- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Supplement Archive
- Cover Archive
- IDSA Guidelines
- IDSA Journals
- The Journal of Infectious Diseases
- Open Forum Infectious Diseases
- Photo Quizzes
- State-of-the-Art Reviews
- Voices of ID
- Author Guidelines
- Open Access
- Why Publish
- Advertising and Corporate Services
- Advertising
- Journals Career Network
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- About Clinical Infectious Diseases
- About the Infectious Diseases Society of America
- About the HIV Medicine Association
- IDSA COI Policy
- Editorial Board
- Self-Archiving Policy
- For Reviewers
- For Press Offices
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Development of next-generation covid-19 vaccines: barda supported phase 2b study designs.
- Article contents
- Figures & tables
- Supplementary Data
Daniel N Wolfe, Elizabeth Arangies, Gloria L David, Brian Armstrong, Theresa Z Scocca, Janel Fedler, Ramya Natarajan, James Zhou, Lakshmi Jayashankar, Ruben Donis, Mirjana Nesin, H Cody Meissner, Laurence Lemiale, Gerald R Kovacs, Shyam Rele, Robin Mason, Huyen Cao, Development of Next-Generation COVID-19 Vaccines: BARDA Supported Phase 2b Study Designs, Clinical Infectious Diseases , 2024;, ciae286, https://doi.org/10.1093/cid/ciae286
- Permissions Icon Permissions
In response to the COVID-19 pandemic, vaccines were quickly and successfully developed and deployed, saving millions of lives globally. While first generation vaccines are safe and effective in preventing disease caused by SARSCoV-2, next-generation vaccines have the potential to improve efficacy and safety. Vaccines delivered by a mucosal route may elicit greater protective immunity at respiratory surfaces thereby reducing transmission. Inclusion of viral antigens in addition to the spike protein may enhance protection against emerging variants of concern. Next-generation vaccine platforms with a new mechanism of action may necessitate efficacy trials to fulfill regulatory requirements. The Biomedical Advanced Research and Development Authority (BARDA) will be supporting Phase 2b clinical trials of candidate next-generation vaccines. The primary endpoint will be improved efficacy in terms of symptomatic disease relative to a currently approved COVID-19 vaccine. In this paper, we discuss the planned endpoints and potential challenges to this complex program.
- antigens, viral
- drug administration routes
- mucous membrane
- pharmacokinetics
- surrogate endpoints
- covid-19 vaccines
- coronavirus pandemic
- spike protein, human
Email alerts
Related articles in pubmed, citing articles via, looking for your next opportunity.
- Recommend to your Library
Affiliations
- Online ISSN 1537-6591
- Print ISSN 1058-4838
- Copyright © 2024 Infectious Diseases Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Scoping Review
- Open access
- Published: 14 November 2021
Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis
- Qiao Liu 1 na1 ,
- Chenyuan Qin 1 , 2 na1 ,
- Min Liu 1 &
- Jue Liu ORCID: orcid.org/0000-0002-1938-9365 1 , 2
Infectious Diseases of Poverty volume 10 , Article number: 132 ( 2021 ) Cite this article
57k Accesses
213 Citations
371 Altmetric
Metrics details
To date, coronavirus disease 2019 (COVID-19) becomes increasingly fierce due to the emergence of variants. Rapid herd immunity through vaccination is needed to block the mutation and prevent the emergence of variants that can completely escape the immune surveillance. We aimed to systematically evaluate the effectiveness and safety of COVID-19 vaccines in the real world and to establish a reliable evidence-based basis for the actual protective effect of the COVID-19 vaccines, especially in the ensuing waves of infections dominated by variants.
We searched PubMed, Embase and Web of Science from inception to July 22, 2021. Observational studies that examined the effectiveness and safety of SARS-CoV-2 vaccines among people vaccinated were included. Random-effects or fixed-effects models were used to estimate the pooled vaccine effectiveness (VE) and incidence rate of adverse events after vaccination, and their 95% confidence intervals ( CI ).
A total of 58 studies (32 studies for vaccine effectiveness and 26 studies for vaccine safety) were included. A single dose of vaccines was 41% (95% CI : 28–54%) effective at preventing SARS-CoV-2 infections, 52% (31–73%) for symptomatic COVID-19, 66% (50–81%) for hospitalization, 45% (42–49%) for Intensive Care Unit (ICU) admissions, and 53% (15–91%) for COVID-19-related death; and two doses were 85% (81–89%) effective at preventing SARS-CoV-2 infections, 97% (97–98%) for symptomatic COVID-19, 93% (89–96%) for hospitalization, 96% (93–98%) for ICU admissions, and 95% (92–98%) effective for COVID-19-related death, respectively. The pooled VE was 85% (80–91%) for the prevention of Alpha variant of SARS-CoV-2 infections, 75% (71–79%) for the Beta variant, 54% (35–74%) for the Gamma variant, and 74% (62–85%) for the Delta variant. The overall pooled incidence rate was 1.5% (1.4–1.6%) for adverse events, 0.4 (0.2–0.5) per 10 000 for severe adverse events, and 0.1 (0.1–0.2) per 10 000 for death after vaccination.
Conclusions
SARS-CoV-2 vaccines have reassuring safety and could effectively reduce the death, severe cases, symptomatic cases, and infections resulting from SARS-CoV-2 across the world. In the context of global pandemic and the continuous emergence of SARS-CoV-2 variants, accelerating vaccination and improving vaccination coverage is still the most important and urgent matter, and it is also the final means to end the pandemic.
Graphical Abstract
Since its outbreak, coronavirus disease 2019 (COVID-19) has spread rapidly, with a sharp rise in the accumulative number of infections worldwide. As of August 8, 2021, COVID-19 has already killed more than 4.2 million people and more than 203 million people were infected [ 1 ]. Given its alarming-spreading speed and the high cost of completely relying on non-pharmaceutical measures, we urgently need safe and effective vaccines to cover susceptible populations and restore people’s lives into the original [ 2 ].
According to global statistics, as of August 2, 2021, there are 326 candidate vaccines, 103 of which are in clinical trials, and 19 vaccines have been put into normal use, including 8 inactivated vaccines and 5 protein subunit vaccines, 2 RNA vaccines, as well as 4 non-replicating viral vector vaccines [ 3 ]. Our World in Data simultaneously reported that 27.3% of the world population has received at least one dose of a COVID-19 vaccine, and 13.8% is fully vaccinated [ 4 ].
To date, COVID-19 become increasingly fierce due to the emergence of variants [ 5 , 6 , 7 ]. Rapid herd immunity through vaccination is needed to block the mutation and prevent the emergence of variants that can completely escape the immune surveillance [ 6 , 8 ]. Several reviews systematically evaluated the effectiveness and/or safety of the three mainstream vaccines on the market (inactivated virus vaccines, RNA vaccines and viral vector vaccines) based on random clinical trials (RCT) yet [ 9 , 10 , 11 , 12 , 13 ].
In general, RNA vaccines are the most effective, followed by viral vector vaccines and inactivated virus vaccines [ 10 , 11 , 12 , 13 ]. The current safety of COVID-19 vaccines is acceptable for mass vaccination, but long-term monitoring of vaccine safety is needed, especially in older people with underlying conditions [ 9 , 10 , 11 , 12 , 13 ]. Inactivated vaccines had the lowest incidence of adverse events and the safety comparisons between mRNA vaccines and viral vectors were controversial [ 9 , 10 ].
RCTs usually conduct under a very demanding research circumstance, and tend to be highly consistent and limited in terms of population characteristics and experimental conditions. Actually, real-world studies differ significantly from RCTs in terms of study conditions and mass vaccination in real world requires taking into account factors, which are far more complex, such as widely heterogeneous populations, vaccine supply, willingness, medical accessibility, etc. Therefore, the real safety and effectiveness of vaccines turn out to be a major concern of international community. The results of a mass vaccination of CoronaVac in Chile demonstrated a protective effectiveness of 65.9% against the onset of COVID-19 after complete vaccination procedures [ 14 ], while the outcomes of phase 3 trials in Brazil and Turkey were 50.7% and 91.3%, reported on Sinovac’s website [ 14 ]. As for the Delta variant, the British claimed 88% protection after two doses of BNT162b2, compared with 67% for AZD1222 [ 15 ]. What is surprising is that the protection of BNT162b2 against infection in Israel is only 39% [ 16 ]. Several studies reported the effectiveness and safety of the COVID-19 vaccine in the real world recently, but the results remain controversial [ 17 , 18 , 19 , 20 ]. A comprehensive meta-analysis based upon the real-world studies is still in an urgent demand, especially for evaluating the effect of vaccines on variation strains. In the present study, we aimed to systematically evaluate the effectiveness and safety of the COVID-19 vaccine in the real world and to establish a reliable evidence-based basis for the actual protective effect of the COVID-19 vaccines, especially in the ensuing waves of infections dominated by variants.
Search strategy and selection criteria
Our methods were described in detail in our published protocol [PROSPERO (Prospective register of systematic reviews) registration, CRD42021267110]. We searched eligible studies published by 22 July 2021, from three databases including PubMed, Embase and Web of Science by the following search terms: (effectiveness OR safety) AND (COVID-19 OR coronavirus OR SARS-CoV-2) AND (vaccine OR vaccination). We used EndNoteX9.0 (Thomson ResearchSoft, Stanford, USA) to manage records, screen and exclude duplicates. This study was strictly performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
We included observational studies that examined the effectiveness and safety of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines among people vaccinated with SARS-CoV-2 vaccines. The following studies were excluded: (1) irrelevant to the subject of the meta-analysis, such as studies that did not use SARS-CoV-2 vaccination as the exposure; (2) insufficient data to calculate the rate for the prevention of COVID-19, the prevention of hospitalization, the prevention of admission to the ICU, the prevention of COVID-19-related death, or adverse events after vaccination; (3) duplicate studies or overlapping participants; (4) RCT studies, reviews, editorials, conference papers, case reports or animal experiments; and (5) studies that did not clarify the identification of COVID-19.
Studies were identified by two investigators (LQ and QCY) independently following the criteria above, while discrepancies reconciled by a third investigator (LJ).
Data extraction and quality assessment
The primary outcome was the effectiveness of SARS-CoV-2 vaccines. The following data were extracted independently by two investigators (LQ and QCY) from the selected studies: (1) basic information of the studies, including first author, publication year and study design; (2) characteristics of the study population, including sample sizes, age groups, setting or locations; (3) kinds of the SARS-CoV-2 vaccines; (4) outcomes for the effectiveness of SARS-CoV-2 vaccines: the number of laboratory-confirmed COVID-19, hospitalization for COVID-19, admission to the ICU for COVID-19, and COVID-19-related death; and (5) outcomes for the safety of SARS-CoV-2 vaccines: the number of adverse events after vaccination.
We evaluated the risk of bias using the Newcastle–Ottawa quality assessment scale for cohort studies and case–control studies [ 21 ]. and assess the methodological quality using the checklist recommended by Agency for Healthcare Research and Quality (AHRQ) [ 22 ]. Cohort studies and case–control studies were classified as having low (≥ 7 stars), moderate (5–6 stars), and high risk of bias (≤ 4 stars) with an overall quality score of 9 stars. For cross-sectional studies, we assigned each item of the AHRQ checklist a score of 1 (answered “yes”) or 0 (answered “no” or “unclear”), and summarized scores across items to generate an overall quality score that ranged from 0 to 11. Low, moderate, and high risk of bias were identified as having a score of 8–11, 4–7 and 0–3, respectively.
Two investigators (LQ and QCY) independently assessed study quality, with disagreements resolved by a third investigator (LJ).
Data synthesis and statistical analysis
We performed a meta-analysis to pool data from included studies and assess the effectiveness and safety of SARS-CoV-2 vaccines by clinical outcomes (rates of the prevention of COVID-19, the prevention of hospitalization, the prevention of admission to the ICU, the prevention of COVID-19-related death, and adverse events after vaccination). Random-effects or fixed-effects models were used to pool the rates and adjusted estimates across studies separately, based on the heterogeneity between estimates ( I 2 ). Fixed-effects models were used if I 2 ≤ 50%, which represented low to moderate heterogeneity and random-effects models were used if I 2 > 50%, representing substantial heterogeneity.
We conducted subgroup analyses to investigate the possible sources of heterogeneity by using vaccine kinds, vaccination status, sample size, and study population as grouping variables. We used the Q test to conduct subgroup comparisons and variables were considered significant between subgroups if the subgroup difference P value was less than 0.05. Publication bias was assessed by funnel plot and Egger’s regression test. We analyzed data using Stata version 16.0 (StataCorp, Texas, USA).
A total of 4844 records were searched from the three databases. 2484 duplicates were excluded. After reading titles and abstracts, we excluded 2264 reviews, RCT studies, duplicates and other studies meeting our exclude criteria. Among the 96 studies under full-text review, 41 studies were excluded (Fig. 1 ). Ultimately, with three grey literatures included, this final meta-analysis comprised 58 eligible studies, including 32 studies [ 14 , 15 , 17 , 18 , 19 , 20 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 ] for vaccine effectiveness and 26 studies [ 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 ] for vaccine safety. Characteristics of included studies are showed in Additional file 1 : Table S1, Additional file 2 : Table S2. The risk of bias of all studies we included was moderate or low.
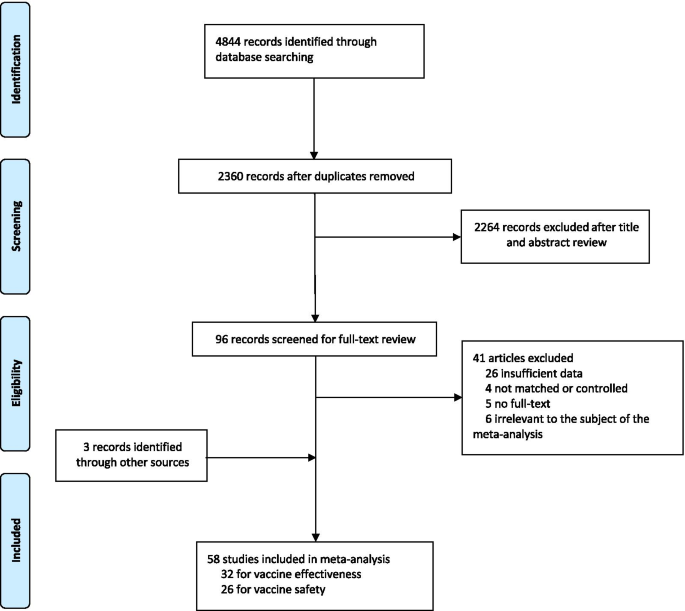
Flowchart of the study selection
Vaccine effectiveness for different clinical outcomes of COVID-19
We separately reported the vaccine effectiveness (VE) by the first and second dose of vaccines, and conducted subgroup analysis by the days after the first or second dose (< 7 days, ≥ 7 days, ≥ 14 days, and ≥ 21 days; studies with no specific days were classified as 1 dose, 2 dose or ≥ 1 dose).
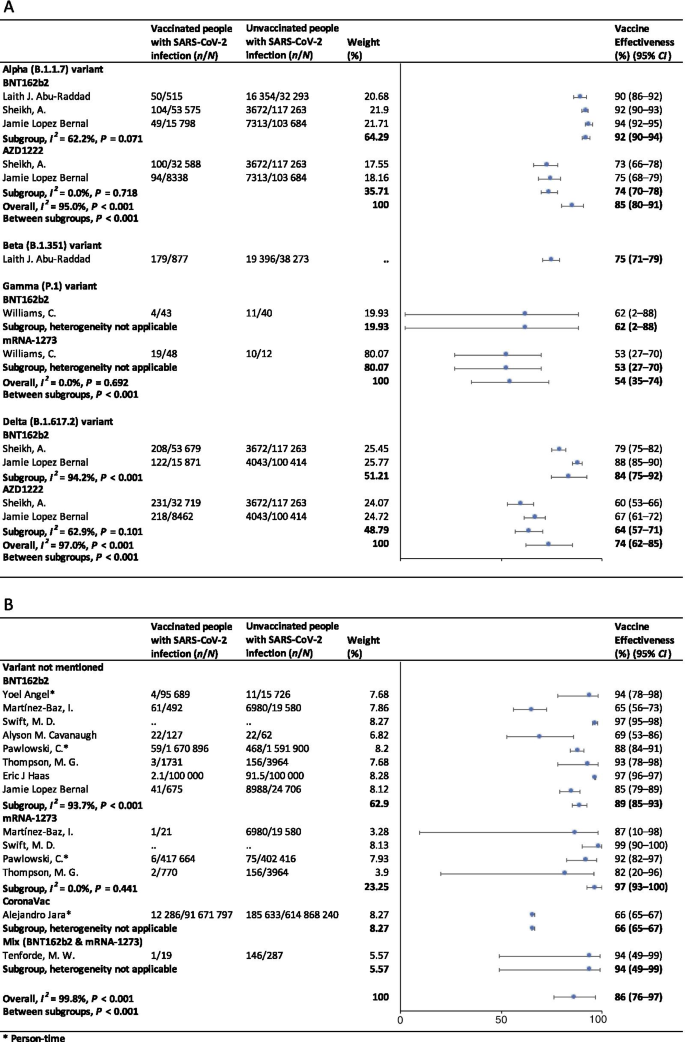
For the first dose of SARS-CoV-2 vaccines, the pooled VE was 41% (95% CI : 28–54%) for the prevention of SARS-CoV-2 infection, 52% (95% CI : 31–73%) for the prevention of symptomatic COVID-19, 66% (95% CI : 50–81%) for the prevention of hospital admissions, 45% (95% CI : 42–49%) for the prevention of ICU admissions, and 53% (95% CI : 15–91%) for the prevention of COVID-19-related death (Table 1 ). The subgroup, ≥ 21 days after the first dose, was found to have the highest VE in each clinical outcome of COVID-19, regardless of ≥ 1 dose group (Table 1 ).
For the second dose of SARS-CoV-2 vaccines, the pooled VE was 85% (95% CI : 81–89%) for the prevention of SARS-CoV-2 infection, 97% (95% CI : 97–98%) for the prevention of symptomatic COVID-19, 93% (95% CI: 89–96%) for the prevention of hospital admissions, 96% (95% CI : 93–98%) for the prevention of ICU admissions, and 95% (95% CI : 92–98%) for the prevention of COVID-19-related death (Table 1 ). VE was 94% (95% CI : 78–98%) in ≥ 21 days after the second dose for the prevention of SARS-CoV-2 infection, higher than other subgroups, regardless of 2 dose group (Table 1 ). For the prevention of symptomatic COVID-19, VE was also relatively higher in 21 days after the second dose (99%, 95% CI : 94–100%). Subgroups showed no statistically significant differences in the prevention of hospital admissions, ICU admissions and COVID-19-related death (subgroup difference P values were 0.991, 0.414, and 0.851, respectively).
Vaccine effectiveness for different variants of SARS-CoV-2 in fully vaccinated people
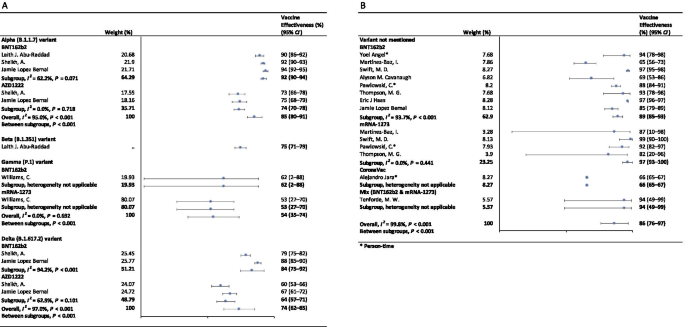
In the fully vaccinated groups (over 14 days after the second dose), the pooled VE was 85% (95% CI: 80–91%) for the prevention of Alpha variant of SARS-CoV-2 infection, 54% (95% CI : 35–74%) for the Gamma variant, and 74% (95% CI : 62–85%) for the Delta variant. There was only one study [ 23 ] focused on the Beta variant, which showed the VE was 75% (95% CI : 71–79%) for the prevention of the Beta variant of SARS-CoV-2 infection. BNT162b2 vaccine had the highest VE in each variant group; 92% (95% CI : 90–94%) for the Alpha variant, 62% (95% CI : 2–88%) for the Gamma variant, and 84% (95% CI : 75–92%) for the Delta variant (Fig. 2 ).
Forest plots for the vaccine effectiveness of SARS-CoV-2 vaccines in fully vaccinated populations. A Vaccine effectiveness against SARS-CoV-2 variants; B Vaccine effectiveness against SARS-CoV-2 with variants not mentioned. SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, COVID-19 coronavirus disease 2019, CI confidence interval
For studies which had not mentioned the variant of SARS-CoV-2, the pooled VE was 86% (95% CI: 76–97%) for the prevention of SARS-CoV-2 infection in fully vaccinated people. mRNA-1273 vaccine had the highest pooled VE (97%, 95% CI: 93–100%, Fig. 2 ).
Safety of SARS-CoV-2 vaccines
As Table 2 showed, the incidence rate of adverse events varied widely among different studies. We conducted subgroup analysis by study population (general population, patients and healthcare workers), vaccine type (BNT162b2, mRNA-1273, CoronaVac, and et al.), and population size (< 1000, 1000–10 000, 10 000–100 000, and > 100 000). The overall pooled incidence rate was 1.5% (95% CI : 1.4–1.6%) for adverse events, 0.4 (95% CI : 0.2–0.5) per 10 000 for severe adverse events, and 0.1 (95% CI : 0.1–0.2) per 10 000 for death after vaccination. Incidence rate of adverse events was higher in healthcare workers (53.2%, 95% CI : 28.4–77.9%), AZD1222 vaccine group (79.6%, 95% CI : 60.8–98.3%), and < 1000 population size group (57.6%, 95% CI : 47.9–67.4%). Incidence rate of sever adverse events was higher in healthcare workers (127.2, 95% CI : 62.7–191.8, per 10 000), Gam-COVID-Vac vaccine group (175.7, 95% CI : 77.2–274.2, per 10 000), and 1000–10 000 population size group (336.6, 95% CI : 41.4–631.8, per 10 000). Incidence rate of death after vaccination was higher in patients (7.6, 95% CI : 0.0–32.2, per 10 000), BNT162b2 vaccine group (29.8, 95% CI : 0.0–71.2, per 10 000), and < 1000 population size group (29.8, 95% CI : 0.0–71.2, per 10 000). Subgroups of general population, vaccine type not mentioned, and > 100 000 population size had the lowest incidence rate of adverse events, severe adverse events, and death after vaccination.
Sensitivity analysis and publication bias
In the sensitivity analyses, VE for SARS-CoV-2 infections, symptomatic COVID-19 and COVID-19-related death got relatively lower when omitting over a single dose group of Maria et al.’s work [ 33 ]; when omitting ≥ 14 days after the first dose group and ≥ 14 days after the second dose group of Alejandro et al.’s work [ 14 ], VE for SARS-CoV-2 infections, hospitalization, ICU admission and COVID-19-related death got relatively higher; and VE for all clinical status of COVID-19 became lower when omitting ≥ 14 days after the second dose group of Eric et al.’s work [ 34 ]. Incidence rate of adverse events and severe adverse events got relatively higher when omitting China CDC’s data [ 74 ]. P values of Egger’s regression test for all the meta-analysis were more than 0.05, indicating that there might not be publication bias.
To our knowledge, this is a comprehensive systematic review and meta-analysis assessing the effectiveness and safety of SARS-CoV-2 vaccines based on real-world studies, reporting pooled VE for different variants of SARS-CoV-2 and incidence rate of adverse events. This meta-analysis comprised a total of 58 studies, including 32 studies for vaccine effectiveness and 26 studies for vaccine safety. We found that a single dose of SARS-CoV-2 vaccines was about 40–60% effective at preventing any clinical status of COVID-19 and that two doses were 85% or more effective. Although vaccines were not as effective against variants of SARS-CoV-2 as original virus, the vaccine effectiveness was still over 50% for fully vaccinated people. Normal adverse events were common, while the incidence of severe adverse events or even death was very low, providing reassurance to health care providers and to vaccine recipients and promote confidence in the safety of COVID-19 vaccines. Our findings strengthen and augment evidence from previous review [ 75 ], which confirmed the effectiveness of the BNT162b2 mRNA vaccine, and additionally reported the safety of SARS-CoV-2 vaccines, giving insight on the future of SARS-CoV-2 vaccine schedules.
Although most vaccines for the prevention of COVID-19 are two-dose vaccines, we found that the pooled VE of a single dose of SARS-CoV-2 vaccines was about 50%. Recent study showed that the T cell and antibody responses induced by a single dose of the BNT162b2 vaccine were comparable to those naturally infected with SARE-CoV-2 within weeks or months after infection [ 76 ]. Our findings could help to develop vaccination strategies under certain circumstances such as countries having a shortage of vaccines. In some countries, in order to administer the first dose to a larger population, the second dose was delayed for up to 12 weeks [ 77 ]. Some countries such as Canada had even decided to delay the second dose for 16 weeks [ 78 ]. However, due to a suboptimum immune response in those receiving only a single dose of a vaccine, such an approach had a chance to give rise to the emergence of variants of SARS-CoV-2 [ 79 ]. There remains a need for large clinical trials to assess the efficacy of a single-dose administration of two-dose vaccines and the risk of increasing the emergence of variants.
Two doses of SARS-CoV-2 vaccines were highly effective at preventing hospitalization, severe cases and deaths resulting from COVID-19, while the VE of different groups of days from the second vaccine dose showed no statistically significant differences. Our findings emphasized the importance of getting fully vaccinated, for the fact that most breakthrough infections were mild or asymptomatic. A recent study showed that the occurrence of breakthrough infections with SARS-CoV-2 in fully vaccinated populations was predictable with neutralizing antibody titers during the peri-infection period [ 80 ]. We also found getting fully vaccinated was at least 50% effective at preventing SARS-CoV-2 variants infections, despite reduced effectiveness compared with original virus; and BNT162b2 vaccine was found to have the highest VE in each variant group. Studies showed that the highly mutated variants were indicative of a form of rapid, multistage evolutionary jumps, which could preferentially occur in the milieu of partial immune control [ 81 , 82 ]. Therefore, immunocompromised patients should be prioritized for anti-COVID-19 immunization to mitigate persistent SARS-CoV-2 infections, during which multimutational SARS-CoV-2 variants could arise [ 83 ].
Recently, many countries, including Israel, the United States, China and the United Kingdom, have introduced a booster of COVID-19 vaccine, namely the third dose [ 84 , 85 , 86 , 87 ]. A study of Israel showed that among people vaccinated with BNT162b2 vaccine over 60 years, the risk of COVID-19 infection and severe illness in the non-booster group was 11.3 times (95% CI: 10.4–12.3) and 19.5 times (95% CI: 12.9–29.5) than the booster group, respectively [ 84 ]. Some studies have found that the third dose of Moderna, Pfizer-BioNTech, Oxford-AstraZeneca and Sinovac produced a spike in infection-blocking neutralizing antibodies when given a few months after the second dose [ 85 , 87 , 88 ]. In addition, the common adverse events associated with the third dose did not differ significantly from the symptoms of the first two doses, ranging from mild to moderate [ 85 ]. The overall incidence rate of local and systemic adverse events was 69% (57/97) and 20% (19/97) after receiving the third dose of BNT162b2 vaccine, respectively [ 88 ]. Results of a phase 3 clinical trial involving 306 people aged 18–55 years showed that adverse events after receiving a third dose of BNT162b2 vaccine (5–8 months after completion of two doses) were similar to those reported after receiving a second dose [ 85 ]. Based on V-safe, local reactions were more frequently after dose 3 (5323/6283; 84.7%) than dose 2 (5249/6283; 83.5%) among people who received 3 doses of Moderna. Systemic reactions were reported less frequently after dose 3 (4963/6283; 79.0%) than dose 2 (5105/6283; 81.3%) [ 86 ]. On August 4, WHO called for a halt to booster shots until at least the end of September to achieve an even distribution of the vaccine [ 89 ]. At this stage, the most important thing we should be thinking about is how to reach a global cover of people at risk with the first or second dose, rather than focusing on the third dose.
Based on real world studies, our results preliminarily showed that complete inoculation of COVID-19 vaccines was still effective against infection of variants, although the VE was generally diminished compared with the original virus. Particularly, the pooled VE was 54% (95% CI : 35–74%) for the Gamma variant, and 74% (95% CI : 62–85%) for the Delta variant. Since the wide spread of COVID-19, a number of variants have drawn extensive attention of international community, including Alpha variant (B.1.1.7), first identified in the United Kingdom; Beta variant (B.1.351) in South Africa; Gamma variant (P.1), initially appeared in Brazil; and the most infectious one to date, Delta variant (B.1.617.2) [ 90 ]. Israel recently reported a breakthrough infection of SARS-CoV-2, dominated by variant B.1.1.7 in a small number of fully vaccinated health care workers, raising concerns about the effectiveness of the original vaccine against those variants [ 80 ]. According to an observational cohort study in Qatar, VE of the BNT162b2 vaccine against the Alpha (B.1.1.7) and Beta (B.1.351) variants was 87% (95% CI : 81.8–90.7%) and 75.0% (95% CI : 70.5–7.9%), respectively [ 23 ]. Based on the National Immunization Management System of England, results from a recent real-world study of all the general population showed that the AZD1222 and BNT162b2 vaccines protected against symptomatic SARS-CoV-2 infection of Alpha variant with 74.5% (95% CI : 68.4–79.4%) and 93.7% (95% CI : 91.6–95.3%) [ 15 ]. In contrast, the VE against the Delta variant was 67.0% (95% CI : 61.3–71.8%) for two doses of AZD1222 vaccine and 88% (95% CI : 85.3–90.1%) for BNT162b2 vaccine [ 15 ].
In terms of adverse events after vaccination, the pooled incidence rate was very low, only 1.5% (95% CI : 1.4–1.6%). However, the prevalence of adverse events reported in large population (population size > 100 000) was much lower than that in small to medium population size. On the one hand, the vaccination population in the small to medium scale studies we included were mostly composed by health care workers, patients with specific diseases or the elderly. And these people are more concerned about their health and more sensitive to changes of themselves. But it remains to be proved whether patients or the elderly are more likely to have adverse events than the general. Mainstream vaccines currently on the market have maintained robust safety in specific populations such as cancer patients, organ transplant recipients, patients with rheumatic and musculoskeletal diseases, pregnant women and the elderly [ 54 , 91 , 92 , 93 , 94 ]. A prospective study by Tal Goshen-lag suggests that the safety of BNT162b2 vaccine in cancer patients is consistent with those previous reports [ 91 ]. In addition, the incidence rate of adverse events reported in the heart–lung transplant population is even lower than that in general population [ 95 ]. On the other hand, large scale studies at the national level are mostly based on national electronic health records or adverse event reporting systems, and it is likely that most mild or moderate symptoms are actually not reported.
Compared with the usual local adverse events (such as pain at the injection site, redness at the injection site, etc.) and normal systemic reactions (such as fatigue, myalgia, etc.), serious and life-threatening adverse events were rare due to our results. A meta-analysis based on RCTs only showed three cases of anaphylactic shock among 58 889 COVID-19 vaccine recipients and one in the placebo group [ 11 ]. The exact mechanisms underlying most of the adverse events are still unclear, accordingly we cannot establish a causal relation between severe adverse events and vaccination directly based on observational studies. In general, varying degrees of adverse events occur after different types of COVID-19 vaccination. Nevertheless, the benefits far outweigh the risks.
Our results showed the effectiveness and safety of different types of vaccines varied greatly. Regardless of SARS-CoV-2 variants, vaccine effectiveness varied from 66% (CoronaVac [ 14 ]) to 97% (mRNA-1273 [ 18 , 20 , 45 , 46 ]). The incidence rate of adverse events varied widely among different types of vaccines, which, however, could be explained by the sample size and population group of participants. BNT162b2, AZD1222, mRNA-1273 and CoronaVac were all found to have high vaccine efficacy and acceptable adverse-event profile in recent published studies [ 96 , 97 , 98 , 99 ]. A meta-analysis, focusing on the potential vaccine candidate which have reached to the phase 3 of clinical development, also found that although many of the vaccines caused more adverse events than the controls, most were mild, transient and manageable [ 100 ]. However, severe adverse events did occur, and there remains the need to implement a unified global surveillance system to monitor the adverse events of COVID-19 vaccines around the world [ 101 ]. A recent study employed a knowledge-based or rational strategy to perform a prioritization matrix of approved COVID-19 vaccines, and led to a scale with JANSSEN (Ad26.COV2.S) in the first place, and AZD1222, BNT162b2, and Sputnik V in second place, followed by BBIBP-CorV, CoronaVac and mRNA-1273 in third place [ 101 ]. Moreover, when deciding the priority of vaccines, the socioeconomic characteristics of each country should also be considered.
Our meta-analysis still has several limitations. First, we may include limited basic data on specific populations, as vaccination is slowly being promoted in populations under the age of 18 or over 60. Second, due to the limitation of the original real-world study, we did not conduct subgroup analysis based on more population characteristics, such as age. When analyzing the efficacy and safety of COVID-19 vaccine, we may have neglected the discussion on the heterogeneity from these sources. Third, most of the original studies only collected adverse events within 7 days after vaccination, which may limit the duration of follow-up for safety analysis.
Based on the real-world studies, SARS-CoV-2 vaccines have reassuring safety and could effectively reduce the death, severe cases, symptomatic cases, and infections resulting from SARS-CoV-2 across the world. In the context of global pandemic and the continuous emergence of SARS-CoV-2 variants, accelerating vaccination and improving vaccination coverage is still the most important and urgent matter, and it is also the final means to end the pandemic.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional information files.
Abbreviations
Coronavirus disease 2019
Severe Acute Respiratory Syndrome Coronavirus 2
Vaccine effectiveness
Confidence intervals
Intensive care unit
Random clinical trials
Preferred reporting items for systematic reviews and meta-analyses
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2021. https://coronavirus.jhu.edu/map.html . Accessed 20 Aug 2021.
Barranco R, Rocca G, Molinelli A, Ventura F. Controversies and challenges of mass vaccination against SARS-CoV-2 in Italy: medico-legal perspectives and considerations. Healthcare (Basel). 2021. https://doi.org/10.3390/healthcare9091163 .
Article Google Scholar
COVID-19 vaccine tracker. 2021. https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/ . Accessed 20 Aug 2021.
Coronavirus (COVID-19) Vaccinations. 2021. https://ourworldindata.org/covid-vaccinations . Accessed 20 Aug 2021.
Kirby T. New variant of SARS-CoV-2 in UK causes surge of COVID-19. Lancet Respir Med. 2021;9(2):e20–1. https://doi.org/10.1016/s2213-2600(21)00005-9 .
Article CAS PubMed PubMed Central Google Scholar
Callaway E. Fast-spreading COVID variant can elude immune responses. Nature. 2021;589(7843):500–1. https://doi.org/10.1038/d41586-021-00121-z .
Article CAS PubMed Google Scholar
Reardon S. How the Delta variant achieves its ultrafast spread. Nature. 2021. https://doi.org/10.1038/d41586-021-01986-w .
Article PubMed Google Scholar
Li R, Liu J, Zhang H. The challenge of emerging SARS-CoV-2 mutants to vaccine development. J Genet Genomics. 2021;48(2):102–6. https://doi.org/10.1016/j.jgg.2021.03.001 .
Article PubMed PubMed Central Google Scholar
Chen M, Yuan Y, Zhou Y, Deng Z, Zhao J, Feng F, Zou H, Sun C. Safety of SARS-CoV-2 vaccines: a systematic review and meta-analysis of randomized controlled trials. Infect Dis Poverty. 2021;10(1):94. https://doi.org/10.1186/s40249-021-00878-5 .
Ling Y, Zhong J, Luo J. Safety and effectiveness of SARS-CoV-2 vaccines: a systematic review and meta-analysis. J Med Virol. 2021. https://doi.org/10.1002/jmv.27203 .
Pormohammad A, Zarei M, Ghorbani S, Mohammadi M, Razizadeh MH, Turner DL, Turner RJ. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9050467 .
Sathian B, Asim M, Banerjee I, Roy B, Pizarro AB, Mancha MA, van Teijlingen ER, Kord-Varkaneh H, Mekkodathil AA, Subramanya SH, et al. Development and implementation of a potential coronavirus disease 2019 (COVID-19) vaccine: a systematic review and meta-analysis of vaccine clinical trials. Nepal J Epidemiol. 2021;11(1):959–82. https://doi.org/10.3126/nje.v11i1.36163 .
Yuan P, Ai P, Liu Y, Ai Z, Wang Y, Cao W, Xia X, Zheng JC. Safety, tolerability, and immunogenicity of COVID-19 vaccines: a systematic review and meta-analysis. medRxiv. 2020. https://doi.org/10.1101/2020.11.03.20224998 .
Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, Pizarro A, Acevedo J, Leo K, Leon F, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2107715 .
Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2108891 .
Israel says Pfizer Covid vaccine is just 39% effective as delta spreads, but still prevents severe illness. 2021. https://www.cnbc.com/2021/07/23/delta-variant-pfizer-covid-vaccine-39percent-effective-in-israel-prevents-severe-illness.html . Accessed 20 Aug 2021.
Zacay G, Shasha D, Bareket R, Kadim I, Hershkowitz Sikron F, Tsamir J, Mossinson D, Heymann AD. BNT162b2 vaccine effectiveness in preventing asymptomatic infection with SARS-CoV-2 virus: a nationwide historical cohort study. Open Forum Infect Dis. 2021;8(6): ofab262. https://doi.org/10.1093/ofid/ofab262 .
Martínez-Baz I, Miqueleiz A, Casado I, Navascués A, Trobajo-Sanmartín C, Burgui C, Guevara M, Ezpeleta C, Castilla J. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection and hospitalisation, Navarre, Spain, January to April 2021. Eurosurveillance. 2021. https://doi.org/10.2807/1560-7917.Es.2021.26.21.2100438 .
Tenforde MW, Olson SM, Self WH, Talbot HK, Lindsell CJ, Steingrub JS, Shapiro NI, Ginde AA, Douin DJ, Prekker ME, et al. Effectiveness of Pfizer-BioNTech and moderna vaccines against COVID-19 among hospitalized adults aged ≥65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):674–9. https://doi.org/10.15585/mmwr.mm7018e1 .
Pawlowski C, Lenehan P, Puranik A, Agarwal V, Venkatakrishnan AJ, Niesen MJM, O’Horo JC, Virk A, Swift MD, Badley AD, et al. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med (N Y). 2021. https://doi.org/10.1016/j.medj.2021.06.007 .
Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp . Accessed 20 Aug 2021.
Rostom A, Dubé C, Cranney A, et al. Celiac Disease. Rockville (MD): Agency for Healthcare Research and Quality (US); 2004 Sep. (Evidence Reports/Technology Assessments, No. 104.) Appendix D. Quality Assessment Forms. Available from: https://www.ncbi.nlm.nih.gov/books/NBK35156/ . Accessed 20 Aug 2021
Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 COVID-19 vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021;385(2):187–9. https://doi.org/10.1056/NEJMc2104974 .
Angel Y, Spitzer A, Henig O, Saiag E, Sprecher E, Padova H, Ben-Ami R. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325(24):2457–65. https://doi.org/10.1001/jama.2021.7152 .
Azamgarhi T, Hodgkinson M, Shah A, Skinner JA, Hauptmannova I, Briggs TWR, Warren S. BNT162b2 vaccine uptake and effectiveness in UK healthcare workers—a single centre cohort study. Nat Commun. 2021;12(1):3698. https://doi.org/10.1038/s41467-021-23927-x .
Bianchi FP, Germinario CA, Migliore G, Vimercati L, Martinelli A, Lobifaro A, Tafuri S, Stefanizzi P. BNT162b2 mRNA COVID-19 vaccine effectiveness in the prevention of SARS-CoV-2 infection: a preliminary report. J Infect Dis. 2021. https://doi.org/10.1093/infdis/jiab262 .
Britton A, Jacobs Slifka KM, Edens C, Nanduri SA, Bart SM, Shang N, Harizaj A, Armstrong J, Xu K, Ehrlich HY, et al. Effectiveness of the Pfizer-BioNTech COVID-19 vaccine among residents of two skilled nursing facilities experiencing COVID-19 outbreaks—Connecticut, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(11):396–401. https://doi.org/10.15585/mmwr.mm7011e3 .
Cavanaugh AM, Fortier S, Lewis P, Arora V, Johnson M, George K, Tobias J, Lunn S, Miller T, Thoroughman D, et al. COVID-19 outbreak associated with a SARS-CoV-2 R1 lineage variant in a skilled nursing facility after vaccination program—Kentucky, March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):639–43. https://doi.org/10.15585/mmwr.mm7017e2 .
Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, Malek JA, Coyle P, Ayoub HH, Al Kanaani Z, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021. https://doi.org/10.1038/s41591-021-01446-y .
Chodick G, Tene L, Patalon T, Gazit S, Ben Tov A, Cohen D, Muhsen K. Assessment of effectiveness of 1 dose of BNT162b2 vaccine for SARS-CoV-2 infection 13 to 24 days after immunization. JAMA Netw Open. 2021;4(6): e2115985. https://doi.org/10.1001/jamanetworkopen.2021.15985 .
Chodick G, Tene L, Rotem RS, Patalon T, Gazit S, Ben-Tov A, Weil C, Goldshtein I, Twig G, Cohen D, et al. The effectiveness of the TWO-DOSE BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab438 .
Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–23. https://doi.org/10.1056/NEJMoa2101765 .
Flacco ME, Soldato G, Acuti Martellucci C, Carota R, Di Luzio R, Caponetti A, Manzoli L. Interim estimates of COVID-19 vaccine effectiveness in a mass vaccination setting: data from an Italian Province. VacCInes (Basel). 2021. https://doi.org/10.3390/vaccines9060628 .
Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–29. https://doi.org/10.1016/s0140-6736(21)00947-8 .
Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, Wellington E, Stowe J, Gillson N, Atti A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–35. https://doi.org/10.1016/s0140-6736(21)00790-x .
Hyams C, Marlow R, Maseko Z, King J, Ward L, Fox K, Heath R, Tuner A, Friedrich Z, Morrison L, et al. Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study. Lancet Infect Dis. 2021. https://doi.org/10.1016/s1473-3099(21)00330-3 .
Khan N, Mahmud N. Effectiveness of SARS-CoV-2 vaccination in a veterans affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology. 2021. https://doi.org/10.1053/j.gastro.2021.05.044 .
Knobel P, Serra C, Grau S, Ibañez R, Diaz P, Ferrández O, Villar R, Lopez AF, Pujolar N, Horcajada JP, et al. COVID-19 mRNA vaccine effectiveness in asymptomatic healthcare workers. Infect Control Hosp Epidemiol. 2021. https://doi.org/10.1017/ice.2021.287 .
Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, Simmons R, Cottrell S, Roberts R, O’Doherty M, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373: n1088. https://doi.org/10.1136/bmj.n1088 .
Mazagatos C, Monge S, Olmedo C, Vega L, Gallego P, Martín-Merino E, Sierra MJ, Limia A, Larrauri A. Effectiveness of mRNA COVID-19 vaccines in preventing SARS-CoV-2 infections and COVID-19 hospitalisations and deaths in elderly long-term care facility residents, Spain, weeks 53, 2020 to 13 2021. Eurosurveillance. 2021. https://doi.org/10.2807/1560-7917.Es.2021.26.24.2100452 .
Pilishvili T, Fleming-Dutra KE, Farrar JL, Gierke R, Mohr NM, Talan DA, Krishnadasan A, Harland KK, Smithline HA, Hou PC, et al. Interim estimates of vaccine effectiveness of Pfizer-BioNTech and Moderna COVID-19 vaccines among health care personnel—33 US Sites, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(20):753–8. https://doi.org/10.15585/mmwr.mm7020e2 .
Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2. https://doi.org/10.1016/s0140-6736(21)01358-1 .
Shrotri M, Krutikov M, Palmer T, Giddings R, Azmi B, Subbarao S, Fuller C, Irwin-Singer A, Davies D, Tut G, et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis. 2021. https://doi.org/10.1016/s1473-3099(21)00289-9 .
Skowronski DM, Setayeshgar S, Zou M, Prystajecky N, Tyson JR, Galanis E, Naus M, Patrick DM, Sbihi H, El Adam S, et al. Single-dose mRNA vaccine effectiveness against SARS-CoV-2, including Alpha and Gamma variants: a test-negative design in adults 70 years and older in British Columbia,Canada. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab616 .
Swift MD, Breeher LE, Tande AJ, Tommaso CP, Hainy CM, Chu H, Murad MH, Berbari EF, Virk A. Effectiveness of mRNA COVID-19 vaccines against SARS-CoV-2 infection in a cohort of healthcare personnel. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab361 .
Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, Olsho LEW, Caban-Martinez AJ, Fowlkes AL, Lutrick K, et al. Prevention and attenuation of COVID-19 with the BNT162b2 and mRNA-1273 Vaccines. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2107058 .
Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, Bedston S, Beggs J, Bradley D, Chuter A, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397(10285):1646–57. https://doi.org/10.1016/s0140-6736(21)00677-2 .
Williams C, Al-Bargash D, Macalintal C, Stuart R, Seth A, Latham J, Gitterman L, Fedsin S, Godoy M, Kozak R, et al. COVID-19 outbreak associated with a SARS-CoV-2 P.1 lineage in a long-term care home after implementation of a vaccination program—Ontario, April–May 2021. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab617 .
Alhazmi A, Alamer E, Daws D, Hakami M, Darraj M, Abdelwahab S, Maghfuri A, Algaissi A. Evaluation of side effects associated with COVID-19 vaccines in Saudi Arabia. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9060674 .
Andrzejczak-Grządko S, Czudy Z, Donderska M. Side effects after COVID-19 vaccinations among residents of Poland. Eur Rev Med Pharmacol Sci. 2021;25(12):4418–21. https://doi.org/10.26355/eurrev_202106_26153 .
Baldolli A, Michon J, Appia F, Galimard C, Verdon R, Parienti JJ. Tolerance of BNT162b2 mRNA COVI-19 vaccine in patients with a medical history of COVID-19 disease: a case control study. Vaccine. 2021;39(32):4410–3. https://doi.org/10.1016/j.vaccine.2021.06.054 .
Cherian S, Paul A, Ahmed S, Alias B, Manoj M, Santhosh AK, Varghese DR, Krishnan N, Shenoy P. Safety of the ChAdOx1 nCoV-19 and the BBV152 vaccines in 724 patients with rheumatic diseases: a post-vaccination cross-sectional survey. Rheumatol Int. 2021;41(8):1441–5. https://doi.org/10.1007/s00296-021-04917-0 .
Chevallier P, Coste-Burel M, Le Bourgeois A, Peterlin P, Garnier A, Béné MC, Imbert BM, Drumel T, Le Gouill S, Moreau P, et al. Safety and immunogenicity of a first dose of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic stem-cells recipients. EJHaem. 2021. https://doi.org/10.1002/jha2.242 .
Connolly CM, Ruddy JA, Boyarsky BJ, Avery RK, Werbel WA, Segev DL, Garonzik-Wang J, Paik JJ. Safety of the first dose of mRNA SARS-CoV-2 vaccines in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021. https://doi.org/10.1136/annrheumdis-2021-220231 .
Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, Zisapel M, Elalouf O, Kaufman I, Meidan R, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021. https://doi.org/10.1136/annrheumdis-2021-220647 .
Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, Nair N, Martin S, Clark T, Markowitz L, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283–8. https://doi.org/10.15585/mmwr.mm7008e3 .
Hashimoto T, Ozaki A, Bhandari D, Sawano T, Sah R, Tanimoto T. High anaphylaxis rates following vaccination with the Pfizer BNT162b2 mRNA vaccine against COVID-19 in Japanese health care workers; a secondary analysis of initial post-approval safety data. J Travel Med. 2021. https://doi.org/10.1093/jtm/taab090 .
Lv G, Yuan J, Xiong X, Li M. Mortality rate and characteristics of deaths following COVID-19 vaccination. Front Med (Lausanne). 2021;8: 670370. https://doi.org/10.3389/fmed.2021.670370 .
McMurry R, Lenehan P, Awasthi S, Silvert E, Puranik A, Pawlowski C, Venkatakrishnan AJ, Anand P, Agarwal V, O’Horo JC, et al. Real-time analysis of a mass vaccination effort confirms the safety of FDA-authorized mRNA COVID-19 vaccines. Med (N Y). 2021. https://doi.org/10.1016/j.medj.2021.06.006 .
Monin L, Laing AG, Muñoz-Ruiz M, McKenzie DR, Del Molino Del Barrio I, Alaguthurai T, Domingo-Vila C, Hayday TS, Graham C, Seow J, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–78. https://doi.org/10.1016/s1470-2045(21)00213-8 .
Pagotto V, Ferloni A, Mercedes Soriano M, Díaz M, Braguinsky Golde N, González MI, Asprea V, Staneloni MI, Zingoni P, Vidal G, et al. Active monitoring of early safety of Sputnik V vaccine in Buenos Aires, Argentina. MediCIna (B Aires). 2021;81(3):408–14.
Google Scholar
Peled Y, Ram E, Lavee J, Sternik L, Segev A, Wieder-Finesod A, Mandelboim M, Indenbaum V, Levy I, Raanani E, et al. BNT162b2 vaccination in heart transplant recipients: Clinical experience and antibody response. J Heart Lung Transplant. 2021. https://doi.org/10.1016/j.healun.2021.04.003 .
Quiroga B, Sánchez-Álvarez E, Goicoechea M, de Sequera P. COVID-19 vaccination among Spanish nephrologists: acceptance and side effects. J Healthc Qual Res. 2021. https://doi.org/10.1016/j.jhqr.2021.05.002 .
Ram R, Hagin D, Kikozashvilli N, Freund T, Amit O, Bar-On Y, Beyar-Katz O, Shefer G, Moshiashvili MM, Karni C, et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy—a single center prospective cohort study. Transplant Cell Ther. 2021. https://doi.org/10.1016/j.jtct.2021.06.024 .
Revon-Riviere G, Ninove L, Min V, Rome A, Coze C, Verschuur A, de Lamballerie X, André N. The BNT162b2 mRNA COVID-19 vaccine in adolescents and young adults with cancer: a monocentric experience. Eur J Cancer. 2021;154:30–4. https://doi.org/10.1016/j.ejca.2021.06.002 .
Riad A, Pokorná A, Mekhemar M, Conrad J, Klugarová J, Koščík M, Klugar M, Attia S. Safety of ChAdOx1 nCoV-19 vaccine: independent evidence from two EU states. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9060673 .
Riad A, Sağıroğlu D, Üstün B, Pokorná A, Klugarová J, Attia S, Klugar M. Prevalence and risk factors of CoronaVac Side effects: an independent cross-sectional study among healthcare workers in Turkey. J Clin Med. 2021. https://doi.org/10.3390/jcm10122629 .
Rosman Y, Lavi N, Meir-Shafrir K, Lachover-Roth I, Cohen-Engler A, Mekori YA, Confino-Cohen R. Safety of BNT162b2 mRNA COVID-19 vaccine in patients with mast cell disorders. J Allergy Clin Immunol Pract. 2021. https://doi.org/10.1016/j.jaip.2021.06.032 .
Signorelli C, Odone A, Gianfredi V, Capraro M, Kacerik E, Chiecca G, Scardoni A, Minerva M, Mantecca R, Musarò P, et al. Application of the “immunization islands” model to improve quality, efficiency and safety of a COVID-19 mass vaccination site. Ann Ig. 2021;33(5):499–512. https://doi.org/10.7416/ai.2021.2456 .
Vallée A, Chan-Hew-Wai A, Bonan B, Lesprit P, Parquin F, Catherinot É, Choucair J, Billard D, Amiel-Taieb C, Camps È, et al. Oxford-AstraZeneca COVID-19 vaccine: need of a reasoned and effective vaccine campaign. Public Health. 2021;196:135–7. https://doi.org/10.1016/j.puhe.2021.05.030 .
Wang J, Hou Z, Liu J, Gu Y, Wu Y, Chen Z, Ji J, Diao S, Qiu Y, Zou S, et al. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): a multicenter study. J Hepatol. 2021. https://doi.org/10.1016/j.jhep.2021.04.026 .
Zhang MX, Zhang TT, Shi GF, Cheng FM, Zheng YM, Tung TH, Chen HX. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Rev Vaccines. 2021. https://doi.org/10.1080/14760584.2021.1925112 .
Shay DK, Gee J, Su JR, Myers TR, Marquez P, Liu R, Zhang B, Licata C, Clark TA, Shimabukuro TT. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine—United States, March–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):680–4. https://doi.org/10.15585/mmwr.mm7018e2 .
Prevention CCfDCa. Information analysis of COVID-19 vaccine adverse reaction monitoring in China. 2021-5-28. http://www.chinacdc.cn/jkzt/ymyjz/ymyjjz_6758/202105/t20210528_230908.html . Accessed 20 Aug 2021.
Kow CS, Hasan SS. Real-world effectiveness of BNT162b2 mRNA vaccine: a meta-analysis of large observational studies. Inflammopharmacology. 2021;29(4):1075–90. https://doi.org/10.1007/s10787-021-00839-2 .
Angyal A, Longet S, Moore S, Payne RP, Harding A et al. T-Cell and Antibody Responses to First BNT162b2 Vaccine Dose in Previously SARS-CoV-2-Infected and Infection-Naive UK Healthcare Workers: A Multicentre, Prospective, Observational Cohort Study. Available at SSRN: https://ssrn.com/abstract=3820576 or https://doi.org/10.2139/ssrn.3820576 . Accessed 20 Aug 2021.
Pimenta D, Yates C, Pagel C, Gurdasani D. Delaying the second dose of covid-19 vaccines. BMJ. 2021;372: n710. https://doi.org/10.1136/bmj.n710 .
Tauh T, Mozel M, Meyler P, Lee SM. An updated look at the 16-week window between doses of vaccines in BC for COVID-19. BC Med J. 2021;63(3):102–3.
Kadire SR, Wachter RM, Lurie N. Delayed second dose versus standard regimen for COVID-19 vaccination. N Engl J Med. 2021;384(9): e28. https://doi.org/10.1056/NEJMclde2101987 .
Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, Mandelboim M, Gal Levin E, Rubin C, Indenbaum V, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2109072 .
Truong TT, Ryutov A, Pandey U, Yee R, Goldberg L, Bhojwani D, Aguayo-Hiraldo P, Pinsky BA, Pekosz A, Shen L, et al. Persistent SARS-CoV-2 infection and increasing viral variants in children and young adults with impaired humoral immunity. medRxiv. 2021. https://doi.org/10.1101/2021.02.27.21252099 .
Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, Solomon IH, Kuo HH, Boucau J, Bowman K, et al. Persistence and evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med. 2020;383(23):2291–3. https://doi.org/10.1056/NEJMc2031364 .
Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med. 2021;385(6):562–6. https://doi.org/10.1056/NEJMsb2104756 .
Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, Mizrahi B, Alroy-Preis S, Ash N, Milo R, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–400. https://doi.org/10.1056/NEJMoa2114255 .
Hause AM, Baggs J, Gee J, Marquez P, Myers TR, Shimabukuro TT, Shay DK. Safety monitoring of an additional dose of COVID-19 vaccine—United States, August 12–September 19, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(39):1379–84. https://doi.org/10.15585/mmwr.mm7039e4 .
Furlow B. Immunocompromised patients in the USA and UK should receive third dose of COVID-19 vaccine. Lancet Rheumatol. 2021. https://doi.org/10.1016/s2665-9913(21)00313-1 .
Flaxman A, Marchevsky NG, Jenkin D, Aboagye J, Aley PK, Angus B, Belij-Rammerstorfer S, Bibi S, Bittaye M, Cappuccini F, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002). Lancet. 2021;398(10304):981–90. https://doi.org/10.1016/s0140-6736(21)01699-8 .
Peled Y, Ram E, Lavee J, Segev A, Matezki S, Wieder-Finesod A, Halperin R, Mandelboim M, Indenbaum V, Levy I, et al. Third dose of the BNT162b2 vaccine in heart transplant recipients: immunogenicity and clinical experience. J Heart Lung Transplant. 2021. https://doi.org/10.1016/j.healun.2021.08.010 .
WHO. WHO press conference on coronavirus disease (COVID-19)—4 August 2021. 2021. https://www.who.int/multi-media/details/who-press-conference-on-coronavirus-disease-(covid-19)---4-august-2021 . Accessed 20 Aug 2021.
Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). In: StatPearls. edn. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
Goshen-Lago T, Waldhorn I, Holland R, Szwarcwort-Cohen M, Reiner-Benaim A, Shachor-Meyouhas Y, Hussein K, Fahoum L, Baruch M, Peer A, et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021. https://doi.org/10.1001/jamaoncol.2021.2675 .
Ou MT, Boyarsky BJ, Motter JD, Greenberg RS, Teles AT, Ruddy JA, Krach MR, Jain VS, Werbel WA, Avery RK, et al. Safety and reactogenicity of 2 doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021. https://doi.org/10.1097/tp.0000000000003780 .
Bookstein Peretz S, Regev N, Novick L, Nachshol M, Goffer E, Ben-David A, Asraf K, Doolman R, Sapir E, Regev Yochay G, et al. Short-term outcome of pregnant women vaccinated by BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol. 2021. https://doi.org/10.1002/uog.23729 .
Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, Marquez PL, Olson CK, Liu R, Chang KT, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273–82. https://doi.org/10.1056/NEJMoa2104983 .
Peled Y, Ram E, Lavee J, Sternik L, Segev A, Wieder-Finesod A, Mandelboim M, Indenbaum V, Levy I, Raanani E, et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. 2021;40(8):759–62. https://doi.org/10.1016/j.healun.2021.04.003 .
Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2110345 .
Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, Neuzil KM, Hahn W, Hunt J, Mulligan MJ, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2105290 .
El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, Campbell TB, Clark J, Jackson LA, Fichtenbaum CJ, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2113017 .
Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, Köse Ş, Erdinç F, Akalın EH, Tabak ÖF, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–22. https://doi.org/10.1016/s0140-6736(21)01429-x .
Kumar S, Saurabh MK, Maharshi V. Efficacy and safety of potential vaccine candidates against coronavirus disease 2019: a systematic review. J Adv Pharm Technol Res. 2021;12(3):215–21. https://doi.org/10.4103/japtr.JAPTR_229_20 .
Burgos-Salcedo J. A rational strategy to support approved COVID-19 vaccines prioritization. Hum Vaccin Immunother. 2021;17(10):3474–7. https://doi.org/10.1080/21645515.2021.1922060 .
Download references
Acknowledgements
This study was funded by the National Natural Science Foundation of China (72122001; 71934002) and the National Science and Technology Key Projects on Prevention and Treatment of Major infectious disease of China (2020ZX10001002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper. No payment was received by any of the co-authors for the preparation of this article.
Author information
Qiao Liu and Chenyuan Qin are joint first authors
Authors and Affiliations
Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, 100191, China
Qiao Liu, Chenyuan Qin, Min Liu & Jue Liu
Institute for Global Health and Development, Peking University, Beijing, 100871, China
Chenyuan Qin & Jue Liu
You can also search for this author in PubMed Google Scholar
Contributions
LQ and QCY contributed equally as first authors. LJ and LM contributed equally as correspondence authors. LJ and LM conceived and designed the study; LQ, QCY and LJ carried out the literature searches, extracted the data, and assessed the study quality; LQ and QCY performed the statistical analysis and wrote the manuscript; LJ, LM, LQ and QCY revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Min Liu or Jue Liu .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Supplementary Information
Additional file 1: table s1..
Characteristic of studies included for vaccine effectiveness.
Additional file 2: Table S2.
Characteristic of studies included for vaccine safety.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Liu, Q., Qin, C., Liu, M. et al. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect Dis Poverty 10 , 132 (2021). https://doi.org/10.1186/s40249-021-00915-3
Download citation
Received : 07 September 2021
Accepted : 01 November 2021
Published : 14 November 2021
DOI : https://doi.org/10.1186/s40249-021-00915-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Effectiveness
- Meta-analysis
Infectious Diseases of Poverty
ISSN: 2049-9957
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
ORIGINAL RESEARCH article
Global research on rna vaccines for covid-19 from 2019 to 2023: a bibliometric analysis.

- 1 Center for Molecular Diagnosis and Precision Medicine, The First Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
- 2 Jiangxi Key Laboratory of Cancer Metastasis and Precision Treatment, the First Hospital of Nanchang, Nanchang, China
- 3 Department of Pathology, Jiangxi Cancer Hospital, Nanchang, China
- 4 Department of Pathology, Jiangxi Provincial Chest Hospital, Nanchang, China
Background: Since the global pandemic of COVID-19 has broken out, thousands of pieces of literature on COVID-19 RNA vaccines have been published in various journals. The overall measurement and analysis of RNA vaccines for COVID-19, with the help of sophisticated mathematical tools, could provide deep insights into global research performance and the collaborative architectural structure within the scientific community of COVID-19 mRNA vaccines. In this bibliometric analysis, we aim to determine the extent of the scientific output related to COVID-19 RNA vaccines between 2019 and 2023.
Methods: We applied the Bibliometrix R package for comprehensive science mapping analysis of extensive bibliographic metadata retrieved from the Web of Science Core Collection database. On January 11th, 2024, the Web of Science database was searched for COVID-19 RNA vaccine-related publications using predetermined search keywords with specific restrictions. Bradford’s law was applied to evaluate the core journals in this field. The data was analyzed with various bibliometric indicators using the Bibliometrix R package.
Results: The final analysis included 2962 publications published between 2020 and 2023 while there is no related publication in 2019. The most productive year was 2022. The most relevant leading authors in terms of publications were Ugur Sahin and Pei-Yong, Shi, who had the highest total citations in this field. The core journals were Vaccines, Frontiers in Immunology, and Viruses-Basel. The most frequently used author’s keywords were COVID-19, SARS-CoV-2, and vaccine. Recent COVID-19 RNA vaccine-related topics included mental health, COVID-19 vaccines in humans, people, and the pandemic. Harvard University was the top-ranked institution. The leading country in terms of publications, citations, corresponding author country, and international collaboration was the United States. The United States had the most robust collaboration with China.
Conclusion: The research hotspots include COVID-19 vaccines and the pandemic in people. We identified international collaboration and research expenditure strongly associated with COVID-19 vaccine research productivity. Researchers’ collaboration among developed countries should be extended to low-income countries to expand COVID-19 vaccine-related research and understanding.
Introduction
Since 2019, the global COVID-19 pandemic has affected the lives of billions of people worldwide ( 1 ). To deal with this situation, countries worldwide began to develop vaccines, including traditional inactivated vaccines, recombinant protein, live-attenuated vaccines, RNA vaccines, etc. ( 2 – 15 ). On October 2nd, 2023, the Nobel Assembly at the Karolinska Institutet decided to award the 2023 Nobel Prize in Physiology or Medicine jointly to Katalin Karikó and Drew Weissman for their discovery of nucleoside base modifications, which made it possible to develop an effective mRNA vaccine against COVID-19 ( 16 ). RNA vaccines have received widespread attention due to their high efficacy, specificity, versatility, rapid and large-scale development capabilities, low-cost production potential, and safety ( 17 , 18 ). RNA vaccines have been developed for several decades ( 19 , 20 ), and since COVID-19 has broken worldly, the RNA vaccines platform has enabled fast vaccine development in response to this pandemic ( 21 ). RNA vaccines provide flexibility in the design and expression of vaccine antigens, simulating the structure and expression of antigens during natural infections. RNA is necessary for protein synthesis and unconformity into the genome, and it is transiently expressed, metabolized, and eliminated by the body’s natural mechanism ( 22 ), so it is considered relatively safe. Many clinical trials have proven RNA-based preventive infectious disease vaccines and RNA therapeutic agents to be safe and well-tolerated ( 23 – 29 ). Generally speaking, vaccination with RNA can trigger a robust innate immune response. RNA guides the expression of vaccine antigens in host cells and has intrinsic adjuvant effects ( 30 – 32 ). One advantage of the RNA vaccine manufacturing platform is that it can quickly produce many vaccines targeting new pathogens, regardless of the encoded pathogen antigen ( 33 ). The bibliometric analysis of published articles provides insights into research prospects, gaps, and future directions in the research field. This study examined scientific publications related to RNA vaccines for COVID-19 through bibliometric analysis and trend analysis.
Search strategy
We conducted a literature search on the Web of Science Core Collection (WoSCC) database ( https://www.webofscience.com/wos/woscc/basic-search ) on January 11th, 2024. The search formula was TS= ((RNA vaccine AND COVID-19) OR (RNA vaccine AND SARS-COV-2)), the published year was set before 2024, and the type of documents was set to articles and reviews. The language filter was set in English ( Figure 1 ). According to our search strategies, there were 2962 studies of RNA vaccines for COVID-19 between 2020 and 2023 (0 publication in 2019), including 1956 articles and 1006 reviews. The analyzed publications were written by 23141 authors (93 with single-authored documents and 23048 with multi-authored documents) from 104 countries and 908 journals.
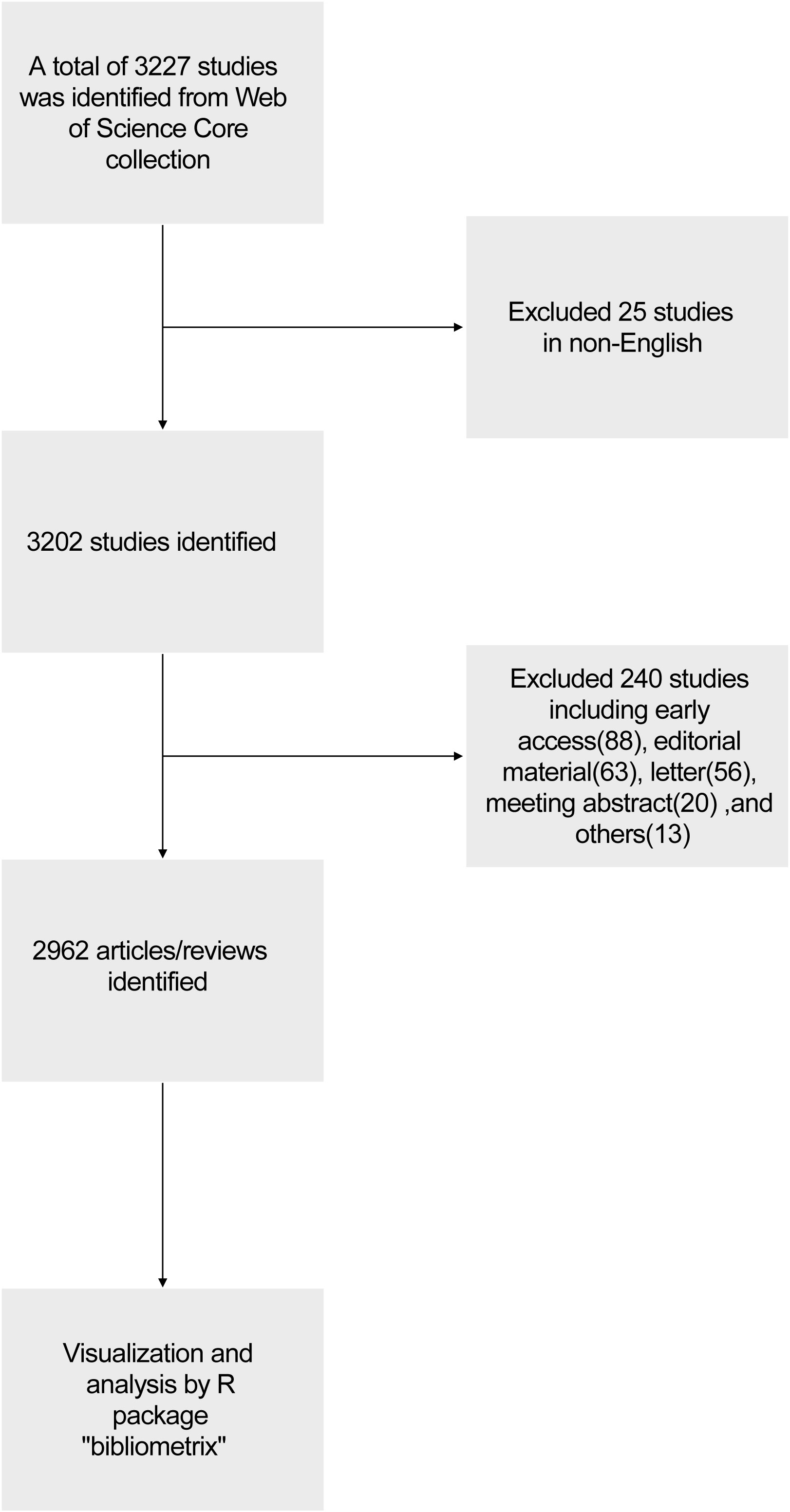
Figure 1 Flowchart of the related data collection and bibliometrics analysis.
Characteristics of the year of publication
Figure 2A shows that the number of annual related publications increased rapidly year by year from 2020 to 2022. In 2020, 271 articles were published, while 795 in 2021, 1144 in 2022, and 752 in 2023. The most productive year was 2022 (n = 1144) with the annual scientific growth rate of 143.9%. The total number of citations per article and the average citations per year have decreased ( Figure 2B ). In 2020, the average number of citations per article was 123.5, 45.9 in 2021, 17.3 in 2022, and 2.48 in 2023. The total average number of citations per year was 24.7 in 2020, 11.5 in 2021, 5.8 in 2022, and 1.2 in 2023.
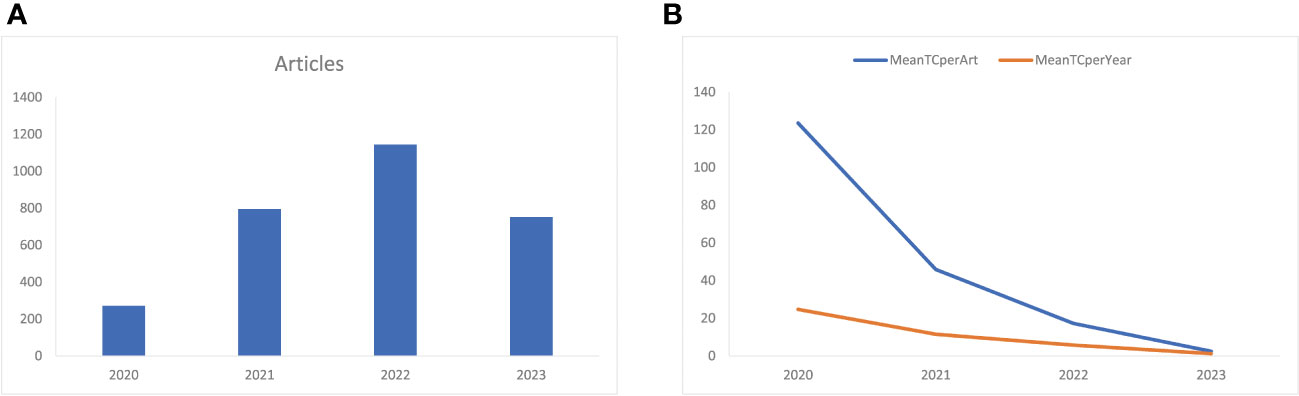
Figure 2 (A) Annual related publication from 2019 to 2023 per year, and (B) average article and average article citations from 2019 to 2023 in COVID-19 RNA vaccine-related research. MeanTCperArt, mean total citation per article; MeanTCperYear, mean total citation per year.
Characteristics of the countries
We filtered and visualized 104 countries that published more than ten articles and constructed a collaborative network based on the number and relationship of publications in each country. From Figure 3A , we can point out that the United States has the highest literature output(n=4163) on COVID-19 RNA vaccines, and the number is significantly higher than that of China(n=1844) and Italy(n=936). Notably, there is much active cooperation between different countries. For example, the United States closely cooperates with China, the United Kingdom, Germany, and Italy; India actively cooperates with Saudi Arabia ( Figure 3B ). It shows that the United States has the most significant number of SCPs and MCPs, which indicates that the United States has the most researches on COVID-19 RNA vaccines and cooperation with other countries in this regard, followed by China on both SCP and MCP ( Figure 3C ).
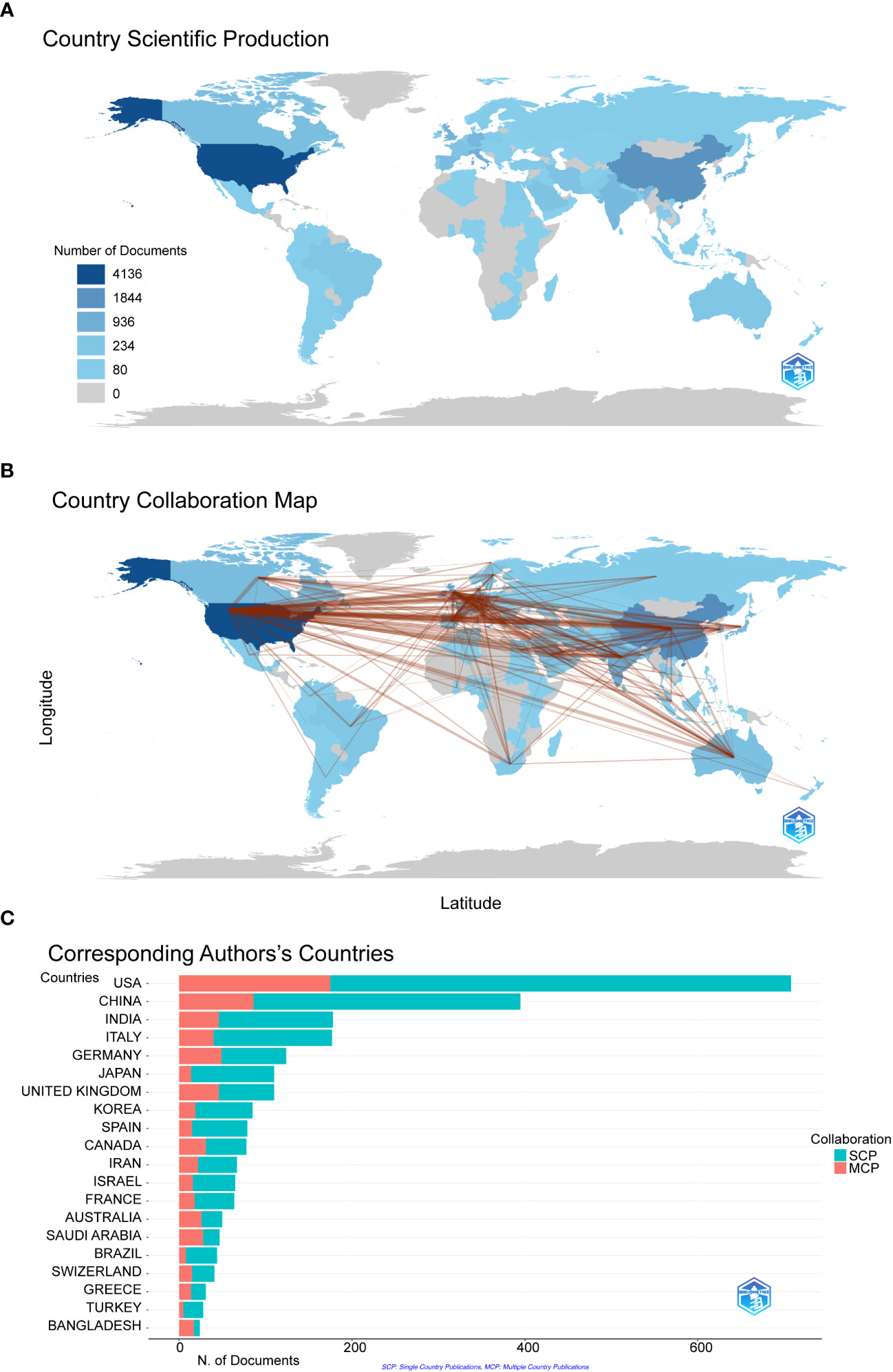
Figure 3 The geographical distribution (A) and visualization (B) of countries on the research of COVID-19 RNA vaccines. A choropleth map detailing the geographic distribution of collaborating countries. The intensity (from light blue to dark blue) is proportional to the number of publications. The number of links (presented as red lines) between any two countries represents the strength of collaboration. (C) Co-authorship analysis of countries in the related research SCP (Single country publications) indicates that the authors of this article are all from the same country, and MCP (Multiple country publications) indicates that the authors of this article are from different countries, indicating international cooperation.
Characteristics of the affiliations
In Figure 4A , Harvard University has the highest number of institutions that receive and publish articles (n=249), followed by the University of California System (n=160) and Harvard Medical School (n=76). Half the top 20 most relevant affiliations were from the United States, followed by the United Kingdom, China, France, and Israel. Subsequently, we selected 34 institutions based on visualization with a minimum number of publications equal to 5. We constructed a collaborative network based on the number of publications and relationships of each affiliation ( Figure 4B ). As shown in Figure 4B , Harvard University and Harvard Medical School cooperated the most, and Tel Aviv University and Sheba Medical Center also had active cooperation. In addition, we noticed that Harvard University had published the most papers and collaborated with the most significant number of affiliations.
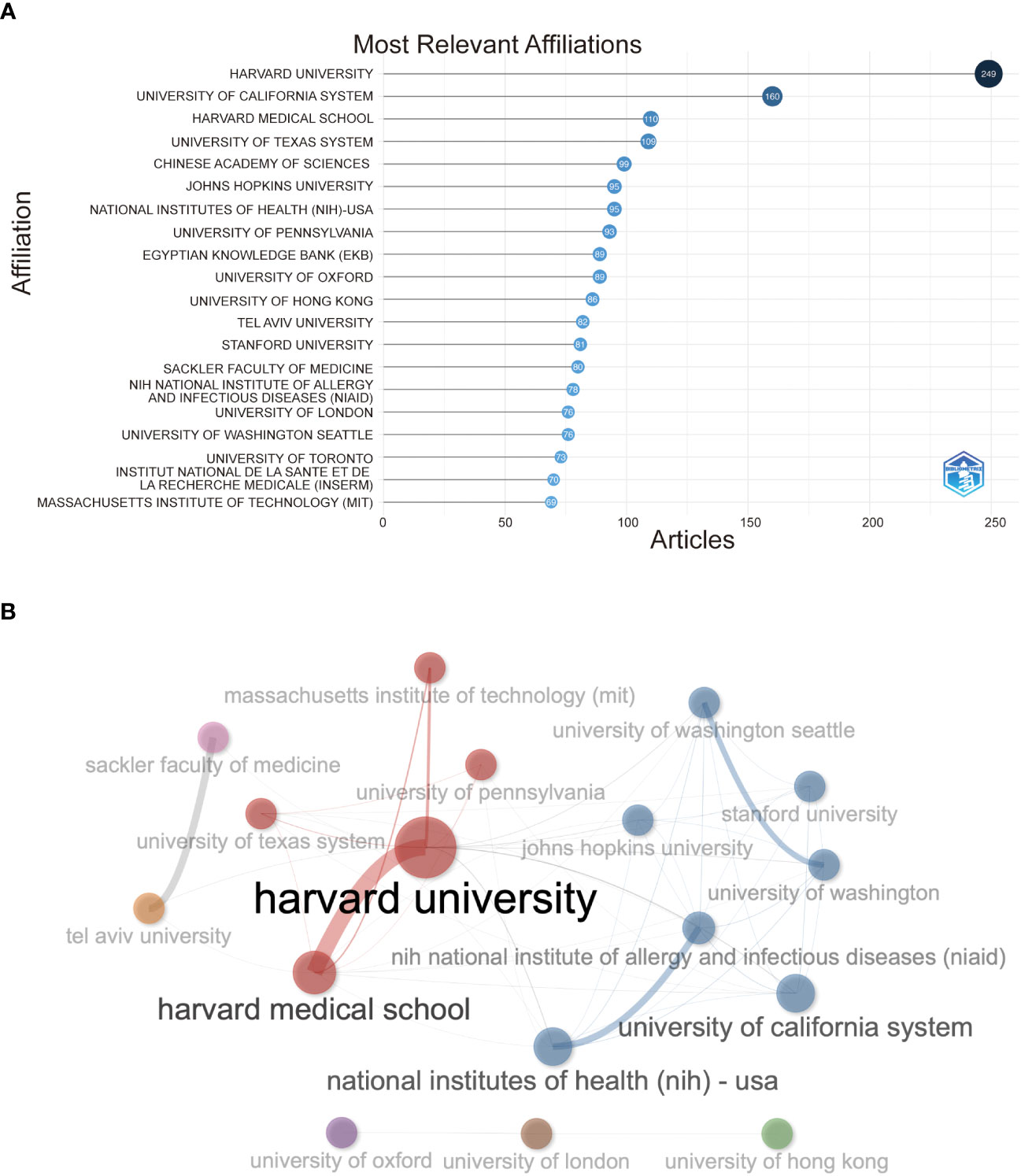
Figure 4 (A) Top 20 most relevant affiliations on the research of COVID-19 RNA vaccines. (B) Network map of co-authorship between affiliations with more than 5 citations.
Characteristics of the top 20 most productive authors
The number of academic publications by an author can represent research activities and contributions in the field to some extent. As shown in Table 1 , Ugur Sahin was the most influential author from University Medical Center, Johannes Gutenberg University, between 2020 and 2023 on COVID-19 RNA vaccines, who had published 14 articles in this field, whose h-index is 9, g-index is 14, m-index is 2.6. He also has the highest number of total citations(n=14203). Pei-Yong Shi’s h-index(n=12) is a close second. Pei-Yong Shi published 16 articles in this field between 2020 and 2023; his g-index is 16, and his m-index is 2.4. Notably, we can find that Pei-Yong Shi and Ugur Sahin had the most significant academic influence on COVID-19 RNA vaccines.
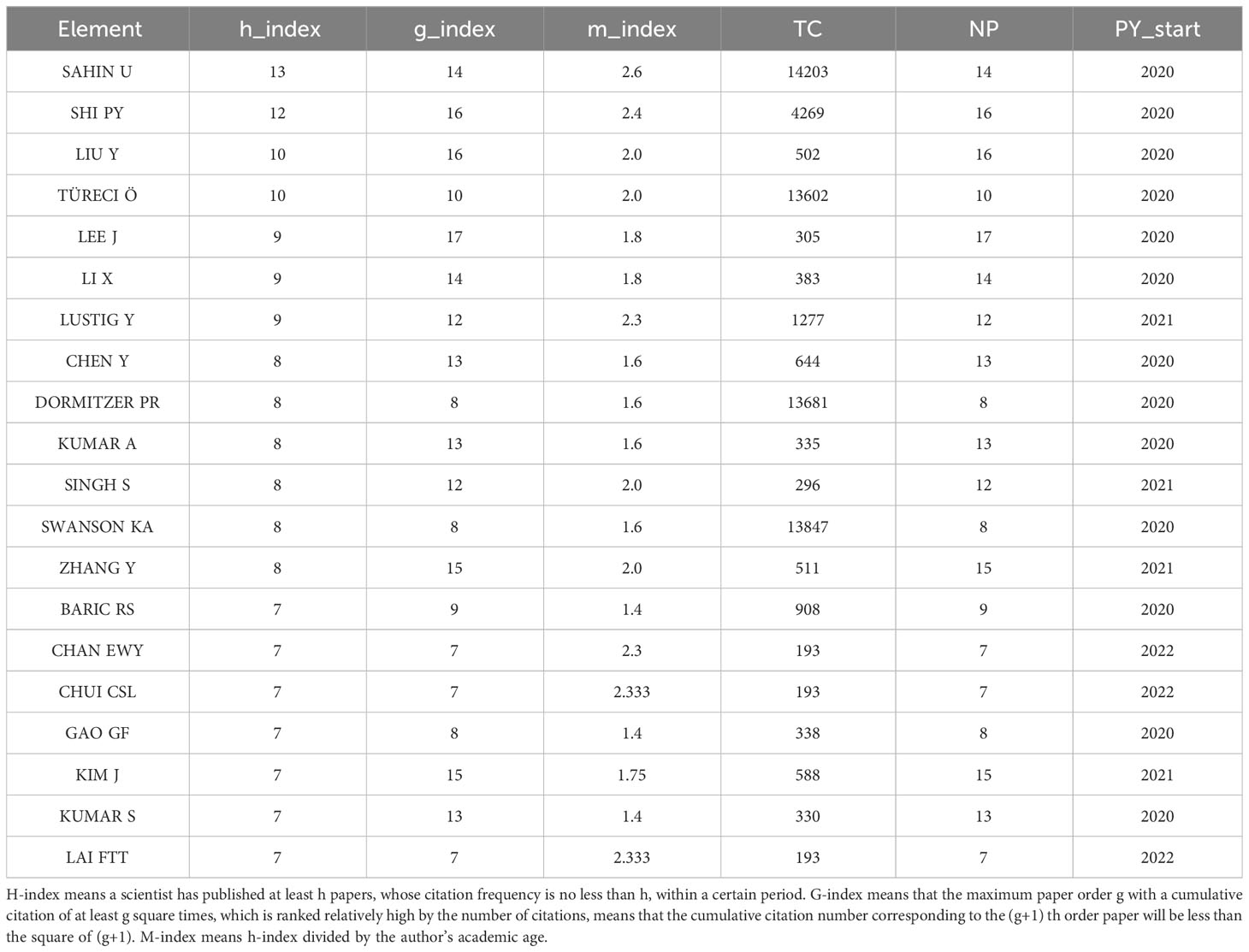
Table 1 The author’s impact in relevant field.
Characteristics of the top 20 journals and co-cited journals
Followed by Frontiers in Immunology (n=105, 3.54%) and Viruses-Basel (n = 58, 2.94%), the Vaccines published the most articles on COVID-19 RNA vaccines (n =171, 5.77%) throughout four years. However, the New England Journal of Medicine, Nature, and Science were the most cited journals. Bradford’s law was applied to assess the core journals in the field of COVID-19 RNA vaccines. As shown in Figure 5A , the core journals in COVID-19 RNA vaccines were Vaccines, Frontiers in Immunology, Viruses-Basel, Clinical Infectious Diseases, Journal of Medical Virology, etc. As for co-cited journals in Figure 5B , journals were categorized into different clusters. The nodes with different colors in the graph represent different clusters. The node size represents the number of articles published in the journal, and the thickness of the lines represents the number of connections between nodes. Frontiers in Immunology, Vaccines, and Journal of Medical Virology were the top three most influential journals in this field. This result can help scholars to select the best-fit journals for submitting their research findings. Also, Table 2 lists the top 20 most-cited publications on COVID-19 RNA vaccines. All these productions were published between 2020 and 2023, and 65% obtained more than 1000 citations. Table 2 shows that the New England Journal of Medicine was the highest-cited journal with the highest h-index, m-index, and total citations. Frontiers in Immunology has the highest g-index. These indexes showed the importance of these two journals on COVID-19 RNA vaccines.
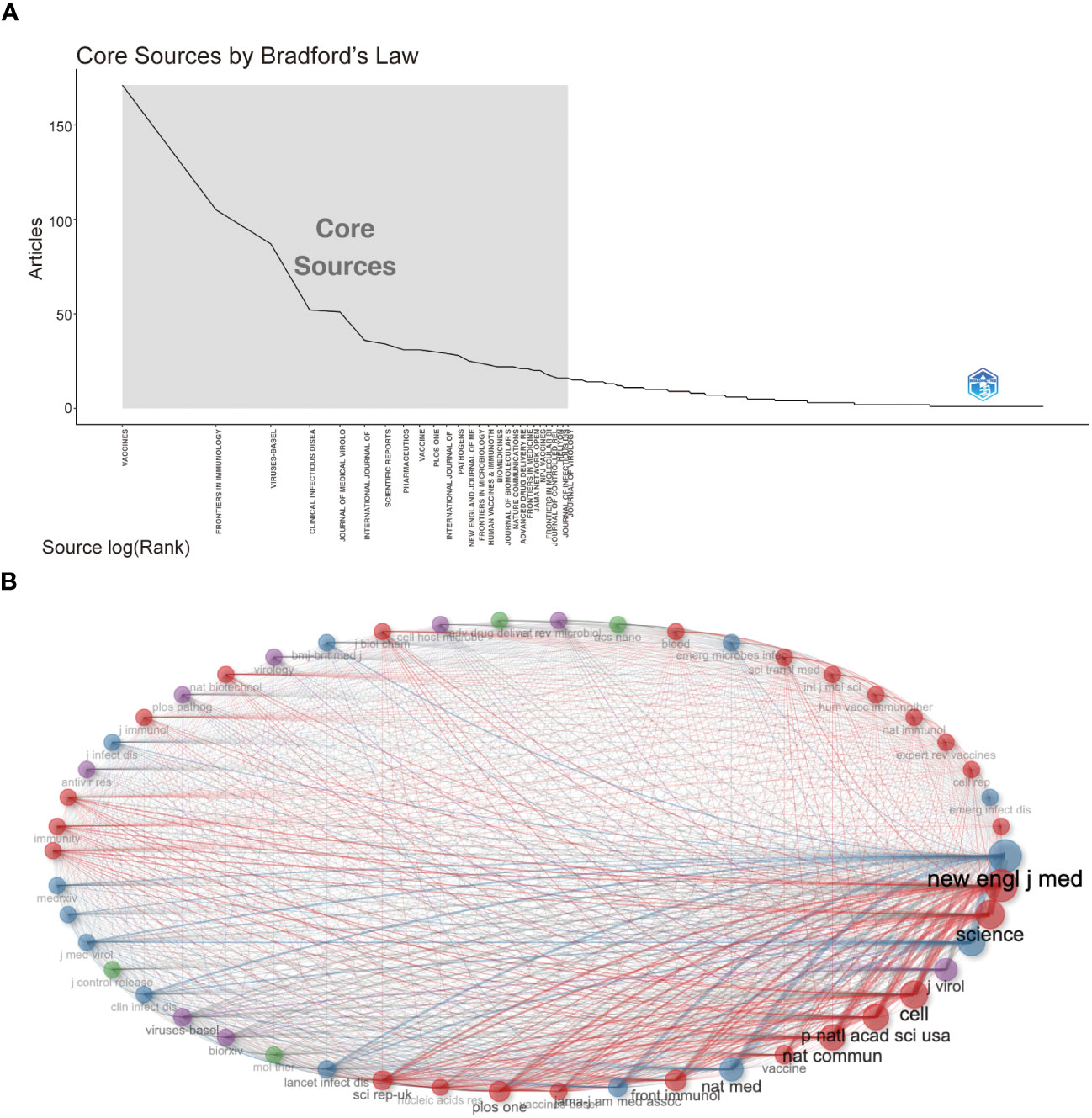
Figure 5 (A) Journals (Sources) clustering through Bradford’s law. (B) Co-cited Journals of COVID-19 RNA vaccines.
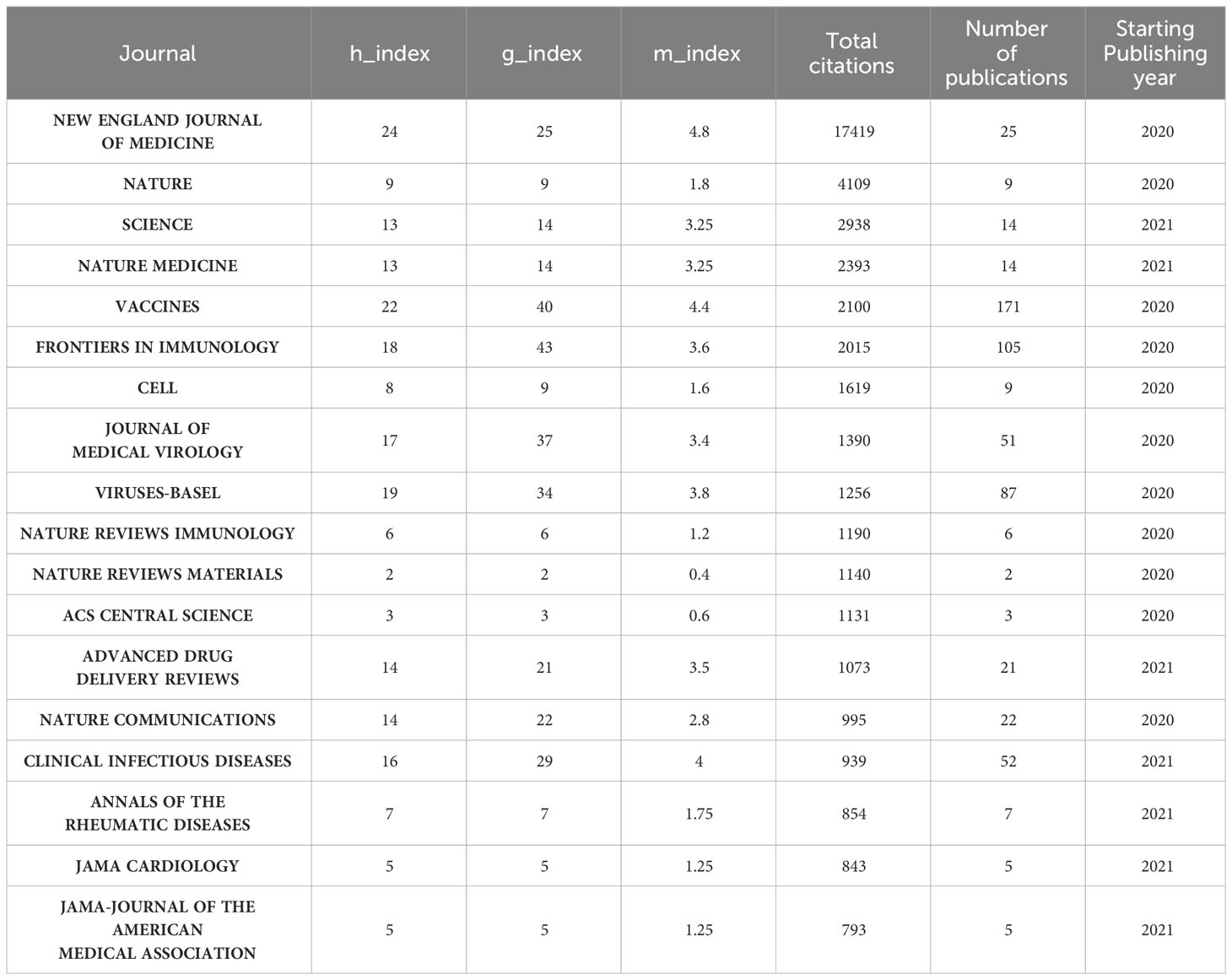
Table 2 The journal’s impact on COVID-19 RNA vaccines.
Relations between journals (left), authors (middle), and affiliations(right)
The relations between journals, authors, and affiliations were visualized using the three-field plot (TFP). In this instance, the significant features were represented in the diagram by rectangles with different colors. The height of the rectangles in the diagram of the TFP depended on the rate or value of the summation of the relations arising between the component the rectangle represents (journals, authors, and affiliations) and the diagram of other elements. The more relations the component or element had, the higher the rectangle represented. Figure 6 shows the TFP analysis of publications on COVID-19 RNA vaccines centered on relations between the journals, authors, and affiliations. The diagram demonstrated the top journals, authors, and affiliations relations in publications on COVID-19 RNA vaccines and their related studies during these four years.
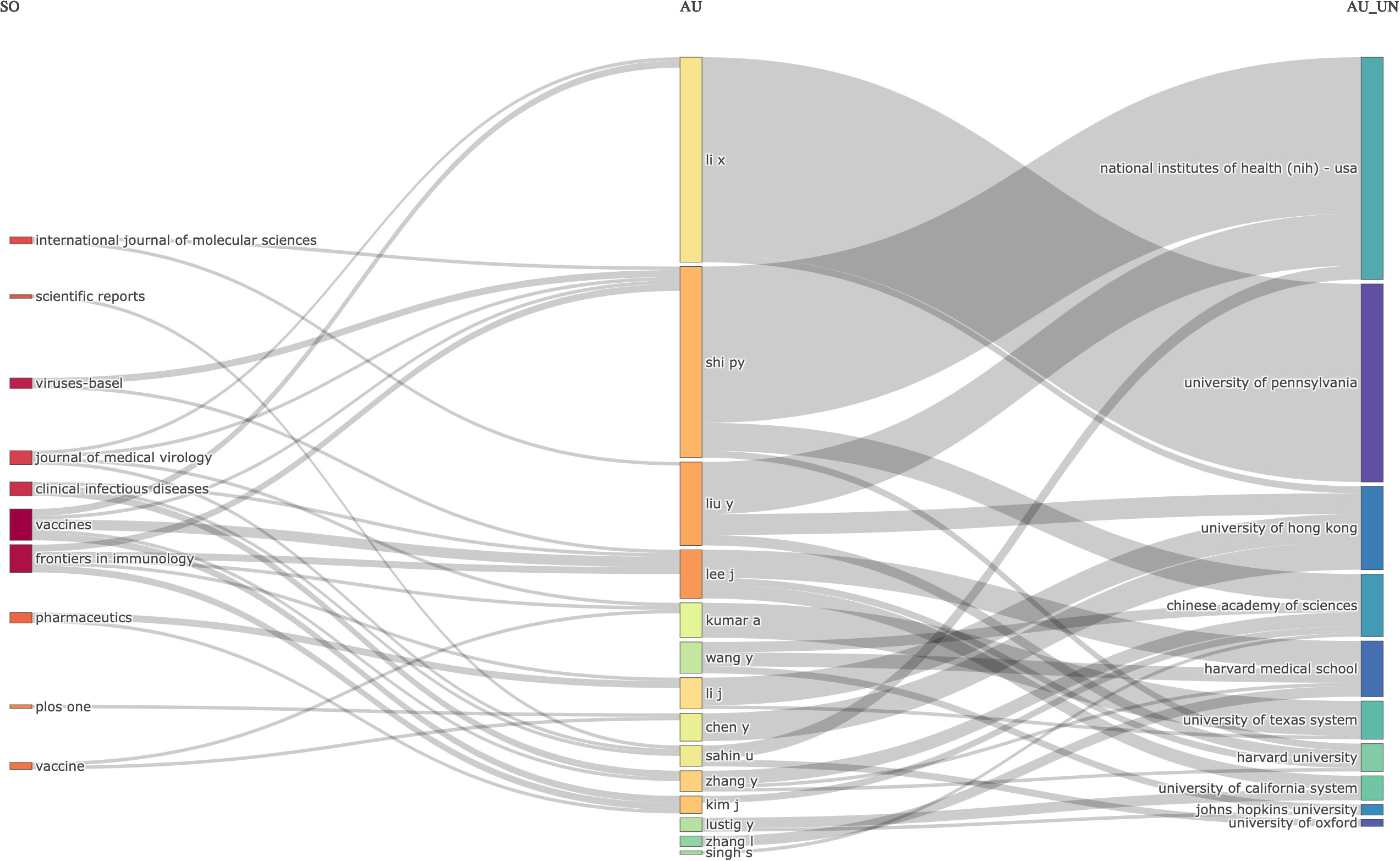
Figure 6 Three-Fields Plot revealed the relations between journals (left), authors (middle), and affiliations (right) for research in COVID-19 RNA vaccines.
Characteristics of the top 20-most cited articles and co-cited references
The top 20 most cited articles were published in 11 journals between 2020 and 2023 ( Table 3 ). Seven articles were published in The New England Journal of Medicine, and four were published in Nature. With 8609 citations, the top-cited article was published by Fernando P Polack from the New England Journal of Medicine in 2020. The total citations per year were 1721.80, and the normalized total citation was 69.69. The following one was published by Edward E Walsh and received 1574 citations, whose total citations per year was 314.80, and the normalized total citation was 12.74.
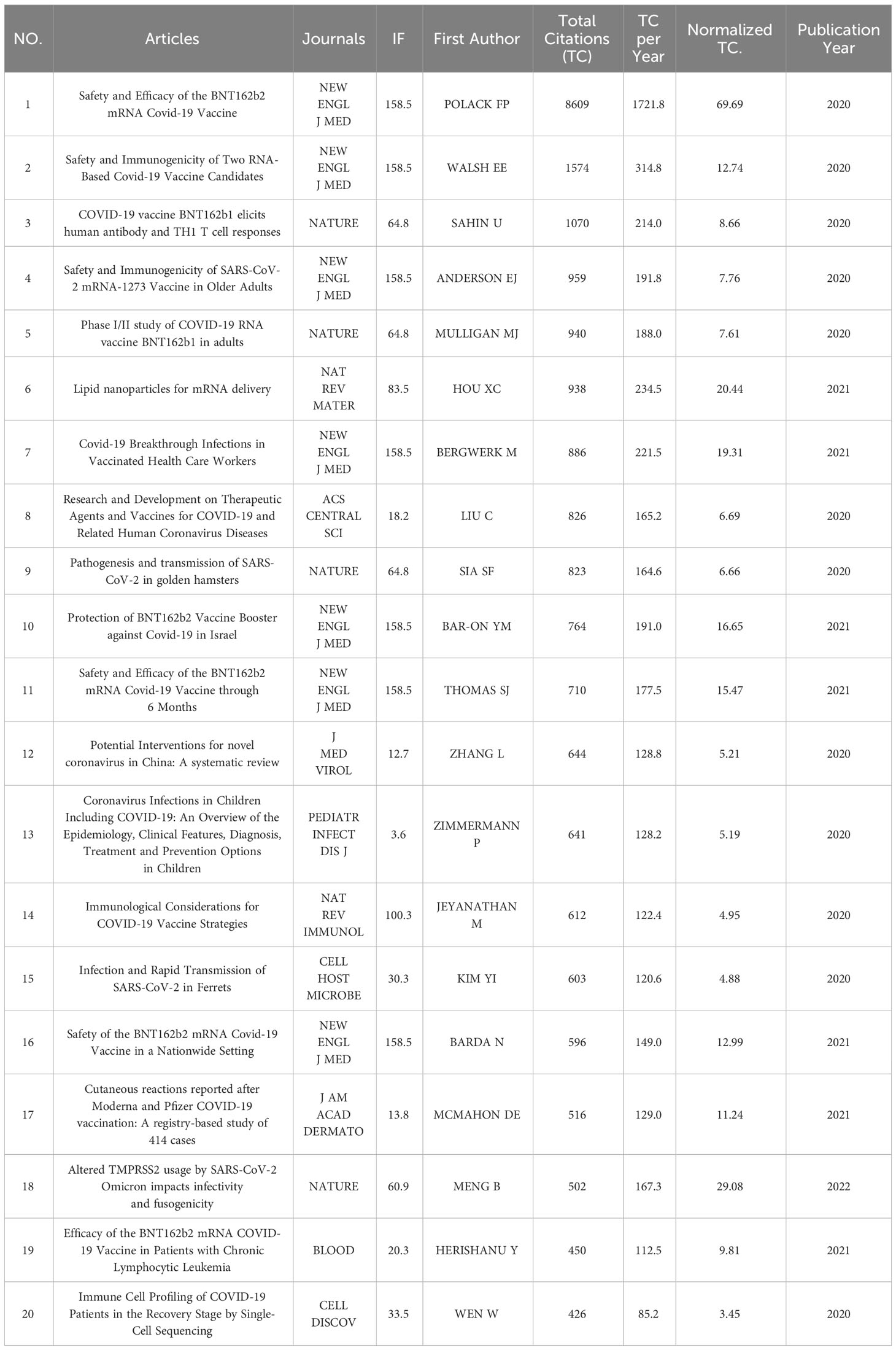
Table 3 Main characteristics of the top 20-most cited articles.
There are 50 references of co-citation with more than five citations. As shown in Figure 7 , Wrapp d 2020 ( 34 ) has the highest number of connections with other references, followed by Hoffmann m 2020 ( 35 ). Polack fp 2020 ( 24 ) has the highest value of PageRank to get other references, which shows the importance of a node to get other nodes, followed by Baden lr 2021 ( 36 ).
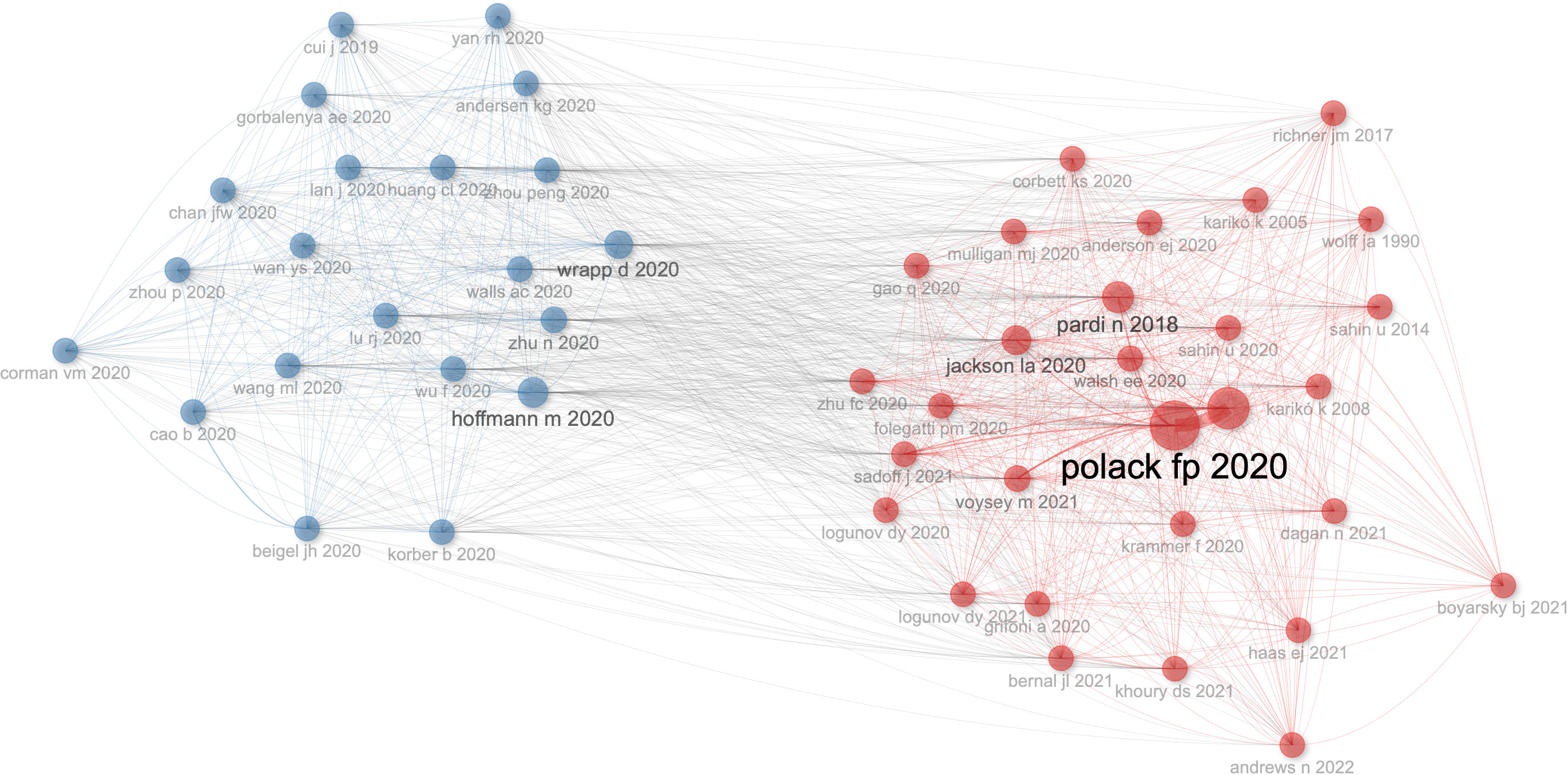
Figure 7 Network map of co-citation between references with more than five citations.
Keyword co-occurrence, clusters
Keywords are always the core research content highly condensed and summarized by researchers, which can reflect the central theme of the research. Therefore, keyword co-occurrence analysis is a crucial way to determine the main research direction and hot research topics of a specific discipline. Among Figure 8B , the most frequent author’s keywords were “covid-19” (n =1166,25%), “sars-cov-2” (n=1054,22%), “vaccine” (n=323,7%), “coronavirus” (n =183,4%), “vaccines” (n =174,4%), and “vaccination” (n = 170,4%). The overall keyword network visualization is presented in Figure 8 . It can be seen that the frequency of the words COVID-19 and SARS-COV-2 has significantly increased from 2020 to 2023.
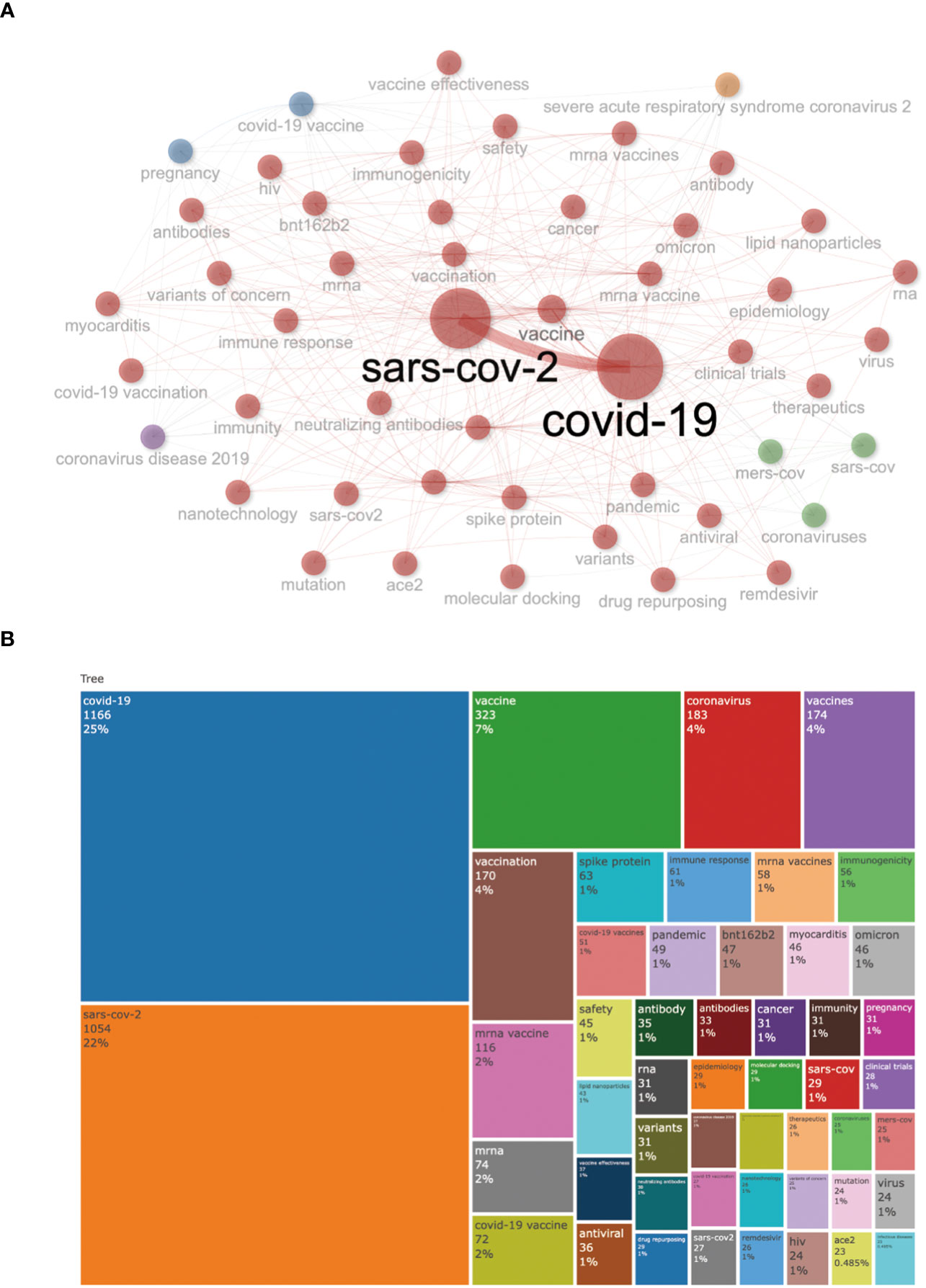
Figure 8 Keyword co-occurrence map (A) and the cluster of COVID-19 RNA vaccines (B) .
In this study, R studio quantitative analysis software was used to analyze the references related to the COVID-19 RNA vaccines and summarize the research results and progress. Quantitative analysis of annual publication quantity, country, author, institution, journal, and other essential information are also included. According to the number of articles published on the COVID-19 RNA vaccines in 2020, the number of documents published in this field is 271, showing an overall increasing trend. The higher the number of citations in a paper, the more excellent its impact on the field and the higher its quality. The total number of citations in this field increases between 2020 and 2022. The number of related articles in 2023 is lower than in 2022.
Through statistical analysis of the number of papers published by countries/regions and institutions, it can be determined that the key countries/regions and research institutions that have published many COVID-19 RNA vaccine literature and have a significant influence can determine their cooperation relationship. The United States and China are major countries conducting research on RNA vaccines for COVID-19, and the United States ranks first. Half of the top 20 research institutions are in the United States, followed by the United Kingdom, China, France and Israel. We noticed the close cooperation among five countries: the United States, China, the United Kingdom, Germany, and Italy. In addition, the United States has active collaborations with China, the United Kingdom, and Germany. The United States is undoubtedly the main driving force for the development of this field. The publications and cooperation between countries are significantly higher in developed countries than in developing countries. Regarding research affiliations, 50% of the top 20 most relevant affiliations were from the United States, which may be one of the essential reasons for the rapid development of the United States in this field. Regarding institutions, Harvard University is the most prolific institution, followed by the University of California System and Harvard Medical School. Affiliations like Tel Aviv University and Sheba Medical Center have an excellent cooperative relationship. Also, we found that Harvard University published the most papers and collaborated with the most institutions, which will be detrimental to the long-term development of academic research. Although some countries have cooperative relations, the frequency, breadth, and intensity of cooperation between institutions are not ideal. For example, there is only a small amount of collaboration between institutions in the United States and China. This situation will hinder the development of the research field in the long run. Therefore, we strongly recommend that research institutions in various countries carry out extensive cooperation and communication to jointly promote the development of RNA vaccines for COVID-19. Close collaboration and communication between countries and institutions are conducive to eliminating academic barriers and further developing research related to the COVID-19 RNA vaccines.
From the perspective of the author, SAHIN U, SHI PY, LIU Y, TÜRECI Ö, and LEE J published the most articles. Professor Uğur Şahin, who had the highest number of total citations, had published 14 papers, 9 of which were concerned with the immunogenicity and effectiveness of COVID-19 mRNA vaccines, and pointed out that BNT162b2 has neutralizing activity on different COVID-19 variants. They also found that BNT162B2 can elicit the response of TH1 cells and antibodies. In addition, the safety of these vaccines has also been proved ( 37 – 46 ). Pei-Yong Shi, whose h-index was second only to Uğur Şahin, has published 16 articles during these four years, most of which pointed out the safety and immunogenicity of COVID-19 RNA vaccines. These vaccines can induce the persistent response of the human germinal center. He also found that some SARS-CoV-2 variants resist these RNA vaccines ( 25 , 27 , 37 , 39 , 47 – 56 ).
Most of the research on COVID-19 RNA vaccines was published in Vaccines (IF=7.8, Q1), indicating it is currently the most productive journal in this research field. Among the journals, the journal with the highest impact factor is the New England Journal of Medicine (IF=158.5, Q1), followed by Nature (IF=64.8, Q1). As for the co-cited journals, we could find that most of them are high-impact Q1 journals. These journals are high-quality international journals providing support for the study of COVID-19 RNA vaccines.
The top 20 most cited articles were mainly published between 2020 and 2021, and all seven were published in the New England Journal of Medicine, indicating the influence of the New England Journal of Medicine in this regard. In addition, the first four articles are all about the safety and effectiveness of the COVID-19 RNA vaccines. It can be seen that the safety and effectiveness of RNA vaccines have always been a hot topic in the discussion of the COVID-19 RNA vaccines.
Vaccines, Frontiers in Immunology, and Virus Basel are the journals that publish the most articles about the COVID-19 RNA vaccines. However, regarding influence, the New England Journal of Medicine has the highest h-index, m-index, and total citations, proving that it currently has the most significant influence in this field. Frontiers in Immunology has the highest g-index, proving its importance in the field of COVID-19 RNA vaccines. Frontiers in Immunology, Vaccines, and Journal of Medical Virology were the top three most influential journals in this field, which may be listed in the journal consideration for the relevant researchers.
According to the keywords, COVID-19, SAR-COV-2, and vaccine are currently the most concerning topics conducive to further research. The research hotspots in this field mainly include COVID-19, SAR-COV-2, and vaccine. We hope this work can provide new ideas for promoting scientific research and clinical applications of COVID-19 RNA vaccines.
In general, this study is the first comprehensive analysis that summarizes the research of the COVID-19 RNA vaccines using literature metrology methods. Our research findings provide valuable information for researchers in this field to understand the basic knowledge landscape, current research hotspots, and future opportunities and identify potential collaborators in the future.
The wide application of the COVID-19 RNA vaccines provides a good platform for the development of RNA vaccine, not only contributes to the research and development of COVID-19 RNA vaccines but also proves the effectiveness and safety of RNA vaccine to a certain extent and provides sufficient theoretical and technical support for the future application of RNA vaccine in other fields, such as cancer treatment.
Limitations
Firstly, to ensure high-quality bibliometric analysis, the analysis of this study is based on articles in the Web of Science database, one of the most commonly used scientific publication databases. However, some studies may be omitted as they are published in non-SCI journals or other databases. Secondly, bibliometric analyses cannot completely replace system retrieval. Third, metrology cannot evaluate the quality of a single study because the citation index is time-dependent, meaning that recent articles may be less cited than earlier, even if they are more valuable. These limitations may slightly impact the overall results but are unlikely to alter the main trends presented in this article. In general, our research has provided a basis for understanding the research topics of the COVID-19 RNA vaccines and the production and application of the RNA vaccine.
Eligibility criteria and data source
In this study, research articles on RNA vaccines for COVID-19 published between 2020 and 2023 as original articles or reviews in English were considered eligible. Web of Science core collection database was used.
In the advanced search option of the Web of Science database, using an appropriate combination of Boolean and wildcard search operators, the following keywords were searched: “Corona Virus Disease 2019”, “COVID-19”, “RNA”, and “vaccines”. The search was performed on January 11th, 2024, and the entire search strategy is presented in TS = ((RNA vaccine AND COVID-19) OR (RNA vaccine AND SARS-COV-2, the type of documents is set to “articles” and “reviews”. The language of articles is set as English only. Then, all the resulted information, including full records and cited references, was downloaded in txt format.
Bibliometric analyses
Data management and bibliometric analyses were conducted using the Bibliometrix package (version 3.1.4) ( 57 ) and Biblioshiny ( 57 ) web apps under R (version 4.0.2). We retrieved all the main information and features included in the study. Publications and citation trends were constructed over four years. From 2020 to 2023, the most influential countries on COVID-19 RNA vaccine research were retrieved and presented as a cluster collaboration network. The cooperative world map represents world research cooperation, with the minimum edge set at 10. In addition, we identified the most productive institutions based on the highest number of paper contributions to the topic over the past four years. We used leading eigenvalue clustering algorithms to construct a collaborative network between institutions with more than five citations. We determined the author with the highest contribution based on the highest number of papers and the top 20 co-citation networks of influential authors. The 20 most cited references and the most influential journals were also identified, and some characteristics were searched, such as h-index, g-index, m-index, the total number of citations, the number of papers on the subject published in the journal, and the year when the journal began to publish COVID-19 RNA vaccine-related topics. In order to observe the inflow and outflow of journals, authors, and affiliated institutions that have contributed to the COVID-19 RNA vaccines in the past four years, a three-field plot was constructed. A tree chart was prepared to display keywords published on this topic from 2020 to 2023.
Conclusions
RNA vaccine has essential research value and application prospects in COVID-19. The rapid increase in the number of publications shows that the research on the RNA vaccine for COVID-19 has attracted more attention from scholars worldwide. The main countries are the United States and China. However, cooperation and communication between countries and institutions still need to be strengthened. On the one hand, studying the immunogenicity and safety of RNA vaccines will help us to prevent COVID-19 variants infection and reduce vaccine side effects ( 58 ). On the other hand, compared with traditional vaccines, RNA vaccines have significant advantages in preventing COVID-19. Therefore, the study of COVID-19 RNA vaccines has essential application value in preventing COVID-19 infection and alleviating symptoms in the future ( 59 ). In addition to the related prevention research of COVID-19, attention can also be paid to the transformation of research achievements, that is, the clinical application of RNA vaccines in other diseases ( 58 ).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
ZC: Conceptualization, Visualization, Writing – original draft. ZL: Software, Validation, Writing – review & editing. YF: Writing – review & editing. AS: Writing – original draft. LW: Writing – review & editing. YS: Conceptualization, Funding acquisition, Supervision, Writing – original draft. CL: Conceptualization, Software, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Jiangxi Provincial Natural Science Foundation of China (20204BCJL23052, 20212ACB216013) and by Nanchang Natural Science Foundation No.129 in 2021.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Center JHUCR. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Available online at: https://coronavirus.jhu.edu/map.html2023 .
Google Scholar
2. Seo SH, Jang Y. Cold-adapted live attenuated SARS-cov-2 vaccine completely protects human ACE2 transgenic mice from SARS-cov-2 infection. Vaccines (2020) 8(4):584. doi: 10.1101/2020.08.04.235689
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Trimpert J, Dietert K, Firsching TC, Ebert N, Thi Nhu Thao T, Vladimirova D, et al. Development of safe and highly protective live-attenuated SARS-CoV-2 vaccine candidates by genome recoding. Cell Rep (2021) 36:109493. doi: 10.1016/j.celrep.2021.109493
4. Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, et al. Effect of an inactivated vaccine against SARS-coV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. Jama (2020) 324:951–60. doi: 10.1001/jama.2020.15543
5. Guebre-Xabier M, Patel N, Tian JH, Zhou B, Maciejewski S, Lam K, et al. NVX-CoV2373 vaccine protects cynomolgus macaque upper and lower airways against SARS-CoV-2 challenge. Vaccine (2020) 38:7892–6. doi: 10.1016/j.vaccine.2020.10.064
6. Wu Y, Huang X, Yuan L, Wang S, Zhang Y, Xiong H, et al. A recombinant spike protein subunit vaccine confers protective immunity against SARS-CoV-2 infection and transmission in hamsters. Sci Trans Med (2021) 13(606):eabg1143. doi: 10.1126/scitranslmed.abg1143
CrossRef Full Text | Google Scholar
7. Yang S, Li Y, Dai L, Wang J, He P, Li C, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis (2021) 21:1107–19. doi: 10.1016/S1473-3099(21)00127-4
8. Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis (2021) 21:39–51. doi: 10.1016/S1473-3099(20)30831-8
9. Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis (2021) 21:637–46. doi: 10.1016/S1473-3099(20)30942-7
10. Kremsner PG, Mann P, Kroidl A, Leroux-Roels I, Schindler C, Gabor JJ, et al. Safety and immunogenicity of an mRNA-lipid nanoparticle vaccine candidate against SARS-CoV-2: A phase 1 randomized clinical trial. Wiener klinische Wochenschrift (2021) 133:931–41. doi: 10.1007/s00508-021-01922-y
11. Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis (2021) 21:181–92. doi: 10.1016/S1473-3099(20)30843-4
12. Corbett KS, Edwards DK, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature (2020) 586:567–71. doi: 10.1038/s41586-020-2622-0
13. Wang Y, Yang C, Song Y, Coleman JR, Stawowczyk M, Tafrova J, et al. Scalable live-attenuated SARS-CoV-2 vaccine candidate demonstrates preclinical safety and efficacy. Proc Natl Acad Sci USA (2021) 118(29):e2102775118. doi: 10.1073/pnas.2102775118
14. Dai L, Zheng T, Xu K, Han Y, Xu L, Huang E, et al. A universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell (2020) 182:722–33.e11. doi: 10.1016/j.cell.2020.06.035
15. Yang J, Wang W, Chen Z, Lu S, Yang F, Bi Z, et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature (2020) 586:572–7. doi: 10.1038/s41586-020-2599-8
16. Prize TN. Press Release: The Nobel Assembly at Karolinska Institutet. Available at: https://www.nobelprize.org/prizes/medicine/2023/press-release/2023 .
17. Pilkington EH, Suys EJA, Trevaskis NL, Wheatley AK, Zukancic D, Algarni A, et al. From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta biomaterialia (2021) 131:16–40. doi: 10.1016/j.actbio.2021.06.023
18. Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer (2021) 20:41. doi: 10.1186/s12943-021-01335-5
19. Zhou X, Berglund P, Rhodes G, Parker SE, Jondal M, Liljeström P. Self-replicating Semliki Forest virus RNA as recombinant vaccine. Vaccine (1994) 12:1510–4. doi: 10.1016/0264-410X(94)90074-4
20. Cagigi A, Loré K. Immune responses induced by mRNA vaccination in mice, monkeys and humans. Vaccines . (2021) 9(1):61. doi: 10.3390/vaccines9010061.
21. Krammer F. SARS-CoV-2 vaccines in development. Nature (2020) 586:516–27. doi: 10.1038/s41586-020-2798-3
22. Cobb M. Who discovered messenger RNA? Curr biol: CB (2015) 25:R526–32. doi: 10.1016/j.cub.2015.05.032
23. Thomas SJ, Moreira ED Jr., Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N Engl J Med (2021) 385:1761–73. doi: 10.1056/NEJMoa2110345
24. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med (2020) 383:2603–15. doi: 10.1056/NEJMoa2034577
25. Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature (2020) 586:589–93. doi: 10.1038/s41586-020-2639-4
26. Li J, Hui A, Zhang X, Yang Y, Tang R, Ye H, et al. Safety and immunogenicity of the SARS-CoV-2 BNT162b1 mRNA vaccine in younger and older Chinese adults: a randomized, placebo-controlled, double-blind phase 1 study. Nat Med (2021) 27:1062–70. doi: 10.1038/s41591-021-01330-9
27. Walsh EE, Frenck RW Jr., Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med (2020) 383:2439–50. doi: 10.1056/NEJMoa2027906
28. Pimpinelli F, Marchesi F, Piaggio G, Giannarelli D, Papa E, Falcucci P, et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative Malignancies on active treatment: preliminary data from a single institution. J Hematol Oncol (2021) 14:81. doi: 10.1186/s13045-021-01090-6
29. Sebastian M, Papachristofilou A, Weiss C, Früh M, Cathomas R, Hilbe W, et al. Phase Ib study evaluating a self-adjuvanted mRNA cancer vaccine (RNActive ® ) combined with local radiation as consolidation and maintenance treatment for patients with stage IV non-small cell lung cancer. BMC Cancer (2014) 14:748. doi: 10.1186/1471-2407-14-748
30. Verbeke R, Lentacker I, De Smedt SC, Dewitte H. Three decades of messenger RNA vaccine development. Nano Today (2019) 28:17. doi: 10.1016/j.nantod.2019.100766
31. Xu S, Yang K, Li R, Zhang L. mRNA vaccine era-mechanisms, drug platform and clinical prospection. Int J Mol Sci (2020) 21(18):6582. doi: 10.3390/ijms21186582
32. Iavarone C, O’Hagan DT, Yu D, Delahaye NF, Ulmer JB. Mechanism of action of mRNA-based vaccines. Expert Rev Vaccines (2017) 16:871–81. doi: 10.1080/14760584.2017.1355245
33. Pardi N, Hogan MJ, Weissman D. Recent advances in mRNA vaccine technology. Curr Opin Immunol (2020) 65:14–20. doi: 10.1016/j.coi.2020.01.008
34. Wrapp D, Wang NS, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Sci (New York NY) (2020) 367:1260. doi: 10.1126/science.abb2507
35. Hoffmann M, Kleine-Weber H, Schroeder S, Krueger N, Herrler T, Erichsen S, et al. SARS-coV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell (2020) 181:271. doi: 10.1016/j.cell.2020.02.052
36. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-coV-2 vaccine. N Engl J Med . (2021) 384:403–16. doi: 10.1056/NEJMoa2035389.
37. Vogel AB, Kanevsky I, Che Y, Swanson KA, Muik A, Vormehr M, et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature (2021) 592:283–9. doi: 10.1038/s41586-021-03275-y
38. Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature (2021) 595:572–7. doi: 10.1038/s41586-021-03653-6
39. Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature (2020) 586:594–9. doi: 10.1038/s41586-020-2814-7
40. Quandt J, Muik A, Salisch N, Lui BG, Lutz S, Krüger K, et al. Omicron BA.1 breakthrough infection drives cross-variant neutralization and memory B cell formation against conserved epitopes. Sci Immunol (2022) 7:eabq2427. doi: 10.1126/sciimmunol.abq2427
41. Muik A, Wallisch AK, Sänger B, Swanson KA, Mühl J, Chen W, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Sci (New York NY) (2021) 371:1152–3. doi: 10.1126/science.abg6105
42. Muik A, Lui BG, Wallisch AK, Bacher M, Mühl J, Reinholz J, et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Sci (New York NY) (2022) 375:678–80. doi: 10.1126/science.abn7591
43. Muik A, Lui BG, Quandt J, Diao H, Fu Y, Bacher M, et al. Progressive loss of conserved spike protein neutralizing antibody sites in Omicron sublineages is balanced by preserved T cell immunity. Cell Rep (2023) 42:112888. doi: 10.1016/j.celrep.2023.112888
44. Muik A, Lui BG, Bacher M, Wallisch AK, Toker A, Finlayson A, et al. Omicron BA.2 breakthrough infection enhances cross-neutralization of BA.2.12.1 and BA.4/BA.5. Sci Immunol (2022) 7:eade2283. doi: 10.1126/sciimmunol.ade2283
45. Muik A, Lui BG, Bacher M, Wallisch AK, Toker A, Couto CIC, et al. Exposure to BA.4/5 S protein drives neutralization of Omicron BA.1, BA.2, BA.2.12.1, and BA.4/5 in vaccine-experienced humans and mice. Sci Immunol (2022) 7:eade9888. doi: 10.1126/sciimmunol.ade9888
46. Rohde CM, Lindemann C, Giovanelli M, Sellers RS, Diekmann J, Choudhary S, et al. Toxicological assessments of a pandemic COVID-19 vaccine-demonstrating the suitability of a platform approach for mRNA vaccines. Vaccines (2023) 11(2):417. doi: 10.3390/vaccines11020417
47. Chen RE, Zhang X, Case JB, Winkler ES, Liu Y, VanBlargan LA, et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med (2021) 27:717–26. doi: 10.1038/s41591-021-01294-w
48. Hajnik RL, Plante JA, Liang Y, Alameh MG, Tang J, Bonam SR, et al. Dual spike and nucleocapsid mRNA vaccination confer protection against SARS-CoV-2 Omicron and Delta variants in preclinical models. Sci Trans Med (2022) 14:eabq1945. doi: 10.1126/scitranslmed.abq1945
49. Liu J, Liu Y, Xia H, Zou J, Weaver SC, Swanson KA, et al. BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants. Nature (2021) 596:273–5. doi: 10.1038/s41586-021-03693-y
50. Liu Y, Liu J, Xia H, Zhang X, Fontes-Garfias CR, Swanson KA, et al. Neutralizing activity of BNT162b2-elicited serum. N Engl J Med (2021) 384:1466–8. doi: 10.1056/NEJMc2102017
51. Pegu A, O’Connell SE, Schmidt SD, O’Dell S, Talana CA, Lai L, et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Sci (New York NY) (2021) 373:1372–7. doi: 10.1101/2021.05.13.444010
52. Schmitz AJ, Turner JS, Liu Z, Zhou JQ, Aziati ID, Chen RE, et al. A vaccine-induced public antibody protects against SARS-CoV-2 and emerging variants. Immunity . (2021) 54:2159–66.e6 doi: 10.1016/j.immuni.2021.08.013
53. Turner JS, O’Halloran JA, Kalaidina E, Kim W, Schmitz AJ, Zhou JQ, et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature (2021) 596:109–13. doi: 10.1038/s41586-021-03738-2
54. Wang L, Kainulainen MH, Jiang N, Di H, Bonenfant G, Mills L, et al. Differential neutralization and inhibition of SARS-CoV-2 variants by antibodies elicited by COVID-19 mRNA vaccines. Nat Commun (2022) 13:4350. doi: 10.1038/s41467-022-31929-6
55. Xia H, Zou J, Kurhade C, Cai H, Yang Q, Cutler M, et al. Neutralization and durability of 2 or 3 doses of the BNT162b2 vaccine against Omicron SARS-CoV-2. Cell Host Microbe (2022) 30:485–8.e3. doi: 10.1016/j.chom.2022.02.015
56. Xie X, Zou J, Kurhade C, Liu M, Ren P, Pei-Yong S. Neutralization of SARS-CoV-2 Omicron sublineages by 4 doses of the original mRNA vaccine. Cell Rep (2022) 41:111729. doi: 10.1016/j.celrep.2022.111729
57. Aria M, Cuccurullo C. bibliometrix: An R-tool for comprehensive science mapping analysis. J Informetrics (2017) 11:959–75. doi: 10.1016/j.joi.2017.08.007.
58. Goel RR, Painter MM, Apostolidis SA, Mathew D, Meng W, Rosenfeld AM, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Sci (New York NY) (2021) 374:abm0829. doi: 10.1126/science.abm0829
59. Andrews N, Stowe J, Kirsebom F, Toffa S, Sachdeva R, Gower C, et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med (2022) 28:831–7. doi: 10.1038/s41591-022-01699-1
Keywords: COVID-19, SARS-CoV-2, RNA vaccines, web of science, bibliometrics
Citation: Chen Z, Liu Z, Feng Y, Shi A, Wu L, Sang Y and Li C (2024) Global research on RNA vaccines for COVID-19 from 2019 to 2023: a bibliometric analysis. Front. Immunol. 15:1259788. doi: 10.3389/fimmu.2024.1259788
Received: 16 July 2023; Accepted: 01 February 2024; Published: 15 February 2024.
Reviewed by:
Copyright © 2024 Chen, Liu, Feng, Shi, Wu, Sang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Sang, [email protected] ; Chenxi Li, [email protected]
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
COVID-19 and vaccine hesitancy: A longitudinal study
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Rady School of Management, University of California San Diego, La Jolla, California, United States of America
Roles Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Roles Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing
- Ariel Fridman,
- Rachel Gershon,
- Ayelet Gneezy

- Published: April 16, 2021
- https://doi.org/10.1371/journal.pone.0250123
- Peer Review
- Reader Comments
How do attitudes toward vaccination change over the course of a public health crisis? We report results from a longitudinal survey of United States residents during six months (March 16 –August 16, 2020) of the COVID-19 pandemic. Contrary to past research suggesting that the increased salience of a disease threat should improve attitudes toward vaccines, we observed a decrease in intentions of getting a COVID-19 vaccine when one becomes available. We further found a decline in general vaccine attitudes and intentions of getting the influenza vaccine. Analyses of heterogeneity indicated that this decline is driven by participants who identify as Republicans, who showed a negative trend in vaccine attitudes and intentions, whereas Democrats remained largely stable. Consistent with research on risk perception and behavior, those with less favorable attitudes toward a COVID-19 vaccination also perceived the virus to be less threatening. We provide suggestive evidence that differential exposure to media channels and social networks could explain the observed asymmetric polarization between self-identified Democrats and Republicans.
Citation: Fridman A, Gershon R, Gneezy A (2021) COVID-19 and vaccine hesitancy: A longitudinal study. PLoS ONE 16(4): e0250123. https://doi.org/10.1371/journal.pone.0250123
Editor: Valerio Capraro, Middlesex University, UNITED KINGDOM
Received: November 12, 2020; Accepted: February 14, 2021; Published: April 16, 2021
Copyright: © 2021 Fridman et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All data and code are publicly available on the Open Science Framework at https://osf.io/kgvdy/ .
Funding: UC San Diego Global Health Initiative (GHI): awarded to all authors; Project number: 1001288. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. https://medschool.ucsd.edu/som/medicine/divisions/idgph/research/Global-Health/grant-recipients/2019-2020/Pages/Faculty-Postdoc-Travel-and-Research.aspx .
Competing interests: The authors have declared that no competing interests exist.
Introduction
Vaccinations are among the most important public health tools for reducing the spread and harm caused by dangerous diseases [ 1 ]. The World Health Organization estimates that vaccines prevented at least 10 million deaths between 2010–2015 worldwide [ 2 ]. Despite considerable evidence showing vaccines are safe [ 3 , 4 ], there is increasing skepticism toward vaccination [ 5 , 6 ]. Vaccine hesitancy has led to a decline in vaccine uptake and to an increase in the prevalence of vaccine-preventable diseases (VPDs) [ 7 , 8 ]. Ironically, the objection to vaccines is commonly a consequence of their effectiveness—because individuals have lower exposure to VPDs, they are less concerned about contracting them [ 9 ], which consequently leads to greater vaccine hesitancy [ 10 ]. The COVID-19 pandemic has created a new reality where individuals are faced with a previously unknown disease and its effects, providing a unique opportunity to investigate vaccine attitudes during a period of heightened disease salience. The present research reports findings from a longitudinal study conducted during the COVID-19 health crisis, in which we measured changes in attitudes toward a prospective vaccine, as well as shifts in vaccine attitudes in general.
Factors influencing vaccine attitudes and behaviors
Past research has identified a variety of situational and individual-level factors that influence vaccine attitudes and behavior, the most prominent of which are risk perceptions and demographic characteristics.
Assessments of risk are influenced by both cognitive evaluations (i.e., objective features of the situation such as probabilities of outcomes) and affective reactions [ 11 ], as well as by contextual factors (e.g., the information that is most available or salient at the time [ 12 ]). For example, research shows that media coverage plays a significant role in determining the extent to which we take threats seriously [ 13 ]. When individuals perceive heightened risk of a threat, they become more favorable toward interventions that mitigate that threat, including vaccination (for a meta-analysis on the effect of perceived risk on intentions and behaviors, see [ 14 ]). In the case of COVID-19, this would suggest more positive attitudes toward a vaccine and greater likelihood to get vaccinated. Indeed, research suggests that individuals should exhibit a greater interest in vaccinations during a pandemic because disease threat is more salient [ 15 ].
Past efforts to improve vaccine attitudes have had limited success or even backfired; for example, messages refuting claims about the link between vaccines and autism, as well as messages featuring images of children who were sick with VPDs, had negative effects on vaccine attitudes among those who were already hesitant to vaccinate [ 16 ]. In contrast, messaging that increases disease threat salience has shown promise in reducing vaccine hesitancy [ 5 ], and there is evidence suggesting that increased threat salience for a particular disease may also increase intentions to vaccinate for other diseases [ 17 ]. Building on these findings, we expected to find an increase in pro-vaccine attitudes and in individuals’ interest in a COVID-19 vaccine when the perceived threat of the COVID-19 virus increased.
Vaccine attitudes are also influenced by a variety of demographic and ideological factors (for a review, see [ 18 ]). For example, perceptions of vaccine risk differ among individuals of different ethnic backgrounds [ 19 ], and there is extant work demonstrating a positive correlation between socioeconomic status (SES) and vaccine hesitancy [ 20 , 21 ]. Socio-demographic factors are also linked to vaccine-related behaviors: among college students, those whose parents have attained a higher level of education are more likely to get immunized [ 22 ], and researchers have identified age as a predictor for receiving the influenza vaccine [ 23 ].
Political ideology is another well-documented determinant of vaccine-related attitudes and behaviors. Despite a common belief that liberals tend toward anti-vaccination attitudes in the United States, there is strong evidence that this trend is more present among conservatives [ 24 , 25 ]. According to a recent Gallup Poll, Republicans are twice as likely to believe the widely debunked myth that vaccines cause autism [ 26 ]. Recent work has shown that exposure to anti-vaccination tweets by President Trump—the first known U.S. president to publicly express anti-vaccination attitudes—has led to increased concern about vaccines among his supporters [ 27 ]. Based on these findings, and in conjunction with the sentiments expressed by the White House that diminished the significance of the pandemic [ 28 ], we expected to find diverging trends between Democrats and Republicans.
The current research
We collected vaccine-related attitudes of individuals living in the U.S. over a six-month period. Beginning in March 2020, we elicited attitudes from a cohort of the same individuals every month. We began data collection before any COVID-19 lockdown measures were in place (i.e., prior to the nation’s first shelter-in-place order [ 29 ]). Hence, our data spans the early phase of the pandemic, when there were fewer than 2,000 total confirmed cases in the U.S., through the following six months, at which point cumulative cases reached over 5.3 million [ 30 ].
Despite our prediction—that a public health crisis would increase disease threat, consequently increasing pro-vaccine attitudes and interest in vaccination—our data show an overall decrease in favorable attitudes toward vaccines. A closer look at the data revealed that political orientation explains more variance than any other socio-demographic variable. Specifically, participants who identify as Republican showed a decrease in their intention to get the COVID-19 vaccine and the influenza vaccine as well as a general decrease in pro-vaccine attitudes, whereas Democrats’ responses to these measures did not show a significant change during this period.
Our work is the first, to our knowledge, to longitudinally measure individuals’ attitudes toward vaccines. In doing so, our findings advance the understanding of how vaccine attitudes might change during an unprecedented public health crisis, revealing a strong association between political party affiliation and vaccine attitudes.
Participants
We recruited a panel of U.S. residents on Amazon’s Mechanical Turk platform to respond to multiple survey waves. To incentivize completion of all waves, we informed participants their payment would increase for subsequent surveys. Participants were paid 30 cents for wave 1, 40 cents for wave 2, and 60 cents for waves 3 and 4, $1.00 for wave 5, and $1.20 for wave 6. In addition, participants were informed that those who completed the first three waves would enter a $100 raffle. The median survey completion time was 5.5 minutes. The sample size for the first wave was 1,018, and the number of participants ranged from 608–762 on subsequent waves (see S1 Table for attrition details). This project was certified as exempt from IRB review by the University of California, San Diego Human Research Protections Program (Project #191273XX).
Our panel represents the broad and diverse population of the United States. The first wave sample included participants from all 50 states (except Wyoming) and Washington D.C., with an age range of 18 to 82 years old (mean = 38.48, median = 35). Approximately half (53%) identified as male, 46% as female, and.6% as other. The racial makeup in our sample was: 80% White, 9% Asian, 6% Black or African American, 4% multiple racial or ethnic identities, and 1% other. Relative to the U.S. Census (2019) [ 31 ] estimates, our sample over-represents White and Asian individuals, and under-represents Black or African American individuals and other racial groups.
We elicited political affiliation using a 6-point Likert scale, ranging from Strongly Republican to Strongly Democratic. In wave 1, 62% identified as Democrats and 38% identified as Republican, which is consistent with results from the most recent General Social Survey (GSS) [ 32 ]. There was no significant change in mean political identity from wave 1 to waves 2–6 (see S2 Table ). We classified participants as Democrats or Republicans using wave 1 political party affiliation. See S2 Appendix for additional details about the correlation of political party affiliation with age, gender, and SES.
Questions and measures
Our primary measure of interest was participants’ stated intention to get the COVID-19 vaccine when it becomes available. We were also interested in their perceptions of COVID-19 threat, general vaccination attitudes, and intention to get the flu shot. For all measures, except flu shot intentions, we combined multiple items to create a composite measure (see S2 Table for specific questions and construct compositions). Questions designed to measure general vaccination attitudes were adapted from prior work [ 33 ].
Additional measures of interest were participants’ trust in broad institutions (media, local government, and federal government). These trust measures followed different trends from each other, and therefore were not combined. At the end of the survey, participants responded to demographic questions. We retained all questions used in wave 1 throughout all six waves (our survey included additional items not reported in this paper; see S2 and S3 Tables for a complete list of measured items).
Data and analysis plan
Only participants with non-missing and non-duplicated responses were included in the analyses (see S1 Appendix for additional details). For all outcomes of interest, we tested for linear trends over time using a fixed effects regression specification [ 34 ]. All regression results include individual-level fixed effects, and standard errors are clustered at the individual level, to adjust for within-person correlation. We used this approach to control for the impact of omitted or unobserved time-invariant variables. P-values are not adjusted for multiple testing (see [ 35 ]). All analyses were conducted using R (version 4.0.2), and regressions were run using the package “fixest” (version 0.6.0). All materials, data, and additional analyses including robustness checks can be found here: https://osf.io/kgvdy/ .
We report results for three different vaccination-related measures: attitudes toward a COVID-19 vaccine, general vaccination attitudes, and flu shot intentions. All measures showed a decreasing trend (Ps < .001, except flu shot intentions where p = .05) for the 6-month duration of the study, indicating a reduction in pro-vaccination attitudes and intention to get vaccinated (COVID-19 and influenza vaccines). See S4 Table for full results of all regressions.
Heterogeneity in trend by political party
To better understand whether the decline in vaccine attitudes over time was driven by a particular factor, we used a data-driven approach, regressing all demographic characteristics on vaccine attitudes, in separate regressions. These demographics included education, income, SES, race, gender, an item measuring whether participants considered themselves to be a minority, whether the participant has children, and political party. Education, income, and SES were median split; race and gender were dummy coded; and political party affiliation was dichotomized into Democrat or Republican. Among all demographic characteristics, separating time trends by political affiliation (by adding an interaction term) attained the greatest adjusted within-R 2 in explaining vaccination attitude measures. In other words, political party affiliation explains the greatest within-individual variation in vaccine attitudes over time.
An analysis of responses by political affiliation revealed that the observed decreasing trend in all three vaccine measures was mostly driven by participants who identified as Republican (all Ps < .05), whereas Democrats’ responses showed either no significant trend (for COVID-19 vaccination and flu shot intentions: Ps >.67) or a significantly less negative time trend (general vaccination: p < .001). For these regressions, and moving forward, all results included interactions between wave and political party as well as interactions for wave and age, and wave and SES, to control for potentially different time trends associated with these variables. In each regression we also tested whether the strength of political affiliation moderates the observed results, and we reported the result when it did. We also conducted ANOVAs to compare mean responses for the outcomes of interest between Democrats and Republicans, separately for each wave (see S5 Table ).
COVID-19 vaccination attitudes ( Fig 1 , Panel A).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
Points represent means, and error bars represent 95% confidence intervals. All scale responses range from 1 to 7.
https://doi.org/10.1371/journal.pone.0250123.g001
A two-item construct ( r = .78) was created, with greater values corresponding to more favorable responses.
In wave 1, Democrats ( M = 5.39, SD = 1.55) had more favorable attitudes toward a COVID-19 vaccine than Republicans ( M = 4.57, SD = 1.76; t = -7.38, p < .001, d = -.48, 95% CI = [-.61, -.35]). Among Democrats, there was no significant time trend ( β = .02, SE = .04, p >.67) whereas Republicans’ responses followed a decreasing time trend ( β = -.09, SE = .05, p = .046). These trends were significantly different from each other ( β = -.11, SE = .02, p < .001).
General vaccination attitudes ( Fig 1 , Panel B).
A ten-item construct ( α = .95) was created, with greater values corresponding to a more positive attitude toward vaccination in general.
In wave 1, Democrats ( M = 5.83, SD = 1.15) expressed more favorable general vaccination attitudes than Republicans ( M = 5.17, SD = 1.31; t = -7.91, p < .001, d = -.52, 95% CI = [-.66, -.39]). Although both Democrats and Republicans had a decreasing time trend (Democrats: β = -.04, SE = .02, p = .029; Republicans: β = -.09, SE = .02, p < .001), the trend for Republicans was significantly more negative ( β = -.04, SE = .01, p < .001).
Flu shot intentions ( Fig 1 , Panel C).
We asked participants whether they plan to get the flu shot next year, with greater values indicating greater intentions.
In wave 1, Democrats ( M = 4.84, SD = 2.34) indicated greater intentions to vaccinate against the flu than Republicans ( M = 4.35, SD = 2.39; t = -3.15, p = .002, d = -.21, 95% CI = [-.34, -.08]). Among Democrats, there was no significant time trend ( β = .01, SE = .04, p = .86), suggesting their vaccination intentions remained largely stable. Republicans’ responses, however, revealed a decreasing time trend ( β = -.12, SE = .04, p = .005), and the two trends were significantly different from each other ( β = -.12, SE = .02, p < .001).
Our analyses revealed an interaction with political affiliation strength among Republicans, whereby participants who identified as more strongly Republican had a more negative time trend ( β = -.05, SE = .02, p = .027). This interaction was not significant for Democrats ( β = -.02, SE = .01, p = .19).
Perceived threat of COVID-19 ( Fig 2 ).
https://doi.org/10.1371/journal.pone.0250123.g002
A three-item construct ( α = .82) was created, with greater perceived threat about COVID-19.
In wave 1, Democrats ( M = 4.26, SD = 1.25) expressed greater perceived threat of COVID-19 than Republicans ( M = 3.90, SD = 1.39; t = -4.14, p < .001, d = -.40, 95% CI = [-.27, -.14]). Democrats’ responses showed an increasing time trend ( β = .08, SE = .04, p = .033), indicating they became increasingly concerned about the threat posed by the virus over time. Among Republicans, there was no significant time trend ( β = -.01, SE = .04, p = .83). These trends were significantly different from each other ( β = -.09, SE = .02, p < .001). While our data does not render causal claims, it is possible that the divergence in COVID-19 threat perceptions over time among Republicans and Democrats contributes to the divergence in vaccine attitudes between these groups over time. We revisit this proposition in the General Discussion.
Our analyses revealed an interaction with political affiliation strength among Democrats—participants who identified as more strongly Democrat had a more positive time trend ( β = .03, SE = .01, p = .019), suggesting an increasing threat perception over time. This interaction was not significant for Republicans ( β = .01, SE = .02, p = .61).
Trust in broad institutions.
The measures of trust in media, local government, and federal government were not highly correlated ( α = .66), and were therefore analyzed separately.
Trust in media ( Fig 3 , Panel A) . In wave 1, Democrats ( M = 3.61, SD = 1.66) reported greater trust in the media than Republicans ( M = 2.73, SD = 1.65; t = -8.12, p < .001, d = -.53, 95% CI = [-.66, -.39]). There was no significant time trend for either Democrats ( β = .02, SE = .04, p = .57) or Republicans ( β = -.05, SE = .04, p = .20). However, the trend for Republicans was significantly more negative ( β = -.07, SE = .02, p < .001). The different trends we observe for Democrats and Republicans with respect to trust in the media may explain the divergence in perceived threat and vaccine attitudes between these groups over time (see General discussion ).
https://doi.org/10.1371/journal.pone.0250123.g003
Trust in local government ( Fig 3 , Panel B) . In wave 1, Democrats ( M = 4.07, SD = 1.60) indicated lower trust in local government than Republicans ( M = 4.28, SD = 1.60; t = 2.01, p = .045, d = .13, 95% CI = [.003,.26]). Among Democrats, there was no significant time trend ( β = -.06, SE = .04, p = .18), though among Republicans, there was a decreasing time trend ( β = -.11, SE = .05, p = .015). These trends were significantly different from each other ( β = -.06, SE = .02, p = .004).
Trust in federal government ( Fig 3 , Panel C) . In wave 1, Democrats ( M = 2.96, SD = 1.67) expressed lower trust in the federal government than Republicans ( M = 4.08, SD = 1.60; t = 10.52, p < .001, d = .68, 95% CI = [.55,.82]). Both Democrats and Republicans had decreasing time trends (Democrats: β = -.08, SE = .04, p = .036; Republicans: β = -.10, SE = .04, p = .025). These trends were not significantly different from each other ( β = -.02, SE = .02, p = .37).
To rule out differential attrition, we tested whether the composition of our sample (i.e., age, gender, and political party) changed over time (see S1 Table ). Specifically, we tested whether participants who responded to waves 2–6 were significantly different at baseline (wave 1) from the full sample at baseline. The only significant change detected (Ps < .05) was with respect to participants’ age, though the differences were small—the average age was 38.5 at baseline, and remained between 39.9 and 40.8 at baseline among participants who responded to subsequent waves. We found no other systematic pattern of attrition among our participants.
General discussion
Over the course of six months of the COVID-19 pandemic, beginning with a relatively early phase prior to any U.S. directives to stay home (March 2020) and continuing through a cumulation of over 5 million cases (August 2020), we found a decrease in pro-vaccine attitudes and COVID-19 vaccination intentions, as well as reduced intentions to get the influenza vaccine. These findings are contrary to our prediction that increased salience of COVID-19 would improve attitudes toward vaccines.
Our analyses identify political ideology as the best predictor of the decreasing time trend across our three vaccine-related attitudes and intentions measures. In particular, we found that while Democrats’ responses remained fairly stable over time, Republicans shifted away from their lower initial responses and from Democrats’ responses, leading to increased polarization throughout the six-month period.
Contrary to the polarization observed in our data, social and behavioral scientists have long argued that groups facing threats often come together, demonstrating stronger social cohesion [ 36 ], and more cooperative behaviors [ 37 , 38 ]. Researchers have also found that individuals’ sense of shared identity plays a role in promoting cooperative behavior in response to threat [ 39 – 41 ]. Considering our results in the context of these findings might suggest that our respondents’ sense of shared identity was dominated by their political ideology, as opposed to a broader (e.g. American) identity.
What might be going on?
Although the nature of our data does not render causal claims, it highlights potential explanations. First, we note that participants’ ratings of perceived COVID-19 threat followed a similar diverging pattern by party affiliation to our three vaccine-related measures during the study period. Democrats perceived COVID-19 threat to be greater at the start of the study than Republicans did, and this gap widened significantly as the study progressed. This trend is consistent with previous research showing that vaccine hesitancy is related to perceived risk of a threat; when a VPD threat level is low, individuals are less motivated to take preventative action (i.e., immunize; for a review, see [ 42 ]).
Our data offers one potential explanation for the polarization of threat perception: Republican and Democratic participants in our study reported consuming different sources of information. The most commonly checked news source for Republicans was Fox News (Republicans: 50%, Democrats: 8%; χ 2 = 164.55, p < .001) and for Democrats was CNN (Democrats: 47%, Republicans: 23%, χ 2 = 43.08, p < .001, see S6 Table ). Corroborating this proposition, a Pew Research Center poll conducted in March 2020 found that 56% of respondents whose main news source is Fox News believed that “the news media have greatly exaggerated the risks about the Coronavirus outbreak,” whereas this was only true for 25% of those whose main news source is CNN [ 43 ]. Of note, Facebook and Instagram, were also in the top four most consumed news sources for participants affiliated with either party. Extant work describes these platforms as echo chambers [ 44 , 45 ], which may exacerbate partisan exposure to news and information.
Another trend highlighted by our data shows that similar to vaccine attitudes, Republicans’ trust in the media decreased significantly more during our study than Democrats’, suggesting these patterns might be related. According to Dr. Heidi Larson, an expert on vaccine hesitancy and founder of the Vaccine Confidence Project, misinformation regarding vaccinations is more likely to take root when individuals do not trust the information source [ 46 ]. Future research might further examine the role of trust in the media on vaccine attitudes.
While trust in media or media exposure may be driving COVID-19 threat perceptions and vaccine attitudes, there are many other possible explanatory factors that are not captured by our data or analyses. For example, it is possible that threat perceptions were influenced by how a respondents’ county or state was affected by COVID-19; up until June 2020, COVID-19 cases were more common in Democrat-leaning states [ 47 ], which might have amplified its salience early on and influenced attitudes and behavior. Further, although we included individual-level fixed-effects which control for all time invariant participant characteristics, and controlled for different trends by age and SES, we cannot rule out the possibility that other factors (e.g., educational attainment or population density) may have influenced the observed trends. Finally, as our data collection began after the onset of COVID-19, it is possible that the trend we observe for Republicans represents a return to a pre-pandemic baseline of vaccine-related attitudes.
Contributions
This work advances our understanding of how health-related attitudes evolve over time. Our focus on vaccine-related attitudes and intentions is important because experts agree that having enough people vaccinate against COVID-19 is key to stemming the pandemic [ 48 ]. More broadly, negative attitudes toward vaccination in general, and reduced vaccine uptake, is increasingly a public health concern [ 49 ]. This research provides insight into the trends of such vaccine hesitancy, underlining the importance of risk salience and its roots in ideology and media exposure.
This work also contributes to our understanding of political parties and polarization. Numerous anecdotes and reports have demonstrated a partisan divide in Americans’ response to the COVID-19 pandemic. For example, research found greater negative affective responses to wearing a face covering among politically right (vs. left) leaning individuals [ 50 ]. Here, we show that although these observations are valid, the reality is more nuanced. For example, our analyses reveal that polarization on vaccine measures—both attitudes and intentions—is driven primarily by self-identified Republicans’ gradual movement away from their initial responses whereas Democrats’ responses remained largely stable. This insight has important practical implications: It informs us about the dynamics of individuals’ attitudes, bringing us closer to understanding the underlying factors that influence attitudes and behaviors. Equipped with this knowledge, one could design more effective communications and interventions.
Note on methodology and data availability
The present study contributes to a small but growing literature in the social sciences using longitudinal data [ 51 ]. Using a longitudinal methodology allowed us to track individual-level changes over time. Merely observing a single point in time would allow us to observe across-group differences, but would lack the bigger picture of how polarization between these groups evolved. Another key advantage of panel data is that it allows us to include individual-level fixed effects, which control for the impact of omitted or unobserved time-invariant variables. Finally, panel data allows for more accurate inference of model parameters [ 52 ].
While the focus of this paper is vaccine attitudes, our broad dataset offers a unique opportunity to understand attitudes and behavior over time. Due to the richness of our data, its unique nature, and its timeliness, we believe it is important to make it available to other researchers interested in exploring it and publishing additional findings. The complete dataset is available at https://osf.io/kgvdy/ (see S2 and S3 Tables for all items collected).
Supporting information
S1 appendix. additional information about sample exclusions..
https://doi.org/10.1371/journal.pone.0250123.s001
S2 Appendix. Additional information about political party affiliation.
https://doi.org/10.1371/journal.pone.0250123.s002
S1 Table. Attrition table.
https://doi.org/10.1371/journal.pone.0250123.s003
S2 Table. Summary table of measures and constructs included in the text.
https://doi.org/10.1371/journal.pone.0250123.s004
S3 Table. Summary table of measures excluded from the text.
https://doi.org/10.1371/journal.pone.0250123.s005
S4 Table. Regression results.
https://doi.org/10.1371/journal.pone.0250123.s006
S5 Table. Outcome measures by political party affiliation.
https://doi.org/10.1371/journal.pone.0250123.s007
S6 Table. Summary of news sources.
https://doi.org/10.1371/journal.pone.0250123.s008
- View Article
- Google Scholar
- 2. World Health Organization. The Power of Vaccines: Still not fully utilized. WHO; 2020. https://www.who.int/publications/10-year-review/vaccines/en/ .
- 4. Immunization Safety Review Committee. Immunization safety review: vaccines and autism. National Academies Press; 2004 Sep 30.
- PubMed/NCBI
- 25. Luton R, Hare C. Conservatives are more likely to believe that vaccines cause autism. The Washington Post. 2015 March 1. https://www.washingtonpost.com/news/monkey-cage/wp/2015/03/01/conservatives-are-more-likely-to-believe-that-vaccines-cause-autism/ .
- 26. Reinhart R. Fewer in US continue to see vaccines as important. 2020 Jan 24. https://news.gallup.com/poll/276929/fewer-continue-vaccines-important.aspx .
- 28. Summers, Juana Timeline: How Trump Has Downplayed The Coronavirus Pandemic. NPR. 2020 October 2, 2020. https://www.npr.org/sections/latest-updates-trump-covid-19-results/2020/10/02/919432383/how-trump-has-downplayed-the-coronavirus-pandemic
- 29. Ortiz J, Hauck G. Coronavirus in the US. USA Today. 2020 March 30, 2020. https://www.usatoday.com/story/news/nation/2020/03/30/coronavirus-stay-home-shelter-in-place-orders-by-state/5092413002/ .
- 30. Elflein J. State of Health. World Health Organization. 2020 October 5. https://www.statista.com/statistics/1103185/cumulative-coronavirus-covid19-cases-number-us-by-day/ .
- 31. United States Census Bureau. Quick Facts. 2019. https://www.census.gov/quickfacts/fact/table/US/PST045219 .
- 32. Smith TW, Davern M, Freese J, Morgan S. General Social Surveys, National Science Foundation. 2020. gssdataexplorer.norc.org
- 34. Wooldridge JM. Econometric analysis of cross section and panel data. MIT press; 2010 Oct 1.
- 46. Anderson J. She Hunts Viral Rumors about Real Viruses. The New York Times. 2020 October 13. https://www.nytimes.com/2020/10/13/health/coronavirus-vaccine-hesitancy-larson.html .
- 47. Bump P. Coronavirus has come to Trump country. The Washington Post. 2020 June 17. https://www.washingtonpost.com/politics/2020/06/17/coronavirus-has-come-trump-country/ .
- 48. Quinn M. Fauci warns U.S. "unlikely" to reach Herd Immunity if too Many Refuse Vaccine. CBS News. 2020 June 29. https://www.cbsnews.com/news/fauci-herd-immunity-coronavirus-vaccine/ .
- 49. World Health Organization. Improving vaccination demand and addressing hesitancy. WHO; 2019. [cited 2020 November 3]. https://www.who.int/immunization/programmes_systems/vaccine_hesitancy/en/ .
Thesis Helpers
Find the best tips and advice to improve your writing. Or, have a top expert write your paper.
170 Vaccination Research Paper Topics For Stellar Students

Research papers are a monumental highlight in your academic journey. They are a critical milestone in your studies that must be tackled with the utmost care and stellar diligence. Vaccination topics are susceptible as you have to show complete mastery of all details.
If you are pursuing a medicine course, then vaccination research topics might be an excellent area of interest. A good research paper starts with a great topic, and we are here to help you nail that. We understand the significance of research papers, and that is why we have handpicked 170 out-of-the-box vaccination research paper topics, titles, and ideas to make your work seamless.
Debate Topics About Vaccination
- What is reverse vaccinology?
- Look at the ways of harnessing the participation of dendritic cells in tolerance and immunity
- What are some of the approaches to advance cancer vaccines to clinical utility?
- Highlight innovative therapeutic and vaccine approaches against respiratory pathogens
- Examine immunity to malaria and vaccine strategies
- Assess molecular vaccines against pathogens in the post-genomic era
- Comprehending the limitations of today’s influenza vaccine strategies and further development of more efficient therapeutic and preventative interventions
- Study HIV-associated persistent inflammation and immune activation
- Analyze recent advances in respiratory virus infection
- What is the novel approach for anti-tumor vaccines
- Unravel the challenges and progress in the development of a B cell-based hepatitis C virus vaccine
- What is the functional relevance of Tatraspanins in the immune system?
- Look at advanced immunization technologies for next-generation vaccines
- Evaluate epitope discovery and synthetic vaccine design
- In what ways can tuberculosis be treated by targeting host immunity
- What are the immunomodulatory effects of drugs in the treatment of immune-related diseases
- Highlight natural antibodies in health and disease
- Discuss different influenza virus vaccines and immunotherapy
- What are some of the shadows of cancer immunotherapy
- Understanding the therapeutical potential of extracellular vesicles
- A review of the ethical theories and problems associated with vaccination in America
- Do vaccines love the Darwinian fitness of immune cells
Vaccination Behavior Research Topics
- Unraveling demand and supply effects on the up-take of influenza vaccinations
- Point out new approaches to the seasonal flu vaccine
- Exploring the impact of vaccination
- Investigating patient experience with, and the use of, an electronic monitoring system to assess vaccination responses
- A meta-analysis of interventions that enhance the use of adult immunization and cancer screening services
- Do vaccines seem to work against bacterial and viral infections, and are they effective?
- Gathering the evidence for the introduction of typhoid vaccine: worldwide vaccine testing
- Explore molecular mimicry to broaden the immune response to carbohydrate antigens for vaccine development
- Tumor-associated glycan and immune surveillance
- Rational design and application of idiotope vaccines
- Assessing the effects of vaccines on immune-deficient people
- What are the impacts of rapid growth and deployment of high-volume vaccines for pandemic response
Anti-vaccination Research Paper Topics
- Should the state impose vaccinations, or should the choice be left up to the child’s parents?
- What is the connection between vaccination and autism?
- Is natural immunity better than immunity through immunization?
- Examining cultural perspectives on vaccination
- Are they worth it? adverse effects of vaccination on children
- To vaccinate or not against HPV? A content analysis of vocabularies of motives
- Vaccines: religious and cultural arguments from an Islamic perspective
- Anti-science populism or biomedicine’s unresolved knots? Comparing views on the movements against mandatory pediatric vaccines
- An anthropological commentary on vaccine hesitancy, decision-making, and interventionism among religious minorities
- Understanding attitudes to vaccination
Research Topics For Covid-19 Vaccination
- Medical mistrust in the context of Covid-19: implications for intended care-seeking and quarantine policy support in the United States
- What is the acceptability of the potential COVID-19 vaccine among smokers and non-smokers?
- COVID-19 vaccine hesitancy in healthcare personnel: are there any differences among classifications
- Discuss various options that one can use to convince people to get the covid-19 vaccine
- Examining COVID-19 vaccine efficacy after the first dose: Pfizer, Moderna, AstraZeneca
- Discuss the impacts of herd immunity during the covid-19 pandemic
- What are some of the effects of covid-19 vaccination on transmission of disease?
- Discuss whether antibodies generated through vaccination recognize all-new variants of covid-19
- Investigate how the intensity of lockdowns accelerate or influence mutation of the COVID virus
- Examine how the new covid-19 strain identified in England will affect the available vaccines.
- Outline which immunoglobulin types can be used as the markers for covid-19 vaccination
- Which is the best way to deal with swaps after completing vaccinations in nursing homes
- How do we curb vaccine hesitancy among healthcare providers?
- Which one is the more dangerous, covid-19 or covid-19 vaccine? What must be the individual decision?
- Analyzing Ebola and the evolving ethics of quarantine
- Break down some of the side effects of covid-19 vaccination
- How long will immunity last after receiving the covid-19 vaccination?
- Will, a covid-19 vaccine work for everyone? Are there people who cannot get vaccinated?
- Is bivalent OPV immunization capable of mitigating the impact of covid-19?
- What are the expected long-term side effects of the vaccination for covid-19?
- Evaluate differences between the first and second doses of the covid-19 mRNA vaccine?
- Examine the ingredients in the covid-19 mRNA vaccine
- Can a person’s DNA change through mRNA vaccines?
- Factors that stops the body from continuing to produce COVID-19 spike protein after getting a COVID-19 mRNA
- Discuss whether a person vaccinated against covid-19 will be able to spread the virus to susceptible people
- Investigating vaccination adverse outcomes and costs of vaccine injury claims(VICs): In the past, present, and during COVID-19.
- Who gets cured: Covid-19 and the development of critical sociology and anthropology of cure
- Development of perception and attitude scales related to COVID-19 pandemic
- Does the mutation of the coronavirus affect the capacity of the vaccines to prevent disease?
- A case-control study: finding a link between pre-existing antibodies got after the childhood vaccinations or past infections and COVID-19?
- Queue questions: ethics of COVID-19 vaccine prioritization
- Disparities between Black and White in H1N1 vaccination among adults in the U.S. in 2009: A cautionary tale for the COVID-19 pandemic
- Autonomy and refusal in pandemics: What to do with those who refuse COVID-19 vaccines
- Knowledge, attitude, and acceptance of a COVID-19 vaccine: a global cross-sectional study
- Prospects of COVID-19 vaccine implementation in the U.S.: Challenges and potential solutions
- What are the effects of COVID-19 vaccines on pregnant women?
- Compare and contrast the efficacy of different covid-19 vaccines.
- Ways to improve covid-19 vaccine acceptance
- Determination of causation between COVID-19 vaccines and potential adverse effects
Vaccination Of Children Topics
- What is the essence of increasing HPV vaccination among children?
- Analyze the primary diseases that vaccines prevent in children
- What will happen if a child’s vaccination schedule is delayed
- Look at the vaccination schedule for children in the U.S.
- Can children receive more than one vaccine at a time?
- Examine revaccination outcomes of children with proximate vaccine seizures
- What are the impacts of measles-containing vaccination in children with the severe underlying neurologic disease?
- Evaluate the challenges involved in measuring immunization activity coverage among measles zero-dose children
- What is the connection between the polio vaccine and the risk of cancer among children?
- Do multiple vaccines affect babies’ health and immune system in an adverse war, or can their bodies handle them?
- What are the various vaccination options available for children, and are they harmful to children’s overall health?
- The case for further research and development: assessing the potential cost-effectiveness of microneedle patches in childhood measles vaccination programs
- Evaluate the accuracy of parental recall of child immunization in an inner-city population
- Evaluating maternal acculturation and childhood immunization levels among children in African-American families in Florida
- Policy analysis: the impact of the vaccine for children’s program on child immunization delivery
- The effect of managed care: investigating access of infant immunizations for poor inner-city families
- Who takes up free flu shots? Investigating the effects of an expansion in coverage
- What are the societal and parental values for the risks and benefits of childhood combination vaccines?
- Looking into trends in vaccination intentions and risk perceptions: a longitudinal study of the first year of the H1N1 pandemic
Healthcare Topics About Vaccination
- Conscious consideration of herd immunity in influenza vaccination decisions
- A case study of ethnic or racial differences in Medicare experiences and immunization
- What preservatives are used in vaccines
- Discuss the relationship between vaccines and autism
- What is the role of epidemiology in infection control?
- How t design and select the most relevant immunogenic peptide sequences
- Discuss why the Zika virus has not had a significant impact in Africa as compared to America
- What are the advantages of using the phage display technology of antibodies versus hybridism technology?
- Analyzing the impact and cost-effectiveness of vaccination programs in a country using mathematical models
- Malaria vaccines: progress and problems
- Malaria: cloning genes for antigens of plasmodium falciparum
- Fighting profits on the pandemic: The fight for vaccines in today’s economic and geopolitical context
- Molecular and biotechnological approaches to fish vaccines
- Immunogenicity of a whole-cell pertussis vaccine with low lipopolysaccharide content in infants
- Immunogrid: an integrative environment for large-scale simulation of the immune system for vaccine discovery, design, and optimization
Thesis Topics In Vaccination
- Investigating challenges and opportunities in vaccine delivery, discovery, and development
- Discuss classic methods of vaccine development
- What are some of the current problems in vaccinology?
- Assess some of the latest tools for vaccine development
- Using cost-effectiveness analysis to support research and development portfolio prioritization for product innovations in measles vaccination
- Communicating vaccine safety during the introduction and development of vaccines
- Highlighting viral vectors for use in the development of biodefense vaccines
- What is the role of US. military research programs in the invention of USA-approved vaccines for naturally occurring infectious diseases
- Curbing outbreaks: utilizing international governmental risk pools to fund research and development of infectious disease medicines and vaccines
- Vaccine stabilization: research commercialization and likely impacts
- Exam the unequal interactions of the role of patient-centered care in the inequitable diffusion of medical innovation, the human papillomavirus(HPV) vaccine
- A case study of the status of development of vaccines and vaccine research for malaria
- Enteric infections vs vaccines: a public health and clinical research agenda for developing countries
- A review of research and vaccine development for industry animals in third world countries
- How the research-based industry approaches vaccine development and establishes priorities
- A look at the status of vaccine research and development of a vaccine for HIV-1
- Modeling a cost-effective vaccination strategy for the prevention of herpes zoster infection
- Using an adequate T.B. vaccination regiment to identify immune responses associated with protection in the murine model
- A systematic analysis of the link between vaccines and atopic dermatitis
- Do vaccines provide better immunity than natural infections?
- Is there a need to be vaccinated against a disease that is not available in your country or community
- How to strengthen adult immunization via coordinated action
- Using the general equilibrium method to assess the value of a malaria vaccine: An application to African countries
- Who should take up free flu shots?
- Evaluate the impact of vaccination among health care personnel
- Retail clinics and their impact on vaccination in the U.S.
- Discuss the societal values for the benefits and risks of childhood combination vaccines
- How safe and effective is the synovial vaccine for people above 60 years
- Evaluating vaccination effectiveness of group-specific fractional-dose strategies
Law Research Topics On Vaccination
- Explain why there are age restrictions for Rotavirus vaccination?
- Vaccination or hygiene : Which factor contributes to the decline of infectious diseases?
- Outline the main factors that cause vaccine failure
- Discuss why HIV is so hard to vaccinate in uninfected people?
- In what ways do maternal vaccinations affect the fetal nervous system development
- How to deliver malaria vaccine effectively and efficiently
- Highlight the vaccines that are specifically licensed in the U.S. for pregnant women
- How does an immune genetic algorithm work?
- Evaluate the relationship between the success of artificial insemination and vaccination
- Outline the reasons why vaccines underperform in low-income countries
- Discuss U.S. immigration and vaccination policy
- Assessing the effectiveness of compelled vaccination
Vaccination Ethical Topics
- What are the requirements for a strain to be used as a vaccine?
- What is the best way to administer vaccines in children?
- Assessing the benefits of maternal vaccination on breastfed infants
- Evaluating the pros and cons of intraperitoneal vaccination
- Examine ways to measure the pattern of vaccination acceptance
- Investigate Covid-19 transmission, vaccination rate, and the fate of resistant strains
- Look into dark web marketplaces and covid-19 vaccines.
- A close look at covid-19 vaccines and kidney diseases
- Contextualizing the impact of covid-19 vaccine misinformation on vaccination intent in the U.S.
- Examining behaviors and attitudes of medical students towards covid-19 vaccines
From the exceptional vaccination debate topics, titles, and other ideas above, you can quickly tell that we have your best interest at heart. We believe that all students deserve decent scores on their papers. As a result, we are more than willing to recommend affordable online help with research papers from the best-rated sites.
Whether you are overwhelmed by other assignments, dealing with a tight deadline, or lack the spark to pull it off, you can count on cheap yet professional thesis help from trusted assignment help sites. They are ready to deliver high-quality papers, and you can confirm that from any sample or example on the platforms.

Make PhD experience your own
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Wiley - PMC COVID-19 Collection

COVID‐19 vaccine research and development: ethical issues
1 Department of Microbiology, Faculty of Medicine Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta Indonesia
2 Medical and Health Research Ethics Committee, Faculty of Medicine Public Health and Nursing, Universitas Gadjah Mada / Dr. Sardjito General Hospital, Yogyakarta Indonesia
The achievements of vaccine research and development bring a hope to our societies that we may cope with the COVID‐19 pandemic. There are two aspects that should be maintained in balance: the immediate necessity for speed of vaccine research and the inherent need for protection of research subjects, which is the foremost concern of research ethics. This narrative review highlights ethical issues in COVID‐19 vaccine research and development that every stakeholder needs to be aware of and to consider.
Introduction
COVID‐19 is a deadly disease which continues to affect many countries in the world. The incidence is higher in the Americas (14 117 714 cases and 486 843 deaths) and Europe (4 515 514 cases and 222 624 deaths) than in South East Asia (4 786 594 cases and 84 541 deaths), Africa (1 088 093 cases and 23 101 deaths) and the Western Pacific (520 012 cases and 11 306 deaths) [ 1 ].
Vaccines are the most important public health measure to protect people from COVID‐19 worldwide, since SARS‐CoV‐2 is highly contagious and infects populations widely and globally [ 2 ]. Traditionally, vaccine development takes years, even decades: from about 40 years for polio to 5 years for Ebola,most vaccines took 15 years on average [ 3 , 4 ]. The trial process for vaccines consists of several steps which need to be conducted systematically and in a measurable stride. The length of this process is correlated with the nature of the vaccine itself, which is to protect healthy people from being infected by pathogens. Adverse events and deleterious effects will not be tolerated, vaccines are not the same as drugs that are consumed by the sick. The risk–benefit analysis for prescription drugs and vaccine administration is different.
The invention of a successful and widely available COVID‐19 vaccine will be a great leap forward for humankind, but there are several challenges to overcome: (1) a lack of understanding of the pathogenesis and the predictive role of vaccines in the clinical pathway of persons being infected by SARS‐CoV‐2 [ 5 , 6 , 7 ], (2) a huge disagreement among experts about how to determine the most immunogenic epitopes and antigens of SARS‐CoV‐2 [ 8 , 9 ], (3) the finding that antibody‐dependent enhancement (ADE) may contribute to the exaggeration of SARS‐CoV‐2 disease [ 10 , 11 ], (4) the lack of established animal models for COVID‐19 vaccine challenge testing, which raises the speculation of using controlled human infection (CHI) as a potential approach [ 3 ], and finally, (5) speculation that the duration of protection by immune response in natural infection is not long enough [ 12 ].
The race for COVID‐19 vaccine invention and development against the spread and catastrophic effects of the disease is real. WHO released a draft list of COVID‐19 candidate vaccines on 3 September 2020. At least 34 vaccine candidates are in clinical evaluation to date [ 13 ]. Several new technologies are used as COVID‐19 vaccine development platforms. Conventional techniques for the development of vaccines such as inactivated, inactivated with adjuvant and live attenuated are still being used. However, reversed vaccinology approaches are also being emplyed, such as a recombinant subunit vaccine, and a more advanced approach using vector delivery systems, along with RNA‐ and DNA‐based vaccines (Table 1 ) [ 4 , 9 , 13 ].
Candidate COVID‐19 Vaccines in Clinical Trial Phases*
The attempts to accelerate vaccine development are associated with efforts to streamline the process. Unfortunately, streamlining may have consequences for the traditional ethics of vaccine research and development, especially the long‐held principles of beneficence and non‐maleficence. This short narrative review summarises the ethical issues that may emerge from the current directions in COVID‐19 vaccine research and development during the pandemic.
Vaccine candidates must fulfil several requirements: safety, efficacy and quality. Because of the current escalation of the global COVID‐19 pandemic, some aspects may change. The speed of vaccine development may push public health ministers, heads of states and the pharmaceutical industry to change their strategy for bulk budget investment for vaccine research. They must decide to prepare mass production events based on the limited data of promising vaccine candidates [ 14 ]. The need to protect billions of earth’s inhabitants pushes governments and societies of the world to a ‘great expectation’ for the new vaccine. The overriding expectation, although with diverse interests, may influence the objective judgement typically required of candidate vaccine safety. Protecting human lives should be the priority.
mRNA‐ [ 15 ] and DNA‐based vaccine technologies [ 9 , 16 ] are being implemented in humans, especially as vaccine candidates. Several concerns about mRNA vaccine safety have been identified besides its promising potential advantages. The most important risks include the possibility that mRNA vaccines may generate strong type I interferon responses that could lead to inflammation and autoimmune conditions [ 17 ]. The safety concerns of DNA‐based vaccines involve the possibility that the targeting of DNA into the chromosomal DNA of the acceptor will trigger mutagenic effects in the functional gene located in the insertion loci [ 18 ]. At present, there are no mRNA‐ and DNA‐based vaccines against any disease authorised to be marketed.
The strategy of DNA vaccines is similar to gene therapy in that a delivery system, such as plasmid, delivers targeted DNA into cells, where it is translated into proteins that induce the acceptors’ immune response to generate targeted T‐cell and antibody responses [ 19 ]. We have experience in using DNA for several gene therapies mostly related to inherited diseases or familial predispositions. Mainstream gene therapy scientists have stated that gene therapy is only suitable for terminally ill patients because the risks are very high [ 20 ]. Vaccine administration is completely different from interventions with gene therapy since the vaccine is for healthy human subjects, and the risk–benefit consideration would be completely different too. Both terminally ill and healthy persons have the same risk for the introduction of foreign DNA into their body, but terminally ill persons may benefit through having a chance to recover from their deadly disease, whereas healthy individuals may not have any benefit because they have never encountered the particular pathogen.
When we perform the risk assessment of new technology, it is based on a theoretical framework without direct evidence concerning to what extent the probability of the risk may occur. Theoretically, DNA vaccine may be able to induce autoimmune diseases and can be inserted into any part of the chromosomes [ 21 ]. Scientists know how the mechanism works and are able to predict the risk if it might happen. But nobody knows for certain how great the probability is of producing mutagenic and deleterious effects in one part of a gene sequence when inserted into another. For example, when a test subject named Jessie Gelsinger was injected with adeno‐associated viruses (AAVs), nobody expected the deadly risk that ultimately occurred in this research subject [ 22 ]. Accordingly, the risk–benefit assessment in the use of new technology should be done carefully. It is true that sometimes we have to deal with a risk possibility that is not immediately present but theoretically possible, and vice versa. Mitigation to the deleterious effect could be started prior to the clinical trial. However, there is always the possible existence of risks that have not been identified yet and will only show in the later phases of clinical trials.
In the current pandemic, all societies expect a breakthrough in medical and health technology. In a situation where understanding of the new disease is poor and no satisfactory medical technology is available for prevention and treatment yet, it is natural to think that ‘doing something is better than nothing’. This is going to make safety judgement among stakeholders more prone to deterioration.
Controlled human infection (CHI)
One of the crucial steps of vaccine development is the challenge test, which is used to measure the potential protection of the candidate. The challenge test is usually part of the pre‐clinical study in an animal model. However, in the case of COVID‐19 and some other diseases, an animal model is not available, although there are candidates that need to be verified [ 3 , 23 , 24 , 25 ]. It seems the pathogen does not produce a similar clinical course in common animal models, which excludes safety and efficacy data from animal models alone. There was a proposal of human challenge testing to replace the pre‐clinical challenge test in animal models, with the use of controlled human infection (CHI). It will solve the problem of the animal models’ unreliability and gain time for the developers especially in phase III [ 3 , 26 ].
To some extent, it is possible to perform these challenge tests with human volunteers. It sounds like an unsafe experimentation, but the choices are extremely limited. The next question is how can we do this experiment with the current ethical review process? The WHO has issued a guideline for CHI [ 27 ]. The guideline is broad and needs local ethics committee approval for its implementation. Considerations of the pros and cons of CHI are widely discussed in COVID‐19 vaccine development. Previously, CHI was used to develop vaccines against malaria [ 28 ], typhoid [ 29 ] and cholera [ 30 ], which are diseases with established treatment [ 31 ]. Subjects who suffered from deleterious effects after experimentation could be rescued by the established treatment. Application of CHI in COVID‐19 is a very different story because there is no standard treatment for this new and highly contagious disease. Nevertheless, there have been thousands of volunteers from 162 countries who declared their willingness to be participants in this CHI [ 32 ]. The need for a vaccine is prevalent in people’s minds and equally necessary from the public health point of view. Without any precedents, it is going to be difficult to judge the risks benefits in this matter [ 33 ].
Controlled human infection could be done in a situation where there is an attenuated virus strain available, for example, using an artificial mutant virus. This approach is to prevent fatal outcomes in trial subjects. But the challenge test results from attenuated virus may not be generalisable – the attenuated strain may not be similar enough to the naturally circulating virus. In addition, producing the attenuated virus may require another step that will take almost as much time to perform as the regular phase III in typical controlled clinical trials. This additional step in an already complicated process will render futile the main purpose to gain more time to develop an effective vaccine [ 34 ].
Location and population
Development sites of COVID‐19 vaccines are involving research subjects from many countries, for example USA, Russia, Argentina, Brazil, Germany, India, Saudi Arabia, Pakistan and others [ 35 ]. The need of multi‐centred research is obvious in the vaccine development. The safety, tolerability, and efficacy of the vaccines should be obtained from different geographic areas, ethnicities, prevalence and varieties of the virus circulating in the areas [ 36 ]. The attempt to fulfil this requirement may result in the involvement of countries with limited resources and whose underdeveloped infrastructure would make the people involved become even more vulnerable as research subjects from the ethical and humane point of view. The possible exploitation of vulnerable people from less developed countries should be reviewed thoroughly. The vaccine trial should give them equitable advantages in trade, such as capacity building, transfer of technology and access to the vaccine during the current pandemic of COVID‐19.
Another concern is the availability of an adequate health facility and system to ensure that trial subjects and their families and/or communities have access to treatment and proper care in case of serious adverse events related to the trial outcomes. This must be assessed before any clinical trials begin. Providing the most comprehensive health services to the trial population will be an added value for population involvement in the trial. The best practice of vaccine clinical trials should have direct benefits for the community, such as improvement and availability of basic health facilities [ 37 ]
Vaccine acceptors are sometimes segmented into target groups, which is related to the host distribution of the target disease, for example by gender, age and specific population in the endemic area. A vaccine clinical trial is usually started in adult subjects and continued to more vulnerable subjects such as infants, young children, the elderly and women. Clinical vaccine trials will recruit vulnerable subjects. Protection measures to safeguard the vulnerable and marginalised populations should be carefully scrutinised during review. Ethical considerations must be adjusted to the individual situation to protect these vulnerable subjects from exploitation and later abandonment [ 38 ].
However, in an emergency pandemic situation, the definition of vulnerability needs to be openly discussed, and emergency calls for exceptions. The exclusion of vulnerable groups may diminish trial validity because of selection bias, so they should not be excluded without reasonable scientific and ethical justification [ 39 ].
Post‐trial access
After clinical vaccine trials, the subjects should have access to the developed vaccine. This is part of their direct advantage for their involvement in the research. While it is mentioned in the international ethical guidelines, not all researchers know and are aware of this important obligation [ 40 ]. The current COVID‐19 vaccine development involves multi‐country and intercontinental research recruiting subjects from different countries and regions. The post‐trial access to COVID‐19 vaccines should be expanded beyond the community where the trial is performed to include the country and region.
Post‐trial access is a matter which must be addressed from the very beginning of research design. Community engagement should be considered prior to the trial and involve all stakeholders: sponsors, industries, developers, investigators, subjects of the trial, communities and the government where the trial is performed.
In summary, the current COVID‐19 vaccine research and development involves people from many countries, which raises ethical issues that must be addressed by all stakeholders. Even in the emergency of a pandemic, the urgency of providing an effective COVID‐19 vaccine for humankind must be balanced with the exigency of research ethics that must be maintained. In any event, the safety and well‐being of research subjects must be protected, especially that of vulnerable subjects.
Sustainable Development Goals (SDGs): SDG 3 (good health and well‐being)
111 Vaccination Research Topics & Best Vaccine Essay Topics
Welcome to our list of vaccination research topics! This page contains only the best vaccine topics for essays and papers on child immunization, COVID-19, and other issues. Feel free to pick the catchiest research title about vaccines from our list!
🏆 Best Essay Topics on Vaccination
🔎 easy vaccination research paper topics, 👍 good vaccination research topics & essay examples, 🎓 most interesting research titles about vaccines, 💡 simple vaccine essay topics.
- Advantages and Disadvantages of Vaccination
- Should Vaccinations Be Required for Public School Students?
- Teaching Plan: Immunizations and Vaccinations
- Vaccinations Against COVID-19 in Canada: A Structural-Functionalism Perspective
- Mandatory Vaccination: Benefits and Reasons
- Vaccination and Immunization Promotion
- The COVID-19 Vaccination Hesitancy: Rhetorical Analysis
- The COVID-19 Vaccination: Side Effects The most significant side effect of the COVID-19 vaccination is severe allergy among patients who have never experienced any other kind of allergy.
- Benefits of Vaccination against Coronavirus Among the numerous recent healthcare advancements, vaccines occupy a specific place for their unique benefits for individuals and society in general.
- COVID-19: Information About Vaccination This work investigates the phenomenon of vaccine development in the context of a coronavirus pandemic and discusses the progress already achieved.
- An Outbreak of the Irrational: Refusion from Measles Disease Vaccination Today people do not face measles disease and its consequences, and for this reason, they are not afraid of it enough. The paper discusses the reasons for refuse from vaccination.
- Childhood Vaccination: Ethical Case Study The problem of moral behavior of nurses in situations of a difficult choice, decision-making, or conflict is actively discussed in the scholarly medical literature.
- COVID-19: Mandatory Vaccination The autonomy of nurses who refuse vaccinations has become a widespread precedent throughout the medical community.
- Quality Improvement Initiative – Influenza Vaccination The main one is vaccination, which is a major concern since only around 60% of healthcare workers worldwide agree with mandatory flu vaccination.
- Q.I. Program for Vaccination Among Healthcare Workers The paper analyzes the necessary QI tools to achieve the initiative of increasing the percentage of healthcare workers given influenza vaccination.
- Poliovirus and Importance of Vaccination The type of vaccination used for poliovirus plays a significant role in its effectiveness. In particular, the oral poliovirus vaccine deems insufficiently effective.
- Vaccination and Associated Advantages Vaccines work with an individual’s natural immunity to build protection against infections, thus reducing the risks of getting infected.
- Public Schools and Vaccination of Youth Vaccines could be described as safety measures to prevent illnesses by stimulating an immune system to produce a prepared defense against a particular sickness.
- The Importance and the Benefits of Vaccinating Children Vaccination is the most effective safe strategy for preventing infectious diseases and reducing disability and mortality from infectious pathology.
- HPV Anti-Vaxxers Policy: Anti-Vaccination Attitudes Smoking and drinking are risk factors for developing OPSCC, and competing illnesses are a likely cause of mortality in this population.
- Vaccination Issue Concerning the COVID-19 Pandemic This paper discusses the current vaccination issue concerning the COVID-19 pandemic. Large numbers of patients worldwide refuse vaccines.
- Mandatory COVID-19 Vaccination for Healthcare Employees Employees must be aware of the danger posed by the disease and undergo any procedures that will benefit the patient.
- The Effect of Vaccinated Travel Lanes in Singapore The recent introduction of Vaccinated Travel Lanes (or VTL) in Singapore allows passengers with recognized vaccination certificates to use airlines on special terms.
- Vaccination and Its Economic Implications The development of vaccines has significant implications for the pharmaceutical market. Disease prevention eliminates the need to create drugs to treat them.
- The COVID-19 Vaccination and Racial Issues The hypothesis for the present research is that even in developed countries, the COVID-19 vaccine doses may be distributed unequally among white people and racial minorities.
- The COVID-19 Anti-Vaccination Decision This paper is about how people argue against the COVID-19 vaccination, how they live with it, and how the rest of society is trying to adapt to the anti-vaccine decision.
- Ineffective COVID-19 Vaccination in Afro-Americans The present work shows the essence of comprehending African American subcultures and history to help raise COVID-19 vaccine awareness and acceptance.
- COVID-19 Debate: Masks for Vaccinated After reviewing credible sources regarding COVID-19 measures of prevention, it is clear that masks should not be mandatory for individuals who are vaccinated.
- Vaccination of Healthcare Workers Vaccination is the most effective way of protection against COVID-19: a highly contagious disease often followed by a set of serious complications.
- COVID-19 Vaccination: Necessity of Excessiveness Despite the risks and rare opposition, mass vaccination continues to be an essential step toward stopping the COVID-19 virus from impacting the world.
- Measles Vaccinations in Colorado State The paper states that with the outbreaks of measles nationwide, it is high time for state legislators to consider measures to combat the disease.
- Should We Get Vaccinated for COVID-19? Vaccinations present the only viable option for preventing the transmission of COVID-19 as well as reducing the risks of adverse symptoms.
- The COVID-19 Vaccination: Resistance and Protests Vaccination remains a pivotal prevention strategy against the COVID-19 pandemic. However, resistance to vaccination continues to dictate the ‘health politics’ of many nations.
- Ethical Principles: Parents’ Vaccination Concerns This case study reviews the ethical and moral choices of doctors who aim to convince parents to vaccinate their children.
- Importance of Employees Getting Vaccinated There is a need to ensure an effective vaccine is administered to employees to ensure that humans can interact freely for effective resumption of normal business activities.
- COVID-19 Measures: Masks for Vaccinated The main point in the essay is that employees should continue to wear a mask in the workplace even after being fully vaccinated.
- Autism and Vaccination: The False Health Claim One of the most popular false health claims concerns the relationship between autism and vaccination, suggesting that the latter causes the former.
- Wearing a Mask after Vaccination Controversial issues regarding collective security during the COVID-19 pandemic often include discussions of if a sanitary mask fulfills the functions assigned to it or not.
- Vaccination Against the Covid-19 Among Healthcare Professionals This paper investigates the adoption of Covid-19 vaccination among academics and resident doctors at the University of Drugs and Pharmacy “Nicolae Testimitanu” in Moldova.
- Disease in Vaccinated Populations This academic work is a laboratory report summarizing the results of a practical simulation of the epidemiological dynamics of a simulated population.
- “Should We Take a Selfie After Getting Vaccinated?” Summary The article’s central point “Should We Take a Selfie After Getting Vaccinated?” is that society is divided into groups with contrasting opinions on the issue.
- COVID-19: Vaccination Program All healthcare workers need to get the COVID-19 vaccine when available because of their increased exposure to this deadly disease.
- Racial Inequalities in the Context of Pandemic Vaccination To concretize the study, a current journalistic article in The New York Times was chosen to highlight racial inequalities in the context of pandemic vaccination.
- Support for Seasonal Influenza Vaccination Requirement Among US Healthcare Personnel The study analyzes an article titled ‘New Approaches for Influenza Vaccination of Healthcare Workers’. The article focuses on the effectiveness of compulsory vaccination.
- Americans’ Readiness to Coronavirus Vaccination The purpose of this paper is to summarize the statistic scales, considering whether Americans are ready to get a COVID-19 vaccine and the reasoning behind them.
- Human Papillomavirus Vaccination in Children The purpose of this paper is the identification of Human Papillomavirus vaccination effectiveness among children of both genders from 9 to 18 years old.
- Human Papillomavirus (HPV) Vaccination for All The health care system in the United States introduced HPV to the public in 2006 following its approval by the Food and Drug Administration.
- Applying Ethical Principles in Determining Vaccination Decisions Vaccination is beneficial regardless of the adverse effects that come along. Communities should be sensitized through seminars on vaccines.
- Vaccination: Child Immunization on the Government Level The experts say that the hesitancy to vaccinate children is one of the biggest threats to public health and the most dangerous mistakes a person can make.
- Compulsory Vaccination for Staff of Nursing Homes Because the COVID-19 vaccine will soon be available for the public, officials must make getting it compulsory for the staff of nursing homes and long-term care facilities.
- Seasonal Influenza Vaccination Program The following paper is focused on the Center for Disease Control (CDC) vaccination program on seasonal influenza.
- The Benefits of Vaccination Outweigh the Risks Medical researchers have succeeded in producing vaccines to help bodies fight pathogens that might overwhelm them.
- Mandatory Swine Flu Vaccination The swine flu vaccine is a vaccine that was developed to control the spread of the swine flu pandemic. These vaccines include Pandemix and Celvapan manufactured by Baxter.
- The H1N1 Influenza Virus: Benefits of Vaccination The basic structure of an effective vaccine and the characteristics of virus mutations will help find a way to immunize against a new flu strain and prevent another epidemic.
- Conflicted State of Mind: Vaccination and Ethics An increasing number of people have come to oppose vaccination on personal or ideological grounds, undermining the protection it provides.
- Why Vaccination Is So Discussed Now? Vaccination is one of the most debatable topics since many arguments for and against immunization confuse young parents who want to take the right decision.
- Vaccination Challenges and New Disease Outbreaks Vaccinations are the subject of heated debate in society because of their effectiveness, philosophical rationale, and religious relevance.
- The Views on HPV Vaccination in Sweeden The purpose of this article is to outline the research on the views on HPV vaccination assessed by school nurses in Sweeden.
- Health of Vaccinated and Unvaccinated Children The article by Mawson et al. evaluates the effectiveness of vaccination concerning improving the health of children when compared to infants who did not receive immunization.
- Vaccinations for Children: Issues and Recommendations for Positive Change This paper discusses vaccinations for childrens, in particular, security, privacy, and confidentiality regulations, laws, and principles related to data management and access.
- Human Papillomavirus Vaccination Among Adolescents Human papillomavirus (HPV) represents a significant threat to global health, which points to the need for implementing effective interventions targeted at its elimination.
- Flu Vaccination Side Effects as a Cause of Refusal Flu vaccinations are considered to be an important part of population health programs in most developed countries.
- Mandatory Vaccination of Children in the USA The issue of child immunization is particularly relevant to nursing, as nurses see the consequences of poor vaccination rates regularly.
- Vaccination Misconceptions in Old Adults This research focuses on the importance of education to improve the older populace’s willingness to get influenza vaccinated.
- Pharma Controversy Presentation: Vaccination and Autism Parents often associate MMR vaccination with autism. No qualitative evidence for the MMR vaccination-autism link.
- Vaccination as a Public Health Policy Issue The controversy surrounding vaccination of children is caused primarily by the miscommunication between the scientific community and the general public.
- 2009 H1N1 Flu Pandemic, Vaccination and Rates Extensive studies of the virus confirmed that it possessed certain characteristics that had not been detected in similar viruses before.
- Childhood Vaccination Policy in Florida This paper discusses the policy issue of childhood vaccination in Florida where parents can exempt their children from vaccinations due to religious reasons.
- Childhood Vaccination: Policy-Priority Issues The issue of vaccinations is often discussed on an international level. Governments do not underestimate the importance of immunization.
- Childhood Vaccination as Healthcare Priority Policy Issue This essay presents the controversial issue of childhood vaccination as a healthcare priority policy issue that requires the immediate attention of legislators.
- Vaccines: Should Parents Avoid Vaccinating Their Children? In this research paper, we will try to establish the benefits of damages of child vaccination and draw a conclusion whether parents should or should not avoid vaccinating their children.
- Vaccination and Its Effects on the Health Care System
- Targeting Head and Neck Cancer by Vaccination
- HPV Vaccination and Cancer Prevention
- Mandatory Vaccination Requirements for School Children
- Vaccination Programs and Its Impact on Public Health
- The Humoral Immune Response to BCG Vaccination
- Renaissance Island Anthrax Test Vaccination
- The Benefits and Effects of the Gardasil Vaccination in Children
- Social Psychological Reasons for Increasing Vaccination Rates
- Ethics, Mandatory Vaccination, and the HPV Vaccine
- Vaccination Crisis and Controversies of Africa
- The MMR Vaccination and Autism
- Anti Vaccination Movement and Its Effects on Children‘s Well
- Vaccination Against Lyme Disease: Past, Present, and Future
- The Ethical Theories and Issues Surrounding Vaccination in America
- Immunological Mechanisms of Vaccination
- Vaccination Coverage and Reasons for Non-vaccination in Istanbul
- Correlates of Protection Induced by Vaccination
- Public Doubts About Vaccination Safety and Resistance Against Vaccination
- COVID-19 Vaccination Hesitancy in the United States: A Rapid National Assessment
- The Issues Preventing the Proper Vaccination of Children in America
- Measles Dynamics and Vaccination Diffusion
- Smallpox Vaccination for Critical Public Service Workers
- Vaccination Myths and Its Effects on the Public Eye
- Childhood Immunizations and Universal Vaccination
- State Required Vaccination and Its Effects on Children
- The Anti Vaccination Movement Decreasing Child Safety
- Vaccination and Effective Smallpox Vaccine
- Prevnar Vaccination Production Process
- Vaccination for Babies 0-6 Months
- Disease Eradication: Private Versus Public Vaccination
- The Ethical Issues and Morality of Flu Vaccination
- Cultural and Social Factors of Vaccination
- Vaccination Can Save the Nation
- Service Members Anthrax Vaccine Vaccination
- Svir Epidemic Models With Vaccination Strategies
- The Contribution of Vaccination to Global Health: Past, Present and Future
- Panuveitis Following Vaccination for COVID-19
- Vaccination Against Coronaviruses in Domestic Animals
- Complex Correlates of Protection After Vaccination
Cite this post
- Chicago (N-B)
- Chicago (A-D)
StudyCorgi. (2022, May 10). 111 Vaccination Research Topics & Best Vaccine Essay Topics. https://studycorgi.com/ideas/vaccination-essay-topics/
"111 Vaccination Research Topics & Best Vaccine Essay Topics." StudyCorgi , 10 May 2022, studycorgi.com/ideas/vaccination-essay-topics/.
StudyCorgi . (2022) '111 Vaccination Research Topics & Best Vaccine Essay Topics'. 10 May.
1. StudyCorgi . "111 Vaccination Research Topics & Best Vaccine Essay Topics." May 10, 2022. https://studycorgi.com/ideas/vaccination-essay-topics/.
Bibliography
StudyCorgi . "111 Vaccination Research Topics & Best Vaccine Essay Topics." May 10, 2022. https://studycorgi.com/ideas/vaccination-essay-topics/.
StudyCorgi . 2022. "111 Vaccination Research Topics & Best Vaccine Essay Topics." May 10, 2022. https://studycorgi.com/ideas/vaccination-essay-topics/.
These essay examples and topics on Vaccination were carefully selected by the StudyCorgi editorial team. They meet our highest standards in terms of grammar, punctuation, style, and fact accuracy. Please ensure you properly reference the materials if you’re using them to write your assignment.
This essay topic collection was updated on January 9, 2024 .
Reexamining Misinformation: How Unflagged, Factual Content Drives Vaccine Hesitancy
Research from the Computational Social Science Lab finds that factual, vaccine-skeptical content on Facebook has a greater overall effect than “fake news,” discouraging millions from the COVID-19 shot.
By Ian Scheffler, Penn Engineering

What threatens public health more, a deliberately false Facebook post about tracking microchips in the COVID-19 vaccine that is flagged as misinformation, or an unflagged, factual article about the rare case of a young, healthy person who died after receiving the vaccine?
According to Duncan J. Watts, Stevens University Professor in Computer and Information Science at Penn Engineering and Director of the Computational Social Science (CSS) Lab , along with David G. Rand, Erwin H. Schell Professor at MIT Sloan School of Management, and Jennifer Allen, 2024 MIT Sloan School of Management Ph.D. graduate and incoming CSS postdoctoral fellow, the latter is much more damaging. “The misinformation flagged by fact-checkers was 46 times less impactful than the unflagged content that nonetheless encouraged vaccine skepticism,” they conclude in a new paper in Science.
Historically, research on “fake news” has focused almost exclusively on deliberately false or misleading content, on the theory that such content is much more likely to shape human behavior. But, as Allen points out, “When you actually look at the stories people encounter in their day-to-day information diets, fake news is a miniscule percentage. What people are seeing is either no news at all or mainstream media.”

“Since the 2016 U.S. presidential election, many thousands of papers have been published about the dangers of false information propagating on social media,” says Watts. “But what this literature has almost universally overlooked is the related danger of information that is merely biased. That’s what we look at here in the context of COVID vaccines.”
In the study, Watts, one of the paper’s senior authors, and Allen, the paper’s first author, used thousands of survey results and AI to estimate the impact of more than 13,000 individual Facebook posts. “Our methodology allows us to estimate the effect of each piece of content on Facebook,” says Allen. “What makes our paper really unique is that it allows us to break open Facebook and actually understand what types of content are driving misinformed-ness.”
One of the paper’s key findings is that “fake news,” or articles flagged as misinformation by professional fact-checkers, has a much smaller overall effect on vaccine hesitancy than unflagged stories that the researchers describe as “vaccine-skeptical,” many of which focus on statistical anomalies that suggest that COVID-19 vaccines are dangerous.
“Obviously, people are misinformed,” says Allen, pointing to the low vaccination rates among U.S. adults, in particular for the COVID-19 booster vaccine, “but it doesn’t seem like fake news is doing it.” One of the most viewed URLs on Facebook during the time period covered by the study, at the height of the pandemic, for instance, was a true story in a reputable newspaper about a doctor who happened to die shortly after receiving the COVID-19 vaccine.
That story racked up tens of millions of views on the platform, multiples of the combined number of views of all COVID-19-related URLs that Facebook flagged as misinformation during the time period covered by the study. “Vaccine-skeptical content that’s not being flagged by Facebook is potentially lowering users’ intentions to get vaccinated by 2.3 percentage points,” Allen says. “A back-of-the-envelope estimate suggests that translates to approximately 3 million people who might have gotten vaccinated had they not seen this content.”
Despite the fact that, in the survey results, fake news identified by fact-checkers proved more persuasive on an individual basis, so many more users were exposed to the factual, vaccine-skeptical articles with clickbait-style headlines that the overall impact of the latter outstripped that of the former.
“Even though misinformation, when people see it, can be more persuasive than factual content in the context of vaccine hesitancy,” says Allen, “it is seen so little that these accurate, ‘vaccine-skeptical’ stories dwarf the impact of outright false claims.”
As the researchers point out, being able to quantify the impact of misleading but factual stories points to a fundamental tension between free expression and combating misinformation, as Facebook would be unlikely to shut down mainstream publications. “Deciding how to weigh these competing values is an extremely challenging normative question with no straightforward solution,” the authors write in the paper.
Allen points to content moderation that involves the user community as one possible means to address this challenge. “Crowdsourcing fact-checking and moderation works surprisingly well,” she says. “That’s a potential, more democratic solution.”
With the 2024 U.S. Presidential election on the horizon, Allen emphasizes the need for Americans to seriously consider these tradeoffs. “The most popular story on Facebook in the lead-up to the 2020 election was about military ballots found in the trash that were mostly votes for Donald Trump,” she notes. “That was a real story, but the headline did not mention that there were nine votes total, seven of them for Trump.”
This study was conducted at the University of Pennsylvania’s School of Engineering and Applied Science, the Annenberg School for Communication and the Wharton School, along with the Massachusetts Institute of Technology Sloan School of Management, and was supported by funding from Alain Rossmann.
This article originally appeared on the Penn Engineering Blog.
- Public Health
- Social Media
Research Areas
- Health Communication
- Science Communication
Related News

Recommendations of APPC Working Group May Be Implemented in 2024 Presidential Debates

Abortion, Not Inflation, Directly Affected Congressional Voting in 2022

No Vacations, No Sleep, but Good Journalism: What It’s Like To Start a Nonprofit Newsroom
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Vaccine Safety Publications
Cdc publications by vaccine safety system, publications about specific vaccine safety topics, covid-19 vaccine safety articles and studies by topic, cdc vaccine safety publications by year.
CDC’s Immunization Safety Office monitors the safety of licensed and authorized vaccines and conducts high-quality vaccine safety research. This research is peer-reviewed and published in reputable scientific outlets.
The vaccine safety articles and studies listed on this page include a full citation, a short summary, and a link to the free PMC article, when available.
- Vaccine Adverse Event Reporting System (VAERS)
- Vaccine Safety Datalink (VSD)
- Clinical Immunization Safety Assessment (CISA) Project
- Human Papillomavirus (HPV) Vaccine Safety [PDF – 2 pages]
- Vaccines and Autism [PDF – 2 pages]
- Thimerosal Publications and References
An asterisk (*) denotes a newly published article added
Shimabukuro T, Nair N. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine JAMA 2021 Feb 23;325(8):780-781 doi: 10.1001/jama.2021.0600.
Pfizer-BioNTech COVID-19 vaccine was authorized by the Food and Drug Administration (FDA) for emergency use in December 2020. CDC and FDA immediately began safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). One health outcome in particular that CDC and FDA monitored for was severe allergic reaction, or anaphylaxis. From December 14–23, 2020, 1.89 million first doses of Pfizer-BioNTech COVID-19 vaccine were administered. The most commonly reported non-anaphylaxis allergic reactions included: rash, itchy skin, itchy and scratchy sensations in the throat, and mild respiratory symptoms. Safety monitoring identified 21 anaphylaxis reports, corresponding to an estimated rate of 11.1 cases per million doses administered; 17 (81% ) had a history of allergies or allergic reactions. No deaths from anaphylaxis were reported. CDC has guidance on the use of mRNA COVID-19 vaccines and management of anaphylaxis.
Shimabukuro T, Cole M, Su JR. Reports of Anaphylaxis After Receipt of mRNA COVID-19 Vaccines in the US—December 14, 2020-January 18, 2021. JAMA 2021 Feb 12; doi:10.1001/jama.2021.1967. Epub ahead of print.
In December 2020, FDA issued Emergency Use Authorizations for two mRNA-based vaccines for prevention of COVID-19 disease: Pfizer-BioNTech COVID-19 vaccine (December 11) and Moderna COVID-19 vaccine (December 18). After implementation of the vaccines, cases of anaphylaxis following both vaccines were reported. Anaphylaxis is a severe, life-threatening allergic reaction that can occur after vaccination. During December 14, 2020 through January 18, 2021, over 9.9 million doses of Pfizer-BioNTech vaccine and over 7.5 million doses of Moderna vaccine were administered. In this same time, CDC identified 66 anaphylaxis cases reported to VAERS: 47 following Pfizer-BioNTech vaccine (rate of 4.7 cases per million doses) and 19 following Moderna vaccine (rate of 2.5 cases per million doses). There were no deaths from anaphylaxis reported after either vaccine. Continued safety monitoring of mRNA COVID-19 vaccines has confirmed anaphylaxis following vaccination is a rare event.
CDC COVID-19 Response Team Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Moderna COVID-19 Vaccine— United States, December 21, 2020-January 10, 2021 MMWR Morb Mortal Wkly Rep. 2021 Jan 22:70(4);125-129.
On December 18, 2020, FDA issued an Emergency Use Authorization for Moderna COVID-19 vaccine to prevent COVID-19. As of January 10, 2021, over 4 million first doses of the vaccine had been administered. Many people did not have any side effects after COVID-19 vaccination. However, some serious adverse reactions were reported, such as the life-threatening allergic reaction, anaphylaxis. From December 21, 20201 through January 10, 2021, VAERS received 108 reports following Moderna vaccine identified as possible allergic reaction, including anaphylaxis. Through case review of medical reports, 10 cases were determined to be anaphylaxis (a rate of 2.5 cases of anaphylaxis per million doses). Of the 10 cases, 9 had a history of allergies or allergic reaction, including 5 who had a history of anaphylaxis. Anaphylaxis following Moderna vaccine appears to be a rare event. CDC and FDA will continue to monitor for anaphylaxis following COVID-19 vaccines.
CDC COVID-19 Response Team Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine — United States, December 14-23, 2020 MMWR Morb Mortal Wkly Rep. 2021 Jan 15:70(2);46-51.
On December 11, 2020, FDA issued an Emergency Use Authorization for Pfizer-BioNTech COVID-19 vaccine to prevent COVID-19. As of December 23, 2020, over 1.8 million first doses of the vaccine had been administered. During this time, CDC and FDA were notified through multiple channels of suspected cases of anaphylaxis following vaccination. Anaphylaxis is a severe, life-threatening allergic reaction that occurs rarely after vaccination. From December 14-23, 2020, VAERS received 175 reports identified as possible allergic reaction, including anaphylaxis. Through case review of medical reports, 21 cases were determined to be anaphylaxis (a rate of 11.1 cases of anaphylaxis per million doses). Most anaphylaxis cases (81%) occurred in persons with a history of allergies or allergic reactions. Anaphylaxis following Pfizer-BioNTech vaccine appears to be a rare event. CDC and FDA will continue to monitor for anaphylaxis following COVID-19 vaccines.
Qian L, Sy LS, Hong V, Glenn SC, Ryan DS, Nelson JC, Hambidge SJ, Crane B, Zerbo O, DeSilva MB, Glanz JM, Donahue JG, Liles E, Duffy J, Xu S. Impact of the COVID-19 Pandemic on Health Care Utilization in the Vaccine Safety Datalink: Retrospective Cohort Study . JMIR Public Health Surveill. 2024 Jan 23:10:e48159. Doi:2196/48159.
Malden DE, Gee J, Glenn S, Li Z, Mercado C, Ogun OA, Kim S, Lewin BJ, Ackerson BK, Jazwa A, Weintraub ES, McNeil MM, Tartof S. Reactions following Pfizer-BioNTech COVID-19 mRNA vaccination and related healthcare encounters among 7,077 children aged 5-11 years within an integrated healthcare system . Vaccine 2023 Jan 9; https://doi.org/10.1016/j.vaccine.2022.10.079. Online ahead of print.
This publication describes data from a digital survey and from electronic health records (EHRs) of children aged 5-11 years who were vaccinated with the Pfizer-BioNTech mRNA COVID-19 vaccine during November 4, 2021, to February 28, 2022. Parents completed a 14-day survey on reactions their children had following vaccination. Self-reported reactions from the survey were combined with the EHRs along with information from parents or guardians who sought medical care for vaccine-related symptoms. Results showed that in most cases, symptoms following Pfizer-BioNTech mRNA COVID-19 vaccination did not require medical attention.
Tompkins LK, Baggs J, Myers TR, Gee JM, Marquez PL, Kennedy SB, Peake D, Dua D, Hause AM, Strid P, Abara W, Rossetti R, Shimabukuro TT, Shay DK. Association between history of SARS-CoV-2 infection and severe systemic adverse events after mRNA COVID-19 vaccination among U.S. adults . Vaccine . 2022 Nov 1;S0264-410X(22)01342-1. Online ahead of print.
This study, published in December 2022, found that after receiving the first of two doses of an mRNA COVID-19 vaccine, patients who had previously tested positive for COVID-19 were somewhat more likely to have a severe systemic reaction to the vaccine compared to those who had not had COVID-19 previously. Though these reactions were rare, people who received the Moderna vaccine had a slightly higher risk of complications compared to participants who received the Pfizer-BioNTech vaccine. The study reviewed data from patients 18 years of age and older who had received a COVID-19 vaccination within the previous seven days and registered on CDC’s V-safe. V-safe is a web-based tool that uses text messages, emails, and web surveys to provide personalized health check-ins for people after receiving a new vaccine.
Tartof SY, Malden DE, Liu ILA, Sy LS, Lewin BJ, Williams JTB, Hambidge SJ, Alpern JD, Daley MF, Nelson JC, McClure D, Zerbo O, Henninger ML, Fuller C, Weintraub E, Saydeh S, Qian L. Health Care Utilization in the 6 Months Following SARS-CoV-2 Infection . JAMA Network . 2022 Aug 12 ;5(8):e2225657. doi:10.1001/jamanetworkopen.2022.25657.
This August 2022 publication presents a study on healthcare use in the six months immediately following COVID-19 infection. Data from the Vaccine Safety Datalink found that COVID-19 infections led to a 4% increase in healthcare use that included a mix of virtual encounters and emergency department visits. This increase shows the potential excess strain on the healthcare system and provides insight into long-term resource planning for patients who were previously infected with COVID-19.
Twentyman E, Wallace M, Roper LE, Anderson TC, Rubis AB, Fleming-Dutra KE, Hall E, Hsu J, Rosenblum HG, Godfrey M, Archer WR, Moulia DL, Daniel L, Brooks O, Talbot HK, Lee GM, Bell BP, Daley M, Meyer S, Oliver SE. Interim Recommendation of the Advisory Committee on Immunization Practices for Use of the Novavax COVID-19 Vaccine in Persons Aged ≥18 years — United States, July 2022 . MMWR Morb Mortal Wkly Rep . 2022 Aug 5;71(31);988–992.
The U.S. Food and Drug Administration issued an Emergency Use Authorization for the Novavax COVID-19 vaccine on July 13, 2022. On July 19, 2022, the Advisory Committee on Immunization Practices (ACIP) made recommendations for the use of the Novavax vaccine in adults ages 18 years and older as a primary series. Since June 2020, ACIP has reviewed data related to COVID-19 and the use of COVID-19 vaccines, including the Novavax vaccine. Data were used from a randomized double blind clinical trial based in the United States and Mexico that enrolled 29,945 participants ages 18 years and older during December 27, 2020, through September 27, 2021. Participants were given doses of either the Novavax vaccine or a saline placebo. Results showed the effectiveness of the Novavax vaccine in adults ages 18 years and older. Additional data sources from the United Kingdom, South Africa, and Australia were considered when looking at the benefits and risks of the Novavax vaccine. Novavax COVID-19 vaccine is an additional option for unvaccinated adults, increasing flexibility for the public and for vaccine providers to prevent severe COVID-19 illness.
Hause AM, Baggs J, Marquez P, Abara WE, Baumblatt J, Blanc PG, Su JR, Hugueley B, Parker C, Myers TR, Gee J, Shimabukuro TT, Shay DK. Safety Monitoring of COVID-19 mRNA Vaccine Second Booster Doses Among Adults Aged ≥50 Years — United States, March 29, 2022–July 10, 2022 . MMWR Morb Mortal Wkly Rep . 2022 Jul 29;71(30);971–976.
This analysis, published July 2022, reviewed vaccine safety data from V-safe and the Vaccine Adverse Event Reporting System after receipt of a second COVID-19 mRNA (Pfizer-BioNTech or Moderna) booster dose among adults ages 50 years and older from March 29–July 10, 2022. Vaccine safety experts identified no new safety concerns following the second mRNA booster dose in this age group. Local (itching, pain, redness, and/or swelling at the injection site) and systemic (fever, headache, joint pain) reactions were observed, and serious adverse events were rare. These findings are consistent with known adverse events after receipt of first booster doses and with the existing body of evidence that mRNA COVID-19 vaccines are safe.
Hause AM, Zhang B, Yue X, Marquez P, Myers TR, Parker C, Gee J, Su J, Shimabukuro TT, Shay DK. Reactogenicity of Simultaneous COVID-19 mRNA Booster and Influenza Vaccination in the US. . JAMA Netw Open . 2022 Jul 1;5(7):e2222241. doi: 10.1001/jamanetworkopen.2022.22241.
A review of vaccine safety data from September 22, 2021, through January 16, 2022, looked at reported reactions following vaccination with both influenza (flu) and an mRNA-based COVID-19 booster dose (third dose administered ≥ 5 months after second dose) during the same healthcare visit. The review found that people who received a booster and flu vaccine simultaneously were slightly more likely to report systemic reactions (i.e., headache, fatigue, and muscle pain) than people who received just the COVID-19 booster. Data were collected and reviewed from V-safe, a web-based tool that uses text messages, emails, and web surveys to provide personalized health check-ins for people after receiving a new vaccine.
Hanson KE, Goddard K, Lewis N, Fireman B, Myers TR, Bakshi N, Weintraub E, Donahue JG, Nelson JC, Xu S, Glanz JM, Williams JTB, Alpern JD, Klein NP. Incidence of Guillain-Barré Syndrome After COVID-19 Vaccination in the Vaccine Safety Datalink. . JAMA Netw Open . 2022 Apr 26; 5(4):e228879. doi: 10.1001/jamanetworkopen.2022.8879.
Post-authorization monitoring of COVID-19 vaccines in large populations may detect rare adverse events (AEs) that were not identified during clinical trials, such as Guillain-Barré syndrome (GBS). This cohort study, published April 2022, was conducted to look at data from the Vaccine Safety Datalink during December 13, 2020, through November 13, 2021, to assess the risk of GBS after COVID-19 vaccination with J&J/Janssen and mRNA (Pfizer-BioNTech or Moderna) vaccines. Data from 10,158,003 COVID-19 vaccine recipients who were at least aged 12 years were analyzed. The study found that cases of GBS were higher among those who received the J&J/Janssen vaccine, which is no longer used in the United States as of May 2023.
Xu S, Hong V, Sy LS, Glenn SC, Ryan DS, Morrissette KL, Nelson JC, Hambidge SJ, Crane B, Zerbo O, DeSilva MB, Glanz JM, Donahue JG, Lile E, Duffy J, Qian L. Changes in incidence rates of outcomes of interest in vaccine safety studies during the COVID-19 pandemic . Vaccine . 2022 April 18;S0264-410X922)00464-9. Online ahead of print.
The COVID-19 pandemic led to increased use of telehealth. Moving from in-person care to telehealth made it harder to identify outcomes in vaccine safety studies that are normally assessed during in-person health visits. Data from eight Vaccine Safety Datalink sites between January 1, 2017, and December 31, 2020, were used to determine changes in incidence rates for 21 outcomes that are traditionally assessed in in-person settings. The study, published May 2022, found that rates of some clinical outcomes changed during the pandemic and should not be used as background rates in vaccine safety studies. Data from 2020 were split into four periods: pre- to early pandemic (January–June), middle (July–September), and later pandemic (October–December). Four corresponding time periods (ranges of months) were used for each year during 2017–2019. Results showed that incidence rates for encephalomyelitis, encephalitis/myelitis/encephalomyelitis/meningoencephalitis, and thrombotic thrombocytopenic purpura cases did not change significantly during 2020. Incidence rates of acute myocardial infarction, anaphylaxis, appendicitis, convulsions/seizures, Guillain-Barré syndrome, immune thrombocytopenia (ITP), narcolepsy/cataplexy, hemorrhagic stroke, ischemic stroke, and venous thromboembolism decreased, and incidence rates of Bell’s palsy, ITP, and narcolepsy/cataplexy were higher. The higher incidence rates of these conditions suggest that telehealth visits should be considered for vaccine studies involving Bell’s palsy, ITP, and narcolepsy/cataplexy.
Kenigsberg TA, Hause AM, McNeil MM, Nelson JC, Shoup JA, Goddard K, Lou Y, Hanson KE, Glenn SC, Weintraub E. Dashboard development for near real-time visualization of COVID-19 vaccine safety surveillance data in the Vaccine Safety Datalink . Vaccine . 2022 May 1;40(22):3064-3071. Epub 2022 Apr 8.
The Vaccine Safety Datalink (VSD) conducts vaccine safety monitoring and vaccine safety research studies. When COVID-19 vaccinations began in the U.S. in December 2020, VSD helped with near real-time safety surveillance. Investigators developed a dashboard to show data metrics on vaccine safety. Dashboard visualizations provide situational awareness on vaccination coverage and the status of safety analysis. This May 2022 report describes the development and implementation of the COVID-19 Vaccine Dashboard, including metrics used to develop the dashboard, which may have application across various public health settings.
Rosenblum HG, Gee JM, Liu R, Marquez PL, Zhang B, Strid P, Abara WE, McNeil MM, Myers TR, Hause AM, Su JR, Baer B, Menschik D, Markowitz LE, Shimabukuro TT, Shay DK. Safety monitoring of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to Vaccine Adverse Events Reporting System and v-safe Lancet Infect Dis .2022 Mar 7;S1473-3099(22)0054-8. Online ahead of print.
In December 2020, two mRNA COVID-19 vaccines were authorized for emergency use in the United States. Clinical trials showed these vaccines to be safe, and post-authorization monitoring is necessary to evaluate their safety in larger and diverse populations. VAERS and v-safe are two national systems CDC uses to monitor COVID-19 vaccine safety. During the first six months of the COVID-19 vaccination program (December 14, 2020 through June 14, 2021) over 298 million doses of mRNA vaccines were administered in the U.S. In that period, over 7.9 million people enrolled in v-safe. Local (pain, redness, swelling at injection site) and systemic (fever, fatigue, and headache) reactions were reported more frequently following dose 2 compared to dose 1. The majority of symptoms were reported as mild, peaked on day 1 following vaccination, and were short-lived. Of the 340,000 adverse event reports to VAERS, the majority (92%) were classified as non-serious (similar to the local and systemic reactions reported to v-safe); 6.6% serious, non-death; 1.3% deaths. An in-depth review of reports of death found rates of death reported to VAERS were lower than expected background rates by age group. This analysis reinforces the safety of COVID-19 vaccines.
Hause AM, Bags J, Myers TR, Su JR, Blanc PG, Girwa Baumblatt JA, Woo EJ, Gee J, Shimabukuro TT, Shay DK. Safety Monitoring of COVID-19 Vaccine Booster Doses Among Adults — United States, September 22, 2021-February 6, 2022 MMWR Morb Mortal Wkly Rep 2022 Feb 11;71. Early Release.
In this February 2022 report, CDC reviewed the safety of a third dose of mRNA COVID-19 vaccine administered ≥ 5 months after the second dose. Safety data on adults ages 18 and older from V-safe and the Vaccine Adverse Event Reporting System showed no unexpected patterns of adverse events and found that for people who received the same mRNA COVID-19 vaccine for dose 3 as they received for doses 1 and 2, local and systemic reactions (such as pain, fatigue, and headache) were less frequently reported after dose 3 than after dose 2. Myocarditis was rarely reported following mRNA COVID-19 vaccine dose 3.
Oliver SE, Wallace M, See I, Mbaeyi S, Godfrey M, Hadler SC, Jatlaoui TC, Twentyman E, Hughes MM, Rao AK, Fiore A, Su JR, Broder KR, Shimabukuro T, Lale A, Shay DK, Markowitz LE, Wharton M, Bell BP, Brooks O, McNally V, Lee GM, Talbot HK, Daley MF. Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine: Updated Interim Recommendations from the Advisory Committee on Immunization Practices – United States, December 2021. Morb Mortal Wkly Rep. 2022 Jan 20;71(3):90-95.
After a thorough review of available vaccine safety and effectiveness data during an emergency meeting in December 2021, the Advisory Committee on Immunization Practices (ACIP) recommended preferential use of mRNA COVID-19 vaccines (Pfizer-BioNTech/Comirnaty and Moderna) over the viral vector COVID-19 vaccine (Johnson & Johnson’s Janssen) for everyone ages 18 years and older in the U.S. CDC endorsed the committee’s decision and updated its recommendations for the prevention of COVID-19, stating a preference for mRNA COVID-19 vaccines over viral vector COVID-19 vaccines, if they are available. The mRNA COVID-19 vaccines are preferred over the viral vector COVID-19 vaccine for primary and booster vaccination. Since the January 2022 publication of this report, the J&J/Janssen COVID-19 vaccine is no longer available for use in the U.S.
Moro PL, McNeil MM. Successes of the CDC monitoring systems in evaluating post-authorization safety of COVID-19 vaccines [Editorial] . Expert Rev Vaccines. 2022 Mar;21(3):284-284. Epub 2022 Jan 5.
The U.S. Food and Drug Administration authorized the first two mRNA COVID-19 vaccines, Pfizer-BioNTech and Moderna, in mid-December 2020, along with the Johnson & Johnson Janssen (J&J/Janssen) vaccine at the end of February 2021, for emergency use in the United States. During pre-emergency use of these vaccines, adverse events consisted of local and systemic reactions. CDC uses three systems to monitor the safety of each of these COVID-19 vaccines in the United States: the Vaccine Adverse Event Reporting System, V-safe, and the Vaccine Safety Datalink. In addition to monitoring the safety of COVID-19 vaccines, these systems provide early safety data for very rare and serious adverse events of anaphylaxis, TTS, myocarditis/pericarditis, and Guillain-Barre syndrome—giving updated information for healthcare providers and vaccine recipients. These are complementary systems that continue to provide critical data about the safety of COVID-19 vaccines. Since the publication of this report, the J&J/Janssen COVID-19 vaccine is no longer available for use in the United States.
Lipkind HS, Vazquez-Benitez G, DeSilva M, Vesco KK, Ackerman-Banks C, Zhu J, Boyce TG, Daley MF, Fuller CC, Getahun D, Irving SA, Jackson LA, Williams JTB, Zerbo O, McNeil MM, Olson CK, Weintraub E, Kharbanda KO. Receipt of COVID-19 Vaccine During Pregnancy and Preterm or Small-for-Gestational-Age at Birth — Eight Integrated Health Care Orgnizations, United States, December 15, 2020-July 22, 2021. MMWR Morb Mort Wkly Rep. 2022 Jan 4:71 Early release.
Pregnant people with COVID-19 are at higher risk for severe illness and adverse birth outcomes such as pre-term and small-for-gestational-age birth, yet many remain reluctant to be vaccinated. COVID-19 vaccines are recommended for pregnant people to prevent maternal morbidity and adverse birth outcomes. This study, published January 2022, analyzed the risk for severe morbidity associated with COVID-19 in pregnancy, along with risk for pre-term birth or small-for-gestational-age at birth babies. Results show that receiving a COVID-19 vaccine during pregnancy was not linked with increased risk for pre-term birth or small-for-gestational-age birth. Risk for severe morbidity associated with COVID-19 disease in pregnancy was low; however, people with symptomatic COVID-19 during pregnancy were at increased risk for intensive care admission, with a 70% increased risk for death, compared with non-pregnant people with symptomatic infections. Results confirm the recommendations and benefits of receiving the COVID-19 vaccine during pregnancy.
Abara WE, Gee J, Mu Y, Deloray M, Ye T, Shay DK, Shimabukuro T. Expected Rates of Select Adverse Events following Immunization for COVID-19 Vaccine Safety Monitoring J Infect Dis. 2021 Dec 27;jiab628. Online ahead of print.
Chapin-Bardales J, Myers T, Gee J, Shay DK, Marquez P, Baggs J, Zhang B, Licata C, Shimabukuro TT. Reactogenicity within 2 weeks after mRNA COVID-19 vaccines: Findings from the CDC v-safe surveillance system. Vaccine 2021 Nov 26;39(48):7066-7073. Epub 2021 Oct 16.
In this review of v-safe vaccine safety data, researchers analyzed surveys of people who received their mRNA vaccine from December 14, 2020, through March 14, 2021. V-safe is a vaccine safety monitoring system that uses text messages and web surveys to collect information on health impacts reported after receipt of COVID-19 vaccines. During this time period, more than 4.7 million participants received one dose of an mRNA vaccine (Pfizer-BioNTech or Moderna), and over 2.9 million received a second dose. Most participants reported either a local reaction at the injection site (68.5% after dose 1; 72.9% after dose 2) or a systemic reaction, such as fever, headache, muscle ache and fatigue (50.6% after dose 1; 69.5% after dose 2). Researchers found that these side effects were reported more frequently among those who received Moderna than those who received Pfizer-BioNTech. An analysis of surveys reported on day 14 after vaccination indicated that new or worsening local and systemic reactions were uncommon during the second week following both dose 1 and dose 2. CDC will continue to closely monitor the safety of COVID-19 vaccines.
Hause AM, Baggs J, Gee J, Marquez P, Myers TR, Shimabukuro TT, Shay DK. Safety Monitoring of an Additional Dose of COVID-19 Vaccine — United States, August 12-September 19, 2021 MMWR Morb Mortal Wkly Rep. epub 2021 Sep 28.
On August 12, 2021, the Food and Drug Administration (FDA) expanded the Emergency Use Authorizations for Pfizer-BioNTech and Moderna (mRNA) COVID-19 vaccines to include an additional dose following the 2-dose vaccination series to those with compromised immune systems. From August 12 through September 19, over 22,000 v-safe enrollees reported an additional COVID-19 dose after completing the primary 2-dose mRNA vaccination series, most with the same vaccine. Among those who completed surveys for all 3 doses, local reactions (like pain or swelling where the shot was given) were reported slightly more after dose 3 compared with after dose 2 (79% vs. 78%), while reported systemic reactions (tiredness, headache) were slightly less common after dose 3 (74% vs. 77%). These side effects were mostly mild to moderate and short-lived. These findings did not show unexpected patterns of adverse events following an additional dose of COVID-19 vaccines. CDC will continue to monitor the safety of additional doses of COVID-19 vaccines and provide data to guide recommendations and protect the public’s health.
Klein NP, Lewis N, Goddard K, Fireman B, Zerbo Q, Hanson KE, Donahue JG, Kharbanda EO, Naleway A, Clark Nelson J, Xu S, Yih WK, Glanz JM, Williams JTB, Hambridge SJ, Lewin BJ, Shimabukuro TT, DeStefano F, Weintraub ES. Surveillance for Adverse Events After COVID-19 mRNA Vaccination JAMA 2021 Sept 3. Doi:10.1001/jama.2021.15072.
The Vaccine Safety Datalink (VSD) has conducted weekly near real-time monitoring, or Rapid Cycle Analysis (RCA), of Pfizer-BioNTech and Moderna mRNA COVID-19 vaccines since those vaccines received emergency use authorization from the Food and Drug Administration in December 2020. Between December 14, 2020 through June 25, 2021, over 11.8 million doses of mRNA were administered to 6.2 million people in the VSD network; 57% received Pfizer-BioNTech and 43% received Moderna. During that time period, VSD monitored 23 pre-specified health outcomes, including myocarditis/pericarditis and anaphylaxis. Researchers identified 34 cases of myocarditis/pericarditis in people ages 12 to 39 years; a majority (85%) were males. Among this age group, there is an increased risk of 6.3 additional myocarditis cases per million mRNA vaccinations administered in the first week following vaccination. The rate of anaphylaxis following vaccination was 4.8 cases per million doses of Pfizer-BioNTech and 5.1 per million doses of Moderna vaccination. VSD monitoring did not detect safety signals for any other pre-specified outcomes. Additional research is ongoing. Getting vaccinated remains the best way to protect against COVID-19 infection.
Rosenblum HG, Hadler SC, Moulia D, Shimabukuro TT, Su JR, Tepper NK, Ess KC, Woo EJ, Mba-Jonas A, Alimchandani M, Nair N, Klein NP, Hanson KE, Markowitz LE, Wharton M, McNally VV, Romero JR, Talbot K, Lee GM, Daley MF, Mbaeyi SA, Oliver SE. Use of COVID-19 Vaccines After Reports of Adverse Events Among Adult Recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 Vaccines (Pfizer-BioNTech and Moderna): Update from the Advisory Committee on Immunization Practices — United States, July 2021 MMWR Morb Mortal Wkly Rep. 2021 Aug 10.
On July 22, 2021, CDC’s Advisory Committee on Immunization Practices (ACIP) reviewed a benefit-risk analysis of Guillain-Barré syndrome (GBS) following Johnson & Johnson’s Janssen (J&J/Janssen) vaccine, as well as the latest information on thrombosis with thrombocytopenia syndrome (TTS) following J&J/Janssen vaccination and myocarditis following mRNA vaccination (Pfizer-BioNTech and Moderna vaccines). As of June 30, 2021, about 12.6 million doses of Janssen vaccine had been administered and 141 million 2nd mRNA vaccine doses had been administered. Overall, there were 7.8 cases of GBS per million J&J/Janssen doses; 3 cases of TTS per million J&J/Janssen doses and 3.5 cases of myocarditis per million 2nd mRNA vaccine doses. After assessing the data, ACIP concluded that the benefits of COVID-19 vaccination in preventing COVID-19 illness, associated hospitalizations, ICU admissions, and death outweigh serious but rare risks of GBS, TTS, and myocarditis.
Pingali C, Meghani M, Razzaghi H, , Lamias MJ, Weintraub E, Kenigsberg TA, Klein NP, Lewis N, Fireman B, Zerbo O, Bartlett J, Goddard K, Donahue J, Hanson K, Naleway A, Kharbanda EO, Yih K, Clark Nelson J, Lewin BJ, Williams JTB, Glanz JM, Singletom JA, Patel SA. COVID-19 Vaccination Coverage Among Insured Persons Aged ≥ 16 years, by Race/Ethnicity and Other Selected Characteristics — Eight Integrated Health Care Organizations, United States, December 14, 2020-May 15, 2021 . MMWR Morb Mortal Wkly Rep. 2021 Jul 16;70(28):985-990.
Data has shown that non-Hispanic Black and Hispanic people have experienced higher COVID-19–associated deaths and serious outcomes; however, COVID-19 vaccination coverage has been lower in these groups. To look into these disparities further, researchers from CDC analyzed data collected from the Vaccine Safety Datalink (VSD) from December 14, 2020, through May 15, 2021. During that time, over 9.6 million people ages 16 years and older were enrolled in the VSD; 48.3% of the population had received at least one vaccine dose and 38.3% were considered fully vaccinated. In non-Hispanic Black and Hispanic populations, only 40.7% and 41.1% had received at least one COVID-19 vaccine dose, respectively. By comparison, non-Hispanic White people and non-Hispanic Asian people had higher coverage rates (54.6% and 57.4%, respectively). CDC will continue its ongoing efforts to improve vaccination coverage in all populations, especially among populations that are most greatly affected by COVID-19.
Gubernot D, Jazwa A, Niu M, Baumblatt J, Gee J, Moro P, Duffy J, Harrington T, McNeil MM, Broder K, Su J, Kamidani S, Olson CK, Panagiotakopoulos L, Shimabukuro T, Forshee R, Anderson S, Bennet S. U.S. Population-Based background incidence rates of medical conditions for use in safety assessment of COVID-19 vaccines Vaccine 2021 Jun 23;39(28):3666-3677. Epub 2021 May 14.
The COVID-19 vaccination campaign is the largest international collaboration of modern times. One aspect of vaccination campaigns that needs consideration is possible side effects or adverse events following vaccination. Serious health outcomes linked to vaccinations are rare, and some outcomes may occur incidentally in the vaccinated population. In this large-scale compilation of U.S. background rates for medical conditions, researchers reviewed scientific literature and calculated the background rates of specific medical conditions that are generally monitored following vaccination. For COVID-19 vaccine safety surveillance, experts at CDC and the Food and Drug Administration assembled a list of 22 potential adverse events that could be monitored following COVID-19 vaccination, including neurological, autoimmune, and cardiovascular conditions, and compiled estimates of the U.S. background rates through scientific literature review gathered from PubMed and other publicly available data. These rates may be useful for future studies that assess adverse events following COVID-19 vaccination.
Hause AM, Gee J, Johnson T, Jazwa A, Marquez P, Miller E, Su J, Shimabukuro TT, Shay DK. Anxiety-Related Adverse Event Cluster After Janssen COVID-19 Vaccination — Five U.S. Mass Vaccination Sites, April 2021 MMWR Morb Mortal Wkly Rep. 2021 April 20. Epub ahead of print.
From April 7-9, 2021, 5 weeks after the J&J/Janssen COVID-19 vaccine was authorized by FDA for emergency use, clusters of anxiety-related events after Janssen vaccination were reported to CDC. The reports came from 5 mass vaccination sites in different states; 4 closed temporarily to investigate the cases. Of the 8,624 Janssen vaccine recipients, there were 64 reports of anxiety-related events, including 17 reports of fainting. Commonly reported symptoms were light-headedness/dizziness (56%), excessive sweating (31%), fainting (27%), nausea or vomiting (25%) and low blood pressure (16%). Additionally, CDC reviewed all reports to VAERS of fainting after Janssen vaccine between March 2 through April 11, 2021 and identified 653 reports out of 8 million doses administered. Review of reports found that fainting occurs in 8 per 100,000 doses administered. Vaccine providers should observe individuals for 15 minutes after COVID-19 vaccination for signs of immediate anxiety-related reactions or fainting.
Shay DK, Gee J, Su JR, Myers TR, Marquez P, Liu R, Zhang B, Licata C, Clark TA, Shimabukuro TT. Safety Monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine — United States, March-April 2021 . MMWR Morb Mortal Wkly Rep. 2021 April 30. Epub ahead of print.
Johnson & Johnson’s Janssen COVID-19 vaccine was authorized by FDA for emergency use on February 27, 2021. By April 21, nearly 8 million doses of the Janssen COVID-19 vaccine had been administered. CDC researchers reviewed safety monitoring data from VAERS and the v-safe after-vaccination health checker, and found 97% of reported reactions after vaccination, such as headache, fever, chills, injection site pain, and fatigue, were nonserious and consistent with clinical trials data. CDC and FDA issued a pause of the Janssen vaccine April 12–23, 2021, after 6 cases of cerebral venous sinus thrombosis (CVST), a serious condition that involves blood clots in the brain, were identified in VAERS. By April 25, a total of 17 thrombotic (blood clots) events with thrombocytopenia (low platelet counts) were reported to VAERS, including 3 thrombotic events not occurring in the brain. CDC and FDA continue to monitor the safety of COVID-19 vaccines, analyzing the risks and benefits of continued use.
Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, Marquez PL, Olson CK, Liu T, Chang KT, Ellington SR, Burke VK, Smoots AN, Green CJ, Licata C, Zhang BC, Alimchandani M, Mba-Jonas A, Martin SW, Gee JM, Meaney-Delman DM. Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons N Engl J Med 2021 April 21. DOI: 10.1056/NEJMoa2104983 Epub ahead of print.
Pregnant people were not included in the messenger RNA (mRNA) COVID-19 vaccine clinical trials. Because of the increased risk of severe illness from COVID-19, CDC has provided guidance to pregnant people who may want to get a COVID-19 vaccine. The safety of mRNA vaccines in pregnant people is monitored through 3 systems: v-safe after vaccination health checker, the v-safe pregnancy registry and VAERS. From December 14, 2020 through February 28, 2021, 35,691 v-safe participants ages 16 to 54 identified as pregnant. Injection site pain was commonly reported. Of those, 3,958 enrolled in the v-safe pregnancy registry: 827 completed pregnancy; 712 (86.1%) had live births, with most vaccinations completed in the 3rd trimester. In the VAERS reports following mRNA vaccinations, 155 (70.1%) were nonpregnancy specific; 66 (29.9%) were pregnancy and neonatal specific events. The analysis of v-safe and VAERS data did not show any safety concerns among pregnant persons who received mRNA COVID-19 vaccines.
Chapin-Bardales J, Gee J, Myers T. Reactogenicity Following Receipt of mRNA-Based COVID-19 Vaccines JAMA Insights 2021 April 5. doi:10.1001/jama.2021.5374 Epub ahead of print.
CDC created v-safe, a smartphone-based tool, to monitor in near-real time the safety of COVID-19 vaccines authorized by FDA for emergency use. V-safe uses text messaging and web surveys to provide personalized health check-ins after COVID-19 vaccination. Researchers reviewed data collected from v-safe from December 14, 2020 to February 28, 2021, including side effects and reactions to the mRNA COVID-19 vaccines. Over 3.6 million v-safe participants completed at least one health check-in after the first dose and over 1.9 million after the second dose. Injection site pain was commonly reported after first (70%) and second doses (75%) of either mRNA vaccine. Systemic reactions, such as fatigue, headache, muscle pain, chills, fever, and joint pain were the top symptoms reported by participants after the first mRNA vaccine dose. These reports increased substantially after the second dose among both mRNA vaccines. People aged 65 years and older reported fewer reactions than younger people. While v-safe is voluntary and includes less than 10% of people vaccinated, reported reactions to the mRNA vaccines were consistent with results observed in clinical trials.
Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, Nair N, Martin S, Clark T, Markowitz L, Lindsey N, Zhang B, Licata C, Jazwa A, Sotir M, Shimabukuro T. First Month of COVID-19 Vaccine Safety Monitoring — United States, December 14, 2020-January 13, 2021 MMWR Morb Mortal Wkly Rep. 2021 Feb 26;70;283-288.
The U.S. FDA authorized two COVID-19 vaccines for emergency use in December 2020: Pfizer-BioNTech and Moderna. During clinical trials, there were reports of local reactions where the shot was given, and systemic reactions affecting other parts of the body. Safety monitoring for these vaccines has been the most intense and comprehensive in U.S. history. From December 14, 2020 through January 13, 2021, almost 14 million vaccine doses were distributed. During that time, over 1.6 million vaccine recipients enrolled in v-safe, and VAERS received 6,994 reports of adverse events following vaccination. About 91% of VAERS reports were non-serious; commonly reported symptoms included headache (22.4%), fatigue (16.5%) and dizziness (16.5%). V-safe enrollees reported similar local and systemic reactions. While deaths were reported to VAERS, available documentation did not suggest a causal link between the vaccine and death. Overall, no unusual or unexpected reporting patterns were detected.
Malden DE, Gee J, Glenn S, Li Z, Mercado C, Ogun OA, Kim S, Lewin BJ, Ackerson BK, Jazwa A, Weintraub ES, McNeil MM, Tartof S. Reactions following Pfizer-BioNTech COVID-19 mRNA vaccination and related healthcare encounters among 7,077 children aged 5-11 years within an integrated healthcare system . Vaccine. 2023 Jan 9; https://doi.org/10.1016/j.vaccine.2022.10.079. Online ahead of print.
Xu S, Huang R, Sy LS, Hong V, Glenn SC, Ryan DS, Morrissette K, Vazquez-Benitez G, Glanz JM, Klein NP, Fireman B, McClure D, Liles EG, Weintraub ES, Tseng HF, Qian L. A Safety Study Evaluating non-COVID-19 Mortality Risk Following COVID-19 Vaccination . Vaccine . 2022 Dec 20; https://doi.org/10.1016/j.vaccine.2022.12.036 Online ahead of print.
A study evaluating the risk of non-COVID-19 mortality after COVID-19 vaccination found that for each vaccine and across age, sex, and race/ethnicity groups, COVID-19 mortality rates were lower among those vaccinated compared to people who were unvaccinated. The study, published December 2022, used data from seven Vaccine Safety Datalink sites from December 14, 2020, through August 31, 2021. Three separate analyses were conducted for each of the three COVID-19 vaccines used in the United States.
Xu S, Huang R, Sy LS, Glenn SC, Ryan DS, Morrissette K, Shay DS, Vazquez-Benitez G, Glanz JM, Klein NP, McClure D, Liles EG, Weintraub ES, Tseng HF, Qian L. COVID-19 Vaccination and Non-COVID-19 Mortality Risk — Seven Integrated Health Care Organizations, United States, December 14, 2020-July 31, 2021 MMWR Morb Mortal Wkly Rep. epub 2021 Oct 22.
Since COVID-19 vaccinations have become available in December 2020, an estimated 182 million people in the United States were fully vaccinated against COVID-19 by September 21, 2021. However, since April 2021, the number of people starting to get COVID-19 vaccines has decreased. People have cited vaccine safety concerns as deterrents to getting a COVID-19 vaccine, concerns that include deaths following COVID-19 vaccination. Although deaths after COVID-19 vaccination have been reported to VAERS, there have been few studies done to evaluate the mortality not associated with COVID-19 among vaccinated and unvaccinated groups. To analyze this, researchers conducted a study using the Vaccine Safety Datalink, comparing those who received COVID-19 vaccines and those who did not between December 2020 through July 2021. This study included data from 11 million people; 6.4 million received either Pfizer-BioNTech, Moderna or Janssen COVID-19 vaccine and 4.6 were unvaccinated. The analysis showed that those who received COVID-19 vaccinations had lower rates of mortality for non-COVID-19 causes than those unvaccinated. These findings provide evidence that COVID-19 vaccines are safe and support current vaccination recommendations.
Cortese MM, Taylor AW, Akinbami LJ, Thames-Allen A, Yousaf AR, Campbell AP, Maloney SA, Harrington TA, Anyalechi EG, Munshi D, Kamidani S, Curtis CR, McCormick DW, Staat MA, Edwards KM, Creech CB, Museru O, Marquez P, Thompson D, Su JR, Schlaudecker EP, Broder KR. Surveillance For Multisystem Inflammatory Syndrome in US Children Aged 5-11 Years Who Received Pfizer-BioNTech COVID-19 Vaccine, November 2021 through March 2022 . J Infect Dis . 2023 Feb 23;jiad051. Online ahead of print.
Multisystem inflammatory syndrome in children (MIS-C) is a rare but serious condition associated with SARS-CoV-2 (the virus that causes COVID-19). MIS-C leads to inflammation of different body parts including the heart, lungs, kidneys, brain, skin, eyes, or gastrointestinal tract. In rare cases, MIS-C has been observed after COVID-19 vaccination. Reporting cases of MIS-C following COVID-19 vaccination is required as part of vaccine safety monitoring in the United States. This February 2023 publication, covering a period when about 7 million children received a COVID-19 vaccine, identified 58 children with MIS-C who also showed evidence of past or recent SARS-CoV-2 infection, along with 4 children with MIS-C who did not show evidence of past or recent SARS-CoV-2 infection. The data collected showed that MIS-C illness in children after receiving a COVID-19 vaccine is rare.
Haq K, Anyalechi EG, Schlaudecker EP, McKay R, Kamidani S, Manos C, Oster ME. Multiple MIS-C Readmissions and Giant Coronary Aneurysm After COVID-19 Illness and Vaccination: A Case Report . The Pediatric Infectious Disease Journal . 2022 Dec 16; DOI: 10.1097/INF.0000000000003801. Online ahead of print.
Multisystem inflammatory syndrome in children (MIS-C) rarely involves delayed giant coronary aneurysms, hospital readmissions, or symptom occurrence after COVID-19 vaccination. In this case report, the authors describe a child with all 3 of these unusual features. The authors discuss his clinical presentation and medical management, review the current literature, and review CDC guidance recommendations regarding further vaccinations. A 5-year-old boy began to show symptoms of MIS-C 55 days after COVID-19 illness and 15 days after receiving the first COVID-19 vaccine dose. This is the only reported case of a patient admitted to the hospital three times for MIS-C complications after COVID-19 vaccination. Whether the child’s MIS-C complications were related to his receiving a COVID-19 vaccine after having COVID-19 illness remains unknown. After consultation with the CDC-funded Clinical Immunization Safety Assessment Project, the patient’s care team decided against further COVID-19 vaccination until at least three months after normalization of inflammatory markers.
Yousaf AR, Cortese MM, Taylor AW, Broder KR, Oster ME, Wong JM, Guh AY, McCormick DW, Kamidani S, Schlaudecker EP, Edwards K, Creech CB, Staat MA, Belay ED, Marquez P, Su JR, Salzman MB, Thompson D, Campbell AP, MIS-C Investigation Authorship Group. Reported cases of multisystem inflammatory syndrome in children aged 12-20 years in the USA who received a COVID-19 vaccine, December, 2020, through August, 2021: a surveillance investigation . Lancet Child Adolesc Health . 2022 May;6(5):303-312. Epub 2022 Feb 23.
Multisystem inflammatory syndrome in children (MIS-C), a condition in children where the heart, liver, kidneys, or other organs of the body become inflamed, is a rare but serious complication of COVID-19 disease in people ages less than 21 years. Because this inflammatory illness occurs after COVID-19 infection, scientists wondered if this same type of inflammatory illness might occur after COVID-19 vaccination. In this report, published May 2022, investigators found that reported cases of MIS-C occurring at some point during the surveillance period after receiving a COVID-19 vaccine were rare; they identified 21 cases in people ages 12–20 years during a period when more than 21 million people these ages had received at least one vaccine dose. Most of the people who developed MIS-C also had laboratory evidence showing past or recent COVID-19 infection. Whether COVID-19 vaccination contributed in any way to them developing MIS-C remains unknown. CDC will continue to monitor reports of MIS-C and report findings, particularly in children and adolescents now authorized to receive the COVID-19 vaccine.
Belay ED, Godfred Cato S, Rao AK, Abrams J, Wilson WW, Lim S, Newton-Cheh C, Melgar M, DeCuir J, Webb B, Marquez P, Su JR, Meng L, Grome HN, Schlaudecker E, Talaat K, Edwards K, Barnett E, Campbell AP, Broder KR, Bamrah Morris S. Multisystem Inflammatory Syndrome in Adults after SARS-CoV-2 infection and COVID-19 vaccination . Clin Infect Dis. 2021 Nov 28;cia963. Online ahead of print.
Multisystem inflammatory syndrome in adults (MIS-A) is a rare but serious complication of COVID-19 disease. Because of the association with COVID-19 illness, MIS-A was included in the list of adverse events to monitor following COVID-19 vaccination. Researchers reviewed reports of MIS-A from December 14, 2020, to April 30, 2021. These MIS-A reports came from different sources, including treating clinicians, state health departments and as well as the Vaccine Adverse Event Reporting System (VAERS). During the analysis period, there were 20 patients who met the CDC case definition for MIS-A. All 20 patients had confirmed past or current COVID-19 infection. Most patients reported gastrointestinal and cardiac issues, low blood pressure, and shock. Seven patients received a COVID-19 vaccine before MIS-A onset, typically 10 days before MIS-A symptoms began; 3 patients received a second COVID-19 vaccine dose 4, 17, and 22 days before MIS-A onset. All vaccinated patients had underlying COVID-19 infection prior to MIS-A onset. MIS-A has not been reported following vaccination alone. Clinicians should report suspected MIS-A cases following COVID-19 vaccination to VAERS.
Goddard K, Hanson KE, Lewis N, Weintraub E, Fireman B, Klein NP. Incidence of Myocarditis/Pericarditis Following mRNA COVID-19 Vaccination Among Children and Younger Adults in the United States. . Annals of Internal Medicine. 2022 Oct 4. doi.org/10.7326/M22-2274.
Vaccine safety monitoring systems have reported cases of myocarditis and pericarditis after mRNA-based COVID-19 vaccination, especially among younger males 0 to 7 days after receiving the second vaccine dose. Using data from the Vaccine Safety Datalink, a population-based surveillance study was conducted on pre-specified outcomes after COVID-19 vaccination among people ages 5 to 39 years. Researchers identified potential cases of myocarditis and pericarditis in emergency department and inpatient settings 1 to 98 days after vaccination and validated initial findings by reviewing medical records. Results found that during December 14, 2020, through May 31, 2022 (among people ages 18–39 years), and through August 20, 2022 (among people ages 5–17 years), there were 320 potential cases of myocarditis or pericarditis after 6,992,340 people were vaccinated. Of 320 potential cases, 224 cases were verified, with 137 cases of myocarditis or pericarditis occurring 0 to 7 days after vaccination: 18 cases after the first dose and 119 cases after the second dose. Adolescent males were shown to have higher incidence of myocarditis and pericarditis. Given the known risk of complications after COVID-19 disease (including myocarditis), the findings of this study support that the benefits of mRNA vaccination outweigh the risks.
Kracalik I, Oster ME, Broder KR, Cortese MM, Glover M, Shields K, Creech CB, Romanson B, Novosad S, Soslow J, Walter EB, Marquez P, Dendy JM, Woo J, Valderrama AL, Ramirez-Cardenas A, Assefa A, Campbell MJ, Su JR, Magill SS, Shay DK, Shimabukuro TT, Basavaraju SV. Outcomes at least 90 days onset of myocarditis after mRNA COVID-19 vaccination in adolescents and young adults in the USA: a follow-up surveillance study . Lancet Child Adolesc Health . 2022 Nov 6;6(11):788-798. Epub 2022 Sept 22.
CDC collected data on individuals with myocarditis after mRNA COVID-19 vaccination through follow-up surveys of people ages 12–29 years for whom a report of myocarditis after mRNA COVID-19 vaccination was made to the Vaccine Adverse Event Reporting System (VAERS) during December 2020 through November 2021. Clinical outcomes and quality of life at least 90 days since onset of myocarditis after mRNA COVID-19 vaccination in adolescents and young adults were assessed. This study, published November 2022, found that approximately 80% of patients diagnosed with myocarditis after receiving an mRNA COVID-19 vaccine were considered recovered by healthcare providers at least 90 days after the onset of myocarditis. CDC is continuing to follow up with patients who have not been considered recovered since myocarditis symptom onset to better understand longer-term outcomes.
Goddard K, Lewis N, Fireman B, Weintraub E, Shimabukuro T, Zerbo O, Boyce TG, Oster ME, Hanson KE, Donahue JG, Ross P, Naleway A, Nelson JC, Lewin B, Glanz JM, Williams JTB, Kharbanda EO, Yih WK, Klein NP. Risk of myocarditis and pericarditis following BNT162b2 and mRNA-1273 COVID-19 vaccination . Vaccine 2022 Aug 19; 40(35):5153-5159. Epub 2022 Jul 12.
Evidence indicates that mRNA COVID-19 vaccination is associated with the risk of myocarditis and possibly pericarditis, especially among adolescent and young adult males. It is unclear if risk differs between mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech). This study, published August 2022, reviewed health records among a diverse population to see if there is a clear difference in risk of myocarditis associated with receiving an mRNA-1273 versus BNT162b2 vaccine. During December 14, 2020, through January 15, 2022, 41 cases of myocarditis and pericarditis were reported after 2,891,498 doses of BNT162b2, and 38 cases of myocarditis and pericarditis were reported after 1,803,267 doses of mRNA-1273. Cases had similar demographic and clinical characteristics. In most cases, patients were hospitalized for one day or less; none required intensive care. Risk of myocarditis and pericarditis was higher after mRNA-1273 vaccine than after BNT162b2 vaccine during the 0–7 days after receiving either vaccine. Both vaccines were associated with increased risk of myocarditis and pericarditis among young males ages 18–39 years.
Weintraub ES, Oster ME, Klein NP. Myocarditis or Pericarditis Following mRNA COVID-19 Vaccination . JAMA 2022 Jun 24; 5(6):e2218512. doi:10.1001/jamanetworkopen.2022.18512
This commentary, published June 2022, discusses a study presenting evidence that a longer time interval between dose 1 and dose 2 of an mRNA COVID-19 vaccine might lower the risk of myocarditis or pericarditis. The commentary also includes data from the Vaccine Safety Datalink, including reported rates of myocarditis or pericarditis after receiving an mRNA COVID-19 vaccine (Moderna or Pfizer-BioNTech). Reported rates of myocarditis or pericarditis were higher after receipt of the Moderna vaccine than the Pfizer-BioNTech vaccine and were higher following dose 2. Vaccine safety monitoring has been ongoing globally, and the risk of myocarditis appears highest among adolescents and young adult males following dose 2 of the primary series. However, the risk of myocarditis after COVID-19 disease remains greater than after COVID-19 vaccination, which remains the most effective way to prevent serious complications from COVID-19 infection.
Block JP, Boehmer TK, Forrest CB, Carton TW, Lee GM, Ajani UA, Christakis DA, Cowell LG, Draper C, Ghildayal N, Harris AM, Kappelman MD, Ko JY, Mayer KH, Nagavedu K, Oster ME, Paranjape A, Puro J, Ritchey MD, Shay DK, Thacker D, Gundlapalli AV. Cardiac Complications After SARS-CoV-2 Infection and mRNA COVID-19 Vaccination — PCORnet, United States, January 2021–January 2022 . MMWR Morb Mortal Wkly Rep. 2022 Apr 8; 71(14);517-523.
Data from 40 U.S. healthcare systems participating in PCORnet, the National Patient-Centered Research Network, were analyzed to identify people ages 5 years and older who developed heart complications after COVID-19 disease or after getting an mRNA COVID-19 vaccination during January 1, 2021, through January 31, 2022. The risk for heart complications after mRNA COVID-19 vaccination was highest among teen boys ages 12–17 years and young men ages 18–29 years after getting the second dose of vaccine. However, the risk of heart complications was higher after COVID-19 disease than after a second dose of vaccine—specifically, 2 to 6 times as high for teen boys and 7 to 8 times as high for young men. These findings support the continued use of recommended mRNA COVID-19 vaccines among all eligible people ages 5 years and older.
Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, Edwards K, Soslow JH, Dendy JM, Schlaudecker E, Lang SM, Barnett ED, Ruberg FL, Smith MJ, Campbell MJ, Lopes RD, Sperling LS, Baumblatt JA, Thompson DL, Marquez PL, Strid P, Woo J, Pugsley R, Reagan-Steiner S, DeStefano F, Shimabukuro TT. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021 . JAMA 2022 Jan 25; 327(4):331-34. Online ahead of print.
A review of vaccine safety data reported during December 2020–August 2021 found that there was a small but increased risk for myocarditis, or inflammation of the heart muscle, following mRNA COVID-19 vaccination (Pfizer-BioNTech and Moderna). After a review of reports submitted to the Vaccine Adverse Event Reporting System, scientists found that the risk of myocarditis was highest following receipt of the second vaccine dose among adolescent and young adult males. This risk should be considered within the context of the significant benefits of COVID-19 vaccination in preventing COVID-19 infection and potential serious complications from COVID-19. The benefits of COVID-19 vaccination continue to outweigh any potential risks, including myocarditis.
Paddock CD, Reagan-Steiner S, Su JR, Oster ME, Martines RB, Bhatnagar J, Shimabukuro TT. Autopsy Histopathologic Cardiac Findings in Two Adolescents Following the Second COVID-19 Vaccine Dose . Arch Pathol Lab Med 2022 Apr 11. Doi: 10.5858/arpa.2022-0084-LE. Online ahead of print.
This letter to the editor, published April 2022, responds to a report describing an investigation of autopsy findings for two adolescents who died after COVID-19 vaccination. The letter to the editor points out that the authors of the original manuscript omitted key findings by CDC. Investigation by CDC found evidence that death was related to C septicum sepsis. C septicum sepsis is a fatal infection that can present with non-specific signs and symptoms. C septicum sepsis is lethal in 60–70% of cases, and death typically occurs within 12–48 hours of symptoms. Information reported to the Vaccine Adverse Event Reporting System showed that the patients described flu-like symptoms for two days before death. The original investigation, by omitting CDC’s additional findings, could be interpreted as evidence that the COVID-19 vaccine was the cause of death.
Oster ME, Shay DK, Su JR, Gee J, Creech B, Broder KR, Edwards K, Soslow JH, Dendy JM, Schlaudecker E, Lang SM, Barnett ED, Ruberg FL, Smith MJ, Campbell MJ, Lopes RD, Sperling LS, Baumblatt JA, Thompson DL, Marquez PL, Strid P, Woo J, Puglsey R, Reagan-Steiner S, DeStefano F, Shimabukuro TT. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US from December 2020 to August 2021 JAMA. 2022 Jan 18;327(4):331-340. Online ahead of print.
Since mRNA-based COVID-19 vaccines were authorized for emergency use in December 2020, there have been reports of myocarditis, or inflammation of the heart muscle, following vaccination. To see if there was an association between mRNA COVID-19 vaccination and myocarditis, researchers reviewed reports submitted to the Vaccine Adverse Event Reporting Systems (VAERS) from December 2020 through August 31, 2021. In that time, more than 192 million people ages 12 years and older have received at least one dose of mRNA COVID-19 vaccines. From this population, VAERS received 1,626 myocarditis reports that met case definition. The review found the rates myocarditis were highest following the second dose of mRNA vaccine among adolescent and young adult males. Myocarditis is a rare but serious adverse event that can occur following mRNA COVID-19 vaccination. The benefits of COVID-19 vaccination continue to outweigh any potential risks, including myocarditis.
Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, Broder KR, Gee J, Weintraub E, Shimabukuro T, Scobie HM, Moulia D, Markowitz LE, Wharton M, McNally VV, Romero JR, Keipp Talbot H, Lee GM, Daley MF, Oliver SE. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices — United States, June 2021 MMWR Morb Mortal Wkly Rep. 2021 Jul 9;70:977-982.
Two mRNA COVID-19 vaccines were given emergency use authorization (EUA) by the Food and Drug Administration (FDA) in December 2020: Pfizer-BioNTech and Moderna COVID-19 vaccines. Pfizer-BioNTech was authorized for individuals 16 years and older, and Moderna for adults 18 years and older. In May 2021, FDA expanded Pfizer-BioNTech vaccine’s authorization to include adolescents aged 12 to 15 years. After reported myocarditis/pericarditis among mRNA vaccine recipients, mostly in younger males after the 2nd dose, the Advisory Committee on Immunization Practices (ACIP) held a meeting to review these reports and conduct a risk-benefit assessment of mRNA COVID-19 vaccination in the U.S. Evidence presented showed that the highest rates of myocarditis were reported in males aged 12-17 and 18-24 (62.8 and 50.5 reported cases of myocarditis per million 2nd mRNA doses administered, respectively). On June 23, after reviewing all the available information, ACIP determined that the benefits of mRNA COVID-19 vaccination under EUA outweighed the risks of myocarditis in all populations. CDC and FDA will continue to monitor cases of myocarditis among mRNA COVID-19 vaccine recipients.
Shay DK, Shimabukuro, TT, DeStefano F. Myocarditis Occurring After Immunization with mRNA-Based COVID-19 Vaccines: Editorial . JAMA Cardiol. Published online June 29, 2021. doi:10.1001/jamacardio.2021.2821.
CDC researchers reviewed several case reports of acute myocarditis occurring in people following mRNA-based COVID-19 vaccinations (Pfizer BioNTech or Moderna). The first report included 4 cases of myocarditis developed 1 to 5 days after getting dose 2 of mRNA-based COVID-19 vaccine. Second report included 23 cases of acute myocarditis within 4 days of vaccination, mostly after dose 2. The last report included 7 cases in adolescents, ages 14-19. All presented with myocarditis or myopericarditis (heart muscle and lining inflammation) within 4 days of dose 2. The review of these cases showed clinical similarities and there were no other known causes for their acute myocarditis, suggesting a likely association with vaccination. Myocarditis following COVID-19 vaccination is rare. Researchers will continue to look into myocarditis following COVID-19 vaccination.
Goddard K, Donahue JG, Lewis N, Hanson KE, Weintraub ES, Fireman B, Klein NP. Safety of COVID-19 mRNA Vaccination Among Young Children in the Vaccine Safety Datalink . Pediatrics . 2023 Jun 6. https://doi.org/10.1542/peds.2023-061894
This June 2023 publication shares results of an examination of medical records of 120,006 children aged 6 months to 5 years within the Vaccine Safety Datalink population who received at least one dose of an mRNA (Pfizer-BioNTech or Moderna) COVID-19 vaccine from June 18, 2022–January 28, 2023, for 23 serious potential health outcomes, including blood clots, seizures, stroke, and brain inflammation. Analyses showed no safety concern for any of the selected serious outcomes. In particular, the evaluation found no concern for seizures after vaccination, something occasionally seen following other routine childhood immunizations in children under 2 years old. Safety monitoring of nearly a quarter million monovalent COVID-19 mRNA vaccine doses given to children aged 6 months to 5 years over a 7-month period showed no increased occurrence of serious negative health outcomes, including no cases of myocarditis (inflammation of the heart muscle) or pericarditis (inflammation of the lining of the heart). The examination’s findings reinforce the safety of COVID-19 vaccination of young children.
Goddard K, Hanson KE, Lewis N, Weintraub E, Fireman B, Klein NP. Incidence of Myocarditis/Pericarditis Following mRNA COVID-19 Vaccination Among Children and Younger Adults in the United States . Annals of Internal Medicine. 2022 Oct 4. doi.org/10.7326/M22-2274.
Hause AM, Marquez P, Zhang B, Myers TR, Gee J, Su JR, Parker C, Thompson D, Panchanathan SS, Shimabukuro TT, Shay DK. COVID-19 mRNA Vaccine Safety Among Children Aged 6 Months–5 Years — United States, June 18, 2022–August 21, 2022 . MMWR Morb Mortal Wkly Rep. 2022 Sep 2;71(35);1115-1120.
This study, published September 2022, found that among children ages 6 months to 5 years who received mRNA COVID-19 vaccines, local and systemic reactions were mostly mild or moderate. Serious reports of adverse events were rare. Safety data from the Vaccine Adverse Event Reporting System and V-safe were used to look at reported reactions in this age group. Most reported reactions—such as injection site pain, irritability, crying, and sleepiness—were consistent with those observed during the vaccines’ preauthorization clinical trials. This study reinforces the safety profile of mRNA COVID-19 vaccination among children in this age group.
Hause AM, Baggs J, Marquez P, Abara WE, Baumblatt JG, Thompson D, Su JR, Myers TR, Gee J, Shimabukuro TT, Shay DK. Safety Monitoring of Pfizer-BioNTech COVID-19 Vaccine Booster Doses Among Children Aged 5–11 Years — United States, May 17–July 31, 2022 . MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33);1047–1051.
A CDC study published August 2022 found that among children ages 5–11 years who received a booster dose of Pfizer-BioNTech’s mRNA COVID-19 vaccine (third dose administered ≥5 months after the second dose), serious reports of adverse events were rare. Data from V-safe and the Vaccine Adverse Event Reporting System were used to look at adverse events reported in this age group. Data showed children that received a third dose had similar adverse events to those reported after receiving a first or second dose. Most reported adverse events—such as injection site pain and headache—were considered mild. The findings are consistent with those observed during the vaccine’s clinical trial and reinforces the safety of mRNA COVID-19 booster dose vaccination among children ages 5–11 years.
Hause AM, Baggs J, Marquez P, Abara WE, Baumblatt JG, Thompson D, Su JR, Myers TR, Gee J, Shimabukuro TT, Shay DK. Safety Monitoring of COVID-19 mRNA Vaccine First Booster Doses Among Persons Aged ≥12 Years with Presumed Immunocompromise Status — United States, January 12, 2022–March 28, 2022 . MMWR Morb Mortal Wkly Rep. 2022 Jul 15; 71(28);899–903.
Immunocompromised people are at risk for severe COVID-19 disease, and additional doses of COVID-19 vaccine are recommended for this population. To characterize the safety of first booster doses among immunocompromised persons ages 12 years and older, CDC reviewed adverse events (AEs) reported to V-safe and the Vaccine Adverse Event Reporting System (VAERS) during the week after receipt of an mRNA COVID-19 first booster dose (fourth dose administered ≥ 3 months after the third) during January 12, 2022–March 28, 2022. Safety data identified no unusual or unexpected patterns of AEs. Mild to moderate reactions, such as injection site pain, fatigue, headache, and muscle pain following a booster dose were similar to those among non-immunocompromised people. Local and systemic reactions were less common following dose 4 compared to dose 3. These findings support evidence that mRNA COVID-19 vaccines are safe for immunocompromised people.
Fleming-Dutra KE, Wallace M, Moulia DL, Twentyman E, Roper LE, Hall E, Link-Gelles R, Godfrey M, Woodworth KR, Anderson TC, Rubis AB, Shanley E III, Jones JM, Morgan RL, Brooks O, Talbot HK, Lee GM, Bell BP, Daley M, Meyer S, Oliver SE. Interim Recommendations of the Advisory Committee on Immunization Practices for Use of Moderna and Pfizer-BioNTech COVID-19 Vaccines in Children Aged 6 Months–5 Years — United States, June 2022 . MMWR Morb Mortal Wkly Rep. 2022 Jul 1; 71(26);859–868.
Vaccination remains the best protection against COVID-19-related hospitalization and death. On June 17, 2022, the U.S. Food and Drug Administration (FDA) granted Emergency Use Authorization for the Moderna COVID-19 vaccine for children ages 6 months–5 years and for the Pfizer-BioNTech COVID-19 vaccine for children ages 6 months–4 years. The Advisory Committee on Immunization Practices (ACIP) determined that the benefits of vaccination for these age groups outweigh the risks. To guide recommendations on the use of vaccines, ACIP used the Evidence to Recommendation Framework. The framework considered the importance of COVID-19 as a public health problem, the benefits and risks of using each vaccine, and parents’ values regarding the use of vaccines in this age group. Studies for each vaccine were conducted as a randomized double-blind study. In both studies, participants in this age group received either two doses of the vaccine (Moderna or Pfizer-BioNTech) or saline placebo. Results showed that both vaccines are safe and effective to prevent severe COVID-19 illness in this age group.
Hause AM, Shay DK, Klein NP, Abara WE, Baggs J, Cortese MM, Fireman B, Gee J, Glanz JM, Goddard K, Hanson KE, Hugueley B, Kenigsberg T, Kharbanda EO, Lewin B, Lewis N, Marquez P, Myers T, Naleway A, Nelson JC, Su JR, Thompson D, Olubajo B, Oster ME, Weintraub ES, Williams JTB, Yousaf AR, Zerbo O, Zhang B, Shimabukuro TT. Safety of COVID-19 Vaccination in United States Children Ages 5 to 11 Years . Pediatrics . 2022 Jul 14. https://doi.org/10.1542/peds.2022-057313.
This study, published July 2022, reviewed adverse events observed following the Pfizer two-dose vaccine administered to children ages 5–11 years and found mild-to-moderate events within the first day or two of vaccination. Researchers analyzed data from three U.S. safety monitoring systems during four months of vaccine administration among children ages 5–11 years to provide insight on adverse events. Among 48,795 children ages 5–11 years enrolled in V-safe—a web-based tool that uses text messages, emails, and web surveys to provide personalized health check-ins for people after receiving a new vaccine—most reported events were mild to moderate, were most frequently reported the day after vaccination, and were more common after the second dose. The most common events reported were injection site pain, fatigue, headache, fever, and muscle soreness. The study also evaluated data from the Vaccine Adverse Events Reporting System (VAERS), the national spontaneous reporting system co-managed by CDC and the U.S. Food and Drug Administration, and the Vaccine Safety Datalink (VSD), an active surveillance system that monitors electronic health records for pre-specified events including myocarditis. VAERS received 7,578 adverse event reports; 97% were non-serious. Reviewing 194 serious VAERS reports, 15 myocarditis cases were verified; 8 occurred in males after dose 2. In VSD, no safety signals were detected in weekly sequential monitoring after administration of 726,820 doses. The authors concluded that the vaccine is safe for children ages 5–11 years and that adverse events are usually clinically mild and resolve quickly.
Paddock CD, Reagan-Steiner S, Su JR, Oster ME, Martines RB, Bhatnagar J, Shimabukuro TT. Autopsy Histopathologic Cardiac Findings in 2 Adolescents Following the Second COVID-19 Vaccine Dose . Arch Pathol Lab Med. 2022 Apr 8; 146 (8): 921–923.
Hause AM, Baggs J, Marquez P, Abara WE, Olubajo B, Myers TR, Su JR, Thompson D, Gee J, Shimabukuro TT, Shay DK. Safety Monitoring of COVID-19 Vaccine Booster Doses Among Persons Aged 12-17 Years — United States, December 9, 2021-February 20, 2022 . MMWR Morb Mortal Wkly Rep . 2022 Mar 4;71(9):347-351.
CDC reviewed safety data for a third dose of Pfizer-BioNTech COVID-19 vaccine administered ≥ 5 months after the second dose among adolescents ages 12–17 years. This review, published March 2022, identified no unusual or unexpected patterns of adverse events. Data from V-safe and the Vaccine Adverse Event Reporting System were used to characterize adverse events reported among this age group. Reactions such as injection site pain, fatigue, headache, and muscle pain following dose 3 vaccinations were mostly mild to moderate in severity and were most frequently reported the day after vaccination. Myocarditis was less frequently reported following a third dose than a second dose. Parents should be advised that local and systemic reactions are expected among adolescents following Pfizer-BioNTech vaccine dose 3 and that serious adverse events, including myocarditis, are rare.
Yousaf AR, Cortese MM, Taylor AW, Broder KR, Oster ME, Wong JM, Guh AY, McCormick DW, Kamidani S, Schlaudecker EP, Edwards K, Creech CB, Staat MA, Belay ED, Marquez P, Su JR, Salzman MB, Thompson D, Campbell AP, MIS-C Investigation Authorship Group. Reported cases of multisystem inflammatory syndrome in children aged 12-20 years in the USA who received a COVID-19 vaccine, December, 2020, through August, 2021: a surveillance investigation . Lancet Child Adolesc Health . 2022 May;6(5):303-312. Epub 2022 Feb 23.
DeSilva MB, Haapal J, Vazquez-Benitez G, Daley MF, Nordin JD, Klein NP, Henninger ML, Williams JTB, Hambidge SJ, Jackson ML, Donahue JG, Qian L, Lindley MC, Gee J, Weintraub ES, Kharbanda EO. Association of the COVID-19 Pandemic with Routine Childhood Vaccination Rates and Proportion Up to Date with Vaccinations Across 8 US Health Systems in the Vaccine Safety Datalink . JAMA Pediatr. 2022 Jan 1;176(1):68-77. Doi: 10.1001/jamapediatrics.2021.4251.
Routine vaccinations in the United States and globally have been affected by the COVID-19 pandemic. This study, published January 2022, used data from eight health systems in California, Oregon, Washington, Colorado, Minnesota, and Wisconsin in the Vaccine Safety Datalink to compare trends in pediatric vaccination before and during the pandemic and to evaluate the proportion of children who were up to date with routine vaccinations in February, May, and September 2020. Children from age groups younger than 24 months and ages 4–6, 11–13, and 16–18 years were included if they had at least 1 week of health system enrollment from January 5, 2020, through October 3, 2020. Results show that as of September 2020, childhood vaccination rates and proportion of children who were up to date on routine vaccinations remained lower than 2019 levels and that intervention measures must be made to promote catch-up vaccinations.
Hause AM, Baggs J, Marquez P, Myers TR, Gee J, Su JR, Zhang B, Thompson D, Shimabukuro TT, Shay DK. COVID-19 Vaccine Safety in Children Ages 5-11 years — United States, November 3-December 19, 2021 . MMWR Morb Mort Wkly Rep. 2021 Dec 31:70(5152);1755-1760.
On October 29, 2021, the FDA expanded emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine to include children ages 5-11 years. Once the EUA was in place, researchers began reviewing vaccine safety data for this age group, collected through the Vaccine Adverse Event Reporting System (VAERS) and v-safe. From November 3 through December 19, 2021, around 8.7 million doses of Pfizer vaccine were administered to children ages 5-11 years. During that time, VAERS received 4,249 reports of adverse events following vaccination for children in that age group, 98% of which were non-serious. There were 11 verified cases of myocarditis. Of the over 42,000 children enrolled in v-safe, 70% recorded a second dose. Local reactions (symptoms around the injection site) and systemic reactions (fever, headache, fatigue) following dose 2 of Pfizer vaccination among this age group were reported less frequently than reactions reported among children ages 12-15 years. The initial safety findings showed no unusual patterns of adverse events and that the benefits of COVID-19 vaccination continue to outweigh the risks. CDC and FDA will continue to monitor COVID-19 vaccine safety, communicate findings, and use vaccine safety data to inform vaccination recommendations.
Hause AM, Gee J, Baggs J, Abara WE, Marquez P, Thompson D, Su JR, Licata C, Rosenblum HG, Myers TR, Shimabukuro TT, Shay DK. COVID-19 Vaccine Safety in Adolescents—United States, December 14, 2020—July 16, 2021 . MMWR Morb Mortal Wkly Rep. 2021 Jul 30.
As of July 2021, Pfizer-BioNTech COVID-19 Vaccine (Pfizer-BioNTech) is the only COVID-19 vaccine authorized for use in adolescents (people aged 12–17 years). To evaluate the safety of Pfizer-BioNTech in adolescents, researchers reviewed data collected from VAERS and v-safe between December 14, 2020 through July 16, 2021. Over 8.9 million Pfizer-BioNTech doses were administered to adolescents ages 12-17. VAERS received 9,246 reports of adverse events in adolescents; over 90% of reports were non-serious. Myocarditis was reported in 4.3% (397) of all VAERS reports. Of the 129,000 adolescents who enrolled in v-safe, the most frequently reported side effects included injection site pain, fatigue, headache, and weakness. With the exception of myocarditis, the safety findings were similar to what was observed during preauthorization trials. CDC and FDA are actively monitoring the safety of COVID-19 vaccines. Serious adverse events after COVID-19 vaccination are rare, and CDC continues to recommend everyone 12 years and older get vaccinated as soon as possible to help protect against COVID-19.
Madni SA, Sharma AJ, Zauche LH, Waters AV, Nahabedian 3rd JF, Johnson T, Olson CK, CDC COVID-19 Vaccine Pregnancy Registry Work Group. CDC COVID-19 Vaccine Pregnancy Registry: Design, data collection, response rates, and cohort description . Vaccine . 2023 Dec 5:S0264-410X(23)01423-8. Doi:10.1016/j.vaccine.2023.11.061. Online ahead of print.
CDC developed and implemented the CDC COVID-19 Vaccine Pregnancy Registry to monitor vaccine safety among pregnant people who received COVID-19 vaccination. Potential participants who received a COVID-19 vaccine during or a month prior to their pregnancy were eligible to participate in the registry, which monitored health outcomes of participants and their infants through phone interviews and review of available medical records. Data for select outcomes, including birth defects, were reviewed by clinicians. In certain cases, medical records were used to confirm and add detail to participant-reported health conditions. Additionally, the authors describe the development and implementation for each data collection aspect of the registry (that is, participant phone interviews, clinical review, and medical record abstraction), data management, and strengths and limitations. The registry continues to provide important information about the safety of COVID-19 vaccination among pregnant people, a population with higher risk of poor outcomes from COVID-19 and who were excluded from the vaccine’s pre-authorization clinical trials. Lessons learned from the registry may guide development and implementation of future vaccine safety monitoring efforts.
Kharbanda EO, Haapala J, Lipkind HS, DeSilva MB, Zhu J, Vesco KK, Daley MF, Donahue JG, Getahun D, Hambidge SJ, Irving SA, Klein NP, Nelson JC, Weintraub ES, Williams JTB, Vazquez-Benitez G. COVID-19 Booster Vaccination in Early Pregnancy and Surveillance for Spontaneous Abortion . JAMA Network Open. 2023 May 19;6(5):e2314350.
This evaluation, published May 2023, found no increased risk of miscarriage among pregnant people who received a monovalent COVID-19 vaccine during early pregnancy. Researchers compared different methods for evaluating associations between monovalent COVID-19 vaccination during early pregnancy and miscarriages. In all analytical approaches, no increased risk for miscarriage was found following COVID-19 vaccination during pregnancy. These findings reinforce the safety of COVID-19 vaccination during pregnancy.
Vazquez-Benitez G, Haapala J, Lipkind HS, DeSilva MB, Zhu J, Daley MF, Getahun D, Klein NP, Vesco KK, Irving SA, Nelson JC, Williams JTB, Hambidge SJ, Donahue J, Fuller CC, Weintraub ES, Olson C, Kharbanda EO. COVID-19 Vaccine Safety Surveillance in Early Pregnancy in the United States: Design Factors Affecting the Association Between Vaccine and Spontaneous Abortion . American Journal of Epidemiology. 2023 Mar 16;kwad059. Online ahead of print.
This evaluation, published August 2023, found no increased risk of miscarriage among pregnant people who received a monovalent COVID-19 vaccine during early pregnancy. Researchers compared different approaches for evaluating associations between monovalent COVID-19 vaccination during early pregnancy and miscarriages, including number of days between COVID-19 vaccination and miscarriage. In all analytical approaches, no increased risk for miscarriage was found following COVID-19 vaccination during pregnancy. These findings reinforce the safety of COVID-19 vaccination during pregnancy.
Moro PL, Olson CK, Zhang B, Marquez P, Strid P. Safety of Booster Doses of Coronavirus Disease 2019 (COVID-19) Vaccine in Pregnancy in the Vaccine Adverse Event Reporting System . Obstet Gynecol. 2022 Sept 1; 40(3):421-427.
A review of reports to the Vaccine Adverse Event Reporting System (VAERS) showed that adverse events (AEs) reported after booster dose mRNA COVID-19 vaccination among pregnant people were similar to AEs reported after primary series mRNA COVID-19 vaccination among pregnant people. During September 22, 2021, through March 24, 2022, VAERS received 323 reports of AEs among pregnant people who received the Pfizer-BioNTech or the Moderna COVID-19 booster dose. The most common pregnancy-specific AE reported after receipt of a booster dose was miscarriage, which is relatively common during pregnancy. This study, published September 2022, did not identify any new or unexpected AEs after receipt of a booster dose of mRNA COVID-19 vaccine among pregnant people.
Irving SA, Crane B, Weintraub E, Kauffman TL, Brooks N, Patel SA, Razzaghi H, Belongia EA, Daley MF, Getahun D, Glenn SC, Hambidge SJ, Jackso LA, Kharbanda E, Klein NP, Zerbo O, Naleway AL. Influenza Vaccination Among Pregnant People Before and During the Coronavirus Disease 2019 (COVID-19) Pandemic . Obstet Gynecol . 2023 Sept 1; 142(3):636-639. Online ahead of print.
Using data from the Vaccine Safety Datalink (VSD), this evaluation, published September 2023, focused on flu vaccination coverage during the 2016-2017 through the 2021-2022 flu seasons among pregnant people aged 18-49 years. Flu vaccination increased from the 2016-2017 season to the 2019-2020 season. After declaration of the COVID-19 pandemic, flu vaccination decreased during the 2021-2022 season. In each season, flu vaccination was lowest among pregnant people in this age group and among non-Hispanic Black pregnant people. These decreases show the need for ongoing efforts to reverse the decline in flu vaccination coverage among pregnant people.
DeSilva MB, Haapla J, Vazquez-Benitez G, Boyce TG, Fuller CC, Daley MF, Getahun D, Hambidge SJ, Lipkind HS, Naleway AL, Nelson JC, Vesco KK, Weintraub ES, Williams JTB, Zerbo O, Kharbanda EO. Medically Attended Acute Adverse Events in Pregnant People After Coronavirus Disease 2019 (COVID-19) Booster Vaccination . Obstet Gynecol . 2023 Jul 1;142(1):125-129.
This publication describes an evaluation that looked at more than 80,000 pregnant people who received a monovalent mRNA COVID-19 booster vaccine during their pregnancy to see if there was an association with adverse events. The data showed no increased risk for thrombocytopenia (low platelet counts), myocarditis (inflammation of the heart muscle), venous thromboembolism (a blood clot that blocks the flow of blood through the veins), ischemic stroke (occurring when there is a lack of blood flow to the brain), or other serious events among pregnant people who received a COVID-19 monovalent mRNA booster dose.
DeSilva M, Haapala J, Vazquez-Benitez G, Vesco KK, Daley MF, Getahun D, Zerbo O, Naleway A, Nelson JC, Williams JTB, Hambidge SJ, Boyce TG, Fuller CC, Lipkind HS, Weintraub E, McNeil MM, Kharbanda EO. Evaluation of Acute Adverse Events after Covid-19 Vaccination during Pregnancy . N Engl J Med. . 2022 Jun 22. DOI: 10.1056/NEJMc2205276. Epub ahead of print.
Pregnant people with COVID-19 symptoms have a higher risk of adverse outcomes than people who are not pregnant. A retrospective study published June 2022 and focusing on pregnant people ages 16–49 years who were either vaccinated or unvaccinated between December 15, 2020, and July 1, 2021, was conducted to show the safety of COVID-19 vaccines and the occurrence of adverse events (AEs). There were 45,232 pregnant people identified in the study who received one or two doses of a COVID-19 vaccine. Less than 1% of pregnant people had to be hospitalized for AEs. There were no serious AEs that occurred more frequently among vaccinated versus unvaccinated pregnant people, showing that the COVID-19 vaccine is not linked to serious AEs in this population.
Razzaghi H, Meghani M, Crane B, Ellington S, Naleway AL, Irving SA, Patel SA. Receipt of COVID-19 Booster Dose Among Fully Vaccinated Pregnant Individuals Aged 18 to 49 Years by Key Demographics . JAMA . 2022 Apr 22; .2354-2351 (23) 327 doi:10.1001/jama.2022.6834.
Data from the Vaccine Safety Datalink showed that fewer than half of pregnant people who were up to date with their COVID-19 vaccines received a COVID-19 booster dose by February 2022. Researchers assessed vaccination trends over time among pregnant people ages 18–49 years beginning the week of August 13, 2021, when additional vaccine doses were authorized, through the week ending February 26, 2022. Out of 71,745 people who were pregnant during the study period, 49,072 were fully vaccinated, with 25,321 of those having received a booster dose. Receipt of a booster dose was highest among pregnant people ages 35–49 years, Asian people, and non-Hispanic White people. Booster dose rates were lower among pregnant people ages 18–24 years, non-Hispanic Black people, and Hispanic people. These findings can help inform methods to increase booster dose vaccinations.
Moro PL, Olson CK, Clark E, Marquez P, Strid P, Ellington S, Zhang B, Mba-Jonas A, Alimchandani M, Cragan J, Moore C. Post-authorization surveillance of adverse events following COVID-19 vaccines in pregnant persons in the Vaccine Adverse Event Reporting System (VAERS), December 2020-October 2021 . Vaccine . 2022 May 26;40(24):3389-3394. Epub 2022 Apr 12.
A CDC study published May 2022 and looking at more than 10 months of vaccine safety data from people who were pregnant and received a COVID-19 vaccine found no concerning patterns of health problems related to vaccination. These findings add to a growing body of evidence that COVID-19 vaccines are safe for people who are pregnant. These findings also reaffirm CDC’s recommendation for pregnant people to get vaccinated to protect themselves and their babies from severe COVID-19 illness.
During December 14, 2020, through October 31, 2021, CDC scientists analyzed more than 3,000 reports that were submitted to the Vaccine Adverse Event Reporting System (VAERS), a passive vaccine safety monitoring system co-managed by CDC and the U.S. Food and Drug Administration (FDA). The study evaluated and summarized reports to VAERS about people who were pregnant and received a COVID-19 vaccine to assess for potential safety problems with the vaccines. Scientists found no concerning patterns of negative outcomes among people who were pregnant and vaccinated or among their babies.
Lipkind HS, Vazquez-Benitez G, DeSilva M, Vesco KK, Ackerman-Banks C, Zhu J, Boyce TG, Daley MF, Fuller CC, Getahun D, Irving SA, Jackson LA, Williams JTB, Zerbo O, McNeil MM, Olson CK, Weintraub E, Kharbanda KO. Receipt of COVID-19 Vaccine During Pregnancy and Preterm or Small-for-Gestational-Age at Birth — Eight Integrated Health Care Organizations, United States, December 15, 2020-July 22, 2021 . MMWR Morb Mort Wkly Rep. 2022 Jan 4:71(1);26-30. Early release.
Moro PL, Panagiotakopoulos L, Oduyebo T, Olson CK, Myers T. Monitoring the safety of COVID-19 vaccines in pregnancy in the US. Human Vaccines & Immunotherapies. 2021 Nov 10. doi.org/10.1080/21645515.2021.1984132.
Zauche LH, Wallace B, Smoots AN, Olson CK, Oduyebo T, Kim SY, Petersen EE, Ju J, Beauregard J, Wilcox AJ, Rose CE, Meaney-Delman DM, Ellington SR, CDC v-safe COVID-19 Pregnancy Registry Team. Receipt of mRNA COVID-19 Vaccines and Risk of Spontaneous Abortion N Engl J Med. 2021 Sept 8. Dpo: 10.1056/NEJMc2113891.
Although pregnant people are at increased risk for severe illness from COVID-19, the COVID-19 vaccination rate among pregnant people has been much lower than that of the general U.S. population. Data about vaccination during pregnancy was initially limited because pregnant participants were excluded from COVID-19 vaccine clinical trials. To evaluate the safety of mRNA vaccines in pregnant people, researchers analyzed data on miscarriage, or a pregnancy loss that occurs before 20 weeks of pregnancy, collected from v-safe COVID-19 Vaccine Pregnancy Registry participants. Over 2,400 registry participants received at least one dose of an mRNA COVID-19 vaccine just before pregnancy or within the first 20 weeks of pregnancy. The cumulative risk of miscarriage among those who received an mRNA COVID-19 vaccine was similar (14.1%) to previously published background rates (11 to 16%) . Therefore, this study demonstrated no increased risk of miscarriage following receipt of COVID-19 mRNA vaccine in early pregnancy. Research will continue on the safety of COVID-19 vaccines in pregnant people.
Kharbanda EO, Haapala J, DeSilva M, Vazquez-Benitez G, Vesco KK, Naleway AL, Lipkind HS. Spontaneous Abortion Following COVID-19 Vaccination During Pregnancy JAMA 2021 Sep 8. Doi:10.1001/jama.2021.15494
Although pregnant people are at increased risk for severe illness from COVID-19, the COVID-19 vaccination rate among pregnant people has been much lower than that of the general U.S. population. Data about vaccination during pregnancy was initially limited because pregnant participants were excluded from vaccine clinical trials. Researchers within the Vaccine Safety Datalink, a collaboration between CDC and 9 health systems, representing approximately 3% of the U.S. population, analyzed data from 8 health systems from December 15, 2020 through June 28, 2021 to evaluate whether there’s an association between COVID-19 vaccine and miscarriage (pregnancy loss that occurs before 20 weeks of pregnancy). This analysis included over 105,000 pregnancies. About 14% received one or more doses of one of the 3 available COVID-19 vaccines during pregnancy before 20 weeks’ gestational age. The analysis found that people who were currently pregnant at the time of COVID-19 vaccination and those who became pregnant after vaccination did not have an increased risk of miscarriage. Research will continue on the safety of COVID-19 vaccines in pregnant people.
Razzaghi H, Meghani M, Pingali C, Crane B, Naleway A, Weintraub E, Kenigsberg TA, Lamias MJ, Irving SA, Kauffman TL, Vesco KK, Daley MF, DeSilva M, Donahue J, Getahun D, Glee S, Hambidge SJ, Jackson LJ, Lipkind HS, Nelson J, Zerbo O, Oduyebo T, Singleton JA, Patel SA. COVID-19 Vaccination Coverage Among Pregnant Women During Pregnancy — Eight Integrated Health Care Organizations, United States, December 14, 2020-May 8, 2021 . MMWR Morb Mortal Wkly Rep . 2021 Jun 18;70(24):895-899.
Pregnant people are at increased risk for severe illness and death from COVID-19; however, current data about vaccination coverage and safety in pregnant people are limited. Researchers reviewed safety data collected from the Vaccine Safety Datalink (VSD) on COVID-19 vaccination among pregnant people. From December 14, 2020 to May 8, 2021, 135,968 pregnant people were identified in the VSD network. Of those, only 16.3% received at least one dose of a COVID-19 vaccine. Researchers identified Hispanic (11.9%) and non-Hispanic Black women (6%) ages 18 to 24 years old as among the groups of pregnant people with the lowest vaccination rate. Non-Hispanic Asian women were the group with the highest vaccination rate (24.7%). Overall, pregnant women ages 35 to 49 years had a high vaccination rate (22.7%). CDC recommends COVID-19 vaccination for anyone who is pregnant or considering becoming pregnant to prevent serious outcomes from COVID-19 illness. Additional outreach, especially for younger pregnant individuals and those from racial and ethnic minority groups, could increase vaccine confidence and COVID-19 vaccination in these populations.
Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, Marquez PL, Olson CK, Liu R, Chang KT, Ellington SR, Burkel VK, Smoots AN, Green CJ, Licata C, Zhang BC, Alimchandani M, Mba-Jonas A, Martin SW, Gee JM, Meaney-Delman DM, CDC v-safe COVID-19 Pregnancy Registry Team. Prelimiary Findings of mRNA COVID-19 Vaccine Safety in Pregnant Persons . N Engl J Med . 2021 Jun 17;384(24):2273-2282. Epub 2021 Apr 21.
Oliver SE, Wallace M, See I, Mbaeyi S, Godfrey M, Hadler SC, Jatlaoui TC, Twentyman E, Hughes MM, Rao AK, Fiore A, Su JR, Broder KR, Shimabukuro T, Lale A, Shay DK, Markowitz LE, Wharton M, Bell BP, Brooks O, McNally V, Lee GM, Talbot HK, Daley MF. Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine: Updated Interim Recommendations from the Advisory Committee on Immunization Practices — United States, December 2021 . MMWR Morb Mortal Wkly Rep. 2022 Jan 21; 71(3);90–95.
See I, Lale A, Marquez P, Streiff MB, Wheeler AP, Tepper NK, Woo EJ, Broder KR, Edwards KM, Gallego R, Geller AI, Jackson KA, Sharma S, Talaat KR, Walter EB, Akpan IJ, Ortel TL, Urrutia VC, Walker S, Yui JC, Shimabukuro TT, Mba-Jonas A, Su JR, Shay DK. Case Series of Thrombosis with Thrombocytopenia Syndrome after COVID-19 vaccination—United States, December 2020 to August 2021 . Ann Intern Med. 2022 Jan 18. Doi: 10.7326/M21-4502 Online ahead of print.
Thrombosis with thrombocytopenia syndrome (TTS) is a rare but potentially life-threatening condition that causes blood clots in large blood vessels and low platelets (blood cells that help form clots). To describe surveillance data and reporting rates of all reported TTS cases after COVID-19 vaccination in the United States, researchers reviewed data from the Vaccine Adverse Event Reporting System (VAERS) reported during December 14, 2020, through August 31, 2021, for patients who reported TTS symptoms after receiving a COVID-19 vaccine. Results showed 57 confirmed TTS cases after receiving a J&J/Janssen, Pfizer-BioNTech, or Moderna vaccine. Reporting rates for TTS were 3.83 per million vaccine doses (Ad26.COV2.S) and 0.00855 per million vaccine doses (mRNA-based COVID-19 vaccines). Of the 3 TTS cases after mRNA-based COVID-19 vaccination (Pfizer-BioNTech or Moderna), two were in men older than 50 years and one was in a woman aged 50 to 59 years. All cases after J&J/Janssen vaccination involved hospitalization. Although rare, TTS is a serious adverse event associated with J&J/Janssen vaccination, which is no longer available in the U.S. as of May 2023.
MacNeil JR, Su JR, Broder KR, Guh AY, Gargano JW, Wallace M, Hadler SC, Scobie HM, Blain AE, Moulia D, Daley MF, McNally VV, Romero JR, Keipp Talbot H, Lee GM, Bell BP, Oliver SE. Updated Recommendations from the Advisory Committee on Immunization Practices for Use of Janssen (Johnson & Johnson) COVID-19 Vaccine After Reports of Thrombosis with Thrombocytopenia Syndrome Among Vaccine Recipients — United States, April 2021 . MMWR Morb Mortal Wkly Rep. 2021 Apr 30;70:651-656.
The Johnson & Johnson/Janssen (Janssen) COVID-19 vaccine was authorized for emergency use on February 27, 2021. On April 13, CDC and the Food and Drug Administration (FDA) recommended pausing the use of Janssen vaccine after thrombosis with thrombocytopenia syndrome (TTS) was reported among vaccine recipients. TTS is a rare syndrome that involves blood clots in large blood vessels with low platelets. The Advisory Committee on Immunization Practices (ACIP) held two emergency meetings to review reports of TTS following Janssen vaccine and conducted a risk-benefit assessment. The estimated reporting rate of TTS was 7 cases of TTS per million Janssen doses administered to women aged 18-49 years. After their review, on April 23, ACIP concluded that the benefits of resuming Janssen COVID-19 vaccination among persons aged 18 years and older outweighed the risks and reaffirmed its interim recommendation under FDA’s Emergency Use Authorization (EUA), which includes a new warning for rare clotting events, primarily in women aged 18-49 years. CDC and FDA will continue to closely monitor reports of TTS following Janssen vaccination.
See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, Streiff MB, Rao AK, Wheeler AP, Beavers SF, Durbin AP, Edwards K, Miller E, Harrington TA, Mba-Jonas A, Nair N, Nguyen DT, Talaat KR, Urrutia VC, Walker SC, Creech B, Clark TA, DeStefano F, Broder KR. US Case Reports of Cerebral Venous Sinus Thrombosis With Thrombocytopenia After Ad26.COV2.S Vaccination, March 2 to April 21, 2021 JAMA 2021 April 30. Doi:10.1001/jama.2021.7517 Epub ahead of print.
Around 7 million doses of Johnson & Johnson’s Janssen (J&J/Janssen) COVID-19 vaccine were given between March 2–April 12, 2021. During this time, VAERS received reports following J&J/Janssen vaccination of cerebral venous sinus thrombosis (CVST) with thrombocytopenia, which involves blood clots in the brain with low platelet counts. By April 21, there were 12 reports of CVST and thrombocytopenia. This serious condition was reported in women between 18 and under 60 years. All were hospitalized; 10 were admitted to intensive care units (ICU). As of April 21, 4 patients were sent home, 2 were moved to hospital units outside of ICU, 3 continued ICU care, and 3 died. The review shows that U.S. cases of CVST and thrombocytopenia after J&J/Janssen vaccination were clinically similar to CVST cases in Europe after Oxford/AstraZeneca COVID-19 vaccination. Investigation of the potential relationship between J&J/Janssen vaccine and CVST with thrombocytopenia is ongoing.
These articles listed were posted on a pre-print server and are in the process of being submitted to a scientific or medical journal. Articles posted on a pre-print server contain preliminary data and are not peer reviewed (reviewed and evaluated by others in the same field but not involved in the study).
The purpose of posting studies on pre-print is to provide the most current data available to the public. When a manuscript is submitted to a peer review journal, additional data may become available and may alter the analysis of the data posted in the pre-print article.
Hause AM, Shay DK, Klein NP, Abara WE, Baggs J, Cortese MM, Fireman B, Gee J, Glanz JM, Goddard K, Hanson KE, Hugueley B, Kenigsberg K, Kharbanda EO, Lewin B, Lewis N, Marquez P, Myers T, Naleway A, Nelson JC, Su JR, Thompson D, Olubajo B, Oster ME, Weintraub ES, Williams JTB, Yousaf AR, Zerbo O, Zhang B, Shimabukuro TT. Safety of COVID-19 Vaccination in US Children Ages 5-11 Years Pediatrics . 2022 May 18. Doi.org/10.1542/peds.2022-057313 Online ahead of print.
Observational Maternal COVID-19 Vaccination Study Principal Investigators: Geeta K Swamy, Karen R Broder, Elizabeth Schlaudecker, Stephen I Pelton Locations: Centers for Disease Control and Prevention, Boston Medical Center, Duke University, Cincinnati Children’s Hospital Medical Center First Posted: April 1, 2021 Summary Recruitment Status: Recruiting
Safety of Simultaneous COVID-19 and IIV4 Vaccination Principal Investigators: Emmanuel B Walter, Kawsar Talaat, Elizabeth Schlaudecker, Karen R Broder Locations: Centers for Disease Control and Prevention, Duke University, Cincinnati Children’s Hospital Medical Center and Johns Hopkins University First Posted: August 31, 2021 Summary Recruitment Status: Recruiting
To search articles on this page by keyword, first click the “open all” tab to list all publications. Then use the “find on this page” function available in most browsers. On desktop, press CTL+F (PC) or CMD+F (Mac) on your keyboard. A “find” text box will open at the top or bottom of your browser. Type the word or phrase you want to search for.
Qian L, Sy LS, Hong V, Glenn SC, Ryan DS, Nelson JC, Hambidge SJ, Crane B, Zerbo O, DeSilva MB, Glanz JM, Donahue JG, Liles E, Duffy J, Xu S. Impact of the COVID-19 Pandemic on Health Care Utilization in the Vaccine Safety Datalink: Retrospective Cohort Study . JMIR Public Health Surveill . 2024 Jan 23:10:e48159. Doi:2196/48159.
Zerbo O, Bartlett J, Fireman B, Lewis N, Goddard K, Dooling K, Duffy J, Glanz J, Naleway A, Donahue JG, Klein N. Effectiveness of Recombinant Zoster Vaccine Against Herpes Zoster in a Real-World Setting . Ann Intern Med. 2024 Jan 9. doi: 10.7326/M23-2023. Online ahead of print.
Kurlandsky KE, Stein AB, Hambidge SJ, Weintraub ES, Williams JTB. Reporting of Race and Ethnicity in the Vaccine Safety Datalink, 2011-2022 . Am J Prev Med . 2024 Jan;66(1) 182-184. Doi: 10.1016/j.amepre.2023.08.019. Online ahead of print. Epub 2023 Sep 3.
Groom HC, Brooks NB, Weintraub ES, Slaughter MT, Mittendorf KF, Naleway AL. Incidence of Adolescent Syncope and Related Injuries Following Vaccination and Routine Venipuncture . J Adolesc Health . 2023 Dec 9:S1054-139X(23)00586-4. Doi: 10.1016/j.jadohealth.2023.11.005. Online ahead of print.
Daley MF, Reifler LM, Shoup JA, Glanz JM, Naleway AL, Nelson JC, Williams JTB, McLean HQ, Vazquez-Benitez G, Goddard K, Lewin BJ, Weintraub ES, McNeil MM, Razzaghi H, Singleton JA. Racial and ethnic disparities in influenza vaccination coverage among pregnant women in the United States: The contribution of vaccine-related attitudes . Prev Med . 2023 Dec, 177:107751 doi: 10.1016/j.ypmed.2023.107751. Online ahead of print .
Kauffman TL, Irving SA, Brooks N, Vesco KK, Slaughter M, Smith N, Tepper NK, Olson CK, Weintraub ES, Naleway AL, Vaccine Safety Datalink Menstrual Irregularities Workgroup. Postmenopausal bleeding after COVID-19 vaccination . Am J Obstet Gynecol , 2023 Sept 17; S0002-9378(23)00613-0. Online ahead of print. https://doi.org/10.1016/j.ajog.2023.09.007 .
This study, published September 2023, focused on people who reported postmenopausal bleeding following COVID-19 vaccination between December 2020 and August 2021. In a population ranging from 75,000 to 83,000 people monthly, there was no significant difference in the reports of postmenopausal bleeding before versus after COVID-19 vaccination. Results concluded that COVID-19 vaccination was not linked to an increase in postmenopausal bleeding. These findings reinforce the safety of COVID-19 vaccination during pregnancy.
Kenigsberg TA, Goddard K, Hanson KE, Lewis N, Klein N, Irving SA, Naleway AL, Crane B, Kauffman TL, Xu S, Daley MF, Hurley LP, Kaiser R, Jackson LA, Jazwa A, Weintraub ES. Simultaneous administration of mRNA COVID-19 bivalent booster and influenza vaccines . Vaccine . 2023 Sep 7; https://doi.org/10.1016/j.vaccine.2023.08.023 . Online ahead of print.
Data from August 31–December 31, 2022, show nearly 1 out of 3 people ages 6 months and older in the Vaccine Safety Datalink (VSD) population received bivalent mRNA COVID-19 and flu vaccines at the same time. About 31.6 percent of adults ages 65 years and older in the VSD population also received bivalent mRNA COVID-19 and flu vaccines at the same time. Among those in the VSD population who received a bivalent mRNA COVID-19 vaccine but not a flu vaccine at the same time, about 13 percent received a flu vaccine before receiving a bivalent mRNA COVID-19 vaccine, and about 40 percent received a flu vaccine after receiving a bivalent mRNA COVID-19 vaccine. This September 2023 publication is informative for future VSD studies and strategies for vaccine promotion and service delivery.
Irving SA, Crane B, Weintraub E, Kauffman TL, Brooks N, Patel SA, Razzaghi H, Belongia EA, Daley MF, Getahun D, Glenn SC, Hambidge SJ, Jackso LA, Kharbanda E, Klein NP, Zerbo O, Naleway AL. Influenza Vaccination Among Pregnant People Before and During the Coronavirus Disease 2019 (COVID-19) Pandemic . Obstet Gynecol. 2023 Sept 1; 142(3):636-639. Online ahead of print
Yih WK, Daley MF, Duffy J, Fireman B, McClure DL, Nelson JC, Qian L, Smith N, Vazquez-Benitez G, Weintraub E, Williams JTB, Xu S, Maro JC. Safety signal identification for COVID-19 bivalent booster vaccination using tree-based scan statistics in the Vaccine Safety Datalink . Vaccine . 2023 Aug 14; https://doi.org/10.1016/j.vaccine.2023.07.10 Online ahead of print.
Traditional active vaccine safety monitoring involves pre-determining specific health outcomes to look for following vaccination, as well as specific timeframes in which to look for these outcomes, limiting the types of adverse events that can be evaluated. In this study, published August 2023, the authors used tree-based scan statistics (the result of a technique used to visualize data) to look broadly for more than 60,000 possible adverse events following bivalent mRNA COVID-19 vaccination. The study population included people aged 5 years and older from the Vaccine Safety Datalink (VSD) who received the Moderna or Pfizer-BioNTech bivalent mRNA COVID-19 vaccine through November 2022 and who were followed for 56 days post-vaccination. Using diagnostic data from inpatient or emergency department settings of those treated for various conditions following vaccination, the authors looked for clusters or trends of common health outcomes. Analysis included 352,509 doses of Moderna and 979,189 doses of Pfizer-BioNTech bivalent mRNA COVID-19 vaccines. No statistically significant clusters were found following Moderna vaccination. Following Pfizer-BioNTech vaccination, clusters of unspecified adverse events, influenza, cough, and COVID-19 were found. The unspecified events were like those seen in other vaccine studies using the same method. The clusters of respiratory illness were likely due to overlap of the follow-up period with the spread of respiratory syncytial virus (RSV), influenza, and COVID-19. Not targeting specific health outcomes following COVID-19 vaccination is a unique advantage of this study’s design, as it allows for many possible diagnoses. Disadvantages, however, include the possibility for missing adverse events not strongly clustered in time or within the data “tree”.
Romanson B, Moro PL, Su JR, Marquez P, Nair N, Day B, DeSantis A, Shimabukuro TT. Notes from the Field: Safety Monitoring of Novavax COVID-19 Vaccine Among Persons Aged ≥ 12 Years – United States, July 13, 2022 – March 13, 2023 . MMWR Morb Mortal Wkly Rep . 2023 Aug 4. 72(31);850-851.
To assess the safety of the Novavax COVID-19 vaccine among individuals aged 12 years and older, CDC reviewed reports made to the Vaccine Adverse Event Reporting System (VAERS) from July 13, 2022, to March 13, 2023. During the review period, the safety profile of the Novavax COVID-19 vaccine was consistent with data from clinical trials. There were no unexpected safety concerns identified in this review, published August 2023. The most commonly reported side effects were not serious and included dizziness, fatigue, and headache.
Moro PL, Zhang B, Marquez P, Reich J. Post-marketing Safety Surveillance of Hexavalent Vaccine in the Vaccine Adverse Event Reporting System . Journal of Pediatrics. 2023 Jul 28. Doi.org/10.1016/j.peds.2023.113643. Online ahead of print.
Combination vaccines involve taking multiple vaccines that can be given individually and putting them into one vaccine. This publication describes data from the Vaccine Adverse Event Reporting System (VAERS) to assess the safety of Vaxelis, a vaccine protecting from diphtheria, tetanus, pertussis, poliovirus, hepatitis B, and Haemophilus influenza b. VAERS received 501 reports involving Vaxelis in the United States during June 26, 2019, to June 16, 2023. In 332 of these reports, Vaxelis was given to patients with one or more other vaccines. Of the 501 reports, vaccination errors (for example, extra dose or wrong product administered) compromised half of all reports. The most common adverse events observed among all reports (nonserious and serious) included fever, vomiting, and injection site reactions. Twenty-one reports were considered serious. This evaluation did not identify new or unexpected safety issues associated with receipt of Vaxelis.
Kenigsberg TA, Hanson KE, Klein NP, Zerbo O, Goddard K, Xu S, Yih WK, Irving SA, Hurley LP, Glanz JM, Kaiser R, Jackson LA, Weintraub ES. Safety of simultaneous vaccination with COVID-19 vaccines in the Vaccine Safety Datalink . Vaccine . 2023 Jul 19; 41(32), 4658–4665. https://doi.org/10.1016/j.vaccine.2023.06.042 .
To assess the safety of receiving COVID-19 vaccines at the same time as other vaccines, data from December 2020 to May 2022 were analyzed using the Vaccine Safety Datalink (VSD). Vaccinations received at the same time as COVID-19 vaccines most frequently were influenza, human papillomavirus (HPV), Tdap, and meningococcal. Results of this assessment, published July 2023, showed pre-specified health outcomes to be rare among those who received a COVID-19 vaccine and another vaccine at the same time and were not different compared to people who did not receive another vaccine at the same time as a COVID-19 vaccine. These findings reinforce the safety of COVID-19 vaccination.
Woo EJ, Gee J, Marquez P, Baggs J, Abara WE, McNeil MM, Dimova RB, Su JR. Post-authorization safety surveillance of Ad.26.COV2.S vaccine: Reports to the Vaccine Adverse Event Reporting System and v-safe, February 2021-February 2022 . Vaccine . 2023 Jul 5; https://doi.org/10.1016/j.vaccine.2023.06.023 Online ahead of print.
In this July 2023 publication, data from February 27, 2021, to February 28, 2022, reported to the Vaccine Adverse Event Reporting System (VAERS) and V-safe were reviewed to evaluate adverse events following receipt of the J&J/Janssen COVID-19 vaccine (no longer available in the United States). More than 17 million vaccine doses were given during this period. Most adverse events reported were non-serious. The evaluation further confirmed safety risks for thrombosis with thrombocytopenia syndrome (a very rare condition in which a person has blood clots as well as low platelet counts) and Guillain-Barré syndrome (a rare disorder in which the body’s immune system attacks its nerves) and identified a potential safety concern for myocarditis (inflammation of the heart muscle) following vaccination.
Schmader KE, Liu CK, Flannery B, Rountree W, Auerbach H, Barnett ED, Schlaudecker EP, Todd CA, Poniewierski M, Staat MA, Harrington T, Li R, Broder KR, Walter EB. Immunogenicity of adjuvanted versus high-dose inactivated influenza vaccines in older adults: a randomized clinical trial . Immun Ageing . 2023 Jul 1; 20(1):30. doi: 10.1186/s12979-023-00355-7.
This U.S. randomized clinical trial directly compared the immune responses of Fluad® and Fluzone® High-Dose in adults aged 65 years and older and found that, overall, the immune responses after receipt of each vaccine were similar. Seroconversion (a type of immune response) to the A(H3N2) virus component of the flu vaccine was not as high after receipt of Fluad® compared with Fluzone® High-Dose; however, the clinical importance of this finding is unclear. This was the first randomized controlled clinical trial in the U.S. directly comparing immune response following vaccination with adjuvanted inactivated influenza vaccine (aIIV) and high-dose inactivated influenza vaccine (IIV-HD) in adults aged 65 years and older. The study, published July 2023, was conducted with trivalent vaccine formulations (aIIV3 and HD-IIV3) before the currently recommended quadrivalent vaccine formulations (aIIV4 and HD-IIV4) were made available.
Zhou ZH, Cortese MM, Fang JL, Wood R, Hummell DS, Risma KA, Norton AE, KuKuruga M, Kirshner S, Rabin RL, Agarabi C, Staat MA, Halasa N, Ware RE, Stahl A, McMahon M, Browning P, Maniatis P, Bolcen S, Edwards KM, Su JR, Dharmarajan S, Forshee R, Broder KR, Anderson S, Kozlowski S. Evaluation of association of anti-PEG antibodies with anaphylaxis after mRNA COVID-19 vaccination . Vaccine . 2023 Jun 23; https://doi.org/10.1016/j.vaccine.2023.05.029 Online ahead of print.
Soon after mRNA COVID-19 vaccines became available for use in the United States, cases of anaphylaxis (an-uh-fuh-LAK-sis), or severe whole body allergic reactions, were reported in vaccine safety monitoring systems, raising concerns that rates of this severe allergic reaction were higher with mRNA vaccines than with other vaccines. Learning the cause of anaphylaxis following mRNA vaccination is important because multiple vaccine doses are required to be considered fully vaccinated against COVID-19. The mRNA COVID-19 vaccines contain polyethylene glycol (PEG), which has been associated with allergic reactions in some medical products. In this publication, authors share results of an evaluation to determine if PEG may cause anaphylaxis following mRNA vaccination. The study compared levels of anti-PEG Immunoglobin E (IgE), a type of antibody, in serum samples of patients who had anaphylaxis after mRNA COVID-19 vaccination to those of patients who did not have any allergic reaction after mRNA vaccination. The evaluation found that anti-PEG IgE antibodies were rare in people with anaphylaxis after mRNA vaccination and that low levels of the antibody were found in people who did not have an allergic reaction after mRNA vaccination. The results suggest that PEG allergy is not a common cause of anaphylaxis following mRNA COVID-19 vaccination.
Miller ER, Moro PL, Shimabukuro TT, Carlock G, Davis SN, Freeborn EM, Roberts AL, Gee J, Taylor AW, Gallego R, Suragh T, Su JR. COVID-19 vaccine safety inquiries to the centers for disease control and prevention immunization safety office . Vaccine . 2023 Jun 19; https://doi.org/10.1016/j.vaccine.2023.05.054 Online ahead of print.
Knowing which vaccine safety topics are important to the public and other partners helps guide CDC’s efforts to better communicate relevant information to its audiences. This June 2023 publication provides a brief overview of the most common questions and concerns about COVID-19 vaccines that were received by the CDC’s Immunization Safety Office. During December 2020 to August 2022, CDC’s Immunization Safety Office received 1,655 inquiries from public health officials, healthcare providers, and the general public about the safety of COVID-19 vaccines. The most common COVID-19 vaccine safety questions were about deaths following vaccination, myocarditis (inflammation of the heart muscle), pregnancy, and reproductive health outcomes; understanding or interpreting data from the Vaccine Adverse Event Reporting System (VAERS); and thrombosis with thrombocytopenia syndrome (a very rare condition in which a person has blood clots as well as low platelet counts).
Naleway AL, Henninger ML, Irving SA, Bianca Salas S, Kauffman TL, Crane B, Mittendorf KF, Harsh S, Elder C, Gee J. Epidemiology of Upper Limb Complex Regional Pain Syndrome in a Retrospective Cohort of Persons Aged 9-30 Years, 2002-2017 . 2023 Jun 15; Perm J , 27(2), 75–86. https://doi.org/10.7812/TPP/22.170 .
This June 2023 publication describes complex regional pain syndrome (CRPS), a form of chronic pain that usually affects an arm or a leg, and rates of CRPS over a period spanning human papillomavirus (HPV) vaccine licensure and reports of CRPS following HPV vaccination. The authors examined CRPS diagnoses in patients aged 9-30 years during January 2002 through December 2017 using electronic medical records, excluding patients with lower limb diagnoses only. Rates of CRPS diagnoses were calculated for three periods: Period 1 (2002-2006: before HPV vaccine licensure), Period 2 (2007-2012: after licensure but before published reports of CRPS), and Period 3 (2013-2017: after published reports of CRPS). A total of 231 individuals received an upper limb or unspecified CRPS diagnosis during the study period; 113 cases were verified through medical record examination. Most verified cases (73 percent) of CRPS were associated with non-vaccine-related injuries or surgical procedures. These data provide a comprehensive assessment of CRPS in children and young adults and provide further reassurance about the safety of HPV vaccination.
Day B, Menschik D, Thompson D, Jankosky C, Su J, Moro P, Zinderman C, Welsh K, Dimova RB, Nair N. Reporting rates for VAERS death reports following COVID-19 vaccination, December 14, 2020-November 17, 2021 . Pharmacoepidemiol Drug Saf . 2023 Jul 9; 32(7):763-772. Online ahead of print.
The Vaccine Adverse Event Reporting System (VAERS) is one of several national vaccine safety monitoring systems that can identify potential health problems (also known as safety signals) after vaccination. Reports submitted to VAERS do not necessarily mean that a vaccine caused the reported health problems–VAERS is designed to look for signals of possible health problems. If vaccine safety experts and scientists detect a safety signal, they conduct follow-up analyses to investigate further.
For COVID-19 vaccines under the U.S. Food and Drug Administration’s (FDA’s) emergency use authorization, healthcare providers were required to report any death following vaccination to VAERS, regardless of the cause of death. Reports of death following vaccination are continuously monitored by vaccine safety experts.
This review of VAERS data, published July 2023, looked at deaths reported after COVID-19 vaccination in the United States between December 14, 2020, and November 17, 2021, in VAERS. There were 9,201 deaths reported among COVID-19 vaccine recipients during this time, still lower than all-cause death rates (the number of deaths reported among people overall) reported within the population. The study’s findings do not suggest an association between vaccination and overall increased rate of death and add to the evidence base supporting safe use of COVID-19 vaccines.
Greenberg V, Vazquez-Benitez G, Kharbanda EO, Daley MF, Tseng HF, Klein NP, Naleway AL, Williams JTB, Donahue J, Jackson L, Weintraub E, Lipkind H, DeSilva MB. Tdap vaccination during pregnancy and risk of chorioamnionitis and related infant outcomes . Vaccine . 2023 May 23;41(22):3429-3435
A new CDC study’s findings provide additional support for the safety of Tdap vaccination during pregnancy. As a result, the Advisory Committee on Immunization Practices has made no changes to its recommendations for routine Tdap immunization among pregnant people. These findings reinforce the safety of Tdap vaccination during pregnancy. Pregnant women should receive one dose of Tdap during each pregnancy, irrespective of their history of receiving the vaccine. Tdap vaccine should be administered at 27 through 36 weeks’ gestation, preferably during the earlier part of this period, although it may be administered at any time during pregnancy.
Hanson KE, Marin M, Daley MF, Groom HC, Jackson LA, Sy LS, Klein NP, DeSilva MB, Panagiotakopoulos L, Weintraub E, Belongia EA, McLean HQ. Safety of measles, mumps, and rubella vaccine in adolescents and adults in the vaccine safety Datalink . Vaccine X : 2023 Feb 4; 13, 100268. https://doi.org/10.1016/j.jvacx.2023.100268 .
The measles, mumps, and rubella vaccine (MMR) is usually given to children; however, some adolescents and adults also receive the MMR vaccine for various reasons. This study, published February 2023, looked at adolescents aged 9-17 years and adults aged 18 years and older who received an MMR vaccine during January 2010 through December 2018. Reactions following vaccination were categorized as clinically serious and for medical review or as non-serious. Results showed that during this period, 276,327 MMR doses were given to adolescents and adults, and serious outcomes following receipt of the vaccine were rare.
Abara WE, Gee J, Marquez P, Woo J, Myers TR, DeSantis A, Baumblatt JAG, Woo EJ, Thompson D, Nair N, Su JR, Shimabukuro TT, Shay DK. Reports of Guillain-Barré Syndrome After COVID-19 Vaccination in the United States . JAMA Network. 2023 Feb 1;6(2):e2253845. doi:10.1001/jamanetworkopen.2022.53845.
Evidence suggests an increased risk of Guillain-Barré Syndrome (GBS) (a rare disorder in which the body’s immune system attacks its nerves) among adults 18 years and older who received the J&J/Janssen COVID-19 vaccine (no longer available in the United States). This publication describes U.S.-based reports of possible GBS made to the Vaccine Adverse Event Reporting System (VAERS) between December 14, 2020, and January 28, 2022. Medical record review was conducted for most of the cases, and this review observed an elevation in GBS among adults who received the J&J/Janssen COVID-19 vaccine with no observed elevation of GBS among adults who received the Pfizer-BioNTech or Moderna mRNA COVID-19 vaccines.
Myers TR, Marquez PL, Gee JM, Hause AM, Panagiotakopoulos L, Zhang B, McCullum I, Licata C, Olson CK, Rahman S, Kennedy SB, Cardozo M, Patel CR, Maxwell L, Kallman JR, Shay DK, Shimabukuro TT. The v-safe after vaccination health checker: Active vaccine safety monitoring during CDC’s COVID-19 pandemic response . Vaccine . 2023 Jan 23; https://doi.org/10.1016/j.vaccine.2022.12.031 Online ahead of print.
CDC’s V-safe vaccine safety monitoring system supplements existing vaccine safety monitoring programs including the Vaccine Adverse Event Reporting System (VAERS) and the Vaccine Safety Datalink (VSD). V-safe provides near real-time reporting of how people feel following vaccination and encourages reports to VAERS from V-safe participants who sought medical care following vaccination. V-safe also supports the identification of candidates for enrollment in a large post-vaccination pregnancy registry. This February 2023 publication provides an overview of how V-safe works, how it is used, and how updates to the platform have been completed.
Yih WK, Daley MF, Duffy J, Fireman B, McClure D, Nelson J, Qian L, Smith N, Vazquez-Benitez G, Weintraub E, Williams JTB, Xu S, Maro JC. A broad assessment of covid-19 vaccine safety using tree-based data-mining in the vaccine safety datalink . Vaccine . 2023 Jan 16; https://doi.org/10.1016/j.vaccine.2022.12.026 . Online ahead of print.
Tree-based data mining is used to look for clusters of a range of health outcomes in health data. The Vaccine Safety Datalink (VSD) applied this methodology to evaluate adverse events (AEs) following COVID-19 vaccines. The data were monitored to identify clusters of potential AEs among COVID-19 vaccines from each manufacturer (Pfizer-BioNTech, Moderna, and J&J/Janssen). Results showed clusters ranging from common vaccine reactions to other rare reactions—such as chest pain, palpitations, and myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining of the heart muscle)—that showed up for specific vaccines such as after receipt of a second dose of Pfizer-BioNTech mRNA COVID-19 vaccine.
Xu S, Huang R, Sy LS, Hong V, Glenn SC, Ryan DS, Morrissette K, Vazquez-Benitez G, Glanz JM, Klein NP, Fireman B, McClure D, Liles EG, Weintraub ES, Tseng HF, Qian L. A safety study evaluating non-COVID-19 mortality risk following COVID-19 vaccination . Vaccine . 2023 Jan 16; https://doi.org/10.1016/j.vaccine.2022.12.036 . Online ahead of print.
Yih WK, Daley MF, Duffy J, Fireman B, McClure D, Nelson J, Qian L, Smith N, Vazquez-Benitez G, Weintraub E, Williams JTB, Xu S, Maro JC. Tree-based data mining for safety assessment of first COVID-19 booster doses in the Vaccine Safety Datalink . Vaccine . 2023 Jan 9; https://doi.org/10.1016/j.vaccine.2022.11.053 . Online ahead of print.
Tree-based data mining is used to look for clusters of a range of health outcomes in electronic health data. This publication describes the data mining methodology that was applied to Vaccine Safety Datalink (VSD) data to assess the safety of first booster doses of Pfizer-BioNTech and Moderna mRNA COVID-19 vaccines and the J&J/Janssen COVID-19 vaccine (no longer available in the United States). Results identified clusters of allergy/rash following receipt of the Moderna booster, common adverse effects following receipt of the Pfizer-BioNTech booster, and no noteworthy clusters following receipt of the J&J/Janssen booster.
Moro PL, Zhang B, Ennulat C, Harris M, McVey R, Woody G, Marquez P, McNeil MM, Su JR. Safety of Co-administration of mRNA COVID-19 and seasonal inactivated influenza vaccines in the Vaccine Adverse Event Reporting System (VAERS) during July 1, 2021 – June 30, 2022 . Vaccine . 2023 Jan 9; https://doi.org/10.1016/j.vaccine.2022.12.069 Online ahead of print.
COVID-19 vaccines can be co-administered with other recommended vaccines, including seasonal flu vaccines. Vaccine Adverse Event Reporting System (VAERS) data were used to look at adverse events (AEs) following co-administration of mRNA COVID-19 and flu vaccines during July 1, 2021, through June 30, 2022. During this period, VAERS received 2,449 reports of AEs consisting of injection site reactions, headaches, pain, dyspnea, COVID-19 infection, and chest pain after co-administration of mRNA COVID-19 and flu vaccines. This review did not reveal unusual or unexpected patterns of AEs.
Malden DE, Gee J, Glenn S, Li Z, Mercado C, Ogun OA, Kim S, Lewin BJ, Ackerson BK, Jazwa A, Weintraub ES, McNeil MM, Tartof S. Reactions following Pfizer-BioNTech COVID-19 mRNA vaccination and related healthcare encounters among 7,077 children aged 5-11 years within an integrated healthcare system . Vaccine . 2023 Jan 9; https://doi.org/10.1016/j.vaccine.2022.10.079 . Online ahead of print.
Tompkins LK, Baggs J, Myers TR, Gee JM, Marquez PL, Kennedy SB, Peake D, Dua D, Hause AM, Strid P, Abara W, Rossetti R, Shimabukuro TT, Shay DK. Association between history of SARS-CoV-2 infection and severe systemic adverse events after mRNA COVID-19 vaccination among U.S. adults . Vaccine . 2022 Dec 12;S0264-410X(22)01342-1. Online ahead of print.
Goddard K, Hanson KE, Lewis N, Weintraub E, Fireman B, Klein NP. Incidence of Myocarditis/Pericarditis Following mRNA COVID-19 Vaccination Among Children and Younger Adults in the United States . Annals of Internal Medicine . 2022 Dec. doi.org/10.7326/M22-2274.
Hause AM, Marquez P, Zhang B, Myers TR, Gee J, Su JR, Blanc PG, Thomas A, Thompson D, Shimabukuro TT, Shay DK. Safety Monitoring of Bivalent COVID-19 mRNA Vaccine Booster Doses Among Persons Aged ≥ 12 Years – United States, August 31 – October 23, 2022 . MMWR Morb Mortal Wkly Rep. 2022 Nov 4; 71(44);1401–1406.
This CDC study, published November 2022, found that among people aged 12 years and older who received a bivalent mRNA COVID-19 vaccine booster dose, serious adverse events were rare. Common side effects were headache, fever, fatigue, pain where the shot was given, and chills. The study’s preliminary findings support the overall safety of bivalent mRNA COVID-19 vaccines. Health impacts associated with the original mRNA and the bivalent mRNA COVID-19 vaccines are less frequent and less serious than COVID-19 illness.
During August 31–October 23, 2022, approximately 4.7 million people aged 12 years and older received a dose of the Pfizer-BioNTech bivalent mRNA COVID-19 booster, and approximately 2.6 million people aged 18 years and older received a dose of the Moderna bivalent mRNA COVID-19 booster. CDC reviewed health impact assessments received by CDC’s V-safe and reviewed reports voluntarily submitted to the Vaccine Adverse Event Reporting System during August 31–October 23, 2022, to characterize the safety of bivalent mRNA COVID-19 booster vaccination among people in this age group. The initial safety findings for bivalent mRNA COVID-19 vaccines were generally like those from pre-authorization clinical trials. Data identified no unusual or unexpected patterns of adverse events following vaccination with the Pfizer-BioNTech or Moderna bivalent mRNA COVID-19 vaccines. Reports of serious adverse events following receipt of bivalent COVID-19 vaccine booster doses were rare. Commonly reported reactions such as headache, fever, fatigue, injection-site pain, and chills, were mild and like those reported following receipt of the monovalent mRNA COVID-19 booster dose.
Nelson JC, Ulloa-Perez E, Yu O, Cook AJ, Jackson ML, Belongia EA, Daley MF, Harpaz R, Kharbanda EO, Klein NP, Naleway AL, Tseng HF, Weintraub ES, Duffy J, Yih WK, Jackson LA. Active Post-Licensure Safety Surveillance for Recombinant Zoster Vaccine Using Electronic Health Record Data . Am J Epidemiol . 2022 Oct 4. doi: 10.1093/aje/kwac170. Online ahead of print.
Recombinant zoster vaccine (RZV) was licensed in 2017 to prevent herpes zoster and its complications in older adults. Vaccine Safety Datalink (VSD) electronic health records data were used to monitor adults ages 50 years and older who received care at VSD healthcare systems in the United States to identify increased risks of 10 pre-specified outcomes potentially related to RZV—including stroke, anaphylaxis, and Guillain-Barré syndrome (GBS). There were 647,833 RZV doses administered during January 2018 through December 2019. During this time, no increased risk of any of these outcomes was detected for RZV recipients who had received the Zoster Vaccine Live (ZVL), a live-attenuated virus vaccine, from 2013–2017, or for non-RZV vaccinated persons who had an annual check-up during the 2018–2019 study period. This study, published October 2022, provides additional reassurance of the safety of recombinant zoster vaccine.
Daley MF, Reifler LM, Glanz JM, Hambidge SJ, Getahun D, Irving SA, Nordin JD, McClure DL, Klein NP, Jackson ML, Duffy J, DeStefano F. Association Between Aluminum Exposure from Vaccines Before Age 24 Months and Persistent Asthma at Age 24-59 Months [PDF – 10 Pages] . Academic Pediatrics . 2022 Sept 27. Online ahead of print
This observational study suggests a possible association between exposure to aluminum in some childhood vaccines and development of persistent asthma in children. The study consisted of 326,991 children and found that cumulative exposure to aluminum from vaccines during the first two years of life was associated with a small increased risk of persistent asthma in children ages 2-5 years. There is overwhelming evidence of the benefits of vaccines. CDC is not changing the current routine childhood vaccination recommendations based on this single study. Small amounts of aluminum are included in many routine childhood vaccines to help the body build a stronger immunity from diseases. Further investigation is needed to explore the potential risk of aluminum exposure from routine childhood vaccines on the development of persistent asthma in children; efforts are underway.
Hause AM, Marquez P, Zhang B, Myers TR, Gee J, Su JR, Parker C, Thompson D, Panchanathan SS, Shimabukuro TT, Shay DK. COVID-19 mRNA Vaccine Safety Among Children Aged 6 Months–5 Years — United States, June 18, 2022–August 21, 2022 . MMWR Morb Mortal Wkly Rep . 2022 Sep 2;71(35);1115-1120.
Hause AM, Baggs J, Marquez P, Myers TR, Su JR, Hugueley B, Thompson D, Gee J, Shimabukuro TT, Shay DK. Safety Monitoring of Pfizer-BioNTech COVID-19 Vaccine Booster Doses Among Children Aged 5–11 Years — United States, May 17–July 31, 2022 . MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33);1047–1051.
Goddard K, Lewis N, Fireman B, Weintraub E, Shimabukuro T, Zerbo O, Boyce TG, Oster ME, Hanson KE, Donahue JG, Ross P, Naleway A, Nelson JC, Lewin B, Glanz JM, Williams JTB, Kharbanda EO, Yih WK, Klein NP. Risk of myocarditis and pericarditis following BNT162b2 and mRNA-1273 COVID-19 vaccination . Vaccine . 2022 Aug 19; 40(35):5153-5159. Epub 2022 Jul 12.
Wong KK, Heilig CM, Hause A, Myers TR, Olson CK, Gee J, Marquez P, Strid P, Shay DK. Menstrual irregularities and vaginal bleeding after COVID-19 vaccination reported to v-safe active surveillance, USA in December, 2020-January, 2022: an observational cohort study . Lancet Digit Health . 2022 Aug 9; S2589-7500(22)00125-X. Online ahead of print.
In this study, published August 2022, CDC vaccine safety experts found that menstrual irregularities and vaginal bleeding have been reported among people who received COVID-19 vaccines. These reports were most often about menstrual cycle timing and the severity of menstrual symptoms. During December 14, 2020, through January 9, 2022, researchers analyzed data from V-safe, a web-based tool that uses text messages, emails, and web surveys to provide personalized health check-ins for people after receiving a new vaccine. Researchers identified 84,943 responses to open-ended survey questions related to menstruation or vaginal bleeding from 63,815 V-safe participants ages 18 years and older. Most respondents reported changes to the timing (i.e., cycle beginning earlier or later than expected, missed cycles, and spotting) and the severity (i.e., heavier flow, more painful than usual, or prolonged bleeding) of menstrual symptoms after COVID-19 vaccination. More respondents reported symptoms after their second vaccine dose compared with their first vaccine dose. While researchers acknowledge that an association between COVID-19 vaccination and menstrual irregularities is plausible, they also note that menstrual irregularities are common without COVID-19 vaccination and suggest that further studies are needed to assess clinical significance of menstrual irregularities after vaccination.
Hause AM, Baggs J, Marquez P, Abara WE, Baumblatt JG, Thompson D, Su JR, Myers TR, Gee J, Shimabukuro TT, Shay DK. Safety Monitoring of COVID-19 mRNA Vaccine First Booster Doses Among Persons Aged ≥12 Years with Presumed Immunocompromise Status — United States, January 12, 2022–March 28, 2022 .. MMWR Morb Mortal Wkly Rep . 2022 Jul 15; 71(28);899–903.
Zerbo O, Modaressi S, Goddard K, Lewis E, Getahun D, Palmsten KK, Fuller CC, Crane B, Donahue JG, Daley MF, Jackson LA, Wodi AP, McNeil MM, Klein NP. Safety of Live-Attenuated Vaccines in Children Exposed to Biologic Response Modifiers in Utero . Pediatrics . 2022 Jul 1; 150(1):e2021056021.
Fleming-Dutra KE, Wallace M, Moulia DL, Twentyman E, Roper LE, Hall E, Link-Gelles R, Godfrey M, Woodworth KR, Anderson TC, Rubis AB, Shanley E III, Jones JM, Morgan RL, Brooks O, Talbot HK, Lee GM, Bell BP, Daley M, Meyer S, Oliver SE. Interim Recommendations of the Advisory Committee on Immunization Practices for Use of Moderna and Pfizer-BioNTech COVID-19 Vaccines in Children Aged 6 Months–5 Years — United States, June 2022 . MMWR Morb Mortal Wkly Rep . 2022 Jul 1; 71(26);859–868.
Hause AM, Zhang B, Yue X, Marquez P, Myers TR, Parker C, Gee J, Su J, Shimabukuro TT, Shay DK. Reactogenicity of Simultaneous COVID-19 mRNA Booster and Influenza Vaccination in the US . JAMA Netw Open 2022 Jul 1;5(7):e2222241.doi: 10.1001/jamanetworkopen.2022.22241.
Weintraub ES, Oster ME, Klein NP. Myocarditis or Pericarditis Following mRNA COVID-19 Vaccination . JAMA . 2022 Jun 24; 5(6):e2218512. doi:10.1001/jamanetworkopen.2022.18512
DeSilva M, Haapala J, Vazquez-Benitez G, Vesco KK, Daley MF, Getahun D, Zerbo O, Naleway A, Nelson JC, Williams JTB, Hambidge SJ, Boyce TG, Fuller CC, Lipkind HS, Weintraub E, McNeil MM, Kharbanda EO. Evaluation of Acute Adverse Events after Covid-19 Vaccination during Pregnancy . N Engl J Med . 2022 Jun 22. DOI: 10.1056/NEJMc2205276. Epub ahead of print.
Moro PL, Olson CK, Clark E, Marquez P, Strid P, Ellington S, Zhang B, Mba-Jonas A, Alimchandani M, Cragan J, Moore C. Post-authorization surveillance of adverse events following COVID-19 vaccines in pregnant persons in the vaccine adverse event reporting system (VAERS), December 2020 – October 2021 . Vaccine . 2022 May 26; 40(24):3389-3394. Epub 2022 Apr 12.
Xu S, Hong V, Sy LS, Glenn SC, Ryan DS, Morrissette KL, Nelson JC, Hambidge SJ, Crane B, Zerbo O, DeSilva MB, Glanz JM, Donahue JG, Liles E, Duffy J, Qian L. Changes in incidence rates of outcomes of interest in vaccine safety studies during the COVID-19 pandemic . Vaccine . 2022 May 20;40(23):3150-3158. Epub 2022 Apr 18.
Kenigsberg TA, Hause AM, McNeil MM, Nelson JC, Shoup JA, Goddard K, Lou Y, Hanson KE, Glenn SC, Weintraub E. Dashboard development for near real-time visualization of COVID-19 vaccine safety surveillance data in the Vaccine Safety Datalink . Vaccine . 2022 May 11;40(22):3064-3071. Epub 2022 Apr 8.
Razzaghi H, Meghani M, Crane B, Ellington S, Naleway AL, Irving SA, Patel SA. Receipt of COVID-19 Booster Dose Among Fully Vaccinated Pregnant Individuals Aged 18 to 49 Years by Key Demographics . JAMA . 2022 Apr 22;327(23):2351-2354. doi:10.1001/jama.2022.683.
Zerbo O, Modaressi S, Goddard K, Lewis E, Fireman B, Daley MF, Irving SA, Jackson LA, Donahue JG, Qian L, Getahun D, DeStefano F, McNeil MM, Klein NP. Safety of measles and pertussis-containing vaccines in children with autism spectrum disorders . Vaccine . 2022 Apr 20; 40(18):2568-2573. Epub 2022 Mar 18.
This study, published April 2022, found no increased risk of adverse events (AEs) after measles or pertussis vaccination among children diagnosed with autism spectrum disorder (ASD) compared to children without an ASD diagnosis. The study included children born between 1995 and 2012 who were ages 4–7 years at the time of vaccination and included members of six healthcare delivery systems within the Vaccine Safety Datalink. The study included 14,947 children with ASD and 1,650,041 children without ASD. Results showed no differences between children with and without ASD for AEs such as fever or reactions that required emergency department visits following their measles or pertussis vaccination.
Paddock CD, Reagan-Steiner S, Su JR, Oster ME, Martines RB, Bhatnagar J, Shimabukuro TT. Autopsy Histopathologic Cardiac Findings in 2 Adolescents Following the Second COVID-19 Vaccine Dose . Arch Pathol Lab Med . 2022 Apr 8; 146 (8): 921–923.
Block JP, Boehmer TK, Forrest CB, Carton TW, Lee GM, Ajani UA, Christakis DA, Cowell LG, Draper C, Ghildayal N, Harris AM, Kappelman MD, Ko JY, Mayer KH, Nagavedu K, Oster ME, Paranjape A, Puro J, Ritchey MD, Shay DK, Thacker D, Gundlapalli AV. Cardiac Complications After SARS-CoV-2 Infection and mRNA COVID-19 Vaccination — PCORnet, United States, January 2021–January 2022 . Morb Mortal Wkly Rep . 2022 Apr 8; 71(14);517-523.
Hanson KE, Goddard K, Lewis N, Fireman B, Myers TR, Bakshi N, Weintraub E, Donahue JG, Nelson JC, Xu S, Glanz JM, Williams JTB, Alpern JD, Klein NP. Incidence of Guillain-Barré Syndrome after COVID-19 Vaccination in the Vaccine Safety Datalink . JAMA Network Open . 2022 Apr 26;5(4):e228879. doi: 10.1001/jamanetworkopen.2022.8879.
Sokolow AG, Stallings AP, Kercsmar C, Harrington T, Jimenez-Truquw N, Zhu Y, Sokolow K, Moody A, Schlaudecker EP, Walter EM, Allen Staat M, Broder KR, Creech CB. Safety of Live Attenuated Influenza Vaccine in Children with Asthma . Pediatrics . 2022 Apr 1;149(4):e2021055432. Epub 2022 Mar 28.
People ages 5 years and older with asthma should receive the quadrivalent live attenuated influenza vaccine (LAIV4) with caution because of concerns for wheezing events. This study, published in April 2022, compared the proportion of children with asthma who experienced asthma-related symptoms after receipt of LAIV4 to the proportion of children with asthma who experienced asthma-related symptoms after receipt of the quadrivalent inactivated influenza vaccine (IIV4) and found that LAIV4 was not associated with increased exacerbations, asthma-related symptoms, or decrease in expiratory flow rate compared with IIV4 among this age group. During two influenza seasons, 142 children with asthma ages 5–17 years were monitored for asthma symptoms for 42 days after IIV4 or LAIV4 vaccination. During the observation period, 18 (13%) of 142 participants had exacerbated symptoms: 8 (11%) who received the LAIV4 and 10 (15%) who received the IIV4 vaccine.
Rosenblum HG, Gee J, Liu R, Marquez PL, Zhang B, Strid P, Abara WE, McNeil MM, Myers TR, Hause AM, Su JR, Markowitz LE, Shimabukuro TT, Shay DK. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the Vaccine Adverse Event Reporting System and v-safe . Lancet Infect Dis . 2022 Mar 7; S1473-3099(22)00054-8. Online ahead of print.
In a comprehensive analysis of mRNA COVID-19 vaccine data (i.e., Pfizer-BioNTech and Moderna), published June 2022, CDC scientists reviewed 6 months of safety data from the Vaccine Adverse Event Reporting System (VAERS), a passive vaccine safety surveillance system co-managed by CDC and the U.S. Food and Drug Administration (FDA), and V-safe, a web-based tool that uses text messages, emails, and web surveys to provide personalized health check-ins for people after receiving a new vaccine. During December 14, 2020, through June 14, 2021, more than 298 million doses of mRNA COVID-19 vaccine were administered. The review found that most reported reactions—such as headache, fatigue, and soreness at the injection site—were mild and short in duration and most reported adverse events were not serious (did not require hospitalization or cause disability, life-threatening illness, or death). These findings reinforce evidence that mRNA COVID-19 vaccines are safe and could reassure those who might be hesitant to get an mRNA vaccine because of safety concerns.
Hause AM, Baggs J, Marquez P, Abara WE, Olubajo B, Myers TR, Su JR, Thompson D, Gee J, Shimabukuro TT, Shay DK. Safety Monitoring of COVID-19 Vaccine Booster Doses Among Persons Aged 12–17 Years — United States, December 9, 2021–February 20, 2022 . MMWR Morb Mortal Wkly Rep . 2022 Mar 1;71(9);347–351.
Moro PL, McNeil MM. Successes of the CDC monitoring systems in evaluating post-authorization safety of COVID-19 vaccines [Editorial] . Expert Rev Vaccines. 2022 Mar;21(3):281-284. Epub 2022 Jan 5.
Irving SA, Groom HC, Dandamudi P, Daley MF, Donahue JG, Gee J, Hechter R, Jackson LA, Klein NP, Liles E, Myers TR, Stokley S. A decade of data: Adolescent vaccination in the vaccine safety datalink, 2007 through 2016 . Vaccine . 2022 Feb 23; 40(9):1246-1252. Epub 2022 Feb 4.
Between May 2005 and March 2007, three vaccines were recommended by the Advisory Committee on Immunization Practices for adolescents in the United States: meningococcal vaccine (MenACWY), pertussis vaccine (Tdap), and human papillomavirus vaccine (HPV). This study, published February 2022, was conducted regarding all vaccines administered to adolescents ages 11 to 18 years in the Vaccine Safety Datalink population during January 1, 2007, through December 31, 2016, to better understand vaccination coverage (the number of vaccine doses administered) for these vaccines. There were 4,884,553 vaccine visits among this age group during the study period. Vaccine coverage for Tdap, MenACWY, and HPV increased across the study period with a variety of vaccine combinations administered among both sexes. Vaccine administration in this population can provide a historical pattern to compare with future vaccination campaigns among this group.
Hause AM, Baggs J, Marquez P, Myers TR, Su JR, Blanc PG, Gwira Baumblatt JA, Woo EJ, Gee J, Shimabukuro TT, Shay DK. Safety Monitoring of COVID-19 Vaccine Booster Doses Among Adults — United States, September 22, 2021–February 6, 2022 . MMWR Morb Mortal Wkly Rep . 2022 Feb 18; 71(7);249–254.
Oster ME, Shay DK, Su JR, Gee J, Creech B, Broder KR, Edwards K, Soslow JH, Dendy JM, Schlaudecker E, Lang SM, Barnett ED, Ruberg FL, Smith MJ, Campbell MJ, Lopes RD, Sperling LS, Baumblatt JA, Thompson DL, Marquez PL, Strid P, Woo J, Puglsey R, Reagan-Steiner S, DeStefano F, Shimabukuro TT. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US from December 2020 to August 2021 JAMA . 2022 Jan 25;327(4):331-340. Online ahead of print.
Oliver SE, Wallace M, See I, Mbaeyi S, Godfrey M, Hadler SC, Jatlaoui TC, Twentyman E, Hughes MM, Rao AK, Fiore A, Su JR, Broder KR, Shimabukuro T, Lale A, Shay DK, Markowitz LE, Wharton M, Bell BP, Brooks O, McNally V, Lee GM, Talbot HK, Daley MF. Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine: Updated Interim Recommendations from the Advisory Committee on Immunization Practices — United States, December 2021 . MMWR Morb Mortal Wkly Rep . 2022 Jan 21; 71(3);90–95.
Navarro RA, Lin CC, Colli B, Qian L, Liu ILA, Sy LS, Jacobsen SJ, Tartof SY. Safety of Influenza Vaccination During Orthopedic Surgery Hospitalizations J Am Acad Orthop Surg . 2022 Jan 15;30(2):e155-e163. Doi: 10.5435/JAAOS-D-21-00101.
Despite national recommendations, flu vaccination rates during hospitalizations remain low. Flu vaccination during hospitalization for orthopedic surgery was studied to address whether there is an increase for infection post discharge. Researchers conducted a study of patients ages ≥ 6 months who were hospitalized for orthopedic surgery between September 1, 2011, and March 31, 2014, to assess the association between flu vaccination during inpatient care for orthopedic surgery and rates of readmission for infections less than seven days post discharge. Results showed 2,395 hospitalizations with inpatient vaccination and 21,708 hospitalizations without inpatient vaccination. Those vaccinated during inpatient care did not show a significant increase in readmission for infection. Data supports the recommendation of vaccinating orthopedic surgery patients against influenza.
Woo EJ, Moro PL. Postmarketing safety surveillance of high-dose quadrivalent influenza vaccine: Reports to the Vaccine Adverse Event Reporting System . Vaccine. 2022 Jan 12. ISSN: 0264-410X. Online ahead of print.
On November 4, 2019, the U.S. Food and Drug Administration approved the high-dose flu vaccine (Fluzone High-Dose Quadrivalent; QIV-HD) for flu prevention in individuals ages 65 years and older. A clinical trial did not show major differences in adverse events (AEs) following vaccination with QIV-HD versus Fluzone High-Dose (trivalent). Researchers reviewed and summarized reports of AEs after QIV-HD vaccination to the Vaccine Adverse Event Reporting System (VAERS) to learn more. During July 30, 2020, through June 30, 2021, VAERS received 2,122 reports after vaccination with QIV-HD. The majority (95.1%) were non-serious and included events that had been observed in the clinical trial such as injection site reactions, fever, headache, and nausea. The most common serious events included Guillain-Barré syndrome, cellulitis or other local reactions, constitutional signs/symptoms (e.g., fever), and cardiovascular events. This review, published February 2022, did not reveal new safety concerns.
Lipkind HS, Vazquez-Benitez G, DeSilva M, Vesco KK, Ackerman-Banks C, Zhu J, Boyce TG, Daley MF, Fuller CC, Getahun D, Irving SA, Jackson LA, Williams JTB, Zerbo O, McNeil MM, Olson CK, Weintraub E, Kharbanda KO. Receipt of COVID-19 Vaccine During Pregnancy and Preterm or Small-for-Gestational-Age at Birth — Eight Integrated Health Care Organizations, United States, December 15, 2020-July 22, 2021 MMWR Morb Mort Wkly Rep. 2022 Jan 4:71 (1);26-30. Early release.
DeSilva MB, Haapal J, Vazquez-Benitez G, Daley MF, Nordin JD, Klein NP, Henninger ML, Williams JTB, Hambidge SJ, Jackson ML, Donahue JG, Qian L, Lindley MC, Gee J, Weintraub ES, Kharbanda EO. Association of the COVID-19 Pandemic with Routine Childhood Vaccination Rates and Proportion Up to Date with Vaccinations Across 8 US Health Systems in the Vaccine Safety Datalink JAMA Pediatr. 2022 Jan 1;176(1):68-77. Doi: 10.1001/jamapediatrics.2021.4251.
Groom HC, Crane B, Naleway AL, Weintraub E, Daley MF, Wain K, Kurilo MB, Burganowski R, DeSilva MB, Donahue JG, Glenn SC, Goddard K, Jackson ML, Kharbanda EO, Lewis N, Lou Y, Lugg M, Scott E, Sy LS, Williams JTB, Irving SA. Monitoring vaccine safety using the Vaccine Safety Datalink: Assessing capacity to integrate data from Immunization Information Systems Vaccine . 2022 Jan 31;40(5):752-756. Epub 2021 Dec 31.
The Vaccine Safety Datalink (VSD) uses vaccination data collected from electronic health records at eight integrated health systems to monitor vaccine safety. To capture additional data about vaccines administered outside traditional health systems, however, vaccine safety researchers looked to the state and local Immunization Information Systems (IIS), which collects vaccination data from non-traditional health settings. Researchers conducted a survey from 2009-2010 to evaluate how VSD incorporates state and local IIS data. Results at that time showed that only three of the then seven VSD sites had received any state or local IIS data. To evaluate the current status of IIS data exchange with VSD, researchers surveyed the now eight VSD sites in January 2021. The survey shows that all eight receive and integrate COVID-19 vaccine data from IIS, which positions the VSD well for conducting quality assessments of vaccine safety.
Hause AM, Baggs J, Marquez P, Myers TR, Gee J, Su JR, Zhang B, Thompson D, Shimabukuro TT, Shay DK. COVID-19 Vaccine Safety in Children Ages 5-11 years — United States, November 3-December 19, 2021 . MMWR Morb Mort Wkly Rep. 2021 Dec 31:70(5152);1755-1760.
Abara WE, Gee J, Mu Y, Deloray M, Ye T, Shay DK, Shimabukuro T. Expected Rates of Select Adverse Events following Immunization for COVID-19 Vaccine Safety Monitoring J Infect Dis . 2021 Dec 27;jiab628. Online ahead of print.
Perez-Vilar S, Dores G, Marquez PL, Ng CS, Cano MV, Rastogi A, Lee L, Su JR, Duffy J. Safety surveillance of meningococcal group B vaccine (Bexsero®), Vaccine Adverse Event Reporting System, 2015-2018 . Vaccine . 2022 Jan 21;40(2):247-254. Epub 2021 Dec 7.
Glanz JM, Clarke CL, Daley MF, Shoup JA, Hambidge SJ, Williams JTB, Groom HC, Kharbanda EO, Klein NP, Jackson LA, Lewin BJ, McClure DL, Xu S, DeStefano F. The Childhood Vaccination Schedule and the Lack of Association with Type 1 Diabetes . Pediatrics. 2021 Dec 1;148(6):e2021051910. Doi: 10.1542/peds.2021-051910 Online ahead of print.
Goud R, Lufkin B, Duffy J, Whitaker B, Wong HL, Liao J, Lo AC, Weintraub E, Kelman JA, Forshee RA. Risk of Guillain-Barré Syndrome Following Recombinant Zoster Vaccine in Medicare Beneficiaries . JAMA Intern Med. 2021 Dec 1;181(12):1623-1630. Doi: 10.1001/jamainternmed.2021.6227. Online ahead of print.
Moro PL, Panagiotakopoulos L, Oduyebo T, Olson CK, Myers T. Monitoring the safety of COVID-19 vaccines in pregnancy in the US . Human Vaccines & Immunotherapies. 2021 Nov 10. doi.org/10.1080/21645515.2021.1984132.
Chapin-Bardales J, Myers T, Gee J, Shay DK, Marquez P, Baggs J, Zhang B, Licata C, Shimabukuro TT. Reactogenicity within 2 weeks after mRNA COVID-19 vaccines: Findings from the CDC v-safe surveillance system . Vaccine. 2021 Nov 26;39(48):7066-7073. Epub 2021 Oct 16.
Pingali C, Meghani M, Razzaghi H, , Lamias MJ, Weintraub E, Kenigsberg TA, Klein NP, Lewis N, Fireman B, Zerbo O, Bartlett J, Goddard K, Donahue J, Hanson K, Naleway A, Kharbanda EO, Yih K, Clark Nelson J, Lewin BJ, Williams JTB, Glanz JM, Singletom JA, Patel SA. COVID-19 Vaccination Coverage Among Insured Persons Aged ≥ 16 years, by Race/Ethnicity and Other Selected Characteristics — Eight Integrated Health Care Organizations, United States, December 14, 2020-May 15, 2021 . MMWR Morb Mortal Wkly Rep . 2021 Jul 16;70(28):985-990.
Shay DK, Shimabukuro TT DeStefano F. Myocarditis Occurring After Immunization with mRNA-Based COVID-19 Vaccines: Editorial . JAMA Cardiol. Published online June 29, 2021. doi:10.1001/jamacardio.2021.2821.
Razzaghi H, Meghani M, Pingali C, Crane B, Naleway A, Weintraub E, Kenigsberg TA, Lamias MJ, Irving SA, Kauffman TL, Vesco KK, Daley MF, DeSilva M, Donahue J, Getahun D, Glee S, Hambidge SJ, Jackson LJ, Lipkind HS, Nelson J, Zerbo O, Oduyebo T, Singleton JA, Patel SA. COVID-19 Vaccination Coverage Among Pregnant Women During Pregnancy — Eight Integrated Health Care Organizations, United States, December 14, 2020-May 8, 2021 . MMWR Morb Mortal Wkly Re p. 2021 Jun 18;70(24):895-899.
Naleway AL, Crane B, Irving SA, Bachman D, Vesco KK, Daley MF, Getahun D, Glenn SC, Hambidge SJ, Jackson LA, Klein NP, McCarthy NL, McClure DL, Panagiotakopoulos L, Panozzo CA, Vazquez-Benitez G, Weintraub E, Zerbo O, Kharbanda EO. Vaccine Safety Datalink infrastructure enhancements for evaluating the safety of maternal vaccination Ther Adv Drug Saf. 2021 Jun 14;12:20420986211021233. eCollection 2021.
Monitoring vaccine safety during pregnancy is important because pregnant women have historically been excluded from vaccine clinical trials. The Vaccine Safety Datalink (VSD) conducts near-real-time surveillance on vaccine safety. When data is collected, researchers use specific algorithms to identify particular subjects. Since 2012, VSD researchers have used an algorithm called Pregnancy Episode Algorithm (PEA) to identify medical records of people who have been vaccinated during pregnancy. In this study, researchers wanted to update and enhance the PEA to include the updated medical codes and incorporate sources of data about how far along the person is in the pregnancy. The researchers did so by developing the Dynamic Pregnancy Algorithm (DPA), which identifies people earlier in their pregnancies. The enhanced PEA and the new DPA will assist researchers in better evaluating the safety of current and future vaccinations administered during or around the time of pregnancy.
Xu S, Clarke Cl, Newcomer SR, Daley MF, Glanz JM. Sensitivity analyses if unmeasured and partially-measure confounders using multiple imputation in a vaccine safety study . Pharmacoepidemiol Drug Saf. 2021 Sept;30(9):1200-1213. Epub 2021 May 31.
Liles E, Irving SA, Dandamudi P, Belongia EA, Daley MF, DeStefano F, Jackson LA, Jacobsen SJ, Kharbanda E, Klein NP, Weintraub E, Naleway AL. Incidence of pediatric inflammatory bowel disease within the Vaccine Safety Datalink network and evaluation of association with rotavirus vaccination Vaccine . 2021 Jun 16;39(27):3614-3620. Epub 2021 May 26.
Multiple studies in the last 20 years have reported an increase of Inflammatory Bowel Disease (IBD) in children. IBD is when the intestines become inflamed, which causes abdominal pain, cramping, and chronic diarrhea. The rotavirus vaccine, a routine pediatric immunization, contains a weakened form of rotavirus that can inflame the lining of the gut. This inflammation could potentially trigger IBD. Researchers wanted to study if there was a connection between pediatric IBD and rotavirus vaccination. From 2007 through 2016, over 2.4 million children ages 10 years and younger from the Vaccine Safety Datalink were included in the analysis. Of the 2.4 million, 333 cases of IBD were identified with rates of IBD higher in children ages 5 to 9 years. The analyzed data suggests there is a small increase of IBD overall in pediatrics with no connection with the rotavirus vaccine. Parents should make sure their children receive their scheduled vaccinations and talk to a healthcare provider about specific concerns.
Gubernot D, Jazwa A, Niu M, Baumblatt J, Gee J, Moro P, Duffy J, Harrington T, McNeil MM, Broder K, Su J, Kamidani S, Olson CK, Panagiotakopoulos L, Shimabukuro T, Forshee R, Anderson S, Bennet S. U.S. Population-Based background incidence rates of medical conditions for use in safety assessment of COVID-19 vaccines . Vaccine. 2021 Jun 23;39(28):3666-3677. Epub 2021 May 14.
Daley MF, Reifler LM, Shoup JA, Narwaney KJ, Kharbanda EO, Groom HC, Jackson ML, Jacobsen SJ, McLean HQ, Klein NP, Williams JTB, Weintraub ES, McNeil MM, Glanz JM. Temporal Trends in Undervaccination: a Population-Based Cohort Study Am J Prev Med . 2021 Jul;61(1):64-72. Epub 2021 Apr 30.
Kharbanda EO, Vazquez-Benitez G, DeSilva MB, Naleway AL, Klein NP, Hechter RC, Glanz JM, Donahue JG, Jackson LA, Sheth SS, Greenberg V, Panagiotakopoulos L, Mba-Jonas A, Lipkind HS. Association of Inadvertent 9-Valent Human Papillomavirus Vaccine in Pregnancy with Spontaneous Abortion and Adverse Birth Outcomes JAMA Netw Open .2021 Apr 1;4(4):e214340.
Woo EK, Moro PL. Postmarketing safety surveillance of quadrivalent recombinant influenza vaccine: Reports to the vaccine adverse event reporting system. Vaccine. 2021 Mar 4;S0264-410X(21)00232-2. Epub ahead of print.
The recombinant hemagglutinin quadrivalent influenza vaccine (Flublok Quadrivalent; RIV4) was approved by FDA in October 2016 for persons 18 years and older to reduce the risk from flu and flu-related complications. To analyze the safety profile of RIV4 since its approval, researchers reviewed adverse events reported to VAERS. From July 1, 2017 through June 30, 2020, VAERS received 849 reports after RIV4 vaccination. A majority of reports (810; 95%) were non-serious; injection site reactions were reported most often. There were 131 reports of allergic reactions. A majority of allergic reactions (127) were reported as non-serious, but required immediate medical care. Reports of allergic reactions do not necessarily suggest that RIV4 is particularly allergenic; some individuals may have a hypersensitivity to drug or vaccine exposure. Among serious adverse event reports, there were 10 cases of Guillain-Barré syndrome. Overall, the analysis did not identify any new safety concerns of RIV4.
Perez-Vilar S, Hu M, Weintraub, Arya D, Lufkin B, Myers T, Woo EJ, Lo AC, Cho S, Swarr M, Liao J, Wernecke M, MaCurdy T, Kelman J, Anderson S, Duffy J, Forshee RA. Guillain-Barré Syndrome After High-Dose Influenza Vaccine Administration in the United States, 2018-2019 Flu Season J Infect Dis . 2021 Feb 13;223(3):416-425. Doi: 10.1093/infdis/jiaa543.
Su JR, McNeil MM, Welsh KJ, Marquez PL, Ng C, Yan M, Cano MV Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018 . Vaccine . 2021 Jan 29; 39(5):839-845. Epub 2021 Jan 6.
Myopericarditis, an inflammation of the heart muscle and tissue around the heart, has many causes including viral infections. While not confirmed as a cause, myopericarditis after vaccination has been periodically reported. Researchers identified reports of myopericarditis following vaccination submitted to the Vaccine Adverse Event Reporting System (VAERS) from 1990–2018. During 1990–2018, VAERS received a total 620,195 reports: 708 (0.1%) met the case definition or were physician-diagnosed as myopericarditis. Most (79%) reports described males, 69% were serious, and 72% had symptom onset within 2 weeks of vaccination. Overall, smallpox (59%) and anthrax (23%) vaccines were most commonly reported, with higher reporting rates only after smallpox vaccine. Myopericarditis remains rarely reported after vaccines licensed for use in the United States. In this analysis, myopericarditis was most commonly reported after smallpox vaccine, and less commonly after other vaccines.
Moro PL, Marquez P. Reports of cell-based influenza vaccine administered during pregnancy in the Vaccine Adverse Event Reporting System (VAERS), 2013-2020. Vaccine. 2021 Jan 22;39(4):678-681. Epub 2020 Dec 25
Flucelvax (ccIIV3 or ccIIV4; ccIIV) was approved by FDA for use in persons aged 18 years and older. There are limited data on the safety of ccIIV in pregnant women or their infants. To assess the safety of ccIIV given during pregnancy, researchers searched VAERS for reports of adverse events (AEs) from July 1, 2013 through May 31, 2020. During that time, VAERS received 4,852 reports following ccIIV, and 391 reports included pregnant women (8%). Of those, 24 (6.1%) were classified as serious. Two neonatal deaths were reported; no maternal deaths occurred. Among the 340 reports with trimester information, ccIIV was administered during the second trimester in 170 (50%). The most frequently reported pregnancy-specific AE was premature delivery (85; 21.7%). There were 62 reports (15.9%) of low birth weight of infants and 15 report of birth defects. While these results are different than previous pregnancy reviews after inactivated influenza vaccines (IIV), no safety concerns were identified.
Kharbanda EO, Vazquez-Benitez G, DeSilva MB, Spaulding AB, Daley MF, Naleway AL, Irving SA, Klein NP, Tseng HF, Jackson LA, Hambridge SJ, Olaiya O, Panozzo CA, Myers TR, Romitti PA. Developing Algorithms for Identifying Major Structural Birth Defects Using Automated Electronic Health Data. Pharmacoepidemiol Drug Saf. 2021 Feb;30(2):266-274. Epub 2020 Dec 3.
Vaccine Safety Datalink (VSD) researchers often rely on electronic health records when conducting observational studies. To improve case identification, researchers use algorithms to accurately identify diagnoses for particular conditions or diseases. Algorithms used in previous studies for selected birth defects were based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. In October 2015, the United States transitioned to the 10 th edition (ICD-10-CM). In this study, researchers updated, validated, and refined algorithms for use with ICD-10-CM codes. Final algorithms were applied to a group of live births delivered between October 2015 through September 2017 at 8 VSD sites and were compared to the original ICD-9-CM algorithms applied to a group of live births in 2004-2013. Results demonstrated that the new ICD-10-CM algorithms can be used for future studies of maternal vaccine safety.
Panagiotakopoulos L, McCarthy NL, Tepper NK, Kharbanda NK, Lipkind HS, Vazquez-Benitez G, McClure DL, Greenberg V, Getahun D, Glanz JM, Naleway AL, Klein NP, Nelson JC, Weintraub ES. Evaluating the Association of Stillbirths After Maternal Vaccination in the Vaccine Safety Datalink. Obstet Gynecol. 2020 Dec;136(6):1086-1094.
The Advisory Committee on Immunization Practices recommends women receive vaccinations against flu and tetanus, diphtheria, and acellular pertussis (Tdap) during each pregnancy. Despite reassuring safety data, pregnant women often have concerns about the safety of vaccines for them and their babies. Researchers used the VSD to evaluate whether vaccinations given during pregnancy were associated with stillbirth (fetal death occurring on or after 20 weeks gestation). The study compared 795 stillbirths (confirmed with medical record review) and 3,180 live birth controls between September 30, 2015 and January 1, 2020. Researchers found 51.7% of stillbirth cases and 52.9% live birth controls were exposed to vaccines during pregnancy, including flu and Tdap vaccines. The findings show that vaccination during pregnancy did not increase the risk of stillbirth, including recommended, non-recommended, and contraindicated vaccines. Overall, the study results support the safety of ACIP recommendations during pregnancy.
Haber P, Tate J, Marquez PL, Moro PL, Parashar U. Safety Profile of rotavirus vaccines among individuals aged ≥8 months of age, United States, vaccine adverse event reporting system (VAERS), 2006-2019. Vaccine. 2020 Nov 29;S0264-410X(20)31466-3. Online ahead of print.
Two live oral rotavirus vaccines, RotaTeq (RV5) and Rotarix (RV1), were introduced into the routine vaccination program in 2006 and 2008, respectively. RV1 is administered at ages 2 and 4 months and RV5 is administered at ages 2, 4, and 6 months. The series is recommended prior to 8 months of age to decrease the risk of intussusception (IS), an intestinal obstruction common in younger children. However, there is limited safety data on the vaccines when given to children older than 8 months. Researchers in the Vaccine Adverse Event Reporting System (VAERS) analyzed reports of adverse events (AEs) following rotavirus vaccination submitted January 2006 through December 2019. A total 344 reports were submitted: 309 reports included children 8 months to 5 years of age, and 35 reports included children 6 years and older. While known AEs were identified – diarrhea, fever and vomiting – no new or unexpected safety concerns were identified for those vaccinated beyond the recommended age.
Perez-Vilar S, Hu M, Weintraub E, Arya D, Lufkin B, Myers T, Woo EJ, Lo A, Chu S, Swarr M, Liao J, Wernecke M, MaCurdy T, Kelman J, Anderson S, Duffy J, Forshee RA. Guillain-Barré Syndrome After High-Dose Influenza Vaccine Administration in the United States, 2018–2019 Season J Infect Dis. 2020 Nov 2; jiaa543. Online ahead of print.
While an association between influenza vaccination and Guillain-Barré syndrome (GBS) was first noticed in 1976, studies in subsequent flu seasons have assessed the risk and found either no or small risk of GBS following influenza vaccination. Early during the 2018-2019 flu season, the Vaccine Safety Datalink (VSD) identified a statistical signal for an increased risk of GBS in days 1–42 following high-dose influenza vaccine (IIV3-HD) administration. The signal was rapidly evaluated using Medicare data by conducting early- and end-of-season analyses. The Medicare analyses, which included more than 7 million IIV3-HD vaccinations, did not detect a statistically significant increased GBS risk. The VSD end-of-season analysis also did not find an increased GBS risk among more than 600,000 IIV3-HD vaccinations. These analyses determined that if a GBS risk existed, it was similar to that from prior seasons.
Duffy J, Marquez P, Dores GM, Ng C, Su J, Cano J, Perez-Vilar S. Safety Surveillance of bivalent meningococcal group B vaccine, Vaccine Adverse Event Reporting System, 2014-2018. Open Forum Infec Dis. 2020 Oct 27. Online ahead of print.
Licensed in October 2014, MenB-FHbp was the first meningococcal group B vaccine approved for use in the United States. The Advisory Committee on Immunization Practices recommends the 3-dose series for individuals aged 10-25 years who are at an increased risk of meningococcal B disease. Researchers reviewed reports of adverse events (AEs) following MenB-FHbp submitted to the Vaccine Adverse Event Reporting System (VAERS) from October 2014 through December 2018. During this time period, VAERS received 2,106 reports involving MenB-FHbp, representing 698 reports per million doses distributed (over 3 million doses were distributed in this analysis period). The most common AEs reported were fever (27%), headache (25%), and pain (16%). Overall, the review did not identify any new safety issues. The most commonly reported AEs following MenB-FHbp were consistent with those identified in clinical trials as described in the package insert.
Miller ER, McNeil MM, Moro PL, Duffy J, Su JR. The reporting sensitivity of the Vaccine Adverse Event Reporting System (VAERS) for anaphylaxis and for Guillain-Barré syndrome. Vaccine. 2020 Nov 3;38(47)7458-7463. Epub 2020 Oct 7.
Underreporting is an important limitation that is common to passive surveillance systems. The number of adverse events (AEs) that occur after vaccination and the percentage of those that get reported to the Vaccine Adverse Event Reporting System (VAERS) is unknown. To determine the sensitivity of VAERS in capturing AE reports, researchers analyzed pre-specified outcomes – anaphylaxis and Guillain-Barré syndrome (GBS) – reported to VAERS and determined if they are similar to previous estimates for other severe AEs. These estimates used were obtained from published studies of the Vaccine Safety Datalink of anaphylaxis and GBS following vaccination. VAERS sensitivity for capturing anaphylaxis after seven different vaccines ranged from 13-76%; sensitivity for capturing GBS after three different vaccines ranged from 12-64%. For anaphylaxis and GBS, VAERS sensitivity is comparable to previous estimates for detecting important AEs following vaccination.
Panagiotakopoulos L, Myers TR, Gee J, Lipkind HS, Kharbanda EO, Ryan DO, Williams, JTB, Naleway AL, Klein NP, Hambridge SJ, Jacobsen SJ, Glanz JM, Jackson LA, Shimabukuro TT, Weintraub ES. SARS-CoV-2 Infection Among Hospitalized Pregnant Women: Reasons for Admission and Pregnancy Characteristics – Eight U.S. Health Care Centers, March 1-May 30, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1355-1359. 2020 Sept 25.
As part of CDC surveillance of COVID-19 hospitalizations, Vaccine Safety Datalink researchers identified 105 pregnant women with SARS-CoV-2 infection from March 1 through May 30, 2020. Of those, 43 (41%) were admitted for COVID-19 illness (e.g., worsening respiratory status) and 62 (59%) were admitted for pregnancy-related treatment or procedures (e.g, delivery) and identified with SARS-CoV-2 infection. More pregnant women with prepregnancy obesity and gestational diabetes were hospitalized for the treatment of COVID-19 illness than pregnant women admitted for pregnancy-related reasons. Intensive care was required in 30% (13/43) of pregnant women admitted for COVID-19 illness, and one pregnant woman died from COVID-19. Adverse birth outcomes, such as preterm delivery and stillbirth, were more common among pregnant women with SARS-CoV-2 infection, regardless of symptoms. Pregnant women should take preventive measures to protect themselves against SARS-CoV-2 infection.
Mbaeyi SA, Bozio CH, Duffy J, Rubin LG, Hariri S, Stephens DS, MacNeil JR. Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020 Sept;69(No. RR-9):1-41.
This report compiles and summarizes all recommendations from CDC’s Advisory Committee on Immunization Practices (ACIP) for use of meningococcal vaccines in the United States; it is intended for use by clinicians and public health providers. A systematic literature search was completed to review all available evidence on the immunogenicity, effectiveness, and safety of U.S. licensed quadrivalent meningococcal conjugate (MenACWY) and serogroup B meningococcal (MenB) vaccines among age groups for which the vaccines were approved. To further assess vaccine safety, data were evaluated from the Vaccine Adverse Event Reporting System (VAERS) and the Vaccine Safety Datalink (VSD), two post-licensure surveillance systems for adverse events.
Myers TR, McNeil MM, NG CS, Li R, Marquez PL, Moro PL, Omer SB, Cano MV. Adverse Events Following Quadrivalent Meningococcal Diphtheria Toxoid Conjugate Vaccine (Menactra ®) Reported to the Vaccine Adverse Event Reporting System (VAERS), 2005-2016. Vaccine. 2020 Sep 11;38(40):6291-6298 Epub 2020 Jul 31.
Licensed in January 2005, Menactra was the first quadrivalent meningococcal conjugate vaccine approved to provide protection against invasive meningococcal disease. It is licensed for use in individuals aged 9 months through 55 years. Researchers reviewed reports of adverse events (AEs) after Menactra to the Vaccine Adverse Event Reporting System (VAERS) from 2005-2016, including serious reports, selected pre-specified outcomes, and use during pregnancy. From January 2005 thought June 2019, VAERS received 13,075 reports of AEs following Menactra vaccination. Most reports (94%) were classified as non-serious; commonly reported AEs included injection site redness and swelling, fever, headache, and dizziness. There were 36 reports of death following Menactra; researchers did not find any evidence to suggest the vaccine caused the deaths. This review did not reveal any new safety concerns and provides further reassurance regarding the safety of Menactra.
Moro PL, Woo EL, Marquez P, Cano M. Monitoring the safety of high-dose, trivalent inactivated influenza vaccine in the vaccine adverse event reporting system (VAERS), 2011-2019. Vaccine. 2020 Aug 18;38(37):5923-5926. Epub 2020 Jul 21.
Older adults are at higher risk of developing serious complications from flu. In December 2009, the high-dose trivalent influenza vaccine (IIV3-HD) was licensed for adults 65 years and older. Using the Vaccine Adverse Event Reporting System, researchers analyzed the 12,320 reports submitted after IIV3-HD vaccination from 2011-2019. Of the total, there were 61 reports of GBS and 13 of anaphylaxis. Nearly 6% of all reports were classified as serious (723). The most commonly reported serious events were fever (30.2%), weakness (28.9%), and shortness of breath (24.9%). There were 55 reports of death following IIV3-HD, and cause of deaths reported were typical for those in this age group with no evidence to suggest the vaccine caused the deaths. There were reports of 13 pregnant women and 59 children who inadvertently received IIV3-HD. Overall, this review of IIV3-HD did not reveal any new safety concerns among individual adults 65 years and older.
Wang SV, Stefanini K, Lewis E, Newcomer SR, Fireman B, Daley MF, Glanz JM, Duffy J, Weintraub E, Kulldorf M. Determining Which of Several Simultaneously Administered Vaccines Increase Risk of an Adverse Event Drug Saf. 2020 Oct;43(10):1057-1065. Epub 2020 Jul 1.
The CDC childhood immunization schedule recommends all children get vaccinated. Children may get multiple vaccinations on the same day. If a child has an adverse event after getting multiple vaccinations, it would be difficult to determine which vaccine, if any, caused the event. Using observed data from two Vaccine Safety Datalink sites, researchers developed a systematic process to determine which of the simultaneously administered vaccine(s) are most likely to have caused an observed increase in risk of an adverse event. From the five scenarios simulated, the process determined which of the vaccines contributed to the simulated excess risk. This process could be used again in the future to provide valuable information on the potential risk of adverse events following individual and simultaneous vaccinations.
Hesse EM, Navarro RA, Daley MF, Getahun D, Henninger ML, Jackson LA, Nordin J, Olson SC, Zerbo O, Zheng C, Duffy J. Risk for Subdeltoid Bursitis After Influenza Vaccination: A Population-Based Cohort Study Ann Intern Med. 2020 Aug 18;173(4):253-261. Epub 2020 Jun 23.
Subdeltoid bursitis, characterized by pain or loss of motion in the shoulder, has been reported as an adverse event following intramuscular vaccination in the upper arm, and most case reports involved the influenza vaccine. With over 160 million U.S. doses distributed annually and recommended to everyone over 6 months of age, researchers wanted to estimate the risk of subdeltoid bursitis following influenza vaccination. In this cohort study using data from 7 Vaccine Safety Datalink sites, researchers included people who received an inactivated influenza vaccine during the 2016–2017 flu season, totaling 2.9 million people. The analysis to calculate risk of bursitis compared cases that appeared 3 days following vaccination to a control period 30-60 days following vaccination. There were an estimated 7.78 (95% CI 2.19-13.38) additional cases of bursitis per one million people vaccinated. While an increased risk of bursitis following vaccination was present, the overall risk was small.
Hause AM, Panagiotakopoulos L, Weintraub E, Sy LS, Glenn SC, Tseng HF, McNeil MM. Adverse Outcomes in Pregnant Women Hospitalized with Respiratory Syncytial Virus Infection: A Case-Series Clin Infect Dis. 2020 Jun 2; ciaa668. Online ahead of print.
Respiratory syncytial virus (RSV) is a common respiratory virus that usually causes mild, cold-like symptoms and can be serious for infants and older adults. RSV infection in pregnant women has not been well described and can be clinically severe and result in hospitalization. CDC has emphasized the need to characterize RSV infection during pregnancy, including burden of the illness, risk factors for severe disease, and pregnancy and neonatal outcomes. In this study, researchers identified 25 pregnant women at Kaiser Permanente Southern California who tested positive for RSV. Ten of those women (40%) were hospitalized: five were diagnosed with pneumonia/atelectasis, two with respiratory failure (one requiring mechanical ventilation), and two with sepsis. Six women had a pregnancy complication during hospitalization, including one induced preterm birth. The information from this study may inform the benefits of maternal vaccination for an RSV vaccine intended to protect infants.
Suragh TA, Hibbs B, Marquez P, McNeil MM. Age inappropriate influenza vaccination in infants less than 6 months old, 2010-2018 Vaccine. 2020 May 6;38(21):3747-3751. Epub Apr 6.
Annual influenza (flu) vaccination is recommended for everyone 6 months or older, and vaccination in infants less than 6 months old is a vaccine error. There are few safety studies in this population. Researchers searched the Vaccine Adverse Event Reporting System (VAERS) for reports of adverse events (AEs) following flu vaccination in infants less than 6 months old from 2010-2018. A total of 114 reports were found; 21 reported a specific AE. Fever, irritability, crying and diarrhea were the most common symptoms. Researchers identified several risk factors: 1) individuals getting vaccinated together resulting in patient mix-ups, 2) healthcare provider not verifying the patient’s information, and 3) provider confusion due to similarities in vaccines’ packaging and names of vaccines that sound alike. This study adds valuable information about the general absence of serious AEs in infants vaccinated with flu vaccine; yet, providers should be vigilant to avoid these preventable errors.
Glanz JM, Clarke CL, Xu S, Daley MF, Shoup JA, Schroeder EB, Lewin BL, McClure DL, Kharbanda E, Klein NP, DeStefano F. Association between Rotavirus Vaccine and Type 1 Diabetes in Children. JAMA Pediatr. 2020 May 1;174(5):455-462. Epub 2020 Mar 9.
Type 1 diabetes mellitus (T1DM) is an autoimmune disease that tends to occur in genetically susceptible individuals and is primarily diagnosed during childhood. Previous research suggests that a live attenuated rotavirus vaccine could either increase or decrease the risk of T1DM in early childhood. Researchers conducted a study of children enrolled in 7 integrated healthcare organizations in the Vaccine Safety Datalink. There were 386,937 children enrolled born between 2006 and 2014. During their infancy, 360,169 children were exposed to the full series of rotavirus vaccination, 15,765 partially exposed and 11,003 unexposed. By the end of 2017, 464 children had developed T1DM. The incidence of T1DM was not significantly different across the vaccination groups, indicating that rotavirus vaccination is not associated with T1DM in children.
Hause AM, Hesse EM, Ng C, Marquez P, McNeil MM, Omer SB. Association Between Vaccine Exemption Policy Change in California and Adverse Event Reporting. Pediatr Infec Dis J., May; 39(5):369-373. Epub 2020 Mar 5.
California Senate Bill 277 (SB277) eliminated non-medical immunization exemptions starting February 19, 2015. Since the bill’s introduction, the rate of medical exemptions in the state has increased. There is a perception that filing a report to the Vaccine Adverse Event Reporting System (VAERS) may aid in applying for a medical exemption. Researchers wanted to describe trends of reporting to VAERS after SB277. From June 2011-July 2018, 6,703 VAERS reports were submitted from California. Parent-submitted reports increased after SB277, from 14% to 23%. The median reporting time by parents increased from 9 days post-vaccination in 2013-2014 to 31 days in 2016-2017. Overall, there was an increase in reports submitted more than 6 months post-vaccination and reports describing behavioral and developmental symptoms. These changes in reporting patterns after SB277’s implementation may indicate more parents are using VAERS to assist in applying for a medical exemption for their child.
Newcomer SR, Daley MF, Marwaney KJ, Xu S, DeStefano F, Groom HC, Jackson ML, Lewin BJ, McLean HQ, Nordin JD, Zerbo O, Glanz JM. Order of Live and Inactivated Vaccines and Risk of Non-vaccine-targeted Infections in US Children 11-23 Months of Age. Pediatr Infect Dis J., 2020 Mar:39(3);247-253.
Children in the United States receive up to 28 vaccine doses against 14 diseases before their 2nd birthday and 3 are live vaccines. Some observational studies suggest that receiving live vaccines may be associated with decreased non-vaccine targeted infection (NVTI) risk. Researchers conducted a retrospective study within the Vaccine Safety Datalink to estimate the risk of NVTIs based on most recent vaccine type received in children 11-23 months of age. Electronic health records and immunization data were reviewed from children born between 2003-2013. Among 428,608 children, 4.9% had more than 1 immunization visit with live vaccines only and 10.3% had a NVTI. Researchers observed modest associations between live vaccine receipt and a decreased risk of NVTIs, which may have been influenced by multiple factors, including healthcare-seeking behavior. In total, the results support the current sequence of live and inactivated vaccines in the U.S. vaccine schedule with respect to NVTI.
Walter EB, Klein NP, Wodi AP, Roundtree W, Todd CA, Wiesner A, Duffy J, Marquez PL, Broder K. Fever after Influenza, Diphtheria-Tetanus-Acellular Pertussis, and Pneumococcal Vaccinations. Pediatrics . 2020 Mar;145(3):e20191909.
A previous CDC study showed that children aged 6-23 months had an increased risk for febrile seizure after simultaneously receiving inactivated influenza vaccine (IIV), pneumococcal conjugate vaccine (PCV13) and diphtheria-tetanus-acellular pertussis vaccine (DTaP). Researchers wanted to see if administering the IIV at a separate visit reduced the risk of post-vaccination fever and potentially febrile seizure. In the 2017-2018 influenza season, 221 children aged 12-16 months were randomized at two CISA sites into 2 groups. Both groups had 2 visits, 2 weeks apart: group 1 (simultaneous) received the PCV13, DTaP, and quadrivalent IIV (IIV4) vaccines at visit 1; no vaccines at visit 2. Group 2 (sequential) received PCV13 and DTaP at visit 1 and IIV4 visit 2. Similar proportions of children in both groups had fever on days 1-2 after visits (simultaneous 8.1%; sequential 9.3%). Delaying IIV4 by 2 weeks in children receiving DTaP and PCV13 did not reduce fever occurrence after vaccination.
Havers FP, Moro PL, Hunter P, Hariri S, Bernstein H. Use of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccines: Updated Recommendations of the Advisory Committee on Immunization Practices – United States, 2019. MMWR Morb Mortal Wkly Rep. 2020 Jan;69:77-83..
In 2005, the Advisory Committee on Immunization Practices recommended a single dose of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine for adolescents and adults. After the initial Tdap vaccine, booster doses of tetanus and diphtheria toxoids (Td) vaccine are recommended every 10 years or when indicated for wound management. During the October 2019 meeting, ACIP updated its recommendation to allow the use of Tdap or Td in situations where only Td was recommended. These situations include the tetanus booster recommended for adults every 10 years, tetanus prophylaxis when indicated for wound management in people who previously received Tdap, and for multiple doses in the catch-up immunization schedule for people 7 years of age and older with an unknown or incomplete vaccination history. This recommendation update allows providers to have flexibility at the point-of-care for patients.
Haber P, Moro PL, Ng C, Dores GM, Perez-Vilar S, Marquez PL, Cano M. Safety review of tetanus toxoid, reduced diphtheria toxoid, acellular pertussis vaccines (Tdap) in adults aged ≥ 65 years, Vaccine Adverse Event Reporting System (VAERS), United States, September 2010 – December 2018. Vaccine. . 2020 Feb 5;38(6):1476-1480. Epub 2019 Dec 28.
The Advisory Committee on Immunization Practices recommends vaccination in adults 65 years of age and older with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap). To date, few studies have assessed the safety of Tdap in this age group. Using the Vaccine Adverse Event Reporting System (VAERS), researchers analyzed reports of adverse events (AEs) following Tdap in adults 65 years and older. From September 2010 to December 2018, VAERS received 1,798 reports; 94% were classified as non-serious. The most common AEs were injection site redness (26%), pain (19%), and swelling (18%). Of 104 serious reports, 7 deaths were reported; none had evidence to suggest the vaccine caused the deaths. Serious non-death reports included nervous system disorders (35.1%; n=34) and infections (18.6%; n=18). Overall, the analysis did not identify any new safety concerns and is consistent with prior post-marketing observations and pre-licensure studies.
Li R, Stewart B, Rose C. A Bayesian approach to sequential analysis in post-licensure vaccine safety surveillance. Pharm Stat. 2020 May;19(3):291-302 Epub 2019 Dec 22.
Bayesian statistics is an approach for learning from evidence as it accumulates. While this analytic method is used in other areas of public health with acknowledged practical benefits, its potential application in vaccine safety monitoring analysis has not been fully realized. In this study, researchers compare the use of a traditional (frequentist) sequential method and a Bayesian method, with simulations and a real-world vaccine safety example. The performance was evaluated using 3 metrics: false positive rate, false negative rate, and average earliest detection time. The authors found that depending on the background rate of adverse events, the Bayesian sequential method could significantly improve performance in terms of the false negative rate and decrease the earliest time to producing a safety signal for further analysis. Overall, the Bayesian sequential approach was found to show promise as an alternative for vaccine safety monitoring.
Su JR, Haber P, Ng CS, Marquez PL, Dores GM, Perez-Vilar S, Cano MV . Erythema multiforme, Stevens Johnson syndrome, and toxic epidermal necrolysis reported after vaccination, 1999-2017. Vaccine . 2020 Feb 11;38(7): 1746-1752. Epub 2019 Dec 20.
While some dermatologic adverse events are common after vaccination (i.e. redness at the injection site), erythema multiforme (EM), Stevens Johnson Syndrome (SJS), Toxic Epidermal Necrolysis (TEN), and SJS/TEN are rare. Since the last review of VAERS data for these conditions, over 37 new vaccines were approved for use in the United States. Of the 466,027 reports to VAERS during 1999–2017, researchers identified and reviewed 984 reports of EM, 89 of SJS, 6 of SJS/TEN, and 7 of TEN. Most reports of EM (91%) were non-serious; 52% of SJS and all reports of SJS/TEN and TEN were serious. Most reports (58%) occurred within 7 days after vaccination. Childhood vaccines were reported most often; 48% of reports were of children younger than 4 years. Of 6 reported deaths, 5 were exposed or potentially exposed to medications known to cause these conditions, and 1 had severe dehydration. Overall, reporting of these conditions after vaccination remained rare, with no new safety concerns identified.
Yu W, Zheng C, Xie F, Chen W, Mercado C, Sy LS, Qian L, Glenn S, Tseng HF, Lee G, Duffy J, McNeil MM, Daley MF, Crane B, McLean HQ, Jackson LA, Jacobsen SJ. The use of natural language processing to identify vaccine-related anaphylaxis at five health care systems in the Vaccine Safety Datalink. Pharmacoepidemiolo Drug Saf. 2020 Feb;29(2): 182-188 Epub 2019 Dec 3.
Anaphylaxis is a rare but serious allergic reaction that can be caused by various triggers, including vaccine components. Natural language processing (NLP) uses computers to analyze large amounts of text. Vaccine Safety Datalink (VSD) researchers developed an NLP application to identify vaccine-related anaphylaxis cases from electronic medical record notes and implemented the method at 5 VSD sites. The NLP system was trained on a dataset of 311 potential anaphylaxis cases and validated on another 731 potential cases. NLP was then applied to the notes of 6.4 million vaccinated patients, and it captured 8 additional true cases confirmed by manual chart review. This study demonstrated the potential to apply NLP to clinical notes to identify anaphylaxis cases and its use to improve sensitivity and efficiency in future vaccine safety studies.
Hesse EM, Atanasoff S, Hibbs BF, Adegoke OJ, Ng C, Marquez P, Osborn M, Su JR, Moro PL, Shimabukuro T, Nair N. Shoulder Injury Related to Vaccine Administration (SIRVA): Petition Claims to the National Vaccine Injury Compensation Program, 2010-2016. Vaccine. 2020 Jan 29;38(5): 1076-1083. Epub 2019 Nov 28.
Petitioner claims for shoulder injury related to vaccine administration (SIRVA) to the National Vaccine Injury Compensation Program (VICP) increased substantially from 2010 to 2016. The Health Resources and Services Administration and the Centers for Disease Control and Prevention initiated a joint scientific review of clinical characteristics of SIRVA petitions to VICP. Researchers queried VICP’s Injury Compensation System database for alleged SIRVA and SIRVA-like injuries and conducted a descriptive analysis of claims recommended by VICP for concession as SIRVA injuries; 476 claims were identified and 400 of them involved influenza vaccine. Of the 476 claims, 227 reported a suspected administration error; 172 reported ‘injection too high’ on the arm. Injection too high on the arm could be a factor due to the risk of injecting into underlying non-muscular tissues. Healthcare providers should be aware of proper injection technique and anatomical landmarks when administering vaccines.
Hibbs BF, Ng CS, Museru O, Moro PL, Marquez P, Woo EJ, Cano MV, Shimabukuro TT. Reports of atypical shoulder pain and dysfunction following inactivated influenza vaccine, Vaccine Adverse Event Reporting System (VAERS), 2010-2017. Vaccine. 2020 Jan 29;38(5):1137-1143. Epub 2019 Nov 26.
Some case reports have suggested that if inactivated influenza vaccine (IIV) is improperly administered, shoulder dysfunction may occur. Researchers reviewed reports of adverse events (AEs) made to the Vaccine Adverse Event Reporting System (VAERS) following IIV from July 2010 to June 2017. During this time, approximately 996 million flu vaccine doses were distributed in the United States. Of the 59,230 reports submitted, 1,220 met analysis criteria of atypical shoulder pain and dysfunction starting within 48 hours following IIV and continuing for more than 1 week. The analysis suggests these reports were not common, averaging 2% of flu vaccine AEs reported each year; most were females (82.6%), median age was 52 years. While the cause of these cases is unknown, vaccines given improperly might be a factor. Proper vaccine administration education and training are preventive measures.
Donahue JG, Kieke BA, Lewis EM, Weintraub ES, Hanson KE, McClure DL, Vickers ER, Gee J, Daley MF, Destefano F, Hechter RC, Jackson LA, Klein NP, Naleway AL, Nelson JC, Belongia EA. Near Real-Time Surveillance to Assess the Safety of the 9-valent Human Papillomavirus Vaccine. Pediatrics. 2019 Dec; 144(6): e20191808. Epub 2019 Nov 18.
Gardasil 9 (human papillomavirus 9-valent vaccine, recombinant; 9vHPV) was approved in 2014 for females and males to protect against 9 types of human papillomavirus infections that can cause cancer. CDC’s Vaccine Safety Datalink (VSD) conducted near real-time post-licensure safety monitoring following 9vHPV for 11 pre-specified adverse events (AEs), including anaphylaxis, allergic reaction, appendicitis, certain neurological disorders, pancreatitis, and stroke. From October 2015 to October 2017, 838,991 9vHPV doses were administered to people aged 9-26 years at 6 VSD sites. Statistical signals were detected for 2 expected AEs: injection site reactions and syncope. Signals were also detected for appendicitis, pancreatitis, and allergic reaction; however, evaluation and medical record reviews did not confirm these to be true associations. Overall, no new safety concerns were identified. The results are consistent with pre-licensure clinical trial data and support the favorable safety profile of 9vHPV.
Shimabukuro TT, Su JR, Marquez PL, Mba-Jonas A, Arana JE, Cano MV. Safety of the 9-Valent Human Papillomavirus Vaccine. Pediatrics 2019 Dec; 144(6). pii: e20191791. Epub 2019 Nov 18.
Gardasil 9 (human papillomavirus 9-valent vaccine, recombinant; 9vHPV) was approved in 2014 for females and males to protect against 9 types of human papillomavirus infections that can cause cancer. Researchers analyzed reports of adverse events (AEs) after 9vHPV to the Vaccine Adverse Event Reporting System (VAERS) from December 2014 to December 2017. During that time, approximately 28 million 9vHPV doses were distributed in the United States. Of the 7,244 reports received, 31% were female, nearly 22% were male, and 47% of reports did not identify gender. Over 97% of reports were classified as non-serious. There were 2 deaths reported; no information in the reports or medical records suggested the deaths were related to vaccination. Overall, the analysis revealed no new or unexpected safety concerns. The 9vHPV safety profile is consistent with pre-licensure clinical trial data, and with the post-marketing safety data of Gardasil, the earlier quadrivalent HPV vaccine.
Moro PL, McNeil MM. Challenges in evaluating post-licensure vaccine safety: observations from the Center for Disease Control and Prevention. Expert Rev Vaccines. 2019 Oct; 18(10): 1091-1101 Epub 2019 Oct 19.
There is overwhelming scientific evidence that supports the safety of vaccines and their proven ability to prevent illness and death caused by infectious diseases. Yet like any medicine, no vaccine can be considered completely safe and completely effective. Prior to licensure, vaccines undergo extensive safety and efficacy evaluations. After licensure, they require follow up studies and continuous monitoring to investigate any new or unexpected adverse events (AEs). This article presents challenges in monitoring U.S. vaccines for AEs after licensure and describes CDC’s post-licensure safety surveillance infrastructure, including the Vaccine Adverse Event Reporting System, the Vaccine Safety Datalink, and the Clinical Immunization Safety Assessment project. The authors describe each system’s unique strengths and limitations, and the harmonized approach they provide in meeting vaccine safety monitoring challenges.
Groom HC, Smith N, Irving SA, Koppolu P, Vazquez-Benitez G, Kharbanda EO, Daley MF, Donahue JG, Getahun D, Jackson LA, Klein NP, McCarthy NL, Nordin JD, Panagiotakopoulos L, Naleway AL. Uptake and safety of hepatitis A vaccination during pregnancy: A Vaccine Safety Datalink study. Vaccine . 2019 Oct 16;37(44):6648-6655. Epub 2019 Sep 20.
Although uncommon, infection with hepatitis A virus during pregnancy is associated with gestational complications and pre-term labor. CDC recommends that pregnant women who are at an increased risk of contracting hepatitis A get the Hepatitis A vaccine (HepA). Current safety data, however, are limited on maternal HepA vaccination. Researchers used the Vaccine Safety Datalink to compare pregnancies with HepA exposure to other vaccine exposures, and those with no exposure, from 2004-2015. Of nearly 667,000 pregnancies, 1,140 had HepA exposure. The rate of maternal HepA vaccination was low, and rarely due to documented risk factors. The results did not show an increased risk of adverse events for HepA vaccination during pregnancy. There was an identified association of maternal HepA exposure and small-for-gestational age (SGA) infants, however, the difference in rates were small (4%), and likely due to other factors. Further research may be needed to further explore this association.
McNeil MM, Paradowska-Stankiewicz I, Miller ER, Marquez PL, Seshadri S, Collins LC Jr, Cano MV. Adverse events following adenovirus type 4 and type 7 vaccine, live, oral in the Vaccine Adverse Event Reporting System (VAERS), United States, October 2011-July 2018. Vaccine . 2019 Oct 16; 37(44): 6760-6767 Epub 2019 Sep 20.
Adenovirus vaccine (adenovirus type 4 and type 7, live, oral) was licensed by FDA in March 2011 for use in U.S. military personnel ages 17-50 years. The vaccine was first routinely given to recruits in October 2011. Researchers reviewed reports of adverse events (AEs) following the adenovirus vaccine from October 2011 to July 2018 using the Vaccine Adverse Event Reporting System (VAERS). VAERS received 100 adverse event reports; 39 were considered serious. While the reporting rate for serious AEs was higher than with other vaccines given in a comparison recruit population (39% versus 18%), no unexpected or concerning pattern of adenovirus vaccine AEs were identified. Reports showed multiple other vaccines (95%) and penicillin G (50%) were given at the same time, and these exposures may have contributed to the higher reporting rate for serious AEs observed with the adenovirus vaccine. Future studies without these exposures would be helpful in clarifying the vaccine’s safety profile.
Donahue JG, Kieke BA, King JP, Mascola MA, Shimabukuro TT, DeStefano F, Hanson KE, McClure DL, Olaiya O, Glanz JM, Hechter RC, Irving SA, Jackson LA, Klein NP, Naleway AL, Weintraub ES, Belongia EA. Inactivated influenza vaccine and spontaneous abortion in the Vaccine Safety Datalink in 2012-13, 2013-14, and 2014-15. Vaccine. 2019 Oct 16;37(44):6673-6681. Epub 2019 Sep 17.
A prior study in the Vaccine Safety Datalink (VSD) covering the two influenza seasons from 2010-2012 reported an association between inactivated influenza vaccine (IIV) and spontaneous abortion (SAB), but only among women who had also been vaccinated in the previous influenza season. In follow-up, VSD researchers conducted a larger case-control study over three more recent influenza seasons (2012-2015). Women with SAB were matched with women who had live births according to VSD site, influenza vaccination status in the previous influenza season, and other factors. The main analysis included 1,236 women. During the three influenza seasons, researchers found no association between IIV and SAB, including among women vaccinated in the previous season. These findings lend support to current recommendations for influenza vaccination at any time during pregnancy, including the first trimester.
Kochhar S, Excler JL, Bok K, Gurwith M, McNeil MM, Seligman SJ, Khuri-Bulos N, Klug B, Laderoute M, Robertson JS, Singh V, Brighton Collaboration Viral Vector Vaccines Safety Working Group (V3SWG). Defining the Interval for Monitoring Potential Adverse Events Following Immunization (AEFIs) After Receipt of Live Viral Vectored Vaccines. Vaccine. 2019 Sep 10;37(38): 5796-5802.
New viral vector vaccines that use live viruses to create an immune response are being developed to fight serious infectious agents like HIV and Ebola. As some live recombinant vectored vaccines may replicate, a key challenge is defining the length of time for monitoring potential adverse events following immunization (AEFI). Potential options include: 1) adapting from the current relevant regulatory guidelines; 2) convening a panel of experts to review the evidence from a systematic literature search to narrow down a list of likely potential or known AEFI and establish the optimal risk window(s); and 3) conducting “near real-time” prospective monitoring for unknown clustering’s of AEFI in validated large linked vaccine safety databases. Depending on the infrastructure, human resources, and databases available in different countries, the authors suggest appropriate options can be determined by regulatory agencies and investigators.
Christianson MS, Wodi P, Talaat K, Halsey N. Primary Ovarian Insufficiency and Human Papilloma Virus Vaccines: A Review of the Current Evidence. Am J Obstet Gynecol . 2020 Mar;222(3):239-244. Epub 2019 Aug 31.
Human papillomavirus (HPV) is the primary cause of cervical cancer, and vaccination is the primary means of preventing cancers caused by HPV infection. Despite HPV vaccine being available for over a decade, coverage rates are lower than other vaccines. Public concerns regarding the vaccine’s safety, including that it may cause primary ovarian insufficiency (POI), have been identified as an important barrier to vaccination. POI-related concerns are driven in part by isolated reports of ovarian failure following the HPV vaccine. In this Clinical Immunization Safety Assessment Project review, researchers summarize published peer-reviewed literature on HPV vaccines and POI. In summary, the current evidence is insufficient to suggest or support a causal relationship between HPV vaccination and POI. Healthcare providers can help address concerns about POI and the HPV vaccine by sharing these findings during consultations with their patients.
DeStefano F, Monk Bodenstab H, Offit PA. Principal Controversies in Vaccine Safety in the United States. Clin Infect Dis. 2019 Aug 1;69(4):726-731.
Concerns about vaccine safety can lead to decreased acceptance of vaccines and resurgence of vaccine-preventable diseases. The authors summarize the key evidence on some of the main current vaccine safety controversies in the United States, including: 1) MMR vaccine and autism; 2) thimerosal, a mercury-based vaccine preservative, and the risk of neurodevelopmental disorders; 3) vaccine-induced Guillain-Barré Syndrome (GBS); 4) vaccine-induced autoimmune diseases; 5) safety of HPV vaccine; 6) aluminum adjuvant-induced autoimmune diseases and other disorders; and 7) too many vaccines given early in life predisposing children to health and developmental problems. A possible small increased risk of GBS following influenza vaccination has been identified, but the magnitude of the increase is less than the risk of GBS following influenza infection. Otherwise, the biological and epidemiologic evidence does not support any of the reviewed vaccine safety concerns.
McNeil MM. Vaccine-Associated Anaphylaxis. Curr Treat Options Allergy. 2019 Sep; 6(3): 297-308. Epub 2019 Jul 16.
Anaphylaxis is a rare, serious hypersensitivity reaction, which can happen within minutes and is characterized by multisystem involvement. Although anaphylaxis may occur after any vaccine, the risk following flu vaccines is important to understand due to the large number of persons vaccinated annually. This review looks at two recent CDC studies that confirm its rarity. In a 25-year review of data from the Vaccine Adverse Event Reporting System, reports in children most commonly followed childhood vaccinations, and in adults most often followed influenza vaccine. In a Vaccine Safety Datalink study, the estimated incidence of anaphylaxis was 1.3 per million vaccine doses administered for all vaccines and 1.6 per million doses for IIV3 (trivalent) influenza vaccine. Despite its rarity, the rapid onset and potentially lethal nature of anaphylaxis requires that all personnel and facilities providing vaccinations have procedures in place to treat it.
Edwards K, Hanquet G, Black S, Mignot E, Jankosky C, Shimabukuro T, Miller E, Nohynek H, Neels P. Meeting Report Narcolepsy and Pandemic Influenza Vaccination: What We Know and What We Need to Know Before the Next Pandemic? A Report From the 2 nd IABS Meeting. Biologicals. 2019 Jul;60:1-7.
Scientific and public health experts and key stakeholders gathered to discuss the state of knowledge on the relationship between adjuvanted monovalent pH1N1 vaccines and narcolepsy. There was consensus that an increased risk of narcolepsy was consistently observed after Pandemrix (AS03-adjuvanted), but similar associations following Arepanrix (AS03) or Focetria (MF59) were not observed. It is not clear whether the differences are due to vaccine composition or other factors such as the timing of large-scale vaccination programs relative to pH1N1 wild-type virus circulation in different geographic regions. Limitations of retrospective observational methodologies could also be contributing to some of the differences across studies. Additional research is needed to further explain the association and possible mechanistic pathways, and to aid in planning and preparation for vaccination programs in advance of the next influenza pandemic.
Hesse EM, Hibbs BF, Cano MV. Notes from the Field: Administration of Expired Injectable Influenza Vaccines Reported to the Vaccine Adverse Event Reporting System — United States, July 2018–March 2019. MMWR Morb Mortal Wkly Rep. 2019; 68: 529–530. 2019 June 14.
During the 2018-2019 flu season, the Vaccine Adverse Event Reporting System received 125 reports (totaling 192 patients) of people receiving expired inactivated influenza vaccine (IIV). During that time, 169.1 million doses of seasonal flu vaccine were distributed. Of those who received the expired IIV, 70% were in high-risks group for influenza (under the age of 5, over the age of 50 and pregnant women). Researchers found the reported adverse events were consistent with adverse events following administration of non-expired seasonal IIV, suggesting no additional safety issues associated with receipt of expired IIV. To avoid inadvertent administration of expired IIV, CDC recommends facilities that administer vaccines follow the guidance in the Vaccine Storage and Handling Toolkit, and make plans for the safe disposal or return of any remaining IIV after the expiration date of June 30 each year.
Weinmann S, Naleway AL, Koppolu P, Baxter R, Belongia EA, Hambidge SJ, Irving SA, Jackson ML, Lewin B, Liles E, Marin M, Smith N, Weintraub E, Chun C. Incidence of Herpes Zoster Among Children: 2003-2014. Pediatrics. 2019 Jul; 144(1). Pii: e20182917. Epub 2019 Jun 10.
After the 1996 introduction of routine varicella (chickenpox) vaccination in the U.S., most studies evaluating the incidence of pediatric herpes zoster (HZ), also known as shingles, reported lower incidence over time, with varying degrees of decline. Researchers used data from 6 integrated health care organizations surveyed by the Vaccine Safety Datalink to examine HZ incidence rate in children from 2003-2014. Using electronic medical records from children aged 0 to 17 years, researchers identified HZ cases and calculated HZ incidence rates for all children and children who were vaccinated versus unvaccinated. Researchers then calculated rates for the 12-year period, examined temporal trends, and compared HZ rates by month and year of age at vaccination. This population-based study confirms the decline in pediatric HZ incidence and the significantly lower incidence among children who are vaccinated, and reinforces the benefit of routine varicella vaccination to prevent pediatric HZ.
Moro PL, Arana J, Marquez PL, Ng C, Barash F, Hibbs BF, Cano M. Is there any harm in administering extra-doses of vaccine to a person? Excess doses of vaccine reported to the Vaccine Adverse Event Reporting System (VAERS), 2007-2017. Vaccine. 2019 Jun 19; 37(28): 3730-3734. Epub 2019 May 30.
The administration of an extra dose of a vaccine may occur due to a vaccination error or when there is need to provide immunization in a person with uncertain vaccination histories (e.g., refugees). There is little data available on the safety of an extra dose of vaccine. Researchers searched for adverse events following the administration of excess doses of vaccines using the Vaccine Adverse Events Reporting System from January 2007 through the end of July 2017. Of 366,815 total reports received, over 5,000 (1.4%) reported an excess dose of vaccine was administered and less than 4,000 (76.9%) did not describe an AE. The top two vaccines reported were trivalent inactivated influenza (15.4%), and varicella (13.9%). The most common events were fever (12.8%), and injection site reaction (9.7%). Among reports where an AE was reported, researchers did not observe any unexpected conditions or clustering of AEs.
Hanson KE, McLean HQ, Belongia EA, Stokley S, McNeil MM, Gee J, VanWormer JJ. Sociodemograhic and clinical correlates of human papillomavirus vaccine attitudes and receipt among Wisconsin adolescents. Papillomavirus Res. 2019 Dec; 8: 100168; Epub 2019 May 25.
Few studies have assessed adolescent human papillomavirus (HPV) vaccine attitudes and whether they are associated with vaccination uptake. The Vaccine Safety Datalink conducted an HPV vaccine study in an integrated healthcare system to identify factors associated with adolescents’ attitude changes and their link to vaccine receipt. Adolescents who had not completed the HPV vaccine series were surveyed using a modified version of the Carolina HPV Immunization Attitudes and Beliefs Scale before and during a campaign to improve HPV vaccination rates. Adolescents’ attitudes to HPV slightly improved during the period of the campaign. However, attitude changes were not associated with receipt of HPV vaccines and adolescents identified as opposed to HPV vaccine before the campaign began were less likely to receive a HPV vaccine dose afterwards. More research is needed to learn how HPV vaccine attitudes form in parents and children, and how best to address concerns about vaccine harms.
Kochhar S, Edwards KM, Ropero Alvarez AM, Moro PL, Ortiz JR. Introduction of new vaccines for immunization in pregnancy – Programmatic, regulatory, safety and ethical considerations . Vaccine . 2019 May 31; 37(25): 3267-3277. Epub 2019 May 6.
Women are encouraged to get immunizations when they are pregnant; but in certain areas of the world, there are no programs to implement vaccine recommendations. Maternal immunization is a promising strategy to reduce infectious disease-related illness and death in pregnant women and their infants. Pre-requisites for introducing immunization during pregnancy include: (1) political commitment and adequate financial resources, (2) healthcare workers to deliver vaccines, (3) combining immunization programs with prenatal care and maternal/child health services, and (4) access to prenatal care for pregnant women in low and middle-income countries where births occur in healthcare facilities. A system to advance a vaccine program from product licensure to successful country-level implementation needs to include evidence of anticipated vaccine program impact, developing supportive policies, and translating policies into local action.
Hechter RC, Qian L, Tartof SY, Sy LS, Klein NP, Weintraub E, Mercado C, Naleway A, McLean HQ, Jacobsen SJ. Vaccine safety in HIV-infected adults within the Vaccine Safety Datalink Project . Vaccine. 2019 May 31; 37(25): 3296-3302. Epub 2019 May 4.
Despite the increased risk of vaccine-preventable infectious diseases in adults with HIV, vaccine coverage among this risk group remains low; safety concerns around side effects or impact on HIV disease may be a factor. Using data from 5 U.S. integrated healthcare sites in the Vaccine Safety Datalink, researchers evaluated the safety of recommended vaccinations among HIV-infected adults. They evaluated 20,417 HIV-infected adults from 2002-2013 and found an elevated risk of cellulitis and infection, particularly among patients with high viral load and those who received bacterial vaccines. These findings were consistent with prior reports in the literature. The analysis did not find an increased risk of other adverse events of interest. Patients with HIV with very high viral load might have elevated risk for stroke and cerebrovascular diseases; future research should examine further. Overall, this study reassures that vaccines currently recommended for HIV-infected adults are safe.
Cook AJ, Wellman RD, Marsh T, Shoaibi A, Tiwari R, Nguyen M, Boudreau D, Weintraub ES, Jackson L, Nelson JS. Applying sequential surveillance methods that use regression adjustment or weighting to control confounding in a multisite, rare-event, distributed setting: Part 2 in-depth example of a reanalysis of the measles-mumps-rubella-varicella combination vaccine and seizure risk. J Clin Epidemiol. 2019 Sep; 113: 114-122. Epub 2019 May 2.
Safety surveillance of newly marketed vaccines is a public health priority. National systems have linked vast amounts of electronic health record (EHR) data across multiple health care organizations and insurers. This allows monitoring of large patient groups for potential safety concerns. Group sequential methods (methods of evaluating data as it is entered) involve routine estimation and testing of vaccine-outcome associations over time. This method can lead to earlier identification of excess risk compared with one-time analysis. Researchers assessed the use of two different sequential methods for safety monitoring: analysis-based confounder adjustment (influential variables) and weighting (the number items or events). Both methods were applied to the FDA’s Sentinel network, that already positively paired the outcome to the vaccine. The estimates from both methods were similar and comparable to prior studies of different designs and are viable alternatives for safety monitoring.
DeStefano F, Shimabukuro TT. The MMR Vaccine and Autism. Annu Rev Virol. 2019 Sep; 6. Epub 2019 Apr 15.
The most damaging vaccine safety controversy of recent years began as an exploration of the possible role of measles and measles vaccines in causing of inflammatory bowel disease (IBD). That work eventually evolved into a report published in 1998, but subsequently retracted by the journal, that suggested Measles-mumps-rubella (MMR) vaccine causes autism. Although numerous scientific studies have since refuted a connection between MMR vaccine and autism, some parents are still hesitant to accept MMR vaccination of their children because they are uncertain about the safety of the vaccine. In this review, the authors summarize the genesis of the controversy and review the scientific evidence against a causal association. Also discussed is the effect of the controversy on MMR vaccine acceptance and the resurgence of measles outbreaks, as well as what can be done to bolster vaccine confidence, including the central role of scientists and healthcare providers.
Zheng C, Yu W, Xie F, Chen W, Mercado C, Sy LS, Qian L, Glenn S, Lee G, Tseng HF, Duffy J, Jackson LA, Daley MF, Crane B, McLean HQ, Jacobsen SJ. The use of natural language processing to identify Tdap-related local reactions at five health care systems in the Vaccine Safety Datalink , International Journal of Medical Informatics , 2019 Jul; 127(1386-5056): 27-34. Epub 2019 Apr 13.
The Vaccine Safety Datalink (VSD) plays a critical role in monitoring adverse events after vaccinations by using the electronic health records. Most studies performed in the VSD rely on diagnosis codes and manual chart review for outcome identification and confirmation. A natural language processing (NLP) system was developed, then deployed and executed at multiple institutions. The system achieved reasonable accuracy in identifying a specific vaccine-related adverse event. This study demonstrates the feasibility of using NLP to reduce the potential burden of conducting manual chart review in future vaccine safety studies. “False negatives” of diagnosis codes are not commonly investigated in vaccine safety studies. NLP can identify cases missed by diagnosis codes. NLP has many potential applications in future vaccine safety studies based on the considerations of the pros and cons of NLP and the specific requirements of the study.
Myers TR, McCarthy NL, Panagiotakopoulos L, Omer SB. Estimation of the Incidence of Guillain-Barré Syndrome During Pregnancy in the United States . Open Forum Infectious Diseases . 2019 Mar; 6(3): ofz071.
Guillain-Barré syndrome (GBS) is an adverse event of interest after vaccination, yet little is known about how frequently this rare neurologic disorder occurs during pregnancy. GBS may be an outcome of particular interest during Zika vaccine trials, because it has been associated with Zika virus infection. In this Vaccine Safety Datalink study, researchers identified potential GBS cases from January 1, 2004 through July 31, 2015 during pregnancy and the 42 days following birth. Of the 1.2 million pregnancies that met inclusion criteria, 35 potential cases of GBS were identified and 2 cases were confirmed as incident GBS during pregnancy. The resulting estimated incidence rate for GBS during pregnancy was 2.8 GBS cases per million person-years. These findings will help inform future safety assessments of Zika and other vaccines in pregnant populations.
Klein NP, Goddard K, Lewis E, Ross P, Gee J, DeStefano F, Baxter R. Long term risk of developing type 1 diabetes after HPV vaccination in males and females. Vaccine . 2019 Mar 28; 37(14):1938-1944. Epub 2019 Mar 1.
Despite scientific evidence, public concerns that the human papillomavirus (HPV) vaccine can cause autoimmune diseases persist. The Vaccine Safety Datalink evaluated whether HPV vaccine is associated with a long-term increased risk of type 1 diabetes at one participating site. This retrospective cohort study identified all potential type 1 diabetes cases from Kaiser Permanente Northern California members who were between 11 and 26 years old any time after June 2006 through December 2015 – over 900,000 individuals. Of the 2,613 cases of type 1 diabetes identified, 338 (123 vaccinated with HPV and 265 unvaccinated) remained in the analysis. Over the 10 years of the study period, comparing vaccinated with unvaccinated persons, researchers did not find an increased risk of type 1 diabetes associated with HPV vaccine receipt.
Haber P, Moro PL, Ng C, Dores GM, Lewis P, Cano M. Post-licensure surveillance of trivalent adjuvanted influenza vaccine (aIIV3; Fluad), Vaccine Adverse Event Reporting System (VAERS), United States, July 2016-June 2018. Vaccine . 2019 Mar 7;37(11):1516-1520. Epub 2019 Feb 7.
Trivalent adjuvanted influenza vaccine (aIIV3; Fluad®) was approved in the U.S. in 2015 for adults aged 65 years and older, and has been in use since the 2016-2017 influenza season. Using the Vaccine Adverse Event Reporting System, researchers analyzed U.S. reports for aIIV3 submitted from July 2016 to June 2018, totaling 630 reports. Of note, there were 79 reports of people under the age of 65 who received the vaccine. The most commonly reported adverse events were consistent with pre-licensure studies, and included injection site pain and redness. Researchers did not identify any new safety concerns associated with aIIV3 among individuals indicated for the vaccine (65 years of age or older). Importantly, vaccine providers should be aware of and follow the prescribing information for the vaccine and administer it only to patients in the recommended age range.
Hesse EM, Shimabukuro TT, Su JR, et al. Postlicensure Safety Surveillance of Recombinant Zoster Vaccine (Shingrix) — United States, October 2017–June 2018 . MMWR Morb Mortal Wkly Rep. 2019 Feb 1; 68(4):91–94.
This is the first report covering post-licensure safety monitoring of the recombinant zoster vaccine (RZV; Shingrix, GSK) in the Vaccine Adverse Event Reporting System (VAERS) during the initial 8 months of use in the United States. From October 2017 to June 2018, VAERS received 4,381 adverse event reports related to Shingrix; 4,251 (97%) were classified as non-serious. During that timeframe, about 3.2 million doses of Shingrix were distributed in the United States. The most common symptoms reported were fever, and injection site pain and redness. These findings are consistent with pre-licensure clinical trial data, and no unexpected patterns were detected. Clinicians should counsel patients to expect common reactions such as pain, swelling, and redness at the injection site, along with possible body aches, fever, and chills. These reactions usually resolve on their own in 2 to 3 days.
Landazabal CS, Moro PL, Lewis P, Omer SB. Safety of 9-valent human papillomavirus vaccine administration among pregnant women: Adverse event reports in the Vaccine Adverse Event Reporting System (VAERS), 2014-2017 . Vaccine . 2019 Feb 21; 37(9):1229-1234. Epub 2019 Jan 16.
9-valent human papillomavirus vaccine (9vHPV) was approved by FDA in December 2014. 9vHPV is not recommended during pregnancy but some women of childbearing age may be inadvertently exposed. This study assessed reports to Vaccine Adverse Event Reporting System (VAERS) of pregnant women vaccinated with 9vHPV in the United States between December 2014-December 2017. Disproportionate reporting of adverse events (AEs) was assessed using proportional reporting ratios. A total of 82 pregnancy reports were identified. Sixty reports (73.2%) did not describe an AE. The most frequently reported AEs were miscarriage and injection site reactions (both n=3; 3.7%). Of note, miscarriage may occur in up to one-third of pregnancies; the observed reports in this study were not unusual or unexpected. No disproportional reporting for any AE was found. Overall, no unexpected AEs were observed among these pregnancy reports.
Su JR, Moro PL, Ng CS, Lewis PW, Said MA, Cano MV. Anaphylaxis after vaccination reported to the Vaccine Adverse Event Reporting System, 1990-2016. J Allergy Clin Immunol . 2019 Apr; 143(4):1465-1473. Epub 2019 Jan 14.
Anaphylaxis is a rare, potentially life-threatening hypersensitivity reaction that can occur after vaccination. During 1990–2016, the Vaccine Adverse Event Reporting System (VAERS) received a total of 467,960 reports. Researchers identified 828 reports describing persons who were physician-diagnosed with or met the Brighton Collaboration case definition for anaphylaxis. Of reports in people aged 18 years or younger, 65% were male; childhood vaccines were most commonly reported. Of reports in people aged 19 years and older, 80% were female, and influenza vaccines were most commonly reported. Over 40% of the 828 reports described persons with no history of hypersensitivity. Of 8 reported deaths, 4 had no history of hypersensitivity. Anaphylaxis after vaccination is rare, but can occur, including among persons with no history of hypersensitivity. Providers who administer vaccines should be prepared to manage severe hypersensitivity reactions.
Tartof SY, Qian L, Liu IA, Tseng HF, Sy LS, Hechter RC, Lewin BJ, Jacobsen SJ. Safety of Influenza Vaccination Administered During Hospitalization . Mayo Clin Proc . 2019 Mar; 94(3):397-407. Epub 2019 Jan 8.
CDC recommends that hospitalized patients who are eligible to receive influenza vaccine be vaccinated before discharge; however, previous data suggest that rates of influenza immunization among hospitalized patients before discharge remain low. In a retrospective cohort study conducted at Kaiser Permanente Southern California, investigators analyzed whether influenza vaccination during hospitalization was associated with an increased risk of outpatient and emergency department visits, readmissions, fever, and clinical laboratory evaluations for infection in the 7 days following discharge. Investigators found no increased risk for these outcomes among those vaccination during hospitalization compared with those who were never vaccinated or were vaccinated at other times. These findings provide reassurance about the safety of influenza vaccination during hospitalization.
McClure DL, Jacobsen SJ, Klein NP, Naleway AL, Kharbanda EO, Glanz JM, Jackson LA, Weintraub ES, McLean HQ. Similar relative risks of seizures following measles containing vaccination in children born preterm compared to full-term without previous seizures or seizure-related disorders . Vaccine . 2019 Jan 3; 37(1):76-79. Epub 2018 Nov 23.
In the United States, measles-mumps-rubella (MMR) and measles-mumps-rubella-varicella (MMRV) vaccines are recommended to children at age 12 months and older. These vaccines are associated with a small increased risk of febrile seizures during the second week after vaccination. This Vaccine Safety Datalink study assessed the relative risk of febrile seizures after MMR/MMRV vaccination in children born preterm and children born full-term. Prior to this study, limited data were available on the safety of vaccinations given during the second year of life in preterm children. Researchers looked at 532,375 children (45,343 preterm and 487,032 full-term) who received their first dose of measles-containing vaccine at age 12 through 23 months. The data showed similar relative risk of seizure in both groups. The results support current Advisory Committee on Immunization Practices recommendations to administer the first dose of these vaccines at age 12 through 15 months for all children, including those born preterm.
McNeil MM, Duderstadt SK, Sabatier JF, Ma GG, Duffy J. Vaccination and Risk of Lone Atrial Fibrillation in the Active Component United States Military. Hum Vaccin Immunother. 2018 Nov 16;15(3): 669-676. Epub 2019 Jan 8.
In this retrospective population-based cohort study of nearly 3 million U.S. military personnel, researchers looked at whether receiving the anthrax vaccine absorbed (AVA) increased the risk of atrial fibrillation in those who did not have identifiable underlying risk factors or structural heart disease (lone atrial fibrillation). The authors used the Defense Medical Surveillance System to review military personnel on active duty from January 1, 1998 through December 31, 2006. Following over 11,000 person-years of service, the study found no elevated risk of diagnosed lone atrial fibrillation associated with AVA (adjusted risk ratio of 0.99), influenza, or smallpox vaccinations given during military service. These findings may be helpful in planning future vaccine safety research.
Moro PL, Lewis P, Cano M Adverse events following purified chick embryo cell rabies vaccine in the Vaccine Adverse Event Reporting System (VAERS) in the United States, 2006-2017 – Correspondence Travel Medicine and Infectious Disease 2019 May-Jun; 29(1477-8939): 80-81. Epub 2018 Oct 26.
Rabies is a viral disease of mammals most often transmitted through the bite of a rabid animal and is life threatening. For those exposed to the virus, the benefits of vaccination outweigh the risks. There are two cell cultures rabies vaccines available in the United States: human diploid cell vaccine (HDCV – licensed in 1980) and purified chick embryo cell vaccine (PCECV – licensed in 1997). A safety study on PCECV has not been done since 2005. Researchers re-assessed the safety of the vaccine in the Vaccine Adverse Event Reporting System (VAERS) from January 2006 through June 2017. Excluding non-U.S. reports and duplicate records, VAERS received 604 reports involving PCECV during the 10 year time frame. Of those, 42 were coded as serious reports. No deaths were reported. Data mining analysis did not reveal disproportional reporting for any adverse event. Adverse events reported were consistent with previous post-licensure study and no new or unexpected adverse events were observed.
Weibel D, Sturkenboom M, Black S, de Ridder M, Dodd C, Bonhoeffer J, Vanrolleghem A, van der Maas N, Lammers GJ, Overeem S, Cauch-Dudek K, Juhasz D, Campitelli M, Datta AN, Kallwei U, Huan WT, Hsu CY, Chen HC, Giner-Soriano M, Morros R, Gaig C, Tió E, Perez-Vilar S, Diez-Domingo J, Puertas FJ, Svenson LW, Mahmud SM, Carleton B, Naus M, Arnheim-Dahlström L, Pedersen L, DeStefano F, Shimabukuro TT. Narcolepsy and Adjuvanted Pandemic Influenza A (H1N1) 2009 Vaccines – Multi-county Assessment. Vaccine. 2018 Oct 1;26(41):6202-6211.
In 2010, a safety signal was detected for narcolepsy in several European countries following vaccination with Pandemrix, a monovalent pandemic H1N1 (pH1N1) vaccine containing AS03 adjuvant. The reports followed large-scale pH1N1 vaccination campaigns during 2009-10. To investigate further, a study team including CDC scientists analyzed vaccine safety data on adjuvanted pH1N1 vaccines (Arenaprix-AS03, Focetria-MF59, and Pandemrix-AS03) from 10 global study sites. Researchers did not detect any new associations between the vaccines and narcolepsy.
Suragh TA, Lamprianou A, MacDonald NE, Loharikar AR, Balakrishnan MR, Benes O, Hyde TB, McNeil MM. Cluster Anxiety-Related Adverse Events Following Immunization (AEFI): An Assessment of Reports Detected in Social Media and Those Identified Using an Online Search Engine. Vaccine. 2018 Sep25;26(40):5949-5954.
Adverse events following immunization (AEFI) that arise from anxiety can occur in clusters and may result in unnecessary medical treatments and disrupted vaccination programs. News of these incidents can spread rapidly via the internet and social media. In this study, researchers used Google and Facebook to identify reports of cluster anxiety-related AEFIs not found in traditional peer-reviewed literature and found 39 reports referring to 18 unique cluster events. The most common vaccine mentioned was human papillomavirus (HPV) vaccine (48.7%). The majority of reports (97.4%) involved children; all occurred in a school setting or as part of vaccination campaigns. Five vaccination programs were reportedly halted despite investigations finding no link between the adverse events and the vaccines. These results demonstrate the potential for using information from the web to supplement traditional sources for identifying cluster anxiety-related AEFIs.
Suragh TA, Lewis P, Arana J, Mba-Jonas A, Li R, Stewart B, Shimabukuro TT, Cano M. Safety of bivalent human papillomavirus vaccine in the US vaccine adverse event reporting system (VAERS), 2009-2017. Br J Clin Pharmacol . 2018 Dec; 84(12):2928-2932. Epub 2018 Sep 21.
In 2009, bivalent human papillomavirus vaccine (2vHPV, Cervarix) was licensed for use in the United States. Due to low use in the marketplace, the manufacturer stopped supplying 2vHPV in the United States in 2016 and withdrew it from the U.S. market completely in late 2017. The vaccine is currently licensed and used in at least 134 other countries worldwide. In this review, reports submitted to the Vaccine Adverse Event Reporting Systems (VAERS) following 2vHPV vaccination during 2009-2017 were analyzed. During this period, over 720,000 2vHPV doses were distributed in the U.S.; VAERS received 241 adverse event reports. Researchers did not identify any new or unexpected safety concerns in their review.
Fortner KB, Swamy GK, Broder KR, Jimenez-Truque N, Zhu Y, Moro PL, Liang J, Walter EB, Heine RP, Moody MA, Yoder S, Edwards KM. Reactogenicity and immunogenicity of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant and nonpregnant women . Vaccine. 2018 Oct 8; 36(42):6354-6360. Epub 2018 Sep 13.
CDC recommends that pregnant women receive Tdap vaccine to protect young infants from pertussis (whooping cough). The CISA Project study enrolled 374 pregnant and 225 nonpregnant women to evaluate safety and immune responses after Tdap; 53% of the pregnant women had received Tdap in the past. Pregnancy and infant health outcomes were also assessed and will be described in a future report. Injection-site and systemic reactions (e.g., fever) were assessed for 7 days after Tdap. Blood was collected from the women before and after Tdap to evaluate immune responses. Researchers found that Tdap was well-tolerated in pregnant and nonpregnant women. Pregnant women were more likely to report moderate or severe injection-site pain (18%) compared with nonpregnant women (11%) but this did not lead to medical visits. Prior Tdap receipt did not increase occurrence of moderate or severe reactions in pregnant women. Immune responses to all Tdap vaccine antigens were robust in both groups.
Groom HC, Irving SA, Koppolu P, Smith N, Vazquez-Benitez G, Kharbanda EO, Daley MF, Donahue JG, Getahun D, Jackson LA, Tse Kawai A, Klein NP, McCarthy NL, Nordin JD, Sukumaran L, Naleway AL. Uptake and safety of Hepatitis B vaccination during pregnancy: A Vaccine Safety Datalink study . Vaccine . 2018 Oct 1; 36(41):6111-6116. Epub 2018 Sep 5.
Hepatitis B virus (HBV) infection acquired during pregnancy can pose a risk to the infant at birth that can lead to significant and lifelong morbidity. Hepatitis B vaccine (HepB) is recommended for anyone at increased risk for contracting HBV infection, including pregnant women. Prior to this study, limited data were available on the safety of HepB administration during pregnancy. In this Vaccine Safety Datalink retrospective cohort study, researchers assessed potential association between maternal HepB vaccinations and pre-specified maternal and infant safety outcomes, looking at pregnancies resulting in live births from 2004-2015. Women were continuously enrolled from 6 months pre-pregnancy to 6 weeks postpartum. Most women who received maternal HepB did not have high-risk indications for vaccination. The study found there was no increased risk for the examined adverse events in women who received maternal HepB or in their offspring.
Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and Control of Seasonal Influenza with Vaccines: Recommendation of the Advisory Committee on Immunization Practices – United States, 2018-2019 Influenza Season. MMWR Recomm Rep. 2018 Aug 24;67(No. RR-3):1-20.
Routine annual influenza vaccination is recommended for all persons 6 months of age and older who do not have contraindications. A licensed, recommended, and age-appropriate vaccine should be used. Inactivated influenza vaccines (IIVs), recombinant influenza vaccine (RIV), and live attenuated influenza vaccine (LAIV) are expected to be available for the 2018–19 season. For adults 65 years and older, any age-appropriate IIV formulation or RIV4 are acceptable options. Given unknown but theoretical concerns of increased reactogenicity when administering two new adjuvant-containing vaccines, selection of a nonadjuvanted influenza vaccine may be considered in situations where influenza vaccine and another vaccine containing a new adjuvant are to be administered concomitantly; vaccination should not be delayed if a specific product is not available. Vaccines with newer adjuvants, like other vaccines, should be administered at separate sites from other vaccines that are given concomitantly.
Naleway AL, Mittendorf KF, Irving SA, Henninger ML, Crane B, Smith N, Daley MF, Gee J. Primary Ovarian Insufficiency and Adolescent Vaccination . Pediatrics. 2018 Sep; 14(3). Epub 2018 Aug 21.
Published case series have suggested a potential association between human papillomavirus (HPV) vaccination and primary ovarian insufficiency (POI). But, no population-based epidemiological studies have been reported. To the authors’ knowledge, this new Vaccine Safety Datalink study – a population-based, retrospective cohort study of nearly 200,000 women – is a first, and overcomes some of the limitations of earlier post-licensure monitoring that relied on passive reporting. Researchers found there was no elevated risk of POI following HPV, Tdap, IIV, and MenACWY vaccination in women of reproductive age. These findings should lessen concern about potential impact on fertility from adolescent vaccination.
Haber P, Amin M, Ng C, Weintraub E, McNeil MM. Reports of lower respiratory tract infection following dose 1 of RotaTeq and Rotarix vaccines to the Vaccine Adverse Event Reporting System (VAERS), 2008-2016 . Hum Vaccin Immunother. 2018 Jul 11:1-5. Epub 2018 Jul 26.
A recent GlaxoSmithKline post-marketing study found a possible association between the administration of the first dose of the rotavirus vaccine Rotarix and lower respiratory tract infections (LRTI) in infants 0-6 days after vaccination. Using Vaccine Adverse Event Reporting System data, this study examined reports of LRTIs in infants 6-15 weeks old who received one of two rotavirus vaccines, Rotarix or RotaTeq, in addition to either the 7-valent (PCV7) or 13-valent (PCV13) pneumococcal conjugate vaccine. Reports of LRTIs occurring in the 0-29 day window following the first dose of the rotavirus vaccination were analyzed between January 2008 and December 2016. Researchers found LRTI rates were not different in those infants from rates of LRTIs in infants receiving other recommended childhood vaccines.
Kharbanda EO, Vazquez-Benitez G, Lipkind HS, Sheth SS, Zhu J, Naleway AL, Klein NP, Hechter R, Daley MF, Donahue JG, Jackson ML, Kawai AT, Sukumaran L, Nordin JD. Risk of Spontaneous Abortion After Inadvertent Human Papillomavirus Vaccination in Pregnancy . Obstet. Gynecol. 2018 Jul; 132(1): 35-44.
Quadrivalent human papillomavirus vaccine (4vHPV) is not recommended during pregnancy but may be given inadvertently when pregnancy status is not known. While data on HPV vaccine exposures during or around the time of pregnancy have not raised concerns, additional safety studies are needed. Using the Vaccine Safety Datalink, researchers conducted a retrospective observational cohort study that evaluated the risk of spontaneous abortion following 4vHPV before and during pregnancy. Between January 2008 and November 2014, 2,800 pregnancies were identified with 4vHPV exposure. The authors found the risk of spontaneous abortion did not increase among women who received 4vHPV before or during pregnancy. These findings are consistent with pre-licensures clinical trials and post-licensure safety studies.
Su JR, Ng C, Lewis PW, Cano MV. Adverse events after vaccination among HIV-positive persons, 1990-2016. PLoS One . 2018 Jun 19; 13(6) e0199229.
Vaccines are especially critical for people with chronic health conditions such as HIV infection, and are recommended by Advisory Committee on Immunization Practices and CDC based on a person’s immune status. Through this study, researchers looked at U.S. reports to Vaccine Adverse Event Reporting System during 1990-2016 to investigate if people living with HIV experienced unexpected adverse events (AEs) or unusual patterns of AEs after vaccination. The analysis found no unexpected or unusual patterns of AEs. These results support the safety of recommended vaccines in people with HIV. Of note, 2 people with HIV with severely compromised immune systems died from widespread infection after receiving live virus vaccines. Healthcare providers should be aware of a patient’s immune status prior to administration of live virus vaccines. Following ACIP best practices can help prevent rare, but life-threatening, AEs.
Walker WL, Hills SL, Miller ER, Fischer M, Rabe IB. Adverse events following vaccination with an inactivated, Vero cell culture-derived Japanese encephalitis vaccine in the United States, 2012-2016 . Vaccine . 2018 Jul 5; 36(29):4369-4374. Epub 2018 Jun 8.
Inactivated Vero cell culture-derived vaccine (JE-VC; IXIARO) was licensed by Food and Drug Administration in 2009 and has a generally favorable safety profile. In this review of adverse events (AEs) following JE-VC reported to Vaccine Adverse Event Reporting System during May 1, 2012 through April 30, 2016, researchers found reporting rates of AEs were similar to those of the previous analysis (2009-2012). Although reporting rates of AEs in children could not be calculated, there were low numbers of reported events in this age group. Safety surveillance for this relatively new vaccine continues to be important to monitor AE reporting rates and identify possible rare serious events.
Moro PL, Perez-Vilar S, Lewis P, Bryant-Genevier M, Kamiya H, Cano M. Safety Surveillance of Diphtheria and Tetanus Toxoids and Acellular Pertussis (DTaP) Vaccines . Pediatrics . 2018 Jul; 142(1). Epub 2018 Jun 4.
Diphtheria, tetanus toxoids and acellular pertussis (DTaP) vaccines were first licensed by the Food and Drug Administration in 1991. To assess the post-licensure safety of DTaP vaccines, researchers reviewed reports of adverse events following vaccination submitted to the Vaccine Adverse Event Reporting System (VAERS). From January 1991 to December 2016, 50,157 reports were submitted to VAERS following DTaP vaccination. The most frequently reported adverse events were injection site redness (25.3%), fever (19.8%), and injection site swelling (15.0%). This assessment did not identify any new or unexpected safety issues and supports the favorable safety profile from pre-clinical trials. Reports of non-serious vaccination errors, such as incorrect vaccine administered or wrong site, call for better education of providers on the specific indications for each of the DTaP vaccines.
Jackson ML, Yu O, Nelson JC, Nordin JD, Tartof SY, Klein NP, Donahue JG, Irving SA, Glanz JM, McNeil MM, Jackson LA. Safety of repeated doses of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine in adults and adolescents . Pharmacoepidemiol. Drug Saf. 2018 Aug; 27(8): 921-925. Epub 2018 Jun 3.
Because protective pertussis immunity may wane within 5 years of Tdap (tetanus toxoid, reduced diphtheria toxoid and acellular pertussis) vaccine receipt, maintaining protection may require repeated vaccination. A possible strategy would be to recommend Tdap in place of decennial Td (tetanus toxoid, reduced diphtheria) doses. This VSD study evaluated the safety of repeated doses of tetanus-containing vaccine at intervals <10 years between doses among a population of 68,915 non-pregnant adults and adolescents. Compared to 7,521 subjects who received a subsequent dose of Td vaccine, 61,394 subjects who received a subsequent dose of Tdap did not have significantly elevated risk of medical visits for seizure, cranial nerve disorders, limb swelling, pain in limb, cellulitis, paralytic syndromes, or encephalopathy/encephalitis/meningitis. These results suggest that repeated Tdap vaccination has acceptable safety relative to Tdap vaccination followed by subsequent Td vaccination.
Tseng HF, Sy LS, Qian L, Liu IA, Mercado C, Lewin B, Tartof SY, Nelson J, Jackson LA, Daley MF, Weintraub E, Klein NP, Belongia E, Liles EG, Jacobsen SJ. Pneumococcal Conjugate Vaccine Safety in Elderly Adults . Open. Forum Infect. Dis. 2018 May 2; 5(6): ofy100. Epub 2018 Jun.
The 13-valent pneumococcal conjugate vaccine (PCV13) and the 23-valent pneumococcal polysaccharide vaccine (PPSV23) are both licensed vaccines recommended for use in adults 65 years of age and older to protect against pneumococcal disease. PPSV23 protects against 23 types of the approximately 90 types of pneumococcal bacteria and was first licensed in 1983; the newer PCV13 vaccine protects against 13 types of pneumococcal bacteria and was licensed in 2010. In this large cohort study using data from 6 Vaccine Safety Datalink sites, researchers compared the risk in adults 65 years of age and older for serious adverse events (AEs) following vaccination with either PCV13 or PPSV23. The analysis did not find an increased risk of adverse events following PCV13 administration compared to PPSV23, and should provide reassurance regarding use of PCV13.
Shimabukuro TT, Miller ER, Strikas RA, Hibbs BF, Dooling K, Goud R, Cano MV Notes from the Field: Vaccine Administration Errors Involving Recombinant Zoster Vaccine — United States, 2017–2018. MMWR Morb Mortal Wkly Rep. 2018 May 25; 67: 585–586.
During the first four months of RZV (Shingrix®) monitoring (October 20, 2017-February 20, 2018), Vaccine Adverse Event Reporting System received a total of 155 reports, of which 13 (8%) documented an administration error, some with more than one type of error. Vaccine providers may be confusing administration procedures and storage requirements between the older ZVL (Zostavax®) vaccine and the newly licensed RZV. Prior experience indicates that reports of administration errors are highest shortly after licensure and recommendation, likely due to lack of familiarity with a new vaccine. To prevent RZV administration errors, vaccine providers should be aware of prescribing information, storage requirements, preparation guidelines, and Advisory Committee on Immunization Practices recommendations for herpes zoster vaccines.
Miller ER, Lewis P, Shimabukuro TT, Su J, Moro P, Woo EJ, Jankosky C, Cano M. Post-licensure safety surveillance of zoster vaccine live (Zostavax®) in the United States, Vaccine Adverse Event Reporting System (VAERS), 2006-2015 . Hum Vaccin Immunother. 2018 Mar 26; 14(8): 1963-1969 Epub 2018 May 18.
Herpes zoster (HZ), or shingles, is caused by reactivation of varicella-zoster virus—the same virus that causes chickenpox. Live-attenuated HZ vaccine (zoster vaccine live, ZVL, Zostavax) was licensed by the Food and Drug Administration in 2006 to prevent shingles and is recommended by CDC for people 60 years and older. Researchers reviewed reports of adverse events following ZVL to the Vaccine Adverse Event Reporting System (VAERS) from May 1, 2006 through January 31, 2015. During this time, close to 22 million ZVL doses were distributed. VAERS received 23,092 reports; 96% were classified as non-serious. The most common adverse events reported included injection site pain (27%), HZ (17%), injection site swelling (17%) and rash (14%). This review did not detect new or unexpected safety signals.
Carter RJ, Idriss A, Widdowson MA, Samai M, Schrag SJ, Legardy-Williams JK, Estivariz CF, Callis A, Carr W, Webber W, Fischer ME, Hadler S, Sahr, Thompson M, Gerby SM, Edem-Hotah J, M’baindu Momoh R, McDonald W, Gee JM, Flagbata Kallon A, Spencer-Walters D, Bresee JS, Cohn A, Hersey S, Gibson L, Schuchat A, Seward JF. Implementing a Multisite Clinical Trial in the Midst of an Ebola Outbreak: Lessons Learned from the Sierra Leone Trial to Introduce a Vaccine Against Ebola. J Infect Dis. 2018 Jen 15;217(suppl_1):S16-S23. Epub 2018 May 18.
Ebola is a highly contagious disease with a high mortality rate, with no licensed vaccine available as of 2018. Vaccine development includes rigorous testing and 3 phases of clinical trials. The Sierra Leone Trial to Introduce a Vaccine Against Ebola (STRIVE) was the second clinical trial phase to study the investigational Ebola virus vaccine rVSV∆-ZEBOV-GP. It was conducted during an unprecedented Ebola epidemic. Even before the outbreak, Sierra Leone had limited infrastructure and staff to conduct the trials. The STRIVE team addressed these challenges by allocating time to renovate the sites; providing ongoing support to maintain the water, electricity, and internet services; and training nearly 350 local staff members without hindering the Ebola response efforts. By strengthening the infrastructure and increasing the number of properly trained staff, Sierra Leone is now better equipped to conduct future clinical trials and in a better position to manage Ebola cases and clusters.
Xu S, Clarke CL, Newcomer SR, Daley MF, Glanz JM. Analyzing self-controlled case series data when case confirmation rates are estimated from an internal validation sample . Biom. J. 2018 Jul; 60(4): 748-760. Epub 2018 May 16.
Vaccine safety studies are often observational studies using electronic health records (EHR), however, these studies face some challenges, including outside influences (confounding) and outcome misclassification. To handle the confounding effect, researchers use self-controlled case series (SCCS) study design and review of EHRs to validate cases. SCCS design is limited to those individuals who experienced the event during or outside of certain times. While SCCS can adjust for some factors, it cannot adjust for others. This review considered 4 approaches for analyzing SCCS data: observed cases, confirmed cases only, known confirmation rate, and multiple imputation. Researchers found through simulation that when misclassification of adverse events is present, multiple imputation analysis should be considered. When only a sample of presumptive cases can be validated, this approach can address the influence of false-positive cases in EHR data.
Zerbo O, Modaressi S, Goddard K, Lewis E, Fireman BH, Daley MF, Irving SA, Jackson LA, Donahue JG, Qian L, Getahun D, DeStefano F, McNeil MM, Klein NP. Vaccination Patterns in Children After Autism Spectrum Disorder Diagnosis and in Their Younger Siblings . JAMA Pediatr. 2018 May 1; 172(5): 469-475.
Recently, several outbreaks of vaccine-preventable diseases generated concerns about the impact of increasing rates of undervaccination. This study investigates whether rates of vaccination in children with autism spectrum disorder (ASD) and their younger siblings differ from rates of vaccination in the general pediatric population. Results show that both children with ASD and their younger siblings are significantly less likely to be fully vaccinated than children in families without a child with ASD. Although the reasons for undervaccination are not fully explored in this study, results suggest that parental refusal of vaccination may play an important role.
Liang JL, Tiwari T, Moro P, Messonnier NE, Reingold A, Sawyer M, Clark TA. Prevention of Pertussis, Tetanus, and Diphtheria with Vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018 Apr 27 ;67(No. RR-2):1–44.
This report compiles and summarizes recommendations from CDC’s Advisory Committee on Immunization Practices on the prevention and control of tetanus, diphtheria, and pertussis in the U.S. This report is a comprehensive summary of previously published recommendations replacing previously published reports and policy notes and does not contain any new recommendations. Infants and young children are recommended to receive a 5-dose series of diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccines, with a booster dose of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine. Adults who never received Tdap are recommended to receive a booster dose. Women are recommended to receive a dose of Tdap during each pregnancy, regardless of previous receipt. Adolescents and adults are recommended to receive a booster tetanus and diphtheria toxoids (Td) vaccine every 10 years to assure ongoing protection against tetanus and diphtheria.
Donahue, J. Response to three Letters to the Editor regarding: Donahue JG, et al. Association of spontaneous abortion with receipt of inactivated influenza vaccine containing H1N1pdm09 in 2010-11 and 2011-12 . Vaccine . 2018 Apr 19; 36(17): 2231-2232.
Summaries are not made for a response to a letter to the editor.
Daley MF, Shoup JA, Newcomer SR, Jackson ML, Groom HC, Jacobsen SJ, McLean HQ, Klein NP, Weintraub ES, McNeil MM, Glanz JM. Assessing Potential Confounding and Misclassification Bias When Studying the Safety of the Childhood Immunization Schedule . Acad. Pediatr. 2018 Sept – Oct; 18(7): 754-762. Epub 2018 Mar 28.
Some parents are concerned the childhood immunization schedule could increase risk for allergic disorders, including asthma. This, along with the overall safety of the schedule, has parents delaying their children’s vaccinations. Researchers wanted to examine if there was a risk of vaccination status misclassification (between parent and health record) and if risk factors of asthma and other allergies varied by status. This survey was conducted among parents of children 19-35 months old at 8 Vaccine Safety Datalink sites. Among a sample of 2,043 parents, 1,209 (59.2%) responded. The observed agreement between parents and health record for no vaccines was 94% and 87.3 % for receiving all vaccines, no delay. Results showed that misclassification of vaccination status was uncommon, and parents’ reports of asthma risk factors generally did not vary by vaccination status. The data from this study will assist future observational studies with measurement and controlling disease risk.
Glanz JM, Newcomer SR, Daley MF, DeStefano F, Groom HC, Jackson ML, Lewin BJ, McCarthy NL, McClure DL, Narwaney KJ, Nordin JD, Zerbo O. Association Between Estimated Cumulative Vaccine Antigen Exposure Through the First 23 Months of Life and Non-Vaccine-Targeted Infections From 24 Through 47 Months of Age . JAMA . 2018 Mar 6; 319(9): 906-913.
Up to 15% of parents delay their children’s immunizations because of concerns that early childhood vaccines may overwhelm the immune system and cause children to be more susceptible to other infections. While a Danish study did not find evidence that multiple vaccine antigen exposure was associated with the risk for non-vaccine-targeted infectious diseases, this type of study has not been completed in the United States. In this case control study, data was collected from 6 Vaccine Safety Datalink sites to compare children with non-vaccine targeted infections to children without such infections. There were 944 children ages 24 through 47 months enrolled (193 cases, and 751 controls) and the results were not different between the two groups in their estimated cumulative vaccine antigen exposure during the first 23 months of life. In summary, exposure to multiple vaccines did not increase a child’s risk of non-vaccine targeted infections.
Irving SA, Groom HC, Stokley S, McNeil MM, Gee J, Smith N, Naleway AL. Human Papillomavirus Vaccine Coverage and Prevalence of Missed Opportunities for Vaccination in an Integrated Healthcare System . Acad. Pediatr. 2018 Mar; 18(2S): S85-S92.
Human papillomavirus (HPV) vaccination has been recommended in the United States for female and male adolescents since 2006 and 2011, respectively. However, vaccination rates for HPV compared to other childhood vaccines are lower. Researchers designed an assessment and provider-feedback intervention to increase HPV vaccine rate and identify missed opportunities for vaccination. The assessment and intervention occurred at 9 Oregon-based Kaiser Permanente Northwest outpatient clinics between April 2015 and June 2016. An average 29,021 adolescents ages 11-17 were included. Researchers collected baseline data four years prior to the intervention and found that vaccination rates were increasing; after intervention, there were no significant increases. Researchers did identify that missed opportunities decreased during the intervention for females 13-17 years old. Increasing HPV rates in large health systems is challenging, but other interventions are worth examining.
Kuntz J, Crane B, Weinmann S, Naleway AL. Myocarditis and pericarditis are rare following live viral vaccinations in adults . Vaccine. 2018 Mar 14; 36(12): 1524-1527. Epub 2018 Feb 15.
Cardiac complications including myocarditis, pericarditis, and arrhythmias following smallpox vaccination have been rarely reported in the United States. However, after 67 cases of myocarditis or pericarditis were reported after a vaccination campaign of military personnel, there was a need to assess these outcomes among adults after live-viral vaccinations. In this study using data from 4 Vaccine Safety Datalink sites from 1996-2007, researchers identified over 400,000 adults who received at least 1 live viral vaccine. Of those, there was only 1 probable case of pericarditis and no cases of myocarditis in 42 days following vaccination. Self-controlled risk interval analysis found there is no increased risk of myopericarditis in the 42 days following vaccination. The study findings suggest that the occurrence of myopericarditis following live viral vaccination is rare, not higher than the background rate, and much lower than rates following smallpox vaccination.
Markowitz LE, Gee J, Chesson H, Stokley S. Ten Years of Human Papillomavirus Vaccination in the United States. Acad Pediatr . 2018 Mar; 18(2S):S3-S10.
Since human papillomavirus (HPV) vaccine was first introduced for females in the United States in 2006, vaccination policy has evolved as additional HPV vaccines were licensed and new data became available. The United States was the first country to adopt a gender-neutral routine HPV immunization policy in 2011. Researchers summarized reviews of the first 10 years of the HPV vaccination program, including the evolution in vaccine policy, the vaccination program and coverage, and post-licensure vaccine safety studies. Reviews show coverage is increasing, although it remains lower than for other vaccines recommended for adolescents. There are various reasons for low coverage, and efforts are ongoing to increase vaccine uptake. The safety profile of HPV vaccine has been well established from 10 years of post-licensure monitoring. Despite low coverage, the early effects of the HPV vaccination program have exceeded expectations.
Arana JE, Harrington T, Cano M, Lewis P, Mba-Jonas A, Rongxia L, Stewart B, Markowitz LE, Shimabukuro TT. Post-licensure safety monitoring of quadrivalent human papillomavirus vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2009-2015 . Vaccine . 2018 Mar 20; 36(13):1781-1788. Epub 2018 Feb 21.
This study reviewed adverse events reported to Vaccine Adverse Event Reporting Systems following Gardasil® (4vHPV) vaccination between January 2009 and December 2015. A previous review did not include males because they were not recommended for vaccination at the time; this study includes both males and females. The analysis found 94.2% of the 19,760 reported adverse events were non-serious, and included headache, nausea, and fatigue. More than 60 million 4vHPV doses were distributed in the United States at the time, making the crude adverse event reporting rate 327 reports per million 4vHPV doses distributed. No unexpected or new safety concerns or reporting patterns were found.
Sukumaran L, McCarthy NL, Kharbanda EO, Vazquez-Benitez G, Lipkind HS, Jackson L, Klein NP, Naleway AL, McClure DL, Hechter RC, Kawai AT, Glanz JM, Weintraub ES. Infant Hospitalizations and Mortality After Maternal Vaccination . Pediatrics. 2018 Mar; 14(3). Epub 2018 Feb 20.
Influenza and Tdap vaccines are recommended for pregnant women. However, there are limited data on long-term outcomes of infants born to mothers vaccinated during pregnancy. This case-control study found that influenza and Tdap vaccines in pregnancy are not associated with an increased risk of hospitalization or death in infants during the first six months of life. These findings contribute to the knowledge of the long-term safety of vaccination during pregnancy.
Li R, Weintraub E, McNeil MM, Kulldorff M, Lewis EM, Nelson J, Xu S, Qian L, Klein NP, DeStefano F. Meningococcal conjugate vaccine safety surveillance in the Vaccine Safety Datalink using a tree-temporal scan data mining method . Pharmacoepidemiol. Drug Saf. 2018 Apr; 27(4): 391-397. Epub 2018 Feb 15.
Traditional pharmacovigilance techniques used in vaccine safety are generally geared to detecting adverse events (AEs) based on pre‐defined sets of conditions or diagnoses. Using a newly developed tree‐temporal scan statistic data mining method, researchers performed a pilot study to evaluate the safety profile of the meningococcal conjugate vaccine Menactra®. The authors detected known AEs following the vaccine; no new safety concerns were raised. The study demonstrates that the tree‐temporal scan statistic data mining method can be successfully applied to screen broadly for a wide range of vaccine‐AE associations within a large health care data network.
Zhou H, Thompson WW, Belongia EA, Fowlkes A, Baxter R, Jacobsen SJ, Jackson ML, Glanz JM, Naleway AL, Ford DC, Weintraub E, Shay DK. Estimated rates of influenza-associated outpatient visits during 2001-2010 in six US integrated health care delivery organizations . Influenza. Other Respir. Viruses. 2018 Jan; 12(1): 122-131. Epub 2018 Feb 15.
Influenza (flu) related illnesses are responsible for many morbidity cases during each flu season, but these illnesses are difficult to count: symptoms are non-specific, diagnostic codes for flu-related symptoms are broad, and lab testing is not routine. This makes population-based estimates of flu-related outpatient visits during flu epidemics or pandemics uncommon. In this study using data from 6 Vaccine Safety Datalink sites from 2001-2010, researchers estimated flu-related outpatient visits. Researchers modeled the rates of outpatient visits with diagnostic codes of pneumonia or acute respiratory visits. Of the nearly 7.7 million people enrolled, children had higher estimated flu-related outpatient rates than adults during pre-pandemic and pandemic seasons. Rates estimated with pneumonia visits plus flu-coded visits were similar to previous studies using confirmed flu cases. These numbers are crucial for measuring the potential benefits of flu prevention and treatment.
McNeil MM, DeStefano F. Vaccine-associated hypersensitivity . J. Allergy Clin. Immunol. 2018 Feb; 141(2): 463-472.
Vaccines are considered one of the most effective public health interventions – resulting in major reductions of vaccine preventable diseases and death. Vaccine-associated hypersensitivity reactions are not infrequent; however, serious acute-onset anaphylaxis reactions are extremely rare. Risk of anaphylaxis after all vaccines is estimated to be 1.31 per million vaccine doses administered. This review focuses on serious hypersensitivity reactions following flu vaccines, given the large number of people vaccinated yearly and the formulation changes the vaccines go through each year to match circulating flu viruses. Recent advances in vaccine technology, along with new vaccines and the universal flu vaccination recommendation (people 6 months of age and older), make continued safety monitoring for hypersensitivity reactions following flu vaccination particularly important.
McNeil MM, Hibbs BF, Miller ER, Cano MV. Notes from the Field: Errors in Administration of an Excess Dosage of Yellow Fever Vaccine – United States, 2017. MMWR Morb Mortal Wkly Rep. 2018 Jan 26; 67:109-110.
Following a March 2017 report to Vaccine Adverse Event Reporting System (VAERS) of four persons receiving incorrect dosages of yellow fever vaccine, CDC conducted a VAERS search and literature review for similar reported administration errors. Reports were few (15 in VAERS; 67 in literature) and most did not involve an adverse event. However, the error was costly in terms of medical follow-up and vaccine wastage. More distinctive single/multi-dose packaging and in-service training might prevent future errors.
Hibbs BF, Miller E, Shi J, Smith K, Lewis P, Shimabukuro TT. Safety of Vaccines That Have Been Kept Outside of Recommended Temperatures: Reports to the Vaccine Adverse Event Reporting System (VAERS), 2008-2012. Vaccine. 2018 Jan 25;36(4):553-558.
This review does not indicate any substantial direct health risk from administration of vaccines kept outside of recommended temperatures. However, there are potential costs and risks, including vaccine wastage, possible decreased protection, and patient inconvenience related to revaccination. Maintaining high vigilance, proper staff training, regular equipment maintenance, and having adequate auxiliary power are important components of comprehensive vaccine cold chain management.
Haber P, Moro PL, Ng C, Lewis PW, Hibbs B, Schillie SF, Nelson NP, Li R, Stewart B, Cano MV. Safety of Currently Licensed Hepatitis B Surface Antigen Vaccines in the United States, Vaccine Adverse Event Reporting System (VAERS), 2005-2015. Vaccine . 2018 Jan 25;26(4):559-564.
This study is based on a national vaccine safety data and reassures the public on the safety of Hepatitis B vaccine(s). Although it reveals increased reports of vaccine storage errors, and incorrect dose or wrong vaccine given to infants or adults, no adverse events are noted. The findings highlight the need for education and training of health providers on prevention of vaccine administration errors.
Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, Nelson NP. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep . 2018 Jan 12; 67(No. RR-1):1-31.
Hepatitis B is a serious disease that affects the liver. The virus is highly infectious and can be transmitted in the absence of visible blood. As part of the recommended immunization schedule for infants and children, Hepatitis B vaccine should be given to children in three doses between birth and 18 months of age. In January 2018, the Advisory Committee on Immunization Practices (ACIP) published new recommendations for the vaccine. These include: 1) administration of the universal hepatitis B vaccination within 24 hours of birth of medically stable infants, 2) testing pregnant women for Hepatitis B, 3) post-vaccination serologic testing for infants whose mother has an unknown hepatitis B status, and 4) the removal of lenient language for delaying the birth dose until after hospital discharge. Vaccine safety information was updated to include data from the pre- and post-licensure studies and report information from the Vaccine Adverse Events Report System from 2005 to 2015.
Newcomer SR, Kulldorff M, Xu S, Daley MF, Fireman B, Lewis E, Glanz JM. Bias from outcome misclassification in immunization schedule safety research . Pharmacoepidemiol. Drug Saf . 2018 Feb; 27(2): 221-228. Epub 2018 Jan 2.
The Institute of Medicine in 2013 recommended conducting observational studies of the childhood immunization schedule safety. However, these studies present a methodical challenge because of bias from misclassification of outcomes in electronic health record data. Using simulations, researchers evaluated the percent of valid diagnoses (positive predictive values, PPVs) as indicators of bias of an exposure-outcome association, and quantitative bias analyses methods used for bias correction. Overall outcome PPVs did not reflect the distribution of false positives by exposure and are poor indicators of bias in individual studies. Quantitative bias analysis was effective in correcting outcome misclassification bias and should be considered in immunization schedule research.
Moro PL, Zheteyeva WY, Barash F, Lewis P, Cano M. Assessing the Safety of Hepatitis B Vaccination During Pregnancy in the Vaccine Adverse Event Reporting System (VAERS), 1990-2016. Vaccine . 2018 Jan 2;26(1):50-54.
Few studies have been done on the safety of hepatitis B vaccine in pregnant women. This review describes adverse events after Hepatitis B vaccination of pregnant women reported to the Vaccine Adverse Event Reporting System (VAERS). During the period from January 1, 1990 to June 30, 2016, VAERS received 192 reports involving pregnant women following Hepatitis B vaccination. No new or unexpected safety concerns were found.
Tamez RL, Tan WV, O’Malley JT, Broder KR, Garzon MC, LaRussa P, Lauren CT. Influenza B Virus Infection and Stevens-Johnson Syndrome. Pediatr Dermatol. 2018 Jan;35(1);e45-e48.
Stevens-Johnson Syndrome (SJS) is a rare, serious disorder that affects the skin and the areas that creates and releases mucus. It starts as flu-like symptoms, and leads to a rash and blisters. Patients who develop SJS require hospitalization to manage the symptoms and identify the cause. This case reviewed SJS in a 2-year-old boy with influenza B infection. He was up to date on his immunizations, including the influenza vaccine 3 months prior to coming to coming to the hospital. He was treated with antiviral oseltamivir and IV treatment’, and his symptoms cleared up. With his diagnosis of influenza type B and SJS, there were still concerns of re-exposure to influenza B antigen during next season’s vaccination. The boy received the quadrivalent inactivated influenza vaccine the following season, was monitored and tolerated the vaccine well without reports of adverse reactions. Medical evaluation concluded that the patient’s influenza B infection was the most likely cause of SJS.
Tamez RL, Tan WV, O’Malley JT, Broder KR, Garzon MC, LaRussa P, Lauren CT. Influenza B virus infection and Stevens-Johnson syndrome. Pediatr Dermatol. 2018 Jan; 35(1):e45-e48. Epub 2017 Dec 28.
Storms AD, Chen J, Jackson LA, Nordin JD, Naleway AL, Glanz JM, Jacobsen SJ, Weintraub ES, Klein NP, Gargiullo PM, Fry AM . Rates and risk factors associated with hospitalization for pneumonia with ICU admission among adults . BMC. Pulm. Med. 2017 Dec 16; 17(1): 208.
Hibbs BF, Miller E, Shi J, Smith K, Lewis P, Shimabukuro TT. Safety of vaccines that have been kept outside of recommended temperatures: Reports to the Vaccine Adverse Event Reporting System (VAERS), 2008-2012 . Vaccine . 2018 Jan 25; 36(4):553-558. Epub 2017 Dec 14.
Haber P, Moro PL, Ng C, Lewis PW, Hibbs B, Schillie SF, Nelson NP, Li R, Stewart B, Cano MV. Safety of currently licensed hepatitis B surface antigen vaccines in the United States, Vaccine Adverse Event Reporting System (VAERS), 2005-2015 . Vaccine . 2018 Jan 25; 36(4):559-564. Epub 2017 Dec 11.
Groom HC, Irving SA, Caldwell J, Larsen R, Beaudrault S, Luther LM, Naleway AL. Implementing a Multipartner HPV Vaccination Assessment and Feedback Intervention in an Integrated Health System . J. Public Health Manag. Pract. 2017 Nov/Dec; 23(6): 589-592.
Loharikar A, Suragh TA, MacDonald NE, Balakrishnan MR, Benes O, Lamprianou S, Hyde TB, McNeil MM. Anxiety-related adverse events following immunization (AEFI): A systematic review of published clusters of illness . Vaccine . 2018 Jan 4; 36(2):299-305. Epub 2017 Nov 29.
Moro PL, Zheteyeva Y, Barash F, Lewis P, Cano M. Assessing the safety of hepatitis B vaccination during pregnancy in the Vaccine Adverse Event Reporting System (VAERS), 1990-2016 . Vaccine . 2018 Jan 2; 36(1):50-54. Epub 2017 Nov 27.
Daley MF, Clarke CL, Glanz JM, Xu S, Hambidge SJ, Donahue JG, Nordin JD, Klein NP, Jacobsen SJ, Naleway AL, Jackson ML, Lee G, Duffy J, Weintraub E. The safety of live attenuated influenza vaccine in children and adolescents 2 through 17 years of age: A Vaccine Safety Datalink study . Pharmacoepidemiol Drug Saf . 2018 Jan; 27(1): 59-68. Epub 2017 Nov 17.
Myers TR, McNeil MM, Current safety issues with quadrivalent meningococcal conjugate vaccines. Hum Vaccin Immunother , 2018 May 4; 14(5): 1175-1178; Epub 2017 Nov 8.
Kemper AR, Barnett ED, Walter EB, Hornik C, Pierre-Joseph N, Broder KR, Silverstein M, Harrington T. Drinking Water to Prevent Postvaccination Presyncope in Adolescents: A Randomized Trial. Pediatrics 2017 Nov; 140(5).
Arana J, Mba-Jonas A, Jankosky C, Lewis P, Moro PL, Shimabukuro TT, Cano M. Reports of Postural Orthostatic Tachycardia Syndrome After Human Papillomavirus Vaccination in the Vaccine Adverse Event Reporting System . J Adolesc Health . 2017 Nov; 61 (5): 577-582.
Stockwell MS, Marchant CD, Wodi AP, Barnett ED, Broder KR, Jakob K, Lewis P, Kattan M, Rezendes AM, Barrett A, Sharma D, Fernandez N, LaRussa P. A multi-site feasibility study to assess fever and wheezing in children after influenza vaccines using text messaging. Vaccine. 2017 Dec 15; 35(50):6941-6948. Epub 2017 Oct 28.
Izurieta HS, Moro PL, Chen RT. Hospital-based collaboration for epidemiological investigation of vaccine safety: A potential solution for low and middle-income countries? Vaccine . 2018 Jan 8; 36(3):345-346. Epub 2017 Oct 21.
McCarthy NL, Sukumaran L, Newcomer S, Glanz J, Daley MF, McClure D, Klein NP, Irving S, Jackson ML, Lewin B, Weintraub E. Patterns of childhood immunization and all-cause mortality . Vaccine. 2017 Dec 4; 35(48 Pt B): 6643-6648. Epub 2017 Oct 20.
Walter EB, Hornik CP, Grohskopf L, McGee CE, Todd CA, Museru OI, Harrington L, Broder KR. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children . Vaccine. 2017 Dec 4; 35(48 Pt B): 6664-6671. Epub 2017 Oct 19.
VanWormer JJ, Bendixsen CG, Vickers ER, Stokley S, McNeil MM, Gee J, Belongia EA, McLean HQ. Association between parent attitudes and receipt of human papillomavirus vaccine in adolescents . BMC. Public Health . 2017 Oct 2; 17(1): 766.
Donahue JG, Kieke BA, King JP, DeStefano F, Mascola MA, Irving SA, Cheetham TC, Glanz JM, Jackson LA, Klein NP, Naleway AL, Weintraub E, Belongia EA. Association of spontaneous abortion with receipt of inactivated influenza vaccine containing H1N1pdm09 in 2010-11 and 2011-12 . Vaccine . 2017 Sep 25; 35(40): 5314-5322.
Gee J, Sukumaran L, Weintraub E. Risk of Guillain-Barre Syndrome following quadrivalent human papillomavirus vaccine in the Vaccine Safety Datalink . Vaccine . 2017 Oct 13; 35(43): 5756-5758. Epub 2017 Sep 19.
Eaton A, Lewis N, Fireman B, Hansen J, Baxter R, Gee J, Klein NP. Birth outcomes following immunization of pregnant women with pandemic H1N1 influenza vaccine 2009-2010 . Vaccine. 2017 May 3; 36(19): 2733-2739. Epub 2017 Sep 13.
Vickers ER, McClure DL, Naleway AL, Jacobsen SJ, Klein NP, Glanz JM, Weintraub ES, Belongia EA . Risk of venous thromboembolism following influenza vaccination in adults aged 50years and older in the Vaccine Safety Datalink . Vaccine. 2017 Oct 13; 35(43): 5872-5877. Epub 2017 Sep 6.
Woo EJ, Moro PL, Cano M, Jankosky C. Postmarketing safety surveillance of trivalent recombinant influenza vaccine: Reports to the Vaccine Adverse Event Reporting System. Vaccine . 2017 Oct 9;35(42):5618-5621. Epub 2017 Sep 5.
Stockwell MS, Broder KR, Lewis P, Jakob K, Iqbal S, Fernandez N, Sharma D, Barrett A, LaRussa P. Assessing Fever Frequency After Pediatric Live Attenuated Versus Inactivated Influenza Vaccination. J Pediatric Infect Dis Soc. 2017 Sep 1; 6(3):e7-e14.
Shaw J, Halsey NA, Weinberg A, Scott Schmid D, George KS, Weldon WC, Jordan M, Bryant PW, LaRussa P, Bradshaw DY, Harrington T, Gershon A. Arm Paralysis After Routine Childhood Vaccinations: Application of Advanced Molecular Methods to the Causality Assessment of an Adverse Event After Immunization . J Pediatric Infect Dis Soc. 2017 Sep 1; 6(3): e161-e164.
Lipkind HS, Vazquez-Benitez G, Nordin JD, Romitti PA, Naleway AL, Klein NP, Hechter RC, Jackson ML, Hambidge SJ, Lee GM, Sukumaran L, Kharbanda EO. Maternal and Infant Outcomes After Human Papillomavirus Vaccination in the Periconceptional Period or During Pregnancy . Obstet. Gynecol. 2017 Sep; 130(3): 599-608.
Duffy J, Hambidge SJ, Jackson LA, Kharbanda EO, Klein NP, Naleway A, Omer SB, Weintraub E. Febrile Seizure Risk after Vaccination in Children One to Five Months of Age . Pediatr. Neurol. 2017 Nov; 76: 72-78. Epub 2017 Aug 23.
Moro PL, Cragan J, Lewis P, Sukumaran L. Major Birth Defects after Vaccination Reported to the Vaccine Adverse Event Reporting System (VAERS) 1990 to 2014 . Birth Defects Res . 2017 Jul 17; 109(13): 1057-1062.
Heininger U, Holm K, Caplanusi I, Bailey SR, CIOMS Working Group on Vaccine Safety. Guide to active vaccine safety surveillance: Report of CIOMS working group on vaccine safety – executive summary . Vaccine . 2017 Jul 13; 35(32):3917-3921. Epub 2017 Jun 20.
DeSilva M, Vazquez-Benitez G, Nordin JD, Lipkind HS, Klein NP, Cheetham TC, Naleway AL, Hambidge SJ, Lee GM, Jackson ML, McCarthy NL, Kharbanda EO. Maternal Tdap vaccination and risk of infant morbidity . Vaccine. 2017 Jun 22; 35(29): 3655-3660. Epub 2017 May 25.
Duffy J, Johnsen 2, Ferris M, Miller M, Leighton K, McGilvray M, McNamara L, Breakwell L, Yu Y, Bhavsar T, Briere E, Patel M . Safety of a meningococcal group B vaccine used in response to two university outbreaks . J Am Coll Health . 2017 Aug-Sep; 65(6):380-388. Epub 2017 May 8.
Stockwell MS, Cano M, Jakob K, Broder KR, Gyamfi-Bannerman C, Castaño PM, Lewis P, Barrett A, Museru OI, Castellanos O, LaRussa PS. Feasibility of Text Message Influenza Vaccine Safety Monitoring During Pregnancy. Am J Prev Med. 2017 Sep; 53(3):282-289. Epub 2017 May 8.
McLean HQ, VanWormer JJ, Chow BDW, Birchmeier B, Vickers E, DeVries E, Meyer J, Moore J, McNeil MM, Stokley S, Gee J, Belongia EA. Improving Human Papillomavirus Vaccine Use in an Integrated Health System: Impact of a Provider and Staff Intervention . J. Adolesc. Health . 2017 Aug; 61(2): 252-258. Epub 2017 Apr 24.
Ray GT, Lewis N, Goddard K, Ross P, Duffy J, DeStefano F, Baxter R, Klein NP. Asthma exacerbations among asthmatic children receiving live attenuated versus inactivated influenza vaccines . Vaccine 2017 May 9; 35(20): 2668-2675. Epub 2017 Apr 9.
Duffy J, Lewis M, Harrington T, Baxter R, Belongia EA, Jackson LA, Jacobsen SJ, Lee GM, Naleway AL, Nordin J, Daley MF . Live attenuated influenza vaccine use and safety in children and adults with asthma . Ann. Allergy Asthma Immunol. 2017 April; 118(4):439-444.
Suragh TA, Miller ER, Hibbs BF, Winiecki SK, Zinderman C, Shimabukuro TT. Cognitive testing to evaluate revisions to the Vaccine Adverse Event Reporting System (VAERS) reporting form . Vaccine . 2017 Apr 25; 35(18):2295-2297. Epub 2017 Mar 25.
Kharbanda EO, Vazquez-Benitez G, Romitti PA, Naleway AL, Cheetham TC, Lipkind HS, Klein NP, Lee G, Jackson ML, Hambidge SJ, McCarthy N, DeStefano F, Nordin JD. First Trimester Influenza Vaccination and Risks for Major Structural Birth Defects in Offspring . J. Pediatr. 2017 Aug; 187: 234-239.e4. Epub 2017 Mar 24.
Daley MF, Glanz JM, Newcomer SR, Jackson ML, Groom HC, Lugg MM, McLean HQ, Klein NP, Weintraub ES, McNeil MM. Assessing misclassification of vaccination status: Implications for studies of the safety of the childhood immunization schedule . Vaccine . 2017 Apr 4; 35(15): 1873-1878. Epub 2017 Mar 9.
Myers TR, McNeil MM, Ng CS, Li R, Lewis PW, Cano MV. Adverse events following quadrivalent meningococcal CRM-conjugate vaccine (Menveo®) reported to the Vaccine Adverse Event Reporting system (VAERS), 2010-2015 . Vaccine . 2017 Mar 27; 35(14):1758-1763. Epub 2017 Mar 3.
Klein NP, Lewis E, McDonald J, Fireman B, Naleway A, Glanz J, Jackson LA, Donahue JG, Jacobsen SJ, Weintraub E, Baxter R. Risk factors and familial clustering for fever 7-10days after the first dose of measles vaccines. Vaccine . 2017 March 14; 35(12): 1615-1621. Epub 2017 Feb 21.
Su JR, Leroy Z, Lewis PW, Haber P, Marin M, Leung J, Woo EJ, Shimabukuro TT. Safety of Second-Dose Single-Antigen Varicella Vaccine . Pediatrics . 2017 Mar; 139(3). Epub 2017 Feb 7.
Narwaney KJ, Breslin K, Ross CA, Shoup JA, Wain KF, Weintraub ES, McNeil,MM, Hambidge SJ. Vaccine adverse events in a safety net healthcare system and a managed care organization . Vaccine . 2017 March 1; 35(9): 1335-1340. Epub 2017 Feb 6.
Moro PL, Sukumaran L. Cholera vaccination: pregnant women excluded no more . Lancet Infect Dis . 2017 May;17(5):469-470. Epub 2017 Feb 2.
Hambidge SJ, Ross C, Shoup JA, Wain K, Narwaney K, Breslin K, Weintraub ES, McNeil MM. Integration of data from a safety net health care system into the Vaccine Safety Datalink . Vaccine. 2017 March 1; 35(9): 1329-1334. Epub 2017 Feb 1.
Moro P, Baumblatt J, Lewis P, Cragan J, Tepper N, Cano M. Surveillance of Adverse Events After Seasonal Influenza Vaccination in Pregnant Women and Their Infants in the Vaccine Adverse Event Reporting System, July 2010-May 2016 . Drug Saf . 2017 Feb; 40(2):145-152.
Kharbanda EO, Vazquez-Benitez G, Romitti PA, Naleway AL, Cheetham TC, Lipkind HS, Sivanandam S, Klein NP, Lee GM, Jackson ML, Hambidge SJ, Olsen A, McCarthy N, DeStefano F, Nordin JD. Identifying birth defects in automated data sources in the Vaccine Safety Datalink . Pharmacoepidemiol. Drug Saf. 2017 Apr; 26(4): 412-420. Epub 2017 Jan 4.
Moro PL, Cragan J, Lewis P, Sukumaran L. Major Birth Defects after Vaccination Reported to the Vaccine Adverse Event Reporting System (VAERS), 1990 to 2014. Birth Defects Res (2017).doi: 10.1002/bdra.23622
Moro PL, Sukumaran L. Cholera vaccination: pregnant women excluded no more. Lancet Infect Dis (2017);17(5):469-470. doi: 10.1016/S1473-3099(17)30055-5.
Bardenheier BH, Duffy J, Duderstadt SK, Higgs JB, Keith MP, Papadopoulos PJ, Gilliland WR, McNeil MM. Anthrax Vaccine and the Risk of Rheumatoid Arthritis and Systemic Lupus Erythematosus in the U.S. Military: A Case-Control Study . Mil Med . 2016 Oct;181(10):1348-1356.
Chen RT, Moro PL, Bauwens J, Bonhoeffer J. Obstetrical and neonatal case definitions for immunization safety data. Vaccine (2016);34(49):5991-5992. doi: 10.1016/j.vaccine.2016.08.026.
Moro PL, Woo EJ, Paul W, Lewis P, Petersen BW, Cano M. Post-Marketing Surveillance of Human Rabies Diploid Cell Vaccine (Imovax) in the Vaccine Adverse Event Reporting System (VAERS) in the United States, 1990‒2015. PLoS Negl Trop Dis (2016);10(7):e0004846. doi: 10.1371/journal.pntd.0004846.
Moro PL, Li R, Haber P, Weintraub E, Cano M. Surveillance systems and methods for monitoring the post-marketing safety of influenza vaccines at the Centers for Disease Control and Prevention. Expert Opin Drug Saf (2016);15(9):1175-83. doi: 10.1080/14740338.2016.1194823.
Moro PL, Cragan J, Tepper N, Zheteyeva Y, Museru O, Lewis P, Broder K. Enhanced surveillance of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccines in pregnancy in the Vaccine Adverse Event Reporting System (VAERS), 2011-2015. Vaccine (2016);34(20):2349-53. doi: 10.1016/j.vaccine.2016.03.049.
Lindsey NP, Rabe IB, Miller ER, Fischer M, Staples JE. Adverse event reports following yellow fever vaccination, 2007-13. J Travel Med . 2016 Jul 4;23(5). doi: 10.1093/jtm/taw045
Miller ER, Moro PL, Cano M, Lewis P, Bryant-Genevier M, Shimabukuro TT. Post-licensure safety surveillance of 23-valent pneumococcal polysaccharide vaccine in the Vaccine Adverse Event Reporting System (VAERS), 1990–2013. Vaccine 2016, 34 (25): 2841-2846. doi: 10.1016/j.vaccine.2016.04.021.
Su JR. Miller ER. Duffy J. Baer BM. Cano MV. Notes from the Field: Administration error involving a meningococcal conjugate vaccine-United States, March 1, 2010-Sept. 22, 2015. MMWR 2016 65(06);161-162. doi: 10.15585/mmwr.mm6506a4.
Moro, P., Baumblatt, J., Lewis, P. et al. Surveillance of Adverse Events After Seasonal Influenza Vaccination in Pregnant Women and Their Infants in the Vaccine Adverse Event Reporting System, July 2010–May 2016. Drug Saf (2016). doi:10.1007/s40264-016-0482-1.
Abrams JY, Weintraub ES, Baggs JM, McCarthy NL, Schonberger LB, Lee GM, et al. Childhood vaccines and Kawasaki disease, Vaccine Safety Datalink, 1996-2006 . Vaccine . 2015 Jan 3;33(2):382-7.
Baker MA, Kaelber DC, Bar-Shain DS, Moro PL, Zambarano B, Mazza M, et al. Advanced clinical decision support for vaccine adverse event detection and reporting . Clin Infect Dis . 2015 Sep 15;61(6):864-70. Epub 2015 Jun 9.
Chen RT, Shimabukuro TT, Martin DB, Zuber PL, Weibel DM, Sturkenboom M. Enhancing vaccine safety capacity globally: A lifecycle perspective . Vaccine. Epub 2015 Sep 30.
Chen RT, Shimabukuro TT, Martin DB, et al. Enhancing vaccine safety capacity globally: A lifecycle perspective . Vaccine . 2015 Nov 27;33 Suppl 4:D46-54.
Datwani H, Moro PL, Harrington T, Broder K. Chorioamnionitis following vaccination in the Vaccine Adverse Event Reporting System . Vaccine . 2015 Jun 17;33(27):3110-3. Epub 2015 May 11.
Duffy J, Weintraub E, Vellozzi C, DeStefano F; Vaccine Safety Datalink. Narcolepsy and influenza A(H1N1) pandemic 2009 vaccination in the United States . Neurology . 2014 Nov 11;83(20):1823-30. Epub 2014 Oct 15.
Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 influenza season . MMWR Morb Mortal Wkly Rep . 2015 Aug 7;64(30):818-25.
Haber P, Parashar UD, Haber M, DeStefano F. Intussusception after monovalent rotavirus vaccine—United States, Vaccine Adverse Event Reporting System (VAERS), 2008–2014 . Vaccine . 2015 Sep 11;33(38):4873-7. Epub 2015 Aug 11.
Hibbs BF, Moro PL, Lewis P, Miller ER, Shimabukuro T. Vaccination errors reported to the Vaccine Adverse Event Reporting System, United States, 2000–2013 . Vaccine . 2015 Jun 22;33(28):3171-8. Epub 2015 May 14.
Iqbal S, Li R, Gargiullo P, Vellozzi C. Relationship between Guillain-Barré syndrome, influenza-related hospitalizations, and influenza vaccine coverage . Vaccine . 2015 Apr 21;33(17):2045-9. Epub 2015 Mar 4.
Iqbal S, Shi J, Seib K, Lewis P, Moro PL, Woo EJ, et al. Preparation for global introduction of inactivated poliovirus vaccine: Safety evidence from the US Vaccine Adverse Event Reporting System, 2000-2012 . Lancet Infect Dis . 2015 Oct;15(10):1175-82. Epub 2015 Aug 16.
Kharbanda EO, Vazquez-Benitez G, Lipkind HS, Klein NP, Cheetham TC, Naleway A, et al. Evaluation of the association of maternal pertussis vaccination with obstetric events and birth outcomes . JAMA . 2014 Nov 12;312(18):1897-904.
Klein NP, Lewis E, Fireman B, Hambidge SJ, Naleway A, Nelson JC, et al. Safety of measles-containing vaccines in 1-Year-old children . Pediatrics . 2015 Feb;135(2):e321-9. Epub 2015 Jan 5.
Lopalco PL, DeStefano F. The complementary roles of Phase 3 trials and post-licensure surveillance in the evaluation of new vaccines . Vaccine . 2015 Mar 24;33(13):1541-8. Epub 2014 Nov 4.
McNamara LA, Shumate AM, Johnsen P, MacNeil JR, Patel M, Bhavsar T, et al. First use of a serogroup B meningococcal vaccine in the us in response to a university outbreak . Pediatrics . 2015 May;135(5):798-804.
McNeil MM, Weintraub E, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP, et al. Risk of anaphylaxis following vaccination in children and adults . J Allergy Clin Immunol. Epub 2015 Sep 28.
Miller ER, Moro PL, Cano M, Shimabukuro T. Deaths following vaccination: What does the evidence show? Vaccine . 2015 Jun 26;33(29):3288-92. Epub 2015 May 23.
Miller ER, Shimabukuro T, Hibbs B, Moro P, Broder K, Vellozzi C. Vaccine safety resources for nurses . Am J Nurs . 2015 Aug; 115(8):55-58.
Moro PL, Arana J, Cano M, Lewis P, Shimabukuro TT. Deaths reported to the Vaccine Adverse Event Reporting System, United States, 1997-2013 . Clin Infect Dis . 2015 Sep 15;61(6):980-7. Epub 2015 May 28.
Moro PL, Jankosky C, Menschik D, Lewis P, Duffy J, Stewart B, et al. Adverse events following Haemophilus influenzae type b vaccines in the Vaccine Adverse Event Reporting System, 1990-2013 . J Pediatr . 2015 Apr;166(4):992-7. Epub 2015 Jan 15.
Moro PL, McNeil MM, Sukumaran L, Broder KR. The Centers for Disease Control and Prevention’s public health response to monitoring Tdap safety in pregnant women in the United States . Hum Vaccin Immunother . Epub 2015 Sep 17.
Moro PL, Zheteyeva Y, Lewis P, Shi J, Yue X, Museru OI, et al. Safety of quadrivalent human papillomavirus vaccine (Gardasil®) in pregnancy: Adverse events among non-manufacturer reports in the Vaccine Adverse Event Reporting System, 2006-2013. Vaccine . 2015 Jan; 33(4): 519-22.
Payne DC, Baggs J, Klein NP, Parashar UD. Does preventing rotavirus infections through vaccination also protect against naturally occurring intussusception over time? Clin Infect Dis . 2015 Jan 1;60(1):163-4.
Petrosky E, Bocchini JA Jr, Hariri S, Chesson H, Curtis CR, Saraiya M, et al; Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices . MMWR Morb Mortal Wkly Rep . 2015 Mar 27;64(11):300-4.
Rabe IB, Miller ER., Fischer M, Hills SL. Adverse events following vaccination with an inactivated, Vero cell culture-derived Japanese encephalitis vaccine in the United States, 2009-2012 . Vaccine . 2015 Jan 29;33(5):708-12.
Rha B, Tate JE, Weintraub E, Haber P, Yen C, Patel M, Cortese MM. Intussusception following rotavirus vaccination: An updated review of the available evidence . Expert Rev Vaccines . 2014 Nov;13(11):1339-48. Epub 2014 Jul 26.
Stratton KG, Cook AJ, Jackson LA, Nelson JC. Simulation study comparing exposure matching with regression adjustment in an observational safety setting with group sequential monitoring . Stat Med . 2015 Mar 30;34(7):1117-33.
Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS) . Vaccine . 2015 Aug 26;33(36):4398-405. Epub 2015 Jul 22.
Sukumaran L, McCarthy NL, Kharbanda EO, McNeil MM, Naleway AL, Klein NP, et al. Association of Tdap vaccination with acute events and adverse birth outcomes among pregnant women with prior tetanus-containing immunizations . JAMA . 2015 Oct 20;314(15):1581-1587.
Sukumaran L, McCarthy NL, Kharbanda EO, Weintraub ES, Vasquez-Benitez G, McNeil MM, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol . 2015 Nov;126(5):1069-74.
Sukumaran L, McCarthy NL, Li R, Weintraub ES, Jacobsen SJ, Hambidge SJ, et al. Demographic characteristics of members of the Vaccine Safety Datalink (VSD): A comparison with the United States population . Vaccine . 2015 Aug 26;33(36):4446-50. Epub 2015 Jul 23.
Sukumaran L, McNeil MM, Moro PL, Lewis PW, Winiecki SK, Shimabukuro TT. Adverse events following measles, mumps, and rubella vaccine in adults reported to the Vaccine Adverse Event Reporting System (VAERS), 2003-2013. Clin Infect Dis . 2015 May 15;60(10):e58-65.
Briere EC, Rubin L, Moro PL, Cohn A, Clark T, Messonnier N. Prevention and control of haemophilus influenzae type b disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) . MMWR. 2014 Feb 28;63(RR-01):1-14.
Daley MF, Yih WK, Glanz JM, Hambidge SJ, Narwaney KJ, Yin R, et al. Safety of diphtheria, tetanus, acellular pertussis and inactivated poliovirus (DTaP-IPV) vaccine . Vaccine. 2014;32(25):3019-3024.
Duffy J, Weintraub, E, Vellozzi, C, DeStefano F. Narcolepsy and influenza A(H1N1) pandemic 2009 vaccination in the United States . Neurology . 2014;83(20):1823-30.
Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2014–15 influenza season . MMWR . 2014 Aug 15;63(32):691-697.
Haber P, Moro PL, McNeil MM, Lewis P, Woo EJ, Hughes H, Shimabukuro TT. Post-licensure surveillance of trivalent live attenuated influenza vaccine in adults, United States, Vaccine Adverse Event Reporting System (VAERS), July 2005-June 2013 . Vaccine. 2014 Nov;32(48):6499-504.
Haber P, Moro PL, McNeil MM, Lewis P, Woo EJ, Hughes H, et al. Post-licensure surveillance of trivalent live attenuated influenza vaccine in children aged 2-18 years, Vaccine Adverse Event Reporting System (VAERS), United States, July 2005-June 2012 . J Ped Infect Dis . Epub 2014 May 7.
Hambidge SJ, Newcomer SR, Narwaney KJ, Glanz JM, Daley MF, Xu S, et al. Timely versus delayed early childhood vaccination and seizures. Pediatrics . 2014 Jun;133(6):e1492-9.
Hibbs BF, Miller ER, Shimabukuro T; Centers for Disease Control and Prevention (CDC). Notes from the field: Rotavirus vaccine administration errors–United States, 2006-2013 . MMWR . 2014 Jan;63(4):81.
Hofstetter AM, Jakob K, Klein NP, Dekker CL, Edwards KM, et al. Live vaccine use and safety in DiGeorge syndrome . Pediatrics . 2014 Apr;133(4):e946-54.
Jackson ML, Peterson D, Nelson JC, Greene SK, Jacobsen SJ, Belongia EA, et al. Using winter 2009-2010 to assess the accuracy of methods which estimate influenza-related morbidity and mortality . Epidemiol Infect . 2014 Dec 12:1-9.
Kawai AT, Li L, Kulldorff M, Vellozzi C, Weintraub E, Baxter R, et al. Absence of associations between influenza vaccines and increased risks of seizures, Guillain-Barre syndrome, encephalitis, or anaphylaxis in the 2012-2013 season . Pharmacoepidemiol Drug Saf . 2014;23(5):548-553.
Kharbanda EO, Vazquez-Benitez G, Lipkind HS, Klein NP, Cheetham TC, Naleway A, et al. Evaluation of the association of maternal pertussis vaccination with obstetric events and birth outcomes. JAMA . 2014 Nov;312(18):1897-904.
Kharbanda EO, Vazquez-Benitez G, Lipkind H, Naleway AL, Klein NP, Cheetham TC, et al. Receipt of pertussis vaccine during pregnancy across 7 Vaccine Safety Datalink sites . Prev Med . 2014 Oct;67:316-9.
Li R, Stewart B, Weintraub E, McNeil MM. Continuous sequential boundaries for vaccine safety surveillance . Stat Med. 2014 Aug 30;33(19):3387-3397.
Lopalco PL, DeStefano F. The complementary roles of Phase 3 trials and post-licensure surveillance in the evaluation of new vaccines . Vaccine . 2015 Mar 24;33(13):1541-8.
McNeil MM, Cano M, Miller ER, Petersen BW, Engler RJ, Bryant-Genevier MG. Ischemic cardiac events and other adverse events following ACAM2000® smallpox vaccine in the Vaccine Adverse Event Reporting System . Vaccine . 2014 Aug 20;32(37):4758-65.
McNeil MM, Gee J, Weintraub ES, Belongia EA, Lee GM, Glanz JM, et al. The Vaccine Safety Datalink: Successes and challenges monitoring vaccine safety . Vaccine. 2014 Sep 22;32(42):5390-5398.
Moro PL, Museru OI, Niu M, Lewis P, Broder K. Reports to the Vaccine Adverse Event Reporting System after hepatitis A and hepatitis AB vaccines in pregnant women . Am J Obstet Gynecol . 2014 Jun;210(6):561.e1-6
Naleway AL, Irving SA, Henninger ML, Li DK, Shifflett P, Ball S, et al. Safety of influenza vaccination during pregnancy: A review of subsequent maternal obstetric events and findings from two recent cohort studies . Vaccine . 2014;32(26):3122-3127.
Naleway AL, Kurosky S, Henninger ML, Gold R, Nordin JD, Kharbanda EO, et al. Vaccinations given during pregnancy, 2002-2009: a descriptive study . Am J Prev Med. 2014;46(2):150-157.
Nelson JC, Shortreed SM, Yu O, Peterson D, Baxter R, Fireman B, et al. Integrating database knowledge and epidemiological design to improve the implementation of data mining methods to evaluate vaccine safety in large healthcare databases . Stat Anal Data Min . 2014 Oct;7(5):337–351.
Nordin JD, Kharbanda EO, Vazquez-Benitez G, Lipkind H, Lee GM, Naleway AL. Monovalent H1N1 influenza vaccine safety in pregnant women, risks for acute adverse events . Vaccine . 2014 Sep 3;32(39):4985-4992.
Nordin JD, Kharbanda EO, Vazquez Benitez G, Lipkind H, Vellozzi C, Destefano F; Vaccine Safety Datalink. Maternal influenza vaccine and risks for preterm or small for gestational age birth. J Pediatr . 2014 May;164(5):1051-1057.e2.
Stockwell MS, Broder K, LaRussa P, Lewis P, Fernandez N, Sharma D, et al. Risk of fever after pediatric trivalent inactivated influenza vaccine and 13-valent pneumococcal conjugate vaccine. JAMA Pediatr . 2014 Mar;168(3):211-9.
Tartof SY, Tseng HF, Liu IL, Qian L, Sy LS, Hechter RC, et al. Inpatient admission for febrile seizure and subsequent outcomes do not differ in children with vaccine-associated versus non-vaccine associated febrile seizures . Vaccine . 2014;32(48):6408-14.
Tartof SY, Tseng HF, Liu AL, Qian L, Sy LS, Hechter RC, et al. Exploring the risk factors for vaccine-associated and non-vaccine associated febrile seizures in a large pediatric cohort . Vaccine . 2014;32(22):2574-2581
Weintraub ES, Baggs J, Duffy J, Vellozzi C, Belongia EA, Irving S, et al. Risk of intussusception after monovalent rotavirus vaccination . N Engl J Med . 2014 Feb; 370:513-519.
Xu S, Newcomer S, Nelson J, Qian L, McClure D, Pan Y, et al. Signal detection of adverse events with imperfect confirmation rates in vaccine safety studies using self-controlled case series design . Biom J . 2014 May;56(3):513-25.
Baxter R, Bakshi N, Fireman B, Lewis E, Ray P, Vellozzi C, et al. Lack of association of Guillain-Barre syndrome with vaccinations . Clin Infect Dis . 2013 Jul;57(2):197-204.
DeStefano F, Price CS, Weintraub ES. Increasing exposure to antibody-stimulating proteins and polysaccharides in vaccines Is not associated with risk of autism . J Pediatr . 2013 Aug;163(2):561-7.
Dodd CN, Romio SA, Black S, Vellozzi C, Andrews N, Sturkenboom M, et al. International collaboration to assess the risk of Guillain Barré Syndrome following influenza A (H1N1) 2009 monovalent vaccines . Vaccine . 2013 Sep;31(40):4448-58.
Glanz JM, Narwaney KJ, Newcomer SR, Daley MF, Hambidge SJ, Rowhani-Rahbar A, et al. Association between undervaccination with Diphtheria. Tetanus Toxoids, and Acellular Pertussis (DTaP) vaccine and risk of pertussis infection in children 3 to 36 months of age. JAMA Pediatr . 2013 Nov;167(11):1060-4.
Glanz JM, Newcomer SR, Narwaney KJ, Hambidge SJ, Daley MF, Wagner NM, et al. A population-based cohort study of undervaccination in 8 managed care organizations across the United States . JAMA Pediatr. 2013;167(3):274-281.
Gold R, Naleway A, Riedlinger K. Factors predicting completion of the human papillomavirus vaccine series . J Adolesc Health. 2013;52(4):427-432.
Greene SK, Li L, Shay DK, Fry AM, Lee GM, Jacobsen SJ, et al. Risk of adverse events following oseltamivir treatment in influenza outpatients, Vaccine Safety Datalink Project, 2007-2010 . Pharmacoepidemiol Drug Saf . 2013;22(4):335-344.
Greene SK, Rett MD, Vellozzi C, Li L, Kulldorff M, Marcy SM, et al. Guillain-Barré syndrome, influenza vaccination, and antecedent respiratory and gastrointestinal infections: A case-centered analysis in the Vaccine Safety Datalink, 2009-2011 . PLoS One . 2013 Jun;8(6):e67185.
Haber P, Patel M, Pan Y, Baggs J, Haber M, Museru O, et al. Intussusception after Rotavirus Vaccines—United States, Vaccine Adverse Event Reporting System (VAERS), 2006-2012 . Pediatrics. 2013 Jun;131(6):1042-9.
Halsey NA, Griffioen M, Dreskin SC, Dekker CL, Wood R, Sharma D, et al. Immediate hypersensitivity reactions following monovalent 2009 pandemic influenza A (H1N1) vaccines: Reports to VAERS. Vaccine . 2013 Dec;31(51):6107-12.
Hechter RC, Qian L, Sy LS, Greene SK, Weintraub ES, Naleway AL, et al. Secular trends in diagnostic code density in electronic healthcare data from health care systems in the Vaccine Safety Datalink Project . Vaccine . 2013 Feb;31(7):1080-85.
Henninger M, Crane B, Naleway A. Trends in influenza vaccine coverage in pregnant women, 2008 to 2012 . Perm J. 2013;17(2):31-36.
Henninger M, Naleway A, Crane B, Donahue J, Irving S. Predictors of seasonal influenza vaccination during pregnancy . Obstet Gynecol. 2013;121(4):741-749.
Iqbal S, Barile JP, Thompson WW, DeStefano F. Number of antigens in early childhood vaccines and neuropsychological outcomes at age 7-10 years . Pharmacoepidemiol Drug Saf . 2013;22(12):1263-1270.
Irving SA, Kieke BA, Donahue JG, Mascola MA, Baggs J, DeStefano F, et al. Trivalent inactivated influenza vaccine and spontaneous abortion . Obstet Gynecol. 2013;121(1):159-165.
Jackson ML, Yu O, Nelson JC, Naleway A, Belongia EA, Baxter R, et al. Further evidence for bias in observational studies of influenza vaccine effectiveness: the 2009 influenza A(H1N1) pandemic . Am J Epidemiol. 2013;178(8):1327-1336.
Jackson LA, Peterson D, Nelson JC, Marcy SM, Naleway AL, Nordin JD, et al. Vaccination site and risk of local reactions in children 1 through 6 years of age . Pediatrics . 2013 Feb;131(2):283-9.
Kharbanda EO, Vazquez-Benitez G, Lipkind H, Naleway A, Lee G, Nordin JD; Vaccine Safety Datalink. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol . 2013 Sep;122(3):659-67.
Kharbanda EO, Vazquez-Benitez G, Shi WX, Lipkind H, Naleway A, Molitor B, et al. Assessing the safety of influenza immunization during pregnancy: the Vaccine Safety Datalink . Am J Obstet Gynecol. 2012;207(3 Suppl):S47-S51.
McCarthy NL, Gee J, Lin ND, Thyagarajan V, Pan Y, Su S, et al. Evaluating the safety of influenza vaccine using a claims-based health system . Vaccine . 2013 Dec;31(50):5975-82.
McCarthy NL, Weintraub E, Vellozzi C, Duffy J, Gee J, Donahue JG, et al. Mortality rates and cause-of-death patterns in a vaccinated population . Am J Prev Med . 2013 Jul;45(1):91-7.
McCarthy NL, Irving S, Donahue JG, Weintraub E, Gee J, Belongia E, et al. Vaccination coverage levels among children enrolled in the Vaccine Safety Datalink. Vaccine . 2013 Dec;31(49):5822.
McNeil MM, Li R, Pickering S, Real TM, Smith PJ, Pemberton MR, et al. Who is unlikely to report adverse events after vaccinations to the Vaccine Adverse Event Reporting System (VAERS)? Vaccine . 2013 May;31(24):2673-9. 2013 Apr.
Moro PL, Harrington T, Shimabukuro T, Cano M, Museru OI, Menschik D, et al. Adverse events after Fluzone® Intradermal vaccine reported to the Vaccine Adverse Event Reporting System (VAERS), 2011-2013 . Vaccine . 2013 Oct;31(43):4984-7.
Moro PL, Museru OI, Broder K, Cragan J, Zheteyeva Y, Tepper N, et al. Safety of influenza A (H1N1) 2009 live attenuated monovalent vaccine in pregnant women . Obstet Gynecol . 2013 Dec;122(6):1271-8.
Naleway AL, Gold R, Kurosky S, Riedlinger K, Henninger ML, Nordin JD, et al. Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink . Vaccine . 2013;31(27):2898-2903.
Nelson JC, Yu O, Dominguez-Islas CP, Cook AJ, Peterson D, Greene SK, et al. Adapting group sequential methods to observational postlicensure vaccine safety surveillance: Results of a pentavalent combination DTaP-IPV-Hib vaccine safety study . Am J Epidemiol . 2013 Jan;177(2):131-41.
Nelson JC, Marsh T, Lumley T, Larson EB, Jackson LA, Jackson M, et al. Validation sampling can reduce bias in health care database studies: an illustration using influenza vaccination effectiveness . J Clin Epidemiol. 2013;66(8 Suppl):S110-S121.
Newcomer SR, Hambidge SJ, McClure DL, Daley MF, Klein NP, Glanz JM. Factors that may explain observed associations between trivalent influenza vaccination and gastrointestinal illness in young children . Vaccine . 2013;31(37):3894-3898.
Nordin JD, Parker ED, Vazquez-Benitez G, Kharbanda EO, Naleway A, Marcy SM, et al. Safety of the yellow fever vaccine: a retrospective study . J Travel Med. 2013;20(6):368-373.
Nordin JD, Kharbanda EO, Benitez GV, Nichol K, Lipkind H, Naleway A, et al. Maternal safety of trivalent inactivated influenza vaccine in pregnant women. Obstet Gynecol . 2013 Mar;121(3):519-25.
Pahud BA, Williams SE, Dekker CL, Halsey N, LaRussa P, et al. Clinical assessment of serious adverse events in children receiving 2009 H1N1 vaccination . Pediatr Infect Dis J . 2013 Feb;32(2):163-168.
Pan Y, Rose CE, Haber M, Ma Y, Carrasco JL, Stewart B, et al. Assessing agreement of repeated binary measurements with an application to the CDC’s anthrax vaccine clinical trial . Int J Biostat . 2013 Jul;9(1). 14.
Poli F, Overeem S, Lammers GJ, et al. Narcolepsy as an adverse event following immunization: case definition and guidelines for data collection, analysis and presentation . Vaccine . 2013 Jan 30;31(6):994-1007.
Qian L, Tseng HF, Sy LS, Jacobsen SJ. Confounder adjustment in vaccine safety studies: Comparing three offset terms for case-centered approach . Vaccine . 2013 Jan;31(2):431-5.
Rowhani-Rahbar A, Fireman B, Lewis E, Nordin J, Naleway A, Jacobsen SJ, et al. Effect of age on the risk of fever and seizures following immunization with measles-containing vaccines in children . JAMA Pediatr . 2013 Dec;167(12):1111-7.
Salmon DA, Proschan M, Forshee R, Gargiullo P, Bleser W, Burwen DR, et al. Association between Guillain-Barre syndrome and influenza A (H1N1) 2009 monovalent inactivated vaccines in the USA: a meta-analysis . Lancet . 2013;381(9876):1461-1468.
Schmidt MA, Gold R, Kurosky SK, Daley MF, Irving SA, Gee J, et al. Uptake, coverage, and completion of quadrivalent human papillomavirus vaccine in the Vaccine Safety Datalink, July 2006-June 2011. J Adolesc Health . 2013 Nov;53(5):637-41.
Thyagarajan V, Su S, Gee J, Duffy J, McCarthy NL, Chan KA, et al. Identification of seizures among adults and children following influenza vaccination using health insurance claims data. Vaccine . 2013 Dec;31(50):5997-6002.
Tseng HF, Sy LS, Qian L, Marcy SM, Jackson LA, Glanz J, et al; Vaccine Safety Datalink. Safety of a tetanus-diphtheria-acellular pertussis vaccine when used off-label in an elderly population. Clin Infect Dis . 2013 Feb;56(3):315-21.
Williams SE, Edwards KM, Baxter RP, Larussa PS, Halsey NA, et al. Comprehensive assessment of serious adverse events following immunization by health care providers . J Pediatr . 2013 Jun;162(6):1276-81, 1281.e1.
Xu S, Hambidge SJ, McClure DL, Daley MF, Glanz JM, et al. A scan statistic for identifying optimal risk windows in vaccine safety studies using self-controlled case series design . Stat Med . 2013;30;32(19):3290-9.
Zeng C, Newcomer SR, Glanz JM, Shoup JA, Daley MF, Hambidge SJ, et al. Bias correction of risk estimates in vaccine safety studies with rare adverse events using a self-controlled case series design . Am J Epidemiol. 2013;178(12):1750-1759.
Zheteyeva Y, Moro PL, Yue X, Broder K. Safety of meningococcal polysaccharide-protein conjugate vaccine in pregnancy: a review of the Vaccine Adverse Event Reporting System. Am J Obstet Gynecol . 2013 Aug;208(6):478.e1-6.
Baxter R, Lewis N, Bakshi N, Vellozzi C, Klein NP; CISA Network. Recurrent Guillain-Barre syndrome following vaccination . Clin Infect Dis . 2012 Mar;54(6):800-4.
Broder KR, Martin DB, Vellozzi C. In the heat of a signal: Responding to a vaccine safety signal for febrile seizures after 2010–11 influenza vaccine in young children, United States. Vaccine . 2012 Mar;30(11):2032-34.
Cano M, Lewis P, Ou AC, Sharma D, Vellozzi C, Broder KR. Bell’s palsy cases following administration of influenza A (H1N1) 2009 monovalent vaccine reported to the Vaccine Adverse Event Reporting System (VAERS) [PDF – 1.84 MB] . J Vaccines Vaccin . 2012;3(2)134.
Greene SK, Rett M, Weintraub ES, Li L, Yin R, Amato AA, et al. Risk of confirmed Guillain-Barre syndrome following receipt of monovalent inactivated influenza A (H1N1) and seasonal influenza vaccines in the Vaccine Safety Datalink project, 2009-2010 . Am J Epidemiol. 2012;175(11):1100-1109.
Greene SK, Shay DK, Yin R, McCarthy NL, Baxter R, Jackson ML, et al. Patterns in influenza antiviral medication use before and during the 2009 H1N1 pandemic, Vaccine Safety Datalink Project, 2000-2010 . Influenza Other Respir Viruses. 2012;6(6):e143-e151.
Halsey NA, Edwards KM, Dekker CL, Klein NP, Baxter R, et al. Algorithm to assess causality after individual adverse events following immunizations . Vaccine. 2012 Aug 24;30(39):5791-8.
Hambidge SJ, Ross C, Glanz J, McClure D, Daley MF, Xu S, et al. Trivalent inactivated influenza vaccine is not associated with sickle cell crises in children . Pediatrics . 2012;129(1):e54-e59.
Hechter RC, Qian L, Sy LS, Greene SK, Weintraub ES, Naleway AL, et al. Secular trends in diagnostic code density in electronic healthcare data from health care systems in the Vaccine Safety Datalink project . Vaccine . 2012;31(7):1080-1085.
Klein NP, Lewis E, Baxter R, Weintraub E, Glanz J, Naleway A, et al. Measles-containing vaccines and febrile seizures in children age 4 to 6 years . Pediatrics . 2012;129(5):809-814.
Leroy Z, Broder K, Menschik D, Shimabukuro T, Martin D. Febrile seizures after 2010–2011 influenza vaccine in young children, United States: A vaccine safety signal from the vaccine adverse event reporting system . Vaccine. 2012;30(11):2020-3.
Loughlin AM, Marchant CD, Adams W, Barnett E, Baxter R, et al. Causality assessment of adverse events reported to the Vaccine Adverse Event Reporting System (VAERS) . Vaccine . 2012 Nov 26;30(50):7253-9.
McClure DL, Xu S, Weintraub E, Glanz JM. An efficient statistical algorithm for a temporal scan statistic applied to vaccine safety analyses . Vaccine . 2012;30(27):3986-3991.
Moro PL, Tepper NK, Grohskopf LA, Vellozzi C, Broder K. Safety of seasonal influenza and influenza A (H1N1) 2009 monovalent vaccines in pregnancy. Expert Rev Vaccines . 2012 Aug;11(8):911-21.
Moro PL, Arana J, Cano M, Menschik D, Yue Lewis P, Haber P, et al. Postlicensure safety surveillance for high-dose trivalent inactivated influenza vaccine in the Vaccine Adverse Event Reporting System, 1 July 2010–31 December 2010 . Clin Infect Dis. 2012;54(11):1608-14.
Naleway AL, Gold R, Drew L, Riedlinger K, Henninger ML, Gee J. Reported adverse events in young women following quadrivalent human papillomavirus vaccination . J Womens Health (Larchmt.) . 2012;21(4):425-432.
Pahud BA, Rowhani-Rahbar A, Glaser C, Gavali S, Salibay CJ, et al. Lack of association between childhood immunizations and encephalitis in California, 1998-2008 . Vaccine . 2012 Jan 5;30(2):247-53.
Rowhani-Rahbar A, Baxter R, Rasgon B, Ray P, Black S, Klein JO, et al. Epidemiologic and clinical features of Bell’s palsy among children in northern California . Neuroepidemiology . 2012;38(4):252-258.
Rowhani-Rahbar A, Fireman B, Lewis N, Ray P, Rasgon B, Klein JO, et al. Rowhani-Rahbar et al. respond to “Immunization and Bell’s palsy in children.” Am J Epidemiol. 2012;175(9):888-889.
Rowhani-Rahbar A, Klein NP, Dekker CL, Edwards KM, Marchant CD, et al. Biologically plausible and evidence-based risk intervals in immunization safety research . Vaccine . 2012 Dec 17;31(1):271-7.
Rowhani-Rahbar A, Klein NP, Lewis N, Fireman B, Ray P, Rasgon B, et al. Immunization and Bell’s palsy in children: a case-centered analysis . Am J Epidemiol. 2012;175(9):878-885.
Shui IM, Baggs J, Patel M, Parashar UD, Rett M, Belongia EA, et al. Risk of intussusception following administration of a pentavalent rotavirus vaccine in US infants . JAMA . 2012;307(6):598-604.
Shui IM, Rett MD, Weintraub E, Marcy M, Amato AA, Sheikh SI, et al. Guillain-Barré syndrome incidence in a large United States cohort (2000-2009) . Neuroepidemiology . 2012;39(2):109-115.
Stewart B, Rose CE, Tokars JI, Martin SW, Keitel WA, Keyserling HL, et al. Health-related quality of life in the CDC Anthrax Vaccine Adsorbed Human Clinical Trial. Vaccine . 2012 Aug;30(40):5875-9.
Tokars JI, Lewis P, DeStefano F, Wise M, Viray M, Morgan O, Gargiullo P, Vellozzi C. The risk of Guillain-Barré syndrome associated with influenza A (H1N1) 2009 monovalent vaccine and 2009-2010 seasonal influenza vaccines: results from self-controlled analyses . Pharmacoepidemiol Drug Saf . 2012 May;21(5):546-52.
Tse A, Tseng HF, Greene SK, Vellozzi C, Lee GM, VSD Rapid Cycle Analysis Influenza Working Group. Signal identification and evaluation for risk of febrile seizures in children following trivalent inactivated influenza vaccine in the Vaccine Safety Datalink Project, 2010-2011 . Vaccine . 2012;30(11):2024-2031.
Wise ME, Viray M, Sejvar JJ, Lewis P, Baughman AL, Connor W, Danila R, Giambrone GP, Hale C, Hogan BC, Meek JI, Murphree R, Oh JY, Reingold A, Tellman N, Conner SM, Singleton JA, Lu PJ, DeStefano F, Fridkin SK, Vellozzi C, Morgan OW. Guillain-Barre syndrome during the 2009-2010 H1N1 influenza vaccination campaign: population-based surveillance among 45 million Americans . Am J Epidemiol . 2012 Jun 1;175(11):1110-9.
Xu S, Zeng C, Newcomer S, Nelson J, Glanz J. Use of fixed effects models to analyze self-controlled case series data in vaccine safety studies . J Biom Biostat. 2012;Suppl 7:006.
Yih WK, Weintraub E, Kulldorff M. No risk of Guillain-Barré syndrome found after meningococcal conjugate vaccination in two large cohort studies . Pharmacoepidemiol Drug Saf . 2012;21(12):1359-1360.
Zheteyeva YA, Moro PL, Tepper NK, Rasmussen SA, Barash FE, Revzina NV, et al. Adverse event reports after tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines in pregnant women . Am J Obstet Gynecol. 2012 Jul; 207(1):59.e1-7.
Baggs J, Gee J, Lewis E, Fowler G, Benson P, Lieu T, et al. The Vaccine Safety Datalink: A model for monitoring immunization safety. Pediatrics . 2011 May;127 Suppl 1:S45-53.
Barile JP, Kuperminc GP, Weintraub ES, Mink JW, Thompson WW, et al. Thimerosal exposure in early life and neuropsychological outcomes 7-10 years later . J Pediatr Psychol. 2011;37(1):106-118.
Gee J, Naleway A, Shui I, Baggs J, Yin R, Li R, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine. 2011 Oct; 29(46):8279-84.
Gidudu J, Sack DA, Pina M, Hudson MJ, Kohl KS, Bishop P, et al. Diarrhea: Case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine . 2011 Jan;29(5): 1053-71.
Glanz JM, Newcomer SR, Hambidge SJ, Daley MF, Narwaney KJ, Xu S, et al. Safety of trivalent inactivated influenza vaccine in children aged 24 to 59 months in the Vaccine Safety Datalink . Arch Pediatr Adolesc Med. 2011;165(8):749-755.
Greene SK, Kulldorff M, Yin R, Yih WK, Lieu TA, Weintraub ES, et al. Near real-time vaccine safety surveillance with partially accrued data. Pharmacoepidemiol Drug Saf . 2011 Jun;20(6):583-90
Haber P, Iskander J, Walton K, Campbell SR, Kohl KS., et al. Internet-based reporting to the vaccine adverse event reporting system: a more timely and complete way for providers to support vaccine safety . Pediatrics . 2011 May;127 Suppl 1: S39-44.
Hambidge SJ, Ross C, McClure D, Glanz J; VSD team. Trivalent inactivated influenza vaccine is not associated with sickle cell hospitalizations in adults from a large cohort . Vaccine . 2011;29(46):8179-8181.
Huang WT, Suh C, Campagna E, Broder KR, Daley MF, Crane LA, et al. Adherence to the advisory committee on immunization practices recommendation to prevent injuries from postvaccination syncope: A national physician survey . Am J Prev Med . 2011 Sep;41(3):317-21.
Jackson LA, Yu O, Nelson JC, Dominguez C, Peterson D, Baxter R, et al. Injection site and risk of medically attended local reactions to acellular pertussis vaccine . Pediatrics . 2011;127(3):e581-e587.
Jackson LA, Peterson D, Dunn J, Hambidge SJ, Dunstan M, Starkovichet P. A randomized placebo-controlled trial of acetaminophen for prevention of post-vaccination fever in infants . PLoS One. 2011;6(6):e20102.
Klein NP, Aukes L, Lee J, Fireman B, Shapira SK, Slade B, et al. Evaluation of immunization rates and safety among children with inborn errors of metabolism. Pediatrics . 2011 May;127(5):e1139-46.
Klein NP, Gidudu J, Qiang Y, Pahud B, Rowhani-Rahbar A, Baxter R, et al. Developing the next generation of vaccinologists. Vaccine . 2011 Nov;29(50):9296-97.
Kulldorff M, Davis RL, Kolczak M, Lewis E, Lieu T, Platt R. A maximized sequential probability ratio test for drug and vaccine safety surveillance. Seq Anal . 2011 Jan;30(1): 58-78.
LaRussa PS, Edwards KM, Dekker CL, Klein NP, Halsey NA, Marchant C, et al. Understanding the role of human variation in vaccine adverse events: The Clinical Immunization Safety Assessment Network. Pediatrics . 2011 May;127 Suppl 1:S65-73.
Lee GM, Greene SK, Weintraub ES, Baggs J, Kulldorff M, Fireman BH. H1N1 and seasonal influenza vaccine safety in the Vaccine Safety Datalink project . Am J Prev Med. 2011;41(2):121-128.
McCarthy NL, Gee J, Weintraub E, Donahue JG, Nordin JD, Daley MF, et al. Monitoring vaccine safety using the Vaccine Safety Datalink: Utilizing immunization registries for pandemic influenza. Vaccine . 2011 Jul; 29(31):4891-96.
Miller EK, Batten B, Hampton L, Campbell SR, Gao J, Iskander J. Tracking vaccine-safety inquiries to detect signals and monitor public concerns. Pediatrics . 2011 May;127 Suppl 1:S87-91.
Miller EK, Dumitrescu L, Cupp C, Dorris S, Taylor S, Sparks R. Atopy history and the genomics of wheezing after influenza vaccination in children 6-59 months of age. Vaccine . 2011 Apr;29(18):3431-37.
Morgan TM, Schlegel C, Edwards KM, Welch-Burke T, Zhu Y, Sparks R. Vaccines are not associated with metabolic events in children with urea cycle disorders. Pediatrics . 2011 May;127(5):e1147-53.
Moro PL, Broder K, Zheteyeva Y, Revzina N, Tepper N, Kissin D, et al. Adverse events following administration to pregnant women of influenza A (H1N1) 2009 monovalent vaccine reported to the Vaccine Adverse Event Reporting System . Am J Obstet Gynecol. 2011; 205(5):473.e1-9.
Moro PL, Yue X, Lewis P, Haber P, Broder K. Adverse events after tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine administered to adults 65 years of age and older reported to the Vaccine Adverse Event Reporting System (VAERS), 2005-2010 . Vaccine . 2011;29(50):9404-8.
Moro PL, Broder K, Zheteyeva Y, Walton K, Rohan P, Sutherland A, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009 . Am J Obstet Gynecol. 2011 Feb; 204(2):146.e1-7.
Mullooly JP, Schuler R, Mesa J, Drew L, DeStefano F; VSD team. Wheezing lower respiratory disease and vaccination of premature infants . Vaccine . 2011;29(44):7611-7617.
Navar-Boggan AM, Halsey NA, Escobar GJ, Golden WC, Klein NP. Underimmunization in the Neonatal Intensive Care Unit . J Perinatol . 2012 May;32(5):363-7.
Nelson JC, Cook AJ, Yu O, et al. Methods for observational post-licensure medical product safety surveillance . Stat Methods Med Res. Epub 2 Dec 2011.
Nordin JD, Yih WK, Kulldorff M, Weintraub E. Tdap and GBS letter . Vaccine . 2011 Feb;29(6):1122.
Pahud BA, Glaser CA, Dekker CL, Arvin AM, Schmid DS. Varicella zoster disease of the central nervous system: Epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis . 2011 Feb;203(3):316-23.
Ray P, Black S, Shinefield H, et al. Risk of rheumatoid arthritis following vaccination with tetanus, influenza and hepatitis B vaccines among persons 15-59 years of age . Vaccine . 2011;29(38):6592-6597.
Salmon DA, Akhtar A, Mergler MJ, Vannice KS, Izurieta H, Ball R, et al. Immunization-safety monitoring systems for the 2009 H1N1 monovalent influenza vaccination program. Pediatrics . 2011 May;127 Suppl 1:S78-86.
Slade B, Gee J, Broder KR, Vellozzi C. Comment on the contribution by Souayah et al., Guillain-Barré syndrome after Gardasil vaccination: Data from Vaccine Adverse Event Reporting System 2006-2009 . Vaccine . 2011 Jan;29(5):865-66.
Tseng HF, Liu A, Sy L, Marcy SM, Fireman B, Weintraub E, et al. Safety of zoster vaccine in adults from a large managed care cohort: a Vaccine Safety Datalink study . J Intern Med. 2011;271(5):510-520.
Williams SE, Pahud BA, Vellozzi C, Donofrio PD, Dekker CL, et al. Causality assessment of serious neurologic adverse events following 2009 H1N1 vaccination . Vaccine . 2011 Oct 26;29(46):8302-8.
Williams SE, Klein NP, Halsey N, Dekker CL, Baxter RP, et al. Overview of the clinical consult case review of adverse events following immunization: Clinical Immunization Safety Assessment (CISA) Network 2004-2009 . Vaccine . 2011 Sep 16;29(40):6920.
Woo EJ, Wise RP, Menschik D, Shadomy SV, Iskander J, Beeler J, et al. Thrombocytopenia after vaccination: Case reports to the US Vaccine Adverse Event Reporting System , 1990-2008. Vaccine . 2011 Feb;29(6):1319-23.
Xu S, Zhang L, Nelson JC, Zeng C, Mullooly J, McClure D, et al. Identifying optimal risk windows for self-controlled case series studies of vaccine safety. Stat Med . 2011 Mar;30(7):742-52.
Yen C, Jakob K, Esona MD, Peckham X, Rausch J, Hull JJ, et al. Detection of fecal shedding of rotavirus vaccine in infants following their first dose of pentavalent rotavirus vaccine. Vaccine . 2011 May;29(24):4151-55.
Yih WK, Kulldorff M, Fireman BH, Shui IM, Lewis EM, Klein NP, et al. Active surveillance for adverse events: The experience of the Vaccine Safety Datalink project. Pediatrics . 2011 May;127 Suppl 1: S54-S64.
Bakare N, Menschik D, Tiernan R, Hua W, Martin D. Severe combined immunodeficiency (SCID) and rotavirus vaccination: Reports to the Vaccine Adverse Events Reporting System (VAERS). Vaccine . 2010 Sep;28(40):6609-12.
Belongia EA, Irving SA, Shui IM, Kulldorf M, Lewis E, Yin R, et al; Vaccine Safety Datalink Real-time surveillance to assess risk of intussusception and other adverse events after pentavalent, bovine-derived rotavirus vaccine . Pedatr Infect Dis J . 2010 Jan;29(1):1-5.
Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010 . MMWR . 2010 Aug;59(RR-8):1-62.
Goodman MJ, Nordin JD, Belongia EA, Mullooly JP, Baggs J. Henoch-Schonlein purpura and polysaccharide meningococcal vaccine. Pediatrics . 2010 Aug;126(2):e325-29.
Greene SK, Kulldorff M, Lewis EM, Li R, Yin R, Weintraub ES, et al. Near real-time surveillance for influenza vaccine safety: Proof of concept in the Vaccine Safety Datalink Project . Am J Epidemiol . 2010 Jan;171(2):177-88. 2009 Dec.
Huang WT, Chang S, Miller ER, Woo EJ, Hoffmaster AR, Gee JE, et al. Safety assessment of recalled Haemophilus influenzae type b (Hib) conjugate vaccines–United States, 2007-2008 . Pharmacoepidemiol Drug Saf . 2010 Mar;19(3):306-310.
Huang WT, Gargiullo PM, Broder KR, Weintraub ES, Iskander JK, Klein NP, et al; Vaccine Safety Datalink. Lack of association between acellular pertussis vaccine and seizures in early childhood. Pediatrics . 2010 Aug;126(2):263-9.
Klein NP, Fireman B, Yih WK, Lewis E, Kulldorff M, Ray P, et al; Vaccine Safety Datalink. Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatrics . 2010;126(1):e1-e8.
Klein NP, Gans HA, Sung P, Yasukawa LL, Johnson J, Sarafanov A, et al. Preterm infants’ T cell responses to inactivated poliovirus vaccine. J Infect Dis . 2010 Jan;201(2):214-22.
Li L, Kulldorff M. A conditional maximized sequential probability ratio test for pharmacovigilance . Stat Med . 2010 Jan;29: 284-95, 2010.
Lin ND, Kleinman K, Chan KA, Soumerai S, Mehta J, Mullooly JP, et al; Vaccine Safety Datalink. Multiple vaccinations and the risk of medically attended fever . Vaccine . 2010 Jun;28(25): 4169-74.
Lindsey NP, Staples JE, Jones JF, Sejvar JJ, Griggs A, Iskander J, et al. Adverse event reports following Japanese encephalitis vaccination in the United States, 1999-2009. Vaccine . 2010 Dec;29(1):58-64.
Marin M, Broder KR, Temte JL, Snider DE, Seward JF; Centers for Disease Control and Prevention (CDC). Use of combination measles, mumps, rubella, and varicella vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR . 2010 May;59(RR-3):1-12.
McNeil MM, Broder KR, Vellozzi C, DeStefano F. Risk of fatal adverse events after H1N1 influenza vaccine: Limitations of passive surveillance data. Clin Infect Dis . 2010;51(7):871–72; author reply 872-3.
Muhammad RD, Haber P, Broder KR, Leroy Z, Ball R, Braun MM, et al. Adverse events following trivalent inactivated influenza vaccination in children: Analysis of the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J . 2010 Jan;30(1):e1-e8.
Navar-Boggan AM, Halsey NA, Golden WC, Escobar GJ, Massolo M, Klein NP. Risk of fever and sepsis evaluations following routine immunizations in the neonatal intensive care unit. J Perinatol . 2010 Sep;30(9):604-9.
Price CS, Thompson WW, Goodson B, Weintraub ES, Croen LA, Hinrichsen VL, et al. Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism . Pediatrics . 2010 Oct;126(4):656-64.
Schmidt MA, Crane B, Mullooly JP, Naleway AL. Frequency of medically attended events following rapid revaccination with trivalent inactivated influenza vaccine . Vaccine . 2010 Nov;28(49):7713-5.
Smith MJ, Woods CR. On-time vaccine receipt in the first year does not adversely affect neuropsychological outcomes. Pediatrics . 2010 Jun;125(6):1134-41.
Sy LS, Liu IL, Solano Z, Cheetham TC, Lugg MM, Greene SK, et al. Accuracy of influenza vaccination status in a computer-based immunization tracking system of a managed care organization. Vaccine . 2010 Jul;28(32):5254-59.
Talbot EA, Brown KH, Kirkland KB, Baughman AL, Halperin SA, Broder KR. The safety of immunizing with tetanus-diphtheria-acellular pertussis vaccine (Tdap) less than 2 years following previous tetanus vaccination: Experience during a mass vaccination campaign of healthcare personnel during a respiratory illness outbreak. Vaccine . 2010 Nov;28(50):8001-7.
Vellozzi C, Broder KR, Haber P, Guh A, Nguyen M, Cano M, et al. Adverse events following influenza A (H1N1) 2009 monovalent vaccines reported to the Vaccine Adverse Event Reporting System, United States, October 1, 2009-January 31, 2010 . Vaccine . 2010 Oct; (45):7248-55.
Wong C, Krashin J, Rue-Cover A, Saraiya M, Unger E, Calugar A, et al. Invasive and in situ cervical cancer reported to the vaccine adverse event reporting system (VAERS). J Womens Health (Larchmt) . 2010 Mar; 19(3):365-370.
Xu S, Gargiullo P, Mullooly J, McClure , Hambidge SJ, Glanz J. Fitting parametric and semi-parametric conditional Poisson regression models with cox’s partial likelihood in self-controlled case series and matched cohort studies. [PDF – 12 pages] J Data Sci . 2010 Apr;8(2):349-60.
Zangwill KM, Yeh SH, Wong EJ, Marcy SM, Eriksen E, Huff KR, et al. Paralytic syndromes in children: Epidemiology and relationship to vaccination. Pediatr Neurol . 2010 Mar;42(3):206-12.
Batra J, Eriksen E, Zangwill K, Lee M, Marcy S, Ward J; Vaccine Safety Datalink. Evaluation of vaccine coverage for low birth weight infants during the first year of life in a large managed care population. Pediatrics . 2009 Mar;123(3):951-958.
Bonhoeffer J, Bentsi-Echnill, Chen R, Fisher M, Gold M, Hartman K, et al; Brighton Collaboration Methods Working Group. Guidelines for collection, analysis, and presentation of vaccine safety data in pre- and post-licensure clinical studies . Vaccine . 2009 Apr;27(16):2282-8
CDC. Safety of influenza A (H1N1) 2009 monovalent vaccines—United States, October 1–November 24, 2009. MMWR . 2009 Dec; 58(48):1351-1356.
Donahue JG, Kieke BA, Yih WK, Berger NR, McCauley JS, Baggs J, et al; Vaccine Safety Datalink. Varicella vaccination and ischemic stroke in children: Is there an association? Pediatrics . 2009 Feb;123(2)e228–34.
Durbin AP, Setse R, Omer SB, Palmer JG, Spaeder JA, Baker J, et al. Monitoring adverse events following yellow fever vaccination using an integrated telephone and internet-based system . Vaccine . 2009 Oct; 27(44):6143-47.
Fiore AE, Shay DK, Broder K, Iskander J, Uyeki TM, Mootrey G, et al. Prevention and control of seasonal influenza. MMWR . 2009 Jul;58(RR-8):1-52.
Greene SK, Shi P, Dutta-Linn MM, Shoup JA, Hinrichsen VL, Ray P, et al. Accuracy of data on influenza vaccination status at four Vaccine Safety Datalink sites. Am J Prev Med . 2009 Dec;37(6):552-5.
Haber P, Sejvar J, Mikaeloff Y, DeStefano F. Vaccines and Guillain-Barré syndrome. Drug Saf . 2009;32(4):309-23.
Hazlehurst B, Naleway A, Mullooly J. Detecting possible vaccine adverse events in clinical notes of the electronic medical record. Vaccine . 2009 Mar;27(14):2077-83.
Hua W, Izurieta HS, Slade B, Belay ED, Haber P, Tiernan R, et al. Kawasaki disease after vaccination: reports to the vaccine adverse event reporting system 1990-2007 . Pediatr Infect Dis J. 2009;28(11):943-7.
Jackson LA, Yu Onchee, Nelson J, Belongia EA, Hambidge SJ, Baxter R, et al. Risk of medically-attended local reactions following diphtheria toxoid containing vaccines in adolescents and young adults: A Vaccine Safety Datalink Study. Vaccine . 2009 Aug;27(36):4912-6.
Jackson LA, Onchee Y, Belongia EA, Hambidge SJ, Nelson J, Baxter R, et al. Frequency of medically attended adverse events following tetanus and diphtheria toxoid vaccine in adolescents and young adults: A Vaccine Safety Datalink study. BMC Infect Dis . 2009 Oct;9:165.
Jackson LA, Baxter R, Naleway AL, Belongia EA, Baggs J. Patterns of pneumococcal vaccination and revaccination in elderly and non-elderly adults: A Vaccine Safety Datalink study. BMC Infect Dis . 2009 Mar;9:37.
Klein NP, Edwards KM, Sparks R, Dekker CL; Clinical Immunization Safety Assessment (CISA) Network. Recurrent sterile abscesses following immunization: A possible association with aluminum adjuvant. BMJ Case Rep . 2009;2009.
Klein NP, Kissner J, Aguirre A, Sparks R, Campbell S, Edwards KM, et al. Differential maternal responses to a newly developed vaccine information pamphlet. Vaccine 2009; 28:323–8.
Naleway AL, Belongia EA, Donahue JG, Kieke BA, Glanz J; Vaccine Safety Datalink. Risk of immune hemolytic anemia in children following immunization. Vaccine . 2009 Dec;27(52):7394-7.
Nelson JC, Bittner RCL, Bounds L, Zhao S, Baggs J, Donahue JG, et al. Compliance with multiple-dose vaccine schedules among older children, adolescents, and adults: results from a Vaccine Safety Datalink Study. Am J Public Health . 2009 Oct;99 Suppl 2:S389-97.
Niu MT, Ball R, Woo EJ, Burwen DR, Knippen M, Braun MM; et al. Adverse events after anthrax vaccination reported to the Vaccine Adverse Event Reporting System (VAERS), 1990-2007 . Vaccine. 2009;27(2):290-7.
Patel MM, Haber P, Baggs J, Zuber P, Bines JE, Parashar UD. Intussusception and rotavirus vaccination: a review of the available evidence . Expert Rev Vaccines. 2009;8(11):1555-64.
Rosenberg M, Sparks R, McMahon A, Iskander J, Campbell JD, Edwards KM. Serious adverse events rarely reported after trivalent inactivated influenza vaccine (TIV) in children 6–23 months of age. Vaccine . 2009 Jul;27(32):4278-83.
Rue-Cover A, Iskander J, Lyn S, Burwen DR, Gargiullo P, Shadomy S, et al. Death and serious illness following influenza vaccination: A multidisciplinary investigation. Pharmacoepidemiol Drug Saf . 2009 Jun;18(6):504-11.
Shui IM, Shi P, Dutta-Linn MM, Weintraub ES, Hambidge SJ, Nordin JD, et al; Vaccine Safety Datalink. Predictive value of seizure ICD-9 codes for vaccine safety research. Vaccine . 2009 Aug;27(39):5307-12.
Slade BA, Leidel L, Vellozzi C, Woo EJ, Hua W, Sutherland A, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA . 2009 Aug;302(7):750-7.
Tate J, Curns A, Cortese M, Weintraub E, Hambidge S, Zangwill K, et al. Burden of acute gastroenteritis hospitalizations and emergency department visits in U.S. children that is totally preventable by rotavirus vaccination: a probe study using the now-withdrawn RotaShield vaccine. Pediatrics . 2009 Mar;123(3):744-9.
Tozzi AE, Bisiacchi P, Tarantino V, De Mei B, D’Elia L, Chariotti F, Salmaso S. Neuropsychological performance 10 years after immunization in infancy with thimerosal-containing vaccines. Pediatrics . 2009 Feb;123(2):475-82.
Vellozzi C, Burwen DR, Dobardzic A, Ball R, Walton K, Haber P. Safety of trivalent inactivated influenza vaccines in adults: Background for pandemic influenza vaccine safety monitoring. Vaccine . 2009 Mar;27(15):2114-20.
Wei F, Mullooly JP, Goodman M, McCarty MC, Hanson AM, Crane B, et al. Identification and characteristics of vaccine refusers. BMC Pediatr . 2009 Mar;9:18.
Yih WK, Nordin JD, Kulldorff M, Lewis E, Lieu TA, Shi P, et al. An assessment of the safety of adolescent and adult tetanus–diphtheria–acellular pertussis (Tdap) vaccine, using active surveillance for adverse events in the Vaccine Safety Datalink. Vaccine . 2009 Jul;27(32):4257-62.
Asatryan A, Pool V, Chen RT, Kohl KS, Davis RL, Iskander JK; VAERS team. Live attenuated measles and mumps viral strain-containing vaccines and hearing loss: Vaccine Adverse Event Reporting System (VAERS), United States, 1990–2003. Vaccine . 2008 Feb 26;26(9):1166-72.
Benson PJ, Jackson LA, Rees TG, Dunn JB. Changes in DT vaccine frequency and indications for use following introduction of DTaP vaccine. Hum Vaccin . 2008 May-Jun;4(3):234-7.
Broder KR, Cohn AC, Schwartz B, Klein JD, Fisher MM, Fishbein DB, et al; Working Group on Adolescent Prevention Priorities. Adolescent immunizations and other clinical preventive services: A needle and a hook? Pediatrics . 2008 Jan;121 Suppl 1:S25-34.
Chapman LE, Iskander JK, Chen RT, Neff J, Birkhead GS, Poland G, et al. A process for sentinel case review to assess causal relationships between smallpox vaccination and adverse outcomes, 2003–2004. Clin Infect Dis . 2008 Mar;46 Suppl 3:S271-93.
Chaves SS, Haber P, Walton K, Wise RP, Izurieta HS, Schmid DS, et al. Safety of varicella vaccine after licensure in the United States: experience from reports to the Vaccine Adverse Event Reporting System, 1995–2005. J Infect Dis . 2008 Mar 1;197 Suppl 2:S170-7.
Clayton HA, Cortese MM, Payne DC, Bartlett DL, Zimmerman LA, Williams WG, Wang M, Stockman LJ, Parashar U, Baggs J. Rotavirus vaccination coverage and adherence to the Advisory Committee on Immunization Practices (ACIP)-recommended vaccination schedule—United States, February 2006–May 2007. MMWR . 2008 Apr;57(15):398-401.
Fishbein DB, Broder KR, Markowitz L, Messonnier N. New, and some not-so-new, vaccines for adolescents and diseases they prevent. Pediatrics . 2008 Jan;121 Suppl 1:S5-14.
France EK, Glanz JM, Xu S, Hambidge S, Yamasaki K, Black SB, et al; Vaccine Safety Datalink. Risk of immune thrombocytopenic purpura after measles-mumps-rubella immunization in children. Pediatrics . 2008 Mar;121(3):e687-92.
Gidudu J, Kohl KS, Halperin S, Hammer SJ, Heath PT, Hennig R, et al; The Brighton Collaboration Local Reactions Working Group for A Local Reaction at or near Injection Site. A local reaction at or near injection site: Case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine . 2008 Dec;26(52):6800-13.
Glanz JM, France EK, Xu S, Hayes T, Hambidge S. A population-based, multisite cohort study of the predictors of chronic idiopathic thrombocytopenic purpura in children. Pediatrics . 2008 Mar;121(3):e506-12.
Haber P, Patel M, Izurieta HS, Baggs J, Gargiullo P, Weintraub E, et al. Postlicensure monitoring of intussusception after RotaTeq vaccination in the United States, February 1, 2006, to September 25, 2007. Pediatrics . 2008 Jun;121(6):1206-12.
Halsey NA. The Human Papillomavirus Vaccine and Risk of Anaphylaxis . [Commentary]. CMAJ . 2008 Sep 9;179(6):509-10. Erratum in: CMAJ. 2008 Sep 23; 179(7):678.
Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR . 2008 Jun;57(RR-5):1-30; quiz CE2-4.
Iskander J, Broder K. Monitoring the safety of annual and pandemic influenza vaccines: lessons from the US experience. Expert Rev Vaccines . 2008 Feb;7(1):75-82.
Iskander J, Gidudu J, Arboleda N, Huang WT. Selected major issues in vaccine safety . Ann Nestlé . 2008;66:93-102.
Klein NP, Massolo ML, Greene J, Dekker CL, Black S, Escobar GJ; Vaccine Safety Datalink. Risk factors for developing apnea after immunization in the neonatal intensive care unit. Pediatrics . 2008 Mar;121(3):463-9.
Kohl K, Magnus M, Ball R, Halsey N, Shadomy S, et al. Applicability, Reliability, Sensitivity, and Specificity of Six Brighton Collaboration Standardized Case Definitions for Adverse Events Following Immunization . Vaccine . 2008 Nov 25; 26(50):6349–60.
Leung J, Rue A, Lopez A, Ortega-Sanchez IR, Harpaz R, Guris D, et al. Varicella outbreak reporting, response, management, and national surveillance. J Infect Dis . 2008 Mar 1;197 Suppl 2:S108-13.
Lindsey NP, Schroeder BA, Miller ER, Braun MM, Hinckley AF, Marano N, et al. Adverse event reports following yellow fever vaccination . Vaccine. 2008;26(48):6077-82.
McClure DL, Glanz JM, Xu S, Hambidge SJ, Mullooly JP, Baggs J. Comparison of epidemiologic methods for active surveillance of vaccine safety. Vaccine . 2008 Jun;26(26):3341-5.
McMahon AW, Iskander JK, Haber P, Braun MM, Ball R. Inactivated influenza vaccine (IIV) in children < 2 years of age: Examination of selected adverse events reported to the Vaccine Adverse Event Reporting System (VAERS) after thimerosal-free or thimerosal-containing vaccine. Vaccine . 2008 Jan;26(3):427-9.
Morgan J, Roper MH, Sperling L, Schieber RA, Heffelfinger JD, Casey CG, et al. Myocarditis, pericarditis, and dilated cardiomyopathy after smallpox vaccination among civilians in the United States, January–October 2003. Clin Infect Dis . 2008 Mar 15;46 Suppl 3:S242-50.
Nachamkin I, Shadomy SV, Moran AP, Cox N, Fitzgerald C, Ung H, et al. Anti-ganglioside antibody induction by swine (A/NJ/1976/H1N1) and other influenza vaccines: Insights into vaccine-associated Guillain-Barre syndrome. J Infect Dis . 2008 Jul 15;198(2):226-33.
Rennels M, Black S, Woo E, Campbell S, Edwards KM. Safety of a fifth dose of diphtheria and tetanus toxoid and acellular pertussis vaccine in children experiencing extensive, local reactions to the fourth dose . Pediatr Infect Dis J . 2008 May;27(5):464-5.
Sutherland A, Izurieta H, Ball R, Braun MM, Miller ER, Broder KR, et al; Centers for Disease Control and Prevention. Syncope after vaccination—United States, January 2005–July 2007. MMWR . 2008 May;57(17):457-60.
Swerdlow DL, Roper MH, Morgan J, Schieber RA, Sperling LS, Sniadack MM, et al; Smallpox Vaccine Cardiac Adverse Events Working Group. Ischemic cardiac events during the Department of Health and Human Services Smallpox Vaccination Program, 2003. Clin Infect Dis . 2008 Mar 15;46 Suppl 3:S234-41.
Tatti KM, Slade BA, Patel M, Messonier NR, Jackson T, Kirkland K, et al. Real-time polymerase chain reaction detection of Bordetella pertussis DNA in acellular pertussis vaccines. Pediatr Infect Dis J . 2008 Jan;27(1):73-4.
Thomas TN, Reef S, Neff L, Sniadack MM, Mootrey GT. A review of the smallpox vaccine adverse events active surveillance system. Clin Infect Dis . 2008 Mar 15;46 Suppl 3:S212-20.
Wood RA, Berger M, Dreskin SC, Setse R, Engler RJM, Dekker CL, et al; Hypersensitivity Working Group of the Clinical Immunization Safety Assessment (CISA) Network. An algorithm for treatment of patients with hypersensitivity reactions after vaccines. Pediatrics . 2008 Sep;122(3):e771-7.
Beigel J, Kohl KS, Brinley F, Graham PL, Khuri-Bulos N, LaRussa PS, et al. Generalized vaccinia as an adverse event following exposure to vaccinia virus: Case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5745–5753.
Beigel J, Kohl KS, Khuri-Bulos N, Bravo L, Nell P, Marcy SM, et al. Rash including mucosal involvement: Case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5697–5706.
Belongia E, Izurieta H, Braun MM, Ball R, Haber P, Baggs J, et al. Postmarketing monitoring of intussusception after RotaTeq vaccination – United States, February 1, 2006-February 15, 2007 . MMWR. 2007 Mar 16;56(10):218-22.
Buettcher M, Baer G, Bonhoeffer J, Schaad UB, Heininger U. Three-year surveillance of intussusception in children in Switzerland. Pediatrics. 2007;120(3):473–480.
Buettcher M, Heininger U, Braun M, Bonhoeffer J, Halperin S, Heijbel H, et al. Hypotonic-hyporesponsive episode (HHE) as an adverse event following immunization in early childhood: Case definition and guidelines for data collection, analysis, and presentation. Vaccine. 2007;25(31):5875–5881.
DeStefano F, Weintraub ES, Chen RT. Hepatitis B vaccine and risk of multiple sclerosis [letter]. Pharmacoepidemiol Drug Saf. 2007 Jun;16(6):705-7, author reply 707-8.
Fiore AE, Shay DK, Haber P, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007 . MMWR Recomm Rep. 2007 Jul 13; 56(RR-6):1-54.
Graham PL, LaRussa PS, Kohl KS; Brighton Collaboration Vaccinia Virus Adverse Event Working Group for Robust Take. Robust take following exposure to vaccinia virus: Case definition and guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007 Aug 1;25(31):5763-70.
Haber MJ, Shay DK, Davis XM, Patel R, Jin X, Weintraub E, et al. Effectiveness of interventions to reduce contact rates during a simulated influenza pandemic. Emerg Infect Dis. 2007 Apr;13(4):581-9.
Haber P, Slade B, Iskander J. Guillain-Barré Syndrome (GBS) after vaccination reported to the United States Vaccine Adverse Event Reporting System (VAERS) in 2004 [Letter] . Vaccine. 2007;25(48):8101.
Halperin S, Kohl KS, Gidudu J, Ball L, Hammer SJ, Heath P, et al. Cellulitis at injection site: Case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5803–5820.
Hirichsen V, Kruskal B, O’Brien MA, Lieu TA, Platt R; Vaccine Safety Datalink Team. Using electronic medical records to enhance detection and reporting of vaccine adverse events. J Am Med Inform Assoc. 2007 Nov-Dec;14(6):731-5.
Jackson LA, Neuzil KM, Nahm MH, Whitney CG, Yu O, Nelson JC, et al. Immunogenicity of varying dosages of 7-valent pneumococcal polysaccharide-protein conjugate vaccine in seniors previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2007;25(20):4029–4037.
Jackson ML, Nelson JC, Chen RT, Davis RL, Jackson LA; Vaccine Safety Datalink team. Vaccines and changes in coagulation parameters in adults on chronic warfarin therapy: a cohort study. Pharmacoepidemiol Drug Saf. 2007 Jul;16(7):790-6.
Jones JF, Kohl KS, Ahmadipour N, Bleijenberg G, Buchwald D, Evengard B, et al. Fatigue: Case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5685–5696.
Jorch G, Tapiainen T, Bonhoeffer J, Fischer TK, Heininger U, Hoet B, et al. Unexplained sudden death, including sudden infant death syndrome (SIDS), in the first and second years of life: Case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5707–5716.
Klein NP, Fireman B, Enright A, Ray P, Black S, Dekker CL; Clinical Immunization Safety Assessment Network. A role for genetics in the immune response to the varicella vaccine. Pediatr Infect Dis J. 2007 Apr;26(4):300-5.
Kohl KS, Ball L, Gidudu J, Hammer SJ, Halperin S, Heath P, et al. Abscess at injection site: Case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5821–5838.
Kohl KS, Gidudu J, Bonhoeffer J, Braun MM, Buettcher M, Chen RT, et al. The development of standardized case definitions and guidelines for adverse events following immunization. Vaccine. 2007;25(31):5671–5674.
Kohl KS, Walop W, Gidudu J, Ball L, Halperin S, Hammer SJ, et al. Induration at or near injection site: Case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5839–5857.
Kohl KS, Walop W, Gidudu J, Ball L, Halperin S, Hammer SJ, et al. Swelling at or near injection site: Case definition and guidelines for collection, analysis and presentation of immunization safety data. Vaccine. 2007;25(31):5858–5874.
Lieu TA, Kulldorff M, Davis RL, Lewis EM, Weintraub E, Yih K, et al. Real-time vaccine safety surveillance for the early detection of adverse events. Med Care. 2007 Oct;45(10 Supl 2):S89-95.
McNeil MM, Ma GW, Aranas A, Payne DC, Rose CE Jr. A comparative assessment of immunization records in the Defense Medical Surveillance System and the Vaccine Adverse Event Reporting System . Vaccine . 2007 Apr 30;25(17):3428-36.
Miller ER, Iskander J, Pickering S, Varricchio F. How can you promote vaccine safety? Nursing. 2007;37(4):58–63.
Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007 Jun 28;25(27):5086-96.
Mullooly JP, Bridges CB, Thompson WW, Chen J, Weintraub E, Jackson LA, et al. Influenza- and RSV-associated hospitalizations among adults. Vaccine. 2007 Jan 15;25(5):846-55.
Mullooly JP, Schuler R, Barrett M, Maher JE. Vaccines, antibiotics, and atopy. Pharmacoepidemiol Drug Saf. 2007 Mar;16(3):275-88.
Nell P, Kohl KS, Graham PL, LaRussa PS, Marcy SM, Fulginiti VA, et al. Progressive vaccinia as an adverse event following exposure to vaccinia virus: Case definition and guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5735–5744.
Nell P, Kohl KS, Graham PL, LaRussa PS, Marcy SM, Fulginiti VA, et al. Eczema vaccinatum as an adverse event following exposure to vaccinia virus: case definition & guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5725–5734.
Payne DC, Aranas A, McNeil MM, Duderstadt S, and Rose Jr. CE. Concurrent vaccinations and U.S. military hospitalizations . Ann. Epidemiol. 2007 Sep;17(9):697-703.
Payne DC, Franzke LH, Stehr-Green PA, Schwartz B, and McNeil MM. Development of the Vaccine Analytic Unit’s research agenda for investigating potential adverse events associated with anthrax vaccine adsorbed . Pharmacoepidemiol Drug Saf. 2007 Jan;16(1):46-54.
Payne DC, Rose Jr. CE, Aranas A, Zhang Y, Tolentino H, Weston E, et al. Assessment of anthrax vaccination data in the Defense Medical Surveillance System, 1998-2004 . Pharmacoepidemiol Drug Saf. 2007 Jun;16(6):605-11.
Rüggeberg JU, Gold MS, Bayas JM, Blum MD, Bonhoeffer J, Friedlander S, et al. Anaphylaxis: Case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5675–5684.
Sejvar JJ, Kohl KS, Bilynsky R, Blumberg D, Cvetkovich T, Galama J, et al. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): Case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007 Aug 1;25(31):5771-92
Tapiainen T, Prevots R, Izurieta HS, Abramson J, Bilynsky R, Bo nhoeffer J, et al. Aseptic meningitis: Case definition and guidelines for collection, analysis and presentation of immunization safety data. Vaccine. 2007 Aug 1;25(31):5793-802.
Thompson WW, Price C, Goodson B, Shay DK, Benson P, Hinrichsen VL, et al. Early thimerosal exposure and neuropsychological outcomes at 7 to 10 years. N Engl J Med. 2007 Sep 27;357(13):1281-92.
Tolentino HD, Matters MD, Walop W, Law B, Tong W, Liu F, Fontelo P, Kohl K, and Payne DC. A UMLS-based spell checker for natural language processing in vaccine safety 2007; 7: 3.
Wenger P, Oleske JM, Kohl KS, Fisher MC, Brien JH, Graham PL, et al. Inadvertent inoculation as an adverse event following exposure to vaccinia virus: Case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5754–5762.
Wise RP, Bonhoeffer J, Beeler J, Donato H, Downie P, Matthews D, et al. Thrombocytopenia: Case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007 Aug 1;25(31):5717-24.
Woo EJ, Ball R, Landa R, Zimmerman AW, Braun MM; VAERS Working Group. Developmental regression and autism reported to the Vaccine Adverse Event Reporting System , Autism. . 2007; 11(4):301-10.
Wood R, Setse R, Halsey NA; Clinical Immunization Safety Assessment Network Hypersensitivity Working Group. Irritant skin test reactions to common vaccines. J Allergy Clin Immunol. 2007 Aug;120(2):478-81.
Yu O, Bohlke K, Hanson CA, Delaney K, Rees TG, Zavitkovsky A, et al. Hepatitis B vaccine and risk of autoimmune thyroid disease: A Vaccine Safety Datalink study . Pharmacoepidemiol Drug Saf. 2007 Jul;16(7):736-45.
Bines JE, Liem NT, Justice F, Son TN, Carlin JB, de Campo M, et al. Validation of a clinical case definition for acute intussusception in infants in Vietnam and Australia. Bull World Health Organ. 2006 Jul;84(7):569-75.
Broder KR, Cortese MM, Iskander JK, Kretsinger K, Slade BA, Brown KH, et al. Preventing tetanus, diphtheria, and pertussis among adolescents: Use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP) . MMWR Recomm Rep. 2006 Mar 24; 55(RR-3):1-34.
CDC. Update: Guillain-Barré syndrome among recipients of Menactra meningococcal conjugate vaccine—United States, October 2005–February 2006. MMWR. 2006;55(13):364–366.
DiMiceli L, Pool V, Kelso JM, Shadomy SV, Iskander J; VAERS Team. Vaccination of yeast sensitive individuals: Review of safety data in the US vaccine adverse event reporting system (VAERS). Vaccine. 2006;24(6):703–707.
Fowler GL, Baggs JM, Weintraub ES, Martin SW, McNeil MM, Gust DA. Factors influencing laboratory workers’ decisions to accept or decline anthrax vaccine adsorbed (AVA): Results of a Ddcision-making study in CDC’s anthrax vaccination program . Pharmacoepidemiol Drug Saf. 2006 Dec;15(12):880-8.
France EK, Smith-Ray R, McClure D, Hambidge S, Xu S, Yamasaki K, et al. Impact of maternal influenza vaccination during pregnancy on the incidence of acute respiratory illness visits among infants. Arch Pediatr Adolesc Med. 2006 Dec;160(12):1277-83.
Glanz JM, McClure DL, Xu S, Hambidge SJ, Lee M, Kolczak MS, et al. Four different study designs to evaluate vaccine safety were equally validated with contrasting limitations. J Clin Epidemiol. 2006 Aug;59(8):808-18.
Hambidge SJ, Glanz JM, France EK, McClure D, Xu S, Yamasaki K, et al. Safety of trivalent inactivated influenza vaccine in children 6 to 23 months old. JAMA. 2006 Oct 25;296(16):1990-7.
Iskander J, Pool V, Zhou W, English-Bullard R; VAERS Team. Data mining in the US using the Vaccine Adverse Event Reporting System. Drug Saf. 2006;29(5):375-84.
Jackson LA, Nelson JC, Whitney CG, Neuzil KM, Benson P, Malais D, et al. Assessment of the safety of a third dose of pneumococcal polysaccharide vaccine in the Vaccine Safety Datalink population. Vaccine. 2006;24(2):151–156.
Jackson LA, Neuzil KM, Baggs J, Davis RL, Black S, Yamasaki KM, et al. Compliance with the recommendations for 2 doses of trivalent inactivated influenza vaccine in children less than 9 years of age receiving influenza vaccine for the first time: A Vaccine Safety Datalink study. Pediatrics. 2006;118(5):2032–2037.
Lin ND, Kleinman K, Chan KA, Yu XJ, France EK, Wei F, et al. Variation in hepatitis B immunization coverage rates associated with provider practices after the temporary suspension of the birth dose. BMC Pediatr. 2006 Nov 13;6:31.
Miller ER, Woo EJ. Time to prevent injuries from postimmunization syncope . Nursing. 2006; 36(12 Pt.1):20.
Muralles AA, Ray P, Black S, Shinefield H, Casey CG, Campbell S, et al. Active telephone surveillance to evaluate adverse events among civilian smallpox vaccine recipients. Vaccine. 2006;24(4):476–484.
Payne DC, Rose CE Jr, Kerrison J, Aranas A, Duderstadt S, McNeil MM. Anthrax vaccination and risk of optic neuritis in the United States military, 1998-2003 . Arch. Neurology 2006;63:871-875.
Prosser LA, Bridges CB, Uyeki TM, Hinrichsen VL, Meltzer MI, Noelle-Angelique MM, et al. Health benefits, risks, and cost-effectiveness of influenza vaccination of children. Emerg Infect Dis. 2006 Oct;12(10):1548-58.
Ray P, Hayward J, Michelson D, Lewis E, Schwalbe J, Black S, et al. Encephalopathy after whole-cell pertussis or measles vaccination: Lack of evidence for a causal association in a retrospective case-control study. Pediatr Infect Dis J. 2006 Sep;25(9):768-73.
Sekaran N, Edwards, K. Extensive swelling reaction associated with diphtheria and tetanus toxoids and acellular pertussis vaccine . Pediatr Infect Dis J . 2006 Apr; 25(4):374-5.
Tapiainen T, Bär G, Bonhoeffer J, Heininger U. Evaluation of The Brighton Collaboration case definition of acute intussusception during active surveillance. Vaccine. 2006 Feb 27;24(9):1483-7.
Varricchio F, Reed J; VAERS Working Group. Follow-up study of medication errors reported to the Vaccine Adverse Event Reporting System (VAERS). South Med J. 2006 May;99(5):486-9.
Vellozzi C, Mitchell T, Miller E, Casey CG, Eidex RB, Hayes EB. Yellow fever vaccine-associated viscerotropic disease (YEL-AVD) and corticosteroid therapy: Eleven United States cases, 1996-2004 . Am J Trop Med Hyg . 2006 Aug;75(2):333-6.
Vermeer-de Bondt PE, Dzaferagić A, David S, van der Maas NA. Performance of The Brighton Collaboration case definition for hypotonic–hyporesponsive episode (HHE) on reported collapse reactions following infant vaccinations in the Netherlands. Vaccine. 2006 Nov 30;24(49-50):7066-70.
Woo EJ, Ball R, Braun M, Clark T, Rosenstein Messonnier N, Wharton M, et al. Update: Guillain-Barré syndrome among recipients of Menactra meningococcal conjugate vaccine—United States, June 2005–September 2006 . MMWR. 2006 Oct 20;55(41):1120-4.
Woo EJ, Ball R, Landa R, Zimmerman AW, Braun MM; VAERS Working Group. Developmental regression and autism reported to the Vaccine Adverse Event Reporting System. Autism. 2007 Jul;11(4):301-10.
Woo EJ, Miller NB, Ball R; VAERS Working Group. Adverse events after hepatitis A B combination vaccine. Vaccine. 2006;24(14):2685–2691.
Baggs J, Chen R, Damon I, Rotz L, Allen C, Fullerton K, et al. Safety profile of smallpox vaccine: Insights from the pre-event smallpox vaccination program . Clin Infect Dis. 2005;40(8):1133-40.
Belongia EA, Naleway A, Kieke B, Qutaishat S, Casey C, Shay DK, et al. Validation of a screening instrument to identify persons for exclusion from smallpox vaccination . Clin Infect Dis. 2005 Feb 15;40(4):620-3.
Bloom S, Rguig A, Berraho A, Zniber L, Bouazzaoui N, Zaghloul Z, et al. Congenital rubella syndrome burden in Morocco: a rapid retrospective assessment . Lancet. 2005 Jan 8;365(9454):135-41.
Casey CG, Iskander JK, Roper MH, Mast EE, Wen XJ, Tӧrӧk TJ, et al. Adverse events associated with smallpox vaccination in the United States, January-October 2003 . JAMA. 2005 Dec 7;294(21):2734-43.
CDC. Guillain-Barré syndrome among recipients of Menactra® meningococcal conjugate vaccine—United States, June–July 2005 . MMWR Dispatch. 2005;54:1-3.
Davis RL, Kolczak M, Lewis E, Nordin J, Goodman M, Shay DK, et al. Active surveillance of vaccine safety: a system to detect early signs of adverse events . Epidemiology. 2005 May;16(3):336-41.
DeStefano F, Weintraub ES, Chen RT. Recombinant hepatitis B vaccine and the risk of multiple sclerosis: a prospective study [letter] . Neurology. 2005 Apr 12;64(7):1317; author reply 1318.
Gust D, Brown C, Sheedy K, Hibbs B, Weaver D, Nowak G. Immunization attitudes and beliefs among parents: Beyond a dichotomous perspective . Am J Health Behav. 2005 Jan-Feb;29(1):81-92.
Gust DA, Kennedy A, Shui I, Smith PJ, Nowak G, Pickering LK. Parent Attitudes Toward Immunizations and Healthcare Providers: The Role of Information . Am J Preventive Health. 2005 Aug;29(2):105-12.
Iskander J, Haber P, Herrera G. Monitoring vaccine safety during an influenza pandemic . Yale J Biol Med. 2005; 78(5):265-75.
Izurieta HS, Haber P, Wise RP, Iskander J, Pratt D, Mink C, et al. Adverse events reported following live, cold-adapted, intranasal influenza vaccine . JAMA . 2005 Dec 7;294(21):2720-5.
Jackson LA, Neuzil KM, Whitney CG, Starkovich P, Dunstan M, Yu O, et al. Safety of varying dosages of 7-valent pneumococcal protein conjugate vaccine in seniors previously vaccinated with 23-valent pneumococcal polysaccharide vaccine . Vaccine. 2005;23(28):3697-703.
Kennedy A, Brown C, Gust D. Vaccine beliefs of parents who oppose compulsory vaccination . Public Health Rep. 2005 May-Jun; 120(3): 252–258.
Kennedy A, Gust D. Parental vaccine beliefs and child’s school type . J Sch Health. 2005 Sep;75(7):276-80.
Khromava AY, Eidex RB, Weld LH, Kohl KS, Bradshaw RD, Chen RT, et al. Yellow fever vaccine: an updated assessment of advanced age as a risk factor for serious adverse events . Vaccine. 2005 May 9;23(25):3256-63.
Kohl KS, Bonhoeffer J, Braun MM, Chen RT, Duclos P, Heijbel H, et al. The Brighton Collaboration: Creating a global standard for case definitions (and guidelines) for adverse events following immunization . In Advances in Patient Safety: From Research to Implementation. Henriksen K, Battles JB, Marks ES, et al., eds. Rockville (MD): Agency for Healthcare Research and Quality (US); 2005 Feb.
Martin SW, Tierney BC, Aranas A, Rosenstein NE, Franzke LH, Apicella L, et al. An overview of adverse events reported by participants in CDC’s anthrax vaccine and antimicrobial availability program. . Pharmacoepidemiol Drug Saf. 2005 Jun;14(6):393-401.
McMahon AW, Iskander J, Haber P, Chang S, Woo EJ, Braun MM, etc. Adverse events after inactivated influenza vaccination among children less than 2 years of age: analysis of reports from the vaccine adverse event reporting system, 1990-2003 . Pediatrics. 2005;115(2):453-60.
Lewis N, Black S, Weintraub E, Baggs J, Thompson W, DeStefano F, et al. Rapid assessment of influenza vaccination coverage among HMO members—Northern California influenza seasons, 2001–02 through 2004–05 . MMWR. 2005;54(27):676-678.
Mandell DS, Thompson WW, Weintraub ES, DeStefano F, Blank MB. Trends in diagnosis rates for autism and ADHD at hospital discharge in the context of other psychiatric diagnoses . Psychiatr Serv. 2005 Jan;56(1):56-62.
McMahon AW, Iskander J, Haber P, Chang S, Woo EJ, Braun MM, et al. Adverse events after inactivated influenza vaccination among children less than 2 years of age: analysis of reports from the vaccine adverse event reporting system, 1990-2003 . Pediatrics. 2005;115(2):453-60.
Papania MJ, Strebel PM. Measles surveillance: the importance of finding the tip of the iceberg [commentary] . Lancet. 2005 Jan 8;365(9454):100-1.
Rollison D, Helzlsouer K, Halsey N, Shah K, and Viscidi R. Markers of past infection with simian virus 40 (SV40) and risk of incident non-Hodgkin lymphoma in a Maryland cohort . Cancer Epidemiol Biomarkers Prev . 2005 June; 14(6) 1448-1452.
Sejvar J, Boneva R, Lane JM, Iskander J. Severe headaches following smallpox vaccination . Headache. 2005 Jan;45(1):87-8.
Sejvar JJ, Labutta RJ, Chapman LE, Grabenstein JD, Iskander J, Lane JM. Neurologic adverse events associated with smallpox vaccination in the United States, 2002-2004 . JAMA. 2005 Dec 7;294(21):2744-50.
Shui I, Kennedy A, Wooten K, Schwartz B, Gust D. Factors influencing African American mothers’ concerns about immunization safety: A summary of focus group findings in Atlanta . J Natl Med Assoc. 2005 May;97(5):657-66.
Vellozzi CJ, Lane MJ, Averhoff F, Mauer T, Norton S, Damon I, Casey C. Generalized vaccinia, progressive vaccinia andeczema vaccinatum are rare following smallpox vaccination: United States Surveillance, 2003 . Clin Infect Dis. 2005 Sep 1;41(5):689-97. Epub 2005 Jul 26.
Verstraeten T, Davis R, Destefano F. Immunity to tetanus is protective against the development of multiple sclerosis . Med Hypotheses . 2005;65(5):966-9.
Yeung LF, Lurie P, Dayan G, Eduardo E, Britz PH, Redd SB, et al. A limited measles outbreak in a highly vaccinated US boarding school . Pediatrics. 2005 Dec;116(6):1287-91.
Begier EM, Burwen DR, Haber P, Ball R; Vaccine Adverse Event Reporting System Working Group. Postmarketing safety surveillance for typhoid fever vaccines from the Vaccine Adverse Event Reporting System, July 1990 through June 2002 . Clin Infect Dis . 2004;38(6):771-9.
Begier EM, Langford CA, Sneller MC, Wise RP, Ball R; VAERS Working Group. Polyarteritis nodosa reports to the Vaccine Adverse Event Reporting System (VAERS): Implications for assessment of suspected vaccine-provoked vasculitis . J Rheumatol. 2004;31(11):2181-8.
Bines JE, Kohl KS, Forster J, Zanardi LR, Davis RL, Hansen J, et al. Acute intussusception in infants and children as an adverse event following immunization: Case definition and guidelines of data collection, analysis, and presentation . Vaccine. 2004 Jan 26;22(5-6):569-74.
Bonhoeffer J, Kohl KS, Chen RT, Duclos P, Heijbel H, Heininger U, et al. Standardized case definitions of adverse events following immunization (AEFI) . Vaccine. 2004 Jan 26;22(5-6):547-50.
Bonhoeffer J, Vermeer P, Halperin S, Kempe A, Music S, Shindman J, et al. Persistent crying in infants and children as an adverse event following immunization: Case definition and guidelines for data collection, analysis, and presentation . Vaccine . 2004 Jan 26;22(5-6):586-91.
Bonhoeffer J, Gold MS, Heijbel H, Vermeer P, Blumberg D, Braun M, et al. Hypotonic-Hyporesponsive Episode (HHE) as an adverse event following immunization: Case definition and guidelines for data collection, analysis, and presentation . Vaccine. 2004 Jan 26;22(5-6):563-8.
Bonhoeffer J, Menkes J, Gold MS, de Souza-Brito G, Fisher MC, Halsey N, et al. Generalized convulsive seizure as an adverse event following immunization: Case definition and guidelines for data collection, analysis, and presentation . Vaccine . 2004 Jan 26;22(5-6):557-62.
Chen R. Evaluation of vaccine safety after the events of 11 September 2001: Role of cohort and case-control studies . Vaccine . 2004 May 7;22(15-16):2047-53.
Freed GL, Clark SJ, Hibbs BH, Santoli JM. Parental vaccine safety concerns. The experience of pediatricians and family physicians . Am J Prev Med . 2004 Jan;26(1):11-4.
Haber P, Zanardi LR, Chen RT, Mootrey GT, Pless R, English R, et al. An analysis of rotavirus vaccine reports to the Vaccine Adverse Event Reporting System: More than intussusception alone? Pediatrics. 2004 Apr;113(4):e353-9.
Haber P, DeStefano F, Angulo FJ, Iskander J, Shadomy SV, Weintraub E, et al. Guillain-Barré syndrome following influenza vaccination . JAMA . 2004 Nov 24;292(20):2478-81
Iskander JK, Miller ER, Chen RT. The role of the Vaccine Adverse Event Reporting system (VAERS) in monitoring vaccine safety . Pediatr Ann. 2004;33(9):599-606.
Kohl KS, Marcy SM, Blum M, Connell Jones M, Dagan R, et al. Fever After Immunization: Current concepts and improved future scientific understanding . Clin Infect Dis . 2004 Aug 1;39(3):389-94.
Marcy SM, Kohl KS, Dagan R, Nalin D, Blum M, Connell Jones M, et al. Fever as an adverse event following immunization: Case definition and guidelines of data collection, analysis, and presentation . Vaccine . 2004 Jan 26;22(5-6):551-6.
Muetsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, et al. Inactivated intranasal influenza vaccine and the risk of Bell’s Palsy in Switzerland . N Engl J Med . 2004 Feb 26;350(9):896-903.
O’Brien MA, Uyeki TM, Shay DK, Thompson WW, Kleinman K, McAdam A, et al. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children . Pediatrics. 2004 Mar;113(3 Pt 1):585-93.
Rothstein E, Kohl KS, Ball L, Halperin SA, Halsey N, Hammer SJ, et al. Nodule at injection site as an adverse event following immunization: Case definition and guidelines for data collection, analysis, and presentation . Vaccine. 2004 Jan 26;22(5-6):575-85.
Steinberg EB, Bishop R, Haber P, Dempsey AF, Hoekstra RM, Nelson JM, et al. Typhoid fever in travelers: Who should be targeted for prevention? Clin Infect Dis . 2004 Jul 15;39(2):186-91. Epub 2004 Jul 1.
Varricchio F, Iskander J, DeStefano F, Ball R, Pless R, Braun MM, et al. Understanding vaccine safety: Information from the Vaccine Adverse Event Reporting System . Pediatr Infect Dis J . 2004 Apr;23(4):287-94.
Wise RP, Iskander J, Pratt RD, Campbell S, Ball R, Pless RP, et al. Postlicensure safety surveillance for 7-valent pneumococcal conjugate vaccine . JAMA. 2004;292(14):1702-10.
Zhou WG, Pool V, Destefano F, Iskander JK, Haber P, Chen RT, and The VAERS Working Group. A potential signal of Bell’s palsy after parenteral inactivated influenza vaccines: Reports to the Vaccine Adverse Event Reporting System (VAERS) – United States, 1991-2001 . Pharmacoepidemiol Drug Saf . 2004 Aug;13(8):505-10.
Bohlke K, Davis RL, Marcy SM, Braun MM, DeStefano F, et al. The risk of anaphylaxis following vaccination of children and adolescents . Pediatrics . 2003;112:815-20.
CDC. Update: Multistate outbreak of monkeypox- Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003 . MMWR. 2003;52:642-646.
Chen RT, Lane JM. Myocarditis: The unexpected return of smallpox vaccine adverse events . Lancet . 2003 Oct 25;362(9393):1345-6.
Cono J, Casey CG, Bell DM. Smallpox vaccination and adverse reactions: Guidance for clinicians . MMWR. 2003;52(No. RR-4):1-28.
DeStefano F, VerstraetenT, Jackson LA, Okoro CA, Benson P, Black SB, et al. Vaccinations and risk of central nervous system demyelinating diseases in adults . Arch Neurol . 2003;60:504-9.
DeStefano F, Verstraeten T, Chen RT. Hepatitis B vaccine and risk of multiple sclerosis . Expert Rev of Vaccines . 2003;2(1):21-29.
Gust DA, Woodruff R, Kennedy A, Brown C, Sheedy K, Hibbs B. Parental perceptions surrounding risks and benefits of immunizations . Semin Pediatr Infect Dis . 2003 Jul;14(3):207-12.
Haber P, DeStefano F. Influenza vaccination and Guillain Barré syndrome . Clin Immunol . 2003; 109(3):359; author reply 360-1.
Hutwagner L, Thompson WW, Seeman GM, Treadwell T. The bioterrorism preparedness and response Early Aberration Reporting System (EARS) . J Urban Health . 2003 Jun;80(2 Suppl 1):i89-96.
Jackson LA, Neuzil KM, Onchee Y, Benson P, Barlow WE, Adams AL, et al. Effectiveness of pneumococcalpolysaccharide vaccine in older adults . N Engl J Med . 2003 May 1;348(18):1747-55.
Jacobson SH, Sewell EC, Allwine DA, Medina EA, Weniger BG. Designing pediatric vaccine formularies and pricing pediatric combination vaccines using operations research models and algorithms . Expert Rev Vaccines . 2003 Feb;2(1):15-9.
Kelly WN. The quality of published adverse drug event reports . Ann Pharmacother . 2003:37(12):1774-1778.
Kohl K, Bonhoeffer J, Chen R, Duclos P, Heijbel H, Heininger U, et al. The Brighton Collaboration: Enhancing comparability of vaccine safety data . Pharmacoepidemiol Drug Saf . 2003 Jun;12(4):335-40.
Lloyd JC, Haber P, Mootrey GT, Braun MM, Chen RT, and VAERS Working Group. Adverse event reporting rates following tetanus-diphtheria and tetanus toxoid vaccinations: Data from the Vaccine Adverse Event Reporting System (VAERS), 1991-1997 . Vaccine . 2003 Sep 8;21(25-26):3746-50.
Mell L, Davis RL, Mullooly JP, Black SB, Shinefield HR, Zangwill KM, et al. Polio extra-immunization in children less than two years old following changes in immunization recommendation [PDF – 650 KB] . Pediatrics. 2003;111:296–301.
Naleway AL, Belongia EA, Greenlee, RT, Kieke BA, Chen RT, Shay DK. Eczematous skin disease and recall of past diagnoses: Implications for smallpox vaccination . Ann Int Med . 2003 Jul 1;139(1):1-7.
Tierney BC, Martin SW, Franzke LH, Marano N, Reissman DB, Louchart RD, et al. Serious adverse events among participants in the Centers for Disease Control and Prevention’s Anthrax Vaccine and Antimicrobial Availability Program for persons at risk for bioterrorism-related inhalational anthrax . Clin Infect Dis. 2003;37:905-911.
Thompson WW, Shay D, Weintraub E, Brammer L, Cox N, Anderson L, et al. Mortality associated with influenza and respiratory syncytial virus in the United States . JAMA. 2003;289:179-186.
Verstraeten T, Davis RL, DeStefano F, Lieu TA, Rhodes PH, Black SB, et al. Safety of thimerosal-containing vaccines: A two-phased study of computerized health maintenance organization databases . Pediatrics 2003;112:1039-48.
Verstraeten T, DeStefano F, Chen RT, Miller E. Vaccine safety surveillance using large linked databases: Opportunities, hazards and proposed guidelines . Expert Rev Vaccines . 2003 Feb;2(1):21-9.
Verstraeten T, Jumaan AO, Mullooly JP, Seward JF, Izurieta HS, DeStefano F, et al. A retrospective cohort study of the association of varicella vaccine failure with asthma, steroid use, age at vaccination, and measles-mumps-rubella vaccination . Pediatrics . 2003 Aug;112(2):e98-103.
Wharton M, Strikas RA, Harpaz R, Rotz LD, Schwartz B, Casey CG, et al. Recommendations for using smallpox vaccine in a pre-event vaccination program: Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC) . MMWR. 2003;52(No. RR-7):1-16.
Yih WK, Lieu TA, Rêgo VH, O’Brien MA, Shay DK, Yokoe DS, et al. Attitudes of healthcare workers in U.S. hospitals regarding smallpox vaccination . BMC Public Health. 2003, 3:20
Zhou W, Pool V, Iskander JK, English-Bullard R, Ball R, Wise RP. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS)— United States, 1991–2001 . MMWR . 2003;52:(SS-1):1-24.
Atkinson WL, Pickering LK, Schwartz B, Weniger BG, Iskander JK. General recommendations on immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP) [sections on jet injection and occupational safety regulations]. MMWR. 2002;51(RR-2):12-13,15.
Black S, Lewis E, Shinefield H, Fireman B, Ray P, et al. Lack of association between receipt of conjugate Haemophilus influenzae type b vaccine (HBOC) in infancy and risk of type 1 (juvenile onset) diabetes: Long term follow up of the HBOC efficacy trial cohort . Pediatr Infect Dis J . 2002 Jun;21(6):568-9.
Bonhoeffer J, Kohl K, Chen R, Duclos P, Heijbel H, et al. The Brighton Collaboration: Addressing the need for standardized case definitions of adverse events following immunization (AEFI) . Vaccine. 2002 Dec 13;21(3-4):298-302.
CDC. Adverse events associated with 17D-derived yellow fever vaccination—United States, 2001–2002 . MMWR 2002;51:989-993.
Cetron MS, Marfin AA, Julian KG, Gubler DJ, Sharp DJ, et al. Yellow fever vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) . MMWR. 2002;51(RR-17):1-10.
Chang EJ, Zangwill KM, Lee H, Ward JI. Lack of association between rotavirus infection and intussusception: Implications for use of attenuated rotavirus vaccines . Pediatr Infect Dis J. 2002 Feb;21(2):97-102.
DeStefano F, Gu D, Kramarz P, Truman BI, Iademarco BF, et al. Childhood vaccinations and risk of asthma . Pediatr Infect Dis J. 2002 Jun;21(6):498-504.
Ekwueme DU, Weniger BG, Chen RT. Model-based estimates of the risks of disease transmission and economic costs of seven injection devices in sub-Saharan Africa . Bull World Health Organ. 2002; 80(11): 859–870.
Gonzalez IM, Averhoff FM, Massoudi MS, et al. Hepatitis b vaccination among adolescents in 3 large health maintenance organizations . Pediatrics. 2002 Nov;110(5):929-34.
Halsey NA. The science of evaluation of adverse effects associated with vaccination . Semin Pediatr Infect Dis. 2002 Jul;13(3):205-14.
Halsey NA. Anthrax vaccine and causality assessment from individual case reports . Pharmacoepidemiol Drug Saf. 2002 Apr-May;11(3):185-7; discussion 203-4.
Jackson LA, Yu O, Heckbert SR, Psaty BM, Malais D, Barlow WE, Thompson WW, and the Vaccine Safety Datalink Study Group. Influenza vaccination is not associated with a reduction in the risk of recurrent coronary events . Am J Epidemiol. 2002;156 (7): 634-640.
Kohl K, Bonhoeffer J, Chen R, Duclos P, Heijbel H, et al. The Brighton Collaboration: Enhancing comparability of vaccine safety data . Pharmacoepidemiol Drug Saf. 2003 Jun;12(4):335-40.
Lathrop S, Ball R, Haber P, Mootrey G, Braun M, et al. Adverse event reports following vaccination for Lyme disease: December 1998-July 2000 . Vaccine. 2002 Feb 22;20(11-12):1603-8.
Mullooly, J, Pearson J, Drew L, Schuler R, Maher J, et al. Wheezing lower respiratory disease and vaccination of full-term infants . Pharmacoepidemiol Drug Saf. 2002 Jan-Feb;11(1):21-30.
Pool V, Braun MM, Kelso JM, Mootrey G, Chen RT, et al. Prevalence of anti-gelatin IgE antibodies in persons with anaphylaxis following measles-mumps-rubella vaccine in the U.S . Pediatrics. 2002 Dec;110(6):e71.
Pless R. Hibbs B. Chiropractic students attitudes about vaccination: A cause for concern? CMAJ. 2002 Jun;166(12):1544-5.
Setia S, Mainzer H, Washington ML, Coil G, Snyder R, et al. Frequency and causes of vaccine wastage . Vaccine. 2002 Jan 15;20(7-8):1148-56.
Barlow WE, Davis RL, Glasser JW, Rhodes PH, Thompson RS, et al. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine . N Engl J Med. 2001;345:656-61.
Chen RT, Pless R, Destefano F. Epidemiology of autoimmune reactions induced by vaccination . J Autoimmun 2001;16:309-18.
Chen RT, Pool V, Takahashi H, Weniger BG, Patel B. Combination vaccines: Postlicensure safety evaluation . Clin Infect Dis 2001;33 (Suppl 4):s327-s333.
Chen RT, DeStefano F, Pless R, Mootrey G, Kramarz P, Hibbs B. Challenges and Controversies in Immunization Safety. In: Peter G, Gardner P (eds). Vaccine recommendations: challenges and controversies. Infect Dis Clinics of North America 2001;15:21-39.
Davis RL, Kramarz P, Bohlke K, Benson P, Thompson RS, et al. Measles-mumps-rubella and other measles-containing vaccines do not increase the risk for inflammatory bowel disease: A case-control study from the Vaccine Safety Datalink project . Arch Pediatr Adolesc Med. 2001 Mar;155(3):354-359.
Davis RL, Lieu TA, Mell LK et al. Impact of the change in polio vaccination schedule on immunization coverage rates: A study in two large health maintenance organizations . Pediatrics 2001;107:671-676.
Destefano F, Davis RL, Chen RT. Autism and MMR vaccination: Controversy laid to rest? CNS Drugs. 2001;15(11):831-7.
DeStefano F, Mullooly JP, Okoro CA, Chen RT, Marcy SM, et al. Childhood vaccinations, vaccination timing, and risk of type 1 diabetes mellitus . Pediatrics. 2001;108:e112-3.
Jackson LA, Austin G, Chen RT, Stout R, DeStefano F, et al. Safety and immunogenicity of varying doses of trivalent inactivated influenza vaccine administered by needle-free jet injectors . Vaccine. 2001;19:4703-4709.
Kramarz P, France EK, Black SB, Shinefield H, Ward JI, et al. Population based study of rotavirus vaccination and intussusception . Ped Infect Dis J. 2001 Apr;20(4):410-6.
Kramarz P, DeStefano F, Gargiullo P, Chen RT, Lieu TA, et al. Does influenza vaccination prevent asthma exacerbations in children? Journal of Pediatrics. 2001;138(3):306-10.
Lewis E, Shinefield HR, Woodruff BA, Black SB, DeStefano F, et al. Safety of neonatal hepatitis B vaccine administration . Pediatr Infect Dis J. 2001; 20: 1049-1054.
Lieu TA, Davis RL, Capra AM, et al. Variation in clinician recommendations for multiple injections during adoptions of inactivated polio vaccine . Pediatrics. 2001; 107:e49.
Meyerhoff AS, Weniger BG, Jacobs RJ. The economic value to parents of reducing the pain and emotional distress of childhood vaccine injections . Pediatr Infect Dis J. 2001;20(11):s57-s62.
Rota JS, Salmon DA, Rodewald LE, Chen RT, Hibbs BF, Gangarosa EJ. Processes for obtaining nonmedical exemptions to State immunization laws . AJPH. 2001;91:645-48.
Sewell EC, Jacobson SH, Weniger BG. “Reverse engineering” a formulary selection algorithm to determine the economic value of pentavalent and hexavalent combination vaccines . Pediatr Infect Dis J. 2001;20(11):s45-s56.
Verstraeten T, Baughman AL, Cadwell B, Zanardi L, Haber P, Chen R and VAERS Team. Enhancing vaccine safety surveillance: A capture-recapture analysis of intussusception after rotavirus vaccination . Am J Epidemiol 2001; 154:1006–12.
Wattigney WA, Mootrey GT, Braun MM, Chen RT. Surveillance for poliovirus vaccine adverse events, 1991-1998: Impact of a sequential vaccination schedule of inactivated poliovirus vaccine followed by oral poliovirus vaccine . Pediatrics. 2001; 107(5):E83.
Zanardi LR, Haber P, Mootrey GT, Niu MT, Wharton M. Intussusception among recipients of rotavirus vaccine: Reports to the vaccine adverse event reporting system . Pediatrics. 2001;107(6):E97.
Belay ED, Holman RC, Clarke MJ, DeStefano F, Shahriari A, et al. The incidence of Kawasaki syndrome in West Coast health maintenance organizations . Ped Infect Dis J . 2000;19:828-32.
Braun MM, Mootrey GT, Salive ME, Chen RT, Ellenberg SS, VAERS Working Group. Infant immunization with acellular pertussis vaccines in the United States: Assessment of the first two years’ data from the Vaccine Adverse Event Reporting System (VAERS) . Pediatrics. 2000;104(4):e51.
Chen RT, DeStefano F, Davis RL, Jackson LA, Thompson RS, et al. The Vaccine Safety Datalink: Immunization research in health maintenance organizations in the USA. Bull WHO. 2000;78:186-194.
Chen RT, Hardy IRB, Rhodes PH, Tyshchenko DK, Moiseeva AV, Marievsky VF. Ukraine, 1992: First assessment of diphtheria vaccine effectiveness during the recent resurgence of diphtheria in the former Soviet Union: Introduction . J Infect Dis 2000;181(suppl 1):S178-S183.
Chen RT, Mootrey GT, DeStefano F. Safety of routine childhood vaccinations. An epidemiological review . Pediatric Drugs. 2000;2(4):273-290.
Collet JP. MacDonald N. Cashman N. Pless R. Monitoring signals for vaccine safety: The assessment of individual adverse event reports by an expert advisory committee. Advisory Committee on Causality Assessment . Bull WHO. 2000;78(2):178-85.
DeStefano F. Opportunities for population-based clinical research using data from managed care organizations . In: Davis JR, editor. Managed care systems and emerging infections. Washington, DC: National Academy Press, 2000;16-8.
Feikin DR, Lezotte DC, Hamman RF, Salmon DA, Chen RT, Hoffman RE. Individual and community risks of measles and pertussis associated with personal exemptions to immunization . JAMA . 2000;284:3145-50.
Izurieta HS, Thompson WW, Kramarz P, Shay DK, Davis RL, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children . N Engl J Med. 2000;342(4):232-239.
Izurieta HS, Thompson WW, Shay DK. Influenza and hospitalizations in children [Letter]. N Engl J Med. 2000;342:1753.
Kramarz P, DeStefano F, Gargiullo PM, Davis RL, Chen RT, et al. Influenza vaccination in children with asthma in health maintenance organizations . Vaccine. 2000;18:2288-2294.
Kramarz P, DeStefano F, Gargiullo PM, Davis RL, Chen RT, et al. Does influenza vaccination exacerbate asthma? Analysis of a large cohort of children with asthma . Arch Fam Med. 2000;9(7):617-623.
Kuppermann M, Nease RF, Ackerson LM, Black SB, Shinefield HR, Lieu TA. Parent preferences for outcomes associated with childhood vaccinations . Pediatr Infect Dis. 2000;19:129-133.
Lieu TA, Black SB, Ray GT, Martin KE, Shinefield HR, Weniger BG. The hidden costs of infant vaccination . Vaccine. 2000;19:33-41.
Pless R. Vaccination benefits, risks and safety: The need for a complete picture . Bull WHO. 2000;78(2):219-221.
Pless R, Risher JF. Mercury, infant neurodevelopment, and vaccination . J Pediatr. 2000;136(5):571-573.
Salmon, DA., Gangarosa EJ, Chen RT. Health consequences of exemptions from immunization laws [letter] . JAMA. 2000; 283: 1141-2.
Takahashi H. Need for improved vaccine safety surveillance . Vaccine. 2000;19(9-10):1004.
Takahashi H, Pool V, Tsai TF, Chen RT. Adverse events after Japanese Encephalitis vaccination: Review of post-marketing surveillance data from Japan and the United States . Vaccine. 2000: 18(26), 2963-2969.
Wise RP, Salive ME, Braun MM, Mootrey GT, Seward JF, Rider LG, Krause PR. Post licensure safety surveillance for varicella vaccine . JAMA. 2000;284:1271-1279.
To receive email updates about this page, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 30, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Novel vaccine concept generates immune responses that could produce multiple types of HIV neutralizing antibodies
by NIH/National Institute of Allergy and Infectious Diseases
Using a combination of cutting-edge immunologic technologies, researchers have successfully stimulated animals' immune systems to induce rare precursor B cells of a class of HIV broadly neutralizing antibodies (bNAbs). The findings, published today in Nature Immunology , are an encouraging, incremental step in developing a preventive HIV vaccine.
HIV is genetically diverse, making the virus difficult to target with a vaccine, but bNAbs may overcome that hurdle because they bind to parts of the virus that remain constant even when it mutates. Germline targeting is an immune system-stimulating approach that guides naïve (precursor) B cells to develop into mature B cells that can produce bNAbs.
A class of bNAbs called 10E8 is a priority for HIV vaccine development because it neutralizes a particularly broad range of HIV variants. The 10E8 bNAb binds to a conserved region of the glycoprotein gp41 on HIV's surface involved in its entry into human immune cells .
Designing an immunogen—a molecule used in a vaccine that elicits a specific immune system response—to stimulate production of 10E8 bNAbs has been challenging because that key region of gp41 is hidden in a recessed crevice on HIV's surface. Prior vaccine immunogens have not generated bNAbs with the physical structure to reach and bind to gp41.
To address this challenge, the researchers engineered immunogens on nanoparticles that mimic the appearance of a specific part of gp41. They vaccinated rhesus macaque monkeys and mice with those immunogens and elicited specific responses from the 10E8 B cell precursors and induced antibodies that showed signs of maturing into bNAbs that could reach the hidden gp41 region. They observed similar responses when they used mRNA-encoded nanoparticles in mice.
The researchers also found that the same immunogens produced B cells that could mature to produce an additional type of gp41-directed bNAb called LN01. Finally, their laboratory analysis of human blood samples found that 10E8-class bNAb precursors occurred naturally in people without HIV, and that their immunogens bound to and isolated naïve human B cells with 10E8-like features.
Together, these observations suggest that the promising immunization data from mice and macaques has the potential for translation to humans.
According to the authors, these findings support the development of the immunogens as the first part of a multi-step vaccine regimen for humans. Their work further supports research in developing a germline-targeting strategy for priming the immune system to elicit a bNAb called VRC01. This bNAb was discovered by NIAID researchers almost 15 years ago. The goal of this line of research is to develop an HIV vaccine that generates multiple classes of bNAbs to prevent HIV.
Explore further
Feedback to editors


Almost 1 in 3 Americans know someone who's died from a drug overdose
2 hours ago

Antibody discovery promises new hope in influenza B battle, paves way for universal vaccine
6 hours ago

Eye-tracking techniques could help primary care providers diagnose autism sooner, more accurately
20 hours ago
This self-powered sensor could make MRIs more efficient
22 hours ago

Not eating can hinder weight loss, study in fruit flies suggests

Neuroscience research suggests ketones can enhance cognitive function and protect brain networks
23 hours ago
New research finds antidepressants may help deliver other drugs into the brain

Mediterranean diet tied to one-fifth lower risk of death in women
May 31, 2024

Scientists find new method to enhance efficacy of bispecific antibodies for solid tumors

Cardiomyocytes study discovers new way to regenerate damaged heart cells
Related stories.
A boost for HIV vaccine research: Studies present comprehensive platform for validating next steps
May 21, 2024
Scientists design and validate promising HIV vaccine strategy
Oct 3, 2022
Groundbreaking HIV vaccine design strategy shows promise in proof-of-principle tests
Nov 7, 2019

Scientists may be closer to effective HIV vaccine
Dec 2, 2022

Tests of HIV vaccine using mRNA technology have begun
Jan 27, 2022

Scientists elicit broadly neutralizing antibodies to HIV in calves
Jul 20, 2017
Recommended for you
AI model confirms vaccination is key to cutting COVID in prisons

Guillain-Barre syndrome 'more common than expected' with RSV vaccine in older people, CDC reiterates

Study shows effectiveness of updated COVID-19 vaccines wanes moderately over time, is lower against current variants

Pharma firm urged to share new 'game-changer' HIV drug

Researchers discover vaccine originally created for HIV may also combat cancer
May 29, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation
- hot-topics Extras
- Newsletters
- Reading room
Tell us what you think. Take part in our reader survey
Celebrating twenty years
- Back to parent navigation item
- Collections
- Water and the environment
- Chemical bonding
- Antimicrobial resistance
- Energy storage and batteries
- AI and automation
- Sustainability
- Research culture
- Nobel prize
- Food science and cookery
- Plastics and polymers
- Periodic table
- Coronavirus
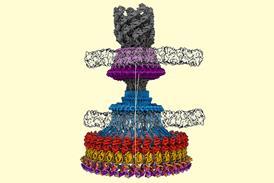
- More from navigation items
Genetic engineering feat coaxes yeast to produce valuable vaccine compound
By James Urquhart 2024-05-30T08:30:00+01:00
- No comments
Yeast has been engineered to perform the complete biosynthesis of QS-21, a potent and highly sought-after saponin-based adjuvant that boosts the immune response to certain vaccines.

Source: © Getty Images
Currently, the important vaccine adjuvant QS-21 is isolated from the soapbark tree that can only be found in Chile
The work represents one of the longest biosynthetic pathways ever transplanted into an organism, introducing 38 enzyme-encoding genes from six different species into yeast. The approach promises to enable a scalable, sustainable and cheaper way to make QS-21, as well as aid the design of new adjuvants.
Researchers have focused on the soap-like compound QS-21 as a vaccine adjuvant since the late 1990s for its ability to activate the immune system. Currently, it’s the only saponin-based vaccine adjuvant approved for clinical use in commercial vaccines, including ones for shingles, malaria and Covid-19, something which prompted concerns about QS-21 availability during the pandemic.
’From a world health perspective, there’s a lot of need for an alternative source of this adjuvant,’ says Jay Keasling at the University of California, Berkeley, US, who spearheaded the work with an international team of collaborators.
QS-21 is usually isolated from the bark of the soapbark tree, Quillaja saponaria , which is only found in Chile. Demand for QS-21 is high but supplies are limited because harvesting the bark requires mature trees and is tightly regulated. What’s more the isolation and purification of QS-21 from the mixture of compounds in bark extract is laborious, costly, uses toxic chemicals and has low yields.
Total synthesis of QS-21 has been previously achieved. However, it required the synthesis of an intermediate chemical first, takes 76 steps owing to the molecule’s complex structure – a glycosylated triterpene scaffold core coupled to a complex glycosylated 18-carbon acyl chain – and yields are poor.
One company, Botanical Solution in California, claims to have solved the supply and cost issues by devising and commercialising a plant tissue culture method to extract QS-21 using soapbark seedlings grown in the lab. Meanwhile, industry-led research published in March this year involving a number of biotech companies, presented another viable production route via cultured plant cells.
Keasling, however, thinks yeast offers the ideal alternative. ‘I want to make everything from a single sugar,’ he says. ‘I’d like to start with glucose, so when the production is performed in large tanks, they’re able to produce QS-21 as easily and inexpensively as possible.’
Pathway to success
The bioengineering feat of making QS-21 in yeast was possible following work published in January this year by some of the same team, including Keasling and Anne Osbourne at the John Innes Centre in Norwich, UK. They identified the complete 20-step biosynthetic pathway of QS-21, reproducing it in tobacco.
To reconstitute this pathway in yeast and make QS-21 from just glucose and galactose, the team first upregulated and fine tuned the passage of metabolites in the yeast strain’s native mevalonate pathway to produce quillaic acid, a key component of QS-21 synthesis. Meanwhile, using Crispr genome editing, enzyme-encoding genes from six other organisms, including plants that produce structurally similar saponins, fungi and bacteria were inserted. In total 38 enzymes spanning seven enzyme families were introduced, while ensuring critical metabolic pathways were unaffected for the yeast’s growth and survival.
‘This is a masterpiece of metabolic engineering, which shows how recent advances in synthetic biology like Crispr–Cas9 can be used to accelerate the discovery and engineering of pathways for the production of highly valuable molecules,’ says synthetic biologist Rodrigo Ledesma Amaro at Imperial College London, UK. ‘This is also a fantastic example of how microbial bioproduction can be an alternative to unsustainable plant extraction practices.’
‘The scale of the work undertaken in the study is quite extraordinary. It’s certainly up there as one of the most complex feats of metabolic engineering,’ says Paul Race , who researches natural product biosynthesis at Newcastle University, UK. ‘Work of this type is fraught with complications and I am in no doubt that a Herculean effort has been needed to get the system working as well as it does.’
However, both Amaro and Race note that low yields are an issue. Currently, it takes three days for the engineered yeast to produce around a third of the amount of QS-21 that the cells of the soapbark tree produces. Nevertheless, Keasling points out that yeast is around 1000 times faster than trees because only mature trees produce QS-21. ‘Even at the levels we’re producing it, it’s cheaper than producing it from the plant.’
‘Significant optimisation of the yeast platform is still required to achieve the yields needed to make this a viable route for QS-21 production at scale,’ Race comments. ‘This study represents the start of a journey that will hopefully result in access to this important molecule via a route that is uncoupled from the well-documented issues of isolation from the soapbark tree.’
Y Liu et al , Nature , 2024, DOI: 10.1038/s41586-024-07345-9
- biosynthesis
- Biotechnology
- Synthetic biology
Related articles
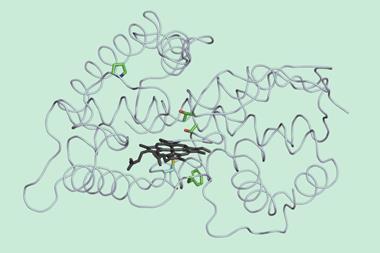
Carbene chemistry built into microbe’s metabolism in first for biosynthesis
2023-05-22T13:30:00Z
By James Urquhart

Soapbark tree’s biosynthetic pathway for vaccine adjuvant saponins
2023-03-31T11:37:00Z
By Anthony King

Yeast engineered to ferment sugars into chemotherapy drug precursors
2022-09-12T09:10:00Z

Nature-inspired crop protection
2022-09-07T14:44:00Z

Renewable rocket fuel made by genetically engineered soil bacteria
2022-07-11T13:46:00Z

Reprogrammed bacterium turns carbon dioxide into chemicals on industrial scale
2022-03-02T14:30:00Z
No comments yet
Only registered users can comment on this article., more from news.

Recycled construction waste could cut cement and steel’s carbon footprint
2024-05-31T11:42:00Z
Conjugate vaccine curbs xylazine effects in mice
2024-05-31T08:51:00Z
By Laura Cooper

Calls to kill off the European Institute of Innovation and Technology mount
2024-05-30T13:30:00Z
By Angeli Mehta

Graduate visa route stays, as UK government proposes ‘crackdown’ on abuses and migration
2024-05-29T13:30:00Z
By Jamie Durrani

Second Nobel prize medal for partition chromatography to be auctioned
2024-05-29T08:50:00Z
By Rebecca Trager

Two explosions in India leave at least 11 dead and dozens injured
2024-05-29T08:36:00Z
By Sanjay Kumar
- Contributors
- Terms of use
- Accessibility
- Permissions
- This website collects cookies to deliver a better user experience. See how this site uses cookies .
- This website collects cookies to deliver a better user experience. Do not sell my personal data .
- Este site coleta cookies para oferecer uma melhor experiência ao usuário. Veja como este site usa cookies .
Site powered by Webvision Cloud
Novel vaccine concept generates immune responses that could produce multiple types of HIV broadly neutralizing antibodies
Using a combination of cutting-edge immunologic technologies, researchers have successfully stimulated animals' immune systems to induce rare precursor B cells of a class of HIV broadly neutralizing antibodies (bNAbs). The findings, published in Nature Immunology , are an encouraging, incremental step in developing a preventive HIV vaccine.
HIV is genetically diverse making the virus difficult to target with a vaccine, but bNAbs may overcome that hurdle because they bind to parts of the virus that remain constant even when it mutates. Germline targeting is an immune system-stimulating approach that guides naïve (precursor) B cells to develop into mature B cells that can produce bNAbs. A class of bNAbs called 10E8 is a priority for HIV vaccine development because it neutralizes a particularly broad range of HIV variants. The 10E8 bNAb binds to a conserved region of the glycoprotein gp41 on HIV's surface involved in its entry into human immune cells. Designing an immunogen -- a molecule used in a vaccine that elicits a specific immune system response -- to stimulate production of 10E8 bNAbs has been challenging because that key region of gp41 is hidden in a recessed crevice on HIV's surface. Prior vaccine immunogens have not generated bNAbs with the physical structure to reach and bind to gp41.
To address this challenge, the researchers engineered immunogens on nanoparticles that mimic the appearance of a specific part of gp41. They vaccinated rhesus macaque monkeys and mice with those immunogens and elicited specific responses from the 10E8 B cell precursors and induced antibodies that showed signs of maturing into bNAbs that could reach the hidden gp41 region. They observed similar responses when they used mRNA-encoded nanoparticles in mice. The researchers also found that the same immunogens produced B cells that could mature to produce an additional type of gp41-directed bNAb called LN01. Finally, their laboratory analysis of human blood samples found that 10E8-class bNAb precursors occurred naturally in people without HIV, and that their immunogens bound to and isolated naïve human B cells with 10E8-like features. Together these observations suggest that the promising immunization data from mice and macaques has the potential for translation to humans.
The research was conducted by the Scripps Consortium for HIV/AIDS Vaccine Development, one of two consortia supported by the National Institutes of Health's (NIH) National Institute of Allergy and Infectious Diseases (NIAID). The research also was supported by collaborating partners including the Bill & Melinda Gates Foundation and other NIH Institutes and Offices. According to the authors, these findings support the development of the immunogens as the first part of a multi-step vaccine regimen for humans. Their work further supports research in developing a germline-targeting strategy for priming the immune system to elicit a bNAb called VRC01. This bNAb was discovered by NIAID researchers almost 15 years ago. The goal of this line of research is to develop an HIV vaccine that generates multiple classes of bNAbs to prevent HIV.
- HIV and AIDS
- Infectious Diseases
- Immune System
- Sexual Health
- Diseases and Conditions
- Antiretroviral drug
- Monoclonal antibody therapy
- Immune system
Story Source:
Materials provided by NIH/National Institute of Allergy and Infectious Diseases . Note: Content may be edited for style and length.
Journal Reference :
- Torben Schiffner, Ivy Phung, Rashmi Ray, Adriana Irimia, Ming Tian, Olivia Swanson, Jeong Hyun Lee, Chang-Chun D. Lee, Ester Marina-Zárate, So Yeon Cho, Jiachen Huang, Gabriel Ozorowski, Patrick D. Skog, Andreia M. Serra, Kimmo Rantalainen, Joel D. Allen, Sabyasachi Baboo, Oscar L. Rodriguez, Sunny Himansu, Jianfu Zhou, Jonathan Hurtado, Claudia T. Flynn, Katherine McKenney, Colin Havenar-Daughton, Swati Saha, Kaitlyn Shields, Steven Schulze, Melissa L. Smith, Chi-Hui Liang, Laura Toy, Simone Pecetta, Ying-Cing Lin, Jordan R. Willis, Fabian Sesterhenn, Daniel W. Kulp, Xiaozhen Hu, Christopher A. Cottrell, Xiaoya Zhou, Jennifer Ruiz, Xuesong Wang, Usha Nair, Kathrin H. Kirsch, Hwei-Ling Cheng, Jillian Davis, Oleksandr Kalyuzhniy, Alessia Liguori, Jolene K. Diedrich, Julia T. Ngo, Vanessa Lewis, Nicole Phelps, Ryan D. Tingle, Skye Spencer, Erik Georgeson, Yumiko Adachi, Michael Kubitz, Saman Eskandarzadeh, Marc A. Elsliger, Rama R. Amara, Elise Landais, Bryan Briney, Dennis R. Burton, Diane G. Carnathan, Guido Silvestri, Corey T. Watson, John R. Yates, James C. Paulson, Max Crispin, Gevorg Grigoryan, Andrew B. Ward, Devin Sok, Frederick W. Alt, Ian A. Wilson, Facundo D. Batista, Shane Crotty, William R. Schief. Vaccination induces broadly neutralizing antibody precursors to HIV gp41 . Nature Immunology , 2024; DOI: 10.1038/s41590-024-01833-w
Cite This Page :
Explore More
- Resting Brain: Neurons Rehearse for Future
- Observing Single Molecules
- A Greener, More Effective Way to Kill Termites
- One Bright Spot Among Melting Glaciers
- Martian Meteorites Inform Red Planet's Structure
- Volcanic Events On Jupiter's Moon Io: High Res
- What Negative Adjectives Mean to Your Brain
- 'Living Bioelectronics' Can Sense and Heal Skin
- Extinct Saber-Toothed Cat On Texas Coast
- Some Black Holes Survive in Globular Clusters
Trending Topics
Strange & offbeat.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 22 December 2020
A guide to vaccinology: from basic principles to new developments
- Andrew J. Pollard ORCID: orcid.org/0000-0001-7361-719X 1 , 2 &
- Else M. Bijker 1 , 2
Nature Reviews Immunology volume 21 , pages 83–100 ( 2021 ) Cite this article
495k Accesses
684 Citations
3667 Altmetric
Metrics details
- Infectious diseases
A Publisher Correction to this article was published on 05 January 2021
This article has been updated
Immunization is a cornerstone of public health policy and is demonstrably highly cost-effective when used to protect child health. Although it could be argued that immunology has not thus far contributed much to vaccine development, in that most of the vaccines we use today were developed and tested empirically, it is clear that there are major challenges ahead to develop new vaccines for difficult-to-target pathogens, for which we urgently need a better understanding of protective immunity. Moreover, recognition of the huge potential and challenges for vaccines to control disease outbreaks and protect the older population, together with the availability of an array of new technologies, make it the perfect time for immunologists to be involved in designing the next generation of powerful immunogens. This Review provides an introductory overview of vaccines, immunization and related issues and thereby aims to inform a broad scientific audience about the underlying immunological concepts.
Similar content being viewed by others

Imprinting of serum neutralizing antibodies by Wuhan-1 mRNA vaccines

Long COVID: major findings, mechanisms and recommendations
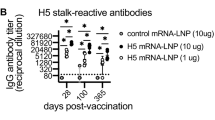
Development of a nucleoside-modified mRNA vaccine against clade 2.3.4.4b H5 highly pathogenic avian influenza virus
Introduction.
Vaccines have transformed public health, particularly since national programmes for immunization first became properly established and coordinated in the 1960s. In countries with high vaccine programme coverage, many of the diseases that were previously responsible for the majority of childhood deaths have essentially disappeared 1 (Fig. 1 ). The World Health Organization (WHO) estimates that 2–3 million lives are saved each year by current immunization programmes, contributing to the marked reduction in mortality of children less than 5 years of age globally from 93 deaths per 1,000 live births in 1990 to 39 deaths per 1,000 live births in 2018 (ref. 2 ).
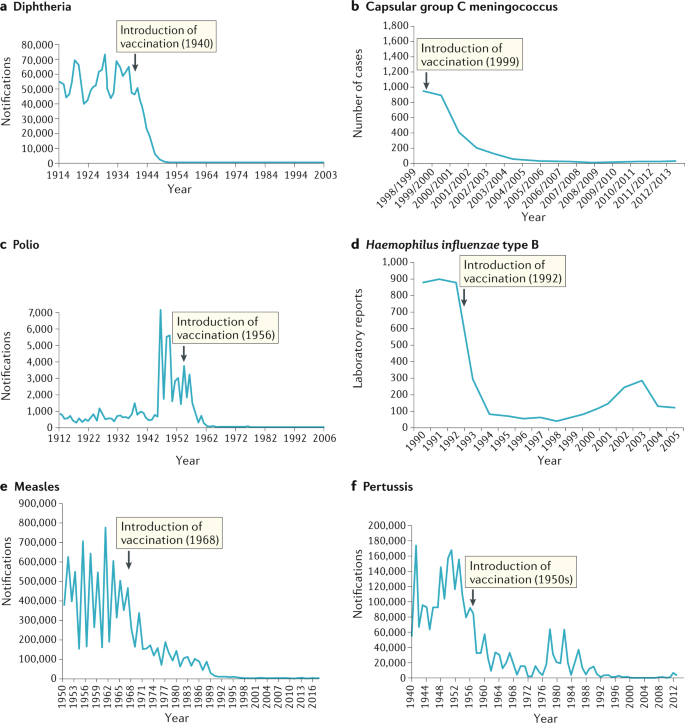
The introduction of vaccination against infectious diseases such as diphtheria (part a ), capsular group C meningococcus (part b ), polio (part c ), Haemophilus influenzae type B (part d ), measles (part e ) and pertussis (part f ) led to a marked decrease in their incidence. Of note, the increase in reports of H. influenzae type B in 2001 led to a catch-up vaccination campaign, after which the incidence reduced. For pertussis, a decline in vaccine coverage led to an increase in cases in the late 1970s and 1980s, but disease incidence reduced again after vaccine coverage increased. Adapted with permission from the Green Book, information for public health professionals on immunisation, Public Health England , contains public sector information licensed under the Open Government Licence v3.0.
Vaccines exploit the extraordinary ability of the highly evolved human immune system to respond to, and remember, encounters with pathogen antigens . However, for much of history, vaccines have been developed through empirical research without the involvement of immunologists. There is a great need today for improved understanding of the immunological basis for vaccination to develop vaccines for hard-to-target pathogens (such as Mycobacterium tuberculosis , the bacterium that causes tuberculosis (TB)) 3 and antigenically variable pathogens (such as HIV) 4 , to control outbreaks that threaten global health security (such as COVID-19 or Ebola) 5 , 6 and to work out how to revive immune responses in the ageing immune system 7 to protect the growing population of older adults from infectious diseases.
In this Review, which is primarily aimed at a broad scientific audience, we provide a guide to the history (Box 1 ), development, immunological basis and remarkable impact of vaccines and immunization programmes on infectious diseases to provide insight into the key issues facing immunologists today. We also provide some perspectives on current and future challenges in continuing to protect the world’s population from common pathogens and emerging infectious threats. Communicating effectively about the science of vaccination to a sceptical public is a challenge for all those engaged in vaccine immunobiology but is urgently needed to realign the dialogue and ensure public health 8 . This can only be achieved by being transparent about what we know and do not know, and by considering the strategies to overcome our existing knowledge gaps.
Box 1 A brief history of vaccination
Epidemics of smallpox swept across Europe in the seventeenth and eighteenth centuries, accounting for as much as 29% of the death rate of children in London 137 . Initial efforts to control the disease led to the practice of variolation, which was introduced to England by Lady Mary Wortley Montagu in 1722, having been used in the Far East since the mid-1500s (see Nature Milestones in Vaccines ). In variolation, material from the scabs of smallpox lesions was scratched into the skin in an attempt to provide protection against the disease. Variolation did seem to induce protection, reducing the attack rate during epidemics, but sadly some of those who were variolated developed the disease and sometimes even died. It was in this context that Edward Jenner wrote ‘An Inquiry into the Causes and Effects of the Variole Vaccinae…’ in 1798. His demonstration, undertaken by scratching material from cowpox lesions taken from the hands of a milkmaid, Sarah Nelms, into the skin of an 8-year-old boy, James Phipps, who he subsequently challenged with smallpox, provided early evidence that vaccination could work. Jenner’s contribution to medicine was thus not the technique of inoculation but his startling observation that milkmaids who had had mild cowpox infections did not contract smallpox, and the serendipitous assumption that material from cowpox lesions might immunize against smallpox. Furthermore, Jenner brilliantly predicted that vaccination could lead to the eradication of smallpox; in 1980, the World Health Assembly declared the world free of naturally occurring smallpox.
Almost 100 years after Jenner, the work of Louis Pasteur on rabies vaccine in the 1880s heralded the beginning of a frenetic period of development of new vaccines, so that by the middle of the twentieth century, vaccines for many different diseases (such as diphtheria, pertussis and typhoid) had been developed as inactivated pathogen products or toxoid vaccines. However, it was the coordination of immunization as a major public health tool from the 1950s onwards that led to the introduction of comprehensive vaccine programmes and their remarkable impact on child health that we enjoy today. In 1974, the World Health Organization launched the Expanded Programme on Immunization and a goal was set in 1977 to reach every child in the world with vaccines for diphtheria, pertussis, tetanus, poliomyelitis, measles and tuberculosis by 1990. Unfortunately, that goal has still not been reached; although global coverage of 3 doses of the diphtheria–tetanus–pertussis vaccine has risen to more than 85%, there are still more than 19 million children who did not receive basic vaccinations in 2019 (ref. 105 ).
What is in a vaccine?
A vaccine is a biological product that can be used to safely induce an immune response that confers protection against infection and/or disease on subsequent exposure to a pathogen. To achieve this, the vaccine must contain antigens that are either derived from the pathogen or produced synthetically to represent components of the pathogen. The essential component of most vaccines is one or more protein antigens that induce immune responses that provide protection. However, polysaccharide antigens can also induce protective immune responses and are the basis of vaccines that have been developed to prevent several bacterial infections, such as pneumonia and meningitis caused by Streptococcus pneumoniae , since the late 1980s 9 . Protection conferred by a vaccine is measured in clinical trials that relate immune responses to the vaccine antigen to clinical end points (such as prevention of infection, a reduction in disease severity or a decreased rate of hospitalization). Finding an immune response that correlates with protection can accelerate the development of and access to new vaccines 10 (Box 2 ).
Vaccines are generally classified as live or non-live (sometimes loosely referred to as ‘inactivated’) to distinguish those vaccines that contain attenuated replicating strains of the relevant pathogenic organism from those that contain only components of a pathogen or killed whole organisms (Fig. 2 ). In addition to the ‘traditional’ live and non-live vaccines, several other platforms have been developed over the past few decades, including viral vectors, nucleic acid-based RNA and DNA vaccines, and virus-like particles (discussed in more detail later).
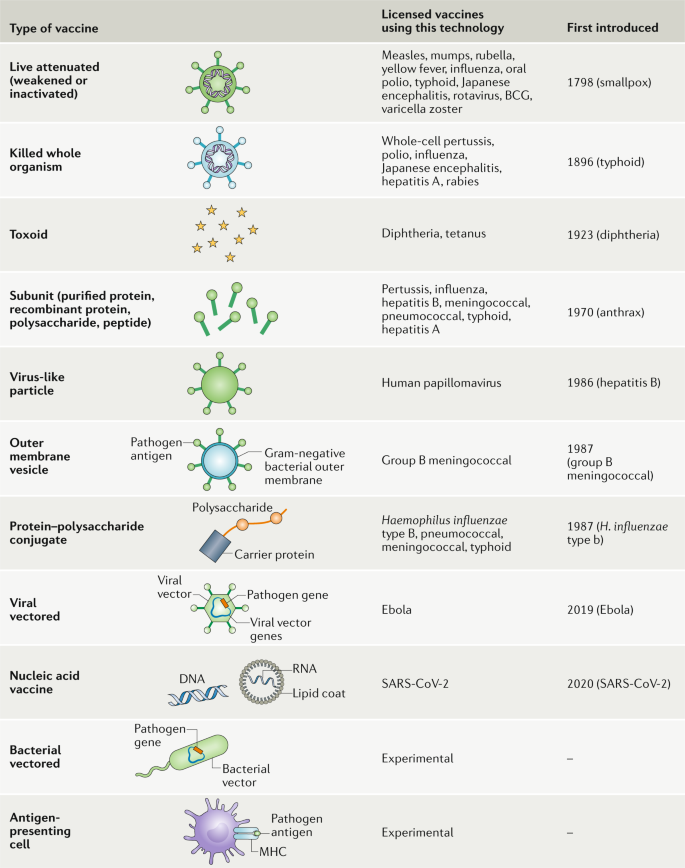
Schematic representation of different types of vaccine against pathogens; the text indicates against which pathogens certain vaccines are licensed and when each type of vaccine was first introduced. BCG, Mycobacterium bovis bacillus Calmette–Guérin.
The distinction between live and non-live vaccines is important. The former may have the potential to replicate in an uncontrolled manner in immunocompromised individuals (for example, children with some primary immunodeficiencies, or individuals with HIV infection or those receiving immunosuppressive drugs), leading to some restrictions to their use 11 . By contrast, non-live vaccines pose no risk to immunocompromised individuals (although they may not confer protection in those with B cell or combined immunodeficiency, as explained in more detail later).
Live vaccines are developed so that, in an immunocompetent host, they replicate sufficiently to produce a strong immune response, but not so much as to cause significant disease manifestations (for example, the vaccines for measles, mumps, rubella and rotavirus, oral polio vaccine, the Mycobacterium bovis bacillus Calmette–Guérin (BCG) vaccine for TB and live attenuated influenza vaccine). There is a trade-off between enough replication of the vaccine pathogen to induce a strong immune response and sufficient attenuation of the pathogen to avoid symptomatic disease. For this reason, some safe, live attenuated vaccines require multiple doses and induce relatively short-lived immunity (for example, the live attenuated typhoid vaccine, Ty21a) 12 , and other live attenuated vaccines may induce some mild disease (for example, about 5% of children will develop a rash and up to 15% fever after measles vaccination) 13 .
The antigenic component of non-live vaccines can be killed whole organisms (for example, whole-cell pertussis vaccine and inactivated polio vaccine), purified proteins from the organism (for example, acellular pertussis vaccine), recombinant proteins (for example, hepatitis B virus (HBV) vaccine) or polysaccharides (for example, the pneumococcal vaccine against S. pneumoniae ) (Fig. 2 ). Toxoid vaccines (for example, for tetanus and diphtheria) are formaldehyde-inactivated protein toxins that have been purified from the pathogen.
Non-live vaccines are often combined with an adjuvant to improve their ability to induce an immune response (immunogenicity). There are only a few adjuvants that are used routinely in licensed vaccines. However, the portfolio of adjuvants is steadily expanding, with liposome-based adjuvants and oil-in-water emulsions being licensed in the past few decades 14 . The mechanism of action of aluminium salts (alum), although extensively used as an adjuvant for more than 80 years, remains incompletely understood 15 , but there is increasing evidence that immune responses and protection can be enhanced by the addition of newer adjuvants that provide danger signals to the innate immune system . Examples of these novel adjuvants are the oil-in-water emulsion MF59, which is used in some influenza vaccines 16 ; AS01 , which is used in one of the shingles vaccines and the licensed malaria vaccine 17 ; and AS04 , which is used in a vaccine against human papillomavirus (HPV) 18 .
Vaccines contain other components that function as preservatives, emulsifiers (such as polysorbate 80) or stabilizers (for example, gelatine or sorbitol). Various products used in the manufacture of vaccines could theoretically also be carried over to the final product and are included as potential trace components of a vaccine, including antibiotics, egg or yeast proteins, latex, formaldehyde and/or gluteraldehyde and acidity regulators (such as potassium or sodium salts). Except in the case of allergy to any of these components, there is no evidence of risk to human health from these trace components of some vaccines 19 , 20 .
Box 2 Correlates of protection
The identification of correlates of protection is helpful in vaccine development as they can be used to compare products and to predict whether the use of an efficacious vaccine in a new population (for example, a different age group, medical background or geographical location) is likely to provide the same protection as that observed in the original setting. There is considerable confusion in the literature about the definition of a correlate of protection. For the purposes of this discussion, it is useful to separate out two distinct meanings. A mechanistic correlate of protection is the specific functional immune mechanism that is believed to confer protection. For example, antitoxin antibodies, which are induced by the tetanus toxoid vaccine, confer protection directly by neutralizing the activity of the toxin. A non-mechanistic correlate of protection does not in itself provide the protective function but has a statistical relationship with the mechanism of protection. An example of a non-mechanistic correlate of protection is total IgG antibody levels against pneumococci. These IgG antibodies contain the mechanistic correlate (thought to be a subset of opsonophagocytic antibodies ) but the mechanism of protection is not being directly measured. Correlates of protection can be measured in clinical trials if there are post-vaccination sera available from individuals who do or do not develop disease, although large-scale serum collection from participants is rarely undertaken in phase III clinical efficacy trials. An alternative approach is to estimate the correlates of protection by extrapolating from sero-epidemiological studies in a vaccinated population and relating the data to disease incidence in the population. Human challenge studies have also been used to determine correlates of protection, although the dose of challenge bacterium or virus and the experimental conditions may not relate closely to natural infection, which can limit the utility of these observations.
Vaccines induce antibodies
The adaptive immune response is mediated by B cells that produce antibodies (humoral immunity) and by T cells (cellular immunity). All vaccines in routine use, except BCG (which is believed to induce T cell responses that prevent severe disease and innate immune responses that may inhibit infection; see later), are thought to mainly confer protection through the induction of antibodies (Fig. 3 ). There is considerable supportive evidence that various types of functional antibody are important in vaccine-induced protection, and this evidence comes from three main sources: immunodeficiency states, studies of passive protection and immunological data.
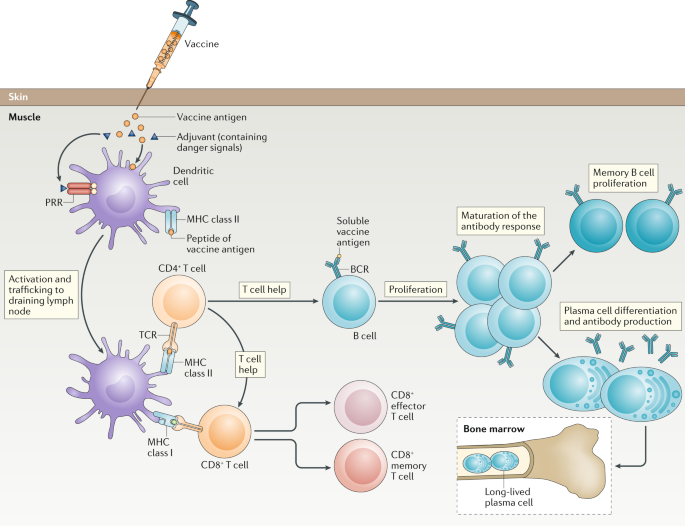
The immune response following immunization with a conventional protein antigen. The vaccine is injected into muscle and the protein antigen is taken up by dendritic cells, which are activated through pattern recognition receptors (PRRs) by danger signals in the adjuvant, and then trafficked to the draining lymph node. Here, the presentation of peptides of the vaccine protein antigen by MHC molecules on the dendritic cell activates T cells through their T cell receptor (TCR). In combination with signalling (by soluble antigen) through the B cell receptor (BCR), the T cells drive B cell development in the lymph node. Here, the T cell-dependent B cell development results in maturation of the antibody response to increase antibody affinity and induce different antibody isotypes. The production of short-lived plasma cells, which actively secrete antibodies specific for the vaccine protein, produces a rapid rise in serum antibody levels over the next 2 weeks. Memory B cells are also produced, which mediate immune memory. Long-lived plasma cells that can continue to produce antibodies for decades travel to reside in bone marrow niches. CD8 + memory T cells can proliferate rapidly when they encounter a pathogen, and CD8 + effector T cells are important for the elimination of infected cells.
Immunodeficiency states
Individuals with some known immunological defects in antibodies or associated immune components are particularly susceptible to infection with certain pathogens, which can provide insight into the characteristics of the antibodies that are required for protection from that particular pathogen. For example, individuals with deficiencies in the complement system are particularly susceptible to meningococcal disease caused by infection with Neisseria meningitidis 21 because control of this infection depends on complement-mediated killing of bacteria, whereby complement is directed to the bacterial surface by IgG antibodies. Pneumococcal disease is particularly common in individuals with reduced splenic function 22 (which may be congenital, resulting from trauma or associated with conditions such as sickle cell disease); S. pneumoniae bacteria that have been opsonized with antibody and complement are normally removed from the blood by phagocytes in the spleen, which are no longer present in individuals with hyposplenism. Antibody-deficient individuals are susceptible to varicella zoster virus (which causes chickenpox) and other viral infections, but, once infected, they can control the disease in the same way as an immunocompetent individual, so long as they have a normal T cell response 23 .
Passive protection
It has been clearly established that intramuscular or intravenous infusion of exogenous antibodies can provide protection against some infections. The most obvious example is that of passive transfer of maternal antibodies across the placenta, which provides newborn infants with protection against a wide variety of pathogens, at least for a few months after birth. Maternal vaccination with pertussis 24 , tetanus 25 and influenza 26 vaccines harnesses this important protective adaptation to reduce the risk of disease soon after birth and clearly demonstrates the role of antibodies in protection against these diseases. Vaccination of pregnant women against group B streptococci 27 and respiratory syncytial virus (RSV) 28 has not yet been shown to be effective at preventing neonatal or infant infection, but it has the potential to reduce the burden of disease in the youngest infants. Other examples include the use of specific neutralizing antibodies purified from immune donors to prevent the transmission of various viruses, including varicella zoster virus, HBV and measles virus 29 . Individuals with inherited antibody deficiency are without defence against serious viral and bacterial infections, but regular administration of serum antibodies from an immunocompetent donor can provide almost entirely normal immune protection for the antibody-deficient individual.
Immunological data
Increasing knowledge of immunology provides insights into the mechanisms of protection mediated by vaccines. For example, polysaccharide vaccines, which are made from the surface polysaccharides of invasive bacteria such as meningococci ( N. meningitidis ) 30 and pneumococci ( S. pneumoniae ) 31 , provide considerable protection against these diseases. It is now known that these vaccines do not induce T cell responses, as polysaccharides are T cell-independent antigens , and thus they must mediate their protection through antibody-dependent mechanisms. Protein–polysaccharide conjugate vaccines contain the same polysaccharides from the bacterial surface, but in this case they are chemically conjugated to a protein carrier (mostly tetanus toxoid, or diphtheria toxoid or a mutant protein derived from it, known as CRM 197 ) 32 , 33 , 34 . The T cells induced by the vaccine recognize the protein carrier (a T cell-dependent antigen ) and these T cells provide help to the B cells that recognize the polysaccharide, but no T cells are induced that recognize the polysaccharide and, thus, only antibody is involved in the excellent protection induced by these vaccines 35 . Furthermore, human challenge studies offer the opportunity to efficiently assess correlates of protection (Box 2 ) under controlled circumstances 36 , and they have been used to demonstrate the role of antibodies in protection against malaria 37 and typhoid 38 .
Vaccines need T cell help
Although most of the evidence points to antibodies being the key mediators of sterilizing immunity induced by vaccination, most vaccines also induce T cell responses. The role of T cells in protection is poorly characterized, except for their role in providing help for B cell development and antibody production in lymph nodes. From studies of individuals with inherited or acquired immunodeficiency, it is clear that whereas antibody deficiency increases susceptibility to acquisition of infection, T cell deficiency results in failure to control a pathogen after infection. For example, T cell deficiency results in uncontrolled and fatal varicella zoster virus infection, whereas individuals with antibody deficiency readily develop infection but recover in the same way as immunocompetent individuals. The relative suppression of T cell responses that occurs at the end of pregnancy increases the severity of infection with influenza and varicella zoster viruses 39 .
Although evidence for the involvement of T cells in vaccine-induced protection is limited, this is likely owing, in part, to difficulties in accessing T cells to study as only the blood is easily accessible, whereas many T cells are resident in tissues such as lymph nodes. Furthermore, we do not yet fully understand which types of T cell should be measured. Traditionally, T cells have been categorized as either cytotoxic (killer) T cells or helper T cells. Subtypes of T helper cells (T H cells) can be distinguished by their profiles of cytokine production. T helper 1 (T H 1) cells and T H 2 cells are mainly important for establishing cellular immunity and humoral immunity, respectively, although T H 1 cells are also associated with generation of the IgG antibody subclasses IgG1 and IgG3. Other T H cell subtypes include T H 17 cells (which are important for immunity at mucosal surfaces such as the gut and lung) and T follicular helper cells (located in secondary lymphoid organs, which are important for the generation of high-affinity antibodies (Fig. 3 )). Studies show that sterilizing immunity against carriage of S. pneumoniae in mice can be achieved by the transfer of T cells from donor mice exposed to S. pneumoniae 40 , which indicates that further investigation of T cell-mediated immunity is warranted to better understand the nature of T cell responses that could be harnessed to improve protective immunity.
Although somewhat simplistic, the evidence therefore indicates that antibodies have the major role in prevention of infection (supported by T H cells), whereas cytotoxic T cells are required to control and clear established infection.
Features of vaccine-induced protection
Vaccines have been developed over the past two centuries to provide direct protection of the immunized individual through the B cell-dependent and T cell-dependent mechanisms described above. As our immunological understanding of vaccines has developed, it has become apparent that this protection is largely manifested through the production of antibody. Another important feature of vaccine-induced protection is the induction of immune memory . Vaccines are usually developed to prevent clinical manifestations of infection. However, some vaccines, in addition to preventing the disease, may also protect against asymptomatic infection or colonization, thereby reducing the acquisition of a pathogen and thus its onward transmission, establishing herd immunity. Indeed, the induction of herd immunity is perhaps the most important characteristic of immunization programmes, with each dose of vaccine protecting many more individuals than the vaccine recipient. Some vaccines may also drive changes in responsiveness to future infections with different pathogens, so called non-specific effects, perhaps by stimulating prolonged changes in the activation state of the innate immune system.
Immune memory
In encountering a pathogen, the immune system of an individual who has been vaccinated against that specific pathogen is able to more rapidly and more robustly mount a protective immune response. Immune memory has been shown to be sufficient for protection against pathogens when the incubation period is long enough for a new immune response to develop (Fig. 4a ). For example, in the case of HBV, which has an incubation period of 6 weeks to 6 months, a vaccinated individual is usually protected following vaccination even if exposure to the virus occurs some time after vaccination and the levels of vaccine-induced antibody have already waned 41 . Conversely, it is thought that immune memory may not be sufficient for protection against rapidly invasive bacterial infections that can cause severe disease within hours or days following acquisition of the pathogen 42 (Fig. 4b ). For example, there is evidence in the case of both Haemophilus influenzae type B (Hib) and capsular group C meningococcal infection that individuals with vaccine-induced immune memory can still develop disease once their antibody levels have waned, despite mounting robust, although not rapid enough, memory responses 43 , 44 . The waning of antibody levels varies depending on the age of the vaccine recipient (being very rapid in infants as a result of the lack of bone marrow niches for B cell survival), the nature of the antigen and the number of booster doses administered. For example, the virus-like particles used in the HPV vaccine induce antibody responses that can persist for decades, whereas relatively short-term antibody responses are induced by pertussis vaccines; and the inactivated measles vaccine induces shorter-lived antibody responses than the live attenuated measles vaccine.
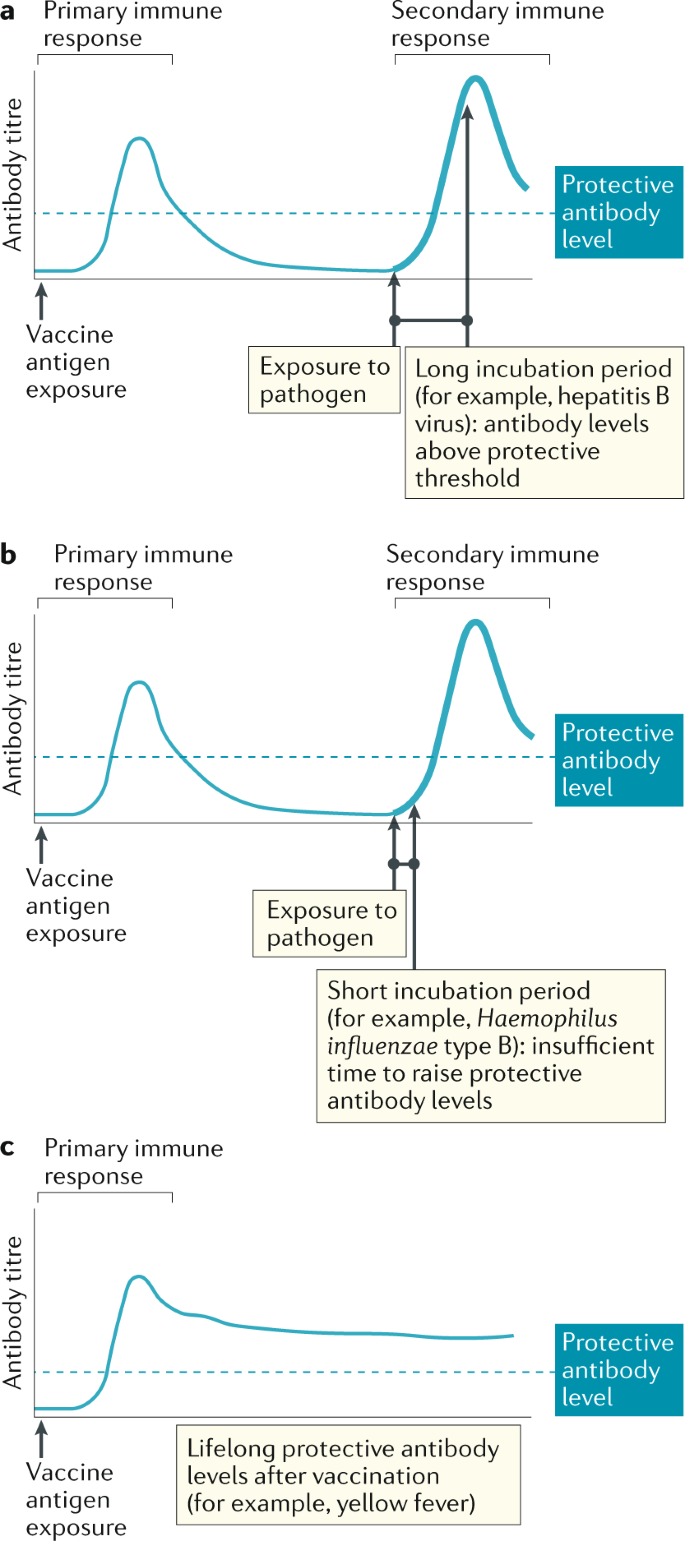
Antibody levels in the circulation wane after primary vaccination, often to a level below that required for protection. Whether immune memory can protect against a future pathogen encounter depends on the incubation time of the infection, the quality of the memory response and the level of antibodies induced by memory B cells. a | The memory response may be sufficient to protect against disease if there is a long incubation period between pathogen exposure and the onset of symptoms to allow for the 3–4 days required for memory B cells to generate antibody titres above the protective threshold. b | The memory response may not be sufficient to protect against disease if the pathogen has a short incubation period and there is rapid onset of symptoms before antibody levels have reached the protective threshold. c | In some cases, antibody levels after primary vaccination remain above the protective threshold and can provide lifelong immunity.
So, for infections that are manifest soon after acquisition of the pathogen, the memory response may be insufficient to control these infections and sustained immunity for individual protection through vaccination can be difficult to achieve. One solution to this is the provision of booster doses of vaccine through childhood (as is the case, for example, for diphtheria, tetanus, pertussis and polio vaccines), in an attempt to sustain antibody levels above the protective threshold. It is known that provision of five or six doses of tetanus 45 or diphtheria 46 vaccine in childhood provides lifelong protection, and so booster doses of these vaccines throughout adult life are not routine in most countries that can achieve high coverage with multiple childhood doses. Given that, for some infections, the main burden is in young children, continued boosting after the second year of life is not undertaken (for example, the invasive bacterial infections including Hib and capsular group B meningococci).
The exception is the pertussis vaccine, where the focus of vaccine programmes is the prevention of disease in infancy; this is achieved both by direct vaccination of infants as well as by the vaccination of other age groups, including adolescents and pregnant women in some programmes, to reduce transmission to infants and provide protection by antibody transfer across the placenta. Notably, in high-income settings, many countries (starting in the 1990s) have switched to using the acellular pertussis vaccine, which is less reactogenic than (and therefore was thought to be preferable to) the older whole-cell pertussis vaccine that is still used in most low-income countries. It is now apparent that acellular pertussis vaccine induces a shorter duration of protection against clinical pertussis and may be less effective against bacterial transmission than is the whole-cell pertussis vaccine 47 . Many high-income countries have observed a rise in pertussis cases since the introduction of the acellular vaccine, a phenomenon that is not observed in low-income nations using the whole-cell vaccine 48 .
By contrast, lifelong protection seems to be the rule following a single dose with some of the live attenuated viral vaccines, such as yellow fever vaccine 49 (Fig. 4c ), although it is apparent that protection is incomplete with others. In the case of varicella zoster and measles–mumps vaccines, some breakthrough cases are described during disease outbreaks among those individuals who have previously been vaccinated, although it is unclear whether this represents a group in whom immunity has waned (and who therefore needed booster vaccination) or a group for whom the initial vaccine did not induce a successful immune response. Breakthrough cases are less likely in those individuals who have had two doses of measles–mumps–rubella vaccine 50 or varicella zoster vaccine 51 , and cases that do occur are usually mild, which indicates that there is some lasting immunity to the pathogen.
An illustration of the complexity of immune memory and the importance of understanding its underlying immunological mechanisms in order to improve vaccination strategies is provided by the concept of ‘original antigenic sin’. This phenomenon describes how the immune system fails to generate an immune response against a strain of a pathogen if the host was previously exposed to a closely related strain, and this has been demonstrated in several infections, including dengue 52 and influenza 53 . This might have important implications for vaccine development if only a single pathogen strain or pathogen antigen is included in a vaccine, as vaccine recipients might then have impaired immune responses if later exposed to different strains of the same pathogen, potentially putting them at increased risk of infection or more severe disease. Strategies to overcome this include the use of adjuvants that stimulate innate immune responses, which can induce sufficiently cross-reactive B cells and T cells that recognize different strains of the same pathogen, or the inclusion of as many strains in a vaccine as possible, the latter approach obviously being limited by the potential of new strains to emerge in the future 54 .
Herd immunity
Although direct protection of individuals through vaccination has been the focus of most vaccine development and is crucial to demonstrate for the licensure of new vaccines, it has become apparent that a key additional component of vaccine-induced protection is herd immunity, or more correctly ‘herd protection’ (Fig. 5 ). Vaccines cannot protect every individual in a population directly, as some individuals are not vaccinated for various reasons and others do not mount an immune response despite vaccination. Fortunately, however, if enough individuals in a population are vaccinated, and if vaccination prevents not only the development of disease but also infection itself (discussed in more detail below), transmission of the pathogen can be interrupted and the incidence of disease can fall further than would be expected, as a result of the indirect protection of individuals who would otherwise be susceptible.
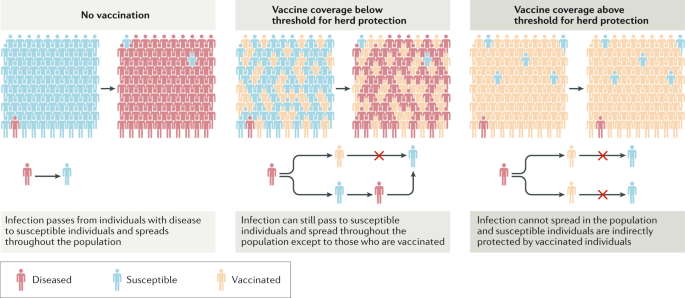
The concept of herd immunity for a highly contagious disease such as measles. Susceptible individuals include those who have not yet been immunized (for example, being too young), those who cannot be immunized (for example, as a result of immunodeficiency), those for whom the vaccine did not induce immunity, those for whom initial vaccine-induced immunity has waned and those who refused immunization.
For highly transmissible pathogens, such as those causing measles or pertussis, around 95% of the population must be vaccinated to prevent disease outbreaks, but for less transmissible organisms a lower percentage of vaccine coverage may be sufficient to have a substantial impact on disease (for example, for polio, rubella, mumps or diphtheria, vaccine coverage can be ≤86%). For influenza, the threshold for herd immunity is highly variable from season to season and is also confounded by the variability in vaccine effectiveness each year 55 . Modest vaccine coverage, of 30–40%, is likely to have an impact on seasonal influenza epidemics, but ≥80% coverage is likely to be optimal 56 . Interestingly, there might be a downside to very high rates of vaccination, as the absence of pathogen transmission in that case will prevent natural boosting of vaccinated individuals and could lead to waning immunity if booster doses of vaccine are not used.
Apart from tetanus vaccine, all other vaccines in the routine immunization schedule induce some degree of herd immunity (Fig. 5 ), which substantially enhances population protection beyond that which could be achieved by vaccination of the individual only. Tetanus is a toxin-mediated disease acquired through infection of breaks in the skin contaminated with the toxin-producing bacteria Clostridium tetani from the environment — so, vaccination of the community with the tetanus toxoid will not prevent an unvaccinated individual acquiring the infection if they are exposed. As an example of the success of herd immunity, vaccination of children and young adults (up to 19 years of age) with capsular group C meningococcal vaccine in a mass campaign in 1999 resulted in almost complete elimination of disease from the UK in adults as well as children 57 . Currently, the strategy for control of capsular groups A, C, W and Y meningococci in the UK is vaccination of adolescents, as they are mainly responsible for transmission and vaccine-mediated protection of this age group leads to community protection through herd immunity 58 . The HPV vaccine was originally introduced to control HPV-induced cervical cancer, with vaccination programmes directed exclusively at girls, but it was subsequently found to also provide protection against HPV infection in heterosexual boys through herd immunity, which led to a marked reduction in the total HPV burden in the population 59 , 60 .
Prevention of infection versus disease
Whether vaccines prevent infection or, rather, the development of disease after infection with a pathogen is often difficult to establish, but improved understanding of this distinction could have important implications for vaccine design. BCG vaccination can be used as an example to illustrate this point, as there is some evidence for the prevention of both disease and infection. BCG vaccination prevents severe disease manifestations such as tuberculous meningitis and miliary TB in children 61 and animal studies have shown that BCG vaccination reduces the spread of M. tuberculosis bacteria in the blood, mediated by T cell immunity 62 , thereby clearly showing that vaccination has protective effects against the development of disease after infection. However, there is also good evidence that BCG vaccination reduces the risk of infection. In a TB outbreak at a school in the UK, 29% of previously BCG-vaccinated children had a memory T cell response to infection, as indicated by a positive interferon-γ release assay , as compared with 47% of the unvaccinated children 63 . A similar effect was seen when studying Indonesian household members of patients with TB, who had a 45% reduced chance of developing a positive interferon-γ release assay response to M. tuberculosis if they had previously been BCG vaccinated 64 . The lack of a T cell response in previously vaccinated individuals indicates that the BCG vaccine induces an innate immune response that results in ‘early clearance’ of the bacteria and prevents infection that induces an adaptive immune response. It will be hugely valuable for future vaccine development to better understand the induction of such protective innate immune responses so that they might be reproduced for other pathogens.
In the case of the current pandemic of the virus SARS-CoV-2, a vaccine that prevents severe disease and disease-driven hospitalization could have a substantial public health impact. However, a vaccine that could also block acquisition of the virus, and thus prevent both asymptomatic and mild infection, would have much larger impact by reducing transmission in the community and potentially establishing herd immunity.
Non-specific effects
Several lines of evidence indicate that immunization with some vaccines perturbs the immune system in such a way that there are general changes in immune responsiveness that can increase protection against unrelated pathogens 65 . This phenomenon has been best described in humans in relation to BCG and measles vaccines, with several studies showing marked reductions in all-cause mortality when these vaccines are administered to young children that are far beyond the expected impact from the reduction in deaths attributed to TB or measles, respectively 66 . These non-specific effects may be particularly important in high-mortality settings, but not all studies have identified the phenomenon. Although several immunological mechanisms have been proposed, the most plausible of which is that epigenetic changes can occur in innate immune cells as a result of vaccination, there are no definitive studies in humans that link immunological changes after immunization with important clinical end points, and it remains unclear how current immunization schedules might be adapted to improve population protection through non-specific effects. Of great interest in the debate, recent studies have indicated that measles disease casts a prolonged ‘shadow’ over the immune system, with depletion of existing immune memory, such that children who have had the disease have an increased risk of death from other causes over the next few years 67 , 68 . In this situation, measles vaccination reduces mortality from measles as well as the unconnected diseases that would have occurred during the ‘shadow’, resulting in a benefit that seems to be non-specific but actually relates directly to the prevention of measles disease and its consequences. This illustrates a limitation of vaccine study protocols: as these are usually designed to find pathogen-specific effects, the possibility of important non-specific effects cannot be assessed.
Factors affecting vaccine protection
The level of protection afforded by vaccination is affected by many genetic and environmental factors, including age, maternal antibody levels, prior antigen exposure, vaccine schedule and vaccine dose. Although most of these factors cannot be readily modified, age of vaccination and schedule of vaccination are important and key factors in planning immunization programmes. The vaccine dose is established during early clinical development, based on optimal safety and immunogenicity. However, for some populations, such as older adults, a higher dose might be beneficial, as has been shown for the influenza vaccine 69 , 70 . Moreover, intradermal vaccination has been shown to be immunogenic at much lower (fractional) doses than intramuscular vaccination for influenza, rabies and HBV vaccines 71 .
Age of vaccination
The highest burden of and mortality from infectious disease occur in the first 5 years of life, with the youngest infants being most affected. For this reason, immunization programmes have largely focused on this age group where there is the greatest benefit from vaccine-induced protection. Although this makes sense from an epidemiological perspective, it is somewhat inconvenient from an immunological perspective as the induction of strong immune responses in the first year of life is challenging. Indeed, vaccination of older children and adults would induce stronger immune responses, but would be of little value if those who would have benefited from vaccination have already succumbed to the disease.
It is not fully understood why immune responses to vaccines are not as robust in early infancy as they are in older children. One factor, which is increasingly well documented, is interference from maternal antibody 72 — acquired in utero through the placenta — which might reduce antigen availability, reduce viral replication (in the case of live viral vaccines such as measles 73 ) or perhaps regulate B cell responses. However, there is also evidence that there is a physiological age-dependent increase in antibody responses in infancy 72 . Furthermore, bone marrow niches to support B cells are limited in infancy, which might explain the very short-lived immune responses that are documented in the first year of life 74 . For example, after immunization with 2 doses of the capsular group C meningococcal vaccine in infancy, only 41% of infants still had protective levels of antibody by the time of the booster dose, administered 7 months later 75 .
In the case of T cell-independent antigens — in other words, plain polysaccharides from Hib, typhoid-causing bacteria, meningococci and pneumococci — animal data indicate that antibody responses depend on development of the marginal zone of the spleen, which is required for the maturation of marginal zone B cells, and this does not occur until around 18 months of age in human infants 76 . These plain polysaccharide vaccines do not induce memory B cells (Fig. 6 ) and, even in adults, provide protection for just 2–3 years, with protection resulting from antibody produced by plasma cells derived from marginal zone B cells 77 . However, converting plain polysaccharide vaccines into T cell-dependent protein–polysaccharide conjugate vaccines, which are immunogenic from 2 months of age and induce immune memory, has transformed prevention of disease caused by the encapsulated bacteria (pneumococci, Hib and meningococci) over the past three decades 78 . These are the most important invasive bacterial pathogens of childhood, causing most cases of childhood meningitis and bacterial pneumonia, and the development of the conjugate vaccine technology in the 1980s has transformed global child health 9 .
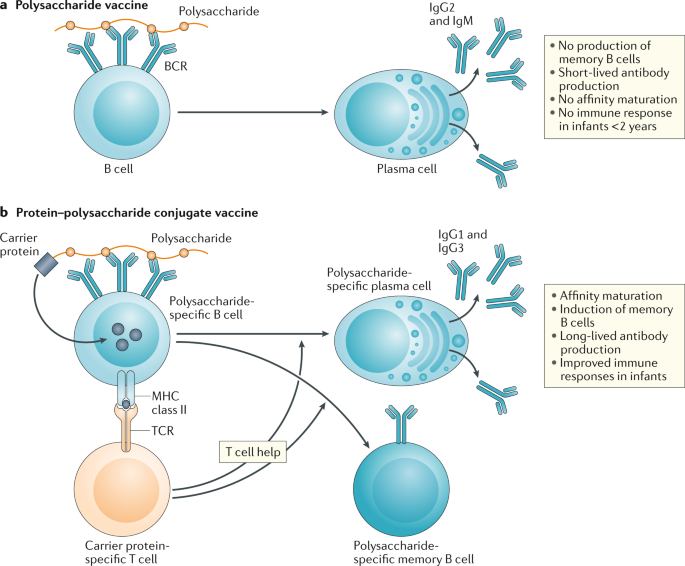
a | Polysaccharide vaccines induce antibody-producing plasma cells by cross-linking the B cell receptor (BCR). However, affinity maturation of the antibody response and the induction of memory B cells do not occur. b | Protein–polysaccharide conjugate vaccines can engage T cells that recognize the carrier protein, as well as B cells that recognize the polysaccharide. T cells provide help to B cells, leading to affinity maturation and the production of both plasma cells and memory B cells. TCR, T cell receptor. Adapted from ref. 35 , Springer Nature Limited.
Immune responses are also poor in the older population and most of the vaccines used in older adults offer limited protection or a limited duration of protection, particularly among those older than 75 years of age. The decline in immune function with age (known as immunosenescence) has been well documented 79 but, despite the burden of infection in this age group and the increasing size of the population, has not received sufficient attention so far amongst immunologists and vaccinologists. Interestingly, some have raised the hypothesis that chronic infection with cytomegalovirus (CMV) might have a role in immunosenescence through unfavourable effects on the immune system, including clonal expansion of CMV-specific T cell populations, known as ‘memory inflation’, and reduced diversity of naive T cells 80 , 81 .
In high-income countries, many older adults receive influenza, pneumococcal and varicella zoster vaccines, although data showing substantial benefits of these vaccines in past few decades in the oldest adults (more than 75 years of age) are lacking. However, emerging data following the recent development and deployment of new-generation, high-dose or adjuvanted influenza vaccines 82 and an adjuvanted glycoprotein varicella zoster vaccine 83 suggest that the provision of additional signals to the immune system by certain adjuvants (such as AS01 and MF59) can overcome immunosenescence. It is now necessary to understand how and why, and to use this knowledge to expand options for vaccine-induced protection at the extremes of life.
Schedule of vaccination
For most vaccines that are used in the first year of life, 3–4 doses are administered by 12 months of age. Conventionally, in human vaccinology, ‘priming’ doses are all those administered at less than 6 months of age and the ‘booster’ dose is given at 9–12 months of age. So, for example, the standard WHO schedule for diphtheria–tetanus–pertussis-containing vaccines (which was introduced in 1974 as part of the Expanded Programme on Immunization 84 ) consists of 3 priming doses at 6, 10 and 14 weeks of age with no booster. This schedule was selected to provide early protection before levels of maternal antibody had waned (maternal antibody has a half-life of around 30–40 days 85 , so very little protection is afforded to infants from the mother beyond 8–12 weeks of age) and because it was known that vaccine compliance is better when doses are given close together. However, infant immunization schedules around the world are highly variable — few high-income or middle-income countries use the Expanded Programme on Immunization schedule — and were largely introduced with little consideration of how best to optimize immune responses. Indeed, schedules that start later at 8–12 weeks of age (when there is less interference from maternal antibody) and have longer gaps between doses (8 weeks rather than 4 weeks) are more immunogenic. A large number of new vaccines have been introduced since 1974 as a result of remarkable developments in technology, but these have generally been fitted into existing schedules without taking into account the optimal scheduling for these new products. The main schedules used globally for diphtheria–tetanus–pertussis vaccine are presented in Supplementary Table 1 , and the changes to the UK immunization schedule since 1963 are presented in Supplementary Table 2 . It should also be noted that surveys show vaccines are rarely delivered on schedule in many countries and, thus, the published schedule may not be how vaccines are actually delivered on the ground. This is particularly the case in remote areas (for example, where health professionals only visit occasionally) and regions with limited or chaotic health systems, leaving children vulnerable to infection.
Safety and side effects of vaccines
Despite the public impression that vaccines are associated with specific safety concerns, the existing data indicate that vaccines are remarkably safe as interventions to defend human health. Common side effects, particularly those associated with the early innate immune response to vaccines, are carefully documented in clinical trials. Although rare side effects might not be identified in clinical trials, vaccine development is tightly controlled and robust post-marketing surveillance systems are in place in many countries, which aim to pick these up if they do occur. This can make the process of vaccine development rather laborious but is appropriate because, unlike most drugs, vaccines are used for prophylaxis in a healthy population and not to treat disease. Perhaps because vaccines work so well and the diseases that they prevent are no longer common, there have been several spurious associations made between vaccines and various unrelated health conditions that occur naturally in the population. Disentangling incorrect claims of vaccine harm from true vaccine-related adverse events requires very careful epidemiological studies.
Common side effects
Licensure of a new vaccine normally requires safety studies involving from 3,000 to tens of thousands of individuals. Thus, common side effects are very well known and are published by the regulator at the time of licensure. Common side effects of many vaccines include injection site pain, redness and swelling and some systemic symptoms such as fever, malaise and headache. All of these side effects, which occur in the first 1–2 days following vaccination, reflect the inflammatory and immune responses that lead to the successful development of vaccine-induced protection. About 6 days after measles–mumps–rubella vaccination, about 10% of 12-month-old infants develop a mild viraemia, which can result in fever and rash, and occasionally febrile convulsions (1 in 3,000) 86 . Although these side effects are self-limiting and relatively mild — and are trivial in comparison with the high morbidity and mortality of the diseases from which the vaccines protect — they can be very worrying for parents and their importance is often underestimated by clinicians who are counselling families about immunization.
Immunodeficiency and vaccination
Most vaccines in current use are inactivated, purified or killed organisms or protein and/or polysaccharide components of a pathogen; as they cannot replicate in the vaccine recipient, they are thus not capable of causing any significant side effects, resulting in very few contraindications for their use. Even in immunocompromised individuals, there is no risk from use of these vaccines, although the induction of immunity may not be possible, depending on the nature of the immune system defect. More caution is required for the use of live attenuated, replicating vaccines (such as yellow fever, varicella zoster, BCG and measles vaccines) in the context of individuals with T cell immunodeficiency as there is a theoretical risk of uncontrolled replication, and live vaccines are generally avoided in this situation 87 . A particular risk of note is from the yellow fever vaccine, which is contraindicated in individuals with T cell immunodeficiency and occasionally causes a severe viscerotropic or neurotropic disease in individuals with thymus disease or after thymectomy, in young infants and adults more than 60 years of age 88 . In individuals with antibody deficiency, there may be some merit in the use of routine live vaccines, as T cell memory may be induced that, although unlikely to prevent future infection, could improve control of the disease if infection occurs.
The myth of antigenic overload
An important parental concern is that vaccines might overwhelm their children’s immune systems. In a telephone survey in the USA, 23% of parents agreed with the statement ‘Children get more immunizations than are good for them’, and 25% indicated that they were concerned that their child’s immune system could be weakened by too many immunizations 89 . However, there is ample evidence to disprove these beliefs. Although the number of vaccines in immunization programmes has increased, the total number of antigens has actually decreased from more than 3,200 to approximately 320 as a result of discontinuing the smallpox vaccine and replacing the whole-cell pertussis vaccine with the acellular vaccine 90 , 91 . Vaccines comprise only a small fraction of the antigens that children are exposed to throughout normal life, with rapid bacterial colonization of the gastrointestinal tract after birth, multiple viral infections and environmental antigens. Moreover, multiple studies have shown that children who received vaccinations had a similar, or even reduced, risk of unconnected infections in the following period 92 , 93 , 94 , 95 . Looking at children who presented to the emergency department with infections not included in the vaccine programme, there was no difference in terms of their previous antigen exposure by vaccination 96 .
Significant rare side effects
Serious side effects from vaccines are very rare, with anaphylaxis being the most common of these rare side effects for parenteral vaccines , occurring after fewer than one in a million doses 97 . Individuals with known allergies (such as egg or latex) should avoid vaccines that may have traces of these products left over from the production process with the specific allergen, although most cases of anaphylaxis are not predictable in advance but are readily managed if vaccines are administered by trained health-care staff.
Very rare side effects of vaccines are not usually observed during clinical development, with very few documented, and they are only recognized through careful surveillance in vaccinated populations. For example, there is a very low risk of idiopathic thrombocytopenic purpura (1 in 24,000 vaccine recipients) after measles vaccination 86 . From 1 in 55,000 to 1 in 16,000 recipients of an AS03-adjuvanted 2009 pandemic H1N1 influenza vaccine 98 , 99 , who had a particular genetic susceptibility (HLA DQB1*0602) 100 , developed narcolepsy , although the debate continues about whether the trigger was the vaccine, the adjuvant or some combination, perhaps with the circulating virus also having a role.
Despite widespread misleading reporting about links between the measles–mumps–rubella vaccine and autism from the end of the 1990s, there is no evidence that any vaccines or their components cause autism 101 , 102 . Indeed, the evidence now overwhelmingly shows that there is no increased risk of autism in vaccinated populations. Thiomersal (also known as thimerosal) is an ethyl mercury-containing preservative that has been used widely in vaccines since the 1930s without any evidence of adverse events associated with it, and there is also no scientific evidence of any link between thiomersal and autism despite spurious claims about this 102 . Thiomersal has been voluntarily withdrawn from most vaccines by manufacturers as a precautionary measure rather than because of any scientific evidence of lack of safety and is currently used mainly in the production of whole-cell pertussis vaccines.
The risk of hospitalization, death or long-term morbidity from the diseases for which vaccines have been developed is so high that the risks of common local and systemic side effects (such as sore arm and fever) and the rare more serious side effects are far outweighed by the massive reductions in disease achieved through vaccination. Continuing assessment of vaccine safety post licensure is important for the detection of rare and longer-term side effects, and efficient reporting systems need to be in place to facilitate this 103 . This is particularly important in a pandemic situation, such as the COVID-19 pandemic, as rapid clinical development of several vaccines is likely to take place and large numbers of people are likely to be vaccinated within a short time.
Challenges to vaccination success
Vaccines only work if they are used. Perhaps the biggest challenge to immunization programmes is ensuring that the strong headwinds against deployment, ranging from poor infrastructure and lack of funding to vaccine hesitancy and commercial priorities, do not prevent successful protection of the most vulnerable in society. It is noteworthy that these are not classical scientific challenges, although limited knowledge about which antigens are protective, which immune responses are needed for protection and how to enhance the right immune responses, particularly in the older population, are also important considerations.
Access to vaccines
The greatest challenge for protection of the human population against serious infectious disease through vaccination remains access to vaccines and the huge associated inequity in access. Access to vaccines is currently limited, to varying degrees in different regions, by the absence of a health infrastructure to deliver vaccines, the lack of convenient vaccine provision for families, the lack of financial resources to purchase available vaccines (at a national, local or individual level) and the marginalization of communities in need. This is perhaps the most pressing issue for public health, with global vaccine coverage having stalled; for example, coverage for diphtheria–tetanus–pertussis-containing vaccines has only risen from 84% to 86% since 2010 (ref. 104 ). However, this figure hides huge regional variation, with near 100% coverage in some areas and almost no vaccinated children in others. For the poorest countries in the world, Gavi, the Vaccine Alliance provides funding to assist with new vaccine introductions and has greatly accelerated the broadening of access to new vaccines that were previously only accessible to high-income countries. However, this still leaves major financial challenges for countries that do not meet the criteria to be eligible for Gavi funding but still cannot afford new vaccines. Inequity remains, with approximately 14 million children not receiving any vaccinations and another 5.7 million children being only partially vaccinated in 2019 (ref. 105 ).
Other important issues can compromise vaccine availability and access. For example, most vaccines must be refrigerated at 2–8 °C, requiring the infrastructure and capacity for cold storage and a cold chain to the clinic where the vaccine is delivered, which is limited in many low-income countries. The route of administration can also limit access; oral vaccines (such as rotavirus, polio or cholera vaccines) and nasal vaccines (such as live attenuated influenza vaccine) can be delivered rapidly on a huge scale by less-skilled workers, whereas most vaccines are injected, which requires more training to administer and takes longer. Nevertheless, these hurdles can be overcome: in Sindh Province, Pakistan, 10 million doses of injected typhoid conjugate vaccine were administered to children to control an outbreak of extensively drug-resistant typhoid in just a few weeks at the end of 2019 (ref. 106 ).
The anti-vaccination movement
Despite access being the main issue affecting global vaccine coverage, a considerable focus is currently on the challenges posed by the anti-vaccination movement, largely as a result of worrying trends of decreasing vaccine coverage in high-income settings, leading to outbreaks of life-threatening infectious diseases, such as measles. In 2018, there were 140,000 deaths from measles worldwide, and the number of cases in 2019 was the highest in any year since 2006 (ref. 107 ). Much has been written about the dangerous role of social media and online search engines in the spread of misinformation about vaccines and the rise of the anti-vaccination movement, but scientists are also at fault for failing to effectively communicate the benefits of vaccination to a lay public. If this is to change, scientists do not need to counter or engage with the anti-vaccination movement but to use their expertise and understanding to ensure effective communication about the science that underpins our remarkable ability to harness the power of the immune system through vaccination to defend the health of our children.
Commercial viability
A third important issue is the lack of vaccines for some diseases for which there is no commercial incentive for development. Typically, these are diseases that have a restricted geographical spread (such as Rift Valley fever, Ebola, Marburg disease or plague) or occur in sporadic outbreaks and only affect poor or displaced communities (such as Ebola and cholera). Lists of outbreak pathogens have been published by various agencies including the WHO 108 , and recent funding initiatives, including those from US and European governments, have increased investment in the development of orphan vaccines . The Coalition for Epidemic Preparedness Innovations (CEPI) is set to have a major role in funding and driving the development of vaccines against these pathogens.
Immunological challenges
For other pathogens, there is likely to be a commercial market but there are immunological challenges for the development of new vaccines. For example, highly variable pathogens, including some with a large global distribution such as HIV and hepatitis C virus, pose a particular challenge. The genetic diversity of these pathogens, which occurs both between and within hosts, makes it difficult to identify an antigen that can be used to immunize against infection. In the case of HIV, antibodies can be generated that neutralize the virus, but the rapid mutation of the viral genome means that the virus can evade these responses within the same host. Some individuals do produce broadly neutralizing antibodies naturally, which target more conserved regions of the virus, leading to viral control, but it is not clear how to robustly induce these antibodies with a vaccine. Indeed, several HIV vaccines have been tested in clinical trials that were able to induce antibody responses (for example, RV144 vaccine showed 31% protection 109 ) and/or T cell responses, but these vaccines have not shown consistent evidence of protection in follow-up studies, and several studies found an increased risk of infection among vaccine recipients 110 .
For other pathogens, such as Neisseria gonorrhoeae (which causes gonorrhoea) and Treponema pallidum (which causes syphilis), antigenic targets for protective immune responses have not yet been determined, partly owing to limited investment and a poor understanding of the mechanisms of immunity at mucosal surfaces, or have thus far only resulted in limited protection. For example, the licensed malaria vaccine, RTSS, provides only 30–40% protection and further work is needed to develop suitable products 111 . New malaria vaccines in development target more conserved antigens on the parasite surface or target different stages of the parasite life cycle. Combinations of these approaches in a vaccine (perhaps targeting multiple stages of the life cycle), together with anti-vector strategies such as the use of genetically modified mosquitoes or Wolbachia bacteria to infect mosquitoes and reduce their ability to carry mosquito parasites 112 , as well as mosquito-bite avoidance, have the potential to markedly reduce malaria parasite transmission.
Seasonal influenza vaccines have, in recent decades, been used to protect vulnerable individuals in high-income countries, including older adults, children and individuals with co-morbidities that increase risk of severe influenza. These vaccines are made from virus that is grown in eggs; purified antigen, split virions or whole virions can be included in the final vaccine product. The vaccines take around 6 months to manufacture and have highly variable efficacy from one season to another, partly owing to the difficulty in predicting which virus strain will be circulating in the next influenza season, so that the vaccine strain may not match the strain causing disease 113 . Another issue that is increasingly recognized is egg adaptation, whereby the vaccine strain of virus becomes adapted to the egg used for production, leading to key mutations that mean it is not well matched to, and does not protect against, the circulating viral strain 114 . Vaccine-induced protection might be improved by the development of mammalian or insect cell-culture systems for growing influenza virus to avoid egg adaptation, and the use of MF59-adjuvanted vaccines and high-dose influenza vaccines to improve immune responses. Because of the cost of purchasing seasonal influenza vaccines annually, and the problem of antigenic variability, the search for a universal influenza vaccine receives considerable attention, with a particular focus on vaccines that induce T H cells or antibodies to conserved epitopes 115 , but there are currently no products in late-stage development.
Although BCG is the most widely used vaccine globally, with 89% of the world population receiving it in 2018 (ref. 105 ), there is still a huge global burden of TB and it is clear that more effective TB vaccines are needed. However, the optimal characteristics of a prophylactic TB vaccine, which antigens should be included and the nature of protective immunity remain unknown, despite more than 100 years of TB vaccine research. A viral vector expressing a TB protein, 85A, has been tested in a large TB-prevention trial in South Africa but this vaccine did not show protection, which was attributed by the authors to poor immunogenicity in the vaccinated children 116 . However, the publication of a study in 2019 showing that a novel TB vaccine, M72/AS01E (an AS01-adjuvanted vaccine containing the M. tuberculosis antigens MTB32A and MTB39A), could limit progression to active TB disease in latently infected individuals with efficacy of 50% over 3 years gives a glimmer of hope that TB control may be realized in the future by novel vaccine approaches 117 . Questions remain about the duration of the effect, but the demonstrated efficacy can now be interrogated thoroughly to determine the nature of protective immunity against TB.
Future vaccine development
There are several important diseases for which new vaccines are needed to reduce morbidity and mortality globally, which are likely to have a market in both high-income and low-income countries, including vaccines for group B Streptococcus (a major cause of neonatal meningitis), RSV and CMV. Group B Streptococcus vaccines are currently in trials of maternal vaccination, with the aim of inducing maternal antibodies that cross the placenta and protect the newborn passively 118 . RSV causes a lower respiratory tract infection, bronchiolitis, in infancy and is the commonest cause of infant hospitalization in developed countries and globally one of the leading causes of death in those less than 12 months of age. As many as 60 new RSV vaccine candidates are in development as either maternal vaccines or infant vaccines, or involving immunization with RSV-specific monoclonal antibodies that have an extended half-life. A licensed RSV vaccine would have a huge impact on infant health and paediatric hospital admissions. CMV is a ubiquitous herpesvirus that is responsible for a significant burden of disease in infants; 15–20% of congenitally infected children develop long-term sequelae, most importantly sensorineural hearing loss, and CMV thus causes more congenital disease than any other single infectious agent. A vaccine that effectively prevents congenital infection would provide significant individual and public health benefits. A lack of understanding of the nature of protective immunity against CMV has hampered vaccine development in the past, but the pipeline is now more promising 119 , 120 .
Another major line of development of new vaccines is to combat hospital-acquired infections, particularly with antibiotic-resistant Gram-positive bacteria (such as Staphylococcus aureus ) that are associated with wound infections and intravenous catheters and various Gram-negative organisms (such as Klebsiella spp. and Pseudomonas aeruginosa ). Progress has been slow in this field and an important consideration will be targeting products to the at-risk patient groups before hospital admission or surgery.
Perhaps the largest area of growth for vaccine development is for older adults, with few products aimed specifically at this population currently. With the population of older adults set to increase substantially (the proportion of the population who are more than 60 years of age is expected to increase from 12% to 22% by 2050 (ref. 121 )), prevention of infection in this population should be a public health priority. Efforts to better understand immunosenescence and how to improve vaccine responses in the oldest adults are a major challenge for immunologists today.
Novel technologies
Important challenges to overcome in the following years are genetic diversity (for example, of viruses such as HIV, hepatitis C virus and influenza), the requirement for a broader immune response including T cells for protection against diseases such as TB and malaria, and the need to swiftly respond to emerging pathogens and outbreak situations. Traditionally, vaccine development takes more than 10 years 122 , but the COVID-19 pandemic has demonstrated the urgency for vaccine technologies that are flexible and facilitate rapid development, production and upscaling 123 .
Novel technologies to combat these hurdles will include platforms that allow for improved antigen delivery and ease and speed of production, application of structural biology and immunological knowledge to aid enhanced antigen design and discovery of better adjuvants to improve immunogenicity. Fortunately, recent advances in immunology, systems biology, genomics and bio-informatics offer great opportunities to improve our understanding of the induction of immune responses by vaccines and to transform vaccine development through increasingly rational design 124 .
New platforms include viral vectored vaccines and nucleic acid-based vaccines. Antigen-presenting cells such as dendritic cells, T cell-based vaccines and bacterial vectors are being explored as well, but are still at early stages of development for use against infectious pathogens. Whereas classic whole-organism vaccine platforms require the cultivation of the pathogen, next-generation viral vectored or nucleic acid-based vaccines can be constructed using the pathogen genetic sequence only, thereby significantly increasing the speed of development and manufacturing processes 125 .
Viral vectored vaccines are based on a recombinant virus (either replicating or not), in which the genome is altered to express the target pathogen antigen. The presentation of pathogen antigens in combination with stimuli from the viral vector that mimic natural infection leads to the induction of strong humoral and cellular immune responses without the need for an adjuvant. A potential disadvantage of viral vectored vaccines is the presence of pre-existing immunity when a vector such as human adenovirus is used that commonly causes infection in humans. This can be overcome by using vectors such as a simian adenovirus, against which almost no pre-existing immunity exists in humans 126 . Whether immune responses against the vector will limit its use for repeated vaccinations with different antigens will need to be investigated.
Nucleic acid-based vaccines consist of either DNA or RNA encoding the target antigen, which potentially allows for the induction of both humoral and cellular immune responses once the encoded antigens are expressed by the vaccine recipient after uptake of the nucleic acid by their cells. A huge advantage of these vaccines is that they are highly versatile and quick and easy to adapt and produce in the case of an emerging pathogen. Indeed, the SARS-CoV-2 mRNA-based vaccine mRNA-1273 entered clinical testing just 2 months after the genetic sequence of SARS-CoV-2 was identified 127 and the BNT162b2 lipid nanoparticle-formulated, nucleoside-modified RNA vaccine was the first SARS-CoV-2 vaccine to be licensed 128 . One of the disadvantages of these vaccines is that they need to be delivered directly into cells, which requires specific injection devices, electroporation or a carrier molecule and brings with it a risk of low transfection rate and limited immunogenicity 129 . Furthermore, the application of RNA vaccines has been limited by their lack of stability and requirement for a cold chain, but constant efforts to improve formulations hold promise to overcome these limitations 130 , 131 .
A beautiful example of how immunological insight can revolutionize vaccine development is the novel RSV vaccine DS-Cav1. The RSV surface fusion (F) protein can exist in either a pre-fusion (pre-F) conformation, which facilitates viral entry, or a post-fusion (post-F) form. Whereas previous vaccines mainly contained the post-F form, insight into the atomic-level structure of the protein has allowed for stable expression of the pre-F protein, leading to strongly enhanced immune responses and providing a proof of concept for structure-based vaccine design 132 , 133 .
In addition to the novel vaccine platforms mentioned above, there are ongoing efforts to develop improved methods of antigen delivery, such as liposomes (spherical lipid bilayers), polymeric particles, inorganic particles, outer membrane vesicles and immunostimulating complexes. These, and other methods such as self-assembling protein nanoparticles, have the potential to optimally enhance and skew the immune response to pathogens against which traditional vaccine approaches have proven to be unsuccessful 129 , 134 . Furthermore, innovative delivery methods, such as microneedle patches, are being developed, with the potential advantages of improved thermostability, ease of delivery with minimal pain and safer administration and disposal 135 . An inactivated influenza vaccine delivered by microneedle patch was shown to be well tolerated and immunogenic in a phase I trial 136 . This might allow for self-administration, although it would be important for professional medical care to be available if there is a risk of severe side effects such as anaphylaxis.
Conclusions and future directions
Immunization protects populations from diseases that previously claimed the lives of millions of individuals each year, mostly children. Under the United Nations Convention on the Rights of the Child, every child has the right to the best possible health, and by extrapolation a right to be vaccinated.
Despite the outstanding success of vaccination in protecting the health of our children, there are important knowledge gaps and challenges to be addressed. An incomplete understanding of immune mechanisms of protection and the lack of solutions to overcome antigenic variability have hampered the design of effective vaccines against major diseases such as HIV/AIDS and TB. Huge efforts have resulted in the licensure of a partially effective vaccine against malaria, but more effective vaccines will be needed to defeat this disease. Moreover, it is becoming clear that variation in host response is an important factor to take into account. New technologies and analytical methods will aid the delineation of the complex immune mechanisms involved, and this knowledge will be important to design effective vaccines for the future.
Apart from the scientific challenges, sociopolitical barriers stand in the way of safe and effective vaccination for all. Access to vaccines is one of the greatest obstacles, and improving infrastructure, continuing education and enhancing community engagement will be essential to improve this, and novel delivery platforms that eliminate the need for a cold chain could have great implications. There is a growing subset of the population who are sceptical about vaccination and this requires a response from the scientific community to provide transparency about the existing knowledge gaps and strategies to overcome these. Constructive collaboration between scientists and between scientific institutions, governments and industry will be imperative to move forwards. The COVID-19 pandemic has indeed shown that, in the case of an emergency, many parties with different incentives can come together to ensure that vaccines are being developed at unprecedented speed but has also highlighted some of the challenges of national and commercial interests. As immunologists, we have a responsibility to create an environment where immunization is normal, the science is accessible and robust, and access to vaccination is a right and expectation.
Change history
05 january 2021.
A Correction to this paper has been published: https://doi.org/10.1038/s41577-020-00497-5.
World Health Organization. Global vaccine action plan 2011–2020. WHO https://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/ (2013).
World Health Organization. Child mortality and causes of death. WHO https://www.who.int/gho/child_health/mortality/mortality_under_five_text/en/ (2020).
Hatherill, M., White, R. G. & Hawn, T. R. Clinical development of new TB vaccines: recent advances and next steps. Front. Microbiol. 10 , 3154 (2019).
Article PubMed Google Scholar
Bekker, L. G. et al. The complex challenges of HIV vaccine development require renewed and expanded global commitment. Lancet 395 , 384–388 (2020).
Matz, K. M., Marzi, A. & Feldmann, H. Ebola vaccine trials: progress in vaccine safety and immunogenicity. Expert Rev. Vaccines 18 , 1229–1242 (2019).
Article CAS PubMed Google Scholar
Ahmed, S. F., Quadeer, A. A. & McKay, M. R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses 12 , 254 (2020).
Article CAS PubMed Central Google Scholar
Pawelec, G. Age and immunity: what is “immunosenescence”? Exp. Gerontol. 105 , 4–9 (2018).
Larson, H. J. The state of vaccine confidence. Lancet 392 , 2244–2246 (2018).
Robbins, J. B. et al. Prevention of invasive bacterial diseases by immunization with polysaccharide–protein conjugates. Curr. Top. Microbiol. Immunol. 146 , 169–180 (1989).
CAS PubMed Google Scholar
Plotkin, S. A. Updates on immunologic correlates of vaccine-induced protection. Vaccine 38 , 2250–2257 (2020). This paper presents a review of immune correlates of protection for specific infections, their immunological basis and relevance for vaccinology .
Rubin, L. G. et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin. Infect. Dis. 58 , e44–e100 (2014).
Milligan, R., Paul, M., Richardson, M. & Neuberger, A. Vaccines for preventing typhoid fever. Cochrane Database Syst. Rev. 5 , CD001261 (2018).
PubMed Google Scholar
WHO. Measles vaccines: WHO position paper — April 2017. Wkly. Epidemiol. Rec. 92 , 205–227 (2017).
Google Scholar
Rappuoli, R., Mandl, C. W., Black, S. & De Gregorio, E. Vaccines for the twenty-first century society. Nat. Rev. Immunol. 11 , 865–872 (2011). This paper presents a review of the role of vaccines in the twenty-first century, with an emphasis on increased life expectancy, emerging infections and poverty .
Article CAS PubMed PubMed Central Google Scholar
Marrack, P., McKee, A. S. & Munks, M. W. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 9 , 287–293 (2009).
Wilkins, A. L. et al. AS03- and MF59-adjuvanted influenza vaccines in children. Front. Immunol. 8 , 1760 (2017).
Article PubMed PubMed Central CAS Google Scholar
Kaslow, D. C. & Biernaux, S. RTS,S: toward a first landmark on the Malaria Vaccine Technology Roadmap. Vaccine 33 , 7425–7432 (2015).
Pedersen, C. et al. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J. Adolesc. Health 40 , 564–571 (2007).
Mitkus, R. J., Hess, M. A. & Schwartz, S. L. Pharmacokinetic modeling as an approach to assessing the safety of residual formaldehyde in infant vaccines. Vaccine 31 , 2738–2743 (2013).
Eldred, B. E., Dean, A. J., McGuire, T. M. & Nash, A. L. Vaccine components and constituents: responding to consumer concerns. Med. J. Aust. 184 , 170–175 (2006).
Fijen, C. A., Kuijper, E. J., te Bulte, M. T., Daha, M. R. & Dankert, J. Assessment of complement deficiency in patients with meningococcal disease in The Netherlands. Clin. Infect. Dis. 28 , 98–105 (1999).
Wara, D. W. Host defense against Streptococcus pneumoniae : the role of the spleen. Rev. Infect. Dis. 3 , 299–309 (1981).
Gershon, A. A. et al. Varicella zoster virus infection. Nat. Rev. Dis. Prim. 1 , 15016 (2015).
Sandmann, F. et al. Infant hospitalisations and fatalities averted by the maternal pertussis vaccination programme in England, 2012–2017: post-implementation economic evaluation. Clin. Infect. Dis. 71 , 1984–1987 (2020).
Demicheli, V., Barale, A. & Rivetti, A. Vaccines for women for preventing neonatal tetanus. Cochrane Database Syst. Rev. 7 , CD002959 (2015).
Madhi, S. A. et al. Influenza vaccination of pregnant women and protection of their infants. N. Engl. J. Med. 371 , 918–931 (2014).
Article PubMed CAS Google Scholar
Madhi, S. A. & Dangor, Z. Prospects for preventing infant invasive GBS disease through maternal vaccination. Vaccine 35 , 4457–4460 (2017).
Madhi, S. A. et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N. Engl. J. Med. 383 , 426–439 (2020).
Young, M. K. & Cripps, A. W. Passive immunization for the public health control of communicable diseases: current status in four high-income countries and where to next. Hum. Vaccin. Immunother. 9 , 1885–1893 (2013).
Patel, M. & Lee, C. K. Polysaccharide vaccines for preventing serogroup A meningococcal meningitis. Cochrane Database Syst. Rev. 3 , CD001093 (2005).
Moberley, S., Holden, J., Tatham, D. P. & Andrews, R. M. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst. Rev. 1 , CD000422 (2013).
Andrews, N. J. et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect. Dis. 14 , 839–846 (2014).
Borrow, R., Abad, R., Trotter, C., van der Klis, F. R. & Vazquez, J. A. Effectiveness of meningococcal serogroup C vaccine programmes. Vaccine 31 , 4477–4486 (2013).
Ramsay, M. E., McVernon, J., Andrews, N. J., Heath, P. T. & Slack, M. P. Estimating haemophilus influenzae type b vaccine effectiveness in England and Wales by use of the screening method. J. Infect. Dis. 188 , 481–485 (2003).
Pollard, A. J., Perrett, K. P. & Beverley, P. C. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat. Rev. Immunol. 9 , 213–220 (2009). This paper presents a review of the mechanism of action of polysaccharide vaccines and their role in establishing long-term protection against invasive bacteria .
Darton, T. C. et al. Design, recruitment, and microbiological considerations in human challenge studies. Lancet Infect. Dis. 15 , 840–851 (2015). This paper presents an overview of human challenge models, their potential use and their role in improving our understanding of disease mechanisms and host responses .
Suscovich, T. J. et al. Mapping functional humoral correlates of protection against malaria challenge following RTS, S/AS01 vaccination. Sci. Transl Med. 12 , eabb4757 (2020).
Jin, C. et al. Efficacy and immunogenicity of a Vi–tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi : a randomised controlled, phase 2b trial. Lancet 390 , 2472–2480 (2017).
Kourtis, A. P., Read, J. S. & Jamieson, D. J. Pregnancy and infection. N. Engl. J. Med. 370 , 2211–2218 (2014).
Malley, R. et al. CD4 + T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl Acad. Sci. USA 102 , 4848–4853 (2005).
Henry, B. & Baclic, O. & National Advisory Committee on Immunization (NACI). Summary of the NACI update on the recommended use of hepatitis B vaccine. Can. Commun. Dis. Rep. 43 , 104–106 (2017).
Kelly, D. F., Pollard, A. J. & Moxon, E. R. Immunological memory: the role of B cells in long-term protection against invasive bacterial pathogens. JAMA 294 , 3019–3023 (2005).
McVernon, J., Johnson, P. D., Pollard, A. J., Slack, M. P. & Moxon, E. R. Immunologic memory in Haemophilus influenzae type b conjugate vaccine failure. Arch. Dis. Child. 88 , 379–383 (2003).
McVernon, J. et al. Immunologic memory with no detectable bactericidal antibody response to a first dose of meningococcal serogroup C conjugate vaccine at four years. Pediatr. Infect. Dis. J. 22 , 659–661 (2003).
World Health Organization. Tetanus vaccines: WHO position paper, February 2017 — recommendations. Vaccine 36 , 3573–3575 (2018).
Article Google Scholar
World Health Organization. Diphtheria vaccine: WHO position paper, August 2017 — recommendations. Vaccine 36 , 199–201 (2018).
Chen, Z. & He, Q. Immune persistence after pertussis vaccination. Hum. Vaccin. Immunother. 13 , 744–756 (2017).
Article PubMed PubMed Central Google Scholar
Burdin, N., Handy, L. K. & Plotkin, S. A. What is wrong with pertussis vaccine immunity? The problem of waning effectiveness of pertussis vaccines. Cold Spring Harb. Perspect. Biol. 9 , a029454 (2017).
WHO. Vaccines and vaccination against yellow fever: WHO Position Paper, June 2013 — recommendations. Vaccine 33 , 76–77 (2015).
Paunio, M. et al. Twice vaccinated recipients are better protected against epidemic measles than are single dose recipients of measles containing vaccine. J. Epidemiol. Community Health 53 , 173–178 (1999).
Zhu, S., Zeng, F., Xia, L., He, H. & Zhang, J. Incidence rate of breakthrough varicella observed in healthy children after 1 or 2 doses of varicella vaccine: results from a meta-analysis. Am. J. Infect. Control. 46 , e1–e7 (2018).
Halstead, S. B., Rojanasuphot, S. & Sangkawibha, N. Original antigenic sin in dengue. Am. J. Trop. Med. Hyg. 32 , 154–156 (1983).
Kim, J. H., Skountzou, I., Compans, R. & Jacob, J. Original antigenic sin responses to influenza viruses. J. Immunol. 183 , 3294–3301 (2009).
Vatti, A. et al. Original antigenic sin: a comprehensive review. J. Autoimmun. 83 , 12–21 (2017).
Statista Research Department. Herd immunity threshold for selected global diseases as of 2013. Statista https://www.statista.com/statistics/348750/threshold-for-herd-immunity-for-select-diseases/ (2013).
Plans-Rubio, P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev. Med. 55 , 72–77 (2012).
Trotter, C. L., Andrews, N. J., Kaczmarski, E. B., Miller, E. & Ramsay, M. E. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 364 , 365–367 (2004).
Trotter, C. L. & Maiden, M. C. Meningococcal vaccines and herd immunity: lessons learned from serogroup C conjugate vaccination programs. Expert. Rev. Vaccines 8 , 851–861 (2009).
Tabrizi, S. N. et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect. Dis. 14 , 958–966 (2014).
Brisson, M. et al. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Public Health 1 , e8–e17 (2016).
Trunz, B. B., Fine, P. & Dye, C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 367 , 1173–1180 (2006).
Barker, L. & Hussey, G. The Immunological Basis for Immunization Series: Module 5: Tuberculosis (World Health Organization, 2011).
Eisenhut, M. et al. BCG vaccination reduces risk of infection with Mycobacterium tuberculosis as detected by γ interferon release assay. Vaccine 27 , 6116–6120 (2009).
Verrall, A. J. et al. Early clearance of Mycobacterium tuberculosis : the INFECT case contact cohort study in Indonesia. J. Infect. Dis. 221 , 1351–1360 (2020).
Pollard, A. J., Finn, A. & Curtis, N. Non-specific effects of vaccines: plausible and potentially important, but implications uncertain. Arch. Dis. Child. 102 , 1077–1081 (2017).
Higgins, J. P. et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ 355 , i5170 (2016).
Mina, M. J. et al. Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science 366 , 599–606 (2019).
Mina, M. J., Metcalf, C. J., de Swart, R. L., Osterhaus, A. D. & Grenfell, B. T. Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science 348 , 694–699 (2015). This paper is an analysis of population-level data from high-income countries, showing a protective effect of measles vaccination on mortality from non-measles infectious diseases .
Falsey, A. R., Treanor, J. J., Tornieporth, N., Capellan, J. & Gorse, G. J. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J. Infect. Dis. 200 , 172–180 (2009).
DiazGranados, C. A. et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 371 , 635–645 (2014).
Schnyder, J. L. et al. Fractional dose of intradermal compared to intramuscular and subcutaneous vaccination—a systematic review and meta-analysis. Travel. Med. Infect. Dis. 37 , 101868 (2020).
Voysey, M. et al. The influence of maternally derived antibody and infant age at vaccination on infant vaccine responses: an individual participant meta-analysis. JAMA Pediatr. 171 , 637–646 (2017).
Caceres, V. M., Strebel, P. M. & Sutter, R. W. Factors determining prevalence of maternal antibody to measles virus throughout infancy: a review. Clin. Infect. Dis. 31 , 110–119 (2000).
Belnoue, E. et al. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood 111 , 2755–2764 (2008).
Pace, D. et al. Immunogenicity of reduced dose priming schedules of serogroup C meningococcal conjugate vaccine followed by booster at 12 months in infants: open label randomised controlled trial. BMJ 350 , h1554 (2015).
Timens, W., Boes, A., Rozeboom-Uiterwijk, T. & Poppema, S. Immaturity of the human splenic marginal zone in infancy. Possible contribution to the deficient infant immune response. J. Immunol. 143 , 3200–3206 (1989).
Peset Llopis, M. J., Harms, G., Hardonk, M. J. & Timens, W. Human immune response to pneumococcal polysaccharides: complement-mediated localization preferentially on CD21-positive splenic marginal zone B cells and follicular dendritic cells. J. Allergy Clin. Immunol. 97 , 1015–1024 (1996).
Claesson, B. A. et al. Protective levels of serum antibodies stimulated in infants by two injections of Haemophilus influenzae type b capsular polysaccharide–tetanus toxoid conjugate. J. Pediatr. 114 , 97–100 (1989).
Crooke, S. N., Ovsyannikova, I. G., Poland, G. A. & Kennedy, R. B. Immunosenescence and human vaccine immune responses. Immun. Ageing 16 , 25 (2019).
Nikolich-Žugich, J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat. Rev. Immunol. 8 , 512–522 (2008).
Kadambari, S., Klenerman, P. & Pollard, A. J. Why the elderly appear to be more severely affected by COVID-19: the potential role of immunosenescence and CMV. Rev. Med. Virol. 30 , e2144 (2020).
Domnich, A. et al. Effectiveness of MF59-adjuvanted seasonal influenza vaccine in the elderly: a systematic review and meta-analysis. Vaccine 35 , 513–520 (2017).
Lal, H. et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 372 , 2087–2096 (2015).
World Health Assembly. The Expanded Programme on Immunization: the 1974 resolution by the World Health Assembly. Assign. Child. 69-72 , 87–88 (1985).
Voysey, M., Pollard, A. J., Sadarangani, M. & Fanshawe, T. R. Prevalence and decay of maternal pneumococcal and meningococcal antibodies: a meta-analysis of type-specific decay rates. Vaccine 35 , 5850–5857 (2017).
Farrington, P. et al. A new method for active surveillance of adverse events from diphtheria/tetanus/pertussis and measles/mumps/rubella vaccines. Lancet 345 , 567–569 (1995).
Pinto, M. V., Bihari, S. & Snape, M. D. Immunisation of the immunocompromised child. J. Infect. 72 (Suppl), S13–S22 (2016).
Seligman, S. J. Risk groups for yellow fever vaccine-associated viscerotropic disease (YEL-AVD). Vaccine 32 , 5769–5775 (2014).
Gellin, B. G., Maibach, E. W. & Marcuse, E. K. Do parents understand immunizations? A national telephone survey. Pediatrics 106 , 1097–1102 (2000).
Offit, P. A. et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics 109 , 124–129 (2002).
Centers for Disease Control and Prevention. Multiple vaccinations at once. CDC https://www.cdc.gov/vaccinesafety/concerns/multiple-vaccines-immunity.html (2020).
Stowe, J., Andrews, N., Taylor, B. & Miller, E. No evidence of an increase of bacterial and viral infections following measles, mumps and rubella vaccine. Vaccine 27 , 1422–1425 (2009).
Otto, S. et al. General non-specific morbidity is reduced after vaccination within the third month of life — the Greifswald study. J. Infect. 41 , 172–175 (2000).
Griffin, M. R., Taylor, J. A., Daugherty, J. R. & Ray, W. A. No increased risk for invasive bacterial infection found following diphtheria–tetanus–pertussis immunization. Pediatrics 89 , 640–642 (1992).
Aaby, P. et al. Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. BMJ 311 , 481–485 (1995).
Glanz, J. M. et al. Association between estimated cumulative vaccine antigen exposure through the first 23 months of life and non-vaccine-targeted infections from 24 through 47 months of age. JAMA 319 , 906–913 (2018).
Bohlke, K. et al. Risk of anaphylaxis after vaccination of children and adolescents. Pediatrics 112 , 815–820 (2003).
Nohynek, H. et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS ONE 7 , e33536 (2012).
Miller, E. et al. Risk of narcolepsy in children and young people receiving AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine: retrospective analysis. BMJ 346 , f794 (2013).
Hallberg, P. et al. Pandemrix-induced narcolepsy is associated with genes related to immunity and neuronal survival. EBioMedicine 40 , 595–604 (2019).
DeStefano, F. & Shimabukuro, T. T. The MMR vaccine and autism. Annu. Rev. Virol. 6 , 585–600 (2019).
DeStefano, F., Bodenstab, H. M. & Offit, P. A. Principal controversies in vaccine safety in the United States. Clin. Infect. Dis. 69 , 726–731 (2019).
Moro, P. L., Haber, P. & McNeil, M. M. Challenges in evaluating post-licensure vaccine safety: observations from the Centers for Disease Control and Prevention. Expert Rev. Vaccines 18 , 1091–1101 (2019).
Peck, M. et al. Global routine vaccination coverage, 2018. MMWR Morb. Mortal. Wkly. Rep. 68 , 937–942 (2019).
World Health Organization. Immunization coverage. WHO https://www.who.int/news-room/fact-sheets/detail/immunization-coverage (2020).
World Health Organization. More than 9.4 million children vaccinated against typhoid fever in Sindh. WHO http://www.emro.who.int/pak/pakistan-news/more-than-94-children-vaccinated-with-typhoid-conjugate-vaccine-in-sindh.html (2019).
World Health Organization. More than 140,000 die from measles as cases surge worldwide. WHO https://www.who.int/news-room/detail/05-12-2019-more-than-140-000-die-from-measles-as-cases-surge-worldwide (2019).
World Health Organization. Disease outbreaks. WHO https://www.who.int/emergencies/diseases/en/ (2020).
Rerks-Ngarm, S. et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361 , 2209–2220 (2009).
Fauci, A. S., Marovich, M. A., Dieffenbach, C. W., Hunter, E. & Buchbinder, S. P. Immunology. Immune activation with HIV vaccines. Science 344 , 49–51 (2014).
Agnandji, S. T. et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 367 , 2284–2295 (2012).
Killeen, G. F. et al. Developing an expanded vector control toolbox for malaria elimination. BMJ Glob. Health 2 , e000211 (2017).
Osterholm, M. T., Kelley, N. S., Sommer, A. & Belongia, E. A. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 12 , 36–44 (2012).
Skowronski, D. M. et al. Low 2012–13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS ONE 9 , e92153 (2014).
Raymond, D. D. et al. Conserved epitope on influenza-virus hemagglutinin head defined by a vaccine-induced antibody. Proc. Natl Acad. Sci. USA 115 , 168–173 (2018).
Tameris, M. D. et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381 , 1021–1028 (2013).
Tait, D. R. et al. Final analysis of a trial of M72/AS01(E) vaccine to prevent tuberculosis. N. Engl. J. Med. 381 , 2429–2439 (2019).
Kobayashi, M. et al. WHO consultation on group B streptococcus vaccine development: report from a meeting held on 27–28 April 2016. Vaccine 37 , 7307–7314 (2019).
Inoue, N., Abe, M., Kobayashi, R. & Yamada, S. Vaccine development for cytomegalovirus. Adv. Exp. Med. Biol. 1045 , 271–296 (2018).
Schleiss, M. R., Permar, S. R. & Plotkin, S. A. Progress toward development of a vaccine against congenital cytomegalovirus infection. Clin. Vaccine Immunol. 24 , e00268–e00317 (2017).
CAS PubMed PubMed Central Google Scholar
World Health Organization. Ageing and health. WHO https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (2018).
Rauch, S., Jasny, E., Schmidt, K. E. & Petsch, B. New vaccine technologies to combat outbreak situations. Front. Immunol. 9 , 1963 (2018).
Jeyanathan, M. et al. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 20 , 615–632 (2020). This paper is an overview of COVID-19 vaccine development, with emphasis on underlying immunological mechanisms and potential scenarios for global development .
Koff, W. C. & Schenkelberg, T. The future of vaccine development. Vaccine 38 , 4485–4486 (2020).
van Riel, D. & de Wit, E. Next-generation vaccine platforms for COVID-19. Nat. Mater. 19 , 810–812 (2020).
Rollier, C. S., Reyes-Sandoval, A., Cottingham, M. G., Ewer, K. & Hill, A. V. Viral vectors as vaccine platforms: deployment in sight. Curr. Opin. Immunol. 23 , 377–382 (2011).
Corbett, K. S. et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 586 , 567–571 (2020).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2034577 (2020).
Wallis, J., Shenton, D. P. & Carlisle, R. C. Novel approaches for the design, delivery and administration of vaccine technologies. Clin. Exp. Immunol. 196 , 189–204 (2019).
Zhang, C., Maruggi, G., Shan, H. & Li, J. Advances in mRNA vaccines for infectious diseases. Front. Immunol. 10 , 594 (2019).
Pardi, N., Hogan, M. J., Porter, F. W. & Weissman, D. mRNA vaccines — a new era in vaccinology. Nat. Rev. Drug Discov. 17 , 261–279 (2018).
Crank, M. C. et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science 365 , 505–509 (2019). This paper presents a phase I trial demonstrating enhanced immunogenicity of the pre-F conformation of RSV surface protein, thereby providing a proof of concept for successful structure-based vaccine design .
Mascola, J. R. & Fauci, A. S. Novel vaccine technologies for the 21st century. Nat. Rev. Immunol. 20 , 87–88 (2020).
Kanekiyo, M., Ellis, D. & King, N. P. New vaccine design and delivery technologies. J. Infect. Dis. 219 , S88–S96 (2019).
Peyraud, N. et al. Potential use of microarray patches for vaccine delivery in low- and middle-income countries. Vaccine 37 , 4427–4434 (2019).
Rouphael, N. G. et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 390 , 649–658 (2017).
Davenport, R. J., Satchell, M. & Shaw-Taylor, L. M. W. The geography of smallpox in England before vaccination: a conundrum resolved. Soc. Sci. Med. 206 , 75–85 (2018).
Download references
Acknowledgements
The authors thank all those whose work in the development, policy and delivery of vaccines underpins immunization programmes to defend our health and the health of our children.
Author information
Authors and affiliations.
Oxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford, UK
Andrew J. Pollard & Else M. Bijker
NIHR Oxford Biomedical Research Centre, Oxford University Hospitals Trust, Oxford, UK
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Correspondence to Andrew J. Pollard .
Ethics declarations
Competing interests.
A.J.P. is Chair of the UK Department of Health and Social Care’s (DHSC) Joint Committee on Vaccination and Immunisation (JCVI), a member of the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization (SAGE) and a National Institute for Health Research (NIHR) Senior Investigator. The views expressed in this article do not necessarily represent the views of the DHSC, JCVI, NIHR or WHO. E.M.B. declares no competing interests. Oxford University has entered into a partnership with AstraZeneca for the development of a viral vectored coronavirus vaccine.
Additional information
Peer review information.
Nature Reviews Immunology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Advisory Committee on Immunization Practices (ACIP): https://www.cdc.gov/vaccines/acip/index.html
Coalition for Epidemic Preparedness Innovations (CEPI): https://cepi.net/
Gavi, the Vaccine Alliance: https://www.gavi.org/
Joint Committee on Vaccination and Immunisation (JCVI): https://www.gov.uk/government/groups/joint-committee-on-vaccination-and-immunisation
Nature Milestones in Vaccines: https://www.nature.com/immersive/d42859-020-00005-8/index.html
The Green Book, information for public health professionals on immunisation, Public Health England : https://www.gov.uk/government/collections/immunisation-against-infectious-disease-the-green-book
Vaccine Knowledge Project: https://vk.ovg.ox.ac.uk/vk/
Vaccines 101: How new vaccines are developed: https://www.youtube.com/watch?v=2t_mQwTY4WQ&feature=emb_logo
Vaccines 101: How vaccines work: https://www.youtube.com/watch?v=4SKmAlQtAj8&feature=emb_logo
Supplementary information
Supplementary information.
Parts of the pathogen (such as proteins or polysaccharides) that are recognized by the immune system and can be used to induce an immune response by vaccination.
The state in which an individual does not develop disease after being exposed to a pathogen.
A reduction in the virulence of a pathogen (through either deliberate or natural changes in virulence genes).
Particles constructed of viral proteins that structurally mimic the native virus but lack the viral genome.
An agent used in a vaccine to enhance the immune response against the antigen.
Molecules that stimulate a more robust immune response together with an antigen. Endogenous mediators that are released in response to infection or injury and that interact with pattern recognition receptors such as Toll-like receptors to activate innate immune cells such as dendritic cells.
The evolutionarily primitive part of the immune system that detects foreign antigens in a non-specific manner.
A liposome-based adjuvant containing 3- O -desacyl-4′-monophosphoryl lipid A and the saponin QS-21. AS01 triggers the innate immune system immediately after vaccination, resulting in an enhanced adaptive immune response.
An adjuvant consisting of aluminium salt and the Toll-like receptor agonist monophosphoryl lipid A.
A network of proteins that form an important part of the immune response by enhancing the opsonization of pathogens, cell lysis and inflammation.
A state of a pathogen in which antibodies or complement factors are bound to its surface.
Antibodies that bind to a pathogen, which subsequently can be eliminated by phagocytosis.
Antigens against which B cells can mount an antibody response without T cell help.
An antigen for which T cell help is required in order for B cells to mount an antibody response.
Studies in which volunteers are deliberately infected with a pathogen, in a carefully conducted study, to evaluate the biology of infection and the efficacy of drugs and vaccines.
The capacity of the immune system to respond quicker and more effectively when a pathogen is encountered again after an initial exposure that induced antigen-specific B cells and T cells.
The period from acquisition of a pathogen to the development of symptomatic disease.
Repeat administration of a vaccine after an initial priming dose, given in order to enhance the immune response.
An assay in which blood is stimulated with Mycobacterium tuberculosis antigens, after which levels of interferon-γ (produced by specific memory T cells if these are present) are measured.
Changes in the expression of genes that do not result from changes in DNA sequence.
A severe and potentially life-threatening reaction to an allergen.
Vaccines that are administered by means avoiding the gastrointestinal tract (for example, by intramuscular, subcutaneous or intradermal routes).
An acquired autoimmune condition characterized by low levels of platelets in the blood caused by antibodies to platelet antigens.
A rare chronic sleep disorder characterized by extreme sleepiness during the day and sudden sleep attacks.
Vaccines that are intended for a limited scope or targeting infections that are rare, as a result of which development costs exceed their market potential.
Blebs made from the outer membrane of Gram-negative bacteria, containing the surface proteins and lipids of the organism in the membrane.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Pollard, A.J., Bijker, E.M. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol 21 , 83–100 (2021). https://doi.org/10.1038/s41577-020-00479-7
Download citation
Accepted : 19 November 2020
Published : 22 December 2020
Issue Date : February 2021
DOI : https://doi.org/10.1038/s41577-020-00479-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Immunomodulatory effects of epiphytic loranthus micranthus leaf extracts collected from two host plants: psidium guajava and parkia biglobosa.
- Ngozi Dorathy Idoko
- Ifeoma Felicia Chukwuma
- Michel de Waard
BMC Complementary Medicine and Therapies (2024)
Ontological representation, modeling, and analysis of parasite vaccines
- Anthony Huffman
- Xumeng Zhang
Journal of Biomedical Semantics (2024)
Efficacy, immunogenicity and safety of respiratory syncytial virus prefusion F vaccine: systematic review and meta-analysis
BMC Public Health (2024)
Immunoinformatics design of a structural proteins driven multi-epitope candidate vaccine against different SARS-CoV-2 variants based on fynomer
- Javad Sarvmeili
- Bahram Baghban Kohnehrouz
- Hamideh Ofoghi
Scientific Reports (2024)
Immunogenicity and efficacy of CNA25 as a potential whole-cell vaccine against systemic candidiasis
- Satya Ranjan Sahu
- Abinash Dutta
- Narottam Acharya
EMBO Molecular Medicine (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.

IMAGES
VIDEO
COMMENTS
Metrics. Vaccines have revolutionized modern medicine by preventing infectious diseases and safeguarding public health. This Collection showcases cutting-edge research on advancements in vaccine ...
Introduction. The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over 192 million cases and 4.1 million deaths as of July 22, 2021. 1 This pandemic has brought along a massive burden in morbidity and mortality in the healthcare systems. Despite the implementation of stringent public health measures, there ...
VOL. 387 NO. 11. The coronavirus disease 2019 (Covid-19) pandemic has claimed an estimated 15 million lives, including more than 1 million lives in the United States alone. The rapid development ...
A two-dose regimen of BNT162b2 (30 μg per dose, given 21 days apart) was found to be safe and 95% effective against Covid-19. The vaccine met both primary efficacy end points, with more than a 99 ...
Vaccination is a powerful method of disease prevention that is relevant to people of all ages and in all countries, as the Covid-19 pandemic illustrates. Vaccination can improve people's chances ...
1. A phase 1/2 randomized, placebo-controlled, multicentre study to evaluate the safety and immunogenicity of COVID-19 vaccine in healthy adults. RBD SARS-CoV-2 HBsAg VLP vaccine, administered at two dose amounts 5 mcg and 25 mcg, by intramuscular injection by investigators during an in-clinic visit. RBD-HBsAg VLPs.
The Biomedical Advanced Research and Development Authority (BARDA) will be supporting Phase 2b clinical trials of candidate next-generation vaccines. ... primary endpoint will be improved efficacy in terms of symptomatic disease relative to a currently approved COVID-19 vaccine. In this paper, we discuss the planned endpoints and potential ...
Our analyses indicate that vaccine effectiveness generally decreases over time against SARS-CoV-2 infections, hospitalisations, and mortality. The baseline vaccine effectiveness levels for the omicron variant were notably lower than for other variants. Therefore, other preventive measures (eg, face-mask wearing and physical distancing) might be necessary to manage the pandemic in the long term.
Amid the staggering amount of suffering and death during this historic pandemic of COVID-19, a remarkable success story stands out. The development of several highly efficacious vaccines against a previously unknown viral pathogen, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in less than 1 year from the identification of the virus is unprecedented in the history of vaccinology.
To date, coronavirus disease 2019 (COVID-19) becomes increasingly fierce due to the emergence of variants. Rapid herd immunity through vaccination is needed to block the mutation and prevent the emergence of variants that can completely escape the immune surveillance. We aimed to systematically evaluate the effectiveness and safety of COVID-19 vaccines in the real world and to establish a ...
There is no question that the current vaccines are effective and safe. The risk of severe reaction to a COVID-19 jab, say researchers, is outweighed by the protection it offers against the deadly ...
Vaccines are a clinical product that is composed of live or dead material from an infectious agent - bacterium, virus, fungus or parasite - that elicit protective immunity against the pathogen ...
Globally, the provision of vaccines is more challenging in many low- and middle- income countries (LMIC), as evidenced by the failure to make the EPI vaccines available to every child by 1990, irrespective of setting (Keja et al., 1988).Central to this is limited financial resources, but other barriers to vaccine introduction include: underappreciation of the value of vaccines locally ...
The Coronavirus Efficacy (COVE) phase 3 trial was launched in late July 2020 to assess the safety and efficacy of the mRNA-1273 vaccine in preventing SARS-CoV-2 infection. An independent data and ...
The total average number of citations per year was 24.7 in 2020, 11.5 in 2021, 5.8 in 2022, and 1.2 in 2023. Figure 2 (A) Annual related publication from 2019 to 2023 per year, and (B) average article and average article citations from 2019 to 2023 in COVID-19 RNA vaccine-related research.
COVID-19 vaccine hesitancy: the five Cs to tackle behavioural and sociodemographic factors. J R Soc Med 2021;114:295-298. Waning vaccine efficacy remains greatest in older individuals and higher-risk groups. Lin DY, Gu Y, Wheeler B, Young H, Holloway S, Sunny SK, et al. Effectiveness of COVID-19 vaccines over a 9-month period in North Carolina.
Vaccines not only afford the best protection against infectious disease but can serve as strong deterrence factors as well. From a bioterrorist perspective, vaccine-resistant agents are more difficult to engineer than drug-resistant agents. But the potential market has been too small and uncertain to encourage the vaccine industry to make large investments in research, development, and ...
The COVID-19 pandemic has created a new reality where individuals are faced with a previously unknown disease and its effects, providing a unique opportunity to investigate vaccine attitudes during a period of heightened disease salience. The present research reports findings from a longitudinal study conducted during the COVID-19 health crisis ...
Vaccination Behavior Research Topics. Unraveling demand and supply effects on the up-take of influenza vaccinations. Point out new approaches to the seasonal flu vaccine. Exploring the impact of vaccination. Investigating patient experience with, and the use of, an electronic monitoring system to assess vaccination responses.
The achievements of vaccine research and development bring a hope to our societies that we may cope with the COVID‐19 pandemic. There are two aspects that should be maintained in balance: the immediate necessity for speed of vaccine research and the inherent need for protection of research subjects, which is the foremost concern of research ethics.
111 Vaccination Research Topics & Best Vaccine Essay Topics. Welcome to our list of vaccination research topics! This page contains only the best vaccine topics for essays and papers on child immunization, COVID-19, and other issues. Feel free to pick the catchiest research title about vaccines from our list!
WHO and its partners are committed to accelerating the development of COVID-19 vaccines while maintaining the highest standards on safety. Vaccines go through various phases of development and testing - there are usually three phases to clinical trials, with the last one designed to assess the ability of the product to protect against disease ...
End-to-End Product Development. Pfizer Vaccine R&D is a fully integrated, global operation that advances assets from discovery to registration and beyond, with talent and technical capabilities in Basic Research, early CMC Development, Clinical Development, Clinical Serology and Diagnostics and Operations. Bioprocess Development.
Types of paper Vaccine publishes primary research papers, review articles, short communications, conference reports and letters on the following topics: Basic Science Review; ... • Title. Concise and informative. Titles are often used in information-retrieval systems. Avoid abbreviations and formulae where possible.
"The misinformation flagged by fact-checkers was 46 times less impactful than the unflagged content that nonetheless encouraged vaccine skepticism," they conclude in a new paper in Science. Historically, research on "fake news" has focused almost exclusively on deliberately false or misleading content, on the theory that such content is ...
CDC's Immunization Safety Office monitors the safety of licensed and authorized vaccines and conducts high-quality vaccine safety research. This research is peer-reviewed and published in reputable scientific outlets. The vaccine safety articles and studies listed on this page include a full citation, a short summary, and a link to the free ...
Designing an immunogen—a molecule used in a vaccine that elicits a specific immune system response—to stimulate production of 10E8 bNAbs has been challenging because that key region of gp41 is ...
Researchers have focused on the soap-like compound QS-21 as a vaccine adjuvant since the late 1990s for its ability to activate the immune system. Currently, it's the only saponin-based vaccine ...
The research was conducted by the Scripps Consortium for HIV/AIDS Vaccine Development, one of two consortia supported by the National Institutes of Health's (NIH) National Institute of Allergy and ...
Tetanus vaccines: WHO position paper, February 2017 — recommendations. Vaccine 36, 3573-3575 ... (SAGE) and a National Institute for Health Research (NIHR) Senior Investigator. The views ...