Advertisement

Recent advances in lung cancer research: unravelling the future of treatment
- Review Article
- Published: 06 April 2024
Cite this article

- Luca Bertolaccini ORCID: orcid.org/0000-0002-1153-3334 1 ,
- Monica Casiraghi 1 , 2 ,
- Clarissa Uslenghi 1 ,
- Sebastiano Maiorca 1 &
- Lorenzo Spaggiari 1 , 2
769 Accesses
2 Citations
1 Altmetric
Explore all metrics
Lung cancer, a multifaceted disease, demands tailored therapeutic approaches due to its diverse subtypes and stages. This comprehensive review explores the intricate landscape of lung cancer research, delving into recent breakthroughs and their implications for diagnosis, therapy, and prevention. Genomic profiling and biomarker identification have ushered in the era of personalised medicine, enabling targeted therapies that minimise harm to healthy tissues while effectively combating cancer cells. The relationship between pulmonary tuberculosis and lung cancer is examined, shedding light on potential mechanisms linking these two conditions. Early detection methods, notably low-dose computed tomography scans, have significantly improved patient outcomes, emphasising the importance of timely interventions. There has been a growing interest in segmentectomy as a surgical intervention for early-stage lung cancer in recent years. Immunotherapy has emerged as a transformative approach, harnessing the body's immune system to recognise and eliminate cancer cells. Combining immunotherapy with traditional treatments, such as chemotherapy and targeted therapies, has shown enhanced efficacy, addressing the disease's heterogeneity and overcoming drug resistance. Precision medicine, guided by genomic profiling, has enabled the development of targeted therapies like tyrosine kinase inhibitors, offering personalised treatments tailored to individual patients. Challenges such as drug resistance and limited accessibility to advanced therapies persist, emphasising the need for collaborative efforts and innovative technologies like artificial intelligence. Despite challenges, ongoing interdisciplinary collaborations and technological advancements offer hope for a future where lung cancer is treatable and preventable, reducing the burden on patients and healthcare systems worldwide.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

The artificial intelligence and machine learning in lung cancer immunotherapy
Application of clinical bioinformatics in lung cancer-specific biomarkers.

Precision Medicine in Lung Cancer
Data availability.
Not applicable.
Cheng B, Xiong S, Li C, Liang H, Zhao Y, Li J et al (2020) An annual review of the remarkable advances in lung cancer clinical research in 2019. J Thorac Dis 12(3):1056–1069
Article PubMed PubMed Central Google Scholar
Ibodeng GO, Uche IN, Mokua R, Galo M, Odigwe B, Galeas JN, Dasgupta S (2023) A snapshot of lung cancer: where are we now?-a narrative review. Ann Transl Med 11(6):261
Article CAS PubMed PubMed Central Google Scholar
Bertolaccini L, Casiraghi M, Petrella F, Rampinelli C, Tessitore A, Spaggiari L (2022) A methodological quality evaluation of the published guidelines and recommendations about the lung cancer screening. Eur J Cancer Prev 31(1):19–25
Article PubMed Google Scholar
Duma N, Santana-Davila R, Molina JR (2019) Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc 94(8):1623–1640
Article CAS PubMed Google Scholar
Hwang SY, Kim JY, Lee HS, Lee S, Kim D, Kim S et al (2022) Pulmonary tuberculosis and risk of lung cancer: a systematic review and meta-analysis. J Clin Med 11(3):765
Yaegashi LB, Baldavira CM, Prieto TG, Machado-Rugolo J, Velosa APP, da Silveira LKR et al (2021) In situ overexpression of matricellular mechanical proteins demands functional immune signature and mitigates non-small cell lung cancer progression. Front Immunol 12:714230
Bourgot I, Primac I, Louis T, Noel A, Maquoi E (2020) Reciprocal interplay between fibrillar collagens and collagen-binding integrins: implications in cancer progression and metastasis. Front Oncol 10:1488
Horne ZD, Jack R, Gray ZT, Siegfried JM, Wilson DO, Yousem SA et al (2011) Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res 171(1):1–5
Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S et al (2017) Tracking the evolution of non-small-cell lung cancer. N Engl J Med 376(22):2109–2121
Oliver AL (2022) Lung cancer: epidemiology and screening. Surg Clin North Am 102(3):335–344
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359(6382):1350–1355
Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M et al (2017) Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547(7662):222–226
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ et al (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371(16):1507–1517
Franzi S, Mattioni G, Rijavec E, Croci GA, Tosi D (2022) Neoadjuvant chemo-immunotherapy for locally advanced non-small-cell lung cancer: a review of the literature. J Clin Med 11(9):2629
Szeto CH, Shalata W, Yakobson A, Agbarya A (2021) Neoadjuvant and adjuvant immunotherapy in early-stage non-small-cell lung cancer, past, present, and future. J Clin Med 10(23):5614
Chai Y, Wu X, Bai H, Duan J (2022) Combined immunotherapy with chemotherapy versus bevacizumab with chemotherapy in first-line treatment of driver-gene-negative non-squamous non-small cell lung cancer: an updated systematic review and network meta-analysis. J Clin Med 11(6):1655
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J et al (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346(2):92–98
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092
Tsuboi M, Herbst RS, John T, Kato T, Majem M, Grohe C et al (2023) Overall Survival with Osimertinib in Resected EGFR-mutated NSCLC. N Engl J Med 389(2):137–147
Dohopolski M, Iyengar P (2021) Oligometastatic non-small cell lung cancer: a narrative review of stereotactic ablative radiotherapy. Ann Palliat Med 10(5):5944–5953
Yuan Z, Wang Y, Zhang J, Zheng J, Li W (2019) A meta-analysis of clinical outcomes after radiofrequency ablation and microwave ablation for lung cancer and pulmonary metastases. J Am Coll Radiol 16(3):302–314
Chen Y, Luo H, Liu R, Tan M, Wang Q, Wu X et al (2023) Efficacy and safety of particle therapy for inoperable stage II–III non-small cell lung cancer: a systematic review and meta-analysis. Radiat Oncol 18(1):86
Harada H, Suefuji H, Mori K, Ishikawa H, Nakamura M, Tokumaru S et al (2023) Proton and carbon ion radiotherapy for operable early-stage lung cancer: 3-year results of a prospective nationwide registry. Int J Radiation Oncol Biol Phys 117(2):23
Article Google Scholar
de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA et al (2020) reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 382(6):503–513
Huo B, Manos D, Xu Z, Matheson K, Chun S, Fris J et al (2023) Screening criteria evaluation for expansion in pulmonary neoplasias (SCREEN). Semin Thorac Cardiovasc Surg 35(4):769–780
Passiglia F, Cinquini M, Bertolaccini L, Del Re M, Facchinetti F, Ferrara R et al (2021) Benefits and harms of lung cancer screening by chest computed tomography: a systematic review and meta-analysis. J Clin Oncol 39(23):2574–2585
Qi SA, Wu Q, Chen Z, Zhang W, Zhou Y, Mao K et al (2021) High-resolution metabolomic biomarkers for lung cancer diagnosis and prognosis. Sci Rep 11(1):11805
Madama D, Martins R, Pires AS, Botelho MF, Alves MG, Abrantes AM, Cordeiro CR (2021) Metabolomic profiling in lung cancer: a systematic review. Metabolites 11(9):630
Planchard D, Kim TM, Mazieres J, Quoix E, Riely G, Barlesi F et al (2016) Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol 17(5):642–650
Araujo DC, Veloso AA, Borges KBG, Carvalho MDG (2022) Prognosing the risk of COVID-19 death through a machine learning-based routine blood panel: a retrospective study in Brazil. Int J Med Inform 165:104835
Chiu HY, Chao HS, Chen YM (2022) Application of artificial intelligence in lung cancer. Cancers (Basel) 14(6):1370
Christie JR, Lang P, Zelko LM, Palma DA, Abdelrazek M, Mattonen SA (2021) Artificial intelligence in lung cancer: bridging the gap between computational power and clinical decision-making. Can Assoc Radiol J 72(1):86–97
Goncalves S, Fong PC, Blokhina M (2022) Artificial intelligence for early diagnosis of lung cancer through incidental nodule detection in low- and middle-income countries-acceleration during the COVID-19 pandemic but here to stay. Am J Cancer Res 12(1):1–16
CAS PubMed PubMed Central Google Scholar
Goldsmith I, Chesterfield-Thomas G, Toghill H (2021) Pre-treatment optimization with pulmonary rehabilitation in lung cancer: making the inoperable patients operable. EClinicalMedicine 31:100663
Shields MD, Chen K, Dutcher G, Patel I, Pellini B (2022) Making the rounds: exploring the role of circulating tumor DNA (ctDNA) in non-small cell lung cancer. Int J Mol Sci 23(16):9006
Abbosh C, Frankell AM, Harrison T, Kisistok J, Garnett A, Johnson L et al (2023) Tracking early lung cancer metastatic dissemination in TRACERx using ctDNA. Nature 616(7957):553–562
Zaman FY, Subramaniam A, Afroz A, Samoon Z, Gough D, Arulananda S, Alamgeer M (2023) Circulating tumour DNA (ctDNA) as a predictor of clinical outcome in non-small cell lung cancer undergoing targeted therapies: a systematic review and meta-analysis. Cancers (Basel) 15(9):2425
Jaffee EM, Dang CV, Agus DB, Alexander BM, Anderson KC, Ashworth A et al (2017) Future cancer research priorities in the USA: a lancet oncology commission. Lancet Oncol 18(11):e653–e706
Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K et al (2022) Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 399(10335):1607–1617
Nakada T, Noda Y, Kato D, Shibasaki T, Mori S, Asano H et al (2019) Risk factors and cancer recurrence associated with postoperative complications after thoracoscopic lobectomy for clinical stage I non-small cell lung cancer. Thorac Cancer 10(10):1945–1952
Bedetti B, Bertolaccini L, Rocco R, Schmidt J, Solli P, Scarci M (2017) Segmentectomy versus lobectomy for stage I non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis 9(6):1615–1623
Bertolaccini L, Prisciandaro E, Bardoni C, Cara A, Diotti C, Girelli L, Spaggiari L (2022) Minimally invasive anatomical segmentectomy versus lobectomy in stage IA non-small cell lung cancer: a systematic review and meta-analysis. Cancers (Basel) 14(24):6157
Wang P, Fu YH, Qi HF, He P, Wang HF, Li C, Liu XC (2023) Evaluation of the efficacy and safety of robot-assisted and video assisted thoracic surgery for early non-small cell lung cancer: a meta-analysis. Technol Health Care 32(2):511–523
Casiraghi M, Galetta D, Borri A, Tessitore A, Romano R, Diotti C et al (2019) Ten years’ experience in robotic-assisted thoracic surgery for early stage lung cancer. Thorac Cardiovasc Surg 67(7):564–572
Wang P, Wang S, Liu Z, Sui X, Wang X, Li X et al (2022) Segmentectomy and wedge resection for elderly patients with stage I non-small cell lung cancer: a systematic review and meta-analysis. J Clin Med 11(2):294
Bertolaccini L, Cara A, Chiari M, Diotti C, Glick N, Mohamed S et al (2023) Real-world survival outcomes of wedge resection versus lobectomy for cT1a/b cN0 cM0 non-small cell lung cancer: a single center retrospective analysis. Front Oncol 13:1226429
Bertolaccini L, Spaggiari L (2023) Is it time to cross the pillars of evidence in favor of segmentectomies in early-stage non-small cell lung cancer? Cancers (Basel) 15(7):1993
Zaraca F, Kirschbaum A, Pipitone MD, Bertolaccini L, Group PS (2023) Prospective randomized study on the efficacy of three-dimensional reconstructions of bronchovascular structures on preoperative chest CT scan in patients who are candidates for pulmonary segmentectomy surgery: the patches (prospective randomized study efficacy of three-dimensional reconstructions segmentecomy) study protocol. Trials 24(1):594
Komarnicki P, Musialkiewicz J, Stanska A, Maciejewski A, Gut P, Mastorakos G, Ruchala M (2022) Circulating neuroendocrine tumor biomarkers: past, present and future. J Clin Med 11(19):5542
Coudray N, Ocampo PS, Sakellaropoulos T, Narula N, Snuderl M, Fenyo D et al (2018) Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med 24(10):1559–1567
Biesinger M, Eicken N, Varga A, Weber M, Brndiar M, Erd G et al (2022) Lymph but not blood vessel invasion is independent prognostic in lung cancer patients treated by VATS-lobectomy and might represent a future upstaging factor for early stages. Cancers 14(8):1893
Asamura H, Nishimura KK, Giroux DJ, Chansky K, Hoering A, Rusch V, et al (2023) IASLC Lung Cancer Staging Project The New Database to Inform Revisions in the Ninth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 18(5): 564–575
Hardenberg MC, Patel B, Matthews C, Califano R, Garcia Campelo R, Grohe C et al (2022) The value of disease-free survival (DFS) and osimertinib in adjuvant non-small-cell lung cancer (NSCLC): an international Delphi consensus report. ESMO Open 7(5):100572
Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M et al (2020) Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med 383(18):1711–1723
Xu H, Baidoo AAH, Su S, Ye J, Chen C, Xie Y et al (2019) A comparison of EGFR mutation status in tissue and plasma cell-free DNA detected by ADx-ARMS in advanced lung adenocarcinoma patients. Transl Lung Cancer Res 8(2):135–143
Zou PC, Wang L, Liu B, Zhang HZ, Liu HC (2011) EGFR-targeted therapies combined with chemotherapy for treating advanced non-small-cell lung cancer: a meta-analysis. Diagnostics 9:38
Google Scholar
Solomon BJ, Bauer TM, Mok TSK, Liu G, Mazieres J, de Marinis F et al (2023) Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir Med 11(4):354–366
Hotta K, Hida T, Nokihara H, Morise M, Kim YH, Azuma K et al (2022) Final overall survival analysis from the phase III J-ALEX study of alectinib versus crizotinib in ALK inhibitor-naive Japanese patients with ALK-positive non-small-cell lung cancer. ESMO Open 7(4):100527
Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G et al (2020) First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med 383(21):2018–2029
Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550
Isaacs J, Stinchcombe TE (2022) Neoadjuvant and adjuvant systemic therapy for early-stage non-small-cell lung cancer. Drugs 82(8):855–863
John AO, Ramnath N (2023) Neoadjuvant versus adjuvant systemic therapy for early-stage non-small cell lung cancer: the changing landscape due to immunotherapy. Oncologist 28(9):752–764
Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Gao S et al (2023) Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med 389(6):491–503
Kogure Y, Hashimoto H, Oki M (2021) A randomized phase iii study of pembrolizumab versus pembrolizumab-carboplatin-pemetrexed for locally advanced or metastatic nonsquamous non-small-cell lung cancer with PD-L1 50% or more (LAPLACE-50): study protocol. Clin Lung Cancer 11:921–924
Download references
Acknowledgements
This work was partially supported by the Italian Ministry of Health with Ricerca Corrente and 5x1000 funds.
Ministero della Salute, 5 × 1000, Ricerca Corrente.
Author information
Authors and affiliations.
Department of Thoracic Surgery, IEO, European Institute of Oncology IRCCS, Via Ripamonti 435, 20141, Milan, Italy
Luca Bertolaccini, Monica Casiraghi, Clarissa Uslenghi, Sebastiano Maiorca & Lorenzo Spaggiari
Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy
Monica Casiraghi & Lorenzo Spaggiari
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Luca Bertolaccini .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Bertolaccini, L., Casiraghi, M., Uslenghi, C. et al. Recent advances in lung cancer research: unravelling the future of treatment. Updates Surg (2024). https://doi.org/10.1007/s13304-024-01841-3
Download citation
Received : 06 March 2024
Accepted : 24 March 2024
Published : 06 April 2024
DOI : https://doi.org/10.1007/s13304-024-01841-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Lung cancer
- Comprehensive review
- Find a journal
- Publish with us
- Track your research
- Lung Neoplasms
- Neoplasms by Site
- Thoracic Neoplasms
- Respiratory Tract Neoplasms
- Lung Cancer
Advances in Lung Cancer Research
Lung cancer cells driven by the KRAS oncogene, which is highlighted in purple.
NCI-funded researchers are working to advance our understanding of how to prevent, detect, and treat lung cancer. In particular, scientists have made progress in identifying many different genetic alterations that can drive lung cancer growth.
This page highlights some of the latest research in non-small cell lung cancer (NSCLC), the most common form of lung cancer, including clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and research findings from recent studies.
Early Detection of Lung Cancer
A great deal of research has been conducted in ways to find lung cancer early. Several methods are currently being studied to see if they decrease the risk of dying from lung cancer.
The NCI-sponsored National Lung Screening Trial (NLST) showed that low-dose CT scans can be used to screen for lung cancer in people with a history of heavy smoking. Using this screening can decrease their risk of dying from lung cancer. Now researchers are looking for ways to refine CT screening to better predict whether cancer is present.
Markers in Blood and Sputum
Scientists are trying to develop or refine tests of sputum and blood that could be used to detect lung cancer early. Two active areas of research are:
- Analyzing blood samples to learn whether finding tumor cells or molecular markers in the blood will help diagnose lung cancer early.
- Examining sputum samples for the presence of abnormal cells or molecular markers that identify individuals who may need more follow-up.
Machine Learning
Machine learning is a method that allows computers to learn how to predict certain outcomes. In lung cancer, researchers are using computer algorithms to create computer-aided programs that are better able to identify cancer in CT scans than radiologists or pathologists. For example, in one artificial intelligence study , researchers trained a computer program to diagnose two types of lung cancer with 97% accuracy, as well as detect cancer-related genetic mutations.
Lung Cancer Treatment
Treatment options for lung cancer are surgery , radiation , chemotherapy , targeted therapy , immunotherapy , and combinations of these approaches. Researchers continue to look for new treatment options for all stages of lung cancer.
Treatments for early-stage lung cancer
Early-stage lung cancer can often be treated with surgery. Researchers are developing approaches to make surgery safer and more effective.
- When lung cancer is found early, people usually have surgery to remove an entire section ( lobe ) of the lung that contains the tumor. However, a recent clinical trial showed that, for certain people with early-stage NSCLC, removing a piece of the affected lobe is as effective as surgery to remove the whole lobe .
- The targeted therapy Osimertinib (Tagrisso ) was approved by the Food and Drug Administration (FDA) in 2021 to be given after surgery—that is, as adjuvant therapy —to people with early-stage NSCLC that has certain mutations in the EGFR gene.
- Two immunotherapy drugs, atezolizumab (Tecentriq) and pembrolizumab (Keytruda) have been approved by the FDA to be used as adjuvant treatments after surgery and chemotherapy, for some patients with early-stage NSCLC.
- The immunotherapy drug nivolumab (Opdivo) is approved to be used, together with chemotherapy, to treat patients with early-stage lung cancer before surgery (called neoadjuvant ). This approval, which came in 2022, was based on the results of the CheckMate 816 trial, which showed that patients at this stage who received neoadjuvant nivolumab plus chemotherapy lived longer than those who received chemotherapy alone .
- In another trial (Keynote-671), patients with early-stage NSCLC who received pembrolizumab plus chemotherapy before surgery and pembrolizumab after surgery had better outcomes than those who received just neoadjuvant or just adjuvant treatment.
Treatments for advanced lung cancer
Newer therapies are available for people with advanced lung cancer. These primarily include immunotherapies and targeted therapies, which continue to show benefits as research evolves.
Immunotherapy
Immunotherapies work with the body's immune system to help fight cancer. They are a major focus in lung cancer treatment research today. Clinical trials are ongoing to look at new combinations of immunotherapies with or without chemotherapy to treat lung cancer.
JAK Inhibitors Boost Immunotherapy in Clinical Trials
The combination shrank lymphoma and lung tumors in people and in mice.
Immune checkpoint inhibitor s are drugs that block an interaction between proteins on immune cells and cancer cells which, in turn, lowers the immune response to the cancer. Several immune checkpoint inhibitors have been approved for advanced lung cancer, including p embrolizumab (Keytruda) , a tezolizumab (Tecentriq) , c emiplimab (Libtayo) , d urvalumab (Imfinzi) , and n ivolumab (Opdivo) .
A key issue with immunotherapies is deciding which patients are most likely to benefit. There is some evidence that patients whose tumor cells have high levels of an immune checkpoint protein called PD-L1 may be more responsive to immune checkpoint inhibitors. Another marker for immunotherapy response is tumor mutational burden , or TMB, which refers to the amount of mutations in the DNA of the cancer cells. In some lung cancer trials, positive responses to immune checkpoint inhibitors have been linked with a high TMB. However, these markers cannot always predict a response and there is ongoing work to find better markers.
To learn more, see Immunotherapy to Treat Cancer .
Targeted Therapies
Targeted treatments identify and attack certain types of cancer cells with less harm to normal cells. In recent years, many targeted therapies have become available for advanced lung cancer and more are in development. Targeted treatments for lung cancer include the below.
Anaplastic lymphoma kinase (ALK) Inhibitors
ALK inhibitors target cancer-causing rearrangements in a protein called ALK. These drugs continue to be refined for the 5% of NSCLC patients who have an ALK gene alteration. Approved treatments include ceritinib (Zykadia) , alectinib (Alecensa) , brigatinib (Alunbrig) , and lorlatinib (Lorbrena) .
These ALK inhibitors are improvements from previous ones in their enhanced ability to cross the blood–brain barrier. This progress is critical because, in non-small cell lung cancer patients with ALK alterations, disease progression tends to occur in the brain. Based on clinical trial results, in 2024 the FDA approved alectinib as adjuvant therapy for people with ALK-positive NSCLC .
EGFR Inhibitors
Lung cancer trial of osimertinib draws praise—and some criticism.
The drug improved survival in a large clinical trial, but some question the trial’s design.
EGFR inhibitors block the activity of a protein called epidermal growth factor receptor (EGFR). Altered forms of EGFR are found at high levels in some lung cancers, causing them to grow rapidly. Osimertinib (Tagrisso) is the most effective and most widely used EGFR inhibitor. It is also used for adjuvant therapy after surgery for resectable NSCLC. Other drugs that target EGFR that are approved for treating NSCLC include afatinib (Gilotrif) , dacomitinib (Vizimpro) , erlotinib (Tarceva) , gefitinib (Iressa) . For people with Exon 20 mutations, amivantamab (Rybrevant) is an approved targeted therapy.
ROS1 Inhibitors
The ROS1 protein is involved in cell signaling and cell growth. A small percentage of people with NSCLC have rearranged forms of the ROS1 gene. Crizotinib (Xalkori) and entrectinib (Rozlytrek) are approved as treatments for patients with these alterations. In late 2023, the FDA approved repotrectinib (Augtyro) for advanced or metastatic NSCLC with ROS1 fusions as an initial treatment and as a second-line treatment in those who previously received a ROS1-targeted drug.
BRAF Inhibitors
The B-Raf protein is involved in sending signals in cells and cell growth. Certain changes in the B-Raf gene can increase the growth and spread of NSCLC cells.
The combination of the B-Raf-targeted drug dabrafenib (Tafinlar) and trametinib (Mekinist ), which targets a protein called MEK, has been approved as treatment for patients with NSCLC that has a specific mutation in the BRAF gene.
Encorafenib (Braftovi) combined with binimetinib (Mektovi) is approved for patients with metastatic NSCLC with a BRAF V600E mutation .
Other Inhibitors
Some NSCLCs have mutations in the genes NRTK-1 and NRTK-2 that can be treated with the targeted therapy larotrectinib (Vitrakvi). Those with certain mutations in the MET gene can be treated with tepotinib (Tepmetko) or capmatinib (Tabrecta) . And those with alterations in the RET gene are treated with selpercatinib (Retevmo) and pralsetinib (Gavreto) . A 2023 clinical trial showed that treatment with selpercatinib led to longer progression-free survival compared with people who received chemotherapy with or without pembrolizumab. Inhibitors of other targets that drive some lung cancers are being tested in clinical trials.
See a complete list of targeted therapies for lung cancer .
NCI-Supported Research Programs
Many NCI-funded researchers at the NIH campus, and across the United States and the world, are seeking ways to address lung cancer more effectively. Some research is basic, exploring questions as diverse as the biological underpinnings of cancer and the social factors that affect cancer risk. And some is more clinical, seeking to translate basic information into improved patient outcomes. The programs listed below are a small sampling of NCI’s research efforts in lung cancer.
- The Pragmatica-Lung Study is a randomized trial that will compare the combination of the targeted therapy ramucirumab (Cyramza) and the immunotherapy pembrolizumab (Keytruda) with standard chemotherapy in people with advanced NSCLC whose disease has progressed after previous treatment with immunotherapy and chemotherapy. In addition to looking at an important clinical question, the trial will serve as a model for future trials because it is designed to remove many of the barriers that prevent people from joining clinical trials.
- Begun in 2014, ALCHEMIST is a multicenter NCI trial for patients with early stage non-small cell lung cancer. It tests to see whether adding a targeted therapy after surgery, based on the genetics of a patient’s tumor, will improve survival.
- The Lung MAP trial is an ongoing multicenter trial for patients with advanced non-small cell lung cancer who have not responded to earlier treatment. Patients are assigned to specific targeted therapies based on their tumor’s genetic makeup.
- The Small Cell Lung Cancer Consortium was created to coordinate efforts and provide a network for investigators who focus on preclinical studies of small-cell lung cancer. The goal of the consortium is to accelerate progress on this disease through information exchange, data sharing and analysis, and face-to-face meetings.
- NCI funds eight lung cancer Specialized Programs of Research Excellence (Lung SPOREs) . These programs are designed to quickly move basic scientific findings into clinical settings. Each SPORE has multiple lung cancer projects underway.
Clinical Trials
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Trials are available for both non-small cell lung cancer treatment and small cell lung cancer treatment .
Lung Cancer Research Results
The following are some of our latest news articles on lung cancer research:
- Lorlatinib Slows Growth of ALK-Positive Lung Cancers, May Prevent Brain Metastases
- Durvalumab Extends Lives of People with Early-Stage Small Cell Lung Cancer
- Alectinib Approved as an Adjuvant Treatment for Lung Cancer
- Repotrectinib Expands Treatment Options for Lung Cancers with ROS1 Fusions
- Tarlatamab Shows Promise for Some People with Small Cell Lung Cancer
- Selpercatinib Slows Progression of RET-Positive Lung, Medullary Thyroid Cancers
View the full list of Lung Cancer Research Results and Study Updates .
- Correspondence
- Open access
- Published: 02 September 2024
Multi-omics and clustering analyses reveal the mechanisms underlying unmet needs for patients with lung adenocarcinoma and identify potential therapeutic targets
- Ken Asada 1 , 2 ,
- Syuzo Kaneko 1 , 2 na1 ,
- Ken Takasawa 1 , 2 na1 ,
- Kouya Shiraishi 3 ,
- Norio Shinkai 1 , 2 ,
- Yoko Shimada 3 ,
- Satoshi Takahashi 1 , 2 ,
- Hidenori Machino 1 , 2 ,
- Kazuma Kobayashi 1 , 2 ,
- Amina Bolatkan 1 , 2 ,
- Masaaki Komatsu 1 , 2 ,
- Masayoshi Yamada 4 ,
- Mototaka Miyake 5 ,
- Hirokazu Watanabe 5 ,
- Akiko Tateishi 6 ,
- Takaaki Mizuno 3 , 6 , 7 ,
- Yu Okubo 8 ,
- Masami Mukai 9 ,
- Tatsuya Yoshida 6 ,
- Yukihiro Yoshida 8 ,
- Hidehito Horinouchi 6 ,
- Shun-Ichi Watanabe 8 ,
- Yuichiro Ohe 6 ,
- Yasushi Yatabe 10 ,
- Takashi Kohno 3 &
- Ryuji Hamamoto 1 , 2
Molecular Cancer volume 23 , Article number: 182 ( 2024 ) Cite this article
Metrics details
The cancer genome contains several driver mutations. However, in some cases, no known drivers have been identified; these remaining areas of unmet needs, leading to limited progress in cancer therapy. Whole-genome sequencing (WGS) can identify non-coding alterations associated with the disease. Consequently, exploration of non-coding regions using WGS and other omics data such as ChIP-sequencing (ChIP-seq) to discern novel alterations and mechanisms related to tumorigenesis have been attractive these days.
Integrated multi-omics analyses, including WGS, ChIP-seq, DNA methylation, and RNA-sequencing (RNA-seq), were conducted on samples from patients with non-clinically actionable genetic alterations (non-CAGAs) in lung adenocarcinoma (LUAD). Second-level cluster analysis was performed to reinforce the correlations associated with patient survival, as identified by RNA-seq. Subsequent differential gene expression analysis was performed to identify potential druggable targets.
Differences in H3K27ac marks in non-CAGAs LUAD were found and confirmed by analyzing RNA-seq data, in which mastermind-like transcriptional coactivator 2 ( MAML2 ) was suppressed. The down-regulated genes whose expression was correlated to MAML2 expression were associated with patient prognosis. WGS analysis revealed somatic mutations associated with the H3K27ac marks in the MAML2 region and high levels of DNA methylation in MAML2 were observed in tumor samples. The second-level cluster analysis enabled patient stratification and subsequent analyses identified potential therapeutic target genes and treatment options.
Conclusions
We overcome the persistent challenges of identifying alterations or driver mutations in coding regions related to tumorigenesis through a novel approach combining multi-omics data with clinical information to reveal the molecular mechanisms underlying non-CAGAs LUAD, stratify patients to improve patient prognosis, and identify potential therapeutic targets. This approach may be applicable to studies of other cancers with unmet needs.
Introduction
Lung cancer is one of the most frequently diagnosed cancers and the second most common cause of death worldwide. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers. Genetic alterations can drive cancer, therefore, genetic testing using next-generation sequencing (NGS) to identify targeted mutations in lung cancer can facilitate strategic decisions regarding cancer therapy [ 1 ]. However, the discovery of genes altered in the coding regions of cancer is expected to reach a plateau. In other words, newly identified genes related to tumorigenesis may essentially be rediscoveries or already reported in cancer research. Therefore, cancer genome studies have gradually shifted from whole-exome sequencing (WES) to whole-genome sequencing (WGS) analysis and analyses of non-coding regions revealed novel mutations, highlighting the feasibility and benefits of WGS. Non-coding or regulatory regions are cis-regulatory elements that include promoters, enhancers, insulators, and 5ʹ- and 3ʹ-untranslated regions (UTRs) as locus control regions. Changes in DNA sequences or functional dysregulation in regulatory regions cause cancer. Thus, focusing on non-coding regions is highly beneficial for cancer studies. As non-coding regions do not code proteins, methods other than genomic sequencing are more desirable. Specifically, multi-omics analysis methods, including chromatin immunoprecipitation sequencing (ChIP-seq), can facilitate genome-wide DNA structure profiling effectively and elucidate cancer traits.
Genomic alterations differ according to race. According to The Cancer Genome Atlas (TCGA) lung adenocarcinoma (LUAD) database, mutations occur in KRAS (32.2%), EGFR (11.3%), and BRAF (7.9%), which are three of the four alterations with available molecular targeting medicines for lung cancer. However, the frequency of EGFR mutations is higher in the East Asian LUAD population (~ 50%) [ 2 ]. This suggests that cancer research using defined cohort datasets is crucial for race-based medicine or precision oncology and will contribute to better decision-making for cancer treatment. Furthermore, driver mutations in 30–50% of patients with NSCLC, including those from East Asian and Caucasian populations, have not yet been identified [ 3 ], leading to limited progress in cancer therapy.
DNA methylation is an epigenetic marker found in the promoter region throughout the gene body and the levels of the DNA methylation are associated with gene expression. Another epigenetics, enhancer activity is an epigenetic landscape, which is characterized by histone modifications associated with chromatin structure, have potential clinical implications. In this study, we conduct multi-omics analysis of patients with non-CAGA LUAD using WGS to identify genomic alterations, ChIP-seq to examine histone modifications, RNA sequencing (RNA-seq) to analyze gene expression, DNA methylation to identify epigenetic modifications, and clinical information to characterize clinical features, reveal the onset of cancer and discover potential therapeutic targets.
Mutational landscape in non-CAGAs LUAD samples
An overview of this study is shown in Fig. S1 . After extracting LUAD, those with sufficient specimens to perform WGS and at least one epigenetic analysis (ChIP-seq and DNA methylation) were used for subsequent analysis ( N = 184). Approximately 40% of the cohort was patients with non-CAGA. Additionally, we analyzed TCGA LUAD dataset and the analysis revealed that approximately 46% samples might be non-CAGA samples. Detailed information on the dataset are provided in the Table S1 -3 and Supplementary Methods.
Previous studies have classified LUAD into driver gene mutation and driver mutation-negative subtypes based on various criteria. The definition of driver mutations in this study was based on three criteria detailed in the Supplementary Methods. Samples that did not contain any of the mutations described in the Supplementary Methods and Table S3 were categorized as driver mutation-negative (hereafter referred to as non-clinically actionable genetic alterations (non-CAGAs)) in this study.
The global landscape of somatic mutations is shown in Fig. S2 A (bin of 1 kb). The mutations in each sample, including non-coding mutations, are summarized in Table S4. Profiling of copy number alterations (CNAs) showed that chromosomes (Chr) 1, 5, 7, and 8 tended to have more gain (Fig. S2 B). One patient showed multiple hetero losses in Chr4, 7, 11, 12, and 13, and the other patient showed duplications in Chr8, 10, 13, and 14 (Fig. S2 C and D). Allele-specific copy number analysis detected patients with a copy neutral loss of heterozygosity (LOH) (Fig. S2 E). Hetero losses and copy neutral LOH were detected as a clonal event, whereas duplications were observed as a subclonal event. However, not all samples had apparent CNAs. Figure S2 C – E shows examples of representative data for CNA in non-CAGA samples. Notably, we recently reported that 1.15% of non-CAGA LUAD cases exhibit chromosomal rearrangement around ERBB2 . This structural variation was linked to the super enhancer formation that was associated with ERBB2 overexpression. We further demonstrated that ERBB2 is a feasible of druggable target in non-CAGA LUAD patients [ 4 ].
Genomic, epigenomic, and transcriptomic differences between adjacent normal and tumor samples
Although we identified the CNAs in a few cases, a study reported that LUAD is generally rich in somatic mutation compared to SV [ 5 ]. We therefore performed a comprehensive study of epigenomic alterations using a combination of WGS, ChIP-seq, RNA-seq, and DNA methylation analyses to investigate the non-coding mutations in enhancer regions, with the aim of revealing the molecular mechanisms underlying non-CAGAs. Here, we compared enhancer activity between normal and non-CAGA samples and found that enhancer activity at the mastermind-like transcriptional coactivator 2 ( MAML2 ) genomic locus (chr11:95976598–96343195) was ablated in a significant fraction of samples (Table S5). MAML is a coactivator of Notch and the MAML complex induced Notch-dependent target genes, including c-MYC , p21 , ERBB2 , CCND3 , HES1 , HEY1 , and NFKB1 . MAML2 has conserved domains, forms stable DNA-binding complexes, and regulates Notch and Wnt/β-catenin signaling pathways by promoting β-catenin turnover independent of Notch signaling [ 6 ]. Several fusion genes, such as YAP1-MAML2 , MECT1-MAML2 , and CRTC1/3-MAML2 have been identified; among these, CRTC1-MAML2 is an oncogenic driver in mucoepidermoid carcinoma (MEC) [ 7 ]. Here, the suppressed enhancer activity was coupled with the down-regulation of MAML2 expression. A comparison of the matched normal adjacent to tumor and tumor tissues samples showed that MAML2 was suppressed in tumors (Fig. 1 A and B). To examine if somatic mutations are associated with enhancer activity, patients with mutations in any of genomic regions of FAM78B (95768953–95789782), CEP57 (CEP57:95790498–95832693), MTMR2 (95832880–95924107), and CCDC82 (96352773–96389912) genomic regions, which are neighboring genes of MAML2 , were examined alongside those of MAML2 . One hundred and seven of 184 patients had mutations in the MAML2 gene locus, whereas other genes showed relatively fewer mutations (Fig. 1 C). H3K27ac ChIP-seq peak of matched normal and tumor samples with genomic mutations are shown in Fig. 1 D. Only two cases were mutated in the coding regions (R60Q and R422L) of the MAML2 gene, indicating that most detected alterations were non-coding regions. Therefore, we further examined whether genomic alterations in those regions affect enhancer activity. We extracted a complete dataset that included WGS, ChIP-seq, and RNA-seq ( N = 113). Forty-nine of the 113 samples had null mutations, whereas 64 samples had alterations at the MAML2 locus. Enhancer activity was suppressed in the mutated samples, which was associated with MAML2 gene expression (Fig. 1 E and F). Analysis of the TCGA non-CAGA-like LUAD dataset revealed that MAML2 was also down-regulated in tumors compared its expression in a relatively large number of normal tissues adjacent to the tumors (Fig. 1 G). This suggests that MAML2 expression was down-regulated in patients with non-CAGA LUAD in both cohorts.
DNA methylation is another epigenetic mechanism that is beneficial for revealing the underlying mechanisms in cancer. The EPIC array includes Functional Annotation of the Mammalian Genome (FANTOM) 5 and Encyclopedia of DNA Elements (ENCODE) enhancer regions for DNA methylation detection, which promotes the study of regulatory regions. Therefore, we decided to use the EPIC array in this study to assess DNA methylation. Analysis using matched normal and tumor samples revealed that 18 of the 102 probes exhibited substantially different patterns in the MAML2 region (Fig. S3 A). Similarly, 46 of 102 probes had different methylation levels in normal to non-CAGA samples. Sixteen probes overlapped in both analyses (Fig. S3 B, left, Venn diagram; right; summary), indicating that these methylation sites may be potential diagnostic markers. We identified low DNA methylation levels in normal samples but high DNA methylation levels in tumor samples, which were inversely correlated with MAML2 gene expression. This finding agrees with those of a previous report, in which high DNA methylation levels in the MAML2 region suppressed gene expression [ 8 ]. To investigate how DNA methylation is regulated in MAML2 , we examined the expression levels of known DNA methyltransferases (DNMTs) and demethylation-related enzymes. In this study, elongator complex protein 3 (ELP3), which plays a role in paternal genome demethylation, and tet methylcytosine dioxygenase (TET) 2, which is involved in the TET dioxygenase-mediated oxidation of 5-methylcyotsine (5mC) pathways, were down-regulated in matched tumor and non-CAGA samples (Fig. S3 C and D). However, we did not observe the up-regulation of DNA methyltransferases, suggesting that demethylation mechanisms play pivotal roles in patients with non-CAGA LUAD. Notably, these mechanisms could be tissue-specific, as DNMT3B is involved in breast cancer [ 8 ]; however, ELP3 and TET2 were associated with patients with non-CAGA LUAD.
MAML2-dependent signaling pathways and genes related to clinical outcomes
To investigate whether known Notch and Wnt/β-catenin targeted genes are associated with MAML2 , we selected seven Notch targeted genes ( BCL2 , CCND3 , CDKN1 , ERBB2 , HERDUP1 , HES1 , HEY1 ), seven Wnt/β-catenin targeted genes ( CD44 , CTNNB1 , FN1 , MMP7 , PMP22 , SMYD3 , VEGFA ), and two common genes ( CCND1 and MYC ) that are expressed in lung cancer (Table S6). We identified genes such as BCL2 (XM_047437733 and NM_000633), CDKN1 (NM_001374511), CD44 (NM_001001390, XM_005253238, and XM_006718390), PMP22 (XM_047436306 and NM_153322) were down-regulated, whereas ERBB2 were overexpressed in non-CAGA samples (Fig. S4A). Next, to identify prognostic biomarkers and potential therapeutic targets associated with MAML2 , we performed a correlation analysis and identified the top 15 positively and negatively correlated genes against MAML2 (Table S7). The RNA-expression levels of these genes were compared to those in normal samples; all 15 genes were significantly down-regulated and most were up-regulated in response to MAML2 down-regulation (Fig. S4B and C). Kaplan–Meier survival analysis revealed poor prognosis in the subgroups with low FAT4 (XM_011532237; isoform X1), HMCN1 (XM_011510038; isoform X1 ) , CD302 (NM_014880; isoform 1 precursor), UTRN (NM_007124; isoform 1), and FOXN3 (NM_001085471; isoform 1) expression (Fig. 1 H-L, Table S8). To validate our findings, we performed survival analysis using a Korean dataset (GSE8894), because the previously published paper showed that the genetic backgrounds of the Japanese and Korean populations were the closest among the populations analyzed [ 9 ]. Consistent with our earlier results, there was a tendency for low expression levels of marker genes that were associated with poor prognosis (Fig. S5), Some of the genes did not show statistical significance, possibly due to the smaller sample size of the Korean dataset ( N = 61) compared to our dataset ( N = 154) and/or the presence of samples with driver mutations (if any), which could affect the results, as genomic information was unavailable in the Korean cohort. To further investigate whether these prognostic marker genes were specific to the Asian cohort, we performed survival analysis using the non-CAGA-like TCGA LUAD dataset, the results of which also revealed significant differences in survival according to CD302 and FOXN3 expression (Fig. 1 M-Q). In summary, CD302 and FOXN3 are prognostic markers in non-CAGA, Korean, and non-CAGA-like TCGA datasets, independent of ethnicity or race.
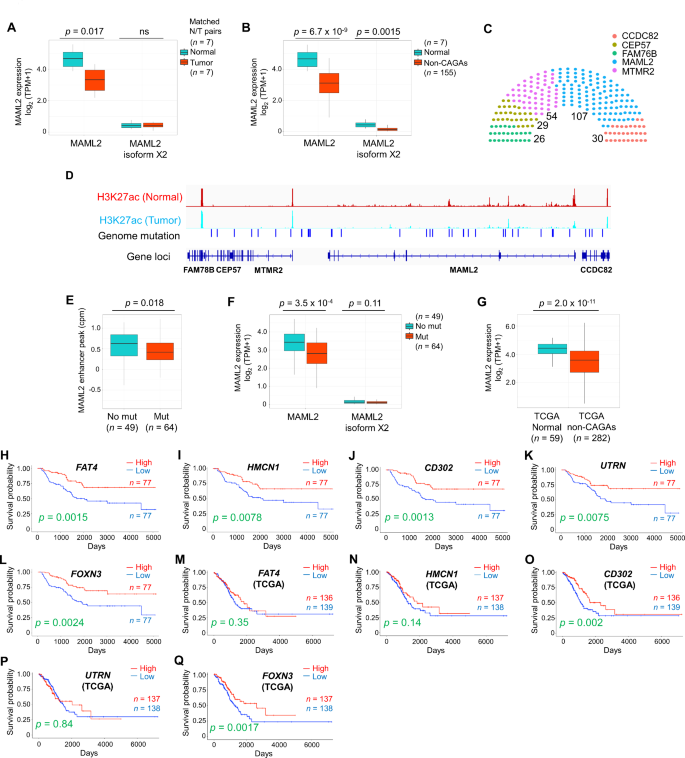
Genetic and epigenetic analysis in non-CAGA lung adenocarcinoma samples. A MAML2 expression in matched normal and tumor tissue samples. B MAML2 expression in normal and non-CAGA samples. C Number of patients with mutations in Chr11 ( FAM78B :95768953-95789782, CEP57 :95790498-95832693, MTMR2 :95832880-95924107, MAML2 :95976598-96343195, and CCDC82 :96352773-96389912). Somatic mutations and small insertions and deletions (INDELs) were analyzed, patients with at least one mutation were counted. D H3K27ac ChIP-seq peak of matched normal and tumor samples with genomic mutations. E Enhancer peak of H3K27ac in null and mutated samples at the MAML2 locus. F MAML2 expression of null and mutated samples at the MAML2 locus. G MAML2 expression analysis using the non-CAGA-like TCGA LUAD dataset. H - L Kaplan–Meier estimates of overall survival (OS) in patient with non-CAGA LUAD. A total of 154 patients were divided in half. H OS of FAT4 . I OS of HMCN1 . J OS of CD302 . K OS of UTRN . L OS of FOXN3 . M - Q Kaplan–Meier estimates of OS using the non-CAGA-like TCGA LUAD dataset. M OS of FAT4 . N OS of HMCN1 . O OS of CD302 . P OS of UTRN . Q OS of FOXN3
Although we identified prognostic markers and potential therapeutic targets, the molecular mechanisms related to MAML2 are unclear. Therefore, to explore the underlying mechanisms, we performed weighted gene correlation network analysis (WGCNA) to identify clusters of highly co-expressed hub genes (Fig. S6). We chose power 10 as the lowest possible power term where topology fits a scale free network (Fig. S6A left and right) and constructed a gene dendrogram to detect modules by hierarchical clustering (Fig. S6B). The PCNX1 gene had the most gene connections at 48, and exhibited greatest co-expression with the RNLS gene, followed by FTO with 19 connections, suggesting that these two genes are hub genes identified in MAML2 -associated subgroups and may orchestrate the signaling pathways (Fig. S6C).
Identification of potential therapeutic target genes via unsupervised learning
Next, we examined whether the prognostic marker genes identified were commonly expressed in all samples. Approximately 30% of patients had a common gene expression profile and were subclassified into either the high expression group or low expression group (Fig. S7A and B). Then, Kaplan–Meier survival analysis was conducted to determine whether commonly expressing subgroups showed improved patient stratification for overall survival (OS) compared with those analyzed using the expression of each gene. This approach failed to achieve better patient stratification (Fig. S7C and D). However, heatmap analysis of the prognostic genes enabled clustering of the samples (Fig. S7E, I – III in samples and A and B in genes), indicating that patient stratification related to OS could be improved. Therefore, we aimed to re-cluster patients by reinforcing existing correlations between the expression levels of prognostic genes and survival associations, thereby inflating the association of these components with survival. The results of the survival analysis for each gene were regarded as a first-level cluster, and patients with low expression were labeled − 1, whereas those with high expression were labeled 1 (Fig. 2 A). Using these labels, hierarchical or non-hierarchical K–means clustering was performed to obtain second-level cluster labels (Fig. S7F-H and Supplementary Method). The Elbow method was used to determine the optimal number of clusters for K–means analysis (Fig. S7G), and the clustering result were plotted (Fig. S7H). Based on the aforementioned results, we performed second-level patient stratification related to prognosis using these labels. Here, we achieved the optimal classification using labels obtained from the hierarchical clustering of CD302 , FAT4 , and FOXN3 genes (Fig. 2 B), rather than hierarchical clustering with weighted average adjustment and K–means cluster labels (Fig. 2 C and D, Table S9 and 10).

Second-level cluster analysis to improve patient stratification. A Workflow of the analysis. B - D Kaplan–Meier estimates of overall survival (OS) with secondlevel cluster analysis. B OS was assessed using hierarchical labels. Second-level cluster labels were obtained from the survival analysis of CD302 , FAT4 , and FOXN3 genes. C OS was assessed using hierarchical labels. Second-level cluster labels were obtained from the survival analysis of CD302 , FAT4 , and FOXN3 genes with weighted average adjustment. D OS was assessed using K–means labels. Second-level cluster labels were obtained from the survival analysis of CD302 , FAT4 , FOXN3 , and UTRN genes. E - F Mutation profiles of high-risk (poor survival) and low-risk (better survival) subgroups. E Low-risk subgroup (cluster 1) from B. F High-risk subgroup (cluster 2) from B. G Patient characteristics in the two subgroups. $ represents p-values obtained from the Mann–Whitney U test. # represents p-values obtained from Fisher’s exact test. H Volcano plot of the genes differentially expressed between high-risk and low-risk subgroups. Up-regulated genes in the poor survival subgroup are represented in red and down-regulated genes are represented in blue.
Genomic features and patient characteristics were examined in both groups. The high-risk group accumulated more mutations than the low-risk group (Fig. 2 E and F). For example, TP53 mutations were found in 72% of high-risk patients, whereas less than half of patients in the low-risk subgroup had TP53 mutations (29%). Other recurrently mutated genes such as TTN and RYR2 were also highly mutated in the high-risk group. A comparison of patient characteristics between the two groups revealed that the occurrence of smoking status, advanced cancer stage, and high tumor mutation burden (TMB) was greater in the high-risk group (Fig. 2 G).
Differentially expressed gene (DEG) analysis revealed 802 up-regulated and 289 down-regulated genes with a threshold of 2-fold difference and false discovery rate (FDR) < 0.05 in the high-risk subgroup (Fig. 2 H). Notably, CD302 , FAT4 , HMCN1 , and UTRN were significantly down-regulated whereas genes including PLK1 , UBE2C , and LYPD3 which are reportedly elevated in LUAD, were up-regulated (Fig. 2 H, Table S11). This finding suggests that second-level stratification, followed by DEG analysis can effectively identify therapeutic target genes. According to gene ontology (GO) biological processes, the up-regulated DEGs were enriched in the mitotic cell cycle, cell cycle process, and cell cycle, whereas down-regulated DEGs were enriched in anatomical structure development, developmental process, and anatomical structure morphogenesis in the poor survival subgroup (Fig. S8A and B, Table S12). To further investigate the global signaling pathways related to the subgroups, we performed Gene Set Enrichment Analysis (GSEA) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses. Blood vessel, circulatory system development, and vasculature development were enriched in GSEA, whereas focal adhesion, platelet activation, actin cytoskeleton regulation, and vascular smooth muscle contraction were enriched in KEGG pathways (Fig. S8C-F).
WGCNA revealed that seven out of 14 modules were significantly correlated with the second-level cluster subgroups (Fig. S9A and B). Among the module eigengenes (MEs), MEblack, MEblue, MEpurple, MEmagenta, and MEbrown were top modules associated with the subgroups. MAML2 , CD302 , FAT4 , FOXN3 , and HMCN1 were clustered as MEblack, whereas UTRN was clustered as MEblue (Fig. S9A and B, Table S13). FAT4 , a human homolog of tumor suppressor gene Fat in Drosophila, modulates Wnt/β-catenin signaling. HMCN1 is associated with the Hippo pathway in cancer. CD302 is associated with cancer-associated fibroblasts (CAFs) and is down-regulated in lung cancer. UTRN inhibits tumor growth by attenuating p38 and JNK/c-Jun signaling and FOXN3 functions as a tumor suppressor by suppressing Wnt/β-catenin signaling. As previously identified by WGCNA for the subgroups with high and low MAML2 expression (Fig. S6), similar genes were discovered as hub genes as well as components of the networks (Fig. S9C, Table S14). This indicates that second-level cluster analysis enabled the re-clustering of patients by reinforcing existing correlations between the expression levels of genes and survival association for more accurate patient stratification.
Although targeted therapies are clinically effective, a more comprehensive understanding of the cancer biology is required for precision oncology because actionable target-negative cancers have hampered progress in the field of cancer therapy for decades. Regulatory elements in non-coding regions are cis-regulatory elements that include promoters, enhancers, insulators, and 5ʹ- and 3ʹ-UTRs as locus control regions. Changes in DNA sequences in regulatory regions cause cancer, and histone modifications govern chromatin remodeling and enhance transcription activity. In this study, investigating the epigenomics revealed the clue of tumorigenesis and the mechanisms underlying non-CAGA LUAD patients. Later, we identified prognostic markers and potential therapeutic targets.
Here, we conducted an integrated multi-omics analysis for regulatory genomics, focusing on samples with non-CAGAs or unmet needs. Genes that were positively correlated with MAML2 expression were considered prognostic maker genes and second-level cluster analysis demonstrated enhanced prognostic predictive power. MAML regulates Notch and Wnt/β-catenin signaling pathways. MAML2 genomic rearrangement has been clinically evaluated in MEC ( https://oncology.testcatalog.org/show/MAMLF ). MAML2-based therapeutic modalities could be approached through several strategies. MAML2 regulates Notch signaling pathways and CTCR1-MAML2 is an oncogenic fusion gene in MEC. CTCR1-MAML2 requires AREG-EGFR signaling for MEC growth; co-targeting of Notch by DBZ and EGFR signaling by Erlotinib was an effective to anti-MEC treatment by attenuating MEC growth [ 7 ]. We found that MAML2 was down-regulated in non-CAGA LUAD samples; therefore, rescuing MAML2 expression serves as a potential therapeutic approach. Putative transcription factor binding sites to MAML2 has been previously predicted using the TransFac program [ 8 ]. Thus, recruiting or enhancing binding affinity of those transcriptional factors to the promoter could induce MAML2 up-regulation. A second therapeutic approach could involve DNA methylation targeting. MAML2 expression negatively correlates with DNA methylation. Hence, DNA methylase inhibition or DNA demethylase activation could induce MAML2 up-regulation. The third therapeutic approach could involve MAML2 expression-related prognostic marker targeting. In this case, positively and negatively correlated genes are considered potential therapeutic targets for the treatment of non-CAGA LUAD. However, further studies are needed to evaluate the efficacy and safety of these approaches.
In our study, Notch target gene BCL2 was down-regulated, and the pro-survival and pro-apoptotic BCL2 family proteins are attractive for the canter treatment. CDKN1 is also one of the Notch targeted gene. Intriguingly, previously published literature demonstrated that knockdown of SOX9 in LUAD resulted in the up-regulation of CDKN1 , suggesting that CDKN1 gene might be a common target of Notch and Wnt/β-catenin [ 6 , 10 ]. A novel aspect of this study is that we regarded the poor and better survival groups as distinct clusters, and second-level cluster analysis using prognosis-related labels led to improved patient stratification. DEGs between groups demonstrated that the identified prognostic markers were down-regulated, whereas potential therapeutic targets for human cancers such as PLK1 and UBE2C were up-regulated, which overexpression represses autophagy, inducing initiation, progression, and metastasis in NSCLC [ 11 , 12 ].
WGCNA identified PCNX1 and FTO as hub genes in both subgroups dichotomized by MAML2 expression and by second-level cluster labels. PCNX1 is an evolutionarily conserved components that activates the Notch signaling. PCNX is a human homolog of Drosophila pecanex (pcx). Currently, the role of PCNX in Notch signaling remains unknown; however, in Drosophila , pcx is a component of Notch signaling and in breast cancer, PCNX expression is associated with post-chemotherapy patient survival [ 13 ]. RNLS , PTEN , and ATAD1 were identified in prostate tumor [ 14 ], suggesting that RNLS plays a pivotal role in tumorigenesis. The other hub gene, FTO is a m 6 A demethylase associated with tumorigenesis in lung cancer and FTO down-regulation promotes epithelial-to-mesenchymal transition (EMT) by regulating Wnt/β-catenin signaling [ 15 ]. From the perspective of targeted therapy using non-CAGAs, we suggest that the identified prognostic marker genes, the genes identified by DEG analysis, and genes in the clinically relevant modules identified by WGCNA according to second-level cluster labels all show considerable promise. We also suggest that the global molecular mechanisms underlying non-CAGAs cancer onset may involve MAML2-related signaling pathways such as Notch and Wnt/β-catenin, however, we cannot exclude other possibilities and further investigation is required using gene knockout studies. Regarding the poor and better survival subgroups in non-CAGA samples divided by second-level cluster labels, immune check point inhibitors might be an option for the poor survival subgroup because patients in this group exhibited high TMB, which is associated with a favorable response to drugs in general.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
Cancer-associated fibroblast
Chromatin-immunoprecipitation sequencing
Copy number alteration
differentially expressed gene
DNA methyltransferase
Encyclopedia of DNA Elements
The Functional Annotation of the Mammalian Genome
Gene Set Enrichment Analysis
Gene ontology
Kyoto Encyclopedia of Genes and Genomes
- Lung adenocarcinoma
Module eigengene
Mucoepidermoid cancer
National Cancer Center
Non-clinically actionable genetic alterations
Non-small cell lung cancer
Overall survival
RNA-sequencing
Structural variation
The Cancer Genome Atlas
Tet methylcytosine dioxygenase
Tumor mutation burden
untranslated region
Whole-exosome sequencing
Weighted gene correlation (co-expression) network analysis
Whole-genome sequencing
Waarts MR, Stonestrom AJ, Park YC, Levine RL. Targeting mutations in cancer. J Clin Invest. 2022;132(8):e154943. https://doi.org/10.1172/JCI154943 .
Article PubMed PubMed Central CAS Google Scholar
Saito M, Shiraishi K, Kunitoh H, Takenoshita S, Yokota J, Kohno T. Gene aberrations for precision medicine against lung adenocarcinoma. Cancer Sci. 2016;107(6):713–20. https://doi.org/10.1111/cas.12941 .
Sholl LM, Aisner DL, Varella-Garcia M, Berry LD, Dias-Santagata D, Wistuba II, Chen H, Fujimoto J, Kugler K, Franklin WA, et al. Multi-institutional Oncogenic Driver Mutation Analysis in Lung Adenocarcinoma: the Lung Cancer Mutation Consortium Experience. J Thorac Oncol. 2015;10(5):768–77. https://doi.org/10.1097/JTO.0000000000000516 .
Kaneko S, Takasawa K, Asada K, Shiraishi K, Ikawa N, Machino H, Shinkai N, Matsuda M, Masuda M, Adachi S, et al. Mechanism of ERBB2 gene overexpression by the formation of super-enhancer with genomic structural abnormalities in lung adenocarcinoma without clinically actionable genetic alterations. Mol Cancer. 2024;23(1):126. https://doi.org/10.1186/s12943-024-02035-6 .
Kumar S, Warrell J, Li S, McGillivray PD, Meyerson W, Salichos L, Harmanci A, Martinez-Fundichely A, Chan CWY, Nielsen MM, et al. Passenger mutations in more than 2,500 Cancer genomes: overall molecular functional impact and consequences. Cell. 2020;180(5):915–e2716. https://doi.org/10.1016/j.cell.2020.01.032 .
Sinha A, Fan VB, Ramakrishnan AB, Engelhardt N, Kennell J, Cadigan KM. Repression of Wnt/beta-catenin signaling by SOX9 and mastermind-like transcriptional coactivator 2. Sci Adv. 2021;7(8):eabe0849. https://doi.org/10.1126/sciadv.abe0849 .
Ni W, Chen Z, Zhou X, Yang R, Yu M, Lu J, Kaye FJ, Wu L. Targeting notch and EGFR signaling in human mucoepidermoid carcinoma. Signal Transduct Target Ther. 2021;6(1):27. https://doi.org/10.1038/s41392-020-00388-0 .
Lubecka K, Kurzava L, Flower K, Buvala H, Zhang H, Teegarden D, Camarillo I, Suderman M, Kuang S, Andrisani O, et al. Stilbenoids remodel the DNA methylation patterns in breast cancer cells and inhibit oncogenic NOTCH signaling through epigenetic regulation of MAML2 transcriptional activity. Carcinogenesis. 2016;37(7):656–68. https://doi.org/10.1093/carcin/bgw048.bgw048 .
Wang Y, Lu D, Chung YJ, Xu S. Genetic structure, divergence and admixture of Han Chinese, Japanese and Korean populations. Hereditas. 2018;155:19. https://doi.org/10.1186/s41065-018-0057-5 .
Article PubMed PubMed Central Google Scholar
Jiang SS, Fang WT, Hou YH, Huang SF, Yen BL, Chang JL, Li SM, Liu HP, Liu YL, Huang CT, et al. Upregulation of SOX9 in lung adenocarcinoma and its involvement in the regulation of cell growth and tumorigenicity. Clin Cancer Res. 2010;16(17):4363–73. https://doi.org/10.1158/1078-0432.CCR-10-0138 .
Article PubMed CAS Google Scholar
Reda M, Ngamcherdtrakul W, Nelson MA, Siriwon N, Wang R, Zaidan HY, Bejan DS, Reda S, Hoang NH, Crumrine NA, et al. Development of a nanoparticle-based immunotherapy targeting PD-L1 and PLK1 for lung cancer treatment. Nat Commun. 2022;13(1):4261. https://doi.org/10.1038/s41467-022-31926-9 .
Guo J, Wu Y, Du J, Yang L, Chen W, Gong K, Dai J, Miao S, Jin D, Xi S. Deregulation of UBE2C-mediated autophagy repression aggravates NSCLC progression. Oncogenesis. 2018;7(6):49. https://doi.org/10.1038/s41389-018-0054-6 .
Al Amri WS, Allinson LM, Baxter DE, Bell SM, Hanby AM, Jones SJ, Shaaban AM, Stead LF, Verghese ET, Hughes TA. Genomic and expression analyses define MUC17 and PCNX1 as predictors of Chemotherapy response in breast Cancer. Mol Cancer Ther. 2020;19(3):945–55. https://doi.org/10.1158/1535-7163.MCT-19-0940 .
Bhandari V, Hoey C, Liu LY, Lalonde E, Ray J, Livingstone J, Lesurf R, Shiah YJ, Vujcic T, Huang X, et al. Molecular landmarks of tumor hypoxia across cancer types. Nat Genet. 2019;51(2):308–18. https://doi.org/10.1038/s41588-018-0318-2 .
Jeschke J, Collignon E, Al Wardi C, Krayem M, Bizet M, Jia Y, Garaud S, Wimana Z, Calonne E, Hassabi B, et al. Downregulation of the FTO m(6)a RNA demethylase promotes EMT-mediated progression of epithelial tumors and sensitivity to wnt inhibitors. Nat Cancer. 2021;2(6):611–28. https://doi.org/10.1038/s43018-021-00223-7 .
Download references
Acknowledgements
We thank Dr. Damiano Fantini for providing helpful suggestions related to the study. We thank Drs. Erik Bergstrom, SM Islam, and Burcak Otlu at the Alexandrov Laboratory for providing technical feedback related to the analysis. We greatly thank Drs. Shinji Kosaka and Kazuya Kanemochi for their support in this study. We thank the members of the Hamamoto laboratory and a member of the Kohno laboratory, Ms. Maiko Matsuda, for their kind assistance.
This work was supported by JSPS KAKENHI (Grant Number JP22K07180) and Takeda Science Foundation to K.A., the Cabinet Office BRIDGE (programs for bridging the gap between R&D and the ideal society (Society 5.0) and generating economic and social value), the AMED Innovative Cancer Medical Practice Research Project (Grant Number JP22ck0106643), JST CREST (Grant Number JPMJCR1689), JSPS Grant-in-Aid for Scientific Research on Innovative Areas (Grant Number JP18H04908), JST AIP-PRISM (Grant Number JPMJCR18Y4), and MEXT subsidy for Advanced Integrated Intelligence Platform to R.H.
Author information
Ken Asada, Syuzo Kaneko and Ken Takasawa contributed equally to this work.
Authors and Affiliations
Division of Medical AI Research and Development, National Cancer Center Research Institute, Tokyo, 104-0045, Japan
Ken Asada, Syuzo Kaneko, Ken Takasawa, Norio Shinkai, Satoshi Takahashi, Hidenori Machino, Kazuma Kobayashi, Amina Bolatkan, Masaaki Komatsu & Ryuji Hamamoto
Cancer Translational Research Team, RIKEN Center for Advanced Intelligence Project, Tokyo, 103-0027, Japan
Division of Genome Biology, National Cancer Center Research Institute, Tokyo, 104-0045, Japan
Kouya Shiraishi, Yoko Shimada, Takaaki Mizuno & Takashi Kohno
Department of Endoscopy, National Cancer Center Hospital, Tokyo, 104-0045, Japan
Masayoshi Yamada
Department of Diagnostic Radiology, National Cancer Center Hospital, Tokyo, 104-0045, Japan
Mototaka Miyake & Hirokazu Watanabe
Department of Thoracic Oncology, National Cancer Center Hospital, Tokyo, 104-0045, Japan
Akiko Tateishi, Takaaki Mizuno, Tatsuya Yoshida, Hidehito Horinouchi & Yuichiro Ohe
Department of Experimental Therapeutics, National Cancer Center Hospital, Tokyo, 104-0045, Japan
Takaaki Mizuno
Department of Thoracic Surgery, National Cancer Center Hospital, Tokyo, 104-0045, Japan
Yu Okubo, Yukihiro Yoshida & Shun-Ichi Watanabe
Division of Medical Informatics, National Cancer Center Hospital, Tokyo, 104-0045, Japan
Masami Mukai
Department of Diagnostic Pathology, National Cancer Center Hospital, Tokyo, 104-0045, Japan
Yasushi Yatabe
You can also search for this author in PubMed Google Scholar
Contributions
K.A., S.K., K.T., K.S., T.K., and R.H. designed the study. K.A., S.K., K.T., K.S., N.S., M.Ma., Y.S., S.T., H.M., K.K., A.B., M.K., M.Y., A.T., T.M., Y.Ok., M.Mu., T.Y., Y.Yo., and H.H. performed the data analysis. S.W., Y.Oh., Y.Ya., T.K., and R.H. supervised this study. K.A. wrote the manuscript, T.K. and R.H. edited the manuscript. All authors contributed to the interpretation of the data and critically revised the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding authors
Correspondence to Ken Asada , Takashi Kohno or Ryuji Hamamoto .
Ethics declarations
Ethics approval and consent to participate.
All methods were performed in accordance with the ethical guidelines for medical and health research involving human subjects. Informed consent was obtained from all patients. This study was approved by the Institutional Review Board of the National Cancer Center (NCC) Hospital (2005 − 109, 2016 − 496, 2019-018). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Consent for publication
All authors revised and approved the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, supplementary material 3, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Asada, K., Kaneko, S., Takasawa, K. et al. Multi-omics and clustering analyses reveal the mechanisms underlying unmet needs for patients with lung adenocarcinoma and identify potential therapeutic targets. Mol Cancer 23 , 182 (2024). https://doi.org/10.1186/s12943-024-02093-w
Download citation
Received : 28 April 2024
Accepted : 16 August 2024
Published : 02 September 2024
DOI : https://doi.org/10.1186/s12943-024-02093-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Epigenomics
- Multi-omics analysis
- Therapeutic target
Online Help
Our 24/7 cancer helpline provides information and answers for people dealing with cancer. We can connect you with trained cancer information specialists who will answer questions about a cancer diagnosis and provide guidance and a compassionate ear.
Chat live online
Select the Live Chat button at the bottom of the page
Call us at 1-800-227-2345
Available any time of day or night
Our highly trained specialists are available 24/7 via phone and on weekdays can assist through online chat. We connect patients, caregivers, and family members with essential services and resources at every step of their cancer journey. Ask us how you can get involved and support the fight against cancer. Some of the topics we can assist with include:
- Referrals to patient-related programs or resources
- Donations, website, or event-related assistance
- Tobacco-related topics
- Volunteer opportunities
- Cancer Information
For medical questions, we encourage you to review our information with your doctor.
Lung Cancer
- What Is Lung Cancer?
- Key Statistics for Lung Cancer
What’s New in Lung Cancer Research?
- Lung Cancer Risk Factors
- What Causes Lung Cancer?
- Can Lung Cancer Be Prevented?
- Can Lung Cancer Be Found Early?
- Lung Nodules
- Signs and Symptoms of Lung Cancer
- Tests for Lung Cancer
- Non-Small Cell Lung Cancer Stages
- Small Cell Lung Cancer Stages
- Lung Cancer Survival Rates
- Questions to Ask About Lung Cancer
- Surgery for Non-Small Cell Lung Cancer
- Radiofrequency Ablation (RFA) for Non-Small Cell Lung Cancer
- Radiation Therapy for Non-Small Cell Lung Cancer
- Chemotherapy for Non-Small Cell Lung Cancer
- Targeted Drug Therapy for Non-Small Cell Lung Cancer
- Immunotherapy for Non-Small Cell Lung Cancer
- Palliative Procedures for Non-Small Cell Lung Cancer
- Treatment Choices for Non-Small Cell Lung Cancer, by Stage
- Chemotherapy for Small Cell Lung Cancer
- Immunotherapy for Small Cell Lung Cancer
- Radiation Therapy for Small Cell Lung Cancer
- Surgery for Small Cell Lung Cancer
- Palliative Procedures for Small Cell Lung Cancer
- Treatment Choices for Small Cell Lung Cancer, by Stage
- Living as a Lung Cancer Survivor
- Second Cancers After Lung Cancer
- If You Have Non-small Cell Lung Cancer
- If You Have Small Cell Lung Cancer
- Lung Cancer Quiz
- Lung Cancer Videos
Research into the prevention, early detection, and treatment of lung cancer is being done in many medical centers worldwide.
Early detection
Prevention offers the greatest opportunity to fight lung cancer. Decades have passed since the link between smoking and lung cancers became clear, but smoking is still responsible for most lung cancer deaths. Research is continuing on:
- Ways to help people quit smoking and stay tobacco-free through counseling, nicotine replacement, and other medicines
- Ways to convince young people to never start smoking
- Inherited differences in genes that may make some people much more likely to get lung cancer if they smoke or are exposed to someone else’s smoke (secondhand smoke)
- Ways to understand why nonsmokers get lung cancer
Environmental causes
Researchers also continue to look into some of the other causes of lung cancer, such as exposure to asbestos , radon , and diesel exhaust . Finding new ways to limit these exposures could possibly save many more lives.
Diet, nutrition, and medicines
Researchers are looking for ways to use vitamins or medicines to prevent lung cancer in people at high risk, but so far none have been shown to clearly reduce risk.
Some studies have suggested that a diet high in fruits and vegetables may offer some protection, but follow-up studies have not confirmed this. While any protective effect of fruits and vegetables on lung cancer risk is likely to be much smaller than the increased risk from smoking, following the American Cancer Society dietary recommendations (such as getting to at a healthy weight and eating a diet high in fruits, vegetables, and whole grains) may still be helpful.
As mentioned in Can Lung Cancer Be Found Early? , low-dose helical CT (LDCT) is used for lung cancer screening. People who used to or continue to smoke tobacco for a long period of time are considered to be at “high risk” and recommended for screening. Screening lowers the risk of death from lung cancer.
Ongoing studies are looking at new ways to improve early detection of lung cancer:
- Ways to use molecular markers from your body fluid (i.e., sputum or blood) for lung cancer screening
- Ways to use new forms of bronchoscopies for lung cancer screening, such as autofluorescence bronchoscopy
At present, a diagnosis of lung cancer is based on tissue biopsy. Researchers are continuing to look for other ways to help patients achieve an earlier diagnosis, for example:
- Ways to look at blood samples to find tumor cells or parts of tumor cells
- Ways to look at sputum samples to find tumor cells or parts of tumor cells
There continues to be focus and interest in looking at how we can better understand each person’s tumor cells to kill the cells more effectively. While we know a lot about targeted therapy and immunotherapy , there is still much to understand about when these treatments should be offered (before and/or after surgery) and if they should be given in combination with chemotherapy. Furthermore, the answers to these questions will vary depending on the stage of lung cancer, as each stage is treated differently. To learn more about ongoing clinical trials for lung cancer, ask your cancer care team for more information.
Lung Cancer Research Highlights
See examples of research we conduct and fund through grants to see how the American Cancer Society is working toward a world without lung cancer.

The American Cancer Society medical and editorial content team
Our team is made up of doctors and oncology certified nurses with deep knowledge of cancer care as well as editors and translators with extensive experience in medical writing.
Caliman E, Fancelli S, Petroni G, Gatta Michelet MR, Cosso F, Ottanelli C, Mazzoni F, Voltolini L, Pillozzi S, Antonuzzo L. Challenges in the treatment of small cell lung cancer in the era of immunotherapy and molecular classification. Lung Cancer. 2023 Jan;175:88-100. doi: 10.1016/j.lungcan.2022.11.014. Epub 2022 Nov 23. PMID: 36493578.
Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, Paz-Ares L. Lung cancer: current therapies and new targeted treatments. Lancet. 2017 Jan 21;389(10066):299-311. doi: 10.1016/S0140-6736(16)30958-8. Epub 2016 Aug 27. PMID: 27574741.
Meijer JJ, Leonetti A, Airò G, Tiseo M, Rolfo C, Giovannetti E, Vahabi M. Small cell lung cancer: Novel treatments beyond immunotherapy. Semin Cancer Biol. 2022 Nov;86(Pt 2):376-385. doi: 10.1016/j.semcancer.2022.05.004. Epub 2022 May 11. PMID: 35568295.
Petrella F, Rizzo S, Attili I, Passaro A, Zilli T, Martucci F, Bonomo L, Del Grande F, Casiraghi M, De Marinis F, Spaggiari L. Stage III Non-Small-Cell Lung Cancer: An Overview of Treatment Options. Curr Oncol. 2023 Mar 7;30(3):3160-3175. doi: 10.3390/curroncol30030239. PMID: 36975452; PMCID: PMC10047909.
Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 2021 Aug;27(8):1345-1356. doi: 10.1038/s41591-021-01450-2. Epub 2021 Aug 12. PMID: 34385702.
Last Revised: January 29, 2024
American Cancer Society medical information is copyrighted material. For reprint requests, please see our Content Usage Policy .
American Cancer Society Emails
Sign up to stay up-to-date with news, valuable information, and ways to get involved with the American Cancer Society.
More in Lung Cancer
- About Lung Cancer
- Causes, Risk Factors, and Prevention
- Early Detection, Diagnosis, and Staging
- Treatment for Non-small Cell Lung Cancer
- Treatment for Small Cell Lung Cancer
- After Treatment
Help us end cancer as we know it, for everyone.

If this was helpful, donate to help fund patient support services, research, and cancer content updates.
- Help & Support
- Lung Health & Diseases
- Lung Disease Lookup
- Lung Cancer
- Lung Cancer Basics
Lung Cancer Research
Why we need research.
Research provides hope and saves lives. This is especially true when it comes to lung cancer research. Lung cancer research can help develop better treatments, increasing the survival and quality of life for patients. Research can provide a better and longer future for those diagnosed with lung cancer as well and can also ultimately increase the number of survivors living with the disease.
The Lung Association supports lung cancer research so we can help prevent lung cancer cases, and failing that, prolong the lives of lung cancer patients. We have made some progress, but we plan to invest more, as lung cancer remains the leading cause of cancer deaths in the United States.
Our Lung Cancer Research Program
The American Lung Association is committed to funding lung cancer research. As part of our Awards and Grants Program , a large part of funds goes toward research on lung cancer prevention, treatment and quality of life. The primary goal of this lung cancer research program is simple: improve and save lives. The secondary goal is almost as important: To fund top-notch lung cancer researchers at important career crossroads to and gain long-term commitment to lung cancer research. Without the life-long dedication of lung cancer researchers and a large and active community of people trying to improve patients' lives, important and much-needed discoveries would be impossible.
What Research Is Being Done?
Thanks to the medical breakthroughs led by Lung Association researchers and their colleagues worldwide, our lung cancer researchers have made significant contributions to the field of lung cancer. For example, biomarker testing and targeted therapies have helped advance the area of personalized treatment (finding the unique genetic makeup of a person's tumor and developing and using drugs that are designed to be most effective for that patient).
Currently funded Lung Association researchers are:
- Finding out why some nonsmokers develop lung cancer
- Discovering new biomarkers as an early warning system to detect the spread of lung cancer
- Decoding the genetic mechanisms which cause lung cancer
- Understanding how the structure and regulation of chromosomes affect lung cancer
- Understanding sex-differences to customize lung cancer treatments
- Using next generation nanotechnology to target lung cancer
- Using a virus to treat lung cancer
- Overcoming obstacles for cellular immunotherapy against lung cancer
- Improving quality of life and access to healthcare for lung cancer patients after completing therapy
- Reversing drug resistance in lung cancers
- Identifying metabolic alterations in lung cancer-associated cachexia
- Testing methods to increase lung cancer screening among Quitline callers
Lung Cancer Researchers
Visit our Meet the Researchers section to view our lung cancer researchers and their studies.
Research Partnerships
Lung cancer interception dream team.
As a collaborative effort with Stand Up To Cancer and the LUNGevity Foundation, the Lung Cancer Interception Dream Team leverages a new approach to lung cancer prevention: cancer interception.
Learn more about the Dream Team .
How You Can Be a Part of Research
Lung cancer registry.
The Lung Cancer Registry is a database of medical information collected from thousands of lung cancer patients. Researchers study this health data to gain a better understanding of the disease, which can ultimately lead to better outcomes for patients. By participating in the Registry, you not only will help advance lung cancer research, but you will also be able to learn about new clinical trial opportunities that may help in your own treatment program.
Learn more about the Lung Cancer Registry and how to sign up.
Lung Cancer Clinical Trials
Read questions and answers about clinical trials and see our Lung Association listing of current trials .
Download our checklist to help you talk with your doctor about clinical trials.
You can also search the Lung Cancer Clinical Trials Matching Service , provided by a partnership between the American Lung Association and EmergingMed. Patients can search for clinical trials that match their specific diagnosis and treatment history.
Find a Clinical Trial
Learn more about clinical trial programs in your area by searching our list and be sure to discuss with your doctor whether a clinical trial is right for you.
Page last updated: June 7, 2024
A Breath of Fresh Air in Your Inbox
Want updates on the latest lung health news, including COVID-19, research, inspiring stories and health information?
You will now receive email updates from the American Lung Association.
Make a Donation
Your tax-deductible donation funds lung disease and lung cancer research, new treatments, lung health education, and more.
Become a Lung Health Insider
Join over 700,000 people who receive the latest news about lung health, including research, lung disease, air quality, quitting tobacco, inspiring stories and more!
Thank you! You will now receive email updates from the American Lung Association.
Select Your Location
Select your location to view local American Lung Association events and news near you.
Change Language
Lung helpline.
Talk to our lung health experts at the American Lung Association. Our service is free and we are here to help you.
1-800-LUNG-USA
(1-800-586-4872)
This week: the arXiv Accessibility Forum
Help | Advanced Search
Quantitative Biology > Genomics
Title: identification of prognostic biomarkers for stage iii non-small cell lung carcinoma in female nonsmokers using machine learning.
Abstract: Lung cancer remains a leading cause of cancer-related deaths globally, with non-small cell lung cancer (NSCLC) being the most common subtype. This study aimed to identify key biomarkers associated with stage III NSCLC in non-smoking females using gene expression profiling from the GDS3837 dataset. Utilizing XGBoost, a machine learning algorithm, the analysis achieved a strong predictive performance with an AUC score of 0.835. The top biomarkers identified - CCAAT enhancer binding protein alpha (C/EBP-alpha), lactate dehydrogenase A4 (LDHA), UNC-45 myosin chaperone B (UNC-45B), checkpoint kinase 1 (CHK1), and hypoxia-inducible factor 1 subunit alpha (HIF-1-alpha) - have been validated in the literature as being significantly linked to lung cancer. These findings highlight the potential of these biomarkers for early diagnosis and personalized therapy, emphasizing the value of integrating machine learning with molecular profiling in cancer research.
| Comments: | This paper has been accepted for publication in the IEEE ICBASE 2024 conference |
| Subjects: | Genomics (q-bio.GN); Artificial Intelligence (cs.AI); Machine Learning (stat.ML) |
| Cite as: | [q-bio.GN] |
| (or [q-bio.GN] for this version) | |
| Focus to learn more arXiv-issued DOI via DataCite |
Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cancer Control
- v.28; Jan-Dec 2021
Cancer Biology, Epidemiology, and Treatment in the 21st Century: Current Status and Future Challenges From a Biomedical Perspective
Patricia piña-sánchez.
1 Oncology Research Unit, Oncology Hospital, Mexican Institute of Social Security, Mexico
Antonieta Chávez-González
Martha ruiz-tachiquín, eduardo vadillo, alberto monroy-garcía, juan josé montesinos, rocío grajales.
2 Department of Medical Oncology, Oncology Hospital, Mexican Institute of Social Security, Mexico
Marcos Gutiérrez de la Barrera
3 Clinical Research Division, Oncology Hospital, Mexican Institute of Social Security, Mexico
Hector Mayani
Since the second half of the 20th century, our knowledge about the biology of cancer has made extraordinary progress. Today, we understand cancer at the genomic and epigenomic levels, and we have identified the cell that starts neoplastic transformation and characterized the mechanisms for the invasion of other tissues. This knowledge has allowed novel drugs to be designed that act on specific molecular targets, the immune system to be trained and manipulated to increase its efficiency, and ever more effective therapeutic strategies to be developed. Nevertheless, we are still far from winning the war against cancer, and thus biomedical research in oncology must continue to be a global priority. Likewise, there is a need to reduce unequal access to medical services and improve prevention programs, especially in countries with a low human development index.
Introduction
During the last one hundred years, our understanding of the biology of cancer increased in an extraordinary way. 1 - 4 Such a progress has been particularly prompted during the last few decades because of technological and conceptual progress in a variety of fields, including massive next-generation sequencing, inclusion of “omic” sciences, high-resolution microscopy, molecular immunology, flow cytometry, analysis and sequencing of individual cells, new cell culture techniques, and the development of animal models, among others. Nevertheless, there are many questions yet to be answered and many problems to be solved regarding this disease. As a consequence, oncological research must be considered imperative.
Currently, cancer is one of the illnesses that causes more deaths worldwide. 5 According to data reported in 2020 by the World Health Organization (WHO), cancer is the second cause of death throughout the world, with 10 million deaths. 6 Clearly, cancer is still a leading problem worldwide. With this in mind, the objective of this article is to present a multidisciplinary and comprehensive overview of the disease. We will begin by analyzing cancer as a process, focusing on the current state of our knowledge on 4 specific aspects of its biology. Then, we will look at cancer as a global health problem, considering some epidemiological aspects, and discussing treatment, with a special focus on novel therapies. Finally, we present our vision on some of the challenges and perspectives of cancer in the 21 st century.
The Biology of Cancer
Cancer is a disease that begins with genetic and epigenetic alterations occurring in specific cells, some of which can spread and migrate to other tissues. 4 Although the biological processes affected in carcinogenesis and the evolution of neoplasms are many and widely different, we will focus on 4 aspects that are particularly relevant in tumor biology: genomic and epigenomic alterations that lead to cell transformation, the cells where these changes occur, and the processes of invasion and metastasis that, to an important degree, determine tumor aggressiveness.
Cancer Genomics
The genomics of cancer can be defined as the study of the complete sequence of DNA and its expression in tumor cells. Evidently, this study only becomes meaningful when compared to normal cells. The sequencing of the human genome, completed in 2003, was not only groundbreaking with respect to the knowledge of our gene pool, but also changed the way we study cancer. In the post-genomic era, various worldwide endeavors, such as the Human Cancer Genome Project , the Cancer Genome ATLAS (TCGA), the International Cancer Genome Consortium, and the Pan-Cancer Analysis Working Group (PCAWG), have contributed to the characterization of thousands of primary tumors from different neoplasias, generating more than 2.5 petabytes (10 15 ) of genomic, epigenomic, and proteomic information. This has led to the building of databases and analytical tools that are available for the study of cancer from an “omic” perspective, 7 , 8 and it has helped to modify classification and treatment of various neoplasms.
Studies in the past decade, including the work by the PCAWG, have shown that cancer generally begins with a small number of driving mutations (4 or 5 mutations) in particular genes, including oncogenes and tumor-suppressor genes. Mutations in TP53, a tumor-suppressor gene, for example, are found in more than half of all cancer types as an early event, and they are a hallmark of precancerous lesions. 9 - 12 From that point on, the evolution of tumors may take decades, throughout which the mutational spectrum of tumor cells changes significantly. Mutational analysis of more than 19 000 exomes revealed a collection of genomic signatures, some associated with defects in the mechanism of DNA repair. These studies also revealed the importance of alterations in non-coding regions of DNA. Thus, for example, it has been observed that various pathways of cell proliferation and chromatin remodeling are altered by mutations in coding regions, while pathways, such as WNT and NOTCH, can be disrupted by coding and non-coding mutations. To the present date, 19 955 genes that codify for proteins and 25 511 genes for non-coding RNAs have been identified ( https://www.gencodegenes.org/human/stats.html ). Based on this genomic catalogue, the COSMIC (Catalogue Of Somatic Mutations In Cancer) repository, the most robust database to date, has registered 37 288 077 coding mutations, 19 396 fusions, 1 207 190 copy number variants, and 15 642 672 non-coding variants reported up to August 2020 (v92) ( https://cosmic-blog.sanger.ac.uk/cosmic-release-v92/ ).
The genomic approach has accelerated the development of new cancer drugs. Indeed, two of the most relevant initiatives in recent years are ATOM (Accelerating Therapeutics for Opportunities in Medicine), which groups industry, government and academia, with the objective of accelerating the identification of drugs, 13 and the Connectivity Map (CMAP), a collection of transcriptional data obtained from cell lines treated with drugs for the discovery of functional connections between genes, diseases, and drugs. The CMAP 1.0 covered 1300 small molecules and more than 6000 signatures; meanwhile, the CMAP 2.0 with L1000 assay profiled more than 1.3 million samples and approximately 400 000 signatures. 14
The genomic study of tumors has had 2 fundamental contributions. On the one hand, it has allowed the confirmation and expansion of the concept of intratumor heterogeneity 15 , 16 ; and on the other, it has given rise to new classification systems for cancer. Based on the molecular classification developed by expression profiles, together with mutational and epigenomic profiles, a variety of molecular signatures have been identified, leading to the production of various commercial multigene panels. In breast cancer, for example, different panels have been developed, such as Pam50/Prosigna , Blue Print , OncotypeDX , MammaPrint , Prosigna , Endopredict , Breast Cancer Index , Mammostrat, and IHC4 . 17
Currently, the genomic/molecular study of cancer is more closely integrated with clinical practice, from the classification of neoplasms, as in tumors of the nervous system, 18 to its use in prediction, as in breast cancer. 17 Improvement in molecular methods and techniques has allowed the use of smaller amounts of biological material, as well as paraffin-embedded samples for genomic studies, both of which provide a wealth of information. 19 In addition, non-invasive methods, such as liquid biopsies, represent a great opportunity not only for the diagnosis of cancer, but also for follow-up, especially for unresectable tumors. 20
Research for the production of genomic information on cancer is presently dominated by several consortia, which has allowed the generation of a great quantity of data. However, most of these consortia and studies are performed in countries with a high human development index (HDI), and countries with a low HDI are not well represented in these large genomic studies. This is why initiatives such as Human Heredity and Health in Africa (H3Africa) for genomic research in Africa are essential. 21 Generation of new information and technological developments, such as third-generation sequencing, will undoubtedly continue to move forward in a multidisciplinary and complex systems context. However, the existing disparities in access to genomic tools for diagnosis, prognosis, and treatment of cancer will continue to be a pressing challenge at regional and social levels.
Cancer Epigenetics
Epigenetics studies the molecular mechanisms that produce hereditable changes in gene expression, without causing alterations in the DNA sequence. Epigenetic events are of 3 types: methylation of DNA and RNA, histone modification (acetylation, methylation, and phosphorylation), and the expression of non-coding RNA. Epigenetic aberrations can drive carcinogenesis when they alter chromosome conformation and the access to transcriptional machinery and to various regulatory elements (promoters, enhancers, and anchors for interaction with chromatin, for example). These changes may activate oncogenesis and silence tumor-suppressor mechanisms when they modulate coding and non-coding sequences (such as micro-RNAs and long-RNAs). This can then lead to uncontrolled growth, as well as the invasion and metastasis of cancer cells.
While genetic mutations are stable and irreversible, epigenetic alterations are dynamic and reversible; that is, there are several epigenomes, determined by space and time, which cause heterogeneity of the “epigenetic status” of tumors during their development and make them susceptible to environmental stimuli or chemotherapeutic treatment. 22 Epigenomic variability creates differences between cells, and this creates the need to analyze cells at the individual level. In the past, epigenetic analyses measured “average states” of cell populations. These studies revealed general mechanisms, such as the role of epigenetic marks on active or repressed transcriptional states, and established maps of epigenetic composition in a variety of cell types in normal and cancerous tissue. However, these approaches are difficult to use to examine events occurring in heterogeneous cell populations or in uncommon cell types. This has led to the development of new techniques that permit marking of a sequence on the epigenome and improvement in the recovery yield of epigenetic material from individual cells. This has helped to determine changes in DNA, RNA, and histones, chromatin accessibility, and chromosome conformation in a variety of neoplasms. 23 , 24
In cancer, DNA hypomethylation occurs on a global scale, while hypermethylation occurs in specific genomic loci, associated with abnormal nucleosome positioning and chromatin modifications. This information has allowed epigenomic profiles to be established in different types of neoplasms. In turn, these profiles have served as the basis to identify new neoplasm subgroups. For example, in triple negative breast cancer (TNBC), 25 and in hepatocellular carcinoma, 26 DNA methylation profiles have helped to the identification of distinct subgroups with clinical relevance. Epigenetic approaches have also helped to the development of prognostic tests to assess the sensitivity of cancer cells to specific drugs. 27
Epigenetic traits could be used to characterize intratumoral heterogeneity and determine the relevance of such a heterogeneity in clonal evolution and sensitivity to drugs. However, it is clear that heterogeneity is not only determined by genetic and epigenetic diversity resulting from clonal evolution of tumor cells, but also by the various cell populations that form the tumor microenvironment (TME). 28 Consequently, the epigenome of cancer cells is continually remodeled throughout tumorigenesis, during resistance to the activity of drugs, and in metastasis. 29 This makes therapeutic action based on epigenomic profiles difficult, although significant advances in this area have been reported. 30
During carcinogenesis and tumor progression, epigenetic modifications are categorized by their mechanisms of regulation ( Figure 1A ) and the various levels of structural complexity ( Figure 1B ). In addition, the epigenome can be modified by environmental stimuli, stochastic events, and genetic variations that impact the phenotype ( Figure 1C ). 31 , 32 The molecules that take part in these mechanisms/events/variations are therapeutic targets of interest with potential impact on clinical practice. There are studies on a wide variety of epidrugs, either alone or in combination, which improve antitumor efficacy. 33 However, the problems with these drugs must not be underestimated. For a considerable number of epigenetic compounds still being under study, the main challenge is to translate in vitro efficacy of nanomolar (nM) concentrations into well-tolerated and efficient clinical use. 34 The mechanisms of action of epidrugs may not be sufficiently controlled and could lead to diversion of the therapeutic target. 35 It is known that certain epidrugs, such as valproic acid, produce unwanted epigenetic changes 36 ; thus the need for a well-established safety profile before these drugs can be used in clinical therapy. Finally, resistance to certain epidrugs is another relevant problem. 37 , 38

Epigenetics of cancer. (A) Molecular mechanisms. (B) Structural hierarchy of epigenomics. (C) Factors affecting the epigenome. Modified from Refs. 31 and 32 .
As we learn about the epigenome of specific cell populations in cancer patients, a door opens to the evaluation of sensitivity tests and the search for new molecular markers for detection, prognosis, follow-up, and/or response to treatment at various levels of molecular regulation. Likewise, the horizon expands for therapeutic alternatives in oncology with the use of epidrugs, such as pharmacoepigenomic modulators for genes and key pathways, including methylation of promoters and regulation of micro-RNAs involved in chemoresponse and immune response in cancer. 39 There is no doubt that integrated approaches identifying stable pharmagenomic and epigenomic patterns and their relation with expression profiles and genetic functions will be more and more valuable in our fight against cancer.
Cancer Stem Cells
Tumors consist of different populations of neoplastic cells and a variety of elements that form part of the TME, including stromal cells and molecules of the extracellular matrix. 40 Such intratumoral heterogeneity becomes even more complex during clonal variation of transformed cells, as well as influence the elements of the TME have on these cells throughout specific times and places. 41 To explain the origin of cancer cell heterogeneity, 2 models have been put forward. The first proposes that mutations occur at random during development of the tumor in individual neoplastic cells, and this promotes the production of various tumor populations, which acquire specific growth and survival traits that lead them to evolve according to intratumor mechanisms of natural selection. 42 The second model proposes that each tumor begins as a single cell that possess 2 functional properties: it can self-renew and it can produce several types of terminal cells. As these 2 properties are characteristics of somatic stem cells, 43 the cells have been called cancer stem cells (CSCs). 44 According to this model, tumors must have a hierarchical organization, where self-renewing stem cells produce highly proliferating progenitor cells, unable to self-renew but with a high proliferation potential. The latter, in turn, give rise to terminal cells. 45 Current evidence indicates that both models may coexist in tumor progression. In agreement with this idea, new subclones could be produced as a result of a lack of genetic stability and mutational changes, in addition to the heterogeneity derived from the initial CSC and its descendants. Thus, in each tumor, a set of neoplastic cells with different genetic and epigenetic traits may be found, which would provide different phenotypic properties. 46
The CSC concept was originally presented in a model of acute myeloid leukemia. 47 The presence of CSCs was later proved in chronic myeloid leukemia, breast cancer, tumors of the central nervous system, lung cancer, colon cancer, liver cancer, prostate cancer, pancreatic cancer, melanoma, and cancer of the head and neck, amongst others. In all of these cases, detection of CSCs was based on separation of several cell populations according to expression of specific surface markers, such as CD133, CD44, CD24, CD117, and CD15. 48 It is noteworthy that in some solid tumors, and even in some hematopoietic ones, a combination of specific markers that allow the isolation of CSCs has not been found. Interestingly, in such tumors, a high percentage of cells with the capacity to start secondary tumors has been observed; thus, the terms Tumor Initiating Cells (TIC) or Leukemia Initiating Cells (LIC) have been adopted. 46
A relevant aspect of the biology of CSCs is that, just like normal stem cells, they can self-renew. Such self-renewal guarantees the maintenance or expansion of the tumor stem cell population. Another trait CSCs share with normal stem cells is their quiescence, first described in chronic myeloid leukemia. 49 The persistence of quiescent CSCs in solid tumors has been recently described in colorectal cancer, where quiescent clones can become dominant after therapy with oxaliplatin. 50 In non-hierarchical tumors, such as melanoma, the existence of slow-cycling cells that are resistant to antimitogenic agents has also been proved. 51 Such experimental evidence supports the idea that quiescent CSCs or TICs are responsible for both tumor resistance to antineoplastic drugs and clinical relapse after initial therapeutic success.
In addition to quiescence, CSCs use other mechanisms to resist the action of chemotherapeutic drugs. One of these is their increased numbers: upon diagnosis, a high number of CSCs are observed in most analyzed tumors, making treatment unable to destroy all of them. On the other hand, CSCs have a high number of molecular pumps that expulse drugs, as well as high numbers of antiapoptotic molecules. In addition, they have very efficient mechanisms to repair DNA damage. In general, these cells show changes in a variety of signaling pathways involved in proliferation, survival, differentiation, and self-renewal. It is worth highlighting that in recent years, many of these pathways have become potential therapeutic targets in the elimination of CSCs. 52 Another aspect that is highly relevant in understanding the biological behavior of CSCs is that they require a specific site for their development within the tissue where they are found that can provide whatever is needed for their survival and growth. These sites, known as niches, are made of various cells, both tumor and non-tumor, as well as a variety of non-cellular elements (extracellular matrix [ECM], soluble cytokines, ion concentration gradients, etc.), capable of regulating the physiology of CSCs in order to promote their expansion, the invasion of adjacent tissues, and metastasis. 53
It is important to consider that although a large number of surface markers have been identified that allow us to enrich and prospectively follow tumor stem cell populations, to this day there is no combination of markers that allows us to find these populations in all tumors, and it is yet unclear if all tumors present them. In this regard, it is necessary to develop new purification strategies based on the gene expression profiles of these cells, so that tumor heterogeneity is taken into account, as it is evident that a tumor can include multiple clones of CSCs that, in spite of being functional, are genetically different, and that these clones can vary throughout space (occupying different microenvironments and niches) and time (during the progression of a range of tumor stages). Such strategies, in addition to new in vitro and in vivo assays, will allow the development of new and improved CSC elimination strategies. This will certainly have an impact on the development of more efficient therapeutic alternatives.
Invasion and Metastasis
Nearly 90% of the mortality associated with cancer is related to metastasis. 54 This consists of a cascade of events ( Figure 2 ) that begins with the local invasion of a tumor into surrounding tissues, followed by intravasation of tumor cells into the blood stream or lymphatic circulation. Extravasation of neoplastic cells in areas distant from the primary tumor then leads to the formation of one or more micrometastatic lesions which subsequently proliferate to form clinically detectable lesions. 4 The cells that are able to produce metastasis must acquire migratory characteristics, which occur by a process known as epithelial–mesenchymal transition (EMT), that is, the partial loss of epithelial characteristics and the acquirement of mesenchymal traits. 55

Invasion and metastasis cascade. Invasion and metastasis can occur early or late during tumor progression. In either case, invasion to adjacent tissues is driven by stem-like cells (cancer stem cells) that acquire the epithelial–mesenchymal transition (EMT) (1). Once they reach sites adjacent to blood vessels, tumor cells (individually or in clusters) enter the blood (2). Tumor cells in circulation can adhere to endothelium and extravasation takes place (3). Other mechanisms alternative to extravasation can exist, such as angiopelosis, in which clusters of tumor cells are internalized by the endothelium. Furthermore, at certain sites, tumor cells can obstruct microvasculature and initiate a metastatic lesion right there. Sometimes, a tumor cells that has just exit circulation goes into an MET in order to become quiescent (4). Inflammatory signals can activate quiescent metastatic cells that will proliferate and generate a clinically detectable lesion (5).
Although several of the factors involved in this process are currently known, many issues are still unsolved. For instance, it has not yet been possible to monitor in vivo the specific moment when it occurs 54 ; the microenvironmental factors of the primary tumor that promote such a transition are not known with precision; and the exact moment during tumor evolution in which one cell or a cluster of cells begin to migrate to distant areas, is also unknown. The wide range of possibilities offered by intra- and inter-tumoral heterogeneity 56 stands in the way of suggesting a generalized strategy that could resolve this complication.
It was previously believed that metastasis was only produced in late stages of tumor progression; however, recent studies indicate that EMT and metastasis can occur during the early course of the disease. In pancreatic cancer, for example, cells going through EMT are able to colonize and form metastatic lesions in the liver in the first stages of the disease. 52 , 57 Metastatic cell clusters circulating in peripheral blood (PB) are prone to generate a metastatic site, compared to individual tumor cells. 58 , 59 In this regard, novel strategies, such as the use of micro-RNAs, are being assessed in order to diminish induction of EMT. 60 It must be mentioned, however, that the metastatic process seems to be even more complex, with alternative pathways that do not involve EMT. 61 , 62
A crucial stage in the process of metastasis is the intravasation of tumor cells (alone or in clusters) towards the blood stream and/or lymphatic circulation. 63 These mechanisms are also under intensive research because blocking them could allow the control of spreading of the primary tumor. In PB or lymphatic circulation, tumor cells travel to distant parts for the potential formation of a metastatic lesion. During their journey, these cells must stand the pressure of blood flow and escape interaction with natural killer (NK) cells . 64 To avoid them, tumor cells often cover themselves with thrombocytes and also produce factors such as VEGF, angiopoietin-2, angiopoietin-4, and CCL2 that are involved in the induction of vascular permeability. 54 , 65 Neutrophils also contribute to lung metastasis in the bloodstream by secreting IL-1β and metalloproteases to facilitate extravasation of tumor cells. 64
The next step in the process of metastasis is extravasation, for which tumor cells, alone or in clusters, can use various mechanisms, including a recently described process known as angiopellosis that involves restructuring the endothelial barrier to internalize one or several cells into a tissue. 66 The study of leukocyte extravasation has contributed to a more detailed knowledge of this process, in such a way that some of the proposed strategies to avoid extravasation include the use of integrin inhibitors, molecules that are vital for rolling, adhesion, and extravasation of tumor cells. 67 , 68 Another strategy that has therapeutic potential is the use of antibodies that strengthen vascular integrity to obstruct transendothelial migration of tumor cells and aid in their destruction in PB. 69
Following extravasation, tumor cells can return to an epithelial phenotype, a process known as mesenchymal–epithelial transition and may remain inactive for several years. They do this by competing for specialized niches, like those in the bone marrow, brain, and intestinal mucosa, which provide signals through the Notch and Wnt pathways. 70 Through the action of the Wnt pathway, tumor cells enter a slow state of the cell cycle and induce the expression of molecules that inhibit the cytotoxic function of NK cells. 71 The extravasated tumor cell that is in a quiescent state must comply with 2 traits typical of stem cells: they must have the capacity to self-renew and to generate all of the cells that form the secondary tumor.
There are still several questions regarding the metastatic process. One of the persisting debates at present is if EMT is essential for metastasis or if it plays a more important role in chemoresistance. 61 , 62 It is equally important to know if there is a pattern in each tumor for the production of cells with the capacity to carry out EMT. In order to control metastasis, it is fundamental to know what triggers acquisition of the migratory phenotype and the intrinsic factors determining this transition. Furthermore, it is essential to know if mutations associated with the primary tumor or the variety of epigenetic changes are involved in this process. 55 It is clear that metastatic cells have affinity for certain tissues, depending on the nature of the primary tumor (seed and soil hypothesis). This may be caused by factors such as the location and the direction of the bloodstream or lymphatic fluid, but also by conditioning of premetastatic niches at a distance (due to the large number of soluble factors secreted by the tumor and the recruitment of cells of the immune system to those sites). 72 We have yet to identify and characterize all of the elements that participate in this process. Deciphering them will be of upmost importance from a therapeutic point of view.
Epidemiology of Cancer
Cancer is the second cause of death worldwide; today one of every 6 deaths is due to a type of cancer. According to the International Agency for Research on Cancer (IARC), in 2020 there were approximately 19.3 million new cases of cancer, and 10 million deaths by this disease, 6 while 23.8 million cases and 13.0 million deaths are projected to occur by 2030. 73 In this regard, it is clear the increasing role that environmental factors—including environmental pollutants and processed food—play as cancer inducers and promoters. 74 The types of cancer that produce the greatest numbers of cases and deaths worldwide are indicated in Table 1 . 6
Total Numbers of Cancer Cases and Deaths Worldwide in 2020 by Cancer Type (According to the Global Cancer Observatory, IARC).
| Cases | ||
|---|---|---|
| Both sexes | Women | Men |
| Breast (2.26 million) | Breast (2.26 million) | Lung (1.43 million) |
| Lung (2.20 million) | Colorectal (865 000) | Prostate (1.41 million) |
| Colorectal (1.93 million) | Lung (770 000) | Colorectal (1.06 million) |
| Prostate (1.41 million) | Cervical (604 000) | Stomach (719 000) |
| Stomach (1.08 million) | Thyroid (448 000) | Liver (632 000) |
| Deaths | ||
|---|---|---|
| Both sexes | Women | Men |
| Lung (1.79 million) | Breast (684 000) | Lung (1.18 million) |
| Colorectal (935 000) | Lung (607 000) | Liver (577 000) |
| Liver (830 000) | Colorectal (419 000) | Colorectal (515 000) |
| Stomach (768 000) | Cervical (341 000) | Stomach (502 000) |
| Breast (684 000) | Stomach (266 000) | Prostate (375 000) |
Data presented on this table were obtained from Ref. 6.
As shown in Figure 3 , lung, breast, prostate, and colorectal cancer are the most common throughout the world, and they are mostly concentrated in countries of high to very high human development index (HDI). Although breast, prostate, and colorectal cancer have a high incidence, the number of deaths they cause is proportionally low, mostly reflecting the great progress made in their control. However, these data also reveal the types of cancer that require further effort in prevention, precise early detection avoiding overdiagnosis, and efficient treatment. This is the case of liver, lung, esophageal, and pancreatic cancer, where the difference between the number of cases and deaths is smaller ( Figure 3B ). Social and economic transition in several countries has had an impact on reducing the incidence of neoplasms associated with infection and simultaneously produced an increase in the types related to reproductive, dietary, and hormonal factors. 75

Incidence and mortality for some types of cancer in the world. (A) Estimated number of cases and deaths in 2020 for the most frequent cancer types worldwide. (B) Incidence and mortality rates, normalized according to age, for the most frequent cancer types in countries with very high/& high (VH&H; blue) and/low and middle (L&M; red) Human Development Index (HDI). Data include both genders and all ages. Data according to https://gco.iarc.fr/today , as of June 10, 2021.
In the past 3 decades, cancer mortality rates have fallen in high HDI countries, with the exception of pancreatic cancer, and lung cancer in women. Nevertheless, changes in the incidence of cancer do not show the same consistency, possibly due to variables such as the possibility of early detection, exposure to risk factors, or genetic predisposition. 76 , 77 Countries such as Australia, Canada, Denmark, Ireland, New Zealand, Norway, and the United Kingdom have reported a reduction in incidence and mortality in cancer of the stomach, colon, lung, and ovary, as well as an increase in survival. 78 Changes in modifiable risk factors, such as the use of tobacco, have played an important role in prevention. In this respect, it has been estimated that decline in tobacco use can explain between 35% and 45% of the reduction in cancer mortality rates, 79 while the fall in incidence and mortality due to stomach cancer can be attributed partly to the control of Helicobacter pylori infection. 80 Another key factor in the fall of mortality rates in developed countries has been an increase in early detection as a result of screening programs, as in breast and prostate cancer, which have had their mortality rates decreased dramatically in spite of an increase in their incidence. 76
Another important improvement observed in recent decades is the increase in survival rates, particularly in high HDI countries. In the USA, for example, survival rates for patients with prostate cancer at 5 years after initial diagnosis was 28% during 1947–1951; 69% during 1975–1977, and 100% during 2003–2009. Something similar occurred with breast cancer, with a 5-year survival rate of 54% in 1947–1951, 75% in 1975–1977, and 90% in 2003–2009. 81 In the CONCORD 3 version, age-standardize 5-year survival for patients with breast cancer in the USA during 2010–2014 was 90%, and 97% for prostate cancer patients. 82 Importantly, even among high HDI countries, significant differences have been identified in survival rates, being stage of disease at diagnosis, time for access to effective treatment, and comorbidities, the main factors influencing survival in these nations. 78 Unfortunately, survival rates in low HDI countries are significantly lower due to several factors, including lack of information, deficient screening and early detection programs, limited access to treatment, and suboptimal cancer registration. 82 It should be noted that in countries with low to middle HDI, neoplasms with the greatest incidence are those affecting women (breast and cervical cancer), which reflects not only a problem with access to health services, but also a serious inequality issue that involves social, cultural, and even religious obstacles. 83
Up to 42% of incident cases and 47% of deaths by cancer in the USA are due to potentially modifiable risk factors such as use of tobacco, physical activity, diet, and infection. 84 It has been calculated that 2.4 million deaths by cancer, mostly of the lung, can be attributed to tobacco. 73 In 2020, the incidence rate of lung cancer in Western Africa was 2.2, whereas in Polynesia and Eastern Asia was 37.3 and 34.4, respectively. 6 In contrast, the global burden of cancer associated with infection was 15.4%, but in Sub-Saharan Africa it was 30%. 85 Likewise, the incidence of cervical cancer in Eastern Africa was 40.1, in contrast with the USA and Canada that have a rate of 6.2. This makes it clear that one of the challenges we face is the reduction of the risk factors that are potentially modifiable and associated with specific types of cancer.
Improvement of survival rates and its disparities worldwide are also important challenges. Five-year survival for breast cancer—diagnosed during 2010-2014— in the USA, for example, was 90%, whereas in countries like South Africa it was 40%. 82 Childhood leukemia in the USA and several European countries shows a 5-year survival of 90%, while in Latin-American countries it is 50–76%. 86 Interestingly, there are neoplasms, such as pancreatic cancer, for which there has been no significant increase in survival, which remains low (5–15%) both in developed and developing countries. 82
Although data reported on global incidence and mortality gives a general overview on the epidemiology of cancer, it is important to note that there are great differences in coverage of cancer registries worldwide. To date, only 1 out of every 3 countries reports high quality data on the incidence of cancer. 87 For the past 50 years, the IARC has supported population-based cancer registries; however, more than one-third of the countries belonging to the WHO, mainly countries of low and middle income (LMIC), have no data on more than half of the 18 indicators of sustainable development goals. 88 High quality cancer registries only cover 4% of the population in Africa, 8% in Asia, and 7% in Latin America, contrasting with 83% in the USA and Canada, and 33% in Europe. 89 In response to this situation, the Global Initiative for Cancer Registry Development was created in 2012 to generate improved infrastructure to permit greater coverage and better quality registries, especially in countries with low and middle HDI. 88 It is expected that initiatives of this sort in the coming years will allow more and better information to guide strategies for the control of cancer worldwide, especially in developing regions. This will enable survival to be measured over longer periods of time (10, 15, or 20 years), as an effective measure in the control of cancer. The WHO has established as a target for 2025 to reduce deaths by cancer and other non-transmissible diseases by 25% in the population between the ages of 30–69; such an effort requires not only effective prevention measures to reduce incidence, but also more efficient health systems to diminish mortality and increase survival. At the moment, it is an even greater challenge because of the effects of the COVID-19 pandemic which has negatively impacted cancer prevention and health services. 90
Oncologic Treatments
A general perspective.
At the beginning of the 20th century, cancer treatment, specifically treatment of solid tumors, was based fundamentally on surgical resection of tumors, which together with other methods for local control, such as cauterization, had been used since ancient times. 91 At that time, there was an ongoing burst of clinical observations along with interventions sustained on fundamental knowledge about physics, chemistry, and biology. In the final years of the 19 th century and the first half of the 20th, these technological developments gave rise to radiotherapy, hormone therapy, and chemotherapy. 92 - 94 Simultaneously, immunotherapy was also developed, although usually on a smaller scale, in light of the overwhelming progress of chemotherapy and radiotherapy. 95
Thus began the development and expansion of disciplines based on these approaches (surgery, radiotherapy, chemotherapy, hormone therapy, and immunotherapy), with their application evolving ever more rapidly up to their current uses. Today, there is a wide range of therapeutic tools for the care of cancer patients. These include elements that emerged empirically, arising from observations of their effects in various medical fields, as well as drugs that were designed to block processes and pathways that form part of the physiopathology of one or more neoplasms according to knowledge of specific molecular alterations. A classic example of the first sort of tool is mustard gas, originally used as a weapon in war, 96 but when applied for medical purposes, marked the beginning of the use of chemicals in the treatment of malignant neoplasms, that is, chemotherapy. 94 A clear example of the second case is imatinib, designed specifically to selectively inhibit a molecular alteration in chronic myeloid leukemia: the Bcr-Abl oncoprotein. 97
It is on this foundation that today the 5 areas mentioned previously coexist and complement one another. The general framework that motivates this amalgam and guides its development is precision medicine, founded on the interaction of basic and clinical science. In the forecasts for development in each of these fields, surgery is expected to continue to be the fundamental approach for primary tumors in the foreseeable future, as well as when neoplastic disease in the patient is limited, or can be limited by applying systemic or regional elements, before and/or after surgical resection, and it can be reasonably anticipated for the patient to have a significant period free from disease or even to be cured. With regards to technology, intensive exploration of robotic surgery is contemplated. 98
The technological possibilities for radiotherapy have progressed in such a way that it is now possible to radiate neoplastic tissue with an extraordinary level of precision, and therefore avoid damage to healthy tissue. 99 This allows administration of large doses of ionizing radiation in one or a few fractions, what is known as “radiosurgery.” The greatest challenges to the efficacy of this approach are related to radio-resistance in certain neoplasms. Most efforts regarding research in this field are concentrated on understanding the underlying biological mechanisms of the phenomenon and their potential control through radiosensitizers. 100
“Traditional” chemotherapy, based on the use of compounds obtained from plants and other natural products, acting in a non-specific manner on both neoplastic and healthy tissues with a high proliferation rate, continues to prevail. 101 The family of chemotherapeutic drugs currently includes alkylating agents, antimetabolites, anti-topoisomerase agents, and anti-microtubules. Within the pharmacologic perspective, the objective is to attain a high concentration or activity of such molecules in specific tissues while avoiding their accumulation in others, in order to achieve an increase in effectiveness and a reduction in toxicity. This has been possible with the use of viral vectors, for example, that are able to limit their replication in neoplastic tissues, and activate prodrugs of normally nonspecific agents, like cyclophosphamide, exclusively in those specific areas. 102 More broadly, chemotherapy also includes a subgroup of substances, known as molecular targeted therapy, that affect processes in a more direct and specific manner, which will be mentioned later.
There is no doubt that immunotherapy—to be explored next—is one of the therapeutic fields where development has been greatest in recent decades and one that has produced enormous expectation in cancer treatment. 103 Likewise, cell therapy, based on the use of immune cells or stem cells, has come to complement the oncologic therapeutic arsenal. 43 Each and every one of the therapeutic fields that have arisen in oncology to this day continue to prevail and evolve. Interestingly, the foreseeable future for the development of cancer treatment contemplates these approaches in a joint and complementary manner, within the general framework of precision medicine, 104 and sustained by knowledge of the biological mechanisms involved in the appearance and progression of neoplasms. 105 , 106
Immunotherapy
Stimulating the immune system to treat cancer patients has been a historical objective in the field of oncology. Since the early work of William Coley 107 to the achievements reached at the end of the 20 th century, scientific findings and technological developments paved the way to searching for new immunotherapeutic strategies. Recombinant DNA technology allowed the synthesis of cytokines, such as interferon-alpha (IFN-α) and interleukin 2 (IL-2), which were authorized by the US Food and Drug Administration (FDA) for the treatment of hairy cell leukemia in 1986, 108 as well as kidney cancer and metastatic melanoma in 1992 and 1998, respectively. 109
The first therapeutic vaccine against cancer, based on the use of autologous dendritic cells (DCs), was approved by the FDA against prostate cancer in 2010. However, progress in the field of immunotherapy against cancer was stalled in the first decade of the present century, mostly due to failure of several vaccines in clinical trials. In many cases, application of these vaccines was detained by the complexity and cost involved in their production. Nevertheless, with the coming of the concept of immune checkpoint control, and the demonstration of the relevance of molecules such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), and programmed cell death molecule-1 (PD-1), immunotherapy against cancer recovered its global relevance. In 2011, the monoclonal antibody (mAb) ipilimumab, specific to the CTLA-4 molecule, was the first checkpoint inhibitor (CPI) approved for the treatment of advanced melanoma. 110 Later, inhibitory mAbs for PD-1, or for the PD-1 ligand (PD-L1), 111 as well as the production of T cells with chimeric receptors for antigen recognition (CAR-T), 112 which have been approved to treat various types of cancer, including melanoma, non-small cell lung cancer (NSCLC), head and neck cancer, bladder cancer, renal cell carcinoma (RCC), and hepatocellular carcinoma, among others, have changed the paradigm of cancer treatment.
In spite of the current use of anti-CTLA-4 and anti-PD-L1 mAbs, only a subgroup of patients has responded favorably to these CPIs, and the number of patients achieving clinical benefit is still small. It has been estimated that more than 70% of patients with solid tumors do not respond to CPI immunotherapy because either they show primary resistance, or after responding favorably, develop resistance to treatment. 113 In this regard, it is important to mention that in recent years very important steps have been taken to identify the intrinsic and extrinsic mechanisms that mediate resistance to CPI immunotherapy. 114 Intrinsic mechanisms include changes in the antitumor immune response pathways, such as faulty processing and presentation of antigens by APCs, activation of T cells for tumor cell destruction, and changes in tumor cells that lead to an immunosuppressive TME. Extrinsic factors include the presence of immunosuppressive cells in the local TME, such as regulatory T cells, myeloid-derived suppressor cells (MDSC), mesenchymal stem/stromal cells (MSCs), and type 2 macrophages (M2), in addition to immunosuppressive cytokines.
On the other hand, classification of solid tumors as “hot,” “cold,” or “excluded,” depending on T cell infiltrates and the contact of such infiltrates with tumor cells, as well as those that present high tumor mutation burden (TMB), have redirected immunotherapy towards 3 main strategies 115 ( Table 2 ): (1) Making T-cell antitumor response more effective, using checkpoint inhibitors complementary to anti-CTLA-4 and anti-PD-L1, such as LAG3, Tim-3, and TIGT, as well as using CAR-T cells against tumor antigens. (2) Activating tumor-associated myeloid cells including monocytes, granulocytes, macrophages, and DC lineages, found at several frequencies within human solid tumors. (3) Regulating the biochemical pathways in TME that produce high concentrations of immunosuppressive molecules, such as kynurenine, a product of tryptophan metabolism, through the activity of indoleamine 2,3 dioxygenase; or adenosine, a product of ATP hydrolysis by the activity of the enzyme 5’nucleotidase (CD73). 116
Current Strategies to Stimulate the Immune Response for Antitumor Immunotherapy.
| Strategies | T cells | Myeloid cells | TME |
|---|---|---|---|
| Lymph node | Anti-CTLA4 | TNF-α | |
| To improve tumor antigen presentation by APCs | Anti-CD137 | IFN-α | |
| To optimize effector T-cell activation | Anti-OX40 | IL-1 | |
| Anti-CD27/CD70 | GM-CSF | ||
| HVEM | CD40L/CD40 | ||
| GITR | CDN | ||
| L-2 | ATP | ||
| IL-12 | HMGB1 | ||
| TLR | |||
| STING | |||
| RIG-1/MDA-5 | |||
| Blood vessel | CX3CL1 | ||
| To improve T-cell traffic to tumors | CXCL9 | ||
| To favor T-cell infiltration into tumors | CXCL10 | ||
| Transference of T cells bearing antigen-specific receptor | CCL5 | ||
| LFA1/ICAM1 | |||
| Selectins | |||
| CAR-T cell | |||
| TCR-T cell | |||
| Tumor | Anti-PD-L1 | Anti-CSF1/CSF1R | Anti-VEGF |
| To improve tumor antigen uptake by APCs | Anti-CTLA-4 | Anti-CCR2 | Inhibitors of IDO anti-CD73 |
| To improve recognition and killing of tumor cells by T cells | Anti-LAG-3 | PI3Kγ | ARs antagonists |
| Anti-TIM-3 | |||
| Anti-TIGIT | |||
| TNFR-agonists | |||
| IL-2 | |||
| IL-10 |
Abbreviations: TME, tumor microenvironment; IL, interleukin; TNF, Tumor Necrosis Factor; TNFR, TNF-receptor; CD137, receptor–co-stimulator of the TNFR family; OX40, member number 4 of the TNFR superfamily; CD27/CD70, member of the TNFR superfamily; CD40/CD40L, antigen-presenting cells (APC) co-stimulator and its ligand; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; STING, IFN genes-stimulator; RIG-I, retinoic acid inducible gene-I; MDA5, melanoma differentiation-associated protein 5; CDN, cyclic dinucleotide; ATP, adenosine triphosphate; HMGB1, high mobility group B1 protein; TLR, Toll-like receptor; HVEM, Herpes virus entry mediator; GITR, glucocorticoid-induced TNFR family-related gene; CTLA4, cytotoxic T lymphocyte antigen 4; PD-L1, programmed death ligand-1; TIGIT, T-cell immunoreceptor with immunoglobulin and tyrosine-based inhibition motives; CSF1/CSF1R, colony-stimulating factor-1 and its receptor; CCR2, Type 2 chemokine receptor; PI3Kγ, Phosphoinositide 3-Kinase γ; CXCL/CCL, chemokine ligands; LFA1, lymphocyte function-associated antigen 1; ICAM1, intercellular adhesion molecule 1; VEGF, vascular endothelial growth factor; IDO, indolamine 2,3-dioxigenase; TGF, transforming growth factor; LAG-3, lymphocyte-activation gene 3 protein; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; CD73, 5´nucleotidase; ARs, adenosine receptors; Selectins, cell adhesion molecules; CAR-T, chimeric antigen receptor T cell; TCR-T, T-cell receptor engineered T cell.
Apart from the problems associated with its efficacy (only a small group of patients respond to it), immunotherapy faces several challenges related to its safety. In other words, immunotherapy can induce adverse events in patients, such as autoimmunity, where healthy tissues are attacked, or cytokine release syndrome and vascular leak syndrome, as observed with the use of IL-2, both of which lead to serious hypotension, fever, renal failure, and other adverse events that are potentially lethal. The main challenges to be faced by immunotherapy in the future will require the combined efforts of basic and clinical scientists, with the objective of accelerating the understanding of the complex interactions between cancer and the immune system, and improve treatment options for patients. Better comprehension of immune phenotypes in tumors, beyond the state of PD-L1 and TME, will be relevant to increase immunotherapy efficacy. In this context, the identification of precise tumor antigenicity biomarkers by means of new technologies, such as complete genome sequencing, single cell sequencing, and epigenetic analysis to identify sites or subclones typical in drug resistance, as well as activation, traffic and infiltration of effector cells of the immune response, and regulation of TME mechanisms, may help define patient populations that are good candidates for specific therapies and therapeutic combinations. 117 , 118 Likewise, the use of agents that can induce specific activation and modulation of the response of T cells in tumor tissue, will help improve efficacy and safety profiles that can lead to better clinical results.
Molecular Targeted Therapy
For over 30 years, and based on the progress in our knowledge of tumor biology and its mechanisms, there has been a search for therapeutic alternatives that would allow spread and growth of tumors to be slowed down by blocking specific molecules. This approach is known as molecular targeted therapy. 119 Among the elements generally used as molecular targets there are transcription factors, cytokines, membrane receptors, molecules involved in a variety of signaling pathways, apoptosis modulators, promoters of angiogenesis, and cell cycle regulators. 120
Imatinib, a tyrosine kinase inhibitor for the treatment of chronic myeloid leukemia, became the first targeted therapy in the final years of the 1990s. 97 From then on, new drugs have been developed by design, and today more than 60 targeted therapies have been approved by the FDA for the treatment of a variety of cancers ( Table 3 ). 121 This has had a significant impact on progression-free survival and global survival in neoplasms such as non-small cell lung cancer, breast cancer, renal cancer, and melanoma.
FDA Approved Molecular Targeted Therapies for the Treatment of Solid Tumors.
| Drug | Therapeutic target | Indications | Biomarkers |
|---|---|---|---|
| Abemaciclib | CDK4/6 inhibitor | Breast cancer | ER+/PR+ |
| Abiraterone | Anti-androgen | Prostate cancer | AR+ |
| Afatinib | TKI anti-ErbB, EGFR (ErbB1), HER2 (ErbB2), ErbB3, ErbB4 | NSCLC | EGFR mutated |
| Deletion of exon 19 | |||
| Substitution in exon 21 (L858R) | |||
| Aflibercept | Anti-VEGF fusion protein | Colorectal cancer | |
| Alectinib | Anti-ALK TKI | NSCLC | ALK+ |
| Alpelisib | PI3K inhibitor | Breast cancer | PI3K mutated |
| Apalutamide | Anti-androgen | Prostate cancer | AR+ |
| Atezolizumab | Anti-PD-L1 mAb | Breast cancer | PD-L1 |
| Hepatocellular carcinoma | |||
| NSCLC | |||
| Bladder cancer | |||
| Avapritinib | Kinase inhibitor | GIST | PDGFRA mutated in exon 18 (D842V) |
| Avelumab | Anti-PD-L1 mAb | Renal cancer | PD-L1 |
| Bladder cancer | |||
| Neuroendocrine tumors | |||
| Axitinib | Anti-VEGF TKI | Renal cancer | |
| Bevacizumab | Anti-VEGF mAb | CNS tumors | |
| Ovarian cancer | |||
| Cervical cancer | |||
| Colorectal cancer | |||
| Hepatocellular carcinoma | |||
| NSCLC | |||
| Renal cancer | |||
| Brigatinib | Anti-ALK TKI | NSCLC | ALK+ |
| Cabozantinib | TKR inhibitor: anti-MET, anti-VEGF, anti-RET, ROS1, MER, KIT | Renal cancer | |
| Hepatocellular carcinoma | |||
| Thyroid cancer | |||
| Ceritinib | Anti-ALK TKI | NSCLC | ALK+ |
| Cetuximab | Anti-EGFR mAb | Colorectal cancer | KRAS |
| Head and Neck cancer | EGFR+ | ||
| Crizotinib | Anti-ALK TKI | NSCLC | ALK+, ROS1+ |
| Dabrafenib | BRAF inhibitor | NSCLC | BRAF-V600E, V600K |
| Thyroid cancer | |||
| Melanoma | |||
| Dacomitinib | Anti-EGFR TKI | NSCLC | EGFR+ |
| Darolutamide | Anti-androgen | Prostate cancer | AR+ |
| Durvalumab | Anti-PD-L1 mAb | NSCLC | PD-L1 |
| Bladder cancer | |||
| Encorafenib | BRAF inhibitor | Colorectal cancer | BRAF-V600E |
| Melanoma | |||
| Entrectinib | Anti-ROS1 TKI | NSCLC | ROS1+ |
| Enzalutamide | Anti-androgen | Prostate cancer | AR+ |
| Erdafitinib | Anti-FGFR-1 TKI | Bladder cancer | |
| Erlotinib | Anti-EGFR TKI | NSCLC | EGFR mutated |
| Pancreatic caner | Deletion of exon 19 | ||
| Substitution in exon 21 (L858R) | |||
| Everolimus | mTOR inhibitor | CNS tumors | |
| Pancreatic cancer | |||
| Breast cancer | |||
| Renal cancer | |||
| Fulvestrant | ER antagonist | Breast cancer | ER+/PR+ |
| Gefitinib | Anti-EGFR TKI | NSCLC | EGFR mutated |
| Deletion of exon 19 | |||
| Substitution in exon 21 (L858R) | |||
| Imatinib | Anti-KIT TKI | GIST | KIT+ |
| Dermatofibroma protuberans | |||
| Ipilimumab | Anti-CTLA-4 mAb | Colorectal cancer | |
| Hepatocellular carcinoma | |||
| NSCLC | |||
| Melanoma | |||
| Renal cancer | |||
| Lapatinib | TKI: anti-EGFR, anti-HER2 | Breast cancer | ERBB2 over-expression or amplification |
| Lenvatinib | TKR: anti-VEGF, VEGFR1 (FLT1), VEGFR2 (KDR) y VEGFR3 (FLT4); (FGF) FGFR1, 2, 3 y 4, PDGF, PDGFRA, KIT, RET | Endometrial cancer | |
| Hepatocellular carcinoma | |||
| Renal cancer | |||
| Thyroid cancer | |||
| Lorlatinib | TKI: anti-ALK, anti-ROS2 | NSCLC | ALK+, ROS1+ |
| Necitumumab | Anti-EGFR mAb | NSCLC | EGFR+ |
| Neratinib | Anti-HER2 TKI | ||
| Anti-EGFR | Breast cancer | ERBB2 over-expression or amplification | |
| Niraparib | PARP inhibitor | Ovarian cancer | BRCA1/2 mutations |
| Fallopian tube cancer | Homologous recombination deficiency | ||
| Peritoneal cancer | |||
| Nivolumab | Anti-PD-1 mAb | Colorectal cancer | PD-1 |
| Esophageal cancer | |||
| Hepatocellular carcinoma | |||
| NSCLC | |||
| Melanoma | |||
| Renal cancer | |||
| Bladder cancer | |||
| Head and Neck cancer | |||
| Olparib | PARP inhibitor | Breast cancer | BRCA1/2 mutations |
| Ovarian cancer | |||
| Pancreatic cancer | |||
| Prostate cancer | |||
| Osimertinib | Anti-EGFR TKI | NSCLC | EGFR-T790M |
| Palbociclib | CDK4/6 inhibitor | Breast cancer | RE+/RP+ |
| Pantitumumab | Anti-EGFR mAb | Colorectal cancer | KRAS |
| EGFR+ | |||
| Pazopanib | TKI: Anti-VEGF, anti-PDGFR, anti-FGFR, anti-cKIT | Renal cancer | |
| Soft tissues sarcoma | |||
| Pembrolizumab | PD-1 inhibitor | Cervical cancer | PD-1 |
| Endometrial cancer | |||
| Esophageal cancer | |||
| Gastric cancer | |||
| Hepatocellular carcinoma | |||
| NSCLC | |||
| Bladder cancer | |||
| Head and Neck cancer | |||
| Pertuzumab | Anti-HER2 mAb | Breast cancer | ERBB2 over-expression or amplification |
| Ramucirumab | Anti-VEGF mAb | Colorectal cancer | |
| Esophageal cancer | |||
| Gastric cancer | |||
| Hepatocellular carcinoma | |||
| NSCLC | |||
| Regorafenib | Anti-cKIT TKI | Colorectal cancer | KIT+ |
| Hepatocellular carcinoma | |||
| GIST | |||
| Ribociclib | CDK4/6 inhibitor | Breast cancer | ER+/PR+ |
| Ripretinib | TKI: anti-KIT, anti-PDGFR | GIST | KIT+ |
| Rucaparib | PARP inhibitor | Prostate cancer | BRCA1/2 mutations |
| Ovarian cancer | |||
| Fallopian tube cancer | |||
| Peritoneal cancer | |||
| Sacituzumab-Govitecan | Conjugated Ab anti-trop-2 | Breast cancer | RE- RP- HER2- |
| Selpercatinib | Kinase inhibitor | NSCLC | RET+ |
| Thyroid cancer | |||
| Sorafenib | Multi-kinase inhibitor: anti-PDGFR, VEGFR, cKIT, TKR | Renal cancer | |
| Hepatocellular carcinoma | |||
| Thyroid cancer | |||
| Sunitinib | Multi-kinase inhibitor: anti-PDGFR, VEGFR, cKIT, TKR | Renal cancer | |
| Pancreatic cancer | |||
| GIST | |||
| Tamoxifeno | SERM | Breast cancer | ER+/PR+ |
| Talazoparib | PARP inhibitor | Breast cancer | BRCA1/2 mutations |
| Temsirolimus | mTOR inhibitor | Renal cancer | |
| Trametinib | BRAF inhibitor | NSCLC | BRAF-V600E, V600K |
| Thyroid cancer | |||
| Melanoma | |||
| Trastuzumab | Anti-HER2 mAb | Gastric cancer | ERBB2 over-expression of amplification |
| Gastro-esophageal junction cancer | |||
| Breast cancer | |||
| Trastuzumab-Deruxtecan | Anti-HER2 conjugated Ab | Breast cancer | ERBB2 over-expression of amplification |
| Trastuzumab-Emtansine | Anti-HER2 conjugated Ab | Breast cancer | ERBB2 over-expression of amplification |
| Tucatinib | Anti-HER2 TKI | Breast cancer | ERBB2 over-expression of amplification |
| Vandetanib | TKI: anti-VEGF, anti-EGFR | Thyroid cancer | EGFR+ |
| Vemurafenib | BRAF inhibitor | Melanoma | BRAF-V600E |
Abbreviations: mAb, monoclonal antibody; ALK, anaplastic lymphoma kinase; CDK, cyclin-dependent kinase; CTLA-4, cytotoxic lymphocyte antigen-4; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; GIST, gastrointestinal stroma tumor; mTOR, target of rapamycine in mammal cells; NSCLC, non-small cell lung carcinoma; PARP, poli (ADP-ribose) polimerase; PD-1, programmed death protein-1; PDGFR, platelet-derived growth factor receptor; PD-L1, programmed death ligand-1; ER, estrogen receptor; PR, progesterone receptor; TKR, tyrosine kinase receptors; SERM, selective estrogen receptor modulator; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor. Modified from Ref. [ 127 ].
Most drugs classified as targeted therapies form part of 2 large groups: small molecules and mAbs. The former are defined as compounds of low molecular weight (<900 Daltons) that act upon entering the cell. 120 Targets of these compounds are cell cycle regulatory proteins, proapoptotic proteins, or DNA repair proteins. These drugs are indicated based on histological diagnosis, as well as molecular tests. In this group there are multi-kinase inhibitors (RTKs) and tyrosine kinase inhibitors (TKIs), like sunitinib, sorafenib, and imatinib; cyclin-dependent kinase (CDK) inhibitors, such as palbociclib, ribociclib and abemaciclib; poli (ADP-ribose) polimerase inhibitors (PARPs), like olaparib and talazoparib; and selective small-molecule inhibitors, like ALK and ROS1. 122
As for mAbs, they are protein molecules that act on membrane receptors or extracellular proteins by interrupting the interaction between ligands and receptors, in such a way that they reduce cell replication and induce cytostasis. Among the most widely used mAbs in oncology we have: trastuzumab, a drug directed against the receptor for human epidermal growth factor-2 (HER2), which is overexpressed in a subgroup of patients with breast and gastric cancer; and bevacizumab, that blocks vascular endothelial growth factor and is used in patients with colorectal cancer, cervical cancer, and ovarian cancer. Other mAbs approved by the FDA include pembolizumab, atezolizumab, nivolumab, avelumab, ipilimumab, durvalumab, and cemiplimab. These drugs require expression of response biomarkers, such as PD-1 and PD-L1, and must also have several resistance biomarkers, such as the expression of EGFR, the loss of PTEN, and alterations in beta-catenin. 123
Because cancer is such a diverse disease, it is fundamental to have precise diagnostic methods that allow us to identify the most adequate therapy. Currently, basic immunohistochemistry is complemented with neoplastic molecular profiles to determine a more accurate diagnosis, and it is probable that in the near future cancer treatments will be based exclusively on molecular profiles. In this regard, it is worth mentioning that the use of targeted therapy depends on the existence of specific biomarkers that indicate if the patient will be susceptible to the effects of the drug or not. Thus, the importance of underlining that not all patients are susceptible to receive targeted therapy. In certain neoplasms, therapeutic targets are expressed in less than 5% of the diagnosed population, hindering a more extended use of certain drugs.
The identification of biomarkers and the use of new generation sequencing on tumor cells has shown predictive and prognostic relevance. Likewise, mutation analysis has allowed monitoring of tumor clone evolution, providing information on changes in canonic gene sequences, such as TP53, GATA3, PIK3CA, AKT1, and ERBB2; infrequent somatic mutations developed after primary treatments, like SWI-SNF and JAK2-STAT3; or acquired drug resistance mutations such as ESR1. 124 The study of mutations is vital; in fact, many of them already have specific therapeutic indications, which have helped select adequate treatments. 125
There is no doubt that molecular targeted therapy is one of the main pillars of precision medicine. However, it faces significant problems that often hinder obtaining better results. Among these, there is intratumor heterogeneity and differences between the primary tumor and metastatic sites, as well as intrinsic and acquired resistance to these therapies, the mechanisms of which include the presence of heterogeneous subclones, DNA hypermethylation, histone acetylation, and interruption of mRNA degradation and translation processes. 126 Nonetheless, beyond the obstacles facing molecular targeted therapy from a biological and methodological point of view, in the real world, access to genomic testing and specific drugs continues to be an enormous limitation, in such a way that strategies must be designed in the future for precision medicine to be possible on a global scale.
Cell Therapy
Another improvement in cancer treatment is the use of cell therapy, that is, the use of specific cells as therapeutic agents. This clinical procedure has 2 modalities: the first consists of replacing and regenerating functional cells in a specific tissue by means of stem/progenitor cells of a certain kind, 43 while the second uses immune cells as effectors to eliminate malignant cells. 127
Regarding the first type, we must emphasize the development of cell therapy based on hematopoietic stem and progenitor cells. 128 For over 50 years, hematopoietic cell transplants have been used to treat a variety of hematologic neoplasms (different forms of leukemia and lymphoma). Today, it is one of the most successful examples of cell therapy, including innovative modalities, such as haploidentical transplants, 129 as well as application of stem cells expanded ex vivo . 130 There are also therapies that have used immature cells that form part of the TME, such as MSCs. The replication potential and cytokine secretion capacity of these cells make them an excellent option for this type of treatment. 131 Neural stem cells can also be manipulated to produce and secrete apoptotic factors, and when these cells are incorporated into primary neural tumors, they cause a certain degree of regression. They can even be transfected with genes that encode for oncolytic enzymes capable of inducing regression of glioblastomas. 132
With respect to cell therapy using immune cells, several research groups have manipulated cells associated with tumors to make them effector cells and thus improve the efficacy and specificity of the antitumor treatment. PB leckocytes cultured in the presence of IL-2 to obtain activated lymphocytes, in combination with IL-2 administration, have been used in antitumor clinical protocols. Similarly, infiltrating lymphocytes from tumors with antitumor activity have been used and can be expanded ex vivo with IL-2. These lymphocyte populations have been used in immunomodulatory therapies in melanoma, and pancreatic and kidney tumors, producing a favorable response in treated patients. 133 NK cells and macrophages have also been used in immunotherapy, although with limited results. 134 , 135
One of the cell therapies with better projection today is the use of CAR-T cells. This strategy combines 2 forms of advanced therapy: cell therapy and gene therapy. It involves the extraction of T cells from the cancer patient, which are genetically modified in vitro to express cell surface receptors that will recognize antigens on the surface of tumor cells. The modified T cells are then reintroduced in the patient to aid in an exacerbated immune response that leads to eradication of the tumor cells ( Figure 4 ). Therapy with CAR-T cells has been used successfully in the treatment of some types of leukemia, lymphoma, and myeloma, producing complete responses in patients. 136

CAR-T cell therapy. (A) T lymphocytes obtained from cancer patients are genetically manipulated to produce CAR-T cells that recognize tumor cells in a very specific manner. (B) Interaction between CAR molecule and tumor antigen. CAR molecule is a receptor that results from the fusion between single-chain variable fragments (scFv) from a monoclonal antibody and one or more intracellular signaling domains from the T-cell receptor. CD3ζ, CD28 and 4-1BB correspond to signaling domains on the CAR molecule.
Undoubtedly, CAR-T cell therapy has been truly efficient in the treatment of various types of neoplasms. However, this therapeutic strategy can also have serious side effects, such as release of cytokines into the bloodstream, which can cause different symptoms, from high fever to multiorgan failure, and even neurotoxicity, leading to cerebral edema in many cases. 137 Adequate control of these side effects is an important medical challenge. Several research groups are trying to improve CAR-T cell therapy through various approaches, including production of CAR-T cells directed against a wider variety of tumor cell-specific antigens that are able to attack different types of tumors, and the identification of more efficient types of T lymphocytes. Furthermore, producing CAR-T cells from a single donor that may be used in the treatment of several patients would reduce the cost of this sort of personalized cell therapy. 136
Achieving wider use of cell therapy in oncologic diseases is an important challenge that requires solving various issues. 138 One is intratumor cell heterogeneity, including malignant subclones and the various components of the TME, which results in a wide profile of membrane protein expression that complicates finding an ideal tumor antigen that allows specific identification (and elimination) of malignant cells. Likewise, structural organization of the TME challenges the use of cell therapy, as administration of cell vehicles capable of recognizing malignant cells might not be able to infiltrate the tumor. This results from low expression of chemokines in tumors and the presence of a dense fibrotic matrix that compacts the inner tumor mass and avoids antitumor cells from infiltrating and finding malignant target cells.
Further Challenges in the 21st Century
Beyond the challenges regarding oncologic biomedical research, the 21 st century is facing important issues that must be solved as soon as possible if we truly wish to gain significant ground in our fight against cancer. Three of the most important have to do with prevention, early diagnosis, and access to oncologic medication and treatment.
Prevention and Early Diagnosis
Prevention is the most cost-effective strategy in the long term, both in low and high HDI nations. Data from countries like the USA indicate that between 40-50% of all types of cancer are preventable through potentially modifiable factors (primary prevention), such as use of tobacco and alcohol, diet, physical activity, exposure to ionizing radiation, as well as prevention of infection through access to vaccination, and by reducing exposure to environmental pollutants, such as pesticides, diesel exhaust particles, solvents, etc. 74 , 84 Screening, on the other hand, has shown great effectiveness as secondary prevention. Once population-based screening programs are implemented, there is generally an initial increase in incidence; however, in the long term, a significant reduction occurs not only in incidence rates, but also in mortality rates due to detection of early lesions and timely and adequate treatment.
A good example is colon cancer. There are several options for colon cancer screening, such as detection of fecal occult blood, fecal immunohistochemistry, flexible sigmoidoscopy, and colonoscopy, 139 , 140 which identify precursor lesions (polyp adenomas) and allow their removal. Such screening has allowed us to observe 3 patterns of incidence and mortality for colon cancer between the years 2000 and 2010: on one hand, an increase in incidence and mortality in countries with low to middle HDI, mainly countries in Asia, South America, and Eastern Europe; on the other hand, an increase in incidence and a fall in mortality in countries with very high HDI, such as Canada, the United Kingdom, Denmark, and Singapore; and finally a fall in incidence and mortality in countries like the USA, Japan, and France. The situation in South America and Asia seems to reflect limitations in medical infrastructure and a lack of access to early detection, 141 while the patterns observed in developed countries reveal the success, even if it may be partial, of that which can be achieved by well-structured prevention programs.
Another example of success, but also of strong contrast, is cervical cancer. The discovery of the human papilloma virus (HPV) as the causal agent of cervical cancer brought about the development of vaccines and tests to detect oncogenic genotypes, which modified screening recommendations and guidelines, and allowed several developed countries to include the HPV vaccine in their national vaccination programs. Nevertheless, the outlook is quite different in other areas of the world. Eighty percent of the deaths by cervical cancer reported in 2018 occurred in low-income nations. This reveals the urgency of guaranteeing access to primary and secondary prevention (vaccination and screening, respectively) in these countries, or else it will continue to be a serious public health problem in spite of its preventability.
Screening programs for other neoplasms, such as breast, prostate, lung, and thyroid cancer have shown outlooks that differ from those just described, because, among other reasons, these neoplasms are highly diverse both biologically and clinically. Another relevant issue is the overdiagnosis of these neoplasms, that is, the diagnosis of disease that would not cause symptoms or death in the patient. 142 It has been calculated that 25% of breast cancer (determined by mammogram), 50–60% of prostate cancer (determined by PSA), and 13–25% of lung cancer (determined by CT) are overdiagnosed. 142 Thus, it is necessary to improve the sensitivity and specificity of screening tests. In this respect, knowledge provided by the biology of cancer and “omic” sciences offers a great opportunity to improve screening and prevention strategies. All of the above shows that prevention and early diagnosis are the foundations in the fight against cancer, and it is essential to continue to implement broader screening programs and better detection methods.
Global Equity in Oncologic Treatment
Progress in cancer treatment has considerably increased the number of cancer survivors. Nevertheless, this tendency is evident only in countries with a very solid economy. Indeed, during the past 30 years, cancer mortality rates have increased 30% worldwide. 143 Global studies indicate that close to 70% of cancer deaths in the world occur in nations of low to middle income. But even in high-income countries, there are sectors of society that are more vulnerable and have less access to cancer treatments. 144 Cancer continues to be a disease of great social inequality.
In Europe, the differences in access to cancer treatment are highly marked. These treatments are more accessible in Western Europe than in its Eastern counterpart. 145 Furthermore, highly noticeable differences between high-income countries have been detected in the cost of cancer drugs. 146 It is interesting to note that in many of these cases, treatment is too costly and the clinical benefit only marginal. Thus, the importance of these problems being approached by competent national, regional, and global authorities, because if these new drugs and therapeutic programs are not accessible to the majority, progress in biomedical, clinical and epidemiological research will have a limited impact in our fight against cancer. We must not forget that health is a universal right, from which low HDI countries must not be excluded, nor vulnerable populations in nations with high HDI. The participation of a well-informed society will also be fundamental to achieve a global impact, as today we must fight not only against the disease, but also against movements and ideas (such as the anti-vaccine movement and the so-called miracle therapies) that can block the medical battle against cancer.
Final Comments
From the second half of the 20th century to the present day, progress in our knowledge about the origin and development of cancer has been extraordinary. We now understand cancer in detail in genomic, molecular, cellular, and physiological terms, and this knowledge has had a significant impact in the clinic. There is no doubt that a patient who is diagnosed today with a type of cancer has a better prospect than a patient diagnosed 20 or 50 years ago. However, we are still far from winning the war against cancer. The challenges are still numerous. For this reason, oncologic biomedical research must be a worldwide priority. Likewise, one of the fundamental challenges for the coming decades must be to reduce unequal access to health services in areas of low- to middle income, and in populations that are especially vulnerable, as well as continue improving prevention programs, including public health programs to reduce exposure to environmental chemicals and improve diet and physical activity in the general population. 74 , 84 Fostering research and incorporation of new technological resources, particularly in less privileged nations, will play a key role in our global fight against cancer.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Hector Mayani https://orcid.org/0000-0002-2483-3782
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Research trend of lung cancer epigenetics research: Bibliometric and visual analysis of top-100 cited documents
Affiliation.
- 1 Chun'an County First People's Hospital, Hangzhou, 311700, People's Republic of China.
- PMID: 39170116
- PMCID: PMC11337132
- DOI: 10.1016/j.heliyon.2024.e35686
Background: Lung cancer is a highly prevalent cancer on a global scale and its oncogenic process is driven by the accumulation of multiple pathological events. Epigenetics has gained significant recognition in recent years as a crucial contributor to the development of lung cancer. Epigenetics include processes such as DNA methylation, histone modification, chromatin remodeling, and RNA modification. These pathways lead to enduring alterations in genetic phenotypes, which are crucial in the advancement and growth of lung cancer. However, the specific mechanisms and roles of epigenetics in lung cancer still need to be further elucidated.
Methods: We obtained publications from the Web of Science databases and applied a rigorous search method to filter them. Ultimately, we gathered high-quality publications that had received the highest 100 number of citations. The data were processed and visualized by various bibliometric tools.
Results: The 100 papers had varying numbers of citations, with the lowest being 491 and the most being 6316. On average, each work received 1119 citations. A total of 1056 co-authors were involved in publishing these papers in 59 journals from 185 institutions in 27 countries. The majority of high-caliber research in the subject of lung cancer epigenetics is conducted in advanced countries, with the United States taking the lead in terms of both the quantity of articles produced and their academic influence. The study of DNA methylation has been a longstanding research priority in the discipline. With the development of next-generation sequencing technology in recent years, research related to non-coding RNA has become a research hotspot. Future research directions may focus more on exploring the mechanisms of action of messenger RNA and circular RNA and developing targeted treatment strategies based on non-coding RNA drugs.
Conclusion: We analyzed 100 top lung cancer and epigenetics documents through various bibliometric analysis tools. This study provides a concise overview of the findings from prior research, anticipates future research directions, and offers potential avenues for additional investigation.
Keywords: Bibliometric analysis; DNA methylation; Epigenetics; Lung cancer; Non-coding RNA.
© 2024 The Authors.
PubMed Disclaimer
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Analysis of published papers. (…
Analysis of published papers. ( A ) Trends in lung cancer and epigenetic…
Keywords related to lung cancer…
Keywords related to lung cancer and epigenetics. ( A ) Mapping of the…
Trends in research topics over…
Trends in research topics over time.
Analysis of journals. ( A…
Analysis of journals. ( A ) Visual analysis of cooperative networks between journals.…
Visualizaiton mapping of co-citation references.…
Visualizaiton mapping of co-citation references. ( A ) Network visualization map. ( B…
Distribution and linkage of country…
Distribution and linkage of country composition related to lung cancer and epigenetics research.…
Analysis of organizations. ( A…
Analysis of organizations. ( A )The 10 most relevant institutions among the 100…
Analysis of authors. ( A…
Analysis of authors. ( A ) The 10 most relevant authors among the…
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. - DOI - PubMed
- Duma N., Santana-Davila R., Molina J.R. Non–small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin. Proc. 2019;94:1623–1640. doi: 10.1016/j.mayocp.2019.01.013. - DOI - PubMed
- Thomas M., Ponce-Aix S., Navarro A., Riera-Knorrenschild J., Schmidt M., Wiegert E., Kapp K., Wittig B., Mauri C., Dómine Gómez M., et al. Immunotherapeutic maintenance treatment with toll-like receptor 9 agonist lefitolimod in patients with extensive-stage small-cell lung cancer: results from the exploratory, controlled, randomized, international phase II IMPULSE study. Ann. Oncol. 2018;29:2076–2084. doi: 10.1093/annonc/mdy326. - DOI - PMC - PubMed
- Bade B.C., Dela Cruz C.S. Lung cancer 2020. Clin. Chest Med. 2020;41:1–24. doi: 10.1016/j.ccm.2019.10.001. - DOI - PubMed
- Chen Z., Fillmore C.M., Hammerman P.S., Kim C.F., Wong K.-K. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat. Rev. Cancer. 2014;14:535–546. doi: 10.1038/nrc3775. - DOI - PMC - PubMed
Related information
Linkout - more resources, full text sources.
- Elsevier Science
- PubMed Central

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 05 April 2023
Lung adenocarcinoma promotion by air pollutants
- William Hill 1 na1 ,
- Emilia L. Lim 1 , 2 na1 na2 ,
- Clare E. Weeden 1 na1 ,
- Claudia Lee ORCID: orcid.org/0000-0003-1715-2265 1 , 2 , 3 ,
- Marcellus Augustine 1 , 2 , 3 , 4 ,
- Kezhong Chen 2 , 5 ,
- Feng-Che Kuan 6 , 7 ,
- Fabio Marongiu 8 , 9 ,
- Edward J. Evans Jr ORCID: orcid.org/0000-0001-8120-3755 8 ,
- David A. Moore ORCID: orcid.org/0000-0002-6296-1312 1 , 2 , 10 ,
- Felipe S. Rodrigues 11 ,
- Oriol Pich ORCID: orcid.org/0000-0002-1956-1882 1 ,
- Bjorn Bakker ORCID: orcid.org/0000-0003-3095-7287 1 ,
- Hongui Cha 2 , 12 ,
- Renelle Myers 13 ,
- Febe van Maldegem 14 , 15 ,
- Jesse Boumelha 14 ,
- Selvaraju Veeriah 2 ,
- Andrew Rowan 1 ,
- Cristina Naceur-Lombardelli 2 ,
- Takahiro Karasaki ORCID: orcid.org/0000-0001-6863-2360 1 , 2 , 16 ,
- Monica Sivakumar 2 ,
- Swapnanil De 2 ,
- Deborah R. Caswell 1 ,
- Ai Nagano ORCID: orcid.org/0000-0002-2354-660X 1 , 2 ,
- James R. M. Black 2 , 17 ,
- Carlos Martínez-Ruiz ORCID: orcid.org/0000-0002-4817-0565 2 , 17 ,
- Min Hyung Ryu 18 ,
- Ryan D. Huff ORCID: orcid.org/0000-0002-6038-8217 18 ,
- Shijia Li 18 ,
- Marie-Julie Favé 19 ,
- Alastair Magness 1 , 2 ,
- Alejandro Suárez-Bonnet ORCID: orcid.org/0000-0003-0296-5896 20 , 21 ,
- Simon L. Priestnall ORCID: orcid.org/0000-0002-6027-1879 20 , 21 ,
- Margreet Lüchtenborg 22 , 23 ,
- Katrina Lavelle 22 ,
- Joanna Pethick 22 ,
- Steven Hardy 22 ,
- Fiona E. McRonald ORCID: orcid.org/0000-0001-7226-9461 22 ,
- Meng-Hung Lin ORCID: orcid.org/0000-0002-8594-3360 24 ,
- Clara I. Troccoli 8 , 25 ,
- Moumita Ghosh 26 ,
- York E. Miller 26 , 27 ,
- Daniel T. Merrick 28 ,
- Robert L. Keith 26 , 27 ,
- Maise Al Bakir ORCID: orcid.org/0000-0002-0846-8332 1 , 2 ,
- Chris Bailey 1 ,
- Mark S. Hill ORCID: orcid.org/0000-0003-0718-8934 1 ,
- Lao H. Saal 29 , 30 ,
- Yilun Chen 29 , 30 ,
- Anthony M. George ORCID: orcid.org/0000-0001-5680-8664 29 , 30 ,
- Christopher Abbosh ORCID: orcid.org/0000-0002-8983-1382 2 ,
- Nnennaya Kanu 2 ,
- Se-Hoon Lee ORCID: orcid.org/0000-0002-9219-3350 12 ,
- Nicholas McGranahan ORCID: orcid.org/0000-0001-9537-4045 2 , 17 ,
- Christine D. Berg 31 ,
- Peter Sasieni ORCID: orcid.org/0000-0003-1509-8744 32 ,
- Richard Houlston ORCID: orcid.org/0000-0002-5268-0242 33 ,
- Clare Turnbull 33 ,
- Stephen Lam 13 ,
- Philip Awadalla ORCID: orcid.org/0000-0001-9946-6393 19 ,
- Eva Grönroos ORCID: orcid.org/0000-0001-8303-5409 1 ,
- Julian Downward ORCID: orcid.org/0000-0002-2331-4729 14 ,
- Tyler Jacks ORCID: orcid.org/0000-0001-5785-8911 34 , 35 ,
- Christopher Carlsten 18 ,
- Ilaria Malanchi ORCID: orcid.org/0000-0003-4867-3311 11 ,
- Allan Hackshaw ORCID: orcid.org/0000-0002-5570-5070 36 ,
- Kevin Litchfield ORCID: orcid.org/0000-0002-3725-0914 2 , 4 ,
- TRACERx Consortium ,
- James DeGregori ORCID: orcid.org/0000-0002-1287-1976 8 na2 ,
- Mariam Jamal-Hanjani ORCID: orcid.org/0000-0003-1212-1259 2 , 16 , 37 na2 &
- Charles Swanton ORCID: orcid.org/0000-0002-4299-3018 1 , 2 , 37
Nature volume 616 , pages 159–167 ( 2023 ) Cite this article
56k Accesses
199 Citations
1953 Altmetric
Metrics details
- Cancer genomics
- Non-small-cell lung cancer
- Preclinical research
- Risk factors
A complete understanding of how exposure to environmental substances promotes cancer formation is lacking. More than 70 years ago, tumorigenesis was proposed to occur in a two-step process: an initiating step that induces mutations in healthy cells, followed by a promoter step that triggers cancer development 1 . Here we propose that environmental particulate matter measuring ≤2.5 μm (PM 2.5 ), known to be associated with lung cancer risk, promotes lung cancer by acting on cells that harbour pre-existing oncogenic mutations in healthy lung tissue. Focusing on EGFR-driven lung cancer, which is more common in never-smokers or light smokers, we found a significant association between PM 2.5 levels and the incidence of lung cancer for 32,957 EGFR-driven lung cancer cases in four within-country cohorts. Functional mouse models revealed that air pollutants cause an influx of macrophages into the lung and release of interleukin-1β. This process results in a progenitor-like cell state within EGFR mutant lung alveolar type II epithelial cells that fuels tumorigenesis. Ultradeep mutational profiling of histologically normal lung tissue from 295 individuals across 3 clinical cohorts revealed oncogenic EGFR and KRAS driver mutations in 18% and 53% of healthy tissue samples, respectively. These findings collectively support a tumour-promoting role for PM 2.5 air pollutants and provide impetus for public health policy initiatives to address air pollution to reduce disease burden.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Cancer incidence trends in New York State and associations with common population-level exposures 2010–2018: an ecological study
Particulate matter promotes cancer metastasis through increased HBEGF expression in macrophages
Outdoor air pollution and risk of incident adult haematologic cancer subtypes in a large US prospective cohort
Data availability.
Duplex-seq data for the PEACE and BDRE cohorts are available at the European Genome–Phenome Archive (EGA) with the identifier EGAS00001006951 . Duplex-seq data generated from PEACE study samples during this study are not publicly available and restrictions apply to the availability of these data. Such Duplex-seq data are available through the Cancer Research UK and University College London Cancer Trials Centre ([email protected]) for academic, non-commercial research purposes upon reasonable request and subject to review of a project proposal that will be evaluated by a PEACE data access committee, entering into an appropriate data access agreement and subject to any applicable ethical approvals. Duplex-seq data generated from the BDRE study are available through J. DeGregori ([email protected]) for academic, non-commercial research purposes upon reasonable request, entering into an appropriate data access agreement and subject to any applicable ethical approvals. The Duplex-seq data for the BDRE and PEACE studies were generated using a larger panel of probes that covered approximately 50 kb of the genome, spanning hotspots frequently mutated in cancers. This full dataset has been provided for the 17 never-smoker individuals from the PEACE study. For all other samples, only data for the EGFR and KRAS regions queried are included in this manuscript. The RNA-seq data for the COPA study are available at the EGA with the identifier EGAS00001006966 . De-identified participant data are available upon reasonable request to C.C. ([email protected]) for academic, non-commercial research purposes. Data availability is subject to a data access agreement and applicable ethical approvals. Mouse WGS data are available at the European Nucleotide Archive (ENA) with the identifier PRJEB58221 (ERP143287). Mouse RNA-seq data are available at the ENA with the identifier PRJEB59269 (ERP144330). Source data are provided with this paper.
Code availability
Code for analysis of epidemiology, RNA-seq and WGS data and processing of healthy lung tissue are available at Zenodo ( https://doi.org/10.5281/zenodo.7705022 ).
Berenblum, I. & Shubik, P. A new, quantitative, approach to the study of the stages of chemical carcinogenesis in the mouse’s skin. Br. J. Cancer 1 , 383–391 (1947).
Article CAS PubMed PubMed Central Google Scholar
Cogliano, V. J. et al. Preventable exposures associated with human cancers. J. Natl Cancer Inst. 103 , 1827–1839 (2011).
Article PubMed PubMed Central Google Scholar
Sun, S., Schiller, J. H. & Gazdar, A. F. Lung cancer in never smokers—a different disease. Nat. Rev. Cancer 7 , 778–790 (2007).
Article CAS PubMed Google Scholar
Midha, A., Dearden, S. & McCormack, R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am. J. Cancer Res. 5 , 2892–2911 (2015).
PubMed PubMed Central Google Scholar
Carrot-Zhang, J. et al. Genetic ancestry contributes to somatic mutations in lung cancers from admixed Latin American populations. Cancer Discov. 11 , 591–598 (2021).
Myers, R. et al. High ambient air pollution exposure among never smokers versus ever smokers with lung cancer. J. Thorac. Oncol. 16 , 1858–1858 (2021).
Article Google Scholar
WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide (World Health Organization, 2021).
Kucab, J. E. et al. A compendium of mutational signatures of environmental agents. Cell 177 , 821–836.e16 (2019).
Riva, L. et al. The mutational signature profile of known and suspected human carcinogens in mice. Nat. Genet. 52 , 1189–1197 (2020).
Moody, S. et al. Mutational signatures in esophageal squamous cell carcinoma from eight countries with varying incidence. Nat. Genet. 53 , 1553–1563 (2021).
Zhang, T. et al. Genomic and evolutionary classification of lung cancer in never smokers. Nat. Genet. 53 , 1348–1359 (2021).
Jamal-Hanjani, M. et al. Tracking the evolution of non-small-cell lung cancer. N. Engl. J. Med. 376 , 2109–2121 (2017).
Chen, Y.-J. et al. Proteogenomics of non-smoking lung cancer in East Asia delineates molecular signatures of pathogenesis and progression. Cell 182 , 226–244.e17 (2020).
Frankell, A. M. et al. The evolution of lung cancer and impact of subclonal selection in TRACERx. Nature https://doi.org/10.1038/s41586-023-05783-5 (2023).
Takahashi, H., Ogata, H., Nishigaki, R., Broide, D. H. & Karin, M. Tobacco smoke promotes lung tumorigenesis by triggering IKKβ- and JNK1-dependent inflammation. Cancer Cell 17 , 89–97 (2010).
Im, H-B. et al. South Korea. Encyclopaedia Britannica. https://www.britannica.com/place/South-Korea (accessed 9 March 2023).
The Ethnic Group. Executive Yuan. https://www.ey.gov.tw/state/99B2E89521FC31E1/2820610c-e97f-4d33-aa1e-e7b15222e45a (accessed 9 March 2023).
Huang, Y. et al. Air pollution, genetic factors, and the risk of lung cancer: a prospective study in the UK Biobank. Am. J. Respir. Crit. Care Med. 204 , 817–825 (2021).
McDaniel Mims, B. & Grisham, M. B. Humanizing the mouse immune system to study splanchnic organ inflammation. J. Physiol. 596 , 3915–3927 (2018).
Hogg, J. C. & Van Eeden, S. Pulmonary and systemic response to atmospheric pollution. Respirology 14 , 336–346 (2009).
Article PubMed Google Scholar
Sutherland, K. D. et al. Multiple cells-of-origin of mutant K-Ras-induced mouse lung adenocarcinoma. Proc. Natl Acad. Sci. USA 111 , 4952–4957 (2014).
Article ADS CAS PubMed PubMed Central Google Scholar
Choi, J. et al. Inflammatory signals induce AT2 cell-derived damage-associated transient progenitors that mediate alveolar regeneration. Cell Stem Cell 27 , 366–382.e7 (2020).
Strunz, M. et al. Alveolar regeneration through a Krt8 + transitional stem cell state that persists in human lung fibrosis. Nat. Commun. 11 , 3559 (2020).
Ryu, M. H. et al. Impact of exposure to diesel exhaust on inflammation markers and proteases in former smokers with chronic obstructive pulmonary disease: a randomized, double-blinded, crossover study. Am. J. Respir. Crit. Care Med. 205 , 1046–1052 (2022).
Ryu, M. H. Effects of Traffic-Related Air Pollution Exposure on Older Adults with and without Chronic Obstructive Pulmonary Disease . PhD thesis, Univ. of British Colombia (2021).
Nolan, E. et al. Radiation exposure elicits a neutrophil-driven response in healthy lung tissue that enhances metastatic colonization. Nat. Cancer 3 , 173–187 (2022).
Dost, A. F. M. et al. Organoids model transcriptional hallmarks of oncogenic KRAS activation in lung epithelial progenitor cells. Cell Stem Cell 27 , 663–678.e8 (2020).
Hiraiwa, K. & van Eeden, S. F. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediators Inflamm. 2013 , 619523 (2013).
Klughammer, B. et al. Examining treatment outcomes with erlotinib in patients with advanced non-small cell lung cancer whose tumors harbor uncommon EGFR mutations. J. Thorac. Oncol. 11 , 545–555 (2016).
Takano, A. P. C. et al. Pleural anthracosis as an indicator of lifetime exposure to urban air pollution: an autopsy-based study in Sao Paulo. Environ. Res. 173 , 23–32 (2019).
Mirsadraee, M. Anthracosis of the lungs: etiology, clinical manifestations and diagnosis: a review. Tanaffos 13 , 1–13 (2014).
Kunzke, T. et al. Patterns of carbon-bound exogenous compounds in patients with lung cancer and association with disease pathophysiology. Cancer Res. 81 , 5862–5875 (2021).
Deprez, M. et al. A single-cell atlas of the human healthy airways. Am. J. Respir. Crit. Care Med. 202 , 1636–1645 (2020).
Sikkema, L. et al. An integrated cell atlas of the human lung in health and disease. Preprint at bioRxiv https://doi.org/10.1101/2022.03.10.483747 (2022).
Tate, J. G. et al. COSMIC: the Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 47 , D941–D947 (2019).
Su, F. et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N. Engl. J. Med. 366 , 207–215 (2012).
Yoshida, K. et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature 578 , 266–272 (2020).
Yokoyama, A. et al. Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature 565 , 312–317 (2019).
Article ADS CAS PubMed Google Scholar
IARC Working Group on the & Evaluation of Carcinogenic Risks to Humans. IARC Monograph Volume105. Diesel And Gasoline Engine Exhausts And Some Nitroarenes (World Health Organization, 2014).
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monograph Volume 109. Outdoor Air Pollution (World Health Organization, 2016).
Turner, M. C. et al. Outdoor air pollution and cancer: an overview of the current evidence and public health recommendations. CA Cancer J. Clin. 70 , 460–479 (2020).
Chung, K. M. et al. Endocrine–exocrine signaling drives obesity-associated pancreatic ductal adenocarcinoma. Cell 181 , 832–847.e18 (2020).
Ridker, P. M. et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390 , 1833–1842 (2017).
Crapo, J. D., Barry, B. E., Gehr, P., Bachofen, M. & Weibel, E. R. Cell number and cell characteristics of the normal human lung. Am. Rev. Respir. Dis. 126 , 332–327 (1982).
CAS PubMed Google Scholar
Doll, R. & Hill, A. B. Smoking and carcinoma of the lung. Preliminary report. 1950. Bull. World Health Organ. 77 , 84–93 (1999).
Kennedy, S. R. et al. Detecting ultralow-frequency mutations by duplex sequencing. Nat. Protoc. 9 , 2586–2606 (2014).
Stoler, N. & Nekrutenko, A. Sequencing error profiles of Illumina sequencing instruments. NAR Genom. Bioinform. 3 , lqab019 (2021).
Valentine, C. C. III et al. Direct quantification of in vivo mutagenesis and carcinogenesis using duplex sequencing. Proc. Natl Acad. Sci. USA 117 , 33414–33425 (2020).
Eeftens, M. et al. Development of land use regression models for PM 2.5 , PM 2.5 absorbance, PM 10 and PM coarse in 20 European study areas; results of the ESCAPE project. Environ. Sci. Technol. 46 , 11195–11205 (2012).
Van Buuren, S. & Groothuis-Oudshoorn, K. mice: multivariate imputation by chained equations in R. J. Stat. Softw. 45 , 1–67 (2011).
Department for Environment Food and Rural Affairs. Modelled Background Pollution Data https://uk-air.defra.gov.uk/data/pcm-data#population_weighted_annual_mean_pm25_data (2021).
British Geological Survey. Radon Data: Indicative Atlas of Radon https://www.bgs.ac.uk/datasets/radon-data-indicative-atlas-of-radon/ (2023).
ONS Postcode Directory (Latest) Centroids (Office for National Statistics; 2021); https://geoportal.statistics.gov.uk/datasets/ons-postcode-directory-november-2022/about (accessed 9 March 2023).
(Air Korea; 2021); https://www.airkorea.or.kr/web (accessed 9 March 2023).
Cancer Registry Statistical Data (National Cancer Center; 2021); https://www.ncc.re.kr/main.ncc?uri=english/sub04_Statistics (accessed 13 March 2023).
Taiwan Air Quality Monitoring Network (Environmental Protection Administration; 2022); https://airtw.epa.gov.tw/ENG/Default.aspx (accessed 23 March 2023).
Politi, K. et al. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev. 20 , 1496–1510 (2006).
Jackson, E. L. et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras . Genes Dev. 15 , 3243–3248 (2001).
Schantz, M. M. et al. Development of two fine particulate matter standard reference materials (<4 μm and <10 μm) for the determination of organic and inorganic constituents. Anal. Bioanal. Chem. 408 , 4257–4266 (2016).
Chan, Y. L. et al. Pulmonary inflammation induced by low-dose particulate matter exposure in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 317 , L424–L430 (2019).
Major, J. et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science 369 , 712–717 (2020).
Pedregosa, F. et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res . 12 , 2825–2830 (2011).
Desai, T. J., Brownfield, D. G. & Krasnow, M. A. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507 , 190–194 (2014).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7 , 16878 (2017).
Article ADS PubMed PubMed Central Google Scholar
Rosenthal, R., McGranahan, N., Herrero, J., Taylor, B. S. & Swanton, C. deconstructSigs: delineating mutational processes in single tumors distinguishes DNA repair deficiencies and patterns of carcinoma evolution. Genome Biol. 17 , 31 (2016).
Ewels, P. A. et al. The nf-core framework for community-curated bioinformatics pipelines. Nat. Biotechnol. 38 , 276–278 (2020).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29 , 15–21 (2013).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics 12 , 323 (2011).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15 , 550 (2014).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102 , 15545–15550 (2005).
Young, M. D. et al. Single cell derived mRNA signals across human kidney tumors. Nat. Commun. 12 , 3896 (2021).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14 , 417–419 (2017).
Dekkers, J. F. et al. Long-term culture, genetic manipulation and xenotransplantation of human normal and breast cancer organoids. Nat. Protoc. 16 , 1936–1965 (2021).
Muiños, F., Martínez-Jiménez, F., Pich, O., Gonzalez-Perez, A. & Lopez-Bigas, N. In silico saturation mutagenesis of cancer genes. Nature 596 , 428–432 (2021).
Article ADS PubMed Google Scholar
Download references
Acknowledgements
This research was conducted using the UK Biobank Resource under application number 82693. This work was supported by the Mark Foundation ASPIRE I Award (grant 21-029-ASP), the Lung Cancer Research Foundation Grant on Disparities in Lung Cancer, Advanced Grant (PROTEUS, grant agreement no. 835297), CRUK EDD (EDDPMA-Nov21\100034) and a Rosetrees Out-of-round Award (OoR2020\100009). W.H. is funded by an ERC Advanced Grant (PROTEUS, grant agreement no. 835297), CRUK EDD (EDDPMA-Nov21\100034), The Mark Foundation (grant 21-029-ASP) and has been supported by Rosetrees. E.L.L. receives funding from the NovoNordisk Foundation (ID 16584), The Mark Foundation (grant 21-029-ASP) and has been supported by Rosetrees. C.E.W. is supported by a RESPIRE4 fellowship from the European Respiratory Society and Marie-Sklodowska-Curie Actions. C.L. is supported by the Agency for Science, Technology & Research, Singapore and the Cancer Research UK City of London Centre and the City of London Centre Clinical Academic Training Programme. M.A. is supported by the City of London Centre Clinical Academic Training Programme (Year 3, SEBSTF-2021\100007). K.C. is supported by the Research Unit of Intelligence Diagnosis and Treatment in Early Non-small Cell Lung Cancer, the Chinese Academy of Medical Sciences (2021RU002), the National Natural Science Foundation of China (no. 82072566) and Peking University People’s Hospital Research and Development Funds (RS2019-01). T.K. receives grant support from JSPS Overseas Research Fellowships Program (202060447). S.-H.L. is supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (no. 2020R1A2C3006535), the National Cancer Center Grant (NCC1911269-3) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number HR20C0025). L.H.S. receives grant support from the Berta Kamprad Foundation, the Swedish Cancer Society and the Swedish Research Council. R.M. and S.L. acknowledge funding from the Terry Fox Research Institute. N.M. is a Sir Henry Dale Fellow, jointly funded by the Wellcome Trust and the Royal Society (grant number 211179/Z/18/Z) and receives funding from Cancer Research UK, the Rosetrees and the NIHR BRC at University College London Hospitals and the CRUK University College London Experimental Cancer Medicine Centre. J. DeGregori, M.G., Y.E.M., D.T.M. and R.L.K. receive funding from the American Association for Cancer Research/Johnson&Johnson (18-90-52-DEGR), and J. DeGregori is supported by the Courtenay C. and Lucy Patten Davis Endowed Chair in Lung Cancer Research and a Merit Award from the Veteran’s Administration (1 I01 BX004495). M.G., Y.E.M., D.T.M. and R.L.K. were supported by the National Cancer Institute (NCI) RO1 CA219893. E.J.E.J. was supported by a NCI Ruth L. Kirschstein National Research Service Award T32-CA190216 and the Blumenthal Fellowship from the Linda Crnic Institute for Down Syndrome. C.I.T. acknowledges funding from UC Anschutz (LHNC T32CA174648). The work at the University of Colorado was also supported by NCI Cancer Center Support Grant P30CA046934. K. Litchfield is funded by the UK Medical Research Council (MR/P014712/1 and MR/V033077/1), the Rosetrees Trust and the Cotswold Trust (A2437) and Cancer Research UK (C69256/A30194). M.J.-H. is a CRUK Career Establishment Awardee has received funding from Cancer Research UK, IASLC International Lung Cancer Foundation, the National Institute for Health Research, the Rosetrees Trust, UKI NETs and the NIHR University College London Hospitals Biomedical Research Centre. C.S. is a Royal Society Napier Research Professor (RSRP\R\210001). His work is supported by the Francis Crick Institute that receives its core funding from Cancer Research UK (CC2041), the UK Medical Research Council (CC2041), and the Wellcome Trust (CC2041). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. C.S. is funded by Cancer Research UK (TRACERx (C11496/A17786), PEACE (C416/A21999) and CRUK Cancer Immunotherapy Catalyst Network); Cancer Research UK Lung Cancer Centre of Excellence (C11496/A30025); the Rosetrees Trust, Butterfield and Stoneygate Trusts; NovoNordisk Foundation (ID16584); Royal Society Professorship Enhancement Award (RP/EA/180007); National Institute for Health Research (NIHR) University College London Hospitals Biomedical Research Centre; the Cancer Research UK-University College London Centre; Experimental Cancer Medicine Centre; the Breast Cancer Research Foundation (US) (BCRF-22-157); Cancer Research UK Early Detection an Diagnosis Primer Award (grant EDDPMA-Nov21/100034); and The Mark Foundation for Cancer Research Aspire Award (grant 21-029-ASP). This work was supported by a Stand Up To Cancer‐LUNGevity-American Lung Association Lung Cancer Interception Dream Team Translational Research Grant (grant number: SU2C-AACR-DT23-17 to S.M. Dubinett and A.E. Spira). Stand Up To Cancer is a division of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the Scientific Partner of SU2C. C.S. is in receipt of an ERC Advanced Grant (PROTEUS) from the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 835297). We acknowledge the PEACE Consortium (PEACE Consortium members are named below) for their expertise and support in putting together the healthy tissue sample cohorts. We thank the clinical and administrative team of the PEACE study for their assistance in data curation (S. Shepherd, Z. Tippu, B. Shum, C. Lewis, M. O’Flaherty, A. Lucanas, E. Carlyle, L. Holt, F. Williams); nursing and biospecimen coordinators for their assistance in sample curation (K. Edmonds, L. Grostate, K. Lingard, D. Kelly, J. Korteweg, L. Terry, J. Biano, A. Murra, K. Kelly, K. Peat, N. Hunter); A. H. -K. Cheung for assistance in pathology review; J. Asklin and C. Forsberg for logistical and technical assistance; staff at the Chang Gung Memorial Hospital for providing Chang Gung Research Database (CGRD) data; staff who provided support at the Flow Cytometry Unit, the Experimental Histopathology Unit, the Advanced Light Microscopy Facility, the Advanced Sequencing Facility and the Biological Resources Unit, especially N. Chisholm and Jay O’Brien, at the Francis Crick Institute; A. Yuen, A. Azhar, K. Lau, C. Schwartz, A. Lee and C. Rider for their logistical support for the human exposure study; and staff at the Centre d’expertise et de services Génome Québec for their sequencing services and support. Data for this study are based on patient-level information collected by the NHS, as part of the care and support of cancer patients. The data are collated, maintained and quality assured by the National Cancer Registration and Analysis Service, which is part of NHS England (NHSE). We extend our thanks to the skilled Cancer Registration Officers (CROs) within the National Disease Registration Service, who abstracted and registered the English tumour and molecular testing data. For the purpose of Open Access, the author has applied for a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. PEACE Consortium members: A. Flanagan, A. Hackshaw, A. Jayaram, A. M. M. Hasan, A. Toncheva, A. Wingate, A. Bunkum, A. Sharp, A. Tookman, A. Murra, A. Magness, A. Coulton, A. M. Frankell, A. Cluroe, A. Kerr, A. Ortega-Franco, A. Lucanas, A. M. Schmitt, A. Furness, A. Rowan, A. Tutt, A. Green, A. Paterson, A.-L. Cattin, A. Fendler, A. Latifoltojar, A. Huebner, A. Thomas, B. T. Alba, B. Chain, B. Naidu, B. Shum, B. Olisemeke, B. Hampton, B. Hanley, B. Tanchel, G. Langman, C. Naceur-Lombardelli, C. Gerard, C. Caldas, C. Martínez-Ruiz, C. Dive, C. Stirling, C. Swanton, C. Ferris, C. Lewis, C. Milner-Watts, C. Spencer, C.-w. Lok, C. Bailey, C. Messiou, C. Wilson, C. Puttick, C. Lee, C. P. Marin, C. Alifrangis, C. Richard, C. T. Hiley, D. Hochhauser, D. Wetterskog, D. A. Moore, D. Deng, D. Marrone, D. Enting, D. Josephs, D. Kelly, D. A. Fennell, D. Biswas, D. Papadatos-Pastos, E. Carlyle, E. Provenzano, E. (L.) Karapanagiotou, E. Pintus, E. L. Lim, E. Beddowes, E. Colliver, E. Nye, F. Gishen, F. Gomes, F. H. Blackhall, F. Byrne, F. Athanasopoulou, G. Attard, G. Stamp, G. Middleton, G. D. Stewart, H. Feng, G. Pulman, G. Leone, H. Bridger, H. Shaw, H. Yan, H. Mudhar, H. Bancroft, H. Pallikonda, I. McNeish, I. Proctor, I. Tomlinson, I. Noorani, I. Lobon, J. Bridgewater, J. L. Reading, J. Black, J. Brenton, J. Larkin, J. Spicer, J. K. Rane, J. Biano, J. Nicod, J. Webb, J. L. Quesne, J. A. Shaw, J. Korteweg, K.-K. Shiu, K. Pearce, K. Young, K. S. S. Enfield, K. Kelly, K. Peat, K. Thol, K. G. Blyth, K. Litchfield, K. Allinson, K. Edmonds, K. Chan, K. Dijkstra, K. Grigoriadis, L. Farrelly, L. Grostate, L. Terry, L. Spain, L. Au, L. Pickering, L. Holt, L. Del Rosario, M. Jamal-Hanjani, M. Linch, M. Sivakumar, M. MacKenzie, M. Al Bakir, M. Collard, M. Forster, M. Falzon, M. Mangwende, M. Carter, M. G. Krebs, M. Emmerich, M. Akay, M. Jimenez-Linan, M. Dietzen, M. Angelova, M. Mitchison, M. O’Flaherty, N. Kanu, N. Magno, N. Yousaf, N. Wright, N. McGranahan, N. Hunter, O. Vainauskas, O. Curtis, O. Lucas, O. Pich, O. Al-Sawaf, P. Prymas, P. Roxburgh, P. Campbell, P. Oliveira, P. Cockcroft, P. Colloby, P. Ellery, P. Hill, P. Parker, P. Van Loo, P. Pawlik, P. Stone, S. Veeriah, R. Vendramin, R. Leslie, R. Fitzgerald, R. Zaidi, R. E. Hynds, R. Salgado, R. Wilson, R. Sinclair, R. Stewart, R. Mitu, S. Beck, S. K. Bola, S. Brandner, S. M. Janes, S. Lise, S. A. Quezada, S. Kuong A. Ung, S. Kadiri, S. Holden, S. Turajlic, S. Gamble, S. Jogai, S. Popat, S. Aitken, S. Benafif, J. Dransfield, S. Howlett, S. Shepherd, S. Ghosh, S. Irshad, S. Phillips-Boyd, S. Baijal, S. Zaccaria, S. Fraser, S. M. Lee, S. Hessey, Y. Ning S. Wong, S. Ward, S. Hazell, T. Enver, T. Karasaki, T. Fernandes, T. Ahmad, T. Sahwangarrom, T. Marafioti, T. B. K. Watkins, T. Maughan, U. Mahadeva, U. McGovern, V. Barbe, W. Drake, W. Hill, W. K. Liu, Y. Summers, Z. Tippu, Z. Rhodes, A. Stewart, A. Alzetani, A. J. Patel, A. Kirk, A. Kerr, A. J. Procter, A. Clipson, A. Rice, A. Bajaj, A. Devaraj, A. Grapa, A. G. Nicholson, A. Robinson, A. Leek, A. Montero, A. Karamani, A. Chaturvedi, A. Nakas, A. Nair, A. Ahmed, A. Osman, A. Sodha-Ramdeen, B. B. Campbell, C. Pilotti, C. Castignani, C. Veiga, C -A. Collins-Fekete, C. Proli, C. Lacson, C. Abbosh, C. E. Weeden, C. R. Lindsay, C. Dick, D. Kaniu, D. Chuter, D. Lawrence, D. R. Pearce, D. Patrini, D. Karagianni, D. Levi, D. G. Rothwell, E. Boleti, E. Borg, E. Kilgour, E. Smith, E. Hoxha, E. L. Cadieux, E. M. Hoogenboom, E. Lim, E. Fontaine, E. Gronroos, F. Granato, F. Galvez-Cancino, F. Monk, F. Fraioli, F. Gimeno-Valiente, G. A. Wilson, G. Royle, G. Kassiotis, G. Stavrou, G. Mastrokalos, G. Price, G. Matharu, H. Zhang, H. Zhai, H. K. Dhanda, H. Bhayani, H. Cheyne, H. Doran, H. L. Lowe, H. Shackleford, H. Chavan, H. Raubenheimer, H. J. W. L. Aerts, I. G. Matos, J. D. Hodgkinson, J. Goldman, J. R. M. Black, J. W. Holding, J. Wilson, J. F. Lester, J. Herrero, J. Richards, J. M. Lam, J. Riley, J. A. Hartley, J. Demeulemeester, J. Tugwood, J. Kisistok, J. Cave, J. Novasio, J. Choudhary, K. S. Peggs, K. Brown, K. Selvaraju, K. Gilbert, K. M. Kerr, K. Rammohan, K. Ang, K. G. Blyth, K. W. Ng, K. Chen, K. AbdulJabbar, K. Thakkar, K. Bhakhri, L. Ensell, L. Joseph, L. Robinson, L. Primrose, L. Scarlett, L. Ambrose, L. Priest, M. Djearaman, M. Hewish, M. N. Taylor, M. Shah, M. Scarci, M. V. Duran, M. Litovchenko, M. W. Sunderland, M. S. Hill, M. D. Forster, M. Hayward, M. Sokac, M. Carter, M. Thomas, M. J. Shackcloth, M. Leung, M. Escudero, M. Diossy, M. Taniƒá, M. Asif, M. Tufail, M. F. Chowdhry, M. Khalil, M. Scotland, M. Malima, N. Fernandes, N. Navani, N. Totten, N. J. Birkbak, N. Gower, N. Panagiotopoulos, N. Kostoulas, O. Ansorge, O. Chervova, P. Ingram, P. Gorman, P. Ashford, P. Bishop, P. De Sousa, P. Russell, P. Crosbie, P. Hobson, P. Shah, R. Rosenthal, R. Shah, R. Boyles, R. Khiroya, R. K. Stone, R. M. Thakrar, R. Bentham, R. C. M. Stephens, R. Bilancia, R. F. Schwarz, S. Saghafinia, S. Lopez, S. Booth, S. Danson, S. Rudman, S. Dulloo, S. Smith, S. Chee, S. Vanloo, S. Richardson, S. Austin, S. I. Buderi, S. Jordan, S. Begum, S. Rathinam, S. Boeing, S. Bandula, S. Moss, S. K. Bola, T. Denner, T. P. Mourikis, T. L. Kaufmann, T. Cruickshank, T. Clark, V. Spanswick, V. Joshi, W.-T. Lu, X. Pan, Y. Wu, Y. Yuan, Y. Naito, Z. Ramsden, Z. Szallasi, A. Dwornik, G. Langman, H. Shackleford, A. Osman, B. Naidu, K. Bowles, C. Grieco, M. L. Le, P. D. D’Arienzo and E. Turay. E.G was supported by an ERC Advanced grant (PROTEUS, grant agreement no 835297).
Author information
These authors contributed equally: William Hill, Emilia L. Lim, Clare E. Weeden
These authors jointly supervised this work: Emilia L. Lim, James DeGregori, Mariam Jamal-Hanjani
Authors and Affiliations
Cancer Evolution and Genome Instability Laboratory, The Francis Crick Institute, London, UK
William Hill, Emilia L. Lim, Clare E. Weeden, Claudia Lee, Marcellus Augustine, David A. Moore, Oriol Pich, Bjorn Bakker, Andrew Rowan, Takahiro Karasaki, Deborah R. Caswell, Ai Nagano, Alastair Magness, Maise Al Bakir, Chris Bailey, Mark S. Hill, Eva Grönroos, Gareth A. Wilson, Rachel Rosenthal, Nicolai J. Birkbak, Alexander M. Frankell, Ariana Huebner, Brittany B. Campbell, Clare Puttick, Crispin T. Hiley, Dhruva Biswas, Emma Colliver, Foteini Athanasopoulou, Haoran Zhai, Jayant K. Rane, Katey S. S. Enfield, Kristiana Grigoriadis, Michelle Dietzen, Michelle Leung, Mihaela Angelova, Olivia Lucas, Othman Al-Sawaf, Sophia Ward, Thomas B. K. Watkins & Charles Swanton
Cancer Research UK Lung Cancer Centre of Excellence, University College London Cancer Institute, London, UK
Emilia L. Lim, Claudia Lee, Marcellus Augustine, Kezhong Chen, David A. Moore, Hongui Cha, Selvaraju Veeriah, Cristina Naceur-Lombardelli, Takahiro Karasaki, Monica Sivakumar, Swapnanil De, Ai Nagano, James R. M. Black, Carlos Martínez-Ruiz, Alastair Magness, Maise Al Bakir, Christopher Abbosh, Nnennaya Kanu, Nicholas McGranahan, Kevin Litchfield, Nicolai J. Birkbak, Abigail Bunkum, Alexander M. Frankell, Antonia Toncheva, Ariana Huebner, Clare Puttick, Corentin Richard, Crispin T. Hiley, Dhruva Biswas, Foteini Athanasopoulou, Francisco Gimeno-Valiente, Haoran Zhai, Jie Min Lam, Kerstin Thol, Kristiana Grigoriadis, Krupa Thakkar, Mariana Werner Sunderland, Michelle Dietzen, Michelle Leung, Olivia Lucas, Othman Al-Sawaf, Paulina Prymas, Robert Bentham, Sadegh Saghafinia, Sergio A. Quezada, Sharon Vanloo, Simone Zaccaria, Sonya Hessey, Sophia Ward, Wing Kin Liu, Martin D. Forster, Siow Ming Lee, Mariam Jamal-Hanjani & Charles Swanton
Division of Medicine, University College London, London, UK
Claudia Lee & Marcellus Augustine
Tumour Immunogenomics and Immunosurveillance Laboratory, University College London Cancer Institute, London, UK
Marcellus Augustine & Kevin Litchfield
Department of Thoracic Surgery and Thoracic Oncology Institute, Peking University People’s Hospital, Beijing, China
Kezhong Chen
Department of Hematology and Oncology, Chang Gung Memorial Hospital, Chiayi Branch, Chiayi, Taiwan
Feng-Che Kuan
Graduate Institute of Clinical Medical Sciences, Chang-Gung University, Taoyuan, Taiwan
Department of Biochemistry and Molecular Genetics, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Fabio Marongiu, Edward J. Evans Jr, Clara I. Troccoli & James DeGregori
Department of Biomedical Sciences, University of Cagliari, Cagliari, Italy
Fabio Marongiu
Department of Cellular Pathology, University College London Hospitals, London, UK
David A. Moore, Teresa Marafioti, Elaine Borg, Mary Falzon & Reena Khiroya
Tumour–Host Interaction Laboratory, The Francis Crick Institute, London, UK
Felipe S. Rodrigues & Ilaria Malanchi
Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
Hongui Cha & Se-Hoon Lee
BC Cancer Research Institute, University of British Columbia, Vancouver, British Columbia, Canada
Renelle Myers & Stephen Lam
Oncogene Biology Laboratory, The Francis Crick Institute, London, UK
Febe van Maldegem, Jesse Boumelha & Julian Downward
Department of Molecular Cell Biology and Immunology, Amsterdam UMC, Amsterdam, The Netherlands
Febe van Maldegem
Cancer Metastasis Laboratory, University College London Cancer Institute, London, UK
Takahiro Karasaki, Abigail Bunkum, Jie Min Lam, Sonya Hessey, Wing Kin Liu & Mariam Jamal-Hanjani
Cancer Genome Evolution Research Group, Cancer Research UK Lung Cancer Centre of Excellence, University College London Cancer Institute, London, UK
James R. M. Black, Carlos Martínez-Ruiz, Nicholas McGranahan, Ariana Huebner, Clare Puttick, Kerstin Thol, Kristiana Grigoriadis, Michelle Dietzen, Michelle Leung & Robert Bentham
Department of Medicine, Division of Respiratory Medicine, Chan-Yeung Centre for Occupational and Environmental Respiratory Disease, Vancouver Coastal Health Research Institute, UBC, Vancouver, British Columbia, Canada
Min Hyung Ryu, Ryan D. Huff, Shijia Li & Christopher Carlsten
Ontario Institute for Cancer Research, Toronto, Ontario, Canada
Marie-Julie Favé & Philip Awadalla
Department of Pathobiology and Population Sciences, The Royal Veterinary College, Hatfield, UK
Alejandro Suárez-Bonnet & Simon L. Priestnall
Experimental Histopathology, The Francis Crick Institute, London, UK
Alejandro Suárez-Bonnet, Simon L. Priestnall, Emma Nye & Richard Kevin Stone
National Disease Registration Service (NDRS), NHS England, Leeds, UK
Margreet Lüchtenborg, Katrina Lavelle, Joanna Pethick, Steven Hardy & Fiona E. McRonald
Centre for Cancer, Society and Public Health, Comprehensive Cancer Centre, School of Cancer and Pharmaceutical Sciences, King’s College London, London, UK
Margreet Lüchtenborg
Health Information and Epidemiology Laboratory, Chang-Gung Memorial Hospital, Chiayi, Taiwan
Meng-Hung Lin
Flagship Biosciences, Boulder, CO, USA
Clara I. Troccoli
Division of Pulmonary Sciences and Critical Care Medicine, Department of Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Moumita Ghosh, York E. Miller & Robert L. Keith
Veterans Affairs Eastern Colorado Healthcare System, Aurora, CO, USA
York E. Miller & Robert L. Keith
Department of Pathology, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Daniel T. Merrick
SAGA Diagnostics, Lund, Sweden
Lao H. Saal, Yilun Chen & Anthony M. George
Division of Oncology, Department of Clinical Sciences, Lund University, Lund, Sweden
Early Cancer Detection Consultant, Bethesda, MD, USA
Christine D. Berg
Comprehensive Cancer Centre, King’s College London, London, UK
Peter Sasieni
Division of Genetics and Epidemiology, Institute of Cancer Research, London, UK
Richard Houlston & Clare Turnbull
David H. Koch Institute for Integrative Cancer Research, Cambridge, MA, USA
Tyler Jacks
Department of Biology, Massachusetts Institute of Technology, Cambridge, MA, USA
Cancer Research UK and UCL Cancer Trials Centre, London, UK
Allan Hackshaw, Abigail Sharp, Sean Smith, Nicole Gower, Harjot Kaur Dhanda, Kitty Chan, Camilla Pilotti & Rachel Leslie
Department of Oncology, University College London Hospitals, London, UK
Mariam Jamal-Hanjani & Charles Swanton
Singleton Hospital, Swansea Bay University Health Board, Swansea, UK
Jason F. Lester, Sarah Benafif, Jie Min Lam, Olivia Lucas, Martin D. Forster, Dionysis Papadatos-Pastos, James Wilson & Tanya Ahmad
University Hospitals of Leicester NHS Trust, Leicester, UK
Amrita Bajaj, Apostolos Nakas, Azmina Sodha-Ramdeen, Keng Ang, Mohamad Tufail, Mohammed Fiyaz Chowdhry, Molly Scotland, Rebecca Boyles, Sridhar Rathinam & Dean A. Fennell
University of Leicester, Leicester, UK
Claire Wilson, Domenic Marrone, Sean Dulloo & Dean A. Fennell
Cancer Research Centre, University of Leicester, Leicester, UK
Gurdeep Matharu, Jacqui A. Shaw, Joan Riley & Lindsay Primrose
Royal Free Hospital, Royal Free London NHS Foundation Trust, London, UK
Ekaterini Boleti
Aberdeen Royal Infirmary NHS Grampian, Aberdeen, UK
Heather Cheyne, Mohammed Khalil, Shirley Richardson & Tracey Cruickshank
Department of Medical Oncology, Aberdeen Royal Infirmary NHS Grampian, Aberdeen, UK
Gillian Price
University of Aberdeen, Aberdeen, UK
Gillian Price & Keith M. Kerr
Department of Pathology, Aberdeen Royal Infirmary NHS Grampian, Aberdeen, UK
Keith M. Kerr
The Whittington Hospital NHS Trust, London, UK
Kayleigh Gilbert
Birmingham Acute Care Research Group, Institute of Inflammation and Ageing, University of Birmingham, Birmingham, UK
University Hospital Birmingham NHS Foundation Trust, Birmingham, UK
Akshay J. Patel, Aya Osman, Christer Lacson, Gerald Langman, Helen Shackleford, Madava Djearaman, Salma Kadiri & Gary Middleton
Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK
Gary Middleton
Manchester Cancer Research Centre Biobank, Manchester, UK
Angela Leek, Jack Davies Hodgkinson & Nicola Totten
Wythenshawe Hospital, Manchester University NHS Foundation Trust, Wythenshawe, UK
Angeles Montero, Elaine Smith, Eustace Fontaine, Felice Granato, Helen Doran, Juliette Novasio, Kendadai Rammohan, Leena Joseph, Paul Bishop, Rajesh Shah, Stuart Moss, Vijay Joshi & Philip Crosbie
Division of Infection, Immunity and Respiratory Medicine, University of Manchester, Manchester, UK
Philip Crosbie
Cancer Research UK Lung Cancer Centre of Excellence, University of Manchester, Manchester, UK
Philip Crosbie, Anshuman Chaturvedi, Lynsey Priest, Pedro Oliveira, Alexandra Clipson, Jonathan Tugwood, Alastair Kerr, Dominic G. Rothwell, Elaine Kilgour & Caroline Dive
The Christie NHS Foundation Trust, Manchester, UK
Fabio Gomes, Kate Brown, Mathew Carter, Anshuman Chaturvedi, Lynsey Priest & Pedro Oliveira
Division of Cancer Sciences, The University of Manchester and The Christie NHS Foundation Trust, Manchester, UK
Colin R. Lindsay, Fiona H. Blackhall, Matthew G. Krebs & Yvonne Summers
Cancer Research UK Manchester Institute Cancer Biomarker Centre, University of Manchester, Manchester, UK
Alexandra Clipson, Jonathan Tugwood, Alastair Kerr, Dominic G. Rothwell, Elaine Kilgour & Caroline Dive
Artificial Intelligence in Medicine (AIM) Program, Mass General Brigham, Harvard Medical School, Boston, MA, USA
Hugo J. W. L. Aerts
Department of Radiation Oncology, Brigham and Women’s Hospital, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA
Radiology and Nuclear Medicine, CARIM and GROW, Maastricht University, Maastricht, The Netherlands
Institute for Computational Cancer Biology, Center for Integrated Oncology (CIO), Cancer Research Center Cologne Essen (CCCE), Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany
Roland F. Schwarz
Berlin Institute for the Foundations of Learning and Data (BIFOLD), Berlin, Germany
Roland F. Schwarz & Tom L. Kaufmann
Berlin Institute for Medical Systems Biology, Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC), Berlin, Germany
Tom L. Kaufmann
Department of Genetics, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Peter Van Loo
Department of Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Cancer Genomics Laboratory, The Francis Crick Institute, London, UK
Peter Van Loo, Jonas Demeulemeester, Carla Castignani & Elizabeth Larose Cadieux
Department of Molecular Medicine, Aarhus University Hospital, Aarhus, Denmark
Nicolai J. Birkbak, Judit Kisistok & Mateo Sokac
Department of Clinical Medicine, Aarhus University, Aarhus, Denmark
Bioinformatics Research Centre, Aarhus University, Aarhus, Denmark
Danish Cancer Society Research Center, Copenhagen, Denmark
Zoltan Szallasi & Miklos Diossy
Computational Health Informatics Program, Boston Children’s Hospital, Boston, MA, USA
Department of Bioinformatics, Semmelweis University, Budapest, Hungary
Zoltan Szallasi
Department of Pathology, ZAS Hospitals, Antwerp, Belgium
Roberto Salgado
Division of Research, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia
Department of Physics of Complex Systems, ELTE Eötvös Loránd University, Budapest, Hungary
Miklos Diossy
Integrative Cancer Genomics Laboratory, Department of Oncology, KU Leuven, Leuven, Belgium
Jonas Demeulemeester
VIB–KU Leuven Center for Cancer Biology, Leuven, Belgium
Computational Cancer Genomics Research Group, University College London Cancer Institute, London, UK
Abigail Bunkum, Olivia Lucas, Simone Zaccaria & Sonya Hessey
The Francis Crick Institute, London, UK
Aengus Stewart, Dina Levi, Jacki Goldman, Mickael Escudero, Philip Hobson, Roberto Vendramin, Stefan Boeing, Tamara Denner, Vittorio Barbè, Wei-Ting Lu, Yutaka Naito & Zoe Ramsden
University College London Cancer Institute, London, UK
Angeliki Karamani, Benny Chain, David R. Pearce, Despoina Karagianni, Elena Hoxha, Felip Gálvez-Cancino, Georgia Stavrou, Gerasimos Mastrokalos, Helen L. Lowe, Ignacio Garcia Matos, James L. Reading, Jayant K. Rane, John A. Hartley, Kayalvizhi Selvaraju, Leah Ensell, Mansi Shah, Marcos Vasquez Duran, Maria Litovchenko, Olga Chervova, Piotr Pawlik, Robert E. Hynds, Saioa López, Samuel Gamble, Seng Kuong Anakin Ung, Supreet Kaur Bola, Thanos P. Mourikis, Victoria Spanswick & Yin Wu
Medical Genomics, University College London Cancer Institute, London, UK
Carla Castignani, Elizabeth Larose Cadieux, Miljana Tanić & Stephan Beck
Bill Lyons Informatics Centre, University College London Cancer Institute, London, UK
Dhruva Biswas & Javier Herrero
Advanced Sequencing Facility, The Francis Crick Institute, London, UK
Foteini Athanasopoulou, Jerome Nicod & Sophia Ward
Retroviral Immunology Group, The Francis Crick Institute, London, UK
George Kassiotis & Kevin W. Ng
Department of Infectious Disease, Faculty of Medicine, Imperial College London, London, UK
George Kassiotis
Department of Haematology, University College London Hospitals, London, UK
Karl S. Peggs
Cancer Immunology Unit, Research Department of Haematology, University College London Cancer Institute, London, UK
Department of Molecular Oncology and Immunology, the Netherlands Cancer Institute, Amsterdam, The Netherlands
Krijn Dijkstra
Oncode Institute, Utrecht, The Netherlands
Experimental Oncology, Institute for Oncology and Radiology of Serbia, Belgrade, Serbia
Miljana Tanić
Othman Al-Sawaf
Immune Regulation and Tumour Immunotherapy Group, Cancer Immunology Unit, Research Department of Haematology, University College London Cancer Institute, London, UK
Sergio A. Quezada
Centre for Medical Image Computing, Department of Medical Physics and Biomedical Engineering, University College London, London, UK
Catarina Veiga
Department of Medical Physics and Bioengineering, University College London Cancer Institute, London, UK
Department of Medical Physics and Biomedical Engineering, University College London, London, UK
Charles-Antoine Collins-Fekete
Institute of Nuclear Medicine, Division of Medicine, University College London, London, UK
Francesco Fraioli
Institute of Structural and Molecular Biology, University College London, London, UK
Paul Ashford
University College London, London, UK
Tristan Clark
Siow Ming Lee
Department of Radiology, University College London Hospitals, London, UK
Alexander James Procter, Asia Ahmed, Magali N. Taylor & Arjun Nair
UCL Respiratory, Department of Medicine, University College London, London, UK
Department of Thoracic Surgery, University College London Hospital NHS Trust, London, UK
David Lawrence & Davide Patrini
Lungs for Living Research Centre, UCL Respiratory, University College London, London, UK
Neal Navani, Ricky M. Thakrar & Sam M. Janes
Department of Thoracic Medicine, University College London Hospitals, London, UK
Neal Navani & Ricky M. Thakrar
University College London Hospitals, London, UK
Emilie Martinoni Hoogenboom, Fleur Monk, James W. Holding, Junaid Choudhary, Kunal Bhakhri, Marco Scarci, Martin Hayward, Nikolaos Panagiotopoulos, Pat Gorman, Robert C. M. Stephens, Yien Ning Sophia Wong & Steve Bandula
The Institute of Cancer Research, London, UK
Anca Grapa, Hanyun Zhang, Khalid AbdulJabbar & Xiaoxi Pan
The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Yinyin Yuan
Independent Cancer Patients’ Voice, London, UK
David Chuter & Mairead MacKenzie
University Hospital Southampton NHS Foundation Trust, Southampton, UK
Serena Chee, Aiman Alzetani, Lydia Scarlett, Jennifer Richards, Papawadee Ingram & Silvia Austin
Department of Oncology, University Hospital Southampton NHS Foundation Trust, Southampton, UK
Judith Cave
Academic Division of Thoracic Surgery, Imperial College London, London, UK
Royal Brompton and Harefield Hospitals, Guy’s and St Thomas’ NHS Foundation Trust, London, UK
Eric Lim, Paulo De Sousa, Simon Jordan, Alexandra Rice, Hilgardt Raubenheimer, Harshil Bhayani, Lyn Ambrose, Anand Devaraj, Hema Chavan, Sofina Begum, Silviu I. Buderi, Daniel Kaniu, Mpho Malima, Sarah Booth, Nadia Fernandes, Pratibha Shah & Chiara Proli
Department of Histopathology, Royal Brompton and Harefield Hospitals, Guy’s and St Thomas’ NHS Foundation Trust, London, UK
Andrew G. Nicholson
National Heart and Lung Institute, Imperial College London, London, UK
Royal Surrey Hospital, Royal Surrey Hospitals NHS Foundation Trust, Guilford, UK
Madeleine Hewish
University of Surrey, Guilford, UK
Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK
Sarah Danson
Liverpool Heart and Chest Hospital, Liverpool, UK
Michael J. Shackcloth
Princess Alexandra Hospital, The Princess Alexandra Hospital NHS Trust, Harlow, UK
Lily Robinson & Peter Russell
School of Cancer Sciences, University of Glasgow, Glasgow, UK
Kevin G. Blyth & John Le Quesne
Cancer Research UK Beatson Institute, Glasgow, UK
Queen Elizabeth University Hospital, Glasgow, UK
Kevin G. Blyth
NHS Greater Glasgow and Clyde, Glasgow, UK
Pathology Department, Queen Elizabeth University Hospital, NHS Greater Glasgow and Clyde, Glasgow, UK
John Le Quesne
Golden Jubilee National Hospital, Clydebank, UK
Alan Kirk, Mo Asif, Rocco Bilancia, Nikos Kostoulas & Mathew Thomas
You can also search for this author in PubMed Google Scholar
TRACERx Consortium
- Jason F. Lester
- , Amrita Bajaj
- , Apostolos Nakas
- , Azmina Sodha-Ramdeen
- , Mohamad Tufail
- , Mohammed Fiyaz Chowdhry
- , Molly Scotland
- , Rebecca Boyles
- , Sridhar Rathinam
- , Claire Wilson
- , Domenic Marrone
- , Sean Dulloo
- , Dean A. Fennell
- , Gurdeep Matharu
- , Jacqui A. Shaw
- , Joan Riley
- , Lindsay Primrose
- , Ekaterini Boleti
- , Heather Cheyne
- , Mohammed Khalil
- , Shirley Richardson
- , Tracey Cruickshank
- , Gillian Price
- , Keith M. Kerr
- , Sarah Benafif
- , Kayleigh Gilbert
- , Babu Naidu
- , Akshay J. Patel
- , Aya Osman
- , Christer Lacson
- , Gerald Langman
- , Helen Shackleford
- , Madava Djearaman
- , Salma Kadiri
- , Gary Middleton
- , Angela Leek
- , Jack Davies Hodgkinson
- , Nicola Totten
- , Angeles Montero
- , Elaine Smith
- , Eustace Fontaine
- , Felice Granato
- , Helen Doran
- , Juliette Novasio
- , Kendadai Rammohan
- , Leena Joseph
- , Paul Bishop
- , Rajesh Shah
- , Stuart Moss
- , Vijay Joshi
- , Philip Crosbie
- , Fabio Gomes
- , Kate Brown
- , Mathew Carter
- , Anshuman Chaturvedi
- , Lynsey Priest
- , Pedro Oliveira
- , Colin R. Lindsay
- , Fiona H. Blackhall
- , Matthew G. Krebs
- , Yvonne Summers
- , Alexandra Clipson
- , Jonathan Tugwood
- , Alastair Kerr
- , Dominic G. Rothwell
- , Elaine Kilgour
- , Caroline Dive
- , Hugo J. W. L. Aerts
- , Roland F. Schwarz
- , Tom L. Kaufmann
- , Gareth A. Wilson
- , Rachel Rosenthal
- , Peter Van Loo
- , Nicolai J. Birkbak
- , Zoltan Szallasi
- , Judit Kisistok
- , Mateo Sokac
- , Roberto Salgado
- , Miklos Diossy
- , Jonas Demeulemeester
- , Abigail Bunkum
- , Aengus Stewart
- , Alastair Magness
- , Alexander M. Frankell
- , Andrew Rowan
- , Angeliki Karamani
- , Antonia Toncheva
- , Ariana Huebner
- , Benny Chain
- , Brittany B. Campbell
- , Carla Castignani
- , Carlos Martínez-Ruiz
- , Charles Swanton
- , Chris Bailey
- , Christopher Abbosh
- , Clare Puttick
- , Clare E. Weeden
- , Claudia Lee
- , Corentin Richard
- , Crispin T. Hiley
- , Cristina Naceur-Lombardelli
- , David A. Moore
- , David R. Pearce
- , Despoina Karagianni
- , Dhruva Biswas
- , Dina Levi
- , Elena Hoxha
- , Elizabeth Larose Cadieux
- , Emilia L. Lim
- , Emma Colliver
- , Eva Grönroos
- , Felip Gálvez-Cancino
- , Foteini Athanasopoulou
- , Francisco Gimeno-Valiente
- , George Kassiotis
- , Georgia Stavrou
- , Gerasimos Mastrokalos
- , Haoran Zhai
- , Helen L. Lowe
- , Ignacio Garcia Matos
- , Jacki Goldman
- , James L. Reading
- , James R. M. Black
- , Javier Herrero
- , Jayant K. Rane
- , Jerome Nicod
- , Jie Min Lam
- , John A. Hartley
- , Karl S. Peggs
- , Katey S. S. Enfield
- , Kayalvizhi Selvaraju
- , Kerstin Thol
- , Kevin Litchfield
- , Kevin W. Ng
- , Kezhong Chen
- , Krijn Dijkstra
- , Kristiana Grigoriadis
- , Krupa Thakkar
- , Leah Ensell
- , Maise Al Bakir
- , Mansi Shah
- , Marcos Vasquez Duran
- , Maria Litovchenko
- , Mariam Jamal-Hanjani
- , Mariana Werner Sunderland
- , Mark S. Hill
- , Michelle Dietzen
- , Michelle Leung
- , Mickael Escudero
- , Mihaela Angelova
- , Miljana Tanić
- , Monica Sivakumar
- , Nicholas McGranahan
- , Nnennaya Kanu
- , Olga Chervova
- , Olivia Lucas
- , Oriol Pich
- , Othman Al-Sawaf
- , Paulina Prymas
- , Philip Hobson
- , Piotr Pawlik
- , Richard Kevin Stone
- , Robert Bentham
- , Robert E. Hynds
- , Roberto Vendramin
- , Sadegh Saghafinia
- , Saioa López
- , Samuel Gamble
- , Selvaraju Veeriah
- , Seng Kuong Anakin Ung
- , Sergio A. Quezada
- , Sharon Vanloo
- , Simone Zaccaria
- , Sonya Hessey
- , Sophia Ward
- , Stefan Boeing
- , Stephan Beck
- , Supreet Kaur Bola
- , Takahiro Karasaki
- , Tamara Denner
- , Teresa Marafioti
- , Thanos P. Mourikis
- , Thomas B. K. Watkins
- , Victoria Spanswick
- , Vittorio Barbè
- , Wei-Ting Lu
- , William Hill
- , Wing Kin Liu
- , Yutaka Naito
- , Zoe Ramsden
- , Catarina Veiga
- , Gary Royle
- , Charles-Antoine Collins-Fekete
- , Francesco Fraioli
- , Paul Ashford
- , Tristan Clark
- , Martin D. Forster
- , Siow Ming Lee
- , Elaine Borg
- , Mary Falzon
- , Dionysis Papadatos-Pastos
- , James Wilson
- , Tanya Ahmad
- , Alexander James Procter
- , Asia Ahmed
- , Magali N. Taylor
- , Arjun Nair
- , David Lawrence
- , Davide Patrini
- , Neal Navani
- , Ricky M. Thakrar
- , Sam M. Janes
- , Emilie Martinoni Hoogenboom
- , Fleur Monk
- , James W. Holding
- , Junaid Choudhary
- , Kunal Bhakhri
- , Marco Scarci
- , Martin Hayward
- , Nikolaos Panagiotopoulos
- , Pat Gorman
- , Reena Khiroya
- , Robert C. M. Stephens
- , Yien Ning Sophia Wong
- , Steve Bandula
- , Allan Hackshaw
- , Abigail Sharp
- , Sean Smith
- , Nicole Gower
- , Harjot Kaur Dhanda
- , Kitty Chan
- , Camilla Pilotti
- , Rachel Leslie
- , Anca Grapa
- , Hanyun Zhang
- , Khalid AbdulJabbar
- , Xiaoxi Pan
- , Yinyin Yuan
- , David Chuter
- , Mairead MacKenzie
- , Serena Chee
- , Aiman Alzetani
- , Judith Cave
- , Lydia Scarlett
- , Jennifer Richards
- , Papawadee Ingram
- , Silvia Austin
- , Paulo De Sousa
- , Simon Jordan
- , Alexandra Rice
- , Hilgardt Raubenheimer
- , Harshil Bhayani
- , Lyn Ambrose
- , Anand Devaraj
- , Hema Chavan
- , Sofina Begum
- , Silviu I. Buderi
- , Daniel Kaniu
- , Mpho Malima
- , Sarah Booth
- , Andrew G. Nicholson
- , Nadia Fernandes
- , Pratibha Shah
- , Chiara Proli
- , Madeleine Hewish
- , Sarah Danson
- , Michael J. Shackcloth
- , Lily Robinson
- , Peter Russell
- , Kevin G. Blyth
- , Craig Dick
- , John Le Quesne
- , Alan Kirk
- , Rocco Bilancia
- , Nikos Kostoulas
- & Mathew Thomas
Contributions
W.H. and E.L.L jointly conceptualized the project, designed the project, performed the experiments analyses and wrote the manuscript. W.H. led and performed the mouse experiments, and E.L.L. led and performed the bioinformatics and epidemiology analyses. C.E.W. performed the mouse experiments, helped write the manuscript and curated the mutation literature. C.L. performed the human RNA-seq analyses and curated the pollution data. M.A. performed the UKBB analyses. K.C. assembled and analysed the TRACERx cohort dataset. F.-C.K. and M.-H.L. performed the Taiwan epidemiological analyses. F. Marongiu, E.J.E.J., C.I.T., M.G., Y.E.M., D.T.M. and R.L.K. generated and analysed the Duplex-seq data. O.P. wrote the Duplex-seq bioinformatics pipeline and performed the mutational signature analyses. H.C. and S.-H.L. performed the Korea epidemiological analyses. F.v.M., J.B., A.M. and D.R.C. were involved in mouse data acquisition. F.S.R. was involved with organoid experiments. S.V., A.R. and C.N.-L. curated and performed DNA extractions on TRACERx and PEACE samples. T.K. helped analyse patient clinical characteristics. D.M., S.D. and M.S. performed pathological assessments of human tissue samples. A.N., B.B., J.R.M.B. and C.M.-R. performed mouse RNA-seq analyses. M.H.R., R.D.H. and S.L. designed and generated data for the human crossover study. A.S.-B. And S.L.P. were involved in mouse pathology analyses. M.L., K. Lavelle, J.P., S.H. and F.E.M. curated England’s National Disease Registration Service data et. R.M. curated the Canadian cohort. M.A.B. and C.B. wrote and ran the MiSeq pipeline. C.A., L.H.S., Y.C. and A.M.G. performed the ddPCR experiments. I.M., J. Downward, T.J., N.K. and E.G. provided supervision for the mouse experiments. M.-J.F., M.H., P.A. and N.M. provided guidance and supervision for the bioinformatics analyses. S.L., P.S., R.H., C.T., C.D.B., A.H. and K. Litchfield provided supervision for epidemiological analyses. C.C. provided supervision for the human crossover study. J. DeGregori designed the BDRE study and supervised the healthy tissue profiling work. M.J.-H. designed the PEACE and TRACERx study protocols, and E.L.L. and M.J.-H. jointly supervised the study and collaborations. C.S. supervised the work, provided strategic oversight and helped write the manuscript.

Corresponding author
Correspondence to Charles Swanton .
Ethics declarations
Competing interests.
M.A.B. has consulted for Achilles Therapeutics. L.H.S., Y.C. and A.M.G. have ownership interest in SAGA Diagnostics. S.V. is a co-inventor to a patent to detecting molecules in a sample (US patent 10578620). D.A.M. reports speaker fees from AstraZeneca, Eli Lilly and Takeda, consultancy fees from AstraZeneca, Thermo Fisher, Takeda, Amgen, Janssen, MIM Software, Bristol-Myers Squibb and Eli Lilly, and has received educational support from Takeda and Amgen. C.A. has received speaking honoraria or expenses from Novartis, Roche, AstraZeneca and Bristol-Myers Squibb and reports employment at AstraZeneca. C.A. is an inventor on a European patent application relating to assay technology to detect tumour recurrence (PCT/GB2017/053289). The patent has been licensed to commercial entities and under their terms of employment, C.A is due a revenue share of any revenue generated from such licence(s). C.A. declares a patent application (PCT/US2017/028013) for methods to detect lung cancer. C.A. is a named inventor on a patent application to determine methods and systems for tumour monitoring (PCT/EP2022/077987). T.J. is a member of the Board of Directors of Amgen and Thermo Fisher Scientific, and a co-Founder of Dragonfly Therapeutics and T2 Biosystems. T.J. serves on the Scientific Advisory Board (SAB) of Dragonfly Therapeutics, SQZ Biotech and Skyhawk Therapeutics. T.J. is also President of Break Through Cancer. K. Litchfield has a patent on indel burden and CPI response pending and speaker fees from Roche tissue diagnostics, research funding from CRUK TDL–Ono–LifeArc alliance, Genesis Therapeutics, and consulting roles with Ellipses Pharma, Monopteros and Kynos Therapeutics. N.M. has received consultancy fees and has stock options in Achilles Therapeutics. N.M. holds European patents relating to targeting neoantigens (PCT/EP2016/ 059401), identifying patient response to immune checkpoint blockade (PCT/EP2016/071471), determining HLA LOH (PCT/GB2018/052004), and predicting survival rates of patients with cancer (PCT/GB2020/050221). C.T. has received honoraria for educational activities and advisory boards from AstraZeneca and Roche (all proceeds donated to registered charity 11511580). C.D.B. has consultantships with GRAIL, LLC, NHS Galleri Trial, IDMC, Mercy BioAnalytics, Lucid DX and Medial EarlySign. J. Downward has acted as a consultant for AstraZeneca, Jubilant, Theras, Roche and Vividion and has funded research agreements with Bristol-Myers Squibb, Revolution Medicines and AstraZeneca. A.H. has received fees for being a member of Independent Data Monitoring Committees for Roche-sponsored clinical trials, and academic projects co-ordinated by Roche. M.J.-H. is a CRUK Career Establishment Awardee and has received funding from CRUK, NIH National Cancer Institute, IASLC International Lung Cancer Foundation, Lung Cancer Research Foundation, Rosetrees Trust, UKI NETs, NIHR, NIHR UCLH Biomedical Research Centre. M.J.-H. has consulted for, and is a member of, the Achilles Therapeutics Scientific Advisory Board and Steering Committee, has received speaker honoraria from Pfizer, Astex Pharmaceuticals, Oslo Cancer Cluster, Bristol Myers Squibb, and is co-inventor on a European patent application relating to methods to detect lung cancer PCT/US2017/028013).M.G., Y.E.M., R.L.K. and D.T.M. acknowledge grant support from Bristol-Myers Squibb. C.S. acknowledges grant support from AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Pfizer, Roche-Ventana, Invitae (previously Archer Dx Inc–collaboration in minimal residual disease sequencing technologies), and Ono Pharmaceutical. C.S. is an AstraZeneca Advisory Board member and Chief Investigator for the AZ MeRmaiD 1 and 2 clinical trials and is also Co-Chief Investigator of the NHS Galleri trial funded by GRAIL and a paid member of GRAIL’s SAB. He receives consultant fees from Achilles Therapeutics (also SAB member), Bicycle Therapeutics (also a SAB member), Genentech, Medicxi, Roche Innovation Centre–Shanghai, Metabomed (until July 2022), and the Sarah Cannon Research Institute. He had stock options in Apogen Biotechnologies and GRAIL until June 2021, and currently has stock options in Epic Bioscience, Bicycle Therapeutics, and has stock options and is co-founder of Achilles Therapeutics. C.S. is an inventor on a European patent application relating to assay technology to detect tumour recurrence (PCT/GB2017/053289), the patent has been licensed to commercial entities, and under his terms of employment, C.S. is due a revenue share of any revenue generated from such licence(s). C.S. holds patents relating to targeting neoantigens (PCT/EP2016/059401), identifying patient response to immune checkpoint blockade (PCT/EP2016/071471), determining HLA LOH (PCT/GB2018/052004), predicting survival rates of patients with cancer (PCT/GB2020/050221), identifying patients who respond to cancer treatment (PCT/GB2018/051912), a US patent relating to detecting tumour mutations (PCT/US2017/28013), methods for lung cancer detection (US20190106751A1) and both a European and US patent related to identifying insertion/deletion mutation targets (PCT/GB2018/051892) and is co-inventor to a patent application to determine methods and systems for tumour monitoring (PCT/EP2022/077987). C.S has received honoraria from Amgen, AstraZeneca, Pfizer, Novartis, GlaxoSmithKline, MSD, Bristol Myers Squibb, Illumina, and Roche-Ventana.
Peer review
Peer review information.
Nature thanks Aaron Cohen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended data fig. 1 study design, dna analysis & epidemiology..
A) Study design schematic featuring the 3 aspects of the paper. LEFT: Epidemiological analysis of cancer incidence and PM2.5. MIDDLE: Pollution exposure in mouse models. RIGHT: Normal lung tissue analysis. B) TX421 Tumours from Smokers. Barplots indicating proportion of SNVs in each tumour attributed to each SBS mutational signature. The barplots (Top: Lung adenocarcinoma (LUAD), Bottom: Lung sqaumous cell carcinoma (LUSC)) reflect the probability that clonal driver mutations in patients, where smoking-related signatures have been detected, are caused by different mutational processes (SBS4 and SBS92 smoking, SBS2 and SBS13 APOBEC, SBS1 and SBS5 ageing). Each observed driver mutation in each patient is given a mutational-signature-causing probability based on the trinucleotide context and the signatures exposure of the patient (see Methods ) and then these probabilities are aggregated. Asterisks represent patients where the smoking-related aggregated probabilities are below 0.5. C) Correlation between PM 2.5 levels and EGFR mutant (EGFRm) adenocarcinoma lung cancer incidence in England. The blue line: robust linear regression line; grey shading: 95% confidence interval. D-E) The Canadian Lung Cancer Cohort. D) Distribution of 3 year and 20 year cumulative PM 2.5 exposure levels for all patients in the Canadian cohort. Red lines mark the thresholds that were used to determine Low, Intermediate and High groups that are used in (D). These are the 1st (6.77 ug/m 3 ) and 5th quintiles (7.27 ug/m 3 ) of the distribution. The full distribution is displayed in the top plot, while the bottom plot displays a narrower range of 4–10 ug/m 3 (for clarity). E) Counts and frequencies of EGFRm in the Canadian Cohort, where 3 year and 20 year cumulative PM 2.5 exposure levels were available. Patients are grouped into high, intermediate and low groups based on thresholds established as described in (D). These groups are defined based on 3 year cumulative PM 2.5 exposure data (left) and based on 20 year cumulative PM 2.5 exposure data (right). The bar plots display the counts and frequency of EGFRm amongst patients within each group. The map was created using DEFRA data in R. The illustrations in A were created using BioRender ( https://biorender.com ).
Extended Data Fig. 2 Effect of PM in multiple mouse models of lung cancer.
A) Schematic of PM exposure and representative huEGFR L858R IHC of ET mice induced with AT2-specific Ad5-SPC-Cre exposed to PM or PBS control and quantification of neoplastic lesions (n = 14 PBS, n = 11 PM). Mann-Whitney test. B) Schematic of PM exposure followed by induction of EGFR L858R and quantification of precancerous lesions/mm 2 of lung tissue (n = 9 PBS; n = 7 5 μg; n = 11 50 μg PM). One-way ANOVA. C) Schematic of PM exposure and representative H&E of a lung adenocarcinoma in a 50 μg PM exposed, doxycycline treated CCSP-rtTa; TetO-EGFR L858R mice; quantification of number of adenocarcinomas per mouse below (n = 9 per group). One-way ANOVA. D) Schematic of PM exposure and representative IHC for red fluorescent protein (RFP, marks tdTomato+ cells) in Rosa26 LSL-tdTomato/+ ; Kras LSL-G12D/+ mouse model in control or 50 μg PM exposed conditions; quantification of number of hyperplastic lesions per mouse (n = 9 control, n = 9 5 μg and n = 12 50 μg). One-way ANOVA. Scale bar 50 μm (C main), 20 μm (C insert), 100 μm A & D.
Source data
Extended data fig. 3 whole genome sequencing analysis of mouse tumours..
WGS analysis of tumours from ET mice exposed to air pollution (n = 5) and those exposed to PBS controls (n = 5). Each mouse tumour is compared vs the corresponding germline from the same mouse. A) Mutational profiles for each tumour sample according to the mutation trinucleotide context. LEFT: PBS Controls, RIGHT: 50 μg PM. B) Barplots indicate the counts of mutations in each sample, where bars are colored based on the base change. C) Boxplot comparing the counts of mutations between tumours from pollution exposed mice (50 μg PM) and tumours from PBS exposed mice (PBS Control). All mutations are summarised in one plot on the left, and are then further divided based on the base change of the mutation (n = 5 mice per group). Two-sided t-test comparing numbers of mutations between PBS and air pollution p-values are displayed. The boxplot line represents the median, the hinges of the box represent the 1st and 3rd quartiles and the limits of the whiskers represent the 1.5 interquartile range. D) Attribution of mutations in each tumour sample to each single base substitution (SBS) mutation signature. The shading indicates the weight of the signature within each sample. Majority of the weights have been assigned to ageing related signatures (SBS40, SBS5, SBS1) Komogolomov-Smirnoff test p-value = 0.26–0.68.
Extended Data Fig. 4 Immune cell profiling in response to PM.
A) Immune cell frequencies in the lungs determined by flow cytometry 24 h post-exposure from induced T and ET mice after 50 μg PM (red) or PBS control (blue) (n = 8 mice per group). Data are presented as the frequency among live CD45+ immune cells. One-way ANOVA. B) Representative immunofluorescent images of CD68+ macrophages (cyan) and tdTomato+ EGFR mutant cells (red) within ET lungs exposed to control or 50 μg PM. Quantification of CD68+ cells per mm 2 of lung tissue (n = 4 mice per group). One-way ANOVA. C) Representative immunofluorescent images of CD68 (red), CD11b (green) and merged images from induced ET mice after 3 weeks of exposure to PBS (top) or 50 μg PM (bottom). Quantification of alveolar macrophages (AMΦ, CD68+CD11b−) and interstitial macrophages (IMΦ, CD68+CD11b+) per mm 2 of lung tissue, selecting 10 x random 500 μm 2 fields of view per mouse (n = 3 mice per group). One-way ANOVA. D) Representative immunofluorescent images of CD68+ macrophages (cyan) within CCSP-rtTA; TetO-EGFR L858R lungs treated with PBS (top) or 50 μg PM (bottom) 10 weeks post oncogene induction; quantification of CD68+ cells per mm 2 of lung tissue, selecting 20 x random 500 μm 2 fields of view per mouse (n = 3 mice per group). Unpaired t-test. E) Representative immunofluorescent images of CD68+ macrophages (cyan) and tdTomato+ Kras G12D mutant cells (red) within KT lungs treated with PBS (top panel) or 50 μg PM (bottom) 10 weeks post oncogene induction; quantification of CD68+ cells per mm 2 of lung tissue, selecting 20 x 500 μm 2 fields of view containing RFP+ cells per mouse (n = 3 mice per group). Unpaired t-test. Scale bar 50 µm B & D, 150 µm C & E. Gating strategies for flow cytometry analysis provided in Extended Data Fig. 6 .
Extended Data Fig. 5 PM-mediated transcriptional changes, effects on AT2 cells and characterising IL-1β.
A-B) Significantly enriched GSEA pathways upregulated in T-PM lung epithelial cells compared to T control mice (A), in ET-PM lung epithelial cells compared to ET control mice (B). For each comparison, barplots indicate the -log10(FDR) of the Komogolomov-Smirnoff test p-value for each pathway. C) AT2 activated progenitor score derived from scRNAseq of bleomycin treated mouse lung used to deconvolute bulk RNA-seq of T and ET mice exposed to 50 μg PM or PBS, (n = 5 mice per group). Welch’s t-test between control and PM. Line represents mean of data. D) Schematic displaying experimental set-up of clinical exposure study in never-smoker volunteers, crossover design with (i) and (ii) in random order separated by 4-week washout. E) Fold change (FC) of significantly upregulated genes (identified in mouse) compared to the fold change of genes changed in the clinical exposure study. Common directionality across species indicated by colour (negative: blue background; positive: red background). F) Schematic of AT2 culture from T or ET mice exposed to 50 μg PM or PBS, with induction of tdTomato or oncogene ex vivo . G) Representative fluorescent images of tdTomato+ AT2 organoids at day 14 from ET mice exposed to PBS or 50 μg PM in vivo. Scale bar 100 μm. H) Quantification of tdTom+ AT2 organoid forming efficiency, data represents averages from 2 technical replicates/mouse; n = 4 mice from T control and PM; n = 5 mice for ET control and PM. One-way ANOVA. I) Representative fluorescent imaging of tdTomato (yellow), Keratin 8 (magenta), SPC (blue) on a wholemount AT2 organoid from an ET mouse treated with 50 μg PM. Scale bar is 20 μm. J) LEFT: Representative IL-1β RNAscope performed on lungs from ET mice treated with PBS or 50 μg PM after 3 weeks of exposure. Scale bar 20 µm. RIGHT: Quantification of IL-1β+ cells per mm 2 of lung tissue from 30 random fields of view (control, n = 3 mice) and 28 fields of view (50 μg PM, n = 3 mice). Mann-Whitney test p-value is displayed. K) LEFT: Representative image of IL-1β RNAscope (green) in CD68 positive (red) macrophages in an ET mouse exposed to 50 μg PM, arrows indicate positive macrophages. n = 3 mice exposed to 50 μg PM. Scale bar 50 μm. RIGHT: Quantification of IL-1β positive CD68+ cells compared to CD68− cells at 3 weeks post induction in ET mice following exposure to PM. Mann-Whitney test. L) LEFT: Representative fluorescent images of EGFR L858R naive (non-PM exposed) AT2 organoids from ET mice treated with control or IL-1β in vitro . tdTomato (yellow) organoids stained with SPC (blue) and Keratin 8 (magenta). Scale bar 50 μm. RIGHT: Quantification of organoid size with each dot representing an organoid at day 14 of control (blue) or IL-1β treated (orange). Organoids derived from n = 2 mice per group. Mann-Whitney test. M) Schematic of anti-IL-1β treatment treatment (black triangles) during PM exposure (black lines) and harvest (red triangle). The illustrations in d and f were created using BioRender ( https://biorender.com ).
Extended Data Fig. 6 Flow cytometry Gating strategy used to identify epithelial and immune cells.
A, B) Example of flow gating strategy to determine frequency of lung (A) alveolar macrophages, interstitial macrophages, neutrophils, dendritic cells and (B) epithelial cells both tdTomato positive and negative. All samples were first gated to exclude debris and doublets, followed by live cell discrimination. C) Representative picture from a tdTomato mouse treated with control PBS for 3 weeks using sort strategy to enrich for for AT2 cells defined in Major et al. 61 and both alveolar and interstitial macrophages defined in Choi et al. 22 .
Extended Data Fig. 7 CONSORT Diagrams for the normal lung tissue profiling cohorts.
TOP: TRACERx study, MIDDLE: PEACE study, BOTTOM: BDRE study.
Extended Data Fig. 8 Normal tissue study design and ddPCR results.
A) Schematic indicating normal lung tissue cohorts analysed by ddPCR and Duplex-seq. B) TRACERx and PEACE Cohort for ddPCR of 5 EGFR mutations. (i) Clinical information for each patient, (ii) Tumour EGFR mutation status, (iii) Normal EGFR mutation status. C) Representative H & E images from anthracotic pigment identification in TRACERx normal tissue. D) Comparing area of normal tissue harbouring anthracotic pigment in never smokers (n = 43) and smokers (n = 138). Each dot represents the ratio of pigmented area respective to total tissue in each anthracosis positive normal lung tissue sample. Two-sided Wilcox test p-value is reported. E) Regression analysis of characteristics influences EGFR mutant (EGFRm) presence in normal lung tissue for ddPCR-TRACERx cohort (n = 195). The illustrations in a were created using BioRender ( https://biorender.com ).
Extended Data Fig. 9 Normal tissue Duplex-seq results.
A) Top: EGFR Mutations detected using Duplex-seq across EGFR exons 18–21 on normal lung samples from the BDRE Study. Bottom: VAFs of each EGFR mutation are displayed. B) Top: KRAS Mutations detected using Duplex-seq across KRAS exons 2-3 on normal lung samples from the BDRE Study. Bottom: VAFs of each KRAS mutation are displayed. A-B) Only cancer-related mutations annotated in the cancer gene census are displayed. Mutations with strong evidence of being a lung cancer driver mutation are indicated in red, while mutations with some evidence of being a lung cancer driver mutation are indicated in pink, all other drivers annotated in COSMIC are indicated in blue. C) VAFs of KRAS mutations across samples of different cancer types. The one patient who received BRAF inhibitor treatment is indicated in purple. D) Comparing VAFs of high confidence (var count >=2, strong evidence) driver mutations in EGFR and KRAS . TOP: Boxplots summarise VAFs across samples. The boxplot line represents the median, the hinges of the box represent the 1st and 3rd quartiles and the limits of the whiskers represent the 1.5 interquartile range. Mutations are grouped according to the gene harbouring the mutation and smoking status of the patient. Two-sided Wilcox test p-values are reported. BOTTOM: dot plots show VAFs of mutations in each sample. Where a sample has 2 mutations (n = 4), they are both indicated. Dots are coloured by the gene harbouring the mutation ( EGFR or KRAS ). A paired t-test was performed between the VAFs of EGFR and KRAS mutations in these 4 cases. (Paired t-test p = 0.015) (Details of driver mutations can be found in Supplementary Table S8 ).
Supplementary information
Reporting summary, supplementary table 1.
Patient Characteristics of the England Cohort . Summary of clinical characteristics from the England lung cancer cohort. The ‘EGFRwt vs EGFRm’ sheet compares patients with and without EGFR mutations in their tumours, whereas the ‘EGFRm Tested vs Non Tested’ sheet compares patients who were tested and untested for EGFR mutations. Chi-squared test P values are reported.
Supplementary Table 2
Patient characteristics of the Korea cohort . Summary of clinical characteristics from the South Korea lung cancer cohort. The ‘EGFRwt vs EGFRm’ sheet compares patients with and without EGFR mutations in their tumours. Chi-squared test P values are reported.
Supplementary Table 3
Patient characteristics of the Taiwan cohort . Summary of clinical characteristics from the Taiwan lung cancer cohort. The ‘EGFRwt vs EGFRm’ sheet compares patients with and without EGFR mutations in their tumours, whereas the ‘EGFRm Tested vs Non Tested’ compares patients who were tested and untested for EGFR mutations. Chi-squared test P values are reported.
Supplementary Table 4
UK Biobank interaction tests and cancer type definitions . Results of multivariable Cox regressions investigating PM 2.5 and cancer incidence in the UKBB. The ‘Lung (main)’ sheet shows the results for all covariates for the analysis on the full cohort. The ‘PanCancer’ sheet features the results for PM 2.5 for all cancer types analysed. The ‘Lung (adenocarcinoma only)’ and ‘Lung (migration)’ sheets contain the results for all covariates when analysing only LUAD and those who remained at their baseline address for at least 3 years before baseline, respectively. Cox-regression P values are reported. The ‘ICD10codes_cancerTypes’ sheet contains the ICD tenth version codes used to define each analysed cancer type.
Supplementary Table 5
Mouse RNA-seq . Results of the differential expression analysis of RNA-seq libraries from reporter tdTomato mice exposed to PBS (T), or particulate matter (T+PM); or tdTomato ; EGFR L858R mice exposed to PBS (ET+PBS) or particulate matter (ET+PM). The ‘Mouse DGE Analysis’ sheet features for each gene, metrics output from DESeq2 and the top two PCs from the PC analysis. The ‘Mouse and Human DGE Analysis Comparison’ sheet features for each gene where human and mouse orthologues can be mapped, the differential expression analysis metrics between air pollution exposed libraries (Mouse: T+PM; Human: Diesel Exhaust (DE)) vs control (Mouse: T; Human: Filtered Air (FA)) libraries.
Supplementary Table 6
Healthy tissue Datasets analysed for EGFR mutations . Published datasets of DNA sequencing of healthy human tissues (skin, lung, oesophagus, colorectal, small intestine, liver, uterus and bladder) describing patient cohorts, sampling, sequencing technology and mutation calling. The presence of any EGFR mutation and EGFR L858R mutation with associated VAFs are reported.
Supplementary Table 7
Cohort clinical characteristics of the TRACERx, PEACE and BDRE studies . Clinical characteristics of each patient included in the healthy lung tissue profiling work. There is one sheet dedicated to each cohort: ‘ddPCR TRACERx’, ‘ddPCR PEACE’, ‘Duplex-seq PEACE’ and ‘Duplex-seq BDRE’.
Supplementary Table 8
Evidence of EGFR or KRAS cancer driver mutation status . Non-silent EGFR and KRAS mutations, detected by Duplex-Seq within PEACE and BDRE cohorts, were researched for published evidence of cancer driver status. For EGFR , literature reports of >1 patient with mutation (not including compound mutations) achieving stable disease (SD), partial response (PR) or complete response (CR) to a clinical EGFR inhibitor combined with supporting evidence (defined from in vitro studies, mouse model data or protein modelling analyses) were classified as ‘strong evidence’ drivers. Reports of 1 patient with SD, PR or CR to clinical EGFR inhibition or the presence of other supporting evidence were defined as ‘some evidence’ drivers. No reported patient sensitivity to EGFR inhibition or little supporting reports were defined as ‘weak evidence’ drivers. For KRAS , literature reports of frequent occurrence in cancer (above or equal to 2% of KRAS mutant cancers), in vitro studies and mouse model data were classified as ‘strong evidence’ drivers; protein modelling analyses were classified as ‘some evidence’ drivers.
Supplementary Table 9
Frequency of EGFR mutations and KRAS mutations in healthy lung tissue . Summaries of the proportion of patients with healthy lung tissues harbouring EGFR or KRAS mutations. Patients are stratified according to diagnosis, sex and smoking status. Proportion test P values are reported.
Supplementary Table 10
List of antibodies . Antibodies used for flow cytometry, immunohistochemical and immunofluorescence analyses of mouse tissue.
Source Data Fig. 2
Source data fig. 3, source data extended data fig. 2, source data extended data fig. 4, source data extended data fig. 5, rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Hill, W., Lim, E.L., Weeden, C.E. et al. Lung adenocarcinoma promotion by air pollutants. Nature 616 , 159–167 (2023). https://doi.org/10.1038/s41586-023-05874-3
Download citation
Received : 17 June 2022
Accepted : 21 February 2023
Published : 05 April 2023
Issue Date : 06 April 2023
DOI : https://doi.org/10.1038/s41586-023-05874-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
- Search Menu
- Sign in through your institution
- Supplements
- Cohort Profiles
- Education Corner
- Author Guidelines
- Submission Site
- Open Access
- About the International Journal of Epidemiology
- About the International Epidemiological Association
- Editorial Team
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Contact the IEA
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
I. mortality and population data, ii. retrospective and prospective studies, iii. studies on pathogenesis, iv. other laboratory investigations, v. interpretation.
- < Previous
Smoking and lung cancer: recent evidence and a discussion of some questions *
- Article contents
- Figures & tables
- Supplementary Data
Jerome Cornfield, William Haenszel, E. Cuyler Hammond, Abraham M. Lilienfeld, Michael B. Shimkin, Ernst L. Wynder, Smoking and lung cancer: recent evidence and a discussion of some questions, International Journal of Epidemiology , Volume 38, Issue 5, October 2009, Pages 1175–1191, https://doi.org/10.1093/ije/dyp289
- Permissions Icon Permissions
Summary This report reviews some of the more recent epidemiologic and experimental findings on the relationship of tobacco smoking to lung cancer, and discusses some criticisms directed against the conclusion that tobacco smoking, especially cigarettes, has a causal role in the increase in broncho-genic carcinoma. The magnitude of the excess lung-cancer risk among cigarette smokers is so great that the results can not be interpreted as arising from an indirect association of cigarette smoking with some other agent or characteristic, since this hypothetical agent would have to be at least as strongly associated with lung cancer as cigarette use; no such agent has been found or suggested. The consistency of all the epidemiologic and experimental evidence also supports the conclusion of a causal relationship with cigarette smoking, while there are serious inconsistencies in reconciling the evidence with other hypotheses which have been advanced. Unquestionably there are areas where more research is necessary, and, of course, no single cause accounts for all lung cancer. The information already available, however, is sufficient for planning and activating public health measures. – J. Nat. Cancer Inst . 22: 173–203, 1959.
“The sum total of scientific evidence establishes beyond reasonable doubt that cigarette smoking is a causative factor in the rapidly increasing incidence of human epidermoid carcinoma of the lung.”
The consideration of the accumulated scientific evidence has led to the acceptance of a similar viewpoint by responsible public health officials in Great Britain, the Netherlands, Norway, and the United States. This consensus of scientific and public health opinion does not mean that all problems, regarding smoking and lung cancer have now been solved or that valid questions and reservations about some aspects of the subject do not remain. An excellent collection of primary references and opinions expressing both “sides” of the question was issued by a committee of the House of Representatives 3 which sought to examine the claims of filter-tip cigarette advertisements.
The general acceptance of the cigarette-lung-cancer relationship has not decreased research interest but has accelerated research in this and in such related fields as respiratory physiology and environmental carcinogens, and on the effect of tobacco smoke in a wide range of physiological and pathological reactions.
The result is that considerably more information has been published or has become available through other media. Included in the recent scientific evidence are the following:
Additional retrospective studies 4 , 5 , 6 on men with lung cancer and on matched controls have appeared. All show an association between cigarette smoking and epidermoid-undifferentiated lung cancer.
Additional retrospective studies on women 7 , 6 also show the association.
The first results of a third large prospective study 8 , which included 200,000 United States veterans who were observed for 30 months, duplicate closely the reported findings of the Hammond-Horn 9 and the Doll-Hill 10 studies.
Analyses by Kreyberg and others 11 , 12 substantiate that, epidemiologically, primary lung cancer must be divided into epidermoid-undifferentiated and adenocarcinoma. The latter is much less related to smoking and, so far as is know at present, to other carcinogenic inhalants.
Additional findings have become available on the impingement of tobacco-smoke particles in the bronchi of animals, ciliary paralysis, and penetration of unidentified fluorescent materials into the bronchial cells. 13 , 14 , 15
Additional data have been published 16 , 17 on the more frequent occurrence of hyperplastic and metaplastic changes in the lungs of smokers as compared with the lungs of nonsmokers. Hyperplastic and metaplastic changes have been produced in bronchi of dogs exposed to direct contact with tobacco “tars” 18 and in bronchi of mice exposed to tobacco smoke. 19
Additional confirmations have been obtained on the induction of cancer of the skin in mice painted with tobacco-smoke condensates. 20 , 21 , 22 , 23 , 24
Progress continues on the isolation and identification of chemical constituents in tobacco smoke, including compounds of the carcinogenic polycyclic type. 23 , 25 , 26 , 27 , 28
The growing and consistent body of evidence has had no noticeable effect upon the viewpoint of a small but important group of individuals who would deny a causal role of cigarette smoking in cancer of the lung. Among these critics are Little 29 and Hartnett 30 , spokesmen for the American tobacco industry. Berkson 31 , 32 has been critical of many aspects of the statistical studies, and his reservations are, in part, also evident in papers by Neyman 33 and Arkin 34 . More general objections by Fisher 35 , 36 , Greene 37 , Hueper 38 , Macdonald 39 . Rigdon 40 , and Rosenblatt 41 have been published.
We have reviewed the criticism that have been made regarding the cigarette-lung cancer relationship in the light of new evidence. In this review we have several objectives: a) to point out recorded facts that directly answer some of the criticisms; b) to define more precisely some inadequacies of information, with the hope that this will lead to further research. The particular references we have used were selected because in our opinion the criticism was well stated; it is not our intention to reply to any specific publication or to any specific critic. Our view is that all valid questions should be answered. However, some questions may not be relevant, or there may be no information presently available for an answer. In the latter case, we believe that a distinction should be made between data that are unavailable and data that have been found to be contradictory.
For convenience, we have divided the criticisms and answers into five major topics, as follows: (I) Mortality and population data; (II) Retrospective and prospective studies; (III) Studies on pathogenesis; (IV) Other laboratory investigations; and (V) Interpretation.
The rising death rate from lung cancer in all countries that have sufficiently detailed mortality statistics is the most striking neoplastic phenomenon of this century. That this increase is a fact and not a spurious result of statistical classification is now commonly accepted An entirely contrary view is held by only a few persons 40 , though there are dissenting opinions 42 , 38 regarding the extent and time relationship of this recorded increase.
Obviously, the case for the etiologic role of cigarette smoking would be seriously compromised if it could be demonstrated that the lung-cancer rate over the past half century had been stationary, particularly after 1920 when much of the rise in cigarette consumption, instead of other forms of tobacco, occurred 43 .
In a recent review, Rigdon and Kirchoff 44 document that primary lung cancer was first recognized as an entity during the early part of the 19 th century, and that its occurrence has increased steadily since then, as manifested by the recorded relative frequency with which it was recognized in the clinic and at necropsy. This is undoubtedly correct but does not constitute evidence against a true increase in the incidence of the disease during the whole, or a more recent part, of the last 100 years.
Hueper 38 , accepting a true increase in the incidence of lung cancer, regards an increase dating back to 1900, or before the widespread use of cigarettes, as evidence against the cigarette-lung-cancer relationship. His contention would have crucial import only if it were maintained that cigarette smoking is the sole cause of lung cancer.
The vital statistics and the necropsy data that support the presumption of a real increase in lung-cancer risk certainly apply to the years after 1920. Because of the uncertainties associated with changes in diagnostic accuracy, no firm conclusions can be reached on whether the rate of increase in lung-cancer mortality has, in truth, accelerated since 1920.
Effect of Aging
Rosenblatt 41 has raised the question about the effect of the aging population on the lung-cancer rate. This particular point has been investigated by the use of age-adjusted rates. Dunn 45 has noted that only one sixth of the over-all increase in lung-cancer mortality among males in the United States (from 4 to 24 deaths per 100,000 males between 1930 and 1951) could be attributed to an aging population. Similar findings 46 have been presented for England and Wales where observations on lung-cancer mortality date back to 1900; the 1953 mortality rate for both sexes, 34 per 100,000 population, was 43 times the corresponding 1900 rate, 0.8 per 100,000 population. Allowance for increased average age of the population could account for only half this rise in lung-cancer mortality, with a 24-fold difference between 1900 and 1953.
Also, an aging population, does not affect the age-specific death rates and cannot account for the phenomenon of increasingly higher lung-cancer mortality at all ages throughout the lifespan, which has occurred among successively younger groups of males born in the United States and England and Wales since 1850. A similar but less pronounced “cohort displacement” has been shown for females.
Diagnostic Factors
Little 29 and others 40 have raised the important question on whether better diagnostic measures and more complete reporting have resulted in a spurious increase in the recorded attack rate. Several special features of the increase in lung-cancer mortality would be difficult to account for on diagnostic grounds. These include the continuous rising ratio of male to female deaths, the increasing lung-cancer mortality rate among successively younger cohorts, and the magnitude of the current, continuing, increase in lung-cancer mortality 46 . By 1955, among white males, 50 to 64 years of age, in the United States, more deaths were attributed to lung cancer than to all other respiratory diseases combined.
Gilliam 42 has made a careful study of the potential effect of improved diagnosis on the course of the lung-cancer death rate. Even assuming that 2 percent of the deaths certified in past years as tuberculosis or other respiratory disease were really due to lung cancer, he concluded that “… all of the increase in mortality attributed to cancer of the lung since 1914 in United States white males and females cannot be accounted for by erroneous death certification to other respiratory diseases without unreasonable assumptions of age and sex differences in diagnostic error.” His computations reduced the respective 26-fold and sevenfold increase in lung-cancer mortality among males and females, between 1914 and 1950, to the more modestly estimated dimensions of fourfold and 30 percent, respectively. These estimates are certainly the lower bound on the magnitude of the true rate of increase during this period.
The Copenhagen Tuberculosis Station data, examined by Clemmesen et al . 47 , provide the greatest measure of control on the diagnostic improvement factor. In a tuberculosis referral service, used extensively by local physicians, where diagnostic standards and procedures including systematic bronchoscopy remained virtually unchanged between 1941 and 1950, the lung-cancer prevalence rate among male examinees increased at a rate comparable to that recorded by the Danish cancer registry for the total male population. This can be regarded as evidence that the reported increase in Danish incidence is not due to diagnostic changes.
Necropsy Data
Most necropsy data agree with mortality data on the increase in lung-cancer risk. To establish this point we referred to a necropsy series summarized by Steiner 48 , and returned to the original sources for evidence on the nature of changes over time. Since an existing compilation was chosen, the results do not represent a culling of autopsy series for data favorable to this thesis. The findings from 13 series are summarized in text- figure 1 as the proportion of lung cancers in relation to all necropsies. The relative frequency in terms of total tumors or total carcinomas yielded results which would lead to substantially the same inferences.
Mortality and necropsy data have their own virtues and weaknesses. Death certificates provide a complete report of deaths, but do not emphasize a high quality of diagnostic evidence, while the reverse holds true for necropsies. However, since both approaches lead to the same inferences, neither great variation in the quality of diagnostic evidence nor the unrepresentative nature of some of the necropsy observations can be viewed as plausible interpretations of the results. The alternative conclusion of a real increase in lung-cancer risk remains.
Urban-Rural Differences
Emphasis has been placed on the alleged incompatibility of the excess lung-cancer mortality, among urban residents, with the cigarette-smoking hypothesis 38 , 49 . Mortality data from several countries indicate strongly that lung-cancer rates are much higher in cities than in rural areas, and the observation that urban males in general have higher lung-cancer mortality than rural males is undoubtedly correct.
The assertion of Macdonald 39 that “ … country people smoke as much, if not more, than do city people …” is not borne out by the facts 50 . Nevertheless, the evidence indicates that adjustment for smoking history could account for only a fraction of this urban-rural difference 51 .
However, this does not establish the converse proposition that control of residence history in the analysis of collected data would account for the excess lung-cancer risk among cigarette smokers. Evidence now in hand weighs strongly against this last assertion. Stocks and Campbell 67 , in their report on lung-cancer mortality among persons in Liverpool, the suburban environs, and rural towns in North Wales, showed that heavy smokers have higher lung-cancer rates when urban and rural males were studied separately. Mills and Porter 52 reported similar findings in Ohio. These results agree with the experience of the Hammond-Horn 9 study, which revealed markedly higher death rates for bronchogenic carcinoma among smokers regardless of whether they lived in cities or in rural areas. No contradictory observations are known to us.
Sex Differences
The sex disparity in lung-cancer mortality has also been cited 35 , 7 as grounds for discarding the cigarette-smoking hypothesis. In this connection it should be noted that persons advocating this line of argument have minimized sex differences in smoking habits to a degree not supported by available facts. A survey of smoking habits in a cross section of the United States population 50 demonstrated that men, on the average, have been smoking for longer periods than women. The sex differences in tobacco use were especially pronounced at ages over 55, when most lung-cancer deaths occur; 0.6 percent of United States females in this age group have been reported as current users of more than 1 pack of cigarettes daily compared to 6.9 percent of United States males. British data 53 also revealed much lower tobacco consumption among females, particularly for the years before World War II.
The present data contrasting the experience by sex would appear to support the cigarette-smoking hypothesis rather than discredit it. When differences in smoking habits are considered, it is possible to reduce the observed fivefold excess lung-cancer mortality among males to the 40 percent excess mortality which prevails for many other causes of death 51 . One intriguing finding from these studies is that the estimated death rates for female nonsmokers agree closely with the death rates derived from retrospective studies on male nonsmokers 7 .
Evidence for Other Etiological Factors
Etiologic factors of industrial origin, such as exposure to chromates and coal gas, are well established 46 . Excess lung-cancer risks among such groups as asbestos workers who develop asbestosis, appear likely 46 . One epidemiologic study 54 of British, World War I, veterans exposed to mustard gas and/or with a wartime history of influenza revealed virtually no excess lung-cancer risk among these groups.
The existence of other important lung-cancer effects associated with such characteristics as socioeconomic class cannot be questioned. Cohart 55 found that the poorest economic class had a 40 percent higher lung-cancer incidence than the remaining population of New Haven, Connecticut. Results from the 10-city morbidity survey 56 have revealed a sharp gradient in lung-cancer incidence, by income class, for white males, which is consistent with Cohart's findings. Since cigarette smoking is not inversely related to socioeconomic status, we can agree with Cohart “… that important environmental factors other than cigarette smoking exist that contribute to the causation of lung cancer.” These and other findings are convincing evidence for multiple causes of lung cancer. It is obviously untenable to regard smoking of tobacco as the sole cause of lung cancer.
Two points should be made: The population exposed to established industrial carcinogens is small, and these agents cannot account for the increasing lung-cancer risk in the remainder of the population. Also, the effects associated with socioeconomic class and related characteristics are smaller than those noted for smoking history, and the smoking-class differences cannot be accounted for in terms of these other effects.
Special population Groups
Haag and Hanmer 57 reported that employees in 9 processing plants of the American Tobacco Company, with an above-average proportion of smokers, had a lower mortality than the general population of Virginia and North Carolina for all causes and for cancer and cardiovascular diseases, but no higher mortality for respiratory cancer and coronary disease. They concluded: “The existence of such a population makes it evident that cigarette smoking per se is not necessarily or invariably associated with a higher risk of lung cancer or cardiovascular diseases or with diminished longevity.”
The group studied by Haag and Hanmer was too small to yield significant results on respiratory cancer. Moreover, a major flaw in the conclusion has been pointed out by Case 58 . It is well known that mortality comparisons cannot be drawn directly between employee groups and the general population, since the death rates for many groups of employed persons are lower than death rates for the general population with age, sex, and race taken into consideration. This is true because there is a strong tendency to exclude from employment those persons who have acute or chronic diseases or who are seriously disabled from any cause and those employees who develop permanent disabilities from disease or other causes are usually discharged, retired, or dropped from the list of regular employees. Reasons of this nature undoubtedly account for the deficit in deaths from all causes noted in the group of employees under consideration.
A different picture is provided by the Society of Actuaries 59 who made a study for 1946 through 1954. The death claims for employees of the tobacco industry were reported to be slightly higher than, and the permanent disability claims were reported to be over three times as high as, those for employees in nonrated industries as a whole. This latter comparison indicates that the basic assumption of the Haag and Hanmer study is incorrect. Also, interpretation of group comparisons in this field should account separately for the experience of smokers and nonsmokers. We hope that Haag and Hanmer will supplement the report to provide data for smokers and nonsmokers in the study population.
The association between smoking and lung cancer has now been investigated and reported by at least 21 independent groups of investigators in 8 different countries, who employed what is known as the retrospective method 1 , 4 , 5 , 6 , 7 , 46 . In these studies, patients with lung cancer, or their relatives, were questioned about their smoking history and other past events, and the answers compared with those of individuals without lung cancer who were selected as controls. Although these 21 studies have certain features in common, they varied greatly in the methods of selecting the groups, the methods of interview, and other important aspects.
The association between smoking and lung cancer was further investigated in two countries by three independent groups 8 , 9 , 10 , using the prospective method. In these studies, large groups were questioned on smoking habits and other characteristics, and the groups were observed for several years for data on mortality and causes of death. The three prospective studies also varied in several important details including the type of subjects, the selection of subjects, and the method of obtaining information on smoking habits.
In each of these studies, an association was found between smoking and lung cancer. In every investigation where the type of smoking and lung cancer. In every investigation where the type of smoking was considered, a higher degree of association was found between lung cancer and cigarette smoking than between lung cancer and pipe or cigar smoking. In every instance where amount of smoking was considered, it was found that the degree of association with lung cancer increased as the amount of smoking increased. When ex-cigarette smokers were compared with current cigarette smokers, it was found that lung-cancer death rates were higher among current cigarette smokers than among ex-cigarette smokers.
A number of investigators 60 have criticized the retrospective method but, for the most part, the specific points of criticism apply only to some of the studies and not to others. Some features of the three prospective studies on smoking also have been criticized. Again, certain of the points of criticism apply to one or another of the three prospective studies but not to all three. Specifically, doubts raised as to the validity of the early findings of the prospective studies have been eliminated by the persistence of the findings in the later phases of the same studies.
The validity of the findings on these extensive investigations has been questioned in regard to two major aspects: 1) the methods of selection of the study groups, and 2) the accuracy of information regarding smoking habits and the diagnosis of lung cancer.
Selection of Study Groups
Neyman 33 pointed out that a study based on a survey of a population at some given instant of time may yield misleading results. Suppose that a study is made on a day when all patients with lung cancer and a group of people without lung cancer are questioned about their smoking habits. If smokers with lung cancer live longer than nonsmokers with lung cancer, there would be a higher proportion of smokers in the lung-cancer group than in the control group – this would follow without questioning the proposition on which the model is based. However, only two of the retrospective studies were conducted in a way approximating an “instantaneous survey” procedure, so that this criticism does not apply to most of the studies. Furthermore, this difficulty is completely avoided in prospective studies.
Berkson 31 indicated that people with two specific complaints are more likely to be hospitalized than people with only one of these complaints. If a retrospective study were conducted exclusively on hospital patients an association would be found between these two specific complaints, even if there were no association between the same two complaints in the general population. This would influence the results if smokers with lung cancer are more likely to be hospitalized than nonsmokers with lung cancer. However, Berkson showed that this difficulty is trivial if a high percentage of people with either one of these two conditions is hospitalized, which is the situation with lung-cancer patients. Furthermore, one retrospective study 67 included all lung cancer patients who were in the study area, including those not hospitalized; another retrospective study 61 was based on individuals who died of lung cancer and other diseases regardless of whether they had been hospitalized or not. This difficulty does not arise in prospective studies.
In all but one of the 21 retrospective studies, the procedure was to compare the smoking habits of lung-cancer patients with the smoking habits of a control group who did not have lung cancer. Hammond 60 , Berkson 31 , and others have pointed out the grave danger of bias if the control group is not selected in such a way as to represent (in respect to smoking habits) the general population which includes the lung-cancer patients. Subsequent events have proved that this criticism is well founded, though the direction of the bias in most studies turned out to yield an underestimate of the degree of association between cigarette smoking and lung cancer. The reason was that in most of the retrospective studies the control group consisted of patients with diseases other than lung cancer. The choice of such a control group is tantamount to assuming that there is no association between smoking and diseases which resulted in hospitalization of the control subjects. This was an incorrect assumption since other studies have indicated an association between smoking and a number of diseases, such as coronary artery disease, thromboangiitis obliterans, and cancer of the buccal cavity.
Doll and Hill 62 , recognizing the possibility of bias in a control group selected from hospital patients, obtained an additional control group by ascertaining the smoking habits of the general population in a random sample of the area in which their hospital was located. The largest percentage of smokers (particularly heavy smokers) was found in the lung-cancer group, the smallest percentage of smokers was found in the general population sample, and an intermediate percentage of smokers was found in the hospital-control group. Similar results have been reported in a recent study of women 7 .
Berkson 31 pointed out that the criticisms in regard to selection bias in the retrospective studies are also applicable to the earlier findings in a prospective study. Suppose that, in selecting subjects for a prospective study, sick smokers are overrepresented in relation to well smokers and/or well non-smokers are overrepresented in relation to sick nonsmokers. In this event, during the earlier period after selection, the death rate of the smokers in the study would be higher than the death rate of the nonsmokers in the study, even if death rates were unrelated to smoking habits of the general population. If smoking is unrelated to death from lung cancer (or other causes), the death rate of the smokers would tend to equalize with that of the nonsmokers as the study progressed. Thus, the bias would diminish with time, and a relationship due to such bias would disappear. This general principle is well known to actuaries and is one of the cornerstones of the life insurance business.
Hammond and Horn 9 , recognizing this possible difficulty, excluded from the study all persons who were obviously ill at the time of selection. As expected, the total death rate of the study population was low and very few deaths from lung cancer occurred during the first 8 months after selection. The total death rate, and particularly the death rate from lung cancer, rose considerably in the subsequent 3 years. What is more important, the observed association between cigarette smoking and lung cancer was considerably higher in the latter part than in the early part of the study, and the association between cigarette smoking and total death rates was also somewhat greater in the latter part of the study. This showed that the original bias in the selection of the subjects was slight and that it yielded an underestimate of the degree of association between smoking and death rates.
This particular problem was not encountered in the prospective studies of Doll and Hill 10 who could observe the death rates of all physicians in Great Britain (nonresponders as well as responders to the smoking questionnaire). The prospective study of Dorn 8 also had a defined population of veterans holding insurance policies, and nonresponders were observed as well as responders. Moreover, these two studies also showed that higher mortality from lung cancer among smokers was more evident during the later period than in the earlier period of observation. Thus, in the course of time, there was no disappearance of any selection bias factors that may have been introduced into the original study groups.
The subjects for the Hammond and Horn prospective study 9 were selected by volunteer workers with specific instructions on how it should be done. Mainland and Herrera 63 have suggested that the volunteer workers may have introduced a bias in the way they selected the subjects. The foregoing evidence of persistence and accentuation of the differences between smokers and nonsmokers, in time, effectively counters purposeful, as well as unknown, sources of such selection.
Accuracy of Information
Berkson 31 , 32 has remarked that the two major variables considered in all these studies – the ascertainment of smoking habits and the diagnosis of disease – are both subject to considerable error. The accuracy of diagnosis is not a major problem in retrospective studies because the investigator can restrict his study to those patients whose diagnosis of lung cancer has been thoroughly confirmed. This feature has been taken into consideration in several retrospective studies. It is more of a problem in prospective studies since all deaths that occur must be included, and certainly some of the diagnoses will be uncertain. However, in all three prospective studies, the total death rate was found to be higher in cigarette smokers than in nonsmokers and found to increase with the amount of cigarette smoking. If some of the excess deaths associated with cigarette smoking and ascribed to lung cancer were actually due to some other disease, then it means that: a) the association between cigarette smoking and lung cancer was somewhat overestimated, but b) the association between smoking and some other disease was somewhat underestimated. The reverse would be true if some of the excess deaths associated with cigarette smoking and ascribed to diseases other than lung cancer were actually due to lung cancer. Hammond and Horn 9 found that the association with cigarette smoking was greater for patients with a well-established diagnosis of lung cancer than for patients with less convincing evidence for a diagnosis of lung cancer. This suggests that inaccuracies in diagnosis resulted somewhat in an underestimate of the degree of association between smoking and lung cancer.
The study on physicians, by Doll and Hill 10 , in which presumably the clinical and pathologic evidence of the cause of death would be somewhat more than in the general population considered by Hammond and Horn and by Dorn, yields almost identical risks to lung cancer by smoking class.
In regard to information about smoking, Finkner et al . 64 have made a thorough study of the accuracy of replies to questionnaires on smoking habits. Their results indicate that replies are not completely accurate but that most of the errors are relatively minor – very few heavy smokers are classified as light smokers. Random and independent errors simply tend to diminish the apparent degree of association between two variables. A national survey of smoking habits in the United States 50 yielded results on tobacco consumption that were consistent with figures on tobacco production and taxation.
On two occasions several years apart, Hammond and Horn 9 and Dorn 8 questioned a proportion of their subjects. The results indicated close reproducibility in the answers.
Hammond 60 and others 39 have questioned the reliability of the retrospective method on the grounds that the illness may bias the responses given by the patient or his family when they are questioned about smoking habits, and that knowledge of the diagnosis may bias the interviewer. This possible difficulty was minimized in several of the 21 retrospective studies on smoking in relation to lung cancer. For example, in the study conducted by Levin 65 , all patients admitted to a hospital during the course of several years were questioned about their smoking habits before a diagnosis was made. Only a small proportion later turned out to have lung cancer, though many had lung disease symptoms or lung diseases other than lung cancer. Doll and Hill 10 also showed that patients whose diagnosis of lung cancer was subsequently established to be erroneous had smoking histories characteristic of the control rather than of the lung-cancer group. Furthermore, a larger percentage of cigarette smokers have been found among patients with epidermoid carcinoma of the lungs than among patients with adenocarcinoma of the lungs 66 . This could hardly have resulted from bias either on the part of the patient or on the part of the interviewer.
Multiple Variables
Arkin 34 , Little 29 , Macdonald 39 , and others have criticized the studies of cigarette-lung cancer relationship on the grounds that only smoking habits were really investigated, and that numerous other possible variables were not considered.
This criticism may seem especially appropriate in view of the accepted fact that no single etiologic factor has been proposed for any neoplastic disease. The criticism may also be valid in relation to any one of the retrospective and prospective studies. However, in the aggregate, quite a number of other variables have been specifically investigated or can be inferentially derived. Of course, all studies considered the basic factors of age and sex; some dealt with geographic distribution 67 , occupation 68 , urban or rural residence 67 , marital and parous status 7 , and some other habits such as coffee consumption 7 .
The Doll and Hill 10 prospective study was confined to a single professional group, physicians. Thus there could be no great variation attributable to occupation or socioeconomic status. Stocks and Campbell 67 put particular emphasis on the study of air pollution and occupational exposure and included a number of other factors in addition to smoking. It is evident, in the Hammond-Horn 9 study and other investigations, that there is a consistent relationship between urban residence and a higher mortality due to lung cancer. The important fact is that in all studies, when other variables are held constant, cigarette smoking retains its high association with lung cancer.
The only factors that may show a higher correlation with lung cancer than heavy cigarette smoking are such occupations as those of the Schneeberg miners and manufacturers of chromate 46 . We are not acquainted with actual studies of these and related occupation groups in which cigarette and other tobacco consumption is also considered. Such studies, we suggest, would be useful additions to our knowledge of other etiologic agents of the interplay between multiple causes in human pulmonary cancer.
Inhalation of Smoke
If cigarette smoking produces cancer of the lungs as a result of direct contact between tobacco smoke and the bronchial mucosa, smokers who inhale cigarette smoke should be exposed to higher concentrations of the carcinogens than noninhalers and therefore have a higher risk to the development of lung cancer. The retrospective study of Doll and Hill 62 , however, elicited no difference between patients with lung cancer and the controls in the proportion of smokers who stated that they inhaled. Fisher 35 , Hueper 38 , and Macdonald 39 have emphasized this point as contradictory to the smoking-lung-cancer relationship, and, of course, it is. Unfortunately, this particular finding was not reinvestigated in the prospective study of Doll and Hill 10 .
Three authors, Lickint 69 , Breslow et al . 68 , and Schwartz and Denoix 4 , however, did find the relative risk of lung cancer to be greater among inhalers than among noninhalers when age, type, and amount of smoking were held constant. It must be admitted that there is no clear explanation of the contradiction posed by the Doll-Hill 62 findings, though a number of plausible hypotheses could be advanced. More experimental work is required, including some objective definition and measurement of the depth and length of inhalation.
Hammond 70 has recently queried male smokers about their inhalation practices. He found that very few pipe and cigar smokers inhale; that most men inhale who smoke only cigarettes; and that there are proportionally fewer inhalers among men who smoke both cigars and cigarettes than among men who smoke only cigarettes. These findings are compatible with the view that differences in inhaling account for the fact that the lung-cancer death rate of cigar and pipe smokers is less than the lung-cancer death rate of cigarette smokers; and that the lung-cancer death rate of men who smoke both cigars and cigarettes is somewhat lower than the lung-cancer death rate of men who smoke only cigarettes.
Upper-Respiratory Cancer
Rosenblatt 41 has drawn attention to the fact that increased consumption of cigarettes has not been accompanied by an increase in upper-respiratory cancer similar to that noted in cancer of the lung and bronchus. Hueper 38 also has expressed doubts about the causative role of cigarette smoking on the basis that cigarette smoking is not associated with cancer of the oral cavity or of the fingers, which are often stained with tobacco tar.
The premise that a carcinogen should act equally on different tissues is not supported by experimental or clinical evidence 71 . Carcinogens, which produce liver tumors in animals, may be noncarcinogenic when applied to the skin. Coal soot, accepted as etiologically related to carcinoma of the scrotum in chimney sweeps, does not increase the risk to cancer of the penis. There is no a priori reason why a carcinogen that produces bronchogenic cancer in man should also produce neoplastic changes in the nasopharynx or in other sites. It is an intriguing fact, deserving further research, that carcinoma of the trachea is a rarity, whereas carcinoma of the bronchus is common among individuals exposed to chromates, as well as among chronic cigarette smokers.
Several studies have established the association of all types of tobacco smoking, including cigarettes, with cancer of the oral cavity 72 . However, the relative risk of developing cancer of the mouth is greater for cigar and pipe smokers than for cigarette smokers. The risk of laryngeal cancer is increased by smoking and an equal risk exists among cigarette, cigar, and pipe smokers 73 . The per capita consumption of cigars and pipe tobacco has decreased since 1920, while cigarette smoking has increased 43 .
These associations contrast sharply with the findings on lung cancer, which have consistently shown that cigarette smokers have much higher risks than either cigar or pipe smokers. Since 1920 the increase in tobacco consumption has been primarily due to the rise in cigarette consumption 43 , and the stabler rates for intra-oral and laryngeal cancer, while the lung-cancer rates have increased steeply, can be considered compatible with the causal role of cigarette smoking in lung cancer.
Effect of Tobacco Smoke on Bronchial Mucosa
Statements by Hartnett 30 , Macdonald 39 , and others 31 , 29 imply that the relationship of cigarette smoking and lung cancer is based exclusively on “statistics” and lacks “experimental” evidence. The differentiation between various methods of scientific inquiry escapes us as being a valid basis for the acceptance or the rejection of facts. Nevertheless it is true that historically the retrospective studies on lung cancer preceded the intensive interest in laboratory investigations stimulated by the statistical findings.
Hilding 13 has shown experimentally that exposure to cigarette smoke inhibited ciliary action in the isolated bronchial epithelium of cows. Kotin and Falk 15 obtained essentially the same results in experiments on rats and rabbits. Hilding 14 further showed that inhibition of ciliary action interfered with the mechanism whereby foreign material is ordinarily removed from the surface of bronchial epithelium. In addition, he found that foreign material deposited on the surface tended to accumulate in any area where the cilia have been destroyed. Auerbach et al . 16 found that the small areas of the bronchial epithelium where ciliated columnar cells were absent appeared more frequently in smokers than in nonsmokers. Chang 17 found that cilia were shorter, on an average, in the bronchial epithelium of smokers than in that of nonsmokers.
These studies have demonstrated the existence of a mechanism whereby foreign material from any source (e.g. tobacco smoke, industrial dusts, fumes from automobile exhausts, general air pollutants, and, perhaps, pathogenic organisms) is likely to remain in contact with the bronchial epithelium for a longer period in smokers than in nonsmokers.
Auerbach and his associates 16 studied the microscopic appearance of the bronchial epithelium of patients who died of lung cancer and patients who died of other diseases. Each of these two groups of patients was classified according to whether they were nonsmokers, light smokers, or heavy cigarette smokers. Among the cancer patients there were no nonsmokers. Approximately 208 sections from all parts of the tracheobronchial tree from each patient were examined. Many areas of basal cell hyperplasia, squamous metaplasia, and marked atypism, with loss of columnar epithelium were found in the tracheo-bronchial tree of men who had died of lung cancer. Almost as many such lesions were found in heavy cigarette smokers who had died of other diseases; somewhat less were found in light cigarette smokers; and much less in nonsmokers. Chang 17 has reported similar findings in the bronchial epithelium of smokers compared with nonsmokers.
The chief criticism of Auerbach's study has concerned terminology. Following the definition previously set forth by Black and Ackerman 74 , Auerbach et al . used the term “carcinoma- in-situ ” to describe certain lesions with marked atypical changes and loss of columnar epithelium. Whether this is an appropriate term may be questioned, but it is not relevant to the validity of the findings. Certainly there are no data to indicate what proportion of these morphologically abnormal areas would progress to invasive carcinoma.
The recent findings of Auerbach et al . and Chang have been reproduced experimentally in animals. Rockey and his associates 75 applied tobacco ”tar” directly to the bronchial mucosa of dogs. Within 3 to 6 weeks, the tar-treated surface became granular and later developed wart like elevations. Upon microscopic examination, hyperplasia, transitional metaplasia, and squamous metaplasia were found in these areas. Leuchtenberger et al . 19 exposed mice to cigarette smoke for periods up to 200 days. The bronchial epithelium was then examined microscopically. Bronchitis, basal-cell hyperplasia, and atypical basal-cell hyperplasia were found in the majority of the animals and squamous metaplasia in a few. Further work and longer periods of observation are necessary to establish whether some of these lesions would progress to frank neoplasia.
Skin Cancer in Rodents
One of the links in the total evidence for the causal relationship of cigarette smoking and lung cancer is the demonstration that tobacco smoke condensates (usually referred to as “tars”) have the biologic property of evoking carcinoma in certain laboratory animals, particularly mice. The production of skin cancer in mice, following repeated, long-term applications of tobacco tar, has now been reported from at least six different laboratories 20 , 21 , 22 , 23 , 24 , 76 . It is undeniable that some investigators did not obtain positive results, perhaps because the dose and other experimental conditions were different, or because the complex tobacco tars probably varied widely in their composition. The negative results of Passey et al . 18 have been quoted by Hueper 38 and others, but a more recent experiment by Passey 24 with Swiss strain mice did lead to the appearance of at least two carcinomas after repeated applications of tobacco-smoke condensate.
Little 29 indicated that “… the extrapolation to the human lung of results obtained by painting of or injection into the skin of mice is decidedly questionable”. Direct extrapolation from one species to another is, of course, not justified. Nevertheless, results in animals are fully consistent with the epidemiologic findings in man. A quotation from Kotin 49 is appropriate: “The chemical demonstration of carcinogenic agents in the environment and their successful use for the production of tumours in experimental animals do not prove or even especially strongly suggest a like relationship in the instance of man. When, however, a demonstrable parallelism exists between epidemiologic data and laboratory findings, greater significance accrues to both. Medical history is replete with examples in which laboratory findings have been proved ultimately to have their counterpart in the human experience. Exceptions have been very few.”
Greene 37 , while discounting the significance of the induction of skin carcinoma in Swiss mice because of the constitutionally “high differential susceptibility” of the strain, believes that the failure to induce neoplasms in embryonic transplants exposed to tobacco tar is more important evidence. Greene's interesting technique does produce positive results when pure chemicals such as benzo[α]pyrene are used, and this chemical has been recovered from some samples of tobacco-smoke condensate. We are not acquainted with reports of neoplasms arising in embryonic tissue that has been exposed in vitro to coal tar, another crude mixture that contains carcinogens.
The high frequency of carcinoma induction reported by Wynder et al . 76 has not been achieved by other investigators, who reported that no more than 20 percent of animals, and usually considerably less, developed carcinoma of the skin. The presence of cocarcinogenic materials in tobacco-smoke condensates has been demonstrated by Gellhorn 22 and by Bock and Moore 20 . To the mouse data are now added the data on the induction of skin cancer in some rabbits painted with tobacco-smoke condensate 77 ; this condensate, when combined with a killed suspension of tubercle bacilli, and introduced into a bronchus, produced a carcinoma of the bronchus in one rat 78 .
Since malignant neoplasms have been obtained in several strains of mice, and a few neoplasms have been produced in rabbits and rats, the issue of strain or species limitation to the reaction is more difficult to maintain. It is, of course, a fact that many agents shown to be carcinogenic to the skin of mice have not been proved carcinogenic to man. In most instances there is simply no experience with such agents in man, so that lack of proof really represents lack of data, pro and con.
The Problem of Dosage
Little 29 has further questioned the applicability of animal data to man, as follows: “Tobacco smoke or smoke condensate has failed to produce cancer even on the skin of susceptible strains of mice when applied in the quantity and at an exposure rate that would simulate conditions of human smoking.”
The differences in species, tissues, and conditions between the induction of neoplasms on the skin of mice and in the bronchi of man, preclude fine comparisons of dose and time relationships.
Bronchogenic Cancer in Animals
The pulmonary adenomatous tumor in mice, rats, and guinea pigs cannot be compared with the bronchogenic carcinoma in man 71 . Until a few years ago, the experimental induction of epidermoid carcinoma had been achieved only in a few mice by passing strings impregnated with carcinogenic hydrocarbons through the lung. Epidermoid carcinoma of the lung was consistently produced in rats by beryllium 79 , by carcinogenic hydrocarbons introduced as fixed pellets into bronchi of rats 80 , and by inhalation of radioactive particles 81 .
Little 29 has noted that “… prolonged exposure of the lungs of rodents to massive doses of cigarette smoke has failed to produce bronchogenic cancer.” This remains true at the time of this report, although it can be questioned whether any animal receives as large a dose of cigarette smoke through indirect exposure as a human being does by voluntary deep inhalation. Therefore the failure may be a technical one, which may be solved by further experimentation. The early results of Leuchtenberger et al . 19 suggested that this may be achieved.
Carcinogens in Tobacco Smoke
The isolation and identification of specific chemical constituents in tobacco smoke, which are carcinogenic for the pulmonary tissue of man, is an important area for research.
It has been clear for some time that combustion or pyrolysis of most organic material, including tobacco, will form higher aromatic polycyclics of established carcinogenic activity 28 . A number of higher aromatic polycyclics have been identified and isolated ( 23 , 25 , 26 , 27 ). These materials include benzo[ e ]pyrene, benzo[ a ]pyrene, dibenz[ a,h ]anthracene, chrysene, and, most recently, a newly established carcinogen, 3,4-benz-fluoranthene. Whether these compounds are equally involved in human pulmonary carcinogenesis is, of course, conjectural.
Little 29 has implied that a specific constituent must be found to account for the biologic activity of tobacco smoke. This is not necessary. The situation is similar to the establishment of the carcinogenic activity of tar, which was accepted before the isolation of benzo[ a ]pyrene by Kennaway and his coworkers. In this instance, also, benzo[ a ]pyrene is most probably not the only carcinogen in the complex mixture called tar, and there are strong indications that some noncarcinogenic components in tar may have cocarcinogenic effects.
Three interpretations of the observed association of lung cancer and cigarette smoking are possible: 1) that cigarette smoking “causes” lung cancer, either (a) through the direct carcinogenic action of smoke on human bronchial epithelium or (b) by a more indirect mode of action such as making the individual susceptible to some other specific carcinogenic agent in the environment; 2) that lung cancer “causes” cigarette smoking, perhaps because a precancerous condition sets up a process which leads to a craving for tobacco; 3) that cigarette smoking and lung cancer both have a common cause, usually specified as a special constitutional make-up, perhaps genetic in origin, which predisposes certain individuals to lung cancer and also makes them cigarette smokers.
The second hypothesis was advanced by Fisher 36 , apparently for the sake of logical completeness, and it is not clear whether it is intended to be regarded as a serious possibility. Since we know of no evidence to support the view that the bronchogenic carcinoma diagnosed after age 50 began before age 18, the median age at which cigarette smokers begin smoking, we shall not discuss it further.
The Constitutional Hypothesis
The first hypothesis may be referred to as the causal hypothesis and the third as the constitutional hypothesis. Nothing short of a series of independently conducted, controlled, experiments on human subjects, continued for 30 to 60 years, could provide a clear-cut and unequivocal choice between them. We nevertheless argue that evidence, in addition to that associating an increased mortality from lung cancer with cigarette smoking, is entirely consistent with the causal hypothesis but inconsistent, in many respects, with the constitutional hypothesis, so that even in the absence of controlled experimentation on human beings the weight of the evidence is for the one and against the other.
The difficulties with the constitutional hypothesis include the following considerations: (a) changes in lung-cancer mortality over the last half century; (b) the carcinogenicity of tobacco tars for experimental animals; (c) the existence of a large effect from pipe and cigar tobacco on cancer of the buccal cavity and larynx but not on cancer of the lung; (d) the reduced lung-cancer mortality among discontinued cigarette smokers. No one of these considerations is perhaps sufficient by itself to counter the constitutional hypothesis ad hoc modification of which can accommodate each additional piece of evidence. A point is reached, however, when a continuously modified hypothesis becomes difficult to entertain seriously.
Changes in Mortality
Mortality from lung cancer has increased continuously in the last 50 years, and considerably more for males than females. Such an increase can be explained either as the result of an environmental change (to which males are more exposed or more sensitive than females, if both are equally exposed) or as the result of a sex-linked mutation. The constitutional hypothesis must be modified in the light of this increase, since an unchanging constitutional make-up cannot by itself explain an increase in mortality. Proponents of the constitutional hypothesis have not indicated the type of modification they would consider. Three suggest themselves to us: 1) differences in constitutional make-up are genetic in origin, but rather than predisposing one to lung cancer, they make one sensitive to some new environmental agent (other than tobacco), which does induce lung cancer; 2) differences in constitutional make-up are not genetic but are the result of differential exposure to some new environmental agent, which both predisposes to lung cancer and creates a craving for cigarette smoke; 3) the mutation has led to a greater susceptibility to lung cancer and a preference for cigarette smoke.
In the first two situations the effect of the postulated constitutional make-up would be mediated through an environmental agent. The modified hypothesis thus requires the existence of an environmental agent other than tobacco, exposure to which would be at least as highly correlated with lung-cancer mortality as exposure to cigarettes, and which also would be highly correlated with cigarette consumption. No such agent has yet been found or even suggested. In view of the magnitude of the increase in mortality from lung cancer, the third situation would require a mutation rate exceeding anything previously observed.
Experimental Carcinogenesis With Tobacco Tar
Condensed tobacco smoke contains substances that are carcinogenic for mouse and rabbit skin. It does not necessarily follow that these substances are also carcinogenic for human lungs nor does it follow that they are not. However, the constitutional hypothesis asserts they are not; and that it is simply a coincidence that these materials which are carcinogenic for experimental animals are also associated with a higher lung-cancer mortality in man.
Types of Tobacco and Cancer Site
A greatly increased lung-cancer risk is associated with increased cigarette consumption but not with increased consumption of pipe and cigar tobacco. Studies on cancer of the buccal cavity and larynx, however, have demonstrated a considerably higher risk among smokers, irrespective of the form or tobacco used. Only two ways of modifying the constitutional hypothesis to take account of this evidence occur to us: 1) There are two different constitutional make-ups, one of which predisposes to cigarettes but not to pipe and cigar consumption and to cancer of the lung, and the other predisposes to cancer of the buccal cavity and larynx but not of the lung and to tobacco consumption in any form. 2) Constitutional make-up predisposes to cigarette consumption and lung cancer only, but tobacco smoke, whether from cigarettes, cigars, or pipes, is carcinogenic for the mucosa of the buccal cavity and the larynx but not for the bronchial epithelium.
Mortality Among Discontinued Smokers
Mortality from lung cancer among discontinued cigarette smokers is less than that among those continuing to smoke 9 , 10 ; the magnitude of the reduction depending on amount previously smoked and the length of the discontinuance. The hypothetical constitutional factor which predisposes to lung cancer and cigarette smoking cannot therefore be a constant characteristic of an individual over his lifetime but must decrease in force at some time in life, thus resulting in the cessation of cigarette smoking and a concomitant, but not causally related, reduction in the lung-cancer risk. Furthermore, since cigarette smoking is rarely begun after age 35 50 , it must be inferred that the constitutional factor cannot increase in force with the passage of time, even though it may decrease.
In summary, the constitutional hypothesis does not provide a satisfactory explanation of all the evidence. It is natural, therefore, to inquire about the positive findings which support it. Even those who regard this hypothesis with favor would agree, we believe, that supporting evidence is quite scanty.
There are a number of characteristics in which cigarette smokers are known to differ from nonsmokers and presumably more will be discovered. Thus, cigarette smokers consume more alcohol, more black coffee, change jobs more often, engage more in athletics, and are more likely to have had at least one parent with hypertension or coronary artery disease 82 . Discontinued cigarette smokers are weaned at a later age than those continuing to smoke 83 . Recently, Fisher 83 reported that 51 monozygotic twins resembled each other more in their smoking habits than 33 dizygotic twins, thus suggesting a genetic determinant.
Two somewhat obvious, but necessary, comments on results of this type are in order: 1) The demonstration that a characteristic is related to smoking status does not by itself create a presumption that it is a common cause. It must also be shown to be related to the development of lung cancer among subgroups of individuals with the same smoking status. Alcohol and coffee fail to meet this test, while none of the other characteristics related to smoking status have been investigated from this point of view. 2) There is a quantitative question. Cigarette smokers have a ninefold greater risk of developing lung cancer than nonsmokers, while over-two-pack-a-day smokers have at least a 60-fold greater risk. Any characteristic proposed as a measure of the postulated cause common to both smoking status and lung-cancer risk must therefore be at least nine-fold more prevalent among cigarette smokers than among nonsmokers and at least 60-fold more prevalent among two-pack-a-day smokers. No such characteristic has yet been produced despite diligent search.
These comments on the quantitative aspects of association apply also to the relationship of certain characteristics with lung cancer. Thus, a possible genetic basis to lung cancer has been suggested to some by the association between gastric cancer and blood group. The difference, in risk of developing gastric cancer, between blood groups A and O, however, is 20 percent, while the only study of lung cancer and blood groups 84 with which we are familiar shows a difference of 27 percent (and is not quite significant at the P = 0.01 level. 1 Such differences are suggestive for further work, but cannot be considered as casting much light on differences of magnitude, ninefold to 60-fold.
Measures of Differences
The comments in the last two paragraphs have utilized a relative measure of differences in lung-cancer risk. Since Berkson 32 has argued that a relative measure is inappropriate in the investigation of smoking and mortality, we now discuss the use of relative and absolute measures of differences in risk. When an agent has an apparent effect on several diseases, the ranking of the diseases by the magnitude of the effect will depend on whether an absolute or a relative measure is used. Thus in Dorn's study 8 of American veterans there were 187 lung-cancer deaths among cigarette smokers compared with an expectation of 20 deaths, based on the rates for nonsmokers. This yields a mortality ratio of 9.35 as a relative measure and an excess of 167 deaths as an absolute measure. For cardiovascular diseases there were 1,780 deaths among cigarette smokers compared to an expectation of 1,165. This gives a relative measure of 1.53 and an absolute measure of 615 deaths. Relatively, cigarettes have much larger effect on lung cancer than on cardiovascular disease, while the reverse is true if an absolute measure is used.
Both the absolute and the relative measures serve a purpose. The relative measure is helpful in 1) appraising the possible noncausal nature of an agent having an apparent effect; 2) appraising the importance of an agent with respect to other possible agents inducing the same effect; and 3) properly reflecting the effects of disease misclassification or further refinement of classification. The absolute measure would be important in appraising the public health significance of an effect known to be causal.
If an agent, A, with no causal effect upon the risk of a disease, nevertheless, because of a positive correlation with some other causal agent, B, shows an apparent risk, r, for those exposed to A, relative to those not so exposed, then the prevalence of B, among those exposed to A, relative to the prevalence among those not so exposed, must be greater than r.
If two uncorrelated agents, A and B, each increase the risk of a disease, and if the risk of the disease in the absence of either agent is small (in a sense to be defined), then the apparent relative risk for A, r, is less than the risk for A in the absence of B.
If a causal agent A increases the risk for disease I and has no effect on the risk for disease II, then the relative risk of developing disease I, alone, is greater than the relative risk of developing disease I and II combined, while the absolute measure is unaffected.
The Causal Hypothesis
When the sexes are compared it is found that lung cancer has been increasing more rapidly in men relatively to women … But it is notorious, and conspicuous in the memory of the most of us, that over the last 50 years the increase of smoking among women has been great, and that among men (even if positive) certainly small. The theory that increasing smoking is ‘the cause’ of the change in apparent incidence of lung cancer is not even tenable in the face of this contrast.
It would thus appear that cigarette smoking is one of the causes of all ills and contributes to the over-all death rate, remembering that this rate includes such causes as accident, homicide, etc. It seems quite clear that cigarette smoking is a symptom, not a cause. It is possible – even though this is a conjecture – that they type of person who is careful of his health is less likely to be a cigarette smoker and that the cigarette smoker is likely to be the person who generally takes greater health risks.
Berkson 32 also has pointed to the multiple findings in both the Hammond-Horn and the Doll-Hill results and concluded that the observed associations may have some other explanation than a causal one. He suggests three: 1) “The observed associations are ‘spurious’ …. 2) The observed associations have a constitutional basis. Persons who are nonsmokers, or relatively light smokers, are the kind of people who are biologically self-protective, and biologically this is correlated with robustness in meeting mortal stress from disease generally. 3) Smoking increases the ‘rate of living’ (Pearl), and smokers at a given age are, biologically, at an age older than their chronologic age.”
One might ask why the finding of an association with a number of diseases, rather than just one, is necessarily contradictory and must be regarded as supporting the constitutional hypothesis. Arkin 34 supplied no answer, while the relevant statements of Berkson 32 on this point were:
When an investigation set up to test the theory, suggested by evidence previously obtained, that smoking causes lung cancer, turns out to indicate that smoking causes or provokes a whole gamut of diseases, inevitably it raises the suspicion that something is amiss. It is not logical to take such a set of results [e.g., an association of smoking with a ‘wide variety of diseases’] as confirming the theory that tobacco smoke contains carcinogenic substances which, by contact with the pulmonary tissues, initiate cancerous changes at the site of contact.
The apparent multiple effects of tobacco do raise a question with respect to the mode of action, however, and since this question is related to another alleged contradiction – the apparent lack of an inhalation effect – we shall discuss them together. What mode of action, it has been asked, can one postulate to explain these diverse effects? Two remarks are in order: 1) The evidence that tobacco is a causal agent in the development of other diseases seems weaker than the evidence for lung cancer simply because the effects are smaller. While we could not exclude the possibility that cigarettes play a causal role in, for instance, the development of arteriosclerotic-coronary heart disease, the possibility that a common third factor will be discovered, which explains a 70 percent elevation in risk from coronary heart disease among cigarette smokers, is less remote than the possibility that the ninefold risk for lung cancer will be so explained. 2) Accepting, for the sake of discussion, the causal role of cigarettes for any disease showing an elevated mortality ratio, no mater how small, the presence of other causes will be manifested in a lowered mortality ratio. Thus, even if cigarette consumption causes an elevation of 70 percent in mortality from coronary heart disease, other causes of great importance must also be present, as is manifested by the high mortality from this disease among nonsmokers. The existence of a small number of nonsmokers who develop lung cancer is a definite indication, by the same token, that cigarettes are not an absolutely necessary condition and that there are other causes of lung cancer.
If tobacco smoke does have multiple effects, each of these effects must be studied separately because of the complex nature of the agent. To postulate in advance that a single mode of action will be found to characterize them all is an unwarranted oversimplification. It is generally accepted, for example, that tobacco smoke causes thromboangiitis obliterans in susceptible humans by interfering with the peripheral circulation, and that it causes tumors when painted on the backs of susceptible mice because of the presence of carcinogenics in the tars. The a priori postulation of a single mode of action for these two effects is no substitute for detailed study of each.
As to the possible mode of action of tobacco smoke in inducing lung cancer, the evidence at this writing suggests direct action of substances in tobacco smoke on susceptible tissues with which they are in contact. Aside from background knowledge derived from experimental carcinogensis which suggests this explanation, the following evidence favors it: 1) Cigarette smoke, which is usually drawn into the lungs is associated with mortality from lung cancer, while smoke from pipes and cigars, which is usually not inhaled, if not. 2) For sites with which smoke is in direct contact, whether or not inhaled, particularly buccal cavity and larynx, the type of tobacco used makes less difference in incidence. 3) In experimental carcinogenesis, which uses tobacco tars, tumors have appeared at the site of application, and their incidence has not yet seriously dependent on the type of tobacco used. 4) The relative risk of lung cancer is higher among cigarette smokers who inhale than among those smoking the same number of cigarettes per day, but who do not inhale.
Several critics 36 , 38 , 39 have stressed the failure of Doll and Hill 62 , in their preliminary report, to find a difference in risk between inhalers and noninhalers, but this finding was contradicted in three other studies 4 , 68 , 69 . Further work on this point is desirable, but would be more convincing if a more objective measure were found of the amount of smoke to which human bronchial epithelium is exposed in the course of smoking a cigarette.
Why, it is sometimes asked, do most heavy cigarette smokers fail to develop lung cancer if cigarettes are in fact a causal agent? We have no answer to this question. But neither can we say why most of the Lübeck babies who were exposed to massive doses of virulent tubercle bacilli failed to develop tuberculosis. This is not a reason, however, for doubting the causal role of the bacilli in the development of the disease.
One cannot discuss the mode of action of tobacco without becoming aware of the necessity of vastly expanded research in the field. The idea that the subject of tobacco and mortality is a closed one requiring no further study is not one we share. As in other fields of science, new findings lead to new questions, and new experimental techniques will continue to cast further light on old ones. This does not imply that judgment must be suspended until all the evidence is in, or that there are hierarchies of evidence, only some types of which are acceptable. The doctrine that one must never assess what has already been learned until the last possible piece of evidence would be a novel one for science.
It would be desirable to have a set of findings on the subject of smoking and lung cancer so clear-cut and unequivocal that they were self-interpreting. The findings now available on tobacco, as in most other fields of science, particularly biologic science, do not meet this ideal. Nevertheless, if the findings had been made on a new agent, to which hundreds of millions of adults were not already addicted, and on one which did not support a large industry, skilled in the arts of mass persuasion, the evidence for the hazardous nature of the agent would be generally regarded as beyond dispute. In the light of all the evidence on tobacco, and after careful consideration of all the criticisms of this evidence that have been made, we find ourselves unable to agree with the proposition that cigarette smoking is a harmless habit with no important effects on health or longevity. The concern shown by medical and public health authorities with the increasing diffusion to ever younger groups of an agent that is a health hazard seems to us to be well founded.
* Cornfield J et al. Smoking and lung cancer: recent evidence and a discussion of some questions. JNCI 1959;22:173–203. Reprinted with permission.
1 Our attention has been called to a summary of three additional studies, which report no association between ABO blood groups and lung cancer, by Roberts JAF. Blood groups and susceptibility to disease. Brit. J. Prev. & Social Med. 11: 107–125, 1957.
Google Scholar
Google Preview
We feel obliged to give proof of the rather obvious statement on the magnitudes of relative risk because it has been suggested that the use of a relative measurement is merely "instinctive" and lacking in rational justification. Let the disease rate for those exposed to the causal agent, B, be r 1 and for those not exposed, r 2 , each rate being unaffected by exposure or nonexposure to the noncausal agent, A. Let r 1 > r 2 . Of those exposed to A, let the proportion exposed to B be p 1 , and of those not exposed to A, let the proportion exposed to B be p 2 . Because of the assumed positive correlation between A and B, p 1 > p 2 . Then
R 1 = rate for those exposed to A = p 1 r 1 + (1 – p 1 ) r 2
The proof again is simple. Let r 11 denote the risk of the disease in the presence of both A and B, r 12 , the risk in the presence of A and absence of B, r 12 , the risk in the absence of A and presence of B, and r 22 the risk in the absence of both A and B. It is reasonable to assume r 22 = 0, but the less restrictive specification r 22 < r 12 r 21 / r 11 is sufficient for what follows. The proportion of the population exposed to B is denoted by p , and this, by hypothesis, is the same whether A is present or absent. Then
R 1 = rate for those exposed to A = pr 11 + (1 – p ) r 12
| Month: | Total Views: |
|---|---|
| December 2016 | 1 |
| January 2017 | 63 |
| February 2017 | 228 |
| March 2017 | 248 |
| April 2017 | 115 |
| May 2017 | 88 |
| June 2017 | 48 |
| July 2017 | 36 |
| August 2017 | 188 |
| September 2017 | 170 |
| October 2017 | 245 |
| November 2017 | 369 |
| December 2017 | 1,272 |
| January 2018 | 1,287 |
| February 2018 | 1,474 |
| March 2018 | 1,444 |
| April 2018 | 1,304 |
| May 2018 | 1,270 |
| June 2018 | 1,004 |
| July 2018 | 621 |
| August 2018 | 721 |
| September 2018 | 762 |
| October 2018 | 826 |
| November 2018 | 890 |
| December 2018 | 537 |
| January 2019 | 453 |
| February 2019 | 642 |
| March 2019 | 883 |
| April 2019 | 660 |
| May 2019 | 717 |
| June 2019 | 584 |
| July 2019 | 664 |
| August 2019 | 483 |
| September 2019 | 520 |
| October 2019 | 477 |
| November 2019 | 401 |
| December 2019 | 707 |
| January 2020 | 477 |
| February 2020 | 456 |
| March 2020 | 363 |
| April 2020 | 628 |
| May 2020 | 369 |
| June 2020 | 504 |
| July 2020 | 345 |
| August 2020 | 261 |
| September 2020 | 473 |
| October 2020 | 441 |
| November 2020 | 398 |
| December 2020 | 386 |
| January 2021 | 415 |
| February 2021 | 519 |
| March 2021 | 640 |
| April 2021 | 563 |
| May 2021 | 448 |
| June 2021 | 348 |
| July 2021 | 321 |
| August 2021 | 419 |
| September 2021 | 495 |
| October 2021 | 563 |
| November 2021 | 519 |
| December 2021 | 370 |
| January 2022 | 506 |
| February 2022 | 506 |
| March 2022 | 854 |
| April 2022 | 745 |
| May 2022 | 559 |
| June 2022 | 327 |
| July 2022 | 302 |
| August 2022 | 339 |
| September 2022 | 457 |
| October 2022 | 469 |
| November 2022 | 598 |
| December 2022 | 439 |
| January 2023 | 489 |
| February 2023 | 435 |
| March 2023 | 471 |
| April 2023 | 402 |
| May 2023 | 413 |
| June 2023 | 267 |
| July 2023 | 290 |
| August 2023 | 268 |
| September 2023 | 404 |
| October 2023 | 369 |
| November 2023 | 428 |
| December 2023 | 467 |
| January 2024 | 525 |
| February 2024 | 466 |
| March 2024 | 549 |
| April 2024 | 519 |
| May 2024 | 379 |
| June 2024 | 329 |
| July 2024 | 297 |
| August 2024 | 304 |
Email alerts
Citing articles via, looking for your next opportunity.
- About International Journal of Epidemiology
- Recommend to your Library
Affiliations
- Online ISSN 1464-3685
- Copyright © 2024 International Epidemiological Association
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- DOI: 10.18332/tid/191857
- Corpus ID: 272143480
Global research trends and hotspots on smoking and lung cancer from 1994–2023: A bibliometric analysis
- Yangfan Xu , Jieqiong Qi , +1 author Yitao Jia
- Published in Tobacco Induced Diseases 28 August 2024
Figures and Tables from this paper

32 References
Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries.
- Highly Influential
Software survey: VOSviewer, a computer program for bibliometric mapping
Citespace ii: visualization and knowledge discovery in bibliographic databases, global research trends in cutaneous neurofibromas: a bibliometric analysis from 2003 to 2022, stakeholders’ voices of lung cancer screening in hong kong: study protocol for a mixed methods study, a bibliometric and visual analysis of low carbohydrate diet, clustering of health and oral health-compromising behaviours in army personnel in central peninsular malaysia, cancer treatment and survivorship statistics, 2022, a quantitative synthesis of eight decades of global multiple sclerosis research using bibliometrics, smokeless tobacco and cigarette smoking: chemical mechanisms and cancer prevention, related papers.
Showing 1 through 3 of 0 Related Papers
- Open access
- Published: 31 August 2024
The links between symptom burden, illness perception, psychological resilience, social support, coping modes, and cancer-related worry in Chinese early-stage lung cancer patients after surgery: a cross-sectional study
- Yingzi Yang ORCID: orcid.org/0000-0003-4242-0444 1 , 2 na1 ,
- Xiaolan Qian 1 na1 ,
- Xuefeng Tang 1 ,
- Chen Shen 2 ,
- Yujing Zhou 3 ,
- Xiaoting Pan 2 &
- Yumei Li 4
BMC Psychology volume 12 , Article number: 463 ( 2024 ) Cite this article
1 Altmetric
Metrics details
This study aims to investigate the links between the clinical, demographic, and psychosocial factors and cancer-related worry in patients with early-stage lung cancer after surgery.
The study utilized a descriptive cross-sectional design. Questionnaires, including assessments of cancer-related worry, symptom burden, illness perception, psychological resilience, coping modes, social support and participant characteristics, were distributed to 302 individuals in early-stage lung cancer patients after surgery. The data collection period spanned from January and October 2023. Analytical procedures encompassed descriptive statistics, independent Wilcoxon Rank Sum test, Kruskal-Wallis- H - test, Spearman correlation analysis, and hierarchical multiple regression.
After surgery, 89.07% had cancer-related worries, with a median (interquartile range, IQR) CRW score of 380.00 (130.00, 720.00). The most frequently cited concern was the cancer itself (80.46%), while sexual issues were the least worrisome (44.37%). Regression analyses controlling for demographic variables showed that higher levels of cancer-related worry (CRW) were associated with increased symptom burden, illness perceptions, and acceptance-rejection coping modes, whereas they had lower levels of psychological resilience, social support and confrontation coping modes, and were more willing to obtain information about the disease from the Internet or applications. Among these factors, the greatest explanatory power in the regression was observed for symptom burden, illness perceptions, social support, and sources of illness information (from the Internet or applications), which collectively explained 52.00% of the variance.
Conclusions
Healthcare providers should be aware that worry is a common issue for early stage lung cancer survivors with a favorable prognosis. During post-operative recovery, physicians should identify patient concerns and address unmet needs to improve patients’ emotional state and quality of life through psychological support and disease education.
Peer Review reports
Introduction
Lung cancer is a common malignant neoplasm that often causes considerable psychological distress to patients and their families [ 1 ]. Due to the increasing public awareness of health screening in recent years and the use and promotion of low-dose computed tomography (CT) screening for early screening and detection of lung cancer, the incidence of early-stage lung cancer has been increasing [ 2 ]. According to the clinical diagnostic criteria for lung cancer, early-stage non-small cell lung cancer refers to a tumor that is confined to the lung and has not metastasized to distant organs or lymph nodes, generally referring to stage I and II [ 3 , 4 ]. For individuals diagnosed with early-stage non-small cell lung cancer, radical surgical resection offers the most beneficial treatment option for extended survival [ 5 , 6 ]. It can be said that after diagnosis and active treatment of early-stage lung cancer patients, the recurrence rate 5 years after surgery is low. Although the survival rate after radical resection has improved, a decline in lung function is inevitable. According to studies [ 7 , 8 ], lung function experienced a steep decline at 1 month after lung resection, partially recovered at 3 months, and stabilized at 6 months after surgery. Patients who have undergone surgery for lung cancer often experience post-treatment symptoms, including pain, dyspnea, and fatigue, which negatively affect their quality of life [ 9 ]. A previous study conducted by our team [ 10 ] found that post-operative lung cancer patients had various unmet needs during their recovery, including physiological, safety, family and social support, and disease information. The psychological distress experienced by patients, including worry, anxiety, and fear, increases due to unmet needs after cancer treatments [ 11 , 12 ].
In recent years, there has been an increasing focus on Cancer-related worry (CRW) as a form of psychological distress experienced by cancer patients. CRW refers to the uncertainty of cancer patients’ future after cancer diagnosis. It encompasses areas of common concern to cancer patients, such as cancer itself, disability, family, work, economic status, loss of independence, physical pain, psychological pain, medical uncertainty, and death. The purpose of CRW is to reflect the unmet needs or concerns of cancer patients [ 13 , 14 , 15 ]. Unlike anxiety, worry primarily reflects the patient’s repetitive thoughts about the uncertainty of the future [ 13 ]. It is also a cognitive manifestation of the uncertainty of disease prognosis [ 16 ].
Currently, measurement scales such as the State Train Anxiety Inventory (STAI) and the Hospital Anxiety and Depression Scale (HADS) are frequently used to evaluate physical symptoms caused by autonomic nervous activity in patients. However, they do not assess patients’ concerns or anxiety content [ 17 ]. Some scholars [ 13 , 17 , 18 ] have developed tools to measure the degree and content of cancer-related worries. The CRW questionnaire is a tool for measuring and evaluating the anxiety status of patients. It can also detect their needs or preferences through convenient means, allowing for the design of personalized care [ 13 ]. These questionnaires were primarily utilized to assess the level and nature of worry among cancer patients diagnosed with breast, prostate, skin, and adolescent cancers [ 19 , 20 , 21 , 22 ], However, it has not been employed in the post-operative population for early-stage lung cancer.
Theory framework
Mishel’s Uncertainty in Illness Theory [ 23 ] defines illness uncertainty as a cognitive state that arises when individuals lack sufficient information to effectively construct or categorize disease-related events. The theory explains how patients interpret the uncertainty of treatment processes and outcomes through a cognitive framework. It consists of three main components (see Fig. 1 ): (a) antecedents of uncertainty, (b) appraisal of uncertainty, and (c) coping with uncertainty. The antecedents of uncertainty include the stimulus frame (such as symptom burden), cognitive capacity (like disease perception), and structure providers (including social support and sources of disease information). Managing uncertainty requires coping modes such as emotional regulation and proactive problem-solving. Studies [ 24 ] have shown that there is a correlation between the way patients manage their emotions and the coping modes they experience when faced with difficulties. Patients with positive emotional coping modes are less likely to worry, while patients with avoidance coping modes are more likely to experience emotional distress. Furthermore, previous research [ 25 ] has shown a strong correlation between psychological resilience and cancer-related worries. Psychological resilience reflected an individual’s ability to adapt and cope with stress or adversity, and was an important indicator of a patient’s psychological traits [ 26 ]. Furthermore, it had a significant impact on their mental state and quality of life. Specifically, higher levels of cancer worries have been linked to lower levels of psychological resilience.
Based on the theoretical framework and literature research presented, it is hypothesized that factors such as psychological resilience, antecedents of disease uncertainty (symptom burden, disease perception, social support and sources of disease information), and coping modes will correlate with the level of cancer-related worries in postoperative patients with early-stage lung cancer. Therefore, the aim of this study was to investigate the potential correlates of cancer-related worry in early-stage lung cancer patients after surgery. This analysis will aid medical professionals in comprehending the worry state and unmet needs of early-stage lung cancer patients after surgery, and in tailoring rehabilitation programs and psychological interventions accordingly.
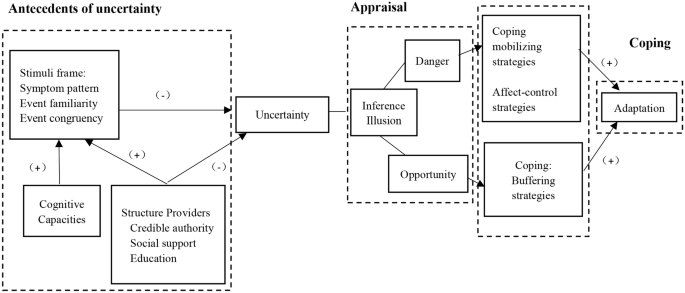
Mishel’s uncertainty in Illness theory framework
Study design
The study was cross-sectional in design. The Stimulating the Reporting of Observational Studies in Epidemiology (STROBE) checklist was completed (see S1 STROBE Checklist).
Setting and participants
This study included patients who underwent surgical treatment for lung cancer at a general hospital in Shanghai between January and October 2023. The study inclusion criteria were: (1) age over 18 years; (2) clinical diagnosis of early-stage primary non-small cell lung cancer (NSCLC), Tumor node metastasis classification (TNM) I to II [ 27 , 28 ], and received video-assisted thoracic surgery; (3) recovery time after surgery was within 1 month. Exclusion criteria were: (1) Postoperative radiotherapy and chemotherapy or second surgery may be required. (2) If other malignant tumors are present, they may also need to be treated. (3) Patients with severe psychological or mental disorders may not be candidates for this survey. (4) Patients with speech communication difficulties or hearing and visual impairments may require additional accommodations.
Sample size was calculated using G*Power software version 3.1.9 [ 29 ]. Calculate power analysis using F-test and linear multiple regression: fixed model, R2 increase as statistical test, and “A priori: Calculate required sample size - given alpha, power, and effect size” as type of power analysis. Cohen’s f2 = 0.15, medium effect size, α = 0.05, power (1-β) = 0.80, number of tested predictors = 8, total number of predictors = 10 as input parameters. The analysis showed that the minimum required sample size was 109 adults. Finally, the required sample size was determined to be over 120 adults with a probability of non-response rate of 10%.
Research team and data collection
The research team consisted of two nursing experts, four nursing researchers (one of whom had extensive experience in nursing psychology research), a group of clinical nurses, and several research assistants. To ensure the scientific quality and rigor of the research, the team was responsible for overseeing the quality of the project design and implementation process. Prior to conducting the formal survey, all research assistants received consistent training and evaluation to ensure consistent interpretation of questionnaire responses.
One month after surgery marks the first stage of the early recovery process for lung cancer patients and is a critical period for psychological adjustment [ 30 ]. Understanding a patient’s psychological state is critical to providing excellent medical care. During this period, patients typically need to visit the outpatient clinic for wound suture removal and dressing changes, further emphasizing the importance of postoperative recovery. Therefore, we chose this time frame for our research to gain a more comprehensive understanding of patients’ psychological well-being.
Prior to the start of the study, the hospital management and department head worked with us to provide comprehensive and standardized training to all participating investigators to ensure the reliability and consistency of the study. Research assistants rigorously screened patients based on predefined inclusion and exclusion criteria, which were designed to ensure a representative sample of lung cancer patients in the early recovery phase.
To obtain informed consent, patients were provided with detailed information and explanations and asked to complete the questionnaire voluntarily. To accommodate the different needs and preferences of patients, we used a combination of electronic and paper questionnaires. The electronic questionnaires were administered via the Questionnaire Star platform ( https://www.wjx.cn/ ), allowing patients to scan a two-dimensional (QR) code to access the survey. For those who preferred paper questionnaires, research assistants provided physical copies and assisted with completion as needed. The questionnaire was designed with consistent instructions to ensure that patients had a full understanding of the questions. Upon completion, each questionnaire was carefully reviewed and checked for accuracy. In addition, to reduce attrition, we obtained patients’ consent to keep their contact information, which allowed us to communicate with them further and collect additional data.
Ethical considerations
The study was approved by the hospital ethics committee. All participants gave informed consent prior to enrollment.
Sociodemographic questionnaire
We developed a sociodemographic survey based on a literature review and expert consultation. The survey includes patient demographic data such as age, sex, residence, lifestyle, education, marital status, childbearing history, religion, insurance, economic status (annual household income), smoking habits, employment status, sources of disease information, and previous psychological counseling. Clinical case information includes physical comorbidity, clinical tumor stage, and cancer type.
Cancer-related worry
The study measured participants’ cancer-related worry using the Brief Cancer-related Worry Inventory (BCWI), which was originally designed by Hirai et al. [ 13 ] to evaluate distinct concerns and anxiety levels among individuals with cancer. For this research, we used the 2019 Chinese edition of the BCWI, as introduced and updated by He et al. [ 31 ] (see Supplementary Table 2 ). The BCWI was comprised of 16 items, which were divided into three domains: (1) future prospects, (2) physical and symptomatic problems, and (3) social and interpersonal problems. Participants were asked to assess their cancer-related worries on a scale ranging from 0 to 100. Worry severity was determined by summing the scores for each item. The higher the total score, the more intense the patient’s cancer-related worry. The BCWI provided a concise evaluation of cancer-related worries in cancer patients. With only 16 items, it was able to differentiate them from symptoms of anxiety, depression, and post-traumatic stress disorder. In this study, the Cronbach’s alpha coefficient for this scale was 0.96.
Symptom burden
The M.D. Anderson Symptom Assessment Scale [ 32 ] was a widely used tool for evaluating symptom burden in cancer patients. The Chinese version of MDASI-C was translated and modified by researchers at the M.D. Anderson Cancer Center. The questionnaire included 13 multidimensional symptom items, such as pain, fatigue, nausea, restless sleep, distress, and shortness of breath, forgetfulness, loss of appetite, lethargy, dry mouth, sadness, vomiting, and numbness. Six additional items were used to evaluate the impact of these symptoms on work, mood, walking, relationships, and daily enjoyment. Patients rated the severity of their symptoms over the past 24 h on a scale of 0 (absent) to 10 (most severe), providing a comprehensive assessment of symptom burden across multiple dimensions. The Cronbach’s alpha coefficient for this study was 0.95.
Illness perception
The Brief Illness Perception Questionnaire (BIPQ) was used to measure participants’ illness perception. The scale, developed by Broadbent [ 33 ], was later revised by Mei [ 34 ] to include a Chinese version. It consisted of eight items divided into cognition, emotion, and comprehension domains as well as one open-ended question (What are the three most important factors in the development of lung cancer, in order of importance? ). The study utilized a 10-point Likert scale to rank items one through eight, with a range of 0–80 points. The ninth item required an open response. A higher total score indicated a greater tendency for individuals to experience negative perceptions and perceive symptoms of illness as more severe. In this study, the Cronbach’s alpha coefficient for this scale of eight scoring items was found to be 0.73.
Psychological resilience
The study measured participants’ psychological resilience using the 10-item Connor Davidson Resilience Scale (CD-RISC-10), originally developed by Connor and Davidson [ 35 ], and later revised by Campbell based on CD-RISC-25. The scale was designed to assess an individual’s level of emotional resilience in a passionate environment. It comprised 10 items, rated on a 5-point Likert scale (0 = never, 1 = rarely, 2 = sometimes, 3 = often, 4 = always). The total score ranged from 0 to 40 points, with higher scores indicating greater resilience. The study utilized the Chinese version of CD-RISC10, which was translated and revised by Ye et al. [ 36 ], to measure psychological resilience. The Cronbach’s alpha coefficient of the scale in this study was 0.96.
Coping modes
The study measured participants’ coping modes using the Medical Coping Modes Questionnaire (MCMQ), a specialized tool for measuring patient coping modes. The MCMQ was first designed by Feifel in 1987 [ 37 ] and was translated and revised into Chinese by Shen S and Jiang Q in 2000 [ 38 ]. It consisted of 20 items and three dimensions: confrontation (eight items), avoidance (seven items), and acceptance-resignation (five items). The study utilized a 4-point scoring system to evaluate coping events, with scores ranging from 1 to 4 based on the strength of each event. Eight items (1, 4, 9, 10, 12, 13, 18, and 19) were negatively scored, resulting in a total score range of 20 to 80 points. A higher score indicated a more frequent use of this coping mode. The three dimensions of this scale can be split into three scales for separate use. The reliability coefficients of the Confrontation Coping Mode Scale, Avoidance Coping Mode Scale, and Acceptance-resignation Coping Mode Scale were 0.69, 0.60, and 0.76.
Social Support
The study used the Perceived Social Support Scale (PSSS) developed by Zimet [ 39 ] to assess the level of self-understanding and perceived social support in postoperative lung cancer patients. The Chinese version of the PSSS, as adapted by Chou [ 40 ], showed satisfactory reliability and validity. The 12-item scale comprised of three dimensions: family, friend, and other support, and was rated using a 7-point Likert scale (1 = strongly disagree, 7 = strongly agree). The scale’s total score ranged from 12 to 84, with a higher score indicating a greater subjective sense of social support received by individuals. The Cronbach’s alpha coefficient for this scale in this study was 0.94.
Statistical analyses
The questionnaire was validated and double-checked before being input into Excel to ensure accuracy. Statistical analysis and processing of the questionnaire data were conducted using Statistical Product and Service Solutions (SPSS) 26.0 software. All statistical tests were two-sided with a significance level of α = 0.05. The quantitative data were presented using means and standard deviations, while the qualitative data were represented by frequency distributions. The data that conforms to normal distribution was analyzed using independent sample t-test for comparison between two groups and one-way ANOVA analysis for comparison between multiple groups. Non-normal distribution data were analyzed using non-parametric Wilcoxon rank sum test for comparison between two groups and Kruskal-Wallis - H -Test for comparison between multiple groups. Additionally, Spearman correlation analysis was used to study the correlations among CRW, MDASI-C, BIPQ, CD-RISC-10, MCMQ, and PSSS. A hierarchical linear regression analysis was performed to identify the multidimensional factors affecting CRW. All variables significantly correlated with the outcome variable ( p < 0.05) were included in the corresponding hierarchical regression analysis. Using Mishel’s Uncertainty in Illness Theory as a framework, a four-step model was adopted to study the factors influencing cancer-related concerns. The model includes individual factors such as sociodemographic and disease-related data, as well as psychological resilience, stimulus frame (symptom burden, illness perception, social support, and sources of disease information), and coping modes.
In this study, 307 questionnaires were distributed (227 electronic, 80 paper). Five paper questionnaires were invalid, resulting in 302 valid questionnaires and a recovery rate of 98.37%. The participants were 302 postoperative lung cancer patients, with 36.75% male and 63.25% female, aged 18–83 years (mean age 52.73, SD 13.07). Most patients (90.07%) were married. Additional demographic and clinical information is in Table 1 .
89.07% of people had cancer-related worries after surgery and median (interquartile range, IQR) score for CRW was 380.00 (130.00, 720.00) with a range of 0-1600. 86.42% reported worry about future prospects, 84.11% worry about physical and symptomatic problems, 79.80% worry about social and interpersonal problems. Among the 16 worry items of BCWI, the highest frequency of patient worries was “About cancer itself” (80.46%), followed by “About whether cancer might get worse in the future” (79.14%); the lowest frequency concern was “about sexual issues” (44.37%). According to the average score of the items in the three dimensions of CRW, it could be seen that patients had the highest standardized score (standardized score = median score/the total score of the dimension*100%) in future prospects (30.00%), the second standardized score in physical and symptomatic problems (20.00%), and the lowest in social and interpersonal problems (15.00%) as it is shown in Table 2 .
On the total score of the CRW scale, patients’ cancer-related worry was significantly correlated with their gender ( p = 0.009) and annual family income ( p = 0.018; see Table 1 ). The results suggested that gender and annual family income were related to the level of concern patients had after developing cancer. Additionally, there was a correlation between patients who received information about their disease from the internet or applications and their level of CRW ( p = 0.024; see Table 1 ).
The correlation analysis between various scales revealed a significant correlation ( p < 0.05; see Table 3 ) between CRW and MDASI-C, BIPQ, CD-RISC-10, and two dimensions of MCMQ (excluding avoidance). It was worth noting that avoidance coping modes did not show a correlation with CRW. In addition, according to the summary of the ninth open-ended question on the BIPQ, patients believed that the main causes of lung cancer were genetics, fatigue, stress from work, family or life, negative emotions (anger, worry) and unhealthy lifestyle (diet, work and rest, smoking), environmental factors (poor air quality, secondhand smoke, cooking fumes), and new coronavirus pneumonia (including vaccination, new coronavirus infection), etc. Further stratified linear regression analysis was conducted to determine the correlation between the dependent and independent variables (Table 4 ).
Table 4 presented the results of the hierarchical linear regression analysis for CRW in early-stage lung cancer patients. First, all scales included in the hierarchical regression analysis were tested for collinearity (variance inflation factor, VIF). The average VIF value was slightly above 1, indicating that the results were acceptable [ 41 ]. Second, the core research variables were divided into four levels according to the theoretical research, and the variables included in each level were analyzed separately. Model 1 included personal characteristics as independent variables and explained 5% of the variance in CRW. The analysis identified only two variables associated with CRW: being female (compared to male) and having low income (compared to high income). In model 2, psychological resilience was identified as an individual psychological characteristic and placed in the second level, resulting in a 9% increase in explanatory power. Model 3 added the antecedents of uncertainty, including symptom burden, illness perception, social support, and source of disease information, at the third level. This significantly increased the explanatory power of the overall regression model by 53%. The addition of coping modes, specifically confrontation coping mode and acceptance-resignation coping mode, to model 4 only increased the explanatory power of the overall regression model by 1%. The overall model demonstrated a total explanatory power of 68%. In Model 4, factors that significantly correlated with CRW included middle income( β = 2.17), psychological resilience( β =-2.42), symptom burden( β = 12.62), illness perception( β = 9.17), social support( β =-3.27), and source of disease( β = 2.01), as well as confrontation coping mode ( β =-1.98) and acceptance-resignation coping mode( β = 2.77), see Table 4 .
This study analyzed data from 302 patients to investigate the clinical, demographic, and psychosocial factors that correlated with cancer-related worry in patients with early-stage lung cancer after surgery. The study extended our understanding of the specific content and relevant factors of psychological distress in post-operative patients with early-stage lung cancer, a relationship that had not been fully investigated.
The study revealed that Chinese patients with early stage lung cancer were primarily concerned about their future prospects related to the disease itself, while sexual life problems caused by cancer were of least concern. This finding was consistent with previous studies [ 42 ], but this study provided more specific information on patients’ cancer-related worries. Lung cancer was widely perceived as a serious illness by the public due to its high cancer-specific mortality rate and low survival rate after diagnosis [ 43 ]. Consequently, patients with lung cancer often experience significant psychological distress after diagnosis. Even if the tumor was successfully removed, patients might still face challenges during recovery [ 44 ]. According to Reese’s research [ 45 ], long-term survivors of lung cancer experienced mild sexual distress. They also noted that sexual distress was significantly associated with physical and emotional symptoms. Although this study found that patients were least concerned about sexual distress, it should be noted that this study was based on the early stages of recovery after cancer surgery. Due to the postoperative repair of their body and emotions, patients were primarily focused on meeting their physiological and safety needs [ 10 ]. Further validation and exploration are required as there are limited studies on sexual distress in post-operative patients with early-stage lung cancer.
This study found that gender and annual family income were associated with the CRW of early-stage lung cancer patients. Among them, women and patients from low-income families had higher CRW scores, which was similar to the results of CRW in other cancer studies [ 14 , 46 ]. The reason might be that women were more conscious about uncomfortable symptoms than men, and women were more concerned about the duration of the disease and subsequent treatment effects than men [ 47 ]. Moreover, lower annual family income might cause patients to face more financial pressure in terms of medical expenses and treatment, thereby increasing their concerns about the consequences of the disease [ 48 ]. In addition, several other studies have found that education level, smoking status, and tumor stage had an impact on cancer-related worry scores [ 14 , 47 ], but this study did not show statistical significance.
After controlling for demographic covariate factors, the study found a significant negative relationship between psychological resilience and CRW. In a study conducted by Chen et al. [ 49 ], lower levels of psychological resilience were observed in post-operative lung cancer patients, which had a direct impact on their emotional state. In the context of treatment and recovery after lung cancer surgery, medical professionals should prioritize enhancing patients’ psychological resilience. This could be achieved through psychological support and appropriate interventions to improve emotional health and quality of life.
The antecedents in the illness uncertainty theoretical framework, such as symptom burden, illness perception, social support, and sources of disease information, were shown to be significantly associated with patients’ cancer worries in this study. The theory of uncertainty in illness [ 50 ] posited that uncertainty was caused by stimulus frames, cognitive abilities, and structural providers. In this study, these antecedents corresponded to symptom burden, illness perception, social support, and sources of disease information, and they correlated with patient worry related to cancer. Patients with a high symptom burden, high illness perception, low social support, and excessive attention to disease information on the Internet were more likely to have high cancer-related concerns. Previous studies [ 40 , 51 ] have verified the relationship between symptom burden, illness perception, and social support with psychological distress in lung cancer patients. However, there have been few studies on this patient group after surgery for early-stage lung cancer, particularly based on the uncertainty theoretical framework. Additionally, when analyzing the antecedents of structured providers, we included the sources from which patients receive information about their disease, particularly statistics from medical staff and online platforms. Our study found that patients who frequently accessed disease information on the internet had higher cancer-related worry scores. This was consistent with previous qualitative studies [ 10 ] which had shown that patients often turn to the internet for disease-related knowledge due to a perceived lack of effective information from medical staff.
This study also explored the association of CRW with coping modes. According to the theoretical framework of uncertainty in illness, coping modes are key for managing uncertainty, as uncertainty influences patients’ coping methods. Previous research [ 51 ] has shown that different coping modes can affect patients’ emotional states. Because this study focused primarily on the factors correlated with CRW, we included coping modes as independent variables in the linear regression analysis. The results indicated that CRW was negatively correlated with the coping mode of confrontation and positively correlated with the coping mode of acceptance-resignation. Acceptance-resignation, a negative coping mode, has been shown [ 52 ] to be associated with patients’ fear of disease progression and negative attitudes toward the disease, which decreases their confidence in treatment. Poręba-Chabros et al. [ 53 ] found that negative coping patterns were significantly associated with depression. When patients adopt an acceptance-resignation coping mode, their compliance behaviors decrease as they succumb to the disease. Conversely, confrontation, a positive coping mode, can enhance patients’ psychosocial adaptability, buffer psychological distress, and improve quality of life [ 52 ]. Interestingly, avoidance coping modes did not show a correlation with CRW in the postoperative population of early stage lung cancer, which is inconsistent with previous studies [ 54 ]. This lack of correlation may be due to several factors. The focus of our study on the first month after surgery may mean that patients are more focused on immediate physical recovery rather than engaging in avoidance behaviors. The nature of avoidance coping may temporarily alleviate worry without addressing underlying concerns, resulting in no measurable effect on CRW. Sample characteristics, measurement limitations, and individual differences in coping modes may also contribute to bias. In addition, strong psychological resilience, robust support systems, positive surgical outcomes, and increased health education may make avoidance modes less relevant or impactful on CRW in this population. Although the two coping modes were found to be associated with cancer-related worry in our study, hierarchical regression analysis showed that their influence on patients’ worry was small. Future research should explore the mechanisms of cancer-related worry and coping modes based on the theoretical framework of illness uncertainty. Given the association between cancer-related worry and coping modes, psychological intervention for patients should be emphasized in clinical practice. Healthcare professionals should conduct comprehensive psychological assessments and provide effective emotional support and education to help cancer patients develop more effective coping modes and reduce worry caused by uncertainty.
Strengths and limitations
This study presented evidence for CRW and the influencing factors that postoperative patients with early-stage lung cancer face. Based on the results, healthcare providers could identify the specific unmet needs of these patients more precisely and develop effective intervention strategies to improve their emotional state and quality of life. While the existing literature has extensively discussed psychological symptoms such as anxiety, depression, and fear in patients with mid-to-late stage lung cancer, relatively little research has been conducted on the mental health of postoperative patients with early-stage lung cancer, who are an important group of long-term lung cancer survivors [ 55 ]. As the number of patients diagnosed with early-stage lung cancer increases, so does concern about their mental health and unmet needs [ 56 , 57 ].
Nonetheless, this study also has some limitations. First, the single-center cross-sectional design and relatively small sample size may limit the generalizability of the findings to the broader population of early-stage lung cancer patients.
Second, while the study adopted a theoretical framework, it primarily conducted basic factor analysis without delving into the interaction mechanisms between the identified factors. This limitation restricts our understanding of how these factors interplay to influence cancer-related worry (CRW) in postoperative patients. In addition, while the CRW variable is largely operationalized in a similar way to the BIPQ and psychological resilience, we found no high correlation between these scales, as indicated by the Variance Inflation Factor (VIF). This means that multicollinearity, or the overlap between the variables, is not an issue in our study. However, we recognize that the scales we used may have limitations and might not fully capture the specific experiences of our sample. Therefore, future research should use different methods to measure CRW and related factors to better understand their individual effects on cancer-related worry.
Third, CRW is a dynamic process [ 20 ], and this study only focused on the patients’ situation within one month after surgery. This snapshot approach may not reflect the evolving nature of CRW over time. Longitudinal studies are needed to provide a more comprehensive understanding of how CRW and its influencing factors change throughout the postoperative recovery period.
These limitations suggest that future research should adopt a longitudinal design to analyze and verify the identified factors over an extended period. Additionally, expanding the study to include multiple centers and larger, more diverse sample sizes would enhance the generalizability of the findings. Exploring the interaction mechanisms between factors using advanced analytical methods could provide deeper insights into the complexities of CRW. By addressing these limitations, future research can build on our findings to offer more robust and generalizable evidence on the psychological distress experienced by postoperative early-stage lung cancer.
Implications for practice
The results of the study indicated that early-stage lung cancer patients in China had significant concerns about their future prospects, particularly regarding the disease itself, with less attention paid to the impact of cancer on sexual life. To facilitate postoperative recovery effectively, healthcare providers must promptly identify and address these specific concerns by incorporating routine psychological assessments and developing tailored intervention strategies, such as cognitive-behavioral therapy and resilience training, to improve patients’ mental health and overall well-being.
Moreover, this study identified psychological resilience, symptom burden, illness perception, social support, sources of disease information (from the Internet or applications), and coping modes of confrontation and acceptance-resignation as key predictors of cancer-related worry in postoperative early-stage lung cancer patients. Managing patients’ postoperative emotional states and enhancing their quality of life requires a deep understanding and proactive intervention by healthcare professionals. Specific strategies include providing psychological assessments, developing individualized care plans, facilitating support groups, and utilizing technology for continuous support.
The study provided insight into cancer-related worry among Chinese patients after surgery for early-stage lung cancer. The results showed that patients were most concerned about their future prospects, particularly the disease itself, while relatively little attention was paid to their sexual distress. The study identified several key factors that correlated with cancer-related worry, including psychological resilience, symptom burden, illness perception, social support, and sources of disease information from the internet, as well as coping modes. These findings emphasized the significance of healthcare providers identifying and addressing the individual needs of patients during post-operative recovery. It is important to improve the emotional state and quality of life of patients through psychological support and disease education. This study provides guidance for post-operative care of patients with early stage lung cancer and suggests avenues for future research. Specifically, further exploration of the mechanisms of these relationships and development of effective interventions are needed.
Data availability
The data that supported the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Meng L, Jiang X, Liang J, Pan Y, Pan F, Liu D. Postoperative psychological stress and expression of stress-related factors HSP70 and IFN-γ in patients with early lung cancer. Minerva Med. 2023;114(1):43–8. https://doi.org/10.23736/s0026-4806.20.06658-6 .
Article PubMed Google Scholar
Zhu S, Yang C, Chen S, Kang L, Li T, Li J, Li L. Effectiveness of a perioperative support programme to reduce psychological distress for family caregivers of patients with early-stage lung cancer: study protocol for a randomised controlled trial. BMJ Open. 2022;12(8):e064416. https://doi.org/10.1136/bmjopen-2022-064416 .
Article PubMed PubMed Central Google Scholar
Rimner A, Ruffini E, Cilento V, Goren E, Ahmad U, Appel S, Bille A, Boubia S, Brambilla C, Cangir AK, et al. The International Association for the study of Lung Cancer Thymic Epithelial tumors Staging Project: an overview of the Central Database Informing Revision of the Forthcoming (Ninth) Edition of the TNM classification of malignant tumors. J Thorac Oncol. 2023;18(10):1386–98. https://doi.org/10.1016/j.jtho.2023.07.008 .
Organization WH. (2023). Lung cancer. Retrieved from https://www.who.int/zh/news-room/fact-sheets/detail/lung-cancer
Association OSCM, House CMAP. Chinese Medical Association guideline for clinical diagnosis and treatment of lung cancer (2023 edition). Chin J Oncol. 2023;45(7):539–74. https://doi.org/10.3760/cma.j.cn112152-20230510-00200 .
Article Google Scholar
Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, Escriu C, Peters S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl4):iv1. https://doi.org/10.1093/annonc/mdx222 .
Gu Z, Wang H, Mao T, Ji C, Xiang Y, Zhu Y, Xu P, Fang W. Pulmonary function changes after different extent of pulmonary resection under video-assisted thoracic surgery. J Thorac Dis. 2018;10(4):2331–7. https://doi.org/10.21037/jtd.2018.03.163 .
Chen L, Gu Z, Lin B, Wang W, Xu N, Liu Y, Ji C, Fang W. Pulmonary function changes after thoracoscopic lobectomy versus intentional thoracoscopic segmentectomy for early-stage non-small cell lung cancer. Transl Lung Cancer Res. 2021;10(11):4141–51. https://doi.org/10.21037/tlcr-21-661 .
Guo X, Zhu X, Yuan Y. Research Progress on the correlation of Psychological disorders in Lung Cancer patients. Med Philos. 2020;41(2):36–9. https://doi.org/10.12014/j.issn.1002-0772.2020.02.09 .
Yang Y, Chen X, Pan X, Tang X, Fan J, Li Y. The unmet needs of patients in the early rehabilitation stage after lung cancer surgery: a qualitative study based on Maslow’s hierarchy of needs theory. Support Care Cancer. 2023;31(12):677. https://doi.org/10.1007/s00520-023-08129-z .
Pongthavornkamol K, Lekdamrongkul P, Pinsuntorn P, Molassiotis A. Physical symptoms, unmet needs, and Quality of Life in Thai Cancer survivors after the completion of primary treatment. Asia Pac J Oncol Nurs. 2019;6(4):363–71. https://doi.org/10.4103/apjon.apjon_26_19 .
Park J, Jung W, Lee G, Kang D, Shim YM, Kim HK, Jeong A, Cho J, Shin DW. Unmet supportive care needs after Non-small Cell Lung Cancer Resection at a Tertiary Hospital in Seoul, South Korea. Healthc (Basel). 2023;11(14). https://doi.org/10.3390/healthcare11142012 .
Hirai K, Shiozaki M, Motooka H, Arai H, Koyama A, Inui H, Uchitomi Y. Discrimination between worry and anxiety among cancer patients: development of a brief Cancer-related worry inventory. Psychooncology. 2008;17(12):1172–9. https://doi.org/10.1002/pon.1348 .
Papaleontiou M, Reyes-Gastelum D, Gay BL, Ward KC, Hamilton AS, Hawley ST, Haymart MR. Worry in thyroid Cancer survivors with a favorable prognosis. Thyroid. 2019;29(8):1080–8. https://doi.org/10.1089/thy.2019.0163 .
Ware ME, Delaney A, Krull KR, Brinkman TM, Armstrong GT, Wilson CL, Mulrooney DA, Wang Z, Lanctot JQ, Krull MR, et al. Cancer-related worry as a predictor of 5-yr physical activity level in Childhood Cancer survivors. Med Sci Sports Exerc. 2023;55(9):1584–91. https://doi.org/10.1249/mss.0000000000003195 .
Mathews A. Why worry? The cognitive function of anxiety. Behav Res Ther. 1990;28(6):455–68. https://doi.org/10.1016/0005-7967(90)90132-3 .
Gotay CC, Pagano IS. Assessment of survivor concerns (ASC): a newly proposed brief questionnaire. Health Qual Life Outcomes. 2007. https://doi.org/10.1186/1477-7525-5-15 . 5.
Andersen MR, Smith R, Meischke H, Bowen D, Urban N. Breast cancer worry and mammography use by women with and without a family history in a population-based sample. Cancer Epidemiol Biomarkers Prev. 2003;12(4):314–20.
PubMed Google Scholar
Wu SM, Schuler TA, Edwards MC, Yang HC, Brothers BM. Factor analytic and item response theory evaluation of the Penn State worry questionnaire in women with cancer. Qual Life Res. 2013;22(6):1441–9. https://doi.org/10.1007/s11136-012-0253-0 .
Jackson Levin N, Zhang A, Reyes-Gastelum D, Chen DW, Hamilton AS, Zebrack B, Haymart MR. Change in worry over time among hispanic women with thyroid cancer. J Cancer Surviv. 2022;16(4):844–52. https://doi.org/10.1007/s11764-021-01078-8 .
McDonnell GA, Brinkman TM, Wang M, Gibson TM, Heathcote LC, Ehrhardt MJ, Srivastava DK, Robison LL, Hudson MM, Alberts NM. Prevalence and predictors of cancer-related worry and associations with health behaviors in adult survivors of childhood cancer. Cancer. 2021;127(15):2743–51. https://doi.org/10.1002/cncr.33563 .
Jones SM, Ziebell R, Walker R, Nekhlyudov L, Rabin BA, Nutt S, Fujii M, Chubak J. Association of worry about cancer to benefit finding and functioning in long-term cancer survivors. Support Care Cancer. 2017;25(5):1417–22. https://doi.org/10.1007/s00520-016-3537-z .
Mishel MH. Uncertainty in illness. Image J Nurs Sch. 1988;20(4):225–32. https://doi.org/10.1111/j1547-50691988tb00082x .
Brand H, Speiser D, Besch L, Roseman J, Kendel F. Making sense of a health threat: illness representations, coping, and psychological distress among BRCA1/2 mutation carriers. Genes (Basel). 2021;12(5). https://doi.org/10.3390/genes12050741 .
Gordon R, Fawson S, Moss-Morris R, Armes J, Hirsch CR. An experimental study to identify key psychological mechanisms that promote and predict resilience in the aftermath of treatment for breast cancer. Psychooncology. 2022;31(2):198–206. https://doi.org/10.1002/pon.5806 .
Harms CA, Cohen L, Pooley JA, Chambers SK, Galvão DA, Newton RU. Quality of life and psychological distress in cancer survivors: the role of psycho-social resources for resilience. Psychooncology. 2019;28(2):271–7. https://doi.org/10.1002/pon.4934 .
National Health Commission, PRC. (2022). Clinical Practice Guideline for Primary Lung Cancer (2022 Version). Medical Journal of Peking Union Medical College Hospital, 13(4), 549–570.Retrieved from https://kns.cnki.net/kcms/detail/11.5882.r.20220629.1511.002.html
Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM Stage groupings in the Forthcoming (Eighth) Edition of the TNM classification for Lung Cancer. J Thorac Oncol. 2016;11(1):39–51. https://doi.org/10.1016/j.jtho.2015.09.009 .
Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G * Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–60. https://doi.org/10.3758/brm.41.4.1149 .
Nelson DB, Mehran RJ, Mena GE, Hofstetter WL, Vaporciyan AA, Antonoff MB, Rice DC. Enhanced recovery after surgery improves postdischarge recovery after pulmonary lobectomy. J Thorac Cardiovasc Surg. 2023;165(5):1731–40. https://doi.org/10.1016/j.jtcvs.2022.09.064 .
He S, Cui G, Liu W, Tang L, Liu J. Validity and reliability of the brief Cancer-related worry inventory in patients with colorectal cancer after operation. Chin Mental Health J. 2020;34(5):463–8. https://doi.org/10.3969/j.issn.1000-6729.2020.5.013 .
Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, Engstrom MC. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–46. https://doi.org/10.1002/1097-0142(20001001)89:7%3C1634::aid-cncr29%3E3.0.co;2-v .
Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception Questionnaire. J Psychosom Res. 2006;60(6):631–7. https://doi.org/10.1016/j.jpsychores.2005.10.020 .
Mei Y, Li H, Yang Y, Su D, Ma L, Zhang T, Dou W. Reliability and validity of Chinese Version of the brief illness perception questionnaire in patients with breast Cancer. J Nurs 2015(24):11–4 https://doi.org/10.16460/j.issn1008-9969.2015.24.011
Connor KM, Davidson JRT. Development of a new resilience scale: the Connor-Davidson Resilience scale (CD-RISC). Depress Anxiety. 2003;18(2):76–82. https://doi.org/10.1002/da.10113 .
Ye Z, Ruan X, Zeng Z, Xie Q, Cheng M, Peng C, Lu Y, Qiu H. Psychometric properties of 10-item Connor-Davidson Resilience scale among nursing students. J Nurs. 2016;23(21):9–13. https://doi.org/10.16460/j.issn1008-9969.2016.21.009 .
Feifel H, Strack S, Nagy VT, DEGREE OF LIFE-THREAT AND DIFFERENTIAL USE OF COPING MODES. J Psychosom Res. 1987;31(1):91–9. https://doi.org/10.1016/0022-3999(87)90103-6 .
Shen S, Jiang Q. Report on application of Chinese version of MCMQ in 701 patients. Chin J Behav Med Brain Sci. 2000;9(1):18. https://doi.org/10.3760/cma.j.issn.1674-6554.2000.01.008 .
Zimet GD, Dahlem NW, Zimet SG, Farley GK. THE MULTIDIMENSIONAL SCALE OF PERCEIVED SOCIAL SUPPORT. J Pers Assess. 1988;52(1):30–41. https://doi.org/10.1207/s15327752jpa5201_2 .
Chou KL. Assessing Chinese adolescents’ social support: the multidimensional scale of perceived social support. Pers Indiv Differ. 2000;28(2):299–307. https://doi.org/10.1016/s0191-8869(99)00098-7 .
Jia JP, He XQ, Jin YJ. Statistics. 7th ed. Beijing: Renmin University of China; 2018.
Google Scholar
Zhao F, Liu L, Zhang F, Kong Q. Analysis of fear of cancer recurrence in patients with lung cancer after surgery and its influencing factors. J Nurses Train. 2023;38(17):1619–22. https://doi.org/10.16821/j.cnki.hsjx.2023.17.018 .
Wang YH, Li JQ, Shi JF, Que JY, Liu JJ, Lappin JM, Leung J, Ravindran AV, Chen WQ, Qiao YL, et al. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry. 2020;25(7):1487–99. https://doi.org/10.1038/s41380-019-0595-x .
Morrison EJ, Novotny PJ, Sloan JA, Yang P, Patten CA, Ruddy KJ, Clark MM. Emotional problems, Quality of Life, and Symptom Burden in patients with Lung Cancer. Clin Lung Cancer. 2017;18(5):497–503. https://doi.org/10.1016/j.cllc.2017.02.008 .
Reese JB, Shelby RA, Abernethy AP. Sexual concerns in lung cancer patients: an examination of predictors and moderating effects of age and gender. Support Care Cancer. 2011;19(1):161–5. https://doi.org/10.1007/s00520-010-1000-0 .
Khoshab N, Vaidya TS, Dusza S, Nehal KS, Lee EH. Factors contributing to cancer worry in the skin cancer population. J Am Acad Dermatol. 2020;83(2):626–8. https://doi.org/10.1016/j.jaad.2019.09.068 .
Chen X, He X. Current status of postoperative psychological distress in patients with lung cancer and its influencing factors. Chin J Mod Nurs. 2021;24:3318–22.
Rogers Z, Elliott F, Kasparian NA, Bishop DT, Barrett JH, Newton-Bishop J. Psychosocial, clinical and demographic features related to worry in patients with melanoma. Melanoma Res. 2016;26(5):497–504. https://doi.org/10.1097/cmr.0000000000000266 .
Chen S, Mei R, Tan C, Li X, Zhong C, Ye M. Psychological resilience and related influencing factors in postoperative non-small cell lung cancer patients: a cross-sectional study. Psychooncology. 2020;29(11):1815–22. https://doi.org/10.1002/pon.5485 .
Mishel MH, Braden CJ. Finding meaning: antecedents of uncertainty in illness. Nurs Res. 1988;37(2):98–103.
Tian X, Jin Y, Chen H, Tang L, Jiménez-Herrera MF. Relationships among Social Support, coping style, perceived stress, and psychological distress in Chinese Lung Cancer patients. Asia Pac J Oncol Nurs. 2021;8(2):172–9. https://doi.org/10.4103/apjon.apjon_59_20 .
Prikken S, Luyckx K, Raymaekers K, Raemen L, Verschueren M, Lemiere J, Vercruysse T, Uyttebroeck A. Identity formation in adolescent and emerging adult cancer survivors: a differentiated perspective and associations with psychosocial functioning. Psychol Health. 2023;38(1):55–72. https://doi.org/10.1080/08870446.2021.1955116 .
Poręba-Chabros A, Kolańska-Stronka M, Mamcarz P, Mamcarz I. Cognitive appraisal of the disease and stress level in lung cancer patients. The mediating role of coping styles. Support Care Cancer. 2022;30(6):4797–806. https://doi.org/10.1007/s00520-022-06880-3 .
Park CL, Cho D, Blank TO, Wortmann JH. Cognitive and emotional aspects of fear of recurrence: predictors and relations with adjustment in young to middle-aged cancer survivors. Psychooncology. 2013;22(7):1630–8. https://doi.org/10.1002/pon.3195 .
Jovanoski N, Bowes K, Brown A, Belleli R, Di Maio D, Chadda S, Abogunrin S. Survival and quality-of-life outcomes in early-stage NSCLC patients: a literature review of real-world evidence. Lung Cancer Manag. 2023;12(3). https://doi.org/10.2217/lmt-2023-0003 .
Jonas DE, Reuland DS, Reddy SM, Nagle M, Clark SD, Weber RP, Enyioha C, Malo TL, Brenner AT, Armstrong C, et al. Screening for Lung Cancer with Low-Dose Computed Tomography: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(10):971–87. https://doi.org/10.1001/jama.2021.0377 .
Ho J, McWilliams A, Emery J, Saunders C, Reid C, Robinson S, Brims F. Integrated care for resected early stage lung cancer: innovations and exploring patient needs. BMJ Open Respir Res. 2017;4(1):e000175. https://doi.org/10.1136/bmjresp-2016-000175 .
Download references
Acknowledgements
The authors would like to thank the nurses (Especially Gu Jingzhi and Liu Jing) and doctors at Shanghai Pulmonary Hospital for facilitating this study and all the patients who kindly participated in the survey.
Scientific clinical research project of Tongji University, JS2210319; Key disciplines of Shanghai’s Three-Year Action Plan to Strengthen Public Health System Construction (2023–2025), GWVI-11.1-28; The National Key Research and Development Plan Project of China, 2022YFC3600903.
Author information
Yingzi Yang and Xiaolan Qian share first authorship.
Authors and Affiliations
Department of Health Care, Shanghai Health and Medical Center, No. 67, Dajishan, Wuxi City, Jiangsu Province, 214063, People’s Republic of China
Yingzi Yang, Xiaolan Qian & Xuefeng Tang
School of Medicine, Tongji University, 1239 Siping Road, Shanghai, 200092, People’s Republic of China
Yingzi Yang, Chen Shen & Xiaoting Pan
Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University, No.507, Zhengmin Road, Shanghai, 200433, People’s Republic of China
Yujing Zhou
Department of Nursing, Shanghai Pulmonary Hospital, Tongji University, No.507, Zhengmin Road, Shanghai, 200433, People’s Republic of China
You can also search for this author in PubMed Google Scholar
Contributions
Y.Y. and Y. L designed the study. Y.Y., Y.Z., C.S and X.P. ran the study and collected the data. Y.Y., X.Q., X.T. and C.S. analyzed the data; Y.Y., and X. Q. interpreted the results and drafted the paper. Y.Y. wrote the main manuscript text and X.Q. prepared Tables 1 , 2 , 3 and 4 . Y.Y., X.T. and Y.L revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Correspondence to Xuefeng Tang or Yumei Li .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the institutional review board at the Shanghai Pulmonary Hospital (Q23–396). The procedures used in this study adhere to the tenets of the Declaration of Helsinki. Consent for publication Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Yang, Y., Qian, X., Tang, X. et al. The links between symptom burden, illness perception, psychological resilience, social support, coping modes, and cancer-related worry in Chinese early-stage lung cancer patients after surgery: a cross-sectional study. BMC Psychol 12 , 463 (2024). https://doi.org/10.1186/s40359-024-01946-9
Download citation
Received : 18 May 2024
Accepted : 12 August 2024
Published : 31 August 2024
DOI : https://doi.org/10.1186/s40359-024-01946-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Lung cancer
- Psychological distress
- Uncertainty
BMC Psychology
ISSN: 2050-7283
- General enquiries: [email protected]
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
August 30, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Long-term conditions could delay lung cancer diagnosis, new study finds
by University of Sussex

A new study from researchers at Brighton and Sussex Medical School (BSMS) reveals that patients living with certain long-term health conditions may face delays in being diagnosed with lung cancer. This is particularly concerning as lung cancer remains the leading cause of cancer-related deaths in the UK for both men and women, with high mortality rates largely due to late-stage diagnosis.
The findings are published in the British Journal of Cancer .
The research highlights how some chronic conditions, such as chronic obstructive pulmonary disease (COPD) and asthma, can obscure early symptoms of lung cancer , leading to delays in diagnosis. The study analyzed GP health records and hospital data from 11,870 lung cancer patients in England, diagnosed between 1990 and 2019.
Dr. Imogen Rogers, who led the analysis, reported that patients with one or more conditions that could provide "alternative explanations" for lung cancer symptoms experienced significant delays in receiving a diagnosis. The study found:
- Patients with one "alternative explanation" condition, such as COPD or asthma, were diagnosed with lung cancer 31 days later on average.
- Patients with two or more such conditions experienced an even longer delay, averaging 74 days.
- COPD was identified as the condition resulting in the longest delay to lung cancer diagnosis, with affected patients being diagnosed 59 days later than those without the condition.
The study also found that conditions placing "competing demands" on a GP's time, such as arthritis or diabetes, did not significantly impact the time to lung cancer diagnosis once factors like age, sex, and smoking history were considered.
The research team suggests that clinical guidelines should be updated to highlight the potential for conditions like COPD to mask early symptoms of lung cancer. By raising awareness of this issue, they hope to reduce diagnostic delays and improve outcomes for patients.
"This research underscores the need for heightened vigilance in patients with chronic respiratory conditions," said Dr. Rogers. "Recognizing that these conditions can mask the symptoms of lung cancer is crucial in ensuring timely diagnosis and treatment."
The NHS in Sussex is already exploring ways to improve early cancer diagnosis, and this study could play a critical role in shaping future strategies. The findings emphasize the importance of comprehensive patient assessments and the need for health care professionals to consider the possibility of lung cancer even in patients with existing respiratory conditions.
This research could pave the way for new guidelines and training for GPs, ultimately aiming to save lives by catching lung cancer earlier, even in patients with complicated health conditions.
Explore further
Feedback to editors

Scientists unlock the secret behind a decades-old dengue mystery
17 minutes ago

Beta cells alone can regulate blood sugar levels, study finds

Novel platform for one-step production of sperm-like micro-robots could enhance precise drug delivery
38 minutes ago

No link found between popular diabetes medication and suicide
2 hours ago

Severe COVID-19 can involve either exacerbated lung inflammation or high viral replication, study finds

'Nudges' plus a little extra staff support help pediatricians deliver secure gun storage program

Analysis finds no increased risk of mental health issues among those using semaglutide for weight loss

Study finds racial and ethnic designation inaccuracies in children's medical records may impede equity efforts

Study explores the cell-type-specific effects of aging and sex on human cortical neurons
3 hours ago

3D-printed mini-tumors mimic human tissue for cancer immunotherapy tests
Related stories.

Lung cancer screening found to prolong lives in real-world study
Jun 10, 2024

Rheumatoid arthritis tied to higher risk for lung cancer
Aug 12, 2024

Raising awareness of lung cancer risk in people with COPD
Feb 18, 2019

Faulty immune system may lead to lung cancer
Jan 24, 2020

COPD linked to heightened risk of lung cancer in people who have never smoked
Apr 2, 2020
Era of hope for patients with lung cancer
Nov 21, 2022
Recommended for you

Gene found in ovarian cancer cells identified as potential new target for treatment
5 hours ago

Novel light-based technique shows 90% accuracy in early prostate cancer detection
Sep 2, 2024

Proof-of-principle study uncovers promising treatment for incurable prostate cancer

Scientists use exosomes secreted by living cells to successfully target TKI-resistant cancer

Two-in-one treatment could hold promise for incurable brain cancer, mouse study shows
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
VIDEO
COMMENTS
Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer-related deaths worldwide with an estimated 2 million new cases and 1·76 million deaths per year. Substantial improvements in our understanding of disease biology, application of predictive biomarkers, and refinements in treatment have led to remarkable progress in the past two decades and transformed ...
The Lung Cancer Master Protocol, or Lung-MAP, is a precision medicine research study for people with advanced non-small cell lung cancer that has continued to grow after treatment. Patients are assigned to different study drug combinations based on the results of genomic profiling of their tumors.
Lung cancer is the leading cause of cancer death in the United States and around the world. Almost as many Americans die of lung cancer every year than die of prostate, breast, and colon cancer combined (Fig. 1). 1 Siegel and colleagues 1 reviewed recent cancer data and estimated a total of 239,320 new cases of lung cancer and 161,250 deaths ...
Lung cancer remains the leading cause of cancer mortality in men and women in the U.S. and worldwide. About 90% of lung cancer cases are caused by smoking and the use of tobacco products. However, other factors such as radon gas, asbestos, air pollution exposures, and chronic infections can contribute to lung carcinogenesis.
Lung cancer is the leading cause of cancer-related mortality in the United States. Investigating epidemiological and clinical parameters can contribute to an improved understanding of disease ...
Tobacco smoking remains the predominant risk factor for lung cancer development. Nontobacco risk factors include environmental and occupational exposures, chronic lung disease, lung infections, and lifestyle factors. Because tobacco remains the leading risk factor for lung cancer, disease prevention is focused on smoking avoidance and cessation ...
Lung cancer is the leading cause of cancer-related death worldwide. However, lung cancer incidence and mortality rates differ substantially across the world, reflecting varying patterns of tobacco ...
Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer-related deaths worldwide with an estimated 2 million new cases and 1·76 million deaths per year. Substantial improvements in our understanding of disease biology, application of predictive biomarkers, and refinements in treatment have led to remarkable ...
Cancer is an international interdisciplinary journal publishing articles on the latest clinical cancer research findings, spanning the breadth of oncology disciplines. Abstract Despite decades of declining mortality rates, lung cancer remains the leading cause of cancer death in the United States. ... Search for more papers by this author ...
The review paper accounts for the novel and current immunotherapy and targeted therapy available for lung cancer in clinical trials and in the research phases in depth. Special attention is being paid to mark out single or multiple genes that are required for malignancy and survival while developing targeted therapies for lung cancer treatment.
Lung cancer is the most common cause of cancer-related deaths globally. Although smoking-related lung cancers continue to account for the majority of diagnoses, smoking rates have been decreasing ...
In 2018, a total of 47,838 people had lung cancer diagnoses with 35,137 deaths recorded. Incidence across the United Kingdom is illustrated in Figure 1. In the past decade, lung cancer age-standardized incidence rates increased by 1% overall with a 15% increase in females and 11% decrease in males ( Fig. 2 ), and lung cancer age-standardized ...
Lung cancer, a multifaceted disease, demands tailored therapeutic approaches due to its diverse subtypes and stages. This comprehensive review explores the intricate landscape of lung cancer research, delving into recent breakthroughs and their implications for diagnosis, therapy, and prevention. Genomic profiling and biomarker identification have ushered in the era of personalised medicine ...
Worldwide in 2012 lung cancer resulted in 1.6 million. deaths. Risk factors include smoking, exposure to radon gas, asbestos, second-hand smoke, air pollution, and. geneticfactors. Pathogenesis is ...
The NCI-sponsored National Lung Screening Trial (NLST) showed that low-dose CT scans can be used to screen for lung cancer in people with a history of heavy smoking. Using this screening can decrease their risk of dying from lung cancer. Now researchers are looking for ways to refine CT screening to better predict whether cancer is present.
New Cases. More than 228,000 people will be diagnosed with lung cancer this year, with the rate of new cases varying by state. The report finds that Utah has the nation's best lung cancer rate while Kentucky has the worst at almost 2.5 times the incidence rate of Utah. Over the last five years, the rate of new cases decreased 9% nationally.
Lung cancer is one of the most frequently diagnosed cancers and the second most common cause of death worldwide. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers. Genetic alterations can drive cancer, therefore, genetic testing using next-generation sequencing (NGS) to identify targeted mutations in lung ...
Screening lowers the risk of death from lung cancer. Ongoing studies are looking at new ways to improve early detection of lung cancer: Ways to use molecular markers from your body fluid (i.e., sputum or blood) for lung cancer screening. Ways to use new forms of bronchoscopies for lung cancer screening, such as autofluorescence bronchoscopy.
The American Lung Association is committed to funding lung cancer research. As part of our Awards and Grants Program, a large part of funds goes toward research on lung cancer prevention, treatment and quality of life. The primary goal of this lung cancer research program is simple: improve and save lives.
Lung cancer. Alesha A Thai, Benjamin J Solomon, Lecia V Sequist, Justin F Gainor, Rebecca S Heist. Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer-related deaths worldwide with an estimated 2 million new cases and 1·76 million deaths per year. Substantial improvements in our understanding of disease ...
Lung cancer remains a leading cause of cancer-related deaths globally, with non-small cell lung cancer (NSCLC) being the most common subtype. This study aimed to identify key biomarkers associated with stage III NSCLC in non-smoking females using gene expression profiling from the GDS3837 dataset. Utilizing XGBoost, a machine learning algorithm, the analysis achieved a strong predictive ...
Up to 42% of incident cases and 47% of deaths by cancer in the USA are due to potentially modifiable risk factors such as use of tobacco, physical activity, diet, and infection. 84 It has been calculated that 2.4 million deaths by cancer, mostly of the lung, can be attributed to tobacco. 73 In 2020, the incidence rate of lung cancer in Western ...
On average, each work received 1119 citations. A total of 1056 co-authors were involved in publishing these papers in 59 journals from 185 institutions in 27 countries. The majority of high-caliber research in the subject of lung cancer epigenetics is conducted in advanced countries, with the United States taking the lead in terms of both the ...
Focusing on EGFR-driven lung cancer, which is more common in never-smokers or light smokers, we found a significant association between PM2.5 levels and the incidence of lung cancer for 32,957 ...
Abstract. Summary This report reviews some of the more recent epidemiologic and experimental findings on the relationship of tobacco smoking to lung cancer, and discusses some criticisms directed against the conclusion that tobacco smoking, especially cigarettes, has a causal role in the increase in broncho-genic carcinoma. The magnitude of the excess lung-cancer risk among cigarette smokers ...
The findings of this study revealed key areas for focus in smoking and lung cancer research, having a view of supplying important data and motivation for further investigations. INTRODUCTION Lung cancer is a significant cause of mortality, especially among smokers. Lung cancer and smoking are strongly associated, according to numerous studies. METHODS Publications related to smoking and lung ...
Lung cancer is a common malignant neoplasm that often causes considerable psychological distress to patients and their families [].Due to the increasing public awareness of health screening in recent years and the use and promotion of low-dose computed tomography (CT) screening for early screening and detection of lung cancer, the incidence of early-stage lung cancer has been increasing [].
1 Introduction. Lung cancer (LC) is the leading cause of cancer-related deaths worldwide, with poor 5-year survival rates of 4-17%. [1, 2] Approximately 85% of patients with LC are diagnosed with non-small cell lung cancer, comprising lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC). []Among the major therapeutic modalities, radiotherapy is crucial in LC treatment, with ≈ ...
The BNT116 lung cancer vaccine trial follows the launch of a vaccine trial for melanoma at the NIHR UCLH Clinical Research Faculty in April this year—another groundbreaking development. Both trials are part of the UK Government's broader efforts to support medical research and accelerate the delivery of clinical trials, reinforcing the UK's status as a globally competitive player in vaccine ...
The research team suggests that clinical guidelines should be updated to highlight the potential for conditions like COPD to mask early symptoms of lung cancer. By raising awareness of this issue ...