- Sign In to save searches and organize your favorite content.
- Not registered? Sign up

Recently viewed (0)
- Save Search
- Subscriptions
- Join E-mail List
Patient Case Studies and Panel Discussion: Plasma Cell Neoplasms
- Get Citation Alerts
- Download PDF to Print
Managing patients with plasma cell neoplasms, diseases in which abnormal plasma cells or myeloma cells form tumors in the bones or soft tissues of the body, poses numerous challenges for clinicians. At the NCCN 2019 Annual Congress: Hematologic Malignancies, a panel of experts discussed evidenced-based approaches for the treatment of patients with these diseases. Moderated by Dr. Andrew D. Zelenetz, the session focused on patients with transplant-ineligible newly diagnosed multiple myeloma, active multiple myeloma, and light chain amyloidosis.
Plasma cell neoplasms are diseases in which abnormal plasma cells or myeloma cells form tumors in the bones or soft tissues of the body. At the NCCN 2019 Annual Congress: Hematologic Malignancies, a panel of experts identified clinical challenges in managing patients with plasma cell neoplasms. Moderated by Dr. Andrew D. Zelenetz, the session focused on 3 case studies, which were used to develop an evidence-based approach for the treatment of these patients.
Patient Case Study 1: Transplant-Ineligible, Newly Diagnosed Multiple Myeloma
In the first case study, a 75-year-old man presented with new back pain and fatigue. The patient was able to perform all activities of daily living and resided approximately 70 miles away from the treatment center. Figure 1 shows results from imaging, laboratory tests, and bone marrow biopsy.

Results from laboratory, imaging, and biopsy studies for the patient in case study 1.
Abbreviations: FISH, fluorescence in situ hybridization; IFE, immunofixation electrophoresis; KLC, kappa light chain; LDH, lactate dehydrogenase; SPEP, serum protein electrophoresis; SUV, standard uptake value; ULN, upper limit of normal.
Citation: Journal of the National Comprehensive Cancer Network J Natl Compr Canc Netw 17, 11.5; 10.6004/jnccn.2019.5034
Although the patient did not harbor high-risk fluorescence in situ hybridization (FISH) changes, his lactate dehydrogenase (LDH) level was elevated at diagnosis. Elevated LDH as a risk factor has been associated with inferior outcomes, 1 , 2 and is also factored into risk stratification by the Revised International Staging System (R-ISS) as the only nongenetic parameter, 3 said Muhamed Baljevic, MD, University of Nebraska Medical Center.
Yvonne A. Efebera, MD, MPH, The Ohio State University Comprehensive Cancer Center – James Cancer Hospital and Solove Research Institute, noted that although this patient is transplant-ineligible, a 3-drug regimen is preferred over 2-drug options.
“This has been shown several times in newly diagnosed patients,” said Dr. Efebera. “In the SWOG SO777 study, bortezomib with lenalidomide and dexamethasone showed better progression-free survival [PFS] and overall survival versus lenalidomide and dexamethasone alone. 4 Furthermore, in the MAIA trial, the addition of daratumumab to lenalidomide and dexamethasone improved PFS versus lenalidomide and dexamethasone alone. 5 However, because we don’t have overall survival data from the MAIA trial, the triplet of bortezomib/lenalidomide/dexamethasone is the only one to show survival benefit,” she said.
Dr. Baljevic noted that bortezomib/lenalidomide/dexamethasone is a category 1 NCCN recommendation for both transplant-eligible and transplant-ineligible patients based on the SWOG SO777 data. Although recent data from the ALYCONE trial suggest that quadruplet regimens may surpass triplet regimens, 6 this particular induction combination may be more relevant to European practice patterns. The combination of daratumumab + bortezomib/melphalan/prednisone led to increased overall response rates and PFS. 6 For transplant-eligible patients, transplant is still preferred to induce the deepest remission possible, Dr. Zelenetz noted, “but we’re not seeing overall survival differences like we used to in the long-term.”
This patient received bortezomib, lenalidomide, and dexamethasone and experienced a very good partial response after 6 cycles. The patient then switched to maintenance lenalidomide, 10 mg daily. Complete responses encompass very deep remissions and clearly detectable disease, said Dr. Zelenetz, who noted that data have shown that undetectable minimal residual disease status is associated with better outcomes. 7 Nevertheless, panelists agreed that minimal residual disease testing to guide therapy for this patient would not have be useful in clinical practice.
“This is a very expensive test, and to make it worthwhile, you want to be able do something with the information obtained,” said Michael M. Green, MD, Kaiser Permanente. “For this patient in particular, I don't think there was much utility.”
- Early-Relapse Disease: No Clear Choice for Treatment
The patient received continuous maintenance therapy with lenalidomide. After 2 years of maintenance, he experienced toxicity from lenalidomide (diarrhea and fatigue) and took a treatment holiday. The first relapse occurred at a PFS time of approximately 39 months. Laboratory results showed a hemoglobin level of 10.2 g/dL and a PET scan showed several new lytic bone lesions. Bone marrow biopsy showed 60% kappa plus plasma cells and FISH identified t(14;16), del(17p), and gain(1q) (4 copies).
Although many new agents are available for relapsed/refractory multiple myeloma, panelists agree that triplet regimens remain the standard, with daratumumab combinations appearing quite effective. The sequencing of agents is still undefined, however. Several novel agents are in development, with early data on venetoclax and B-cell maturation antigen–targeted therapies showing promise.
“There are lots of positive trials and lots of options in this setting, but there is no clear, unequivocal correct answer, which is an important point,” said Dr. Zelenetz.
“Oftentimes, when we’re making a decision about what the next line of treatment will be at first relapse, we're really thinking about what the third-line option will be down the road,” Dr. Green added. “Whatever decision we make up front is going to impact the kinds of options available later on.”
- Patient Case Study 2: Active Multiple Myeloma
In the second case study, a 53-year-old man presented with persistent swelling and pain around the left shoulder for several months. A small mass was detected on his left clavicle on physical examination. Medical history showed hypertension, schizoaffective disorder, and chronic hepatitis B, and a diagnosis of active multiple myeloma was the panelists’ consensus. Figure 2 shows test results for this patient. The patient was treated with lenalidomide, bortezomib, and dexamethasone (continued for 15 months). Approximately 1 month after starting treatment, he received radiotherapy to his left clavicle for 2 weeks.

Results from laboratory, imaging, and biopsy studies for the patient in case study 2.
Abbreviations: FISH, fluorescence in situ hybridization; SUV, standard uptake value.
Nina Shah, MD, UCSF Hellen Diller Family Comprehensive Cancer Center, noted that a carfilzomib + lenalidomide and dexamethasone (KRD) combination may have been the better option because of the patient’s 17p deletion and high-risk disease.
“Data from the University of Chicago seem to show that when you gave patients at high risk more-aggressive induction of KRD followed by transplant and KRD consolidation and lenalidomide maintenance, their outcomes are similar to those with standard risk,” said Dr. Shah. “That’s a very small trial subset and there are a lot of caveats, but we have to do something different for patients at high risk than we're doing for those at standard risk.”
Dr. Efebera added that for patients at high risk, 2-drug maintenance may yield better outcomes than single-agent maintenance. “We don’t have any prospective studies, and only 15% of patients are high-risk, but retrospective analysis at Emory has shown that 2-drug maintenance is feasible and possibly better. The jury is still out,” she said.
Dr. Baljevic noted that the field is moving toward 4-drug combinations for both standard- and high-risk disease, which raises legitimate questions regarding financial toxicity. “The possibility of moving toward pentads (5-drug combinations) with molecularly adapted regimens has even been discussed,” he said. “Provided safety profiles would not be prohibitory, the idea would be to treat patients for a shorter period of time upfront, and see if less therapy in the long term is possible.”
Although autologous stem cell transplantation (ASCT) was discussed, this patient elected not to move forward with it due to social concerns. Approximately 1 month after stopping treatment, he experienced disease recurrence characterized by progressive disease in the sternum and rib.
“Patients may be initially resistant to transplant for a number of reasons, but after the disease comes back early, they may be more amenable,” said Dr. Efebera, who noted that insurance companies are more frequently paying for collection of stem cells and storage.
This patient was started on daratumumab, pomalidomide, and dexamethasone, which he continued for 10 months before experiencing cytopenias with daratumumab and pomalidomide. Approximately 1 week after starting therapy, he received radiotherapy to the sternum (for 2 weeks).
“Radiotherapy should be used for symptomatic lesions and should not delay systemic therapy,” said Dr. Zelenetz. He noted that 3 weeks after the patient started therapy, bone marrow biopsy showed <5% atypical plasma cells. However, major complications and adverse events occurred during treatment, leading to drug disruptions and treatment delays. The patient’s disease recurred 10 months after starting therapy. He was admitted for altered mental status, hypercalcemia, back pain, and was treated with carfilzomib, cyclophosphamide, and dexamethasone.
“There are other treatment options for extramedullary disease, but there really is no right answer,” said Dr. Shah. “This patient should go to a clinical trial for CAR-T cell therapy or should undergo a transplant.”
“For high-risk disease and for people who are young, undergoing transplant upfront is strongly recommended,” added Dr. Green. “It could save the patient from getting into these situations.”
- Patient Case Study 3: Light Chain Amyloidosis
For the final case study, a 63-year-old man presented with easy bruising, foamy urine, and red tongue, with no prior medical history. Figure 3 shows his test results.

Results from laboratory and imaging studies for the patient in case study 3.
Based on the diagnosis of light chain amyloidosis, panelists agreed that urine immunofixation should be performed for optimal diagnostic sensitivity. As Dr. Zelenetz reported, one study found that not performing urine immunofixation led to missing 6% of amyloidogenic clones. 8
“For patients with light chain amyloidosis, I really want to stress the importance of mass spectroscopy in terms of identifying the clone and the type of amyloid,” Dr. Efebera added. “It’s been shown that 13% of patients with TTR [transthyretin] familial amyloidosis are treated as having light chain amyloidosis due to having monoclonal gammopathy of undetermined significance [MGUS] unrelated to their amyloidosis 9 ; 3% of the general population aged ≥50 years have MGUS. It’s very important that mass spectroscopy is incorporated into your tissue biopsy.”
“Fat pad biopsies are easy and cheap,” said Dr. Zelenetz. “If you’re not sure, it’s worth it to do a biopsy.”
Following work-up, the Mayo Clinic Staging System for light chain amyloidosis was used. For those inexperienced with this system, Michaela Liedtke, MD, Stanford Cancer Institute, reassured the audience that this system is relatively straightforward and is routinely used in practice. “You basically plug in the numbers for the N -terminal pro–B-type natriuretic peptide and troponin, and the differential of the free light chains,” she said. “The ratio is not as important as the difference between the involved light chain that produces the amyloid deposits and the uninvolved light chain.”
“Although this is a prognostic tool, it also provides a lot of information about whether somebody is a transplant candidate or not,” Dr. Baljevic explained. “After induction therapy and tumor reduction, recent data show that it’s actually possible to make someone who was not initially a transplant candidate into a candidate.” 10
“Patients with amyloid differ significantly from patients with myeloma,” Dr. Baljevic continued. “With light chain amyloidosis, it’s even more important to try to get patients to transplant. It’s one of the most important measures in terms of long-term outcomes that we can offer them.” 11
This patient was treated with 4 cycles of cyclophosphamide, bortezomib, and dexamethasone and experienced a hematologic very good partial response. “Depth of response is important in myeloma, but even more important in amyloidosis because you want to reduce the light-chain burden as much as possible,” said Dr. Liedtke.
According to Dr. Zelenetz, the key to making the diagnosis of light chain amyloidosis is suspecting the diagnosis in the first place. “This is the heart and soul of what we do as physicians,” he concluded. “When you approach the patient, you have to have the right differential diagnosis. You’ve got to suspect, because you can’t make the right diagnosis you’re not thinking about.”
Dimopoulos MA , Barlogie B , Smith TL , Alexanian R . High serum lactate dehydrogenase level as a marker for drug resistance and short survival in multiple myeloma . Ann Intern Med 1991 ; 115 : 931 – 935 .
- Search Google Scholar
- Export Citation
Terpos E , Katodritou E , Roussou M , et al. . High serum lactate dehydrogenase adds prognostic value to the international myeloma staging system even in the era of novel agents . Eur J Haematol 2010 ; 85 : 114 – 119 .
Palumbo A , Avet-Loiseau H , Oliva S , et al. . Revised international staging system for multiple myeloma: a report from International Myeloma Working Group . J Clin Oncol 2015 ; 33 : 2863 – 2869 .
Durie BG , Hoering A , Abidi MH , et al. . Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial . Lancet 2017 ; 389 : 519 – 527 .
Facon T , Kumar S , Plesner T , et al. . Daratumumab plus lenalidomide and dexamethasone for untreated myeloma . N Engl J Med 2019 ; 380 : 2104 – 2115 .
Mateos MV , Dimopoulos MA , Cavo M , et al. . Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma . N Engl J Med 2018 ; 378 : 518 – 528 .
Munshi NC , Avet-Loiseau H , Rawstron AC , et al. . Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: a meta-analysis . JAMA Oncol 2017 ; 3 : 28 – 35 .
Dispenzieri A , Merlini G , Comenzo RL . Amyloidosis 2008 BMT tandem meetings (February 13-17, San Diego) . Biol Blood Marrow Transplant 2008 ; 14 ( Suppl 1 ): 6 – 11 .
Lachmann HJ , Booth DR , Booth SE , et al. . Misdiagnosis of hereditary amyloidosis as AL (primary) amyloidosis . N Engl J Med 2002 ; 346 : 1786 – 1791 .
Manwani R , Hegenbart U , Mahmood S , et al. . Deferred autologous stem cell transplantation in systemic AL amyloidosis . Blood Cancer J 2018 ; 8 : 101 .
Dispenzieri A , Kyle RA , Lacy MQ , et al. . Superior survival in primary systemic amyloidosis patients undergoing peripheral blood stem cell transplantation: a case-control study . Blood 2004 ; 103 : 3960 – 3963 .
Disclosures: Dr. Zelenetz has disclosed that he received consulting fees from AbbVie, Inc., Amgen Inc., AstraZeneca Pharmaceuticals LP, Celgene Corporation, Gilead Sciences, Inc., Janssen Pharmaceutica Products, LP, Novartis Pharmaceuticals Corporation, Adaptive Biotechnologies Corporation, Genentech, Inc./Roche Laboratories, Inc., and Pharmacyclics; is a scientific advisor for AbbVie, Inc., AstraZeneca Pharmaceuticals LP, and MorphoSys AG; and receives grant/research support from BeiGene, Gilead Sciences, Inc., MEI Pharma Inc., and Roche Laboratories, Inc. Dr. Baljevic has disclosed that he received consulting fees from Cardinal Health and Takeda Pharmaceuticals North America, Inc., and served as an internal review committee member for Karyopharm Therapeutics and a scientific advisor for Takeda Pharmaceuticals North America, Inc. Dr. Efebera has disclosed that she has received honoraria from Janssen Pharmaceutica Products, LP, and Takeda Pharmaceuticals North America, Inc, and is a scientific advisor for Akcea Therapeutics. Dr. Green has disclosed that he has no financial interests, arrangement, affiliations, or commercial interests with any manufacturers of any products discussed in this article or their competitors. Dr. Liedtke has disclosed that she receives grant/research support from Agios, Inc., Amgen Inc., Celator Pharmaceuticals, Celgene Corporation, Genentech, Inc., Gilead Sciences, Inc., Janssen Pharmaceutica Products, LP, bluebird bio, Inc., Prothena, Pfizer Inc., and Takeda Pharmaceuticals North America, Inc; received consulting fees from Amgen Inc.; and is a scientific advisor for Celgene Corporation, Janssen Pharmaceutica Products, LP, and Jazz Pharmaceuticals Inc. Dr. Shah has disclosed that she receives grant/research support from Celgene Corporation, Janssen Pharmaceutica Products, LP, bluebird bio, Inc., Sutro Biopharma; is a scientific advisor for Genentech, Inc., Seattle Genetics, Oncopeptides, Karoypharm, Surface Oncology, Precision Biosciences, Glaxo Smith Kline, Nektar, Amgen, Indapta Therapeutics, and Sanofi; and owns stock in Indapta Therapeutics.
Article Sections
- View raw image
- Download Powerpoint Slide
Article Information
- Get Permissions
- Similar articles in PubMed
Google Scholar
Related articles.
- Advertising
- Terms of Use
- Privacy Policy
- Permissions

© 2019-2024 National Comprehensive Cancer Network
Powered by:
- [66.249.64.20|195.216.135.184]
- 195.216.135.184
Character limit 500 /500

Journal of the Association of Physicians of India is a monthly scientific journal started in 1952. The journal reaches over 22000 members of the Association of Physicians of India every month and benefits millions through the online version.
Social Media
Contact info.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Browse by collection
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 11, Issue 10
- Clinical features and diagnosis of multiple myeloma: a population-based cohort study in primary care
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0003-2997-5867 Anouchka Seesaghur 1 ,
- Natalia Petruski-Ivleva 2 ,
- Victoria Louise Banks 1 ,
- Jocelyn Ruoyi Wang 2 ,
- Ali Abbasi 1 ,
- David Neasham 1 ,
- Karthik Ramasamy 3
- 1 Center for Observational Research (CfOR) , Amgen Ltd , Uxbridge , UK
- 2 Science , Aetion, Inc , Boston , Massachusetts , USA
- 3 Department of Haematology , Oxford University Hospitals NHS Foundation Trust , Oxford , UK
- Correspondence to Dr Karthik Ramasamy; karthik.ramasamy{at}ndcls.ox.ac.uk
Objectives Patients with multiple myeloma (MM) experience significant delays in diagnosis due to non-specific symptomatology. The aim of this study was to characterise the frequency and timing of clinical features in the primary care setting prior to MM diagnosis.
Design Population-based cohort study.
Setting Electronic health records data of approximately 17 million patients (2006–2016) within the UK Clinical Practice Research Datalink.
Participants Patients aged ≥18 years with newly diagnosed MM (NDMM), no history of solid tumours and ≥2 years registration in a primary care practice prior to MM diagnosis.
Main outcome measures Clinical features and symptoms including bone pain, skeletal-related events (SREs), investigation and confirmation of MM diagnostic CRAB criteria (hyperCalcaemia, Renal impairment, Anaemia, Bone lesions) during the 2 years prior to MM diagnosis; time between symptom manifestation and/or relevant investigation and diagnosis of MM.
Results Among 2646 patients with NDMM, 47.5% had a bone pain record during the 2-year period prior to MM diagnosis, mainly affecting the back. Regardless of baseline bone pain, investigations for serum calcium level were used in 36.4% of patients prior to MM diagnosis, followed by haemoglobin (65.6%) or renal function (74.1%). Median (Q1, Q3) time from first-recorded bone pain to MM diagnosis was 220 (80, 476) days. Median (Q1, Q3) time from first-recorded hypercalcaemia, renal impairment or anaemia to MM diagnosis was 23 (12, 46), 58 (17, 254) and 73 days (28, 232), respectively. An imaging investigation or referral for imaging was recorded for 60.0% of patients with bone pain/SRE and 32% without.
Conclusions Nearly half of patients diagnosed with NDMM presented with bone pain approximately 7 months prior to MM diagnosis. Investigations to evaluate all CRAB criteria, including targeted imaging, were underused. Early recognition of myeloma clinical features and optimised use of investigations in primary care may potentially expedite MM diagnosis.
- primary care
- epidemiology
Data availability statement
Data may be obtained from a third party and are not publicly available. Data sharing agreements under licence from CPRD prohibit the patient-level data to be publicly available. No additional data available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjopen-2021-052759
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Strengths and limitations of this study
Our study provides more clarity to the occurrence and timing of key myeloma clinical features and use of diagnostic investigations prior to the diagnosis of multiple myeloma (MM).
Our study is the first attempt to provide key diagnostic information such as patient symptoms and laboratory testing on a large representative sample of patients who are newly diagnosed with MM in primary care over a 10-year period.
Demographic characteristics, comorbidities, symptoms, clinical events, drug exposures and laboratory investigation definitions have been validated during a reproducibility study and published separately.
This study relies on the quality and completeness of data collected in the Clinical Practice Research Datalink database.
In this study, medications, investigations or events occurring typically within the hospital settings may be under-reported.
Introduction
Multiple myeloma (MM) is the second most common haematological malignancy in Europe, with an estimated age-standardised incidence of 9.6 per 100 000 people in the UK for the year 2017, projected to increase to 12 per 100 000 people by 2035. 1 2 The UK-based Haematological Malignancy Research Network (HMRN) raised awareness on potential diagnostic delays in primary care, recognising non-specific symptomatology as a main barrier to MM diagnosis. 3 4 Patients with myeloma have one of the longest time-to-diagnosis intervals among cancers, with an average time between symptom onset and MM diagnosis of 99 days. 5 6 Compared with patients with other cancers, they also have the most repeated consultations occurring in primary care before referral to a specialist, with 50% experiencing three or more repeat consultations. 7 While 57% of patients are ultimately diagnosed through general practitioner (GP) referral, timely recognition and diagnosis of MM are challenging; 8 patients typically present to their GPs or family physicians with a myriad of non-specific symptoms such as bone pain or aches occurring at multiple sites, and fatigue. 9 Because the average age of MM presentation is 70 years, 10 these clinical signs may be overlooked as gradual ageing. Furthermore, the average primary care physician in an individual clinical practice may see fewer than ten patients with MM throughout their career given the rare cancer status of MM.
Despite the presentation of non-specific symptoms, MM causes disabling complications including skeletal-related conditions (destructive lytic lesions, osteoporosis and hypercalcaemia, skeletal-related events (SREs)), renal impairment, infection, neurological complications and anaemia. 9 11 12 The 1-year survival of patients diagnosed through GP referral or emergency presentation after MM diagnosis was 70% and 42%, respectively. 13 Early detection is a high priority for patients and improves survival; 84% of patients with myeloma survive for >5 years if diagnosed at the earliest stage, compared with only 26% if diagnosed at advanced stage. 14 Early diagnosis and subsequent management of myeloma improve patients’ quality of life and reduce symptom burden and serious complications of the disease. 15 The International Myeloma Working Group (IMWG) recommends a series of laboratory and imaging investigations to evaluate patients with a suspected diagnosis of MM, namely diagnostic imaging and blood tests to assess the CRAB (hyperCalcaemia, Renal impairment, Anaemia, Bone lesions) diagnostic criteria for MM. 8 In the primary care setting, access to laboratory testing (eg, haemoglobin, calcium levels, kidney function, paraprotein and light chains) is readily available and diagnostic testing can identify underlying cause of clinical features following evidence gained through the physical examination (signs and symptoms). The presence of bone pain in combination with laboratory abnormalities, such as anaemia, hypercalcaemia or unexplained renal impairment, have a high diagnostic certainty for MM. 16
The extent to which these common clinical features have been used to diagnose MM in the primary care setting has not been widely investigated. Most existing studies on MM have represented a population with more advanced disease in clinical secondary care settings. 9 As the first point of contact for patients, primary care practices provide an opportunity to investigate patients presenting with clinical features underlying MM and direct the diagnostic pathway for patients with suspected MM. In our study, we used primary care electronic medical records (EMRs) to characterise early clinical features of patients newly diagnosed with MM in the UK and describe investigations for the diagnostic CRAB criteria undergone by patients prior to MM diagnosis.
Study design and data source
This study was a population-based cohort study of newly diagnosed MM (NDMM) patients using the UK Clinical Practice Research Datalink (CPRD) GOLD database. The CPRD is based on standardised EMR systems in UK primary care. 17 The database contains routinely collected GP data from patients registered in over 600 primary care practices. The geographical distribution of GP practices has been shown to be representative of the UK and the patients are broadly representative of the UK general population in terms of age and sex distributions as reported by the national population census. 18
Study population
The study population included NDMM patients over the age of 18 at diagnosis who were registered with GP practices across the UK and contributed to the CPRD database. Patients who were continuously registered with GP practices during a minimum 2-year baseline period prior to (and not including) the MM diagnosis date (index date) were included in the cohort on their first record of MM diagnosis between 1 January 2006 and 31 December 2016. Patients were eligible for inclusion if their record was labelled as acceptable by CPRD quality control. Patients were excluded if they had one or more record of a solid tumour (including skin cancer) diagnosis during any time prior to (and including) the index date to avoid the inclusion of patients experiencing bone pain due to metastases of their tumour to the bone. Figure 1 presents the study design diagram. 19
- Download figure
- Open in new tab
- Download powerpoint
Study design. The study design diagram visually displays study design implementation. The vertical line represents the cohort entry date (index date), which is the first-order temporal anchor. The boxes represent second-order temporal anchors (time windows). The brackets in the boxes show time intervals anchored on day 0. Dx, diagnosis; EXCL, exclusion; MM, multiple myeloma.
Defining MM and clinical features
Primary care Read codes were used to identify MM diagnosis, comorbidities, bone pain and SREs from clinical and referral records. 20 Product codes were used to identify prescribed medications. Laboratory investigations and confirmation of the CRAB criteria, including serum calcium, haemoglobin and creatine level, were identified using Read codes from clinical and referral records and Entity type from Test records. Investigation and confirmation of bone lesions was identified through Read codes from clinical and referral records, Entity type and Medcodes from Test records ( figure 2 ). Testing of interest included haemoglobin level, blood calcium level, serum creatinine level and diagnostic imaging (bone scan, CT scan, MRI scan, positron emission tomography scan and any X-ray). Reasons for imaging procedures were not available and patients may have received those for reasons not related to the CRAB criteria work-up. Details and lists of Read code have been previously described. 21
CRAB criteria for multiple myeloma investigation and confirmation. *Plain radiographs coded as a record of X-ray; other imaging studies coded as a record of bone scan or CT scan or MRI or positron emission tomography scan; diagnostic imaging investigations.
Symptoms of MM, including bone pain and SREs prior to diagnosis, and clinical features of the CRAB criteria during the baseline period were retrieved.
Statistical analysis
Patients were described in terms of demographic characteristics at baseline, prevalent comorbidities, clinical features and symptoms. Prescribed medications related to bone health and pain management including bisphosphonates, considered standard of care for bone disease, were also described. 22 Summary statistics included frequencies (%) for categorical variables and mean (SD) and median (Q1, Q3) for continuous variables such as time of the diagnostic interval from first recorded bone pain, SRE and CRAB investigations. Time of symptom presentation or relevant diagnostic CRAB criteria to the diagnosis of MM were also evaluated. Results were stratified by the presence of a record for bone pain and/or SRE at baseline (symptomatic) or absence of bone pain and/or SRE at baseline (asymptomatic).
All analyses were conducted using the Aetion Evidence Platform (V.3.12), a rapid-cycle analytic tool which has been validated in a range of studies and therapeutic areas including oncology. 23
Patient and public involvement
Although, there has been no specific patient or public involvement (contact) in this retrospective database study, CPRD works diligently and independently with contributing practices to ensure patients are aware of how their anonymised data are used and of their right to opt out of their data being shared for research ( https://www.cprd.com/public ).
At the time of analysis, the CPRD database contained 17 756 119 patients. Among 4823 patients with NDMM, 2177 patients were excluded for not meeting the eligibility criteria, leading to a total of 2646 NDMM patients between 2006 and 2016 included in our analysis ( online supplemental figure S1 ). Among all patients with NDMM, the median (Q1, Q3) age was 71 (63, 79) years and 54.7% were men. On average, patients were observable in the CPRD database for 11.5 years prior to their initial MM diagnosis and for 2.7 years post MM diagnosis. Overall, 43.8% of patients with NDMM had at least one musculoskeletal comorbid condition (29.2% with osteoarthritis and 10.8% with osteoporosis); 45.0% of all patients had hypertension, 21.2% chronic kidney disease, 20.2% cardiovascular disease.
Supplemental material
Figure 3 shows the baseline demographic and clinical characteristics of the NDMM patients by the presence (symptomatic) or absence (asymptomatic) of bone pain/SREs at baseline. Musculoskeletal comorbidities were observed among symptomatic patients and asymptomatic patients, including osteopenia (6.2% and 4.7%), osteoporosis (14.8% and 7.0%) and osteoarthritis (32.8% and 25.7%), respectively. Both symptomatic and asymptomatic patients frequently received analgesics during baseline, including non-opioid analgesics (88.1% and 62.4%, respectively), weak opioid (73.4% and 40.9%) and strong opioid use (41.8% and 14.6%). A prescription for bisphosphonates was observed for 17.3% of symptomatic patients during baseline ( online supplemental table S1 ). Among a subgroup of 361 patients with NDMM and a prescription of oral bisphosphonates in the 2 years prior to MM diagnosis, 62.3% (n=225) had a record of bone pain or SRE and 37.7% (n=136) did not.
Baseline clinical characteristics and CRAB-related presentation prior to multiple myeloma (MM) diagnosis among patients with symptomatic and asymptomatic MM. Percentage of patients for each characteristic is shown among symptomatic patients with bone pain and/or SRE (red bars) and asymptomatic patients without bone pain and/or SRE (orange bars). For CRAB diagnostic investigations, the percentages of patients tested for hypercalcaemia, renal impairment, anaemia and bone lesions are shown for symptomatic patients (red bars) and asymptomatic patients (orange bars). Percentages of patients with confirmation are shown for symptomatic (pink bars) and asymptomatic patients (light orange bars). CRAB criteria investigations and confirmations prior to MM diagnosis between 2006 and 2016. A maximum of 2 years minus 90 days before (and not including) the MM index date was used. Patients were required to be continuously enrolled throughout each time window to be included into these subgroups. CRAB, hyperCalcaemia, Renal impairment, Anaemia, Bone lesions; NDMM, newly diagnosed MM; SRE, skeletal-related event.
Combination of CRAB investigations for hypercalcaemia, renal impairment, anaemia and diagnostic imaging for bone lesion. The frequency of CRAB criteria testing was assessed in the 12 months prior to and not including the cohort entry date. The testing includes laboratory tests only; total tests measure the number of patients who had the specific test or combination of tests, alone or with additional tests. CRAB investigation categories are not mutually exclusive. Patients included in each category were required to have, at minimum, the tests indicated by the black circles; they may or may not have had the tests indicated by the white circles. CRAB, hyperCalcaemia, Renal impairment, Anaemia, Bone lesions.
Clinical features and CRAB investigation
Overall, 49.1% of the patients with NDMM were symptomatic with either bone pain and/or SRE during baseline. Among patients with NDMM, 47.5% of patients had a baseline bone pain record, mainly affecting the back (33.7%) or other joints (17.3%). Only 4.8% of patients with NDMM had a record of an SRE, mostly captured as pathological fracture (3.7%). Records of spinal cord compression and surgery to bone were rare (<1%) ( online supplemental table S2 ). An imaging investigation or referral for an imaging investigation was recorded for 60.0% of symptomatic bone pain/SRE and 31.7% of asymptomatic patients. Among NDMM patients who had an imaging investigation, 19.0% had an MRI and 22.1% had a CT scan ( online supplemental table S3 ). Confirmed bone lesions were recorded in 8.1% of symptomatic patients and 2.2% of asymptomatic patients ( online supplemental table S4 ).
During the baseline period, most patients with NDMM received a laboratory investigation for renal impairment (approximately 74%) or anaemia (approximately 65%), regardless of being symptomatic or asymptomatic ( figure 3 , online supplemental table S4 ). Confirmation of hypercalcaemia was infrequently observed prior to MM diagnosis regardless of the presence or absence of bone pain/SRE ( figure 3 , online supplemental table S4 ). The proportion of patients who met any one of the CRAB criteria was 0.8% and 0.7% for hypercalcaemia, 3.4% and 7.3% for renal impairment, 11.9% and 15.6% for anaemia among symptomatic and asymptomatic patients, respectively ( online supplemental table S4 ).
During the 12 months prior to MM diagnosis, the proportion of CRAB-related diagnostic test combinations received by patients were 75.7% for renal impairment and anaemia, 50.4% for hypercalcaemia and renal impairment, 50.2% for hypercalcaemia and anaemia, and 48.9% for hypercalcaemia, renal impairment and anaemia. Only 18.9% of all patients with NDMM underwent investigations for all four CRAB criteria ( figure 4 , online supplemental table S5 ). We observed complete CRAB criteria testing with all four components in 26.7% of symptomatic patients and 11.5% of asymptomatic patients ( online supplemental table S5 ).
Timing of clinical features and CRAB investigation
Among all patients with NDMM, the median time (Q1, Q3) between MM diagnosis and the initial laboratory diagnostic workup to ascertain renal impairment or anaemia were 488 (203, 626) and 380 (95, 594) days, respectively. We observed a 6-month interval between MM diagnosis and the initial investigation for hypercalcaemia (median (Q1, Q3) of 176 (44, 507) days) ( online supplemental table S6 ). The median time (Q1, Q3) between date of ascertainment of renal impairment or anaemia (via the first record of a confirmed abnormal test result) and date of MM diagnosis were 58 (17, 254) and 73 (28, 232) days, respectively. The median (Q1, Q3) diagnostic interval between a confirmed hypercalcaemia and the MM diagnosis was only 23 (12, 46) days ( figure 5 , online supplemental table S7 ).
Timing of clinical CRAB (hyperCalcaemia, Renal impairment, Anaemia, Bone lesions) criteria between confirmation to diagnosis of MM. The timing and event occurrence were measured during baseline period, 730 days to 1 day prior to the MM diagnosis date; the time periods were counted starting from the first ever laboratory investigation or diagnosis during the 730 days prior to and including the cohort entry date. MM, multiple myeloma.
Overall, we observed a 6-month interval between the initial investigation for bone lesion (median (Q1, Q3) of 195 (59, 452) days) and MM diagnosis ( online supplemental table S6 ), and a median (Q1, Q3) diagnostic interval of 105 (30, 346) days between confirmed imaging results for bone lesions and the MM diagnosis. Among symptomatic patients, the median (Q1, Q3) time from the initial bone pain record to MM diagnosis was 220 (80, 476) days. The median (Q1, Q3) time from bone pain to investigations with bone X-ray, MRI scan, bone scans or CT scan was 34 (8, 175) days, 93 (38, 256) days, 112 (44, 259) and 181 (58, 406) days, respectively ( online supplemental table S8 ).
This population-based cohort study using real-world data revealed that nearly half of 2646 patients with NDMM had a record of symptomatic bone pain, approximately 7 months prior to MM diagnosis in primary care. Approximately 71% of symptomatic patients presented with back pain. Diagnostic intervals (ie, the time from investigation to MM diagnosis) ranged from 6 months to over 12 months among both symptomatic and asymptomatic patients. Abnormal laboratory results for the CRAB criteria were observed closer to MM diagnosis time, with a median time of 1–2 months. Investigations for hypercalcaemia were uncommon in patients presenting with bone pain, and diagnostic tests to identify CRAB criteria were underused. Among symptomatic patients (with bone pain/SRE), advanced bone imaging investigation recommended by IMWG and National Institute for Health and Care Excellence (NICE) 24 with MRI or CT scan was limited; 20% of symptomatic patients had a record of MRI or CT imaging.
Strengths and limitations
The main strength of our study is that it fills the knowledge gap about key clinical features and diagnostic timing leading up to the diagnosis of myeloma in a primary care setting by providing a comprehensive picture for both symptomatic and asymptomatic patients. Our study is based on electronic health records data from a large representative sample of the UK population registered with GPs in the primary care setting with a wide geographic coverage. 16 Over a 10-year study period, our study captured a large number of newly diagnosed patients with MM. Additionally, to minimise misclassification, a group of clinical experts and epidemiologists developed algorithms to identify conditions of interest. We also investigated the frequency of bone pain recording on an annual basis over the study period and found consistency in recording of the symptom across the different years.
The study also has some limitations. First, since our study relies on recorded diagnoses in electronic health records, conditions or comorbidities not reported to the GPs might not be captured. Similarly, CPRD data are collected at the time of GP clinical care and not for research purposes; therefore, the completeness of medical information from specialists and inpatient care, is not known. Our study only focused on the CRAB diagnostic criteria and did not investigate other relevant tests such as protein electrophoresis, Bence-Jones protein urine test, serum free light chain test or erythrocyte sedimentation rate, which may also be conducted to assess myeloma. In addition, plain radiographs and other imaging studies were part of the CRAB criteria for bone lesions; however, patients may have received a chest X-ray for other reasons not related to the CRAB criteria work-up. Reasons for imaging procedures were not available and patients may have received those for reasons not related to the CRAB criteria work-up. Finally, the study only looked at bone pain in the 2 years prior to diagnosis and patients may have had bone pain prior to the start of the baseline assessment period.
Comparison with existing literature
Previous studies have reported that most patients with MM complained about their bone pain at presentation. In a comprehensive review of the literature, Nador et al showed that 59% of patients with MM presented with bone pain. 11 Goldschmidt et al conducted an analysis using EMRs and Israeli Health Maintenance Organization data and reported that back pain was the most common complaint during the 2 years before MM diagnosis. 25 In our study, the frequency of bone pain recorded in primary care was consistent with these studies despite differences in geographic region and data source. As previous studies highlighted, findings about diagnostic delays in MM can vary from one study to another due to differences in data sources, data collection methods, study design and study periods. For example, Howell et al , used data from the UK-based HMRN, and estimated that the total interval between time to help-seeking (self-reported symptoms) was 163 days. 4 In a systematic review and meta-analysis of seven studies, Koshiaris et al reported that the median diagnostic interval between first presentation to primary care and MM diagnosis was approximately 109 days. 26 Our observational findings support substantial delays between recorded symptoms and MM diagnosis in a primary care setting. However, there is considerable heterogeneity in symptoms (patient-reported vs predefined symptoms) and time periods reported in previous studies, 26 which makes comparing findings across studies difficult. 4
Clinical and policy implications
The current pathway to diagnosing myeloma is recognised as being complex, with multiple GP appointments and significant delay before diagnosis. There is limited evidence regarding the clinical scenarios in which MM should be suspected. 13 16 Our study provides more clarity on the occurrence and timing of key clinical features and diagnostic investigations leading to the diagnosis of MM.
Our findings suggest that GPs could have significant input in improving the time to diagnosis of MM. The first National Audit of Cancer Diagnosis in Primary Care collected data on primary care referrals submitted voluntarily by GPs on their patients diagnosed with cancer in England. This audit showed avoidable delays in 27% of patients with MM receiving their diagnosis in primary care in England. 5 During the COVID-19 pandemic, there have been additional delays in myeloma diagnosis due to markedly reduced CT and MRI imaging, longer waiting times for investigations, and a fall in urgent cancer referrals. 27 28 At the end of September 2020, there were a total of 215 463 patients waiting for a diagnostic MRI, and the number of patients waiting 6 weeks or more for an MRI increased by 20% compared with September 2019. 29 30 Further research is warranted to quantify the impact of delays in MM diagnosis and treatment on patient quality of life and outcomes.
To reduce delays in diagnosing of MM in the primary care, there is a need for improved diagnostic safety netting, that is, the process of managing diagnostic uncertainty during the GP consultation and communicating to patients when and how to follow-up on potential symptoms. 31 Various stages of the pathway to MM diagnosis would benefit from tailored safety nets to manage diagnosis uncertainty and timely evaluation. Reflecting on insights from our study and other published studies, we propose a plethora of actions in different settings to be undertaken by both the patient and the GPs, using the action, actor, context, target, time framework ( figure 6 ). 32
Proposed action, actor, context, target, time specification to improve diagnosis of multiple myeloma (MM) (adapted from Presseau et al ). 32 CRAB, hyperCalcaemia, Renal impairment, Anaemia, Bone lesions; GP, general practitioner.
One aspect of safety netting is to provide advice on potential red-flag symptoms and on accessing further medical care. Targeted awareness campaigns co-produced with patients and the public on the clinical features and symptom profiles of MM may help reduce delays in MM diagnosis in primary care. Tailored GP education programmes on MM diagnosis through their regular channels would be an enabler for early diagnosis. Back pain combined with other symptoms such as fatigue and weight loss, or back pain combined with abnormal blood tests warrant definitive investigation for MM. 33 34 Such focused approach may be more impactful to address the delays in seeking help, representation and diagnosis. Such delays, with myeloma patients taking half a month to 7 months from initial symptom/health change to first seeking help, were recently reported. 35
In the case of patients without bone pain and/or SRE (asymptomatic), further research is required ( figure 6 ) 36 to identify biomarkers/precursors of MM, and to help identify those who may benefit from early screening haematological investigations. For example, Koshiaris et al used CPRD data to develop a prediction tool based on patient characteristics, symptoms and blood tests to identify patients at risk of MM in primary care. 37 Patients with monoclonal gammopathy of unknown significance (MGUS), however, were excluded from the analysis. Since premalignant plasma cell disorders such as MGUS often precedes MM, research is currently underway to monitor MGUS in community or secondary care, identify biomarkers and better predict patients who progress from MGUS to myeloma.
Another aspect of safety netting includes the follow-up and management of investigations. Improved access to diagnostic facilities may enable GPs to request timely laboratory and advanced imaging investigations, thereby accelerating the time to MM diagnosis. There is already a call for one stop shop diagnostic services directly within the community, closer to patients’ homes. 38 39 This cross-collaboration aligns with efforts across the UK, as outlined in the National Health Service Long Term Plan, to focus on a radical overhaul of services for the diagnosis of suspected cancer, including the introduction of Rapid Diagnostic Centres, and the organisation of imaging networks with better access to MRI and CT scanners. 40 41
Early recognition of red-flag symptoms and self or GP referral to these diagnostic services with rapid turnaround time for results will result in early diagnosis and prompt treatment. Further research, in close partnership with the patient, their support networks and the public, is required on the development, implementation and effectiveness of these potential safety netting. 42
Nearly half of patients with NDMM presented with a bone pain symptom in primary care, approximately 7 months prior to MM diagnosis. Diagnostic tests to explore evidence of the CRAB criteria were underused. Investigations for hypercalcaemia and advanced imaging were not frequent in patients presenting with bone pain. Increased awareness of clinical features of MM, including its early presentation as bone pain, may lead to early recognition and testing of MM in primary care, thereby potentially accelerating disease diagnosis and timely medical care.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
The research protocol was reviewed and approved by the Independent Scientific Advisory Committee (reference ISAC, protocol No 18_292). In this study, all data were completely anonymised and no participant’s consent was required.
Acknowledgments
We wish the acknowledge Edwin Hoeben, George Kafatos, Joe Maskell, Shannon Reynolds and Andrew Weckstein for their contribution to the research project and Pattra Mattox, CMPP, Aetion, for her editorial assistance to the manuscript. We also acknowledge Dr William Murk, J L Novosad and Aditya Rajan for their contribution to the data visualisations. The authors thank the peer-reviewers for their helpful comments on an earlier version of the manuscript.
- Cancer Research UK
- Smittenaar CR ,
- Petersen KA ,
- Stewart K , et al
- Howell DA ,
- Smith AG , et al
- Jack A , et al
- McPhail S ,
- Witt J , et al
- Friese CR ,
- Magazu LS , et al
- Lyratzopoulos G ,
- Barbiere JM , et al
- National Cancer Registration and Analysis Service (NCRAS)
- Kariyawasan CC ,
- Hughes DA ,
- Jayatillake MM , et al
- Wisloff F ,
- Samson D , et al
- Ramasamy K ,
- Panitsas F , et al
- Asmussen JT , et al
- Elliss-Brookes L ,
- Ives A , et al
- Blood Cancer UK
- Wilkenfeld P ,
- Shephard EA ,
- Rose P , et al
- Herrett E ,
- Gallagher AM ,
- Bhaskaran K , et al
- Schneeweiss S ,
- Rassen JA ,
- Brown JS , et al
- Seesaghur A ,
- Petruski-Ivleva N ,
- Banks V , et al
- Dimopoulos MA , et al
- Verpillat P ,
- Rassen JA , et al
- National Institute for Health and Care Excellence (NICE)
- Goldschmidt N ,
- Poperno A , et al
- Koshiaris C ,
- Abel L , et al
- UK Parliament
- Watt I , et al
- Presseau J ,
- McCleary N ,
- Lorencatto F , et al
- Appleton S , et al
- Kolovos S ,
- Kishore B , et al
- Smith A , et al
- Van den Bruel A ,
- Nicholson BD , et al
- Wilkinson E
- Greenwood E , et al
- Diaz-delCastillo M ,
- Andrews RE ,
- Mandal A , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
Contributors AS, NP-I, VLB, DN made substantial contributions to the design of the work. NP-I, VLB and JRW contributed to the analysis of the data. AS, NP-I, JRW, AA and KR contributed to the interpretation of data. AS, NP-I, AA drafted the work; AS, NP-I, VLB, JRW, AA, DN and KR made substantial contributions to substantively revise the manuscript. All authors provided final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AS is the guarantor and accepts full responsibility for the work and/or the conduct of the study, had access to the data and controlled the decision to publish.
Funding This work was supported by Amgen. Award/Grant number is not applicable.
Competing interests AS and DN are employees of and hold stock options in Amgen. AA is a contract worker at Amgen. During the study conduct and reporting, VLB was a contract worker for Amgen. NP-I and JRW were employees of Aetion at the time the study was conduct and reporting and hold equity in Aetion. KR reports honoraria, research grant and advisory board from Janssen, Celgene, Takeda and Amgen.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
Early-onset multiple myeloma: an illustrative case report
Affiliation.
- 1 Southern California University of Health Sciences, Whittier, CA, USA.
- PMID: 17870425
- DOI: 10.1016/j.jmpt.2007.07.002
Objective: This case study describes a patient diagnosed with early manifestations of multiple myeloma and illustrates relevant aspects of differential diagnosis and the use of laboratory, radiologic, and advanced imaging techniques to aid in establishing the diagnosis and issues of management.
Clinical features: A 36-year-old male student experienced midback pain that occurred primarily at night in conjunction with fever and unexplained weight loss. Minor trauma induced a significant fracture and an occult fracture in the upper extremity. Physical examination revealed an elevated temperature indicating a fever of undetermined etiology. Plain radiographs revealed diffuse osteoporosis of the thoracic spine. Laboratory tests revealed anemia, hypercalcemia, and abnormal monoclonal paraprotein. Magnetic resonance imaging revealed a fracture with poor healing and an occult fracture in the upper extremity.
Intervention and outcome: The patient was initially assessed for fever of undetermined etiology in association with nocturnal midback pain. Although considered a disease of the geriatric population, subsequent laboratory and radiologic evaluations established a diagnosis of early-onset multiple myeloma. Early recognition and referral with comanagement by an oncologist provided optimum care. Early-onset cases of multiple myeloma tend to have a more favorable response to treatment as compared with cases diagnosed in the geriatric population.
Conclusion: Multiple myeloma should be a consideration when a patient presents with nocturnal back pain and fever of undetermined etiology. Differentiating multiple myeloma from other causes of back pain is especially important in making management decisions. With a precise history and physical diagnosis, the diagnosis may be suspected, but confirmation must rely on ancillary investigations. Multiple myeloma is frequently accompanied by a poor prognosis, but early-onset cases generally respond more favorably to interventions.
Publication types
- Case Reports
- Back Pain / diagnosis
- Back Pain / etiology
- Diagnosis, Differential
- Fever / etiology
- Fracture Healing
- Multiple Myeloma / complications
- Multiple Myeloma / diagnosis*
- Multiple Myeloma / therapy
- Scaphoid Bone / injuries
- Thoracic Vertebrae
- Treatment Outcome
- Weight Loss
- Wrist Injuries / complications
- Wrist Injuries / diagnosis
- Wrist Injuries / therapy
- Case report
- Open access
- Published: 10 September 2007
An unusual presentation of multiple myeloma: a case report
- Catherine B. Molloy 1 , 2 ,
- Rahul A. Peck 2 ,
- Stephen J. Bonny 3 ,
- Simon N. Jowitt 3 , 4 ,
- John Denton 5 ,
- Anthony J. Freemont 5 &
- Abbas A. Ismail 2
Journal of Medical Case Reports volume 1 , Article number: 84 ( 2007 ) Cite this article
17k Accesses
9 Citations
2 Altmetric
Metrics details
Multiple myeloma can occasionally manifest with joint disease. We report the case of an individual with a progressive bilateral carpal syndrome and a symmetrical severe seronegative polyarthritis and joint swelling. Investigations revealed an erosive seronegative inflammatory arthritis in association with bilateral carpal tunnel syndrome, anaemia, hepatic impairment and nephrotic-range proteinuria. Synovial fluid cytology demonstrated plasmablasts and multinucleated cells with products of chondrolysis. The diagnosis of multiple myeloma (with secondary amyloidosis) was made on serum protein electrophoresis and bone marrow biopsy.
The relationship between myeloma and joint disease is discussed, highlighted by the presence in this case of all three pathogenic features associated with arthritis in myeloma patients- an erosive arthritis, carpal tunnel syndrome and an invasive tumoural arthritis.
Peer Review reports
Multiple myeloma is a malignant proliferation of plasma cells producing a monoclonal paraprotein. Multiple myeloma can present in a range of ways, for example, hypercalcaemia, hyperviscosity, renal failure and bone pains/fractures. We report an unusual presentation of multiple myeloma in the form of symmetrical severe polyarthritis and joint swelling.
Case presentation
A 55 year old lady referred to the rheumatology clinic with a 3 month history of progressive disabling polyarthralgia and joint swelling, a 5 kg weight loss and fatigue. The predominant joints affected were her knees, shoulders, wrists and small hand joints; her hand function was so impaired at the time of presentation that she was no longer able to feed herself. She denied joint stiffness, thigh pain, a history of skin rash, gastrointestinal or genitourinary symptoms.
On examination she was pale and cachectic. She had generalised soft tissue swelling of her hands, with markedly reduced wrist movements, but without synovitis. Tinel's and Phalen's tests were strongly positive bilaterally consistent with carpal tunnel syndrome. Moderate cool effusions were present in both knees. No synovitis was present elsewhere and the rest of her systemic examination was normal.
She had a normochromic anaemia with a borderline leucopaenia (Hb 65 g/l, MCV 80 fl, WCC 3.9 × 10 9 /l, platelets 200 × 10 9 /l) and a grossly raised ESR (>140 mm/hr). She was hypercalcaemic (corrected calcium 3.15 mmol/l, phosphate 1.82 mmol/l, alkaline phosphatase 102 U/l) with deranged liver function (LDH 1085 U/l, AST 46 U/l, normal bilirubin, albumin and globulin levels). Significant renal disease was evident (urea 22 mmol/l, creatinine 407 μmol/l), +1 of blood and protein on urinalysis, a creatinine clearance of 16 ml/min and nephrotic range proteinuria (5.29 g/d). Hand radiographs showed wrist joint space narrowing with juxta-articular erosions.
Left knee synovial fluid cytology revealed atypical cells resembling plasmablasts and multinucleate cells, as well as changes consistent with chondrolysis, figure 1 . It was felt this was due to malignant infiltration of cartilage, with bone and cartilage degradation products present in the fluid. Wrist aspiration was dry.
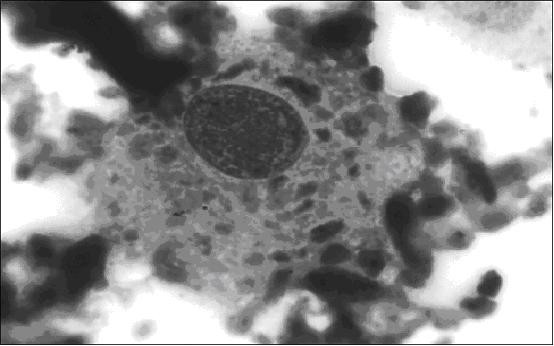
Knee synovial fluid: plasmablast-like cell containing particles of phagocytosed degenerate articular cartilage surrounded by suspended degenerate cartilage (Jenner Giemsa, ×1000). Informed consent was given for publication from the patient's next-of-kin.
Subsequently, rheumatoid factor, ANA, ENA and ANCA were all negative and a non-contrast CT scan of her thorax, abdomen and pelvis did not identify any abnormalities of the viscera or the skeleton.
A panhypogammaglobulinaemia was identified [IgG was 3.7 g/l (8–16), IgA and IgM were both 0.1 g/l (1.4–4, 0.5–2)]. Electrophoresis identified a small paraprotein band (2 g/l), and a large amount of free kappa light chains in both the serum and the urine (8.8 mg/l). Haematological advice was sought and bone marrow biopsies were undertaken, demonstrating a heavy (>90%) infiltration by plasma cells including atypical forms, with a marked reduction in granulopoiesis and erythropoiesis. Amyloid protein was also identified in the walls of blood vessels within the trephine biopsy.
Thus a diagnosis of aggressive multiple myeloma was made (stage IIIB) and the patient was treated with aggressive VCADVCAD chemotherapy (vincristine, cyclophosphamide, adriamycin and dexamethasone). Unfortunately, she died from pneumonia seven weeks after presentation.
We have described the initial presentation of an aggressive multiple myeloma with an erosive seronegative polyarthritis due to direct myelomatous joint infiltration. On review of the literature, a few case reports have described articular presentations of the plasma cell dyscrasias-multiple myeloma (MM) [ 1 , 2 ], monoclonal gammopathy of uncertain significance (MGUS) [ 1 , 2 ] and Waldenström's macroglobulinaemia [ 3 ].
Joint involvement in myeloma is typically an oligoarthritis [ 1 ] or a polyarticular rheumatoid-like pattern, as seen in this case. Though individuals with myeloma are at greater risk of both septic arthritis and gouty arthritis [ 3 ], other pathophysiological mechanisms have been postulated to account for joint disease. Firstly, local synovial precipitation of cryoprecipitable paraproteins [ 1 , 4 ] or immunoglobulin crystals [ 4 ] may activate the inflammatory response resulting in an erosive arthritis [ 2 ]. Secondly, a carpal tunnel syndrome may develop from intrasynovial deposition of amyloid protein or immunoglobulins [ 5 ]. Finally, juxta-articular plasmacytic lesions may infiltrate the synovium and synovial fluid resulting in a 'tumoural arthritis'. This direct tumour invasion of the joint has been identified in other primary haematological malignancies [ 3 , 6 – 8 ], however it is an extremely rare manifestation of the plasma cell dyscrasias, having only previously been described in 2 individuals with myeloma [ 3 , 8 ]. This case demonstrated all of these three pathogenic features- an erosive arthritis, carpal tunnel syndrome and an invasive tumoural arthritis.
This case is also unique in that the synovial fluid analysis yielded the ultimate diagnosis. In a case series of 9 individuals with a monoclonal gammopathy (MGUS or MM) and arthritis, the majority [ 5 ] were diagnosed with the plasma dyscrasia first, synchronous diagnoses were made in 3, and arthritis was the presenting feature in only 1 case [ 1 ]. There is no information on the prognosis of cases presenting in this manner, but based on the presence of anaemia, hypercalcaemia, renal impairment, advanced lytic bone lesions and high tissue M-component levels in this case, a high myeloma tissue mass was present, related to a poor prognosis [ 9 ].
We report the case of a patient presenting with tumoural arthritis and carpal tunnel syndrome from an aggressive myeloma. This case stresses the importance of analysing the synovial fluid of any patient with an atypical joint disease or a suspected plasma cell dyscrasia for cytology and immunohistochemistry, micro-organisms, crystals, and also for immunoglobulins and amyloid.
Jorgensen C, Guerin B, Ferrazzi V, Bologna C, Sany J: Arthritis associated with monoclonal gammopathy: clinical characteristics. Br J Rheumatol. 1996, 35: 241-243. 10.1093/rheumatology/35.3.241.
Article CAS PubMed Google Scholar
Vitalli C, Baglioni P, Vivaldi I, Cacialli R, Tavoni A, Bombardieri S: Erosive arthritis in monoclonal gammopathy of unknown significance: report of four cases. Arthritis Rheum. 1991, 34: 1600-1605.
Article Google Scholar
Roux S, Fermand JP, Brechignac S, Mariette X, Kahn MF, Brouet JC: Tumoral joint involvement in multiple myeloma and Waldenströms macroglobulinaemia- report of 4 cases. J Rheumatol. 1996, 23: 2175-2178.
CAS PubMed Google Scholar
Langlands D, Dawkins R, Matz I: Arthritis associated with a crystallizing cryoprecipitable IgG paraprotein. Am J Med. 1980, 68: 461-465. 10.1016/0002-9343(80)90122-9.
Wiernik P: Amyloid joint disease. Medicine (Baltimore). 1972, 51: 465-478. 10.1097/00005792-197211000-00003.
Article CAS Google Scholar
Evans T, Nercessian B, Sanders K: Leukaemic arthritis. Arthritis Rheum. 1994, 24: 48-56. 10.1016/0049-0172(94)90099-X.
Rice D, Semble E, Ahl E, Bohrer S, Rothberger H: Primary lymphoma of bone presenting as monoarthritis. J Rheumatol. 1984, 11: 851-854.
Villiaumey J, Larget-Piet B, Pointud P: Les complications articulaires de la maladie de Kahler. Résultats d'une enquête portant sur 1953 dossiers de myélomes plasmocytaires. Rev Rhum Mal Osteoartic. 1975, 42: 25-34.
Durie BGM, Salmon SE: A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment and survival. Cancer. 1975, 36: 842-854. 10.1002/1097-0142(197509)36:3<842::AID-CNCR2820360303>3.0.CO;2-U.
Download references
Acknowledgements
The authors declare no funding was required for the writing and submission of the manuscript. Informed written consent was received from the patient for publication of the manuscript.
Author information
Authors and affiliations.
Rheumatology, St. Michaels Hospital, Toronto, Canada
Catherine B. Molloy
Rheumatology, Stockport NHS Trust, UK
Catherine B. Molloy, Rahul A. Peck & Abbas A. Ismail
Haematology, Stockport NHS Trust, UK
Stephen J. Bonny & Simon N. Jowitt
Haematology, Salford Royal NHS Trust, UK
Simon N. Jowitt
Osteoarticular Pathology, Manchester University, UK
John Denton & Anthony J. Freemont
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Catherine B. Molloy .
Additional information
Competing interests.
The author(s) declare that they have no competing interests.
Authors' contributions
The authors were involved in the writing of the manuscript or patient clinical care. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Molloy, C.B., Peck, R.A., Bonny, S.J. et al. An unusual presentation of multiple myeloma: a case report. J Med Case Reports 1 , 84 (2007). https://doi.org/10.1186/1752-1947-1-84
Download citation
Received : 16 June 2007
Accepted : 10 September 2007
Published : 10 September 2007
DOI : https://doi.org/10.1186/1752-1947-1-84
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Multiple Myeloma
- Carpal Tunnel Syndrome
- Plasma Cell Dyscrasia
- Erosive Arthritis
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Considerations in Multiple Myeloma: Case Studies in Front-line Therapy

It is with great pleasure that I present the first issue of our third annual “Considerations in Multiple Myeloma” newsletter series. The goal of this case-based curriculum is to help clinicians integrate the latest advances in the treatment of multiple myeloma (MM) into the clinical setting. Each issue will contain 2 case studies, featuring faculty perspectives by an oncologist, a pharmacist, and a nurse from a leading cancer institution. In this first newsletter, faculty from Winship Cancer Institute of Emory University discuss the multidisciplinary treatment of 2 newly diagnosed patients with MM. Subsequent issues in this series will focus on maintenance therapy, retreatment for relapsed/refractory disease, stem cell transplantation in the era of novel agents, the management of MM in the nontransplant setting, the evolving role of cytogenetic testing, side-effect management, and the latest advances in improving bone health. It is my sincere hope that the information presented in t...
Related Papers
Leukemia & Lymphoma
Martin Kropff
The prognosis for patients multiple myeloma (MM) has improved substantially over the past decade with the development of new, more effective chemotherapeutic agents and regimens that possess a high level of anti-tumor activity. In spite of this important progress, however, nearly all MM patients ultimately relapse, even those who experience a complete response to initial therapy. Management of relapsed MM thus represents a vital aspect of the overall care for patients with MM and a critical area of ongoing scientific and clinical research. This comprehensive manuscript from the International Myeloma Working Group provides detailed recommendations on management of relapsed disease, with sections dedicated to diagnostic evaluation, determinants of therapy, and general approach to patients with specific disease characteristics. In addition, the manuscript provides a summary of evidence from clinical trials that have significantly impacted the field, including those evaluating conventio...
Update on Multiple Myeloma [Working Title]
Khalid Al-Anazi
Belgian Journal of Hematology
Marie-christiane Vekemans
Cancer Nursing Practice
Seminars in Hematology
Efstathios Kastritis
Journal of the advanced practitioner in oncology
Beth Faiman
Multiple myeloma (MM) is a relapsing disease for many patients with multiple myeloma. At relapse, patients have many options for treatment once disease has progressed. Advanced practitioners are well suited to set expectations for ongoing therapy and underscore the importance of continued disease monitoring. Criteria for relapsed myeloma rely on biomarker and radiologic imaging, as well as physical exam and awareness of new bone pain or changes in physiologic function. The treatment of patients with relapsed MM requires a personalized approach and considers patient desires in regard to aggressiveness of therapy and willingness to participate in a clinical trial. The prognosis of patients with relapsed MM depends upon disease characteristics at baseline or throughout, as patients may acquire adverse cytogenetic abnormalities through various lines of treatment. Empowering patients to understand their diagnosis, interpret labs, and take an active role in treatment selection through shared decision-making can improve patients' quality of life and enhance adherence.
Mayo Clinic proceedings
Hasib Sidiqi
Life expectancy in patients with multiple myeloma is increasing because of the availability of an increasing number of novel agents with various mechanisms of action against the disease. However, the disease remains incurable in most patients because of the emergence of resistant clones, leading to repeated relapses of the disease. In 2015, 5 novel agents were approved for therapy for relapsed multiple myeloma. This surfeit of novel agents renders management of relapsed multiple myeloma more complex because of the occurrence of multiple relapses, the risk of cumulative and emergent toxicity from previous therapies, as well as evolution of the disease during therapy. A group of physicians at Mayo Clinic with expertise in the care of patients with multiple myeloma regularly evaluates the evolving literature on the biology and therapy for multiple myeloma and issues guidelines on the optimal care of patients with this disease. In this article, the latest recommendations on the diagnost...
Bone Marrow Transplantation
David Vesole
Pharmaceuticals
Weam Elbezanti
Multiple myeloma (MM) is a challenging hematological cancer which typically grows in bone marrow. MM accounts for 10% of hematological malignancies and 1.8% of cancers. The recent treatment strategies have significantly improved progression-free survival for MM patients in the last decade; however, a relapse for most MM patients is inevitable. In this review we discuss current treatment, important pathways for proliferation, survival, immune suppression, and resistance that could be targeted for future treatments.

RELATED PAPERS
Izabela Sykta
Research Journal of Seed Science
Saleh Seadh
Josianne Lima
Egyptian Journal of Aquatic Biology and Fisheries
Safwat Salama
sunny kumar
Journal of Surgical Oncology
Pranab Sinha
Computer Assisted Mechanics and Engineering Sciences
Flavio Bussamra
Current Developments in Nutrition
Fahmida Tofail
Paper in rivista scientifica di classe A
Andrea Germani
Current Psychology
jordan rodriguez
Disability and rehabilitation
Sigrid Østensjø
Handbuch Filmtheorie
Christian Pischel
arXiv: History and Philosophy of Physics
Elio Antonello
Θεόδωρος Φωτιάδης
Proceedings of the National Academy of Sciences
Amir hosein Behzadi bigdeli
Tamara Rebeiz , Hadi Antoun
ULB Institutional Repository
Shlomo Weber
Czechoslovak Mathematical Journal
Hubert Gollek
Nierówności społeczne a wzrost gospodarczy
Robert Nowacki
Pham Dang Quyet
Tạp chí Nghiên cứu Y học
Trần Quế Sơn
Geological Magazine
Rosolino Cirrincione
André Bueno
Irish Journal of Medical Science (1971 -)
Deniz Dülgeroğlu
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Br J Cancer
- v.57(5); 1988 May
Multiple myeloma--a case-control study.
A total of 399 patients with multiple myeloma and an equal number of match controls were interviewed about factors possibly related to the causes of their disease. Factors studied included occupation, chemical exposure, radiation exposure, prior diseases, immunizations, chronic infections and markers for defects in immune regulation. A strong risk associated with agriculture/food processing was observed (RR = 1.8, P = 0.002). The risk could not be restricted to those exposed to animals or meat products, or those exposed to pesticides. Significant excesses were also noted for reported exposures to chemicals and gases/fumes, but no specific agent or group of agents could be identified. Cases had fewer tonsillectomies above the age of 10 (P = 0.01). A large excess of shingles (herpes zoster) was observed in cases (P less than 0.001), but most of the excess cases occurred within 10 years of diagnosis, suggesting this was a preclinical manifestation of disease rather than a cause of it.
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (806K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References .
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein AM. Occupational mortality: cancer. Ann Occup Hyg. 1972 Apr; 15 (1):53–59. [ PubMed ] [ Google Scholar ]
- Brinton LA, Stone BJ, Blot WJ, Fraumeni JF., Jr Nasal cancer in U.S. furniture industry countries. Lancet. 1976 Sep 18; 2 (7986):628–628. [ PubMed ] [ Google Scholar ]
- Burmeister LF, Everett GD, Van Lier SF, Isacson P. Selected cancer mortality and farm practices in Iowa. Am J Epidemiol. 1983 Jul; 118 (1):72–77. [ PubMed ] [ Google Scholar ]
- Cuzick J. Radiation-induced myelomatosis. N Engl J Med. 1981 Jan 22; 304 (4):204–210. [ PubMed ] [ Google Scholar ]
- Cuzick J, Velez R, Doll R. International variations and temporal trends in mortality from multiple myeloma. Int J Cancer. 1983 Jul 15; 32 (1):13–19. [ PubMed ] [ Google Scholar ]
- Dörken H, Vollmer I. Die Epidemiologie des multiplen Myeloms. Untersuchung von 149 Fällen. Arch Geschwulstforsch. 1968; 31 (1):18–38. [ PubMed ] [ Google Scholar ]
- Gallagher RP, Spinelli JJ, Elwood JM, Skippen DH. Allergies and agricultural exposure as risk factors for multiple myeloma. Br J Cancer. 1983 Dec; 48 (6):853–857. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ichimaru M, Ishimaru T, Mikami M, Matsunaga M. Multiple myeloma among atomic bomb survivors in Hiroshima and Nagasaki, 1950-76: relationship to radiation dose absorbed by marrow. J Natl Cancer Inst. 1982 Aug; 69 (2):323–328. [ PubMed ] [ Google Scholar ]
- Linos A, Kyle RA, O'Fallon WM, Kurland LT. Incidence and secular trend of multiple myeloma in Olmsted County, Minnesota: 1965-77. J Natl Cancer Inst. 1981 Jan; 66 (1):17–20. [ PubMed ] [ Google Scholar ]
- Pearce NE, Smith AH, Howard JK, Sheppard RA, Giles HJ, Teague CA. Case-control study of multiple myeloma and farming. Br J Cancer. 1986 Sep; 54 (3):493–500. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Turesson I, Zettervall O, Cuzick J, Waldenstrom JG, Velez R. Comparison of trends in the incidence of multiple myeloma in Malmö, Sweden, and other countries, 1950-1979. N Engl J Med. 1984 Feb 16; 310 (7):421–424. [ PubMed ] [ Google Scholar ]
- Velez R, Beral V, Cuzick J. Increasing trends of multiple myeloma mortality in England and Wales; 1950-79: are the changes real? J Natl Cancer Inst. 1982 Aug; 69 (2):387–392. [ PubMed ] [ Google Scholar ]
Triclonal Gammopathy in Multiple Myeloma with Bleeding Diathesis: A Case Report
- CASE REPORT
- Published: 22 April 2024
Cite this article

- H. R. Samruddhi 1 ,
- Srikantiah Rukmini Mysore ORCID: orcid.org/0000-0001-7357-3772 1 ,
- Sindhu Harish 1 ,
- Prashantha Balanthimogru 2 &
- Sharada Rai 3
1 Altmetric
Introduction
Gammopathies have clinical and diagnostic significance for lymphoproliferative disorders and plasma cell dyscrasias such as multiple myeloma which is distinguished by the malignant growth of a plasma cell clone that generates monoclonal immunoglobulin. A 70-year old patient was evaluated for recurrent epistaxis and bleeding per rectum along with severe anemia in the past two months. A general physical examination revealed pallor. Blood tests showed pancytopenia.
Hemogram, biochemical investigations, SPE, Serum-free light chain assay, Immunofixation electrophoresis, Bone marrow aspiration cytology & Immunophenotyping, and flow cytometry.
Immunofixation electrophoresis showed triclonal gammopathy IgM Kappa, IgG Kappa, and IgA Lambda and SPE quantitation of M band—5.6 gm%. Serum kappa and lambda light chains were 204 mg/L and 11.22 mg/L respectively, and the kappa/lambda ratio-18.18. Bone marrow aspiration cytology showed extensive rouleaux formation of RBCs, scattered myeloid and erythroid cells, and a few abnormal plasmacytoid cells accounting for approximately 4% of all nucleated cells. The flow cytometry revealed clotted aspirate and the smear showed occasional scattered plasma cells. The bone marrow- immunophenotyping showed CD38/CD138 bright plasma cells (4.7%). The plasma cells showed co-expression of CD81, and CD27 and were dim for CD45 with variable CD56 expression. A severely altered A/G ratio was observed.
In plasma cell dyscrasias and lymphoproliferative diseases, monoclonal gammopathies are often observed, while the biclonal variety is less frequent. However, tri clonal gammopathy having a combination of IgM Kappa, IgG Kappa, and IgA Lambda is extremely rare and their clinical significance is unknown.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
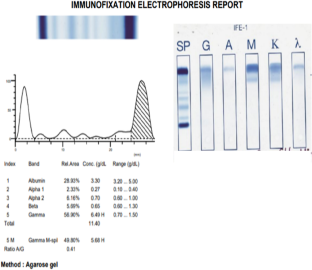
Aksungar FB, Ayer M, Serteser M, Coskun A, Unsal I. A tri clonal gammopathy in a relapsing multiple myeloma patient, detected by immunosubtraction method. Ann Clin Biochem. 2014;51(5):606–10.
Article PubMed Google Scholar
Tirelli A, Guastafierro S, Cava B, Lucivero G. Triclonal gammopathy in an extranodal non-Hodgkin lymphoma patient. Am J Hematology. 2003;73(4):273–5.
Article Google Scholar
Myrlande M, Vikram P. Triclonal gammopathy in a patient with smoldering plasma cell myeloma (PCM). Arch Hematology Case Rep Rev. 2021;6(1):0137.
Google Scholar
Gwathmey TM, Willis MS, Tatreau J, Wang S, McCudden CR. Clinical relevance of trace bands on serum electrophoresis in patients without a history of gammopathy. EJIFCC. 2015;26(2):114–24.
CAS PubMed PubMed Central Google Scholar
Michels TC, Petersen KE, Myeloma M. Diagnosis and Treatment. Am Fam Physician. 2017;95(6):373–84.
PubMed Google Scholar
Download references
Author information
Authors and affiliations.
Department of Biochemistry, Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal, India
H. R. Samruddhi, Srikantiah Rukmini Mysore & Sindhu Harish
Department of Hematooncology, Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal, India
Prashantha Balanthimogru
Department of Pathology, Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal, India
Sharada Rai
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Srikantiah Rukmini Mysore .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Samruddhi, H.R., Srikantiah, R.M., Harish, S. et al. Triclonal Gammopathy in Multiple Myeloma with Bleeding Diathesis: A Case Report. Ind J Clin Biochem (2024). https://doi.org/10.1007/s12291-024-01224-w
Download citation
Received : 24 December 2023
Accepted : 07 April 2024
Published : 22 April 2024
DOI : https://doi.org/10.1007/s12291-024-01224-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Tri clonal gammopathy
- Find a journal
- Publish with us
- Track your research
- In-person and virtual events just for HCPs

- Conferences
- Publications
- Biomarker Consortium
Case Study 2: An Elderly Patient With Multiple Myeloma
Transcript:
Thomas G. Martin, MD: In this case, an 81-year-old woman presented with fatigue and was found to have anemia. In fact, she had some pancytopenia. She had low neutrophils, she had low platelets, she had some anemia, and then she was found to have a monoclonal protein. On x-rays, she was found to have bone lesions and advanced bone disease, and she was found to have an elevation in her creatinine with a lower creatinine clearance. Now, it is typical in an older patient who has multiple myeloma that they have comorbidities: pancytopenia and renal insufficiency.
I would have, for this case, done a 24-hour urine test to see if, are they spilling myeloma protein in the urine. Do they have renal insufficiency from myeloma? Or is their creatinine elevated just because of their age? In this patient, it might just be because of their age. We may make some treatment decisions based on that. Now, this patient presented with about 20% of plasma cells in the bone marrow and the most common chromosomal abnormality: translocation (11;14). That happens in about 20% of patients with multiple myeloma and puts her in a standard-risk group of patients.
Now, if we look at risk in assessing the stage of this patient, she has an elevation in her beta-2 microglobulin: 6.2 mcg/mL. By the International Staging System, that would make her stage 3. She did have preserved albumin of 3.8 g/dL, but she’s still international stage 3. There’s now a revised International Staging System that takes into consideration cytogenetics. So, she would be revised international stage 2. Now, if we look at just the regular Durie-Salmon Staging System, which takes into account calcium, hemoglobin, the level of M protein, and the volume of bone disease, because she has advanced bone disease she would have Durie-Salmon stage 3 disease.
Rafael Fonseca, MD: In 2017, as we approach patients who have newly diagnosed myeloma but are of advanced age—that would be someone over the age of 80—we still consider risk status. I think I’ve mentioned in other venues that we don’t want to undertreat patients who could potentially benefit from important drugs, such as a proteasome inhibitor. So, for a patient who is elderly who has a t(4;14), we would probably start on treatment with VRd. There are some patients who have such comorbidities that we may start on a doublet, something like Rd. This case is very interesting, and I will allude to that as we talk about therapy for the relapse.
This patient had t(11;14), which is present in about 15% of myeloma patients, and this has turned out to be more tricky than we thought. Fifteen years ago, t(11;14) was a good prognostic marker, and the reason for that is most patients were being treated with alkylators. As all myelomas have improved over the years, t(4;14) has remained stagnant. Nowadays, you’re better off if you have a t(4;14) than if you have a t(11;14) at the time of diagnosis. We’ll talk about that, about the options for relapse in a patient like this, but I would say if the person is otherwise fit and willing and capable, a triplet would be indicated as first-line therapy for their disease.
One of the things we have learned in myeloma is that chronicity of therapy is important. Maybe we’ll have a future, hopefully, where a patient can be treated for a week, we’ll close the book, and it will all be done because there’s a high cure rate. But nowadays, that’s not the situation. In fact, there are a number of studies, like the MM-015 study or the FIRST study, that have shown chronicity of therapy is important. We have several studies that now show the importance of maintenance post stem cell transplant.
Most recently, a meta-analysis was published in the Journal of Clinical Oncology by Dr. McCarthy. Those same principles have been used for patients who are elderly who received induction therapy. So, if a patient received 8 cycles of VRd, we don’t just stop therapy. For most of those patients, we would actually transition to a maintenance approach, very much like you would do post transplant. There are different opinions for how long you would do that. I say there’s the American way, which is usually indefinite until there is either progression or intolerance, or the European way, which is more time limited to maybe 1 or 2 years. I would say at the bare minimum, I would try to shoot for a year. We understand it’s difficult sometimes, although much easier being the doctor. But if a patient is going great, they’re tolerating their medication, and they’re under control, l would be inclined to continue therapy.
Transcript Edited for Clarity

FDA Grants Breakthrough Therapy Designation to Ziftomenib in NPM1-Mutant AML

Revisit the OncLive On Air Episodes From February 2024

FAK Inhibition Emerges as a Potential Complementary Treatment Pathway

Lunning and Jacobson Dissect the Role of CAR T-Cell Therapy in Follicular Lymphoma

Revisit Every OncLive On Air Episode from March 2024

FDA Requires Boxed Warning for Risk of T-Cell Malignancies With Approved CAR T-Cell Therapies
Latest Conference Coverage

Navigating the Intersection of Radiation Therapy and Immunotherapy in Endometrial Cancer

As Orthopedic Oncology Evolves, Caring for the Clinician Must Be a Priority

Belumosudil Produces Long-Term Responses Without New Safety Concerns in cGVHD

Prophylactic Itacitinib May Safely Mitigate CRS Following Axi-Cel Administration in Lymphoma
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 19 April 2024
Single-cell multiomic dissection of response and resistance to chimeric antigen receptor T cells against BCMA in relapsed multiple myeloma
- Michael Rade ORCID: orcid.org/0000-0001-6247-9289 1 na2 ,
- Nora Grieb 2 , 3 na2 ,
- Ronald Weiss 1 , 4 ,
- Jaren Sia 5 ,
- Luise Fischer 2 ,
- Patrick Born 2 ,
- Andreas Boldt 4 ,
- Stephan Fricke 4 ,
- Paul Franz 4 ,
- Jonathan Scolnick 5 ,
- Lakshmi Venkatraman 5 ,
- Stacy Xu 5 ,
- Christina Kloetzer 2 ,
- Simone Heyn 2 ,
- Anne Sophie Kubasch 2 ,
- Ronny Baber 6 , 7 ,
- Song Yau Wang ORCID: orcid.org/0000-0001-5433-8750 2 ,
- Enrica Bach 2 ,
- Sandra Hoffmann 2 ,
- Jule Ussmann 2 ,
- Birthe Schetschorke 2 ,
- Saskia Hell 2 ,
- Sebastian Schwind ORCID: orcid.org/0000-0002-1315-2332 2 na1 ,
- Klaus H. Metzeler 2 ,
- Marco Herling 2 ,
- Madlen Jentzsch 2 ,
- Georg-Nikolaus Franke ORCID: orcid.org/0000-0001-8239-002X 2 ,
- Ulrich Sack 4 ,
- Ulrike Köhl 1 , 4 ,
- Uwe Platzbecker ORCID: orcid.org/0000-0003-1863-3239 2 ,
- Kristin Reiche ORCID: orcid.org/0000-0002-4452-4872 1 , 4 , 8 na3 ,
- Vladan Vucinic 2 na3 &
- Maximilian Merz ORCID: orcid.org/0000-0002-2805-5973 2 na3
Nature Cancer ( 2024 ) Cite this article
325 Accesses
5 Altmetric
Metrics details
- Cancer immunotherapy
- Cancer microenvironment
- Immunotherapy
Markers that predict response and resistance to chimeric antigen receptor (CAR) T cells in relapsed/refractory multiple myeloma are currently missing. We subjected mononuclear cells isolated from peripheral blood and bone marrow before and after the application of approved B cell maturation antigen-directed CAR T cells to single-cell multiomic analyses to identify markers associated with resistance and early relapse. Differences between responders and nonresponders were identified at the time of leukapheresis. Nonresponders showed an immunosuppressive microenvironment characterized by increased numbers of monocytes expressing the immune checkpoint molecule CD39 and suppressed CD8 + T cell and natural killer cell function. Analysis of CAR T cells showed cytotoxic and exhausted phenotypes in hyperexpanded clones compared to low/intermediate expanded clones. We identified potential immunotherapy targets on CAR T cells, like PD1, to improve their functionality and durability. Our work provides evidence that an immunosuppressive microenvironment causes resistance to CAR T cell therapies in multiple myeloma.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
111,21 € per year
only 9,27 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Cellular dynamics following CAR T cell therapy are associated with response and toxicity in relapsed/refractory myeloma
Luise Fischer, Nora Grieb, … Maximilian Merz
Anti-BCMA CAR T-cell therapy in multiple myeloma: can we do better?
Mattia D’Agostino & Noopur Raje
Development of CAR-T cell therapies for multiple myeloma
Nico Gagelmann, Kristoffer Riecken, … Nicolaus Kröger
Data availability
RNA-seq and scRNA-seq, BCR sequencing and TCR sequencing data that support the findings of this study have been deposited in the Gene Expression Omnibus under accession code GSE234261 . Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
Code availability
Processing and analysis code related to this study is deposited in the GitHub repository at https://github.com/fraunhofer-izi/Rade_Grieb_et_al_2023 .
Merz, M. et al. Adjusted comparison of outcomes between patients from CARTITUDE-1 versus multiple Myeloma Patients with Prior Exposure to PI, Imid and Anti-CD-38 from a German Registry. Cancers 13 , 5996 (2021).
Article CAS PubMed PubMed Central Google Scholar
Munshi, N. C. et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med. 384 , 705–716 (2021).
Article CAS PubMed Google Scholar
Berdeja, J. G. et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 398 , 314–324 (2021).
Martin, T. et al. Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J. Clin. Oncol. 41 , 1265–1274 (2022).
Da Vià, M. C. et al. Homozygous BCMA gene deletion in response to anti-BCMA CAR T cells in a patient with multiple myeloma. Nat. Med. 27 , 616–619 (2021).
Article PubMed Google Scholar
Samur, M. K. et al. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat. Commun. 12 , 868 (2021).
Gagelmann, N. et al. Access to and affordability of CAR T-cell therapy in multiple myeloma: an EBMT position paper. Lancet Haematol. 9 , e786–e795 (2022).
Gazeau, N. et al. Effective anti-BCMA retreatment in multiple myeloma. Blood Adv. 5 , 3016–3020 (2021).
Deng, H. et al. Efficacy of humanized anti-BCMA CAR T cell therapy in relapsed/refractory multiple myeloma patients with and without extramedullary disease. Front. Immunol. 12 , 720571 (2021).
Article PubMed PubMed Central Google Scholar
Haradhvala, N. J. et al. Distinct cellular dynamics associated with response to CAR-T therapy for refractory B cell lymphoma. Nat. Med. 28 , 1848–1859 (2022).
Sheih, A. et al. Clonal kinetics and single-cell transcriptional profiling of CAR-T cells in patients undergoing CD19 CAR-T immunotherapy. Nat. Commun. 11 , 219 (2020).
Bai, Z. et al. Single-cell antigen-specific landscape of CAR T infusion product identifies determinants of CD19-positive relapse in patients with ALL. Sci. Adv. 8 , eabj2820 (2022).
Boiarsky, R. et al. Single cell characterization of myeloma and its precursor conditions reveals transcriptional signatures of early tumorigenesis. Nat. Commun. 13 , 7040 (2022).
Cohen, Y. C. et al. Identification of resistance pathways and therapeutic targets in relapsed multiple myeloma patients through single-cell sequencing. Nat. Med . 27 , 491–503 (2021).
Dutta, A. K. et al. Single-cell profiling of tumour evolution in multiple myeloma—opportunities for precision medicine. Nat. Rev. Clin. Oncol . 19 , 223–236 (2022).
Ledergor, G. et al. Single cell dissection of plasma cell heterogeneity in symptomatic and asymptomatic myeloma. Nat. Med. 24 , 1867–1876 (2018).
Merz, M. et al. Deciphering spatial genomic heterogeneity at a single cell resolution in multiple myeloma. Nat. Commun. 13 , 807 (2022).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184 , 3573–3587 (2021).
Phipson, B. et al. propeller: testing for differences in cell type proportions in single cell data. Bioinformatics 38 , 4720–4726 (2022).
van der Leun, A. M., Thommen, D. S. & Schumacher, T. N. CD8 + T cell states in human cancer: insights from single-cell analysis. Nat. Rev. Cancer 20 , 218–232 (2020).
Cohen, A. D. et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Invest. 129 , 2210–2221 (2019).
Nakamura, K., Smyth, M. J. & Martinet, L. Cancer immunoediting and immune dysregulation in multiple myeloma. Blood 136 , 2731–2740 (2020).
Merz, M. et al. Spatiotemporal assessment of immunogenomic heterogeneity in multiple myeloma. Blood Adv . 7 , 718–733 (2022).
Zavidij, O. et al. Single-cell RNA sequencing reveals compromised immune microenvironment in precursor stages of multiple myeloma. Nat. Cancer 1 , 493–506 (2020).
Clements, A. N. & Warfel, N. A. Targeting PIM kinases to improve the efficacy of immunotherapy. Cells 11 , 3700 (2022).
Keane, N. A., Reidy, M., Natoni, A., Raab, M. S. & O’Dwyer, M. Targeting the Pim kinases in multiple myeloma. Blood Cancer J. 5 , e325 (2015).
Chatterjee, S. et al. Targeting PIM kinase with PD1 inhibition improves immunotherapeutic antitumor T-cell response. Clin. Cancer Res. 25 , 1036–1049 (2019).
Moesta, A. K., Li, X.-Y. & Smyth, M. J. Targeting CD39 in cancer. Nat. Rev. Immunol. 20 , 739–755 (2020).
Vucinic, V. et al. S287: factors influencing autologous lymphocyte collections for chimeric antigen receptor (CAR) T-cells—the role of T-cell senescence. HemaSphere 6 , 188 (2022).
Article Google Scholar
Chen, P.-H. et al. Activation of CAR and non-CAR T cells within the tumor microenvironment following CAR T cell therapy. JCI Insight 5 , e134612 (2020).
Dhodapkar, K. M. et al. Changes in bone marrow tumor and immune cells correlate with durability of remissions following BCMA CAR T therapy in myeloma. Blood Cancer Discov. 3 , 490–501 (2022).
Mathewson, N. D. et al. Inhibitory CD161 receptor identified in glioma-infiltrating T cells by single-cell analysis. Cell 184 , 1281–1298 (2021).
Melenhorst, J. J. et al. Decade-long leukaemia remissions with persistence of CD4 + CAR T cells. Nature 602 , 503–509 (2022).
Wang, B. et al. Chimeric antigen receptor T cell therapy in the relapsed or refractory multiple myeloma with extramedullary disease—a single institution observation in China. Blood 136 , 6 (2020).
García-Guerrero, E. et al. All- trans retinoic acid works synergistically with the γ-secretase inhibitor crenigacestat to augment BCMA on multiple myeloma and the efficacy of BCMA-CAR T cells. Haematologica 108 , 568–580 (2023).
Merz, M. et al. Cytogenetic subclone formation and evolution in progressive smoldering multiple myeloma. Leukemia 34 , 1192–1196 (2020).
Merz, M. et al. Prognostic significance of cytogenetic heterogeneity in patients with newly diagnosed multiple myeloma. Blood Adv. 2 , 1–9 (2017).
Kumar, S. et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 17 , e328–e346 (2016).
Campbell, T. et al. Uses of anti-BCMA chimeric antigen receptors. Worldwide patent WO2021091978A1 (2021).
Schecter, J. M. & Fan, X. BCMA-targeted CAR-T cell therapy for multiple myeloma. Worldwide patent WO2022116086A1 (2022).
Germain, P.-L., Lun, A., Meixide, C. G., Macnair, W. & Robinson, M. D. Doublet identification in single-cell sequencing data using scDblFinder. F1000Res. 10 , 979 (2022).
Aran, D. et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 20 , 163–172 (2019).
Koh, W. & Hoon, S. MapCell: learning a comparative cell type distance metric with Siamese neural nets with applications toward cell-type identification across experimental datasets. Front. Cell Dev. Biol. 9 , 767897 (2021).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177 , 1888–1902 (2019).
Andreatta, M. et al. Interpretation of T cell states from single-cell transcriptomics data using reference atlases. Nat. Commun. 12 , 2965 (2021).
Andreatta, M., Berenstein, A. J. & Carmona, S. J. scGate: marker-based purification of cell types from heterogeneous single-cell RNA-seq datasets. Bioinformatics 38 , 2642–2644 (2022).
Fu, R. et al. clustifyr: an R package for automated single-cell RNA sequencing cluster classification. F1000Res. 9 , 223 (2020).
Tirosh, I. et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352 , 189–196 (2016).
Borcherding, N., Bormann, N. L. & Kraus, G. scRepertoire: an R-based toolkit for single-cell immune receptor analysis. F1000Res. 9 , 47 (2020).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16 , 1289–1296 (2019).
Wu, T. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation 2 , 100141 (2021).
CAS PubMed PubMed Central Google Scholar
Tickle, T., Tirosh, I., Brown, M. & Haas, B. InferCNV: inferring copy number alterations from tumor single cell RNA-seq data. GitHub https://github.com/broadinstitute/inferCNV/wiki (2023).
Wang, Y. et al. iTALK: an R package to characterize and illustrate intercellular communication. Preprint at bioRxiv https://doi.org/10.1101/507871 (2019).
Dimitrov, D. et al. Comparison of methods and resources for cell–cell communication inference from single-cell RNA-seq data. Nat. Commun. 13 , 3224 (2022).
Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566 , 496–502 (2019).
Blache, U. et al. Advanced flow cytometry assays for immune monitoring of CAR-T cell applications. Front. Immunol. 12 , 658314 (2021).
Download references
Acknowledgements
We thank S. Scharf from the Leipzig Medical Biobank for handling cryopreserved samples and D. Bretschneider, C. Mueller, K. Wildenberger and C. Wilhelm for performing fluorescence in situ hybridization analyses on clinical samples. We express our gratitude toward the participants and their families who participated in this analysis. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SPP µbone) and EU HORIZON Project CERTAINTY (101136379), and M.M. received financial support from grants from the International Myeloma Society, SpringWorks and Janssen.
Author information
Deceased: Sebastian Schwind.
These authors contributed equally: Michael Rade, Nora Grieb.
These authors jointly supervised this work: Kristin Reiche, Vladan Vucinic, Maximilian Merz.
Authors and Affiliations
Fraunhofer Institute for Cell Therapy and Immunology IZI, Leipzig, Germany
Michael Rade, Ronald Weiss, Ulrike Köhl & Kristin Reiche
Department of Hematology, Cellular Therapy and Hemostaseology, University Hospital of Leipzig, Leipzig, Germany
Nora Grieb, Luise Fischer, Patrick Born, Christina Kloetzer, Simone Heyn, Anne Sophie Kubasch, Song Yau Wang, Enrica Bach, Sandra Hoffmann, Jule Ussmann, Birthe Schetschorke, Saskia Hell, Sebastian Schwind, Klaus H. Metzeler, Marco Herling, Madlen Jentzsch, Georg-Nikolaus Franke, Uwe Platzbecker, Vladan Vucinic & Maximilian Merz
Innovation Center Computer Assisted Surgery, University Hospital of Leipzig, Leipzig, Germany
Institute for Clinical Immunology, University Hospital of Leipzig, Leipzig, Germany
Ronald Weiss, Andreas Boldt, Stephan Fricke, Paul Franz, Ulrich Sack, Ulrike Köhl & Kristin Reiche
Singleron Biotechnologies, Cologne, Germany
Jaren Sia, Jonathan Scolnick, Lakshmi Venkatraman & Stacy Xu
Institute for Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics, University Hospital Leipzig, Leipzig, Germany
Ronny Baber
Leipzig Medical Biobank, University Leipzig, Leipzig, Germany
Center for Scalable Data Analytics and Artificial Intelligence (ScaDS.AI), Dresden, Leipzig, Germany
Kristin Reiche
You can also search for this author in PubMed Google Scholar
Contributions
Conception and design: M.R., N.G., R.W., V.V. and M.M. Acquisition of data (acquired and managed participants, provided facilities, fluorescence in situ hybridization, flow cytometry, biobanking, in vitro studies and so on): L.F., P.B., A.B., S.F., P.F., C.K., S. Heyn, A.S.K., R.B., S.Y.W., E.B., S. Hoffmann, J.U., B.S., S. Hell, M.J., S.S., K.H.M., G.-N.F., M.H., U.S., K.R., U.K., U.P., V.V. and M.M. Analysis and interpretation of data (for example, statistical analysis, biostatistics and computational analysis): M.R., N.G., R.W., J. Sia, S.X., L.F., J. Scolnick, L.V., K.R., V. V. and M.M. Writing, review and/or revision of the manuscript: all authors.
Corresponding author
Correspondence to Maximilian Merz .
Ethics declarations
Competing interests.
S.F.: consultant and/or speaker fees from Novartis Pharma, Janssen-Cilag, Vertex Pharmaceuticals (Germany), Kite/Gilead Sciences, MSGO and Bristol-Myers Squibb. U.K.: consultant and/or speaker fees from AstraZeneca, Affimed, Glycostem, GammaDelta, Zelluna, Miltenyi Biotec and Novartis Pharma and Bristol-Myers Squibb. M.M.: advisory boards/honoraria/research support from Amgen, BMS, Celgene, Gilead, Janssen, Stemline, Springworks and Takeda. K.H.M.: BMS (consultancy and honoraria), AbbVie (honoraria, research funding), Pfizer (honoraria), Otsuka (honoraria), Janssen (honoraria) and Novartis (consultancy). U.P.: Syros (consultancy, honoraria, research funding), MDS Foundation (membership on an entity’s Board of Directors or advisory committees), Silence Therapeutics (consultancy, honoraria, research funding), Celgene (honoraria), Takeda (consultancy, honoraria, research funding), Fibrogen (research funding), Servier (consultancy, honoraria, research funding), Roche (research funding), Merck (research funding), Amgen (consultancy, research funding), Novartis (consultancy, honoraria, research funding), AbbVie (consultancy), Curis (consultancy, research funding), Janssen Biotech (consultancy, research funding), Jazz (consultancy, honoraria, research funding), BeiGene (research funding), Geron (consultancy, research funding) and Bristol-Myers Squibb (consultancy, honoraria, membership on an entity’s Board of Directors or advisory committees, other, travel support, medical writing support, research funding). M.J.: Novartis (honoraria), Amgen (honoraria), Pfizer (honoraria), Blueprint Medicine (honoraria), BMS (honoraria) and Jazz (honoraria). The remaining authors declare no competing interests.
Peer review
Peer review information.
Nature Cancer thanks Marco Davila and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 enrichment analysis for post-infusional bmmcs and pbmcs..
GO term enrichment analysis of significantly differentially expressed genes (non-responders vs. responders) for BMMCs and PBMCs. Terms are ranked by rich factor, which is the number of DE genes in the term divided by the number of background genes in that term. Dot plot depict the highest ranked significantly enriched GO terms for biological processes (FDR < 0.05). The dot size indicates the z-score, which is the number of DE genes with logFC >0 minus the number of DE genes with logFC <0 divided by the square root of the number of term-associated genes. Grey/white dots indicate the same number of genes with a logFC >0 and <0. Cell type abbreviations: NK = natural killer; Mono = monocyte; cDC = classical dendritic cell.
Source data
Extended data fig. 2 comparing post- and pre-infusional bmmcs and pbmcs in noncr and cr..
a , Comparison between post- and pre-infusional cell types in PBMCs from responders (CR) and non-responders (nonCR). The number of biologically independent samples for the contrasts is as follows. CR: n = 4 for post- and n = 5 for pre-infusional groups. nonCR: n = 5 for post- and n = 5 for pre-infusional groups. Significant differences (unadjusted p-values) were estimated using the empirical Bayes moderated t-statistics (two-sided) implemented in the speckle package (* p = 0.1, ** p = 0,05, *** p = 0.01, **** p = 0.001, p < 0.0001 = *****). The exact p-values for CR are as follows: NK (p = 0.00210), NK_CD56bright (p = 0.01849), B memory (p = 0.08501) and pDC (p = 0,02643), B intermediate (p = 0.074792) and HSPC (p = 0,09701). The exact p-value for nonCR, is as follows: CD14 Mono (p = 0.03526). The center of the box plots indicates the median, and the upper and lower bounds of the boxes indicate first and third interquartile ranges. Whiskers extends to 1.5 x interquartile range. b , Differential gene expression analysis comparing post- with pre-infusional BMMCs and PBMCs in CR and nonCR Only cell types with significant genes are shown (FDR < 0.05 and fold change <1.5 or >1.5). A positive log fold change indicates upregulation in post-infusional samples. c , GO term enrichment analysis of significantly differentially expressed genes (post- vs. pre-infusional). Terms are ranked by rich factor, which is the number of DE genes in the term divided by the number of background genes in that term. Dot plots depict the highest ranked significantly enriched GO terms for biological processes (FDR < 0.05). The dot size indicates the z-score, which is the number of DE genes with logFC >0 minus the number of DE genes with logFC <0 divided by the square root of the number of term-associated genes. Grey/white dots indicate the same number of genes with a logFC >0 and <0. Cell type abbreviations: NK = natural killer; gdT-Cell = gamma delta T cell; Mono = monocyte; cDC = classical dendritic cell; pDC = plasmacytoid dendritic cell; Cilta cel = Ciltacabtagene autoleucel; Ide cel = Idecabtagene vicleucel; GMP = granulocyte-monocyte progenitor, HSPC = Hematopoietic stem and progenitor cell.
Extended Data Fig. 3 Comparing post- with pre-infusional T cell subtypes in nonCR and CR.
a , Differences in T cell subtype composition between post- and pre-infusional PBMCs from CR and nonCR. For each cell type, the log fold change in mean cell fraction between post- and pre-infusional samples was calculated with the R package speckle based on arcsin square root transformation. The cell fraction calculation includes all cell types (the denominator is the sum of all cells analyzed). For clarity, only cell types with a fold change >1.5 are shown. b , GO term enrichment analysis for DEGs comparing post- with pre-infusional PBMCs in CR and nonCR. GO terms are ranked by rich factor, which is the number of DE genes in the term divided by the number of background genes in that term. Dot plot depict the highest ranked significantly enriched GO terms for biological processes (FDR < 0.05). Cell type abbreviations: CM = central memory; EM = effector memory; TEMRA = effector memory cells re-expressing CD45RA; TPEX = precursor exhausted T cells; TEX = exhausted T cell; MAIT = Mucosal-associated invariant T cell; CTL_EOMES = eomesodermin expressing cytotoxic lymphocytes; CTL_GNLY = granulysin expressing cytotoxic lymphocytes; CTL_Exh = exhausted cytotoxic lymphocytes; Tfh T follicular helper cells; Treg = regulatory T cells; gdT = gamma delta T cell.
Extended Data Fig. 4 Enrichment analysis in CAR T cells (CD4 and CD8) compared to pre-infusional T cells from patients treated with Ide-cel.
GO term enrichment analysis of significantly differentially expressed genes (CAR vs. pre-infusional T cells) in participants 12 ( a ) and 14 ( b ). Terms are ranked by rich factor, which is the number of DE genes in the term divided by the number of background genes in that term. Dot plot depict the highest ranked significantly enriched GO terms for biological processes (FDR < 0.05).
Extended Data Fig. 5 Enrichment analysis in CAR T cells (T cell subtypes) compared to pre-infusional T cells from patients treated with Ide-cel.
a , GO term enrichment analysis of significantly differentially expressed genes (CAR vs. pre-infusional T cells) in PBMCs for patient 12. No significantly enriched GO terms were found for patient 14. b , Differential cell surface protein expression for CD4 and CD8 T-cells when comparing CAR+ from PBMCs with CAR+ from BMMCs for patient 12 (BMMCs CD4 n = 41; CD8 430 // PBMCs CD4 n = 214; CD8 4699). Genes with an FDR < 0.05 and fold change <1.5 or >1.5 were considered as significantly differentially expressed. Cell type abbreviations: CM = central memory; EM = effector memory; TEMRA = effector memory cells re-expressing CD45RA; TEX = exhausted T cell.
Extended Data Fig. 6 Analysis of CD38 surface protein expression in patient 01 with samples available after exposure/becoming refractory to a CD38 antibody as well as after discontinuing the antibody.
Patient 01 had progressed with a plasma cell leukemia after frontline treatment with 4 cycles bortezomib/cyclophosphamide/dexamethasone (VCD), high-dose melphalan and autologous transplantation (HDM + ASCT) and lenalidomide (Len) maintenance. Circulating malignant plasma cells (n = 7071) showed homogeneous CD38 protein expression, which was significantly decreased 4 days after starting Daratumumab/Carfilzomib/Dexamethasone (Dara/Carf/Dex). The patient relapsed 9 months later. CD38 expression on circulating malignant plasma cells significantly increased after 4 days of 3rd line treatment with Elotuzumab/Pomalidomide/Dexamethasone (Elo/Pom/Dex). CD38 expression levels increased further upon relapsed from treatment with Idecabtagene vicleucel (Ide-cel) 2 months after the last dose of Daratumumab.
Extended Data Fig. 7 Marker genes for T cell identities.
DE genes for each T cell identity were determined using the Wilcoxon rank-sum test. Only CAR negative cells were analyzed. Genes with an FDR < 0.05 and an absolute fold change >1.5 were considered statistically significant. Genes are ranked according to FDR. For each cell identity, 5 significant genes are shown. The color intensity indicates the standardized average expression level in a cell identity. The dot size indicates the percentage of expressing cells within each cell identity of the corresponding genes.
Supplementary information
Supplementary information.
Supplementary Figs. 1–8 (pseudotime trajectory analysis of preinfusional and postinfusional CD4 + CD8 + T cells and CAR T cells) and Supplementary Fig. 9 (gating strategy).
Reporting Summary
Supplementary tables.
Supplementary Table 1. Summary of ten individuals treated with Ide-cel or Cilta-cel (participants P7 and P8). Except for participants P8 and P10, all individuals had at least one high-risk cytogenetic feature detected by fluorescence in situ hybridization (mostly chromosome 1 abnormalities). Bridging therapy consisted mainly of antibody-based three-drug combinations. Participant P9 was treated with high-dose chemotherapy and autologous stem cell transplantation before CAR T cell infusion. Abbreviations: m, male; f, female; FISH, fluorescence in situ hybridization; Elo/Pom/Dex, elotuzumab/pomalidomid/dexamethasone; Benda/Carf/Dex, bendamustine/carfilzomib/dexamethasone; Dara/Pom/Dex, daratumumab/pomalidomide/dexamethasone; HD-Melphalan, high-dose melphalan; ASCT, autologous stem cell transplantation; Benda/Dex, bendamustine/dexamethasone; IsaPomDex, isatuximab/pomalidomide/dexamethasone; Dara/Len/Dex, daratumumab/lenalidomide/dexamethasone; Isa/Carf/Dex, isatuximab/carfilzomib/dexamethasone. Supplementary Table 2. Summary of samples and quality control data from scRNA-seq, TCR and BCR sequencing and surface proteomics. Abbreviations: PR, partial remission; PD, progressive disease; UMI, unique molecular identifier. Supplementary Table 3. Feature reference .csv file declaring antibody capture constructs and associated barcodes. The CellRanger multipipeline requires this configuration .csv file as input. Supplementary Table 4. Summary of differential gene expression analyses. Testing for differential gene expression was performed using the function FindMarkers (Wilcoxon rank-sum test) implemented in Seurat. Genes with an FDR of <0.05 (Benjamini–Hochberg correction) and fold change of <1.5 or >1.5 were considered DEGs. Summaries include comparisons of different cell types between nonresponders and responders from PBMCs after CAR T cell therapy (post-PBMC; non-CR versus CR), BMMCs after CAR T cell therapy (post-BMMC; non-CR versus CR), PBMCs collected at apheresis (pre-PBMC; non-CR versus CR) and comparisons of PBMCs after CAR T cell therapy versus PBMCs at apheresis in responders (CR; post versus pre) and nonresponders (non-CR; post versus pre). Supplementary Table 5. Summary of differential gene expression analyses for T cell identities. Testing for differential gene expression was performed using the function FindMarkers (Wilcoxon rank-sum test) implemented in Seurat. Genes with an FDR of <0.05 (Benjamini–Hochberg correction) and fold change of <1.5 or >1.5 were considered DEGs. Summaries include comparisons of T cell identities between nonresponders and responders from PBMCs at apheresis (pre-PB; T cell; non-CR versus CR), PBMCs after CAR T cell therapy (post-PB; T cell; non-CR versus CR), BMMCs after CAR T cell therapy (post-BM; T cell; non-CR versus CR) and comparisons of PBMCs after CAR T cell therapy versus PBMCs at apheresis in responders (CR; PB; T cell; post versus pre) and nonresponders (non-CR; PB; T cell post versus pre), CAR T cells versus preinfusional T cells in participant P12 (CAR versus pre-T cell) and CAR T cells versus preinfusional T cells in participant P14 (CAR versus pre-T cell).
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Source data fig. 3, source data fig. 4, source data fig. 5, source data fig. 6, source data fig. 7, source data fig. 8, source data extended data fig. 1, source data extended data fig. 2, source data extended data fig. 3, source data extended data fig. 4, source data extended data fig. 5, source data extended data fig. 6, source data extended data fig. 7, rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Rade, M., Grieb, N., Weiss, R. et al. Single-cell multiomic dissection of response and resistance to chimeric antigen receptor T cells against BCMA in relapsed multiple myeloma. Nat Cancer (2024). https://doi.org/10.1038/s43018-024-00763-8
Download citation
Received : 24 February 2023
Accepted : 26 March 2024
Published : 19 April 2024
DOI : https://doi.org/10.1038/s43018-024-00763-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.

IMAGES
VIDEO
COMMENTS
Light-chain multiple myeloma (LCMM) is a less frequent type of multiple myeloma (MM), with a more aggressive course and poorer prognosis. It is characterized by the inability of the malignant plasma cells to produce heavy chains, resulting in the exclusive production of light chains. Therefore, no M-spike is visible in serum protein ...
Daratumumab is a human IgGκ monoclonal antibody targeting CD38 with direct on-tumor 5-8 and immunomodulatory 9-11 mechanisms of action. Daratumumab has been approved for use in combination with ...
Managing patients with plasma cell neoplasms, diseases in which abnormal plasma cells or myeloma cells form tumors in the bones or soft tissues of the body, poses numerous challenges for clinicians. At the NCCN 2019 Annual Congress: Hematologic Malignancies, a panel of experts discussed evidenced-based approaches for the treatment of patients with these diseases. Moderated by Dr. Andrew D ...
presentation of multiple myeloma: a case series. Ann Med Health Sci Res 2019;9:586-590. 7. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003;78(1):21-33. 8.important. Lazarus HM, Kellermeyer RW, Aikawa M, et al. Multiple myeloma in young men clinical course and electron
multiple myeloma incidence and mortality by age, sex, country, and regions and their association with common lifestyle and metabolic risk factors around the world. Our study shows a wide variation in the burden of multiple myeloma, with higher incidence and mortality observed in men and countries with higher human development index.
Objectives Patients with multiple myeloma (MM) experience significant delays in diagnosis due to non-specific symptomatology. The aim of this study was to characterise the frequency and timing of clinical features in the primary care setting prior to MM diagnosis. Design Population-based cohort study. Setting Electronic health records data of approximately 17 million patients (2006-2016 ...
Objective: This case study describes a patient diagnosed with early manifestations of multiple myeloma and illustrates relevant aspects of differential diagnosis and the use of laboratory, radiologic, and advanced imaging techniques to aid in establishing the diagnosis and issues of management. Clinical features: A 36-year-old male student experienced midback pain that occurred primarily at ...
The goal of this case study was to show that it could be interesting to think about the association of myeloma ... Kim YJ, Kim SJ, Min K, Kim HY, Kim HJ, Lee YK, Zang DY: Multiple myeloma with myelomatous pleural effusion: a case report and review of the literature. Acta Haematol. 2008, 120:108-11. 10.1159/000165694
We report here one of the rare variants of multiple myeloma, namely IgD-kappa multiple myeloma. Its clinical course, response to therapy and outcome is highlighted. CASE REPORT A 71-year-old man, with a background history of diabetes mellitus, hypertension and chronic kidney disease, was referred to our center for multiple myeloma work-up as ...
PDF | A total of 399 patients with multiple myeloma and an equal number of match controls were interviewed about factors possibly related to the causes... | Find, read and cite all the research ...
The diagnosis of multiple myeloma is based on the presence of at least 10% plasma cells in the bone marrow sample and the presence of monoclonal protein in serum or urine. In patients with Nonsecretory myeloma, the diagnosis is based on the presence of 30% plasma cells or evidence of plasmacytoma in the bone marrow [3].
Multiple myeloma can occasionally manifest with joint disease. We report the case of an individual with a progressive bilateral carpal syndrome and a symmetrical severe seronegative polyarthritis and joint swelling. Investigations revealed an erosive seronegative inflammatory arthritis in association with bilateral carpal tunnel syndrome, anaemia, hepatic impairment and nephrotic-range ...
The diagnostic criteria are summarized in Table 1. The International Myeloma Working Group produced updated guidelines in 2014, which now include patients with > 60% bone marrow involvement of plasma cells, serum FLC ratio of > 100, and > 1 focal lesions on an MRI study as symptomatic MM. 5, 6.
Pulmonary parenchyma is an uncommon site. of extramedullary involvement in multiple. myeloma; on ly isolated cases with h istologica l. proofs have been reported in the literature. One study ...
For the patient in this case study, prophylaxis with an LMWH or full-dose 12 February 2010 warfarin should be initiated as she possessed multiple risk factors for VTE, such as advanced age, obesity, immobility, history of breast cancer, and newly diagnosed myeloma.
Download book PDF. Download book EPUB ... The following case studies on multiple myeloma demonstrate these alterations (Annexure). Download chapter PDF. 1 Case Study 11.1: Multiple Myeloma with IgH Gene Rearrangement, t(11;14) Brief Clinical History. A 77-year-old male patient presented with complaints of backache for a few weeks. Bone marrow ...
Abstract. A total of 399 patients with multiple myeloma and an equal number of match controls were interviewed about factors possibly related to the causes of their disease. Factors studied included occupation, chemical exposure, radiation exposure, prior diseases, immunizations, chronic infections and markers for defects in immune regulation.
PDF | Multiple myeloma (MM) is the second most common hematological cancer and causes significant mortality and morbidity. ... alcohol, epidemiology, family case-control study, multiple myeloma ...
Multiple Myeloma Case Studies (Tandem 2017) (Final)(1) - Free download as PDF File (.pdf), Text File (.txt) or view presentation slides online. multiple myeloma
What is Multiple Myeloma? Multiple myeloma starts in the plasma cells in bone mar-row. The bone marrow is the soft, spongy tissue found in the center of many bones where blood cells are produced. When the plasma cells become malignant, they grow out of control, crowd out the normal blood cells that help fight infection and disease, and form a ...
Myrlande M, and Vikram P presented a rare case of triclonal gammopathy in a patient with smoldering Plasma Cell Myeloma . Multiple myeloma is an unusual malignancy seen in nearly 10% of all hematological malignancies. According to Aksungar FB, et al., the most common triclonal pattern of M-protein includes IgG κ, IgG λ, IgA κ. In the present ...
This case is very interesting, and I will allude to that as we talk about therapy for the relapse. This patient had t(11;14), which is present in about 15% of myeloma patients, and this has turned ...
Request PDF | A Case Study on Multiple Myeloma | Introduction: Multiple myeloma (MM), also known as plasma cell myeloma and simply myeloma, is a cancer of plasma cells, a type of white blood cell ...
Markers that predict response and resistance to chimeric antigen receptor (CAR) T cells in relapsed/refractory multiple myeloma are currently missing. We subjected mononuclear cells isolated from ...
Multiple Myeloma Case Study - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free.