DSpace JSPUI
Dspace preserves and enables easy and open access to all types of digital content including text, images, moving images, mpegs and data sets.
- Newcastle University eTheses
- Newcastle University
- Faculty of Science, Agriculture and Engineering
- School of Chemical Engineering and Advanced Materials
| Title: | Stress corrosion crackling of pipeline steels in contaminated aqueous CO₂ environments |
| Authors: | |
| Issue Date: | 2016 |
| Publisher: | Newcastle University |
| Abstract: | This work addresses the risk of Stress Corrosion Cracking (SCC) in CO₂transport pipelines. The susceptibility of X80 pipeline steels in aqueous CO₂environments in the presence of nitrates and sulphites is investigated using electrochemical potentiodynamic tests and Slow Strain Rate Tests (SSRT) at 23 and 75°C. The electrochemical measurements showed that in CO2-free and CO₂-saturated systems, the material presents an active-passive transition in bicarbonate / carbonate solutions with nitrate and sulphite. This indicated that SCC is possible in all the test environments. SCC occurred in bicarbonate / carbonate solutions with nitrates and sulphites at 75°C, both under CO₂-free and CO₂-saturated conditions. SCC severity declined as the potential moved towards the free corrosion potential. Cracking was still observed at +50 mV from Ecorr. The cracking mode in the active domain was transgranular for all the systems. In CO2-free systems, the severity and cracking mode in the HCO₃--CO₃² --H₂O and NaNO₃-HCO₃--CO₃² --H₂O systems was similar in the active-passive transition domain at 75°C. Crack growth was controlled by anodic dissolution and the crack mode was intergranular for both systems. At high pH (>9), the overall cracking mechanism remains dominated by the HCO₃--CO₃² --H₂O system even in the presence of nitrates. The addition of sulphites to bicarbonate / carbonate solutions however decreased the severity of cracking and shifted the cracking mode to transgranular. In CO₂-saturated systems, the SCC susceptibility in all test environments decreased with lower pH. Yet the highest susceptibility to cracking in the active-passive domain was identified in the nitrate-containing systems. With the drop in pH, nitrate SCC becomes the dominant mechanism when nitrates are present in the HCO₃--CO₃² --H₂O system. The addition of CO2 shifted the mode of cracking to transgranular in the active-passive domain in the pure bicarbonate / carbonate solution. |
| Description: | PhD Thesis |
| URI: | |
| Appears in Collections: | |
| File | Description | Size | Format | |
|---|---|---|---|---|
| Thesis | 48.5 MB | Adobe PDF | ||
| Licence | 43.82 kB | Adobe PDF |
Items in DSpace are protected by copyright, with all rights reserved, unless otherwise indicated.
- Architecture and Design
- Asian and Pacific Studies
- Business and Economics
- Classical and Ancient Near Eastern Studies
- Computer Sciences
- Cultural Studies
- Engineering
- General Interest
- Geosciences
- Industrial Chemistry
- Islamic and Middle Eastern Studies
- Jewish Studies
- Library and Information Science, Book Studies
- Life Sciences
- Linguistics and Semiotics
- Literary Studies
- Materials Sciences
- Mathematics
- Social Sciences
- Sports and Recreation
- Theology and Religion
- Publish your article
- The role of authors
- Promoting your article
- Abstracting & indexing
- Publishing Ethics
- Why publish with De Gruyter
- How to publish with De Gruyter
- Our book series
- Our subject areas
- Your digital product at De Gruyter
- Contribute to our reference works
- Product information
- Tools & resources
- Product Information
- Promotional Materials
- Orders and Inquiries
- FAQ for Library Suppliers and Book Sellers
- Repository Policy
- Free access policy
- Open Access agreements
- Database portals
- For Authors
- Customer service
- People + Culture
- Journal Management
- How to join us
- Working at De Gruyter
- Mission & Vision
- De Gruyter Foundation
- De Gruyter Ebound
- Our Responsibility
- Partner publishers

Your purchase has been completed. Your documents are now available to view.

Localized corrosion and stress corrosion cracking of stainless steels in halides other than chlorides solutions: a review
Mariano Kappes obtained his Bachelor’s degree in Materials Science and Engineering at the Instituto Sabato in Argentina in 2006 and then obtained his PhD in 2011 at the Ohio State University. His PhD thesis was distinguished with the Morris Cohen Award, awarded annually by The Electrochemical Society to outstanding graduate research in the field of Corrosion. Since 2014, he is a research scientist at the National Agency of Atomic Energy in Argentina and a Professor at the National University of General San Martin. He holds a position at the National Scientific and Technical Research Council as scientist since 2015.
Fluorides, bromides, and iodides, despite being less common than chlorides, are present in various environments of industrial relevance. Stainless steels suffer pitting corrosion in solutions of all halides except fluorides, which can be understood considering that fluoride is the anion of a weak acid. The aggressiveness of the rest of the halides for pitting corrosion is on the order Cl − > Br − > I − for stainless steels with Mo content below 3 wt.%. Mo is not as effective in inhibiting Br − pitting corrosion as it is for inhibiting Cl − pitting corrosion. Most of those observations were rationalized based on the effect of anions on pit growth kinetics. Sensitized austenitic stainless steel suffers stress corrosion cracking (SCC) in solutions of all halides, albeit chlorides seem to be the most aggressive. Fluoride SCC is relevant for SCC under insulation of stainless steels, and standards and regulations developed to mitigate this problem consider this ion as aggressive as chloride. For the solubilized stainless steels, aggressiveness toward SCC is in the order Cl − > Br − . The SCC of solubilized stainless steels was not observed in solutions of F − and I − , and the possible reasons for this fact are discussed.
1 Introduction
Chlorides are present in a wide variety of industrial environments ( Kolotyrkin, 1963 ; Brown, 1977 ), including fossil and nuclear power plants, food, paper, pulp, and chemical industry and petroleum refineries. Therefore, pitting, crevice corrosion, and stress corrosion cracking (SCC) of stainless steels were extensively studied in chloride solutions ( Brown, 1977 ; Cragnolino et al., 1981 ; Cragnolino & Macdonald, 1982 ; Haruyama, 1982 ; Sedriks, 1996 ; Frankel, 1998 ; Szklarska-Smialowska, 2005 ). Given those considerations, this review will be focused on SCC and pitting corrosion of stainless steels in solutions of halides other than chlorides, in particular, at a temperature below 100°C.
The SCC of stainless steel was observed in solutions of all halide ions. The SCC in F − and I − solutions was only reported for alloys in the sensitized condition ( Table 1 ). Sensitization occurs by exposure of stainless steels to elevated temperatures during welding, heat treatment, or service conditions, causing chromium carbide precipitation at grain boundaries and depletion in chromium close to grain boundaries, thus, favoring intergranular (IG) attack and intergranular stress corrosion cracking (IGSCC). A remarkable observation is that IGSCC was observed in laboratory tests even when F − concentration in the solutions was as low as 1 ppm ( Ward et al., 1969 ; Theus & Cels, 1974 ). Pitting corrosion of stainless steels, on the other hand, occurs in solutions of all halides except F − ( Table 1 ). Moreover, F − can act as an inhibitor of pitting and crevice corrosion in chloride solutions ( Yamazaki, 1994 , 1996 ), and this was explained based on the fact that it is the anion of a weak acid.
Selected properties of the halides and overview of observed modes of corrosion of stainless steel in halide solutions.
| Halide ion, X | F | Cl | Br | I | References |
|---|---|---|---|---|---|
| Radius of X (nm) | 0.133 | 0.181 | 0.196 | 0.219 | |
| (kJ/mol) | −472 | −351 | −326 | −294 | |
| pKa of HX | 3.17 | −7 | −9 | −10 | ; |
| Concentration in seawater (ppm) | 1.3 | 19×10 | 65 | 0.05 (present as I and ) | ; ; ; |
| Pitting of stainless steel in X solutions | No | Yes | Yes | Yes | ; ; |
| SCC of stainless steel in X solutions | Only in sensitized condition | Yes | Yes | Only in sensitized condition | Rhodes, 1969; ; ; Griess et al., 1985; Takemoto et al., 1985; ; ; ; ; ; ; |
2 Abundance of halogens in nature
Fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At) are the elements of the halogen family that occur naturally on Earth. Astatine is the scarcest of the naturally occurring elements ( Greenwood & Earnshaw, 1997 ), and it is radioactive with a half-life of 8.1 h for its most stable isotope ( Lide, 2005 ). Therefore, At is not further discussed in this review. The rest of the elements in the halogen family achieve a stable configuration by forming diatomic molecules: F 2 and Cl 2 are gases, Br 2 is a liquid, and I 2 is solid at ambient temperature and pressure. However, due to their reactivity, halogens occur in nature as halides (X − ), and iodine also occurs as iodate ( IO 3 − ) ( Greenwood & Earnshaw, 1997 ). The abundance of elements in crustal rocks decreases with an increase in the atomic mass of the halogen element ( Greenwood & Earnshaw, 1997 ). While fluoride is more abundant than chloride in crustal rocks, fluoride is mainly present in minerals that are scarcely soluble in water, like fluorite or fluorspar (CaF 2 ), cryolite (Na 3 AlF 6 ), and fluorapatite (CaF 2 ·3Ca 3 (PO 4 ) 2 ), whereas chloride is mainly present in water-soluble rock salt (NaCl) ( Chambers & Holliday, 1975 ; Greenwood & Earnshaw, 1997 ). Chloride is the dominant anion in ocean water, where its concentration is 1.9 wt.% ( Greenwood & Earnshaw, 1997 ), about 15,000 times higher than F − ( Warner, 1971 ) and 290 times higher than Br − ( Stine, 1929 ). Iodine is less abundant than lighter halogens both in the earth’s crust and in ocean water. Iodine is a micronutrient necessary for various organisms ( Ito, 1988 ). In seawater, it can be present as I − and IO 3 − , with total inorganic iodine in ocean water at around 0.05 ppm ( Ito, 1988 ). The concentration of halide ions in seawater is summarized in Table 1 . Chloride dominates in aerosols in the atmosphere, and they can contact metals by direct deposition or after dissolution in rainwater. The main sources of chlorides in aerosols are the ocean, road deicing salts, or the products of the combustion of fossil fuels and residues ( Willison et al., 1989 ).
3 Stability of halides in water
The stable oxidation number of fluorine and chlorine throughout the range of stability of water is −1 ( Pourbaix, 1966 ). In dilute solutions of hydrofluoric acid (HF), F − dominate at pH >3.17 ( Mccoubrey, 1955 ), which is the pk a of HF ( Table 1 ). Below this pH, HF dominates ( Pourbaix, 1966 ). In more concentrated solutions (greater than 3.8 g F/l), the bifluoride ion, HF 2 − , predominates in the pH region close to pk a ( Pourbaix, 1966 ). Hydrochloric acid, HCl, is a strong acid; hence, it is completely dissociated in water, and the stable form of chlorine in water solutions is as Cl − . Bromine is stable as Br − in almost the entire range of water stability, except for very low pH and oxidizing conditions, where it can react according to ( Pourbaix, 1966 ):
Iodine has the lowest standard reduction potential of the halogen group ( E 0 =0.62 V SHE ), and aqueous I − solutions are thermodynamically unstable in the presence of dissolved oxygen, reacting to give IO 3 − ( Pourbaix, 1966 ):
IO 3 − is the thermodynamically stable form of iodine in seawater, but I − is produced by biologically mediated reduction of IO 3 − or under reducing conditions ( Ito, 1988 ). In acid media, the intermediate formation of I 2 or I 3 − (triiodide) occurs according to ( Pourbaix, 1966 ):
4 Presence of halides in industrial environments
4.1 fluorides.
In industrial applications of stainless steels, some relevant sources of F − are thermal insulation ( Takemoto et al., 1985 ; Whorlow et al., 1997 ) and electrode coatings and fluxes used during welding operation ( Ward et al., 1969 ; Takemoto et al., 1985 ). Fluorinated hydrocarbons used in lubricants and gaskets can liberate F − at the temperature of operation of pressurized or boiling water reactors ( Brown, 1977 ). Fluorides are present in pickling solutions of stainless steels. Pickling is a surface process that removes metallic contamination, besides welding and heat treatment scales. This process is typically performed by immersion in nitric and hydrofluoric acid solutions, but it is explicitly not recommended for sensitized stainless steels by ASTM A380 ( ASTM A380-17, 2017 ), due to possible intergranular attack and SCC even under ambient conditions ( Berry et al., 1973 ). Fluoridation is the addition of F − to a public water supply to prevent tooth decay, and it is targeted to maintain a concentration of about 1 ppm in water ( Greenwood & Earnshaw, 1997 ). Cryolite is used in the electrolysis of alumina for metallic aluminum production ( Greenwood & Earnshaw, 1997 ). Fluorine has a key role in the nuclear fuel cycle ( Crouse, 2015 ), where it is used to produce gaseous UF 6 from which fissionable isotopes can be separated by different technologies.
4.2 Bromides
Industrial applications of bromine compounds used to be dominated by 1,2-dibromoethane or ethylene dibromide, a compound added to gasoline as a lead scavenger, until environmental legislation limited the use of lead-based anti-knock additives ( Greenwood & Earnshaw, 1997 ; Thomas et al., 1997 ). Ethylene dibromide and methyl bromide have applications as pesticides as well ( Greenwood & Earnshaw, 1997 ). Other major applications of bromine compounds are as flame retardants, in plastics, fibers, rugs, and carpets. Silver bromide, AgBr, is an active compound in photographic films. Some catalysts for chemical industries contain bromides, and this caused pitting of a type 316L stainless steel component handling organic acids ( Ohtsu & Miyazawa, 2012 ). Bromides are present in high-density brine completion fluids, applied to deep oil and gas wells to balance the high pressure of the well and maintain the borehole stability ( Liu et al., 2014 ). Those fluids typically contain bromides or chlorides of Ca, Zn, and Na ( Sridhar et al., 2017 ). Calcium bromide is used for controlling mercury emissions of coal-fired power plants ( Ladwig & Blythe, 2017 ). The salt decomposes in the furnace to yield bromine or hydrogen bromide, and they react with elemental mercury. Oxidized mercury compounds are then more easily captured in downstream wet scrubbers for flue gas desulfurization. However, bromides can cause localized corrosion problems of materials used for wet scrubbers ( Ozturk & Grubb, 2012 ).
Concentrated LiBr brines have applications in absorption refrigeration systems ( Itzhak & Elias, 1994 ; Srikhirin et al., 2001 ). The main advantage of those systems is that a compressor is not used. Water is used as the refrigerant, and when it evaporates at low pressure, heat is absorbed from the environment. This water vapor is absorbed by concentrated LiBr brines. The brine, now diluted, is heated in a generator. The brine becomes concentrated, and evaporated water from it is condensed in a heat exchanger. The condensed water and concentrated brine are now ready to start a new cycle. Stainless steels are frequently used in metallic parts of those systems, which motivated corrosion studies in concentrated (above 50%) LiBr brines at temperature in the range from 80°C to 160°C ( Griess et al., 1985 ; Guiñon et al. 1994 ; Itzhak & Elias, 1994 ; Itzhak et al., 1996 ).
4.3 Iodides
Iodine solutions are commonly used in redox titrations. Iodine is scarcely soluble in water, but solubility increases in iodide solutions by the following complexation reaction ( Harris, 2007 ):
I 3 − solutions can be used to titrate solutions of reducing agents, using starch as an indicator. Oxidizing solutions are treated with I − to produce an excess of I 3 − , which is then titrated with thiosulfate ( Harris, 2007 ).
Triiodide solutions have applications as antiseptic for cuts and wounds ( Chambers & Holliday 1975 ). Stainless steels 304L and 316L exhibit pitting in iodine solutions or solutions exposed to iodine vapor, as reported by Tsukaue et al. (1993 , 1994a , b ) at temperatures in the range from 50°C to 80°C. Triiodide is involved in the cathodic reaction (Eq. (3). Iodides produced in this reaction favor the solubility of iodine so that the reactant of the cathodic reaction is regenerated by Eq. (5). This causes the accumulation of triiodide in stainless steels, resulting in stable pit growth ( Tsukaue et al., 1994a ), b ). Iodine is a dangerous fission product that could be released in accidents of nuclear power plants ( Wren et al., 1999 ). The amount of iodine released to the environment is monitored with sampling systems that have stainless steel lines that transport gases through filters and absorbers where the presence and concentration of radionuclides are analyzed ( Evans & Nugraha, 2002 ). It was shown that with water vapor content above 75% and above 10 −9 m I 2 in the gas, aqueous pitting corrosion by iodine is possible ( Evans & Nugraha, 2002 ). Besides the integrity problem in the material of the sampling line, the amount of iodine transmitted through the line is reduced, causing errors in the monitoring process ( Evans & Nugraha, 2002 ).
5 Pitting corrosion in halides other than chloride solutions
Pitting corrosion of stainless steels involves breakdown of the passive film, metastable pitting, and stable growth of pits ( Frankel et al., 2017 ). Breakdown of the passive film was explained by halide adsorption and thinning of the passive film, halide penetration, and the film-breaking mechanism, as reviewed in-depthly elsewhere ( Frankel, 1998 ; Szklarska-Smialowska, 2005 ; Soltis, 2015 ). Halide ions affect properties of the passive film, for example, XPS studies show that the thickness of the passive film of iron in buffer solution decreases as the concentration of Cl − , Br − , and I − increases ( Khalil et al., 1985 ), and Cl − is the most aggressive for a given concentration. The point defect model was also applied to analyze passivity breakdown in solutions of the different halide ions ( Macdonald & Lei, 2016 ). The model successfully predicts that the ability of halide ions to cause passivity breakdown is in the order F − ≪Cl − >Br − >I − . According to this model, passivity breakdown first requires absorption of the halide ion into the passive film. In short, halide ions absorb by occupying surface oxygen anion vacancies, and this process requires expansion of the oxygen vacancy to accommodate the halide ion with a larger diameter, dehydration of the halide ion, and insertion of the halide ion into the expanded oxygen vacancy. By considering the Gibbs free energy required for each of those processes, a minimum in breakdown potential was predicted for Cl − solutions. The exceptionally high stability of hydrated F − ions is elucidated in Table 1 by the Gibbs free energy values of halide anion hydration, Δ G 0 hyd , ( Macdonald & Lei, 2016 ). The high energy required for F − dehydration results in the absence of passivity breakdown in F − solutions.
Despite those effects of halide ions on properties and breakdown of the passive film, it is known that breakdown events of the passive film can occur at a very high rate, as evidenced during metastable pitting at potentials below the stable pitting potential, E pit ( Frankel et al., 2017 ). Those frequent events of passive film breakdown and metastable growth are not a problem for structural integrity if repassivation of the passive film is rapid ( Frankel et al., 2017 ). Furthermore, stainless steels for industrial applications usually have appreciable fractions of inclusions or second phase particles, which provide favorable sites for pit initiation and possibly sulfur-containing aggressive species to the local environment ( Frankel, 1998 ). Therefore, the main attention is often targeted to understanding the conditions required for stable growth, or in other words, those that make a metastable pit grow stably ( Frankel, 1998 ; Newman, 2001 ; Li et al., 2018 ). Like many other variables that affect pitting corrosion resistance ( Newman, 2001 ), the aggressiveness of the different halide ions can be understood in terms of their effect on stable pit growth rather than on pit nucleation.
The aggressiveness of the different halide anions can be ranked by comparison of pitting potential ( E pit ), the repassivation potential ( E rp ) or the critical pitting temperature (CPT) at a given molar concentration of the anion, or by evaluating the minimum amount required for stable pit growth ( Szklarska-Smialowska, 2005 ).
5.1 Fluoride solutions
Hydrofluoric acid is a weak acid, with a pK a of 3.17 ( Mccoubrey, 1955 ) ( Table 1 ). The rest of the acids are strong, meaning that they completely dissociate in water. The localized acidification theory of pitting corrosion ( Galvele, 1976 ) predicts that the anions of weak acids act as inhibitors. In accord with those considerations, pitting corrosion of stainless steels was not observed in fluoride solutions at room temperature ( Streicher, 1956 ; Koch, 1993 ). Studied materials where this was verified include quenched and tempered martensitic 403 stainless steel ( Pahlavan et al., 2016 ), supermartensitic 13 Cr stainless steel ( Macdonald & Lei, 2016 ), sensitized 304 stainless steel ( Zucchi et al., 1988 ), 17 wt.% Cr, 12 wt.% Mn and 0.61 wt.% N stainless steel, and 18 wt.% Cr with 9 wt.% Ni stainless steels ( Tzaneva et al., 2006 ). The absence of pitting was confirmed by either observation of specimens after the test or absence of hysteresis in the return potential scanning curve.
While type 304 sensitized stainless steel suffer intergranular attack and SCC in the presence of F − ions ( Ward et al., 1969 ; Theus & Cels, 1974 ), breakdown of the passive layer was not observed in the absence of stress at room temperature ( Trabanelli et al., 1988 ; Zucchi et al., 1988 ). The Cr content near the grain boundary of sensitized stainless steel is controlled by the bulk chemical composition of the stainless steel, time, and temperature in the carbide precipitation region, and grain size. For type 304 stainless steel, scanning transmission electron microscope (STEM) studies coupled with X-ray energy-dispersive spectroscopy (EDS) revealed that the Cr content near the grain boundary can decrease to 13 wt.% or less ( Rao, 1979 ; Ford & Silverman, 1980 ; Thorvaldsson & Salwén, 1984 ). Despite this depletion in Cr at the grain boundary, as the potential was increased in the anodic direction, polarization curves of sensitized type 304 stainless steel in F − solutions at room temperature exhibited a passive behavior, followed by a current increase in the potential range corresponding to oxygen evolution ( Zucchi et al., 1988 ). No hysteresis was observed on the potential scan in the noble direction, confirming that localized corrosion did not occur even at the most positive potentials ( Zucchi et al., 1988 ). In accord with those studies, anodic polarization in F − solutions did not cause breakdown of the passive layer in martensitic stainless steels ( Pahlavan et al. 2016 ), where the bulk Cr content was close to the Cr content near the grain boundary of sensitized 304 stainless steel.
Despite stable pitting is not observed in fluoride solutions, Macdonald and Lei (2016 ) reported metastable pitting for a 13 Cr martensitic stainless steel in 0.1 m NaF solution. The rate of metastable pitting events and the average current density increased in the order F − <I − <Br − <Cl − . The average current density of metastable pits in F − solutions was one order of magnitude lower than in Cl − solutions ( Macdonald & Lei 2016 ). The low frequency of those events and their low current might explain why other authors working in similar systems reported the absence of metastable pitting in F − solutions ( Pahlavan et al., 2016 ).
5.2 Fluorides as inhibitors of Cl − pitting and crevice corrosion
It was shown that F − increases the pitting initiation ( Yamazaki, 1994 ) and crevice corrosion initiation and repassivation ( Yamazaki, 1996 , 1997 ) potential of type 304 stainless steel in Cl − solutions. This increase in the pitting potential when F − is added to Cl − solutions was also reported by Pahlavan et al. (2016 ) for type 403 martensitic stainless steel. Interestingly, when F − was added to Cl − solutions, the rate of nucleation of metastable pits increased ( Pahlavan et al., 2016 ). Therefore, F − appear to inhibit the stable growth of pits, and this was explained on the basis of Galvele’s localized acidification theory ( Galvele, 1976 ). The high pK a of HF results in F − bonding to H + at pit bottoms, therefore, inhibiting pit growth ( Pahlavan et al., 2016 ).
5.3 Pitting corrosion in halides and strength of the hydrohalide acid
Stable pitting of stainless steels was observed in solutions of the rest of the halides. The strength of the acid increases from HCl to HI ( Table 1 ) ( Chambers & Holliday, 1975 ), but because they are all strong and completely dissociated in water, this difference in strength can only be observed in solvents more acidic than water, for example, acetic acid. In aqueous solution, this difference in strength is irrelevant because they are all leveled out to H 3 O + ( Chambers & Holliday, 1975 ), and in fact, the aggressiveness of the different anions for pitting corrosion of stainless steels and passive iron in aqueous solutions generally increases from I − <Br − <Cl − ( Tousek, 1975 ; Janik-Czachor, 1979 ; Szklarska-Smialowska, 2005 ; Tzaneva et al., 2006 ; Pahlavan et al., 2016 ). A notable exception to this rule is observed in Mo alloyed stainless steels, where it was found that Br − ions are more aggressive than Cl − ( Guo & Ives, 1990 ; Kaneko & Isaacs, 2002 ) above a certain content of Mo, as discussed in a subsequent section.
5.4 Effect of halide ion concentration on the pitting potential
Pitting potentials ( E pit ) decrease with an increase in the concentration of the aggressive ion, [X − ], according to ( Galvele, 1976 ; Szklarska-Smialowska, 2005 ):
where a and B are constants. The B value is controlled by the charge of the aggressive anion and complexation reactions between metal cations and anions inside the pit ( Nguyen et al., 2019 ). Without complexation, a B value of 59 mV is predicted for all halide ions at room temperature ( Galvele, 1976 ; Nguyen et al., 2019 ). For pure iron in borate buffer solutions containing Cl − , Br − , or I − , Janik-Czachor (1979 ) reported a B value of 100 mV, irrespective of the nature of the halide ion. For pure iron, this is an unusually high value, considering other studies ( Galvele, 1976 ; Nguyen et al., 2019 ) in Cl − solutions, where it was reported to be close to 59 mV. On the other hand, for stainless steels that suffer pitting at room temperature, the reported B values in Cl − solutions are close to 90 mV ( Galvele, 1976 ; Laycock & Newman, 1997 ; Nguyen et al., 2019 ). The B value increases with the stability constant of the metal-chloride complex, and stainless steels contain chromium, which forms complexes more stable than iron ( Nguyen et al., 2019 ). Measurements in martensitic stainless steels ( Pahlavan et al., 2016 ) revealed that the B value for Cl − , Br − , and I − was 130, 80, and 76 mV, respectively. For type 316 austenitic stainless steel, a slope of 91 mV was reported for Cl − solutions and of 75 mV in Br − solutions ( Pahlavan et al., 2019a ). In other words, an equal increase in halide ion concentration will cause a higher decrease in pitting potential for Cl − than for the rest of the halides.
5.5 Adsorption of the halides and pitting corrosion
The halide ion radius and the strength of the adsorbed halide-metal bond increase with atomic mass ( Table 1 ) ( de Castro & Wilde, 1979 ; Szklarska-Smialowska, 2005 ) so that larger iodide ion adsorption is thermodynamically more favored than fluoride adsorption. Therefore, the anions that adsorb more strongly to the metal are less aggressive for pitting ( Szklarska-Smialowska, 2005 ). A plausible explanation for this fact could be that adsorption of halide ions to the bare metal surface exposed to an acid solution can inhibit active dissolution of the metal, under certain conditions. Cl − , Br − , and I − additions to a H 2 SO 4 solution result in lower rates of active metal dissolution, as it is observed for mild steel ( Jesionek & Szklarska-Smialowska, 1983 ), pure nickel ( Abd El Rehim et al., 1986 ), and 18 Cr-8 Ni stainless steel ( Asawa, 1971 ). An inhibiting effect on carbon steel corrosion is also observed when KF is added to 0.01 m H 2 SO 4 solutions ( Sekine et al., 1994 ), but this is probably related to a buffering effect of F − ions.
The strength of adsorption of halides to active metal, the surface coverage of halides and the inhibition efficiency increase with increasing halide ion size ( Jesionek & Szklarska-Smialowska, 1983 ; Abd El Rehim et al., 1986 ). However, this inhibiting effect of halides on active dissolution of metals in acid solutions is observed below a concentration that is specific for each halide ion and material ( Asawa, 1971 ; Jesionek & Szklarska-Smialowska, 1983 ; Abd El Rehim et al., 1986 ) and around 10 −2 m ( Abd El Rehim et al., 1986 ). For Ni in sulfuric acid solutions ( Abd El Rehim et al., 1986 ), when halide ions are present at a concentration above 10 −2 m , they accelerate anodic dissolution and shift the active to passive transition to higher potential, and the aggressiveness increases in the order I − <Br − <Cl − . A similar effect is observed in 18 Cr-8 Ni stainless steels in 4 N H 2 SO 4 ( Asawa, 1971 ), for concentrations of halide ions up to 1 m . At the bottom of a pit under sustained growth, the concentration of halide ions can be much higher, on the order of 8 m or more ( Mankowski & Szklarska-Smialowska, 1975 ). The effect of halide ion on metal dissolution at those concentrations was studied with the artificial pit electrode, as will be discussed below.
Pitting corrosion studies with wire electrodes showed an “anomalous” higher aggressiveness of Br − vs. Cl − ( Carroll & Lynskey, 1994 ), under a wide range of experimental variables. The pitting potential was lower in Br − than in Cl − solutions for types 316, 304L, and 302 stainless steel wires, measured in solutions of pH ranging from 3 to 9 and halide concentration ranging from 0.1 m to 1 m . This anomalous effect was associated to a refining effect of the wire-drawing process on inclusions like sulfides ( Pistorius & Burstein, 1992 ), which act as favorable sites for pit initiation of stainless steels in Cl − solutions ( Szklarska-Smialowska, 2005 ). On the other hand, pitting potentials in bromide solutions were similar whether they were measured with wire electrodes or electrodes cut from bars, suggesting that pitting initiation in Br − solutions relies more on the adsorption of Br − ions than on interaction with inclusions. According to the authors ( Carroll & Lynskey, 1994 ), Br − has a greater tendency to adsorb on the metal surface than Cl − , and this has a greater effect on pit initiation when there is a low population of sulfides. Measurements of pitting potential of 316L stainless steels electrodes cut from bars, with a regular population of sulfides, showed the higher aggressiveness of Cl − vs. Br − usually observed for low Mo stainless steels.
5.6 Pit growth in Cl − vs. Br − solutions
Pit growth can be studied independently of pit initiation with the “lead-in-pencil” or artificial pit electrode technique ( Laycock & Newman, 1997 ; Kaneko & Isaacs, 2000 ; Ernst & Newman, 2008 ). A survey of the literature published up to date reveals that this technique was used to study pit growth kinetics in Br − vs. Cl − solutions ( Kaneko & Isaacs, 2000 ; Pahlavan et al., 2019a , b ), but no studies were reported in I − solutions. In this technique, a wire of the metal or alloy to be studied is embedded in epoxy, and after dissolving back the metal at an anodic potential, a one-dimensional artificial pit is eventually formed after the conglomeration of smaller pits ( Laycock & Newman, 1997 ). The electrochemical potential is then scanned in the active direction, while the current is measured. Figure 1 shows the polarization curves of artificial pit electrodes obtained in Br − vs. Cl − solutions, for an 18 wt.% Cr-12 wt.% Ni austenitic stainless steel ( Kaneko & Isaacs, 2000 ). Similar results were obtained by Pahlavan et al.(2019a) for a type 316 austenitic stainless steel. A region where current is independent of potential is observed in Figure 1 . In this region, a salt film is precipitated on the metal surface, and the current density of the unidimensional pit is diffusion limited ( i lim ) ( Laycock & Newman, 1997 ):

Anodic polarization curves of 18 wt.% Cr–12 wt.% Ni stainless steel, obtained for a 0.44-mm deep artificial pit electrode in 1 m LiCl and 1 m LiBr bulk solutions. The electrochemical potential was scanned at a rate of 5 mV/s in the active direction. E T is the transition potential, where anodic dissolution shifts from diffusion control to activation/ohmic control. Adapted from Corrosion Science, 42(1), Kaneko and Isaacs, Pitting of stainless steel in bromide, chloride and bromide/chloride solutions, 67–78, Copyright (2000), with permission from Elsevier.
where n is the average charge of metal ions, F is Faraday’s constant, D is the effective or average diffusion coefficient of metal cations, C sat is the molar concentration of metal ions in the saturated halide salt solution, and x is the depth of the unidimensional pit. Notice that Eq. (8) is based on Fick’s first law of diffusion and, therefore, is strictly valid during steady state. Because of metal dissolution, x increases with time. However, relative changes of x during the characteristic time of diffusion, x 2 / D , are small enough for the approximation to be valid ( Laycock & Newman, 1997 ). As a consequence, despite x increases as potential is scanned in the active direction, i lim is independent of potential for scan rates in the range of 1 mV/s to 10 mV/s, typically used in this experiment ( Laycock & Newman 1997 ; Li et al. 2019 ).
It is expected that the most prominent element in the alloy will precipitate first ( Bocher et al., 2010 ). For stainless steel, ferrous halide should dominate in the salt film. However, how chromium, nickel, and the rest of the alloying and impurity elements affect the precipitation of iron halides is not known in depth. Kaneko and Isaacs (2000 ) argue that the concentration of saturated FeBr 2 should be similar to saturated FeCl 2 and around 5 m . In that case, the reason for the higher diffusion-limited current density reported in the literature ( Kaneko & Isaacs, 2000 ; Pahlavan et al., 2019a ) and schematized in Figure 1 resides in a higher diffusion coefficient of metallic cations in Br − vs. Cl − solutions, which is roughly estimated to be within a factor of 1.5. As potential is decreased, eventually, the salt film is dissolved, which is indicated by a small hump in the i vs. E curve ( Ernst & Newman, 2008 ) ( Figure 1 ). Current then decreases sharply with potential, due to ohmic drop and activation control.
The transition potential, E T , is the minimum applied potential required for salt film precipitation at the pit bottom. From Figure 1 , this potential can be estimated as the potential, where E=i lim , on the left of the hump associated with salt film dissolution ( Laycock & Newman, 1997 ; Ernst & Newman, 2008 ). The actual potential at the pit bottom might be lower due to an ohmic drop in potential, and it can be estimated by different procedures detailed in the literature ( Gaudet et al., 1986 ; Laycock & Newman 1997 ; Li et al., 2019 ). An increase in E T measured with pencil electrodes correlated with an increase in E pit measured on flat electrodes, as the Br − /Cl − concentration ratio increased in solutions with a total halide concentration of 0.2 m ( Pahlavan et al. 2019b ). Kaneko and Isaacs (2000 ) reported similar results for 1 m Br − and 1 m Cl − solutions. According to Laycock and Newman (1997 ), stable pitting requires the precipitation of a salt film at the pit bottom, thus, explaining the correlation between E T and E pit . While a solution saturated in metallic cations is the most aggressive solution attainable at the pit bottom at a given temperature, recent studies confirm that stable pitting can be sustained in less concentrated solutions ( Srinivasan & Kelly, 2017 ; Li et al., 2019 ). For example, according to Srinivasan and Kelly (2017 ), stable pitting of a 316L stainless steel at room temperature requires a concentration around 50% of the saturated solution at the pit bottom ( Srinivasan & Kelly, 2017 ), otherwise the pit repassivates.
A deeper understanding of how measurements with the artificial pit electrode can explain E pit differences in Br − vs. Cl − solutions can be achieved with a suitable model for pit growth ( Li et al., 2018 ; Pahlavan et al., 2019b ). During pitting corrosion, metallic cations are produced by anodic dissolution at the pit bottom and diffuse out of the pit down a concentration gradient. Mathematically, a stable growth of pits requires that ( Li et al., 2018 )
where i diss,max is the maximum anodic dissolution current at the pit bottom for a given temperature, pit solution concentration, and potential, and i diff,crit is the critical current density for diffusion of metal cations out of the pit. In other words, the rate of production of metallic ions at the pit bottom must compensate its loss by diffusion out of the pit, otherwise, dilution and repassivation occur. The expression for i diff,crit for unidimensional pits is similar to Eq. (8), but C crit is a fraction of C sat ( Li et al., 2018 ):
From artificial pit experiments and a diffusion model ( Gaudet et al., 1986 ), Kaneko and Isaacs (2000 ) argue that to prevent pit repassivation, a higher concentration of metallic ions is required in Br − vs. Cl − solutions, i.e. C crit in Br − will be higher than C crit in Cl − in Eq. (10). As previously discussed, the diffusion coefficient of metallic cations is higher in Br − vs. Cl − solutions, according to experiments conducted by Kaneko and Isaacs (2000 ). Therefore, from Eqs. (9) and (10) and for a given pit depth x , pit stability in Br − solutions requires a higher dissolution current i diss,max at the pit bottom. Finally, considering the curves presented in Figure 1 , it is inferred that for a given current density, a higher potential is required to attain it in Br − vs. Cl − solutions. Notice that this analysis was made considering unidimensional pits, but it can be extended because similar expressions hold for hemispherical pits ( Li et al., 2018 ). All those contributions explain the higher E pit observed in Br − vs. Cl − solutions ( Kaneko & Isaacs, 2000 ; Pahlavan et al., 2019a , b ), confirming that the higher aggressiveness of Br − vs. Cl − ions can be explained on the basis of pit growth kinetics.
5.7 The Mo effect in Br − vs. Cl − solutions
Ferritic ( Kaneko & Isaacs, 2002 ) and austenitic ( Guo & Ives, 1990 ; Kaneko & Isaacs, 2002 ) stainless steels alloyed with Mo can be more susceptible to pitting in Br − vs. Cl − solutions. A higher pitting potential was measured in Br − vs. Cl − solutions when Mo content was low ( Guo & Ives, 1990 ; Kaneko & Isaacs, 2002 ; Pahlavan et al., 2019a ); however, Mo additions had a stronger inhibiting effect on the pitting potential in Cl − than in Br − solutions ( Horvath & Uhlig, 1968 ). Hence, above a certain Mo content that depended on stainless steel microstructure ( Kaneko & Isaacs, 2002 ), Br − became more aggressive than Cl − , causing stable pitting at lower potentials. Those results were in good correlation with anodic polarization curves measured with the artificial pit electrode ( Kaneko & Isaacs, 2002 ). In Cl − solutions, studies with the artificial pit electrode reveal that Mo increases pitting resistance by shifting the bare metal anodic dissolution curve to higher potentials ( Laycock & Newman, 1997 ). This displacement of the anodic curve for a given increment of Mo is lower in Br − solutions vs. Cl − solutions ( Kaneko & Isaacs, 2002 ). This lower effect of Mo in preventing Br − pitting was attributed to the formation of soluble molybdenum complexes ( Newman, 2001 ), a lower production of inhibiting polymolybdate species in bromide solutions ( Domínguez-Aguilar & Newman, 2006 ), or to the fact that when Br − displaces the anodic dissolution curve to a higher potential, as schematized in Figure 1 , Mo dissolves more easily, and it is less effective as an inhibitor ( Ernst & Newman, 2008 ).
For commercial steels, E pit in Cl − is lower than E pit in Br − for type 304 stainless steel ( Ernst & Newman, 2008 ), type 301 stainless steel (0.19% Mo as impurity) ( Guo & Ives, 1990 ), type 316 stainless steel (2.5% Mo) ( Guo & Ives, 1990 ; Ernst & Newman, 2008 ; Pahlavan et al., 2019a ), and type UNS S31260 (3% Mo) duplex stainless steel ( Yamamoto & Hosoya, 1995 ). The reverse behavior is observed for type 904L (UNS N08904) with 4.5 wt.% Mo ( Guo & Ives, 1990 ; Abd El Meguid, 1997 ) and for 6% Mo superaustenitic stainless steel (UNS S31254) ( Guo & Ives, 1990 ). Some of those results are summarized in Figure 2 for measurements of E pit near room temperature.

Effect of molybdenum content on the pitting potential, E pit , in Cl − (filled symbols) vs. Br − (hollow symbols) solutions near room temperature for various commercial alloys as indicated in the top scale ( Guo & Ives, 1990 ; Abd El Meguid, 1997 ; Ernst & Newman, 2008 ; Pahlavan et al., 2019a ).
5.8 PRE N and localized corrosion resistance in Br − solutions
With the exception of Mo, how effective the different alloying elements are for preventing localized corrosion in Br − and I − solutions was not as deeply and systematically studied as in Cl − solutions. For Cl − solutions, the beneficial effect of Cr, Mo, W, and N on pitting and crevice corrosion resistance of stainless steels is often summarized with the PRE N (pitting resistance equivalent with nitrogen) number ( ISO 2010 ):
PRE N = wt . % Cr + 3.3 ( wt . % Mo + 0.5 wt . % W ) + 16 wt . % N
In the literature, there are similar versions of this equation that do not consider the beneficial effect of W or with different factors for N (Malik et al., 1994 , 1995 ; Sedriks, 1996 ; Szklarska-Smialowska, 2005 ). E pit in seawater ( Malik et al., 1994 , 1995 ) and CPT in ferric chloride solutions ( Sedriks, 1996 ) both increase with an increase in PRE N . Similar correlations exist for crevice corrosion critical parameters ( Sedriks, 1996 ). CPT increases with PRE N in 35,500 ppm Br − solutions, but the slope in the CPT vs. PRE N graph was lower in Br − than in Cl − solutions ( Ozturk & Grubb, 2012 ). Considering this finding, thumb or engineering rules valid in chloride solutions, like PRE N >40 for resistance to localized corrosion in seawater at room temperature ( Norsok, 2014 ; Francis & Hebdon, 2015 ), will not be valid for bromide solutions of similar concentrations. The critical pitting temperature of austenitic UNS N08904 (PRE N =34.9) and UNS S31254 (PRE N =43.5) stainless steel is near room temperature in Br − solutions ( Guo & Ives, 1990 ; Abd El Meguid, 1997 ), i.e. more than 30°C lower than values measured in Cl − solutions ( Guo & Ives, 1990 ). Given those results, the use of high PRE N (and in particular, high Mo) stainless steels for mitigation of pitting corrosion is less effective in Br − than in Cl − solutions.
6 Stress corrosion cracking in halides solutions
Austenitic stainless steels suffer SCC in the presence of Cl − ions ( Scully, 1968 ; Hänninen, 1979 ; Cragnolino & Macdonald, 1982 ; Sedriks, 1996 ; Streicher, 2011 ), both in the fully solubilized and sensitized microstructures. The crack path in Cl − solutions can be either transgranular (TG) or intergranular (IG), as reviewed by Cragnolino and Macdonald (1982 ). Similar to the situation for pitting corrosion, the SCC in halides other than Cl − solutions received comparatively less attention. Some cases of SCC were reported for austenitic ( Griess et al., 1985 ; Itzhak & Elias, 1994 ; Itzhak et al., 1996 ) and martensitic ( Downs et al., 2007 ) stainless steels in the presence of bromides or other bromine species ( Lee et al., 1983 ; Nordin, 1983 ). Similar to Cl − solutions, sensitization is not required for SCC occurrence in Br − solutions. Finally, a number of researchers conclude that I − act as an inhibitor of Cl − SCC of austenitic stainless steels ( Overman, 1966 ; Uhlig & Cook, 1969 ; O’Dell & Brown, 1978 ; O’Dell et al., 1980 ; Pinkus et al., 1981 ; Itzhak & Eliezer, 1983 ).
While pitting corrosion was not observed in F − solutions, sensitized stainless steels suffer IGSCC in this environment ( Ward et al., 1969 ; Theus & Cels, 1974 ; Takemoto et al., 1985 ; Trabanelli et al., 1988 ; Zucchi et al., 1988 ; Shibata et al., 1993a , b ; Whorlow et al., 1997 ), a problem that was researched mainly because the presence of F − is common in thermal insulation materials ( Whorlow & Hutto, 1997 ) and in fluxes used in welding processes ( Ward et al., 1969 ; Takemoto et al., 1985 ). In contrast to Cl − solutions, no instances of F − -induced SCC were reported up to date in fully solubilized microstructures, as will be discussed in depth below.
6.1 SCC testing techniques and apparent “thresholds” for SCC
A short discussion of different tests used to quantify the aggressiveness of the different halide anions toward the SCC of stainless steel is presented. It has first to be noticed that sensitized type 304 suffers IGSCC in slow strain rate tests (SSRT) even in oxygen-containing “pure water” at a temperature above 50°C (Ford & Povich, 1979; Ford & Silverman, 1980 ; Cragnolino & Macdonald, 1982 ; Congleton & Sui, 1992 ). By “pure water,” it is meant a solution with less than 5 ppb Cl − ( Congleton & Sui, 1992 ) or conductivity <0.3 μS/cm ( Ford & Silverman, 1980 ). It was proposed that strain can expose bare metal to the environment, and if the potential is in a range where repassivation of grain boundaries is slower than the matrix, crack propagation can proceed even in the absence of aggressive ions ( Ford & Povich, 1979 ; Ford & Silverman, 1980 ; Cragnolino & Macdonald, 1982 ). The presence of halide ions increases the SCC susceptibility, for example, the minimum oxygen concentration and the minimum potential for SCC occurrence in SSRT decrease with an increase in Cl − concentration ( Congleton & Sui, 1992 ).
Halide ions are typically required for cracking in constant deflection tests, like those depicted in Figure 3 . Dana and Delong (1956 ) and Dana (1957 ) proposed in the 1950s the SCC test shown in Figure 3 , left. The test mimics the leaching of chlorides in the insulation followed by concentration by evaporation at the stainless steel surface, simulating the environment in contact with a stainless steel pipe under thermal insulation. This test was standardized in 1971 in the ASTM C692 standard, and it is one of the alternatives listed in the current version of this standard ( ASTM C692-13, 2018 ). This test, however, is limited to “wicking-type” thermal insulation. Those materials wet completely when partially immersed in water. In this test, chlorides and other ions leach in the deionized (DI) water and concentrate on the surface of a U-bend stainless steel specimen. Temperature is controlled by the Joule effect with a transformer, connected either to a resistance heater taped to the specimen, as originally proposed by Dana (1957 ) or directly to the specimen, as later standardized by ASTM C692 standard ( Figure 3 , left). The 304 stainless steel is sensitized by heating for 3 h at 649°C. This temperature is close to the nose of the time-temperature-sensitization curve of type 304 stainless steel ( Sedriks, 1996 ). The specimen is then bent into a U shape and stressed to 30 ksi (207 MPa) with a bolt and nut, and placed in the testing apparatus depicted in Figure 3 , left. An insulation specimen passes the test if there are no cracks in the stainless steel surface after a 28-day period. In an alternative method ( Hutto et al., 1985 ; Whitaker et al., 1990 ), temperature is controlled with a steam-heated pipe, and the DI water is dripped over the insulating material with a peristaltic pump ( Figure 3 , right). This alternative method was incorporated to ASTM C692 in the 1990 edition, and it has the advantage that it is applicable to both wicking and non-wicking thermal insulators ( Hutto et al., 1985 ), while also reproducing more closely the type of wetting of an insulator most likely to be encountered in “real life” service. Prior to using either of the tests depicted in Figure 3 for qualification of insulating materials, the standard ( ASTM C692-13, 2018 ) requires that the test method and sensitized stainless steel must be tested with pure water (less than 0.1 ppm Cl − ) and with a 1500-ppm Cl − solution. Four coupons must crack in the Cl − solution, and none of the four coupons should crack in pure water. Notice that similar solution conditions than those of the “blank” test might produce cracking of a sensitized stainless steel tested in a SSRT. In other words, the observed or apparent threshold conditions for cracking are dependent on the test method used.

Alternative tests for laboratory studies of stress corrosion cracking under thermal insulation, after ASTM C692 ( ASTM C692-13, 2018 ).
“Thresholds” for SCC, i.e. a threshold aggressive anion concentration, potential, temperature, and stress intensity factor, are supposedly critical parameters above which SCC is possible and below which immunity is granted. Threshold values are usually defined considering material performance in short-term laboratory tests, performed under aggressive conditions ( Andresen, 2019 ). Immunity is associated with successful performance in an accelerated laboratory test, which often implies the absence of crack initiation after a certain time. For constant load and constant deflection tests, exposure time is often set to 30 days ( Sridhar et al., 2017 ). After finishing the test, specimens are analyzed; however, there could be ambiguity in the minimum dimensions that a flaw or defect must have to be considered an initiated crack. In addition, it could be argued that cracks could have initiated if the exposure time was longer than the selected test duration.
It was proposed that rather than attempting to define thresholds for SCC, dependencies of crack growth rate with SCC variables should be characterized ( Andresen, 2013 , 2019 ). The SCC crack growth rate has a complex dependence on many variables, i.e. temperature, species in solution and their concentration, pH, stress, electrochemical potential, material microstructure, and strength ( Staehle & Gorman, 2003 ). Careful experiments of in situ crack-growth rate measurements of stainless steels and nickel-based alloys in regions of supposed “immunity” to SCC in high-temperature water revealed in many instances crack propagation at a low growth rate ( Andresen, 2019 ). It was proposed that a similar situation might be valid in other material-environment systems. If crack growth rate dependency with SCC variables and the actual defect size (or resolution of non-destructive testing methods) are known ( Andresen & Ford, 1988 ), the residual life of the component or the optimum inspection interval can be assessed.
Much of the research conducted on the effect of F − , I − , and Br − on SCC was based on accelerated tests, where the effect of one of those variables is explored while keeping the others constant. The identified region of “immunity” or crack growth at a low rate will not guarantee crack propagation at a low rate if one or more of the rest of the SCC variables or loading conditions are changed. Ideally, the dependency of crack growth rate with each of the variables that control SCC should be studied, but the equipment required is more sophisticated, and tests are more expensive.
6.2 SCC in sulfuric acid+halide ion solutions
Most cases of SCC occur when the bulk surface of the stainless steel is in the passive state. However, it has been shown ( Acello & Greene, 1962 ); Asawa, 1971 , 1987 ) that dilute additions of Cl − , Br − , and I − to a H 2 SO 4 solution cause the SCC of the solution annealed type 304 stainless steel. This occurs in a specific range of halide ion concentration and at a potential close to the corrosion potential, where the alloy is actively dissolving. Outside this potential range, the alloy failed by uniform attack or water-line corrosion ( Asawa, 1987 ). Cl − , Br − , and I − acted as dissolution inhibitors when their concentrations were below 10 −1 , 2×10 −3 , and 3×10 −4 m , respectively; beyond these concentrations, they promoted uniform corrosion( Asawa, 1987 ). SCC was observed in a potential and halide ion concentration where the uniform dissolution rate was lower than 1 nm/s, regardless of the nature of the halide ion ( Asawa, 1987 ).
6.3 Effect of halides other than Cl − on SCC in high-temperature water
A few studies addressed the effect of halides other than Cl − on high temperature (around 300°C) water, an environment relevant for cooling water of nuclear power plants. The SCC in high-temperature water of types 304 and 316 stainless steel in the presence of Br − ions ( Kumada, 1996 ) is characterized by a transgranular crack path, and the reported threshold amounts of Br − and dissolved oxygen required for cracking in accelerated tests at 250°C were 50 ppm and 0.1 ppm, respectively. Fluorides at a concentration level of 1–10 ppm favored intergranular cracking in constant load tests of thermally sensitized type 304 and type 316 stainless steel in high-temperature water with 10 ppm O 2 ( Berry et al., 1973 ). Finally, it was reported ( Chung et al., 1996 ) that fluorides favor IGSCC of irradiated type 304 stainless steel, where Cr depletion at the grain boundary was caused by irradiation.
6.4 Critical potential for SCC in halide solutions
The rest of this review will mainly address SCC of stainless steels in halide solutions at lower temperatures and at bulk pH values where a passive film is stable, but might rupture locally giving localized corrosion phenomena like pitting or crevice corrosion. Under such conditions, Cl − SCC initiates from localized attack in pits and crevices ( Newman, 2001 ). It was proposed that a lower bound for the critical potential for Cl − SCC of stainless steels and other corrosion-resistant alloys is the repassivation potential for localized corrosion, E rp ( Tsujikawa et al., 1994 ; Cragnolino et al., 1996 ; Sridhar et al., 2017 ). E rp might correspond to the pitting or crevice repassivation potential, depending on the type of localized corrosion process that is occurring. Electrochemical techniques for measuring E rp are described in the literature ( Sridhar et al., 2017 ). Below this potential, any initiated crack or flaw in the passive film would be arrested by repassivation of the film ( Cragnolino et al., 1994 ). This condition of E corr > E rp for SCC crack propagation is necessary, but not a sufficient condition. Notice that an excessively high potential would cause crack blunting by pitting or crevice corrosion ( Tsujikawa et al., 1994 ; Newman, 2001 ). Crack velocity has to be larger than the rate of localized corrosion, and because an increase in temperature causes a higher increase in crack velocity due to a larger activation energy ( Che-Sheng Chen et al., 1997 ; Newman, 2001 ), this results in a critical temperature for SCC ( Che-Sheng Chen et al., 1997 ). Finally, plastic strain rate is required at the crack tip, for a local disruption of the passive film ( Andresen & Ford, 1988 ; Sridhar et al., 2017 ; Andresen, 2019 ).
Extensive testing by Tsujikawa et al. (1994 ), using spot-welded specimens of various austenitic stainless steels exposed to Cl − solutions confirmed that initiation of cracks required a potential greater than E rp . In those tests, a small sheet of stainless steel was spot-welded on top of a larger coupon, generating both a crevice and weld nuggets with residual stresses around them. The SSRT of Fe-Ni-Cr-Mo alloys 316L and 825 in Cl − solutions confirmed that at potentials below E rp , specimens failed in a ductile fashion ( Cragnolino et al., 1996 ; Pan et al., 2000 ). For the same system, using pre-cracked wedge-loaded fracture mechanics specimens, crack growth was only detected when the potential was above E rp ( Pan et al., 2000 ), and no cracks were observed below E rp in constant deflection tests ( Cragnolino et al., 1996 ). Recently, in situ measurement of crack growth rate on pre-cracked specimens confirmed ( Gui et al., 2014 ; Sridhar et al., 2017 ) a decrease in crack growth rate of almost two orders of magnitude as the potential decreased below E rp , for a 13-Cr supermartensitic stainless steel in a 0.3 m NaCl solution at 85°C.
Summarizing, depending on the test method used, it is inferred that for stainless steels in Cl − solutions at a potential below E rp , SCC initiation or crack growth rate is extremely difficult or decreases abruptly, respectively. This dependence of SCC with potential, assuming a similar cracking mechanism in solutions of the other halides, is useful for interpreting SCC results reported for the rest of the halides. For stainless steels with no or little content of Mo, the E rp ( Tzaneva et al., 2006 ) in halide solutions increases in the same order as E pit , i.e. Cl − <Br − <I − . In this regard, chlorides are often reported ( Scully, 1968 ; Davis, 1994 ; Whorlow et al., 1997 ) as the most effective of the halide ions to promote SCC of stainless steels. Likewise, considering the absence of stable localized corrosion of stainless steels in F − solutions ( Tzaneva et al., 2006 ; Macdonald & Lei, 2016 ; Pahlavan et al., 2016 ), SCC should not be expected in F − solutions. As previously mentioned, fluoride SCC is exclusively observed in sensitized microstructures ( Zucchi et al., 1988 ), probably because the chromium-depleted region near the grain boundary repassivates slower than the matrix in the presence of F − ions.
6.5 IGSCC in F − solutions
Sensitized stainless steels suffer IGSCC in the presence of F − ions ( Ward et al., 1969 ; Theus & Cels, 1974 ; Takemoto et al., 1985 ; Trabanelli et al., 1988 ; Zucchi et al., 1988 ; Shibata et al., 1993a , b ), even in dilute 5×10 −5 m (1 ppm) F − solutions at room temperature, as determined by the SSRT ( Trabanelli et al., 1988 ; Zucchi et al., 1988 ). IGSCC was also reported in constant load tests in 10 −4 m (2 ppm) F − solutions at room temperature ( Trabanelli et al., 1988 ). By comparison of results available in the literature ( Takemoto et al., 1985 ; Trabanelli et al., 1988 ; Zucchi et al., 1988 ), it is observed that IGSCC occurrence requires more aggressive conditions (higher T or higher F − concentration) in constant load or constant deflection tests than in SSRT.
6.5.1 Effect of potential on F − IGSCC
The breakdown of the passive layer was not observed in conventional polarization curves of sensitized stainless steel in F − solutions ( Zucchi et al., 1988 ), as previously discussed. In contrast, a breakdown potential ( E b ) associated to intergranular cracking was observed when stressed specimens were anodically polarized in F − solutions ( Theus & Cels, 1974 ) at 65°C. According to the authors ( Theus & Cels, 1974 ), IGSCC occurred when E corr > E b . E b decreased with pH and with F − concentration in the range of 1–1000 ppm. E corr decreased with pH, but it was fairly independent of F − concentration. Therefore, a decrease in pH or an increase in F − concentration increased cracking susceptibility. Notice the difference of this cracking condition with E corr > E rp for the SCC of stainless steel ( Tsujikawa et al., 1994 ; Cragnolino et al., 1996 ; Sridhar et al., 2017 ). The E b determined by Theus and Cels (1974 ) is a potentiodynamically determined potential for initiation , and it might vary with experimental parameters like crack incubation time and potential scanning rate. While specimens exhibited cracks when the potential was above E b , some specimens exhibited cracking when polarized below E b . A more conservative potential to prevent cracking might be the repassivation potential, E rp , measured during a backward scan in the active direction after controlled localized corrosion propagation ( Szklarska-Smialowska, 2005 ; Sridhar et al., 2017 ). Theus and Cels (1974 ) did not conduct cyclic potentiodynamic tests for E rp determination, but the existence of an arrest potential for intergranular attack in F − solutions is hypothesized. The existence of a crack arrest or critical potential in F − solutions can be inferred from tests conducted by Zucchi et al. (1988 ). The authors performed an SSRT of sensitized stainless steel wires and then after crack initiation; the deformation was kept constant. Crack propagation could be detected by monitoring the load as a function of time: crack propagation caused a decrease in load, and the load remained constant when the crack was arrested ( Zucchi et al., 1988 ). It was verified that crack propagated and arrested as the potential was switched within or outside the crack propagation potential range, respectively. For example, for a 200-ppm F − solution at 25°C, crack propagation occurred within −0.4 and +0.4 V SCE . The upper potential limit might correspond to crack blunting, but this was not discussed in depth by the authors. Finally, the minimum crack propagation rate that can be resolved with this technique was not reported in the paper.
6.5.2 Effect of sensitization on F − IGSCC
In contrast to Cl − SCC, a solution annealing heat treatment completely prevents F − IGSCC ( Ward et al., 1969 ; Theus & Cels, 1974 ). Transgranular branching and pitting attack commonly observed in tests of Cl − SCC of sensitized type 304 SS are not observed in F − solutions ( Ward et al., 1969 ). Those results suggest that F − attack is restricted to the intergranular region. Theus and Cels (1974 ) argue that the dependence of IGSCC with parameters of the sensitizing thermal cycle is difficult to assess. This issue could be solved with a systematic study of the dependence of F − IGSCC with the degree of sensitization (DOS). The thermal cycle affects DOS, which can be defined as the extent of Cr depletion near the grain boundary ( Parvathavarthini & Mudali, 2014 ). DOS can be quantified by coverage (fraction of total grain boundary depleted in Cr), depth (minimum concentration of Cr), and width (distance from grain boundary with a depleted Cr content) ( Parvathavarthini & Mudali, 2014 ). DOS can be estimated with electrochemical techniques, as recently reviewed by Parvathavarthini and Mudali (2014 ).
6.5.3 Effect of temperature and F − concentration on F − IGSCC
Similar to the case for Cl − SCC ( Speidel, 1981 ), an increase in temperature from 25°C to 80°C increases the kinetics of the attack, according to Zucchi et al. (1988 ) and Ward et al. (1969 ). Measurements of time to failure at constant load and the ductility loss in the SSRT suggest that the aggressiveness of F − increases with its concentration in the solutions, from 10 −5 to 10 −2 m (0.2–200 ppm F − ) ( Trabanelli et al., 1988 ; Zucchi et al. 1988 ). This is in accord with the decrease in breakdown potential in anodic polarization curves of stressed specimens reported by Theus and Cels (1974 ), from 5×10 −5 to 5×10 −2 m (1–1000 ppm F − ). Some authors claim that a maximum susceptibility to IGSCC occurs at an intermediate F − concentration. Using SSRT, Shibata et al. (1993 b ) showed that the region of maximum susceptibility is around 450 ppm F − (0.02 m ). Likewise, Ward et al. (1969 ) reported that solutions with a concentration around 1 m F − (20,000 ppm F − ) do not cause IGSCC. Adding Cl − ions to a the F − solution did not change the kinetics or mode of attack; hence, a synergism between those ions was discarded ( Ward et al., 1969 ). Whorlow et al. (1997 ) drew similar conclusions, after adding F − ions to a Cl − solution. This solution had a Cl − concentration just below the threshold amount required for SCC in constant deflection tests, as will be discussed in detail in the next section.
6.5.4 SCC of stainless steels under thermal insulation
SCC in F − and F − +Cl − solutions were mainly studied ( Takemoto et al., 1985 ; Whorlow et al., 1997 ; Whorlow and Hutto, 1997 ) because those ions are common impurities found in materials for thermal insulation. This failure mechanism is also known as ESCC, where the “ E ” means that the water and halides involved in the cracking mechanism are external to the pipe, tank, or vessel. Pipes conveying high-temperature fluids are commonly wrapped with thermal insulation to minimize heat losses and to protect nearby personnel. Water from rain, nearby processes, or arising from steam condensation can wet the thermal insulation ( Dana, 1957 ; Ahluwalia, 2006 ). Alternatively, vapor from the atmosphere can condensate on a surface that is temporarily below the dew point ( Ahluwalia, 2006 ). Water lixiviates impurities in the thermal insulation, and upon contact with the hot stainless steel surface, those impurities are concentrated by evaporation of the solvent ( Dana, 1957 ; Ahluwalia, 2006 ). If aggressive species, namely, F − +Cl − , are originally present in the thermal insulation material, this leaching and concentration process can create optimum environmental conditions for the SCC of the stainless steel. Most failures occur in equipment with the stainless steel surface at a temperature between 50°C and 175°C ( Ahluwalia, 2006 ; NACE, 2017 ). Below this temperature, the concentration by evaporation is not significant, and the kinetics of SCC is low, and above 175°C, liquid water presence on the stainless steel surface is less frequent. More conservatively ( McIntyre, 1985 ), the upper temperature is listed as 260°C, the temperature below which some chloride salts retain their hydration water. However, even in equipment operating above the maximum temperature, it is necessary to consider possible SCC occurrence during start-up and shutdown ( McIntyre, 1985 ).
The SCC of stainless steel under insulation, like any other SCC problem, requires tensile stresses on susceptible materials exposed to a given environment. Most stainless steel products contain sufficient residual stresses to cause SCC in an aggressive environment even without applied stresses ( NACE, 2017 ). This situation worsens if welding or cold work is involved in the fabrication process. Decreasing stress can be impractical, and changing the material is economically infeasible, especially in operating plants. Therefore, most strategies for prevention of ESCC focus on the environment. Standard specifications, like ASTM C795 ( ASTM C795-08, 2018 ), provide limits for the maximum concentration of Cl − and F − in thermal insulation in contact with stainless steel. Thermal insulation has to be qualified in a preproduction standard test (ASTM C692-13, 2018), Figure 3, where it is tested to determine if the amount of leachable Cl − , F − , and inhibitors in the thermal insulator can cause SCC in a sensitized type 304 stainless steel stressed specimen at high temperature. Once the chemical composition and production process of the insulator is thus qualified, standard test method ASTM C871 ( ASTM C871-182018 ) is used to measure the chemical composition, specifically the concentration of chlorides, fluorides, sodium, and silicates of subsequent production lots. The maximum acceptable concentrations of Cl − and F − in the insulator are indicated in standard ASTM C795 ( ASTM C795-08, 2018 ), as a function of the concentration of inhibiting species (sodium and silicates). Regulatory guide US NRC 1.36 ( US Nuclear Regulatory Commission 2015 ), applicable to thermal insulation to be used in nuclear power plants, contains similar guidelines as those of ASTM C795. The historical evolution of those standards is briefly discussed below, and it is of interest because it shows changes in perception on the aggressiveness of F − .
6.5.5 Historical evolution of standards related to SCC of stainless steels under thermal insulation
Karnes in the 1960s determined that SCC can be inhibited if a certain amount of sodium and silicates are present in the thermal insulation material ( Whitaker et al., 1990 ), and the results were reported in a Cl − vs. Na + +silicates graph, known as the acceptability curve ( Figure 4 ). In this graph, an arbitrary line separates the compositions of the insulator that cause the SCC in the stainless steel from those that do not. The line was traced considering that if no more than one out of four specimens failed, the test was considered a “pass” ( Whitaker et al., 1990 ). While Karnes originally published the acceptability curve with Cl − in the ordinate, the United States Atomic Energy Commission (USAEC) published the Regulatory Guide (RG) 1.36 in 1973 ( US Atomic Energy Commission, 1973 ), where the ordinate was changed to Cl − +F − . Accordingly, the maximum Cl − +F − content in the insulator was limited to 600 ppm. According to Whitaker et al. (1990 ), F − was added to this regulatory guide because of the “chemical similarity and normally greater chemical aggressiveness of the fluoride,” rather than based on experimentally measured effects of F − on SCC. The United States military standard MIL-I-24244 ( MIL-I-24244A 1974 ) issued in 1974 did not contain the F − requirement, but this was added in a subsequent revision, to be consistent with RG 1.36 ( Whitaker et al., 1990 ). Furthermore, the minimum sodium+silicate content was fixed at 50 ppm. ASTM C795, first published in 1977, did not contain the fluoride requirement in the acceptability curve ( Figure 4 ), until the 2008 version, where it was added to “be consistent with other standards” (ASTM C795-08, 2018).

Acceptability curve of thermal insulation material, based on analysis of leachable halides (Cl − or Cl − +F − , depending on the standard or specification as indicated) and leachable inhibitors (sodium+silicate). The diagonal line was first proposed by Karnes ( Whitaker et al., 1990 ), and below this line, one out of four or zero out of four stainless steel specimens failed in an accelerated SCC test. Karnes line was adopted by subsequent standards and specifications, with major modifications as indicated.
The F − +Cl − vs. Cl − controversy in the ordinate of the Karnes acceptability graph was studied in depth by Whorlow and Hutto (1997 ), in a report prepared for the US Nuclear Regulatory Commission and later published by ASTM ( Whorlow et al., 1997 ). The experimental setup was similar to the one depicted in Figure 3 (right), but instead of dripping DI water to leach ions from the insulator block, different solutions were dripped directly over the type 304 sensitized stainless steel surface, at a fixed flow rate for 28 days. The authors ( Whorlow & Hutto, 1997 ) provided a chart to convert the concentration of ions in the solution (in mg/l) to the equivalent concentration in the insulator (in mg/kg), considering that in a real qualification test, the same flow rate would drip over the insulator but with DI water. In the absence of inhibiting species like sodium and silicates, the authors concluded that F − ions cause SCC when they are in a concentration above 20 mg/kg (20 ppm) in the insulator. This is equivalent ( Whorlow & Hutto, 1997 ) to 0.8 ppm of F − in solution, which is further concentrated at the stainless steel surface by water evaporation. Therefore, this susceptibility to SCC of type 304 sensitized stainless steel in F − solutions is in accord to the results reported elsewhere ( Ward et al., 1969 ; Theus & Cels, 1974 ; Zucchi et al., 1988 ) with different experimental setups.
In comparison to Cl − ions, SCC in F − can be inhibited with considerably lower amounts of sodium and silicates, as summarized in Figure 5 ( Whorlow & Hutto, 1997 ). Furthermore, when F − ions were added to a solution of Cl − , sodium, and silicate originally below the Karnes acceptability curve, SCC was not observed, despite the total Cl − +F − content of the solution was in the cracking region of the acceptability graph ( Figure 6 ). A single exception was the test run at 1000 ppm sodium+silicate and 100 ppm Cl − +35 ppm F − , marked with an arrow in Figure 6 . Based on those results, the authors ( Whorlow & Hutto, 1997 ) discarded a synergistic effect between Cl − and F − , in accord with research previously published by Ward et al. (1969 ). In other words, the amount of silicate and sodium required to inhibit Cl − SCC seems to be sufficient to inhibit the effect of further additions of F − ( Whorlow & Hutto, 1997 ). Despite those results, when the US Nuclear Regulatory Commission (NRC) revised RG 1.36 in 2015 ( US Nuclear Regulatory Commission, 2015 ), both Cl − and F − were considered as equally aggressive species and placed in the ordinate of Karnes acceptability graph. By this time, ASTM had already included F − in the ordinate of Karnes graph, in the 2008 version of ASTM C795 ( ASTM C795-08, 2018 ). The International Atomic Energy Agency (IAEA) published in 2011 the report “Stress corrosion cracking in light water reactors: good practices and lessons learned” ( International Atomic Energy Agency, 2011 ), where the Karnes figure with Cl − +F − in the ordinates was reproduced. In summary, current standards and regulatory guides on ESCC of austenitic stainless steels consider F − as aggressive as Cl − , despite contrary conclusions drawn in laboratory tests.

SCC of type 304 sensitized stainless steel in fluoride and sodium+silicate solutions; results are presented superimposed to ASTM C795 criteria for acceptability of thermal insulators ( Whorlow & Hutto, 1997 ).

Effect of fluoride addition to a chloride, sodium, and silicate solution that passed the 28-day SCC tests. Karnes line given as reference. Except for the solution concentration marked with an arrow, no SCC was observed in Cl − +F − solutions after 28 days. Adapted from Whorlow KM, Woolridge E0, and Hutto FB, Effect of halogens and inhibitors on the external stress corrosion cracking of type 304 austenitic stainless steel, Insulation materials: testing and applications: third volume, ASTM STP 1320, R.S. Graves and R.R. Zarr, Eds., copyright ASTM International, 100 Barr Harbor Drive, West Conshohocken PA19428, www.astm.org .
6.5.6 Some limitations of standards related to SCC of stainless steels under thermal insulation
Whorlow and coworkers ( Whorlow & Hutto, 1997 ; Whorlow et al., 1997 ) reported that the inhibitor effectiveness was dependent on the type of silicate; the order of increasing effectiveness was sodium disilicate (Na 2 Si 2 O 5 ), sodium metasilicate (Na 2 SiO 3 ) and sodium orthosilicate (Na 4 SiO 4 ). In other words, summarizing criticism on the acceptability curve of thermal insulation material ( Figure 4 ), neither the aggressive ions plotted in ordinate or the inhibiting ions in the abscissa seem to be equally effective in promoting or preventing ESCC, respectively. Whorlow and coworkers reported the SCC of sensitized stainless steels in environments with chloride and sodium+silicate concentrations below the Karnes line, in other words, in the zone of acceptable analysis ( Whorlow & Hutto 1997 ; Whorlow et al., 1997 ). The occurrence of SCC failures below the Karnes curve is not surprising because this curve was traced following the criterion that if none or one out of four SCC specimens failed for a given thermal insulator composition, the point was considered a pass ( McIntyre, 1985 ; Whitaker et al., 1990 ). Current recommendations of ASTM C 795 require that the preproduction SCC test (ASTM C692-13, 2018) is passed if none of the specimens exhibit cracks. Lots produced with this validated production method and using similar ingredients are acceptable if their chemical composition falls inside the acceptable region ( Figure 4 ) or if their chemical composition was validated with a pass in a preproduction SCC test. US REG 1.36 ( US Nuclear Regulatory Commission, 2015 ) is more conservative because it not only requires that the chemical composition of the insulator falls in the acceptable region ( Figure 4 ) but also that the chemical analysis of the Cl − +F − content of the lot does not exceed 150% of the average values measured during preproduction qualification tests and that the sodium and silicate content is not below 50% of the average amount of those inhibitors measured during successful preproduction qualification tests.
Takemoto et al. (1985 ) questioned the 600 ppm high chloride limit in the acceptability graph ( Figure 4 ). Using constant deflection tests with an initial stress above that required by ASTM C692 ( ASTM C692-13, 2018 ), they proposed a threshold of 2000 ppm for a sensitized type 304 stainless steel and 3000 ppm for a solution annealed stainless steel, exposed to 95°C solutions. The difference in apparent Cl − threshold concentration values could be explained considering its strong dependence with the rest of the testing variables.
In contrast to other studies ( Ward et al., 1969 ; Whorlow & Hutto, 1997 ), Takemoto et al. (1985 ) suggest a synergistic effect of F − and Cl − , indicating that the 600 ppm high Cl − limit can be much lower in the presence of F − , decreasing to 200 ppm when the F − concentration is 100 ppm. The authors state that F − aggressiveness is maximum at 50°C, which is in conflict with results later published by Zucchi et al. (1988 ), where aggressiveness of F − was higher at 80°C. Figure 7 summarizes the effect of F − and Cl − on the SCC of sensitized stainless steels at 50°C, according to Takemoto et al. (1985 ). The line that separates the SCC vs. No SCC zone was constructed based on laboratory tests in NaF and NaCl solutions without added silicates.

Summary of the effect of fluorides and chlorides on SCC at 50°C, for a solution without silicates and a type 304 sensitized stainless steel under constant deflection. Adapted from Takemoto et al. (1985 ). © NACE International, 1985.
A major limitation of ASTM standards for ESCC management is that they only measure the capacity of inhibitors in the insulating material to guard against SCC failures caused by aggressive species in the insulating material. As reviewed by McIntyre (1985), Takemoto et al. (1985 ), and Hutto et al. (1985 ), and NACE International Standard SP0198-2017 ( NACE, 2017 ), other potential sources of Cl − of industrial relevance are the atmosphere, especially near coastal or industrial areas, or rainwater, processed, or potable water that might accidentally contact the thermal insulation. Insulation materials might have an excess of inhibitors to mitigate the effect of aggressive ions introduced from external sources. The ability of the insulation material to inhibit the effect of aggressive ions from outside sources can be tested using any of the experimental setups in Figure 3 , if a Cl − solution is used instead of DI water. In the “accelerated Dana test” a 1500-ppm (mg/l) Cl − solution is used ( Hutto et al., 1985 ; Whitaker et al., 1990 ), and an additional benefit is that the testing time is reduced from 28 to 6 days. However, it has not yet been included as an alternative procedure in ASTM C692. While inhibitors in the thermal insulator can offset the effect of externally introduced aggressive ions, it has to be noticed that if the equipment is severely wetted, the inhibitor might be leached and transported away from the surfaces needing inhibition ( NACE, 2017 ).
If water reaches the stainless steel surface through cracks in the thermal insulator without soaking through the insulation ( McIntyre, 1985 ), the amount of leachable aggressive and inhibiting species in the insulator is not so relevant, and cracking can be controlled by the Cl − or F − concentration in the water. Guidelines for preventing water ingress to the thermal insulation are listed in the NACE International Standard SP0198-2017 ( NACE, 2017 ). This standard addresses corrosion under insulation (CUI) under both stainless and carbon steels. Despite differences in their corrosion mechanisms, preventing water contact to the external surface of equipment is a common corrosion control strategy. An additional ESCC control strategy is the use of coatings or aluminum foil wrapping ( Richardson & Fitzsimmons, 1985 ; NACE, 2017 ). The aluminum foil provides an additional barrier that prevents water and ions to be in contact with the stainless steel surface ( Richardson & Fitzsimmons, 1985 ). More important, aluminum provides cathodic protection and decreases the corrosion potential of the stainless steel surface ( Richardson & Fitzsimmons, 1985 ), which is effective for preventing Cl − and F − SCC ( Smialowski & Rychcik, 1967 ; Rhodes, 1969 ; Uhlig & Cook, 1969 ; Theus & Cels, 1974 ).
Finally, it is noticed that the methodology of ESCC management with ASTM standards was developed based on results obtained with accelerated tests with smooth stainless steel coupons. However, if flaws or cracks are detected on a stainless steel component, it cannot be assured that they will not propagate during further use, even if new thermal insulation is installed, and its composition lays within the acceptability zone of Figure 4 . Under such a scenario, knowledge of dependence of SCC growth rate with stress and environmental and material variables would be of much greater use for assessing the remaining life of the component ( Andresen & Ford, 1988 ).
6.6 Br − stress corrosion cracking
Pitting and crevice corrosion of stainless steels is observed in Br − solutions, even in the fully solubilized condition. Therefore, considering a similar mechanism for SCC in Br − vs. Cl − , one of the necessary ( Tsujikawa et al., 1994 ; Che-Sheng Chen et al., 1997 ; Newman, 2001 ; Sridhar et al., 2017 ) conditions for SCC would be fulfilled if E Corr > E rp . Br − SCC was reported in environments of industrial interest, like completion fluids for the oil and gas industry ( Downs et al., 2007 ; Sridhar et al., 2017 ) and brines for absorption refrigeration systems ( Griess et al., 1985 ; Itzhak & Elias, 1994 ; Itzhak et al., 1996 ). With regard to laboratory tests, Rhodes (1969 ) reported SCC in 304 stainless steel exposed to 59% MgBr 2 solutions at 150°C, but cracking was not observed when the solution was more dilute than 36%. As a comparison, the SCC of 304 stainless steels occurs readily in accelerated laboratory tests in 20% MgCl 2 at 105°C ( Brauns & Ternes, 1968 ), and more generally, at a temperature above 100°C, just a few ppm of Cl − can cause SCC ( Haruyama 1982 ) in industrial equipment. Therefore, albeit probably not as aggressive as Cl − , the evidence reported suggests that concentrated, hot Br − brines can cause SCC of stainless steels even in the fully solubilized condition.
Mo increases resistance to both localized corrosion and SCC of stainless steels in Cl − solutions ( Speidel, 1981 ; Sedriks, 1996 ; Prosek et al., 2009 ). This higher resistance is reflected in material selection guidelines; for example, to prevent Cl − SCC in marine atmospheric environments, Norsok standard M-001 ( Norsok 2014 ) sets the maximum operating temperature of type 6 Mo stainless steel in 120°C, higher than the 60°C set for a type 316 stainless steel (2.5 wt.% Mo). As illustrated in Figure 2 , for alloys with a high concentration of Mo like stainless steel type 904L, Br − can be more aggressive toward pitting than Cl − . This higher aggressiveness of Br − is also evidenced by a large decrease in CPT and CCT ( Guo & Ives, 1990 ; Abd El Meguid, 1997 ; Ozturk & Grubb, 2012 ). An unsolved question is whether the higher aggressiveness of Br − vs. Cl − toward pitting and crevice corrosion in high Mo stainless steels implies also a higher SCC susceptibility in Br − vs. Cl − solutions.
6.6.1 SCC in Br − containing completion fluids for oil and gas wells
Completion fluids used in high pressure, high-temperature oil, and gas wells might contain large concentrations of Br − . When contaminated with O 2 , or acid gases like CO 2 and H 2 S, those brines can cause SCC in corrosion-resistant alloys like martensitic stainless steels (13 Cr-1Mo, 13 Cr-2Mo) ( Downs et al., 2007 ). SCC was reported in laboratory studies of 13 Cr-2Mo stainless steel exposed to CO 2 and H 2 S solutions at both 160°C and 40°C in concentrated Br − solutions. Formate brines are often used in replacement of bromide brines to mitigate this problem ( Downs et al., 2007 ; Sridhar et al., 2017 ).
6.6.2 SCC in Br − brines for absorption refrigeration systems
LiBr commercial brines for use in absorption refrigeration systems can contain lithium chromates and lithium hydroxide ( Griess et al., 1985 ; Igual Muñoz et al., 2003 ), added as corrosion inhibitors. These systems operate under deaerated conditions, and besides the problem of integrity of materials, the release of hydrogen by the cathodic reaction at the corrosion potential produces non-condensable gases that decrease efficiency ( Chandler, 1999 ). Desired corrosion rates in LiBr brines are below 1 mpy (25 μm/year). Various stainless steels and carbon steels in presence of chromates or other inhibitors fulfill this constraint ( Chandler, 1999 ). The presence of chromates and deaerated conditions contribute to lower the corrosion potential ( Igual Muñoz et al., 2003 ) and mitigate pitting corrosion. However, the high operating temperature (~150°C) might favor SCC.
With regard to SCC of stainless steels in LiBr brines, in a preliminary report, Griess et al. (1985 ) studied the SCC of austenitic type 304 stainless steel in 68% LiBr solutions at 160°C, conditions similar to those encountered in absorption refrigeration systems. The presence of oxygen was required for cracking of U-bend specimens, and none out of 20 specimens cracked under deaerated conditions ( Griess et al., 1985 ). On the other hand, the SCC of types 304 and 316 stainless steels was reported by Itzhak et al. in 55% LiBr brines at 120°C and 140°C in deaerated conditions, both with the constant load ( Itzhak & Elias, 1994 ) and slow strain rate technique ( Itzhak et al., 1996 ). Specimens were studied in the “as-received” state ( Itzhak & Elias, 1994 ; Itzhak et al., 1996 ), which included a 15% cold work estimated by comparison to fully annealed specimens of the same heat. The SCC crack path was generally transgranular, but depending on loading mode and temperature, mixed IG and TG cracks were observed for type 316 stainless steel. Itzhak et al. studied SCC in brines with pH varying from 6 to 11.6, and time to failure generally increased with increasing pH. The content of chlorides present as potential impurities in LiBr brines was not measured, and two different grades of LiBr salts were used by the authors, i.e. “analytical” ( Itzhak & Elias, 1994 ) and “commercial” ( Itzhak et al., 1996 ) grade.
Considering the concentration of LiBr solutions used in studies of evaluation of stainless steel for absorption refrigeration systems, it could be argued that trace amounts of chlorides in the salt could explain the SCC process. For example, Griess et al. (1985 ) reported that 37 ppm of chlorides were present in the brine as impurities from the LiBr salt, enough to cause chloride SCC at 160°C even in absence of Br − ( Sedriks, 1996 ). Despite this, the authors argue that Cl − was not responsible for cracking because the Cl − SCC inhibitors like chromate did not inhibit cracking in the concentrated LiBr salt ( Griess et al., 1985 ). However, the role of chromate as a Cl − SCC inhibitor is disputed ( O’Dell & Brown, 1978 ) because despite stabilizing the passive film, it can raise the electrochemical potential. Stronger evidence to support the observation that cracking was actually due to Br − is that according to U-bend constant deflection SCC tests conducted by Rhodes (1969 ), intentional additions of Cl − to 36% MgBr 2 solutions did not induce cracking in type 304 stainless steel specimen, but SCC was observed in 59% MgBr 2 solutions at 150°C.
6.6.3 Br − SCC under thermal insulation
The effect of Br − on SCC of stainless steel under thermal insulation was not as deeply studied as the effect of F − and Cl − , probably because they are not as widely found in insulation as the lighter halides ( Whorlow et al., 1997 ). The SCC of sensitized type 304 stainless steels in Br − solutions was reported with an experimental setup similar to that shown in Figure 3 (right) ( Whorlow et al., 1997 ). The presence of SCC cracks and pits was reported in sensitized type 304 stainless steel U-bend coupons heated to 100°C and exposed to 1500 ppm Br − solutions (added as KBr), which was directly dripped over the stainless steel coupon. Under this experimental setup, the solution was concentrated by water evaporation at the hot stainless steel surface, so it is difficult to compare those experiments with those conducted in LiBr brines. The Br − SCC of sensitized stainless steel could be inhibited with sodium orthosilicate, with a concentration of inhibitor that was much lower than required to inhibit Cl − SCC ( Whorlow & Hutto, 1997 ). Br − ions were never included in ordinates of the acceptability curve of thermal insulation material ( Figure 4 ). However, some analytical techniques ( ASTM C871-18, 2018 ) to determine the Cl − concentration in thermal insulation cannot discriminate between Cl − , Br − , and I − , thus providing a false higher concentration of Cl − . The concentration of inhibitor required in the thermal insulator would be increased accordingly, and considering that Cl − is the most effective of those halides to cause ESCC in sensitized stainless steel, the possible effect of Br − and I − would be inhibited as well ( Whorlow & Hutto, 1997 ).
6.6.4 SCC and localized corrosion in bromine compounds in desalination plants
The presence of Br − ions in seawater can lead to corrosion issues in stainless steels used for multi-stage flash (MSF) desalination plants ( Oldfield & Todd, 1981 ). Despite Cl − being more concentrated in seawater than Br − , Table 1, two different mechanisms ( Oldfield & Todd, 1981 ; Lee et al., 1983 ) were proposed for the enrichment of bromine species in the vapor phase. Chlorine (Cl 2 ) is usually added to feed-water as a biocide, and it can oxidize Br − to Br 2 , a reaction that is favored when pH is lower than 6 ( Oldfield & Todd, 1981 ). Alternatively, bromamines can evolve from chlorinated seawater, if ammonia is present in seawater ( Lee et al., 1983 ). Bromine and bromamines are then removed from the MSF plant together with non-condensable gases. Bromamines can decompose to hydrobromic acid and free bromine ( Lee et al., 1983 ). Bromine is more oxidizing than oxygen ( Lee et al., 1983 ), thus it raises the corrosion potential and favors pitting, crevice corrosion, and SCC. The SCC of type 316L stainless steel due to the presence of bromine species was reported in air ejector condenser systems ( Black & Morris, 1981 ; Nordin, 1983 ) and venting pipes of MSF plants ( Lee et al., 1983 ). Pitting and uniform corrosion of 316L were also reported in Br 2 solutions ( Hodgkiess et al., 1985 ), and it was related to bromine reduction reaction, Eq. (1) that yields bromides. To prevent this type of failure, it was proposed to use higher alloyed stainless steel like 904L ( Black & Morris, 1981 ; Nordin, 1983 ) or strict control of pH, chlorination level, and ammonia content in feedwater ( Oldfield & Todd, 1981 ; Lee et al., 1983 ).
6.7 SCC in the presence of iodides and iodine
The SCC of zirconium alloys used for nuclear power reactor fuel cladding in iodine, a byproduct of the uranium fission reaction, is a well-documented problem ( Cox, 1972 ; Wood, 1972 ; Sidky, 1998 ). While zirconium alloys are widely used as cladding in water-cooled reactors operating at temperatures close to 300°C, studies ( Lobb & Jones, 1976 ; Lobb & Nicholson 1976 ; Lobb, 1978 ; Kiselevskii et al., 1993 ) conducted at 650°C and 750°C report that iodine vapor decreases creep resistance of austenitic stainless steels, a problem relevant for fuel cladding integrity of fast and advanced cooled reactors. Likewise, I 2 and I − can be generated in aqueous homogeneous reactors, where uranium salts are dissolved in an aqueous solution that serves as moderator and coolant ( Lillard, 2010 ). As reviewed by Lillard (2010 ), pitting and SCC of stainless steel in iodine species can affect the integrity of the off-gas extraction system of those reactors, required to remove the gases produced by radiolysis.
E pit and E rp of stainless steels in I − solutions are larger than in Br − or Cl − solutions ( Rhodes, 1969 ; Tzaneva et al., 2006 ; Macdonald & Lei, 2016 ). Therefore, the E Corr > E rp ( Tsujikawa et al., 1994 ; Che-Sheng Chen et al., 1997 ; Sridhar et al., 2017 ) criterion predicts SCC resistance in a wider range of potential in I − vs. the rest of the halide solutions. Furthermore, under open-circuit conditions, the requirement that E Corr > E rp ( Tsujikawa et al., 1994 ; Che-Sheng Chen et al., 1997 ; Sridhar et al., 2017 ) is usually fulfilled by the presence of oxygen in the solution. However, iodide solutions are unstable in the presence of oxygen, reacting with it to give iodates or iodine (Eqs. (2) and (11) ( Pinkus et al., 1981 ; Itzhak & Eliezer, 1983 ):
Given those considerations, in the case of I − solutions, the reactants required for halide stress cracking might not be present simultaneously under common open-circuit conditions.
Nevertheless, SCC was observed in sensitized type 304 stainless steel. Whorlow and Hutto (1997 ), using an experimental setup similar to the one in Figure 3 (right), reported SCC in I − solutions, added as KI. However, cracks were much shorter than those observed in chlorides, and cracking could be inhibited with silicates. In a previous attempt, SCC was not observed in iodide solutions ( Whitaker et al., 1990 ), but this was explained based on a possible low carbon content of the stainless steel used. Current recommendations of ASTM C692 ( ASTM C692-13, 2018 ) for evaluation of thermal insulation require a solution annealed type 304 stainless steel with carbon content in the range of 0.05–0.06%, which is then sensitized for 3 h at 649°C.
6.8 Inhibition of Cl − SCC by I − and I 2
Some researchers propose that iodide and iodine can act as inhibitors of SCC in Cl − and Br − solutions. Slow strain rate test (SSRT) studies ( Huang et al., 1993 ) of solution annealed Ti-stabilized 321 stainless steel (UNS S32100) concluded that I − and I 2 inhibit SCC of stainless steels in 0.5 m NaCl+0.5 m HCl solutions at 55°C, with I − being more effective than I 2 . The fracture surface in the presence of 5 mmol/l KI was ductile with the presence of dimples. Similarly ( Uhlig & Cook, 1969 ), additions of NaI to the boiling MgCl 2 SCC test solution increased the time to failure of solution annealed and cold-worked specimens of 304 stainless steel under constant load conditions, and no cracks were observed after 200 h of testing when more than 3.7% of NaI were added to the MgCl 2 solution. According to Itzhak et al. ( Pinkus et al., 1981 ; Itzhak & Eliezer 1983 ), I − acts as a cathodic inhibitor of chloride SCC, reacting with oxygen and protons according to Eq. (11). Evidence for the occurrence of this reaction was that the solution turned to yellow-brown color. The corrosion potential in boiling 38% MgCl 2 +15% NaI was 200 mV lower than the corrosion potential in boiling 38% MgCl 2 ( Pinkus et al., 1981 ). A decrease in corrosion potential should contribute to inhibition of chloride SCC in boiling MgCl 2 solutions ( Smialowski & Rychcik, 1967 ; Rhodes, 1969 ; Uhlig & Cook, 1969 ). Additions of 1% KI to a 55% LiBr brine of pH=4 also inhibited Br − SCC of type 316 stainless steel ( Itzhak et al., 1996 ). Finally, some constant deflection tests performed on type 304 stainless steel with a setup similar to that shown in Figure 3 (right) ( Whorlow et al., 1997 ) suggest that I − is an ineffective inhibitor of Br − and F − SCC when the stainless steel is sensitized.
7 Summary and conclusions
Pitting and SCC of stainless steels is possible in halides other than chlorides. Several examples where this problem manifested in the industry were presented. Chloride is not only the halide most commonly encountered in environments of industrial relevance but also in most conditions the most aggressive. There is general agreement that the localized corrosion initiation ( E pit ) and repassivation potential ( E rp ) of stainless steels increase in the following order, Cl − <Br − <I − . The intensity of SCC in halide solutions decreases in the same order, which can be understood considering that a necessary condition for SCC is that E Corr > E rp . A notable exception is that pitting in Br − solutions can be more aggressive than in Cl − solutions, for alloys with a high content of molybdenum. However, it is yet unsolved if high molybdenum stainless steels are more susceptible to SCC in Br − than in Cl − solutions. Detailed knowledge of the effect of alloying elements on Br − localized corrosion and SCC would allow more rationality in the selection of stainless steels for bromide service.
For solubilized stainless steels, SCC was not observed in F − and I − solutions. For F − solutions, this could be related to the pitting and localized corrosion immunity in those solutions. However, a sensitizing heat treatment decreases chromium content at the grain boundary, favoring localized rupture, and intergranular SCC is possible in the presence of stress. Passivity breakdown of stainless steels in iodide solutions would require excessively high potentials, and furthermore, iodides are unstable in the presence of dissolved oxygen, reacting with it to yield iodates and iodine. Hence, several researchers propose that iodides act as inhibitors for SCC of stainless steels in bromides and chlorides.
About the author

Acknowledgments
The author thanks Dr. Martín Rodríguez for helpful comments on the manuscript.
Abd El Meguid EA. Pitting corrosion behavior of type 904L stainless steel in sodium bromide solutions. Corrosion 1997; 53: 623–630. 10.5006/1.3290295 Search in Google Scholar
Abd El Rehim SS, Abd El Wahaab SM, Abdel Maguid EA. Electrochemical behaviour of nickel anode in H 2 SO 4 solutions and the effect of halide ions. Mater Corros 1986; 37: 550–555. 10.1002/maco.19860371006 Search in Google Scholar
Acello SJ, Greene ND. Anodic protection of austenitic stainless steels in sulfuric acid-chloride media. Corrosion 1962; 18: 286t–290t. 10.5006/0010-9312-18.8.286 Search in Google Scholar
Ahluwalia HS. Corrosion under insulation. In: Cramer SD, Covino BS, editors. ASM handbook Vol. 13C: corrosion: environments and industries. Materials Park, OH: ASM International, 2006: 654–658. Search in Google Scholar
Andresen PL. Stress corrosion cracking of current structural materials in commercial nuclear power plants. Corrosion 2013; 69: 1024–1038. 10.5006/0801 Search in Google Scholar
Andresen PL. A brief history of environmental cracking in hot water. Corrosion 2019; 75: 240–253. 10.5006/2881 Search in Google Scholar
Andresen PL, Ford PF. Life prediction by mechanistic modeling and system monitoring of environmental cracking of iron and nickel alloys in aqueous systems. Mater Sci Eng A 1988; A103: 167–184. 10.1016/0025-5416(88)90564-2 Search in Google Scholar
Asawa M. Stress corrosion cracking of 18-8 austenitic stainless steel in sulfuric acid. Tetsu-to-Hagane 1971; 57: 1340–1349. 10.2355/tetsutohagane1955.57.8_1340 Search in Google Scholar
Asawa M. Stress corrosion cracking regions on contour maps of dissolution rates for AISI 304 stainless steel in sulfuric acid solutions with chloride, bromide, or iodide. Corrosion 1987; 43: 198–203. 10.5006/1.3583136 Search in Google Scholar
ASTM A380-17. Standard practice for cleaning, descaling, and passivation of stainless steel parts, equipment, and systems. West Conshohocken, PA: ASTM, 2017. Search in Google Scholar
ASTM C692-13. Standard test method for evaluating the influence of thermal insulations on external stress corrosion cracking tendency of austenitic stainless steel. West Conshohocken, PA: ASTM, 2018. Search in Google Scholar
ASTM C795-08. Standard specification for thermal insulation for use in contact with austenitic stainless steel. West Conshohocken, PA: ASTM, 2018. Search in Google Scholar
ASTM C871-18. Standard test methods for chemical analysis of thermal insulation materials for leachable chloride, fluoride, silicate, and sodium ions. West Conshohocken, PA: ASTM, 2018. Search in Google Scholar
Berry WE, White EL, Boyd WK. Stress corrosion cracking of sensitized stainless steel in oxygenated high temperature water. Corrosion 1973; 29: 451–469. 10.5006/0010-9312-29.12.451 Search in Google Scholar
Black DW, Morris RM. Experience in commissioning large desalination plants in the middle east. Desalination 1981; 39: 229–239. 10.1016/S0011-9164(00)86127-5 Search in Google Scholar
Bocher F, Huang R, Scully JR. Prediction of critical crevice potentials for Ni-Cr-Mo alloys in simulated crevice solutions as a function of molybdenum content. Corrosion 2010; 66: 055002-1–055002-15. 10.5006/1.3430462 Search in Google Scholar
Brauns E, Ternes H. Untersuchungen Über Die Transkristalline Spannungsrißkorrosion Austenitischer Chrom-Nickel-Stähle in Heißen Chloridlösungen. Werkstoffe Korros 1968; 19: 1–19. 10.1002/maco.19680190102 Search in Google Scholar
Brown BF. Stress corrosion cracking control measures (NBS Monograph 156). Washington, DC: National Bureau of Standards, 1977. 10.21236/ADA043000 Search in Google Scholar
Carroll WM, Lynskey EE. A crevice-free electrode assembly for the determination of reproducible breakdown potentials for stainless steels in halide environments. Corros Sci 1994; 36: 1667–1678. 10.1016/0010-938X(94)90061-2 Search in Google Scholar
Chambers C, Holliday AK. Modern inorganic chemistry. London, U.K.: Butterworth & Co., 1975. Search in Google Scholar
Chandler T. Absorption chiller corrosion protection-SBIR Phase I Final Report – DOE Grant DE-FG03-98ER82658, 1999. Search in Google Scholar
Che-Sheng Chen P, Shinohara T, Tsujikawa S. Applicability of the competition concept in determining the stress corrosion cracking behavior of austenitic stainless steels in chloride solutions. Zairyo-to-Kankyo 1997; 46: 313–320. 10.3323/jcorr1991.46.313 Search in Google Scholar
Chung HM, Ruther WE, Sanecki JE, Hins A, Zaluzec NJ, Kassner TF. Irradiation-assisted stress corrosion cracking of austenitic stainless steels: recent progress and new approaches. J Nucl Mater 1996; 239: 61–79. 10.1016/S0022-3115(96)00677-0 Search in Google Scholar
Congleton J, Sui G. The stress corrosion cracking of heavily sensitized type 316 stainless steel in water in the temperature range 50–100°C. Corros Sci 1992; 33: 1691–1717. 10.1016/0010-938X(92)90003-L Search in Google Scholar
Cox B. Environmentally induced cracking of zirconium alloys. Corrosion 1972; 28: 207–217. 10.5006/0010-9312-28.6.207 Search in Google Scholar
Cragnolino G, Macdonald DD. Intergranular stress corrosion cracking of austenitic stainless steel at temperatures below 100°C – a review. Corrosion 1982; 38: 406–424. 10.5006/1.3577354 Search in Google Scholar
Cragnolino G, Lin LF, Szklarska-Smialowska Z. Stress corrosion cracking of sensitized type 304 stainless steel in sulfate and chloride solutions at 250°C and 100°C. Corrosion 1981; 37: 312–320. 10.5006/1.3577279 Search in Google Scholar
Cragnolino GA, Dunn DS, Sridhar N. Environmental effects on stress corrosion cracking of type 316l stainless steel and alloy 825 as high-level nuclear waste container materials, Prepared for Nuclear Regulatory Commission, CNWRA 94-028. San Antonio, TX: Center for Nuclear Waste Regulatory Analyses, 1994. Search in Google Scholar
Cragnolino G, Dunn DS, Sridhar N. Environmental factors in the stress corrosion cracking of type 316l stainless steel and alloy 825 in chloride solutions. Corrosion 1996; 52: 194–203. 10.5006/1.3292114 Search in Google Scholar
Crouse PL. Fluorine: a key enabling element in the nuclear fuel cycle. J South Afr Inst Min Met 2015; 115: 931–935. 10.17159/2411-9717/2015/v115n10a5 Search in Google Scholar
Dana AW. Stress-corrosion cracking of insulated austenitic stainless steel. ASTM Bull 1957; 225: 46–52. Search in Google Scholar
Dana AW, Delong WB. Topic of the month – stress-corrosion cracking test. Corrosion 1956; 12: 19–20. 10.5006/0010-9312-12.7.19 Search in Google Scholar
Davis JR. Stainless steels ASM specialty handbook. Materials Park, OH: ASM International, 1994. Search in Google Scholar
de Castro MAC, Wilde BE. The corrosion and passivation of iron in the presence of halide ions in aqueous solution. Corros Sci 1979; 19: 923–936. 10.1016/S0010-938X(79)80084-0 Search in Google Scholar
Domínguez-Aguilar MA, Newman RC. Detection of deleterious phases in duplex stainless steel by weak galvanostatic polarization in alkaline solution. Corros Sci 2006; 48: 2577–2591. 10.1016/j.corsci.2005.08.017 Search in Google Scholar
Downs JD, Harris M, Benton W, Howard SK, Billingham M. New insights into the potential for environmental cracking of corrosion resistant alloys in high-density formate and bromide well completion brines at high temperature. Corrosion/2007, Paper 07097. Houston, TX: NACE International, 2007. Search in Google Scholar
Ernst P, Newman RC. The interaction between alloyed molybdenum and dissolved bromide in the pitting corrosion of stainless steels. Electrochem Solid-State Lett 2008; 11: C1–C4. 10.1149/1.2801877 Search in Google Scholar
Evans GJ, Nugraha T. A study of the kinetics of I 2 deposition on stainless steel sampling lines. Nucl Technol 2002; 140: 315–327. 10.13182/NT02-A3342 Search in Google Scholar
Ford FP, Povich MJ. The effect of oxygen temperature combinations on the stress corrosion susceptibility of sensitized type 304 stainless steel in high purity water. Corrosion 1979; 35: 569–574. 10.5006/0010-9312-35.12.569 Search in Google Scholar
Ford FP, Silverman M. The prediction of stress corrosion cracking of sensitized 304 stainless steel in 0.01 M Na 2 SO 4 at 97°C. Corrosion 1980; 36: 558–565. 10.5006/0010-9312-36.10.558 Search in Google Scholar
Francis R, Hebdon S. The selection of stainless steels for seawater pumps. Corrosion/2015, Paper C2015-5446. Houston, TX: NACE International, 2015. Search in Google Scholar
Frankel GS. Pitting corrosion of metals – a review of the critical factors. J Electrochem Soc 1998; 145: 2186–2198. 10.1149/1.1838615 Search in Google Scholar
Frankel GS, Li T, Scully JR. Localized corrosion: passive film breakdown vs pit growth. J Electrochem Soc 2017; 164: 180–181. 10.1149/MA2017-02/11/753 Search in Google Scholar
Galvele JR. Transport processes and the mechanism of pitting of metals. J Electrochem Soc 1976; 123: 464–474. 10.1149/1.2132857 Search in Google Scholar
Gaudet GT, Mo WT, Hatton TA, Tester JW, Tilly J, Isaacs HS, Newman RC. Mass transfer and electrochemical kinetic interactions in localized pitting corrosion. AIChe J 1986; 32: 949–958. 10.1002/aic.690320605 Search in Google Scholar
Greenwood NN, Earnshaw A. Chemistry of the elements, 2nd ed . Oxford, UK: Butterworth-Heinemann, 1997. Search in Google Scholar
Griess JC, DeVan JH, Perez Blanco H. ORNL/TM-9646 Corrosion of materials in absorption heating and refrigeration fluids. Oak Ridge, TN: Oak Ridge National Laboratory, 1985. 10.2172/5415292 Search in Google Scholar
Gui F, Cao L, Thodla R, Sridhar N. Localized corrosion and stress corrosion cracking of corrosion resistant alloys in H 2 S containing environment. Corrosion/2014, Paper 4482. Houston, TX: NACE International, 2014. Search in Google Scholar
Guiñon JL, Garcia-Anton J, Pérez-Herranz V, Lacoste G. Corrosion of carbon steels, stainless steels, and titanium in aqueous lithium bromide solution. Corrosion 1994; 50: 240–246. 10.5006/1.3293516 Search in Google Scholar
Guo R, Ives MB. Pitting susceptibility of stainless steels in bromide solutions at elevated temperatures. Corrosion 1990; 46: 125–129. 10.5006/1.3585076 Search in Google Scholar
Hänninen HE. Influence of metallurgical variables on environment-sensitive cracking of austenitic alloys. Int Met Rev 1979; 24: 85–136. 10.1179/095066079790136372 Search in Google Scholar
Harris DC. Quantitative chemical analysis, 7th ed . New York: Freeman and Company, 2007. Search in Google Scholar
Haruyama S. Stress corrosion cracking by cooling water of stainless steel shell and tube heat exchangers. Mater Perform 1982; 21: 14–19. Search in Google Scholar
Hodgkiess T, Ng NK, Argyropoulos P. The corrosion behaviour of a number of materials exposed to bromine-containing environments. Desalination 1985; 55: 229–246. 10.1016/0011-9164(85)80075-8 Search in Google Scholar
Horvath J, Uhlig HH. Critical Potentials for pitting corrosion of Ni, Cr-Ni, Cr-Fe, and related stainless steels. J Electrochem Soc 1968; 115: 791–795. 10.1149/1.2411433 Search in Google Scholar
Huang YL, Cao CN, Lu M, Lin HC. Inhibition effects of I − and I 2 on stress corrosion cracking of stainless steel in acidic chloride solutions. Corrosion 1993; 49: 644–649. 10.5006/1.3316095 Search in Google Scholar
Hutto FB, Tissot RG, Whitaker TE. A new apparatus and test procedure for running ASTM C 692 stress corrosion cracking tests. In: Pollock WI, Barnhart JM, editors. Corrosion of Metals Under Thermal Insulation, ASTM STP 880. Philadelphia, PA: American Society for Testing and Materials, 1985: 211–219. 10.1520/STP39171S Search in Google Scholar
IAEA-Nuclear Energy Series. Stress corrosion cracking in light water reactors: good practices and lessons learned. Vienna, Austria: International Atomic Energy Agency, 2011. Search in Google Scholar
Igual Muñoz A, García Antón J, Guiñón JL, Pérez Herranz V. Corrosion behavior and galvanic coupling of stainless steels, titanium, and alloy 33 in lithium bromide solutions. Corrosion 2003; 59: 606–615. 10.5006/1.3277591 Search in Google Scholar
ISO 21457:2010(E) International Standard . Petroleum, petrochemical and natural gas industries – materials selection and corrosion control for oil and gas production systems. Geneva, Switzerland: ISO, 2010. Search in Google Scholar
Ito K. Determination of iodide in natural water by ion chromatography. Anal Chem 1988; 69: 3628–3632. 10.1021/ac9700787 Search in Google Scholar
Itzhak D, Elias O. Behavior of type 304 and type 316 austenitic stainless steels in 55 % lithium bromide heavy brine environments. Corrosion 1994; 50: 131–137. 10.5006/1.3293501 Search in Google Scholar
Itzhak D, Eliezer D. The stress corrosion cracking of welded austenitic stainless steels in MgCl 2 solutions in the presence of NaI additions. Corros Sci 1983; 23: 1285–1291. 10.1016/0010-938X(83)90078-1 Search in Google Scholar
Itzhak D, Elias O, Greenberg Y. Behavior of type 316 austenitic stainless steel under slow strain rate technique conditions in lithium bromide heavy brine environments. Corrosion 1996; 52: 72–78. 10.5006/1.3292098 Search in Google Scholar
Janik-Czachor M. Effect of halide ions on the nucleation of corrosion pits in iron. Mater Corros 1979; 30: 255–257. 10.1002/maco.19790300405 Search in Google Scholar
Jesionek M, Szklarska-Smialowska Z. The inhibition of the dissolution of iron in sulfuric acid by halide ions. Corros Sci 1983; 23: 183–187. 10.1016/0010-938X(83)90115-4 Search in Google Scholar
Kaneko M, Isaacs HS. Pitting of stainless steel in bromide, chloride and bromide/chloride solutions. Corros Sci 2000; 42: 67–78. 10.1016/S0010-938X(99)00056-6 Search in Google Scholar
Kaneko M, Isaacs HS. Effects of molybdenum on the pitting of ferritic- and austenitic-stainless steels in bromide and chloride solutions. Corros Sci 2002; 44: 1825–1834. 10.1016/S0010-938X(02)00003-3 Search in Google Scholar
Khalil W, Haupt S, Strehblow HH. The thinning of the passive layer of iron by halides. Werkstoffe Korros 1985; 36: 16–21. 10.1002/maco.19850360104 Search in Google Scholar
Kiselevskii VN, Kovalev VV, Neklyudov IM, Ozhigov LS. Corrosion-cracking resistance of austenitic stainless steel under stress in an iodine medium. Strength Mater 1993; 25: 864–869. 10.1007/BF00774631 Search in Google Scholar
Koch GH. Localized corrosion in halides other than chlorides. Mater Perform 1993; 32: 54–58. Search in Google Scholar
Kolotyrkin JAM. Pitting corrosion of metals. Corrosion 1963; 19: 261t–268t. 10.5006/0010-9312-19.8.261 Search in Google Scholar
Kumada M. Bromide stress corrosion cracking of stainless steels in high-temperature water. Zairyo-To-Kankyo 1996; 45: 284–291. 10.3323/jcorr1991.45.284 Search in Google Scholar
Ladwig KJ, Blythe GM. Flue-gas desulfurization products and other air emissions controls. In: Robl T, Oberlink A, Jones R, editors. Coal combustion products (CCP’s) characteristics, utilization and beneficiation. Duxford, United Kingdom: Woodhead Publishing, 2017: 67–95. Search in Google Scholar
Laycock NJ, Newman RC. Localised dissolution kinetics, salt films and pitting potentials. Corros Sci 1997; 39: 1771–1790. 10.1016/S0010-938X(97)00049-8 Search in Google Scholar
Lee WSW, Oldfield OW, Todd B. Corrosion problems caused by bromine formation in additive dosed MSF desalination plants. Desalination 1983; 44: 209–221. 10.1016/0011-9164(83)87120-3 Search in Google Scholar
Li T, Scully JR, Frankel GS. Localized corrosion: passive film breakdown vs. pit growth stability: Part III. A unifying set of principal parameters and criteria for pit stabilization and salt film formation. J Electrochem Soc 2018; 165: 762–770. 10.1149/2.0251811jes Search in Google Scholar
Li T, Scully JR, Frankel GS. Localized corrosion: passive film breakdown vs. pit growth stability: Part V. Validation of a new framework for pit growth stability using one-dimensional artificial pit electrodes. J Electrochem Soc 2019; 166: C3341–C3354. 10.1149/2.0431911jes Search in Google Scholar
Lide DR, editor. CRC handbook of chemistry and physics. Boca Raton, FL: CRC Press, 2005. Search in Google Scholar
Lillard RS. A review of corrosion issues related to uranyl nitrate base aqueous homogeneous reactors. Corros Eng Sci Technol 2010; 45: 194–203. 10.1179/147842210X12659647007121 Search in Google Scholar
Liu Y, Xu LN, Zhu JY, Meng Y. Pitting corrosion of 13Cr steel in aerated brine completion fluids. Mater Corros 2014; 65: 1096–1102. 10.1002/maco.201307489 Search in Google Scholar
Lobb RC. The effect of iodine vapour on creep rupture properties of nitride 20% Cr/25% Ni/Nb/1.5 Ti stainless steel. J Nucl Mater 1978; 74: 212–220. 10.1016/0022-3115(78)90360-4 Search in Google Scholar
Lobb RC, Jones RB. The influence of iodine vapour on creep rupture properties Of20%Cr/25%Ni/Nb stabilised stainless steel. J Nucl Mater 1976; 59: 280–292. 10.1016/0022-3115(76)90060-X Search in Google Scholar
Lobb RC, Nicholson RD. The effect of iodine vapour on the creep rupture properties of M316 stainless steel. Mater Sci Eng 1976; 22: 157–165. 10.1016/0025-5416(76)90148-8 Search in Google Scholar
Macdonald DD, Lei X. Theoretical interpretation of anion size effects in passivity. J Electrochem Soc 2016; 163: 738–744. 10.1149/2.0571613jes Search in Google Scholar
Malik AU, Siddiqi NA, Andijani IN. Corrosion behavior of some highly alloyed stainless steels in seawater. Desalination 1994; 97: 189–197. 10.1016/0011-9164(94)00086-7 Search in Google Scholar
Malik AU, Siddiqi NA, Ahmad S, Andijani IN. The effect of dominant alloy additions on the corrosion behavior of some conventional and high alloy stainless steels in seawater. Corros Sci 1995; 37: 1521–1535. 10.1016/0010-938X(95)00043-J Search in Google Scholar
Mankowski J, Szklarska-Smialowska Z. Studies on accumulation of chloride ions in pits growing during anodic polarization. Corros Sci 1975; 15: 493–501. 10.1016/0010-938X(75)90015-3 Search in Google Scholar
Mccoubrey JC. The acid strength of the hydrogen halides. Trans Faraday Soc 1955; 51: 743–747. 10.1039/tf9555100743 Search in Google Scholar
McIntyre D. Factors affecting the stress corrosion cracking of austenitic stainless steels under thermal insulation. In: Pollock WI, Barnhart JM, editors. Corrosion of metals under thermal insulation, ASTM STP 880. Philadelphia, PA: American Society for Testing and Materials, 1985: 27–41. 10.1520/STP39154S Search in Google Scholar
MIL-I-24244A. Insulation materials, thermal, with special corrosion and chloride requirements. Military Specification, 1974. Search in Google Scholar
NACE International SP0198. Standard practice control of corrosion under thermal insulation and fireproofing materials – a systems approach. Houston, TX: NACE International, 2017. Search in Google Scholar
Newman RC. 2001 W.R. Whitney Award Lecture: understanding the corrosion of stainless steel. Corrosion 2001; 57: 1030–1041. 10.5006/1.3281676 Search in Google Scholar
Nguyen VA, Carcea AG, Ghaznavi M, Newman RC. The effect of cation complexation on the predicted “B” value in Galvele’s pit model. J Electrochem Soc 2019; 166: C3297–C3304. 10.1149/2.0281911jes Search in Google Scholar
Nordin S. Studies on stainless steels for service in desalination plants. Desalination 1983; 44: 255–263. 10.1016/0011-9164(83)87124-0 Search in Google Scholar
Norsok Standard M-001-Materials Selection. Standards Norway, Lysaker, Norway, 2014. Search in Google Scholar
O’Dell CS, Brown BF. Control of stress corrosion cracking by inhibitors (a review of the literature). Washington, DC: Chemistry Department, The American University, 1978. 10.6028/NBS.MONO.156 Search in Google Scholar
O’Dell CS, Brown BF, Foley RT. An exploratory study of inhibition of intergranular stress corrosion cracking in sensitized type 304 stainless steel. Corrosion 1980; 36: 183–200. 10.5006/0010-9312-36.4.183 Search in Google Scholar
Ohtsu T, Miyazawa M. Materials selection and corrosion management in a process containing halides, Corrosion/2012, Paper C2012-0001352. Houston, TX: NACE International, 2012. Search in Google Scholar
Oldfield JW, Todd B. Corrosion problems caused by bromine formation in MSF desalination plants. Desalination 1981; 38: 233–245. 10.1016/S0011-9164(00)86070-1 Search in Google Scholar
Overman RF. Using radioactive tracers to study chloride stress corrosion cracking of stainless steels. Corrosion 1966; 22: 48–52. 10.5006/0010-9312-22.2.48 Search in Google Scholar
Ozturk B, Grubb JF. Corrosion of stainless steels and titanium in bromide-containing solutions. Corrosion/2012, Paper C2012-0001253. Houston, TX: NACE International, 2012. Search in Google Scholar
Pahlavan S, Moazen S, Taji I, Saffar K, Hamrah M, Moayed MH, Mollazadeh Beidokhti S. Pitting Corrosion of martensitic stainless steel in halide bearing solutions. Corros Sci 2016; 112: 233–240. 10.1016/j.corsci.2016.07.008 Search in Google Scholar
Pahlavan S, Moayed MH, Mirjalili M. The contrast between the pitting corrosion of 316 SS in NaCl and NaBr solutions: Part I. Evolution of metastable pitting and stable pitting. J Electrochem Soc 2019a; 166: C65–C75. 10.1149/2.0811902jes Search in Google Scholar
Pahlavan S, Moayed MH, Mirjalili M. The contrast between the pitting corrosion of 316 SS in NaCl and NaBr solutions: Part II. Morphology, chemistry, and stabilization of the pits. J Electrochem Soc 2019b; 166: C321–C331. 10.1149/2.0481912jes Search in Google Scholar
Pan YM, Dunn DS, Cragnolino GA. Effects of environmental factors and potential on stress corrosion cracking of Fe-Ni-Cr-Mo Alloys in chloride solutions. In: Kane RD, editor. Environmentally assisted cracking: predictive methods for risk assessment and evaluation of materials, equipment, and structures, ASTM STP 1401. West Conshohocken, PA: American Society for Testing and Materials, 2000: 273–288. Search in Google Scholar
Parvathavarthini N, Mudali UK. Electrochemical techniques for estimating the degree of sensitization in austenitic stainless steels. Corros Rev 2014; 32: 183–225. 10.1515/corrrev-2014-0029 Search in Google Scholar
Pinkus P, Eliezer D, Itzhak D. The influence of alkali-halide additions on the stress corrosion cracking of an austenitic stainless steel in MgCl 2 solution. Corros Sci 1981; 21: 417–423. 10.1016/0010-938X(81)90040-8 Search in Google Scholar
Pistorius PC, Burstein GT. Metastable pitting corrosion of stainless steel and the transition to stability. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 1992; 341: 531–559. Search in Google Scholar
Pourbaix M. Atlas of electrochemical equilibria in aqueous solutions. New York: Pergamon Press, 1966. Search in Google Scholar
Prosek T, Iversen A, Taxén C, Thierry D. Low-temperature stress corrosion cracking of stainless steels in the atmosphere in the presence of chloride deposits. Corrosion 2009; 65: 105–117. 10.5006/1.3319115 Search in Google Scholar
Rao P. Microstructural and microchemical studies in weld sensitized austenitic stainless steels. In: Abrams H, Maniar GN, Nail DA, Solomon HD, editors. MiCon 78: optimization of processing, properties, and service performance through microstructural control, ASTM STP 672. Philadelphia, PA: American Society for Testing and Materials, 1979: 321–333. 10.1520/STP36877S Search in Google Scholar
Rhodes PR. Mechanism of chloride stress corrosion cracking of austenitic stainless steels. Corrosion 1969; 25: 462–472. 10.5006/0010-9312-25.11.462 Search in Google Scholar
Richardson JA, Fitzsimmons T. 1985. Use of aluminum foil for prevention of stress corrosion cracking of austenitic stainless steel under thermal insulation. In: Pollock WI, Barnhart JM, editors. Corrosion of metals under thermal insulation, ASTM STP 880. Philadelphia, PA: American Society for Testing and Materials, 1985: 188–198. 10.1520/STP39168S Search in Google Scholar
Scully JC. The electrochemical parameters of stress-corrosion cracking. Corros Sci 1968; 8: 513–523. 10.1016/S0010-938X(68)80006-X Search in Google Scholar
Sedriks AJ. Corrosion of stainless steels, 2nd ed . New York: John Wiley & Sons, 1996. Search in Google Scholar
Sekine I, Usui H, Kitagawa S, Yuasa M, Silao L. The effect of fluoride ions on the corrosion of steel materials in H 2 SO 4 and CH3COOH solutions. Corros Sci 1994; 36: 1411–1424. 10.1016/0010-938X(94)90189-9 Search in Google Scholar
Shibata T, Haruna T, Oki T. Initiation and growth of intergranular stress corrosion cracks for sensitized 304 stainless steel depending on NaF concentration of aqueous solution. Tetsu-to-Hagane 1993a; 79: 721–725. 10.2355/tetsutohagane1955.79.6_721 Search in Google Scholar
Shibata T, Oki T, Haruna T. Stress corrosion cracking susceptibility of sensitized type 304 stainless steel in NaF solution evaluated by SSRT. Zairyo-to-Kankyo 1993b; 42: 15–19. 10.3323/jcorr1991.42.15 Search in Google Scholar
Sidky PS. Iodine stress corrosion cracking of zircaloy reactor cladding : iodine chemistry (a review). J Nucl Mater 1998; 256: 1–17. 10.1016/S0022-3115(98)00044-0 Search in Google Scholar
Smialowski M, Rychcik M. Effect of potential and stress on time to failure of austenitic stainless steels in magnesium chloride solutions. Corrosion 1967; 23: 218–221. 10.5006/0010-9312-23.7.218 Search in Google Scholar
Soltis J. passivity breakdown, pit initiation and propagation of pits in metallic materials – review. Corros Sci 2015; 90: 5–22. 10.1016/j.corsci.2014.10.006 Search in Google Scholar
Speidel MO. Stress Corrosion cracking of stainless steels in NaCI solutions. Metall Trans A 1981; 12A: 779–789. 10.1007/BF02648342 Search in Google Scholar
Sridhar N, Thodla R, Gui F, Cao L, Anderko A. Corrosion-resistant alloy testing and selection for oil and gas production. Corros Eng Sci Technol 2017; 53: 75–89. 10.1080/1478422X.2017.1384609 Search in Google Scholar
Srikhirin P, Aphornratana S, Chungpaibulpatana S. A review of absorption refrigeration technologies. Renew Sustain Energy Rev 2001; 5: 343–372. 10.1016/S1364-0321(01)00003-X Search in Google Scholar
Srinivasan J, Kelly RG. On a recent quantitative framework examining the critical factors for localized corrosion and its impact on the Galvele pit stability criterion. Corrosion 2017; 73: 613–633. 10.5006/2334 Search in Google Scholar
Staehle RW, Gorman JA. Quantitative assessment of submodes of stress corrosion cracking on the secondary side steam generator tubing in pressurized water reactors: Part 1. Corrosion 2003; 59: 931–994. 10.5006/1.3277522 Search in Google Scholar
Stine CMA. Recovery of bromine from sea water. Ind Eng Chem 1929; 21: 434–442. 10.1021/ie50233a010 Search in Google Scholar
Streicher MA. Pitting corrosion of 18Cr–8Ni stainless steel. J Electrochem Soc 1956; 103: 375–390. 10.1149/1.2430359 Search in Google Scholar
Streicher MA. Austenitic and ferritic stainless steels. In: Revie W, editor. Uhlig’s corrosion handbook, 3rd ed. Hoboken, New Jersey: Wiley, 2011: 657–693. 10.1002/9780470872864.ch51 Search in Google Scholar
Szklarska-Smialowska Z. Pitting and crevice corrosion. Houston, TX: NACE International, 2005. Search in Google Scholar
Takemoto M, Shonohara T, Shirai M, Shinogaya T. External stress corrosion cracking (ESCC) of austenitic stainless steel. Mater Perform 1985; 24: 26–32. Search in Google Scholar
Theus GJ, Cels JR. Fluoride induced intergranular stress corrosion cracking of sensitized stainless steel. In: Tedmon CS, editor. Corrosion problems in energy conversion and generation. Princeton, New Jersey: Corrosion Division, Electrochemical Society, 1974: 384–396. Search in Google Scholar
Thomas VM, Bedford JA, Cicerone RJ. Bromine emissions from leaded gasoline. Geophys Res Lett 1997; 24: 1371–1374. 10.1029/97GL01243 Search in Google Scholar
Thorvaldsson T, Salwén A. Measurement of diffusion coefficients for Cr at low temperatures in a type 304 stainless steel. Scr Metall 1984; 18: 739–742. 10.1016/0036-9748(84)90331-4 Search in Google Scholar
Tousek J. Eisenlochfrass in Alkalischen Halogenidlösungen. Corros Sci 1975; 15: 147–154. 10.1016/S0010-938X(75)80005-9 Search in Google Scholar
Trabanelli G, Zucchi F, Demertzis G. Intergranular stress corrosion cracking of sensitized AISI 304 by fluoride ions and its inhibition. Key Eng Mater 1988; 20–28: 1905–1912. 10.4028/www.scientific.net/KEM.20-28.1905 Search in Google Scholar
Tsujikawa S, Shinohara T, Lichang W. Spot-welded specimen maintained above the crevice-repassivation potential to evaluate stress corrosion cracking susceptibility of stainless steels in NaCl solutions. In: Cragnolino G, Sridhar N, editors. Application of accelerated corrosion tests to service life prediction of materials, ASTM STP 1194. Philadelphia, PA: American Society for Testing and Materials, 1994: 340–354. 10.1520/STP24893S Search in Google Scholar
Tsukaue Y, Kudo A, Nakao G, Yamasaki H, Kimura S. Accumulation of trihalide ions caused by corrosion. Corrosion 1993; 49: 220–234. 10.5006/1.3316043 Search in Google Scholar
Tsukaue Y, Nakao G, Takimoto Y, Yoshida K. Initiation behavior of pitting in stainless steels by accumulation of triiodide ions in water droplets. Corrosion 1994a; 50: 755–760. 10.5006/1.3293465 Search in Google Scholar
Tsukaue Y, Yamasaki H, Nakao G. Characteristics of pit initiation of stainless steel in triiodide aqueous solution. Zairyo-to-Kankyo 1994b; 43: 487–492. 10.3323/jcorr1991.43.487 Search in Google Scholar
Tzaneva BR, Fachikov LB, Raicheff RG. Effect of halide anions and temperature on initiation of pitting in Cr–Mn–N and Cr–Ni steels. Corros Eng Sci Technol 2006; 41: 62–66. 10.1179/174327806X94027 Search in Google Scholar
Uhlig HH, Cook EW. Mechanism of inhibiting stress corrosion cracking of 18-8 stainless steel in MgCl 2 by acetates and nitrates. J Electrochem Soc 1969; 116: 173–177. 10.1149/1.2411789 Search in Google Scholar
US Atomic Energy Commission-Regulatory Guide 1.36 – Nonmetallic Thermal Insulation for Austenitic Stainless Steel. Washington, DC: US Atomic Energy Commission – Directorate of Regulatory Standards, 1973. Search in Google Scholar
US Nuclear Regulatory Commission – Regulatory Guide 1.36 – Nonmetallic Thermal Insulation for Austenitic Stainless Steel. Washington, DC: U.S. Nuclear Regulatory Commission – Office of Nuclear Regulatory Research, 2015. Search in Google Scholar
Ward CT, Mathis DL, Staehle RW. Research in progress intergranular attack of sensitized austenitic stainless steel by water containing fluoride ions. Corrosion 1969; 25: 394–396. 10.5006/0010-9312-25.9.394 Search in Google Scholar
Warner TB. Normal fluoride content of seawater. Deep-Sea Res 1971; 18: 1255–1263. 10.1016/0011-7471(71)90030-1 Search in Google Scholar
Whitaker TE, Whorlow KM, Hutto FB. New developments in test technology for ASTM C 692 (preproduction corrosion test for insulation to be used on austenitic stainless steel). In: McElroy DL, Kimpflen JF, editors. Insulation materials, testing, and applications, ASTM STP 1030. Philadelphia, PA: American Society for Testing and Materials, 1990: 688–698. Search in Google Scholar
Whorlow KM, Hutto FB. NUREG/CR-6539 – effects of fluoride and other halogen ions on the external stress corrosion cracking of type 304 austenitic stainless steel. Washington, DC: U.S. Nuclear Regulatory Commission, 1997. 10.2172/505259 Search in Google Scholar
Whorlow KM, Woolridge EO, Hutto FB. Effect of halogens and inhibitors on the external stress corrosion cracking of type 304 austenitic stainless steel. In: Graves RS, Zarr RR, editors. Insulation materials: testing and applications: Third Volume, ASTM STP 1320. Philadelphia, PA: American Society for Testing and Materials, 1997: 485–497. 10.1520/STP12294S Search in Google Scholar
Willison MJ, Clarke AG, Zeki EM. Chloride aerosols in central Northern England. Atmos Environ 1989; 23: 2231–2239. 10.1016/0004-6981(89)90185-6 Search in Google Scholar
Wood JC. Factors affecting stress corrosion cracking of zircaloy in iodine vapour. J Nucl Mater 1972; 45: 105–122. 10.1016/0022-3115(72)90178-X Search in Google Scholar
Wren JC, Glowa GA, Merritt J. Corrosion of stainless steel by gaseous I 2 . J Nucl Mater 1999; 265: 161–177. 10.1016/S0022-3115(98)00504-2 Search in Google Scholar
Yamamoto K, Hosoya K. Corrosivity of Br − and Cl − on duplex stainless steel. Mater Sci Eng A 1995; 198: 239–243. 10.1016/0921-5093(95)80079-A Search in Google Scholar
Yamazaki O. Effect of fluoride ion on the pitting corrosion of type 304 stainless steel in neutral NaCl solution. Zairyo-to-Kankyo 1994; 43: 265–271. 10.3323/jcorr1991.43.265 Search in Google Scholar
Yamazaki O. Effect of fluoride ion on the crevice corrosion for type 304 stainless steel in neutral NaCl solution. Zairyo-to-Kankyo 1996; 45: 365–369. 10.3323/jcorr1991.45.365 Search in Google Scholar
Yamazaki O. Repassivation potential E R for crevice corrosion of type 304 stainless steel/FPM-crevice in neutral NaCl/NaF solutions. Zairyo-To-Kankyo 1997; 46: 419–423. 10.3323/jcorr1991.46.419 Search in Google Scholar
Zucchi F, Trabanelli G, Demertzis G. The intergranular stress corrosion cracking of a sensitized AISI 304 in NaF and NaCl solutions. Corros Sci 1988; 28: 69–79. 10.1016/0010-938X(88)90008-X Search in Google Scholar
©2020 Walter de Gruyter GmbH, Berlin/Boston
- X / Twitter
Supplementary Materials
Please login or register with De Gruyter to order this product.
Journal and Issue
Articles in the same issue.

Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Author Biographies
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Review of prediction of stress corrosion cracking in gas pipelines using machine learning.

1. Introduction
2. methodology and analysis, 2.1. research framework, 2.2. research definition, 2.3. search strategy.
| (“Machine Learning” <OR> “Machine Learning Application”) |
| [AND] |
| (“Oil and Gas” <OR> “Energy Industry” <OR> “Energy”) |
| [AND] |
| (“Stress Corrosion Cracking” <OR> “SCC”) |
| [AND] |
| (“Prediction Techniques” <OR> “SCC Prediction” <OR> “Pipeline Condition Assessment”) |
| [AND] |
| (“Non-Destructive Testing” <OR> “Pipeline Integrity Management” <OR> “Integrity Management”) |
2.4. Analysis of Publications
3. critical research and analysis, 3.1. stress corrosion cracking (scc) failure events, 3.2. statistics of corrosion incidents and factors leading to scc in energy pipelines.
- Uniform corrosion;
- Pitting corrosion;
- Cavitation and erosion–corrosion;
- Stray current corrosion;
- Microbiologically influenced corrosion;
- Stress corrosion cracking (SCC);
- Selective seam corrosion (SSC).
- Metal metallurgy;
- Metal physical properties;
- Manufacturing process;
- Process and operating conditions;
- Protecting coating conditions;
- Soil conditions.
3.3. Stress Corrosion Cracking (SCC) Susceptibility Parameters
3.4. stress corrosion cracking (scc) management program, 3.5. stress corrosion cracking (scc) detection techniques.
| Detection Method | Brief Details |
|---|---|
| Linear polarization resistance (LPR) | This method quantifies the electrochemical resistance of a corroding metal working electrode in close proximity to its open circuit potential. The process entails the polarization of a voltage range of ±10 mV relative to the corrosion potential [ , ]. |
| In-line inspection (ILI) | ILI tools, commonly known as “smart pigs”, are devices that are inserted into the pipeline and travel with the product flow. They use various technologies such as magnetic flux leakage (MFL) or ultrasonic sensors to detect anomalies, including SCC, within the pipeline. These tools provide a comprehensive assessment of the pipeline’s condition [ ]. Currently, there are two primary methods used for crack detection: ultrasonic testing and test using electromagnetic acoustic transducers (EMATs) [ ]. They are readily accessible for the on-line inspection of SCC in commercial settings. Further ILI technologies can be seen in , and their merits and demerits are in [ ]. |
| Electrochemical noise (EN) | This technique continually monitors corrosion potential and variations in current. It is utilized to acquire corrosion current by measuring noise resistance [ ]. |
| Acoustic emission (AE) | AE monitoring involves detecting and analyzing the high-frequency acoustic signals emitted by crack growth or propagation. It can provide real-time information on the occurrence and progression of SCC [ ]. AE sensors are placed on the pipeline, and any acoustic emissions resulting from crack activity are captured and analyzed [ ]. |
| Electromagnetic testing | Electromagnetic techniques, such as eddy current testing (ECT) and magnetic particle inspection (MPI), can be employed to detect SCC [ ]. ECT utilizes electromagnetic induction to detect surface and near-surface cracks, while MPI uses magnetic fields and iron particles to locate cracks or defects that are magnetically visible. |
| Ultrasonic testing (UT) | UT uses high-frequency sound waves to detect internal defects or cracks in the pipeline. It involves transmitting ultrasonic waves into the material and analyzing the reflected waves to identify any indications of SCC [ ]. UT can be performed on both the external and internal surfaces of the pipeline. |
| Cyclic potentiodynamic polarization | This process entails applying an over-potential greater than the corrosion potential toward the noble side until a current of 5 mA is reached. Then, the potential is reversed until the corrosion potential is achieved [ ]. |
| Radiographic testing (RT) | RT uses X-rays or gamma rays to detect internal defects in the pipeline. It involves passing the radiation through the material and capturing the transmitted radiation on a film or detector. Any cracks or indications of SCC can be identified by examining the resulting radiographic image [ ]. |
| Electrochemical impedance spectroscopy (EIS) | This process entails the use of an alternating current (AC) potential with a magnitude of ±10 mV around the corrosion potential. This is achieved throughout a broad range of frequencies, generally spanning 0.1 to 106 Hz. The purpose of this is to obtain the corrosion current [ ]. |
| Electromagnetic acoustic transducer (EMAT) | The electromagnetic acoustic transducer (EMAT) is a modern non-destructive testing (NDT) device employed in in-line inspection (ILI) equipment to detect SCC in gas pipelines [ ]. EMATs operate by utilizing a magnetic field to create an ultrasonic compression wave on the inner surface of the pipe wall [ ]. |
| Hydrostatic testing | Hydrostatic testing is a method employed to detect SCC in pipelines. When conducted correctly, this approach ensures that any significant flaws present during the test are discovered. Hydrostatic testing is a frequently employed technique to ensure the preservation of pipeline integrity in the presence of developing flaws, such as pitting corrosion, fatigue, corrosion fatigue, or SCC [ , ]. |
| Magnetic flux leakage | Magnetic flux leakage (MFL) is a non-destructive testing (NDT) method employed for the identification of SCC. A high-strength magnet is employed to magnetize the steel in areas prone to corrosion or potential metal degradation. This method has been employed to identify corrosion flaws, fractures, and mechanical impairments [ ]. |
ILI Technologies Used to Detect SCC
Click here to enlarge figure
| Technology | Pros | Cons | Run in Operating | |
|---|---|---|---|---|
| Oil | Gas | |||
| Shear Wave (Liquid-coupled) Ultrasound | No | Yes | ||
| EMAT | Yes | Yes | ||
| FMFL | Yes | Yes | ||
| LWUT | Yes | Yes | ||
| New Technologies | Yes | Yes | ||
| SEEC (Self-Excited Eddy Current) | Yes | Yes | ||
3.6. Machine Learning (ML)
3.7. scc prediction through machine learning, 3.8. research analysis, 3.9. critical review analysis, 3.10. gaps and challenges in implementing machine learning.
| Gap/Challenge | Brief Details |
|---|---|
| Data Availability | ML models require large amounts of high-quality data to effectively learn and make accurate predictions. Obtaining sufficient labeled data related to SCC in pipelines to validate ML models is a major challenge. |
| Data Quality | A lack of good quality data is one of the major problems that machine learning experts are facing in obtaining the required outcomes. Data quality is an issue in developing a good model to predict SCC in energy pipelines. As a result, we must make sure that data pre-processing is carried out to achieve the highest degree of accuracy possible, which involves eliminating outliers, the imputation of missing values, and eliminating undesired characteristics. |
| Data Variability | The data collected for SCC detection can vary in terms of pipeline materials, environmental conditions, stress levels, and other factors. This variability makes it challenging to develop a machine learning model that can effectively handle different data types. Ensuring a diverse and representative dataset is crucial for training models that can handle the various conditions encountered in pipeline systems. |
| Data Privacy | Data protection, data security, and privacy are some of the issues connected with the application of machine learning. For instance, the General Data Protection Regulation (GDPR) was developed in 2016 to provide people with more control over their data while also protecting the personal information of those living in the European Union and the European Economic Area. The California Consumer Privacy Act (CCPA) launched in 2018 mandates businesses to tell customers about the acquisition of their data. It is one example of a state policy being developed in the United States [ ]. Process data, operation data, and other inspection and maintenance data are some of the most important information that pipeline companies need to secure to avoid any interruption in their businesses and operations. |
| Required Skillset | To obtain the best results from the data collected over the years, the oil and gas sector is facing difficulty in obtaining the right skills. Machine learning techniques and approaches are relatively new to people working in the energy pipeline industry. There are not enough ML specialists in this field, which hinders the potential to develop successful models that will bring benefits to business or predict issues to control unwanted events. |
| Affordability | To develop a significantly advanced data analytics system in order to use machine learning techniques, pipeline owners will require data engineers/scientists with sound technical knowledge of data analytics, modeling, and mathematics. Without these skills, companies are not able to start with a good digital transformation system. |
| Understanding the Algorithms | Given the complexity of machine learning, data scientists are required to have expertise in this particular field and an in-depth understanding of science, technology, and mathematics to develop ML models to achieve the best results. Many businesses lack the internal expertise necessary to comprehend algorithms and how they operate, which can cause them to lose out on crucial insights. |
| Class Imbalance | SCC occurrences in pipelines are typically rare events in the overall dataset. This class imbalance can lead to biased models that struggle to accurately detect SCC instances. Techniques such as oversampling, undersampling, or synthetic data generation can be employed to address the class imbalance issue and ensure that the model is trained on a balanced dataset. |
| ML Model Generalization | Developing a generalized ML model that can be applied to the detection of unseen pipeline conditions is difficult. A model should be capable of detecting SCC across different pipeline sections, varying stress levels, and diverse corrosion environments. Adequate model evaluation and validation of unseen data are necessary to assess the generalization capability of a model. |
| Obtaining the Right Data/Information | Obtaining the appropriate data to train ML models is one of the biggest challenges we are facing. ML models may not perform as well as they should since data are frequently siloed, erroneous, or incomplete. Therefore, this requires careful data gathering, processing, and curation for the purpose of model training. |
| Lack of Training Data | The most crucial step in ML model development is training the model using enough data in order to let the model obtain reliable outputs. Less training data will result in model outputs that are biased or erroneous. |
| Infrastructure Requirements | In some oil and gas companies, the data infrastructure is inadequate, which makes it difficult to find the required data in the data retrieval process. Therefore, it is an essential requirement to maintain an appropriate data management infrastructure in a company for the easy use of available data to dig out embedded values. This will make testing various tools easier and also make data transfers easier. |
| Feature Selection | Identifying the most informative features or input parameters for predicting SCC in gas pipelines can be a challenge. Different factors, such as pipe material, temperature, pressure, pH, environmental variables, and pipe geometry, can influence SCC. |
| Incorporating Time-Dependent Factors | SCC in gas pipelines is a complex phenomenon that can evolve over time because of various factors, including aging, environmental changes, and operational conditions. Capturing and incorporating the temporal aspect of SCC into ML models can be a research gap. |
| Lack of Labeled Data | ML models typically require labeled data for training and validation. However, obtaining labeled data for SCC in oil and gas pipelines can be difficult given the complex and expensive nature of conducting inspections and assessments. |
4. Future Perspective
- Design data;
- Field data;
- Maintenance history;
- Experimental data;
- Simulated data.
- Available models need to be tested in the field, and a model’s accuracy needs to be verified in a controlled environment. This might be achieved through collaborations between model developers and plant/industry research teams in data collection and model testing.
- Data selection can be made better by including more details about the methods used for data collection, generation, and pre-processing. The literature lacks good-quality data, especially for machine learning where labeled data are required. This subject needs a good review in order to dig up more relevant data and clean that data.
- Both field testing and laboratory experiments must be used to evaluate SCC in terms of its severity and frequency of occurrence. Forecasting and management become critical for unveiling external corrosion-provoked deterioration events where machine learning might help to predict SCC and, hence, aid in the determination of remaining useful life based on identified SCC anomalies and their growth. To achieve this, it is of great interest to develop a framework applied to the detection of SCC, which is referred to in Figure 10 .
Proposed Framework to Identify SCC in Energy Pipelines
5. conclusions, author contributions, data availability statement, conflicts of interest.
| Author Names | Reference No. | Year | Brief Details | Comments |
|---|---|---|---|---|
| Al-Sabaeei et al. | [ ] | 2023 | A systematic review highlights the complexity and effectiveness of ML methods in predicting pipeline failures, emphasizing factors such as dataset variations, data sources, and model complexity. It underscores the success of ANNs, SVMs, and HML in detecting defects, focusing on corrosion while also identifying a need for more diverse research on other failure types. | |
| Ma et al. | [ ] | 2023 | A novel hybrid approach is presented to effectively estimate the burst pressure of corroded pipelines. It incorporates a feature space with physical importance and a fusion mechanism that combines empirical formula and collective learning. The suggested model, which uses the light gradient-boosting machine, exhibits better interpretability through feature importance analysis. | |
| Alamri A. H. | [ ] | 2022 | This review summarizes the current state of ML applications in SCC for risk assessment. It identifies existing knowledge gaps, discusses challenges, and outlines future perspectives on utilizing ML and AI in corrosion risk assessment. | |
| Liu and Bao | [ ] | 2022 | Explores the application of ML in automated pipeline condition assessment, leveraging advanced sensing technologies to analyze routine operations, NDT, and computer vision data. | |
| Soomro et al. | [ ] | 2022 | Emphasizes the limitations of existing probabilistic models; this research advocates for Bayesian network approaches, offering insights, methodologies, and dataset considerations for risk analysis in evaluating corroded hydrocarbon pipelines. | |
| Soomro et al. | [ ] | 2022 | Emphasizes the emerging role of machine learning in predicting pipeline corrosion, mainly through hybrid models like ANNs and SVMs, while also addressing current research gaps and proposing future directions for enhancing accuracy and validation in this evolving field. | |
| Coelho et al. | [ ] | 2022 | This study emphasizes that localized corrosion and inhibition efficiency prediction is recommended, requiring large, high-quality training data and collaboration for systematic ML integration into the corrosion community. | |
| Wasim and Djukic | [ ] | 2022 | This review includes an analysis of monitoring tools; models for corrosion prevention, prediction, failure occurrence, and remaining life; and insights into external corrosion management, reliability-based and risk-based models, and integrity assessment using machine learning and fuzzy logic approaches. | |
| Khakzad et al. | [ ] | 2022 | Using a Bayesian network and an empirical corrosion simulation model, this research estimates corrosion rates based on factors like pipe diameter and flow conditions, subsequently converting these predictions into a distribution of failure probabilities. | |
| Seghier et al. | [ ] | 2022 | Presents a robust ensemble learning approach for accurate internal corrosion rate prediction in oil and gas pipelines, utilizing four models: random forest, adaptive boosting, gradient boosting regression tree, and extreme gradient boosting. | |
| Soomro et al. | [ ] | 2021 | This study proposes ML-based algorithms to estimate the probability of failure, leveraging extensive simulations to generate a rich dataset for comprehensive validation and providing insights into improved reliability assessment in the industry. | |
| Sheikh et al. | [ ] | 2021 | Employing a hybrid approach integrating machine learning techniques, this research successfully predicts corrosion severity levels with high accuracy based on distinct features extracted from acquired acoustic emission data. | |
| Rachman et al. | [ ] | 2021 | Explores the integration of ML in pipeline integrity management (PIM). This review covers ML applications across PIM elements such as inspection, monitoring, maintenance, and analysis techniques and addresses current challenges while also highlighting future research opportunities. | |
| Reddy et al. | [ ] | 2021 | Emphasizing the importance of early detection and prevention, this review explores sensor technologies employing physical and electrochemical techniques, discussing their recent developments, sensitivity, selectivity, and standard inspection methods for corrosion monitoring. | |
| Ossai, C. I. | [ ] | 2020 | This study uses a data-driven methodology to estimate increased corrosion defect depth (CDD) in oil and gas pipelines using a subspace clustering neural network (SSCN) and particle swarm optimization (PSO). | |
| Jiang, P. | [ ] | 2018 | This thesis addresses the growing global demand for risk analysis in corrosion- and SCC-related failure events. It introduces an innovative method utilizing machine learning, including ensemble methods and support vector machines (SVMs) for automatic risk analysis. | |
| Ratnayake and Antosz | [ ] | 2017 | Presents a novel approach to risk-based maintenance (RBM) analysis using fuzzy logic. The proposed approach extends the traditional RBM framework by incorporating fuzzy sets to represent the uncertainty associated with risk factors. | |
| Aljaroudi et al. | [ ] | 2016 | Addresses the critical issue of offshore pipeline leak-detection system failures, emphasizing their potential operational and environmental consequences. Introduces a risk-based assessment methodology to evaluate system integrity, quantify associated risks, and guide decision-makers in determining appropriate preventive measures based on an acceptable risk threshold. | |
| Hasan, A. | [ ] | 2016 | Introduces a risk-based security management method utilizing an analytic hierarchy process (AHP) model to assess the likelihood of pilferage in different pipeline sections, aiding in prioritizing security measures for effective prevention. | |
| Guo et al. | [ ] | 2016 | Introduces a robust risk evaluation method utilizing a fuzzy Petri net (FPN) model to assess potential hazards in long-distance oil and gas transportation pipelines. | |
| Parvizsedghy and Zayed | [ ] | 2016 | This work employs a neuro-fuzzy technique; the study develops a model utilizing historical data to predict and assess the financial consequences of potential failures, offering an 80% accurate tool for practitioners and academics involved in the risk assessment of gas pipelines. | |
| Zhou et al. | [ ] | 2016 | Provides an analytical model based on fuzzy logic to determine the probability of corrosion-related issues in energy pipelines, considering corrosion cracking and thinning to be important variables. This model offers important insights into corrosion failure likelihood by considering variables like inspection efficacy and timing. | |
| Lu et al. | [ ] | 2015 | This study offers a new method of assessing the possible risks related to natural gas pipeline leaks. The approach makes use of a risk matrix in addition to a bowtie model. | |
| Zhou et al. | [ ] | 2015 | Provides a novel strategy for estimating the service time of subterranean gas pipelines before corroding under the cyclically loading condition. The methodology employs cumulative damage rates, models corrosion defect depths as an exponential function of elapsed time, and computes remaining life by using an iterative approach. | |
| De Masi et al. | [ ] | 2015 | Addresses the growing challenge of maintaining the integrity of hydrocarbon pipelines over long distances because of aging plants and components in the oil and gas industry. By leveraging an ensemble of artificial neural networks (ANNs), the proposed ML approach demonstrates promising results in predicting the complex evolution of corrosion, outperforming traditional deterministic models and single-ANN models. | |
| El-Abbasy et al. | [ ] | 2015 | Proposes a condition assessment model and uses both an analytic network process and a Monte Carlo simulation to consider the uncertainty of factors affecting pipeline conditions and the interdependency relationships between them. | |
| De Masi et al. | [ ] | 2014 | Highlights the role of reliable corrosion predictions in pipeline integrity management, reducing economic impact, and preventing environmental damage. | |
| Ismail et al. | [ ] | 2011 | Explores SCC in austenitic stainless steel in high-temperature aquatic surroundings, employing fact-based techniques such as classical statistics, machine learning, and fuzzy logic. The decision tree approach was found to be highly effective, demonstrating superior performance and intelligibility in addressing the investigated problem. |
- Overholt, M. The Importance of Oil and Gas in Today’s Economy. Tiger Gen. 2016 . Available online: https://www.tigergeneral.com/the-importance-of-oil-and-gas-in-today-s-economy/ (accessed on 2 July 2023).
- Roberge, P.R. Handbook of Corrosion Engineering ; McGraw-Hill Education: Chicago, IL, USA, 2019. [ Google Scholar ]
- Parkins, R.N. A review of stress corrosion cracking of high pressure gas pipelines. In Proceedings of the CORROSION 2000, Orlando, FL, USA, 26–31 March 2000; OnePetro: Richardson, TX, USA, 2000. [ Google Scholar ]
- Hussain, M.; Zhang, T. Potential of Big Data analytics for energy pipeline integrity management. Corros. Manag. 2023 , 2023 , 31–33. [ Google Scholar ]
- Koch, G.H.; Brongers, M.P.; Thompson, N.G.; Virmani, Y.P.; Payer, J.H. Corrosion Cost and Preventive Strategies in the United States ; Federal Highway Administration: Washington, DC, USA, 2002. [ Google Scholar ]
- Gupta, N.K.; Verma, C.; Salghi, R.; Lgaz, H.; Mukherjee, A.; Quraishi, M. New phosphonate based corrosion inhibitors for mild steel in hydrochloric acid useful for industrial pickling processes: Experimental and theoretical approach. New J. Chem. 2017 , 41 , 13114–13129. [ Google Scholar ] [ CrossRef ]
- Dublin. Global Corrosion Monitoring Strategic Business Report 2023: Rising Corrosion Costs in Oil and Gas Production Industries Augments Demand for Corrosion Monitoring Solutions. 23 March 2023. Source: Research and Markets. Available online: https://www.globenewswire.com/en/news-release/2023/03/23/2633526/28124/en/Global-Corrosion-Monitoring-Strategic-Business-Report-2023-Rising-Corrosion-Costs-in-Oil-and-Gas-Production-Industries-Augments-Demand-for-Corrosion-Monitoring-Solutions.html (accessed on 2 July 2023).
- Wei, X.; Fu, D.; Chen, M.; Wu, W.; Wu, D.; Liu, C. Data mining to effect of key alloying elements on corrosion resistance of low alloy steels in Sanya seawater environment. J. Mater. Sci. Technol. 2021 , 64 , 222–232. [ Google Scholar ] [ CrossRef ]
- Völker, C.; Kruschwitz, S.; Ebell, G. A machine learning-based data fusion approach for improved corrosion testing. Surv. Geophys. 2020 , 41 , 531–548. [ Google Scholar ] [ CrossRef ]
- Coelho, L.B.; Zhang, D.; Van Ingelgem, Y.; Steckelmacher, D.; Nowé, A.; Terryn, H. Reviewing machine learning of corrosion prediction in a data-oriented perspective. Npj Mater. Degrad. 2022 , 6 , 8. [ Google Scholar ] [ CrossRef ]
- Chen, W. Modeling and prediction of stress corrosion cracking of pipeline steels. In Trends in Oil and Gas Corrosion Research and Technologies ; Elsevier: Amsterdam, The Netherlands, 2017; pp. 707–748. [ Google Scholar ]
- Khalifeh, A. Stress Corrosion Cracking Behavior of Materials ; IntechOpen: London, UK, 2020. [ Google Scholar ]
- Alamri, A.H. Application of machine learning to stress corrosion cracking risk assessment. Egypt. J. Pet. 2022 , 31 , 11–21. [ Google Scholar ] [ CrossRef ]
- Jiang, P. Machine Learning Methods for Corrosion and Stress Corrosion Cracking Risk Analysis of Engineered Systems. Ph.D. Thesis, Science Department, The University of New South Wales, Sydney, Australia, 2018. [ Google Scholar ]
- Ren, C.-Y.; Qiao, W.; Tian, X. Natural gas pipeline corrosion rate prediction model based on BP neural network. In Proceedings of the Fuzzy Engineering and Operations Research, Babolsar, Iran, 25–26 October 2012; Springer: Berlin/Heidelberg, Germany, 2012; pp. 449–455. [ Google Scholar ]
- Soomro, A.A.; Mokhtar, A.A.; Kurnia, J.C.; Lashari, N.; Lu, H.; Sambo, C. Integrity assessment of corroded oil and gas pipelines using machine learning: A systematic review. Eng. Fail. Anal. 2022 , 131 , 105810. [ Google Scholar ] [ CrossRef ]
- Ning, J.; Zheng, Y.; Brown, B.; Young, D.; Nešić, S. A thermodynamic model for the prediction of mild steel corrosion products in an aqueous hydrogen sulfide environment. Corrosion 2015 , 71 , 945–960. [ Google Scholar ] [ CrossRef ]
- Dong, C.; Ji, Y.; Wei, X.; Xu, A.; Chen, D.; Li, N.; Kong, D.; Luo, X.; Xiao, K.; Li, X. Integrated computation of corrosion: Modelling, simulation and applications. Corros. Commun. 2021 , 2 , 8–23. [ Google Scholar ] [ CrossRef ]
- De Waard, C.; Lotz, U.; Dugstad, A. Influence of liquid flow velocity on CO 2 corrosion: A semi-empirical model. In Corrosion 95: The NACE International Annual Conference and Corrosion Show ; NACE International: Houston, TX, USA, 1995. [ Google Scholar ]
- Garber, J.D.; Farshad, F.; Reinhardt, J.R.; Li, H.; Yap, K.M.; Winters, R. A corrosion predictive model for use in flowline and pipeline integrity management. In Proceedings of the CORROSION 2008, New Orleans, LO, USA, 16–20 March 2008; OnePetro: Richardson, TX, USA, 2008. [ Google Scholar ]
- Lazzari, L.; Kopliku, A.; Hoxha, G.; Cabrini, M.; Pietro, P. Prediction of CO 2 corrosion in oil and gas wells: Analysis of some case histories. In Proceedings of the CORROSION 98, San Diego, CA, USA, 22–27 March 1998; OnePetro: Richardson, TX, USA, 1998. [ Google Scholar ]
- Crolet, J.-L.; Bonis, M. Prediction of the risks of CO 2 corrosion in oil and gas wells. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 7–10 May 1990; OnePetro: Richardson, TX, USA, 1990. [ Google Scholar ]
- Nesic, S.; Nyborg, R.; Stangeland, A.; Nordsveen, M. Mechanistic modeling for CO 2 corrosion with protective iron carbonate films. In Proceedings of the CORROSION 2001, Houston, TX, USA, 11–16 March 2001; OnePetro: Richardson, TX, USA, 2001. [ Google Scholar ]
- Nesic, S.; Postlethwaite, J.; Olsen, S. An electrochemical model for prediction of corrosion of mild steel in aqueous carbon dioxide solutions. Corrosion 1996 , 52 , 280–294. [ Google Scholar ] [ CrossRef ]
- Wang, Q.; Song, Y.; Zhang, X.; Dong, L.; Xi, Y.; Zeng, D.; Liu, Q.; Zhang, H.; Zhang, Z.; Yan, R.; et al. Evolution of corrosion prediction models for oil and gas pipelines: From empirical-driven to data-driven. Eng. Fail. Anal. 2023 , 146 , 107097. [ Google Scholar ] [ CrossRef ]
- Qin, G.; Cheng, Y.F. A review on defect assessment of pipelines: Principles, numerical solutions, and applications. Int. J. Press. Vessel. Pip. 2021 , 191 , 104329. [ Google Scholar ] [ CrossRef ]
- Zakikhani, K.; Nasiri, F.; Zayed, T. A review of failure prediction models for oil and gas pipelines. J. Pipeline Syst. Eng. Pract. 2020 , 11 , 03119001. [ Google Scholar ] [ CrossRef ]
- Ngai, E.W.; Hu, Y.; Wong, Y.H.; Chen, Y.; Sun, X. The application of data mining techniques in financial fraud detection: A classification framework and an academic review of literature. Decis. Support Syst. 2011 , 50 , 559–569. [ Google Scholar ] [ CrossRef ]
- Rachman, A.; Zhang, T.; Ratnayake, R.C. Applications of machine learning in pipeline integrity management: A state-of-the-art review. Int. J. Press. Vessel. Pip. 2021 , 193 , 104471. [ Google Scholar ] [ CrossRef ]
- Tawfik, G.M.; Dila, K.A.S.; Mohamed, M.Y.F.; Tam, D.N.H.; Kien, N.D.; Ahmed, A.M.; Huy, N.T. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop. Med. Health 2019 , 47 , 1–9. [ Google Scholar ] [ CrossRef ]
- Drucker, A.M.; Fleming, P.; Chan, A.-W. Research techniques made simple: Assessing risk of bias in systematic reviews. J. Investig. Dermatol. 2016 , 136 , e109–e114. [ Google Scholar ] [ CrossRef ]
- Seers, K. Qualitative systematic reviews: Their importance for our understanding of research relevant to pain. Br. J. Pain 2015 , 9 , 36–40. [ Google Scholar ] [ CrossRef ]
- Nguyen, T.; Gosine, R.G.; Warrian, P. A systematic review of big data analytics for oil and gas industry 4.0. IEEE Access 2020 , 8 , 61183–61201. [ Google Scholar ] [ CrossRef ]
- Sinclair, S.; Rockwell, G. Voyant-Tools (v.2.6.10). 2023. Available online: https://voyant-tools.org (accessed on 22 July 2023).
- Alhudithi, E. Review of voyant tools: See through your text. Lang. Learn. Technol. 2021 , 25 , 43–50. [ Google Scholar ]
- Yahi, S.; Bensmaili, A.; Haddad, A.; Benmohamed, M. Experimental approach to monitoring the degradation status of pipelines transporting hydrocarbons. Eur. J. Eng. Sci. Technol. 2021 , 4 , 34–44. [ Google Scholar ] [ CrossRef ]
- Mingjiang, X.; Zhigang, T. A review on pipeline integrity management utilizing in-line inspection data. Eng. Fail. Anal. 2018 , 92 , 222–239. [ Google Scholar ]
- Khasanova, A. Corrosion cracking under main pipelines stress. J. Phys. Conf. Ser. 2022 , 2176 , 012051. [ Google Scholar ] [ CrossRef ]
- Leis, B.; Eiber, R. Stress-corrosion cracking on gas-transmission pipelines: History, causes, and mitigation. In Proceedings of the First International Business Conference on Onshore Pipelines, Berlin, Germany, 8–9 December 1997. [ Google Scholar ]
- Wright, R.F.; Ziomek-Moroz, M.; Ohodnicki, P.R. Fe thin film coated optics for monitoring internal corrosion in natural gas pipelines. In Proceedings of the CORROSION 2018, Phoenix, AZ, USA, 15–19 April 2018. [ Google Scholar ]
- Wright, R.F.; Lu, P.; Devkota, J.; Lu, F.; Ziomek-Moroz, M.; Ohodnicki, P.R., Jr. Corrosion sensors for structural health monitoring of oil and natural gas infrastructure: A review. Sensors 2019 , 19 , 3964. [ Google Scholar ] [ CrossRef ]
- Baboian, R. Corrosion Tests and Standards: Application and Interpretation ; ASTM International: Washington, DC, USA, 2005; Volume 20. [ Google Scholar ]
- Hussain, M.; Hussain, I.; Hussain, A.; Kousar, S. Failure due to Cl-SCC of austenitic stainless steels. Mater. Perform. 2019 , 58 , 42–45. [ Google Scholar ]
- Hussain, M.; Zhang, T.; Khan, S.; Hassan, N. Stress corrosion cracking is a threat to pipeline integrity management. In Proceedings of the Corrosion and Prevention Conference, Perth, Australia, 24–27 November 2020; Available online: https://www.researchgate.net/publication/344781593 (accessed on 23 July 2023).
- Galvão, T.L.; Novell-Leruth, G.; Kuznetsova, A.; Tedim, J.O.; Gomes, J.R. Elucidating structure–property relationships in aluminum alloy corrosion inhibitors by machine learning. J. Phys. Chem. C 2020 , 124 , 5624–5635. [ Google Scholar ] [ CrossRef ]
- Jamil, I.; Bano, H.; Castano, J.G.; Mahmood, A. Characterization of atmospheric corrosion near the coastal areas of Arabian sea. Mater. Corros. 2018 , 69 , 898–907. [ Google Scholar ] [ CrossRef ]
- Shabarchin, O.; Tesfamariam, S. Internal corrosion hazard assessment of oil & gas pipelines using Bayesian belief network model. J. Loss Prev. Process Ind. 2016 , 40 , 479–495. [ Google Scholar ]
- Li, X.; Zhang, D.; Liu, Z.; Li, Z.; Du, C.; Dong, C. Materials science: Share corrosion data. Nature 2015 , 527 , 441–442. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Canadian Energy Pipeline Association. Stress Corrosion Cracking: Recommended Practices , 2nd ed.; An Industry Learding Document Detailing the Management of Transgranular SCC; Cadanian Energy Pipeline Association: Calgary, AB, Canada, 2007. [ Google Scholar ]
- ROSEN. The Framework You Can Trust—A Reasoned and Systematic Approach to Crack Management. 21 September 2017. Available online: https://www.rosen-group.com/global/company/insight/news/2017/The-Framework-you-can-trust.html (accessed on 2 August 2023).
- Alexander, R.; Altamirano, M.; Batdorj, S.; Brooks, M.; Brun, P.; Del Angel, A.; de la Rosa, A.; Brewer, C.; Deible, M. System for corrosion inspection and monitoring. Ind. Syst. Eng. Rev. 2016 , 4 , 82–87. [ Google Scholar ] [ CrossRef ]
- Coleman, G.A. Self excited eddy currents for the detection of SCC. In Proceedings of the International Pipeline Conference, Atlanta, Georgia, 22–27 July 2008; pp. 463–469. [ Google Scholar ]
- Butusova, Y.N.; Mishakin, V.; Kachanov, M. On monitoring the incubation stage of stress corrosion cracking in steel by the eddy current method. Int. J. Eng. Sci. 2020 , 148 , 103212. [ Google Scholar ] [ CrossRef ]
- Abubakar, S.A.; Mori, S.; Sumner, J. A review of factors affecting SCC initiation and propagation in pipeline carbon steels. Metals 2022 , 12 , 1397. [ Google Scholar ] [ CrossRef ]
- Heselmans, J.; Hladky, K.; Holdefer, M.; Wessels, R. New corrosion monitoring probe combines ER, LPR, HDA, floating B-constant, electrochemical noise and conductivity measurements. In Proceedings of the NACE CORROSION, Orlando, FL, USA, 17–21 March 2013. [ Google Scholar ]
- ElBatanouny, M.K.; Mangual, J.; Ziehl, P.H.; Matta, F. Early corrosion detection in prestressed concrete girders using acoustic emission. J. Mater. Civ. Eng. 2014 , 26 , 504–511. [ Google Scholar ] [ CrossRef ]
- Parlak, B.O.; Yavasoglu, H.A. A comprehensive analysis of in-line inspection tools and technologies for steel oil and gas pipelines. Sustainability 2023 , 15 , 2783. [ Google Scholar ] [ CrossRef ]
- Culbertson, D.L. Use of intelligent pigs to detect stress corrosion cracking in gas pipelines. In Proceedings of the NACE CORROSION, Denver, CO, USA, 24–29 March 1996. [ Google Scholar ]
- Lowe, A.; Eren, H.; Tan, Y.-J.; Kinsella, B.; Bailey, S. Continuous corrosion rate measurement by noise resistance calculation. IEEE Trans. Instrum. Meas. 2001 , 50 , 1059–1065. [ Google Scholar ] [ CrossRef ]
- Calabrese, L.; Proverbio, E. A review on the applications of acoustic emission technique in the study of stress corrosion cracking. Corros. Mater. Degrad. 2020 , 2 , 1–30. [ Google Scholar ] [ CrossRef ]
- Yang, H. Improvement of Acoustic Emission Technology for Stress Corrosion Cracking ; Brunel University London: London, UK, 2023. [ Google Scholar ]
- Zhang, Y. Electric and Magnetic Contributions and Defect Interactions in Remote Field Eddy Current Techniques ; Queen’s University at Kingston: Kingston, ON, Canada, 1997. [ Google Scholar ]
- Farhangdoust, S.; Mehrabi, A. Health monitoring of closure joints in accelerated bridge construction: A review of non-destructive testing application. J. Adv. Concr. Technol. 2019 , 17 , 381–404. [ Google Scholar ] [ CrossRef ]
- Groysman, A. Nondestructive Testing and Corrosion Monitoring. In Non-Destructive Evaluation of Corrosion and Corrosion-Assisted Cracking ; Wiley: Hoboken, NJ, USA, 2019; pp. 261–409. [ Google Scholar ]
- Dwivedi, S.K.; Vishwakarma, M.; Soni, A. Advances and researches on non destructive testing: A review. Mater. Today Proc. 2018 , 5 , 3690–3698. [ Google Scholar ] [ CrossRef ]
- Beuker, T.; Alers, R.; Brown, B.; Alers, G. SCC detection and coating disbondment detection improvements using the high resolution EMAT ILI-technology. In Proceedings of the International Pipeline Conference, San Diego, CA, USA, 1–4 August 2004. [ Google Scholar ]
- Aanes, M.; Haas, M.; Andersen, K.K.; Talberg, A.S. Inline-inspection crack detection for gas pipelines using a novel technology. In Proceedings of the International Pipeline Conference, Alberta, CA, USA, 26–30 September 2022; American Society of Mechanical Engineers: New York, NY, USA, 2022. [ Google Scholar ]
- Yang, Z.; Kan, B.; Li, J.; Su, Y.; Qiao, L. Hydrostatic pressure effects on stress corrosion cracking of X70 pipeline steel in a simulated deep-sea environment. Int. J. Hydrogen Energy 2017 , 42 , 27446–27457. [ Google Scholar ] [ CrossRef ]
- Beavers, J.A.; Jaske, C.E. Near-neutral pH SCC of pipelines: Effects of pressure fluctuations on crack propagation. In Proceedings of the NACE CORROSION, San Diego, CA, USA, 22–27 March 1998. [ Google Scholar ]
- Niaz, U.; Hussain, M. The threat to pipeline integrity from soil corrosion. Corros. Manag. 2021 , 25–27. [ Google Scholar ] [ CrossRef ]
- Dral, P.O.; von Lilienfeld, O.A.; Thiel, W. Machine learning of parameters for accurate semiempirical quantum chemical calculations. J. Chem. Theory Comput. 2015 , 11 , 2120–2125. [ Google Scholar ] [ CrossRef ]
- Ser, C.T.; Žuvela, P.; Wong, M.W. Prediction of corrosion inhibition efficiency of pyridines and quinolines on an iron surface using machine learning-powered quantitative structure-property relationships. Appl. Surf. Sci. 2020 , 512 , 145612. [ Google Scholar ] [ CrossRef ]
- Hussain, M.; Zhang, T.; Naseer, M.S.; Hussain, I. Impact of COVID-19 and needs of digital transformation to protect assets from corrosion. Corros. Manag. 2022 , 2022 , 31–33. [ Google Scholar ] [ CrossRef ]
- Michie, D.; Spiegelhalter, D.J.; Taylor, C.C.; Campbell, J. Machine Learning, Neural and Statistical Classification ; Ellis Horwood: Los Angeles, CA, USA, 1995. [ Google Scholar ]
- Ayodele, T.O. Machine learning overview. New Adv. Mach. Learn. 2010 , 2 , 9–18. [ Google Scholar ]
- Chen, A.; Zhang, X.; Zhou, Z. Machine learning: Accelerating materials development for energy storage and conversion. InfoMat 2020 , 2 , 553–576. [ Google Scholar ] [ CrossRef ]
- Liu, Y.; Guo, B.; Zou, X.; Li, Y.; Shi, S. Machine learning assisted materials design and discovery for rechargeable batteries. Energy Storage Mater. 2020 , 31 , 434–450. [ Google Scholar ] [ CrossRef ]
- Luo, Z.; Yang, X.; Wang, Y.; Liu, W.; Liu, S.; Zhu, Y.; Huang, Z.; Zhang, H.; Dou, S.; Xu, J. A survey of artificial intelligence techniques applied in energy storage materials R&D. Front. Energy Res. 2020 , 8 , 116. [ Google Scholar ]
- Gao, T.; Lu, W. Machine learning toward advanced energy storage devices and systems. Iscience 2021 , 24 , 101936. [ Google Scholar ] [ CrossRef ]
- Salian, I. Supervize Me: What’s the Difference between Supervised, Unsupervised, Semi-Supervised and Reinforcement Learning? Nvidia. 2 August 2018. Available online: https://blogs.nvidia.com/blog/supervised-unsupervised-learning/ (accessed on 2 August 2023).
- Soomro, A.A.; Mokhtar, A.A.; Kurnia, J.C.; Lu, H. Deep learning-based reliability model for oil and gas pipeline subjected to stress corrosion cracking: A review and concept. J. Hunan Univ. Nat. Sci. 2021 , 48 , 189–198. [ Google Scholar ]
- Li, R.; Verhagen, W.J.; Curran, R. A comparative study of data-driven prognostic approaches: Stochastic and statistical models. In Proceedings of the 2018 IEEE International Conference on Prognostics and Health Management (ICPHM), Seattle, WA, USA, 11–13 June 2018. [ Google Scholar ]
- Zhang, Y.; Xiong, R.; He, H.; Liu, Z. A LSTM-RNN method for the lithuim-ion battery remaining useful life prediction. In Proceedings of the 2017 Prognostics and System Health Management Conference (PHM-Harbin), Harbin, China, 9–12 July 2017. [ Google Scholar ]
- Li, X.; Zhang, W.; Ding, Q. Deep learning-based remaining useful life estimation of bearings using multi-scale feature extraction. Reliab. Eng. Syst. Saf. 2019 , 182 , 208–218. [ Google Scholar ] [ CrossRef ]
- Fentaye, A.D.; Ul-Haq Gilani, S.I.; Baheta, A.T.; Li, Y.-G. Performance-based fault diagnosis of a gas turbine engine using an integrated support vector machine and artificial neural network method. Proc. Inst. Mech. Eng. Part A J. Power Energy 2019 , 233 , 786–802. [ Google Scholar ] [ CrossRef ]
- Chang, H.-H.; Liu, L.; Yi, Y. Deep echo state Q-network (DEQN) and its application in dynamic spectrum sharing for 5G and beyond. IEEE Trans. Neural Netw. Learn. Syst. 2020 , 33 , 929–939. [ Google Scholar ] [ CrossRef ]
- School of Information, University of California, Berkeley. What Is Machine Learning (ML)? 26 June 2020. Available online: https://ischoolonline.berkeley.edu/blog/what-is-machine-learning/ (accessed on 2 August 2023).
- Cai, J.; Cottis, R.; Lyon, S. Phenomenological modelling of atmospheric corrosion using an artificial neural network. Corros. Sci. 1999 , 41 , 2001–2030. [ Google Scholar ] [ CrossRef ]
- Cheng, Y.; Huang, W.; Zhou, C. Artificial neural network technology for the data processing of on-line corrosion fatigue crack growth monitoring. Int. J. Press. Vessel. Pip. 1999 , 76 , 113–116. [ Google Scholar ] [ CrossRef ]
- Abbas, M.H.; Norman, R.; Charles, A. Neural network modelling of high pressure CO 2 corrosion in pipeline steels. Process Saf. Environ. Prot. 2018 , 119 , 36–45. [ Google Scholar ] [ CrossRef ]
- Arzaghi, E.; Abbassi, R.; Garaniya, V.; Binns, J.; Chin, C.; Khakzad, N.; Reniers, G. Developing a dynamic model for pitting and corrosion-fatigue damage of subsea pipelines. Ocean. Eng. 2018 , 150 , 391–396. [ Google Scholar ] [ CrossRef ]
- De Masi, G.; Vichi, R.; Gentile, M.; Bruschi, R.; Gabetta, G. A neural network predictive model of pipeline internal corrosion profile. In Proceedings of the 1st International Conference on Systems Informatics, Modeling and Simulation, Cambridge, UK, 26–28 March 2014. [ Google Scholar ]
- Askari, M.; Aliofkhazraei, M.; Ghaffari, S.; Hajizadeh, A. Film former corrosion inhibitors for oil and gas pipelines—A technical review. J. Nat. Gas Sci. Eng. 2018 , 58 , 92–114. [ Google Scholar ] [ CrossRef ]
- Cheng, A.; Chen, N.-Z. Corrosion fatigue crack growth modelling for subsea pipeline steels. Ocean. Eng. 2017 , 142 , 10–19. [ Google Scholar ] [ CrossRef ]
- Dann, M.R.; Maes, M.A. Stochastic corrosion growth modeling for pipelines using mass inspection data. Reliab. Eng. Syst. Saf. 2018 , 180 , 245–254. [ Google Scholar ] [ CrossRef ]
- Velázquez, J.; Cruz-Ramirez, J.; Valor, A.; Venegas, V.; Caleyo, F.; Hallen, J. Modeling localized corrosion of pipeline steels in oilfield produced water environments. Eng. Fail. Anal. 2017 , 79 , 216–231. [ Google Scholar ] [ CrossRef ]
- Jančíková, Z.; Zimný, O.; Koštial, P. Prediction of metal corrosion by neural networks. Metalurgija 2013 , 52 , 379–381. [ Google Scholar ]
- Kenny, E.D.; Paredes, R.S.; de Lacerda, L.A.; Sica, Y.C.; de Souza, G.P.; Lázaris, J. Artificial neural network corrosion modeling for metals in an equatorial climate. Corros. Sci. 2009 , 51 , 2266–2278. [ Google Scholar ] [ CrossRef ]
- Ossai, C.I. A data-driven machine learning approach for corrosion risk assessment—A comparative study. Big Data Cogn. Comput. 2019 , 3 , 28. [ Google Scholar ] [ CrossRef ]
- Bhandari, J.; Khan, F.; Abbassi, R.; Garaniya, V.; Ojeda, R. Modelling of pitting corrosion in marine and offshore steel structures–A technical review. J. Loss Prev. Process Ind. 2015 , 37 , 39–62. [ Google Scholar ] [ CrossRef ]
- Wu, K.-Y.; Mosleh, A. Effect of temporal variability of operating parameters in corrosion modelling for natural gas pipelines subject to uniform corrosion. J. Nat. Gas Sci. Eng. 2019 , 69 , 102930. [ Google Scholar ] [ CrossRef ]
- Keshtegar, B.; Seghier, M.E.A.B.; Zhu, S.-P.; Abbassi, R.; Trung, N.-T. Reliability analysis of corroded pipelines: Novel adaptive conjugate first order reliability method. J. Loss Prev. Process Ind. 2019 , 62 , 103986. [ Google Scholar ] [ CrossRef ]
- Liu, X.; Zheng, J.; Fu, J.; Ji, J.; Chen, G. Multi-level optimization of maintenance plan for natural gas pipeline systems subject to external corrosion. J. Nat. Gas Sci. Eng. 2018 , 50 , 64–73. [ Google Scholar ] [ CrossRef ]
- Zelmati, D.; Bouledroua, O.; Hafsi, Z.; Djukic, M.B. Probabilistic analysis of corroded pipeline under localized corrosion defects based on the intelligent inspection tool. Eng. Fail. Anal. 2020 , 115 , 104683. [ Google Scholar ] [ CrossRef ]
- Muthanna, B.G.N.; Bouledroua, O.; Meriem-Benziane, M.; Setvati, M.R.; Djukic, M.B. Assessment of corroded API 5L X52 pipe elbow using a modified failure assessment diagram. Int. J. Press. Vessel. Pip. 2021 , 190 , 104291. [ Google Scholar ] [ CrossRef ]
- Ben Seghier, M.e.A.; Bettayeb, M.; Correia, J.; De Jesus, A.; Calçada, R. Structural reliability of corroded pipeline using the so-called Separable Monte Carlo method. J. Strain Anal. Eng. Des. 2018 , 53 , 730–737. [ Google Scholar ] [ CrossRef ]
- Qin, G.; Cheng, Y.F. Failure pressure prediction by defect assessment and finite element modelling on natural gas pipelines under cyclic loading. J. Nat. Gas Sci. Eng. 2020 , 81 , 103445. [ Google Scholar ] [ CrossRef ]
- Seghier, M.E.A.B.; Höche, D.; Zheludkevich, M. Prediction of the internal corrosion rate for oil and gas pipeline: Implementation of ensemble learning techniques. J. Nat. Gas Sci. Eng. 2022 , 99 , 104425. [ Google Scholar ] [ CrossRef ]
- Reddy, M.S.B.; Ponnamma, D.; Sadasivuni, K.K.; Aich, S.; Kailasa, S.; Parangusan, H.; Ibrahim, M.; Eldeib, S.; Shehata, O.; Ismail, M. Sensors in advancing the capabilities of corrosion detection: A review. Sens. Actuators A Phys. 2021 , 332 , 113086. [ Google Scholar ] [ CrossRef ]
- Wang, F.; Wang, F.; He, H. Parametric electrochemical deposition of controllable morphology of copper micro-columns. J. Electrochem. Soc. 2016 , 163 , E322. [ Google Scholar ] [ CrossRef ]
- Muhlbauer, W.K. Pipeline Risk Management Manual: Ideas, Techniques, and Resources ; Elsevier: Amsterdam, The Netherlands, 2004. [ Google Scholar ]
- Cai, J.; Jiang, X.; Lodewijks, G.; Pei, Z.; Wu, W. Residual ultimate strength of damaged seamless metallic pipelines with combined dent and metal loss. Mar. Struct. 2018 , 61 , 188–201. [ Google Scholar ] [ CrossRef ]
- Arumugam, T.; Karuppanan, S.; Ovinis, M. Residual strength analysis of pipeline with circumferential groove corrosion subjected to internal pressure. Mater. Today Proc. 2020 , 29 , 88–93. [ Google Scholar ] [ CrossRef ]
- Guo, Y.; Meng, X.; Wang, D.; Meng, T.; Liu, S.; He, R. Comprehensive risk evaluation of long-distance oil and gas transportation pipelines using a fuzzy Petri net model. J. Nat. Gas Sci. Eng. 2016 , 33 , 18–29. [ Google Scholar ] [ CrossRef ]
- Mai, W.; Soghrati, S. A phase field model for simulating the stress corrosion cracking initiated from pits. Corros. Sci. 2017 , 125 , 87–98. [ Google Scholar ] [ CrossRef ]
- Taylor, C.D.; Tossey, B.M. High temperature oxidation of corrosion resistant alloys from machine learning. Npj Mater. Degrad. 2021 , 5 , 38. [ Google Scholar ] [ CrossRef ]
- Kong, L.-W.; Fan, H.-W.; Grebogi, C.; Lai, Y.-C. Machine learning prediction of critical transition and system collapse. Phys. Rev. Res. 2021 , 3 , 013090. [ Google Scholar ] [ CrossRef ]
- Cha, Y.J.; Choi, W.; Suh, G.; Mahmoudkhani, S.; Büyüköztürk, O. Autonomous structural visual inspection using region-based deep learning for detecting multiple damage types. Comput.-Aided Civ. Infrastruct. Eng. 2018 , 33 , 731–747. [ Google Scholar ] [ CrossRef ]
- Gao, Y.; Mosalam, K.M. Deep transfer learning for image-based structural damage recognition. Comput.-Aided Civ. Infrastruct. Eng. 2018 , 33 , 748–768. [ Google Scholar ] [ CrossRef ]
- Chatterjee, S.; Sarkar, S.; Hore, S.; Dey, N.; Ashour, A.S.; Balas, V.E. Particle swarm optimization trained neural network for structural failure prediction of multistoried RC buildings. Neural Comput. Appl. 2017 , 28 , 2005–2016. [ Google Scholar ] [ CrossRef ]
- Lu, Q.; Parlikad, A.K.; Woodall, P.; Don Ranasinghe, G.; Xie, X.; Liang, Z.; Konstantinou, E.; Heaton, J.; Schooling, J. Developing a digital twin at building and city levels: Case study of West Cambridge campus. J. Manag. Eng. 2020 , 36 , 05020004. [ Google Scholar ] [ CrossRef ]
- Liu, M.; Fang, S.; Dong, H.; Xu, C. Review of digital twin about concepts, technologies, and industrial applications. J. Manuf. Syst. 2021 , 58 , 346–361. [ Google Scholar ] [ CrossRef ]
- Aljaroudi, A.; Khan, F.; Akinturk, A.; Haddara, M.; Thodi, P. Risk-based assessment of offshore crude oil pipelines and condition-monitoring systems. J. Pipeline Eng. 2016 , 15 , 57. [ Google Scholar ]
- Hasan, A. Security of cross-country oil and gas pipelines: A risk-based model. J. Pipeline Syst. Eng. Pract. 2016 , 7 , 04016006. [ Google Scholar ] [ CrossRef ]
- Lu, L.; Liang, W.; Zhang, L.; Zhang, H.; Lu, Z.; Shan, J. A comprehensive risk evaluation method for natural gas pipelines by combining a risk matrix with a bow-tie model. J. Nat. Gas Sci. Eng. 2015 , 25 , 124–133. [ Google Scholar ] [ CrossRef ]
- Kabir, G.; Sadiq, R.; Tesfamariam, S. A fuzzy Bayesian belief network for safety assessment of oil and gas pipelines. Struct. Infrastruct. Eng. 2016 , 12 , 874–889. [ Google Scholar ] [ CrossRef ]
- Parvizsedghy, L.; Zayed, T. Consequence of failure: Neurofuzzy-based prediction model for gas pipelines. J. Perform. Constr. Facil. 2016 , 30 , 04015073. [ Google Scholar ] [ CrossRef ]
- Zhou, Q.; Wu, W.; Liu, D.; Li, K.; Qiao, Q. Estimation of corrosion failure likelihood of oil and gas pipeline based on fuzzy logic approach. Eng. Fail. Anal. 2016 , 70 , 48–55. [ Google Scholar ] [ CrossRef ]
- Dundulis, G.; Žutautaitė, I.; Janulionis, R.; Ušpuras, E.; Rimkevičius, S.; Eid, M. Integrated failure probability estimation based on structural integrity analysis and failure data: Natural gas pipeline case. Reliab. Eng. Syst. Saf. 2016 , 156 , 195–202. [ Google Scholar ] [ CrossRef ]
- Nessim, M.; Zhou, W.; Zhou, J.; Rothwell, B. Target reliability levels for design and assessment of onshore natural gas pipelines. J. Press. Vessel Technol. 2009 , 131 , 061701. [ Google Scholar ] [ CrossRef ]
- Zhou, W.G.; Liu, D.J.; Wang, H.; Pan, X.X. Remaining-life prediction and reliability assessment of buried gas pipelines under corrosion and alternating loads. J. Pipeline Syst. Eng. Pract. 2015 , 6 , 05014002. [ Google Scholar ]
- Khan, F.I.; Abbasi, S. Techniques and methodologies for risk analysis in chemical process industries. J. Loss Prev. Process Ind. 1998 , 11 , 261–277. [ Google Scholar ] [ CrossRef ]
- Khan, F.; Rathnayaka, S.; Ahmed, S. Methods and models in process safety and risk management: Past, present and future. Process Saf. Environ. Prot. 2015 , 98 , 116–147. [ Google Scholar ] [ CrossRef ]
- Li, X.; Zhang, L.; Khan, F.; Han, Z. A data-driven corrosion prediction model to support digitization of subsea operations. Process Saf. Environ. Prot. 2021 , 153 , 413–421. [ Google Scholar ] [ CrossRef ]
- Khan, F.I.; Abbasi, S. Risk analysis of a typical chemical industry using ORA procedure. J. Loss Prev. Process Ind. 2001 , 14 , 43–59. [ Google Scholar ] [ CrossRef ]
- Khan, F.I.; Haddara, M.M. Risk-based maintenance (RBM): A quantitative approach for maintenance/inspection scheduling and planning. J. Loss Prev. Process Ind. 2003 , 16 , 561–573. [ Google Scholar ] [ CrossRef ]
- Khan, F.; Yarveisy, R.; Abbassi, R. Risk-based pipeline integrity management: A road map for the resilient pipelines. J. Pipeline Sci. Eng. 2021 , 1 , 74–87. [ Google Scholar ] [ CrossRef ]
- Rachman, A.; Ratnayake, R.C. Machine learning approach for risk-based inspection screening assessment. Reliab. Eng. Syst. Saf. 2019 , 185 , 518–532. [ Google Scholar ] [ CrossRef ]
- Samarakoon, S.M.; Ratnayake, R.C. Strengthening, modification and repair techniques’ prioritization for structural integrity control of ageing offshore structures. Reliab. Eng. Syst. Saf. 2015 , 135 , 15–26. [ Google Scholar ] [ CrossRef ]
- Ratnayake, R.C.; Antosz, K. Development of a risk matrix and extending the risk-based maintenance analysis with fuzzy logic. Procedia Eng. 2017 , 182 , 602–610. [ Google Scholar ] [ CrossRef ]
- Askari, M.; Aliofkhazraei, M.; Afroukhteh, S. A comprehensive review on internal corrosion and cracking of oil and gas pipelines. J. Nat. Gas Sci. Eng. 2019 , 71 , 102971. [ Google Scholar ] [ CrossRef ]
- Jamshidi, A.; Yazdani-Chamzini, A.; Yakhchali, S.H.; Khaleghi, S. Developing a new fuzzy inference system for pipeline risk assessment. J. Loss Prev. Process Ind. 2013 , 26 , 197–208. [ Google Scholar ] [ CrossRef ]
- Zhang, W.; Bao, Z.; Jiang, S.; He, J. An artificial neural network-based algorithm for evaluation of fatigue crack propagation considering nonlinear damage accumulation. Materials 2016 , 9 , 483. [ Google Scholar ] [ CrossRef ]
- Wasim, M.; Djukic, M.B. External corrosion of oil and gas pipelines: A review of failure mechanisms and predictive preventions. J. Nat. Gas Sci. Eng. 2022 , 100 , 104467. [ Google Scholar ] [ CrossRef ]
- El-Abbasy, M.S.; Senouci, A.; Zayed, T.; Mosleh, F. A condition assessment model for oil and gas pipelines using integrated simulation and analytic network process. Struct. Infrastruct. Eng. 2015 , 11 , 263–281. [ Google Scholar ] [ CrossRef ]
- Lahiri, S.; Ghanta, K. Development of an artificial neural network correlation for prediction of hold-up of slurry transport in pipelines. Chem. Eng. Sci. 2008 , 63 , 1497–1509. [ Google Scholar ] [ CrossRef ]
- Marhavilas, P.-K.; Koulouriotis, D.; Gemeni, V. Risk analysis and assessment methodologies in the work sites: On a review, classification and comparative study of the scientific literature of the period 2000–2009. J. Loss Prev. Process Ind. 2011 , 24 , 477–523. [ Google Scholar ] [ CrossRef ]
- Nataraj, S. Analytic hierarchy process as a decision-support system in the petroleum pipeline industry. Issues Inf. Syst. 2005 , 6 , 16–21. [ Google Scholar ]
- Shahriar, A.; Sadiq, R.; Tesfamariam, S. Risk analysis for oil & gas pipelines: A sustainability assessment approach using fuzzy based bow-tie analysis. J. Loss Prev. Process Ind. 2012 , 25 , 505–523. [ Google Scholar ]
- Sinha, S.K.; Pandey, M.D. Probabilistic neural network for reliability assessment of oil and gas pipelines. Comput.-Aided Civ. Infrastruct. Eng. 2002 , 17 , 320–329. [ Google Scholar ] [ CrossRef ]
- Han, Z.Y.; Weng, W.G. Comparison study on qualitative and quantitative risk assessment methods for urban natural gas pipeline network. J. Hazard. Mater. 2011 , 189 , 509–518. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ismail, M.A.; Sadiq, R.; Soleymani, H.R.; Tesfamariam, S. Developing a road performance index using a Bayesian belief network model. J. Frankl. Inst. 2011 , 348 , 2539–2555. [ Google Scholar ] [ CrossRef ]
- Liu, G.; Ayello, F.; Vera, J.; Eckert, R.; Bhat, P. An exploration on the machine learning approaches to determine the erosion rates for liquid hydrocarbon transmission pipelines towards safer and cleaner transportations. J. Clean. Prod. 2021 , 295 , 126478. [ Google Scholar ] [ CrossRef ]
- Lu, H.; Peng, H.; Xu, Z.-D.; Qin, G.; Azimi, M.; Matthews, J.C.; Cao, L. Theory and machine learning modeling for burst pressure estimation of pipeline with multipoint corrosion. J. Pipeline Syst. Eng. Pract. 2023 , 14 , 04023022. [ Google Scholar ] [ CrossRef ]
- Sheikh, M.F.; Kamal, K.; Rafique, F.; Sabir, S.; Zaheer, H.; Khan, K. Corrosion detection and severity level prediction using acoustic emission and machine learning based approach. Ain Shams Eng. J. 2021 , 12 , 3891–3903. [ Google Scholar ] [ CrossRef ]
- De Masi, G.; Gentile, M.; Vichi, R.; Bruschi, R.; Gabetta, G. Machine learning approach to corrosion assessment in subsea pipelines. In Proceedings of the OCEANS 2015-Genova, Genova, Italy, 18–21 May 2015; pp. 1–6. [ Google Scholar ]
- Peng, X.; Anyaoha, U.; Liu, Z.; Tsukada, K. Analysis of magnetic-flux leakage (MFL) data for pipeline corrosion assessment. IEEE Trans. Magn. 2020 , 56 , 1–15. [ Google Scholar ] [ CrossRef ]
- Papamarkou, T.; Guy, H.; Kroencke, B.; Miller, J.; Robinette, P.; Schultz, D.; Hinkle, J.; Pullum, L.; Schuman, C.; Renshaw, J. Automated detection of pitting and stress corrosion cracks in used nuclear fuel dry storage canisters using residual neural networks. arXiv 2020 , arXiv:2003.03241. [ Google Scholar ]
- Sturrock, C.; Bogaerts, W. Empirical learning investigations of the stress corrosion cracking of austenitic stainless steels in high-temperature aqueous environments. Corrosion 1997 , 53 , NACE-97040333. [ Google Scholar ] [ CrossRef ]
- Khakzad, S.; Yang, M.; Lohi, A.; Khakzad, N. Probabilistic failure assessment of oil pipelines due to internal corrosion. Process Saf. Prog. 2022 , 41 , 793–803. [ Google Scholar ] [ CrossRef ]
- Habib, K.; Fakhral-Deen, A. Risk assessment and evaluation of materials commonly used in desalination plants subjected to pollution impact of the oil spill and oil fires in marine environment. Desalination 2001 , 139 , 249–253. [ Google Scholar ] [ CrossRef ]
- Choi, B.-H.; Chudnovsky, A. Observation and modeling of stress corrosion cracking in high pressure gas pipe steel. Metall. Mater. Trans. A 2011 , 42 , 383–395. [ Google Scholar ] [ CrossRef ]
- Zukhrufany, S. The Utilization of Supervised Machine Learning in Predicting Corrosion to Support Preventing Pipelines Leakage in Oil and Gas Industry. Master’s Thesis, University of Stavanger, Stavanger, Norway, 2018. [ Google Scholar ]
- Tan, W.C.; Goh, P.C.; Chua, K.H.; Chen, I.-M. Learning with corrosion feature: For automated quantitative risk analysis of corrosion mechanism. In Proceedings of the 2018 IEEE 14th International Conference on Automation Science and Engineering (CASE), Munich, Germany, 20–24 August 2018; pp. 1290–1295. [ Google Scholar ]
- Qasim, A.; Khan, M.S.; Lal, B.; Shariff, A.M. A perspective on dual purpose gas hydrate and corrosion inhibitors for flow assurance. J. Pet. Sci. Eng. 2019 , 183 , 106418. [ Google Scholar ] [ CrossRef ]
- IBM. Design for AI−Machine Learning. 6 December 2022. Available online: https://www.ibm.com/design/ai/basics.ml (accessed on 21 August 2023).
- Al-Sabaeei, A.M.; Alhussian, H.; Abdulkadir, S.J.; Jagadeesh, A. Prediction of oil and gas pipeline failures through machine learning approaches: A systematic review. Energy Rep. 2023 , 10 , 1313–1338. [ Google Scholar ] [ CrossRef ]
- Ma, H.; Wang, H.; Geng, M.; Ai, Y.; Zhang, W.; Zheng, W. A new hybrid approach model for predicting burst pressure of corroded pipelines of gas and oil. Eng. Fail. Anal. 2023 , 149 , 107248. [ Google Scholar ] [ CrossRef ]
- Liu, Y.; Bao, Y. Review on automated condition assessment of pipelines with machine learning. Adv. Eng. Inform. 2022 , 53 , 101687. [ Google Scholar ] [ CrossRef ]
- Soomro, A.A.; Mokhtar, A.A.; Kurnia, J.C.; Lashari, N.; Sarwar, U.; Jameel, S.M.; Inayat, M.; Oladosu, T.L. A review on Bayesian modeling approach to quantify failure risk assessment of oil and gas pipelines due to corrosion. Int. J. Press. Vessel. Pip. 2022 , 200 , 104841. [ Google Scholar ] [ CrossRef ]
- Ossai, C.I. Corrosion defect modelling of aged pipelines with a feed-forward multi-layer neural network for leak and burst failure estimation. Eng. Fail. Anal. 2020 , 110 , 104397. [ Google Scholar ] [ CrossRef ]
| Searching Index | Specific Content |
|---|---|
| Article Type | Publications in books, journals, and conferences |
| Database | Web of Science, IEEE Xplore, Elsevier, Springer |
| Classification | By the type of publication (i.e., concept, case study, and review), nationalities, application segments, enabling technologies, and affiliations (i.e., universities and industries) |
| Focus | Determine opportunities and challenges related to SCC detection and prediction in the context of oil and gas |
| Types of Learning | Data | Goal |
|---|---|---|
| Supervised | Labeled | Learn a mapping function |
| Unsupervised | Unlabeled | Find patterns |
| Semi-supervised | Labeled and unlabeled | Define a mapping function |
| Reinforcement | Trial and error | Maximize rewards |
| Corrosion Sensor Detection Technique | Type of Corrosion | Corrosion Phenomena/Parameter Assessed | Sensitivity | Field Monitoring Use |
|---|---|---|---|---|
| Acoustic emission (AE) | Stress corrosion cracks, pitting corrosion | Acoustic energy (impingement, leaks, and cracks) | Medium | Yes |
| Image processing techniques (IPT) | General, localized corrosion, SCC, erosion–corrosion | Morphology of the corroded surface (image color, texture, and shape characteristics) | High | Yes |
| Electrochemical noise (EN) | Uniform corrosion, localized corrosion (pitting, crevice), SCC | Electrical noise on the corrosion potential or current | High | Yes |
| Hydrogen monitoring (HM) | Erosion–corrosion, stress corrosion cracking | Hydrogen diffusion through metal | High | Yes |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Hussain, M.; Zhang, T.; Chaudhry, M.; Jamil, I.; Kausar, S.; Hussain, I. Review of Prediction of Stress Corrosion Cracking in Gas Pipelines Using Machine Learning. Machines 2024 , 12 , 42. https://doi.org/10.3390/machines12010042
Hussain M, Zhang T, Chaudhry M, Jamil I, Kausar S, Hussain I. Review of Prediction of Stress Corrosion Cracking in Gas Pipelines Using Machine Learning. Machines . 2024; 12(1):42. https://doi.org/10.3390/machines12010042
Hussain, Muhammad, Tieling Zhang, Muzaffar Chaudhry, Ishrat Jamil, Shazia Kausar, and Intizar Hussain. 2024. "Review of Prediction of Stress Corrosion Cracking in Gas Pipelines Using Machine Learning" Machines 12, no. 1: 42. https://doi.org/10.3390/machines12010042
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

- {{subColumn.name}}
AIMS Materials Science
- {{newsColumn.name}}
- Share facebook twitter google linkedin

Stress corrosion cracking of pipeline steels in near-neutral pH solutions: the role of mechanochemical and chemomechanical effects
- Roman I. Bogdanov 1 ,
- Yaakov B. Unigovski 2 , , ,
- Emmanuel M. Gutman 2 ,
- Iliya V. Ryakhovskikh 1 ,
- Roni Z. Shneck 2
- 1. Institute of Natural Gases and Gas Technologies-Gazprom VNIIGAZ LLC, Moscow Oblast, Razvilka Settlement, Russia
- 2. Dept. of Materials Engineering, Ben-Gurion University of the Negev, Beer-Sheva, Israel
- Received: 28 July 2019 Accepted: 09 October 2019 Published: 31 October 2019
- Full Text(HTML)
- Download PDF
- near-neutral pH SCC (NNpHSCC) ,
- mechanochemical ,
- chemomechanical effects
Citation: Roman I. Bogdanov, Yaakov B. Unigovski, Emmanuel M. Gutman, Iliya V. Ryakhovskikh, Roni Z. Shneck. Stress corrosion cracking of pipeline steels in near-neutral pH solutions: the role of mechanochemical and chemomechanical effects[J]. AIMS Materials Science, 2019, 6(6): 1065-1085. doi: 10.3934/matersci.2019.6.1065
Supplements
Access History
- Corresponding author: Ben-Gurion University of the Negev;
Reader Comments
- © 2019 the Author(s), licensee AIMS Press. This is an open access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/4.0 )
通讯作者: 陈斌, [email protected]
沈阳化工大学材料科学与工程学院 沈阳 110142

Article views( 4386 ) PDF downloads( 510 ) Cited by( 4 )
Figures and Tables
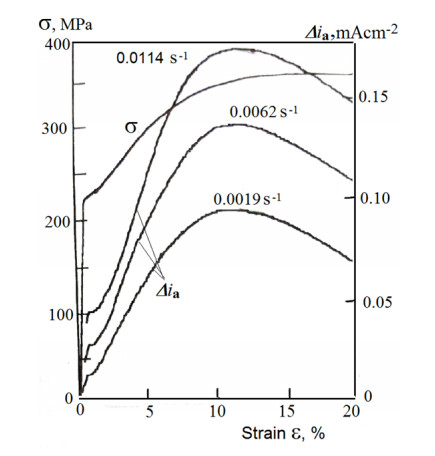
Figures( 3 )

Associated material
Other articles by authors.
- Roman I. Bogdanov
- Yaakov B. Unigovski
- Emmanuel M. Gutman
- Iliya V. Ryakhovskikh
- Roni Z. Shneck
Related pages
- on Google Scholar
- Email to a friend
- Order reprints
Export File

- Figure 1. The effect of strain and strain rate $\dot{\varepsilon}$ on anodic current density i a for low-carbon wire steel in 3.5 M H 2 SO 4 (Reprinted with permission from Ref. [ 19 ] )
- Figure 2. Schematic illustration of the effect of time upon SCC velocity (Reprinted with permission from Ref. [ 49 ] )
- Figure 3. Effect of cathodic potential on reduction-in-elongation in NS4 solution and pH 4 solution as compared to air (Reprinted with permission from Ref. [ 67 ] )

- ‹ Previous Chapter
- Next Chapter ›
32 Fatigue Crack Growth, Fatigue, and Stress Corrosion Crack Growth: Section XI Evaluation
- Published: 2020
- Cite Icon Cite
- Permissions
- Search Site
Bamford, WH. "Fatigue Crack Growth, Fatigue, and Stress Corrosion Crack Growth: Section XI Evaluation." Online Companion Guide to the ASME Boiler & Pressure Vessel Codes. ASME Press, 2020.
Download citation file:
- Ris (Zotero)
- Reference Manager
Fatigue has often been described as the most common cause of failure in engineering structures, and designers of pressure vessels and piping have incorporated fatigue considerations into design requirements since the first edition of Section III in 1963. The development of this technology and its application in Section III are discussed in Chapter 39 of the third edition of this publication. Its application in Section XI is discussed in Section 32.3 of this chapter.
With the advancement of the state of the art, the ability to recognize how the growth and size of a crack can lead to failure has been enhanced. This technology has been a key aspect of the Section XI flaw evaluation procedures since the 1974 edition was published and will be discussed thoroughly herein.
Further advancements in the state-of-the-art in fracture evaluation resulted in the introduction of models for stress corrosion cracking into Section XI in 2001, and since that time additional models have been added and more are planned. This addition gives Section XI flaw evaluations a complete treatment of all the potential modes of cracking that can occur in components, and details will be provided in Section 32.2.
Since additional crack growth data, for both fatigue and stress corrosion cracking, is periodically made available on a regular basis from additional research testing, the models contained in Section XI are regularly reviewed and updated to ensure their applicability, accuracy, and reflection of the latest known material behaviors. This improved knowledge may lead to more conservative models for crack growth over time. To accommodate such situations, Section XI has adopted an approach to conservatively account for potential increases in crack growth predictions with augmented examinations. The rules of IWB-3600 require that augmented (additional) examinations must be performed at an accelerated pace for situations where a flaw indication was accepted by analytical evaluation. This rule also protects against NDE uncertainties at the location of interest.
The nomenclature used here will be identical to that used in Section XI; any nomenclature that is not present in Section XI will be explained as it appears.
This chapter has been updated using the 2019 Code edition.
Warren H. Bamford is the original author of this chapter and has since revised it for all of the subsequent editions including the current online edition.
Email alerts
Related chapters, related articles, related proceedings papers, asme journals.
- About ASME Journals
- Information for Authors
- Submit a Paper
- Call for Papers
- Title History
ASME Conference Proceedings
- About ASME Conference Publications and Proceedings
- Conference Proceedings Author Guidelines
ASME eBooks
- About ASME eBooks
- ASME Press Advisory & Oversight Committee
- Book Proposal Guidelines
- Frequently Asked Questions
- Publication Permissions & Reprints
- ASME Membership
Opportunities
- Faculty Positions
- ASME Instagram

- Accessibility
- Privacy Statement
- Terms of Use
- Get Adobe Acrobat Reader
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Oxford Thesis Collection
- CC0 version of this metadata
Understanding the mechanisms controlling stress corrosion cracking through high-resolution characterization
Austenitic alloys have been extensively used in the nuclear industry as structural components due to their combination of excellent mechanical properties and high corrosion resistance. Although these alloys have a good service record, many can become susceptible to stress corrosion cracking (SCC) under pressurized water reactor (PWR) primary water conditions. After several decades of study, a considerable number of factors have been revealed that affect SCC susceptibility, such as material...
Email this record
Please enter the email address that the record information will be sent to.
Please add any additional information to be included within the email.
Cite this record
Chicago style, access document.
- Understanding the mechanisms controlling stress corrosion crac... ( Preview , pdf, 15.6MB, Terms of use )
Why is the content I wish to access not available via ORA?
Content may be unavailable for the following four reasons.
- Version unsuitable We have not obtained a suitable full-text for a given research output. See the versions advice for more information.
- Recently completed Sometimes content is held in ORA but is unavailable for a fixed period of time to comply with the policies and wishes of rights holders.
- Permissions All content made available in ORA should comply with relevant rights, such as copyright. See the copyright guide for more information.
- Clearance Some thesis volumes scanned as part of the digitisation scheme funded by Dr Leonard Polonsky are currently unavailable due to sensitive material or uncleared third-party copyright content. We are attempting to contact authors whose theses are affected.
Alternative access to the full-text
Request a copy.
We require your email address in order to let you know the outcome of your request.
Provide a statement outlining the basis of your request for the information of the author.
Please note any files released to you as part of your request are subject to the terms and conditions of use for the Oxford University Research Archive unless explicitly stated otherwise by the author.
Contributors
Bibliographic details, item description, terms of use, views and downloads.
If you are the owner of this record, you can report an update to it here: Report update to this record
Report an update
We require your email address in order to let you know the outcome of your enquiry.
OBSERVATIONS OF STRESS CORROSION CRACKING BEHAVIOUR IN SUPER DUPLEX STAINLESS STEEL
- Mohammed Al-Rabie
- Department of Materials
Student thesis : Phd
| Date of Award | 31 Dec 2011 |
|---|---|
| Original language | English |
| Awarding Institution |
File : application/pdf, -1 bytes
Type : Thesis

- Create Account
- Login
- Home
UR Research > Materials Science Program > Materials Science Ph.D. Theses >
Experiments to explore the mechanisms of stress corrosion cracking., url to cite or link to: http://hdl.handle.net/1802/14819.
| 3.70 MB (No. of downloads : 3420) | PDF of thesis. | |
| You can try | Zipped archive of source files (Admin only) |



IMAGES
VIDEO
COMMENTS
To capture the stress corrosion cracking (SCC) in metallic materials, a phase-field framework considering localized plastic deformation and stress states is established. A new function of critical energy release rate is proposed to describe the degradation of cracking resistance of materials in the SCC process. This proposed framework can ...
Stress corrosion reactions only affect the cement; they do not affect the grains. Therefore, each parallel bond is a potential reaction site. (b) Stress corrosion reactions occur at the bond surface and remove bond material at a uniform rate that is proportional to the crack velocity in Eq. (3), which is, in turn, proportional to the reaction ...
Factors Affecting Stress Assisted Corrosion Cracking of Carbon Steel under Industrial Boiler Conditions A Dissertation Presented to The Academic Faculty by Dong Yang In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy in the School of Mechanical Engineering Georgia Institute of Technology Atlanta, GA August, 2008
Stress corrosion cracking (SCC) is a form of material damage which relies on coupling between a microstructure, a chemical (and physical) environment and a stress state; it results in cracking of the material but a certain number of characteristics differentiates it from pure mechanical rupture. ... PhD thesis. Université de Toulouse (2015 ...
Stress Corrosion Cracking and Corrosion of Carbon Steel in Ethanolic Environments Doctor of Philosophy, 2022 Ali Ashrafriahi Department of Chemical Engineering and Applied Chemistry University of Toronto Several studies have recently claimed that the Stress Corrosion Cracking (SCC) of carbon steel in
This work addresses the risk of Stress Corrosion Cracking (SCC) in CO₂transport pipelines. The susceptibility of X80 pipeline steels in aqueous CO₂environments in the presence of nitrates and sulphites is investigated using electrochemical potentiodynamic tests and Slow Strain Rate Tests (SSRT) at 23 and 75°C. ... PhD Thesis: URI: http ...
Machine learning methods for corrosion and stress corrosion cracking risk analysis of engineered systems Author: Jiang, Peng ... A thesis in fulfillment of the requirements for the degree of ... First name: Peng . Other name/s: Abbreviation for degree as given in the University calendar: PhD . School: Materials Science and Engineering ...
His PhD thesis was distinguished with the Morris Cohen Award, awarded annually by The Electrochemical Society to outstanding graduate research in the field of Corrosion. ... Stress corrosion cracking of sensitized stainless steel in oxygenated high temperature water. Corrosion 1973; 29: 451-469. 10.5006/0010-9312-29.12.451 Search in Google ...
Stress corrosion cracking of P235 and P265 steels is investigated through slow strain rate and constant deformation tests on samples exposed in a cell reproducing the underground conditions of the French deep geological nuclear disposal, at 25 and 90°C. ... [PhD thesis]. France: Université Technologique de Compiègne; 2008 December 7. ...
Abstract: In this chapter, we present a few examples of stress corrosion cracking (SCC) studies with an emphasis on the various approaches implemented to reproduce, understand and propose solutions. Based on a few material/environment couples, we attach particular importance to the various study methodologies aiming to reproduce, at laboratory ...
Pipeline integrity and safety depend on the detection and prediction of stress corrosion cracking (SCC) and other defects. In oil and gas pipeline systems, a variety of corrosion-monitoring techniques are used. The observed data exhibit characteristics of nonlinearity, multidimensionality, and noise. Hence, data-driven modeling techniques have been widely utilized. To accomplish intelligent ...
Thesis (PhD)--University of Pretoria, 2013. tm2015. Materials Science and Metallurgical Engineering. PhD. ... The critical stress intensity for stress-corrosion cracking - K1scc - was determined by measuring the current flow from an external cathode, with comparisons with the crack growth rate. Crack propagation was measured with the potential ...
The review presents brief theoretical foundations of the mechanochemical and chemomechanical surface effects (MCE and CME) associated with corrosion-mechanical destruction of metals, considers the results of various scientific publications that studied the mechanochemical behavior of steels in near-neutral pH media, and analyzes the application of the results of such studies with the purpose ...
PhD School: Mining Engineering Faculty: Engineering Title: Laboratory-Based Investigation of Stress Corrosion Cracking Of Cable Bolts Abstract 350 words maximum Premature failure of cable bolts due to stress corrosion cracking (SCC) in underground excavations is a worldwide problem with limited cost-effective solutions at present.
Materials and Corrosion is a leading journal at the interface of materials science, metallurgy, and metallurgical engineering, with a focus on corrosion science. Modelling of intergranular stress corrosion cracking (SCC) in pipelines is an important field of study as predictive techniques are integral to pipeline integrity and management.
Further advancements in the state-of-the-art in fracture evaluation resulted in the introduction of models for stress corrosion cracking into Section XI in 2001, and since that time additional models have been added and more are planned. ... PhD Dissertation, Ohio State University, 1978. 49. Jones, R. H., Stress-Corrosion Cracking, ASM ...
Although these alloys have a good service record, many can become susceptible to stress corrosion cracking (SCC) Logos Skip to main NEW SEARCH Collections About Deposit HELP 0. Back to Search. CONTACT. Name. Email-Comment. Send message Actions ... [PhD thesis]. University of Oxford. Copy APA Style MLA Style. Shen, Z. Understanding the ...
Student thesis: Phd. Abstract The new generation of highly alloyed super duplex stainless steels such as Zeron 100 are preferable materials for industrial applications demanding high strength, toughness and superior corrosion resistance, especially against stress corrosion cracking (SCC). SCC is an environmentally assisted failure mechanism ...
Type II hot corrosion combined with static stress in Ni-based superalloys has not been extensively studied. However, stress corrosion cracking (SCC) is a well-documented failure mechanism especially in aqueous systems [ 12,13 ]. Studies have been conducted on the effects of stress on corrosion pitting growth in aluminium alloys [ 14 ].
It assumes the existence of a threshold of stress intensity factor. In the local model for intergranular stress corrosion cracking, Couvant et al. [CAB 16, COU 17] propose that the propagation step of a long crack can be modeled using a sigmoid law: [14.2] da / dt = A × K n 1 + exp − λ × K − K 0 × exp − Q RT.
Description : Thesis (Ph. D.)--University of Rochester. Materials Science Program, 2011. Abstract : Stress corrosion cracking (SCC) is a type of subcritical cracking of materials that occurs when a SCC susceptible material is simultaneously stressed in tension (applied or residual) and exposed to a specific corrosive environment.
Stress Corrosion Cracking of High-Strength Pipeline Steels. Y. Frank Cheng, Y. Frank Cheng. Search for more papers by this author. Book Author(s): Y. Frank Cheng, ... Mechanoelectrochemical Effect of Corrosion of Pipelines Under Strain. REFERENCES, () , , , - ...
This paper presents an analysis of Stress Corrosion Cracking (SCC) based on the Theory of Critical Distances (TCD). The research is based on an experimental program composed of fracture specimens with notch radius varying from 0 mm (crack-like defect) up to 1 mm, and tensile specimens.
Corrosion fatigue and stress corrosion cracking of magnesium alloys in a simulated physiological environment. Magnesium (Mg) alloys have attracted great attention as potential materials for temporary implants in uses such as pins, screws, plates and stents. ... Accordingly, this PhD thesis has attempted to evaluate CF and SCC resistance of one ...