Balanced Diet Essay for Students and Children
500 words essay on balanced diet.
We have grown up listening to the term ‘balanced diet’ in science. It refers to a diet that has all the essential nutrients and minerals that will keep us healthy. Having a balanced diet has been encouraged by our childhood. After all, it is important in keeping our health well.

A person intakes appropriate amounts of proteins, minerals, and nutrients in a balanced diet. It is quite necessary for the smooth functioning of our body. If we consume a balanced diet regularly, we will always remain healthy. It lessens any chances of falling ill. Moreover, a balanced diet also boosts our immunity system.

Importance of a Balanced Diet
Most people believe that a balanced diet is definitely the key to a healthy lifestyle. It is rightly believed as even scientists say so. When we always consume a balanced diet, we will maintain our physical as well as mental health. A balanced diet must contain the proper foods that are consumed in apt quantities. A perfect balanced diet is composed of carbohydrates, proteins, fats, minerals, high fiber content, vitamins, and more.
Get the huge list of more than 500 Essay Topics and Ideas
Moreover, nowadays the trend of junk food is here to stay. People are not taking a balanced diet rather eating all sorts of harmful foods. It is more important than ever to tell people about the importance of a balanced diet. You cannot merely exercise and expect your body to stay fit. A balanced diet is crucial for that.
Most importantly, it is called a ‘balanced’ diet because it requires all the foods to be eaten in a balanced manner. For instance, if you intake large amounts of carbohydrates and a little amount of protein, then that will not be called a balanced diet, even if you are eating the right foods. The balance needs to be maintained for that.
How to Have a Balanced Diet?
One can always adopt a healthy lifestyle by starting to consume a balanced diet. Firstly, one must definitely increase the amount of liquid to consume in a day. Fluids are very important for the human body to function healthily. As almost 80% of our body is filled with water, we need it for good metabolism. Thus, start with drinking at least two to three liters of water every day. Moreover, try cutting down on the consumption of tea, coffee, alcohol, and other such addictive liquids.
Furthermore, one must always eat fresh vegetables and fruits. As fresh fruits and vegetables are great sources of fiber and vitamins, we must consume them for good body growth. Try to avoid eating deep-fried or overcooked food as it loses all its nutrients. The balanced diet must have the five essential elements, i.e. bitter, sour, sweet, pungent and salty. Also, the emphasis is on fresh fruits because the processed or packed ones do not have nutrients.
Most importantly, always chew your food patiently. Do not just swallow it after chewing for four-five times. This way your food won’t get digested properly. Savor the food slowly and steadily. Next, do not eat in excess. You must know when to draw the line and stop when you don’t have the appetite. Therefore, we see how a balanced diet will keep you healthy and fit. It will improve the quality of your life and keep all the illnesses away.
FAQs on Balanced Diet Essay
Q.1 Why is a balanced diet important?
A.1 Balanced diet is important because it keeps us fit and fine. It also prevents any illnesses or diseases.
Q.2 How can we have a balanced diet?
A.2 One can have a balanced diet by having a good amount of water. Furthermore, one must always consume fresh foods and chew slowly for proper digestion.
Customize your course in 30 seconds
Which class are you in.

- Travelling Essay
- Picnic Essay
- Our Country Essay
- My Parents Essay
- Essay on Favourite Personality
- Essay on Memorable Day of My Life
- Essay on Knowledge is Power
- Essay on Gurpurab
- Essay on My Favourite Season
- Essay on Types of Sports
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App

- CBSE Class 10th
- CBSE Class 12th
- UP Board 10th
- UP Board 12th
- Bihar Board 10th
- Bihar Board 12th
- Top Schools in India
- Top Schools in Delhi
- Top Schools in Mumbai
- Top Schools in Chennai
- Top Schools in Hyderabad
- Top Schools in Kolkata
- Top Schools in Pune
- Top Schools in Bangalore
Products & Resources
- JEE Main Knockout April
- Free Sample Papers
- Free Ebooks
- NCERT Notes
- NCERT Syllabus
- NCERT Books
- RD Sharma Solutions
- Navodaya Vidyalaya Admission 2024-25
- NCERT Solutions
- NCERT Solutions for Class 12
- NCERT Solutions for Class 11
- NCERT solutions for Class 10
- NCERT solutions for Class 9
- NCERT solutions for Class 8
- NCERT Solutions for Class 7
- JEE Main 2024
- JEE Advanced 2024
- BITSAT 2024
- View All Engineering Exams
- Colleges Accepting B.Tech Applications
- Top Engineering Colleges in India
- Engineering Colleges in India
- Engineering Colleges in Tamil Nadu
- Engineering Colleges Accepting JEE Main
- Top IITs in India
- Top NITs in India
- Top IIITs in India
- JEE Main College Predictor
- JEE Main Rank Predictor
- MHT CET College Predictor
- AP EAMCET College Predictor
- GATE College Predictor
- KCET College Predictor
- JEE Advanced College Predictor
- View All College Predictors
- JEE Main Question Paper
- JEE Main Mock Test
- JEE Main Registration
- JEE Main Syllabus
- Download E-Books and Sample Papers
- Compare Colleges
- B.Tech College Applications
- GATE 2024 Result
- MAH MBA CET Exam
- View All Management Exams
Colleges & Courses
- MBA College Admissions
- MBA Colleges in India
- Top IIMs Colleges in India
- Top Online MBA Colleges in India
- MBA Colleges Accepting XAT Score
- BBA Colleges in India
- XAT College Predictor 2024
- SNAP College Predictor
- NMAT College Predictor
- MAT College Predictor 2024
- CMAT College Predictor 2024
- CAT Percentile Predictor 2023
- CAT 2023 College Predictor
- CMAT 2024 Registration
- TS ICET 2024 Registration
- CMAT Exam Date 2024
- MAH MBA CET Cutoff 2024
- Download Helpful Ebooks
- List of Popular Branches
- QnA - Get answers to your doubts
- IIM Fees Structure
- AIIMS Nursing
- Top Medical Colleges in India
- Top Medical Colleges in India accepting NEET Score
- Medical Colleges accepting NEET
- List of Medical Colleges in India
- List of AIIMS Colleges In India
- Medical Colleges in Maharashtra
- Medical Colleges in India Accepting NEET PG
- NEET College Predictor
- NEET PG College Predictor
- NEET MDS College Predictor
- DNB CET College Predictor
- DNB PDCET College Predictor
- NEET Application Form 2024
- NEET PG Application Form 2024
- NEET Cut off
- NEET Online Preparation
- Download Helpful E-books
- LSAT India 2024
- Colleges Accepting Admissions
- Top Law Colleges in India
- Law College Accepting CLAT Score
- List of Law Colleges in India
- Top Law Colleges in Delhi
- Top Law Collages in Indore
- Top Law Colleges in Chandigarh
- Top Law Collages in Lucknow
Predictors & E-Books
- CLAT College Predictor
- MHCET Law ( 5 Year L.L.B) College Predictor
- AILET College Predictor
- Sample Papers
- Compare Law Collages
- Careers360 Youtube Channel
- CLAT Syllabus 2025
- CLAT Previous Year Question Paper
- AIBE 18 Result 2023
- NID DAT Exam
- Pearl Academy Exam
Animation Courses
- Animation Courses in India
- Animation Courses in Bangalore
- Animation Courses in Mumbai
- Animation Courses in Pune
- Animation Courses in Chennai
- Animation Courses in Hyderabad
- Design Colleges in India
- Fashion Design Colleges in Bangalore
- Fashion Design Colleges in Mumbai
- Fashion Design Colleges in Pune
- Fashion Design Colleges in Delhi
- Fashion Design Colleges in Hyderabad
- Fashion Design Colleges in India
- Top Design Colleges in India
- Free Design E-books
- List of Branches
- Careers360 Youtube channel
- NIFT College Predictor
- UCEED College Predictor
- NID DAT College Predictor
- IPU CET BJMC
- JMI Mass Communication Entrance Exam
- IIMC Entrance Exam
- Media & Journalism colleges in Delhi
- Media & Journalism colleges in Bangalore
- Media & Journalism colleges in Mumbai
- List of Media & Journalism Colleges in India
- CA Intermediate
- CA Foundation
- CS Executive
- CS Professional
- Difference between CA and CS
- Difference between CA and CMA
- CA Full form
- CMA Full form
- CS Full form
- CA Salary In India
Top Courses & Careers
- Bachelor of Commerce (B.Com)
- Master of Commerce (M.Com)
- Company Secretary
- Cost Accountant
- Charted Accountant
- Credit Manager
- Financial Advisor
- Top Commerce Colleges in India
- Top Government Commerce Colleges in India
- Top Private Commerce Colleges in India
- Top M.Com Colleges in Mumbai
- Top B.Com Colleges in India
- IT Colleges in Tamil Nadu
- IT Colleges in Uttar Pradesh
- MCA Colleges in India
- BCA Colleges in India
Quick Links
- Information Technology Courses
- Programming Courses
- Web Development Courses
- Data Analytics Courses
- Big Data Analytics Courses
- RUHS Pharmacy Admission Test
- Top Pharmacy Colleges in India
- Pharmacy Colleges in Pune
- Pharmacy Colleges in Mumbai
- Colleges Accepting GPAT Score
- Pharmacy Colleges in Lucknow
- List of Pharmacy Colleges in Nagpur
- GPAT Result
- GPAT 2024 Admit Card
- GPAT Question Papers
- NCHMCT JEE 2024
- Mah BHMCT CET
- Top Hotel Management Colleges in Delhi
- Top Hotel Management Colleges in Hyderabad
- Top Hotel Management Colleges in Mumbai
- Top Hotel Management Colleges in Tamil Nadu
- Top Hotel Management Colleges in Maharashtra
- B.Sc Hotel Management
- Hotel Management
- Diploma in Hotel Management and Catering Technology
Diploma Colleges
- Top Diploma Colleges in Maharashtra
- UPSC IAS 2024
- SSC CGL 2024
- IBPS RRB 2024
- Previous Year Sample Papers
- Free Competition E-books
- Sarkari Result
- QnA- Get your doubts answered
- UPSC Previous Year Sample Papers
- CTET Previous Year Sample Papers
- SBI Clerk Previous Year Sample Papers
- NDA Previous Year Sample Papers
Upcoming Events
- NDA Application Form 2024
- UPSC IAS Application Form 2024
- CDS Application Form 2024
- CTET Admit card 2024
- HP TET Result 2023
- SSC GD Constable Admit Card 2024
- UPTET Notification 2024
- SBI Clerk Result 2024
Other Exams
- SSC CHSL 2024
- UP PCS 2024
- UGC NET 2024
- RRB NTPC 2024
- IBPS PO 2024
- IBPS Clerk 2024
- IBPS SO 2024
- Top University in USA
- Top University in Canada
- Top University in Ireland
- Top Universities in UK
- Top Universities in Australia
- Best MBA Colleges in Abroad
- Business Management Studies Colleges
Top Countries
- Study in USA
- Study in UK
- Study in Canada
- Study in Australia
- Study in Ireland
- Study in Germany
- Study in China
- Study in Europe
Student Visas
- Student Visa Canada
- Student Visa UK
- Student Visa USA
- Student Visa Australia
- Student Visa Germany
- Student Visa New Zealand
- Student Visa Ireland
- CUET PG 2024
- IGNOU B.Ed Admission 2024
- DU Admission
- UP B.Ed JEE 2024
- DDU Entrance Exam
- IIT JAM 2024
- IGNOU Online Admission 2024
- Universities in India
- Top Universities in India 2024
- Top Colleges in India
- Top Universities in Uttar Pradesh 2024
- Top Universities in Bihar
- Top Universities in Madhya Pradesh 2024
- Top Universities in Tamil Nadu 2024
- Central Universities in India
- CUET PG Admit Card 2024
- IGNOU Date Sheet
- CUET Mock Test 2024
- CUET Application Form 2024
- CUET PG Syllabus 2024
- CUET Participating Universities 2024
- CUET Previous Year Question Paper
- CUET Syllabus 2024 for Science Students
- E-Books and Sample Papers
- CUET Exam Pattern 2024
- CUET Exam Date 2024
- CUET Syllabus 2024
- IGNOU Exam Form 2024
- IGNOU Result
- CUET PG Courses 2024
Engineering Preparation
- Knockout JEE Main 2024
- Test Series JEE Main 2024
- JEE Main 2024 Rank Booster
Medical Preparation
- Knockout NEET 2024
- Test Series NEET 2024
- Rank Booster NEET 2024
Online Courses
- JEE Main One Month Course
- NEET One Month Course
- IBSAT Free Mock Tests
- IIT JEE Foundation Course
- Knockout BITSAT 2024
- Career Guidance Tool
Top Streams
- IT & Software Certification Courses
- Engineering and Architecture Certification Courses
- Programming And Development Certification Courses
- Business and Management Certification Courses
- Marketing Certification Courses
- Health and Fitness Certification Courses
- Design Certification Courses
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
- UG Degree Courses
- PG Degree Courses
- Short Term Courses
- Free Courses
- Online Degrees and Diplomas
- Compare Courses
Top Providers
- Coursera Courses
- Udemy Courses
- Edx Courses
- Swayam Courses
- upGrad Courses
- Simplilearn Courses
- Great Learning Courses
Access premium articles, webinars, resources to make the best decisions for career, course, exams, scholarships, study abroad and much more with
Plan, Prepare & Make the Best Career Choices
Importance Of A Balanced Diet Essay - 100, 200, 500 Words
- Essay on The Importance of A Balanced Diet -
A balanced diet is a diet that includes a variety of different types of foods and provides the body with the nutrients it needs to function properly. It includes foods from all the different food groups in the right amounts to suit our bodies. This typically includes fruits, vegetables, whole grains, lean proteins, and healthy fats . Here are a few sample essays on Importance Of A Balanced Diet.
100 Words Essay on The Importance of A Balanced Diet
200 words essay on the importance of a balanced diet, 500 words essay on the importance of a balanced diet.

A balanced diet is essential for good health and well-being. It can help you maintain a healthy weight and get the nutrients you need to support your body's functions. A balanced diet comprises a variety of foods from all the different food groups in the right amounts. Th is includes fruits and vegetables, proteins, carbohydrates, and healthy fats. A balanced diet helps to reduce your risk of chronic diseases, such as heart disease, diabetes, and cancer . In addition, eating a balanced diet can support your mental health and give you the energy you need to stay active and engaged in your daily life.
A balanced diet is a vital ingredient for good health and well-being . It includes a variety of foods from all the different food groups in the right amounts. This means eating a mix of fruits and vegetables, proteins, carbohydrates, and healthy fats, getting enough fibre and staying hydrated by drinking enough water.
Eating a balanced diet can help you live a healthy lifestyle and support your mental health by giving you the energy you need to stay active and engaged in your daily life. It reduces the risk of diseases in a person's body. Furthermore, a balanced diet can also support your immune system and help you fight off illness and infection. By getting the right nutrients, your body can maintain a strong and healthy immune system, which can help you stay healthy and avoid getting sick.
Overall, the importance of a balanced diet cannot be overstated. It is essential for good health and well-being and can support your body's functions, reduce your risk of chronic diseases, and support your immune system. By eating a variety of healthy foods, you can ensure that your body gets all the nutrients it needs to stay strong and healthy, which improves your way of living and even your life span.
Good health and well-being depends heavily on a balanced diet. In this type of diet, you will find that there are a variety of foods from all the different food groups, consumed in the right amounts from all of them. This means eating a mix of fruits and vegetables, proteins, carbohydrates, and healthy fats . It also means getting enough fibre and staying hydrated by drinking enough water.
The benefits of eating a balanced diet are numerous . It is one of the best ways to keep a healthy weight. Keeping your calorie intake under control and avoiding excess weight can be accomplished by eating the right types and amounts of food. It is important to maintain a healthy weight because being overweight or obese increases your risk of chronic diseases, such as heart disease, diabetes, and cancer.
A balanced diet not only helps you maintain a healthy weight, but it also ensures that your body gets the nutrients it needs to function properly. Different nutrients have different roles in the body, and it is important to eat a variety of nutrients to support your overall health. For example, carbohydrates provide energy, while protein helps build and repair tissues . Vitamins and minerals are also crucial for maintaining healthy bodily functions.
Eating a balanced diet can also support your mental health. Certain foods, such as fruits and vegetables, are rich in vitamins and minerals that can support brain function and improve mood . In addition, eating a balanced diet can provide your body with the energy it needs to stay active and engaged in your daily life. This can help you feel more productive and energised and can improve your overall quality of life.
My Uncle’s Experience
In January 2020, my uncle started to consult a nutritionist for a balanced diet plan in order to lose some weight as he was getting obese and started to accumulate a number of diseases. The nutritionist gave him a diet plan, which was designed in such a way that it only had good fats, high protein, the right amounts of carbohydrates and proper meals at all times. The plan only had foods that could be prepared at home and were not expensive. After following the diet plan for about a month, and regularly exercising, he lost about 12 kg, which was highly surprising for everyone. He was living a healthy life, with good habits, and great body health. It came to me as a shock how changes in our daily food routine and making our diet a healthy and balanced diet, can bring up so many benefits in one’s life.
A balanced diet is highly necessary for modern-day life . It is crucial for good health and well-being and can help you maintain a healthy weight, provide your body with the nutrients it needs, support your mental health, and support your immune system. By eating a variety of healthy foods, you can ensure that your body gets all the nutrients it needs to stay strong and healthy.
Explore Career Options (By Industry)
- Construction
- Entertainment
- Manufacturing
- Information Technology
Bio Medical Engineer
The field of biomedical engineering opens up a universe of expert chances. An Individual in the biomedical engineering career path work in the field of engineering as well as medicine, in order to find out solutions to common problems of the two fields. The biomedical engineering job opportunities are to collaborate with doctors and researchers to develop medical systems, equipment, or devices that can solve clinical problems. Here we will be discussing jobs after biomedical engineering, how to get a job in biomedical engineering, biomedical engineering scope, and salary.
Data Administrator
Database professionals use software to store and organise data such as financial information, and customer shipping records. Individuals who opt for a career as data administrators ensure that data is available for users and secured from unauthorised sales. DB administrators may work in various types of industries. It may involve computer systems design, service firms, insurance companies, banks and hospitals.
Ethical Hacker
A career as ethical hacker involves various challenges and provides lucrative opportunities in the digital era where every giant business and startup owns its cyberspace on the world wide web. Individuals in the ethical hacker career path try to find the vulnerabilities in the cyber system to get its authority. If he or she succeeds in it then he or she gets its illegal authority. Individuals in the ethical hacker career path then steal information or delete the file that could affect the business, functioning, or services of the organization.
Data Analyst
The invention of the database has given fresh breath to the people involved in the data analytics career path. Analysis refers to splitting up a whole into its individual components for individual analysis. Data analysis is a method through which raw data are processed and transformed into information that would be beneficial for user strategic thinking.
Data are collected and examined to respond to questions, evaluate hypotheses or contradict theories. It is a tool for analyzing, transforming, modeling, and arranging data with useful knowledge, to assist in decision-making and methods, encompassing various strategies, and is used in different fields of business, research, and social science.
Geothermal Engineer
Individuals who opt for a career as geothermal engineers are the professionals involved in the processing of geothermal energy. The responsibilities of geothermal engineers may vary depending on the workplace location. Those who work in fields design facilities to process and distribute geothermal energy. They oversee the functioning of machinery used in the field.
Remote Sensing Technician
Individuals who opt for a career as a remote sensing technician possess unique personalities. Remote sensing analysts seem to be rational human beings, they are strong, independent, persistent, sincere, realistic and resourceful. Some of them are analytical as well, which means they are intelligent, introspective and inquisitive.
Remote sensing scientists use remote sensing technology to support scientists in fields such as community planning, flight planning or the management of natural resources. Analysing data collected from aircraft, satellites or ground-based platforms using statistical analysis software, image analysis software or Geographic Information Systems (GIS) is a significant part of their work. Do you want to learn how to become remote sensing technician? There's no need to be concerned; we've devised a simple remote sensing technician career path for you. Scroll through the pages and read.
Geotechnical engineer
The role of geotechnical engineer starts with reviewing the projects needed to define the required material properties. The work responsibilities are followed by a site investigation of rock, soil, fault distribution and bedrock properties on and below an area of interest. The investigation is aimed to improve the ground engineering design and determine their engineering properties that include how they will interact with, on or in a proposed construction.
The role of geotechnical engineer in mining includes designing and determining the type of foundations, earthworks, and or pavement subgrades required for the intended man-made structures to be made. Geotechnical engineering jobs are involved in earthen and concrete dam construction projects, working under a range of normal and extreme loading conditions.
Cartographer
How fascinating it is to represent the whole world on just a piece of paper or a sphere. With the help of maps, we are able to represent the real world on a much smaller scale. Individuals who opt for a career as a cartographer are those who make maps. But, cartography is not just limited to maps, it is about a mixture of art , science , and technology. As a cartographer, not only you will create maps but use various geodetic surveys and remote sensing systems to measure, analyse, and create different maps for political, cultural or educational purposes.
Budget Analyst
Budget analysis, in a nutshell, entails thoroughly analyzing the details of a financial budget. The budget analysis aims to better understand and manage revenue. Budget analysts assist in the achievement of financial targets, the preservation of profitability, and the pursuit of long-term growth for a business. Budget analysts generally have a bachelor's degree in accounting, finance, economics, or a closely related field. Knowledge of Financial Management is of prime importance in this career.
Product Manager
A Product Manager is a professional responsible for product planning and marketing. He or she manages the product throughout the Product Life Cycle, gathering and prioritising the product. A product manager job description includes defining the product vision and working closely with team members of other departments to deliver winning products.
Underwriter
An underwriter is a person who assesses and evaluates the risk of insurance in his or her field like mortgage, loan, health policy, investment, and so on and so forth. The underwriter career path does involve risks as analysing the risks means finding out if there is a way for the insurance underwriter jobs to recover the money from its clients. If the risk turns out to be too much for the company then in the future it is an underwriter who will be held accountable for it. Therefore, one must carry out his or her job with a lot of attention and diligence.
Finance Executive
Operations manager.
Individuals in the operations manager jobs are responsible for ensuring the efficiency of each department to acquire its optimal goal. They plan the use of resources and distribution of materials. The operations manager's job description includes managing budgets, negotiating contracts, and performing administrative tasks.
Bank Probationary Officer (PO)
Investment director.
An investment director is a person who helps corporations and individuals manage their finances. They can help them develop a strategy to achieve their goals, including paying off debts and investing in the future. In addition, he or she can help individuals make informed decisions.
Welding Engineer
Welding Engineer Job Description: A Welding Engineer work involves managing welding projects and supervising welding teams. He or she is responsible for reviewing welding procedures, processes and documentation. A career as Welding Engineer involves conducting failure analyses and causes on welding issues.
Transportation Planner
A career as Transportation Planner requires technical application of science and technology in engineering, particularly the concepts, equipment and technologies involved in the production of products and services. In fields like land use, infrastructure review, ecological standards and street design, he or she considers issues of health, environment and performance. A Transportation Planner assigns resources for implementing and designing programmes. He or she is responsible for assessing needs, preparing plans and forecasts and compliance with regulations.
An expert in plumbing is aware of building regulations and safety standards and works to make sure these standards are upheld. Testing pipes for leakage using air pressure and other gauges, and also the ability to construct new pipe systems by cutting, fitting, measuring and threading pipes are some of the other more involved aspects of plumbing. Individuals in the plumber career path are self-employed or work for a small business employing less than ten people, though some might find working for larger entities or the government more desirable.
Construction Manager
Individuals who opt for a career as construction managers have a senior-level management role offered in construction firms. Responsibilities in the construction management career path are assigning tasks to workers, inspecting their work, and coordinating with other professionals including architects, subcontractors, and building services engineers.
Urban Planner
Urban Planning careers revolve around the idea of developing a plan to use the land optimally, without affecting the environment. Urban planning jobs are offered to those candidates who are skilled in making the right use of land to distribute the growing population, to create various communities.
Urban planning careers come with the opportunity to make changes to the existing cities and towns. They identify various community needs and make short and long-term plans accordingly.
Highway Engineer
Highway Engineer Job Description: A Highway Engineer is a civil engineer who specialises in planning and building thousands of miles of roads that support connectivity and allow transportation across the country. He or she ensures that traffic management schemes are effectively planned concerning economic sustainability and successful implementation.
Environmental Engineer
Individuals who opt for a career as an environmental engineer are construction professionals who utilise the skills and knowledge of biology, soil science, chemistry and the concept of engineering to design and develop projects that serve as solutions to various environmental problems.
Naval Architect
A Naval Architect is a professional who designs, produces and repairs safe and sea-worthy surfaces or underwater structures. A Naval Architect stays involved in creating and designing ships, ferries, submarines and yachts with implementation of various principles such as gravity, ideal hull form, buoyancy and stability.
Orthotist and Prosthetist
Orthotists and Prosthetists are professionals who provide aid to patients with disabilities. They fix them to artificial limbs (prosthetics) and help them to regain stability. There are times when people lose their limbs in an accident. In some other occasions, they are born without a limb or orthopaedic impairment. Orthotists and prosthetists play a crucial role in their lives with fixing them to assistive devices and provide mobility.
Veterinary Doctor
Pathologist.
A career in pathology in India is filled with several responsibilities as it is a medical branch and affects human lives. The demand for pathologists has been increasing over the past few years as people are getting more aware of different diseases. Not only that, but an increase in population and lifestyle changes have also contributed to the increase in a pathologist’s demand. The pathology careers provide an extremely huge number of opportunities and if you want to be a part of the medical field you can consider being a pathologist. If you want to know more about a career in pathology in India then continue reading this article.
Speech Therapist
Gynaecologist.
Gynaecology can be defined as the study of the female body. The job outlook for gynaecology is excellent since there is evergreen demand for one because of their responsibility of dealing with not only women’s health but also fertility and pregnancy issues. Although most women prefer to have a women obstetrician gynaecologist as their doctor, men also explore a career as a gynaecologist and there are ample amounts of male doctors in the field who are gynaecologists and aid women during delivery and childbirth.
An oncologist is a specialised doctor responsible for providing medical care to patients diagnosed with cancer. He or she uses several therapies to control the cancer and its effect on the human body such as chemotherapy, immunotherapy, radiation therapy and biopsy. An oncologist designs a treatment plan based on a pathology report after diagnosing the type of cancer and where it is spreading inside the body.
Audiologist
The audiologist career involves audiology professionals who are responsible to treat hearing loss and proactively preventing the relevant damage. Individuals who opt for a career as an audiologist use various testing strategies with the aim to determine if someone has a normal sensitivity to sounds or not. After the identification of hearing loss, a hearing doctor is required to determine which sections of the hearing are affected, to what extent they are affected, and where the wound causing the hearing loss is found. As soon as the hearing loss is identified, the patients are provided with recommendations for interventions and rehabilitation such as hearing aids, cochlear implants, and appropriate medical referrals. While audiology is a branch of science that studies and researches hearing, balance, and related disorders.
Hospital Administrator
The hospital Administrator is in charge of organising and supervising the daily operations of medical services and facilities. This organising includes managing of organisation’s staff and its members in service, budgets, service reports, departmental reporting and taking reminders of patient care and services.
For an individual who opts for a career as an actor, the primary responsibility is to completely speak to the character he or she is playing and to persuade the crowd that the character is genuine by connecting with them and bringing them into the story. This applies to significant roles and littler parts, as all roles join to make an effective creation. Here in this article, we will discuss how to become an actor in India, actor exams, actor salary in India, and actor jobs.
Individuals who opt for a career as acrobats create and direct original routines for themselves, in addition to developing interpretations of existing routines. The work of circus acrobats can be seen in a variety of performance settings, including circus, reality shows, sports events like the Olympics, movies and commercials. Individuals who opt for a career as acrobats must be prepared to face rejections and intermittent periods of work. The creativity of acrobats may extend to other aspects of the performance. For example, acrobats in the circus may work with gym trainers, celebrities or collaborate with other professionals to enhance such performance elements as costume and or maybe at the teaching end of the career.
Video Game Designer
Career as a video game designer is filled with excitement as well as responsibilities. A video game designer is someone who is involved in the process of creating a game from day one. He or she is responsible for fulfilling duties like designing the character of the game, the several levels involved, plot, art and similar other elements. Individuals who opt for a career as a video game designer may also write the codes for the game using different programming languages.
Depending on the video game designer job description and experience they may also have to lead a team and do the early testing of the game in order to suggest changes and find loopholes.
Radio Jockey
Radio Jockey is an exciting, promising career and a great challenge for music lovers. If you are really interested in a career as radio jockey, then it is very important for an RJ to have an automatic, fun, and friendly personality. If you want to get a job done in this field, a strong command of the language and a good voice are always good things. Apart from this, in order to be a good radio jockey, you will also listen to good radio jockeys so that you can understand their style and later make your own by practicing.
A career as radio jockey has a lot to offer to deserving candidates. If you want to know more about a career as radio jockey, and how to become a radio jockey then continue reading the article.
Choreographer
The word “choreography" actually comes from Greek words that mean “dance writing." Individuals who opt for a career as a choreographer create and direct original dances, in addition to developing interpretations of existing dances. A Choreographer dances and utilises his or her creativity in other aspects of dance performance. For example, he or she may work with the music director to select music or collaborate with other famous choreographers to enhance such performance elements as lighting, costume and set design.
Videographer
Multimedia specialist.
A multimedia specialist is a media professional who creates, audio, videos, graphic image files, computer animations for multimedia applications. He or she is responsible for planning, producing, and maintaining websites and applications.
Social Media Manager
A career as social media manager involves implementing the company’s or brand’s marketing plan across all social media channels. Social media managers help in building or improving a brand’s or a company’s website traffic, build brand awareness, create and implement marketing and brand strategy. Social media managers are key to important social communication as well.
Copy Writer
In a career as a copywriter, one has to consult with the client and understand the brief well. A career as a copywriter has a lot to offer to deserving candidates. Several new mediums of advertising are opening therefore making it a lucrative career choice. Students can pursue various copywriter courses such as Journalism , Advertising , Marketing Management . Here, we have discussed how to become a freelance copywriter, copywriter career path, how to become a copywriter in India, and copywriting career outlook.
Careers in journalism are filled with excitement as well as responsibilities. One cannot afford to miss out on the details. As it is the small details that provide insights into a story. Depending on those insights a journalist goes about writing a news article. A journalism career can be stressful at times but if you are someone who is passionate about it then it is the right choice for you. If you want to know more about the media field and journalist career then continue reading this article.
For publishing books, newspapers, magazines and digital material, editorial and commercial strategies are set by publishers. Individuals in publishing career paths make choices about the markets their businesses will reach and the type of content that their audience will be served. Individuals in book publisher careers collaborate with editorial staff, designers, authors, and freelance contributors who develop and manage the creation of content.
In a career as a vlogger, one generally works for himself or herself. However, once an individual has gained viewership there are several brands and companies that approach them for paid collaboration. It is one of those fields where an individual can earn well while following his or her passion.
Ever since internet costs got reduced the viewership for these types of content has increased on a large scale. Therefore, a career as a vlogger has a lot to offer. If you want to know more about the Vlogger eligibility, roles and responsibilities then continue reading the article.
Individuals in the editor career path is an unsung hero of the news industry who polishes the language of the news stories provided by stringers, reporters, copywriters and content writers and also news agencies. Individuals who opt for a career as an editor make it more persuasive, concise and clear for readers. In this article, we will discuss the details of the editor's career path such as how to become an editor in India, editor salary in India and editor skills and qualities.
Linguistic meaning is related to language or Linguistics which is the study of languages. A career as a linguistic meaning, a profession that is based on the scientific study of language, and it's a very broad field with many specialities. Famous linguists work in academia, researching and teaching different areas of language, such as phonetics (sounds), syntax (word order) and semantics (meaning).
Other researchers focus on specialities like computational linguistics, which seeks to better match human and computer language capacities, or applied linguistics, which is concerned with improving language education. Still, others work as language experts for the government, advertising companies, dictionary publishers and various other private enterprises. Some might work from home as freelance linguists. Philologist, phonologist, and dialectician are some of Linguist synonym. Linguists can study French , German , Italian .
Public Relation Executive
Travel journalist.
The career of a travel journalist is full of passion, excitement and responsibility. Journalism as a career could be challenging at times, but if you're someone who has been genuinely enthusiastic about all this, then it is the best decision for you. Travel journalism jobs are all about insightful, artfully written, informative narratives designed to cover the travel industry. Travel Journalist is someone who explores, gathers and presents information as a news article.
Quality Controller
A quality controller plays a crucial role in an organisation. He or she is responsible for performing quality checks on manufactured products. He or she identifies the defects in a product and rejects the product.
A quality controller records detailed information about products with defects and sends it to the supervisor or plant manager to take necessary actions to improve the production process.
Production Manager
Merchandiser.
A QA Lead is in charge of the QA Team. The role of QA Lead comes with the responsibility of assessing services and products in order to determine that he or she meets the quality standards. He or she develops, implements and manages test plans.
Metallurgical Engineer
A metallurgical engineer is a professional who studies and produces materials that bring power to our world. He or she extracts metals from ores and rocks and transforms them into alloys, high-purity metals and other materials used in developing infrastructure, transportation and healthcare equipment.
Azure Administrator
An Azure Administrator is a professional responsible for implementing, monitoring, and maintaining Azure Solutions. He or she manages cloud infrastructure service instances and various cloud servers as well as sets up public and private cloud systems.
AWS Solution Architect
An AWS Solution Architect is someone who specializes in developing and implementing cloud computing systems. He or she has a good understanding of the various aspects of cloud computing and can confidently deploy and manage their systems. He or she troubleshoots the issues and evaluates the risk from the third party.
Computer Programmer
Careers in computer programming primarily refer to the systematic act of writing code and moreover include wider computer science areas. The word 'programmer' or 'coder' has entered into practice with the growing number of newly self-taught tech enthusiasts. Computer programming careers involve the use of designs created by software developers and engineers and transforming them into commands that can be implemented by computers. These commands result in regular usage of social media sites, word-processing applications and browsers.
ITSM Manager
Information security manager.
Individuals in the information security manager career path involves in overseeing and controlling all aspects of computer security. The IT security manager job description includes planning and carrying out security measures to protect the business data and information from corruption, theft, unauthorised access, and deliberate attack
Business Intelligence Developer
Applications for admissions are open..

JEE Main Important Chemistry formulas
As per latest 2024 syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters

Aakash iACST Scholarship Test 2024
Get up to 90% scholarship on NEET, JEE & Foundation courses

Resonance Coaching
Enroll in Resonance Coaching for success in JEE/NEET exams

TOEFL ® Registrations 2024
Thinking of Studying Abroad? Think the TOEFL® test. Register now & Save 10% on English Proficiency Tests with Gift Cards

ALLEN JEE Exam Prep
Start your JEE preparation with ALLEN

NEET 2024 Most scoring concepts
Just Study 32% of the NEET syllabus and Score upto 100% marks
Everything about Education
Latest updates, Exclusive Content, Webinars and more.
Download Careers360 App's
Regular exam updates, QnA, Predictors, College Applications & E-books now on your Mobile
Cetifications
We Appeared in

Essay on Balanced Diet
Students are often asked to write an essay on Balanced Diet in their schools and colleges. And if you’re also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic.
Let’s take a look…
100 Words Essay on Balanced Diet
The importance of a balanced diet.
A balanced diet is crucial for good health. It refers to eating a variety of foods in the right proportions. This helps us get all the nutrients our body needs.
Components of a Balanced Diet
A balanced diet includes proteins, carbohydrates, fats, vitamins, and minerals. Proteins build muscles, carbohydrates provide energy, fats store energy, and vitamins and minerals keep us healthy.
Benefits of a Balanced Diet
Eating a balanced diet helps us grow, stay active, and fight diseases. It also helps us maintain a healthy weight.
Remember, a balanced diet is key to a healthy life!
Also check:
- 10 Lines on Balanced Diet
- Paragraph on Balanced Diet
- Speech on Balanced Diet
250 Words Essay on Balanced Diet
Introduction.
A balanced diet is not just a meal plan, but a lifestyle choice that promotes optimal health and well-being. It is a diet that includes an appropriate proportion of nutrients such as proteins, vitamins, minerals, carbohydrates, and fats, derived from a variety of food sources.
The human body requires a diverse range of nutrients for its complex functions and processes. A balanced diet supplies these nutrients in the right quantities, thereby ensuring the body’s physiological functions are maintained. It boosts the immune system, supports growth and development, and reduces the risk of chronic diseases such as obesity, diabetes, and cardiovascular diseases.
A balanced diet comprises of five main food groups: fruits and vegetables, proteins, dairy, grains, and fats. Fruits and vegetables provide essential vitamins, minerals, and fiber. Proteins, found in meat, fish, and legumes, are vital for growth and repair. Dairy products supply calcium for strong bones and teeth. Grains, particularly whole grains, are a great source of energy and provide fiber, iron, and B-vitamins. Fats, though often vilified, are necessary in moderate amounts for energy and absorption of fat-soluble vitamins.
In conclusion, a balanced diet is a cornerstone of good health. It requires mindful choices and variety in food consumption. It’s not about strict limitations, but rather about improving overall health, feeling good, having more energy, and boosting mood. It is an investment in one’s health and well-being, and its benefits extend beyond the individual to society at large.
500 Words Essay on Balanced Diet
Introduction to balanced diet.
A balanced diet is a cornerstone of health. It is the key to our body’s proper functioning and well-being. It refers to the intake of appropriate types and adequate amounts of foods and drinks to supply nutrition and energy for the maintenance of body cells, tissues, and organs, and to support normal growth and development.
The significance of a balanced diet cannot be overstated. It provides the necessary nutrients required for the body to function effectively. Nutrients such as proteins, carbohydrates, fats, vitamins, and minerals are critical for various body functions. For instance, proteins are essential for growth and repair, carbohydrates provide energy, fats act as energy reserves, and vitamins and minerals are crucial for various metabolic processes.
A balanced diet also plays a pivotal role in maintaining optimal body weight, enabling overall physical well-being and boosting mental health. It can prevent various chronic diseases such as obesity, heart disease, diabetes, and cancer.
A balanced diet consists of a variety of foods from different food groups. These include:
1. Fruits and Vegetables: They are rich in vitamins, minerals, and fiber. They boost immunity and help in digestion.
2. Proteins: Sources include meat, fish, eggs, dairy, and plant-based proteins like legumes and nuts. They are vital for growth and repair.
3. Carbohydrates: Foods like bread, rice, and potatoes provide energy.
4. Fats: While excessive fat can lead to obesity, a certain amount is necessary for insulation, protection, and energy production. Sources include oils, butter, and fatty fish.
5. Dairy: Milk and dairy products are an excellent source of calcium for bone health.
The Concept of Moderation and Variety
In the context of a balanced diet, the principles of moderation and variety are crucial. Moderation refers to controlling portion sizes and not overindulging in particular foods to prevent excess calorie intake and associated health issues. Variety, on the other hand, pertains to consuming a wide array of foods across and within each food group. This ensures the intake of a broad spectrum of different nutrients.
In conclusion, a balanced diet is integral to good health and well-being. It provides the body with essential nutrients required for optimal functioning. Remember, it’s not about short-term dietary changes but rather about a long-term lifestyle modification. Eating a variety of foods, in the right proportions, and consuming the right amount of food and drink to achieve and maintain a healthy body weight are the key components of a balanced diet. A balanced diet, coupled with regular exercise, can lead to a better quality of life and contribute to a longer and healthier future.
That’s it! I hope the essay helped you.
If you’re looking for more, here are essays on other interesting topics:
- Essay on Health and Fitness
- Essay on Solar Panel
- Essay on Plastic Waste Management
Apart from these, you can look at all the essays by clicking here .
Happy studying!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
A Healthy Lifestyle and a Well Balanced Diet Essay
A healthy lifestyle and a well-balanced diet are both essential for the well-being of modern society. Nevertheless, obesity, malnutrition, and eating disorders are becoming increasingly alarming problems in today’s world. Moreover, an unhealthy diet can accompany and exacerbate conditions such as stress, depression, and overwork. By applying for the Dietetic Internship at Florida International University, I am looking forward to integrating my knowledge of balanced nutrition into counseling people to gain healthier eating habits.
Coming from Middle Eastern origins, gatherings mostly revolved around food. Whenever there was a celebration food had to be present. Although my dad’s side of the family suffered from obesity and diabetes, they did not have the tools to understand how nutrition could improve regarding chronic diseases. Seeing some of my family members pass away from these diseases made me want to provide a better and well-rounded understanding of nutrition. Facilitating people is a goal that I would like to achieve while educating people that do not have enough knowledge or any background in nutrition. Having food is a necessity but in some countries, food and water are not available. I aim to achieve availability and accessibility concerning nutrition education in third-world countries.
Throughout my life, I have been interested in a healthy lifestyle. I have often shared what I learned with my cousins and other family members. The information that I knew was inaccessible to those without such education, and it made me think about how much society influences what is right and wrong to eat. With my own family as an example, I can assert that both extreme low-calorie diets and overeating do not improve a person’s mental and physical condition. Currently, my primary goal is to help people get better in their nutritional patterns and come into harmony with themselves, letting them know that food is not bad to eat, but that it fuels a healthier life. Therefore, my strongest motivation and aim to become a registered dietitian is to educate people about the incredible opportunities for proper nutrition and a healthy lifestyle.
I believe that a dietitian can genuinely make a difference in changing the situation with a growing number of diagnosed overweight and improper eating habits for the better. My personal and academic experience demonstrate enthusiasm in this internship. My initial goal was to translate personal interests into a professional skill, which I hope to achieve in my career. Over the past academic years, I have participated in various nutrition-related activities to increase and expand my knowledge and different areas of the topic such as community nutrition and clinical nutrition. Attending courses and workshops on dietetics deepened my knowledge and understanding of a professional dietician’s role in society. In addition, I actively performed group and individual assignments and demonstrated my motivation and knowledge of the topics. My high inspiration and the ability to work individually and in a team is a traits that can be well-implemented in this sphere of work. While my work allows me to self-motivate and manage, I can also adapt to the leadership or work styles of my colleagues.
Organizing and playing a leading role in many extracurricular events and meetings dedicated to poor eating habits and lack of nutritional knowledge in society, in both academic and clinical settings, have allowed me to achieve good results by expanding my knowledge and doing extra research. I have developed methods and behaviors that assist in my management of responsibilities when it comes to nutrition-related activities. In my working experience with patients and athletes, I have demonstrated advertency and empathy to be my main advantages for this position. Since people with eating disorders often require compassion, additional attention, and involvement in the treatment process, I believe mindfulness and a caring attitude are vital in dealing with patients. Additionally, I find that continued work and interaction with a patient is essential even after initial treatment, to observe favorable results.
Although academic skills are valuable to me, I believe that practical applications of knowledge are an integral part of the training. I also realize that to be entirely successful in my profession, I critically need to gain more practical experience in a clinical setting. I have strengths in leadership, individual work, and technical knowledge, but I understand that I need further improvement in my practical experience, teamwork, and contentious learning of dietetics. By applying for FIU Dietetic Internship, I am looking forward to gaining new expertise, employing it in practice, and developing communication skills for further advancement in my chosen profession. As a future registered dietitian, I hope to serve people and promote the most appropriate health and lifestyle choices. I believe that it is vital to recommend diet and nutrition methods, depending on individual preferences, requirements, and health indications. As I move forward in my profession, I will develop individualized approaches to patient health and be considerate and respectful concerning their potential conditions. I hope to use this internship to perform according to professional instructions, receive and implement criticism, gain experience, and continue working with others in the dietetics field. In this regard, FIU Dietetic Internship is also an excellent opportunity to develop my professional goals.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2022, November 2). A Healthy Lifestyle and a Well Balanced Diet. https://ivypanda.com/essays/a-healthy-lifestyle-and-a-well-balanced-diet/
"A Healthy Lifestyle and a Well Balanced Diet." IvyPanda , 2 Nov. 2022, ivypanda.com/essays/a-healthy-lifestyle-and-a-well-balanced-diet/.
IvyPanda . (2022) 'A Healthy Lifestyle and a Well Balanced Diet'. 2 November.
IvyPanda . 2022. "A Healthy Lifestyle and a Well Balanced Diet." November 2, 2022. https://ivypanda.com/essays/a-healthy-lifestyle-and-a-well-balanced-diet/.
1. IvyPanda . "A Healthy Lifestyle and a Well Balanced Diet." November 2, 2022. https://ivypanda.com/essays/a-healthy-lifestyle-and-a-well-balanced-diet/.
Bibliography
IvyPanda . "A Healthy Lifestyle and a Well Balanced Diet." November 2, 2022. https://ivypanda.com/essays/a-healthy-lifestyle-and-a-well-balanced-diet/.
- Childhood Obesity in Context of Dietetics
- Dietetics Care Plan: Gluten-Free Diet
- The FIU Modesto Maidique Campus: Transportation System
- Healthcare Institution Nutrition Strategy and Management
- International Digital Dental Academy Meeting of 2015
- Florida International University and Its Aspects
- Nutrition Instructor's Teaching Philosophy
- Addressing Nutrition-Related Problems in Miami-Dade, Zip Code 33193
- Importance of Health Diet for Modern Person
- The Fast Food Danger Awareness Among the Young People
- Consequences of Pandemic COVID-19: The Psychological Climate in the Family
- The Public Health Intervention Wheel
- How to Establish a Habit of Doing Exercise
- A Biographical Sketch Michael Charles Jackson
- Playing Videogames vs Crocheting and Blogging
Why a balanced diet is important for your health
Wondering why a balanced diet is important? These nutritionists weigh in

What is a balanced diet?
Why is eating a balanced diet so important, tips for having a balanced diet everyday.
You may be wondering why a balanced diet is important. The simple answer is eating a healthy, balanced diet is a vital part of maintaining good health and helping you to feel your best. While some groups of people, such as athletes, may require additional support by way of the best protein powders and the best protein bars to fuel muscle growth, the majority of us can get everything we need by ensuring we’re eating a healthy and varied range of foods.
A balanced diet supplies the fuel your body needs to work effectively. Without balanced nutrition, your body is more prone to illnesses such as heart disease, diabetes, and cancer. Eating a variety of foods and consuming less salt, sugars, and saturated fats are essential to ensure your body functions at its best.
If your diet isn’t balanced and you consume foods that don’t provide enough nutritional value, your nutrient levels will start to decline. Research has found that 31% of the U.S. population is at risk of at least one vitamin deficiency. There are many dangers of a nutrient deficiency, including digestion problems, anemia, and skin problems.
In this article, you will learn about what you need for a balanced diet, why a balanced diet is so important, and tips for ensuring you meet your nutritional needs every day.
“A balanced diet ideally includes five food groups,” Isabel Maples , registered dietitian, and spokesperson for the Academy of Nutrition and Dietetics told Live Science. “The individual food groups each supply certain groups of nutrients. One group is not more important than the other - each provides key vitamins, minerals, fiber, and calories. But when one food group is eaten less, then it becomes the weakest link in maintaining balance. More focus on it might help bring the diet back into balance.”
The Dietary Guidelines for Americans, set out by the U.S Department of Agriculture (USDA) recommend nutrient-dense foods that provide vitamins, minerals, and other health-promoting components and have no or little added sugars, saturated fat, and sodium.

The core elements that make up a healthy dietary pattern include:
- Vegetables of all types - dark green; red and orange; beans, peas, and lentils; starchy; and other vegetables.
- Fruits, especially whole fruit (rather than fruit juices).
- Grains, at least half of which are whole grain.
- Dairy, including fat-free or low-fat milk, yogurt, and cheese, and/or lactose-free versions and fortified soy beverages and yogurt as alternatives.
- Protein foods, including lean meats, poultry, and eggs; seafood; beans, peas, and lentils; and nuts, seeds, and soy products.
- Oils, including vegetable oils and oils in food, such as seafood and nuts.
Nutritionist Lamorna Hollingsworth says variety is key when it comes to eating fruit and vegetables. “Go for at least five portions of fruit and vegetables a day,” she says. “Fresh, frozen, canned, and dried all count. Eating a diverse range of plant-based foods is great news for our gut health and microbiome which thrives best when we consume a wide variety - aiming for 30+ different plant-based foods a week is a great target.”
The dietary guidelines also advise limiting foods and beverages higher in added sugars, saturated fat, and sodium and limiting alcoholic beverages. The recommended limits are:
- Added sugars: Less than 10% of your calories per day.
- Saturated fat: Less than 10% of your calories per day.
- Sodium: Less than 2,300 milligrams per day (and even less for children younger than age 14).
- Alcoholic beverages: Adults of legal drinking age can choose not to drink, or to drink in moderation by limiting intake to two drinks or less in a day for men and one drink or less in a day for women when alcohol is consumed. Drinking less is better for health than drinking more.
The foods we eat have a profound impact on physical and mental health. The scientific connection between food and health is well documented, with substantial evidence showing that following a healthy diet can help people achieve and maintain good health and reduce the risk of chronic diseases.
A balanced diet supplies the nutrients your body needs to work well. Without balanced nutrition, your body is more prone to disease, infection, and fatigue.
According to the Center for Science in the Public Interest , four of the top 10 leading causes of death in the United States - heart disease, cancer, stroke, and type 2 diabetes - are directly linked to diet.

Some evidence suggests a close relationship between diet and mood. In 2016, research published in the journal Appetite found that diets with a high glycemic load may trigger increased symptoms of depression and fatigue. Foods with a high glycemic load include many refined carbohydrates, often found in soft drinks, cakes, white bread, and biscuits. Vegetables, whole fruit, and whole grains have a lower glycemic load.
A healthy diet may help maintain brain health too. A 2015 study published in the journal of Neurology, Psychiatry and Brain Research identified nutrients and foods that protect against cognitive decline and dementia. The researchers found the following beneficial - vitamin D, vitamin C, and vitamin E, omega-3 fatty acids, and fish.
A healthy diet will combine all the recommended nutrients and food groups mentioned, but you need to balance them too.
The plate method is a handy way to remember how much of each food group to eat. Maples endorses the USDA’s ' ChooseMyPlate ' initiative, which recommends:
- Filling half your plate with fruits and vegetables.
- Filling just over one quarter with grains.
- Filling just under one quarter with protein foods.
- Adding dairy on the side (or a non-dairy replacement).
But individual needs will vary, so the USDA also provides an interactive tool, ' MyPlate Plan ', where you can enter your own details to determine your personal needs.
Hollingsworth believes that proper balance comes when you view food on a spectrum, as labeling foods ‘good’ or ‘bad’ may lead to unhealthy restrictive habits. She told LiveScience: “It could be argued that a balanced diet that includes healthy and occasional not-so-healthy foods is more important than aiming for perfection with all our food choices.
“Taking this approach allows individuals to fuel their bodies with healthy options but also provides a positive place for our mental health too. Having this kind of mindset prevents guilt that could be felt upon eating the occasional unhealthy food.”
Bird, J., Murphy, R., Ciappio, E., & McBurney, M. (2017). Risk of Deficiency in Multiple Concurrent Micronutrients in Children and Adults in the United States. Nutrients, 9(7), 655. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5537775/
Breymeyer, K. L., Lampe, J. W., McGregor, B. A., & Neuhouser, M. L. (2016). Subjective mood and energy levels of healthy weight and overweight/obese healthy adults on high-and low-glycemic load experimental diets. Appetite, 107, 253–259. https://www.sciencedirect.com/science/article/abs/pii/S0195666316303221
Strasser, B., & Fuchs, D. (2015). Role of physical activity and diet on mood, behavior, and cognition. Neurology, Psychiatry and Brain Research, 21(3), 118–126. http://www.barbara-strasser.at/wp-content/uploads/Neurology-Psychiatry-and-Brain-Research-2015.pdf
U.S. Department of Agriculture. (2020). Dietary Guidelines for Americans 2020 - 2025. https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf
Why Good Nutrition is Important. (2018, May 17). Center for Science in the Public Interest. Retrieved April 14, 2022, from https://www.cspinet.org/eating-healthy/why-good-nutrition-important
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.

Catherine is a freelance journalist writing across titles such as Verywell Health, Healthline, The Daily Telegraph, Refinery29, Elle, and Vogue. She specializes in content covering health, fitness, wellness, and culture. Catherine worked in healthcare administration and communications for a decade, producing easy-to-understand patient information for a wide variety of health conditions.
8-hour intermittent fasting tied to 90% higher risk of cardiovascular death, early data hint
PFAS 'forever chemicals' to officially be removed from food packaging, FDA says
Where does the solar system end?
Most Popular
By Anna Gora December 27, 2023
By Anna Gora December 26, 2023
By Anna Gora December 25, 2023
By Emily Cooke December 23, 2023
By Victoria Atkinson December 22, 2023
By Anna Gora December 16, 2023
By Anna Gora December 15, 2023
By Anna Gora November 09, 2023
By Donavyn Coffey November 06, 2023
By Anna Gora October 31, 2023
By Anna Gora October 26, 2023
- 2 MIT scientists have just figured out how to make the most popular AI image generators 30 times faster
- 3 James Webb telescope confirms there is something seriously wrong with our understanding of the universe
- 4 'You could almost see and smell their world': Remnants of 'Britain's Pompeii' reveal details of life in Bronze Age village
- 5 How to safely record the April 8 eclipse with your phone
- 2 9,000-year-old rock art discovered among dinosaur footprints in Brazil
- 3 The 7 most powerful supercomputers in the world right now
- 4 Single enormous object left 2 billion craters on Mars, scientists discover
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- News & Views
- Food and mood: how do...
Food and mood: how do diet and nutrition affect mental wellbeing?
Read our food for thought 2020 collection.
- Related content
- Peer review
This article has a correction. Please see:
- Food and mood: how do diet and nutrition affect mental wellbeing? - November 09, 2020
- Joseph Firth , research fellow 1 2 ,
- James E Gangwisch , assistant professor 3 4 ,
- Alessandra Borsini , researcher 5 ,
- Robyn E Wootton , researcher 6 7 8 ,
- Emeran A Mayer , professor 9 10
- 1 Division of Psychology and Mental Health, Faculty of Biology, Medicine and Health, Oxford Road, University of Manchester, Manchester M13 9PL, UK
- 2 NICM Health Research Institute, Western Sydney University, Westmead, Australia
- 3 Department of Psychiatry, Columbia University Vagelos College of Physicians and Surgeons, New York, USA
- 4 New York State Psychiatric Institute, New York, NY, USA
- 5 Section of Stress, Psychiatry and Immunology Laboratory, Institute of Psychiatry, Psychology and Neuroscience, Department of Psychological Medicine, King’s College London, London, UK
- 6 School of Psychological Science, University of Bristol, Bristol, UK
- 7 MRC Integrative Epidemiology Unit, Oakfield House, Bristol, UK
- 8 NIHR Biomedical Research Centre, University Hospitals Bristol NHS Foundation Trust and University of Bristol, Bristol, UK
- 9 G Oppenheimer Center for Neurobiology of Stress and Resilience, UCLA Vatche and Tamar Manoukian Division of Digestive Diseases, UCLA, Los Angeles, CA, USA
- 10 UCLA Microbiome Center, David Geffen School of Medicine, UCLA, Los Angeles, CA, USA
- Correspondence to: J Firth joseph.firth{at}manchester.ac.uk
Poor nutrition may be a causal factor in the experience of low mood, and improving diet may help to protect not only the physical health but also the mental health of the population, say Joseph Firth and colleagues
Key messages
Healthy eating patterns, such as the Mediterranean diet, are associated with better mental health than “unhealthy” eating patterns, such as the Western diet
The effects of certain foods or dietary patterns on glycaemia, immune activation, and the gut microbiome may play a role in the relationships between food and mood
More research is needed to understand the mechanisms that link food and mental wellbeing and determine how and when nutrition can be used to improve mental health
Depression and anxiety are the most common mental health conditions worldwide, making them a leading cause of disability. 1 Even beyond diagnosed conditions, subclinical symptoms of depression and anxiety affect the wellbeing and functioning of a large proportion of the population. 2 Therefore, new approaches to managing both clinically diagnosed and subclinical depression and anxiety are needed.
In recent years, the relationships between nutrition and mental health have gained considerable interest. Indeed, epidemiological research has observed that adherence to healthy or Mediterranean dietary patterns—high consumption of fruits, vegetables, nuts, and legumes; moderate consumption of poultry, eggs, and dairy products; and only occasional consumption of red meat—is associated with a reduced risk of depression. 3 However, the nature of these relations is complicated by the clear potential for reverse causality between diet and mental health ( fig 1 ). For example, alterations in food choices or preferences in response to our temporary psychological state—such as “comfort foods” in times of low mood, or changes in appetite from stress—are common human experiences. In addition, relationships between nutrition and longstanding mental illness are compounded by barriers to maintaining a healthy diet. These barriers disproportionality affect people with mental illness and include the financial and environmental determinants of health, and even the appetite inducing effects of psychiatric medications. 4

Hypothesised relationship between diet, physical health, and mental health. The dashed line is the focus of this article.
- Download figure
- Open in new tab
- Download powerpoint
While acknowledging the complex, multidirectional nature of the relationships between diet and mental health ( fig 1 ), in this article we focus on the ways in which certain foods and dietary patterns could affect mental health.
Mood and carbohydrates
Consumption of highly refined carbohydrates can increase the risk of obesity and diabetes. 5 Glycaemic index is a relative ranking of carbohydrate in foods according to the speed at which they are digested, absorbed, metabolised, and ultimately affect blood glucose and insulin levels. As well as the physical health risks, diets with a high glycaemic index and load (eg, diets containing high amounts of refined carbohydrates and sugars) may also have a detrimental effect on psychological wellbeing; data from longitudinal research show an association between progressively higher dietary glycaemic index and the incidence of depressive symptoms. 6 Clinical studies have also shown potential causal effects of refined carbohydrates on mood; experimental exposure to diets with a high glycaemic load in controlled settings increases depressive symptoms in healthy volunteers, with a moderately large effect. 7
Although mood itself can affect our food choices, plausible mechanisms exist by which high consumption of processed carbohydrates could increase the risk of depression and anxiety—for example, through repeated and rapid increases and decreases in blood glucose. Measures of glycaemic index and glycaemic load can be used to estimate glycaemia and insulin demand in healthy individuals after eating. 8 Thus, high dietary glycaemic load, and the resultant compensatory responses, could lower plasma glucose to concentrations that trigger the secretion of autonomic counter-regulatory hormones such as cortisol, adrenaline, growth hormone, and glucagon. 5 9 The potential effects of this response on mood have been examined in experimental human research of stepped reductions in plasma glucose concentrations conducted under laboratory conditions through glucose perfusion. These findings showed that such counter-regulatory hormones may cause changes in anxiety, irritability, and hunger. 10 In addition, observational research has found that recurrent hypoglycaemia (low blood sugar) is associated with mood disorders. 9
The hypothesis that repeated and rapid increases and decreases in blood glucose explain how consumption of refined carbohydrate could affect psychological state appears to be a good fit given the relatively fast effect of diets with a high glycaemic index or load on depressive symptoms observed in human studies. 7 However, other processes may explain the observed relationships. For instance, diets with a high glycaemic index are a risk factor for diabetes, 5 which is often a comorbid condition with depression. 4 11 While the main models of disease pathophysiology in diabetes and mental illness are separate, common abnormalities in insulin resistance, brain volume, and neurocognitive performance in both conditions support the hypothesis that these conditions have overlapping pathophysiology. 12 Furthermore, the inflammatory response to foods with a high glycaemic index 13 raises the possibility that diets with a high glycaemic index are associated with symptoms of depression through the broader connections between mental health and immune activation.
Diet, immune activation, and depression
Studies have found that sustained adherence to Mediterranean dietary patterns can reduce markers of inflammation in humans. 14 On the other hand, high calorie meals rich in saturated fat appear to stimulate immune activation. 13 15 Indeed, the inflammatory effects of a diet high in calories and saturated fat have been proposed as one mechanism through which the Western diet may have detrimental effects on brain health, including cognitive decline, hippocampal dysfunction, and damage to the blood-brain barrier. 15 Since various mental health conditions, including mood disorders, have been linked to heightened inflammation, 16 this mechanism also presents a pathway through which poor diet could increase the risk of depression. This hypothesis is supported by observational studies which have shown that people with depression score significantly higher on measures of “dietary inflammation,” 3 17 characterised by a greater consumption of foods that are associated with inflammation (eg, trans fats and refined carbohydrates) and lower intakes of nutritional foods, which are thought to have anti-inflammatory properties (eg, omega-3 fats). However, the causal roles of dietary inflammation in mental health have not yet been established.
Nonetheless, randomised controlled trials of anti-inflammatory agents (eg, cytokine inhibitors and non-steroidal anti-inflammatory drugs) have found that these agents can significantly reduce depressive symptoms. 18 Specific nutritional components (eg, polyphenols and polyunsaturated fats) and general dietary patterns (eg, consumption of a Mediterranean diet) may also have anti-inflammatory effects, 14 19 20 which raises the possibility that certain foods could relieve or prevent depressive symptoms associated with heightened inflammatory status. 21 A recent study provides preliminary support for this possibility. 20 The study shows that medications that stimulate inflammation typically induce depressive states in people treated, and that giving omega-3 fatty acids, which have anti-inflammatory properties, before the medication seems to prevent the onset of cytokine induced depression. 20
However, the complexity of the hypothesised three way relation between diet, inflammation, and depression is compounded by several important modifiers. For example, recent clinical research has observed that stressors experienced the previous day, or a personal history of major depressive disorders, may cancel out the beneficial effects of healthy food choices on inflammation and mood. 22 Furthermore, as heightened inflammation occurs in only some clinically depressed individuals, anti-inflammatory interventions may only benefit certain people characterised by an “inflammatory phenotype,” or those with comorbid inflammatory conditions. 18 Further interventional research is needed to establish if improvements in immune regulation, induced by diet, can reduce depressive symptoms in those affected by inflammatory conditions.
Brain, gut microbiome, and mood
A more recent explanation for the way in which our food may affect our mental wellbeing is the effect of dietary patterns on the gut microbiome—a broad term that refers to the trillions of microbial organisms, including bacteria, viruses, and archaea, living in the human gut. The gut microbiome interacts with the brain in bidirectional ways using neural, inflammatory, and hormonal signalling pathways. 23 The role of altered interactions between the brain and gut microbiome on mental health has been proposed on the basis of the following evidence: emotion-like behaviour in rodents changes with changes in the gut microbiome, 24 major depressive disorder in humans is associated with alterations of the gut microbiome, 25 and transfer of faecal gut microbiota from humans with depression into rodents appears to induce animal behaviours that are hypothesised to indicate depression-like states. 25 26 Such findings suggest a role of altered neuroactive microbial metabolites in depressive symptoms.
In addition to genetic factors and exposure to antibiotics, diet is a potentially modifiable determinant of the diversity, relative abundance, and functionality of the gut microbiome throughout life. For instance, the neurocognitive effects of the Western diet, and the possible mediating role of low grade systemic immune activation (as discussed above) may result from a compromised mucus layer with or without increased epithelial permeability. Such a decrease in the function of the gut barrier is sometimes referred to as a “leaky gut” and has been linked to an “unhealthy” gut microbiome resulting from a diet low in fibre and high in saturated fats, refined sugars, and artificial sweeteners. 15 23 27 Conversely, the consumption of a diet high in fibres, polyphenols, and unsaturated fatty acids (as found in a Mediterranean diet) can promote gut microbial taxa which can metabolise these food sources into anti-inflammatory metabolites, 15 28 such as short chain fatty acids, while lowering the production of secondary bile acids and p-cresol. Moreover, a recent study found that the ingestion of probiotics by healthy individuals, which theoretically target the gut microbiome, can alter the brain’s response to a task that requires emotional attention 29 and may even reduce symptoms of depression. 30 When viewed together, these studies provide promising evidence supporting a role of the gut microbiome in modulating processes that regulate emotion in the human brain. However, no causal relationship between specific microbes, or their metabolites, and complex human emotions has been established so far. Furthermore, whether changes to the gut microbiome induced by diet can affect depressive symptoms or clinical depressive disorders, and the time in which this could feasibly occur, remains to be shown.
Priorities and next steps
In moving forward within this active field of research, it is firstly important not to lose sight of the wood for the trees—that is, become too focused on the details and not pay attention to the bigger questions. Whereas discovering the anti-inflammatory properties of a single nutrient or uncovering the subtleties of interactions between the gut and the brain may shed new light on how food may influence mood, it is important not to neglect the existing knowledge on other ways diet may affect mental health. For example, the later consequences of a poor diet include obesity and diabetes, which have already been shown to be associated with poorer mental health. 11 31 32 33 A full discussion of the effect of these comorbidities is beyond the scope of our article (see fig 1 ), but it is important to acknowledge that developing public health initiatives that effectively tackle the established risk factors of physical and mental comorbidities is a priority for improving population health.
Further work is needed to improve our understanding of the complex pathways through which diet and nutrition can influence the brain. Such knowledge could lead to investigations of targeted, even personalised, interventions to improve mood, anxiety, or other symptoms through nutritional approaches. However, these possibilities are speculative at the moment, and more interventional research is needed to establish if, how, and when dietary interventions can be used to prevent mental illness or reduce symptoms in those living with such conditions. Of note, a recent large clinical trial found no significant benefits of a behavioural intervention promoting a Mediterranean diet for adults with subclinical depressive symptoms. 34 On the other hand, several recent smaller trials in individuals with current depression observed moderately large improvements from interventions based on the Mediterranean diet. 35 36 37 Such results, however, must be considered within the context of the effect of people’s expectations, particularly given that individuals’ beliefs about the quality of their food or diet may also have a marked effect on their sense of overall health and wellbeing. 38 Nonetheless, even aside from psychological effects, consideration of dietary factors within mental healthcare may help improve physical health outcomes, given the higher rates of cardiometabolic diseases observed in people with mental illness. 33
At the same time, it is important to be remember that the causes of mental illness are many and varied, and they will often present and persist independently of nutrition and diet. Thus, the increased understanding of potential connections between food and mental wellbeing should never be used to support automatic assumptions, or stigmatisation, about an individual’s dietary choices and their mental health. Indeed, such stigmatisation could be itself be a casual pathway to increasing the risk of poorer mental health. Nonetheless, a promising message for public health and clinical settings is emerging from the ongoing research. This message supports the idea that creating environments and developing measures that promote healthy, nutritious diets, while decreasing the consumption of highly processed and refined “junk” foods may provide benefits even beyond the well known effects on physical health, including improved psychological wellbeing.
Contributors and sources: JF has expertise in the interaction between physical and mental health, particularly the role of lifestyle and behavioural health factors in mental health promotion. JEG’s area of expertise is the study of the relationship between sleep duration, nutrition, psychiatric disorders, and cardiometabolic diseases. AB leads research investigating the molecular mechanisms underlying the effect of stress and inflammation on human hippocampal neurogenesis, and how nutritional components and their metabolites can prevent changes induced by those conditions. REW has expertise in genetic epidemiology approaches to examining casual relations between health behaviours and mental illness. EAM has expertise in brain and gut interactions and microbiome interactions. All authors contributed to, read, and approved the paper, and all the information was sourced from articles published in peer reviewed research journals. JF is the guarantor.
Competing interests: We have read and understood BMJ policy on declaration of interests and declare the following: JF is supported by a University of Manchester Presidential Fellowship and a UK Research and Innovation Future Leaders Fellowship and has received support from a NICM-Blackmores Institute Fellowship. JEG served on the medical advisory board on insomnia in the cardiovascular patient population for the drug company Eisai. AB has received research funding from Johnson & Johnson for research on depression and inflammation, the UK Medical Research Council, the European Commission Horizon 2020, the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust, and King’s College London. REW receives funding from the National Institute for Health Research Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol. EAM has served on the external advisory boards of Danone, Viome, Amare, Axial Biotherapeutics, Pendulum, Ubiome, Bloom Science, Mahana Therapeutics, and APC Microbiome Ireland, and he receives royalties from Harper & Collins for his book The Mind Gut Connection. He is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, and the US Department of Defense. The views expressed are those of the authors and not necessarily those of the organisations above.
Provenance and peer review: Commissioned; externally peer reviewed.
This article is part of series commissioned by The BMJ. Open access fees are paid by Swiss Re, which had no input into the commissioning or peer review of the articles. T he BMJ thanks the series advisers, Nita Forouhi, Dariush Mozaffarian, and Anna Lartey for valuable advice and guiding selection of topics in the series.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
- Friedrich MJ
- Johnson J ,
- Weissman MM ,
- Lassale C ,
- Baghdadli A ,
- Siddiqi N ,
- Koyanagi A ,
- Gangwisch JE ,
- Salari-Moghaddam A ,
- Larijani B ,
- Esmaillzadeh A
- de Jong V ,
- Atkinson F ,
- Brand-Miller JC
- Seaquist ER ,
- Anderson J ,
- American Diabetes Association ,
- Endocrine Society
- Towler DA ,
- Havlin CE ,
- McIntyre RS ,
- Nguyen HT ,
- O’Keefe JH ,
- Gheewala NM ,
- Kastorini C-M ,
- Milionis HJ ,
- Esposito K ,
- Giugliano D ,
- Goudevenos JA ,
- Panagiotakos DB
- Teasdale SB ,
- Köhler-Forsberg O ,
- N Lydholm C ,
- Hjorthøj C ,
- Nordentoft M ,
- Yahfoufi N ,
- Borsini A ,
- Horowitz MA ,
- Kiecolt-Glaser JK ,
- Fagundes CP ,
- Andridge R ,
- Osadchiy V ,
- Martin CR ,
- O’Brien C ,
- Sonnenburg ED ,
- Sonnenburg JL
- Rampelli S ,
- Jeffery IB ,
- Tillisch K ,
- Kilpatrick L ,
- Walsh RFL ,
- Wootton RE ,
- Millard LAC ,
- Jebeile H ,
- Garnett SP ,
- Paxton SJ ,
- Brouwer IA ,
- MooDFOOD Prevention Trial Investigators
- Francis HM ,
- Stevenson RJ ,
- Chambers JR ,
- Parletta N ,
- Zarnowiecki D ,
- Fischler C ,
- Sarubin A ,
- Wrzesniewski A
- Harrington D ,
Home — Essay Samples — Nursing & Health — Diet — Diet Paragraph
Diet Paragraph
- Categories: Diet
About this sample

Words: 777 |
Published: Mar 14, 2024
Words: 777 | Pages: 2 | 4 min read


Cite this Essay
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Verified writer
- Expert in: Nursing & Health

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
2 pages / 919 words
2 pages / 947 words
3 pages / 1497 words
1 pages / 371 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online
Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Related Essays on Diet
The Three Day Diet, also known as the Military Diet, is a popular fad diet that claims to help individuals lose up to 10 pounds in just three days. This diet is said to be a quick and effective way to shed unwanted pounds, but [...]
Our dietary choices play a pivotal role in our overall health and well-being. What we eat not only affects our physical health but can also impact our energy levels, mood, and long-term health outcomes. To gain insight into the [...]
Diet analysis is a crucial aspect of maintaining a healthy lifestyle. It involves the assessment of an individual's dietary intake and the subsequent evaluation of its impact on their overall health. In this essay, I will [...]
Ketogenic (keto) diets are nutrition plans which promote consumption of a 4:1 or similar ratio of dietary fat to combined proteins and carbohydrates. The primary motivation for following a ketogenic diet in the therapeutic sense [...]
What is a good diet? Is it good if it makes you lose weight? Perhaps it’s considered a great diet if you drop weight rapidly or does that harm us in ways we don’t even consider? These are some of the questions we will be asking [...]
Are you tired of struggling with your weight and feeling sluggish all the time? Have you tried countless diets and exercise routines without seeing the results you desire? It may be time to consider the benefits of sticking to a [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
A healthy, balanced diet
What is a healthy balanced diet.
The key to a healthy eating is having a balance of different foods and nutrients in your diet.
In the UK, the healthy eating model is called the Eatwell Guide .
There are a range of different types of eating (e.g. vegan and vegetarian) but the key principles of a healthy dietary pattern should run through all of these.
The six principles of healthy eating
Although there is no ‘one size fits all’ healthy diet, there are six principles we recommend.

At least 5 A DAY
Remember to include at least five portions of fruit and vegetables a day.

Include plenty of fibre-rich foods, especially wholegrains.

Include a range of protein foods, especially beans, peas & lentils.

Dairy and alternatives
Include some dairy foods or fortified alternatives.

Choose the right fats
Mainly unsaturated fats and oils.

Eat fewer foods and drinks that are high in fat, salt and sugars.
Key facts about healthy, balanced diets
- Fruit and vegetables are a key part of a healthy diet , and we should aim to get our 5 A DAY.
- Starchy foods like bread, potatoes, pasta, rice and other grains are part of a healthy diet , but the quality of what we choose is important.
- Plant-based protein foods , like beans and lentils, are naturally low in saturated fat and are sources of protein and fibre . We should aim to include these regularly in the diet.
- Animal protein foods like fish, eggs, meat and dairy provide a range of vitamins and minerals .
- Dairy foods provide protein, calcium, B vitamins and iodine .If choosing plant-based dairy alternatives, then look for those that are fortified with calcium and ideally other vitamins and minerals.

Why do I need a balanced diet?
Following a healthy, balanced diet helps make sure that our bodies get all the nutrients needed to work well and feel good. There’s plenty of evidence that shows eating healthily can also reduce the risk of diseases like heart disease, stroke, type 2 diabetes and some types of cancer.
Around the world, governments provide guidance on the balance of foods and drinks that make up a healthy diet. The guidelines vary in how they are presented but the message is similar; a healthy diet is about getting a balance and variety of foods from the main food groups.
What are the current healthy eating guidelines in the UK?
In the UK, our healthy eating model is called the Eatwell Guide. The guide has been developed scientifically, using modelling to look at the balance of different foods and drinks that provides the nutrients the body needs in the recommended amounts .
The Eatwell Guide shows the proportions that each of the food groups should make up in our diet and is designed to apply to most of us (although not to children under 2 years old as their dietary needs are different).
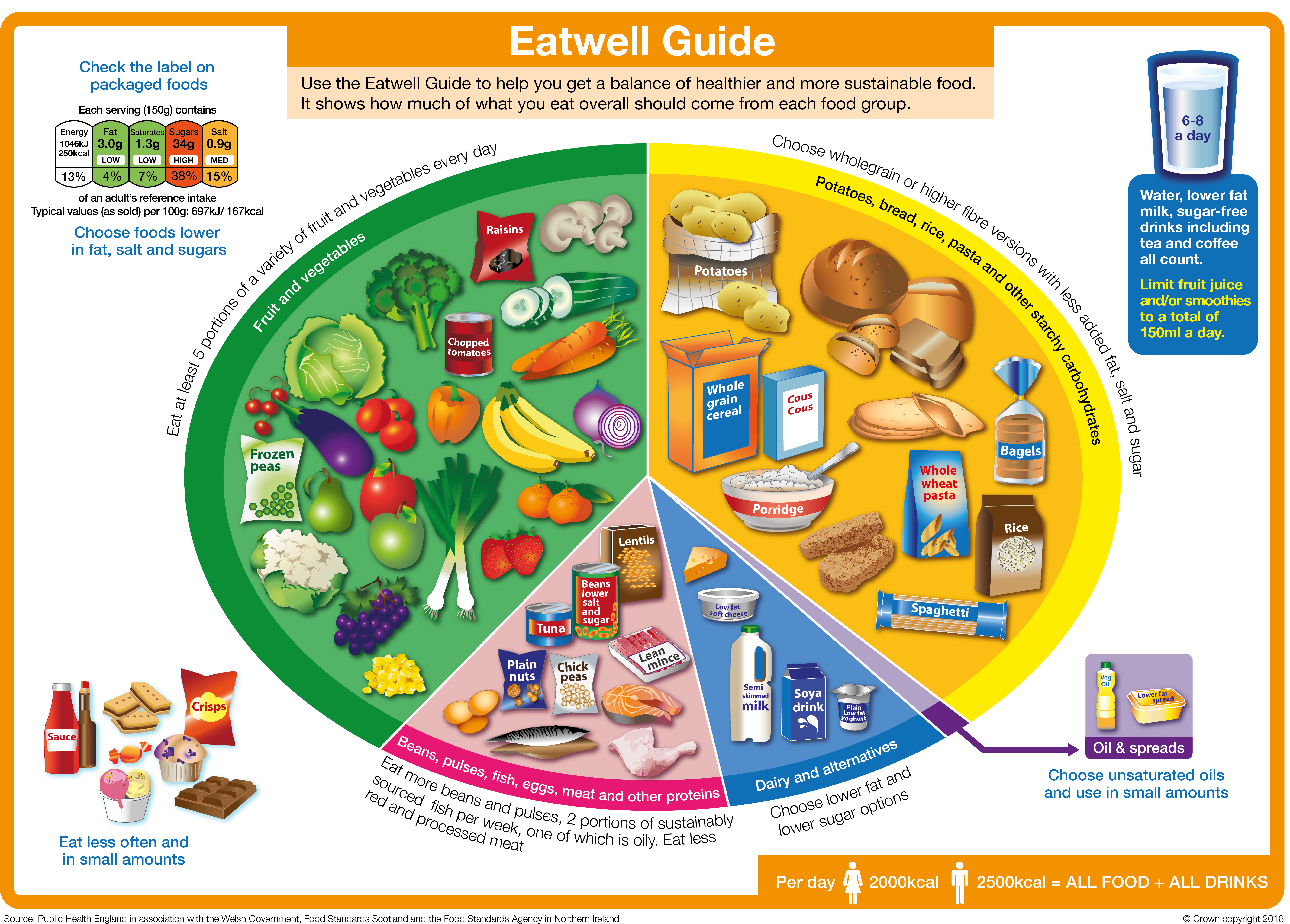
You can learn more about the Eatwell Guide on this page from the NHS .
The main food groups that feature in the Eatwell Guide, are outlined below. Each section has a useful guide to that food group.
- Fruit and vegetables
- Starchy foods
- Protein foods
- Dairy foods and alternatives
- Fats and oils
- Foods high in fat, salt and sugar
Fruit and vegetables - eat more!
The fruit and vegetables group is the biggest in the Eatwell Guide and we are recommended to eat at least 5 A DAY. Diets high in fruit and vegetables are linked to a lower risk of diseases like heart disease, stroke and some types of cancer.
Fruit and vegetables provide a range of essential nutrients and fibre, as well as chemical compounds that occur naturally in plants that may have health benefits. Fruit and vegetables can also help you maintain a healthy weight as they are generally low in calories, so you can have plenty for relatively few calories.
Only 1 in 3 adults and 1 in 10 11-18 year olds are getting their 5 A DAY
Helena Gibson-Moore, Nutrition Scientist, British Nutrition Foundation
To get the most nutritional benefit out of your 5 A DAY it’s important to have a variety of fruits and vegetables. This is because different types and colours of fruits and vegetables contain different combinations of important nutrients such as:
- Vitamin C - important for keeping body tissues, such as skin and cartilage healthy.
- Vitamin A - important for normal vision, skin and the immune system.
- Folate - important for making red blood cells and supporting the immune system
- Potassium – important for healthy blood pressure and to support the nervous system
- Fibre – helps to maintain a healthy gut and can reduce the risk of diseases like type 2 diabetes and heart disease.
Did you know? Fresh, frozen, dried and canned fruits and vegetables all count towards our 5 A DAY.
Table 1: 5 A DAY. What counts as a portion?
You can find out more about 5 A DAY portion sizes by reading this NHS page .
5 Top Tips For Eating More Fruit & Vegetables
- Add fresh or dried fruit to breakfast cereal or porridge
- Snack on fresh fruit or vegetable sticks
- Experiment with salads – you could try using red cabbage, adding brightly coloured vegetables such as grated carrot or sliced pepper and including leftover cooked vegetables like broccoli or peas in your salads.
- Add plenty of vegetables to dishes like pasta sauces, stews or curries – frozen or canned vegetables can be a quick and easy way to do this.
- Try fruit-based puddings like fruit salad or canned/dried fruits with plain yogurt
Starchy foods - go for wholegrain and higher fibre!
Also known as ‘carbs,’ starchy foods like bread, pasta, potatoes, rice and other grains are one of the main food groups included in healthy dietary guidelines all over the world.
These foods are sometimes (incorrectly) thought of as ‘fattening’ but what’s important is the types and portion sizes we eat
Sara Stanner, Science Director, British Nutrition Foundation
Starchy foods are a key source of fibre as well as vitamins and minerals such as iron, calcium, folate and B vitamins. For a healthier diet, we should choose more wholegrains and higher fibre starchy foods, such as wholemeal breads, wholemeal pasta, wholegrain breakfast cereals or oats and potatoes with skins.
Top tip! Try swapping white versions of bread, pasta or rice for wholegrain versions, go for wholegrains cereals or oats and try other types of wholegrains such as bulgur wheat, quinoa, freekeh, barley and spelt.
Looking for more information? Download: A guide to the food group 'Potatoes, bread, rice, pasta and other starchy carbohydrates'.
Protein foods - variety is key.
In the Eatwell Guide, this food group is called ‘Beans, pulses, fish, eggs, meat and other proteins'. This group of foods are a source of protein as well as other vitamins and minerals. It is a good idea to eat a variety of different types, and to include more plant-based sources of protein, such as beans, lentils or chickpeas, as these are higher in fibre and naturally low in fat.
Nuts and seeds (plain, unsalted) are included in this food group and contain vitamins, minerals and fibre. They are also high in fat but the majority of this is ‘healthier’ fat (unsaturated) and are a nutritious option in moderation (keeping portion sizes to just a small handful).
It’s recommended that we eat at least two portions (2 x 140g cooked weight) per week of sustainably sourced fish (fresh, frozen or canned), including a portion of oily fish. Oily fish includes salmon, sardines, mackerel and trout. Fish are sources of lots of vitamins and minerals. In particular, oily fish are natural sources of vitamin D and are the richest source of a special type of fat called long chain omega-3’s, which may help to prevent heart disease.
Meat can be part of a healthy diet and can be a source of several vitamins and minerals including iron, zinc and selenium. We are advised not to eat too much red or processed meat as high consumption has been linked with a higher risk of bowel cancer. You can cut down the fat content of meat by choosing leaner cuts such as lower fat mince, cutting off visible fat and taking the skin off poultry and using less fat when cooking, such as grilling instead of frying.
Looking for more information? Download: Beans, pulses, fish, eggs, meat and other proteins
Dairy foods and alternatives – go for lower sugar.
This food group includes milk, yogurt and cheese as well as plant-based alternatives to these. Dairy foods are an important source of calcium as well as protein, iodine and B vitamins. The nutritional content of dairy alternatives varies depending on what they are made from (such as soya, rice or oats) and whether they are fortified. If having dairy alternatives such as soya or oat milk, it’s best to choose those that are fortified with calcium and ideally other vitamins and minerals.
Dairy foods contain saturated fat, which we’re advised to eat less of (see below). Some studies suggest that despite their saturated fat content, dairy foods like milk, cheese and yogurt have a neutral effect on heart health. However, lower-fat versions of milk, cheese and plain yogurt are also lower in energy (calories) and so can be helpful if you are trying to manage your weight.
Fats and oils - choose unsaturated types!
There are different types of fats and oils in the diet – those that are mostly saturated such as butter, coconut oil, ghee, lard and palm oil, and those that are mostly unsaturated such as vegetable (usually rapeseed), sunflower and olive oils and spreads made from these. High intakes of saturated fat are linked to higher blood cholesterol and swapping saturated for unsaturated fats has been shown to reduce blood cholesterol and risk of heart disease. So it is a good idea to choose unsaturated fats and oils most of the time for cooking and spreading.
All fats are high in calories, even unsaturated fats, so it is important to use them in small amounts to avoid adding more calories than you need.
Looking for more information? Download: A guide to oils and spreads in the diet
Foods high in fat, salt and sugar – keep portions small.
Foods high in saturated fat, salt and sugar such as crisps, sweets, biscuits, cakes, chocolate and sugary drinks are not within the main food groups of the Eatwell Guide as they are not needed as part of a healthy diet. Sometimes called ‘treat foods’ we probably all know that these are foods to eat less of. If you do include them, then it is best to have small portions – for example, those that provide about 100-150kcal such as a small chocolate biscuit bar, 4 small squares of chocolate, 2 small biscuits, a small multipack bag of crisps, a mini muffin or a small chocolate mousse.
When it comes to sugary drinks it is best to swap these for water or sugar free versions.
What is the healthy eating guidance for different dietary patterns ?
The main food groups above are the building blocks of a healthy, balanced diet but they can be put together in different ways, based on our culture, preferences and dietary requirements. There are a whole range of different types of eating but the key principles of a healthy dietary pattern run through all of these
Applying these principles to your diet will help make sure it is balanced and healthy. There are a whole range of diets out there in books, in the press and on social media, some of which claim to have specific effects on health or to help with weight loss. It is not always easy to work out whether these diets are healthy – they may be promoted by doctors or mention scientific studies.
Diets that do not follow the healthy eating principles, for example those that cut out whole food groups, are probably going to be difficult to stick with and not likely to be good for your health in the longer term.
Zoe Hill, Nutrition Scientist, British Nutrition Foundation
The Mediterranean diet
The Mediterranean diet is often thought of as one of the healthiest eating patterns and features plenty of fruit, vegetables, pulses, wholegrains, olive oil, fish and smaller amounts of meat, dairy, eggs and sugary foods. A Mediterranean diet contains a higher proportion of fat than other healthy eating patterns, but most of this is unsaturated fats from olive oil, nuts and seeds and oily fish. This style of eating may reduce the risk of heart disease and have other potential health benefits. If this way of eating works for you then that’s great! However, it is not the only way to eat healthily, and may not work for everyone.
Vegetarian and vegan diets
Vegetarian and vegan diets have had a lot of interest and some research suggests that these diets may reduce the risk of heart disease. A healthy, balanced vegetarian or vegan diet will typically provide plenty of vegetables, pulses and wholegrains and so be rich in fibre and low in saturated fat.
Looking for more information? Read our page on vegetarian and vegan diets to find out more.
Plant-based diets.
The term ‘plant-based diet’ is increasingly popular but there is some confusion about what it means. Some people think this refers to a vegetarian or vegan diet, but many authoritative bodies agree that plant-based eating means proportionately choosing more of your foods from plant sources and so is a diet mainly made up of plant foods, but may still include some meat, fish, eggs and dairy foods. Most healthy eating guidelines, including the Eatwell Guide recommend a mainly plant-based diet. The two biggest food groups; fruit and vegetables and starchy foods, are both plant-based and we are also encouraged to eat more beans and pulses and to use plant-based oils and spreads. So you can make your diet more ‘plant-based’ by including a wider variety of fruits and vegetables, including wholegrains as well as choosing more plant-based sources of protein.
Looking for more information? Read our information on how to put a healthy diet into practice including planners, tips and information on portion sizes.
At a glance:.
- Research looking at the relationship between diet and health outcomes has shown a positive relationship between healthier dietary patterns and better health outcomes.
- To help people achieve a healthy balanced diet, governments around the world have developed food-based dietary guidance to illustrate what a healthy diet means in practice.
- Food-based dietary guidelines around the world vary in their presentation and format but the messages about the characteristics of healthy and balanced diets are relatively consistent.
- The UK Eatwell Guide was developed using optimisation modelling to find the proportions of the main food groups needed to fulfil current dietary recommendations.
- It has been estimated that following a dietary pattern consistent with the Eatwell Guide would benefit population health as well as being more environmentally sustainable.

What are dietary guidelines?
While nutrition recommendations often focus on individual nutrients or food components, food-based advice is important to help people put nutrition recommendations into practice.
Governments around the world have developed food-based dietary guidelines to communicate healthy eating recommendations in a nationally and culturally appropriate and practical way. The guidelines typically divide foods into food groups and provide advice on the proportion each of these groups should make up in the diet. Most guidelines also include a range of advice for people on how to put this into practice, including guidance on making healthier choices within the food groups (such as choosing wholegrain foods), how often to eat specific types of food (such as 5 A DAY), information on appropriate portion sizes as well tools such as planners or recipes.
While presentation of national food-based dietary guidance varies from country to country, the dietary pattern they present is broadly similar, with fruit and vegetables and starchy foods making up the largest groups, often with a particular focus on wholegrains. Dairy foods and protein food groups make up a smaller proportion, along with advice to limit foods high in fat, salt and sugar.
Redevelopment of UK food-based dietary guidance
UK food-based guidance has been through several iterations over the decades. Most recently, following changes to recommendations on fibre and free sugars in 2015, the government redeveloped the UK model the Eatwell Guide (then the Eatwell Plate) to take these changes into account.
Modelling was carried out by scientists at the University of Oxford using data on dietary intakes from the National Diet and Nutrition Survey (NDNS). Different scientific methods to recalculate the proportions of the food groups in the guide were considered and optimisation modelling using linear programming was selected. This used statistical techniques to model how current diets would need to change to meet current nutrition recommendations – both nutrient recommendations, such as those for fibre, and food-based recommendations, including 5 A DAY, were incorporated.
The results are summarised in Figure 1 below, showing that the proportion of foods from the fruit and vegetables and starchy foods groups would have to increase significantly and that the proportion of foods from all other groups would have to decrease. The food categories within the food groups did not always follow this pattern – in the ‘proteins’ food group, the modelling found that beans and pulses would have to increase by 90% and red meat would have to decrease by 78%.
Studies that have looked at the effect of following the Eatwell Guide compared to the average diet in the UK have found that it could significantly improve population health. One analysis carried out in 2016 when the Eatwell Guide was revised found that following the guidance (without increasing energy intake) could potentially avoid 17.8 million years of ill-health or early death (disability adjusted life years or DALYs) over the lifetime of the population.
Much of the benefit seen was due to prevention of type 2 diabetes as well as prevention of cancer and cardiovascular disease. Another analysis carried out in 2020, found that following at least five of the nine Eatwell Guide recommendations reduced mortality risk by approximately 7% as well as reducing carbon emissions by 30% compared to following 2 or fewer of the recommendations.
While there is increasing evidence for the benefits following healthier dietary patterns as set out in the Eatwell Guide and other models, we are a long way from making this a reality. As shown in the studies mentioned above, significant changes in eating habits are needed for current average UK diets to meet Eatwell Guide recommendations. It has been estimated that less than 1% of the population meet all current dietary guidelines and that only about 30% are meeting five or more of the nine specific recommendations in the Eatwell Guide.
Current UK diets are a long way from following the Eatwell Guide. Significant changes to diets are needed including increases in fruit and vegetable, fish and wholegrain consumption and reductions in foods high in saturated fat, salt and sugar
Anne de la Hunty, Senior Nutrition Scientist, British Nutrition Foundation
While trend data from the NDNS from 2008-2017 shows that there has been some progress in reducing intakes of free sugars and sugary drinks in recent years, intakes of free sugars, saturated fat and salt remain higher than recommended. There has been no appreciable increase in fruit and vegetable, fish, or fibre intakes. The broad principles of what makes up a healthy diet are well established but the challenge of how to encourage people in the population to take up this advice remains.
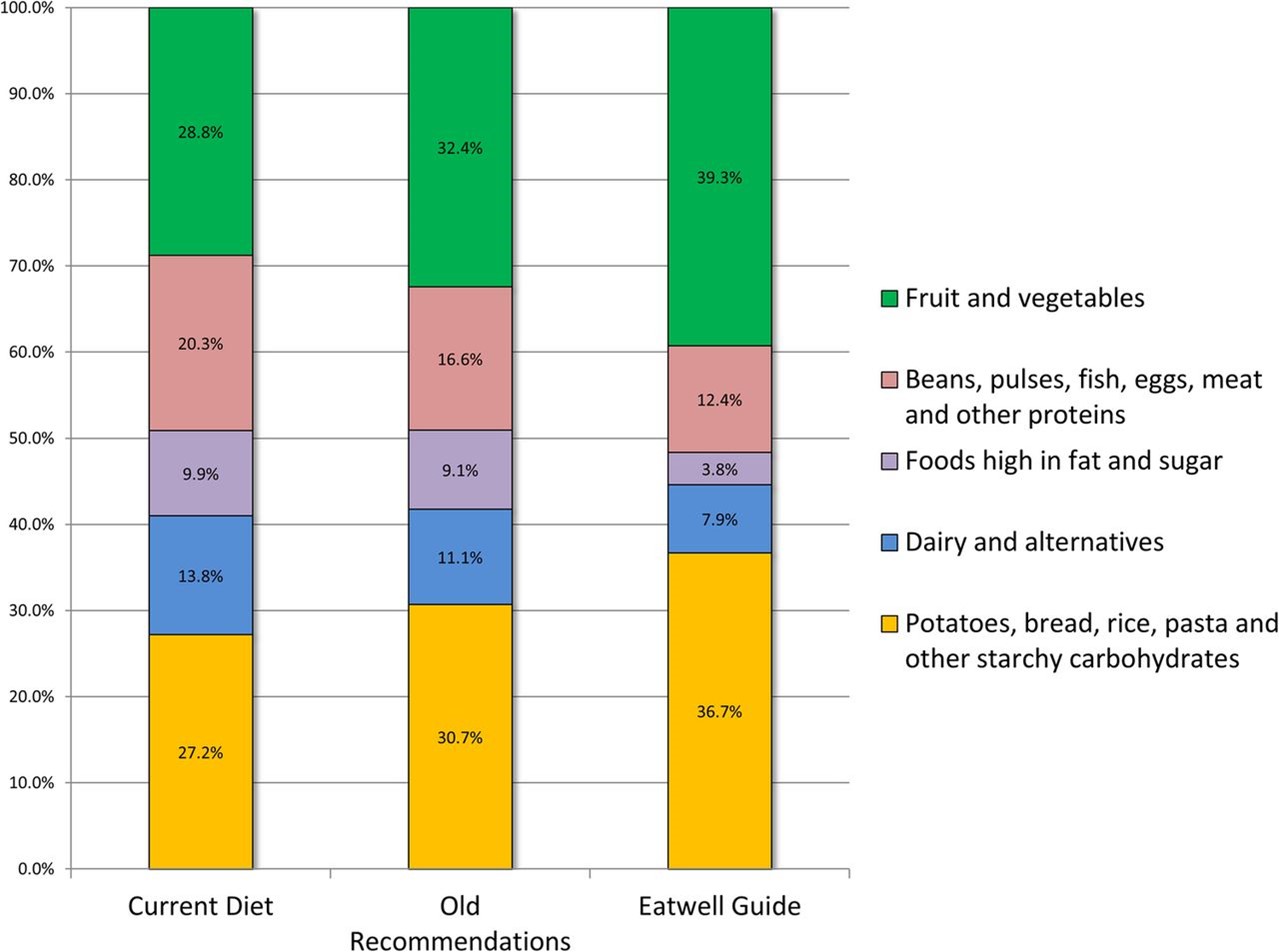
Breakdown of the diet by Eatwell Guide food group categories for current consumption, the ‘Eatwell Guide’ scenario and the ‘old recommendations’ (using previous recommendations for sugars and fibre) scenario
Scarborough et al. 2016
Research on dietary patterns
In recognition of the importance of dietary patterns rather than single foods or nutrients, researchers have developed methods for measuring healthy diets as part of cohort or intervention studies. This section will look at some examples of these measures and studies that have used them.
The Healthy Eating Index (HEI) is a measure of diet quality used to assess how well a set of foods aligns with key recommendations of the Dietary Guidelines for Americans.
The HEI-2015 includes 13 components that reflect the key recommendations in the 2015-2020 guidelines. There are two groups, ‘adequacy components’ and ‘moderation components’.
‘Adequacy components’ represent the elements of the diet that are encouraged in the guidelines. For these components, higher scores reflect higher intakes, because higher intakes are desirable.
The adequacy components are:
- Total fruit
- Whole fruit
- Total vegetables
- Greens and beans
- Wholegrains
- Total protein foods
- Seafood and plant proteins
‘Moderation components’ represent the foods or nutrients where there are recommended limits to consumption. In this case higher scores reflect lower intakes because lower intakes are more desirable.
The moderation components are:
- Refined grains
- Added sugars
- Saturated fats
Overall, a higher total HEI score suggests a diet that aligns better with the US dietary recommendations. Studies have found that greater adherence to the HEI is associated with lower risk of all-cause, cardiovascular and cancer mortality.
Alternative Healthy Eating Index (AHEI)
The AHEI was developed as an alternative to the HEI and focussed on foods and nutrients associated with reducing the risk of chronic disease. It is based on 11 components: six where the highest intakes are considered ideal (vegetables, fruit, whole grains, nuts and legumes, long chain omega-3 fats [docosahexaenoic acid and eicosapentaenoic acid], and polyunsaturated fatty acids), alcohol, for which moderate intake was considered ideal, and four components for which avoidance was recommended (sugar sweetened drinks and fruit juice, red and processed meat, trans fat and sodium). Each component is given a score between zero and ten, all the components adding up to a potential maximum score of 110, with higher scores suggesting better dietary quality.
The DASH diet
The DASH diet was developed to help lower blood pressure and it emphasises higher consumption of fruit, vegetables, legumes and nuts, wholegrains and low-fat dairy and limits sugary drinks, meat and sodium. The DASH score quantifies the level of adherence to the diet.
A systematic review and meta-analysis looking at dietary quality as assessed by the HEI, AHEI and the Dietary Approaches to Stop Hypertension (DASH) scores and health outcomes found that higher diet quality according to these methods was associated with reduced risk of all-cause mortality, reduced risk of cardiovascular and cancer incidence and mortality as well as reduced risk of type 2 diabetes and neurodegenerative disease.
The Nordic diet
The Nordic diet focuses on healthier foods that are locally produced in the Nordic region, and which are considered of importance to cultural and gastronomic identity in these countries. A Nordic style diet generally includes fruits such as apples, pears and berries, vegetables including roots, cruciferous vegetables and cabbages, as well as wholegrain and rye breads, a high intake of fish, low-fat dairy products, potatoes and vegetable fats.
Different scores have been developed to define a healthy Nordic diet, including the Baltic Sea Diet score (from Finland), Healthy Nordic Food Index (from Denmark) and the New Nordic Diet score (from Norway). Evidence from observational studies suggest that a Healthy Nordic is associated with lower risk of type 2 diabetes, stroke and a reduced risk of mortality (particularly from cardiovascular disease). Results from a small number of trials also suggest that a Nordic diet may help improve body weight, blood pressure and blood lipids. However, there is a general lack of evidence in non-Nordic populations to support these potentially beneficial effects if such a dietary pattern is adopted more widely.
Mediterranean diet
The Mediterranean diet, which is usually used to refer to the types of diets traditionally consumed in countries such as Italy and Greece, has been the subject of a lot of research over recent decades. Researchers have developed several different ways of measuring adherence to a Mediterranean dietary pattern. While these differ in exactly what is measured and how the score is calculated, higher consumption of fruit, vegetables, legumes, cereals, olive oil and fish and lower intakes of meat, dairy foods eggs and sugar are generally included. Research suggests that greater adherence to a Mediterranean diet may be associated with lower risk of coronary heart disease, lower levels of inflammation and a reduced risk of cognitive decline.
Plant-based diets have been the subject of much popular and scientific discussion in recent years. A set of plant-based diet indices have been developed as a research tool to investigate the health effects of such diets. The plant-based diet index (PDI) looks at the consumption of plant-based vs animal-based foods in the diet overall. The ‘healthful plant-based diet index’ (hPDI) emphasises plant-based foods that were considered to be healthy, including wholegrains, fruits, vegetables, nuts, legumes and tea and coffee. The ‘unhealthful plant-based diet index’ (uPDI) includes plant foods they considered as less healthy including fruit juices, refined grains, potatoes, sugary drinks and sweets. In each case, diets were scored according to their content of plant- vs animal-based foods and drinks, with an emphasis on healthy/less healthy plant-based elements for the hPDI and uPDI scores, respectively.
In a study looking at body weight in three large cohort studies, researchers found that a higher PDI score was associated with greater weight loss, but that this effect was greater with the hPDI score than the uPDI score. Another study looked at the association between these plant-based indices and risk of type 2 diabetes. The overall PDI score was associated with reduced risk although this was attenuated when it was controlled for body mass index (BMI). The hPDI score was associated with reduced risk of type 2 diabetes and the relationship remained after controlling for BMI. Conversely the uPDI score was associated with a higher risk of type 2 diabetes. A similar pattern was seen in a study looking at risk of coronary heart disease (CHD). The overall PDI score was associated with reduced risk of CHD, with a greater reduction seen with the hPDI score. Whereas the uPDI score was associated with increased risk of CHD.
Key references:
- EFSA (2010) Scientific Opinion on establishing Food-Based Dietary Guidelines https://www.efsa.europa.eu/en/efsajournal/pub/1460
- Morze et al. (2020) Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: A Second Update of a Systematic Review and Meta-Analysis of Cohort Studies. Journal of the Academy of Nutrition and Dietetics https://doi.org/10.1016/j.jand.2020.08.076
- PHE (2016) From Plate to Guide: What, why and how for the Eatwell model https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/579388/eatwell_model_guide_report.pdf
- Scarborough et al. (2016) Eatwell Guide: modelling the dietary and cost implications of incorporating new sugar and fibre guidelines. BMJ Open 6:e013182. http://dx.doi.org/10.1136/bmjopen-2016-013182
More on vitamins and minerals
Calcium counts
This resource looks at the calcium content in dairy and non-dairy foods that may contribute calcium to your diet.
Calcium Counts

A healthy balanced diet
We can probably all agree that eating a healthy, balanced diet is a good thing, but what does this mean in practice?

Vital Vitamin D
There are a limited number of foods naturally containing or fortified with vitamin D.
Healthy Diet FAQs
Is a healthy diet more sustainable?
As well as thinking about how what we eat affects our health, it’s also good to consider how it affects the planet. The good news is that healthier diets also tend to be more environmentally sustainable.
Should I try a different diet if I am overweight?
There are a whole range of other popular diets such as keto, paleo or raw food diets, some of which claim to have specific effects on health or to help with weight loss. However, those diets which do not follow the healthy eating principles are typically harder to stick to and may be detrimental to your overall health in the longer term.
To lose weight, you need to create a calorie deficit in your diet. That means you need to burn more calories than you consume. To learn more about how to lose weight healthily read our page on healthy weight loss here.
Last reviewed October 2023. Next review due October 2026.
Did you find this page useful?
We'd love to hear your feedback. If you would like a response, please contact us. Please note that advice provided on our website about nutrition and health is general in nature. We do not provide any individualised advice on prevention, treatment and management for patients or their family members.
English that goes straight to the heart
Importance of Healthy Diet essay
A healthy diet is one that helps to maintain or improve overall health. We should consume a balanced diet consisting of essential nutrition. A healthy and balanced diet reduces stress levels and promotes healthy life without any suffering.

Importance of Healthy Diet essay (350+ Words)
People consume junk foods and unhealthy items solely for taste, neglecting the importance of nourishing their bodies. A healthy and balanced diet reduces stress and promotes a suffering-free life, highlighting its utmost significance.
Daily Test - Attempt Now
A healthy diet maintains or improves overall health through essential nutrition: liquids, proteins, fatty acids, vitamins, minerals, and calories. To maintain a healthy body, we should consume fresh fruits, salad, green leafy vegetables, milk, eggs, yogurt, etc., on time.
Green vegetables and fruits provide minerals like iron, calcium, sodium, potassium , iodine, copper, etc. Fish oil, butter, carrot, papaya, etc., contain Vitamin A, while green leafy vegetables, wheat grain, etc., contain Vitamin B.
Vitamin C is found in green chili, green vegetables, amla, lemon, and citric fruits. Vitamin D is present in fish oil, butter, and sun rays. Vitamins E and K are necessary for our health, and milk is a well-balanced diet on its own.
We should only eat fresh, well-washed, and well-cooked food that is free from dust and flies. Harmful are fried foods and foods with excess fat, spices, and chilies. Eating on the roadside should be avoided. The last meal should be taken two or three hours before going to bed. Our stomach needs a good time gap between two meals for proper digestion.
In addition to proper nutrition, a healthy body requires daily physical activities, adequate rest and sleep, cleanliness, a healthy environment, fresh air, and water, as well as personal hygiene. Furthermore, it is important to drink a sufficient amount of water, at least 7-8 glasses. This not only balances blood pressure but also supplies essential nutrients rapidly to the body. An individual who is fit and healthy develops a higher resistance to infections and diseases.
While wealth holds some significance, it is not as important as health. Spending a large amount of money on junk food in five-star hotels or other entertainment sources, such as watching films for a day, yields no advantages except for self-satisfaction.
Physical and mental well-being enables an individual to be socially and financially healthy. A healthy person is more active, lively, and energetic, working with utmost efficiency. Conversely, a wealthy but unhealthy person easily succumbs to fatigue, ultimately losing the true wealth of life, namely, health.
You Asked, We Listened – Get Free Access to All Writing Lists 😍😍

10 Famous Moral Stories
Read More »

How to Write a Report?

How to Write a letter to the Editor
Daily reading comprehension test - attempt now, discover more from english luv.
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
The relationship between nutrition and the immune system
Camelia munteanu.
1 Department of Plant Culture, Faculty of Agriculture, University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca, Cluj-Napoca, Romania
Betty Schwartz
2 Robert H. Smith Faculty of Agriculture, Food and Environment, The School of Nutritional Sciences, The Institute of Biochemistry, Food Science and Nutrition, The Hebrew University of Jerusalem, Rehovot, Israel
Nutrition plays an essential role in the regulation of optimal immunological response, by providing adequate nutrients in sufficient concentrations to immune cells. There are a large number of micronutrients, such as minerals, and vitamins, as well as some macronutrients such as some amino acids, cholesterol and fatty acids demonstrated to exert a very important and specific impact on appropriate immune activity. This review aims to summarize at some extent the large amount of data accrued to date related to the modulation of immune function by certain micro and macronutrients and to emphasize their importance in maintaining human health. Thus, among many, some relevant case in point examples are brought and discussed: (1) The role of vitamin A/all-trans-retinoic-acids (ATRA) in acute promyelocytic leukemia, being this vitamin utilized as a very efficient therapeutic agent via effective modulation of the immune function (2) The involvement of vitamin C in the fight against tumor cells via the increase of the number of active NK cells. (3) The stimulation of apoptosis, the suppression of cancer cell proliferation, and delayed tumor development mediated by calcitriol/vitamin D by means of immunity regulation (4) The use of selenium as a cofactor to reach more effective immune response to COVID vaccination (5). The crucial role of cholesterol to regulate the immune function, which is demonstrated to be very sensitive to the variations of this macronutrient concentration. Other important examples are reviewed as well.
Introduction
Food, nutrition and health are highly interrelated and consumption of specific nutrients have a profound impact on human health. The amount and type of nutrients consumed are tightly linked to the metabolic stage and the immune health and thus, inappropriate nutrient consumption is associated with development of major human diseases due to an immune system not properly functioning ( 1 ).
The inflammatory mechanisms that compose the innate immunity are strongly influenced by nutrition, and this interaction, when perturbed, can profoundly affect disease development. The immune system is able to destroy antigens through both innate and adaptive immune cells and finally through antibodies that are specific for each pathogen ( 2 ).
The number of studies related to the impact of nutrition on immune system is continuously increasing. The initial studies published were related to nutritional-modulation of the immune function were mostly based on the effects of micro and macronutrients ( 3 ). Lately, a wide variety of phytochemicals and other chemical biocomponents found in nutrients has been added to the list of nutritional-immuno-modulators. These biocomponents affect the immune function but are not crucial for maintaining normal cell metabolism and function ( 3 ). Cases in point are several phytochemicals demonstrated to exert impressive positive immune effects ( 3 ).
In light of the strong effects, that we will list in the following paragraphs, nutrients have on the immune system it can be concluded that a rich-nutrient diet is rigorously required in order to maintain an adequate health status. This is in addition to the fact that nutrients are the main factors for survival, including cell proliferation, specialization, development of tissue and organs growth, energy supply, and the immune defense function ( 4 ).
To clarify all these aspects, it is essential to understand the meaning of an adequate diet and concomitantly to recognize the harmful effect of processed foods that impact the immune system ( Figure 1 ).

The importance of understanding the meaning of a healthy diet and concomitantly to recognize the harmful effect of pro-inflammatory foods that impact the immune system.
Moreover, nutritional deficiencies are closely associated with impaired immune response and loss of the host resistance to infection ( 3 ). On the one hand, in less developed regions malnutrition continues to be a major health problem ( 5 – 7 ) since it is associated with a higher incidence of morbidity and mortality usually linked with the higher prevalence of bacterial and parasitic infection diseases in these regions ( 3 ). In contrast, developed countries confront with inadequate diet consumption, with no real nutritional value, accompanied by excess calories ( 8 ). Therefore, malnutrition due to undernutrition or to consumption of poor diets, deficient in macro- and micronutrients, reduce the effectiveness of the immune system, not only by causing a deterioration of the immune protection but also reducing its efficacy in appropriate elimination of the pathogens, thus making people unprotected to a vast variety of diseases.
In addition to food consumption, an important question that arises is regarding the bioavailability of nutrients. Could we treat certain deficiencies using supplements if we are aware of them? Or should we try to build an adequate and complex menu that ensures the proper and desired bioavailability? Another important question refers to where does the absorption of the nutrients take place? It is important to know that the pathogenic agents such as bacterial products, bacteria and some toxic alimentary particles from food and the intestinal microbiota are often responsible for triggering an immune response ( 9 ). This is very important because prevention against pathogens is mainly maintained through the intestinal epithelial barrier. The gastrointestinal tract has an essential role due to its lymphoid tissue and for this reason, represents an essential part of the immune system. It has been demonstrated that the epithelial barrier contains cells that present antigens to dendritic cells (DCs) in the lamina propria. The most important are CD103 + CX3CR1- DCs. These imprint the intestinal lymphocytes to stimulate the development of regulatory T cells, the production of IgA, and dendritic cells responsible for the development of Th17 cells and the production of TNFα ( 10 ). Additionally, nutrients can control the expression of pro and anti-inflammatory cytokines via interaction with Toll-like receptors (TLRs), which are proteins known to play a key role in the control of the innate immune system. They are located in cells such as macrophages and dendritic cells and as such they control immune cell activity via appropriate crosstalk and signaling. As a result, immune cell’s enzymatic activity is affected and therefore molecular and chemical changes linked to oxidative stress and inflammation take place finally affecting the immune function. Most of the activities are associated with oxidative reactions, affecting neutralized cytotoxicity ( 11 ).
The normal standard diets of human beings (except for vegetarians and vegans), include vegetables, eggs, milk, dairy products, and meat. From a biochemical perspective, the foods can be converted into micronutrients and macronutrients that ensure the organism’s well-functioning ( 12 ).
This review aims to summarize at some extent the large amount of data accrued to date related to the modulation of immune function by certain micro and macronutrients and to emphasize their importance in maintaining human health.
In order to achieve this goal, research articles and reviews, found in several international databases, have been researched using phrases and keywords. We used the following keywords: nutrition, health, immunity, nutrients, vitamins, minerals, amino acids, and cholesterol.
The review material covers an extended period of time from 1973 to 2022. To achieve this aim some of the issues addressed were: the correlation of nutrition with the immune system in order to obtain good health, certain nutrients involved in the modulation of immune function through mediating pro-and anti-inflammatory responses, and cholesterols’ role in the immune response.
The effect of nutrition on optimal immune response
As alluded to earlier, nutrition plays an essential role in the regulation of optimal immunological response, by providing adequate nutrients in sufficient concentrations to the immune cells. In such a manner, the immune system can initiate effective responses against pathogens. In order to avoid chronic inflammation, nutrients stemmed from the diet exert significant effects in initiating this quick response ( 13 ). When the dietary nutrients are insufficient or inefficient, the supply of these elements to the immune system cells is significantly spared and immunity is compromised.
There are certain micronutrients such as vitamins and minerals as well as some macronutrients such as specific amino acids demonstrated to exert a very important and particular impact on immune modulation. Amino acids such as L-arginine and L-tryptophan are responsible and critical for macrophages’ appropriate immune activity. Macrophages are characterized by variations in their plasticity and polarization in response to changes in the intracellular environment. They are capable to transform into different subtypes depending on the intracellular microenvironment and to the different signaling molecules ( Figure 2 ). L-arginine is associated with a well-known immunoregulatory mechanism exploited by M2 macrophages. The mechanism involves arginase 1, which consumes L-arginine and the genes responsible for M1inhibition, concomitantly with M2 promotion ( 14 ). Furthermore, arginine and methionine together, are in charge of the synthesis of polyamines. Due to their ability to maintain cell membrane stability and keep DNA homeostasis, they stimulate cell proliferation ( Figure 2 ). In addition, many studies show the involvement of these kinds of amino acids in tumor cell growth metabolic pathways as well as in immune antitumor response ( 15 ). Through their degradation, these kinds of amino acids supply chemical precursors for a number of biological reactions ( 16 ). Regarding insulin-like growth factor -I and insulin growth hormone these can use as strong secretagogue arginine. Metabolic syndrome and type 2 diabetes as well are major worldwide public health problems which are strongly related to nutrition. There are some amino acids implicated in the synergistic stimulation of insulin release from pancreatic β-cells. One of the most well-known mechanisms is related to the fact that arginine in the presence of glucose can depolarize the plasma membrane at a neutral pH. This gating mechanism is called cationic ( 17 ).

The relationship between nutrition and the immune system. Macronutrients such as arginine and tryptophan are involved in cell proliferation and macrophages’ adequate activities. Micronutrients like Vitamin A and Zinc can promote cell proliferation; inhibit the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway; decrease the pro-inflammatory cytokines IL-1β and tumor necrosis factor-α (TNF-α); regulate the Th17 and Th9 cell differentiation; initiate the growth Treg cell population.
Indoleamine 2,3-dioxygenase 1 (IDO1) is a powerful immunosuppressive enzyme involved in the catalysis of the first and rate limiting step of L-tryptophan catabolism. IDO1 depletes L-tryptophan storage and induces the production of immunoregulatory molecules interferon-γ (IFN-γ), tumor-necrosis factor (TNF) and IL-1 ( 18 ). High IDO1 expression and catalytic activity occur in dendritic cells (DCs)— in response to IFN-γ ( 18 ). Tryptophan metabolism leads to the synthesis of NAD +, which is known as a cofactor capable of redox reactions. In immune tolerance, arginine catabolism may determine the initiation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway. Arginine may also provide a substrate for the growth and survival of the cells and concomitantly exerts a key role in differentiation and appropriate gene expression ( 16 ).
The decline of protein metabolism that is related to the diminishing concentration of certain amino acids, leads to the endoplasmic reticulum (ER) stress. As a result, the T cells which produce pro-inflammatory cytokines are activated ( 19 ). The deficiency of Arg is correlated with reduced T cell ability to trigger tumor immunity ( 15 ).
Vitamins and minerals such as vitamin A and Zn in addition to their involvement in cell division and proliferation ( Figure 2 ) are involved in immune-modulation. For example, the rate of antibody synthesis can be modified by these micronutrients ( 20 ).
Vitamin A ( Figure 2 ), which is involved in biosynthesis of carotenoids and retinyl esters, molecules well known to affect appropriate immune function ( 21 ). It can also exert a role as transcription factor if it is bound to retinoic acid receptors (RARs). As a result, it can be responsible for lipid homeostasis, cell division, growth, and specialization by regulating the expression of certain specific genes ( 21 ). Vitamin A deficiency has repercussions on immune functions, such as impaired neutrophil function, suppressing the activity of natural killer (NK) cells, as well as a decline in their number, and damaged capacity of phagocytosing of macrophages. In addition, it may affect the growth and differentiation of B cells ( 2 ). In this way, the predisposition for infection disease can increase ( 22 ).
Zinc represents another example of the micronutrient group. The transcription factor NF-κB ( 23 ) can be inhibited by Zinc ( Figure 2 ). Also, the pro-inflammatory cytokines IL-1β and tumor necrosis factor (TNF-α) production ( 24 ) may be repressed, as a result of modulation of the Toll-Like Receptor 4 (TLR4) signaling pathway. Moreover, the pro-inflammatory specific Th17 and Th9 cell differentiation pathway ( 25 , 26 ) can be moderated by Zn. Treg cell population can be increased after Zinc administration ( 27 , 28 ) thus Zn is considered an important factor for immune cell development. Specific effects include impaired lymphocyte proliferation, Delayed-Type Hypersensitivity (DTH) response, and natural killer (NK) cell activity ( 29 – 31 ). As we mentioned before, there are strong and dynamic relationships between nutrition and the immune system, which are important for maintaining good health. We will discuss further in more details the role of specific nutrients in the mediation of pro-and anti-inflammatory responses.
Nutrients involved in mediation of pro-and anti-inflammatory responses
The immune system consists of cells belonging to the two types of immune responses, i.e., the innate and adaptive mechanisms. Once the pathogens enter the body, the first reaction is mediated by the cells belonging to the innate immunity system. This system consists phagocytes, dendritic cells, eosinophils, neutrophils, mast cells, and some additional cells ( 32 ). In this case, the immune response acts quickly. The difference between the innate immunity system and the adaptive response is that the former is unspecialized and less efficient ( 13 ). In contrast, the adaptive response is capable to recognize each pathogen, and furthermore, remember if it has been encountered before, therefore T cells being the most important in antigen identification. They are also involved in immune response regulation. Furthermore, there are two kinds: cytotoxic T cells/T8 (CD8 inducer), which are implied directly in killing infected cells ( 33 ) and tumor cells, and the T4 helper cells (CD4 inducer), which are useful in modulating other cell’s responses. Furthermore, in the function of type cytokines produce by them, there are some subtypes of T helper cells: Th1, Th2, Th17 ( 13 , 34 ). Th1 cells are responsible for fighting against bacteria and viruses. The main role of these cells is to produce Interferon γ (IFNγ) and IL-2. IFNγ, like IL2, is a cytokine created by both immune adaptive and innate cells like Th1, T8 lymphocytes as well as innate lymphoid cells and NK ( 35 ). At the same time, the immune function is activated by Th2 cells. They are capable to produce other interleukins (ILs) ( 36 ). Induced cells apoptosis can be caused by activated macrophages and cytotoxic CD8 + T. Interestingly, the other immunity regulatory T cells are involved in the suppression or the blockage of cytokine secretion by the immune response ( 37 ). In light of this information, they have a crucial role in peripheral tolerance through the initiation and continuance of this stage ( 38 ).
B lymphocytes, which also belong to the adaptive immune system are involved in the synthesis of antibodies. Similar to T cells, they have the ability to specifically respond to each antigen ( 39 ). Antigens can actually produce damage to the tissue that they attack. It makes sense that the pathogens in the tissues and around this region promote an inflammatory response. Its main role is to repair the damage tissue in certain ways that can eliminate the antigens and their effects, and decrease their extension ( 40 ). After that this process takes place, some physiological changes occur which are responsible for increasing the phagocytes number in the place where this process is happening. As a result, pro-and anti-inflammatory cytokines, prostaglandins, and complements are delivered, especially through the activation of phagocytes. All these changes cause the growth of the inflammatory response ( 1 ). Based on the knowledge of which cells are involved in each inflammatory response pathway, it is now feasible to shed light on the effects of specific nutrients on each of these processes.
To this end, we selected to discuss the influence of certain vitamins such as: A, B1, B2, B3, B12, C, and D, minerals like: Zinc, and Selenium as well as certain amino acids such as arginine and tryptophan and some fatty acids.
Vitamin A plays an essential role in the regulation of innate and cell-mediated immunity, and antibody responsiveness through the activity of either all-trans retinoic acid, 9-cis retinoic acid or other metabolites and nuclear retinoic acid receptors ( 41 ). Vitamin A and associated retinoid metabolites exert an important regulatory function of the immune system. This essential role is evidenced when Vitamin A is deficient and an augmented susceptibility to infections is evident ( 42 ). Vitamin A deficiency affects processes related to appropriate cytokines release and antibody production. Additionally, vitamin A deficiency is associated with a reduced production of natural killer cells, monocytes or macrophages, and impaired maturation and proliferation of T- and β-lymphocytes. Vitamin A deficiency impairs innate immunity by impeding normal regeneration of mucosal barriers damaged by infection, and by diminishing the function of neutrophils, macrophages, and natural kill cells. Vitamin A supplementation cuts down morbidity and mortality in various infectious diseases ( 43 ). In the case of vitamin A deficiency, the integrity of the mucosal epithelium is altered, resulting in enhanced accessibility to various pathogens to the gastrointestinal tracts and other organs, being children the most affected population ( 44 ). In children, severe vitamin A deficiency causes almost the disappearance of goblet cells present in the upper layer of the epithelial line, therefore the production of mucus by these cells is compromised, and bacterial adherence to the epithelial lining is reinforced thus becoming the major factor for the development of the bacterial disease ( 45 ). Additionally, vitamin A deficiency is associated with diminished phagocytic activity and macrophage oxidative breakdown that takes place during the process of inflammation along with a reduction in the number of natural killer (NK) cells ( 42 ).
It has been demonstrated that vitamin A (Tab.1) stimulates the expansion and differentiation of Th1 and Th2. Thus, vitamin A is capable of promoting the Th2 anti-inflammatory response by repression of IL-12 and IFNγ which are synthesized by Th1 lymphocytes ( 46 ). In addition, some studies suggest a positive relationship between vitamin A and mitogen-induced pro-inflammatory cytokine (IFN-γ) and anti-inflammatory cytokine (IL-10) ( 47 ). It is important to know that retinol, retinoic acid (RA), and retinal are the three forms of vitamin A. It has been shown that RA is involved in a lot of biological activities ( 48 ). According to Rampal et al. ( 49 ) under inflammatory conditions, RA might sustain or cause stimulation of intestinal inflammation ( 50 ). Moreover, through the release of certain cytokines, such as: IL-1, IL-6, IL-12, and nitric oxide, RA can affect the macrophages’ activity ( 51 ). When it comes to hypovitaminosis in children, vitamin A administration reduces mortality caused by diarrheal diseases ( 52 ). Vitamin A might be responsible for antitumor effects on human pancreatic cell lines ( 53 ). In metastasis of renal carcinoma, it seems that all-trans-RA (ATRA) have a similar effect ( 54 ). ATRA represents a nutrient that is required in small quantities and it is synthesized in the human body from the A vitamin ( 55 ). In acute promyelocytic leukemia (APL), ATRA is utilized as a very efficient therapeutic agent. Furthermore, together with arsenic trioxide (ATO), they are able to increase life expectancy. Due to this combination, the recovery of this disease is approximately 95% of cases ( 56 ). It was observed that, in breast cancer, after the administration of vitamin A, cytotoxic effects have been seen, but the healthy cells weren’t influenced. As a result, vitamin A is capable of reducing some negative chemotherapy effects ( 57 ). The next question worth asking would be whether there are more nutrients involved in mediating pro- and anti-inflammatory responses.
Vitamins B1, B2, B3, and B12
The group of B-vitamins comprise eight water soluble vitamins in charged to carry out essential, inter-related roles for appropriate cellular functioning. These vitamins act as efficient co-enzymes in a vast array of catabolic and anabolic enzymatic reactions and they are essential cofactors for many important cellular metabolic pathways. We therefore cannot refer to life or to cellular life without referring to the B vitamins. In this respect, the crucial enzymes responsible for the regulation of vital functions in cells use specific cofactors such as: Nicotinamide Adenine Dinucleotide/B3, Flavin Mono Nucleotide/Flavin Adenine Dinucleotide/B2, and Thiamine Pyro Phosphate/B1 ( 58 ). However, an important aspect to be considered in terms of B vitamins, is that when it comes to the human body, an important source of vitamins B is determined by the activity of the gut microbiota except for some that may be ingested by the diet. The absorption of B vitamins takes place in two different intestinal locations, the large and small intestines. The large intestine represents the main absorption place for most bacterial-produced B vitamins. At the same time, the small intestine represents the place where dietary B vitamins are absorbed. It is tentatively to surmise whether two specific immune responses result from the two different absorption places ( 59 ). We surmise that the immune activities at the two specific locations are different since the population of gut immune cells are different ( 59 ).
Vitamin B1 or Thiamine ( Table 1 ), exerts an anti-oxidative role due to its protective action on sulfhydryl groups from the surface of neutrophils. As a result, the synthesis of cytokines from macrophages is blocked furthermore. Regarding the stimulation of antimicrobial oxidative reactions myeloperoxidase (MPO), H 2 O 2 , and a halide (HRP/H 2 O 2 /Nal) determined by the activation of polymorphonuclear leukocytes, thiamine together with other compounds can prevent and inhibit this oxidative system of PMNL ( 60 ). The NF-κB pathway involved in the control of the oxidative stress, is prevented by Thiamine. This role is highlighted by suppressing the phosphorylation and catabolism of inhibitory kappa B (IκB), which subsequently inhibits the nuclear translocation of the transcription factor-sensitive redox NF-κB ( 58 , 61 ). From a biochemical and immunological point of view, we can conclude that thiamine derivates are involved in the control of immune metabolism through the regulation of cells’ immune activities. These properties are a result of its function in maintaining an equilibrium between glycolysis and the TCA cycle ( 62 ). As we mentioned before, they are cofactors for enzymes participating in these pathway’s. The TCA energy cycle represents the main source of naïve T cells, rest macrophages, and T-regulatory cells. Interestingly, activated T helper cells need energy from aerobic glycolysis because the amount of energy from TCA is not sufficient ( 63 ). Due to the significant effects of thiamine on these pathways, B1 deficiencies have so significant side effects. One of the side effects is linked to the stimulation of IL-1, IL-6, and TNF-α (pro-inflammatory cytokines) expression and neuro-inflammation. Finally, neuronal death may occur due to the inhibition of CD 40 and CD 40L regulation ( 64 ). It was observed that B1 could be used in the treatment of neurodegenerative diseases through its involvement in the suppression of the pro-oxidative activity of microglial cells ( 65 ). Additionally, in regards to B vitamins, we should pay attention to their role in oncogenesis and more over is extremely important to clearly make a distinction between healthy and sick individuals. Some speculations exist regarding the role of B1 in cancer due to its involvement as a cofactor in proliferation and energy pathways that are essential in the development of tumor cells. Further research is needed in order to clearly distinguish Thiamine’s possible oncogenic effects ( 58 , 66 ).
Vitamins with pro-and anti-inflammatory effects as well as pro-tumor and anti-tumor effects.
ATRA, all-trans-RA; HIF-1α, hypoxia-inducible factor 1-alpha; IL, interleukin; IFNγ, Interferon γ; NK, natural killer; NF-κB, pro-inflammatory factor Kappa B; hs-CRP high-sensitivity C-reactive protein; RA, retinoic acid; ROS, reactive oxygen species; Th, helper T cell; TNF-α, tumor necrosis factor.
Riboflavin, or vitamin B2 is crucial for energy metabolism through its function as a cofactor ( 67 ). It also plays an important role as an anti-inflammatory and anti-oxidant modulator, especially in lungs ( 68 , 69 ). Some specific aspects regarding the link between major histocompatibility complex (MHC) and B2 bacterial compounds are worth mentioning. This function on the innate mucosal results in the stimulation of invariant T cells. Riboflavin and its precursors selectively activate mucosa-associated invariant T cells (MAIT) that represent the largest population of innate-like T cells in humans. Their synthesis as well as the link with the major histocompatibility complex through the major histocompatibility complex-protein (MR1) are not fully understood. It was observed that the activation of MAIT cells is dependent on genes that encode enzymes responsible for the formation of intermediate compounds in the synthesis of bacterial riboflavin. ( 70 ). These types of cells are known for their function in the inflammation and defense activity in gut mucosal due to their production of IL-17 and IFN-γ ( 71 ). The proliferation of neutrophils and monocytes as well as the stimulation of macrophages and neutrophils activities might be boosted by the activity of riboflavin ( 72 , 73 ). The catabolism of inhibitory kappa B (IκB) is responsible for the activation of the pro-inflammatory factor Kappa B (NF-κB). Following this catabolic pathway, the inflammatory signaling pathway becomes activated. At the end of this signaling pathway, the activation of pro-inflammatory cytokines, such as TNF-α and ILs, takes place. In this signaling process vitamin B2, act as an anti-inflammatory suppressor and it may block the activation of the NF-κB ( 74 ). Furthermore, through the overexpression of catalase and nitric oxide synthase vitamin B2 could reduce oxidative stress ( 75 ).
Vitamin B3, niacin ( Table 1 ) is known as NADP and NAD precursor. Similarly, to all B vitamins, it is a cofactor for a wide variety of enzymes involved in several metabolic pathways. In contrast to other B vitamin groups, human cells can synthesize NADP and NAD cofactors through independent pathways. From a biochemical point of view, niacin and the resulting cofactors are involved in redox reactions. NAD is responsible for genomic equilibrium and epigenetic regulation may represent its mechanism of action ( 76 ). Additionally, there is a positive correlation between high concentrations of NAD and the blockage of ROS synthesis ( 77 ). Furthermore, NAD can be considered an anti-inflammatory micronutrient due to its inhibitory and deacetylation actions, which were observed in the NF-κB pathway ( 78 ). Also, it has an inhibitory effect on inflammatory cytokines as well as on animal tumor cells ( 79 ). NAD is also considered an efficient anti-inflammatory component since it induces the reduction of certain cytokines released from alveolar macrophages ( 80 ).
B12, cobalamin ( Table 1 ) affects pro- and anti-inflammatory responses. A negative correlation has been observed between vitamin B12 and TNF-α ( 81 ). It has been demonstrated that an increase of TNF-α induce the exhaustion of antioxidants involved in the defense against free radicals ( 82 ). As a result, pro-inflammatory cytokines and some other pro-inflammatory compounds are activated ( 83 ). Interestingly, in human anemia with cobalamin deficiency, the number of CD8 + T cells decreases compared to the levels in healthy individuals. In contrast, an increase in the number of CD4 + T cells has been observed in patients with cobalamin deficiencies, which differed compared to healthy people. In these cases, the CD4 + /CD8 + ratio is pathological higher. Additionally, in these patients, the activity of NK cells is decreased ( 84 ). Interestingly, hyperhomocysteinemia is the result of vitamin B12 deficiency ( 85 ), leading to chronic diseases such as insulin resistance ( 86 ) and coronary heart disease ( 87 ) through the expansion of inflammatory processes. Since vitamin B12 deficiency is associated with abnormal TNF-α activity, it can also lead to insulin resistance ( 88 , 89 ). Regarding cancer activity, a study by Cheng et al. ( 90 ) from a genetic perspective found no correlation between B12 and certain types of cancers such as squamous cell carcinoma, prostate, breast, and colorectal cancer. In the case of lung cancer, B12 administration was not considered a risk factor ( 91 ). On the contrary, a higher intake of B12 was considered dangerous for many types of cancer as indicated in a big meta-analysis of cancer patients ( 92 ).
Vitamin C ( Table 1 ), is considered an essential micronutrient ( 93 ) in humans since they cannot synthesize it. Human absorption of vitamin C is higher compared to other species that are capable to synthesized it ( 94 , 95 ). Vitamin C is involved in the modulation of a wide variety of immune functions and play a role as a regulator of cell-signaling. Vitamin C is also, involved in gene transcription as well as in hydroxylation reactions ( 96 ). Through its main function as an antioxidant, it became capable to defend the body against reactive oxygen species that are the result of the activity of toxins and pollution ( 97 ).
Vitamin C is responsible for discontinuing the action of the pro-inflammatory cytokines and inhibiting the initiation of the NF-κB reaction ( 98 ). In peripheral blood cultures that are stimulated with LPS (lipopolysaccharide), after vitamin C administration, an enhancement of IL-10 and a reduction of TNF-α and IFN-γ has been observed ( 99 ). Moreover, as a result of ROS accumulation in microbial infections, vitamin C causes neutrophils displacement into infected sites ( 100 ). Additionally, vitamin C might be useful as a cofactor in the synthesis pathways for vasopressin and norepinephrine in severe infections. This has a noticeable effect on the infection response of the cardiovascular system when the pathological state represents a danger ( 101 ). It appears that vitamin C may be considered as an antioxidant protector for the skin in the fight against ROS as a result of external factors’ synergistic work, particularly of pollutants ( 102 ). In this case, the effect is more pronounced if vitamin C is administrated in combination with vitamin E ( 103 ). Ellulu et al. ( 104 ) have demonstrated in hypertensive and/or diabetic adults that following C vitamin treatment a decreased inflammation associated with a moderate decline in inflammatory markers such as: the high-sensitivity C-reactive protein (hs-CRP) and IL-6 is observed. Vitamin C is also involved in the regulation of hypoxia-inducible factor 1-alpha (HIF-1α) activity, which makes neutrophil viability under hypoxic conditions possible ( 105 ), and in this way, neutrophil apoptosis is delayed ( 106 ). Furthermore, it is thought that vitamin C is involved in the fight against tumor cells through the increase in the number of NK cells ( 107 ).
Vitamin D, ( Table 1 ) exerts many anti-inflammatory roles ( 108 ) since receptors to this vitamin are expressed in different organs throughout the human body. The best known and established effects are linked to mineral and bone metabolism ( 109 ). Wöbke et al. ( 108 ) demonstrated that vitamin D effects are mediated through either nuclear and cytosolic signaling control pathways involving also pro-inflammatory components. Vitamin D binds its receptors (VDR) resulting in a complex of vitamin D-VDR that may contribute to the formation of homodimer with an additional VDR or formation of a heterodimer compound with the nuclear retinoid X receptor (RXR). Also, the nuclear role is demonstrated following the formation of heterodimers with steroid hormone receptors ( 110 ). Vitamin D bound to/VDR/RXR can cross the nuclear membrane, and then binds to a response element and start its specific gene regulation action ( 111 ) through activation of expression of its responsive genes.
Vitamin D is apparently involved in the adaptive immunity since immune cells such B and T cells express a high number of VDR’s ( 112 ). From the immunological regulatory aspect, vitamin D can block the secretion of the pro-inflammatory cytokines IL-6 or TNFα in monocytes ( 113 ). Additionally, the same effect has been observed in prostate cells ( 114 ). These effects are caused by the inhibition on P-38 MAP kinase (a subclass of mitogen-activated protein kinase) as a response to pro-inflammatory cytokines ( 113 ). Moreover, there is an interesting relationship between VDR/RXR and MAP kinase signaling path in terms of activation or inhibition. The results are closely related to the specificity of cells, their response, and effects of triggering factors ( 115 ). Furthermore, the complex VDR/RXR can bind to other compounds involved in the transcription process, such as glucocorticoid receptor (GCR) and NF-κB. Thus, vitamin D may be considered an anti-inflammatory micronutrient as a result of these interactions.
The vitamin D bound VDR becomes active and thus exerts inhibitory effects on NF-κB, which is also a heterodimer compound ( 116 ). Additionally, some studies suggest anti-inflammatory role of D vitamin is mediated also through the inhibition of specific pro-inflammatory Th1 cell cytokines such as TNF-α, IFN-γ, IL-6, IL-2, and IL-17 ( 117 , 118 ). Additionally, vitamin D is capable of increasing the concentration of cytokines such as IL-10, IL-4, and IL-5 as a result of an increase in the activity of Th2 cells ( 119 ). At the same time, it may induce the amplification of Treg cells as well as a reduction of the number of Th17 cells ( 120 , 121 ).
From a medical perspective, vitamin D has an important effect on the lung defense system against microbial pathogens. This function represents the result of the antimicrobial peptides activation expression in monocytes, epithelial cells lining the respiratory tract, monocytes, neutrophils, and NK cells ( 122 ). Lower levels of vitamin D in the serum are correlated with higher infection risks ( 123 ). Particularly, the administration of vitamin D induce a decline in acute respiratory infections ( 124 ).
The anti-cancer effect of vitamin D in tumor cells is mediated by calcitriol which is the biologically active molecule of vitamin D ( 125 ). The stimulation of apoptosis, the suppression of cancer cell proliferation, and associated delayed tumor development in cancer are the main effects mediated by calcitriol ( 125 , 126 ). At a molecular level, through the suppression of pro-inflammatory cytokines and prostaglandins (PG) activity as well as by preventing the NF-κB signaling pathway, calcitriol is considered an anti-inflammatory nutrient ( 127 ). From this point of view, calcitriol may be used as a preventive and therapeutic agent in cancer ( 128 ).
The minerals-zinc and selenium
When inflammatory cytokines are maintained at a high level, chronic inflammation takes place ( 129 ). This process is closely linked to the action of some minerals. In this way, it is important to test what is the role of Zinc in this essential process. The process is mediated ( Table 2 ) by the activity of several signaling pathways that are triggered due to the action of some changes produced by antigens and their metabolites. The main compound involved in inflammatory responses as a result of its role in cell proliferation, cell apoptosis, and the release of certain cytokines like IL-6, IL-8, and IL-1β are mediated by the activity of the NF-κB factor ( 130 ). The role of Zinc in this regard is controversial. In vitro studies demonstrate that the zinc effects can be either anti-or-pro- inflammatory ( 131 ). The NF-κB signaling pathway can be blocked by distinctive intracellular membrane chelator such as TPEN (N, N, N’, N’-tetrakis (2-pyridinylmethyl)-1,2-ethanediamine) ( 132 ). From one side the apoptosis effect is evident following the binding of the chelator and heavy metals ( 133 ). From the other side, other studies indicate a strong relationship between the initiation of LPS-induced NF-κB and zinc ( 132 ). Moreover, after a decline in the release of IL-1β, zinc is able to inhibit pro-inflammatory actions ( 134 ).
Selenium and Zinc with pro-and anti-inflammatory effects as well as anti-tumor.
IL, interleukin; IFNγ, Interferon γ; NF-κB, pro-inflammatory factor Kappa B; ROS, reactive oxygen species; Th, helper T cell; TNF-α, tumor necrosis factor; TPEN, N,N,N’,N’-tetrakis (2-pyridinylmethyl)-1,2-ethanediamine.
Furthermore, it has been reported that cytokine synthesis is dependent on Zinc status and this is closely related to chronic inflammation. In this regard, it has been observed that obese people having low zinc plasma concentrations over-express IL-1β, IL-1α and IL-6 genes ( 135 ). Zinc exerts beneficial effects on the proliferation and differentiation of T lymphocytes ( 46 ). The strong relation between a high number of cytokines and the decline in zinc plasma levels in infections and trauma-associated conditions has been demonstrated in cross-sectional studies. In patients with severe head injuries, upregulated cytokine production genes have been observed ( 136 ). In addition, the production of cytokines is elevated in patients that are in a critical state due to their decrease in plasma zinc concentration ( 137 ). Moreover, zinc antioxidant effects help the body to defend against reactive nitrogen species (RNS) and reactive oxygen species (ROS) ( 138 ). Zinc is able to induce the initiation of the CD8 + T cells proliferation ( 36 , 139 ). Zinc also can be capable to support the integrity of skin and the mucous membrane ( 36 ). To summarize, zinc has an essential effect on the proliferation and development of cells belonging to the immune system, such as T lymphocytes, CD8 + T cells, etc., which are known for their quick turnover. In this regard, it was observed that deficiency of this mineral negatively impacts health by decreasing resistance to infectious diseases, dermatitis, growth diseases, and genetic disorders ( 140 ).
Another crucial micronutrient is selenium ( Table 2 ), which is involved in the functioning of the thyroid metabolism and the cardiovascular system as well as in ensuring a functional immune system and preventing cancer. From the cellular point of view, there are still discrepancies regarding the exact dose that may be translated into deficiency or toxicity, even if these stages do not commonly take place in the human body ( 141 , 142 ). It is well known that when selenium is present within the amino acid selenocysteine is able to control certain metabolic reactions that may lead to lipoxygenase synthesis that finally, can be involved in the production of inflammatory mediators ( 143 , 144 ). In mice, selenium, due to stimulation of T cell receptor complexes (TCR) activity and conversion of Th1 from T0 cells, may improve the regulation of cellular immunity ( 145 ). Selenium can also contribute to the defense against pathogens as a result of its effects on redox signaling activities ( 146 ). It was recently demonstrated that in COVID-19 patients, selenium together with zinc exert a protective role and they are associated with a higher chance of survival ( 147 ). During vaccination against COVID-19, it has been demonstrated that the response may increase after selenium administration as well as the increase of titers antibodies. It is assumed that selenium may act as a cofactor in immunity response that is mediated by the vaccine ( 148 ).
Additionally, in women that are infertile as a result of polycystic ovary syndrome, to whom fertilization in vitro has been recommended, a decline in IL-1 and TNF-α gene expression was observed as a result of selenium treatment ( 149 ). This effect suggests that selenium has an anti-inflammatory role in the human body. Furthermore, in patients with cancer, the supplementation of selenium increased antibody titers of IgA and IgG as well as the number of neutrophils ( 150 ). We can say that selenium is involved in the regulation of the inflammatory mediators’ synthesis and, also, it might increase the activity of phagocytic cells as well. Selenium is capable to enhance the immune response of Th1 cells and the stimulation of T cells. Selenium has a positive relationship with the number of B cells. The innate immune system may be strengthened after selenium administration. A similar effect has been observed on cellular immunity ( 11 ). Increased titers of antibodies were measured due to selenium supplements that can cause an enhancement of vaccine effects ( 145 , 146 ). In the brain, both neurogenesis and hippocampal neural precursor cells are increased after selenium infusion ( 151 ).
Macronutrients: Amino acids arginine and tryptophan
Besides micronutrients, macronutrients, such as proteins and amino acids, also play an important role in the activity of the immune system. Proteins are formed from amino acids that are essential in the construction of other proteins among which antibodies and cytokines that are typical proteins belonging to the immune system ( 20 ).
Arginine ( Table 3 ) contribute with the production of nitric oxide in macrophage cells. Nitric oxide (NO) resulting from arginine under the action of nitric oxide synthase (iNOS) determines the cytotoxicity of macrophages in the fight against antigens such as pathogenic bacteria and parasites. Moreover, M1 macrophages use arginine to produce NO ( 152 ). Even though that arginine was initially considered a non-essential amino acid ( 153 ), after one decade, some papers have proven that arginine is essential for embryonic outliving, ontogenetic fetal development, and for constant hemodynamics and vascular parameters ( 154 ). Moreover, the induction of the NF-κB pathway has been linked to the arginine degradation pathway ( 16 ). As we presented previously, arginine through cations dependent mechanism can improve the release of insulin from pancreatic β cells. In addition, in β-pancreatic cells, arginine causes an increase in Ca 2+ concentration due to electron transport through a mechanism dependent on the amino acid mCAT2A transporter. When the membrane was depolarized, the Ca 2+ voltage-dependent channels are opened, followed by the increase in the intracytoplasmic concentration of Calcium and finally the stimulation of insulin secretion. However, clinical evaluations have shown that the beneficial effects of arginine administration are limited, probably due to the fact that it is very quickly transformed into ornithine or citrulline in epithelial cells ( 17 ). In addition, the polyamines, compounds which are also derived from arginine degradation, are involved in balanced levels of membrane, mRNA and DNA. Thus polyamines are capable to control the proliferation of cells ( 155 ). In vitro , polyamines can modify the inflammatory process ( 156 ). Furthermore, it has been demonstrated that higher concentrations of intracellular polyamines may change the in vitro cytotoxicity regulated by macrophage cells ( 157 ). The inflammation regulation and identification of pathogens are closely related to polyamines through their binding manner to receptor-ligand complexes ( 155 ).
Macronutrients implied in mediate pro-and anti-inflammatory responses.
Arg-1, type-I arginase; IDO, indoleamine 2,3-dioxygenase; Kyn/Trp, kynurenine/tryptophan; 5-methoxytryptophan, 5-MTP; IL, interleukin; NF-κB, pro-inflammatory factor Kappa B; Th, helper T cell.
In the last decades, it has been discovered that arginine is more beneficial than it has been supposed to be in the past, for example arginine induces the decline of oxidative stress and causes the reduction of the intestine’s inflammation ( 158 ).
Arginine is capable of diminishing intestinal damage and reestablishing mucosal immune equilibrium in humans and mammalians’ intestinal diseases ( 159 ). In an in vitro intestinal system in Caco-2 cells, arginine is able to induce the inhibition of IL-1β-mediated NF-κB pathway ( 160 ). However, the mechanism of reducing the inflammatory pathways is still unknown. Perhaps, it is linked to the activity of Arginase-1 (Arg-1), which, in this case, is stimulated by L-arginine as a substrate. The arginase-1 is an enzyme involved in the end of the urea cycle with the aim of forming l-ornithine and urea from l-arginine ( 161 ). Some studies suggest that the Arg-1 has positive effects in certain inflammatory diseases through an anti-inflammatory action ( 162 , 163 ). In contrast, there are studies which have shown that higher metabolism of arginine in tumors cells, together with their particular environment, create conditions that are intermediary, and at the same time, crucial for the maintenance and development of cancer cells. The result of these actions is translated into proper immunosuppression ( 164 , 165 ). One thing is certain, that the relationship between arginine and cancer cells is controversial. On the one hand there is data suggesting that arginine deprivation is correlated with a delay in the development of some tumor cells ( 166 ). On the other hand, arginine can have antitumoral actions which are observed through the enhancement of immune response ( 167 ). Furthermore, Al-Koussa et al. ( 168 ) have shown that certain kinds of cancer cells need arginine to develop. In this sense, some authors suggest that arginine deprivation may downregulate the migration of cancer cells. In physiological conditions, the movement process is useful for embryonic growth and immune function. But when it comes to cancer cells, things are different. This happens since certain kinds of cancer cells can use this property with the aim to stimulate metastasis ( 169 , 170 ). Therefore, arginine deprivation in cancer cells is capable of reducing metastatic activity ( 168 ). Unfortunately, the exact mechanism remains unknown.
Tryptophan (Trp) is clearly essential for the activity of the immune system ( Table 3 ). Since Trp is necessary for protein synthesis, it becomes to be indispensable for cell division and development ( 171 ). Since Trp is not synthesized by the human body, it is required to be obtained from the diet ( 172 ). Trp serves as a substrate for the biosynthesis and formation of serotonin (5-HT), kynurenine (Kyn), and indoles ( 173 ). The most useful and active Trp metabolism is the Kyn path which is related to the formation of nicotinamide adenine dinucleotide (NAD) and kynurenic acid. Of course, similarly to all pathways, this type takes place due to the involvement of two types of enzymes indoleamine 2,3-dioxygenase (IDO and IDO2) and tryptophan 2,3-dioxygenase (TDO) ( 174 , 175 ). Additionaly to the Trp metabolism, we brought information on its role in the regulation of inflammation through its initiators, starting with IDO, which exerts an insignificant effect on healthy and normal conditions. Things are changed by some cytokines, including interferons which represent the result of the triggered inflammatory process ( 176 ). The highly potent and amply used cytokine interferon-gamma (IFN-γ). It is linked to the promotor-region of IDO and it is capable to express itself in many types of cells. However, the highest expressive grade is found in dendritic cells and macrophages, but there are some other places where it was manifest such as epithelial and connective tissues ( 177 – 180 ). When it comes to cancer, infections, auto-immune diseases, or cardiovascular problems, the serum ratio Kyn/Trp may represent a marker for inflammation which is related to IDO activity ( 181 ). As we discussed before, inflammation and chronic immune tolerance are regulated by Trp biochemistry. This being affected by the ability of IDO to change the Kyn/Trp ratio. IDO-competent cells may trigger an anti-inflammatory action through the Kyn/Trp equilibrium, which has the ability to influence some immune signaling and certain metabolic pathways ( 182 ). The last step metabolite of the Kyn pathway is represented by the NAD +, which is known for initiating CD8 + and CD4 + lymphocytes programmed death ( 183 ). In tumor cells, an important step for metabolic reprogramming is represented by amino acids metabolism. Some authors suggest that, in the case of glioma, there is a strong link between the two because the metabolic amino acid pathway could be used as a predictor for survival as well as certain clinical characteristics ( 184 ). As we mentioned before, amino acids and their metabolites are responsible for both controlling malignant cells as well as for changing the microenvironment. In this way, the results are translated into the improvement of immunosuppression and malignancy state ( 185 ). Kynurenine metabolism is capable of stimulating an oxidative stress resistance pathway, and, in this way, creating an opportunity to make changes in the tumor microenvironment that helps the development of the tumor ( 186 ). However, another metabolite of tryptophan; 5-methoxytryptophan (5-MTP) has the ability to suppress the development of tumors and the displacement of cancer cells in other tissues. Wu et al. ( 187 ) consider that these effects are due to suppressing the activity of cyclooxygenase-2 (COX-2). This type of inflammation-associated enzyme is very abundant in tumor cells and also contributes to development process of cancer ( 187 ).
The role of cholesterol in the immune response
Cholesterol has a key function on cellular membranes functionality, especially in the plasma membrane of the cell where it is found at higher concentrations. Its special location at the lipid bilayer allows optimal interaction with other lipids and displays a significant role in membrane fluidity. Cholesterol points its structure mainly into the lipid bilayer leaving only the hydroxyl group facing the external environment. Thus, the steroid rings are in close vicinity to the hydrocarbon chains of adjacent lipids ( 188 ). Cholesterol is vital for the many physiological roles that the plasma membrane is involved. The cells keep their lipid bilayer appropriate functionality due to cholesterol molecules, otherwise, microenvironment, endocytosis, signaling pathways, as well as other functions, would be altered. Cholesterol is involved in membrane integrity and it is responsible for receptors arrangement and bilayer fluidity ( 189 , 190 ). For a better understanding of the information provided later-on, we will describe the cholesterol synthesis and pathway in a schematic frame. Cholesterol biosynthesis of is characterized by a complex pathway, nonetheless the pathways involved have been clearly elucidated ( 191 ). Its synthesis involves more than 20 metabolic-specific actions, which include enzymatic reactions belonging to the mevalonate pathway of and additional synthesis pathway of cholesterol. Enzymes involved in cholesterol biosynthesis are mainly detected in the membranes of the endoplasmic reticulum (ER). These enzymes are the target of several molecular reactions which, closely controlled in order to not allow cellular damage ( 191 , 192 ). However, cholesterol is non-uniformly disseminated in the plasmalemma. In the cell, the plasma membrane represents the main pool/storage. It has been observed that each pool is corresponded to an exclusive function in the plasma membrane physiology ( 193 – 195 ).
It is clear that cholesterol equilibrium involves a transport mechanism by virtue of the concentration gradient from high concentration cholesterol places to regions where cholesterol has been lost or has a low level. The transport of cholesterol is dependent on proteins due to its hydrophobic conformation, thus it cannot be transported through the blood. Thus cholesterol binds to different proteins and forms distinct lipoprotein compounds such as low-density (LDL) and high-density (HDL). As expected, regulatory mechanisms for the formation of each lipoprotein are specific ( 196 ). The surplus of cholesterol can be transported through the efflux process or deposited as intracellular lipid droplets ( 191 ) because of the incapacity of most human cells to efficiently degrade it. The deposition of lipid droplet plaques in the bloodstream causes the release of inflammatory cytokines which create later an inflammatory process. The consequence of this event is associated with inflammation triggered by the cytokine interleukin-1β (IL-1β) ( 197 ). Furthermore, IL-1β is considered an important marker in the inflammatory process ( 198 ).
The cholesterol signaling pathway plays a role in the immune response we therefore will highlight these pathways. Sterol response element-binding protein (SREBP) exerts an essential role in the signaling pathway of cholesterol ( Figure 3 ). Normally, these proteins are located in the membrane of the ER, which is capable of binding with additional two complex proteins such as the cleavage-activating protein and generating (SCAP) ( 199 ) and the insulin-inducible genes (INSIGs) ( 200 ). The shift of SCAP from ER to the Golgi apparatus plays a key role in its activity. SREBPs proteins are composed of three variants SREBP1a, SREBP1c, and SREBP2, being the latter the most important ( 199 ). SREBP2 is a protein complex structure that seems to be capable to regulate the expression of all the enzymes that are involved in cholesterol biosynthesis ( 201 ). Its most important activity is its specific response to high concentration of sterols which are able to efficiently induce a decrease in cholesterol synthesis. SREBP2 fulfills its function when the sterols concentration decrease. This change in sterol concentration due to SREBP2 activity will generate afterward the shifting the complex SCAP from ER to the Golgi apparatus ( 200 ). In this organelle, the SCAP molecule is changed ( 199 ). Once the SCAP reaches the Golgi apparatus, proteases sit 1 and 2 cut this complex ( Figure 3 ). As a result, the transcription factor (TF) is created and stimulated ( 202 ). Then the TF enters the nucleus where it is responsible for the regulation of the cholesterol synthetic pathway enzymes ( 203 ). Regarding immune-related mechanisms, SREBP2 may be activated in the immune cells’ T cell receptor (TCR) and B cell receptor (BCR) through their signaling pathways. All these pathways may stimulate the flux of cholesterol biosynthesis ( 204 , 205 ).

Schematic frame of cholesterol biosynthesis. In the signaling pathway of cholesterol, sterol response element-binding protein SREBP2 has an essential role. SREBP2 is located in RE, where it forms a complex with the protein like cleavage-activating protein and generating (SCAP); Its most important activity is to reduce the cellular cholesterol concentration when this is higher. Then, SCAP is shifted from ER to the Golgi apparatus. Once SCAP reaches the Golgi apparatus, proteases sit 1 and 2 digest this complex and subsequently, the transcription factor (TF) is formed and is activated ( 202 ). Then, TF moves into the nucleus where it becomes active and control the transcription of the enzymes of cholesterol biosynthetic pathway.
Additionally, it is important to mention that all cholesterol associated pathways involving synthesis, influx, efflux, and esterification take place through mechanisms closely related to each other allowing well-adjusted whole mechanistic biochemical pathways. All these tightly controlled mechanisms highlight the crucial role of cholesterol in life span, and clarify the potential risks when the concentrations are diverted from the optimal range. In this regards, Luo et al. ( 191 ) have demonstrated that there is a correlation between certain metabolic diseases such as familial hypercholesterolemia, Schnyder corneal disease and altered cholesterol metabolism ( 191 ). Moreover, in several diseases such as various types of cancer, infections and allergies, cholesterol biochemical equilibrium is severely altered through inflammation-associated consequences. Regarding the relationship between cholesterol and macrophages, counter-regulatory mechanisms oppose macrophage inflammation and at the same time cholesterol cellular accumulation. When the concentration of cellular cholesterol increases, specific sterols are formed. With their help, the transcription factors liver X receptor (LXR)–retinoid X receptor (RXR) are activated. These heterodimers have anti-inflammatory roles, including controlling the expression of ATP-binding cassette transporters (ABC transporters), which are ABC subfamily A member 1 (ABCA1) and ABCG1. They are also involved in stimulating the efflux of cholesterol from macrophages. In this way, they can suppress the activation of TLR signaling given by the increased intracellular cholesterol concentration. When TLRs are activated, LXR genes are inhibited, thus decreasing the cholesterol efflux from macrophages. Activation of cholesterol efflux by ABCA1 and ABCG1 is via apolipoprotein A1 (APOA1), forming HDL and initiating the process of transporting cholesterol back to the liver via blood vessels and lymphatics. Therefore, as a way of amplifying the inflammatory response, the immune system can alter cholesterol homeostasis ( 206 ). When the control of cholesterol biosynthesis is disturbed resulting in high cholesterol levels it can be translated into metabolic diseases such as atherosclerosis and dyslipidemia ( Figure 4 ). In some of these cases, both the innate and the adaptive immune functions have the ability to regulate this phenomenon ( 207 ). In this way, ApoB-containing lipoproteins are originated immediately after atherosclerosis damages. These are generated, developed and stored in the endothelial compartment ( 208 ). Interestingly, these molecules exert pro-inflammatory effects through acetylation, oxidation, and especially induce aggregation with additional molecules ( 209 ). The modifications provoked by the accumulation of ApoB-containing lipoproteins ( Figure 4 ) in endothelial location results in the growth of adherence, hold, and mobility in this place of immune cells ( 210 ). In summary, the inflammation is supported through the generation of ROS and certain cytokines such as (TNF) α, IL6, and (IL)1β ( 208 , 210 ). When it comes to the inflammatory stage, the Treg cells exert an anti-inflammatory effect through the inhibition action of CD8 + Th and CD4 + T cells. At the same time, Th17 and Th1 are involved in the pro-inflammatory process ( 209 , 211 ). Long-term inflammation ( Figure 4 ) and the development of atherosclerosis are strongly linked with the decline of the Treg/Th17 ratio ( 212 – 214 ).

Cholesterol rate in the modulation of immune function. Some diseases, such as cancer, infections, and allergies, are capable of modifying the cholesterol biochemical equilibrium through their own state of inflammation; Atherosclerosis and dyslipidemia are negative effects of the increased cholesterol biosynthesis; The ApoB-containing lipoproteins are accumulated in endothelial place; This phenomenon has pro-inflammatory effects; The inflammation is correlated with the generation of certain cytokines like (TNF) α, IL6, and (IL)1β; The Treg cells have an anti-inflammatory effect; Th17 cells are involved in the pro-inflammatory process; The decline of Treg/Th17 ratio is linked with long-term inflammation.
The polyunsaturated fatty acids effects on immunomodulation
Polyunsaturated fatty acids (PUFAs) are essential fatty acids that contain more than one double bond in their backbone. PUFAs are divided into two main groups: omega-3 and omega-6. The structural chemical difference between the two groups is represented by the location of the cis double bonds ( 215 ). Together with cholesterol, PUFAs are essential for cell membrane integrity, development and maintenance in the homeostasis of cell function. Moreover, they are used by certain structures in cells and they stimulate cell proliferation ( 216 ). Calder and Grimble demonstrated that the changes in fatty acids’ membrane composition can affect membrane fluidity, mostly due to their modification in the enzymes’ affinity for substrates, which can change the signaling pathways. In this way, the sensitivity of the immune function can be modified ( 217 ). The most representative polyunsaturated fatty acids are eicosapentaenoic acid (EPA), alpha-linolenic acid (ALA), and docosahexaenoic acid (DHA), all defined as omega-3 fatty acids ( 215 ). They are very intensively studied since they are involved in many essential vital activities and more interestingly in immunomodulation pathways. In addition, the ALA is important due to the fact that it is a precursor of other fatty acids ( 218 ). Omega-3 PUFAs have a role in immunomodulation by decreasing pro-inflammatory eicosanoids. They represent a substrate for AA cascade enzymes, in this way certain prostanoids and leukotrienes are produced. Some lipid mediators such as maresins have omega-3 PUFAs as precursors. They promote the ending of the inflammatory process ( 219 ). In human breast cancer cells ALA produce inhibition of cell proliferation and activate apoptosis ( 220 ). In diabetic rats, ALA increases insulin sensitivity and restored lipid and glucose metabolic abnormalities ( 221 ). ALA is considered essential because it cannot be synthesized by the human body. In these regards, from the omega-6 group, an essential is considered linoleic acid (LA). Following LA ingestion, this fatty acid is quickly converted into arachidonic acid (ARA), which is responsible for the fluidity as well as the flexibility of the cell membrane. Free ARA is involved in the modulation of ion channels, enzymes, and receptors through stimulation or suppression of their function ( 222 ). Free unesterified ARA exerts antitumoral activity in vitro as well in vivo . It can be used as an anti-cancer drug. ( 223 ). Moreover, ARA can cause the death of tumor cells through the suppression of proliferation determining in this way, the death via stimulation of neutral sphingomyelinase (nSMase) mechanism ( 224 ).
Omega-3, as well as omega-6, participate in immunomodulation. According to Simonetto et al. ( 225 ) they have antagonist effects. Omega-3 from the PUFAs group is involved in anti-inflammatory reactions through the inhibition of ARA from the membrane, which is the main precursor for pro-inflammatory eicosanoids ( 225 ). They are capable to modulate immune and inflammatory responses through intensity and duration. On the one hand, pro-inflammatory effects are linked to fever, vasodilatation and intensification of pain. On the other hand, they could have anti-inflammatory effects by blocking natural killer activity and lymphocyte proliferation. Also, they are capable to inhibit IL-6, IL-2, and TNF-α ( 217 ). However, most importantly is the ratio between the 2 groups of PUFAs. Simopoulos, tried to shade light in this regards and proposed that a low omega-6/omega-3 ratio in women is responsible for the decrease in breast cancer risk. She additionally concluded that a lower ratio is associated with a general decrease in very common chronic diseases in the Western society ( 226 ).
Conclusion and future perspectives
The nutrients which have major effects on normal immune cell function and immune homeostasis.
As mentioned throughout all this review, there is a strong and dynamic link between nutrition and immune function, as a direct consequence of the modulation of the immune function through the pro-inflammatory and anti-inflammatory effects of certain nutrients including cholesterol who exerts a crucial impact in these complex biological settings and holds a great capacity to regulate immune function, tightly related to its concentration. Certain micronutrients mentioned in this review: A, B1, B2, B3, B12, C, and D vitamins and minerals such as Zinc, and Selenium affect innate as well as adaptive immunity specifically through genetic, biochemical, and signaling pathways. All these may be translated into the modulation of proliferation, cell division, cell mobilization, and physiology of immune cells.
Additionally, we provide evidence that some macro-nutrients such as tryptophan, arginine, cholesterol and PUFAs may be involved in the prevention and therapy of some immune-related diseases. Also, is very important to note that some vitamins such as A and D are fat soluble ( 227 ). That is why when we consume fat-free (light) products, we are practically deprived of fat-soluble vitamins and the immune function can be affected. So, western diets should contain the accurate class of healthy fats, such as PUFAs, in a correct ratio, otherwise the edible products become poor in nutrients. A good example is the Mediterranean diet. In addition, the fats are much more satiating and give food a much better taste ( 228 ).
The nutrients implicated in inflammation and immunopathologies
We highlight the difference in response to micro- and macro nutrients between healthy and sick population. We provided evidence that the response in pathophysiologic stages are very different to normal physiologic stages additionally to interindividual variations. As a result, the immune response is different and variable ( 11 , 229 , 230 ). We also presented some evidences and speculations on the roles of some vitamins, as well as certain amino acids, in cancer patients, due to their involvement as cofactors in proliferation and energy-related pathways finally leading to the development of tumor cells. We foresee that further research needs to be done in order to clearly distinguish the possible oncogenic effects of thiamin, cobalamin, and arginine ( 58 , 66 ).
We additionally provide evidence that the inflammatory responses in general, and the changes in immune functions are modified by the lack of an accurate cholesterol metabolism. The alterations in the cholesterol biosynthetic pathway may have both positive and negative immune health-related repercussions. Alterations in the cholesterol biosynthetic pathway may directly impinge and interfere with antimicrobial responses, as well as with antiviral effects ( 192 ). Thus, an immediate action is required in order to adjust the cholesterol metabolism.
Perspectives on the role of nutrients in ameliorating severity of specific inflammatory diseases, autoimmunity
Moreover, we refer to the bioavailability of macro- and micro nutrients from food. We ask whether foods contain enough amounts of macro- and micro nutrients. Does the soil and then the foods today still have the same nutritional value as before for example in fruits and vegetables? These led us to question under what conditions can artificial supplementation with macro or micronutrients be done? And how should be done? Should they be taken alone or as a complex? The question is the synergistic and complementary action of taking supplements of vitamin complexes, results in a better or worst outcome? We surmise that the administration of nutrients (micro and macro) would exert distinct effects on each person. We know that each individual is different, and thus their immune responses will differ from each other. To add more complexity, we referred also to the absorption capability of nutrients in the different compartments of the digestive system. From all the information provided above we can establish that malnutrition and/or supplementation strongly affect the immune system.
We finally provided evidence that for each stage of the immune process, both micro and macronutrients are needed for the proper functioning of this important system.
Author contributions
BS and CM wrote the manuscript after a rigorous investigation, interpretation, systematization, and conceptualization of current data. Both authors agreed to publish the present manuscript, contributed to the article, and approved the submitted version.
This study was supported by funds from the National Research Development Projects to finance excellence (PFE)-14/2022-2024 granted by the Romanian Ministry of Research and Innovation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 11 March 2024
Effects of dietary intervention on human diseases: molecular mechanisms and therapeutic potential
- Yu-Ling Xiao ORCID: orcid.org/0000-0002-3684-0816 1 , 2 na1 ,
- Yue Gong 1 , 2 na1 ,
- Ying-Jia Qi ORCID: orcid.org/0009-0006-9878-4019 1 , 2 na1 ,
- Zhi-Ming Shao 1 , 2 &
- Yi-Zhou Jiang 1 , 2
Signal Transduction and Targeted Therapy volume 9 , Article number: 59 ( 2024 ) Cite this article
4027 Accesses
1 Citations
56 Altmetric
Metrics details
- Cancer imaging
- Cancer metabolism
Immunotherapy
Diet, serving as a vital source of nutrients, exerts a profound influence on human health and disease progression. Recently, dietary interventions have emerged as promising adjunctive treatment strategies not only for cancer but also for neurodegenerative diseases, autoimmune diseases, cardiovascular diseases, and metabolic disorders. These interventions have demonstrated substantial potential in modulating metabolism, disease trajectory, and therapeutic responses. Metabolic reprogramming is a hallmark of malignant progression, and a deeper understanding of this phenomenon in tumors and its effects on immune regulation is a significant challenge that impedes cancer eradication. Dietary intake, as a key environmental factor, can influence tumor metabolism. Emerging evidence indicates that dietary interventions might affect the nutrient availability in tumors, thereby increasing the efficacy of cancer treatments. However, the intricate interplay between dietary interventions and the pathogenesis of cancer and other diseases is complex. Despite encouraging results, the mechanisms underlying diet-based therapeutic strategies remain largely unexplored, often resulting in underutilization in disease management. In this review, we aim to illuminate the potential effects of various dietary interventions, including calorie restriction, fasting-mimicking diet, ketogenic diet, protein restriction diet, high-salt diet, high-fat diet, and high-fiber diet, on cancer and the aforementioned diseases. We explore the multifaceted impacts of these dietary interventions, encompassing their immunomodulatory effects, other biological impacts, and underlying molecular mechanisms. This review offers valuable insights into the potential application of these dietary interventions as adjunctive therapies in disease management.
Similar content being viewed by others

Fasting-mimicking diet causes hepatic and blood markers changes indicating reduced biological age and disease risk
Sebastian Brandhorst, Morgan E. Levine, … Valter D. Longo

Metformin and feeding increase levels of the appetite-suppressing metabolite Lac-Phe in humans
Barry Scott, Emily A. Day, … Lydia Lynch
Life expectancy can increase by up to 10 years following sustained shifts towards healthier diets in the United Kingdom
Lars T. Fadnes, Carlos Celis-Morales, … John C. Mathers
Introduction
Nutrients play a crucial role in regulating various physiological processes. 1 The main source of nutrients is usually considered to be diet. The quantity, quality, and composition of the food consumed, as well as the timing of meals, directly impact human health by influencing the availability of nutrients. 2 Although there have been advancements in understanding the link between diet and disease in recent years, there is still much to learn about how specific dietary components affect disease risk and prevention. 3
Epidemiological studies have linked various dietary patterns to cancer and other diseases. 4 For instance, diets high in saturated fats and sugars have been associated with an increased risk of cardiovascular diseases (CVD) and type 2 diabetes. 5 Conversely, diets rich in fiber, fruits, and vegetables are associated with a lower risk of these conditions. 6 Similarly, conditions such as osteoporosis and certain neurological disorders have also shown links to dietary patterns, highlighting the broad influence of diet on overall health. 7 , 8 In the context of cancer, increased consumption of alcohol and red or processed meat is associated with a heightened risk of cancer, whereas adherence to a Mediterranean dietary pattern—characterized by high intake of fruits, vegetables, whole grains, legumes, fish, and olive oil, along with moderate consumption of dairy products such as yogurt—may confer protective effects against carcinogenesis. 9 , 10 Similarly, a strong adherence to the plant-based Paleolithic diet and a Paleolithic-like lifestyle has been found to significantly reduce the risk of colorectal cancer (CRC), especially in individuals with a body mass index (BMI) less than 30. 11 Although many cancer patients are interested in using dietary intervention to improve cancer therapy outcomes or even using it as a key component of the therapeutic process, 12 there is currently no solid evidence showing that any nutrition-related regimen can be a primary treatment for cancer. 13 However, preclinical studies suggest that calorie and energy restrictions can hinder tumor growth and progression and increase the efficacy of chemotherapy and radiotherapy. 14 , 15 A rising number of clinical trials are exploring the impact of dietary interventions or nutritional supplements in conjunction with standard antitumor therapies, with some showing clinical benefits. 16 , 17
Diet is a crucial source of nutrients for tumors and has emerged as a key component in determining whole-body metabolism. 18 The nutrients in the tumor microenvironment (TME) largely regulate tumor cell and immune cell metabolism. 19 Recent evidence suggests that metabolic reprogramming, a crucial hallmark of cancer, involves several metabolic adaptations by tumor cells to sustain proliferation and metastasis in the TME. 19 , 20 , 21 The TME constitutes a multifaceted and dynamic ecosystem comprising an assortment of cell types, including tumor cells, immune cells, and stromal cells, in addition to components of the extracellular matrix. The interplay among these constituents, along with the challenging environmental conditions, exerts a significant influence on the growth trajectory and progression of tumors. 22 For example, oxygen levels within the TME can vary due to increased metabolic demand from rapidly proliferating tumor cells, resulting in low oxygen tension, known as hypoxia, in tissues. In addition, nutrient availability, including the availability of glucose, fatty acids, and amino acids, can vary within the TME, impacting metabolic processes and energy production. The accumulation of metabolic waste products and alterations in pH can further contribute to a hostile TME, which can impair immune function and promote tumor progression. 23 These factors, along with dynamic interactions within the TME, play crucial roles in influencing tumor proliferation and the effectiveness of antitumor immune responses. 24
As our understanding of the complex relationships between diet, metabolic reprogramming, and various diseases continues to evolve, it becomes increasingly evident that dietary components and patterns significantly influence disease risk, prevention, and progression. This review delves into the unique metabolic characteristics and nutrient availability of tumors. Furthermore, we investigate recent evidence and emerging trends concerning the effects of dietary interventions on both cancer and other diseases, underscoring the potential therapeutic benefits these dietary strategies may offer to a wide range of patients (Fig. 1 ).
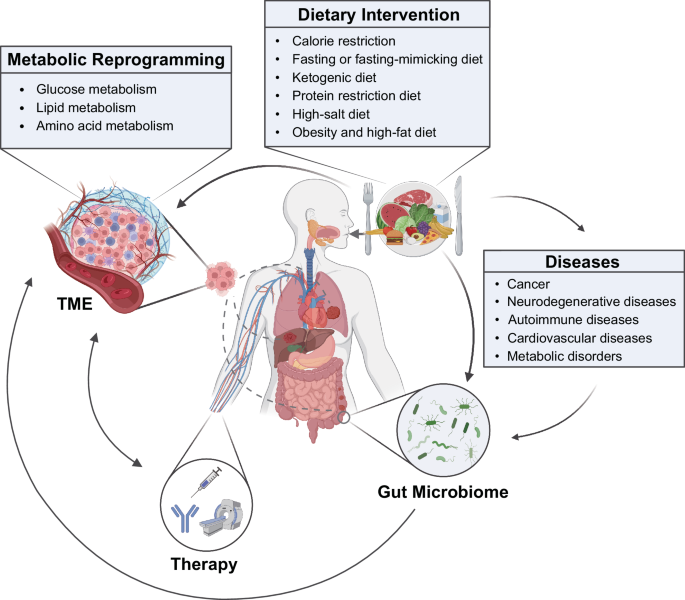
Overview of the relationship between dietary interventions and diseases. The cellular microenvironment, including the tumor microenvironment (TME), plays a crucial role in disease biology, and diet serves as a vital source of nutrients that can influence these microenvironments. Metabolic reprogramming, a prominent feature associated with disease progression, can affect cell metabolism and immune function. Dietary interventions, such as caloric restriction (CR), fasting-mimicking diet (FMD), and ketogenic diet (KD), can modulate the progression and treatment sensitivity of various diseases, including cancer. Additionally, dietary interventions can alter the composition and functional capacity of the gut microbiome, thereby indirectly influencing the progression and treatment of diseases. These direct and indirect effects of dietary interventions can influence metabolic reprogramming, modulate immune responses, and potentially enhance the clinical efficacy of treatments for various diseases. This figure was created with BioRender.com
Metabolic characteristics and nutrient availability in the tumor
Cellular metabolism encompasses a complex array of biochemical reactions that utilize specific nutrients, including carbohydrates, fatty acids, and amino acids. These nutrients are the primary sources for maintaining energy homeostasis and synthesizing macromolecules. 25 Our focus here is on cancer metabolism, which differs from that in corresponding healthy tissues in terms of nutrient levels and metabolic demands. 26 Within the TME, cancer cells can establish an immunosuppressive metabolic microenvironment by depriving immune cells of vital metabolites such as glucose and oxygen while also elevating the levels of mediators such as lactate and adenosine that limit the function of immune cells. 27 Therefore, different subsets of immune cells undergo metabolic reprogramming in tumors, and specific nutrients are required for these metabolic programs. 28 , 29 Generally, the metabolic programs that play vital roles in immune cells include glycolysis, the tricarboxylic acid (TCA) cycle, oxidative phosphorylation (OXPHOS), the pentose phosphate pathway (PPP), fatty acid oxidation (FAO), fatty acid synthesis (FAS) and the amino acid metabolic pathway 30 (Fig. 2 ).
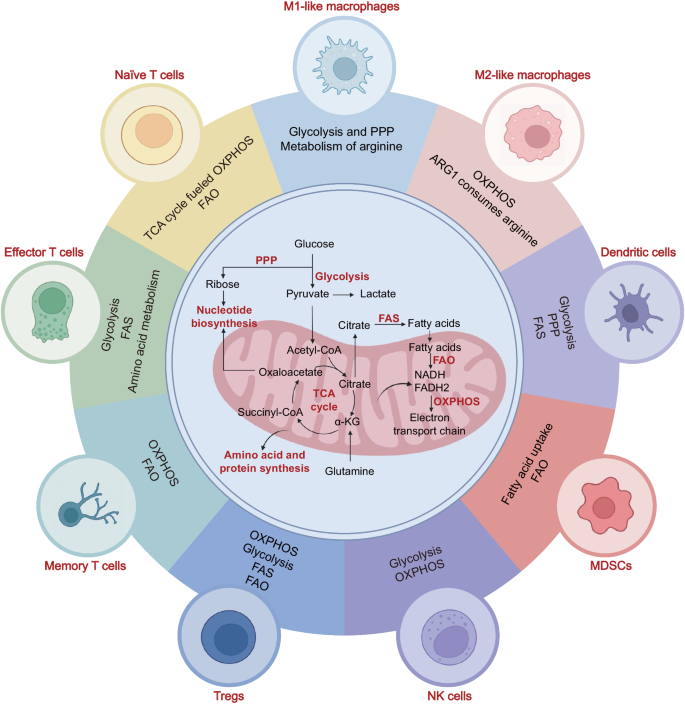
Major metabolic pathways associated with different immune cell subtypes within the tumor microenvironment (TME). Summary of the main metabolic pathways of immune cells, highlighting the distinctive metabolic characteristics and requirements of different subsets of immune cells. This figure was created with BioRender.com
Glucose metabolism
Glucose serves as a vital energy source, facilitating the functioning of immune cells. Once transported across the plasma membrane, glucose is metabolically processed via three distinct pathways: glycolysis, the PPP, and the TCA cycle. Glycolysis, which occurs in the cytosol, transforms glucose into pyruvate and lactate, simultaneously generating adenosine triphosphate (ATP). Under aerobic conditions, pyruvate is channeled into the TCA cycle, where OXPHOS occurs, yielding additional ATP. Moreover, glucose-6-phosphate, a derivative of glycolysis, fuels the PPP, culminating in the production of ribose-5-phosphate and nicotinamide adenine dinucleotide phosphate (NADPH). Recent research has indicated a marked disparity in energy consumption between immune cells in resting and activated states. 18 Although glycolysis does not generate as many ATP molecules as OXPHOS, glycolysis produces ATP more rapidly, which is important to metabolically active immune cells.
Cancer cells are characterized by their rapid proliferation, primarily fueled by the consumption of glucose as an energy source. Intriguingly, these cells continue to rely on glycolysis for energy production even in the presence of ample oxygen, a phenomenon referred to as the “Warburg effect”. 31 This unique phenomenon leads to glucose depletion and lactic acid (LA) accumulation in the microenvironment, ultimately inhibiting antitumor responses. 32 High glycolytic rates in triple-negative breast cancer cells promote the infiltration of myeloid-derived suppressor cells (MDSCs) and suppress T-cell function, while suppressing glycolysis inhibits tumor colony-stimulating factor (CSF) expression and MDSC development. 33 Cancer cells produce LA through glycolysis, which reduces the antitumor activity of CD8 + T cells and natural killer (NK) cells. However, the activation of LA metabolism pathways in regulatory T cells (Tregs) is increased, and these cells adapt to high-LA conditions. 34 , 35 Furthermore, cancer cells can take advantage of immune cells by utilizing their metabolic byproducts. LA can shift tumor-associated macrophages (TAMs) from a proinflammatory (M1-like) to an anti-inflammatory (M2-like) phenotype in the TME. Notably, lactate-activated TAMs enhance cancer cell adhesion, migration, invasion in vitro, and promote metastasis in vivo. 36
T cells play crucial roles in the TME. Upon activation, these cells undergo metabolic reprogramming, which subsequently yields diverse functional outcomes. Naïve T cells, which are metabolically quiescent, exhibit basic nutrient intake rates and low glycolysis rates. They primarily generate ATP through TCA cycle-fueled OXPHOS. 37 The activation of specific membrane receptors triggers the differentiation of naïve T cells into effector T cells, also known as T eff cells. This process is accompanied by a pronounced increase in both energy demand and biosynthetic activity within T eff cells. In T eff cells, the metabolic state is changed to increasingly rely on glycolysis, as these cells upregulate GLUT1, increase glucose intake. 38 , 39 , 40 , 41 Simultaneously, this metabolic alteration benefits T eff cells by reducing their reliance on oxygen for energy production, which enables them to maintain cytokine production and cytolytic activity even when they migrate into microenvironments within solid tumors that have low oxygen levels. 42 In contrast to naïve and T eff cells, memory T cells undergo a metabolic rewiring process that leads them to enter a quiescent state characterized by elevated OXPHOS rates compared to the glycolysis rate. 43 Tregs, known for their suppressive function, exhibit decreased glycolysis rates and primarily rely on OXPHOS to support their function, while glycolysis is crucial for their migration. 44 It has been reported that the Treg-specific transcription factor FOXP3 reprograms Treg metabolism by suppressing Myc expression and glycolysis while promoting OXPHOS and NAD(H) oxidation. This adaptation enables Tregs to be more adaptable to low-glucose and/or lactate-rich microenvironments. 45
There are several other types of cells within the TME that exhibit distinct metabolic functions. In the case of NK cells, glycolysis and OXPHOS play important roles in maintaining their cytotoxicity, as indicated by the inhibition of these processes leading to diminished expression of IFNγ and Fas ligands. 46 Researchers have shown that transcription factor-controlled glucose metabolism, specifically by sterol regulatory element-binding proteins (SREBPs), which conventionally control lipid synthesis, is essential for metabolic reprogramming in activated NK cells. 47 Dendritic cells (DCs), on the other hand, rely on glycolysis and the PPP for energy production to sustain their function, including cytokine production, antigen processing and presentation, and the stimulation of T cells. 48 Furthermore, different subsets of macrophages present distinct metabolic functions. M1-like macrophages predominantly utilize anabolic metabolism, specifically glycolysis and the PPP, to generate energy and synthesize cellular components, whereas M2-like macrophages are more reliant on OXPHOS, particularly through the enhancement of FAO. 49
Lipid metabolism
Lipids, such as fatty acids, triglycerides, cholesterol, phospholipids, and sphingolipids, play crucial roles as precursors to many important biological molecules. 50 Lipids, including substances such as cholesterol and fatty acids that are widely distributed in organelles, are key components of internal cellular membranes. Moreover, lipids are essential biological molecules that provide energy during nutrient deficiency, participate in the synthesis of complex fat-containing substances, and aid in cellular signal transmission as second messengers. 51 Lipids within the microenvironment profoundly influence the proliferation of cancer cells and regulate the functional activity of immune cells.
Cancer cells undergo metabolic reprogramming of lipids in the tumor niche. The activation of adipocytes triggers the lipolysis of stored triglycerides and secretion of fatty acids. Cancer cells can then take up these fatty acids to fulfill their lipid requirements for rapid growth. 52 Research has also demonstrated that ovarian cancer cells stimulate membrane cholesterol efflux from TAMs, fostering an environment that promotes tumor growth by enhancing interleukin (IL)-4-mediated reprogramming and suppressing IFNγ-induced gene expression. The deletion of ABC transporters, responsible for cholesterol efflux, reversed the tumor-promoting functions of TAMs, leading to reduced tumor progression. 53
Furthermore, elevated cholesterol levels in the microenvironment stimulate the expression of immune checkpoints, including PD-1, 2B4, TIM-3, and LAG-3, in T cells, driving T-cell exhaustion via the activation of the endoplasmic reticulum stress response. 54 In contrast to the negative effects of reprogramming T-cell lipid metabolism on antitumor immunity, the inhibition of ACAT1, a pivotal enzyme responsible for cholesterol esterification in CD8 + T cells, results in elevated cholesterol levels in the plasma membrane. This increase subsequently amplifies TCR signaling and promotes antitumor activity. These findings highlight the complex mechanisms through which cholesterol regulates T-cell function. 55
For efficient tumor antigen processing and presentation to T cells, activated DCs need high rates of cell surface or secretory protein biosynthesis, which is partly regulated by FAS-induced increases in cytokine production. 56 T eff cells depend mainly on FAS to support inflammatory cytokine secretion and proliferation, while naïve T cells and memory T cells maintain their basic functions by increasing the FAO rate. 57 , 58 , 59 Although T eff cells rely mainly on glycolysis for energy, CD8 + T cells that undergo enhanced FAO exhibit stable antitumor functions even under conditions of low glucose and oxygen levels. By promoting fatty acid catabolism, CD8 + T cells exhibit increased functionality, and the efficacy of immunotherapy in patients with melanoma can thus increase. 60
While these studies indicate a positive influence of lipids on the functionality and metabolism of CD8 + T cells in the TME, it is important to note that alterations to T-cell lipid metabolism might attenuate their antitumoral effects. In obesity-related breast cancer murine models, the activation of STAT3 triggered an increase in FAO in CD8 + T cells, which suppressed glycolysis and weakened their tumor-suppressing ability. 61 Moreover, enhanced lipid uptake and peroxidation can result in high oxidative stress, which leads to CD8 + T cell dysfunction. CD36, a fatty acid scavenger receptor, facilitates the incorporation of arachidonic acid into CD8 + T cells. This process subsequently triggers lipid peroxidation and ferroptosis, events that cumulatively attenuate the antitumor immune response and reduce the efficacy of immunotherapy. 62 , 63 , 64
Lipid metabolism also plays an active role in regulating Treg function. Fatty acid synthase (FASN)-mediated FAS contributes to the proliferation and maturation of Tregs, and FAO provides the energy crucial for Treg infiltration into the TME. 65 Research has shown that OX40 plays a role in modifying the lipid composition of Tregs, leading to the proliferation of OX40 + Tregs in the TME. This effect is achieved through increased FAS expression and glycolysis rate in Tregs. 66 CD36, via the peroxisome proliferator-activated receptor-β (PPAR) signaling pathway, maintains the mitochondrial fitness of Tregs, promoting Treg viability and inhibitory functions. 67 SREBPs have been found to show increased activity in Tregs that infiltrate tumors. Inhibiting FAS and metabolic signaling by targeting SREBPs has been shown to effectively activate the antitumor immune response without causing autoimmune toxicity. When the SREBP-SCAP axis was inhibited, in addition to tumor growth attenuation, immunotherapy effectiveness was boosted. These findings suggest that SREBPs may be promising targets for cancer therapy. 68
High expression of FASN in TAMs promotes the accumulation of fatty acids, leading to enhanced tumor immune tolerance via the FAO pathway. 69 Notably, lipid metabolism differs between M1-like and M2-like macrophages. M1-like macrophages prevalently engage the FAS pathway, while M2-like macrophages predominantly utilize the mitochondrial FAO pathway for their bioenergetic demands. 70 , 71 Receptor-interacting protein kinase 3 (RIPK3), which is crucial for necroptosis, is found to be diminished in hepatocellular carcinoma (HCC)-associated macrophages, leading to inhibited caspase1-mediated cleavage of PPAR, a process vital for enhancing fatty acid metabolism, including FAO. This metabolic shift results in increased accumulation and polarization of M2-like macrophages in the TME, contributing to accelerated HCC growth. 72
MDSCs also exert a substantial influence in suppressing antitumor immunity in the microenvironment, and they can be categorized into monocytic MDSCs (M-MDSCs) and granulocytic MDSCs (PMN-MDSCs). 73 Tumor-infiltrating MDSCs increase fatty acid uptake and induce FAO. 74 The accumulation of lipids in MDSCs increases oxidative metabolism, resulting in MDSC acquisition of an immunosuppressive and anti-inflammatory phenotype. 75
Amino acid metabolism
Amino acids are the primary substrates for protein biosynthesis, and recent evidence emphasizes the critical role of amino acid availability and metabolism in the regulation of antitumor immunity.
Glutamine is the most abundant amino acid and a crucial energy substrate, as well as an important nitrogen and carbon donor for various biosynthetic precursors. 76 T eff cells require higher levels of glutamine than naïve T cells due to their rapid proliferation and demand for sufficient raw materials for macromolecule synthesis and cytokine secretion. 77 Cancer cells have been shown to exhibit the highest glutamine uptake capacity and consume most of the glutamine in the microenvironment. 76 In turn, elevated glutamine consumption by cancer cells diminishes the glutamine supply necessary for T cells, consequently impeding the antitumor immune response. 78 In the microenvironment, cancer cells consume glutamine to synthesize γ-aminobutyric acid (GABA) via glutamate decarboxylase 1 (GAD1). By activating the GABA B receptor, GABA inhibits GSK-3β activity, which enhances β-catenin signaling, promoting cancer cell proliferation while suppressing intratumoral infiltration of CD8 + T cells. 79 Furthermore, elimination of glutaminase, a vital enzyme for glutamine metabolism, within tumor cells stimulates T-cell activation and augments the efficacy of antitumor immune responses. The compound V-9302, an inhibitor of the glutamine transporter, selectively impedes glutamine uptake in cancer cells while simultaneously enhancing both glutamine assimilation and glutathione synthesis in T eff cells, ultimately enhancing their function. 80
Tryptophan is another essential amino acid. Following its entry into eukaryotic cells via the transport proteins SLC1A5 or SLC7A5, tryptophan is primarily subjected to three primary metabolic pathways: incorporation into protein synthesis, metabolism via the kynurenine (Kyn) pathway, or conversion through the serotonin pathway. 81 Notably, a substantial fraction of tryptophan is directed through the Kyn pathway, culminating in the production of a suite of metabolites with significant physiological implications. 82 Tryptophan plays a crucial role in determining the strength and effectiveness of the T cell response by affecting its availability in the microenvironment. 83 However, within the tumor niche, cancer cells, MDSCs, TAMs, suppressive DCs, and cancer-associated fibroblasts, among other cell types, exhibit upregulated expression of indoleamine 2,3-dioxygenase (IDO), which metabolizes tryptophan into suppressive kynurenine to promote Tregs and suppress CD8 + T cell function. 84 , 85 , 86 Most cancer cells overexpress IDO, and the level of kynurenine in the microenvironment is associated with poor prognosis in multiple solid and hematological malignancies. 87 Kynurenine has been found to bind to the aryl hydrocarbon receptor (AHR) in naïve CD4 + T cells, which promotes Treg differentiation. 87
An additional metabolite generated through the Kyn pathway is the essential redox cofactor nicotinamide adenine dinucleotide (NAD+), a molecule of fundamental importance for the maintenance of cellular homeostasis. 88 In particular, cancer cells heavily depend on NAD+ to promote metabolic reprogramming and meet higher demands for ATP. Elevated NAD+ levels have been demonstrated to promote the proliferation of cancer cells. 89 Although the majority of studies suggest that an increase in NAD+ drives cellular proliferation, prior investigations have proposed that a decrease in NAD+ levels can lead to genomic instability, subsequently instigating liver tumorigenesis. 90 Moreover, tryptophan metabolism mediated by IDO affects not only the Kyn pathway but also other pathways, such as the purine, nicotinamide, and pyrimidine metabolism pathways, ultimately leading to decreased T-cell function. 91 In addition to IDO, another enzyme, tryptophan 2,3-dioxygenase (TDO), is involved in tryptophan catabolism. High TDO expression has been shown to impair T-cell antitumor immunity and to be correlated with poor clinical prognosis. Suppressing TDO expression can increase the antitumor efficacy of immune checkpoint inhibitors (ICIs). 92
In addition to the aforementioned amino acids, other amino acids play crucial roles in regulating tumor metabolism. T-cell proliferation relies heavily on arginine consumption. L-arginine supplementation has been shown to facilitate the metabolic shift from glycolysis to OXPHOS, enhancing T-cell survival and boosting antitumor responses of CD8 + tumor infiltrating lymphocytes (TILs). 93 Notably, the functional differences resulting from TAM polarization partially depend on arginine metabolism. In macrophages with the M1-like phenotype, arginine is converted into nitric oxide (NO) and citrulline via inducible nitric oxide synthase (iNOS), and this anabolic pathway is closely associated with macrophage cytotoxicity and antitumor effects. Conversely, in macrophages with the M2-like phenotype, arginine is hydrolyzed to yield ornithine and urea through arginase 1 (Arg1). 94 This metabolic shift affects arginine availability, which in turn impacts the activation and proliferation of T cells and NK cells, leading to immune suppression within the microenvironment. Notably, Arg1 expression in MDSCs contributes to arginine depletion in the microenvironment, further inhibiting T-cell antitumor function and reducing their survival. 95 , 96 In addition, depletion of cystine and cysteine is also linked to the immunosuppressive effect of MDSCs. T cells are unable to synthesize the essential amino acid cysteine from substances such as cystine or methionine, necessitating its import from external sources for their functionality. 97 MDSCs import cystine but do not release cysteine, thus the levels of cysteine in the microenvironment are regulated, inhibiting T-cell activation. 98 Asparagine is another amino acid that significantly boosts CD8 + T-cell activation and antitumor responses. Restricting dietary asparagine or inhibiting its uptake impaired T-cell activation and differentiation into memory-like cells. 99 Cancer cells consume higher levels of methionine due to increased expression of its transporter (SLC43A2), which inhibits methionine metabolism and function in CD8 + T cells by altering histone methylation patterns. 100
Organ-specific metabolic profiles
Understanding the metabolic differences between various organs is critical for developing targeted therapeutic strategies in cancer treatment. Each organ has unique metabolic demands and pathways that can be dysregulated in cancer, leading to distinct metabolic profiles for different types of tumors. 101 This organ-specific metabolic reprogramming plays a key role in cancer progression and survival, and its understanding could be leveraged for therapeutic benefits.
Consider primary brain tumors as an example. These tumors, often found nestled within the intricate neural networks of the brain, exhibit a remarkable metabolic flexibility. 102 They are known to express elevated levels or alternative isoforms of glycolytic enzymes, a trait that points towards a potential therapeutic opportunity. 103 Specifically, the therapeutic strategy of glucose deprivation could selectively starve brain tumor cells while sparing healthy neurons, which are capable of surviving on alternative fuels such as ketone bodies. 104 Similarly, HCC cells undergo a significant metabolic shift from glucose production (a state known as gluconeogenesis) to glucose usage. 105 HCC cells also exhibit a marked increase in amino acid metabolism, particularly in the metabolism of glutamine. 106 Additionally, studies have shown that HCC cells often exhibit abnormal lipid accumulation, increased FAS, and enhanced cholesterol metabolism. These changes contribute to the aggressive and metastatic behaviors of HCC. 107
Moreover, hormone-sensitive tissues such as the breast, endometrium, and prostate also exhibit significant metabolic fluctuations in response to hormone levels. 101 Hyperactivation of the PI3K pathway, a lipid kinase that promotes proliferation and nutrient uptake in response to growth signals, has been implicated in breast and endometrial cancers, providing a possible mechanism for hormonal therapy evasion. 107 This pathway could be a potential target for therapeutic interventions, particularly in hormone therapy-resistant cancers.
In summary, understanding organ-specific metabolic profiles and their dysregulation in cancer can open up new avenues for targeted cancer therapy. By exploiting these unique metabolic dependencies of tumors, more effective and personalized treatment strategies can be developed.
Targeted dietary interventions and mechanistic insights into their impact on cancer
Understanding the metabolic pathways of glucose, lipids, and amino acids lays a crucial foundation for exploring the effects of various dietary restrictions. Macronutrients, including carbohydrates, fats, and proteins, are the primary sources of energy for our bodies, and they each follow distinct metabolic pathways. By manipulating the relative intake of these macronutrients, we can influence the metabolic pathways they utilize and thereby exert control over our systemic metabolism. This concept forms the basis for various dietary restrictions and special diets, such as caloric restriction (CR), fasting or fasting-mimicking diet (FMD), ketogenic diet (KD), high-fat diet (HFD), or amino acid-defined diet. Moreover, high-salt diet (HSD), although not directly involving macronutrients, is noteworthy due to its potential impact on tumor biology. Therefore, an in-depth discussion on the role of HSD in cancer research and treatment is included in our exploration.
The connections between various dietary patterns and cancer risk are likely rooted in several biological mechanisms, such as inflammation and immune function; specific factors, such as the gut microbiota and their metabolites; unfavorable events, such as certain epigenetic changes and metabolic or hormonal disruptions; and stress, such as oxidative stress. 108 Alterations in dietary composition impact not only the availability of nutrients within tumor cells but also the surrounding microenvironment, thereby offering potential opportunities to impede tumor growth 109 (Table 1 ).
Calorie restriction
Effective CR is a dietary intervention that reduces energy intake by approximately 15–30% while maintaining a balanced proportion of macronutrients and preventing malnutrition. 110 CR has been shown to prolong life and reduce age-related diseases, including cancer, in experimental models. 111
Although the antitumor effect of CR has been confirmed, the underlying mechanism remains unclear. Nonetheless, it is believed that the tumor-inhibiting effect is partially mediated by several biological changes, such as increased apoptosis rates in cancer cells, decreased circulating blood glucose levels, inhibited insulin-like growth factor 1 (IGF-1) signaling, reduced insulin levels, and mediators that regulate metabolic pathway activation and inhibit angiogenesis. 112 In particular, controlling IGF-1 signal transduction is a critical component underlying the antitumor effects of CR. The IGF-1 signaling pathway is frequently activated in cancer cells, and it shifts metabolic resources toward growth and proliferation. Therefore, the reduction in IGF-1 levels in response to CR leads to attenuated tumor growth and progression. 113 The impact of CR on cancer is also interconnected with mutations and oncogenic pathways. A study showed that CR results in a reduction of insulin levels, thereby diminishing tumor PI3K signaling. 114 CR has also been found to suppress xenograft tumor growth by upregulating the aldolase A (ALDOA)/DNA-PK/p53 pathway, with ALDOA acting as a potential oncogene that can also activate the tumor suppressor p53. 115 Moreover, CR has been shown to modify the cancer stem cell (CSC) phenotype, reducing their carcinogenic and metastatic potential. Notably, in MMTV-ErbB2 transgenic mice, the CSC subpopulation was most affected by CR, as shown by a reduction of luminal cells (CD24 high /CD49f low ), putative mammary reconstituting unit subpopulations (CD24 high /CD49f high ) and luminal progenitor cells (CD61 high /CD49f high ). These effects were largely attributed to the concurrent inhibition of estrogen receptor and ErbB2 signaling. 116
CR has been shown to shape the TME in several ways, including through the specific reduction in the number of TAMs, increase in the formation of CD8 + cytotoxic T cells and memory T cells, and negative modulation of immunosuppressive Treg cell activity and immunosuppressive cytokine levels. 117 Additionally, CR promotes favorable changes in the immune signature, providing enhanced protection against tumor growth and metastasis, possibly in part by remodeling the TME. In mice, no impact of a CR diet was observed on the number of CD4 + or CD8 + cells in the TME; however, the cytotoxic killing potential of these cells was elevated. Notably, higher expression of CD103 + , a marker of crucial tissue-resident memory T cells that possess enhanced cytotoxic capacity and can contribute to tissue protection against tumor cell invasion, was found. Additionally, a downward trend in the frequency of Tregs was observed, and a significant reduction in the total number of MDSCs was detected. 118 Hence, it was concluded that CR not only inhibits cancer cell proliferation but also helps maintain antitumor immunity.
Furthermore, research has shown that fasting, CR, and caloric restriction mimetics (CRMs) can promote T-cell-mediated tumor cytotoxicity, alter NK cell function, and potentially trigger immunogenic cell death, thereby stimulating cancer immunosurveillance pathways. 119 CRMs are pharmacological agents or natural compounds that imitate the biochemical effects of CR by reducing the lysine acetylation rates of cellular proteins. 120 Examples of CRMs include hydroxycitrate (an inhibitor of ATP citrate lyase), spermidine (an inhibitor of EP300 acetyl transferase activity), and resveratrol (an activator of sirtuin-1 deacetylase activity). 121 Treatment with CRMs has been found to decrease the concentration of free IGF-1, promote autophagy in cancer cells, and improve the antitumor immune response, resulting in a reduction in tumor growth when combined with immunogenic chemotherapeutics. 119 CRM hydroxycitrate has been found to stimulate autophagy in U2OS osteosarcoma cells in vitro, thereby increasing antitumor immunosurveillance and reducing tumor mass in mice with autophagy-competent mutant KRAS-induced lung cancers. 122 Moreover, in vitro treatment with resveratrol inhibits mitochondrial respiration in breast cancer cell lines through a SIRT1-dependent mechanism, diminishes the expression of markers associated with breast CSCs, and promotes their differentiation. 123 Collectively, these findings suggest that CRMs may enhance antitumor immunosurveillance in preclinical models.
Moderate physical activity, energy restriction, and their combination can also affect tumor growth. In fact, the combined effects of moderate physical activity and 10% energy restriction (PA + ER) have been shown to significantly delay primary tumor growth, reduce spontaneous metastases, and prolong survival. These effects on tumor progression and survival are accompanied by beneficial changes in immune cell infiltrates within the microenvironment. Specifically, the PA + ER combination leads to an increase in the percentage of CD8 + T cells and a decrease in the percentage of total MDSCs and MDSC subsets within tumors. 124
Nevertheless, it is crucial to emphasize that there are established nutritional recommendations for cancer care, and the weight loss or reduction in protein intake often associated with CR may conflict with these guidelines. 125 These dietary practices could exacerbate the risk of malnutrition, sarcopenia, fatigue, delayed wound healing, and impaired immunity, particularly in cancer patients who are already at an increased age-associated risk for these conditions. 126 Therefore, while exploring dietary interventions for cancer treatment, the potential adverse effects on overall patient health and nutritional status must be carefully considered.
Fasting or fasting-mimicking diet
In addition to CR, alternative approaches such as intermittent fasting (IF), including short-term fasting (STF), intake of an FMD, and time-restricted feeding (TRF), which limits food consumption to a specific time window each day, are being condisered. 127 , 128 The term “fasting” has a broad definition, encompassing a range of eating patterns, including complete and voluntary deprivation of food with no restriction on drinking water. 129 An FMD is based on a regimen of low-calorie and low-protein foods that mimics the effects of fasting but induces fewer side effects. This approach retains the benefits of traditional fasting methods while minimizing their potential drawbacks. 130
Fasting or intake of an FMD can cause various metabolic changes, including alterations in the systemic levels of hormones and growth factors such as insulin, glucagon, growth hormone, IGF-1, glucocorticoids or adrenaline. 131 In response to these changes, normal cells activate protective mechanisms against stress and toxic insults, thereby reducing their metabolic requirements and cell division rate. On the other hand, because fasting or FMDs reduce tumor growth-promoting nutrients and factors, cancer cells struggle to manage metabolite deprivation and thus develop greater sensitivity to cancer therapies. 132 In obesity-driven postmenopausal cancer mouse models, TRF was shown to delay the onset of tumors and reduce lung metastasis. Moreover, TRF was found to increase systemic insulin sensitivity and decrease hyperinsulinemia. Importantly, TRF could also restore the circadian rhythm of gene expression within tumors while attenuating both tumor growth and insulin signal transduction. 133 Fasting can cause an “anti-Warburg effect” by reducing aerobic glycolysis and glutaminolysis while increasing OXPHOS uncoupled from ATP synthesis. 134 In cancer cells, OXPHOS increases reactive oxygen species (ROS) production and leads to oxidative stress, activation of p53 signaling and DNA damage, particularly when combined with chemotherapy or other cancer therapies. 135 Therefore, the unique metabolic vulnerabilities of cancer cells, which differ from those of normal cells, can be strategically targeted to develop novel and effective therapeutic interventions. According to a recent study, the combination of chemical treatment with an FMD reduces the expression of heme oxygenase-1 (HO-1), which is a stress-responsive enzyme that protects cancer cells against oxidative damage and apoptosis in vivo. Interestingly, this combination treatment resulted in upregulated HO-1 expression in normal cells. The downregulation of HO-1 production in cancer cells, in part, facilitated FMD-induced chemosensitization of cancer cells by boosting CD8 + TIL-dependent cytotoxicity, which was possibly facilitated by decreased Tregs. 136 A separate study conducted with mouse models of colon cancer indicated that alternate day fasting for 2 weeks triggered autophagy in cancer cells, which in turn downregulated CD73 expression. As a result, the production of immunosuppressive adenosine in cancer cells was reduced, ultimately preventing macrophages from acquiring an M2 immunosuppressive phenotype. 137
Clinical experiments have suggested that intake of an FMD can induce metabolic changes and increase antitumor immunity in cancer patients. In fact, the final outcomes of an FMD-treated clinical trial (NCT03340935) demonstrated that a severely calorie-restricted, five-day FMD regimen was well tolerated and resulted in substantial systemic metabolic changes in patients with different tumor types who were concurrently receiving antitumor therapies. 138 , 139 In another clinical trial called DigesT (NCT03454282), a five-day FMD regimen was found to broadly reshape intratumor immunity in breast cancer patients. Specifically, the FMD was shown to promote the infiltration of activated and cytotoxic immune cell populations, including total and activated intratumoral CD8 + T cells, M1-like macrophages, aDCs, and NK cells. These changes were paralleled by an increase in immune signatures associated with improved clinical outcomes in cancer patients. 138
Ketogenic diet
A KD comprises a high-fat component, very low carbohydrate levels, and low to moderate protein levels, as explained in a recent study. 140 A traditional KD is typically formulated at a 4:1 ratio of fat:carbohydrate plus protein. 141 In this classical formulation, 80–85% of calories are derived from fat, 10–15% from protein, and less than 5% from carbohydrates. 142 A KD is known to be effective at treating epilepsy, lowering glucose levels, and producing ketone bodies in vivo. 143 There is increasing evidence to support the use of KD as a potential tumor treatment or prevention method, either as a standalone approach or in combination with other medicines. 144
The Warburg effect indicates that lower intratumoral glucose levels can impede tumor growth, which can be achieved through pharmacological intervention and dietary changes such as a KD. Cancer cells, unable to utilize ketone bodies produced by KD for energy due to their aberrant mitochondrial function and diminished enzyme activity, can essentially be “starved” of glucose. Hence, KD emerges as a potentially promising strategy for cancer prevention. 145 One of the primary ways in which a KD potentially promotes potential anticancer effects is by increasing the levels of β-hydroxybutyrate (β-HB), which is the most abundant ketone body. 146 For instance, β-HB has been proven to inhibit CRC by activating the transcriptional regulator Hopx through the surface receptor Hcar2, thereby reducing the proliferation of colonic crypt cells and suppressing tumor growth. 147 Another antitumoral effect of KD is upregulating the expression of the circadian clock gene Per (Period) by activating AMPK and upregulating SIRT1 (Sirtuin1), resulting in enhanced apoptosis and growth delay in tumor cells. 148 KD also decreases insulin-regulated PI3K-Akt-mTOR signaling, which is overactivated in pancreatic neuroendocrine tumors (PanNETs), resulting in decreased blood glucose levels and a suppressive effect on the development and progression of PanNETs. 149
Emerging evidence suggests that a KD may be a valuable clinical tool to enhance T-cell-mediated antitumor immune responses. In vitro and in vivo studies have shown that KD intake markedly increased the specific responses of human T cells, resulting in enhanced CD4 + , CD8 + , and Treg capacity, as well as augmented T memory cell formation. Under conditions of KD intake, CD8 + T cells undergo metabolic reprogramming to rely on OXPHOS in response to increased ketone bodies, leading to enhanced cellular energy and respiratory reserve, potentially improving their functionality. 150 In addition, KD intake prevented the progression of colon tumors by inducing tumor cell oxidative stress, inhibiting MMP-9 expression, and promoting M2 to M1 TAM polarization. 151 In a mouse model of malignant glioma, KD feeding led to significantly enhanced innate and adaptive tumor-specific immune responses. Mice fed a KD showed increased cytokine production (IFNγ, TNF, and IL-2) and greater tumor-reactive CD8 + T-cell cytotoxicity. Moreover, the mice maintained on a KD presented with a higher number of immune cells and a higher ratio of CD4 + T cells to Tregs, while the functionality of the Tregs was weakened. Feeding mice with the KD resulted in a noteworthy decrease in the expression of immune inhibitory receptors (PD-1 and CTLA-4) on CD8 + TILs, as well as a reduction in the expression of inhibitory ligands (CD86 and PD-L1) on cancer cells. 152 These findings suggest that a KD has the potential to attenuate tumor-induced T-cell suppression by decreasing the population of cells susceptible to the inhibitory PD-1 pathway.
Although KD has shown various potential benefits to tumor patients with its promising effects of inhibiting tumor cell growth and activating immune response, there is still limitation in its clinical application owing to its inevitable side effects. 153 It should be considered that KD also presents some risks, as they are typically high in saturated fats and may lack a substantial amount of nutrients, specifically carbohydrates and dietary fiber, as well as micronutrients such as calcium, magnesium, potassium and vitamins A, B and B6. 154 , 155 According to a recent research, KD delayed tumor growth but meanwhile accelerated cachexia onset, therefore shortening survival in a mouse model of IL-6-producing cancer. Excitingly, the same research group found that applying dexamethasone during KD treatment might delay cachexia onset without affecting the inhibition of tumor growth, providing fundamental insight into reversing the limitations of the clinical application of KD. 156
Protein restriction diet
The prevailing notion suggests that high protein intake, particularly among individuals under the age of 65, potentially escalates the risk of overall and cancer-related mortality. 157 To establish a protein restriction diet, either dietary protein intake or the number of amino acids can be reduced. 140 Recent research has demonstrated that dietary protein restriction is linked with a reduced incidence of tumor occurrence and a decreased risk of mortality. 158
Dietary restriction of protein and certain amino acids, including serine, methionine, and branched-chain amino acids (BCAAs) such as leucine, isoleucine, and valine, has been shown to impede tumor growth. 159 One mechanism through which protein restriction may inhibit tumor growth is via the IGF-1 signaling pathway. In melanoma and breast cancer mouse models, it has been observed that mice fed a low-protein diet (4% kcal protein) exhibit reduced IGF-1 levels and slower tumor progression compared to those fed a high-protein diet (18% kcal protein). A low-protein diet has been associated with reduced IGF-1 levels in patients aged 50–65 years, subsequently decreasing their risk of death from cancer. Conversely, a low-protein diet has been linked with an increased mortality rate in older patients (aged 65 and above), suggesting that a life-stage-specific approach to protein intake could optimize healthspan and longevity. 157 Other potential mechanisms for cancer prevention that are mediated by protein restriction could involve mTOR signaling, amino acid metabolic programming, FGF21, and autophagy. 158 In addition to these general effects, specific dietary restrictions on certain amino acids, such as serine and glycine, have been associated with prolonged survival in mouse models of various tumor types. The mechanisms underlying this observed survival benefit could include the correction of abnormal cellular nucleotide, protein, and lipid synthesis; improved mitochondrial function; and changes in epigenetic modifications. 160 , 161
The antitumoral effect of a low-protein diet also hinges on promoting immunosurveillance against cancer, while the dietary restriction of amino acids may adversely affect the metabolic reprogramming of the TME in various ways. In multiple mouse models, reducing dietary methionine inhibited tumor growth and boosted antitumor immunity by increasing the quantity and cytotoxicity of tumor-infiltrating CD8 + T cells. 162 Moreover, restricted intake of dietary protein or methionine/cystine has been shown to modify the infiltration and tumoricidal capacity of TAMs, leading to a significant increase in tumor-infiltrating CD8 + T cells and a decrease in the number of infiltrating MDSCs. Mechanistically, a protein-restricted diet inhibited mTOR pathway activation and increased macrophage acquisition of an antitumor phenotype by increasing the number of macrophages undergoing polarization to the M1 type. 163 Macrophages might sense diet-derived cytosolic amino acids via the GTPase Rag, which subsequently regulates the expression of TFEB, TFE3 and mTORC1 when activated. 164 Furthermore, an isocaloric diet that moderately reduced protein intake (by 25%) was shown to trigger an unfolded protein response (UPR) that depended on IRE1α in cancer cells. The increase in UPR activation, in turn, led to an increase in the recruitment of CD8 + T cells and enhanced antitumor immunosurveillance. Notably, intake of a low-carbohydrate diet did not exert the same effect. 165 Although a low-protein isocaloric diet has been proven to reduce the concentration of amino acids in tumor tissues, it remains uncertain whether this reduction is limited to certain amino acids. Thus, further research is needed to explore the correlation between a low-protein isocaloric diet and the decrease in the levels of specific amino acids in tumors.
Interestingly, several studies have shown that high-protein diets may also benefit the restriction of tumor growth or clinical outcoming of cancer patients, which seem contradictory to the findings of the protein restriction diet discussed above. However, the underlying mechanisms are totally different. A high-protein diet increased the production of urinary urea in a tumor protein 53 (TP53)-mutated orthotopic bladder tumor mouse model, leading to the cascade modulation of ammonia in tumor cells, which induces tumor apoptosis. 166 These findings challenge the former hypothesis that high urinary urea concentrations caused by a high-protein diet might serve as a potential carcinogenic factor in the bladder, suggesting the urgent need for further investigation. 167 Applying a high-protein diet may improve the overall survival of older outpatients with advanced gastrointestinal cancer, which may improve the nutritional state of these patients with poor digestive system function. 168
Moreover, there have been efforts to develop a series of drugs that mimic amino acid restriction. One focus of researchers in the cancer therapy field has been on glutamine metabolism, as cancer cells rely heavily on glutamine. Glutaminase inhibitors, for instance, have been shown to decrease tumor burden. 169 , 170 The use of 6-diazo-5-L-oxo-norleucine (DON) promoted antitumor immunity by greatly favoring OXPHOS over glycolysis in CD8 + T cells while disrupting the metabolism of cancer cells. 171 Notably, DON showed the ability to significantly inhibit the generation and recruitment of MDSCs and to reprogram M2-like TAMs into proinflammatory TAMs, which increased tumor antigen cross-presentation to T cells and enhanced the efficacy of immune checkpoint blockade (ICB). 172 In addition, CB-839, which is considered the most effective glutaminase inhibitor, can be utilized alone or in combination with PD-1 inhibitors to treat solid or hematological malignancies. 173 , 174 , 175 As previously mentioned, IDO and TDO are tryptophan catabolism enzymes, and inhibitors of these enzymes have been developed and evaluated in various clinical trials. 176 For example, epacadostat is a novel compound that serves as an IDO1 inhibitor, suppressing systemic tryptophan catabolism. 177 Both in vitro and in vivo studies have demonstrated that epacadostat can reduce tumor growth and promote the proliferation of T cells and NK cells. 178 Furthermore, cyst(e)inase, a glutathione inhibitor that degrades cysteine and cystine, reduces tumor progression by elevating ROS levels and inducing tumor cell-selective ferroptosis. 179 , 180
High-salt diet
HSD has long been considered as a risk factor and trigger of malignancies. However, recent studies have provided new insights into the effect of sodium intake. As research continues, it is becoming increasingly clear that salt can accumulate in the interstitium and modulate immune cell differentiation, activation, and function through the effects of extracellular hypersalinity. 181 In addition, consumption of a HSD can lead to elevated tissue sodium concentrations and affect immune responses within microenvironments, ultimately impacting the development of immune-regulated diseases such as infections and cancer. 182
HSD, comprising 4% sodium chloride (NaCl), is recognized as a robust immunomodulator that is capable of eliciting a substantial inflammatory response. 183 Indeed, research has shown that high salt conditions can inhibit tumor growth by enhancing antitumor immunity, particularly through the modulation of MDSC functions. 184 According to a recent study, an HSD reduced the production of cytokines essential for the expansion of MDSCs and thus attenuated the accumulation of MDSCs within the tumor niche. As a result, the two primary types of MDSCs acquired different phenotypes: M-MDSCs differentiated into antitumor macrophages, and PMN-MDSCs adopted a proinflammatory phenotype, which led to the reactivation of T-cell antitumor functions. 185 Furthermore, a high salt level has been found to induce the transformation of anti-inflammatory Tregs into proinflammatory Th1 cells, which led to the secretion of the inflammatory cytokine IFNγ. 186 In another study, salt functioned as an adjuvant that enhanced the effectiveness of anti-PD-1 immunotherapy in tumor regression. Specifically, an HSD induces NK cell-mediated tumor immunity by suppressing PD-1 expression while increasing IFNγ levels and the serum hippurate concentration. Notably, hippurate is a microbial benzoate metabolism product that has been identified as a metabolic marker of effective PD-1 immunotherapy in responsive patients. 183 Although the major antitumoural effect of HSD is modulating immune cell function, mechanisms other than immunomodulation have also been discovered. For instance, HSD suppressed tumor growth and lung metastasis in a murine model of breast cancer, possibly by inducing hyperosmotic stress or through mimicking CR. 187
Nevertheless, despite the potential benefits of salt intake on cancer treatment effectiveness, high salt intake can also lead to the development of a proinflammatory state, which can negatively impact cancer outcomes. 188 High salt intake is a risk factor for various types of cancer in humans, including lung, testicular, bladder, renal cell, pancreatic, esophageal, and gastric cancer. 182 HSD has been shown to induce chronic inflammation, which may in turn incite continuous cell proliferation, DNA damage, or cancer transformation. However, whether there is a connection remains uncertain. 188 IL-17, specifically IL-17A, plays an important role in the mechanism of action of HSD. Evidence suggests that high salt intake can induce the differentiation of Th17 cells, a prominent source of IL-17A. 189 The overproduction of IL-17A can lead to inflammation and other immune responses that contribute to various pathologies. Furthermore, in the case of breast cancer, an HSD has been found to promote tumor progression and lung metastasis, increase the proportion of Th17 cells, and activate the MAPK/ERK signaling pathway in breast cancer cells through the secretion of IL-17F. The increase in the secreted IL-17F level results in the unregulated expression of protumor genes and the induced inflammatory responses, ultimately accelerating the proliferation, migration and invasion of breast tumors. 190 In addition, the combination of high NaCl concentrations with subeffective IL-17 has been proven to reduce reactive nitrogen and oxygen species (RNS/ROS) levels and enhance the growth of breast cancer cells. 191 , 192 Recent research has also demonstrated that intake of an HSD can disrupt the development and function of NK cells in mice. 193 Therefore, it can be concluded that dietary salt may exert dual effects on tumorigenesis, and the contradictory results obtained may be due to variations in the effects of high salt concentrations on tumors in different tissues and during different phases of tumor development.
Obesity and high-fat diet
Obesity, a serious health issue characterized by excessive body fat, is a known risk factor for multiple types of cancer. It can be induced or exacerbated by HFD, characterized by the consumption of foods rich in saturated fats and cholesterol. 194 Obesity can induce systemic metabolic disruptions within the body, leading to dyslipidemia, hypercholesterolemia, insulin resistance, alterations in hormone levels, and changes in the baseline inflammation status. 195 Conversely, a low-fat diet, typically associated with reduced total fat intake, can potentially lower the risk of certain types of cancer. 196 , 197 Given that both HFD and obesity are major factors influencing cancer risk, the forthcoming discussion will primarily focus on these aspects. By diving deeper into the mechanisms by which HFD and obesity affect cancer development and progression, we aim to provide a more comprehensive understanding of this intricate relationship.
Dietary obesity is associated with multiple factors related to cancer occurrence and exacerbation of immune suppression in tumor niches. 198 In the context of obesity, increased hepatic expression of the unconventional prefoldin RPB5 interactor (URI) has been shown to couple nutrient surplus with inflammation, leading to nonalcoholic steatohepatitis (NASH) and consequent HCC. This process involves URI-induced DNA damage in hepatocytes triggering Th17 lymphocyte-mediated inflammation, and subsequent IL-17A-induced adipose tissue neutrophil infiltration, which promotes insulin resistance and hepatic fat accumulation, thereby inducing NASH and HCC. 199 Notably, obesity also accelerates Helicobacter felis -induced gastric carcinogenesis by enhancing the trafficking of immature myeloid cells and the Th17 response. This exacerbates proinflammatory immune responses, characterized by cross-talk between inflamed gastric and adipose tissues, thereby contributing to a protumorigenic gastric microenvironment. 200
Diet-induced obesity has been shown to elevate nitric oxide (NO) production, which enhances tumor growth. This is primarily due to the recruitment of macrophages and the overexpression of inducible NO synthase as a result of HFD. 201 Additionally, in response to HFD intake, IL-6-mediated inflammation has been shown to accelerate prostate cancer tumor growth and increase the fraction of MDSCs and the M2/M1 macrophage ratio. 202 The effects of diet-induced obesity extend to the microenvironment of colitis-associated CRC. Here, diet-induced obesity has been shown to increase IL-6 expression and promote the polarization of macrophages into M2-like macrophages, enhancing the production of CC-chemokine-ligand (CCL) 20. CCL20 recruits CC-chemokine receptor 6 (CCR6)-expressing B cells and γδ T cells, ultimately leading to colitis-associated CRC progression. 203 In animal models of HFD-induced obesity, the infiltration rate of TAMs and the expression of cytokines in M2-like macrophages were increased, enhancing tumor growth and metastasis. However, ablation of VEGFR-1 signaling can reverse the abnormal TME associated with obesity and reprogram TAMs to promote their acquisition of the M1 phenotype. 204
The intake of an HFD has been shown to significantly increase the incidence of oral squamous cell carcinoma (OSCC) by expanding MDSCs within the local immune microenvironment. 205 Obesity induced by diet can also trigger the accumulation of PMN-MDSCs, leading to Fas/FasL-mediated apoptosis of tumor-infiltrating CD8 + T cells and causing resistance to immunotherapy in breast cancer treatment. 206 Obesity has been shown to suppress the infiltration and function of CD8 + T cells, which was linked to decreased chemokine production, reduced fatty acid availability, and alterations in amino acid metabolism. 207 , 208 Moreover, based on findings from mouse models, obesity reduced the number and function of CD4 + T cells in the TME of CRC, leading to a compromised antitumor response of both CD4 + and CD8 + T cells and ultimately accelerating disease progression. 209 Furthermore, considerable evidence shows that obesity-associated adipocytes in pancreatic ductal adenocarcinoma can secrete IL-1β to attract tumor-associated neutrophils (TANs), which subsequently activate pancreatic stellate cells and contribute to tumor growth. 210
HFD or diet-induced obesity may induce tumor metastasis. HFD has been proven to increase palmitate secretion from alveolar type 2 cells and nuclear factor-kappaB subunit p65 acetylation in the lung to prepare a premetastatic niche. 211 HFD-induced fatty liver may promote liver metastasis by facilitating the secretion of hepatocyte-derived extracellular vesicles (EVs), which transfer Yes-associated protein (YAP) signaling-regulating microRNAs, hence elevating nuclear YAP expression, CYR61 expression, and M2-like macrophage infiltration. 212 Another mechanism of HFD-induced liver metastasis is the upregulation of NOD-like receptor C4 (NLRC4), which further induces M2-like macrophage activation and IL-1β processing. An alteration from an indolent to a metastatic state may be stimulated by HFD-induced lipid accumulation in prostate tumors, the mechanism of which may be related to the sterol regulatory element-binding protein (SREBP)-related prometastatic lipogenic program. 213 In addition, it is widely acknowledged that the fatty acid receptor CD36 plays an important role in HFD-related metastasis promotion by enhancing the metastatic potential of CD36 + metastasis-initiating cells. 214 However, a recent study revealed that CD36 may prevent palmitate-induced lipotoxicity rather than facilitating HFD-driven metastasis, suggesting that further investigations of the dual effects of CD36 are needed. 215
An elevated cholesterol level is an obesity comorbidity, and studies suggest that the effects of obesity on cancer may be partly mediated by increased cholesterol levels. 216 In fact, a high-cholesterol diet (HCD) alone has been shown to promote macrophage infiltration and significantly enhance the growth of CRC tumors. 217 One mechanism by which HCD promotes CRC progression is through the inhibition of the CD8 + T-cell response. Specifically, macrophages with infiltration driven by HCD can secrete CCL5, which obstructs the activation of CD8 + T cells, thereby facilitating the evasion of immune system surveillance by CRC cells. 218 27-Hydroxycholesterol (27-HC) is a crucial mediator of the effects of dietary cholesterol on cancer metastasis. This oxysterol is synthesized through the action of the CYP27A1 enzyme and is present at high levels in the circulatory system. 219 Oxysterol has been shown to modulate the TME by recruiting immunosuppressive neutrophils to the metastatic niche, facilitating cancer progression. 220 However, some studies have reported conflicting findings regarding the effects of high serum cholesterol levels on cancer progression. For instance, one study showed that high serum levels of cholesterol attributed to HCD intake increased the accumulation of NK cells and promoted their effector functions to reduce the growth of liver tumors in mice. 221 However, further studies are needed to understand these conflicting findings.
In expanding on the relationship between HFD and tumor promotion, it is worth noting that the tumor-promoting effect of HFD is not universal and depends largely on the subtype of fatty acids involved. Mouse models of breast cancer developed comparable obesity levels from an HFD of either cocoa butter or fish oil. However, the consumption of the cocoa butter HFD, which is high in saturated fatty acids, led to faster mammary tumor growth and increased protumor macrophages and IL-10 expression while reducing B-cell and CD8 + T-cell infiltration. On the other hand, the fish oil HFD, which is rich in omega-3 fatty acids, disrupted the typical obesity-tumor growth link and reduced the number of protumor macrophages. 222 This effect of dietary omega-3 fatty acids is mediated by host GPR120 and has also been shown to inhibit prostate cancer. 223 Moreover, oleic acid (OA) and linoleic acid (LA) are the most common unsaturated fatty acids in dietary oils. While both an HFD rich in OA and an HFD rich in LA can similarly induce obesity in mice, a diet high in LA specifically encourages the growth of mammary tumors. Furthermore, an LA-rich HFD can impair antitumor T-cell responses via the induction of mitochondrial dysfunction. 224 Based on these findings, it appears that modulating dietary oil composition may constitute a promising strategy for enhancing immune function in both the prevention and treatment of obesity-associated cancers. By carefully selecting and balancing the types of fatty acids in HFDs, it may be possible to reduce the tumor-promoting effects of obesity while simultaneously increasing immune responses against tumors. Further research in this area may help to identify more precise dietary interventions that can ultimately improve outcomes for individuals at risk of developing obesity-associated cancers.
Potential role of dietary factors in cancer treatment
Recent studies have highlighted the pivotal influence of the TME on the efficacy of immunotherapy in cancer treatment. 225 Immunotherapy, recognized as a substantial advance in cancer treatment, has revolutionized the field of oncology by augmenting the body’s innate defenses to effectively target and eliminate malignant cells. 226 Various forms of cancer immunotherapy have been developed, including oncolytic virus therapies, cancer vaccines, cytokine therapies, adoptive cell transfer, and ICIs, all of which have shown promise in clinical practice. 227 Among these therapies, ICIs are perhaps the most important, as they are antibody-based drugs that can eliminate the influence of tumor-specific CD8 + T cells. 228 In particular, ICIs targeting PD-1 or its ligand PD-L1 have demonstrated notable clinical efficacy in the treatment of various advanced cancers. 229
Extensive research has been conducted to identify the effects of various dietary substances and patterns on tumor growth, metastasis and TME reprogramming, which has led to the consideration of nutritional intervention as a possible strategy for increasing the efficacy of tumor treatment 230 , 231 (Tables 2 , 3 ). The decline in T-cell functionality with aging, a widely documented phenomenon, is linked to a reduced efficacy of anti-OX40 immunotherapy in murine models. 232 CR not only preserves T-cell function but also improves the response of aged CD4 + T-cell populations to anti-OX40 therapy. 233 When used in combination with immunogenic cell death (ICD)-inducing chemotherapy and immunotherapy, CRMs potentially enhance the efficacy of cancer treatments through synergistic effects. 234 Preclinical studies have shown that STF, which serves as an adjunct to various cancer treatments, may bolster antitumor immunity by attenuating immunosuppressive conditions and amplifying CD8 + T-cell cytotoxicity. 235 For example, an experimental study of non-small cell lung cancer demonstrated that STF sensitized cancer cells to anti-PD-1 therapy. The antitumor efficacy of combination therapy was achieved by inhibiting IGF-1-IGF-1R signaling in cancer cells, boosting the intratumoral CD8 cell: Treg ratio in the TME. 132 Furthermore, intake of an FMD has been shown to enhance the effectiveness of immunotherapy against triple-negative breast cancer with low immunogenicity by affecting the TME. Specifically, intake of an FMD has been shown to reactivate T eff cells that underwent early exhaustion, shift cancer metabolism from glycolytic to OXPHOS, and reduce the collagen deposition rate. 236 These effects led to the increased efficacy of anti-PD-L1 and anti-OX40 immunotherapy. These results suggest that combining immunotherapy with dietary restriction may lead to profound synergistic effects.
KD also enhances the antitumor effects of PD-1 blockade alone or in combination with anti-CTLA-4 antibodies. Mechanistically, the principal ketone body 3-hydroxybutyrate (3HB) in a KD prevented the ICB-mediated upregulation of PD-L1 on myeloid cells while simultaneously promoting the expansion of CXCR3 + T cells. 237 Similarly, KD enhanced the effectiveness of anti-CTLA-4 immunotherapy by reducing PD-L1 protein levels and augmenting the expression of interferons and antigen presentation-related genes. When combined with immunotherapy, the intake of a KD can reshape the TME by increasing the population of CD8 + TILs, macrophages and CD86 + DCs. Mechanistically, the activation of AMPK via KD intake is the key molecular event that promotes immunotherapy efficacy. This activated AMPK phosphorylates PD-L1 on Ser283, which interrupts its association with CMTM4 and results in PD-L1 degradation. Furthermore, AMPK phosphorylates EZH2, which impedes polycomb repressive complex 2 (PRC2), leading to an increase in interferons and antigen-presenting gene expression. 238
Combining a protein-restricted diet with a vaccine or anti-PD-1 therapy has been shown to significantly inhibit tumor growth and prolong survival. 239 Notably, treatment with a methionine-/cystine-restricted diet significantly increased the number of tumor-infiltrating CD8 + T cells and cytotoxic granzyme B + CD8 + T cells, which was further enhanced when combined with immunotherapy. 163 Another study confirmed the inhibitory effect of dietary methionine restriction on tumor growth and its ability to synergize with PD-1 blockers to increase tumor control. Mechanistically, this dietary approach reduced the number of metabolites, such as S-adenosylmethionine (SAM), which controls N6-methyladenosine (m6A) methylation reactions, in cancer cells. A reduction in the SAM level altered the m6A modification rate and decreased the expression of PD-L1 and V-domain Ig suppressor of T-cell activation (VISTA) in cancer cells. 162 Moreover, the enzyme cyst(e)inase breaks down cystine and cysteine, thereby bolstering T-cell-mediated antitumor immunity and inducing ferroptosis in tumor cells when combined with PD-L1 blockade. 240 IDO1 is a critical enzyme in the tryptophan–kynurenine pathway and has been identified as a promising immunomodulatory target. 241 A phase 1/2 (ECHO-202/KEYNOTE-037) trial evaluating the effectiveness of the IDO1 inhibitor epacadostat combined with pembrolizumab on advanced solid tumors showed a high objective response rate (ORR) of 40.3% overall and 61.9% in malignant melanoma patients, demonstrating promising antitumor efficacy. 242 Unfortunately, phase 3 trials failed to confirm these benefits. The ECHO-301/KEYNOTE-252 trial showed that combining epacadostat with pembrolizumab failed to prolong progression-free survival (PFS) or overall survival (OS) compared to pembrolizumab alone in patients with advanced melanoma. 243
Despite being linked to T-cell dysfunction and poor cancer prognosis, obesity has paradoxically been shown to enhance the response to anti-PD-1/PD-L1 immunotherapy. 244 Recent research suggests that immunotherapy yielded superior outcomes in obese patients, evidenced by an improved response rate and extended PFS and OS, in comparison to lean patients. 245 However, obesity also promoted tumor growth and T-cell exhaustion, leading to increased PD-1 expression and dysfunction, partly due to high leptin levels. Despite this outcome, PD-1-mediated T-cell dysfunction in individuals with obesity was found to significantly enhance tumor responsiveness to PD-1/PD-L1 inhibitors, as confirmed by preclinical and clinical data. 246 Therefore, obesity seems to be a double-edged sword for cancer immunotherapy, and the underlying mechanisms remain unclear and require further investigation.
Chemotherapy
Chemotherapy, a cornerstone of traditional cancer treatment, employs drugs to destroy rapidly dividing cells, a defining characteristic of cancer. 247 Despite its widespread use and undeniable efficacy in many cases, chemotherapy often has substantial side effects due to its impact on healthy cells. 248 Additionally, individual responses to chemotherapy can vary greatly and are influenced by a multitude of factors, including genetics, tumor characteristics, and, intriguingly, diet. 15 A growing body of research now highlights the role of dietary interventions in modulating the effectiveness of chemotherapy, emphasizing the need to further understand these interactions for improved therapeutic outcomes.
Due to their expression of oncogenes, cancer cells are more susceptible to the effects of fasting and CR than are normal cells, an effect termed ‘differential stress resistance’. 14 , 249 , 250 Based on this characteristic, CRM hydroxycitrate has been shown to increase sensitivity to chemotherapy by eliciting an adaptive cellular immune response, resulting in a decrease in the number of tumor-infiltrating Tregs into the tumor niche in various tumor models. 122
Emerging research also suggests a profound influence of fasting or FMD on the efficacy of chemotherapy. In vitro studies indicate that fasting cycles not only retard tumor growth but also sensitize a wide array of cancer cell types to chemotherapy. 14 This heightened sensitivity has been observed in various contexts, including the enhancement of gemcitabine efficacy in mice with prostate cancer xenografts and the increased efficacy of chemotherapy in triple-negative breast cancer via the upregulation of ROS. 251 , 252 FMD combined with vitamin C can potentially increase the effectiveness of chemotherapy for treating KRAS-mutant cancer cells by reversing the vitamin C-induced upregulation of HO-1 and ferritin. 253 Furthermore, when combined with a ferroptosis inducer, FMD can effectively eliminate slow-cycling, chemotherapy-resistant cells, suggesting a potential strategy for enhancing the sensitivity of certain difficult-to-treat cancers to chemotherapy through dietary interventions. 254 Interestingly, fasting can also counteract certain adverse effects of chemotherapy. For instance, it has been demonstrated to enhance self-renewal in hematopoietic stem cells and mitigate the immunosuppression induced by cyclophosphamide chemotherapy in mice. 255 In tumor-bearing mice, both prolonged fasting and FMDs can induce specific stress resistance responses, enhancing chemotoxicity in cancer cells while protecting normal cells. 256 This dual action is partly mediated by the reduction in IGF-1 and glucose levels, thus shielding normal cells and organs from chemical toxicity. 250 The potential of FMD in clinical settings has been supported by the ‘DIRECT’ study involving HER2-negative stage II/III breast cancer patients. This study revealed that treatment with FMD, administered three days prior to and during neoadjuvant chemotherapy, enhanced therapeutic efficacy without increasing toxicity or reducing chemotherapy-induced DNA damage in T cells. 257 Collectively, these findings highlight the potential of fasting and FMD as adjuncts to chemotherapy, warranting further exploration and clinical testing.
In addition to slowing tumor growth, KD also sensitizes tumor cells to classic chemotherapy. For example, the combination of KD with metronomic cyclophosphamide significantly enhances antitumor effects, resulting in the regression of neuroblastoma tumors. 258 , 259 Similarly, in pancreatic cancer, cotreatment with KD and cytotoxic chemotherapy substantially elevates tumor NADH levels, synergistically suppressing tumor growth and tripling survival benefits compared to chemotherapy alone. 260
Radiotherapy
Dietary interventions have emerged as promising strategies for enhancing the efficacy of radiotherapy in cancer treatment. For instance, CR combined with radiotherapy, has been shown to modulate the TME in a triple-negative breast cancer model by decreasing the number of intratumoral Tregs, increasing the CD8 + cell: Treg ratio, and upregulating PD-1 expression on CD8 + T cells. Furthermore, compared with patients who received radiotherapy alone, breast cancer patients who underwent CR concurrently with radiotherapy exhibited a significant reduction in the serum levels of immunosuppressive cytokines, suggesting potential benefits of CR in mitigating radiation-induced immunosuppression. 261
When combined with radiation or radiochemotherapy, KD slows tumor growth in lung cancer xenografts, potentially through a mechanism involving increased oxidative stress. 262 Additionally, KD was shown to enhance radiation sensitivity in a pancreatic cancer xenograft model, suggesting potential improvements in therapeutic outcomes. However, phase 1 clinical trials in patients with locally advanced non-small cell lung cancer and pancreatic cancer showed suboptimal compliance with the diet, indicating challenges in practical application. 263
Moreover, other dietary restrictions, such as methionine deprivation, have shown promising results in enhancing the efficacy of radiation and antimetabolite chemotherapy. In patient-derived xenograft and autochthonous tumor mouse models, methionine restriction sensitized tumor cells to these treatments, possibly via alterations in one-carbon metabolism. 264
Other therapies
In hormone receptor-positive breast cancer mouse models, periodic fasting or an FMD can enhance the therapeutic effects of endocrine agents such as tamoxifen and fulvestrant. This enhancement is believed to occur through a reduction in circulating IGF1, insulin, and leptin levels and suppression of AKT-mTOR signaling. Concurrent administration of these dietary strategies with a therapeutic regimen of fulvestrant and palbociclib has been associated with prolonged tumor regression and reversal of treatment resistance. Analogous metabolic alterations found in patients on an FMD during estrogen therapy suggest the potential of diet as an adjuvant in treating hormone receptor-positive breast cancer. 265
In addition to their effects on hormone-driven cancers, fasting or FMD has also been shown to enhance the efficacy of tyrosine kinase inhibitors (TKIs) across different cancer cell lines. Mechanistically, these effects are attributed to the increased ability of TKIs to block cancer cell growth and inhibit the MAPK signaling pathway under starvation conditions. 266 Another study reported that in HCC cells, xenografts, and patient-derived organoids, fasting improved the therapeutic response to sorafenib through the regulation of glucose transporters and proapoptotic protein expression by p53. 267
KD has also shown promise in supporting the effectiveness of phosphatidylinositol 3 kinase (PI3K) inhibitors and overcoming drug resistance in various mouse cancer models, including pancreatic, bladder, endometrial, and breast cancer models, as well as acute myeloid leukemia. 145 KD appears to enhance this effectiveness by decreasing hyperglycemia and reducing insulin secretion, actions correlated with a decrease in mTORC1 signaling within the tumor. 268
Finally, the combination of serine deprivation and biguanide treatment, such as phenformin and metformin, can lead to metabolic stress in cancer cells. This stress arises from the forced upregulation of glycolysis due to the biguanide-induced reduction in OXPHOS. Under conditions of serine deficiency, this stress may exceed the metabolic flexibility of cancer cells, leading to their potential death and, consequently, enhanced anticancer effects. 269
In summary, these findings underscore the potential of dietary interventions to modulate the therapeutic landscape of cancer treatment, enhancing the effectiveness of drugs and potentially overcoming resistance mechanisms. However, it should be viewed with cautious optimism. The biological plausibility of diet modifying treatment efficacy and resistance is compelling; however, the translation of this concept into clinical practice requires rigorous validation. It is critical to remain grounded in evidence-based medicine, recognizing that dietary strategies are adjuncts, not replacements, for established therapeutic regimens. Further exploration and clinical validation are necessary to fully understand these interactions and to integrate dietary strategies into standard cancer care effectively and safely.
Diet changes the gut microbiome in conjunction with antitumor effects and cancer treatment
The gut microbiome encompasses the genetic makeup of all species within the gut, such as bacteria, viruses, yeasts, protozoans, fungi, and archaea, and can be affected by a range of internal and external factors. 270 The gut microbiota plays a significant role in influencing the health and disease status of the host. The constituents of the gut microbiome and their interactions with the host immune system can impact the development of tumors and carcinogenesis. 271 Various dietary patterns have been found to significantly influence the composition and functionality of the gut microbiome. 272 , 273 It is through these changes in the gut microbiome that dietary patterns can indirectly influence the outcomes of cancer patients. 274
In recent early studies, several interventional strategies, ranging from dietary interventions to fecal microbiome transplant (FMT) and prebiotic, probiotic and antibiotic treatments, have shown promise in altering the composition or functional capacity of the gut microbiome. 275 Two prospective cohort studies have suggested that diet-related inflammation can alter the gut microbiome, leading to the development of CRC by suppressing adaptive antitumor immune responses. 276 , 277 Other prospective cohort studies have revealed the associations between prudent diets (rich in whole grains and dietary fiber) and Western diets (rich in red and processed meat, refined grains, and desserts) with CRC risk and indicated that the effect of these diets may differ based on the presence of Fusobacterium nucleatum in tumor tissue. 278 , 279 Specifically, these studies showed that, compared with a Western diet, adhering to a long-term prudent diet is associated with a reduced risk of F. nucleatum -positive CRC; however, it does not appear to mitigate the risk of F. nucleatum -negative CRC. 278 A recent study investigated the impact of the gut microbiota and dietary patterns on the response to ICIs in patients with melanoma. The present study revealed that patients with microbiomes dominated by the Ruminococcaceae family had greater response rates than did those with microbiomes dominated by the Bacteroidaceae family. Furthermore, another finding revealed that a poor response was associated with decreased intake of fiber and omega-3 fatty acids. 280 These results suggest that dietary interventions may be promising for improving cancer treatment outcomes.
Accumulating data suggest that alterations in the gut microbiome primarily contribute to the progression, prognosis, and treatment of cancer, primarily through interactions with the immune system. Metabolites produced by the microbiota play important roles in modulating antitumor immunity. 281 , 282 Microbiota-derived metabolites have been demonstrated to influence the efficacy of tumor immunotherapy. Short-chain fatty acids (SCFAs) are produced primarily by the fermentation of nondigestible carbohydrates, such as dietary fiber, by the microbiota. The main SCFAs include acetate, propionate, and butyrate. 283 , 284 The gut microbiota, which is mediated by SCFAs, can potentiate the antitumor activity of CD8 + T cells, thereby influencing the efficacy of tumor immunotherapy both in vitro and in vivo. 285 Metabolic and epigenetic reprogramming enables pentanoate and butyrate to enhance the effectiveness of cancer immunotherapy by boosting the antitumor activity of antigen-specific cytotoxic T lymphocytes and ROR1-targeting chimeric antigen receptor (CAR)-T cells. 286 Inosine is another important metabolite produced by the microbiome and is closely associated with immunotherapy. Intestinal Bifidobacterium pseudolongum promoted Th1 cell transcriptional differentiation and antitumor activity to increase the efficacy of immunotherapy, mainly through the action of inosine. 287 Inosine is instrumental in enhancing antitumor therapy by serving as a carbon source for CD8 + T cells in glucose-restricted microenvironments, facilitating their growth and optimal functioning. 288 Moreover, engineered bacteria can modify the concentration of metabolites in the microenvironment, thereby altering the composition of the TME. For instance, the genetically engineered probiotic strain Escherichia coli Nissle 1917 colonizes tumor sites and continuously converts ammonia metabolites into L-arginine. When injected into the tumor, this strain has been shown to increase the concentration of L-arginine within the microenvironment, leading to increased infiltration of tumor-infiltrating T cells, sustained effector T-cell functions, increased tumor-specific T-cell memory formation, and enhanced efficacy of PD-L1-blocking antibodies. 289
Recent research has highlighted the role of the gut microbiota in the antitumor effects of dietary intervention (Fig. 3 ). Specifically, enrichment of Bifidobacterium bifidum after CR increases acetate levels, which in turn elevates IFNγ + CD8 + T cells in the TME. In contrast, the antitumor effect of IF was not mediated by the gut microbiome, as it was not abrogated after the microbiota was depleted. 290 Similarly, recent studies have revealed that KD significantly influences the gut microbiota, inducing a shift from a population dominated by tolerogenic bacteria ( Lactobacilli spp., Clostridium asparagiforme ) toward a population dominated by an increase in immunogenic bacteria (such as Akkermansia muciniphila ). 237 It has been reported that a shift in the gut microbiota is partially attributable to the host’s production of ketone bodies due to the intake of a KD. Among these ketone bodies, β-HB selectively suppresses the proliferation of Bifidobacterium . This suppression subsequently leads to a reduction in intestinal Th17 immune cells. 291 Dietary methionine/cystine restriction has been shown to alter the gut microbiota and potentially contribute to immune system alterations. Specifically, this type of diet restriction promoted a significant decrease in the relative abundance of multiple Ruminococcaceae and Prevotellaceae families while increasing the presence of members of the Lactobacillaceae family. 163 Consumption of an HSD promotes an increase in the abundance of Bifidobacterium , which, due to enhanced gut permeability, infiltrates tumors, subsequently augmenting the functionality of NK cells and ultimately contributing to tumor regression. These results suggest that HSD intake modulates the gut microbiome, which may stimulate NK cell-dependent tumor immunity, thereby providing potential implications for the development of novel therapeutic interventions. 183 The intake of HSD has also been shown to inhibit enterotoxigenic Bacteroides fragilis (ETBF)-promoted colon carcinogenesis by decreasing the expression of IL-17A and iNOS, thereby inhibiting inflammation. 292 However, intake of an HSD can exacerbate Helicobacter pylori infection, contributing to gastric carcinogenesis. 293 In a mouse model of Barrett’s esophagus, feeding an HFD was observed to promote dysplasia and carcinogenesis by modulating the esophageal microenvironment and gut microbiome, thereby inducing inflammation and promoting stem cell proliferation. 294 The bile salt hydrolase (BSH) enzyme expressed by Bacteroides was also found to play a crucial role in CRC progression in overweight patients and in model mice with HFD-induced CRC. High BSH activity activates the β-catenin/CCL28 axis, resulting in an increase in immunosuppressive Tregs and accelerated CRC progression. 295 Moreover, HFD feeding can reduce the level of SCFA-producing bacteria and the rate of SCFA production, leading to decreased levels of SCFAs that can activate the MCP-1/CCR2 axis. This effect promotes M2 TAM recruitment and polarization, ultimately contributing to CRC progression. 296
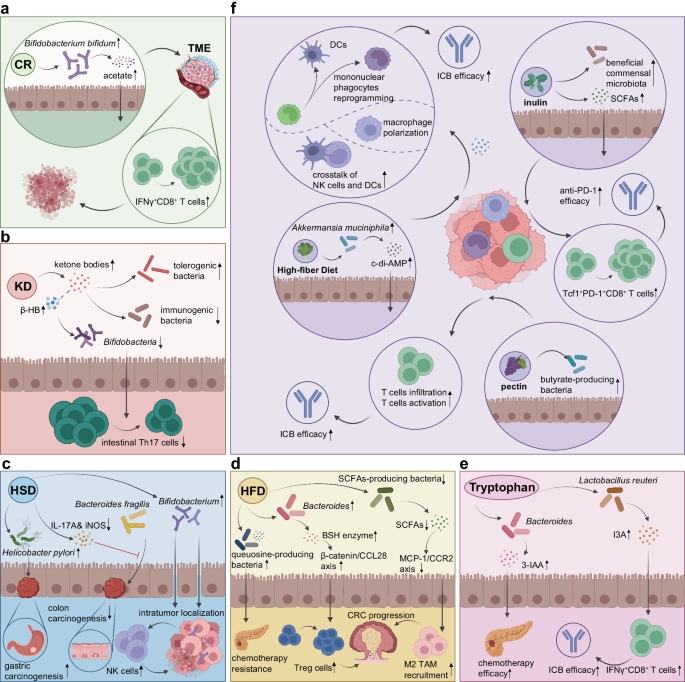
Mechanisms by which diet modulates antitumor effects and cancer treatment via modulation of the gut microbiome. a Calorie restriction (CR) elevates IFNγ + CD8 + T cells in the tumor microenvironment (TME) by enriching Bifidobacterium bifidum and increasing acetate levels. b Ketogenic diet (KD) induces a shift from tolerogenic ( Lactobacilli spp., Clostridium asparagiforme ) toward immunogenic bacteria (such as Akkermansia muciniphila ) driven by host production of ketone bodies, of which β-HB selectively inhibits the growth of bifidobacteria, resulting in KD-associated decreases in intestinal Th17 cell levels. c High-salt diet (HSD) increases the abundance of Bifidobacterium and leads to intratumoral localization of Bifidobacterium , further enhancing NK cell functions and tumor regression. HSD decreases the expression of IL-17A and iNOS and inhibits inflammation, which reduces enterotoxigenic Bacteroides fragilis (ETBF)-promoted colon carcinogenesis. HSD exacerbates Helicobacter pylori infection and promotes gastric carcinogenesis. d High-fat diet (HFD), through augmentation of queuosine-producing gut bacteria, can incite chemotherapy resistance in pancreatic cancer patients. HFD reduces SCFA-producing bacteria and SCFA production, leading to decreased levels of short-chain fatty acids (SCFAs) that activate the MCP-1/CCR2 axis, which promotes M2 TAM recruitment and polarization, ultimately contributing to colorectal cancer (CRC) progression. High bile salt hydrolase (BSH) enzyme activity in an HFD mouse model activates the β-catenin/CCL28 axis, further inducing immunosuppressive Tregs and accelerating CRC progression. e Dietary intake rich in tryptophan stimulates certain Bacteroides to produce the metabolite indole-3-acetic acid (3-IAA). Increased levels of 3-IAA enhance the efficacy of chemotherapy treatment. Dietary intake rich in tryptophan, through the action of the probiotic Lactobacillus reuteri (Lr), leads to the production of the metabolite indole-3-aldehyde (I3A). This metabolite promotes the production of IFNγ from CD8 + T cells, thereby enhancing antitumor immunity and the efficacy of immune checkpoint inhibitors (ICIs). f High-fiber diet enriches Akkermansia muciniphila which produces the microbiota-derived STING agonist c-di-AMP, inducing type I interferon (IFN-I) production by intratumoural monocytes, resulting in various TME modulation pathways, including reprogramming of mononuclear phagocytes into immunostimulatory monocytes and DCs, promoting macrophage polarization toward an antitumor phenotype and stimulating crosstalk between NK cells and DCs, further enhancing the therapeutic effect of immunotherapy. Dietary fiber inulin can enhance the effectiveness of anti-PD-1 therapy by increasing the abundance of beneficial commensal microbes (e.g., Akkermansia , Lactobacillus and Roseburia ) and SCFAs, further increasing the number of stem-like T-cell factor-1 (Tcf1) + PD-1 + CD8 + T cells numbers. Dietary fiber pectin can improve the effectiveness of anti-PD-1 therapy by increasing the abundance of butyrate-producing bacteria, further promoting T-cell infiltration and activation in the TME. This figure was created with BioRender.com
Studies suggest that the gut microbiota plays a crucial role in modulating the therapeutic response to immunotherapy. 297 , 298 In fact, specific gut microbial signatures have been shown to differentiate responders from nonresponders across various epithelial tumor types in cohorts treated with ICB. 299 Considering the profound impact of the gut microbiota on the immune system, research investigating the modulation of the gut microbiota via dietary interventions to optimize cancer treatment efficacy has been predominantly centered around immunotherapy. A high-fiber dietary intervention has been associated with significantly prolonged PFS in melanoma patients receiving ICB treatment. 300 Microbiota-derived STING agonists, specifically c-di-AMP, induce the production of type I interferon (IFN-I) in intratumoral monocytes. This activation results in the transformation of mononuclear phagocytes within the TME into immunostimulatory monocytes and DCs. Additionally, it promotes the polarization of macrophages to antitumor macrophages and stimulates crosstalk between NK cells and DCs. A high-fiber diet can trigger this mechanism by enriching the population of Akkermansia muciniphila , which produces c-di-AMP and enhances the therapeutic effect of ICB in melanoma patients. 301 The presence of Akkermansia , a mucin-degrading bacterium, is strongly associated with favorable outcomes in cancer patients. 302 Moreover, inulin, a polysaccharide dietary fiber, can enhance the effectiveness of anti-PD-1 therapy by increasing the abundance of beneficial commensal microbiota genera (e.g., Akkermansia , Lactobacillus and Roseburia ) and SCFAs, further increasing the number of stem-like T-cell factor-1 (Tcf1) + PD-1 + CD8 + T cells. 303 Similarly, oral administration of pectin, another dietary polysaccharide fiber, can largely improve the efficacy of anti-PD-1 mAbs by increasing the number of butyrate-producing bacteria, which is sufficient to promote T-cell infiltration and activation in the TME. 304
Although research into the antitumor or protumor effects of the intratumor microbiome is still in its early stages, recent studies have started to focus on how the intratumor microbiome can influence the effectiveness of immunotherapy. The colonization of Bifidobacterium in the microenvironment, combined with anti-CD47 monoclonal antibody treatment, stimulates the STING signaling pathway and enhances the cross-priming of DCs to upregulate CD8 + T cells. 305 The probiotic Lactobacillus reuteri (Lr) within melanoma promotes the local generation of IFNγ by CD8 + T cells through the release of its tryptophan breakdown metabolite, indole-3-aldehyde (I3A), thus enhancing ICI efficacy. Dietary intake rich in tryptophan boosts the antitumor immunity induced by Lr and ICI, which is dependent on the CD8 + T-cell AhR signaling pathway. 306
Apart from immunotherapy, recent research has also started to investigate how diet, by influencing the gut microbiota, could affect other forms of cancer treatment. By enriching the gut microbiome with queuosine-producing bacteria, HFD can induce chemotherapy resistance in pancreatic cancer through the upregulation of the oxidative stress protector PRDX1. This resistance can be counteracted by SAM, which is typically produced by bacteria in lean diets, highlighting the influence of diet on chemotherapy effectiveness via gut microbiome adjustments. 307 Expanding on the theme of diet’s influence on chemotherapy effectiveness in pancreatic cancer, another study revealed that the microbiota-derived tryptophan metabolite indole-3-acetic acid (3-IAA) is enriched in patients responsive to chemotherapy. Through dietary manipulation of tryptophan, an increase in 3-IAA production enhances chemotherapy efficacy by disrupting cancer cell metabolic fitness via increased reactive oxygen species and reduced autophagy. 308 These findings further emphasize the crucial role of gut microbiota modulation via dietary interventions in cancer treatment outcomes.
Despite the significant progress in this field, the complex relationships among dietary factors, the gut microbiota, and cancer treatment still need to be understood. Each individual’s microbiome is unique, influenced by genetics, diet, environment, and lifestyle, which adds layers of complexity to the task of identifying universally beneficial interventions. Additionally, the development of high-throughput technologies and bioinformatics tools for microbiome analysis will be vital in deciphering these complex interactions. These advancements could enable the identification of biomarkers for microbiome-related treatment responses and the customization of diet-based interventions to enhance the efficacy of cancer therapies. The identification of specific dietary factors and gut microbiota constituents that can enhance the effectiveness of cancer therapies may lead to the development of personalized treatments to improve therapeutic outcomes for cancer patients.
Implications of dietary intervention for other diseases
Dietary interventions may induce, prevent or delay the progression of various diseases in addition to cancer, which also influence human health and longevity. Healthy dietary patterns that are rich in fiber and beneficial nutrients may reduce the risk of disease, while unhealthy dietary patterns may increase the risk of disease and worsen clinical outcomes. 309 Here, we summarize preclinical and human studies revealing the implications and mechanisms of various dietary patterns on other diseases in addition to cancer, including neurodegenerative diseases, autoimmune diseases, CVD, and metabolic disorders.
Neurodegenerative diseases
Several neurodegenerative diseases (NDs), such as epilepsy, Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS), which feature chronic progressive damage to the nervous system, have been proven to be tightly connected with nutrient availability and dietary patterns. 310 The underlying mechanisms of various dietary interventions mainly include altering neurotransmitters, remodeling, interfering with brain energy metabolism and mitochondrial function, and altering inflammation and oxidative stress. The underlying mechanisms also include altering the composition and balance of the gut microbiome, which further influence the process of neurodegeneration via the gut-brain axis (Fig. 4 ).
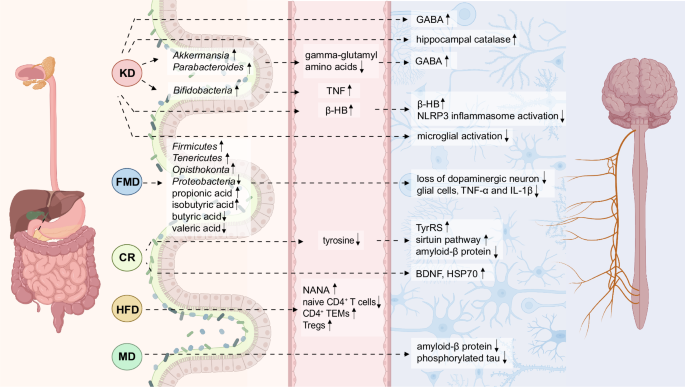
Impact of different diets on neurodegenerative diseases. The ketogenic diet (KD) can enhance inhibitory neurotransmission and anti-inflammatory effects in epilepsy, influence the gut microbiota, and elevate beneficial metabolites. KD is particularly beneficial for treating pediatric drug-resistant epilepsy with elevated specific Bifidobacteria and TNF. In Alzheimer’s disease (AD) and Parkinson’s disease (PD), KD could counteract decreased β-HB levels, inhibit the NLRP3 inflammasome, reduce pathology, and alleviate symptoms by inhibiting microglial activation. Fasting mimicking diet (FMD) enhances the gut microbiota composition and metabolites, inhibiting neuroinflammation. This results in the attenuated loss of dopaminergic neurons in the substantia nigra in patients with PD. Caloric restriction (CR) may prevent AD by lowering serum tyrosine levels, reversing the exhaustion of tyrosyl-tRNA synthetase (TyrRS), and upregulating the sirtuin pathway, which attenuates the amyloidogenic processing of amyloid-β protein precursor (APP). Dietary restriction can increase brain-derived neurotrophic factor (BDNF) and chaperone heat-shock protein-70 (HSP70) levels in the striatum and cortex, which are relevant to Huntington’s disease (HD). High-fat diet (HFD) can accelerate recognition-memory impairment in an AD mouse model by increasing blood N-acetylneuraminic acid (NANA) levels, leading to systemic immune exhaustion. Conversely, the Mediterranean diet (MD) may protect against memory decline and mediotemporal atrophy by lowering amyloid-β protein and phosphorylated tau levels, reducing AD risk. This figure was created with BioRender.com
KD has been clinically applied for nearly a century as alternative therapy for childhood intractable epilepsy, but there is sufficient evidence that a modified Atkins diet (MAD) is more tolerable and has a greater probability of causing seizure reduction than a classical KD according to a systematic review. 311 , 312 , 313 Increased levels of the inhibitory neurotransmitter GABA can be observed in preclinical KD models and patient cerebrospinal fluid (CSF), dampening neuronal excitability. 314 , 315 , 316 An increase in peroxisome proliferator activated receptor gamma 2 (PPARγ2) and upregulation of hippocampal catalase in KD-fed rats are observed, which may increase anti-inflammatory and antioxidant activity. 317 In addition, a KD may upregulate potassium channels that are sensitive to ATP opening, reducing the electrical excitability of the brain and increasing the seizure threshold. 318 The gut microbiota, which includes Akkermansia , Parabacteroides , and Bifidobacteria , also contributes to the neuroprotective effects of KD on epilepsy. 319 , 320
Epidemiologic evidence indicates that obesity is an independent risk factor for AD, while HFD is closely associated with an increased risk of obesity. 321 Recognition-memory impairment in an AD mouse model (5xFAD) can be accelerated by high-fat obesogenic diet by increasing blood levels of the metabolite N-acetylneuraminic acid (NANA), which results in systemic immune exhaustion. 322 HFD may also enhance neuroinflammation by increasing circulating free fatty acids and cytokines, which may lead to cognitive impairment. 323 Conversely, healthy dietary interventions, including the Mediterranean diet (MD), CR, and KD, may prevent AD progression. 324 , 325 , 326 Adhering to MD may act as a protective factor against memory decline and mediotemporal atrophy, as indicated by decreased levels of amyloid-β protein and phosphorylated tau, reducing the risk of AD. 327 CR may prevent AD by lowering serum tyrosine levels to reverse the exhaustion of tyrosyl-tRNA synthetase (TyrRS) and upregulating the sirtuin pathway, which attenuates the amyloidogenic processing of amyloid-β protein precursor (APP), as confirmed by in vivo and in vitro models. 328 , 329 KD may reverse the decreased β-HB levels in red blood cells and the brain parenchyma of AD patients, hence inhibiting NLRP3 inflammasome activation and reducing AD pathology. 330 In addition, diet can influence AD by modulating the gut microbiome and metabolites. For instance, a Mediterranean-ketogenic diet (MMKD) is associated with improved AD biomarkers in CSF, as indicated by increased Akkermansia muciniphila levels, which modulate GABA levels and gut transit time. 331 , 332
Gut microenvironmental changes may trigger the development of PD through the gut-brain axis, as determined by the presence of α-synuclein and Lewy bodies in the enteric nervous system and the convincing association between PD and gut inflammation. 333 , 334 Research has revealed changes in the gut microbiome in PD patients compared to healthy volunteers, highlighting the potential benefits of dietary interventions in treating PD patients. 335 High serum sodium is associated with cognitive decline, as observed in the aged population. 336 However, a recent study denies the association between HSD and neurodegeneration or α-synuclein accumulation in a PLP-hαSyn model, suggesting that the mechanism of HSD needs further exploration. 337 Adhering to MD is associated with a decreased incidence of PD, the mechanisms of which may include reducing neuroinflammation, similar to AD. 338 , 339 KD ameliorates motor and nonmotor symptoms in PD patients by inhibiting microglial activation 340 . FMD promotes a favorable gut microbiota composition and metabolites and inhibits neuroinflammation, consequently attenuating the loss of dopaminergic neurons in the substantia nigra in a PD model. 341
Other neurodegenerative diseases with lower incidence rates are also relevant to dietary interventions. A clinical trial suggested that increased consumption of dairy products may increase the risk of phenoconversion, resulting in earlier onset of HD. 342 In addition, high antigliadin antibody titers in patients with HD suggest the potential value of applying gluten-free diet in HD patients. 343 A dietary restriction regimen retarded the progression of neuropathological, behavioral, and metabolic abnormalities in an HD model, resulting in an extension of life span by increasing brain-derived neurotrophic factor and chaperone heat-shock protein-70 (HSP70) levels in the striatum and cortex, the mechanisms of which still need further explanation. 344 A cross-sectional baseline analysis revealed that a higher intake of antioxidants and carotenes may result in greater ALS function. 345 Another meta-analysis revealed that a greater intake of ω-3 PUFAs is associated with a reduced risk of ALS. 346 Although weight loss has been identified as a negative prognostic factor, high-calorie fatty acid diet provides a significant survival benefit for patients in the subgroup of fast-progressing ALS patients only. 347
Autoimmune diseases
Different types of autoimmune diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), inflammatory bowel disease (IBD), Hashimoto’s thyroiditis (HT), and multiple sclerosis (MS), can cause distinct clinical features from abnormal activation of the immune system that erroneously attacks healthy host cells and tissues. Impaired gut barrier function, also referred to as a “leaky gut”, which may disrupt the balance between tolerance and immunity to non-self-antigens, is often observed in autoimmune diseases. 348 This finding suggested a close relationship between diet, the gut, and autoimmune diseases. Dietary interventions may influence the susceptibility, progression and treatment response of these autoimmune diseases through various mechanisms, from adjusting inflammation levels and immune cell composition to adjusting the gut microbiome composition (Fig. 5 ).
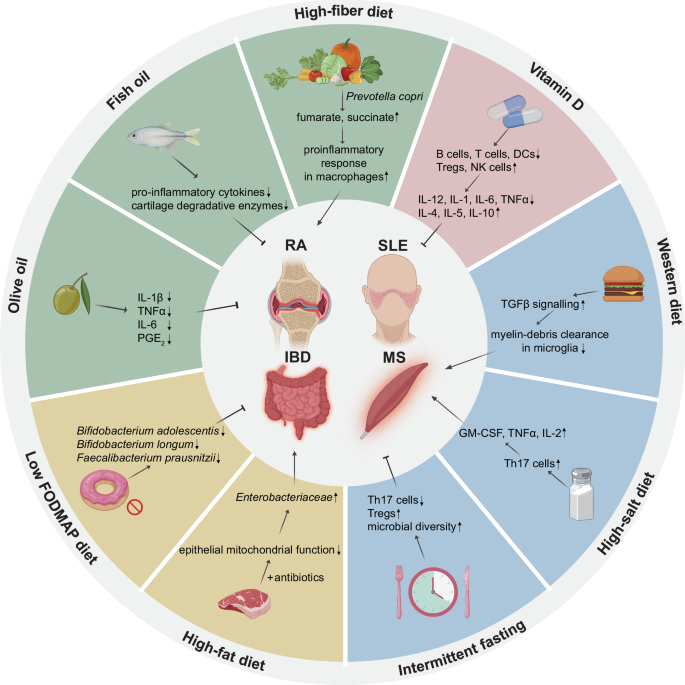
Impact of different diets on autoimmune diseases. Extravirgin olive oil (EVOO) can reduce joint inflammation and degradation in rheumatoid arthritis (RA) due to its phenolic compounds. However, the protective effects of a high-fiber diet can be reversed by Prevotella copri colonization, which promotes proinflammatory responses. Fish oil supplementation can suppress proinflammatory cytokines and cartilage degradation, improving RA outcomes. Vitamin D can inhibit the proliferation, differentiation, and function of B and T cells, potentially reducing inflammatory cytokine expression in systemic lupus erythematosus (SLE) patients. A diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) can alleviate gut symptoms in quiescent inflammatory bowel disease (IBD) patients, possibly by regulating the immune response through reducing fecal microbial abundance. However, a high-fat diet (HFD) can exacerbate pre-IBD inflammation by impairing epithelial mitochondrial bioenergetics and triggering microbiota disruptions, especially when combined with antibiotics. High salt diet (HSD) can exacerbate autoimmune conditions such as multiple sclerosis (MS) by promoting the induction of pathogenic Th17 cells. Intermittent fasting (IF) can improve MS by reducing the number of IL-17-producing T cells, increasing the number of Tregs in the gut, and enhancing antioxidative microbial metabolic pathways. However, the Western diet can impair myelin-debris clearance in microglia, hindering lesion recovery after demyelination and potentially contributing to MS induction. This figure was created with BioRender.com
A healthy MD may benefit RA by reducing inflammatory activity and increasing physical function. 349 Phenolic compounds in extravirgin olive oil (EVOO), an essential component of the MD, can decrease joint edema, cell migration, cartilage degradation and bone erosion by reducing the levels of proinflammatory cytokines and prostaglandin E2 in the joint. 350 However, the protective effect of high-fiber diet may be reversed if there exists colonization of Prevotella copri , which leads to the overproduction of organic acids, including fumarate and succinate, during the digestion of complex fibers and the promotion of proinflammatory responses in macrophages, exacerbating arthritis in an RA model. 351 In addition, abundant supplementation of fish oil benefits the clinical outcome of RA by suppressing the production of proinflammatory cytokines and cartilage degradative enzymes. 352 The erythrocyte level of ω-6 PUFAs acts as a biomarker that inverses the risk of RA, and the remission rate of RA increases when ω-3 PUFAs are added to disease-modifying anti-rheumatic drug (DMARD) treatment. 353 , 354
Dysbiosis of the gut microbiome can be observed in SLE patients, including a decreased richness and diversity of the gut microbiota and a reduced proportion of Firmicutes/Bacteroides (F/B); the latter may promote lymphocyte activation and Th17 differentiation from naïve CD4 + lymphocytes. 355 , 356 Blooming of Ruminococcus (blautia) gnavus occurs at times of high disease activity and during lupus nephritis, indicating that it is the driver of often remitting-relapsing SLE. 357 Another analysis showed that Veillonella dispar has a positive association with the activity of SLE. 358 According to a systematic review, nutritional support in the SLE population is focused mainly on interventions involving ω-3 and vitamin D. 359 The anti-inflammatory effect of ω-3 may contribute to its clinical function, similar to that of RA. 360 Vitamin D blocks the proliferation, differentiation and function of B cells and T cells, which may attenuate the expression of inflammatory cytokines in patients with SLE. 361 Inadequate levels of serum vitamin D have been observed in SLE patients, suggesting the importance of supplementing their diet with vitamin D 362 . Dietary patterns other than single nutrients as supplementary treatments for SLE still require further investigation. 363
Ulcerative colitis (UC) and Crohn’s disease (CD) are the two major clinical phenotypes of IBD. Dietary management and microbiota modulation have been clinically recommended for IBD treatment according to clinical guidelines. 364 Obesity is a risk factor for IBD, especially for CD. 365 As a potential trigger of obesity, HFD, together with antibiotics, exacerbates inflammation in pre-IBDs by impairing epithelial mitochondrial bioenergetics and triggering microbiota disruptions in mouse models. 366 However, IBD increases the risk of malnutrition, which triggers inflammatory responses and subsequently leads to poor clinical outcomes. 367 Therefore, dietary interventions and nutritional care should be planned according to the precise nutritional assessment and dietary assessment for IBD patients. 368 Exclusive enteral nutrition (EEN), the first-line therapy in pediatric patients with active CD, can effectively decrease clinical activity and reduce the complications of CD simultaneously, but its benefit in adults still lacks competent evidence. 369 Similarly, CD exclusion diet (CDED) positively correlates with the clinical remission of pediatric patients with active CD. 370 In addition, diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) can relieve the gut symptoms of patients with quiescent IBD, possibly reducing the fecal abundance of microbes and thereby regulating the immune response of the host. 371
Dietary interventions may also influence the risk and clinical outcome of other autoimmune diseases. A recent study on HT suggested that low intake of animal foods, mainly meat, has a protective effect on thyroid autoimmunity and potentially has a positive influence on redox balance, which further reduces oxidative stress-related disorders. 372 Improvement in HT has also been observed in other dietary interventions, including elimination of gluten or lactose, energy restriction, and consumption of Nigella sativa, suggesting the potential benefit of diet as a complementary treatment for HT. 373 MS is more common in western countries, suggesting diet as a potential risk factor. 374 Western diet triggers impaired myelin-debris clearance in microglia, thereby impairing lesion recovery after demyelination, which may explain its role in MS induction. 375 Moreover, an elevated intake of dietary salt can exacerbate autoimmune conditions by promoting the induction of pathogenic Th17 cells, contributing to MS. 376 Conversely, IF diet ameliorates the clinical course and pathology of MS by reducing the number of IL-17-producing T cells, increasing the number of Tregs in the gut and increasing the richness of gut bacteria, which enhance antioxidative microbial metabolic pathways. 377 Vitamin D supplementation has been shown to lower the incidence and benefit MS patients with sufficient evidence, and a “Coimbra Protocol” referring to daily doses up to 1000 I.U. vitamin D3 per kg body weight is clinically applied to treat patients with MS. 378 , 379
Cardiovascular diseases (CVD)
According to epidemiological studies, obesity and unhealthy diet are risk factors for CVD. Greater dietary fiber intake from cereal, vegetables and fruits is associated with a lower risk of CVD, suggesting that high-fiber diet is a potential protective factor. 380 An experimental model fed with diet lack of prebiotic fiber induces hypertension through inducing deficiency of SCFA production and GPR43/109A signaling, suggesting the underlying mechanisms of dietary fiber. 381 Besides, high-fiber diet and acetate supplementation can lead to changes in the gut microbiota, particularly an increase in Bacteroides acidifaciens , which is protective against the development of CVD. 382 Other healthy dietary patterns, including the Nordic diet, the Dietary Approaches to Stop Hypertension (DASH) diet, the MD, and the vegetarian diet, also have protective effects on CVD risk. 383 High sodium intake is the leading dietary risk factor for CVD. 384 High salt load may induce persistent hepatic steatosis and inflammation by inhibiting SIRT3 expression, thereby contributing to cardiovascular damage. 385 Conversely, a low-sodium diet may dampen the risk of CVD, which is highly recommended by current dietary guidelines. 386 Amino acids play different roles in the progression of CVD. Diet with high-unsaturated fatty acid composition and less saturated fat might be cardioprotective. 387 In contrast, higher intake of BCAAs is associated with increased platelet activity and arterial thrombosis formation; therefore, BCAA levels are associated with the risk of CVD. 388
Therapeutic implications of diet for CVD treatment have also been a focus of recent studies. CR attenuates hypertension, left ventricular remodeling and diastolic dysfunction in DS/obese rats by reducing cardiac oxidative stress and inflammation. 389 In addition, a combination of CR and exercise can improve cardiac mitochondrial dynamics, decrease cardiac apoptosis, and maintain cardiac [Ca 2+ ] i homeostasis in obese insulin-resistant rats. 390 CR also helps to maintain the iron homeostasis of cardiomyocytes. 391 These findings suggest the function of CR in cardiac protection. However, strictly adhering to CR is very difficult for most patients. IF is easier to perform than CR and has similar potential clinical value. 392 FMD, a 5-day fasting dietary pattern, increases cardiac vascularity and function and resistance to cardiotoxins in a high-fat, high-calorie diet (HFCD) mouse model, thereby postponing the process of cardiac aging. 393 Alternate day fasting (ADF) improves cardiovascular marker levels, including reduced fat mass, an improved fat-to-lean ratio, and increased β-HB-hydroxybutyrate levels, suggesting its clinical relevance for CVD intervention. 394 KD has a beneficial effect on the blood lipid profile, the NLRP3 inflammasome, myocardial energy metabolism, and the vascular endothelium, benefiting CVD patients. 395 However, research on healthy individuals has reported that lipid profiles deteriorate in response to a KD, suggesting that its role in preventing CVD in the normal population needs further inquiry (Fig. 6 ). 396
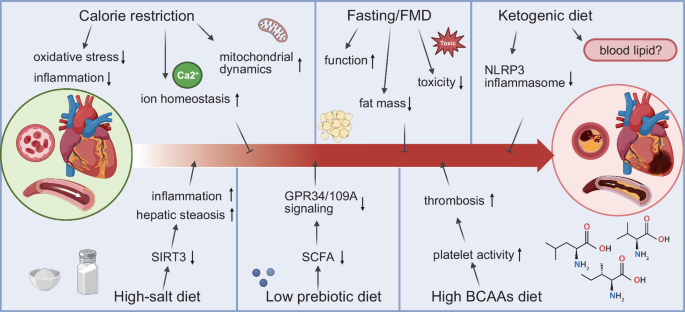
Impact of different diets on cardiovascular diseases. Calorie restriction (CR) can reduce cardiac oxidative stress and inflammation, improve cardiac mitochondrial dynamics and maintain cardiac ion homeostasis, which may be protective against cardiovascular disease (CVD) in obese and/or insulin-resistant models. Fast-mimicking diet (FMD) increases cardiac vascularity and function and resistance to cardiotoxins in a high-fat, high-calorie diet (HFCD) mouse model. Alternate day fasting (ADF) improves cardiovascular markers, for example, reduced fat mass. Ketogenic diet (KD) inhibits the NLRP3 inflammasome and improves the blood lipid profile but may lead to impaired blood lipid profiles in healthy individuals. High-salt diet (HSD) can inhibit SIRT3 expression and induce persistent hepatic steatosis and inflammation, thereby contributing to cardiovascular damage. A diet lacking prebiotic fiber induces hypertension through inducing a deficiency in short-chain fatty acid (SCFA) production and GPR43/109A signaling. High branched-chain amino acid (BCAA) intake is associated with increased platelet activity and arterial thrombosis formation. This figure was created with BioRender.com
Metabolic disorders
Overnutrition is a driving factor for obesity and related metabolic disorders, mainly including type 2 diabetes mellitus (T2DM), metabolic syndrome, nonalcoholic fatty liver disease (NAFLD), and polycystic ovarian syndrome (PCOS). 397 In addition, these metabolic disorders have a complicated internal relation, for instance, T2DM and NAFLD are independent factors for each other, and PCOS is closely related to insulin resistance and T2DM. 398 , 399 These epidemiological characteristics suggest a high correlation between dietary patterns and multiple metabolic disorders (Fig. 7 ). Changes in the gut microbiome may also explain the etiology of metabolic disorders by altering the levels of metabolites, such as SCFAs and succinate. 400
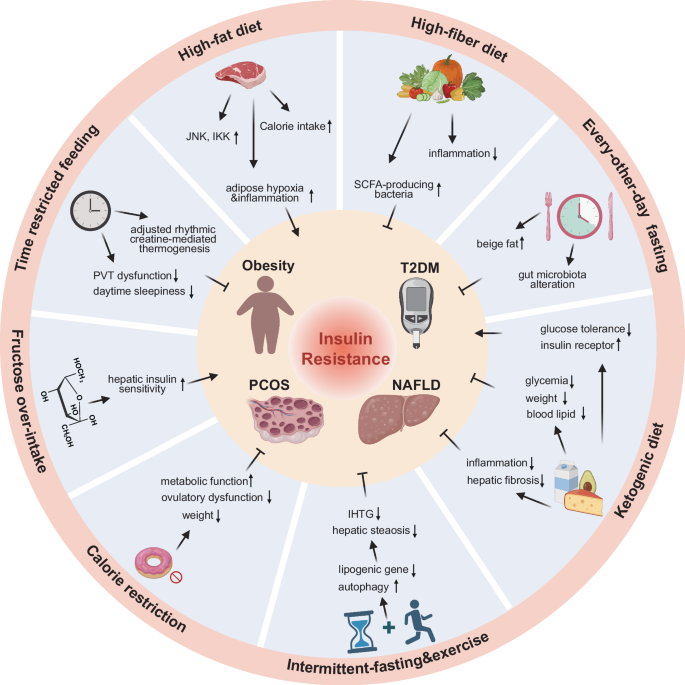
Impact of different diets on metabolic disorders. High-fat diet (HFD) can directly increase caloric intake, induce inflammatory mediators such as JNK and IκB kinase (IKK) to promote hypothalamic inflammation, and contribute to adipose tissue hypoxia and inflammation, which all lead to the development of obesity and/or insulin resistance. Over-intake of fructose can also increase caloric intake and induce obesity by impairing hepatic insulin sensitivity. However, time-restricted feeding (TRF) with equivalent caloric intake from HFD can adjust various signaling pathways and rhythmic creatine-mediated thermogenesis and reverse excessive daytime sleepiness induced by paraventricular thalamic nucleus (PVT) dysfunction, resulting in a protective effect on HFD-induced obesity. High-fiber diet can reduce inflammation and insulin resistance by influencing the gut microbiota and associated molecules, for instance, SCFA-producing bacteria. Every-other-day fasting (EODF) regimen can also shift the gut microbiota composition and stimulate beige fat development within white adipose tissue to inhibit insulin resistance. Ketogenic diet (KD) is clinically beneficial for the glycemic control of type 2 diabetes mellitus (T2DM) and nonalcoholic fatty liver disease (NAFLD). However, in experimental models, KD can decrease sensitivity to peripheral insulin by upregulating insulin receptors. Intermittent fasting (IF) alone or combined with exercise can reduce intrahepatic triglyceride (IHTG) levels and hepatic steatosis in NAFLD patients by downregulating hepatic inflammatory pathways, modifying lipogenic gene expression and inducing autophagy. Calorie restriction (CR) can be effective at reducing weight loss and reversing ovulatory/metabolic dysfunction in polycystic ovarian syndrome (PCOS) patients. This figure was created with BioRender.com
HFD is the standard method to induce obesity in animal models and results from the overconsumption of fat, which directly increases caloric intake. The elevation of inflammatory mediators such as JNK and IκB kinase (IKK) in hypothalamic inflammation may also explain the obesity induced by HFD. 401 Interestingly, a TRF with equivalent caloric intake from HFD has been shown to have a protective effect on HFD-induced obesity and associated complications by adjusting various signaling pathways and causing rhythmic creatine-mediated thermogenesis, which may further improve nutrient utilization and energy expenditure and reverse excessive daytime sleepiness induced by paraventricular thalamic nucleus (PVT) dysfunction. 402 , 403 , 404 Adipose tissue hypoxia and inflammation may lead to adipocyte dysfunction and obesity-induced insulin resistance in HFD-fed models, as indicated by increased infiltration of adipose tissue macrophages (ATMs), activation of the NLRP3 inflammasome and increased levels of proinflammatory cytokines. 405 , 406 , 407 In addition to fat intake, the overintake of fructose may also impair hepatic insulin sensitivity, and several metabolic pathways are independent of increased weight gain and caloric intake. 408 Within this complex interplay of diet, metabolism, and inflammation, IL-17 has been identified as a key player in metabolic dysregulation associated with HFD, where inhibiting IL-17A production or blocking its receptor can attenuate obesity by enhancing adipose tissue browning and energy dissipation. 409 Complementarily, IL-17F promote the expression of TGFβ1 in adipocytes, which fosters sympathetic innervation and suggests a novel therapeutic target for obesity that could stimulate thermogenic activity in fat tissue, thereby improving metabolic health and providing a potential treatment strategy for obesity and its related metabolic disorders. 410
Cohort studies have demonstrated that healthy diets, including the Portfolio diet, DASH diet, and MD, are associated with a decreased risk of T2DM. 411 , 412 , 413 The promotion of SCFA-producing bacteria induced by dietary fibers observed in T2DM patients suggests the potential value of fiber supplementation in clinical practice. 414 In addition, increased fiber consumption is associated with decreased insulin resistance, the mechanism of which mainly includes the gut microbiota and associated molecules. 415 , 416 IF is an effective strategy for controlling weight and increasing insulin sensitivity in patients with diabetes and can also improve cardiometabolic outcomes. 417 , 418 The every-other-day fasting (EODF) regimen selectively stimulates beige fat development within white adipose tissue and shifts the gut microbiota composition in experimental models, explaining the mechanism through which IF ameliorates obesity, insulin resistance, and hepatic steatosis. 419 KD has therapeutic effects on glycemia, lipid control, and weight reduction in T2DM patients. 420 However, KD may contribute to decreased sensitivity to peripheral insulin and impaired glucose tolerance by upregulating insulin receptors, as determined by previous studies, which contradicts clinical findings. 421
NAFLD features hepatic steatosis or adiposity with a potential risk of developing into inflammation, fibrosis, and cancer. MD, as the most recommended dietary pattern for NAFLD, can reduce liver steatosis and improve insulin sensitivity even without weight loss in an insulin-resistant population. 422 Reduced liver fat may be associated with ameliorated inflammation induced by antioxidants, low glycemic response induced by dietary fiber, and improved hepatic lipid metabolism. 423 KD is more clinically meaningful for glycemic control in individuals with T2DM and NAFLD than low-calorie diet or high-carbohydrate, low-fat (HCLF) diet. 424 , 425 Mechanistically, ketone bodies may modulate inflammation and fibrosis in hepatic cells. 426 IF alone or combined with exercise is effective at lowering intrahepatic triglyceride (IHTG) levels and reducing hepatic steatosis in patients with NAFLD, possibly by downregulating hepatic inflammatory pathways, modifying lipogenic gene expression and increasing levels of autophagy. 427 , 428
PCOS features a series of metabolic irregularities, mainly androgen excess and ovarian dysfunction. A meta-analysis showed that women with PCOS have a lower overall diet quality with higher cholesterol, lower magnesium and lower zinc intake. 429 Dietary modification with lower caloric intake to achieve weight loss is recommended as a first-line therapy for managing PCOS, and higher supplementary nutrient intake, including vitamin D, chromium and ω-3, may also benefit patients suffering from PCOS. 430 MD, KD and their combination can all lead to significant improvements in body weight, metabolic function and ovulatory dysfunction in PCOS patients. 431 , 432 , 433 In addition, IF may be beneficial for treating anovulatory PCOS by reducing body fat and improving menstruation, hyperandrogenemia, insulin resistance and chronic inflammation. 434 CR may also improve weight and metabolic disorders in patients with PCOS, alone or in combination with supplementation. 435 However, the exact mechanisms of these dietary interventions remain unclear and need further exploration.
While the potential of dietary interventions to influence systemic diseases of the whole body is supported by various studies, a critical outlook reveals the necessity for more rigorous, long-term clinical trials to validate these findings. It is essential to approach these interventions with caution, considering individual differences and the intricate balance of potential benefits against nutritional deficiencies or other risks.
Conclusions and perspectives
Our review provides compelling evidence that dietary interventions, including calorie restriction, fasting or FMD, KD, protein restriction diet, HSD, HFD, and high-fiber diet, have substantial potential for modulating metabolism, redirecting disease progression, and enhancing therapeutic responses. These findings highlight the pivotal role of diet, an important environmental factor, in influencing tumor metabolism and the course of various diseases, such as cancer, neurodegenerative diseases, autoimmune diseases, CVD, and metabolic disorders.
Despite compelling evidence, the potential impact of dietary interventions on disease treatment, particularly cancer treatment, is not fully understood. 436 The latest American Society of Clinical Oncology (ASCO) guidelines suggest that “there is currently insufficient evidence to recommend for or against dietary interventions such as ketogenic or low-carbohydrate diets, low-fat diets, functional foods, or fasting to improve outcomes related to quality of life (QoL), treatment toxicity, or cancer control”. 437 The intricate relationship between dietary interventions and treatment outcomes can be influenced by numerous factors, such as overall lifestyle habits, health status, specific disease type and its corresponding treatment, degree of dietary alterations, and patient adherence. A comprehensive assessment of these variables is crucial for understanding the precise impact of diet on treatment efficacy. 438 , 439
With the recognition of metabolic reprogramming inherent in disease progression, particularly in malignancies, it is becoming essential to explore the value of implementing dietary interventions and translating the evidence into practice. Future research should focus on unraveling the specific molecular mechanisms involved, which will enable the development of more effective, personalized dietary interventions that serve as adjunct therapies in comprehensive disease management.
Building upon the initial observation, it is crucial to interpret and apply these findings with caution due to potential variations and discrepancies. The efficacy of dietary interventions may vary significantly, for instance, depending on the mouse model used. 440 Each model might have unique metabolic and immune responses that could influence the outcome of dietary interventions. Similarly, the type of cancer cells used to induce tumor formation, whether primary cells derived directly from patient tissues or cultured cell lines, can have profound impacts on the experimental results. 441 Orthotopic or heterotopic transplantation technique is another significant factor that can influence how tumors respond to dietary interventions. Furthermore, the duration of treatment and the specifics of dietary interventions can substantially influence the results, as short-term interventions might not yield the same results as long-term interventions, and different dietary components could have varying effects on tumor growth and progression. 120 Therefore, future research in this field should carefully consider the design of animal models and the specifics of dietary interventions to ensure that the findings are robust and translatable to human cancer treatment.
Additionally, clinical trials with larger sample sizes and longer follow-up periods are needed to further validate the efficacy of these strategies and to identify potential side effects and contraindications. It is important for these trials to be designed to represent diverse population groups, including elderly and obese individuals, as these groups may respond differently to dietary modifications. The safety of dietary interventions is another key consideration. While dietary changes generally cause fewer side effects than pharmacological treatments, potential risks should not be overlooked. For instance, severe dietary restrictions may lead to malnutrition or other health complications, particularly in vulnerable population groups. Therefore, in addition to efficacy, these trials should systematically evaluate the safety of dietary interventions, identifying any potential side effects and contraindications.
In conclusion, dietary interventions hold great promise as a novel approach to disease management. However, to realize their full potential, it is essential to continue rigorous scientific investigations into their mechanisms of action, safety profiles, and efficacy in different patient populations. With further research, dietary interventions could become integral components of personalized medicine, providing a new avenue for the prevention and treatment of a myriad of diseases.
Collins, N. & Belkaid, Y. Control of immunity via nutritional interventions. Immunity. 55 , 210–223 (2022).
Article CAS PubMed Google Scholar
Wu, Q., Gao, Z. J., Yu, X. & Wang, P. Dietary regulation in health and disease. Signal Transduct. Target. Ther. 7 , 252 (2022).
Article PubMed Central PubMed Google Scholar
Wiseman, M. J. Nutrition and cancer: prevention and survival. Br. J. Nutr. 122 , 481–487 (2019).
Jochems, S. H. et al. Impact of dietary patterns and the main food groups on mortality and recurrence in cancer survivors: a systematic review of current epidemiological literature. BMJ Open 8 , e014530 (2018).
Schwab, U. et al. Dietary fat intakes and cardiovascular disease risk in adults with type 2 diabetes: a systematic review and meta-analysis. Eur. J. Nutr. 60 , 3355–3363 (2021).
Partula, V. et al. Associations between consumption of dietary fibers and the risk of cardiovascular diseases, cancers, type 2 diabetes, and mortality in the prospective NutriNet-Santé cohort. Am. J. Clin. Nutr. 112 , 195–207 (2020).
Article PubMed Google Scholar
Ellouze, I., Sheffler, J., Nagpal, R. & Arjmandi, B. Dietary patterns and Alzheimer’s disease: an updated review linking nutrition to neuroscience. Nutrients 15 , 3204 (2023).
Article CAS PubMed Central PubMed Google Scholar
Muñoz-Garach, A., García-Fontana, B. & Muñoz-Torres, M. Nutrients and dietary patterns related to osteoporosis. Nutrients 12 , 1986 (2020).
Ubago-Guisado, E. et al. Evidence update on the relationship between diet and the most common cancers from the European prospective investigation into cancer and nutrition (EPIC) study: a systematic review. Nutrients 13 , 3582 (2021).
Shan, Z. et al. Healthy eating patterns and risk of total and cause-specific mortality. JAMA Intern. Med. 183 , 142–153, (2023).
Xiao, Y. et al. Adherence to the Paleolithic diet and Paleolithic-like lifestyle reduce the risk of colorectal cancer in the United States: a prospective cohort study. J. Transl Med. 21 , 482 (2023).
Jia, T. et al. Association of healthy diet and physical activity with breast cancer: lifestyle interventions and oncology education. Front. Public Health 10 , 797794 (2022).
Vernieri, C. et al. Diet and supplements in cancer prevention and treatment: clinical evidences and future perspectives. Crit. Rev. Oncol. Hematol. 123 , 57–73 (2018).
Lee, C. et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl Med. 4 , 124ra127 (2012).
Article Google Scholar
Mercier, B. D. et al. Dietary interventions in cancer treatment and response: a comprehensive review. Cancers 14 , 5149 (2022).
Anic, K. et al. Intermittent fasting-short- and long-term quality of life, fatigue, and safety in healthy volunteers: a prospective, clinical trial. Nutrients 14 , 4216 (2022).
Ibrahim, E. M., Al-Foheidi, M. H. & Al-Mansour, M. M. Energy and caloric restriction, and fasting and cancer: a narrative review. Support Care Cancer 29 , 2299–2304 (2021).
Xia, L. et al. The cancer metabolic reprogramming and immune response. Mol. Cancer 20 , 28 (2021).
Chen, P. H. et al. Metabolic diversity in human non-small cell lung cancer cells. Mol. Cell 76 , 838–851.e835 (2019).
Fan, C. et al. Emerging role of metabolic reprogramming in tumor immune evasion and immunotherapy. Sci. China Life Sci. 64 , 534–547 (2021).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12 , 31–46 (2022).
Petitprez, F. et al. The tumor microenvironment in the response to immune checkpoint blockade therapies. Front. Immunol. 11 , 784 (2020).
Duan, Q., Zhang, H., Zheng, J. & Zhang, L. Turning cold into hot: firing up the tumor microenvironment. Trends Cancer 6 , 605–618 (2020).
Pansy, K. et al. Immune regulatory processes of the tumor microenvironment under malignant conditions. Int. J. Mol. Sci. 22 , 13311 (2021).
Ahmad, F., Cherukuri, M. K. & Choyke, P. L. Metabolic reprogramming in prostate cancer. Br. J. Cancer 125 , 1185–1196 (2021).
Sanderson, S. M., Gao, X., Dai, Z. & Locasale, J. W. Methionine metabolism in health and cancer: a nexus of diet and precision medicine. Nat. Rev. Cancer 19 , 625–637 (2019).
Jia, Q. et al. Heterogeneity of the tumor immune microenvironment and its clinical relevance. Exp. Hematol. Oncol. 11 , 24 (2022).
Andrejeva, G. & Rathmell, J. C. Similarities and distinctions of cancer and immune metabolism in inflammation and tumors. Cell Metab. 26 , 49–70 (2017).
Biswas, S. K. Metabolic reprogramming of immune cells in cancer progression. Immunity 43 , 435–449 (2015).
Domínguez-Amorocho, O., Takiishi, T., da Cunha, F. F. & Camara, N. O. S. Immunometabolism: a target for the comprehension of immune response toward transplantation. World J. Transplant. 9 , 27–34 (2019).
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23 , 27–47 (2016).
Ping, Y., Shen, C., Huang, B. & Zhang, Y. Reprogramming T-Cell metabolism for better anti-tumor immunity. Cells 11 , 3103 (2022).
Li, W. et al. Aerobic glycolysis controls myeloid-derived suppressor cells and tumor immunity via a specific CEBPB isoform in triple-negative breast cancer. Cell Metab. 28 , 87–103.e106 (2018).
Watson, M. J. et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 591 , 645–651 (2021).
Article ADS CAS PubMed Central PubMed Google Scholar
Apostolova, P. & Pearce, E. L. Lactic acid and lactate: revisiting the physiological roles in the tumor microenvironment. Trends Immunol. 43 , 969–977 (2022).
Chen, P. et al. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc. Natl Acad. Sci. USA 114 , 580–585 (2017).
Pearce, E. L., Poffenberger, M. C., Chang, C. H. & Jones, R. G. Fueling immunity: insights into metabolism and lymphocyte function. Science 342 , 1242454 (2013).
Rangel Rivera, G. O. et al. Fundamentals of T Cell metabolism and strategies to enhance cancer immunotherapy. Front. Immunol. 12 , 645242 (2021).
Abdel-Wahab, A. F., Mahmoud, W. & Al-Harizy, R. M. Targeting glucose metabolism to suppress cancer progression: prospective of anti-glycolytic cancer therapy. Pharmacol. Res. 150 , 104511 (2019).
Siska, P. J. et al. Suppression of glut1 and glucose metabolism by decreased Akt/mTORC1 signaling drives T Cell impairment in B cell leukemia. J. Immunol. 197 , 2532–2540 (2016).
Ho, P. C. et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162 , 1217–1228 (2015).
Turbitt, W. J., Buchta Rosean, C., Weber, K. S. & Norian, L. A. Obesity and CD8 T cell metabolism: implications for anti‐tumor immunity and cancer immunotherapy outcomes. Immunol Rev. 295 , 203–219 (2020).
Corrado, M. & Pearce, E. L. Targeting memory T cell metabolism to improve immunity. J. Clin. Investig. 132 , e148546 (2022).
Yan, Y. et al. Metabolic profiles of regulatory T cells and their adaptations to the tumor microenvironment: implications for antitumor immunity. J. Hematol. Oncol. 15 , 104 (2022).
Angelin, A. et al. Foxp3 reprograms T Cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 25 , 1282–1293.e1287 (2017).
Wu, S. Y., Fu, T., Jiang, Y. Z. & Shao, Z. M. Natural killer cells in cancer biology and therapy. Mol. Cancer 19 , 120 (2020).
Assmann, N. et al. Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat. Immunol. 18 , 1197–1206 (2017).
Peng, X. et al. Metabolism of dendritic cells in tumor microenvironment: for immunotherapy. Front. Immunol. 12 , 613492 (2021).
Singer, K. et al. Immunometabolism in cancer at a glance. Dis. Model. Mech. 11 , dmm034212 (2018).
Martin-Perez, M., Urdiroz-Urricelqui, U., Bigas, C. & Benitah, S. A. The role of lipids in cancer progression and metastasis. Cell Metab. 34 , 1675–1699 (2022).
Cockcroft, S. Mammalian lipids: structure, synthesis and function. Essays Biochem. 65 , 813–845 (2021).
Chae, H. S. & Hong, S. T. Overview of cancer metabolism and signaling transduction. Int. J. Mol. Sci. 24 , 12 (2022).
Article ADS PubMed Central PubMed Google Scholar
Goossens, P. et al. Membrane cholesterol efflux drives tumor-associated macrophage reprogramming and tumor progression. Cell Metab. 29 , 1376–1389.e1374 (2019).
Ma, X. et al. Cholesterol induces CD8(+) T cell exhaustion in the tumor microenvironment. Cell Metab. 30 , 143–156.e145 (2019).
Yang, W. et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature 531 , 651–655 (2016).
Du, X., Chapman, N. M. & Chi, H. Emerging roles of cellular metabolism in regulating dendritic cell subsets and function. Front. Cell. Dev. Biol. 6 , 152 (2018).
Yin, Z. et al. Targeting T cell metabolism in the tumor microenvironment: an anti-cancer therapeutic strategy. J. Exp. Clin. Cancer Res. 38 , 403 (2019).
O’Sullivan, D. The metabolic spectrum of memory T cells. Immunol. Cell Biol. 97 , 636–646 (2019).
Raynor, J. L., Chapman, N. M. & Chi, H. Metabolic control of memory T-cell generation and stemness. Cold Spring Harb. Perspect. Biol. 13 , a037770 (2021).
Zhang, Y. et al. Enhancing CD8(+) T cell fatty acid catabolism within a metabolically challenging tumor microenvironment increases the efficacy of melanoma immunotherapy. Cancer Cell 32 , 377–391.e379 (2017).
Zhang, C. et al. STAT3 activation-induced fatty acid oxidation in CD8(+) T effector cells is critical for obesity-promoted breast tumor growth. Cell Metab. 31 , 148–161.e145 (2020).
Prendeville, H. & Lynch, L. Diet, lipids, and antitumor immunity. Cell Mol. Immunol. 19 , 432–444 (2022).
Ma, X. et al. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 33 , 1001–1012.e1005 (2021).
Xu, S. et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8(+) T cells in tumors. Immunity 54 , 1561–1577.e1567 (2021).
Zhang, M., Wei, T., Zhang, X. & Guo, D. Targeting lipid metabolism reprogramming of immunocytes in response to the tumor microenvironment stressor: a potential approach for tumor therapy. Front. Immunol. 13 , 937406 (2022).
Pacella, I. et al. Fatty acid metabolism complements glycolysis in the selective regulatory T cell expansion during tumor growth. Proc. Natl Acad. Sci. USA 115 , E6546–e6555 (2018).
Wang, H. et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat. Immunol. 21 , 298–308 (2020).
Lim, S. A. et al. Lipid signalling enforces functional specialization of T(reg) cells in tumours. Nature 591 , 306–311 (2021).
Wang, J. et al. Metabolism and polarization regulation of macrophages in the tumor microenvironment. Cancer Lett. 543 , 215766 (2022).
Ocaña, M. C., Martínez-Poveda, B., Quesada, A. R. & Medina, M. Metabolism within the tumor microenvironment and its implication on cancer progression: an ongoing therapeutic target. Med. Res. Rev. 39 , 70–113 (2019).
Luo, Q. et al. Lipid accumulation in macrophages confers protumorigenic polarization and immunity in gastric cancer. Cancer Sci. 111 , 4000–4011 (2020).
Wu, L. et al. RIPK3 orchestrates fatty acid metabolism in tumor-associated macrophages and hepatocarcinogenesis. Cancer Immunol. Res. 8 , 710–721 (2020).
Yang, X. et al. Lactate-modulated immunosuppression of myeloid-derived suppressor cells contributes to the radioresistance of pancreatic cancer. Cancer Immunol. Res. 8 , 1440–1451 (2020).
Hossain, F. et al. Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol. Res. 3 , 1236–1247 (2015).
Yan, D. et al. Lipid metabolic pathways confer the immunosuppressive function of myeloid-derived suppressor cells in tumor. Front. Immunol. 10 , 1399 (2019).
Reinfeld, B. I. et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature. 593 , 282–288 (2021).
Ma, G. et al. Reprogramming of glutamine metabolism and its impact on immune response in the tumor microenvironment. Cell Commun. Signal 20 , 114 (2022).
Lian, X. et al. Immunometabolic rewiring in tumorigenesis and anti-tumor immunotherapy. Mol. Cancer 21 , 27 (2022).
Huang, D. et al. Cancer-cell-derived GABA promotes β-catenin-mediated tumour growth and immunosuppression. Nat. Cell Biol. 24 , 230–241 (2022).
Edwards, D. N. et al. Selective glutamine metabolism inhibition in tumor cells improves antitumor T lymphocyte activity in triple-negative breast cancer. J. Clin. Investig. 131 , e140100 (2021).
Perez-Castro, L. et al. Tryptophan and its metabolites in normal physiology and cancer etiology. FEBS J. 290 , 7–27 (2023).
Gouasmi, R. et al. The kynurenine pathway and cancer: why keep it simple when you can make it complicated. Cancers 14 , 2793 (2022).
Leone, R. D. & Powell, J. D. Metabolism of immune cells in cancer. Nat. Rev. Cancer 20 , 516–531 (2020).
DePeaux, K. & Delgoffe, G. M. Metabolic barriers to cancer immunotherapy. Nat. Rev. Immunol. 21 , 785–797 (2021).
Liu, M. et al. Targeting the IDO1 pathway in cancer: from bench to bedside. J. Hematol. Oncol. 11 , 100 (2018).
Liang, F. et al. Tobacco carcinogen induces tryptophan metabolism and immune suppression via induction of indoleamine 2,3-dioxygenase 1. Signal Transduct. Target. Ther. 7 , 311 (2022).
Heintzman, D. R., Fisher, E. L. & Rathmell, J. C. Microenvironmental influences on T cell immunity in cancer and inflammation. Cell Mol. Immunol. 19 , 316–326 (2022).
Savitz, J. The kynurenine pathway: a finger in every pie. Mol. Psychiatry 25 , 131–147 (2020).
Chiarugi, A., Dölle, C., Felici, R. & Ziegler, M. The NAD metabolome–a key determinant of cancer cell biology. Nat, Rev, Cancer 12 , 741–752 (2012).
Tummala, K. S. et al. Inhibition of de novo NAD(+) synthesis by oncogenic URI causes liver tumorigenesis through DNA damage. Cancer Cell 26 , 826–839 (2014).
Amobi-McCloud, A. et al. IDO1 expression in ovarian cancer induces PD-1 in T cells via aryl hydrocarbon receptor activation. Front. Immunol. 12 , 678999 (2021).
Schramme, F. et al. Inhibition Of tryptophan-dioxygenase activity increases the antitumor efficacy of immune checkpoint inhibitors. Cancer Immunol. Res. 8 , 32–45 (2020).
Geiger, R. et al. L-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167 , 829–842.e813 (2016).
Arlauckas, S. P. et al. Arg1 expression defines immunosuppressive subsets of tumor-associated macrophages. Theranostics 8 , 5842–5854 (2018).
Grzywa, T. M. et al. Myeloid cell-derived arginase in cancer immune response. Front. Immunol. 11 , 938 (2020).
Sosnowska, A. et al. Inhibition of arginase modulates T-cell response in the tumor microenvironment of lung carcinoma. Oncoimmunology 10 , 1956143 (2021).
Xu, H. et al. Ferroptosis in the tumor microenvironment: perspectives for immunotherapy. Trends Mol. Med. 27 , 856–867 (2021).
Srivastava, M. K. et al. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 70 , 68–77 (2010).
Wu, J. et al. Asparagine enhances LCK signalling to potentiate CD8(+) T-cell activation and anti-tumour responses. Nat.Cell Biol. 23 , 75–86 (2021).
Bian, Y. et al. Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature 585 , 277–282 (2020).
Taylor, S. R., Falcone, J. N., Cantley, L. C. & Goncalves, M. D. Developing dietary interventions as therapy for cancer. Nat. Rev. Cancer 22 , 452–466 (2022).
Badr, C. E., Silver, D. J., Siebzehnrubl, F. A. & Deleyrolle, L. P. Metabolic heterogeneity and adaptability in brain tumors. Cell Mol. Life Sci. 77 , 5101–5119 (2020).
Venneti, S. & Thompson, C. B. Metabolic reprogramming in brain tumors. Annu. Rev. Pathol. 12 , 515–545 (2017).
Maurer, G. D. et al. Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy. BMC Cancer 11 , 315 (2011).
Elia, I., Schmieder, R., Christen, S. & Fendt, S. M. Organ-specific cancer metabolism and its potential for therapy. Handb. Exp. Pharmacol. 233 , 321–353 (2016).
Dai, W. et al. OGDHL silencing promotes hepatocellular carcinoma by reprogramming glutamine metabolism. J. Hepatol. 72 , 909–923 (2020).
Sangineto, M. et al. Lipid metabolism in development and progression of hepatocellular carcinoma. Cancers 12 , 1419 (2020).
Steck, S. E. & Murphy, E. A. Dietary patterns and cancer risk. Nat. Rev. Cancer 20 , 125–138 (2020).
Kanarek, N., Petrova, B. & Sabatini, D. M. Dietary modifications for enhanced cancer therapy. Nature 579 , 507–517 (2020).
Article ADS CAS PubMed Google Scholar
Lean, M. E. J., Astrup, A. & Roberts, S. B. Making progress on the global crisis of obesity and weight management. Bmj 361 , k2538 (2018).
Li, Z. et al. Aging and age-related diseases: from mechanisms to therapeutic strategies. Biogerontology 22 , 165–187 (2021).
Article PubMed Central Google Scholar
O’Flanagan, C. H., Smith, L. A., McDonell, S. B. & Hursting, S. D. When less may be more: calorie restriction and response to cancer therapy. BMC Med. 15 , 106 (2017).
Salvadori, G., Mirisola, M. G. & Longo, V. D. Intermittent and periodic fasting, hormones, and cancer prevention. Cancers 13 , 4587 (2021).
Kalaany, N. Y. & Sabatini, D. M. Tumours with PI3K activation are resistant to dietary restriction. Nature 458 , 725–731 (2009).
Ma, D. et al. Upregulation of the ALDOA/DNA-PK/p53 pathway by dietary restriction suppresses tumor growth. Oncogene 37 , 1041–1048 (2018).
Ma, Z. et al. Caloric restriction inhibits mammary tumorigenesis in MMTV-ErbB2 transgenic mice through the suppression of ER and ErbB2 pathways and inhibition of epithelial cell stemness in premalignant mammary tissues. Carcinogenesis 39 , 1264–1273 (2018).
Vidoni, C. et al. Calorie restriction for cancer prevention and therapy: mechanisms, expectations, and efficacy. J. Cancer Prev. 26 , 224 (2021).
Pomatto-Watson, L. C. D. et al. Daily caloric restriction limits tumor growth more effectively than caloric cycling regardless of dietary composition. Nat. Commun. 12 , 6201 (2021).
Pistollato, F. et al. Effects of caloric restriction on immunosurveillance, microbiota and cancer cell phenotype: possible implications for cancer treatment. Semin. Cancer Biol. 73 , 45–57 (2021).
Madeo, F., Carmona-Gutierrez, D., Hofer, S. J. & Kroemer, G. Caloric restriction mimetics against age-associated disease: targets, mechanisms, and therapeutic potential. Cell Metab. 29 , 592–610 (2019).
Zitvogel, L., Pietrocola, F. & Kroemer, G. Nutrition, inflammation and cancer. Nat. Immunol. 18 , 843–850 (2017).
Pietrocola, F. et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 30 , 147–160 (2016).
Deus, C. M. et al. Sirtuin 1-dependent resveratrol cytotoxicity and pro-differentiation activity on breast cancer cells. Arch. Toxicol. 91 , 1261–1278 (2017).
Turbitt, W. J. et al. Physical activity plus energy restriction prevents 4T1.2 mammary tumor progression, MDSC accumulation, and an immunosuppressive tumor microenvironment. Cancer Prev. Res. 12 , 493–506 (2019).
Article CAS Google Scholar
Caccialanza, R., Aprile, G., Cereda, E. & Pedrazzoli, P. Fasting in oncology: a word of caution. Nat. Rev. Cancer 19 , 177 (2019).
Castejón, M. et al. Energy restriction and colorectal cancer: a call for additional research. Nutrients 12 , 114 (2020).
Petersen, M. C. et al. Complex physiology and clinical implications of time-restricted eating. Physiol. Rev. 102 , 1991–2034 (2022).
Turbitt, W. J., Demark-Wahnefried, W., Peterson, C. M. & Norian, L. A. Targeting glucose metabolism to enhance immunotherapy: emerging evidence on intermittent fasting and calorie restriction mimetics. Front. Immunol. 10 , 1402 (2019).
Isaac-Lam, M. F. & DeMichael, K. M. Calorie restriction and breast cancer treatment: a mini-review. J. Mol. Med. 100 , 1095–1109 (2022).
Zhang, J., Deng, Y. & Khoo, B. L. Fasting to enhance cancer treatment in models: the next steps. J. Biomed. Sci. 27 , 58 (2020).
Nencioni, A., Caffa, I., Cortellino, S. & Longo, V. D. Fasting and cancer: molecular mechanisms and clinical application. Nat. Rev. Cancer 18 , 707–719 (2018).
Ajona, D. et al. Short-term starvation reduces IGF-1 levels to sensitize lung tumors to PD-1 immune checkpoint blockade. Nat. Cancer 1 , 75–85 (2020).
Das, M. et al. Time-restricted feeding normalizes hyperinsulinemia to inhibit breast cancer in obese postmenopausal mouse models. Nat. Commun. 12 , 565 (2021).
Bianchi, G. et al. Fasting induces anti-Warburg effect that increases respiration but reduces ATP-synthesis to promote apoptosis in colon cancer models. Oncotarget 6 , 11806–11819 (2015).
Blaževitš, O., Di Tano, M. & Longo, V. D. Fasting and fasting mimicking diets in cancer prevention and therapy. Trends Cancer 9 , 212–222 (2023).
Di Biase, S. et al. Fasting-mimicking diet reduces HO-1 to promote T cell-mediated tumor cytotoxicity. Cancer Cell 30 , 136–146 (2016).
Sun, P. et al. Fasting inhibits colorectal cancer growth by reducing M2 polarization of tumor-associated macrophages. Oncotarget 8 , 74649–74660 (2017).
Vernieri, C. et al. Fasting-mimicking diet is safe and reshapes metabolism and antitumor immunity in patients with cancer. Cancer Discov. 12 , 90–107 (2022).
Ligorio, F. et al. Exceptional tumour responses to fasting-mimicking diet combined with standard anticancer therapies: a sub-analysis of the NCT03340935 trial. Eur. J. Cancer 172 , 300–310 (2022).
Zhang, X. et al. Impact of diets on response to immune checkpoint inhibitors (ICIs) Therapy against tumors. Life 12 , 409 (2022).
Article ADS MathSciNet CAS PubMed Central PubMed Google Scholar
Thau-Zuchman, O. et al. A new ketogenic formulation improves functional outcome and reduces tissue loss following traumatic brain injury in adult mice. Theranostics 11 , 346 (2021).
Bandera-Merchan, B. et al. Ketotherapy as an epigenetic modifier in cancer. Rev. Endocr. Metab. Disord. 21 , 509–519 (2020).
Simeone, T. A., Simeone, K. A., Stafstrom, C. E. & Rho, J. M. Do ketone bodies mediate the anti-seizure effects of the ketogenic diet? Neuropharmacology 133 , 233–241 (2018).
Talib, W. H. et al. Ketogenic diet in cancer prevention and therapy: molecular targets and therapeutic opportunities. Curr. Issues Mol. Biol. 43 , 558–589 (2021).
Zhu, H. et al. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Signal Transduct. Target. Ther. 7 , 11 (2022).
Shah, U. A. & Iyengar, N. M. Plant-based and ketogenic diets as diverging paths to address cancer: a review. JAMA Oncol. 8 , 1201–1208 (2022).
Dmitrieva-Posocco, O. et al. β-Hydroxybutyrate suppresses colorectal cancer. Nature 605 , 160–165 (2022).
Li, B. et al. Glucose restriction induces AMPK-SIRT1-mediated circadian clock gene per expression and delays NSCLC progression. Cancer Lett. 576 , 216424 (2023).
Chen, Y. et al. Metabolic intervention by low carbohydrate diet suppresses the onset and progression of neuroendocrine tumors. Cell Death Dis. 14 , 597 (2023).
Hirschberger, S. et al. Very-low-carbohydrate diet enhances human T-cell immunity through immunometabolic reprogramming. EMBO Mol. Med. 13 , e14323 (2021).
Zhang, N. et al. Ketogenic diet elicits antitumor properties through inducing oxidative stress, inhibiting MMP-9 expression, and rebalancing M1/M2 Tumor-associated macrophage phenotype in a mouse model of colon cancer. J. Agric. Food Chem. 68 , 11182–11196 (2020).
Lussier, D. M. et al. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer 16 , 310 (2016).
Kim, A. J., Hong, D. S. & George, G. C. Dietary influences on symptomatic and non-symptomatic toxicities during cancer treatment: a narrative review. Cancer Treat. Rev. 108 , 102408 (2022).
Kenig, S. et al. Assessment of micronutrients in a 12-wk ketogenic diet in obese adults. Nutrition 67 - 68 , 110522 (2019).
Manolis, A. S., Manolis, T. A., Manolis, A. A. & Melita, H. Diet and sudden death: how to reduce the risk. Curr. Vasc. Pharmacol. 20 , 383–408 (2022).
Ferrer, M. et al. Ketogenic diet promotes tumor ferroptosis but induces relative corticosterone deficiency that accelerates cachexia. Cell Metab. 35 , 1147–1162.e1147 (2023).
Levine, M. E. et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 19 , 407–417 (2014).
Article CAS PubMed Central Google Scholar
Yin, J. et al. Protein restriction and cancer. Biochim. Biophys. Acta Rev. Cancer 1869 , 256–262 (2018).
Jiménez-Alonso, J. J. & López-Lázaro, M. Dietary manipulation of amino acids for cancer therapy. Nutrients 15 , 2879 (2023).
Shunxi, W. et al. Serine metabolic reprogramming in tumorigenesis, tumor immunity, and clinical treatment. Adv. Nutr. 14 , 1050–1066 (2023).
Maddocks, O. D. K. et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 544 , 372–376 (2017).
Li, T. et al. Methionine deficiency facilitates antitumour immunity by altering m(6)A methylation of immune checkpoint transcripts. Gut 72 , 501–511 (2023).
Orillion, A. et al. Dietary protein restriction reprograms tumor-associated macrophages and enhances immunotherapy. Clin. Cancer Res. 24 , 6383–6395 (2018).
Zhang, X. et al. Reprogramming tumour-associated macrophages to outcompete cancer cells. Nature 619 , 616–623 (2023).
Rubio-Patiño, C. et al. Low-protein diet induces IRE1α-dependent anticancer immunosurveillance. Cell Metab. 27 , 828–842.e827 (2018).
Jing, W. et al. Metabolic modulation of intracellular ammonia via intravesical instillation of nanoporter-encased hydrogel eradicates bladder carcinoma. Adv. Sci. 10 , e2206893 (2023).
Liu, M. et al. Elevated urinary urea by high-protein diet could be one of the inducements of bladder disorders. J. Transl Med. 14 , 53 (2016).
Pimentel, G. D., Pichard, C., Laviano, A. & Fernandes, R. C. High protein diet improves the overall survival in older adults with advanced gastrointestinal cancer. Clin. Nutr. 40 , 1376–1380 (2021).
Lieu, E. L., Nguyen, T., Rhyne, S. & Kim, J. Amino acids in cancer. Exp. Mol. Med. 52 , 15–30 (2020).
Du, H. et al. Detachable MOF-based core/shell nanoreactor for cancer dual-starvation therapy with reversing glucose and glutamine metabolisms. Small 19 , e2303253 (2023).
Leone, R. D. et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 366 , 1013–1021 (2019).
Oh, M. H. et al. Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J. Clin. Investig. 130 , 3865–3884 (2020).
Gross, M. I. et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol. Cancer Ther. 13 , 890–901 (2014).
Tannir, N. M. et al. CANTATA: a randomized phase 2 study of CB-839 in combination with cabozantinib vs. placebo with cabozantinib in patients with advanced/metastatic renal cell carcinoma. J. Clin. Oncol. 36 , TPS4601–TPS4601 (2018).
Wu, Q. et al. Metabolic regulation in the immune response to cancer. Cancer Commun. 41 , 661–694 (2021).
Guo, Y. et al. Indoleamine 2,3-dioxygenase (Ido) inhibitors and their nanomedicines for cancer immunotherapy. Biomaterials 276 , 121018 (2021).
Tang, K., Wu, Y. H., Song, Y. & Yu, B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J. Hematol. Oncol. 14 , 68 (2021).
Jochems, C. et al. The IDO1 selective inhibitor epacadostat enhances dendritic cell immunogenicity and lytic ability of tumor antigen-specific T cells. Oncotarget 7 , 37762–37772 (2016).
Cramer, S. L. et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat. Med. 23 , 120–127 (2017).
Badgley, M. A. et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 368 , 85–89 (2020).
Wilck, N. et al. The role of sodium in modulating immune cell function. Nat. Rev. Nephrol. 15 , 546–558 (2019).
Li, X. et al. The modulatory effect of high salt on immune cells and related diseases. Cell Prolif. 55 , e13250 (2022).
Rizvi, Z. A. et al. High-salt diet mediates interplay between NK cells and gut microbiota to induce potent tumor immunity. Sci. Adv. 7 , eabg5016 (2021).
Willebrand, R. et al. High salt inhibits tumor growth by enhancing anti-tumor immunity. Front. Immunol. 10 , 1141 (2019).
He, W. et al. High-salt diet inhibits tumour growth in mice via regulating myeloid-derived suppressor cell differentiation. Nat. Commun. 11 , 1732 (2020).
Hernandez, A. L. et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J. Clin. Investig. 125 , 4212–4222 (2015).
Xu, Y. et al. High salt intake attenuates breast cancer metastasis to lung. J. Agric. Food Chem. 66 , 3386–3392 (2018).
Allu, A. S. & Tiriveedhi, V. Cancer salt nostalgia. Cells 10 , 1285 (2021).
Wu, C. et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496 , 513–517 (2013).
Article ADS CAS PubMed Central Google Scholar
Chen, J. et al. High salt diet may promote progression of breast tumor through eliciting immune response. Int. Immunopharmacol. 87 , 106816 (2020).
Amara, S., Ivy, M. T., Myles, E. L. & Tiriveedhi, V. Sodium channel γENaC mediates IL-17 synergized high salt induced inflammatory stress in breast cancer cells. Cell Immunol. 302 , 1–10 (2016).
Huangfu, L., Li, R., Huang, Y. & Wang, S. The IL-17 family in diseases: from bench to bedside. Signal Transduct. Target. Ther. 8 , 402 (2023).
Zeng, X. et al. A high-salt diet disturbs the development and function of natural killer cells in mice. J. Immunol. Res. 2020 , 6687143 (2020).
Yu, W. et al. Contradictory roles of lipid metabolism in immune response within the tumor microenvironment. J. Hematol. Oncol. 14 , 187 (2021).
Deng, T. et al. Obesity, inflammation, and cancer. Annu. Rev. Pathol. 11 , 421–449 (2016).
Peng, L. et al. Association between low-fat diet and liver cancer risk in 98,455 participants: results from a prospective study. Front. Nutr. 9 , 1013643 (2022).
Chlebowski, R. T. et al. Low-fat dietary pattern and breast cancer mortality in the women’s health initiative randomized controlled trial. J. Clin. Oncol. 35 , 2919–2926 (2017).
Barbi, J. et al. Visceral obesity promotes lung cancer progression-toward resolution of the obesity paradox in lung cancer. J. Thorac. Oncol. 16 , 1333–1348 (2021).
Gomes, A. L. et al. Metabolic inflammation-associated IL-17A causes non-alcoholic steatohepatitis and hepatocellular carcinoma. Cancer Cell 30 , 161–175 (2016).
Ericksen, R. E. et al. Obesity accelerates helicobacter felis-induced gastric carcinogenesis by enhancing immature myeloid cell trafficking and TH17 response. Gut 63 , 385–394 (2014).
Yadav, A. K. et al. Activity-based NIR bioluminescence probe enables discovery of diet-induced modulation of the tumor microenvironment via nitric oxide. ACS Cent. Sci. 8 , 461–472 (2022).
Hayashi, T. et al. High-fat diet-induced inflammation accelerates prostate cancer growth via IL6 signaling. Clin. Cancer Res. 24 , 4309–4318 (2018).
Wunderlich, C. M. et al. Obesity exacerbates colitis-associated cancer via IL-6-regulated macrophage polarisation and CCL-20/CCR-6-mediated lymphocyte recruitment. Nat. Commun. 9 , 1646 (2018).
Incio, J. et al. PlGF/VEGFR-1 signaling promotes macrophage polarization and accelerated tumor progression in obesity. Clin. Cancer Res. 22 , 2993–3004 (2016).
Peng, J. et al. Diet-induced obesity accelerates oral carcinogenesis by recruitment and functional enhancement of myeloid-derived suppressor cells. Cell Death Dis. 12 , 946 (2021).
Gibson, J. T. et al. Obesity-associated myeloid-derived suppressor cells promote apoptosis of tumor-infiltrating CD8 T cells and immunotherapy resistance in breast cancer. Front. Immunol. 11 , 590794 (2020).
Ringel, A. E. et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell 183 , 1848–1866.e1826 (2020).
Dyck, L. et al. Suppressive effects of the obese tumor microenvironment on CD8 T cell infiltration and effector function. J. Exp. Med. 219 , e20210042 (2022).
Yamada, K. et al. Reduced number and immune dysfunction of CD4+ T cells in obesity accelerate colorectal cancer progression. Cells 12 , 86 (2022).
Incio, J. et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 6 , 852–869 (2016).
Altea-Manzano, P. et al. A palmitate-rich metastatic niche enables metastasis growth via p65 acetylation resulting in pro-metastatic NF-κB signaling. Nat. Cancer 4 , 344–364 (2023).
Wang, Z. et al. Extracellular vesicles in fatty liver promote a metastatic tumor microenvironment. Cell Metab. 35 , 1209–1226.e1213 (2023).
Chen, M. et al. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nat. Genet. 50 , 206–218 (2018).
Pascual, G. et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541 , 41–45 (2017).
Terry, A. R. et al. CD36 maintains lipid homeostasis via selective uptake of monounsaturated fatty acids during matrix detachment and tumor progression. Cell Metab. 35 , 2060–2076.e2069 (2023).
Garcia-Estevez, L. & Moreno-Bueno, G. Updating the role of obesity and cholesterol in breast cancer. Breast Cancer Res. 21 , 35 (2019).
Du, Q. et al. Dietary cholesterol promotes AOM-induced colorectal cancer through activating the NLRP3 inflammasome. Biochem. Pharmacol. 105 , 42–54 (2016).
Liu, C. et al. Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway. Cell Death Differ. 27 , 1765–1781 (2020).
Asghari, A. & Umetani, M. Obesity and cancer: 27-Hydroxycholesterol, the missing link. Int. J. Mol. Sci. 21 , 4822 (2020).
Baek, A. E. et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat. Commun. 8 , 864 (2017).
Qin, W. H. et al. High serum levels of cholesterol increase antitumor functions of nature killer cells and reduce growth of liver tumors in mice. Gastroenterology 158 , 1713–1727 (2020).
Liu, L. et al. Consumption of the fish oil high-fat diet uncouples obesity and mammary tumor growth through induction of reactive oxygen species in protumor macrophages. Cancer Res. 80 , 2564–2574 (2020).
Liang, P. et al. Role of host GPR120 in mediating dietary omega-3 fatty acid inhibition of prostate cancer. J. Natl Cancer Inst. 111 , 52–59 (2019).
Jin, R. et al. Dietary fats high in linoleic acids impair antitumor T-cell responses by inducing E-FABP-mediated mitochondrial dysfunction. Cancer Res. 81 , 5296–5310 (2021).
Plesca, I. et al. Characteristics of tumor-infiltrating lymphocytes prior to and during immune checkpoint inhibitor therapy. Front. Immunol. 11 , 364 (2020).
Marin-Acevedo, J. A., Kimbrough, E. O. & Lou, Y. Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol. 14 , 45 (2021).
Luo, C. et al. Progress and prospect of immunotherapy for triple-negative breast cancer. Front. Oncol. 12 , 919072 (2022).
Shergold, A. L., Millar, R. & Nibbs, R. J. Understanding and overcoming the resistance of cancer to PD-1/PD-L1 blockade. Pharmacol. Res. 145 , 104258 (2019).
Xia, L., Liu, Y. & Wang, Y. PD-1/PD-L1 blockade therapy in advanced non-small-cell lung cancer: current status and future directions. Oncologist 24 , S31–S41 (2019).
Spyrou, N., Vallianou, N., Kadillari, J. & Dalamaga, M. The interplay of obesity, gut microbiome and diet in the immune check point inhibitors therapy era. Semin. Cancer Biol. 73 , 356–376 (2021).
Zitvogel, L. & Kroemer, G. Boosting the immunotherapy response by nutritional interventions. J. Clin. Investig. 132 , e161483 (2022).
Coleman, M. F. et al. Cell intrinsic and systemic metabolism in tumor immunity and immunotherapy. Cancers 12 , 852 (2020).
Farazi, M. et al. Caloric restriction maintains OX40 agonist-mediated tumor immunity and CD4 T cell priming during aging. Cancer Immunol. Immunother. 63 , 615–626 (2014).
Lévesque, S. et al. A synergistic triad of chemotherapy, immune checkpoint inhibitors, and caloric restriction mimetics eradicates tumors in mice. Oncoimmunology 8 , e1657375 (2019).
de Gruil, N., Pijl, H., van der Burg, S. H. & Kroep, J. R. Short-term fasting synergizes with solid cancer therapy by boosting antitumor immunity. Cancers 14 , 1390 (2022).
Cortellino, S. et al. Fasting renders immunotherapy effective against low-immunogenic breast cancer while reducing side effects. Cell Rep. 40 , 111256 (2022).
Ferrere, G. et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight 6 , e145207 (2021).
Dai, X. et al. Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade. Mol. Cell 81 , 2317–2331.e2316 (2021).
Yue, T. et al. Hydrogen sulfide creates a favorable immune microenvironment for colon cancer. Cancer Res. 83 , 595–612 (2023).
Wang, W. et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569 , 270–274 (2019).
Fujiwara, Y. et al. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat. Rev. 110 , 102461 (2022).
Mitchell, T. C. et al. Epacadostat plus pembrolizumab in patients with advanced solid tumors: phase I results from a multicenter, open-label phase I/II trial (ECHO-202/KEYNOTE-037). J. Clin. Oncol. 36 , 3223–3230 (2018).
Long, G. V. et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 20 , 1083–1097 (2019).
Assumpção, J. A. F. et al. The ambiguous role of obesity in oncology by promoting cancer but boosting antitumor immunotherapy. J. Biomed. Sci. 29 , 12 (2022).
Murphy, W. J. & Longo, D. L. The surprisingly positive association between obesity and cancer immunotherapy efficacy. JAMA 321 , 1247–1248 (2019).
Wang, Z. et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 25 , 141–151 (2019).
Zhao, B. et al. Research progress of conjugated nanomedicine for cancer treatment. Pharmaceutics 14 , 1522 (2022).
Schirrmacher, V. From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 54 , 407–419 (2019).
Raffaghello, L. et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc. Natl Acad. Sci. USA 105 , 8215–8220 (2008).
Lee, C. et al. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 70 , 1564–1572 (2010).
D’Aronzo, M. et al. Fasting cycles potentiate the efficacy of gemcitabine treatment in in vitro and in vivo pancreatic cancer models. Oncotarget 6 , 18545–18557 (2015).
Pateras, I. S. et al. Short term starvation potentiates the efficacy of chemotherapy in triple negative breast cancer via metabolic reprogramming. J. Transl Med. 21 , 169 (2023).
Di Tano, M. et al. Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers. Nat. Commun. 11 , 2332 (2020).
Liu, X. et al. Fasting-mimicking diet synergizes with ferroptosis against quiescent, chemotherapy-resistant cells. EBioMedicine 90 , 104496 (2023).
Cheng, C. W. et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell 14 , 810–823 (2014).
Green, C. L., Lamming, D. W. & Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. 23 , 56–73 (2022).
de Groot, S. et al. Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 DIRECT trial. Nat. Commun. 11 , 3083 (2020).
Morscher, R. J. et al. Combination of metronomic cyclophosphamide and dietary intervention inhibits neuroblastoma growth in a CD1-nu mouse model. Oncotarget 7 , 17060–17073 (2016).
Aminzadeh-Gohari, S. et al. A ketogenic diet supplemented with medium-chain triglycerides enhances the anti-tumor and anti-angiogenic efficacy of chemotherapy on neuroblastoma xenografts in a CD1-nu mouse model. Oncotarget 8 , 64728–64744 (2017).
Yang, L. et al. Ketogenic diet and chemotherapy combine to disrupt pancreatic cancer metabolism and growth. Med. 3 , 119–136, (2022).
Manukian, G. et al. Caloric restriction impairs regulatory T cells within the tumor microenvironment after radiation and primes effector T cells. Int. J. Radiat. Oncol. Biol. Phys. 110 , 1341–1349 (2021).
Allen, B. G. et al. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin. Cancer Res. 19 , 3905–3913 (2013).
Zahra, A. et al. Consuming a ketogenic diet while receiving radiation and chemotherapy for locally advanced lung cancer and pancreatic cancer: the university of iowa experience of two phase 1 clinical trials. Radiat. Res. 187 , 743–754 (2017).
Gao, X. et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 572 , 397–401 (2019).
Caffa, I. et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 583 , 620–624 (2020).
Caffa, I. et al. Fasting potentiates the anticancer activity of tyrosine kinase inhibitors by strengthening MAPK signaling inhibition. Oncotarget 6 , 11820–11832 (2015).
Krstic, J. et al. Fasting improves therapeutic response in hepatocellular carcinoma through p53-dependent metabolic synergism. Sci. Adv. 8 , eabh2635 (2022).
Hopkins, B. D. et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560 , 499–503 (2018).
Gravel, S. P. et al. Serine deprivation enhances antineoplastic activity of biguanides. Cancer Res. 74 , 7521–7533 (2014).
Lee, K. A. et al. Role of the gut microbiome for cancer patients receiving immunotherapy: dietary and treatment implications. Eur. J. Cancer 138 , 149–155 (2020).
Ogunrinola, G. A., Oyewale, J. O., Oshamika, O. O. & Olasehinde, G. I. The human microbiome and its impacts on health. Int. J. Microbiol. 2020 , 8045646 (2020).
Greathouse, K. L. et al. Diet-microbiome interactions in cancer treatment: opportunities and challenges for precision nutrition in cancer. Neoplasia 29 , 100800 (2022).
Zeng, X. et al. Gut bacterial nutrient preferences quantified in vivo. Cell 185 , 3441–3456.e3419 (2022).
Soldati, L. et al. The influence of diet on anti-cancer immune responsiveness. J. Transl Med. 16 , 75 (2018).
Duan, H. et al. Antibiotic-induced gut dysbiosis and barrier disruption and the potential protective strategies. Crit. Rev. Food Sci. Nutr. 62 , 1427–1452 (2022).
Liu, L. et al. Association between inflammatory diet pattern and risk of colorectal carcinoma subtypes classified by immune responses to tumor. Gastroenterology 153 , 1517–1530.e1514 (2017).
Liu, L. et al. Diets that promote colon inflammation associate with risk of colorectal carcinomas that contain fusobacterium nucleatum. Clin. Gastroenterol. Hepatol. 16 , 1622–1631.e1623 (2018).
Mehta, R. S. et al. Association of dietary patterns with risk of colorectal cancer subtypes classified by fusobacterium nucleatum in tumor tissue. JAMA Oncol. 3 , 921–927 (2017).
Shimomura, Y. et al. Mediation effect of intestinal microbiota on the relationship between fiber intake and colorectal cancer. Int. J. Cancer 152 , 1752–1762 (2023).
Simpson, R. C. et al. Diet-driven microbial ecology underpins associations between cancer immunotherapy outcomes and the gut microbiome. Nat. Med. 28 , 2344–2352 (2022).
Lu, Y. et al. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J. Hematol. Oncol. 15 , 47 (2022).
Klement, R. J. & Pazienza, V. Impact of different types of diet on gut microbiota profiles and cancer prevention and treatment. Medicina 55 , 84 (2019).
Luu, M. & Visekruna, A. Short-chain fatty acids: bacterial messengers modulating the immunometabolism of T cells. Eur. J. Immunol. 49 , 842–848 (2019).
Martin-Gallausiaux, C. et al. SCFA: mechanisms and functional importance in the gut. Proc. Nutr. Soc. 80 , 37–49 (2021).
Dong, Y. et al. Gut microbiota-derived short-chain fatty acids regulate gastrointestinal tumor immunity: a novel therapeutic strategy? Front. Immunol. 14 , 1158200 (2023).
Luu, M. et al. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 12 , 4077 (2021).
Mager, L. F. et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 369 , 1481–1489 (2020).
Wang, T. et al. Inosine is an alternative carbon source for CD8(+)-T-cell function under glucose restriction. Nat. Metab. 2 , 635–647 (2020).
Canale, F. P. et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 598 , 662–666 (2021).
Mao, Y. Q. et al. The antitumour effects of caloric restriction are mediated by the gut microbiome. Nat. Metab. 5 , 96–110 (2023).
Ang, Q. Y. et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell 181 , 1263–1275.e1216 (2020).
Hwang, S. et al. Dietary salt administration decreases enterotoxigenic bacteroides fragilis (ETBF)-promoted tumorigenesis via inhibition of colonic inflammation. Int. J. Mol. Sci. 21 , 8034 (2020).
Gaddy, J. A. et al. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect. Immun. 81 , 2258–2267 (2013).
Münch, N. S. et al. High-fat diet accelerates carcinogenesis in a mouse model of Barrett’s esophagus via interleukin 8 and alterations to the gut microbiome. Gastroenterology 157 , 492–506.e492 (2019).
Sun, L. et al. Bile salt hydrolase in non-enterotoxigenic bacteroides potentiates colorectal cancer. Nat. Commun. 14 , 755 (2023).
Liu, T. et al. High-fat diet-induced dysbiosis mediates MCP-1/CCR2 axis-dependent M2 macrophage polarization and promotes intestinal adenoma-adenocarcinoma sequence. J. Cell Mol. Med. 24 , 2648–2662 (2020).
Villemin, C. et al. The heightened importance of the microbiome in cancer immunotherapy. Trends Immunol. 44 , 44–59 (2023).
Singh, A., Alexander, S. G. & Martin, S. Gut microbiome homeostasis and the future of probiotics in cancer immunotherapy. Front. Immunol. 14 , 1114499 (2023).
Park, E. M. et al. Targeting the gut and tumor microbiota in cancer. Nat. Med. 28 , 690–703 (2022).
Spencer, C. N. et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 374 , 1632–1640 (2021).
Lam, K. C. et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell 184 , 5338–5356.e5321 (2021).
Paden, H. et al. Dietary impacts on changes in diversity and abundance of the murine microbiome during progression and treatment of cancer. Nutrients 15 , 724 (2023).
Han, K. et al. Generation of systemic antitumour immunity via the in situ modulation of the gut microbiome by an orally administered inulin gel. Nat. Biomed. Eng. 5 , 1377–1388 (2021).
Zhang, S. L. et al. Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice humanized with gut microbiota from patients with colorectal cancer. Theranostics 11 , 4155–4170 (2021).
Shi, Y. et al. Intratumoral accumulation of gut microbiota facilitates CD47-based immunotherapy via STING signaling. J. Exp. Med. 217 , e20192282 (2020).
Bender, M. J. et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell 186 , 1846–1862.e1826 (2023).
Kesh, K. et al. Obesity enriches for tumor protective microbial metabolites and treatment refractory cells to confer therapy resistance in PDAC. Gut Microbes 14 , 2096328 (2022).
Tintelnot, J. et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature 615 , 168–174 (2023).
Kurowska, A., Ziemichód, W., Herbet, M. & Piątkowska-Chmiel, I. The role of diet as a modulator of the inflammatory process in the neurological diseases. Nutrients. 15 , 1436 (2023).
Chu, C. Q. et al. Can dietary patterns prevent cognitive impairment and reduce Alzheimer’s disease risk: exploring the underlying mechanisms of effects. Neurosci. Biobehav. Rev. 135 , 104556 (2022).
Ułamek-Kozioł, M., Czuczwar, S. J., Januszewski, S. & Pluta, R. Ketogenic diet and epilepsy. Nutrients. 11 , 2510 (2019).
Neal, E. G. et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 7 , 500–506 (2008).
Devi, N. et al. Efficacy and safety of dietary therapies for childhood drug-resistant epilepsy: a systematic review and network meta-analysis. JAMA Pediatr. 177 , 258–266 (2023).
Calderón, N., Betancourt, L., Hernández, L. & Rada, P. A ketogenic diet modifies glutamate, gamma-aminobutyric acid and agmatine levels in the hippocampus of rats: a microdialysis study. Neurosci. Lett. 642 , 158–162 (2017).
Rudy, L. et al. Anticonvulsant mechanisms of the ketogenic diet and caloric restriction. Epilepsy Res. 168 , 106499 (2020).
Napolitano, A. et al. The ketogenic diet increases in vivo glutathione levels in patients with epilepsy. Metabolites 10 , 504 (2020).
Knowles, S. et al. Ketogenic diet regulates the antioxidant catalase via the transcription factor PPARγ2. Epilepsy Res. 147 , 71–74 (2018).
Yellen, G. Ketone bodies, glycolysis, and KATP channels in the mechanism of the ketogenic diet. Epilepsia 49 , 80–82 (2008).
Olson, C. A. et al. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173 , 1728–1741.e1713 (2018).
Dahlin, M. et al. Higher levels of Bifidobacteria and tumor necrosis factor in children with drug-resistant epilepsy are associated with anti-seizure response to the ketogenic diet. EBioMedicine 80 , 104061 (2022).
Nianogo, R. A. et al. Risk factors associated with alzheimer disease and related dementias by sex and race and ethnicity in the US. JAMA Neurol. 79 , 584–591, (2022).
Suzzi, S. et al. N-acetylneuraminic acid links immune exhaustion and accelerated memory deficit in diet-induced obese Alzheimer’s disease mouse model. Nat. Commun. 14 , 1293 (2023).
Pan, W. et al. Dimethyl itaconate ameliorates cognitive impairment induced by a high-fat diet via the gut-brain axis in mice. Microbiome 11 , 30 (2023).
Phillips, M. C. L. et al. Randomized crossover trial of a modified ketogenic diet in Alzheimer’s disease. Alzheimers Res. Ther. 13 , 51 (2021).
Coelho-Júnior, H. J., Trichopoulou, A. & Panza, F. Cross-sectional and longitudinal associations between adherence to Mediterranean diet with physical performance and cognitive function in older adults: a systematic review and meta-analysis. Ageing Res. Rev. 70 , 101395 (2021).
Elias, A., Padinjakara, N. & Lautenschlager, N. T. Effects of intermittent fasting on cognitive health and Alzheimer’s disease. Nutr. Rev. 81 , 1225–1233 (2023).
Ballarini, T. et al. Mediterranean diet, Alzheimer disease biomarkers and brain atrophy in old age. Neurology. 96 , e2920–e2932 (2021).
Jhanji, M. et al. Cis- and trans-resveratrol have opposite effects on histone serine-ADP-ribosylation and tyrosine induced neurodegeneration. Nat. Commun. 13 , 3244 (2022).
Bonda, D. J. et al. The sirtuin pathway in ageing and Alzheimer disease: mechanistic and therapeutic considerations. Lancet Neurol. 10 , 275–279 (2011).
Shippy, D. C. et al. β-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer’s disease pathology. J. Neuroinflammation 17 , 280 (2020).
Nagpal, R. et al. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 47 , 529–542 (2019).
Dilmore, A. H. et al. Effects of a ketogenic and low-fat diet on the human metabolome, microbiome, and foodome in adults at risk for Alzheimer’s disease. Alzheimers Dement. 19 , 4805–4816 (2023).
Travagli, R. A., Browning, K. N. & Camilleri, M. Parkinson disease and the gut: new insights into pathogenesis and clinical relevance. Nat. Rev. Gastroenterol. Hepatol. 17 , 673–685 (2020).
Augustin, A. et al. Faecal metabolite deficit, gut inflammation and diet in Parkinson’s disease: Integrative analysis indicates inflammatory response syndrome. Clin. Transl Med. 13 , e1152 (2023).
Lin, C. H. et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflammation 16 , 129 (2019).
Nowak, K. L. et al. Serum sodium and cognition in older community-dwelling men. Clin. J. Am. Soc. Nephrol. 13 , 366–374 (2018).
Heras-Garvin, A. et al. High-salt diet does not boost neuroinflammation and neurodegeneration in a model of α-synucleinopathy. J. Neuroinflammation 17 , 35 (2020).
Sofi, F. et al. Adherence to Mediterranean diet and health status: meta-analysis. Bmj 337 , a1344 (2008).
Sampson, T. R. et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167 , 1469–1480.e1412 (2016).
Phillips, M. C. L. et al. Low-fat versus ketogenic diet in Parkinson’s disease: a pilot randomized controlled trial. Mov. Disord. 33 , 1306–1314 (2018).
Zhou, Z. L. et al. Neuroprotection of fasting mimicking diet on MPTP-induced Parkinson’s disease mice via gut microbiota and metabolites. Neurotherapeutics 16 , 741–760 (2019).
Marder, K. et al. Relationship of Mediterranean diet and caloric intake to phenoconversion in huntington disease. JAMA Neurol. 70 , 1382–1388 (2013).
PubMed Central PubMed Google Scholar
Bushara, K. O., Nance, M. & Gomez, C. M. Antigliadin antibodies in huntington’s disease. Neurology 62 , 132–133 (2004).
Duan, W. et al. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc. Natl Acad. Sci. USA 100 , 2911–2916 (2003).
Nieves, J. W. et al. Association between dietary intake and function in amyotrophic lateral sclerosis. JAMA Neurol. 73 , 1425–1432, (2016).
Fitzgerald, K. C. et al. Dietary ω-3 polyunsaturated fatty acid intake and risk for amyotrophic lateral sclerosis. JAMA Neurol. 71 , 1102–1110, (2014).
Ludolph, A. C. et al. Effect of high-caloric nutrition on survival in amyotrophic lateral sclerosis. Ann. Neurol. 87 , 206–216 (2020).
Fasano, A. Leaky gut and autoimmune diseases. Clin. Rev. Allergy Immunol. 42 , 71–78 (2012).
Sköldstam, L., Hagfors, L. & Johansson, G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann. Rheum. Dis. 62 , 208–214 (2003).
Rosillo, M. et al. Anti-inflammatory and joint protective effects of extra-virgin olive-oil polyphenol extract in experimental arthritis. J. Nutr. Biochem. 25 , 1275–1281 (2014).
Jiang, L. et al. A high-fiber diet synergizes with Prevotella copri and exacerbates rheumatoid arthritis. Cell Mol. Immunol . 19 , 1414–1424 (2022).
Cleland, L. G., James, M. J. & Proudman, S. M. The role of fish oils in the treatment of rheumatoid arthritis. Drugs 63 , 845–853 (2003).
de Pablo, P. et al. High erythrocyte levels of the n-6 polyunsaturated fatty acid linoleic acid are associated with lower risk of subsequent rheumatoid arthritis in a southern European nested case-control study. Ann. Rheum. Dis. 77 , 981–987 (2018).
Proudman, S. M. et al. Fish oil in recent onset rheumatoid arthritis: a randomised, double-blind controlled trial within algorithm-based drug use. Ann. Rheum. Dis. 74 , 89–95 (2015).
Toumi, E. et al. Gut microbiota in systemic lupus erythematosus patients and lupus mouse model: a cross species comparative analysis for biomarker discovery. Front. Immunol. 13 , 943241 (2022).
López, P. et al. Th17 responses and natural IgM antibodies are related to gut microbiota composition in systemic lupus erythematosus patients. Sci. Rep. 6 , 24072 (2016).
Azzouz, D. F. et al. Longitudinal gut microbiome analyses and blooms of pathogenic strains during lupus disease flares. Ann. Rheum. Dis. 82 , 1315–1327 (2023).
Li, Y. et al. Disordered intestinal microbes are associated with the activity of systemic lupus erythematosus. Clin Sci. 133 , 821–838 (2019).
de Medeiros, M. C. S. et al. Dietary intervention and health in patients with systemic lupus erythematosus: a systematic review of the evidence. Crit. Rev. Food Sci. Nutr. 59 , 2666–2673 (2019).
Duarte-García, A. et al. Effect of omega-3 fatty acids on systemic lupus erythematosus disease activity: a systematic review and meta-analysis. Autoimmun. Rev. 19 , 102688 (2020).
Shoenfeld, Y. et al. Vitamin D and systemic lupus erythematosus - the hype and the hope. Autoimmun. Rev. 17 , 19–23 (2018).
Islam, M. A. et al. Vitamin D status in patients with systemic lupus erythematosus (SLE): a systematic review and meta-analysis. Autoimmun. Rev. 18 , 102392 (2019).
Hsieh, C. C. & Lin, B. F. Dietary factors regulate cytokines in murine models of systemic lupus erythematosus. Autoimmun. Rev. 11 , 22–27 (2011).
Bischoff, S. C. et al. ESPEN guideline on clinical nutrition in inflammatory bowel disease. Clin. Nutr. 42 , 352–379 (2023).
Chan, S. S. M. et al. Obesity is associated with increased risk of Crohn’s disease, but not ulcerative colitis: a pooled analysis of five prospective cohort studies. Clin. Gastroenterol. Hepatol. 20 , 1048–1058 (2022).
Lee, J. Y. et al. High-fat diet and antibiotics cooperatively impair mitochondrial bioenergetics to trigger dysbiosis that exacerbates pre-inflammatory bowel disease. Cell Host Microbe 28 , 273–284.e276 (2020).
Massironi, S. et al. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 8 , 579–590 (2023).
Lamb, C. A. et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 68 , s1–s106 (2019).
Hansen, T. & Duerksen, D. R. Enteral nutrition in the management of pediatric and adult Crohn’s disease. Nutrients. 10 , 537 (2018).
Sigall Boneh, R. et al. Dietary therapies induce rapid response and remission in pediatric patients with active Crohn’s disease. Clin. Gastroenterol. Hepatol. 19 , 752–759 (2021).
Cox, S. R. et al. Effects of low FODMAP diet on symptoms, fecal microbiome, and markers of inflammation in patients with quiescent inflammatory bowel disease in a randomized trial. Gastroenterology 158 , 176–188.e177 (2020).
Ruggeri, R. M. et al. Influence of dietary habits on oxidative stress markers in Hashimoto’s Thyroiditis. Thyroid 31 , 96–105 (2021).
Osowiecka, K. & Myszkowska-Ryciak, J. The influence of nutritional intervention in the treatment of Hashimoto’s Thyroiditis-a systematic review. Nutrients. 15 , 1041 (2023).
Manzel, A. et al. Role of “Western diet” in inflammatory autoimmune diseases. Curr. Allergy Asthma Rep. 14 , 404 (2014).
Bosch-Queralt, M. et al. Diet-dependent regulation of TGFβ impairs reparative innate immune responses after demyelination. Nat. Metab. 3 , 211–227 (2021).
Kleinewietfeld, M. et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496 , 518–522 (2013).
Cignarella, F. et al. Intermittent fasting confers protection in CNS Autoimmunity by altering the gut microbiota. Cell Metab. 27 , 1222–1235.e1226 (2018).
Evans, E., Piccio, L. & Cross, A. H. Use of vitamins and dietary supplements by patients with multiple sclerosis: a review. JAMA Neurol. 75 , 1013–1021 (2018).
Lemke, D. et al. Vitamin D resistance as a possible cause of autoimmune diseases: a hypothesis confirmed by a therapeutic high-dose vitamin D protocol. Front. Immunol. 12 , 655739 (2021).
Threapleton, D. E. et al. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. Bmj 347 , f6879 (2013).
Kaye, D. M. et al. Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation 141 , 1393–1403 (2020).
Marques, F. Z. et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 135 , 964–977 (2017).
Zampelas, A. & Magriplis, E. Dietary patterns and risk of cardiovascular diseases: a review of the evidence. Proc. Nutr. Soc. 79 , 68–75 (2020).
GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 393 , 1958–1972 (2019).
Gao, P. et al. Salt-induced hepatic inflammatory memory contributes to cardiovascular damage through epigenetic modulation of SIRT3. Circulation 145 , 375–391 (2022).
Cook, N. R., Appel, L. J. & Whelton, P. K. Lower levels of sodium intake and reduced cardiovascular risk. Circulation 129 , 981–989 (2014).
Jayachandran, M., Chung, S. S. M. & Xu, B. A critical review on diet-induced microbiota changes and cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 60 , 2914–2925 (2020).
Xu, Y. et al. Branched-chain amino acid catabolism promotes thrombosis risk by enhancing Tropomodulin-3 Propionylation in platelets. Circulation 142 , 49–64 (2020).
Takatsu, M. et al. Calorie restriction attenuates cardiac remodeling and diastolic dysfunction in a rat model of metabolic syndrome. Hypertension 62 , 957–965 (2013).
Palee, S. et al. Combination of exercise and calorie restriction exerts greater efficacy on cardioprotection than monotherapy in obese-insulin resistant rats through the improvement of cardiac calcium regulation. Metabolism 94 , 77–87 (2019).
An, H. S. et al. Caloric restriction reverses left ventricular hypertrophy through the regulation of cardiac iron homeostasis in impaired leptin signaling mice. Sci. Rep. 10 , 7176 (2020).
Fontana, L. Interventions to promote cardiometabolic health and slow cardiovascular ageing. Nat. Rev. Cardiol. 15 , 566–577 (2018).
Mishra, A. et al. Fasting-mimicking diet prevents high-fat diet effect on cardiometabolic risk and lifespan. Nat. Metab. 3 , 1342–1356 (2021).
Stekovic, S. et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 30 , 462–476.e466 (2019).
Dyńka, D., Kowalcze, K., Charuta, A. & Paziewska, A. The ketogenic diet and cardiovascular diseases. Nutrients 15 , 3368 (2023).
Burén, J., Ericsson, M., Damasceno, N. R. T. & Sjödin, A. A Ketogenic low-carbohydrate high-fat diet increases LDL cholesterol in healthy, young, normal-weight women: a randomized controlled feeding trial. Nutrients 13 , 814 (2021).
Chen, X. W., Ding, G., Xu, L. & Li, P. A glimpse at the metabolic research in China. Cell Metab. 33 , 2122–2125 (2021).
Targher, G., Corey, K. E., Byrne, C. D. & Roden, M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 18 , 599–612 (2021).
Wang, C. et al. Mendelian randomization analyses for PCOS: evidence, opportunities, and challenges. Trends Genet. 38 , 468–482 (2022).
Article MathSciNet CAS PubMed Google Scholar
Canfora, E. E., Meex, R. C. R., Venema, K. & Blaak, E. E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 15 , 261–273 (2019).
Jais, A. & Brüning, J. C. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Investig. 127 , 24–32 (2017).
Hatori, M. et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 15 , 848–860 (2012).
Hepler, C. et al. Time-restricted feeding mitigates obesity through adipocyte thermogenesis. Science 378 , 276–284 (2022).
Wang, X. et al. Time-restricted feeding is an intervention against excessive dark-phase sleepiness induced by obesogenic diet. Natl Sci. Rev. 10 , nwac222 (2023).
Lee, Y. S. et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell 157 , 1339–1352 (2014).
Cao, S. et al. EGFR-mediated activation of adipose tissue macrophages promotes obesity and insulin resistance. Nat. Commun. 13 , 4684 (2022).
Rheinheimer, J. et al. Current role of the NLRP3 inflammasome on obesity and insulin resistance: a systematic review. Metabolism 74 , 1–9 (2017).
Softic, S. et al. Fructose and hepatic insulin resistance. Crit. Rev. Clin. Lab. Sci. 57 , 308–322 (2020).
Teijeiro, A. et al. Inhibition of the IL-17A axis in adipocytes suppresses diet-induced obesity and metabolic disorders in mice. Nat. Metab. 3 , 496–512 (2021).
Hu, B. et al. γδ T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature 578 , 610–614 (2020).
Glenn, A. J. et al. The portfolio diet and incident type 2 diabetes: findings from the women’s health initiative prospective cohort study. Diabetes Care 46 , 28–37 (2023).
Hashemi, R., Rahimlou, M., Baghdadian, S. & Manafi, M. Investigating the effect of DASH diet on blood pressure of patients with type 2 diabetes and prehypertension: randomized clinical trial. Diabetes Metab. Syndr. 13 , 1–4 (2019).
Salas-Salvadó, J. et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann. Intern. Med. 160 , 1–10 (2014).
Zhao, L. et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359 , 1151–1156 (2018).
Tucker, L. A. Fiber intake and insulin resistance in 6374 adults: the role of abdominal obesity. Nutrients 10 , 237 (2018).
Deehan, E. C. et al. Elucidating the role of the gut microbiota in the physiological effects of dietary fiber. Microbiome 10 , 77 (2022).
Herz, D. et al. Efficacy of fasting in type 1 and type 2 diabetes mellitus: a narrative review. Nutrients 15 , 3525 (2023).
Patikorn, C. et al. Intermittent fasting and obesity-related health outcomes: an umbrella review of meta-analyses of randomized clinical trials. JAMA Netw. Open 4 , e2139558 (2021).
Li, G. et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 26 , 672–685.e674 (2017).
Yuan, X. et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr. Diabetes 10 , 38 (2020).
Kinzig, K. P., Honors, M. A. & Hargrave, S. L. Insulin sensitivity and glucose tolerance are altered by maintenance on a ketogenic diet. Endocrinology 151 , 3105–3114 (2010).
Ryan, M. C. et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 59 , 138–143 (2013).
Godos, J., Federico, A., Dallio, M. & Scazzina, F. Mediterranean diet and nonalcoholic fatty liver disease: molecular mechanisms of protection. Int. J. Food Sci. Nutr. 68 , 18–27 (2017).
Browning, J. D. et al. Short-term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am. J. Clin. Nutr. 93 , 1048–1052 (2011).
Hansen, C. D. et al. Effect of calorie-unrestricted low-carbohydrate, high-fat diet versus high-carbohydrate, low-fat diet on type 2 diabetes and nonalcoholic fatty liver disease : a randomized controlled trial. Ann. Intern. Med. 176 , 10–21 (2023).
Watanabe, M. et al. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: a comprehensive review of the literature. Obes. Rev. 21 , e13024 (2020).
Ezpeleta, M. et al. Effect of alternate day fasting combined with aerobic exercise on non-alcoholic fatty liver disease: a randomized controlled trial. Cell Metab. 35 , 56–70.e53 (2023).
Marjot, T., Tomlinson, J. W., Hodson, L. & Ray, D. W. Timing of energy intake and the therapeutic potential of intermittent fasting and time-restricted eating in NAFLD. Gut 72 , 1607–1619 (2023).
Kazemi, M. et al. Comparison of dietary and physical activity behaviors in women with and without polycystic ovary syndrome: a systematic review and meta-analysis of 39 471 women. Hum. Reprod. Update 28 , 910–955 (2022).
Faghfoori, Z., Fazelian, S., Shadnoush, M. & Goodarzi, R. Nutritional management in women with polycystic ovary syndrome: a review study. Diabetes Metab. Syndr. 11 , S429–s432 (2017).
Barrea, L. et al. Adherence to the Mediterranean diet, dietary patterns and body composition in women with polycystic ovary syndrome (PCOS). Nutrients 11 , 2278 (2019).
Magagnini, M. C. et al. Does the ketogenic diet improve the quality of ovarian function in obese women? Nutrients 14 , 4147 (2022).
Paoli, A. et al. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J. Transl Med. 18 , 104 (2020).
Li, C. et al. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J. Transl Med. 19 , 148 (2021).
Tabrizi, F. P. F., Farhangi, M. A., Vaezi, M. & Hemmati, S. The effects of spinach-derived thylakoid supplementation in combination with calorie restriction on anthropometric parameters and metabolic profiles in obese women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled clinical trial. Nutr. J. 19 , 82 (2020).
Goncalves, M. D. & Maddocks, O. D. Engineered diets to improve cancer outcomes. Curr. Opin. Biotechnol. 70 , 29–35 (2021).
Ligibel, J. A. et al. Exercise, diet, and weight management during cancer treatment: ASCO guideline. J. Clin. Oncol. 40 , 2491–2507 (2022).
McQuade, J. L., Daniel, C. R., Helmink, B. A. & Wargo, J. A. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 20 , e77–e91 (2019).
Mayne, S. T., Playdon, M. C. & Rock, C. L. Diet, nutrition, and cancer: past, present and future. Nat. Rev. Clin. Oncol. 13 , 504–515 (2016).
Preguiça, I. et al. Diet-induced rodent models of obesity-related metabolic disorders- a guide to a translational perspective. Obes. Rev. 21 , e13081 (2020).
Fenton, J. I. & Hord, N. G. Stage matters: choosing relevant model systems to address hypotheses in diet and cancer chemoprevention research. Carcinogenesis 27 , 893–902 (2006).
Liu, Y. et al. Host obesity alters the ovarian tumor immune microenvironment and impacts response to standard of care chemotherapy. J. Exp. Clin. Cancer Res. 42 , 165 (2023).
Download references
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (82103369) and the China Postdoctoral Science Foundation (2022M710757). The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript. The figures were created with Biorender.com.
Author information
These authors contributed equally: Yu-Ling Xiao, Yue Gong, Ying-Jia Qi
Authors and Affiliations
Key Laboratory of Breast Cancer in Shanghai, Department of Breast Surgery, Fudan University Shanghai Cancer Center, Shanghai, China
Yu-Ling Xiao, Yue Gong, Ying-Jia Qi, Zhi-Ming Shao & Yi-Zhou Jiang
Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, 200032, China
You can also search for this author in PubMed Google Scholar
Contributions
Y.-Z.J., Z.-M.S., Y.-L.X., and Y.G. designed and finalized the study. Y.-L.X., Y.G., and Y.-J.Q. wrote and edited the paper and generated the figures. All authors have read and approved the article.
Corresponding author
Correspondence to Yi-Zhou Jiang .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Consent for publication
The content of this manuscript has not been previously published and is not under consideration for publication elsewhere. All authors are aware of and agree to the content of the paper and are listed as coauthors of the paper.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Xiao, YL., Gong, Y., Qi, YJ. et al. Effects of dietary intervention on human diseases: molecular mechanisms and therapeutic potential. Sig Transduct Target Ther 9 , 59 (2024). https://doi.org/10.1038/s41392-024-01771-x
Download citation
Received : 01 August 2023
Revised : 05 February 2024
Accepted : 18 February 2024
Published : 11 March 2024
DOI : https://doi.org/10.1038/s41392-024-01771-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
Try to avoid eating deep-fried or overcooked food as it loses all its nutrients. The balanced diet must have the five essential elements, i.e. bitter, sour, sweet, pungent and salty. Also, the emphasis is on fresh fruits because the processed or packed ones do not have nutrients. Most importantly, always chew your food patiently.
A balanced diet gives us the nutrients we need. Good nutrition helps our body work well. An unhealthy diet can cause health problems. So, it's vital to eat a balanced diet for a healthy life. 500 Words Essay on Diet And Nutrition Understanding Diet and Nutrition. Diet and nutrition are two important words that we often hear about.
Long Essay on Balanced Diet 500 words in English. Balanced Diet Essay is usually given to classes 7, 8, 9, and 10. A balanced diet is a diet that contains all essential food groups. There are five primary nutrients that our body requires for proper functioning. The carbohydrates are the energy-giving food that contains starch and sugar ...
A balanced diet is essential for good health and well-being. It can help you maintain a healthy weight and get the nutrients you need to support your body's functions. A balanced diet comprises a variety of foods from all the different food groups in the right amounts. This includes fruits and vegetables, proteins, carbohydrates, and healthy fats.
A healthy diet includes the following: Fruit, vegetables, legumes (e.g. lentils and beans), nuts and whole grains (e.g. unprocessed maize, millet, oats, wheat and brown rice). At least 400 g (i.e. five portions) of fruit and vegetables per day (2), excluding potatoes, sweet potatoes, cassava and other starchy roots.
2. Components of a Healthy Diet and Their Benefits. A healthy diet is one in which macronutrients are consumed in appropriate proportions to support energetic and physiologic needs without excess intake while also providing sufficient micronutrients and hydration to meet the physiologic needs of the body [].Macronutrients (i.e., carbohydrates, proteins, and fats) provide the energy necessary ...
Published: Feb 8, 2022. Nutrition is the process of providing your body nourishment in the form of food which is necessary for your health. The food choices you make every day can affect your health and your mood in a drastic way. Good nutrition is an important part of living a healthy lifestyle because it ultimately boils down to life and death.
A balanced diet provides the body with energy, proteins, fats, vitamins and minerals, which should be in right proportions to avoid excesses and inadequacies that lead to diseases. Deficiency diseases such as osteoporosis result from an extended period of time without taking the right amounts of nutrients in food (Meier & Kranzlin 2011).
A balanced diet consists of a variety of foods from different food groups. These include: 1. Fruits and Vegetables: They are rich in vitamins, minerals, and fiber. They boost immunity and help in digestion. 2. Proteins: Sources include meat, fish, eggs, dairy, and plant-based proteins like legumes and nuts.
4. Diet and Culture for Healthy and Long Life. What elevates food to become diet and a meal is the manner and the context in which that food is consumed [].Numerous traditional and socio-cultural facets of dietary habits can be even more significant than their molecular, biochemical, and physiological concerns regarding their nutritional ingredients and composition.
A Healthy Lifestyle and a Well Balanced Diet Essay. Exclusively available on IvyPanda. A healthy lifestyle and a well-balanced diet are both essential for the well-being of modern society. Nevertheless, obesity, malnutrition, and eating disorders are becoming increasingly alarming problems in today's world.
A balanced diet supplies the fuel your body needs to work effectively. Without balanced nutrition, your body is more prone to illnesses such as heart disease, diabetes, and cancer. Eating a ...
Poor nutrition may be a causal factor in the experience of low mood, and improving diet may help to protect not only the physical health but also the mental health of the population, say Joseph Firth and colleagues ### Key messages Depression and anxiety are the most common mental health conditions worldwide, making them a leading cause of disability.1 Even beyond diagnosed conditions ...
In conclusion, the importance of food and nutrition cannot be overstated. Their impact on physical health, mental well-being, and overall quality of life is significant. It is crucial to prioritize food and nutrition in order to achieve optimal health and well-being. By making informed food choices and ensuring a balanced diet, individuals can ...
In today's fast-paced world, the topic of diet and nutrition has never been more important or more confusing. With so much information available, it can be overwhelming to navigate the best choices for your health and well-being. In this essay, we will explore the importance of a balanced diet and how it can impact various aspects of our lives ...
Fruit and vegetables are a key part of a healthy diet, and we should aim to get our 5 A DAY. Starchy foods like bread, potatoes, pasta, rice and other grains are part of a healthy diet, but the quality of what we choose is important. Plant-based protein foods, like beans and lentils, are naturally low in saturated fat and are sources of protein ...
The effects of certain foods or dietary patterns on glycaemia, immune activation, and the gut microbiome may play a role in the relationships between food and mood. More research is needed to understand the mechanisms that link food and mental wellbeing and determine how and when nutrition can be used to improve mental health. 3 fig 1 4.
Diet and Nutrition Essay. A person eats to gain energy from food that allows our body to perform various functions that allows us to survive. The food that people take in will go through their digestive system that then allows them to harness the energy within food. That energy that is taken from the food is then sent, via the bloodstream, to ...
Drinking 7-8 glasses of water is crucial. It balances blood pressure and quickly supplies vital nutrients to the body. Being fit and healthy boosts resistance to infections and diseases. Health is more important than wealth. Spending money on junk food or entertainment sources like films offers no benefits except self-satisfaction.
We should consume a balanced diet consisting of essential nutrition. Importance of Healthy Diet essay: A healthy diet is one that helps to maintain or improve overall health. ... We should consume a balanced diet consisting of essential nutrition. A healthy and balanced diet reduces stress levels and promotes healthy life without any suffering.
In a proper balanced diet 50-60% of. total calories should come from carbohydrates, mostly. from complex carbohydrates, 10-15% from proteins, and 20-30% calorie from both visible and invisible fat ...
Introduction. Food, nutrition and health are highly interrelated and consumption of specific nutrients have a profound impact on human health. The amount and type of nutrients consumed are tightly linked to the metabolic stage and the immune health and thus, inappropriate nutrient consumption is associated with development of major human diseases due to an immune system not properly ...
Diet, serving as a vital source of nutrients, exerts a profound influence on human health and disease progression. Recently, dietary interventions have emerged as promising adjunctive treatment ...
Unit 7 - Diet Scenarios. A balanced diet is important because it provides the body with the nutrients it needs to operate and function properly. If we don't have balanced nutrition, our bodies can be more prone to disease, infection, fatigue and all over low performance.
Snacktime. Q's go-to snacks include whole fruits, nuts, and smoothies. "I don't eat bars or crackers, or store-bought bread or any snack that comes in a bag," she says. But that doesn't ...