
Presentations made painless
- Get Premium

119 Alzheimer’s Disease Essay Topic Ideas & Examples
Inside This Article
Alzheimer's Disease is a devastating neurodegenerative condition that affects millions of people worldwide. As researchers continue to search for a cure, raising awareness about the disease becomes crucial. One effective way to do this is through the written word, which allows for a deeper exploration of the topic. If you are tasked with writing an essay on Alzheimer's Disease but are struggling to come up with a topic, fear not! Here are 119 Alzheimer's Disease essay topic ideas and examples to inspire your writing:
- The history and discovery of Alzheimer's Disease.
- The impact of Alzheimer's Disease on families and caregivers.
- The role of genetics in Alzheimer's Disease development.
- Exploring the connection between Alzheimer's Disease and other neurodegenerative disorders.
- The societal and economic burden of Alzheimer's Disease.
- Alzheimer's Disease and its impact on the aging population.
- Understanding the stages of Alzheimer's Disease progression.
- The diagnostic methods and tools used to detect Alzheimer's Disease.
- The role of neuroimaging in diagnosing and monitoring Alzheimer's Disease.
- The physiological changes in the brain during Alzheimer's Disease.
- Investigating the link between Alzheimer's Disease and inflammation.
- The importance of early detection and intervention in Alzheimer's Disease.
- The impact of lifestyle choices on Alzheimer's Disease risk.
- The potential role of nutrition in preventing or managing Alzheimer's Disease.
- The relationship between Alzheimer's Disease and cardiovascular health.
- The impact of environmental factors on Alzheimer's Disease development.
- The role of oxidative stress in Alzheimer's Disease.
- Investigating the connection between sleep disturbances and Alzheimer's Disease.
- The potential benefits of exercise in preventing or slowing down Alzheimer's Disease.
- The impact of chronic stress on Alzheimer's Disease risk.
- Exploring the connection between type 2 diabetes and Alzheimer's Disease.
- The role of hormonal changes in Alzheimer's Disease.
- Investigating the impact of traumatic brain injuries on Alzheimer's Disease risk.
- The potential benefits of cognitive training in Alzheimer's Disease management.
- The use of medication in treating Alzheimer's Disease symptoms.
- Investigating alternative therapies for Alzheimer's Disease.
- The ethical implications of using animal models in Alzheimer's Disease research.
- The impact of Alzheimer's Disease on memory and cognition.
- The potential role of stem cells in Alzheimer's Disease treatment.
- The relationship between Alzheimer's Disease and depression.
- Exploring the impact of Alzheimer's Disease on language and communication.
- The use of music therapy in improving the quality of life for Alzheimer's Disease patients.
- The psychological impact of Alzheimer's Disease on patients and their families.
- The potential benefits of art therapy in Alzheimer's Disease management.
- Investigating the impact of social isolation on Alzheimer's Disease progression.
- The challenges of providing care for Alzheimer's Disease patients in rural areas.
- The impact of Alzheimer's Disease on sensory perception.
- Exploring the connection between Alzheimer's Disease and personality changes.
- The potential benefits of aromatherapy in managing Alzheimer's Disease symptoms.
- The use of virtual reality in improving cognitive function in Alzheimer's Disease patients.
- Investigating the impact of Alzheimer's Disease on motor skills.
- The role of inflammation in Alzheimer's Disease-related behavioral changes.
- The potential benefits of mindfulness meditation in Alzheimer's Disease management.
- The impact of Alzheimer's Disease on the sense of self and identity.
- Exploring the relationship between Alzheimer's Disease and sleep disorders.
- The potential benefits of animal-assisted therapy for Alzheimer's Disease patients.
- The role of the immune system in Alzheimer's Disease progression.
- Investigating the impact of Alzheimer's Disease on visual perception.
- The potential benefits of reminiscence therapy in Alzheimer's Disease management.
- The impact of Alzheimer's Disease on executive function and decision-making.
- Exploring the connection between Alzheimer's Disease and aggression.
- The potential benefits of dance therapy in improving motor function in Alzheimer's Disease patients.
- The role of neuroplasticity in Alzheimer's Disease treatment.
- Investigating the impact of Alzheimer's Disease on the sense of time.
- The potential benefits of horticultural therapy in Alzheimer's Disease management.
- The impact of Alzheimer's Disease on spatial orientation and navigation.
- Exploring the connection between Alzheimer's Disease and hallucinations.
- The potential benefits of laughter therapy in improving mood in Alzheimer's Disease patients.
- The role of neuroinflammation in Alzheimer's Disease progression.
- Investigating the impact of Alzheimer's Disease on taste and smell.
- The potential benefits of pet therapy for Alzheimer's Disease patients.
- The impact of Alzheimer's Disease on social cognition and empathy.
- Exploring the connection between Alzheimer's Disease and apathy.
- The potential benefits of light therapy in managing sleep disturbances in Alzheimer's Disease.
- The role of neurodegeneration in Alzheimer's Disease.
- Investigating the impact of Alzheimer's Disease on emotional processing.
- The potential benefits of intergenerational programs for Alzheimer's Disease patients.
- The impact of Alzheimer's Disease on the ability to recognize faces and objects.
- Exploring the connection between Alzheimer's Disease and delusions.
- The potential benefits of humor therapy in improving well-being in Alzheimer's Disease patients.
- The role of neurovascular dysfunction in Alzheimer's Disease progression.
- Investigating the impact of Alzheimer's Disease on the ability to navigate familiar environments.
- The potential benefits of art classes for Alzheimer's Disease patients.
- The impact of Alzheimer's Disease on the ability to understand and express emotions.
- Exploring the connection between Alzheimer's Disease and anxiety.
- The potential benefits of yoga in managing stress in Alzheimer's Disease.
- The role of neurofibrillary tangles in Alzheimer's Disease.
- Investigating the impact of Alzheimer's Disease on the ability to perform daily tasks.
- The potential benefits of gardening programs for Alzheimer's Disease patients.
- The impact of Alzheimer's Disease on social interactions and relationships.
- Exploring the connection between Alzheimer's Disease and wandering behavior.
- The potential benefits of massage therapy in improving relaxation in Alzheimer's Disease patients.
- The role of amyloid plaques in Alzheimer's Disease progression.
- Investigating the impact of Alzheimer's Disease on the ability to recognize and interpret emotions in others.
- The potential benefits of cooking classes for Alzheimer's Disease patients.
- The impact of Alzheimer's Disease on the ability to engage in hobbies and interests.
- Exploring the connection between Alzheimer's Disease and sleep disturbances.
- The potential benefits of reminiscence therapy in improving memory in Alzheimer's Disease patients.
- The role of immune dysfunction in Alzheimer's Disease.
- Investigating the impact of Alzheimer's Disease on the ability to engage in conversation.
- The potential benefits of music classes for Alzheimer's Disease patients.
- The impact of Alzheimer's Disease on the ability to maintain personal hygiene.
- Exploring the connection between Alzheimer's Disease and emotional lability.
- The potential benefits of drama therapy in improving communication skills in Alzheimer's Disease patients.
- The role of mitochondrial dysfunction in Alzheimer's Disease progression.
- Investigating the impact of Alzheimer's Disease on the ability to recognize familiar places.
- The potential benefits of pet ownership for Alzheimer's Disease patients.
- The impact of Alzheimer's Disease on the ability to engage in leisure activities.
- Exploring the connection between Alzheimer's Disease and repetitive behaviors.
- The potential benefits of puzzle-solving activities in improving cognitive function in Alzheimer's Disease patients.
- The role of neuroinflammation in Alzheimer's Disease-related psychosis.
- Investigating the impact of Alzheimer's Disease on the ability to plan and organize.
- The potential benefits of dance classes for Alzheimer's Disease patients.
- The impact of Alzheimer's Disease on the ability to navigate new environments.
- Exploring the connection between Alzheimer's Disease and hoarding behavior.
- The potential benefits of aroma classes in managing anxiety in Alzheimer's Disease.
- The role of tau protein in Alzheimer's Disease progression.
- Investigating the impact of Alzheimer's Disease on the ability to recognize familiar faces.
- The potential benefits of knitting or crochet classes for Alzheimer's Disease patients.
- The impact of Alzheimer's Disease on the ability to engage in social activities.
- Exploring the connection between Alzheimer's Disease and sleep-wake cycle disturbances.
- The potential benefits of nature walks in improving well-being in Alzheimer's Disease patients.
- The role of neuroinflammation in Alzheimer's Disease-related depression.
- Investigating the impact of Alzheimer's Disease on the ability to follow directions.
- The potential benefits of cooking therapy in improving nutrition in Alzheimer's Disease patients.
- The impact of Alzheimer's Disease on the ability to recognize spatial relationships.
- Exploring the connection between Alzheimer's Disease and sundowning syndrome.
- The potential benefits of mindfulness classes in managing stress in Alzheimer's Disease.
- The role of neurogenesis in Alzheimer's Disease treatment.
These 119 essay topic ideas and examples should provide you with a starting point to delve into the complex and multifaceted world of Alzheimer's Disease. Remember to choose a topic that resonates with you and allows you to explore the aspects of the disease that interest you the most. Through your writing, you can contribute to raising awareness, promoting understanding, and supporting the ongoing efforts to find a cure for Alzheimer's Disease.
Want to create a presentation now?
Instantly Create A Deck
Let PitchGrade do this for me
Hassle Free
We will create your text and designs for you. Sit back and relax while we do the work.
Explore More Content
- Privacy Policy
- Terms of Service
© 2023 Pitchgrade
Alzheimers Disease - Free Essay Examples And Topic Ideas
When writing an essay on Alzheimer’s disease, it is crucial to understand the complexities of this condition. It is necessary to effectively convey its impact on individuals’ memory and overall health. Alzheimer’s is a form of dementia that primarily affects older Americans.
Crafting a comprehensive essay introduction and conclusion for Alzheimer’s disease requires careful consideration of the key aspects of the topic. To begin, familiarize yourself with Alzheimer’s disease essay examples. This way, you can understand how to accurately present your research and content. These examples will help you choose relevant essay topics and formulate thesis statements reflecting your paper’s main arguments. In addition, ensure your paper has a well-structured outline that organizes your ideas logically. Devote separate paragraphs to each aspect of Alzheimer’s disease. It can be symptoms, diagnosis, and available treatments.
In your introduction, provide a brief overview of Alzheimer’s disease, its prevalence, and the brain changes that occur. Involve the reader by highlighting the significance of age, which plays a significant role in the development and progression of the disease. In the body of your essay, explore the causes, risk factors, and progression of the illness. Discuss the impact on neurons in the brain and the resulting cognitive decline. Also, don’t forget to mention how it accelerates memory loss. Highlight the need for improved orientation and care for patients. Support your arguments with evidence from Alzheimer’s disease research paper works and reputable sources. Remember to cite any sources used in your essay and consider including a list of free essays on Alzheimer’s disease or additional resources for readers seeking further information.

A Research Paper on Alzheimer’s Disease
Abstract In this paper, Alzheimer's disease will be delved into, investigated and dissected. This will include all that is known about the disease as much of it is unknown still, despite increasing efforts from the medical community to uncover its origin. The disease's causes, symptoms and stages will be discussed and illuminated. The effects on other body systems, its signs and symptoms and any other complications will be highlighted as well. Additionally, advancements in treating this disease are carefully examined. […]
Alzheimer’s Disease Still Alice
Alzheimer's Disease is a devastating illness which affects 30 million people worldwide. This disease develops gradually over time and worsens with every year; it destroys past memories and cognitive functions. AD impairs its victim and gradually makes them less of a person and more of a hollowed shell with no life experiences or knowledge of how to perform daily tasks (of which they can remember). Alzheimer's Disease is one of the most difficult mental illnesses a person can face; it […]
Alzheimer’s is a Disease and the Nervous System
Alzheimer's is a disease that is associated with the Nervous System. It was discovered by Alois Alzheimer in 1906. It results in loss of memory and cognitive abilities. These cognitive declines disrupt daily life due to their severity. The following with describe the Nervous System and the several aspects of the disease itself. The Nervous System is composed of numerous complex characteristics and it enables humans to process information and a reaction based on this information. It also allows humans […]
We will write an essay sample crafted to your needs.
Risk Factors from Alzheimer’s Disease
About 40 million people are affected by dementia, with the majority of these individuals being over the age of 60. This number is expected to double in the next 20 years, reaching around 80 million by 2050. Approximately 60-70% of all dementia cases are caused by Alzheimer's disease, affecting between 2.17 and 4.78 million people. Out of those, 46% have a moderate or severe form of the disease. It is estimated that by 2050, between 7.98 and 12.95 million people […]
Alzheimer’s Disease: Preserving Memories through Lifestyle Adjustments
Memories are made to be with us and make us happy every time we talk about it, but Alzheimer’s disease makes people forget their memories. Alzheimer’s disease is a type of dementia disease, causing an issue with memory, thinking, and behavior. There are many things cause Alzheimer ’s, but the main causing is age and some genes and gets worse and worse when people became older. Currently, there is no specific treatment and medicine for Alzheimer ’s, and the modern […]
Alzheimer’s Disease Memory and Cognition
Alzheimer's Disease (AD) affects memory and cognition. It is most common in the elderly, but symptoms may begin to show during mid-adulthood. There are three different stages of AD; mild, moderate, and severe. As we age our memory starts to diminish, we are no longer able to recognize certain places and people, we forget to do everydays tasks such as eat, shower, change, we start to present verbal problems, and odd behaviors such as disorientation and wandering. According to the […]
Alzheimer’s Disease and the Symptoms
Alzheimer's disease is a common dementia that slowly causes problem with your memory and behavior. Since the symptoms are not noticeable in the beginning the disease is hard to detect. alzheimers is said to affect people who are in their middle age or old age. Alzheimers disease was discovered by german physician dr. Alois alzheimer in 1906 when he discovered changes in patients brain tissues and changes in their behavior and the patient having a difficult time recalling memories. In […]
Research of Alzheimer’s Disease
Alzheimer's is one of the fastest working diseases. Eighty-three thousand people die each year from this disease. Two-thirds of the Alzheimer's population are women. Scientist believe that for most people, Alzheimer's is caused by a combination of genetic, lifestyle, and environmental factors. The seven stages of Alzheimer's are no impairment, very mild decline, mild decline, moderate decline, moderately severe decline, severe decline, and last is very severe decline. Education can lower your risk such as, taking classes, learning languages, and […]
Alzheimer’s Disease – Disease of the Brain
Alzheimer’s disease is a type of dementia where the nerves are damaged resulting in memory loss and behavioral changes. It affects people from the age of 40 or usually 65 and it worsens with the years. Patients with Alzheimer’s disease lose thinking skills and the ability to carry out even the easiest tasks. Experts say that it is more common in women than men. The main factors involved are amyloid plaques (abnormal clumps) and neurofibrillary (tangled bundles of fibers). Dr. […]
I was Here: Alzheimer’s Disease
Alzheimer's is an irreversible brain disorder that destroys memory and thinking skills over time. The sickness is the most common cause of dementia and death among older people, making it one of the few diseases that are deadly for older adults in the United States. The sickness is responsible for at least 500,000 annual deaths in the Country, but it affects many more people than that. In fact, it affects us all. So many people are affected by Alzheimer's every […]
Memories about my Past and High School
I have gone over my memories for the past several days; I am not sure how to explain my thoughts. I struggle on a day–to-day basis. I forget simple things and have for quite some time. I know a lot of it could be due to absent-mindedness or that I am trying to multi-task, but recently I have been getting nervous. My aunt and dad both suffer from Alzheimer’s. My aunt has been battling this disease for a few years, […]
Alzheimer’s Disease is a Growing Problem
With age comes wisdom, a growing family, and a chance to experience life in a different way. For some, growing older means being able to make more memories and share more experiences, but for others remembering those memories or experiences is only half of the struggle. What if recognizing family or enjoying the changes associated with growing older presented the challenges such as losing memories, not being able to complete simple everyday tasks, or even making decisions? These are the […]
The Physiology and Genetics Behind Alzheimer Disease
Alzheimer disease is a progressive and ultimately fatal brain disorder, in which communication between cells are halted and eventually lost. It is the most common form of dementia, and is generally (though not exclusively) diagnosed in patients over the age of 65. As communication amongst neurons is lost, symptoms such as inability to recall memories, make appropriate judgment, and proper motor function are lost and worsen over time. Affecting an estimated 2.4 million to 4.5 million Americans, with the number […]
What is Alzheimer’s Disease?
Alzheimer's is a structure of dementia that motives shrinking of the Genius in the affected person is susceptible to memory. Side effects through a large develop step through step and deteriorate after some time, getting to be extreme sufficient to block from each and every day undertakings. By large, sufferers with malady"those with the late-beginning compose"indications at first show up in their mid-60s. Early-beginning Alzheimer's occurs between a man's 30s and mid-60s and is extraordinarily uncommon. Alzheimer's sickness is the […]
Alzheimer’s an Unforgettable Disease
Memory, the gift of holding onto the things that we love, the things that define us, and the things we never want to neglect. Positive and negative, memories accompany us through life. Consider time passing, consider losing your ability to consciously store your memories. Additionally, consider the inability to communicate verbally, and recognize familiar places or individuals. This genetic shortcoming haunts you, depresses you and negatively impacts your quality of life. Within Kelly Cherry's expressive poem, "Alzheimer's" the relationship between […]
Causes of the Alzheimer’s Progress
Nutrition is vital to the health of an individual. It provides the necessary nutrients that enhance one's immunity thus preventing the body from infections. Additionally, it equips the body with the necessary mechanism that can fight infections even after development. Subsequently, nutrients are instrumental to the health of human beings. Alzheimer disease is a condition that progressively attacks the mental functions including memory and other cognitive aspects (Jevtic et al., 2017). Often, it starts as confusion and short memory loss. […]
MRI Based Techniques in Diagnosing Alzheimer’s Disease
ABSTRACT With an aging population comes associated health complications. Senior Citizens aged 65 years and older have many health deficits but the most feared is Alzheimer's disease. Alzheimer's Disease is a neurodegenerative brain disorder. A brain disorder of this kind causes significant alterations to normal brain functions as well as structural. Magnetic Resonance Imaging, also known as MRI, is a diagnostic imaging modality that is used to diagnose and stage this disease as well as multiple other age-specific brain disorders. […]
Two Main Types of Alzheimer’s Disease
Alzheimer's Disease (AD) is a degenerative brain disease that causes a significant amount of damage to neurons and eventually other parts of the brain. As the disease progresses the individual afflicted will lose the ability to perform basic functions such as walking and swallowing. The final stages of Alzheimer's leave the individual bed-ridden and in need of constant care until death occurs (Alzheimer's Association, 2016). AD has been recognized as the most common cause of dementia, which is the loss […]
Alzheimer’s is a Form of Dementia
What happens as you get older? What things occur physically, emotionally, and mentally as you leave your youth and adulthood and enter into the elderly stage of life? As humans progress into elderly hood, they have a greater risk of getting conditions such as arthritis, kidney and bladder problems, glaucoma, and alzheimer's. Alzheimer's is a form of dementia that causes problems with memory, thinking, and behavior. Symptoms usually develop slowly and get worse over time, becoming severe enough to interfere […]
Main Information Regarding Alzheimer’s Disease
Introduction The disease I will be discussing is Alzheimer's Disease "AD". AD affects the brain, nervous and vascular system. As AD progresses to later stages of the disease more organ systems are affected. Alzheimer's Disease is a "progressive and irreversible neurodegenerative brain disorder that causes a significant disruption of normal brain structure and functions" Lane, Hardy, & Schott, (2018). Alzheimer's is the most common form of "Dementia", it is steady decline in cognitive, behavioral and physical abilities, that can become […]
Etiology of Alzheimer’s Disease
Alzheimer's disease is a debilitating and, unfortunately, common illness that is characterized by extensive neuronal atrophy, memory loss, personality changes, and is the most common form of dementia. The atrophy is especially prevalent in the cerebral cortex and hippocampus. Patients are found to have severe loss of acetylcholine (a neurotransmitter) in these areas along with a corresponding loss of neurons in the basal nucleus and nearby cholinergic cell groups (Vanderah, Nolte, & Gould, 2016). With these being the general affected […]
Pesticides Exposure and the Risk of Alzheimer’s Disease
Abstract This literature review discusses the possible connection between pesticide exposure and the risk of developing Alzheimer's disease (AD). After a thorough examination of peer-reviewed articles and a recent literature review, it was revealed that there is an association between the risk of Alzheimer's disease and pesticide exposure, primarily limited to those with a history of occupational pesticide exposure. Only brief evidence of environmental pesticide exposure and risk of Alzheimer's disease was found. While each article touched on the aforementioned […]
How Epigenetics May Affect Alzheimer’s Disease
Abstract Alzheimer's disease (AD) is a neurodegenerative disease affecting approximately 5.5 million people. Each year, more and more information is uncovered about AD and, recently, studies are attempting to validate the hypothesis that epigenetics significantly affects AD pathology. Recognizing the need for these studies the National Institute on Aging and Alzheimer's Association (NIA-AA) published a new research framework in an effort to redefine the disease based on biological marker, as opposed to syndromal markers. This review considers two published works, […]
The Healthcare Epidemic of Alzheimer’s Disease
Alzheimer's Disease is a subset of dementia, which is classified by issues with memory function, thinking, and behavior. It is the most common cause of dementia in the United States, with cases of the disease continuing to grow exponentially as time goes on. Currently, there are over five and a half million Americans with Alzheimer's Disease, and it appears that these numbers will worsen in the coming decades. The disease is known to make patients experience progressively worsening memory function, […]
Alzheimer’s Disease and Relate Dementia Reform Health Care
Executive Summary The "model minority" stereotype and the lack of disaggregated data foster inaccurate representation of the Asian Americans and Pacific Islander (AAPI) community. This causes older adults with Alzheimer's disease and related dementias in particular to experience language and cultural discriminatory barriers, impacting their access to appropriate healthcare services. Advocating for linguistically and culturally appropriate healthcare services will aid in meeting the health needs of older AAPI adults through the implementation of in-language resources by healthcare providers and educating […]
The Relationship between Cholesterol and AD
Abstract: As many know cholesterol is a risk factor for many disease and illness. Not surprisingly many studies nationwide have proved that it contributes to the development of Alzheimer's Disease (AD). Many studies have been conducted to find the relationship between cholesterol and AD; in this review the studies focused on the apolipoprotein E (APOE), its different encodings: e4 allele, e3 allele, and e2 allele. It will also explain how APOE could neuronal cholesterol could help produce neurofibrillary tangles (NFTs) […]
The Benefits of the Ketogenic Diet on People with AD
Non-communicable diseases only continue to increase across the nation. When thinking about the drastic escalation in health issues, it leaves the population questioning where these diseases are stemming from and what really is considered a non-communicable disease? A non-communicable disease cannot be contracted as they are considered a medical condition, slowly progressing over an individual's life time, so essentially, they are lifelong. Furthermore, these non-communicable diseases that a given person may develop tend to be due to genetics, physiological, environmental, […]
Diagnosis of Alzheimer’s Disease
There isn't a specialized trial for Alzheimer's dementia, instead using a number of approaches and tools to help form a diagnosis. These approaches and tools consist of: Acquiring a medical and family history from the individual, and history of behavioral changes. Asking a family member to offer their view about alterations in intellectual capabilities and behavior. Conducting cognitive tests and physical and neurologic examinations. Having the individual undertake blood tests and brain imaging to rule out other impending causes of […]
Traumatic Brain Injury and Alzheimer’s Disease
Abstract This literature review investigates traumatic brain injury and Alzheimer's disease using various experimental methods. Participants were drawn from a range of demographic areas and were either observed or experimented upon. Blunt force trauma to the cranium has shown an association with late-onset Alzheimer's disease in both animal models and human observations. The results in all studies are consistent with the previously accepted notion that traumatic brain injury is highly correlated with the development of Alzheimer's disease. Methodologies included experiments […]
Additional Example Essays
- Tuberculosis research
- Benefits of Swimming
- Childhood obesity parents are the blame
- Black Death DBQ
- The Mental Health Stigma
- Psychiatric Nurse Practitioner
- Substance Abuse and Mental Illnesses
- PTSD in Veterans
- A Reflection on Mental Health Awareness and Overcoming Stigma
- Mandatory Organ Donation: Ethical or Unethical
- Compare And Contrast In WW1 And WW2
- Why College Should Not Be Free
How to Write an Essay About Alzheimers Disease
Understanding alzheimer's disease.
Before you start writing an essay about Alzheimer's disease, it's essential to understand what this disease is and its impact on individuals and society. Alzheimer's disease is a progressive neurological disorder that causes brain cells to degenerate and die, leading to a continuous decline in thinking, behavioral, and social skills. Begin your essay by explaining the nature of Alzheimer's, including its symptoms, stages, and the way it affects cognitive functions like memory and decision-making. Discuss the prevalence of the disease, particularly among older populations, and its status as one of the leading causes of dementia. An understanding of the biological and genetic factors contributing to Alzheimer's disease can also provide a solid foundation for your essay.
Developing a Thesis Statement
A strong essay on Alzheimer's disease should be anchored by a clear, concise thesis statement. This statement should present a specific viewpoint or argument about Alzheimer's disease. For example, you might examine the challenges of caregiving for Alzheimer's patients, analyze the current state of Alzheimer's research and treatment options, or discuss the social and economic impacts of the disease. Your thesis will guide the direction of your essay and provide a structured approach to your topic.
Gathering Supporting Evidence
To support your thesis, gather evidence from credible sources such as medical journals, healthcare reports, and case studies. This might include data on the effectiveness of different treatment methods, research findings on the progression of Alzheimer's disease, or testimonies from healthcare professionals and family members of Alzheimer's patients. Use this evidence to support your thesis and build a persuasive argument. Remember to address different perspectives and potential counterarguments in your essay.
Analyzing the Impact of Alzheimer's Disease
Dedicate a section of your essay to analyzing the impact of Alzheimer's disease. Discuss how the disease affects not only the patients but also their families, caregivers, and the broader healthcare system. Explore the emotional, financial, and societal challenges posed by Alzheimer's disease, including the strain on healthcare resources and the need for increased support and awareness.
Concluding the Essay
Conclude your essay by summarizing the main points of your discussion and restating your thesis in light of the evidence provided. Your conclusion should tie together your analysis and emphasize the significance of understanding and addressing Alzheimer's disease. You might also suggest areas for future research or policy development to better support Alzheimer's patients and their caregivers.
Reviewing and Refining Your Essay
After completing your essay, review and refine it for clarity and coherence. Ensure that your arguments are well-structured and supported by evidence. Check for grammatical accuracy and ensure that your essay flows logically from one point to the next. Consider seeking feedback from peers, educators, or medical professionals to further improve your essay. A well-crafted essay on Alzheimer's disease will not only demonstrate your understanding of the topic but also your ability to engage critically with medical and social issues.
1. Tell Us Your Requirements
2. Pick your perfect writer
3. Get Your Paper and Pay
Hi! I'm Amy, your personal assistant!
Don't know where to start? Give me your paper requirements and I connect you to an academic expert.
short deadlines
100% Plagiarism-Free
Certified writers
A cultural approach to dementia prevention
- An Introduction to Alzheimer’s Disease: What is it?

By: Adrianna Fusco
Introduction: Alzheimer’s disease, something we hear about online, in commercials, on news stations, and in many other parts of life. However, we are never told much about Alzheimer’s disease other than the devastating impacts it has. What is Alzheimer’s disease? What are the symptoms or signs to look out for? How does it progress? What causes it? How can it be prevented?
What is it? Alzheimer’s disease is a form of dementia, which is just an umbrella term used to describe loss of memory, language, problem solving, and other thinking abilities. More specifically, Alzheimer’s diseaseis a progressive, neurodegenerative disease that is categorized by a loss of memory, along with basic life skills like eating, bathing, talking, etc.
Symptoms: Common symptoms include: memory loss, paranoia, depression, anger, aggression, anxiety, apathy, loneliness, and psychosis. These symptoms vary from person to person.
Progress: As mentioned above, Alzheimer’s disease is a progressive disease. This means that it develops and gets worse over time. In the first stages of Alzheimer’s disease, there is usually very mild memory loss or problems with thinking abilities. The person may have a hard time remembering where they placed something or have a hard time recalling the right word to say. However, they still are independent, meaning they can still take care of themselves and do things like driving.
During the middle stages of Alzheimer’s disease, the cognitive processes get worse. Now the person may not be able to remember their personal history, like their address or phone number. They also may have a hard time recalling memories or remembering something from their past. The person is no longer able to take care of themselves because in this stage, they tend to forget where they are and often have a hard time using the bathroom or getting dressed appropriately for the day. An example of this is the person wearing shorts in the winter. Along with the cognitive changes, the person may begin to feel sad, lonely, anxious, and paranoid. The symptoms vary from person to person.
When the person hits stage 2, they will need a caregiver to assist them with their tasks and the caregiving will increase as the disease progresses. However, it’s important to help them without trying to do everything for them. They are still adults and they want to be treated as such, so it’s important to still let them have at least some control over their life. Whether that’s letting them do simply chores, like folding clothes, or doing activities, like arts and crafts. This will help provide a sense of normalcy.
The final stage of Alzheimer’s disease is when people begin to lose sense and control of the environment around them. By this point, the cognitive abilities of the individual have tremendously decreased. They can no longer speak in long formulated sentences, instead they speak in short fragments or words. They have trouble completing everyday tasks like walking, sitting, eating, and drinking. This means that they require around the clock assistance to make sure that they are remembering to eat and to help them eat. In general, the assistance is meant to make sure the person is safe and is living to their best ability. At this point, the individuals are very susceptible to infections. When the symptoms and daily conditions get really bad, usually, families turn to hospice care, so that the patient is comfortable at the end of their life. Hospice care also provides emotional support to loved ones, which is vital. Losing a loved one can cause serious emotional and mental strain, so that support is important.
The cause of Alzheimer’s disease is still being researched, but researchers have identified what they believe to be the main culprits of the disease: plaques and tangles.
Plaques are deposits of amyloid beta that forms between nerve cells that blocks the signals and stops the right materials from being sent to the nerve for survival. In a healthy brain, amyloid beta is used to help support neural repair and growth. However, in Alzheimer’s disease, there is an overproduction of this amyloid beta protein that disturbs these cells and eventually causes the death of the cells. The death of the old cells causes the loss of old memories and information. The blocking of nerve cells can stop the production of new connections, which means short term memories are not being accurately encoded in the brain to become long term memories.
Tangles are made up of twisted tau that builds up between cells. In a healthy brain, tau is used to help support neural strength and is important in keeping stability in the cells. However, a build up leads to the cells not being able to receive signals and the supplies it needs to function (i.e. energy). These lead to death of the cells, leading to loss of information and life skills.
There is also a biomarker known as APOE-4, that is thought to predispose people to Alzheimer’s disease. This gene along with some environmental stressors could affect whether someone gets the disease and the progression of it. However, a lot of research is still being conducted on this topic and we are constantly rerouting what we know, as new information is found.
Alzheimer’s disease is a terrible disease that claims the lives of a lot of people every year. It’s important to know the signs and to check up with your doctor when anything seems unusual. Alzheimer’s disease and dementia are not a normal part of aging, so see your doctor if you notice any issues with your memory. The earlier the disease is detected, the better it can be treated.
Stay tuned for more blog posts about Alzheimer’s disease, including a look into the mental health of caregivers, prevention, treatment, and more! We also will be writing posts about interviews with doctors, as well as posts about brain health!
Thank you for reading!
References:
“Alzheimer’s Caregivers: 8 Tips for People Caring for a Loved One With Alzheimer’s Disease or Dementia: Caregivers.” 30Seconds Health ,
30seconds.com/health/tip/14389/Alzheimers-Caregivers-8-Tips-for-People-Caring-for-a-Loved-One-With -Alzheimers-Disease-or-Dementia.
Mayeux, Richard, et al. “Treatment of Alzheimer’s Disease: NEJM.” Edited by Alastair J.J. Wood, New England Journal of Medicine , 16 Mar. 2000, www.nejm.org/doi/pdf/10.1056/NEJM199911253412207.
NHS Choices, NHS, 10 May 2018,
www.nhs.uk/conditions/alzheimers-disease/causes/#:~:text=Alzheimer’s%20disease%20is%20thought%2 0to,form%20tangles%20within%20brain%20cells.
Porsteinsson, Anton P., et al. “Neuropsychiatric Symptoms in Dementia: A Cause or Consequence?” American Journal of Psychiatry , American Psychiatric Association Publishing, 30 Apr. 2015, ajp.psychiatryonline.org/doi/10.1176/appi.ajp.2015.15030277#:~:text=The%20term%20neuropsychiatric %20symptoms%20describes%20heterogeneous%20behavioral%20or,agitation%2C%20anxiety%2C%20 apathy%2C%20depression%2C%20psychosis%2C%20and%20sleep%20disturbance.
“Stages of Alzheimer’s.” Alzheimer’s Disease and Dementia , www.alz.org/alzheimers-dementia/stages.
“What Is Alzheimer’s?” Alzheimer’s Disease and Dementia ,
www.alz.org/alzheimers-dementia/what-is-alzheimers.
Home — Essay Samples — Nursing & Health — Neurology & Nervous System Diseases — Alzheimer's Disease
Essay Examples on Alzheimer's Disease
What makes a good alzheimer's disease essay topics.
When it comes to writing an essay on Alzheimer's Disease, choosing the right topic is crucial. An engaging and thought-provoking topic can make your essay stand out and leave a lasting impression on your readers. But What Makes a Good Alzheimer's Disease essay topic? Here are a few recommendations on how to brainstorm and choose an essay topic:
- Consider your interests and passions: Think about what aspects of Alzheimer's Disease you find most intriguing. Whether it's the latest research developments, caregiving challenges, or the impact on society, choosing a topic that aligns with your interests will make the writing process more enjoyable and the final product more engaging.
- Brainstorm ideas: Take some time to brainstorm potential essay topics. Consider the latest trends and developments in Alzheimer's Disease research, as well as the impact of the disease on individuals, families, and communities. You can also explore controversial issues or ethical dilemmas related to Alzheimer's Disease to spark ideas for your essay topic.
- Research potential topics: Once you have a list of potential essay topics, take the time to research each one. Consider the availability of credible sources, the depth of information on the topic, and its relevance to the current discourse on Alzheimer's Disease. This will help you narrow down your options and choose a topic that is well-supported and relevant.
- Choose a unique angle: Instead of rehashing common topics, try to approach Alzheimer's Disease from a unique angle. Consider how you can shed new light on a familiar topic or explore a lesser-known aspect of the disease. This will make your essay more compelling and help it stand out from the rest.
In summary, a good Alzheimer's Disease essay topic is one that aligns with your interests, is well-researched, and offers a unique perspective on the subject. By following these recommendations, you can ensure that your essay topic is engaging, thought-provoking, and well-supported.
Best Alzheimer's Disease Essay Topics
When it comes to choosing the best Alzheimer's Disease essay topics, it's important to think outside the box and choose topics that are not only relevant but also creative and thought-provoking. Here are some of the best Alzheimer's Disease essay topics that are sure to stand out:
- The Role of Genetics in Alzheimer's Disease
- The Impact of Alzheimer's Disease on Family Caregivers
- Treating Alzheimer's Disease: A Comprehensive Review
- Ethical Considerations in Alzheimer's Disease Research
- The Stigma of Alzheimer's Disease in Society
- The Link Between Alzheimer's Disease and Lifestyle Factors
- Innovative Approaches to Alzheimer's Disease Treatment
- Alzheimer Disease: Effects on Patients and Families
- Alzheimer's Disease in the Aging Population
- The Economic Burden of Alzheimer's Disease on Society
- The Intersection of Alzheimer's Disease and Mental Health
- The Future of Alzheimer's Disease Research and Treatment
These essay topics offer a fresh perspective on Alzheimer's Disease and are sure to capture the attention of your readers. By choosing a creative and thought-provoking topic, you can set your essay apart and make a lasting impression.
Alzheimer's Disease essay topics Prompts
Looking for some creative prompts to inspire your Alzheimer's Disease essay? Here are five engaging prompts to get you started:
- Imagine a world where Alzheimer's Disease is completely eradicated. How would this impact society, healthcare, and the lives of individuals and families affected by the disease?
- Write a personal reflection on your experience with Alzheimer's Disease, whether as a caregiver, a healthcare professional, or a researcher. What have you learned from this experience, and how has it shaped your perspective on the disease?
- Explore the ethical implications of using artificial intelligence and technology to diagnose and treat Alzheimer's Disease. What are the potential benefits and drawbacks of these advancements?
- Consider the impact of Alzheimer's Disease on different cultural and ethnic communities. How does cultural diversity influence the experience of the disease, as well as access to care and support?
- Imagine a day in the life of someone living with Alzheimer's Disease. What challenges do they face, and how do they navigate their daily routines and interactions with others?
These prompts are designed to spark creativity and encourage you to explore the complexities of Alzheimer's Disease from a fresh perspective. Whether you're writing an essay for a class assignment or for personal exploration, these prompts can help you delve into the many facets of Alzheimer's Disease and create a compelling and engaging essay.
Disease: Research Project Outline
The impact of alzheimer's disease: individuals, loved ones, and society, made-to-order essay as fast as you need it.
Each essay is customized to cater to your unique preferences
+ experts online
Alzheimers Disease
The nature of alzheimer's disease, alzheimer's disease and its influence on the brain center, a report on alzheimer's disease, let us write you an essay from scratch.
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Background on Alzheimer's Disease
Musical memory and alzheimer's disease, the positive impact of exercise in protecting the brain from alzheimer's disease, risk factors and symptoms of alzheimers, get a personalized essay in under 3 hours.
Expert-written essays crafted with your exact needs in mind
Alzheimer’s: How It Affects Both The Patient and The Caregiver
The link between amyloid beta and alzheimer’s disease, the effect on human feelings caused by predictive testing methods for degenerative illnesses like alzheimer’s and huntington’s disease, denial and acceptance in alzheimer's diagnosis , neurodegenerative diseases and the most prevalent of them, alzheimer’s and dementia with lewy bodies, a study on how alzheimer’s disease connects to the human immune system, the effects of theanine consumption on individuals with alzheimer’s disease, the analysis of the article "precision medicine offers a glimmer of hope for alzheimer's disease" by melissa healy, nicotine and black sesame pigment, food for alzheimer’s thought, understanding alzheimer's disease: causes, symptoms, and treatment options, relevant topics.
- Post Traumatic Stress Disorder
- Postpartum Depression
- Sleep Deprivation
- Cerebral Palsy
- Multiple Sclerosis
- Psychological Disorders
- Mental Retardation
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NATURE INDEX
- 13 March 2024
Researchers call for a major rethink of how Alzheimer’s treatments are evaluated
- Esther Landhuis 0
Esther Landhuis is a science journalist in the San Francisco Bay Area, California.
You can also search for this author in PubMed Google Scholar

Credit: Taj Francis
In January 2023, the US Food and Drug Administration (FDA) approved lecanemab — an antibody medication that decreases β-amyloid protein build-up in the brain — as a treatment for Alzheimer’s disease. Pivotal evidence came from a large, randomized trial of people with early-stage Alzheimer’s , which afflicts around 32 million people worldwide. By the end of that 18-month study 1 , patients in the placebo group scored on average 1.66 points worse than their performance at baseline on a standard dementia test, which assesses cognitive and functional changes over time through interviews with a patient and their caregiver. The mean score of treated participants, by comparison, worsened by 1.21 points — a 27% slowing of cognitive decline.
But is this improvement meaningful for patients and their families?

Nature Index 2024 Health sciences
There are two major categories of drugs used to treat Alzheimer’s disease and other progressive conditions: symptomatic drugs, which treat the symptoms, and disease-modifying drugs, which target the root cause. Donepezil and rivastigmine, for example, are symptomatic drugs that boost the activity of chemicals in the brain to compensate for declines in cognitive and memory function caused by Alzheimer’s disease, but they cannot stop its progression . Lecanemab, developed jointly by Japanese pharmaceutical company Eisai and American biotechnology firm Biogen, targets the underlying issue of amyloid build-up in the brain, and in doing so, could fundamentally change the course of the disease.
An important feature of disease-modifying drugs is that their benefits are cumulative. Studies of patients with multiple sclerosis , for example, have shown the benefits of starting disease-modifying drugs earlier in the course of the disease compared with later, including improved mortality rates and reduced disability in the long term. Being able to quantify how long a disease-modifying drug can delay or halt the progression of Alzheimer’s disease could change how researchers understand — and communicate — its benefits.
In studies of potential disease-modifying drugs for Alzheimer’s disease, there has always been a tension between being able to produce a treatment effect and being able to measure it, says Suzanne Hendrix, statistician and founder of the clinical trials consulting firm Pentara in Salt Lake City, Utah. Clinical trials generally enrol early-stage patients — those with mild cognitive impairment and evidence of brain amyloid — because amyloid-targeting therapies have the best chance of working if given well before the disease takes hold. But in the early stages, patients deteriorate so gradually that it can be difficult to perceive the impact of a disease-modifying drug using standardized tests.
At a scientific meeting in 2009, Hendrix recalls being pulled aside by an executive at Eisai, who told her: “Nobody’s measuring this disease right. Until we measure the most progressive aspects of disease, we’re not going to be able to see treatment effects.”
Source: Institute for Health Metrics and Evaluation; Cummings, J. L., Goldman, D. P., Simmons-Stern, N. R., Ponton, E. Alzheimers Dement. 18 , 469–477 (2022)
Hendrix and other researchers are exploring time-based metrics as a new approach. Savings of time, measured as prolonged quality of life after 18 months of treatment, for example, is “much easier to talk about” than point differences on cognitive and functional scales, says Lars Rau Raket, a statistician at the Copenhagen, Denmark, branch of US pharmaceutical company Eli Lilly. For early-stage Alzheimer’s patients , says Raket, “it’s about how much you can extend the time in the ‘good parts’ — in the milder stages of disease”.
Straight line to time
To come up with a time-based approach, Hendrix and her colleagues pooled parts of several rating scales from standard dementia tests to develop a new tool called that picks up on subtle changes that occur in early Alzheimer’s. By zeroing in on where changes are more pronounced in these early stages, such as a diminished ability to juggle tasks or to recall past events, the team could track the progression of several key features of the disease.
To measure the effectiveness of disease-modifying treatments on these key features as units of time, the researchers used clinical outcomes from placebo and treated participants in a phase II trial of another amyloid-lowering therapy, donanemab . They calculated that over the 76-week duration of the trial, overall disease progression was delayed by 5.2 months.
In a paper published last year 2 , when he was working for Danish firm Novo Nordisk, in a lab just outside Copenhagen, Raket took a similar approach to calculating treatment effects in terms of time. But their methods differed in some ways. Whereas Hendrix’s work focused on calculating time savings across multiple outcomes, Raket used multiple models to calculate time savings for each outcome measure.
The idea of time-based models seems to be gaining traction. They were used as exploratory measures in a phase III trial of donanemab, conducted by Eli Lilly and Company, and published in JAMA last year 3 . Eisai also showed a time-based analysis in a 2022 presentation of its phase III lecanemab data at the Clinical Trials on Alzheimer’s Disease meeting in San Francisco. In those analyses, participants treated with lecanemab took 25.5 months to reach the same degree of worsening on a common dementia test as the placebo group did at 18 months — a time saving of 7.5 months.
Raket says he has been approached by several people in the pharmaceutical industry and academia, and some are working with him to apply the concept to their research. At the 2023 Alzheimer’s Association International Conference in Amsterdam, Raket and his collaborators in the United States, Canada and Europe compared time-based models with conventional statistical approaches for progressive diseases, and analysed how delays in disease progression calculated with time-based methods translate to treatment differences on standard cognitive tests. “I haven’t experienced this kind of interest in my work before,” he says. Raket predicts that an increasing number of trials in the neurodegeneration space will be reporting time-savings estimates in the years to come.
Broad impacts
Beyond Alzheimer’s disease, time-saved models could be applied to other progressive conditions, including Parkinson’s disease and amyotrophic lateral sclerosis (ALS). Cancer and cardiovascular disease studies, which tend to focus on events — delaying relapse or death, or cutting the risk of heart attacks, for instance — are less suited to models that track progression. If, however, heart disease were conceptualized as a gradual worsening of blood pressure or cholesterol over time, and treatment could be shown to slow the rate of deterioration, the time-saved approach could be used to measure the treatment benefit, says Hendrix.
One benefit of time-based methods is that they could help make clinical trials less prone to being skewed by outliers , says Geert Molenberghs, a biostatistician at KU Leuven and Hasselt University, both in Belgium, who collaborates with Hendrix. For example, a small subset of people with early Alzheimer’s disease deteriorate unusually quickly. If these rapid decliners are in the treated group, they could potentially mask a drug benefit, says Molenberghs. The details become “very technical”, he says, but with time-based approaches, these rare individuals “are less influential. They have less capacity to overturn the statistics.”
Time-based metrics could impact broader conversations with health economists and policymakers. “The idea that you could take somebody who’s already in their senior years and keep them functional and not needing 24/7 care — that’s incredibly valuable information for making estimates about the true burden or cost of the disease to caregivers and society,” says John Harrison, chief science officer at Scottish Brain Sciences, a research institute in Edinburgh, Scotland. “It’s a very neat communications tool which feeds into estimates of progression, cost, strategy and, one hopes, legislation and planning.”
There are open questions that might need to be addressed before time-saved models are more widely applied in clinical trials. One is that, although time progresses linearly, not all points on that line are equally meaningful. For example, the anti-amyloid mechanism might only be beneficial in the early stages of Alzheimer’s disease, says Ron Petersen, a neurologist at Mayo Clinic in Rochester, Minnesota. “By the time the person progresses to, say, moderate dementia, modifying amyloid probably isn’t going to make any difference.”
Hendrix is hopeful that the time-saved idea can be further developed and applied to clinical trials in the future, because it could make a big difference in tracking not only how effective new disease-modifying drugs are, but also in helping Alzheimer’s patients and their families to better understand the progression of the disease and how they can plan for it.
Ultimately, as more studies “start focusing on how much time we’ve saved people, all of the effects that we see will be more relevant” to people’s daily lives, Hendrix says.
Nature 627 , S18-S20 (2024)
doi: https://doi.org/10.1038/d41586-024-00756-8
This article is part of Nature Index 2024 Health sciences , an editorially independent supplement. Advertisers have no influence over the content.
Van Dyck, C. H. et al. N. Engl. J. Med. 388 , 9–21 (2023).
Article PubMed Google Scholar
Raket, L. L. Stat. Med. 41 , 5537–5557 (2022).
Sims, J. R. et al. JAMA 330 , 512–527 (2023).
Download references
Related Articles

Partner content: A holistic approach to health in Japan
Partner content: Can we increase the safety of carbon nanotubes?
Partner content: Building for a digitized, personalized future
Partner content: Translating basic research into medical advances
Partner content: Meds, meals and microbes: gut health on the cancer front line
Partner content: A hotspot for research and development of medical AI
Partner content: What are we learning from the world’s myopia capital?
Partner content: Systems biology: a network view of disease could unearth hidden targets
Partner content: Learning from the pupil: the power of pupillometry
Partner content: Does green tea offer cognitive and sleep benefits?
Partner content: A team effort delivers a new tool for fighting neuroblastoma
Partner content: Shaping the future of medical science - A multidisciplinary paradigm
Partner content: Harnessing AI to see a patient’s unique patterns
Partner content: Improving the outlook for chronic kidney disease
Partner content: From medical robots to a mouse metaverse
Partner content: What comes after clinical trials?
Partner content: Making vitamin D detection much more accessible
Partner content: A new injectable gel could seal wounds after cancer surgery
Partner content: The surprising interplay between metabolism and mind
Partner content: Detecting atrial fibrillation while there’s still time to act
- Alzheimer's disease
- Medical research
- Health care

Hacking the immune system could slow ageing — here’s how
News Feature 07 MAY 24

‘Orangutan, heal thyself’: First wild animal seen using medicinal plant
News 02 MAY 24

Genomics reveal unknown mutation-promoting agents at global sites
News & Views 01 MAY 24

How ignorance and gender inequality thwart treatment of a widespread illness
Outlook 09 MAY 24

Bird flu in US cows: where will it end?
News 08 MAY 24

US funders to tighten oversight of controversial ‘gain-of-function’ research
News 07 MAY 24
Postdoctoral Associate- Neuronal Resilience and Regeneration
Houston, Texas (US)
Baylor College of Medicine (BCM)
Research Positions in China Spallation Neutron Source
We are seeking 23 researchers with a proven track record of conducting advanced research and demonstrating outstanding academic achievements.
Dongguan, Guangdong, China
Spallation Neutron Source Science Center
Postdoctoral Associate- Electrophysiology
Postdoctoral scholar - clinical pharmacy & translational science.
Memphis, Tennessee
The University of Tennessee Health Science Center (UTHSC)
Postdoctoral Scholar - Pathology
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Open access
- Published: 07 May 2024
Promoting Alzheimer’s disease research and therapy with stem cell technology
- Zimeng Cao 1 na1 ,
- Fanshu Kong 1 na1 ,
- Jiaqi Ding 1 na1 ,
- Chunxia Chen 1 ,
- Fumei He 1 , 2 &
- Wenbin Deng ORCID: orcid.org/0000-0003-2552-2553 1
Stem Cell Research & Therapy volume 15 , Article number: 136 ( 2024 ) Cite this article
Metrics details
Alzheimer’s disease (AD) is a prevalent form of dementia leading to memory loss, reduced cognitive and linguistic abilities, and decreased self-care. Current AD treatments aim to relieve symptoms and slow disease progression, but a cure is elusive due to limited understanding of the underlying disease mechanisms.
Main content
Stem cell technology has the potential to revolutionize AD research. With the ability to self-renew and differentiate into various cell types, stem cells are valuable tools for disease modeling, drug screening, and cell therapy. Recent advances have broadened our understanding beyond the deposition of amyloidβ (Aβ) or tau proteins in AD to encompass risk genes, immune system disorders, and neuron–glia mis-communication, relying heavily on stem cell-derived disease models. These stem cell-based models (e.g., organoids and microfluidic chips) simulate in vivo pathological processes with extraordinary spatial and temporal resolution. Stem cell technologies have the potential to alleviate AD pathology through various pathways, including immunomodulation, replacement of damaged neurons, and neurotrophic support. In recent years, transplantation of glial cells like oligodendrocytes and the infusion of exosomes have become hot research topics.
Although stem cell-based models and therapies for AD face several challenges, such as extended culture time and low differentiation efficiency, they still show considerable potential for AD treatment and are likely to become preferred tools for AD research.

Introduction
Alzheimer’s disease (AD) is a common, chronic neurodegenerative disorder characterized by the extensive distribution of neuronal tangles and amyloid plaques in the brain along with astrogliosis, neuroinflammation, synaptotoxicity, neuronal loss, and vascular alterations [ 28 ]. In recent decades, AD has been a subject of intensive research, however, no cure has been developed to date due to a limited understanding of AD pathogenesis. Fortunately, stem cells—as a category of cells with multi-directional differentiation potential—present new and powerful tools for AD disease modeling, drug screening, and cell therapy.
The conventional animal models used to study AD, which is mainly constructed through transgenesis or induced aging, have considerable limitations due to the complexity of the human cerebellum, such as neuronal sub-type changes [ 45 ]. Stem cell-based models show significant potential for solving these problems. By reprogramming donor somatic cells with disease morphology, one can obtain induced pluripotent stem cells (iPSCs) with the ability to expand and differentiate, thereby freeing researchers from reliance on animal models. Moreover, the ability to culture 3D iPSC-based organoids and construct humanized AD models has enabled researchers to explore other possible factors related to pathogenesis, such as immune system disorders, impaired synapses, abnormal mitochondrial structure and function, and mutations in risk genes (Figure 1 ). As the amyloid hypothesis continues to be investigated [ 99 ], understanding the contributions of these pathological phenomena to disease etiology and neuronal death is of great importance for understanding AD pathogenesis and developing new therapies.
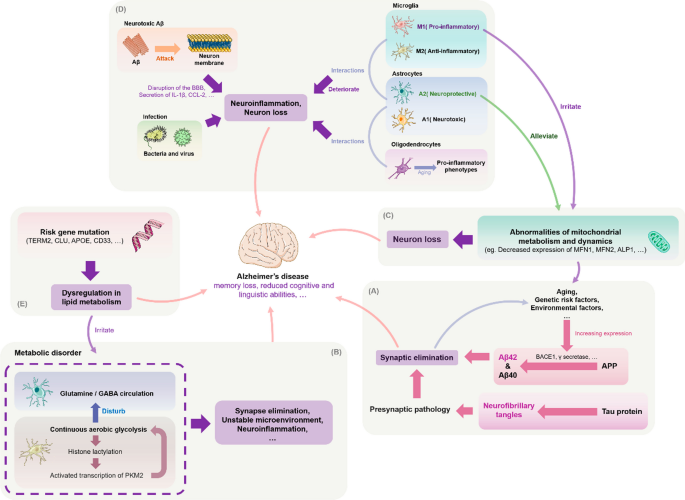
Possible pathogenesis of Alzheimer's disease. A Amyloid-beta precursor protein (APP) is cleaved by BACE1 and other enzymes in the amyloid pathogenic pathway to form different isoforms of Aβ (this process can also be affected by gene mutations), which rapidly aggregate to form neurotoxic oligomers that can induce synaptic loss and further cause cognitive impairment and dementia. Simultaneously, Aβ can contribute to tau pathology by increasing abnormal phosphorylation and the formation of toxic neurofibrillary tangles (NFTs), which can lead to neurodegeneration. According to the synaptic defect hypothesis, synaptic damage is the underlying cause of AD and defective synapses may lead to the formation of neurotoxic Aβ. B One potential mechanism of AD progression is dysregulation of multiple metabolic pathways. Microglia glucose metabolism disruption promotes a vicious cycle of "glycolysis-histone lactation-PKM2", leading to an imbalance of microglia homeostasis and neuroinflammation. Astrocyte metabolic failure also impairs the neurotransmitter glutamate/GABA glutamine cycle and causes synaptic dysfunction. C Dysfunctional mitochondrial dynamics can result in aberrant mitochondrial fusion fission, in turn causing synaptic degeneration and neuronal injury. Such dynamics include diminished expression of the proteins that control this process. By transferring healthy mitochondria, astrocytes could reduce the negative effects of damaged mitochondria on neurons. This function is blocked in chronic inflammatory situations, which may hasten AD progression. The removal of Aβ and emergence of tau disease may also be impacted by aberrant mitochondrial autophagy. D Microglia, astrocytes, and oligodendrocytes have pro-inflammatory phenotypes that promote neuroinflammation and the development of AD. The three types of glial cells interact with one another to speed up neuroinflammation. For example, activated microglia secrete IL-1a, TNF-a, and C1q, which cause reactive astrocytes to develop into the A1 neurotoxic phenotype, whereas oligodendrocytes cause astrocytes to develop into the NF-B signaling or A1 phenotype. Other harmful processes in AD include the attack of Aβ on its own neuronal cell membranes and bacterial-viral infection. E Mutations in common risk genes, including APOE, triggering receptor expressed on myeloid cells 2 (TREM2), CLU, and CD33, may disrupt normal functions—particularly lipid metabolism in glial cells—thereby increasing the risk of AD development
The development of stem cell modeling technology has also launched a new era of personalized pharmaceuticals. Current drug screening methods are expensive, time-consuming, and complicated. In contrast, stem cell-based models can accurately simulate pathological changes in the AD brain, providing more possibilities for AD-related drug screening. Using stem cell technology, we can understand the effects of drugs at the earliest stage of analytical development and testing and make accurate assessments of their side effects and efficacy.
Currently, Aβ and tau pathologies remain the focus of new therapies undergoing clinical trials for AD, which include monoclonal antibodies targeting Aβ[ 50 , 88 , 109 , 131 ], the β-secretase (BACE1) inhibitor that inhibits secretase activity to lessen Aβ deposition [ 35 ], and drugs that target tau protein [ 94 ]. Unfortunately, most clinical trials have not achieved good results and some have been terminated due to serious side effects [ 193 ]. Such cases demonstrate the challenges of sub-cellular therapies while highlighting the advantages of stem cell therapies, such as the possibility of reversing the entire pathological process of AD [ 136 , 142 ]. Transplantation of stem cells in animal models has been shown to provide significant benefits by affecting multiple pathological mechanisms and ultimately improving cognitive function [ 103 ]. Targeting ganglion stem cells in the body to activate and generate new glial cells or neurons is another strategy for stem cell therapy. New targets have been recently identified in animal models to produce these pro-regenerative effects [ 77 ].
However, the heterogeneity of stem cells and their survival and migration rates after transplantation limit their applications. Researchers have attempted to address these issues through methods like modifying stem cell encapsulation, pretreatment, or co-delivery. Alternatively, direct use of stem cell-derived exosomes for AD treatment may achieve better results than various complex stem cell modification methods [ 128 ], as they show significantly improved pathology and cognitive function in preclinical experiments. Their therapeutic potential may be even further exploited after engineering. For example, the production of exosomes can be increased by 3D culture or co-culture with chloroquine, NH4Cl, and other molecules. Also, by changing the content of oxygen, hydrogen ions, and nutrients in stem cell culture medium, the release of exosomes can be altered, however, this may require exploration through further experiments. Fusion of protein sequences or peptides with exosome membranes can also be used to build devices for targeted delivery, and electroporation is the most widely used method for membrane fusion [ 181 ].
In this review, we summarize progress in stem cell-derived AD models and the principles and applications of stem cells and derived therapies to alleviate AD pathology. We then discuss the prospects of stem cells as models for further investigation of AD pathogenesis and drug screening, as well as application prospects and development directions for stem cells and derived therapies targeting AD.
Stem cell-derived disease models in AD research
Stem cells are a category of cells with the potential of self-renewal and multidirectional differentiation. They originate from various tissues such as placenta, adipose tissue, and dental pulp and have numerous potentially useful functions such as tissue and organ regeneration and treatment of diseases (especially severe diseases).
One crucial application of stem cells in AD research is the creation of patient-derived human cellular models. Examples of these are organoids and assembled spheroids, which are multicellular models composed of different brain cell types. These models can be used to test the impact of genetic, chemical, and environmental factors on AD, as well as to evaluate new genetic engineering and genome editing techniques to unravel disease pathogenesis. Another area where stem cells are used is the development of in vivo models that more accurately reflect human physiology and disease, such as humanized animal models. As transplanted stem cells or their derived tissues/organs retain their original features and functions, humanized models constructed by transplanting stem cells of human origin into animals can circumvent ethical constraints associated with human experimentation [ 164 ]. Utilizing information on AD-related risk genes, biological processes and pathways, and biomarkers provided by advanced multi-omics technologies, such as single-cell and spatial transcriptomics, long-range sequencing, next-generation proteomics, and other high-throughput cellular phenotyping platforms, stem-cell-derived disease models can provide comprehensive insights into the pathogenesis, progression, and drug screening of AD [ 182 , 185 , 186 , 194 , 196 , 199 ].
2D culture systems
The in vitro model of human iPSCs was first established in 2007 in the laboratory of Prof. Shinya Yamanaka [ 125 ]. More than a decade later, iPSCs have been differentiated into various pathogenic cell types including neurons and glial cells to mimic central nervous system (CNS) developmental processes and profile disease phenotypes. To promote the differentiation of iPSCs into the above cell types in 2D culture and prohibit their differentiation to mesoderm or endoderm, dual TGF-β (SMAD) inhibition is utilized to generate neural stem cells (NSCs) with higher purity [ 19 ]. By inhibiting the expression of sonic hedgehog (SHH) signaling through enhanced Wnt signaling, [ 66 , 129 ], NSCs can develop into cortical neurons. Cortical neurons, such as cortical glutamatergic neurons, are mainly distributed in the neocortex and hippocampus, which are early affected tissues in AD [ 78 ]. By further manipulating extrinsic signaling conditions, NSCs can differentiate into other disease-related cell types such as cholinergic neurons [ 11 ]. Substantial loss of cholinergic neurons has been observed in the brains of early-stage AD patients. After prolonged culture, neurogenic cultures can undergo gliogenesis conversion to produce various glial cells [ 18 , 42 ]. Dysfunction of such glial cells is also considered a cause of AD pathology. As a result, it is increasingly important to study interactions between various neuronal subtypes and glial cells using such models. Moreover, compared to using human-derived stem cells of limited origin or rodent cell lines from widely varying species, 2D co-culture systems using iPSCs yield highly scalable cell models that enable rapid exploration of cellular pathophysiology, including transcriptional, genetic, and signaling pathways [ 136 , 142 ].
3D culture systems
To solve the problem of simulating AD pathology in vivo, 3D neuronal culture technology, has been developed based on 2D cell culture systems. Unlike traditional 2D cultures, 3D neuronal cultures better simulate the natural environment for cell survival in organisms, thereby achieving cell–cell interactions and more realistic biochemical and physiological responses. To date, many rigid scaffolds have been developed to support neuronal culture, including super-porous/non-porous hydrogels, polydimethylsiloxane with micrometric cavities [ 14 ], sintered titanium [ 70 ], and hydrogels or matrigels [ 59 ]. 3D soft matrix scaffolds can also be constructed using hydrogels, fibronectin-bound differentiated iPSCs, and 3D printing techniques. Not only do these approaches maintain the electrophysiological activity of neural tissue compared to rigid scaffolds, but they also allow the mimics to be made into different shapes as needed to accommodate multiple platform applications.
Although scaffolds can somewhat better mimic the developing brain, they still lack the cellular diversity, structural complexity, and physical structure visible in vivo [ 5 ]. The development of 3D organoid models and microfluidic organoids has effectively addressed some of these deficiencies. 3D organoids are grown from iPSCs generated from human stem cells or adult cells and have a similar composition and structure as primary tissues as well as ease of manipulation and cryopreservation. Organoids have been shown to have well-defined radial glial cells, astrocytes, and neurons that better mimic human cortical structure during development or in disease states [ 22 , 102 , 170 ]. Early brain organoids derived from directed neuroectodermal differentiation did not contain mesodermal lineage cells, such as myeloid microglia or endothelial cells [ 213 ]. However, this can now be achieved by co-culturing human umbilical vein endothelial cells (HUVECs) and microglia-like cells isolated from primary tissues or differentiated from human embryonic stem cells (hESCs) with brain organoids [ 209 ]. Functional microglia can also be directly generated from modified unguided protocols [ 201 ] or hESCs in human cortical organoids (hCOs) [ 167 ]. The multiple types of human brain cells contained in the above organoids play an important role in neurodevelopment and the occurrence of neurodegenerative diseases [ 206 ]. As a result, 3D organoids can be used to study human diseases that are difficult to simulate using animal models, such as AD.
Microfluidic organ-on-a-chip technology, which is a “human-on-a-chip” cell culture system for studying physiological processes, can be used to grow neurons, glial cells, endothelial cells, and skeletal muscle cells while providing a highly fluidic extracellular environment and maintaining fluid isolation with a multi-lumen device to better outline organ-like structures [ 47 ]. This approach also allows one to manipulate biological specimens and cells and simulate pathological conditions with extraordinary spatial and temporal resolution to reveal mechanistic insights into the disease, thereby accelerating drug discovery, screening, and toxicology research [ 118 , 159 ].
Humanized animal models
Humanized animals are animals carrying functional human genes, cells, tissues and/or organs, immune system, or microorganisms that are used in biomedical research. As they can fully simulate the pathological phenotypes and disease mechanisms of human AD, humanized AD animal models play an important role in AD research. There have been recent developments of genetically humanized AD animal models. For example, Pang et al. [ 202 ] successfully developed a gene knock-in rat model capable of fully simulating AD and more accurately simulating AD pathogenesis in the brain. Andersen et al. [ 162 ] used CRISPR-Cas9-based gene editing to develop a haploid insufficiency model of sortilin-related receptor 1 (SORL1) (which encodes the endosomal recycling receptor) in Gögenting’s miniature pigs, providing support for studying the pathological characteristics of AD caused by SORL1 haploinsufficiency. However, at present, the use of stem cell transplantation technology to establish cellular humanized animal models—also known as chimeras—is mainly applied in the research fields of immunology autoimmunity, transplantation, infectious diseases, and tumors, and is not widely used to study neurodegenerative diseases like AD. To create a humanized AD model, [ 16 ] transplanted cortical neurons produced from human iPSCs into the brains of transgenic Tg (APP/PS1-21) mice. Their goal was to reveal species-specific vulnerability of human neurons to AD pathology. Disease processes and treatment advancements in AD have been clarified by this model. Another humanized AD model was constructed by Espuny-Camacho et al. [ 177 ], who transplanted healthy human neural precursor cells (NPCs) from iPSCs into the frontal cortex of immunodeficient neonatal transgenic APP/PS1-21 mice [ 177 ]. Their study revealed significant degeneration of transplanted healthy human neurons upon exposure to Aβ, microglia, and astrocytes [ 101 ]. Chimeric models based on stem cell technology can offer further insights into AD pathogenesis. For example, by constructing a chimeric mouse model with grafted Clusterin (CLU) C (CLU rs11136000 C mutation) medial ganglionic eminence progenitors (CLU hiMGEs), Chen et al. clarified that over-activation of the sphingolipid pathway may be the underlying mechanism of CLU AD. Using CLU C humanized mice, the study shed light on AD pathogenesis, and highlighted the need for more research on GABA transmitter synthesis abnormalities [ 169 ].
Humanized AD animal models constructed by the stem cell transplantation technique described above have significant advantages over 3D models. Most importantly, they provide longer latency to capture more critical AD neuropathological features including astrocyte proliferation, microglia proliferation, synaptic deficiency, dendritic degeneration, neuronal cytoskeleton abnormalities, progressive 4R tau expression, and tau hyperphosphorylation [ 16 ]. These advantages allow researchers to understand the relative timing of the appearance of different pathological features of AD, thus providing a valuable platform for studying how aging causes or affects neurodegeneration (Fig. 2 ).
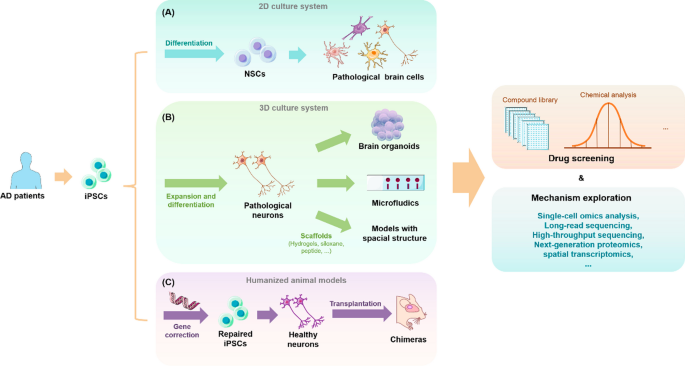
Stem cell-derived disease models in AD research. A iPSCs obtained from AD patients are used in 2D stem cell culturing systems to generate pathological neurons for drug screening and mechanism exploration. B Stem cell 3D culture system: using iPSC from AD patients to construct organoid and microfluidic organ-on-a-chip models for drug screening and mechanism exploration. C iPSCs from AD patients with gene alterations resulting in the production of normal neurons, which were then used to create a humanized animal model
Contributions of stem cell technology to AD research and treatment
Great progress has been made in iPSCs in neural differentiation in recent years, and there are now many protocols for inducing or reprogramming stem cells to differentiate into different types of cells and neurons involved in AD pathogenesis, such as neural progenitor cells [ 9 ], microglia [ 1 ], astrocytes [ 134 ], oligodendrocytes [ 138 ], endothelial pericytes [ 3 ], glutamatergic, aminobutyric acidergic neurons [ 23 , 41 ], and basal forebrain neurons [ 90 ], resulting in the possibility to create physiologically relevant in vitro models and more promising means of AD treatment. In the following sections, the value and potential of stem cells and their derived 2D, 3D, and humanized animal models in AD mechanism research, drug screening, and cell therapy are further explored.
Stem cell-derived platforms for studying AD pathogenesis
With the rapid development and application of the above-described stem cell-derived disease models, understanding of AD has improved. The emergence of these new models has helped scientists study the pathological mechanisms of AD from different perspectives, such as “risk genes”, “immunity”, and “mitochondria”, and propose new pathological hypotheses of AD while improving classic hypotheses.
Amyloid hypothesis
In 1991, John Hardy and David Alsop proposed the β-amyloid cascade hypothesis [ 180 ], which suggested that extracellular β-amyloid (Aβ) in the cerebral cortex is the central component of brain senile plaques in AD patients and that its deposition is the central cause of patient morbidity. Aβ is a short neurotoxic peptide produced by successive cleavage of APP by β-secretase and γ-secretase enzymes [ 115 ]. It can rapidly aggregate to form oligomers that are recognized as potent synaptotoxins, inducing synaptic loss and memory impairment to some extent [ 135 ]. In contrast, autosomal dominant mutations in APP, presenilin-1 (PSEN1), and presenilin-2 (PSEN2) have been observed in patients with familial AD (fAD), and these mutations are responsible for the pathogenic aggregation of Aβ peptides into neuritis plaques [ 130 ]. Even without such mutations, similar pathological changes can be observed in sporadic AD (sAD). Thus, it is presumed that insufficient brain Aβ clearance is the key event leading to its aggregation [ 84 ].
Stem cell-derived disease models have played a key role in the formation and development of the above hypothesis. In 2011, Yagi et al. established the first fAD-iPSC-derived neuron model carrying PSEN1 and PSEN2 mutations. These neurons were found to have increased Aβ42 secretion, while PSEN fAD mutations impaired γ-secretase activity, thus confirming the pathogenic link between fAD and PS1 and PS2 mutations [ 149 ]. To further identify the phenotypes common to fAD, [ 116 ] selected cortical neurons produced by iPSCs carrying different APP and/or PSEN1 mutations as a model. They found that endosomal transport defects in AD were caused by APP cleavage by-product β C-terminal fragment (β-CTF), as shown by analysis of their transcriptome and translatome. This finding suggests that the pathological mechanism of AD is not limited to Aβ, and that future research should target other APP metabolites (such as β-CTF) to prevent neurodegeneration [ 116 ]. Using this disease model, future research could also test whether endosomal changes are related to neuronal death and whether blocking the accumulation of other APP metabolites such as β-CTF can prevent neurodegeneration. It is worth mentioning that humanized AD mouse models have also played a key role in evaluating the amyloid hypothesis [ 208 ].
In addition to the 2D models described above, 3D cell culture systems and humanized AD models have been used to assess this hypothesis. Li et al. [ 190 ] constructed a 3D hydrogel culture system and observed many extracellular Aβ deposits in fAD ReN cells after 6 weeks of differentiation. Choi, Park, and Lee [ 171 ] established a 3D neurospheroid model consisting of prenatal rat (not human) cortical neurons and synthetic Aβ42 to test Aβ toxicity in a microfluidic platform [ 171 ]. In 2017, researchers generated a humanized AD model by transplanting human iPSC-derived cortical neurons into the brains of transgenic Tg (APP/PS1-21) mice [ 16 ]. In the comparison of human and mouse neurons, it was found that the Aβ plaque morphology in human neuron clusters was more diffuse than the dense core plaques in mouse brains, further revealing the species-specific vulnerability of human neurons to AD pathology. This model has provided key insights for the development of the AD amyloid hypothesis.
Hyperphosphorylation of tau protein
Given the failure of amyloid-based therapies for AD, researchers’ interest in tau has increased. Tau is a protein with roles in the stabilization and assembly of neuronal microtubules (MAP) that is mainly expressed in neurons, oligodendrocytes, and astrocytes in the CNS and peripheral nervous system [ 127 ]. Tau proteins control microtubule stability through heterodimerization and phosphorylation. Hyperphosphorylation of tau leads to abnormal function and aggregation, further forming neurofibrillary tangles (NFTs). The combination of toxicity acquired by aggregates or their precursors and the deleterious effects of loss of normal tau function in disease states may be an important cause of neurodegeneration [ 8 ].
Scientists are currently attempting to use stem cell-derived pathological models to address an important but unresolved question in AD: the relationship between tau pathology and Aβ, i.e., whether tau protein aggregation is secondary to Aβ deposition or an independent factor directly contributing to neurodegeneration. In 2012, Israel et al. [ 53 ] reprogrammed primary fibroblasts from two patients with fAD and found that tau phosphorylation was increased in neurons carrying APP repeat mutations and that β-secretase inhibitors significantly reduced tau phosphorylation by blocking Aβ production [ 53 ]. Wang et al. [ 137 , 215 , 216 ] found that the elevated level of hyperphosphorylated tau in iPSC-derived neurons carrying apolipoprotein E4 (APOE4) was not dependent on Aβ in APOE4 , paving the way for studies on other factors underlying tau phosphorylation. A more extensive study was conducted by [ 121 ]. who performed shRNA knockdown screening of more than 50 AD risk genes in iPSC-derived neurons to examine the effects on Aβ deposition and tau hyperphosphorylation and their interconnections. They found that FERM domain containing kindlin 2 (FERMT2) expression in neurons promoted late-onset AD (LOAD) formation and deterioration by increasing Aβ accumulation and tau levels [ 121 ].
Taken together, these findings demonstrate that human iPSC-derived neurons can effectively reflect the early features of AD pathogenesis, including the accumulation of pathogenic Aβ species and early tau disease. These results have important implications for further research and refinement of hypotheses and interrelationships between tau pathology and Aβ. Notably, both 3D culture models and humanized organoid models better show extracellular β-amyloid aggregation and NFT pathology than 2D neuronal cultures, providing a more robust model for AD research [ 121 ].
Risk gene mutations
Genome-wide association studies (GWAS) have identified many risk genes associated with different types of AD. Examples of these risk genes are summarized in Table 1 .
Stem cell technology combined with gene editing techniques can shed light on the role of risk genes in AD and the interplay between environmental factors and genetic background. CRISPR-Cas9-based genome editing and its derivatives have enabled efficient genetic modification of hiPSCs. The resulting “homozygous pairs” have greatly improved the resolution of disease-specific phenotypes and circumvented the need to collect patient cell lines with different genetic backgrounds, thereby facilitating research on the role of risk genes in AD [ 37 ].
Apolipoprotein E (APOE) is the strongest genetic risk site for LOAD [ 25 ].It also plays an important role in maintaining membrane homeostasis, supporting synaptic integrity, and healing brain injury. In humans, APOE has three isoforms: APOE2, APOE3, and APOE4. [ 158 ] demonstrated that human iPSC-derived astrocytes are a powerful platform for exploring the role of APOE ε4 isoforms in AD. They found that, when co-cultured with iPSC-derived neurons, human iPSC-derived APOE ε4/ε4 astrocytes promoted neuronal survival and synaptogenesis, whereas APOE ε4/ε4 astrocytes were not as effective as astrocytes with the APOE ε3/ε3 genotype in supporting these neurotrophic functions [ 158 ]. Sienski et al. (2021) also used this platform to explore the relationship between lipid metabolism and APOEε4 mutations. They demonstrated that APOE4, but not APOE3, disrupts the lipidome of iPSC-derived astrocytes [ 113 ]. 3D brain organoids from AD patients have been used in related studies. Zhao et al. [ 223 ] found that brain organoids from AD patients carrying APOE ε4/ε4 showed greater apoptosis and lower synaptic integrity and that APOE4-related pathways were directly associated with pathological tau protein accumulation. CRISPR/Cas9 technology has also contributed greatly to research on APOE4. For example, using CRISPR/Cas9 and gene expression analysis, Lin et al. observed that the APOE4 variant affects a series of genes that regulate lipid metabolism and transport and that the effect is cell-specific. These findings provide a reference for the design of future gene therapies targeting AD risk genes [ 68 ].
TREM2 , another important AD risk gene, has been extensively studied using stem cell-derived disease models. TREM2 encodes the myeloid trigger receptor 2, which is a cell surface transmembrane protein produced in the brain by microglia that primarily regulates microglial cell survival and activation [ 75 ]. Mutations in this gene increase the chance of developing AD by 200–400% above the normal level [ 43 , 61 ]. McQuade et al. [ 195 ] analyzed microglia differentiated in CRISPR-modified TREM2 knockdown iPSC lines [ 195 ]. By combining multi-omics analysis with a chimeric AD mouse model, they found that TREM2 deletion reduced microglia survival, impaired phagocytosis of key substrates including APOE, and inhibited SDF-1α/CXCR4-mediated chemotaxis, ultimately leading to impaired uptake of β-amyloid plaques in vivo. By combining chronic demyelination paradigms and cell sorting with RNA sequencing and lipidomics, Nugent et al. [ 200 ] elucidated the causal link between abnormal cholesterol transport and metabolism in TREM2-deficient microglia and the accumulation of toxic proteins in the brain, confirming that TREM2 deficiency exacerbates AD pathology.
In summary, stem cell technology can also be used to conduct GWAS. The genomes of iPSCs are more stable and reflect the genetic information of individuals more realistically than the traditional cell lines used in GWAS research. Furthermore, since iPSCs can differentiate into various cell types associated with AD, they can better mimic GWAS-related diseases. CRISPR-based gene editing technology can introduce knockouts or single-nucleotide polymorphism (SNPs)-targeted mutations into iPSCs, thus providing a high-throughput platform to functionally validate AD-related SNPs and genes [ 46 ].
Abnormal mitochondrial structure and function
There is growing evidence that impaired mitochondrial biogenesis and function are involved in many neurodegenerative diseases and can impair synaptic neurotransmission, which is essential for normal cognitive function. As a neurodegenerative disease [ 114 ], aging is a major risk factor for AD. However, the reprogramming step of iPSC technology resets age-related features (e.g., mitochondrial dysfunction, telomere shortening, etc.) back to the fetal stage, [ 74 ], meaning that stem cell models are useful for not only studying disease mechanisms but also disease processes—especially early biological and pathological events that disrupt neurological maintenance. Stem cells and their derived models thus provide a highly exploitable platform to study structural-functional changes in mitochondria early in AD development. They can also help us better understand the molecular basis of mitochondrial heterogeneity in structure and function. Stem cells with normal or abnormal mitochondria can be generated from healthy people and patients and then differentiated into various cell lineages. This approach allows us to investigate how the state of mitochondria affects stem cells’ ability to generate different cell types and regulate remodeling in disease-specific cells. As proof of concept, we can use patient-derived stem cell platforms to investigate the role of mitochondria in AD development. This approach represents a paradigm shift in understanding the role of mitochondria in human disease and opens up new avenues for AD research and therapy.
Using stem cell-derived models, many studies have shown that mitochondrial dysfunction, including metabolic disorders, increased ROS production, altered mitochondrial morphology, and dysregulation of mitochondrial quality control (QC) [ 211 , 218 ], are early signs of AD progression and contribute to disease progression. GWAS have identified variants in the ABCA7 gene encoding ATP-binding cassette (ABC) subfamily A member 7 that are strong risk factors for late-onset AD [ 183 ]. By constructing ABCA7-deficient iPSC-derived cortical organoids, Kawatani et al. proposed a new mechanism of AD-related neuronal injury caused by ABCA7 function loss: ABCA7 deficiency interferes with mitochondrial lipid metabolism, damages mitochondrial respiration, produces excessive ROS, enlarges mitochondrial morphology, and leads to disruption of mitochondrial homeostasis and synaptic function of neurons [ 188 ]. Mitochondrial QC includes fission and fusion processes, mitochondrial trafficking, and mitophagy [ 214 ]. The constant change of mitochondrial structure and shape through continuous fusion and fission is a process called mitochondrial dynamics [ 165 ]. There is growing evidence that dysfunction of mitochondrial dynamics may be the pathological cause of delayed onset neurodegenerative diseases such as AD [ 165 ]. When the contents and organelles of mitochondria are damaged, mitochondrial autophagy is triggered.[ 191 ]. Mitochondrial autophagy is a process that selectively removes damaged mitochondria, plays an essential role in regulating the number of intracellular mitochondria and maintaining normal mitochondrial function [ 161 ]. In the early course of AD, Fang et al. [ 178 ] observed widespread impaired mitochondrial autophagy in iPSC human AD neurons.[ 178 ] To investigate the effect of APP cleavage products on mitochondria function, Lee et al. (2022) used induced neural stem cells (iNSC) derived from fibroblasts of an AD patient origin as a model. They concluded that the accumulation of APP-derived C-terminal fragments (APP-CTFs) within the structural domain of mitochondria may be responsible for dysfunction such as impaired mitochondrial autophagy [ 64 ]. Martin-Maestro et al. [ 80 ] observed that dysfunctional mitochondria accumulated due to impaired mitochondrial autophagy in skin fibroblast iPSC-derived neurons carrying fAD -associated mutations. They further determined that the defective autophagic process was caused by a reduced autophagic degradation phase due to lysosomal abnormalities [ 80 ]. They also constructed hiPSC-derived NSCs carrying the fAD-associated PS1 M146L mutation and identified mitochondrial respiratory chain defects, such as low expression levels of key enzymes like cytochrome c oxidase (complex IV), cytochrome c reductase (complex III), fusion proteins like mitofusin-2 (Mfn2) and optic atrophy 1 (OPA1), and autophagy-associated proteins light chain 3 (LC3) . Reduced expression of lysosomal associated membrane protein 1 (LAMP1) and transcription factor EB (TFEB) may further contribute to the accumulation of defective mitochondria. These findings suggest that early metabolic alterations and impaired mitochondrial QC may have consequences for the pathophysiology of AD.
Because autopsy does not provide relevant information about disease progression, it is critical to develop model systems to study early molecular changes. AD human iPSC-derived models are thus complementary tools to study the degenerative process. Researchers have used such models to demonstrate the critical role of early events like mitochondrial dysfunction and impaired mitochondrial autophagy on AD pathogenesis [ 81 ]. Besides DNA in nuclei, cDNA mutations in mitochondria are another potentially important cause of AD, as well as the root of aging, and thus deserve our attention [ 222 ]. Researchers should focus on mitochondria signal pathways and mitochondria-targeted therapy to improve obstacle stress and energy metabolism in AD, ultimately improving our understanding of the interaction between genetic and environmental factors that contribute to mitochondria dysfunction in AD.
Immune system disorders
There is an intricate link between the immune system and CNS; when this balance is broken, it can cause neuroinflammation and, ultimately, AD. Neuroinflammation is an inflammatory response in the CNS caused by various pathological injuries. In this process, CNS glial cells, such as microglia and astrocytes, produce various pro-inflammatory cytokines, such as Interleukin-1β (IL-1β), IL-6, IL-18, tumor necrosis factor (TNF), and chemokines, as well as small molecule messengers and reactive oxygen species [ 31 ]. CNS glial cells thus play an important role in the progression of inflammation.
Stem cell technology allows researchers to study the interplay between immune function and AD using models like co-culture of immune cells from a single patient with their organoids [ 82 ]. As stem cell-derived 3D cultures can generate human oligodendrocyte spheroids, astrocyte spheroids, and neurons, these models can be applied to investigate glial cell functional abnormalities, different glial cell interactions, neuronal interaction networks, and the mechanisms of AD immune disorders. Bianco et al. [ 10 ] used a microfluidic platform to investigate the complex roles of different regional astrocytes in neuroinflammation. They inoculated astrocytes from the cortex and hippocampus, as well as primary hippocampal neuron cell types, from rat embryos in different chambers of the microfluidic network. By examining the effects on neuronal viability in two neuroinflammatory injury models (i.e., metabolic stress and exposure to amyloid β protofibrils), they clarified the differential contributions of brain region-specific neuroglia in two in vitro models. This study reflects the superiority of 3D microfluidic organ chips with multiple chambers to independently control culture conditions and selectively differentiate stem cells into different cell types with different stimuli. As this is also an open-ended method, it allows close monitoring of the specific contribution of each cell type by analyzing morphology, viability, calcium kinetic, and electrophysiological parameters when different chambers are micro fluidically connected [ 10 ]. Such models can reveal the complexity of cell–cell interactions in neuroinflammation, which is of great importance for elucidating the molecular mechanisms involved. To study the effect of the immune system on neuronal signaling, Guttikonda et al. [ 44 ] established a well-defined human pluripotent stem cells (hPSC)-derived tri-culture system containing pure populations of hPSC-derived microglia, astrocytes, and neurons. Using this culture system, they were able to demonstrate that increased complement C3 production in the brain is partly caused by microglia activation and its interaction with astrocytes, which in turn worsens neuroinflammation [ 44 ].
Synthetic models with AD features have also been developed. For example, Park et al. [ 95 ] used this technique to create a human ternary culture model containing NPC-derived neurons and astrocytes as well as immortalized human microglia. This method reflected the phenomena of microglia recruitment, neurotoxic activity, and nitrogen monoxide (NO) release damaging AD neurons and astrocytes and is suitable for further investigating the complex molecular mechanisms of neuroinflammation that underlie AD pathology [ 95 ]. Human pluripotent stem cell-derived brain-like Organoids co-cultured with immune cells from AD patients can also be used to better understand inter-glial interactions and provide an ideal experimental platform to explore the mechanisms of AD immune dysfunction under an in vivo growth environment.
As a proof of concept, we can use patient-derived stem cell platforms to study the role of the immune system and various glial cell communication disorders in the development of AD. This represents a paradigm shift in understanding the role of immunity in human disease and opens new avenues for AD research and treatment. In the future, new models could be developed to evaluate novel therapeutical interventions for AD such as immunomodulatory therapy, anti-inflammatory drugs, and immunoglobulin therapy.
In summary, reprogramming somatic cells from AD patients back to stem cells that can be re-differentiated into in vitro disease models to probe disease progression and pathogenesis provides a new approach for understanding the pathogenesis of human neurodegenerative diseases. This approach also takes advantage of the reverse engineering platform to monitor and determine key pathogenetic events during disease development. The hallmark of degeneration of the aging brain is selective cell vulnerability in disease-specific patterns many decades after the birth of long-lived brain cells. The traditional paradigm for investigating neurodegeneration has been to define the biological processes and pathways mediating brain cell dysfunction and death during the adult life; neurodegenerative diseases have thus been considered a discrete pathological entity rather than a continuum of a common pathogenic process. The new perspective considers neurodegenerative diseases as a fundamental disorder of neural development in which subtle impairment in the regional programming of neural development results in selective neural and brain network vulnerability to a spectrum of late-life stressors. Indeed, emerging data support the possibility of pathogenic associations between abnormalities in the birth and death of vulnerable brain cell subtypes [ 210 ].
Development of stem cell technology platforms for drug screening
Good disease models can be used to explore the diverse pathogenesis of diseases and support drug screening and development. However, drugs to stop AD progression are currently lacking. One important reason for this is that current drug screening methods typically rely on holistic animal models and cell lines as pharmacological and toxicological testing systems. Animal models of AD have species differences and cannot fully represent human responses, while most human cell lines are immortalized tumor cell lines that hardly reflect the reactivity of normal human cells.
Alternatively, in vitro differentiation of stem cells as a model of normal human development can be used for drug screening or to obtain multiple normal somatic cell types to test the toxicological effects of drugs on specific cells. The use of iPSC in vitro differentiated cells to screen new drugs can greatly reduce the number of experimental animals required for drug experiments. The use of stem cells to establish models that comprehensively reflect the pathological phenotype and disease mechanisms of human AD is thus of great significance for AD drug development and screening.
In 2017, Kondo et al. presented a platform for drug discovery and development using human iPSCs and compound screening. They used 13 iPSC-differentiated pure cortical neurons from AD patients. After two rounds of screening, the final screen q yielded six potential compounds that could minimize Aβ40 and Aβ42 levels. Such models can help overcome the drawbacks encountered in drug development and screening using standard human iPSC platforms [ 56 , 123 ].
Simple models of iPSC-derived neurons have failed to account for interactions with vascular cells, glial cells, and the blood-brain barrier (BBB). The BBB is a major factor limiting the action of AD drugs. Moreover, BBB dysfunction and catabolism have been observed in AD, which in turn triggers a series of inflammatory responses and neuronal loss and neurodegeneration. The development of specific AD BBB models and their application in preclinical studies are therefore important for improving the success of AD drug clinical trials. Many recent studies have reported successfully applying combined iPSC and microfluidic technology to mimic the BBB in a neuro-neuroglial interaction model [ 132 ]. The use of iPSC-derived BBB models in combination with gene editing (e.g., CRISPR/Cas-9 technology) to mimic disease-associated mutations or introduce artificial mutations (e.g., gene knockout) can better reflect altered brain dynamics in disease states and help screen new drugs that can reach deeper neural tissues through the BBB [ 4 , 112 ]. Gene-edited iPSCs expanded and differentiated in vitro into different cell types can also be transplanted into AD animal models to generate human-animal chimeras for drug screening [ 136 , 142 ].
Potential of stem cell-based therapies for AD
In addition to their value as disease models and for drug screening, stem cells have played a significant role in treating AD. The transplantation of stem cells to treat AD is mainly based on their ability to differentiate into neurons and glial cells that can replace damaged cells, release cytokines to activate endogenous neurogenesis, and regulate immune cell status. Transplanted stem cells also can be induced to differentiate into glial cells or corresponding precursor cells to improve the neuronal environment by repairing the functions of supporting cells, ultimately repairing overall neural network function. Stem cells can also act therapeutically through the contents of their secretomes, e.g. exosomes. Unlike monoclonal antibodies or enzyme inhibitors that target Aβ to slow down the pathological process, stem cells have the potential to rebuild the integrity of the CNS. Moreover, given the interconnectedness of the various mechanisms of AD pathology, targeting just one for treatment may not be as effective as therapies targeting various factors—stem cells have the potential to treat AD via multiple mechanisms (Fig. 3 ).
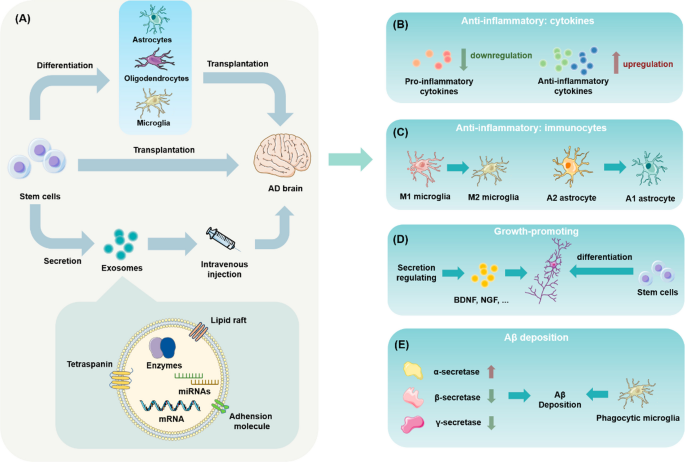
The role of stem cell-based therapies in the treatment of AD. A Through direct transplantation, retransplantation following differentiation into neural cells, and secretory exosome infusion, stem cells offer various benefits for treating AD. B Stem cells with their differentiated cells and derived exosomes regulate the release of a range of cytokines, reducing levels of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) and increasing levels of anti-inflammatory cytokines (IL-1 receptor antagonist (IL-1ra), IL-10) in cerebrospinal fluid. C The phenotypes of microglia and astrocytes are changed by stem cells through their differentiated cells and exosomes, shifting them from a pro-inflammatory to anti-inflammatory phenotype and decreasing the inflammatory response in the brain and promoting neuroactivity. D Stem cells with their differentiated cells and derived exosomes are upregulated with Brain-Derived Neurotrophic Factor (BDNF), Nerve Growth Factor (NGF), etc. The stem cells transplanted through exogenous pathways are integrated into the neural network to regenerate synapses. E As MSCs with their differentiated cells and exosomes activate microglia, they specifically phagocytose Aβ or regulate the expression levels of Aβ-related enzymes (e.g., upregulate α-secretase activity or downregulate β-secretase activity) to clear existing Aβ deposits
Stem cell transplantation therapy
The formation of Aβ and its damage to the neurological system is one of the best known pathological processes in AD [ 141 ]. Clearing Aβ from the brain is thus thought to be one of the most promising ways to treat AD. Stem cell transplantation could reduce Aβ deposition through the following mechanisms: activation of microglia to eliminate Aβ through specific phagocytosis [ 62 , 63 ], regulation of the expression levels of enzymes related to Aβ synthesis or degradation (e.g., upregulation of α-secretase activity or downregulation of γ-secretase or β-secretase activity) [ 224 ], or removal of existing Aβ deposits through immunotherapy. These effects on Aβ could occur separately or simultaneously [ 189 ]. The clearance of Aβ is frequently accompanied by microglia, however, this does not mean that microglia activation is essential for stem cell transplantation therapy to achieve Aβ clearance [ 144 ].
Hyperphosphorylation of tau protein and NFT production are also significant pathological aspects of AD, and different tau structures may mediate different pathogenic processes. However, more extensive research on how stem cells can improve tau pathology is required [ 36 , 38 ]. Nevertheless, it is important to investigate potential improvement in tau pathology by stem cell therapy given the current assumption that tau pathology may develop independently of Aβ pathology. There is adequate evidence that stem cell transplantation can improve the activity of cells damaged by abnormal tau protein neurotoxicity. For example, Zilka et al. [ 226 ] co-cultured AT tau cells, a model of AD cells developed from human truncated tau protein (Tau151-391, 4R), with mesenchymal stem cells (MSCs) and found that this greatly enhanced cell viability, demonstrating that MSC therapy rescued cells from toxic tau protein. The researchers suggested that this effect of MSCs is partly attributed to the complex bioactive components in their secretomes. They also reported that MSCs encouraged neurite development in cells expressing truncated tau. These findings demonstrate that stem cell therapy can reduce cellular damage caused by toxic tau proteins—at least in in vitro experiments [ 226 ]. However, the therapeutic effect of stem cell transplantation on tau-oligomers-burdened models still needs to be explored, for tau oligomers is now observed to cause serious synapse elimination [ 212 ].
One of the most significant therapeutic functions of stem cells in neurodegenerative disorders is replacement of senescent, damaged neurons. When stem cells like iPSC-NPCs are pre-induced in vitro to differentiate into cholinergic neurons and then reimplanted they could improve memory and cognitive function. They have been proven to survive and differentiate into cholinergic and GABAergic neurons when directly transplanted to lesion sites. The positive effects of stem cells on synaptic regeneration can be described as endogenous or exogenous: exogenous synaptic regeneration is realized through the integration of stem cells implanted into neural networks, whereas endogenous synaptic regeneration is realized through the promotion of synaptic regeneration via upregulation of factors positively associated with neurogenesis, e.g., BDNF, NGF, etc.
Many experiments have demonstrated enhancement of synaptic integrity and elevation of neuronal activity after injection of stem cells, and Basal forebrain-like cholinergic neurons (BFCN) progenitor cells are frequently utilized in experiments [ 184 , 221 ], indicating the possibility of rebuilding neuronal circuits utilizing exogenous neural stem cells.
As an alternative to exogenous implantation, numerous studies have sought to achieve brain repair by promoting the expression of endogenous growth factors and, subsequently, repairing neuronal circuits. In many studies, the upregulation of proteins associated with antioxidation, synaptic connectivity, and neurogenesis such as sirtuin1 (Sirt1), BDNF, synuclein (SYN), and β-nerve growth factor (β-NGF), has been observed. [ 157 , 172 , 175 ] Stem cells’ secretomes or the trophic factors produced after their differentiation into glial cells also act as sources of neurotrophic factors. For instance, using co-culture experiments, Peng et al. [ 203 ] showed that BM-MSCs can differentiate into Schwann cells and promote the quantity and length of sprouting neurons of dorsal root ganglia neurons.
However, other studies suggest that the delivery of neurotrophic factors is not the primary benefit of stem cells for treating neurodegenerative illnesses [ 34 ]. Although delivery of neurotrophic factors may appear to be less effective at rescuing neurodegeneration, this technique can be utilized to enhance the therapeutic efficacy of stem cell transplantation.
For example, using BDNF-transfected MSCs for transplantation, Song et al. [ 117 ] showed that the neuroprotective effect of BDNF increased the activity and synaptic integrity of primary cultured neurons in APP/PS1 mice. The transplanted BDNF-MSCs differentiated into neuronal cells, increased the number of local cholinergic neurons, promoted Acetylcholine (Ach) secretion, and improved cognitive function [ 117 ]. Overall, synaptic regeneration may be more effectively promoted by combining the secretion of neurotrophic factors with the exogenous replacement of neurons.
Given increasing emphasis and support for the “infection theory” of AD, neuroinflammation mitigation has become an innovative treatment approach [ 51 , 85 ], and the modulatory effects of stem cell infusion therapies on immunological function in the CNS are becoming focal areas.
As one of the most common immune cell types in the CNS, microglia maintain and protect neural cells by controlling inflammation and preserving the integrity of the microenvironment, among other methods [ 166 ]. In AD, related gene expression in certain microglia changes during the course of the disease [ 83 ], the expression of these genes in certain microglia has been shown to rescue neurodegeneration [ 61 ]. Thus, altering microglia activity is considered a potential method for treating AD. In APP/PS1 mice treated with human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs), [ 63 ] demonstrated an increase in microglia in the alternative activation state and a decrease in neuroinflammation. In addition, Yokokawa [ 220 ] showed that MSCs transplantation into the brains of APdE9 mice induced microglia to convert from the M1 phenotype to the M2 phenotype and engage in phagocytosis, while also reducing neuroinflammation and enhancing the redox state. Besides, the transition between different microglia phenotypes, lower expression of pro-inflammatory factors (TNF-, IL-6, and Monocyte chemoattractant protein-1 (MCP-1)) and decreases in the quantity and volume of microglia in the cortex have been observed in stem cell transplantation studies [ 197 ].
Rather than a one-way influence, there is mutual action between neural stem cells and microglia [ 207 ]. Thus, the efficacy of stem cell transplantation is likely to be influenced by the microglia’s state of activation. In support of this, a study by Popova et al. [ 204 ] showed that microglia could induce transcriptional changes in neural stem cells and reduce interferon signaling response genes, while interferon-induced transmembrane protein 3 (IFITM3) was previously shown to upregulate γ-secretase activity to increase Aβ production. By co-culturing activated microglia with NPC, Lilienberg et al. [ 67 ] demonstrated that naïve microglia do not affect the proliferation of NPCs and enhanced neurosynaptic growth. Using this co-culture system, the authors also observed that the nitro blebbistatin-triggered synaptic development was enhanced by naïve microglia. [ 67 ]. Popova et al. [ 204 ] showed that microglia can induce transcriptional changes in neural stem cells and accelerate the emergence of synchronized oscillatory network activity in brain organoids, which has implications for the integration of stem cells into the original neural network following transplantation. These findings have revealed the potential of using drug induction in stem cell therapy and the important effect the environment’s microglia status has on neural stem cells. More importantly, not all instances of activated microglia cause damage to neurons. Thus, pre-regulating the state of microglia in the environment or combining it with drug induction may need to be considered when designing stem cell transplantation therapies.
In terms of other parts of the immunoregulation system is the CNS, the glymphatic system, which is related to various diseases like Parkinson’s [ 122 ], diabetes [ 55 ], hypertension [ 89 ], and aging [ 57 ], has recently attracted attention for its role in brain homeostasis. The glymphatic system controls the clearance of various toxic compounds that develop with aging or other changes from the brain, including Aβ proteins linked to AD. Astrocytes play a significant role in the glymphatic system, such as removing waste proteins by regulating cerebrospinal fluid shunting via aquaporin-4 (AQP-4). A promising approach to treating AD thus involves altering astrocyte activity through stem cell transplantation.
Previous studies have reported that stem cells can inhibit the proliferation of astrocytes and encourage the development of non-reactive astrocytes, which have the capacity to support synaptic regeneration, heal injured neurons, and engage in phagocytosis and Aβ catabolism [ 6 ]. Because abnormal cerebrospinal fluid-mediated immune function has significant implications for AD pathology and stem cell transplantation has been shown to improve some causes of glymphatic system disorders—including upregulation of aquaporin (AQP-4) expression and attenuation of astrocyte proliferation—the beneficial effects of stem cells on the lymphoid-like system warrant further investigation [ 97 , 143 ]. One novel avenue for future stem cell research in AD may be stem cell manipulation of astrocyte protein expression, which in turn influences the effectiveness of material exchange and waste clearance in the lymphoid system.
In summary, stem cell transplantation therapy has been shown to not only improve typical pathology indicators of AD, such as Aβ and Tau fiber tangles, but also to have a reciprocal modulatory effect on numerous important immune system cell types, including microglia and astrocytes. These various efficacies most likely work together to achieve the neuronal regeneration and cognitive improvement observed after transplantation. Pre-conditioning the microenvironment of the treated area or combining neural stem cells with certain glial precursor cells (GPCs) may be a workable therapeutic approach when considering the interactions between cells and their environments.
Stem cell-derived glial cell therapy and models
Although neurons may be directly responsible for cognitive function, their sustained activity relies largely on support from glial cells, which account for the majority of brain cell populations. The repeated failures of strategies to eliminate abnormal protein aggregates in AD patients—such as Aβ aggregation inhibitors, Aβ antigens, anti-Aβ monoclonal/polyclonal antibodies, γ-secretase inhibitors, γ-secretase modulators, and β-site amyloid precursor protein cleaving enzyme (BACE) inhibitors—may teach us a lesson: although glial cells have long been ignored, they make important contributions to the onset and progression of AD.
Glial cells are now widely recognized to have potential for AD research and therapy. Using disease models based on glial cells, it is possible to investigate AD pathogenesis in a more systemic way. With rapid developments in stem cell technology, more attention is being paid to glial cell transplantation therapies. The three major approaches to transplanting glial cells derived from stem cells are microglia transplantation, astrocyte transplantation, and oligodendrocyte transplantation. NSCs, GPCs, and pluripotent stem cells (PSCs) are some sources of stem cells used for glial cell transplantation. GPCs can also be obtained from PSCs. Given the potential of stem cell-derived neuroglia platforms and therapies, it is necessary to discuss possible approaches to glia-based platforms and transplantation therapy.
Stem cell-derived glia in disease modeling
Given the important role of glial cells in maintaining a stable brain environment and neuronal health, it is necessary to involve multiple glial cell types in the construction of disease models. Accordingly, glial cell-based models of AD need to be developed using different glia types to establish in vitro and in vivo models. Such models will enable researchers to study AD in a more controlled and systemic manner to identify novel treatments.
Many studies using AD disease models containing glial cells have provided new understandings of pathological processes. Complement C3 is a protein that is increased under inflammatory conditions and implicated in synaptic loss. Guttikonda et al. [ 44 ] identified the main reason for increased C3 in AD and the main cell types involved being microglia versus astrocytes using a hPSC-derived microglia and tri-culture system model [ 44 ]. By using neurons, astrocytes, and microglia in a 3D microfluidic platform, Park et al. [ 95 ]. presented a 3D human AD tri-culture model that mirrors microglial recruitment and important neurotoxic activities in AD [ 95 ].
To summarize, to deepen our understanding of AD pathogenesis and promote AD therapy, more research is needed to help design glia-based AD models that mirror typical activity in AD. A tri-culture system comprising all three kinds of neuroglia may be a more promising approach than using a single glial cell type. This suggests that human brain organoids comprising neurons and several types of glial cells should be an ideal platform for AD research. However, additional studies about the different glia subtypes are warranted, since the subtypes have different functions and may thus have different effects on AD pathology.
Stem cell-derived glia in AD therapy
Traditional brain cell therapies tend to use ectogenic neurons to replace damaged ones. However, this type of transplantation has numerous drawbacks, many of which are caused by neurons’ ability to be excited. In some cases, neuron transplantation can cause convulsion in mice, which can eventually lead to death. Ectogenic neurons are thought to be responsible for these severe side effect because they are more excitable [ 105 ]. Further research is clearly needed to explore additional cell types for cell replacement therapies for AD. Glial cells, including microglia, astrocytes, and oligodendrocytes, play crucial roles in maintaining the environment in the human brain and regulating processes over the lifecycle of neurons. Glial cells are now considered a promising choice because they positively influence neurons in many ways, including secretion of factors, phagocytosis, and direct interaction between cells. Glial cells are also comparatively safe for transplantation because they are non-excitable, thereby protecting the recipient from hypo-excitation.
Microglia transplantation for AD
Due to their ability to activate and escalate neuroinflammatory responses, microglia have long been considered one of the main contributors to damage caused by neuroinflammation. However, based on in-depth studies on microglia function and state, current microglia therapies no longer advocate comprehensive suppression of their functions, but rather distinct strategies to promote alleviation of neuroinflammation that are tailored to the microenvironment of the specific disease. Due to the extended longevity of microglia and the fact that the gradual loss of homeostasis is associated with AD pathogenesis, the low efficiency of nascent microglia in removing aggregates of toxic proteins may encourage the pathological processes of AD. To restore their natural immunological filtering function, senescent microglia should be rejuvenated or healthy microglia could be replenished endogenously or exogenously as part of an AD treatment strategy. Exogenous microglia supplementation or stem cell-derived microglia transplantation are potential approaches to reestablish central immune clearance. In bone marrow transplantation (BMT), microglia-like cells produced from iPSCs, ESCs, or monocytes are the main cell sources for microglia transplantation. Several studies have reported successful transplantation of microglia into the mouse brain and validation of functional integration of microglia after transplantation [ 145 , 145 , 147 , 147 ]. Notably, the microglia showed oligodendrocyte phagocytic and synaptic phagocytic functions characteristic of normal microglia. Microglia transplanted into mice brains are stimulated by Aβ to alter expression groups, i.e., gene expression that tends to be in an activated state [ 76 ]. However, proof of their mitigating effects on AD pathology is limited. It has also been shown that TREM2 expression is lower in hPSC-derived microglia [ 32 ]. Given that low TREM2 expression was thought to have a positive effect on maintaining microglia homeostasis, human pluripotent stem cell-derived microglia may help maintain a stable phenotype that is beneficial to remission of neuroinflammation after transplantation into the brain.
Although there is insufficient research on microglia transplantation to treat AD pathogenesis, transplanting microglia could theoretically help reduce harmful protein buildup from pre-existing, hard-to-remove senescent microglia and help carry out typical synaptic pruning and oligodendrocyte phagocytosis. Transplantation of a single microglia type (or equivalent precursor cell, such as a primitive macrophage progenitor cell (PMP) might not result in significant changes in AD pathology; transplantation combining several stem cell types might be preferable. Depending on the lesion location, the appropriate subtype of microglia can be selected and paired with astrocytes or neural stem cells to more accurately reflect the environment that supports neurons in vivo.
Astrocyte transplantation
The properties and activities of astrocytes are altered in many neurological illnesses, including AD, which may worsen the degenerative processes. It is well established that astrocytes can be generated from stem cells or histocytes. Transplanting astrocytes to achieve recovery of functions is regarded as one of the most promising directions for AD treatment.
At present, astrocyte transplantation therapy uses human embryonic stem cell (hESC) differentiated astrocytes, human iPSC differentiated astrocytes, human fetal brain-derived astrocytes, or human fetal spinal cord-derived astrocytes. The best source of astrocytes is currently differentiation using stem cells (e.g., iPSCs, hESCs), as it is difficult to achieve the quantity needed for transplantation therapy using astrocytes derived from the human fetal spinal cord and human fetal brain, which is crucial for efficacy [ 157 ].
The feasibility of transplanting astrocytes requires integration of glial cells into existing circuits. This was demonstrated by Zhang et al. [ 224 ] in their study showing that NSC-derived glial precursor cells could differentiate into astrocytes and integrate into existing circuits in the brain after transplantation, with a survival time of more than one year. Initially proposed to treat spinal cord damage, astrocyte transplantation has been proven to protect host spinal cord neurons, encourage axonal regeneration, reduce glutamate toxicity, and support restoration of motor, sensory, and respiratory functions [ 160 ].
The neurotrophic effects of astrocytes, microglia activation, and oligodendrocyte activation all support astrocyte transplantation for treating AD. Although there are only a few studies using astrocytes for AD therapy, the existing research provides preliminary proof that intracerebral transplanted astrocytes undergo morphological changes in response to Aβ deposition and that this morphology varies with AD stage [ 101 ]. It has also been demonstrated that human ESC-derived astrocytes stimulate the IL-4 signaling cascade by controlling the microglia phenotype and serving as neuroprotectants, which is consistent with previous findings on the clearance of Aβ by astroglia [ 98 , 136 , 142 ]. As demonstrated by increased AQP4 polarization facilitating deposit clearance and improved neuronal function [ 151 ], implantation of GPC-derived astrocytes was recently shown to result in long-term functional integration and sensory enhancement in elderly mice.
In addition to astrocyte transplantation alone, astrocyte transplantation has been proposed in combination with neuronal transplantation. Because astrocytes facilitate neuronal maturation and increase synaptic plasticity, combined cellular therapies may be advantageous for both maintaining astrocyte function and promoting neuronal differentiation [ 160 ].
Oligodendrocyte transplantation
Oligodendrocytes are the core cell type involved in myelination. They can be differentiated from oligodendrocyte precursor cells (OPC) capable of migration, expansion, and protecting axons and neurons. The degenerative process in AD frequently involves oligodendrocyte dysfunction, which can result in further neuronal loss and dysfunction. OPC transplantation has mainly been investigated for treatment of multiple sclerosis (MS). However, its potential for AD treatment should not be disregarded since OPCs play a crucial role in remyelination by producing new oligodendrocytes to replace damaged ones and recovering lost cells after injury. Additionally, the biphasic communication of OPCs with astrocytes makes their function closely related to that of the central immune system. Theoretically, OPCs can be transplanted alone to promote myelination and supporting axons, or transplanted with neurons to repair loss of functional cells in the brain.
Oligodendrocyte transplantation is less common and is typically intended to heal neurological impairment rather than to treat AD. For example, Windrem et al. [ 217 ] transplanted human glial precursor cells (hGPCs) for differentiation into oligodendrocytes and observed myelin repair in the brain of myelin-deficient shiverer mice; the researchers thought the cells would subsequently function as remyelinating oligodendrocytes and be normally recruited. This study proves that integration of transplanted GPCs differentiated into oligodendrocytes in the brain is possible. Notably, OPC transplantation may be a more durable approach to external regeneration of oligodendrocytes due to the potential of GPCs to differentiate into other glial cells.
Stem cell-derived exosome therapy
Although stem cell therapy has shown superiority over traditional drug therapy for AD, it still faces many problems. First, since stem cells have a low degree of differentiation and strong ability to divide, they are prone to tumors. Second, intranasal administration is often used to administer drugs to the brain, but is challenging with stem cells. Using MSCs (with a diameter of 30–60 μm) as an example, the large size of stem cells may block the pulmonary capillaries and induce immune rejection [ 87 ]. Thus, delivering transplanted stem cells to the brain is challenging and carries some risk [ 27 , 96 ]. Autologous transplantation of MSCs is challenging in patients with fulminant disease due to the complex cell preparation process and time needed for cell transplantation [ 48 ].
Exosomes derived from stem cells have become a promising new cell-free strategy in the decades-long pursuit to improve the therapeutic efficacy of stem cells. Exosomes, as a part of stem cell secretomes, are diverse membrane-bound vesicles that function as intercellular messengers. They carry different loads including DNA, RNA, proteins, and lipids, among others. Stem cell-derived exosomes show molecular specificity due to engineering and drug accumulation and can induce potent action mediated by the activation of factors and molecules with variable concentrations and signaling cascades. These actions can be easily modified and enriched by engineering exosomes [ 24 ].
The physiological roles of extracellular vesicles (EVs), which are abundant in most body fluids and released by cells. In fact, transplanted stem cells function in the body mainly due to their paracrine effect. As important parts of the stem cell secretion group, the physiological functions of exosomes are similar to those of their stem cell counterparts [ 153 ].
Compared with stem cell transplantation, exosome therapy has low immunogenicity and a lower likelihood of forming tumors. Nanoparticles (NPs) between 10 and 1000 nm in size can encapsulate therapeutic molecules by targeting the transport process in the CNS and then effectively migrate to target organs after infusion without blocking pulmonary capillaries and causing immune rejection. Cell-secreted exosomes, which are lipid-membraned vesicles, can easily permeate the BBB to exert their physiological effects. Exosome therapy has also shown advantages for AD patients with acute onset.
Because stem cell-derived exosomes generally show comparable biological activity as stem cells and have many advantages, they have become a hot topic along with cell therapy in the treatment of neurodegenerative diseases—exosomes are believed to be able to clear Aβ protein in the brain, restore synaptic activity in the brain, reduce inflammatory responses in the brain, enhance neural network connectivity, and alter metabolism. They thus have great potential for treating a variety of diseases including AD.
Researchers in Taiwan [ 22 ] Chen, et al. recently established a mouse FAD model and showed that the level of Aβ in mouse cells after exosome treatment was significantly reduced. In this experiment, exosome therapy was also found to improve glucose metabolism in the brain and restore the protein-modifying enzyme histone deacetylase 4 (HDAC4), representing improvement in memory and cognitive function.
Some researchers have improved exosomes’ efficacy at reducing Aβ in the brain through combination with other drugs. [ 140 ] used BM-MSC-derived exosomes in combination with sphingosine kinase inhibitor (SKI-II) or sphingosine-1-phosphate-1 receptor blocker (VPC23019). They found that exosomes inhibited Aβ1-40, Aβ1-42, BACE1, and PS1 in AD mouse cortex and hippocampus, thereby improving spatial learning and memory.
Some studies have found that stem cell-derived exosomes have therapeutic effects on neurological function [ 73 ]. A proteomic analysis of brain tissue from AD mice treated with MSC-EVs showed that a variety of proteins in EVs exert neuroprotective, synaptic growth, and neurogenesis effects. In the above study, EVs were also found to have therapeutic effects on some risk genes that can cause AD. Compared with the control group, 1094 genes were upregulated and 267 genes were downregulated in the brains of AD mice after exosome treatment. Some of these upregulated genes—such as piccolo (PCLO), TENM1 , and Neurite extension and migration factor ( NEXMIF )—are positive for synaptic function and memory improvement, while some downregulated genes are associated with cell death, such as BAD . These results suggest that EVs can relieve nerve damage and significantly increase neurogenesis in AD mice.
Regarding inhibition of inflammation in the brain, controlling M1/M2 phenotypic switching of microglia is significant for treatment of AD. Exosomes derived from MSCs can convert from the M1 phenotype with a pro-inflammatory effect into the M2 phenotype with an anti-inflammatory effect, which can effectively reduce neuroinflammation and relieve AD symptoms [ 30 , 71 , 96 , 119 ].
Many previous studies have used AD mouse models to prove the effect of exosomes on inhibiting inflammation. However, due to differences in molecular pathways and signaling functions between rodents and human microglia, a research group from Stanford University [ 40 ] chose to study the role of MSC-EVs in the human microglial cell line (HMC3). They showed that EV treatment reduced the expression of CD11b in cells and the number of proteolysis and exerted an anti-inflammatory effect by promoting M2 phenotypic transformation of microglia. Notably, these results are similar to findings in AD mice.
Recent studies have shown that stem cell-derived exosomes can act on astrocytes to reduce their activation, thereby reducing neuroinflammation and improving cognitive impairment in AD patients. The most intuitive use of stem cell-derived exosomes on astrocytes is to reduce the proportion of active astrocytes, which is manifested as reduced glial fibrillary acidic protein (GFAP) levels in the brain and colocalization of GFAP and amyloid plaques [ 22 ] [ 24 , 137 , 215 , 216 ])
Some findings for stem cell-derived exosomes in AD therapy over the last five years are listed below (Table 2 ):
Challenges and strategies for stem cell technology in AD research and treatment
(Fig. 4 ).
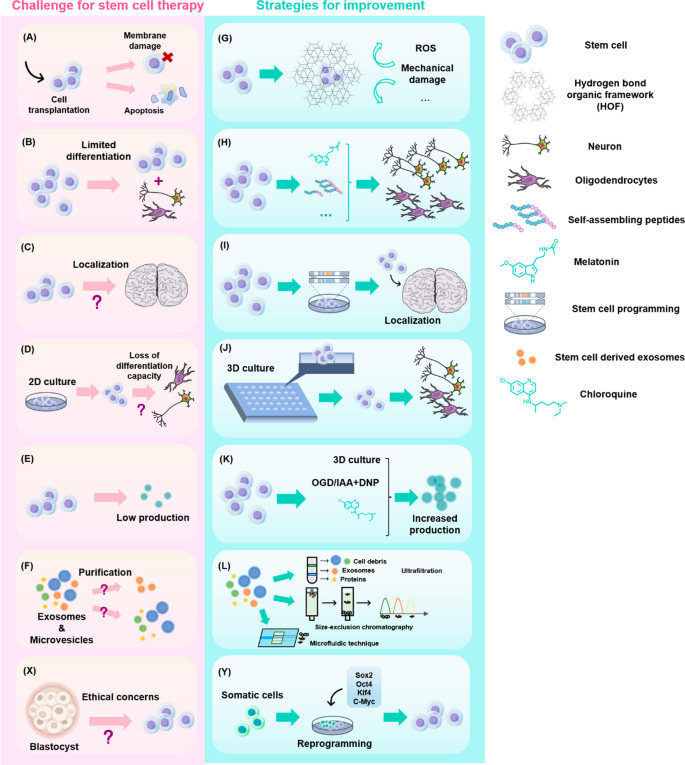
Examples of current challenges for stem cell-based AD therapies and strategies for improvement. A Bare stem cell transplantation results in challenges such as disruption of cell membranes and apoptosis. B Delivery after encapsulation with a protective organic framework protects stem cells from oxidative damage and mechanical damage. C Stem cell transplantation therapy may have limited efficacy due to low differentiation efficiency. D Co-culture of certain biomolecules with stem cells can enhance stem cell differentiation. E The efficiency of migration and localization of stem cells in the body after transplantation is difficult to guarantee. F Regulating the expression of specific genes can promote targeted migration of stem cells. G Traditional 2D culture methods may result in reduced or even lost differentiation potential of stem cells over time. H Beneficial characteristics, such as differentiation potential, can be better retained by using a 3D culture strategy. I Poor production of stem cell-derived exosomes. J Altered environmental conditions, such as oxygen–glucose deprivation, the addition of specific small molecules or 3D culture, can be effective for increasing exosome yields. K The extracted cellular secretion components are complex and it is difficult to isolate the therapeutic exosomes. L Exosomes of a certain particle size can be effectively separated using methods like ultrafiltration, volume exclusion method, microfluidics, etc. X Ethical concerns about the production of stem cells. Y The use of iPSCs for stem cell-based therapy
Limitations and strategies of using stem cells in disease modeling and drug screening
Although human iPSCs have become a powerful tool for human disease modeling and have great potential for translational research in target discovery and drug development, various challenges remain. Human iPSC-derived neurons are sensitive and require extended culture time (80 days) to develop mature neuronal features; it is challenging to do this consistently and at a large scale by manually changing the culture medium [ 111 ]. Several strategies exist to overcome these challenges. Notch and γ-secretase inhibitors have been shown to shorten the maturation period of most neuronal cell types [ 13 , 20 ]. Another approach includes overexpression of neuro-specific genes. For example, overexpression of Ngn2 accelerates the generation of forebrain and motor neuron cells to within 10 days [ 52 , 56 ]. In addition to shortening the time, the use of a high-throughput screening platform for aged neurons may be more translatable. Although scientists have differentiated stem cells into neurons, these neurons are functionally immature—similar to embryonic or early postnatal neurons. The limited maturity of neurons obtained using stem cell culture techniques diminishes their potential for neurodegeneration research. However, recent studies have found that differentiating human iPSCs into motor and cortical neurons and then culturing them on synthetic nanofiber coatings with high internal supramolecular motion can enrich them into more mature neurons [ 2 ]. Furthermore, since sAD is influenced not only by risk genes but also epigenetics, aging and environmental factors may contribute to its development. The ability of iPSC models to accurately mimic the sAD phenotype is another challenge in disease modeling and is thought to be an important reason why numerous AD therapies effective in preclinical trials have poor clinical outcomes [ 33 ]. The development of more effective sAD models is thus an important goal for the future.
Compared to 2D culture systems, 3D culture platforms are better suited to study AD pathophysiological mechanisms involving cell–cell interactions, controlled flow dynamics, circulating blood cells, and brain-specific microenvironments [ 100 ]. However, they still cannot mimic the intrinsic immune rejection reactions encountered in practical clinical applications. There are also many technical challenges facing organoid culture and microfluidic technology. For example, human brain organoids lack the vasculature of brain tissue in vivo, which plays a facilitating role for both nutritional supply and neuronal cell differentiation. This lack of vascularity leads to necrosis in brain-like organ centers, further interfering with normal development and neuronal migration routes. Although co-culture with vascular stem cells provides a partial solution to these problems, the issues of nutrient supply and the true blood microenvironment remain. hESCs expressing human ETS variant 2 (ETV2) have been used to successfully construct brain cortical organoids (hCOs) with vascular-like structures, including tight junctions, increased expression of nutrient transport proteins, and transendothelial resistance, thereby addressing the challenge of missing blood vessels [ 17 ]. However, further development is needed for their application in neurodegenerative disease. Regarding microfluidic technology, the requirements for reagent volume and reaction conditions are so stringent that small changes in the volume of reaction components can lead to changes in the results [ 118 ]. Therefore, the clinical usefulness of this method needs to be further explored.
For drug screening, it is extremely important to select the appropriate early-onset predominant phenotype as a marker to measure the effect of the tested drug. In AD, the dominance of tau hyperphosphorylation or Aβ aberrant deposition phenotypes has still not been elucidated. As a result, extensive mechanistic studies are needed before applying the findings to drug screening [ 21 ]. Since neurons, astrocytes, and microglia are highly specialized and disease-relevant cell types, the long time required for their differentiation limits the application of iPSC-derived glial cells in disease modeling and drug screening. The discovery of novel strategies to promote gliogenesis is thus crucial for large-scale drug screening. Novel gene editing tools and biomolecular technologies, such as single-cell RNA sequencing, could also enable more accurate disease modeling and drug screening processes.
To summarize, stem cells are widely used in disease modeling, drug screening, and cell therapy because of their unlimited self-renewal ability and potential to differentiate into all cell types. By using stem cell-based platforms, AD research can better study the fundamental biological mechanisms of the disease, develop new technologies, and innovate on therapeutic concepts. In the future, interdisciplinary collaborations should be fostered to build organoids or assemble spheroids and other multicellular models of human tissues comprising diverse cell types (e.g., excitatory/inhibitory neurons, astrocytes, oligodendrocytes, and microglia). This could enable rapid testing of genetic, chemical, and environmental perturbations to develop genetic engineering and genome editing tools to study the pathogenesis and treatment of the disease and develop in vivo models that more accurately recapitulate human physiology. We must develop multi-omics technologies, such as single cell and spatial transcriptomics, long read sequencing, next-generation proteomics, and other high-throughput cellular phenotyping platforms. We also need to develop software, data mining, and machine learning methods to dissect biological complexity in aging and disease and to perform functional and genomic analyses of multicellular models (e.g., bulk or single cell transcriptomic characterization, live cell imaging, and electrophysiology) and chemical biology-enabled multi-omics or chemical library-based drug screening studies.
Limitations and strategies of stem cells and derived therapies
AD research that uses stem cell technology faces several significant challenges. These include: (1) limited capacity for self-renewal and differentiation of stem cells, (2) heterogeneity of stem cell populations and their derived cell types, and (3) ethical concerns surrounding the use of embryonic stem cells. However, ongoing efforts to overcome these limitations hold great promise for improving the quality and effectiveness of stem cell-based approaches. These efforts include: (1) the development of new techniques to promote differentiation, (2) preventing pre-mature death of stem cells and improving the therapeutic effect, and (3) investigating alternative, ethically acceptable sources of stem cells. Further research and innovation will therefore be critical to realize the full potential of stem cell technology in the fight against AD. The development and implementation of new strategies will improve the quality and utility of stem cell-based platforms for AD research and therapy, ultimately leading to better outcomes for patients with AD and related dementias.
Limitations of current stem cell therapies
Limited capacity for self-renewal and differentiation.
The ability of stem cells to differentiate into specific cell types that carry out corresponding functions is frequently what determines how effective they are. As a result, inadequate differentiation rates or unexpected differentiation can significantly impede therapy. In certain cases, uncontrolled stem cell differentiation can potentially result in the development of tumors [ 12 ]. For these reasons, many studies using stem cells for therapeutic purposes pre-differentiate stem cells such as iPSCs into certain progenitor cells before transplantation [ 126 ]. Another factor that negatively affects stem cell therapy is the loss of beneficial properties that occur during processes such as culturing, including the differentiation potential of stem cells. The loss of these beneficial properties is partly due to traditional culture systems that do not accurately reflect the natural environment of the body. In response to this limitation, many studies aim to maximize the original properties of stem cells through methods like 3D culture or the addition of specific small molecules.
Large-scale clinical translation is also thought to be hampered by stem cells’ capacity for in vitro amplification. Since stem cells have a unique ability to differentiate, it is essential that they maintain their original characteristics during expansion. However, at present, additional generations of expansion are more suitable for small-scale operations in laboratories than large-scale clinical applications.
Heterogeneity of stem cell populations and their derived cell types
Other important factors affecting the efficacy of stem cells is heterogeneity [ 60 ] and derived cell types. Stem cell heterogeneity is universal,for example, NSCs contain different cell populations with both neurogenic and gliogenic potential [ 58 ]. MSCs are inherently heterogeneous cell populations, although the International Society for Cellular Therapy has clear restrictions on the types and proportions of molecules expressed on their cell surfaces. Explicit quantification of differentiation capacity and cell surface molecular expression serves to reduce the possible impact of stem cell heterogeneity on efficacy. It is well known that stem cells derived from different tissue sources can differ in the number and type of cytokines secreted, cell proliferation capacity, immunomodulatory capacity, etc. [ 39 , 72 , 120 ]. Stem cell heterogeneity is also related to tumorigenic potential. Notably, some studies have observed sustained proliferation and tumorigenic mass production after transplantation of hPSC neuronal derivatives in rodent models of PD [ 106 , 107 ]. Stem cell heterogeneity must thus be accounted for in AD therapy research. [ 156 ] concluded that the ideal stem cell therapy for AD would transplant cells with different differentiation potentials precisely to the corresponding functional sites to achieve overall restoration of the functional network of the brain, which relies heavily on the identification and isolation of stem cell heterogeneity. [ 156 ] Besides stem cell heterogeneity, the heterogeneity of their derived cell types can greatly affect efficacy. For example, MSCs derived from healthy controls and patients can exhibit differences in certain functions. There are also unresolved questions whether MSCs of the same tissue origin have different epigenetic imprints. While current research on stem cells and heterogeneity of origin has focused on tumor therapy, we believe it is also valuable to consider this issue in the context of neural stem cell use for AD therapy. First, identifying and isolating subpopulations of neural stem cells in different states or with different differentiation potentials can both help determine the precise efficacy of specific subpopulations of stem cells and provide more possibilities for precise AD treatment. Second, emphasis on stem cell source heterogeneity could lead to better quality control of stem cells used for treatment, which in turn will provide assurance of efficacy.
Ethical and safety concerns surrounding the use of embryonic stem cells and cerebral organoids
Ethical issues have been widely discussed along with the development of stem cell technology, most of which are regarding the sources of stem cells. In the development of therapies using hESCs, the rationale of destroying early human embryos to treat diseases is controversial. Other current ethical issues concern the unlimited differentiation potential of iPSCs, which might be used for human cloning and thus the generation of human-animal chimeras and human embryos [ 133 ]. It is worth noting that human-animal chimeras are one of the most promising research platforms for AD pathology research. However, these models have been ethically questioned due to their similarity to the human brain. The crucial question is: does this technology have the potential to produce a human-like mind? Although the likelihood of human brain-like organs generating consciousness is currently considered minimal, we cannot exclude the possibility of future brain-like organs developing consciousness. To better understand AD pathology, we will likely need to develop more complex, organ-like models that more accurately reflect the human brain. [ 108 ] concluded that although there is currently no chance that cerebral organoids may develop typical consciousness, questions surrounding the presence or absence of consciousness may plague the development of brain-like organs. We thus need more precise protocols for the production, use, and destruction of different levels of organoids that consider ethical norms and safety.
At present, the primary source of ESCs is human embryos (blastocysts), which has limited the development of derivative therapies and poses considerable difficulties for clinical applications because of the difficulty of mass production. Although it seems promising to use iPSCs as an alternative source of ESCs, there are safety concerns because of the genomic instability of iPSCs—even with improved protocols for differentiation. As a result, before transplantation, many studies first promote in vitro differentiation into progenitor cells of specific neuronal types. The use of iPSC-derived gametes has also raised concerns about the potential exploitation of developed embryos, human nuclear transfer, and the risk of altering natural reproduction, including the potential to derive gametes for same-sex and asexual reproduction.
Strategies to overcome existing limitations and improve the quality of stem cell-based therapy and platforms
Several studies have sought to overcome the current limitations and improve the quality of stem cell-based therapies and platforms through methods like the development of new stem cell lines and new direct differentiation methods. The success of new encapsulation techniques, stem cell pretreatment, and co-delivery with small compounds also provides inspiration for further stem cell research.
Development of new techniques to promote differentiation
Co-administration of small molecules to promote differentiation
The process of differentiation in vivo , which is thought to play a significant role in efficacy, occurs in a variety of stem cell therapies, regardless of whether the cells are anticipated to differentiate into the target neuronal type in vivo or are infused after initial differentiation in vitro. The recent discovery that chiral small molecules promote stem cell differentiation in vivo has provided a new idea for improving the efficacy of stem cell therapy: co-administration of small molecules that encourage stem cell differentiation [ 110 ]. Observed that, when exposed to near-infrared light, nanoparticles with strong chirality can significantly speed up the differentiation of mouse NSCs into neurons. This finding suggests that strong chiral nanomaterials may be utilized to direct cell development and improve differentiation to achieve better efficacy in stem cell therapy. The use of near-infrared light might also lead to better control of the pro-differentiation effect [ 110 ].
Furthermore, [ 104 ] demonstrated that nanoparticle assemblies exerted circularly polarized light-dependent forces on the cytoskeleton and that light-induced periodic mechanical deformation of actin nanofibers at a frequency of 50 Hz stimulated neural stem cell differentiation into a neuronal phenotype. When implanted in the hippocampus of a mouse AD model, neural stem cells irradiated according to a polarity-optimized scheme substantially reduced the formation of amyloid plaques. These findings provide novel ideas for improving the efficacy of stem cells and a reference for the biological effects of circularly polarized light. Muñoz et al. [ 90 ] developed a differentiation scheme that successfully generated basal forebrain-like cholinergic neurons (BFCN) from iPSCs using small molecule inhibitors and growth factors. The study used LDN193189 and TGF-β inhibitors SB431542, FGF-2, SHH, FGF-8, BDNF, and NGF (separately or in combination) at different stages of differentiation to achieve a facilitative effect on the differentiation of iPSCs to BFCNs. These results also indicate that the strategy of adding small molecule inhibitors and growth factors at different periods has a regulatory effect on stem cell differentiation, providing theoretical support for promoting the differentiation of stem cells after transplantation. Since this experiment was conducted in vitro, further research is needed to determine whether the effects extend to intracerebral transplantation. We cannot rule out the possibility that the aforementioned differentiation-promoting small molecules could have unintended effects on the brain [ 90 ].
Pretreatment of stem cells
Since stem cells are sensitive to environmental changes, pretreatment is one of the most efficient strategies to change their phenotype and improve therapeutic efficiency. Stem cell transplantation can influence the number or type of surface receptors of certain types of somatic cells, enzyme expression levels, and secretome composition and have other effects that influence therapeutic efficacy by modifying environmental factors. Alternatively, small molecules can be added to the cell co-culture medium. It has been demonstrated that cytokines (such as IL-3, IL-6, HGF, etc.) increase the expression of CXCR4, which in turn encourages targeted migration of MSCs. Hypoxic preconditioning is another method frequently used to change the culture environment. In a study by [ 146 ], culture under hypoxic conditions induced an increase in SDF-1/CXCR4 signaling, which promoted MSCs migration. Similarly, [ 65 ] observed that hypoxia-induced MSCs had better viability, stronger proliferation potential, and better resistance to hypoxia and reactive oxygen species (ROS) stress. The proliferation and viability of neural stem cells have also been shown to be enhanced by hypoxia culture [ 15 ], which is a proven way to improve the survival rate of stem cells after transplantation.
Since transplanted neural stem cells must differentiate to be therapeutic, it is necessary to promote their differentiation at the lesion site. A study by [ 86 ] demonstrated that, after melatonin treatment with neurosphere culture, an inhibitory effect on NSC proliferation at a pharmacological concentration (25 μM) was observed: differentiation of NSCs into neurons—as well as oligodendrocytes—was promoted, while differentiation of NSCs into GFAP-expressing astrocytes was unaffected. Melatonin also enhanced the bioenergetic activity of mitochondria in NSCs and exhibited a dose-dependent pattern, suggesting that this stimulation method provides energetically pertinent support for the differentiation process. Melatonin therapy thus promotes NSC differentiation and neural replacement using NSCs combining pharmacological melatonin dosages is a potential regenerative therapy for neurological diseases. Studies on chemically created self-assembling peptides have also been performed to encourage NSC differentiation. [ 26 ] established a self-assembling peptide (DSP) that can spontaneously assemble into ordered nanofibers and contains a functional structural domain made of the laminin-derived amino acids Tyr-Ile-Gly-Ser-Arg (YIGSR); not only did this peptide increase cell viability in normal culture conditions, it also lowered the number of apoptotic cells induced by Aβ in vitro. NSC transplantation in AD rats treated with DSP was observed to enhance survival and neuronal differentiation of transplanted NSCs; increase secretion of anti-inflammatory and neurotrophic factors such as IL-10, brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), and insulin-like growth factor 1 (IGF-1); and reduce increased expression of pro-inflammatory factors such as TNF-α and IL-1β. These experimental findings are consistent with the hypothesis that the biomaterial DSP ameliorates AD pathophysiology by promoting NSC graft survival and development.
Brn-4 protein in MSCs also plays a positive role in treatment of AD, but miR-937 in stem cells hinders translation of Brn-4. Thus, administration of specially pretreated anti-miR-937 MSCs can contribute to the reduction and clearance of brain Aβ deposition [ 69 ].
3D culture of stem cells
Improving stem cell culture methods is another potential strategy to improve efficacy. Traditional in vitro 2D culture conditions may result in loss of stemness and other favorable qualities, as 2D culture mitigation does not accurately represent the ecological niche of stem cells in vivo. Supporting cells, extracellular matrix, and growth factors make up the microenvironment inhabited by stem cells in vivo. In contrast, 3D culture can better simulate intercellular and cell–matrix interactions. The natural environment of stem cells can be more accurately recreated in 3D culture and their favorable qualities can be maintained. Thus, one of the most promising methods currently being examined to support clinical translation of stem cells is 3D cultivation technologies. For example, Frith, Thomson, and Genever (2010) performed 3D culture of MSCs and demonstrated that dynamic 3D culture promoted MSCs to form aggregates with good cell viability. This effect was attributed to the combination of 3D culture with dynamic factors like increased cell–cell contact, which has been shown to improve stem cell subdifferentiation potential. Similarly, [ 139 ] designed culture substrates with micropatterns to culture MSC multicellular spheroids in 3D culture, finding that this method maintained cell function and improved differentiation potential in the longer term.
In conclusion, as 3D culture can better mimic the microenvironment of stem cells in vivo and help maintain stemness, it is of great value for advancing clinical translation of stem cell therapies.
Preventing premature death of stem cells and improving therapeutic effects
Encapsulation of stem cells
Many encapsulation approaches are designed to improve the low survival rate and prevent premature death of stem cells. Hydrogel encapsulation followed by medication administration is a common technique. Hydrogel materials are biocompatible, can be safely dissolved and absorbed in vivo, can remain stem cells aggregated and increase cell viability [ 92 , 93 ]. Since MSCs have superior healing and anti-inflammatory potential when aggregated into spheres for administration, aggregation of stem cells may support efficacy [ 92 , 93 ]. Multiple studies have shown that encapsulation with hydrogel materials improves the survival as well as the function of stem cells [ 49 , 91 ].
However, after drug delivery, the volume of hydrogel molecules may restrict molecule diffusion. This was recently addressed by a single-cell encapsulation scheme using alginate microgels designed by [ 79 ] in which MSCs were encapsulated in a thin layer of alginate material using a microfluidic approach to form microgels. The reduced size of the encapsulated material allowed for improved diffusive drug transport. This method can enable intravenous drug delivery. The half-life of encapsulated cells is also considerably longer than that of naked cells.
As early as 2016, studies have shown that hydrogel-encapsulated stem cells can be used to treat AD mice. Other materials like DSP (a self-assembled peptide) can promote neuroprotection and exert anti-neuroinflammation and paracrine effects by increasing the survival rate and differentiation ratio of transplanted stem cells, thereby maximizing the therapeutic effect of NSC transplantation for AD [ 26 ].
The potential for widespread application or commercialization of such technology is enhanced by the fact that encapsulants can be preserved using normal cryopreservation techniques and defrosted cells have similar persistence of to freshly encapsulated cells after removing the encapsulants. [ 155 ] established a novel hydrogen-bonded (HOFs) encapsulation technique for NSCs in organic frameworks. They concluded that the main causes of the suboptimal efficiency of transplantation are “stemness” loss, cell membrane damage during transplantation, and apoptosis induced by oxidative stress after transplantation. This study was the first to encapsulate NSCs using HOFs and showed that this method can improve NSC viability, encourage neurogenesis, and reduce cognitive damage. Protective encapsulation of stem cells may be a viable means to prevent loss during transplantation and guarantee efficiency. [ 154 ] established a delivery strategy for encapsulated NSC drugs that integrates antioxidant nanoenzymes (e.g., ceria) into metal–organic frameworks (MOFs). This method combines the antioxidant capacity of nanoenzymes with the effective drug delivery of MOFs, resulting in lower oxidative damage to stem cells while the reactive framework releases encapsulated stem cells in the lesion area. Targeted delivery with a reactive framework while loading enzymes to prevent oxidative damage is instructive as a delivery route.
MOFs have also recently attracted attention due to their potential in developing new AD diagnostic methods. MOFs are assessed by fluorescence and electrochemiluminescence (ECL) detection of AD biomarkers. Fluorescence detection of major metal ions in the brain (Zn 2+ , Cu 2+ , Fe 3+ , and Al 3+ ) can be performed as part of magnetic resonance imaging (MRI) [ 124 ].
In summary, encapsulation methods and their modifications appear to be viable and adaptable strategies to achieve targeted release of stem cells and increase the survival rate. The reactive design of the encapsulating framework enables targeted release of stem cells, while the varying loads (enzymes, etc.) provide the potential to withstand oxidative damage. What is currently needed is to adjust the size of the formed package carriers (e.g., to form microgels) with reference to existing studies to create an encapsulating vehicle that is appropriate for intravenous drug delivery while avoiding issues like vascular blockage. The optimum method of encapsulation should enable post-thaw use in addition to conventional cryopreservation.
Regulation of gene expression in stem cells
A commonly used method to alter cellular phenotypes is the introduction of genes encoding specific products. At present, many studies use viruses as vectors for this process. For stem cell therapy for neurological diseases, a promising option is to upregulate gene expression to help localize post-transplanted stem cells to the lesion sire. [ 152 ] demonstrated in vitro that overexpression of Wnt3a promoted the migration and neural differentiation of MSCs through the Wnt/PCP pathway. Although in vivo experiments are needed to demonstrate whether upregulating expression of this pathway has the same effect, this method has the potential to promote MSC migration to achieve better efficacy. [ 152 ]
Investigation of alternative, ethically-acceptable sources of stem cells
At present, the main sources of stem cells can be grouped into four categories: embryonic tissue, fetal tissue, specific locations in adult organisms, and differentiated somatic cells after being genetically reprogrammed [ 7 ]. A significant proportion of these sources involve the destruction of embryos or fetuses, which has historically been a source of ethical controversy in stem cell therapy. Of these, only morula-derived ESCs are totipotent, i.e., can differentiate into all cell types in an organism. In contrast, stem cells from fetuses are generally pluripotent and can differentiate into a limited number of specific cell types. To circumvent the ethical issues of extracting stem cells, iPSCs seem to be a promising source of stem cells. However, there is the issue of overcoming possible immune rejection caused by allogeneic transplantation. Using a patient’s own somatic cells as a source of stem cells would result in an increased time cost and is not suitable for large-scale clinical application. Currently, adipose-derived stem cells (ASCs) are one of the best choices for stem cell therapy and tissue engineering due to their easy accessibility and proliferative capacity relative to BM-MSCs. In the treatment of neurodegenerative diseases, ASCs have been observed to outperform various other stem cells in the secretion of trophic factors such as BDNF. Therefore, adipose tissue appears to be a good source of stem cells for stem cell-based therapy considering ease of access, ethical issues, and overall efficacy.
Overcoming the current limitations of stem cell technology is crucial for the advancement of AD research and treatment. The improvement and implementation of new strategies will enhance the quality and utility of stem cell-based platforms for AD research and therapy, leading to better outcomes for patients with AD and related dementias.
Limitations and strategies of stem cell-derived exosome therapy
Despite the great potential of exosome-based therapies, many difficulties must still be overcome to translate these therapies into clinical practice. First, traditional EV production processes based on pricey 2D cell culture protocols require a lot of cell culture plastic, media, and space. Furthermore, as exosomes are secretory products of cells, their yield is low and they are difficult to produce on an industrial scale. Another significant obstacle is how to accurately identify exosomes from other extracellular vesicles (EVs), particularly functional microvesicles. The techniques that aim to address these challenges are described in the following section [ 29 ].
Increasing exosome secretion
Traditional 2D culture techniques are less efficient in terms of space, material, and yield. In contrast, 3D culture offers healthier culture conditions and has the potential to produce higher EV secretion rates. MSC-EVs from conventional 2D monolayer cell cultures, 3D suspended droplet spheroids, and 3D aggregates were compared by Harrel et al. [ 48 ]. Their results showed that EV secretion was three-fold higher using 3D culture compared to 2D culture. Thus, an effective way to improve exosome production is by 3D culture.
A recent study that treated AD mice with 3D-MSC-EVs found that when 6E10 antibodies were used as the criterion to assess Aβ density, there was no significant difference in intracellular levels of HPCs between the control group and 3D-MSC-EV group; however, the levels of some inflammatory markers in mice’s brains were significantly reduced and behavioral experiments yielded positive results [ 24 ].
Chemical molecular interference
Inducing metabolic changes and other disruptions in cells through the administration of chemical molecules can also impact exosome generation. By blocking both oxidative phosphorylation and glycolysis, sodium iodoacetate (IAA) and 2,4-dinitrophenol (DNP) were shown to effectively reduce the cellular energy charge. Exosome production can also be increased using chemicals like chloroquine, lipocalin (APN), and NH4Cl [ 24 ].
Environmental factors
According to previous studies [ 54 , 148 ], exosomes can be promoted by hypoxia, pH changes, and low glucose levels. EV secretion was slightly enhanced after moderate hypoxic treatment of MSCs (0.5–1% oxygen), while no increase in exosome release was observed when MSCs were stimulated with 5% hypoxia, although the nature of the exosomes was altered. Many researchers are interested in acidity as a potential way to control exosome generation. A recent study demonstrated that the environmental factors of excessive acid and alkali resulted in decreased exosome formation compared to normal pH levels [ 24 ].
Studies have also shown that MSCs in a hypoxic state present stronger activity, while oxygen-rich environments lead to lower stage viability of MSCs. [ 69 ] administered hypoxic-conditioned MSC-derived miR-21-riched exosomes to APP/PS1 mice. Their results showed that hypoxia-treated MSCs had obvious activity, while upregulation of miR-21 enhanced memory and reduced Aβ plaque deposition. [ 69 ]
Improvement in separation methods
Exosomes, which are a part of stem cell secretomes that differs from microvesicles and apoptotic vesicles, occur through direct germination of the plasma membrane, whose biogenesis starts with inward growth of the plasma membrane to create early endosomes. These endosomes then mature into multivesicular bodies (MVBs), including inward germination through the endosomal membrane, to create intraluminal vesicles (ILVs).
The emerging role of exosomes in AD research and therapy has created the need for their large-scale isolation. However, this is costly and difficult using conventional methods like ultra-centrifugation. Ultrafiltration-based exosome separation is a good substitute for conventional ultracentrifugation because it involves a shorter processing time and requires no specialized equipment. Additionally, by varying the size of the filter, ultrafiltration enables separation and collection of tiny extracellular vesicles of a certain particle size, including exosomes. Tangential flow filtration is another efficient method to eliminate vesicle blockage caused by ultrafiltration in combination with pretreatment with protease, resulting in decreased viscosity of the fluid sample. Microfluidic devices are ideal tools for exosome separation and are beginning to achieve acceptance as superior exosome detection tools. These compact platforms make it possible to process nanovesicles quickly and affordably—even in small quantities of liquid samples.
A different technique for separating exosomes while maintaining the biological activity of isolated exosomes is volumetric exclusion (SEC). Unlike ultra-ionization and ultrafiltration, SEC is achieved by passive gravity flow with no effects on vesicle structure and integrity. High resolution and repeatability are additional benefits of SEC [ 150 , 219 ].
Conclusions and future directions
This review provides a comprehensive overview of the latest research advancements in stem cell-derived models for AD and discusses the fundamental mechanisms and applications of stem cells and their derived therapies for mitigating AD pathology. Stem cell technology is helpful for the discovery of such mechanisms and potential therapeutic options, including cell transplantation and generating exosomes for disease treatment.
Our understanding of the pathogenic mechanisms of AD has improved with ongoing research, progressing from the original amyloid theory to higher levels including immunological and genetic theories. Increased knowledge of pathogenic mechanisms has helped researchers identify new drug targets, and the rapid development of stem cell technology has made it possible to reveal underlying mechanisms of AD pathology previously unavailable. For example, iPSC-derived disease models, as well as humanized AD animal models, have great potential for drug development and AD screening. Due to the limitations of 2D and 3D stem cell model protocols, there are still no models that can fully simulate the brains of fAD patients. Thus, in further research, aging and environmental factors should be included in iPSC-based models to explore whether phenotypes occur only in patient-specific cells.
To generate cell–cell circuits of different cell types, co-culture systems, microfluidic chips, and 3D organoids containing neurons, glial cells, and immune cells with complex neuronal networks mimicking the brain should be developed. Researchers need to clarify the key principles of delaying aging to reverse AD through epigenetic remodeling. Combined with multi-dimensional phenotypic analysis and single-cell transcriptome sequencing, the interactive effects of tissue injury, inflammation, and regeneration on the structure of the nervous system and systemic vasculature, aging, and immune challenge need to be systematically studied. A systematic view of AD needs to be achieved through complex data modeling. AD organoid models constructed using gene editing tools to assess the association between risk genes and disease phenotypes require further research. Although CRISPR has recently been useful in gene treatment of AD, its applications in humans are mainly limited to cancer research.
Notably, most existing attempts to treat AD with stem cells have used a single type of stem cell. Given the complexity of human neural networks, stem cell transplantation may need to be performed in conjunction with small molecule drug induction. For example, in the case of neurogenesis, there is a reciprocal regulation between drug-induced regeneration and stem cell-induced regeneration, and the state of supporting cells in the environment and the use of stem cells to induce glial cells for transplantation therapy also show considerable potential. Combined application of stem cells and glial cells is also a promising direction. In the future, it seems likely that stem cell therapies will be combined with small molecule drugs (that promote neuroregeneration or regulate differentiation) or glial cell precursors. Such combination therapies can be studied accurately and efficiently using the above-described multi-level and multi-faceted models.
Many recently developed omics technologies already have clinical applications. AD is a complex disease, and its pathogenesis is caused by protein alterations as well as DNA, mRNA, and even miRNA alterations. Research on the pathogenesis and treatment of AD is thus increasingly adopting a multi-omics approach. Take stem cell-derived exosomes as an example. The exosomes contain proteins, mRNAs, miRNAs, and other substances, and their contents can be changed in a targeted manner through exosome engineering, resulting in a therapeutic effect on AD from a multi-omics perspective. Researchers have identified many proteins that contribute to neuroprotection in MSC-derived exosomes, and many miRNAs that can be encapsulated in exosomes—such as miR-29a, miR-126-3p, miR-137, miR-144-3p, miR-188-5p, miR-425-3p, and miR-451a—can have therapeutic effects on AD. Such findings represent new advancement in multi-omics research in the exploration of AD mechanisms and therapeutic approaches.
The majority of drugs and therapies for AD focus on the late stage of AD onset, which is characterized by memory loss and aphasia that seriously impact the lives of AD patients and their families. Medication for advanced AD mainly aims to alleviate symptoms, reduce clinical progression, and delay disability. However, drugs with the potential to prevent AD are being tested using stem cell-derived disease models. Stem cell models make it possible to demonstrate the entire disease process, reflecting effects of environmental factors and indicators of pathogenic risks. Regular physical examinations before clinical symptoms appear and behavioral diagnosis may indicate AD development. Stem cells and their derivative therapies may also be able to intervene at the early stages of AD to provide a real cure rather than just alleviate symptoms and delay progression, providing a new way of thinking about treating AD and reducing the burden of this disease on the elderly and society.
The life sciences have entered the era of big data, big platforms, and big discoveries. Big data technology has greatly enhanced the efficiency of research and biotechnology for the treatment of AD. Modern software, machine learning, and comprehensive data analysis methods can aid in the analysis of complex biological processes involved in aging and disease and help evaluate the efficacy and safety of pharmacological interventions and cell or cell-derived exosome therapies. Thus, interdisciplinary collaborations between researchers from different fields are crucial.
Personalized medicine and precision medicine of biomarker development have also radically changed the conventional paradigm of disease diagnosis and treatment, resulting in transformation of the pharmaceutical industry in the field of AD. Stem cells and regenerative medicine have opened up new paths for disease treatment. Novel research methods, such as single-cell technology, directed proteomics technology, genome editing technology, and optogenetics technology, have enabled exploration of AD in a more precise and real-time manner.
In conclusion, stem cell technology has the potential to significantly advance the understanding and treatment of AD by providing new disease models, drug development opportunities, and cell therapy options. In the future, stem cell technology will develop in the direction of informatization, manipulation, and multi-omics and play a positive role at different levels to achieve better therapeutic results for AD.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CHH, Newman SA, Yeromin AV, Scarfone VM, Marsh SE, Fimbres C, Caraway CA, Fote GM, Madany AM, Agrawal A, Kayed R, Gylys KH, Cahalan MD, Cummings BJ, Antel JP, Mortazavi A, Carson MJ, Poon WW, Blurton-Jones M. iPSC-derived human microglia-like cells to study neurological diseases. Neuron. 2017;94:278-93.e9.
Article CAS PubMed PubMed Central Google Scholar
Álvarez Z, Ortega JA, Sato K, Sasselli IR, Kolberg-Edelbrock AN, Qiu R, Marshall KA, Nguyen TP, Smith CS, Quinlan KA, Papakis V, Syrgiannis Z, Sather NA, Musumeci C, Engel E, Stupp SI, Kiskinis E. Artificial extracellular matrix scaffolds of mobile molecules enhance maturation of human stem cell-derived neurons. Cell Stem Cell. 2023;30:219-38.e14.
Article PubMed Google Scholar
Appelt-Menzel A, Cubukova A, Metzger M. Establishment of a human blood-brain barrier co-culture model mimicking the neurovascular unit using induced pluripotent stem cells. Curr Protoc Stem Cell Biol. 2018;47: e62.
Appelt-Menzel A, Oerter S, Mathew S, Haferkamp U, Hartmann C, Jung M, Neuhaus W, Pless O. Human iPSC-derived blood-brain barrier models: valuable tools for preclinical drug discovery and development? Curr Protoc Stem Cell Biol. 2020;55: e122.
Article CAS PubMed Google Scholar
Arber C, Lovejoy C, Wray S. Stem cell models of Alzheimer’s disease: progress and challenges. Alzheimers Res Ther. 2017;9:42.
Article PubMed PubMed Central Google Scholar
Arranz AM, De Strooper B. The role of astroglia in Alzheimer’s disease: pathophysiology and clinical implications. Lancet Neurol. 2019;18:406–14.
Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, Kasalkova NS, Svorcik V, Kolska Z, Motarjemi H, Molitor M. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—a review. Biotechnol Adv. 2018;36:1111–26.
Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–72.
Bardy C, van den Hurk M, Eames T, Marchand C, Hernandez RV, Kellogg M, Gorris M, Galet B, Palomares V, Brown J, Bang AG, Mertens J, Böhnke L, Boyer L, Simon S, Gage FH. Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro. Proc Natl Acad Sci USA. 2015;112:E2725–34.
Bianco F, Tonna N, Lovchik RD, Mastrangelo R, Morini R, Ruiz A, Delamarche E, Matteoli M. Overflow microfluidic networks: application to the biochemical analysis of brain cell interactions in complex neuroinflammatory scenarios. Anal Chem. 2012;84:9833–40.
Bissonnette CJ, Lyass L, Bhattacharyya BJ, Belmadani A, Miller RJ, Kessler JA. The controlled generation of functional basal forebrain cholinergic neurons from human embryonic stem cells. Stem Cells. 2011;29:802–11.
Blasco MA, Serrano M, Fernandez-Capetillo O. Genomic instability in iPS: time for a break. Embo j. 2011;30:991–3.
Borghese L, Dolezalova D, Opitz T, Haupt S, Leinhaas A, Steinfarz B, Koch P, Edenhofer F, Hampl A, Brüstle O. Inhibition of notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells. 2010;28:955–64.
Bosi S, Rauti R, Laishram J, Turco A, Lonardoni D, Nieus T, Prato M, Scaini D, Ballerini L. From 2D to 3D: novel nanostructured scaffolds to investigate signalling in reconstructed neuronal networks. Sci Rep. 2015;5:9562.
Bürgers HF, Schelshorn DW, Wagner W, Kuschinsky W, Maurer MH. Acute anoxia stimulates proliferation in adult neural stem cells from the rat brain. Exp Brain Res. 2008;188:33–43.
Cahill MK, Huang EJ. Testing the amyloid hypothesis with a humanized AD mouse model. Neuron. 2017;93:987–9.
Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, Kang YJ, Chapeton K, Patterson B, Yuan Y, He CS, Raredon MSB, Dengelegi J, Kim KY, Sun P, Zhong M, Lee S, Patra P, Hyder F, Niklason LE, Lee SH, Yoon YS, Park IH. Engineering of human brain organoids with a functional vascular-like system. Nat Methods. 2019;16:1169–75.
Canals I, Ginisty A, Quist E, Timmerman R, Fritze J, Miskinyte G, Monni E, Hansen MG, Hidalgo I, Bryder D, Bengzon J, Ahlenius H. Rapid and efficient induction of functional astrocytes from human pluripotent stem cells. Nat Methods. 2018;15:693–6.
Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–80.
Chambers SM, Qi Y, Mica Y, Lee G, Zhang XJ, Niu L, Bilsland J, Cao L, Stevens E, Whiting P, Shi SH, Studer L. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol. 2012;30:715–20.
Chang CY, Ting HC, Liu CA, Su HL, Chiou TW, Lin SZ, Harn HJ, Ho TJ. Induced pluripotent stem cell (iPSC)-based neurodegenerative disease models for phenotype recapitulation and drug screening. Molecules. 2020;25:2000.
Chen YA, Lu CH, Ke CC, Chiu SJ, Jeng FS, Chang CW, Yang BH, Liu RS. Mesenchymal stem cell-derived exosomes ameliorate Alzheimer’s disease pathology and improve cognitive deficits. Biomedicines. 2021;9:594.
Chuang JH, Yang WC, Lin Y. Glutamatergic neurons differentiated from embryonic stem cells: an investigation of differentiation and associated diseases. Int J Mol Sci. 2021;22:4592.
Cone AS, Yuan X, Sun L, Duke LC, Vreones MP, Carrier AN, Kenyon SM, Carver SR, Benthem SD, Stimmell AC, Moseley SC, Hike D, Grant SC, Wilber AA, Olcese JM, Meckes DG Jr. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer’s disease-like phenotypes in a preclinical mouse model. Theranostics. 2021;11:8129–42.
Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–3.
Cui GH, Shao SJ, Yang JJ, Liu JR, Guo HD. Designer self-assemble peptides maximize the therapeutic benefits of neural stem cell transplantation for Alzheimer’s disease via enhancing neuron differentiation and paracrine action. Mol Neurobiol. 2016;53:1108–23.
Cui GH, Wu J, Mou FF, Xie WH, Wang FB, Wang QL, Fang J, Xu YW, Dong YR, Liu JR, Guo HD. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. Faseb j. 2018;32:654–68.
De Strooper B, Karran E. The cellular phase of Alzheimer’s disease. Cell. 2016;164:603–15.
Debbi L, Guo S, Safina D, Levenberg S. Boosting extracellular vesicle secretion. Biotechnol Adv. 2022;59: 107983.
Ding M, Shen Y, Wang P, Xie Z, Xu S, Zhu Z, Wang Y, Lyu Y, Wang D, Xu L, Bi J, Yang H. Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in Alzheimer’s disease. Neurochem Res. 2018;43:2165–77.
DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139(Suppl 2):136–53.
Douvaras P, Sun B, Wang M, Kruglikov I, Lallos G, Zimmer M, Terrenoire C, Zhang B, Gandy S, Schadt E, Freytes DO, Noggle S, Fossati V. Directed differentiation of human pluripotent stem cells to microglia. Stem Cell Rep. 2017;8:1516–24.
Article CAS Google Scholar
Drummond E, Wisniewski T. Alzheimer’s disease: experimental models and reality. Acta Neuropathol. 2017;133:155–75.
Duncan T, Valenzuela M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res Ther. 2017;8:111.
Egan MF, Kost J, Voss T, Mukai Y, Aisen PS, Cummings JL, Tariot PN, Vellas B, van Dyck CH, Boada M, Zhang Y, Li W, Furtek C, Mahoney E, Harper Mozley L, Mo Y, Sur C, Michelson D. Randomized trial of verubecestat for prodromal Alzheimer’s disease. N Engl J Med. 2019;380:1408–20.
Falcon B, Zivanov J, Zhang W, Murzin AG, Garringer HJ, Vidal R, Crowther RA, Newell KL, Ghetti B, Goedert M, Scheres SHW. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature. 2019;568:420–3.
Fernando MB, Ahfeldt T, Brennand KJ. Modeling the complex genetic architectures of brain disease. Nat Genet. 2020;52:363–9.
Fitzpatrick AWP, Falcon B, He S, Murzin AG, Murshudov G, Garringer HJ, Crowther RA, Ghetti B, Goedert M, Scheres SHW. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature. 2017;547:185–90.
Friedman R, Betancur M, Boissel L, Tuncer H, Cetrulo C, Klingemann H. Umbilical cord mesenchymal stem cells: adjuvants for human cell transplantation. Biol Blood Marrow Transpl. 2007;13:1477–86.
Article Google Scholar
Garcia-Contreras M, Thakor AS. Human adipose tissue-derived mesenchymal stem cells and their extracellular vesicles modulate lipopolysaccharide activated human microglia. Cell Death Discov. 2021;7:98.
Gonzalez-Ramos A, Waloschková E, Mikroulis A, Kokaia Z, Bengzon J, Ledri M, Andersson M, Kokaia M. Human stem cell-derived GABAergic neurons functionally integrate into human neuronal networks. Sci Rep. 2021;11:22050.
Gorris R, Fischer J, Erwes KL, Kesavan J, Peterson DA, Alexander M, Nöthen MM, Peitz M, Quandel T, Karus M, Brüstle O. Pluripotent stem cell-derived radial glia-like cells as stable intermediate for efficient generation of human oligodendrocytes. Glia. 2015;63:2152–67.
Gratuze M, Leyns CEG, Holtzman DM. New insights into the role of TREM2 in Alzheimer’s disease. Mol Neurodegener. 2018;13:66.
Guttikonda SR, Sikkema L, Tchieu J, Saurat N, Walsh RM, Harschnitz O, Ciceri G, Sneeboer M, Mazutis L, Setty M, Zumbo P, Betel D, de Witte LD, Pe’er D, Studer L. Fully defined human pluripotent stem cell-derived microglia and tri-culture system model C3 production in Alzheimer’s disease. Nat Neurosci. 2021;24:343–54.
Haldipur P, Aldinger KA, Bernardo S, Deng M, Timms AE, Overman LM, Winter C, Lisgo SN, Razavi F, Silvestri E, Manganaro L, Adle-Biassette H, Guimiot F, Russo R, Kidron D, Hof PR, Gerrelli D, Lindsay SJ, Dobyns WB, Glass IA, Alexandre P, Millen KJ. Spatiotemporal expansion of primary progenitor zones in the developing human cerebellum. Science. 2019;366:454–60.
Han Y, Tan L, Zhou T, Yang L, Carrau L, Lacko LA, Saeed M, Zhu J, Zhao Z, Nilsson-Payant BE, Lira Neto FT, Cahir C, Giani AM, Chai JC, Li Y, Dong X, Moroziewicz D, Paull D, Zhang T, Koo S, Tan C, Danziger R, Ba Q, Feng L, Chen Z, Zhong A, Wise GJ, Xiang JZ, Wang H, Schwartz RE, tenOever BR, Noggle SA, Rice CM, Qi Q, Evans T, Chen S. A human iPSC-array-based GWAS identifies a virus susceptibility locus in the NDUFA4 gene and functional variants. Cell Stem Cell. 2022;29:1475-90.e6.
Haring AP, Sontheimer H, Johnson BN. Microphysiological human brain and neural systems-on-a-chip: potential alternatives to small animal models and emerging platforms for drug discovery and personalized medicine. Stem Cell Rev Rep. 2017;13:381–406.
Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. 2019;8:1605.
Ho SS, Murphy KC, Binder BY, Vissers CB, Leach JK. Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels. Stem Cells Transl Med. 2016;5:773–81.
Honig LS, Vellas B, Woodward M, Boada M, Bullock R, Borrie M, Hager K, Andreasen N, Scarpini E, Liu-Seifert H, Case M, Dean RA, Hake A, Sundell K, Poole Hoffmann V, Carlson C, Khanna R, Mintun M, DeMattos R, Selzler KJ, Siemers E. Trial of Solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med. 2018;378:321–30.
Hur JY, Frost GR, Wu X, Crump C, Pan SJ, Wong E, Barros M, Li T, Nie P, Zhai Y, Wang JC, Tcw J, Guo L, McKenzie A, Ming C, Zhou X, Wang M, Sagi Y, Renton AE, Esposito BT, Kim Y, Sadleir KR, Trinh I, Rissman RA, Vassar R, Zhang B, Johnson DS, Masliah E, Greengard P, Goate A, Li YM. The innate immunity protein IFITM3 modulates γ-secretase in Alzheimer’s disease. Nature. 2020;586:735–40.
Imamura K, Izumi Y, Watanabe A, Tsukita K, Woltjen K, Yamamoto T, Hotta A, Kondo T, Kitaoka S, Ohta A, Tanaka A, Watanabe D, Morita M, Takuma H, Tamaoka A, Kunath T, Wray S, Furuya H, Era T, Makioka K, Okamoto K, Fujisawa T, Nishitoh H, Homma K, Ichijo H, Julien JP, Obata N, Hosokawa M, Akiyama H, Kaneko S, Ayaki T, Ito H, Kaji R, Takahashi R, Yamanaka S, Inoue H. The Src/c-Abl pathway is a potential therapeutic target in amyotrophic lateral sclerosis. Sci Transl Med. 2017;9:3962.
Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS, Carson CT, Laurent LC, Marsala M, Gage FH, Remes AM, Koo EH, Goldstein LS. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature. 2012;482:216–20.
Jiang H, Zhao H, Zhang M, He Y, Li X, Xu Y, Liu X. Hypoxia induced changes of exosome cargo and subsequent biological effects. Front Immunol. 2022;13: 824188.
Jiang Q, Zhang L, Ding G, Davoodi-Bojd E, Li Q, Li L, Sadry N, Nedergaard M, Chopp M, Zhang Z. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab. 2017;37:1326–37.
Kondo T, Imamura K, Funayama M, Tsukita K, Miyake M, Ohta A, Woltjen K, Nakagawa M, Asada T, Arai T, Kawakatsu S, Izumi Y, Kaji R, Iwata N, Inoue H. iPSC-based compound screening and in vitro trials identify a synergistic anti-amyloid β combination for Alzheimer’s disease. Cell Rep. 2017;21:2304–12.
Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845–61.
Lam M, Sanosaka T, Lundin A, Imaizumi K, Etal D, Karlsson FH, Clausen M, Cairns J, Hicks R, Kohyama J, Kele M, Okano H, Falk A. Single-cell study of neural stem cells derived from human iPSCs reveals distinct progenitor populations with neurogenic and gliogenic potential. Genes Cells. 2019;24:836–47.
Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–9.
Latta CH, Brothers HM, Wilcock DM. Neuroinflammation in Alzheimer’s disease a source of heterogeneity and target for personalized therapy. Neuroscience. 2015;302:103–11.
Lee CYD, Daggett A, Gu X, Jiang LL, Langfelder P, Li X, Wang N, Zhao Y, Park CS, Cooper Y, Ferando I, Mody I, Coppola G, Xu H, Yang XW. Elevated TREM2 gene dosage reprograms microglia responsivity and ameliorates pathological phenotypes in Alzheimer’s disease models. Neuron. 2018;97:1032-48.e5.
Lee JK, Jin HK, Bae JS. Bone marrow-derived mesenchymal stem cells reduce brain amyloid-beta deposition and accelerate the activation of microglia in an acutely induced Alzheimer’s disease mouse model. Neurosci Lett. 2009;450:136–41.
Lee JK, Schuchman EH, Jin HK, Bae JS. Soluble CCL5 derived from bone marrow-derived mesenchymal stem cells and activated by amyloid β ameliorates Alzheimer’s disease in mice by recruiting bone marrow-induced microglia immune responses. Stem Cells. 2012;30:1544–55.
Lee SE, Kwon D, Shin N, Kong D, Kim NG, Kim HY, Kim MJ, Choi SW, Kang KS. Accumulation of APP-CTF induces mitophagy dysfunction in the iNSCs model of Alzheimer’s disease. Cell Death Discov. 2022;8:1.
Li B, Li C, Zhu M, Zhang Y, Du J, Xu Y, Liu B, Gao F, Liu H, Cai J, Yang Y. Hypoxia-induced mesenchymal stromal cells exhibit an enhanced therapeutic effect on radiation-induced lung injury in mice due to an increased proliferation potential and enhanced antioxidant ability. Cell Physiol Biochem. 2017;44:1295–310.
Li XJ, Zhang X, Johnson MA, Wang ZB, Lavaute T, Zhang SC. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136:4055–63.
Lilienberg J, Apáti Á, Réthelyi JM, Homolya L. Microglia modulate proliferation, neurite generation and differentiation of human neural progenitor cells. Front Cell Dev Biol. 2022;10: 997028.
Lin YT, Seo J, Gao F, Feldman HM, Wen HL, Penney J, Cam HP, Gjoneska E, Raja WK, Cheng J, Rueda R, Kritskiy O, Abdurrob F, Peng Z, Milo B, Yu CJ, Elmsaouri S, Dey D, Ko T, Yankner BA, Tsai LH. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron. 2018;98:1141-54.e7.
Liu Z, Wang C, Wang X, Xu S. Therapeutic effects of transplantation of As-MiR-937-expressing mesenchymal stem cells in murine model of Alzheimer’s disease. Cell Physiol Biochem. 2015;37:321–30.
Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev. 2013;19:485–502.
Losurdo M, Pedrazzoli M, D’Agostino C, Elia CA, Massenzio F, Lonati E, Mauri M, Rizzi L, Molteni L, Bresciani E, Dander E, D’Amico G, Bulbarelli A, Torsello A, Matteoli M, Buffelli M, Coco S. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer’s disease. Stem Cells Transl Med. 2020;9:1068–84.
Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, Han ZB, Xu ZS, Lu YX, Liu D, Chen ZZ, Han ZC. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017–26.
CAS PubMed Google Scholar
Ma X, Huang M, Zheng M, Dai C, Song Q, Zhang Q, Li Q, Gu X, Chen H, Jiang G, Yu Y, Liu X, Li S, Wang G, Chen H, Lu L, Gao X. ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer’s disease. J Control Release. 2020;327:688–702.
Mahmoudi S, Brunet A. Aging and reprogramming: a two-way street. Curr Opin Cell Biol. 2012;24:744–56.
Malik M, Parikh I, Vasquez JB, Smith C, Tai L, Bu G, LaDu MJ, Fardo DW, Rebeck GW, Estus S. Genetics ignite focus on microglial inflammation in Alzheimer’s disease. Mol Neurodegener. 2015;10:52.
Mancuso R, Van Den Daele J, Fattorelli N, Wolfs L, Balusu S, Burton O, Liston A, Sierksma A, Fourne Y, Poovathingal S, Arranz-Mendiguren A, Sala Frigerio C, Claes C, Serneels L, Theys T, Perry VH, Verfaillie C, Fiers M, De Strooper B. Stem-cell-derived human microglia transplanted in mouse brain to study human disease. Nat Neurosci. 2019;22:2111–6.
Maniglier M, Vidal M, Bachelin C, Deboux C, Chazot J, Garcia-Diaz B, Baron-Van Evercooren A. Satellite glia of the adult dorsal root ganglia harbor stem cells that yield glia under physiological conditions and neurons in response to injury. Stem Cell Reports. 2022;17:2467–83.
Mann DM. Pyramidal nerve cell loss in Alzheimer’s disease. Neurodegeneration. 1996;5:423–7.
Mao AS, Özkale B, Shah NJ, Vining KH, Descombes T, Zhang L, Tringides CM, Wong SW, Shin JW, Scadden DT, Weitz DA, Mooney DJ. Programmable microencapsulation for enhanced mesenchymal stem cell persistence and immunomodulation. Proc Natl Acad Sci U S A. 2019;116:15392–7.
Martín-Maestro P, Gargini R, A. Sproul A, García E, Antón LC, Noggle S, Arancio O, Avila J, García-Escudero V. Mitophagy failure in fibroblasts and iPSC-derived neurons of Alzheimer’s disease-associated presenilin 1 mutation. Front Mol Neurosci 2017; 10: 291.
Martín-Maestro P, Sproul A, Martinez H, Paquet D, Gerges M, Noggle S, Starkov AA. Autophagy Induction by Bexarotene promotes Mitophagy in Presenilin 1 Familial Alzheimer’s disease iPSC-derived neural stem cells. Mol Neurobiol. 2019;56:8220–36.
Marton RM, Miura Y, Sloan SA, Li Q, Revah O, Levy RJ, Huguenard JR, Pașca SP. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat Neurosci. 2019;22:484–91.
Mathys H, Adaikkan C, Gao F, Young JZ, Manet E, Hemberg M, De Jager PL, Ransohoff RM, Regev A, Tsai LH. Temporal tracking of microglia activation in neurodegeneration at single-cell resolution. Cell Rep. 2017;21:366–80.
Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774.
Meier-Stephenson FS, Meier-Stephenson VC, Carter MD, Meek AR, Wang Y, Pan L, Chen Q, Jacobo S, Wu F, Lu E, Simms GA, Fisher L, McGrath AJ, Fermo V, Barden CJ, Clair HDS, Galloway TN, Yadav A, Campágna-Slater V, Hadden M, Reed M, Taylor M, Kelly B, Diez-Cecilia E, Kolaj I, Santos C, Liyanage I, Sweeting B, Stafford P, Boudreau R, Reid GA, Noyce RS, Stevens L, Staniszewski A, Zhang H, Mrvs Murty P, Lemaire S, Chardonnet CD, Richardson V, Gabelica E, DePauw R, Brown S, Darvesh OA, Weaver DF. Alzheimer’s disease as an autoimmune disorder of innate immunity endogenously modulated by tryptophan metabolites. Alzheimers Dement. 2022;8: e12283.
Mendivil-Perez M, Soto-Mercado V, Guerra-Librero A, Fernandez-Gil BI, Florido J, Shen YQ, Tejada MA, Capilla-Gonzalez V, Rusanova I, Garcia-Verdugo JM, Acuña-Castroviejo D, López LC, Velez-Pardo C, Jimenez-Del-Rio M, Ferrer JM, Escames G. Melatonin enhances neural stem cell differentiation and engraftment by increasing mitochondrial function. J Pineal Res. 2017;63:12415.
Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transpl. 2019;54:789–92.
Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, Shcherbinin S, Sparks J, Sims JR, Brys M, Apostolova LG, Salloway SP, Skovronsky DM. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384:1691–704.
Mortensen KN, Sanggaard S, Mestre H, Lee H, Kostrikov S, Xavier ALR, Gjedde A, Benveniste H, Nedergaard M. Impaired glymphatic transport in spontaneously hypertensive rats. J Neurosci. 2019;39:6365–77.
Muñoz SS, Engel M, Balez R, Do-Ha D, Cabral-da-Silva MC, Hernández D, Berg T, Fifita JA, Grima N, Yang S, Blair IP, Nicholson G, Cook AL, Hewitt AW, Pébay A, Ooi L. A simple differentiation protocol for generation of induced pluripotent stem cell-derived basal forebrain-like cholinergic neurons for Alzheimer’s disease and frontotemporal dementia disease modeling. Cells. 2020;9:2018.
Murphy KC, Fang SY, Leach JK. Human mesenchymal stem cell spheroids in fibrin hydrogels exhibit improved cell survival and potential for bone healing. Cell Tissue Res. 2014;357:91–9.
Murphy KC, Whitehead J, Falahee PC, Zhou D, Simon SI, Leach JK. Multifactorial experimental design to optimize the anti-inflammatory and proangiogenic potential of mesenchymal stem cell spheroids. Stem Cells. 2017;35:1493–504.
Murphy KC, Whitehead J, Zhou D, Ho SS, Leach JK. Engineering fibrin hydrogels to promote the wound healing potential of mesenchymal stem cell spheroids. Acta Biomater. 2017;64:176–86.
Novak P, Schmidt R, Kontsekova E, Zilka N, Kovacech B, Skrabana R, Vince-Kazmerova Z, Katina S, Fialova L, Prcina M, Parrak V, Dal-Bianco P, Brunner M, Staffen W, Rainer M, Ondrus M, Ropele S, Smisek M, Sivak R, Winblad B, Novak M. Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol. 2017;16:123–34.
Park J, Wetzel I, Marriott I, Dréau D, D’Avanzo C, Kim DY, Tanzi RE, Cho H. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat Neurosci. 2018;21:941–51.
Passeri E, Elkhoury K, Morsink M, Broersen K, Linder M, Tamayol A, Malaplate C, Yen FT, Arab-Tehrany E. Alzheimer’s disease: treatment strategies and their limitations. Int J Mol Sci. 2022;23:13954.
Piehl N, van Olst L, Ramakrishnan A, Teregulova V, Simonton B, Zhang Z, Tapp E, Channappa D, Oh H, Losada PM, Rutledge J, Trelle AN, Mormino EC, Elahi F, Galasko DR, Henderson VW, Wagner AD, Wyss-Coray T, Gate D. Cerebrospinal fluid immune dysregulation during healthy brain aging and cognitive impairment. Cell. 2022;185:5028-39.e13.
Pihlaja R, Koistinaho J, Malm T, Sikkilä H, Vainio S, Koistinaho M. Transplanted astrocytes internalize deposited beta-amyloid peptides in a transgenic mouse model of Alzheimer’s disease. Glia. 2008;56:154–63.
Piller C. Blots on a field? Science. 2022;377:358–63.
Prasanna P, Rathee S, Rahul V, Mandal D, Chandra Goud MS, Yadav P, Hawthorne S, Sharma A, Gupta PK, Ojha S, Jha NK, Villa C, Jha SK. Microfluidic platforms to unravel mysteries of Alzheimer’s disease: how far have we come? Life. 2021;11:1022.
Preman P, Tcw J, Calafate S, Snellinx A, Alfonso-Triguero M, Corthout N, Munck S, Thal DR, Goate AM, De Strooper B, Arranz AM. Human iPSC-derived astrocytes transplanted into the mouse brain undergo morphological changes in response to amyloid-β plaques. Mol Neurodegener. 2021;16:68.
Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, Yoon KJ, Jeang W, Lin L, Li Y, Thakor J, Berg DA, Zhang C, Kang E, Chickering M, Nauen D, Ho CY, Wen Z, Christian KM, Shi PY, Maher BJ, Wu H, Jin P, Tang H, Song H, Ming GL. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165:1238–54.
Qin C, Lu Y, Wang K, Bai L, Shi G, Huang Y, Li Y. Transplantation of bone marrow mesenchymal stem cells improves cognitive deficits and alleviates neuropathology in animal models of Alzheimer’s disease: a meta-analytic review on potential mechanisms. Transl Neurodegener. 2020;9:20.
Qu A, Sun M, Kim JY, Xu L, Hao C, Ma W, Wu X, Liu X, Kuang H, Kotov NA, Xu C. Stimulation of neural stem cell differentiation by circularly polarized light transduced by chiral nanoassemblies. Nat Biomed Eng. 2021;5:103–13.
Qu S, Liu W, Yang H, Wang Z, Yang Y, Liu F, Sharma A, Sharma H, Luan Z. Clinical observation of electroencephalographic changes and risk of convulsion occurrence in children receiving neural precursor cell transplantation. CNS Neurol Disord Drug Targets. 2018;17:233–9.
Rhee YH, Ko JY, Chang MY, Yi SH, Kim D, Kim CH, Shim JW, Jo AY, Kim BW, Lee H, Lee SH, Suh W, Park CH, Koh HC, Lee YS, Lanza R, Kim KS, Lee SH. Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. J Clin Invest. 2011;121:2326–35.
Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–68.
Sawai T, Sakaguchi H, Thomas E, Takahashi J, Fujita M. The ethics of cerebral organoid research: being conscious of consciousness. Stem Cell Rep. 2019;13:440–7.
Selkoe DJ. Alzheimer disease and aducanumab: adjusting our approach. Nat Rev Neurol. 2019;15:365–6.
Shi B, Zhao J, Xu Z, Chen C, Xu L, Xu C, Sun M, Kuang H. Chiral nanoparticles force neural stem cell differentiation to alleviate Alzheimer’s disease. Adv Sci. 2022;9: e2202475.
Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15(477–86): s1.
PubMed Google Scholar
Shin Y, Choi SH, Kim E, Bylykbashi E, Kim JA, Chung S, Kim DY, Kamm RD, Tanzi RE. Blood-brain barrier dysfunction in a 3D in vitro model of Alzheimer’s disease. Adv Sci. 2019;6:1900962.
Sienski G, Narayan P, Bonner JM, Kory N, Boland S, Arczewska AA, Ralvenius WT, Akay L, Lockshin E, He L, Milo B, Graziosi A, Baru V, Lewis CA, Kellis M, Sabatini DM, Tsai LH, Lindquist S. APOE4 disrupts intracellular lipid homeostasis in human iPSC-derived glia. Sci Transl Med. 2021;13:4564.
Singh M, Denny H, Smith C, Granados J, Renden R. Presynaptic loss of dynamin-related protein 1 impairs synaptic vesicle release and recycling at the mouse calyx of held. J Physiol. 2018;596:6263–87.
Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari SM, Wang S, Walker D, Zhao J, McConlogue L, John V. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–40.
Small SA, Simoes-Spassov S, Mayeux R, Petsko GA. Endosomal traffic jams represent a pathogenic hub and therapeutic target in Alzheimer’s disease. Trends Neurosci. 2017;40:592–602.
Song MS, Learman CR, Ahn KC, Baker GB, Kippe J, Field EM, Dunbar GL. In vitro validation of effects of BDNF-expressing mesenchymal stem cells on neurodegeneration in primary cultured neurons of APP/PS1 mice. Neuroscience. 2015;307:37–50.
Sonnen KF, Merten CA. Microfluidics as an emerging precision tool in developmental biology. Dev Cell. 2019;48:293–311.
Spellicy SE, Stice SL. Tissue and stem cell sourced extracellular vesicle communications with microglia. Stem Cell Rev Rep. 2021;17:357–68.
Subramanian A, Fong CY, Biswas A, Bongso A. Comparative characterization of cells from the various compartments of the human umbilical cord shows that the Wharton’s jelly compartment provides the best source of clinically utilizable mesenchymal stem cells. PLoS ONE. 2015;10: e0127992.
Sullivan SE, Liao M, Smith RV, White C, Lagomarsino VN, Xu J, Taga M, Bennett DA, De Jager PL, Young-Pearse TL. Candidate-based screening via gene modulation in human neurons and astrocytes implicates FERMT2 in Aβ and TAU proteostasis. Hum Mol Genet. 2019;28:718–35.
Sundaram S, Hughes RL, Peterson E, Müller-Oehring EM, Brontë-Stewart HM, Poston KL, Faerman A, Bhowmick C, Schulte T. Establishing a framework for neuropathological correlates and glymphatic system functioning in Parkinson’s disease. Neurosci Biobehav Rev. 2019;103:305–15.
Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133–50.
Tajahmadi S, Molavi H, Ahmadijokani F, Shamloo A, Shojaei A, Sharifzadeh M, Rezakazemi M, Fatehizadeh A, Aminabhavi TM, Arjmand M. Metal-organic frameworks: a promising option for the diagnosis and treatment of Alzheimer’s disease. J Control Release. 2023;353:1–29.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72.
Thompson LH, Björklund A. Reconstruction of brain circuitry by neural transplants generated from pluripotent stem cells. Neurobiol Dis. 2015;79:28–40.
Trojanowski JQ, Schuck T, Schmidt ML, Lee VM. Distribution of tau proteins in the normal human central and peripheral nervous system. J Histochem Cytochem. 1989;37:209–15.
Upadhya R, Madhu LN, Attaluri S, Gitaí DLG, Pinson MR, Kodali M, Shetty G, Zanirati G, Kumar S, Shuai B, Weintraub ST, Shetty AK. Extracellular vesicles from human iPSC-derived neural stem cells: miRNA and protein signatures, and anti-inflammatory and neurogenic properties. J Extracell Vesicles. 2020;9:1809064.
van de Leemput J, Boles NC, Kiehl TR, Corneo B, Lederman P, Menon V, Lee C, Martinez RA, Levi BP, Thompson CL, Yao S, Kaykas A, Temple S, Fasano CA. CORTECON: a temporal transcriptome analysis of in vitro human cerebral cortex development from human embryonic stem cells. Neuron. 2014;83:51–68.
van der Kant R, Goldstein LSB, Ossenkoppele R. Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat Rev Neurosci. 2020;21:21–35.
van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9–21.
Vatine GD, Barrile R, Workman MJ, Sances S, Barriga BK, Rahnama M, Barthakur S, Kasendra M, Lucchesi C, Kerns J, Wen N, Spivia WR, Chen Z, Van Eyk J, Svendsen CN. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell. 2019;24:995-1005.e6.
Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 2018;15:36–45.
Voulgaris D, Nikolakopoulou P, Herland A. Generation of human iPSC-derived astrocytes with a mature star-shaped phenotype for CNS modeling. Stem Cell Rev Rep. 2022;18:2494–512.
Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–9.
Wang J, Jiang P, Deng W, Sun Y, Liu Y. Grafted human ESC-derived astroglia repair spinal cord injury via activation of host anti-inflammatory microglia in the lesion area. Theranostics. 2022;12:4288–309.
Wang L, Pei S, Han L, Guo B, Li Y, Duan R, Yao Y, Xue B, Chen X, Jia Y. Mesenchymal stem cell-derived exosomes reduce A1 astrocytes via downregulation of phosphorylated NFκB P65 subunit in spinal cord injury. Cell Physiol Biochem. 2018;50:1535–59.
Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M, Goldman SA. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12:252–64.
Wang W, Itaka K, Ohba S, Nishiyama N, Chung UI, Yamasaki Y, Kataoka K. 3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells. Biomaterials. 2009;30:2705–15.
Wang X, Yang G. Bone marrow mesenchymal stem cells-derived exosomes reduce Aβ deposition and improve cognitive function recovery in mice with Alzheimer’s disease by activating sphingosine kinase/sphingosine-1-phosphate signaling pathway. Cell Biol Int. 2021;45:775–84.
Wang YJ, Zhou HD, Zhou XF. Clearance of amyloid-beta in Alzheimer’s disease: progress, problems and perspectives. Drug Discov Today. 2006;11:931–8.
Wang ZB, Wang ZT, Sun Y, Tan L, Yu JT. The future of stem cell therapies of Alzheimer’s disease. Ageing Res Rev. 2022;80: 101655.
Wu TT, Su FJ, Feng YQ, Liu B, Li MY, Liang FY, Li G, Li XJ, Zhang Y, Cai ZQ, Pei Z. Mesenchymal stem cells alleviate AQP-4-dependent glymphatic dysfunction and improve brain distribution of antisense oligonucleotides in BACHD mice. Stem Cells. 2020;38:218–30.
Xie ZH, Liu Z, Zhang XR, Yang H, Wei LF, Wang Y, Xu SL, Sun L, Lai C, Bi JZ, Wang XY. Wharton’s Jelly-derived mesenchymal stem cells alleviate memory deficits and reduce amyloid-β deposition in an APP/PS1 transgenic mouse model. Clin Exp Med. 2016;16:89–98.
Xu R, Li X, Boreland AJ, Posyton A, Kwan K, Hart RP, Jiang P. Human iPSC-derived mature microglia retain their identity and functionally integrate in the chimeric mouse brain. Nat Commun. 2020;11:1577.
Xu W, Xu R, Li Z, Wang Y, Hu R. Hypoxia changes chemotaxis behaviour of mesenchymal stem cells via HIF-1α signalling. J Cell Mol Med. 2019;23:1899–907.
Xu Z, Rao Y, Huang Y, Zhou T, Feng R, Xiong S, Yuan TF, Qin S, Lu Y, Zhou X, Li X, Qin B, Mao Y, Peng B. Efficient strategies for microglia replacement in the central nervous system. Cell Rep. 2020;32: 108041.
Yaghoubi S, Najminejad H, Dabaghian M, Karimi MH, Abdollahpour-Alitappeh M, Rad F, Mahi-Birjand M, Mohammadi S, Mohseni F, Sobhani Lari M, Teymouri GH, Rigi Yousofabadi E, Salmani A, Bagheri N. How hypoxia regulate exosomes in ischemic diseases and cancer microenvironment? IUBMB Life. 2020;72:1286–305.
Yagi T, Ito D, Okada Y, Akamatsu W, Nihei Y, Yoshizaki T, Yamanaka S, Okano H, Suzuki N. Modeling familial Alzheimer’s disease with induced pluripotent stem cells. Hum Mol Genet. 2011;20:4530–9.
Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, Larcher LM, Chen S, Liu N, Zhao Q, Tran PHL, Chen C, Veedu RN, Wang T. Progress, opportunity, and perspective on exosome isolation—efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684–707.
Yang Z, Gong M, Jian T, Li J, Yang C, Ma Q, Deng P, Wang Y, Huang M, Wang H, Yang S, Chen X, Yu Z, Wang M, Chen C, Zhang K. Engrafted glial progenitor cells yield long-term integration and sensory improvement in aged mice. Stem Cell Res Ther. 2022;13:285.
Yao P, Yu Q, Zhu L, Li J, Zhou X, Wu L, Cai Y, Shen H, Zhou L. Wnt/PCP pathway regulates the migration and neural differentiation of mesenchymal stem cells in vitro. Folia Histochem Cytobiol. 2022;60:44–54.
Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142–57.
Yu D, Ma M, Liu Z, Pi Z, Du X, Ren J, Qu X. MOF-encapsulated nanozyme enhanced siRNA combo: control neural stem cell differentiation and ameliorate cognitive impairments in Alzheimer’s disease model. Biomaterials. 2020;255: 120160.
Yu D, Zhang H, Liu Z, Liu C, Du X, Ren J, Qu X. Hydrogen-bonded organic framework (HOF)-based single-neural stem cell encapsulation and transplantation to remodel impaired neural networks. Angew Chem Int Ed Engl. 2022;61: e202201485.
Yue C, Feng S, Chen Y, Jing N. The therapeutic prospects and challenges of human neural stem cells for the treatment of Alzheimer’s disease. Cell Regen. 2022;11:28.
Zhang K, Chen X. Sensory response in host and engrafted astrocytes of adult brain in Vivo. Glia. 2017;65:1867–84.
Zhao J, Davis MD, Martens YA, Shinohara M, Graff-Radford NR, Younkin SG, Wszolek ZK, Kanekiyo T, Bu G. APOE ε4/ε4 diminishes neurotrophic function of human iPSC-derived astrocytes. Hum Mol Genet. 2017;26:2690–700.
Zheng F, Fu F, Cheng Y, Wang C, Zhao Y, Gu Z. Organ-on-a-chip systems: microengineering to biomimic living systems. Small. 2016;12:2253–82.
Zheng X, Wang W. Astrocyte transplantation for repairing the injured spinal cord. J Biomed Res. 2022;36:312–20.
Zhong Z, Lemasters JJ. A unifying hypothesis linking hepatic adaptations for ethanol metabolism to the proinflammatory and profibrotic events of alcoholic liver disease. Alcohol Clin Exp Res. 2018;42:2072–89.
Andersen OM, Bøgh N, Landau AM, Pløen GG, Jensen AMG, Monti G, Ulhøi BP, Nyengaard JR, Jacobsen KR, Jørgensen MM, Holm IE, Kristensen ML, Alstrup AKO, Hansen ESS, Teunissen CE, Breidenbach L, Droescher M, Liu Y, Pedersen HS, Callesen H, Luo Y, Bolund L, Brooks DJ, Laustsen C, Small SA, Mikkelsen LF, Sørensen CB. A genetically modified minipig model for Alzheimer’s disease with SORL1 haploinsufficiency. Cell Rep Med. 2022;3: 100740.
Andrade-Guerrero J, Santiago-Balmaseda A, Jeronimo-Aguilar P, Vargas-Rodríguez I, Cadena-Suárez AR, Sánchez-Garibay C, Pozo-Molina G, Méndez-Catalá CF, Cardenas-Aguayo MD, Diaz-Cintra S, Pacheco-Herrero M, Luna-Muñoz J, Soto-Rojas LO. Alzheimer’s disease: an updated overview of its genetics. Int J Mol Sci. 2023;24:3754.
Brody H. Alzheimer’s disease. Nature. 2011;475:S1.
Burté F, Carelli V, Chinnery PF, Yu-Wai-Man P. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat Rev Neurol. 2015;11:11–24.
Cai Y, Liu J, Wang B, Sun M, Yang H. Microglia in the neuroinflammatory pathogenesis of Alzheimer’s disease and related therapeutic targets. Front Immunol. 2022;13:856376.
Cakir B, Tanaka Y, Kiral FR, Xiang Y, Dagliyan O, Wang J, Lee M, Greaney AM, Yang WS, duBoulay C, Kural MH, Patterson B, Zhong M, Kim J, Bai Y, Min W, Niklason LE, Patra P, Park IH. Expression of the transcription factor PU.1 induces the generation of microglia-like cells in human cortical organoids. Nat Commun. 2022;13:430.
Castranio EL, Hasel P, Haure-Mirande JV, Ramirez Jimenez AV, Hamilton BW, Kim RD, Glabe CG, Wang M, Zhang B, Gandy S, Liddelow SA, Ehrlich ME. Microglial INPP5D limits plaque formation and glial reactivity in the PSAPP mouse model of Alzheimer’s disease. Alzheimers Dement. 2023;19:2239–52.
Chen C, Tang X, Lan Z, Chen W, Su H, Li W, Li Y, Zhou X, Gao H, Feng X, Guo Y. GABAergic signaling abnormalities in a novel CLU mutation Alzheimer’s disease mouse model. Transl Res. 2023;1(260):32–45.
Chen X, Sun G, Tian E, Zhang M, Davtyan H, Beach TG, Reiman EM, Blurton-Jones M, Holtzman DM, Shi Y. Modeling sporadic Alzheimer’s disease in human brain organoids under serum exposure. Adv Sci. 2021;8: e2101462.
Choi YJ, Park J, Lee SH. Size-controllable networked neurospheres as a 3D neuronal tissue model for Alzheimer’s disease studies. Biomaterials. 2013;34:2938–46.
Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006;198:54–64.
Cuccaro ML, Carney RM, Zhang Y, Bohm C, Kunkle BW, Vardarajan BN, Whitehead PL, Cukier HN, Mayeux R, St George-Hyslop P, Pericak-Vance MA. SORL1 mutations in early- and late-onset Alzheimer disease. Neurol Genet. 2016;2: e116.
Cui GH, Guo HD, Li H, Zhai Y, Gong ZB, Wu J, Liu JS, Dong YR, Hou SX, Liu JR. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun Ageing. 2019;16:10.
Cui Y, Ma S, Zhang C, Cao W, Liu M, Li D, Lv P, Xing Q, Qu R, Yao N, Yang B, Guan F. Human umbilical cord mesenchymal stem cells transplantation improves cognitive function in Alzheimer’s disease mice by decreasing oxidative stress and promoting hippocampal neurogenesis. Behav Brain Res. 2017;320:291–301.
Elia CA, Tamborini M, Rasile M, Desiato G, Marchetti S, Swuec P, Mazzitelli S, Clemente F, Anselmo A, Matteoli M, Malosio ML, Coco S. Intracerebral injection of extracellular vesicles from mesenchymal stem cells exerts reduced Aβ plaque burden in early stages of a preclinical model of Alzheimer’s disease. Cells. 2019;8:1059.
Espuny-Camacho I, Arranz AM, Fiers M, Snellinx A, Ando K, Munck S, Bonnefont J, Lambot L, Corthout N, Omodho L, Vanden Eynden E, Radaelli E, Tesseur I, Wray S, Ebneth A, Hardy J, Leroy K, Brion JP, Vanderhaeghen P, De Strooper B. Hallmarks of Alzheimer’s disease in stem-cell-derived human neurons transplanted into mouse brain. Neuron. 2017;93:1066-81.e8.
Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, Lautrup S, Hasan-Olive MM, Caponio D, Dan X, Rocktäschel P, Croteau DL, Akbari M, Greig NH, Fladby T, Nilsen H, Cader MZ, Mattson MP, Tavernarakis N, Bohr VA. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci. 2019;22:401–12.
Fernández-Calle R, Konings SC, Frontiñán-Rubio J, García-Revilla J, Camprubí-Ferrer L, Svensson M, Martinson I, Boza-Serrano A, Venero JL, Nielsen HM, Gouras GK, Deierborg T. APOE in the bullseye of neurodegenerative diseases: impact of the APOE genotype in Alzheimer’s disease pathology and brain diseases. Mol Neurodegener. 2022;17:62.
Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12:383–8.
Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748–59.
Hidalgo M, Amant F, Biankin AV, Budinská E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Mælandsmo GM, Roman-Roman S, Seoane J, Trusolino L, Villanueva A. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4:998–1013.
Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, Abraham R, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Jones N, Stretton A, Thomas C, Richards A, Ivanov D, Widdowson C, Chapman J, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Beaumont H, Warden D, Wilcock G, Love S, Kehoe PG, Hooper NM, Vardy ER, Hardy J, Mead S, Fox NC, Rossor M, Collinge J, Maier W, Jessen F, Rüther E, Schürmann B, Heun R, Kölsch H, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Gallacher J, Hüll M, Rujescu D, Giegling I, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel KH, Klopp N, Wichmann HE, Pankratz VS, Sando SB, Aasly JO, Barcikowska M, Wszolek ZK, Dickson DW, Graff-Radford NR, Petersen RC, van Duijn CM, Breteler MM, Ikram MA, DeStefano AL, Fitzpatrick AL, Lopez O, Launer LJ, Seshadri S, Berr C, Campion D, Epelbaum J, Dartigues JF, Tzourio C, Alpérovitch A, Lathrop M, Feulner TM, Friedrich P, Riehle C, Krawczak M, Schreiber S, Mayhaus M, Nicolhaus S, Wagenpfeil S, Steinberg S, Stefansson H, Stefansson K, Snaedal J, Björnsson S, Jonsson PV, Chouraki V, Genier-Boley B, Hiltunen M, Soininen H, Combarros O, Zelenika D, Delepine M, Bullido MJ, Pasquier F, Mateo I, Frank-Garcia A, Porcellini E, Hanon O, Coto E, Alvarez V, Bosco P, Siciliano G, Mancuso M, Panza F, Solfrizzi V, Nacmias B, Sorbi S, Bossù P, Piccardi P, Arosio B, Annoni G, Seripa D, Pilotto A, Scarpini E, Galimberti D, Brice A, Hannequin D, Licastro F, Jones L, Holmans PA, Jonsson T, Riemenschneider M, Morgan K, Younkin SG, Owen MJ, O’Donovan M, Amouyel P, Williams J. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43:429–35.
Hu W, Feng Z, Xu J, Jiang Z, Feng M. Brain-derived neurotrophic factor modified human umbilical cord mesenchymal stem cells-derived cholinergic-like neurons improve spatial learning and memory ability in Alzheimer’s disease rats. Brain Res. 2019;1710:61–73.
Johnson ECB, Carter EK, Dammer EB, Duong DM, Gerasimov ES, Liu Y, Liu J, Betarbet R, Ping L, Yin L, Serrano GE, Beach TG, Peng J, De Jager PL, Haroutunian V, Zhang B, Gaiteri C, Bennett DA, Gearing M, Wingo TS, Wingo AP, Lah JJ, Levey AI, Seyfried NT. Large-scale deep multi-layer analysis of Alzheimer’s disease brain reveals strong proteomic disease-related changes not observed at the RNA level. Nat Neurosci. 2022;25:213–25.
Johnson ECB, Dammer EB, Duong DM, Ping L, Zhou M, Yin L, Higginbotham LA, Guajardo A, White B, Troncoso JC, Thambisetty M, Montine TJ, Lee EB, Trojanowski JQ, Beach TG, Reiman EM, Haroutunian V, Wang M, Schadt E, Zhang B, Dickson DW, Ertekin-Taner N, Golde TE, Petyuk VA, De Jager PL, Bennett DA, Wingo TS, Rangaraju S, Hajjar I, Shulman JM, Lah JJ, Levey AI, Seyfried NT. Large-scale proteomic analysis of Alzheimer’s disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat Med. 2020;26:769–80.
Jonas LA, Jain T, Li YM. Functional insight into LOAD-associated microglial response genes. Open Biol. 2022;12: 210280.
Kawatani K, Holm ML, Starling SC, Martens YA, Zhao J, Lu W, Ren Y, Li Z, Jiang P, Jiang Y, Baker SK. ABCA7 deficiency causes neuronal dysregulation by altering mitochondrial lipid metabolism. Mol Psychiatry. 2023;22:1–1.
Google Scholar
Kim KS, Kim HS, Park JM, Kim HW, Park MK, Lee HS, Lim DS, Lee TH, Chopp M, Moon J. Long-term immunomodulatory effect of amniotic stem cells in an Alzheimer’s disease model. Neurobiol Aging. 2013;34:2408–20.
Li H, Wijekoon A, Leipzig ND. 3D differentiation of neural stem cells in macroporous photopolymerizable hydrogel scaffolds. PLoS ONE. 2012;7: e48824.
Liang J, Wang C, Zhang H, Huang J, Xie J, Chen N. Exercise-induced benefits for Alzheimer’s disease by stimulating mitophagy and improving mitochondrial function. Front Aging Neurosci. 2021;13: 755665.
Liu S, Fan M, Xu JX, Yang LJ, Qi CC, Xia QR, Ge JF. Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J Neuroinflammation. 2022;19:35.
Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179:312–39.
Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, Menon M, He L, Abdurrob F, Jiang X, Martorell AJ, Ransohoff RM, Hafler BP, Bennett DA, Kellis M, Tsai LH. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature. 2019;570:332–7.
McQuade A, Kang YJ, Hasselmann J, Jairaman A, Sotelo A, Coburn M, Shabestari SK, Chadarevian JP, Fote G, Tu CH, Danhash E, Silva J, Martinez E, Cotman C, Prieto GA, Thompson LM, Steffan JS, Smith I, Davtyan H, Cahalan M, Cho H, Blurton-Jones M. Gene expression and functional deficits underlie TREM2-knockout microglia responses in human models of Alzheimer’s disease. Nat Commun. 2020;11:5370.
Morabito S, Miyoshi E, Michael N, Shahin S, Martini AC, Head E, Silva J, Leavy K, Perez-Rosendahl M, Swarup V. Single-nucleus chromatin accessibility and transcriptomic characterization of Alzheimer’s disease. Nat Genet. 2021;53:1143–55.
Naaldijk Y, Jäger C, Fabian C, Leovsky C, Blüher A, Rudolph L, Hinze A, Stolzing A. Effect of systemic transplantation of bone marrow-derived mesenchymal stem cells on neuropathology markers in APP/PS1 Alzheimer mice. Neuropathol Appl Neurobiol. 2017;43:299–314.
Nakano M, Kubota K, Kobayashi E, Chikenji TS, Saito Y, Konari N, Fujimiya M. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer’s disease model by increasing the expression of microRNA-146a in hippocampus. Sci Rep. 2020;10:10772.
Nativio R, Lan Y, Donahue G, Sidoli S, Berson A, Srinivasan AR, Shcherbakova O, Amlie-Wolf A, Nie J, Cui X, He C, Wang LS, Garcia BA, Trojanowski JQ, Bonini NM, Berger SL. An integrated multi-omics approach identifies epigenetic alterations associated with Alzheimer’s disease. Nat Genet. 2020;52:1024–35.
Nugent AA, Lin K, van Lengerich B, Lianoglou S, Przybyla L, Davis SS, Llapashtica C, Wang J, Kim DJ, Xia D, Lucas A, Baskaran S, Haddick PCG, Lenser M, Earr TK, Shi J, Dugas JC, Andreone BJ, Logan T, Solanoy HO, Chen H, Srivastava A, Poda SB, Sanchez PE, Watts RJ, Sandmann T, Astarita G, Lewcock JW, Monroe KM, Di Paolo G. TREM2 regulates microglial cholesterol metabolism upon chronic phagocytic challenge. Neuron. 2020;105:837-54.e9.
Ormel PR, Vieira de Sá R, van Bodegraven EJ, Karst H, Harschnitz O, Sneeboer MAM, Johansen LE, van Dijk RE, Scheefhals N, Berdenis van Berlekom A, Ribes Martínez E, Kling S, MacGillavry HD, van den Berg LH, Kahn RS, Hol EM, de Witte LD, Pasterkamp RJ. Microglia innately develop within cerebral organoids. Nat Commun. 2018;9:4167.
Pang K, Jiang R, Zhang W, Yang Z, Li LL, Shimozawa M, Tambaro S, Mayer J, Zhang B, Li M, Wang J, Liu H, Yang A, Chen X, Liu J, Winblad B, Han H, Jiang T, Wang W, Nilsson P, Guo W, Lu B. An App knock-in rat model for Alzheimer’s disease exhibiting Aβ and tau pathologies, neuronal death and cognitive impairments. Cell Res. 2022;32:157–75.
Peng J, Wang Y, Zhang L, Zhao B, Zhao Z, Chen J, Guo Q, Liu S, Sui X, Xu W, Lu S. Human umbilical cord Wharton’s jelly-derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Res Bull. 2011;84:235–43.
Popova G, Soliman SS, Kim CN, Keefe MG, Hennick KM, Jain S, Li T, Tejera D, Shin D, Chhun BB, McGinnis CS, Speir M, Gartner ZJ, Mehta SB, Haeussler M, Hengen KB, Ransohoff RR, Piao X, Nowakowski TJ. Human microglia states are conserved across experimental models and regulate neural stem cell responses in chimeric organoids. Cell Stem Cell. 2021;28:2153-66.e6.
Reza-Zaldivar EE, Hernández-Sapiéns MA, Gutiérrez-Mercado YK, Sandoval-Ávila S, Gomez-Pinedo U, Márquez-Aguirre AL, Vázquez-Méndez E, Padilla-Camberos E, Canales-Aguirre AA. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen Res. 2019;14:1626–34.
Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23:1018–27.
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705.
Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, O’Gorman J, Qian F, Arastu M, Li M, Chollate S, Brennan MS, Quintero-Monzon O, Scannevin RH, Arnold HM, Engber T, Rhodes K, Ferrero J, Hang Y, Mikulskis A, Grimm J, Hock C, Nitsch RM, Sandrock A. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537:50–6.
Shi Y, Sun L, Wang M, Liu J, Zhong S, Li R, Li P, Guo L, Fang A, Chen R, Ge WP, Wu Q, Wang X. Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol. 2020;18: e3000705.
Siehr MS, Massey CA, Noebels JL. Arx expansion mutation perturbs cortical development by augmenting apoptosis without activating innate immunity in a mouse model of X-linked infantile spasms syndrome. Dis Models Mech. 2020;13(3):dmm042515.
Singh A, Kukreti R, Saso L, Kukreti S. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules. 2019;24(8):1583.
Taddei RN, Perbet R, Mate de Gerando A, Wiedmer AE, Sanchez-Mico M, Connors Stewart T, Gaona A, Melloni A, Amaral AC, Duff K, Frosch MP, Gómez-Isla T. Tau oligomer-containing synapse elimination by microglia and astrocytes in Alzheimer disease. JAMA Neurol. 2023;80:1209–21.
Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, Quadrato G, Paulsen B, Nguyen L, Adiconis X, Regev A, Levin JZ, Arlotta P. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature. 2019;570:523–7.
Wai T, Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab. 2016;27:105–17.
Wang C, Najm R, Xu Q, Jeong DE, Walker D, Balestra ME, Yoon SY, Yuan H, Li G, Miller ZA, Miller BL, Malloy MJ, Huang Y. Gain of toxic apolipoprotein E4 effects in human iPSC-derived neurons is ameliorated by a small-molecule structure corrector. Nat Med. 2018;24:647–57.
Wang SS, Jia J, Wang Z. Mesenchymal stem cell-derived extracellular vesicles suppresses iNOS expression and ameliorates neural impairment in Alzheimer’s disease mice. J Alzheimers Dis. 2018;61:1005–13.
Windrem MS, Schanz SJ, Zou L, Chandler-Militello D, Kuypers NJ, Nedergaard M, Lu Y, Mariani JN, Goldman SA. Human Glial Progenitor Cells Effectively Remyelinate the Demyelinated Adult Brain. Cell Rep. 2020;31(7):107658. https://doi.org/10.1016/j.celrep.2020.107658
Xu LL, Shen Y, Wang X, Wei LF, Wang P, Yang H, Wang CF, Xie ZH, Bi JZ. Mitochondrial dynamics changes with age in an APPsw/PS1dE9 mouse model of Alzheimer’s disease. NeuroReport. 2017;28:222–8.
Yang L, Zhai Y, Hao Y, Zhu Z, Cheng G. The regulatory functionality of exosomes derived from hUMSCs in 3D culture for Alzheimer’s disease therapy. Small. 2020;16: e1906273.
Yokokawa K, Iwahara N, Hisahara S, Emoto MC, Saito T, Suzuki H, Manabe T, Matsumura A, Matsushita T, Suzuki S, Kawamata J, Sato-Akaba H, Fujii HG, Shimohama S. Transplantation of mesenchymal stem cells improves Amyloid-β pathology by modifying microglial function and suppressing oxidative stress. J Alzheimers Dis. 2019;72:867–84.
Yue W, Li Y, Zhang T, Jiang M, Qian Y, Zhang M, Sheng N, Feng S, Tang K, Yu X, Shu Y, Yue C, Jing N. ESC-derived basal forebrain cholinergic neurons ameliorate the cognitive symptoms associated with Alzheimer’s disease in mouse models. Stem Cell Reports. 2015;5:776–90.
Zeviani M, Viscomi C. Mitochondrial neurodegeneration. Cells. 2022;11(4):637.
Zhao J, Fu Y, Yamazaki Y, Ren Y, Davis MD, Liu CC, Lu W, Wang X, Chen K, Cherukuri Y, Jia L, Martens YA, Job L, Shue F, Nguyen TT, Younkin SG, Graff-Radford NR, Wszolek ZK, Brafman DA, Asmann YW, Ertekin-Taner N, Kanekiyo T, Bu G. APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer’s disease patient iPSC-derived cerebral organoids. Nat Commun. 2020;11:5540.
Zheng XY, Wan QQ, Zheng CY, Zhou HL, Dong XY, Deng QS, Yao H, Fu Q, Gao M, Yan ZJ, Wang SS, You Y, Lv J, Wang XY, Chen KE, Zhang MY, Xu RX. Amniotic mesenchymal stem cells decrease Aβ deposition and improve memory in APP/PS1 transgenic mice. Neurochem Res. 2017;42:2191–207.
Zhong L, Xu Y, Zhuo R, Wang T, Wang K, Huang R, Wang D, Gao Y, Zhu Y, Sheng X, Chen K, Wang N, Zhu L, Can D, Marten Y, Shinohara M, Liu CC, Du D, Sun H, Wen L, Xu H, Bu G, Chen XF. Soluble TREM2 ameliorates pathological phenotypes by modulating microglial functions in an Alzheimer’s disease model. Nat Commun. 2019;10:1365.
Zilka N, Zilkova M, Kazmerova Z, Sarissky M, Cigankova V, Novak M. Mesenchymal stem cells rescue the Alzheimer’s disease cell model from cell death induced by misfolded truncated tau. Neuroscience. 2011;193:330–7.
Download references
Acknowledgements
Not applicable.
The authors acknowledge the funding support from Shenzhen Science and Technology Program (Grant No. KQTD20190929173853397) and National Natural Science Foundation of China (Grant No. 81971081).
Author information
Zimeng Cao, Fanshu Kong and Jiaqi Ding contributed equally to this work and should be considered co-first authors.
Authors and Affiliations
School of Pharmaceutical Sciences, Shenzhen Campus of Sun Yat-Sen University, Shenzhen, 518107, China
Zimeng Cao, Fanshu Kong, Jiaqi Ding, Chunxia Chen, Fumei He & Wenbin Deng
School of Pharmaceutical Sciences, Dali University, Dali, 671000, China
You can also search for this author in PubMed Google Scholar
Contributions
WBD, ZMC, FSK and JQD were responsible for the conception and design of the review and revised the article. ZMC, FSK and JQD accomplished the first draft of the manuscript and prepared all the figures. ZMC, FSK and JQD contributed equally and should be considered co-first authors. WBD, FMH and CXC participated the revision of manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Correspondence to Chunxia Chen , Fumei He or Wenbin Deng .
Ethics declarations
Ethical approval and consent to participate.
No human participants, human data or human tissue are involved in this work.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Cao, Z., Kong, F., Ding, J. et al. Promoting Alzheimer’s disease research and therapy with stem cell technology. Stem Cell Res Ther 15 , 136 (2024). https://doi.org/10.1186/s13287-024-03737-w
Download citation
Received : 12 October 2023
Accepted : 17 April 2024
Published : 07 May 2024
DOI : https://doi.org/10.1186/s13287-024-03737-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Alzheimer’s disease
- Stem cell technology
- Disease modeling
- Pathogenesis
- Neuron–glia interaction
- Stem cell-derived therapy
Stem Cell Research & Therapy
ISSN: 1757-6512
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Psychology Issues: Alzheimer’s Disease Research Paper
Introduction.
Alzheimer’s disease (AD) is a psychological disorder that involves the progressive destruction of brain cells and reduction in the proper functioning of the brain (Altman, 2000). Alzheimer’s disease is a type of dementia that is characterized by degeneration of brain functions such as memory, speech, and comprehension. It affects people at a young age but signs and symptoms appear in old age.
The first sign of the disease is memory deterioration, which is characterized by memory loss and poor concentration (Altman, 2000). The disease is difficult to diagnose because its early signs can be easily confused with the effects of aging.
On the other hand, early symptoms of the disease can also be observed in other conditions such as depression, fatigue, continued use of certain medications, and grief. Alzheimer’s disease usually results in death due to the lack of a cure.
Even though certain medications are used, they only slow down the development of the disease and reduce the severity of symptoms. The pattern of AD’s development is different in individuals. However, its symptoms and effects are similar.
The main cause of AD has not yet identified. However, scientists have presented four main hypotheses to explain the causes. These include genetics, cholinergic hypotheses, tau hypotheses, and amyloid hypotheses.
Genetics and physiological contributions
Scientists have linked genetic inheritance to AD. According to scientists, three types of gene mutations cause the disease. They include mutations that encode presenilins 1, presenilins 2, and amyloid precursor protein (Foster, 2004). These mutations are responsible for the greatest percentage of autosomal familial Alzheimer’s disease.
The aforementioned mutations increase the secretion of a protein known as Aβ42, which plays an important role in the formation of senile plaques. Other mutations alter the ration of the protein to other forms of proteins without affecting the level of Aβ42. For that reason, presenilin mutations can cause AD without altering the level of protein Aβ42.
Cases that do not involve autosomal-dominant inheritance are referred to as sporadic AD. In those cases, the disease is caused by the inheritance of the ε4 allele. The genes are contained in a protein referred to as the apolipoprotein E. Studies have established that approximately 40-80 percent of people with Alzheimer’s disease possess the APOEε4 allele gene, which increases the risk factor of developing the disease (Foster, 2004).
Research has also found out that the TREM2 gene causes AD. Its mutation results in uneven regulation of beta amyloid in the brain that leads to accumulation of the protein in the brain.
Cholinergic hypothesis
According to the cholinergic hypothesis, Alzheimer’s disease results from low production of acetylcholine. Acetylcholine is a neurotransmitter that functions in the peripheral, autonomic, and central nervous systems (Brill, 2005).
The neurotransmitter is produced by the cholinergic system, whose damage results in Alzheimer’s disease. The hypothesis is not widely supported because medications that enhance production o acetylcholine do not alleviate the symptoms of the disease.
Amyloid and physiological contributions
According to the amyloid hypothesis, deposits of beta-amyloid (Aβ) cause Alzheimer’s disease. The hypothesis was based on the discovery that a large percentage of individuals with Down syndrome develop the disease. In addition, it was supported by the discovery that apolipoprotein4 was a risk factor for the disease. Apolipoprotein plays an important role in the dissolution of beta-amyloid in the brain.
However, certain forms of the protein promote the accumulation of the protein to toxic levels. The buildup of beta-amyloid damages brain cells and alters the proper functioning of the brain. Research studies have led to the development of a hypothesis that predicts that non-plaque Aβ aggregates are the cause of AD (Brill, 2005). Scientists have predicted that they bind to neuron receptors and inhibit communication.
Further studies suggested that a protein related to the beta-amyloid protein could be the cause of the disease. Even though scientists have not agreed regarding the cause of AD, studies show the involvement of beta-amyloid in the development of Alzheimer’s disease. Clumps of the protein interfere with neuronal communication between brain cells leading to their decline and death.
Tau and physiological contributions
According to the tau hypothesis, the tau protein is responsible for Alzheimer’s disease. The human brain depends on an internal system that transports nutrients and other essential elements to various brain cells and structures. This system relies on a protein knows as tau. The hyperphosphorylation of tau initiates the loss of its ability to bind to microtubules.
As a result, tau proteins bind to other tau filaments resulting in the formation of neurofibrillary twists that alter the transport and communication systems. Phosphorylation of tau protein is necessary for the proper function of the brain. However, uncontrolled rates of phosphorylation affect the proper functioning of the brain.
Hyperphosphorylation of tau proteins alters certain processes that maintain the activity of brain cells. For instance, hyperphosphorylated tau does not bind to neurotransmitters and does not initiate the assembly of microtubules thus causing their disintegration. Twisting of tau protein threads interferes with the proper functioning of the nutrient transport system thus leading to the death of brain cells (Lillrank & Collins, 2007).
Symptoms of AD are mild, moderate, and severe depending on the stage of development. Mild symptoms include mood swings, trouble completing daily tasks, problems with speech and language, and prevalent episodes of forgetting (Altman, 2000).
Moderate symptoms include sleep problems, delusions, difficulties with problem-solving, confusion, and restlessness (Brill, 2005). Severe symptoms include loss of ability to communicate, major confusion, intense mood swings, weight loss, and hallucinations (Budson & Kowall, 2011). Each group of symptoms appears at a certain stage in the development of the disease.
Pathophysiology
The main characteristics of AD include loss of communication between cells in the cerebral cortex, death of affected areas, and reduction in the size of certain brain parts (Budson & Kowall, 2011). Individuals with the disease possess amyloid plaques and tangles of tau protein in their brains.
The biochemistry of Alzheimer’s disease involves the accumulation of beta-amyloid and tau proteins to levels alter certain brain functions. Hyperphosphorylation of tau proteins results in abnormal aggregations that kill brain cells (Grossberg & Kamat, 2010). On the other hand, the accumulation of beta-amyloid proteins also kills brain cells.
Alzheimer’s disease is diagnosed through a comprehensive medical evaluation. Diagnosis involves an analysis of a patient’s medical history and mental status, as well as physical and neurological examination (Gauthier, 2006). In addition, blood tests and brain imaging are conducted to aid in proper diagnosis.
The main symptoms that all patients exhibit include confusion, poor memory, the decline in communication skills, and difficulties in executing daily activities. Medical practitioners conduct thorough assessments in order to rule out related or unrelated conditions that have similar symptoms (Grossberg & Kamat, 2010). Mental tests involve answering a set of questions in order to test a patient’s memory and thinking capabilities (Lillrank & Collins, 2007).
Physical examination and blood tests aid in the identification conditions that could be the cause of certain symptoms (Gauthier, 2006). Brain imaging identifies conditions such as strokes and tumors that could be the cause of memory loss and confusion. Major types of brain scans include computerized axial tomography, magnetic resonance imaging, and single photon emission computerized tomography (Gauthier, 2006).
AD is incurable. However, certain medications slow the development of the disease. Medications that are commonly used include Cognex, Donepezil, Exelon, Razadyne, and Memantine (Grossberg & Kamat, 2010). Cognex is rarely used because it causes liver damage. Memantine prevents nerve damage and improves mental function.
Prevention and management
Prevention of AD involves medication, lifestyle changes, and choosing the right diet. Research has revealed that people who use non-steroidal anti-inflammatory drugs are at a lower risk of developing Alzheimer’s disease compared to people who do not use the drugs (Shankle & Amen, 2005). In addition, the drugs reduce inflammations that are caused by reactions of beta-amyloid proteins.
Before technological advancements, hormone replacement therapy was used to prevent AD. However, research studies later revealed that the remedy increases the risk of developing the disease. Lifestyle is an important factor in the prevention of AD. Individuals who develop the habits of reading, socializing, and completing intellectually challenging puzzles lower the risk of the disease (Grossberg & Kamat, 2010).
According to the cognitive reserve theory, working the brain enhances the performance of the neural system. Finally, physical exercise keeps the brain active and healthy. Proper diet plays a vital role in the prevention of Alzheimer’s disease. Legumes, fruits, vegetables, unrefined cereals, fish, and diets rich in flavonoids are recommended (Lillrank & Collins, 2007). In addition, moderate consumption of wine and dairy products reduces the risk of AD.
Alzheimer’s disease (AD) is a psychological disorder that alters the proper functioning of the brain. The main symptoms of the disease are memory loss and language problems. It is most common among old people even though its onset occurs during the middle ages.
Causes of AD include genetics, accumulation of beta-amyloid proteins, and twisting of tau proteins. Also, scientists predict that low production of acetylcholine and mutations that encode presenilins 1, presenilins 2, and amyloid precursor protein contributed towards the development of AD.
Symptoms of the disease include mood swings, difficulty in completing daily tasks, problems with speech and language, prevalent episodes of forgetfulness, sleep problems, delusions, difficulties in solving problems, confusion, and restlessness. Other symptoms include loss of ability to communicate, major confusion, intense mood swings, weight loss, and hallucinations.
The hyperphosphorylation of tau results in the loss of the protein’s ability to bind to microtubules. As a result, it binds to other tau filaments resulting in the formation of neurofibrillary twists that initiate the breakdown of microtubules. Mutations that encode presenilins 1, presenilins 2, and amyloid precursor protein increase the secretion of a protein known as Aβ42, which plays an important role in the formation of senile plaques.
Correct diagnosis of AD involves analysis of a patient’s medical history, physical and neurological examination, and evaluation of mental status. In addition, tests such as blood tests and brain imaging are carried out to aid in proper diagnosis. Prevention and management of AD involve medication, lifestyle changes, and eating a healthy diet.
Altman, L. J. (2000). Alzheimer’s Disease . New York: Lucent Books.
Brill, M. T. (2005). Alzheimer’s Disease . New York: Marshall Cavendish.
Budson, A. E., & Kowall, N. W. (2011). The Handbook of Alzheimer’s Disease and Other Dementias . New York: John Wiley & Sons.
Foster, H. D. (2004). What really Causes Alzheimer’s Disease . New York: Trafford Publishing.
Gauthier, S. (2006). Clinical Diagnosis and Management of Alzheimer’s Disease . New York: CRC Press.
Grossberg, G., & Kamat, S. (2010). Alzheimer’s: The Latest Assessment & Treatment Strategies . New York: Jones & Bartlett Learning.
Lillrank, S., & Collins, C. E. (2007). Psychological Disorders: Alzheimer’s Disease and Other Dementias . London: Infobase Publishing.
Shankle, W. R., & Amen, D. G. (2005). Preventing Alzheimer’s: Ways to Help Prevent, Delay, Detect, and Even Halt Alzheimer’s Disease and Other Forms of Memory Loss . New York: Penguin.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2023, December 31). Psychology Issues: Alzheimer’s Disease. https://ivypanda.com/essays/psychology-issues-alzheimers-disease/
"Psychology Issues: Alzheimer’s Disease." IvyPanda , 31 Dec. 2023, ivypanda.com/essays/psychology-issues-alzheimers-disease/.
IvyPanda . (2023) 'Psychology Issues: Alzheimer’s Disease'. 31 December.
IvyPanda . 2023. "Psychology Issues: Alzheimer’s Disease." December 31, 2023. https://ivypanda.com/essays/psychology-issues-alzheimers-disease/.
1. IvyPanda . "Psychology Issues: Alzheimer’s Disease." December 31, 2023. https://ivypanda.com/essays/psychology-issues-alzheimers-disease/.
Bibliography
IvyPanda . "Psychology Issues: Alzheimer’s Disease." December 31, 2023. https://ivypanda.com/essays/psychology-issues-alzheimers-disease/.
- Empirical Support for Mindfulness Based Therapy
- Bioinformatics of Enzyme Kinase
- Post Traumatic Stress Disorder or Combat Fatigue
- Psychology Issues: Conduct Disorders
- Psychology: Attention Deficit and Hyperactivity Disorder
- Personality Theories: Yesterday, Today, and Tomorrow
- Refugees and Mental Health
- Vaccination as a Cause Autism
- Share full article
Advertisement
Supported by
Study Suggests Genetics as a Cause, Not Just a Risk, for Some Alzheimer’s
People with two copies of the gene variant APOE4 are almost certain to get Alzheimer’s, say researchers, who proposed a framework under which such patients could be diagnosed years before symptoms.

By Pam Belluck
Scientists are proposing a new way of understanding the genetics of Alzheimer’s that would mean that up to a fifth of patients would be considered to have a genetically caused form of the disease.
Currently, the vast majority of Alzheimer’s cases do not have a clearly identified cause. The new designation, proposed in a study published Monday, could broaden the scope of efforts to develop treatments, including gene therapy, and affect the design of clinical trials.
It could also mean that hundreds of thousands of people in the United States alone could, if they chose, receive a diagnosis of Alzheimer’s before developing any symptoms of cognitive decline, although there currently are no treatments for people at that stage.
The new classification would make this type of Alzheimer’s one of the most common genetic disorders in the world, medical experts said.
“This reconceptualization that we’re proposing affects not a small minority of people,” said Dr. Juan Fortea, an author of the study and the director of the Sant Pau Memory Unit in Barcelona, Spain. “Sometimes we say that we don’t know the cause of Alzheimer’s disease,” but, he said, this would mean that about 15 to 20 percent of cases “can be tracked back to a cause, and the cause is in the genes.”
The idea involves a gene variant called APOE4. Scientists have long known that inheriting one copy of the variant increases the risk of developing Alzheimer’s, and that people with two copies, inherited from each parent, have vastly increased risk.
The new study , published in the journal Nature Medicine, analyzed data from over 500 people with two copies of APOE4, a significantly larger pool than in previous studies. The researchers found that almost all of those patients developed the biological pathology of Alzheimer’s, and the authors say that two copies of APOE4 should now be considered a cause of Alzheimer’s — not simply a risk factor.
The patients also developed Alzheimer’s pathology relatively young, the study found. By age 55, over 95 percent had biological markers associated with the disease. By 65, almost all had abnormal levels of a protein called amyloid that forms plaques in the brain, a hallmark of Alzheimer’s. And many started developing symptoms of cognitive decline at age 65, younger than most people without the APOE4 variant.
“The critical thing is that these individuals are often symptomatic 10 years earlier than other forms of Alzheimer’s disease,” said Dr. Reisa Sperling, a neurologist at Mass General Brigham in Boston and an author of the study.
She added, “By the time they are picked up and clinically diagnosed, because they’re often younger, they have more pathology.”
People with two copies, known as APOE4 homozygotes, make up 2 to 3 percent of the general population, but are an estimated 15 to 20 percent of people with Alzheimer’s dementia, experts said. People with one copy make up about 15 to 25 percent of the general population, and about 50 percent of Alzheimer’s dementia patients.
The most common variant is called APOE3, which seems to have a neutral effect on Alzheimer’s risk. About 75 percent of the general population has one copy of APOE3, and more than half of the general population has two copies.
Alzheimer’s experts not involved in the study said classifying the two-copy condition as genetically determined Alzheimer’s could have significant implications, including encouraging drug development beyond the field’s recent major focus on treatments that target and reduce amyloid.
Dr. Samuel Gandy, an Alzheimer’s researcher at Mount Sinai in New York, who was not involved in the study, said that patients with two copies of APOE4 faced much higher safety risks from anti-amyloid drugs.
When the Food and Drug Administration approved the anti-amyloid drug Leqembi last year, it required a black-box warning on the label saying that the medication can cause “serious and life-threatening events” such as swelling and bleeding in the brain, especially for people with two copies of APOE4. Some treatment centers decided not to offer Leqembi, an intravenous infusion, to such patients.
Dr. Gandy and other experts said that classifying these patients as having a distinct genetic form of Alzheimer’s would galvanize interest in developing drugs that are safe and effective for them and add urgency to current efforts to prevent cognitive decline in people who do not yet have symptoms.
“Rather than say we have nothing for you, let’s look for a trial,” Dr. Gandy said, adding that such patients should be included in trials at younger ages, given how early their pathology starts.
Besides trying to develop drugs, some researchers are exploring gene editing to transform APOE4 into a variant called APOE2, which appears to protect against Alzheimer’s. Another gene-therapy approach being studied involves injecting APOE2 into patients’ brains.
The new study had some limitations, including a lack of diversity that might make the findings less generalizable. Most patients in the study had European ancestry. While two copies of APOE4 also greatly increase Alzheimer’s risk in other ethnicities, the risk levels differ, said Dr. Michael Greicius, a neurologist at Stanford University School of Medicine who was not involved in the research.
“One important argument against their interpretation is that the risk of Alzheimer’s disease in APOE4 homozygotes varies substantially across different genetic ancestries,” said Dr. Greicius, who cowrote a study that found that white people with two copies of APOE4 had 13 times the risk of white people with two copies of APOE3, while Black people with two copies of APOE4 had 6.5 times the risk of Black people with two copies of APOE3.
“This has critical implications when counseling patients about their ancestry-informed genetic risk for Alzheimer’s disease,” he said, “and it also speaks to some yet-to-be-discovered genetics and biology that presumably drive this massive difference in risk.”
Under the current genetic understanding of Alzheimer’s, less than 2 percent of cases are considered genetically caused. Some of those patients inherited a mutation in one of three genes and can develop symptoms as early as their 30s or 40s. Others are people with Down syndrome, who have three copies of a chromosome containing a protein that often leads to what is called Down syndrome-associated Alzheimer’s disease .
Dr. Sperling said the genetic alterations in those cases are believed to fuel buildup of amyloid, while APOE4 is believed to interfere with clearing amyloid buildup.
Under the researchers’ proposal, having one copy of APOE4 would continue to be considered a risk factor, not enough to cause Alzheimer’s, Dr. Fortea said. It is unusual for diseases to follow that genetic pattern, called “semidominance,” with two copies of a variant causing the disease, but one copy only increasing risk, experts said.
The new recommendation will prompt questions about whether people should get tested to determine if they have the APOE4 variant.
Dr. Greicius said that until there were treatments for people with two copies of APOE4 or trials of therapies to prevent them from developing dementia, “My recommendation is if you don’t have symptoms, you should definitely not figure out your APOE status.”
He added, “It will only cause grief at this point.”
Finding ways to help these patients cannot come soon enough, Dr. Sperling said, adding, “These individuals are desperate, they’ve seen it in both of their parents often and really need therapies.”
Pam Belluck is a health and science reporter, covering a range of subjects, including reproductive health, long Covid, brain science, neurological disorders, mental health and genetics. More about Pam Belluck
The Fight Against Alzheimer’s Disease
Alzheimer’s is the most common form of dementia, but much remains unknown about this daunting disease..
How is Alzheimer’s diagnosed? What causes Alzheimer’s? We answered some common questions .
A study suggests that genetics can be a cause of Alzheimer’s , not just a risk, raising the prospect of diagnosis years before symptoms appear.
Determining whether someone has Alzheimer’s usually requires an extended diagnostic process . But new criteria could lead to a diagnosis on the basis of a simple blood test .
The F.D.A. has given full approval to the Alzheimer’s drug Leqembi. Here is what to know about i t.
Alzheimer’s can make communicating difficult. We asked experts for tips on how to talk to someone with the disease .

COMMENTS
1. Introduction. Alzheimer's disease (AD) (named after the German psychiatric Alois Alzheimer) is the most common type of dementia and can be defined as a slowly progressive neurodegenerative disease characterized by neuritic plaques and neurofibrillary tangles (Figure 1) as a result of amyloid-beta peptide's (Aβ) accumulation in the most affected area of the brain, the medial temporal ...
A Research Paper on Alzheimer's Disease. Abstract. In this paper, Alzheimer's disease will be delved into, investigated and dissected. This will include all that is known about the disease as much of it is unknown still, despite increasing efforts from the medical community to uncover its origin. The disease's causes, symptoms and stages ...
and effects of Alzheimer's Disease. This finding from the pre-existing research sparked the goal of this research study: to examine the extent to which Connecticut facilities that care for residents with Alzheimer's Disease are incorporating aspects of the Mediterranean Diet into meal plans.
Late-onset Alzheimer's disease is an irreversible neurodegenerative disorder which accounts for the majority of dementia cases 1.Despite major private and public investments in research, there ...
Introduction. Alzheimer disease (AD) is one of the greatest medical care challenges of our century and is the main cause of dementia. In total, 40 million people are estimated to suffer from dementia throughout the world, and this number is supposed to become twice as much every 20 years, until approximately 2050. 1 Because dementia occurs mostly in people older than 60 years, the growing ...
Alzheimer's disease (AD) is the primary cause of dementia, affecting ~45.0 million individuals worldwide and is ranked as the fifth leading cause of death globally [].In the United States alone ...
Alzheimer's disease is still a field of research with lots of open questions. The complexity of the disease prevents the early diagnosis before visible symptoms regarding the individual's ...
Alzheimer's disease (AD) is a progressive neurodegenerative disease and the leading cause of dementia in the elderly [ 1 ]. Worldwide, around 50 million people have dementia, and 50-70% of cases are attributed to AD [ 2, 3 ]. Both the prevalence and incidence of AD increase with age. Globally, the population aged 65 years or older is ...
Alzheimer's disease is a progressive neurodegenerative disease that causes brain cells to waste away and die. It is characterized by progressive cognitive deterioration and continuous decline in ...
Alzheimer's Disease: History, Mechanisms and Treatment. Nevertheless, researchers state that the development of Alzheimer's is impacted by the formation of protein plaques and tangles in the brain. Alzheimer's Disease: Causes and Treatment. AD is associated with different changes, both cognitive and behavioral.
The Nature paper has been cited in about 2300 scholarly articles—more than all but four other Alzheimer's basic research reports published since 2006, according to the Web of Science database. Since then, annual NIH support for studies labeled "amyloid, oligomer, and Alzheimer's" has risen from near zero to $287 million in 2021.
1907 and named after Alzheimer in 1910. Alzheimer's Disease vs. Dementia and Normal Aging Alzheimer's disease is often confused with normal aging and dementia. Severe memory loss, characteristic of AD, is not a symptom of normal aging. Healthy aging may involve the gradual loss of hair, weight, height and muscle mass.
New data confirm that APOE4 homozygosity is a major genetic cause of Alzheimer's disease, warranting the development of specialized research strategies, treatment approaches and clinical trials.
Alzheimer's Disease is a devastating neurodegenerative condition that affects millions of people worldwide. As researchers continue to search for a cure, raising awareness about the disease becomes crucial. One effective way to do this is through the written word, which allows for a deeper exploration of the topic.
Its greatest effects are that it affects the overall memory ability of a patient, thinking and ultimately behavior. Alzheimer's disease results into a wide range of diagnosis features, including memory impairment, poor judgment ability, personality problems, improper decision-making and language problems (Turkington & Mitchell, 2009 ...
A Research Paper on Alzheimer's Disease Words: 1424 Pages: 5 11167. Abstract In this paper, Alzheimer's disease will be delved into, investigated and dissected. This will include all that is known about the disease as much of it is unknown still, despite increasing efforts from the medical community to uncover its origin.
Alzheimer's disease is a form of dementia, which is just an umbrella term used to describe loss of memory, language, problem solving, and other thinking abilities. More specifically, Alzheimer's diseaseis a progressive, neurodegenerative disease that is categorized by a loss of memory, along with basic life skills like eating, bathing ...
Introduction Alzheimer's disease is a devastating neurodegenerative disorder that has become increasingly common in recent years. The disease primarily affects elderly individuals, and it is estimated that nearly six million people in the United States alone are living with Alzheimer's disease. This essay aims to...
For example, a small subset of people with early Alzheimer's disease deteriorate unusually quickly. If these rapid decliners are in the treated group, they could potentially mask a drug benefit ...
Alzheimer's disease (AD) is a prevalent form of dementia leading to memory loss, reduced cognitive and linguistic abilities, and decreased self-care. Current AD treatments aim to relieve symptoms and slow disease progression, but a cure is elusive due to limited understanding of the underlying disease mechanisms. Stem cell technology has the potential to revolutionize AD research.
Introduction. Alzheimer's disease (AD) is a psychological disorder that involves the progressive destruction of brain cells and reduction in the proper functioning of the brain (Altman, 2000). Alzheimer's disease is a type of dementia that is characterized by degeneration of brain functions such as memory, speech, and comprehension.
Scientists are proposing a new way of understanding the genetics of Alzheimer's that would mean that up to a fifth of patients would be considered to have a genetically caused form of the disease.