When you choose to publish with PLOS, your research makes an impact. Make your work accessible to all, without restrictions, and accelerate scientific discovery with options like preprints and published peer review that make your work more Open.
- PLOS Biology
- PLOS Climate
- PLOS Complex Systems
- PLOS Computational Biology
- PLOS Digital Health
- PLOS Genetics
- PLOS Global Public Health
- PLOS Medicine
- PLOS Mental Health
- PLOS Neglected Tropical Diseases
- PLOS Pathogens
- PLOS Sustainability and Transformation
- PLOS Collections

Welcome to the PLOS Writing Center
Your source for scientific writing & publishing essentials.
A collection of free, practical guides and hands-on resources for authors looking to improve their scientific publishing skillset.
ARTICLE-WRITING ESSENTIALS
Your title is the first thing anyone who reads your article is going to see, and for many it will be where they stop reading. Learn how to write a title that helps readers find your article, draws your audience in and sets the stage for your research!
The abstract is your chance to let your readers know what they can expect from your article. Learn how to write a clear, and concise abstract that will keep your audience reading.
A clear methods section impacts editorial evaluation and readers’ understanding, and is also the backbone of transparency and replicability. Learn what to include in your methods section, and how much detail is appropriate.
In many fields, a statistical analysis forms the heart of both the methods and results sections of a manuscript. Learn how to report statistical analyses, and what other context is important for publication success and future reproducibility.
The discussion section contains the results and outcomes of a study. An effective discussion informs readers what can be learned from your experiment and provides context for the results.
Ensuring your manuscript is well-written makes it easier for editors, reviewers and readers to understand your work. Avoiding language errors can help accelerate review and minimize delays in the publication of your research.
The PLOS Writing Toolbox
Delivered to your inbox every two weeks, the Writing Toolbox features practical advice and tools you can use to prepare a research manuscript for submission success and build your scientific writing skillset.
Discover how to navigate the peer review and publishing process, beyond writing your article.
The path to publication can be unsettling when you’re unsure what’s happening with your paper. Learn about staple journal workflows to see the detailed steps required for ensuring a rigorous and ethical publication.
Reputable journals screen for ethics at submission—and inability to pass ethics checks is one of the most common reasons for rejection. Unfortunately, once a study has begun, it’s often too late to secure the requisite ethical reviews and clearances. Learn how to prepare for publication success by ensuring your study meets all ethical requirements before work begins.
From preregistration, to preprints, to publication—learn how and when to share your study.
How you store your data matters. Even after you publish your article, your data needs to be accessible and useable for the long term so that other researchers can continue building on your work. Good data management practices make your data discoverable and easy to use, promote a strong foundation for reproducibility and increase your likelihood of citations.
You’ve just spent months completing your study, writing up the results and submitting to your top-choice journal. Now the feedback is in and it’s time to revise. Set out a clear plan for your response to keep yourself on-track and ensure edits don’t fall through the cracks.
There’s a lot to consider when deciding where to submit your work. Learn how to choose a journal that will help your study reach its audience, while reflecting your values as a researcher.
Are you actively preparing a submission for a PLOS journal? Select the relevant journal below for more detailed guidelines.
How to Write an Article
Share the lessons of the Writing Center in a live, interactive training.
Access tried-and-tested training modules, complete with slides and talking points, workshop activities, and more.
Scientific Writing: Structuring a scientific article
- Resume/Cover letter
- Structuring a scientific article
- AMA Citation Style This link opens in a new window
- APA Citation Style This link opens in a new window
- Scholarly Publishing This link opens in a new window
How to Structure a Scientific Article
Many scientific articles include the following elements:
I. Abstract: The abstract should briefly summarize the contents of your article. Be sure to include a quick overview of the focus, results and conclusion of your study.
II. Introduction: The introduction should include any relevant background information and articulate the idea that is being investigated. Why is this study unique? If others have performed research on the topic, include a literature review.
III. Methods and Materials: The methods and materials section should provide information on how the study was conducted and what materials were included. Other researchers should be able to reproduce your study based on the information found in this section.
IV. Results: The results sections includes the data produced by your study. It should reflect an unbiased account of the study's findings.
V. Discussion and Conclusion: The discussion section provides information on what researches felt was significant and analyzes the data. You may also want to provide final thoughts and ideas for further research in the conclusion section.
For more information, see How to Read a Scientific Paper.
Scientific Article Infographic
- Structure of a Scientific Article
- << Previous: Resume/Cover letter
- Next: AMA Citation Style >>

- Last Updated: Jan 23, 2023 11:48 AM
- URL: https://guides.himmelfarb.gwu.edu/scientific-writing

- Himmelfarb Intranet
- Privacy Notice
- Terms of Use
- GW is committed to digital accessibility. If you experience a barrier that affects your ability to access content on this page, let us know via the Accessibility Feedback Form .
- Himmelfarb Health Sciences Library
- 2300 Eye St., NW, Washington, DC 20037
- Phone: (202) 994-2850
- [email protected]
- https://himmelfarb.gwu.edu

Scientific Reports
What this handout is about.
This handout provides a general guide to writing reports about scientific research you’ve performed. In addition to describing the conventional rules about the format and content of a lab report, we’ll also attempt to convey why these rules exist, so you’ll get a clearer, more dependable idea of how to approach this writing situation. Readers of this handout may also find our handout on writing in the sciences useful.
Background and pre-writing
Why do we write research reports.
You did an experiment or study for your science class, and now you have to write it up for your teacher to review. You feel that you understood the background sufficiently, designed and completed the study effectively, obtained useful data, and can use those data to draw conclusions about a scientific process or principle. But how exactly do you write all that? What is your teacher expecting to see?
To take some of the guesswork out of answering these questions, try to think beyond the classroom setting. In fact, you and your teacher are both part of a scientific community, and the people who participate in this community tend to share the same values. As long as you understand and respect these values, your writing will likely meet the expectations of your audience—including your teacher.
So why are you writing this research report? The practical answer is “Because the teacher assigned it,” but that’s classroom thinking. Generally speaking, people investigating some scientific hypothesis have a responsibility to the rest of the scientific world to report their findings, particularly if these findings add to or contradict previous ideas. The people reading such reports have two primary goals:
- They want to gather the information presented.
- They want to know that the findings are legitimate.
Your job as a writer, then, is to fulfill these two goals.
How do I do that?
Good question. Here is the basic format scientists have designed for research reports:
- Introduction
Methods and Materials
This format, sometimes called “IMRAD,” may take slightly different shapes depending on the discipline or audience; some ask you to include an abstract or separate section for the hypothesis, or call the Discussion section “Conclusions,” or change the order of the sections (some professional and academic journals require the Methods section to appear last). Overall, however, the IMRAD format was devised to represent a textual version of the scientific method.
The scientific method, you’ll probably recall, involves developing a hypothesis, testing it, and deciding whether your findings support the hypothesis. In essence, the format for a research report in the sciences mirrors the scientific method but fleshes out the process a little. Below, you’ll find a table that shows how each written section fits into the scientific method and what additional information it offers the reader.
Thinking of your research report as based on the scientific method, but elaborated in the ways described above, may help you to meet your audience’s expectations successfully. We’re going to proceed by explicitly connecting each section of the lab report to the scientific method, then explaining why and how you need to elaborate that section.
Although this handout takes each section in the order in which it should be presented in the final report, you may for practical reasons decide to compose sections in another order. For example, many writers find that composing their Methods and Results before the other sections helps to clarify their idea of the experiment or study as a whole. You might consider using each assignment to practice different approaches to drafting the report, to find the order that works best for you.
What should I do before drafting the lab report?
The best way to prepare to write the lab report is to make sure that you fully understand everything you need to about the experiment. Obviously, if you don’t quite know what went on during the lab, you’re going to find it difficult to explain the lab satisfactorily to someone else. To make sure you know enough to write the report, complete the following steps:
- What are we going to do in this lab? (That is, what’s the procedure?)
- Why are we going to do it that way?
- What are we hoping to learn from this experiment?
- Why would we benefit from this knowledge?
- Consult your lab supervisor as you perform the lab. If you don’t know how to answer one of the questions above, for example, your lab supervisor will probably be able to explain it to you (or, at least, help you figure it out).
- Plan the steps of the experiment carefully with your lab partners. The less you rush, the more likely it is that you’ll perform the experiment correctly and record your findings accurately. Also, take some time to think about the best way to organize the data before you have to start putting numbers down. If you can design a table to account for the data, that will tend to work much better than jotting results down hurriedly on a scrap piece of paper.
- Record the data carefully so you get them right. You won’t be able to trust your conclusions if you have the wrong data, and your readers will know you messed up if the other three people in your group have “97 degrees” and you have “87.”
- Consult with your lab partners about everything you do. Lab groups often make one of two mistakes: two people do all the work while two have a nice chat, or everybody works together until the group finishes gathering the raw data, then scrams outta there. Collaborate with your partners, even when the experiment is “over.” What trends did you observe? Was the hypothesis supported? Did you all get the same results? What kind of figure should you use to represent your findings? The whole group can work together to answer these questions.
- Consider your audience. You may believe that audience is a non-issue: it’s your lab TA, right? Well, yes—but again, think beyond the classroom. If you write with only your lab instructor in mind, you may omit material that is crucial to a complete understanding of your experiment, because you assume the instructor knows all that stuff already. As a result, you may receive a lower grade, since your TA won’t be sure that you understand all the principles at work. Try to write towards a student in the same course but a different lab section. That student will have a fair degree of scientific expertise but won’t know much about your experiment particularly. Alternatively, you could envision yourself five years from now, after the reading and lectures for this course have faded a bit. What would you remember, and what would you need explained more clearly (as a refresher)?
Once you’ve completed these steps as you perform the experiment, you’ll be in a good position to draft an effective lab report.
Introductions
How do i write a strong introduction.
For the purposes of this handout, we’ll consider the Introduction to contain four basic elements: the purpose, the scientific literature relevant to the subject, the hypothesis, and the reasons you believed your hypothesis viable. Let’s start by going through each element of the Introduction to clarify what it covers and why it’s important. Then we can formulate a logical organizational strategy for the section.
The inclusion of the purpose (sometimes called the objective) of the experiment often confuses writers. The biggest misconception is that the purpose is the same as the hypothesis. Not quite. We’ll get to hypotheses in a minute, but basically they provide some indication of what you expect the experiment to show. The purpose is broader, and deals more with what you expect to gain through the experiment. In a professional setting, the hypothesis might have something to do with how cells react to a certain kind of genetic manipulation, but the purpose of the experiment is to learn more about potential cancer treatments. Undergraduate reports don’t often have this wide-ranging a goal, but you should still try to maintain the distinction between your hypothesis and your purpose. In a solubility experiment, for example, your hypothesis might talk about the relationship between temperature and the rate of solubility, but the purpose is probably to learn more about some specific scientific principle underlying the process of solubility.
For starters, most people say that you should write out your working hypothesis before you perform the experiment or study. Many beginning science students neglect to do so and find themselves struggling to remember precisely which variables were involved in the process or in what way the researchers felt that they were related. Write your hypothesis down as you develop it—you’ll be glad you did.
As for the form a hypothesis should take, it’s best not to be too fancy or complicated; an inventive style isn’t nearly so important as clarity here. There’s nothing wrong with beginning your hypothesis with the phrase, “It was hypothesized that . . .” Be as specific as you can about the relationship between the different objects of your study. In other words, explain that when term A changes, term B changes in this particular way. Readers of scientific writing are rarely content with the idea that a relationship between two terms exists—they want to know what that relationship entails.
Not a hypothesis:
“It was hypothesized that there is a significant relationship between the temperature of a solvent and the rate at which a solute dissolves.”
Hypothesis:
“It was hypothesized that as the temperature of a solvent increases, the rate at which a solute will dissolve in that solvent increases.”
Put more technically, most hypotheses contain both an independent and a dependent variable. The independent variable is what you manipulate to test the reaction; the dependent variable is what changes as a result of your manipulation. In the example above, the independent variable is the temperature of the solvent, and the dependent variable is the rate of solubility. Be sure that your hypothesis includes both variables.
Justify your hypothesis
You need to do more than tell your readers what your hypothesis is; you also need to assure them that this hypothesis was reasonable, given the circumstances. In other words, use the Introduction to explain that you didn’t just pluck your hypothesis out of thin air. (If you did pluck it out of thin air, your problems with your report will probably extend beyond using the appropriate format.) If you posit that a particular relationship exists between the independent and the dependent variable, what led you to believe your “guess” might be supported by evidence?
Scientists often refer to this type of justification as “motivating” the hypothesis, in the sense that something propelled them to make that prediction. Often, motivation includes what we already know—or rather, what scientists generally accept as true (see “Background/previous research” below). But you can also motivate your hypothesis by relying on logic or on your own observations. If you’re trying to decide which solutes will dissolve more rapidly in a solvent at increased temperatures, you might remember that some solids are meant to dissolve in hot water (e.g., bouillon cubes) and some are used for a function precisely because they withstand higher temperatures (they make saucepans out of something). Or you can think about whether you’ve noticed sugar dissolving more rapidly in your glass of iced tea or in your cup of coffee. Even such basic, outside-the-lab observations can help you justify your hypothesis as reasonable.
Background/previous research
This part of the Introduction demonstrates to the reader your awareness of how you’re building on other scientists’ work. If you think of the scientific community as engaging in a series of conversations about various topics, then you’ll recognize that the relevant background material will alert the reader to which conversation you want to enter.
Generally speaking, authors writing journal articles use the background for slightly different purposes than do students completing assignments. Because readers of academic journals tend to be professionals in the field, authors explain the background in order to permit readers to evaluate the study’s pertinence for their own work. You, on the other hand, write toward a much narrower audience—your peers in the course or your lab instructor—and so you must demonstrate that you understand the context for the (presumably assigned) experiment or study you’ve completed. For example, if your professor has been talking about polarity during lectures, and you’re doing a solubility experiment, you might try to connect the polarity of a solid to its relative solubility in certain solvents. In any event, both professional researchers and undergraduates need to connect the background material overtly to their own work.
Organization of this section
Most of the time, writers begin by stating the purpose or objectives of their own work, which establishes for the reader’s benefit the “nature and scope of the problem investigated” (Day 1994). Once you have expressed your purpose, you should then find it easier to move from the general purpose, to relevant material on the subject, to your hypothesis. In abbreviated form, an Introduction section might look like this:
“The purpose of the experiment was to test conventional ideas about solubility in the laboratory [purpose] . . . According to Whitecoat and Labrat (1999), at higher temperatures the molecules of solvents move more quickly . . . We know from the class lecture that molecules moving at higher rates of speed collide with one another more often and thus break down more easily [background material/motivation] . . . Thus, it was hypothesized that as the temperature of a solvent increases, the rate at which a solute will dissolve in that solvent increases [hypothesis].”
Again—these are guidelines, not commandments. Some writers and readers prefer different structures for the Introduction. The one above merely illustrates a common approach to organizing material.
How do I write a strong Materials and Methods section?
As with any piece of writing, your Methods section will succeed only if it fulfills its readers’ expectations, so you need to be clear in your own mind about the purpose of this section. Let’s review the purpose as we described it above: in this section, you want to describe in detail how you tested the hypothesis you developed and also to clarify the rationale for your procedure. In science, it’s not sufficient merely to design and carry out an experiment. Ultimately, others must be able to verify your findings, so your experiment must be reproducible, to the extent that other researchers can follow the same procedure and obtain the same (or similar) results.
Here’s a real-world example of the importance of reproducibility. In 1989, physicists Stanley Pons and Martin Fleischman announced that they had discovered “cold fusion,” a way of producing excess heat and power without the nuclear radiation that accompanies “hot fusion.” Such a discovery could have great ramifications for the industrial production of energy, so these findings created a great deal of interest. When other scientists tried to duplicate the experiment, however, they didn’t achieve the same results, and as a result many wrote off the conclusions as unjustified (or worse, a hoax). To this day, the viability of cold fusion is debated within the scientific community, even though an increasing number of researchers believe it possible. So when you write your Methods section, keep in mind that you need to describe your experiment well enough to allow others to replicate it exactly.
With these goals in mind, let’s consider how to write an effective Methods section in terms of content, structure, and style.
Sometimes the hardest thing about writing this section isn’t what you should talk about, but what you shouldn’t talk about. Writers often want to include the results of their experiment, because they measured and recorded the results during the course of the experiment. But such data should be reserved for the Results section. In the Methods section, you can write that you recorded the results, or how you recorded the results (e.g., in a table), but you shouldn’t write what the results were—not yet. Here, you’re merely stating exactly how you went about testing your hypothesis. As you draft your Methods section, ask yourself the following questions:
- How much detail? Be precise in providing details, but stay relevant. Ask yourself, “Would it make any difference if this piece were a different size or made from a different material?” If not, you probably don’t need to get too specific. If so, you should give as many details as necessary to prevent this experiment from going awry if someone else tries to carry it out. Probably the most crucial detail is measurement; you should always quantify anything you can, such as time elapsed, temperature, mass, volume, etc.
- Rationale: Be sure that as you’re relating your actions during the experiment, you explain your rationale for the protocol you developed. If you capped a test tube immediately after adding a solute to a solvent, why did you do that? (That’s really two questions: why did you cap it, and why did you cap it immediately?) In a professional setting, writers provide their rationale as a way to explain their thinking to potential critics. On one hand, of course, that’s your motivation for talking about protocol, too. On the other hand, since in practical terms you’re also writing to your teacher (who’s seeking to evaluate how well you comprehend the principles of the experiment), explaining the rationale indicates that you understand the reasons for conducting the experiment in that way, and that you’re not just following orders. Critical thinking is crucial—robots don’t make good scientists.
- Control: Most experiments will include a control, which is a means of comparing experimental results. (Sometimes you’ll need to have more than one control, depending on the number of hypotheses you want to test.) The control is exactly the same as the other items you’re testing, except that you don’t manipulate the independent variable-the condition you’re altering to check the effect on the dependent variable. For example, if you’re testing solubility rates at increased temperatures, your control would be a solution that you didn’t heat at all; that way, you’ll see how quickly the solute dissolves “naturally” (i.e., without manipulation), and you’ll have a point of reference against which to compare the solutions you did heat.
Describe the control in the Methods section. Two things are especially important in writing about the control: identify the control as a control, and explain what you’re controlling for. Here is an example:
“As a control for the temperature change, we placed the same amount of solute in the same amount of solvent, and let the solution stand for five minutes without heating it.”
Structure and style
Organization is especially important in the Methods section of a lab report because readers must understand your experimental procedure completely. Many writers are surprised by the difficulty of conveying what they did during the experiment, since after all they’re only reporting an event, but it’s often tricky to present this information in a coherent way. There’s a fairly standard structure you can use to guide you, and following the conventions for style can help clarify your points.
- Subsections: Occasionally, researchers use subsections to report their procedure when the following circumstances apply: 1) if they’ve used a great many materials; 2) if the procedure is unusually complicated; 3) if they’ve developed a procedure that won’t be familiar to many of their readers. Because these conditions rarely apply to the experiments you’ll perform in class, most undergraduate lab reports won’t require you to use subsections. In fact, many guides to writing lab reports suggest that you try to limit your Methods section to a single paragraph.
- Narrative structure: Think of this section as telling a story about a group of people and the experiment they performed. Describe what you did in the order in which you did it. You may have heard the old joke centered on the line, “Disconnect the red wire, but only after disconnecting the green wire,” where the person reading the directions blows everything to kingdom come because the directions weren’t in order. We’re used to reading about events chronologically, and so your readers will generally understand what you did if you present that information in the same way. Also, since the Methods section does generally appear as a narrative (story), you want to avoid the “recipe” approach: “First, take a clean, dry 100 ml test tube from the rack. Next, add 50 ml of distilled water.” You should be reporting what did happen, not telling the reader how to perform the experiment: “50 ml of distilled water was poured into a clean, dry 100 ml test tube.” Hint: most of the time, the recipe approach comes from copying down the steps of the procedure from your lab manual, so you may want to draft the Methods section initially without consulting your manual. Later, of course, you can go back and fill in any part of the procedure you inadvertently overlooked.
- Past tense: Remember that you’re describing what happened, so you should use past tense to refer to everything you did during the experiment. Writers are often tempted to use the imperative (“Add 5 g of the solid to the solution”) because that’s how their lab manuals are worded; less frequently, they use present tense (“5 g of the solid are added to the solution”). Instead, remember that you’re talking about an event which happened at a particular time in the past, and which has already ended by the time you start writing, so simple past tense will be appropriate in this section (“5 g of the solid were added to the solution” or “We added 5 g of the solid to the solution”).
- Active: We heated the solution to 80°C. (The subject, “we,” performs the action, heating.)
- Passive: The solution was heated to 80°C. (The subject, “solution,” doesn’t do the heating–it is acted upon, not acting.)
Increasingly, especially in the social sciences, using first person and active voice is acceptable in scientific reports. Most readers find that this style of writing conveys information more clearly and concisely. This rhetorical choice thus brings two scientific values into conflict: objectivity versus clarity. Since the scientific community hasn’t reached a consensus about which style it prefers, you may want to ask your lab instructor.
How do I write a strong Results section?
Here’s a paradox for you. The Results section is often both the shortest (yay!) and most important (uh-oh!) part of your report. Your Materials and Methods section shows how you obtained the results, and your Discussion section explores the significance of the results, so clearly the Results section forms the backbone of the lab report. This section provides the most critical information about your experiment: the data that allow you to discuss how your hypothesis was or wasn’t supported. But it doesn’t provide anything else, which explains why this section is generally shorter than the others.
Before you write this section, look at all the data you collected to figure out what relates significantly to your hypothesis. You’ll want to highlight this material in your Results section. Resist the urge to include every bit of data you collected, since perhaps not all are relevant. Also, don’t try to draw conclusions about the results—save them for the Discussion section. In this section, you’re reporting facts. Nothing your readers can dispute should appear in the Results section.
Most Results sections feature three distinct parts: text, tables, and figures. Let’s consider each part one at a time.
This should be a short paragraph, generally just a few lines, that describes the results you obtained from your experiment. In a relatively simple experiment, one that doesn’t produce a lot of data for you to repeat, the text can represent the entire Results section. Don’t feel that you need to include lots of extraneous detail to compensate for a short (but effective) text; your readers appreciate discrimination more than your ability to recite facts. In a more complex experiment, you may want to use tables and/or figures to help guide your readers toward the most important information you gathered. In that event, you’ll need to refer to each table or figure directly, where appropriate:
“Table 1 lists the rates of solubility for each substance”
“Solubility increased as the temperature of the solution increased (see Figure 1).”
If you do use tables or figures, make sure that you don’t present the same material in both the text and the tables/figures, since in essence you’ll just repeat yourself, probably annoying your readers with the redundancy of your statements.
Feel free to describe trends that emerge as you examine the data. Although identifying trends requires some judgment on your part and so may not feel like factual reporting, no one can deny that these trends do exist, and so they properly belong in the Results section. Example:
“Heating the solution increased the rate of solubility of polar solids by 45% but had no effect on the rate of solubility in solutions containing non-polar solids.”
This point isn’t debatable—you’re just pointing out what the data show.
As in the Materials and Methods section, you want to refer to your data in the past tense, because the events you recorded have already occurred and have finished occurring. In the example above, note the use of “increased” and “had,” rather than “increases” and “has.” (You don’t know from your experiment that heating always increases the solubility of polar solids, but it did that time.)
You shouldn’t put information in the table that also appears in the text. You also shouldn’t use a table to present irrelevant data, just to show you did collect these data during the experiment. Tables are good for some purposes and situations, but not others, so whether and how you’ll use tables depends upon what you need them to accomplish.
Tables are useful ways to show variation in data, but not to present a great deal of unchanging measurements. If you’re dealing with a scientific phenomenon that occurs only within a certain range of temperatures, for example, you don’t need to use a table to show that the phenomenon didn’t occur at any of the other temperatures. How useful is this table?
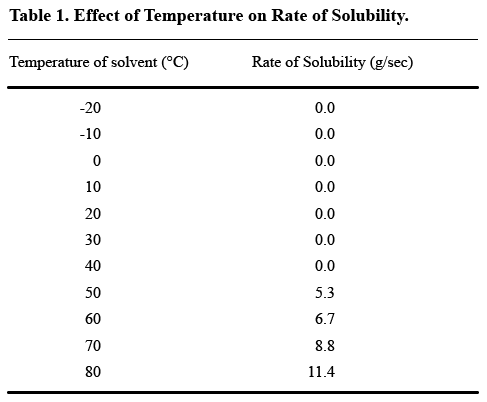
As you can probably see, no solubility was observed until the trial temperature reached 50°C, a fact that the text part of the Results section could easily convey. The table could then be limited to what happened at 50°C and higher, thus better illustrating the differences in solubility rates when solubility did occur.
As a rule, try not to use a table to describe any experimental event you can cover in one sentence of text. Here’s an example of an unnecessary table from How to Write and Publish a Scientific Paper , by Robert A. Day:
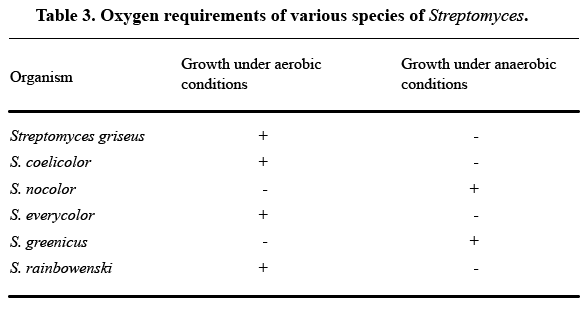
As Day notes, all the information in this table can be summarized in one sentence: “S. griseus, S. coelicolor, S. everycolor, and S. rainbowenski grew under aerobic conditions, whereas S. nocolor and S. greenicus required anaerobic conditions.” Most readers won’t find the table clearer than that one sentence.
When you do have reason to tabulate material, pay attention to the clarity and readability of the format you use. Here are a few tips:
- Number your table. Then, when you refer to the table in the text, use that number to tell your readers which table they can review to clarify the material.
- Give your table a title. This title should be descriptive enough to communicate the contents of the table, but not so long that it becomes difficult to follow. The titles in the sample tables above are acceptable.
- Arrange your table so that readers read vertically, not horizontally. For the most part, this rule means that you should construct your table so that like elements read down, not across. Think about what you want your readers to compare, and put that information in the column (up and down) rather than in the row (across). Usually, the point of comparison will be the numerical data you collect, so especially make sure you have columns of numbers, not rows.Here’s an example of how drastically this decision affects the readability of your table (from A Short Guide to Writing about Chemistry , by Herbert Beall and John Trimbur). Look at this table, which presents the relevant data in horizontal rows:
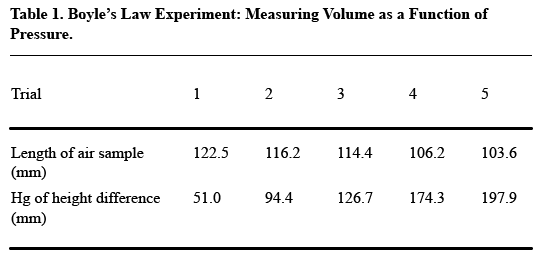
It’s a little tough to see the trends that the author presumably wants to present in this table. Compare this table, in which the data appear vertically:
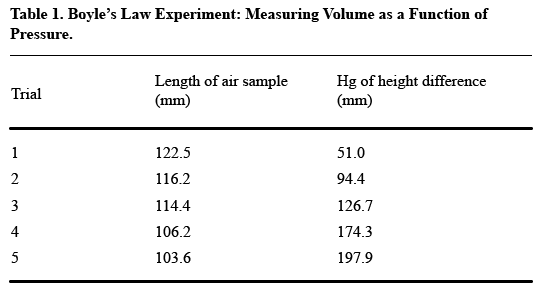
The second table shows how putting like elements in a vertical column makes for easier reading. In this case, the like elements are the measurements of length and height, over five trials–not, as in the first table, the length and height measurements for each trial.
- Make sure to include units of measurement in the tables. Readers might be able to guess that you measured something in millimeters, but don’t make them try.
- Don’t use vertical lines as part of the format for your table. This convention exists because journals prefer not to have to reproduce these lines because the tables then become more expensive to print. Even though it’s fairly unlikely that you’ll be sending your Biology 11 lab report to Science for publication, your readers still have this expectation. Consequently, if you use the table-drawing option in your word-processing software, choose the option that doesn’t rely on a “grid” format (which includes vertical lines).
How do I include figures in my report?
Although tables can be useful ways of showing trends in the results you obtained, figures (i.e., illustrations) can do an even better job of emphasizing such trends. Lab report writers often use graphic representations of the data they collected to provide their readers with a literal picture of how the experiment went.
When should you use a figure?
Remember the circumstances under which you don’t need a table: when you don’t have a great deal of data or when the data you have don’t vary a lot. Under the same conditions, you would probably forgo the figure as well, since the figure would be unlikely to provide your readers with an additional perspective. Scientists really don’t like their time wasted, so they tend not to respond favorably to redundancy.
If you’re trying to decide between using a table and creating a figure to present your material, consider the following a rule of thumb. The strength of a table lies in its ability to supply large amounts of exact data, whereas the strength of a figure is its dramatic illustration of important trends within the experiment. If you feel that your readers won’t get the full impact of the results you obtained just by looking at the numbers, then a figure might be appropriate.
Of course, an undergraduate class may expect you to create a figure for your lab experiment, if only to make sure that you can do so effectively. If this is the case, then don’t worry about whether to use figures or not—concentrate instead on how best to accomplish your task.
Figures can include maps, photographs, pen-and-ink drawings, flow charts, bar graphs, and section graphs (“pie charts”). But the most common figure by far, especially for undergraduates, is the line graph, so we’ll focus on that type in this handout.
At the undergraduate level, you can often draw and label your graphs by hand, provided that the result is clear, legible, and drawn to scale. Computer technology has, however, made creating line graphs a lot easier. Most word-processing software has a number of functions for transferring data into graph form; many scientists have found Microsoft Excel, for example, a helpful tool in graphing results. If you plan on pursuing a career in the sciences, it may be well worth your while to learn to use a similar program.
Computers can’t, however, decide for you how your graph really works; you have to know how to design your graph to meet your readers’ expectations. Here are some of these expectations:
- Keep it as simple as possible. You may be tempted to signal the complexity of the information you gathered by trying to design a graph that accounts for that complexity. But remember the purpose of your graph: to dramatize your results in a manner that’s easy to see and grasp. Try not to make the reader stare at the graph for a half hour to find the important line among the mass of other lines. For maximum effectiveness, limit yourself to three to five lines per graph; if you have more data to demonstrate, use a set of graphs to account for it, rather than trying to cram it all into a single figure.
- Plot the independent variable on the horizontal (x) axis and the dependent variable on the vertical (y) axis. Remember that the independent variable is the condition that you manipulated during the experiment and the dependent variable is the condition that you measured to see if it changed along with the independent variable. Placing the variables along their respective axes is mostly just a convention, but since your readers are accustomed to viewing graphs in this way, you’re better off not challenging the convention in your report.
- Label each axis carefully, and be especially careful to include units of measure. You need to make sure that your readers understand perfectly well what your graph indicates.
- Number and title your graphs. As with tables, the title of the graph should be informative but concise, and you should refer to your graph by number in the text (e.g., “Figure 1 shows the increase in the solubility rate as a function of temperature”).
- Many editors of professional scientific journals prefer that writers distinguish the lines in their graphs by attaching a symbol to them, usually a geometric shape (triangle, square, etc.), and using that symbol throughout the curve of the line. Generally, readers have a hard time distinguishing dotted lines from dot-dash lines from straight lines, so you should consider staying away from this system. Editors don’t usually like different-colored lines within a graph because colors are difficult and expensive to reproduce; colors may, however, be great for your purposes, as long as you’re not planning to submit your paper to Nature. Use your discretion—try to employ whichever technique dramatizes the results most effectively.
- Try to gather data at regular intervals, so the plot points on your graph aren’t too far apart. You can’t be sure of the arc you should draw between the plot points if the points are located at the far corners of the graph; over a fifteen-minute interval, perhaps the change occurred in the first or last thirty seconds of that period (in which case your straight-line connection between the points is misleading).
- If you’re worried that you didn’t collect data at sufficiently regular intervals during your experiment, go ahead and connect the points with a straight line, but you may want to examine this problem as part of your Discussion section.
- Make your graph large enough so that everything is legible and clearly demarcated, but not so large that it either overwhelms the rest of the Results section or provides a far greater range than you need to illustrate your point. If, for example, the seedlings of your plant grew only 15 mm during the trial, you don’t need to construct a graph that accounts for 100 mm of growth. The lines in your graph should more or less fill the space created by the axes; if you see that your data is confined to the lower left portion of the graph, you should probably re-adjust your scale.
- If you create a set of graphs, make them the same size and format, including all the verbal and visual codes (captions, symbols, scale, etc.). You want to be as consistent as possible in your illustrations, so that your readers can easily make the comparisons you’re trying to get them to see.
How do I write a strong Discussion section?
The discussion section is probably the least formalized part of the report, in that you can’t really apply the same structure to every type of experiment. In simple terms, here you tell your readers what to make of the Results you obtained. If you have done the Results part well, your readers should already recognize the trends in the data and have a fairly clear idea of whether your hypothesis was supported. Because the Results can seem so self-explanatory, many students find it difficult to know what material to add in this last section.
Basically, the Discussion contains several parts, in no particular order, but roughly moving from specific (i.e., related to your experiment only) to general (how your findings fit in the larger scientific community). In this section, you will, as a rule, need to:
Explain whether the data support your hypothesis
- Acknowledge any anomalous data or deviations from what you expected
Derive conclusions, based on your findings, about the process you’re studying
- Relate your findings to earlier work in the same area (if you can)
Explore the theoretical and/or practical implications of your findings
Let’s look at some dos and don’ts for each of these objectives.
This statement is usually a good way to begin the Discussion, since you can’t effectively speak about the larger scientific value of your study until you’ve figured out the particulars of this experiment. You might begin this part of the Discussion by explicitly stating the relationships or correlations your data indicate between the independent and dependent variables. Then you can show more clearly why you believe your hypothesis was or was not supported. For example, if you tested solubility at various temperatures, you could start this section by noting that the rates of solubility increased as the temperature increased. If your initial hypothesis surmised that temperature change would not affect solubility, you would then say something like,
“The hypothesis that temperature change would not affect solubility was not supported by the data.”
Note: Students tend to view labs as practical tests of undeniable scientific truths. As a result, you may want to say that the hypothesis was “proved” or “disproved” or that it was “correct” or “incorrect.” These terms, however, reflect a degree of certainty that you as a scientist aren’t supposed to have. Remember, you’re testing a theory with a procedure that lasts only a few hours and relies on only a few trials, which severely compromises your ability to be sure about the “truth” you see. Words like “supported,” “indicated,” and “suggested” are more acceptable ways to evaluate your hypothesis.
Also, recognize that saying whether the data supported your hypothesis or not involves making a claim to be defended. As such, you need to show the readers that this claim is warranted by the evidence. Make sure that you’re very explicit about the relationship between the evidence and the conclusions you draw from it. This process is difficult for many writers because we don’t often justify conclusions in our regular lives. For example, you might nudge your friend at a party and whisper, “That guy’s drunk,” and once your friend lays eyes on the person in question, she might readily agree. In a scientific paper, by contrast, you would need to defend your claim more thoroughly by pointing to data such as slurred words, unsteady gait, and the lampshade-as-hat. In addition to pointing out these details, you would also need to show how (according to previous studies) these signs are consistent with inebriation, especially if they occur in conjunction with one another. To put it another way, tell your readers exactly how you got from point A (was the hypothesis supported?) to point B (yes/no).
Acknowledge any anomalous data, or deviations from what you expected
You need to take these exceptions and divergences into account, so that you qualify your conclusions sufficiently. For obvious reasons, your readers will doubt your authority if you (deliberately or inadvertently) overlook a key piece of data that doesn’t square with your perspective on what occurred. In a more philosophical sense, once you’ve ignored evidence that contradicts your claims, you’ve departed from the scientific method. The urge to “tidy up” the experiment is often strong, but if you give in to it you’re no longer performing good science.
Sometimes after you’ve performed a study or experiment, you realize that some part of the methods you used to test your hypothesis was flawed. In that case, it’s OK to suggest that if you had the chance to conduct your test again, you might change the design in this or that specific way in order to avoid such and such a problem. The key to making this approach work, though, is to be very precise about the weakness in your experiment, why and how you think that weakness might have affected your data, and how you would alter your protocol to eliminate—or limit the effects of—that weakness. Often, inexperienced researchers and writers feel the need to account for “wrong” data (remember, there’s no such animal), and so they speculate wildly about what might have screwed things up. These speculations include such factors as the unusually hot temperature in the room, or the possibility that their lab partners read the meters wrong, or the potentially defective equipment. These explanations are what scientists call “cop-outs,” or “lame”; don’t indicate that the experiment had a weakness unless you’re fairly certain that a) it really occurred and b) you can explain reasonably well how that weakness affected your results.
If, for example, your hypothesis dealt with the changes in solubility at different temperatures, then try to figure out what you can rationally say about the process of solubility more generally. If you’re doing an undergraduate lab, chances are that the lab will connect in some way to the material you’ve been covering either in lecture or in your reading, so you might choose to return to these resources as a way to help you think clearly about the process as a whole.
This part of the Discussion section is another place where you need to make sure that you’re not overreaching. Again, nothing you’ve found in one study would remotely allow you to claim that you now “know” something, or that something isn’t “true,” or that your experiment “confirmed” some principle or other. Hesitate before you go out on a limb—it’s dangerous! Use less absolutely conclusive language, including such words as “suggest,” “indicate,” “correspond,” “possibly,” “challenge,” etc.
Relate your findings to previous work in the field (if possible)
We’ve been talking about how to show that you belong in a particular community (such as biologists or anthropologists) by writing within conventions that they recognize and accept. Another is to try to identify a conversation going on among members of that community, and use your work to contribute to that conversation. In a larger philosophical sense, scientists can’t fully understand the value of their research unless they have some sense of the context that provoked and nourished it. That is, you have to recognize what’s new about your project (potentially, anyway) and how it benefits the wider body of scientific knowledge. On a more pragmatic level, especially for undergraduates, connecting your lab work to previous research will demonstrate to the TA that you see the big picture. You have an opportunity, in the Discussion section, to distinguish yourself from the students in your class who aren’t thinking beyond the barest facts of the study. Capitalize on this opportunity by putting your own work in context.
If you’re just beginning to work in the natural sciences (as a first-year biology or chemistry student, say), most likely the work you’ll be doing has already been performed and re-performed to a satisfactory degree. Hence, you could probably point to a similar experiment or study and compare/contrast your results and conclusions. More advanced work may deal with an issue that is somewhat less “resolved,” and so previous research may take the form of an ongoing debate, and you can use your own work to weigh in on that debate. If, for example, researchers are hotly disputing the value of herbal remedies for the common cold, and the results of your study suggest that Echinacea diminishes the symptoms but not the actual presence of the cold, then you might want to take some time in the Discussion section to recapitulate the specifics of the dispute as it relates to Echinacea as an herbal remedy. (Consider that you have probably already written in the Introduction about this debate as background research.)
This information is often the best way to end your Discussion (and, for all intents and purposes, the report). In argumentative writing generally, you want to use your closing words to convey the main point of your writing. This main point can be primarily theoretical (“Now that you understand this information, you’re in a better position to understand this larger issue”) or primarily practical (“You can use this information to take such and such an action”). In either case, the concluding statements help the reader to comprehend the significance of your project and your decision to write about it.
Since a lab report is argumentative—after all, you’re investigating a claim, and judging the legitimacy of that claim by generating and collecting evidence—it’s often a good idea to end your report with the same technique for establishing your main point. If you want to go the theoretical route, you might talk about the consequences your study has for the field or phenomenon you’re investigating. To return to the examples regarding solubility, you could end by reflecting on what your work on solubility as a function of temperature tells us (potentially) about solubility in general. (Some folks consider this type of exploration “pure” as opposed to “applied” science, although these labels can be problematic.) If you want to go the practical route, you could end by speculating about the medical, institutional, or commercial implications of your findings—in other words, answer the question, “What can this study help people to do?” In either case, you’re going to make your readers’ experience more satisfying, by helping them see why they spent their time learning what you had to teach them.
Works consulted
We consulted these works while writing this handout. This is not a comprehensive list of resources on the handout’s topic, and we encourage you to do your own research to find additional publications. Please do not use this list as a model for the format of your own reference list, as it may not match the citation style you are using. For guidance on formatting citations, please see the UNC Libraries citation tutorial . We revise these tips periodically and welcome feedback.
American Psychological Association. 2010. Publication Manual of the American Psychological Association . 6th ed. Washington, DC: American Psychological Association.
Beall, Herbert, and John Trimbur. 2001. A Short Guide to Writing About Chemistry , 2nd ed. New York: Longman.
Blum, Deborah, and Mary Knudson. 1997. A Field Guide for Science Writers: The Official Guide of the National Association of Science Writers . New York: Oxford University Press.
Booth, Wayne C., Gregory G. Colomb, Joseph M. Williams, Joseph Bizup, and William T. FitzGerald. 2016. The Craft of Research , 4th ed. Chicago: University of Chicago Press.
Briscoe, Mary Helen. 1996. Preparing Scientific Illustrations: A Guide to Better Posters, Presentations, and Publications , 2nd ed. New York: Springer-Verlag.
Council of Science Editors. 2014. Scientific Style and Format: The CSE Manual for Authors, Editors, and Publishers , 8th ed. Chicago & London: University of Chicago Press.
Davis, Martha. 2012. Scientific Papers and Presentations , 3rd ed. London: Academic Press.
Day, Robert A. 1994. How to Write and Publish a Scientific Paper , 4th ed. Phoenix: Oryx Press.
Porush, David. 1995. A Short Guide to Writing About Science . New York: Longman.
Williams, Joseph, and Joseph Bizup. 2017. Style: Lessons in Clarity and Grace , 12th ed. Boston: Pearson.
You may reproduce it for non-commercial use if you use the entire handout and attribute the source: The Writing Center, University of North Carolina at Chapel Hill
Make a Gift

- Langson Library
- Science Library
- Grunigen Medical Library
- Law Library
- Connect From Off-Campus
- Accessibility
- Gateway Study Center

Email this link
Writing a scientific paper.
- Writing a lab report
- INTRODUCTION
Writing a "good" results section
Figures and Captions in Lab Reports
"Results Checklist" from: How to Write a Good Scientific Paper. Chris A. Mack. SPIE. 2018.
Additional tips for results sections.
- LITERATURE CITED
- Bibliography of guides to scientific writing and presenting
- Peer Review
- Presentations
- Lab Report Writing Guides on the Web
This is the core of the paper. Don't start the results sections with methods you left out of the Materials and Methods section. You need to give an overall description of the experiments and present the data you found.
- Factual statements supported by evidence. Short and sweet without excess words
- Present representative data rather than endlessly repetitive data
- Discuss variables only if they had an effect (positive or negative)
- Use meaningful statistics
- Avoid redundancy. If it is in the tables or captions you may not need to repeat it
A short article by Dr. Brett Couch and Dr. Deena Wassenberg, Biology Program, University of Minnesota
- Present the results of the paper, in logical order, using tables and graphs as necessary.
- Explain the results and show how they help to answer the research questions posed in the Introduction. Evidence does not explain itself; the results must be presented and then explained.
- Avoid: presenting results that are never discussed; presenting results in chronological order rather than logical order; ignoring results that do not support the conclusions;
- Number tables and figures separately beginning with 1 (i.e. Table 1, Table 2, Figure 1, etc.).
- Do not attempt to evaluate the results in this section. Report only what you found; hold all discussion of the significance of the results for the Discussion section.
- It is not necessary to describe every step of your statistical analyses. Scientists understand all about null hypotheses, rejection rules, and so forth and do not need to be reminded of them. Just say something like, "Honeybees did not use the flowers in proportion to their availability (X2 = 7.9, p<0.05, d.f.= 4, chi-square test)." Likewise, cite tables and figures without describing in detail how the data were manipulated. Explanations of this sort should appear in a legend or caption written on the same page as the figure or table.
- You must refer in the text to each figure or table you include in your paper.
- Tables generally should report summary-level data, such as means ± standard deviations, rather than all your raw data. A long list of all your individual observations will mean much less than a few concise, easy-to-read tables or figures that bring out the main findings of your study.
- Only use a figure (graph) when the data lend themselves to a good visual representation. Avoid using figures that show too many variables or trends at once, because they can be hard to understand.
From: https://writingcenter.gmu.edu/guides/imrad-results-discussion
- << Previous: METHODS
- Next: DISCUSSION >>
- Last Updated: Aug 4, 2023 9:33 AM
- URL: https://guides.lib.uci.edu/scientificwriting
Off-campus? Please use the Software VPN and choose the group UCIFull to access licensed content. For more information, please Click here
Software VPN is not available for guests, so they may not have access to some content when connecting from off-campus.

Scientific Writing
Ucla writing center.
Vistit the UCLA Writing Center to make an appointment to review your Cover letters, CVs, or papers.
COVER LETTERS AND CVs
Sample Cover Letter & CV
THE ABSTRACT
How to Write an Abstract
What is a Research Abstract
THE RESEARCH PAPER
A Paper Planner to break down your writing timeline prepared by UCLA’s Undergraduate Science Journal
A guide to science writing prepared by UCLA’s Undergraduate Science Journal
Introductions and conclusions for scientific papers by the George Mason University Writing Center
The Science of Scientific Writing by American Scientist
Scientific Writing Booklet compiled by Marc Tischler, Ph.D. University of Arizona
ORCID provides a digital identifier to help track all published work and help distinguish yourself from other researchers
WI+RE Reading Strategies compile tutorials to help students learn to analyze research papers and academic articles.

- Research Process
Writing a Scientific Research Project Proposal
- 5 minute read
- 100.4K views
Table of Contents
The importance of a well-written research proposal cannot be underestimated. Your research really is only as good as your proposal. A poorly written, or poorly conceived research proposal will doom even an otherwise worthy project. On the other hand, a well-written, high-quality proposal will increase your chances for success.
In this article, we’ll outline the basics of writing an effective scientific research proposal, including the differences between research proposals, grants and cover letters. We’ll also touch on common mistakes made when submitting research proposals, as well as a simple example or template that you can follow.
What is a scientific research proposal?
The main purpose of a scientific research proposal is to convince your audience that your project is worthwhile, and that you have the expertise and wherewithal to complete it. The elements of an effective research proposal mirror those of the research process itself, which we’ll outline below. Essentially, the research proposal should include enough information for the reader to determine if your proposed study is worth pursuing.
It is not an uncommon misunderstanding to think that a research proposal and a cover letter are the same things. However, they are different. The main difference between a research proposal vs cover letter content is distinct. Whereas the research proposal summarizes the proposal for future research, the cover letter connects you to the research, and how you are the right person to complete the proposed research.
There is also sometimes confusion around a research proposal vs grant application. Whereas a research proposal is a statement of intent, related to answering a research question, a grant application is a specific request for funding to complete the research proposed. Of course, there are elements of overlap between the two documents; it’s the purpose of the document that defines one or the other.
Scientific Research Proposal Format
Although there is no one way to write a scientific research proposal, there are specific guidelines. A lot depends on which journal you’re submitting your research proposal to, so you may need to follow their scientific research proposal template.
In general, however, there are fairly universal sections to every scientific research proposal. These include:
- Title: Make sure the title of your proposal is descriptive and concise. Make it catch and informative at the same time, avoiding dry phrases like, “An investigation…” Your title should pique the interest of the reader.
- Abstract: This is a brief (300-500 words) summary that includes the research question, your rationale for the study, and any applicable hypothesis. You should also include a brief description of your methodology, including procedures, samples, instruments, etc.
- Introduction: The opening paragraph of your research proposal is, perhaps, the most important. Here you want to introduce the research problem in a creative way, and demonstrate your understanding of the need for the research. You want the reader to think that your proposed research is current, important and relevant.
- Background: Include a brief history of the topic and link it to a contemporary context to show its relevance for today. Identify key researchers and institutions also looking at the problem
- Literature Review: This is the section that may take the longest amount of time to assemble. Here you want to synthesize prior research, and place your proposed research into the larger picture of what’s been studied in the past. You want to show your reader that your work is original, and adds to the current knowledge.
- Research Design and Methodology: This section should be very clearly and logically written and organized. You are letting your reader know that you know what you are going to do, and how. The reader should feel confident that you have the skills and knowledge needed to get the project done.
- Preliminary Implications: Here you’ll be outlining how you anticipate your research will extend current knowledge in your field. You might also want to discuss how your findings will impact future research needs.
- Conclusion: This section reinforces the significance and importance of your proposed research, and summarizes the entire proposal.
- References/Citations: Of course, you need to include a full and accurate list of any and all sources you used to write your research proposal.
Common Mistakes in Writing a Scientific Research Project Proposal
Remember, the best research proposal can be rejected if it’s not well written or is ill-conceived. The most common mistakes made include:
- Not providing the proper context for your research question or the problem
- Failing to reference landmark/key studies
- Losing focus of the research question or problem
- Not accurately presenting contributions by other researchers and institutions
- Incompletely developing a persuasive argument for the research that is being proposed
- Misplaced attention on minor points and/or not enough detail on major issues
- Sloppy, low-quality writing without effective logic and flow
- Incorrect or lapses in references and citations, and/or references not in proper format
- The proposal is too long – or too short
Scientific Research Proposal Example
There are countless examples that you can find for successful research proposals. In addition, you can also find examples of unsuccessful research proposals. Search for successful research proposals in your field, and even for your target journal, to get a good idea on what specifically your audience may be looking for.
While there’s no one example that will show you everything you need to know, looking at a few will give you a good idea of what you need to include in your own research proposal. Talk, also, to colleagues in your field, especially if you are a student or a new researcher. We can often learn from the mistakes of others. The more prepared and knowledgeable you are prior to writing your research proposal, the more likely you are to succeed.
Language Editing Services
One of the top reasons scientific research proposals are rejected is due to poor logic and flow. Check out our Language Editing Services to ensure a great proposal , that’s clear and concise, and properly referenced. Check our video for more information, and get started today.

- Manuscript Review
Research Fraud: Falsification and Fabrication in Research Data

Research Team Structure
You may also like.

Descriptive Research Design and Its Myriad Uses

Five Common Mistakes to Avoid When Writing a Biomedical Research Paper

Making Technical Writing in Environmental Engineering Accessible


To Err is Not Human: The Dangers of AI-assisted Academic Writing

When Data Speak, Listen: Importance of Data Collection and Analysis Methods

Choosing the Right Research Methodology: A Guide for Researchers

Why is data validation important in research?

Writing a good review article
Input your search keywords and press Enter.
Writing an Introduction for a Scientific Paper
Dr. michelle harris, dr. janet batzli, biocore.
This section provides guidelines on how to construct a solid introduction to a scientific paper including background information, study question , biological rationale, hypothesis , and general approach . If the Introduction is done well, there should be no question in the reader’s mind why and on what basis you have posed a specific hypothesis.
Broad Question : based on an initial observation (e.g., “I see a lot of guppies close to the shore. Do guppies like living in shallow water?”). This observation of the natural world may inspire you to investigate background literature or your observation could be based on previous research by others or your own pilot study. Broad questions are not always included in your written text, but are essential for establishing the direction of your research.
Background Information : key issues, concepts, terminology, and definitions needed to understand the biological rationale for the experiment. It often includes a summary of findings from previous, relevant studies. Remember to cite references, be concise, and only include relevant information given your audience and your experimental design. Concisely summarized background information leads to the identification of specific scientific knowledge gaps that still exist. (e.g., “No studies to date have examined whether guppies do indeed spend more time in shallow water.”)
Testable Question : these questions are much more focused than the initial broad question, are specific to the knowledge gap identified, and can be addressed with data. (e.g., “Do guppies spend different amounts of time in water <1 meter deep as compared to their time in water that is >1 meter deep?”)
Biological Rationale : describes the purpose of your experiment distilling what is known and what is not known that defines the knowledge gap that you are addressing. The “BR” provides the logic for your hypothesis and experimental approach, describing the biological mechanism and assumptions that explain why your hypothesis should be true.
The biological rationale is based on your interpretation of the scientific literature, your personal observations, and the underlying assumptions you are making about how you think the system works. If you have written your biological rationale, your reader should see your hypothesis in your introduction section and say to themselves, “Of course, this hypothesis seems very logical based on the rationale presented.”
- A thorough rationale defines your assumptions about the system that have not been revealed in scientific literature or from previous systematic observation. These assumptions drive the direction of your specific hypothesis or general predictions.
- Defining the rationale is probably the most critical task for a writer, as it tells your reader why your research is biologically meaningful. It may help to think about the rationale as an answer to the questions— how is this investigation related to what we know, what assumptions am I making about what we don’t yet know, AND how will this experiment add to our knowledge? *There may or may not be broader implications for your study; be careful not to overstate these (see note on social justifications below).
- Expect to spend time and mental effort on this. You may have to do considerable digging into the scientific literature to define how your experiment fits into what is already known and why it is relevant to pursue.
- Be open to the possibility that as you work with and think about your data, you may develop a deeper, more accurate understanding of the experimental system. You may find the original rationale needs to be revised to reflect your new, more sophisticated understanding.
- As you progress through Biocore and upper level biology courses, your rationale should become more focused and matched with the level of study e ., cellular, biochemical, or physiological mechanisms that underlie the rationale. Achieving this type of understanding takes effort, but it will lead to better communication of your science.
***Special note on avoiding social justifications: You should not overemphasize the relevance of your experiment and the possible connections to large-scale processes. Be realistic and logical —do not overgeneralize or state grand implications that are not sensible given the structure of your experimental system. Not all science is easily applied to improving the human condition. Performing an investigation just for the sake of adding to our scientific knowledge (“pure or basic science”) is just as important as applied science. In fact, basic science often provides the foundation for applied studies.
Hypothesis / Predictions : specific prediction(s) that you will test during your experiment. For manipulative experiments, the hypothesis should include the independent variable (what you manipulate), the dependent variable(s) (what you measure), the organism or system , the direction of your results, and comparison to be made.
If you are doing a systematic observation , your hypothesis presents a variable or set of variables that you predict are important for helping you characterize the system as a whole, or predict differences between components/areas of the system that help you explain how the system functions or changes over time.
Experimental Approach : Briefly gives the reader a general sense of the experiment, the type of data it will yield, and the kind of conclusions you expect to obtain from the data. Do not confuse the experimental approach with the experimental protocol . The experimental protocol consists of the detailed step-by-step procedures and techniques used during the experiment that are to be reported in the Methods and Materials section.
Some Final Tips on Writing an Introduction
- As you progress through the Biocore sequence, for instance, from organismal level of Biocore 301/302 to the cellular level in Biocore 303/304, we expect the contents of your “Introduction” paragraphs to reflect the level of your coursework and previous writing experience. For example, in Biocore 304 (Cell Biology Lab) biological rationale should draw upon assumptions we are making about cellular and biochemical processes.
- Be Concise yet Specific: Remember to be concise and only include relevant information given your audience and your experimental design. As you write, keep asking, “Is this necessary information or is this irrelevant detail?” For example, if you are writing a paper claiming that a certain compound is a competitive inhibitor to the enzyme alkaline phosphatase and acts by binding to the active site, you need to explain (briefly) Michaelis-Menton kinetics and the meaning and significance of Km and Vmax. This explanation is not necessary if you are reporting the dependence of enzyme activity on pH because you do not need to measure Km and Vmax to get an estimate of enzyme activity.
- Another example: if you are writing a paper reporting an increase in Daphnia magna heart rate upon exposure to caffeine you need not describe the reproductive cycle of magna unless it is germane to your results and discussion. Be specific and concrete, especially when making introductory or summary statements.
Where Do You Discuss Pilot Studies? Many times it is important to do pilot studies to help you get familiar with your experimental system or to improve your experimental design. If your pilot study influences your biological rationale or hypothesis, you need to describe it in your Introduction. If your pilot study simply informs the logistics or techniques, but does not influence your rationale, then the description of your pilot study belongs in the Materials and Methods section.
How will introductions be evaluated? The following is part of the rubric we will be using to evaluate your papers.
- The Scientist University
How to Write a Good Introduction Section
A strong narrative is as integral a part of science writing as it is for any other form of communication..

Nathan Ni holds a PhD from Queens University. He is a science editor for The Scientist’s Creative Services Team who strives to better understand and communicate the relationships between health and disease.
View full profile.
Learn about our editorial policies.

First impressions are important. Scientists need to make their work stand out among a sea of others. However, many mistakenly believe that first impressions are formed based only on titles and abstracts. In actuality, the introduction section is critical to making a real impression on the audience. The introduction is where authors outline their research topic and describe their study. It is where they provide background information and showcase their writing and argumentation styles. For these reasons, the introduction engages the audience in a deeper way than the formalities and rigidities of the title and abstract can afford. To use a fishing analogy: if the title and the abstract serve as the hook and the bait, then the introduction is the process of actually reeling the fish into the boat.
Good Introductions Are Important Guides
In contrast to the constraints placed on the title and abstract, the introduction is the first real opportunity for the scientist to engage with their audience and showcase and convey their passions and motivations for the study in question. This opportunity is somewhat of a double-edged sword. Study authors inevitably have a treasure trove of knowledge and expertise when it comes to their projects and their fields. However, they must remember that the audience does not necessarily have this background information—and that they are only engaging with their audience for a finite amount of time. Despite the urge to excitedly write about all of the different aspects and intricacies of the project, it is very important that authors keep their introductions simple and well organized.
Therefore, the introduction should move from broad scopes to narrow focuses as the audience reads further. The author should direct the reader along this journey, focusing on topics with direct relevance to what was investigated in the study. A broad fact introduced early on should be linked or paired with a more specific fact along the same lines of thought, eventually culminating in how this information led to the motivation behind the study itself. It is vital to not go off on tangents or talk about things that are too esoteric. A confused audience is an audience that tends not to read further.
Applying Common Principles Across Well-Known and Niche Subjects
Writers can apply these principles in more specialized manuscripts focusing on a single entity rather than a well-known pathology. Consider the following example from a manuscript by cell biologist Luis R. Cruz-Vera’s research team from the University of Alabama in Huntsville, published in the Journal of Biological Chemistry. 1
Here, they divide the opening paragraph of their introduction into four distinct sections. First, they explain what ribosome arresting peptides (RAPs) are and what they do.
Ribosome arresting peptides (RAPs) are nascent polypeptides that act in cis on the translating ribosome to control the expression of genes by inducing ribosome arrest during translation elongation or termination. RAPs commonly sense external forces or low molecular weight compounds in the environment that spatially and temporally contribute to the expression of genes.
Then they introduce the two different types of RAPs.
RAPs such as SecM that sense external forces on the ribosome are typically large, because these nascent peptides have a domain that functions outside of the ribosome. In contrast, those that sense small molecules inside of the ribosome, such as TnaC are smaller.
They describe how each type works via a different mechanism.
Typically, larger RAPs interact with cellular factors that can control their capacity for arresting ribosomes. Because of their size and proximity to ribosomal components, large RAPs clearly show two structural domains, a sensor domain and an arresting domain. At the moment of the arrest for the large RAPs, the sensor domain is located outside the ribosome exit tunnel, whereas the arresting domain remains inside the tunnel. The short RAPs currently characterized interact with the compounds that they sense by using the ribosome exit tunnel as a binding surface. For these short RAPs, it has been determined that conserved amino acid residues are necessary to induce arrest by either directly binding the effector molecule or by acting at the peptidyl-transferase center (PTC) during ribosome arrest.
And finally, they conclude by highlighting a knowledge gap in how small RAPs operate versus what is already known about large RAPs.
However, because the size of short RAPs ranges from only a few to a couple of dozen amino acids, as in the case of TnaC, it has remained unclear whether short RAPs are constituted by the two independent sensor and stalling domains, as it has been observed with larger RAPs.
In this way, the authors make a natural progression from “why this topic is important” to “what is known about this topic,” setting the stage for “what is unknown about this topic and why it should be studied.”
Gradually Moving from Broad to Narrow

These principles can be further transferred towards the introductory section as a whole. The first paragraph should serve as an introduction to the field and the topic. The middle paragraph(s) provide exposition and detail regarding what is known and unknown, and what has already been done and still remains to do, and the final paragraph outlines the study and its principle findings, providing a transition into either the materials and methods or the results section.
For example, this work by radiation oncologist Eric Deutsch’s group at Université Paris-Saclay, published in PLoS One , 2 opens by succinctly explaining a scientific problem: “ the threat of extensive dispersion of radioactive isotopes within populated areas that would have an unfortunate effect on human health has increased drastically .” It then offers the call to action necessitated by this problem: “ the development of a decorporating agent capable of effectively mitigating the effects of a wide range of isotopes is critical .”
In the next two paragraphs, the study authors provide information on how and why dispersion of radioactive isotopes are a problem—“ the FDA has approved only three compounds (only one of which is used as a preventative therapy) for the treatment of exposure to specific radioactive elements ”—and highlights the strengths and weaknesses of what is currently available. They then introduce the focal point of their own work, chitosan@DOTAGA, within this context, explaining its potential as a solution to the problem they previously introduced: “ After oral administration to rodents over several days, no signs of acute or chronic toxicity were observed, and DOTAGA did not enter the blood stream and was fully eliminated from the gastrointestinal tract within 24 hours of administration. ”
Finally, the introduction concludes by listing the study objective—“ explore the potential of this polymer for use in the decorporation of a wide range of radioactive isotopes ”—and the motivations and rationale behind the study objective—“ there are no suitable countermeasures available for uranium poisoning. […] This innovative approach aims to directly chelate the radioactive cations, specifically uranium, within the gastrointestinal tract prior to their systemic absorption, which ensures their prompt elimination and mitigation of the associated toxicities. ”
The Introduction Engages with the Reader
The introduction section is often overlooked in favor of the title and the abstract, but it serves two important functions. First, it gives the audience all of the information that it needs to contextualize the yet-to-be-presented data within the context of the problem that needs to be solved or the scientific question that needs to be addressed. Second, and more importantly, it justifies the importance of the study, of its initiative, rationale, and purpose. The introduction is the author’s best—and arguably only real—opportunity to convince the audience that their study is worth reading.
Looking for more information on scientific writing? Check out The Scientist’s TS SciComm section. Looking for some help putting together a manuscript, a figure, a poster, or anything else? The Scientist’s Scientific Services may have the professional help that you need.
- Judd HNG, et al. Functional domains of a ribosome arresting peptide are affected by surrounding nonconserved residues . J Biol Chem . 2024;300(3):105780.
- Durand A, et al. Enhancing radioprotection: A chitosan-based chelating polymer is a versatile radioprotective agent for prophylactic and therapeutic interventions against radionuclide contamination . PLoS One . 2024;19(4):e0292414.
Related community Research Resources

Building a Scientific Narrative

Where Books Meet Bacteria

The Fundamentals of Academic Science Writing
May 1, 2024
18 min read
Can Scientific Thinking Save the World?
A physicist, a philosopher and a psychologist are working together to bring better, smarter decision-making to the masses
By Lee Billings
Kislev/Getty Images
A physicist, a philosopher and a psychologist walk into a classroom.
Although it sounds like a premise for a joke, this was actually the origin of a unique collaboration between Nobel Prize–winning physicist Saul Perlmutter, philosopher John Campbell and the psychologist Rob MacCoun. Spurred by what they saw as a perilously rising tide of irrationality, misinformation and sociopolitical polarization, they teamed up in 2011 to create a multidisciplinary course at the University of California, Berkeley, with the modest goal of teaching undergraduate students how to think—more specifically, how to think like a scientist . That is, they wished to show students how to use scientific tools and techniques for solving problems, making decisions and distinguishing reality from fantasy . The course proved popular, drawing enough interest to run for more than a decade (and counting) while sparking multiple spin-offs at other universities and institutions.
Now the three researchers are bringing their message to the masses with a new book, Third Millennium Thinking: Creating Sense in a World of Nonsense . And their timing is impeccable: Our world seems to have only become more uncertain and complex since their course began, with cognitive biases and information overload all too easily clouding debates over high-stakes issues such as climate change , global pandemics , and the development and regulation of artificial intelligence . But one need not be an academic expert or policymaker to find value in this book’s pages. From parsing the daily news to treating a medical condition, talking with opposite-minded relatives at Thanksgiving or even choosing how to vote in an election, Third Millennium Thinking offers lessons that anyone can use—individually and collectively—to make smarter, better decisions in everyday life.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Scientific American spoke with Perlmutter, Campbell and MacCoun about their work—and whether it’s wishful thinking to believe logic and evidence can save the world.
[ An edited transcript of the interview follows .]
How did all of this begin, and what motivated each of you to take on such an ambitious project?
PERLMUTTER: In 2011 I was looking at our society making big decisions: “Should we raise the debt ceiling?”—things like that. And surprisingly enough, we were not doing it in a very sensible way. The conversations I was hearing about these political decisions weren’t like those I’d have over lunch with a bunch of scientists at the lab—not because of politics, but rather because of the style of how scientists tend to think about solving problems. And I thought, “Well, where did scientists learn this stuff? And is it possible for us to articulate what these concepts are and teach them in a way that people would apply them in their whole lives, not just in a lab? And can we empower them to think for themselves using the best available cognitive tools rather than teaching them to ‘just trust scientists?’”
So that was the starting point of it. But that’s not the whole story. If you put a bunch of physicists together in a faculty meeting, they don’t necessarily act much more rational than any other faculty members, right? So it was clear we really needed expertise from other fields, too, such as John’s expertise in philosophy and Rob’s expertise in social psychology. We actually put a little sign up looking for people who’d want to help develop the course. It said something like, “Are you embarrassed watching our society make decisions? Come help invent our course; come help save the world.”
MacCOUN: When Saul approached me about the course, I was delighted to work with him. Even back in 2011 I was filled with angst about the inefficacy of policy debates; I had spent years working on two big hot-button issues: drug legalization and open military service for gay and lesbian individuals. I worked with policymakers and advocates on both sides, just trying to be an honest broker in these debates to help clarify the truth—you know, “What do we actually know, and what don’t we know?” And the quality of debate for both of those issues was so bad, with so much distortion of research findings. So when Saul mentioned the course to me, I just jumped at the chance to work on this.
CAMPBELL: It was obvious to me that this was philosophically very interesting. I mean, we’re talking about how science inputs into decision-making. And in decision-making, there are always questions of value, as well as questions of fact; questions about where you want to go, as well as questions about how do we get there; and questions about what “the science” can answer. And it’s very interesting to ask, “Can we tease apart facts and values in decision-making? Does the science have anything to tell us about values?” Well, likely not. Scientists always shy away from telling us about values. So we need to know something about how broader effective concerns can be woven in with scientific results in decision-making.
Some of this is about how science is embedded in the life of a community. You take a village—you have the pub, you have the church, you know clearly what they are for and how they function in the whole community. But then the science, what is that? Is it just this kind of shimmering thing that produces telephones, TVs and stuff? How does it fit into the life of the community? How does it embed in our civilization? Classically, it’s been regarded as a “high church” kind of thing. The scientists are literally in an ivory tower and do as they please. And then occasionally, they produce these gadgets, and we’re not sure if we should like them or not. But we really need a more healthy, grounded conception of how science plays into our broader society.
I’m glad you brought up the distinction between facts and values. To me, that overlaps with the distinction between groups and individuals—“values” feel more personal and subjective and thus more directly applicable to a reader, in a way. And the book is ultimately about how individuals can empower themselves with so-called scientific thinking—presumably to live their best lives based on their personal values. But how does that accord with this other assertion you’ve just made, saying science likely doesn’t have anything to tell us about values in the first place?
PERLMUTTER: Well, I think what John was getting at is: even once we develop all these ways to think through facts, we don’t want to stop thinking through values, right? One point here is that we’ve actually made progress together thinking about values over centuries. And we have to keep talking to each other. But it’s still very helpful to separate the values and the facts because each requires a slightly different style of thinking, and you want people to be able to do both.
MacCOUN: That’s right. Scientists can’t tell us and shouldn’t tell us, in fact, what values to hold. Scientists get in trouble when they try that. We talk in the book about “pathologies” of science that sometimes happen and how those can be driven by values-based thinking. Regarding values, where science excels is in clarifying where and how they conflict so that in public policy analysis, you can inform the trade-offs to make sure that the stakeholders in a debate empirically understand how its various outcomes advance certain values while impeding others. Usually what happens next is finding solutions that minimize those trade-offs and reduce the friction between conflicting values.
And let’s be clear: when we talk about values, we sometimes talk as if people are either one thing or another. You know, someone may ask, “Are you for or against ‘freedom?’” But in reality, everyone values freedom. It’s just a question of how much, of how we differ in our rankings of such things. And we’re all looking for some way to pursue more than one value at a time, and we need other people to help us get there.
PERLMUTTER: And let’s remember that we’re not even consistent within our own selves about our individual rankings of values, which tend to fluctuate a lot based on the situation.
I love how our discussion is now reflecting the style of the book: breezy and approachable but also unflinching in talking about complexity and uncertainty. And in it, you’re trying to give readers a “tool kit” for navigating such things. That’s great, yet it can be challenging for readers who might assume it’s, say, a science-infused self-help book offering them a few simple rules about how to improve their rational thinking. This makes me wonder: If you did have to somehow reduce the book’s message to something like a series of bullet points on a note card, what would that be? What are the most essential tools in the kit?
CAMPBELL: This may be a bit ironic, but I was reading somewhere recently that where AI programs such as ChatGPT really go wrong is in not giving sources. Most of these tools don’t tell you what evidence they’re using for their outputs. And you’d think, of course, we should always show what evidence we have for anything we’re gonna say. But really, we can’t do that. Most of us can’t remember the evidence for half of what we know. What we can usually recall is how likely we thought some assertion was to be true, how probable we thought it was. And keeping track of this is a worthwhile habit of mind: if you’re going to act on any belief you might have, you need to know the strength with which you can hold that belief.
PERLMUTTER: We spend a fair amount of time on this in the book because it allows you to see that the world doesn’t come to us with certainty in almost anything. Even when we’re pretty sure of something, we’re only pretty sure, and there’s real utility in having a sense of the possibility for something contradicting what we think or expect. Many people do this naturally all the time, thinking about the odds for placing a bet on their favorite sports team or about the chance of a rain shower spoiling a picnic. Acknowledging uncertainty puts your ego in the right place. Your ego should, in the end, be attached to being pretty good at knowing how strong or weak your trust is in some fact rather than in being always right. Needing to always be right is a very problematic way to approach the world. In the book, we compare it to skiing down a mountain with all your weight rigid on both legs; if you don’t ever shift your stance to turn and slow down, you might go very fast, but you usually don’t get very far before toppling over! So instead you need to be able to maneuver and adjust to keep track of what it is that you really do know versus what you don’t. That’s how to actually get wherever you’re trying to go, and it’s also how to have useful conversations with other people who may not agree with you.
MacCOUN: And that sense of working together is important because these habits of mind we’re discussing aren’t just about your personal decision-making; they’re also about how science works in a democracy. You know, scientists end up having to work with people they disagree with all the time. And they cultivate certain communal ways of doing that—because it’s not enough to just be a “better” thinker; even people well-trained in these methods make mistakes. So you also need these habits at a communal level for other people to keep you honest. That means it’s okay, and necessary even, to interact with people who disagree with you—because that’s how you find out when you’re making mistakes. And it doesn’t necessarily mean you’ll change your mind. But it’ll improve your thinking about your own views.

So in summary:
Try to rank your confidence in your beliefs.
Try to update your beliefs based on new evidence and don’t fear being (temporarily) wrong.
Try to productively engage with others who have different beliefs than you.
That’s a pretty good “top three” list, I think! But, pardon my cynicism, do you worry that some of this might come off as rather quaint? We mentioned at the outset how this project really began in 2011, not much more than a decade ago. Yet some would probably argue that social and technological changes across that time have now effectively placed us in a different situation, a different world. It seems—to me at least—on average much harder now than it was 10 years ago for people with divergent beliefs and values to have a pleasant, productive conversation. Are the challenges we face today really things that can be solved by everyone just getting together and talking?
CAMPBELL: I agree with you that this sort of cynicism is now widespread. Across the past few decades we seem to have forgotten how to have a conversation across a fundamental divide, so now we take for granted that it’s pointless to try to convert those holding different views. But the alternative is to run society by coercion. And just beating people down with violent subjugation is not a long-term tenable solution. If you’re going to coerce, you have to at least show your work. You have to engage with other people and explain why you think your policies are good.
MacCOUN: You can think of cynicism as this god-awful corrosive mix of skepticism and pessimism. At the other extreme, you have gullibility, which, combined with optimism, leads to wishful thinking. And that’s really not helpful either. In the book we talk about an insight Saul had, which is that scientists tend to combine skepticism with optimism—a combo I’d say is not generally cultivated in our society. Scientists are skeptical, not gullible, but they’re optimistic, not pessimistic: they tend to assume that problems have a solution. So scientists sitting around the table are more likely to be trying to figure out fixes for a problem rather than bemoaning how terrible it is.
PERLMUTTER: This is something we’ve grappled with, and there are a couple of elements, I think, that are important to transmit about it. One is that there are good reasons to be disappointed when you look at the leaders of our society. They’ve structurally now gotten themselves into a fix, where they seem unable to even publicly say what they believe, let alone find real compromises on divisive issues. Meanwhile you can find lots of examples of “citizen assembly” events where a random selection of average people who completely disagree and support the opposite sides of the political spectrum sit down together and are much more able to have a civil, thoughtful conversation than their sociopolitical leaders can. That makes me think most of the [people in the] country (but not all!) could have a very reasonable conversation with each other. So clearly there’s an opportunity that we haven’t taken advantage of to structurally find ways to empower those conversations, not just the leaders trying to act for us. That’s something to be optimistic about. Another is that the daily news portrays the world as a very scary and negative place—but we know the daily news is not offering a very good representative take on the true state of the world, especially regarding the huge improvements in human well-being that have occurred over the past few decades.
So it feels to me that many people are living in “crisis” mode because they’re always consuming news that’s presenting us crises every moment and driving us apart with wedge issues. And I think there’s optimism to be found in looking for ways to talk together again. As John says, that’s the only game in town: to try to work with people until you learn something together, as opposed to just trying to win and then having half your population being unhappy.
CAMPBELL: We are maybe the most tribal species on the planet, but we are also perhaps the most amazingly flexible and cooperative species on the planet. And as Saul said, in these almost town-hall-style deliberative citizen assemblies you see this capacity for cooperation coming out, even among people who’d be bitterly divided and [belong to] opposite tribes otherwise—so there must be ways to amplify that and to escape being locked into these tribal schisms.
MacCOUN: And it’s important to remember that research on cooperation suggests you don’t need to have everybody cooperating to get the benefits. You do need a critical mass, but you’re never going to get everyone, so you shouldn’t waste your time trying to reach 100 percent. [Political scientist] Robert Axelrod and others studying the evolution of cooperation have shown that if cooperators can find each other, they can start to thrive and begin attracting other cooperators, and they can become more robust in the face of those who are uncooperative or trying to undermine cooperation. So somehow getting that critical mass is probably the best you can hope for.
I’m sure it hasn’t escaped anyone’s notice that as we discuss large-scale social cooperation, we’re also in an election year in the U.S., ostensibly the world’s most powerful democracy. And sure, part of the equation here is breaking down walls with basic acts of kindness and humility: love thy neighbor, find common ground, and so on. But what about voting? Does scientific decision-making give us some guidance on “best practices” there?
PERLMUTTER: Well, clearly we want this to be something that transcends election years. But in general, you should avoid making decisions—voting included—purely based on fear. This is not a time in the world where fear should be the dominant thing driving our individual or collective actions. Most of our fears divide us, yet most of our strength is found in working together to solve problems. So one basic thing is not to let yourself be flustered into voting for anyone or anything out of fear. But another is to look for leaders who use and reflect the scientific style of thinking, in which you’re open to being wrong, you’re bound by evidence, and you’re able to change your mind if it turns out that you were pursuing a bad plan. And that’s something that unfortunately we very rarely see.
CAMPBELL: At the moment we have an abundance of free speech—everyone can get on to some kind of social media and explain their views to the entire country. But we seem to have forgotten that the whole point of free speech was the testing of ideas. That was why it seemed like such a good thing: through free speech, new ideas can be generated and discussed and tested. But that idea of testing the ideas you freely express has just dropped out of the culture. We really need to tune back in to that in how we teach and talk about free speech and its value. It’s not just an end in itself, you know?
MacCOUN: And let’s be mindful of some lessons from history, too. For a lot of these issues that are so polarizing and divisive, it’s probably going to turn out that neither side was completely right, and there was some third possibility that didn’t occur to most, if any, of us. This happens in science all the time, with each victorious insight usually being provisional until the next, better theory or piece of evidence comes along. And in the same way, if we can’t move past arguing about our current conception of these problems, we’re trapping ourselves in this one little region of conceptual space when the solution might lie somewhere outside. This is one of very many cognitive traps we talk about in the book. Rather than staking out our hill to die on, we should be more open to uncertainty and experimentation: we test some policy solution to a problem, and if it doesn’t work, we’re ready to rapidly make adjustments and try something else.
Maybe we can practice what we preach here, this idea of performing evidence-based testing and course correction and escaping various sorts of cognitive traps. While you were working on this book, did you find and reflect on any irrational habits of mind you might have? And was there a case where you chose a hill to die on, and you were wrong, and you begrudgingly adjusted?
MacCOUN: Yeah, in the book we give examples of our own personal mistakes. One from my own research involves the replicability crisis and people engaging in confirmation bias. I had written a review paper summarizing evidence that seemed to show that decriminalizing drugs—that is, removing criminal penalties for them—did not lead to higher levels of use. After writing it, I had a new opportunity to test that hypothesis, looking at data from Italy, where in the 1970s they’d basically decriminalized personal possession of small quantities of all drugs. And then they recriminalized them in 1990. And then they redecriminalized in 1993. So it was like a perfect opportunity. And the data showed drug related deaths actually went down when they reinstituted penalties and went back up again when the penalties were removed. And this was completely opposite of what I had already staked my reputation on! And so, well, I had a personal bias, right? And that’s really the only reason I went and did more research, digging deeper on this Italian thing, because I didn’t like the findings. So across the same span of time I looked at Spain (a country that had decriminalized without recriminalizing) and at Germany (a country that never decriminalized during that time), and all three showed the same death pattern. This suggests that the suspicious pattern of deaths in fact had nothing to do with penalties. Now, I think that leads to the correct conclusion—my original conclusion, of course! But the point is: I’m embarrassed to admit I had fallen into the trap of confirmation bias—or, really, of its close cousin called disconfirmation bias, where you’re much tougher on evidence that seems to run counter to your beliefs. It’s a teachable moment, for sure.
CAMPBELL: It takes a lot of courage to admit these sorts of things and make the necessary transitions. One cognitive trap that affects many of us is what’s called the implicit bias blind spot, where you can be really subtle and perceptive in spotting other people’s biases but not your own. You can often see a bias of some sort in an instant in other people. But what happens when you look at yourself? The reaction is usually, “Na, I don't do that stuff!” You know, I must have been through hundreds and hundreds of student applications for admission or searches for faculty members, and I never spotted myself being biased at all, not once. “I just look at the applications straight,” right? But that can’t always be true because the person easiest to fool is yourself! Realizing that can be such a revelation.
PERLMUTTER: And this really informs one of the book’s key points: that we need to find better ways to work with people with whom we disagree—because one of the very best ways to get at your own biases is to find somebody who disagrees with you and is strongly motivated to prove you wrong. It’s hard, but you really do need the loyal opposition. Thinking back, for instance, to the big race for measuring the cosmological expansion of the universe that led to the discovery of dark energy, it was between my team and another team. Sometimes my colleagues and I would see members of the other team showing up to do their observations at the telescopes just as we were leaving from doing ours, and it was uncomfortable knowing both teams were chasing the same thing. On the other hand, that competition ensured we’d each try to figure out if the other team was making mistakes, and it greatly improved the confidence we collectively had in our results. But it’s not good enough just to have two opposing sides—you also need ways for them to engage with each other.
I realize I’ve inadvertently left probably the most basic question for last. What exactly is “third millennium thinking?”
PERLMUTTER: That’s okay, we actually leave explaining this to the book’s last chapter, too!
MacCOUN: Third millennium thinking is about recognizing a big shift that’s underway. We all have a sense of what the long millennia predating science must have been like, and we all know the tremendous advances that gradually came about as the modern scientific era emerged—from the practices of various ancient civilizations to the Renaissance and the Enlightenment, all those shifts in thinking that led to the amazing scientific revolution that has so profoundly changed our world here in what, until the end of the 20th century, was the second millennium. But there’s also been disenchantment with science, especially recently. And there’s validity to concerns that science was sometimes just a handmaiden of the powerful and that scientists sometimes wield more authority than they deserve to advance their own personal projects and politics. And sometimes science can become pathological; sometimes it can fail.
A big part of third millennium thinking is acknowledging science’s historic faults but also its capacity for self-correction, some of which we’re seeing today. We think this is leading us into a new era in which science is becoming less hierarchical. It’s becoming more interdisciplinary and team-based and, in some cases, more approachable for everyday people to be meaningfully involved—think of so-called citizen science projects. Science is also becoming more open, where researchers must show their work by making their data and methods more readily available so that others can independently check it. And we hope these sorts of changes are making scientists more humble: This attitude of “yeah, I’ve got the Ph.D., so you listen to me,” that doesn’t necessarily work anymore for big, divisive policy issues. You need a more deliberative consultation in which everyday people can be involved. Scientists do need to stay in their lane to some extent and not claim authority just based on their pedigree—the authority comes from the method used, not from the pedigree.
We see these all connected in their potential to advance a new way of doing science and of being scientists, and that’s what third millennium thinking is about.
CAMPBELL: With the COVID pandemic, I think we’ve all sadly become very familiar with the idea that the freedom of the individual citizen is somehow opposed to the authority of the scientist. You know, “the scientist is a person who will boss you around, diminish your freedom and inject you with vaccines laced with mind-controlling nanobots” or whatever. And it’s such a shame. It’s so debilitating when people use or see science like that. Or alternatively, you might say, “Well, I’m no scientist, and I can’t do the math, so I’ll just believe and do whatever they tell me.” And that really is relinquishing your freedom. Science should be an enabler of individual power, not a threat to your freedom. Third millennium thinking is about achieving that, allowing as many people as possible to be empowered—to empower themselves—by using scientific thinking.
PERLMUTTER: Exactly. We're trying to help people see that this combination of trends we’re now seeing around the world is actually a very fertile opportunity for big, meaningful, positive change. And if we lean into this, it could set us in a very good position on the long-term path to a really great millennium. Even though there are all these other forces to worry about at the moment, by applying the tools, ideas and processes from the culture of science to other parts of our lives, we can have the wind at our back as we move toward a brighter, better future.
Special Features
Vendor voice.
Some scientists can't stop using AI to write research papers
If you read about 'meticulous commendable intricacy' there's a chance a boffin had help.
Linguistic and statistical analyses of scientific articles suggest that generative AI may have been used to write an increasing amount of scientific literature.
Two academic papers assert that analyzing word choice in the corpus of science publications reveals an increasing usage of AI for writing research papers. One study , published in March by Andrew Gray of University College London in the UK, suggests at least one percent – 60,000 or more – of all papers published in 2023 were written at least partially by AI.
A second paper published in April by a Stanford University team in the US claims this figure might range between 6.3 and 17.5 percent, depending on the topic.
Both papers looked for certain words that large language models (LLMs) use habitually, such as “intricate,” “pivotal,” and “meticulously." By tracking the use of those words across scientific literature, and comparing this to words that aren't particularly favored by AI, the two studies say they can detect an increasing reliance on machine learning within the scientific publishing community.
In Gray's paper, the use of control words like "red," "conclusion," and "after" changed by a few percent from 2019 to 2023. The same was true of other certain adjectives and adverbs until 2023 (termed the post-LLM year by Gray).
In that year use of the words "meticulous," "commendable," and "intricate," rose by 59, 83, and 117 percent respectively, while their prevalence in scientific literature hardly changed between 2019 and 2022. The word with the single biggest increase in prevalence post-2022 was “meticulously”, up 137 percent.
The Stanford paper found similar phenomena, demonstrating a sudden increase for the words "realm," "showcasing," "intricate," and "pivotal." The former two were used about 80 percent more often than in 2021 and 2022, while the latter two were used around 120 and almost 160 percent more frequently respectively.
- Beyond the hype, AI promises leg up for scientific research
- AI researchers have started reviewing their peers using AI assistance
- Boffins deem Google DeepMind's material discoveries rather shallow
- Turns out AI chatbots are way more persuasive than humans
The researchers also considered word usage statistics in various scientific disciplines. Computer science and electrical engineering were ahead of the pack when it came to using AI-preferred language, while mathematics, physics, and papers published by the journal Nature, only saw increases of between five and 7.5 percent.
The Stanford bods also noted that authors posting more preprints, working in more crowded fields, and writing shorter papers seem to use AI more frequently. Their paper suggests that a general lack of time and a need to write as much as possible encourages the use of LLMs, which can help increase output.
Potentially the next big controversy in the scientific community
Using AI to help in the research process isn't anything new, and lots of boffins are open about utilizing AI to tweak experiments to achieve better results. However, using AI to actually write abstracts and other chunks of papers is very different, because the general expectation is that scientific articles are written by actual humans, not robots, and at least a couple of publishers consider using LLMs to write papers to be scientific misconduct.
Using AI models can be very risky as they often produce inaccurate text, the very thing scientific literature is not supposed to do. AI models can even fabricate quotations and citations, an occurrence that infamously got two New York attorneys in trouble for citing cases ChatGPT had dreamed up.
"Authors who are using LLM-generated text must be pressured to disclose this or to think twice about whether doing so is appropriate in the first place, as a matter of basic research integrity," University College London’s Gray opined.
The Stanford researchers also raised similar concerns, writing that use of generative AI in scientific literature could create "risks to the security and independence of scientific practice." ®
Narrower topics
- Large Language Model
- Machine Learning
- Neural Networks
- Tensor Processing Unit
Broader topics
- Self-driving Car
Send us news
Other stories you might like
With run:ai acquisition, nvidia aims to manage your ai kubes, google search results polluted by buggy ai-written code frustrate coders, intel's neuromorphic 'owl brain' swoops into sandia labs, easing the cloud migration journey.
Forget the AI doom and hype, let's make computers useful
Don't rent out that container ship yet: cios and biz buyers view ai pcs with some caution, law prof predicts generative ai will die at the hands of watchdogs, politicians call for ban on 'killer robots' and the curbing of ai weapons, us, japan announce joint ai research projects funded by nvidia, microsoft, others, what's up with alphabet and microsoft lately profits, sales – and ai costs, jensen huang and sam altman among tech chiefs invited to federal ai safety board, intel, ampere show running llms on cpus isn't as crazy as it sounds.
- Advertise with us
Our Websites
- The Next Platform
- Blocks and Files
Your Privacy
- Cookies Policy
- Privacy Policy
- Ts & Cs

Copyright. All rights reserved © 1998–2024
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 30, Number 7—July 2024
Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024
Suggested citation for this article
We report highly pathogenic avian influenza A(H5N1) virus in dairy cattle and cats in Kansas and Texas, United States, which reflects the continued spread of clade 2.3.4.4b viruses that entered the country in late 2021. Infected cattle experienced nonspecific illness, reduced feed intake and rumination, and an abrupt drop in milk production, but fatal systemic influenza infection developed in domestic cats fed raw (unpasteurized) colostrum and milk from affected cows. Cow-to-cow transmission appears to have occurred because infections were observed in cattle on Michigan, Idaho, and Ohio farms where avian influenza virus–infected cows were transported. Although the US Food and Drug Administration has indicated the commercial milk supply remains safe, the detection of influenza virus in unpasteurized bovine milk is a concern because of potential cross-species transmission. Continued surveillance of highly pathogenic avian influenza viruses in domestic production animals is needed to prevent cross-species and mammal-to-mammal transmission.
Highly pathogenic avian influenza (HPAI) viruses pose a threat to wild birds and poultry globally, and HPAI H5N1 viruses are of even greater concern because of their frequent spillover into mammals. In late 2021, the Eurasian strain of H5N1 (clade 2.3.4.4b) was detected in North America ( 1 , 2 ) and initiated an outbreak that continued into 2024. Spillover detections and deaths from this clade have been reported in both terrestrial and marine mammals in the United States ( 3 , 4 ). The detection of HPAI H5N1 clade 2.3.4.4b virus in severe cases of human disease in Ecuador ( 5 ) and Chile ( 6 ) raises further concerns regarding the pandemic potential of specific HPAI viruses.
In February 2024, veterinarians were alerted to a syndrome occurring in lactating dairy cattle in the panhandle region of northern Texas. Nonspecific illness accompanied by reduced feed intake and rumination and an abrupt drop in milk production developed in affected animals. The milk from most affected cows had a thickened, creamy yellow appearance similar to colostrum. On affected farms, incidence appeared to peak 4–6 days after the first animals were affected and then tapered off within 10–14 days; afterward, most animals were slowly returned to regular milking. Clinical signs were commonly reported in multiparous cows during middle to late lactation; ≈10%–15% illness and minimal death of cattle were observed on affected farms. Initial submissions of blood, urine, feces, milk, and nasal swab samples and postmortem tissues to regional diagnostic laboratories did not reveal a consistent, specific cause for reduced milk production. Milk cultures were often negative, and serum chemistry testing showed mildly increased aspartate aminotransferase, gamma-glutamyl transferase, creatinine kinase, and bilirubin values, whereas complete blood counts showed variable anemia and leukocytopenia.
In early March 2024, similar clinical cases were reported in dairy cattle in southwestern Kansas and northeastern New Mexico; deaths of wild birds and domestic cats were also observed within affected sites in the Texas panhandle. In > 1 dairy farms in Texas, deaths occurred in domestic cats fed raw colostrum and milk from sick cows that were in the hospital parlor. Antemortem clinical signs in affected cats were depressed mental state, stiff body movements, ataxia, blindness, circling, and copious oculonasal discharge. Neurologic exams of affected cats revealed the absence of menace reflexes and pupillary light responses with a weak blink response.
On March 21, 2024, milk, serum, and fresh and fixed tissue samples from cattle located in affected dairies in Texas and 2 deceased cats from an affected Texas dairy farm were received at the Iowa State University Veterinary Diagnostic Laboratory (ISUVDL; Ames, IA, USA). The next day, similar sets of samples were received from cattle located in affected dairies in Kansas. Milk and tissue samples from cattle and tissue samples from the cats tested positive for influenza A virus (IAV) by screening PCR, which was confirmed and characterized as HPAI H5N1 virus by the US Department of Agriculture National Veterinary Services Laboratory. Detection led to an initial press release by the US Department of Agriculture Animal and Plant Health Inspection Service on March 25, 2024, confirming HPAI virus in dairy cattle ( 7 ). We report the characterizations performed at the ISUVDL for HPAI H5N1 viruses infecting cattle and cats in Kansas and Texas.
Materials and Methods
Milk samples (cases 2–5) and fresh and formalin-fixed tissues (cases 1, 3–5) from dairy cattle were received at the ISUVDL from Texas on March 21 and from Kansas on March 22, 2024. The cattle exhibited nonspecific illness and reduced lactation, as described previously. The tissue samples for diagnostic testing came from 3 cows that were euthanized and 3 that died naturally; all postmortem examinations were performed on the premises of affected farms.
The bodies of 2 adult domestic shorthaired cats from a north Texas dairy farm were received at the ISUVDL for a complete postmortem examination on March 21, 2024. The cats were found dead with no apparent signs of injury and were from a resident population of ≈24 domestic cats that had been fed milk from sick cows. Clinical disease in cows on that farm was first noted on March 16; the cats became sick on March 17, and several cats died in a cluster during March 19–20. In total, >50% of the cats at that dairy became ill and died. We collected cerebrum, cerebellum, eye, lung, heart, spleen, liver, lymph node, and kidney tissue samples from the cats and placed them in 10% neutral-buffered formalin for histopathology.
At ISUVDL, we trimmed, embedded in paraffin, and processed formalin-fixed tissues from affected cattle and cats for hematoxylin/eosin staining and histologic evaluation. For immunohistochemistry (IHC), we prepared 4-µm–thick sections from paraffin-embedded tissues, placed them on Superfrost Plus slides (VWR, https://www.vwr.com ), and dried them for 20 minutes at 60°C. We used a Ventana Discovery Ultra IHC/ISH research platform (Roche, https://www.roche.com ) for deparaffinization until and including counterstaining. We obtained all products except the primary antibody from Roche. Automated deparaffination was followed by enzymatic digestion with protease 1 for 8 minutes at 37°C and endogenous peroxidase blocking. We obtained the primary influenza A virus antibody from the hybridoma cell line H16-L10–4R5 (ATCC, https://www.atcc.org ) and diluted at 1:100 in Discovery PSS diluent; we incubated sections with antibody for 32 minutes at room temperature. Next, we incubated the sections with a hapten-labeled conjugate, Discovery anti-mouse HQ, for 16 minutes at 37°C followed by a 16-minute incubation with the horse radish peroxidase conjugate, Discovery anti-HQ HRP. We used a ChromoMap DAB kit for antigen visualization, followed by counterstaining with hematoxylin and then bluing. Positive controls were sections of IAV-positive swine lung. Negative controls were sections of brain, lung, and eyes from cats not infected with IAV.
We diluted milk samples 1:3 vol/vol in phosphate buffered saline, pH 7.4 (Gibco/Thermo Fisher Scientific, https://www.thermofisher.com ) by mixing 1 unit volume of milk and 3 unit volumes of phosphate buffered saline. We prepared 10% homogenates of mammary glands, brains, lungs, spleens, and lymph nodes in Earle’s balanced salt solution (Sigma-Aldrich, https://www.sigmaaldrich.com ). Processing was not necessary for ocular fluid, rumen content, or serum samples. After processing, we extracted samples according to a National Animal Health Laboratory Network (NAHLN) protocol that had 2 NAHLN-approved deviations for ISUVDL consisting of the MagMax Viral RNA Isolation Kit for 100 µL sample volumes and a Kingfisher Flex instrument (both Thermo Fisher Scientific).
We performed real-time reverse transcription PCR (rRT-PCR) by using an NAHLN-approved assay with 1 deviation, which was the VetMAX-Gold SIV Detection kit (Thermo Fisher Scientific), to screen for the presence of IAV RNA. We tested samples along with the VetMAX XENO Internal Positive Control to monitor the possible presence of PCR inhibitors. Each rRT-PCR 96-well plate had 2 positive amplification controls, 2 negative amplification controls, 1 positive extraction control, and 1 negative extraction control. We ran the rRT-PCR on an ABI 7500 Fast thermocycler and analyzed data with Design and Analysis Software 2.7.0 (both Thermo Fisher Scientific). We considered samples with cycle threshold (Ct) values <40.0 to be positive for virus.
After the screening rRT-PCR, we analyzed IAV RNA–positive samples for the H5 subtype and H5 clade 2.3.4.4b by using the same RNA extraction and NAHLN-approved rRT-PCR protocols as described previously, according to standard operating procedures. We performed PCR on the ABI 7500 Fast thermocycler by using appropriate controls to detect H5-specific IAV. We considered samples with Ct values <40.0 to be positive for the IAV H5 subtype.
We conducted genomic sequencing of 2 milk samples from infected dairy cattle from Texas and 2 tissue samples (lung and brain) from cats that died at a different Texas dairy. We subjected the whole-genome sequencing data to bioinformatics analysis to assemble the 8 different IAV segment sequences according to previously described methods ( 8 ). We used the hemagglutinin (HA) and neuraminidase (NA) sequences for phylogenetic analysis. We obtained reference sequences for the HA and NA segments of IAV H5 clade 2.3.4.4 from publicly available databases, including GISAID ( https://www.gisaid.org ) and GenBank. We aligned the sequences by using MAFFT version 7.520 software ( https://mafft.cbrc.jp/alignment/server/index.html ) to create multiple sequence alignments for subsequent phylogenetic analysis. We used IQTree2 ( https://github.com/iqtree/iqtree2 ) to construct the phylogenetic tree from the aligned sequences. The software was configured to automatically identify the optimal substitution model by using the ModelFinder Plus option, ensuring the selection of the most suitable model for the dataset and, thereby, improving the accuracy of the reconstructed tree. We visualized the resulting phylogenetic tree by using iTOL ( https://itol.embl.de ), a web-based platform for interactive tree exploration and annotation.
Gross Lesions in Cows and Cats
All cows were in good body condition with adequate rumen fill and no external indications of disease. Postmortem examinations of the affected dairy cows revealed firm mammary glands typical of mastitis; however, mammary gland lesions were not consistent. Two cows that were acutely ill before postmortem examination had grossly normal milk and no abnormal mammary gland lesions. The gastrointestinal tract of some cows had small abomasal ulcers and shallow linear erosions of the intestines, but those observations were also not consistent in all animals. The colon contents were brown and sticky, suggesting moderate dehydration. The feces contained feed particles that appeared to have undergone minimal ruminal fermentation. The rumen contents had normal color and appearance but appeared to have undergone minimal fermentation.
The 2 adult cats (1 intact male, 1 intact female) received at the ISUVDL were in adequate body and postmortem condition. External examination was unremarkable. Mild hemorrhages were observed in the subcutaneous tissues over the dorsal skull, and multifocal meningeal hemorrhages were observed in the cerebrums of both cats. The gastrointestinal tracts were empty, and no other gross lesions were observed.
Microscopic Lesions in Cows and Cats
Figure 1 . Mammary gland lesions in cattle in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. A, B) Mammary gland...
The chief microscopic lesion observed in affected cows was moderate acute multifocal neutrophilic mastitis ( Figure 1 ); however, mammary glands were not received from every cow. Three cows had mild neutrophilic or lymphocytic hepatitis. Because they were adult cattle, other observed microscopic lesions (e.g., mild lymphoplasmacytic interstitial nephritis and mild to moderate lymphocytic abomasitis) were presumed to be nonspecific, age-related changes. We did not observe major lesions in the other evaluated tissues. We performed IHC for IAV antigen on all evaluated tissues; the only tissues with positive immunoreactivity were mastitic mammary glands from 2 cows that showed nuclear and cytoplasmic labeling of alveolar epithelial cells and cells within lumina ( Figure 1 ) and multifocal germinal centers within a lymph node from 1 cow ( Table 1 ).
Figure 2 . Lesions in cat tissues in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Tissue sections were stained with...
Both cats had microscopic lesions consistent with severe systemic virus infection, including severe subacute multifocal necrotizing and lymphocytic meningoencephalitis with vasculitis and neuronal necrosis, moderate subacute multifocal necrotizing and lymphocytic interstitial pneumonia, moderate to severe subacute multifocal necrotizing and lymphohistiocytic myocarditis, and moderate subacute multifocal lymphoplasmacytic chorioretinitis with ganglion cell necrosis and attenuation of the internal plexiform and nuclear layers ( Table 2 ; Figure 2 ). We performed IHC for IAV antigen on multiple tissues (brain, eye, lung, heart, spleen, liver, and kidney). We detected positive IAV immunoreactivity in brain (intracytoplasmic, intranuclear, and axonal immunolabeling of neurons), lung, and heart, and multifocal and segmental immunoreactivity within all layers of the retina ( Figure 2 ).
PCR Data from Cows and Cats
We tested various samples from 8 clinically affected mature dairy cows by IAV screening and H5 subtype-specific PCR ( Table 3 ). Milk and mammary gland homogenates consistently showed low Ct values: 12.3–16.9 by IAV screening PCR, 17.6–23.1 by H5 subtype PCR, and 14.7–20.0 by H5 2.3.4.4 clade PCR (case 1, cow 1; case 2, cows 1 and 2; case 3, cow 1; and case 4, cow 1). We forwarded the samples to the National Veterinary Services Laboratory, which confirmed the virus was an HPAI H5N1 virus strain.
When available, we also tested tissue homogenates (e.g., lung, spleen, and lymph nodes), ocular fluid, and rumen contents from 6 cows by IAV and H5 subtype-specific PCR ( Table 3 ). However, the PCR findings were not consistent. For example, the tissue homogenates and ocular fluid tested positive in some but not all cows. In case 5, cow 1, the milk sample tested negative by IAV screening PCR, but the spleen homogenate tested positive by IAV screening, H5 subtype, and H5 2.3.4.4 PCR. For 2 cows (case 3, cow 1; and case 4, cow 1) that had both milk and rumen contents available, both samples tested positive for IAV. Nevertheless, all IAV-positive nonmammary gland tissue homogenates, ocular fluid, and rumen contents had markedly elevated Ct values in contrast to the low Ct values for milk and mammary gland homogenate samples.
We tested brain and lung samples from the 2 cats (case 6, cats 1 and 2) by IAV screening and H5 subtype-specific PCR ( Table 3 ). Both sample types were positive by IAV screening PCR; Ct values were 9.9–13.5 for brain and 17.4–24.4 for lung samples, indicating high amounts of virus nucleic acid in those samples. The H5 subtype and H5 2.3.4.4 PCR results were also positive for the brain and lung samples; Ct values were consistent with the IAV screening PCR ( Table 3 ).
Phylogenetic Analyses
We assembled the sequences of all 8 segments of the HPAI viruses from both cow milk and cat tissue samples. We used the hemagglutinin (HA) and neuraminidase (NA) sequences specifically for phylogenetic analysis to delineate the clade of the HA gene and subtype of the NA gene.

Figure 3 . Phylogenetic analysis of hemagglutinin gene sequences in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Colors indicate different...
For HA gene analysis, both HA sequences derived from cow milk samples exhibited a high degree of similarity, sharing 99.88% nucleotide identity, whereas the 2 HA sequences from cat tissue samples showed complete identity at 100%. The HA sequences from the milk samples had 99.94% nucleotide identities with HA sequences from the cat tissues, resulting in a distinct subcluster comprising all 4 HA sequences, which clustered together with other H5N1 viruses belonging to clade 2.3.4.4b ( Figure 3 ). The HA sequences were deposited in GenBank (accession nos. PP599465 [case 2, cow 1], PP599473 [case 2, cow 2], PP692142 [case 6, cat 1], and PP692195 [case 6, cat 2]).

Figure 4 . Phylogenetic analysis of neuraminidase gene sequences in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Colors indicate different...
For NA gene analysis, the 2 NA sequences obtained from cow milk samples showed 99.93% nucleotide identity. Moreover, the NA sequences derived from the milk samples exhibited complete nucleotide identities (100%) with those from the cat tissues. The 4 NA sequences were grouped within the N1 subtype of HPAI viruses ( Figure 4 ). The NA sequences were deposited in GenBank (accession nos. PP599467 [case 2, cow 1], PP599475 [case 2, cow 2], PP692144 [case 6, cat 1], and PP692197 [case 6, cat 2]).
This case series differs from most previous reports of IAV infection in bovids, which indicated cattle were inapparently infected or resistant to infection ( 9 ). We describe an H5N1 strain of IAV in dairy cattle that resulted in apparent systemic illness, reduced milk production, and abundant virus shedding in milk. The magnitude of this finding is further emphasized by the high death rate (≈50%) of cats on farm premises that were fed raw colostrum and milk from affected cows; clinical disease and lesions developed that were consistent with previous reports of H5N1 infection in cats presumably derived from consuming infected wild birds ( 10 – 12 ). Although exposure to and consumption of dead wild birds cannot be completely ruled out for the cats described in this report, the known consumption of unpasteurized milk and colostrum from infected cows and the high amount of virus nucleic acid within the milk make milk and colostrum consumption a likely route of exposure. Therefore, our findings suggest cross-species mammal-to-mammal transmission of HPAI H5N1 virus and raise new concerns regarding the potential for virus spread within mammal populations. Horizontal transmission of HPAI H5N1 virus has been previously demonstrated in experimentally infected cats ( 13 ) and ferrets ( 14 ) and is suspected to account for large dieoffs observed during natural outbreaks in mink ( 15 ) and sea lions ( 16 ). Future experimental studies of HPAI H5N1 virus in dairy cattle should seek to confirm cross-species transmission to cats and potentially other mammals.
Clinical IAV infection in cattle has been infrequently reported in the published literature. The first report occurred in Japan in 1949, where a short course of disease with pyrexia, anorexia, nasal discharge, pneumonia, and decreased lactation developed in cattle ( 17 ). In 1997, a similar condition occurred in dairy cows in southwest England leading to a sporadic drop in milk production ( 18 ), and IAV seroconversion was later associated with reduced milk yield and respiratory disease ( 19 – 21 ). Rising antibody titers against human-origin influenza A viruses (H1N1 and H3N2) were later again reported in dairy cattle in England, which led to an acute fall in milk production during October 2005–March 2006 ( 22 ). Limited reports of IAV isolation from cattle exist; most reports occurred during the 1960s and 1970s in Hungary and in the former Soviet Union, where H3N2 was recovered from cattle experiencing respiratory disease ( 9 , 23 ). Direct detection of IAV in milk and the potential transmission from cattle to cats through feeding of unpasteurized milk has not been previously reported.
An IAV-associated drop in milk production in dairy cattle appears to have occurred during > 4 distinct periods and within 3 widely separated geographic areas: 1949 in Japan ( 17 ), 1997–1998 and 2005–2006 in Europe ( 19 , 21 ), and 2024 in the United States (this report). The sporadic occurrence of clinical disease in dairy cattle worldwide might be the result of changes in subclinical infection rates and the presence or absence of sufficient baseline IAV antibodies in cattle to prevent infection. Milk IgG, lactoferrin, and conglutinin have also been suggested as host factors that might reduce susceptibility of bovids to IAV infection ( 9 ). Contemporary estimates of the seroprevalence of IAV antibodies in US cattle are not well described in the published literature. One retrospective serologic survey in the United States in the late 1990s showed 27% of serum samples had positive antibody titers and 31% had low-positive titers for IAV H1 subtype-specific antigen in cattle with no evidence of clinical infections ( 24 ). Antibody titers for H5 subtype-specific antigen have not been reported in US cattle.
The susceptibility of domestic cats to HPAI H5N1 is well-documented globally ( 10 – 12 , 25 – 28 ), and infection often results in neurologic signs in affected felids and other terrestrial mammals ( 4 ). Most cases in cats result from consuming infected wild birds or contaminated poultry products ( 12 , 27 ). The incubation period in cats is short; clinical disease is often observed 2–3 days after infection ( 28 ). Brain tissue has been suggested as the best diagnostic sample to confirm HPAI virus infection in cats ( 10 ), and our results support that finding. One unique finding in the cats from this report is the presence of blindness and microscopic lesions of chorioretinitis. Those results suggest that further investigation into potential ocular manifestations of HPAI H5N1 virus infection in cats might be warranted.
The genomic sequencing and subsequent analysis of clinical samples from both bovine and feline sources provided considerable insights. The HA and NA sequences derived from both bovine milk and cat tissue samples from different Texas farms had a notable degree of similarity. Those findings strongly suggest a shared origin for the viruses detected in the dairy cattle and cat tissues. Further research, case series investigations, and surveillance data are needed to better understand and inform measures to curtail the clinical effects, shedding, and spread of HPAI viruses among mammals. Although pasteurization of commercial milk mitigates risks for transmission to humans, a 2019 US consumer study showed that 4.4% of adults consumed raw milk > 1 time during the previous year ( 29 ), indicating a need for public awareness of the potential presence of HPAI H5N1 viruses in raw milk.
Ingestion of feed contaminated with feces from wild birds infected with HPAI virus is presumed to be the most likely initial source of infection in the dairy farms. Although the exact source of the virus is unknown, migratory birds (Anseriformes and Charadriiformes) are likely sources because the Texas panhandle region lies in the Central Flyway, and those birds are the main natural reservoir for avian influenza viruses ( 30 ). HPAI H5N1 viruses are well adapted to domestic ducks and geese, and ducks appear to be a major reservoir ( 31 ); however, terns have also emerged as an important source of virus spread ( 32 ). The mode of transmission among infected cattle is also unknown; however, horizontal transmission has been suggested because disease developed in resident cattle herds in Michigan, Idaho, and Ohio farms that received infected cattle from the affected regions, and those cattle tested positive for HPAI H5N1 ( 33 ). Experimental studies are needed to decipher the transmission routes and pathogenesis (e.g., replication sites and movement) of the virus within infected cattle.
In conclusion, we showed that dairy cattle are susceptible to infection with HPAI H5N1 virus and can shed virus in milk and, therefore, might potentially transmit infection to other mammals via unpasteurized milk. A reduction in milk production and vague systemic illness were the most commonly reported clinical signs in affected cows, but neurologic signs and death rapidly developed in affected domestic cats. HPAI virus infection should be considered in dairy cattle when an unexpected and unexplained abrupt drop in feed intake and milk production occurs and for cats when rapid onset of neurologic signs and blindness develop. The recurring nature of global HPAI H5N1 virus outbreaks and detection of spillover events in a broad host range is concerning and suggests increasing virus adaptation in mammals. Surveillance of HPAI viruses in domestic production animals, including cattle, is needed to elucidate influenza virus evolution and ecology and prevent cross-species transmission.
Dr. Burrough is a professor and diagnostic pathologist at the Iowa State University College of Veterinary Medicine and Veterinary Diagnostic Laboratory. His research focuses on infectious diseases of livestock with an emphasis on swine.
Acknowledgment
We thank the faculty and staff at the ISUVDL who contributed to the processing and analysis of clinical samples in this investigation, the veterinarians involved with clinical assessments at affected dairies and various conference calls in the days before diagnostic submissions that ultimately led to the detection of HPAI virus in the cattle, and the US Department of Agriculture National Veterinary Services Laboratory and NAHLN for their roles and assistance in providing their expertise, confirmatory diagnostic support, and communications surrounding the HPAI virus cases impacting lactating dairy cattle.
- Caliendo V , Lewis NS , Pohlmann A , Baillie SR , Banyard AC , Beer M , et al. Transatlantic spread of highly pathogenic avian influenza H5N1 by wild birds from Europe to North America in 2021. Sci Rep . 2022 ; 12 : 11729 . DOI PubMed Google Scholar
- Bevins SN , Shriner SA , Cumbee JC Jr , Dilione KE , Douglass KE , Ellis JW , et al. Intercontinental movement of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4 virus to the United States, 2021. Emerg Infect Dis . 2022 ; 28 : 1006 – 11 . DOI PubMed Google Scholar
- Puryear W , Sawatzki K , Hill N , Foss A , Stone JJ , Doughty L , et al. Highly pathogenic avian influenza A(H5N1) virus outbreak in New England seals, United States. Emerg Infect Dis . 2023 ; 29 : 786 – 91 . DOI PubMed Google Scholar
- Elsmo EJ , Wünschmann A , Beckmen KB , Broughton-Neiswanger LE , Buckles EL , Ellis J , et al. Highly pathogenic avian influenza A(H5N1) virus clade 2.3.4.4b infections in wild terrestrial mammals, United States, 2022. Emerg Infect Dis . 2023 ; 29 : 2451 – 60 . DOI PubMed Google Scholar
- Bruno A , Alfaro-Núñez A , de Mora D , Armas R , Olmedo M , Garcés J , et al. First case of human infection with highly pathogenic H5 avian influenza A virus in South America: a new zoonotic pandemic threat for 2023? J Travel Med. 2023 ;30:taad032.
- Pulit-Penaloza JA , Brock N , Belser JA , Sun X , Pappas C , Kieran TJ , et al. Highly pathogenic avian influenza A(H5N1) virus of clade 2.3.4.4b isolated from a human case in Chile causes fatal disease and transmits between co-housed ferrets. Emerg Microbes Infect . 2024 ; 2332667 . DOI PubMed Google Scholar
- United States Department of Agriculture Animal and Plant Health Inspection Service . Federal and state veterinary, public health agencies share update on HPAI detection in Kansas, Texas dairy herds. 2024 [ cited 2024 Mar 29 ]. https://www.aphis.usda.gov/news/agency-announcements/federal-state-veterinary-public-health-agencies-share-update-hpai
- Sharma A , Zeller MA , Souza CK , Anderson TK , Vincent AL , Harmon K , et al. Characterization of a 2016–2017 human seasonal H3 influenza A virus spillover now endemic to U.S. swine. MSphere . 2022 ; 7 : e0080921 . DOI PubMed Google Scholar
- Sreenivasan CC , Thomas M , Kaushik RS , Wang D , Li F . Influenza A in bovine species: a narrative literature review. Viruses . 2019 ; 11 : 561 . DOI PubMed Google Scholar
- Sillman SJ , Drozd M , Loy D , Harris SP . Naturally occurring highly pathogenic avian influenza virus H5N1 clade 2.3.4.4b infection in three domestic cats in North America during 2023. J Comp Pathol . 2023 ; 205 : 17 – 23 . DOI PubMed Google Scholar
- Klopfleisch R , Wolf PU , Uhl W , Gerst S , Harder T , Starick E , et al. Distribution of lesions and antigen of highly pathogenic avian influenza virus A/Swan/Germany/R65/06 (H5N1) in domestic cats after presumptive infection by wild birds. Vet Pathol . 2007 ; 44 : 261 – 8 . DOI PubMed Google Scholar
- Keawcharoen J , Oraveerakul K , Kuiken T , Fouchier RAM , Amonsin A , Payungporn S , et al. Avian influenza H5N1 in tigers and leopards. Emerg Infect Dis . 2004 ; 10 : 2189 – 91 . DOI PubMed Google Scholar
- Kuiken T , Rimmelzwaan G , van Riel D , van Amerongen G , Baars M , Fouchier R , et al. Avian H5N1 influenza in cats. Science . 2004 ; 306 : 241 . DOI PubMed Google Scholar
- Herfst S , Schrauwen EJA , Linster M , Chutinimitkul S , de Wit E , Munster VJ , et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science . 2012 ; 336 : 1534 – 41 . DOI PubMed Google Scholar
- Agüero M , Monne I , Sánchez A , Zecchin B , Fusaro A , Ruano MJ , et al. Highly pathogenic avian influenza A(H5N1) virus infection in farmed minks, Spain, October 2022. Euro Surveill . 2023 ; 28 : 2300001 . DOI PubMed Google Scholar
- Leguia M , Garcia-Glaessner A , Muñoz-Saavedra B , Juarez D , Barrera P , Calvo-Mac C , et al. Highly pathogenic avian influenza A (H5N1) in marine mammals and seabirds in Peru. Nat Commun . 2023 ; 14 : 5489 . DOI PubMed Google Scholar
- Saito K . An outbreak of cattle influenza in Japan in the fall of 1949. J Am Vet Med Assoc . 1951 ; 118 : 316 – 9 . PubMed Google Scholar
- Gunning RF , Pritchard GC . Unexplained sporadic milk drop in dairy cows. Vet Rec . 1997 ; 140 : 488 . PubMed Google Scholar
- Brown IH , Crawshaw TR , Harris PA , Alexander DJ . Detection of antibodies to influenza A virus in cattle in association with respiratory disease and reduced milk yield. Vet Rec . 1998 ; 143 : 637 – 8 . PubMed Google Scholar
- Crawshaw TR , Brown I . Bovine influenza. Vet Rec . 1998 ; 143 : 372 . PubMed Google Scholar
- Gunning RF , Brown IH , Crawshaw TR . Evidence of influenza A virus infection in dairy cows with sporadic milk drop syndrome. Vet Rec . 1999 ; 145 : 556 – 7 . DOI PubMed Google Scholar
- Crawshaw TR , Brown IH , Essen SC , Young SCL . Significant rising antibody titres to influenza A are associated with an acute reduction in milk yield in cattle. Vet J . 2008 ; 178 : 98 – 102 . DOI PubMed Google Scholar
- Lopez JW , Woods GT . Influenza virus in ruminants: a review. Res Commun Chem Pathol Pharmacol . 1984 ; 45 : 445 – 62 . PubMed Google Scholar
- Jones-Lang K , Ernst-Larson M , Lee B , Goyal SM , Bey R . Prevalence of influenza A virus (H1N1) antibodies in bovine sera. New Microbiol . 1998 ; 21 : 153 – 60 . PubMed Google Scholar
- Briand FX , Souchaud F , Pierre I , Beven V , Hirchaud E , Hérault F , et al. Highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus in domestic cat, France, 2022. Emerg Infect Dis . 2023 ; 29 : 1696 – 8 . DOI PubMed Google Scholar
- Frymus T , Belák S , Egberink H , Hofmann-Lehmann R , Marsilio F , Addie DD , et al. Influenza virus infections in cats. Viruses . 2021 ; 13 : 1435 . DOI PubMed Google Scholar
- Songserm T , Amonsin A , Jam-on R , Sae-Heng N , Meemak N , Pariyothorn N , et al. Avian influenza H5N1 in naturally infected domestic cat. Emerg Infect Dis . 2006 ; 12 : 681 – 3 . DOI PubMed Google Scholar
- Thiry E , Zicola A , Addie D , Egberink H , Hartmann K , Lutz H , et al. Highly pathogenic avian influenza H5N1 virus in cats and other carnivores. Vet Microbiol . 2007 ; 122 : 25 – 31 . DOI PubMed Google Scholar
- Lando AM , Bazaco MC , Parker CC , Ferguson M . Characteristics of U.S. consumers reporting past year intake of raw (unpasteurized) milk: results from the 2016 Food Safety Survey and 2019 Food Safety and Nutrition Survey. J Food Prot . 2022 ; 85 : 1036 – 43 . DOI PubMed Google Scholar
- Fourment M , Darling AE , Holmes EC . The impact of migratory flyways on the spread of avian influenza virus in North America. BMC Evol Biol . 2017 ; 17 : 118 . DOI PubMed Google Scholar
- Guan Y , Smith GJD . The emergence and diversification of panzootic H5N1 influenza viruses. Virus Res . 2013 ; 178 : 35 – 43 . DOI PubMed Google Scholar
- de Araújo AC , Silva LMN , Cho AY , Repenning M , Amgarten D , de Moraes AP , et al. Incursion of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus, Brazil, 2023. Emerg Infect Dis . 2024 ; 30 : 619 – 21 . DOI PubMed Google Scholar
- American Veterinary Medical Association . States with HPAI-infected dairy cows grows to six. USDA provides guidance for veterinarians, producers on protecting cattle from the virus. 2024 [ cited 2024 Apr 10 ]. https://www.avma.org/news/states-hpai-infected-dairy-cows-grows-six
- Figure 1 . Mammary gland lesions in cattle in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. A, B) Mammary...
- Figure 2 . Lesions in cat tissues in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Tissue sections were stained...
- Figure 3 . Phylogenetic analysis of hemagglutinin gene sequences in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Colors indicate...
- Figure 4 . Phylogenetic analysis of neuraminidase gene sequences in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Colors indicate...
- Table 1 . Microscopic lesions observed in cattle in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024
- Table 2 . Microscopic lesions observed in cats in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024
- Table 3 . PCR results from various specimens in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024
Suggested citation for this article : Burrough ER, Magstadt DR, Petersen B, Timmermans SJ, Gauger PC, Zhang J, et al. Highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Emerg Infect Dis. 2024 Jul [ date cited ]. https://doi.org/10.3201/eid3007.240508
DOI: 10.3201/eid3007.240508
Original Publication Date: April 29, 2024
Table of Contents – Volume 30, Number 7—July 2024
Please use the form below to submit correspondence to the authors or contact them at the following address:
Eric R. Burrough, Iowa State University Veterinary Diagnostic Laboratory, 1937 Christensen Dr, Ames, IA 50011, USA
Comment submitted successfully, thank you for your feedback.
There was an unexpected error. Message not sent.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Metric Details
Article views: 24125.
Data is collected weekly and does not include downloads and attachments. View data is from .
What is the Altmetric Attention Score?
The Altmetric Attention Score for a research output provides an indicator of the amount of attention that it has received. The score is derived from an automated algorithm, and represents a weighted count of the amount of attention Altmetric picked up for a research output.

Chemical pollutants can change your skin bacteria and increase your eczema risk − new research explores how
Chief, Epithelial Therapeutics Unit, National Institute of Allergy and Infectious Diseases
Disclosure statement
Ian Myles receives funding from the Department of Intramural Research at the National Institute of Allergy and Infectious Diseases. He is the author of, and receives royalties for, the book GATTACA Has Fallen: How population genetics failed the populace. Although he is the co-discoverer of Roseomonas mucosa RSM2015 for eczema, he has donated the patent to the public and has no current conflict of interest for its sales.
View all partners
“We haven’t had a full night’s sleep since our son was born eight years ago,” said Mrs. B, pointing to her son’s dry, red and itchy skin.
Her son has had eczema his entire life. Also known as atopic dermatitis , this chronic skin disease affects about 1 in 5 children in the industrialized world. Some studies have found rates of eczema in developing nations to be over thirtyfold lower compared with industrialized nations.
However, rates of eczema didn’t spike with the Industrial Revolution , which began around 1760. Instead, eczema in countries such as the U.S. , Finland and other countries started rapidly rising around 1970.
What caused eczema rates to spike?
I am an allergist and immunologist working with a team of researchers to study trends in U.S. eczema rates. Scientists know that factors such as diets rich in processed foods as well as exposure to specific detergents and chemicals increase the risk of developing eczema. Living near factories, major roadways or wildfires increase the risk of developing eczema. Environmental exposures may also come from inside the house through paint, plastics , cigarette smoke or synthetic fabrics such as spandex, nylon and polyester.
While researchers have paid a lot of attention to genetics , the best predictor of whether a child will develop eczema isn’t in their genes but the environment they lived in for their first few years of life.
There’s something in the air
To figure out what environmental changes may have caused a spike in eczema in the U.S., we began by looking for potential eczema hot spots – places with eczema rates that were much higher than the national average. Then we looked at databases from the U.S. Environmental Protection Agency to see which chemicals were most common in those areas.
For eczema, along with the allergic diseases that routinely develop with it – peanut allergy and asthma – two chemical classes leaped off the page: diisocyanates and xylene .
Diisocyanates were first manufactured in the U.S. around 1970 for the production of spandex, nonlatex foam, paint and polyurethane. The manufacture of xylene also increased around that time, alongside an increase in the production of polyester and other materials.

The chemically active portion of the diisocyanates and xylene molecules are also found in cigarette smoke and wildfires . After 1975, when all new cars became outfitted with a new technology that converted exhaust gas to less toxic chemicals, isocyanate and xylene both became components of automobile exhaust .
Research has found that exposing mice to isocyanates and xylene can directly cause eczema , itch and inflammation by increasing the activity of receptors involved in itch, pain and temperature sensation. These receptors are also more active in mice placed on unhealthy diets . How directly exposing mice to these toxins compares to the typical levels of exposure in people is still unclear.
How and why might these chemicals be linked to rising rates of eczema?
Skin microbiome and pollution
Every person is coated with millions of microorganisms that live on the skin, collectively referred to as the skin microbiome . While researchers don’t know everything about how friendly bacteria help the skin, we do know that people need these organisms to produce certain types of lipids, or oils , that keep the skin sealed from the environment and stave off infection.
You’ve probably seen moisturizers and other skin products containing ceramides, a group of lipids that play an important role in protecting the skin. The amount of ceramides and related compounds on a child’s skin during their first few weeks of life is a consistent and significant predictor of whether they will go on to develop eczema . The less ceramides they have on their skin, the more likely they’ll develop eczema.

To see which toxins could prevent production of the beneficial lipids that prevent eczema, my team and I used skin bacteria as canaries in the coal mine. In the lab, we exposed bacteria that directly make ceramides (such as Roseomonas mucosa ), bacteria that help the body make its own ceramides (such as Staphylococcus epidermidis ) and bacteria that make other beneficial lipids (such as Staphylococcus cohnii ) to isocyanates and xylene. We made sure to expose the bacteria to levels of these chemicals that are similar to what people might be exposed to in the real world, such as the standard levels released from a factory or the fumes of polyurethane glue from a hardware store.
We found that exposing these bacteria to isocyanates or xylene led them to stop making ceramides and instead make amino acids such as lysine. Lysine helps protect the bacteria from the harms of the toxins but doesn’t provide the health benefits of ceramides.
We then evaluated how bed sheets manufactured using isocyanates or xylene affect the skin’s bacteria. We found that harmful bacteria such as Staphylococcus aureus proliferated on nylon, spandex and polyester but could not survive on cotton or bamboo. Bacteria that help keep skin healthy could live on any fabric, but, just as with air pollution, the amount of beneficial lipids they made dropped to less than half the levels made when grown on fabrics like cotton.
Addressing pollution’s effects on skin
What can be done about the connection between pollution and eczema?
Detectors capable of sensing low levels of isocyanate or xylene could help track pollutants and predict eczema flare-ups across a community. Better detectors can also help researchers identify air filtration systems that can scrub these chemicals from the environment. Within the U.S., people can use the EPA Toxics Tracker to look up which pollutants are most common near their home.
In the meantime, improving your microbial balance may require avoiding products that limit the growth of healthy skin bacteria. This may include certain skin care products, detergents and cleansers. Particularly for kids under 4, avoiding cigarette smoke, synthetic fabrics, nonlatex foams, polyurethanes and some paints may be advised.
Replacing bacteria that has been overly exposed to these chemicals may also help. For example, my research has shown that applying Roseomonas mucosa , a ceramide-producing bacterium that lives on healthy skin, can lead to a monthslong reduction in typical eczema symptoms compared with placebo . Researchers are also studying other potential probiotic treatments for eczema.
Evaluating the environmental causes of diseases that have become increasingly common in an increasingly industrialized world can help protect children from chemical triggers of conditions such as eczema. I believe that it may one day allow us to get back to a time when these diseases were uncommon.
- Environmental health
- Air pollution
- Dermatology
- New research
- Skin microbiome
- Atopic dermatitis
- STEEHM new research

Scheduling Analyst

Assistant Editor - 1 year cadetship

Executive Dean, Faculty of Health

Lecturer/Senior Lecturer, Earth System Science (School of Science)

Sydney Horizon Educators (Identified)

- Latest News
View Press Releases
- Post a Press Release
- Reprint Articles
- Digital Advertising
- Lead Generation
- Editorial Calendar
- Whitepapers
- Inside eBooks
- Industry Events
- IT Conferences
- Bio-IT World Expo
- Bio-IT World Expo Europe
- Editorial Profile
- Editorial Calender
- Submit a story
- Innovative Practices Awards
- Linking Policy
- Bio-IT World Boston
- Bio-IT World Europe
Bioscript Group acquires Enzyme Communications to bolster its medical writing, scientific content creation and storytelling capabilities
Bioscript Group , a leading provider of medical communications, market access, and regulatory writing services, has today announced the acquisition of science communication experts, Enzyme Communications.
Committed to excellence in healthcare communications, this acquisition builds on Bioscript Group’s extensive experience in this sector. Known for its innovative approach to scientific storytelling, Enzyme Communications brings a new dimension to Bioscript Group by providing expertise in building scientific stories from data, and helping clients effectively articulate how their products change lives.
The latest addition to Bioscript Group, Enzyme Communications enhances Bioscript Group’s expertise in complex diseases, adding specialist knowledge in oncology and rare diseases. They also bring a wealth of experience in strategy and planning, creativity, and content production.
The Enzyme Communications team will sit within the medical and scientific communications division of Bioscript Group and will continue to be led by co-founders Neil Kumar and Adam Goodband.
Gabrielle Silver, CEO of Bioscript Group, said: “Enzyme Communications is a very welcome addition to our portfolio as we continue our journey to become leaders in scientific communications for the life sciences sector.
“The synergies between our two organisations are clear. Enzyme’s distinctive approach to strategic planning and scientific storytelling bolsters the group’s capabilities and will help our clients think even more creatively about how to define their scientific proposition across target audiences.”
Enzyme Communications’ co-founder and client services director, Neil Kumar, said: “Enzyme was built on the foundations of quality, creativity, and authenticity and we recognise these same values in Bioscript Group.
“Joining Bioscript Group not only allows us to scale and enhance the services we currently offer to our clients, it also brings new opportunities for growth and development for our talented team. We can’t wait to see them thrive and progress in this partnership.”
Science Communication Director and Co-founder Adam Goodband highlighted: “Ultimately, we are a team of people who get excited about working on cutting edge science and repackaging it in ways that are strategically relevant, creative, and new. In Bioscript Group, we recognise those shared passions, and compliment each other’s skill set which will enhance our offerings to existing clients on both sides.”
Enzyme Communications is the latest addition to Bioscript Group’s portfolio which is formed from multiple, specialist businesses focused across scientific and medical communications and market access consultancy. These companies include Bioscript Regulatory Writing, Valid Insight, Fortis, Meridian HealthComms, and Bioscript Medical Communications.
About Bioscript Group
Established in 2005, Bioscript Group is a global scientific and medical communications and market access consulting partner dedicated to supporting pharmaceutical clients at critical points in the product life cycle. With a comprehensive suite of services across medical communications, regulatory writing, and market access consulting, Bioscript Group helps clients navigate across rare and complex disease, oncology, and immunology. Headquartered in Macclesfield, UK, with a global presence, the company prides itself on its deep therapeutic knowledge and commitment to scientific excellence.
www.bioscriptgroup.com
About Enzyme Communications
Enzyme Communications stands at the forefront of scientific communications, specialising in the art of scientific storytelling. From brand strategy to product launch and communications materials, Enzyme Communications turns science into stories and data into evidence-based arguments for commercial teams. With a focus on rare and complex diseases, Enzyme Communications combines strategic planning with creative and production excellence to deliver impactful communications. Renowned for its fast growth and distinctive culture, Enzyme Communications is committed to quality, authenticity, and delivering meaningful patient outcomes.
www.enzymecomms.com
Media enquiries:

IMAGES
VIDEO
COMMENTS
Basic Recommendations for Scientific Writing. Prospective authors need to know and tailor their writing to the audience. When writing for scientific journals, 4 fundamental recommendations are: clearly stating the usefulness of the study, formulating a key message, limiting unnecessary words, and using strategic sentence structure.
We describe here the basic steps to follow in writing a scientific article. We outline the main sections that an average article should contain; the elements that should appear in these sections, and some pointers for making the overall result attractive and acceptable for publication. 1.
Scientific writing, while an indispensable step of the scientific process, is often overlooked in undergraduate courses in favor of maximizing class time devoted to scientific concepts. However, the ability to effectively communicate research findings is crucial for success in the biological sciences.
A clear format will ensure that your research paper is understood by your readers. Follow: 1. Context — your introduction. 2. Content — your results. 3. Conclusion — your discussion. Plan ...
In each paragraph, the first sentence defines the context, the body contains the new idea and the final sentence offers a conclusion. For the whole paper, the introduction sets the context, the ...
Choose a research paper topic. Conduct preliminary research. Develop a thesis statement. Create a research paper outline. Write a first draft of the research paper. Write the introduction. Write a compelling body of text. Write the conclusion. The second draft.
Delivered to your inbox every two weeks, the Writing Toolbox features practical advice and tools you can use to prepare a research manuscript for submission success and build your scientific writing skillset. Discover how to navigate the peer review and publishing process, beyond writing your article.
Many scientific articles include the following elements: I. Abstract: The abstract should briefly summarize the contents of your article. Be sure to include a quick overview of the focus, results and conclusion of your study.
Then, writing the paper and getting it ready for submission may take me 3 to 6 months. I like separating the writing into three phases. The results and the methods go first, as this is where I write what was done and how, and what the outcomes were. In a second phase, I tackle the introduction and refine the results section with input from my ...
Table of contents. Step 1: Introduce your topic. Step 2: Describe the background. Step 3: Establish your research problem. Step 4: Specify your objective (s) Step 5: Map out your paper. Research paper introduction examples. Frequently asked questions about the research paper introduction.
Start the manuscript preparation by describing the materials and methods, including the planned statistical analysis (~1,000 words or less). This can often be copied from the study protocol. The second step is to describe the results (~350 words). The methods and results are the most important parts of the paper.
What this handout is about. This handout provides a general guide to writing reports about scientific research you've performed. In addition to describing the conventional rules about the format and content of a lab report, we'll also attempt to convey why these rules exist, so you'll get a clearer, more dependable idea of how to approach ...
AUTHORS. 1. The person who did the work and wrote the paper is generally listed as the first author of a research paper. 2. For published articles, other people who made substantial contributions to the work are also listed as authors. Ask your mentor's permission before including his/her name as co-author.
After writing the paper comes the time of reading your paper a few times in order to get everything perfect.In this section you will learn how to remove a lot of mistakes you might have been writing. In the end, you will have to build your own checklist corresponding to your own problems you want to avoid.
Present the results of the paper, in logical order, using tables and graphs as necessary. Explain the results and show how they help to answer the research questions posed in the Introduction. Evidence does not explain itself; the results must be presented and then explained. Avoid: presenting results that are never discussed; presenting ...
A guide to science writing prepared by UCLA's Undergraduate Science Journal. Introductions and conclusions for scientific papers by the George Mason University Writing Center. The Science of Scientific Writing by American Scientist. Scientific Writing Booklet compiled by Marc Tischler, Ph.D. University of Arizona
Developing such a skill takes practice. Here is an exercise to help you develop this skill. Pick a scientific article in your field. Read the paper with the abstract covered. Then try to write an abstract based on your reading. Compare your abstract to the author's. Repeat until you feel confident.
The main purpose of a scientific research proposal is to convince your audience that your project is worthwhile, and that you have the expertise and wherewithal to complete it. The elements of an effective research proposal mirror those of the research process itself, which we'll outline below. Essentially, the research proposal should ...
Dr. Michelle Harris, Dr. Janet Batzli,Biocore. This section provides guidelines on how to construct a solid introduction to a scientific paper including background information, study question, biological rationale, hypothesis, and general approach. If the Introduction is done well, there should be no question in the reader's mind why and on ...
First impressions are important. Scientists need to make their work stand out among a sea of others. However, many mistakenly believe that first impressions are formed based only on titles and abstracts. In actuality, the introduction section is critical to making a real impression on the audience. The introduction is where authors outline ...
But it'll improve your thinking about your own views. So in summary: Try to rank your confidence in your beliefs. Try to update your beliefs based on new evidence and don't fear being ...
Fri 3 May 2024 // 10:28 UTC. Linguistic and statistical analyses of scientific articles suggest that generative AI may have been used to write an increasing amount of scientific literature. Two academic papers assert that analyzing word choice in the corpus of science publications reveals an increasing usage of AI for writing research papers.
Those findings strongly suggest a shared origin for the viruses detected in the dairy cattle and cat tissues. Further research, case series investigations, and surveillance data are needed to better understand and inform measures to curtail the clinical effects, shedding, and spread of HPAI viruses among mammals.
For example, my research has shown that applying Roseomonas mucosa, a ceramide-producing bacterium that lives on healthy skin, can lead to a monthslong reduction in typical eczema symptoms ...
Bioscript Group, a leading provider of medical communications, market access, and regulatory writing services, has today announced the acquisition of science communication experts, Enzyme Communications.. Committed to excellence in healthcare communications, this acquisition builds on Bioscript Group's extensive experience in this sector.