as lungs. The RBCs are biconcave, oval, and do not contain most cell organelles; this creates more space for hemoglobin packaging (Starr, 143). RBCs are adapted to carry oxygen and carbon (IV) oxide due to the presence of Hemoglobin; it is an Iron-molecule that binds to oxygen and carbon dioxide to allow for its transport (Starr, 144). The RBCs are manufactured in the bone marrow; they move around the body for about 120 days before being broken down and recycled (Starr, 145). Other functions of RBCs include the ability of the RBCs to release energy when subjected to stress, which allows for ease movement of blood in the vessels.
The Eosinophils are a very important part of the white blood cells synthesized in the bone marrow during hematopoiesis (Starr, 143). They are brick-like transparent cells, they are nucleated, they are found in both the blood serum and body organs outside the blood vessels; their size is about 17 micrometers. They function in offering the body defense against infections, multicellular parasites, and play a role in controlling allergic reactions in the body such as asthma. They are about six percent of the white blood cells; they exist for up to 12 hours in the blood and 12 days in the tissues. The Eosinophils are an important part of the instinctive immune system as they destroy any foreign invasion in the body non-specifically. The functions of these blood cells are activated by the cytokines from the helper T cells. Once they are active, they produce reactive substances such as superoxide, hypobromite, and peroxide; enzymes among many other compounds that help them in effecting their defensive function (Starr, 143). Increased production of the Eosinophils causes hypersensitivity reactions such as allergic reactions, these results due to parasite infections especially intra-tissue infestation, these effects body defense (Starr, 143).
There are four different types of blood found in human beings; these are O, B, A, and AB (Starr, 146). The letters are used to indicate the type of antigen found on the RBCs; blood group ‘O’ lacks antigen on the RBCs. The most complex of all blood types is type AB; it contains all the two antigens but lack blood antibodies ‘a’ and ‘b’. People with this type of blood are universal recipients as they can accommodate blood from any other type of donation; this is possible because no antibodies are released to cause agglutination in blood (Starr, 146). This blood type is not compatible with any other when it comes to donation, the presence of all the antigens will cause the other blood types to produce antibodies hence causing agglutination.
The Rhesus factor (Rh) is another very important aspect of blood found on the surface of the RBCs (Starr, 148). Blood is either Rh negative where it lacks the Rh antigen (Rh-) or Rh positive where it has the Rh antigen (Rh+). If a Rh+ man and a Rh- woman get a baby, the baby might suffer health implications if the baby is Rh+; this happens during birth, as the mothers blood intermingle with the babies producing antibodies that cause agglutination of the child’s blood, commonly known as hemolytic disease of the newborn (Starr, 148)
Works Cited
Starr, Cecie, and Beverly McMillan. Human Biology . 9th ed. Stamford, Connecticut, U.S.: Cengage Learning. 2012. Print.
Cite this paper
- Chicago (N-B)
- Chicago (A-D)
StudyCorgi. (2022, May 3). The Red Blood Cells and Types of Blood. https://studycorgi.com/the-red-blood-cells-and-types-of-blood/
"The Red Blood Cells and Types of Blood." StudyCorgi , 3 May 2022, studycorgi.com/the-red-blood-cells-and-types-of-blood/.
StudyCorgi . (2022) 'The Red Blood Cells and Types of Blood'. 3 May.
1. StudyCorgi . "The Red Blood Cells and Types of Blood." May 3, 2022. https://studycorgi.com/the-red-blood-cells-and-types-of-blood/.
Bibliography
StudyCorgi . "The Red Blood Cells and Types of Blood." May 3, 2022. https://studycorgi.com/the-red-blood-cells-and-types-of-blood/.
StudyCorgi . 2022. "The Red Blood Cells and Types of Blood." May 3, 2022. https://studycorgi.com/the-red-blood-cells-and-types-of-blood/.
This paper, “The Red Blood Cells and Types of Blood”, was written and voluntary submitted to our free essay database by a straight-A student. Please ensure you properly reference the paper if you're using it to write your assignment.
Before publication, the StudyCorgi editorial team proofread and checked the paper to make sure it meets the highest standards in terms of grammar, punctuation, style, fact accuracy, copyright issues, and inclusive language. Last updated: May 3, 2022 .
If you are the author of this paper and no longer wish to have it published on StudyCorgi, request the removal . Please use the “ Donate your paper ” form to submit an essay.

Iron is an important mineral that helps maintain healthy blood. A lack of iron is called iron-deficiency anemia, which affects about 4-5 million Americans yearly. [1] It is the most common nutritional deficiency worldwide, causing extreme fatigue and lightheadedness. It affects all ages, with children, women who are pregnant or menstruating, and people receiving kidney dialysis among those at highest risk for this condition.
Iron is a major component of hemoglobin, a type of protein in red blood cells that carries oxygen from your lungs to all parts of the body. Without enough iron, there aren’t enough red blood cells to transport oxygen, which leads to fatigue. Iron is also part of myoglobin, a protein that carries and stores oxygen specifically in muscle tissues. Iron is important for healthy brain development and growth in children, and for the normal production and function of various cells and hormones.
Iron from food comes in two forms: heme and non-heme. Heme is found only in animal flesh like meat, poultry, and seafood. Non-heme iron is found in plant foods like whole grains, nuts, seeds, legumes, and leafy greens. Non-heme iron is also found in animal flesh (as animals consume plant foods with non-heme iron) and fortified foods.
Iron is stored in the body as ferritin (in the liver, spleen, muscle tissue, and bone marrow) and is delivered throughout the body by transferrin (a protein in blood that binds to iron). A doctor may sometimes check blood levels of these two components if anemia is suspected.
Recommended Amounts
RDA: The Recommended Dietary Allowance (RDA) for adults 19-50 years is 8 mg daily for men, 18 mg for women, 27 mg for pregnancy, and 9 mg for lactation. [2] The higher amounts in women and pregnancy are due to blood loss through menstruation and because of the rapid growth of the fetus requiring extra blood circulation during pregnancy. Adolescents 14-18 years actively growing also need higher iron: 11 mg for boys, 15 mg for girls, 27 mg for pregnancy, and 10 mg for lactation. The RDA for women 51+ years drops to 8 mg with the assumption that cessation of menstruation has occurred with menopause. It may be noted that menopause occurs later for some women, so they should continue to follow the RDA for younger women until menopause is confirmed.
UL: The Tolerable Upper Intake Level is the maximum daily intake unlikely to cause harmful effects on health. The UL for iron is 45 mg daily for all males and females ages 14+ years. For younger ages, the UL is 40 mg.
Food Sources
Meats, poultry, and seafood are richest in heme iron. Fortified grains, nuts, seeds, legumes, and vegetables contain non-heme iron. In the U.S. many breads, cereals, and infant formulas are fortified with iron.
Heme iron is better absorbed by the body than non-heme iron. Certain factors can improve or inhibit the absorption of non-heme iron. Vitamin C and heme iron taken at the same meal can improve the absorption of non-heme iron. Bran fiber, large amounts of calcium particularly from supplements, and plant substances like phytates and tannins can inhibit the absorption of non-heme iron. [3]
Sources of heme iron:
- Oysters, clams, mussels
- Beef or chicken liver
- Organ meats
- Canned sardines
- Canned light tuna
Sources of non-heme iron:
- Fortified breakfast cereals
- Dark chocolate (at least 45%)
- Potato with skin
- Nuts , seeds
- Enriched rice or bread
What about iron supplements?
Confusion with iron supplements.
There are several types of iron available as over-the-counter supplements, e.g., ferrous sulfate, ferrous fumarate, ferrous gluconate. Confusion is also caused by two number amounts listed on the label, a higher number and a lower number. What is the difference among supplement forms and which number should you refer to for the right amount to take?
Elemental versus chemical form of iron. If two iron amounts are listed on the label, the larger number is the chemical compound form because iron is bound to salts (e.g., ferrous sulfate), whereas the smaller number refers only to the amount of iron in the compound, also called the elemental iron. Elemental iron is the more important number because this is the amount available for the body to absorb. However, a physician may not specify in a prescription if the iron amount is the chemical form or the elemental iron. For example, a ferrous sulfate iron supplement may list a total of 325 mg of ferrous sulfate on the front of the label but 65 mg of elemental iron in smaller print on the back. If a physician prescribed 65 mg of iron, would you take five pills to equal 325 mg, or just one pill, assuming the prescription referred to elemental iron?
Different types. All types of supplemental iron help to increase red blood cell production but vary in cost and amounts of elemental iron. Ferrous gluconate is usually sold in liquid form and some clinical studies have shown that it is better absorbed than ferrous sulfate tablets. However, ferrous gluconate contains less elemental iron than ferrous sulfate, so a greater dosage may be needed to correct a deficiency. It is also more expensive than ferrous sulfate. Newer slow-release forms of iron have been introduced, which may help reduce gastrointestinal side effects, but they are more expensive and usually contain less iron.
Any confusion with iron supplement types and amounts can be resolved by asking your doctor to specify both the elemental amount and the chemical compound amount. You can also ask a store pharmacist for assistance in interpreting a doctor’s prescription or to recommend an appropriate amount if you do not have a prescription.
Signs of Deficiency and Toxicity
An iron deficiency is seen most commonly in children, women who are menstruating or pregnant, and those eating a diet lacking in iron.
Iron deficiency occurs in stages. [4] The mild form begins with a decrease in stored iron, usually either from a low-iron diet or from excessive bleeding. If this does not resolve, the next stage is a greater depletion of iron stores and a drop in red blood cells. Eventually this leads to iron-deficiency anemia (IDA) where iron stores are used up and there is significant loss of total red blood cells. Typically, a doctor screens for anemia by first checking a complete blood count (including hemoglobin, hematocrit, and other factors that measure red blood cell volume and size). If this is below normal, ferritin and transferrin levels may be measured to determine if the type of anemia is IDA (there are other forms of anemia not caused specifically by an iron deficiency). All of these measures would decrease with IDA.
Signs of IDA:
- Fatigue, weakness
- Lightheadedness
- Confusion, loss of concentration
- Sensitivity to cold
- Shortness of breath
- Rapid heartbeat
- Hair loss, brittle nails
- Pica: cravings for dirt, clay, ice, or other non-food items
IDA is usually corrected with oral iron supplements of up to 150-200 mg of elemental iron daily. Those at high risk of IDA may be prescribed 60-100 mg daily. Blood levels should be rechecked periodically, and supplements discontinued or taken at a lower dosage if levels return to normal, as long-term high dosages can lead to constipation or other digestive upset.
Groups at risk for IDA:
- Pregnant women —during pregnancy a woman produces much greater amounts of red blood cells for the fetus, increasing the need for additional dietary or supplemental iron. IDA during pregnancy can lead to premature birth or low birth weight so iron is routinely included in prenatal vitamins. The Centers for Disease Control and Prevention recommend that all pregnant women begin taking 30 mg daily of supplemental iron. [3]
- Menstruating women —women who experience heavy bleeding during menstruation (lasting longer than 7 days or soaking through tampons or pads once every hour) can develop IDA.
- Children —infants and children have high iron needs due to their rapid growth.
- Elderly —older ages are associated with a higher risk of poor nutrition and chronic inflammatory diseases that can lead to anemia. [1]
- Vegetarians —those who eat a diet without heme iron from meats, fish, and poultry may develop IDA if they do not include adequate non-heme iron foods in the diet. Because non-heme iron is not well-absorbed, either greater quantities of these foods my be required or careful attention is needed in how they are eaten to improve absorption (consuming with vitamin C-rich foods while avoiding eating with calcium-rich foods , calcium supplements, or tea).
- Endurance athletes —running can cause trace amounts of gastrointestinal bleeding and a condition called “foot-strike” hemolysis that breaks down red blood cells at a faster rate. Female endurance athletes who are also menstruating are at greatest risk for IDA. [4]
- People with chronic kidney failure on dialysis —the kidneys make a hormone called erythropoietin (EPO) that signals the body to make red blood cells. Kidney failure reduces the production of EPO and therefore blood cells. In addition, there is some blood loss during hemodialysis.
Anemia of chronic disease (AOCD) occurs not from a low iron intake but with conditions that cause inflammation in the body, such as infections, cancer, kidney disease, inflammatory bowel disease, heart failure, lupus, and rheumatoid arthritis. The body may actually contain normal amounts of iron, but levels in the blood are very low. Inflammation changes the body’s immune function, preventing the body from being able to use available stored iron to make red blood cells and also causing blood cells to die out more quickly.
Treatment for AOCD focuses on treating the inflammatory condition. Increasing iron in the diet typically does not help. If the inflammation or condition improves, the anemia will usually decrease as well. In rare severe cases, a blood transfusion can be given to quickly boost the amount of hemoglobin in the blood.
Toxicity is rare because the body regulates iron absorption and will absorb less if iron stores are adequate. [2] Excessive iron occurs most often from taking high-dosage supplements when not needed or from having a genetic condition that stores too much iron.
Common signs:
- Constipation
- Upset stomach
- Nausea, vomiting
- Abdominal pain
Some people have a hereditary condition called hemochromatosis that causes an excessive buildup of iron in the body. Treatments are given periodically to remove blood or excess iron in the blood. People with hemochromatosis are educated to follow a low-iron diet and to avoid iron and vitamin C supplements. If left untreated, iron can build up in certain organs so that there is a higher risk of developing conditions like liver cirrhosis, liver cancer, or heart disease.
Did You Know?
It is possible to obtain enough iron in a vegetarian/vegan diet with careful planning. Try this easy dish that can boost iron levels by combining foods rich in non-heme iron and vitamin C:
- In a large bowl, combine cooked beans or lentils with diced fresh tomatoes, raw baby spinach, pumpkin seeds or cashews, and raisins or dried chopped apricots. Drizzle with a simple lemon vinaigrette made from 2 tablespoons lemon juice, ½ teaspoon Dijon mustard, 3 tablespoons olive oil, and 1 teaspoon of honey (optional). Stir ingredients well and allow to sit for at least 15 minutes to incorporate the flavors.
Vitamins and Minerals
- Le CH. The prevalence of anemia and moderate-severe anemia in the US population (NHANES 2003-2012). PLoS One . 2016 Nov 15;11(11):e0166635.
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc : a Report of the Panel on Micronutrients . Washington, DC: National Academy Press; 2001.
- National Institutes of Health Office of Dietary Supplements: Iron Fact Sheet for Health Professionals https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/ . Accessed 9/2/2019.
- Powers JM, Buchanan GR. Disorders of Iron Metabolism: New Diagnostic and Treatment Approaches to Iron Deficiency. Hematology/Oncology Clinics . 2019 Jun 1;33(3):393-408.
Last reviewed March 2023
Terms of Use
The contents of this website are for educational purposes and are not intended to offer personal medical advice. You should seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The Nutrition Source does not recommend or endorse any products.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Risk Factors
- Providing Care
- Living with Diabetes
- Clinical Guidance
- DSMES for Health Care Providers
- Prevent Type 2 Diabetes: Talking to Your Patients About Lifestyle Change
- Employers and Insurers
- Community-based Organizations (CBOs)
- Toolkits for Diabetes Educators and Community Health Workers
- National Diabetes Statistics Report
- Reports and Publications
- Current Research Projects
- National Diabetes Prevention Program
- State, Local, and National Partner Diabetes Programs for Public Health
- Diabetes Self-Management Education and Support (DSMES) Toolkit
About Insulin Resistance and Type 2 Diabetes
- Insulin resistance leads to prediabetes and type 2 diabetes.
- Find out more about what it is, what causes it, and how you can reverse it.
What is insulin?
Insulin is a key hormone that regulates your blood sugar levels. It's so important that you can't live without it. Here's how insulin works when everything is working properly:
- When you eat, food is broken down into sugars that circulate in your blood.
- This increase in blood sugar causes an internal organ called the pancreas to release insulin.
- Insulin acts like a key to let blood sugar into your body cells, which use the sugar as energy.
- After this happens, your blood sugar returns to a normal level.
Insulin also signals your liver to store extra blood sugar as energy for later. If you haven't eaten recently, your liver releases stored blood sugar so energy is always available.
What is insulin resistance?
Sometimes this finely tuned system can get out of balance. When your body is exposed to too much blood sugar over an extended period of time, you can develop insulin resistance. Here's what that means:
- A l ot of blood sugar enters your bloodstream.
- Your pancreas pumps out high levels of insulin to get more blood sugar into your cells.
- Over time, your cells stop responding well to insulin, which is known as resistance.
- Your blood sugar remains high, which causes your pancreas to keep releasing more insulin to make your cells respond.
- Eventually, your pancreas can't keep up and your blood sugar keeps increasing.
High blood sugar is damaging to the body, so your body keeps trying to remove excess blood sugars. It stores extra sugar in your liver and muscles. When they're full, the liver sends the remaining sugar to be stored as body fat, causing weight gain. Insulin resistance sets the stage for prediabetes and type 2 diabetes.
What about type 1 diabetes?
Do you have insulin resistance.
You can't tell if someone has insulin resistance by looking at them. Although overweight and obesity are risk factors, you do not have to have them to have insulin resistance. Other risk factors for insulin resistance include:
- High blood sugar levels.
- High triglycerides (a kind of fat found in the blood).
- High LDL (bad) cholesterol.
- Low HDL (good) cholesterol.
- Family history of type 2 diabetes.
- Physical inactivity.
Talk to your doctor if you think you might have insulin resistance, or want to find out more.
Reverse insulin resistance
If you have insulin resistance, there's good news. You can reverse it by making your cells more sensitive to insulin.
Physical activity makes you more sensitive to insulin, one reason it's important for diabetes management (and good health in general!). Don't wait until you're diagnosed with prediabetes or type 2 diabetes to start moving more. The earlier you take action, the better off you'll be.
Weight loss if you have overweight or obesity is another great way to relieve strain on your body and increase your insulin sensitivity.
Eating a balanced diet with non-starchy vegetables, fruits, whole grains and lean proteins helps to reduce your blood sugar. This will decrease the amount of insulin your pancreas releases to help reverse insulin resistance.
Other lifestyle changes like managing stress, and getting enough sleep will also help you reach your health goals.
Diabetes is a chronic disease that affects how your body turns food into energy. About 1 in 10 Americans has diabetes.
For Everyone
Health care providers, public health.

What Is Hypereosinophilic Syndrome?
Hypereosinophilic syndromes (HES) are a rare group of blood disorders associated with an abnormally high number of white blood cells called eosinophils. Eosinophils are important for your immune system’s response to infections and allergic reactions. In people with HES, chronically elevated eosinophil levels can infiltrate the body’s tissues, causing inflammation and organ damage. This may affect your skin, heart, lungs, brain, and digestive tract.
People of all ages can develop HES, but it is most common in people assigned male at birth between ages 30 and 50. Various factors can trigger the development of HES, including genetics, parasitic infections, drug hypersensitivity, blood cancers, and immunodeficiency disorders.
Symptoms of HES vary depending on the organ system affected but commonly include skin rash, fever, fatigue, muscle aches, weakness, and weight loss. Treatment focuses on reducing the number of eosinophils in the blood and may involve corticosteroids, chemotherapy drugs, or targeted therapies, depending on the underlying cause.
Hypereosinophilic syndrome is classified based on the underlying cause. Understanding the type of HES you have helps determine the most effective treatment.
- Myeloid hypereosinophilic syndrome (MHES): Develops when an overgrowth of myeloid cells in the bone marrow leads to the overproduction of eosinophils.
- Lymphocytic variant hypereosinophilic syndrome (LHES): Develops when abnormal lymphocytes (white blood cells) called T-cells contribute to excessive eosinophil production.
- Overlap hypereosinophilic syndromes : Includes eosinophilic diseases that affect a single organ system, such as eosinophilic granulomatosis with polyangiitis (EGPA), or share features with idiopathic HES.
- Associated hypereosinophilic syndromes: Develops as a complication of known HES triggers, such as parasitic infections , drug hypersensitivity, or underlying immune deficiencies.
- Familial HES: Certain gene mutations, passed down through families, cause this type.
- Idiopathic: When there is no identifiable cause of elevated eosinophils, HES is referred to as “idiopathic,” which is a term used when a condition occurs spontaneously or does not have a known cause.
Hypereosinophilic Syndrome Symptoms
Symptoms of hypereosinophilic syndromes vary, depending on which organs and tissues are affected by excess eosinophils. Symptoms common with all types of HES include:
- Skin rash, which may be red, itchy bumps ( urticaria/hives ) or swollen lumps under the skin (angioedema)
- Fatigue
- Muscle aches
- Loss of appetite and/or unintentional weight loss
- Shortness of breath
- Memory problems
What Causes HES?
A normal eosinophil blood count is less than 500 cells per microliter (mL) of blood—typically less than 5% of all white blood cells circulating in your blood. Temporary increases in eosinophil counts can occur when your body is fighting a parasitic or viral infection or during an allergic reaction.
Hypereosinophilic syndrome is when eosinophil counts are higher than 1,500/mL for six months or longer. Gene changes and underlying conditions can contribute to the development of hypereosinophilic syndrome, though the exact cause is not always clear.
Gene Changes
Gene changes can cause hypereosinophilic syndromes in two ways: gene fusions and inherited gene mutations.
- Gene fusions occur when two formerly separate genes merge into one hybrid (fusion) gene with features from both original genes. In HES, the fusion of the FIP1L1 and PDGFRA genes leads to the creation of proteins that trigger uncontrolled growth of eosinophils.
- Inherited gene mutations run in families. Familial HES occurs when mutations in the 5q31-q33 region of chromosome 5 lead to abnormal levels of a pro-inflammatory cytokine (a type of inflammatory protein) called interleukin-5, which controls the growth and mobilization of eosinophils.
Underlying Conditions
Certain conditions can activate immune cells, causing your body to release inflammatory substances that can contribute to excess eosinophil production. These conditions include:
- Parasitic, fungal, and viral infections
- Allergic reactions and allergy-related disorders, including asthma and atopic dermatitis
- Autoimmune disorders, including inflammatory bowel disease (IBD) and connective tissue disorders
- Blood cancers, including leukemia and lymphoma
- Drug hypersensitivity (allergic reaction to over-the-counter or prescription medications or dietary supplements)
Risk Factors
Anyone can develop hypereosinophilic syndrome, but it is more common in people assigned male at birth between the ages of 30 and 50.
Diagnosing hypereosinophilic syndrome can be a challenge because the symptoms are similar to those in other conditions, such as autoimmune disorders and allergic reactions. Typically, a multi-step approach is taken to rule out other possible causes of your symptoms before diagnosing HES.
Your healthcare provider will review your medical history, including medication use, previous infections, current medical conditions, and family history. They will ask about your symptoms and perform a physical examination to look for signs of HES, such as skin rash or shortness of breath.
Diagnostic tests help provide an accurate diagnosis and determine the underlying cause of HES. Depending on your symptoms, they may order:
- Blood tests to measure your complete blood cell count (CBC) and eosinophil count. Blood tests can also measure substances that monitor liver and kidney function, vitamin B12, and tryptase levels (an enzyme that contributes to allergic reactions).
- Imaging scans , such as X-rays , computed tomography (CT) scans , and ultrasounds , allow your healthcare provider to views your internal organs to check for damage. A chest X-ray, for example, can show lung damage, and an echocardiogram (ultrasound) shows how your heart is functioning.
- Skin biopsy , which can be used to assess the cause of a rash. Your healthcare provider may take a small skin sample and send it to the lab, where a technician will examine it under a microscope to confirm the presence of eosinophils in the tissue.
- Bone marrow biopsy , which is done by removing a small amount of bone (usually from the hip bone) to look for abnormalities in white blood cells and measure the amount of eosinophils in the bone marrow.
- Allergy testing , such as skin prick or blood tests, can reveal allergies to specific substances (e.g., pollen) and diagnose allergic diseases.
Related: Biopsy: What You Need To Know
Treatments for Hypereosinophilic Syndrome
Treatment for hypereosinophilic syndrome focuses on decreasing high eosinophil levels in your blood, reducing inflammation to prevent organ damage, and slowing disease progression. The treatment your healthcare provider recommends will depend on which organs are involved and the underlying cause of HES.
Corticosteroids
Corticosteroids , such as prednisone, are a first-line treatment for HES. These potent anti-inflammatory drugs are used to suppress eosinophil production and reduce inflammation to prevent organ damage. Steroid therapy is highly effective, returning eosinophil counts to normal or near-normal levels in 85% of those with HES.
Corticosteroids do not cure HES, and eosinophil levels will return to elevated levels when you stop taking the medication. Long-term steroid use can cause side effects, including weight gain, mood changes, high blood pressure, and bone loss.
Interferon Alpha (IFNa)
Interferon Alpha (IFNa) is an immunomodulating medication, meaning it helps change your body's natural immune response. They are delivered via injection into fatty tissue under the skin 3 to 5 times a week.
INFa suppresses HES symptoms, but initially causes flu-like symptoms, such as fever, joint pain, and muscle aches, which will subside over time. Long-term use of INFa can cause serious side effects, including elevated liver enzymes, low blood cell counts, depression and mood disorders, and heart attack .
Monoclonal Antibodies
Monoclonal antibodies are a type of biologic medication. They are lab-made immune system proteins that may be prescribed to people with HES when other treatments are ineffective. Two monoclonal antibodies can treat HES:
- Nucala (mepolizumab): Targets and blocks interleukin-5, a cytokine that controls eosinophil growth.
- Lemtrada (lemtuzumab): Targets and binds to a protein on the surface of eosinophils (CD52), effectively killing them.
Tyrosine Kinase Inhibitors
Tyrosine kinase inhibitors are enzymes that play a role in activating inflammatory immune cells. Tyrosine kinase inhibitors like Gleevac (imatinib) may help reduce eosinophil counts and control HES symptoms with minimal side effects in people with HES associated with specific gene changes.
Chemotherapy
Chemotherapy drugs, such as hydroxyurea, cyclophosphamide, vincristine, etoposide, and methotrexate, kill fast-growing cells like eosinophils. These are potent drugs with serious side effects (e.g., nausea, vomiting, hair loss, anemia, increased risk of infection), and healthcare providers typically only prescribe them for the most advanced cases of HES.
The causes of hypereosinophilic syndromes are diverse and complex, and there is no known way to prevent the condition. If you have risk factors for or a condition related to HES, regular appointments with your healthcare provider and close monitoring of your eosinophil levels can help detect HES early. Timely diagnosis and treatment can help prevent extensive organ damage and slow disease progression.
Related Conditions
People with HES often have additional health conditions, many of which develop as a complication of eosinophils infiltrating and damaging the body’s organs.
- Eosinophilic gastrointestinal diseases (EGIDs): Eosinophil infiltration of the gastrointestinal tract can cause symptoms such as abdominal pain, nausea, vomiting, diarrhea, bloating , and weight loss.
- Allergic diseases: Many people with HES have allergic diseases, such as asthma, food allergies, and atopic dermatitis. Shared genetic factors and excessive inflammatory responses may play a role in the connection.
- Heart disease: Eosinophil infiltration can damage heart muscles and valves, leading to arrhythmias (irregular heartbeat), heart murmurs, thrombus ( blood clots ), mitral valve insufficiency, or heart failure .
- Organ enlargement: The build-up of eosinophils in certain organs, most commonly the spleen and liver, can inflame and enlarge organs in people with HES.
- Lung damage: When eosinophils infiltrate lung tissues, lung function can decline and lead to respiratory difficulties like shortness of breath, coughing, and chest pain.
- Lungs: Eosinophils can infiltrate and inflame lung tissues, leading to respiratory problems, asthma-like symptoms, coughing, and even interstitial lung disease, impacting how well your lungs function and use oxygen.
- Neurological complications: Eosinophils infiltrating the nervous system can lead to seizures , neuropathy (nerve damage), and encephalopathy (e.g., memory problems and confusion).
Living With HES
While there’s no cure for hypereosinophilic syndrome, taking a proactive approach to your health and following your treatment plan can help manage symptoms, slow disease progression, and lower your risk of extensive organ damage and complications. With treatment, most people with HES enjoy productive, fulfilling lives. Without treatment, HES can cause life-threatening complications.
Symptoms of HES can have a significant impact on your daily life, and you may need to make adjustments to your routine. Ak family and friends for support on your more difficult days or talk to your healthcare provider about your challenges. They may have support resources you can access or adjust your treatment plan if your symptoms worsen.
Frequently Asked Questions
What is the life expectancy of someone with HES?
The life expectancy of someone with HES depends on the type and severity of HES, which organs are involved, and their response to treatment. Thanks to advancements in HES treatments, more than 80% of people with HES live five years or longer after diagnosis.
What happens if HES is left untreated?
Untreated HES can cause extensive damage to the heart, lungs, gastrointestinal tract, and other organs, increasing your risk of life-threatening complications.
Is hypereosinophilic syndrome a type of leukemia?
There are several types of hypereosinophilic syndrome, and most are not a type of leukemia. However, a subtype of HES—chronic eosinophilic leukemia—is a rare form of leukemia that is a type of idiopathic HES.
For more Health.com news, make sure to sign up for our newsletter!
Read the original article on Health.com .

- Open access
- Published: 17 May 2024
Brain-targeted drug delivery - nanovesicles directed to specific brain cells by brain-targeting ligands
- Ricardo Moreira 1 , 2 , 3 ,
- Clévio Nóbrega 4 , 5 ,
- Luís Pereira de Almeida 1 , 2 , 3 , 6 &
- Liliana Mendonça 1 , 2 , 6
Journal of Nanobiotechnology volume 22 , Article number: 260 ( 2024 ) Cite this article
273 Accesses
1 Altmetric
Metrics details
Neurodegenerative diseases are characterized by extensive loss of function or death of brain cells, hampering the life quality of patients. Brain-targeted drug delivery is challenging, with a low success rate this far. Therefore, the application of targeting ligands in drug vehicles, such as lipid-based and polymeric nanoparticles, holds the promise to overcome the blood-brain barrier (BBB) and direct therapies to the brain, in addition to protect their cargo from degradation and metabolization. In this review, we discuss the barriers to brain delivery and the different types of brain-targeting ligands currently in use in brain-targeted nanoparticles, such as peptides, proteins, aptamers, small molecules, and antibodies. Moreover, we present a detailed review of the different targeting ligands used to direct nanoparticles to specific brain cells, like neurons (C4-3 aptamer, neurotensin, Tet-1, RVG, and IKRG peptides), astrocytes (Aquaporin-4, D4, and Bradykinin B2 antibodies), oligodendrocytes (NG-2 antibody and the biotinylated DNA aptamer conjugated to a streptavidin core Myaptavin-3064), microglia (CD11b antibody), neural stem cells (QTRFLLH, VPTQSSG, and NFL-TBS.40–63 peptides), and to endothelial cells of the BBB (transferrin and insulin proteins, and choline). Reports demonstrated enhanced brain-targeted delivery with improved transport to the specific cell type targeted with the conjugation of these ligands to nanoparticles. Hence, this strategy allows the implementation of high-precision medicine, with reduced side effects or unwanted therapy clearance from the body. Nevertheless, the accumulation of some of these nanoparticles in peripheral organs has been reported indicating that there are still factors to be improved to achieve higher levels of brain targeting. This review is a collection of studies exploring targeting ligands for the delivery of nanoparticles to the brain and we highlight the advantages and limitations of this type of approach in precision therapies.
The World Health Organization (WHO) estimates that 1 in every 5 humans suffers from Central Nervous System (CNS) diseases [ 1 ]. Neurodegenerative diseases, such as Alzheimer’s disease (AD) or Parkinson’s disease (PD), are becoming more prevalent in today’s increasingly aged societies and are a social and financial burden worldwide [ 2 , 3 , 4 ]. Despite the increasing awareness for this problem and the efforts of the scientific community to develop therapeutic strategies, this research field has the poorest success rates in terms of effective drug development [ 5 ].
The complex physiology of the human brain, the Blood-Brain Barrier (BBB), and the substantial limitations of most animal models used to study human CNS diseases [ 6 ] play an important role in the lack of success in the development of new therapies to treat brain diseases. Considering these hurdles, the rational design of nanoparticles (NPs) prone to be administered in minimally invasive ways (e.g. intravenous administration [IV]) can be a promising approach to overcome some of these limitations [ 7 , 8 ].
NPs comprise materials with size in the nanoscale in at least one dimension [ 9 , 10 ]. Such nanomaterials can be part of Nanomedicines that, according to the European Commission’s recommendation, are between 1 and 100 nm in size for at least 50% of the particles [ 11 ]. NPs can load a great variety of drugs (small molecules, proteins, nucleic acids, etc.), protecting them from metabolization and elimination from the body, and increasing their half-life in the systemic circulation, raising the probability of drugs to reach their target tissue/organ [ 7 , 8 , 12 , 13 ]. The materials to be used in the NPs composition must be, whenever possible, biocompatible and biodegradable in order to reduce immunogenicity and toxicity [ 14 ]. Furthermore, NPs’ charge, size, and surface chemistry can be manipulated to improve biodistribution [ 15 , 16 ]. An important functionalization of NPs is the attachment of hydrophilic polymers to their surface, such as polyethylene glycol (PEG). This hydrophilic polymer creates a “cloud” of water molecules on the surface of the NPs, reducing the opsonization effect and the consequent NPs elimination from bloodstream, increasing their time in blood circulation [ 17 , 18 ] and their ability to efficiently reach the target cells after IV administration. Additionally, the functionalization of NPs surface by adding targeting ligands makes it possible to direct them to a specific cell type or tissue, increasing the accumulation of the NPs in the tissue/cells and reducing the off-target effects [ 19 , 20 ].
The identification of brain-specific ligands that might be employed in the development of brain-targeted NPs is also a critical aspect. Such ligands might specifically direct the NPs to the brain tissue, avoiding unspecific interactions in other compartments, reducing off-target effect and peripheral drug elimination, and consequently enhancing the bioavailability in the brain of the delivered drug. There are different types of targeting ligands that may be employed in the development of NPs (Fig. 1 ), such as proteins, antibodies, peptides, small molecules, and aptamers, and each of them presents advantages and disadvantages (Table 1 ) [ 21 , 22 , 23 ].
Types of targeting ligands. Several different types of molecules have been employed to achieve specific cellular targeting depending on the characteristics of the NPs used, the goal of the delivery, cost-benefit, and the characteristics of the targeting ligands. Such targeting ligands include antibodies, small molecules, aptamers (RNA/DNA sequences that recognize proteins and receptors with affinity and specificity), proteins, and peptides
Blood-brain barrier composition and crossing
BBB comprises endothelial cells, pericytes, and astrocytes, building a tight barrier that selectively limits the entry of molecules into the CNS (Fig. 2 ) [ 49 ]. Furthermore, this barrier is characterized by (1) the absence of fenestrations and (2) the presence of tight junctions between endothelial cells and the brain microvasculature formed by claudin, occludin, and junction adhesion molecules [ 49 ]. The presence of these molecular tight junctions results in a high transendothelial electrical resistance (1500 Ω/cm 2 in in vivo measurements [ 50 , 51 ]), limiting the entry of pathogens and undesired molecules and cells from peripheral circulation into the CNS. However crucial for the maintenance of brain homeostasis, this barrier also hampers the effectiveness of therapies to the brain by limiting their entrance [ 52 , 53 ]. Less than 1% of the macromolecules and no more than 2% of small molecules are able to cross the BBB by paracellular diffusion [ 54 ]. Small hydrophilic and hydrophobic molecules need to have a molecular mass inferior to 150 Da and 400–600 Da, respectively, to be able to cross the BBB by passive diffusion. Consequently, most molecules enter the BBB endothelial cells by endocytosis [ 55 ]. After endocytosis, the molecules accumulate in late endosomes, which eventually fuse with lysosomes (forming the phagolysosome), where they can be destroyed by the low pH and hydrolytic enzymes [ 56 ]. Thus, the endosomal escape is a key step in the success of therapies that reach the CNS by crossing the BBB [ 57 ].

Cellular structure of the Blood-Brain Barrier (BBB). The endothelial cells (red cells) that compose the brain microvasculature are attached to each other by Tight Junctions that bring these cells close together, limiting the passage of unspecific molecules between them. Pericytes (purple cells) are important regulatory cells that involve the endothelial cells. Finally, the endfeet of astrocytes (yellow/orange cells) also involve this structure, providing regulatory support. The BBB strongly suppresses the entry of unwanted pathogens and cells into the brain parenchyma, protecting the resident cells from insults
Furthermore, the “enzymatic BBB”, which is a complex set of enzymes from brain endothelial cells, promotes chemical compounds degradation [ 55 ]. Another key issue regarding the transcytosis of the BBB is the presence of highly efficient efflux pumps in these cells. These efflux pumps, mediated by p-glycoprotein, are responsible for the recognition of molecules that are unnecessary for the brain and transport them back to the vascular lumen, preventing their entry into the brain parenchyma [ 58 ]. Accordingly, some studies indicate that the concentration of several drugs is increased in the CNS upon blockage of these efflux transporters [ 59 , 60 ]. The paracellular aqueous and the transcellular lipophilic pathways allow the passage of very small molecules in between the endothelial cells of the BBB or through them, respectively. Besides these mechanisms, there are other pathways required for large macromolecules to enter the CNS, such as the proteins that enter via receptor-mediated or adsorptive transcytosis (Fig. 3 ) [ 61 , 62 ].
Pathways for molecular transport across the BBB. The cellular and molecular structure of the BBB makes this barrier highly restrictive and selective to molecules that can only cross the BBB through specific mechanisms. Small molecules like glucose are able to enter the brain using for example the glucose transporter Glut-1 as carrier in a Carrier-Mediated Transport. Small lipophilic molecules are able to overcome the BBB via passive diffusion in the Transcellular Lipophilic Pathway. Small hydrophilic molecules, unable to cross through the endothelial cells, are small enough to pass through the Tight Junctions into the brain parenchyma by the Paracellular Pathway. Some cationic molecules are able to interact with the negative charges on the surface of the endothelial cells and cross this barrier in a low capacity and non-specific mechanism called Adsorptive Transcytosis. Finally, large molecules, such as transferrin and insulin, enter the brain parenchyma via specific receptors expressed on the surface of endothelial cells in a mechanism called Receptor-Mediated Transcytosis
In Carrier-Mediated Transport, macromolecules such as glucose, essential fatty acids, and aminoacids, take advantage of transport proteins inserted in the endothelium and use them to transpose the BBB along or against concentration rates. While in receptor-mediated transcytosis, macromolecules such as insulin, epidermal growth factor, LDL, and transferrin bind to specific receptors on the surface of endothelial cells, which activates their endocytosis in the basolateral side of the cells [ 61 , 62 ]. Finally, in adsorptive transcytosis (non-specific), positively charged ligands interact with the negatively charged cell surface and this interaction promotes endocytosis (Fig. 3 ).
Overcoming the blood-brain barrier (BBB)
The most direct way to surpass the BBB is by intraventricular, intrathecal, or intraparenchymal injection of the drugs in the brain or intranasal administration. Several publications demonstrated the successful use of these administration routes when aiming at the delivery of molecular therapies to the brain, which are reviewed elsewhere [ 63 , 64 ]. However, some of these approaches, namely intraventricular, intraparenchymal, and intrathecal, are highly invasive, requiring very delicate brain surgeries and can cause complications such as spinal cord lesions, seizures, encephalopathy, meningitis, cerebral infection, or subdural empyema [ 65 , 66 , 67 ]. In particular, intraventricular injection is associated with a bulk flow of CSF from the ventricles to the subarachnoid space (where major arteries are located), thus causing fast clearance of the injected therapies from the brain [ 68 , 69 ]. This fast clearance results in the need of frequent dosing, which may impair patient compliance and tolerance to the treatment [ 63 ]. The limited drug penetration from CSF to the brain parenchyma, especially for macromolecules is another handicap of this approach [ 63 ]. These limitations, and complications related to the devices, namely severe infections, leakage, and immune system activation (presence of white cells in the CSF), have reduced the use of this strategy for brain therapies [ 63 , 70 ]. As for intraparenchymal administration, the distribution of the therapies in the brain is frequently limited to the site of injection, constraining the therapeutic effect [ 63 , 64 ], and complications associated with such an invasive surgery have been described [ 64 , 71 ]. Intrathecal (IT) administration allows access through the perivascular spaces but this approach is highly dependent on the size of the therapy administered [ 72 ], and serious adverse effects have been reported related to blood and lymphatic system disorders due to malfunction of port devices for IT which need to be imbedded in the patients for repeated administration [ 73 ]. Intranasal administration is a less invasive approach (and more patient-friendly) that allows access to the brain through the nasal epithelium at the level of the cribriform plate, bypassing the BBB, with minimal serum clearance and peripheral metabolism [ 63 , 64 ]. This promising administration route to deliver therapies into the brain is challenging due to the physicochemical proprieties of the therapies to be delivered that determine their ability to efficiently cross the nasal epithelium and avoid systemic distribution, and the design of the administration device which is crucial to access the specific location in the cribriform plate and allow a controlled administration to both nostrils [ 63 , 64 ]. A second approach is the use of strategies that transiently promote BBB leakage using compounds to biochemically modulate tight junctions (such as cereport, mannitol, or borneol) or physical methods like hyperosmotic arabinose solutions, electroconvulsive stimulation, laser-induced thermal therapy, or focused ultrasound [ 5 , 74 , 75 , 76 , 77 ]. Nevertheless, this approach carries the risk of brain edema and it also facilitates the invasion of pathogens from the bloodstream [ 78 ]. In a third approach, the receptors overexpressed in the BBB have been explored as an entrance gate for the brain, by developing brain-targeting NPs incorporating ligands that target these overexpressed receptors [ 79 ], such as the Transferrin receptor (TfR) and the Low-Density Lipoprotein Receptor (LDLR) (Table 2 ).
The TfR is a glycoprotein widely expressed in several cell types including the BBB endothelial cells, which, although lacks cell-specificity, has been extensively used to target NPs to the brain, especially in cancer [ 30 , 80 , 81 , 122 ], given the overexpression of this receptor by cancer cells. Despite the straightforward use of this receptor to target NPs, the high levels of circulating transferrin, which will compete for the TfR, may hamper the targeting of NPs to the BBB. In order to overcome this issue, monoclonal antibodies against TfR, such as OX26, 8D3, and RI7217, were developed to deliver drugs into the brain [ 82 , 83 ].
Low-Density Lipoprotein Receptor (LDLR) has been tested for both direct- and indirect-brain targeting. Regarding indirect-brain targeting, Kreuter and colleagues observed that coating poly(butyl cyanoacrylate)-NPs, encapsulating loperamide or dalargin (drugs with analgesic properties), with polysorbate 80 enables the adsorption of apolipoprotein E (ApoE) from circulation in their surface, allowing these NPs to target LDLR on the BBB and cross it via receptor-mediated transcytosis [ 85 ]. For the direct brain-targeting approach, ApoE was covalently bound to human serum albumin NPs (ApoE-NPs) and IV-injected into SV 129 mice. After 15 and 30 min the animals were sacrificed, their brains removed and evaluated by transmission electron microscopy. Interestingly, only ApoE-NPs were observed inside the brain parenchyma and associated with neurons, while unbound NPs were undetected, demonstrating the targeted delivery of NPs using ApoE [ 86 ]. Angiopep-2 is a 19 amino acid peptide that has been shown to target LDLR and to improve brain uptake [ 87 , 88 ]. Angiopep-2 was conjugated with 3 molecules of the anti-cancer drug paclitaxel and this system tested for breast cancer brain metastasis targeting, since this receptor is overexpressed both in the BBB and brain tumors. The Angiopep-2-conjugated paclitaxel and free drug was tested in mice by IV administration. A 161-fold increase in the brain accumulation and a 12-fold increase in the brain metastasis accumulation of the Angiopep-2-conjugated drug were reported. These results suggest an improved brain and brain metastasis delivery of the drug conjugated with Angiopep-2, compared with free drug [ 89 ].
Insulin and monoclonal antibodies targeting the insulin receptor have also been used to direct NPs into the brain. Ulbrich and colleagues prepared human serum albumin NPs covalently bound to insulin or to the anti-insulin receptor monoclonal antibody 29B4 to deliver loperamide (an opiate receptor agonist unable to cross the BBB) into the brain after IV administration in mice [ 90 ]. The targeted NPs loaded with loperamide were able to induce significant nociceptive effects in mice evaluated by the tail flick test, as compared with NPs attached to an unspecific IgG. Moreover, a pre-injection of free 29B4 anti-insulin receptor antibody, 30 min prior to insulin-targeted NPs administration, inhibited the antinociceptive effects previously observed with these NPs [ 90 ]. Thus, data showed that the use of ligands targeting the insulin receptor enables crossing of the BBB.
The high expression of the choline transporter in the BBB has also been explored for brain targeting. Choline is an essential amino acid and a precursor of the neurotransmitter acetylcholine produced by cholinergic neurons that play an important role in learning and memory [ 123 ]. Choline is able to transpose the BBB through the choline transporter present on the surface of brain microvascular endothelial cells [ 123 ]. Li and colleagues took advantage of the high expression of Choline transporter in the BBB and glioma cells to achieve a dual targeting with a single ligand [ 91 ]. Authors complexed a plasmid encoding for human tumor necrosis factor-related apoptosis-inducing ligand (Trail) and the chemotherapeutic drug doxorubicin (DOX) with dendrigraft poly-L-lysine to establish NPs capable to mediate gene therapy and chemotherapy to tackle glioma. Moreover, a choline derivate ligand, designed with the bis-quaternary ammonium compound isoquinoline that has demonstrated high affinity to the choline transporter in the BBB [ 92 ], was used as targeting ligand to overcome the BBB. The higher cellular uptake and therapeutic efficiency of the choline transporter-targeted NPs, compared to the non-targeted NPs, was demonstrated in the U87 MG glioma cell line. U87 MG glioma cells were injected in the right striatum of male Balb/c nude mice, and the choline transporter-targeted and non-targeted NPs were intravenously injected 18 days after the cells’ implantation. NIR images, taken 2 h after NPs administration, demonstrated a preferential accumulation of the choline transporter-targeted NPs in the brain, as compared to non-targeted NPs. However, both types of NPs revealed high accumulation in peripheral organs, especially in the liver and spleen [ 91 ].
Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is another membrane bound receptor widely expressed in the cerebral blood vessel endothelia, neurons, and glial cells [ 124 ]. It has been demonstrated that the carrier protein CRM197 is able to mediate the BBB-targeted delivery using receptor-mediated endocytosis via HB-EGF [ 125 ]. CRM197 is a mutated form of the diphtheria toxin produced by the bacteria Corynebaterium diphtheriae that when released in the bloodstream may cause neuritis [ 126 ]. CRM197 targeting ligand has been used with success [ 93 , 127 ]. For example, using an in vitro BBB model composed of human brain-microvascular endothelial cells (HBMEC) seeded on the top (Polyester membrane) of a transwell and human astrocytes seeded on the bottom, Kuo and colleagues investigated the ability of polybutylcyanoacrylate (PBCA) NPs conjugated with CRM197 to deliver zidovudine (AZT). The NPs were loaded with dextran-FITC and their uptake in HBMEC was demonstrated by fluorescent microscopy [ 93 ]. Similarly, the ability of CRM197 to deliver polymeric poly-lactide (PLGA) NPs to the brain of CD1 wild-type mice after IV administration was assessed [ 127 ]. CRM197-targeted NPs loaded with the rhodamine B dye were administered to the mice, which were sacrificed 30 and 60 min after the administration. For both time points, red spots were observed in whole brain parenchyma, indicating the presence of the NPs. It was also reported significant accumulation of the CRM197-NPs in the liver and spleen and limited uptake in the kidneys and lungs. The cellular tropism of the CRM197-NPs was evaluated 30 min, 6 and 48 h after administration. A preferential accumulation in NeuN-positive cells (neurons) was detected. Additionally, over time there was an increased accumulation of these NPs, being reported that 40%, 48%, and 63% of the cells co-localized with the NPs for each time point, respectively. GFAP-positive cells (astrocytes) presented 35% of co-localization with NPs at 30 min, but their presence was decreased to 15% and 2% for 6 and 48 h, respectively. Furthermore, CRM197-NPs loaded with loperamide were intravenously injected in mice to test their ability to trigger nociceptive effects. Five hours post administration, the analgesic effect reached 35% and remained high for 2 days. Whereas, the control groups, namely free loperamide and unloaded CRM197-NPs, were unable to trigger analgesic effect. The untargeted loperamide-loaded NPs showed reduced analgesic activity with maximum possible effect (MPE) values between 5 and 10% [ 127 ].
Brain inflammation is a critical condition observed in most neurodegenerative diseases [ 128 , 129 , 130 ], promotes significant alterations in the BBB, including enhanced leakage of this structure, further increasing neuroinflammation and brain edema [ 95 , 131 , 132 ]. Some studies explored this inflammatory status to target therapies to the brain, namely by targeting specific markers of inflammation in the endothelium. In particular, Marcos-Conteras and colleagues developed NPs loaded with mRNA of thrombomodulin (a factor produced by endothelial cells that is responsible for inhibiting thrombosis, vascular leakage, and inflammation) using as targeting ligand an antibody to vascular adhesion molecule 1 (anti-VCAM-1) and compared their delivery capacity to TfR- and anti-intracellular adhesion molecule 1 (anti-ICAM1)-targeted liposomes [ 95 ]. ICAM1 is expressed in endothelial cells, including vascular endothelial cells, as a surface receptor and its expression is described to be enhanced in pathological conditions [ 133 ]. Regarding VCAM-1, this receptor is specifically expressed on the surface of vascular endothelial cells and was described as overexpressed in neuroinflammation, serving as one of the initial players to this process [ 134 ]. The delivery capacity of the NPs was tested in C57Bl/6 mice with acute brain inflammation induced by microinjection of TNFα in the striatum. The brain accumulation of liposomes using anti-VCAM-1 as targeting ligand was 27- and 8-fold enhanced compared to liposomes with anti-TfR and anti-ICAM1, respectively. Additionally, lipid NPs conjugated with anti-VCAM-1 and loaded with mRNA of thrombomodulin selectively accumulated in the inflamed brain and the de novo expression of the cargo mRNA resulted in alleviation of TNFα-induced brain edema [ 95 ]. Additionally, to improve the targeted delivery, after overcoming the BBB it is important to direct NPs to specific cells in the brain parenchyma. In this regard, several strategies exploring the specific recognition by targeting ligands of the different resident cells in the brain, namely neurons, astrocytes, microglia, oligodendrocytes, and neural stem cells, (Fig. 4 ) have been developed and will be discussed in the following sections.
Cell specific targeting. The presence of specific receptors or overexpression of certain receptors on the cell surface may be explored to promote a targeted delivery of the NPs to such cells. NPs formulated with a specific targeting ligand are unable to enter cells lacking the specific receptor for the targeting ligand as illustrated by the purple cell. On the other hand, the NPs are able to specifically deliver its cargo to the cells expressing the receptor specific for the targeting ligand, illustrated by the gray cell
Targeting brain tumors
The most common primary malignancy in the CNS is glioma, which, due to its infiltrative growth and difficulty to be removed surgically, is associated with poor prognosis and short survival rates [ 135 , 136 ]. In this regard, extensive work has been done aiming at the development of anti-cancer medicines capable to overcome the BBB and target glioma using NPs as drug carriers [ 137 , 138 , 139 , 140 ]. Interestingly, TfR and LDLR are described to be overexpressed in glioma cells and in endothelial cells of the BBB, marking them attractive targets in the development of such therapies [ 141 , 142 , 143 , 144 , 145 ]. Beside the challenge to overcome the BBB, glioma therapy also faces the hurdle to penetrate the tumor. As so, Zhu and colleagues developed docetaxel-loaded nanomicelles coupled with two targeting ligands, Angiopep-2 and TAT [ 146 ]. As discussed above, Angiopep-2 is a peptide that targets LDLR, while TAT is a cell penetrating peptide (CPP). TAT was linked to a short PEG 2000 , shielded by a longer PEG 6000 to avoid unspecific cell penetration during circulation in the bloodstream. Authors argue that after coupling of Angiopep-2 to its target receptor, the close contact between NPs and endothelial cells triggers the effect of TAT, enhancing the crossing of the BBB and further accumulation in the glioma [ 146 ]. Several different ratios of the two ligands in the NPs were tested and the combination of 20 mol% of Angiopep-2 with 10 mol% of TAT resulted in higher cell uptake of the NPs compared to single targeted Angiopep-2 micelles and non-targeted micelles. To study the pharmacokinetics of the NPs, authors labeled the docetaxel-loaded micelles with Cy-5 and injected them into Balb/C mice. Comparing to free drug, all micelles (non-, double- or single-targeted) presented over 10-fold higher circulation times. Moreover, the double-targeted NPs exhibited more pronounced drug delivery to the brain. Importantly, the accumulation observed in peripheral organs for double-targeted NPs was relatively low, indicating that the shielding of TAT with PEG was successful. Regarding antitumor efficacy of the double-targeted NPs, the formulation was injected in Balb/C nude mice bearing an orthotopic U87MG glioma. The docetaxel loaded double-targeted NPs were more efficient in inhibiting tumor growth, resulting in pronounced reduction of body weight loss, and increase in survival time up to 2-fold, with residual damage of peripheral organs [ 146 ].
Zhu and colleagues also established a formulation based in reduction-sensitive Polycaprolactone (PCL) micelles, functionalized with cyclic RGD peptide, to deliver DOX to U87MG glioma xenografts [ 147 ]. cRGD has high affinity for α v β 3 integrins, which are described to be highly expressed on malignant tumor cells like U87MG [ 148 , 149 ]. Beside the lack of a targeted approach, the slow drug release from their vehicle also causes poor efficacy of antitumor therapy [ 150 , 151 , 152 ]. Hence, the authors took advantage of the reductive environment in cancer cells [ 153 , 154 ], to develop micelles with a S-S (disulfide) linker between PCL and PEG in order to enhance the NPs destabilization once inside the cancer cells and consequently promote DOX release. DOX release in U87MG cells was 2.3- and 4-fold increased for cRGD/PEG-SS-PCL micelles compared to non-targeted PEG-SS-PCL and reduction insensitive cRGD/PEG-PCL micelles, respectively [ 147 ]. In nude mice xenotransplanted with U87MG cells, cRGD/PEG-SS-PCL and cRGD/PEG-PCL micelles exhibited 2.2-fold increase accumulation in the tumor site compared to non-targeted PEG-SS-PCL micelles (4.38% ID/g and 4.12% ID/g VS 1.99% ID/g, respectively), with lower DOX accumulation in liver and heart. Moreover, the DOX signal at the tumor site for cRGD/PEG-PCL micelles was weaker than the signal for cRGD/PEG-SS-PCL, indicating an enhanced DOX release promoted by the latter micelles. Regarding tumor growth, cRGD/PEG-SS-PCL significantly inhibited tumor growth by 50% compared to cRGD/PEG-PCL and PEG-SS-PCL micelles [ 147 ], demonstrating the therapeutic efficiency of DOX delivered by the cRGD/PEG-SS-PCL micelles.
The dysregulation of gene expression in glioblastoma cells, namely of microRNAs like miR-21, has been associated with tumor development and progression [ 155 ]. As so, modulation of these miRNAs with oligonucleotides (ODNs) has been demonstrated to reduce migration and proliferation of glioblastoma cells and increase the cytotoxic effect of anticancer drugs [ 156 , 157 ]. With this in mind, Costa and colleagues developed stable nucleic acid lipid particles (SNALPs) loaded with anti-miR-21 ODNs and using chlorotoxin (CTX) as targeting ligand [ 158 ]. CTX is reported to bind to matrix metalloproteinase 2 (MMP-2), which is considerably overexpressed in glioblastoma compared to normal tissues [ 159 ]. Using FAM-labeled anti-miR-21 ODNs in CTX-targeted and non-targeted SNALPs, the authors observed an almost 10-fold increase in fluorescence signal for CTX-SNALPs compared to non-targeted NPs, indicating that CTX significantly increases the internalization of SNALPs by U87MG cells. Furthermore, CTX-SNALPs promoted a 5-fold reduction in miR-21 expression in these cells compared to non-targeted SNALPs, which had no effect on miR-21 expression. Interestingly, miR-21 silencing resulted in increased expression of PTEN and PDCD4, two tumor suppressors modulated by miR-21 [ 160 , 161 ]. Moreover, a reduction in the antiapoptotic effect, by a 2-fold increase in caspase 3/7 activity, was also observed. For in vivo experiments, CTX- and non-targeted SNALPs were administered into a glioblastoma mouse model, established through GL261 cell (mouse glioblastoma cell line) injection in the mice brain. A 2-fold accumulation of CTX-SNALPs compared to non-targeted particles was observed in the transplanted glioblastoma cells [ 158 ].
Up to 20% of cancer patients will develop brain metastases, leading to poor prognosis and reduced survival rates with current state-of-the-art treatments [ 162 , 163 , 164 ]. Pharmacological access to these brain metastases is a major hurdle, with reported drug concentrations 10 times lower in brain metastases compared to other metastases, which is explained in part by the presence of the BBB [ 165 , 166 ]. Prostate-specific membrane antigen (PSMA) is a receptor described to be overexpressed in BBB endothelial cells of newly formed vasculature feeding the brain metastases, while PSMA detection on regular endothelial cells of the BBB is residual [ 167 , 168 ]. Taking advantage of this different PSMA expression, Ni and colleagues developed PLGA-NPs employing a double-targeting system approach. Thus, NPs were conjugated to the small molecule ACUPA, which has been described as an efficient targeting ligand for PSMA [ 169 , 170 ], to target the brain metastases endothelial vasculature, and the peptide cyclic TT1 (cTT1) which has demonstrated tumor-targeting abilities [ 162 , 171 ]. The in vivo evaluation of the NPs was performed in mice bearing breast cancer cell metastases (BCBM), induced by intracardiac injection of 231Br cells (human breast cancer cell line). The NPs were loaded with DOX or Lapatinib (LAP); both types of NPs were co-injected to achieve synergistic activity between both drugs. After injection in BCBM mice, ACUPA (A)-NPs and A-NPs-cTT1 enhanced brain accumulation, while no significant accumulation was observed in peripheral organs. Moreover, treatment with DOX and LAP loaded A-NPs-cTT1 led to tumor growth reduction compared to free drug and non-targeted NPs. Finally, animals treated with A-NPs-cTT1 had an extended median survival time (44 days) compared to saline (25 days), free combination (29 days), non-targeted NPs (29 days), A-NPs (33 days), and NPs-cTT1 (32 days) [ 162 ].
Targeting neurons
Neurons are specialized brain cells responsible to process and transmit information to other cells via electrical and chemical signals [ 172 ]. Therapies that specifically target these cells are particularly important since they are the major cell type affected in neurodegenerative diseases [ 173 , 174 ]. Typically, neurodegenerative diseases affect one specific subset of neurons, leading to the dysfunction of specific brain regions [ 174 ]. For example, neurons from the hippocampus and the cerebral cortex, which mostly express M1 and M2 muscarinic acetylcholine receptors, are the most affected in AD [ 175 ], while neurons from the striatum, which in turn express more M4 muscarinic acetylcholine receptors, are more affected in PD [ 176 ]. Given these differences between neurons of different brain regions, it is important to select an appropriate ligand that is able to target the specific cells in the brain aimed to be treated [ 20 ]. Although challenging, some work has been done in order to develop NPs that specifically target neurons in the context of several neurodegenerative diseases [ 20 , 177 , 178 , 179 ].
Neurotensin neuropeptide has been demonstrated to be specifically internalized by neurons via receptor-mediated uptake [ 96 ]. To target neurons, Hsieh and colleagues coupled Neurotensin to graphene oxide NPs, functionalized with polyethyleneimine (PEI) in order to obtain positively charged NPs [ 97 ]. Taking advantage of external destabilization of the cellular membrane using near-infrared (NIR) laser irradiation, the mentioned NPs were used for plasmid DNA (pDNA) delivery specifically into neurons. In vitro, the described system was able to deliver pDNA in PC-12 cells differentiated into neuron-like cells. Upon intracerebral injection in the caudate nucleus of C57Bl/6 mice, the NPs not coupled to neurotensin transfected mostly glial cells. Whereas, neurotensin-coupled NPs transfected mostly neurons [ 97 ].
Park and colleagues compared PEGylated neurotensin-coated PEI NPs (NT-PEI) with Tet-1-coated NPs [ 98 ]. Tet-1 is a peptide with the binding characteristics of the tetanus toxin, which interacts specifically with motor neurons and has the ability to undertake retrograde transport to the cell soma [ 98 ]. The NPs (NT-PEI, Tet1-PEI, and PEI (control)) labeled with the YOYO-1 fluorophore were added to neuron-like differentiated PC-12 cells. Flow cytometry analysis revealed that the PEI-treated cells had a similar fluorescence profile as untreated cells (0.6% of cells). While cells treated with the targeted NT-PEI and Tet1-PEI NPs presented 12.7% and 16.3% higher fluorescence levels, respectively. Furthermore, as the Tet1-PEI NPs revealed higher binding affinity to neuron-like cells, it was also demonstrated, through confocal microscopy, that neuronal cultures internalize the Tet1-PEI NPs [ 98 ].
The Tropomyosin receptor kinase B (TrkB) is a receptor abundantly expressed by neurons, being activated by BDNF and internalized upon activation. This receptor is key to neuronal survival, plasticity, and neuroregeneration [ 180 ]. Therefore, it might be an interesting entrance gate in neurons. Accordingly, Huang and associates developed a screening platform for aptamers that target this receptor [ 99 ]. The C4-3 aptamer was identified as an agonist for TrkB and was tested in primary cultures of embryonic rat cortical neurons. Data revealed an increase in phosphorylated TrkB (p-TrkB) (the activated form of this receptor), as well as increased neuroprotection when the cells were deprived of supplements in their culture media [ 99 ]. To test the agonist activity of C4-3 in vivo, this aptamer or a scrambled (control) aptamer were injected into the hippocampus of adult mice. Increased p-TrkB levels were observed in the hippocampus of C4-3-injected mice, which was not detected in mice injected with the scrambled aptamer, demonstrating the agonist activity of C4-3 in vivo [ 99 ]. In line with this work, Xu and colleagues developed IKRG, a tetra peptide that mimics BDNF function and interacts with TrkB promoting its internalization, to be used as a targeting ligand for neurons in polymeric polycaprolactone (PCL) NPs functionalized with PEG [ 100 ]. In a proof-of-concept study, the authors started to evaluate the uptake of PEG-PCL NPs functionalized with IKRG to selectively target TrkB. The ability of these NPs to be internalized by TrkB-expressing (PC-12) and non-expressing (HeLa) cells was tested. Data indicated that IKRG-NPs were only internalized by TrkB-expressing cells. Furthermore, the authors evaluated the ability of these NPs to deliver VO-OHpic, an inhibitor of PTEN (Phosphatase and tension homolog deleted on chromosome 10), in order to promote neuroregeneration in peripheral neuropathies. For this, the NPs were tested in primary cell cultures obtained from the dorsal root ganglion of C57Bl/6 mice, composed of neurons, Schwann cells, fibroblasts, and glial cells. Successful and preferential internalization of the IKRG-NPs in neurons was reported, as demonstrated by the 2-fold increase in the co-labeling of NPs with TUJ-1 (a neuron-specific marker), compared to untargeted NPs [ 100 ].
Lopes and colleagues tested a non-toxic carboxylic fragment of the tetanus neurotoxin heavy chain with 54 kDa and neurotropic properties, which is able to undergo active retrograde transport after peripheral administration [ 101 , 102 , 103 ]. In this work, the authors took advantage of the neuron-targeting properties of this fragment to direct polymeric NPs composed by thiolated trimethyl chitosan, loaded with pDNA encoding for BDNF. These NPs were tested in a mouse model of peripheral nerve injury, in order to restore enervation and neuroregeneration after intramuscular administration [ 103 ]. This delivery system promoted a significant expression of BDNF in neurons, compared to vehicle or non-targeted NPs, followed by neuroregeneration and functional recovery after injury. Additionally, data revealed an increase in the expression of neurofilament heavy chain (associated with neuroregeneration) and GAP-43 (a protein associated with axonal growth) proteins in the site of injury, a significantly higher density of myelinated axons, increased pAKT expression, and enhanced neurite outgrowth and density [ 103 ], demonstrating the targeted delivery potential of this fragment of the tetanus neurotoxin.
Cell-penetrating peptides (CPP) are small, relatively non-toxic peptides (with less than 30 aminoacids) that were discovered 30 years ago and since then have been used to deliver different kinds of cargo to cells, including pDNA, small interfering RNA (siRNA), viruses, small molecules, and even therapeutic proteins and NPs [ 181 ]. These peptides can be derived from natural proteins (such as viral and antimicrobial proteins), chimeric, or completely synthetic [ 181 ]. The exact mechanism of how CPP are able to enter the cell is still a matter of debate. Endocytosis and direct penetration of the cell membrane are the two more likely cell entry mechanisms for CPP and are highly dependent on the type of CPP, its concentration, cargo, and the cell type [ 181 ]. For example, one of the first CPP to demonstrate the ability to enter differentiated neurons was a DNA-binding peptide, a 60 aminoacids region of the antennapedia homeobox protein (pAntp) from Drosophila [ 182 ]. Moreover, Santos Rodrigues and co-workers tested the ability of liposomes functionalized with transferrin and CPP to accumulate in different cell types (endothelial cells, astrocytes, and neurons) [ 104 ]. In this experiment, three different CPPs, Mellitin (Mel), Kaposi fibroblast growth factor (kFGF), and a conjugation of the penetration accelerating sequence (Pas) with the arginine-rich peptide R8 (PasR8), were tested together or not with transferrin. Mel is a 26 aminoacids cationic peptide derived from bee venom, which causes the rearrangement of the cell’s plasma membrane to form pores upon contact, facilitating the entry of cargo into the cell [ 183 , 184 ]. kFGF is a hydrophobic peptide with the ability to non-covalently bind to DNA by complexation, protecting the cargo from nucleases, and successfully delivering it to cells [ 185 , 186 ]. Pas is also a hydrophobic peptide (FFLIPKG) that when added to the arginine-rich R8 peptide forms a hybrid peptide with enhanced carrier abilities and capacity to evade lysosomes [ 187 , 188 ]. The ability of the functionalized liposomes to efficiently deliver pDNA encoding for green fluorescent protein (GFP) to neurons isolated from newborn rats was evaluated 48 h after incubation. Interestingly, liposomes with dual functionalization (conjugated with two ligands) transfected more cells than single-functionalized liposomes conjugated with one of the CPPs. Neurons displayed 5% of transfection after incubation with non-functionalized liposomes vs. 7%, 18%, 8%, 20%, 6%, and 10% of transfection with liposomes functionalized with Mel, Mel + Tf, kFGF, kFGF + Tf, PasR8, and PasR8 + Tf, respectively. Furthermore, liposomes were loaded with lissamine rhodamine, administered in the tail vein of C56Bl/6 mice, and biodistribution was evaluated through the relative fluorescence intensity measured using near-infrared (NIR) imaging. The fluorescence in the brain of the mice injected with the liposomes functionalized with kFGF + Tf was increased. The latter NPs resulted in higher brain accumulation (5.7% of the injected dose/g [ID/g]), as compared with 2.3%, 2.7%, 3.2%, 2.1%, and 3.7% of brain accumulation obtained for liposomes conjugated with kFGF, Mel, Mel + Tf, PasR8, and PasR8 + Tf, respectively. Despite these encouraging results, a significant accumulation of the NPs in the liver (14.6% ID/g), kidneys and lungs (4.8–10.4%), hearth (5.4%), and spleen (3.2%) was also reported [ 104 ]. These authors also tested the conjugation of liposomes with Tf and other CPPs, such as the vascular endothelial-cadherin-derived peptide (pVec), the pentapeptide QLPVM (QL), and the HIV-1 trans-activating protein (TAT) [ 105 ]. pVec is an 18 aminoacids amphipathic peptide, which presents a hydrophilic end that interacts with the cell membrane and another hydrophobic end that destabilizes the membrane allowing the entry of the CPP into the cell [ 189 , 190 ]. QL is a hydrophobic pentapeptide derived from the Bax-binding domain of the Ku-70 protein that has cell permeability ability [ 191 , 192 ]. TAT is a cationic peptide that was the first CPP to be characterized [ 193 ]. TAT owes its cell-penetrating capacity to its positive charges that interact with the negative charges of glycosaminoglycans present at the cell surface [ 194 ]. Using the same methodology, it was evaluated the transfection ability of single-functionalized (with one of the 3 CCPs) and dual-functionalized (Tf and one of the 3 CPPs) liposomes loaded with pDNA encoding for GFP. As previously observed, the dual-functionalized liposomes outperformed their single-functionalized counterparts. Moreover, TAT (single and dual) functionalized liposomes demonstrated the best delivery capacity. The number of transfected neurons was 4% for non-functionalized liposomes compared with 7%, 10%, 6%, 8%, 9%, and 13% for liposomes functionalized with pVec, pVec + Tf, QL, QL + Tf, TAT, and TAT + Tf, respectively [ 105 ]. In vivo, a brain accumulation of 7.7% for TAT + Tf-liposomes and 3.1% for TAT-liposomes was reported. Additionally, the authors also reported a considerable accumulation of these liposomes in the liver and kidneys, and TAT-liposomes were also found accumulated in the lungs [ 105 ]. Despite interesting, the poor tissue specificity observed when applying CPPs in delivery systems [ 195 ] raises concerns regarding accumulation in off-target cells.
The glycopeptide 7 (g7) has brain-targeting ability. This peptide was engineered from the opioid peptide MMP-2200 through the replacement of the aminoacid Tyr (responsible for the opioid effect) by Phe [ 107 , 196 ]. Thus, g7 peptide conjugated in PLGA NPs was tested to overcome the BBB and accumulate in neurons [ 106 ]. These NPs were injected, via intraperitoneal (i.p.) administration, in C57Bl/6 mice and a brain accumulation of up to 10% of the injected dose was reported. Furthermore, neurons were the main cell type targeted by the NPs, although affinity to microglia and minor co-localization of the NPs with astrocytes was also detected. Interestingly, region-specific brain accumulation of the NPs was reported, namely into some subtypes of neurons, such as neuropeptide Y (NPY) and glutamic acid decarboxylase (GAD) positive interneurons. Moreover, interaction studies revealed a clathrin-dependent internalization mechanism in the NPs’ internalization by the neurons [ 106 ].
The rabies virus glycoprotein (RVG) is the glycoprotein responsible for the neurotrophic nature of the rabies virus [ 197 ]. The receptor of the nervous system responsible for the interaction with RVG is still a matter of debate; nevertheless, the nicotinic acetylcholine receptor (nAChR) was the first receptor identified to play a key role in this interaction [ 198 ]. Derivates of the RVG have been explored to target NPs to the brain. These peptides are shorter versions of the original RVG, which retain the capacity to target and be internalized by neurons. For example, in the context of Machado-Joseph disease (MJD), a neurodegenerative disease presenting extensive neuronal death caused by the mutant ataxin-3 presence in neurons, our group developed RVG-9r-conjugated liposomes encapsulating siRNA to silence mutant ataxin-3 [ 108 ]. RVG-9r, a ligand derived from RVG with 9 arginine residues, was used as a brain-targeting ligand that enables the BBB transpose. The biodistribution data of RVG-9r-liposomes (encapsulating the near-infrared dye (NIR) indocyanine green (ICG)) IV injected in mice showed that the RVG-9r targeting ligand increased by 20% the brain accumulation of liposomes, compared to a control ligand. The RVG-9r targeting ligand also led to 25% and 30% decrease in liposomes accumulation in the heart and lungs, respectively, compared to the control ligand. Furthermore, the administration of RVG-9r-liposomes encapsulating siRNA for mutant ataxin-3 silencing, in an MJD transgenic mouse model [ 199 ], resulted in 30% reduction in mutant ATXN3 mRNA, as compared with RVG-9r-liposomes encapsulating a control siRNA [ 108 ]. These data indicate that RVG-9r-mediated delivery of liposomes encapsulating gene silencing therapies is an efficient approach to silence mutant ataxin-3 in MJD. Moreover, a peptide derived from RVG with 29 amino-acids (RVG-29) was used by Chen and colleagues to target human serum albumin NPs loaded with the antifungal drug itraconazole (ITZ) to treat brain fungal infections [ 109 ]. NPs conjugated or not with RVG-29 were injected into the caudal vein of adult mice. Significantly increased levels of ITZ in the group of animals injected with RVG29-conjugated NPs, as compared to control/untargeted liposomes, was reported. Namely, 2 h post-injection, 100 ng of ITZ/g of brain tissue was detected for the untargeted NPs. Whereas, 200 ng of ITZ/g of brain tissue was detected for the RVG-29-conjugated NPs. These data showed that RVG-29-conjugated NPs could be exploited as a brain delivery system [ 109 ].
Despite the encouraging data reported for brain-targeted NPs, these reports also highlight the need to develop more specific brain-targeting ligands and/or NPs to avoid their accumulation in peripheral organs, which results in loss of NPs in undesired sites and also to potential off-target effects (Table 3 ).
Targeting astrocytes
Astrocytes have key functions in neurotrophic, physical, and metabolic maintenance to neurons, and are indispensable in neurotransmission, namely in supporting and modulating synapses [ 200 , 201 , 202 , 203 , 204 ]. Additionally, astrocytes contribute to immune surveillance in the brain becoming activated in insults, infections, and brain diseases, releasing inflammatory mediators [ 205 , 206 ]. Several neurodegenerative diseases, such as AD, PD, Huntington’s disease (HD), and Amyotrophic Lateral Sclerosis (ALS), affect astrocytes (reviewed in [ 207 ]), requiring their treatment and consequently drug targeting. The most employed delivery system targeted to astrocytes are viral vectors, since virus can be engineered to have pseudo-tropism for astrocytes and astrocyte-specific promoters can be used to guarantee the gene expression in these cells [ 208 , 209 ]. Although the development of drug-delivery NPs that specifically target astrocytes is still limited, astrocytes present a rich repertoire of receptors, which may be used to specifically target drugs and NPs to them.
Aquaporin 4 (AQP4) is a water channel preferentially expressed on astrocytes and displays a wide range of functions, namely, regulation of potassium and calcium concentrations, osmotic pressure, waste clearance, neuroinflammation, and cell migration and synaptic plasticity [ 210 , 211 ]. Interestingly, AQP4 is strongly expressed on the surface of astrocytes in the context of neurodegeneration [ 212 ]. Taking advantage of the preferential expression of this water channel on astrocytes, an anti-AQP4 antibody was conjugated with polymeric poly(glycidyl methacrylate) (PGMA) NPs to deliver the anti-oxidant resveratrol to tackle oxidative stress in the context of neurodegenerative diseases [ 110 ]. Resveratrol has shown poor bioavailability and rapid metabolization in vivo [ 213 , 214 ]. Thus, the authors reported the accumulation of the AQP4-targeted NPs loaded with rhodamine B in GFAP-positive astrocytes, demonstrating the anti-AQP4 antibody targeting to astrocytes. AQP4-targeted NPs loaded with resveratrol were then administered in situ after optic nerve injury induction in adult female Piebald Viral Glaxo rats. The AQP4-targeted NPs were found to accumulate inside astrocytes and to effectively deliver resveratrol when administered to the site of injury. Furthermore, the targeted NPs were also able to rescue oxidative damage in the site of injury, as demonstrated by the reduction of immunoreactivity of 8-hydroxy-2’-deoxyguanosine (8OHdG) (a hallmark of oxidative damage in nuclear and mitochondrial DNA), as compared to non-targeted or non-loaded NPs [ 110 ]. Therefore, this study demonstrates the ability of the anti-AQP4 antibody to target NPs to astrocytes.
In another approach, the D4 monoclonal antibody that recognizes the GFAP protein preferentially expressed by astrocytes [ 215 , 216 ], was linked to PEGylated liposomes [ 111 ]. The DiI fluorescent dye was integrated into the liposome’s bilayer allowing the visualization of the targeted NPs interaction with the astrocytes in vitro, through fluorescence microscopy. The specificity of the D4 antibody-conjugated liposomes to specifically interact with astrocytes was confirmed, since non-targeted or liposomes conjugated with a Control (non-specific) antibody were not visualized in the astrocytes. However, when administered to male Wistar rats by IV administration in the femoral vein, these NPs were unable to reach CNS astrocytes, mainly due to their inability to cross the BBB [ 111 ]. This work opens the avenue to speculate that these NPs may be useful in the context of diseases that present a weakened BBB or, furthermore, to functionalize these NPs with a second targeting ligand to allow their BBB crossing. In line with the former example, chitosan NPs functionalized with two commercially available antibodies, one targeting the transferrin receptor (widely expressed on BBB endothelial cells) and another targeting the bradykinin B2 receptor. Bradykinin B2 receptor (B2R) is associated with vasodilatation, neuroinflammation, and glucose uptake [ 217 , 218 ]. B2R is not exclusive to astrocytes but is highly expressed in these cells [ 219 , 220 ]. Therefore, an antibody anti-BR2, which is rapidly internalized after binding with a specific ligand, was employed in combination with transferrin in chitosan NPs to aid in overcoming the BBB [ 112 ]. These double-targeted chitosan NPs were tested in a BBB in vitro model to deliver siRNA to inhibit HIV-1 replication in astrocytes. SiRNA anti-SART3 and -hCycT1 genes, both important for HIV-1 replication in astrocytes, were employed. It was reported that the dual-targeted NPs penetrated across the human cerebral microvascular endothelial cells (hMCEC/D3) and accumulated in the human astrocytoma cells (U138-MG). This cell targeting resulted in a 6 times higher accumulation of siRNA in U138-MG cells, as compared to non-targeted NPs. Furthermore, the presence of the siRNA in these cells resulted in a gene knockdown of 81% and 67% for SART3 and hCycT1 mRNA, respectively [ 112 ].
Considering the small development of NPs specifically targeting astrocytes, it is of great interest to further explore more receptors that are exclusively or preferentially expressed by astrocytes in order to use them in NPs. For example, the N-acetylaspartylglutamate (NAAG) receptor, also known as metabotropic glutamate receptor 3 (mGluR3), is expressed in both neurons and astrocytes but their expression is enriched in astrocytes [ 221 ]. This receptor is activated by the neurotransmitter NAAG peptidase released by stimulated neurons [ 222 ] and its activation is believed to influence neuron and neurovascular stimulation in the context of schizophrenia and other neuropathies [ 222 ]. Moreover, a recent review highlighted the importance of some astrocyte receptors and transporters in the context of AD [ 223 ]. In particular, the excitatory aminoacid transporters EAAT1 and EAAT2, which although not exclusively expressed by astrocytes are in much larger amount in these cells [ 224 ]. In fact, EAAT are more active on astrocytes since they are responsible for 80% of the glutamate uptake [ 225 ]. Targeting these receptors would not only be promising to direct NPs to astrocytes but represents as well an opportunity to treat excitotoxicity in the context of neurodegenerative diseases [ 223 , 226 , 227 ]. Furthermore, the protein S100β is a calcium-binding protein abundantly expressed by mature astrocytes with the ability to be internalized [ 228 , 229 ]. Thus, the coupling of targeting ligands for S100β to NPs may also present a capable strategy to target astrocytes for drug delivery. Finally, the active targeting of the cannabinoid receptors CB1 and CB2 present in glial cells, such as astrocytes and microglia, may help to control the neuroinflammation characteristic of several neurodegenerative diseases, by modulating the expression of inflammatory cytokines in these cells and their migration [ 230 ]. However interesting, NPs with targeting ligands that direct them to these receptors are yet to be explored.
Targeting microglia
Despite being CNS resident immune cells, microglia do not develop from the neuroectoderm like other neural cells. They are derived from the yolk sac primitive macrophages and migrate to the CNS during embryonic development [ 231 ], representing 5 to 12% of all cells in the healthy CNS [ 232 ]. Physiologically, microglia have surveillance phenotype characterized by a ramified morphology and are the first line of defense against pathogens, promoting brain homeostasis and repair [ 233 , 234 ]. Moreover, these cells have key functions in several processes such as neurogenesis, neural circuits refinement, and mediation of neurotransmission and synaptic pruning [ 235 , 236 ]. However, in situations where the homeostasis in the brain is compromised, such as neurodegeneration or sustained inflammation, microglia changes their phenotype to an ameboid-like structure and alters their secretome, upregulating the expression of several cytokines, interleukins, and complement factors, enhancing and perpetuating neuroinflammation [ 232 , 237 ]. Considering the characteristics of microglia as first responders to changes in brain homeostasis and their role in neuroinflammation, they appear as an interesting target for brain therapies. Indeed, several publications demonstrate a high internalization ability of activated microglia compared to non-activated [ 238 , 239 , 240 ]. Nonetheless, given the intrinsic phagocytic nature of microglia, concerns have been raised considering the specificity of microglial uptake of NPs, since NPs may just be recognized as pathogens [ 241 , 242 ].
Microglia present a wide range of receptors, due to their surveillance function, so NPs can be tailored to take advantage of these receptors. Innate immune cells, such as microglia, have Pattern Recognition Receptors (PRRs) that have been used to target them [ 243 ]. These include Toll-Like Receptors (TLR), Receptors for Advanced Glycation Endproducts (RAGE), and Scavenger Receptors [ 244 , 245 , 246 , 247 ].
Choi and colleagues designed ceria-zirconia NPs (composed of Cerium and Zirconium) that specifically targeted microglia by conjugation with antibodies anti-CD11b (a receptor expressed on the surface of microglia and macrophages [ 248 , 249 ]). In this work, the authors hypothesized that oxidative stress and inflammatory activation of microglia plays a role in neuropathic pain by sensitizing neurons, and tackled this by taking advantage of the anti-oxidant proprieties of ceria, particularly Ce 3+ [ 250 ]. CD11b-targeted and non-targeted NPs labeled with FITC were incubated with microglia cells isolated from C57Bl/6 pups. Authors reported a higher percentage of FITC-positive cells with targeted NPs compared with non-targeted NPs, 80% and 40%, respectively. Regarding the induction of oxidative stress in microglia using tert -butyl hydroperoxide, a more pronounced reduction of ROS was observed when the CD11b-targeted NPs were added to the culture medium as compared to non-targeted NPs. Additionally, in cells pre-treated with lipoteichoic acid to induce the expression of iNOS, IL-6, and IL-1β (related to oxidative stress and inflammation) the treatment with CD11b-targeted NP, led to a 95%, 86%, and 91%, respectively, reduction in the mRNA levels of these genes. While the treatment with non-targeted NPs was only able to achieve reduction levels of 82%, 63%, and 71%, respectively. Moreover, CD11b-targeted and non-targeted NPs were administered using intrathecal injection in a neuropathic pain C57Bl/6 mouse model (spinal nerve transection). It was described a strong correlation between the FITC signal and the microglia-specific marker Iba-1, with co-localization observed in 84% of cells. Whereas, co-localization with the astrocyte marker GFAP and the neuron marker MAP2 was only detected in 26% and 11% of cells, respectively. Finally, the authors also observed a reduction in the hypersensitivity of these animals after treatment with CD11b-targeted NPs, compared with animals treated with non-targeted NPs [ 113 ], demonstrating the targeting ability of these NPs to microglia.
Despite these promising results, more targeting receptors and proteins specific to microglia are required to be explored in NPs development. For example, scavenger receptors are receptors present in cells of the immune system, having a wide range of functions, such as cargo transport inside the cell, lipid transport, recognition and removal of altered lipoproteins, and pathogen clearance [ 251 ]. Examples of scavenger receptors expressed by microglia are SR-A1 and CD36, which are used by microglia to bind and clear β-amyloid fibrils in the context of Alzheimer´s disease [ 252 , 253 ]. However, these receptors are not fully specific of microglia since they are also expressed in macrophages, platelets, and endothelial cells. Therefore, careful consideration must be done when considering these receptors as targets for NPs targeting [ 254 , 255 ].
Other interesting target is the transmembrane lectin sialic acid-binding immunoglobulin-like lectin H (Siglec-H) that in mice is able to discriminate microglia from CNS-bound macrophages and monocytes more accurately than CD11b or Iba-1 [ 256 ]. Further characterization (e.g. binding ligands and specificity, internalization mechanisms, etc.) and the discovery of a human homolog of this receptor may create the opportunity to design NPs to deliver therapies specifically to microglia [ 256 ]. Another receptor widely characterized and acknowledged to be microglia specific is the Cx3Cr1 receptor, also known as fractalkine receptor or G-protein coupled receptor 13 (GPR13) [ 257 ]. This receptor binds to the chemokine CX3CL1, also known as neurotactin or fractalkine. Moreover, the receptor P 2 × 4 is also an interesting potential target, since it is widely expressed in microglia and neurons but has a 3-fold increased expression in microglia under pathological conditions, such as neuroinflammation, hypoxia, and neuropathic pain [ 258 , 259 ]. Although widely expressed in microglia, to this day there are no NPs developed to specifically target these receptors in these cells.
Targeting oligodendrocytes
Oligodendrocytes are specialized cells of the CNS responsible for the myelination of neurons [ 260 , 261 ]. The myelin sheath is a highly complex structure composed of 80% lipids and 20% proteins [ 261 , 262 ] that provides insulating properties to neuronal axons which facilitate electrical signals transmission [ 261 ].
Given their unique characteristics, oligodendrocytes are among the most vulnerable cells in the CNS, and demyelination of axons is one of the hallmarks of neurodegeneration [ 260 , 262 ]. As so, one potential therapeutic approach is to promote remyelination by inducing oligodendrocyte progenitor cells (OPC) to mature into oligodendrocytes and remyelinate the axons [ 263 ]. In order to promote remyelination by targeting OPC, Rittchen and colleagues developed PLGA NPs loaded with leukemia inhibitory factor (LIF), a robust pro-remyelination factor [ 114 ]. To achieve targeted delivery of the NPs to OPC, the authors used as targeting moiety antibodies anti-NG-2 chondroitin sulfate proteoglycan, a proteoglycan predominately expressed in OPC [ 264 ]. Three days after a 24 h treatment with PLGA-LIF NPs targeted to NG-2, rat OPC cultures presented a 33% increase in cells expressing myelin basic protein (MBP), a marker of mature oligodendrocytes, compared to non-targeted PLGA-LIF NPs. The remyelination potential of these NPs in vivo was tested in a mouse model of focal demyelinating lesion, in which the myelin toxin lysophosphatidylcholine (LPC) was administered to the corpus callosum by stereotaxic injection [ 114 ]. Eight days after the lesion, NG-2-targeted and non-targeted PLGA-LIF NPs were injected in the animals, and the effects were assessed 10- and 17-days post-administration. Using electron microscopy, a significant increase in the percentage of myelinated fibers per lesion and significantly thicker myelin sheaths were observed in animals treated with NG-2 targeted PLGA-LIF NPs compared to animals that received non-targeted NPs [ 114 ].
Interestingly, immunoglobulin M antibodies demonstrated the ability to target reactive oligodendrocytes and promote remyelination in a multiple sclerosis (MS) mouse model [ 265 ]. Inspired by this work, Tuerk and colleagues tried to identify DNA aptamers with the same binding affinity to myelin as the immunoglobulin M antibodies [ 266 ]. Authors identified a 40-nucleotide guanosine-rich DNA aptamer with anti-myelin proprieties when in a G-quadruplex structure (LJM-3064) [ 267 ]. In order to obtain the G-quadruplex structure, the biotinylated DNA aptamer was conjugated to a streptavidin core [ 268 ], resulting in a structure the authors called Myaptavin-3064 [ 267 ]. The capacity of this structure to promote remyelination in a mouse model of MS was demonstrated, but the specific interaction with oligodendrocytes was not tested [ 267 ]. In a recent work, the same group tested the affinity of Myaptavin-3064 to a human oligodendroglioma cell line (HOG) and mature oligodendrocytes differentiated from HOG cells [ 115 ]. Flow-cytometry data demonstrated that the binding of Myaptavin-3064 to HOG was increased upon differentiation with almost 90% of differentiated oligodendrocytes positive for Myaptavin-3064, while only 50% of HOG cells bound to Myaptavin-3064 with the same dose. The specificity of Myaptavin-3064 for oligodendrocytes was further confirmed with lung (L2) and kidney (BHK) cells, since flow-cytometry results indicated a residual affinity to these cells. Moreover, in primary cultures of adult rat cortical tissue, the authors identified that 97% of cells positive for the O4, an oligodendrocytes marker, were also positive for anti-streptavidin when co-cultured with Myaptavin-3064, while the co-staining was residual after culture with a control conjugate with a non-specific aptamer (LJM-3060) [ 115 ].
Another group linked the same aptamer (LJM-3064) to the surface of mouse mesenchymal stem cell-derived Exosomes to deliver cargo to oligodendrocytes [ 116 ]. In this work, LJM-3064 was employed not only as a targeting ligand for oligodendrocytes but also for the remyelinating capacity that it had demonstrated before as well [ 267 ]. The binding affinity of the exosome-aptamer conjugate (Exo-APT) was demonstrated in vitro in an oligodendrocytes cell line (OLN93). Exosomes, either targeted or untargeted with the aptamer, were then labeled with ATTO647N. Through flow cytometry analysis an increase in cell fluorescence was observed after incubation with Exo-APT compared to untargeted exosomes. Moreover, Exo-APT also promoted a significant increase in OLN93 proliferation compared to untargeted exosomes, assessed by BrdU cell proliferation assay [ 116 ]. Exo-APT or untargeted exosomes were administrated intravenously in mice before the induction of autoimmune encephalomyelitis (a mouse model commonly used to study MS [ 269 ]). A strong reduction in demyelination, a robust suppression in inflammation, and a reduction in the disease severity in animals administered with the Exo-APT were reported [ 116 ].
Taken together, despite promising, the work done so far to specifically target NPs to oligodendrocytes to treat brain diseases is still very scarce.
Targeting neural stem cells
The loss of neurons is a major hallmark of neurodegenerative diseases; thus, an approach to tackle these diseases is the replacement of dead or impaired neurons. This can be achieved by stimulating neurogenesis, a process in which new mature neural cells are produced from neural stem cells (NSC) present in endogenous niches or engrafted by cell transplantation [ 270 , 271 ]. The adult brain presents regions where NSC reside, the so-called neurogenic niches. The subgranular zone (SGZ) of the dentate gyrus and the subventricular zone (SVZ) of the lateral ventricles are two well-studied niches of NSC. The activity of these niches is crucial for neuroplasticity and learning. However, studies suggest that with aging a reduction of the proliferative, migratory, and integrative capacity of NSC takes place, which severely hampers neuroplasticity [ 271 , 272 ]. Therefore, targeting endogenous NSC with drugs that promote their ability to proliferate, differentiate, migrate, and integrate may be advantageous to promote the replacement of the lost neural cells [ 273 , 274 ]. However, NPs targeting the neurogenic niches and NSC is a field of research poorly explored and there is a demand to find targeting ligands that specifically direct drugs to NSC.
Schmidt and associates identified ligands by phage display technology with the ability to target neural progenitor cells (NPC) [ 118 ]. In this study, the ability of random peptides from a 7mer phage library commercially available to bind and be internalized by neurosphere cultures derived from the hippocampus of adult C57Bl/6 mice was evaluated. The authors tested 130 candidates for their binding efficiency for Nestin-positive cells in vitro. QTRFLLH and VPTQSSG peptides showed 10 to 20-fold increased binding to NPC compared with other peptides. Moreover, regarding cell specificity, QTRFLLH binding to NPC was significantly higher compared to Pan02 (pancreatic cancer cells), NIH3T3 (fibroblasts), H1299 (lung cancer cells), and HEK293 (human embryonic kidney cells). As for VPTQSSG, it exhibited lower binding affinity to NPC but higher cell specificity compared to QTRFLLH, with binding affinities 10 times lower to Pan02 and NIH3T3 and residual binding to H1299 and HEK293. QTRFLLH and VPTQSSG also revealed strong uptake by NPC. As adenoviruses present low infection efficiency of NPC [ 117 ], QTRFLLH and VPTQSSG were covalently linked to an adenoviral vector (wild-type capsid) expressing red fluorescent protein (RFP) to improve the viral delivery to NPC. Through immunofluorescence microscopy, it was observed the expression of RFP inside the NPC, supporting the hypothesis that these ligands can mediate adenovirus binding and uptake by NPC. Then, these viral vectors coding for RFP and linked with either peptide were injected into the hippocampus of a transgenic mouse model expressing GFP in Nestin-positive cells (pNestin-GFP) [ 275 ]. A strong specific co-localization of GFP and RFP was detected, suggesting that the peptides are efficient in guiding the adenovirus to Nestin-positive cells; whereas, the same adenovirus but linked to an unspecific peptide, led to almost no co-localization of RFP and GFP. The percentage of cells with RFP and GFP co-localization was 83.5% for the QTRFLLH peptide and 85.6% for the VPTQSSG peptide, whereas this percentage was 15.5% for the wild-type vector without any peptide and 8.6% for the adenovirus with the unspecific peptide [ 118 ]. Thus, these data indicate that these peptides mediate specific targeting to Nestin-expressing NPC.
The neurofilament light subunit (NFL) is known to present a strong interaction with NSC of the SVZ, showing a preferential accumulation in these cells in vivo after intra-lateral ventricular injections and the ability to induce their differentiation in vitro [ 276 , 277 ]. Accordingly, it was demonstrated that the tubulin-binding site of the NFL (NFL-TBS.40–63), adsorbed to the surface of lipid nanocapsules (NFL-LNC), is able to guide lipid nanocapsules specifically to NSC in the SVZ [ 120 ].
Interestingly, besides being used to target the BBB, transferrin has as well been used to target NSC. In the work by Praca and colleagues, gold nanoparticles and gold nanorods were functionalized with medium density of transferrin peptides (between 169 and 230 transferrin peptides per NP) to direct the particles to the NSC [ 121 ]. Gold-NPs, with and without transferrin functionalization, were injected in the tail vein of adult (8 weeks old) C57Bl/6 mice. Irradiation with near-infrared light (NIR) was applied 1 h after administration to transiently open the BBB. Then, the animals were sacrificed 2 h after the NPs administration and their presence in the different brain regions was analyzed using mass spectrometry. As expected, the gold-NPs functionalized with transferrin preferentially accumulated in the brain compared to non-functionalized gold-NPs. Interestingly, after NIR irradiation, gold-NPs functionalized with transferrin were significantly accumulated in the SVZ (almost 0.2% of the injected dose). Without radiation, the percentage of these NPs accumulated in the SVZ was less than 0.1% of the injected dose and the NPs were more scattered in the brain and found preferentially in non-neurogenic areas. Gold-NPs without functionalization were found only residually in some non-neurogenic regions [ 121 ].
Altogether, these data demonstrate that although interesting, the targeting of NPs to NSC and NPC is still a very unexplored field.
Limitations, successes, and strategies of brain-targeted NPs development for drug delivery
Despite the great potential of brain-targeted NPs to deliver therapeutic molecules to the brain, there is a need to better study the limitations and challenges of this strategy. In this regard, the concept of critical quality attributes (CQAs) has been established by the regulatory authorities to guide their development, characterization, and stability [ 278 , 279 ]. The lipid composition of the NPs is a critical parameter to determine their proprieties and safety [ 280 ]. Therefore, the implementation of biocompatible and biodegradable materials [ 280 , 281 , 282 ] to develop safe NPs to be used in long-term therapies is key. The best composition of NPs is highly dependent on the intended use and, especially, on the cargo drug to be encapsulated [ 283 , 284 ]. Physical characteristics, such as morphology, size, size distribution, surface-to-size ratio, and zeta potential are of the utmost importance to their safety and efficiency as delivery vehicles. Nonetheless, these characteristics are also highly dependent on their intended application, cargo drug, and composition. In general, the regulatory advice is that NPs should have a size lower to 100 nm [ 11 ]. In fact, smaller NPs are more easily eliminated by the kidneys, while larger NPs tend to be trapped in the lungs [ 282 ]. Regarding the size distribution, a polydispersity index (PDI) of 0.3 or below is considered adequate and reflective of a homogenous NPs population [ 285 ]. A large surface-to-size ratio (small size and a very large surface area) may lead to problems like limited drug loading, particle aggregation or friction, and a high clearance ratio [ 281 ]. The large surface area also increases their chemical reactivity, which may cause toxicity, namely through increased reactive oxygen species (ROS) production, neuroinflammation, and DNA damage [ 281 , 286 ]. The zeta potential of NPs is determined by the presence of ionic lipids and/or charged surface ligands in their composition, which influences particle repulsion, aggregation tendency, and biodistribution [ 287 ]. Values between − 30 mV and + 30 mV are considered to keep stable particles suspension and enough inter-particle repulsion [ 278 , 279 ]. Finally, the physical stability over time, namely particle fusion or aggregation, drug leakage, and chemical degradation of the lipids and their cargo, are also critical parameters that must be clearly evaluated several months post formulation [ 278 , 279 ].
Alongside these issues, another limitation is the lack of specific targeting ligands to be used in NPs. In fact, although a preferential accumulation of the targeted NPs exists in the intended cells, some non-specific and potentially toxic accumulation in peripheral organs persists, especially in metabolizing organs such as the liver, lungs, and kidneys (Table 3 ). This may cause off-target adverse effects and hinder the therapeutic efficacy of the targeted therapies. The use of targets that are ubiquitously expressed through the body enhances this non-specific targeting. Ideally, a targeted drug-delivery approach for the brain using NPs should be able to overcome the BBB, specifically recognize the target cells in the brain aimed for treatment, enable endosomal escape after internalization, and release the cargo drug [ 288 ]. Thus, future research needs to focus on identifying more tissue and cell specific markers to be implemented in targeted therapies. An interesting approach to overcome this non-specificity may be the use of a double-targeting strategy. As seen in some reports targeting cancer cells [ 146 , 162 ], the coupling of two different targeting-ligands to NPs can enhance their active targeting and improve their cargo delivery. Such dual targeting, where a targeting ligand is used to overcome the BBB and a second ligand to deliver the NPs to a specific cell population in the brain, can drastically improve therapeutic efficacy. Zhang and colleagues applied this strategy in the context of AD, using a peptide to target the BBB (TGN) and a second peptide (QSH) that binds to Aβ 1-42 in poly(lactic acid) PLA NPs. Authors reported increased brain concentration and distribution in the Aβ 1-42 plaques using the dual-targeted NPs [ 94 ]. These encouraging results, indicate that a dual targeting strategy to tackle brain diseases is a promising strategy. Nonetheless, whether it is the best therapeutic option or not it will have to be assessed on a case-by-case basis, as in some situations it will not be necessary, particularly given that some ligands may trigger this double targeting.
Furthermore, the production of targeted NPs with defined targeting, high quality and adequate for translational reproducibility remains a challenge. One of the key advantages of NPs is that their surface is highly tunable from a chemistry standpoint. In this regard the conjugation of the targeting ligand to the surface of the NPs becomes a critical aspect in the formulation [ 289 , 290 ]. Two main strategies exist for the addition of targeting moieties to NPs, one-pot assembly and post-insertion through surface modifications [ 289 ]. In the one-pot assembly strategy, the targeting ligand is directly added to the lipid mixture prior to NPs formation. This is only feasible for targeting moieties able to endure exposure to organic solvents and high temperatures used during NPs production. Despite quite simple, this strategy presents a major hurdle, the orientation and density of the targeting ligand is completely unpredictable, resulting in a high percentage of ligand in the inside surface of the NPs. In the post-insertion strategy, the NPs are firstly formed and then a surface modification is performed by conjugation of the targeting ligand [ 289 , 290 ]. This conjugation requires a chemical modification on the surface of the NPs by addition of functional groups that will react with reactive groups on the targeting ligands [ 290 ]. An important limitation of this strategy is the frequently observed low insertion yields of ligands in NPs [ 289 ]. Such low yields present a scalability problem since very high amounts of ligand are required to produce small amounts of targeted NPs, resulting in a very expensive manufacturing process.
Targeting ligand density in NPs defines much of the targeting abilities of the NPs since, if there is a too high ligand density, it may result in off-target binding in tissues with lower expression levels of the target receptor. On the other hand, if the density is too low, target cell uptake may be limited. Hence, targeting ligand density in NPs should match the receptor density in target cells [ 105 ]. Additionally, in vivo stability of the targeting ligands must also be addressed during NPs development [ 290 , 291 , 292 ]. In vivo, targeting ligands are subjected to a hostile environment promoted by degrading enzymes, pH, hypoxia, redox, and temperature variations. These are very important factors that might significantly impact targeted NPs therapeutic success [ 289 ].
To be successfully marketed, targeted NPs formulations need to have a large-scale manufacturing process. In addition to the issues related to ligand-NPs conjugation mentioned above, batch-to-batch variations in ligand density and stability, the choice of raw materials, synthesis processes, batch sizes, stability analyses, and documentation needs to be carefully considered [ 293 ].
The translation of a new therapy from a pre-clinical to a clinical investigation setting is always challenging [ 294 ]. For example, the most popular liposome production method is the lipid film hydration method, but the scaling up of this method from milliliters to liters batches, maintaining formulations with similar physicochemical proprieties is demanding [ 295 ]. Other methods like ethanol injection or reverse-phase evaporation are more easily adapted to an industrial setting; nevertheless, these methods face other challenges, such as optimization of particles size reduction, formulation homogeneity, and the removal of organic solvents and detergents [ 294 , 295 ]. Attention must be drawn to several aspects in the early development stages to facilitate transition to an industrial setting. These include use of affordable and high grade raw materials; avoid low-yield and long synthetic reactions; avoid difficult to remove solvents and catalysts; use automation and closed circuit systems for improved safety, cost reduction, and evading errors; establish rigorous and adequate in-process and end-product quality controls; consider production risk assessment for hazardous batch contaminations and interference with the NPs formulation; use adequate methods for stability and shelf-life estimation; give special attention to formulations with a biological product, such as antibodies or proteins; and a cost-effective industrial production [ 293 , 294 , 295 ]. Overall, a multiparameter evaluation of the targeted NPs production process is needed to achieve successful scale-up manufacturing [ 293 , 296 ], based in adequate in-process and final quality controls to ensure homogenous characteristics between batches and cost-effectiveness.
Conclusions
Nanovesicles hold the promise to efficiently and precisely deliver diverse therapies into the brain to tackle neurodegenerative diseases. Nonetheless, these nanoparticles need to be specifically and efficiently delivered to the brain in order to potentiate their therapeutic outcomes without causing major side effects due to their accumulation in peripheral tissues. In this review, we summarized the different targeting ligands identified to deliver nanoparticles to specific cells in the brain. The research done so far in the development of brain-targeted NPs shows promising results in the targeted delivery and treatment of brain cells. In the future, this may result in high precision medicine, with reduced adverse side effects or unwanted therapy clearance from the body. However, much room for improvement still exists for these therapies to reach their full potential in the context of neurodegenerative diseases. For example, the identification of specific cell receptors expressed exclusively by each one of the different cell types would certainly prompt this field to the desired targeted drug delivery. Hence, we believe that it is important to keep focusing research endeavors on the screening of brain cell-specific receptors and in the design of high-affinity targeting ligands to be employed in the development of brain-targeted NPs carrying therapeutic molecules.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
Alzheimer’s disease
Amyotrophic Lateral Sclerosis
apolipoprotein E
Aquaporin 4
Bradykinin B2 receptor
blood-brain barrier
brain-derived neurotrophic factor
cannabinoid receptors
central nervous system
Cell-penetrating peptides
excitatory aminoacid transporters
glycopeptide 7
glutamic acid decarboxylase
green fluorescent protein
Heparin-binding epidermal growth factor-like growth factor
Huntington’s disease
human immunodeficiency virus
immortalized human cerebral microvascular endothelial cell line
human oligodendroglioma cell line
intraperitoneal
indocyanine green
itraconazole
intravenous administration
kaposi fibroblast growth factor
low density lipoprotein
Low-Density Lipoprotein Receptor
leukaemia inhibitory factor
lysophosphatidylcholine
myelin basic protein
metabotropic glutamate receptor 3
Machado-Joseph disease
multiple sclerosis
not applicable
N-acetylaspartylglutamate
nicotinic acetylcholine receptor
neurofilament light subunit
near infrared
neural progenitor cells
nanoparticles
neuropeptide Y
neural stem cells
oligodendrocytes cell line
oligodendrocyte progenitor cells
penetration accelerating sequence
poly(n-butyl cyanoacrylate)
Polycaprolactone
Parkinson’s disease
polyethylene glycol
polyethyleneimine
poly(glycidyl methacrylate)
poly(lactic- co -glycolic acid)
Pattern Recognition Receptors
vascular endothelial-cadherin-derived peptide
pentapeptide QLPVM
arginine-rich peptide
Receptors for Advanced Glycation Endproducts
red fluorescent protein
reactive oxygen species
rabies virus glycoprotein
subgranular zone
transmembrane lectin sialic acid-binding immunoglobulin-like lectin H
subventricular zone
HIV-1 trans-activating protein
transferrin
Transferrin receptor
Toll-Like Receptors
Tropomyosin receptor kinase B
human astrocytoma cell line
world health organization
Hartl N, Adams F, Merkel OM. From adsorption to covalent bonding: apolipoprotein E functionalization of polymeric nanoparticles for drug delivery across the blood-brain barrier. Adv Ther (Weinh). 2021;4(1).
Raz L, Knoefel J, Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab. 2016;36(1):172–86.
Article PubMed PubMed Central Google Scholar
Racette BA, Willis AW. Time to change the blind men and the elephant approach to Parkinson disease? Neurology. 2015;85(2):190–6.
Frahm-Falkenberg S, Ibsen R, Kjellberg J, Jennum P. Health, social and economic consequences of dementias: a comparative national cohort study. Eur J Neurol. 2016;23(9):1400–7.
Article CAS PubMed Google Scholar
Dong X. Current strategies for Brain Drug Delivery. Theranostics. 2018;8(6):1481–93.
Article CAS PubMed PubMed Central Google Scholar
Mendonca LS, Onofre I, Miranda CO, Perfeito R, Nobrega C, de Almeida LP. Stem cell-based therapies for Polyglutamine diseases. Adv Exp Med Biol. 2018;1049:439–66.
Alam MI, Beg S, Samad A, Baboota S, Kohli K, Ali J, et al. Strategy for effective brain drug delivery. Eur J Pharm Sci. 2010;40(5):385–403.
Hoshyar N, Gray S, Han H, Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomed (Lond). 2016;11(6):673–92.
Article CAS Google Scholar
Murthy SK. Nanoparticles in modern medicine: state of the art and future challenges. Int J Nanomed. 2007;2(2):129–41.
CAS Google Scholar
Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20.
Soares S, Sousa J, Pais A, Vitorino C. Nanomedicine: principles, properties, and Regulatory issues. Front Chem. 2018;6:360.
Boado RJ, Pardridge WM. The trojan horse Liposome Technology for Nonviral Gene transfer across the blood-brain barrier. J Drug Deliv. 2011;2011:296151.
Mendonça LS, De Pedroso MC, Simöes S. Targeted lipid-based systems for siRNA delivery. J Drug Deliv Sci Technol. 2012;22(1):65–73.
Article Google Scholar
Su S, Kang PM. Systemic review of biodegradable nanomaterials in Nanomedicine. Nanomaterials (Basel). 2020;10(4).
Jo DH, Kim JH, Lee TG, Kim JH. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomedicine. 2015;11(7):1603–11.
Kang JH, Jang WY, Ko YT. The Effect of Surface charges on the Cellular Uptake of liposomes investigated by live cell imaging. Pharm Res. 2017;34(4):704–17.
Xu W, Ling P, Zhang T. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J Drug Deliv. 2013;2013:340315.
Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99(Pt A):28–51.
Khan AR, Yang X, Fu M, Zhai G. Recent progress of drug nanoformulations targeting to brain. J Control Release. 2018;291:37–64.
Zhang F, Lin YA, Kannan S, Kannan RM. Targeting specific cells in the brain with nanomedicines for CNS therapies. J Control Release. 2016;240:212–26.
Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol. 2019;71(8):1185–98.
Yoo J, Park C, Yi G, Lee D, Koo H. Active targeting strategies using Biological ligands for Nanoparticle Drug Delivery systems. Cancers (Basel). 2019;11(5).
Zhao Z, Ukidve A, Kim J, Mitragotri S. Targeting strategies for tissue-specific drug delivery. Cell. 2020;181(1):151–67.
Juan A, Cimas FJ, Bravo I, Pandiella A, Ocana A, Alonso-Moreno C. Antibody conjugation of nanoparticles as therapeutics for breast Cancer Treatment. Int J Mol Sci. 2020;21(17).
Pietersz GA, Wang X, Yap ML, Lim B, Peter K. Therapeutic targeting in nanomedicine: the future lies in recombinant antibodies. Nanomed (Lond). 2017;12(15):1873–89.
Juan A, Cimas FJ, Bravo I, Pandiella A, Ocana A, Alonso-Moreno C. An overview of antibody conjugated polymeric nanoparticles for breast Cancer therapy. Pharmaceutics. 2020;12(9).
Alibakhshi A, Abarghooi Kahaki F, Ahangarzadeh S, Yaghoobi H, Yarian F, Arezumand R, et al. Targeted cancer therapy through antibody fragments-decorated nanomedicines. J Control Release. 2017;268:323–34.
Kholodenko RV, Kalinovsky DV, Doronin II, Ponomarev ED, Kholodenko IV. Antibody fragments as potential biopharmaceuticals for Cancer Therapy: Success and limitations. Curr Med Chem. 2019;26(3):396–426.
Eloy JO, Petrilli R, Trevizan LNF, Chorilli M, Immunoliposomes. A review on functionalization strategies and targets for drug delivery. Colloids Surf B Biointerfaces. 2017;159:454–67.
Clark AJ, Davis ME. Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core. Proc Natl Acad Sci U S A. 2015;112(40):12486–91.
Choi CH, Alabi CA, Webster P, Davis ME. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc Natl Acad Sci U S A. 2010;107(3):1235–40.
Daniels TR, Bernabeu E, Rodriguez JA, Patel S, Kozman M, Chiappetta DA, et al. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim Biophys Acta. 2012;1820(3):291–317.
Zhou J, Li M, Lim WQ, Luo Z, Phua SZF, Huo R, et al. A transferrin-conjugated Hollow Nanoplatform for Redox-controlled and targeted chemotherapy of Tumor with reduced inflammatory reactions. Theranostics. 2018;8(2):518–32.
Deshpande P, Jhaveri A, Pattni B, Biswas S, Torchilin V. Transferrin and octaarginine modified dual-functional liposomes with improved cancer cell targeting and enhanced intracellular delivery for the treatment of ovarian cancer. Drug Deliv. 2018;25(1):517–32.
Chi L, Na MH, Jung HK, Vadevoo SM, Kim CW, Padmanaban G, et al. Enhanced delivery of liposomes to lung tumor through targeting interleukin-4 receptor on both tumor cells and tumor endothelial cells. J Control Release. 2015;209:327–36.
Lu Z, Long Y, Cun X, Wang X, Li J, Mei L, et al. A size-shrinkable nanoparticle-based combined anti-tumor and anti-inflammatory strategy for enhanced cancer therapy. Nanoscale. 2018;10(21):9957–70.
Liu M, Fang X, Yang Y, Wang C. Peptide-enabled targeted Delivery systems for therapeutic applications. Front Bioeng Biotechnol. 2021;9:701504.
Accardo A, Aloj L, Aurilio M, Morelli G, Tesauro D. Receptor binding peptides for target-selective delivery of nanoparticles encapsulated drugs. Int J Nanomed. 2014;9:1537–57.
Google Scholar
Guan B, Zhang X. Aptamers as versatile ligands for Biomedical and Pharmaceutical Applications. Int J Nanomed. 2020;15:1059–71.
Stein CA, Castanotto D. FDA-Approved Oligonucleotide therapies in 2017. Mol Ther. 2017;25(5):1069–75.
Parashar A. Aptamers in therapeutics. J Clin Diagn Res. 2016;10(6):BE01–6.
CAS PubMed PubMed Central Google Scholar
Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov. 2010;9(7):537–50.
Eilers A, Witt S, Walter J. Aptamer-modified nanoparticles in Medical Applications. Adv Biochem Eng Biotechnol. 2020;174:161–93.
CAS PubMed Google Scholar
Catuogno S, Esposito CL, de Franciscis V. Aptamer-mediated targeted delivery of therapeutics: an update. Pharmaceuticals (Basel). 2016;9(4).
Ledermann JA, Canevari S, Thigpen T. Targeting the folate receptor: diagnostic and therapeutic approaches to personalize cancer treatments. Ann Oncol. 2015;26(10):2034–43.
Lv Y, Cao Y, Li P, Liu J, Chen H, Hu W et al. Ultrasound-Triggered Destruction of Folate-Functionalized Mesoporous silica nanoparticle-loaded Microbubble for targeted Tumor Therapy. Adv Healthc Mater. 2017;6(18).
Srinivasarao M, Low PS. Ligand-targeted drug delivery. Chem Rev. 2017;117(19):12133–64.
Li H, Li Y, Ao H, Bi D, Han M, Guo Y, et al. Folate-targeting annonaceous acetogenins nanosuspensions: significantly enhanced antitumor efficacy in HeLa tumor-bearing mice. Drug Deliv. 2018;25(1):880–7.
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25.
Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol. 1990;429:47–62.
Butt AM, Jones HC. Effect of histamine and antagonists on electrical resistance across the blood-brain barrier in rat brain-surface microvessels. Brain Res. 1992;569(1):100–5.
Urayama A. [The blood-brain barrier and neurodegenerative lysosomal storage diseases]. Brain Nerve. 2013;65(2):153–63.
Warren KE. Beyond the blood:Brain Barrier: the importance of Central Nervous System (CNS) pharmacokinetics for the Treatment of CNS Tumors, including diffuse intrinsic pontine glioma. Front Oncol. 2018;8:239.
van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat. 2015;19:1–12.
Article PubMed Google Scholar
Perez-Martinez FC, Guerra J, Posadas I, Cena V. Barriers to non-viral vector-mediated gene delivery in the nervous system. Pharm Res. 2011;28(8):1843–58.
Swanson JA, Baer SC. Phagocytosis by zippers and triggers. Trends Cell Biol. 1995;5(3):89–93.
Suk JS, Suh J, Choy K, Lai SK, Fu J, Hanes J. Gene delivery to differentiated neurotypic cells with RGD and HIV Tat peptide functionalized polymeric nanoparticles. Biomaterials. 2006;27(29):5143–50.
Misra A, Ganesh S, Shahiwala A, Shah SP. Drug delivery to the central nervous system: a review. J Pharm Pharm Sci. 2003;6(2):252–73.
Shalgunov V, Xiong M, L’Estrade ET, Raval NR, Andersen IV, Edgar FG, et al. Blocking of efflux transporters in rats improves translational validation of brain radioligands. EJNMMI Res. 2020;10(1):124.
Batrakova EV, Li S, Vinogradov SV, Alakhov VY, Miller DW, Kabanov AV. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: contributions of energy depletion and membrane fluidization. J Pharmacol Exp Ther. 2001;299(2):483–93.
Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC. The blood-brain barrier: an engineering perspective. Front Neuroeng. 2013;6:7.
Strazielle N, Ghersi-Egea JF. Physiology of blood-brain interfaces in relation to brain disposition of small compounds and macromolecules. Mol Pharm. 2013;10(5):1473–91.
Yue W, Shen J. Local delivery strategies for peptides and proteins into the CNS: Status Quo, challenges, and future perspectives. Pharmaceuticals (Basel). 2023;16(6).
Yi X, Manickam DS, Brynskikh A, Kabanov AV. Agile delivery of protein therapeutics to CNS. J Control Release. 2014;190:637–63.
Kwong YL, Yeung DY, Chan JC. Intrathecal chemotherapy for hematologic malignancies: drugs and toxicities. Ann Hematol. 2009;88(3):193–201.
Glascock JJ, Osman EY, Coady TH, Rose FF, Shababi M, Lorson CL. Delivery of therapeutic agents through intracerebroventricular (ICV) and intravenous (IV) injection in mice. J Vis Exp. 2011(56).
Shofty B, Neuberger A, Naffaa ME, Binawi T, Babitch T, Rappaport ZH, et al. Intrathecal or intraventricular therapy for post-neurosurgical gram-negative meningitis: matched cohort study. Clin Microbiol Infect. 2016;22(1):66–70.
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra11.
Noguchi Y, Kato M, Ozeki K, Ishigai M. Pharmacokinetics of an intracerebroventricularly administered antibody in rats. MAbs. 2017;9(7):1210–5.
Schulz A, Ajayi T, Specchio N, de Los Reyes E, Gissen P, Ballon D, et al. Study of Intraventricular Cerliponase Alfa for CLN2 disease. N Engl J Med. 2018;378(20):1898–907.
Sampson JH, Akabani G, Archer GE, Berger MS, Coleman RE, Friedman AH, et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol. 2008;10(3):320–9.
Pizzo ME, Wolak DJ, Kumar NN, Brunette E, Brunnquell CL, Hannocks MJ, et al. Intrathecal antibody distribution in the rat brain: surface diffusion, perivascular transport and osmotic enhancement of delivery. J Physiol. 2018;596(3):445–75.
C ID, Sevin C, Krageloh-Mann I, Giugliani R, Sakai N, Wu J, et al. Safety of intrathecal delivery of recombinant human arylsulfatase A in children with metachromatic leukodystrophy: results from a phase 1/2 clinical trial. Mol Genet Metab. 2020;131(1–2):235–44.
Dorovini-Zis K, Bowman PD, Betz AL, Goldstein GW. Hyperosmotic arabinose solutions open the tight junctions between brain capillary endothelial cells in tissue culture. Brain Res. 1984;302(2):383–6.
Ito M, Bolati K, Kinjo T, Ichimura K, Furuta A, McLoughlin DM, et al. Electroconvulsive stimulation transiently enhances the permeability of the rat blood-brain barrier and induces astrocytic changes. Brain Res Bull. 2017;128:92–7.
Zhang S, Gong P, Zhang J, Mao X, Zhao Y, Wang H, et al. Specific frequency electroacupuncture stimulation transiently enhances the permeability of the blood-brain barrier and induces tight Junction Changes. Front Neurosci. 2020;14:582324.
Luo H, Shusta EV. Blood-brain barrier modulation to improve Glioma Drug Delivery. Pharmaceutics. 2020;12(11).
Jahnke K, Kraemer DF, Knight KR, Fortin D, Bell S, Doolittle ND, et al. Intraarterial chemotherapy and osmotic blood-brain barrier disruption for patients with embryonal and germ cell tumors of the central nervous system. Cancer. 2008;112(3):581–8.
Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13.
Lalani J, Raichandani Y, Mathur R, Lalan M, Chutani K, Mishra AK, et al. Comparative receptor based brain delivery of tramadol-loaded poly(lactic-co-glycolic acid) nanoparticles. J Biomed Nanotechnol. 2012;8(6):918–27.
Mendonca LS, Firmino F, Moreira JN, Pedroso de Lima MC, Simoes S. Transferrin receptor-targeted liposomes encapsulating anti-BCR-ABL siRNA or asODN for chronic myeloid leukemia treatment. Bioconjug Chem. 2010;21(1):157–68.
Fornaguera C, Dols-Perez A, Caldero G, Garcia-Celma MJ, Camarasa J, Solans C. PLGA nanoparticles prepared by nano-emulsion templating using low-energy methods as efficient nanocarriers for drug delivery across the blood-brain barrier. J Control Release. 2015;211:134–43.
Ulbrich K, Hekmatara T, Herbert E, Kreuter J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB). Eur J Pharm Biopharm. 2009;71(2):251–6.
Abo-Krysha N, Rashed L. The role of iron dysregulation in the pathogenesis of multiple sclerosis: an Egyptian study. Mult Scler. 2008;14(5):602–8.
Kreuter J, Shamenkov D, Petrov V, Ramge P, Cychutek K, Koch-Brandt C, et al. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J Drug Target. 2002;10(4):317–25.
Zensi A, Begley D, Pontikis C, Legros C, Mihoreanu L, Wagner S, et al. Albumin nanoparticles targeted with apo E enter the CNS by transcytosis and are delivered to neurones. J Control Release. 2009;137(1):78–86.
Demeule M, Currie JC, Bertrand Y, Che C, Nguyen T, Regina A, et al. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J Neurochem. 2008;106(4):1534–44.
Demeule M, Regina A, Che C, Poirier J, Nguyen T, Gabathuler R, et al. Identification and design of peptides as a new drug delivery system for the brain. J Pharmacol Exp Ther. 2008;324(3):1064–72.
Thomas FC, Taskar K, Rudraraju V, Goda S, Thorsheim HR, Gaasch JA, et al. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm Res. 2009;26(11):2486–94.
Ulbrich K, Knobloch T, Kreuter J. Targeting the insulin receptor: nanoparticles for drug delivery across the blood-brain barrier (BBB). J Drug Target. 2011;19(2):125–32.
Li J, Guo Y, Kuang Y, An S, Ma H, Jiang C. Choline transporter-targeting and co-delivery system for glioma therapy. Biomaterials. 2013;34(36):9142–8.
Li J, Zhou L, Ye D, Huang S, Shao K, Huang R, et al. Choline-derivate-modified nanoparticles for brain-targeting gene delivery. Adv Mater. 2011;23(39):4516–20.
Kuo YC, Chung CY. Transcytosis of CRM197-grafted polybutylcyanoacrylate nanoparticles for delivering zidovudine across human brain-microvascular endothelial cells. Colloids Surf B Biointerfaces. 2012;91:242–9.
Zhang C, Zheng X, Wan X, Shao X, Liu Q, Zhang Z, et al. The potential use of H102 peptide-loaded dual-functional nanoparticles in the treatment of Alzheimer’s disease. J Control Release. 2014;192:317–24.
Marcos-Contreras OA, Greineder CF, Kiseleva RY, Parhiz H, Walsh LR, Zuluaga-Ramirez V, et al. Selective targeting of nanomedicine to inflamed cerebral vasculature to enhance the blood-brain barrier. Proc Natl Acad Sci U S A. 2020;117(7):3405–14.
Faure MP, Alonso A, Nouel D, Gaudriault G, Dennis M, Vincent JP, et al. Somatodendritic internalization and perinuclear targeting of neurotensin in the mammalian brain. J Neurosci. 1995;15(6):4140–7.
Hsieh TY, Huang WC, Kang YD, Chu CY, Liao WL, Chen YY, et al. Neurotensin-conjugated reduced Graphene Oxide with Multi-stage Near-Infrared-triggered synergic targeted neuron gene transfection in Vitro and in vivo for neurodegenerative Disease Therapy. Adv Healthc Mater. 2016;5(23):3016–26.
Park IK, Lasiene J, Chou SH, Horner PJ, Pun SH. Neuron-specific delivery of nucleic acids mediated by Tet1-modified poly(ethylenimine). J Gene Med. 2007;9(8):691–702.
Huang YZ, Hernandez FJ, Gu B, Stockdale KR, Nanapaneni K, Scheetz TE, et al. RNA aptamer-based functional ligands of the neurotrophin receptor, TrkB. Mol Pharmacol. 2012;82(4):623–35.
Xu J, Chau Y. Polymeric nanoparticles decorated with BDNF-derived peptide for neuron-targeted delivery of PTEN inhibitor. Eur J Pharm Sci. 2018;124:37–45.
Lopes CD, Oliveira H, Estevao I, Pires LR, Pego AP. In vivo targeted gene delivery to peripheral neurons mediated by neurotropic poly(ethylene imine)-based nanoparticles. Int J Nanomed. 2016;11:2675–83.
Lopes CD, Gomes CP, Neto E, Sampaio P, Aguiar P, Pego AP. Microfluidic-based platform to mimic the in vivo peripheral administration of neurotropic nanoparticles. Nanomed (Lond). 2016;11(24):3205–21.
Lopes CDF, Goncalves NP, Gomes CP, Saraiva MJ, Pego AP. BDNF gene delivery mediated by neuron-targeted nanoparticles is neuroprotective in peripheral nerve injury. Biomaterials. 2017;121:83–96.
Dos Santos Rodrigues B, Lakkadwala S, Kanekiyo T, Singh J. Dual-modified liposome for targeted and enhanced gene delivery into mice Brain. J Pharmacol Exp Ther. 2020;374(3):354–65.
Dos Santos Rodrigues B, Lakkadwala S, Kanekiyo T, Singh J. Development and screening of brain-targeted lipid-based nanoparticles with enhanced cell penetration and gene delivery properties. Int J Nanomed. 2019;14:6497–517.
Vilella A, Tosi G, Grabrucker AM, Ruozi B, Belletti D, Vandelli MA, et al. Insight on the fate of CNS-targeted nanoparticles. Part I: Rab5-dependent cell-specific uptake and distribution. J Control Release. 2014;174:195–201.
Tosi G, Fano RA, Bondioli L, Badiali L, Benassi R, Rivasi F, et al. Investigation on mechanisms of glycopeptide nanoparticles for drug delivery across the blood-brain barrier. Nanomed (Lond). 2011;6(3):423–36.
Conceicao M, Mendonca L, Nobrega C, Gomes C, Costa P, Hirai H, et al. Intravenous administration of brain-targeted stable nucleic acid lipid particles alleviates Machado-Joseph disease neurological phenotype. Biomaterials. 2016;82:124–37.
Chen W, Zhan C, Gu B, Meng Q, Wang H, Lu W, et al. Targeted brain delivery of itraconazole via RVG29 anchored nanoparticles. J Drug Target. 2011;19(3):228–34.
Lozic I, Hartz RV, Bartlett CA, Shaw JA, Archer M, Naidu PS, et al. Enabling dual cellular destinations of polymeric nanoparticles for treatment following partial injury to the central nervous system. Biomaterials. 2016;74:200–16.
Chekhonin VP, Zhirkov YA, Gurina OI, Ryabukhin IA, Lebedev SV, Kashparov IA, et al. PEGylated immunoliposomes directed against brain astrocytes. Drug Deliv. 2005;12(1):1–6.
Gu J, Al-Bayati K, Ho EA. Development of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood-brain barrier as a strategy for inhibiting HIV replication in astrocytes. Drug Deliv Transl Res. 2017;7(4):497–506.
Choi B, Soh M, Manandhar Y, Kim D, Han SI, Baik S, et al. Highly selective microglial uptake of ceria-zirconia nanoparticles for enhanced analgesic treatment of neuropathic pain. Nanoscale. 2019;11(41):19437–47.
Rittchen S, Boyd A, Burns A, Park J, Fahmy TM, Metcalfe S, et al. Myelin repair in vivo is increased by targeting oligodendrocyte precursor cells with nanoparticles encapsulating leukaemia inhibitory factor (LIF). Biomaterials. 2015;56:78–85.
Fereidan-Esfahani M, Yue WY, Wilbanks B, Johnson AJ, Warrington AE, Howe CL et al. Remyelination-promoting DNA aptamer conjugate Myaptavin-3064 binds to adult oligodendrocytes in Vitro. Pharmaceuticals (Basel). 2020;13(11).
Hosseini Shamili F, Alibolandi M, Rafatpanah H, Abnous K, Mahmoudi M, Kalantari M, et al. Immunomodulatory properties of MSC-derived exosomes armed with high affinity aptamer toward mylein as a platform for reducing multiple sclerosis clinical score. J Control Release. 2019;299:149–64.
Schmidt A, Bockmann M, Stoll A, Racek T, Putzer BM. Analysis of adenovirus gene transfer into adult neural stem cells. Virus Res. 2005;114(1–2):45–53.
Schmidt A, Haas SJ, Hildebrandt S, Scheibe J, Eckhoff B, Racek T, et al. Selective targeting of adenoviral vectors to neural precursor cells in the hippocampus of adult mice: new prospects for in situ gene therapy. Stem Cells. 2007;25(11):2910–8.
Carradori D, Saulnier P, Preat V, des Rieux A, Eyer J. NFL-lipid nanocapsules for brain neural stem cell targeting in vitro and in vivo. J Control Release. 2016;238:253–62.
Carradori D, Dos Santos AG, Masquelier J, Paquot A, Saulnier P, Eyer J, et al. The origin of neural stem cells impacts their interactions with targeted-lipid nanocapsules: potential role of plasma membrane lipid composition and fluidity. J Control Release. 2018;292:248–55.
Praca C, Rai A, Santos T, Cristovao AC, Pinho SL, Cecchelli R, et al. A nanoformulation for the preferential accumulation in adult neurogenic niches. J Control Release. 2018;284:57–72.
Mendonca LS, Moreira JN, de Lima MC, Simoes S. Co-encapsulation of anti-BCR-ABL siRNA and imatinib mesylate in transferrin receptor-targeted sterically stabilized liposomes for chronic myeloid leukemia treatment. Biotechnol Bioeng. 2010;107(5):884–93.
Inazu M. Functional expression of Choline transporters in the blood-brain barrier. Nutrients. 2019;11(10).
Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim Biophys Acta. 1997;1333(3):F179–99.
Gaillard PJ, de Boer AG. A novel opportunity for targeted drug delivery to the brain. J Control Release. 2006;116(2):e60–2.
O’Brien P, Wong RW. Optic neuritis following diphtheria, tetanus, pertussis, and inactivated poliovirus combined vaccination: a case report. J Med Case Rep. 2018;12(1):356.
Tosi G, Vilella A, Veratti P, Belletti D, Pederzoli F, Ruozi B, et al. Exploiting bacterial pathways for BBB crossing with PLGA nanoparticles modified with a mutated form of Diphtheria Toxin (CRM197): in vivo experiments. Mol Pharm. 2015;12(10):3672–84.
Singh J, Habean ML, Panicker N. Inflammasome assembly in neurodegenerative diseases. Trends Neurosci. 2023;46(10):814–31.
Calsolaro V, Edison P. Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement. 2016;12(6):719–32.
Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences. Neurosci Res. 2014;79:1–12.
Candelario-Jalil E, Dijkhuizen RM, Magnus T, Neuroinflammation. Stroke, blood-brain barrier dysfunction, and Imaging modalities. Stroke. 2022;53(5):1473–86.
Yang C, Hawkins KE, Dore S, Candelario-Jalil E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol. 2019;316(2):C135–53.
Hsu J, Rappaport J, Muro S. Specific binding, uptake, and transport of ICAM-1-targeted nanocarriers across endothelial and subendothelial cell components of the blood-brain barrier. Pharm Res. 2014;31(7):1855–66.
Ailuno G, Zuccari G, Baldassari S, Lai F, Caviglioli G. Anti-vascular cell adhesion Molecule-1 nanosystems: a Promising Strategy against Inflammatory Based diseases. J Nanosci Nanotechnol. 2021;21(5):2793–807.
Cuddapah VA, Robel S, Watkins S, Sontheimer H. A neurocentric perspective on glioma invasion. Nat Rev Neurosci. 2014;15(7):455–65.
Huse JT, Holland EC. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer. 2010;10(5):319–31.
Munir MU. Nanomedicine Penetration to Tumor: challenges, and Advanced strategies to Tackle this issue. Cancers (Basel). 2022;14(12).
Zhao M, van Straten D, Broekman MLD, Preat V, Schiffelers RM. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics. 2020;10(3):1355–72.
Tang W, Fan W, Lau J, Deng L, Shen Z, Chen X. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem Soc Rev. 2019;48(11):2967–3014.
Lee C, Hwang HS, Lee S, Kim B, Kim JO, Oh KT et al. Rabies virus-inspired silica-coated gold nanorods as a Photothermal Therapeutic platform for treating brain tumors. Adv Mater. 2017;29(13).
Mojarad-Jabali S, Farshbaf M, Hemmati S, Sarfraz M, Motasadizadeh H, Shahbazi Mojarrad J, et al. Comparison of three synthetic transferrin mimetic small peptides to promote the blood-brain barrier penetration of vincristine liposomes for improved glioma targeted therapy. Int J Pharm. 2022;613:121395.
Liu C, Zhao Z, Gao H, Rostami I, You Q, Jia X, et al. Enhanced blood-brain-barrier penetrability and tumor-targeting efficiency by peptide-functionalized poly(amidoamine) dendrimer for the therapy of gliomas. Nanotheranostics. 2019;3(4):311–30.
Choudhury H, Pandey M, Chin PX, Phang YL, Cheah JY, Ooi SC, et al. Transferrin receptors-targeting nanocarriers for efficient targeted delivery and transcytosis of drugs into the brain tumors: a review of recent advancements and emerging trends. Drug Deliv Transl Res. 2018;8(5):1545–63.
Cui Y, Xu Q, Chow PK, Wang D, Wang CH. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials. 2013;34(33):8511–20.
Xin H, Sha X, Jiang X, Zhang W, Chen L, Fang X. Anti-glioblastoma efficacy and safety of paclitaxel-loading angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials. 2012;33(32):8167–76.
Zhu Y, Jiang Y, Meng F, Deng C, Cheng R, Zhang J, et al. Highly efficacious and specific anti-glioma chemotherapy by tandem nanomicelles co-functionalized with brain tumor-targeting and cell-penetrating peptides. J Control Release. 2018;278:1–8.
Zhu Y, Zhang J, Meng F, Deng C, Cheng R, Feijen J, et al. cRGD-functionalized reduction-sensitive shell-sheddable biodegradable micelles mediate enhanced doxorubicin delivery to human glioma xenografts in vivo. J Control Release. 2016;233:29–38.
Zhong Y, Wang C, Cheng R, Cheng L, Meng F, Liu Z, et al. cRGD-directed, NIR-responsive and robust AuNR/PEG-PCL hybrid nanoparticles for targeted chemotherapy of glioblastoma in vivo. J Control Release. 2014;195:63–71.
Miura Y, Takenaka T, Toh K, Wu S, Nishihara H, Kano MR, et al. Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood-brain tumor barrier. ACS Nano. 2013;7(10):8583–92.
Talelli M, Barz M, Rijcken CJ, Kiessling F, Hennink WE, Lammers T. Core-Crosslinked Polymeric micelles: principles, Preparation, Biomedical Applications and clinical translation. Nano Today. 2015;10(1):93–117.
Venditto VJ, Szoka FC Jr. Cancer nanomedicines: so many papers and so few drugs! Adv Drug Deliv Rev. 2013;65(1):80–8.
Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12(11):991–1003.
Sun H, Meng F, Cheng R, Deng C, Zhong Z. Reduction-sensitive degradable micellar nanoparticles as smart and intuitive delivery systems for cancer chemotherapy. Expert Opin Drug Deliv. 2013;10(8):1109–22.
Meng F, Hennink WE, Zhong Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials. 2009;30(12):2180–98.
Novakova J, Slaby O, Vyzula R, Michalek J. MicroRNA involvement in glioblastoma pathogenesis. Biochem Biophys Res Commun. 2009;386(1):1–5.
Costa PM, Cardoso AL, Nobrega C, Pereira de Almeida LF, Bruce JN, Canoll P, et al. MicroRNA-21 silencing enhances the cytotoxic effect of the antiangiogenic drug sunitinib in glioblastoma. Hum Mol Genet. 2013;22(5):904–18.
Dong CG, Wu WK, Feng SY, Wang XJ, Shao JF, Qiao J. Co-inhibition of microRNA-10b and microRNA-21 exerts synergistic inhibition on the proliferation and invasion of human glioma cells. Int J Oncol. 2012;41(3):1005–12.
Costa PM, Cardoso AL, Mendonca LS, Serani A, Custodia C, Conceicao M, et al. Tumor-targeted chlorotoxin-coupled nanoparticles for nucleic acid delivery to Glioblastoma cells: a Promising System for Glioblastoma Treatment. Mol Ther Nucleic Acids. 2013;2(6):e100.
Deshane J, Garner CC, Sontheimer H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem. 2003;278(6):4135–44.
Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133(2):647–58.
Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, et al. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27(31):4373–9.
Ni J, Miao T, Su M, Khan NU, Ju X, Chen H, et al. PSMA-targeted nanoparticles for specific penetration of blood-brain tumor barrier and combined therapy of brain metastases. J Control Release. 2021;329:934–47.
Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, et al. Brain metastases. Nat Rev Dis Primers. 2019;5(1):5.
Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–7.
Taskar KS, Rudraraju V, Mittapalli RK, Samala R, Thorsheim HR, Lockman J, et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm Res. 2012;29(3):770–81.
Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664–78.
Kasoha M, Unger C, Solomayer EF, Bohle RM, Zaharia C, Khreich F, et al. Prostate-specific membrane antigen (PSMA) expression in breast cancer and its metastases. Clin Exp Metastasis. 2017;34(8):479–90.
Nomura N, Pastorino S, Jiang P, Lambert G, Crawford JR, Gymnopoulos M, et al. Prostate specific membrane antigen (PSMA) expression in primary gliomas and breast cancer brain metastases. Cancer Cell Int. 2014;14(1):26.
Meher N, VanBrocklin HF, Wilson DM, Flavell RR. PSMA-Targeted nanotheranostics for imaging and radiotherapy of prostate Cancer. Pharmaceuticals (Basel). 2023;16(2).
Xu X, Wu J, Liu Y, Saw PE, Tao W, Yu M, et al. Multifunctional envelope-type siRNA delivery nanoparticle platform for prostate Cancer therapy. ACS Nano. 2017;11(3):2618–27.
Paasonen L, Sharma S, Braun GB, Kotamraju VR, Chung TD, She ZG, et al. New p32/gC1qR ligands for targeted Tumor Drug Delivery. ChemBioChem. 2016;17(7):570–5.
Lovinger DM. Communication networks in the brain: neurons, receptors, neurotransmitters, and alcohol. Alcohol Res Health. 2008;31(3):196–214.
PubMed PubMed Central Google Scholar
Gorman AM. Neuronal cell death in neurodegenerative diseases: recurring themes around protein handling. J Cell Mol Med. 2008;12(6A):2263–80.
Saxena S, Caroni P. Selective neuronal vulnerability in neurodegenerative diseases: from Stressor thresholds to degeneration. Neuron. 2011;71(1):35–48.
Fisher A. Cholinergic modulation of amyloid precursor protein processing with emphasis on M1 muscarinic receptor: perspectives and challenges in treatment of Alzheimer’s disease. J Neurochem. 2012;120(Suppl 1):22–33.
Ztaou S, Maurice N, Camon J, Guiraudie-Capraz G, Kerkerian-Le Goff L, Beurrier C, et al. Involvement of Striatal Cholinergic Interneurons and M1 and M4 muscarinic receptors in motor symptoms of Parkinson’s Disease. J Neurosci. 2016;36(35):9161–72.
Garcia-Chica J, WK DP, Tanabe S, Serra D, Herrero L, Casals N, et al. An overview of nanomedicines for neuron targeting. Nanomed (Lond). 2020;15(16):1617–36.
Babazadeh A, Mohammadi Vahed F, Jafari SM. Nanocarrier-mediated brain delivery of bioactives for treatment/prevention of neurodegenerative diseases. J Control Release. 2020;321:211–21.
Hernando S, Gartziandia O, Herran E, Pedraz JL, Igartua M, Hernandez RM. Advances in nanomedicine for the treatment of Alzheimer’s and Parkinson’s diseases. Nanomed (Lond). 2016;11(10):1267–85.
Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. BDNF function and intracellular signaling in neurons. Histol Histopathol. 2010;25(2):237–58.
Ramsey JD, Flynn NH. Cell-penetrating peptides transport therapeutics into cells. Pharmacol Ther. 2015;154:78–86.
Joliot A, Pernelle C, Deagostini-Bazin H, Prochiantz A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc Natl Acad Sci U S A. 1991;88(5):1864–8.
Pino-Angeles A, Lazaridis T. Effects of peptide charge, orientation, and concentration on Melittin Transmembrane pores. Biophys J. 2018;114(12):2865–74.
Qian S, Heller WT. Melittin-induced cholesterol reorganization in lipid bilayer membranes. Biochim Biophys Acta. 2015;1848(10 Pt A):2253–60.
Upadhya A, Sangave PC. Hydrophobic and electrostatic interactions between cell penetrating peptides and plasmid DNA are important for stable non-covalent complexation and intracellular delivery. J Pept Sci. 2016;22(10):647–59.
Bolhassani A, Jafarzade BS, Mardani G. In vitro and in vivo delivery of therapeutic proteins using cell penetrating peptides. Peptides. 2017;87:50–63.
Takayama K, Nakase I, Michiue H, Takeuchi T, Tomizawa K, Matsui H, et al. Enhanced intracellular delivery using arginine-rich peptides by the addition of penetration accelerating sequences (pas). J Control Release. 2009;138(2):128–33.
Takayama K, Hirose H, Tanaka G, Pujals S, Katayama S, Nakase I, et al. Effect of the attachment of a penetration accelerating sequence and the influence of hydrophobicity on octaarginine-mediated intracellular delivery. Mol Pharm. 2012;9(5):1222–30.
Guidotti G, Brambilla L, Rossi D. Cell-penetrating peptides: from Basic Research to clinics. Trends Pharmacol Sci. 2017;38(4):406–24.
Milletti F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov Today. 2012;17(15–16):850–60.
Yoshida T, Tomioka I, Nagahara T, Holyst T, Sawada M, Hayes P, et al. Bax-inhibiting peptide derived from mouse and rat Ku70. Biochem Biophys Res Commun. 2004;321(4):961–6.
Gomez JA, Gama V, Yoshida T, Sun W, Hayes P, Leskov K, et al. Bax-inhibiting peptides derived from Ku70 and cell-penetrating pentapeptides. Biochem Soc Trans. 2007;35(Pt 4):797–801.
Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55(6):1189–93.
Simon MJ, Gao S, Kang WH, Banta S, Morrison B. 3rd. TAT-mediated intracellular protein delivery to primary brain cells is dependent on glycosaminoglycan expression. Biotechnol Bioeng. 2009;104(1):10–9.
Kristensen M, Birch D, Morck Nielsen H. Applications and challenges for Use of cell-penetrating peptides as delivery vectors for peptide and protein cargos. Int J Mol Sci 2016;17(2).
Tosi G, Ruozi B, Belletti D, Vilella A, Zoli M, Vandelli MA, et al. Brain-targeted polymeric nanoparticles: in vivo evidence of different routes of administration in rodents. Nanomed (Lond). 2013;8(9):1373–83.
Yan X, Mohankumar PS, Dietzschold B, Schnell MJ, Fu ZF. The Rabies virus glycoprotein determines the distribution of different rabies virus strains in the brain. J Neurovirol. 2002;8(4):345–52.
Lafon M. Rabies virus receptors. J Neurovirol. 2005;11(1):82–7.
Torashima T, Koyama C, Iizuka A, Mitsumura K, Takayama K, Yanagi S, et al. Lentivector-mediated rescue from cerebellar ataxia in a mouse model of spinocerebellar ataxia. EMBO Rep. 2008;9(4):393–9.
Verkhratsky A, Nedergaard M. Physiology of Astroglia. Physiol Rev. 2018;98(1):239–389.
Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci. 2010;11(4):227–38.
Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22(5):208–15.
McCall MA, Gregg RG, Behringer RR, Brenner M, Delaney CL, Galbreath EJ, et al. Targeted deletion in astrocyte intermediate filament (gfap) alters neuronal physiology. Proc Natl Acad Sci U S A. 1996;93(13):6361–6.
Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001;63:795–813.
Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50(4):427–34.
Pekny M, Wilhelmsson U, Pekna M. The dual role of astrocyte activation and reactive gliosis. Neurosci Lett. 2014;565:30–8.
Phatnani H, Maniatis T. Astrocytes in neurodegenerative disease. Cold Spring Harb Perspect Biol. 2015;7(6).
Colin A, Faideau M, Dufour N, Auregan G, Hassig R, Andrieu T, et al. Engineered lentiviral vector targeting astrocytes in vivo. Glia. 2009;57(6):667–79.
Delzor A, Escartin C, Deglon N. Lentiviral vectors: a powerful tool to target astrocytes in vivo. Curr Drug Targets. 2013;14(11):1336–46.
Nagelhus EA, Ottersen OP. Physiological roles of aquaporin-4 in brain. Physiol Rev. 2013;93(4):1543–62.
Hubbard JA, Szu JI, Binder DK. The role of aquaporin-4 in synaptic plasticity, memory and disease. Brain Res Bull. 2018;136:118–29.
Wells J, Kilburn MR, Shaw JA, Bartlett CA, Harvey AR, Dunlop SA, et al. Early in vivo changes in calcium ions, oxidative stress markers, and ion channel immunoreactivity following partial injury to the optic nerve. J Neurosci Res. 2012;90(3):606–18.
Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem. 2003;36(1):79–87.
Walle T, Hsieh F, DeLegge MH, Oatis JE Jr., Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32(12):1377–82.
Zhang Z, Ma Z, Zou W, Guo H, Liu M, Ma Y, et al. The appropriate marker for astrocytes: comparing the distribution and expression of three astrocytic markers in different mouse cerebral regions. Biomed Res Int. 2019;2019:9605265.
Li D, Liu X, Liu T, Liu H, Tong L, Jia S, et al. Neurochemical regulation of the expression and function of glial fibrillary acidic protein in astrocytes. Glia. 2020;68(5):878–97.
Jean M, Gera L, Charest-Morin X, Marceau F, Bachelard H. In vivo effects of Bradykinin B2 receptor agonists with varying susceptibility to Peptidases. Front Pharmacol. 2015;6:306.
PubMed Google Scholar
Gregnani MF, Hungaro TG, Martins-Silva L, Bader M, Araujo RC. Bradykinin B2 receptor signaling increases glucose uptake and oxidation: evidence and open questions. Front Pharmacol. 2020;11:1162.
Cholewinski AJ, Stevens G, McDermott AM, Wilkin GP. Identification of B2 bradykinin binding sites on cultured cortical astrocytes. J Neurochem. 1991;57(4):1456–8.
Stephens GJ, Cholewinski AJ, Wilkin GP, Djamgoz MB. Calcium-mobilizing and electrophysiological effects of bradykinin on cortical astrocyte subtypes in culture. Glia. 1993;9(4):269–79.
Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, et al. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science. 2013;339(6116):197–200.
Baslow MH. The astrocyte surface NAAG receptor and NAAG peptidase signaling complex as a therapeutic target. Drug News Perspect. 2008;21(5):251–7.
Zhang X, Lao K, Qiu Z, Rahman MS, Zhang Y, Gou X. Potential astrocytic receptors and transporters in the pathogenesis of Alzheimer’s Disease. J Alzheimers Dis. 2019;67(4):1109–22.
Liang J, Takeuchi H, Doi Y, Kawanokuchi J, Sonobe Y, Jin S, et al. Excitatory amino acid transporter expression by astrocytes is neuroprotective against microglial excitotoxicity. Brain Res. 2008;1210:11–9.
Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105.
Rudy CC, Hunsberger HC, Weitzner DS, Reed MN. The role of the tripartite glutamatergic synapse in the pathophysiology of Alzheimer’s disease. Aging Dis. 2015;6(2):131–48.
Dezsi L, Tuka B, Martos D, Vecsei L. Alzheimer’s disease, astrocytes and kynurenines. Curr Alzheimer Res. 2015;12(5):462–80.
Rickmann M, Wolff JR. S100 protein expression in subpopulations of neurons of rat brain. Neuroscience. 1995;67(4):977–91.
Zhang Y, Zhu J, Xu H, Yi Q, Yan L, Ye L, et al. Time-Dependent internalization of S100B by mesenchymal stem cells via the pathways of Clathrin- and lipid raft-mediated endocytosis. Front Cell Dev Biol. 2021;9:674995.
Walter L, Stella N. Cannabinoids and neuroinflammation. Br J Pharmacol. 2004;141(5):775–85.
Tay TL, Savage JC, Hui CW, Bisht K, Tremblay ME. Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J Physiol. 2017;595(6):1929–45.
Szepesi Z, Manouchehrian O, Bachiller S, Deierborg T. Bidirectional microglia-neuron communication in Health and Disease. Front Cell Neurosci. 2018;12:323.
Fan Y, Xie L, Chung CY. Signaling pathways Controlling Microglia Chemotaxis. Mol Cells. 2017;40(3):163–8.
Xu Y, Jin MZ, Yang ZY, Jin WL. Microglia in neurodegenerative diseases. Neural Regen Res. 2021;16(2):270–80.
Presumey J, Bialas AR, Carroll MC. Complement system in neural synapse elimination in Development and Disease. Adv Immunol. 2017;135:53–79.
Borst K, Schwabenland M, Prinz M. Microglia metabolism in health and disease. Neurochem Int. 2019;130:104331.
Hickman S, Izzy S, Sen P, Morsett L, El Khoury J. Microglia in neurodegeneration. Nat Neurosci. 2018;21(10):1359–69.
Zhang F, Mastorakos P, Mishra MK, Mangraviti A, Hwang L, Zhou J, et al. Uniform brain tumor distribution and tumor associated macrophage targeting of systemically administered dendrimers. Biomaterials. 2015;52:507–16.
Papa S, Ferrari R, De Paola M, Rossi F, Mariani A, Caron I, et al. Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury. J Control Release. 2014;174:15–26.
Nance E, Porambo M, Zhang F, Mishra MK, Buelow M, Getzenberg R, et al. Systemic dendrimer-drug treatment of ischemia-induced neonatal white matter injury. J Control Release. 2015;214:112–20.
Duffy CM, Ahmed S, Yuan C, Mavanji V, Nixon JP, Butterick T. Microglia as a surrogate Biosensor to determine nanoparticle neurotoxicity. J Vis Exp 2016(116).
Yang Z, Liu ZW, Allaker RP, Reip P, Oxford J, Ahmad Z, et al. A review of nanoparticle functionality and toxicity on the central nervous system. J R Soc Interface. 2010;7(Suppl 4):S411–22.
Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91(2):461–553.
Wilkinson K, El Khoury J. Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer’s disease. Int J Alzheimers Dis. 2012;2012:489456.
Lue LF, Walker DG, Brachova L, Beach TG, Rogers J, Schmidt AM, et al. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: identification of a cellular activation mechanism. Exp Neurol. 2001;171(1):29–45.
Teismann P, Sathe K, Bierhaus A, Leng L, Martin HL, Bucala R, et al. Receptor for advanced glycation endproducts (RAGE) deficiency protects against MPTP toxicity. Neurobiol Aging. 2012;33(10):2478–90.
Fiebich BL, Batista CRA, Saliba SW, Yousif NM, de Oliveira ACP. Role of Microglia TLRs in Neurodegeneration. Front Cell Neurosci. 2018;12:329.
Honarpisheh P, Lee J, Banerjee A, Blasco-Conesa MP, Honarpisheh P, d’Aigle J, et al. Potential caveats of putative microglia-specific markers for assessment of age-related cerebrovascular neuroinflammation. J Neuroinflammation. 2020;17(1):366.
Jurga AM, Paleczna M, Kuter KZ. Overview of General and discriminating markers of Differential Microglia phenotypes. Front Cell Neurosci. 2020;14:198.
Soh M, Kang DW, Jeong HG, Kim D, Kim DY, Yang W, et al. Ceria-Zirconia nanoparticles as an enhanced multi-antioxidant for Sepsis Treatment. Angew Chem Int Ed Engl. 2017;56(38):11399–403.
Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol. 2013;13(9):621–34.
Chung H, Brazil MI, Irizarry MC, Hyman BT, Maxfield FR. Uptake of fibrillar beta-amyloid by microglia isolated from MSR-A (type I and type II) knockout mice. NeuroReport. 2001;12(6):1151–4.
El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, et al. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197(12):1657–66.
Shannahan JH, Bai W, Brown JM. Implications of scavenger receptors in the safe development of nanotherapeutics. Receptors Clin Investig. 2015;2(3):e811.
Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2(72):re3.
Konishi H, Kobayashi M, Kunisawa T, Imai K, Sayo A, Malissen B, et al. Siglec-H is a microglia-specific marker that discriminates microglia from CNS-associated macrophages and CNS-infiltrating monocytes. Glia. 2017;65(12):1927–43.
Murai N, Mitalipova M, Jaenisch R. Functional analysis of CX3CR1 in human induced pluripotent stem (iPS) cell-derived microglia-like cells. Eur J Neurosci. 2020;52(7):3667–78.
Duveau A, Bertin E, Boue-Grabot E. Implication of Neuronal Versus Microglial P2X4 Receptors in Central Nervous System Disorders. Neurosci Bull. 2020;36(11):1327–43.
Zabala A, Vazquez-Villoldo N, Rissiek B, Gejo J, Martin A, Palomino A et al. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol Med. 2018;10(8).
Kuhn S, Gritti L, Crooks D, Dombrowski Y. Oligodendrocytes in development, myelin Generation and Beyond. Cells. 2019;8(11).
Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):37–53.
Jin GZ, Chakraborty A, Lee JH, Knowles JC, Kim HW. Targeting with nanoparticles for the therapeutic treatment of brain diseases. J Tissue Eng. 2020;11:2041731419897460.
Munzel EJ, Williams A. Promoting remyelination in multiple sclerosis-recent advances. Drugs. 2013;73(18):2017–29.
Somkuwar SS, Staples MC, Galinato MH, Fannon MJ, Mandyam CD. Role of NG2 expressing cells in addiction: a new approach for an old problem. Front Pharmacol. 2014;5:279.
Warrington AE, Asakura K, Bieber AJ, Ciric B, Van Keulen V, Kaveri SV, et al. Human monoclonal antibodies reactive to oligodendrocytes promote remyelination in a model of multiple sclerosis. Proc Natl Acad Sci U S A. 2000;97(12):6820–5.
Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249(4968):505–10.
Nastasijevic B, Wright BR, Smestad J, Warrington AE, Rodriguez M, Maher LJ. 3rd. Remyelination induced by a DNA aptamer in a mouse model of multiple sclerosis. PLoS ONE. 2012;7(6):e39595.
Sedlak SM, Schendel LC, Gaub HE, Bernardi RC. Streptavidin/biotin: tethering geometry defines unbinding mechanics. Sci Adv. 2020;6(13):eaay5999.
Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol. 2011;164(4):1079–106.
Goncalves JT, Schafer ST, Gage FH. Adult neurogenesis in the Hippocampus: from stem cells to Behavior. Cell. 2016;167(4):897–914.
Valero J, Bernardino L, Cardoso FL, Silva AP, Fontes-Ribeiro C, Ambrosio AF, et al. Impact of Neuroinflammation on hippocampal neurogenesis: relevance to aging and Alzheimer’s Disease. J Alzheimers Dis. 2017;60(s1):S161–8.
Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging Cell. 2006;5(2):139–52.
Zhu SZ, Szeto V, Bao MH, Sun HS, Feng ZP. Pharmacological approaches promoting stem cell-based therapy following ischemic stroke insults. Acta Pharmacol Sin. 2018;39(5):695–712.
Vukovic J, Blackmore DG, Jhaveri D, Bartlett PF. Activation of neural precursors in the adult neurogenic niches. Neurochem Int. 2011;59(3):341–6.
Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. NeuroReport. 2000;11(9):1991–6.
Lepinoux-Chambaud C, Barreau K, Eyer J. The neurofilament-derived peptide NFL-TBS.40–63 targets neural stem cells and affects their Properties. Stem Cells Transl Med. 2016;5(7):901–13.
Lepinoux-Chambaud C, Eyer J. The NFL-TBS.40–63 anti-glioblastoma peptide enters selectively in glioma cells by endocytosis. Int J Pharm. 2013;454(2):738–47.
Fan Y, Marioli M, Zhang K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J Pharm Biomed Anal. 2021;192:113642.
Peltonen L. Practical guidelines for the characterization and quality control of pure drug nanoparticles and nano-cocrystals in the pharmaceutical industry. Adv Drug Deliv Rev. 2018;131:101–15.
Vinod C, Jena S, Nano-Neurotheranostics. Impact of nanoparticles on neural dysfunctions and strategies to reduce toxicity for Improved Efficacy. Front Pharmacol. 2021;12:612692.
Naqvi S, Panghal A, Flora SJS. Nanotechnology: a Promising Approach for Delivery of neuroprotective drugs. Front Neurosci. 2020;14:494.
Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6(4):268–86.
Kelly IB 3rd, Fletcher RB, McBride JR, Weiss SM, Duvall CL. Tuning composition of polymer and porous Silicon Composite nanoparticles for early endosome escape of Anti-microRNA peptide nucleic acids. ACS Appl Mater Interfaces. 2020;12(35):39602–11.
Rodenak-Kladniew B, Islan GA, de Bravo MG, Duran N, Castro GR. Design, characterization and in vitro evaluation of linalool-loaded solid lipid nanoparticles as potent tool in cancer therapy. Colloids Surf B Biointerfaces. 2017;154:123–32.
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A et al. Impact of particle size and Polydispersity Index on the clinical applications of Lipidic Nanocarrier systems. Pharmaceutics. 2018;10(2).
Vega-Villa KR, Takemoto JK, Yanez JA, Remsberg CM, Forrest ML, Davies NM. Clinical toxicities of nanocarrier systems. Adv Drug Deliv Rev. 2008;60(8):929–38.
Smith MC, Crist RM, Clogston JD, McNeil SE. Zeta potential: a case study of cationic, anionic, and neutral liposomes. Anal Bioanal Chem. 2017;409(24):5779–87.
Luo Y, Yang H, Zhou YF, Hu B. Dual and multi-targeted nanoparticles for site-specific brain drug delivery. J Control Release. 2020;317:195–215.
Marques-Gallego P, de Kroon AI. Ligation strategies for targeting liposomal nanocarriers. Biomed Res Int. 2014;2014:129458.
Friedman AD, Claypool SE, Liu R. The smart targeting of nanoparticles. Curr Pharm Des. 2013;19(35):6315–29.
Choi Y, Cho BK, Seok SH, Kim C, Ryu JH, Kwon IC. Controlled spatial characteristics of ligands on nanoparticles: determinant of cellular functions. J Control Release. 2023;360:672–86.
Rana S, Yeh YC, Rotello VM. Engineering the nanoparticle-protein interface: applications and possibilities. Curr Opin Chem Biol. 2010;14(6):828–34.
Paliwal R, Babu RJ, Palakurthi S. Nanomedicine scale-up technologies: feasibilities and challenges. AAPS PharmSciTech. 2014;15(6):1527–34.
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102.
Wagner A, Vorauer-Uhl K. Liposome technology for industrial purposes. J Drug Deliv. 2011;2011:591325.
Kwon HJ, Shin K, Soh M, Chang H, Kim J, Lee J, et al. Large-scale synthesis and medical applications of uniform-sized metal oxide nanoparticles. Adv Mater. 2018;30(42):e1704290.
Download references
Acknowledgements
Not applicable.
This work was funded by the European Regional Development Fund (ERDF) through the Centro 2020 Regional Operational Programme under BrainHealth2020 projects (CENTRO-01-0145-FEDER-000008), through the COMPETE 2020 - Operational Programme for Competitiveness and Internationalization and Portuguese national funds via FCT – Fundação para a Ciência e a Tecnologia , under projects - UIDB/04539/2020 and UIDP/04539/2020, POCI-01-0145-FEDER-030737 (NeuroStemForMJD, PTDC/BTM-ORG/30737/2017), CEECIND/04242/2017, and PhD Scholarship 2020.04751.BD.
Author information
Authors and affiliations.
CNC - Center for Neuroscience and Cell Biology, University of Coimbra, Rua Larga, polo 1, Coimbra, FMUC, 3004-504, Portugal
Ricardo Moreira, Luís Pereira de Almeida & Liliana Mendonça
CIBB - Center for Innovative Biomedicine and Biotechnology, University of Coimbra, Coimbra, 3004-504, Portugal
Faculty of Pharmacy, University of Coimbra, Coimbra, 3000-548, Portugal
Ricardo Moreira & Luís Pereira de Almeida
Algarve Biomedical Center Research Institute (ABC-RI), University of Algarve, Faro, 8005-139, Portugal
Clévio Nóbrega
Faculty of Medicine and Biomedical Sciences, University of Algarve, Faro, 8005-139, Portugal
Institute of Interdisciplinary Research, University of Coimbra, Coimbra, 3030-789, Portugal
Luís Pereira de Almeida & Liliana Mendonça
You can also search for this author in PubMed Google Scholar
Contributions
Manuscript writing and editing: RM; manuscript revision: CN, LPA, and LM; funding acquisition: LPA and LM; corresponding author: LM. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Liliana Mendonça .
Ethics declarations
Ethics approval and consent to participate, consent for publication.
All authors gave their consent for the publication of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Moreira, R., Nóbrega, C., de Almeida, L.P. et al. Brain-targeted drug delivery - nanovesicles directed to specific brain cells by brain-targeting ligands. J Nanobiotechnol 22 , 260 (2024). https://doi.org/10.1186/s12951-024-02511-7
Download citation
Received : 15 February 2024
Accepted : 29 April 2024
Published : 17 May 2024
DOI : https://doi.org/10.1186/s12951-024-02511-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Brain delivery
- Nanoparticles
- Brain-targeting ligands
- Targeting nanoparticles to specific brain cells
Journal of Nanobiotechnology
ISSN: 1477-3155
- Submission enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Functions. Transport oxygen from the lungs to the cells of the body. Pick up carbon dioxide from other tissues and unload it in the lungs. 2. White Blood Cells (Leukocytes) Account for only about 1% of the blood. 4500-11,000/mm 3. They are the cells that make up the majority of the immune system.
Each type of white blood cell plays a specific role in defense. For example, some white blood cells are involved in engulfing and breaking down pathogens, while others recognize specific microorganisms and launch immune responses against them. Different types of white blood cells have different lifetimes, ranging from hours to years, and new ...
Blood (histological slide) In this article we will describe the different types of blood cells. Blood is specialized fluid connective tissue.It travels through the circulatory system transporting gases, nutrients, wastes, and other macromolecules throughout the body. The main gases traveling in the blood are oxygen and carbon dioxide.
Recall that blood is a connective tissue. Like all connective tissues, it is made up of cellular elements and an extracellular matrix. The cellular elements—referred to as the formed elements —include red blood cells (RBCs), white blood cells (WBCs), and cell fragments called platelets.The extracellular matrix, called plasma, makes blood unique among connective tissues because it is fluid.
Chapter 1. Blood and the cells it contains. The average human adult has more than 5 liters (6 quarts) of blood in his or her body. Blood carries oxygen and nutrients to living cells and takes away their waste products. It also delivers immune cells to fight infections and contains platelets that can form a plug in a damaged blood vessel to ...
Red Blood Cells. Red blood cells, or erythrocytes (erythro- = "red"; -cyte = "cell"), are specialized cells that circulate through the body delivering oxygen to cells; they are formed from stem cells in the bone marrow. In mammals, red blood cells are small biconcave cells that at maturity do not contain a nucleus or mitochondria and are only 7-8 µm in size.
In the human embryo, the first site of blood formation is the yolk sac.Later in embryonic life, the liver becomes the most important red blood cell-forming organ, but it is soon succeeded by the bone marrow, which in adult life is the only source of both red blood cells and the granulocytes.Both the red and white blood cells arise through a series of complex, gradual, and successive ...
Blood makes up about 8% of the human body weight. It contains erythrocytes, leucocytes, thrombocytes (platelets) and plasma. The volume percentage of all blood cells in the whole blood is about 45% of adults (hematocrit). The rest consists of liquid plasma (e.g. water, plasma proteins, electrolytes etc.). The blood is composed of:
Blood is made up of about 55% blood plasma and about 45% different types of blood cells. Blood plasma is a light yellow, slightly cloudy liquid. Over 90% of blood plasma is water, while less than 10% consists of dissolved substances, mostly proteins. Blood plasma also contains electrolytes, vitamins and nutrients such as glucose and amino acids.
Oxygen is needed for our cells to burn sugars, fats and proteins in a controlled way. This provides us with the energy we need to live. Outside our bodies, we know that when iron is exposed to ...
In general, Type AB can be transfused with Types A, B, AB and O. Type A can be transfused with Types A and O. Type B can be transfused with Types B and O. Type O can only be transfused with Type O. There are actually many antigens or molecules on a blood cells that can cause an immune reaction, but these major antigens are the most important ...
A person's blood type is determined by the antigens on the red blood cells. Antigens are protein molecules on the surface of these cells. Antibodies are proteins in plasma that alert the immune ...
Blood Cells and Their Functions Essay. Blood is the fluid that transports oxygen nutrients through the whole body and carries away the waste products of the organism. An average human adult has about five liters of blood, which constitutes 8% of the entire body weight (Shier et al., 2019). Due to its complex nature and transport function, a ...
Essay # 3. Blood Cells in Humans: There are three types of blood cells which are floating in blood plasma. The amount is about 45% of the total blood volume. These cells are: (1) Erythrocytes or Red Blood Cells (2) Leukocytes or White Blood Cells (3) Thrombocytes or Blood Platelets. 1. Erythrocytes:
The blood is made up of more than 10 different cell types. Each of these cell types falls into one of three broad categories: 1. Red blood cells (erythrocytes): These transport oxygen and ...
What is a normal white blood cell count? It is normal for you to produce nearly 100 billion white blood cells each day. After completing a blood draw, a test counts your white blood cells, which equals number of cells per microliter of blood. The normal white blood cell count ranges between 4,000 and 11,000 cells per microliter.
Hematopoiesis is blood cell production. Your body continually makes new blood cells to replace old ones. Hematopoiesis ensures you have a healthy supply of blood cells to supply oxygen to your tissue (red blood cells), fight infection (white blood cells) and clot your blood when you're injured (platelets). Most blood cells get made in your ...
Updated on July 28, 2019. Red blood cells, also called erythrocytes, are the most abundant cell type in the blood. Other major blood components include plasma, white blood cells, and platelets. The primary function of red blood cells is to transport oxygen to body cells and deliver carbon dioxide to the lungs. A red blood cell has what is known ...
Blood is a highly complex fluid which is composed of two parts—a liquid, called the plasma and different types of cells which remain suspended in the plasma. The cells are called the blood corpuscles. The plasma constitutes about 55 percent, and the cells about 45 percent, of the total volume of human blood. Essay # 2.
ADVERTISEMENTS: The differentiation of mammalian red blood cells in bone marrow (a process called erythropoiesis) is one of the most striking examples of specialization occurring in nature. In adults, erythropoies is begins with a pluripotent (i.e., multi-potent) stem cell that gives rise in 7 to 10 days to mature, hemoglobin-filled erythrocytes. Differentiation and specialization of […]
Blood is a fluid connective tissue which comprises RBC, WBC, platelets and plasma. The main function of blood is to deliver oxygen and nutrients to various cells and tissues of the body. 2. State the types of blood cells found in human blood. Blood cells are classified into the following types:
Types of Blood. There are four main types of blood: A, B, AB, and O. Each type is determined by the presence or absence of certain substances on the surface of the red blood cells. People with type A blood have A antigens, those with type B have B antigens, those with type AB have both, and those with type O have neither. Importance of Blood
The red blood cells (RBCs) are oxygen and carbon dioxide carrying cells found in the blood serum of vertebrate organisms (Starr, 143). They transport oxygen from the lungs to the tissues, and carbon dioxide from the tissues to organs that get rid of them, such as lungs. The RBCs are biconcave, oval, and do not contain most cell organelles; this ...
Introduction. This lab report is about blood typing. Blood typing as the name implies is simply a system or method of knowing the type of blood one has. The purpose of blood typing is to identify the blood group/type that may be safe for blood transfusion into patients with different ABO type. Prior knowledge of one's blood group or type is ...
Iron is a major component of hemoglobin, a type of protein in red blood cells that carries oxygen from your lungs to all parts of the body. Without enough iron, there aren't enough red blood cells to transport oxygen, which leads to fatigue. Iron is also part of myoglobin, a protein that carries and stores oxygen specifically in muscle tissues.
The COVID-19 pandemic has made assessing vaccine efficacy more challenging. Besides neutralizing antibody assays, systems vaccinology studies use omics technology to reveal immune response mechanisms and identify gene signatures in human peripheral blood mononuclear cells (PBMCs). However, due to their low proportion in PBMCs, profiling the immune response signatures of dendritic cells (DCs ...
High blood sugar is damaging to the body, so your body keeps trying to remove excess blood sugars. It stores extra sugar in your liver and muscles. When they're full, the liver sends the remaining sugar to be stored as body fat, causing weight gain. Insulin resistance sets the stage for prediabetes and type 2 diabetes.
The U.S. health regulator on Wednesday approved the expanded use of Bristol Myers Squibb's (BMY.N) cancer cell therapy Breyanzi for the treatment of adults with a type of blood cancer called ...
A normal eosinophil blood count is less than 500 cells per microliter (mL) of blood—typically less than 5% of all white blood cells circulating in your blood.
Neurodegenerative diseases are characterized by extensive loss of function or death of brain cells, hampering the life quality of patients. Brain-targeted drug delivery is challenging, with a low success rate this far. Therefore, the application of targeting ligands in drug vehicles, such as lipid-based and polymeric nanoparticles, holds the promise to overcome the blood-brain barrier (BBB ...