- Get new issue alerts Get alerts

Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
The effectiveness of yoga to prevent diabetes mellitus type 2
A protocol for systematic review and meta-analysis.
Ramamoorthi, Ramya BVSc, GCTLHE a,∗ ; Gahreman, Daniel PhD a ; Moss, Simon PhD a ; Skinner, Timothy MTEM, PhD b
a College of Health and Human Sciences, Charles Darwin University, Darwin, Northern Territory, Australia
b Københavns Universitet, Institut for Psykologi, Center for Sundhed og Samfund, Københavns Universitet, Øster Farimagsgade, København, Denmark.
∗Correspondence: Ramya Ramamoorthi, Associate Lecturer in Clinical Sciences, Blue 5.1.43, College of Health and Human Sciences, Charles Darwin University, Ellengowan Drive, Darwin, Northern Territory 0909, Australia (e-mail: [email protected] ).
Abbreviations: CMA = comprehensive meta-analysis, DBP = diastolic blood pressure, FBG = fasting blood glucose, HbA1c = hemoglobin A1C, HDL = high-density lipoprotein, LDL = low-density lipoprotein, PPBG = postprandial blood glucose, PRISMA-P = Preferred Reporting Items for Systematic Reviews and Meta-Analysis for Protocols, PROSPERO = the protocol in the International Prospective Register of Systematic Reviews, SBP = systolic blood pressure, SMD = standardized mean difference, T2DM = type 2 diabetes mellitus, TC = total cholesterol, VLDL = very low-density lipoproteins.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website ( www.md-journal.com ).
This is an open access article distributed under the Creative Commons Attribution License 4.0 (CCBY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. http://creativecommons.org/licenses/by/4.0
Background:
Type 2 diabetes mellitus (T2DM) is becoming a leading problem worldwide. Emerging reports reveal alarming evidence of increasing prevalence of T2DM that has reached pandemic levels. Despite the significant incidence, there are limited reliable data resources and comprehensive systematic review and meta-analysis on the effects of yoga on people who are a prediabetic or high risk for developing T2DM.
Objective:
The objective of this protocol is to conduct a full-scale systematic review and meta-analyses on the effects of yoga on people who are prediabetes or high risk of developing T2DM.
Methods:
The articles enrolled in the study will be retrieved from the online databases between 2002 and the date the searches are executed. The searches will be repeated just before the final analyses and further relevant studies for inclusion. We will conduct a bibliographic search in databases: Medline/PubMed, Scopus, Cochrane Library, EBSCO, and IndMED using keywords including prediabetes state, high risk for diabetes, metabolic syndrome, and yoga. A defined search strategy will be implemented along with selection criteria to obtain full-text articles of relevant studies. This study protocol was prepared according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis for Protocols 2015 guidelines. There will be no language restrictions.
Ethics and dissemination:
The proposed study will be based on published studies and publicly available anonymized data without directly involving human participants and therefore requires neither formal human ethical review nor approval by a human research ethics committee. We published an outline of the protocol in the International Prospective Register of Systematic Reviews (PROSPERO) in 2018. We plan to disseminate the findings of this systematic review and meta-analysis through publication in a peer-reviewed journal and presentation at relevant conference proceedings. In addition, we believe the results of the systematic review will have implications for policy and practice. We will prepare policymaker summary using a validated format, disseminate through social media and email discussion groups.
Review registration number:
PROSPERO registration number CRD 42018106657
1 Introduction
Type 2 diabetes mellitus (T2DM) is one of the greatest public health challenges in today's world. [1] The body becomes either resistant to insulin or gradually loses the ability to produce insulin. [2] According to the World Health Organization, global report on diabetes incidence, an estimated 422 million people were found to be living with diabetes in 2014. [3] International Diabetes Federation reported that it is estimated that there will be 629 million people with diabetes by the end of 2045, and diabetes-related health expenditure will exceed US$776 billion. [4]
The development and maintenance of T2DM are attributed to sedentary lifestyle, [5] unhealthy diet, and psychologic stress. Psychologic stress has a strong correlation with both the risk factors [6–8] and maintenance of the disease. [9,10] Several acquired risk factors such as prediabetic state contribute to the development of T2DM apart from the genetic background. [5,11]
Many complementary and alternative practices are explored by people in both the prevention and treatment of diabetes. [12,13] Yoga is one such Eastern practice that originated in India over 5000 years ago principally to develop mental faculties. [14,15] Yoga advocates that a healthy body is a by-product of healthy mind. [16,17] Most importantly, a growing body of research suggests that the practice of yoga may reduce insulin resistance syndrome and may attenuate signs, reduce complications, and improve the prognosis of diabetes. [18–23] Also, studies have shown that the progression of diabetic condition from prediabetes could be either delayed or halted with regular physical activity, [24–27] healthy diet, [27] and effective stress management. [28,29]
It is proposed yoga intervenes T2DM by 2 proposed mechanisms downregulation of both the hypothalamic pituitary adrenal axis and the sympathetic nervous system. [30–32]
2 Rationale
2.1 what is the issue and how will our study address this.
Several studies in the prediabetic population show the effectiveness of yoga in reducing the risk of progression to diabetic state. [33–36] There is not a single review to show the benefits of yoga in prediabetes. This will be the 1st systematic review that will show evidence that yoga significantly affects the prediabetic state and will summarize the results of these available studies. This study will announce the gaps in present research and will set directions for future research. The study will further provide evidence for people to adopt yoga practise as an attractive alternative to other forms of physical training especially for people who are discouraged by the perceived rigor of other exercises.
2.2 Review questions
The aim of our systematic review and meta-analysis protocol is to describe the methodologic approach for conducting a systematic review and meta-analysis to examine the effects of yoga on people who are prediabetic or high risk for developing T2DM.
The questions for this review are as follows:
- 1. Does yoga delay or prevent the progression of diabetes in prediabetic population?
- 2. What is the significance of yoga compared with exercise in a prediabetic population?
- 3. How much does the effect size of physiologic outcomes vary across studies and subgroups?
3.1 Search strategy and study selection
The proposed systematic review and meta-analysis will be performed according to the guidelines of Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement issued in 2015. The authors will consider the published studies explaining the effectives of yoga in prediabetic and metabolic syndrome with no restrictions on study participant's age, ethnicity, morbidity, and occupation. There will be no language restrictions. The authors will perform a literature search using 5 computerized English and Indian scientific electronic bibliographic databases: PubMed, Scopus, Cochrane Library, EBSCO, and IndMED. The search strategy will include only search terms related to “yoga” and “cardiovascular disease risk factors” and adapted for each database as necessary. Studies published between 2002 and the date the searches executed will be sought. The searches will be repeated just before the final analyses. Many searches in the proposed study will be undertaken to ensure the identification of eligible studies using one of the several search term combinations for the effectiveness of yoga on the high risk of diabetes or prediabetic population or metabolic syndrome (prediabetes state, high risk for diabetes, metabolic syndrome, and yoga). Keywords used were: “yoga [abstract],” “prediabetes [abstract],” and “glucose [text].” Keywords used included yoga + type 2 diabetes [in the title of the article], yoga + type II diabetes [in the title of the article], and exercise therapy + type 2 diabetes patients [in the title of the article].
3.2 Search strategy
A draft search strategy for the databases and search string that will be used to identify the studies describing effects of yoga in prediabetic and metabolic syndrome is shown in Table 1 .

3.3 Study selection criteria
3.3.1 inclusion criteria.
- 1. Study examining yoga intervention (including at least one of asana, pranayama, meditation) to promote T2DM management and comparing yoga intervention with other usual care or physical exercise or nontherapeutic intervention.
- 2. Study that is randomized control trial, randomized cross-over studies, cluster-randomized trials, or quasi-experimental design will be included.
- 3. Studies evaluating the primary outcome measure-glycemic control, measured in both the intervention and control group conditions as well as other measures such as HbA1c, blood pressure, or fasting blood sugar, and lipid profile (triglycerides, high-, and low-density lipoprotein [HDL and LDL] cholesterol, systolic blood pressure [SBP], and diastolic blood pressure [DBP]) will be included.
- 4. Study participants must be prediabetic or designated as high risk for diabetes because of physiologic measures, and the outcomes must be reported specifically for each group
3.3.2 Exclusion criteria
- 1. Studies will be excluded if participants were members of a specific age group, such as adolescents or geriatric age groups.
- 2. Studies will be excluded if participants were all in a transient state, such as pregnancy or menopause.
- 3. Studies will be excluded if the yoga intervention was modified to a dance program.
- 4. Conference proceedings, editorials, commentaries, and book chapters/book reviews will be excluded.
3.4 Data extraction and management
Two authors will be involved in data extraction and independently evaluate the published studies with the selection criteria, and corresponding authors will be contacted for missing information in the studies. Data will be extracted on study design and methods, demographic characteristics of study participants, as well as details of yoga interventions, control interventions, and outcome measures PRISMA guidelines will be used to prepare the data extraction form using MS Excel data extraction form. This form will be utilized to standardize the data collection process.
3.5 Selection process
The relevant titles and abstracts will be screened with the selection criteria and PRISMA guidelines for eligibility by the first author. Potential full-text eligible articles will be downloaded and reviewed independently by the authors. The corresponding author will perform a final review for the double check to recover any omitted articles in the analysis. The references of the selected articles will be imported into EndNote file to form an initial list of eligible studies following that duplicates will then be removed. All the authors will be involved in the selection process, and a file will be removed only when there is an agreement that it did not fulfill the eligibility criteria. Any discrepancies associated with selection of the studies will be resolved by mutual discussions involving the third reviewer. The entire selection process is illustrated in Figure 1 .

3.6 Data items
The authors will extract 6 categories of data:
- 1. Bibliometric data (1st author, year of publication, country, a journal of publication, the study period).
- 2. Study design (a type of research, details of randomised control trial, randomized cross-over studies and cluster-randomized trials or quasi-experimental design and the validity of confirmative diagnosis and method of data collection).
- 3. Study participants characteristics (condition, age, gender, race, sample size, and sampling procedures).
- 4. Yoga interventions characteristics (yoga type: asana, pranayama, meditation, components, frequency, duration).
- 5. Control interventions characteristics (type: usual care or physical exercise or nontherapeutic intervention, frequency, duration).
- 6. Outcome measures: SBP, DBP; heart rate; respiratory rate; abdominal obesity (waist circumference, waist-hip ratio, index of central obesity); blood lipid levels (triglycerides, HDL, and LDL cholesterol); glycemic control (both the intervention and control group conditions, such as HbA1c, blood pressure, or fasting blood sugar).
3.7 Study outcomes
3.7.1 primary outcome.
The primary outcome is to measure the glycemic control (HbA1c, fasting blood glucose [FBG], and postprandial glucose [PPBG]) in both the intervention and control group conditions.
3.7.2 Secondary outcomes
The secondary outcomes are to measure other markers of diabetes management including triglycerides, HDL, LDL, SBP, DBP, body composition, and fasting cortisol.
3.8 Assessment of risk of bias
Risk of bias of included studies will be assessed using the Cochrane Risk of Bias Assessment tool that contains several items under 7 categories such as random sequence generation, allocation concealment, blinding of participants and investigators, the blindness of outcome assessments, incomplete outcome data, selective outcome reporting, and other biases. Based on the assessment, the studies will be evaluated as low, unclear, or high bias. The Jadad scale will be used to evaluate the quality of each trial where three domains in the scale cover Randomization (0–2 points), blinding (0–2 points), and dropouts and withdrawals (0–1 point). A trial with a score ≤2 indicates low quality while a score of ≥3 indicates high quality. Assessment of publication bias will be performed using funnel plots generated by Comprehensive Meta-Analysis (CMA) 3.0 software.
3.9 Data synthesis
Data will be synthesised into 3 different steps:
- 1. Step 1 will provide a descriptive overview (qualitative data synthesis or Systematic Review based on selection criteria and PRISMA guidelines).
- 2. Step 2 will provide a quantitative analysis of the characteristics of the selected studies (calculation of pooled estimates and meta-analysis). Meta-analysis will calculate pooled estimates of the study findings on the effectiveness of yoga interventions on the glycemic status of the prediabetic population.
- 3. Step 3 will examine the influence of study, participant, and outcome characteristics based on the difference in intervention among the prediabetic population by conducting subgroup and meta-regression analyses.
3.10 Meta-analysis
Meta-analyses on the effectiveness of yoga interventions on the glycemic status of the prediabetic population will be performed using CMA 3.0 for the obtained pooled estimates, standardized mean difference (SMD) and 95% confidence intervals from the included studies. Forest plots will be generated to show the pooled effect size of the study findings and with random-effects models of meta-analysis due to between-study heterogeneity into the model. Heterogeneity will be calculated using Cochrane Q test and I 2 statistic. Q statistics will be estimated for each outcome and provides a test of the null hypothesis that all studies in the proposed meta-analysis share a common effect size. If all studies shared the same effect size, the expected value of Q would be equal to the degrees of freedom (the number of studies minus 1). I 2 statistics informs what proportion of the observed variance reflects the difference in true effects sizes rather than sampling error. Z -statistic will be performed to assess heterogeneity. FBG, PPBG, TC, LDL-c, VLDL-c, HDL-c, and TG are reported as mg/dL, where studies reported as mmol/L a numerical conversion to mg/dL will be done. HbA1c is reported.
3.11 Publication bias
Publication bias of the included studies will be assessed using Egger bias indicator test, Orwin and Classic fail-safe N test, Begg and Mazumdar rank collection test, Duval and Tweedie trim and fill [37] calculation. An inverted funnel plot will be constructed simultaneously alongside the forest plot, with the aid of SMD (SMD values used in the meta-analysis) and the standard error. The symmetrical funnel plots will indicate low risk, and asymmetrical funnel plots will indicate a high risk of publication bias.
3.12 Subgroup analyses
Subgroup analyses will be performed according to study, participant and outcome characteristics and methodologic factors if sufficient studies and retrieved data are identified and available. We plan to investigate specific subgroup analyses according to differences in intervention and key features of identified study participants such as condition, age, gender, race, sample size, and sampling procedures, follow-up, clinical setting, of prediabetic participants. Further, specific subgroup analyses will be performed based on the outcome measures such as blood pressure (systolic, diastolic); heart rate; respiratory rate; abdominal obesity (waist circumference, waist-hip ratio, index of central obesity); blood lipid levels (triglycerides, HDL, and LDL cholesterol); glycemic control (both the intervention and control group conditions, such as HbA1c, blood pressure, or fasting blood sugar) (if sufficient additional information is identified and available). Tables, flowchart, and figures will be plotted to depict the results appealingly.
4 Meta-regression
Prediabetic participant characteristics such as gender, methods of data collection, sample size, research quality, and sampling procedure will be evaluated. A random-effects model will be selected and assigned to weight for each study by calculating R 2 with the quantity of the proposed variance. The heterogeneity of intervention associations with one or more study variables will be explained using meta-regression analysis.
5 Reporting of this review and its findings
The findings will be published as per PRISMA guidelines. [38] A flowchart will be employed to outline the selection process ( Fig. 1 ). Text description will be used to review the qualitative data of the included studies. Outputs of meta-analyses will be depicted in a forest plot. Publication bias will be represented in the inverted funnel plot. The search strategy will be provided in Supplement Table 1 (Supplemental Digital Content, https://links.lww.com/MD/C749 ).
Author contributions
Conceptualization: Ramya Ramamoorthi, Daniel Gahreman, Simon Moss, Timothy Skinner.
Data curation: Ramya Ramamoorthi, Daniel Gahreman, Simon Moss.
Investigation: Ramya Ramamoorthi.
Methodology: Ramya Ramamoorthi.
Project administration: Ramya Ramamoorthi.
Resources: Ramya Ramamoorthi.
Supervision: Daniel Gahreman, Simon Moss, Timothy Skinner.
Validation: Ramya Ramamoorthi, Daniel Gahreman.
Visualization: Ramya Ramamoorthi.
Writing – original draft: Ramya Ramamoorthi.
Writing – review & editing: Ramya Ramamoorthi, Daniel Gahreman, Simon Moss, Timothy Skinner.
- Cited Here |
- PubMed | CrossRef |
- Google Scholar
- View Full Text | PubMed | CrossRef |
meta-analyses; prediabetes; protocol; systematic review; yoga
Supplemental Digital Content
- MD_2018_12_21_RAMAMOORTHI_MD-D-18-08782_SDC1.doc; [Word] (36 KB)
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Effects of high-intensity interval training versus moderate-intensity..., effect of physical activity interventions on quality of life in older adults: a ..., aging-related changes in knee flexor muscle strength and cross-sectional area, multidimensional analyses of the effect of exercise on women with depression: a ..., the effects of high impact exercise intervention on bone mineral density,....
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Can Yoga Reduce Blood Sugar Levels for People With Type 2 Diabetes?
Thomas Barwick / Getty Images
Key Takeaways
- Mind-body practices like yoga may help lower blood sugar levels in people with type 2 diabetes, according to a new review of studies.
- The reduction in A1C levels from yoga was significant, only 0.1% behind metformin.
- Adding mind-body practices to a doctor-prescribed regimen could help some people better manage their condition.
Yoga may significantly lower blood sugar in people with type 2 diabetes, according to a new study by the University of California’s Keck School of Medicine, which was the first to rigorously quantify the impact of mind-body practices on blood sugar.
After analyzing 28 studies conducted between 1993 and 2022, the researchers found a link between practicing yoga and reduced hemoglobin A1C levels. Specifically, people who practiced yoga were able to lower A1C levels by 1%.
While this number seems small, it is comparable to the reduction provided by metformin, the most common diabetes drug, the researchers noted. Metformin typically reduces hemoglobin A1C levels by an average of 1.1%.
What Is A1C?
A1C measures average blood sugar levels over the last three months. An A1C of 6.5% or higher is indicative of diabetes, and keeping levels under 7% is considered good control. Levels of 9% and higher are considered dangerous.
In addition to yoga, researchers looked at the impact of meditation, qigong, and mindfulness-based stress reduction techniques. All led to reductions in blood sugar levels, yielding a cumulative 0.84% reduction in A1C levels.
The researchers noted that a general target for diabetes control is to get A1C levels under 7%, but only about half of people who are diagnosed with type 2 diabetes meet this goal. The study results suggest that adding mindfulness practices to an existing medication regimen could help people more effectively manage their condition.
“Type 2 diabetes is a major chronic health problem and we are not doing a good enough job at controlling it,” lead study author Fatimata Sanogo, a PhD student in the Department of Population and Public Health Sciences at University of California’s Keck School of Medicine, said in a press release . She added that the team was taken by surprise by how successful these practices appear to be at managing blood sugar.
“We expected there to be a benefit, but never anticipated it would be this large,” Sanogo said.
Live Well With Diabetes
We're launching a new newsletter to help you manage your type 2 diabetes. Every week, we'll share symptom management advice, medication news, and more.
Sign Up Now
Why Does This Benefit Exist?
Marisa Gefen, MD, a Philadelphia-based physician who treats patients with diabetes and other chronic conditions at Oak Street Health, told Verywell that the reason mind-body practices may be so beneficial in this population is due to the impact that stress can have on blood sugar.
In the body, adrenal glands respond to stress by releasing a the hormone cortisol, which can raise blood sugar levels. Further, people with diabetes tend to have high baseline levels of cortisol. Mind-body practices can work against this, providing both physical and mental health benefits that can reduce cortisol levels.
“When you’re exercising, your body is able to decrease blood sugar because the muscles are utilizing it,” Gefen said. “So the physical practice of something like yoga and Tai chi will lower blood sugar that way, and the mindfulness component will decrease levels of cortisol.”
How long will the benefits last? Gefen said results seem to depend on how diligent a person is about their workout or meditative regimen.
“If you’re doing it once in a blue moon, I think it will benefit you while you’re doing it and a couple hours afterwards,” Gefen said. “If you do it consistently on a regular basis, it provides a more cumulative effect that will benefit you in the long term.”
“Diabetes Doctor” Stephanie Redmond, PharmD, CDE, BC-ADM , wrote in an email to Verywell that in addition to reducing cortisol and blood sugar levels, mindfulness practices may indirectly impact other lifestyle behaviors for the better. This can include helping a person not to overeat, or promoting healthy sleep, she said.
Medication Still Matters
Still, mind-body practices aren’t a replacement for prescription medication, insulin, proper diet, or other doctor-recommended strategies for managing type 2 diabetes.
“It is not ‘instead of’ medication,” Gefen said of practices like yoga. “It’s very important to follow your doctor’s orders and make sure that you’re taking any medication as prescribed. If you [practice yoga] consistently and you do see some blood sugar lowering, there is a possibility your doctor might lower some medication doses over time, but it’s absolutely not ‘instead of’ prescribed modalities for blood-sugar lowering.”
What This Means For You
Mind-body practices like yoga could help lower blood sugar levels in people with type 2 diabetes. While it can be a good idea to incorporate these practices into your diabetes management routine, yoga is not a replacement for prescribed medications or other physician-prescribed care.
Sanogo F, Xu K, Cortessis VK, Weigensberg MJ, Watanabe RM. Mind- and body-based interventions improve glycemic control in patients with type 2 diabetes: a systematic review and meta-analysis . J Integr Complement Med . Published online September 7, 2022. doi:10.1089/jicm.2022.0586
Dias JP, Joseph JJ, Kluwe B, et al. The longitudinal association of changes in diurnal cortisol features with fasting glucose: MESA . Psychoneuroendocrinology . 2020;119:104698. doi:10.1016/j.psyneuen.2020.104698
Joseph JJ, Golden SH. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus . Ann N Y Acad Sci . 2017;1391(1):20-34. doi:10.1111/nyas.13217
By Claire Wolters Claire Wolters is a former staff reporter covering health news for Verywell. She is most passionate about stories that cover real issues and spark change.
- Short report
- Open access
- Published: 23 December 2021
Diabetic yoga protocol improves glycemic, anthropometric and lipid levels in high risk individuals for diabetes: a randomized controlled trial from Northern India
- Navneet Kaur 1 , 4 ,
- Vijaya Majumdar 2 ,
- Raghuram Nagarathna 2 ,
- Neeru Malik 3 ,
- Akshay Anand 4 &
- Hongasandra Ramarao Nagendra 2
Diabetology & Metabolic Syndrome volume 13 , Article number: 149 ( 2021 ) Cite this article
3191 Accesses
10 Citations
194 Altmetric
Metrics details
To study the effectiveness of diabetic yoga protocol (DYP) against management of cardiovascular risk profile in a high-risk community for diabetes, from Chandigarh, India.
The study was a randomized controlled trial, conducted as a sub study of the Pan India trial Niyantrita Madhumeha Bharath (NMB) . The cohort was identified through the Indian Diabetes Risk Scoring (IDRS) (≥ 60) and a total of 184 individuals were randomized into intervention (n = 91) and control groups (n = 93). The DYP group underwent the specific DYP training whereas the control group followed their daily regimen. The study outcomes included changes in glycemic and lipid profile. Analysis was done under intent-to-treat principle.
The 3 months DYP practice showed diverse results showing glycemic and lipid profile of the high risk individuals. Three months of DYP intervention was found to significantly reduce the levels of post-prandial glucose levels (p = 0.035) and LDL-c levels (p = 0.014) and waist circumference (P = 0.001).
The findings indicate that the DYP intervention could improve the metabolic status of the high-diabetes-risk individuals with respect to their glucose tolerance and lipid levels, partially explained by the reduction in abdominal obesity. The study highlights the potential role of yoga intervention in real time improvement of cardiovascular profile in a high diabetes risk cohort.
Trial registration: CTRI, CTRI/2018/03/012804. Registered 01 March 2018—Retrospectively registered, http://www.ctri.nic.in/ CTRI/2018/03/012804.
Introduction
The rise of diabetes in the developing world poses a threat to meager health budgets. Owing to the strong association between various morbidity and mortality outcomes as complications of this dreaded disease, early detection of diabetes risk through non-invasive parameters is a primary requisite. Observational studies show that the risk reduction for diabetes can be decreased by 58% or 63–65% if risk factors could be controlled [ 1 , 2 ]. Many argue that such experimental strategies for the possible halting of conversion of prediabetes into diabetes must continue to include pharmacological interventions even though the rates have not been compared [ 3 ]. Identification of individuals at increased risk for the disease with invasive measurements of fasting and post challenge (postprandial) blood glucose are costly and time consuming. Hence, it has been advocated that the realistic prevention of diabetes should identify high-risk subjects with the use of the non-invasive risk scores [ 4 ]. Such studies should also target subjects with normoglycemia and prevent their progression to poor glycemic status [ 4 ].
Yoga plays a promising role in minimizing the risk of Diabetes for high-risk individuals with prediabetes [ 5 , 6 ]. It reduces body weight, glucose, and lipid levels, though, most of these studies comply with the guidelines of randomized controlled trials adhered to the CONSORT statements [ 7 , 8 , 9 , 10 , 11 ] whereas majority of studies have not reported as per CONSORT statements [ 12 , 13 , 14 , 15 ]. Several review of published studies, in people with diabetes and prediabetes, have concluded that the practice of yoga may reduce insulin resistance and related cardiovascular disease (CVD) risk factors and improve clinical outcomes [ 16 ]. Specifically, reports suggest that a yoga-based lifestyle intervention reduces body weight, glucose and lipid levels that should reduce diabetes risk. Keeping in view the high transition rates of diabetes in India, we selected a high-risk cohort from Chandigarh, one of the most affluent Union Territories of India with highest reported prevalence of diabetes in order to establish the efficacy of yoga to alleviate the cardiovascular disease. Indian Diabetes Risk Score (IDRS), specific for Indian ethnicity a validated tool was used for identification of the high-risk population [ 17 ]. We developed a national consensus ‘Diabetes Yoga protocol’ based on published reports and classical literature with an aim to stimulate weight reduction by combination of postures and meditation techniques [ 18 , 19 ]. Additionally, cardiometabolic risk reduction has also been recognized as one of the potential outcomes of yoga-based interventions [ 20 ]. Yoga has been shown to be regulating the risk parameters of diabetes, waist circumference (WC), body mass index (BMI), oxidative stress, fasting blood sugar (FBS) and systolic blood pressure (SBP) respectively [ 21 ]. Hence, in this study we tested the efficacy of diabetic yoga protocol (DYP) on alleviation of glycemic and lipid imbalances in individuals at high risk of diabetes.
Materials and methods
Study population.
Under the multi-region survey of Niyantarita Maduhmeha Bharat (NMB-2017) a door-to-door screening was carried out for the identification of high risk individuals among the population of Chandigarh (U.T) and Panchkula (District in Haryana state) on the basis of Indian Diabetes Risk Score (IDRS). The data collection was carried out by well trained yoga volunteers for diabetes management (YVDMs). Written informed consents were taken from every subject during door to door screening as well as at the time of registration. All the experimental protocol, methods and procedures were approved by Ethics committee of Indian Yoga Association (IYA) (ID: RES/IEC-IYA/001). All experiments methods and procedures were carried out in accordance with relevant guidelines and regulations of ethics committee. The study was registered at clinical trial registry of India, CTRI/2018/03/012804 (dated: 01/03/2018).
Study design
The present study is the two-armed randomized controlled trial conducted in the population of Chandigarh and Panchkula regions of northern India. Indian Diabetes Risk Score (IDRS) was used for detection of high risk (≥ 60 score) individuals from the study. Self-declared diabetics and low (< 30 score) and moderate [between 30–50 score] risk individuals were excluded from the study. As evident from the flow of patients presented in the flowchart, out of 1214 eligible subjects, there was approximately 50% loss of sample data due to error in the sampling. Further out of 564, we had to exclude as they were self-declared patients with diabetes and did not further participate in the study. However, this led to final participation of only 184 subjects in the study and allocation of these subjects diminishing the random selection of the study cohort. A cohort of high diabetes-risk cohort consisting of n = 184 participants was randomized into the interventional and control groups (n = 91:93). After excluding the dropouts from the study, based on CONSORT guidelines, the remaining subjects in the DYP and control group were further assessed for selected anthropometric, glycemic and lipid parameters. The intervention group was given the Diabetic Yoga Protocol for three months and control group continued with their daily routine activities. The detailed categorization of the samples is shown in Fig. 1 . The control group was waitlisted for yoga.
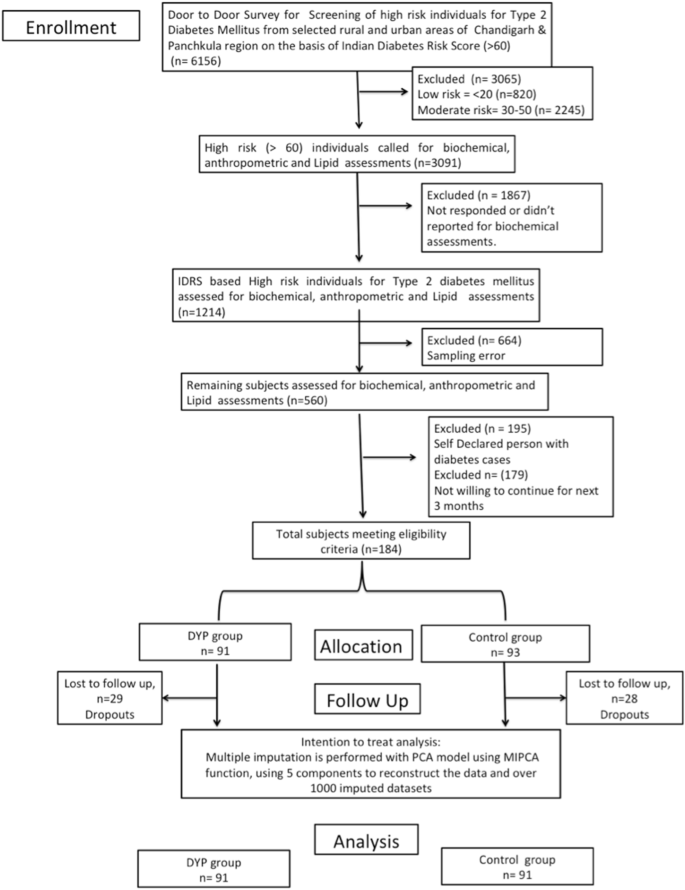
Flowchart of study design. PCA principal component analysis, MIPCA multiple imputations with PCA
Randomization
Simple randomization technique was used to allocate participants into the intervention and the control groups. An independent statistician generated a computer-generated random number sequence and the sequence was given to an external staff who had no involvement in the study procedures. The participants were allocated their consecutive numbers, after baseline measurements. Blinding of the participants was not possible due to the nature of the intervention. However, the outcome assessors were blinded.
Risk assessment
To identify the individuals at high-risk of diabetes, Indian Diabetes Risk Score (IDRS) was administered as proposed by Mohan et al. [ 22 ]. It consisted of two unmodifiable (i.e. age, and family history) and two modifiable (physical activity and waist circumference) risk factors for diabetes, which can predict the level of risk for the development of diabetes in the community. The IDRS is one of the easily accessible and budget friendly questionnaire to be administered. The aggregate score of the unmodifiable and modifiable risk used to probe the level of risk among the population (i.e. High risk > 60, Moderate risk-30–50, Low risk < 30).
Sample size
Sample size estimation for the main Pan India study was focused for prediabetes subjects [ 23 ]. However, for the present pilot scale study we calculated sample size assuming a small effect size 0.3 [ 5 ] of DYP vs waitlist control 0.25, α = 0.80 as 180 (n = 90:90). Further, assuming an attrition rate of 20%, the final sample size was n = 220.
Study outcomes
Changes in the glycemic and other metabolic variables (anthropometric and lipid) over 3 months were documented. The fasting blood sample was withdrawn. For glucose analysis, fasting samples for 10–12 h were taken early in the morning for the estimation of FBS and afterwards 75 g glucose was given to the participants. The blood sampling was repeated after 2 h. for estimation of OGTT.
Biochemical analysis
For the estimation of biochemical parameters viz. FBS (Fasting Blood Sugar, Rxl-Max 500), OGTT (Oral Glucose Tolerance Test), HbA1c (Bio-Rad D-10), Triglycerides, Cholesterol, HDL, LDL, Chol/HDL ratio, HDL/LDL ratio (Rxl-Max 500) and VLDL about 9 ml of blood was drawn and analyzed by phlebotomist of Sisco Research Laboratories (SRL) of Chandigarh. Anthropometric measurements were also obtained (i.e. height, weight, waist circumference) by trained researcher. The waist circumference (WC) was reported in centimeters. The BMI was obtained by using the formula (weight in kg/height (meter) 2 ).
Interventions
The study protocol consisted of Diabetic Yoga Protocol (DYP) approved by the Ministry of AYUSH and Quality Council of India as shown in Table 1 . This is the first protocol to be made specifically for the prediabetics and diabetics. The complete sequence of prayer, yogic postures, breathing and meditative techniques, along with specified time, was shown in previously published paper [ 24 ]. The Yogic practices were performed for 3 months for 60 min. Certified yoga instructors took the yoga classes and they recorded regular attendance. Randomization was done through a computer-generated list of random numbers and allocation was concealed to the participants until the completion of the baseline assessment.
Statistical analysis
For the analysis of data SPSS for Windows (version 22; IBM SPSS Inc., Chicago IL) 0 and R statistical package were used. The normality of data was analyzed using Kolmogorov–Smirnov test. The paired t-test was used to estimate the Baseline and posttest differences of DYP, and control group and the significant level was set at ≤ 0.05. The trial outcomes were analyzed according to the intention-to-treat principle; hence multiple imputation was carried for the missing variables accounting for the loss to follow up. We used absolute change (time and treatment interaction), to estimate intervention effects refers to the difference in the outcome of the intervention and control over different time-points of assessment. Absolute change was determined as follows: absolute change = [(intervention group follow-up) – (intervention group baseline)] – [(control group follow-up) – (control group baseline)]. The percentage change, also called the relative change was determined as relative change = (absolute change / intervention group baseline) × 100%. To evaluate the influence of missing data, we applied multiple imputations to the data using missMDA R package (v1.13) based on the principal component analysis method [ 25 ] from the package, using 5 components to reconstruct the data and over 1000 imputed datasets. One-way multivariate analysis of covariance (MANCOVA) was conducted to compare the effects of the DYP with control group glycemic and metabolic measures, while controlling for the age, gender and baseline values of the covariates.
Baseline characteristics
The data used in this study was collected in (NMB-2017) the northern region of India i.e. Chandigarh and Panchkula. The age range of participants was 3–70 years; [mean age 48.51 (SD 10.08) years]with baseline characteristics of the yoga and control groups as shown in Table 2 . Mean HbA1c of the high-risk cohort was 5.64% (0.38), mean FBS was 97.13 mg/dl (SD 11.10), and mean PPBS were 108.40 mg/dl (SD 28.79). Distributions of age and gender was similar between the intervention and the control groups. The IDRS and anthropometric values were also similarly distributed between the groups. Overall, there was no significant difference in the distribution of demographic, anthropometric, or biochemical parameters between the DYP and the control groups at the baseline.
When analyzed by multivariate analysis of covariance (MANCOVA), adjusting for age, gender and status of diabetes/prediabetes/normoglycemia, and baseline values of the covariates, yoga intervention was found to have significant influence on few cardinal parameters related to glycemic control (PPBS), and lipid control (LDL-C) as shown in Table 3 . We also observed a significant influence of DPP on waist circumference reduction [relative changes, − 1.94%. Compared to the control, DYP also resulted in significant reductions in LDL-C and, − 0.16% and − 2.81%, for LDL-Cholesterol and post-prandial blood glucose levels from baseline to 3 months [absolute changes, − 0.18% and − 3.08%, respectively and relative changes, − 0.16% and − 2.81%, respectively].
We examined the effect of Diabetic Yoga Protocol on baseline and post (3 months) levels of HbA1c and other glycemic (OGTT and FBS), Lipid (Total cholesterol, triglycerides, HDL-c, LDL-c, and VLDL-c, CDL/HDL, LDL/HDL) and anthropometric parameters (BMI). In the present study, we show the efficacy of DYP in substantial improvement in the waist circumference in a high-risk diabetes population from Chandigarh (relative change of 1.94 cm). We could also demonstrate a significant decline in the worsening of post prandial glucose levels with yoga intervention as compared to the wait-list control group (relative change of 2.82 mg/ml). However, for LDL-c levels, there were clinically significant improvements by 0.16 units. Notably, over 3 months study duration there was an overall increase in the levels of total cholesterol, triglyceride and VLDL means in the study cohort, while HDL levels had decreased. In particular TG levels have gone from normal range to mildly high (> 150 mg/dl) [ 26 ] which draws our attention towards accelerated pace of metabolic dysfunction in the high risk population. These findings comply with Chandigarh being an affluent union territory of India with high per-capita GDP and has been documented to have highest prevalence of diabetes 13.6%, 12.8–15·2 as compared to other Indian states [ 27 ]. As mentioned above, there was a significant influence of DYP on the waist circumference, one of the two important modifiable parameters of Indian Diabetes Risk Score [ 17 ]. The relevance of WC reduction in context of reduced risk of CVD is well established; a 1 cm increase in WC has been associated with a 2% increase in the relative risk of future CVD [ 28 ]. The visceral adipose tissue is a primary source of cytokine production and insulin resistance (IR) [ 29 ]. Given the higher susceptibility towards visceral fat accumulation and insulin resistance in Asian populations as compared to their Caucasian counterparts, the observed influence of DYP on WC is of particular relevance to the metabolically obese phenotype of Asian Indians [ 30 ].
In relation to the glucose metabolism, we could also demonstrate a significant decline in the worsening of post prandial glucose levels with DYP as compared to the wait-list control group (relative change = − 2.81%, P < 0.05); however, no significant influence could be established for fasting blood glucose concentration. These findings could be justified by the phenotypic differences underlying fasting and post-challenge hyperglycemia that represent distinct natural histories in the evolution of type 2 diabetes [ 31 ]. Postprandial glucose disposal is the primary pathogenic manifestation in impaired glucose tolerance (IGT), and impaired fasting glucose (IFG) merely signifies an abnormal glucose set point [ 31 , 32 ]. Our relevance of the study findings is further underlined by the previous results wherein PPG has been reported to contribute more than FBS to overall hyperglycemia and its control was found essential either to decrease or to obtain HbA1c goals of < 7 [ 33 ]. Several epidemiological studies have suggested that increased glycemic exposure, especially post challenge or postprandial hyperglycemia, is an independent risk factor for macrovascular disease with no apparent upper or lower threshold. Our results indicate a significant influence of yoga on glycemic control integrating postprandial glycemic alterations in the high diabetes risk group. Since in the present study the high-risk cohort was selected through A1c based diagnosis, and IGT was not a primary manifestation in the cohort, hence, the overall improvement in postprandial glucose should be specifically tested in an IGT cohort. The findings of the current study with a 3-month intervention of yoga on postprandial measures of glucose at-risk population deserves clinical attention. Increase in the glucose concentration even in the prediabetes stage, manifests as a chronic inflammatory condition and predisposes an individual to the risk of pathogenic infections [ 32 , 34 , 35 ].
The simultaneous reduction in waist circumference observed in the cohort, is also consistent with the observation of an association between abdominal obesity and the risk of IGT. Based on a significant association between IGT and CVD risk [ 32 , 33 , 36 ], we note a significant improvement in lipid concentrations [LDL-c] by the DYP protocol as compared to the control group. These results are consistent with the previously reported overall beneficial effect of yoga in the management of hyperlipidemia [ 36 ]. These results need validation at larger scale and to ascertain the mechanistic insights into the action of yoga, the indices of monocyte chemotaxis, endothelial inflammation, oxidation, nitric oxide production, and thrombosis should also be explored [ 37 ], including animal models, invitro systems and other approaches [ 38 , 39 , 40 , 41 , 42 , 43 , 44 ].
The findings of the present study indicate that identification of high-risk group through IDRS and consequent intervention of Yoga based lifestyle protocol could be an effective strategy to combat the metabolic perturbations associated with diabetes, whose co-morbidity is also being reported to be associated with increasing vulnerability to the emerging viral pandemic of COVID-19. Lifestyle interventions are reported to reduce the risk of Type 2 diabetes in high-risk individuals after mid and long-term follow-up. Information on determinants of intervention outcome, adherence and the mechanisms underlying diabetes progression are valuable for a more targeted implementation. Weight loss is a major contributor in the prevention and management of type 2 diabetes. In many of the earlier lifestyle intervention group of the DPP, weight loss was the dominant predictor of reduced diabetes risk, with a 16% reduction observed for every kilogram of weight loss during the 3.2-year follow-up [ 45 ]. Though we failed to observe a significant weight loss over 3 months of DYP intervention, the significant reductions in WC indicate the plausibility of significant weight loss on longer interventions and follow ups.
Whether Yoga alters the conversion of prediabetics into healthy status and if it helps in maintenance of glycemic index can be assessed by longitudinal studies. There was a significant improvement in the glycemic status of the high risk population at administration of DYP. The analysis shows the aptness of Diabetic protocol which is apparently superior to previous studies where no standardized protocols were used for intervention [ 46 , 47 ]. The findings suggest that there is potential of DYP to manage glucose levels in diabetes patients if public intervention is planned through forthcoming wellness centers in India. There are additional studies showing beneficial effects of Yoga on FBS [ 48 ], PPBS [ 49 , 50 , 51 ], HbA1c [ 50 , 51 ], total cholesterol, LDL [ 50 , 51 ]. The analysis of the yoga protocols used in above said studies reveal the incorporation of some common and important postures in DYP, which seem to be important in managing the disease. It is also the possible that the beneficial effects of mind body techniques are sensitive to mental disposition of subjects and has been characterized by various measures like psychometric analysis [ 52 , 53 ], namely, Tridosha and Triguna scoring [ 54 , 55 ]. These were not analyzed in this study.
Briefly, DYP’s promising efficacy on glycemic and metabolic parameters requires mechanistic insights. This can be examined by further studies, and long term follow up which was not possible in this study. As DYP is a non-pharmacological, cost-effective method to halt the conversion of early diabetes into prediabetes and/or healthy individuals, the success of its integration into public health policy will depend on its wider acceptability and perception of benefits by both public as well as healthcare workers [ 56 , 57 , 58 , 59 ]. Yoga’s benefits in maintaining and regulation of the glycemic status are supported by several other studies [ 49 , 50 ], which might enable its inclusion in the National Ayushman Bharat scheme or as part COVID pandemic management protocol in which a large number of individuals with diabetes and heart disease are falling prey [ 60 , 61 ]. This will further encourage molecular and Ayurgenomic studies which presumably underlie the stated clinical outcome.
Limitations
Moreover, there are some limitations of our study that we only studied in two regions of North India and thus the result of this study cannot be generalized on the remaining population. Further, in this study, the socio economic status and psychological assessments were not carried out. We were not able to control for the dietary habits and psychological status of the study participants. However, the small sample size and absence of long term evaluations limit the strength of the study.
Availability of data and materials
The datasets used during the present study are available from the corresponding author on reasonable request.
Abbreviations
American Diabetes Association
Body mass Index
Cardiovascular disease
- Diabetic yoga protocol
Fasting blood sugar
- Glycated hemoglobin
High density lipid-cholesterol
Indian Diabetes Risk Score
Impaired fasting glucose
Impaired glucose tolerance
Indian Yoga Association
Low density lipid-cholesterol
Niyantarita Maduhmeha Bharat
Oral glucose tolerance test
Postprandial blood glucose
Systolic blood pressure
Very low density lipid-cholesterol
Waist circumference
Yoga volunteers for diabetes management
Laaksonen DE, Lindström J, Lakka TA, Eriksson JG, Niskanen L, Wikström K, Aunola S, Keinänen-Kiukaanniemi S, Laakso M, Valle TT, Ilanne-Parikka P. Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes. 2005;54(1):158–65.
CAS PubMed Google Scholar
Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, Hämäläinen H, Härkönen P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368(9548):1673–9.
PubMed Google Scholar
DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368(9541):1096–105.
Google Scholar
Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care. 2003;26(3):725–31.
McDermott KA, Rao MR, Nagarathna R, Murphy EJ, Burke A, Nagendra RH, Hecht FM. A yoga intervention for type 2 diabetes risk reduction: a pilot randomized controlled trial. BMC Complement Altern Med. 2014;14(1):1–4.
Jyotsna VP. Prediabetes and type 2 diabetes mellitus: evidence for effect of yoga. Indian J Endocrinol Metab. 2014;18(6):745.
PubMed PubMed Central Google Scholar
Thind H, Lantini R, Balletto BL, Donahue ML, Salmoirago-Blotcher E, Bock BC, Scott-Sheldon LA. The effects of yoga among adults with type 2 diabetes: a systematic review and meta-analysis. Prev Med. 2017;1(105):116–26.
Singh VP, Khandelwal B. Effect of yoga and exercise on glycemic control and psychosocial parameters in type 2 diabetes mellitus: a randomized controlled study. Int J Yoga. 2020;13(2):144.
Chen N, Xia X, Qin L, Luo L, Han S, Wang G, Zhang R, Wan Z. Effects of 8-week Hatha yoga training on metabolic and inflammatory markers in healthy, female Chinese subjects: a randomized clinical trial. BioMed Res Int. 2016. https://doi.org/10.1155/2016/5387258 .
Article PubMed PubMed Central Google Scholar
Ramamoorthi R, Gahreman D, Skinner T, Moss S. Development of Sham yoga poses to assess the benefits of yoga in future randomized controlled trial studies. Life. 2021;11(2):130.
Sreedevi A, Gopalakrishnan UA, Ramaiyer SK, Kamalamma L. A randomized controlled trial of the effect of yoga and peer support on glycaemic outcomes in women with type 2 diabetes mellitus: a feasibility study. BMC Complement Altern Med. 2017;17(1):1–8.
Ramamoorthi R, Gahreman D, Skinner T, Moss S. The effect of yoga practice on glycemic control and other health parameters in the prediabetic state: a systematic review and meta-analysis. PLoS ONE. 2019;14(10): e0221067.
CAS PubMed PubMed Central Google Scholar
Chimkode SM, Kumaran SD, Kanhere VV, Shivanna R. Effect of yoga on blood glucose levels in patients with type 2 diabetes mellitus. J Clin Diagn Res JCDR. 2015;9(4):CC01.
Kacker S, Saboo N, Sharma S, Sorout J. Quasi prospective comparative study on effect of yoga among prediabetics on progression of cardiovascular risk factors. Int J Yoga. 2019;12(2):114.
Ebrahimi M, Guilan-Nejad TN, Pordanjani AF. Effect of yoga and aerobics exercise on sleep quality in women with Type 2 diabetes: a randomized controlled trial. Sleep Sci. 2017;10(2):68.
Innes KE, Vincent HK. The influence of yoga-based programs on risk profiles in adults with type 2 diabetes mellitus: a systematic review. Evid-Based Complementary Altern Med. 2007;4(4):469–86.
Mohan V, Anbalagan VP. Expanding role of the Madras diabetes research foundation-Indian diabetes risk score in clinical practice. Indian J Endocrinol Metab. 2013;17(1):31.
Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D’Agostino RB. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med. 2007;167(10):1068–74.
Chen L, Magliano DJ, Balkau B, Colagiuri S, Zimmet PZ, Tonkin AM, Mitchell P, Phillips PJ, Shaw JE. AUSDRISK: an Australian Type 2 Diabetes Risk Assessment Tool based on demographic, lifestyle and simple anthropometric measures. Med J Aust. 2010;192(4):197–202.
Yang K, Bernardo LM, Sereika SM, Conroy MB, Balk J, Burke LE. Utilization of 3-month yoga program for adults at high risk for type 2 diabetes: a pilot study. Evid-Based Complementary Altern Med. 2011;1:2011.
Hegde SV, Adhikari P, Shetty S, Manjrekar P, D’Souza V. Effect of community-based yoga intervention on oxidative stress and glycemic parameters in prediabetes: a randomized controlled trial. Complement Ther Med. 2013;21(6):571–6.
Mohan V, Deepa R, Deepa M, Somannavar S, Datta M. A simplified Indian Diabetes Risk Score for screening for undiagnosed diabetic subjects. J Assoc Phys India. 2005;53:759–63.
CAS Google Scholar
Nagarathna R, Rajesh SK, Amit S, Patil S, Anand A, Nagendra HR. Methodology of Niyantrita Madhumeha Bharata Abhiyaan-2017, a nationwide multicentric trial on the effect of a validated culturally acceptable lifestyle intervention for primary prevention of diabetes: Part 2. Int J Yoga. 2019;12(3):193.
Nagarathna Raghuram VR, Vijaya Majumdar RS, Amit Singh SP, Akshay Anand IJ, Srikanta Bhaskara JR. Effectiveness of a yoga-based lifestyle protocol (YLP) in preventing diabetes in a high-risk Indian cohort: a multicenter cluster-randomized controlled trial (NMB-trial). Front Endocrinol. 2021. https://doi.org/10.3389/fendo.2021.664657 .
Article Google Scholar
Josse J, Husson F. missMDA: a package for handling missing values in multivariate data analysis. J Stat Softw. 2016;70(1):31.
Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al., ICMR–INDIAB Collaborative Study Group. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54(12):3022–7. https://doi.org/10.1007/s00125-011-2291-5 . Epub 2011 Sep 30. PMID: 21959957.
Rygiel K. Hypertriglyceridemia—common causes, prevention and treatment strategies. Curr Cardiol Rev. 2018;14(1):67–76.
De Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007;28(7):850–6.
McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96(11):E1756–60.
Jeon J, Jung KJ, Jee SH. Waist circumference trajectories and risk of type 2 diabetes mellitus in Korean population: the Korean genome and epidemiology study (KoGES). BMC Public Health. 2019;19(1):1–1.
Roumen C, Corpeleijn E, Feskens EJ, Mensink M, Saris WH, Blaak EE. Impact of 3-year lifestyle intervention on postprandial glucose metabolism: the SLIM study. Diabet Med. 2008;25(5):597–605.
Bock G, Dalla Man C, Campioni M, Chittilapilly E, Basu R, Toffolo G, et al. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 2006;55:3536–49.
Gerich JE. The importance of tight glycemic control. Am J Med. 2005;118(9):7–11.
Buysschaert M, Medina JL, Bergman M, Shah A, Lonier J. Prediabetes and associated disorders. Endocrine. 2015;48(2):371–93.
Diabetes D. Learning about prediabetes. American Diabetes Association website http://www.diabetes.org/diabetes-basics/diagnosis . 2014.
Shantakumari N, Sequeira S. Effects of a yoga intervention on lipid profiles of diabetes patients with dyslipidemia. Indian Heart J. 2013;65(2):127–31.
DeGoma EM, Degoma RL, Rader DJ. Beyond high-density lipoprotein cholesterol levels: evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J Am Coll Cardiol. 2008;51(23):2199–211.
Anand A, Saraf MK, Prabhakar S. Sustained inhibition of brotizolam induced anterograde amnesia by norharmane and retrograde amnesia by l-glutamic acid in mice. Behav Brain Res. 2007;182(1):12–20.
Anand A, Saraf MK, Prabhakar S. Antiamnesic effect of B. monniera on L-NNA induced amnesia involves calmodulin. Neurochem Res. 2010;35(8):1172–81.
Goyal K, Koul V, Singh Y, Anand A. Targeted drug delivery to central nervous system (CNS) for the treatment of neurodegenerative disorders: trends and advances. Cent Nerv Syst Agents Med Chem (Formerly Current Medicinal Chemistry-Central Nervous System Agents). 2014;14(1):43–59.
Gupta PK, Prabhakar S, Abburi C, Sharma NK, Anand A. Vascular endothelial growth factor-A and chemokine ligand (CCL2) genes are upregulated in peripheral blood mononuclear cells in Indian amyotrophic lateral sclerosis patients. J Neuroinflammation. 2011;8(1):1–6.
Kumar S, Modgil S, Bammidi S, Minhas G, Shri R, Kaushik S, Singh V, Anand A. Allium cepa exerts neuroprotective effect on retinal ganglion cells of pterygopalatine artery (PPA) ligated mice. J Ayurveda Integr Med. 2020;11(4):489–94.
Saraf MK, Prabhakar S, Anand A. Neuroprotective effect of Bacopa monniera on ischemia induced brain injury. Pharmacol Biochem Behav. 2010;97(2):192–7.
Singh T, Prabhakar S, Gupta A, Anand A. Recruitment of stem cells into the injured retina after laser injury. Stem Cells Dev. 2012;21(3):448–54.
Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–86.
Hegde SV, Adhikari P, Kotian S, Pinto VJ, D’Souza S, D’Souza V. Effect of 3-month yoga on oxidative stress in type 2 diabetes with or without complications: a controlled clinical trial. Diabetes Care. 2011;34(10):2208–10.
Sharma M, Knowlden AP. Role of yoga in preventing and controlling type 2 diabetes mellitus. J Evid-Based Complementary Altern Med. 2012;17(2):88–95.
Keerthi GS, Pal P, Pal GK, Sahoo JP, Sridhar MG, Balachander J. Effect of 12 Weeks of yoga therapy on quality of life and Indian diabetes risk score in normotensive Indian young adult prediabetics and diabetics: randomized control trial. J Clin Diagn Res. 2017;11(9):CC10.
Sahay BK. Role of yoga in diabetes. JAPI. 2007;55:121–6.
Balaji PA. Effects of yoga-pranayama practices on metabolic parameters and anthropometry in type 2 diabetes. Int Multidiscipl Res J. 2011;1(10).
Cui J, Yan JH, Yan LM, Pan L, Le JJ, Guo YZ. Effects of yoga in adults with type 2 diabetes mellitus: a meta-analysis. J Diabet Investig. 2017;8(2):201–9.
Sedlmeier P, Eberth J, Schwarz M, Zimmermann D, Haarig F, Jaeger S, Kunze S. The psychological effects of meditation: a meta-analysis. Psychol Bull. 2012;138(6):1139.
Bonura KB, Tenenbaum G. Effects of yoga on psychological health in older adults. J Phys Act Health. 2014;11(7):1334–41.
Rastogi S. Development and validation of a Prototype Prakriti Analysis Tool (PPAT): Inferences from a pilot study. AYU. 2012;33(2):209.
Govindaraj P, Nizamuddin S, Sharath A, Jyothi V, Rotti H, Raval R, Nayak J, Bhat BK, Prasanna BV, Shintre P, Sule M. Genome-wide analysis correlates Ayurveda Prakriti. Sci Rep. 2015;5(1):1–2.
Singh AK, Kaur N, Kaushal S, Tyagi R, Mathur D, Sivapuram MS, Metri K, Bammidi S, Podder V, Modgil S, Khosla R. Partitioning of radiological, stress and biochemical changes in pre-diabetic women subjected to Diabetic Yoga Protocol. Diabetes Metab Syndr. 2019;13(4):2705–13.
Pal DK, Bhalla A, Bammidi S, Telles S, Kohli A, Kumar S, Devi P, Kaur N, Sharma K, Kumar R, Malik N. Can yoga-based diabetes management studies facilitate integrative medicine in India current status and future directions. Integr Med Int. 2017;4(3–4):125–41.
Bali P, Kaur N, Tiwari A, Bammidi S, Podder V, Devi C, Kumar S, Sivapuram MS, Ghani A, Modgil S, Malik N. Effectiveness of yoga as the public health intervention module in the management of diabetes and diabetes associated dementia in South East Asia: a narrative review. Neuroepidemiology. 2020;54(4):287–303.
Anand A. Narendra Modi’s citizen centered Yoga-Diabetes Management Program: will Indian state install integrative medicine in premier institutes? Ann Neurosci. 2019;26(2):47–8.
Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14(3):247–50.
Peric S, Stulnig TM. Diabetes and COVID-19. Wien Klin Wochenschr. 2020;132(13):356–61.
Download references
Acknowledgements
The authors would like to thank Central Council for Research in Yoga & Naturopathy (CCRYN) for their support for man power, Ministry of Health and Family Welfare (MOHFW) for support the cost of investigations and Indian Yoga Association (IYA) for the overall project implementation. The authors also like to thank to thank Yoga Volunteer for Diabetes Management (YVDMs) for helping in collection of data and also for training participants for yoga.
The Project was funded by Ministry of AYUSH, Government of India (grant number 16-63/2016-17/CCRYN/RES/Y&D/ MCT/).
Author information
Authors and affiliations.
Department of Physical Education, Panjab University, Chandigarh, 160014, India
Navneet Kaur
Division of Life Sciences, Swami Vivekananda Yoga Anusandhana Samsathana, Bengaluru, Karnataka, 560106, India
Vijaya Majumdar, Raghuram Nagarathna & Hongasandra Ramarao Nagendra
Dev Samaj College of Education, Sector 36B, Chandigarh, 160036, India
Neeru Malik
Department of Neurology, Neuroscience Research Lab, Postgraduate Institute of Medical Education and Research, Chandigarh, 160012, India
Navneet Kaur & Akshay Anand
You can also search for this author in PubMed Google Scholar
Contributions
NK: writing of manuscript, collection of data. VM: writing of manuscript, analysis. RN: conceptualization of manuscript, supervision and study design. NM: co-conceptualization of manuscript. AA: conceptualization of manuscript. HRN: supervision. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Raghuram Nagarathna or Akshay Anand .
Ethics declarations
Ethics approval and consent to participate.
Written informed consents were taken from every subject during door to door screening as well as at the time of registration. All the experimental protocol, methods and procedures were approved by Ethics committee of Indian Yoga Association (IYA) (ID: RES/IEC-IYA/001). All experiments methods and procedures were carried out in accordance with relevant guidelines and regulations of ethics committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kaur, N., Majumdar, V., Nagarathna, R. et al. Diabetic yoga protocol improves glycemic, anthropometric and lipid levels in high risk individuals for diabetes: a randomized controlled trial from Northern India. Diabetol Metab Syndr 13 , 149 (2021). https://doi.org/10.1186/s13098-021-00761-1
Download citation
Received : 26 August 2021
Accepted : 17 November 2021
Published : 23 December 2021
DOI : https://doi.org/10.1186/s13098-021-00761-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Indian diabetes risk score
- Prediabetes
Diabetology & Metabolic Syndrome
ISSN: 1758-5996
- Submission enquiries: [email protected]
Welcome, Please Sign In
1. Make sure you typed your email correctly.
2. Did you recently update your email address? If so, use the new email address to log in.
3. Did you forget your email address? Click here to recover your email address using your phone number.
4. Still having issues? Click here to contact someone on our team.

Scientific Research on Yoga > Disease and Disorders > Diabetes and Endocrine
Other Research Categories
Diabetes and endocrine type 1 and 2 diabetes, metabolic syndrome/prediabetes, endocrine conditions.

Our hope is for yoga schools and yoga teachers to utilize this impactful content in their teachings to promote and highlight yoga's evident multi-faceted ability to improve lives. Let us know how research on yoga is important or valuable to you on social media (@YogaAlliance) or by emailing us at [email protected] . We honor and value your personal experiences and look forward to featuring your stories.
These citations were curated by Yoga Alliance's Director of Yoga Research, Dr. Sat Bir Singh Khalsa .
Main Research Categories

Review Papers (What's this?)
Effectiveness of Yoga as the Public Health Intervention Module in the Management of Diabetes and Diabetes Associated Dementia in South East Asia: A Narrative Review. Bali P, Kaur N, Tiwari A, Bammidi S, Podder V, Devi C, Kumar S, Sivapuram MS, Ghani A, Modgil S, Malik N, Anand A. Neuroepidemiology. 2020 Feb 19:1-17. [ full text ]
The effect of yoga practice on glycemic control and other health parameters in the prediabetic state: A systematic review and meta-analysis. Ramamoorthi R, Gahreman D, Skinner T, Moss S. PLoS One. 2019 Oct 16;14(10):e0221067. [ full text ]
Therapeutic Role of Yoga in Type 2 Diabetes. Raveendran AV, Deshpandae A, Joshi SR. Endocrinol Metab (Seoul). 2018 Sep;33(3):307-317. [ full text ]
The benefits of yoga practice compared to physical exercise in the management of type 2 Diabetes Mellitus: A systematic review and meta-analysis. Jayawardena R, Ranasinghe P, Chathuranga T, Atapattu PM, Misra A. Diabetes Metab Syndr. 2018 Sep;12(5):795-805. [ abstract ]
The effects of yoga among adults with type 2 diabetes: A systematic review and meta-analysis. Thind H, Lantini R, Balletto BL, Donahue ML, Salmoirago-Blotcher E, Bock BC, Scott-Sheldon LAJ. Prev Med. 2017 Dec;105:116-126. [ abstract ]
A narrative review on role of Yoga as an adjuvant in the management of risk factor, disease progression and the complications of type 2 diabetes mellitus. Mooventhan A. Diabetes Metab Syndr. 2017 Nov;11 Suppl 1:S343-S346. [ abstract ]
The effect of yoga practice on glycemic control and other health parameters in Type 2 diabetes mellitus patients: A systematic review and meta-analysis. Vizcaino M, Stover E. Complement Ther Med. 2016 Oct;28:57-66. doi: 10.1016/j.ctim.2016.06.007. [ abstract ]
Yoga for metabolic syndrome: A systematic review and meta-analysis. Cramer H, Langhorst J, Dobos G, Lauche R. Eur J Prev Cardiol. 2016 Dec;23(18):1982-1993. [ abstract ]
Role of yoga for patients with type II diabetes mellitus: A systematic review and meta-analysis. Kumar V, Jagannathan A, Philip M, Thulasi A, Angadi P, Raghuram N. Complement Ther Med. 2016 Apr;25:104-12. [ abstract ]
Yoga for Adults with Type 2 Diabetes: A Systematic Review of Controlled Trials. Innes KE, Selfe TK. J Diabetes Res. 2016;2016:6979370. [ full text ]
Psycho-neuro-endocrine-immune mechanisms of action of yoga in type II diabetes. Singh VP, Khandelwal B, Sherpa NT. Anc Sci Life. 2015 Jul-Sep;35(1):12-7. [ full text ]
The benefits of yoga for adults with type 2 diabetes: a review of the evidence and call for a collaborative, integrated research initiative. de G R Hansen E, Innes KE. Int J Yoga Therap. 2013;(23):71-83. [ full text ]
Male reproductive health and yoga. Sengupta P, Chaudhuri P, Bhattacharya K. Int J Yoga. 2013 Jul;6(2):87-95. [ full text ]
Notable Publications (What's this?)
Effect of an Integrated Naturopathy and Yoga Program on Long-Term Glycemic Control in Type 2 Diabetes Mellitus Patients: A Prospective Cohort Study. Bairy S, Rao MR, Edla SR, Manthena SR, Tatavarti NVGD. Int J Yoga. 2020 Jan-Apr;13(1):42-49. doi: 10.4103/ijoy.IJOY_32_19. [ full text ]
Randomized Controlled Trial of A 12-Week Yoga-Based (Including Diet) Lifestyle vs. Dietary Intervention on Cardio-Metabolic Risk Factors and Continuous Risk Score in Indian Adults with Metabolic Syndrome. Yadav R, Yadav RK, Khadgawat R, Pandey RM, Upadhyay AD, Mehta N. Behav Med. 2020 Jan-Mar;46(1):9-20. [ abstract ]
Efficacy of a Validated Yoga Protocol on Dyslipidemia in Diabetes Patients: NMB-2017 India Trial. Nagarathna R, Tyagi R, Kaur G, Vendan V, Acharya IN, Anand A, Singh A, Nagendra HR. Medicines (Basel). 2019 Oct 11;6(4). [ full text ]
Partitioning of radiological, stress and biochemical changes in pre-diabetic women subjected to Diabetic Yoga Protocol. Singh AK, Kaur N, Kaushal S, Tyagi R, Mathur D, Sivapuram MS, Metri K, Bammidi S, Podder V, Modgil S, Khosla R, Sharma K, Anand A, Malik N, Boroiah V, Nagarathna R, Nagendra HR, Anand A. Diabetes Metab Syndr. 2019 Jul - Aug;13(4):2705-2713. [ abstract ]
Comparative efficacy of a 12 week yoga-based lifestyle intervention and dietary intervention on adipokines, inflammation, and oxidative stress in adults with metabolic syndrome: a randomized controlled trial. Yadav R, Yadav RK, Khadgawat R, Pandey RM. Transl Behav Med. 2019 Jul 16;9(4):594-604. [ full text ]
Effectiveness of Adjuvant Yoga Therapy in Diabetic Lung: A Randomized Control Trial. Balaji R, Ramanathan M, Bhavanani AB, Ranganadin P, Balachandran K. Int J Yoga. 2019 May-Aug;12(2):96-102. [ full text ]
"I can do almost anything": The experience of adults with type 2 diabetes with a yoga intervention. Thind H, Guthrie KM, Horowitz S, Conrad M, Bock BC. Complement Ther Clin Pract. 2019 Feb;34:116-122. [ full text ]
Feasibility of yoga as a complementary therapy for patients with type 2 diabetes: The Healthy Active and in Control (HA1C) study. Bock BC, Thind H, Fava JL, Dunsiger S, Guthrie KM, Stroud L, Gopalakrishnan G, Sillice M, Wu W. Complement Ther Med. 2019 Feb;42:125-131. [ full text ]
Effect of Ayurveda intervention, lifestyle modification and Yoga in prediabetic and type 2 diabetes under the National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke (NPCDCS)-AYUSH integration project. Sharma R, Shahi VK, Khanduri S, Goyal A, Chaudhary S, Rana RK, Singhal R, Srikanth N, Dhiman KS. Ayu. 2019 Jan-Mar;40(1):8-15. [ full text ]
One Year of Yoga Training Alters Ghrelin Axis in Centrally Obese Adults With Metabolic Syndrome. Yu AP, Ugwu FN, Tam BT, Lee PH, Lai CW, Wong CSC, Lam WW, Sheridan S, Siu PM. Front Physiol. 2018 Sep 20;9:1321. [ full text ]
Influence of Time of Yoga Practice and Gender Differences on Blood Glucose Levels in Type 2 Diabetes Mellitus and Normal Healthy Adults. Vijayakumar V, Mooventhan A, Raghuram N. Explore (NY). 2018 Jul - Aug;14(4):283-288. [ abstract ]
Multidimensional Improvements in Health Following Hatha Yoga for Individuals with Diabetic Peripheral Neuropathy. Van Puymbroeck M, Atler K, Portz JD, Schmid AA. Int J Yoga Therap. 2018 Nov;28(1):71-78. [ abstract ]
Effect of 12 Weeks of Yoga Therapy on Quality of Life and Indian Diabetes Risk Score in Normotensive Indian Young Adult Prediabetics and Diabetics: Randomized Control Trial. Keerthi GS, Pal P, Pal GK, Sahoo JP, Sridhar MG, Balachander J. J Clin Diagn Res. 2017 Sep;11(9):CC10-CC14. [ full text ]
Effect of yoga and aerobics exercise on sleep quality in women with Type 2 diabetes: a randomized controlled trial. Ebrahimi M, Guilan-Nejad TN, Pordanjani AF. Sleep Sci. 2017 Apr-Jun;10(2):68-72. doi: 10.5935/1984-0063.20170012. [ full text ]
A Randomized controlled trial of the effect of yoga and peer support on glycaemic outcomes in women with type 2 diabetes mellitus: a feasibility study. Sreedevi A, Gopalakrishnan UA, Karimassery Ramaiyer S, Kamalamma L. BMC Complement Altern Med. 2017 Feb 7;17(1):100. [ full text ]
Yoga for Risk Reduction of Metabolic Syndrome: Patient-Reported Outcomes from a Randomized Controlled Pilot Study. Sohl SJ, Wallston KA, Watkins K, Birdee GS. Evid Based Complement Alternat Med. 2016;2016:3094589. [ full text ]
Impact of individualized yoga therapy on perceived quality of life performance on cognitive tasks and depression among Type II diabetic patients. Satish L, Lakshmi VS. Int J Yoga. 2016 Jul-Dec;9(2):130-6. [ full text ]
Effect of 6 months intense Yoga practice on lipid profile, thyroxine medication and serum TSH level in women suffering from hypothyroidism: A pilot study. Nilakanthan S, Metri K, Raghuram N, Hongasandra N. J Complement Integr Med. 2016 Jun 1;13(2):189-93. [ abstract ]
Effects of a 12-Week Hatha Yoga Intervention on Metabolic Risk and Quality of Life in Hong Kong Chinese Adults with and without Metabolic Syndrome. Lau C, Yu R, Woo. PLoS One. 2015 Jun 25;10(6):e0130731. [ full text ]
Effects of 1-year yoga on cardiovascular risk factors in middle-aged and older adults with metabolic syndrome: a randomized trial. Siu PM, Yu AP, Benzie IF, Woo J. Diabetol Metab Syndr. 2015 Apr 30;7:40. [ full text ]
Completion report: Effect of Comprehensive Yogic Breathing program on type 2 diabetes: A randomized control trial. Jyotsna VP, Dhawan A, Sreenivas V, Deepak KK, Singla R. Indian J Endocrinol Metab. 2014 Jul;18(4):582-4. [ full text ]
Effect of restorative yoga vs. stretching on diurnal cortisol dynamics and psychosocial outcomes in individuals with the metabolic syndrome: the PRYSMS randomized controlled trial. Corey SM, Epel E, Schembri M, Pawlowsky SB, Cole RJ, Araneta MR, Barrett-Connor E, Kanaya AM. Psychoneuroendocrinology. 2014 Nov;49:260-71. [ full text ]
A yoga intervention for type 2 diabetes risk reduction: a pilot randomized controlled trial. McDermott KA, Rao MR, Nagarathna R, Murphy EJ, Burke A, Nagendra RH, Hecht FM. BMC Complement Altern Med. 2014 Jul 1;14:212. [ full text ]
Restorative yoga and metabolic risk factors: the Practicing Restorative Yoga vs. Stretching for the Metabolic Syndrome (PRYSMS) randomized trial. Kanaya AM, Araneta MR, Pawlowsky SB, Barrett-Connor E, Grady D, Vittinghoff E, Schembri M, Chang A, Carrion-Petersen ML, Coggins T, Tanori D, Armas JM, Cole RJ. J Diabetes Complications. 2014 May-Jun;28(3):406-12. [ full text ]
Effect of regular yogic training on growth hormone and dehydroepiandrosterone sulfate as an endocrine marker of aging. Chatterjee S, Mondal S. Evid Based Complement Alternat Med. 2014;2014:240581. [ full text ]
Stay Connected
Have questions.
Visit our Help Center .
Media Inquiries
Stay up-to-date on the latest from Yoga Alliance, subscribe to our newsletters.

- Previous Article
- Next Article
Case Presentation
Case study: a patient with uncontrolled type 2 diabetes and complex comorbidities whose diabetes care is managed by an advanced practice nurse.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Geralyn Spollett; Case Study: A Patient With Uncontrolled Type 2 Diabetes and Complex Comorbidities Whose Diabetes Care Is Managed by an Advanced Practice Nurse. Diabetes Spectr 1 January 2003; 16 (1): 32–36. https://doi.org/10.2337/diaspect.16.1.32
Download citation file:
- Ris (Zotero)
- Reference Manager
The specialized role of nursing in the care and education of people with diabetes has been in existence for more than 30 years. Diabetes education carried out by nurses has moved beyond the hospital bedside into a variety of health care settings. Among the disciplines involved in diabetes education, nursing has played a pivotal role in the diabetes team management concept. This was well illustrated in the Diabetes Control and Complications Trial (DCCT) by the effectiveness of nurse managers in coordinating and delivering diabetes self-management education. These nurse managers not only performed administrative tasks crucial to the outcomes of the DCCT, but also participated directly in patient care. 1
The emergence and subsequent growth of advanced practice in nursing during the past 20 years has expanded the direct care component, incorporating aspects of both nursing and medical care while maintaining the teaching and counseling roles. Both the clinical nurse specialist (CNS) and nurse practitioner (NP) models, when applied to chronic disease management, create enhanced patient-provider relationships in which self-care education and counseling is provided within the context of disease state management. Clement 2 commented in a review of diabetes self-management education issues that unless ongoing management is part of an education program, knowledge may increase but most clinical outcomes only minimally improve. Advanced practice nurses by the very nature of their scope of practice effectively combine both education and management into their delivery of care.
Operating beyond the role of educator, advanced practice nurses holistically assess patients’ needs with the understanding of patients’ primary role in the improvement and maintenance of their own health and wellness. In conducting assessments, advanced practice nurses carefully explore patients’ medical history and perform focused physical exams. At the completion of assessments, advanced practice nurses, in conjunction with patients, identify management goals and determine appropriate plans of care. A review of patients’ self-care management skills and application/adaptation to lifestyle is incorporated in initial histories, physical exams, and plans of care.
Many advanced practice nurses (NPs, CNSs, nurse midwives, and nurse anesthetists) may prescribe and adjust medication through prescriptive authority granted to them by their state nursing regulatory body. Currently, all 50 states have some form of prescriptive authority for advanced practice nurses. 3 The ability to prescribe and adjust medication is a valuable asset in caring for individuals with diabetes. It is a crucial component in the care of people with type 1 diabetes, and it becomes increasingly important in the care of patients with type 2 diabetes who have a constellation of comorbidities, all of which must be managed for successful disease outcomes.
Many studies have documented the effectiveness of advanced practice nurses in managing common primary care issues. 4 NP care has been associated with a high level of satisfaction among health services consumers. In diabetes, the role of advanced practice nurses has significantly contributed to improved outcomes in the management of type 2 diabetes, 5 in specialized diabetes foot care programs, 6 in the management of diabetes in pregnancy, 7 and in the care of pediatric type 1 diabetic patients and their parents. 8 , 9 Furthermore, NPs have also been effective providers of diabetes care among disadvantaged urban African-American patients. 10 Primary management of these patients by NPs led to improved metabolic control regardless of whether weight loss was achieved.
The following case study illustrates the clinical role of advanced practice nurses in the management of a patient with type 2 diabetes.
A.B. is a retired 69-year-old man with a 5-year history of type 2 diabetes. Although he was diagnosed in 1997, he had symptoms indicating hyperglycemia for 2 years before diagnosis. He had fasting blood glucose records indicating values of 118–127 mg/dl, which were described to him as indicative of “borderline diabetes.” He also remembered past episodes of nocturia associated with large pasta meals and Italian pastries. At the time of initial diagnosis, he was advised to lose weight (“at least 10 lb.”), but no further action was taken.
Referred by his family physician to the diabetes specialty clinic, A.B. presents with recent weight gain, suboptimal diabetes control, and foot pain. He has been trying to lose weight and increase his exercise for the past 6 months without success. He had been started on glyburide (Diabeta), 2.5 mg every morning, but had stopped taking it because of dizziness, often accompanied by sweating and a feeling of mild agitation, in the late afternoon.
A.B. also takes atorvastatin (Lipitor), 10 mg daily, for hypercholesterolemia (elevated LDL cholesterol, low HDL cholesterol, and elevated triglycerides). He has tolerated this medication and adheres to the daily schedule. During the past 6 months, he has also taken chromium picolinate, gymnema sylvestre, and a “pancreas elixir” in an attempt to improve his diabetes control. He stopped these supplements when he did not see any positive results.
He does not test his blood glucose levels at home and expresses doubt that this procedure would help him improve his diabetes control. “What would knowing the numbers do for me?,” he asks. “The doctor already knows the sugars are high.”
A.B. states that he has “never been sick a day in my life.” He recently sold his business and has become very active in a variety of volunteer organizations. He lives with his wife of 48 years and has two married children. Although both his mother and father had type 2 diabetes, A.B. has limited knowledge regarding diabetes self-care management and states that he does not understand why he has diabetes since he never eats sugar. In the past, his wife has encouraged him to treat his diabetes with herbal remedies and weight-loss supplements, and she frequently scans the Internet for the latest diabetes remedies.
During the past year, A.B. has gained 22 lb. Since retiring, he has been more physically active, playing golf once a week and gardening, but he has been unable to lose more than 2–3 lb. He has never seen a dietitian and has not been instructed in self-monitoring of blood glucose (SMBG).
A.B.’s diet history reveals excessive carbohydrate intake in the form of bread and pasta. His normal dinners consist of 2 cups of cooked pasta with homemade sauce and three to four slices of Italian bread. During the day, he often has “a slice or two” of bread with butter or olive oil. He also eats eight to ten pieces of fresh fruit per day at meals and as snacks. He prefers chicken and fish, but it is usually served with a tomato or cream sauce accompanied by pasta. His wife has offered to make him plain grilled meats, but he finds them “tasteless.” He drinks 8 oz. of red wine with dinner each evening. He stopped smoking more than 10 years ago, he reports, “when the cost of cigarettes topped a buck-fifty.”
The medical documents that A.B. brings to this appointment indicate that his hemoglobin A 1c (A1C) has never been <8%. His blood pressure has been measured at 150/70, 148/92, and 166/88 mmHg on separate occasions during the past year at the local senior center screening clinic. Although he was told that his blood pressure was “up a little,” he was not aware of the need to keep his blood pressure ≤130/80 mmHg for both cardiovascular and renal health. 11
A.B. has never had a foot exam as part of his primary care exams, nor has he been instructed in preventive foot care. However, his medical records also indicate that he has had no surgeries or hospitalizations, his immunizations are up to date, and, in general, he has been remarkably healthy for many years.
Physical Exam
A physical examination reveals the following:
Weight: 178 lb; height: 5′2″; body mass index (BMI): 32.6 kg/m 2
Fasting capillary glucose: 166 mg/dl
Blood pressure: lying, right arm 154/96 mmHg; sitting, right arm 140/90 mmHg
Pulse: 88 bpm; respirations 20 per minute
Eyes: corrective lenses, pupils equal and reactive to light and accommodation, Fundi-clear, no arteriolovenous nicking, no retinopathy
Thyroid: nonpalpable
Lungs: clear to auscultation
Heart: Rate and rhythm regular, no murmurs or gallops
Vascular assessment: no carotid bruits; femoral, popliteal, and dorsalis pedis pulses 2+ bilaterally
Neurological assessment: diminished vibratory sense to the forefoot, absent ankle reflexes, monofilament (5.07 Semmes-Weinstein) felt only above the ankle
Lab Results
Results of laboratory tests (drawn 5 days before the office visit) are as follows:
Glucose (fasting): 178 mg/dl (normal range: 65–109 mg/dl)
Creatinine: 1.0 mg/dl (normal range: 0.5–1.4 mg/dl)
Blood urea nitrogen: 18 mg/dl (normal range: 7–30 mg/dl)
Sodium: 141 mg/dl (normal range: 135–146 mg/dl)
Potassium: 4.3 mg/dl (normal range: 3.5–5.3 mg/dl)
Lipid panel
• Total cholesterol: 162 mg/dl (normal: <200 mg/dl)
• HDL cholesterol: 43 mg/dl (normal: ≥40 mg/dl)
• LDL cholesterol (calculated): 84 mg/dl (normal: <100 mg/dl)
• Triglycerides: 177 mg/dl (normal: <150 mg/dl)
• Cholesterol-to-HDL ratio: 3.8 (normal: <5.0)
AST: 14 IU/l (normal: 0–40 IU/l)
ALT: 19 IU/l (normal: 5–40 IU/l)
Alkaline phosphotase: 56 IU/l (normal: 35–125 IU/l)
A1C: 8.1% (normal: 4–6%)
Urine microalbumin: 45 mg (normal: <30 mg)
Based on A.B.’s medical history, records, physical exam, and lab results, he is assessed as follows:
Uncontrolled type 2 diabetes (A1C >7%)
Obesity (BMI 32.4 kg/m 2 )
Hyperlipidemia (controlled with atorvastatin)
Peripheral neuropathy (distal and symmetrical by exam)
Hypertension (by previous chart data and exam)
Elevated urine microalbumin level
Self-care management/lifestyle deficits
• Limited exercise
• High carbohydrate intake
• No SMBG program
Poor understanding of diabetes
A.B. presented with uncontrolled type 2 diabetes and a complex set of comorbidities, all of which needed treatment. The first task of the NP who provided his care was to select the most pressing health care issues and prioritize his medical care to address them. Although A.B. stated that his need to lose weight was his chief reason for seeking diabetes specialty care, his elevated glucose levels and his hypertension also needed to be addressed at the initial visit.
The patient and his wife agreed that a referral to a dietitian was their first priority. A.B. acknowledged that he had little dietary information to help him achieve weight loss and that his current weight was unhealthy and “embarrassing.” He recognized that his glucose control was affected by large portions of bread and pasta and agreed to start improving dietary control by reducing his portion size by one-third during the week before his dietary consultation. Weight loss would also be an important first step in reducing his blood pressure.
The NP contacted the registered dietitian (RD) by telephone and referred the patient for a medical nutrition therapy assessment with a focus on weight loss and improved diabetes control. A.B.’s appointment was scheduled for the following week. The RD requested that during the intervening week, the patient keep a food journal recording his food intake at meals and snacks. She asked that the patient also try to estimate portion sizes.
Although his physical activity had increased since his retirement, it was fairly sporadic and weather-dependent. After further discussion, he realized that a week or more would often pass without any significant form of exercise and that most of his exercise was seasonal. Whatever weight he had lost during the summer was regained in the winter, when he was again quite sedentary.
A.B.’s wife suggested that the two of them could walk each morning after breakfast. She also felt that a treadmill at home would be the best solution for getting sufficient exercise in inclement weather. After a short discussion about the positive effect exercise can have on glucose control, the patient and his wife agreed to walk 15–20 minutes each day between 9:00 and 10:00 a.m.
A first-line medication for this patient had to be targeted to improving glucose control without contributing to weight gain. Thiazolidinediones (i.e., rosiglitizone [Avandia] or pioglitizone [Actos]) effectively address insulin resistance but have been associated with weight gain. 12 A sulfonylurea or meglitinide (i.e., repaglinide [Prandin]) can reduce postprandial elevations caused by increased carbohydrate intake, but they are also associated with some weight gain. 12 When glyburide was previously prescribed, the patient exhibited signs and symptoms of hypoglycemia (unconfirmed by SMBG). α-Glucosidase inhibitors (i.e., acarbose [Precose]) can help with postprandial hyperglycemia rise by blunting the effect of the entry of carbohydrate-related glucose into the system. However, acarbose requires slow titration, has multiple gastrointestinal (GI) side effects, and reduces A1C by only 0.5–0.9%. 13 Acarbose may be considered as a second-line therapy for A.B. but would not fully address his elevated A1C results. Metformin (Glucophage), which reduces hepatic glucose production and improves insulin resistance, is not associated with hypoglycemia and can lower A1C results by 1%. Although GI side effects can occur, they are usually self-limiting and can be further reduced by slow titration to dose efficacy. 14
After reviewing these options and discussing the need for improved glycemic control, the NP prescribed metformin, 500 mg twice a day. Possible GI side effects and the need to avoid alcohol were of concern to A.B., but he agreed that medication was necessary and that metformin was his best option. The NP advised him to take the medication with food to reduce GI side effects.
The NP also discussed with the patient a titration schedule that increased the dosage to 1,000 mg twice a day over a 4-week period. She wrote out this plan, including a date and time for telephone contact and medication evaluation, and gave it to the patient.
During the visit, A.B. and his wife learned to use a glucose meter that features a simple two-step procedure. The patient agreed to use the meter twice a day, at breakfast and dinner, while the metformin dose was being titrated. He understood the need for glucose readings to guide the choice of medication and to evaluate the effects of his dietary changes, but he felt that it would not be “a forever thing.”
The NP reviewed glycemic goals with the patient and his wife and assisted them in deciding on initial short-term goals for weight loss, exercise, and medication. Glucose monitoring would serve as a guide and assist the patient in modifying his lifestyle.
A.B. drew the line at starting an antihypertensive medication—the angiotensin-converting enzyme (ACE) inhibitor enalapril (Vasotec), 5 mg daily. He stated that one new medication at a time was enough and that “too many medications would make a sick man out of me.” His perception of the state of his health as being represented by the number of medications prescribed for him gave the advanced practice nurse an important insight into the patient’s health belief system. The patient’s wife also believed that a “natural solution” was better than medication for treating blood pressure.
Although the use of an ACE inhibitor was indicated both by the level of hypertension and by the presence of microalbuminuria, the decision to wait until the next office visit to further evaluate the need for antihypertensive medication afforded the patient and his wife time to consider the importance of adding this pharmacotherapy. They were quite willing to read any materials that addressed the prevention of diabetes complications. However, both the patient and his wife voiced a strong desire to focus their energies on changes in food and physical activity. The NP expressed support for their decision. Because A.B. was obese, weight loss would be beneficial for many of his health issues.
Because he has a sedentary lifestyle, is >35 years old, has hypertension and peripheral neuropathy, and is being treated for hypercholestrolemia, the NP performed an electrocardiogram in the office and referred the patient for an exercise tolerance test. 11 In doing this, the NP acknowledged and respected the mutually set goals, but also provided appropriate pre-exercise screening for the patient’s protection and safety.
In her role as diabetes educator, the NP taught A.B. and his wife the importance of foot care, demonstrating to the patient his inability to feel the light touch of the monofilament. She explained that the loss of protective sensation from peripheral neuropathy means that he will need to be more vigilant in checking his feet for any skin lesions caused by poorly fitting footwear worn during exercise.
At the conclusion of the visit, the NP assured A.B. that she would share the plan of care they had developed with his primary care physician, collaborating with him and discussing the findings of any diagnostic tests and procedures. She would also work in partnership with the RD to reinforce medical nutrition therapies and improve his glucose control. In this way, the NP would facilitate the continuity of care and keep vital pathways of communication open.
Advanced practice nurses are ideally suited to play an integral role in the education and medical management of people with diabetes. 15 The combination of clinical skills and expertise in teaching and counseling enhances the delivery of care in a manner that is both cost-reducing and effective. Inherent in the role of advanced practice nurses is the understanding of shared responsibility for health care outcomes. This partnering of nurse with patient not only improves care but strengthens the patient’s role as self-manager.
Geralyn Spollett, MSN, C-ANP, CDE, is associate director and an adult nurse practitioner at the Yale Diabetes Center, Department of Endocrinology and Metabolism, at Yale University in New Haven, Conn. She is an associate editor of Diabetes Spectrum.
Note of disclosure: Ms. Spollett has received honoraria for speaking engagements from Novo Nordisk Pharmaceuticals, Inc., and Aventis and has been a paid consultant for Aventis. Both companies produce products and devices for the treatment of diabetes.
Email alerts
- Advanced Practice Care: Advanced Practice Care in Diabetes: Epilogue
- Advanced Practice Care: Advanced Practice Care in Diabetes: Preface
- Online ISSN 1944-7353
- Print ISSN 1040-9165
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association

This Feature Is Available To Subscribers Only
Sign In or Create an Account
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Role of yoga in diabetes
Affiliation.
- 1 Osmania Medical College, Hyderabad.
- PMID: 17571741
The science of yoga is an ancient one. It is a rich heritage of our culture. Several older books make a mention of the usefulness of yoga in the treatment of certain diseases and preservation of health in normal individuals. The effect of yogic practices on the management of diabetes has not been investigated well. We carried out well designed studies in normal individuals and those with diabetes to assess the role of yogic practices on glycaemic control, insulin kinetics, body composition exercise tolerance and various co-morbidities like hypertension and dyslipidemia. These studies were both short term and long-term. These studies have confirmed the useful role of yoga in the control of diabetes mellitus. Fasting and postprandial blood glucose levels came down significantly. Good glycaemic status can be maintained for long periods of time. There was a lowering of drug requirement and the incidence of acute complications like infection and ketosis was significantly reduced. There were significant changes in the insulin kinetics and those of counter-regulatory hormones like cortisol. There was a decrease in free fatty acids. There was an increase in lean body mass and decrease in body fat percentage. The number of insulin receptors was also increased. There was an improvement in insulin sensitivity and decline in insulin resistance. All these suggest that yogic practices have a role even in the prevention of diabetes. There is a beneficial effect on the co-morbid conditions like hypertension and dyslipidemia.
PubMed Disclaimer
Similar articles
- The beneficial effect of yoga in diabetes. Malhotra V, Singh S, Tandon OP, Sharma SB. Malhotra V, et al. Nepal Med Coll J. 2005 Dec;7(2):145-7. Nepal Med Coll J. 2005. PMID: 16519085
- Secondary diabetes associated with principal endocrinopathies: the impact of new treatment modalities. Resmini E, Minuto F, Colao A, Ferone D. Resmini E, et al. Acta Diabetol. 2009 Jun;46(2):85-95. doi: 10.1007/s00592-009-0112-9. Epub 2009 Mar 26. Acta Diabetol. 2009. PMID: 19322513 Review.
- Beta-cell function and mass in type 2 diabetes. Larsen MO. Larsen MO. Dan Med Bull. 2009 Aug;56(3):153-64. Dan Med Bull. 2009. PMID: 19728971
- Insulin sensitivity and cardiac autonomic function in young male practitioners of yoga. Chaya MS, Ramakrishnan G, Shastry S, Kishore RP, Nagendra H, Nagarathna R, Raj T, Thomas T, Vaz M, Kurpad AV. Chaya MS, et al. Natl Med J India. 2008 Sep-Oct;21(5):217-21. Natl Med J India. 2008. PMID: 19320319
- Initiating insulin in patients with type 2 diabetes. Aoki TJ, White RD. Aoki TJ, et al. J Fam Pract. 2007 Aug;56(8 Suppl Hot Topics):S12-20. J Fam Pract. 2007. PMID: 18667139 Review.
- A Study on Yoga-Based Lifestyle Intervention versus Dietary Intervention Alone on Cardiometabolic Risk Factors among People with Prediabetes. Saboo N, Kacker S. Saboo N, et al. Ann Afr Med. 2024 Apr 1;23(2):202-212. doi: 10.4103/aam.aam_56_23. Epub 2024 May 1. Ann Afr Med. 2024. PMID: 39028170 Free PMC article. Clinical Trial.
- Influence of 24-Week Yoga Intervention on Cardiovascular Risk Factors and Inflammatory Markers in Type 2 Diabetes. Sharma S, Bhardwaj S, Gupta A, Katoch VM, Sharma KK, Gupta R. Sharma S, et al. Int J Yoga. 2023 Jan-Apr;16(1):27-33. doi: 10.4103/ijoy.ijoy_176_22. Epub 2023 Jul 10. Int J Yoga. 2023. PMID: 37583542 Free PMC article.
- Elastic band resistance combined with modified Thai yoga exercise to alleviate oxidative stress and airway inflammation in type 2 diabetes mellitus. Promsrisuk T, Kongsui R, Sriraksa N, Boonla O, Srithawong A. Promsrisuk T, et al. J Exerc Rehabil. 2023 Apr 27;19(2):114-125. doi: 10.12965/jer.2346040.020. eCollection 2023 Apr. J Exerc Rehabil. 2023. PMID: 37163180 Free PMC article.
- An Observational Study on the Effect of Yoga and Sudarshan Kriya in Type 2 Diabetes Mellitus Patients. Verma MR, Langade DG, Rao RD, Shivangi S, Khedkar S, Kanchibhotla D. Verma MR, et al. Cureus. 2022 Aug 12;14(8):e27951. doi: 10.7759/cureus.27951. eCollection 2022 Aug. Cureus. 2022. PMID: 36120271 Free PMC article.
- The Effect of Yoga on the Lipid Profile: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Ghazvineh D, Daneshvar M, Basirat V, Daneshzad E. Ghazvineh D, et al. Front Nutr. 2022 Jul 14;9:942702. doi: 10.3389/fnut.2022.942702. eCollection 2022. Front Nutr. 2022. PMID: 35911119 Free PMC article.
- Search in MeSH
Related information
- PubChem Compound
- PubChem Compound (MeSH Keyword)
- PubChem Substance
LinkOut - more resources
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

6 Best Yoga Poses To Cure Diabetes And Case Study [2022]
Table of content.

Written By : Kirti Tayal Writes On: Yoga & Fitness

Medically reviewed by Ashmit Choudhary MS, Yoga Instructor By Punjab University

I have completed my Bachelors of Arts, Bachelors of Education(Yoga) from Panjab University and Masters degree in Yoga from Himalayiya University, Uttarakhand.
I started my journey with Fitelo as a Yoga coach and Have experience of handling over 500+ clients with positive results.
After working as a yoga coach for 1 year I have been Promoted to an assistant manager, Currently I am leading a team of 10 coaches and polishing my leadership skills for better client satisfaction.
With this i love to Travel and Paint, Painting provides me relaxation and Travel gives me new perspective.
- Bachelors of Arts
- Bachelors of Education (Yoga) Panjab University
- Masters degree (Yoga) Himalayiya University, Uttarakhand.

Reviewed By
Ashmit Choudhary, a Certified Yoga Coach, currently holds the role of Yoga Coach within Fitelo's esteemed Department of Wellness.
START YOUR FAT TO FIT JOURNEY WITH FITELO
- 50,000+ Happy Customers
- Serving in 70+ countries globally
- 10,000+ online reviews with 4.5+ rating
Yoga has played a vital role in everyone’s life for ages. But in today’s world, because of the lifestyle people have no time for their health. Moreover, our sedentary lifestyle and food habits lead to many issues like diabetes, constipation, chronic diseases, etc. However, yoga is a natural treatment that fits easily into everyone’s busy life schedule and can help to overcome these diseases. Although people are not very aware that yoga not only helps in daily well-being but also targets specific health concerns like yoga for constipation, and yoga for diabetes, and does show a positive improvement in blood sugar levels and body composition. Diabetes is the most common problem people are suffering from.
Let’s find out through this article, how to cure diabetes with yoga and learn how simple yoga asanas can help to improve your overall health.
How Can Yoga Help With Diabetes?
If you are diabetic, eating healthy and daily exercise is the best way to keep your sugar levels in control and yoga can be considered one of the best forms of exercise to fight diabetes. Instead of going for medicine, you can try yoga for diabetes as it increases glucose which helps to lower blood sugar levels and blood pressure. Like yoga for constipation helps to cure constipation, yoga for weight loss helps you shed kilos. So, the same is for diabetic patients. Let’s find out the best yoga poses or asanas for diabetes.
Yoga Poses For Diabetes
Although there are various asanas for Diabetes. Here are seven asanas that are considered the most effective to treat diabetes :
1. Vakrasana [Twisted Pose]
Vakra means twisted in Sanskrit hence the name is vakrasana. This is a spine-twisted pose for flexibility.
Benefits of Vakrasana
- Vakrasana is a spinal twist pose.
- This posture improves the functioning of the pancreas.
- This asana also massages the abdominal organs.
- Moreover, it increases the production of insulin in the body.
2. Mastyendrasana [Lord Of The Fish Pose]
It is a seated twist asana that is practiced to improve body posture.
Benefits Of Mastyendrasana
- It helps improve the function of the liver, pancreas, and spleen.
- Moreover, this asana improves the production of insulin in the body.
- It also improves digestion and removes toxins from the body.
- Besides It even helps to reduce obesity.
3. Bhujangasana [Cobra Pose]
It is a lying down pose as a part of sun salutation, it is considered best for the spine.
Benefits of Bhujangsana
- It is a prone posture.
- This asana also stimulates abdominal muscles.
- It also reduces your blood sugar levels.
4. Virabhadrasana [Warrior Pose]
Warrior Pose is a half-standing asana for stability and muscle strength.

Benefits of Virabhadrasana
- This yoga pose provides good blood circulation.
- It also gives energy to the whole body.
- Moreover, it helps in stretching the whole body, especially the belly.
5. Malasana [Garland Pose]
It is also known as a yogic squat as its a full bend squat, and it helps stretch the entire lower body.
Benefits of Malasana
- This is a sitting posture.
- Malasana helps to stretch the abdominal muscle and also strengthens the abdominal organs.
- It also prevents pregnant women from getting diabetic.
Yoga Tips For Diabetes
Let’s read out the following yoga tips listed below:

- Sufficient Intake Of Protein And Fats
Sufficient protein intake reduces the risk of diabetes and also improves metabolism which helps to reduce body weight. Healthy fats help to lower the blood sugar level and improve insulin production in the body.
- Eat More Fibers
Fibers help to control the level of blood sugar and also help to keep the blood sugar level in the proper range. Fiber-rich food can keep your stomach fuller for a longer time and also reduce the level of blood glucose level.
- Daily Exercise
Doing daily exercises and yoga helps lower the blood sugar level and boost the body. It also helps the body to be sensitive to insulin.
- Changes In Lifestyle
However, making some changes in your daily routine help to control the level of diabetes like doing daily yoga practice and eating healthy and focusing on eating according to your body’s needs, and limiting the food which is high in fat and sugar. Therefore, these small daily lifestyle changes can be very helpful with yoga in a long run.
- Avoid Sugar
Your body gains weight when you eat too many foods containing added sugar and because of that, the chances of getting type 2 diabetes increase. Besides you need to avoid such food items and switch to healthier options like fruits and dry nuts.
Case Study Of Diabetic Patient
- Name Meenakshi Goel.
- Age: 45 yr.
- Location: Chandigarh
Issue: Diagnosed with Prediabetes
Symptoms: Frequent urination, fatigue, increased thirst.
With age, many of us feel various changes in our health. The same thing happened with Meenakshi. She was facing many problems like fatigue, increased thirst, weight gain, etc. Then, as per her sister’s suggestion, she went for a proper body test, and she found out that she was suffering from a prediabetic condition. The doctor suggested making changes to her lifestyle. The doctor also suggested she take some medications and asked her to incorporate yoga into her daily routine. So, she started taking yoga classes, and after some time, she found yoga very helpful and relaxing for her physical and mental health. She also followed yoga very diligently, and after almost 7-8 months, was able to overcome diabetes. Now she is happier and healthier than before. Moreover, she also made yoga a vital part of her daily routine to have a fit and happy future.
Yoga Poses She Followed :
- Bhujangasana
- Matsyendrasana
- Markatasana
Outcomes : Here are the outcomes:
Psychological Changes
- Good Mood.
- Increased Life quality.
- No Stress.
Physiological Changes
- Losing Body weight
- Blood pressure control.
- Less Cholesterol
- Decreased Blood glucose
- Increased production of Insulin in the body.
- Improvement Endothelial function
So, this is how yoga can play an important role in our life. Any disease can be treated with proper diet, yoga, and guidance.
Frequently Asked Questions
Q. what are the benefits of yoga.
Ans . Yoga has numerous health benefits. It helps in mental and physical well-being. Moreover, yoga practice over time becomes a way of living. It helps fight many health issues such as high blood pressure, constipation, etc.
Q. What Are the Side Effects of Yoga?
Ans . You need proper guidance if you are a beginner. Moreover, yoga does not have any side – effects if followed properly by someone who is experienced.
People are very well aware of Yoga’s benefits for health and skin, however, yoga can treat several health issues and it even helps to recover from diabetes faster. Following yoga on daily basis can really help fights all type of health issues like blood pressure and heart diseases and it is beneficial in the long run for a happy and healthy life.
Contact Us Today
Your search to find the right guidance for any disorder and healthy lifestyle ends here. So, contact us today , if you are looking for a psychologist or mind coach. Also, we will discuss how we can help you to lead a stress-free and happy life.
This blog is for information purposes only. If you find any of the above health concerns mentioned in this blog, please contact a professional. Even you can book a consultation with Fitelo to start a healthy journey.
Creamy White Sauce Pasta Recipe: A Delicious & Easy Pasta Dish

Indulge in the creamy goodness of white sauce pasta, a …
Leave A Comment Cancel Reply
Some articles from our experts.

Refreshing Kakdi Koshimbir: A Cooling Maharashtrian Cucumber Salad
5 min read May 27, 2024 502 Views

Let’s Discover The Power Of Pranayama For Weight Loss
19 min read Feb 5, 2024 2370 Views

Power Yoga For Diabetes Management: Empower Your Health
15 min read Aug 21, 2023 2847 Views

What Are The Types Of Cardio Yoga For Weight Loss?
13 min read Aug 17, 2023 4819 Views
The first step to a healthier you starts here. Talk to our experts now
- Whatsapp Us
Talk to our health experts now for immediate guidance
- Book an appointment
Schedule a call with our team at a time that works best for you
Get access to 1000+ healthy and tasty recipes, fitness tips and more. Subscribe to our newsletter

Expand Offer
Start your fat to fit journey with fitelo.
- Diabetes & Primary Care
- Vol:26 | No:04
Interactive case study: MODY – a strong family history of diabetes
Share this article + Add to reading list – Remove from reading list ↓ Download pdf

Diabetes & Primary Care ’s series of interactive case studies is aimed at all healthcare professionals in primary and community care who would like to broaden their understanding of diabetes.
These two scenarios review the most common subtypes of maturity-onset diabetes of the young (MODY), signs and symptoms, differential diagnosis and management.
The format uses typical clinical scenarios as tools for learning. Information is provided in short sections, with most ending in a question to answer before moving on to the next section.
Working through the case studies will improve our knowledge and problem-solving skills in diabetes care by encouraging us to make evidence-based decisions in the context of individual cases.
Readers are invited to respond to the questions by typing in their answers. In this way, we are actively involved in the learning process, which is hopefully a much more effective way to learn.
By actively engaging with these case histories, readers will feel more confident and empowered to manage such presentations effectively in the future.
George , a 31-year-old chef, comes to the surgery asking to be tested for diabetes. He reports symptoms of thirst and explains that there is a strong family history of diabetes. His BMI is 25.2 kg/m 2 and a capillary blood glucose reading is 13.4 mmol/L. How would you proceed from here?
Nadia , 27 years old, has, amongst a set of otherwise normal routine blood investigations, a mildly elevated fasting blood glucose level, confirmed on repeat testing, and is diagnosed with diabetes. Her BMI is 23.2 kg/m 2 and her HbA 1c is 49 mmol/mol (6.6%). She has no relevant past medical history and her only medication is the combined contraceptive pill. Her father was diagnosed with type 2 diabetes at the age of 43, and this is controlled by diet. What type of diabetes might you suspect?
By working through this interactive case study, we will consider the signs, symptoms, differential diagnosis and management of maturity-onset diabetes of the young (MODY).
Editorial: The importance of getting the correct diabetes diagnosis
Pcds committee elections (2024): call for candidates, conference over coffee: diabetes and obesity within multiple long-term conditions, q&a: lipid management – part 3: triglycerides and use of non-statin drugs, lada – assessing diabetes in a non-overweight younger person, challenges and opportunities in reducing risk of diabetes-related cardiovascular disease: making every contact count.

Jane Diggle tackles the challenges of getting the diabetes diagnosis right.

How to stand for election to the PCDS Committee.

The interactions between diabetes, obesity and long-term conditions, including cardiovascular disease, chronic kidney disease and cancer.
Claire Davies answers questions on triglycerides and non-statin drugs.
Sign up to all DiabetesontheNet journals
- CPD Learning
- Journal of Diabetes Nursing
- Diabetes Care for Children & Young People
- The Diabetic Foot Journal
- Diabetes Digest
Useful information
- Terms and conditions
- Privacy policy
- Editorial policies and ethics

By clicking ‘Subscribe’, you are agreeing that DiabetesontheNet.com are able to email you periodic newsletters. You may unsubscribe from these at any time. Your info is safe with us and we will never sell or trade your details. For information please review our Privacy Policy .
Are you a healthcare professional? This website is for healthcare professionals only. To continue, please confirm that you are a healthcare professional below.
We use cookies responsibly to ensure that we give you the best experience on our website. If you continue without changing your browser settings, we’ll assume that you are happy to receive all cookies on this website. Read about how we use cookies .
- Open access
- Published: 09 September 2024
Association of free fatty acid in first trimester with the risk of gestational diabetes mellitus: a nested case-control study
- Liuyan Pu 1 ,
- Haibo Zhou 2 ,
- Hui Liu 2 ,
- Jinhua Wu 1 ,
- Wen Jiang 1 ,
- Shuting Si 2 ,
- Haoyue Cheng 2 ,
- Wenliang Luo 2 ,
- Zhicheng Peng 2 ,
- Xing Xin 2 ,
- Danqing Chen 3 &
- Yunxian Yu 2
BMC Endocrine Disorders volume 24 , Article number: 182 ( 2024 ) Cite this article
Metrics details
Accumulating evidence shows that free fatty acids (FFA) are associated with gestational diabetes mellitus (GDM). However, most of the studies focus on a few specific types of FFA, such as α-linolenic acid (C18:3n3) and Arachidonic acid (C20:4n6) or a total level of FFA.
This study aimed to test the association between a variety of FFAs during the first trimester and the risk of GDM.
The participants came from the Zhoushan Pregnant Women Cohort (ZWPC). A 1:2 nested case-control study was conducted: fifty mothers with GDM were matched with 100 mothers without GDM by age, pre-pregnancy body mass index (BMI), month of oral glucose tolerance test (OGTT) and parity. Thirty-seven FFAs (including 17 saturated fatty acids (SFA), 8 monounsaturated fatty acids (MUFA), 10 polyunsaturated fatty acids (PUFA) and 2 trans fatty acids (TFA)) in maternal plasma during the first trimester were tested by Gas Chromatography–Mass Spectrometry (GC-MS). Conditional logistic regression models were performed to assess the associations of FFA with the risk of GDM.
Nine FFAs were respectively associated with an increased risk of GDM ( P < 0.05), and four FFAs were respectively associated with a decreased risk of GDM ( P < 0.05). SFA risk score was associated with a greater risk of GDM (OR = 1.34, 95% CI: 1.12–1.60), as well as UFA risk score (OR = 1.26, 95% CI: 1.11–1.44), MUFA risk score (OR = 1.70, 95%CI: 1.27–2.26), PUFA risk score (OR = 1.32, 95%CI: 1.09–1.59) and TFA risk score (OR = 2.51, 95%CI: 1.23–5.13). Moreover, joint effects between different types of FFA risk scores on GDM were detected. For instance, compared with those with low risk scores of SFA and UFA, women with high risk scores of SFA and UFA had the highest risk of GDM (OR = 8.53, 95%CI: 2.41–30.24), while the Odds ratio in those with a low risk score of SFA and high risk score of UFA and those with a high risk score of SFA and low risk score of UFA was 6.37 (95%CI:1.33– 30.53) and 4.25 (95%CI: 0.97–18.70), respectively.
Maternal FFAs during the first trimester were positively associated with the risk of GDM. Additionally, there were joint effects between FFAs on GDM risk.
Condensation
Elevated FFA levels in the first trimester increased the risk of GDM.
Peer Review reports
Introduction
It is estimated that 1-30% of pregnant women would suffer from gestational diabetes mellitus (GDM) [ 1 ]. As is reported in previous studies, GDM is often associated with adverse outcomes for both the mother and the baby, such as macrosomia, preterm, cesarean section, pre-eclampsia, hypertension, and type-2 diabetes mellitus (T2DM) [ 2 ]. However, the risk factors of GDM are not clarified completely. Research has shown that the maternal dietary pattern is associated with the risk of gestational diabetes mellitus (GDM) [ 3 , 4 ]. What’s more, one study [ 5 ] pointed out that dietary intake might influence the FFAs. Therefore, FFA may be involved in the development of GDM.
Fatty acid (FA) is a hydrocarbon chain carboxylic acid and can be divided into SFAs and UFAs. Synthesis of FAs occurs in the endoplasmic reticulum and cytoplasm. SFAs can be synthesized by all mammals, and the final products are usually stearic acid (C18:0) and palmitic acid (C16:0). Long-chain FAs are transformed by fatty acid synthase (FAS) from Malonyl-CoA. Palmitic acid is the primary fatty acid that is synthesized by FAS and then palmitic acid will go through elongation to synthesize longer chain SFAs by elongases (ELOVL). MUFAs and PUFAs are then transformed by fatty acid desaturates (FADS). The -CH 3 is called omega (ω) carbon. Depending on the first double bond from the methyl end of molecule backbone, UFAs can be divided into omega-3 (n3), omega-6 (n6), and omega-9 (n9) UFAs. As mentioned above, SFAs can be synthesized to generate omega-9 MUFAs, but SFAs can not be used to generate the precursors of omega-6 and omega-3 series of PUFAs [ 6 ]. Thus, two parent fatty acids of omega-3 and omega-6 fatty acids are known to be essential fatty acids: alpha-linoleic acid (C18:3n3) and linoleic acid (C18:2 cis -n6) [ 7 ]. When the FAs are circulating in the plasma rather than in easter form, fatty acids are also known as non-esterified fatty acids (NEFAs) or free fatty acids (FFAs).
During pregnancy, maternal lipid metabolism will change to adapt to fetal growth and development, including the accumulation of adipose tissue in the first trimester, accompanied by insulin resistance, enhanced lipolysis in the third trimester, and elevated FFA levels [ 8 ]. Increased blood FFA levels are associated with insulin resistance and impaired glucose tolerance [ 9 ]. However, some studies show that FFAs such as Palmitoleic acid, Oleic acid, Linoleic acid and alpha-Linolenic acid are negatively connected with homeostatic model assessment of insulin resistance [ 5 ]. These studies indicate a controversial role that FFAs might play in the process of GDM. An elevated level of FFAs was discovered in individuals diagnosed with normal glucose tolerance, impaired glucose tolerance and type 2 diabetes [ 10 ].However, these studies measured either an overall level of FFAs or only a few types of FFAs. A detailed relationship between different FFAs and GDM needs to be discovered.
This study aimed to explore the associations of both the concentration of various FFAs in the first trimester and their risk scores with the risk of GDM by a nested case-control study. In addition, the joint effects and interactions analysis of different types of FFAs on the risk of GDM were also evaluated.
Materials and methods
Participants.
Zhoushan Pregnant Cohort (ZWPC) is a prospective cohort that was initiated in 2011 at Zhoushan Maternal and Child Care Hospital in Zhoushan (N30°). Under the ZWPC study, women who met the following conditions were included: (1) enrollment at the gestational age of 8-12th week; (2) accomplishment of perinatal examination and delivery of infants in Zhoushan Maternal and Child Care Hospital; (3) Women who were between 18 and 45 years old; (4) No family history of mental disorder (5) Agreement on participation in the study. Exclusion criteria included (1) a history of serious chronic or acute disease; (2) a psychic disorder before pregnancy; (3) threatened abortion; (4) fetal malformations or fetal development abnormalities; (5) incapability of completing the questionnaire due to intellectual problems. The detailed information about this cohort has been previously described [ 11 , 12 ]. Briefly, up to May 2018, the cohort recruited 3431 women who had taken the OGTT test. The study protocol was approved by the Medical Ethical Committee of the School of Medicine, Zhejiang University. A nested case-control study was conducted to detect the effect of FFA on the risk of GDM. In the current study, 50 pregnant women diagnosed with GDM were randomly selected, and 100 healthy pregnant women were matched with GDM cases by maternal age (± 3 years), pre-pregnancy BMI (± 1 kg/m 2 ), OGTT month (± 1 month) and parity.
Information and blood sample collection
After pregnant women provided the informed consent form, a face-to-face interview would be conducted by a well-trained nurse to collect socio-demographic, lifestyle and health behavior information using a structured questionnaire in 8th -14th gestational week, and a 5 ml fasting venous blood sample would be drawn, and centrifuged under 4 °C, then the plasma and white blood cell were stored under − 80 °C until use. Each pregnant woman would be followed up in the 24th -28th gestational week, 32th -36th gestational week and 42nd day postpartum, respectively. The corresponding questionnaire was investigated, and a 5 ml fasting venous blood sample would also be drawn at each visit.
Diagnosis of GDM
Diagnosis of GDM was determined with criteria proposed by the International Association of Diabetes and Pregnancy Study Groups [ 13 ]. A 75 g oral glucose tolerance test (OGTT) was performed during gestational age of 24–28 weeks. Pregnant women who had not been previously diagnosed with diabetes, and then GDM was diagnosed if one of the following conditions was met: fasting plasma glucose ≥ 5.1 mmol/L, 1 h glucose ≥ 10.0 mmol/L or 2 h plasma glucose ≥ 8.5 mmol/L.
Measurement of FFA and data management
A total of 37 FFAs were selected including SFAs (C4:0, C6:0, C8:0, C10:0, C11:0, C12:0, C13:0, C14:0, C15:0, C16:0, C17:0, C18:0, C20:0, C21:0, C22:0, C23:0, C24:0), MUFAs (C14:1, C15:1, C16:1, C17:1, C18:1 cis -n9, C20:1, C22:1n9, C24:1), PUFAs (C18:2 cis -n6, C18:3n6, C18:3n3, C20:2, C20:3n6, C20:3n3, C20:4n6, C22:2, C20:5n3, C22:6n3), TFAs(C18:1 trans -n9, C18:2 trans -n6).
Concentrations of 37 types of FFAs during the first trimester were measured using Gas chromatography–mass spectrometry (SHIMADZU, GC-MS), which allows analysis and detection of a small amount of substance [ 14 ], ranging from nanogram (10 − 9 g) to femtogram (10 − 15 g). In order to control the quality of measurement, ten of the blood samples were tested twice to test the stability of the result. The inter assay coefficient of variation (CV) for FFA is 6.42%.
Outliers were defined as values that deviated by three times the standard deviation and marked as missing values; then (x-min)/(max-min) was used for the standardization of each FFA. This allows for the integration of variables on different scales into a single risk assessment model. Similarly, by standardizing FFA concentrations, we aimed to facilitate the combined analysis of FFAs with varying concentrations, ensuring that each contributes proportionately to the risk assessment. Out of 37 FFAs, 27 have missing values; the highest missing rate was less than 5%. A detailed description of the missing rate of each fatty acid was summarized in Supplement Table 1 . Missing values of FFA were filled using multiple imputation with R software package mice (3.9.0).
Statistical analysis
Shapiro-Wilk test of normality was performed to determine whether the variables met a normal distribution. Mean ± SD or median (Q1, Q3) were used to present variables of normal and abnormal distribution, respectively; and comparison of corresponding variables between GDM and no-GDM group were conducted with student’s t-test and Kruskal-Wallis Rank Sum Test, respectively. Comparison of categorical variables between two groups was conducted using the chi-square test or Fisher exact test.
Firstly, multivariable conditional logistic regression was performed to detect the association of the original value of each FFA concentration with a risk of GDM. Secondly, due to very different concentrations between FFAs, ranging from less than 1 nmol/mL to almost 4000 nmol/mL (Supplement Table 2 ), in order to detect the comprehensive effect of each specific category of FFAs and all FFAs, the standardized concentration of each FFA was generated by formula: (x-min)/(max-min), then standardized regression coefficient (β) of each FFA with GDM was evaluated. If their association (β) was negative, the standardized concentration was furtherly transferred by 1- standardized concentration to ensure each standardized FFA positively correlates with GDM risk. Then, the standardized regression coefficient (β) of each FFA with GDM was used to generate the weighted risk scores. The conditional logistic regression model was used to evaluate the association of the weighted risk score with GDM risk.
In addition, all the FFA risk scores were divided into high and low by median; crossover analysis was used to detect the joint effect of risk scores among different types of FFAs. All the models were adjusted for weight gain from pregnancy to 24th gestational weeks and exercise during pregnancy.
Besides, one previous study [ 4 ] with a case-control study design indicated that dietary factors and GDM history may influence GDM. Therefore, the frequency of dietary intake of protein, fiber, and carbohydrates and the history of diabetes diagnosis were also included as covariates. Our questionnaire collected the intake frequency of sugar drinks, sweets, meat, seafood, milk, eggs, vegetables, and fruit. They were divided into three categories (< 1 time a week, 1–4 times a week, ≥ 5 times a week). Main food intake frequency was divided into three categories (< 200 g a day, 200–400 a day, and > 400 g a day). Supplement intake frequency was divided into 3 categories (Never, 1–3 times a week, ≥ times a week). Finally, carbohydrate intake frequency score was calculated as the sum of the main food, sugar drink and sweets intake. Protein intake frequency score was calculated as the sum of meat, milk, bean products, and egg intake. Fiber intake frequency score was calculated as the sum of vegetable and fruit intake. All the intake frequencies were used to represent the intake level of the nutrients. Detailed distribution of all the nutrient intake was in Supplement Table 3 .
All the analysis was based on R version 3.6.3. P value less than 0.05 was regarded as statistically significant.
Population characteristics
Table 1 summarizes the baseline traits of participants by GDM status. GDM-Control pairs were perfectly matched in maternal age, pre-pregnancy BMI, parity, OGTT month, and exercise during pregnancy. There is also no difference in carbohydrate intake, protein intake, and supplement intake. A slight difference occurred in fiber intake between the GDM and the control group.
Plasma fatty acids and GDM
Women with GDM were more likely to have higher levels of FFAs except for C18:1 trans -n9 (Supplement Table 2 ). Even-chain SFAs especially increased the risk of GDM (C8:0, OR = 1.42, 95%CI: 1.14–1.76, P = 0.0028, Table 2 ). C10:0 is also associated with an elevated risk of GDM. Other even-chain SFAs did not show a significant connection with GDM. On the other hand, odd-chain SFAs reduced the risk for GDM (C11:0, OR = 0.08, 95%CI: 0.01–0.50, P = 0.0089 Table 2 ). Other SFAs with an odd number of carbon atoms (C13:0, C23:0) also served as protecting factors against GDM.
Besides, almost all of the MUFAs, including C14:1, C16:1, and C20:1, showed an adverse effect on GDM (C14:1, OR = 1.23, 95%CI: 1.04–1.46, P = 0.0153, Table 2 ). Nevertheless, C24:1 showed a protective effect on GDM (OR = 0.96, 95%CI: 0.92–0.99, P = 0.0214).
Similar to the result of MUFA, a higher level of PUFA was linked to a higher risk of GDM (C18:3 n6, OR = 1.03, 95%CI: 1.00-1.06, P = 0.0248). Other PUFAs, such as C20:3n6, C20:5n3 and C22:6n3, were all associated with a higher risk of GDM.
Specially, one trans fatty acid C18:1 trans -n9 (OR = 0.91, 95%CI: 0.85–0.98, P = 0.0145) protected women from GDM, and the other TFA showed no statistical significance (C18:2 trans -n6, OR = 1.17, 95%CI: 0.67–2.04, P = 0.5775).
FFAs weighted risk score and GDM
The associations of FFAs weight risk score with GDM risk were presented in Table 4 . Weighted risk score of SFA (OR = 1.34, 95% CI: 1.12–1.60), UFA (OR = 1.26, 95%CI: 1.11–1.44), MUFA (OR = 1.70, 95%CI: 1.27–2.26), PUFA (OR = 1.32, 95%CI: 1.09–1.59), TFA (OR = 2.51, 95%CI: 1.23–5.13) and overall (OR = 1.19, 95%CI: 1.09–1.31) was significantly associated with GDM, respectively.
Joint effect of FFA and GDM
Since most FFAs’ concentrations were highly correlated with each other (Supplement Fig. 1 ), a crossover analysis was implemented to explore the joint effect of different types of FFAs (Table 3 ). Compared with women with both lower MUFA Risk score and PUFA risk score, women with higher MUFA risk score (OR = 4.44, 95%CI = 1.05–18.74, P = 0.0426) had a higher risk of GDM. Furthermore, a joint effect of FFA risk scores emerged in women with both higher risk scores (OR = 6.46, 95%CI = 2.02–20.61, P = 0.0016). Joint effects of other risk scores are similar, including PUFA and TFA, SFA and PUFA, SFA and UFA.
Principal findings
FFAs in the first trimester changed the risk of GDM. There was synergistic effect on the risk of GDM between different FFAs.
Strengths and weaknesses of the study
A major strength of this study was using data collected from a longitudinal cohort, thus reducing the risk of recall bias. Besides, this is a study integrating thirty-seven FFAs tested with GC-MS, which is an accurate technique for FFA detection. Employment of this technology, together with the amount of FFAs, is an assurance of depicting the relationship of FFA and GDM.
However, our study has several limitations. Variables such as annual income and education should be considered as adjustments in the regression models. Allowing for the power of the regression, we only adjusted the exercise after pregnancy and weight gain from pre-pregnancy to 24 weeks of gestational age. This could lead to an underfit problem, reducing the accuracy of the study. Besides, the sample size was not big enough to explore the associations of FFAs with the risk of GDM subgroup. Thus, Multi-center research is needed to increase sample size and avoid selection bias. Finally, we controlled the general dietary intake frequency, including carbohydrates, fiber, protein, and supplements, rather than a detailed intake of dietary ingredients.
Results in the context of what is known
There were very few studies investigating plasma levels of FFA and GDM. FFAs were often taken as an insulin resistance marker in nonpregnant individuals. FFAs were thought to support 30–50% of basic insulin secretion, which allowed obese people to compensate for peripheral insulin resistance [ 15 ]. In women diagnosed with GDM, the plasma FFA level is usually higher in the first trimester [ 16 ], which is in line with our study.
One hypothesis is that FFAs serve as energy producers since Oxidation of 1 g FA generates 37 kJ energy. FAs are considered to provide energy for the fetus after crossing the placenta [ 17 ]. However, an Acute exposure of FFAs leads to insulin secretion and a chronic exposure suppresses insulin secretion [ 18 ]. Thus, during the period of pregnancy, as FFA concentration grows higher, insulin resistance comes along.
A study conducted by Zhu et al. [ 19 ]. revealed a positive relationship between plasma phospholipid SFA at the gestational age of 10 to 14 weeks and GDM and a negative relationship between odd-chain SFA. A similar trend of odd-chain and even-chain SFA also appeared in our study. However, in Zhu et al.’s research, C16:0 was related to a higher risk for GDM, and C17:0 protected women from GDM, while in our study, C16:0 and C17:0 did not reduce or increase the risk for GDM. Given the literature mentioned above, a deduction was made that the number of carbons of SFA might influence its biological function.
Gouaref et al. [ 19 ] suggested that total MUFA concentration was higher in the T2DM group compared with healthy individuals. Our study revealed a similar pattern of MUFA in GDM women. Furthermore, a higher level of C18:1n9 and C14:1n9 was detected in GDM women. Consistent with the finding found by Amélie et al. [ 20 ]. , a higher level of FFA was observed in T2DM patients compared with healthy people.
In addition to MUFA, serum PUFAs were also higher in the GDM group [ 21 ], and the same increase in PUFA levels in the GDM group in the first trimester was also detected. Interestingly, essential FFAs did not show a clear tendency of protection from GDM. To be specific, the concentration of essential FFA C20:4n6 did not differ between the GDM group and the control group. Literature also showed that C20:4n6 was either the same in healthy people and T2DM patients or a little bit higher in the T2DM group [ 20 ]. This is also close to one study that suggests no correlation between serum FFA and T2DM [ 21 ]. An elevation in C22:6n3 (Docosahexaenoic Acid, DHA) and C20:5n3 (Eicosapentaenoic Acid, EPA) was also observed. Previous study suggest that a higher level of DHA and EPA in serum was associated with markers of insulin sensitivity [ 22 ]. Another meta-analysis indicated that omega-3 supplementation was not associated with GDM but was slightly relevant to insulin resistance [ 23 ]. This could be due to the anti-inflammatory properties of DHA and EPA, which might help reduce systemic inflammation and, consequently, insulin resistance [ 24 ]. DHA and EPA can also alter cell membrane fluidity [ 25 ], enhancing insulin receptor function and promoting better insulin sensitivity.
In particular, one of the trans-FFA (C18:1 trans -n9) was higher in healthy control, meaning that it could be linked with lower GDM risk. On the other hand, it may also be for the small sample size, since the result was different from most of the studies’ opinion on trans FFAs. When converted into risk scores, TFA risk score has a positive relationship with GDM. To our knowledge, there were few studies demonstrating the beneficial effect of specific TFAs [ 23 ], and the function of TFAs still needs to be studied.
Except for seeking links between one specific FFA risk score and GDM, we investigated that the total risk score of the FFAs had a robust positive relationship with GDM. However, in the crossover analysis, it seems the TFA risk score had a suppressive effect with other risk scores of FFAs on the risk of GDM. This conclusion differed from most studies that investigate the dietary intake of TFAs, but it may be out of the small sample size. In addition, a joint adverse effect of the FFA risk score was detected, indicating that the risk may increase with the FFA risk score growing higher.
Previous studies indicated that dietary products might influence the risk of GDM [ 26 ]. Therefore, we additionally controlled the effect of dietary factors, including protein, carbohydrate, fiber and supplements intake. The results remained similar, suggesting that FFA may have an independent effect on GDM during pregnancy.
Clinical implications
The progress of GDM involves multiple factors; this paper put a spotlight on FFA in the first trimester, which has been studied by few investigations previously. Our study highlights the importance of FFA in the first trimester in order to identify potential risk factors of GDM. The intervention of FFA in early pregnancy would protect the mothers from GDM.
Research implications
There was a high correlation between FFAs in early pregnant women. Hence, which FFA was really associated with GDM must be further explored, and the detailed molecular mechanism is still unclear.
Our study found that most FFAs increased the risk of GDM, and there were joint effects on GDM risk between different FFAs.
Data availability
The data presented in this study are available on request from the corresponding author. The data are not publicly available because they contain information that could compromise the privacy of research participants.
Abbreviations
Body Mass Index
Docosahexaenoic Acid
Eicosapentaenoic Acid
Fatty Acid Desaturases
Fatty Acid Synthase
Free Fatty Acids
Gestational Diabetes Mellitus
Gas Chromatography–Mass Spectrometry
Monounsaturated fatty acids
Non-esterified fatty acids
Oral Glucose Tolerance Test
Polyunsaturated Fatty Acid
Saturated Fatty Acid
Type-2 Diabetes Mellitus
Trans Fatty Acids
Unsaturated Fatty Acid
Zhoushan Pregnant Women Cohort
McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5:47.
Article PubMed Google Scholar
Johns EC, Denison FC, Norman JE, Reynolds RM. Gestational diabetes Mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab. 2018;29:743–54.
Article PubMed CAS Google Scholar
Abdollahi S, Soltani S, de Souza RJ, Forbes SC, Toupchian O, Salehi-Abargouei A. Associations between maternal dietary patterns and perinatal outcomes: a systematic review and Meta-analysis of Cohort studies. Adv Nutr. 2021;12:1332–52.
Article PubMed PubMed Central CAS Google Scholar
Daneshzad E, Tehrani H, Bellissimo N, Azadbakht L. Dietary total antioxidant capacity and gestational diabetes Mellitus: a case-control study. Oxid Med Cell Longev. 2020;2020:5471316.
Article PubMed PubMed Central Google Scholar
Chen X, Stein TP, Steer RA, Scholl TO. Individual free fatty acids have unique associations with inflammatory biomarkers, insulin resistance and insulin secretion in healthy and gestational diabetic pregnant women. BMJ Open Diabetes Res Care. 2019;7:e000632.
Chavan-Gautam P, Rani A, Freeman DJ. Distribution of fatty acids and lipids during pregnancy. Adv Clin Chem. 2018;84:209–39.
Essential Fatty Acids. Linus Pauling Institute. 2014. https://lpi.oregonstate.edu/mic/other-nutrients/essential-fatty-acids . Accessed 10 Apr 2024.
Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care. 2007;30(Suppl 2):S112–119.
Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23.
Gastaldelli A, Abdul Ghani M, DeFronzo RA. Adaptation of insulin clearance to metabolic demand is a key determinant of glucose tolerance. Diabetes. 2021;70:377–85.
Yang Y, Wang Z, Mo M, Muyiduli X, Wang S, Li M, et al. The association of gestational diabetes mellitus with fetal birth weight. J Diabetes Complications. 2018;32:635–42.
Shao B, Mo M, Xin X, Jiang W, Wu J, Huang M, et al. The interaction between prepregnancy BMI and gestational vitamin D deficiency on the risk of gestational diabetes mellitus subtypes with elevated fasting blood glucose. Clin Nutr. 2020;39:2265–73.
American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(Suppl 1):S11–24.
Article Google Scholar
Medeiros PM. Gas chromatography–Mass Spectrometry (GC–MS). In: White WM, editor. Encyclopedia of Geochemistry: a comprehensive reference source on the Chemistry of the Earth. Cham: Springer International Publishing; 2018. pp. 530–5.
Chapter Google Scholar
Wuesten O, Balz CH, Bretzel RG, Kloer H-U, Hardt PD. Effects of oral fat load on insulin output and glucose tolerance in healthy control subjects and obese patients without diabetes. Diabetes Care. 2005;28:360–5.
Villafan-Bernal JR, Acevedo-Alba M, Reyes-Pavon R, Diaz-Parra GA, Lip-Sosa DL, Vazquez-Delfin HI, et al. Plasma levels of free fatty acids in women with gestational diabetes and its intrinsic and extrinsic determinants: systematic review and Meta-analysis. J Diabetes Res. 2019;2019:7098470.
Herrera E, Desoye G. Maternal and fetal lipid metabolism under normal and gestational diabetic conditions. Horm Mol Biol Clin Investig. 2016;26:109–27.
Haber EP, Ximenes HMA, Procópio J, Carvalho CRO, Curi R, Carpinelli AR. Pleiotropic effects of fatty acids on pancreatic beta-cells. J Cell Physiol. 2003;194:1–12.
Zhu Y, Tsai MY, Sun Q, Hinkle SN, Rawal S, Mendola P, et al. A prospective and longitudinal study of plasma phospholipid saturated fatty acid profile in relation to cardiometabolic biomarkers and the risk of gestational diabetes. Am J Clin Nutr. 2018;107:1017–26.
Sobczak IS, A Blindauer A, Stewart CJ. Changes in plasma free fatty acids Associated with Type-2 diabetes. Nutrients. 2019;11:2022.
Article CAS Google Scholar
Barre DE, Mizier-Barre KA, Griscti O, Hafez K. Flaxseed oil supplementation manipulates correlations between serum individual mol % free fatty acid levels and insulin resistance in type 2 diabetics. Insulin resistance and percent remaining pancreatic β-cell function are unaffected. Endocr Regul. 2016;50:183–93.
England JA, Jain J, Holbrook BD, Schrader R, Qualls C, Mozurkewich E. Effect of prenatal EPA and DHA on maternal and cord blood insulin sensitivity: a secondary analysis of the mothers, omega 3, and mental health study. BMC Pregnancy Childbirth. 2019;19:452.
Devarshi PP, Grant RW, Ikonte CJ, Hazels Mitmesser S. Maternal Omega-3 Nutrition, placental transfer and fetal Brain Development in Gestational Diabetes and Preeclampsia. Nutrients. 2019;11:1107.
Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851:469–84.
Jacobs ML, Faizi HA, Peruzzi JA, Vlahovska PM, Kamat NP. EPA and DHA differentially modulate membrane elasticity in the presence of cholesterol. Biophys J. 2021;120:2317–29.
Lambert V, Muñoz SE, Gil C, Román MD. Maternal dietary components in the development of gestational diabetes mellitus: a systematic review of observational studies to timely promotion of health. Nutr J. 2023;22:15.
Download references
Acknowledgements
We extend our deepest gratitude to all participants and their families. The team at the Zhoushan Maternal and Child Care Hospital is thanked.
This study was funded by the Chinese National Natural Science Foundation (81973055), Major research and development projects of the Zhejiang science and Technology Department (2018C03010), Zhejiang Medical and health science and Technology Project (2015RCA026) and Zhoushan Medical and health science and technology project (2015A02).
Author information
Authors and affiliations.
Zhoushan Maternal and Child Care Hospital, Zhoushan, China
Liuyan Pu, Jinhua Wu & Wen Jiang
Department of Epidemiology & Health Statistics, School of Public Health, School of Medicine, Zhejiang University, Hangzhou, China
Haibo Zhou, Hui Liu, Shuting Si, Haoyue Cheng, Wenliang Luo, Zhicheng Peng, Xing Xin & Yunxian Yu
Department of Obstetrics and Gynaecology, Woman’s Hospital, School of Medicine, Zhejiang University, Hangzhou, China
Danqing Chen
You can also search for this author in PubMed Google Scholar
Contributions
Haibo Zhou and Liuyan Pu analyzed the data. Hui Liu, Wen Jiang, Jinhua Wu, Yunxian Yu reviewed the manuscript. Haoyue Cheng, Wenliang Luo, Zhicheng Peng prepared the manuscript. Xing Xin, Danqing Chen, and Shuting Si prepared all the tables in the paper.
Corresponding author
Correspondence to Yunxian Yu .
Ethics declarations
Ethics approval and consent to participate.
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the institutional review board of Zhejiang University School of Medicine on 2 March 2016 ((2016) Lun Shen Yan (Shen 017)). Informed consent was obtained from all the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Pu, L., Zhou, H., Liu, H. et al. Association of free fatty acid in first trimester with the risk of gestational diabetes mellitus: a nested case-control study. BMC Endocr Disord 24 , 182 (2024). https://doi.org/10.1186/s12902-024-01714-1
Download citation
Received : 24 September 2023
Accepted : 30 August 2024
Published : 09 September 2024
DOI : https://doi.org/10.1186/s12902-024-01714-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Gestational diabetes mellitus
- Free fatty acids
- Joint effect
BMC Endocrine Disorders
ISSN: 1472-6823
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Diabetol Metab Syndr

Diabetic yoga protocol improves glycemic, anthropometric and lipid levels in high risk individuals for diabetes: a randomized controlled trial from Northern India
Navneet kaur.
1 Department of Physical Education, Panjab University, Chandigarh, 160014 India
4 Department of Neurology, Neuroscience Research Lab, Postgraduate Institute of Medical Education and Research, Chandigarh, 160012 India
Vijaya Majumdar
2 Division of Life Sciences, Swami Vivekananda Yoga Anusandhana Samsathana, Bengaluru, Karnataka 560106 India
Raghuram Nagarathna
Neeru malik.
3 Dev Samaj College of Education, Sector 36B, Chandigarh, 160036 India
Akshay Anand
Hongasandra ramarao nagendra, associated data.
The datasets used during the present study are available from the corresponding author on reasonable request.
To study the effectiveness of diabetic yoga protocol (DYP) against management of cardiovascular risk profile in a high-risk community for diabetes, from Chandigarh, India.
The study was a randomized controlled trial, conducted as a sub study of the Pan India trial Niyantrita Madhumeha Bharath (NMB) . The cohort was identified through the Indian Diabetes Risk Scoring (IDRS) (≥ 60) and a total of 184 individuals were randomized into intervention (n = 91) and control groups (n = 93). The DYP group underwent the specific DYP training whereas the control group followed their daily regimen. The study outcomes included changes in glycemic and lipid profile. Analysis was done under intent-to-treat principle.
The 3 months DYP practice showed diverse results showing glycemic and lipid profile of the high risk individuals. Three months of DYP intervention was found to significantly reduce the levels of post-prandial glucose levels (p = 0.035) and LDL-c levels (p = 0.014) and waist circumference (P = 0.001).
The findings indicate that the DYP intervention could improve the metabolic status of the high-diabetes-risk individuals with respect to their glucose tolerance and lipid levels, partially explained by the reduction in abdominal obesity. The study highlights the potential role of yoga intervention in real time improvement of cardiovascular profile in a high diabetes risk cohort.
Trial registration: CTRI, CTRI/2018/03/012804. Registered 01 March 2018—Retrospectively registered, http://www.ctri.nic.in/ CTRI/2018/03/012804.
Introduction
The rise of diabetes in the developing world poses a threat to meager health budgets. Owing to the strong association between various morbidity and mortality outcomes as complications of this dreaded disease, early detection of diabetes risk through non-invasive parameters is a primary requisite. Observational studies show that the risk reduction for diabetes can be decreased by 58% or 63–65% if risk factors could be controlled [ 1 , 2 ]. Many argue that such experimental strategies for the possible halting of conversion of prediabetes into diabetes must continue to include pharmacological interventions even though the rates have not been compared [ 3 ]. Identification of individuals at increased risk for the disease with invasive measurements of fasting and post challenge (postprandial) blood glucose are costly and time consuming. Hence, it has been advocated that the realistic prevention of diabetes should identify high-risk subjects with the use of the non-invasive risk scores [ 4 ]. Such studies should also target subjects with normoglycemia and prevent their progression to poor glycemic status [ 4 ].
Yoga plays a promising role in minimizing the risk of Diabetes for high-risk individuals with prediabetes [ 5 , 6 ]. It reduces body weight, glucose, and lipid levels, though, most of these studies comply with the guidelines of randomized controlled trials adhered to the CONSORT statements [ 7 – 11 ] whereas majority of studies have not reported as per CONSORT statements [ 12 – 15 ]. Several review of published studies, in people with diabetes and prediabetes, have concluded that the practice of yoga may reduce insulin resistance and related cardiovascular disease (CVD) risk factors and improve clinical outcomes [ 16 ]. Specifically, reports suggest that a yoga-based lifestyle intervention reduces body weight, glucose and lipid levels that should reduce diabetes risk. Keeping in view the high transition rates of diabetes in India, we selected a high-risk cohort from Chandigarh, one of the most affluent Union Territories of India with highest reported prevalence of diabetes in order to establish the efficacy of yoga to alleviate the cardiovascular disease. Indian Diabetes Risk Score (IDRS), specific for Indian ethnicity a validated tool was used for identification of the high-risk population [ 17 ]. We developed a national consensus ‘Diabetes Yoga protocol’ based on published reports and classical literature with an aim to stimulate weight reduction by combination of postures and meditation techniques [ 18 , 19 ]. Additionally, cardiometabolic risk reduction has also been recognized as one of the potential outcomes of yoga-based interventions [ 20 ]. Yoga has been shown to be regulating the risk parameters of diabetes, waist circumference (WC), body mass index (BMI), oxidative stress, fasting blood sugar (FBS) and systolic blood pressure (SBP) respectively [ 21 ]. Hence, in this study we tested the efficacy of diabetic yoga protocol (DYP) on alleviation of glycemic and lipid imbalances in individuals at high risk of diabetes.
Materials and methods
Study population.
Under the multi-region survey of Niyantarita Maduhmeha Bharat (NMB-2017) a door-to-door screening was carried out for the identification of high risk individuals among the population of Chandigarh (U.T) and Panchkula (District in Haryana state) on the basis of Indian Diabetes Risk Score (IDRS). The data collection was carried out by well trained yoga volunteers for diabetes management (YVDMs). Written informed consents were taken from every subject during door to door screening as well as at the time of registration. All the experimental protocol, methods and procedures were approved by Ethics committee of Indian Yoga Association (IYA) (ID: RES/IEC-IYA/001). All experiments methods and procedures were carried out in accordance with relevant guidelines and regulations of ethics committee. The study was registered at clinical trial registry of India, CTRI/2018/03/012804 (dated: 01/03/2018).
Study design
The present study is the two-armed randomized controlled trial conducted in the population of Chandigarh and Panchkula regions of northern India. Indian Diabetes Risk Score (IDRS) was used for detection of high risk (≥ 60 score) individuals from the study. Self-declared diabetics and low (< 30 score) and moderate [between 30–50 score] risk individuals were excluded from the study. As evident from the flow of patients presented in the flowchart, out of 1214 eligible subjects, there was approximately 50% loss of sample data due to error in the sampling. Further out of 564, we had to exclude as they were self-declared patients with diabetes and did not further participate in the study. However, this led to final participation of only 184 subjects in the study and allocation of these subjects diminishing the random selection of the study cohort. A cohort of high diabetes-risk cohort consisting of n = 184 participants was randomized into the interventional and control groups (n = 91:93). After excluding the dropouts from the study, based on CONSORT guidelines, the remaining subjects in the DYP and control group were further assessed for selected anthropometric, glycemic and lipid parameters. The intervention group was given the Diabetic Yoga Protocol for three months and control group continued with their daily routine activities. The detailed categorization of the samples is shown in Fig. 1 . The control group was waitlisted for yoga.

Flowchart of study design. PCA principal component analysis, MIPCA multiple imputations with PCA
Randomization
Simple randomization technique was used to allocate participants into the intervention and the control groups. An independent statistician generated a computer-generated random number sequence and the sequence was given to an external staff who had no involvement in the study procedures. The participants were allocated their consecutive numbers, after baseline measurements. Blinding of the participants was not possible due to the nature of the intervention. However, the outcome assessors were blinded.
Risk assessment
To identify the individuals at high-risk of diabetes, Indian Diabetes Risk Score (IDRS) was administered as proposed by Mohan et al. [ 22 ]. It consisted of two unmodifiable (i.e. age, and family history) and two modifiable (physical activity and waist circumference) risk factors for diabetes, which can predict the level of risk for the development of diabetes in the community. The IDRS is one of the easily accessible and budget friendly questionnaire to be administered. The aggregate score of the unmodifiable and modifiable risk used to probe the level of risk among the population (i.e. High risk > 60, Moderate risk-30–50, Low risk < 30).
Sample size
Sample size estimation for the main Pan India study was focused for prediabetes subjects [ 23 ]. However, for the present pilot scale study we calculated sample size assuming a small effect size 0.3 [ 5 ] of DYP vs waitlist control 0.25, α = 0.80 as 180 (n = 90:90). Further, assuming an attrition rate of 20%, the final sample size was n = 220.
Study outcomes
Changes in the glycemic and other metabolic variables (anthropometric and lipid) over 3 months were documented. The fasting blood sample was withdrawn. For glucose analysis, fasting samples for 10–12 h were taken early in the morning for the estimation of FBS and afterwards 75 g glucose was given to the participants. The blood sampling was repeated after 2 h. for estimation of OGTT.
Biochemical analysis
For the estimation of biochemical parameters viz. FBS (Fasting Blood Sugar, Rxl-Max 500), OGTT (Oral Glucose Tolerance Test), HbA1c (Bio-Rad D-10), Triglycerides, Cholesterol, HDL, LDL, Chol/HDL ratio, HDL/LDL ratio (Rxl-Max 500) and VLDL about 9 ml of blood was drawn and analyzed by phlebotomist of Sisco Research Laboratories (SRL) of Chandigarh. Anthropometric measurements were also obtained (i.e. height, weight, waist circumference) by trained researcher. The waist circumference (WC) was reported in centimeters. The BMI was obtained by using the formula (weight in kg/height (meter) 2 ).
Interventions
The study protocol consisted of Diabetic Yoga Protocol (DYP) approved by the Ministry of AYUSH and Quality Council of India as shown in Table Table1. 1 . This is the first protocol to be made specifically for the prediabetics and diabetics. The complete sequence of prayer, yogic postures, breathing and meditative techniques, along with specified time, was shown in previously published paper [ 24 ]. The Yogic practices were performed for 3 months for 60 min. Certified yoga instructors took the yoga classes and they recorded regular attendance. Randomization was done through a computer-generated list of random numbers and allocation was concealed to the participants until the completion of the baseline assessment.
Diabetic yoga protocol (DYP)
| S. No. | Name of practice | Duration (min) |
|---|---|---|
| 1 | Starting prayer: Asatoma Sat Gamaya | 2 |
| 2 | Preparatory Sukshma Vyayamas and Shithililarna Practices 1. 2. (3 rounds)
3. Sarvangapushti (3 rounds clockwise, 3 rounds anticlockwise) | 6 |
| 3 | Surya Namaskara (SN) 10 step fast 6 rounds 12 step slow 1 round Modified version Chair SN 7 rounds | 9 |
| 4 | Asanas (1 min per Asana) 1 Trikonasana, Parvritta Trikonasana, Prasarita Padhastasana 2 Jatara Parivartanasana, Pawanmuktasana, Viparitakarani 3 Bhujangasana, Dharuasana followed by Pawanmuktasana 4 Mandukasana, Vakrasana/ Ardhamatsayendrasana, Paschimatanasana, Ardha Ushtrasana At the end, relaxation with abdominal breathing in supine position (vishranti), 10–15 rounds (2 min) | 15 |
| 5 | Kriya a. :1 min b. | 3 |
| 6 | Pranayama ( )
| 9 |
| 7 | Meditation (for Stress, for deep relaxation and silencing of mind) Cyclic Meditation | 15 |
| 8 | Resolve (I am Completely Healthy) | 1 |
| 9 | Closing Prayer: Sarvebhavantu Sukhina………… | 1 |
| Total duration | 60 |
Statistical analysis
For the analysis of data SPSS for Windows (version 22; IBM SPSS Inc., Chicago IL) 0 and R statistical package were used. The normality of data was analyzed using Kolmogorov–Smirnov test. The paired t-test was used to estimate the Baseline and posttest differences of DYP, and control group and the significant level was set at ≤ 0.05. The trial outcomes were analyzed according to the intention-to-treat principle; hence multiple imputation was carried for the missing variables accounting for the loss to follow up. We used absolute change (time and treatment interaction), to estimate intervention effects refers to the difference in the outcome of the intervention and control over different time-points of assessment. Absolute change was determined as follows: absolute change = [(intervention group follow-up) – (intervention group baseline)] – [(control group follow-up) – (control group baseline)]. The percentage change, also called the relative change was determined as relative change = (absolute change / intervention group baseline) × 100%. To evaluate the influence of missing data, we applied multiple imputations to the data using missMDA R package (v1.13) based on the principal component analysis method [ 25 ] from the package, using 5 components to reconstruct the data and over 1000 imputed datasets. One-way multivariate analysis of covariance (MANCOVA) was conducted to compare the effects of the DYP with control group glycemic and metabolic measures, while controlling for the age, gender and baseline values of the covariates.
Baseline characteristics
The data used in this study was collected in (NMB-2017) the northern region of India i.e. Chandigarh and Panchkula. The age range of participants was 3–70 years; [mean age 48.51 (SD 10.08) years]with baseline characteristics of the yoga and control groups as shown in Table Table2. 2 . Mean HbA1c of the high-risk cohort was 5.64% (0.38), mean FBS was 97.13 mg/dl (SD 11.10), and mean PPBS were 108.40 mg/dl (SD 28.79). Distributions of age and gender was similar between the intervention and the control groups. The IDRS and anthropometric values were also similarly distributed between the groups. Overall, there was no significant difference in the distribution of demographic, anthropometric, or biochemical parameters between the DYP and the control groups at the baseline.
Baseline characteristics of the participants in the intervention and control group
| Characteristics | DYP Group N = 91 | Control group N = 93 | P value |
|---|---|---|---|
| Gender | |||
| Male, n (%) | 19 (20.88) | 30 (32.26) | 0.096 |
| Age (years) | 47.77 (9.59) | 49.24 (10.53) | 0.323 |
| Weight, Kg | 70.93 (10.90) | 70.80 (12.44) | 0.936 |
| Waist circumference, cm | 99.34 (9.05) | 99.72 (9.05) | 0.794 |
| BMI, Kg/m | 28.59 (5.75) | 28.53 (5.01) | 0.949 |
| IDRS | 74.07 (10.43) | 75.27 (9.95) | 0.425 |
| Biochemical variables | |||
| FBG, mg/dl | 96.89 (9.95) | 97.36 (12.20) | 0.776 |
| PPBG, mg/dl | 102.88 (21.91) | 113.78 (33.47) | 0.012* |
| HbA1c (%) | 5.61 (0.38) | 5.66 (0.38) | 0.400 |
| Total cholesterol mg/dl | 186.88 (37.64) | 179.98 (34.98) | 0.199 |
| Triglycerides, mg/dl | 131.93 (68.59) | 138.44 (68.89) | 0.522 |
| HDL-c, mg/dl | 47.76 (9.16) | 48.33 (17.43) | 0.780 |
| LDL-c, mg/dl | 112.75 (31.02) | 104.38 (31.70) | 0.072 |
| VLDL, mg/dl | 26.39 (13.72) | 28.00 (13.50) | 0.423 |
Continuous variables are represented as mean (SD) and compared using independent t-test. Categorical variables are represented as number (percentages) and compared using chi-square test. P value < 0.05 were considered significant. FBS fasting blood sugar, PPBG postprandial blood glucose, HbA1c glycated hemoglobin, HDL-c high density lipid-cholesterol, LDL-c low density lipid-cholesterol, VLDL very low density lipid-cholesterol, IDRS Indian diabetes risk score
When analyzed by multivariate analysis of covariance (MANCOVA), adjusting for age, gender and status of diabetes/prediabetes/normoglycemia, and baseline values of the covariates, yoga intervention was found to have significant influence on few cardinal parameters related to glycemic control (PPBS), and lipid control (LDL-C) as shown in Table Table3. 3 . We also observed a significant influence of DPP on waist circumference reduction [relative changes, − 1.94%. Compared to the control, DYP also resulted in significant reductions in LDL-C and, − 0.16% and − 2.81%, for LDL-Cholesterol and post-prandial blood glucose levels from baseline to 3 months [absolute changes, − 0.18% and − 3.08%, respectively and relative changes, − 0.16% and − 2.81%, respectively].
Comparative assessment of influence of DYP on biochemical and weight related variables with the control group
| Variables | Baseline mean (SD) | After 3 months mean (SD) | Absolute change | Relative change | P value | Partial η2 |
|---|---|---|---|---|---|---|
| Waist circumference (cm) | ||||||
| DYP | 99.34 (9.05) | 98.14 (6.88) | − 1.93 | − 1.94 | 0.032 | 0.029 |
| Control | 99.72 (9.05) | 100.25 (7.72) | ||||
| BMI, kg/m2 | ||||||
| DYP | 28.59 (5.75) | 28.00 (6.84) | − 0.4 | − 1.40 | 0.622 | 0.002 |
| Control | 28.53 (5.01) | 28.34 (4.98) | ||||
| Weight, Kg | ||||||
| DYP | 70.93 (10.90) | 69.04 (9.13) | − 1.04 | − 1.47 | 0.397 | 0.005 |
| Control | 70.80 (12.44) | 69.95 (10.44) | ||||
| Postprandial blood glucose, mg/dl | ||||||
| DYP | 102.88 (21.91) | 118.32 (29.89) | − 1.51 | − 1.47 | 0.006 | 0.046 |
| Control | 113.78 (33.47) | 130.73 (36.98) | ||||
| Fasting blood glucose, mg/dl | ||||||
| DYP | 96.89 (9.95) | 99.82 (9.49) | 1.44 | 1.49 | 0.287 | 0.007 |
| Control | 97.36 (12.20) | 98.85 (9.26) | ||||
| HBA1c (%) | ||||||
| DYP | 5.61 (0.38) | 5.61 (0.39) | − 0.02 | − 0.36 | 0.077 | 0.020 |
| Control | 5.66 (0.38) | 5.68 (0.38) | ||||
| Total Cholesterol, mg/dl | ||||||
| DYP | 186.88(37.64) | 189.01 (25.64) | − 0.4 | − 0.21 | 0.130 | 0.014 |
| Control | 179.98 (34.98) | 182.51(20.82) | ||||
| Triglycerides, TG, mg/dl | ||||||
| DYP | 131.93 (68.59) | 148.14 (54.92) | − 13.98 | − 10.60 | 0.138 | 0.014 |
| Control | 138.44 (68.89) | 168.63 (75.06) | ||||
| HDL-C, mg/dl | ||||||
| DYP | 47.76 (9.16) | 47.01 (9.16) | 2.2 | 4.61 | 0.097 | 0.017 |
| Control | 48.33 (17.43) | 45.38 (12.57) | ||||
| LDL-C, mg/dl | ||||||
| DYP | 112.75 (31.02) | 103.39 (21.44) | − 17.56 | − 15.57 | 0.044* | 0.025 |
| Control | 104.38 (31.70) | 112.58 (21.99) | ||||
| VLDL, mg/dl | ||||||
| DYP | 26.39 (13.72) | 28.85 (10.47) | − 1.23 | − 4.66 | 0.229 | 0.009 |
| Control | 28.00 (13.50) | 31.69 (10.57) |
Absolute change = [(intervention group follow-up) – (intervention group baseline)] – [(control group follow-up) – (control group baseline)]. Relative change = (absolute change / intervention group baseline) × 100%; p value for difference between the intervention and the control groups by MANCOVA adjusting for age, gender, status of diabetes/prediabetes/normoglycemia baseline values of glycemic and lipid variables, length of time having had prior exposure of yoga
We examined the effect of Diabetic Yoga Protocol on baseline and post (3 months) levels of HbA1c and other glycemic (OGTT and FBS), Lipid (Total cholesterol, triglycerides, HDL-c, LDL-c, and VLDL-c, CDL/HDL, LDL/HDL) and anthropometric parameters (BMI). In the present study, we show the efficacy of DYP in substantial improvement in the waist circumference in a high-risk diabetes population from Chandigarh (relative change of 1.94 cm). We could also demonstrate a significant decline in the worsening of post prandial glucose levels with yoga intervention as compared to the wait-list control group (relative change of 2.82 mg/ml). However, for LDL-c levels, there were clinically significant improvements by 0.16 units. Notably, over 3 months study duration there was an overall increase in the levels of total cholesterol, triglyceride and VLDL means in the study cohort, while HDL levels had decreased. In particular TG levels have gone from normal range to mildly high (> 150 mg/dl) [ 26 ] which draws our attention towards accelerated pace of metabolic dysfunction in the high risk population. These findings comply with Chandigarh being an affluent union territory of India with high per-capita GDP and has been documented to have highest prevalence of diabetes 13.6%, 12.8–15·2 as compared to other Indian states [ 27 ]. As mentioned above, there was a significant influence of DYP on the waist circumference, one of the two important modifiable parameters of Indian Diabetes Risk Score [ 17 ]. The relevance of WC reduction in context of reduced risk of CVD is well established; a 1 cm increase in WC has been associated with a 2% increase in the relative risk of future CVD [ 28 ]. The visceral adipose tissue is a primary source of cytokine production and insulin resistance (IR) [ 29 ]. Given the higher susceptibility towards visceral fat accumulation and insulin resistance in Asian populations as compared to their Caucasian counterparts, the observed influence of DYP on WC is of particular relevance to the metabolically obese phenotype of Asian Indians [ 30 ].
In relation to the glucose metabolism, we could also demonstrate a significant decline in the worsening of post prandial glucose levels with DYP as compared to the wait-list control group (relative change = − 2.81%, P < 0.05); however, no significant influence could be established for fasting blood glucose concentration. These findings could be justified by the phenotypic differences underlying fasting and post-challenge hyperglycemia that represent distinct natural histories in the evolution of type 2 diabetes [ 31 ]. Postprandial glucose disposal is the primary pathogenic manifestation in impaired glucose tolerance (IGT), and impaired fasting glucose (IFG) merely signifies an abnormal glucose set point [ 31 , 32 ]. Our relevance of the study findings is further underlined by the previous results wherein PPG has been reported to contribute more than FBS to overall hyperglycemia and its control was found essential either to decrease or to obtain HbA1c goals of < 7 [ 33 ]. Several epidemiological studies have suggested that increased glycemic exposure, especially post challenge or postprandial hyperglycemia, is an independent risk factor for macrovascular disease with no apparent upper or lower threshold. Our results indicate a significant influence of yoga on glycemic control integrating postprandial glycemic alterations in the high diabetes risk group. Since in the present study the high-risk cohort was selected through A1c based diagnosis, and IGT was not a primary manifestation in the cohort, hence, the overall improvement in postprandial glucose should be specifically tested in an IGT cohort. The findings of the current study with a 3-month intervention of yoga on postprandial measures of glucose at-risk population deserves clinical attention. Increase in the glucose concentration even in the prediabetes stage, manifests as a chronic inflammatory condition and predisposes an individual to the risk of pathogenic infections [ 32 , 34 , 35 ].
The simultaneous reduction in waist circumference observed in the cohort, is also consistent with the observation of an association between abdominal obesity and the risk of IGT. Based on a significant association between IGT and CVD risk [ 32 , 33 , 36 ], we note a significant improvement in lipid concentrations [LDL-c] by the DYP protocol as compared to the control group. These results are consistent with the previously reported overall beneficial effect of yoga in the management of hyperlipidemia [ 36 ]. These results need validation at larger scale and to ascertain the mechanistic insights into the action of yoga, the indices of monocyte chemotaxis, endothelial inflammation, oxidation, nitric oxide production, and thrombosis should also be explored [ 37 ], including animal models, invitro systems and other approaches [ 38 – 44 ].
The findings of the present study indicate that identification of high-risk group through IDRS and consequent intervention of Yoga based lifestyle protocol could be an effective strategy to combat the metabolic perturbations associated with diabetes, whose co-morbidity is also being reported to be associated with increasing vulnerability to the emerging viral pandemic of COVID-19. Lifestyle interventions are reported to reduce the risk of Type 2 diabetes in high-risk individuals after mid and long-term follow-up. Information on determinants of intervention outcome, adherence and the mechanisms underlying diabetes progression are valuable for a more targeted implementation. Weight loss is a major contributor in the prevention and management of type 2 diabetes. In many of the earlier lifestyle intervention group of the DPP, weight loss was the dominant predictor of reduced diabetes risk, with a 16% reduction observed for every kilogram of weight loss during the 3.2-year follow-up [ 45 ]. Though we failed to observe a significant weight loss over 3 months of DYP intervention, the significant reductions in WC indicate the plausibility of significant weight loss on longer interventions and follow ups.
Whether Yoga alters the conversion of prediabetics into healthy status and if it helps in maintenance of glycemic index can be assessed by longitudinal studies. There was a significant improvement in the glycemic status of the high risk population at administration of DYP. The analysis shows the aptness of Diabetic protocol which is apparently superior to previous studies where no standardized protocols were used for intervention [ 46 , 47 ]. The findings suggest that there is potential of DYP to manage glucose levels in diabetes patients if public intervention is planned through forthcoming wellness centers in India. There are additional studies showing beneficial effects of Yoga on FBS [ 48 ], PPBS [ 49 – 51 ], HbA1c [ 50 , 51 ], total cholesterol, LDL [ 50 , 51 ]. The analysis of the yoga protocols used in above said studies reveal the incorporation of some common and important postures in DYP, which seem to be important in managing the disease. It is also the possible that the beneficial effects of mind body techniques are sensitive to mental disposition of subjects and has been characterized by various measures like psychometric analysis [ 52 , 53 ], namely, Tridosha and Triguna scoring [ 54 , 55 ]. These were not analyzed in this study.
Briefly, DYP’s promising efficacy on glycemic and metabolic parameters requires mechanistic insights. This can be examined by further studies, and long term follow up which was not possible in this study. As DYP is a non-pharmacological, cost-effective method to halt the conversion of early diabetes into prediabetes and/or healthy individuals, the success of its integration into public health policy will depend on its wider acceptability and perception of benefits by both public as well as healthcare workers [ 56 – 59 ]. Yoga’s benefits in maintaining and regulation of the glycemic status are supported by several other studies [ 49 , 50 ], which might enable its inclusion in the National Ayushman Bharat scheme or as part COVID pandemic management protocol in which a large number of individuals with diabetes and heart disease are falling prey [ 60 , 61 ]. This will further encourage molecular and Ayurgenomic studies which presumably underlie the stated clinical outcome.
Limitations
Moreover, there are some limitations of our study that we only studied in two regions of North India and thus the result of this study cannot be generalized on the remaining population. Further, in this study, the socio economic status and psychological assessments were not carried out. We were not able to control for the dietary habits and psychological status of the study participants. However, the small sample size and absence of long term evaluations limit the strength of the study.
Acknowledgements
The authors would like to thank Central Council for Research in Yoga & Naturopathy (CCRYN) for their support for man power, Ministry of Health and Family Welfare (MOHFW) for support the cost of investigations and Indian Yoga Association (IYA) for the overall project implementation. The authors also like to thank to thank Yoga Volunteer for Diabetes Management (YVDMs) for helping in collection of data and also for training participants for yoga.
Abbreviations
| ADA | American Diabetes Association |
| BMI | Body mass Index |
| CVD | Cardiovascular disease |
| DYP | Diabetic yoga protocol |
| FBS | Fasting blood sugar |
| HbA1c | Glycated hemoglobin |
| HDL-c | High density lipid-cholesterol |
| IDRS | Indian Diabetes Risk Score |
| IFG | Impaired fasting glucose |
| IGT | Impaired glucose tolerance |
| IYA | Indian Yoga Association |
| LDL-c | Low density lipid-cholesterol |
| NMB | |
| OGTT | Oral glucose tolerance test |
| PPBG | Postprandial blood glucose |
| SBP | Systolic blood pressure |
| VLDL | Very low density lipid-cholesterol |
| WC | Waist circumference |
| YVDM | Yoga volunteers for diabetes management |
Authors' contributions
NK: writing of manuscript, collection of data. VM: writing of manuscript, analysis. RN: conceptualization of manuscript, supervision and study design. NM: co-conceptualization of manuscript. AA: conceptualization of manuscript. HRN: supervision. All authors read and approved the final manuscript.
The Project was funded by Ministry of AYUSH, Government of India (grant number 16-63/2016-17/CCRYN/RES/Y&D/ MCT/).
Availability of data and materials
Declarations.
Written informed consents were taken from every subject during door to door screening as well as at the time of registration. All the experimental protocol, methods and procedures were approved by Ethics committee of Indian Yoga Association (IYA) (ID: RES/IEC-IYA/001). All experiments methods and procedures were carried out in accordance with relevant guidelines and regulations of ethics committee.
Not applicable.
The authors declare that they have no competing interests.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Raghuram Nagarathna, Email: moc.liamg@antaraganr .
Akshay Anand, Email: moc.liamffider@dnana1yahska .

IMAGES
VIDEO
COMMENTS
Abstract. A growing body of evidence suggests yogic practices may benefit adults with type 2 diabetes (DM2). In this systematic review, we evaluate available evidence from prospective controlled trials regarding the effects of yoga-based programs on specific health outcomes pertinent to DM2 management. To identify qualifying studies, we ...
Effect of Yoga on Blood Glucose Levels in Patients with ...
Therapeutic Role of Yoga in Type 2 Diabetes - PMC
Objectives: This study:Healthy Active and in Control (HA1C), examined the feasibility and acceptability of yoga as a complementary therapy for adults with Type-2 Diabetes (T2DM). Design: A 2-arm randomized clinical trial comparing Iyengar yoga with a supervised walking program. Setting: Hospital based gym-type facility and conference rooms.
We excluded uncontrolled trials, cross-sectional studies, case series, and case studies, as well as trials that were published only in dissertation or abstract form. We also excluded articles that did not specifically target adults with diabetes, involve an intervention focused on yoga as the major component, study a yoga program of at least ...
The Healthy, Active, and in Control (HA1C) study is designed to examine the feasibility and acceptability of yoga among adult patients with T2DM. In this pilot randomized controlled trial, adults with T2DM were randomly assigned to either a 12-week Iyengar Yoga intervention given twice weekly, or a twice-weekly 12-week program of traditional ...
Other studies applied a non-randomized study design that could affect the final outcomes 11-13. Thus, in the present study, we carried out a meta-analysis of randomized controlled trials (RCTs) to determine the effectiveness of yoga in patients with type 2 diabetes mellitus. Materials and Methods
3.3 Study selection criteria 3.3.1 Inclusion criteria. 1. Study examining yoga intervention (including at least one of asana, pranayama, meditation) to promote T2DM management and comparing yoga intervention with other usual care or physical exercise or nontherapeutic intervention.; 2. Study that is randomized control trial, randomized cross-over studies, cluster-randomized trials, or quasi ...
A growing body of evidence suggests yogic practices may benefit adults with type 2 diabetes (DM2). In this systematic review, we evaluate available evidence from prospective controlled trials regarding the efects of yoga-based programs on specific health outcomes pertinent to DM2 management.
YOGA & DIABETES BROMBERG, DAHL, PRADA, SUGAI 1 Case Studies on the Impact of Yogatherapy on Diabetes, Prediabetes & Metabolic Syndrome ... on specific case studies and highlights how the approaches can vary along more complicated lines than simply the type of diabetes. We targeted 13 patients, including 12 female and 1 male participant.
Study design. The Healthy Active and in Control (HA1C) study is designed to examine the feasibility and acceptability of yoga among adult patients with type 2 diabetes (T2DM). In this pilot randomized controlled trial, adult diabetics are randomly assigned to either; (1) a 12-week Iyengar yoga intervention given twice weekly, or (2) a twice-weekly 12-week program of traditional exercise (e.g ...
Objectives: To assess the beneficial effects of yoga on blood glucose levels in normal and T2DM volunteers. Materials and methods: A prospective case-control study was conducted in the Department of Physiology and Diabetic clinic of a tertiary care teaching hospital over period of two years. The study subjects consisted of 30 male diabetic ...
Key Takeaways. Mind-body practices like yoga may help lower blood sugar levels in people with type 2 diabetes, according to a new review of studies. The reduction in A1C levels from yoga was significant, only 0.1% behind metformin. Adding mind-body practices to a doctor-prescribed regimen could help some people better manage their condition.
Purpose To study the effectiveness of diabetic yoga protocol (DYP) against management of cardiovascular risk profile in a high-risk community for diabetes, from Chandigarh, India. Methods The study was a randomized controlled trial, conducted as a sub study of the Pan India trial Niyantrita Madhumeha Bharath (NMB). The cohort was identified through the Indian Diabetes Risk Scoring (IDRS) (≥ ...
In this review, we briefly describe the role of various yoga practices in the management of diabetes based on evidence from various clinical studies. Showing the mechanisms of benefits of yoga ...
A narrative review on role of Yoga as an adjuvant in the management of risk factor, disease progression and the complications of type 2 diabetes mellitus. Mooventhan A. Diabetes Metab Syndr. 2017 Nov;11 Suppl 1:S343-S346. [ abstract ] The effect of yoga practice on glycemic control and other health parameters in Type 2 diabetes mellitus ...
The purpose of this meta-analysis was to examine the effects of yoga for glycemic control among adults with type 2 diabetes (T2DM). Comprehensive electronic databases searches located 2559 unique studies with relevant key terms. Studies were included if they (1) evaluated a yoga intervention to promote T2DM management, (2) used a comparison ...
Citation: Saha S. Deal with Diabetes in Yogic Way -A Case Study. Ann Yoga Phys Ther. 2023; 6(1): 1049. Annals of Yoga and Physical Therapy Open Access Introduction Diabetes is one of the major non-communicable killer dis-eases at the present time. This lifestyle disease has become a common occurrence for past few years worldwide. It is a con-
Case Study: A Patient With Uncontrolled Type 2 Diabetes and ...
These studies have confirmed the useful role of yoga in the control of diabetes mellitus. Fasting and postprandial blood glucose levels came down significantly. Good glycaemic status can be maintained for long periods of time. There was a lowering of drug requirement and the incidence of acute complications like infection and ketosis was ...
Yoga Poses For Diabetes. Although there are various asanas for Diabetes. Here are seven asanas that are considered the most effective to treat diabetes : 1. Vakrasana [Twisted Pose] Vakra means twisted in Sanskrit hence the name is vakrasana. This is a spine-twisted pose for flexibility. Benefits of Vakrasana.
In case of TG, LDL, and VLDL, the decrease also ... et al. Impact of lifestyle-related factors on all-cause and cause-specific mortality in patients with type 2 diabetes: The Taichung Diabetes Study. Diabetes Care. 2012; 35:105-12. [PMC free article] ... Yoga and Diabetes. Bombay: Publ. Health Care Communications; 1994. pp. 159-67. ...
Diabetes & Primary Care's series of interactive case studies is aimed at all healthcare professionals in primary and community care who would like to broaden their understanding of diabetes.. These two scenarios review the most common subtypes of maturity-onset diabetes of the young (MODY), signs and symptoms, differential diagnosis and management.
Accumulating evidence shows that free fatty acids (FFA) are associated with gestational diabetes mellitus (GDM). However, most of the studies focus on a few specific types of FFA, such as α-linolenic acid (C18:3n3) and Arachidonic acid (C20:4n6) or a total level of FFA. This study aimed to test the association between a variety of FFAs during the first trimester and the risk of GDM.
Yoga's benefits in maintaining and regulation of the glycemic status are supported by several other studies [49, 50], which might enable its inclusion in the National Ayushman Bharat scheme or as part COVID pandemic management protocol in which a large number of individuals with diabetes and heart disease are falling prey [60, 61]. This will ...