- Share full article
Advertisement
Supported by

Tough Decisions About Dementia and End-of-Life Care
Readers discuss Sandeep Jauhar’s guest essay about dementia and advance directives.

To the Editor:
Re “ My Father Didn’t Want to Live if He Had Dementia. But Then He Had It ,” by Sandeep Jauhar (Opinion guest essay, Oct. 28):
As a hale elder, about to become 90, I have a terrible fear of getting dementia. I can relate entirely to the way Dr. Jauhar’s father felt in his healthier days, but I think Dr. Jauhar is missing something that his father likely felt, which he may not have articulated in his advance directive.
I suspect that he never wanted to be a burden on his children. That is certainly what underlies my urgency to beseech my children to allow me to die if I get dementia. The thought of the burden that it would impose on those who love me is unbearable.
Please listen to those advance directives. What good does it do to merely exist without the zest and delight of awareness? I think that a father who loved his sons dearly and expressed those early thoughts would bless them for allowing him to die with dignity, as he wished in his sentient days.
Carol Landau-Meyerson Floral Park, N.Y.
Sandeep Jauhar’s essay should make us think more deeply about assessing quality of life. My father, Eugene Lang , a brilliant philanthropist and entrepreneur, developed outward signs of Alzheimer’s in his late 80s. He remained physically strong and healthy otherwise.
He knew he was diminished. “I think I reduced myself from one thing to something else,” he randomly observed one day.
My father never wept or complained of pain. He claimed to be “consciously content” — more genial than before his illness and rarely angry. He couldn’t read a book or remember what happened either long ago or five minutes before, but he could sing, word for word, all the verses of songs he taught me when I was a toddler.
This is how my father lived in the Alzheimer’s stage of his life. Had I described it to him before the disease took hold, he would have suggested that I shoot him; he was very worried about losing his marbles, as he put it. And so I signed the do-not-resuscitate order with confidence that it was what he had wanted.
But as the months went on, I no longer believed that my father’s wishes from a time before he had experienced his life with Alzheimer’s were dispositive.
Dr. Jauhar’s quandary reminded me of all this. My father never suggested that he didn’t wish to live. He continued to appreciate the pleasures of food, music and company. The circumstances of his life — a walker, fractured words, imbalance, confusion, 24-hour nursing assistance — would have horrified him at one time, but they rarely perturbed him in the moment.
I hear you had a good day, I said to him one night. “I didn’t have a good day,” he replied. “I had a damn good day.”
My father died at home in his sleep in 2017 at 98.
Jane Lang Washington
The loss of a beloved parent is wrenching, but I wonder if Dr. Sandeep Jauhar’s deeply felt ambivalence about respecting his father’s advance directive doesn’t reflect his own inability to accept his father’s decline and death rather than ethical qualms.
Since his father had clearly stated his wishes before he became too impaired to review them, it seems both infantilizing and disrespectful for his children to second-guess these desires when he can no longer defend them himself.
Surely the ability to enjoy a mouthful of ice cream is not a meaningful benchmark for continuing the diminished existence his father had clearly feared and rejected?
Jane Zimmerman Palo Alto, Calif.
Advance care planning is inherently problematic given that we cannot predict our future ailments and, importantly, cannot predict how we will feel about our quality of life when we’re afflicted with serious illness. Discussions with loved ones help, and the legal durable power of attorney is important. However, loving families may disagree about what your wishes are.
Dr. Barak Gaster has published a now widely used Advance Directive for Dementia , which addresses life-support choices in the various stages of dementia. Also, End of Life Washington has developed a more flexible and extensive set of Dementia Directives , allowing revocation and changes by the individual affected.
There ultimately comes a time to “let go” for all of us. Because advance directive documents are always nuanced, the deep discussions we have with our loved ones are critical to help them support our wishes at the end.
Jim deMaine Seattle The writer, a former pulmonary and critical care physician, is the author of “Facing Death: Finding Dignity, Hope and Healing at the End.”
Dr. Sandeep Jauhar’s thoughtful analysis of the conundrum facing caretakers of a loved one with Alzheimer’s disease overlooks what, in my book, is the most important consideration: how my loved ones remember me.
My memory of my father, once a powerful intellectual with a commanding presence, is now overlaid by memories of him lashing out in anger and frustration at everyone around him as his condition worsened. Our relationship was always complex, but he left me with indelible sadness when, in his last somewhat lucid moments, he selfishly demanded that I kill him, with no concern for the possible imprisonment I would face.
My earlier memories of him are now buried under the vision of a man slumped in a wheelchair in diapers, looking at me with empty eyes.
I’ve had seven decades of life; a few more months at the end are nothing compared to what I’ve achieved and how I want to be remembered. I want to die with my dignity intact. I want to be remembered as a whole human, not a hollowed shell. I don’t want the last memories of my loved ones to be of me in a diaper, unable to answer the simplest questions.
Craig James Santa Cruz, Calif.
Both my parents made me promise never to let them linger, like so many of their friends. I’m a retired neurologist who has withdrawn support for hundreds of people. My colleagues told me it would be different when it happened to my parents. No, it wasn’t.
When my parents became incapacitated, my mother from a rapidly progressive dementia, my father from multi-organ failure, I kept them comfortable and did nothing more, offering food and water but no tubes. Both died within six weeks of their beginning to fail.
I was at their bedside when they died. Of course, it hurt, but I kept my promise. It was the second best thing I have ever done in my life (marrying my wife was the best).
I realize today that if I gave any gift at all to medicine, it was not in cures for patients. I was no super doc miracle worker. No, my gift was in facing the end of life, facing reality, which meant using the words death, die and dying, and doing what I could do — comfort, relieve pain and be present at the end.
Michael S. Smith Eugene, Ore.

Presentations made painless
- Get Premium
108 Dementia Essay Topic Ideas & Examples
Inside This Article
Dementia is a complex and challenging condition that affects millions of people worldwide. Writing an essay on dementia can be a great way to raise awareness about this condition, explore its causes and symptoms, and discuss potential treatments and care strategies. However, coming up with a unique and engaging topic can sometimes be a daunting task. To help you get started, here are 108 dementia essay topic ideas and examples:
- The impact of dementia on individuals and their families.
- The role of genetics in the development of dementia.
- Exploring the different stages of dementia.
- The ethical considerations surrounding the care of individuals with dementia.
- The importance of early diagnosis and intervention in dementia.
- The challenges faced by caregivers of individuals with dementia.
- The impact of dementia on cognitive functions.
- Investigating the link between dementia and Alzheimer's disease.
- The role of nutrition in preventing or managing dementia.
- The social stigma associated with dementia and its effects on individuals and families.
- The potential benefits of music therapy for individuals with dementia.
- Examining the role of exercise in improving cognitive function in dementia patients.
- Exploring the impact of sleep disturbances on dementia progression.
- The influence of environmental factors on dementia risk.
- Investigating the effectiveness of non-pharmacological interventions in managing dementia symptoms.
- The economic burden of dementia on healthcare systems.
- The impact of dementia on the quality of life of individuals and their caregivers.
- The role of technology in supporting individuals with dementia.
- The potential benefits and risks of pharmacological interventions in dementia treatment.
- Examining the relationship between cardiovascular health and dementia risk.
- The impact of dementia on language and communication abilities.
- Exploring the relationship between depression and dementia.
- The importance of person-centered care in dementia management.
- The role of art therapy in improving emotional well-being in individuals with dementia.
- The potential benefits of reminiscence therapy in dementia care.
- Investigating the impact of social isolation on dementia progression.
- The role of occupational therapy in supporting individuals with dementia.
- The impact of dementia on sensory perception.
- Examining the effectiveness of cognitive stimulation therapy in improving cognitive function in individuals with dementia.
- The potential benefits of aromatherapy in managing behavioral symptoms of dementia.
- Investigating the impact of dementia on motor function and mobility.
- The role of spirituality in supporting individuals with dementia.
- The influence of cultural factors on dementia care.
- The impact of dementia on decision-making abilities.
- Exploring the relationship between diabetes and dementia.
- The potential benefits of pet therapy for individuals with dementia.
- Investigating the impact of traumatic brain injury on dementia risk.
- The role of neuroimaging in the early detection of dementia.
- The impact of dementia on sleep patterns and circadian rhythms.
- Examining the relationship between hearing loss and dementia.
- The potential benefits of mindfulness-based interventions in dementia care.
- Investigating the impact of dementia on social relationships and interactions.
- The role of respite care in supporting caregivers of individuals with dementia.
- The impact of dementia on executive functions and problem-solving abilities.
- Exploring the relationship between dementia and visual perception.
- The potential benefits of horticulture therapy in dementia care.
- Investigating the impact of dementia on emotional regulation.
- The role of nutrition in preventing or delaying dementia onset.
- The impact of dementia on the sense of self and identity.
- Examining the relationship between inflammation and dementia.
- The potential benefits of dance therapy for individuals with dementia.
- Investigating the impact of dementia on the sense of smell.
- The role of mindfulness meditation in reducing caregiver stress in dementia.
- The impact of dementia on personality and behavior.
- Exploring the relationship between traumatic childhood experiences and dementia risk.
- The potential benefits of light therapy in managing sleep disturbances in individuals with dementia.
- Investigating the impact of dementia on the ability to recognize faces.
- The role of laughter therapy in improving emotional well-being in individuals with dementia.
- The impact of dementia on the ability to perform daily living activities.
- Examining the relationship between dementia and social inequality.
- The potential benefits of virtual reality interventions in dementia care.
- Investigating the impact of dementia on the ability to navigate and orient in space.
- The role of cognitive rehabilitation in improving cognitive function in individuals with dementia.
- The impact of dementia on the ability to perceive and interpret emotions.
- Exploring the relationship between dementia and substance abuse.
- The potential benefits of animal-assisted therapy in dementia care.
- Investigating the impact of dementia on the ability to recognize objects and symbols.
- The role of humor therapy in improving emotional well-being in individuals with dementia.
- The impact of dementia on the ability to plan and execute complex tasks.
- Examining the relationship between dementia and post-traumatic stress disorder.
- The potential benefits of drama therapy for individuals with dementia.
- Investigating the impact of dementia on the ability to understand and produce language.
- The role of mindfulness-based stress reduction in supporting caregivers of individuals with dementia.
- The impact of dementia on the ability to learn and remember new information.
- Exploring the relationship between dementia and anxiety disorders.
- The potential benefits of creative writing therapy in dementia care.
- Investigating the impact of dementia on the ability to reason and make logical judgments.
- The role of cognitive-behavioral therapy in managing behavioral symptoms of dementia.
- The impact of dementia on the ability to recognize and interpret music.
- Examining the relationship between dementia and personality disorders.
- The potential benefits of art therapy for individuals with dementia.
- Investigating the impact of dementia on the ability to process and understand visual information.
- The role of mindfulness-based cognitive therapy in improving emotional well-being in individuals with dementia.
- The impact of dementia on the ability to recognize and interpret non-verbal cues.
- Exploring the relationship between dementia and sleep disorders.
- The potential benefits of poetry therapy in dementia care.
- Investigating the impact of dementia on the ability to problem-solve and make decisions.
- The role of cognitive training in improving cognitive function in individuals with dementia.
- The impact of dementia on the ability to recognize and interpret facial expressions.
- Examining the relationship between dementia and obsessive-compulsive disorder.
- The potential benefits of dance/movement therapy for individuals with dementia.
- Investigating the impact of dementia on the ability to process and understand auditory information.
- The role of acceptance and commitment therapy in improving emotional well-being in individuals with dementia.
- The impact of dementia on the ability to recognize and interpret body language.
- Exploring the relationship between dementia and eating disorders.
- The potential benefits of storytelling therapy in dementia care.
- Investigating the impact of dementia on the ability to focus and sustain attention.
- The role of cognitive reserve in delaying cognitive decline in individuals with dementia.
- The impact of dementia on the ability to recognize and understand emotions in others.
- Examining the relationship between dementia and bipolar disorder.
- The potential benefits of gardening therapy for individuals with dementia.
- Investigating the impact of dementia on the ability to inhibit impulsive behaviors.
- The role of social engagement in promoting cognitive health in individuals with dementia.
- The impact of dementia on the ability to recognize and interpret humor.
- Exploring the relationship between dementia and schizophrenia.
- The potential benefits of pet therapy for individuals with advanced dementia.
- Investigating the impact of dementia on the ability to switch between tasks and mental states.
- The role of cognitive enhancers in improving cognitive function in individuals with dementia.
Remember, these topics are just a starting point, and you can modify or combine them to suit your interests and research goals. Whether you choose to explore the biological, psychological, social, or environmental aspects of dementia, writing an essay on this topic can contribute to the understanding and improvement of dementia care and support.
Want to create a presentation now?
Instantly Create A Deck
Let PitchGrade do this for me
Hassle Free
We will create your text and designs for you. Sit back and relax while we do the work.
Explore More Content
- Privacy Policy
- Terms of Service
© 2023 Pitchgrade
131 Dementia Essay Topic Ideas & Examples
🏆 best dementia topic ideas & essay examples, 💡 interesting topics to write about dementia, ⭐ good research topics about dementia, 📝 simple & easy dementia essay titles, ❓ research questions about dementia.
- Care For a Client Suffering From Moderate Dementia One of the problems may be connected to hearing; in this case, it is recommended to arrange clients in positions closer to the caregiver to enhance their ability to hear and follow the narration of […]
- Caring for Clients With Dementia These include Alzheimer’s disease, which is the most common, followed by vascular dementia and dementia, with Lewy bodies as the least common of the three. We will write a custom essay specifically for you by our professional experts 808 writers online Learn More
- Care of the Elderly With Dementia When speaking of the ethical issue of autonomy and restraints, it is vital to recognize how Deontology emphasizes respect and support of autonomy when it is the right decision to make.
- The Frontal Lobe and the Impact of Dementia on It When discussing the frontal lobe, it is essential to mention the prefrontal cortex, which is the front part of the frontal lobe.
- Dementia: Non-Drug and Pharmacological Treatment The problem of dementia remains relevant in modern times, and the issue is especially acute in nursing homes. Accordingly, the following organizations should monitor this issue to improve the non-drug and pharmacological treatment of dementia […]
- Dementia in Older Adults: Effects and Prevention As a result, the research questions for the topic of dementia are as follows: How does the body deteriorate with dementia, and how strong can these changes be for the person diagnosed with dementia?
- Therapeutic Dogs, Dementia, Alzheimer’s and Fluid Intelligence It is worth noting that with dementia, the patient has a speech disorder and a personality change in the early stages of the pathology.
- The Alzheimer’s Association Dementia Care Practice Therefore, achieving the philosophy and recommendations of the association is a shared responsibility between doctors, patients, and caregivers. Ultimately, CAPD tests the functionalities of the patient ranging from the psychomotor activities, perceptions, awareness, and orientations, […]
- Dementia, Alzheimer, and Delirium in an Elderly Woman Additionally, she struggles with identifying the appropriate words to use in dialogue and changes the topic. Timing: While in the middle of conversations and public places like supermarkets.
- Diagnosis of Dementia by Machine Learning Methods in Epidemiological Studies Therefore, epidemiological studies directly impact the diagnosis, prognosis, and clinical treatment by presenting medical practitioners with relevant data on the course, presentation, and treatment of an illness.
- The Clock Drawing Test: Dementia Diagnosis Firstly, one should draw attention to the fact that the diagnosis of dementia was made in 2011, and the patient did not experience any evident symptoms of the condition for the next three years.
- Non- and Pharmacological Dementia Care Methods The analysis of the importance of non-pharmacological versus pharmacological methods in providing care for individuals living with dementia formulates the objectives of the health policy.
- Pharmacological Methods to Provide Care to Dementia Patients The aim of this paper is to discuss the non-pharmacological and pharmacological methods of providing care to dementia patients in nursing homes.
- Toxic Environmental Factors and Development of Dementia As a result, at the moment, the study of the influence of environmental particles on the development of diseases in humans is relevant.
- Managing Dementia and Alzheimer’s Disease The PICOT question is “In the care of Alzheimer’s and dementia patients, does integrated community-based care as compared to being in a long-term care facility improve outcome throughout the remainder of their lives”.
- Nursing Physical Assessment of Dementia Patient As a nurse, I must care for the patient and provide patient education to the wife and his close relatives. It would help him forget his worries and trigger the brain to function.
- Therapy Approaches to Aphasia and Dementia Aphasia also illustrates various emotional and social impacts, with people facing these issues, and their families, describing the experience as a journey.
- Health Care Within Aging White Veterans With Dementia Since this condition is heavily linked with damage to the brain, these people should be addressed in a friendly manner to avoid misunderstanding.
- Person-Centered Strategy of Diabetes and Dementia Care The population of focus for this study will be Afro-American women aged between sixty and ninety who have diabetes of the second type and dementia or are likely to develop dementia in the future.
- Therapy of Dementia Elderly People The aging process is characterized by a progressive decrease in the functionality of all vital organs, as a result of which elderly patients are more sensitive to both therapeutic and side effects of drugs taken.
- Analysis of Dementia Treatment Cognitive, biographical pieces of training contribute to the tone of memory and intelligence. Furthermore, using these types of therapies will contribute to health education and a decrease in hospitalization.
- Delirium, Dementia and Immobility Disorders The issues of the inability of patients to function properly, the difficulties of identifying the causes of the symptoms and their relation to the disorder, and insufficient research influence the situation in general.
- Frontotemporal Dementia vs. Alzheimer’s Disease in a Patient Moreover, Alzheimer’s disease affects hypertrophies in the hippocampus as the initial part is involved in the brain’s memory areas and spatial orientation.
- A Report on Assessing Aged Patients With Dementia Since assessment forms the main part of treatment and care of patients with dementia, this report gives several assessment tools that could be used in finding the degree of pain, depression and ability to feed […]
- Dementia: Relaxing Music at Mealtime in Nursing Homes Agitated Patients To reinforce the evidence in support of this modality, and supplementing work carried out by Goddaer and Abraham, the present study scrutinizes the relationship between agitation and soothing music in an assembly of aged residents […]
- Dementia in Residential Aged Care Setting Dementia is a health condition which is defined by Bidewell & Chang, as the progressive decline in cognitive function or, simply, the worsening of a person’s ability to process thought.
- Planning Care Delivery in Dementia According to Chinn and Kramer, the failure to address the requirements of each phase undermines the quality of care. The care planning process begins with the assessment of the client’s needs and preferences.
- Dementia: How Individuals Cope With Condition In most cases, individuals living with dementia find it difficult to successfully cope with the situation mainly because they lose their autonomy and are forced to depend on their relatives and friends.
- Urinary Tract Infections and Dementia Management Importance Reporting the History of Dementia Many patients residing in hospitals after being diagnosed with dementia are, usually, very vulnerable to other infections such as pneumonia and UTI. These illnesses take advantage of the weak immunity in the bodies of the patients since most of them are 81 years and above (Fortinash & Holoday-Worret, 2012). […]
- Management of Dementia Condition Dementia is one of the most common disorders in society that is associated with the loss of cognitive ability in aged adults.
- Dementia and Memory Retention Art therapy is an effective intervention in the management of dementia because it stimulates reminiscence and enhances memory retention among patients with dementia.
- The Middle Range Theory and Care to Patients Suffering From Dementia This paper applies the Lazarus and Folkman Stress and Coping Theory to a family providing health support to a family member by the name Martin. From the exercise, I learned that the family members found […]
- Inter-Professional Healthcare Collaboration: 72-Year-Old Dementia Patient The conversational difficulties in Loretta were caused by the decline of the mental processes essential to the communicative functions, including the functions of recognition and usage of language signs.
- Human Disorders: Alzheimer’s Disease and Dementia The brain shows notable changes in Alzheimer’s disease notably, development of tangles in deep areas of the brain and also formation of plagues in other areas.
- Family Theory Use With Dementia The theories of the family include the historical theory, the stress theory, the functional-structural theory, and of course the attachment theory.
- Neurological Disorders and Management of TIA, CVA, Delirium and Dementia In the course of the diagnostic it is advisable to handle the patients with care as some patients tend to be bluntly combative or highly agitated and thus may require the use of chemical restraints.
- The Causes Dementia in Older Adults The purpose of this report is to investigate the causes of dementia and explore the role of a mental health nurse in helping patients to manage the condition.
- Pharmacotherapy for Dementia The prevalence of the disease is yet relatively low but is projected to grow, at least in the United States. The individual set of symptoms usually is the basis for the prescription of drug therapy.
- Mental Health Nursing: Dementia Statistics relating to dementia, as a mental health issue, suggest that there will be an increase in the number of patients diagnosed with the disease as more people seek help for their mental health issues […]
- Vulnerable Population: Elderly With Dementia The purpose of this paper is to describe the ways of supporting older people with dementia with the use of several strategies.
- Changes in the Brain: Types of Dementia According to Cavanaugh and Blanchard-Fields, dementia is a “family of disorders” that involves behavioral and cognitive deficits due to permanent adverse changes to the brain structure and its functioning.
- Dementia: Disease Analysis and Treatment Strategies The purpose of this paper is to research this mental condition and present evidence-based ideas that different professionals can utilize to meet the changing health demands of more patients.
- Alcoholic Dementia and the Wernicke-Korsakoff Syndrome However, this situation can be problematic because of the nature of the two conditions as well as their interactions. As such, medical practitioners struggle to prescribe treatments that are appropriate to the patient’s situation.
- Dementia in Survivors of Ischemic or Hemorrhagic Stroke The incidence of stroke is the highest in older adults, and this condition is also among the leading causes of long-term disability in the country.
- Elderly Kit Business Plan for Dementia in the UAE The research of the situation has opened an opportunity to think about a product that could improve the quality of life of people with dementia in the UAE.
- Frontotemporal Dementia: Causes and Etymology These findings demonstrate that the enhanced tendency to develop Frontotemporal Dementia in these people is not due to a shared environment but to shared genetic material.”One of the major criteria used for distinguishing frontal variant […]
- Reminiscing Group Therapy for Dementia Patients The elderly population which is increasing rapidly due to the rise in longevity is a group that requires plenty of attention by way of therapy and interventions for their several problems of health and disability. […]
- Dementia: Non-Pharmacologic Interventions Inappropriate behaviors in any disease are very common and in dementia different behaviors are common as in this disease memory function involves that’s why patient behaves abnormally.
- Dementia: Ethical Dilemmas Opting to withdraw the tube may lead to the physiological deprivation of the patient and as a result, the worst-case scenario is the death of the patient.
- Geriatric Dementia, Delirium, and Depression I talked to the patient’s daughter to get additional information about the patient’s medical history and symptoms. In the future, I will consider more therapies and lifestyle changes to offer to the patient.
- Dementia, Delirium, and Depression in Older Adults The comparison is no pharmacological treatment or placebo to exclude the use of other medications, and the outcome is the reduction of delirium severity.
- “Knowing Residents With Dementia” by Kasin and Kautz The research works to eliminate all of the unique aspects of the environment in order to apply the results to the largest possible number of subjects and experiments.
- Dementia in Elderly Population While the condition is common for people over 65, dementia is not a part of the aging process. The drugs of dementia symptoms are expensive and are often reported as a source of financial hardships […]
- Diagnosing Neurological Disorders: Dementia The needs of patients with memory issues are quite difficult to address due to the increase in the levels of stress experienced by both a patient and their family members.
- Dementia, Delirium, and Depression in Frail Elders The patient’s daughter should be educated about the necessity of contact with the patient and possible mobility and other aids to help her with ADL.
- Elderly Dementia: Holistic Approaches to Memory Care The CMAI is a nursing-rated questionnaire that evaluates the recurrence of agitation in residents with dementia. Since the research focuses on agitation, the CMAI was utilized to evaluate the occurrence of agitation at baseline.
- Music Intervention’s Effect on Falls in a Dementia Unit That is why the authors investigate the issue of the relation between music and dementia in order to find the best solution to the existing problem.
- Dementia, Aging, Gerontology: Theories and Care Proponents of the theory, Elaine Cumming and William Henry take the psychosocial perspective in explaining the unhealthy collective relationships the aging person’s experience in the latest phases of their lives.
- Down Syndrome and Dementia: Theories and Treatment The genetic material in the chromosome 21 is responsible for the development of the disorder, and its symptoms appear at the infantry stage of development.
- Age Ailment: Dementia and Alzheimer’s Disease It is a time for one to clean the mind and take time to do what matters most in life. With an increased level of technological advancements, a digital sabbatical is mandatory to lower the […]
- Dementia Life Expectancy: Developed vs. Developing Countries Analysis of Economic Aspects Influencing the Lifespan of People with Dementia in Developing and Developed Countries On the one hand, the previously discussed studies point to the direct influence of age on life of people […]
- Dementia and Its Connection With Memory Loss
- Behavioral and Psychiatric Symptoms of Dementia and Rate of Decline in Alzheimer’s Disease
- Altered High-Density Lipoprotein Composition in Behavioral Variant Frontotemporal Dementia
- Children With Dementia and Parkinson’s Disease
- Early Dementia, Alzheimer’s and Parkinson’s Disease Pathology
- Determining Modifiable Risk Factors of Dementia
- Accountable Practitioner Consent and Application to Practice Dementia and Ability to Give Informed Consent
- Burden Among Family Caregivers of Dementia in the Oldest-Old: An Exploratory Study
- Learning Language and Acoustic Models for Identifying Alzheimer’s Dementia From Speech
- Music Therapy and Dementia
- Depression and Missed Work Among Informal Caregivers of Older Individuals With Dementia
- Lifestyle and Dietary Factors Associated With Dementia Status in the Elderly Aged 65 and Older
- Cognitive and Neuropsychiatric Manifestations of COVID-19 and Effects on Elderly Individuals With Dementia
- Acoustic and Language-Based Deep Learning Approaches for Alzheimer’s Dementia Detection From Spontaneous Speech
- Links Between Adiponectin and Dementia: From Risk Factors to Pathophysiology
- Dementia and Its Effects on Society
- Anger Management Therapy for Dementia Patients
- Causes, Symptoms, and Treatment of Dementia
- Amidated and Ibuprofen-Conjugated Kyotorphins and Neuronal Rescue and Memory Recovery in Cerebral Hypoperfusion Dementia Model
- Dementia and Its Effects on Mental Health
- Difference Between Dementia, Delirium and Alzheimer’s
- Enable Rights and Choices of Individuals With Dementia
- Big Data and Dementia: Charting the Route-Ahead for Research, Ethics, and Policy
- Depression: Psychology and Subsequent Vascular Dementia
- Alzheimer’s Disease for Dementia With Lewy Bodies
- Dementia Care Pathway-People With Learning Disability
- Dementia and the Different Parts of the Brain Affected
- Equality, Diversity, and Inclusion in Dementia Care
- Background Information About Dementia and Home Care Services
- Cognitive Stimulation and Cognitive and Functional Decline in Alzheimer’s Disease: The Cache County Dementia Progression Study
- Caring for Patients With Dementia
- Affective and Engagement Issues in the Conception and Assessment of a Robot-Assisted Psychomotor Therapy for Persons With Dementia
- Dementia and Its Effect on the Function of the Brain
- Anti-neurotrophic Effects From Autoantibodies in Adult Diabetes Having Primary Open-Angle Glaucoma or Dementia
- Association Between Cortical Superficial Siderosis and Dementia in Patients With Cognitive Impairment: A Meta-Analysis
- Nutritional Status, Oxidative Stress, and Dementia: The Role of Selenium in Alzheimer’s Disease
- Body Weight Variability Increases Dementia Risk Among Older Adults: A Nationwide Population-Based Cohort Study
- Caring for Persons Living With Dementia During the COVID-19 Pandemic
- Biomarkers for Dementia, Fatigue, and Depression in Parkinson’s Disease
- Dementia and Evidence-Based Practice
- What Are Antipsychotic Drugs, and Why Are They Used on Dementia Patients?
- What Are the Nurses’ Experiences in Caring for Dementia Patients With Challenging Behavior?
- What Causes Juvenile Dementia?
- Where Would You Turn for Help for Dementia Care?
- How Does Art Therapy Affect a Patient With Dementia?
- How Does Dementia Onset in Parents Influence Unmarried Adult Children’s Wealth?
- How Exercise Delays Onset of Dementia in Alzheimer’s Patients?
- Can Cognitive Training Slow Down the Progression of Dementia?
- Can Doll Therapy Preserve or Promote Attachment in People With Dementia?
- Can Medication Alter the Course of Dementia?
- Can Mobile Technology Help Prevent the Burden of Dementia in Low- And Mid-Income Countries?
- Adult ADHD: Risk Factor for Dementia or Phenotypic Mimic?
- Are Anticholinergic Medications Associated With Increased Risk of Dementia and Behavioral and Psychological Symptoms of Dementia?
- Dementia: How and Whom Does It Affect?
- Healthy Aging and Dementia: Two Roads Diverging in Midlife?
- Informal and Formal Care: Substitutes or Complements in Care for People With Dementia?
- Vascular Dementia: Are There Any Differences From Vascular Aging?
- Nitrendipine and Dementia: Forgotten Positive Facts?
- What Are the First Signs of Having Dementia?
- What Are the Five Types of Dementia?
- What Does Dementia Do to a Person?
- Do People With Dementia Know They Have It?
- Does a Person With Dementia Know They Are Confused?
- Does Dementia Run in Families?
- What Is the Leading Cause of Dementia?
- Can a Person Recover From Dementia?
- How Long Do Dementia Patients Live?
- Is There a Way to Prevent Dementia?
- What Vitamins Help Prevent Dementia?
- Does Lack Sleep Cause Dementia?
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, March 2). 131 Dementia Essay Topic Ideas & Examples. https://ivypanda.com/essays/topic/dementia-essay-topics/
"131 Dementia Essay Topic Ideas & Examples." IvyPanda , 2 Mar. 2024, ivypanda.com/essays/topic/dementia-essay-topics/.
IvyPanda . (2024) '131 Dementia Essay Topic Ideas & Examples'. 2 March.
IvyPanda . 2024. "131 Dementia Essay Topic Ideas & Examples." March 2, 2024. https://ivypanda.com/essays/topic/dementia-essay-topics/.
1. IvyPanda . "131 Dementia Essay Topic Ideas & Examples." March 2, 2024. https://ivypanda.com/essays/topic/dementia-essay-topics/.
Bibliography
IvyPanda . "131 Dementia Essay Topic Ideas & Examples." March 2, 2024. https://ivypanda.com/essays/topic/dementia-essay-topics/.
- ADHD Essay Ideas
- Caregiver Topics
- Alzheimer’s Disease Research Ideas
- Delirium Essay Ideas
- Alcoholism Essay Titles
- Elder Abuse Ideas
- Nervous System Research Topics
- Parkinson’s Disease Questions
- Nursing Home Questions
- Occupational Therapy Titles
- Gerontology Titles
- Schizophrenia Essay Topics
- Aging Ideas
- Sleep Disorders Research Topics
- Arthritis Titles
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Dementia articles from across Nature Portfolio
Dementia is a syndrome that involves severe loss of cognitive abilities as a result of disease or injury. Dementia caused by traumatic brain injury is often static, whereas dementia caused by neurodegenerative disorders, such as Alzheimer's disease, is usually progressive and can eventually be fatal.
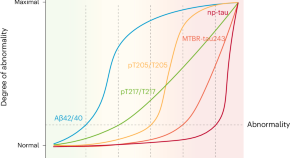
Five biomarkers from one cerebrospinal fluid sample to stage Alzheimer’s disease
Staging Alzheimer’s disease on the basis of the disease’s biological underpinnings might help with stratification and prognostication, both in the clinical setting and in clinical trials. We propose a staging model based on only five biomarkers, which are related to amyloid-β and tau pathologies in different ways and can be measured with a single sample of cerebrospinal fluid.
Related Subjects
- Alzheimer's disease
Latest Research and Reviews
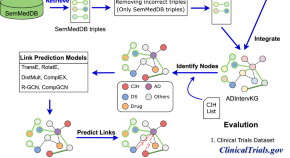
Repurposing non-pharmacological interventions for Alzheimer's disease through link prediction on biomedical literature
- Yongkang Xiao
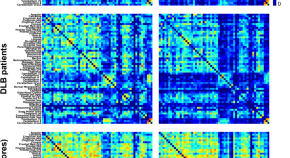
Grey matter networks in women and men with dementia with Lewy bodies
- Annegret Habich
- Javier Oltra
- Daniel Ferreira
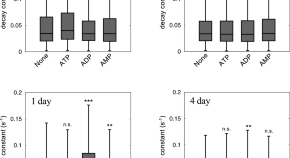
Adenosine triphosphate induces amorphous aggregation of amyloid β by increasing Aβ dynamics
- Masahiro Kuramochi
- Momoka Nakamura
- Kazuaki Yoshimune
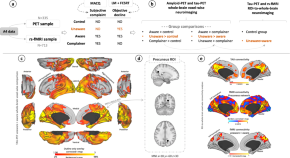
Multi-modal Neuroimaging Phenotyping of Mnemonic Anosognosia in the Aging Brain
Bueichekú et al. use multimodal in vivo neuroimaging to investigate the brain characteristics of individuals presenting unawareness of memory loss who are at risk of Alzheimer’s disease due to age. They find unawareness of memory decline is an early behavioral sign that a person might develop Alzheimer’s disease.
- Elisenda Bueichekú
- Patrizia Vannini
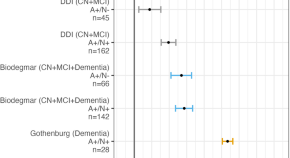
Plasma brain-derived tau is an amyloid-associated neurodegeneration biomarker in Alzheimer’s disease
The authors investigated associations of brain-derived-tau (BD-tau) with Aβ pathology, changes in cognition and MRI signatures. Staging Aβ-pathology according to neurodegeneration, using BD-tau, identifies individuals at risk of near-term cognitive decline and atrophy.
- Fernando Gonzalez-Ortiz
- Bjørn-Eivind Kirsebom
- Kaj Blennow
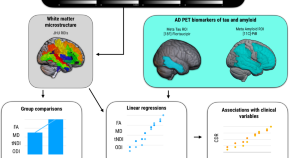
Influences of amyloid-β and tau on white matter neurite alterations in dementia with Lewy bodies
- Robert I. Reid
- Kejal Kantarci
News and Comment
Neuronal activity drives glymphatic waste clearance.
Two new studies show that clearance of waste, including pathogenic amyloid, through the glymphatic system is driven by synchronized neuronal activity.
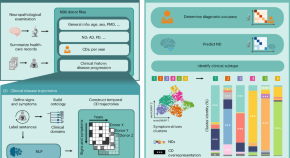
Revealing clinical heterogeneity in a large brain bank cohort
Clinical disease trajectories that describe neuropsychiatric symptoms were identified using natural language processing for 3,042 brain donors diagnosed with various neurodegenerative disorders. Trajectories revealed distinct temporal patterns that result in the identification of new clinical subtypes, and a subset of misdiagnosed donors.
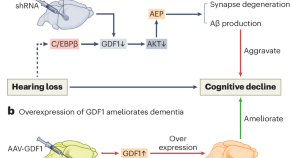
Hearing loss promotes Alzheimer’s disease
Epidemiological studies reveal a correlation between hearing loss and the development and progression of Alzheimer’s disease (AD), but the underlying causal mechanisms remain unclear. A study now provides experimental evidence that hearing loss can promote AD via the growth differentiation factor 1 (GDF1) pathway, which may aid in developing potential AD therapeutic strategies.
- Hong-Bo Zhao
Marker provides 10-year warning of dementia
Changes in plasma levels of specific proteins could predict the development of dementia more than 10 years before clinical diagnosis.
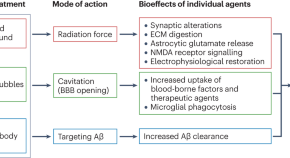
Ultrasound and antibodies — a potentially powerful combination for Alzheimer disease therapy
Success in a trial of low-intensity ultrasound combined with an amyloid-β antibody represents a major stride towards integrating pharmacological and nonpharmacological approaches to reduce the amyloid-β load in patients with mild Alzheimer disease. This trial also highlights the potential of therapeutic ultrasound modalities to combat neurodegenerative diseases.
- Jürgen Götz
- Pranesh Padmanabhan
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

Dementia prevention, intervention, and care: 2020 report of the Lancet Commission
Gill livingston.
a Division of Psychiatry, University College London, London, UK
d Camden and Islington NHS Foundation Trust, London, UK
Jonathan Huntley
Andrew sommerlad.
f National Ageing Research Institute and Academic Unit for Psychiatry of Old Age, University of Melbourne, Royal Melbourne Hospital, Parkville, VIC, Australia
Clive Ballard
g University of Exeter, Exeter, UK
Sube Banerjee
h Faculty of Health: Medicine, Dentistry and Human Sciences, University of Plymouth, Plymouth, UK
Carol Brayne
i Cambridge Institute of Public Health, University of Cambridge, Cambridge, UK
Alistair Burns
j Department of Old Age Psychiatry, University of Manchester, Manchester, UK
Jiska Cohen-Mansfield
k Department of Health Promotion, School of Public Health, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
l Heczeg Institute on Aging, Tel Aviv University, Tel Aviv, Israel
m Minerva Center for Interdisciplinary Study of End of Life, Tel Aviv University, Tel Aviv, Israel
Claudia Cooper
Sergi g costafreda.
n Department of Preventive and Social Medicine, Goa Medical College, Goa, India
b Dementia Research Centre, UK Dementia Research Institute, University College London, London, UK
o Institute of Neurology, National Hospital for Neurology and Neurosurgery, University College London Hospitals NHS Foundation Trust, London, UK
Laura N Gitlin
p Center for Innovative Care in Aging, Johns Hopkins University, Baltimore, MA, USA
Robert Howard
Helen c kales.
r Department of Psychiatry and Behavioral Sciences, UC Davis School of Medicine, University of California, Sacramento, CA, USA
Mika Kivimäki
c Department of Epidemiology and Public Health, University College London, London, UK
Eric B Larson
s Kaiser Permanente Washington Health Research Institute, Seattle, WA, USA
Adesola Ogunniyi
t University College Hospital, Ibadan, Nigeria
Vasiliki Orgeta
Karen ritchie.
u Inserm, Unit 1061, Neuropsychiatry: Epidemiological and Clinical Research, La Colombière Hospital, University of Montpellier, Montpellier, France
v Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, UK
Kenneth Rockwood
w Centre for the Health Care of Elderly People, Geriatric Medicine Dalhousie University, Halifax, NS, Canada
Elizabeth L Sampson
e Barnet, Enfield, and Haringey Mental Health Trust, London, UK
Quincy Samus
q Department of Psychiatry and Behavioral Sciences, Johns Hopkins University, Baltimore, MA, USA
Lon S Schneider
x Department of Psychiatry and the Behavioural Sciences and Department of Neurology, Keck School of Medicine, Leonard Davis School of Gerontology of the University of Southern California, Los Angeles, CA, USA
Geir Selbæk
y Norwegian National Advisory Unit on Ageing and Health, Vestfold Hospital Trust, Tønsberg, Norway
z Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway
aa Geriatric Department, Oslo University Hospital, Oslo, Norway
ab Department Psychosocial and Community Health, School of Nursing, University of Washington, Seattle, WA, USA
Naaheed Mukadam
Associated data, executive summary.
The number of older people, including those living with dementia, is rising, as younger age mortality declines. However, the age-specific incidence of dementia has fallen in many countries, probably because of improvements in education, nutrition, health care, and lifestyle changes. Overall, a growing body of evidence supports the nine potentially modifiable risk factors for dementia modelled by the 2017 Lancet Commission on dementia prevention, intervention, and care: less education, hypertension, hearing impairment, smoking, obesity, depression, physical inactivity, diabetes, and low social contact. We now add three more risk factors for dementia with newer, convincing evidence. These factors are excessive alcohol consumption, traumatic brain injury, and air pollution. We have completed new reviews and meta-analyses and incorporated these into an updated 12 risk factor life-course model of dementia prevention. Together the 12 modifiable risk factors account for around 40% of worldwide dementias, which consequently could theoretically be prevented or delayed. The potential for prevention is high and might be higher in low-income and middle-income countries (LMIC) where more dementias occur.
Our new life-course model and evidence synthesis has paramount worldwide policy implications. It is never too early and never too late in the life course for dementia prevention. Early-life (younger than 45 years) risks, such as less education, affect cognitive reserve; midlife (45–65 years), and later-life (older than 65 years) risk factors influence reserve and triggering of neuropathological developments. Culture, poverty, and inequality are key drivers of the need for change. Individuals who are most deprived need these changes the most and will derive the highest benefit.
Policy should prioritise childhood education for all. Public health initiatives minimising head injury and decreasing harmful alcohol drinking could potentially reduce young-onset and later-life dementia. Midlife systolic blood pressure control should aim for 130 mm Hg or lower to delay or prevent dementia. Stopping smoking, even in later life, ameliorates this risk. Passive smoking is a less considered modifiable risk factor for dementia. Many countries have restricted this exposure. Policy makers should expedite improvements in air quality, particularly in areas with high air pollution.
We recommend keeping cognitively, physically, and socially active in midlife and later life although little evidence exists for any single specific activity protecting against dementia. Using hearing aids appears to reduce the excess risk from hearing loss. Sustained exercise in midlife, and possibly later life, protects from dementia, perhaps through decreasing obesity, diabetes, and cardiovascular risk. Depression might be a risk for dementia, but in later life dementia might cause depression. Although behaviour change is difficult and some associations might not be purely causal, individuals have a huge potential to reduce their dementia risk.
In LMIC, not everyone has access to secondary education; high rates of hypertension, obesity, and hearing loss exist, and the prevalence of diabetes and smoking are growing, thus an even greater proportion of dementia is potentially preventable.
Amyloid-β and tau biomarkers indicate risk of progression to Alzheimer's dementia but most people with normal cognition with only these biomarkers never develop the disease. Although accurate diagnosis is important for patients who have impairments and functional concerns and their families, no evidence exists to support pre-symptomatic diagnosis in everyday practice.
Our understanding of dementia aetiology is shifting, with latest description of new pathological causes. In the oldest adults (older than 90 years), in particular, mixed dementia is more common. Blood biomarkers might hold promise for future diagnostic approaches and are more scalable than CSF and brain imaging markers.
Wellbeing is the goal of much of dementia care. People with dementia have complex problems and symptoms in many domains. Interventions should be individualised and consider the person as a whole, as well as their family carers. Evidence is accumulating for the effectiveness, at least in the short term, of psychosocial interventions tailored to the patient's needs, to manage neuropsychiatric symptoms. Evidence-based interventions for carers can reduce depressive and anxiety symptoms over years and be cost-effective.
Keeping people with dementia physically healthy is important for their cognition. People with dementia have more physical health problems than others of the same age but often receive less community health care and find it particularly difficult to access and organise care. People with dementia have more hospital admissions than other older people, including for illnesses that are potentially manageable at home. They have died disproportionately in the COVID-19 epidemic. Hospitalisations are distressing and are associated with poor outcomes and high costs. Health-care professionals should consider dementia in older people without known dementia who have frequent admissions or who develop delirium. Delirium is common in people with dementia and contributes to cognitive decline. In hospital, care including appropriate sensory stimulation, ensuring fluid intake, and avoiding infections might reduce delirium incidence.
Key messages
- • New evidence supports adding three modifiable risk factors—excessive alcohol consumption, head injury, and air pollution—to our 2017 Lancet Commission on dementia prevention, intervention, and care life-course model of nine factors (less education, hypertension, hearing impairment, smoking, obesity, depression, physical inactivity, diabetes, and infrequent social contact).
- • Modifying 12 risk factors might prevent or delay up to 40% of dementias.
- • Prevention is about policy and individuals. Contributions to the risk and mitigation of dementia begin early and continue throughout life, so it is never too early or too late. These actions require both public health programmes and individually tailored interventions. In addition to population strategies, policy should address high-risk groups to increase social, cognitive, and physical activity; and vascular health.
- • Aim to maintain systolic BP of 130 mm Hg or less in midlife from around age 40 years (antihypertensive treatment for hypertension is the only known effective preventive medication for dementia).
- • Encourage use of hearing aids for hearing loss and reduce hearing loss by protection of ears from excessive noise exposure.
- • Reduce exposure to air pollution and second-hand tobacco smoke.
- • Prevent head injury.
- • Limit alcohol use, as alcohol misuse and drinking more than 21 units weekly increase the risk of dementia.
- • Avoid smoking uptake and support smoking cessation to stop smoking, as this reduces the risk of dementia even in later life.
- • Provide all children with primary and secondary education.
- • Reduce obesity and the linked condition of diabetes. Sustain midlife, and possibly later life physical activity.
- • Addressing other putative risk factors for dementia, like sleep, through lifestyle interventions, will improve general health.
- • Many risk factors cluster around inequalities, which occur particularly in Black, Asian, and minority ethnic groups and in vulnerable populations. Tackling these factors will involve not only health promotion but also societal action to improve the circumstances in which people live their lives. Examples include creating environments that have physical activity as a norm, reducing the population profile of blood pressure rising with age through better patterns of nutrition, and reducing potential excessive noise exposure.
- • Dementia is rising more in low-income and middle-income countries (LMIC) than in high-income countries, because of population ageing and higher frequency of potentially modifiable risk factors. Preventative interventions might yield the largest dementia reductions in LMIC.
For those with dementia, recommendations are:
- • Post-diagnostic care for people with dementia should address physical and mental health, social care, and support. Most people with dementia have other illnesses and might struggle to look after their health and this might result in potentially preventable hospitalisations.
- • Specific multicomponent interventions decrease neuropsychiatric symptoms in people with dementia and are the treatments of choice. Psychotropic drugs are often ineffective and might have severe adverse effects.
- • Specific interventions for family carers have long-lasting effects on depression and anxiety symptoms, increase quality of life, are cost-effective and might save money.
Acting now on dementia prevention, intervention, and care will vastly improve living and dying for individuals with dementia and their families, and thus society.
Introduction
Worldwide around 50 million people live with dementia, and this number is projected to increase to 152 million by 2050, 1 rising particularly in low-income and middle-income countries (LMIC) where around two-thirds of people with dementia live. 1 Dementia affects individuals, their families, and the economy, with global costs estimated at about US$1 trillion annually. 1
We reconvened the 2017 Lancet Commission on dementia prevention, intervention, and care 2 to identify the evidence for advances likely to have the greatest impact since our 2017 paper and build on its work. Our interdisciplinary, international group of experts presented, debated, and agreed on the best available evidence. We adopted a triangulation framework evaluating the consistency of evidence from different lines of research and used that as the basis to evaluate evidence. We have summarised best evidence using, where possible, good- quality systematic reviews, meta-analyses, or individual studies, where these add important knowledge to the field. We performed systematic literature reviews and meta-analyses where needed to generate new evidence for our analysis of potentially modifiable risk factors for dementia. Within this framework, we present a narrative synthesis of evidence including systematic reviews and meta-analyses and explain its balance, strengths, and limitations. We evaluated new evidence on dementia risk in LMIC; risks and protective factors for dementia; detection of Alzheimer's disease; multimorbidity in dementia; and interventions for people affected by dementia.
Nearly all the evidence is from studies in high-income countries (HIC), so risks might differ in other countries and interventions might require modification for different cultures and environments. This notion also underpins the critical need to understand the dementias related to life-course disadvantage—whether in HICs or LMICs.
Our understanding of dementia aetiology is shifting. A consensus group, for example, has described hippocampal sclerosis associated with TDP-43 proteinopathy, as limbic-predominant age-related TDP-43 encephalopathy (LATE) dementia, usually found in people older than 80 years, progressing more slowly than Alzheimer's disease, detectable at post-mortem, often mimicking or comorbid with Alzheimer's disease. 3 This situation reflects increasing attention as to how clinical syndromes are and are not related to particular underlying pathologies and how this might change across age. More work is needed, however, before LATE can be used as a valid clinical diagnosis.
The fastest growing demographic group in HIC is the oldest adults, those aged over 90 years. Thus a unique opportunity exists to focus on both human biology, in this previously rare population, as well as on meeting their needs and promoting their wellbeing.
Prevention of dementia
The number of people with dementia is rising. Predictions about future trends in dementia prevalence vary depending on the underlying assumptions and geographical region, but generally suggest substantial increases in overall prevalence related to an ageing population. For example, according to the Global Burden of Diseases, Injuries, and Risk Factors Study, the global age-standardised prevalence of dementia between 1990 and 2016 was relatively stable, but with an ageing and bigger population the number of people with dementia has more than doubled since 1990. 4
However, in many HIC such as the USA, the UK, and France, age-specific incidence rates are lower in more recent cohorts compared with cohorts from previous decades collected using similar methods and target populations 5 ( figure 1 ) and the age-specific incidence of dementia appears to decrease. 6 All-cause dementia incidence is lower in people born more recently, 7 probably due to educational, socio-economic, health care, and lifestyle changes. 2 , 5 However, in these countries increasing obesity and diabetes and declining physical activity might reverse this trajectory. 8 , 9 In contrast, age-specific dementia prevalence in Japan, South Korea, Hong Kong, and Taiwan looks as if it is increasing, as is Alzheimer's in LMIC, although whether diagnostic methods are always the same in comparison studies is unclear. 5 , 6 , 7

Incidence rate ratio comparing new cohorts to old cohorts from five studies of dementia incidence 5
IIDP Project in USA and Nigeria, Bordeaux study in France, and Rotterdam study in the Netherlands adjusted for age. Framingham Heart Study, USA, adjusted for age and sex. CFAS in the UK adjusted for age, sex, area, and deprivation. However, age-specific dementia prevalence is increasing in some other countries. IID=Indianapolis–Ibadan Dementia. CFAS=Cognitive Function and Ageing Study. Adapted from Wu et al, 5 by permission of Springer Nature.
Modelling of the UK change suggests a 57% increase in the number of people with dementia from 2016 to 2040, 70% of that expected if age-specific incidence rates remained steady, 10 such that by 2040 there will be 1·2 million UK people with dementia. Models also suggest that there will be future increases both in the number of individuals who are independent and those with complex care needs. 6
In our first report, the 2017 Commission described a life-course model for potentially modifiable risks for dementia. 2 Life course is important when considering risk, for example, obesity and hypertension in midlife predict future dementia, but both weight and blood pressure usually fall in later life in those with or developing dementia, 9 so lower weight and blood pressure in later life might signify illness, not an absence of risk. 11 , 12 , 13 , 14 We consider evidence on other potential risk factors and incorporate those with good quality evidence in our model.
Figure 2 summarises possible mechanisms of protection from dementia, some of which involve increasing or maintaining cognitive reserve despite pathology and neuropathological damage. There are different terms describing the observed differential susceptibility to age-related and disease-related changes and these are not used consistently. 15 , 16 A consensus paper defines reserve as a concept accounting for the difference between an individual's clinical picture and their neuropathology. It, divides the concept further into neurobiological brain reserve (eg, numbers of neurones and synapses at a given timepoint), brain maintenance (as neurobiological capital at any timepoint, based on genetics or lifestyle reducing brain changes and pathology development over time) and cognitive reserve as adaptability enabling preservation of cognition or everyday functioning in spite of brain pathology. 15 Cognitive reserve is changeable and quantifying it uses proxy measures such as education, occupational complexity, leisure activity, residual approaches (the variance of cognition not explained by demographic variables and brain measures), or identification of functional networks that might underlie such reserve. 15 , 16 , 17 , 18 , 19 , 20

Possible brain mechanisms for enhancing or maintaining cognitive reserve and risk reduction of potentially modifiable risk factors in dementia
Early-life factors, such as less education, affect the resulting cognitive reserve. Midlife and old-age risk factors influence age-related cognitive decline and triggering of neuropathological developments. Consistent with the hypothesis of cognitive reserve is that older women are more likely to develop dementia than men of the same age, probably partly because on average older women have had less education than older men. Cognitive reserve mechanisms might include preserved metabolism or increased connectivity in temporal and frontal brain areas. 17 , 18 , 19 , 20 , 21 People in otherwise good physical health can sustain a higher burden of neuropathology without cognitive impairment. 22 Culture, poverty, and inequality are important obstacles to, and drivers of, the need for change to cognitive reserve. Those who are most deprived need these changes the most and will derive the highest benefit from them.
Smoking increases air particulate matter, and has vascular and toxic effects. 23 Similarly air pollution might act via vascular mechanisms. 24 Exercise might reduce weight and diabetes risk, improve cardiovascular function, decrease glutamine, or enhance hippocampal neurogenesis. 25 Higher HDL cholesterol might protect against vascular risk and inflammation accompanying amyloid-β (Aβ) pathology in mild cognitive impairment. 26
Dementia in LMIC
Numbers of people with dementia in LMIC are rising faster than in HIC because of increases in life expectancy and greater risk factor burden. We previously calculated that nine potentially modifiable risk factors together are associated with 35% of the population attributable fraction (PAFs) of dementia worldwide: less education, high blood pressure, obesity, hearing loss, depression, diabetes, physical inactivity, smoking, and social isolation, assuming causation. 2 Most research data for this calculation came from HIC and there is a relative absence of specific evidence of the impact of risk factors on dementia risk in LMIC, particularly from Africa and Latin America. 27
Calculations considering country-specific prevalence of the nine potentially modifiable risk factors indicate PAF of 40% in China, 41% in India and 56% in Latin America with the potential for these numbers to be even higher depending on which estimates of risk factor frequency are used. 28 , 29 Therefore a higher potential for dementia prevention exists in these countries than in global estimates that use data predominantly from HIC. If not currently in place, national policies addressing access to education, causes and management of high blood pressure, causes and treatment of hearing loss, socio-economic and commercial drivers of obesity, could be implemented to reduce risk in many countries. The higher social contact observed in the three LMIC regions provides potential insights for HIC on how to influence this risk factor for dementia. 30 We could not consider other risk factors such as poor health in pregnancy of malnourished mothers, difficult births, early life malnutrition, survival with heavy infection burdens alongside malaria and HIV, all of which might add to the risks in LMIC.
Diabetes is very common and cigarette smoking is rising in China while falling in most HIC. 31 A meta-analysis found variation of the rates of dementia within China, with a higher prevalence in the north and lower prevalence in central China, estimating 9·5 million people are living with dementia, whereas a slightly later synthesis estimated a higher prevalence of around 11 million. 30 , 32 These data highlight the need for more focused work in LMIC for more accurate estimates of risk and interventions tailored to each setting.
Specific potentially modifiable risk factors for dementia
Risk factors in early life (education), midlife (hypertension, obesity, hearing loss, traumatic brain injury, and alcohol misuse) and later life (smoking, depression, physical inactivity, social isolation, diabetes, and air pollution) can contribute to increased dementia risk ( table 1 ). Good evidence exists for all these risk factors although some late-life factors, such as depression, possibly have a bidirectional impact and are also part of the dementia prodrome. 33 , 34
PAF for 12 dementia risk factors
Data are relative risk (95% CI) or %. Overall weighted PAF=39·7%. PAF=population attributable fraction.
In the next section, we briefly describe relevant newly published and illustrative research studies that add to the 2017 Commission's evidence base, including risks and, for some, mitigation. We have chosen studies that are large and representative of the populations, or smaller studies in areas where very little evidence exists. We discuss them in life-course order and within the life course in the order of magnitude of population attributable factor.
Education and midlife and late-life cognitive stimulation
Education level reached.
Higher childhood education levels and lifelong higher educational attainment reduce dementia risk. 2 , 35 , 36 , 37 New work suggests overall cognitive ability increases, with education, before reaching a plateau in late adolescence, when brain reaches greatest plasticity; with relatively few further gains with education after age 20 years. 38 This suggests cognitive stimulation is more important in early life; much of the apparent later effect might be due to people of higher cognitive function seeking out cognitively stimulating activities and education. 38 It is difficult to separate out the specific impact of education from the effect of overall cognitive ability, 38 , 39 and the specific impact of later-life cognitive activity from lifelong cognitive function and activity. 39 , 40
Cognitive maintenance
One large study in China tried to separate cognitive activity in adulthood from activities for those with more education, by considering activities judged to appeal to people of different levels of education. 40 It found people older than 65 years who read, played games, or bet more frequently had reduced risk of dementia (n=15 882, odds ratio [OR]=0·7, 95% CI 0·6–0·8). The study excluded people developing dementia less than 3 years after baseline to reduce reverse causation.
This finding is consistent with small studies of midlife activities which find them associated with better late-life cognition; so for example, in 205 people aged 30–64 years, followed up until 66–88 years, travel, social outings, playing music, art, physical activity, reading, and speaking a second language, were associated with maintaining cognition, independent of education, occupation, late-life activities, and current structural brain health. 41 Similarly, engaging in intellectual activity as adults, particularly problem solving, for 498 people born in 1936, was associated with cognitive ability acquisition, although not the speed of decline. 42
Cognitive decline
The use it or lose it hypothesis suggests that mental activity, in general, might improve cognitive function. People in more cognitively demanding jobs tend to show less cognitive deterioration before, and sometimes after retirement than those in less demanding jobs. 43 , 44 One systematic review of retirement and cognitive decline found conflicting evidence. 45 Subsequently, a 12-year study of 1658 people found older retirement age but not number of years working, was associated with lower dementia risk. 46 Those retiring because of ill health had lower verbal memory and fluency scores than those retiring for other reasons. 47 Another study found a two-fold increase in episodic memory loss attributable to retirement (n=18 575, mean age 66 years), compared to non-retirees, adjusting for health, age, sex, and wealth. 48 Similarly, in a cohort of 3433 people retiring at a mean age of 61 years, verbal memory declined 38% (95% CI 22–60) faster than before retirement. 44 In countries with younger compared to higher retirement ages, average cognitive performance drops more. 49
Cognitive interventions in normal cognition and mild cognitive impairment
A cognitive intervention or cognition-orientated treatment comprises strategies or skills to improve general or specific areas of cognition. 50 Computerised cognitive training programmes have increasingly replaced tasks that were originally paper-and-pencil format with computer-based tasks for practice and training. 51
Three systematic reviews in the general population found no evidence of generalised cognition improvement from specific cognitive interventions, including computerised cognitive training, although the domain trained might improve. 52 , 53 , 54
A meta-analysis of 17 controlled trials of at least 4 hours of computerised cognitive training, (n=351, control n=335) for mild cognitive impairment, found a moderate effect on general cognition post-training (Hedges' g=0·4, 0·2–0·5); 55 however few high quality studies and no long-term high quality evidence about prevention of dementia currently exists. A meta-analysis of 30 trials of computerised, therapy-based and multimodal interventions for mild cognitive impairment found an effect on activities of daily living (d=0·23) and metacognitive outcomes (d=0·30) compared to control. 56 A third systematic review identified five high quality studies, four group-delivered and one by computer, and concluded the evidence for the effects of cognitive training in mild cognitive impairment was insufficient to draw conclusions. 53 A comprehensive, high quality, systematic overview of meta-analyses of cognitive training in healthy older people, those with mild cognitive impairment and those with dementia, found that most were of low standard, were positive and most reached statistical significance but it was unclear whether results were of clinical value because of the poor standard of the studies and heterogeneity of results ( figure 3 ). 51

Pooled results of meta-analyses investigating objective cognitive outcomes of cognition-oriented treatment in older adults with and without cognitive impairment
K represents the number of primary trials included in the analysis. If a review reported several effect sizes within each outcome domain, a composite was created and k denotes the range of the number of primary trials that contributed to the effect estimate. AMSTAR=A MeaSurement Tool to Assess systematic Reviews (max score 16). Adapted from Gavelin et al, 51 by permission of Springer Nature.
In the only randomised controlled trial (RCT) of behavioural activation (221 people) for cognition in amnestic mild cognitive impairment, behavioural activation versus supportive therapy was associated with a decreased 2-year incidence of memory decline (relative risk [RR] 0·12, 0·02–0·74). 57
Hearing impairment
Hearing loss had the highest PAF for dementia in our first report, using a meta-analysis of studies of people with normal baseline cognition and hearing loss present at a threshold of 25 dB, which is the WHO threshold for hearing loss. In the 2017 Commission, we found an RR of 1·9 for dementia in populations followed up over 9–17 years, with the long follow-up times making reverse causation bias unlikely. 2 A subsequent meta-analysis using the same three prospective studies measuring hearing using audiometry at baseline, found an increased risk of dementia (OR 1·3, 95% CI 1·0–1·6) per 10 dB of worsening of hearing loss. 58 A cross-sectional study of 6451 individuals designed to be representative of the US population, with a mean age of 59·4 years, found a decrease in cognition with every 10 dB reduction in hearing, which continued to below the clinical threshold so that subclinical levels of hearing impairment (below 25 dB) were significantly related to lower cognition. 59
Although the aetiology still needs further clarification, a small US prospective cohort study of 194 adults without baseline cognitive impairment, (baseline mean age 54·5 years), and at least two brain MRIs, with a mean of 19 years follow-up, found that midlife hearing impairment measured by audiometry, is associated with steeper temporal lobe volume loss, including in the hippocampus and entorhinal cortex. 60
Hearing aids
A 25-year prospective study of 3777 people aged 65 years or older found increased dementia incidence in those with self-reported hearing problems except in those using hearing aids. 61 Similarly, a cross–sectional study found hearing loss was only associated with worse cognition in those not using hearing aids. 62 A US nationally representative survey of 2040 people older than 50 years, tested every two years for 18 years, found immediate and delayed recall deteriorated less after initiation of hearing aid use, adjusting for other risk factors. 63 Hearing aid use was the largest factor protecting from decline (regression coefficient β for higher episodic memory 1·53; p<0·001) adjusting for protective and harmful factors. The long follow-up times in these prospective studies suggest hearing aid use is protective, rather than the possibility that those developing dementia are less likely to use hearing aids. Hearing loss might result in cognitive decline through reduced cognitive stimulation.
Traumatic brain injury (TBI)
The International Classification of Disease (ICD) defines mild TBI as concussion and severe TBI as skull fracture, oedema, brain injury or bleed. Single, severe TBI is associated in humans, and mouse models, with widespread hyperphosphorylated tau pathology, and mice with APOE ε4 compared to APOE ε3 allele have more hippocampal hyper-phosphorylated tau after TBI. 64 , 65 TBI is usually caused by car, motorcycle, and bicycle injuries; military exposures; boxing, horse riding, and other recreational sports; firearms; and falls. 66 A nationwide Danish cohort study of nearly 3 million people aged 50 years or older, followed for a mean of 10 years, found an increased dementia (HR 1·2, 95% CI 1·2–1·3) and Alzheimer's disease risk (1·2, 1·1–1·2). 67 Dementia risk was highest in the 6 months after TBI (4·1, 3·8–4·3) and increased with number of injuries in people with TBI (one TBI 1·2, 1·2–1·3; ≥5 TBIs 2·8, 2·1–3·8). Risk was higher for TBI than fractures in other body areas (1·3, 1·3–1·3) and remained elevated after excluding those who developed dementia within 2 years after TBI, to reduce reverse causation bias. 67
Similarly, a Swedish cohort of over 3 million people aged 50 years or older, found TBI increased 1-year dementia risk (OR 3·5, 95% CI 3·2–3·8); and risk remained elevated, albeit attenuated over 30 years (1·3, 1·1–1·4). 68 ICD defined single mild TBI increased the risk of dementia less than severe TBI and multiple TBIs increased the risk further (OR 1·6, 95% CI 1·6–1·7 for single TBI; 2·1, 2·0–2·2 for more severe TBI; and 2·8, 2·5–3·2 for multiple TBI). A nested case control study of early onset clinically diagnosed Alzheimer's disease within an established cohort also found TBI was a risk factor, increasing with number and severity. 69 A stronger risk of dementia was found nearer the time of the TBI, leading to some people with early-onset Alzheimer's disease.
Military veterans have a high risk of occupational TBI, and formal record keeping allows long-term follow-up. A study of 178 779 veterans with TBI with propensity-matched veterans without TBI found dementia risk was associated with TBI severity (HR 2·4, 95% CI 2·1–2·7 for mild TBI without loss of consciousness; 2·5, 2·3–2·8 for mild TBI with loss of consciousness; and 3·8, 3·6–3·9 for moderate to severe TBI). 70 Similarly women veterans with TBI had increased risk of dementia compared to those without TBI (1·5, 1·0–2·2). 71
A cohort study of 28 815 older adults with concussion, found the risk of dementia doubled, with 1 in 6 developing dementia over a mean follow-up of 3·9 years, although those taking statins had a 13% reduced risk of dementia compared to those who were statin-free. They suggest future RCTs as statins might mitigate injury-related brain oedema, oxidative stress, amyloid protein aggregation, and neuroinflammation. 72
The term chronic traumatic encephalopathy describes sports head injury, which is not yet fully characterised and covers a broad range of neuropathologies and outcomes, with current views largely conjecture. 73 The evidence has subsequently been strengthened by a study on Scottish former soccer players reporting that they are more likely than controls to have Alzheimer's disease specified on their death certificates (HR 5·1, 95% CI 2·9–8·8) and to have been prescribed any dementia-related medications (OR 4·9, 95% CI 3·8–6·3) but not on medical records. 74 The study controlled for socio-economic class based on residential address, which in footballers might be less linked to level of education.
Hypertension
Persistent midlife hypertension is associated with increased risk of a late life dementia. In the Framingham Offspring cohort comprising 1440 people, elevated systolic blood pressure (≥140 mm Hg in midlife; mean age 55 years) was associated with an increased risk of developing dementia (HR 1·6, 95% CI 1·1–2·4) over an 18 year follow-up period. 12 In this study risk increased further if hypertension persisted into later life (mean age 69 years; HR 2·0, 95% CI 1·3–3·1). In the same cohort, people in late midlife (mean age 62 years) with ideal cardiovascular parameters (current non-smoker, body mass index [BMI] 18·5–25 kg/m 2 , regular physical activity, healthy diet, optimum blood pressure <120/<80 mm Hg, cholesterol, and normal fasting blood glucose) were compared to people with at least one of these risks. 75 Those with ideal cardiovascular parameters had a lower 10-year risk of all-cause dementia (HR 0·8, 95% CI 0·1–1·0), vascular dementia (0·5, 0·3–0·8) and clinically diagnosed Alzheimer's disease (0·8, 0·6–1·0). In a UK cohort study of 8639 civil servants, a single measure of systolic blood pressure of 130 mm Hg or higher at age 50 years but not at age 60 or 70 years was associated with increased risk of dementia (1·4, 1·1–1·7). 13 In those with persistent systolic blood pressure of 130 mm Hg or higher, from mean age 45 to 61 years, dementia risk is increased even if free of cardiovascular disease relative to those without hypertension (1·3, 1·0–1·7).
A further cohort study has provided potential insights into mechanisms, reporting that midlife hypertension, defined as from age 40 years, was associated with reduced brain volumes and increased white matter hyperintensity volume but not amyloid deposition. 76 Of note, blood pressure declines in later life and this decline is associated with and, potentially caused by, dementia development (HR 2·4, 95% CI 1·4–4·2). 12 , 13 , 77
Antihypertensive drugs, aspirin, and statins
The US and Puerto Rico Systolic Blood Pressure Intervention Trial (SPRINT) in 9361 hypertensive adults aged 50 years and older, was stopped early because of significantly fewer cardiovascular events and deaths occurring in the intensive treatment arm (aiming for systolic <120 mm Hg, n=4678) in comparison with standard treatment (systolic <140 mm Hg, n=4683). 78 Cognitive assessment continued after stopping the trial intervention in SPRINT MIND. 79 In the intensive compared with the standard treatment group, there were 7·2 dementia cases as opposed to 8·6 cases/1000 person-years (HR 0·8; 95% CI 0·7–1·0) within on average 2 years from the end of the intervention period and 5 years after baseline. Pre-specified secondary outcomes were also reduced in the intensive arm for mild cognitive impairment (14·6 vs 18·3 cases/1000 person-years; HR 0·8, 95% CI 0·7–1·0), combined mild cognitive impairment or dementia (20·2 vs 24·1 cases/1000 person-years; HR 0·9, 95% CI 0·7–1·0) 79 making this the first trial to suggest reduction of risk for mild cognitive impairment. Those who were lost to follow-up were at greater risk of dementia than those who continued but follow-up rates did not differ according to intervention group. 80
Four meta-analyses of blood pressure medications to lower high blood pressure with six studies overlap have provided combined estimates of effects. All meta-analyses suggest reduced dementia in those in the interventions arms for outcomes of any dementia as well as clinically diagnosed Alzheimer's disease. The first included randomised controlled trials (RCTs) of any drug to lower blood pressure and reported a reduction in risk of around 10% at marginal significance (RR 0·9, 95% CI 0·9–1·0). 81 Meta-regression showed risk lowered more if the achieved systolic pressure differential was larger between the intervention and control group. The second included 15 trials and observational studies of diuretics involving 52 599 people (median age 76 years) with 6·1 years median follow-up (dementia HR 0·8, 95% CI 0·8–0·9 and Alzheimer's disease 0·8, 0·7–0·9). 82 The third included used individual participant data from six observational studies; (dementia 0·9, 0·8–1·0 and Alzheimer's disease 0·8, 0·7–1·0; figure 4 ). 83 The fourth focused on people prescribed calcium channel blocker only, included 10 RCTs and observational studies comprising 75 239 hypertensive older adults (median age 72 years, median follow-up 8·2 years) found lowered dementia risk (RR 0·7, 95% CI 0·6–0·9). 84 A 2019 meta-analysis addressing which class of anti-hypertensive drug to use to lower risk of either incident dementia or cognitive decline, found over 50 000 participants in 27 studies and reported no consistent difference in effect according to which class of drug was used. 85

Associations of antihypertensive medication use with incident dementia in those with high blood pressure
Adapted from Ding et al, 83 by permission of Elsevier.
A Cochrane review reported good evidence that statins given to older people at risk of vascular disease do not prevent cognitive decline or dementia. 86 One RCT found 100 mg aspirin versus placebo in 19 114 healthy adults older than 65 years did not reduce dementia (HR 1·0, 95% CI 0·8–1·2), death, physical disability, or cardiovascular disease over a period of 4·7 years. 87
Physical inactivity, exercise, and fitness
Studies of physical activity are complex. Patterns of physical activity change with age, generation, and morbidity and are different across sex, social class, and cultures. The studies suggest a complicated relationship with the potential for both risk reduction and reverse causation.
Meta-analyses of longitudinal observational studies of 1–21 years duration showed exercise to be associated with reduced risk of dementia. 2 A further overview of systematic reviews concluded that there is convincing evidence for physical activity protecting against clinically diagnosed Alzheimer's disease. 88
Since the 2017 Commission, the HUNT study of 28 916 participants aged 30–60 years has been published, reinforcing the previous literature in this area. At least weekly midlife moderate-to-vigorous physical activity (breaking into a sweat) was associated with reduced dementia risk over a 25-year period of follow-up (HR 0·8, 95% CI 0·6–1·1) but the confidence intervals were wide. 89 In contrast the Whitehall Study reporting on the 28-year follow-up of 10 308 people, found that more than 2·5 hours of self-reported moderate-to-vigorous physical activity per week, lowered dementia risk over 10, but not 28 years. 33 Very long-term studies are unusual; however, one 44-year study recruited 191 women (mean age 50) purposively to be representative of the Swedish population and reported that 32% of the participants with low baseline peak fitness, 25% with medium, and 5% with high fitness developed dementia (high vs medium HR 0·1, 95% CI 0·03–0·5, low vs medium 1·4, 0·7–2·8). 90
An individual-level meta-analysis of 19 observational studies of relatively younger adults included 404 840 participants' data (mean baseline age 45·5 years; mean follow-up duration 14·9 years), reporting an increased incidence of all-cause dementia (HR 1·4, 95% CI 1·2–1·7) and clinically diagnosed Alzheimer's disease (1·4, 1·1–1·7) in those who were physically inactive in the 10-year period before diagnosis. 91 Notably, however, no difference in dementia risk measured 10–15 years before time of dementia incidence was found except in those with comorbid cardio-metabolic disease (RR 1·3, 95% CI 0·8–2·1).
People might stop exercising due to prodromal dementia so inactivity might be either a consequence or a cause or both in dementia and might be more of a risk in those with cardiovascular morbidity. As with other outcomes, exercise might be required to be sustained and continue nearer the time of risk. 92
Trials of exercise
Since the 2017 Commission several meta-analyses and systematic reviews have been published with three high quality meta-analyses which we include. The first included 39 RCTs with an unclear total number of participants examining moderate or vigorous exercise of any frequency lasting 45–60 min per session in cognitively normal adults aged older than 50 years. This analysis reported global cognitive improvements (standard mean difference [SMD]=0·3, 95% CI 0·2–0·4) for moderate or vigorous resistance (13 studies) or aerobic exercise (18 studies) lasting 45–60 min per session with no difference between them but no effect found for yoga. 93 A second meta-analysis of RCTs in people with mild cognitive impairment found global cognition improved in the intervention group (0·3, 0·1–0·5) with aerobic exercise having a higher effect (0·6, 0·5–0·6). 94 This study did not have dementia as an outcome measure. A third meta-analysis of RCTs of longer term exercise found five studies (four lasting 12 months and one 24 months) with 2878 participants with normal baseline cognition. 95 The incidence of dementia was 3·7% (n=949) for exercisers and 6·1% (n=1017) for controls (random effect RR 0·6, 95% CI 0·3–1·1; fixed effect as no evidence of heterogeneity 0·7, 0·4–1·0). The authors concluded that the study showed no significant effect of exercise for reducing dementia, mild cognitive impairment, or clinically significant cognitive decline but was underpowered. WHO guidelines have been published since the 2017 Commission, suggesting specific activity levels drawing on these, and one further systematic review which considered sex differences on the effect of exercise. 96 , 97 It concluded the evidence points towards physical activity having a small, beneficial effect on normal cognition, with a possible effect in mild cognitive impairment, mostly due to aerobic exercise. 97 Evidence about the effect of specific types of exercise, such as progressive muscle resistance training, on dementia risk is scarce.
In the 2017 Commission we reported on diabetes as a risk factor for dementia. Distinguishing between treated and untreated diabetes as a risk factor for dementia is challenging in observational studies. In a pooled meta-analysis from over 2·3 million individuals with type 2 diabetes across 14 cohort studies, including 102 174 with dementia, diabetes was associated with an increased risk of any dementia (RR 1·6, 95% CI 1·5–1·8 for women and 1·6, 1·4–1·8 for men). 98 The risk of dementia increased with the duration and severity of diabetes. The effect of different diabetic medications on cognition or dementia outcomes remains unclear as few studies have investigated this area. 99 However, one meta-analysis of cohort studies of diabetes reported that, cross sectionally, people with diabetes taking metformin had lower prevalence of cognitive impairment (three studies OR 0·6, 95% CI 0·4–0·8) and, longitudinally, reduced dementia incidence (six studies HR 0·8, 95% CI 0·4–0·9) compared with those taking other medications or no medication. 100 However another analysis did not find a protective effect of metformin for incident dementia (three studies, RR 1·1, 95% CI 0·5–2·4) with possible harm with insulin therapy (1·2, 1·1–1·4); but this did not account for severity of diabetes of those with type 2 diabetes on insulin. 99 A Cochrane review reported intensive compared to standard diabetes control trials with 5 year follow up (n=11 140), showing no impact on cognitive decline (1·0, 95% CI 0·9–1·1) or dementia (1·3, 0·9–1·9). 101
Overall type 2 diabetes is a clear risk factor for development of future dementia; however, whether any particular medication ameliorates this risk is unclear. Intensive diabetic control does not decrease the risk of dementia.
Combined cardiovascular risk factors
Studies of individual cardiovascular risk factors usually control for other cardiovascular risks, which cluster in individual people. This does not take into account the combinations and contexts in which risk occurs. A UK study of 7899 people aged 50 years followed up for 25 years, calculated a cardiovascular health score based on four behaviour-related (smoking, diet, physical activity, BMI) and three biological (fasting glucose, blood cholesterol, blood pressure) metrics each coded on a three-point scale (0, 1, 2). 100 A better score was associated with a lower risk of dementia (HR 0·9, 95% CI 0·9–1·0 per 1 point scale increment), for both behaviour-related (HR/1 point increment in subscales 0·9, 95% CI 0·8–0·9) and biological subscales (0·9, 0·8–1·0), maintained in people free of cardiovascular disease over the follow-up (0·9, 95% CI 0·8–1·0). These authors also reported an association of the score on the scale with hippocampal atrophy and total brain volume but not white matter hyperintensities. This finding underlines the importance of clustering of cardiovascular risk factors in midlife, as studies of individual risk factors in this sample had not shown a significant association, when controlling for other individual risks. 33
Excessive alcohol consumption
Heavy drinking is associated with brain changes, cognitive impairment, and dementia, a risk known for centuries. 102 An increasing body of evidence is emerging on alcohol's complex relationship with cognition and dementia outcomes from a variety of sources including detailed cohorts and large-scale record based studies. Alcohol is strongly associated with cultural patterns and other sociocultural and health-related factors, making it particularly challenging to understand the evidence base.
A French 5-year longitudinal study of over 31 million people admitted to hospital, found alcohol use disorders (harmful use or dependence as defined in ICD) were associated with increased dementia risk, calculated separately for men and women (women HR 3·3, 95% CI 3·3–3·4, men 3·4, 3·3–3·4). 103 The relationship of dementia with alcohol use disorders was particularly clear in the earlier onset dementias (age less than 65 years) in which 56·6% had an alcohol use disorder noted in their records (n=57 353; 5·2% all dementias).
A systematic review incorporating 45 studies of light to moderate drinking using a variety of definitions reported a reduced risk of dementia compared with not drinking (RR 0·7; 95% CI 0·6–0·91). 104 Risk was not reported separately for men and women. Drinking less than 21 units of alcohol per week (1 unit of alcohol=10 mL or 8 g pure alcohol) might be associated with a lower risk of dementia. 105 , 106 A 5-year follow-up study of 13 342 men and women volunteers from UK biobank aged 40–73 years who drank, included few heavy drinkers and did not analyse abstainers. 106 The study reported that those who drank more than 12 units per week declined slightly more in reaction time in a perceptual matching task than those who drank less (β2=−0·07, 95% CI −0·09 to −0·04). 106 The UK Whitehall study with 23 years follow-up, included 9087 participants aged 35–55 years at baseline. 107 Drinking more than 21 units per week and long-term abstinence were both associated with a 17% (95% CI 4–32 and 13–23 respectively) increase in dementia compared to drinking less than 14 units. Drinking more than 14 units was also associated with right sided hippocampal atrophy on MRI. 108
Weight control and obesity
Overweight is an emerging concern, given the changing BMI across the world's ageing population. New evidence supports the relationship between increased BMI and dementia from a review of 19 longitudinal studies including 589 649 people aged 35 to 65 years, followed up for up to 42 years. It reported obesity (BMI ≥30; RR 1·3, 95% CI 1·1–1·6) but not being overweight (BMI 25–30; 1·1, 1·0–1·2) was associated with late-life dementia. 109 In a further meta-analysis of individual level data from 1·3 million adults (aged ≥18 years), which included two studies from the meta-analysis cited above, 109 higher body mass measured before probable preclinical and prodromal dementia was associated with increased dementia risk (RR 1·3, 1·1–1·7/5-unit increase in BMI). 11
Weight loss in midlife and dementia risk
A meta-analysis of seven RCTs (468 participants) and 13 longitudinal studies (551 participants) of overweight and obese adults without dementia, mean age 50 years, found weight loss of 2 kg or more in people with BMI greater than 25 was associated with a significant improvement in attention and memory. All but one of the studies included participants aged younger than 65 years. The RCTs reported memory improvement over 8–48 weeks (SMD=0·4, 95% CI 0·2–0·6) and short-term longitudinal studies found improvement over a median of 24 weeks (SMD=0·7, 95% CI 0·5–0·8); however, data about the long-term effects or the effect of weight loss in preventing dementia are absent. 110
Smokers are at higher risk of dementia than non-smokers, 2 and at a higher risk of premature death before the age at which they might have developed dementia, introducing some bias and uncertainty in the association between smoking and risk of dementia. 111 , 112 Stopping smoking, even when older, reduces this risk. Among 50 000 men aged older than 60 years, stopping smoking for more than 4 years, compared to continuing, substantially reduced dementia risk over the subsequent 8 years (HR 0·9; 95% CI 0·7–1·0). 113 Worldwide, 35% of non-smoking adults and 40% of children are estimated to be exposed to second-hand smoke; 114 although literature on the impact of this exposure and dementia risk is scarce. One study indicated that in women aged 55–64 years, second-hand smoke exposure was associated with more memory deterioration and the risk increased with exposure duration even after controlling for other confounding factors. 115
Depression is associated with dementia incidence, with a variety of possible psychological or physiological mechanisms. It is also part of the prodrome and early stages of dementia. Reverse causation is possible whereby depressive symptoms result from dementia neuropathology that occurs years before clinical dementia onset. These explanations are not mutually exclusive. As in diabetes, few studies considering depression as a risk factor for dementia have distinguished between treated and untreated depression. In a meta-analysis of 32 studies, with 62 598 participants, with follow-up from 2 to 17 years, a depressive episode was a risk factor for dementia (pooled effect size 2·0, 95% CI 1·7–2·3). 116 Meta-regression analysis revealed a non-significant trend for the association between depression and incident dementia to be weaker when the length of follow-up was longer. The Norwegian HUNT study, suggested that symptoms of psychological distress predicted dementia 25 years later however with wide bounds of uncertainty (HR 1·3, 95% CI 1·0–1·7). 89 Two further studies differentiate between late-life and earlier life depressive symptoms. The UK Whitehall study, in a follow-up of 10 189 people, reports that in late life these symptoms increase dementia risk but not at younger ages (follow-up 11 years HR 1·7; 95% CI 1·2–2·4; follow-up 22 years 1·0, 0·7–1·4). 34 , 117 A 14-year longitudinal study of 4922 initially cognitively healthy men, aged 71–89 years, found depression was associated with 1·5 (95% CI 1·2- 2·0) times the incidence of dementia but this association was accounted for by people developing dementia within 5 years of depression. 118 The use of antidepressants did not decrease this risk.
A study of 755 people with mild cognitive impairment and with a history of depression from the Australian longitudinal Alzheimer's Disease Neuroimaging Initiative, considered the effect of selective serotonin-reuptake inhibitor (SSRI) treatment, such as citalopram, known to reduce amyloid plaque generation and plaque formation in animal models. 119 The study found that more than 4 years of such treatment was associated with delayed progression to clinically diagnosed Alzheimer's disease. People treated with antidepressants seem likely to differ from those who are not treated. Thus, the question of whether antidepressant treatment mitigates dementia risk remains open.
Social contact
Social contact, now an accepted protective factor, enhances cognitive reserve or encourages beneficial behaviours, although isolation might also occur as part of the dementia prodrome. Several studies suggest that less social contact increases the risk of dementia. Although most people in mid and later life are married, by the time they reach older age, disproportionate numbers of women are widowed as they outlive their husbands, thus reducing their social contact. In these generations, marital status is therefore an important contributor to social engagement. Additionally, most marriages are in the relatively young, and married people usually have more interpersonal contact than do single people—this gives a long-term estimate of the effect of social contact. A systematic review and meta-analysis including 812 047 people worldwide found dementia risk to be elevated in lifelong single (RR 1·4, 95% CI 1·1–1·9) and widowed people (1·2, 1·0–1·4), compared with married people and the association was consistent in different sociocultural settings. 120 Studies adjusted for sex and we do not know if a differential risk between men and women exists. Differences persisted in studies that adjusted for education and physical health so might be attributable to married people having more social contact, rather than solely because they tend to have better physical health and more education, although residual confounding is possible. A systematic review and meta-analysis of 51 longitudinal cohort studies of social isolation and cognition included 102 035 participants aged 50 or more years at baseline, with follow-up of 2–21 years. 121 High social contact (measured through either or both of social activity and social network) was associated with better late-life cognitive function (r=0·05, 95% CI: 0·04–0·065) and no differences according to sex or length of time followed up.
A new meta-analysis found that in long-term studies (≥10 years), good social engagement was modestly protective (n=8876, RR=0·9, 95% CI 0·8–1·0); but loneliness was not associated with dementia risk. 122 No long term (>10 years) studies of loneliness and dementia outcomes have been done.
A UK 28-year follow-up study of 10 308 people found that more frequent social contact at age 60 years was associated with lower dementia risk over 15 years of follow-up (HR for one standard deviation social contact frequency 0·9, 95% CI 0·8–1·0). This finding suggests more frequent social contact during late middle age is associated with a modest reduction in dementia risk, independent of socio-economic and other lifestyle factors. 123 A Japanese longitudinal cohort study of 13 984 adults aged older than 65 years with a mean of 10 years follow-up calculated a five-point social contact scale based on: marital status; exchanging support with family members; having contact with friends; participating in community groups; and engaging in paid work. It found the score to be linearly associated with reduced dementia risk; those who scored highest on the five-point scale were 46% less likely to develop incident dementia compared with those in the lowest category. 124
Despite clear cultural variation in the meaning and perception of social isolation, findings of protective effect of more social contact are largely consistent in different settings and for either sex across the studies and meta-analyses. 118 , 120 , 121
Social interventions
Little evidence of the effects of social interventions on dementia exists but a systematic review of low quality RCTs of 576 adults aged 60 or more years with normal cognition found facilitated meeting and discussion groups were associated with improved global cognition and increased brain volume at follow-up. 118
Air pollutants
Air pollution and particulate pollutants are associated with poor health outcomes, including those related to non-communicable diseases. Attention has turned to their potential effect on the brain. Animal models suggest airborne particulate pollutants accelerate neurodegenerative processes through cerebrovascular and cardiovascular disease, Aβ deposition, and amyloid precursor protein processing. 125 , 126 Although the higher levels of dementia from air pollutants are still subject to the potential for residual confounding, the effects on animal models are evidence of physiological effects over and above those driven by life-course deprivation.
High nitrogen dioxide (NO 2 ) concentration (>41·5 μg/m 3 ; adjusted HR 1·2, 95% CI 1·0–1·3), fine ambient particulate matter (PM) 2·5 from traffic exhaust (1·1, 1·0–1·2) 127 , 128 , 129 and PM 2·5 from residential wood burning (HR=1·6, 95% CI 1·0–2·4 for a 1 μg/m 3 increase) are associated with increased dementia incidence. Traffic often produces NO 2 and PM 2·5 and it is hard to separate their effects, although evidence for additive effects of different pollutants exists. 127 , 128 , 129 A systematic review of studies until 2018 including 13 longitudinal studies with 1–15 years follow-up of air pollutants exposure and incident dementia, found exposure to PM 2·5, NO 2 , and carbon monoxide were all associated with increased dementia risk. 24 The attributable burden of dementia and excess death from PM 2·5 in one large 10-year US study was particularly high in Black or African American individuals and socio-economically disadvantaged communities and related to particulate PM 2·5 concentrations above the US guidelines. 130
Mechanisms by which sleep might affect dementia remain unclear, but sleep disturbance has been linked with β-amyloid (Aβ) deposition, 131 , 132 reduced glymphatic clearance pathways activation, 133 low grade inflammation, increased Tau, hypoxia 132 , 134 and cardiovascular disease. 135 Sleep disturbance is hypothesised to increase inflammation which raises Aβ burden, leading to Alzheimer's disease and further sleep disturbance. 136
Two meta-analyses showed similar findings. The first was a synthesis of longitudinal studies with an average of 9·5 years follow-up and the second reported cross-sectional and prospective cohort studies of mixed quality with different methods of measuring sleep. Sleep disturbances were defined broadly, often self-reported and including short and long sleep duration, poor sleep quality, circadian rhythm abnormality, insomnia, and obstructive sleep apnoea. All these disturbances were associated with a higher risk of all-cause dementia (RR 1·2; 95% CI 1·1–1·3) 137 and clinically diagnosed Alzheimer's disease (1·6, 1·3–1·9) compared with no sleep disturbance, although not all cohort studies excluded those with cognitive impairment or dementia at baseline from their analyses. 138 A U-shaped association has been reported between sleep duration and risk of mild cognitive impairment or dementia with higher risks of dementia with less than 5 hours (HR=2·6; 95% CI 1·4–5·1) compared with more than 5 and less than 7 and more than 10 hours sleep (2·2, 1·4–3·5) and risks for all-cause dementia and clinically diagnosed Alzheimer's disease being similar. 135 , 139 , 140 , 141
The postulated mechanisms of reduced sleep leading to accumulation of Alzheimer's type pathology is inconsistent with the evidence that both more sleep and less sleep are associated with increased risk of dementia. New onset late-life sleep disturbance, a few years before clinical dementia, might be part of the natural history of the dementia syndrome, appearing to be a risk factor, or reflect other disorders, for example, mood disturbances or cardiovascular disease. 135 , 142 Hypnotic use might increase risks although this is unclear and a 2018 study 139 suggests that findings of a connection were related to reverse causality and confounders. 143 When benzodiazepine use was considered, in one study, sleep length was no longer significant 139 but not in all studies. 135 Those taking hypnotics were at greater risk of dementia than those who did not regardless of sleep duration. 139 Medication for sleep disturbance might be harmful and benzodiazepines are associated with falls, hospital admissions, and possibly dementia. 139 , 144
Nutrition and dietary components are challenging to research with controversies still raging around the role of many micronutrients and health outcomes in dementia. Observational studies have focused on individual components ranging from folate and B vitamins, Vitamin C, D, E, and selenium amongst others as potential protective factors. 88 There has been a move towards considering the evidence base for whole diets in the last 5 years, particularly high plant intake such as in the Mediterranean diet (high intake of vegetables, legumes, fruits, nuts, cereals, and olive oil; low intake of saturated lipids and meat) or the similar Nordic diet, rather than individual nutrients, which might reduce cognitive decline and dementia. 145 One example is a longitudinal cohort study of 960 participants, ages 58–99 years, in which those reporting the highest intake of green leafy vegetables, equivalent to 1·3 servings per day, had less cognitive decline over 4·7 years than those reporting the lowest intake (β=0·05 standardised units 95% CI 0·02–0·07). 146 The authors report this difference as being equivalent to being 11 years younger. A further prospective cohort study with three midlife dietary assessments in 8255 people, followed up for a mean of nearly 25 years, found neither healthy dietary pattern nor Mediterranean diet protected from dementia, except in those with cardiovascular disease, suggesting that diet might influence dementia risk by protecting from the excess risk of cardiovascular risk factors. 147
Dietary interventions
As well as whole diets, there has been some interest in multi-nutrient interventions. A systematic review and a Cochrane review including RCTs of supplements (A, B, C, D, and E; calcium, zinc, copper, and multivitamins trials, n-3 fatty acids, antioxidant vitamins, and herbs) found a lack of evidence for supplement use to preserve cognitive function or prevent dementia in middle-aged (45–64 years) or older people (aged 65 years and older). 148 , 149 Cochrane reviews found no evidence for beneficial effects on cognition of those with mild cognitive impairment of supplementation with B vitamins for 6 to 24 months 150 or with vitamin E in preventing progression from mild cognitive impairment to dementia. 151 A 24-month RCT of 311 people of a multi-nutrient drink containing docosahexaenoic acid, vitamins B12, B6, folic acid, and other nutrients; found no significant effect on preventing cognitive deterioration in prodromal Alzheimer's disease. 152 The authors comment that the control group's cognitive decline was much lower than expected, leading to an inadequately powered trial.
Meta-analysis of two RCTs with 471 participants with normal cognition found the Mediterranean diet improved global cognition compared to controls (SMD 0·2, 95% CI 0·0–0·4). 153 A further meta-analysis identified five RCTs (n=1888) with a weak effect on global cognition (SMD 0·2, 95% Cl 0·0–0·5) 154 but no benefit of Mediterranean diet for incident cognitive impairment or dementia.
The WHO guidelines recommend a Mediterranean diet to reduce the risk of cognitive decline or dementia, as it might help and does not harm, but conclude Vitamins B and E, polyunsaturated fatty acid, and multicomplex supplementation should not be recommended. 97
Trials of combination strategies to prevent dementia
The FINGER RCT was a 2-year multidomain intervention to prevent cognitive decline and dementia in 1260 people with cardiovascular risk factors aged 60–77 years, recruited from a Finnish national survey. Similar multidomain studies were discussed in the 2017 Commission. 2 FINGER found a small group reduction in cognitive decline in the intervention group compared with control (comprehensive neuropsychological test battery Z score 0·02, 95% Cl 0·00–0·04) regardless of baseline sociodemographic, socio-economic, cognitive, or cardiovascular status. 155 However, in a subgroup analysis, greater beneficial effects were observed on processing speed in individuals with higher baseline cortical thickness in Alzheimer's disease areas. 156
The Healthy Ageing Through Internet Counselling in the Elderly (HATICE) study recruited 2724 older people (≥65 years) in the Netherlands, Finland, and France with two or more cardiovascular risk factors. 157 , 158 It compared an interactive internet platform plus remote support by a coach, aiming to improve self-management of vascular risk factors, with a non-interactive control platform with basic health information. A small improvement in the cardiovascular risk composite primary outcome was observed in the intervention group compared with the control group at 18 months, mainly through weight loss, and the dementia risk score was slightly lower in those who received the intervention (mean difference −0·15, 95% CI −0·3 to −0·0). A larger effect was observed in the younger age group (65–70 years) and those with the lowest level of education, who had a higher baseline risk, suggesting that targeting high-risk populations might be more effective. Several multidomain preventive trials are ongoing—for example, World Wide FINGERS .
Total PAF calculation
We incorporated excessive alcohol consumption, TBI, and air pollution into our life-course model of dementia, as well as the original nine risk factors, because of the updated evidence. To calculate new RRs for excessive alcohol consumption, TBI and air pollution, we systematically reviewed the literature and did new meta-analyses for excessive alcohol consumption and TBI. For the other nine factors, we used values for RR and risk factors prevalence from our previous analysis and calculated communality using the same method as in the 2017 Commission. 2
PAF calculation
We used a representative sample of over 10 000 UK community-dwelling adults, to calculate communality (clustering of risk factors) of 11 risk factors for which data existed, 159 to allow calculation of each factor's unique risk. As we could find no datasets measuring TBI, with the other 11 risk factors of interest, we could not calculate its communality. We therefore used the mean of the other 11 communalities to calculate a weighted PAF, so we could include TBI. We used cohabitation as a proxy measure for social contact, and urbanicity for air pollution exposure. Our analysis found four principal components, explaining 55% of the total variance between the eleven risk factors, suggesting substantial overlap. The appendix (p 2) shows the PAF formula and the steps in calculating communality and we detail our new meta-analyses next, which we used to update the figure and perform our new calculations.
Incorporation of the new chosen risks in new systematic reviews
We searched, from inception to Oct 29, 2019, Embase, Allied, and Complementary Medicine, MEDLINE, and PsycINFO terms “dementia” OR “dement*” OR “AD” OR “VaD”, “Alzheimer*” AND “alcohol” OR “ethanol” OR “alcohol*” OR “drink*” OR “drunk*” to update an earlier review. 160 We used inclusion criteria: original population-based cohort studies measuring drinking during midlife, as alcohol intake tends to fall with age; 161 alcohol consumption quantified at baseline by units or number of drinks (one drink, 1·5 units) per week; and all-cause dementia ascertained at follow-up using validated clinical measures. We contacted authors for additional data. 162 Three studies met our inclusion criteria. 107 , 162 , 163 We converted HRs to RRs 164 and used raw data 162 to calculate RR, 165 for our random effects meta-analysis using Generic Inverse Variance Methods. The RR associated with drinking—more than 21 units (168 g) of alcohol weekly—compared with lighter drinking was 1·18 (95% Cl 1·06–1·31; figure 5 ). We used Health Survey England figures for heavier drinking prevalence to calculate PAF as we could not find a worldwide estimate. The weighted PAF was 0·8.

Meta-analysis of relative risk of dementia associated with drinking more than 21 units of alcohol per week in midlife compared to lighter consumption of alcohol
To estimate the RR of TBI of all severities for all cause dementia, we searched Embase, Medline, and PsycINFO from Jan 1, 2016, to Oct 21, 2019, updating an earlier search, 166 using terms (“traumatic brain injury” or “head injury” or “brain injury” or TBI) AND (neurodegeneration or “cognitive dysfunction” or dementia or “Alzheimer's disease” or “Parkinson's disease” or “frontotemporal dementia”). We converted HR figures to RR. 164 , 167 We used inclusion criteria: original population-based cohort studies, baseline TBI of all severities reported, and all-cause dementia ascertained at follow-up using validated clinical measures. We combined four new studies meeting inclusion criteria 67 , 68 , 71 , 168 with the four studies meeting criteria from the original review in a random effects meta-analysis. 166 The pooled RR was 1·84 (95% CI 1·54–2·20) for all cause dementia from all severities of TBI ( figure 6 ) although there was heterogeneity in study-specific estimates, possibly because of different populations. We used the TBI adult population prevalence of 12·1% from a meta-analysis to calculate PAF. 173 The weighted PAF was 3·4.

Meta-analysis of relative risk of all-cause dementia associated with all severity midlife traumatic brain injury
A 2019 systematic review synthesised observational studies, finding consistently increased risk of dementia from air pollution, but heterogeneous comparator groups precluded meta-analysis. 24 We updated the search, using the same search terms and searching MEDLINE, Embase, and PsycINFO from Sept 20, 2018, (the end date of the last search) to Oct 22, 2019. We included longitudinal studies with assessment of all cause air pollution exposure; use of formal assessment of cognitive function at baseline; report of incident all-cause dementia, data from adults (age ≥18 years); and a minimum follow-up of 6 months. As meta-analysis was not possible, we used data from the only study of all-cause air pollution with the outcome of all-cause dementia, with low-moderate risk of bias. This population-based, observational cohort was from Canada, where pollutant concentrations are among the lowest in the world and examined 2 066 639 people, with a mean baseline age of 67 years. 174 We calculated the RR of dementia for those in the three highest quartiles compared to the lowest was 1·09 (1·07–1·11). The attributable fraction for exposure to the highest three quartiles versus the lowest quartile of PM 2·5 and NO 2 was 6·1% (4·8–7·5). The weighted PAF was 2·3.
Table 1 displays the prevalence, communality, relative risk, unweighted and weighted PAFs adjusted for communality. Figure 7 shows the updated life-course model of potentially modifiable risk factors for dementia, including the three new risk factors.

Population attributable fraction of potentially modifiable risk factors for dementia
Strengths and limitations
This Commission is the most comprehensive analysis to date and updates the 2017 Commission with emerging risk factor evidence convincing enough to calculate PAF for potentially reversible risk factors. We reviewed the literature systematically for the chosen risk factors and provided illustrative new literature to update our synthesis and identify data to calculate communality. We find a hopeful picture with an estimate of around 40% of all cases of dementia being associated with 12 potentially modifiable risk factors.
We have made assumptions to calculate this new model. We used global figures for dementia risk although we know the risk factors prevalence varies between countries and most global research is from HIC, so LMIC are under-represented because of lack of data. We have assumed a causal relationship between risk factors and dementia, although we have been cautious and not included risk factors with less good evidence. No single database exists with all 12 risk factors together, but we found 11 of the factors in a UK database and used the mean figure for communality calculations for TBI. We calculated communality for the other 11. We do not know how far findings of communality in other geographical populations might differ, or in those with a differing distribution of age groups or sex. We found that social isolation was not explicitly measured and had to use proxies, such as cohabitation when considering prevalence, which are approximate.
Specifically, evidence for the association of alcohol misuse with dementia comes from HIC and future studies from LMIC are needed to complete the picture. Exposure to air pollution changes over a lifetime and is inextricably linked to poverty and deprivation. However, the effects on animal models suggests specific physiological effects over and above those driven by life-course deprivation. We also considered the overlap with education for this and other risk factors and the correction for education, strongly inversely linked to deprivation, will address at least some of the confounding. However, the results in one study which reported the effect of air pollution on incident dementia showed very little difference in estimates before and after adjustment for education and other risk factors, suggesting little residual confounding exists. 174 We were also unable to meta-analyse data on pollution and thus unlike the other relative risks, the figure comes from only one study, from an area of low pollution so is likely to be an underestimate.
The longitudinal evidence linking potentially modifiable risk factors to dementia generally fulfils causality criteria in observational data (strength, consistency, biological plausibility, temporality, dose–response, coherence, and quasi-experimental studies, for example, more education or using hearing aids). When measuring a risk nearer to the age of dementia onset, then it is more likely that prodromal change affects, or even causes it. Alternatively, a risk factor might act on preclinical pathology or even cause dementia near the time of exposure. Thus, excessive alcohol, and TBI are particularly important in young-onset dementia, although many early onset dementias relate to genetic risks. Risk factors might also matter more at a time of higher biological vulnerability, which the studies we have drawn on cannot establish. The length of exposure required for risk or protection effect, and their inter-relationships as they change across life is unclear—it seems probable that longer or more intense exposure has stronger effects. Additionally, as our communality figures show, risk factors overlap. We cannot establish from these data if having multiple risk factors has an additive or synergistic effect. Association does not prove causation, however, as already noted, the reductions in prevalence and incidence in several HIC suggests that at least some of the risk factors estimated here do have a causal relationship with the clinical expression of dementia.
Key points and recommendations
We judge that sufficient new evidence supports adding three additional modifiable risk factors for dementia to our 2017 Commission model (excessive alcohol, traumatic brain injury, and air pollution). We have been able to add updated evidence on the nine risk factors implicated in the 2017 Commission (education, hypertension, hearing impairment, smoking, obesity, depression, inactivity, diabetes, and social contact). Reduction of these risk factors might be protective for people with or without a genetic risk, although study findings have not been entirely consistent. 175 , 176 , 177 , 178 As we noted in the 2017 Commission, others have previously calculated an estimate of the risk associated with APOε4 at 7% taking into account some other risk factors and this estimate highlights how relatively important potentially modifiable risk factors are in dementia. 2 , 179
For some risk factors, the pattern of risk and the individual's other health, both physical and mental, might be especially important. Currently, the evidence suggests a Mediterranean or Scandinavian diet might have value in preventing cognitive decline in people with intact cognition, particularly as one component of a healthy lifestyle, although how long the exposure has to be or during which ages is unclear. We do not recommend taking additional vitamins, oils, or mixed dietary supplements as a means of preventing dementia as extensive testing in trials has not led to signals of beneficial effects.
Data from RCTs on interventions to prevent cognitive decline, all-cause dementia, or Alzheimer's disease are few. For some key life influences, only observational data, particularly related to natural experiments such as changing the statutory education age, are possible. These influences should be investigated systematically wherever possible. Others can theoretically be investigated but the long follow-up required for midlife risk and protective factors and non-random attrition in longer studies are challenging. Using intermediate endpoints, such as cognition, and dementia onset in research remains uncertain because no intermediate markers with such a close relationship to dementia outcomes exist that it would be possible to predict with certainty for any given individual, age, and sex. Overall, the evidence for treating hypertension is strongest and high blood pressure throughout midlife increases the risk of dementia even without stroke.
Although a need for more evidence is apparent, recommendations should not wait, as clear indications of ways to reduce the chances of developing dementia without causing harm will also lead to other health and wellbeing benefits.
Our recommended strategies for dementia risk reduction include both population-wide and targeted interventions ( panel ). It is important to remember that more socially disadvantaged groups, including Black, Asian, and minority ethnic groups, are particularly at risk.
Recommended strategies for dementia risk reduction
Risks are particularly high in more socially disadvantaged populations including in Black, Asian, and minority ethnic groups.
Population-wide
- • Prioritise childhood education for all, worldwide
- • Implement social public health policies that reduce hypertension risk in the entire population
- • Develop policies that encourage social, cognitive, and physical activity across the life course for all (with no evidence for any specific activities being more protective)
- • Scrutinise the risks for hearing loss throughout the life course, to reduce the risk of exposure to this risk factor
- • Reduce the risk of serious brain trauma in relevant settings, including occupational and transport
- • National and international policies to reduce population exposure to air pollution
- • Continue to strengthen national and international efforts to reduce exposure to smoking, both for children and adults, and to reduce uptake and encourage cessation
Targeted on individuals
- • Treat hypertension and aim for SBP <130 mm Hg in midlife
- • Use hearing aids for hearing loss; we need to help people wear hearing aids as many find them unacceptable, too difficult to use, or ineffective
- • Avoid or discourage drinking 21 or more units of alcohol per week
- • Prevent head trauma where an individual is at high risk
- • Stopping smoking is beneficial regardless of age
- • Reduce obesity and the linked condition of diabetes by healthy food availability and an environment to increase movement
- • Sustain midlife, and possibly late-life physical activity
Although we have more to learn about effectiveness, avoiding or delaying even a proportion of potentially modifiable dementias should be a national priority for all.
Interventions and care in dementia
Not all dementia will be preventable and we present the latest evidence on intervention and care for dementia. To date the emphasis has been on specific subtypes of dementia, most notably on Alzheimer's disease, which has been conceptualised over the years in a variety of changing diagnostic criteria—eg, DSM IV and DSM V. 180 , 181 Intense efforts have been put into biomarkers for early preclinical detection of the disease process before it becomes dementia. Biomarkers need to show reliability and validity, and for dementias they also need to be very closely and clearly related to clinical syndrome outcomes in the way that, for example, human papillomavirus is for cervical cancer, and hypertension has been for stroke.
Biomarkers and detection of Alzheimer's disease
Markers of neurodegeneration linked to clinical dementia include brain volume loss—ie, hippocampal volume loss and entorhinal cortex and medial temporal cortical thinning—seen in structural imaging. The most studied molecular markers are in Alzheimer's disease and are amyloid and tau, which PET and CSF detect clinically. The prevalence of particular pathologies at different ages is important in interpretation of such studies. So, for example, population derived studies show increases in plaques in the population from less than 3% at age 50–59 years to around 40% at age 80–89 years. 182
Amyloid imaging
Amyloid imaging detects amyloid in the brain with high sensitivity and specificity in both cognitively normal and people with Alzheimer's disease when the gold-standard comparison is either neuropathology or clinical diagnosis, distinguishing Alzheimer's disease from other neurodegenerative conditions. 183 Amyloid imaging is not a diagnostic test for dementia. A US study of randomly selected older people from the community recruited 1671 people (mean age of 71 years). 182 The prevalence of PET detected amyloid positivity increased from 2·7% (95% CI 0·5–4·9) of people without cognitive impairment aged 50–59 years to 41·3% (95% CI 33·4–49·2%) aged 80–89 years. 182 In 10-year follow-up PET positivity was associated with a higher probability of developing Alzheimer's disease compared with those who were amyloid negative (HR 2·6, 95% CI 1·4–4·9). In participants with mild cognitive impairment who were amyloid positive the probability (HR 1·9, 95% CI 0·9–3·9) was not very different to those who were amyloid negative (1·6, 0·8–3·4).
Similarly, an 8-year follow-up study of 599 volunteers (average age 70 years) in Australia found that cognitively normal PET amyloid-positive people had an elevated risk of developing Alzheimer's disease compared with amyloid negative (17·7% vs 8·1%; OR 2·4, 95% CI 1·5–4·0). 184 Over 80% of the 266 people who were PET amyloid-positive did not go onto develop a cognitive impairment within 8 years, showing positive status does not predict impairment for most people in a timeframe that might be a useful prognostic window. Follow-up at 5 years of amyloid-positive participants with normal cognition or mild cognitive impairment versus amyloid negative people found the same pattern of increased risk (2·6, 1·4–4·9). Risk also increases per 1 year of age (HR 1·05, 95% CI 0·55–2·0/year), and APOEε4 status (2·6, 1·4–5·0). 184
Most people who are amyloid positive with no other markers have not developed Alzheimer's disease dementia during their lifetime. A model of lifetime risks of people who are amyloid positive without any other biomarkers finds it to be 8·4% for a 90-year-old woman who is cognitively normal at baseline, 23·5% for a 75-year-old woman and 29·3% for a 65-year-old woman. 185 The 10-year risk is considerably less, so a 65-year-old woman with only amyloid biomarkers but who is cognitively normal and has no neurodegeneration has a 10-year Alzheimer's disease risk of 2·5% and a man 2·3%, but the risk is higher with accompanying neurodegeneration ( table 2 ). 185
Ten-year risks by age of developing Alzheimer's disease for women based on amyloidosis alone and in the presence of neurodegeneration and mild cognitive impairment
Data are relative risk (95% CI) or %. Reproduced from Brookmeyer and Abdalla 185 by permission of Elsevier.
Overall, the knowledge of PET-measured amyloid and tau status and MRI-derived cortical thickness in a general population derived sample, only adds a small improvement, which might not be clinically important for predicting memory decline over a model with clinical and genetic variables. 186
Using amyloid PET in patients with cognitive impairment of uncertain causes, results in changes to the clinical diagnosis of Alzheimer's disease 187 and sometimes to medication prescription. We do not know whether PET use improves patient care or decreases care costs. Many people have a mixed cause of dementia and a positive result does not indicate only Alzheimer's disease.
Fluid biomarkers
PET imaging is very costly (US$3000 in the USA) and although used in some clinical settings remains the topic of research to understand its usefulness in broader populations. Fluid biomarkers—ie, blood and cerebrospinal fluid tests—have become a more practical focus of interest since it has become possible to measure specific proteins linked to the proteins associated with the neuropathologies of Alzheimer's disease. 188 A composite blood biomarker for amyloid tested in a discovery dataset and then a validation cohort of participants aged 60–90 years who were already taking part in studies in Japan or Australia had areas under the receiver operating characteristic curves of 96·7% for discovery and 94·1% for validation. The blood biomarker had sensitivity and specificity above 80% against amyloid PET measurement 188 and correlated with CSF concentrations of Aβ1–42. These results are similar to other amyloid blood biomarkers 189 , 190 and harmonisation to a common reference standard is now vital. Although CSF Aβ1–42/1–40 ratio and amyloid PET are now considered interchangeable, 191 CSF tau biomarkers have only correlated weakly with brain tau as currently measured by radioligands. 192 Neurofilament light protein is measured in many cohorts; however, it is non-specific. People with Huntington's disease, multiple sclerosis, mild cognitive impairment, and Alzheimer's disease might have raised blood neurofilament light concentrations, which are a marker of neurodegeneration. 193 , 194 , 195
Key points and conclusions
To be useful in clinical practice biomarkers must be well understood in the populations to which they are going to be applied, including the effects of age and sex on results. There is now reasonable evidence that amyloid and tau measured by PET or in fluid indicate increased risk for development of cognitive impairment in older adults but at the individual level prognostication is not possible as most cognitively normal people with these markers do not develop dementia within a clinically relevant timeframe. Negative amyloid results can be useful for ruling out current Alzheimer's pathology in people with cognitive impairment when the cause is uncertain and show an individual is unlikely to develop Alzheimer's disease during the next few years. High neurofilament light concentrations indicate a neurodegenerative process but not its cause. The value of biomarkers, in terms of diagnostic value, has not been addressed in different representative populations and particularly not in those from LMIC. The potential advantages of blood biomarkers are their low cost and their wider acceptability and applicability in many settings. In many areas of medicine more reliable diagnostic tests have improved research, including epidemiological and public health research and trials, to help distinguish cause from symptom (tuberculosis from a fever) or assess risk factor and disease (hypercholesterolaemia and ischaemic heart disease). Those biomarkers developed for the underlying biology of the dementia syndrome are subject to the same assessment of value.
Principles of intervention in people with dementia
In the 2017 Commission, we discussed that when concerns are raised by patients or family, an accurate diagnosis is helpful. Such a diagnosis provides a gateway to intervention and services where available, for planning for possible futures, and support for family, as well as to research. Unfortunately, these services are not always available. National plans for dementia support timely diagnosis and offer help to individuals and their families.
We did not address screening of those not presenting with concerns but rigorous systematic reviews by the US Task Force on Prevention have found an absence of evidence of benefit and harm. 196 The first trial of population screening took place in the USA, screening 4005 primary care patients aged 65 years or older. No clear benefit or harm in terms of quality of life, mood, or increasing diagnostic rates was found. 197 Other strategies might become more valuable in time such as sensitive awareness of risk factors, when routine records suggest an individual might be deteriorating cognitively. 198
People with dementia have complex problems with symptoms in many domains. Those providing support and any interventions must consider the person as a whole, as well as their context and their close carers, whether family or friends. Individuals' medical, cognitive, psychological, environmental, cultural, and social needs must be given consideration. 2 In the context of under provision of services, this notion is and will continue to be a challenge. Dementia, as an illness which affects cognition by definition, affects the ability to organise activities and people with dementia often need help to do what they enjoy—for example, listen to music, or go to gardens and parks. Wellbeing is one of the goals of dementia care.
Interventions once a diagnosis has been made
Cholinesterase inhibitors have a useful, modest role in improving cognition and activities of daily living in patients with mild-to-moderate Alzheimer's disease and memantine can be prescribed in combination or each drug used separately for moderate and severe Alzheimer's disease. 2 , 199 , 200 However, although available in most countries these drugs are no longer remunerated in France because it is felt that they offer only a small benefit while shifting clinician's attention from other interventions. Whether non-prescribing of this drug will help patients by removing an intervention with known benefit or be detrimental to them is unknown. 201 No advances have been reported in Aβ therapeutics, with negative results from phase 3 trials of monoclonal antibodies (eg, solanezumab, crenezumab) and inhibitors of β-secretase, a protease involved in the production of Aβ peptides. 202 Aducanumab previously abandoned as futile now has further unpublished results. Three 5HT6 antagonists and the calcium channel blocker nilvadipine 203 , 204 have also been ineffective. These drugs also show substantial impact during treatments at so-called therapeutic concentrations on the leakiness of blood vessels. The long-term impact of such side-effects is unknown. Anti-tau, anti-amyloid, and anti-inflammatory drugs continue to be in focus and some argue that pre-symptomatic interventions are necessary, especially if targeting Aβ production, but no evidence of efficacy 205 and some evidence of worsening target symptoms currently exists. 206
Cognitive training in people with dementia
A meta-analysis of 12 controlled trials of 389 people with mild dementia, completing 4 or more hours of group-based computerised cognitive training (mean age 66–81 years, 63·5% female participants), found a small, statistically significant beneficial effect on overall cognition, driven by two trials of virtual reality or Video games (SMD=0·3, 95% CI 0·0–0·5), one with a low and one with a high risk of bias. 55
A Cochrane review 207 found 33 trials of cognitive training, only one of which overlapped with the study above, with around 2000 participants with mild-to-moderate dementia, most with a high or uncertain risk of bias. 207 People completing cognitive training, compared with usual treatment or non-specific activities, had small-to-moderate effects on overall cognition (SMD 0·4, 95% CI 0·2–0·6) and specific cognitive abilities such as verbal fluency and improvements lasted for a few months to 1 year. No direct evidence was observed to suggest that cognitive training was better than cognitive stimulation therapy.
Exercise and physical activity
The Dementia and Physical Activity RCT 208 found moderate-to-high intensity aerobic and strength exercise training did not slow cognitive impairment in people with mild-to-moderate dementia but improved physical fitness. The US Reducing Disability in Dementia study 209 implemented an at-home multicomponent intervention including exercise education, training to increase pleasant events, and activator-behaviour-consequence problem-solving approach over 6 weeks by case managers in 255 community dwelling people with dementia older than 60 years and their family carer and were able to follow up 140 (54·9%). The study found increased physical activity; days of taking 30 or more minutes of exercise (effect size 0·6, 95% CI 0·4–0·8 after the treatment and 0·3, 0·1–0·5 at 13 months) in a before and after intervention comparison.
Interventions for neuropsychiatric symptoms of dementia
Neuropsychiatric symptoms are common and often clustered in people with dementia. These symptoms might precede dementia and are associated with tau and amyloid neuropathology. 210 This suggests that underlying neurobiological mechanisms might underpin neuropsychiatric symptoms. However, other drivers relating to the personal history and the environment of the person with dementia are also likely to exist. Neurodegeneration could lead to increased vulnerability to stressors or triggers. Genetics, cognitive reserve, resilience, medical comorbidities, and environment including responses of carers might modify these relationships. Needs and responses will also be individual and relate to a person's own social, cultural, and historical context. First-line assessment and management of neuropsychiatric symptoms should focus on basic health: describe and diagnose symptoms; look for causes such as pain (using validated pain assessments might help), illness, discomfort, hunger, loneliness, boredom, lack of intimacy and worry that could cause the behaviours and alleviate these while considering risks of harm. 2
No new evidence of medication effectiveness for these symptoms exists; risperidone in low doses (0·5 mg daily) and some other antipsychotics are sometimes effective but often ineffective and have adverse effects. 2 Specific initiatives have led to a decrease in antipsychotic prescriptions for people with dementia, although often replaced with other psychotropics ( figure 8 ), such as benzodiazepines, antidepressants, and mood stabilisers. 211 These psychotropics lack evidence of efficacy for neuropsychiatric symptoms but show clear evidence of possible harm; for example, trazodone and benzodiazepines increase fall-related injuries. 144 Major policy changes should be assessed carefully, within and across countries for unintended consequences (and perhaps unexpected benefits) and their costs.

Proportion of patients with a diagnosis of dementia prescribed an antipsychotic drug (A) and those prescribed an anxiolytic, hypnotic, or antidepressant (B)
CPRD=Clinical Practice Research Datalink. Reproduced from Donegan et al, 211 by permission of Elsevier.
Evidence is slowly accumulating for the effectiveness, at least in the short term, of person-centred evidence-based psychosocial interventions. In Germany, a 6-month cluster RCT of nurse-delivered, supervised dementia care management used a computer-assisted nurse assessment to determine personalised intervention modules, then a multi-disciplinary team discussion and agreement with the physician for 634 people (mean age 80 years) with dementia living at home with a primary carer or alone. 212 The mean mini mental state examination (MMSE) was 23, only 38% had a formal diagnosis of dementia; the majority of participants (51%) had mild dementia but some had moderate and some severe dementia. The intervention consisted of psychosocial management of treatment and care, medication management and carer support, and education and discussion with a psychiatrist or neurologist. The intervention, compared with care as usual, was associated with better outcomes for neuropsychiatric symptoms (Neuropsychiatric Inventory [NPI] score −7·5, 95% CI −11·1 to −3·8), however this effect could be because of deterioration in care as usual (in the care as usual group NPI increased from 7·2 to 15·2; in the intervention group NPI increased from 7·6 to 8·2). This between-group reduction in neuropsychiatric symptoms was greater than that expected, extrapolating from other study results, with antipsychotic medication. Effects on quality of life were only apparent for those people living with a carer.
An eight-session home-based tailored activity programme RCT, tailored both to the person with dementia living at home and to a family member compared with eight telephone-based education sessions, recruited 160 participants with 64% follow-up, imputing values for the rest. 213 The study reported a large reduction in overall neuropsychiatric symptoms immediately after the intervention, which were better in the group receiving home-based tailored activity programme on the neuropsychiatric inventory (mean difference in score 24·3, 95% CI 3·1–45·6), and on functional dependence and pain but this was not sustained 4 months later. Non-completers had more severe neuropsychiatric symptoms.
Since the 2017 Commission two new systematic reviews of antidepressants to treat depression in dementia reported moderate quality evidence that antidepressant treatment for people with dementia does not lead to better control of symptomatology compared with placebo. 214 , 215
Agitation is distressing for people with dementia and those around them, and contributes substantially to the overall costs as the level of agitation increases. 216 The body of evidence on this key behaviour is growing, mostly focused on care-home settings. These findings are valuable as these populations are most affected; however, because many people with dementia reside at home a major gap in knowledge remains.
Care home residents with agitation often find sitting still difficult and therefore might not be included in activities. 217 , 218 Two new cluster RCTs of professionals delivering multicomponent, interdisciplinary, interventions in care homes successfully reduced agitation. The WHELD study 219 included participants with or without neuropsychiatric symptoms and provided person-centred care, aiming to improve communication with people with dementia. It implemented social, sensory experiences or other activities; educated about antipsychotic review; and addressed physical problems, finding lower Cohen Mansfield Agitation Inventory (CMAI) at 9 months (MD −4·3 points, 95% CI −7·3 to −1·2). 219 The TIME study 220 for people with moderate-to-high levels of agitation consisted of a manual-based comprehensive assessment of the resident and structured case conference for the staff and doctor, to create a tailored plan, and then implement it. This intervention led to reduced agitation at 8 weeks (NPI −1·1 points, 95% CI −0·1 to −2·1; CMAI −4·7 points, −0·6 to −8·8) and 12 weeks (NPI −1·6, −0·6 to −2·7; CMAI −5·9, −1·7 to −10·1). 220 These effect sizes are similar to those seen for medications, but without harmful side-effects. 2 , 221 A further RCT studied a six-session intervention with staff in groups, teaching staff to understand agitation as related to medical, psychological, or social unmet needs and to implement strategies to meet these needs, using the describe, investigate, create, and evaluate approach. 222 The intervention did not reduce agitation symptoms, although it was cost-effective, improving quality of life. 223 Overall, the current evidence for agitation in care homes favours multi-component interventions by clinical staff, including considering if drugs might harm, and not drug interventions. Thus a major gap remains in knowledge about people living at home who comprise the majority of those with dementia.
Psychotic symptoms in dementia
People with dementia might be wrongly thought to have delusions when they misremember, and new psychotic symptoms are often due to delirium, thus thorough assessment of symptoms is essential. 2 Management of psychosis in dementia should start with non-pharmacological interventions; however, evidence for effectiveness of these interventions for psychosis in dementia is weaker than for agitation. 224 Antipsychotics for psychosis in dementia should be prescribed in as low a dose and for the shortest duration possible. 2 However, a Cochrane review of antipsychotics withdrawal found two trials with participants with dementia who had responded to antipsychotic treatment. These reported that stopping antipsychotics was associated with symptomatic relapse 225 suggesting the need for caution in any medication withdrawal in this group. There was low-quality evidence that, in general, discontinuation might make little or no difference to overall neuropsychiatric symptoms, adverse events, quality of life or cognitive function. 226
Apathy might be conceptualised as the opposite of engagement, comprising reduced interest, initiative, and activity. Like people without dementia, those with dementia engage more in preferred activities, but require additional support to do so. 227 A study in care homes observed engagement increased during activities in those who attended the groups. 228 A Cochrane review of the few people who had been in drug RCTs of methylphenidate versus placebo for apathy in dementia found small improvements on the apathy evaluation scale (MD −5·0, 95% CI −9·6 to−0·4, n=145, three studies, low-quality evidence) but not on the NPI apathy subscale (MD −0·1, 95% CI −3·9 to 3·7, n=85, two studies). 229
There is no evidence that medication for sleep in dementia is effective 230 and considerable evidence for harm—ie, earlier death, increased hospitalisation, and falls—exists. 139 , 144 Testing of non-pharmacological interventions is ongoing. 231
Carer distress related to neuropsychiatric symptoms rather than the dementia symptoms was associated in one study with increased use and costs of health services, 232 highlighting the need for effectively identifying, educating, and supporting distressed carers. An RCT 233 reporting 6-year follow-up after the eight session STrAtegies for RelaTives intervention—manual-based coping intervention delivered by supervised psychology graduates—found continuing effectiveness for depressive symptoms in carers (adjusted MD −2·00; 95% CI −3·4 to −0·6) and risk of case-level depression, with patient-related cost being approximately 3 times lower than those who did not receive the intervention (median £5759 vs £16 964 in the final year; p=0·07). 233 Another US study 234 followed up 663 people, mean age 77 years, 55% women. Caregiver depression rather than symptoms of people with dementia predicted emergency department use for people with dementia, with a 73% (RR 1·73, 95% CI 1·3–2·3) increase. 234
Functioning
A UK RCT of 14 sessions of cognitive rehabilitation focused on individual goal attainment with therapy delivered at home by an occupational therapist or nurse to 475 participants with mild-to-moderate dementia (MMSE ≥18 for inclusion; mean 24) and a family carer. 235 Individuals had two or three goals; the most common was engaging in activities (21% of goals). The intervention group reported increased goal attainment over 3 and 9 months compared with usual treatment (effect size 0·8, 95% CI 0·6–1·0 at both 3 and 9 months). 235 The treatment did not improve participants' quality of life, mood, self-efficacy, cognition, carer stress, or health status and was not cost-effective. A systematic review 236 of RCTs without meta-analysis for overall effect size, concluded that all interventions which had improved functioning in people living with dementia in the community have been individual rather than group interventions. These were: in-home physiotherapist delivered aerobic exercise (two studies, larger one positive, 140 people with Alzheimer's disease; smaller study negative, 30 people with Alzheimer's disease), individualised cognitive rehabilitation (mild or moderate dementia; two studies; 257 cognitive reserve intervention groups and 255 controls), and in-home activities-focused occupational therapy (people with mild to moderate dementia, three studies, 201 intervention, 191 controls) reduced functional decline compared to controls but group-exercise and reminiscence therapies were ineffective. 236
People with dementia have other illnesses
Multimorbidity is a huge challenge in dementia, not only because people with dementia have increased rates of other illnesses, but also because they often find it particularly difficult to organise care. People with dementia might forget to tell their family or health professionals of symptoms, struggle to understand or follow agreed plans, and are more likely to forget to drink and eat, increasing falling and infection rates. 237 People with dementia consult primary care less often 238 and have fewer dental visits 239 than those without dementia and their family members, if involved, often feel they lack knowledge to assist. 240 Health-care professionals need education to be more comfortable, understanding, and positive in communicating with people with dementia. 241
Around 70–80% of people diagnosed with dementia in primary care have at least two other chronic illnesses. 242 , 243 People who are physically more frail are more likely to have dementia, but the relationship between pathology and symptoms in these people is comparatively weak suggesting that dementia might be from other causes. 22 Compared to the general older population, people with dementia have increased rates of cerebrovascular disease, 243 , 244 , 245 , 246 stroke, 247 Parkinson's disease, 243 , 245 diabetes, 245 , 247 skin ulcers, anxiety and depression, 243 , 245 pneumonia, incontinence, and electrolyte disturbance. 245 Multimorbidity in people with dementia is associated with faster functional decline 248 and worse quality of life for people with dementia and their family carers. 249
Dementia and COVID-19
Severe acute respiratory syndrome coronavirus 2, was first identified in patients with viral pneumonia in Hubei province, China. 250 Severity and mortality of the associated disease (COVID-19) worsen with increasing age 251 and with pre-existing illnesses such as hypertension and diabetes, 252 and thus many people with dementia are at particular risk. Death certificates from the UK indicate that dementia and Alzheimer's disease were the most common underlying conditions, specified in 11 950 deaths (25·6% of all deaths involving COVID-19) in March to May, 2020. 253 Many charities, practitioners, and academics supporting people with dementia have issued guidance based on current evidence and best practice, including advance consideration of whether people would wish to be hospitalised if they develop severe COVID-19. Concern has been expressed that the illness and consequent distancing might increase family carer stress, loneliness, neuropsychiatric symptoms and use of psychotropic medication, and lead to complications, including future dementia. Interventions delivered remotely through technology have also been implemented in some places. 254 , 255 , 256 , 257
People with dementia might struggle to adhere to measures to reduce virus transmission, as they might not understand or remember about required changes to behaviour, such as physical distancing and hygiene, leading to increased risk to themselves and their carers. 258 They might additionally be vulnerable if they depend on others for daily activities or personal care, as this necessitates close personal contact.
This situation is particularly concerning in those care homes, where many residents have dementia and where many COVID-19 deaths have occurred in many countries 259 , 260 , 261 with reports of more than half of residents being admitted to hospital. In US nursing homes, among 10 576 people with confirmed COVID-19, residents living with dementia made up 52% of COVID-19 cases; yet, accounted for 72% of all deaths (an increased risk of 1·7). 262 The number of people living together in care homes means that the infection of an individual, either staff or resident, could endanger more people than in traditional or family households. Although evidence exists that if staff are sufficiently and rigorously protected they are unlikely to develop COVID-19, many staff have become unwell and some have died. 263 , 264 Illness means that there are fewer people to care for residents at a time when they need particularly high levels of care. This situation is particularly relevant in the care of residents with dementia, if they are expected to remain in their own rooms, rather than eating and participating in activities with others. Staff or residents might also be moved between care homes and increase risk in other homes. 261 Restrictions on visitors to private homes, care homes, and hospitals might cause greater distress for people with dementia and they might not understand why people are wearing masks, recognise who is behind it, or understand speech when lips are covered. Lack of restrictions means that the visitors might also be at elevated risk. 261
The impacts of COVID-19 on people with dementia might be particularly severe in LMICs, due to smaller health budgets for testing and protective equipment, capacity of health-care systems, quality of care home provision and patterns of workforce mobility. 264
Thus, people with dementia are particularly vulnerable to COVID-19 because of their age, multimorbidity, and difficulties in maintaining physical distancing. 250 , 251 , 252
We recommend rigorous public health measures of protective equipment and hygiene, including not moving staff or residents between care homes or admitting new residents when their COVID-19 status is unknown, should mitigate impacts on people with dementia. It is also imperative that there is frequent and regular testing of staff in care homes for infection, ensuring staff have sick pay so that they do not come in when symptomatic and interim care is being set up for people discharged from hospital so that only those who are COVID-19 free come to live in care homes. Resident testing should encompass asymptomatic as well as symptomatic people, when there is exposure within the home to COVID-19. In the future, many homes might be able to start to provide oxygen therapy so that those who do not want to be admitted to hospital are still able to access oxygen therapy. In addition, it is also important to reduce isolation by providing the necessary equipment and a brief training to relatives on how to protect themselves and others from COVID-19; so that they can visit their relatives with dementia in nursing homes safely when it is allowed. Further evidence is needed to inform responses to this and future public health emergencies.
Hospital admissions
Hospitalisation in people with dementia is associated with adverse, unintended consequences, including distress, functional and cognitive decline, and high economic costs. 265 , 266 , 267 People with dementia have 1·4 to 4 times more hospital admissions than others with similar illnesses. 266 , 268 , 269 , 270
A systematic review and meta-analysis including 34 studies of 277 432 people with dementia found that in the six studies which compared the two groups, people with dementia had increased hospital admissions compared with those without dementia, after adjusting for age, sex, and physical comorbidity (RR 1·4, 95% CI 1·2–1·7; figure 9 ). 271 Hospitalisation rates in people with dementia ranged from 0·37 to 1·26 per person-year in high-quality studies. Admissions are often for conditions that might be manageable in the community (potentially preventable hospitalisations). 268 People with dementia experience longer and more frequent admissions and readmissions; health-care expenditure for people with moderate-severe dementia is around double that of people without dementia. 269 , 272 , 273 Early detection and management of physical ill-health in people with dementia, particularly of pain, falls, diabetes, incontinence, and sensory impairment, is important. 199 , 274 , 275 However, no intervention has successfully reduced number of hospital admissions of community-dwelling people with dementia, 276 although education, exercise, rehabilitation, and telemedicine have reduced admissions for older people without dementia. 277

Systematic review and meta-analysis of hospitalisation rates of people with dementia compared to those without dementia controlled for age and sex
Reproduced from Shepherd et al, 271 by permission of Springer Nature.
High-quality care for people with dementia takes longer than caring for others with the same condition. 278 Recognition of dementia in hospital inpatients is necessary for optimum care, 279 but dementia is often undetected or unrecorded. 280 In the UK however, detection rates have increased over the past 10 years. 281
Physical illness, delirium, and dementia
Dementia and delirium frequently occur together. In one hospital inpatients' survey nearly 35% of those older than 80 years experienced delirium; those with prior cognitive impairment had 15 times the risk of developing delirium than those without (OR 15·3, 95% CI 5·2–45·4). 282 People with delirium without known dementia are more likely to be diagnosed with dementia in the future than others, either because of pre-existing undiagnosed dementia or cognitive impairment, present in 20·7% (95% CI 11·9–29·5) and 37·8% (27·3–88·3) respectively of one cohort, or because delirium has neurotoxic effects and so precipitates dementia. 283 People with similar neuropathology show faster cognitive decline if they develop delirium than if they do not. 284 Additionally, older people without dementia declined cognitively more than twice as fast after an emergency hospital admission for any cause, compared with those not admitted, suggesting any severe illness is associated with cognitive decline. 285 Risk factors for delirium in dementia include sensory impairment, pain, polypharmacy, dehydration, intercurrent illnesses, such as urinary tract infections or faecal impaction, and an unfamiliar or changing environment. 286 Delirium in older people should prompt consideration of underlying dementia.
Most research on delirium prevention has been in people without dementia. It suggests targeting hydration, stopping medication predisposing to delirium, monitoring the depth of anaesthesia, and sleep promotion. However, no evidence for medication efficacy, including cholinesterase inhibitors, antipsychotic medication, or melatonin exists. 287 , 288 , 289 The Hospital Elder Life Program 290 —an intervention to prevent delirium in those admitted to hospital—reduces delirium incidence and includes people who are cognitively impaired. This multidisciplinary treatment consists of daily visits, orientation, therapeutic activities, sleep enhancement, early mobilisation, vision and hearing adaptation, fluid repletion, infection prevention and management of constipation, pain, and hypoxia, and feeding assistance. 290
A network meta-analysis of drugs for prevention and treatment of delirium did not include studies of people with dementia, thus we cannot use this to recommend drugs for people with dementia and delirium as this research might be inapplicable to them. 291
Little high-quality research exists on managing delirium in dementia. One RCT compared care at a specialist medical and mental health unit to usual care for 600 confused people older than 65 years, acutely admitted to hospital and found no difference in the primary outcome of days spent at home or in hospital, but increased family satisfaction. 292 A further RCT of cognitively stimulating activities for people with delirium in dementia did not improve the delirium. 293 No definitive evidence that any medication improves delirium in people with dementia exists: cholinesterase inhibitors, antipsychotics, and sedating benzodiazepines are ineffective and antipsychotics and benzodiazepines are associated with mortality and morbidity. 265 , 288 , 294 , 295 , 296 , 297 Given the risk of dementia in people who develop delirium, its prevention, and possibly advances in its management, might offer a means for dementia prevention. 298
Link between very old age, frailty, and dementia
The fastest growing demographic group in most advanced countries are people aged 90 years and older. One well characterised post-mortem cohort of the oldest old (n=1079; mean age 90 years) dying with dementia, found that neuropathological features of Alzheimer's disease account for about half of the cognitive decline seen as people diagnosed with Alzheimer's disease had mixed causes of dementia. 299 Although Alzheimer's disease neuropathology was the commonest cause of dementia, Alzheimer's disease changes rarely occurred on their own, so only 9% of people with dementia had pure Alzheimer's disease pathology. 300 People who have Alzheimer's disease pathology without developing dementia tend to have fewer age-related health deficits than those who develop it with even low concentrations of plaques and tangles. 301 A moderation analysis showed that the relationship between Alzheimer's disease pathology and dementia status differed according to level of frailty (adjusted for age, sex, and education) with increasing frailty weakening the relationship between Alzheimer's disease pathology and dementia ( figure 10 ). 22 As with delirium, some of this additional health risk might be modifiable. This approach suggests a new type of therapy focus on specific age-related processes that underpin many diseases of late life might reduce the incidence or severity of dementia.

Moderation analyses of the relationship between Alzheimer's disease pathology and clinical diagnosis of Alzheimer's dementia (adjusted for age, sex, and education)
As frailty increased, the odds of a neuropathological diagnosis of Alzheimer disease corresponding to a clinical diagnosis decreased. Reproduced from Wallece et al, 22 by permission of Elsevier.
End-of-life care in dementia
The numbers of people dying with dementia are increasing but the evidence for the best end-of-life care is scarce. Trends in age-standardised death rates (3·6%) for dementia increased slightly between 1990–2016, with pronounced increases in the USA and Japan and decreases in western Europe and central Latin America. 4 Dementia is more readily being included on death certificates, which accounts for some of the rise. The increase might be related to dementia manifesting at later ages, with higher physical frailty 22 leading to a faster decline.
Most people with dementia might die while still in the mild-to-moderate stages whereas only about a quarter of those dying with dementia have severe dementia. 302 , 303 The trajectory of dementia is often unpredictable 304 and palliative care initiation should reflect need not prognosis.
Decision making about end of life is complex and simple rules of thumb, co-designed with staff and carers, provided clarity in some small studies. 304 One RCT testing decision-aids about families' and doctors' goals of care for people with advanced dementia led to increased palliative care content in care plans. 305 , 306 In a 9-month UK prospective study, 85 care home residents with advanced dementia from 14 homes were likely to be living with distressing symptoms, specifically agitation (54%) or pain (61% on movement). 304
Capacity to make abstract decisions, including about the future, might be lost early in dementia. 307 Therefore, advance care planning, designed to empower people with dementia and improve quality of dying, might theoretically be something everyone should do before developing dementia. 308 However, people might not be able to predict their future wishes. This might explain why family carer proxies show only low-to-moderate agreement with stated end-of-life treatment preferences of people with dementia. 309 Advance care planning might, however, reduce carers' uncertainty in decision making and improve perceptions of quality of care. 310
Partners of people dying with dementia experience poorer mental health than those facing bereavement from other causes 311 possibly because of long and difficult caring responsibilities. This might be ameliorated through sensitive and timely information, particularly regarding the progression of dementia, 312 individually or through family and staff case-conferencing. 313 , 314

Conclusions
Knowledge about risk factors and potential prevention, detection, and diagnosis of dementia is improving although significant gaps remain. 315 In this Commission report, we have specified policy and individual changes to delay the onset of cognitive impairment and dementia and better ways to support and treat people with dementia and their families and to improve their quality of life.
Interventions, including organisation of the complex physical illness and social needs, to support people affected by dementia can have a huge effect when taken as a whole. Our ambition is for worldwide provision of resources for an adequate level of wellbeing to people with dementia and their carers with a better evidence base to guide individual care and policy making alike. With good quality care, people can live well with dementia and families can feel supported.
Acknowledgments
We are partnered by University College London (UCL), the Alzheimer's Society, UK, the Economic and Social Research Council, and Alzheimer's Research UK, and would like to thank them for financial help. These organisations funded the fares, accommodation, and food for the Commission meeting but had no role in the writing of the manuscript or the decision to submit it for publication. We would like to thank Bernadette Courtney, Jacques Gianino, and Nuj Monowari, from UCL, London, UK, for their administrative help, including managing finances, booking rooms and food, and setting up a website supported by the University College London Hospitals National Institute for Health Research Biomedical Research Centre. We would like thank Henrik Zetterberg for advice on biomarkers and dementia.
Contributors
GL, JH, AS, and NM contributed to literature searches and quality assessments for systematic reviews. JH and NM performed meta-analyses. GL, JH, AS, and NM conceived the new PAF calculation and NM led the statistical analysis. GL, JH, AS, NM, DA, CLB, SB, AB, JC-M, CC, SGC, NF, RH, HCK, EBL, VO, KRi, KRo, ELS, QS, LSS, and GS attended the conference to discuss the content. GL, JH, EBL, AS, DA, and ELS wrote first drafts of sections of the paper. GL wrote the first draft of the whole paper and revisions of drafts. CBa reviewed and contributed to revision of the final drafts. All authors contributed to sections of the reports and all revised the paper for important intellectual content.
Declaration of interests
AS reports grants from Wellcome Trust (200163/Z/15/Z), outside the submitted work. DA reports grants from Eli Lilly, during the conduct of the study. CBa reports grants and personal fees from Aca-dia and Lundbeck; and personal fees from Roche, Otsuka, Biogen, Eli Lilly, and Pfizer, outside the sub-mitted work. SB reports grants and personal fees from AbbVie, personal fees and non-financial sup-port from Eli Lilly, and personal fees from Eleusis, Daval International, Boehringer Ingelheim, Axovant Sciences, Lundbeck, and Nutricia, outside the submitted work; and he has been employed by the Department of Health for England. NF reports non-financial support from Eli Lilly, outside the submitted work. LNG and her institutions (Johns Hopkins University, Baltimore, MD, USA, Drexel University, Philadelphia, PA, USA, and Thomas Jefferson University, Philadelphia, PA, USA) are entitled to receive royalties from fees associated with online training for the tailored activity program, which is an evidence-based program referenced in the Review. RH reports grants from Department of Health, NIHR HTA Programme, outside the submitted work; and he is a Scientific Trustee of the charity Alzheimer's Research UK. MK reports grants from the UK Medical Research Council (S011676, R024227), NordForsk (the Nordic Programme on Health and Welfare, 75021) and the Academy of Finland (311492), outside the submitted work. EBL reports other (royalties) from UpToDate, outside the submitted work. KRo reports personal fees from Clinical Cardio Day-Cape Breton University, Sydney, NS, Canada, CRUIGM-Montreal, Jackson Laboratory, Bar Harbor, MA, USA (speaker fees), MouseAge, Rome, Italy (speaker fees), Lundbeck, Frontemporal Dementia Study-Group, SunLife Insurance, Japan, outside the submitted work. He is a President and Chief Science Officer of DGI Clinical, which in the last 5 years has contracts with pharma and device manufacturers (Baxter, Baxalta, Shire, Hollister, Nutricia, Roche, Otsuka) on individualised outcome measurement. In 2017, he attended an advisory board meeting with Lundbeck. He is also Associate Director of the Canadian Consortium on Neurodegeneration in Aging, which is funded by the Canadian Institutes of Health Research, and with additional funding from the Alzheimer Society of Canada and several other charities, as well as, in its first phase (2013-2018), from Pfizer Canada and Sanofi Canada. He receives career support from the Dalhousie Medical Research Foundation as the Kathryn Allen Weldon Professor of Alzheimer Research, and research support from the Canadian Institutes of Health Research, the QEII Health Science Centre Foundation, the Capital Health Research Fund and the Fountain Family Innovation Fund of the QEII Health Science Centre Foundation. LSS reports grants and personal fees from Eli Lilly, Merck, and Roche/Genentech; personal fees from Avraham, Boehringer Ingelheim, Neurim, Neuronix, Cognition, Eisai, Takeda, vTv, and Abbott; and grants from Biogen, Novartis, Biohaven, and Washington University DIAN-TU, outside the submitted work. The remaining authors declare no conflict of interests.
Supplementary Material
Uncited references.

What Effect Does Alzheimer’s Disease Have on Writing?
Observations on dementia's impact on creative writing and diagnostic potential..
Posted February 7, 2022 | Reviewed by Tyler Woods
- Agraphia is very common in Alzheimer's disease but is of limited diagnostic value.
- The earlier diagnosis of Alzheimer's disease has led to a new wave of writers who have movingly described what it is like to lose memory.
- Computerized textual analysis may be able to distinguish early writing changes due to incipient dementia, especially in creative writers.
In the earliest stages of dementia , literate individuals are able to compensate for their written mistakes by double-checking what they have put down on paper and then using word processing software to further correct spelling, grammar, and punctuation. Uncharacteristic substitution of homophones such as "there" for "their" or the jumbling of syllables are sometimes early voiced concerns, but it is impossible for a neurologist to diagnose Alzheimer’s disease from a letter received from a person who is worried about their memory . On the other hand, the copious written accounts running to several pages that are often handed over during the medical consultation by people worried they might be developing dementia and by those with functional cognitive disorder (pseudodementia) can be of considerable diagnostic value.
Characteristics of Writing in Early Alzheimer's
When writing by individuals in the early stages of Alzheimer’s disease is compared with their written text from ten years earlier it may be found to have an impoverished vocabulary less grammatical complexity with fewer subjunctive clauses, and a lack of punctuation. Frequent perseverations, unnecessary gaps between letters and words and an inappropriate mixing of lower- and upper-case letters may also be present. Poorly constructed or illegible letters and omission or over-repetition of letter strokes are other characteristic findings. Written expression is more affected than spoken language in many people with dementia, but despite this many people with Alzheimer’s retain their ability to produce simplified but coherent written sentences for many years after diagnosis. Ronald Reagan's handwritten letter composed without assistance that announced he had been diagnosed with Alzheimer's disease is of interest in this regard. Although his writing had become crabbed and the strikingly uneven margins raise the possibility of new visuo-spatial difficulties, the grammar, spelling and punctuation were accurate.

As the pathology of Alzheimer’s disease spreads from the hippocampus to involve the angular gyrus and Wernicke’s and Broca’s language areas, word-finding difficulties increase, and agraphia, an acquired inability to write, is inevitable. In 1906, Alois Alzheimer reported that, when attempting to write, his patient Auguste D tended to hold the paper as if she had a right-visual-field defect and that she would duplicate some letters while omitting others. Later in the course of her illness, when she was asked to write her name, she would get as far as writing "Frau" and then come to an abrupt halt because she could not remember what came next. Lynn Brophy, in her 2015 blog "Boomerang-Parents," includes a sample of her mother’s writing a few weeks before she died with Alzheimer’s disease. On the second line, Brophy deciphers an attempt by her mother to write her Christian name, Eleanor in capital letters, but which she was unable to complete with her script dilapidating into an illegible scribble ( see below)
There are a few outstanding individuals with Alzheimer's disease who have written about their own cognitive impairments in autobiographies, newsletters, and, increasingly, on blogs. One of these, the late Thomas de Baggio, an herb farmer from the Washington, D.C., area described his difficulties writing:
With failing memory, it is difficult to write long passages without getting lost in words. Where does the story go? Words come when I sit down to write, but they dance away seductively.... I am having trouble reading the writing I do with a pencil or pen. It used to be sharp; now it wobbles and is full of uncertainty. The words come normally but the letters are sometimes not in proper order.
The words that are lost first are those that tend to appear less frequently in everyday English, so that sentences become formulaic and infantile with a preponderance of "low-image" verbs like "come," "do," "get," and "have," and indefinite nouns like "thing."
Regular writing and reading are recommended as therapy for people with episodic memory difficulties. Lists, notebooks, diaries, calendars, and labels for items whose name and function are not reliably accessible become over time essential aide-memoires . Christine Boden, in her pathography Who Will I Be When I Die writes:
Without a shopping list, it is pointless me venturing to the shop. Without my diary, I don’t remember what day it is, what anyone is doing, where they are and so on. I don’t seem to have space in my brain for that sense of "Thursday-ness"...or "April-ness" or "1998-ness."
Gabriel García Márquez
Eighteen years ago, the Colombian Nobel Laureate Gabriel García Márquez, best remembered for popularizing a style of writing called magical realism, wrote his last book after a long-anticipated wait of more than 10 years. Memories of My Melancholy Whores was a rather terse 115-page novella that received a mixed reception. Alberto Manguel, a literary critic for The Guardian, considered the work to be flat and conventional lacking Márquez’s characteristic color, inventiveness, and trademark quotable snippets of wisdom . In the Literary Review, Sam Leith complained that the book was seeded with odd little paradoxes that were tense, careful, deadpan, and often baffling, while Michiko Kakutani writing in the New York Times had this to say:
The fertile inventiveness that animated his masterpiece ''One Hundred Years of Solitude'' is decidedly muted in these pages, and the reverence for the mundane realities of ordinary life, showcased in more recent works, seems attenuated as well. As a result, ''Memories of My Melancholy Whores'' feels like a brittle little fable composed on automatic pilot. The trajectory of this narrative turns out to be highly predictable, leading to a banal ending to a banal story that's quite unworthy of the great Gabriel García Márquez's prodigious talents.
Five years before the book’s publication, García Márquez had been diagnosed with cancer of the lymphatic system and, despite going into remission with chemotherapy, concerns about his health continued to be raised from time to time over the next decade in the Colombian press. Finally, in 2012, Jaime García Márquez announced that his brother had been suffering with dementia and that he would be unable to complete his autobiography. Gabriel García Márquez died two years later of a chest infection in Mexico City at the age of 87.

In his masterpiece One Hundred Years of Solitude, Marquez provided a detailed description of a type of memory impairment that was eventually characterized eight years later by the clinical neuropsychologist Elizabeth Warrington and many years later became known as semantic dementia. Marquez wrote that those living in the Pueblo of Mirrors who sucked on Úrsula Iguarán's homemade sweets became unable to sleep and developed a difficulty in remembering the names and uses of everyday items. To compensate for this, the villagers started to mark the name with an inked brush on every important object. For example, one hung a sign around a cow’s neck saying, "this is the cow which must be milked every morning so that she will produce milk and the milk must then be boiled in order that it can be mixed with coffee."
It is conceivable that in the course of his work as a journalist, Márquez heard about la bobera ("the foolishness"), a pestilence known to cause amnesia in certain families who lived in and around the Andean municipality of Yarumal in North West Colombia. Before Márquez’s death, it was discovered that the cause of this familial dementia which went back for hundreds of years was a mutation in the presenilin 1 gene , a known genetic cause of early-onset Alzheimer’s disease. In his announcement to the press, Jaime García Márquez had reported that their mother and other brother had also suffered from Alzheimer’s disease.
An interrogation of the texts of the "dementia writers," like Thomas DeBaggio, Christine Boden, Greg O’Brien, and Daniel Gibbs reveals none of the recognized handwriting abnormalities reported in Alzheimer’s disease, perhaps, in part, because of editorial assistance, but it is worth at least considering whether the lukewarm reception of Márquez’s last novel might have been justified and explained by incipient Alzheimer’s disease rather than its "smutty" subject matter, the author’s general ill health and increasing age, or the lapse in time from the publication of his previous book.
Jackson’s Dilemma by Iris Murdoch was published in 1995, four years before her death. As with Memories of My Melancholy Whores, the reviews were not what she or her publisher had hoped for. The writer A.S. Byatt commented that the structure of the novel was akin to an "Indian Rope Trick" in which the characters "have no selves and therefore there is no story and no novel," and Penelope Fitzgerald noted that the economy of the writing made it appear "as though Murdoch had let her fiction wear through almost to transparency."
Computerized Language Analysis
In 2005, clinical neuroscientist Peter Garrard used a computerized textual and "word typing" program to compare the writing of Murdoch’s last book with two of her earlier novels, Under the Net , written in her 30s, and the Booker Prize-winning The Sea . Garrard’s analysis indicated that the vocabulary used in Jackson’s Dilemma was more stunted compared with her other books. A postmortem examination had confirmed clinical suspicions of Alzheimer’s disease, and a biography by Murdoch’s husband, the writer John Bayley, provided moving detail of her mental difficulties during her final years.
If computerized language analysis of the sort used by Garrard can be refined further and be made less time-consuming to perform, it might one day become a useful adjunct in the neuropsychological evaluation of people with early memory impairment. Detailed case study analyses, similar to the one carried out with Iris Murdoch’s writing, have also been attempted in the paintings of artists like Willem de Kooning and William Utermohlen, serially describing the changes in style and technique that occurred after the diagnosis of Alzheimer's disease was made.
Rascovsky, K., Growdon, M. E., Pardo, I.R., Grossman, S., Miller, B.L. "The quicksand of forgetfulness": semantic dementia in One Hundred Years of Solitude . Brain. 2009;132(Pt 9):2609–2616.
Garrard, P., Maloney, L. M., Hodges, J.R., Patterson, K. The effects of very early Alzheimer's disease on the characteristics of writing by a renowned author. Brain. 2005;128(Pt 2):250–260.

Andrew Lees, M.D., is a Professor of Neurology at The National Hospital, Queen Square and University College London. He is in the top three most highly cited Parkinson’s disease researchers in the world and included in Thomson Reuters 2015 List of the World's Most Scientific Minds.
- Find Counselling
- Find a Support Group
- Find Online Therapy
- Richmond - Tweed
- Newcastle - Maitland
- Canberra - ACT
- Sunshine Coast
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Therapy Center NEW
- Diagnosis Dictionary
- Types of Therapy

Understanding what emotional intelligence looks like and the steps needed to improve it could light a path to a more emotionally adept world.
- Coronavirus Disease 2019
- Affective Forecasting
- Neuroscience
147 Dementia Essay Topics
🏆 best essay topics on dementia, ✍️ dementia essay topics for college, 👍 good dementia research topics & essay examples, 🎓 most interesting dementia research titles, 💡 simple dementia essay ideas, ❓ research questions about dementia.
- Pain Management in Patients With Dementia
- Alzheimer and Dementia Patients Nursing Care
- Diagnosis and Management of Dementia
- Dementia: Treatment and Management
- Dementia – Health Issues and Caregiver Burden
- Alzheimer’s Disease and Dementia Description
- Effects of Music Therapy on the Behavioral and Psychological Symptoms of Dementia
- Communication Strategies in Dementia Patients The study on communication strategies to tailor the needs of dementia patients is proposed for submission to the International Journal of Nursing Studies.
- Participatory Horticultural Therapy for Dementia Horticultural therapy is effective at reducing agitated behavior, stabilizing emotions, and increasing social interaction in patients diagnosed with dementia.
- Dementia and Its Controversial Treatment Dementia is a progressive decline in cognitive ability, an ailment commonly thought to be inseparable from the concept of the elderly.
- Dementia: Ertha Williams’ Case Analysis Dementia is one of the most common brain dysfunctions that predominantly occur in older adults by diminishing their quality of life and chances for independent functioning.
- Dementia Patients: Communication Strategy and Techniques The specialists advise using slow and simple gestures not to produce the feeling of agitation in a person with dementia.
- Care Services for Elderly People with Dementia in China This paper is a critical review of the report that determines the state of the supportive and health services available to older people with dementia in Rural China.
- Dementia: Dangers and Complications Memory lapses, lack of control over one’s actions, and decreased brain function do not let one underestimate the dangers of dementia.
- Dementia and Alzheimer’s Disease While Alzheimer’s disease can be found in every state, Texas’ statistics indicate the special prevalence of the condition, making dementia a permeating public health issue.
- Feeding Patients With Dementia or Alzheimer’s Disease The effectiveness of probes in feeding persons with dementia/Alzheimer’s disease remains high. However, because of the risks, other less invasive methods are recommended.
- Care for Dementia Patients in Nursing Homes Unlike pharmacological dementia management, the non-pharmacological approach help reduces pain without risks associated with drug administration.
- Healthcare Program: Informational Campaign on Dementia The role of nurses shall not be underestimated when it comes to designing and implementing healthcare programs aimed to improve the well-being of the general population.
- Cognitive-Behavioral Therapy: Dementia and Geriatric Cognitive Disorders Group therapy is an evidence-based psychotherapy method that helps solve many problems, including relationship issues and personal difficulties.
- Patient-Centered Care in a Dementia Unit Patient-centered care implies the consideration of the individual needs, preferences, and abilities of each individual who seeks assistance.
- Dementia-Associated Pain Management Guidelines People suffering from dementia experience physical pain; however, they may not be able to communicate this due to their declining brain functionalities.
- Communication Challenges in Vascular Dementia and Dysphagia When residents do not understand me, I can become more creative by limiting potential distractions to them from my response concerning a given issue.
- Preparing a Podcast on Dementia and Alzheimer Alzheimer’s disease (AD) and dementia is the podcast’s topic, and the objective is to inform the aged group, which is the target group, about the disease.
- Lewy Body Dementia: Diagnosis/Condition LBD is a condition that leads to the development of dementia and which predominantly affects people over the age of 50.
- Lewy Body Disease in Aging Patients With Dementia By using extensive professional support, as well as active education of family members, one can improve the quality of Lewi Body Disease patients’ lives significantly.
- Dementia Disease and Its Physiological Effects The rarest manifestations for people with dementia are euphoria and hallucinations. The significant clinical presentations of this condition are depression, apathy, and anxiety.
- Dementia, Bladder Infection and Other Nursing Issues This paper discusses five major issues for nurses, which are dementia, bladder infection, immobility, anxiety, blindness, and discusses a nursing process for each.
- Therapeutic Interventions for the Older Adult With Depression and Dementia The paper researches the therapeutic interventions which relevant for the older people with depression and dementia nowadays.
- Alzheimer’s Disease and Dementia Dementia is considered a general term for impairments in remembering, moving, and thinking that serve as obstacles in a person’s everyday activity.
- Mindfulness Interventions for Dementia Patients Mindfulness-based interventions for patients with dementia are expected to develop their flexibility and broaden their attention, leading to positive emotions and stress reduction.
- Dementia of Alzheimer’s Type and Diagnostic Criteria Alzheimer’s disease is one of the major debilitating brain diseases whose effects are loss of memory and important mental functions among patients.
- Behavioral Disturbances in Dementia Delirium and agitated depression can occur with dementia. Delirium and dementia have similar symptoms; however, delirium is a confusion that occurs and goes away rather quickly.
- Dementia of Alzheimer’s Type: 10 Warning Signs Dementia is a protracted deterioration in memory, thinking, and reasoning competence. Alzheimer’s disease usually manifests in patients after the age of 60.
- Dementia of Alzheimer’s Type: Signs and Symptoms This paper seeks to analyze dementia that comes about as a result of Alzheimer’s disease. The signs and symptoms of the disease will also be discussed, as well as its stages.
- The Case of Dementia of the Vascular Type The paper submits a brief review of needs and rationales for each of the patterns for this case of dementia of the vascular type.
- Dementia – The Disease of the Older Generation The research paper explores the ways in which the quality of life of patients with dementia could be improved.
- Donepezil for Dementia Due to Alzheimer’s Disease by Govind The article ‘Donepezil for Dementia Due to Alzheimer’s Disease’ discusses the implementation of donepezil for the treatment of Alzheimer’s disease and dementia in general.
- Mother-Adult Daughter Relationships Within Dementia Care The research is devoted to the mother-daughter relationships and the perspectives of women who receive care from their adult daughters.
- Women With Dementia Receiving Their Daughters’ Care The article is devoted to the necessity of home care for people with dementia and provides interviews of people who have to live with relatives suffering from dementia.
- Diagnosing Dementia in Older Patients Many older people of various occupations receive the ‘dementia’ diagnosis. The patient has trouble recalling the names of his family members, remembering his room number, etc.
- Frontotemporal Dementia and Alzheimer Diseases The study measured and compared the balance and gait characteristics of patients with possible FTD and AD to those of a control group.
- Falls in Alzheimer’s and Dementia Older Patients The authors introduced new methods to prevent falls among older patients with Alzheimer’s disease and frontotemporal dementia.
- Patients with Frontotemporal Dementia and Alzheimer Diseases The article discusses the research study aimed at the identification of the influence of gait and balance parameters on the condition of people with Alzheimer’s disease and frontotemporal dementia.
- Falls in Patients with Dementia and Alzheimer’s In their study, Velayutham et al. consider the opportunities for reducing the threat of falls among patients with Alzheimer’s disease.
- Balance and Gait in Dementia and Alzheimer’s Patients The study by Velayutham et al. aims to investigate how Alzheimer’s disease (AD) and Frontotemporal dementia (FTD) affect elderly patients’ gait and balance.
- Dementia: Evaluation of an Epidemiological Problem Individuals with dementia experience memory loss and the reduction of cognitive abilities caused by their brain’s degenerative processes.
- Dementia Symptoms and Awareness in Nurses The present paper discusses a study that intends to improve the awareness of the Behavioral and Psychological Symptoms of Dementia (BPSD) in nurses.
- Patients with Dementia: Communication Techniques Dementia is a difficult condition and can complicate the process of receiving care. The techniques provided in the article appear to be effective.
- Falls Prevention in Patients With Dementia in Nursing Homes The problem of falls in the older adults with dementia remains the complicated issue despite considerable efforts aiming to improve the situation.
- Hospital Staff-Dementia Patients Interactions Some healthcare providers do not use effective approaches to communicate with patients who have dementia. To improve communication, one can use a range of techniques.
- Communication with Patients with Dementia In their article “Pilot testing an educational intervention to improve communication with patients with dementia,” the authors suggest certain communication techniques.
- Lewy Body Dementia, Its Symptoms and Treatment LBD occurs as the so-called Lewi bodies, i.e. alpha-synuclein aggregates of protein, start developing in nerve cells in the substantia nigra or cortex of the brain.
- Diabetes and Dementia Relationships and Nursing The article discusses the possible links between the two illnesses, as well as the risk of developing one of the conditions when already having the other.
- Client-Oriented Approach in Dementia Diagnosis The cultural and demographic backgrounds affect the diagnostic procedures. It takes a different amount of time to diagnose dementia in different patients.
- Health and Social Care for Older People Suffer From Dementia in the UK This paper analyzes the impacts of the reduction of the fund on older people and the unmet social care services, particularly the nursing home services in the UK.
- Dementia with Lewy Bodies and Its Treatment This paper is devoted to dementias and the ways of treating them. Particular attention is paid to dementia with Lewy bodies (DLB).
- Dementia of the Alzheimer’s Type This essay explores the Dementia of the Alzheimer’s type by providing an insight into aspects such as age of disease onset, warning signs, disease stages.
- Falls Among Older Persons With Dementia
- Dementia: Alzheimer’s Disease and Brain Changes
- Comparison Between Dementia and Delirium
- Alzheimer’s Disease and Its Relation With Dementia
- Blood Sugar Levels and Dementia
- Gibbs Reflective Cycle Dementia Care
- Equality Diversity and Inclusion in Dementia Care Practice
- Patient-Centered Pain Control of Elderly People With Dementia
- Can Cognitive Training Slow Down the Progression of Dementia?
- Risk Factors for Incident Dementia Among Older Cubans
- Dementia Problem Behavior and the Production of Informal Caregiving Services
- Dementia and Its Effects on Mental Health
- Physical Restraints for Dementia Patients
- Challenges for Professional Care of Advanced Dementia
- Bilingualism Delays the Onset of Dementia
- Dementia vs. Alzheimer’s Disease
- Anger Management Therapy for Dementia Patients
- Can Medication Alter the Course of Dementia?
- Patients With Dementia and Cognitive Impairment
- Dementia and Its Effect on the Function of the Brain
- Children With Dementia and Parkinson‘s Disease
- Dementia and the Ageing Population
- Indicated Causes and Symptoms of Senile Dementia
- Music and Dementia and Alzheimer‘s Disease
- Mindfulness Training for Carers of People With Dementia
- End-Of-Life Decision Making for Nursing Home Residents With Dementia
- Dementia, Help With Financial Management, and Well-Being
- Person-Centered Care For Dementia Patients
- Dementia Patients and Sexual Compulsion
- Dementia Effects on the Elderly and Their Caregivers
- Both Autism and Dementia in Terms of Diagnosis and Treatment
- The Alzheimer’s Disease and the Frontotemporal Dementia
- Determining Modifiable Risk Factors of Dementia
- Communication With Individuals Who Have Dementia
- Dementia: Brain and Self-Care Activities
- Pathways Connecting Late-Life Depression and Dementia
- Imaging Mild Cognitive Impairment and Dementia in Parkinson’s Disease
- Dementia Care Aging Innovation
- Enhancing Cognition Before Clinical Symptoms of Dementia
- Caring for Patients With Dementia
- Biomarkers for Early Diagnosis of Dementia
- Alzheimer’s Disease and Relate Dementia Reform Health Care
- Health Organisations for Dementia in New Zealand
- Dementia and Its Connection With Memory Loss
- Biomarkers for Dementia, Fatigue, and Depression in Parkinson’s Disease
- Alzheimer’s Dementia Posterior Cortical Atrophy
- Behavioral and Psychological Symptoms of Dementia
- Longitudinal Associations Between Serum Cytokine Levels and Dementia
- Neuro Protective Effect and Attenuation of Dementia
- Dementia and the Different Parts of the Brain Affected
- Observational Pain Assessment Scales for People With Dementia
- The Care for Dementia Patients and Its Positive Impact on Their Lives
- Genetic Dementia Alzheimer’s Disease Gene
- Technology for the Elderly With Dementia
- Cerebrum and Dementia Care
- Exploration Deficits Under Ecological Conditions as a Marker of Apathy in Frontotemporal Dementia
- Dementia Care Pathway-People With Learning Disability
- Environmental Factors That Affect the Risk of Developing Dementia
- Dementia: Alzheimer’s Disease and Loved Ones
- Brain Injury and Dementia in Pakistan: Current Perspectives
- Are Many Retirees With Dementia Lacking Help?
- What Autoimmune Diseases Cause Dementia?
- Why Does Early Retirement Increase the Risk of Dementia?
- Do Retirees With Dementia Need Financial Assistance?
- What Are Pharmacological Interventions for Dementia?
- Why Is Early Diagnosis for Dementia Important?
- Can Dementia Be Cured if Caught Early?
- What Is an Early Indicator of Dementia?
- Does a Brain Scan Show Dementia?
- What Is the Most Important Thing in Caring for Dementia Patients?
- Why Does a Head Injury Cause Dementia?
- How Does Medication Affect Dementia?
- What Medication Worsens the Symptoms of Dementia?
- Can Dementia Be Brought on by Medication?
- What Are the Social Needs of a Dementia Patient?
- Is There a Link Between Mental Health and Dementia?
- What Are the Three Behavioral Problems Associated With Dementia?
- Is Dementia a Psychiatric or Neurological Disorder?
- What Does a Psychiatrist Do for Dementia?
- Can Dementia Cause Nasty Behavior?
- What Are Signs That Dementia Is Getting Worse?
- How Do Dementia Patients Deal With Inappropriate Behavior?
- At What Stage of Dementia Does Aggression Occur?
- What Are Some Common Behaviors Seen in a Person With Dementia?
- Should a Dementia Patient See a Psychiatrist?
- At What Stage Do Dementia Patients Forget Family Members?
- What Is the Life Expectancy of Someone With Parkinson’s and Dementia?
- How Do You Know When Someone With Dementia Is Close to Death?
- Is There a Link Between Parkinson’s Disease and Dementia?
- How Does Dementia Influence a Person’s Care Needs?
Cite this post
- Chicago (N-B)
- Chicago (A-D)
StudyCorgi. (2022, March 1). 147 Dementia Essay Topics. https://studycorgi.com/ideas/dementia-essay-topics/
"147 Dementia Essay Topics." StudyCorgi , 1 Mar. 2022, studycorgi.com/ideas/dementia-essay-topics/.
StudyCorgi . (2022) '147 Dementia Essay Topics'. 1 March.
1. StudyCorgi . "147 Dementia Essay Topics." March 1, 2022. https://studycorgi.com/ideas/dementia-essay-topics/.
Bibliography
StudyCorgi . "147 Dementia Essay Topics." March 1, 2022. https://studycorgi.com/ideas/dementia-essay-topics/.
StudyCorgi . 2022. "147 Dementia Essay Topics." March 1, 2022. https://studycorgi.com/ideas/dementia-essay-topics/.
These essay examples and topics on Dementia were carefully selected by the StudyCorgi editorial team. They meet our highest standards in terms of grammar, punctuation, style, and fact accuracy. Please ensure you properly reference the materials if you’re using them to write your assignment.
This essay topic collection was updated on January 5, 2024 .
- Research Article
- Open access
- Published: 20 January 2024
Dementia care pathways in prisons – a comprehensive scoping review
- Samantha Treacy 1 ,
- Steven Martin 2 ,
- Nelum Samarutilake 3 ,
- Veronica Phillips 4 ,
- Ben R. Underwood 3 , 5 &
- Tine Van Bortel ORCID: orcid.org/0000-0003-0467-6393 2 , 3
Health & Justice volume 12 , Article number: 2 ( 2024 ) Cite this article
1038 Accesses
1 Altmetric
Metrics details
The number of older people in prison is growing. As a result, there will also be more prisoners suffering from dementia. The support and management of this population is likely to present multiple challenges to the prison system.
To examine the published literature on the care and supervision of people living in prison with dementia and on transitioning into the community; to identify good practice and recommendations that might inform the development of prison dementia care pathways.
A scoping review methodology was adopted with reporting guided by the PRISMA extension for scoping reviews checklist and explanation.
Sixty-seven papers were included. Most of these were from high income countries, with the majority from the United Kingdom ( n = 34), followed by the United States ( n = 15), and Australia ( n = 12). One further paper was from India.
The literature indicated that there were difficulties across the prison system for people with dementia along the pathway from reception to release and resettlement. These touched upon all aspects of prison life and its environment, including health and social care. A lack of resources and national and regional policies were identified as important barriers, although a number of solutions were also identified in the literature, including the development of locally tailored policies and increased collaboration with the voluntary sector.
To our knowledge, this is the most comprehensive and inclusive review of the literature on dementia care pathways in prison to date. It has identified a number of important areas of concern and opportunities for future research across the prison system, and its operations. This will hopefully lead to the identification or adaptation of interventions to be implemented and evaluated, and facilitate the development of dementia care pathways in prisons.
The number of older people (defined here as those over 50 Footnote 1 ) being held in prison in England and Wales has almost tripled over the last 20 years, and they now represent 17.1% of that population (Ministry of Justice, 2022a ). The growing number of older people has brought with it an increasing number of health and social care problems, reportedly affecting around 85% of older people in prison, with associated costs (Di Lorito, et al., 2018 ; Hayes et al., 2012 , 2013 ; Senior, et al., 2013 ). It has been estimated that 8.1% of those over the age of 50 in prison have mild cognitive impairment or dementia, which is much higher than estimates for this age group in the general population (Dunne et al., 2021 ; Forsyth et al., 2020 ). This pattern of poor health also increased the vulnerability of older people in prison during the pandemic (Kay, 2020 ).
Prison policy and legislation mandates that health and social care be ‘equivalent’ to that provided in the community (Care Act, 2014 ; Department of Health, 1999 ). Despite this, provisions are reportedly inconsistent, and the government has been described as ‘failing’ in its duty of care (Health and Social Care Committee, 2018 ; HM Inspectorate of Prisons & Care Quality Commission, 2018 ). This is likely exacerbated by the suspension and limiting of healthcare services during the pandemic, noted to have had a ‘profound’ impact on people’s health and wellbeing (HM Inspectorate of Prisons, 2021 ). This may be particularly so for people living in prison with dementia (PLiPWD), whereby the difficulties of delivering health and social care are compounded by inappropriate buildings, environments, and prison regimes (rules and regulations). In addition, PLiPWDs may experience an increase in social isolation, including separation from friends and family, all of which may make their time in prison more challenging (Moll, 2013 ; Peacock et al., 2019 ).
There is no current national strategy for older people in prison in England and Wales, including PLiPWD, although the British government recently agreed that there is a need for one (Justice Committee, 2020 ). A ‘Model for Operational Delivery’ for older people has been published by Her Majesty's Prison & Probation Service ( 2018 ) in England and Wales, though this is guidance only and the “properly resourced and coordinated strategy” previously called for has not been produced (Prisons & Probation Ombudsman, 2017 , p7; Brooke and Rybacka, 2020 ; HM Inspectorate of Prisons, 2019 ; Justice Committee, 2020 ). One way of attempting to standardise and improve the quality of treatment and care in the community has been through the use of care pathways (Centre for Policy on Ageing, 2014 ; Schrijvers et al., 2012 ). Care pathways have been defined as “a complex intervention for the mutual decision-making and organisation of care processes for a well-defined group of patients during a well-defined period”, involving an articulation of goals and key aspects of evidence-based care, coordination and sequencing of activities and outcomes evaluation (Vanhaecht, et al., 2007 , p137).
The development of care pathways within the prison system lags behind that of the community, but the National Institute for Health and Care Excellence (NICE) has produced a pathway for prisoner health for England and Wales (National Institute for Health and Care Excellence, 2019 ), and there is a care pathway for older prisoners in Wales (Welsh Government & Ministry of Justice, 2011 ). There has also recently been an overall care pathway developed for people in prison with mild cognitive impairment and dementia, although this has not been implemented as yet, and it does not include any details regarding release and resettlement (Forsyth et al, 2020 ). It has been recommended that care pathways should be developed locally, as they are context-sensitive, should be viewed as processual and flexible, and the needs of the person, their experiences and characteristics need to be taken into account – such as age, gender and race (Centre for Policy on Ageing, 2014 ; Pinder, et al., 2005 ).
Here we review the current literature on people living in prison with dementia. There have been two recent systematic literature reviews conducted on PLiPWD, both of which only included primary research studies that were small in number (Brooke and Rybacka, 2020 ( n = 10); Peacock et al., 2019 ( n = 8)), and focused on prevalence, identification (screening and diagnosis), and the need for tailored programming and staff training. Peacock et al., ( 2019 ) identified dementia as a concern and suggested recommendations for improved screening and care practices. Brooke et al. ( 2020 ) noted that, whilst the prevalence of dementia in prison populations was largely unknown, there was a need for national policies and local strategies that support a multi-disciplinary approach to early detection, screening and diagnosis. Neither paper, however, reported on the much more extensive and rich grey literature in this area (Brooke and Rybacka, 2020 ), to help comprehensively identify the systemic and operational problems, barriers and potential solutions that would be useful to consider in developing local dementia care pathways. Therefore, the aim of this paper is to conduct a comprehensive systematic scoping review of the available published literature on the support and management of PLiPWD in prison and upon transitioning into the community, and to identify practice and recommendations that would be useful to consider in the development of a local prison dementia care pathway.
A scoping review methodology using Arksey and O’Malley’s ( 2005 ) five-stage framework was adopted for this review. Reporting was guided by the PRISMA extension for scoping reviews checklist and explanation (Tricco et al., 2018 ). The completed checklist for this review is available in Additional file 1 : Appendix 1.
Identification of relevant reports
The search strategy was formulated by the research team, and included an electronic database search and subsequent hand search. The electronic search involved searching twelve electronic databases: Applied Social Sciences Index and Abstract, Criminal Justice Abstracts, Embase, Medline (OVID), National Criminal Justice Reference Service, Open Grey, Psycinfo, Pubmed, Scopus, Social Services Abstracts, Sociological Abstracts, and Web of Science. The search combined condition-related terms (dementia OR Alzheimer*) AND context-related ones (prison OR jail OR gaol OR penitentia* OR penal OR correctional* OR incarcerat*), with no date or language restrictions, and covered the full range of publications up until April 2022. Additional file 2 : Appendix 2 has an example of the search strategy used.
Electronic searches were supplemented by comprehensive hand searching and reference mining. Searches were also undertaken using: search engines; websites related to prisons and/or dementia (for example, Prison Reform Trust); a database from a previous related literature review (Lee et al, 2019 ); recommendations from academic networking sites; contacting prominent authors in the field directly; government-related websites (for example Public Health England, now called Health Security Agency); recent inspection reports for all prisons in England and Wales from Her Majesty’s Inspectorate of Prisons and the Independent Monitoring Board.
Inclusion and exclusion criteria
Papers were considered suitable for inclusion in this review if they met the following criteria:
Setting: Papers should primarily be set in, or pertain to, prisons. Documents solely referring to community services, hospitals or medical facilities that are not part of the prison system were excluded.
People: Papers involving PLiPWD. Research focused only on older people in prison more generally was excluded, as was research which described the disorienting effects of imprisonment more generally, but which was not related to dementia.
Intervention: Some consideration of the treatment, care, support or management of PLiPWD; this can be health or social-care associated, as well as related to the prison overall, and to any individuals, groups or agencies who visit or work with individuals during their time in prison (including family, friends, charities, probation services). Papers which mostly describe prevalence studies, sentencing practices or profiles were excluded.
Study design: All designs were considered for inclusion. Editorials, book reviews, online blogs, press releases, announcements, summaries, newspaper and magazine articles, abstracts and letters were excluded.
The titles, abstracts and full-text of the papers identified by the searches were screened for inclusion in the review. The screening was undertaken by two independent researchers (ST and NS) for inter-rater reliability purposes (Rutter et al., 2010 ). Any differences of opinion on inclusion were resolved between the researchers (ST, NS and SM), and with the Principle Investigator (TVB).
Charting the data
An extraction template was developed for the review, guided by the PICO formula (Richardson et al., 1995 ) and informed by pathway stages and key areas highlighted in the older prisoner pathways toolkit for England and Wales (Department of Health, 2007 ), and the older prisoner pathway formulated for Wales (Welsh Government & Ministry of Justice, 2011 ). Using this extraction template, all of the data was extracted from the included papers by one member of the research team (ST), with a second researcher extracting data from a third of the papers as a check for consistency (SM). Any unresolved issues were related to the Principle Investigator (TVB) for resolution.
Collating, summarising and reporting results
The review was deliberately inclusive of a wide variety of types of papers, which meant that taking a meta-analytic approach to the data was not feasible. Therefore, a narrative approach to summarising and synthesising the findings and recommendations of the included papers was adopted (Popay et al, 2006 ).
Sixty-seven papers were included in this scoping review. The screening process phases conducted by the research team are shown in Fig. 1 .
PRISMA flow diagram
A brief overview of the key features of each of the papers is presented in Table 1 . All but one of the included papers were from high income countries, with the majority from the United Kingdom ( n = 34), and then the United States ( n = 15), Australia ( n = 12), Canada ( n = 4), Italy ( n = 1) and India ( n = 1). The papers were split into types, with twenty-two guidance and inspection documents, and twenty-seven discussion and intervention description papers. Of the eighteen research and review articles with a defined methodology included there were four literature reviews (one was systematic), nine qualitative studies, four mixed-methods studies (one which followed participants up), and one survey-based study.
Areas to consider in the support and management of PLiPWD during their time in prison and upon their release
The pathway through the prison is shown in Fig. 2 , and typically involves: (i) reception into prison; (ii) assessments, and allocation of the person within prison; (iii) time held in prison; (iv) transfers between prisons, and between prisons and other services such as time spent in hospital; and (v) release and preparations for resettlement in the community. There were also a number of (vi) cross-cutting themes which could potentially impact people with dementia living in prison at each stage across the prison pathway.
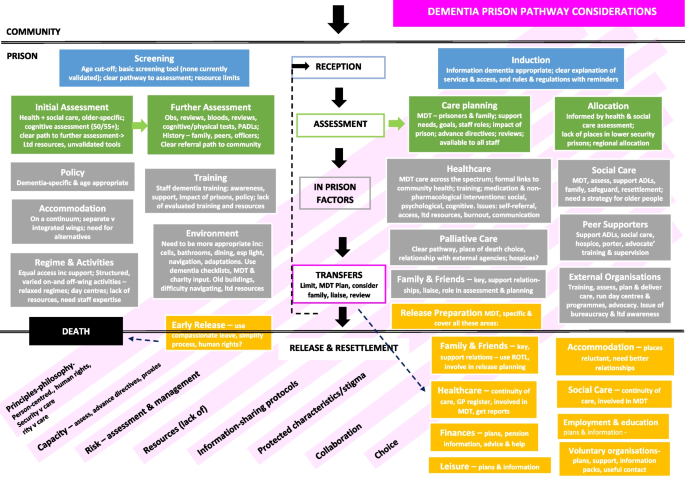
Dementia prison pathway considerations
(i) Reception
Upon entry into prison, prisoners are subject to an initial reception screening to identify and support immediate health and social care problems, and those in need of further assessment. An induction to prison rules and regulations also typically occurs at this step.
All papers reported that reception screening with appropriate screening tools was important in identifying cognitive difficulties and in establishing a baseline, but implementation seemed to vary (Peacock et al., 2019 ). One study in England and Wales found only 30% of prisons contacted routinely did this (Forsyth et al., 2020 ). Supporting policy and a service/person to refer to directly for further assessment were also highlighted as useful (Brooke & Jackson, 2019 ; Brooke et al., 2018 ; Gaston & Axford, 2018 ; Inspector of Custodial Services, 2015 ; Patterson et al., 2016 ). Proposed cut-offs for this screening were either 50 years of age ( n = 7), under 55 years ( n = 1), or 55 years of age ( n = 7). One paper reported that only a third of prisoners who were offered this screening accepted it, although the reasons for this were not stated (Patel & Bonner, 2016 ). Another paper suggested that a screening programme could have unintended adverse consequences, that could damage already fragile relationships between staff and people living in prison (Moore & Burtonwood, 2019 ). Whilst many screening tools were mentioned, there are currently no tools validated for use in prisons, and many of those used in the community may be inappropriate (Baldwin & Leete, 2012 ; Brooke et al., 2018 ; du Toit et al., 2019 ; Feczko, 2014 ; Forsyth et al., 2020 ; Moore & Burtonwood, 2019 ; National Institute for Health and Care Excellence, 2017 ; Turner, 2018 ; Williams et al., 2012 ). One validation study found that the Six-item Cognitive Impairment Test (6CIT) was not suitably sensitive for use (Forsyth et al., 2020 ). Other difficulties included the limited amount of time and resources available to screen at reception (Christodoulou, 2012 ; Patterson et al., 2016 ; Peacock et al., 2019 ), and that staff lacked ‘familiarity’ with screening tools (Peacock et al., 2019 ).
Only two papers mentioned the induction process (Her Majesty's Prison & Probation Service, 2018 ; Welsh Government and Ministry of Justice, 2011 ) as important. A need for clearly explained information in a dementia-appropriate format (written and verbal) particularly regarding healthcare, and a recommendation that PLiPWD should be regularly reminded of rules and regulations, were suggested.
(ii) Assessment
Following the screening process, the current recommendation is that an initial healthcare assessment takes place in the first seven days after entering prison. During this initial assessment period, although not necessarily within this timeframe, care plans and allocation decisions may also be made regarding where the prisoner is placed within the prison.
An initial older-person-specific health and/or social care assessment or standard process for assessment has been recommended by ten papers, six of which were from government or related bodies. It was also suggested by some papers, that a cognitive assessment should take place at either 50 years ( n = 6) or 55 years ( n = 2), which should be repeated every three months ( n = 3), six months ( n = 5) or annually ( n = 12), with the latter including recommendations from NICE guidelines (National Institute for Health and Care Excellence, 2017 ). One study set in England and Wales found that most prisons (60%) that screened older people, did so between 7–12 months (Forsyth et al., 2020 ). Brief and affordable tools were considered more useful (Garavito, 2020 ; Turner, 2018 ), although the Montreal Cognitive Assessment (MOCA) was recommended in the care pathway developed by Forsyth et al. ( 2020 ).
Typically, assessments were conducted by healthcare staff, GPs or a psychologist ( n = 6), a specialist in-house assessment unit ( n = 2), or a specific dementia admissions assessment unit ( n = 4). For further assessment, some prisons had internal teams to refer to ( n = 5). Forsyth et al. ( 2020 ) recommend referral to external Memory Assessment Services for assessment. A case finding tool was being piloted in one prison (Sindano & Swapp, 2019 ). Assessments included can be found in Table 2 .
Assessments also explored risk and safeguarding (National Institute for Health and Care Excellence, 2017 ; Patterson et al., 2016 ; Welsh Government and Ministry of Justice, 2011 ), environmental impact (National Institute for Health and Care Excellence, 2017 ), capacity (Prison & Probation Ombudsman, 2016 ), work, education, and drug and alcohol use (Welsh Government and Ministry of Justice, 2011 ) and a person’s strengths (Hamada, 2015 ; National Institute for Health and Care Excellence, 2017 ). Prison staff contributed to some assessments of activities of daily living (ADLs) or prison-modified ADLs (Brooke et al., 2018 ; Brown, 2016 ; Dillon et al., 2019 ; Department of Health, 2007 ; Feczko, 2014 ; Forsyth et al., 2020 ; Gaston, 2018 ; Gaston & Axford, 2018 ; Patterson et al., 2016 ; Turner, 2018 ; Welsh Government and Ministry of Justice, 2011 ; Williams et al., 2012 ). Challenges to Assessment can be found in Table 3 .
Twelve papers described or recommended care planning post-assessment, in collaboration with PLiPWD and primary care, or a multi-disciplinary team (MDT) of health, social care and prison staff with external specialists healthcare proxies charities or family (Brown, 2016 ; Dillon et al., 2019 ; du Toit & Ng, 2022 ; Hamada, 2015 ; Her Majesty's Inspectorate of Prisons, 2014 ; Her Majesty's Prison & Probation Service, 2018 ; Moll, 2013 ; National Institute for Health and Care Excellence, 2017 ; Patterson et al., 2016 ; Prisons and Probation Ombudsman, 2016 ; Welsh Government and Ministry of Justice, 2011 ). However, it was suggested that prison staff be removed from the decision-making process as the dementia progresses, and be part of the ‘duty of care’ of healthcare staff and services (du Toit & Ng, 2022 ). It was recommended too that care plans be disseminated to prison wing staff (Forsyth et al., 2020 ) and peer supporters (Goulding, 2013 ), and that consent be sought for this (Goulding, 2013 ; Her Majesty's Inspectorate of Prisons, 2014 ) An ombudsman report in England and Wales noted that care plans for PLiPWD who had died in prison were inadequate (Peacock et al., 2018 ), and of the varying degrees of care planning found by Forsyth et al ( 2020 ), it was described typically as “rudimentary” (p26). Care plans are described further in Table 4 .
Many papers reported that prisons did or should make decisions about where people should be accommodated within the prison after health assessments (Brown, 2016 ; Feczko, 2014 ; Forsyth et al., 2020 ; Hodel & Sanchez, 2013 ; Inspector of Custodial Services, 2015 ; Mistry & Muhammad, 2015 ; Turner, 2018 ; Welsh Government and Ministry of Justice, 2011 ; Williams et al., 2012 ), taking age and health into account. However, despite recommendations that PLiPWD should be placed on the ground floor on low bunks for instance (Baldwin & Leete, 2012 ; Department of Health, 2007 ; Welsh Government and Ministry of Justice, 2011 ), there were reports that this was not happening (Inspector of Custodial Services, 2015 ). There were also recommendations for allocations to be made across a region to ensure people are appropriately placed in the prison system (Baldwin & Leete, 2012 ; Booth, 2016 ; Gaston & Axford, 2018 ; Welsh Government and Ministry of Justice, 2011 ). Concerns were expressed about the lack of lower category places for PLiPWD (Department of Health, 2007 ), and the lack of guidance regarding placement of people with high support needs (Sindano & Swapp, 2019 ) in England and Wales.
(iii) Within-prison issues
A number of papers reported on a need for policies or frameworks to support staff to identify, assess and support people who may be living with dementia (Brooke et al., 2018 ; Brooke & Jackson, 2019 ; Department of Health, 2007 ; Feczko, 2014 ; Gaston, 2018 ; Gaston & Axford, 2018 ; Patterson et al., 2016 ; Turner, 2018 ; Welsh Government and Ministry of Justice, 2011 ), without which staff have faced difficulties in providing quality care and support (Feczko, 2014 ; Prisons and Probation Ombudsman, 2016 ). Whilst there were some examples of guidance for dementia (Hamada, 2015 ; Patterson et al., 2016 ; Treacy et al., 2019 ; Turner, 2018 ), it was suggested that all policies should be reviewed and amended to ensure that they are appropriate for older people and people living with dementia (Department of Health, 2007 ; Lee et al., 2019 ; Treacy et al., 2019 ). Specific policy areas are described in Table 5 .
Issues around staff training on dementia were discussed in the majority of papers ( n = 54) Many of these reported that prison staff either lacked training on dementia, or that training was limited ( n = 16), with one study in England and Wales reporting that only a quarter of prison staff had received such training (Forsyth et al., 2020 ). Perhaps consequently, a number of papers identified that prison staff required some dementia training ( n = 19). Staff working on a specialist dementia unit reportedly had a comprehensive 40-h training (Brown, 2014 , 2016 ; Gaston & Axford, 2018 ; Hodel & Sanchez, 2013 ; Moll, 2013 ), and it was suggested that more comprehensive training be facilitated for officers, particularly those working with PLiPWD ( n = 18) and offender managers ( n = 2). A need for all staff working with PLiPWD to be supervised was also suggested (Gaston & Axford, 2018 ; Maschi et al., 2012 ). Despite a lack of consensus on content and duration (du Toit et al, 2019 ), typically, the staff training undertaken and recommended was in four areas (Table 6 ). It was also recommended that training for healthcare could be more comprehensive and focused on screening, identification, assessment, diagnoses, supervision and intervention training (Baldwin & Leete, 2012 ; Brooke & Jackson, 2019 ; Brown, 2014 ; Gaston & Axford, 2018 ; Her Majesty's Inspectorate of Prisons, 2014 ; Moll, 2013 ; Moore & Burtonwood, 2019 ; National Institute for Health and Care Excellence, 2017 ; Peacock et al, 2019 ; Treacy et al, 2019 ; Turner, 2018 ; Williams, 2014 ). It is of note that only 21% of healthcare staff in one study in England and Wales reported attending training to identify dementia (Forsyth et al., 2020 ), similar to the figures regarding prison staff in the same study.
Much of the training described in the included papers had been formulated and delivered by dementia- or older people-specific voluntary organisations (Alzheimer’s Society, 2018 ; Brooke et al. 2018 ; Brown, 2016 ; Gaston & Axford, 2018 ; HMP Hull, 2015 ; Her Majesty's Prison & Probation Service, 2018 ; Hodel & Sanchez, 2013 ; Moll, 2013 ; Peacock et al., 2018 ; Prisons and Probation Ombudsman, 2016 ; Sindano & Swapp, 2019 ; Tilsed, 2019 ; Treacy et al., 2019 ). Although it has also been recommended to involve health and social care (Goulding, 2013 ; Her Majesty's Prison & Probation Service, 2018 ; Ministry of Justice, 2013 ; Treacy et al., 2019 ; Turner, 2018 ), and officers and peer supporters (Brooke & Jackson, 2019 ; Masters et al., 2016 ; National Institute for Health and Care Excellence, 2017 ; Treacy et al., 2019 ) in developing the training. In one study, prison staff were also trained to deliver dementia information sessions to their peers (Treacy et al., 2019 ). A suggestion of video-training packages was also made (du Toit et al., 2019 ). Dementia training typically lacked robust evaluation (Brooke et al., 2018 ), although those available generally reported benefits in their understanding of dementia, relationships, and diagnoses (Goulding, 2013 ; HMP Littlehey, 2016 ; Masters et al., 2016 ; Sindano & Swapp, 2019 ; Treacy et al., 2019 ). It was also reported that some prison staff were resistant to working with PLiPWD (Moll, 2013 ), and that resource limitations resulted in training cuts (HMP Hull, 2015 ; Treacy et al., 2019 ).
Offering healthcare across the spectrum for PLiPWDs, from acute to chronic care, with a focus on preventative and long-term care as well as palliative care was recommended by some papers (Brown, 2014 ; du Toit & Ng, 2022 ; Gaston, 2018 ; Maschi et al., 2012 ; Mistry & Muhammad, 2015 ; Peacock et al, 2018 ; Welsh Government and Ministry of Justice, 2011 ; Williams et al., 2012 ). The development of care pathways to guide this were also recommended or formulated (du Toit et al., 2019 ; Forsyth et al., 2020 ; Peacock et al., 2019 ), although the majority (69%) of prisons in one study in England and Wales did not have one (Forsyth et al., 2020 ). Clear and formal links with local hospitals, memory clinics, forensic and community teams for planning, training, advice, support and in-reach were also present or recommended by sixteen research and guidance papers. The amount of healthcare cover in prisons in England and Wales reportedly varied with the function of the prison with largely only local prisons having 24-h healthcare staff (Treacy et al., 2019 ), and most other forms of prison having office-type hours’ healthcare cover – including sex offender prisons where the majority of older prisoners are held (Brown, 2016 ; Correctional Investigator Canada, 2019 ; Goulding, 2013 ; Inspector of Custodial Services, 2015 ; Treacy et al., 2019 ). While specialist services or units for PLiPWD exist in a number of jurisdictions (Baldwin & Leete, 2012 ; Brown, 2016 ; Cipriani et al., 2017 ; Gaston & Axford, 2018 ; Goulding, 2013 ; Hodel & Sanchez, 2013 ; Inspector of Custodial Services, 2015 ; Maschi et al., 2012 ; Mistry & Muhammad, 2015 ; Treacy et al, 2019 ), more are reportedly needed (Brooke et al., 2018 ; du Toit et al., 2019 ; Forsyth et al., 2020 ; Welsh Government and Ministry of Justice, 2011 ).
Most healthcare teams were reportedly MDT, or this was recommended, alongside joint health and social care working ( n = 16). A number of healthcare staff acted as the lead for older people in prisons (Department of Health, 2007 ; Her Majesty's Inspectorate of Prisons, 2014 ; Her Majesty's Inspectorate of Prisons, 2016 ; Moll, 2013 ; Welsh Government and Ministry of Justice, 2011 ), with a recommendation that a dementia-trained nurse should lead any dementia care pathways (Forsyth et al., 2020 ) and indeed it was suggested that healthcare staff in general have training and experience in working with older people (Her Majesty's Inspectorate of Prisons, 2014 ; Her Majesty's Inspectorate of Prisons, 2017b ; Moll, 2013 ; Patterson et al., 2016 ; Public Health England, 2017b ; Treacy et al., 2019 ; Turner, 2018 ; Welsh Government and Ministry of Justice, 2011 ). Whilst one of the recommended roles for healthcare was the prescription and monitoring of medication (Feczko, 2014 ; Her Majesty's Inspectorate of Prisons, 2017b ; Moll, 2013 ), much of the focus was on early identification and diagnosis, and keeping a dementia register (Department of Health, 2007 ; Moll, 2013 ; Patterson et al., 2016 ; Welsh Government and Ministry of Justice, 2011 ), and the use of non-pharmacological approaches. These broadly included: psychological interventions (Goulding, 2013 ; Hamada, 2015 ; Moll, 2013 ; Wilson & Barboza, 2010 ); assistance with ADLs and social care (Feczko, 2014 ; Hamada, 2015 ; Hodel & Sanchez, 2013 ; Maschi, et al., 2012 ; Murray, 2004 ; Prisons and Probation Ombudsman, 2016 ); development and delivery of specialist dementia prison programmes (Brown, 2014 , 2016 ; Hodel & Sanchez, 2013 ; Mistry & Muhammad, 2015 ; Moll, 2013 ; Peacock et al., 2018 ; Wilson & Barboza, 2010 ); reablement and rehabilitation (Welsh Government and Ministry of Justice, 2011 ); relaxation (Wilson & Barboza, 2010 ); safeguarding (Hodel & Sanchez, 2013 ); and cognitive stimulation groups (Moll, 2013 ; Williams, 2014 ). Other possible roles included: training or supporting staff and peer supporters, as reported in fourteen papers, as well as advocacy (Feczko, 2014 ; Peacock et al., 2018 ; Welsh Government and Ministry of Justice, 2011 ), allocation, assessment for offending behaviour groups, risk assessments and disciplinary hearings (Booth, 2016 ; Department of Health, 2007 ; Her Majesty's Prison & Probation Service, 2018 ; Murray, 2004 ; Prisons and Probation Ombudsman, 2016 ). Challenges to Healthcare are noted in Table 7 .
Palliative care
A care pathway for dying people that meets community standards was recommended (Department of Health, 2007 ; Her Majesty's Prison & Probation Service, 2018 ; Welsh Government and Ministry of Justice, 2011 ), as was ensuring that people could choose a preferred place to die (Her Majesty's Prison & Probation Service, 2018 ). Some prisoners were moved to community hospices or hospitals (Brooke & Jackson, 2019 ; Inspector of Custodial Services, 2015 ), or it was felt that they should be (Her Majesty's Prison & Probation Service, 2018 ). Although it was noted that some prisons lack relationships with community hospices or palliative care services and need to foster them (Brooke & Jackson, 2019 ; Brown, 2016 ; Correctional Investigator Canada, 2019 ; Department of Health, 2007 ; Her Majesty's Prison & Probation Service, 2018 ).
A number of prisons also reportedly had hospices, particularly in the United States (Brooke et al., 2018 ; Brown, 2016 ; Feczko, 2014 ; Goulding, 2013 ; Williams et al., 2012 ), although these have not been comprehensively evaluated (Williams et al., 2012 ). It was recommended that these be staffed by MDTs (Her Majesty's Prison & Probation Service, 2018 ), including chaplains and nutritionists (Her Majesty's Prison & Probation Service, 2018 ; Goulding, 2013 ), and many included prisoner peer supporters (Brooke et al., 2018 ; Goulding, 2013 ). The use of independent contractors was also suggested as staff-prisoner relationships were considered problematic in some prisons (Williams et al., 2012 ). Regarding family, many hospices were described as allowing more visits (Brooke & Jackson, 2019 ; Goulding, 2013 ; Her Majesty's Prison & Probation Service, 2018 ), including one prison with family accommodation (Her Majesty's Prison & Probation Service, 2018 ). Whilst re-engaging with family was reportedly encouraged (Brown, 2016 ), a lack of support was noted (Correctional Investigator Canada, 2019 ). Suggested improvements include a family liaison officer, providing a list of counselling options, and hosting memorial services (Her Majesty's Prison & Probation Service, 2018 ).
Social care
A social care strategy for older prisoners and a social care lead for all prisons in England and Wales has been recommended (Department of Health, 2007 ; Prisons and Probation Ombudsman, 2016 ). It was reported that MDTs working with PLiPWD should and increasingly do include social workers including specialist units and hospices (Baldwin & Leete, 2012 ; Brooke et al., 2018 ; Brown, 2016 ; Cipriani et al., 2017 ; Goulding, 2013 ; HMP Littlehey, 2016 ; Her Majesty's Prison & Probation Service, 2018 ; Maschi et al., 2012 ; Prisons and Probation Ombudsman, 2016 ; Sindano & Swapp, 2019 ; Treacy et al., 2019 ; Welsh Government and Ministry of Justice, 2011 ). Social care roles can be found in Table 8 .
The work may be direct or may be through co-ordinating external agencies or peer supporters (Brooke & Jackson, 2019 ; Department of Health, 2007 ; Her Majesty's Prison & Probation Service, 2018 ; Prisons and Probation Ombudsman, 2016 ; Tilsed, 2019 ; Treacy et al., 2019 ; Welsh Government and Ministry of Justice, 2011 ). Clarity in these roles was considered paramount, particularly as uncertainty reportedly continues to exist over who is responsible for meeting prisoners’ social care needs in some prisons in England and Wales despite the passing of the Care Act, 2014 (Dementia Action Alliance, 2017 ; Tilsed, 2019 ; Welsh Government and Ministry of Justice, 2011 ). There was also some ambiguity around the threshold PLiPWD were expected to meet in order to access social care (Forsyth et al., 2020 ). In some instances, personal care was delivered informally by untrained and unsupported prison staff and peer supporters in lieu of suitably trained social care workers (Treacy et al., 2019 ), with issues raised about the unavailability of social care through the night (Forsyth et al., 2020 ). Where social care staff were involved in coordinating personal care for prisoners, it was reported as positive for prisoners and prison staff (Her Majesty's Inspectorate of Prisons, 2016 ; Treacy et al., 2019 ), particularly, in one prison, where social care staff were prison-based (Forsyth et al., 2020 ).
Peer supporters
Prisoner peer supporters were operating in a number of prisons, as reported in 22 papers, and their employment was recommended by a further fourteen. Typically, these were people who had ‘good’ disciplinary and mental health records, and certainly in the US, were longer-serving prisoners. A number of papers indicated the need for peer supporters to receive training in dementia, including awareness and support (Brooke et al., 2018 ; Brooke & Jackson, 2019 ; Brown, 2016 ; Correctional Investigator Canada, 2019 ; Department of Health, 2007 ; Dillon et al., 2019 ; du Toit & Ng, 2022 ; Gaston, 2018 ; Gaston & Axford, 2018 ; Goulding, 2013 ; HMP Hull, 2015 ; HMP Littlehey, 2016 ; Her Majesty's Prison & Probation Service, 2018 ; Inspector of Custodial Services, 2015 ; Maschi et al., 2012 ; Mistry & Muhammad, 2015 ; Sindano & Swapp, 2019 ; Tilsed, 2019 ; Treacy et al., 2019 ). Comprehensive 36–40 h training on dementia was delivered for those working on specialist units, including one leading to a qualification (Brooke & Jackson, 2019 ; Brown, 2016 ; Gaston & Axford, 2018 ; Her Majesty's Prison & Probation Service, 2018 ; Moll, 2013 ). Much of the training was developed and delivered by charities, particularly dementia-related ones, as reported in eleven papers. Ongoing support and supervision was offered or recommended by some prisons, provided largely by health or social care staff or charities (Brooke & Jackson, 2019 ; Brown, 2016 ; Correctional Investigator Canada, 2019 ; Department of Health, 2007 ; Her Majesty's Prison & Probation Service, 2018 ; Gaston & Axford, 2018 ; Maschi et al., 2012 ; Prisons and Probation Ombudsman, 2016 ; Sindano & Swapp, 2019 ; Treacy et al., 2019 ), with informal peer-to-peer support also described (Brown, 2016 ; Gaston & Axford, 2018 ; Treacy et al., 2019 ). The support and supervision received was found to be valuable (Brooke & Jackson, 2019 ; Brown, 2016 ; Treacy et al., 2019 ). Peer-supporter roles are listed in Table 9 .
A number of benefits to: (a) the peer supporters, (b) the prisoners they supported and, (c) the prison, were described, although formal evaluations were lacking (Brown, 2016 ; Christodoulou, 2012 ; Department of Health, 2007 ; du Toit et al., 2019 ; Gaston, 2018 ; Gaston & Axford, 2018 ; Goulding, 2013 ; Treacy et al., 2019 ; Welsh Government and Ministry of Justice, 2011 ). This included: payment, development of skills which could be used on release, positive impact on progression through the system, and on self-confidence and compassion, and the creation of a more humane environment. However, frustration and distress amongst peer supporters largely when untrained and unsupported was also reported (Brooke & Jackson, 2019 ; Brown, 2016 ; Correctional Investigator Canada, 2019 ; Inspector of Custodial Services, 2015 ; Prisons and Probation Ombudsman, 2016 ; Treacy et al., 2019 ), and concerns raised in relation to an over-reliance on peers to do work that it is the statutory duty of health and social care to provide (Prisons and Probation Ombudsman, 2016 ; Treacy et al., 2019 ). This was a particular problem in light of personal care being prohibited for peer supporters in England and Wales (Her Majesty's Prison & Probation Service, 2018 ; Moll, 2013 ). It is also of note that the role of peer supporter may also attract the opprobrium of other prisoners, with reports that they have been seen as ‘snitches’ or ‘dogs’ in some areas (Brown, 2016 ; Goulding, 2013 ). In addition, in some prisons, the peer supporter role was not advocated due to: fear of litigation; fear of replacing staff with peers; belief that people should be acquiring more transferable skills, since many would be unable to undertake care work in the community due to their offence history (Brown, 2016 ; Goulding, 2013 ).
Accommodation
There were mixed views regarding accommodation for PLiPWD. A continuum of prison accommodation was suggested from independent to 24-h care (including assisted living) (Forsyth et al., 2020 ; Gaston & Axford, 2018 ; Williams et al., 2012 ). A number of papers ( n = 18) recommended that there should be some form of alternative, more appropriate accommodation developed, potentially regional, including secure facilities possibly with a palliative orientation (Hodel & Sanchez, 2013 ; Mistry & Muhammad, 2015 ; Sfera et al., 2014 ). However, there were concerns about the availability, costs and staffing of specialist units, and distances that family would have to travel to visit despite potential benefits (du Toit et al., 2019 ; Moore & Burtonwood, 2019 ). It was also suggested that PLiPWD should be released to live in the community instead (Correctional Investigator Canada, 2019 ).
Within prisons, there was a debate evident within the papers about whether PLiPWD should be accommodated in separate units or integrated within the general prison population, which had generated little clear evidence and mixed views (Brooke & Jackson, 2019 ; Dillon et al., 2019 ; Her Majesty's Prison & Probation Service, 2018 ; Treacy et al., 2019 ). Authors have suggested that specialist or separate wings focused on older people or those with dementia were safer, met peoples’ needs better, and offered better care, support and programmes than integrated units (Brown, 2014 ; Dillon et al., 2019 ; du Toit & Ng, 2022 ; du Toit et al., 2019 ; Goulding, 2013 ; Maschi et al., 2012 ; Murray, 2004 ; Treacy et al., 2019 ; Williams et al., 2012 ), as long as they were ‘opt-in’ for prisoners and staff (Correctional Investigator Canada, 2019 ; Moll, 2013 ; Treacy et al., 2019 ; Williams et al., 2012 ), and opportunities to get off the wing to socialise with others are provided (Treacy et al., 2019 ). The types of ‘specialist’ accommodation that PLiPWD were living in are reported in Table 10 . It is of note that papers reported a highly limited number of beds available in specialist units (Inspector of Custodial Services, 2015 ; Patterson et al., 2016 ; Turner, 2018 ), and that a number of older prisoner-specific prisons were being closed due to costs (Turner, 2018 ).
Four papers described the benefits of older people and those PLiPWD residing within the general prison population (Dillon et al., 2019 ; Her Majesty's Prison & Probation Service, 2018 ; Treacy et al., 2019 ; Williams et al., 2012 ). Those living with dementia reported a benefit from socialising with, and being cared for by, younger people (Dillon et al., 2019 ; Her Majesty's Prison & Probation Service, 2018 ; Williams et al., 2012 ). The presence of older people also reportedly calmed younger prisoners (Dillon et al., 2019 ; Her Majesty's Prison & Probation Service, 2018 ; Williams et al., 2012 ). Importantly, removing people from their prison social networks may have a detrimental effect (Williams et al., 2012 ), and living on specialist units can be stigmatising (Treacy et al., 2019 ).
Regime and activities
The maintenance of prisons regimes is the primary focus of prison officers (Brooke & Jackson, 2019 ). However, there was a reported need ( n = 19) for PLiPWD to have equal access to activities and services including work, education, gym, library and day centres where they exist, as well as a structured and varied regime on the wing on which they were accommodated, and support to access these. This support could include providing adequate seating (Welsh Government and Ministry of Justice, 2011 ), or giving prisoners more time to accomplish activities, and to assist if needed (Brooke & Jackson, 2019 ; Goulding, 2013 ; Her Majesty's Prison & Probation Service, 2018 ; Hodel & Sanchez, 2013 ). Other recommendations included an overall relaxation of regimes (Gaston & Axford, 2018 ; Treacy et al., 2019 ), an ‘open door’ policy (Brown, 2016 ; Cipriani et al., 2017 ; Goulding, 2013 ; Her Majesty's Inspectorate of Prisons, 2014 ; Her Majesty's Inspectorate of Prisons, 2017b ; Her Majesty's Prison & Probation Service, 2018 ; Treacy et al., 2019 ), more visible staff (The King's Fund, 2013 ), and creating a more communal social environment (Christodoulou, 2012 ). On-wing social activities are described in Table 11 .
Having on-wing work available or alternative means for prisoners who are unable to work to make money was also reportedly important (Christodoulou, 2012 ; Department of Health, 2007 ; Gaston, 2018 ; Gaston and Axford, 2018 ; Her Majesty's Inspectorate of Prisons, 2014 , 2016 , 2017b ; Her Majesty's Prison & Probation Service, 2018 ; Moll, 2013 ; Murray, 2004 ; Treacy et al., 2019 ; Welsh Government and Ministry of Justice, 2011 ). It was suggested that people with dementia should have the chance to work if wanted, and adaptations could be made to work programmes or working days made shorter to facilitate this. Some prisons had specific roles which involved lighter, simple, repetitive tasks such as gardening (Baldwin & Leete, 2012 ; Brooke & Jackson, 2019 ; Inspector of Custodial Services, 2015 ; Moll, 2013 ; Treacy et al., 2019 ). Day centres existed in some prisons, or were thought to be feasible (Department of Health, 2007 ; Her Majesty's Prison & Probation Service, 2018 ; Moll, 2013 ; Treacy et al., 2019 ; Welsh Government and Ministry of Justice, 2011 ), and it was suggested that attendance at these could constitute meaningful paid activity (Her Majesty's Prison & Probation Service, 2018 ). The centres were largely developed and facilitated by charities, and ran a wide variety of social, therapeutic, recreational, arts and advice-centred activities (Her Majesty's Prison & Probation Service, 2018 ; Moll, 2013 ).
Equal access to educational activities, including rehabilitation and offending behaviour programmes, was highlighted as important, particularly where attendance is needed to facilitate people’s progression through the system (Booth, 2016 ; Brooke & Jackson, 2019 ; Dillon et al., 2019 ; Department of Health, 2007 ; Her Majesty's Prison & Probation Service, 2018 ). Some prisons provided, or felt there was a need for, particular educational activities for PLiPWD and adaptations may be, or have been, made to learning materials and equipment, content and pace (Brooke & Jackson, 2019 ; Department of Health, 2007 ; Gaston, 2018 ; Gaston & Axford, 2018 ; Her Majesty's Prison & Probation Service, 2018 ; Treacy et al., 2019 ; Welsh Government and Ministry of Justice, 2011 ). Dedicated library sessions have been designated in some prisons, and some libraries can and do stock specialist resources including books, audiobooks, reminiscence packs and archives of local photos, music and DVDs (Department of Health, 2007 ; Her Majesty's Prison & Probation Service, 2018 ;Treacy et al., 2019 ; Williams, 2014 ). Educational materials could and have been available between sessions to aid memory with distance learning also possible (Brooke & Jackson, 2019 ; Her Majesty's Prison & Probation Service, 2018 ). Suggestions for alternatives for PLiPWD focused on activity and stimulation (du Toit & Ng, 2022 ; Gaston, 2018 ; Her Majesty's Prison & Probation Service, 2018 ), preparing for retirement classes (Department of Health, 2007 ), health promotion (Brooke et al., 2018 ; Christodoulou, 2012 ; Gaston & Axford, 2018 ; Her Majesty's Prison & Probation Service, 2018 ; Maschiet al., 2012 ; Murray, 2004 ; Welsh Government and Ministry of Justice, 2011 ), the arts (Brooke & Jackson, 2019 ) and IT classes (Her Majesty's Prison & Probation Service, 2018 ). Prisoner forums or representative could also be consulted regarding regimes and activities (Moll, 2013 ; Her Majesty's Prison & Probation Service, 2018 ; Welsh Government and Ministry of Justice, 2011 ). Challenges to regimen and activities are described in Table 12 .
Environment
A large number ( n = 42) of the included papers discussed changes that prisons had made, or should make, to the built environment in order to be more suitable for PLiPWD – in one study in England and Wales, around half of prisons surveyed had made such environmental modifications (Forsyth et al., 2020 ). These focused on: (i) prisoners’ cells, (ii) bathrooms, (iii) dining hall, (iv) outside space and recreation areas, and (v) overall general prison environment (Table 13 ).
Problematically, the age and dementia-inappropriateness of buildings are considered a challenge (Baldwin & Leete, 2012 ; Brown, 2016 ; Dementia Action Alliance, 2017 ; Forsyth et al., 2020 ; Goulding, 2013 ; Inspector of Custodial Services, 2015 ; Mistry & Muhammad, 2015 ; Prisons and Probation Ombudsman, 2016 ; Treacy et al., 2019 ). Difficulties in navigating prisons where everywhere looks the same (Dementia Action Alliance, 2017 ; Murray, 2004 ; Treacy et al., 2019 ), and the lack of budget (HMP Littlehey, 2016 ; HMP Littlehey, 2016 ; Inspector of Custodial Services, 2015 ; Treacy et al., 2019 ) were also reported issues. It was suggested that the use of dementia-friendly environmental checklists could be useful, potentially with input from occupational therapists, health and social care, and dementia charities and in-house education, work and estates departments (Brown, 2014 ; Christodoulou, 2012 ; Dillon et al., 2019 ; Goulding, 2013 ; HMP Littlehey, 2016 ; Her Majesty's Prison & Probation Service, 2018 ; Hodel & Sanchez, 2013 ; Peacock et al., 2018 ; Sindano & Swapp, 2019 ; Treacy et al., 2019 ). Hope was expressed that newly built prisons would be more dementia-friendly (Dementia Action Alliance, 2017 ; Her Majesty's Prison & Probation Service, 2018 ; Williams et al., 2012 ).
Formal policies and procedures should be in place to help maintain links between family and prisoners, and to foster an understanding of the central importance of families particularly for PLiPWD (Her Majesty's Inspectorate of Prisons, 2016 ; Treacy et al., 2019 ). Some papers described how prisons could support contact by: giving help and additional time to make telephone calls and arranging visits in quieter spaces (Her Majesty's Prison & Probation Service, 2018 ; Prisons and Probation Ombudsman, 2016 ; Treacy et al., 2019 ); increasing the number of visits (Jennings, 2009 ); and allowing for accumulated visits or transfers to other prisons for visits closer to home (Her Majesty's Prison & Probation Service, 2018 ). Family communication – additional information can be found in Table 14 .
External organisations
One review suggested that external voluntary agencies were not often contacted or referred to, despite their potential benefits in terms of costs and support for staff and PLiPWDs (du Toit et al., 2019 ). However, other papers reported that charities for PLiPWD, or older people, were involved in (or were recommended to be involved in): designing and/or delivering dementia training; being part of MDTs; informing the design of referral processes, screening, assessment and case finding tools; consulting on environmental design; creating and delivering social care plans (including running activity centres); advice and support; advocacy and; co-facilitating a cognitive stimulation therapy group (Alzheimer’s Society 2018 ; Brooke et al., 2018 ; Brown, 2014 , 2016 ; Correctional Investigator Canada, 2019 ; Department of Health, 2007 ; du Toit & Ng, 2022 ; du Toit et al., 2019 ; Gaston, 2018 ; Gaston & Axford, 2018 ; Goulding, 2013 ; Her Majesty's Inspectorate of Prisons, 2014 ; HMP Hull, 2015 ; Her Majesty's Prison & Probation Service, 2018 ; Hodel & Sanchez, 2013 ; Moll, 2013 ; Peacock et al., 2018 ; Prisons and Probation Ombudsman, 2016 ; Sindano & Swapp, 2019 ; Tilsed, 2019 ; Treacy et al., 2019 ; Williams, 2014 ). It was also recommended that external organisations need to have a better knowledge and understanding of prisons and people living in prison, in order to better manage risk, and for clear information sharing protocols (du Toit & Ng, 2022 ).
(iv) Transfers
During the course of their sentence, people in prison may be transferred to other prisons for various reasons or to receive treatment in hospital. The need for MDT transfer plans to be developed was reported (Welsh Government and Ministry of Justice, 2011 ), as was the need to limit the number of prisoner transfers as moving accommodation is likely to have an adverse effect (Her Majesty's Prison & Probation Service, 2018 ; Patterson et al., 2016 ). It was recommended that transfers should take the distance from family and friends into account (Her Majesty's Prison & Probation Service, 2018 ), and that the ‘receiving’ facility (prison or healthcare setting) should be liaised with regarding health and social care, and risk (Her Majesty's Prison & Probation Service, 2018 ; Welsh Government and Ministry of Justice, 2011 ) to ensure continuity of care (Cipriani et al., 2017 ). A standard document transfer protocol was also postulated as useful, as documents need to be forwarded quickly as well (Brown, 2016 ; Tilsed, 2019 ; Welsh Government and Ministry of Justice, 2011 ). At the receiving facility, it was suggested that assessments and care plans should be reviewed on the day of the transfer (Brown, 2016 ; Her Majesty's Prison & Probation Service, 2018 ; National Institute for Health and Care Excellence, 2017 ; Welsh Government, 2014 ), and for re-inductions to be facilitated for prison transfers (Her Majesty's Prison & Probation Service, 2018 ).
(v) Release and resettlement
Most prisoners will be released from prison at the end of their sentence, although a number may die before their time is served. A number of areas were highlighted regarding the release and resettlement of PLiPWD, including the possibility of early release due to dementia.
Early release
A number of papers advocated for compassionate release policies and their actual use, or alternative custodial placements such as halfway houses or secure nursing homes, that would effectively result in the early release of PLiPWD (Brown, 2016 ; Cipriani et al., 2017 ; Correctional Investigator Canada, 2019 ; Dementia Action Alliance, 2017 ; Department of Health, 2007 ; du Toit & Ng, 2022 ; du Toit et al., 2019 ; Fazel et al., 2002 ; Gaston & Axford, 2018 ; Goulding, 2013 ; Her Majesty's Prison & Probation Service, 2018 ; Hodel & Sanchez, 2013 ; Inspector of Custodial Services, 2015 ; Maschi et al., 2012 ; Mistry & Muhammad, 2015 ; Pandey et al., 2021 ; Turner, 2018 ; Williams et al., 2012 ). Although, it has also been noted that early release may not be a popular idea for some sections of the community (du Toit et al., 2019 ; Garavito, 2020 ), it was also suggested that raising community awareness of dementia may ameliorate this (du Toit & Ng, 2022 ). It was reported that prisoners with dementia should be considered in any criteria set forth for early release, particularly given the high cost/low risk ratio which they represent (Baldwin & Leete, 2012 ; Correctional Investigator Canada, 2019 ; Department of Health, 2007 ; Goulding, 2013 ; Her Majesty's Prison & Probation Service, 2018 ; Inspector of Custodial Services, 2015 ; Maschi et al., 2012 ; Murray, 2004 ; Williams et al., 2012 ). For prisoners who do not understand the aims of prison, continuing to hold them may be a contravention of human rights and equality laws – particularly where health and social care is inadequate (Baldwin & Leete, 2012 ; Dementia Action Alliance, 2017 ; Fazel et al., 2002 ; Gaston & Axford, 2018 ; Murray, 2004 ). It was also emphasised that the existence of units and programmes for PLiPWD should not be used to legitimise prison as an appropriate place for PLiPWD (Correctional Investigator Canada, 2019 ). More information can be found in Table 15 .
Resettlement
Ten different areas were identified in the literature which related to the issues PLiPWD leaving prison may face on their release and resettlement into the community, these were:
(a) In-prison release preparation
Specific pre-release programmes or services for older people or those living with dementia may be required (Department of Health, 2007 ; Williams et al., 2012 ), with prisoners being cognitively screened prior to release (Goulding, 2013 ), although the latter was only found in 10% of prisons in one study (Forsyth et al., 2020 ). Other suggestions for programme content included: self-efficacy, health, staving off dementia and associated anxiety, accessing services, addressing institutionalisation, setting up email addresses, and the provision of information packs on national, regional and local services and resources (Department of Health, 2007 ; Her Majesty's Prison & Probation Service, 2018 ; Williams et al., 2012 ).
It has been suggested that release plans and transitions be facilitated by an MDT including prisoners, the voluntary sector, offender managers, and other appropriate community-based organisations (du Toit et al., 2019 ; Feczko, 2014 ; Goulding, 2013 ; Her Majesty's Prison & Probation Service, 2018 ; Inspector of Custodial Services, 2015 ; Moll, 2013 ; Welsh Government and Ministry of Justice, 2011 ). Recommended plan content included: risk management strategies, health, social care, housing, finance, employment, leisure and voluntary sector considerations (Welsh Government and Ministry of Justice, 2011 ). It was also suggested that Circles of Support and Accountability (CoSA), primarily associated with sex offenders, could be set up for PLiPWD as a means to support those leaving prison and settling back into the community particularly without family support (Her Majesty's Prison & Probation Service, 2018 ).
Challenges to release preparation were identified as: a lack of resources, (Turner, 2018 ) the lack of clarity regarding staff resettlement roles (Inspector of Custodial Services, 2015 ), and the lack of resettlement provision offered at sex offender prisons in England and Wales (Her Majesty's Prison & Probation Service, 2018 ).
A number of papers reported the key role that family and friends can or do play in supporting PLiPWD leaving prison, and that this should be supported or facilitated by prison staff (Brown, 2016 ; Her Majesty's Prison & Probation Service, 2018 ; Goulding, 2013 ). Initially this could include encouraging diagnosis disclosure (Dillon et al., 2019 ), using prison leave to maintain relationships (Her Majesty's Prison & Probation Service, 2018 ), involvement in discharge planning (Welsh Government and Ministry of Justice, 2011 ), and placing prison leavers close to family upon release and ensuring family are supported (Correctional Investigator Canada, 2019 ; Gaston & Axford, 2018 ). Where PLiPWD lack family, setting up CoSAs as described above may be useful (Her Majesty's Prison & Probation Service, 2018 ).
(c) Probation
It was suggested that probation staff should have training to work with older people, and that some offender managers could specialise in this work (Department of Health, 2007 ; Welsh Government and Ministry of Justice, 2011 ). Probation officers or offender managers are or can be involved in resettlement planning, (Her Majesty's Prison & Probation Service, 2018 ; Welsh Government and Ministry of Justice, 2011 ), arranging accommodation (Inspector of Custodial Services, 2015 ), liaising with agencies such as health care or social services, checking that PLiPWD are accessing these services and disseminating reports of to-be released prisoners to relevant parties (Department of Health, 2007 ; Moll, 2013 ; Welsh Government and Ministry of Justice, 2011 ). Importantly, the forwarding of important documents to offender managers by the prison should be routine (Department of Health, 2007 ; Moll, 2013 ). It was also recommended that probation staff should visit people in prison before release if they live out of area (Department of Health, 2007 ). The work of probation services was reportedly hampered by limited resources (Brown, 2016 ).
Continuity of care upon release can be difficult, and it was suggested that it could be a role of prison healthcare to ensure this (including registering with the local GP and dentist (Cipriani et al., 2017 ; Department of Health, 2007 ; Gaston, 2018 ; Gaston & Axford, 2018 ; Her Majesty's Prison & Probation Service, 2018 ; Inspector of Custodial Services, 2015 ; Welsh Government and Ministry of Justice, 2011 ). There appeared to be some differences regarding the distribution of full healthcare reports to offender managers and other appropriate agencies with some prisons sending them, some only if requested, and some not providing them on grounds of confidentiality (Moll, 2013 ). Typically, it was recommended that it was better for to-be released older prisoners if these reports were disseminated (Department of Health, 2007 ). It was also suggested that healthcare staff in prison and from the community form part of multi-disciplinary release planning, and that these plans include health considerations and healthcare staff advice on issues of accommodation (du Toit & Ng, 2022 ; Inspector of Custodial Services, 2015 ; Moll, 2013 ; Welsh Government and Ministry of Justice, 2011 ).
(e) Social care
Some papers reported that social workers can and should be involved in the process of resettlement (Department of Health, 2007 ; Welsh Government and Ministry of Justice, 2011 ) and release preparation (Goulding, 2013 ). Continuity of social care arranged with the local authority was also recommended (Her Majesty's Prison & Probation Service, 2018 ; Welsh Government and Ministry of Justice, 2011 ).
(f) Accommodation
Release planning should include plans for accommodation, and involve housing agencies or care services in the community in that planning (Welsh Government and Ministry of Justice, 2011 ). Importantly, people in prison may need help in registering for housing, and their homes may be in need of adaptation in response to their health or social care needs (Department of Health, 2007 ; Her Majesty's Prison & Probation Service, 2018 ). Nursing homes and other care providing facilities were reported to be reluctant to accommodate people who have been in prison (Brown, 2014 ; Brown, 2016 ; Booth, 2016 ; Correctional Investigator Canada, 2019 ; du Toit et al., 2019 ; Gaston, 2018 ; Garavito, 2020 ; Goulding, 2013 ; Inspector of Custodial Services, 2015 ). This was described as particularly the case for those who were living with dementia (Brown, 2014 ; Correctional Investigator Canada, 2019 ; Dillon et al., 2019 ), with further issues reported in accommodating those who have committed sex offences (Brown, 2014 , 2016 ; Dillon et al., 2019 ; Garavito, 2020 ; Inspector of Custodial Services, 2015 ). Concerns regarding the safety of other residents and the views of their families, and the rights of victims in general, were cited as reasons behind these placement difficulties (Brown, 2014 ; Goulding, 2013 ) – one paper reported that there had been community protests (Brown, 2016 ).
It was suggested that prisons need to build better relationships with care providers in the community, which had reportedly been forged by some (Brown, 2016 ; Goulding, 2013 ; Inspector of Custodial Services, 2015 ), and that they could also provide education and support to these services (Booth, 2016 ). However, it was also noted that there may be a need for specialist residential units to be created in the community for people released from prison with dementia (Inspector of Custodial Services, 2015 ), with an example of a state-run facility for ex-prisoners in the United States (Goulding, 2013 ), and particular attention for younger ex-prisoners with dementia (Brown, 2014 ). A number of papers reported that if accommodation could not be arranged for people, this largely resulted in them remaining in prison until it was (Correctional Investigator Canada, 2019 ; Goulding, 2013 ; Inspector of Custodial Services, 2015 ; Peacock et al., 2018 ; Soones et al., 2014 ).
(g) Finance
Imprisonment likely leads to a loss of income, meaning that older prisoners who may have served more lengthy sentences are likely to be poorer, particularly if unable to work in prison (Baldwin & Leete, 2012 ; Gaston, 2018 ). Therefore, it was suggested that release planning ought to include issues of finance (Welsh Government and Ministry of Justice, 2011 ). Given that it has been suggested that people in prison should be given advice on pensions and welfare benefits, and help to arrange these (Department of Health, 2007 ; Her Majesty's Prison & Probation Service, 2018 ; Goulding, 2013 ), addressing this would seem to be an area of particular use for older people leaving prison who may have additional problems in these areas, and for those who may need assistance in arranging their financial affairs because of their deteriorating health problems.
(h) Employment and education
People’s employment prospects are likely to be impacted upon release from prison, particularly for older people who may have served long sentences (Gaston, 2018 ). Where appropriate, it was recommended that release planning should include issues around employment (Welsh Government and Ministry of Justice, 2011 ), that information packs for people should include sections on education and employment, and that it could be useful to help people make links with the Department for Work and Pensions (Her Majesty's Prison & Probation Service, 2018 ).
(i) Leisure
Leisure activities and resources could be considered in release planning, and included in pre-release information packs for prisoners (Her Majesty's Prison & Probation Service, 2018 ; Welsh Government and Ministry of Justice, 2011 ).
(j) Charities and voluntary sector organisations
It was recommended in a number of papers that charity and voluntary sector organisations working with PLiPWD be involved in release planning (Department of Health, 2007 ; du Toit et al., 2019 ; Her Majesty's Prison & Probation Service, 2018 ; Moll, 2013 ; Welsh Government and Ministry of Justice, 2011 ), continuity of care (Moll, 2013 ), and in providing support during the transition and after (du Toit & Ng, 2022 ; Welsh Government and Ministry of Justice, 2011 ). It was also suggested that in general it would be useful for PLiPWD to have contact with these organisations (Department of Health, 2007 ; Her Majesty's Prison & Probation Service, 2018 ; Inspector of Custodial Services, 2015 ), and that they may be well-placed to develop information packs for prisoners on release regarding local amenities, services and resources (Her Majesty's Prison & Probation Service, 2018 ).
(vi) Cross-cutting themes
Eight more generalised concerns were also described which had a clear impact on the passage of PLiPWD through prison, on release and resettlement in the community, and on the issues raised thus far in the review.
Principles-philosophy
The principles suggested to underpin the support of PLiPWD are that it should be person-centred, holistic, adhere to human rights and dignity principles, proactive, health promoting, and enabling – making choices but supported if needed (Brown, 2014 , 2016 ; Christodoulou, 2012 ; Cipriani et al., 2017 ; Correctional Investigator Canada, 2019 ; Department of Health, 2007 ; Dillon et al., 2019 ; du Toit & Ng, 2022 ; Gaston & Axford, 2018 ; Her Majesty's Inspectorate of Prisons, 2017b ; Her Majesty's Prison & Probation Service, 2018 ; Mackay, 2015 ; Maschi et al., 2012 ; Treacy et al., 2019 ; Welsh Government and Ministry of Justice, 2011 ; Wilson & Barboza, 2010 ). Conversely, clashes in philosophies between prison staff, and health and social care staff have been reported with security trumping care in many cases, which can have a negative impact (du Toit & Ng, 2022 ; Gaston, 2018 ; Gaston & Axford, 2018 ; Goulding, 2013 ; Mackay, 2015 ; Murray, 2004 ; Patterson et al., 2016 ; Prisons and Probation Ombudsman, 2016 ; Treacy et al., 2019 ; Williams, 2014 ). It was suggested that positioning dementia as more than just a health issue and fostering a whole-prison care-custody model or approach, with clearly defined roles for ‘care’ and ‘custody’, may be useful in resolving this (du Toit & Ng, 2022 ; Public Health England, 2017b ; Welsh Government and Ministry of Justice, 2011 ).
A number of papers ( n = 15) reported that budget and resource limitations had a variety of negative impacts including difficulties in providing: appropriate assessment, support and accommodation to PLiPWD; specialist accommodations, plans for which were then curtailed; delivering programmes and activities; healthcare cover; and, staff training (Booth, 2016 ; Christodoulou, 2012 ; Correctional Investigator Canada, 2019 ; Dementia Action Alliance, 2017 ; Dillon et al., 2019 ; du Toit et al., 2019 ; du Toit & Ng, 2022 ; Goulding, 2013 ; HMP Hull, 2015 ; Jennings, 2009 ; Mackay, 2015 ; Moll, 2013 ; Moore & Burtonwood, 2019 ; Pandey et al., 2021 ; Patterson et al., 2016 ; Peacock et al., 2018 ; Treacy et al., 2019 ; Turner, 2018 ). Ultimately, lack of resources has reportedly led to a system that is not able to cope appropriately with PLiPWD (Moll, 2013 ; Williams et al., 2012 ; Wilson & Barboza, 2010 ), with associated problems transferring out of the prison system into probation and care systems when people are released (Williams et al., 2012 ).
It has been suggested that PLiPWD in prison should be treated as if they have capacity to make decisions such as giving or withholding consent for treatment, unless it is proven otherwise. This is consistent with legislation such as the Mental Capacity Act (Prisons and Probation Ombudsman, 2016 ). It has been recommended that healthcare staff should conduct capacity assessments if there are concerns (National Institute for Health and Care Excellence, 2017 ; Welsh Government and Ministry of Justice, 2011 ), and be trained to do so (Maschi et al., 2012 ; Welsh Government, 2014 ). It is of note that an ombudsman report showed that PLiPWD who died lacked access to mental capacity assessments (Peacock et al., 2018 ). For PLiPWD, who are likely to lack capacity as their condition progresses, early education about, and development of, advance directives has been advocated (Brown, 2016 ; Cipriani et al., 2017 ; Inspector of Custodial Services, 2015 ; Maschi et al., 2012 ; Prisons and Probation Ombudsman, 2016 ), and staff should be trained on this (Maschi et al., 2012 ). It has also been suggested that family members, independent mental capacity advocates or healthcare proxies could or should be used for PLiPWD who lack capacity in making care, welfare and financial decisions (Brown, 2016 ; Soones et al., 2014 ), supported by legislation and oversight, as opposed to prison or healthcare staff making decisions (Correctional Investigator Canada, 2019 ).
The issue of ‘risk’ related to PLiPWD revolves around four areas: (i) assessment, (ii) management, (iii) disciplinary procedures, and (iv) safeguarding. Full details can be found in Table 16 .
There were a number of additional facets to risk concerns regarding PLiPWD described in the papers. There were concerns that the lack of understanding of the impact of dementia on people’s behaviour could ultimately lead to people being held in prison for longer periods on account of seemingly transgressive or aggressive behaviour that could in fact be related to their dementia difficulties (Dementia Action Alliance, 2017 ; Mistry & Muhammad, 2015 ; Treacy et al., 2019 ). In one study, a prisoner with dementia was transferred to another prison because staff felt that they were ‘grooming’ an officer (Treacy et al., 2019 ), likely lengthening their overall prison stay. There was also a recurring issue in fatal incidents investigations in England and Wales of prisoners being restrained whilst dying in hospital, a practice described as unnecessary in light of their likely frail state (Peacock et al., 2018 ; Prisons and Probation Ombudsman, 2016 ). One paper suggested linking future accommodation options and considerations for Release on Temporary Licence to a PLiPWD’s risk of reoffending, as well as the severity of their symptoms (Forsyth et al., 2020 ). Moore and Burtonwood ( 2019 ) also observed that a lack of risk assessment protocols was a barrier to release of PLiPWD., and as Table 16 suggests, a comprehensive risk assessment, applied by appropriately trained staff should make health and its impact on future offending more salient to aid this.
There were recommendations that PLiPWD should have the opportunity to make choices in their treatment and care. This included input into care plans or making informed decisions about their care (Department of Health, 2007 ; du Toit & Ng, 2022 ; National Institute for Health and Care Excellence, 2017 ; Welsh Government and Ministry of Justice, 2011 ), as well as developing advance directives particularly early in a person’s sentence (Brown, 2016 ; Cipriani et al., 2017 ; Inspector of Custodial Services, 2015 ; Maschi et al., 2012 ; Pandey et al., 2021 ; Peacock et al., 2019 ; Prisons and Probation Ombudsman, 2016 ), and choosing ‘preferred’ places to die (Her Majesty's Prison & Probation Service, 2018 ).
Protected characteristics
There was a reported need for culturally appropriate assessments, treatment and activities (Brooke et al., 2018 ; Department of Health, 2007 ; Hamada, 2015 ; Welsh Government and Ministry of Justice, 2011 ), spiritual support (Welsh Government and Ministry of Justice, 2011 ), multilingual information (Welsh Government and Ministry of Justice, 2011 ), and the recognition of gender differences in dementia healthcare needs (Brown, 2014 ; Department of Health, 2007 ; Williams et al., 2012 ). It was also highlighted that racism makes the experience of living with dementia in prison more problematic (Brooke et al., 2018 ; Brown, 2014 ; Correctional Investigator Canada, 2019 ). There were some examples of policy and practice within prisons which considered some protected characteristics: assessment tools in different languages (Patterson et al., 2016 ), additional support for PLiPWD to plan care (Department of Health, 2007 ; Welsh Government and Ministry of Justice, 2011 ), and the development of culturally appropriate care planning (Hamada, 2015 ). Hamada ( 2015 ) also advocated assessment and treatment that was culturally ‘competent’ and respectful, and which acknowledged the importance of culture and diversity.
An overall need to tackle dementia- and age-related stigma was also reported in some papers, and the need to foster cultures that are age-respectful should be reflected in staff training (Department of Health, 2007 ; Treacy et al., 2019 ; Welsh Government and Ministry of Justice, 2011 ), In addition, practices which openly discriminate such as the lack of: dedicated dementia resources (Turner, 2018 ), appropriate lower category prison places (Department of Health, 2007 ; Welsh Government and Ministry of Justice, 2011 ), and appropriate accommodation on release, which at times prevents release, should also be challenged (Correctional Investigator Canada, 2019 ; Forsyth et al., 2020 ; Ministry of Justice, 2013 ; Prisons and Probation Ombudsman, 2016 ). There was also a lack of research into the interaction between protected characteristics and dementia in prison (Brooke & Jackson, 2019 ; Treacy et al., 2019 ; Williams et al., 2012 ).
Collaboration
Many papers advocated the need for prisons and specialist dementia units to adopt a collaborative MDT approach drawing from staff teams across the prison regarding: the identification and support of prisoners with dementia, care planning, the disciplinary process, the development, dissemination and implementation of policy, and in environmental change and the building of new prisons (Brooke et al., 2018 ; Brown, 2014 , 2016 ; Christodoulou, 2012 ; Cipriani et al., 2017 ; Dillon et al., 2019 ; Department of Health, 2007 ; Feczko, 2014 ; Forsyth et al., 2020 ; Gaston & Axford, 2018 ; Her Majesty's Inspectorate of Prisons, 2014 , 2016 ; HMP Hull, 2015 ; HMP Littlehey, 2016 ; Her Majesty's Prison & Probation Service, 2018 ; Inspector of Custodial Services, 2015 ; Moll, 2013 ; Patterson et al., 2016 ; Peacock et al., 2018 ; Peacock, 2019 ; Prisons and Probation Ombudsman, 2016 ; Sindano & Swapp, 2019 ; The King’s Fund 2013 ; Tilsed, 2019 ; Treacy et al., 2019 ; Welsh Government and Ministry of Justice, 2011 , 2014 ; Williams, 2014 ). There were examples of prisoners collaborating with staff in the care of PLiPWD as peer supporters, and having joint staff-prisoner supervision and training (Brooke & Jackson, 2019 ), of joint staff-prisoner wing meetings in one prison (Treacy et al., 2019 ), and of the co-designing of services and activities in others (Her Majesty's Prison & Probation Service, 2018 ; Treacy et al., 2019 ). It was suggested that this collaborative way of working should be supported by an information sharing protocol, clear definitions of staff and peer supporter roles and responsibilities, and training (Brooke & Jackson, 2019 ; Dillon et al., 2019 ; du Toit & Ng, 2022 ; HMP Littlehey, 2016 ; Turner, 2018 ). It was reported that there had been a lack of communication and coordination of this process in some prisons which had a negative impact on all involved (Brooke & Rybacka, 2020 ; Forsyth et al., 2020 ; Moll, 2013 ; Prisons and Probation Ombudsman, 2016 ).
It was also suggested that the prisons collaborate with healthcare, hospice and dementia specialists in the community and with external charitable organisations (Brooke et al., 2018 ; Brown, 2014 ; Cipriani et al., 2017 ; du Toit & Ng, 2022 ; Gaston, 2018 ; Gaston & Axford, 2018 ; Goulding, 2013 ; HMP Hull, 2015 ; HMP Littlehey, 2016 ; Her Majesty's Prison & Probation Service, 2018 ; Moll, 2013 ; Peacock, 2019 ; Prisons and Probation Ombudsman, 2016 ; Sindano & Swapp, 2019 ; Tilsed, 2019 ; Treacy et al., 2019 ; Welsh Government and Ministry of Justice, 2011 ; Williams, 2014 ). In addition, inter-prison networks were recommended to be developed to share good practice across prisons (Dementia Action Alliance, 2017 ; Moll, 2013 ; Peacock et al., 2019 ; Prisons and Probation Ombudsman, 2016 ).
Information-sharing
A number of papers ( n = 7) recommended the need for a clear information sharing protocol regarding the assessment and support of PLiPWD (Brooke et al., 2018 ; Dillon et al., 2019 ; Department of Health, 2007 ; Goulding, 2013 ; Moll, 2013 ; Tilsed, 2019 ; Welsh Government and Ministry of Justice, 2011 ), or a register (Forsyth et al., 2020 ). Particular attention to the interface between healthcare and prison staff and peer supporters was suggested, where it has been reported that privacy regulations have sometimes prevented contributions to collateral histories (Feczko, 2014 ) and the sharing of care plans, impairing their ability to offer appropriate support (Inspector of Custodial Services, 2015 ). Also, it may be against the wishes of the person with dementia, and informed consent should be sought (Forsyth et al., 2020 ; Moll, 2013 ). This lack of information can have a detrimental effect on a person’s health and wellbeing (Brown, 2014 , 2016 ; Feczko, 2014 ; Inspector of Custodial Services, 2015 ), and so discussion of this was highlighted as important, particularly where the safety of the person or others were concerned (National Institute for Health and Care Excellence, 2017 ). A care plan which gives only very basic information to staff and peer supporters was used in a couple of prisons (Goulding, 2013 ; Williams, 2014 ).
There also appeared to be variance with respect to whether healthcare staff disclose a dementia diagnosis to the person diagnosed with dementia. A couple of prisons’ policy was to share a diagnosis and involve family in doing so (Maschi et al., 2012 ; Welsh Government and Ministry of Justice, 2011 ; Wilson & Barboza, 2010 ), however, in one prison disclosed if a person was judged to be able to cope with it, and another only disclosed if asked (Brown, 2016 ). The importance of disclosure to family allowing them to contribute to assessments, planning and support was also emphasised in some papers (Brown, 2016 ; Dillon et al., 2019 ; National Institute for Health and Care Excellence, 2017 ; Welsh Government and Ministry of Justice, 2011 ).
This review has explored the literature regarding all parts of the custodial process and its impact on people living in prison with cognitive impairment and dementia, which includes: reception, assessment, allocation, training, policy, healthcare, accommodation, adaptation, routine, access to family and external agencies, transfer and resettlement. We found evidence that problems had been identified in each of these parts of the process. We also identified a number of cross-cutting themes which interacted with the issues identified across the prison journey including: principles or philosophy regarding care; capacity; resources; considerations of risk; scope for choice; peoples’ protected characteristics; collaboration; and, information sharing. Broadly, our findings were similar to those found in previous reviews, regarding the problems with the prison process identified, and the lack of robust outcomes, and policy guidance regarding PLiPWD (Brooke and Rybacka, 2020 ; Peacock et al., 2019 ).
The aim of this review was to identify areas of good practice and for recommendations that could inform the development of prison dementia care pathways. There is a considerable breadth to the findings, but the main recommendations that have arisen from the review are:
To screen prisoners for cognitive difficulties at reception, from either 50 or 55 years
An initial older-person specific health and social care assessment, post-screening – from either 50 or 55 years, and repeated (from 3 – 12 months)
A spectrum of healthcare to be delivered including preventative, long-term and palliative care, with continuity of care upon release, and in tandem with social care
Mixed views about appropriate accommodation, but it needs to run along a continuum from independent living to 24-h care, with decisions possibly made after health assessments
Environments need to be made more older-person or dementia friendly, using checklists available, and with the voluntary sector as potential partners
A need for prison staff training on dementia, and further training for healthcare staff
The use of peer supporters was broadly reported positively, and were seemingly frequently used. However, there needs to be adequate training and support, and not to be used to do the work that is the statutory duty of health and social care staff
Equal access to activities and services, especially programmes which help people move through the system (such as offending behaviour), as well as opportunities to earn additional monies, and that provide structure and routine on wings
The maintenance of family links, and for families to be supported, are important for PLiPWD, and may be particularly so on release and resettlement
Prisons may also need to work with external care agencies to ensure placements upon release, or alternative specialist care facilities may need to be created
The main barriers to implementing these recommendations are a lack of policy or guidance at local, regional and national levels to support staff in working with PLiPWD, and also the lack of budget and resources available. The latter would also include infrastructure issues, such that a number of prisons are not appropriate for people living with dementia, and could be expensive to modify to become so, coupled with a lack of currently available alternative facilities for PLiPWD to be released to in the community. The lack of use of compassionate release is also an issue here, including during the COVID-19 pandemic, with only 54 people released (Halliday & Hewson, 2022 ). Lastly, the roles that each professional and peer group had regarding PLiPWD needed clarification in some prisons, including some resolution of the ‘clash’ of philosophies (control v care) underpinning this.
In terms of ‘solutions’, multiple organisations have advocated for years for the need for national policy to assist prisons with older people in prison, including those living with dementia (Cornish et al., 2016 ; HM Inspectorate of Prisons, 2004 , 2019 ; Prisons & Probation Ombudsman, 2016 , 2017 ). This was eventually accepted and commissioned by the UK government, although it has not been released as yet (Justice Committee, 2020 ). It has also been suggested that at a more local level, existing policies could be adapted to be more appropriate for PLiPWD – such as restraint policies for frail prisoners, and disciplinary procedures which reflect the impact that dementia may have on behaviour (Department of Health, 2007 ; Treacy et al., 2019 ). Considerations around capacity and consent would need to be weaved in, as well as a focus on the intersection with other protected characteristics. These adaptations would also need to extend to services and activities to ensure that people have equal access and opportunities. A number of reports highlighted the contribution that greater collaboration with partners in external health and social care teams could have, as well as partnerships with the voluntary sector. These could potentially assist in multiple areas including training staff and peer supporters, providing activities, assisting release preparation, at a relatively low cost, to high benefit. There were some recommendations that prisons adopt a whole-prison approach to dementia that focuses on being person-centred, health and human rights focused that may help to ameliorate some differences in philosophical approach between various staff and peer groups in prisons.
A number of potential areas for future research were also indicated by the literature, which would also support the development of prison pathways. These would include: (i) induction to prison, and (ii) release and resettlement from prison, which are important beginning and end-points, but which are under-researched; (iii) the validation of a screening tool for use in prisons, and the development or adaptation of prison-specific health and social care assessments; (iv) the interaction of protected characteristics and dementia, and the need for more culturally and gender aware pathways; (v) the paucity of research conducted in low and middle-income countries, that needs to be addressed; (vi) dementia and age-related stigma in prisons; and (vii) evaluations of all elements of the prison pathway for PLiPWD to undertaken including training, the role of peer supporters, and targeted programmes.
Strengths and limitations of the review
One key strength of this review is its comprehensiveness, particularly as it includes much grey literature. Given the lack of robust evaluation in this area, it was felt that this was necessary to represent the volume of work that has nonetheless taken place. There are, however, a number of limitations of this review. Firstly, despite the use of broad search terms, there may be the possibility that some relevant research was missed, either because of deficiencies in our searches or because of publication bias. Additionally, whilst there are twenty-two guidance and inspection documents included in this review, it is possible that some grey literature might also remain unidentified, particularly outside of the UK where the review was undertaken. Secondly, this review may be subject to a selection bias, as the yielded search results might have included literature that were excluded but which may have indirectly impacted upon the care pathways elements explored in the review. There is also a language bias, and whilst this may reflect the languages spoken by the review team members, it is also reflective of the “northern epistemic hegemony” (Aas, 2012 ), that also may have resulted in the review being largely populated by papers from high income countries. Thirdly, no formal assessment of study quality was undertaken. This is in keeping with scoping review methodology which focuses on breadth, but is nonetheless an important shortcoming inherent in scoping reviews more generally (Arksey & O’Malley, 2005 ).
We have completed the most comprehensive review of the literature on PLiPWD in prisons to date that we have found, including a synthesis of the extensive grey literature, and found important gaps in the literature. Our review includes a mixture of academic research, policy and position papers which identified an increasing number of prisoners with dementia or cognitive impairment as an issue, but there were more limited descriptions of what should be done, and even less describing implementation of these. Most of the literature came from developed nations where extensive assessment and care services are in place for PWD in the community, although a key question is whether prison populations are given easy access to these existing services or whether bespoke services for prisoners are required. We suggest this literature now needs to be drawn together to inform interventions for PLiPWD in the criminal justice system which can be piloted and evaluated, and inform the development of robust dementia care pathways for prisons.
Availability of data and materials
All data and materials used in this review are included in this article and its appendices.
There is no standard cut-off age for older people living in prison, but it is typically set at least ten to fifteen years lower than the general population. People in prison are thought to age more rapidly due to both pre- and post-imprisonment chaotic lifestyles, substance misuse and less healthcare access and use, as well as the ‘pains of imprisonment’. See Williams et al., ( 2012 ) for further discussion.
Aas, K. F. (2012). ‘The earth is one, but the world is not’: Criminological theory and its geopolitical divisions. Theoretical Criminology., 16(1), 5–20. https://doi.org/10.1177/1362480611433433
Article Google Scholar
Ahalt, C., Haney, C., Rios, S., Fox, M. P., Farabee, D., & Williams, B. (2017). Reducing the use and impact of solitary confinement in corrections. International Journal of Prisoner Health, 13(1), 41–48. https://doi.org/10.1108/IJPH-08-2016-0040
Alzheimer’s Society. (2018). Dementia: A guide for prison officers . Alzheimer’s Society.
Google Scholar
Arksey, H., & O’Malley, L. (2005). Scoping studies: Towards a methodological framework. International Journal of Social Research Methodology, 8(1), 19–23. https://doi.org/10.1080/1364557032000119616
Baldwin, J., & Leete, J. (2012). Behind bars: The challenge of an ageing prison population. Australian Journal of Dementia Care, 1(2), 16–19.
Booth, B. D. (2016). Elderly sexual offenders. Current Psychiatry Reports, 18(4), 34. https://doi.org/10.1007/s11920-016-0678-1
Brooke, J., Diaz-Gil, A., & Jackson, D. (2018). The impact of dementia in the prison setting: A systematic review. Dementia , 1471301218801715. https://doi.org/10.1177/1471301218801715
Brooke, J., & Jackson, D. (2019). An exploration of the support provided by prison staff, education, health and social care professionals, and prisoners for prisoners with dementia. The Journal of Forensic Psychiatry & Psychology , 1–17. https://doi.org/10.1080/14789949.2019.1638959
Brooke, J., & Rybacka, M. (2020). Development of a dementia education workshop for prison staff, prisoners, and health and social care professionals to enable then to support prisoners with dementia. Journal of Correctional Health Care, 26(2), 159–167. https://doi.org/10.1177/1078345820916444
Brown, J. (2014). Dementia in prison. Discussion Paper #9. Alzheimer’s Australia NSW. https://www.dementia.org.au/files/20140423-NSW-REP-DementiaInPrison.pdf .
Brown, J. (2016). Living with dementia in prison: To investigate effective care programs for people living with dementia in prison . https://www.churchilltrust.com.au/media/fellows/Brown_J_2015_Living_with_dementia_in_prison.pdf .
Care Act UK. (2014). The Stationary Office . https://www.legislation.gov.uk/ukpga/2014/23/contents/enacted .
Centre for Policy on Ageing. (2014). The effectiveness of care pathways in health and social care: Rapid review . Centre for Policy on Ageing. https://www.ageuk.org.uk/Documents/EN-GB/For-professionals/Research/CPA-Effectiveness_of_care_pathways.pdf?dtrk=true .
Christodoulou, M. (2012). Locked up and at risk of dementia. The Lancet Neurology, 11(9), 750–751. https://doi.org/10.1016/S1474-4422%2812%2970195-3
Cipriani, G., Danti, S., Carlesi, C., & Di Fiorino, M. (2017). Old and dangerous: Prison and dementia. Journal of Forensic & Legal Medicine, 51, 40–44. https://doi.org/10.1016/j.jflm.2017.07.004
Cornish, N., Edgar, K., Hewson, A., & Ware, S. (2016). Social care or systemic neglect? Older people on release from prison . Prison Reform Trust. https://prisonreformtrust.org.uk/publication/social-care-or-systemic-neglect-older-people-on-release-from-prison/ .
Dementia Action Alliance. (2017). Meeting the challenges of dementia: Roundtable discussion briefing paper . Dementia Action Alliance. https://www.dementiaaction.org.uk/assets/0003/4619/Prisons_and_Dementia_-_DAA_briefing_paper.pdf .
Department of Health. (1999). The future organisation of prison health care . Department of Health. https://www.legislation.gov.uk/ukpga/2014/23/contents/enacted .
Department of Health. (2007). A pathway to care for older offenders: A toolkit for good practice . Department of Health. http://webarchive.nationalarchives.gov.uk/20130123192716tf_/http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_079928 .
Di Lorito, C., Vӧllm, B., & Dening, T. (2018). Psychiatric disorders among older prisoners: A systematic review and comparison study against older people in the community. Aging & Mental Health, 22(1), 1–10. https://doi.org/10.1080/13607863.2017.1286453
Dillon, G., Vinter, L. P., Winder, B., & Finch, L. (2019). “The guy might not even be able to remember why he’s here and what he’s in here for and why he’s locked in”: Residents and prison staff experiences of living and working alongside people with dementia who are serving prison sentences for a sexual offence. Psychology, Crime & Law, 25(5), 440–457. https://doi.org/10.1080/1068316X.2018.1535063
du Toit, S. H. J., & McGrath, M. (2018). Dementia in prisons - enabling better care practices for those ageing in correctional facilities. British Journal of Occupational Therapy, 81(8), 460–462. https://doi.org/10.1177/0308022617744509
du Toit, S., & Ng, S. (2022). Improving care for older prisoners living with dementia in Australian prisons: Perspectives of external organizations. The Gerontologist, 62(4), 543–555. https://doi.org/10.1093/geront/gnab077
du Toit, S. H. J., Withall, A., O’Loughlin, K., Ninaus, N., Lovarini, M., Snoyman, P., Butler, T., Forsyth, K., & Surr, C. A. (2019). Best care options for older prisoners with dementia: A scoping review. International Psychogeriatrics, 31(8), 1081–1097.
Dunne, R. A., Aarsland, D., O’Brien, J. T., Ballard, C., Banerjee, S., Fox, N. C., Isaacs, J. D., Underwood, B. R., Perry, R. J., Chan, D., Dening, T., Thomas, A. J., Schryer, J., Jones, A. M., Evans, A. R., Alessi, C., Coulthard, E. J., Pickett, J., Elton, P., … Burns, A. (2021). Mild cognitive impairment: The Manchester consensus. Age & Ageing., 50 ( 1), 72–80. https://doi.org/10.1093/ageing/afaa228
Fazel, S., McMillan, J., & O’Donnell, I. (2002). Dementia in prison: Ethical and legal implications. Journal of Medical Ethics, 28(3), 156–159. https://doi.org/10.1136/jme.28.3.156
Feczko, A. (2014). Dementia in the incarcerated elderly adult: Innovative solutions to promote quality care. Journal of the American Association of Nurse Practitioners, 26(12), 640–648. https://doi.org/10.1002/2327-6924.12189
Forsyth, K., Heathcote, L., Senior, J., Malik, B., Meacock, R., Perryman, K., Tucker, S., Domone, R., Carr, M., Hayes, H., et al. (2020). Dementia and mild cognitive impairment in prisoners aged over 50 years in England and Wales: A mixed-methods study. Health Services and Delivery Research, 8 ( 27). Southampton: National Institute for Health Research.
Garavito, D. M. N. (2020). The prisoner’s dementia: Ethical and legal issues regarding dementia and healthcare in prison. Cornell Journal of Law and Public Policy., 29, 211–235. /intechopen.73161.
Gaston, S., & Axford, A. (2018). Re-framing and re-thinking dementia in the correctional setting. In H. F. Sibat (Ed.), Cognitive disorders. IntechOpen. https://www.intechopen.com/books/cognitive-disorders/re-framing-and-re-thinking-dementia-in-the-correctional-setting . 10.5772
Gaston, S. (2018). Vulnerable prisoners: Dementia and the impact on prisoners, staff and the correctional setting. Collegian, 25(2), 241–246. https://doi.org/10.1016/j.colegn.2017.05.004
Goulding, P. (2013). “Silver bullet” or confused greying fox? Best practice support model for older prisoners . Wintringham. https://www.wintringham.org.au/file/2016/I/Best_practice_support_model_for_older_prisoners.pdf .
Halliday, M., & Hewson, A. (2022). Bromley briefings prison factfile: Winter 2022 . Prison Reform Trust. https://prisonreformtrust.org.uk/wp-content/uploads/2022/02/Winter-2022-Factfile.pdf .
Hamada, J. N. (2015). ATPEACE with Dementia. American Jails, 29(2), 34–40.
Hayes, A. J., Burns, A., Turnbull, P., & Shaw, J. J. (2012). The health and social needs of older male prisoners. International Journal of Geriatric Psychiatry, 27(11), 1155–1162. https://doi.org/10.1002/gps.3761
Hayes, A. J., Burns, A., Turnbull, P., & Shaw, J. J. (2013). Social and custodial needs of older adults in prison. Age and Ageing, 42(5), 589–593. https://doi.org/10.1093/ageing/aft066
Health and Social Care Committee. (2018). Prison health: Twelfth report of session 2017–19. House of Commons. https://publications.parliament.uk/pa/cm201719/cmselect/cmhealth/963/963.pdf .
Her Majesty's Inspectorate of Prisons. (2014). Expectations: Criteria for assessing the treatment of and conditions for women in prison. Her Majesty’s Inspectorate of Prisons. https://www.justiceinspectorates.gov.uk/hmiprisons/wp-content/uploads/sites/4/2014/02/final-womens-expectation_web-09-14-2.pdf .
Her Majesty's Inspectorate of Prisons. (2015). HMP Isle of Wight Her Majesty's Inspectorate of Prisons. https://www.justiceinspectorates.gov.uk/hmiprisons/wp-content/uploads/sites/4/2015/09/Isle-of-Wight-2015-web.pdf .
Her Majesty's Inspectorate of Prisons. (2016). HMP Stafford . Her Majesty's Inspectorate of Prisons. https://www.justiceinspectorates.gov.uk/hmiprisons/wp-content/uploads/sites/4/2016/06/Stafford-Web-2016.pdf .
Her Majesty's Inspectorate of Prisons. (2017b). HMP Erlestoke . Her Majesty's Inspectorate of Prisons. https://www.justiceinspectorates.gov.uk/hmiprisons/wp-content/uploads/sites/4/2017/11/HMP-Erlestoke-Web-2017.pdf .
Her Majesty's Inspectorate of Prisons. (2017a). Expectations: Criteria for assessing the treatment of and conditions for men in prisons. Her Majesty’s Inspectorate of Prisons. = https://www.justiceinspectorates.gov.uk/hmiprisons/wp-content/uploads/sites/4/2018/02/Expectations-for-publication-FINAL.pdf .
Her Majesty’s Prison & Probation Service. (2018). Model for operational delivery: Older prisoners - supporting effective delivery in prisons . Her Majesty’s Prison & Probation Service.
HM Inspectorate of Prisons. (2004). ‘ No problems – old and quiet’: Older prisoners in England and Wales. HM Inspectorate of Prisons. https://www.justiceinspectorates.gov.uk/hmiprisons/wp-content/uploads/sites/4/2014/08/OlderPrisoners-2004.pdf .
HM Inspectorate of Prisons and Care Quality Commission. (2018). Social care in prisons in England and Wales: A thematic report . HM Inspectorate of Prisons. https://www.justiceinspectorates.gov.uk/hmiprisons/wp-content/uploads/sites/4/2018/10/Social-care-thematic-2018-web.pdf .
HM Inspectorate of Prisons. (2019). HM Chief Inspector of Prisons for England and Wales: Annual report 2018–19 . HM Inspectorate of Prisons. https://www.justiceinspectorates.gov.uk/hmiprisons/wp-content/uploads/sites/4/2019/07/6.5563_HMI-Prisons-AR_2018-19_WEB_FINAL_040719.pdf .
HM Inspectorate of Prisons. (2021). What happens to prisoners in a pandemic?: A thematic review. HM Inspectorate of Prisons. https://www.justiceinspectorates.gov.uk/hmiprisons/wp-content/uploads/sites/4/2021/02/What-happens-to-prisoners-in-a-pandemic.pdf .
HMP Hull. (2015). HM Prison Hull - Dementia action plan. Dementia Action Alliance. https://www.dementiaaction.org.uk/members_and_action_plans/4507-hm_prison_hull .
HMP Littlehey. (2016). HM Prison Littlehey - Dementia action plan. Dementia Action Alliance . https://www.dementiaaction.org.uk/members_and_action_plans/5356-hm_prison_littlehey .
Hodel, B., & Sanchez, H. G. (2013). The Special Needs Program for Inmate-Patients with Dementia (SNPID): A psychosocial program provided in the prison system. Dementia, 12(5), 654–660. https://doi.org/10.1177/1471301211432952
Inspector of Custodial Services. (2015). Old and inside: Managing aged offenders in custody. Inspector of Custodial Services: NSW Government. http://www.custodialinspector.justice.nsw.gov.au/Documents/Old%20and%20inside%20Managing%20aged%20offenders%20in%20custody.pdf .
Jennings, L. K. (2009). Aging in a confined place: An exploration of elder inmate health andhHealthcare. PhD: Univeristy of Alabama, Tuscaloosa. http://acumen.lib.ua.edu/content/u0015/0000001/0000026/u0015_0000001_0000026.pdf .
Justice Committee. (2020). Ageing prison population. House of Commons. https://committees.parliament.uk/publications/2149/documents/19996/default/ .
Kay, C. (2020). COVID-19 in custody: Responding to pandemics in prisons in England and Wales. British Journal of Community Justice, 16 (1). https://mmuperu.co.uk/bjcj/wp-content/uploads/sites/2/2020/09/BJCJ_Kay_2020.pdf .
Lee, C., Treacy, S., Haggith, A., Wickramasinghe, N. D., Cater, F., Kuhn, I., & van Bortel, T. (2019). A systematic integrative review of programmes addressing the social care needs of older prisoners. HEalth and Justice, 7, 9. https://doi.org/10.1186/s40352-019-0090-0
Mackay, A. (2015). Human rights protections for people with mental health and cognitive disability in prisons. Psychiatry, Psychology and Law, 22(6), 842–868. https://doi.org/10.1080/13218719.2015.1015207
Maschi, T., Kwak, J., Ko, E., & Morrissey, M. B. (2012). Forget me not: Dementia in prison. The Gerontologist, 52(4), 441–451. https://doi.org/10.1093/geront/gnr131
Masters, J. L., Magnuson, T. M., Bayer, B. L., Potter, J. F., & Falkowski, P. P. (2016). Preparing corrections staff for the future: Results of a 2-day training about aging inmates. Journal of Correctional Health Care, 22(2), 118–128. https://doi.org/10.1177/1078345816634667
Ministry of Justice. (2022a). Offender management statistics quarterly: January to March 2022 – Annual prison population 2022 . Ministry of Justice. https://www.gov.uk/government/statistics/offender-management-statistics-quarterly-january-to-march-2022 .
Ministry of Justice. (2013). Hidden disabilities: Dementia - essential guide for prison officers . Ministry of Justice.
Mistry, P., & Muhammad, L. (2015). Dementia in the incarcerated: Ready or not? Corrections Forum, 24(5), 8–12.
Moll, A. (2013). Losing track of time: Dementia and the ageing prison population . Mental Health Foundation. https://www.mentalhealth.org.uk/sites/default/files/losing-track-of-time-2013.pdf .
Moore, K. J., & Burtonwood, J. (2019). Are we failing to meet the healthcare needs of prisoners with dementia? International Psychogeriatrics, 31(8), 1071–1074. https://doi.org/10.1017/S104161021900108X
Murray, A. (2004). Prisoners who develop dementia: What we need to know. Journal of Dementia Care, 12(1), 29–33.
National Institute for Health and Care Excellence. (2017). Mental health of adults in contact with the criminal justice system . National Institute for Health and Care Excellence. https://www.nice.org.uk/guidance/ng66/evidence/full-guideline-pdf-4419120205
National Institute for Health and Care Excellence. (2019). Health of people in the criminal justice system. National Institute for Health and Care Excellence. https://pathways.nice.org.uk/pathways/health-of-people-in-the-criminal-justice-system .
Pandey, P., Varshney, P., Gajera, G. V., Nirisha, P. L., Malathesh, B. C., Manjunatha, N., Sivakumar, P. T., Kumar, C. N., & Math, S. B. (2021). Criminal responsibility in geropsychiatry: Competence, culpability, and care. Indian Journal of Psychological Medicine, 43(5), S97–S106. https://doi.org/10.1177/02537176211030993
Patel, S., & Bonner, D. (2016). Dementia screening service in a prison population . Central & North West London NHS Foundation Trust.
Patterson, K., Newman, C., & Doona, K. (2016). Improving the care of older persons in Australian prisons using the Policy Delphi method. Dementia, 15(5), 1219–1233. https://doi.org/10.1177/1471301214557531
Peacock, S., Burles, M., Hodson, A., Kumaran, M., MacRae, R., Peternelj-Taylor, C., & Holtslander, L. (2019). Older persons with dementia in prison – an integrative review. International Journal of Prisoner Health, 16(1), 1–16. https://doi.org/10.1108/IJPH-01-2019-0007
Peacock, S., Hodson, A., MacRae, R., & Peternelj-Taylor, C. (2018). Living with dementia in correctional settings: A case report. Journal of Forensic Nursing, 14(3), 180–184. https://doi.org/10.1097/jfn.0000000000000194
Pinder, R., Petchey, R., Shaw, S., & Carter, Y. (2005). What’s in a care pathway? Towards a cultural cartography of the new NHS. Sociology of Health & Illness, 27(6), 759–779. https://doi.org/10.1111/j.1467-9566.2005.00473.x
Popay, J., Roberts, H., Sowden, A., Petticrew, M., Arai, L., Rodgers, M., Britten, N., Roen, K., & Duffy, S. (2006). Guidance on the conduct of narrative synthesis in systematic reviews: A product from the ESRC methods programme . Lancaster University. https://www.lancaster.ac.uk/media/lancaster-university/content-assets/documents/fhm/dhr/chir/NSsynthesisguidanceVersion1-April2006.pdf .
Prisons and Probation Ombudsman. (2016). Dementia learning lessons bulletin: Fatal Incidents Investigation. Prisons and Probation Ombudsman . http://www.ppo.gov.uk/app/uploads/2016/07/PPO-Learning-Lessons-Bulletins_fatal-incident-investigations_issue-11_Dementia_WEB_Final.pdf .
Prisons & Probation Ombudsman. (2017). Learning from PPO investigations: Older prisoners . Prisons & Probation Ombudsman. https://s3-eu-west-2.amazonaws.com/ppo-prod-storage-1g9rkhjhkjmgw/uploads/2017/06/6-3460_PPO_Older-Prisoners_WEB.pdf .
Public Health England. (2017b). Physical health checks in prisons: Programme guidance. Public Health England. https://www.healthcheck.nhs.uk/commissioners-and-providers/national-guidance/ .
Public Health England. (2017a). Health and social care needs assessment of older people in prison. Public Health England. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/662677/Health_and_social_care_needs_assessments_of_the_older_prison_population.pdf .
Richardson, W. S., Wilson, M. C., Nishikawa, J., & Hayward, R. S. (1995). The well-built clinical question: A key to evidence-based decisions. ACP Journal Club, 123(3), A12–A13.
Rutter, D., Francis, J., Coren, E., & Fisher, M. (2010). SCIE research resource 1: SCIE systematic research reviews: guidelines , 2 nd edition. Social Care Institute for Excellence. https://www.scie.org.uk/publications/researchresources/rr01.asp .
Schrijvers, G., van Hoorn, A., & Huiskes, N. (2012). The care pathway: Concepts and theories – an introduction. Special edition: Integrated care pathways), e192. doi:10.5334.ijic.812
Senior, J., Forsyth, K., Walsh, E., O’Hara, K., Stevenson, C., Hayes, A., Short, V., Webb, R., Challis, D., Fazel, S., Burns, A., & Shaw, J. (2013). Health and social care services for older male adults in prison: The identification of current service provision and piloting of an assessment and care planning model. Health Services and Delivery Research, 1 (5). National Institute for Health Research. https://www.ncbi.nlm.nih.gov/books/NBK259270/pdf/Bookshelf_NBK259270.pdf .
Sfera, A., Osorio, C., Gradini, R., & Price, A. (2014). Neurodegeneration behind bars: From molecules to jurisprudence. Frontiers in Psychiatry, 5, (no pagination) (Article 115). https://doi.org/10.3389/fpsyt.2014.00115 .
Sindano, N., & Swapp, J. (2019). Prison inreach: Dementia support provision . Paper presented at the Addressing the Challenges of Dementia in Prisons, London. https://www.dementiaaction.org.uk/assets/0004/2759/Addressing_the_Challenges_of_Dementia_in_Prisons_-_Prison_Inreach_Dementia_Support_Provision_-_Natasha_Sindano.pdf .
Soones, T., Ahalt, C., Garrigues, S., Faigman, D., & Williams, B. A. (2014). “My older clients fall through every crack in the system”: Geriatrics knowledge of legal professionals. Journal of the American Geriatrics Society, 62(4), 734–739. https://doi.org/10.1111/jgs.12751
The King's Fund. (2013). Developing supportive design for people with dementia: The King’s Fund’s Enhancing the Healing Environment programme 2009–2012 . The King’s Fund. https://www.kingsfund.org.uk/publications/developing-supportive-design-people-dementia .
The Correctional Investigator Canada. (2019). Aging and dying in prison: An investigation into the experiences of older individuals in federal custody. Office of the Correctional Investigator. https://publications.gc.ca/collections/collection_2019/bec-oci/PS104-17-2019-eng.pdf .
Tilsed, S. (2019). From seldom heard to seen and heard . Paper presented at the Addressing the Challenges of Dementia in Prisons London. https://www.dementiaaction.org.uk/assets/0004/2756/Addressing_the_Challenges_of_Dementia_in_Prisons_-_From_Seldom_Heard_to_Seen_and_Heard_-_Sarah_Tilsed.pdf .
Treacy, S., Haggith, A., Wickramasinghe, N. D., & Van Bortel, T. (2019). Dementia-friendly prisons: A mixed-methods evaluation of the application of dementia-friendly community principles to two prisons in England. British Medical Journal Open, 9(8), e030087. https://doi.org/10.1136/bmjopen-2019-030087 .
Tricco, A. C., Lillie, E., Zarin, W., O’Brien, K. K., Colquhoun, H., Levac, D., Moher, D., Peters, M. D. J., Horsley, T., Weeks, L., Hempel, S., Akl, E. A., Chang, C., McGowan, J., Stewart, L., Hartling, L., Aldcroft, A., Wilson, M. G., Garritty, C., (…) Straus, S. E. (2018). PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Annals of Internal Medicine, 169(7), 467–473. https://doi.org/10.7326/M18-0850
Turner, E. K. (2018). A study of dementia assessment practices in Ohio prisons. ( Doctor of Psychology), Wright State University, Dayton, Ohio. https://etd.ohiolink.edu/!etd.send_file?accession=wsupsych1530901309258281&disposition=inline .
Vanhaecht, K., Sermeus, W., & De Witte, W. (2007). The impact of clinical pathways on the organisation of care processes . PHD Thesis. Leuven: Katholieke Universiteit Leuven. https://limo.libis.be/primo-explore/fulldisplay?docid=LIRIAS1718750&context=L&vid=Lirias&search_scope=Lirias&tab=default_tab&lang=en_US&fromSitemap=1#:~:text=Thirdly%2C%20in%20a%20study%20with,up%20of%20the%20care%20process.&text=Organisations%20using%20clinical%20pathways%20had,five%20subscales%20of%20the%20CPSET .
Vogel, R. (2016). Dementia in prison: An argument for training correctional officers. (PhD), . University of Denver. https://digitalcommons.du.edu/cgi/viewcontent.cgi?article=1219&context=capstone_masters .
Welsh Government and Ministry of Justice. (2011). A pathway to care for older prisoners: A guide to improving health, well-being and healtcare of older prisoners . Welsh Government. http://www.wales.nhs.uk/document/168109/info/ .
Welsh Government. (2014). Policy implementation guidance: Mental health services for prisoners . Welsh Government.
Williams, G. (2014). Running a cognitive stimulation therapy group in a prison environment . The Network. https://www.seapn.org.uk/uploads/files/Norfolk-RUNNING-A-COGNITIVE-STIMULATION-THERAPY-GROUP-IN-A-PRISON-ENVIRONMENT.pdf .
Williams, B. A., Stern, M. F., Mellow, J., Safer, M., & Greifinger, R. B. (2012). Aging in correctional custody: Setting a policy agenda for older prisoner health care. American Journal of Public Health, 102(8), 1475–1481. https://doi.org/10.2105/AJPH.2012.300704
Wilson, J., & Barboza, S. (2010). The looming challenge of dementia in corrections. Correct Care, 24(2), 12–14. https://www.ncchc.org/filebin/images/Website_PDFs/24-2.pdf .
Download references
Acknowledgements
We would like to thank all the funders for their contributions towards this review. We also would like to thank the key stakeholders, especially the prison advisors and old age psychiatry and care advisors, who contributed towards shaping and contextualising this evidence review.
This is a summary of research which was partly funded by the National Institute for Health Research (NIHR) Applied Research Collaboration East of England - previously, the Collaboration for Leadership in Applied Health Research and Care East of England – and the Cambridgeshire and Peterborough NHS Foundation Trust (CPFT), as part of the wider prison care programme. The views expressed are those of the author(s).
Author information
Authors and affiliations.
Faculty of Humanities and Social Sciences, Department of Criminology, Sociology & Social Policy, Swansea University, Swansea, UK
Samantha Treacy
Leicester School of Allied Health Sciences, De Montfort University, Leicester, UK
Steven Martin & Tine Van Bortel
Department of Psychiatry, School of Clinical Medicine, University of Cambridge, Cambridge, UK
Nelum Samarutilake, Ben R. Underwood & Tine Van Bortel
Cambridge Medical Library, School of Clinical Medicine, University of Cambridge, Cambridge, UK
Veronica Phillips
Cambridgeshire and Peterborough NHS Foundation Trust, Cambridge, UK
Ben R. Underwood
You can also search for this author in PubMed Google Scholar
Contributions
ST was the lead researcher and conceptualised, designed, searched, analysed and interpreted data, and led on writing the manuscript. VP provided crucial and extensive library support. SM and NS were involved in screening and extracting data as well as analysis (SM), reviewing and editing various versions of the manuscript. TVB was the Principle Investigator/Study Lead and contributed towards conceptualisation, design, data quality control, manuscript reviewing and editing, and supervising all aspects of the study. BRU was Co-Principle Investigator and provided clinical advice input. ST, SM and TVB revised the peer-reviewed manuscript. All authors read and approved the final submitted manuscript.
Corresponding author
Correspondence to Tine Van Bortel .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist.
Additional file 2. Appendix 2:
Example search strategy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Treacy, S., Martin, S., Samarutilake, N. et al. Dementia care pathways in prisons – a comprehensive scoping review. Health Justice 12 , 2 (2024). https://doi.org/10.1186/s40352-023-00252-7
Download citation
Received : 07 September 2023
Accepted : 16 November 2023
Published : 20 January 2024
DOI : https://doi.org/10.1186/s40352-023-00252-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- People living in prison
- Care pathways
Health & Justice
ISSN: 2194-7899
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Antipsychotics May Do Great Harm to People With Dementia: Report
By Dennis Thompson HealthDay Reporter

THURSDAY, April 18, 2024 (HealthDay News) -- Antipsychotics can substantially increase dementia patients’ risk of many serious health problems, a new study warns.
Dementia patients prescribed antipsychotics have increased risk of stroke, blood clots, heart attack, heart failure, bone fractures , pneumonia and kidney damage, researchers reported April 17 in the BMJ .
“A move away from the overprescription of antipsychotics is overdue,” concluded the research team led by Pearl Mok , a research fellow with the University of Manchester in England.
The study adds impetus to an ongoing investigation by the U.S. Centers for Medicare and Medicaid Services into the overuse of antipsychotic drugs in nursing homes.
U.S. Cities With the Most Homelessness

The investigation, announced last year, was launched in response to reports that some nursing homes might be falsely labeling patients as schizophrenic so they can be given antipsychotic drugs.
For the new study, researchers analyzed data on nearly 174,000 people in England diagnosed with dementia between January 1988 and May 2018, at an average age of 82.
More than 35,500 of those dementia patients had been prescribed an antipsychotic, and their health profiles were compared against up to 15 randomly selected patients who hadn’t used an antipsychotic.
Antipsychotic use more than doubled the risk of pneumonia among dementia patients, researchers found.
About 4.5% of dementia patients on antipsychotic drugs wound up developing pneumonia within three months of starting the meds, versus 1.5% of non-users.
The drugs were also associated with a 72% increased risk of kidney injury, a 62% increased risk of blood clots, a 61% increased risk of stroke, a 43% increased risk of bone fractures, a 28% increased risk of heart attack and a 27% increased risk of heart failure.
For all these outcomes, risks were highest during the first week on antipsychotics, particularly for pneumonia, researchers said.
The most commonly prescribed antipsychotics were risperidone , quetiapine , haloperidol , and olanzapine , researchers said. Together, these accounted for almost 80% of all prescriptions.
International guidelines advise restricting antipsychotic use to dementia patients with severe behavioral and psychological symptoms, the researchers noted.
However, the rate of antipsychotic prescriptions has risen in recent years, partly due to a scarcity of effective non-drug alternatives, as well as the substantial resources required to implement the alternatives that do exist, researchers said.
Antipsychotics can cause side effects like drowsiness, confusion, shaking and unsteadiness, the Alzheimer’s Society says. People taking them also have a higher risk of swollen lower limbs, infections and blood clots.
More information
The Alzheimer’s Society has more on antipsychotics and dementia .
SOURCE: BMJ , news release, April 17, 2024
Copyright © 2024 HealthDay . All rights reserved.
Join the Conversation
America 2024

Health News Bulletin
Stay informed on the latest news on health and COVID-19 from the editors at U.S. News & World Report.
Sign in to manage your newsletters »
Sign up to receive the latest updates from U.S News & World Report and our trusted partners and sponsors. By clicking submit, you are agreeing to our Terms and Conditions & Privacy Policy .
You May Also Like
The 10 worst presidents.
U.S. News Staff Feb. 23, 2024

Cartoons on President Donald Trump
Feb. 1, 2017, at 1:24 p.m.

Photos: Obama Behind the Scenes
April 8, 2022

Photos: Who Supports Joe Biden?
March 11, 2020

What to Know: Trump Trial Day One
Lauren Camera April 22, 2024

The Week in Cartoons April 22-26
April 22, 2024, at 2:29 p.m.

Protests Boil Over on College Campuses

GDP, Inflation Highlight Economic Data
Tim Smart April 22, 2024

House Passes $95B Foreign Aid Package
Aneeta Mathur-Ashton April 20, 2024

Title IX or Student Debt Relief?
Lauren Camera April 19, 2024

- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
How to Thrive as You Age
A cheap drug may slow down aging. a study will determine if it works.

Allison Aubrey

A drug taken by millions of people to control diabetes may do more than lower blood sugar.
Research suggests metformin has anti-inflammatory effects that could help protect against common age-related diseases including heart disease, cancer, and cognitive decline.
Scientists who study the biology of aging have designed a clinical study, known as The TAME Trial, to test whether metformin can help prevent these diseases and promote a longer healthspan in healthy, older adults.
Michael Cantor, an attorney, and his wife Shari Cantor , the mayor of West Hartford, Connecticut both take metformin. "I tell all my friends about it," Michael Cantor says. "We all want to live a little longer, high-quality life if we can," he says.
Michael Cantor started on metformin about a decade ago when his weight and blood sugar were creeping up. Shari Cantor began taking metformin during the pandemic after she read that it may help protect against serious infections.

Shari and Michael Cantor both take metformin. They are both in their mid-60s and say they feel healthy and full of energy. Theresa Oberst/Michael Cantor hide caption
Shari and Michael Cantor both take metformin. They are both in their mid-60s and say they feel healthy and full of energy.
The Cantors are in their mid-60s and both say they feel healthy and have lots of energy. Both noticed improvements in their digestive systems – feeling more "regular" after they started on the drug,
Metformin costs less than a dollar a day, and depending on insurance, many people pay no out-of-pocket costs for the drug.
"I don't know if metformin increases lifespan in people, but the evidence that exists suggests that it very well might," says Steven Austad , a senior scientific advisor at the American Federation for Aging Research who studies the biology of aging.
An old drug with surprising benefits
Metformin was first used to treat diabetes in the 1950s in France. The drug is a derivative of guanidine , a compound found in Goat's Rue, an herbal medicine long used in Europe.
The FDA approved metformin for the treatment of type 2 diabetes in the U.S. in the 1990s. Since then, researchers have documented several surprises, including a reduced risk of cancer. "That was a bit of a shock," Austad says. A meta-analysis that included data from dozens of studies, found people who took metformin had a lower risk of several types of cancers , including gastrointestinal, urologic and blood cancers.
Austad also points to a British study that found a lower risk of dementia and mild cognitive decline among people with type 2 diabetes taking metformin. In addition, there's research pointing to improved cardiovascular outcomes in people who take metformin including a reduced risk of cardiovascular death .
As promising as this sounds, Austad says most of the evidence is observational, pointing only to an association between metformin and the reduced risk. The evidence stops short of proving cause and effect. Also, it's unknown if the benefits documented in people with diabetes will also reduce the risk of age-related diseases in healthy, older adults.
"That's what we need to figure out," says Steve Kritchevsky , a professor of gerontology at Wake Forest School of Medicine, who is a lead investigator for the Tame Trial.
The goal is to better understand the mechanisms and pathways by which metformin works in the body. For instance, researchers are looking at how the drug may help improve energy in the cells by stimulating autophagy, which is the process of clearing out or recycling damaged bits inside cells.

Shots - Health News
Scientists can tell how fast you're aging. now, the trick is to slow it down.

You can order a test to find out your biological age. Is it worth it?
Researchers also want to know more about how metformin can help reduce inflammation and oxidative stress, which may slow biological aging.
"When there's an excess of oxidative stress, it will damage the cell. And that accumulation of damage is essentially what aging is," Kritchevsky explains.
When the forces that are damaging cells are running faster than the forces that are repairing or replacing cells, that's aging, Kritchevsky says. And it's possible that drugs like metformin could slow this process down.
By targeting the biology of aging, the hope is to prevent or delay multiple diseases, says Dr. Nir Barzilai of Albert Einstein College of Medicine, who leads the effort to get the trial started.
The ultimate in preventative medicine
Back in 2015, Austad and a bunch of aging researchers began pushing for a clinical trial.
"A bunch of us went to the FDA to ask them to approve a trial for metformin,' Austad recalls, and the agency was receptive. "If you could help prevent multiple problems at the same time, like we think metformin may do, then that's almost the ultimate in preventative medicine," Austad says.
The aim is to enroll 3,000 people between the ages of 65 and 79 for a six-year trial. But Dr. Barzilai says it's been slow going to get it funded. "The main obstacle with funding this study is that metformin is a generic drug, so no pharmaceutical company is standing to make money," he says.
Barzilai has turned to philanthropists and foundations, and has some pledges. The National Institute on Aging, part of the National Institutes of Health, set aside about $5 million for the research, but that's not enough to pay for the study which is estimated to cost between $45 and $70 million.
The frustration over the lack of funding is that if the trial points to protective effects, millions of people could benefit. "It's something that everybody will be able to afford," Barzilai says.
Currently the FDA doesn't recognize aging as a disease to treat, but the researchers hope this would usher in a paradigm shift — from treating each age-related medical condition separately, to treating these conditions together, by targeting aging itself.
For now, metformin is only approved to treat type 2 diabetes in the U.S., but doctors can prescribe it off-label for conditions other than its approved use .
Michael and Shari Cantor's doctors were comfortable prescribing it to them, given the drug's long history of safety and the possible benefits in delaying age-related disease.
"I walk a lot, I hike, and at 65 I have a lot of energy," Michael Cantor says. I feel like the metformin helps," he says. He and Shari say they have not experienced any negative side effects.
Research shows a small percentage of people who take metformin experience GI distress that makes the drug intolerable. And, some people develop a b12 vitamin deficiency. One study found people over the age of 65 who take metformin may have a harder time building new muscle.

Millions of women are 'under-muscled.' These foods help build strength
"There's some evidence that people who exercise who are on metformin have less gain in muscle mass, says Dr. Eric Verdin , President of the Buck Institute for Research on Aging. That could be a concern for people who are under-muscled .
But Verdin says it may be possible to repurpose metformin in other ways "There are a number of companies that are exploring metformin in combination with other drugs," he says. He points to research underway to combine metformin with a drug called galantamine for the treatment of sarcopenia , which is the medical term for age-related muscle loss. Sarcopenia affects millions of older people, especially women .
The science of testing drugs to target aging is rapidly advancing, and metformin isn't the only medicine that may treat the underlying biology.
"Nobody thinks this is the be all and end all of drugs that target aging," Austad says. He says data from the clinical trial could stimulate investment by the big pharmaceutical companies in this area. "They may come up with much better drugs," he says.
Michael Cantor knows there's no guarantee with metformin. "Maybe it doesn't do what we think it does in terms of longevity, but it's certainly not going to do me any harm," he says.
Cantor's father had his first heart attack at 51. He says he wants to do all he can to prevent disease and live a healthy life, and he thinks Metformin is one tool that may help.
For now, Dr. Barzilai says the metformin clinical trial can get underway when the money comes in.

7 habits to live a healthier life, inspired by the world's longest-lived communities
This story was edited by Jane Greenhalgh
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
How Pew Research Center will report on generations moving forward
Journalists, researchers and the public often look at society through the lens of generation, using terms like Millennial or Gen Z to describe groups of similarly aged people. This approach can help readers see themselves in the data and assess where we are and where we’re headed as a country.
Pew Research Center has been at the forefront of generational research over the years, telling the story of Millennials as they came of age politically and as they moved more firmly into adult life . In recent years, we’ve also been eager to learn about Gen Z as the leading edge of this generation moves into adulthood.
But generational research has become a crowded arena. The field has been flooded with content that’s often sold as research but is more like clickbait or marketing mythology. There’s also been a growing chorus of criticism about generational research and generational labels in particular.
Recently, as we were preparing to embark on a major research project related to Gen Z, we decided to take a step back and consider how we can study generations in a way that aligns with our values of accuracy, rigor and providing a foundation of facts that enriches the public dialogue.
A typical generation spans 15 to 18 years. As many critics of generational research point out, there is great diversity of thought, experience and behavior within generations.
We set out on a yearlong process of assessing the landscape of generational research. We spoke with experts from outside Pew Research Center, including those who have been publicly critical of our generational analysis, to get their take on the pros and cons of this type of work. We invested in methodological testing to determine whether we could compare findings from our earlier telephone surveys to the online ones we’re conducting now. And we experimented with higher-level statistical analyses that would allow us to isolate the effect of generation.
What emerged from this process was a set of clear guidelines that will help frame our approach going forward. Many of these are principles we’ve always adhered to , but others will require us to change the way we’ve been doing things in recent years.
Here’s a short overview of how we’ll approach generational research in the future:
We’ll only do generational analysis when we have historical data that allows us to compare generations at similar stages of life. When comparing generations, it’s crucial to control for age. In other words, researchers need to look at each generation or age cohort at a similar point in the life cycle. (“Age cohort” is a fancy way of referring to a group of people who were born around the same time.)
When doing this kind of research, the question isn’t whether young adults today are different from middle-aged or older adults today. The question is whether young adults today are different from young adults at some specific point in the past.
To answer this question, it’s necessary to have data that’s been collected over a considerable amount of time – think decades. Standard surveys don’t allow for this type of analysis. We can look at differences across age groups, but we can’t compare age groups over time.
Another complication is that the surveys we conducted 20 or 30 years ago aren’t usually comparable enough to the surveys we’re doing today. Our earlier surveys were done over the phone, and we’ve since transitioned to our nationally representative online survey panel , the American Trends Panel . Our internal testing showed that on many topics, respondents answer questions differently depending on the way they’re being interviewed. So we can’t use most of our surveys from the late 1980s and early 2000s to compare Gen Z with Millennials and Gen Xers at a similar stage of life.
This means that most generational analysis we do will use datasets that have employed similar methodologies over a long period of time, such as surveys from the U.S. Census Bureau. A good example is our 2020 report on Millennial families , which used census data going back to the late 1960s. The report showed that Millennials are marrying and forming families at a much different pace than the generations that came before them.
Even when we have historical data, we will attempt to control for other factors beyond age in making generational comparisons. If we accept that there are real differences across generations, we’re basically saying that people who were born around the same time share certain attitudes or beliefs – and that their views have been influenced by external forces that uniquely shaped them during their formative years. Those forces may have been social changes, economic circumstances, technological advances or political movements.
When we see that younger adults have different views than their older counterparts, it may be driven by their demographic traits rather than the fact that they belong to a particular generation.
The tricky part is isolating those forces from events or circumstances that have affected all age groups, not just one generation. These are often called “period effects.” An example of a period effect is the Watergate scandal, which drove down trust in government among all age groups. Differences in trust across age groups in the wake of Watergate shouldn’t be attributed to the outsize impact that event had on one age group or another, because the change occurred across the board.
Changing demographics also may play a role in patterns that might at first seem like generational differences. We know that the United States has become more racially and ethnically diverse in recent decades, and that race and ethnicity are linked with certain key social and political views. When we see that younger adults have different views than their older counterparts, it may be driven by their demographic traits rather than the fact that they belong to a particular generation.
Controlling for these factors can involve complicated statistical analysis that helps determine whether the differences we see across age groups are indeed due to generation or not. This additional step adds rigor to the process. Unfortunately, it’s often absent from current discussions about Gen Z, Millennials and other generations.
When we can’t do generational analysis, we still see value in looking at differences by age and will do so where it makes sense. Age is one of the most common predictors of differences in attitudes and behaviors. And even if age gaps aren’t rooted in generational differences, they can still be illuminating. They help us understand how people across the age spectrum are responding to key trends, technological breakthroughs and historical events.
Each stage of life comes with a unique set of experiences. Young adults are often at the leading edge of changing attitudes on emerging social trends. Take views on same-sex marriage , for example, or attitudes about gender identity .
Many middle-aged adults, in turn, face the challenge of raising children while also providing care and support to their aging parents. And older adults have their own obstacles and opportunities. All of these stories – rooted in the life cycle, not in generations – are important and compelling, and we can tell them by analyzing our surveys at any given point in time.
When we do have the data to study groups of similarly aged people over time, we won’t always default to using the standard generational definitions and labels. While generational labels are simple and catchy, there are other ways to analyze age cohorts. For example, some observers have suggested grouping people by the decade in which they were born. This would create narrower cohorts in which the members may share more in common. People could also be grouped relative to their age during key historical events (such as the Great Recession or the COVID-19 pandemic) or technological innovations (like the invention of the iPhone).
By choosing not to use the standard generational labels when they’re not appropriate, we can avoid reinforcing harmful stereotypes or oversimplifying people’s complex lived experiences.
Existing generational definitions also may be too broad and arbitrary to capture differences that exist among narrower cohorts. A typical generation spans 15 to 18 years. As many critics of generational research point out, there is great diversity of thought, experience and behavior within generations. The key is to pick a lens that’s most appropriate for the research question that’s being studied. If we’re looking at political views and how they’ve shifted over time, for example, we might group people together according to the first presidential election in which they were eligible to vote.
With these considerations in mind, our audiences should not expect to see a lot of new research coming out of Pew Research Center that uses the generational lens. We’ll only talk about generations when it adds value, advances important national debates and highlights meaningful societal trends.
- Age & Generations
- Demographic Research
- Generation X
- Generation Z
- Generations
- Greatest Generation
- Methodological Research
- Millennials
- Silent Generation

How Teens and Parents Approach Screen Time
Who are you the art and science of measuring identity, u.s. centenarian population is projected to quadruple over the next 30 years, older workers are growing in number and earning higher wages, teens, social media and technology 2023, most popular.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Coronavirus (COVID-19)
- Economy & Work
- Family & Relationships
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Politics & Policy
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
Terms & Conditions
Privacy Policy
Cookie Settings
Reprints, Permissions & Use Policy
AI Index: State of AI in 13 Charts
In the new report, foundation models dominate, benchmarks fall, prices skyrocket, and on the global stage, the U.S. overshadows.

This year’s AI Index — a 500-page report tracking 2023’s worldwide trends in AI — is out.
The index is an independent initiative at the Stanford Institute for Human-Centered Artificial Intelligence (HAI), led by the AI Index Steering Committee, an interdisciplinary group of experts from across academia and industry. This year’s report covers the rise of multimodal foundation models, major cash investments into generative AI, new performance benchmarks, shifting global opinions, and new major regulations.
Don’t have an afternoon to pore through the findings? Check out the high level here.
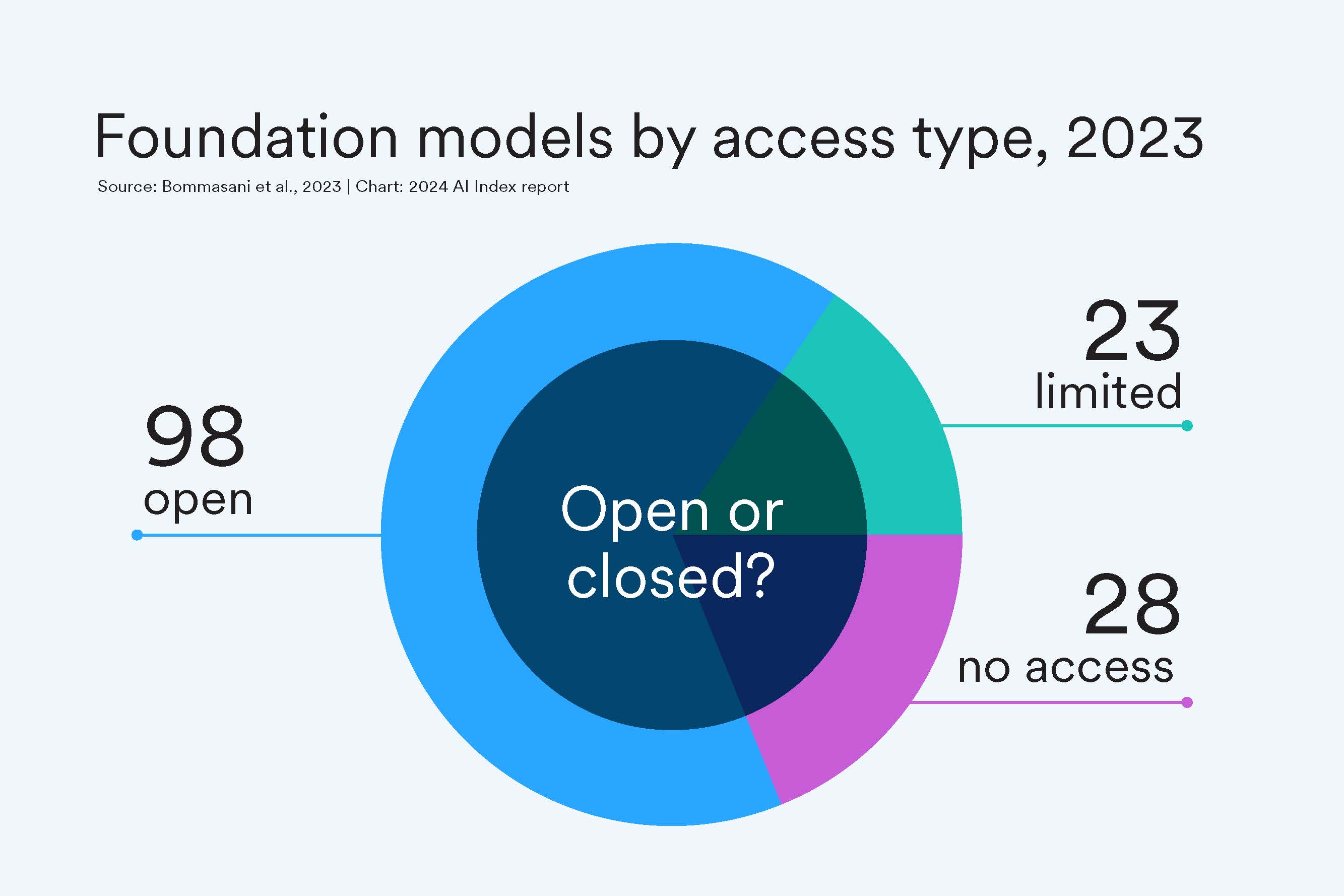
A Move Toward Open-Sourced
This past year, organizations released 149 foundation models, more than double the number released in 2022. Of these newly released models, 65.7% were open-source (meaning they can be freely used and modified by anyone), compared with only 44.4% in 2022 and 33.3% in 2021.
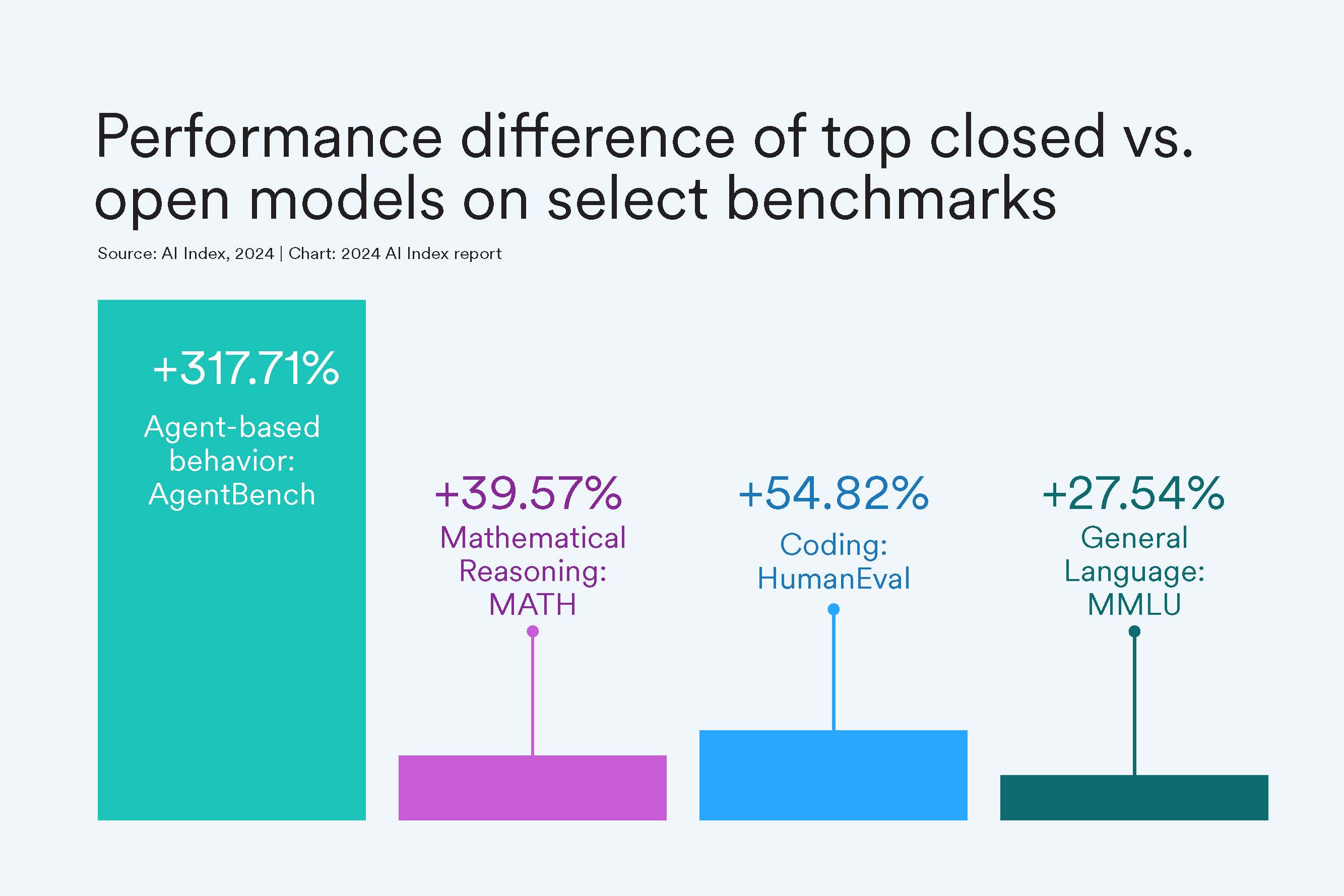
But At a Cost of Performance?
Closed-source models still outperform their open-sourced counterparts. On 10 selected benchmarks, closed models achieved a median performance advantage of 24.2%, with differences ranging from as little as 4.0% on mathematical tasks like GSM8K to as much as 317.7% on agentic tasks like AgentBench.
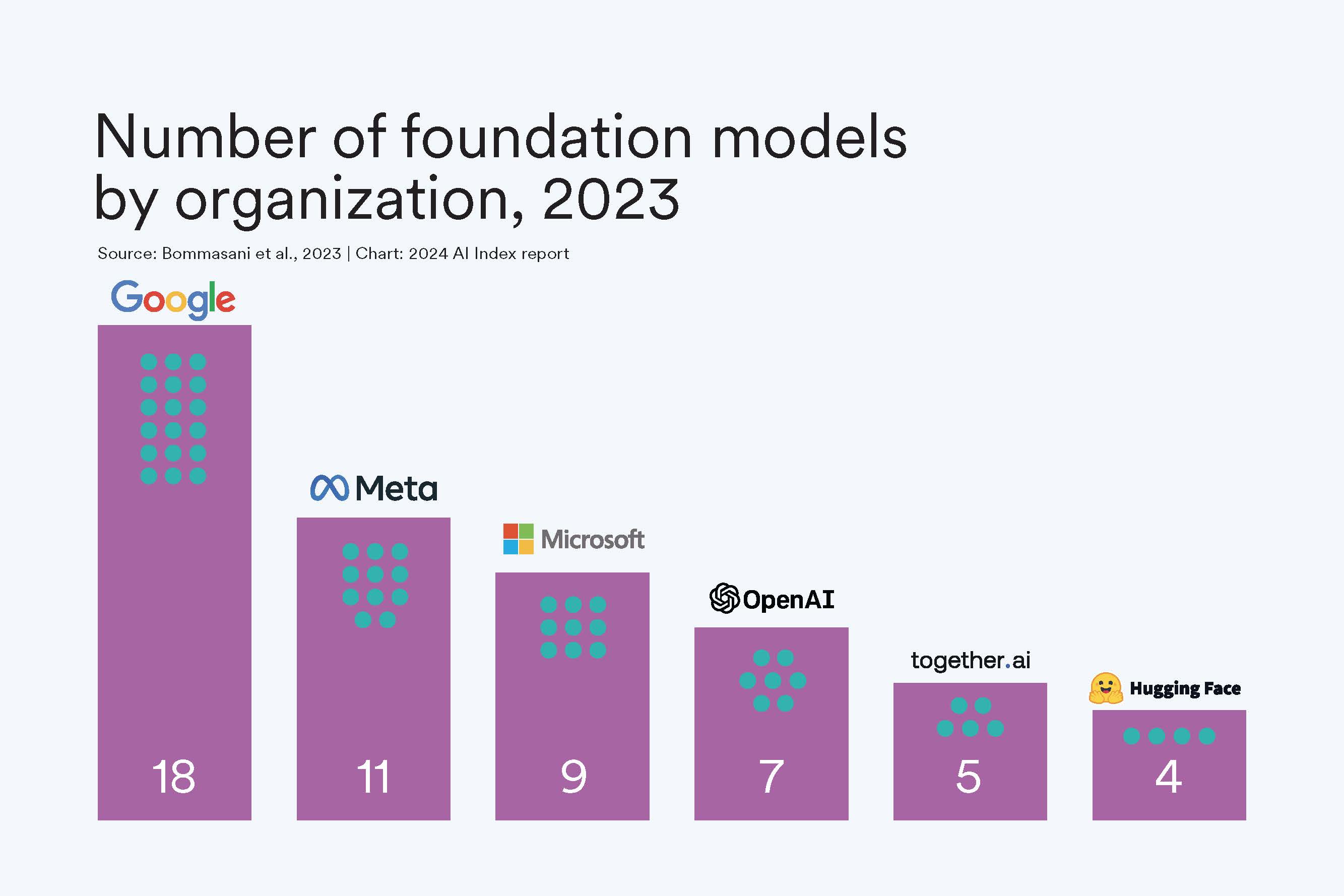
Biggest Players
Industry dominates AI, especially in building and releasing foundation models. This past year Google edged out other industry players in releasing the most models, including Gemini and RT-2. In fact, since 2019, Google has led in releasing the most foundation models, with a total of 40, followed by OpenAI with 20. Academia trails industry: This past year, UC Berkeley released three models and Stanford two.
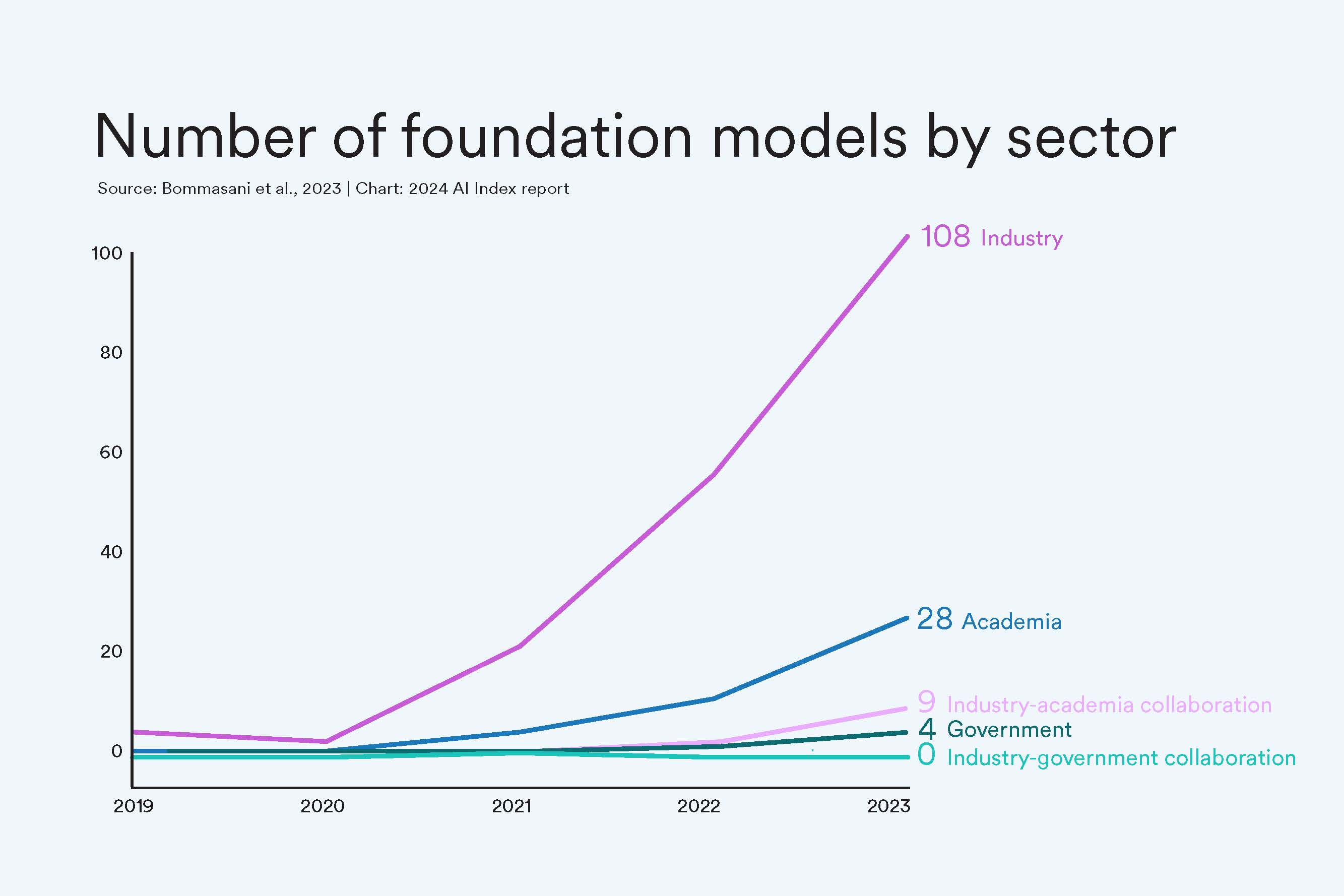
Industry Dwarfs All
If you needed more striking evidence that corporate AI is the only player in the room right now, this should do it. In 2023, industry accounted for 72% of all new foundation models.
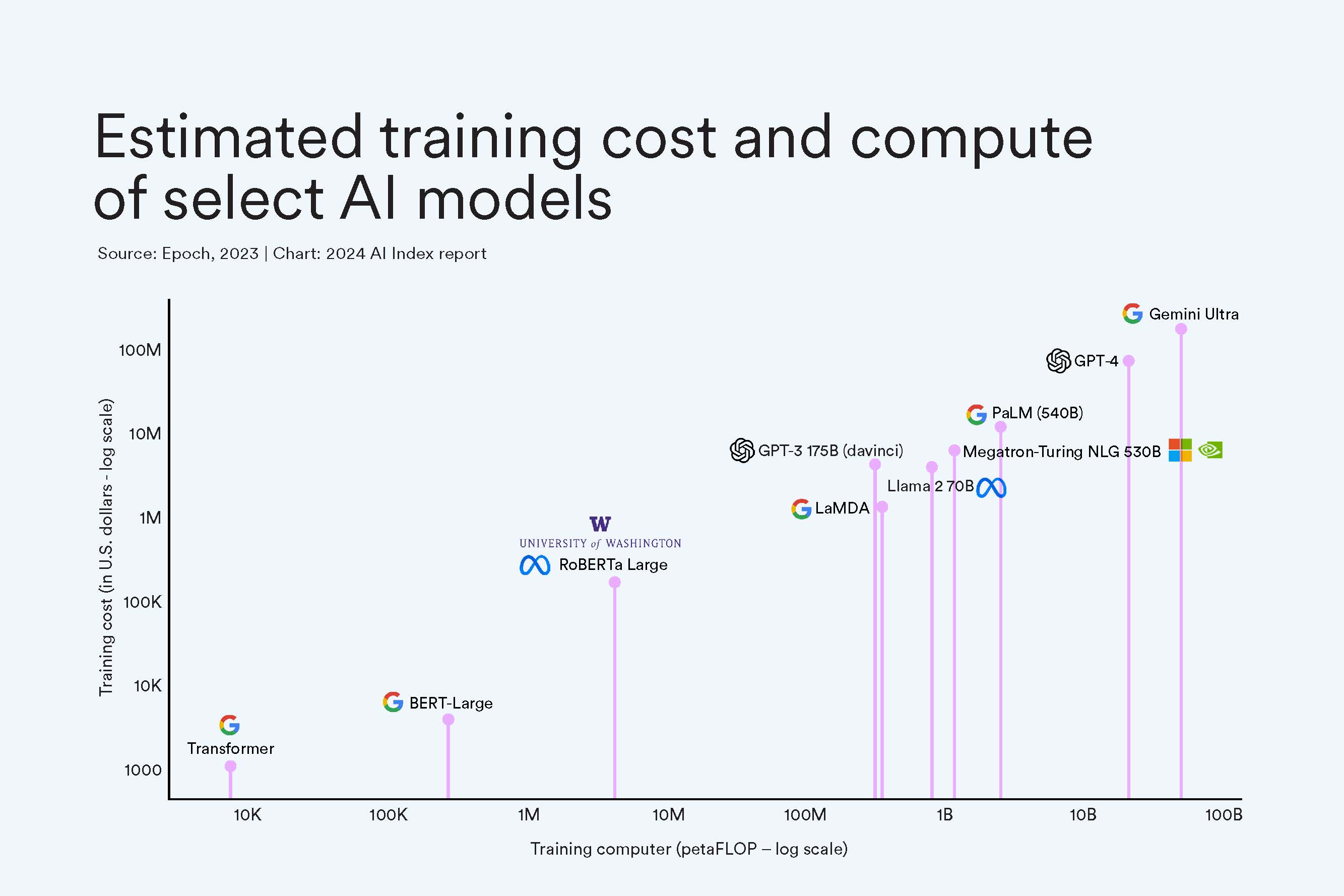
Prices Skyrocket
One of the reasons academia and government have been edged out of the AI race: the exponential increase in cost of training these giant models. Google’s Gemini Ultra cost an estimated $191 million worth of compute to train, while OpenAI’s GPT-4 cost an estimated $78 million. In comparison, in 2017, the original Transformer model, which introduced the architecture that underpins virtually every modern LLM, cost around $900.
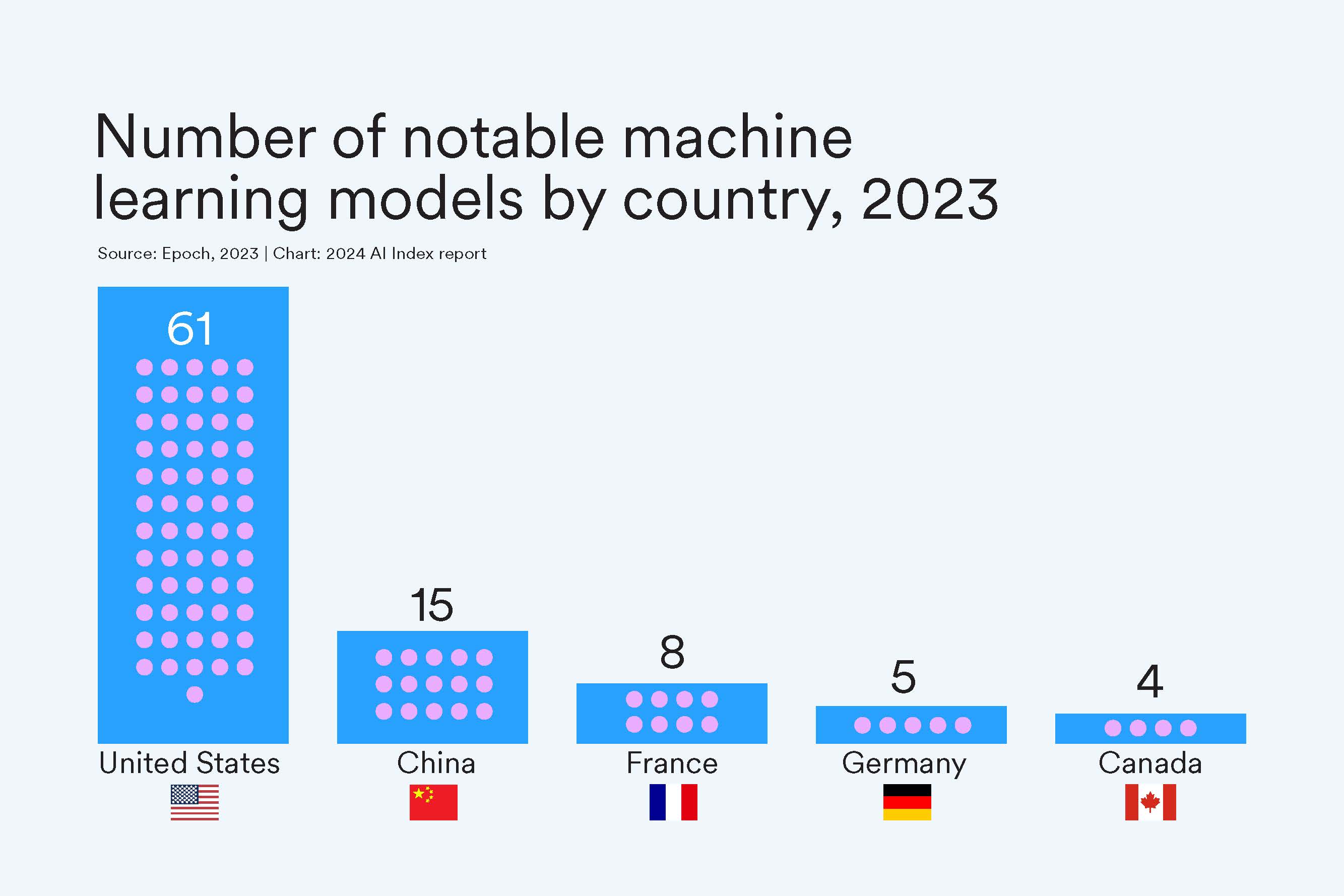
What AI Race?
At least in terms of notable machine learning models, the United States vastly outpaced other countries in 2023, developing a total of 61 models in 2023. Since 2019, the U.S. has consistently led in originating the majority of notable models, followed by China and the UK.
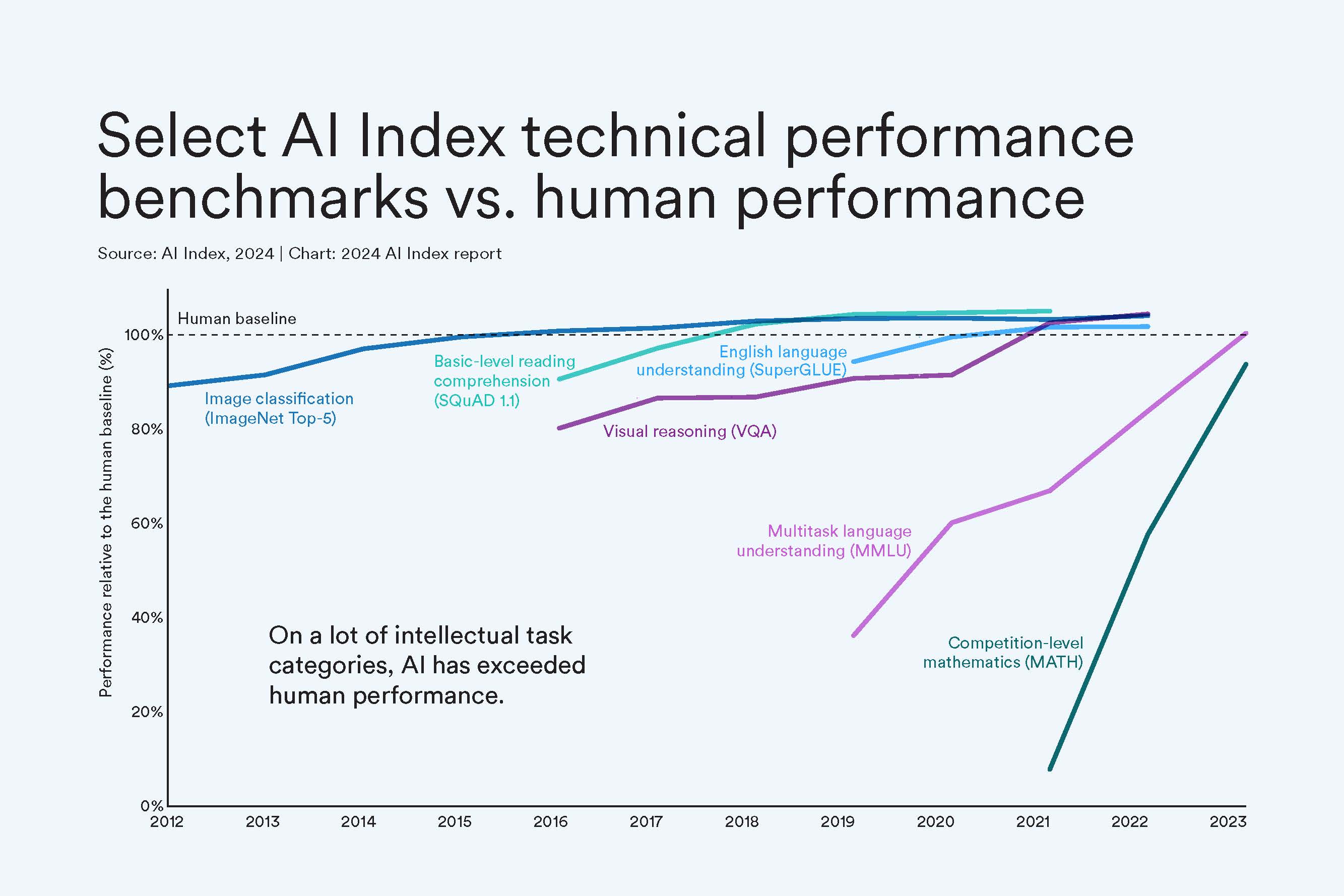
Move Over, Human
As of 2023, AI has hit human-level performance on many significant AI benchmarks, from those testing reading comprehension to visual reasoning. Still, it falls just short on some benchmarks like competition-level math. Because AI has been blasting past so many standard benchmarks, AI scholars have had to create new and more difficult challenges. This year’s index also tracked several of these new benchmarks, including those for tasks in coding, advanced reasoning, and agentic behavior.
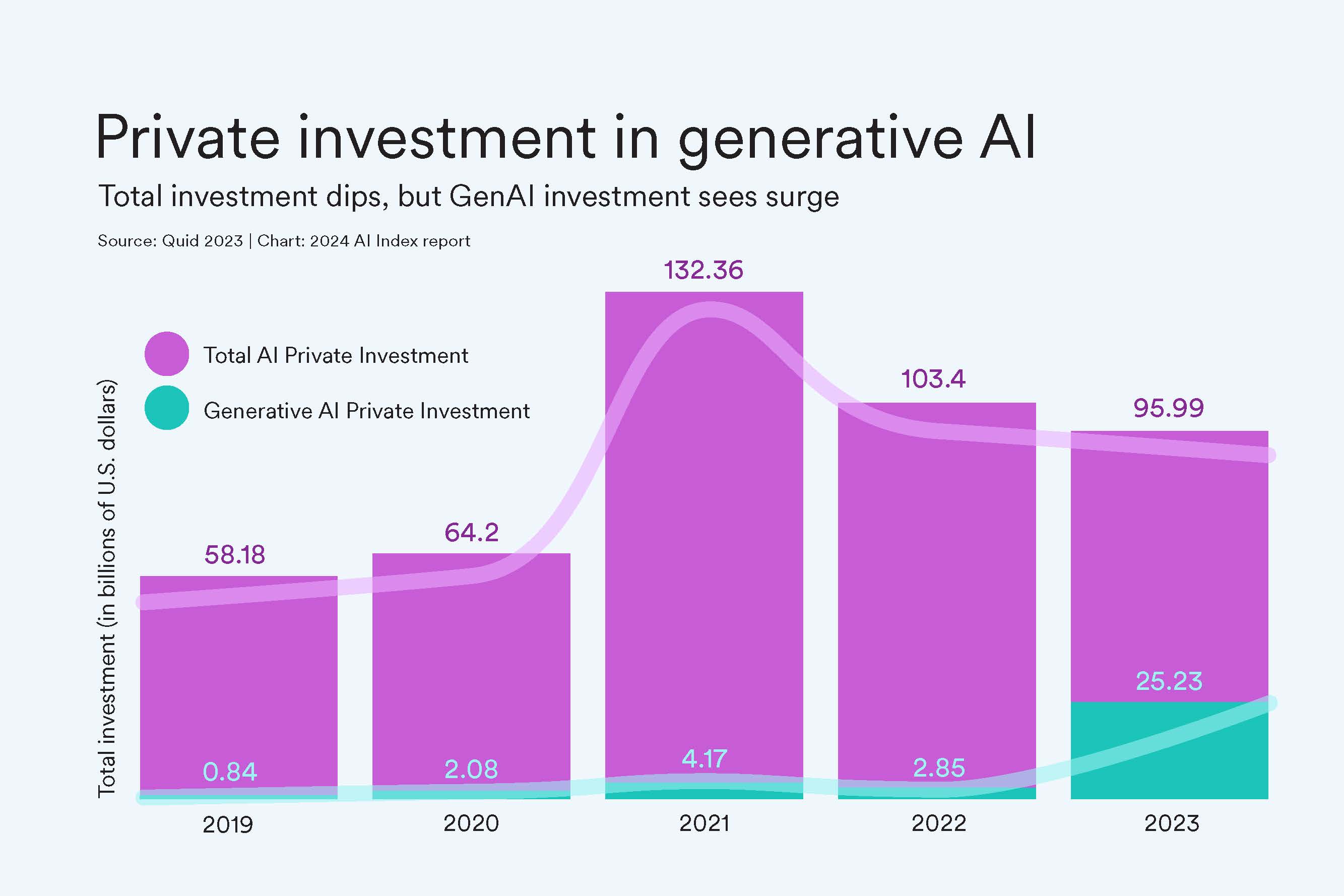
Private Investment Drops (But We See You, GenAI)
While AI private investment has steadily dropped since 2021, generative AI is gaining steam. In 2023, the sector attracted $25.2 billion, nearly ninefold the investment of 2022 and about 30 times the amount from 2019 (call it the ChatGPT effect). Generative AI accounted for over a quarter of all AI-related private investments in 2023.
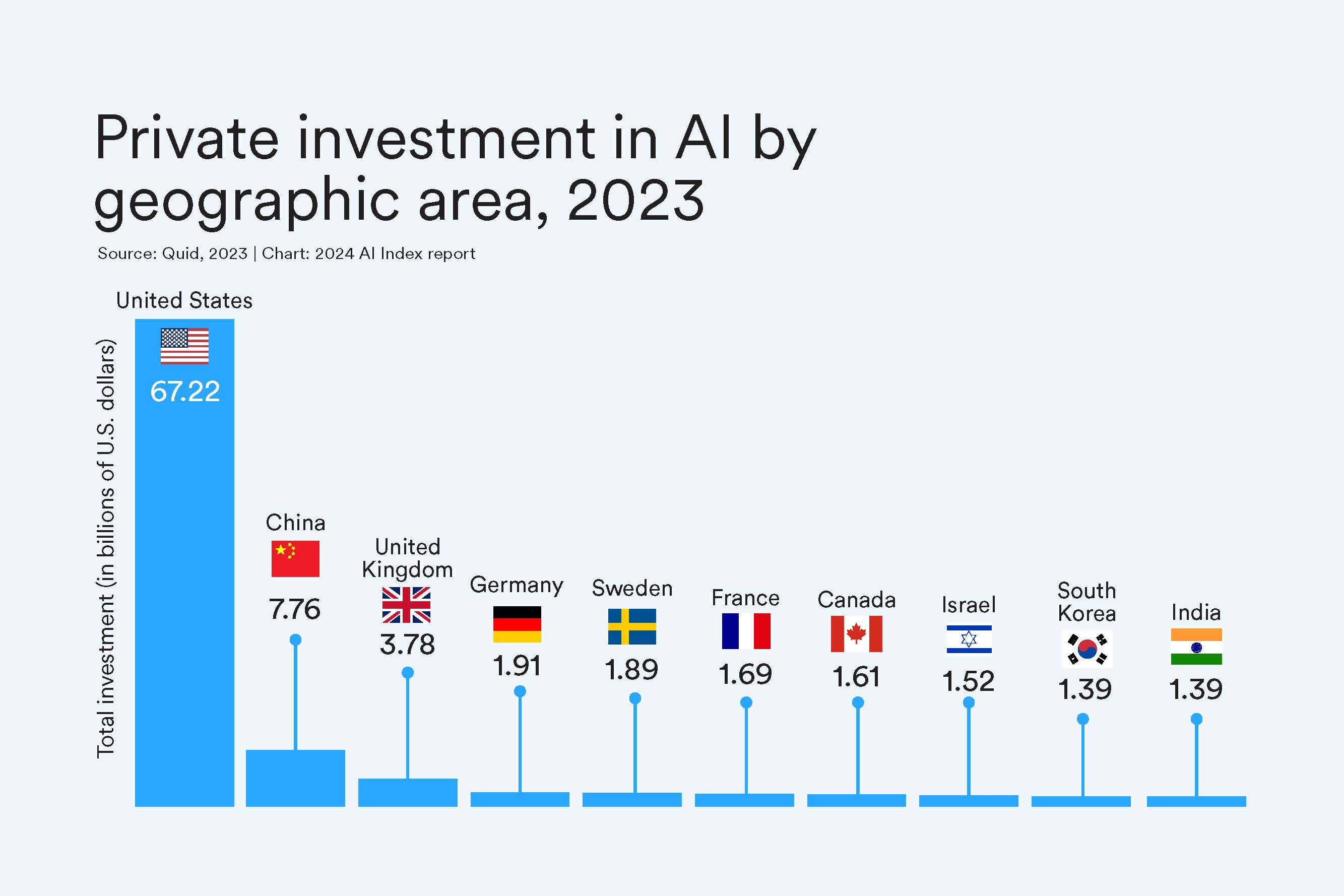
U.S. Wins $$ Race
And again, in 2023 the United States dominates in AI private investment. In 2023, the $67.2 billion invested in the U.S. was roughly 8.7 times greater than the amount invested in the next highest country, China, and 17.8 times the amount invested in the United Kingdom. That lineup looks the same when zooming out: Cumulatively since 2013, the United States leads investments at $335.2 billion, followed by China with $103.7 billion, and the United Kingdom at $22.3 billion.
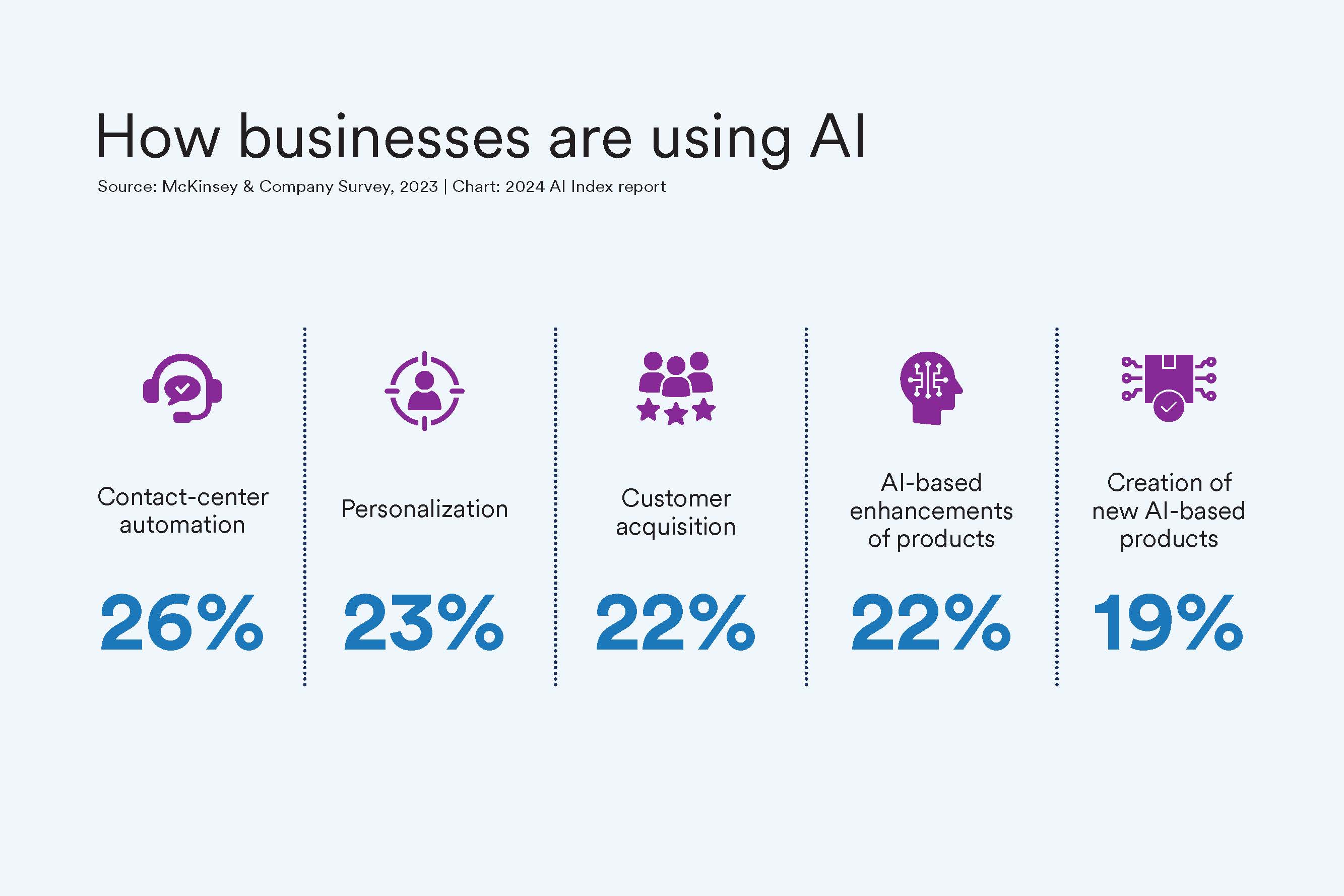
Where is Corporate Adoption?
More companies are implementing AI in some part of their business: In surveys, 55% of organizations said they were using AI in 2023, up from 50% in 2022 and 20% in 2017. Businesses report using AI to automate contact centers, personalize content, and acquire new customers.
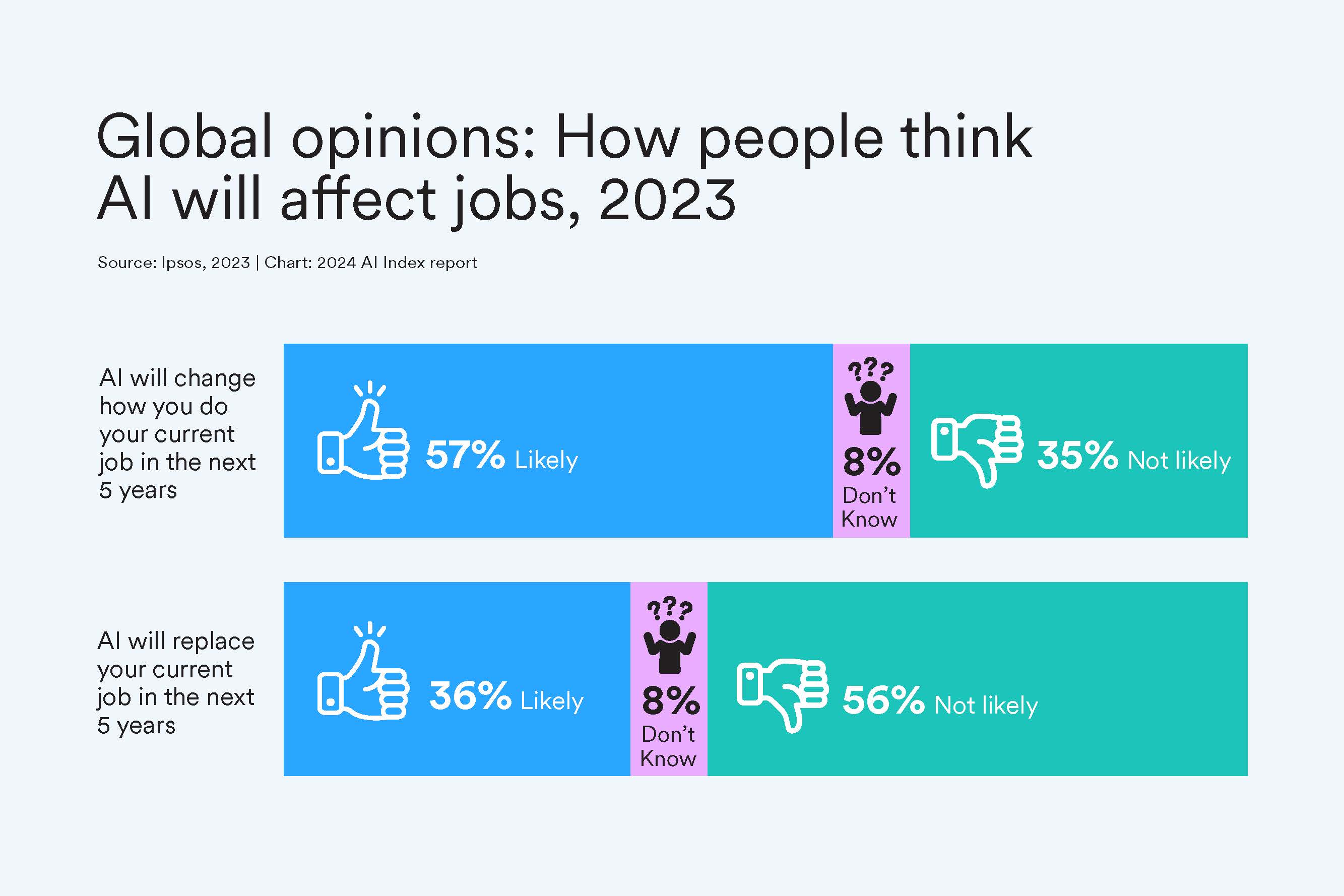
Younger and Wealthier People Worry About Jobs
Globally, most people expect AI to change their jobs, and more than a third expect AI to replace them. Younger generations — Gen Z and millennials — anticipate more substantial effects from AI compared with older generations like Gen X and baby boomers. Specifically, 66% of Gen Z compared with 46% of boomer respondents believe AI will significantly affect their current jobs. Meanwhile, individuals with higher incomes, more education, and decision-making roles foresee AI having a great impact on their employment.
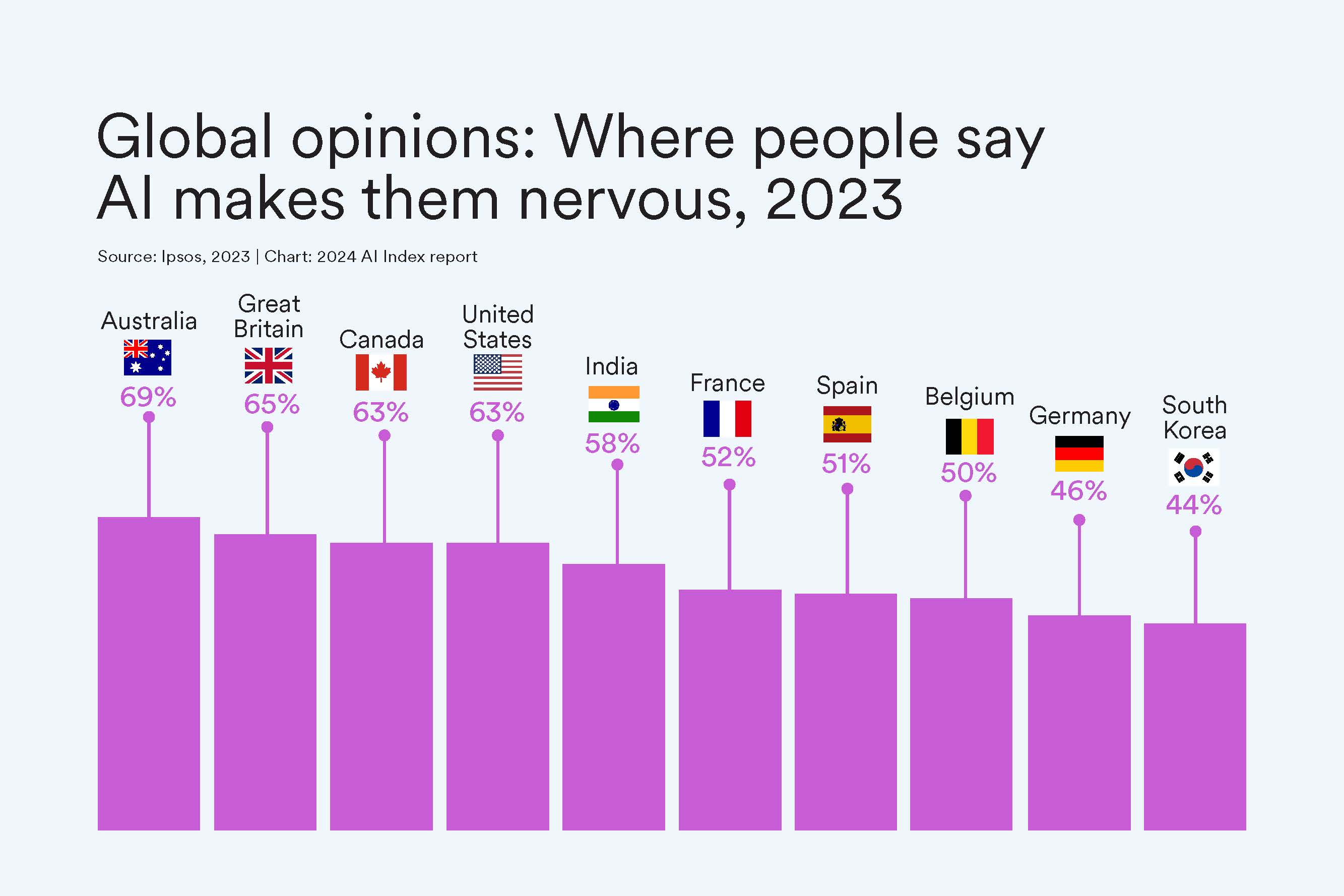
While the Commonwealth Worries About AI Products
When asked in a survey about whether AI products and services make you nervous, 69% of Aussies and 65% of Brits said yes. Japan is the least worried about their AI products at 23%.
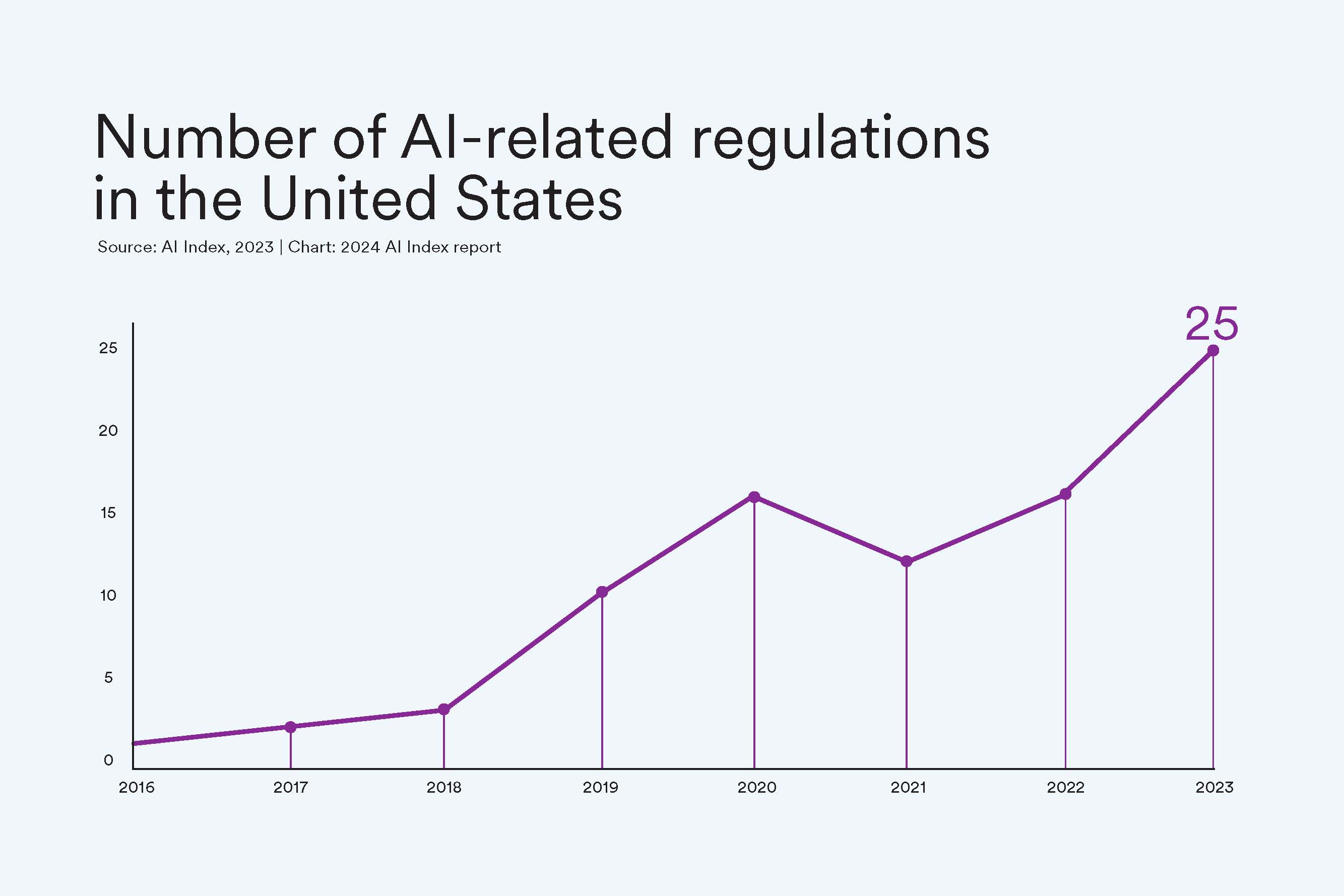
Regulation Rallies
More American regulatory agencies are passing regulations to protect citizens and govern the use of AI tools and data. For example, the Copyright Office and the Library of Congress passed copyright registration guidance concerning works that contained material generated by AI, while the Securities and Exchange Commission developed a cybersecurity risk management strategy, governance, and incident disclosure plan. The agencies to pass the most regulation were the Executive Office of the President and the Commerce Department.
The AI Index was first created to track AI development. The index collaborates with such organizations as LinkedIn, Quid, McKinsey, Studyportals, the Schwartz Reisman Institute, and the International Federation of Robotics to gather the most current research and feature important insights on the AI ecosystem.
More News Topics

IMAGES
COMMENTS
The number of older people, including those living with dementia, is rising, as younger age mortality declines. However, the age-specific incidence of dementia has fallen in many countries, probably because of improvements in education, nutrition, health care, and lifestyle changes. Overall, a growing body of evidence supports the nine potentially modifiable risk factors for dementia modelled ...
The National Dementia Strategy (Department of Health 2009) sets out the aim that ^All people with dementia and their carers should live well with dementia. The aim of this research is to examine what it means to live well with dementia, and to further our understanding of the social factors that enable people to live well with dementia.
INTRODUCTION. Dementia is a common public health problem. 1 Worldwide, approximately 47 million people have dementia and this number is expected to increase to 131 million by 2050. 1 Reductions in age-adjusted incidence of dementia have occurred in the United States (US) and other developed countries over the last 20 years, perhaps associated with increasing formal educational attainment.
NIH has released Advancing Alzheimer's Disease and Related Dementias Research for All Populations: Prevent.Diagnose. Treat. Care (PDF, 17.5M), a 2022 scientific progress report. This report provides a comprehensive overview of the meaningful progress researchers made from April 2021 through March 2022 to address the enormous challenges of Alzheimer's and related dementia diseases.
1. Introduction. Alzheimer's disease (AD) (named after the German psychiatric Alois Alzheimer) is the most common type of dementia and can be defined as a slowly progressive neurodegenerative disease characterized by neuritic plaques and neurofibrillary tangles (Figure 1) as a result of amyloid-beta peptide's (Aβ) accumulation in the most affected area of the brain, the medial temporal ...
An estimated 6.7 million Americans are currently living with Alzheimer's disease.. Worldwide, more than 50 million people have dementia, a diagnosis that may include Alzheimer's or a related disorder such as frontotemporal disorders, Lewy body dementia, or vascular dementias.This public health challenge takes a tremendous emotional, physical, and financial toll on those living with these ...
and improve quality of life for patients and carers living with dementia in the community, including care homes?' 1.3 REPORT STRUCTURE This report of the literature review consists of three further sections covering: Literature search, study selection and data retrieval; Results from the literature reviewed;
Global efforts to summarise the prevalence and burden of dementia, including the World Dementia Report and the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD), can serve to guide resource allocation and health policy decision making. ... General GBD methods can be found in the GBD 2019 summary papers and in the appendix (pp 10 ...
The WH Dementia report estimates there ere .7 million ne cases o dementia in the ear 2010, or one ne case ever our seconds . hat is alread three times as man as IV/AID 2. million per ear . Assumin that incidence ill increase in line ith prevalence, since lobal agein is drivin oth numbers, b 2050 the incidence ill have increased to 24. million
Essay On A Word: A Lived Experience Of Alzheimer—s Disease. February 2002. Dementia 1 (1):7-10. DOI: 10.1177/147130120200100103. Authors: Gloria J. Sterin. To read the full-text of this research ...
Tips and templates for producing dementia-friendly documents, ... Example of report updating on progress during an ongoing project . This is a pdf of a presentation which was shared in whole, or in part, according to people's access needs and preferences. It tells the story of an ongoing project - to develop this resource - so that people can ...
to the disease that was shattering my grandparents' world. It wasn't until my senior year of high school that my world started to really expand and I started to pay attention to and respect my
The signs and symptoms can vary depending on the type and may include: Experiencing memory loss, poor judgment, and confusion. Difficulty speaking, understanding and expressing thoughts, or reading and writing. Wandering and getting lost in a familiar neighborhood. Trouble handling money responsibly and paying bills.
Tough Decisions About Dementia and End-of-Life Care. Readers discuss Sandeep Jauhar's guest essay about dementia and advance directives. To the Editor: Re " My Father Didn't Want to Live if ...
108 Dementia Essay Topic Ideas & Examples. Dementia is a complex and challenging condition that affects millions of people worldwide. Writing an essay on dementia can be a great way to raise awareness about this condition, explore its causes and symptoms, and discuss potential treatments and care strategies. However, coming up with a unique and ...
The purpose of this report is to investigate the causes of dementia and explore the role of a mental health nurse in helping patients to manage the condition. Pharmacotherapy for Dementia. The prevalence of the disease is yet relatively low but is projected to grow, at least in the United States.
Dementia is a syndrome that involves severe loss of cognitive abilities as a result of disease or injury. Dementia caused by traumatic brain injury is often static, whereas dementia caused by ...
Modelling of the UK change suggests a 57% increase in the number of people with dementia from 2016 to 2040, 70% of that expected if age-specific incidence rates remained steady, 10 such that by 2040 there will be 1·2 million UK people with dementia.
The aim of the nurse is to have a person-centred dementia care, a holistic approach on caring for someone as a whole person and endeavouring to meet all their needs instead of focusing only on the physical aspect (Newton, 1991). The nurse should involve the patient and family on making a plan of care, as this preserve the dignity and respect of ...
Before Márquez's death, it was discovered that the cause of this familial dementia which went back for hundreds of years was a mutation in the presenilin 1 gene, a known genetic cause of early ...
Dementia is a protracted deterioration in memory, thinking, and reasoning competence. Alzheimer's disease usually manifests in patients after the age of 60. Dementia of Alzheimer's Type: Signs and Symptoms. This paper seeks to analyze dementia that comes about as a result of Alzheimer's disease.
A brief overview of the key features of each of the papers is presented in Table 1.All but one of the included papers were from high income countries, with the majority from the United Kingdom (n = 34), and then the United States (n = 15), Australia (n = 12), Canada (n = 4), Italy (n = 1) and India (n = 1).The papers were split into types, with twenty-two guidance and inspection documents, and ...
Alzheimer's & Dementia is delighted to announce a Call for Papers for a Special Issue of Alzheimer's & Dementia dedicated to advancing the science of Alzheimer's disease and related dementias (AD/ADRD) care and caregiving and reflecting the multifaceted and pressing challenges within this domain. This issue aims to encapsulate the diverse ...
More than half a million people with dementia could go undiagnosed and untreated in the UK by 2040, without a radical shift in detection of the disease, a major report shows. Regulators are ...
For the new study, researchers analyzed data on nearly 174,000 people in England diagnosed with dementia between January 1988 and May 2018, at an average age of 82.
Austad also points to a British study that found a lower risk of dementia and mild cognitive decline among people with type 2 diabetes taking metformin. In addition, there's research pointing to ...
Ongoing NIH-funded microbiome research may also help explain why subgroups of people with Alzheimer's respond differently to dietary and other interventions. Research also points to a gut-liver-brain connection in dementia through the production of bile acids as well as key lipids that are needed by the brain.
Mission. The AI Index report tracks, collates, distills, and visualizes data related to artificial intelligence (AI). Our mission is to provide unbiased, rigorously vetted, broadly sourced data in order for policymakers, researchers, executives, journalists, and the general public to develop a more thorough and nuanced understanding of the complex field of AI.
How Pew Research Center will report on generations moving forward. Journalists, researchers and the public often look at society through the lens of generation, using terms like Millennial or Gen Z to describe groups of similarly aged people. This approach can help readers see themselves in the data and assess where we are and where we're ...
This year's AI Index — a 500-page report tracking 2023's worldwide trends in AI — is out.. The index is an independent initiative at the Stanford Institute for Human-Centered Artificial Intelligence (HAI), led by the AI Index Steering Committee, an interdisciplinary group of experts from across academia and industry. This year's report covers the rise of multimodal foundation models ...