Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals

Cardiovascular diseases articles from across Nature Portfolio
Cardiovascular diseases are pathological conditions affecting the heart and/or blood vessels that is, the cardiovascular system.
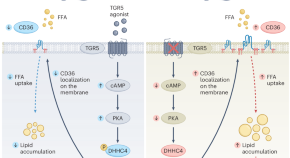
Bile acids for diabetic cardiomyopathy
Diabetes can cause heart failure by a toxic accumulation of lipids in cardiac myocytes, which impairs their function. This work shows that stimulation of the bile acid receptor TGR5 limits fatty acid uptake into cardiac myocytes and prevents the development of diabetic cardiomyopathy.
- David Weissman
- Christoph Maack
Directions for promoting patency of arterial grafts
By dissecting the cell composition and function of arterial grafts derived from the internal thoracic, radial and right gastroepiploic arteries, we identified factors that might promote patency rates of arterial grafts, including combating lipid deposition, disturbances in wall shear stress, smooth muscle cell proliferation, fibrosis and spasm.
Related Subjects
- Acute coronary syndromes
- Arrhythmias
- Cardiomyopathies
- Congenital heart defects
- Dyslipidaemias
- Heart failure
- Hypertension
- Valvular disease
- Vascular diseases
Latest Research and Reviews
Spontaneous coronary artery dissection: a clinically oriented narrative review
- Sonya Burgess
- Sarah Zaman
A novel score for early prediction of urinary tract infection risk in patients with acute ischemic stroke: a nomogram-based retrospective cohort study
- Qinqin Zhao
- Pinpin Feng
The pan-PPAR agonist lanifibranor improves cardiometabolic health in patients with metabolic dysfunction-associated steatohepatitis
Cardiovascular events are the main cause of mortality in patients with metabolic dysfunctionassociated steatohepatitis (MASH). Here, the authors show that lanifibranor improves cardiometabolic health - insulin sensitivity, lipid and glucose metabolism, systemic inflammation and hepatic steatosis.
- Michael P. Cooreman
- Javed Butler
- Sven M. Francque
Development and multinational validation of an algorithmic strategy for high Lp( a ) screening
Elevated Lp( a ) is an independent atherosclerosis risk factor that is not routinely measured in the general population. Aminorroaya et al. develop and validate a machine learning model, ARISE, that allows for the detection of elevated Lp( a ) using commonly available clinical features from electronic records.
- Arya Aminorroaya
- Lovedeep S. Dhingra
- Rohan Khera
Discovery of potent small-molecule inhibitors of lipoprotein(a) formation
Biochemical screening and optimization identify small molecules that inhibit the formation of lipoprotein(a), and these inhibitors reduce the levels of Lp(a) in several animal models, suggesting that they could provide a therapeutic option in humans.
- Carlos Perez
- Laura F. Michael
Illusion of revascularization: does anyone achieve optimal revascularization during percutaneous coronary intervention?
In 1993, Lincoff and Topol claimed that the thrombolytic treatment of ST-segment elevation myocardial infarction was suboptimal in many patients and gave an ‘illusion of reperfusion’. In this Perspective article, the authors propose that a similar illusion of revascularization exists for contemporary percutaneous revascularization in patients with coronary artery disease and ischaemia, and identify how outcomes might be improved.
- Simone Fezzi
- Daixin Ding
- William Wijns
News and Comment

SASP in peripartum cardiomyopathy and preeclampsia
- Anna Kriebs
No benefit of apoA-I infusion after myocardial infarction
In the AEGIS-II trial, infusion of apolipoprotein A-I to increase cholesterol efflux capacity did not improve outcomes in patients with acute myocardial infarction.
- Gregory B. Lim
Screening for Helicobacter pylori infection in patients with cardiovascular and gastrointestinal disease
- Jonatan Wärme
- Martin O. Sundqvist
- Robin Hofmann
Reply to ‘Screening for Helicobacter pylori infection in patients with cardiovascular and gastrointestinal disease’
- Azita H. Talasaz
- Behnood Bikdeli
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Research article
- Open access
- Published: 20 June 2018
Cardiovascular disease (CVD) and associated risk factors among older adults in six low-and middle-income countries: results from SAGE Wave 1
- Ye Ruan 1 na1 ,
- Yanfei Guo 1 na1 ,
- Yang Zheng 1 ,
- Zhezhou Huang 1 ,
- Shuangyuan Sun 1 ,
- Paul Kowal 2 , 3 ,
- Yan Shi 1 &
BMC Public Health volume 18 , Article number: 778 ( 2018 ) Cite this article
25k Accesses
121 Citations
134 Altmetric
Metrics details
Cardiovascular disease (CVD) is one of the leading causes of death worldwide. Our study aimed to investigate the prevalence of two conditions, angina and stroke, and relevant risk factors among older adults in six low- and middle- income countries(LMICs).
The data was from World Health Organization (WHO) Study on global AGEing and adult Health (SAGE) Wave 1 in China, Ghana, India, Mexico, Russian Federation and South Africa. Presence of CVD was based on self-report of angina and stroke. Multivariate logistic regression was performed to examine the relationship between CVD and selected variables, including age, sex, urban/rural setting, household wealth, and risk factors such as smoking, alcohol drinking, fruit/vegetable intake, physical activity and BMI.
The age standardized prevalence of angina ranged from 9.5 % (South Africa) to 47.5 % (Russian Federation), and for stoke from 2.0% (India) to 6.1 % (Russia). Hypertension was associated with angina in China, India and Russian Federation after adjustment for age, sex, urban/rural setting, education and marital status (OR ranging from 1.3 [1.1-1.6] in India to 3.8 [2.9-5.0] in Russian Federation), furthermore it was a risk factor of stroke in five countries except Mexico. Low or moderate physical activity were also associated with angina in China, and were also strongly associated with stroke in all countries except Ghana and India. Obesity had a stronger association with angina in Russian Federation and China(ORs were 1.5[1.1-2.0] and 1.2 [1.0-1.5] respectively), and increased the risk of stroke in China. Smoking was associated with angina in India and South Africa(ORs were 1.6[1.0-2.4] and 2.1 [1.2-3.6] respectively ), and was also a risk factor of stroke in South Africa. We observed a stronger association between frequent heavy drinking and stroke in India. Household income was associated with reduced odds of angina in China, India and Russian Federation, however higher household income was a risk factor of angina in South Africa.
While the specific mix of risk factors contribute to disease prevalence in different ways in these six countries – they should all be targeted in multi-sectoral efforts to reduce the high burden of CVD in today’s society.
Peer Review reports
Cardiovascular diseases (CVDs) are by far the leading cause of death in the world. An estimated 17.9 million people died from CVDs in 2015. Ischemic heart disease (IHD) and stroke were the top two leading causes of CVD health lost in each world region [ 1 , 2 ]. By 2030 more than 22.2 million people will die annually from CVDs. Populations in low and middle income countries (LMICs) now contribute 75% of the CVD deaths, which leads to 7% reduction of gross domestic product(GDP) in these countries [ 3 ].
A larger proportion of the global burden of CVDs is now borne by LMICs than in high income countries, this is despite a comparatively lower burden from risk factors in low compared to high income countries [ 4 , 5 , 6 ]. Given the high prevalence of CVD among older adults in LMIC, the projected increases in this population will be a major challenge for the health care system. Twenty-three percent of the total global burden of disease(GBD) was attributed to disorders in people aged 60 years and older. The main contributors to disease burden were CVDs, accounting for 30.3% of the total burden in older people in 2010 [ 7 ]. Reliable and comparable analysis of risks to CVD is especially important for projecting future disease burden and for shaping disease prevention efforts.
A number of population-based studies from lower income countries have suggested that socio-demographic characteristics are associated with CVD, with increasing age, female sex and lower education consistently associated with higher prevalence of CVD. Some epidemiological evidence also suggests that CVD is associated with behavioral risk factors such as smoking, alcohol use, low physical activity levels, and insufficient vegetable and fruit intake, hypertension is also regarded as a very important risk factor for CVD. Independently or in combination, these risk factors present an opportunity for interventions to reduce future CVD burdens in ageing populations in LMIC.A number of large recent studies have compared CVD risks in higher and lower income countries, providing valuable and needed information about CVD and CVD risks [ 4 , 5 , 6 ]. However, the results of these studies may not be representative of the older adult population. For example, the Prospective Urban Rural Epidemiology (PURE) study sampling strategy and distributions provide less reliable estimates at older ages [ 8 ]. The World Health Organization Study on global AGEing and adult health (SAGE) is focused on older adults and use similar methodology across countries to improve comparability of important covariates and disease prevalence. Three of the countries overlap in PURE and SAGE (China, India and South Africa) where SAGE includes three additional middle income countries (Ghana, Mexico and the Russian Federation).
The aim of the present study was to investigate the prevalence of two main CVDs (angina, stroke) and behavioural risk factors and associated social-economic status (SES) factors among older adults using a unique data set with nationally representative samples in six low and middle income countries.
Sample and procedure
The data was from World Health Organization (WHO) Study on Global AGEing and adult health (SAGE) Wave 1, a longitudinal cohort study of ageing and older adults from 2007 to 2010 in six low- and middle-income countries (China, Ghana, India, Mexico, Russian Federation and South Africa) [ 9 ]. SAGE Wave 1 used face-to-face individual interviews to capture data. All six countries implemented multistage cluster sampling strategies which resulted in nationally representative cohorts of older adults ( http://www.who.int/healthinfo/sage/SAGEWorkingPaper5_Wave1Sampling.pdf?ua=1 ). Response rates for SAGE countries were Mexico 51%, India 68%, Ghana 80%, Russian Federation 83%, South Africa 77% and China 93%. Examination of non-respondent data suggested non-significant differences on some covariates (data not shown). Data were obtained following application for access through http://apps.who.int/healthinfo/systems/surveydata/index.php/catalog .
SAGE has been approved by the World Health Organization's Ethical Review Board. Additionally, each partner organization obtained ethical clearance through their respective review bodies. All study participants signed informed consent.
CVDs conditions
Two methods of assessing presence or absence of CVD were used. One was based on self-report of angina or stroke; and the second used an algorithm based on validated symptom-reporting methods to estimate and compare prevalence rates.
Sociodemographic variables
Socio-demographic variables contain age, sex, education, rural/urban residence, and income quintiles. Age was categorized into four groups: 50 to 59 years; 60 to 69 years; 70 to 79 years; and 80 years or older. Education level was classified into seven categories for analysis using an international classification scheme [ 10 ]. The income quintiles were generated using an asset-based approach- possession of assets and dwelling characteristics [ 11 ], with quintile 1(Q1) the quintile of the poorest households and quintile 5(Q5) the quintile of the richest.
Risk factors
Tobacco use.
Tobacco use was assessed by self-report and included different forms (manufactured or hand-rolled cigarettes, cigars, cheroots or whether tobacco is smoked, chewed, sucked or inhaled), and frequency of smoking, snuffing or chewing in each day over the week before interview[ 12 ], classified into four groups: never smoker, not current smokers, smokers(not daily) and current daily smokers.
Alcohol consumption
Alcohol consumption was categorized into four groups: life time abstainer, non-heavy drinkers, infrequent heavy drinkers and frequent heavy drinkers according to the consumption number of standard drinks of beer, wine and or spirit, fermented cider, and other alcoholic drinks during the week before interview.
Physical activity
Physical activity was measured by the Global Physical Activity Questionnaire (GPAQ) and assessed intensity, duration, and frequency of physical activity in three domains: occupational, transport-related, and discretionary or leisure time. Based on a standard classification scheme, three categories were generated: low, moderate and high levels [ 13 ].
Fruit and vegetable consumption
Fruit and vegetable consumption was assessed according to the number of daily servings eaten – with each serving approximating 80 grams. Five or more servings were defined as sufficient daily intake (at least 400 grams per day), fewer than five servings were categorized as insufficient [ 14 ].
Hypertension
The definition of hypertension used was systolic blood pressure ≥140mmHg and/or diastolic blood pressure ≥ 90mmHg and/or self-reported treatment with antihypertensive medication during the two weeks before interview. Blood pressure measurements were conducted three times on the right arm of the seated respondent with an automated recording device (OMRON R6 Wrist Blood Pressure Monitor, HEM-6000-E, Omron Healthcare Europe), and calculated as an average of the latter two measurements.
According to the classification criteria proposed by the WHO [ 15 ], body mass index (BMI) of <18.5 kg/m 2 , 25–29.9 kg/m 2 and ≥30 kg/m 2 are used to define underweight, overweight and obesity, respectively. Modified BMI cutoffs for China and India were used to perform an additional set of analyses that describes overweight (BMI 23.0-27.5) and obesity (BMI >27.5) in Asian populations [ 16 ].
Statistical methods
Statistic analyse were conducted using STATA SE version 11 (Stata Corp, College Station, TX). The prevalence of angina and stroke were calculated by using normalized weights in each country. Weights were based on selection probability, non-response, and post-stratification adjustments. To improve comparability across countries, the prevalence rates were age-standardized using the WHO World Standard Population Distribution based on world average population 2000-2025 [ 17 ]. Multivariate logistic regression was performed to examine the relationship between CVD and selected variables, including the socio-demographics such as age, sex, urban/rural setting, education, household wealth, and health risk factors such as smoking, alcohol drinking, fruit/vegetable intake, physical activity, hypertension and obesity. P < 0.05 from two-sided statistical tests was considered statistically significant.
A total of 34,114 individuals were included in the final analyses. Table 1 shows the sample distribution and demographic, socioeconomic and lifestyle characteristics by countries. The proportions of women are higher than men in four countries, except Ghana and India. The majority of older Indian lived in rural locations, while compared to urban areas in the other countries. The 50-59 age groups had the largest proportions in all countries, but the SAGE sample population distributions match those of the United Nations and US Census Bureau’s International Data Base estimates [ 18 ]. The percentage of respondents with no formal education were higher in Ghana (54.0%) and India (51.2%). In contrast, Russian Federation had the highest educational level with only 0.5% with no formal education and over 20% with a college degree or higher.
The rate of daily smoking ranged from 7.6% (Ghana) to 46.9% (India), frequent heavy drinker was the highest in China (6.4%) and lowest in Mexico (0.1%), and the highest rate of low physical activity was in South Africa (59.5%). Insufficient fruit and vegetable intake was more common in India, the Russian Federation and Mexico (90.6, 81.0 and 81.4%, respectively) compared with China, South Africa and Ghana (35.7, 68.5 and 68.9%, respectively).
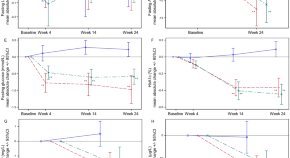
The age standardized prevalence of angina ranged from 9.5 % (South Africa) to 47.5 % (Russian Federation). It was higher in women than in men in all six countries. The rates were higher in rural than in urban locations other than in China. Angina rose with age in each country except Mexico, and a slight drop was seen in the highest age group in Ghana, India, Russian Federation and South Africa. The lowest prevalence of angina was found in individuals with the highest household income in China, Ghana, India and Russian Federation, respectively (see Table 2 ).
The prevalence of stroke was 6.1% in Russian Federation, which was higher than the other SAGE countries, while India had the lowest prevalence of 2.0%. In Russian Federation, the prevalence of stroke in men was almost twice that of women. Stroke was higher in urban than in rural locations in all six countries. Stroke prevalence tended to increase with age in all SAGE countries, but a slight drop in 80+ age group in Mexico and Russian Federation. In China, the wealthiest older adults had the lowest stroke prevalence (see Table 3 ).
Table 4 shows the Odds ratios for likelihood of angina by risk factors. Hypertension was associated with angina in China, India and Russian Federation after adjustment for age, sex, urban/rural setting and education (OR ranging from 1.32 [1.13-1.55] in India to 3.80 [2.91-4.96] in Russian Federation). Low and moderate physical activity was also associated with angina in China (ORs were 1.46 [1.22-1.76] and 1.66[1.39-1.99], respectively). Obesity had a stronger association with angina in Russian Federation and China (ORs were 1.48[1.08-2.02] and 1.24[1.01-1.53], respectively). Smoking was associated with angina in India and South Africa (ORs were 1.56[1.02-2.36] and 2.11 [1.23-3.61], respectively). Non-heavy drinking was a protective factor for angina in China (OR was 0.67[0.51-0.87]). The OR (1.56[1.19-2.05]) for insufficient fruits and vegetables intake was highest in Ghana. Household income was associated with reduced odds ratios of angina in China, India and Russian Federation, however higher household income was a risk factor of angina in South Africa (see Table 4 ).
In all six LIMCs except Mexico, hypertension was associated with stroke (OR ranging from 1.98[1.04-3.80] in Ghana to 3.16[1.72-5.83] in Russian Federation). Low, moderate physical activity were also strongly associated with stroke in four LMICs apart from Ghana and India. In China, Obesity increased the risk of stroke (OR was 1.66[1.20-2.28]). Smoking was also a risk factor of stroke in South Africa. We observed a stronger association between frequent heavy drinking and stroke in India (OR 6.64[1.39 – 31.82]). Insufficient fruit and vegetable intake and household income were not significantly associated with stroke in any of the countries (see Table 5 ).
This study reports the prevalence of two common cardiovascular diseases, angina and stroke, and the relevant risk factors among older adults in six LIMCs. Globally, the age-adjusted CVDs mortality continues to be unevenly distributed: where it has decreased in high income countries(HICs) by 43% in recent decades [ 19 ], while LIMCs are drowning in a rising tide of CVD. Although age-standardized rates of death attributable to CVD declined 13% in LMICs from 381 per 100000 in 1990 to 332 per 100000 in 2013, the number of deaths increased 66% from 7.21 million to 12 million in 2013 with ageing and population growth ascribed as the main drivers [ 19 ]. Ischaemic heart disease and cerebrovascular disease (stroke) combined accounted for more than 85.1% of all cardiovascular disease deaths in 2016[ 20 ]. Our study indicated that CVDs(angina, stroke) were prevalent and variable among older adults in six countries. Angina and stroke were both highest in Russian Federation(47.5%, 6.1% respectively). Women were more likely to have angina than men in all six countries. Stroke was more prevalent in urban than in rural. Angina and stroke both tended to increase with age in China.
Prevalence of CVDs generally appeared to be most closely linked to a country’s stage of epidemiological transition [ 21 ], especially when high disease rates in middle age carry through into older ages. Underlying social, environmental, and economic shifts in many countries have led to increasing levels of predominant causes such as tobacco and alcohol use, sedentary lifestyle, unhealthy diets, and suboptimum levels of weight, blood pressure, cholesterol, and plasma glucose. The high and growing prevalence of CVD in LIMCs largely reflects the burden of these key risk factors. Our study revealed that hypertension, high BMI, decreased physical activity, frequent heavy drinking and lower household health were key risk factors of angina and stroke. However, the distribution of risk factors in six counties was unequal, for example, the factor with highest OR of angina in China and Russian Federation was hypertension, whereas it was smoking in India and South Africa.
Hypertension has been shown to be an independent risk factor for acute myocardial infarction and stroke in older people [ 22 , 23 ]. We found that hypertension was associated with angina in China, India and Russian Federation, in addition it was a risk factor of stroke in five of the six countries in this study (not Mexico). Between 1980 and 2008, blood pressure decreased by 2.0mmHg or more (for men) and 3.5 mmHg or more (for women) per decade in western Europe and Australia but increased by up to 2.7 mmHg over this same period in Oceania, East and West Africa and South and Southeast Asia [ 24 ]. Systematic review revealed that blood pressure lowering greatly reduced the major cardiovascular disease events and all-cause mortality, irrespective of starting blood pressure [ 25 ].However among these six LIMCs 66% hypertensives were undiagnosed before the survey, 73% untreated and 90% uncontrolled. Although the proportions of undiagnosed and untreated were lowest in Russia (30% and 35%), the uncontrolled rate was higher (83%) [ 26 ], low level of health care (primary and secondary prevention) and irregular treatment continued to be a major problem [ 27 ]. Hence, further research on early screening strategies, available health care and effective treatment of hypertension may be critical for improving outcomes.
Our study also showed that low physical activity and obesity besides hypertension were both associated with angina and stroke in China, and insufficient fruit and vegetable intake was risk factor of angina in Ghana. Compared with data from 1997, total physical activity in 2009 has decreased by 29% in males and by 38% in females in China [ 28 ], and physical inactivity was estimated the third leading risk factor for coronary heart disease [ 29 ]. As the relation between physical and obesity well recognized, obesity was an important risk factor of CVD. People are becoming more and more obese. Global age-standardised mean BMI increased from 21.7 kg/m 2 in 1975 to 24.2 kg/m 2 in 2014 in men, and from 22.1 kg/m 2 in 1975 to 24.4 kg/m 2 in 2014 in women. Over this period, age-standardised prevalence of obesity increased from 3.2% in 1975 to 10.8% in 2014 in men, and from 6·4 to 14.9% in women [ 30 ]. More than 50% of the obese individuals in the world lived in just 10 countries (listed in order of number of obese individuals): USA, China, India, Russia, Brazil, Mexico, Egypt, Pakistan, Indonesia, and Germany, and China and India jointly accounting for 15% in 2013[ 31 ]. China has moved from 60th place for men and 41st place for women in 1975 to second for both men and women in 2014 in the worldwide ranking of the number of severely obese individuals [ 30 ]. Unfortunately, the prevalence of obesity among children and adolescents are both on the rise. In comparison with obesity rate in 1985, it increased by 8.7 times for children and 38.1 times for adolescents [ 32 ]. In the World Health Survey 2002-2003, prevalence of low fruits and vegetable consumption among individuals aged 18-99 years in Ghana was the lowest among 52 countries [ 33 ]. However, the prevalence was higher (68.9%) among persons aged 50 years and older [ 34 ]. We also found that insufficient fruits and vegetables intake was associated with angina in Ghana. All of these contribute to the increasing burden of CVD.
We observed a relationship between smoking and angina, frequent heavy drinking and stroke in India. The prevalence of angina was 19.6% (95%CI:16.5-23.0) in India, the second highest for these six countries. CVD-related conditions contributed nearly two-thirds of the burden of NCD mortality in India [ 35 ], with ischemic heart disease(IHD) and stroke contributing substantially to CVD mortality in India (83%) [ 36 ].Up to 35% of adults in India consume tobacco [ 37 ], with the rate of daily tobacco use was highest(46.9%) among the six LIMCs in this study, highest in younger individuals (20–35 years) [ 38 ].The relation between alcohol consumption and CVD has been widely studied. Several analyses showed that low-moderate levels of alcohol consumption had cardio protective effects, while heavy drinking is harmful, usually described as “U-shaped” or “J-shaped” relationship [ 39 , 40 ]. Aside from alcohol consumption, drinking pattern (binge-pattern drinking) played an important role in elevating the risk of CVD [ 41 , 42 ]. Another cohort study showed that heterogeneous associations exist between level of alcohol consumption and CVD: compared with moderate drinking, heavy drinking raised risk of coronary death, heart failure, cardiac arrest, ischaemic stroke but a lower risk of myocardial infarction or stable angina [ 43 ]. We found that in China non-heavy drinking was a protective factor for angina and stroke, and frequent heavy drinking showed a dangerous effect for stroke in India.
There were a few limitations in our study. Firstly, although SAGE assembled nationally representative cohorts from six countries, the response rates were different across the countries, ranging from 51% in Mexico to 93% in China. The low response rate in Mexico was for specific reasons related to timing of the survey and inability to engage in repeat visits to households to maintain the sample and we note this introduces the potential for selection bias into the results for Mexico. Secondly, the data for stroke and some risk factors were based on self-reports, which may lead to recall bias. However, validated symptom-reporting methods were also used in these analyses to estimate and compare prevalence rates for angina to improve prevalence estimates. Thirdly, the question on stroke in SAGE did not distinguish between ischemic stroke and hemorrhagic stroke. Last, these results are based on cross-sectional data and as such, cannot be sure of the direction of the associations we identified.
Conclusions
In conclusion, our study provided representative prevalence of angina and stroke and relevant risk factors in elders in six LIMCs. Due to the variation pattern of prevalence and risk factors distribution, policies and health interventions will need to be targeted and tailored for a broad range of local conditions to achieve the health goals set by the United Nations for 2025.
Abbreviations
Body mass index
Cardiovascular disease
Global burden of disease
Gross national income
Global Physical Activity Questionnaire
High income countries
Ischemic heart disease
Low- and middle- income countries
Study on global AGEing and adult Health
Social-economic status
World Health Organization
Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am College Cardiol. 2017;70(1):1–25.
Article Google Scholar
Cardiovascular diseases (CVDs) Fact sheet. Available at: http://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) . Accessed 15 Mar 2017.
World Health Organization. Burden: mortality, morbidity and risk factors. In: Alwan A, editor. Global Status Report on Noncommunicable Diseases 2010. Geneva: World Health Organization; 2011.
Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing Epidemic of Coronary Heart Disease in Low- and Middle-Income Countries. Curr Probl Cardiol. 2010;35:72–115.
Article PubMed PubMed Central Google Scholar
Yusuf S, Rangarajan S, Teo K, Islam S, Li W, Liu L, et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med. 2014;371:818–27.
Article PubMed CAS Google Scholar
Teo KK, Dokainish H. The Emerging Epidemic of Cardiovascular Risk Factors and Atherosclerotic Disease in Developing Countries. Can J Cardiol. 2017;33:358–65.
Article PubMed Google Scholar
Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O'Donnell M, Sullivan R, et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385:549–62.
Corsi DJ, Subramanian SV, Chow CK, et al. Prospective Urban Rural Epidemiology (PURE) study: Baseline characteristics of the household sample and comparative analyses with national data in 17 countries. American Heart Journal. 2013;166:636–46.
Kowal P, Chatterji S, Naidoo N, Biritwum R, Fan W, Lopez Ridaura R, et al. Data resource profile: the World Health Organization Study on global AGEing and adult health (SAGE). Int J Epidemiol. 2012;41:1639–49.
United Nations Educational Scientific and Cultural Organization. International Standard Classification of Education (ISCED). 1997. ISBN 92-9189-035-9. Available at: http://uis.unesco.org/sites/default/files/documents/international-standard-classification-of-education-1997-en_0.pdf .
Ferguson BD, Tandon A, Gakidou E, Murray CJL. Estimating permanent income using asset and indicator variables. In: Evans DE, Murray CJL, editors. Health systems performance assessment debates, methods and empiricism. Geneva: World Health Organization; 2003. p. 747–60.
Google Scholar
World Health Organization. Guidelines for controlling and monitoring the tobacco epidemic. Geneva: World Health Organization; 1998. p. 76–101.
World Health Organization. Global Physical Activity Questionnaire (GPAQ) Analysis Guide. Available at: http://www.who.int/chp/steps/resources/GPAQ_Analysis_Guide.pdf?ua=1,2017-08-08 . Accessed 15 Mar 2017.
World Health Organization. Diet, nutrition and the prevention of chronic diseases. Report of a joint WHO/FAO expert consultation. In: WHO Technical Report Series No 916. Geneva: World Health Organization; 2003.
World Health Organization. Obesity: preventing and managing the global epidemic. In: WHO Technical Report Series 894. Geneva: World Health Organization; 2000.
Consultation WE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63.
Ahmad OB, Boschi Pinto C, D. Lopez A, Jl Murray C, Lozano R, Inoue M. Age Standardization of Rates: A New WHO Standard, vol. No. 31; 2001.
He W, Muenchrath MN, Kowal P, U.S. Census Bureau. Shades of Gray: A Cross-Country Study of Health and Well-Being of the Older Populations in SAGE Countries, 2007–2010. Washington, DC: U.S. Government Printing Office; 2012.
Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. 2015;132:1667–78.
GBD 2016 Causes of Death Collaborators. Global, Regional, and National Age-Sex Specific Mortality for 264 Causes of Death, 1980–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–210.
Omran AR. The epidemiologic transition: a theory of the epidemiology of population change. Milbank Mem Fund Q. 1971;49:509–38.
Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52.
O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, Rangarajan S, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study.): a case-control study. Lancet. 2010;376:112–23.
Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, et al. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5.4 million participants. Lancet. 2011;377:568–77.
Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–67.
Lloyd-Sherlock P, Beard J, Minicuci N, Ebrahim S, Chatterji S. Hypertension among older adults in low- and middle-income countries: prevalence, awareness and control. Int J Epidemiol. 2014;43:116–28.
Roberts B, Stickley A, Balabanova D, Haerpfer C, McKee M. The persistence of irregular treatment of hypertension in the former Soviet Union. J Epidemiol Community Health. 2012;66:1079–82.
Su C, Huang H, Wang HJ, Wang ZH, Zhang JG, Du WW, et al. Study on status and trend of physical activity among Chinese adults aged 18-49 years old in 9 provinces from 1997 to 2009. Chin J Health Educ. 2013;186:5926–32.
Li Y, Wang DD, Ley SH, Howard AG, He Y, Lu Y, et al. Potential impact of time trend of life-style factors on cardiovascular disease burden in China. J Am Coll Cardiol. 2016;68:818–33.
NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377–96.
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–81.
Ma J, Cai XH, Wang HJ, Dong B, Song Y, Hu PJ, et al. The trend analysis of overweight and obesity in Chinese students during 1985–2010. Chin J Prev Med. 2012;46:776–80.
Hall JN, Moore S, Harper SB, Lynch JW. Global variability in fruit and vegetable consumption. Am J Prev Med. 2009;36:402–9.
Wu F, Guo Y, Chatterji S, Zheng Y, Naidoo N, Jiang Y, et al. Common risk factors for chronic non-communicable diseases among older adults in China, Ghana, Mexico, India, Russia and South Africa: the study on global AGEing and adult health (SAGE) wave 1. BMC Public Health. 2015; https://doi.org/10.1186/s12889-015-1407-0 .
Patel V, Chatterji S, Chisholm D, Ebrahim S, Gopalakrishna G, Mathers C, et al. Chronic diseases and injuries in India. Lancet. 2011;377:413–28.
Institute of Health Metrics and Evaluation. GBD Compare 2010. Available at: http://vizhub.healthdata.org/gbd-compare/ . Accessed 30 Apr 2017.
Giovino GA, Mirza SA, Samet JM, Gupta PC, Jarvis MJ, Bhala N, et al. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet. 2012;380:668–79.
Bhan N, Srivastava S, Agrawal S, Subramanyam M, Millett C, Selvaraj S, et al. Are socioeconomic disparities in tobacco consumption increasing in India? A repeated cross-sectional multilevel analysis. BMJ Open. 2012. https://doi.org/10.1136/bmjopen-2012-001348 .
Fernández-Solà J. Cardiovascular risks and benefits of moderate and heavy alcohol consumption. Nat Rev Cardiol. 2015;12:576–87.
Roerecke M, Rehm J. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 2014;12:182.
Britton A, McKee M. The relation between alcohol and cardiovascular disease in Eastern Europe: explaining the paradox. J Epidemiol Community Health. 2000;54:328–32.
Article PubMed PubMed Central CAS Google Scholar
Leong DP, Smyth A, Teo KK, McKee M, Rangarajan S, Pais P, et al. Patterns of alcohol consumption and myocardial infarction risk: observations from 52 countries in the INTERHEART case control study. Circulation. 2014;130:390–8.
Bell S, Daskalopoulou M, Rapsomaniki E, George J, Britton A, Bobak M, et al. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: population based cohort study using linked health records. BMJ. 2017. https://doi.org/10.1136/bmj.j909 .
Download references
Acknowledgements
The authors would like to thank the respondents and interviewers from all six SAGE countries for their contributions and hard work.
This work was supported by WHO, the US National Institutes on Aging through Interagency Agreements [OGHA 04034785; YA1323-08-CN-0020; Y1-AG-1005-01] and through a research grant (R01-AG034479), and Three-year Action Plan on Public Health, Phase IV, Shanghai, China[15GWZK0801;GWIV-22].
Availability of data and materials
The datasets supporting the conclusions of this article are available upon request in the website of WHO ( http://apps.who.int/healthinfo/systems/surveydata/index.php/catalog/sage ).
Author information
Ye Ruan and Yanfei Guo contributed equally to this work.
Authors and Affiliations
Shanghai Municipal Center for Disease Control and Prevention (Shanghai CDC), Shanghai, China
Ye Ruan, Yanfei Guo, Yang Zheng, Zhezhou Huang, Shuangyuan Sun, Yan Shi & Fan Wu
World Health Organization, Geneva, Switzerland
Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand
You can also search for this author in PubMed Google Scholar
Contributions
FW, PK, YFG and YZ designed, implemented the conduct of this study. YR and YFG conceived of the analysis, and drafted the manuscript. YR, YFG, YS, ZZH, YZ and SYS contributed to the statistical analyses. ZZH and SYS contributed to the editing of initial draft. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Yan Shi or Fan Wu .
Ethics declarations
Ethics approval and consent to participate.
WHO’s Ethical Review Committee approved SAGE (RPC146), and each country obtained local ethical approval to conduct the study. Written informed consent was obtained from each respondent.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Ruan, Y., Guo, Y., Zheng, Y. et al. Cardiovascular disease (CVD) and associated risk factors among older adults in six low-and middle-income countries: results from SAGE Wave 1. BMC Public Health 18 , 778 (2018). https://doi.org/10.1186/s12889-018-5653-9
Download citation
Received : 04 December 2017
Accepted : 01 June 2018
Published : 20 June 2018
DOI : https://doi.org/10.1186/s12889-018-5653-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cardiovascular diseases
- Risk Factors
- Low and middle income countries
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 08 May 2024
The cGAS-STING pathway in cardiovascular diseases: from basic research to clinical perspectives
- Cheng An 1 na1 ,
- Zhen Li 2 na1 ,
- Yao Chen 1 na1 ,
- Shaojun Huang 1 ,
- Fan Yang 3 ,
- Ying Hu 4 ,
- Chengxin Zhang 1 &
- Shenglin Ge ORCID: orcid.org/0000-0002-0691-5936 1
Cell & Bioscience volume 14 , Article number: 58 ( 2024 ) Cite this article
133 Accesses
Metrics details
The cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) synthase-stimulator of interferon genes (cGAS-STING) signaling pathway, an important component of the innate immune system, is involved in the development of several diseases. Ectopic DNA-induced inflammatory responses are involved in several pathological processes. Repeated damage to tissues and metabolic organelles releases a large number of damage-associated molecular patterns (mitochondrial DNA, nuclear DNA, and exogenous DNA). The DNA fragments released into the cytoplasm are sensed by the sensor cGAS to initiate immune responses through the bridging protein STING. Many recent studies have revealed a regulatory role of the cGAS-STING signaling pathway in cardiovascular diseases (CVDs) such as myocardial infarction, heart failure, atherosclerosis, and aortic dissection/aneurysm. Furthermore, increasing evidence suggests that inhibiting the cGAS-STING signaling pathway can significantly inhibit myocardial hypertrophy and inflammatory cell infiltration. Therefore, this review is intended to identify risk factors for activating the cGAS-STING pathway to reduce risks and to simultaneously further elucidate the biological function of this pathway in the cardiovascular field, as well as its potential as a therapeutic target.
Introduction
Cardiovascular diseases (CVDs) are the leading cause of death worldwide and the number one cause of death in the United States [ 1 ]. The 2020 American Heart Association statistics show that approximately 19.05 million people died from CVDs worldwide, which was an increase of 18.7% from 2010 [ 2 ]. Previous surveys have shown that with the progress of industrialization and dietary changes, 37.4% of men and 35.9% of women over the age of 20 years in the United States have some form of CVDs, and men account for 50.6% of deaths from CVDs [ 1 ]. Innate and adaptive immunity play an important roles in CVDs, leading to inflammatory infiltration, abnormalities including apoptotic cells and autoantigen enhancement, and ultimately organ and functional damage [ 3 , 4 ]. Notably, exogenous, host-damaged, and ectopic DNA enhances autoimmunity, leading to an enhanced inflammatory response and further exacerbation of cardiovascular injury [ 5 , 6 ]. Therefore, finding a DNA sensor that recognizes DNA to intervene and simultaneously prevent risk factors for DNA damage are necessary.
Cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS), a cytoplasmic DNA sensor, is activated upon detection and binding of double-stranded DNA (dsDNA), thus catalyzing the synthesis of 2′3′-cGAMP. As a secondary messenger, 2′3’-cGAMP activates the stimulator of interferon genes (STING) and the transcription factor type-I interferon regulatory factor 3 (IRF3), which induces strong innate immunity [ 7 ]. cGAS is involved in important biological processes such as macular degeneration, cellular senescence, and myocardial infarction (MI), heart failure(HF), and cardiac hypertrophy [ 8 , 9 , 10 ]. The regulatory role of this signaling pathway in CVDs has attracted widespread attention. For example, Yan et al. [ 10 ] found that diabetic cardiomyopathy triggers cellular pyroptosis and activates the cGAS-STING signaling pathway, which promotes the production of type I interferon (IFN-I) and nucleotide-binding oligomerization domain-like receptor pyrin domain containing 3 (NLRP3) inflammasome. However, inhibition of this signaling pathway prevents the secretion of IFN-I and other proinflammatory cytokines [ 11 ]. In addition, studies have confirmed the success of the small-molecule inhibitor evolocumab, a proprotein convertase subtilisin/kexin type 9 (PSCK9) inhibitor, in treating hyperlipidemia in patients with CVD in phase IV trials (Clinical Trials.gov Identifier: NCT02867813 and NCT03080935), suggesting that targeted inhibition of an intracellular signaling pathway can treat CVDs [ 12 ]. Therefore, targeted small-molecule inhibitors designed against cGAS-STING signaling pathway may be a new strategy for the treatment of CVDs.
In this review, we describe the mechanism of the cGAS-STING signaling pathway in detail and highlight its role in different CVDs. Furthermore, the risk factors for the activation of cGAS-STING and the most clinically valuable specific small-molecule inhibitors developed in the cardiovascular field are reviewed.
Overview of the cGAS-STING pathway
First reported in 2013, cGAS, also known as MB21D1, is predominantly distributed in the cytoplasm, with studies showing it in the cell membrane and nucleus [ 13 ]. Structural studies have shown that cGAS cannot function alone in the free state and must be combined with dsDNA [ 14 ]. DNA, the most fundamental carrier of life, is restricted to the nucleus and some organelles (e.g., mitochondria). Ectopic DNA is rapidly recognized and subsequently degraded by scavenger cells and extracellular or intracellular ribonucleases. However, tissue damage leads to persistent accumulation of dsDNA as well as exogenous dsDNA invasion, e.g., from pathogens such as viruses, bacteria, transcellular vesicles, or ruptured dying cells, which can be internalized into the cytosol to activate cGAS in various ways [ 15 , 16 , 17 ]. Further research has revealed that not all free dsDNA binds to cGAS; only dsDNA longer than 20 bp can combine with cGAS, and dsDNA > 40 bp can form more stable complexes to activate the biological activity of the cGAS protein [ 18 , 19 ]. dsDNA binds mainly to the surface of the A site of cGAS, whereas the B site binds to another dsDNA as a complementary site. This unique structure facilitates the formation of a 2:2 cGAS:dsDNA bidirectional complex, leading to a conformational change in cGAS and rearrangement of the enzymatic catalytic pocket [ 20 ]. Activated cGAS synthesizes the endogenous second messenger cyclic dinucleotide (CDN) cGAMP by catalyzing the synthesis of the substrates guanosine triphosphate (GTP) and adenosine triphosphate (ATP), which then activate STING signaling [ 21 ]. Interestingly, STING, whose common names include TMEM173, MITA, and ERIS, is an endoplasmic reticulum (ER) membrane protein that was discovered in 2008 [ 22 , 23 ]. In its resting state, STING neither directly performs its biological function and nor directly recognizes DNA; instead, STING binds to cGAMP in mammalian cells to activate its biological effects [ 14 ]. Upon binding to cGAMP, STING undergoes conformational changes to form a stable tetramer that translocates from the ER to the Golgi apparatus via the ER-Golgi intermediate compartment (ERGIC), recruits TANK-binding kinase 1 (TBK1), and promotes the phosphorylation of TBK1 [ 24 ]. Phosphorylated TBK1 is essential for IRF3 and I-kappa B kinase complex (IKK) activity. On the one hand, phosphorylated TBK1 promotes IRF3 phosphorylation, leading to its dimerization and translocation into the nucleus; induces IFN-I expression; and promotes gene expression of inflammatory mediators and chemokines [ 25 ]. On the other hand, the IKK-promoted activation of nuclear factor-kappa B (NF-κB), the p50/p65 complex, which acts as a transcription factor translocated into the nucleus, also promotes the expression of inflammatory mediator genes and binds to the IFN-I promoter to aid in its expression [ 22 , 23 ]. STING binds to cGAMP and translocates from the ER to the Golgi compartment, further activating downstream signaling and regulating multiple biological functions (Fig. 1 ).
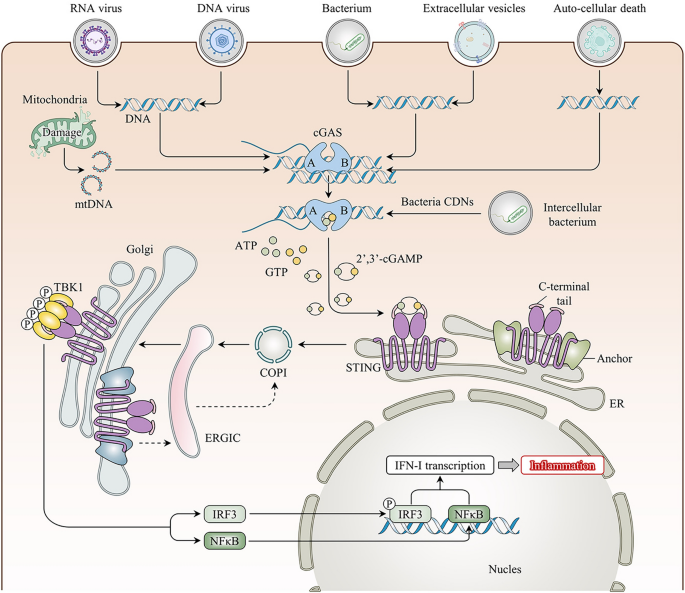
Overview of the cGAS-STING pathway. Exogenous (viral, bacterial) or endogenous (carried by extracellular vesicles, released by auto-cell death, intracellular mitochondrial damage) DNA binds to free cGAS in the cytoplasm and activates its biological dynamic, catalyzing the synthesis of 2′3′-cGAMP from ATP and GTP. Then, cGAMP binds to stimulator of interferon gene (STING) on the endoplasmic reticulum (ER) membrane resulting in a conformational change to activate its function. Activated STING transports to the Golgi apparatus through the coatomer protein complex I (COP I) and the ER–Golgi intermediate compartment (ERGIC), promoting the phosphorylation of TBK1 to form a stable tetramer. Phosphorylation of TBK1 exerts its effects by prompting IRF3 and NF-κB to migrate to the nucleus to trigger type I interferon transcription and activate inflammatory pathways
Increasing evidence suggests that the activation of the cGAS-STING signaling pathway is involved in a variety of cellular processes, such as pyroptosis, apoptosis, autophagy, senescence, and IFN-I inflammatory signaling. Furthermore, some research teams have found that activating the cGAS-STING pathway leads to increased IFN-I expression, with a strong, positive association [ 26 ]. In contrast, STING knockdown reduces the IFN-I inflammatory signaling pathway activity and inhibits cardiomyocyte apoptosis and inflammatory infiltration in myocardial toxicology experiments [ 27 ]. These results suggest that the cGAS-STING signaling pathway plays a key role in the induction of inflammation. Recently, the cGAS-STING signaling pathway was found to be activated in various CVDs to participate in the regulation of immune-inflammatory responses [ 28 ], suggesting that it may be a therapeutic target for the treatment of CVDs.

Overview of CVDs
CVDs such as atherosclerosis (AS) affect tens of thousands of people, are progressively occurring at a younger age, and are triggered by a combination of internal and external influences such as genetic and environmental factors [ 1 , 29 , 30 ]. Several studies have shown that CVDs are associated with age and sex [ 31 , 32 ]. Specifically, age has been shown to be an independent risk factor for CVDs [ 33 ]. Interestingly, men are more susceptible to CVDs, with women developing the disease an average of 10–15 years later [ 34 ]. The most common CVDs encountered in clinical practice include atherosclerotic heart disease, congenital heart disease, aortic coarctation/aneurysm, and valvular diseases. Aortic coarctation/aneurysm is the deadliest surgical CVD, with mortality increasing 1–2% per hour after onset and 50% of patients dying outside the hospital [ 35 ]. This suggests an urgent need to elucidate the intrinsic mechanisms of CVDs to better prevent and intervene in their development. Substantial evidence confirms that CVD development is related to both innate and adaptive immunity, in which dysregulation of immunity is a major trigger in pathogenesis and for the induction of an acute inflammatory response to tissue injury and infection [ 36 ]. Unfortunately, host damage leading to ectopic DNA and invasion by exogenous DNA can cause dysregulation of immune homeostasis, triggering an inflammatory response that can lead to CVDs, making detecting and promptly eliminating the effects of DNA particularly important [ 37 ]. Three-prime repair exonuclease 1 (TREX1) is a DNA enzyme that degrades single- and double-stranded DNA. Importantly, TREX1 can degrade DNA in cytoplasmic lysates, thereby preventing accumulation in the cytosol, activating the IFN-I inflammatory response pathway, and protecting the host from inappropriate damage [ 38 ]. Morita et al. [ 39 ] demonstrated that TREX1 deletion (TREX −/− ) leads to increased mortality in mice through the IFN-1 pathway, and mice are more likely to develop inflammatory myocarditis, leading to progressive cardiomyopathy and circulatory failure. Similarly, the inability to clear DNA from apoptotic cells in mice lacking macrophage lysosomal DNAase activates an IFN-I-driven inflammatory response [ 40 ]. Fortunately, the discovery of a new DNA receptor signal, cGAS-STING, can reverse this phenomenon and potently modulate immune inflammation. Gray et al. [ 41 ] found that TREX −/− mice lacking cGAS exhibit complete protection from lethality, have significantly reduced IFN-I-mediated tissue inflammation, and fail to produce autoantibodies. Importantly, in cGAS-deficient (cGAS −/− ) mice, disrupting IFN-I-dependent signaling results in reduced cardiac expression of inflammatory cytokines and chemokines, decreased cardiac inflammatory cell infiltration, attenuated ventricular dilatation, and improved cardiac function [ 8 ]. Therefore, cGAS-STING acts as a DNA receptor signaling pathway to provide novel support for host defense against inflammatory responses resulting from abnormalities in immune homeostasis, thereby providing new targets for treating CVDs.
Functional role of the cGAS-STING pathway in CVDs
Recent studies have shown that the cGAS-STING pathway activated is closely associated with CVDs, such as AS [ 42 ], aortic aneurysms and dissections (AAD) [ 43 ], and heart failure (HF) [ 44 ] (Table 1 ). In contrast, this signaling pathway downregulates IFN-I-mediated inflammatory factor release when inhibited, suggesting that it plays an instrumental role in CVD development. Here, we summarize the regulatory role of the cGAS-STING signaling pathway in various CVDs.
Inflammatory infiltration, lipid deposition, and foam cell formation in atherosclerosis (AS) predominantly affect the medium- and large-sized arteries, causing luminal narrowing and tissue ischemia and resulting in severe clinical symptoms and complications with a poor prognosis. AS is currently the main killer and the incidence is increasing worldwide. Experimental data suggest that IFN-I is involved in the entire process of atherogenesis, affecting macrophages to enhance phagocytosis and the formation of foam cells and extracellular traps; in addition, IFN-1 can change the phenotype of dendritic, T cells, and B cells, causing immune responses that accelerate further AS deterioration [ 45 ]. Recent studies have shown that the cGAS-STING signaling pathway involves immunoinflammatory recognition signals that senses ectopic DNA to activate IFN-I, which provides a new vision for understanding the pathogenesis and continued progression of AS [ 20 , 46 ]. Wan et al. [ 42 ] found that cGAS-STING regulates phenotypic switching, proliferation, and apoptosis in vascular smooth muscle (VSMC), which can be directly activated by intracellular Ca 2+ (i.e., an inflammatory activator) concentrations to promote AS plaque rupture by activating STING expression. Similarly, Bi et al. [ 47 ] found that cGAS or STING deficiency blocks plaque vulnerability by inhibiting the IFN-I pathway-induced premature senescence and phenotypic transition of VSMC, attenuating VSMC fibrous cap loss and thinning. The rupture of AS plaques seriously jeopardizes health and causes irreversible organic damage. Therefore, inhibiting the cGAS-STING signaling pathway may be a potential target for the treatment of AS.
AAD are aggressive and one of the most difficult surgically treated conditions for cardiac and macrovascular surgeons. In the United States, a study of residents of Olmsted County, Minnesota found that the incidence of aortic lesions was approximately 7.7/1,000,000 [ 48 ]. In addition, the incidence of AAD is twice as high in men as in women and increases with age [ 49 ]. Furthermore, the disease onset is accompanied by a variety of clinical manifestations such as coma, crushing pain in the anterior thoracic region, elevated blood pressure, pericardial tamponade, and renal failure, resulting in poor prognosis. The mechanism of AAD development is predominantly progressive smooth muscle cell (SMC) loss and extracellular matrix (ECM) degradation and depletion, leading to aortic aneurysm, dissection, and rupture; however, the underlying mechanisms are still being explored [ 50 ]. Recent studies have revealed the role of the cGAS-STING signaling pathway in the pathogenesis of AAD [ 43 , 51 ]. Specifically, Luo et al. [ 43 ] showed that increased cytoplasmic DNA release from SMCs and macrophages in AAD patient tissues activates STING. DNA from damaged SMCs in sporadic AAD induced by a high-fat diet and angiotensin (Ang) in mice is phagocytosed by macrophages, activating STING and its downstream IRF3, which directly induces matrix metalloproteinase-9 expression and ECM degeneration, leading to aortic thickening, dissection, and rupture. Compared with wild-type mice, this phenomenon is inhibited in STING-deficient mice (STING gt/gt ). In addition, reactive oxygen species (ROS)-mediated DNA damage results in the progressive release of DNA fragments, which subsequently stimulate the cGAS-TING-TBK1-IRF3 signaling pathway and promote aortic SMC death [ 46 ]. In a single-cell study, STING-IRF3 signaling induced chromatin remodeling that drove SMC from a contractile to an inflammatory phenotype; however, in STING −/− mice, the aortic stress-induced shift of SMC to an inflammatory phenotype was blocked, and the SMC population was preserved [ 51 ]. Therefore, the cGAS-STING signaling pathway is a new target class for future AAD therapies.
HF is characterized by systolic or diastolic dysfunction of the heart, insufficient peripheral ejection, and inability to supply enough oxygen to meet the metabolic requirements of the entire organism, resulting in clinical symptoms [ 52 ]. The main clinical manifestations are edema of the lower extremities, dyspnea, pink foamy expectorations, pulmonary edema, pleural effusion, and progressive exertional failure that interferes with daily life [ 53 ]. HF has become an epidemic in the modern world, affecting approximately 1–2% of adults [ 54 ]. In Europe and North America, the lifetime risk of developing HF is approximately one in five for people over 40 years of age [ 55 ]. A growing body of research supports the idea that myocardial hypertrophy and the activation of cardiac inflammation, in which cGAS-STNG plays an important role, are key triggers of HF in humans and animals [ 56 , 57 ]. In a study by Hu et al.[ 9 ], pathological myocardial remodeling and ventricular dysfunction occurred in transverse aortic constriction (TAC) treatment-induced HF, where cGAS-STING signaling was activated. Interestingly, when the upstream expression of cGAS was decreased, the survival of mice after TAC improved, with preserved myocardial contractility and attenuated myocardial hypertrophy, fibrosis, and pyroptosis. Similarly, Zhang et al. [ 44 ] found that STING-deficient mice undergoing aortic banding (AB) surgery have attenuated AB-induced cardiac hypertrophy and an inhibited macrophage infiltration and IFN-I-mediated inflammatory response. Interestingly, in another study of AB surgery, STING overexpression was shown to improve cardiac function and significantly attenuate cardiac hypertrophy, fibrosis, and inflammation; the in vitro STING overexpression in Ang II-induced cardiomyocytes significantly suppressed the cardiomyocyte cross-sectional area and atrial natriuretic peptide (ANP) mRNA levels [ 57 ]. Thus, the cGAS-STING signaling pathway clearly plays an important role in HF. Although the exact mechanism has remained controversial, the pathway may still be targeted for the treatment of other CVDs; further clarification may be needed to develop small-molecule inhibitors for applications in HF.
MI, a disease that develops in the elderly, has a sudden onset, high lethality, and high disability [ 58 ]. Once the attack intensifies to persistent, severe retrosternal pain, patients experience fear, anxiety, and other disturbing emotions, especially a near-death feeling, resulting in lasting psychological trauma [ 59 ]. The interruption of blood flow in the area of MI can simultaneously lead to massive cardiac cell death, releasing a large number of molecules associated with the pattern of injury and causing an inflammatory response and infiltration of inflammatory cells [ 60 ]. Phagocytosis of injured cardiac cells by inflammatory cells and some matrix debris activates repair pathways to form a fibrotic scar and maintain cardiac integrity but sclerotizes the ventricular wall to further compromise cardiac function [ 61 ]. Complex mechanisms exist during this period, and prolonged activation of inflammatory signaling and infiltration of inflammatory cells exacerbate adverse remodeling and concomitant injuries. Recent studies have shown that cGAS-STING is involved in inflammatory activation pathways after MI. King et al. [ 8 ] found that the phagocytosis of necrotic cells and cellular debris by macrophages after MI promotes the lethal process of MI by activating the STING-IRF3 mutation. I-IFN induction is inhibited in cGAS −/− , STING gt/gt , and IRF3 −/− mice, with STING gt/gt and IRF3 −/− mice showing the same expression patterns and survival rates of 82% (39/47), 77% (24/31), and 98% (44/45), respectively. Cao et al. [ 62 ] found that the loss of cGAS function eliminates the induction of key inflammatory programs, such as inducible nitric oxide synthase (iNOS), and promotes macrophage conversion to a reparative phenotype, thereby enhancing repair and improving hemodynamic performance. Thus, inhibiting the cGAS-STING pathway could be a new pharmacological target for the treatment of MI.
Cardiomyopathy
Cardiomyopathy, a relatively rare cardiac disease with multiple causes, can be divided into primary cardiomyopathies, which include hereditary, mixed, and acquired types, and secondary cardiomyopathies, which include dilated, hypertrophic, and restrictive types [ 63 ]. However, with a poor prognosis and limited therapeutic options, cardiomyopathy has been reported in nearly 50% of children and adolescents who die suddenly or undergo heart transplantation [ 64 ]. The pathogenesis of cardiomyopathies remains unclear. The most widely studied is hypertrophic cardiomyopathy, for which there is now extensive evidence of the involvement of cGAS-STING in the development. For example, in a mouse model of diabetic cardiomyopathy, Yan et al. [ 10 ] and Ma et al. [ 65 ] found increased levels of ROS in the myocardial tissue by dihydroethidium (DHE) staining, an increase in ectopic DNA by co-localization of dsDNA with mitochondrial somatic tracking, and an increase in cardiomyocyte death by dUTP nick end labeling (TUNEL) staining. Mechanistic studies revealed that it was associated with the activation of the cGAS-STING-induced cardiomyocyte focal death, worsening the progression of diabetic cardiomyopathy and leading to myocardial hypertrophy. Myocardial hypertrophy is closely associated with certain types of cardiomyopathy and is a major marker in patients with chronic kidney disease (CKD). Han et al. [ 66 ] developed a CKD model using cGAS-deficient (cGAS −/− ) or STING-deficient (STING −/− ) mice and were able to inhibit cardiac hypertrophy through the NF-KB pathway.
As previously mentioned, cGAS-STING is closely related to CVDs, and targeting this pathway may be a new means of treating CVDs. In addition, once CVDs occur, they impose a huge economic and psychological burden on patients and their families; the best way to address this is through primary prevention, understanding the risk factors that may activate the pathway daily, and early-stage prevention.
Risk factors for activating cGAS-STING in daily life
Disease complexity and heterogeneity are closely related to genetic, lifestyle, and environmental factors [ 67 ]. Although genetics are critical to disease development, some studies have shown that lifestyle and environmental factors can alter genetic behavior [ 68 ]. CVD triggers include risk factors such as smoking [ 69 ], obesity [ 70 ], and radiation [ 71 ] (Fig. 2 ). Understanding the role that lifestyle and environmental factors play in activating the cGAS-STING pathway is critical and provides a basis for guiding us to live healthier lives and avoid exposure.
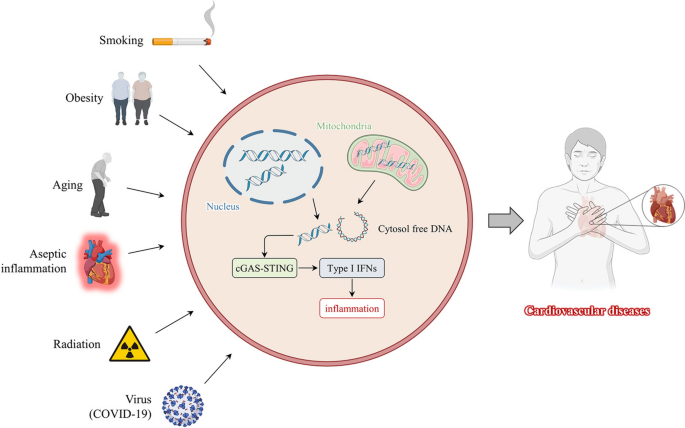
Risk factors for activating cGAS-STING in daily life. Smoking, obesity, aging, radiation, aseptic inflammation, and viruses (COVID-19) contribute to organismal damage. DNA from mitochondria or nucleus is released into the cytoplasm, activating the cGAS-STING signaling pathway and type I interferon transcription, causing an excessive inflammatory response resulting in cardiovascular diseases and poor prognosis
Although smoking kills approximately 6 million people annually, the deaths are preventable [ 72 ]. Thousands of chemicals in cigarette smoke contribute to the development of CVDs through a variety of mechanisms such as inflammation, dysfunctional hemostasis, and endothelial dysfunction [ 73 ]. Smoking is a leading cause of CVDs, with 140,000 premature deaths from CVDs each year [ 72 ]. The smoking population is relatively small in developed countries. Of note, the smoking population in Japan is 29.4% male and 7.2% female, and unfortunately, the average smoking prevalence rate of middle-aged males remains approximately 40% [ 74 ]. Smoking is an important underlying trigger for CVD development, and cardiovascular vigilance will need to be a permanent concern in the coming decades. Ueda et al. [ 75 ] found increased levels of free DNA in the serum of AS patients in a smoking population, and overexpression of free DNA was also detected in AS plaques, which activated the cGAS-STING pathway and became a AS marker. Clinical and experimental data suggest that cigarette smoking and secondhand smoke are equally detrimental in CVDs such as coronary artery disease, hypertension, and cardiac systolic and diastolic abnormalities. Liu et al. [ 69 ] found that exposing wild-type mice to sidestream cigarette smoke caused mtDNA release and activation of cGAS-STING signaling induced myocardial autophagy to disrupt cardiac homeostasis, and this effect removing in Beclin1 haplotype-sufficient (Beclin1 ± ) mice. Long-term chronic obstructive pulmonary disease (COPD) is one of the most important causes of cardiac impairment and failure. In COPD induced by exposing mice to high levels of cigarette smoke, mitochondrial DNA (mtDNA) release from respiratory tract injury activates the cGAS-STING-IRF3 pathway to cause an excessive inflammatory response and exacerbate lung injury; however, the pathway is inhibited in cGAS-deficient (cGAS −/− ) and STING-deficient (STING −/− ) mice [ 76 ]. Therefore, smoking cessation is an urgent issue for patients with CVDs, and avoiding secondhand smoke to reduce exposure may lead to a better prognosis for patients with CVDs.
Obesity and aging
People with a body mass index (BMI) > 25.0 kg/m 2 are classified as obese [ 77 ]. During the last decades of the twentieth century, changes in the dietary structure of the population have resulted in a shift from having a standard weight or being underweight to being overweight or obese. Each 1 kg/m 2 increase in BMI in obese patients increases the risk of HF and coronary heart disease by 12% and 7%, respectively, and a BMI reduction to that of the 1980s is needed to mitigate the ongoing weight damage [ 78 , 79 ]. The mechanisms through which obesity affects CVDs are currently being investigated. Recently, cGAS-STING has been shown to play a role in high-fat-induced myocardial injury in mice [ 80 ]. For example, Mao et al. [ 70 ] found that metabolic stress in obese patients induces endothelial cell inflammation, and the cGAS-STING pathway is active in PA-induced aortic endothelium, whereas in vivo experiments have shown that STING-deficient (STING gt/gt ) mice prevent high-fat diet-induced vascular inflammation, insulin resistance, and glucose intolerance. Similarly, Gong et al. [ 81 ] found that cGAS-STING is activated in mice with high-fat diet-induced cardiac abnormalities, leading to impaired myocardial contractile function. However, aging is commonly accompanied by metabolic disorders (e.g., obesity), which induce chronic inflammation and lead to cardiomyocyte hypertrophy, fibrosis, and death, thereby increasing the CVD risk [ 82 ]. Takahashi et al. [ 83 ] found that when DNA enzymes (i.e., DNase2 and TREX1) are progressively downregulated in senescent cells, the aberrant accumulation of cytosolic DNA activates cGAS-STING, which induces senescence-associated secretory phenotypes (SASPs) via IFN-β. Notably, mouse senescent myocardium is triggered by the inflammatory cytokines interleukin (IL)-1β, IL-6, and IL-18 to release mtDNA, which activates cGAS-STING to induce SASP development [ 84 ]. Hu et al. [ 85 ] found that immunohistochemical staining of aortic tissue sections from aging patients revealed increased expression of cGAS, STING, and p-IRF3 relative to that in younger patients; the in vitro inhibition of cGAS attenuates the aortic endothelial senescence markers p53, p21, and β-galactosidase, demonstrating the potential value in the prevention of CVDs in the elderly. Thus, obesity and aging contribute to activating the cGAS-STING pathway. Obesity can be controlled through healthy means such as exercise and reduced food intake, while normal aging is a natural process that is not overly concerning.
Aseptic inflammation
Aseptic inflammation is the absence of microbial infection and features of intractable chronic inflammation that promote the development of autoimmune, metabolic, neurodegenerative, and cardiovascular diseases [ 86 ]. Inflammation requires either exogenous (e.g., microorganisms, bacteria, and viruses) or endogenous (e.g., autologous DNA, proteins, and lipids) ligands that activate downstream inflammatory signals [ 87 ]. The sources of inflammatory cytokine production in CVDs include almost all cardiac cells such as endothelial cells, cardiomyocytes, and resident macrophages [ 88 , 89 , 90 ]. Recent reports have suggested that the cGAS-STING signaling pathway plays an important role in cardiovascular inflammation [ 91 ]. The PA-induced release of mtDNA from endothelial cells activates the cGAS-STING-IRF3 signaling pathway to promote increased levels of inflammatory cytokines IL-1β, IL-6, and NLRP3 inflammasome, leading to pyroptosis onset and affecting the formation of AS; using small interfering RNAs cGAS or STING inhibits this process [ 92 ]. Guo et al. [ 56 ] found that iNOS deficiency inhibits mtDNA release to attenuate stress-induced aseptic cardiac inflammation; however, activated cGAS-STING attenuates its cardioprotective function, suggesting that preventing sterile inflammation is particularly critical for CVDs. However, sterile inflammation can be incomprehensible in the daily lives of the less cognizant general population, which is a major challenge. Better popularization of scientific materials and easy-to-understand language is required to publicize the dangers of aseptic inflammation, avoid overexercising to increase cardiorespiratory injury, control blood pressure, and reduce heat, acidity, and other factors that may cause inflammatory lesions.
With the development of medical technology, radiation therapy and diagnosis have become essential medical support tools for cancer patients [ 93 ]. Different cancer types may require different radiation therapy doses, and clinically significant CVDs may occur when radiation therapy doses exceed 30 Gy [ 94 ]. The breast cancer radiation treatment group has been shown to have a 27% increase in mortality from heart disease compared to that in the non-radiation group, and a case–control study of Hodgkin's lymphoma survivors found an increased risk of coronary heart disease of 7.4% per Gy [ 95 , 96 ]. Radiation therapy induces cardiovascular ROS, and chronic inflammation leads to cardiac remodeling, including fibrosis, apoptosis, and hypertrophy. Elevated inflammatory markers and enhanced immune response modulation have been found in cancer survivors [ 97 ]. Philipp et al.[ 71 ] observed the in vitro radiation of human coronary artery endothelial cells; proteomics revealed that few protein changes are induced by low (0.25 Gy) and medium (0.5 Gy) doses of radiation, but DNA damage, increased ROS levels, and inflammatory reactions occur after high doses of radiation (2 and 10 Gy). Interestingly, the protein expression in the cGAS-STING pathway has been associated with an increased radiation dose, with a strong effect at 10 Gy after one week. Pain in patients with cancer seriously affects their psychological and physical well-being, and paying attention to the radiation dose during treatment and diagnosis is even more important to avoid complications due to improper activation of the cGAS-STING pathway, which further damages the cardiovascular system.
Viral (i.e., severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2])
Both RNA and DNA viruses are strongly associated with CVDs; however, the ability of RNA viruses to rapidly mutate and recombine is more dangerous [ 98 ]. Unfortunately, the December 2019 outbreak in Wuhan was caused by a novel coronavirus that became a global epidemic and was named coronavirus disease 2019 (COVID-19) [ 99 ]. As research progressed, COVID-19, identified as a type B coronavirus that was closely related to SARS-CoV and a bat coronavirus, was renamed SARS-CoV-2 [ 100 ]. Patients with SARS-CoV-2 pneumonia presenting with cardiovascular complications have a variety of clinical manifestations, with initial symptoms of palpitations and chest tightness [ 101 ]. SARS-CoV-2 causes myocardial injury, arrhythmia, and HF, with incidences as high as 19.7%, 16.7%, and 23%, respectively [ 102 ]. Cardiovascular complications due to SARS-CoV-2 are associated with systemic inflammatory storms, oxidative stress, and DNA damage, and the cGAS-STING pathway can activate these factors [ 103 , 104 ]. Mechanistically, Elahi et al. [ 105 ] also described that, in theory, the cGAS-STING signaling pathway leads to SARS-CoV-2-induced endothelial dysfunction and coagulation disorders, which may require more experimental evidence. Fang et al. [ 106 ] found that the aberrant activation of cGAS-STING signaling in viral myocarditis leads to an inflammatory infiltrate of I-IFN transcription that damages the myocardium, which is inhibited in T-cell receptor interacting molecule (TRIM)-deficient mice. Although SARS-CoV-2 is still present and constantly mutating, it does not cause anxiety or panic. Now that vaccines and drugs are available, the virus has become less pathogenic and has a symbiotic relationship. However, older populations and those with underlying medical conditions such as high blood pressure, diabetes, and immune deficiencies still need to take precautions.
Therapeutic targets of the cGAS-STING pathway in CVDs
As described previously, the cGAS-STING signaling pathway is involved in the development of multiple CVDs and risk factors to avoid overexposure. Notably, therapeutic agents are needed for patients with CVDs in order to target the pathway to block the inflammatory cascade response, especially I-IFN transcription, which is difficult and requires a long-term commitment. In a phase II clinical trial of squamous cell carcinoma of the head and neck, the cGAS inhibitor MK-1454 was well tolerated clinically, providing great confidence in its clinical applications (clinicaltrials.gov: NCT04220866). Therefore, small-molecule inhibitors targeting the cGAS-STING signaling pathway in CVDs are promising. In this section, we summarize the most clinically promising small-molecule inhibitors of cGAS and STING that are used in cardiovascular research (Tables 2 and 3 ).
cGAS inhibitors
Based on the molecular structure and DNA recognition of cGAS, several compounds have been developed that inhibit the catalytic activity of cGAS or compete with cGAS for DNA binding. Briefly, cGAS is not sensed when small-molecule inhibitors that are unable to bind to ATP and GTP substrates or their product (i.e., cGAMP) compete for the cGAS active site and lose catalytic activity or when DNA competes with other small molecules for binding [ 107 , 108 ]. Representative compounds include the RU series compounds [ 109 ], antimalarials [ 110 ], and PF Series Compounds [ 81 ].
RU series compounds
The RU family of small-molecule compounds includes RU.521 and RU.365. The most studied cardiovascular RU compound is RU.521, possibly because of its minimal toxicity at ≥ 50% loss of cell viability at IC50; RU.521 competes with ATP and GTP for the active site of cGAS [ 111 ]. Wu et al. [ 112 ] found a significant prolongation of the graft time in mouse hearts using cGAS −/− mouse heart donors, and the same results were obtained by intraperitoneal injection of 10 mg/kg/day RU.521 (dimethyl sulfoxide using corn oil dilution) in recipient mice, starting 1 d before surgery. The results are related to the reduction of inflammatory factors IL-6, TNF-α, and IFN-γ and leukocyte infiltration. Yu et al. [ 85 ] found that in six-month-old mice, continuous administration of RU.521 for six months inhibits cGAS expression and I-IFN transcription to reduce aortic endothelial cell senescence. Interestingly, a study linking neuroinflammation in the paraventricular nucleus of mice with hypertensive heart disease found that an intra-feeding perfusion of RU.521 blocked microglia autophagic fluxes to attenuate the hypertensive myocardial injury caused by neuroinflammation and sympathetic overactivation [ 113 ]. Meanwhile, RU.521 plays a critical role in attenuating sepsis-induced cardiac dysfunction [ 109 ], endothelial focal death-induced AS [ 92 ], ischemia–reperfusion-induced myocardial apoptosis, and cardiac dysfunction [ 114 ]. These findings provide a new phase in the clinical application of RU.521 for the treatment of CVDs.
New targets of antimalarial drugs
Until quinine (QN), a valuable synthetic antimalarial drug, was synthesized and purified during World War II, malaria was treated empirically using the bark of the cinchona tree, a Latin American tropical plant [ 115 ]. Recent studies have shown that some classical antimalarial drugs such as quinoline (i.e., chloroquine [CQ] and hydroxychloroquine [HCQ]) and acridine (QC) can treat several inflammatory diseases [ 116 , 117 ]. Importantly, they block the triggering of cGAS activity using dsDNA via embedding, active groove binding, and covalent binding interactions [ 118 ]. In a study of ultraviolet (UV)-induced acute inflammation, B6 mice were continuously injected with HCQ (25 mg/kg/day) for three weeks. The I-IFN expression decreased in the skin and blood after six hours of UV irradiation, and the early IFN-I response in the skin and infiltration of inflammatory cells were dependent on the presence of cGAS [ 119 ]. Interestingly, An et al. [ 120 ] found that oral administration of X6 or HCQ (25 mg/kg/day) in TREX1-deficient mice from birth was able to treat cGAS-STING pathway active-mediated autoimmune myocarditis attenuating endomyocardial fibrosis and inflammation, and compared to HCQ, X6 significantly reduced cGAMP expression in the heart in an AGS model, with a therapeutic effect of better. These studies suggest that antimalarial drugs have new clinical applications and are significant for treating the cGAS-STING signaling pathway, a pharmacological CVD target.
PF series compounds
Hall et al. [ 121 ] used a high-throughput drug screening technique to identify PF-06928215 among the PF series of compounds. Using a novel fluorescence polarization assay, they also found that PF-06928215 interacted with Lys362 and Lys350 in cGAS, inhibited the catalytic activity of cGAS by ATP and GTP, and interfered with the production of cGAMP. Notably, their study also showed that PF-06928215 inhibited the activity of cGAS using as little as 200 nmmol/L, which demonstrated a high affinity. In studies investigating the effects of PM2.5 on senescence of lung cells, Wang et al. [ 122 ] showed that PF-06928215 was able to inhibit lung epithelial cell senescence induced by a cGAS-STING signaling pathway-mediated release of inflammatory factors IL-6, IL-8, and TNF-α. In a mechanistic study of high-fat diet-induced cardiac remodeling and contractile dysfunction, Gong et al. [ 81 ] discovered that PF-06928215 inhibited cGAS activity, prolonged palmitic acid-induced peak contraction and maximal shortening velocities of cardiomyocytes (+ dL/dt), and treated myocardial contractile dysfunction without eliciting any mechanical response on its own. Treatment effects of PF-06928215 on cardiovascular disease through inhibition of the cGAS-STING signaling pathway have not been studied in vivo. However, indirect evidence provides an essential research base for in vivo applied therapy. Further research with PF-06928215 may make it possible to treat cardiovascular diseases through inhibition of the cGAS-STING signaling pathway.
STING inhibitors
STING, an intermediate pathway protein, has been associated with several CVDs. Competitive antagonists have been designed based on molecules occupying CDN binding sites according to molecular crystal structure dynamics or palmitoylation of the STING protein structural domains Cys88 or Cys91 that alter molecular properties.
Nitrofuran derivatives
Nitrofuran derivatives include C-176, C-171, C-178, and C-170 [ 20 ]. C-176 has become the most studied compound in cardiovascular research. Nitrofuran derivatives can palmitoylate Cys99 on STING proteins to alter their molecular properties and affect their response-mediated I-IFN transcription [ 123 ]. Hua et al.[ 124 ] showed that in α-MyHC-induced autoimmune myocarditis (EAM), consecutive intraperitoneal injection of 1 μmol of C-176 for 14 days results in an inflammatory response in EAM, with IFN-β, TNF-α, CCL2, and F4/80 expression that is ameliorated by blocking macrophage STING expression. In an in vitro study, C-176 blocked the PA-induced NLRP3-mediated endothelial cell pyroptosis and I-IFN transcription. Pham et al.[ 125 ] identified that consecutive 1 μmol intraperitoneal injections of C-176, three times a week for 12 weeks in an AS model inhibits STING-associated vascular inflammation and macrophage activation to attenuate AS formation. Importantly, C-176 also has a therapeutic role in MI [ 126 ], diabetic cardiomyopathy [ 10 ], and plaque stability [ 47 ]. In addition, the 3-acylaminoindole derivative H-151 has the same target as the nitrofuran derivative. In a mouse model of myocardial ischemia–reperfusion injury using preoperative intraperitoneal injection of 750 nmol of H-151, blocking STING attenuates the I-IFN-mediated inflammatory response and macrophage infiltration, increases the left heart ejection fraction, and reduces the extent of infarcts to maintain cardiac function [ 11 , 127 ]. Therefore, developing pharmacological targets against STING for CVD treatment is essential.
Li et al. [ 128 ] isolated and purified the cyclic peptide Astin C from the Chinese herb, Aster. In subsequent studies, they also found that, owing to the specificity of its molecular crystal structure, Astin C specifically binds to the C-terminal activation pocket of STING and inhibits inflammatory signaling by preventing the recruitment and activation of IRF3 on STING signalosomes through CDN displacement. Furthermore, mice injected intravenously with Astin C compound formulations (2 or 4 mg/kg) have a 100% survival rate within seven days, and decreased levels of the pro-inflammatory factor, IFN-β, as detected by serum enzyme-linked immunosorbent assay (ELISA) assay after treatment [ 128 ]. Significantly, Astin C attenuates in vitro myocardial contractile dysfunction in palmitate-induced cardiomyocytes by inhibiting the cGAS-STING signaling pathway in experiments of high-fat diet-induced cardiac remodeling and contractile abnormalities [ 81 ]. This in vivo and in vitro evidence of the safety and applicability of Astin C provides confidence and suggests that with further research, the use of Astin C as a pharmacological target for STING in the treatment of CVDs may become a reality.
Future expectations
Continued basic and clinical research has demonstrated that the cGAS-STING signaling pathway plays a vital role in CVDs. Recently, this pathway has been shown to be activated in MI, AS, and HF, affecting I-IFN transcription to promote an inflammatory cascade response. These findings suggest that the cGAS-STING pathway is of broad interest in the cardiovascular field, including risk factor investigation and the development of pharmacological targets against its molecular crystallographic properties. As a result, we anticipate future cardiovascular clinical applications of cGAS-STING based on these characteristics, new technological features, and achievements.
In terms of the current therapeutic developments in CVDs, no optimal method to eradicate lesions exists. Diseases that do not require surgical intervention include mild or moderate stenosis of the coronary artery, AS, hypertension, and early HF that requires continuous drug therapy. However, pharmacological interventions are not specific and may strain other organs. Surgical treatments are also necessary, including those for severe congenital heart disease, heart transplants, aortic dissection, and genetically related Marfan syndrome. Surgery eradicates these lesions, but with physical and financial burdens, as well as the possibility that the most severe complications can lead to loss of life. This highlights the urgent need for more precise therapies to intervene effectively and promptly in CVDs to prevent deterioration and bodily damage. Sequencing technologies are evolving rapidly. Traditional sequencing microarrays and RNA sequencing, for example, involve averaging gene expression differences in different cell populations in heterogeneous populations reported as a single data point, which is insufficiently precise. This is avoided by single-cell sequencing, which uses fluorescence-activated or conjugated magnetic bead-assisted methods to categorize target cell populations and analyze them individually [ 129 ]. In a mouse aortic aneurysm model, single-cell sequencing revealed that increased STING expression in smooth muscle and macrophage subpopulations led to smooth muscle death and dsDNA release, which activated the macrophage STING-TBK1-IRF3 pathway, leading to increased expression of matrix metalloproteinases (MMPs), which degrade the ECM. However, this phenomenon can be inhibited by the STING pathway inhibitor amlexanox [ 43 ]. Clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated nuclease 9 (Cas9), a simple and easy-to-operate gene editing technology that has a wide range of applications in targeting and preventing severe diseases, including CVDs, has been one of the greatest advances in biomedicine in the last two decades [ 130 ]. Zhao et al. [ 131 ] revealed that CRISPR-Cas9 gene editing delivered by an adeno-associated virus ameliorates familial hypercholesterolemia due to low-density lipoprotein receptor (LDLR) mutations, reduces the aortic AS plaque area, and attenuates inflammatory infiltration. Human papillomavirus (HPV) infection is closely associated with CVD development [ 132 ], but the underlying mechanisms are unknown. Using CRISPR-Cas9, Gusho et al. [ 133 ] discovered that HPV-infected cells can block STING and IRF3 activation after cGAS knockdown. These results illustrate that single-cell sequencing and CRISPR-Cas9 technology can be applied for the development of pharmacological targets for the cGAS-STING signaling pathway, making precise treatment of CVDs possible.
Neutrophils are one of the most common effector cells of the innate immune system and play an instrumental role in cardiovascular pathophysiology, in addition to their anti-infective function [ 134 , 135 ]. Neutrophil extracellular traps (NETs) released by activated neutrophils play a crucial role in inflammatory injury [ 136 ]. Numerous recent studies have shown that NETs are associated with CVDs, such as AS [ 137 ], myocarditis [ 138 ], and MI [ 139 ], which may be related to the unique cell death program of neutrophils called "NETosis,” “NETosis” releases granules, cytoplasmic guanosine-conjugated chromatin, and bactericidal proteins to form a reticulum during cell death that plays a crucial role in host defense, autoimmunity, and blood coagulation [ 140 ]. The "NETosis" structure also includes many neutrophil derivatives such as dsDNA, myeloperoxidase (MPO)-binding DNA, citrullinated histones, and neutrophil elastase, which lead to ROS bursts that mediate tissue damage [ 141 ]. Importantly, the neutrophil derivatives in NETs are associated with clinical variables; in 2013, Borissoff et al. [ 142 ] showed a positive correlation between the degree of coronary AS by imaging NETs markers. Kang et al. [ 143 ] demonstrated that NETs can activate the STING pathway, and inhibiting the I-IFN response enhances neovascularization and vascular repair. However, the cGAS-STING signaling pathway acts as a DNA receptor that senses cytoplasmic DNA to mediate I-IFN production. Based on the above results, we speculate that the cGAS-STING signaling pathway can mediate the function of NETs to influence CVD development, providing a new direction for future therapy.
In summary, an in-depth study of cGAS-STING in CVDs can combine basic and clinical research. New technologies and cutting-edge developments can be combined, which may provide completely new therapeutic approaches to serve patients with CVDs in the future to alleviate the suffering caused by the disease.
The cGAS-STING signaling pathway is closely related to CVD development. This primer enables readers to better understand the role of cGAS-STING in CVDs, not through over-exploration of the mechanism but rather by serving as a tool to better focus on and develop health, avoid exposure, and learn about the emergence of new therapeutic cardiovascular targets. Recently developed small-molecule inhibitors targeting this pathway have achieved some success in animal experiments. We expect more investigators to study this pathway, including in fields other than CVDs. Simultaneously, we intend to conduct additional, faster clinical trials involving these small-molecule inhibitors or their derivatives to obtain more evidence and provide better clinical treatment for patients.
Availability of data and materials
No new data sets were used for analysis in this review.
Abbreviations
- Cardiovascular diseases
Cyclic guanosine monophosphate-adenosine monophosphate synthase
Stimulator of interferon gene
Type I interferon
Nucleotide-binding oligomerization domain-like receptor pyrin domain containing 3
Endoplasmic reticulum
TANK-binding kinase 1
Atherosclerosis
Three-prime repair exonuclease 1
Aortic aneurysms and dissection
Heart failure
Transverse aortic constriction
Myocardial infarction
Chronic obstructive pulmonary disease
Absent in melanoma 2
Low-density lipoprotein receptor
Neutrophil extracellular traps
Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. https://doi.org/10.1038/s41572-019-0106-z .
Article PubMed Google Scholar
Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart disease and stroke statistics-2023 update: a report from the American heart association. Circulation. 2023;147:e93–621. https://doi.org/10.1161/CIR.0000000000001123 .
Golia E, Limongelli G, Natale F, Fimiani F, Maddaloni V, Pariggiano I, et al. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep. 2014;16:435. https://doi.org/10.1007/s11883-014-0435-z .
Article CAS PubMed Google Scholar
Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124:315–27. https://doi.org/10.1161/CIRCRESAHA.118.313591 .
Article CAS PubMed PubMed Central Google Scholar
Dabravolski SA, Khotina VA, Sukhorukov VN, Kalmykov VA, Mikhaleva LM, Orekhov AN. The role of mitochondrial DNA mutations in cardiovascular diseases. Int J Mol Sci. 2022;23:952. https://doi.org/10.3390/ijms23020952 .
Roy P, Orecchioni M, Ley K. How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nat Rev Immunol. 2022;22:251–65. https://doi.org/10.1038/s41577-021-00584-1 .
Yan X, Yao C, Fang C, Han M, Gong C, Hu D, et al. Rocaglamide promotes the infiltration and antitumor immunity of NK cells by activating cGAS-STING signaling in non-small cell lung cancer. Int J Biol Sci. 2022;18:585–98. https://doi.org/10.7150/ijbs.65019 .
King KR, Aguirre AD, Ye Y, Sun Y, Roh JD, Ng RP, et al. IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat Med. 2017;23:1481–7. https://doi.org/10.1038/nm.4428 .
Hu D, Cui YX, Wu MY, Li L, Su LN, Lian Z, et al. Cytosolic DNA sensor cGAS plays an essential pathogenetic role in pressure overload-induced heart failure. Am J Physiol Heart Circ Physiol. 2020;318:H1525–37. https://doi.org/10.1152/ajpheart.00097.2020 .
Yan M, Li Y, Luo Q, Zeng W, Shao X, Li L, et al. Mitochondrial damage and activation of the cytosolic DNA sensor cGAS-STING pathway lead to cardiac pyroptosis and hypertrophy in diabetic cardiomyopathy mice. Cell Death Discov. 2022;8:258–258. https://doi.org/10.1038/s41420-022-01046-w .
Hu S, Gao Y, Gao R, Wang Y, Qu Y, Yang J, et al. The selective STING inhibitor H-151 preserves myocardial function and ameliorates cardiac fibrosis in murine myocardial infarction. Int Immunopharmacol. 2022;107:108658. https://doi.org/10.1016/j.intimp.2022.108658 .
O’Donoghue ML, Giugliano RP, Wiviott SD, Atar D, Keech A, Kuder JF, et al. Long-term evolocumab in patients with established atherosclerotic cardiovascular disease. Circulation. 2022;146:1109–19. https://doi.org/10.1161/CIRCULATIONAHA.122.061620 .
Hopfner K, Hornung V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat Rev Mol Cell Biol. 2020;21:501–21. https://doi.org/10.1038/s41580-020-0244-x .
Kim J, Kim H, Chung JH. Molecular mechanisms of mitochondrial DNA release and activation of the cGAS-STING pathway. Exp Mol Med. 2023;55:510–9. https://doi.org/10.1038/s12276-023-00965-7 .
Chamilos G, Gregorio J, Meller S, Lande R, Kontoyiannis DP, Modlin RL, et al. Cytosolic sensing of extracellular self-DNA transported into monocytes by the antimicrobial peptide LL37. Blood. 2012;120:3699–707. https://doi.org/10.1182/blood-2012-01-401364 .
Klarquist J, Hennies CM, Lehn MA, Reboulet RA, Feau S, Janssen EM. STING-mediated DNA sensing promotes antitumor and autoimmune responses to dying cells. J Immunol. 2014;193:6124–34. https://doi.org/10.4049/jimmunol.1401869 .
Schoggins JW, MacDuff DA, Imanaka N, Gainey MD, Shrestha B, Eitson JL, et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505:691–5. https://doi.org/10.1038/nature12862 .
Barnett KC, Coronas-Serna JM, Zhou W, Ernandes MJ, Cao A, Kranzusch PJ, et al. Phosphoinositide interactions position cGAS at the plasma membrane to ensure efficient distinction between self- and viral DNA. Cell. 2019;176:1432-1446.e11. https://doi.org/10.1016/j.cell.2019.01.049 .
Luecke S, Holleufer A, Christensen MH, Jonsson KL, Boni GA, Sorensen LK, et al. cGAS is activated by DNA in a length-dependent manner. EMBO Rep. 2017;18:1707–15. https://doi.org/10.15252/embr.201744017 .
Decout A, Katz JD, Venkatraman S, Ablasser A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021;21:548–69. https://doi.org/10.1038/s41577-021-00524-z .
Ergun SL, Fernandez D, Weiss TM, Li L. STING polymer structure reveals mechanisms for activation, hyperactivation, and inhibition. Cell. 2019;178:290-301.e10. https://doi.org/10.1016/j.cell.2019.05.036 .
Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–8. https://doi.org/10.1038/nature07317 .
Zhong B, Yang Y, Li S, Wang Y, Li Y, Diao F, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–50. https://doi.org/10.1016/j.immuni.2008.09.003 .
Zhang B, Nandakumar R, Reinert LS, Huang J, Laustsen A, Gao Z, et al. STEEP mediates STING ER exit and activation of signaling. Nat Immunol. 2020;21:868–79. https://doi.org/10.1038/s41590-020-0730-5 .
Tanaka Y, Chen ZJ. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signal. 2012;5:ra20–ra20. https://doi.org/10.1126/scisignal.2002521 .
Article PubMed PubMed Central Google Scholar
Hu Y, Chen B, Yang F, Su Y, Yang D, Yao Y, et al. Emerging role of the cGAS-STING signaling pathway in autoimmune diseases: Biologic function, mechanisms and clinical prospection. Autoimmun Rev. 2022;21:103155. https://doi.org/10.1016/j.autrev.2022.103155 .
Li N, Zhou H, Wu H, Wu Q, Duan M, Deng W, et al. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019;24:101215. https://doi.org/10.1016/j.redox.2019.101215 .
Khan MS, Khan SU, Khan SU, Suleman M, Shan AR, Khan MU, et al. Cardiovascular diseases crossroads: cGAS-STING signaling and disease progression. Curr Probl Cardiol. 2024;49:102189. https://doi.org/10.1016/j.cpcardiol.2023.102189 .
Münzel T, Hahad O, Sørensen M, Lelieveld J, Duerr GD, Nieuwenhuijsen M, et al. Environmental risk factors and cardiovascular diseases: a comprehensive expert review. Cardiovasc Res. 2022;118:2880–902. https://doi.org/10.1093/cvr/cvab316 .
Prasher D, Greenway SC, Singh RB. The impact of epigenetics on cardiovascular disease. Biochem Cell Biol. 2020;98:12–22. https://doi.org/10.1139/bcb-2019-0045 .
Booth LK, Redgrave RE, Tual-Chalot S, Spyridopoulos I, Phillips HM, Richardson GD. Heart disease and ageing: the roles of senescence, mitochondria, and telomerase in cardiovascular disease. Subcell Biochem. 2023;103:45–78. https://doi.org/10.1007/978-3-031-26576-1_4 .
Regitz-Zagrosek V, Gebhard C. Gender medicine: effects of sex and gender on cardiovascular disease manifestation and outcomes. Nat Rev Cardiol. 2023;20:236–47. https://doi.org/10.1038/s41569-022-00797-4 .
Dhingra R, Vasan RS. Age as a risk factor. Med Clin North Am. 2012;96:87–91. https://doi.org/10.1016/j.mcna.2011.11.003 .
Rossouw JE. Hormones, genetic factors, and gender differences in cardiovascular disease. Cardiovasc Res. 2002;53:550–7. https://doi.org/10.1016/S0008-6363(01)00478-3 .
Dib B, Seppelt PC, Arif R, Weymann A, Veres G, Schmack B, et al. Extensive aortic surgery in acute aortic dissection type A on outcome—insights from 25 years single center experience. J Cardiothorac Surg. 2019;14:187. https://doi.org/10.1186/s13019-019-1007-7 .
Passos LSA, Lupieri A, Becker-Greene D, Aikawa E. Innate and adaptive immunity in cardiovascular calcification. Atherosclerosis. 2020;306:59–67. https://doi.org/10.1016/j.atherosclerosis.2020.02.016 .
Rehman A, Kumari R, Kamthan A, Tiwari R, Srivastava RK, van der Westhuizen FH, et al. Cell-free circulating mitochondrial DNA: an emerging biomarker for airborne particulate matter associated with cardiovascular diseases. Free Radical Biol Med. 2023;195:103–20. https://doi.org/10.1016/j.freeradbiomed.2022.12.083 .
Article CAS Google Scholar
Thomas CA, Tejwani L, Trujillo CA, Negraes PD, Herai RH, Mesci P, et al. Modeling of TREX1-dependent autoimmune disease using human stem cells highlights L1 accumulation as a source of neuroinflammation. Cell Stem Cell. 2017;21:319-331.e8. https://doi.org/10.1016/j.stem.2017.07.009 .
Morita M, Stamp G, Robins P, Dulic A, Rosewell I, Hrivnak G, et al. Gene-targeted mice lacking the trex1 (DNase III) 3′→5′ DNA exonuclease develop inflammatory myocarditis. Mol Cell Biol. 2004;24:6719–27. https://doi.org/10.1128/MCB.24.15.6719-6727.2004 .
Krieser RJ, MacLea KS, Longnecker DS, Fields JL, Fiering S, Eastman A. Deoxyribonuclease IIα is required during the phagocytic phase of apoptosis and its loss causes perinatal lethality. Cell Death Differ. 2002;9:956–62. https://doi.org/10.1038/sj.cdd.4401056 .
An J, Woodward JJ, Sasaki T, Minie M, Elkon KB. Cutting edge: antimalarial drugs inhibit IFN-beta production through blockade of cyclic GMP-AMP synthase-DNA interaction. J Immunol. 2015;194:4089–93. https://doi.org/10.4049/jimmunol.1402793 .
Wan X, Tian J, Hao P, Zhou K, Zhang J, Zhou Y, et al. cGAS-STING pathway performance in the vulnerable atherosclerotic plaque. Aging Dis. 2022;13:1606. https://doi.org/10.14336/AD.2022.0417 .
Luo W, Wang Y, Zhang L, Ren P, Zhang C, Li Y, et al. Critical role of cytosolic DNA and its sensing adaptor STING in aortic degeneration, dissection, and rupture. Circulation. 2020;141:42–66. https://doi.org/10.1161/CIRCULATIONAHA.119.041460 .
Zhang Y, Chen W, Wang Y. STING is an essential regulator of heart inflammation and fibrosis in mice with pathological cardiac hypertrophy via endoplasmic reticulum (ER) stress. Biomed Pharmacother. 2020;125:110022. https://doi.org/10.1016/j.biopha.2020.110022 .
Chen H, Tas SW, de Winther MPJ. Type-I interferons in atherosclerosis. J Exp Med. 2020;217:e20190459. https://doi.org/10.1084/jem.20190459 .
Oduro PK, Zheng X, Wei J, Yang Y, Wang Y, Zhang H, et al. The cGAS–STING signaling in cardiovascular and metabolic diseases: future novel target option for pharmacotherapy. Acta Pharmaceutica Sinica B. 2022;12:50–75. https://doi.org/10.1016/j.apsb.2021.05.011 .
Bi X, Du C, Wang X, Wang XY, Han W, Wang Y, et al. Mitochondrial damage-induced innate immune activation in vascular smooth muscle cells promotes chronic kidney disease-associated plaque vulnerability. Adv Sci. 2021;8:2002738. https://doi.org/10.1002/advs.202002738 .
DeMartino RR, Sen I, Huang Y, Bower TC, Oderich GS, Pochettino A, et al. Population-based assessment of the incidence of aortic dissection, intramural hematoma, and penetrating ulcer, and its associated mortality from 1995 to 2015. Circulation Cardiovasc Quality Outcomes. 2018;11:e004689. https://doi.org/10.1161/CIRCOUTCOMES.118.004689 .
Article Google Scholar
Smedberg C, Steuer J, Leander K, Hultgren R. Sex differences and temporal trends in aortic dissection: a population-based study of incidence, treatment strategies, and outcome in Swedish patients during 15 years. Eur Heart J. 2020;41:2430–8. https://doi.org/10.1093/eurheartj/ehaa446 .
Vilacosta I, San Román JA, di Bartolomeo R, Eagle K, Estrera AL, Ferrera C, et al. Acute aortic syndrome revisited. J Am Coll Cardiol. 2021;78:2106–25. https://doi.org/10.1016/j.jacc.2021.09.022 .
Chakraborty A, Li Y, Zhang C, Li Y, Rebello KR, Li S, et al. Epigenetic induction of smooth muscle cell phenotypic alterations in aortic aneurysms and dissections. Circulation. 2023;12:959–77. https://doi.org/10.1161/CIRCULATIONAHA.123.063332 .
Boorsma EM, Ter Maaten JM, Damman K, Dinh W, Gustafsson F, Goldsmith S, et al. Congestion in heart failure: a contemporary look at physiology, diagnosis and treatment. Nat Rev Cardiol. 2020;17:641–55. https://doi.org/10.1038/s41569-020-0379-7 .
Ekman I, Cleland JGF, Andersson B, Swedberg K. Exploring symptoms in chronic heart failure. Eur J Heart Fail. 2005;7:699–703. https://doi.org/10.1016/j.ejheart.2005.07.003 .
Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–46. https://doi.org/10.1136/hrt.2003.025270 .
Tanai E, Frantz S. Pathophysiology of heart failure. Compr Physiol. 2015;6:187–214. https://doi.org/10.1002/cphy.c140055 .
Guo Y, You Y, Shang F, Wang X, Huang B, Zhao B, et al. iNOS aggravates pressure overload-induced cardiac dysfunction via activation of the cytosolic-mtDNA-mediated cGAS-STING pathway. Theranostics. 2023;13:4229–46. https://doi.org/10.7150/thno.84049 .
Xiong R, Li N, Chen L, Wang W, Wang B, Jiang W, et al. STING protects against cardiac dysfunction and remodelling by blocking autophagy. Cell Commun Signal. 2021;19:109. https://doi.org/10.1186/s12964-021-00793-0 .
Murphy A, Goldberg S. Mechanical complications of myocardial infarction. Am J Med. 2022;135:1401–9. https://doi.org/10.1016/j.amjmed.2022.08.017 .
Lu L, Liu M, Sun R, Zheng Y, Zhang P. Myocardial infarction: symptoms and treatments. Cell Biochem Biophys. 2015;72:865–7. https://doi.org/10.1007/s12013-015-0553-4 .
Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018;15:203–14. https://doi.org/10.1038/nrcardio.2017.161 .
Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis. Circ Res. 2016;118:1021–40. https://doi.org/10.1161/CIRCRESAHA.115.306565 .
Cao DJ, Schiattarella GG, Villalobos E, Jiang N, May HI, Li T, et al. Cytosolic DNA sensing promotes macrophage transformation and governs myocardial ischemic injury. Circulation. 2018;137:2613–34. https://doi.org/10.1161/CIRCULATIONAHA.117.031046 .
Brieler J, Breeden MA, Tucker J. Cardiomyopathy: an overview. Am Fam Physician. 2017;96:640–6.
PubMed Google Scholar
McKenna WJ, Maron BJ, Thiene G. Classification, epidemiology, and global burden of cardiomyopathies. Circ Res. 2017;121:722–30. https://doi.org/10.1161/CIRCRESAHA.117.309711 .
Ma XM, Geng K, Law BY, Wang P, Pu YL, Chen Q, et al. Lipotoxicity-induced mtDNA release promotes diabetic cardiomyopathy by activating the cGAS-STING pathway in obesity-related diabetes. Cell Biol Toxicol. 2023;39:277–99. https://doi.org/10.1007/s10565-021-09692-z .
Han W, Du C, Zhu Y, Ran L, Wang Y, Xiong J, et al. Targeting myocardial mitochondria-sting-polyamine axis prevents cardiac hypertrophy in chronic kidney disease. JACC Basic Trans Sci. 2022;7:820–40. https://doi.org/10.1016/j.jacbts.2022.03.006 .
Lin Y, Chen J, Shen B. Interactions between genetics lifestyle, and environmental factors for healthcare. Trans Inform Smart Healthcare. 2017;1005:167–91. https://doi.org/10.1007/978-981-10-5717-5_8 .
Grimes DA, Schulz KF. Cohort studies: marching towards outcomes. Lancet. 2002;359:341–5. https://doi.org/10.1016/S0140-6736(02)07500-1 .
Liu F, Liu Y, Zhuang Z, Ma J, Xu X, Zhang W, et al. Beclin1 haploinsufficiency accentuates second-hand smoke exposure-induced myocardial remodeling and contractile dysfunction through a STING-mediated mechanism. J Mol Cell Cardiol. 2020;148:78–88. https://doi.org/10.1016/j.yjmcc.2020.08.016 .
Mao Y, Luo W, Zhang L, Wu W, Yuan L, Xu H, et al. STING–IRF3 triggers endothelial inflammation in response to free fatty acid-induced mitochondrial damage in diet-induced obesity. Arterioscler Thromb Vasc Biol. 2017;37:920–9. https://doi.org/10.1161/ATVBAHA.117.309017 .
Philipp J, Le Gleut R, Toerne CV, Subedi P, Azimzadeh O, Atkinson MJ, et al. Radiation response of human cardiac endothelial cells reveals a central role of the cGAS-STING pathway in the development of inflammation. Proteomes. 2020;8:30. https://doi.org/10.3390/proteomes8040030 .
Burns DM. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis. 2003;46:11–29. https://doi.org/10.1016/S0033-0620(03)00079-3 .
Breitling LP. Current genetics and epigenetics of smoking/tobacco-related cardiovascular disease. Arterioscler Thromb Vasc Biol. 2013;33:1468–72. https://doi.org/10.1161/ATVBAHA.112.300157 .
Kondo T, Nakano Y, Adachi S, Murohara T. Effects of tobacco smoking on cardiovascular disease. Circ J. 2019;83:1980–5. https://doi.org/10.1253/circj.CJ-19-0323 .
Ueda K, Sakai C, Ishida T, Morita K, Kobayashi Y, Horikoshi Y, et al. Cigarette smoke induces mitochondrial DNA damage and activates cGAS-STING pathway: application to a biomarker for atherosclerosis. Clin Sci. 2023;137:163–80. https://doi.org/10.1042/CS20220525 .
Nascimento M, Gombault A, Lacerda-Queiroz N, Panek C, Savigny F, Sbeity M, et al. Self-DNA release and STING-dependent sensing drives inflammation to cigarette smoke in mice. Sci Rep. 2019;9:14848. https://doi.org/10.1038/s41598-019-51427-y .
Lorenzo AD. New obesity classification criteria as a tool for bariatric surgery indication. World J Gastroenterol. 2016;22:681. https://doi.org/10.3748/wjg.v22.i2.681 .
McPherson K. Reducing the global prevalence of overweight and obesity. Lancet. 2014;384:728–30. https://doi.org/10.1016/S0140-6736(14)60767-4 .
Rosengren A. Obesity and cardiovascular health: the size of the problem. Eur Heart J. 2021;42:3404–6. https://doi.org/10.1093/eurheartj/ehab518 .
Bai J, Cervantes C, He S, He J, Plasko GR, Wen J, et al. Mitochondrial stress-activated cGAS-STING pathway inhibits thermogenic program and contributes to overnutrition-induced obesity in mice. Commun Biol. 2020;3:257. https://doi.org/10.1038/s42003-020-0986-1 .
Gong Y, Li G, Tao J, Wu NN, Kandadi MR, Bi Y, et al. Double knockout of Akt2 and AMPK accentuates high fat diet-induced cardiac anomalies through a cGAS-STING-mediated mechanism. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165855. https://doi.org/10.1016/j.bbadis.2020.165855 .
Bartlett D, Miller R, Thiesfeldt S, Lakhani H, Shapiro J, Sodhi K. The role of Na/K-ATPase signaling in oxidative stress related to aging: implications in obesity and cardiovascular disease. Int J Mol Sci. 2018;19:2139. https://doi.org/10.3390/ijms19072139 .
Takahashi A, Loo TM, Okada R, Kamachi F, Watanabe Y, Wakita M, et al. Downregulation of cytoplasmic DNases is implicated in cytoplasmic DNA accumulation and SASP in senescent cells. Nat Commun. 2018;9:1249. https://doi.org/10.1038/s41467-018-03555-8 .
Rech L, Rainer PP. The innate immune cGAS-STING-pathway in cardiovascular diseases—a mini review. Front Cardiovas Med. 2021;8:715903. https://doi.org/10.3389/fcvm.2021.715903 .
Yu H, Liao K, Hu Y, Lv D, Luo M, Liu Q, et al. Role of the cGAS-STING pathway in aging-related endothelial dysfunction. Aging Dis. 2022;13:1901. https://doi.org/10.14336/AD.2022.0316 .
Du Y, Zhang H, Nie X, Qi Y, Shi S, Han Y, et al. Link between sterile inflammation and cardiovascular diseases: focus on cGAS-STING pathway in the pathogenesis and therapeutic prospect. Front Cardiovasc Med. 2022;9:965726. https://doi.org/10.3389/fcvm.2022.965726 .
Nakayama H, Otsu K. Mitochondrial DNA as an inflammatory mediator in cardiovascular diseases. Biochem J. 2018;475:839–52. https://doi.org/10.1042/BCJ20170714 .
Liu Y, Lian K, Zhang L, Wang R, Yi F, Gao C, et al. TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2014;109:415. https://doi.org/10.1007/s00395-014-0415-z .
Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, et al. Revisiting cardiac cellular composition. Circulat Res. 2016;118:400–9. https://doi.org/10.1161/CIRCRESAHA.115.307778 .
Sandanger O, Ranheim T, Vinge LE, Bliksoen M, Alfsnes K, Finsen AV, et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2013;99:164–74. https://doi.org/10.1093/cvr/cvt091 .
Hong Z, Mei J, Guo H, Zhu J, Wang C. Intervention of cGAS-STING signaling in sterile inflammatory diseases. J Mol Cell Biol. 2022;14:mjac005. https://doi.org/10.1093/jmcb/mjac005 .
An C, Sun F, Liu C, Huang S, Xu T, Zhang C, et al. IQGAP1 promotes mitochondrial damage and activation of the mtDNA sensor cGAS-STING pathway to induce endothelial cell pyroptosis leading to atherosclerosis. Int Immunopharmacol. 2023;123:110795. https://doi.org/10.1016/j.intimp.2023.110795 .
Puukila S, Lemon JA, Lees SJ, Tai TC, Boreham DR, Khaper N. Impact of ionizing radiation on the cardiovascular system: a review. Radiat Res. 2017;188:539–46. https://doi.org/10.1667/RR14864.1 .
Cutter DJ, Schaapveld M, Darby SC, Hauptmann M, van Nimwegen FA, Krol ADG, et al. Risk for valvular heart disease after treatment for Hodgkin lymphoma. JNCI J Natl Cancer Inst. 2015;107:djv008. https://doi.org/10.1093/jnci/djv008 .
Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–106. https://doi.org/10.1016/S0140-6736(05)67887-7 .
Mazzola R, Giaj Levra N, Alongi F. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol. 2016;34:2940–1. https://doi.org/10.1200/JCO.2016.66.7840 .
Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–53. https://doi.org/10.1161/01.RES.0000130526.20854.fa .
Cheng VCC, Lau SKP, Woo PCY, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and Reemerging infection. Clin Microbiol Rev. 2007;20:660–94. https://doi.org/10.1128/CMR.00023-07 .
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China JAMA. 2020;323:1061. https://doi.org/10.1001/jama.2020.1585 .
Chung MK, Zidar DA, Bristow MR, Cameron SJ, Chan T, Harding CV, et al. COVID-19 and cardiovascular disease. Circ Res. 2021;128:1214–36. https://doi.org/10.1161/CIRCRESAHA.121.317997 .
Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–60. https://doi.org/10.1038/s41569-020-0360-5 .
Liu F, Liu F, Wang L. COVID-19 and cardiovascular diseases. J Mol Cell Biol. 2021;13:161–7. https://doi.org/10.1093/jmcb/mjaa064 .
Panico P, Ostrosky-Wegman P, Salazar AM. The potential role of COVID-19 in the induction of DNA damage. Mutat Res Rev Mutat Res. 2022;789:108411. https://doi.org/10.1016/j.mrrev.2022.108411 .
Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–41. https://doi.org/10.1016/j.immuni.2020.05.002 .
Elahi R, Hozhabri S, Moradi A, Siahmansouri A, Jahani Maleki A, Esmaeilzadeh A. Targeting the cGAS-STING pathway as an inflammatory crossroad in coronavirus disease 2019 COVID-19. Immunopharmacol Immunotoxicol. 2023. https://doi.org/10.1080/08923973.2023.2215405 .
Fang M, Zhang A, Du Y, Lu W, Wang J, Minze LJ, et al. TRIM18 is a critical regulator of viral myocarditis and organ inflammation. J Biomed Sci. 2022;29:55. https://doi.org/10.1186/s12929-022-00840-z .
Lama L, Adura C, Xie W, Tomita D, Kamei T, Kuryavyi V, et al. Development of human cGAS-specific small-molecule inhibitors for repression of dsDNA-triggered interferon expression. Nat Commun. 2019;10:2261. https://doi.org/10.1038/s41467-019-08620-4 .
Padilla-Salinas R, Sun L, Anderson R, Yang X, Zhang S, Chen ZJ, et al. Discovery of Small-molecule cyclic GMP-AMP synthase inhibitors. J Org Chem. 2020;85:1579–600. https://doi.org/10.1021/acs.joc.9b02666 .
Xu Q, Xiong H, Zhu W, Liu Y, Du Y. Small molecule inhibition of cyclic GMP-AMP synthase ameliorates sepsis-induced cardiac dysfunction in mice. Life Sci. 2020;260:118315. https://doi.org/10.1016/j.lfs.2020.118315 .
Han J, Li X, Luo X, He J, Huang X, Zhou Q, et al. The mechanisms of hydroxychloroquine in rheumatoid arthritis treatment: inhibition of dendritic cell functions via Toll like receptor 9 signaling. Biomed Pharmacother. 2020;132:110848. https://doi.org/10.1016/j.biopha.2020.110848 .
Vincent J, Adura C, Gao P, Luz A, Lama L, Asano Y, et al. Small molecule inhibition of cGAS reduces interferon expression in primary macrophages from autoimmune mice. Nat Commun. 2017;8:750. https://doi.org/10.1038/s41467-017-00833-9 .
Wu Z, Miao X, Jiang Y, Kong D, Liu H, Xie W, et al. Cardiomyocytic cyclic GMP-AMP synthase is critical for the induction of experimental cardiac graft rejection. J Thorac Cardiovasc Surg. 2023. https://doi.org/10.1016/j.jtcvs.2023.03.005 .
Han C, Qian X, Ren X, Zhang S, Hu L, Li J, et al. Inhibition of cGAS in paraventricular nucleus attenuates hypertensive heart injury via regulating microglial autophagy. Mol Neurobiol. 2022;59:7006–24. https://doi.org/10.1007/s12035-022-02994-1 .
Li J, Song Z, Hou X. Scutellarin ameliorates ischemia/reperfusion injury-induced cardiomyocyte apoptosis and cardiac dysfunction via inhibition of the cGAS-STING pathway. Exp Ther Med. 2023;25:155. https://doi.org/10.3892/etm.2023.11854 .
Flannery EL, Chatterjee AK, Winzeler EA. Antimalarial drug discovery—approaches and progress towards new medicines. Nat Rev Microbiol. 2013;11:849–62. https://doi.org/10.1038/nrmicro3138 .
Ehsanian R, Van Waes C, Feller SM. Beyond DNA binding - a review of the potential mechanisms mediating quinacrine’s therapeutic activities in parasitic infections, inflammation, and cancers. Cell Commun Signal. 2011;9:13. https://doi.org/10.1186/1478-811X-9-13 .
Gurova K. New hopes from old drugs: revisiting DNA-binding small molecules as anticancer agents. Future Oncol. 2009;5:1685–704. https://doi.org/10.2217/fon.09.127 .
An J, Minie M, Sasaki T, Woodward JJ, Elkon KB. Antimalarial drugs as immune modulators: new mechanisms for old drugs. Annu Rev Med. 2017;68:317–30. https://doi.org/10.1146/annurev-med-043015-123453 .
Skopelja-Gardner S, An J, Tai J, Tanaka L, Sun X, Hermanson P, et al. The early local and systemic Type I interferon responses to ultraviolet B light exposure are cGAS dependent. Sci Reports. 2020. https://doi.org/10.1038/s41598-020-64865-w .
An J, Woodward JJ, Lai W, Minie M, Sun X, Tanaka L, et al. Inhibition of cyclic GMP-AMP synthase using a novel antimalarial drug derivative in Trex1-deficient mice. Arthr Rheumatol. 2018;70:1807–19. https://doi.org/10.1002/art.40559 .
Hall J, Brault A, Vincent F, Weng S, Wang H, Dumlao D, et al. Discovery of PF-06928215 as a high affinity inhibitor of cGAS enabled by a novel fluorescence polarization assay. PLoS ONE. 2017;12:e0184843. https://doi.org/10.1371/journal.pone.0184843 .
Wang X, Lu W, Xia X, Zhu Y, Ge C, Guo X, et al. Selenomethionine mitigate PM2.5-induced cellular senescence in the lung via attenuating inflammatory response mediated by cGAS/STING/NF-κB pathway. Ecotoxicol Environ Safety. 2022;247:114266. https://doi.org/10.1016/j.ecoenv.2022.114266 .
Wan X, Tian J, Hao P, Zhang J, Zhou Y, Ge C, et al. The cGAS-STING pathway: a ubiquitous checkpoint perturbing myocardial attributes. Curr Vasc Pharmacol. 2023;21:152–62. https://doi.org/10.2174/1570161121666230501201756 .
Hua X, Bao M, Mo H, Sun Z, Xu M, Chen X, et al. STING regulates the transformation of the proinflammatory macrophage phenotype by HIF1A into autoimmune myocarditis. Int Immunopharmacol. 2023;121:110523. https://doi.org/10.1016/j.intimp.2023.110523 .
Pham PT, Fukuda D, Nishimoto S, Kim-Kaneyama J, Lei X, Takahashi Y, et al. STING, a cytosolic DNA sensor, plays a critical role in atherogenesis: a link between innate immunity and chronic inflammation caused by lifestyle-related diseases. Eur Heart J. 2021;42:4336–48. https://doi.org/10.1093/eurheartj/ehab249 .
Rech L, Abdellatif M, Pöttler M, Stangl V, Mabotuwana N, Hardy S, et al. Small molecule STING inhibition improves myocardial infarction remodeling. Life Sci. 1973;2022(291):120263–120263. https://doi.org/10.1016/j.lfs.2021.120263 .
Xiong Y, Leng Y, Tian H, Deng X, Li W, Li W, et al. Decreased MFN2 activates the cGAS-STING pathway in diabetic myocardial ischaemia–reperfusion by triggering the release of mitochondrial DNA. Cell Commun Signal. 2023;21:192. https://doi.org/10.1186/s12964-023-01216-y .
Li S, Hong Z, Wang Z, Li F, Mei J, Huang L, et al. The cyclopeptide Astin C specifically inhibits the innate immune CDN sensor STING. Cell Rep. 2018;25:3405-3421.e7. https://doi.org/10.1016/j.celrep.2018.11.097 .
Paik DT, Cho S, Tian L, Chang HY, Wu JC. Single-cell RNA sequencing in cardiovascular development, disease and medicine. Nat Rev Cardiol. 2020;17:457–73. https://doi.org/10.1038/s41569-020-0359-y .
Khoshandam M, Soltaninejad H, Mousazadeh M, Hamidieh AA, Hosseinkhani S. Clinical applications of the CRISPR/Cas9 genome-editing system: delivery options and challenges in precision medicine. Genes Dis. 2024;11:268–82. https://doi.org/10.1016/j.gendis.2023.02.027 .
Zhao H, Li Y, He L, Pu W, Yu W, Li Y, et al. In Vivo AAV-CRISPR/Cas9–mediated gene editing ameliorates atherosclerosis in familial hypercholesterolemia. Circulation. 2020;141:67–79. https://doi.org/10.1161/CIRCULATIONAHA.119.042476 .
Liang X, Chou OHI, Cheung BMY. The effects of human papillomavirus infection and vaccination on cardiovascular diseases, NHANES 2003–2016. Am J Med. 2023;136:294-301.e2. https://doi.org/10.1016/j.amjmed.2022.09.021 .
Gusho E, Laimins LA. Human papillomaviruses sensitize cells to DNA damage induced apoptosis by targeting the innate immune sensor cGAS. PLoS Pathog. 2022;18:e1010725. https://doi.org/10.1371/journal.ppat.1010725 .
Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–70. https://doi.org/10.1016/j.immuni.2010.11.011 .
Döring Y, Libby P, Soehnlein O. Neutrophil extracellular traps participate in cardiovascular diseases. Circ Res. 2020;126:1228–41. https://doi.org/10.1161/CIRCRESAHA.120.315931 .
Zhao J, Zhen N, Zhou Q, Lou J, Cui W, Zhang G, et al. NETs promote inflammatory injury by activating cGAS-STING pathway in acute lung injury. Int J Mol Sci. 2023;24:5125. https://doi.org/10.3390/ijms24065125 .
Han H, Liu C, Li M, Wang J, Liu Y, Zhou Y, et al. Increased intracellular Cl− concentration mediates neutrophil extracellular traps formation in atherosclerotic cardiovascular diseases. Acta Pharmacol Sin. 2022;43:2848–61. https://doi.org/10.1038/s41401-022-00911-9 .
Carai P, Gonzalez LF, Van Bruggen S, Spalart V, De Giorgio D, Geuens N, et al. Neutrophil inhibition improves acute inflammation in a murine model of viral myocarditis. Cardiovasc Res. 2023;118:3331–45. https://doi.org/10.1093/cvr/cvac052 .
Zhang Z, Ding S, Wang Z, Zhu X, Zhou Z, Zhang W, et al. Prmt1 upregulated by Hdc deficiency aggravates acute myocardial infarction via NETosis. Acta Pharmaceutica Sinica B. 2022;12:1840–55. https://doi.org/10.1016/j.apsb.2021.10.016 .
Ling S, Xu J. NETosis as a pathogenic factor for heart failure. Oxid Med Cell Longev. 2021;2021:1–24. https://doi.org/10.1155/2021/6687096 .
Biermann MHC, Podolska MJ, Knopf J, Reinwald C, Weidner D, Maueröder C, et al. Oxidative burst-dependent NETosis is implicated in the resolution of necrosis-associated sterile inflammation. Front Immunol. 2016;7:557. https://doi.org/10.3389/fimmu.2016.00557 .
Borissoff JI, Joosen IA, Versteylen MO, Brill A, Fuchs TA, Savchenko AS, et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol. 2013;33:2032–40. https://doi.org/10.1161/ATVBAHA.113.301627 .
Kang L, Yu H, Yang X, Zhu Y, Bai X, Wang R, et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun. 2020;11:2488. https://doi.org/10.1038/s41467-020-16191-y .
Download references
Acknowledgements
This study was supported by the Natural Science Foundation of Anhui Province (2208085MH203).
Author information
Cheng An, Zhen Li and Yao Chen contributed equally to this work.
Authors and Affiliations
Department of Cardiovascular Surgery, The First Affiliated Hospital of Anhui Medical University, No.218 Jixi Road, Hefei, 230032, Anhui, China
Cheng An, Yao Chen, Shaojun Huang, Chengxin Zhang & Shenglin Ge
Department of Orthopedics, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China
Department of Ophthalmology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China
State Key Laboratory of Pharmaceutical Biotechnology, School of Life Sciences, Nanjing University, Nanjing, Jiangsu, China
Inflammation and Immune Mediated Diseases Laboratory of Anhui Province, Anhui Institute of Innovative Drugs, School of Pharmacy, Anhui Medical University, Hefei, Anhui, China
You can also search for this author in PubMed Google Scholar
Contributions
C.A. and Z.L. writing-original draft. S.H. and Y.C. Investigation. F.Y. and Y.H. visualization. T.X. writing—review & editing. C.Z. and S.G. conceptualization, project administration. All authors reviewed the final manuscript and agreed to submit it.
Corresponding authors
Correspondence to Chengxin Zhang or Shenglin Ge .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
An, C., Li, Z., Chen, Y. et al. The cGAS-STING pathway in cardiovascular diseases: from basic research to clinical perspectives. Cell Biosci 14 , 58 (2024). https://doi.org/10.1186/s13578-024-01242-4
Download citation
Received : 11 October 2023
Accepted : 29 April 2024
Published : 08 May 2024
DOI : https://doi.org/10.1186/s13578-024-01242-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- cGAS-STING pathway
- Inflammatory response
- Risk factors
Cell & Bioscience
ISSN: 2045-3701
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Twenty Years of Cardiovascular Complications and Risk Factors in Patients With Type 2 Diabetes: A Nationwide Swedish Cohort Study
Affiliations.
- 1 Institute of Cardiovascular and Medical Sciences, British Heart Foundation Glasgow Cardiovascular Research Centre, UK (N.S., J.M.).
- 2 Department of Molecular and Clinical Medicine (J.B., A.R., E.O., N.B., J.H., K.S., A.R.), Institute of Medicine, University of Gothenburg, Sweden.
- 3 Wallenberg Laboratory for Cardiovascular and Metabolic Research (J.B., A.R., E.O., N.B., A.R.), Institute of Medicine, University of Gothenburg, Sweden.
- 4 Region Västra Götaland, Department of Specialist Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden (B.E.).
- 5 Population Health Research Institute, McMaster University and Hamilton Health Sciences, Hamilton, ON, Canada (H.C.G.).
- 6 Department of Internal Medicine, University of Texas Southwestern Medical Center, and Parkland Health and Hospital System, Dallas (D.K.M.).
- 7 Brigham and Women's Hospital Heart and Vascular Center, Harvard Medical School, Boston, MA (D.B.).
- PMID: 37154040
- DOI: 10.1161/CIRCULATIONAHA.122.063374
Background: The goal of this work was to investigate trends (2001-2019) for cardiovascular events and cardiometabolic risk factor levels in individuals with type 2 diabetes (T2D) and matched control subjects.
Methods: This study included 679 072 individuals with T2D from the Swedish National Diabetes Register and 2 643 800 matched control subjects. Incident outcomes comprised coronary artery disease, acute myocardial infarction, cerebrovascular disease, and heart failure (HF). Trends in time to first event for each outcome were analyzed with Cox regression and standardized incidence rates. In the group with T2D, Cox regression was also used to assess risk factor levels beyond target and outcomes, as well as the relative importance of each risk factor to each model.
Results: Among individuals with T2D, incidence rates per 10 000 person-years in 2001 and 2019 were as follows: acute myocardial infarction, 73.9 (95% CI, 65.4-86.8) and 41.0 (95% CI, 39.5-42.6); coronary artery disease, 205.1 (95% CI, 186.8-227.5) and 80.2 (95% CI, 78.2-82.3); cerebrovascular disease, 83.9 (95% CI, 73.6-98.5) and 46.2 (95% CI, 44.9-47.6); and HF, 98.3 (95% CI, 89.4-112.0) and 75.9 (95% CI, 74.4-77.5). The incidence for HF plateaued around 2013, a trend that then persisted. In individuals with T2D, glycated hemoglobin, systolic blood pressure, estimated glomerular filtration rate, and lipids were independently associated with outcomes. Body mass index alone potentially explained >30% of HF risk in T2D. For those with T2D with no risk factor beyond target, there was no excess cardiovascular risk compared with control subjects except for HF, with increased hazard with T2D even when no risk factor was above target (hazard ratio, 1.50 [95% CI, 1.35-1.67]). Risk for coronary artery disease and cerebrovascular disease increased in a stepwise fashion for each risk factor not within target. Glycated hemoglobin was most prognostically important for incident atherosclerotic events, as was body mass index for incident of HF.
Conclusions: Risk and rates for atherosclerotic complications and HF are generally decreasing among individuals with T2D, although HF incidence has notably plateaued in recent years. Modifiable risk factors within target levels were associated with lower risks for outcomes. This was particularly notable for systolic blood pressure and glycated hemoglobin for atherosclerotic outcomes and body mass index for heart failure.
Keywords: cardiometabolic risk factors; cardiovascular disease; cerebrovascular disorders; coronary artery disease; diabetes mellitus, type 2; heart failure; myocardial infarction.
Publication types
- Research Support, Non-U.S. Gov't
- Atherosclerosis* / complications
- Cerebrovascular Disorders* / complications
- Cerebrovascular Disorders* / diagnosis
- Cerebrovascular Disorders* / epidemiology
- Cohort Studies
- Coronary Artery Disease* / complications
- Coronary Artery Disease* / diagnosis
- Coronary Artery Disease* / epidemiology
- Diabetes Mellitus, Type 2* / diagnosis
- Diabetes Mellitus, Type 2* / epidemiology
- Glycated Hemoglobin
- Heart Failure* / diagnosis
- Heart Failure* / epidemiology
- Heart Failure* / etiology
- Myocardial Infarction* / complications
- Myocardial Infarction* / epidemiology
- Risk Factors
- Sweden / epidemiology
American College of Cardiology
Site improvements in progress., thank you for your patience..
This site is undergoing maintenance from Friday, May 10 until Tuesday, May 21 to modernize and improve the College's digital infrastructure. Please visit JACC.org for access to clinical guidelines and related resources, as well as the latest science and research from all 10 JACC journals. For assistance during this period, please reach out to the ACC Member Care team at [email protected] . Please be aware that our customer service may be limited until our systems are fully restored.
Featured Clinical Reviews
- Screening for Atrial Fibrillation: US Preventive Services Task Force Recommendation Statement JAMA Recommendation Statement January 25, 2022
- Evaluating the Patient With a Pulmonary Nodule: A Review JAMA Review January 18, 2022
Select Your Interests
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Exercise Might Lower Heart Disease Risk in Part By Decreasing Stress in the Brain
Physical activity might reduce the risk of cardiovascular disease partly by decreasing activity in parts of the brain related to stress, a study in the Journal of the American College of Cardiology suggests. Researchers looked at data from more than 50 300 adults in Massachusetts who completed an exercise survey and more than 700 who underwent brain imaging. People who exercised more tended to have fewer cardiovascular disease events and lower activity in stress-related brain regions, the researchers reported.
People with depression had larger reductions in cardiovascular disease risk from physical activity than those without depression and had even greater reductions if they exercised for more than the recommended 150 weekly minutes.
The results may help clinicians highlight the importance of physical activity for lowering cardiovascular disease risk, decreasing stress, and improving brain health, the researchers noted.
Published Online: May 10, 2024. doi:10.1001/jama.2024.7730
See More About
Harris E. Exercise Might Lower Heart Disease Risk in Part By Decreasing Stress in the Brain. JAMA. Published online May 10, 2024. doi:10.1001/jama.2024.7730
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Reviewer Login |

Journal of Cardiovascular Disease Research

For DOI Activation: [email protected]
Aim and scope.
Journal of cardiovascular Disease Research (JCDR) (J Cardiovasc. Dis. Res.) [www.jcdronline.org] J Cardiovasc. Dis. Res. [ISSN: Print -0975-3583, Online - 0976-2833] is a double-blind peer-reviewed, open access international circulating professional journal led by a group of research scientists,
JCDR publishes a wide range of topics covering basic and clinical research on cardiovascular disease, including but not limited to.
- cardiovascular disease mechanisms,
- translational and clinical research,
- cardiac electrophysiology,
- cardiovascular surgery,
- resuscitation,
- hypertension,
- vascular disease,
- molecular biology,
- biophysics,
- pharmacology,
- physiology,
- stem cells.
2021, Vol: 8, Issue: 8
Research Article
- BORDERLAND IDENTITY: A CRITICAL DISCOURSE ANALYSIS OF THE MINISTRY OF UTMOST HAPPINESS (2017) Masooma Ishtiaq,Shazir Hassan,Dr. Saeed ur Rahman JCR. 2021; 8(8): 1863-1867 » Abstract » PDF » doi: 10.31838/jcr.08.02.189
'Surprisingly' high number of adults in the U.S. at risk of heart syndrome
Nearly 90% of adults over age 20 in the United States are at risk of developing heart disease , an alarming new study suggests.
While the unexpectedly high number doesn't mean that the majority of adults in the U.S. have full-blown heart disease, it does indicate that many are at risk of developing the condition, even younger people.
Researchers identified people at high risk using a recently defined syndrome that takes into account the strong links between heart disease, obesity, diabetes and kidney disease, according to the research published Wednesday in JAMA.
The American Heart Association alerted doctors in October about cardiovascular-kidney-metabolic (CKM) syndrome , a condition which affects major organs in the body, including the brain, heart, liver and kidneys. CKM is diagnosed in stages ranging from zero — no risk factors for heart disease — to 4 — people with diagnosed heart disease plus excess body fat, metabolic risk factors such as hypertension and diabetes, or kidney disease.
For the new study, researchers analyzed almost a decade’s worth of data from more than 10,000 people who were participating in the National Health and Nutrition Examination Survey (NHANES).
“We absolutely were surprised that almost 90% of people met the criteria,” said study co-author Dr. Rahul Aggarwal, a cardiology fellow at Brigham and Women’s Hospital, Harvard Medical School, in Boston. “It was much higher than we anticipated in a database that included younger adults.”
Especially concerning was the finding that almost 50% of the NHANES participants were at stage 2 of CKM, meaning that they were at moderate risk because they had either high blood sugar, hypertension, high cholesterol or chronic kidney disease, Aggarwal said.
Just more than a quarter of the group — people listed as stage 1 — were at increased risk of developing heart disease because of being obese or overweight, having excess belly fat and fat around their organs, but didn't have specific symptoms.
The researchers found that 15% of the participants had advanced disease, a number that remained fairly constant between 2011 and 2020.
“I think one of the biggest factors contributing to the fact that the percentage of people in advanced stages is not improving is obesity, which is very prevalent in the U.S.,” Aggarwal said, adding that 40% of people in America are obese. Another 32% are overweight based on body mass index calculations , according to the Centers for Disease Control and Prevention.
Carrying excess pounds increases the likelihood a person will have high blood pressure, high blood sugar and high cholesterol, although some have metabolic risk factors even if they are at a healthy weight.
Participants older than 65 were more likely to be at an advanced stage than people between 45 to 64. But being young wasn’t as protective as one might assume. Only 18% of people ages 20 through 44 were at stage zero. That is, they had no risk factors.
The new findings show that health care providers need to be picking up on these conditions earlier “before they lead to downstream effects,” such as increased risk of heart attack, heart failure and stroke, Aggarwal said. “We need to diagnose earlier and be more aggressive at treating people.”
Latest news on heart health
- Science shows how a surge of anger could raise heart attack risk.
- Intermittent fasting linked to a higher risk of cardiovascular death, new analysis finds.
- High levels of a common B vitamin linked to heart disease.
Adopting lifestyle changes, such as improved diets and increased activity, can help protect against heart attack and stroke.
The findings also show that “young adults, those younger than 45, are not as healthy as we thought they were,” Aggarwal said.
Experts were also surprised by the high rates of CKM.
“It is alarming that 90% of the population is at least stage 1 and only 10% have no risk factors,” said Dr. Sripal Bangalore, a professor of medicine and director of invasive and interventional cardiology at NYU Langone Health in New York City.
He blames the epidemic of overweight and obesity for those numbers.
“We have a lot of work to do to reduce the rates of overweight and obesity,” Bangalore said. “If we can do that, then hopefully we can reduce the number of people who progress to stage 2 and also move the needle down for higher stages.”
The inclusion of kidney disease in the risk assessments for cardiovascular disease makes a lot of sense, said Dr. Adriana Hung, a kidney specialist and epidemiologist and a professor of medicine at the Vanderbilt University Medical Center in Nashville, Tennessee.
“Kidney disease magnifies cardiovascular disease,” she said. “Some studies show that a patient has as much as six times the risk of dying from cardiovascular disease if kidney disease is also present.”
The new, broader approach to heart disease is likely to help identify more people who are at risk, said Dr. Robert Rosenson, director of lipids and metabolism for the Mount Sinai Health System in New York City.
“The main message from this study should be that many common behaviors are leading to an accumulation of diseases over one’s lifetime, which will impact quality of life and survival,” he said.
The large numbers of people with CKM in this study are related to overweight and obesity, insulin resistance and a diet that is high in fat and salt, Rosenson added.
People need to realize that it’s not just the heart that is being harmed by unhealthy diets and lack of exercise, he said, but that lifestyle factors also have an effect on cognition.
Linda Carroll is a regular health contributor to NBC News. She is coauthor of "The Concussion Crisis: Anatomy of a Silent Epidemic" and "Out of the Clouds: The Unlikely Horseman and the Unwanted Colt Who Conquered the Sport of Kings."
- International edition
- Australia edition
- Europe edition

Chemicals in vapes could be highly toxic when heated, research finds
AI analysis of 180 vape flavours finds that products contain 127 ‘acutely toxic’ chemicals, 153 ‘health hazards’ and 225 ‘irritants’
Chemicals used to produce vapes could be acutely toxic when heated and inhaled, according to research .
Vaping devices heat the liquid flavouring to high temperatures to form an aerosol that is then inhaled. They contain chemicals including vegetable glycerin, propylene glycol, nicotine and flavourings, blended in various amounts.
Previous experiments have shown that some fruit-flavoured vapes – such as strawberry, melon and blueberry – produce dangerous compounds called volatile carbonyls due to this heating process.
These compounds are known to have health implications for chronic obstructive pulmonary disease (COPD), cardiovascular disease and cancers.
With so many chemicals used in tens of thousands of different vape products, conducting experiments to test every brand and flavour for toxicity could take decades of research.
Instead, the study used AI to analyse the chemical composition of 180 vape flavours and simulate how they decompose when heated. The research, published in Scientific Reports , predicted that vapes produce 127 “acutely toxic” chemicals, 153 “health hazards” and 225 “irritants”.
Nearly every flavour put through the AI predictor showed at least one product that was classified as a health hazard, with many predicting several. The toxins were associated with vapes containing no nicotine, as well as those with.
The research team at RCSI University of Medicine and Health Sciences, Dublin , conclude there is a “potential public health threat facing the 4.5 million vapers in the UK” and an urgent need for “enhanced restrictions” on flavours and regulations that are reflective of the health risks of vaping, especially for young people.
In January, the government announced that it would ban disposable vapes and restrict sweet and fruity flavours . Lead author Donal O’Shea, professor of chemistry at RCSI, said that the UK government should go further and remove all flavours from vapes.
It is crucial to understand the impact of flavoured vapes on health “before it’s too late”, he added.
“It is plausible that we are on the cusp of a new wave of chronic diseases that will emerge 15 to 20 years from now due to these exposures.”
Given the popularity of flavoured vapes among non-smoking teenagers and young adults, understanding the long-term effects of these products on public health, morbidity and mortality is crucial, the study concludes.
“Without comprehensive regulation, as we try to treat the nicotine addictions of older tobacco smokers, there is a substantial risk of transferring new health issues to younger generations.”
Responding to the findings, a Department of Health and Social Care spokesperson said: “The health advice is clear – if you don’t smoke, don’t vape and children should never vape.
“That’s why we are banning disposable vapes and our tobacco and vapes bill includes powers to limit flavours, packaging and displays of vapes to reduce the appeal to children.
“It is clear that flavours like cotton candy and cherry cola are deliberately being targeted at children, not adult smokers trying to quit, which is completely unacceptable. That is why we are taking decisive action and will be restricting vape flavours.”
Prof Sanjay Agrawal, the Royal College of Physicians’ special adviser on tobacco, said that while vaping can be a very effective way to break the addiction to tobacco, it should only be used for this purpose.
“Vaping is not risk-free, so those who don’t smoke, including children and young people, should not vape either,” he said.
John Dunne, director general at the trade body the UK Vaping Industry Association, said: “The science on vaping is very clear, it is the most effective way for smokers to quit and is at least 95% less harmful than smoking. Every chemical used in vaping e-liquid in the UK is stringently tested, including analysing chemicals when heated, and is only approved for use by the UK government if it is deemed safe.”
Most viewed

COMMENTS
Abstract. Cardiovascular disease has become a growing global and public health concern among non-communicable diseases (NCDs). The purpose of the study was to focus on the increasing prevalence of the risk factors of cardiovascular diseases (CVD), irrespective of age and gender, and its effect on public health worldwide.
Cardiovascular diseases are the most common noncommunicable conditions worldwide and account for approximately one third of all deaths globally. 1 Modifiable risk factors such as body-mass index ...
Apolipoprotein A1 Infusions and Cardiovascular Outcomes after Acute Myocardial Infarction. C.M. Gibson and OthersN Engl J Med 2024;390:1560-1571. In patients with acute MI, multivessel coronary ...
Read the latest Research articles in Cardiovascular diseases from Nature Reviews Cardiology. ... Patients with cardiovascular disease (CVD) have an increased risk of cancer, and patients with ...
This scientific statement supersedes the 2006 American Heart Association (AHA) scientific statement on diet and lifestyle recommendations. 1 The evidence documenting aspects of diet that improve cardiovascular health and reduce cardiovascular risk is summarized, focusing on dietary patterns and food-based guidance. Poor diet quality is strongly associated with elevated risk of cardiovascular ...
Cardiovascular disease (CVD) stubbornly retains its reputation as a leading cause of morbidity and mortality. Deaths from CVD remain alarmingly high and are projected to be an astounding 23 million worldwide in 2030, although overall mortality rates have reduced over recent decades [1,2]. Nonetheless, the burden of CVD remains unchanged, owing to an increasing number of patients surviving into ...
Research is revealing the causes of heart disease and what can be done to tackle the world's biggest killer. ... During the time it takes to read this brief article, ischaemic heart disease, in ...
2,831Views. Treatment With Sacubitril/Valsartan in Patients With Advanced Heart Failure and Reduced Ejection Fraction. Explore the latest advances in PCI and device therapy, treatment of myocardial infarction, dyslipidemia, heart failure, afib & rhythm disorders, TAVR, and.
Cardiovascular diseases articles from across Nature Portfolio. Atom; RSS Feed; Definition. Cardiovascular diseases are pathological conditions affecting the heart and/or blood vessels that is, the ...
Cardiovascular disease, including stroke, is the leading cause of illness and death in the United States. There are an estimated 62 million people with cardiovascular disease and 50 million people ...
This methodology avoided double counting of costs for patients with multiple cardiovascular conditions. By 2030, 40.5% of the US population is projected to have some form of CVD. Between 2010 and 2030, real (2008$) total direct medical costs of CVD are projected to triple, from $273 billion to $818 billion.
Symptoms are subjective experiences that may indicate disease or significant change in health status. Symptoms have been linked to cardiovascular disease (CVD) since Egyptian physicians and Hippocrates described fatigue and dyspnea, respectively, as being related to the failing heart. 1,2 In a contemporary view of CVD, symptoms often are critical elements of the diagnosis, evaluation ...
Ischaemic heart disease and cerebrovascular disease (stroke) combined accounted for more than 85.1% of all cardiovascular disease deaths in 2016. Our study indicated that CVDs(angina, stroke) were prevalent and variable among older adults in six countries. Angina and stroke were both highest in Russian Federation(47.5%, 6.1% respectively).
To curb high rates of heart disease and stroke, experts urge prevention and innovation . Jan 24, 2024. The latest statistics report from the American Heart Association details persistent cardiovascular disease challenges, emphasizing the need for new approaches and targeted strategies to address risk factors.
Cardiovascular diseases (CVDs) are the leading cause of death worldwide and the number one cause of death in the United States [].The 2020 American Heart Association statistics show that approximately 19.05 million people died from CVDs worldwide, which was an increase of 18.7% from 2010 [].Previous surveys have shown that with the progress of industrialization and dietary changes, 37.4% of ...
1 Institute of Cardiovascular and Medical Sciences, British Heart Foundation Glasgow Cardiovascular Research Centre, UK (N.S., J.M.). ... cerebrovascular disease, and heart failure (HF). Trends in time to first event for each outcome were analyzed with Cox regression and standardized incidence rates. In the group with T2D, Cox regression was ...
These connections between well-being and cardiac health are evident across many types and manifestations of cardiovascular disease. Perhaps the clearest example is that of stress-induced or takotsubo cardiomyopathy, characterized by transient and sometimes severe systolic dysfunction following an acute stressor. 1 This neurocardiogenic syndrome is increasingly recognized and often has a ...
Physical activity might reduce the risk of cardiovascular disease partly by decreasing activity in parts of the brain related to stress, a study in the Journal of the American College of Cardiology suggests. Researchers looked at data from more than 50 300 adults in Massachusetts who completed an exercise survey and more than 700 who underwent brain imaging.
Short bursts of anger may temporarily damage the ability of blood vessels to properly dilate, a function believed to be pivotal in preventing arteries from hardening, new research suggests. The findings, published Wednesday in the Journal of the American Heart Association, may help explain how anger contributes to the risk of having a heart attack.
Res. [ISSN: Print -0975-3583, Online - 0976-2833] is a double-blind peer-reviewed, open access international circulating professional journal led by a group of research scientists, JCDR publishes a wide range of topics covering basic and clinical research on cardiovascular disease, including but not limited to. stem cells. This is an open ...
By Linda Carroll. Nearly 90% of adults over age 20 in the United States are at risk of developing heart disease, an alarming new study suggests. While the unexpectedly high number doesn't mean ...
Background. Sickle cell disease is an inherited blood disorder which can lead to severe complications, particularly in the cardiovascular and respiratory systems, potentially resulting in arrhythmias, pulmonary hypertension (PH), and cardiomegaly. This study aims to investigate the risk of PH and arrhythmias in adult SCD patients.
Last modified on Wed 8 May 2024 21.31 EDT. Chemicals used to produce vapes could be acutely toxic when heated and inhaled, according to research. Vaping devices heat the liquid flavouring to high ...