- Privacy Policy

Read Chemistry
Concentration of solutions: definitions, formulas, solved problems.
Analytical Chemistry , General Chemistry
– In this subject, we will discuss the Concentration of Solutions Concentration of Solutions (Definitions, Formulas, Solved Problems).

Concentration of Solutions
– For history, measurements and their corresponding units were invented at the local level.
– By necessity of primitive communication and local technology, standards were nearly nonexistent, and conversions among the many systems were difficult.
– The result was many hundreds of distinct ways of expressing concentrations of solutions.
– Fortunately for us, the advent of rapid communications technology and the development of efficient travel have forced the globalization of measurement science and, along with it, the definition of global measurement standards.
– No field has enjoyed more benefit in this regard than chemistry in general and analytical chemistry in particular.
– Even so, we use several methods for expressing concentration.
Ways for Expressing Concentration of Solutions
– In this subject, we describe the four fundamental ways of expressing solution concentration:
(1) molar concentration
(2) percent concentration
(3) solution-diluent volume ratio
(4) p-functions.
(1) Molar Concentration
– The molar concentration c x of a solution of a solute species X is the number of moles of that species that is contained in 1 liter of the solution (not 1 L of the solvent).
– In terms of the number of moles of solute, n, and the volume, V, of solution, we write:
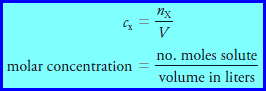
– The unit of molar concentration is molar, symbolized by M, which has the dimensions of mol/L, or mol L -1 .
– Molar concentration is also the number of millimoles of solute per milliliter of solution.
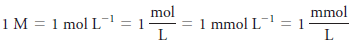
Example (1): Calculate the molar concentration of ethanol in an aqueous solution that contains 2.30 g of C 2 H 5 OH (46.07 g/mol) in 3.50 L of solution.
– To calculate molar concentration, we must find both the amount of ethanol and the volume of the solution.
– The volume is given as 3.50 L, so all we need to do is convert the mass of ethanol to the corresponding amount of ethanol in moles:
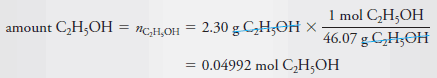
– To obtain the molar concentration, c C2H5OH , we divide the amount by the volume. Thus:
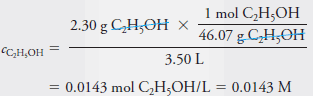
– We will see that there are two ways of expressing molar concentration:
- molar analytical concentration
- molar equilibrium concentration.
– The distinction between these two expressions is in whether the solute undergoes a chemical change in the solution process.
(A) Molar Analytical Concentration
– The molar analytical concentration, or for the sake of brevity, just analytical concentration, of a solution gives the total number of moles of a solute in 1 liter of the solution (or the total number of millimoles in 1 mL).
– In other words, the molar analytical concentration specifies a recipe by which the solution can be prepared regardless of what might happen to the solute during the solution process.
– Note that in Example (1), the molar concentration that we calculated is also the molar analytical concentration c C2H5OH = 0.0143 M because the solute ethanol molecules are intact following the solution process.
– In another example, a sulfuric acid solution that has an analytical concentration of c H2SO4 = 1.0 M can be prepared by dissolving 1.0 mole, or 98 g, of H 2 SO 4 in water and diluting the acid to exactly 1.0 L.
– As we shall see, there are important differences between the ethanol and sulfuric acid examples.
(B) Molar Equilibrium Concentration
– The molar equilibrium concentration, or just equilibrium concentration, refers to the molar concentration of a particular species in a solution at equilibrium.
– To specify the molar equilibrium concentration of a species, it is necessary to know how the solute behaves when it is dissolved in a solvent.
– For example, the molar equilibrium concentration of H 2 SO 4 in a solution with a molar analytical concentration c H2SO4 = 1.0 M is actually 0.0 M because the sulfuric acid is completely dissociated into a mixture of H + , HSO 4 – , SO 4 – ions.
– There are essentially no H 2 SO 4 molecules in this solution.
– The equilibrium concentrations of the ions are 1.01, 0.99, and 0.01M, respectively.
– Equilibrium molar concentrations are usually symbolized by placing square brackets around the chemical formula for the species.
– So, for our solution of H 2 SO 4 with an analytical concentration of c H2SO4 = 1.0 M, we write:
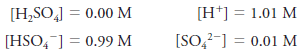
Solved Problems on Molar Concentration
Example (2): Calculate the analytical and equilibrium molar concentrations of the solute species in an aqueous solution that contains 285 mg of trichloroacetic acid, Cl 3 CCOOH (163.4 g/mol), in 10.0 mL (the acid is 73% ionized in water).
– As in Example (1), we calculate the number of moles of Cl 3 CCOOH, which we designate as HA, and divide by the volume of the solution, 10.0 mL, or 0.0100 L. Therefore:
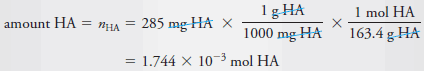
– The molar analytical concentration, c HA , is then:
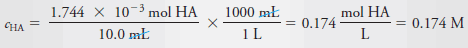
– In this solution, 73% of the HA dissociates, giving H + and A – :
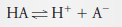
– The equilibrium concentration of HA is then 27% of c HA . Thus,
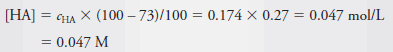
– The equilibrium concentration of A – is equal to 73% of the analytical concentration of HA, that is
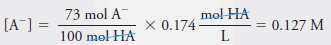
– Because 1 mole of H + is formed for each mole of A – , we can also write:
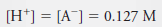
Example (3): Describe the preparation of 2.00 L of 0.108 M BaCl 2 from BaCl 2 .2H 2 O (244.3 g/mol).
– To determine the number of grams of solute to be dissolved and diluted to 2.00 L, we note that 1 mole of the dihydrate yields 1 mole of BaCl 2 .
– Therefore, to produce this solution we will need:
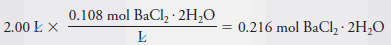
– The mass of BaCl 2 .2H 2 O is then:
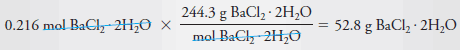
– Dissolve 52.8 g of BaCl 2 .2H2O in water and dilute to 2.00 L.
Example (4): Describe the preparation of 500 mL of 0.0740 M Cl 2 solution from solid BaCl 2 .2H 2 O (244.3 g/mol).
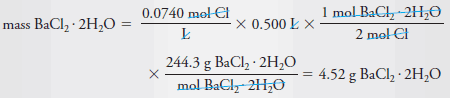
– Dissolve 4.52 g of BaCl 2 .2H 2 O in water and dilute to 0.500 L or 500 mL.
(2) Percent Concentration
– Chemists frequently express concentrations in terms of percent (parts per hundred).
– Unfortunately, this practice can be a source of ambiguity because the percent composition of a solution can be expressed in several ways.
– Three common methods are:
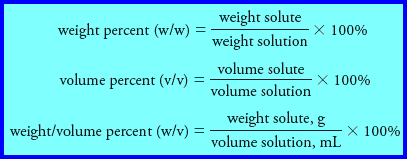
(1) the denominator in each of these expressions is the mass or volume of solution rather than the mass or volume of the solvent.
(2) the first two expressions do not depend on the units used for weight (mass) as long as the same units are used in the numerator and the denominator.
(3) In the third expression, units must be defined because the numerator and denominator have different units that do not cancel.
(4) Of the three expressions, only weight percent has the advantage of being temperature independent.
– Weight percent is often used to express the concentration of commercial aqueous reagents.
– For example, nitric acid is sold as a 70% (w/w) solution, meaning that the reagent contains 70 g of HNO 3 per 100 g of solution (see Example 8).
– Volume percent is commonly used to specify the concentration of a solution prepared by diluting a pure liquid compound with another liquid.
– For example, a 5% (v/v) aqueous solution of methanol usually describes a solution prepared by diluting 5.0 mL of pure methanol with enough water to give 100 mL.
– Weight or volume percent is often used to indicate the composition of dilute aqueous solutions of solid reagents.
– For example, 5% (w/v) aqueous silver nitrate often refers to a solution prepared by dissolving 5 g of silver nitrate in sufficient water to give 100 mL of solution.
– To avoid uncertainty, always specify explicitly the type of percent composition being discussed.
– If this information is missing, the investigator must decide intuitively which of the several types is to be used.
– The potential error resulting from a wrong choice is considerable.
– For example, commercial 50% (w/w) sodium hydroxide contains 763 g NaOH per liter, which corresponds to 76.3% (w/v) sodium hydroxide.
Parts per Million and Parts per Billion
– For very dilute solutions, parts per million ( ppm ) is a convenient way to express concentration:

– where c ppm is the concentration in parts per million.
– The units of mass in the numerator and denominator must agree so that they cancel.
– For even more dilute solutions, 10 9 ppb rather than 10 6 ppm is used in the previous equation to give the results in parts per billion (ppb).
– The term parts per thousand (ppt) is also used, especially in oceanography.
Example (5): What is the molar concentration of K + in a solution that contains 63.3 ppm of K 3 Fe(CN) 6 (329.3 g/mol)?
– Because the solution is so dilute, it is reasonable to assume that its density is 1.00 g/mL. therefore:
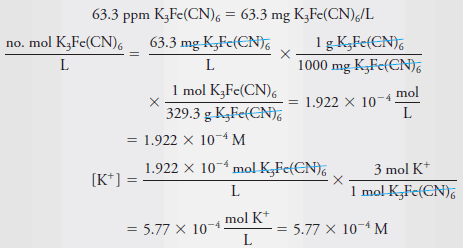
(3) Solution-Diluent Volume Ratios
– The composition of a dilute solution is sometimes specified in terms of the volume of a more concentrated solution and the volume of solvent used in diluting it.
– The volume of the former is separated from that of the latter by a colon.
– Thus, a 1:4 HCl solution contains four volumes of water for each volume of concentrated hydrochloric acid.
– This method of notation is frequently ambiguous in that the concentration of the original solution is not always obvious to the reader.
– Moreover, under some circumstances, 1:4 means diluting one volume with three volumes.
– Because of such uncertainties, you should avoid using solution-diluent ratios.
(4) p-Functions
– Scientists frequently express the concentration of a species in terms of its p-function, or p-value.
– The p-value is the negative logarithm (to the base 10) of the molar concentration of that species. Thus, for the species X:
As shown by the following examples, p-values offer the advantage of allowing concentrations that vary over ten or more orders of magnitude to be expressed in terms of small positive numbers.
Example (6): Calculate the p-value for each ion in a solution that is 2.00 × 10 -3 M in NaCl and 5.4 × 10 -4 M in HCl.
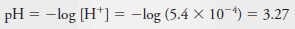
– To obtain pNa, we write:
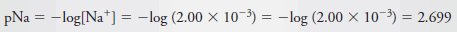
– The total Cl 2 concentration is given by the sum of the concentrations of the two solutes:
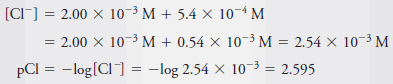
Example (7): Calculate the molar concentration of Ag + in a solution that has a pAg of 6.372.
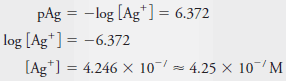
Density and Specific Gravity of Solutions
– Density and specific gravity are related terms often found in the analytical literature.
– The density of a substance is its mass per unit volume, and its specific gravity is the ratio of its mass to the mass of an equal volume of water at 4°C.
– Density has units of kilograms per liter or grams per milliliter in the metric system. Specific gravity is dimensionless and so is not tied to any particular system of units. For this reason, specific gravity is widely used in describing items of commerce.
– The following Figure shows the Label from a bottle of reagent-grade hydrochloric acid.
– Note that the specific gravity of the acid over the temperature range of 60° to 80°F is specified on the label:

– Since the density of water is approximately 1.00 g/mL and since we use the metric system throughout this text, we use density and specific gravity interchangeably.
– The specific gravities of some concentrated acids and bases are given in the following Table:
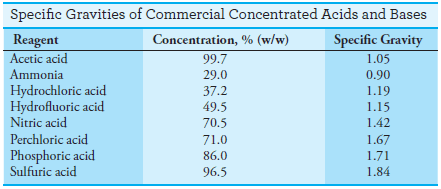
Solved Problems on Concentration
Example (8): Calculate the molar concentration of HNO 3 (63.0 g/mol) in a solution that has a specific gravity of 1.42 and is 70.5% HNO 3 (w/w).
Let us first calculate the mass of acid per liter of concentrated solution:
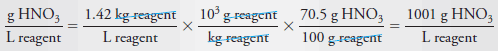
Example (9): Describe the preparation of 100 mL of 6.0 M HCl from a concentrated solution that has a specific gravity of 1.18 and is 37% (w/w) HCl (36.5 g/mol).
– Proceeding as in Example (8), we first calculate the molar concentration of the concentrated reagent.
– We then calculate the number of moles of acid that we need for the diluted solution.
– Finally, we divide the second figure by the first to obtain the volume of concentrated acid required.
– Thus, to obtain the concentration of the reagent, we write:
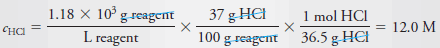
– The number of moles HCl required is given by:
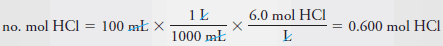
– Finally, to obtain the volume of concentrated reagent, we write:
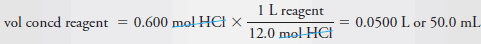
– Therefore, dilute 50 mL of the concentrated reagent to 600 mL.
– The solution to Example (9) is based on the following useful relationship, which we will be using countless times:
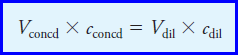
– where the two terms on the left are the volume and molar concentration of a concentrated solution that is being used to prepare a diluted solution having the volume and concentration given by the corresponding terms on the right.
– This equation is based on the fact that the number of moles of solute in the diluted solution must equal the number of moles in the concentrated reagent.
– Note that the volumes can be in milliliters or liters as long as the same units are used for both solutions.
Reference: Fundamentals of analytical chemistry / Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch. (ninth edition), 2014. USA
Related Articles
Titration Curves in Analytical Chemistry : Definition, Types
11 hours ago

Gravimetric Titrations | Definition, Calculations & Advantages
12 hours ago

Some Terms Used in Volumetric Titration
Applications of Gravimetric methods
Precipitation Gravimetry
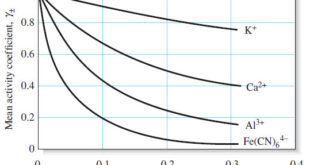
Activity Coefficients : Definition, Equation, Examples, Properties
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Concentration of Solutions
This is a series of lectures in videos covering Chemistry topics taught in High Schools. These lessons look at calculating the concentration of a solution.
Related Pages IGCSE Chemistry High School Chemistry More Lessons for Chemistry
Concentrations Calculations (% weight/volume) A tutorial on calculating the concentration of a solution in % weight-volume.
Example: You dissolve 2.5 moles of strantium acetate into 1.0 L of water. Determine the concentration of the solution in %W/C.
Concentration of Solutions: Volume/Volume % (v/v) A volume/volume percent (v/v) gives the volume of solute divided by the volume of the solution (expressed as a percent). The following video looks at calculating concentration of solutions. We will look at a sample problem dealing with volume/volume percent (v/v)%
Example: Rubbing alcohol is commonly used as an antiseptic for small cuts. It is sold as 70% (v/v) solution of isopropyl alcohol in water. What volume of isopropyl alcohol is used to make 500 mL of rubbing alcohol?
Concentration of Solutions: mass/volume % (m/v)% The following video looks at calculating concentration of solutions. We will look at a sample problem dealing with mass/volume percent (m/v)%.
Example: Many people use a solution of sodium phosphate (Na 3 PO 4 - commonly called TSP), to clean walls before putting up wallpaper. The recommended concentration is 1.7%(m/v). What mass of TSP is needed to make 2.0 L of solution?
Concentration of Solutions: Mass/Mass % (m/m)% A mass/mass percent gives the mass of a solute divided by the mass of solution (expressed as a percent) The following video looks at calculating concentration of solutions. We will look at a sample problem dealing with mass/mass percent (m/m)%
Example: CaCl 2 is used to melt ice on roads. To determine how much CaCl 2 has been used, you take a sample of slush to analyze. The sample had a mass of 23.47g. When the solution was evaporated, the residue had a mass of 4.58g. What was the mass/mass percent of CaCl 2 in the slush? How many grams of CaCl 2 were present in 100g of solution?

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.
- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- Browse Articles
- Learn Something New
- Quizzes Hot
- This Or That Game
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications
- Chemistry Calculations
How to Calculate the Concentration of a Solution
Last Updated: March 20, 2024 Fact Checked
This article was co-authored by Chris Hasegawa, PhD and by wikiHow staff writer, Hunter Rising . Dr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona. There are 8 references cited in this article, which can be found at the bottom of the page. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed 2,131,194 times.
In chemistry, a solution’s concentration is how much of a dissolvable substance, known as a solute, is mixed with another substance, called the solvent. The standard formula is C = m/V, where C is the concentration, m is the mass of the solute dissolved, and V is the total volume of the solution. If you have a small concentration, find the answer in parts per million (ppm) to make it easier to follow. In a lab setting, you may be asked to find the molarity , or molar concentration, of the solution instead.
Using the Mass per Volume Equation

- If the solute you’re using is a liquid, then you can also calculate the mass using the density formula, where density D = m/V, where m is the mass of the liquid and V is the volume. To find the mass, multiply the density of the liquid by the volume.
Tip: If you need to use a scale, subtract the mass of the container you’re using to hold the solute or else your calculations will be off.

- If you aren’t measuring the volume yourself, you may need to convert the mass of the solute into volume using the density formula.
- For example, if you’re finding the concentration of 3.45 grams of salt in 2 liters of water, you would find the volume of salt using the density formula. Look up the density of salt either in a textbook or online and solve the formula for m. In this case, the density of salt is 2.16 g/mL. The formula would read 2.16 g/mL = (3.45 g)/V. Multiply each side by V to get V(2.16 g/mL) = 3.45 g. Then divide the each side by 2.16 to find the volume, or V = (3.45 g)/(2.16 g/mL) = 1.60 mL.
- Add the volume of the solute to the volume of your solvent, ma. So in this example, 2 L + 1.6 mL = 2,000 mL + 1.6 mL = 2,001.6 mL. You can either leave the measurement in milliliters or convert it back to liters to get 2.002 L.

- In our example for the concentration of 3.45 grams of salt in 2 liters of water, your equation would be C = (3.45 g)/(2.002 L) = 1.723 g/L.
- Certain problems may ask for your concentration in specific units. Be sure to convert the units before putting them in your final formula.
Finding Concentration in Percentage or Parts per Million

- If your solute is a liquid, you may need to calculate the mass using the formula D = m/V, where D is the liquid’s density, m is the mass, and V is the volume. Look up the density of the liquid in a textbook or online and then solve the equation for the mass.

- For example, if you want to find the concentration of 10 g of cocoa powder mixed with 1.2 L of water, you would find the mass of the water using the density formula. The density of water is 1,000 g/L, so your equation would read 1,000 g/L = m/(1.2 L). Multiply each side by 1.2 L to solve the mass in grams, so m = (1.2 L)(1,000 g/L) = 1,200 g. Add the mass of the cocoa powder to get 1,210 g.

- In our example, C = (10 g)/(1,210 g) = 0.00826.

- In this example, the percent concentration is (0.00826)(100) = 0.826%.

- In our example, the ppm = (0.00826)(1,000,000) = 8,260 ppm.
Tip: Parts per million is usually used for very small concentrations since it’s easier to write and understand than a percentage.
Calculating Molarity

- For example, if your solute is potassium hydroxide (KOH), find the atomic masses for potassium, oxygen, and hydrogen and add them together. In this case molar mass = 39 +16 + 1 = 56 g/mol.
- Molarity is used mainly in chemistry when you know the chemical makeup of the solute you’re using.

- For example, if you want to find the number of moles in 25 g of potassium hydroxide (KOH), then the equation is mol = (25 g)/(56 g/mol) = 0.45 mol
- Convert the mass of your solute to grams if it isn’t already listed in grams.
- Moles are used to represent the number of atoms in the solution.

- In this example, if you’re using 400 mL of water, then divide it by 1,000 to convert it to liters, which is 0.4 L.
- If your solvent is already listed in liters, then you can skip this step.
Tip: You don’t need to include the volume of the solute since it doesn’t usually affect the volume that much. If there is a visible change in volume when you mix the solute with the solvent, then use the total volume instead.

- In this example, M = (0.45 mol)/(0.4 L) = 1.125 M.
Calculator, Practice Problems, and Answers

Community Q&A
- If you are in a lab and don’t know how much of a solute was added, you can perform a titration test using other reactive chemicals. You do need to learn how to balance chemical equations with stoichiometry . Thanks Helpful 0 Not Helpful 0

You Might Also Like

- ↑ https://www.physiologyweb.com/calculators/mass_per_volume_solution_concentration_calculator.html
- ↑ https://www.omnicalculator.com/conversion/ppm
- ↑ https://sciencing.com/calculate-concentration-ppm-6935286.html
- ↑ https://chem.libretexts.org/Courses/Los_Angeles_Trade_Technical_College/Chem_51/15%3A_Solutions/15.03%3A_Solution_Concentration_-_Molality_Mass_Percent_ppm_and_ppb
- ↑ https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_-_The_Central_Science_(Brown_et_al.)/04%3A_Reactions_in_Aqueous_Solution/4.05%3A_Concentration_of_Solutions
- ↑ Chris Hasegawa, PhD. Retired Science Professor & Dean. Expert Interview. 29 July 2021.
- ↑ https://www.khanacademy.org/science/chemistry/states-of-matter-and-intermolecular-forces/mixtures-and-solutions/a/molarity
- ↑ https://www.inchcalculator.com/convert/milliliter-to-liter/
About This Article

To calculate the concentration of a solution, start by converting the solute, or the substance being dissolved, into grams. If you're converting from milliliters, you may need to look up the solute's density and then multiply that by the volume to convert to grams. Next, convert the solvent to liters. Finally, divide the solvent by the solute to find the concentration of the solution. To learn how to calculate the concentration of a solution as a percentage or parts per million, scroll down! Did this summary help you? Yes No
- Send fan mail to authors
Reader Success Stories
Brenda Horn
Oct 19, 2020
Did this article help you?
Jan 9, 2017
Nov 19, 2017
Ejiga Victor
Jun 28, 2017
Katelyn Bosh
May 15, 2017

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
Don’t miss out! Sign up for
wikiHow’s newsletter
8.010 m means 8.010 mol / 1 kg of solvent (8.010 mol) (98.0768 g/mol) = 785.6 g of solute 785.6 g + 1000 g = 1785.6 g total for solute and solvent in the 8.010 m solution. 1785.6 g / 1.354 g/mL = 1318.76 mL 8.01 moles / 1.31876 L = 6.0739 M 6.074 M (to four sig figs)
1 L of solution = 1000 mL = 1000 cm 3 1.329 g/cm 3 times 1000 cm 3 = 1329 g (the mass of the entire solution) 1329 g minus 571.4 g = 757.6 g = 0.7576 kg (the mass of water in the solution) 571.4 g / 98.0768 g/mol = 5.826 mol of H 2 SO 4 5.826 mol / 0.7576 kg = 7.690 m
mass of acetone: (3.30 mL) (0.789 g/mL) = 2.6037 g moles of acetone: 2.6037 g / 58.0794 g/mol = 0.04483 mol mass of solution: (75.0 mL) (0.993 g/mL) = 74.475 g mass of water in the solution: 74.475 g - 2.6037 g = 71.8713 g moles of water: 71.8713 g / 18.015 g/mol = 3.9896 mol
0.04483 mol / 0.0750 L = 0.598 M
0.04483 mol / 0.0718713 kg = 0.624 m
0.04483 mol / (0.04483 mol + 3.9896 mol) = 0.0111
(15.00 mol/L) (1.000 L) = 15.00 mole of HCl 15.00 mol times 36.4609 g/mol = 546.9135 g of HCl
(1000. mL) (1.0745 g/cm 3 ) = 1074.5 g of solution 1074.5 g minus 546.9135 g = 527.5865 g of water = 0.5275865 kg
15.00 mol / 0.5275865 kg = 28.43 m (to four sig figs)
0.449 mol/kg = x / 2.92 kg x = 1.31108 mol of KBr
(1.31108 mol) (119.0023 g/mol) = 156 g KBr
156 g KBr + 2920 g water = 3076 g total If you wanted to be real technical about it, then use three sig figs to obtain 3080 g.
(0.391 mol) ( 86.1766 g/mol) = 33.6950 g 33.6950 g + 1000 g = 1033.6950 g In other words, every 1033.6950 g of 0.391 m solution delivers 33.6950 g of hexane 33.6950 is to 1033.6950 as 247 is to x x = 7577.46446 g to three sig figs, 7.58 kg of solution
1.42 m means 1.42 mole of C 6 H 6 in 1 kg of tetrahydrofuran (1.42 mol) ( 78.1134 g/mol) = 110.921 g 110.921 g + 1000 g = 1110.921 g 110.921 is to 1110.921 as x is to 1630 x = 162.75 g To check, do this: 162.75 g / 78.1134 g/mol = 2.08351 mol 1630 g − 162.75 g = 1467.25 g 2.08351 mol / 1.46725 kg = 1.42 m
A mole fraction of 0.100 for NaCl means the mole fraction of water is 0.900. Let us assume a solution is present made up of 0.100 mole of NaCl and 0.900 mole of water. mass of water present ---> (0.900 mol) (18.015 g/mol) = 16.2135 g molality of solution ---> 0.100 mol / 0.0162135 kg = 6.1677 m to three sig figs, 6.17 m
mass solvent ---> 7550 g − 929 g = 6621 g = 6.621 kg moles solute ---> 929 g/ 84.93 g/mol = 10.9384 mol molality = 10.9384 mol / 6.621 kg = 1.65 m
(1000 mL) (1.230 g/mL) = 1230 g
(3.75 mol) (98.0768 g/mol) = 367.788 g
1230 − 367.788 = 862.212 g
3.75 mol / 0.862212 kg = 4.35 molal (to three sig figs)
1.00 L of this solution contains 4.20 mole of NaCl. (1.00 L) (1050 g/L) = 1050 g of solution.
(4.20 mol) (58.443 g/mol) = 245.4606 g 1050 g - 245.4606 g = 804.5394 g
4.20 mol / 0.8045394 kg = 5.22 m (to three sig figs)
3.58 mole of RbCl in 1000 g of water.
(3.58 mol) (120.921 g/mol) = 432.89718 g 1000 g + 432.89718 g = 1432.89718 g
1432.89718 g / 1.12 g/mL = 1279.37 mL
3.58 mol / 1.27937 L = 2.80 M
molality = moles of naphthalene / kilograms of benzene (16.5 g / 128.1732 g/mol) / 0.0543 kg = 2.37 m
(1.34 mL) (1.59 g/mL) = 2.1306 g 2.1306 g / 153.823 g/mol = 0.013851 mol
(65.0 mL) (1.33 g/mL) = 86.45 g = 0.08645 kg
0.013851 mol / 0.08645 kg = 0.160 m (to three sig figs)
MV = mass / molar mass (x) (0.4500 L) = 0.825 g / 141.9579 g/mol x = 0.0129 M
0.825 g / 141.9579 g/mol = 0.00581158 mol 0.00581158 mol / 0.4500 kg = 0.0129 m
Na 2 HPO 4 ---> 0.825 g / 141.9579 g/mol = 0.00581158 mol H 2 O ---> 450.0 g / 18.015 g/mol = 24.97918401 mol mole fraction of the water ---> 24.97918401 mol / (24.97918401 mol + 0.00581158 mol) = 0.9998
water ---> (450 g / 450.825 g) (100) = 99.8%
ppm means the number of grams of solute per 1,000,000 grams of solution 0.825 is to 450.825 as x is to 1,000,000 x = 1830 ppm
mol solute m = ––––––––– kg solvent
mass solute n = ––––––––––––––––– molar mass of solute
I'm going to use the kg/mol amount and the reason will show up in a moment. mass solute n = –––––––––––– 0.0580794 kg/mol
mass of solution = mass of solvent + mass of solute = 450.0 g = 0.4500 kg I'll ignore those two trailing zeros for the moment. We write this: mass of solvent = 0.45 − mass of solute Here's the reason why I must use the kg/mol unit: In the subtraction just above, the 0.45 is in kilograms. That means the mass of solute must also be in kilograms. You can't subtract two numbers using different units. Also, the bottom unit must be in kilograms because the 0.75 molal value is determined with kg in the denominator. Using grams in the denominator is not done with molality.
x –––––– 0.0580794 0.75 = –––––––––––– 0.45 − x x 0.3375 − 0.75x = ––––––––– 0.0580794 x = 0.019602 − 0.04356x 1.04356x = 0.019602 x = 0.01878 kg = 18.78 g (to 4 sig figs)
0.7500 molal means 0.7500 mole of solute (the acetone) per 1000 g of water mass of acetone ---> 58.0794 g/mol times 0.7500 mol = 43.56 g mass of solution ---> 1000 g + 43.56 g = 1043.56 g 43.56 is to 1043.56 as x is to 450 x = 18.78 g
Calculating the Concentration of a Chemical Solution
How to Calculate Concentration
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution . There are multiple units of concentration . Which unit you use depends on how you intend to use the chemical solution. The most common units are molarity, molality, normality, mass percent, volume percent, and mole fraction. Here are step-by-step directions for calculating concentration, with examples showing the math and tips on when to use the units.
How to Calculate Molarity of a Chemical Solution
Yucel Yilmaz / Getty Images
Molarity is one of the most common units of concentration. It is used when the temperature of an experiment won't change. It's one of the easiest units to calculate. You get the mass of solute for the solution, mix the solute with a known volume of solvent, and divide mass by volume for concentration.
Calculate Molarity : moles solute per liter of solution ( not volume of solvent added since the solute takes up some space)
M = moles / liter
Example : What is the molarity of a solution of 6 grams of NaCl (~1 teaspoon of table salt) dissolved in 500 milliliters of water?
First, convert grams of NaCl to moles of NaCl.
From the periodic table:
- Na = 23.0 g/mol
- Cl = 35.5 g/mol
- NaCl = 23.0 g/mol + 35.5 g/mol = 58.5 g/mol
- Total number of moles = (1 mole / 58.5 g) * 6 g = 0.62 moles
Now determine moles per liter of solution:
M = 0.62 moles NaCl / 0.50 liter solution = 1.2 M solution (1.2 molar solution)
Note that I assumed dissolving the 6 grams of salt did not appreciably affect the volume of the solution. When you prepare a molar solution, avoid this problem by adding solvent to your solute to reach a specific volume.
How to Calculate Molality of a Solution
Molality is used to express the concentration of a solution when you are performing experiments that involve temperature changes or are working with colligative properties. Note that with aqueous solutions at room temperature, the density of water is approximately 1 kg/L, so M and m are nearly the same.
Calculate Molality : moles solute per kilogram solvent
m = moles / kilogram
Example : What is the molality of a solution of 3 grams of KCl (potassium chloride) in 250 ml of water?
First, determine how many moles are present in 3 grams of KCl. Start by looking up the number of grams per mole of potassium and chlorine on a periodic table . Then add them together to get the grams per mole for KCl.
- K = 39.1 g/mol
- KCl = 39.1 + 35.5 = 74.6 g/mol
For 3 grams of KCl, the number of moles is:
(1 mole / 74.6 g) * 3 grams = 3 / 74.6 = 0.040 moles
Express this as moles per kilogram solution. Now, you have 250 ml of water, which is about 250 g of water (assuming a density of 1 g/ml), but you also have 3 grams of solute, so the total mass of the solution is closer to 253 grams than 250. Using 2 significant figures, it's the same thing. If you have more precise measurements, don't forget to include the mass of solute in your calculation!
- 250 g = 0.25 kg
- m = 0.040 moles / 0.25 kg = 0.16 m KCl (0.16 molal solution)
How to Calculate Normality of a Chemical Solution
Normality is similar to molarity, except it expresses the number of active grams of a solute per liter of solution. This is the gram equivalent weight of solute per liter of solution.
Normality is often used in acid-base reactions or when dealing with acids or bases.
Calculate Normality : grams active solute per liter of solution
Example : For acid-base reactions, what would be the normality of 1 M solution of sulfuric acid (H 2 SO 4 ) in water?
Sulfuric acid is a strong acid that completely dissociates into its ions, H + and SO 4 2- , in aqueous solution. You know there are 2 moles of H+ ions (the active chemical species in an acid-base reaction) for every 1 mole of sulfuric acid because of the subscript in the chemical formula. So, a 1 M solution of sulfuric acid would be a 2 N (2 normal) solution.
How to Calculate Mass Percent Concentration of a Solution
Mass percent composition (also called mass percent or percent composition) is the easiest way to express the concentration of a solution because no unit conversions are required. Simply use a scale to measure the mass of the solute and the final solution and express the ratio as a percentage. Remember, the sum of all percentages of components in a solution must add up to 100%
Mass percent is used for all sorts of solutions but is particularly useful when dealing with mixtures of solids or anytime physical properties of the solution are more important than chemical properties.
Calculate Mass Percent : mass solute divided by mass final solution multiplied by 100%
Example : The alloy Nichrome consists of 75% nickel, 12% iron, 11% chromium, 2% manganese, by mass. If you have 250 grams of nichrome, how much iron do you have?
Because the concentration is a percent, you know a 100-gram sample would contain 12 grams of iron. You can set this up as an equation and solve for the unknown "x":
12 g iron / 100 g sample = x g iron / 250 g sample
Cross-multiply and divide:
x= (12 x 250) / 100 = 30 grams of iron
How to Calculate Volume Percent Concentration of a Solution
Volume percent is the volume of solute per volume of solution. This unit is used when mixing together volumes of two solutions to prepare a new solution. When you mix solutions, the volumes aren't always additive , so volume percent is a good way to express concentration. The solute is the liquid present in a smaller amount, while the solution is the liquid present in a larger amount.
Calculate Volume Percent : volume of solute per volume of solution ( not volume of solvent), multiplied by 100%
symbol : v/v %
v/v % = liters/liters x 100% or milliliters/milliliters x 100% (doesn't matter what units of volume you use as long as they are the same for solute and solution)
Example : What is the volume percent of ethanol if you dilute 5.0 milliliters of ethanol with water to obtain a 75-milliliter solution?
v/v % = 5.0 ml alcohol / 75 ml solution x 100% = 6.7% ethanol solution, by volume.
How to Calculate Mole Fraction of a Solution
Mole fraction or molar fraction is the number of moles of one component of a solution divided by the total number of moles of all chemical species. The sum of all mole fractions adds up to 1. Note that moles cancel out when calculating mole fraction, so it is a unitless value. Note some people express mole fraction as a percent (not common). When this is done, the mole fraction is multiplied by 100%.
symbol : X or the lower-case Greek letter chi, χ, which is often written as a subscript
Calculate Mole Fraction : X A = (moles of A) / (moles of A + moles of B + moles of C...)
Example : Determine the mole fraction of NaCl in a solution in which 0.10 moles of the salt is dissolved in 100 grams of water.
The moles of NaCl is provided, but you still need the number of moles of water , H 2 O. Start by calculating the number of moles in one gram of water, using periodic table data for hydrogen and oxygen:
- H = 1.01 g/mol
- O = 16.00 g/mol
- H 2 O = 2 + 16 = 18 g/mol (look at the subscript to note there are 2 hydrogen atoms)
Use this value to convert the total number of grams of water into moles:
(1 mol / 18 g ) * 100 g = 5.56 moles of water
Now you have the information needed to calculate mole fraction.
- X salt = moles salt / (moles salt + moles water)
- X salt = 0.10 mol / (0.10 + 5.56 mol)
- X salt = 0.02
More Ways to Calculate and Express Concentration
There are other easy ways to express the concentration of a chemical solution. Parts per million and parts per billion are used primarily for extremely dilute solutions.
g/L = grams per liter = mass of solute / volume of solution
F = formality = formula weight units per liter of solution
ppm = parts per million = ratio of parts of solute per 1 million parts of the solution
ppb = parts per billion = ratio of parts of solute per 1 billion parts of the solution.
- Calculating Concentrations with Units and Dilutions
- Molarity Definition in Chemistry
- Concentration Definition (Chemistry)
- Osmolarity and Osmolality
- How to Prepare a Solution
- Molarity Example Problem
- What is the Difference Between Molarity and Molality?
- Convert Molarity to Parts Per Million Example Problem
- The Difference Between Molality and Molarity
- How to Calculate Normality (Chemistry)
- Molality and Concentration of a Chemical Solution
- How to Calculate Molarity of a Solution
- Determine Concentration and Molarity
- Why Is Molality Used Instead of Molarity?
- Mass Percent Composition Problem
- How to Calculate Mass Percent Composition
Talk to our experts
1800-120-456-456
- Concentration of Solution

An introduction to Concentration of Solution
Everyone talks about the concentration of solutions. They may also talk about the concentration of coffee or tea. Everyone has a particular view of what is meant by the concentration of a solution. You must have noticed that whenever you make coffee, if you add a lot of powder, you will end up with a concentrated drink, whereas if you add little, it will result in a dilute solution. Therefore, it is essential that you understand what the concentration of the solution is. In this chapter, we will learn about what is meant by the concentration of a solution; we will also see how to find the concentration of a solution and the different methods of expressing the concentration of the solution.
What is Concentration of a Solution?
In an aqueous solution, two parts exist, namely solute and solvent. They are the two basic solution concentration terms that you need to know. We always need to keep an account of the amount of solute in the solution. In chemistry, we define the concentration of solution as the amount of solute in a solvent. When a solution has more solute in it, we call it a concentrated solution. Whereas when the solution has more solvent in it, we call it a dilute solution.
(Image will be uploaded soon)
Now that you understand the concept of what is the concentration of solution let's move on to the different methods of expressing concentration.
Methods of Expressing the concentration of Solution
There are various methods of expressing the concentration of a solution. You will usually see Chemists working with the number of moles. Pharmacists will use percentage concentrations instead of the number of moles. Hence it is important to understand all the methods of expressing the concentration of solutions.
The concentration of the solution formula is given as follows.
Concentration of solution = \[\frac{\text{Weight of the solute in gram}}{\text{volume in Litres}}\]
We will also see other methods on how to calculate the concentration of a solution based on the different methods of expressing concentrations.
Concentration in Parts per Million
It is expressed in terms of weight. The formula for parts per million is given as follows:
ppm(A)= \[\frac{\textrm{Mass of A}}{\textrm{Total mass of the solution}}\]x10\[^{6}\]
Mass Percentage (w/w)
It is expressed in terms of mass percentage of solute to the solution. The formula for mass percentage is given as follows.
Mass percentage of A = \[\frac{\text{Mass of component A}}{\text{Total mass of the soution}}\]x100
e.g. CH 3 COOH 33% w/w, and H 2 SO 4 98.0% w/w.
Volume Percentage (V/V)
It is expressed in terms of the volume percentage of solute to the solvent. The formula for volume percentage is given as follows.
Volume percentage of A = \[\frac{\text{Volume of component A}}{\text{Total volume of the solution}}\]x100
Mass by Volume Percentage (w/V)
Percentage weight in volume expresses the number of grams of solute in 100 ml of product.
e.g. BaCl 2 solution 10% w/v, and H 2 O 2 solution 5-7% w/v.
Molarity (M)
It is the number of moles of solute contained in 1000 ml of solution. It is a commonly used method for expressing concentrations.
Molarity = \[\frac{\text{Mass of solute}}{\text{volume of solution in litres}}\]
Molality (m)
The molality is expressed as the number of moles of a solute contained in 1000 gm of a solvent. The formula for molality is given as follows.
Molality (m) = \[\frac{\text{Mass of solute}}{\text{Mass of solvent in Kg}}\]
Normality (N)
We can define it as the number of equivalents of the solute present in the solution, and it is also called equivalent concentration. The formula for normality is given as follows.
Normality (N) = \[\frac{\text{Weight of solute in grams}}{\text{Equivalent mass} \times \text{Volume in litre}}\]
Mole Fraction:
The mole fraction (X) of a component in a solution is defined as the ratio of the number of moles of that component to the total number of moles of all components in the solution. The mole fraction of A is expressed as X A with the help of the following equation in a solution consisting of A, B, C, … we can calculate X A .
X\[_{A}\] = \[\frac{\text{moles of A}}{\text{mole of A + mole of B + momle of C +.... }}\]
Similarly, we can calculate the mole fraction of B, X B with the help of the following formula.
X\[_{B}\] = \[\frac{\text{moles of B}}{\text{mole of A + mole of B + momle of C +.... }}\]
Now that you know how to find the concentration of a solution using various concentrations of solution formulas, we will try to solve some concentrations of solution questions.
Solved Problems
Question 1) 2 ml of water is added to 4 g of a powdered drug. The final volume is 3ml. Find the mass by volume percentage of the solution?
Answer 1) Given, Mass of solute = 4g
Volume of solution = 3ml
Mass by volume percentage = \[\frac{\text{Mass of solute}}{\text{Volume of solution}}\]x100 = \[\frac{4g}{3ml}\] = 133%
Therefore, the mass by volume percentage is 133 %.
Question 2) Many people use a solution of Na 3 PO 4 to clean walls before putting up wallpaper. The recommended concentration is 1.7 % (m/v). Find the mass of Na 3 PO 4 needed to make 2.0 L of the solution?
Answer 2) Given,
Mass/Volume percentage = 1.7 %
Volume of Solution = 2000 ml
Mass by volume percentage = \[\frac{\text{Mass of solute}}{\text{Volume of solution}}\] × 100
1.7 % = \[\frac{\text{Mass of solute}}{2000ml}\] ×100
Mass of solute = 34 g
Therefore, the mass required is 34 g.
In chemistry, we are often required to calculate the concentration of the solution. The above-mentioned methods of expressing the concentration of a solution are important. The solved examples are helpful for a better understanding of the concept of concentration of a solution.

FAQs on Concentration of Solution
1. Does solution concentration change when solution volume changes?
The answer to this question depends on how we define concentration. If we talk about molarity, then yes it does change. If we take concentration by mass into consideration, it will still change, unless the substance is with an undefined density. That's because the mass of a substance will change with its volume, and so the concentration changes. But, if both the solute and solvent are either increasing or decreasing in volume/mass/moles in an equal ratio, the concentration and molarity will remain the same.
2. How do I convert from molarity to a weight percentage?
The first step is to multiply the molarity by the molar mass of the solute to get grams of solute per litre. The second step is to divide the concentration expressed as grams of solute per litre by the density of the solution in grams per litre. Finally, multiply it by 100% to convert it to percentage.
- Thermodynamics
- Analytical Chemistry
- Atomic Structure
- Classification of Organic Compounds
- Periodic Table of Elements
- Nuclear Force
- Importance of Chemistry
- Chemistry Notes Class 11
- Chemistry Notes Class 8
- Chemistry Notes Class 9
- Chemistry Notes Class 10
- Chemistry Notes Class 12
- CBSE Class 9 Chemistry Notes
Chapter 1: Matter in our Surroundings
- Matter is Made of Tiny Particles
- Why Solids, Liquids and Gases Have Different Properties
- Classification of Matter
- Brownian Movement
- States of Matter: Solid, Liquid, Gas and Plasma
- Evaporation
- Effects of Relative Humidity and Wind Speed
- How Does Evaporation Cause Cooling?
- Effect of Change of Temperature
- Melting Point
- What is Vaporization?
- Condensation
- Effects of Change of Pressure
- Difference between Rigidity and Fluidity of Matter
- Prove That Liquids have No fixed Shape but have a Fixed Volume
- Diffusion in Solids, Liquids, and Gases
- What is the Unit of Temperature?
- What is the Relationship Between Celsius and Kelvin Scale of Temperature?
- Liquification of Gases
- How to demonstrate the Presence of Water Vapour in Air?
- What is Plasma and Bose-Einstein Condensate?
Chapter 2: Is Matter Around Us Pure?
- Solution: Properties of Solution
- Saturated and Unsaturated Solutions
Concentration of a Solution
- Suspensions
- How will you distinguish a Colloid from a Solution?
- Classification of Colloids
- Tyndall Effect
- Separation of Mixtures
- How to separate a Mixture of Two Solids?
- Separation by a suitable solvent
- Separation of Mixtures using Sublimation and Magnets
- How to Separate a Mixture of a Solid and a Liquid?
- Filtration: Definition, Process, Diagram and Examples
- Water Purification
- Centrifugation
- How to Separate Cream from milk?
- Difference Between Homogeneous and Heterogeneous Mixture
- Difference Between Compound and Mixture
- Factors affecting Solubility
- Separation by Evaporation
- Crystallization
- Chromatography
- Distillation
- Separation of Mixtures of Two or More Liquids
- Fractional Distillation
- Pure and Impure Substances
- What is an Element?
- Metals, Non-Metals and Metalloids
- Properties of Metals and Non-Metals
Chapter 3: Atoms and Molecules
- Laws of Chemical Combination
- Law of Conservation of Mass
- Verification of the Law of Conservation of Mass in a Chemical Reaction
- Law of Constant Proportions
- What is Atom?
- Atomic Mass
- How Do Atoms Exist?
- Cations vs Anions
- What are Ionic Compounds?
- What are Monovalent Ions?
- What are Divalent Ions?
- Trivalent Ions - Cations and Anions
- Polyatomic Ions
- Formulas of Ionic Compounds
- Chemical Formula
- Chemical Formula of Common Compounds
- Molecular Mass
- Mole Concept
- Problems Based on Mole Concepts
- Dalton's Atomic Theory
- Drawbacks of Dalton's Atomic Theory
- Significance of the Symbol of Elements
- Difference Between Molecules and Compounds
- How to Calculate Valency of Radicals?
- What is the Significance of the Formula of a Substance?
- Gram Atomic and Gram Molecular Mass
Chapter 4: Structure of the Atom
- Charged Particles in Matter
- Thomson's Atomic Model
- Rutherford Atomic Model
- Drawbacks of Rutherford's Atomic Model
- Bohr's Model of an Atom
- Valence Electrons
- Mass Number
- Relation Between Mass Number and Atomic Number
- Why do all the Isotopes of an Element have similar Chemical Properties?
- Why Isotopes have different Physical Properties?
- What is Fractional Atomic Mass?
- Radioactive Isotopes
- Discovery of Electrons
- What is a Proton?
- Rutherford's Alpha Scattering Experiment
- Atomic Nucleus
- How did Neil Bohr explained the Stability of Atom?
- Electron Configuration
- Potassium and Calcium - Atomic Structure, Chemical Properties, Uses
- What is meant by Chemical Combination?
- Difference between Electrovalency and Covalency
Concentration of Solution is a measure of the amount of solute that has been dissolved in the given amount of solvent. In simple words, it means determining how much of one substance is mixed with another substance. As Concentration is a frequently used term in chemistry and other relevant fields, although it is most commonly used in the context of solutions, where it refers to the quantity of solute dissolved in a solvent. Concentration can be expressed in both qualitative or quantitative (numerically) terms.
Concentration of a Solution Definition
Concentration of a solution is defined as the amount of solute dissolved in the solution. It is given by the ratio of the amount of solute to the amount of solution or solvent sometimes. However, we can express it in percentages, Parts per Million, and several other ways. The concentration of a solution can be expressed both qualitatively and quantitatively which we will see in the below topics. Before learning more about concentration let’s understand some of the general types of solution
Solution of Solid and Liquid
Solutions of solids and liquids involve the dissolution of a solid solute in a liquid solvent or a liquid solute in a liquid solvent. Examples include:
a. Solid in Liquid: Dissolving table salt (NaCl) in water.
b. Liquid in Liquid: Mixing ethanol and water to form a homogeneous solution.
Solution of Gas
Solutions of gases involve the dissolution of a gas in a liquid solvent or another gas. Examples include:
a. Gas in Liquid: Dissolving carbon dioxide (CO 2 ) in water to form carbonated water.
b. Gas in Gas: Mixing different gases, such as oxygen and nitrogen, in the atmosphere to form a homogeneous mixture.
Qualitative Expressions of Concentration
To qualitatively express concentration, a solution can be classified as a dilute solution or a concentrated solution, which are explained as follows:
Dilute Solution
Dilute Solution contains a smaller proportion of solute than the proportion of solvent. For example, if 2 grams of salicylic acid is dissolved in 100 ml of water and in another container, 8 grams of salicylic acid is dissolved in the same amount of water then a 2-gram solution of salicylic acid is a dilute solution compared to 8 grams solution of salicylic acid.
Concentrated Solution
Concentrated Solution contains a much greater proportion of solute than the proportion of solvent. For example, if 2 grams of salicylic acid is dissolved in 100 ml of water and in another container, 8 grams of salicylic acid is dissolved in the same amount of water then 8 grams solution of salicylic acid is a concentrated solution compared to 2 grams solution of salicylic acid.

Figure 1. Dilute (left) and Concentrated (right) solutions
Semi-Qualitative Expressions of Concentration
To semi-qualitatively express concentration, a solution can be classified as a saturated solution or an unsaturated solution , which are explained as follows:

Saturated Solution
A saturated solution is one in which the greatest quantity of solute is dissolved in a solvent at a given temperature. When a solution reaches saturation, it can no longer dissolve any more solute at that temperature. Undissolved chemicals settle to the bottom. The saturation point is determined as the point at which no more solute can be dissolved in the solvent.
Unsaturated Solution
An unsaturated solution is one that contains less solute than the maximum possible solute it can dissolve before the solution reaches the saturation level. When more solute is dissolved in this solution, there are no residual substances at the bottom, indicating that all of the solutes have been dissolved in the solvent. An unsaturated solution is a chemical solution in which the solute concentration is less than the corresponding equilibrium solubility.

Figure 2. Unsaturated (left) and Saturated (right) solutions
Solubility is defined as the greatest amount of solute that may dissolve in a certain quantity of solvent at a given temperature.
A solution is a liquid that is a homogeneous combination of one or more solutes and a solvent. A typical example of a solution is, sugar cubes added to a cup of tea or coffee. Here, solubility is the characteristic that allows sugar molecules to dissolve.
As a result, the term solubility may be defined as a substance’s (solute’s) ability to dissolve in a particular solvent.
Quantitative Expressions of Concentration
Qualitative expressions of concentration are relative terms, which do not provide the exact concentration of the solution. To characterize the concentrations of various solutions around us in an accurate and precise manner, we require quantitative expressions of concentration.
Generally, concentration is represented in both ways: Concentration = Quantity of Solute / Quantity of Solution or Concentration = Quantity of Solute / Quantity of Solvent
To quantitatively express concentration, we use the following terms:
- Mass Percentage
- Volume Percentage
- Mass by Volume Percentage
- Parts per Million and Parts per Billion
Mole Fraction
Mass percentage (w/w%).
Mass percentage which is also called weight by weight concentration of solute and is defined as the amount of solute (in grams) present in 100 gm of the solution.
Mass Percentage = (Mass of Solute / Mass of Solution) × 100
Mass percentage has no unit as it is the ratio of the mass of solute and solution.
Volume Percentage (v/v%)
Volume Percentage which is also called volume by volume concentration of solute and is defined as the amount of solute (in ml) present in 100 ml of the solution.
Volume Percentage = (Volume of Solute / Volume of Solution) × 100
Volume percentage has no unit as it is the ratio of the volume of solute and solution.
Mass by Volume Percentage(w/v %)
It is defined as the amount of solute (in grams) present in the 100ml of the solution.
Mass by Volume Percentage = (Mass of Solute(in gm) / Volume of Solution(in ml) × 100
Unit of mass by volume percentage is gram per milliliter as it is the ratio of the mass of the solute and volume of the solution.
Parts per Million (PPM)
Parts Per Million or PPM is used to measure the very small amount of solute dissolved in the Solvent. Drinking water, air, soils, etc. are the solvents that have very fewer amounts of solutes in them, which can’t be measured in percentage as a percentage only calculates the concentration out of 100. If we represent concentrations of these solvents in percentage it looks like 0.00002 %, which is not an effective way. That’s why parts per million were introduced to make a representation of these concentrations.
PPM of solute = Mass of solute (in milligrams)/Mass of solution(In Kg)
Parts per Billion
Like, Parts per million, Parts Per Billion are also used to represent solute available in trace quantities. Parts Per Billion represents the amount of solute in 1 billion parts of the solution.
PPB of solute = Mass of solute (in micrograms)/Mass of solution(In Kg)
Molarity of a given solution is defined as the number of moles present in the 1 liter of solution. For example, if 2 moles of NaCl are dissolved in 1 liter of water, the molarity of the resulting solution would be 2M, and the Formula for Molarity is given as follows:
Molarity (M) = Moles of solute /Volume of solution(in Liter)
Molality of a given solution is defined as the number of moles present in 1 kg of solution. For example, if 3 moles of KOH are dissolved in 3 Liters of water (density of water 1 kg/L), the molality of the resulting solution would be 1 m, as there is 1 mole of KOH present in each Kg of water. The formula for molality is given as follows:
Molality (m) = Moles of solute / Mass of solvent(in Kg)
Mole fraction i.e., X is defined as the ratio of the number of moles of one component to the total number of moles present in the solution. It is a dimensionless quantity. The mole fraction of solute A in a solution containing n components such as A, B, C, . . ., N can be calculated using the following formula:
Mole fraction of A (X A ) = Moles of A / (Moles of A + Moles of B + . . . + Moles of N)
The mole fraction of other solvents (B, C, D, . . .N) in a solution can be calculated using a similar formula.
Normality is a measure of concentration equivalent to the number of equivalents per liter of solution. It is often used for reactions that involve acid-base neutralization, precipitation reactions, or redox reactions, and it takes into account the stoichiometry of the reaction.
Normality (N) = Equivalents of solute / Volume of solution in liters
For example, for an acid-base reaction, normality can be calculated using:
Normality = Molarity × Basicity or Acidity of the compound
Where basicity or acidity is the number of hydrogen ions (H⁺) or hydroxide ions (OH⁻) that can be released per molecule of the compound.
Formality is similar to molarity in that it measures the number of moles of solute per liter of solution. However, formality is used when the solute undergoes a reaction or dissociation in solution. Formality measures the concentration based on the initial composition of the solution, not the final dissolved state.
Formality (F) = Moles of solute / Volume of solution in liters
Temperature Dependence of Quantitative Expressions of Concentration
The following table shows the temperature dependence of the Quantitative Expressions of Concentration.
Mole Concept Heterogeneous and Homogeneous Mixtures Ideal and Non-Ideal Solutions
Sample Problems on Concentration of Solution
Problem 1: 15 g of common salt is dissolved in 400 g of water. Calculate the concentration of the solution by expressing it in Mass by Mass percentage (w/w%).
Given that, Mass of solute (common salt) = 15 g …(1) Mass of Solvent (water) = 400 g …(2) It is known that, Mass of Solution = Mass of Solute + Mass of Solvent …(3) So, Substituting (1) and (2) in (3), we obtain the following, Mass of Solution = 15 g + 400 g = 415 g …(4) From Figure 4, we know Mass by Mass Percentage = ( Mass of Solute / Mass of Solution ) × 100 …(5) Substituting (1) and (4) in (5), we obtain the following, Mass by Mass Percentage = ( 15 g / 415 g ) × 100 = ( 0.0361 ) × 100 = 3.61 Answer is: ( w / w % ) = 3.61
Problem 2: 15 g of common salt is dissolved in a solution of 300 mL, calculate the Mass by Volume percentage (w/v%).
Given that, Mass of solute (common salt) = 15 g . . . (1) Mass of Solution (salt solution) = 300 mL . . . (2) From Figure 5, we know Mass by Volume Percentage = ( Mass of Solute / Volume of Solution ) × 100 . . . (3) Substituting (1) and (2) in (3), we obtain the following, Mass by Volume Percentage = ( 15 g / 300 mL ) × 100 = ( 0.05 ) × 100 = 5 g/mL Answer is: ( w / v % ) = 5 g/mL
Problem 3: Richard dissolved 70 g of sugar in 750 mL of sugar solution. Calculate the Mass by Volume percentage (w/v%).
Given that, Mass of solute (common salt) = 70 g . . . (1) Mass of Solution (salt solution) = 750 mL . . . (2) From Figure 5, we know Mass by Volume Percentage = ( Mass of Solute / Volume of Solution ) × 100 . . . (3) Substituting (1) and (2) in (3), we obtain the following, Mass by Volume Percentage = ( 70 g / 750 mL ) * 100 = ( 0.933 ) × 100 = 93.3 g/mL Answer is: ( w / v % ) = 93.3 g/mL
Problem 4: What is the molarity of a solution containing 0.5 moles of NaCl dissolved in 500 mL of water?
Given, Moles of NaCl = 0.5, Volume of Solution = 500 mL = 0.5 Liter As, Molarity (M) = moles of solute / liters of solution ⇒ M = 0.5 moles / 0.5 liters = 1 M So the molarity of the solution is 1 M.
Problem 5: What is the molality of a solution containing 20 g of glucose dissolved in 500 g of water?
Given: Mass of Glucose = 20 g, Mass of water = 500 g = 0.5 kg, Molar mass of glucose (C 6 H 12 O 6 ) = 180 g/mol Number of Moles = Mass/Molar Mass ⇒ Moles of Glucose = 20 / 180 = 1/9 ≈ 0.111 moles of glucose As, Molality (m) = moles of solute / kilograms of solvent ⇒ m = 0.111 / 0.5 = 0.222 mol/kg So the molality of the solution is 0.222 mol/kg.
Problem 6:How many moles of HCl are present in 250 mL of a 0.2 M HCl solution?
Given: Molarity of solution = 0.2 M, Volume of solution = 250 mL = 0.25 liters Molarity (M) = moles of solute / liters of solution ⇒ Moles of solute = Molarity x liters of solution ⇒ Moles of HCl = 0.2 M x 0.25 L = 0.05 moles So there are 0.05 moles of HCl present in 250 mL of the solution.
Problem 7:What is the ppm of lead in a sample that contains 20 mg of lead in 10 L of water?
Given : mass of solute(in mg) = 20 mg and Volution of solvent = 10 L Mass of solution = Mass of water = 10 L x 1 Kg/L = 10 Kg (density of water is 1Kg/L or 1g/mL) ppm (parts per million) = Mass of solute(in mg)/Mass of solution (in Kg) ⇒ ppm = 20 / 10 = 2 So the ppm of lead in the sample is 2ppm.
Problem 8: What is the ppb of mercury in a sample that contains 0.01 g of mercury in 1000 L of air?
Given : mass of solute(in mg) = 0.01 g = 10,000 μg and Volution of solvent = 1000 L Mass of solution = Mass of water = 1000 L x 1 Kg/L = 1000 Kg (density of water is 1Kg/L or 1g/mL) ppb (parts per million) = Mass of solute(in μg)/Mass of solution (in Kg) ⇒ ppm = 10000 / 1000 = 10 So the ppm of lead in the sample is 10 ppb.
FAQs on Concentration of Solution
What is solubility, what is a dilute solution.
A dilute solution is solution which contains a smaller proportion of solute as compared to the proportion of solvent.
What is the difference between PPM and PPB?
PPM and PPB are both represents the concentration of very small scale and only difference between them is that PPM is 1000 times greater scale then PPB or PPB is 1000 times smaller scale then PPM i.e., 1 PPM = 1000 PPB
What is the difference between Molarity and Molality?
Molarity is the number of moles of solute per liter of solution, whereas molality is the number of moles of solute per kilogram of solvent.
Please Login to comment...
Similar reads.
- Chemistry-Class-11
- Chemistry-Formulas
- Physical-Chemistry
- School Chemistry
- School Learning

Improve your Coding Skills with Practice
What kind of Experience do you want to share?

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Dilution Example Problems

In most laboratory settings, a stock solution is created when a compound is used over and over. This stock solution will have a high concentration. If lower concentrations are needed, a dilution is performed.
A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. These dilution example problems show how to perform the calculations needed to make a diluted solution.
The key idea behind a dilution is the number of moles of solute in the solutions does not change as the solvent is added.
moles of solute prior to dilution = moles solute after dilution
The concentration of a solution can be expressed in molarity (M).
Solve for moles and get:
Since moles of solute prior to dilution = moles solute after dilution,
M i V i = M D V D where: M i = initial concentration V i = initial volume M D = diluted concentration V D = diluted volume
Example Problem 1:
Problem: What volume of 5 M NaOH is needed to create a 100 mL solution of 1 M NaOH?
Solution: Use the formula M i V i = M D V D .
M i = 5 M V i = initial volume M D = 1 M V D = 100 mL
Answer: 20 mL of 5 M NaOH is needed to create a 100 mL solution of 1 M NaOH. Water is added to the 20 mL solution until there is 100 mL.
Example Problem 2:
Problem: If you have 300 mL of 1.5 M NaCl, how many mL of 0.25 M NaCl can you make?
Solution: Use the formula M i V i = M D V D .
M i = 1.5 M V i = 300 mL M D = 0.25 M V D = final volume
Answer: You can make 1800 mL of 0.25 M NaCl solution from 300 mL of 1.5 M NaCl solution.
Related Posts
- Chemistry Articles
- Concentration Of Solution And The Calculation
Expression of Concentration of Solutions
We always discuss a solution being diluted or concentrated; this is a qualitative way of expressing the concentration of the solution. A dilute solution means the quantity of solute is relatively very small, and a concentrated solution implies that the solution has a large amount of solute. But these are relative terms and do not give us the quantitative concentration of the solution.
Table of Contents
Concentration.
- Recommended Video
- Concentration in parts per million
- Mass percentage
- Volume percentage
- Mass by Volume percentage
- Mole Fraction
- Solutions of Solid in Liquid
Solubility of Gases
Frequently asked questions – faqs.
So, to quantitatively describe the concentrations of various solutions around us, we commonly express levels in the following way:
It is the amount of solute present in one litre of solution. It is denoted by C or S.
Recommended Videos
Introduction to solutions – concentration terms.

Is Matter Around us Pure?

Effect of Change in Concentration

1. Concentration in Parts Per Million (ppm)
The parts of a component per million parts (10 6 ) of the solution.
2. Mass Percentage (w/w):
When the concentration is expressed as the percent of one component in the solution by mass it is called mass percentage (w/w). Suppose we have a solution containing component A as the solute and B as the solvent, then its mass percentage is expressed as:
Mass % of A = \(\begin{array}{l} \frac {Mass \space of \space component \space A ~ in ~the ~ solution}{Total ~ mass ~of~ the~ solution } × 100 \end{array} \)
3. Volume Percentage (V/V):
Sometimes we express the concentration as a percent of one component in the solution by volume, it is then called as volume percentage and is given as:
volume % of A = \(\begin{array}{l} \frac {Volume ~of~ component~ A~ in~ the ~solution}{Total ~ volume ~ of ~ the ~ solution } × 100 \end{array} \)
For example, if a solution of NaCl in water is said to be 10 % by volume that means a 100 ml solution will contain 10 ml NaCl.
4. Mass by Volume Percentage (w/V):
This unit is majorly used in the pharmaceutical industry. It is defined as the mass of a solute dissolved per 100mL of the solution.
% w/V = (Mass of component A in the solution/ Total Volume of the Solution)x 100
5. Molarity (M):
One of the most commonly used methods for expressing the concentrations is molarity. It is the number of moles of solute dissolved in one litre of a solution. Suppose a solution of ethanol is marked 0.25 M, this means that in one litre of the given solution 0.25 moles of ethanol is dissolved.
Molarity (M) = Moles of Solute/Volume of Solution in litres
6. Molality (m):
Molality represents the concentration regarding moles of solute and the mass of solvent. It is given by moles of solute dissolved per kg of the solvent . The molality formula is as given-
\(\begin{array}{l} Molality (m) = \frac {Moles~ of ~solute}{Mass~ of~ solvent~ in~ kg}\end{array} \)
7. Normality
It is the number of gram equivalents of solute present in one litre of the solution and it is denoted by N.
The relation between normality and molarity.
- N x Eq.Wt = Molarity x Molar mass
- N = Molarity x Valency
- N = Molarity x Number of H + or OH – ion.
8. Formality
It is the number of gram formula units present in one litre of solution. It is denoted by F.
It is applicable in the case of ionic solids like NaCl.
9. Mole Fraction:
If the solution has a solvent and the solute, a mole fraction gives a concentration as the ratio of moles of one component to the total moles present in the solution. It is denoted by x. Suppose we have a solution containing A as a solute and B as the solvent. Let n A and n B be the number of moles of A and B present in the solution respectively. So, mole fractions of A and B are given as:
\(\begin{array}{l}~~~~~~~~~~~~~~~\end{array} \) \(\begin{array}{l} x_A = \frac {n_A}{n_A + n_B}\end{array} \) \(\begin{array}{l}~~~~~~~~~~~~~~~\end{array} \) \(\begin{array}{l} x_B = \frac {n_B}{n_A + n_B} \end{array} \)
The above-mentioned methods are commonly used ways of expressing the concentration of solutions. All the methods describe the same thing that is, the concentration of a solution, each of them has its own advantages and disadvantages. Molarity depends on temperature while mole fraction and molality are independent of temperature. All these methods are used on the basis of the requirement of expressing the concentrations.
Solutions of Solids in Liquids
- A saturated solution is a solution that remains in contact with an excess of solute.
- The amount of solute dissolved per 100g of solvent in a saturated solution at a specific temperature represents the solubility of the solute.
- For exothermic substances such as KOH, CaO, Ca(OH) 2 , M 2 CO 3 , M 2 SO 4 , etc, solubility is inversely proportional to temperature.
- For endothermic substances such as NaCl, KNO 3 , NaNO 3 , glucose, etc., solubility is directly proportional to temperature.
The solubility of gases is mostly expressed in terms of the absorption coefficient,k that is the volume of the gas dissolved by unit volume of solvent at 1 atm pressure and a specific temperature.
The solubility of a gas in a liquid depends upon
- Temperature solubility is inversely proportional to temperature as the dissolution of gas is exothermic in most cases.
- Nature of gas – Gases having a higher value of van der Waals force of attraction that is gases that are more easily liquefied are more soluble. For example, SO 2 and CO 2 are more soluble in water than O 2 , N 2 , and H 2 .
- Nature of solvent – Gases which can ionise in an aqueous solution are more stable in water as compared to the other solvents.
How do you change the concentration of a solution?
Sometimes, by modifying the quantity of solvent, a worker would need to modify the concentration of a solution. Dilution is the addition of a solvent that reduces the solute concentration of the solution. Concentration is solvent elimination, which increases the solute concentration in the solution.
What is a high concentration?
A concentration of persons means that in one place there are more of them. A high concentration of a material in a solution means there’s a lot of it compared to the volume: because of the high concentration of salt, the Great Salt Lake has very little fish.
Is dilute acid dangerous?
In general, a mild condensed acid is more harmful than a solid diluted acid. Although concentrated acetic acid is much less reactive, you do not want to touch the skin or mucous membranes because it is corrosive.
What is the concentration of a solution?
A solution concentration is a measure of the quantity of solute that has been dissolved in a given quantity of solvent or solution. One that contains a relatively high volume of dissolved solute is a concentrated solution. That that contains a relatively minimal volume of dissolved solute is a dilute solution.
How do you prepare a solution of known concentration?
Solutions of known concentration can be prepared either by dissolving the known mass of the solvent solution and diluting it to the desired final volume or by diluting it to the desired final volume by diluting the acceptable volume of the more concentrated solution (the stock solution).

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

- SUGGESTED TOPICS
- The Magazine
- Newsletters
- Managing Yourself
- Managing Teams
- Work-life Balance
- The Big Idea
- Data & Visuals
- Reading Lists
- Case Selections
- HBR Learning
- Topic Feeds
- Account Settings
- Email Preferences
AI’s Trust Problem
- Bhaskar Chakravorti

As AI becomes more powerful, it faces a major trust problem. Consider 12 leading concerns: disinformation, safety and security, the black box problem, ethical concerns, bias, instability, hallucinations in LLMs, unknown unknowns, potential job losses and social inequalities, environmental impact, industry concentration, and state overreach. Each of these issues is complex — and not easy to solve. But there is one consistent approach to addressing the trust gap: training, empowering, and including humans to manage AI tools.
Twelve persistent risks of AI that are driving skepticism.
With tens of billions invested in AI last year and leading players such as OpenAI looking for trillions more, the tech industry is racing to add to the pileup of generative AI models. The goal is to steadily demonstrate better performance and, in doing so, close the gap between what humans can do and what can be accomplished with AI.
- Bhaskar Chakravorti is the Dean of Global Business at The Fletcher School at Tufts University and founding Executive Director of Fletcher’s Institute for Business in the Global Context . He is the author of The Slow Pace of Fast Change .
Partner Center

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

11.3: Solution Concentration - Molarity
- Last updated
- Save as PDF
- Page ID 465574
Learning Objectives
- To describe the concentrations of solutions quantitatively
Many people have a qualitative idea of what is meant by concentration . Anyone who has made instant coffee or lemonade knows that too much powder gives a strongly flavored, highly concentrated drink, whereas too little results in a dilute solution that may be hard to distinguish from water. In chemistry, the concentration of a solution is the quantity of a solute that is contained in a particular quantity of solvent or solution. Knowing the concentration of solutes is important in controlling the stoichiometry of reactants for solution reactions. Chemists use many different methods to define concentrations, some of which are described in this section.
The most common unit of concentration is molarity , which is also the most useful for calculations involving the stoichiometry of reactions in solution. The molarity (M) is defined as the number of moles of solute present in exactly 1 L of solution . It is, equivalently, the number of millimoles of solute present in exactly 1 mL of solution:
\[ molarity = \dfrac{moles\: of\: solute}{liters\: of\: solution} = \dfrac{mmoles\: of\: solute} {milliliters\: of\: solution} \label{4.5.1} \]
The units of molarity are therefore moles per liter of solution (mol/L), abbreviated as \(M\). An aqueous solution that contains 1 mol (342 g) of sucrose in enough water to give a final volume of 1.00 L has a sucrose concentration of 1.00 mol/L or 1.00 M. In chemical notation, square brackets around the name or formula of the solute represent the molar concentration of a solute. Therefore,
\[[\rm{sucrose}] = 1.00\: M \nonumber \]
is read as “the concentration of sucrose is 1.00 molar.” The relationships between volume, molarity, and moles may be expressed as either
\[ V_L M_{mol/L} = \cancel{L} \left( \dfrac{mol}{\cancel{L}} \right) = moles \label{4.5.2} \]
\[ V_{mL} M_{mmol/mL} = \cancel{mL} \left( \dfrac{mmol} {\cancel{mL}} \right) = mmoles \label{4.5.3} \]
Figure \(\PageIndex{1}\) illustrates the use of Equations \(\ref{4.5.2}\) and \(\ref{4.5.3}\).
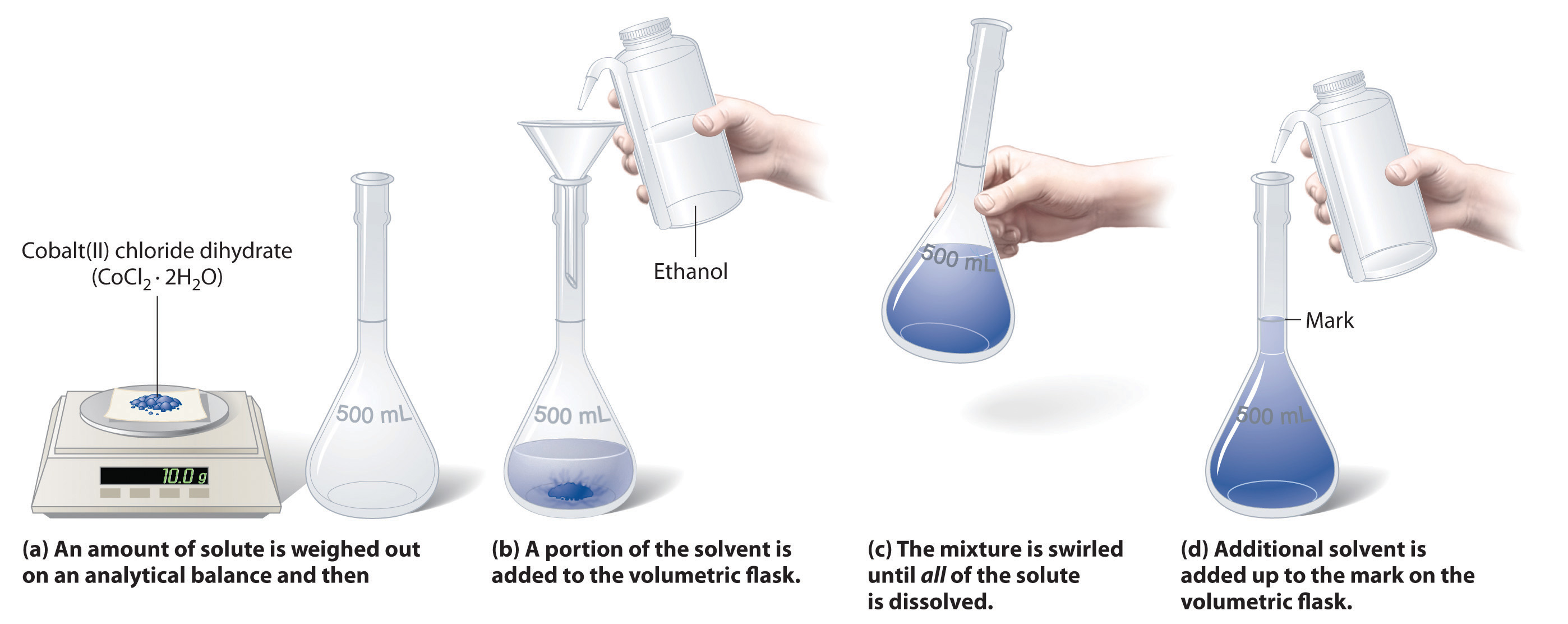
Example \(\PageIndex{1}\): Calculating Moles from Concentration of NaOH
Calculate the number of moles of sodium hydroxide (NaOH) in 2.50 L of 0.100 M NaOH.
Given: identity of solute and volume and molarity of solution
Asked for: amount of solute in moles
Use either Equation \ref{4.5.2} or Equation \ref{4.5.3}, depending on the units given in the problem.
Because we are given the volume of the solution in liters and are asked for the number of moles of substance, Equation \ref{4.5.2} is more useful:
\( moles\: NaOH = V_L M_{mol/L} = (2 .50\: \cancel{L} ) \left( \dfrac{0.100\: mol } {\cancel{L}} \right) = 0 .250\: mol\: NaOH \)
Exercise \(\PageIndex{1}\): Calculating Moles from Concentration of Alanine
Calculate the number of millimoles of alanine, a biologically important molecule, in 27.2 mL of 1.53 M alanine.
Calculations Involving Molarity (M): Calculations Involving Molarity (M), YouTube(opens in new window) [youtu.be]
Concentrations are also often reported on a mass-to-mass (m/m) basis or on a mass-to-volume (m/v) basis, particularly in clinical laboratories and engineering applications. A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution; a concentration on an m/v basis is the number of grams of solute per milliliter of solution. Each measurement can be expressed as a percentage by multiplying the ratio by 100; the result is reported as percent m/m or percent m/v. The concentrations of very dilute solutions are often expressed in parts per million ( ppm ), which is grams of solute per 10 6 g of solution, or in parts per billion ( ppb ), which is grams of solute per 10 9 g of solution. For aqueous solutions at 20°C, 1 ppm corresponds to 1 μg per milliliter, and 1 ppb corresponds to 1 ng per milliliter. These concentrations and their units are summarized in Table \(\PageIndex{1}\).
The Preparation of Solutions
To prepare a solution that contains a specified concentration of a substance, it is necessary to dissolve the desired number of moles of solute in enough solvent to give the desired final volume of solution. Figure \(\PageIndex{1}\) illustrates this procedure for a solution of cobalt(II) chloride dihydrate in ethanol. Note that the volume of the solvent is not specified. Because the solute occupies space in the solution, the volume of the solvent needed is almost always less than the desired volume of solution. For example, if the desired volume were 1.00 L, it would be incorrect to add 1.00 L of water to 342 g of sucrose because that would produce more than 1.00 L of solution. As shown in Figure \(\PageIndex{2}\), for some substances this effect can be significant, especially for concentrated solutions.
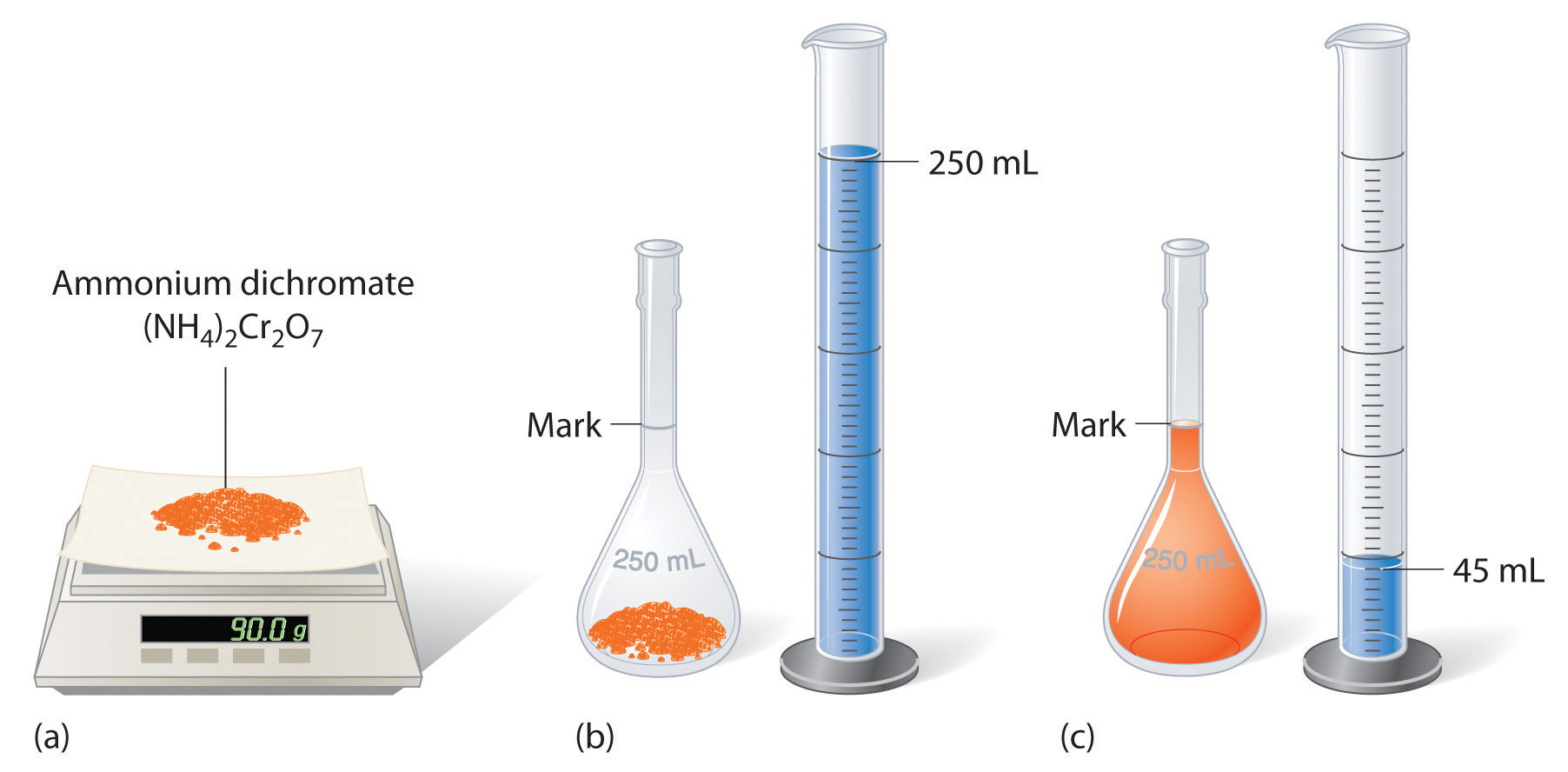
Example \(\PageIndex{2}\)
The solution contains 10.0 g of cobalt(II) chloride dihydrate, CoCl 2 •2H 2 O, in enough ethanol to make exactly 500 mL of solution. What is the molar concentration of \(\ce{CoCl2•2H2O}\)?
Given: mass of solute and volume of solution
Asked for: concentration (M)
To find the number of moles of \(\ce{CoCl2•2H2O}\), divide the mass of the compound by its molar mass. Calculate the molarity of the solution by dividing the number of moles of solute by the volume of the solution in liters.
The molar mass of CoCl 2 •2H 2 O is 165.87 g/mol. Therefore,
\[ moles\: CoCl_2 \cdot 2H_2O = \left( \dfrac{10.0 \: \cancel{g}} {165 .87\: \cancel{g} /mol} \right) = 0 .0603\: mol \nonumber \]
The volume of the solution in liters is
\[ volume = 500\: \cancel{mL} \left( \dfrac{1\: L} {1000\: \cancel{mL}} \right) = 0 .500\: L \nonumber \]
Molarity is the number of moles of solute per liter of solution, so the molarity of the solution is
\[ molarity = \dfrac{0.0603\: mol} {0.500\: L} = 0.121\: M = CoCl_2 \cdot H_2O \nonumber \]
Exercise \(\PageIndex{2}\)
The solution shown in Figure \(\PageIndex{2}\) contains 90.0 g of (NH 4 ) 2 Cr 2 O 7 in enough water to give a final volume of exactly 250 mL. What is the molar concentration of ammonium dichromate?
\[(NH_4)_2Cr_2O_7 = 1.43\: M \nonumber \]
To prepare a particular volume of a solution that contains a specified concentration of a solute, we first need to calculate the number of moles of solute in the desired volume of solution using the relationship shown in Equation \(\ref{4.5.2}\). We then convert the number of moles of solute to the corresponding mass of solute needed. This procedure is illustrated in Example \(\PageIndex{3}\).
Example \(\PageIndex{3}\): D5W Solution
The so-called D5W solution used for the intravenous replacement of body fluids contains 0.310 M glucose. (D5W is an approximately 5% solution of dextrose [the medical name for glucose] in water.) Calculate the mass of glucose necessary to prepare a 500 mL pouch of D5W. Glucose has a molar mass of 180.16 g/mol.
Given: molarity, volume, and molar mass of solute
Asked for: mass of solute
- Calculate the number of moles of glucose contained in the specified volume of solution by multiplying the volume of the solution by its molarity.
- Obtain the mass of glucose needed by multiplying the number of moles of the compound by its molar mass.
A We must first calculate the number of moles of glucose contained in 500 mL of a 0.310 M solution:
\( V_L M_{mol/L} = moles \)
\( 500\: \cancel{mL} \left( \dfrac{1\: \cancel{L}} {1000\: \cancel{mL}} \right) \left( \dfrac{0 .310\: mol\: glucose} {1\: \cancel{L}} \right) = 0 .155\: mol\: glucose \)
B We then convert the number of moles of glucose to the required mass of glucose:
\( mass \: of \: glucose = 0.155 \: \cancel{mol\: glucose} \left( \dfrac{180.16 \: g\: glucose} {1\: \cancel{mol\: glucose}} \right) = 27.9 \: g \: glucose \)
Exercise \(\PageIndex{3}\)
Another solution commonly used for intravenous injections is normal saline, a 0.16 M solution of sodium chloride in water. Calculate the mass of sodium chloride needed to prepare 250 mL of normal saline solution.
A solution of a desired concentration can also be prepared by diluting a small volume of a more concentrated solution with additional solvent. A stock solution is a commercially prepared solution of known concentration and is often used for this purpose. Diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is difficult to carry out with a high degree of accuracy. Dilution is also used to prepare solutions from substances that are sold as concentrated aqueous solutions, such as strong acids.
The procedure for preparing a solution of known concentration from a stock solution is shown in Figure \(\PageIndex{3}\). It requires calculating the number of moles of solute desired in the final volume of the more dilute solution and then calculating the volume of the stock solution that contains this amount of solute. Remember that diluting a given quantity of stock solution with solvent does not change the number of moles of solute present. The relationship between the volume and concentration of the stock solution and the volume and concentration of the desired diluted solution is therefore
\[(V_s)(M_s) = moles\: of\: solute = (V_d)(M_d)\label{4.5.4} \]
where the subscripts s and d indicate the stock and dilute solutions, respectively. Example \(\PageIndex{4}\) demonstrates the calculations involved in diluting a concentrated stock solution.
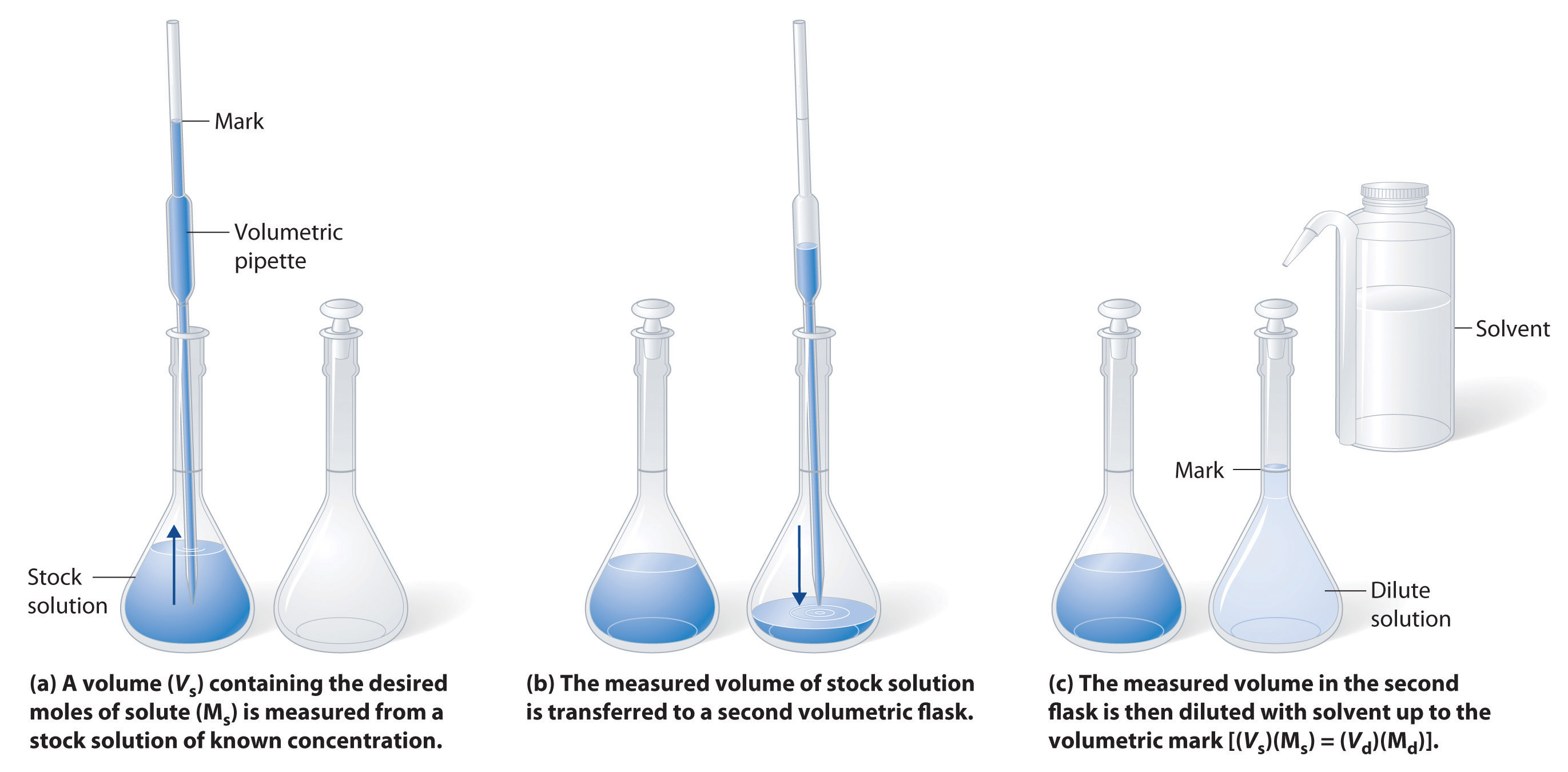
Example \(\PageIndex{4}\)
What volume of a 3.00 M glucose stock solution is necessary to prepare 2500 mL of the D5W solution in Example \(\PageIndex{3}\)?
Given: volume and molarity of dilute solution
Asked for: volume of stock solution
- Calculate the number of moles of glucose contained in the indicated volume of dilute solution by multiplying the volume of the solution by its molarity.
- To determine the volume of stock solution needed, divide the number of moles of glucose by the molarity of the stock solution.
A The D5W solution in Example 4.5.3 was 0.310 M glucose. We begin by using Equation 4.5.4 to calculate the number of moles of glucose contained in 2500 mL of the solution:
\[ moles\: glucose = 2500\: \cancel{mL} \left( \dfrac{1\: \cancel{L}} {1000\: \cancel{mL}} \right) \left( \dfrac{0 .310\: mol\: glucose} {1\: \cancel{L}} \right) = 0 .775\: mol\: glucose \nonumber \]
B We must now determine the volume of the 3.00 M stock solution that contains this amount of glucose:
\[ volume\: of\: stock\: soln = 0 .775\: \cancel{mol\: glucose} \left( \dfrac{1\: L} {3 .00\: \cancel{mol\: glucose}} \right) = 0 .258\: L\: or\: 258\: mL \nonumber \]
In determining the volume of stock solution that was needed, we had to divide the desired number of moles of glucose by the concentration of the stock solution to obtain the appropriate units. Also, the number of moles of solute in 258 mL of the stock solution is the same as the number of moles in 2500 mL of the more dilute solution; only the amount of solvent has changed . The answer we obtained makes sense: diluting the stock solution about tenfold increases its volume by about a factor of 10 (258 mL → 2500 mL). Consequently, the concentration of the solute must decrease by about a factor of 10, as it does (3.00 M → 0.310 M).
We could also have solved this problem in a single step by solving Equation 4.5.4 for V s and substituting the appropriate values:
\[ V_s = \dfrac{( V_d )(M_d )}{M_s} = \dfrac{(2 .500\: L)(0 .310\: \cancel{M} )} {3 .00\: \cancel{M}} = 0 .258\: L \nonumber \]
As we have noted, there is often more than one correct way to solve a problem.
Exercise \(\PageIndex{4}\)
What volume of a 5.0 M NaCl stock solution is necessary to prepare 500 mL of normal saline solution (0.16 M NaCl)?
Ion Concentrations in Solution
In Example \(\PageIndex{2}\), the concentration of a solution containing 90.00 g of ammonium dichromate in a final volume of 250 mL were calculated to be 1.43 M. Let’s consider in more detail exactly what that means. Ammonium dichromate is an ionic compound that contains two NH 4 + ions and one Cr 2 O 7 2 − ion per formula unit. Like other ionic compounds, it is a strong electrolyte that dissociates in aqueous solution to give hydrated NH 4 + and Cr 2 O 7 2 − ions:
\[ (NH_4 )_2 Cr_2 O_7 (s) \xrightarrow {H_2 O(l)} 2NH_4^+ (aq) + Cr_2 O_7^{2-} (aq)\label{4.5.5} \]
Thus 1 mol of ammonium dichromate formula units dissolves in water to produce 1 mol of Cr 2 O 7 2 − anions and 2 mol of NH 4 + cations (see Figure \(\PageIndex{4}\)).
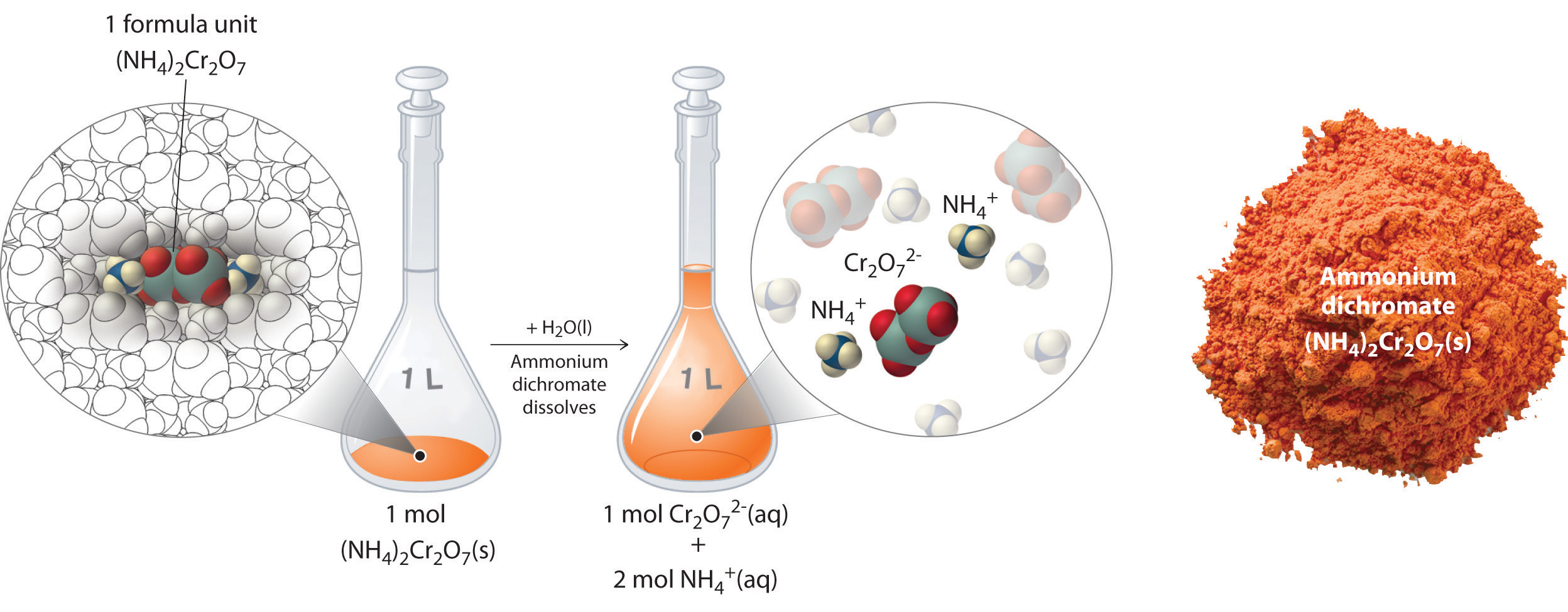
When carrying out a chemical reaction using a solution of a salt such as ammonium dichromate, it is important to know the concentration of each ion present in the solution. If a solution contains 1.43 M (NH 4 ) 2 Cr 2 O 7 , then the concentration of Cr 2 O 7 2 − must also be 1.43 M because there is one Cr 2 O 7 2 − ion per formula unit. However, there are two NH 4 + ions per formula unit, so the concentration of NH 4 + ions is 2 × 1.43 M = 2.86 M. Because each formula unit of (NH 4 ) 2 Cr 2 O 7 produces three ions when dissolved in water (2NH 4 + + 1Cr 2 O 7 2 − ), the total concentration of ions in the solution is 3 × 1.43 M = 4.29 M.
Concentration of Ions in Solution from a Soluble Salt: Concentration of Ions in Solution from a Soluble Salt, YouTube(opens in new window) [youtu.be]
Example \(\PageIndex{5}\)
What are the concentrations of all species derived from the solutes in these aqueous solutions?
- 0.21 M NaOH
- 3.7 M (CH 3 ) 2 CHOH
- 0.032 M In(NO 3 ) 3
Given: molarity
Asked for: concentrations
A Classify each compound as either a strong electrolyte or a nonelectrolyte.
B If the compound is a nonelectrolyte, its concentration is the same as the molarity of the solution. If the compound is a strong electrolyte, determine the number of each ion contained in one formula unit. Find the concentration of each species by multiplying the number of each ion by the molarity of the solution.
B Because each formula unit of NaOH produces one Na + ion and one OH − ion, the concentration of each ion is the same as the concentration of NaOH: [Na + ] = 0.21 M and [OH − ] = 0.21 M.
B The only solute species in solution is therefore (CH 3 ) 2 CHOH molecules, so [(CH 3 ) 2 CHOH] = 3.7 M.
\( In(NO _3 ) _3 (s) \xrightarrow {H_ 2 O(l)} In ^{3+} (aq) + 3NO _3^- (aq) \)
B One formula unit of In(NO 3 ) 3 produces one In 3 + ion and three NO 3 − ions, so a 0.032 M In(NO 3 ) 3 solution contains 0.032 M In 3 + and 3 × 0.032 M = 0.096 M NO 3 – —that is, [In 3 + ] = 0.032 M and [NO 3 − ] = 0.096 M.
Exercise \(\PageIndex{5}\)
- 0.0012 M Ba(OH) 2
- 0.17 M Na 2 SO 4
- 0.50 M (CH 3 ) 2 CO , commonly known as acetone
\([Ba^{2+}] = 0.0012\: M; \: [OH^-] = 0.0024\: M\)
\([Na^+] = 0.34\: M; \: [SO_4^{2-}] = 0.17\: M\)
\([(CH_3)_2CO] = 0.50\: M\)
Solution concentrations are typically expressed as molarities and can be prepared by dissolving a known mass of solute in a solvent or diluting a stock solution.
- definition of molarity: \[ molarity = \dfrac{moles\: of\: solute}{liters\: of\: solution} = \dfrac{mmoles\: of\: solute} {milliliters\: of\: solution} \nonumber \]
- relationship among volume, molarity, and moles : \[ V_L M_{mol/L} = \cancel{L} \left( \dfrac{mol}{\cancel{L}} \right) = moles \nonumber \]
- relationship between volume and concentration of stock and dilute solutions : \[(V_s)(M_s) = moles\: of\: solute = (V_d)(M_d) \nonumber \]
The concentration of a substance is the quantity of solute present in a given quantity of solution. Concentrations are usually expressed in terms of molarity , defined as the number of moles of solute in 1 L of solution. Solutions of known concentration can be prepared either by dissolving a known mass of solute in a solvent and diluting to a desired final volume or by diluting the appropriate volume of a more concentrated solution (a stock solution ) to the desired final volume.
Contributors and Attributions
Modified by Joshua Halpern ( Howard University )
Why RAG won’t solve generative AI’s hallucination problem

Hallucinations — the lies generative AI models tell, basically — are a big problem for businesses looking to integrate the technology into their operations.
Because models have no real intelligence and are simply predicting words, images, speech, music and other data according to a private schema , they sometimes get it wrong. Very wrong. In a recent piece in The Wall Street Journal, a source recounts an instance where Microsoft’s generative AI invented meeting attendees and implied that conference calls were about subjects that weren’t actually discussed on the call.
As I wrote a while ago, hallucinations may be an unsolvable problem with today’s transformer-based model architectures. But a number of generative AI vendors suggest that they can be done away with, more or less, through a technical approach called retrieval augmented generation, or RAG.
Here’s how one vendor, Squirro, pitches it :
At the core of the offering is the concept of Retrieval Augmented LLMs or Retrieval Augmented Generation (RAG) embedded in the solution … [our generative AI] is unique in its promise of zero hallucinations. Every piece of information it generates is traceable to a source, ensuring credibility.
Here’s a similar pitch from SiftHub:
Using RAG technology and fine-tuned large language models with industry-specific knowledge training, SiftHub allows companies to generate personalized responses with zero hallucinations. This guarantees increased transparency and reduced risk and inspires absolute trust to use AI for all their needs.
RAG was pioneered by data scientist Patrick Lewis, researcher at Meta and University College London, and lead author of the 2020 paper that coined the term. Applied to a model, RAG retrieves documents possibly relevant to a question — for example, a Wikipedia page about the Super Bowl — using what’s essentially a keyword search and then asks the model to generate answers given this additional context.
“When you’re interacting with a generative AI model like ChatGPT or Llama and you ask a question, the default is for the model to answer from its ‘parametric memory’ — i.e., from the knowledge that’s stored in its parameters as a result of training on massive data from the web,” David Wadden, a research scientist at AI2, the AI-focused research division of the nonprofit Allen Institute, explained. “But, just like you’re likely to give more accurate answers if you have a reference [like a book or a file] in front of you, the same is true in some cases for models.”
RAG is undeniably useful — it allows one to attribute things a model generates to retrieved documents to verify their factuality (and, as an added benefit, avoid potentially copyright-infringing regurgitation ). RAG also lets enterprises that don’t want their documents used to train a model — say, companies in highly regulated industries like healthcare and law — to allow models to draw on those documents in a more secure and temporary way.
But RAG certainly can’t stop a model from hallucinating. And it has limitations that many vendors gloss over.
Wadden says that RAG is most effective in “knowledge-intensive” scenarios where a user wants to use a model to address an “information need” — for example, to find out who won the Super Bowl last year. In these scenarios, the document that answers the question is likely to contain many of the same keywords as the question (e.g., “Super Bowl,” “last year”), making it relatively easy to find via keyword search.
Things get trickier with “reasoning-intensive” tasks such as coding and math, where it’s harder to specify in a keyword-based search query the concepts needed to answer a request — much less identify which documents might be relevant.
Even with basic questions, models can get “distracted” by irrelevant content in documents, particularly in long documents where the answer isn’t obvious. Or they can — for reasons as yet unknown — simply ignore the contents of retrieved documents, opting instead to rely on their parametric memory.
RAG is also expensive in terms of the hardware needed to apply it at scale.
That’s because retrieved documents, whether from the web, an internal database or somewhere else, have to be stored in memory — at least temporarily — so that the model can refer back to them. Another expenditure is compute for the increased context a model has to process before generating its response. For a technology already notorious for the amount of compute and electricity it requires even for basic operations, this amounts to a serious consideration.
That’s not to suggest RAG can’t be improved. Wadden noted many ongoing efforts to train models to make better use of RAG-retrieved documents.
Some of these efforts involve models that can “decide” when to make use of the documents, or models that can choose not to perform retrieval in the first place if they deem it unnecessary. Others focus on ways to more efficiently index massive datasets of documents, and on improving search through better representations of documents — representations that go beyond keywords.
“We’re pretty good at retrieving documents based on keywords, but not so good at retrieving documents based on more abstract concepts, like a proof technique needed to solve a math problem,” Wadden said. “ Research is needed to build document representations and search techniques that can identify relevant documents for more abstract generation tasks. I think this is mostly an open question at this point.”
So RAG can help reduce a model’s hallucinations — but it’s not the answer to all of AI’s hallucinatory problems. Beware of any vendor that tries to claim otherwise.

IMAGES
VIDEO
COMMENTS
PROBLEM 6.1.1.6 6.1.1. 6. Calculate the molarity of each of the following solutions: (a) 0.195 g of cholesterol, C 27 H 46 O, in 0.100 L of serum, the average concentration of cholesterol in human serum. (b) 4.25 g of NH 3 in 0.500 L of solution, the concentration of NH 3 in household ammonia.
CHEMFILE MINI-GUIDE TO PROBLEM SOLVING General Plan for Solving Molarity Problems Mass of solute in g 1 Amount of solute in mol M moles solute liter solution 2 Volume of solution in L 3 Molar concentration, M 4 Convert using the molar mass of the solute. MOLARITY Molarity is the most common way to express concentration in chemistry.
Example (1): Calculate the molar concentration of ethanol in an aqueous solution that contains 2.30 g of C2H5OH (46.07 g/mol) in 3.50 L of solution. Solution: - To calculate molar concentration, we must find both the amount of ethanol and the volume of the solution. - The volume is given as 3.50 L, so all we need to do is convert the mass ...
The following video looks at calculating concentration of solutions. We will look at a sample problem dealing with mass/volume percent (m/v)%. Example: Many people use a solution of sodium phosphate (Na 3 PO 4 - commonly called TSP), to clean walls before putting up wallpaper. The recommended concentration is 1.7% (m/v).
Concentration of a solution. 15 g KCl is added to 45 g water to form an aqueous solution. Calculate the mass percent of the KCl solution. % . Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of providing a free, world ...
3. Divide the mass of the solute by the total mass of the solution. Set up your equation so the concentration C = mass of the solute/total mass of the solution. Plug in your values and solve the equation to find the concentration of your solution. [6] In our example, C = (10 g)/ (1,210 g) = 0.00826. 4.
Problem #15: Determine concentration of a solution that contains 825 mg of Na 2 HPO 4 dissolved in 450.0 mL of water in (a) molarity, (b) molality, (c) mole fraction, (d) mass %, and (e) ppm. Assume the density of the solution is the same as water (1.00 g/mL). Assume no volume change upon the addition of the solute. Solution: 1) Molarity:
Concentrations of Solutions. Concentrations of Solutions. There are a number of ways to express the relative amounts of solute and solvent in a solution. This page describes calculations for four different units used to express concentration: Percent Composition (by mass) Molarity. Molality.
Calculating concentration of a solution. A solution of fructose ( C 6 H 12 O 6 ) in water is 15 % (w/w) . What is the mole fraction of each of the components of the solution? Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the ...
Practice Problems - Concentrations of Solutions. 1. What is the mass percent of a solution of 7.6 grams sucrose in 83.4 grams of water? 2. How many grams of sucrose must be added to 375 grams of water to prepare a 2.75 % by mass solution of sucrose? 3. A saline solution, NaCl in water, is 0.92 % (m/v). How many grams of NaCl are required to ...
Because the concentration is a percent, you know a 100-gram sample would contain 12 grams of iron. You can set this up as an equation and solve for the unknown "x": 12 g iron / 100 g sample = x g iron / 250 g sample. Cross-multiply and divide: x= (12 x 250) / 100 = 30 grams of iron.
Use either Equation 4.5 or Equation 4.6, depending on the units given in the problem. Solution: Because we are given the volume of the solution in liters and are asked for the number of moles of substance, Equation 4.5 is more useful: moles NaOH = V L M mol/L = (2 .50 L) ( 0 .100 mol L) = 0 .250 mol NaOH. Exercise.
Molarity. The most common way to express solution concentration is molarity (M), which is defined as the amount of solute in moles divided by the volume of solution in liters: M = moles of solute/liters of solution. A solution that is 1.00 molar (written 1.00 M) contains 1.00 mole of solute for every liter of solution. Created by Sal Khan.
Concentration in Parts per Million. It is expressed in terms of weight. The formula for parts per million is given as follows: ppm (A)=. Mass of A Total mass of the solution Mass of A Total mass of the solution. x10. 6 6. Mass Percentage (w/w) It is expressed in terms of mass percentage of solute to the solution.
Sample Problems on Concentration of Solution. Problem 1: 15 g of common salt is dissolved in 400 g of water. Calculate the concentration of the solution by expressing it in Mass by Mass percentage (w/w%). Solution: Given that, Mass of solute (common salt) = 15 g ….
V i = 300 mL. M D = 0.25 M. V D = final volume. Solve for V D. V D = 1800 mL = 1.8 L. Answer: You can make 1800 mL of 0.25 M NaCl solution from 300 mL of 1.5 M NaCl solution. A dilution is where the concentration of a solution is lowered by adding solvent to the solution. These dilution example problems show how to dilute a solution.
A dilute solution means the quantity of solute is relatively very small, and a concentrated solution implies that the solution has a large amount of solute. But these are relative terms and do not give us the quantitative concentration of the solution. Table of Contents. Concentration; Recommended Video; Concentration in parts per million; Mass ...
06 May 2024 0. The decision by the ANC to not proceed with former president Jacob Zuma's disciplinary hearing underscores how the governing party has found it impossible to manage the situation ...
As AI becomes more powerful, it faces a major trust problem. Consider 12 leading concerns: disinformation, safety and security, the black box problem, ethical concerns, bias, instability ...
WASHINGTON—The May edition of AARP Bulletin offers readers professional tips to solve 26 everyday problems.Bulletin editors sought guidance from dozens of top professional field readers' burning questions.Their tips and solutions cover the gamut of life's sources of daily annoyances and confusion - from health, to finances, to home and tech-related issues.
Therefore, the solution pH was adjusted to 3, the concentration of Pinus patula BC was 13.5 g/L in 200 mL of WW, and the BC particle size was 300-450 µm. A number of 10 mL volume aliquots were taken at 5, 15, 30, 60 and 120 min, and filtered with a nylon syringe filter (0.45 µm of pore diameter) to analyse the performance of the elimination ...
Molarity. The most common unit of concentration is molarity, which is also the most useful for calculations involving the stoichiometry of reactions in solution.The molarity (M) is defined as the number of moles of solute present in exactly 1 L of solution.It is, equivalently, the number of millimoles of solute present in exactly 1 mL of solution:
RAG is being pitched as a solution of sorts to generative AI hallucinations. But there's limits to what the technique can do. ... like a proof technique needed to solve a math problem," Wadden said.