- Chikungunya
- Hepatitis A
- Hepatitis B
- Hib (Haemophilus influenzae Type B)
- HPV (Human Papillomavirus)
- Japanese Encephalitis
- Meningococcal ACWY
- Meningococcal B
- Pneumococcal
- RSV (Respiratory Syncytial Virus)
- TBE (Tick-borne Encephalitis)
- Varicella (Chickenpox)
- Yellow Fever
- Zoster (Shingles)
- VISs Overview
- DTaP (Diphtheria, Tetanus, Pertussis)
- Hib ( Haemophilus Influenzae Type B)
- Influenza (Inactivated or Recombinant)
- Influenza (Live Intranasal)
- MMR (Measles, Mumps, Rubella)
- MMRV (Measles, Mumps, Rubella, Varicella)
- Multi-Vaccine
- PCV (Pneumococcal Conjugate)
- PPSV (Pneumococcal Polysaccharide)
- RSV Preventive Antibody – IIS
- Smallpox/Monkeypox
- Td (Tetanus, Diphtheria)
- Tdap (Tetanus, Diphtheria, Pertussis)
- VIS Translations Vaccine-specific VISs translated into dozens of languages
- Vaccine History Timeline Notable milestones in immunization going back to 400 BC
- CDC Recommended Schedules Current child/adolescent and adult immunization schedules from CDC; updated annually
- ACIP Vaccine Recommendations From the Advisory Committee on Immunization Practices, as endorsed and published by CDC
- Search All Clinical Resources
- All Patient Handouts
- All Resources for Staff & Providers
- Addressing Vaccination Anxiety
- Administering Vaccines
- Adolescent Vaccination
- Adult Vaccination
- Contraindications & Precautions
- Documenting Vaccination
- Healthcare Personnel
- Managing Vaccine Reactions
- Parent Handouts
- Pregnancy and Vaccines
- Q&As by Diseases and Vaccines
- Schedules for Patients
- Screening Checklists
- Standing Orders Templates
- Storage & Handling
- Strategies & Policies
- Vaccine Confidence
- Vaccine Recommendations
- Vaccine Confidence Overview
- Alternative Medicine
- Alternative Schedules
- Importance of Vaccines
- Improving the Vaccination Experience
- MMR Vaccine
- Religious Concerns
- Responding to Parents
- Unprotected People Stories
- Vaccine Hesitancy
- Vaccine Safety
- Image Library Overview
- Meningococcal
- Vaccination
- Vaccine Manufacturers
- Vaccine Apps
- Email News Services
- Immunize.org Partners
- Webinars & Videos Our library of educational videos and webinar recordings for providers
- Ask the Experts Overview
- View All Questions

Vaccine Topics
- Hib (Haemophilus Influenzae Type B)
- MMR (Measles, Mumps, and Rubella)
General Topics
- Billing & Reimbursement
- Combination Vaccines
- Scheduling Vaccines
- Travel Vaccines
- CDC Recommended Schedules
- ACIP Vaccine Recommendations
- Additional CDC Resources
- Package Inserts & EUAs
- Additional FDA Resources
- Vaccine-Specific Requirements
- State Exemptions
- State Immunization Websites
- Additional State Resources
- WHO Connect to position papers on vaccines and other key vaccine-related resources from the World Health Organization
- About IZ Express
- Current Issue
- Browse All Issues
- Website Updates
- Press Releases
- Official Release Repository Chronological listing of announcements of vaccine licensures, recommendations, and resources from ACIP, CDC, and FDA
- Calendar of Events Immunization-related events, including conferences and training programs from CDC and other organizations, plus upcoming Immunize.org webinars
- Publication Archives A repository of our news and educational publications going back to 1994, including IAC Express (now IZ Express), Needle Tips, Vaccinate Adults, and more
- Board of Directors
- Recognition
- Our Founder
- Publication Archives
- Our History Through Film
- Corporate Membership Program
Patient Safety Honor Rolls
- Influenza Vaccination Honor Roll
- Hepatitis B Birth Dose Honor Roll
- MenB Vaccination Honor Roll
- Honor Rolls Honorees
- The Immunize.org Becky Payne Award
- Immunize.org In the News
Home / Calendar of Events
Calendar of Events

Powered by Events Manager
This page was updated on December 20, 2023 .
Our Affiliated Sites
Jagannath Chatterjee
The eternal problem....
Childhood Illnesses – Road to Health
- Standard childhood illnesses, such as measles, mumps, and even whooping cough, may be of key benefit to a child's developing immune system and it may be inadvisable to suppress these illnesses with immunisations. – Dr Phillip Incao, MD
- Childhood illnesses are a standard feature of childhood because the young body needs them. - Rudolf Steiner, Austrian Scientist
- All acute inflammatory childhood illness--measles, mumps, rubella, chicken pox, scarlatina, or whooping cough--develops the cell-mediated immune system – the deeper immunity
- Childhood illnesses create immunity for life. Vaccines can push diseases to an age where it is dangerous.
Childhood Illnesses Can Prevent Serious Conditions Later in Life
- In early 1997, a team of British physicians writing in Science noted : "Childhood infections may, therefore,�paradoxically protect against asthma.“ These infections�have a purpose in building general immunity.
- Danish physician Tove Ronne in The Lancet in 1985:�"Measles virus infection in childhood can prevent disease in adult life." Among these, Dr. Ronne listed skin disease, immune dysfunctions, degenerative diseases of bone and cartilage, and certain cancers.
- Measles can cure eczema. Induced measles can cause cancer to reduce (remission). Having measles, mumps, rubella in early childhood can prevent against allergies, eczema, asthma , cardiovascular disease mortality and cancer. Polio virus used for cancer remission
- Association of measles and mumps with cardiovascular disease: The Japan Collaborative Cohort (JACC) study.
Kubota Y, et al. Atherosclerosis. 2015. http://www.ncbi.nlm.nih.gov/m/pubmed/26122188/
Childhood Illnesses Protect Against Cancer
- Albonico et al found that adults are significantly protected against non-breast cancers -- genital, prostate, gastrointestinal, skin, lung, ear-nose-throat, and others -- if they contracted measles (odds ratio, OR = 0.45), rubella (OR = 0.38) or chickenpox (OR = 0.62) earlier in life. [ Med Hypotheses 1998; 51(4): 315-20].
- Montella et al found that contracting measles in childhood reduces the risk of developing lymphatic cancer in adulthood [ Leuk Res 2006; 30(8): 917-22].
- Alexander et al found that infection with measles during childhood is significantly protective -- it cuts the risk in half -- against developing Hodgkin's disease (OR = 0.53) [ Br J Cancer 2000; 82(5): 1117-21].
- Glaser et al also found that lymph cancer is significantly more likely in adults who were not infected with measles, mumps or rubella in childhood [ In J Cancer 2005; 115(4): 599-605].
- Gilham et al found that infants with the least exposure to common infections have the greatest risk of developing childhood leukemia [ BMJ 2005; 330: 1294].
- Urayama et al also found that early exposure to infections is protective against leukemia [ Int J Cancer 2011; 128(7): 1632-43].
- In the world of science, it is quite well known that having infections in early life protects against various cancers in later life
Disease Mortality Declined Before Vaccines Introduced – Australian Data . Data from USA, UK, New Zealand & Canada also reflect the same.
Disease Mortality Decline In US (1900-1980) Due to Better Living Conditions, Sanitation, Medical care – JAMA 1999. Vaccines not mentioned. 30 diseases studied. No widespread vaccinations in that period.
US – Major diseases
USA – Diphtheria, Measles
UK & France
Safety of Vaccines Questioned
- A steep rise in cases of Autism (moderate to very severe and mostly permanent disability in children) after more vaccines introduced in 1990’s raised questions over vaccine safety. Parents could know deterioration in children after receiving vaccine shots.
- Doubts raised over use of mercury in vaccines (Thiomersal) and links to Autism. Mercury contamination had led to similar symptoms in Japan and Iraq.
- It became known that Aluminum, Formalin, Polysorbate 80, Phenol, MSG, Neomycin, Squalene, human and animal matter was contained in vaccines.
- More and more parents started reporting vaccination injury and Autism (a severe disorder) after vaccines.
Vaccine Ingredients – a secret well kept
Vaccines and Autism
- Vehemently denied, yet link impossible to hide
- CDC studies trying to disprove link have run into controversies for hiding the link. Scandals during reign of Julie Gerberding
- Scientists openly discussed vaccine autism link – and more dangers – at Simpsonwood (2000) while trying to hide autism link in CDC studies but minutes of meeting were exposed
- Two CDC studies called Verstraeten studies showed very high vaccine autism link. One study was manipulated to show no link and published. The other showing considerable link still lies unpublished
- Another group of CDC studies called Danish studies came crashing down after its Principal Coordinator Dr Poul Thorsen was figured in most wanted list of US Police for having misappropriated the research funds
- Recently the De-Stefano study of CDC came under a storm as its co-author Dr William Thompson revealed data manipulation to hide huge vaccine autism link (340%). Scientists tried to destroy data in 2002.
- CDC now faces Congress investigation. It’s research division “paralyzed with fear”. Congressman Bill Posey submitted on 29 th July 2015
How do vaccines cause Autism?
- As per original CDC study findings, mercury in vaccines is responsible for causing Autism in children. Studies have now shown that mercury even in trace amounts can be responsible
- Independent researchers have also pointed out the clear causative role played by Aluminum present in vaccines
- As per award winning journalist Janine Roberts, the aluminum in vaccines is in form of nano particles so that vaccine ingredients can permeate each cell of the body. Obviously this has a devastating impact on the body.
- Both mercury and aluminum have the ability to cross both blood brain barrier as well as placenta barrier. Thus vaccines given to pregnant women can kick start autism in the infants
- Researchers have also pointed out that the use of animal and human matter in vaccines can cause severe autoimmune reactions and lead to autism
- Vaccines like DPT, DTaP, MMR, Chicken Pox, Hep-B are implicated. Graphs show increase in autism after these vaccines were introduced
Japan – MMR Vac & Autism
Japan – Autism drops as MMR temporarily suspended 1993
AAP Fellow Quits in Protest....
How are vaccines tested for safety?
- Vaccines are tested by manufacturers or persons or agencies under their control. No other agency usually tests them. Phase I to Phase III. Phase IV after release.
- Vaccines are tested on extremely healthy subjects for a very short time (7 to 14) days or till the time adverse events start appearing
- Often serious adverse events are explained away or not reported
- Vaccines are not tested against any genuine placebo. They are tested against the same vaccine without the antigen or some other equally toxic substance
- In India, the Supreme Court and even Parliament has questioned trials but vaccines continue.
- Official vaccinated vs un-vaccinated study never conducted
Vaccines can NEVER be safe for children!
What tests are needed?
- Tests by independent agencies lacking conflict of interest
- Testing against genuine inert placebo and not another vaccine or toxic substance.
- Multiple vaccines given at one visit; never tested!
- Effects of entire vaccine schedule never studied!
- Long term effects never studied! Never studied for causing cancer, infertility, admitted in literature!
- No official studies of vaccinated vs non vaccinated
- Studies required on three generations of mice. Research shows toxicity carried down across generations through breast milk, sperm, epigenetic change
- What is the impact on the human gene? Has it been already altered by vaccines?
1967 study – Trauma of vaccination
Epigenetic Study - 2013
2008: Genes Activated
Independent Vaccinated Vs Non Vaccinated
UK Data – Vaccine Reactions
Adverse reactions – US Data (published MedAlert)
Adverse effects Encountered (MedAlert)
USA, for the year 2013
HPV Vaccines: Deaths now 232 (June 2015)
HPV Vaccine – Reactions Reported
Baby Ian – Allergic Reaction to Hep-B Vaccine
Mercury in Vaccines – Reduced in Developed Nations
- Thiomersal (mercury compound) used in vaccines since 1930. Never tested by FDA. One human study on 21 meningitis patients (1929); all died in short period, attributed to meningitis.
- "As it turns out, we are injecting our children with 400 times the amount of mercury that FDA or EPA considers safe.” - Robert F. Kennedy, Jr. on vaccine-autism-mercury link, 6/22/05
- In June 22, 2000 FDA formally declared that mercury should be removed from vaccines in USA. However mercury still remains as trace amount. Some vaccines still contain mercury in high amounts
- In India and other developing countries full dose mercury remains in all non live virus vaccines �
Mercury – Neurotoxin & Genotoxin
- Mercury -second most poisonous element (second only to uranium). Brain neurons rapidly and permanently disintegrate in the presence of mercury within 30 minutes of exposure. Mercury is known to change a body’s chromosomes. Used as decontaminant in vaccines. Can cross blood brain and placenta barrier
- “Symptoms of high exposure to this class of mercury based compounds include: long term neurological disorders, liver disorders, injuries to the cardiovascular system and hematopoietic system, deafness and ataxia, death.” As genotoxin, mercury is more dangerous in smaller doses.
- Acute poisoning may cause gastrointestinal irritation and renal failure, coordination problems, tremors, mental disorders among many others. Mercury vapour can cause damage.
- Causes Autism as per CDC unpublished study (Relative Risk – 7.6!)
Rat Study – Thiomersal (Mercury Compound in vaccines)
Folia Neuropathol. 2010;48(4):258-69.
Lasting neuropathological changes in rat brain after intermittent neonatal administration of thimerosal.
Olczak M 1 , Duszczyk M , Mierzejewski P , Wierzba-Bobrowicz T , Majewska MD .
Thimerosal, an organomercurial added as a preservative to some vaccines, is a suspected iatrogenic factor, possibly contributing to paediatric neurodevelopmental disorders including autism . We examined the effects of early postnatal administration of thimerosal (four i.m. injections, 12 or 240 μg THIM-Hg/kg, on postnatal days 7, 9, 11 and 15) on brain pathology in Wistar rats. Numerous neuropathological changes were observed in young adult rats which were treated postnatally with thimerosal. They included: ischaemic degeneration of neurons and "dark" neurons in the prefrontal and temporal cortex, the hippocampus and the cerebellum, pathological changes of the blood vessels in the temporal cortex, diminished synaptophysin reaction in the hippocampus, atrophy of astroglia in the hippocampus and cerebellum, and positive caspase-3 reaction in Bergmann astroglia. These findings document neurotoxic effects of thimerosal, at doses equivalent to those used in infant vaccines or higher, in developing rat brain, suggesting likely involvement of this mercurial in neurodevelopmental disorders.
PMID: 21225508 [PubMed - indexed for MEDLINE]
- http://www.ncbi.nlm.nih.gov/pubmed/21225508
“Since excessive accumulation of extracellular glutamate is linked with excitotoxicity, our data imply that neonatal exposure to thimerosal-containing vaccines might induce excitotoxic brain injuries, leading to neurodevelopmental disorders..” – Quoted in subsequent study. (2012) http://www.ncbi.nlm.nih.gov/pubmed/22015977
Thiomersal Bottle – Notice the skull & bones warning
In the USA incidence of autism started to dip as mercury in vaccines were reduced by Order but the flu vaccine with mercury was introduced, and aluminum uptake increased in other vaccines
Aluminum – Cause of brain damage
- Aluminum is a suspected carcinogen. It is a cardiovascular or blood toxicant, neurotoxicant, and respiratory toxicant. It has been implicated as a cause of brain damage, and is a suspected factor in Alzheimer’s Disease, dementia, convulsions, and comas. Aluminum can cross the blood brain barrier .
- It suffers synergistic toxicity with mercury and can increase the toxicity of mercury 100 times. Aluminum and mercury are both present in vaccines.
- In humans, there have been reports of a chronic inflammation syndrome called macrophagic myofascitis (MMF) being induced by alum-based vaccines. Symptoms included myalgias, arthralgias, marked asthenia, muscle weakness, and fever. In cats, this vaccine ingredient causes cancers.�
Aluminum in Vaccines & Autism
Formaldehyde - Carcinogen
- Formaldehyde (Formalin in vaccines) is ranked as one of the most hazardous compounds on ecosystems and human health - Environmental Defense Fund. These findings are for environmental exposure, and therefore, the dangers are much greater for the formaldehyde included in vaccines, since it is injected directly into the blood (should not be). Declared carcinogen-Twelfth Report on Carcinogens (2011).
- Toxic symptoms are more than a hundred. Can cause cancer, asthma, eczema, attention and memory problems, damage to reproductive organs, jaundice, kidney pain, menstrual irregularities, schizophrenia like symptoms, sterility, blood vomiting etc
- Formaldehyde, when present with mercury and aluminum, increases toxicity of mercury 1000 times .
Beats logic..
Package Insert - MMR
Package Insert – Hep B Vaccine
Package Insert – Chicken Pox Vaccine
Some other issues with vaccines
- According to medical texts, infants should not be given anything else except breast milk; not even water. Then how do they allow the extremely toxic vaccine injections? – Dr Suzanne Humphries
- Vaccinated mothers no longer have natural antibodies that they can pass on to children via breast milk. Therefore infants have become more vulnerable creating a vicious cycle
- Vaccines are an industrial product, manufactured for profits. Vaccine markets are created to generate more revenue. Effects on children are overlooked.
- Vaccines are now being mandated in the USA and Australia. This is in gross violation of parental rights and also of the Nuremburg Code and AMA’s own guidelines. The Senators pushing these mandates were exposed,paid by pharma as part of their lobbying
- The CDC of USA which recommends vaccines has a for-profit wing which receives generous funds from vaccine industry. Even WHO depends on industry funding. They cannot be trusted.
Are vaccines effective?
- Vaccines are supposed to produce humoral (blood related antibodies) that theoretically protect us against infections? Is this theory correct?
- It is known that, in many instances, antigen-specific antibody titers do not correlate with protection. In addition, very little is known on parameters of cell-mediated immunity which could be considered as surrogates of protection. (Del Giudice G et al, 2001)
- In a study published in Neurology it was demonstrated that titers (the measurement of the levels of antibodies in the bloodstream) were poor indicators of immunity. (Nathan E Crone et al, 1992)
- A titer test does not and cannot measure immunity, because immunity to specific viruses is reliant not on antibodies, but on memory cells – Expert testimony before European Court of Human Rights, 2006
Vaccine Failures!
- Diseases vaccinations are supposed to prevent regularly occur in fully vaccinated populations.
- A measles outbreak in early 1989 among approximately 4200 vaccinated students at a high school and two intermediate schools in suburban Houston, TX, was investigated to evaluate reasons for vaccine failure. (Pubmed)
- Mumps Outbreaks in Vaccinated Populations: Are Available Mumps Vaccines Effective Enough to Prevent Outbreaks? (Pubmed)
- Disease outbreaks are concentrated in highest-vaccinated population – Council on Foreign Relations Graph
- Whooping cough outbreaks are HIGHER among vaccinated children compared with unvaccinated children- Dr. David Witt, infectious disease specialist at the Kaiser Permanente Medical Center in California.
- In India measles vaccine is failing to prevent measles - Report �
Vaccines increase susceptibility!
- Vaccinated individuals are often made more susceptible to the diseases they are vaccinated against and are more likely to die from it.
- Dr R P Garrow reports in the British Medical Journal, Jan 14, 1928, that a person vaccinated against Small Pox was five times more likely to die of the disease than the unvaccinated.
- He also reported, the vaccination was causing a great surge in cases of Small Pox in areas where it was widely given
- Using RTI data doctors in India reported that children given the Oral Polio Vaccine are 6.26 times more likely to suffer paralysis than the unvaccinated.
- Measles after vaccination is more deadly.
Vaccines Cause Serious Side Effects
- The DaPT vaccine Tripedia has listed Autism and ADHD as a side effect. The DPT vaccine is linked to asthma, provocative polio, hyperactivity and learning disorders in children. A 1948 study by Dr Byers et al linked it to deaths, blindness, deafness, spasticity, convulsions, and other severe neurological disorders.
- The Hepatitis B vaccine has been linked to serious adverse effects including cancer of liver. Combination vaccines have more adverse effects; MMR, DPT, Pentavalent etc
- Vaccines are known to cause- Allergies * Asthma * Attention Deficit Disorder *Autism * Auto-immune Diseases * Blindness * Brain Cell Loss *Cancer * Central Nervous System Damage* Deafness * Developmental Damage *DEATH * Diabetes * Epilepsy * Learning Disabilities * Leukemia * Multiple Sclerosis * Neurological Disease * Organ Disease * Psoriasis * Seizures * Shaken Baby Syndrome * SIDS * Total Paralysis * All Diseases in Internal Medicine
- All vaccines can do harm – Dr Andrew Moulden, MD. Vaccines can never be safe – Dr Suzanne Humphries, MD
200 adverse effects compiled from published research papers � http://www.greenmedinfo.com/blog/200-evidence-based-reasons-not-vaccinate-free-research-pdf-download
Vaccine victims are mocked..
Vaccines being opposed by Dr’s and Medical Scientists in India
- BCG – Tested in 15 year trial in India. 0% effective!
- Hep-B vaccine – Meant for promiscuous adults in developed nations, now given to children in developing countries because they refused
- Oral Polio Vaccine – Known to cause polio; both individual cases and epidemics. Court case filed (CCF).
- Pentavalent vaccine – Very high death rate & hospitalization in all countries introduced. CCF
- HPV vaccine – Trial in India conducted unethically killing 7 (9?) tribal adolescent girls. Supreme Court
- Rota Vaccine – Rota diarrhea can be controlled by ORS. The vaccine has serious adverse effect. Recent Indian vaccine to be launched hiding trial data. CCF
How vaccines ‘eradicate disease’
- Disease rates are inflated 100 to 1000 times before vaccines are introduced
- Disease definition is changed when vaccines are introduced making it difficult to report the disease
- Doctors are advised that, ‘vaccinated children cannot come down with the disease vaccinated against’. So they change disease names when such cases come up
- Symptoms (for the same disease) are bifurcated to create new disease names and show drop in cases
- Pathology samples can be checked in only pre-selected laboratories so that positive cases can be suppressed
- Low level health workers or officials cannot report disease, they have to go through ‘experts’ who can then suppress cases
How Polio Was “Eradicated”
- 32,419 cases of the disease were inflated to 3,50,000. In India the figure was 12,000
- Definition of polio was changed three times to bring down number of cases
- A disease name called “Non Polio Acute Flaccid Paralysis” was created to bifurcate polio figures. Polio is traditionally infantile paralysis. It was changed to viral polio. Earlier 20 days duration was required to identify polio, it was increased to 60 days.
- Pathological tests could be carried out only in select WHO certified laboratories
- Cases of polio caused due the vaccine were suppressed
- Paralysis in children increased from 1005 in 1996 to 60,992 in 2012 after India was declared polio free!
Polio Vac – Controversial since 1955!
Oral Polio Vac and Cancer!
Polio Incidence & Pesticide (DDT)
Change in Polio Definition
- Traditionally infantile paralysis of any kind lasting more than 24 hours was recorded as polio
- After vaccine introduced only polio caused by 3 enteroviruses were considered polio. Other paralysis was given different names
- Later paralysis had to last more than 20 days
- This was increased to 60 days
- In addition, stools had to confirm presence of PV
- Only WHO accredited laboratories can confirm
- Polio caused by vaccine or vaccine strain virus turned virulent cannot be called polio
- Polio recorded as “Non Polio Acute Flaccid Paralysis”
- “Clinically indistinguishable and twice as deadly” –Dr’s
Polio – Controlled by vaccine?
You think Small Pox Was Eradicated by Vaccines?
- The practice of inoculation (against small pox) manifestly tends to spread the contagion, for a contagious disease is produced by innoculation where it would not otherwise have produced. The Gentleman’s Magazine and Historical Chronicle, vol.34, 1764,p.333
- In the 38 years after the start of innoculation, deaths from small pox relative to the number born increased to 127 per 1,000 (a 41 % increase) and relative to the number of burials (deaths) 81 per 1,000 (a 27% increase). The Great Small Pox Epidemic of 1775-82, History Today, July 20, 2003, p.12
- Since the late 1700s, the medical profession has supported vaccination without comparing vaccinated and unvaccinated. MMWR, vol.50, CDC, June 22, 2001, pp1-25
- ...the level of antibody that protects against small pox is unknown. ibid
Why did Gandhi revolt against vaccines?
- Gandhiji revolted against vaccines and declared it a ‘filthy process’. Why?
- “When we recall that vaccine lymph is derived, in the first place, either from a small pox corpse, the ulcerated udder of a cow, or the running sores of a sick horse’s heels...it has far reaching ill effects on the human constitution”. Studies in Vaccinia, The Lancet, vol. 1999, no.5150, May 13, 1922
- No practitioner knows whether the lymph he employs is derived from small pox, rabbit pox, ass-pox or mule pox. Ibid. (What viruses were in the vaccines? Even CDC does not know! Admitted after genetic assay of Dryvax)
- Some of the animals that have been used to passage today’s vaccine virus include rabbits, mice, goats, cows, pigs, horses, sheep, dogs, birds and humans.�
Small Pox Vaccine & Cancer
What else did the vaccine cause?
- “In these cases it is highly likely that acute infectious hepatitis was a result of contamination of human lymph derived vaccine. Other infectious diseases attributed to vaccination includes tuberculosis & syphillis” New York Times, Sept 26, 1869
- In the year 1981, a WHO consultant released to the media, that his investigation into the AIDS epidemic under the instruction of WHO, clearly revealed that HIV spread to humans from primates due to the small pox vaccine. WHO suppressed the report.
- The vaccine was linked to eczema vaccinatum with a fatality rate of 4-40%. Also linked to erisypelus epidemics which had high mortality rates. CNS damage.
- Huge epidemics of small pox in 1872-73 (Boston) started AFTER introduction of vaccine.
Leicester Small Pox Spike After Mandatory Vaccination. This UK state eliminated small pox with isolation, sanitation and nutrition
Testimonial of a Nobel Prize Winner
Blind Belief in Doctors – Not Recommended!
Educate before you vaccinate!
- Neither government or doctors will inform you about vaccine dangers
- If your child dies, becomes disabled or diseased it will be denied by authorities and marked ‘coincidence’
- You will not receive any free treatment, compensation or any rehabilitation help for your child
- The very doctor who vaccinated your child will become your enemy
- Doctors receive commissions, political parties receive funds, bureaucrats receive perks and promotions. You gain a dead, sick or disabled child!
- As per a law signed into effect in the USA in 1986 vaccine manufacturers and even individual vaccines are protected from law suits
- Vaccine industry & doctors protected, not your child!
Profitable industrial product
To Protect Your Child
- Ensure adequate age for marriage
- Provide proper nutrition and care for pregnant mother
- Homeopathic treatment during pregnancy will ensure a healthy child. Search for senior homeopath.
- Avoid drugs and vaccines during pregnancy. The tetanus vaccine being given during pregnancy is also used to sterilize girls and women in developing nations (used in India, Kenya). It can cause abortions and premature birth.
- Go in for natural child birth. Delay cord clamping. Do not clean child immediately after birth. Learn from natural birth movement. Aisharwaya Rai Bachhan did this!
- Give the first yellow milk – colostrum- to your child. You can breastfeed up to 2 years & beyond for benefits and emotional bonding
- Give baby homeopathic treatment during illnesses
What do other systems recommend?
- Ayurveds flay village practice of giving even a drop of honey to infants
- Homeopathic texts talk about giving medicines to mothers so that children get it through breast milk
- Pregnancy, infancy and childhood are to be respected
- General practitioners (GP’s) were wary about medicating these groups. Vaccination was selective based upon child’s health. Many also conducted skin tests before administration. Seniors were more cautious and even skipped them if they perceived no threat
- Things changed after paediatricians arrived on the scene. They are reckless about vaccination and prescribing antibiotics etc for resultant conditions
Role of civil society
- Medicine is too important to be left at the hands of doctors. Civil society should not forget its watchdog role
- Civil society representatives should be a part of the National Technical Advisory Group on Immunizations that advises GoI on vaccinations
- The civil society should sensitize doctors on vaccine dangers. The industry keeps them in the dark
- Awareness about the murky world of vaccines ought to be created among the general populations
- Should demand a vaccine adverse event reporting system with compulsory reporting and monitor the same and seek compensation and rehabilitation for victims
- Vaccines crimes need to be referred to civil and criminal courts. Protecting vaccines is the goal of medical boards.
Be scientific...
Vaccine Information Websites
- www.nvic.org
- www.sanevax.org
- www.mercola.com
- www.greenmedinfo.com
- www.vaccineresearchlibrary.com
- www.vactruth.com
- www.vaccinetruth.org
- www.currenthealthscenario.blogspot.in
- www.vaccinationinformationnetwork.org
- www.naturalnews.com
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- GMS J Med Educ
- v.37(4); 2020

Language: English | German
Evaluation of a vaccination seminar in regard to medical students' attitudes and their theoretical and practical vaccination-specific competencies
Evaluation eines impfseminares im hinblick auf einstellungen sowie theoretische und praktische fähigkeiten von medizinstudierenden.
1 Universitätsklinikum Frankfurt, Betriebsärztlicher Dienst, Frankfurt a. M., Germany
Björn Steffen
2 Universitätsklinikum Frankfurt, Zentrum der Inneren Medizin, Hämatologie und Onkologie, Frankfurt a. M., Germany
Sabine Wicker
Aim: Despite having a generally positive attitude toward vaccinations, medical students show gaps in their own immunization histories and knowledge about vaccinations. Future practicing physicians will be confronted with the need to evaluate protective immunity and make vaccination recommendations. This study aims to investigate the extent to which a seminar on the topic of vaccination can improve students’ attitudes, knowledge and practical skills in interpreting vaccination certificates.
Project description: Two different one-hour seminars were developed and integrated into the required clinical curriculum. A third of the students attended a theory-based seminar; the other two-thirds completed a predominantly practice-based seminar. The theoretical seminar consisted of a lecture on the principles and theoretical aspects of immunization. In the practical seminar, the curricular content was case-based and taught using fictive examples of vaccination certificates. Before the seminar was held, a voluntary and anonymous survey of the students was conducted regarding their attitudes toward and knowledge of immunization. At the conclusion of the seminar, the students’ ability to understand vaccination certificates was tested. After completing the seminar, all of the participants received a link to participate in a voluntary online survey to evaluate the seminar.
Results: Of the 149 seminar attendees in the 2017/18 winter semester, 148 participated in the study.
Attitude: Students have a positive attitude toward vaccinations. Regardless of the type of seminar attended, the agreement with statements on vaccination could be significantly increased primarily among students who already at the start of the seminar expressed a high degree of agreement. Students vaccinated against influenza showed significantly stronger agreement than unvaccinated students.
Knowledge: Regardless of teaching format, students’ knowledge about vaccination topics could be increased. For those vaccinated against influenza, the mean value for agreement with the statement, “The vaccination of healthcare workers prevents nosocomial transmission of diseases,” saw an increase on a five-point Likert scale from 3.97 to 4.4 (p<0.001; R=0.67). For the unvaccinated students, the mean value rose from 4.04 to 4.19 (p=0.06; R=0.29).
Practical skills: The students who attended the theory-based seminar tended to score higher on interpreting vaccination certificates than those who attended the practical seminar; however, this difference was not statistically significant.
Seminar evaluation: The online evaluation was completed by 18% of the participants. The theoretical seminar received the grade of 2.9 based on the conventional German academic grading scale; the practical seminar received 1.9. This difference is statistically significant (p=0.02).
Conclusion: Precisely for skeptical students it was only possible to minimally change existing views with a seminar that offers very brief instruction. Attendees of the theoretical seminar tended to score somewhat higher on interpreting vaccination certificates than those who took the practical seminar. The practical seminar was rated significantly better on the course evaluation than the theoretical one. The advantage that the students attending the theoretical seminar had can be explained best by the structured review of the current vaccination recommendations as part of the seminar, which should, as a consequence, be integrated into the practical seminar.
Zusammenfassung
Zielsetzung: Trotz der generell positiven Einstellung von Medizinstudierenden gegenüber Impfungen sind ihre Impfquoten und ihr Wissen zu Impfungen lückenhaft. In der ärztlichen Praxis werden die zukünftigen ÄrztInnen mit der Beurteilung von Impfschutz und Impfempfehlung konfrontiert sein. In dieser Studie sollte untersucht werden, inwieweit ein Seminar zum Thema Impfen Einstellungen, Wissen und praktische Fertigkeiten der Studierenden im Umgang mit dem Impfpass verbessern kann.
Projektbeschreibung: Zwei verschiedene einstündige Impfseminare wurden entwickelt und in das klinische Pflichtcurriculum integriert. Ein Drittel der Studierenden besuchte ein theoretisches Seminar, die verbleibenden zwei Drittel absolvierten ein überwiegend praktisch aufgebautes Seminar. Das theoretische Seminar bestand aus einem Vortrag zu Grundlagen und theoretischen Aspekten des Impfens. Im praktischen Seminar wurden die Lehrinhalte fallbezogen anhand fiktiver Beispiel-Impfpässe vermittelt. Vor Seminarbeginn erfolgte eine freiwillige und anonyme Befragung der Studierenden zu Einstellungen und Wissen zum Thema Impfungen, am Kursende wurden die Fähigkeiten im Umgang mit dem Impfpass überprüft. Nach Abschluss des Kurses wurde an alle Teilnehmer ein Link zu einer freiwilligen Online-Evaluation des Kurses versendet, in der die Studierenden den Kurs bewerten sollten.
Ergebnisse: Von den 149 SeminarteilnehmerInnen des Wintersemesters 2017/2018 nahmen 148 an der Studie teil.
Einstellung: Studierende sind Impfungen gegenüber positiv eingestellt. Unabhängig vom besuchten Kursformat konnte die Zustimmung zu Impfthemen vor allem bei Studierenden, die bereits zu Seminarbeginn einen hohen Zustimmungsgrad aufwiesen, signifikant gesteigert werden. Gegen Influenza geimpfte Studierende wiesen eine signifikant höhere Zustimmung auf als nicht geimpfte.
Wissen: Unabhängig vom Lehrformat konnte das Wissen der Studierenden zu Impfthemen gesteigert werden. In der Gruppe der Influenza-geimpften Studierenden stieg der Mittelwert der Zustimmung zur Aussage „Die Impfung von Gesundheitspersonal verhindert die nosokomiale Übertragung von Krankheiten“ auf einer fünfstufigen Skala vom Likert-Typ von 3,97 auf 4,4 (p<0,001; R=0,67). Bei den nicht geimpften Studierenden stieg der Mittelwert von 4,04 auf 4,19 (p=0,06; R=0,29).
Praktische Fähigkeiten: Die TeilnehmerInnen des theoretischen Seminars schnitten im Umgang mit dem Impfpass tendenziell besser ab als die TeilnehmerInnen des praktischen Seminars, der Unterschied war jedoch nicht statistisch signifikant.
Kursevaluation: Die Online-Evaluation wurde von 18% der TeilnehmerInnen abgeschlossen. Das theoretische Seminar wurde im Durchschnitt mit der Schulnote 2,9 bewertet; das praktische Seminar mit der Note 1,9. Dieser Unterschied ist statistisch signifikant (p=0,02).
Schlussfolgerungen: Gerade bei skeptisch eingestellten Studierenden können bestehende Ansichten durch ein Seminar mit recht kurzer Unterrichtsdauer nur geringfügig verändert werden. TeilnehmerInnen des theoretischen Seminars schnitten im Umgang mit dem Impfpass tendenziell etwas besser ab als die TeilnehmerInnen des praktischen Seminars. Das praktische Seminar wurde signifikant besser bewertet als das theoretische. Der Vorsprung der TeilnehmerInnen des theoretischen Seminars im Bereich der praktischen Fertigkeiten erklärt sich am ehesten durch die strukturierte Rekapitulation der aktuellen Impfempfehlungen im Rahmen des Seminars, die folglich auch in das praktische Seminar integriert werden sollte.
1. Introduction
As future physicians, medical students will be responsible for imparting pertinent information and administering vaccines to patients. Physicians have a significantly higher credibility with patients when it comes to positive health behaviors if it is known whether or not they themselves are vaccinated [ 1 ]. There is greater probability that physicians who have been vaccinated against influenza will recommend the influenza vaccine to their patients than physicians who have not been vaccinated [ 2 ], [ 3 ]. The medical recommendations for a certain vaccine is in many cases significantly associated with the actual administration of that vaccine or at least the intention to receive it [ 4 ], [ 5 ], [ 6 ], [ 7 ], [ 8 ]: A higher percentage of the patients of physicians who are vaccinated against influenza are vaccinated against influenza than are the patients of unvaccinated physicians [ 8 ]. Despite the generally positive attitude of medical students toward vaccinations [ 9 ], their own protective immunity has gaps [ 10 ], [ 11 ], [ 12 ], [ 13 ], [ 14 ], [ 15 ], [ 16 ], [ 17 ], [ 18 ], [ 19 ], [ 20 ], [ 21 ] and does not have the necessary percentages to achieve herd immunity [ 22 ]. The National Catalogue of Competency-based Learning Objectives for Undergraduate Medical Education (NKLM), which was adopted in 2015, provides for interdisciplinary teaching of vaccination topics [ http://www.nklm.de ]: At graduation, medical students should, among other things, be able to weigh the risks and benefits of vaccinations, know the indications and contraindications, advise patients regarding immunization and administer vaccinations. The professional approach to immunization is based on three pillars: theoretical knowledge, practical skills and communication skills to inform about the importance of vaccinations and acknowledge the doubts and uncertainties of parents and patients. Medical students‘ knowledge of vaccinations is, however, incomplete [ 23 ], [ 24 ], [ 25 ]. Depending on the survey, only 39.8% to 77.9% of students are familiar with the general recommendations for healthcare workers regarding influenza vaccination [ 10 ], [ 25 ], [ 26 ]. Practical and communication skills in respect to the topic of vaccination are covered only late or not at all in a medical degree program.
At the medical school of the Goethe-Universität in Frankfurt am Main, the different aspects of immunization are integrated into many different courses. For instance, the scientific principles underlying immune system function are taught in the preclinical subjects of biology, biochemistry and physiology. During the clinical phase of study, additional theoretical bases for vaccination are included in lectures on microbiology and virology, general practice, internal medicine, and pediatrics. Focus is placed here on vaccine-preventable diseases, the types of vaccines and the corresponding vaccination schedules. However, when practicing medicine, a physician will be confronted with more or less well-documented vaccination certificates. The interpretation of such vaccination histories and the resulting determination of immune status and which vaccines are needed is not part of the medical curriculum.
Afonso et al. [ 27 ] have already been able to show that a two-hour, interactive vaccination seminar for first-semester medical students leads to a significant improvement in attitude toward the topic of immunization: Agreement with the statement, “It is important to be vaccinated against Influenza” , increased from 71% to 93% (p<0.01). Also, it was seen that students who were vaccinated against influenza viewed the influenza vaccination as more important than their unvaccinated peers did. In contrast to this US study, our study focuses on medical students at more advanced semester levels. Instead of administering vaccines, the practical focus was placed on making sense of vaccination certificates. The aim of our study was to investigate the extent to which a newly implemented vaccination seminar at the Goethe-Universität can contribute to improving students’ attitudes, knowledge, and practical skills in terms of understanding vaccination certificates. Furthermore, we wished to clarify if a theory-based seminar or a predominantly practice-based seminar is better suited to achieve this.
2. Project description
The vaccination seminar was integrated into the mandatory medical curriculum in cooperation with the Center for Internal Medicine (Zentrum der Inneren Medizin). Students complete a three-week-long block practicum in Internal Medicine during the second or third semester of clinical study. The first week of this practicum is the “central instruction week” and held as a seminar with practical exercises (e.g. ECG, sonography, doctor/patient consultations, evaluations of findings). Each semester, approximately 150 students attend this central week of instruction which takes place in small groups. For each small group, a one-hour time slot was found in which to hold the vaccination seminar. Due to the time constraint, it was necessary to limit the learning objectives and seminar content. Since administering vaccines is covered later in the block practicum on General Practice, we did not cover this topic in our seminar.
The following learning objectives were chosen:
- Students know the current vaccination recommendations of the STIKO (Standing Committee on Vaccination at the Robert Koch Institute in Berlin) and the different vaccination categories (standard, booster, indicated) and can recite this information;
- Students can identify indicated vaccines based on a vaccination certificate and correctly document an administered vaccine.
During the 2017/18 winter semester, 34.2% of the students were taught in a theory-based seminar; the remaining 65.8% in a practice-based seminar. The theoretical seminar consisted of a lecture on the principles and theoretical aspects of immunization, contraindications, side effects, and possible complications. The STIKO recommendations, communicating with patients about vaccinations, and correct documentation of a vaccination were also covered in this seminar. In the practice-based seminar, the learning content was imparted using four fictive patient cases reflecting different vaccination-related concerns. The students were given a fictive vaccination certificate and the corresponding patient’s medical history. Groups of three to four students were assigned the task of evaluating the status of the patient’s immunity based on the STIKO recommendations. Figure 1 (Fig. 1) contains one of the example patient cases used in this seminar.

Anamnesis: Polly Ester is 30 years old. She would like to get pregnant and wants to have her health status checked so as not to cause damage to the child. As a child she had a strong reaction to a vaccination, the reason why her mother did not allow her to be immunized further. Polly Ester can no longer recall which vaccination caused the reaction or how old she was when it happened.
Task: Does the patient have a complete standard immunity? If not: Which catch-up vaccinations need to be given to achieve the standard protective immunity? Are additional vaccinations necessary or a good idea?
Student surveys were conducted at different time points – before and directly after the seminar and after conclusion of the first week of the practicum; an overview is presented in figure 2 (Fig. 2) . At the beginning of the seminar a voluntary and anonymous student survey was conducted using a questionnaire (“pre-test”). This pre-test (see figure 3 (Fig. 3) ) asked for demographic information (age, gender), influenza vaccination status in the 2017/18 flu season, and questions about the three focal areas of this study (attitude, knowledge, practical skills). A total of four questions were asked about attitude toward vaccinations (see figure 3 (Fig. 3) , questions 6a-6c, 6e); the responses were given on five-point or seven-point Likert scales. Questions were also asked about the reasons for and against an influenza vaccination (see figure 3 (Fig. 3) , questions 4, 5). The pre-test contained a question on knowledge (see figure 3 (Fig. 3) , questions 6d): Students were asked to rate their level of agreement with the statement, “The vaccination of healthcare workers prevents nosocomial transmission of diseases,” on a five-point Likert scale. To gather data on practical skills prior to the seminar, students were asked to assess their own practical skills regarding immunization (e.g. ability to identify necessary vaccinations on the basis of a vaccination certificate) using the conventional German academic grading scale (see figure 3 (Fig. 3) , questions 7a-d). The “post-test” was administered after completion of the seminar (see figure 4 (Fig. 4) ); the same questions were asked again about attitude and knowledge. To measure practical skills, a fictive vaccination certificate was also handed out as part of the post-test. Students were asked to determine which series of standard immunizations had not been fully completed and to identify additional indicated vaccines for the fictional patient (a secondary school graduate prior to beginning a nursing internship). The answers were recorded using single- or multiple-choice responses. As a final question, students were asked if they now felt more confident in understanding and interpreting vaccination certificates. A brief evaluation was conducted at the conclusion of the seminar giving students the opportunity to rate the seminar based on the German grading scale. A link to the voluntary online evaluation was then sent to the participants after conclusion of the first practicum week. Within the scope of this final survey, there was a more detailed evaluation of the seminar (e.g. relevance to future medical practice);table 1 (Tab. 1) contains the questions of the online evaluation.

Statistical analysis was performed using the software program “BiAS.” for Windows (program version 11.02). The test methods applied include: chi 2 test, Mann-Whitney U test, Wilcoxon matched pairs test, Spearman rank correlation, Friedman test.
An “agreement score” was calculated to better analyze the questions about attitude by adding the individual point values for the responses on the Likert scale to yield a score that reflects the agreement with the vaccination topic; values ranged from a minimum of 4 to a maximum of 22 points. This score is not to be confused with the “overall attitude” of the students which represents a subjective assessment by the students (response to the statement: “Overall, I am completely for/mostly for/more for/neutral/more against/mostly against/completely against vaccination” ). To better differentiate between attitudes, the response options were placed on a seven-point Likert scale, rather than a five-point one.
To analyze students’ skill in handling vaccination certificates, a “vaccination certificate score” was calculated, with a possible maximum of 12 points. To calculate this, a point was given for each correctly ticked box on the two post-test questions about a specific vaccination certificate; a point was also given for each box that was correctly left unticked. The scores were developed in collaboration with a colleague at the Institute for Biostatistics and Mathematical Modeling at the Goethe-Universität Medical School.
Of the 149 seminar attendees, 148 participated in the study (99.3%). Table 2 (Tab. 2) provides an overview of the participants’ demographic data and their vaccination status. The distribution according to gender, age groups, and those vaccinated against influenza did not significantly differ from each other in the two seminar formats.

3.1. Attitude

Subjective self-assessment of the students before and after the seminar (response to the question: “Overall I am completely for/mostly for/more for/neutral/more against/mostly against/completely against vaccination”). n=147

To better analyze attitudes toward the topic of vaccination, an “agreement score” was calculated from the responses to the four questions about attitudes by adding the individual point values of the responses on the Likert scale to yield a score that reflects the agreement with the vaccination topic, with values ranging from a minimum of 4 to a maximum of 22 points.
3.2. Knowledge
Students‘ knowledge of vaccination topics could be increased by the seminar, regardless of format, age, gender or influenza vaccination status: In the pre-test, 115 students (79.3%) completely agreed or mostly agreed with the statement, “The vaccination of healthcare workers prevents nosocomial transmission of diseases” ; in the post-test the number was 128 students (86.4%) (see table 5 (Tab. 5) , section a). In the pre-test there was a mean value of 4.0 on the five-point Likert scale; in the post-test the mean value was at 4.28. This difference is statistically significant with p<0.001. For the group of vaccinated students, agreement with this statement increased more strongly than for the unvaccinated group (see table 5 (Tab. 5) , section b): The mean value for agreement on the five-point Likert scale increased from 3.97 to 4.4 (p<0.001; R=0.67). For the unvaccinated students, the mean value increased from 4.04 to 4.19 (p=0.06; R=0.29).

3.3. Practical skills
The students assessed their skills in handling vaccination certificates as being “good” to “satisfactory.” The assessment of whether they could evaluate a vaccination certificate or identify the missing vaccinations was significantly (p<0.001) poorer than their assessment of other skills (administering and documenting a vaccination). The self-assessments of the students aged at least 25 years old and the students vaccinated against influenza were in part significantly better than the self-assessments of the younger or unvaccinated students (see table 6 (Tab. 6) ). In the post-test the students achieved an average vaccination certificate score of 8.76 points (SD 1.36). Table 7 (Tab. 7) shows the vaccination certificate score for the different groups. Participants in the theory-based seminar tended to score better with an average of 8.92 points (SD 1.34) than participants in the practice-based seminar (8.68 points; SD 1.38); however, this difference was not statistically significant (p=0.36). Age and influenza vaccination status also showed no significant influence on the vaccination certificate score. Participants who attended the practice-based seminar made mistakes significantly more often (p=0.04) than participants in the theory-based seminar and misidentified primary vaccinations as missing when they were not.

3.4. Self-assessment
No student felt less confident after the seminar than before when it came to understanding and working with vaccination certificates. A total of 72.6% felt a little more confident and 17.8% much more confident than before the seminar. Participants who attended the practical seminar felt more confident after the seminar than participants who attended the theoretical seminar (p<0.01). There was no correlation between a perception of greater confidence in handling vaccination certificates and the vaccination certificate score. Furthermore, contradictory results were visible regarding the self-assessment and the actual skills demonstrated: The participants who attended the practice-based seminar misidentified primary vaccinations as missing when they were not, despite having a greater feeling of confidence. Still, students with a high level of confidence recommended significantly fewer vaccinations that were not in fact indicated (see figure 7 (Fig. 7) ).

Given in percentages. Participants in the theory-based seminar misidentified vaccines as missing on an average of 0.41 (SD 0.67), even though they had been given. Participants in the practice-based seminar misidentified vaccines as missing on an average of 0.72 (SD 0.87), even though they had been given. This difference is statistically significant with p=0.04.
3.5. Evaluation
The online evaluation was completed by 27 students (18% of participants). Sixteen (59.3%) attended the practice-based seminar and 11 (40.7%) the theory-based seminar, a distribution which corresponds with the assignment of the students to the two teaching formats. The participants in the theoretical seminar gave an average rating of 2.9 (SD 1.0; median 3); the practical seminar was rated on average with 1.9 (SD 0.7; median 2). This difference is statistically significant (p=0.02; R=0.5; Mann-Whitney U test).
4. Discussion
To our knowledge, this study is the first of its kind in the German-speaking countries to investigate the influence of a vaccination seminar on the attitude, knowledge and practical skills of medical students. In 2011/12, Afonso et al. [ 27 ] held a two-hour vaccination seminar for first-semester medical students and were able to significantly improve the students’ attitude toward the topic of immunization as a result. It was also shown that students who were vaccinated against influenza viewed the influenza vaccine as more important than their unvaccinated peers did. In contrast to this US study, our study focused on medical students in the later clinical phase of study. The practical focus was placed on understanding and using vaccination certificates. Our study was also able to determine an improvement in the attitude of medical students toward the topic of vaccination. Since at the beginning of the seminar 92.5% of the students were already overall “mostly for” or “completely for” vaccination, the increase was not so dramatic than that seen in Afonso et al. A significant improvement could be shown only for the group of students who already had a high level of agreement with pre-formulated statements at the beginning of the seminar. Our study also shows that students who are vaccinated against influenza have a significantly higher level of agreement with pro-vaccination statements than unvaccinated students. The attitude of unvaccinated students improved significantly as result of the seminar, but remained behind the attitude of the students who were vaccinated against influenza. Influenza vaccination status can be seen as an expression of a positive attitude toward vaccinations [ 28 ]: Vaccinated individuals view protective immunity against influenza as important enough to make the effort to get vaccinated. Conversely, having a positive attitude toward vaccination does not automatically result in getting vaccinated against influenza: Over 90% of the students have a very positive attitude toward immunization, but only less than half are vaccinated against influenza. In 1975 Ajzen & Fishbein first described their theory of reasoned action postulating that human behavior is determined by intention to act which, in turn, is influenced by personal attitudes and social norms [ 29 ]. The discrepancy between the intended and actual behaviors is referred to as the intention-behavior gap [ 30 ]. There are probably different reasons for the discrepancy uncovered in our study between having a positive attitude toward vaccination and actual influenza vaccination behavior on the part of the students. A survey conducted by Petersen et al. showed, for instance, that only 46.4% of the surveyed students knew of the general recommendation that healthcare workers be vaccinated against influenza [ 10 ]. Many students judge the importance, safety [ 31 ] and efficacy [ 32 ], [ 33 ] of the influenza vaccination as deficient.
The data in this study allow speculation that students’ gain in knowledge is higher if at the beginning of the seminar they already have a positive attitude: The agreement with the factually correct statement, “The vaccination of healthcare workers prevents nosocomial transmission of diseases,” could be increased by the seminar. A significant increase could be observed among the students who were vaccinated against influenza; in the group of unvaccinated students the increase in agreement with this statement was not significant. The practice-based seminar was rated significantly better than the theory-based seminar and the content was viewed significantly more often as being relevant to future medical practice. Participants who attended the theory-based seminar tended to score higher on understanding vaccination certificates than those who attended the practice-based seminar. This is possibly due to the fact that it is easier for students to recall knowledge if it has been previously reviewed by means of structured teaching. A review of the current vaccination recommendations took place during the theoretical seminar; in the practical seminar the recommendations were handed out but not discussed.
The percentage of women in the theory-based seminar was 76.5% and 60.8% in the practical seminar. No statistically significant difference in connection with the gender distribution between the two course formats was found using the chi 2 test (p=0.08). Since the agreement score in the pre-test did not differ between the genders, we are not assuming an influence of gender on the different results for the two seminar formats.
Although the participants attending the practice-based seminar felt significantly more confident after the seminar than the participants attending the theoretical seminar, they did not score higher when it came to interpreting and understanding the vaccination certificate. No correlation was found between a high degree of perceived confidence with vaccination certificates and the vaccination certificate score; however, students with a greater feeling of confidence recommended non-indicated vaccinations significantly less often. These results are indeed contradictory. One explanation for this could be a lack in the students’ ability to self-assess, although a weakness in the questionnaires concerning the questions on practical skills cannot be ruled out.
Limitations and strengths of the study
At the beginning of the seminar the students were informed and asked to fill out the survey. In the case that students were not interested in participating, they were asked to turn in the survey at the same time as the willing participants to ensure the voluntary and anonymous nature of the survey. Despite this, it cannot be ruled out that responses were given in an effort to produce the socially desired ones. To gain the most unbiased picture possible of the actual attitudes of students, multiple surveys were conducted and a score was calculated based on all of them. A biased depiction of the actual attitude is, however, still conceivable. For reasons pertaining to data protection, the seminar evaluation had to take place as an online survey and was not integrated into the official course evaluation for the first week of the block practicum. This was certainly disadvantageous for the response rate: Only 27 students (18% of participants) completed the online evaluation. Among the strengths of this study is the required participation of all medical students in a vaccination seminar. As a result, it was possible to capture a comprehensive view of student attitudes and knowledge. An elective vaccination seminar would probably have been attended primarily by students interested in the topic of immunization and would have yielded even more positive attitudes than are actually present in the full student cohort.
5. Conclusion
The observations of this study lead to the assumption that among medical students at the time of the seminar there were already fixed views on vaccinations that can only be changed minimally by a seminar with limited time for instruction. Questions on personal views are often loaded with emotional components. Precisely with the topic of immunization is this strongly pronounced, something which is repeatedly evident in public debates between those for and those against vaccination [ 34 ]. It must be assumed that, even among medical students who view immunization skeptically, the opinions and stances on topics related to vaccination are grounded more in emotion than in science. There is a discrepancy between the positive attitudes and the very prevalent intention to get vaccinated against influenza and the actual vaccination behavior. Despite the easy availability of information and opportunities to receive the annual influenza vaccine at the University Hospital in Frankfurt, less than half of the students are vaccinated. It remains to be seen if the implementation of the vaccination seminar contributes in the long term to an increase in the vaccination rate for influenza or other occupationally indicated vaccines. Further research should look at which measures are effective in turning the positive attitude toward vaccinations and the real intentions to be vaccinated against influenza into high rates of actual vaccination against influenza. To compensate for the deficiency seen in the participants who attended the practice-based seminar regarding their ability to interpret and understand vaccination certificates, a structured review of the STIKO recommendations should be integrated into the practice-based seminar prior to the group work with fictive vaccination certificates.
Acknowledgements
We wish to thank Prof. Ochsendorf (MME, Dermatology Clinic, University Hospital, Frankfurt) for his valuable advice on the design for the seminar and the study. Our thanks also go to Mr. Scherzer (Teaching Office, Internal Medicine) for providing the evaluation data and assisting with the organization of the seminar. Finally, we wish to express our gratitude to all of the students who participated in the seminar and this study.
Competing interests
Sabine Wicker is a member of the Standing Committee on Vaccination. The authors declare that there are no financial or economic conflicts of interest.
- Open access
- Published: 13 November 2023
The faces behind vaccination: unpacking the attitudes, knowledge, and practices of staff of Cameroon’s Expanded program on Immunization
- Yauba Saidu ORCID: orcid.org/0000-0002-0571-0074 1 , 2 ,
- Jessica Gu 3 ,
- Budzi Michael Ngenge 1 ,
- Sangwe Clovis Nchinjoh 1 ,
- Amani Adidja 4 ,
- Nadege Edwidge Nnang 1 ,
- Nkwain Jude Muteh 5 ,
- Vouking Marius Zambou 6 ,
- Clarence Mvalo Mbanga 1 ,
- Valirie Ndip Agbor 7 ,
- Diaby Ousmane 8 ,
- Andreas Ateke Njoh 9 , 10 ,
- Junie Flegere 4 ,
- Demba Diack 4 ,
- Owens Wiwa 11 ,
- Emanuele Montomoli 2 , 12 , 13 ,
- Sue Ann Costa Clemens 2 , 14 &
- Ralf Clemens 2
Human Resources for Health volume 21 , Article number: 88 ( 2023 ) Cite this article
902 Accesses
2 Altmetric
Metrics details
Immunization is regarded as one of the most cost-effective public health interventions in global health. However, its cost-effectiveness depends greatly on the knowledge and skills of vaccinators. With the growing complexity of immunization programs, the need for a well-trained vaccination workforce cannot be overemphasized. In this study, we assessed the knowledge, attitudes, and practices among vaccination staff in Cameroon.
Through a descriptive cross-sectional design, we used structured questionnaires and observation guides to collect data from vaccination staff in health facilities that were selected by a multistage sampling method. Data were analyzed using STATA 13 software.
Overall, we collected data from Expanded Program on Immunization focal staff in 265 health facilities across 68 health districts. Over half (53%) of the surveyed facilities were found in rural areas. Nearly two-thirds of health facilities had immunization focal staff with knowledge gaps for each of the four basic immunization indicators assessed. In other words, only 37% of staff knew how to estimate coverages, 36% knew how to inteprete the EPI monitoring curve, 35% knew how to prepare vaccine orders, and 37% knew how to estimate vaccine wastage. In terms of practices, staff waited for more than ten children to be present before opening a 20-dose vaccine vial in 63% of health facilities, and more than five children to be present before opening a 10-dose vaccine vial in 80% of surveyed facilities. Provision of vaccine-specific information (informing caregiver about vaccine received, explanation of benefits and potential side effects) during immunization sessions was suboptimal for the most part.
This study suggests marked deficits in immunization knowledge among vaccination staff and exposes common attitudes and practices that could contribute to missed opportunities for vaccination and hinder vaccination coverage and equity in Cameroon. Our findings highlight the urgent need to invest in comprehensive capacity building of vaccination staff in Cameroon, especially now that the immunization program is becoming increasingly complex.
Peer Review reports
Introduction
Immunization is regarded as one of the most cost-effective public health interventions in modern public health history. This intervention alone averts between 3.5 to 5 million deaths annually [ 1 ] and has substantially contributed to the observed reduction in global child mortality, from 12.5 million under-five deaths in 1990 to 5.3 million deaths in 2018 [ 2 ]. Over the past decades, great strides have been made globally in expanding the reach of immunization programs; however, the coverage of the third dose of the diphtheria–tetanus–pertussis, containing vaccine (DTP-3) has not gone above 86% since 2018 [ 3 ]. Similarly, despite strides in improving the performance of routine vaccinations, coverage in the WHO African region has stagnated for about a decade, with significant inter- and intra-country disparities [ 4 , 5 , 6 ].
The expanded program of immunization (EPI) in Cameroon, which was launched in 1976, has remarkably contributed to increased vaccination coverage over the past four decades [ 7 ]. In 2010, Cameroon was one of the three Central African countries with an immunization coverage rate of over 80% [ 8 ]. Despite this remarkable progress, national immunization coverage still falls below set targets. In Cameroon's 2015 to 2019 comprehensive multi-year plan (cMYP), the EPI envisaged raising the DTP-3 coverage from 89% in 2013 to 92% in 2019. Unfortunately, the EPI did not only fail to attain this goal, the program registered a 22 percent point decrease, with DTP-3 coverage plummeting from 89% in 2013 to 67% in 2019 [ 6 , 9 ]. This drop in immunization coverage has left many children without life-saving vaccines. Indeed, according to the most recent Demographic and Health Survey (DHS) in Cameroon, only 52% of children aged 12–23 months have received all essential vaccinations (one dose of Bacille Calmette-Guerin, BCG and measles vaccines and three doses of DTP and poliomyelitis vaccines) [ 10 ]. In addition, in 2019, Cameroon was among the top 10 countries contributing to 86% of the world's 7.3 million estimated zero-dose children [ 11 ].
Immunization coverage has been shown to be driven by several factors [ 12 , 13 ]. Indeed, a well-functioning routine immunization system relies on interactions of several components, including robust cold chain and logistics management systems, sustainable financing, strong managerial and technical leadership, and quality service delivery [ 14 ]. In addition, a well-functioning vaccination system with quality vaccination services anchors on effectiveness as a core guiding principle, defined by the World Health Organization as providing evidence-based vaccination services based on scientific rigor to achieve the best possible outcomes [ 15 ]. While the community needs to collaborate with healthcare providers to improve coverage, the quality of vaccination services provided by health personnel is imperative for the success of vaccination programs [ 16 ]. This success has been shown to significantly depend on the knowledge, attitudes, and skills of vaccination and managerial staff at healthcare facilities [ 17 ]. Indeed, the need for a well-trained and competent health workforce for vaccination cannot be overemphasized, particularly in recent years where immunization programs are bent on "leaving no one behind" and expanding the benefits of vaccination to every individual, irrespective of who he/she is and where he/she lives [ 18 ]. Meeting this noble goal will require significant improvements in providers' knowledge, attitudes, and practices, as these could positively or negatively influence parental decisions to seek vaccination services or return for subsequent vaccinations. For example, Musa et al. reported increased immunization service utilization in settings where health workers displayed positive attitudes and practices [ 19 ]. However, such evidence is limited in sub-Saharan Africa. As a result, evaluating the knowledge, attitudes, and practices of vaccination staff may serve as a standpoint for improving the quality of immunization service delivery, which in turn can improve immunization coverage and equity in many settings.
In Cameroon, several studies have examined specific drivers of declining routine immunization (RI) performance [ 20 , 21 , 22 , 23 , 24 ]. However, none of the work focused on assessing immunization knowledge among vaccination staff and their attitudes and practices during vaccination sessions. Thus, this study aimed to generate preliminary data on this neglected area of immunization.
Materials and methods
Study design.
This descriptive cross-sectional study was based on data from a national baseline assessment that was implemented by the EPI in collaboration with the Clinton Health Access Initiative (CHAI). The study aimed at identifying and characterizing potential factors contributing to declining immunization coverage in Cameroon.
Study setting
The study was conducted in Cameroon, a country that is located in the Gulf of Guinea. The country has a population of approximately 28 million inhabitants and a total surface area of 475,440 km 2 [ 25 ]. The country is divided into ten administrative regions: Adamawa (AD), Center (CE), East (ES), Far-North (EN), Littoral (LT), North (NO), North West (NW), West (OU), South (SU) and South West (SW) regions [ 26 ].
Cameroon's health sector is organized into three main levels (central, intermediate, and peripheral), each having specific competencies, administrative, health, and dialogue structures. The health structure lies under the leadership of the Minister of Public Health. The central level is led by various directorates under the leadership of the Minister of Public Health and focuses mainly on developing policies, strategies, and coordination. The intermediate level is led by the 10 Regional Delegates and provides technical support to the 189 health districts nationwide. District Medical Officers manage the health districts at the third level, the operation or implementation level for primary health care in Cameroon. Preventive services, including, immunization activities, are incorporated into all health system levels [ 27 ].
A multistage sampling technique was used to select health facilities. Before sampling, the total number of districts was allocated proportionately to the total number of districts per region in the ten regions. Then, the number of urban and rural districts was assigned within each region based on the region-specific breakdown and health facilities were allocated across regions in proportion to the national distribution.
The districts were then randomly selected within the specified region's urban or rural strata in the first stage. Health facilities were randomly selected within the identified rural/urban districts in the second stage. This selection was made while ensuring that the same number of facilities was selected within each district.

Study procedures
Administrative approval.
Administrative approval was obtained from the ministry of public health before data collection. Additionally, written approvals were also obtained from all regional delegations of public health, who in turn issued administrative letters to district heads requesting full support for the data collection process.
Training was carried out in all regions to provide regional supervisors and data collectors with the necessary knowledge and skills to undertake the baseline assessment. The training consisted of theoretical presentations and practical sessions on data collection, entry, and transmission processes. During practical sessions, assessors were split into groups and accompanied by the assessment management team to health facilities, where they were closely observed as they completed questionnaires and observation guides.
Data collection
Data collection was conducted by trained assessors selected from regional and district staff. The assessment management team and regional EPI teams developed data collection plans for target districts and health facilities. To prevent unproductive visits during data collection (e.g., visiting a health facility when there was no vaccination session), assessors contacted the health facilities via phone to remind them of planned visits. Upon arrival in the facilities, the purpose of the assessment was explained to the facility head or their representative. Then the assessor first obtained informed written consent before proceeding to interview the health provider in charge of immunization service delivery in each facility and observed an ongoing fixed post-vaccination session.
Study tools
The tools used for this study were:
A health facility questionnaire designed to assess the knowledge, attitudes and practices of immunization staff and their knowledge of key immunization indicators.
Vaccination Services Observation Guide, which included several prompts to assess the attitudes and practices of vaccinators during immunization sessions.
These study tools were developed in English and French and pre-tested in four facilities in Yaounde prior to study initiation.
Data management and analysis
Before data entry, a comprehensive database was built, pre-tested, and validated by an expert data manager. Each assessor entered data from the filled questionnaires and observation forms into the database and transmitted the files to a secure server within three days of data collection. Data were exported and cleaned in Microsoft Excel 2016 and analyzed with STATA 13 software (StataCorp. 2013. Stata Statistical Software : Release 13. College Station, TX: StataCorp LP). Frequencies and proportions were used to summarize variables of interest, and the unit of analysis was the health facility. Districts and facilities were sampled proportional to national distributions, and no post-stratification weights were applied.
Operational definition of variables
Immunization staff knowledge: This was defined as the knowledge of the health provider in charge of immunization service delivery (EPI focal point) on four key immunization indicators (vaccination coverage estimation, Interpretation of the EPI curve, preparation of a vaccine order, and estimation of vaccine wastage). Knowledge on each of these indicators was assessed separately, as either correct or incorrect based on EPI recommendations.
Immunization staff attitudes and practices: This was based on assessors' observation during fixed post-immunization sessions and the responses from the EPI focal point's interview.
Immunization staff: Included all health staff working in the immunization unit. Only the head of the immunization unit (or their representative) was interviewed for knowledge assessment.
General characteristics of health facilities
A total of 265 health facilities in 68 health districts were assessed nationwide during the study period. Over half (53%) of the facilities were in rural areas. Of all the facilities surveyed, the Center (21%), Littoral (13%), and North West (13%) regions were most represented, as shown in Table 1 .
The majority (84%) of the health facilities had two or more staff assigned to the immunization service unit, though with notable regional disparities. Notably, in the South region, 31% of health facilities had only one health provider assigned to vaccination services. Over two-thirds (93%) of the facilities had no trained immunization staff, with facilities in the South (85%) and East (100%) having very high proportions of untrained staff.
Vaccine-provider knowledge on key immunization indicators
Figure 1 provides level of awareness of selected immunization indicators. Overall, only 37% of health facilities had immunization staff who knew how to estimate vaccination coverage. In most regions, less than a third of health facilities had staff knowledgeable on vaccination coverage estimation. It is worth noting that in the South region, no surveyed facility had staff who had knowledge on estimating vaccination coverage. Figure 1 also shows that at national level, only 36% of health facilities had staff who could interpret the EPI monitoring curve, with the West (58%) and Adamawa (56%) regions having the highest proportion of such staff. It was also noted that only 35% and 37% of health facilities had staff knowledgeable on preparing a vaccination order and estimating vaccine wastage, respectively (Fig. 1 ).
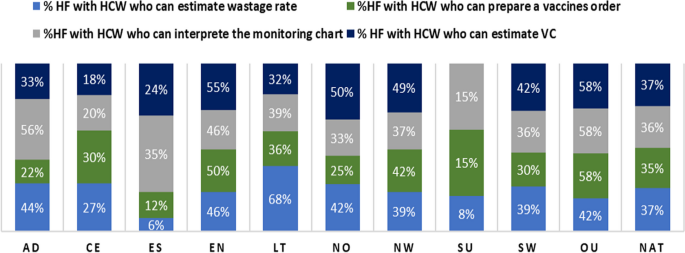
Regional distribution of vaccine-provider knowledge on key immunization indicators. NAT National, AD Adamawa, CE Center, ES East, EN Extreme North, LT Littoral, NO North, NW North West, SU South, SW South West, OU West, HCW health care worker, HF health facility, VC vaccination coverage
Healthcare workers attitudes and practices
Table 2 provides a battery of attitudes and practices of immunization staff that were observed by study staff during immunization sessions in the 265 surveyed facilities. In terms of vaccine handling and utilization, HCWs in 46% of facilities waited for 10 to 15 children to be present for vaccination sessions before a 20-dose BCG vial was opened, while in 17% of facilities, staff waited for more than 15 children to be present. Similarly, in 68% of health facilities, HCWs waited for five to nine children to be present before opening a 10-dose pentavalent or measles vaccine vial, while 21% of facilities waited for at least nine children before opening a 10-dose vial. In all facilities in the Far North and North regions, HCW did not open a vial if less than 5 children were present for vaccination.
Practices regarding handling of multi-dose vials were concerning. Overall, HCW in 18% of the surveyed facilities noted the date that a WHO-MDVP was opened—an observation that was consistent across all 10 regions (Table 2 ). Another harmful practice was placing vials on icepacks. Indeed, in 44% of surveyed facilities, HCWs placed vials on ice packs during immunization sessions, and this practice was particularly prominent in the North (65%), Center (55%), Far North (54%) and East (53%) regions.
Table 2 also illustrates some key parameters that were checked by HCW before vaccine administration. As illustrated, HCW in 94% of surveyed facilities verified the ages of children before administering a vaccine—a finding that was consistent across the 10 regions. Similarly, HCWs in nearly 90% of facilities verified the vaccines that the child had previously received before administering the next one. Despite these positive practices, HCW in 49% of health facilities, did not inform caregivers about the vaccine their child was receiving. Similarly, HCW in 51% of surveyed health facilities did not educate caregivers about the benefits of vaccination. Similarly, HCWs did not inform the caregivers about normal and potential side effects of vaccination in 68% and 69% of surveyed facilities, respectively. Last, but not least, HCWs in 14% of facilities did not request caregivers to return for follow-up vaccinations.
This study, which was nested in a national baseline assessment of Cameroon's immunization system, aimed at examining the immunization knowledge of vaccination staff in Cameroon as well as their attitudes and practices during vaccination sessions. We found that the knowledge of immunization staff on vaccination in practice was limited, with remarkable regional disparities. In assessing staff attitudes and practices during vaccination sessions, we noted significant gaps in health worker-to-caregiver communication and the utilization and handling of multi-dose vaccines. To the best of our knowledge, this is the first study evaluating immunization staff's knowledge, attitudes, and practices on a national scale in Cameroon.
We found that for the four basic immunization indicators assessed (vaccination coverage estimation, EPI monitoring chart interpretation, vaccine order preparation, and vaccine wastage estimation), nearly two-thirds of health facilities had focal immunization staff with suboptimal knowledge. This observation corroborates the findings of a study carried out in one district in Cameroon, which noted limited vaccination knowledge of health personnel using a different set of immunization indicators [ 16 ]. Another study in Nigeria reported that only 55% of vaccinators in 54 surveyed facilities were familiar with WHO-MDVP for minimizing vaccine wastage [ 28 ]. A poor knowledge base could limit immunization staff's ability to plan and deliver quality vaccination services, thus hindering improvement in vaccination coverage. One of the factors that could be at the root of this knowledge deficit in our study is the limited capacity building of staff working in vaccination services, as up to 67% of the surveyed facilities had no staff trained on immunization. This issue highlights the need for regular capacity building of the vaccination workforce as immunization programs become more complex with the increasing number of vaccines and recommendations [ 14 ]. Training programs have been shown, in different settings, to increase the knowledge of primary care health workers involved in vaccination and improve vaccination coverage [ 29 , 30 ].
As pertains to the utilization of multi-dose vaccines, vaccine providers waited for more than 10 children to be present before opening 20-dose vaccine vials in over three-quarters (75%) of health facilities, and more than 5 children to be present before opening 10-dose vaccine vials in a great majority (91%) of surveyed facilities. This practice is comparable to findings from a study in Nigeria which found that, on average, vaccinators waited for a minimum of six children to be present before opening a 10-dose measles-containing vaccine (MCV) [ 28 ]. Similarly, in a multi-country qualitative study, some healthcare workers in Senegal and Zambia reported sending unvaccinated children back home because not enough children were present to necessitate the opening of a new 10-dose vial [ 31 ]. These attitudes and practices with multi-dose vaccines could lead to increased caregiver waiting time, influencing their decisions for future vaccinations, and contribute to missed opportunities for vaccination (MOV) either directly or indirectly, which in turn may impact vaccination coverage [ 32 ]. Mindful that the objective usually driving these practices is to minimize vaccine wastage and prevent stockouts, these findings underscore the need to put policies and strategies in place to ensure reducing MOV is prioritized over wastage concerns [ 30 ]. As a result, many countries are considering switching to products with smaller dose vials; however, such a switch could overwhelm the cold chain and supply chain capacity of the vaccination system and significantly increase the cost of vaccination per child [ 33 ]. This finding was even more surprising for vaccine products that meet the four critical criteria for the WHO Multi-Dose Vial Policy that allows for the storage of open vials for up to 28 days [ 34 ]. However, this could be accounted for by high staff attrition, particularly in private facilities in urban areas—further highlighting the importance of putting in place a system for continuous learning, including onboarding, e-learning, coaching, and supportive supervision.
Another remarkable finding was that vaccine providers placed vials on ice packs during vaccination sessions in nearly half (44%) of health facilities. This practice could compromise the potency of freeze-sensitive vaccines. Exposure of vaccines to negative temperature is pervasive in Cameroon, not only limited to immunization sessions but across the entire supply chain, from central vaccine stores to outreaches [ 23 ]. Again, this is a capacity issue that should be corrected with training to improve their vaccine handling knowledge and practices.
We also found that providing vaccine-specific information (informing caregiver about vaccine received, explanation of benefits and potential side effects) during immunization sessions was suboptimal for the most part. Our findings are discordant with that of Al-Salihi et al. in Iraq, who reported that up to 96% of primary healthcare staff informed caregivers about potential side effects during vaccination sessions [ 35 ]. This difference could be because up to 87% of immunization staff received at least one formal training course on vaccination, unlike in our study, where over two-thirds of surveyed facilities (67%) had no staff trained on immunization. A vast majority of caregivers consider health workers as their primary source of immunization information, and their recommendations are known to influence parental decisions [ 14 , 36 , 37 ]. Vaccination sessions offer a unique opportunity to interact with health workers and gain basic vaccine-specific information. However, immunization staff commonly need more training on interpersonal skills and their contribution to improving vaccination uptake [ 14 ].
While our study has revealed significant gaps in the knowledge, attitudes and practices of immunization staff in Cameroon, certain limitations must be considered while interpreting our results. First, the data collected from observation sessions may have been subject to some bias because EPI personnel were aware of being observed and may have performed differently from a regular unobserved day. Secondly, the knowledge indicators assessed were not comprehensive as this was done in the context of a larger assessment, narrowing the extent of the knowledge assessment. Last, but not least, given that the unit of analysis was the health facility, individual provider level variations present at facility level could have been missed. However, the nationwide coverage of our study increases the generalizability of our study findings and highlights specific regional deficits in vaccination workforce knowledge, attitudes and practices.
This study highlights marked deficiencies in immunization staff training and knowledge of basic EPI indicators. It also exposes several gaps in knowledge, attitudes and practices in vaccine handling, utilization, and caregiver information sharing that could contribute to MOV, which may impact vaccination coverage and equity in Cameroon. This challenge prompts a great need to invest in systematic, comprehensive capacity building of immunization staff in Cameroon while strengthening supportive supervision and formulation of policies and strategies to minimize vaccine wastage without creating MOV.
Availability of data and materials
Data used for this research are available from the corresponding author upon reasonable request.
Abbreviations
Clinton Health Access Initiative
Expanded Program on Immunization
Healthcare workers
Health facility
Measles containing vaccine
Missed Opportunities for Vaccination
WHO Multi-dose Vaccine Policy
World Health Organisation. Vaccines and immunization [Internet]. 2022 [cited 2022 Dec 29]. Available from: https://www.who.int/health-topics/vaccines-and-immunization .
United Nations Children’s Fund (UNICEF). Levels & Trends in Child Mortality: Report 2019, Estimates developed by the United Nations Inter-agency Group for Child Mortality Estimation (UN IGME). New York; 2019.
World Health Organisation. Global Vaccine Action Plan 2011–2020. 2013.
Okeibunor JC, Akanmori BD, Balcha GM, Mihigo R, Vaz RM, Nshimirimana D. Enhancing access to immunization services and exploiting the benefits of recent innovations in the African region. Vaccine. 2013;31(37):3772–6.
Article CAS PubMed Google Scholar
Mihigo R, Okeibunor J, Anya B, Mkanda P, Zawaira F. Challenges of immunization in the African Region. Pan Afr Med J. 2017;27(Suppl 3):12.
PubMed PubMed Central Google Scholar
World Health Organisation. Diphtheria tetanus toxoid and pertussis (DTP3) immunization coverage among 1-year-olds, Global Health Observatory. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/diphtheria-tetanus-toxoid-and-pertussis-(dtp3)-immunization-coverage-among-1-year-olds-(-) .
Programme Elargie de Vaccination(PEV), Ministère de la Santé, Cameroon. PEV :: Historiques. Available from: https://pevcameroon.cm/apropos/histories .
LaFond A, Kanagat N, Steinglass R, Fields R, Sequeira J, Mookherji S. Drivers of routine immunization coverage improvement in Africa: findings from district-level case studies. Health Policy Plan. 2015;30(3):298–308.
Article PubMed Google Scholar
Programme Elargie de Vaccination (PEV), Ministère de la Santé, Cameroon. Plan Pluriannuel Complet 2015–2019. 2014. Available from: https://extranet.who.int/countryplanningcycles/file-repository/CMR .
National Institute of Statistics (Cameroon) and ICF. 2018 Cameroon DHS Summary Report. Maryland; 2020. Available from: www.DHSprogram.com .
World Health Organization. Regional Office for Africa. Framework for the implementation of the immunization agenda 2030 in the WHO African Region: report of the Secretariat. 2021.
Bobo JK, Gale JL, Thapa PB, Wassilak SG. Risk factors for delayed immunization in a random sample of 1163 children from Oregon and Washington. Pediatrics. 1993;91(2):308–14.
Biset G, Woday A, Mihret S, Tsihay M. Full immunization coverage and associated factors among children age 12–23 months in Ethiopia: systematic review and meta-analysis of observational studies. Hum Vaccin Immunother. 2021;17(7):2326–35.
Article PubMed PubMed Central Google Scholar
Shen AK, Fields R, McQuestion M. The future of routine immunization in the developing world: challenges and opportunities. Glob Health Sci Pract. 2014;2(4):381–94.
World Health Organisation. Quality immunization services: a planning guide. 2022.
EbileAkoh W, Ateudjieu J, Nouetchognou JS, Yakum MN, DjoumaNembot F, NafackSonkeng S, et al. The expanded program on immunization service delivery in the Dschang health district, west region of Cameroon: a cross sectional survey. BMC Public Health. 2016;16(1):801.
Article Google Scholar
World Health Organisation. Global Routine Immunization Strategies and Practices (GRISP): a companion document to the Global Vaccine Action Plan (GVAP). 2016.
Gavi, The Vaccine Alliance. New 2021–2025 high level strategy to leave no-one behind with immunisation approved by Gavi Board. 2022. Available from: https://www.gavi.org/news/media-room/new-2021-2025-high-level-strategy-leave-no-one-behind-immunisation-approved-gavi .
Musa S, Skrijelj V, Kulo A, Habersaat KB, Smjecanin M, Primorac E, et al. Identifying barriers and drivers to vaccination: a qualitative interview study with health workers in the Federation of Bosnia and Herzegovina. Vaccine. 2020;38(8):1906–14.
Russo G, Miglietta A, Pezzotti P, Biguioh RM, BoutingMayaka G, Sobze MS, et al. Vaccine coverage and determinants of incomplete vaccination in children aged 12–23 months in Dschang, West Region, Cameroon: a cross-sectional survey during a polio outbreak. BMC Public Health. 2015;15(1):630.
Ateudjieu J, Kenfack B, Nkontchou BW, Demanou M. Program on immunization and cold chain monitoring: the status in eight health districts in Cameroon. BMC Res Notes. 2013;6(1):101.
Yakum MN, Ateudjieu J, Walter EA, Watcho P. Vaccine storage and cold chain monitoring in the North West region of Cameroon: a cross sectional study. BMC Res Notes. 2015;8(1):145.
Yauba S, Sobngwi J, Jude N, Tracy B, Kobela M, Charles N, et al. Temperature monitoring in the vaccine cold chain in Cameroon. J Vacc Vacc. 2017;1:09.
Google Scholar
Yauba S, Harmelle EE, Marius VZ, Jude N, Delphine K, Calvin T, et al. Availability and status of vaccine cold chain equipment in Cameroon. J Vacc Vacc. 2019;10(2):1–6.
World Bank Group. Population, total-Cameroon|Data. 2021. Available from: https://data.worldbank.org/indicator/SP.POP.TOTL?locations=CM .
Presidency of the Republic of Cameroon. Cameroon. Available from: https://www.prc.cm/en/cameroon .
Ministry of Public Health, Cameroon. Health Sector Strategy 2016–2027.
Wallace AS, Willis F, Nwaze E, Dieng B, Sipilanyambe N, Daniels D, et al. Vaccine wastage in Nigeria: An assessment of wastage rates and related vaccinator knowledge, attitudes and practices. Vaccine. 2017;35(48 Pt B):6751–8.
Uskun E, Uskun SB, Uysalgenc M, Yagız M. Effectiveness of a training intervention on immunization to increase knowledge of primary healthcare workers and vaccination coverage rates. Public Health. 2008;122(9):949–58.
Siddiqui FA, Padhani ZA, Salam RA, Aliani R, Lassi ZS, Das JK, et al. Interventions to improve immunization coverage among children and adolescents: a meta-analysis. Pediatrics. 2022;149(Supplement 6):e2021053852D.
Kanagat N, Krudwig K, Wilkins KA, Kaweme S, Phiri G, Mwansa FD, et al. Health care worker preferences and perspectives on doses per container for 2 lyophilized vaccines in Senegal, Vietnam, and Zambia. Glob Health Sci Pract. 2020;8(4):680–8.
World Health Organization. Reducing Missed Opportunities for Vaccination (MOV). Available from: https://www.who.int/teams/immunization-vaccines-and-biologicals/essential-programme-on-immunization/implementation/reducing-missed-opportunities-for-vaccination-(mov) .
Assi TM, Brown ST, Djibo A, Norman BA, Rajgopal J, Welling JS, et al. Impact of changing the measles vaccine vial size on Niger’s vaccine supply chain: a computational model. BMC Public Health. 2011;11(1):425.
World Health Organization. WHO Policy Statement: Multi-dose Vial Policy (MDVP)- Handling of multi-dose vaccine vials after opening. Geneva; 2014. Available from: https://apps.who.int/iris/bitstream/handle/10665/135972/WHO_IVB_14.07_eng.pdf;sequence=1 .
Al-Salihi LG, Aakef IR, Al-Shuwaili SJ, ZakiHadi WM. Primary health-care staff barriers to immunization. Indian J Community Med. 2019;44(3):256–60.
Filia A, Bella A, D’Ancona F, Fabiani M, Giambi C, Rizzo C, et al. Childhood vaccinations: knowledge, attitudes and practices of paediatricians and factors associated with their confidence in addressing parental concerns, Italy, 2016. Euro Surveill. 2019;24(6):1800275.
Paterson P, Meurice F, Stanberry LR, Glismann S, Rosenthal SL, Larson HJ. Vaccine hesitancy and healthcare providers. Vaccine. 2016;34(52):6700–6.
Download references
Acknowledgements
Regional delegates and districts staff for facilitating the conduct of the study in their respective districts and regions and the facility staff for their collaboration.
This study was funded by Bill and Melinda Gates Foundation (BMGF); however the outcome of the study does not necessarily represent the views of BMGF.
Author information
Authors and affiliations.
Clinton Health Access Initiative Inc., PO Box 2664, Yaounde, Cameroon
Yauba Saidu, Budzi Michael Ngenge, Sangwe Clovis Nchinjoh, Nadege Edwidge Nnang & Clarence Mvalo Mbanga
Institute for Global Health, University of Siena, 53100, Siena, Italy
Yauba Saidu, Emanuele Montomoli, Sue Ann Costa Clemens & Ralf Clemens
Global Vaccine Delivery, Clinton Health Access Initiative Inc, Boston, MA, 02127, United States of America
Faculty of Medicine and Biomedical Sciences, University of Yaoundé 1, Yaoundé, Cameroon
Amani Adidja, Junie Flegere & Demba Diack
Gavi, The Vaccine Alliance, Geneva, Switzerland
Nkwain Jude Muteh
UNICEF, Yaoundé, Cameroun
Vouking Marius Zambou
Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, United Kingdom
Valirie Ndip Agbor
Department of Projects, Ministry of Public Health, Yaounde, Cameroon
Diaby Ousmane
Expanded Program on Immunization, Cameroon Ministry of Public Health, PO Box 2084, Yaoundé, Cameroon
Andreas Ateke Njoh
School of Global Health and Bioethics, Euclid University, PO Box 157, Bangui, Central African Republic
Clinton Health Access Iniative Inc., Abuja, Nigeria
Department Molecular Medicine, University of Siena, Via Aldo Moro 3, 53100, Siena, Italy
Emanuele Montomoli
VisMederi Srl, Via Ferrini 53, 53035, Siena, Italy
Department of Pediatrics, University of Oxford, Oxford, United Kingdom
Sue Ann Costa Clemens
You can also search for this author in PubMed Google Scholar
Contributions
Conception and design: YS, JG, AA, NJM, VMZ; statistical analysis: VNA, NJM; initial draft preparation: YS, SCN, BMN, CM; review and editing: YS, NNE, NJM, VMZ, CM, VNA, DO, DO, AAN, JF, DD, OW, EM, SACC; supervision: YS, OW, JG, SACC, EM, RC. All authors read and approved the submission of the final article.
Corresponding author
Correspondence to Yauba Saidu .
Ethics declarations
Ethics approval and consent to participate.
This study was conducted in compliance with the Helsinki Declaration and all applicable national laws and institutional rules and has been approved by the author’s institutional review board. Ethical approval was granted by the Cameroon National Ethics Committee for Human Health Research.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Saidu, Y., Gu, J., Ngenge, B.M. et al. The faces behind vaccination: unpacking the attitudes, knowledge, and practices of staff of Cameroon’s Expanded program on Immunization. Hum Resour Health 21 , 88 (2023). https://doi.org/10.1186/s12960-023-00869-7
Download citation
Received : 03 July 2023
Accepted : 03 October 2023
Published : 13 November 2023
DOI : https://doi.org/10.1186/s12960-023-00869-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Expanded Program of Immunization
- Vaccination staff
Human Resources for Health
ISSN: 1478-4491
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
COVID-19 Vaccine Webinar Series
COVID-19 vaccine recommendations have been updated as of October 3, 2023 to add 2023-2024 updated Novavax COVID-19 vaccine. The content on this page will be updated to align with the new recommendations. Learn more .

CDC is offering a series of brief (15-20 minute) webinars addressing topics around COVID-19 vaccination. These interactive, web-based training modules offer a real-world perspective on different issues around COVID-19 vaccines. Topics range from routine clinical and vaccine safety information to guidance for on-site clinic vaccination activities and having conversations with vaccine recipients. Each webinar includes self-test practice questions and lists additional resources related to the topic discussed.
Immunization providers: Physicians, nurses, nurse practitioners, pharmacists, physician assistants, DoD paraprofessionals, medical students, state and local immunization programs, and others authorized to prepare and administer COVID-19 vaccine.
Continuing Education
Continuing Education is no longer available for this series.
Description
Key Additional Resources
Recommendations for Bivalent COVID-19 Booster Doses
This module discusses recommendations for a bivalent COVID-19 booster dose, the interim COVID-19 immunization schedule to date, COVID-19 vaccine products, and recommended actions for addressing bivalent and monovalent COVID-19 vaccine administration errors.
Purpose: To describe and apply bivalent COVID-19 booster recommendations.
Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States
Interim COVID-19 Immunization Schedule
Storing and Handling Vaccines: Expiration Date, Beyond-Use Date, and Beyond-Use Time
This module discusses how to determine the expiration date, beyond-use date, and beyond-use time; when to use the expiration date and when to use the beyond-use date; and where to find comprehensive vaccine storage and preparation resources for clinical staff.
Purpose: To differentiate the expiration date and beyond-use date or time and apply best practices to assure vaccine is given safely and effectively.
- 2021–2022 Influenza Season Vaccine Label Examples
- Vaccine Label Examples
U.S. COVID-19 Vaccine Product Information
- Beyond-Use Date (BUD) video
- Multidose Vial (MDV) video
- Reconstitute Lyophilized Vaccine video
- CDC Immunization Education and Training
- Immunization Action Coalition’s Vaccines with Diluents: How to Use Them
- Manufacturer package inserts
- EUA Fact Sheets for Healthcare Providers
Administering More Than One Vaccine on the Same Day: Clinical Considerations
This module discusses simultaneous administration or co-administration of vaccines. This is an important clinical strategy because it helps ensure patients receive all vaccines they need are up-to-date.
Purpose: To review the clinical considerations, best practices and clinical resources for simultaneous administration.
Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized in the U.S.
Vaccine Administration: Needle Gauge and Length (You Call the Shots)
Vaccine Administration: Intramuscular (IM) Injection, Children 7 through 18 years of age (You Call the Shots)
Vaccine Administration: Intramuscular (IM) injections: Adults 19 years of age and older (cdc.gov)
What Healthcare Providers Need to Know about COVID-19 Vaccines and Becoming a Vaccination Provider
This module discusses how to become a COVID-19 vaccination provider and how to comply with COVID-19 vaccination program requirements. Learning about how COVID-19 vaccines work allows healthcare workers to better educate their patients during the vaccination appointment.
Purpose: To discuss essential information about COVID-19 vaccines and what is necessary to become a COVID-19 vaccinator.
How to Enroll as a COVID-19 Vaccination Provider
CARES Act Provider Relief Fund: For Providers
U.S COVID-19 Vaccine Product Information
CDC COVID-19 Vaccination Program Provider Requirements and Support
COVID-19 Vaccine Effectiveness Research
CDC Training and Education
CDC COVID-19 Vaccine Training Modules
Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized in the United States
Prevaccination Checklist for COVID-19 Vaccines
V-safe After Vaccination Health Checker
Vaccines.gov: COVID-19 Vaccine Information for Jurisdictions and Healthcare Providers
COVID-19 Vaccines Authorized for Emergency Use
Ensuring Vaccine Safety in the United States: A Primer for Healthcare Workers
This module reviews multiple aspects of vaccine safety so that healthcare professionals can educate their patients and answer questions. Vaccine life cycle and safety monitoring is described throughout as well as how adverse events are monitored and reported. Finally, the roles of federal agencies in monitoring vaccine safety is also discussed.
Purpose : To understand the systems that are in place to ensure vaccine safety.
About Smallpox
Polio Photos
- Vaccine Adverse Event Reporting System (VAERS)
Safety Information by Vaccine
Common Vaccine Safety Questions and Concerns
Vaccine Safety Information for Healthcare Providers
Information for Parents and Caregivers
Vaccine Safety Research and Safety Studies
About the Immunization Safety Office (ISO)
Ensuring Vaccine Safety
Emergency Preparedness and Vaccine Safety
Getting Shots in Arms: A Primer for Healthcare Workers on Storing and Transporting Vaccines
This module discusses how healthcare professionals can apply best practices to store and transport vaccines once they are received. Best practices include maintaining cold chain through staff training and establishing a standard operating procedure. Special considerations related to transporting and preparing vaccines are also shared.
Purpose: To understand how to properly store, handle and transport vaccines so that vaccines remain safe and effective.
Pink Book: Storage and Handling
At A Glance Resource Guide: Vaccine Administration and Storage and Handling
Vaccine Storage and Handling Resources
Hosting Off-Site COVID-19 Vaccination Clinics: A Primer for Healthcare Workers
This module discusses how healthcare professionals can successfully host an off-site COVID-19 vaccination clinic. Considerations address preventing exposure to SARS-CoV2, optimizing limited vaccine supply, and maximizing the number of patients going through the clinic.
Purpose: To apply considerations for hosting off-site COVID-19 vaccination clinics so that these vaccination clinics are as effective as possible.
COVID-19 Vaccine Information for Specific Groups
Guidance for Planning Vaccination Clinics Held at Satellite, Temporary or Off-Site Locations
Considerations for Planning Curbside/Drive-Through Vaccination Clinics
Resources for Hosting a Vaccination Clinic
Satellite, Temporary, and Off-Site Vaccination Clinic Supply Checklists
Checklist of Best Practices for Vaccination Clinics Held at Satellite, Temporary, or Off-Site Locations
After Action Report Template
Ten Principles for Holding a Safe Vaccination Clinic
Hosting Off-Site Vaccination Clinics: A Primer for Healthcare Workers
This module describes how healthcare professionals can effectively use off-site clinics as part of their vaccination strategy. Four phases of planning and hosting an off-site vaccination clinic are discussed. Recommended practices for planning and executing off-site vaccination clinics are included for each phase.
Purpose: To learn recommendations for planning and hosting effective off-site vaccinations clinics.
Infection Control Guidance for Healthcare Professionals about Coronavirus (COVID-19)
Timing and Spacing of Routine Vaccines
Planning Activities for Temporary, Satellite, or Off-Site Vaccination Clinics
Immunization Education and Training Homepage
Preventing Vaccine Administration Errors: A Primer for Healthcare Workers
This module reviews how healthcare professionals can utilize best practices to prevent common errors in vaccine administration. Causes that lead to common vaccine administration errors are addressed as well as different error types and how to prevent them.
Purpose: To recognize common vaccine administration errors and learn how to prevent them from occurring.
Pink Book: Vaccine Administration
COVID-19 Vaccines Emergency Use Authorization
You Call the Shots: Vaccine Administration Training Course
Vaccine Administration: Preventing Vaccine Administration Errors (You Call the Shots )
Recommended and Minimum Ages and Intervals Between Doses of Routinely Recommended Vaccines (Pink Book)
Vaccinating Adolescents: Injectable Vaccines
This module shares adolescent specific strategies for pain management and vaccine administration best practices. Managing and preventing syncope after vaccination is also important with adolescent patients.
Purpose: To apply a planned approach to decreasing adolescents’ anxiety before, during and after immunization as well as minimizing pain from the injection.
Screening Checklist for Contraindications to Vaccines for Children and Teens
Vaccines for Your Children: Before, During, and After Shots
Fainting (Syncope) after Vaccination
Vaccinate with Confidence: Routinely Recommended Vaccines
Vaccinate with Confidence: COVID-19 Vaccine
- CDC Vaccine Storage and Handling Toolkit
- CDC Pink Book
- CDC Pink Book Webinar Series
- You Call the Shots: Web-based Training Courses
- CDC Immunization Schedules
- Resources for Healthcare Providers about Immunizations Schedules
- Vaccine Administration Resource Library
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

IMAGES
VIDEO
COMMENTS
Immunization Webinars are 1-hour presentations with question and answer sessions to reinforce presentation content. Internet access is needed to participate. These presentations, collectively titled "Current Issues in Immunization," are scheduled 4 to 5 times per year. Specific topic (s) will be announced prior to each occurrence.
Immunization Education & Training. CDC offers numerous education and training programs for healthcare personnel. A variety of topics and formats are available. All are based on vaccine recommendations made by the Advisory Committee on Immunization Practice (ACIP). Physicians, nurses, health educators, pharmacists, and other healthcare ...
This annual event has been designed to provide up-to-date education to health care providers in order to improve health outcomes in the field of immunization and vaccine preventable diseases. New recommendations and guidelines will be addressed through didactic lectures and panel discussion.
Calendar of Events. Advisory Committee on Immunization Practices (ACIP) Meeting. Centers for Disease Control and Prevention (CDC) February 28-29, 2024. Atlanta, Georgia and virtually. More information. 16th National Conference for Immunization Coalitions and Partnerships (NCICP) Pennsylvania Immunization Coalition (PAIC) April 9-11, 2024.
The Texas Immunization Conference (TIC) is a chance for immunization partners to come together to share information, discuss current issues, and recommend strategies for improving immunization rates across Texas. TIC 2022 was held virtually June 14-15, 2022. As follows are recordings and presentations from the two-day event.
The Global Vaccine and Immunization Research Forum, the central forum for research related to Immunization Agenda 2030, brings together the entire vaccine and immunization research community every two years and is organized by the U.S. National Institute of Allergy and Infectious Diseases, the Bill & Melinda Gates Foundation, and the World ...
Overview. Module 1: Target diseases and vaccines. Module 2: The vaccine cold chain. Module 3: Ensuring safe injections. Module 4: micro planning for reaching every community. Module 5: Managing an immunization session. Module 6: Monitoring and surveillance. Module 7: Partnering with communities.
Aluminum is a suspected carcinogen. It is a cardiovascular or blood toxicant, neurotoxicant, and respiratory toxicant. It has been implicated as a cause of brain damage, and is a suspected factor in Alzheimer's Disease, dementia, convulsions, and comas. Aluminum can cross the blood brain barrier.
Childhood and Adolescent Immunization Topics. School Immunization Requirements 2019-20 Webinar Original broadcast date: Thursday, March 12, 2020; School Immunization Requirements Webinar for the 2019-20 School Year Original broadcast date: Monday, May 13, 2019; School Immunization Requirements 2018-19: Frequently Asked Questions Webinar: April 2018
The Essential Programme on Immunization aims to strengthen vaccine programmes, supply, and delivery, and ensure universal access to all relevant vaccines for all populations across the life course. Immunization campaigns. Vaccination campaigns are a delivery strategy used to quickly reach large numbers of children/individuals with one or more ...
Centers for Disease Control and Prevention (CDC) Date: October 22-23, 2025 (Save the Dates!) Location: Atlanta, Georgia and virtually. Top of Page. Last Reviewed: March 13, 2024. Source: National Center for Immunization and Respiratory Diseases. Calendar of meetings and events related to vaccines and immunizations.
GVIRF 2021 Presentations. Introduction to GVIRF (B. Fenton Hall, NIAID) Keynote Speakers. Vaccinology in the context of pandemic preparedness: The COVID-19 experience (Anthony Fauci, NIAID) The lessons of COVID-19 for vaccine development (Bill Gates, BMGF) Innovating from research to impact at scale: the role of the Gavi Alliance (Anuradha ...
7,000 to 26,000 estimated flu-related hospitalizations per season in children aged <5 years during 2010-2011 to 2019-2020. 37 to 199 reported flu-related deaths in children per season during 2004-2005 to 2019-2020. Flu vaccination is the best way to prevent flu in children.
Step 2: Calculate requirements. Total vaccine volume is the space needed to store or transport vaccines in the cold chain. It is determined by: the presentation of the vaccine product (primary container of vaccine and diluent, secondary packaging, and packaging material) the packed vaccine volume per dose.
Training for immunization staff is one of the most important factors in enhancing immunization performance and effectively introducing new vaccines, technologies, practices and policies. Staff working on each level-national, sub national and health facility need to be adequately trained. This section provides standard training materials that ...
Pre-assessment (Answer key) Select the correct response. Step 3: Plan vaccine management. By the end of this module, participants will be able to: Calculate the storage space needed for COVID-19 vaccines with different characteristics. Specify the elements of plans for storing COVID-19 vaccines and ancillary supplies.
Summary and timeline. Dr. M Kobayashi. Page last reviewed: June 25, 2021. Content source: National Center for Immunization and Respiratory Diseases. ACIP Presentation slides from June 23-25, 2021 meeting. Advisory Committee on Immunization Practices (ACIP).
Aim: Despite having a generally positive attitude toward vaccinations, medical students show gaps in their own immunization histories and knowledge about vaccinations.Future practicing physicians will be confronted with the need to evaluate protective immunity and make vaccination recommendations. This study aims to investigate the extent to which a seminar on the topic of vaccination can ...
Immunization is regarded as one of the most cost-effective public health interventions in global health. However, its cost-effectiveness depends greatly on the knowledge and skills of vaccinators. With the growing complexity of immunization programs, the need for a well-trained vaccination workforce cannot be overemphasized. In this study, we assessed the knowledge, attitudes, and practices ...
CDC is offering a series of brief (15-20 minute) webinars addressing topics around COVID-19 vaccination. These interactive, web-based training modules offer a real-world perspective on different issues around COVID-19 vaccines. Topics range from routine clinical and vaccine safety information to guidance for on-site clinic vaccination ...
September 13 2023, 3-6:30 pm (CEST), 9-12 am (EDT) 6-9:30 am (PDT) - Virtual webinarPurpose This webinar is to discuss issues related to global demand for vaccine adjuvants and the associated research and development, as well as facilitating global access to novel adjuvants and formulation technologies for vaccine developers.Audience Scientists, product developers, regulators, policy makers ...
Neuroscience Seminar Series: Jacob Nordman, PhD (SIU School of Medicine) Apr 1, 2024, 4:00 - 5:00 PM . Apr 2. MS Project Presentation - Environmental Science and Policy. Apr 2, 2024, 10:00 AM - 12:00 PM . Johnson Center 334, Meeting Room E. View Calendar. Mason Vision Day 2024.