- Department of Health and Human Services
- National Institutes of Health


COVID-19 Research Studies
More information, about clinical center, clinical trials and you, participate in a study, referring a patient.
About Clinical Research
Research participants are partners in discovery at the NIH Clinical Center, the largest research hospital in America. Clinical research is medical research involving people The Clinical Center provides hope through pioneering clinical research to improve human health. We rapidly translate scientific observations and laboratory discoveries into new ways to diagnose, treat and prevent disease. More than 500,000 people from around the world have participated in clinical research since the hospital opened in 1953. We do not charge patients for participation and treatment in clinical studies at NIH. In certain emergency circumstances, you may qualify for help with travel and other expenses Read more , to see if clinical studies are for you.
Medical Information Disclaimer
Emailed inquires/requests.
Email sent to the National Institutes of Health Clinical Center may be forwarded to appropriate NIH or outside experts for response. We do not collect your name and e-mail address for any purpose other than to respond to your query. Nevertheless, email is not necessarily secure against interception. This statement applies to NIH Clinical Center Studies website. For additional inquiries regarding studies at the National Institutes of Health, please call the Office of Patient Recruitment at 1-800-411-1222

Find NIH Clinical Center Trials
The National Institutes of Health (NIH) Clinical Center Search the Studies site is a registry of publicly supported clinical studies conducted mostly in Bethesda, MD.


- Policy & Compliance
- Clinical Trials
NIH Definition of Clinical Trial Case Studies
The case studies provided below are designed to help you identify whether your study would be considered by NIH to be a clinical trial. Expect the case studies and related guidance to evolve over the upcoming year. For continuity and ease of reference, case studies will retain their original numbering and will not be renumbered if cases are revised or removed.
The simplified case studies apply the following four questions to determine whether NIH would consider the research study to be a clinical trial:
- Does the study involve human participants?
- Are the participants prospectively assigned to an intervention?
- Is the study designed to evaluate the effect of the intervention on the participants?
- Is the effect being evaluated a health-related biomedical or behavioral outcome?
If the answer to all four questions is “yes,” then the clinical study would be considered a clinical trial according to the NIH definition.
See this page for more information about the NIH definition of a clinical trial.
General Case Studies
Institute or center specific case studies.
The study involves the recruitment of research participants who are randomized to receive one of two approved drugs. It is designed to compare the effects of the drugs on the blood level of a protein.
- Does the study involve human participants? Yes, the study involves human participants.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, one of two drugs.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the drugs on the level of the protein in the participants’ blood.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, the level of a protein, is a health-related biomedical outcome.
The study involves the recruitment of research participants with condition Y to receive a drug that has been approved for another indication. It is designed to measure the drug’s effects on the level of a biomarker associated with the severity of condition Y.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, the approved drug.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the drug’s effect on the level of the biomarker.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, the level of a biomarker, is a health-related biomedical outcome.
The study involves the recruitment of research participants with condition X to receive investigational compound A. It is designed to assess the pharmacokinetic properties of compound A.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, compound A.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate how the body interacts with compound A
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, pharmacokinetic properties, is a health-related biomedical outcome.
The study involves the recruitment of research participants with disease X to receive an investigational drug. It is designed to assess safety and determine the maximum tolerated dose of the drug.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, the investigational drug.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to assess safety and determine the maximum tolerated dose of the investigational drug.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, safety and maximum tolerated dose, is a health-related biomedical outcome.
The study involves the recruitment of research participants with disease X to receive a chronic disease management program. It is designed to assess usability and to determine the maximum tolerated dose of the chronic disease program (e.g., how many in-person and telemedicine visits with adequate adherence).
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, the chronic disease management program.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to determine the maximum tolerated dose of the program to obtain adequate adherence.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, tolerable intensity and adequate adherence of the intervention, is a health-related outcome.
The study involves the recruitment of research participants with disease X to receive either an investigational drug or a placebo. It is designed to evaluate the efficacy of the investigational drug to relieve disease symptoms.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, the investigational drug or placebo.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the investigational drug on the participants’ symptoms.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, relief of symptoms, is a health-related outcome.
The study involves the recruitment of research participants with disease X to receive an investigational drug. It is designed to assess whether there is a change in disease progression compared to baseline. There is no concurrent control used in this study.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the investigational drug on the subject’s disease progression.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, disease progression, is a health-related outcome.
The study involves the recruitment of research participants with disease X to test an investigational in vitro diagnostic device (IVD). It is designed to evaluate the ability of the device to measure the level of an antibody in blood.
- Are the participants prospectively assigned to an intervention? No, in this context the IVD would not be considered an intervention. The IVD is being used to test its ability to measure antibody levels, but not to test its effects on any health-related biomedical or behavioral outcomes.
The study involves the recruitment of research participants with disease X to be evaluated with an investigational in vitro diagnostic device (IVD). The study is designed to evaluate how knowledge of certain antibody levels impacts clinical management of disease.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, measurement of an antibody level, with the idea that knowledge of that antibody level might affect clinical management.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate how knowledge of the level of an antibody might inform treatment.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being measured, how blood antibody levels inform treatment, is a health-related outcome.
The study involves the recruitment of healthy volunteers who will be randomized to different durations of sleep deprivation (including no sleep deprivation as a control) and who will have stress hormone levels measured. It is designed to determine whether the levels of stress hormones in blood rise in response to different durations of sleep deprivation.
- Does the study involve human participants? Yes, the healthy volunteers are human participants.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, different durations of sleep deprivation followed by a blood draw.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to measure the effect of different durations of sleep deprivation on stress hormone levels.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, stress hormone levels, is a health-related biomedical outcome.
The study involves the analysis of de-identified, stored blood samples and de-identified medical records of patients with disease X who were treated with an approved drug. The study is designed to evaluate the level of a protein in the blood of patients that is associated with therapeutic effects of the drug.
- Does the study involve human participants? No, the study does not involve human participants because only de-identified samples and information are used.
The study involves the analysis of identifiable, stored blood samples and identified medical records of patients with disease X who were treated with an approved drug. The study is designed to evaluate the level of a protein in the blood of patients that is associated with therapeutic effects of the drug.
- Does the study involve human participants? Yes, patients are human participants because the blood and information are identifiable.
- Are the participants prospectively assigned to an intervention? No, secondary research with biospecimens or health information is not a clinical trial.
The study involves the recruitment of a healthy volunteers whose blood is drawn for genomic analysis. It is designed to identify the prevalence of a genetic mutation in the cohort and evaluate potential association between the presence of the mutation and the risk of developing a genetic disorder.
- Are the participants prospectively assigned to an intervention? No, sample collection (blood draw) is not an intervention in this context.
Physicians report that some patients being treated with drug A for disease X are also experiencing some improvement in a second condition, condition Y. The study involves the recruitment of research participants who have disease X and condition Y and are being treated with drug A. The participants are surveyed to ascertain whether they are experiencing an improvement in condition Y.
- Are the participants prospectively assigned to an intervention? No, participants are not prospectively assigned to receive an intervention as they are receiving drugs as part of their clinical care. The surveys are being used for measurement, not to modify a biomedical or behavioral outcome.
The study involves the recruitment of patients with disease X who are receiving one of three standard therapies as part of their clinical care. It is designed to assess the relative effectiveness of the three therapies by monitoring survival rates using medical records over a few years.
- Are the participants prospectively assigned to an intervention? No, there is no intervention. The therapies are prescribed as part of clinical care; they are not prospectively assigned for the purpose of the study. The study is observational.
The study involves the recruitment of research participants with disease X vs. healthy controls and comparing these participants on a range of health processes and outcomes including genomics, biospecimens, self-report measures, etc. to explore differences that may be relevant to the development of disease X.
- Are the participants prospectively assigned to an intervention? No, the measures needed to assess the outcomes are not interventions in this context, as the study is not intended to determine whether the measures modify a health-related biomedical or behavioral outcome.
The study involves the recruitment of healthy volunteers for a respiratory challenge study; participants are randomized to receive different combinations of allergens. The study evaluates the severity and mechanism of the immune response to different combinations of allergens introduced via inhalation.
- Does the study involve human participants? Yes, healthy volunteers are human participants.
- Are the participants prospectively assigned to an intervention? Yes, healthy volunteers are prospectively assigned to randomly selected combinations of allergens.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of different combinations of allergens on the immune response in healthy individuals.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the study evaluates the severity and mechanism of the immune reaction to allergens, which are health-related biomedical outcomes.
The study involves the recruitment of research participants with Alzheimer’s disease (AD) to evaluate the effects of an investigational drug on memory, and retention and recall of information.
- Are the participants prospectively assigned to an intervention? Yes, participants are prospectively assigned to receive the investigational drug.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of the drug on participants’ memory.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the study evaluates memory, and retention and recall of information in the context of AD.
The study involves the recruitment of individuals to receive a new behavioral intervention for sedentary behavior. It is designed to measure the effect of the intervention on hypothesized differential mediators of behavior change.
- Are the participants prospectively assigned to an intervention? Yes, participants are prospectively assigned to receive a behavioral intervention.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of the intervetion on mediators of behavior change.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, mediators of behavior change, are behavioral outcomes relevant to health.
The study involves the recruitment of patients with disease X to be evaluated with a new visual acuity task. It is designed to evaluate the ability of the new task to measure visual acuity as compared with the gold standard Snellen Test
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, the new visual acuity test.
- Is the study designed to evaluate the effect of the intervention on the participants? No, the study is designed to evaluate the ability of the new visual acuity test to measure visual acuity as compared to the gold standard Snellen Test, but not to modify visual acuity.
The study involves the recruitment of research participants with CHF who were hospitalized before or after implementation of the Medicare incentives to reduce re-hospitalizations. Morbidity, mortality, and quality of life of these participants are evaluated to compare the effects of these Medicare incentives on these outcomes.
- Are the participants prospectively assigned to an intervention? No, the intervention (incentives to reduce re-hospitalization) were assigned by Medicare, not by the research study.
The study involves the recruitment of healthcare providers to assess the extent to which being provided with genomic sequence information about their patients informs their treatment of those patients towards improved outcomes.
- Does the study involve human participants? Yes, both the physicians and the patients are human participants.
- Are the participants prospectively assigned to an intervention? Yes, physicians are prospectively assigned to receive genomic sequence information, which is the intervention.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of intervening with physicians, on the treatment they provide to their patients.
- Is the effect being evaluated a health-related, biomedical, or behavioral outcome? Yes, the effect being evaluated, the extent to which providing specific information to physicians informs the treatment of patients, is a health-related outcome.
The study involves the recruitment of research participants with a behavioral condition to receive either an investigational behavioral intervention or a behavioral intervention in clinical use. It is designed to evaluate the effectiveness of the investigational intervention compared to the intervention in clinical use in reducing the severity of the obsessive compulsive disorder.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, either the investigational intervention or an intervention in clinical use.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate whether the investigational intervention is as effective as the standard intervention, at changing behavior.
- Is the effect being evaluated a health-related, biomedical, or behavioral outcome? Yes, the effect being evaluated, the interventions’ effectiveness in reducing the severity of the condition, is a health-related behavioral outcome.
The study involves the recruitment of physicians who will be randomly assigned to use a new app or an existing app, which cues directed interviewing techniques. The study is designed to determine whether the new app is better than the existing app at assisting physicians in identifying families in need of social service support. The number of community service referrals will be measured.
- Does the study involve human participants? Yes, both the physicians and the families are human participants.
- Are the participants prospectively assigned to an intervention? Yes, physicians are prospectively assigned to use one of two apps, which are the interventions.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of intervening with physicians, on social service support referral for families.
- Is the effect being evaluated a health-related, biomedical, or behavioral outcome? Yes, the effect being evaluated, the number of referrals, is a health-related outcome.
The study involves the recruitment of parents to participate in focus groups to discuss topics related to parental self-efficacy and positive parenting behaviors. It is designed to gather information needed to develop an intervention to promote parental self-efficacy and positive parenting behaviors.
- Does the study involve human participants? Yes, the parents are human participants.
- Are the participants prospectively assigned to an intervention? No, a focus group is not an intervention.
The study involves the recruitment of healthy volunteers to test a new behavioral intervention. It is designed to evaluate the effect of a meditation intervention on adherence to exercise regimens and quality of life to inform the design of a subsequent, fully-powered trial.
- Does the study involve human participants? Yes, study participants are human participants.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to a behavioral intervention.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the intervention on adherence, and quality of life.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, adherence and quality of life are health-related outcomes.
A study will test the feasibility a mobile phone app designed to increase physical activity. A group of sedentary individuals will use the app for a week while their interactions with the app are monitored. The number of interactions with the app will be measured, as well as any software issues. Participants will also complete a survey indicating their satisfaction with and willingness to use the app, as well as any feedback for improvement. The app’s effect on physical activity, weight, or cardiovascular fitness will not be evaluated.
- Does the study involve human participants? Yes, sedentary individuals will be enrolled.
- Are the participants prospectively assigned to an intervention? The participants will interact with the app for a week.
- Is the study designed to evaluate the effect of the intervention on the participants? No. While the participants’ interactions are monitored (steps or heart rate may be recorded in this process), the study is NOT measuring the effect of using the app ON the participant. The study is only measuring the usability and acceptability of the app, and testing for bugs in the software. The effect on physical activity is NOT being measured.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? N/A
The study involves the recruitment of healthy family members of patients hospitalized for disease X to test two CPR training strategies. Participants will receive one of two training strategies. The outcome is improved CPR skills retention.
- Does the study involve human participants? Yes, family members of patients are human participants.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to one of two CPR educational strategies.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of educational strategies on CPR skills.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, retention of CPR skills is a health-related behavioral outcome.
The study involves the recruitment of research participants in three different communities (clusters) to test three CPR training strategies. The rate of out-of- hospital cardiac arrest survival will be compared.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive one of three types of CPR training, which is the intervention.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of different CPR training strategies on patient survival rates post cardiac arrest.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, out-of-hospital cardiac arrest survival is a health-related outcome.
A study involves the recruitment of school children to evaluate two different tools for monitoring food intake. Food consumption behavior will be measured by asking children to activate a pocket camera during meals and to use a diary to record consumed food. The accuracy of the two food monitoring methods in measuring energy intake will be assessed.
- Does the study involve human participants? Yes, children are human participants.
- Are the participants prospectively assigned to an intervention? No, in this context the monitoring methods would not be considered an intervention. The study is designed to test the accuracy of two monitoring methods, but not to test the effect on any health-related biomedical or behavioral outcomes.
A study involves the recruitment of school children to evaluate two different tools for monitoring food intake. Food consumption behavior will be measured by asking children to activate a pocket camera during meals and to use a diary to record consumed food. Changes to eating behavior will be assessed.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to two food monitoring methods.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to determine whether using the monitoring methods changes eating behavior.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, eating behavior is a health-related outcome.
A study involves the recruitment of children at two schools to monitor eating behavior. Children’s food choices will be monitored using a remote food photography method. Food consumption and the accuracy of food monitoring methods will be assessed.
- Does the study involve human participants? Yes, the children participating in this study are human participants.
- Are the participants prospectively assigned to an intervention? No, not in this context. The study involves observing and measuring eating behavior, but not modifying it. This is an observational study.
A study involves the recruitment of children at two schools to evaluate their preferences for graphics and colors used in healthy food advertisements. Children will be presented with multiple health advertisements and their preferences for graphics and colors will be assessed.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to see different advertisements.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the advertisements.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? No, preferences are not health-related biomedical or behavioral outcomes.
The study involves ambulatory patients who have new-onset stable angina and who are recruited from community practices. They are randomized to undergo CT angiography or an exercise stress test of the doctor’s choice. To keep the trial pragmatic, the investigators do not prescribe a protocol for how physicians should respond to test results. The study is designed to determine whether the initial test (CT angiography or stress test) affects long-term rates of premature death, stroke, or myocardial infarctions.
- Are the participants prospectively assigned to an intervention? Yes, the participants are randomized to undergo CT angiography or an exercise stress test.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to determine whether the initial test done affects long-term rates of certain clinical events.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, premature death, stroke, and myocardial infarction are health-related biomedical outcomes.
The study involves patients who present with stable angina to community practices. As part of their routine care some of their physicians refer them for CT angiography, while others refer them for exercise stress tests. The study is designed to see whether or not there's an association between the type of test that is chosen and long-term risk of death, stroke, or myocardial infarction.
- Are the participants prospectively assigned to an intervention? No, the intervention is not prospectively assigned by the investigators. Rather, the intervention, in this case diagnostic study, occurs as part of routine clinical care.
The investigators conduct a longitudinal study of patients with schizophrenia. Their physicians, as part of their standard clinical care, prescribe antipsychotic medication. The investigators conduct an imaging session before starting treatment; they repeat imaging 4-6 weeks later.
- Does the study involve human participants? Yes.
- Are the participants prospectively assigned to an intervention? No, not in this context. Antipsychotic medications are given as part of clinical care, not as part of a prospective, approved research protocol.
The investigators conduct a longitudinal study of patients with schizophrenia. Their physicians, as part of their standard clinical care, prescribe antipsychotic medication. As part of the research protocol, all participants will be prescribed the same dose of the antipsychotic medication. The investigators conduct an imaging session before starting treatment; they repeat imaging 4-6 weeks later.
- Are the participants prospectively assigned to an intervention? Yes, although participants are all receiving antipsychotic medication as part of their standard medical care, the dose of the antipsychotic medication is determined by the research protocol, rather than individual clinical need.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of a dose of antipsychotic medication on brain function.
- Is the effect being evaluated a health-related biomedical or behavioral outcome ? Yes, brain function measured by imaging is a health-related outcome.
The study involves recruitment of healthy volunteers who will wear a thermal compression device around their legs. This pilot study is designed to examine preliminary performance and safety of a thermal compression device worn during surgery. Investigators will measure core temperature, comfort, and presence of skin injury in 15-minute intervals.
- Are the participants prospectively assigned to an intervention? Yes, participants are assigned to wear a thermal compression device.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the thermal compression device on participant core temperature, comfort, and presence of skin injury.
- Is the effect being evaluated a health-related biomedical or behavioral outcome ? Yes, participant core temperature, comfort, and presence of skin injury are health-related biomedical outcomes.
The study involves collection of data on hospitalizations for various acute illnesses among people who live close to a border between two states that have recently implemented different laws related to public health (e.g. smoking regulations, soda taxes). The investigators want to take advantage of this “natural experiment” to assess the health impact of the laws.
- Does the study involve human participants? Yes, the study involves human participants.
- Are the participants prospectively assigned to an intervention? No, the interventions were assigned by state laws and state of residence, not by the research study.
The study involves recruitment of healthy volunteers to engage in working memory tasks while undergoing transcranial magnetic stimulation (TMS) to induce competing local neuronal activity. The study is measuring task performance to investigate the neural underpinnings of working memory storage and processing.
- Are the participants prospectively assigned to an intervention? Yes, healthy volunteers are prospectively assigned to receive TMS stimulation protocols during a working memory task.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of local TMS stimulation on working memory performance and oscillatory brain activity in healthy individuals.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the study evaluates working memory processes, which are health-related biomedical outcomes.
The study involves recruitment of healthy volunteers to engage in a social valuation task while dopamine tone in the brain is manipulated using tolcapone, an FDA-approved medication. The study aims to understand the role of dopamine in social decision-making and to search for neural correlates of this valuation using fMRI.
- Are the participants prospectively assigned to an intervention? Yes, healthy volunteers are prospectively assigned to receive tolcapone during a social valuation task.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of modulating dopamine tone on social decision-making. Although this study uses an FDA-approved drug to modulate dopamine tone, the goal of this intervention is to understand the role of dopamine in a fundamental phenomenon (social valuation), and not to study the mechanism of action of the drug or its clinical effects.
The career development candidate proposes to independently lead a study to test a new drug A on patients with disease X. Patients will be randomized to a test and control group, with the test group receiving one dose of drug A per week for 12 months and controls receiving placebo. To assess presence, number, and type of any polyps, a colonoscopy will be performed. To assess biomarkers of precancerous lesions, colon mucosal biopsies will be collected. Complete blood count will be measured, and plasma will be stored for potential biomarker evaluation.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, drug A or placebo.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of drug A and placebo on the presence and type of polyps.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, the presence and type of polyps, is a health-related biomedical outcome.
Ancillary Study to Case Study #42a: Some types of drug A being evaluated in Case Study #42a have been reported to impact renal function. An internal medicine fellow performs an ancillary study where stored plasma from Case Study #42a will be evaluated for multiple biomarkers of renal function.
- Does the study involve human participants? Yes, patients are human participants because the plasma and information are identifiable.
- Are the participants prospectively assigned to an intervention? No, because the assignment of participants to an intervention occurs as part of an existing, separately funded clinical trial. This proposal would be considered an ancillary study that is not an independent clinical trial.
Ancillary Study to Case Study #42a: An internal medicine fellow designs an independent ancillary trial where a subset of patients from the parent trial in Case Study #42a will also receive drug B, based on the assumption that a two-drug combination will work significantly better than a single drug at both improving renal function and reducing polyps. The test subjects will be evaluated for renal function via plasma clearance rates at 6 and 12 months after initiation of drugs A and B.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, drugs A and B.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of drugs A and B on renal function.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, renal function, is a health-related biomedical outcome.
A group of healthy young adults will perform a Go/No-Go task while undergoing fMRI scans. The purpose of the study is to characterize the pattern of neural activation in the frontal cortex during response inhibition, and the ability of the participant to correctly withhold a response on no-go
- Does the study involve human participants? Yes, healthy young adults will be enrolled in this study.
- Are the participants prospectively assigned to an intervention? Yes, the participants will be prospectively assigned to perform a Go/No-Go task, which involves different levels of inhibitory control.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the Go/No-Go task on neural activation in the frontal cortex. The study will measure inhibitory control and the neural systems being engaged. In this study, the Go/No-Go task is the independent variable, and behavioral performance and the associated fMRI activations are the dependent variables.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the neural correlates of inhibitory control and behavioral performance are health-related biomedical outcomes.
A group of adolescents will participate in a longitudinal study examining changes in executive function over the course of a normal school year. Color naming performance on the standard version of the Stroop test will be obtained. All measures will be compared at multiple time points during the school year to examine changes in executive function. The purpose is to observe changes in executive function and to observe if differences exist in the Stroop effect over the course of the school year for these adolescents.
- Does the study involve human participants? Yes, adolescents will be enrolled in this study.
- Are the participants prospectively assigned to an intervention? No, there is no intervention in this study and no independent variable manipulated. The adolescents are not prospectively assigned to an intervention, but instead the investigator will examine variables of interest (including the Stroop test) over time. The Stroop effect is used as a measurement of point-in-time data.
- Is the study designed to evaluate the effect of the intervention on the participants? No, there is no intervention. Performance on the Stroop test is a well-established measure of executive function and the test is not providing an independent variable of interest here. It is not being used to manipulate the participants or their environment. The purpose is simply to obtain a measure of executive function in adolescents over the course of the school year.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? N/A. No effect of an intervention is being evaluated.
A group of participants with social anxiety will perform an experimentally manipulated Stroop test. In this variant of the Stroop test, the stimuli presented are varied to include emotional and neutral facial expressions presented in different colors. Participants are instructed to name the colors of the faces presented, with the expectation that they will be slower to name the color of the emotional face than the neutral face. The purpose of the study is to examine the degree to which participants with social anxiety will be slower to process emotional faces than neutral faces.
- Does the study involve human participants? Yes, participants with social anxiety will be enrolled in this study.
- Are the participants prospectively assigned to an intervention? Yes, the participants will be prospectively assigned to perform a modified Stroop test using different colored emotional/neutral faces to explore emotional processing in people with social anxiety. Note that the independent variable is the presentation of emotional vs neutral faces.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to measure the effect of emotional valence (i.e. emotional faces) on participant response time to name the color. The purpose is to determine whether the response time to emotional faces is exaggerated for people with social anxiety as compared to neutral faces. Note that the response time to name the colors is the dependent variable in this study.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the processing of emotional information is a health-related biomedical outcome.
The study involves healthy volunteers and compares temporal SNR obtained with a new fMRI pulse sequence with that from another sequence.
- Are the participants prospectively assigned to an intervention? No, in this context the different pulse sequences would not be considered an intervention. The pulse sequences are not being used to modify any biomedical or behavioral outcome; rather the investigator is comparing performance characteristics of the two pulse sequences.
The study is designed to demonstrate that a new imaging technology (e.g. MRI, PET, ultrasound technologies, or image processing algorithm) is equivalent to, or has better sensitivity/specificity than a standard of care imaging technology. Aim one will use the new imaging technology and the gold standard in ten healthy volunteers. Aim Two will use the new imaging technology and the gold standard before and after a standard care procedure in ten patients. In both aims the performance of the new technology will be compared to the gold standard. No clinical care decisions will be made based on the use of the device in this study.
- Does the study involve human participants? YES. Aim one will study ten healthy volunteers, and aim two will study ten patient volunteers.
- Are the participants prospectively assigned to an intervention? Yes, participants will be prospectively assigned to be evaluated with a new imaging technology and the gold standard technology.
- Is the study designed to evaluate the effect of the intervention on the participants? No, the study is not measuring the effect of the technologies ON the human subjects. The study is determining if the new technology is equivalent or better than the gold standard technology. No effect on the participant is being measured.
An investigator proposes to add secondary outcomes to an already funded clinical trial of a nutritional intervention. The trial is supported by other funding, but the investigator is interested in obtaining NIH funding for studying oral health outcomes. Participants in the existing trial would be assessed for oral health outcomes at baseline and at additional time points during a multi-week dietary intervention. The oral health outcomes would include measures of gingivitis and responses to oral health related quality of life questionnaires. Oral fluids would be collected for analysis of inflammatory markers and microbiome components.
- Are the participants prospectively assigned to an intervention? No, because the assignment of participants to an intervention (and the administration of the intervention) occur as part of an existing, separately funded clinical trial. This proposal would be considered an ancillary study that leverages an already existing clinical trial.
The goal of the project is to use functional neuroimaging to distinguish patients with temporomandibular disorders (TMD) who experience TMD pain through centralized pain processes from those with TMD related to peripheral pain. Pain processing in a study cohort of TMD patients and healthy controls will be measured through functional magnetic resonance neuroimaging (fMRI) following transient stimulation of pain pathways through multimodal automated quantitative sensory testing (MAST QST). TMD patients will receive study questionnaires to better correlate the extent to which TMD pain centralization influences TMD prognosis and response to standard of care peripherally targeted treatment (prescribed by physicians, independently of the study).
- Are the participants prospectively assigned to an intervention? No, not in this context. The transient stimulation of pain pathways and the fMRI are being performed to measure and describe brain activity, but not to modify it.
An investigator proposes to perform a study of induced gingivitis in healthy humans, to study microbial colonization and inflammation under conditions of health and disease. During a 3-week gingivitis induction period, each study participant will use a stent to cover the teeth in one quadrant during teeth brushing. A contralateral uncovered quadrant will be exposed to the individual's usual oral hygiene procedures, to serve as a control. Standard clinical assessments for gingivitis will be made and biospecimens will be collected at the point of maximal induced gingivitis, and again after normal oral hygiene is resumed. Biospecimens will be assessed for microbial composition and levels of inflammation-associated chemokines.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, abstaining from normal oral hygiene for a portion of the mouth, to induce gingivitis.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the induced gingivitis on microbial composition and levels of inflammatory chemokines in oral samples.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the microbial composition and chemokine levels in oral samples are health-related biomedical outcomes.
The study will enroll older adults with hearing loss, comparing the effectiveness of enhanced hearing health care (HHC) to usual HHC. In addition to routine hearing-aid consultation and fitting, participants randomized to enhanced HCC will be provided patient-centered information and education about a full range of hearing assistive technologies and services. Study outcomes include the utilization of technology or services, quality of life, communication abilities, and cognitive function.
- Does the study involve human participants? Yes, the study enrolls older adults with hearing loss.
- Are the participants prospectively assigned to an intervention? Yes, participants are randomized to receive enhanced HCC or usual HCC interventions.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study will evaluate enhanced HCC’s effectiveness in modifying participant behavior and biomedical outcomes.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, rate of technology/service utilization is a behavioral outcome and quality of life, communications, and cognition are biomedical outcomes that may be impacted by the interventions.
The study involves the recruitment of obese individuals who will undergo a muscle biopsy before and after either exercise training or diet-induced weight loss. Sarcolemmal 1,2-disaturated DAG and C18:0 ceramide species and mitochondrial function will be measured. Levels will be correlated with insulin sensitivity.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to either exercise training or a diet.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to compare the effects of the interventions on muscle metabolism.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, muscle metabolism/signaling is a health-related outcome.
The study involves the recruitment of participants with type 2 diabetes who will undergo a muscle biopsy before and after a fast to measure acetylation on lysine 23 of the mitochondrial solute carrier adenine nucleotide translocase 1 (ANT1). Levels will be related to rates of fat oxidation.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to undergo a fast.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to compare the effects of the fast on molecular parameters of metabolism.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, metabolism is a health-related outcome.
Insulin-resistant and insulin-sensitive nondiabetic adults who have a parent with type 2 diabetes will be followed over time to understand the role of mitochondrial dysfunction in the development of diabetes. Oral glucose tolerance tests will be performed annually to measure insulin sensitivity and glycemic status. Participants will also undergo a brief bout of exercise, and mitochondrial ATP synthesis rates will be measured by assessing the rate of recovery of phosphocreatine in the leg muscle, using 31P magnetic resonance spectroscopy.
- Are the participants prospectively assigned to an intervention? No, the participants are not assigned to an intervention; the OGTT and 31P MRS are measures.
Participants with chronic kidney disease will be recruited to receive one of two drug agents. After 6 weeks of therapy, subjects will undergo vascular function testing and have measures of oxidative stress evaluated in their plasma and urine. Results of the function testing and the oxidative stress biomarkers will be related to drug treatment.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to receive two different drugs.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to compare the effects of the drugs on vascular function.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, vascular function is a health-related outcome.
Participants with Autosomal Dominant Polycystic Kidney Disease will be recruited to receive an oral curcumin therapy or placebo and the participants will undergo vascular function testing, renal imaging to assess kidney size, and assessment of oxidative stress biomarkers in urine and plasma after an ascorbic acid challenge. Changes in these outcomes will be related to oral therapy.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to receive medication or placebo.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to compare the effects of the drugs on vascular function and kidney size.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, vascular function and kidney size are health-related outcomes.
Kidney transplant recipients will be recruited to undergo an experimental imaging procedure at several timepoints up to 4 months post-transplantation. Output from the images will be related to pathological assessments of the transplant as well as clinical measures of renal function.
- Are the participants prospectively assigned to an intervention? No, the participants are not assigned to receive an intervention. They undergo transplantation as part of their routine clinical care. The imaging procedure is a measure and not an intervention.
The study proposes the development of a novel probe to assess clearance of a nutritional metabolite in a given disease state. The probe is a GMP grade, deuterated, intravenously administered tracer and clearance is assessed by mass spectrometry analysis of serial blood draws. Participants will either receive a micronutrient supplement or will receive no supplementation. The clearance rate of the probe will be compared in the two groups, to understand the performance of the probe.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to receive either a micronutrient supplement or nothing.
- Is the study designed to evaluate the effect of the intervention on the participants? No, the intervention is being used to assess the performance of the probe and is not looking at an effect on the participant.
In order to assess the contribution of ingested glycolate to oxalate production, healthy participants will be recruited to a study involving the consumption of a controlled diet for three days, followed by an infusion of 13C2-glycolate. Blood and urine will be collected during the subsequent 24 hours to assess the amount of labeled glycolate in plasma and urine oxalate.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to receive a controlled diet for three days.
- Is the study designed to evaluate the effect of the intervention on the participants? No, the intervention (controlled diet) is being used to minimize exogenous dietary sources of oxalate in the participants prior to the labeled tracer infusion. The study will not be evaluating the effect of the diet on the participants.
This page last updated on: April 28, 2021
- Bookmark & Share
- E-mail Updates
- Privacy Notice
- Accessibility
- National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
- NIH... Turning Discovery Into Health
Clinical Trials at Stanford Medicine
Join our community of volunteers leading the way in transformative research

Stanford Cancer Institute offers leading-edge research and compassionate care with over 250 actively recruiting clinical trials, investigating a broad spectrum of new preventative, diagnostic, and treatment strategies.
More about the Cancer Institute

Stanford Pediatric Clinical Trials play a vital role in developing new therapies for a large range of conditions that affect children. These trials can help pave the way for a brighter healthier future for our youngest generation.
More about Stanford Children's Health

Healthy volunteers play a vital role in clinical studies, helping researchers learn how to keep people well. Some studies compare healthy people to those who have a specific disease or condition.
More about being a healthy volunteer

What is a clinical trial?
Clinical trials are research studies that explore whether a medical strategy, treatment or device is safe and effective for humans. These studies may also show which medical approaches work best for certain illnesses or groups of people. Clinical trials produce information that helps patients and their health-care providers make better health-related decisions.
We're looking for healthy volunteers
Stanford research registry.
The Stanford Research Registry connects people like you, with teams conducting research, to improve health care. If you are eligible for a study, researchers may contact you to see if you would like to learn more.
COVID-19 Clinical Studies
Explore COVID-19 Clinical Studies . Stanford Medicine researchers and scientists have launched dozens of research projects as part of the global response to COVID-19. By participating in our COVID-19 clinical research, you help accelerate medical science by providing valuable insights into potential treatments and methods of prevention.
Stanford Diabetes Research Center
The Stanford Diabetes Research Center (SDRC) is looking for participants, including healthy volunteers, to join the various diabetes-related studies being conducted at Stanford. Join the SDRC research registry
Project Baseline
Project Baseline is a broad effort designed to develop a well-defined reference, or “baseline,” of good health. Its rich data platform will be used to better understand the transition from health to disease and identify additional risk factors for disease.
Stanford Well for Life
Stanford WELL for Life wants to help you improve your health, wellness, and well-being through challenges, resources and tips to improve your well-being from Stanford experts.
Latest Clinical Trials News

Stanford Medicine offers gene therapy for a devastating pediatric neurologic disease

Existing high blood pressure drugs may prevent epilepsy, Stanford Medicine-led study finds

Stanford Medicine trial: 15-day Paxlovid regimen safe but adds no clear long-COVID benefit

Christopher Garcia is the 2024 Passano Award winner

Janice ‘Wes’ Brown, infectious disease researcher and physician, dies at 63
Clinicial trial faq.
Why should I participate in a clinical trial?
Clinical trials are critical to progressing medical advancements and helping people live longer. Many of the treatments used today would not be available if they were not first tested in clinical trials.
At Stanford, our physician-researchers and scientists perform collaborative research to improve diagnosis and treatment options for people worldwide. Because of their level of expertise, some of the trials and innovative treatments we offer are not available elsewhere in the world.
How do I know if a clinical trial is right for me?
To determine if a clinical trial is right for you, talk to your doctor. He or she can refer you to a study coordinator for more information on research studies that may be suitable for your specific condition.
You can also find the guidelines for who can participate in a particular clinical trial online. However, it is best to work with your doctor to decide the right care approach for your needs.
Why are clinical trials done in phases?
Clinical trials are executed in phases to determine their safety and effectiveness. Specific scientific questions are answered in each phase to demonstrate the potential of a new drug, device, or medical approach.
Is there a cost associated with participating in a clinical trial?
As a study participant, you receive a new drug, device, medical approach, or other treatment for free.
Why are some clinical trials closed and others open?
Open trials refer to studies currently accepting participants. Closed trials are not currently enrolling, but may open in the future for enrollment.
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms
Masks Strongly Recommended but Not Required in Maryland, Starting Immediately
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
Understanding Clinical Trials
Clinical research: what is it.

Your doctor may have said that you are eligible for a clinical trial, or you may have seen an ad for a clinical research study. What is clinical research, and is it right for you?
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn which new ideas may help people.
Every drug, device, tool, diagnostic test, technique and technology used in medicine today was once tested in volunteers who took part in clinical research studies.
At Johns Hopkins Medicine, we believe that clinical research is key to improve care for people in our community and around the world. Once you understand more about clinical research, you may appreciate why it’s important to participate — for yourself and the community.
What Are the Types of Clinical Research?
There are two main kinds of clinical research:
Observational Studies
Observational studies are studies that aim to identify and analyze patterns in medical data or in biological samples, such as tissue or blood provided by study participants.
Clinical Trials
Clinical trials, which are also called interventional studies, test the safety and effectiveness of medical interventions — such as medications, procedures and tools — in living people.
Clinical research studies need people of every age, health status, race, gender, ethnicity and cultural background to participate. This will increase the chances that scientists and clinicians will develop treatments and procedures that are likely to be safe and work well in all people. Potential volunteers are carefully screened to ensure that they meet all of the requirements for any study before they begin. Most of the reasons people are not included in studies is because of concerns about safety.
Both healthy people and those with diagnosed medical conditions can take part in clinical research. Participation is always completely voluntary, and participants can leave a study at any time for any reason.
“The only way medical advancements can be made is if people volunteer to participate in clinical research. The research participant is just as necessary as the researcher in this partnership to advance health care.” Liz Martinez, Johns Hopkins Medicine Research Participant Advocate
Types of Research Studies
Within the two main kinds of clinical research, there are many types of studies. They vary based on the study goals, participants and other factors.
Biospecimen studies
Healthy volunteer studies.
Clinical trials study the safety and effectiveness of interventions and procedures on people’s health. Interventions may include medications, radiation, foods or behaviors, such as exercise. Usually, the treatments in clinical trials are studied in a laboratory and sometimes in animals before they are studied in humans. The goal of clinical trials is to find new and better ways of preventing, diagnosing and treating disease. They are used to test:
Drugs or medicines

New types of surgery

Medical devices

New ways of using current treatments

New ways of changing health behaviors

New ways to improve quality of life for sick patients

Goals of Clinical Trials
Because every clinical trial is designed to answer one or more medical questions, different trials have different goals. Those goals include:
Treatment trials
Prevention trials, screening trials, phases of a clinical trial.
In general, a new drug needs to go through a series of four types of clinical trials. This helps researchers show that the medication is safe and effective. As a study moves through each phase, researchers learn more about a medication, including its risks and benefits.
Is the medication safe and what is the right dose? Phase one trials involve small numbers of participants, often normal volunteers.
Does the new medication work and what are the side effects? Phase two trials test the treatment or procedure on a larger number of participants. These participants usually have the condition or disease that the treatment is intended to remedy.
Is the new medication more effective than existing treatments? Phase three trials have even more people enrolled. Some may get a placebo (a substance that has no medical effect) or an already approved treatment, so that the new medication can be compared to that treatment.
Is the new medication effective and safe over the long term? Phase four happens after the treatment or procedure has been approved. Information about patients who are receiving the treatment is gathered and studied to see if any new information is seen when given to a large number of patients.
“Johns Hopkins has a comprehensive system overseeing research that is audited by the FDA and the Association for Accreditation of Human Research Protection Programs to make certain all research participants voluntarily agreed to join a study and their safety was maximized.” Gail Daumit, M.D., M.H.S., Vice Dean for Clinical Investigation, Johns Hopkins University School of Medicine
Is It Safe to Participate in Clinical Research?
There are several steps in place to protect volunteers who take part in clinical research studies. Clinical Research is regulated by the federal government. In addition, the institutional review board (IRB) and Human Subjects Research Protection Program at each study location have many safeguards built in to each study to protect the safety and privacy of participants.
Clinical researchers are required by law to follow the safety rules outlined by each study's protocol. A protocol is a detailed plan of what researchers will do in during the study.
In the U.S., every study site's IRB — which is made up of both medical experts and members of the general public — must approve all clinical research. IRB members also review plans for all clinical studies. And, they make sure that research participants are protected from as much risk as possible.
Earning Your Trust
This was not always the case. Many people of color are wary of joining clinical research because of previous poor treatment of underrepresented minorities throughout the U.S. This includes medical research performed on enslaved people without their consent, or not giving treatment to Black men who participated in the Tuskegee Study of Untreated Syphilis in the Negro Male. Since the 1970s, numerous regulations have been in place to protect the rights of study participants.
Many clinical research studies are also supervised by a data and safety monitoring committee. This is a group made up of experts in the area being studied. These biomedical professionals regularly monitor clinical studies as they progress. If they discover or suspect any problems with a study, they immediately stop the trial. In addition, Johns Hopkins Medicine’s Research Participant Advocacy Group focuses on improving the experience of people who participate in clinical research.
Clinical research participants with concerns about anything related to the study they are taking part in should contact Johns Hopkins Medicine’s IRB or our Research Participant Advocacy Group .
Learn More About Clinical Research at Johns Hopkins Medicine
For information about clinical trial opportunities at Johns Hopkins Medicine, visit our trials site.
Video Clinical Research for a Healthier Tomorrow: A Family Shares Their Story
Clinical Research for a Healthier Tomorrow: A Family Shares Their Story

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 19 June 2024
Apolipoprotein E in Alzheimer’s disease trajectories and the next-generation clinical care pathway
- Sneha Narasimhan 1 ,
- David M. Holtzman ORCID: orcid.org/0000-0002-3400-0856 2 ,
- Liana G. Apostolova 3 , 4 , 5 ,
- Carlos Cruchaga 6 , 7 ,
- Colin L. Masters 8 ,
- John Hardy 9 ,
- Victor L. Villemagne 10 ,
- Joanne Bell 1 ,
- Min Cho ORCID: orcid.org/0000-0003-4696-0173 1 &
- Harald Hampel ORCID: orcid.org/0000-0003-0894-8982 1
Nature Neuroscience ( 2024 ) Cite this article
1112 Accesses
22 Altmetric
Metrics details
- Alzheimer's disease
Alzheimer’s disease (AD) is a complex, progressive primary neurodegenerative disease. Since pivotal genetic studies in 1993, the ε4 allele of the apolipoprotein E gene ( APOE ε4 ) has remained the strongest single genome-wide associated risk variant in AD. Scientific advances in APOE biology, AD pathophysiology and ApoE-targeted therapies have brought APOE to the forefront of research, with potential translation into routine AD clinical care. This contemporary Review will merge APOE research with the emerging AD clinical care pathway and discuss APOE genetic risk as a conduit to genomic-based precision medicine in AD, including ApoE’s influence in the ATX(N) biomarker framework of AD. We summarize the evidence for APOE as an important modifier of AD clinical–biological trajectories. We then illustrate the utility of APOE testing and the future of ApoE-targeted therapies in the next-generation AD clinical–diagnostic pathway. With the emergence of new AD therapies, understanding how APOE modulates AD pathophysiology will become critical for personalized AD patient care.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others

APOE4 homozygosity represents a distinct genetic form of Alzheimer’s disease
Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies
Prediction of clinical diagnosis of Alzheimer’s disease, vascular, mixed, and all-cause dementia by a polygenic risk score and APOE status in a community-based cohort prospectively followed over 17 years
Alzheimer’s Association. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 18 , 700–789 (2022).
Hampel, H. et al. The amyloid-β pathway in Alzheimer’s disease. Mol. Psychiatry 26 , 5481–5503 (2021).
Article CAS PubMed PubMed Central Google Scholar
Hampel, H. et al. Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat. Rev. Neurol. 17 , 580–589 (2021).
Article PubMed Google Scholar
Yamazaki, Y., Zhao, N., Caulfield, T. R., Liu, C. C. & Bu, G. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat. Rev. Neurol. 15 , 501–518 (2019).
Genin, E. et al. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol. Psychiatry 16 , 903–907 (2011).
Corder, E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261 , 921–923 (1993).
Article CAS PubMed Google Scholar
Belloy, M. E. et al. APOE genotype and Alzheimer disease risk across age, sex, and population ancestry. JAMA Neurol. 80 , 1284–1294 (2023).
Article PubMed PubMed Central Google Scholar
Farrer, L. A. et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. JAMA 278 , 1349–1356 (1997).
Neu, S. C. et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurol. 74 , 1178–1189 (2017).
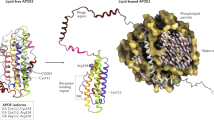
Chen, Y., Strickland, M. R., Soranno, A. & Holtzman, D. M. Apolipoprotein E: structural insights and links to Alzheimer disease pathogenesis. Neuron 109 , 205–221 (2021).
Linton, M. F. et al. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J. Clin. Invest. 88 , 270–281 (1991).
Mahley, R. W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240 , 622–630 (1988).
Stuchell-Brereton, M. D. et al. Apolipoprotein E4 has extensive conformational heterogeneity in lipid-free and lipid-bound forms. Proc. Natl Acad. Sci. USA 120 , e2215371120 (2023).
Reiman, E. M. et al. Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nat. Commun. 11 , 667 (2020).
Kaup, A. R. et al. Cognitive resilience to apolipoprotein E ε4: contributing factors in Black and white older adults. JAMA Neurol. 72 , 340–348 (2015).
Zheng, L. et al. Gender specific factors contributing to cognitive resilience in APOE ε4 positive older adults in a population-based sample. Sci. Rep. 13 , 8037 (2023).
Utermann, G., Hees, M. & Steinmetz, A. Polymorphism of apolipoprotein E and occurrence of dysbetalipoproteinaemia in man. Nature 269 , 604–607 (1977).
Corder, E. H. et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat. Genet. 7 , 180–184 (1994).
Naslavsky, M. S. et al. Global and local ancestry modulate APOE association with Alzheimer’s neuropathology and cognitive outcomes in an admixed sample. Mol. Psychiatry 27 , 4800–4808 (2022).
Chen, Q., Wang, T., Kang, D. & Chen, L. Protective effect of apolipoprotein E ε3 on sporadic Alzheimer’s disease in the Chinese population: a meta-analysis. Sci. Rep. 12 , 13620 (2022).
Nishita, Y. et al. Effects of APOEε4 genotype on age-associated change in cognitive functions among Japanese middle-aged and older adults: a 20-year follow-up study. Exp. Gerontol. 171 , 112036 (2023).
Ali, M. et al. Large multi-ethnic genetic analyses of amyloid imaging identify new genes for Alzheimer disease. Acta Neuropathol. Commun. 11 , 68 (2023).
Serrano-Pozo, A., Das, S. & Hyman, B. T. APOE and Alzheimer’s disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 20 , 68–80 (2021).
Cacace, R., Sleegers, K. & Van Broeckhoven, C. Molecular genetics of early-onset Alzheimer’s disease revisited. Alzheimers Dement. 12 , 733–748 (2016).
van Duijn, C. M. et al. Apolipoprotein E4 allele in a population-based study of early-onset Alzheimer’s disease. Nat. Genet. 7 , 74–78 (1994).
Polsinelli, A. J. et al. APOE ε4 is associated with earlier symptom onset in LOAD but later symptom onset in EOAD. Alzheimers Dement. 19 , 2212–2217 (2023).
Polsinelli, A. J. et al. APOE ε4 carrier status and sex differentiate rates of cognitive decline in early- and late-onset Alzheimer’s disease. Alzheimers Dement. 19 , 1983–1993 (2023).
Bu, G. APOE targeting strategy in Alzheimer’s disease: lessons learned from protective variants. Mol. Neurodegener. 17 , 51 (2022).
Arboleda-Velasquez, J. F. et al. Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: a case report. Nat. Med. 25 , 1680–1683 (2019).
Sepulveda-Falla, D. et al. Distinct tau neuropathology and cellular profiles of an APOE3 Christchurch homozygote protected against autosomal dominant Alzheimer’s dementia. Acta Neuropathol. 144 , 589–601 (2022).
Fagan, A. M. et al. Human and murine ApoE markedly alters Aβ metabolism before and after plaque formation in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 9 , 305–318 (2002).
Holtzman, D. M. et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA 97 , 2892–2897 (2000).
Tiraboschi, P. et al. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology 62 , 1977–1983 (2004).
Migliore, L. & Coppede, F. Gene–environment interactions in Alzheimer disease: the emerging role of epigenetics. Nat. Rev. Neurol. 18 , 643–660 (2022).
Hampel, H. et al. Designing the next-generation clinical care pathway for Alzheimer’s disease. Nat. Aging 2 , 692–703 (2022).
Barthelemy, N. R. et al. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer’s disease. Nat. Med. 26 , 398–407 (2020).
Therriault, J. et al. Association of phosphorylated tau biomarkers with amyloid positron emission tomography vs tau positron emission tomography. JAMA Neurol. 80 , 188–199 (2022).
Article PubMed Central Google Scholar
Gonzalez-Ortiz, F. et al. Brain-derived tau: a novel blood-based biomarker for Alzheimer’s disease-type neurodegeneration. Brain 146 , 1152–1165 (2023).
Hampel, H. et al. Blood-based biomarkers for Alzheimer’s disease: current state and future use in a transformed global healthcare landscape. Neuron 111 , 2781–2799 (2023).
Bradley, J. et al. Genetic architecture of plasma Alzheimer disease biomarkers. Hum. Mol. Genet. 32 , 2532–2543 (2023).
Elias-Sonnenschein, L. S., Viechtbauer, W., Ramakers, I. H., Verhey, F. R. & Visser, P. J. Predictive value of APOE - ε4 allele for progression from MCI to AD-type dementia: a meta-analysis. J. Neurol. Neurosurg. Psychiatry 82 , 1149–1156 (2011).
Vermunt, L. et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 15 , 888–898 (2019).
Leonenko, G. et al. Genetic risk for Alzheimer disease is distinct from genetic risk for amyloid deposition. Ann. Neurol. 86 , 427–435 (2019).
Tomassen, J. et al. Amyloid-β and APOE genotype predict memory decline in cognitively unimpaired older individuals independently of Alzheimer’s disease polygenic risk score. BMC Neurol. 22 , 484 (2022).
Emrani, S., Arain, H. A., DeMarshall, C. & Nuriel, T. APOE4 is associated with cognitive and pathological heterogeneity in patients with Alzheimer’s disease: a systematic review. Alzheimers Res. Ther. 12 , 141 (2020).
Buckley, R. F. et al. Sex, amyloid, and APOE ε4 and risk of cognitive decline in preclinical Alzheimer’s disease: findings from three well-characterized cohorts. Alzheimers Dement . 14 , 1193–1203 (2018).
Kumar, A. et al. Genetic effects on longitudinal cognitive decline during the early stages of Alzheimer’s disease. Sci. Rep. 11 , 19853 (2021).
Shi, Y. et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549 , 523–527 (2017).
Steward, A. et al. ApoE4 and connectivity-mediated spreading of tau pathology at lower amyloid levels. JAMA Neurol. 80 , 1295–1306 (2023).
Morris, J. C. et al. APOE predicts amyloid-β but not tau Alzheimer pathology in cognitively normal aging. Ann. Neurol. 67 , 122–131 (2010).
Burnham, S. C. et al. Impact of APOE - ε4 carriage on the onset and rates of neocortical Aβ-amyloid deposition. Neurobiol. Aging 95 , 46–55 (2020).
Jansen, W. J. et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 313 , 1924–1938 (2015).
Lim, Y. Y., Mormino, E. C. & Alzheimer’s Disease Neuroimaging Initiative. APOE genotype and early β-amyloid accumulation in older adults without dementia. Neurology 89 , 1028–1034 (2017).
Serrano-Pozo, A., Qian, J., Monsell, S. E., Betensky, R. A. & Hyman, B. T. APOEε2 is associated with milder clinical and pathological Alzheimer disease. Ann. Neurol. 77 , 917–929 (2015).
Deming, Y. et al. Genome-wide association study identifies four novel loci associated with Alzheimer’s endophenotypes and disease modifiers. Acta Neuropathol. 133 , 839–856 (2017).
West, T. et al. A blood-based diagnostic test incorporating plasma Aβ 42/40 ratio, ApoE proteotype, and age accurately identifies brain amyloid status: findings from a multi cohort validity analysis. Mol. Neurodegener. 16 , 30 (2021).
Castellano, J. M. et al. Human ApoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 3 , 89ra57 (2011).
Liu, C. C. et al. ApoE4 accelerates early seeding of amyloid pathology. Neuron 96 , 1024–1032 (2017).
Koffie, R. M. et al. Apolipoprotein E4 effects in Alzheimer’s disease are mediated by synaptotoxic oligomeric amyloid-β. Brain 135 , 2155–2168 (2012).
Hashimoto, T. et al. Apolipoprotein E, especially apolipoprotein E4, increases the oligomerization of amyloid β peptide. J. Neurosci. 32 , 15181–15192 (2012).
Hori, Y., Hashimoto, T., Nomoto, H., Hyman, B. T. & Iwatsubo, T. Role of apolipoprotein E in β-amyloidogenesis: isoform-specific effects on protofibril to fibril conversion of Aβ in vitro and brain Aβ deposition in vivo. J. Biol. Chem. 293 , 7267 (2018).
Kanekiyo, T., Xu, H. & Bu, G. ApoE and Aβ in Alzheimer’s disease: accidental encounters or partners? Neuron 81 , 740–754 (2014).
Deane, R. et al. ApoE isoform-specific disruption of amyloid β peptide clearance from mouse brain. J. Clin. Invest. 118 , 4002–4013 (2008).
Castellano, J. M. et al. Low-density lipoprotein receptor overexpression enhances the rate of brain-to-blood Aβ clearance in a mouse model of β-amyloidosis. Proc. Natl Acad. Sci. USA 109 , 15502–15507 (2012).
Hawkes, C. A. et al. Disruption of arterial perivascular drainage of amyloid-β from the brains of mice expressing the human APOE ε4 allele. PLoS ONE 7 , e41636 (2012).
Huang, Y. A., Zhou, B., Wernig, M. & Sudhof, T. C. ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and Aβ secretion. Cell 168 , 427–441 (2017).
Lin, Y. T. et al. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron 98 , 1141–1154 (2018).
Article Google Scholar
Nuriel, T. et al. The endosomal–lysosomal pathway is dysregulated by APOE4 expression in vivo. Front. Neurosci. 11 , 702 (2017).
Hou, X. et al. Differential and substrate-specific inhibition of γ-secretase by the C-terminal region of ApoE2, ApoE3, and ApoE4. Neuron 111 , 1898–1913 (2023).
Lim, Y. Y. et al. Association of β-amyloid and apolipoprotein E ε4 with memory decline in preclinical Alzheimer disease. JAMA Neurol. 75 , 488–494 (2018).
Ghisays, V. et al. Brain imaging measurements of fibrillar amyloid-β burden, paired helical filament tau burden, and atrophy in cognitively unimpaired persons with two, one, and no copies of the APOE ε4 allele. Alzheimers Dement. 16 , 598–609 (2020).
Cruchaga, C. et al. GWAS of cerebrospinal fluid tau levels identifies risk variants for Alzheimer’s disease. Neuron 78 , 256–268 (2013).
Farfel, J. M., Yu, L., De Jager, P. L., Schneider, J. A. & Bennett, D. A. Association of APOE with tau-tangle pathology with and without β-amyloid. Neurobiol. Aging 37 , 19–25 (2016).
Goldberg, T. E., Huey, E. D. & Devanand, D. P. Association of APOE e2 genotype with Alzheimer’s and non-Alzheimer’s neurodegenerative pathologies. Nat. Commun. 11 , 4727 (2020).
Wang, Y. T. et al. Interactive rather than independent effect of APOE and sex potentiates tau deposition in women. Brain Commun. 3 , fcab126 (2021).
Google Scholar
Dincer, A. et al. APOE ε4 genotype, amyloid-β, and sex interact to predict tau in regions of high APOE mRNA expression. Sci. Transl. Med. 14 , eabl7646 (2022).
Yan, S. et al. Sex modifies APOE ε4 dose effect on brain tau deposition in cognitively impaired individuals. Brain 144 , 3201–3211 (2021).
Ferrari-Souza, J. P. et al. APOEε4 associates with microglial activation independently of Aβ plaques and tau tangles. Sci. Adv. 9 , eade1474 (2023).
Shi, Y. et al. Microglia drive APOE-dependent neurodegeneration in a tauopathy mouse model. J. Exp. Med. 216 , 2546–2561 (2019).
Wang, C. et al. Selective removal of astrocytic APOE4 strongly protects against tau-mediated neurodegeneration and decreases synaptic phagocytosis by microglia. Neuron 109 , 1657–1674 (2021).
Seo, D. O. et al. ApoE isoform- and microbiota-dependent progression of neurodegeneration in a mouse model of tauopathy. Science 379 , eadd1236 (2023).
Koutsodendris, N. et al. Neuronal APOE4 removal protects against tau-mediated gliosis, neurodegeneration and myelin deficits. Nat. Aging 3 , 275–296 (2023).
Parhizkar, S. & Holtzman, D. M. APOE mediated neuroinflammation and neurodegeneration in Alzheimer’s disease. Semin. Immunol. 59 , 101594 (2022).
Shi, Y. & Holtzman, D. M. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat. Rev. Immunol. 18 , 759–772 (2018).
Ulrich, J. D. et al. ApoE facilitates the microglial response to amyloid plaque pathology. J. Exp. Med. 215 , 1047–1058 (2018).
Serrano-Pozo, A. et al. Effect of APOE alleles on the glial transcriptome in normal aging and Alzheimer’s disease. Nat. Aging 1 , 919–931 (2021).
Stephen, T. L. et al. APOE genotype and sex affect microglial interactions with plaques in Alzheimer’s disease mice. Acta Neuropathol. Commun. 7 , 82 (2019).
Rodriguez, G. A., Tai, L. M., LaDu, M. J. & Rebeck, G. W. Human APOE4 increases microglia reactivity at Aβ plaques in a mouse model of Aβ deposition. J. Neuroinflammation 11 , 111 (2014).
Parhizkar, S. et al. Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat. Neurosci. 22 , 191–204 (2019).
Gratuze, M. et al. Activated microglia mitigate Aβ-associated tau seeding and spreading. J. Exp. Med. 218 , e20210542 (2021).
Fitz, N. F. et al. Phospholipids of APOE lipoproteins activate microglia in an isoform-specific manner in preclinical models of Alzheimer’s disease. Nat. Commun. 12 , 3416 (2021).
Chen, X. et al. Microglia-mediated T cell infiltration drives neurodegeneration in tauopathy. Nature 615 , 668–677 (2023).
Shi, Y. et al. Overexpressing low-density lipoprotein receptor reduces tau-associated neurodegeneration in relation to ApoE-linked mechanisms. Neuron 109 , 2413–2426 (2021).
Litvinchuk, A. et al. Apolipoprotein E4 reduction with antisense oligonucleotides decreases neurodegeneration in a tauopathy model. Ann. Neurol. 89 , 952–966 (2021).
Nugent, A. A. et al. TREM2 regulates microglial cholesterol metabolism upon chronic phagocytic challenge. Neuron 105 , 837–854 (2020).
Victor, M. B. et al. Lipid accumulation induced by APOE4 impairs microglial surveillance of neuronal-network activity. Cell Stem Cell 29 , 1197–1212 (2022).
CAS Google Scholar
Tcw, J. et al. Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell 185 , 2213–2233 (2022).
Qi, G. et al. ApoE4 impairs neuron–astrocyte coupling of fatty acid metabolism. Cell Rep. 34 , 108572 (2021).
Blanchard, J. W. et al. APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature 611 , 769–779 (2022).
Chatterjee, P. et al. Diagnostic and prognostic plasma biomarkers for preclinical Alzheimer’s disease. Alzheimers Dement. 18 , 1141–1154 (2022).
Stevenson-Hoare, J. et al. Plasma biomarkers and genetics in the diagnosis and prediction of Alzheimer’s disease. Brain 146 , 690–699 (2023).
Bonomi, C. G. et al. Cerebrospinal fluid sTREM-2, GFAP, and β-S100 in symptomatic sporadic Alzheimer’s disease: microglial, astrocytic, and APOE contributions along the Alzheimer’s disease continuum. J. Alzheimers Dis. 92 , 1385–1397 (2023).
Greenberg, S. M. et al. Cerebral amyloid angiopathy and Alzheimer disease — one peptide, two pathways. Nat. Rev. Neurol. 16 , 30–42 (2020).
Jakel, L., De Kort, A. M., Klijn, C. J. M., Schreuder, F. & Verbeek, M. M. Prevalence of cerebral amyloid angiopathy: a systematic review and meta-analysis. Alzheimers Dement. 18 , 10–28 (2022).
Tai, L. M. et al. The role of APOE in cerebrovascular dysfunction. Acta Neuropathol. 131 , 709–723 (2016).
Montagne, A. et al. APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature 581 , 71–76 (2020).
Barisano, G. et al. A ‘multi-omics’ analysis of blood–brain barrier and synaptic dysfunction in APOE4 mice. J. Exp. Med. 219 , e20221137 (2022).
Liu, C. C. et al. Peripheral ApoE4 enhances Alzheimer’s pathology and impairs cognition by compromising cerebrovascular function. Nat. Neurosci. 25 , 1020–1033 (2022).
Fryer, J. D. et al. Apolipoprotein E markedly facilitates age-dependent cerebral amyloid angiopathy and spontaneous hemorrhage in amyloid precursor protein transgenic mice. J. Neurosci. 23 , 7889–7896 (2003).
Fryer, J. D. et al. Human apolipoprotein E4 alters the amyloid-β 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J. Neurosci. 25 , 2803–2810 (2005).
Xiong, M. et al. Astrocytic APOE4 removal confers cerebrovascular protection despite increased cerebral amyloid angiopathy. Mol. Neurodegener. 18 , 17 (2023).
Chwalisz, B. K. Cerebral amyloid angiopathy and related inflammatory disorders. J. Neurol. Sci. 424 , 117425 (2021).
Filippi, M. et al. Amyloid-related imaging abnormalities and β-amyloid-targeting antibodies: a systematic review. JAMA Neurol. 79 , 291–304 (2022).
Hampel, H. et al. Amyloid-related imaging abnormalities (ARIA): radiological, biological and clinical characteristics. Brain 146 , 4414–4424 (2023).
Liu, C. C., Liu, C. C., Kanekiyo, T., Xu, H. & Bu, G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 9 , 106–118 (2013).
Cruchaga, C. et al. Proteogenomic analysis of human cerebrospinal fluid identifies neurologically relevant regulation and informs causal proteins for Alzheimer’s disease. Preprint at Reseach Square https://doi.org/10.21203/rs.3.rs-2814616/v1 (2023).
Tible, M. et al. Dissection of synaptic pathways through the CSF biomarkers for predicting Alzheimer disease. Neurology 95 , e953–e961 (2020).
Roberts, J. S. & Uhlmann, W. R. Genetic susceptibility testing for neurodegenerative diseases: ethical and practice issues. Prog. Neurobiol. 110 , 89–101 (2013).
Largent, E. A. et al. Disclosing genetic risk of Alzheimer’s disease to cognitively unimpaired older adults: findings from the study of knowledge and reactions to APOE testing (SOKRATES II). J. Alzheimers Dis. 84 , 1015–1028 (2021).
Blasco, D. & Roberts, J. S. Editorial: implications of emerging uses of genetic testing for Alzheimer’s disease. J. Prev. Alzheimers Dis. 10 , 359–361 (2023).
CAS PubMed PubMed Central Google Scholar
Goldman, J. S. et al. ADDENDUM: genetic counseling and testing for Alzheimer disease: joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genet. Med. 21 , 2404 (2019).
Galluzzi, S. et al. Disclosure of genetic risk factors for Alzheimer’s disease to cognitively healthy individuals—from current practice towards a personalised medicine scenario. Biomedicines 10 , 3177 (2022).
Batra, P. & Huang, K. L. Genotype concordance and polygenic risk score estimation across consumer genetic testing data. Ann. Hum. Genet. 84 , 352–356 (2020).
Cummings, J. et al. Lecanemab: appropriate use recommendations. J. Prev. Alzheimers Dis. 10 , 362–377 (2023).
Bertram, L. & Hampel, H. The role of genetics for biomarker development in neurodegeneration. Prog. Neurobiol. 95 , 501–504 (2011).
Tolar, M., Abushakra, S., Hey, J. A., Porsteinsson, A. & Sabbagh, M. Aducanumab, gantenerumab, BAN2401, and ALZ-801—the first wave of amyloid-targeting drugs for Alzheimer’s disease with potential for near term approval. Alzheimers Res. Ther. 12 , 95 (2020).
Abushakra, S. et al. Clinical effects of tramiprosate in APOE4/4 homozygous patients with mild Alzheimer’s disease suggest disease modification potential. J. Prev. Alzheimers Dis. 4 , 149–156 (2017).
CAS PubMed Google Scholar
Walsh, T. et al. Outreach, screening, and randomization of APOE ε4 carriers into an Alzheimer’s prevention trial: a global perspective from the API Generation Program. J. Prev. Alzheimers Dis. 10 , 453–463 (2023).
Solomon, A. et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 75 , 462–470 (2018).
Stocker, H., Mollers, T., Perna, L. & Brenner, H. The genetic risk of Alzheimer’s disease beyond APOE ε4 : systematic review of Alzheimer’s genetic risk scores. Transl. Psychiatry 8 , 166 (2018).
Jung, S. H. et al. Transferability of Alzheimer disease polygenic risk score across populations and its association with Alzheimer disease-related phenotypes. JAMA Netw. Open 5 , e2247162 (2022).
Green, R. C. et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N. Engl. J. Med . 361 , 245–254 (2009).
Lineweaver, T. T., Bondi, M. W., Galasko, D. & Salmon, D. P. Effect of knowledge of APOE genotype on subjective and objective memory performance in healthy older adults. Am. J. Psychiatry 171 , 201–208 (2014).
Chao, S. et al. Health behavior changes after genetic risk assessment for Alzheimer disease: the REVEAL Study. Alzheimer Dis. Assoc. Disord. 22 , 94–97 (2008).
Zick, C. D. et al. Genetic testing for Alzheimer’s disease and its impact on insurance purchasing behavior. Health Aff. 24 , 483–490 (2005).
Cook, L. et al. Tools for communicating risk for Parkinson’s disease. NPJ Parkinsons Dis. 8 , 164 (2022).
Hampel, H. et al. The foundation and architecture of precision medicine in neurology and psychiatry. Trends Neurosci. 46 , 176–198 (2023).
Jansen, I. E. et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 51 , 404–413 (2019).
Kunkle, B. W. et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 51 , 414–430 (2019).
Xiong, M. et al. APOE immunotherapy reduces cerebral amyloid angiopathy and amyloid plaques while improving cerebrovascular function. Sci. Transl. Med. 13 , eabd7522 (2021).
Huynh, T. V. et al. Age-dependent effects of ApoE reduction using antisense oligonucleotides in a model of β-amyloidosis. Neuron 96 , 1013–1023 (2017).
Pankiewicz, J. E. et al. Blocking the ApoE/Aβ interaction ameliorates Aβ-related pathology in APOE ε2 and ε4 targeted replacement Alzheimer model mice. Acta Neuropathol. Commun. 2 , 75 (2014).
PubMed PubMed Central Google Scholar
Zhao, N. et al. APOE ε2 is associated with increased tau pathology in primary tauopathy. Nat. Commun. 9 , 4388 (2018).
Margeta, M. A. et al. Association of APOE with primary open-angle glaucoma suggests a protective effect for APOE ε4 . Invest. Ophthalmol. Vis. Sci. 61 , 3 (2020).
Marino, C. et al. APOE Christchurch-mimetic therapeutic antibody reduces APOE-mediated toxicity and tau phosphorylation. Alzheimers Dement. 20 , 819–836 (2023).
Jack, C. R. Jr. et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 9 , 119–128 (2010).
Gratuze, M. et al. APOE antibody inhibits Aβ-associated tau seeding and spreading in a mouse model. Ann. Neurol. 91 , 847–852 (2022).
Hudry, E. et al. Gene transfer of human APOE isoforms results in differential modulation of amyloid deposition and neurotoxicity in mouse brain. Sci. Transl. Med. 5 , 212ra161 (2013).
Rosenberg, J. B. et al. AAVrh.10-mediated APOE2 central nervous system gene therapy for APOE4 -associated Alzheimer’s disease. Hum. Gene Ther. Clin. Dev. 29 , 24–47 (2018).
Download references
Acknowledgements
Figure rendering under the direction and schema devised by the authors was provided by Y. Henderson of MediTech Media and was funded by Eisai, in accordance with Good Publication 20 Practice (GPP3) guidelines.
Author information
Authors and affiliations.
Eisai Inc., Nutley, NJ, USA
Sneha Narasimhan, Joanne Bell, Min Cho & Harald Hampel
Department of Neurology, Hope Center for Neurological Disorders, Knight ADRC, Washington University in St. Louis, St. Louis, MO, USA
David M. Holtzman
Department of Neurology, Indiana University School of Medicine, Indianapolis, IN, USA
Liana G. Apostolova
Department of Radiology and Imaging Neurosciences, Indiana University School of Medicine, Indianapolis, IN, USA
Department of Medical and Molecular Genetics, Indiana University School of Medicine, Indianapolis, IN, USA
Department of Psychiatry, Washington University School of Medicine, St. Louis, MO, USA
Carlos Cruchaga
NeuroGenomics and Informatics Center, Washington University School of Medicine, St. Louis, MO, USA
Florey Institute and the University of Melbourne, Parkville, Victoria, Australia
Colin L. Masters
Department of Neurodegenerative Disease and Dementia Research Institute, Reta Lila Weston Research Laboratories, UCL Institute of Neurology, Queen Square, London, UK
Department of Psychiatry, the University of Pittsburgh, Pittsburgh, PA, USA
Victor L. Villemagne
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Harald Hampel .
Ethics declarations
Competing interests.
H.H. is an employee of Eisai. He serves as a reviewing editor for the journal Alzheimer’s & Dementia . He is an inventor of 11 patents and has received no royalties: In Vitro Multiparameter Determination Method for the Diagnosis and Early Diagnosis of Neurodegenerative Disorders, patent number 8916388; In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases, patent number 8298784; Neurodegenerative Markers for Psychiatric Conditions, publication number 20120196300; In Vitro Multiparameter Determination Method for the Diagnosis and Early Diagnosis of Neurodegenerative Disorders, publication number 20100062463; In Vitro Method for the Diagnosis and Early Diagnosis of Neurodegenerative Disorders, publication number 20100035286; In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases, publication number 20090263822; In Vitro Method for the Diagnosis of Neurodegenerative Diseases, patent number 7547553; CSF Diagnostic In Vitro Method for Diagnosis of Dementias and Neuroinflammatory Diseases, publication number 20080206797; In Vitro Method for the Diagnosis of Neurodegenerative Diseases, publication number 20080199966; Neurodegenerative Markers for Psychiatric Conditions, publication number 20080131921; Method for diagnosis of dementias and neuroinflammatory diseases based on an increased level of procalcitonin in cerebrospinal fluid, publication number: United States patent 10921330. D.M.H. cofounded, has equity in and is on the scientific advisory board of C2N Diagnostics. He is on the scientific advisory board of Denali, Cajal Neuroscience and Genentech and consults for Alector. He is an inventor on US patent application US-20190270794-A1, ‘Anti-ApoE antibodies’. Anti-ApoE antibodies and this patent application have been licensed by Washington University the NextCure. L.G.A. receives research support from the NIH, the Alzheimer Association, AVID Pharmaceuticals, Life Molecular Imaging, Roche Diagnostics and Eli Lilly. L.G.A. has served as a consultant for Biogen, Two Labs, IQVIA, the NIH, the Florida Department of Health, Siemens, Corium, the NIH Biobank, Eli Lilly, Eisai, GE Healthcare, Roche Diagnostics, Alnylam and Genentech. L.G.A. is a member of various data and safety monitoring boards and advisory boards for IQVIA, NIA R01 AG061111, the UAB Nathan Schick Center, the FDA PCNS Advisory Board and the University New Mexico ADRC. L.G.A. serves in a leadership or fiduciary role at the Medical and Scientific Council of the Alzheimer’s Association Greater Indiana Chapter, the Alzheimer’s Association Science Program Committee, the FDA PCNS Advisory Committee and the Beeson Program Committee. L.G.A. has received equipment, materials, drugs, medical writing, gifts or other services from AVID Pharmaceuticals, Life Molecular Imaging and Roche Diagnostics. L.G.A. owns stock in Cassava Neurosciences and Golden Seeds. C.C. has received research support from GSK. C.C. is a member of the advisory board of Vivid Genomics and Circular Genomics and owns stocks. C.L.M. has no competing interests to declare. J.H. has consulted for Eisai and Eli Lilly. V.L.V. serves as a senior associate editor for the Journal of Neurochemistry . He has been a consultant for IXICO and Life Molecular Imaging and has received speaker honoraria from GE Healthcare, Piramal Lifesciences and Eli Lilly. M.C., J.B. and S.N. are employees of Eisai.
Peer review
Peer review information.
Nature Neuroscience thanks David Bennett and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Narasimhan, S., Holtzman, D.M., Apostolova, L.G. et al. Apolipoprotein E in Alzheimer’s disease trajectories and the next-generation clinical care pathway. Nat Neurosci (2024). https://doi.org/10.1038/s41593-024-01669-5
Download citation
Received : 14 August 2023
Accepted : 18 April 2024
Published : 19 June 2024
DOI : https://doi.org/10.1038/s41593-024-01669-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Health Tech
- Health Insurance
- Medical Devices
- Gene Therapy
- Neuroscience
- H5N1 Bird Flu
- Health Disparities
- Infectious Disease
Mental Health
- Cardiovascular Disease
- Chronic Disease
- Alzheimer's
- Coercive Care
- The Obesity Revolution
- The War on Recovery
- Adam Feuerstein
- Matthew Herper
- Jennifer Adaeze Okwerekwu
- Ed Silverman
- CRISPR Tracker
- Breakthrough Device Tracker
- Generative AI Tracker
- Obesity Drug Tracker
- 2024 STAT Summit
- Wunderkinds Nomination
- STAT Madness
- STAT Brand Studio
Don't miss out
Subscribe to STAT+ today, for the best life sciences journalism in the industry
Turning a ‘no’ vote into progress: How to advance MDMA-assisted psychotherapy for PTSD
By Andrew D. Forsyth , Mallory O. Johnson , and Jae M. Sevelius June 25, 2024

M any people have interpreted as a setback the “no” vote by an advisory committee to the Food and Drug Administration on Lykos Therapeutics’ new drug application for MDMA-assisted psychotherapy for post-traumatic stress disorder. We believe it is an opportunity to build upon Lykos’ success and rally support for better-funded, well-controlled clinical trials to determine the safety and efficacy of a promising PTSD treatment.
Upon reviewing the FDA’s and Lykos’ briefing documents, the Psychopharmacologic Drugs Advisory Committee deemed the study data insufficient to approve the application, and it determined that the company’s risk evaluation and mitigation strategy did not fully address potential harms of this therapy.
advertisement
The committee’s concerns about study design and data limitations are not uncommon in clinical trials, especially those on psychedelics. However, Lykos’ reliance on philanthropy and donations may have limited its capacity to ensure the rigor needed to meet the agency’s evidentiary standards , particularly in neglecting to properly investigate and quantify the specific contributions of the psychotherapy component. These issues raise the possibility that better study planning and coordination , enabled by more extensive funding, may have mitigated these challenges.
The potential of MDMA-assisted psychotherapy for PTSD treatment
As the FDA noted in its briefing documents , PTSD is a condition affecting an estimated 13 million Americans that may arise following intensely distressing life events, such as threats of death, severe injury, or sexual violence. Besides intrusive memories, hyperarousal, and avoidant behavior, persons with PTSD have a higher risk of suicidal ideation and behavior. Although 60% respond to FDA-approved medications such as sertraline and paroxetine, only 20% to 30% achieve remission, underscoring a vast unmet need for effective PTSD treatments, particularly for those most likely to experience trauma (e.g., combat veterans, women, racial, sexual, and gender minorities).
Related: The inside story of how Lykos’ MDMA research went awry
First synthesized in the early 1900s, MDMA gained notoriety as the 1980s street drug ecstasy before being banned as a Schedule I substance . It resurfaced in the 1990s when clinical researchers noted its capacity to facilitate profound empathy and compassion toward self and others, making it a promising adjunct to psychotherapy . Of relevance to PTSD and other avoidance-based disorders is MDMA’s capacity to diminish fear responses and facilitate lasting shifts from avoidance to acceptance behaviors.
By reducing the aversiveness of unpleasant thoughts and emotions, MDMA enables greater tolerance of traumatic memories and enhances empathetic acceptance.
Understanding the MDMA trials and the committee’s decision
Lykos submitted data from two Phase 3 clinical trials, known as MAPP1 and MAPP2 , which compared the effects of MDMA-assisted psychotherapy for severe and at least moderate PTSD versus inactive placebo plus psychotherapy. Three cycles of treatment for each participant, provided over four months, consisted of preparatory sessions, an eight-hour medication session, and integrative therapy. Endpoints measured changes in symptoms and functional impairment at 18 weeks.
Both studies reported significant improvement with MDMA-assisted psychotherapy and limited evidence of adverse events.
After careful deliberation, the Psychopharmacologic Drugs Advisory Committee endorsed several concerns raised in the FDA’s briefing documents :
First, committee members were concerned that many participants guessed correctly their assignment to the MDMA-assisted and placebo conditions, which may have affected patient-reported outcomes. No additional analyses were reported to examine this possibility.
Second, the committee pointed out missing lab tests and underreported drug reactions like euphoria and elation, which are essential for assessing side effects and abuse potential.
Related: Oral ketamine tablets effective for treating depression, new study finds
Third, the panelists shared FDA’s concerns that there were insufficient safeguards to protect patients from coercion, misconduct, and injury following improper discharge after MDMA dosing sessions.
Fourth, the committee concluded that the proposed strategies to mitigate potential harms needed more provisions for therapist training and potential misconduct, external monitoring, on-site medical supervision, and ways for patients to report issues.
A need for improved clinical trials infrastructure
The MAPP1 and MAPP2 data exhibit many common shortcomings of clinical trials on psychedelics that make it challenging to be sure that the findings reflect unbiased estimates of the underlying phenomena, namely that MDMA-assisted psychotherapy for PTSD, as implemented by Lykos Therapeutics, is safe and effective.
Given the methodological noise, the reported findings suggest a promising signal that is hard to decipher. While acknowledging the challenges raised by these studies, the committee’s “no” vote does not discourage research on MDMA-assisted psychotherapy for PTSD. Rather, in line with the National Academies of Sciences, Engineering, and Medicine’s recommendations , it suggests the need for public sector collaboration and investment to support more rigorous clinical trials to advance knowledge and translate findings into new tools for mental health.
Two examples of such public investment are noteworthy. First, the National Institutes of Health’s HIV Clinical Trials Networks models how to support high-quality clinical research worldwide, with coordinated leadership and operations, laboratory, and statistical and data management centers collaborating with advocates, government, academia, industry, and nongovernmental organizations. These networks develop and test novel HIV treatments and cure strategies with state-of-the-science protocol development, research design, and data safety and monitoring.
A second example is the U.S. Department of Veterans Affairs’ National Center for PTSD , which seeks to improve clinical care for military veterans through interdisciplinary research on stress-related disorders. Recently, the VA issued its first request for applications since the 1960s to study psychedelic compounds for treating PTSD and depression. This initiative aligns with extant NIH efforts to establish a psychedelic science and medicine interest group , co-sponsor workshops , and a $7.3 million portfolio of predominantly preclinical psychedelic studies. Although the VA’s investments primarily benefit veterans, they highlight the potential for federal investment in clinical trials to identify safe and effective PTSD treatments.
Sign up for Morning Rounds
Understand how science, health policy, and medicine shape the world every day
A call to action
The Psychopharmacologic Drugs Advisory Committee’s vote on Lykos Therapeutics’ new drug application is a call to action for advocates, academics, political leaders, funding agencies, foundations, and the scientific community. Public investment is driven by affected communities advocating for their needs and resources to address morbidity and mortality risks. In the 1990s, HIV advocates in the U.S. drastically increased federal and state investment in HIV science, shortened NIH funding cycles, and accelerated access to experimental drugs, bringing control of the global pandemic within reach. Advocates for those with PTSD should follow their lead in pushing for the rapid development of safe and effective new therapies for a persistent threat to well-being.
The time is right. There is a receptive audience in Washington, D.C., for public investment in psychedelic-assisted psychotherapy: the bipartisan Congressional Psychedelics Advancing Clinical Treatments (PACT) Caucus , led by Reps. Lou Correa (D-Calif.) and Jack Bergman (R-Mich.), which aims to fund research on the safety, efficacy, and durability of these treatments to address the national mental health crisis. In the footsteps of Australia and the Netherlands , the U.S. must redouble its efforts to fund more rigorous clinical trials to determine the safety and efficacy of a promising PTSD treatment for those who stand to benefit from it.
Andrew D. Forsyth, Ph.D., is an independent consultant and former NIH program officer based in Berkeley, Calif. Mallory O. Johnson, Ph.D., is a professor of medicine and nursing at the University of California, San Francisco. Jae M. Sevelius, Ph.D., is a professor of medical psychology in the Department of Psychiatry at New York Presbyterian/Columbia University Irving Medical Center.
LETTER TO THE EDITOR
Have an opinion on this essay submit a letter to the editor here ., about the authors reprints, andrew d. forsyth, mallory o. johnson, jae m. sevelius.
psychedelics
STAT encourages you to share your voice. We welcome your commentary, criticism, and expertise on our subscriber-only platform, STAT+ Connect
To submit a correction request, please visit our Contact Us page .

Recommended

Recommended Stories

To secure the U.S.’s biotech supply chain, pay attention to domestic companies

Medicare’s Part D policy is blocking progress needed to achieve Black health equity

STAT Plus: What the Sarepta decision means for Duchenne patients, the company, and the FDA

STAT Plus: Grail, aiming to market a blood test for cancer, faces host of challenges as it debuts on Nasdaq

STAT Plus: Ateev Mehrotra, the researcher the telehealth lobby loves to hate, isn’t backing down

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
What Are Clinical Trials and Studies?
On this page:
What is clinical research?
Why participate in a clinical trial, what happens in a clinical trial or study, what happens when a clinical trial or study ends, what are the different phases of clinical trials, questions to ask before participating in clinical research, how do researchers decide who will participate, clinical research needs participants with diverse backgrounds.
By participating in clinical research, you can help scientists develop new medications and other strategies to treat and prevent disease. Many effective treatments that are used today, such as chemotherapy, cholesterol-lowering drugs, vaccines, and cognitive-behavioral therapy, would not exist without research participants. Whether you’re healthy or have a medical condition, people of all ages and backgrounds can participate in clinical trials. This article can help you learn more about clinical research, why people choose to participate, and how to get involved in a study.
Mr. Jackson's story
Mr. Jackson is 73 years old and was just diagnosed with Alzheimer’s disease . He is worried about how it will affect his daily life. Will he forget to take his medicine? Will he forget his favorite memories, like the births of his children or hiking the Appalachian Trail? When Mr. Jackson talked to his doctor about his concerns, she told him about a clinical trial that is testing a possible new Alzheimer’s treatment. But Mr. Jackson has concerns about clinical trials. He does not want to feel like a lab rat or take the chance of getting a treatment that may not work or could make him feel worse. The doctor explained that there are both risks and benefits to being part of a clinical trial, and she talked with Mr. Jackson about research studies — what they are, how they work, and why they need volunteers. This information helped Mr. Jackson feel better about clinical trials. He plans to learn more about how to participate.
Clinical research is the study of health and illness in people. There are two main types of clinical research: observational studies and clinical trials.

Observational studies monitor people in normal settings. Researchers gather information from people and compare changes over time. For example, researchers may ask a group of older adults about their exercise habits and provide monthly memory tests for a year to learn how physical activity is associated with cognitive health . Observational studies do not test a medical intervention, such as a drug or device, but may help identify new treatments or prevention strategies to test in clinical trials.
Clinical trials are research studies that test a medical, surgical, or behavioral intervention in people. These trials are the primary way that researchers determine if a new form of treatment or prevention, such as a new drug, diet, or medical device (for example, a pacemaker), is safe and effective in people. Often, a clinical trial is designed to learn if a new treatment is more effective or has less harmful side effects than existing treatments.
Other aims of clinical research include:
- Testing ways to diagnose a disease early, sometimes before there are symptoms
- Finding approaches to prevent a health problem, including in people who are healthy but at increased risk of developing a disease
- Improving quality of life for people living with a life-threatening disease or chronic health problem
- Studying the role of caregivers or support groups
Learn more about clinical research from MedlinePlus and ClinicalTrials.gov .

People volunteer for clinical trials and studies for a variety of reasons, including:
- They want to contribute to discovering health information that may help others in the future.
- Participating in research helps them feel like they are playing a more active role in their health.
- The treatments they have tried for their health problem did not work or there is no treatment for their health problem.
Whatever the motivation, when you choose to participate in a clinical trial, you become a partner in scientific discovery. Participating in research can help future generations lead healthier lives. Major medical breakthroughs could not happen without the generosity of clinical trial participants — young and old, healthy, or diagnosed with a disease.
Where can I find a clinical trial?
Looking for clinical trials related to aging and age-related health conditions? Talk to your health care provider and use online resources to:
- Search for a clinical trial
- Look for clinical trials on Alzheimer's, other dementias, and caregiving
- Find a registry for a particular diagnosis or condition
- Explore clinical trials and studies supported by NIA
After you find one or more studies that you are interested in, the next step is for you or your doctor to contact the study research staff and ask questions. You can usually find contact information in the description of the study.
Let your health care provider know if you are thinking about joining a clinical trial. Your provider may want to talk to the research team to make sure the study is safe for you and to help coordinate your care.
Joining a clinical trial is a personal decision with potential benefits and some risks. Learn what happens in a clinical trial and how participant safety is protected . Read and listen to testimonials from people who decided to participate in research.
Here’s what typically happens in a clinical trial or study:
- Research staff explain the trial or study in detail, answer your questions, and gather more information about you.
- Once you agree to participate, you sign an informed consent form indicating your understanding about what to expect as a participant and the various outcomes that could occur.
- You are screened to make sure you qualify for the trial or study.
- If accepted into the trial, you schedule a first visit, which is called the “baseline” visit. The researchers conduct cognitive and/or physical tests during this visit.
- For some trials testing an intervention, you are assigned by chance (randomly) to a treatment group or a control group . The treatment group will get the intervention being tested, and the control group will not.
- You follow the trial procedures and report any issues or concerns to researchers.
- You may visit the research site at regularly scheduled times for new cognitive, physical, or other evaluations and discussions with staff. During these visits, the research team collects data and monitors your safety and well-being.
- You continue to see your regular physician(s) for usual health care throughout the study.
How do researchers decide which interventions are safe to test in people?
Before a clinical trial is designed and launched, scientists perform laboratory tests and often conduct studies in animals to test a potential intervention’s safety and effectiveness. If these studies show favorable results, the U.S. Food and Drug Administration (FDA) approves the intervention to be tested in humans. Learn more about how the safety of clinical trial participants is protected.
Once a clinical trial or study ends, the researchers analyze the data to determine what the findings mean and to plan the next steps. As a participant, you should be provided information before the study starts about how long it will last, whether you will continue receiving the treatment after the trial ends (if applicable), and how the results of the research will be shared. If you have specific questions about what will happen when the trial or study ends, ask the research coordinator or staff.
Clinical trials of drugs and medical devices advance through several phases to test safety, determine effectiveness, and identify any side effects. The FDA typically requires Phase 1, 2, and 3 trials to be conducted to determine if the drug or device can be approved for further use. If researchers find the intervention to be safe and effective after the first three phases, the FDA approves it for clinical use and continues to monitor its effects.
Each phase has a different purpose:
- A Phase 1 trial tests an experimental drug or device on a small group of people (around 20 to 80) to judge its safety, including any side effects, and to test the amount (dosage).
- A Phase 2 trial includes more people (around 100 to 300) to help determine whether a drug is effective. This phase aims to obtain preliminary data on whether the drug or device works in people who have a certain disease or condition. These trials also continue to examine safety, including short-term side effects.
- A Phase 3 trial gathers additional information from several hundred to a few thousand people about safety and effectiveness, studying different populations and different dosages, and comparing the intervention with other drugs or treatment approaches. If the FDA agrees that the trial results support the intervention’s use for a particular health condition, it will approve the experimental drug or device.
- A Phase 4 trial takes place after the FDA approves the drug or device. The treatment’s effectiveness and safety are monitored in large, diverse populations. Sometimes, side effects may not become clear until more people have used the drug or device over a longer period of time.
Clinical trials that test a behavior change, rather than a drug or medical device, advance through similar steps, but behavioral interventions are not regulated by the FDA. Learn more about clinical trials , including the types of trials and the four phases.
Choosing to participate in research is an important personal decision. If you are considering joining a trial or study, get answers to your questions and know your options before you decide. Here are questions you might ask the research team when thinking about participating.
- What is this study trying to find out?
- What treatment or tests will I have? Will they hurt? Will you provide me with the test or lab results?
- What are the chances I will be in the experimental group or the control group?
- If the study tests a treatment, what are the possible risks, side effects, and benefits compared with my current treatment?
- How long will the clinical trial last?
- Where will the study take place? Will I need to stay in the hospital?
- Will you provide a way for me to get to the study site if I need it, such as through a ride-share service?
- Will I need a trial or study partner (for example, a family member or friend who knows me well) to come with me to the research site visits? If so, how long will he or she need to participate?
- Can I participate in any part of the trial with my regular doctor or at a clinic closer to my home?
- How will the study affect my everyday life?
- What steps are being taken to ensure my privacy?
- How will you protect my health while I participate?
- What happens if my health problem gets worse during the trial or study?
- Can I take my regular medicines while participating?
- Who will be in charge of my care while I am in the trial or study? Will I be able to see my own doctors?
- How will you keep my doctor informed about my participation?
- If I withdraw from the trial or study, will this affect my normal care?
- Will it cost me anything to be in the trial or study? If so, will I be reimbursed for expenses, such as travel, parking, lodging, or meals?
- Will my insurance pay for costs not covered by the research, or must I pay out of pocket? If I don’t have insurance, am I still eligible to participate?
- Will my trial or study partner be compensated for his or her time?
- Will you follow up on my health after the end of the trial or study?
- Will I continue receiving the treatment after the trial or study ends?
- Will you tell me the results of the research?
- Whom do I contact if I have questions after the trial or study ends?

To be eligible to participate, you may need to have certain characteristics, called inclusion criteria. For example, a clinical trial may need participants to have a certain stage of disease, version of a gene, or family history. Some trials require that participants have a study partner who can accompany them to clinic visits.
Participants with certain characteristics may not be allowed to participate in some trials. These characteristics are called exclusion criteria. They include factors such as specific health conditions or medications that could interfere with the treatment being tested.
Many volunteers must be screened to find enough people who are eligible for a trial or study. Generally, you can participate in only one clinical trial at a time, although this is not necessarily the case for observational studies. Different trials have different criteria, so being excluded from one trial does not necessarily mean you will be excluded from another.

When research only includes people with similar backgrounds, the findings may not apply to or benefit a broader population. The results of clinical trials and studies with diverse participants may apply to more people. That’s why research benefits from having participants of different ages, sexes, races, and ethnicities.
Researchers need older adults to participate in clinical research so that scientists can learn more about how new drugs, tests, and other interventions will work for them. Many older adults have health needs that are different from those of younger people. For example, as people age, their bodies may react differently to certain drugs. Older adults may need different dosages of a drug to have the intended result. Also, some drugs may have different side effects in older people than in younger individuals. Having older adults enrolled in clinical trials and studies helps researchers get the information they need to develop the right treatments for this age group.
Researchers know that it may be challenging for some older adults to join a clinical trial or study. For example, if you have multiple health problems, can you participate in research that is looking at only one condition? If you are frail or have a disability, will you be strong enough to participate? If you no longer drive, how can you get to the research site? Talk to the research coordinator or staff about your concerns. The research team may have already thought about some of the potential obstacles and have a plan to make it easier for you to participate.
Read more about diversity in clinical trials .
You may also be interested in
- Learning more about the benefits, risks, and safety of clinical research
- Finding out about participating in Alzheimer's disease research
- Downloading or sharing an infographic with the benefits of participating in clinical research
Sign up for email updates on healthy aging
For more information about clinical trials.
Alzheimers.gov www.alzheimers.gov Explore the Alzheimers.gov website for information and resources on Alzheimer’s and related dementias from across the federal government.
Clinical Research Trials and You National Institutes of Health www.nih.gov/health-information/nih-clinical-research-trials-you
ClinicalTrials.gov www.clinicaltrials.gov
This content is provided by the NIH National Institute on Aging (NIA). NIA scientists and other experts review this content to ensure it is accurate and up to date.
Content reviewed: March 22, 2023
nia.nih.gov
An official website of the National Institutes of Health
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Individual case studies in clinical research
Affiliation.
- 1 Department of Psychology, University of Newcastle upon Tyne, UK.
- PMID: 9839641
- DOI: 10.1046/j.1365-2753.1998.00011.x
Case studies have acquired an unmerited reputation as being anecdotal, unscientific and intrinsically inferior to group studies. The subsequent disregarding of individual patients as the focus of investigation has led to the neglect of an extremely useful clinical research method, and has probably impaired the pace of therapeutic innovation. The purpose of this paper is to clarify the scope and nature of case studies and promote their rehabilitation. Case studies can, in principle, be used to test any theory that has implications for individual patients. There are two crucial methodological stages. The first is to identify scientifically plausible general theories and derive from them specific hypotheses or models of sufficient precision to have implications for individual cases. The second is to test these hypothetical models against 'pure' cases, selected so as to exclude interfering variables. There are two main types of case study--those made by serendipity (unplanned case observations which challenge an implicit theoretical framework); and formal case studies (designed prospectively to collect pure cases to test a prior hypothesis). The difference between serendipity and planned case studies roughly corresponds to the difference between surveillance and screening. A worked-example of a formal case study is described here in order to illustrate the method. Individual case studies deserve fresh consideration by researchers, since they are a clinician-friendly method with a unique potential for incorporation into routine practice.
PubMed Disclaimer
Similar articles
- Methodological and conceptual issues regarding occupational psychosocial coronary heart disease epidemiology. Burr H, Formazin M, Pohrt A. Burr H, et al. Scand J Work Environ Health. 2016 May 1;42(3):251-5. doi: 10.5271/sjweh.3557. Epub 2016 Mar 9. Scand J Work Environ Health. 2016. PMID: 26960179
- The effectiveness of internet-based e-learning on clinician behavior and patient outcomes: a systematic review protocol. Sinclair P, Kable A, Levett-Jones T. Sinclair P, et al. JBI Database System Rev Implement Rep. 2015 Jan;13(1):52-64. doi: 10.11124/jbisrir-2015-1919. JBI Database System Rev Implement Rep. 2015. PMID: 26447007
- Candida and candidaemia. Susceptibility and epidemiology. Arendrup MC. Arendrup MC. Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
- Analyzing the "nature" and "specific effectiveness" of clinical empathy: a theoretical overview and contribution towards a theory-based research agenda. Neumann M, Bensing J, Mercer S, Ernstmann N, Ommen O, Pfaff H. Neumann M, et al. Patient Educ Couns. 2009 Mar;74(3):339-46. doi: 10.1016/j.pec.2008.11.013. Epub 2009 Jan 4. Patient Educ Couns. 2009. PMID: 19124216 Review.
- [Methodological aspects of the reconstitution and evaluation of the behavioral theories that underlie population policy]. Leeuw F. Leeuw F. Politiq Popul. 1991 Sep;4(4):5-42. Politiq Popul. 1991. PMID: 12285025 French.
- Uneventful Coadministration of Seasonal Influenza and COVID-19 BNT162b2 Vaccines Two Weeks Post-Influenza Vaccination in an Egg-Allergic Subject: A Case Report. Sayed AA. Sayed AA. Vaccines (Basel). 2023 May 5;11(5):950. doi: 10.3390/vaccines11050950. Vaccines (Basel). 2023. PMID: 37243054 Free PMC article.
- Extended time window mechanical thrombectomy for pediatric acute ischemic stroke. Aburto-Murrieta Y, Méndez B, Marquez-Romero JM. Aburto-Murrieta Y, et al. J Cent Nerv Syst Dis. 2022 Apr 24;14:11795735221098140. doi: 10.1177/11795735221098140. eCollection 2022. J Cent Nerv Syst Dis. 2022. PMID: 35492739 Free PMC article. Review.
- Vaccine-Hesitant Justifications: "Too Many, Too Soon," Narrative Persuasion, and the Conflation of Expertise. Rodriguez NJ. Rodriguez NJ. Glob Qual Nurs Res. 2016 Aug 12;3:2333393616663304. doi: 10.1177/2333393616663304. eCollection 2016 Jan-Dec. Glob Qual Nurs Res. 2016. PMID: 28508015 Free PMC article.
- Clinical research and medical care: towards effective and complete integration. Sacristán JA. Sacristán JA. BMC Med Res Methodol. 2015 Jan 9;15:4. doi: 10.1186/1471-2288-15-4. BMC Med Res Methodol. 2015. PMID: 25575454 Free PMC article.
- Advance modern medicine with clinical case reports. Wáng YX. Wáng YX. Quant Imaging Med Surg. 2014 Dec;4(6):439-43. doi: 10.3978/j.issn.2223-4292.2014.11.10. Quant Imaging Med Surg. 2014. PMID: 25525572 Free PMC article.
- Search in MeSH
LinkOut - more resources
Full text sources.

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Health Topics
- Brochures and Fact Sheets
- Help for Mental Illnesses
- Clinical Trials
Clinical Research Trials and You: Questions and Answers

- Download PDF
- Order a free hardcopy
What is a clinical trial?
A clinical trial is a research study that involves people like you. Researchers conduct clinical trials to find new or better ways to prevent, detect, or treat health conditions. Often, researchers want to find out if a new test, treatment, or preventive measure is safe and effective. Tests can include ways to screen for, diagnose, or prevent a disease or condition. Treatments and preventive measures can include medications, surgeries, medical devices, and behavioral therapies.
Clinical trials are important because they serve as the foundation for most medical advances. Without clinical trials, many of the medical treatments and cures we have today wouldn’t exist.
Why should I volunteer for a clinical trial?
People volunteer for clinical trials for many reasons. Some want to advance science or help doctors and researchers learn more about disease and improve health care. Others, such as those with an illness, may join to try new or advanced treatments that aren’t widely available.
Whatever your reason for joining a clinical trial, researchers generally need two types of volunteers: those without specific illnesses or conditions and those with them.
A healthy volunteer is someone in a clinical trial with no known related health problems. Researchers need healthy volunteers to establish a healthy or optimal reference point. They use data from healthy volunteers to test new treatments or interventions, not to provide direct benefit to participants.
A patient volunteer is someone in a clinical trial who has the condition being studied. Researchers need patient volunteers to learn if new tests, treatments, or preventive measures are safe and effective. Not all trial participants will receive experimental medications or treatments; sometimes, participants may receive a placebo. Researchers need to vary medications and treatments so they can compare results and learn from their differences.
While a study’s treatment or findings may help patients directly, sometimes participants will receive no direct benefit. However, in many cases, study results can still serve as building blocks that are used to help people later.
What would I experience during a clinical trial?
During a clinical trial, the study team will track your health. Participating in a clinical trial may take more time than standard treatment, and you may have more tests and treatments than you would if you weren’t in a clinical trial. The study team also may ask you to keep a log of symptoms or other health measures, fill out forms about how you feel, or complete other tasks. You may need to travel or reside away from home to take part in a study.

What are the risks and benefits of my participation in a clinical trial?
Clinical trials can provide many benefits to participants and society. However, before volunteering for a clinical trial, you should talk with your health care provider and the study team about the risks and benefits.
Potential Risks
When weighing the risks of volunteering, you should consider:
- The likelihood of any harm occurring
- How much harm could result from your participation in the study
Researchers try to limit patient discomfort during clinical trials. However, in some cases, volunteers have complications that require medical attention. In rare cases, volunteers have died when participating in clinical trials.
Potential Benefits
The benefits of volunteering can include:
- Treatment with study medications that may not be available elsewhere
- Care from health care professionals who are familiar with the most advanced treatments available
- The opportunity to learn more about an illness and how to manage it
- Playing an active role in your health care
- Helping others by contributing to medical research
Where can I find a mental health clinical trial?
The National Institute of Mental Health (NIMH) is the lead federal agency for research on mental disorders. While NIMH supports research around the world, it also conducts many clinical trials at the National Institutes of Health (NIH) campus in Bethesda, Maryland.
To learn more about NIMH studies conducted on the NIH campus, visit NIMH's Join a Study webpage . These studies enroll volunteers from the local area and across the nation. In some cases, participants receive free study-related evaluations, treatment, and transportation to NIH.
To learn more about NIMH-funded clinical trials at universities, medical centers, and other institutions, visit NIMH's clinical trials webpage .
What is the next step after I find a clinical trial?
To learn more about a specific clinical trial, contact the study coordinator. You can usually find this contact information in the trial’s description.
If you decide to join a clinical trial, let your health care provider know. They may want to talk to the study team to coordinate your care and ensure the trial is safe for you. Find tips to help prepare for and get the most out of your visit .
How do I know if I can join a clinical trial?
People of all ages, ethnicities, and racial backgrounds can volunteer for clinical trials. If you want to join a clinical trial, you must be eligible to participate in that specific trial. Your eligibility can usually be determined by phone or online screening.
All clinical trials have eligibility guidelines called inclusion and exclusion criteria. These criteria may include:
- The type and stage of an illness
- Treatment history
- Other medical conditions
Researchers use these guidelines to find suitable study participants, maximize participant safety, and ensure trial data are accurate.
What kinds of questions should I ask the study team before deciding if I want to take part in a clinical trial?
It can be helpful to write down any questions or concerns you have. When you speak with the study team, you may want to take notes or ask to record the conversation. Bringing a supportive friend or family member may also be helpful.
The following topics may give you some ideas for questions to ask:
- The study’s purpose and duration
- The possible risks and benefits
- Your participation and care
- Personal and cost concerns
For a list of specific questions, check out Questions to Ask About Volunteering for a Research Study from the U.S. Department of Health and Human Services’ Office for Human Research Protections.
How is my safety protected if I choose to take part in a clinical trial?
Strict rules and laws help protect participants in research studies, and the study team must follow these rules to conduct research. Below are some measures that can help ensure your safety.
Ethical Guidelines
Ethical guidelines protect volunteers and ensure a study’s scientific integrity. Regulators created these guidelines primarily in response to past research errors and misconduct. Federal policies and regulations require that researchers conducting clinical trials obey these ethical guidelines.
Informed Consent
Before joining a trial, you should understand what your participation will involve. The study team will provide an informed consent document with detailed information about the study. The document will include details about the length of the trial, required visits, medications, and medical procedures. It will also explain the expected outcomes, potential benefits, possible risks, and other trial details. The study team will review the informed consent document with you and answer any questions you have. You can decide then or later if you want to take part in the trial.
If you choose to join the trial, you will be asked to sign the informed consent document. This document is not a contract; it verifies you understand the study and describes what your participation will include and how your data will be used. Your consent in a clinical trial is ongoing and your participation is voluntary. You may stop participating at any time.
Institutional Review Board Review
Institutional review boards (IRBs) review and monitor most clinical trials in the United States. An IRB works to protect the rights, welfare, and privacy of human subjects. An IRB usually includes a team of independent doctors, scientists, and community members. The IRB’s job is to review potential studies, weigh the risks and benefits of studies, and ensure that studies are safe and ethical.
If you’re thinking about volunteering for a clinical trial, ask if an IRB reviewed the trial.
What happens when a clinical trial ends?
When a clinical trial ends, researchers will analyze the data to help them determine the results. After reviewing the findings, researchers often submit them to scientific journals for others to review and build on.
Before your participation ends, the study team should tell you if and how you’ll receive the results. If this process is unclear, be sure to ask about it.
Where can I find more information?
This fact sheet covers the basics of clinical trials. To find more details and resources, visit NIMH's clinical trials webpage .
For More Information
MedlinePlus (National Library of Medicine) ( en español )
ClinicalTrials.gov ( en español )
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health NIH Publication No. 23-MH-4379 Revised 2023
The information in this publication is in the public domain and may be reused or copied without permission. However, you may not reuse or copy images. Please cite the National Institute of Mental Health as the source. Read our copyright policy to learn more about our guidelines for reusing NIMH content.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- For Patients
- Clinical Trials: What Patients Need to Know
What Are the Different Types of Clinical Research?
Different types of clinical research are used depending on what the researchers are studying. Below are descriptions of some different kinds of clinical research.
Treatment Research generally involves an intervention such as medication, psychotherapy, new devices, or new approaches to surgery or radiation therapy.
Prevention Research looks for better ways to prevent disorders from developing or returning. Different kinds of prevention research may study medicines, vitamins, vaccines, minerals, or lifestyle changes.
Diagnostic Research refers to the practice of looking for better ways to identify a particular disorder or condition.
Screening Research aims to find the best ways to detect certain disorders or health conditions.
Quality of Life Research explores ways to improve comfort and the quality of life for individuals with a chronic illness.
Genetic studies aim to improve the prediction of disorders by identifying and understanding how genes and illnesses may be related. Research in this area may explore ways in which a person’s genes make him or her more or less likely to develop a disorder. This may lead to development of tailor-made treatments based on a patient’s genetic make-up.
Epidemiological studies seek to identify the patterns, causes, and control of disorders in groups of people.
An important note: some clinical research is “outpatient,” meaning that participants do not stay overnight at the hospital. Some is “inpatient,” meaning that participants will need to stay for at least one night in the hospital or research center. Be sure to ask the researchers what their study requires.
Phases of clinical trials: when clinical research is used to evaluate medications and devices Clinical trials are a kind of clinical research designed to evaluate and test new interventions such as psychotherapy or medications. Clinical trials are often conducted in four phases. The trials at each phase have a different purpose and help scientists answer different questions.
Phase I trials Researchers test an experimental drug or treatment in a small group of people for the first time. The researchers evaluate the treatment’s safety, determine a safe dosage range, and identify side effects.
Phase II trials The experimental drug or treatment is given to a larger group of people to see if it is effective and to further evaluate its safety.
Phase III trials The experimental study drug or treatment is given to large groups of people. Researchers confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the experimental drug or treatment to be used safely.
Phase IV trials Post-marketing studies, which are conducted after a treatment is approved for use by the FDA, provide additional information including the treatment or drug’s risks, benefits, and best use.
Examples of other kinds of clinical research Many people believe that all clinical research involves testing of new medications or devices. This is not true, however. Some studies do not involve testing medications and a person’s regular medications may not need to be changed. Healthy volunteers are also needed so that researchers can compare their results to results of people with the illness being studied. Some examples of other kinds of research include the following:
A long-term study that involves psychological tests or brain scans
A genetic study that involves blood tests but no changes in medication
A study of family history that involves talking to family members to learn about people’s medical needs and history.
- Open access
- Published: 27 June 2011
The case study approach
- Sarah Crowe 1 ,
- Kathrin Cresswell 2 ,
- Ann Robertson 2 ,
- Guro Huby 3 ,
- Anthony Avery 1 &
- Aziz Sheikh 2
BMC Medical Research Methodology volume 11 , Article number: 100 ( 2011 ) Cite this article
788k Accesses
1063 Citations
37 Altmetric
Metrics details
The case study approach allows in-depth, multi-faceted explorations of complex issues in their real-life settings. The value of the case study approach is well recognised in the fields of business, law and policy, but somewhat less so in health services research. Based on our experiences of conducting several health-related case studies, we reflect on the different types of case study design, the specific research questions this approach can help answer, the data sources that tend to be used, and the particular advantages and disadvantages of employing this methodological approach. The paper concludes with key pointers to aid those designing and appraising proposals for conducting case study research, and a checklist to help readers assess the quality of case study reports.
Peer Review reports
Introduction
The case study approach is particularly useful to employ when there is a need to obtain an in-depth appreciation of an issue, event or phenomenon of interest, in its natural real-life context. Our aim in writing this piece is to provide insights into when to consider employing this approach and an overview of key methodological considerations in relation to the design, planning, analysis, interpretation and reporting of case studies.
The illustrative 'grand round', 'case report' and 'case series' have a long tradition in clinical practice and research. Presenting detailed critiques, typically of one or more patients, aims to provide insights into aspects of the clinical case and, in doing so, illustrate broader lessons that may be learnt. In research, the conceptually-related case study approach can be used, for example, to describe in detail a patient's episode of care, explore professional attitudes to and experiences of a new policy initiative or service development or more generally to 'investigate contemporary phenomena within its real-life context' [ 1 ]. Based on our experiences of conducting a range of case studies, we reflect on when to consider using this approach, discuss the key steps involved and illustrate, with examples, some of the practical challenges of attaining an in-depth understanding of a 'case' as an integrated whole. In keeping with previously published work, we acknowledge the importance of theory to underpin the design, selection, conduct and interpretation of case studies[ 2 ]. In so doing, we make passing reference to the different epistemological approaches used in case study research by key theoreticians and methodologists in this field of enquiry.
This paper is structured around the following main questions: What is a case study? What are case studies used for? How are case studies conducted? What are the potential pitfalls and how can these be avoided? We draw in particular on four of our own recently published examples of case studies (see Tables 1 , 2 , 3 and 4 ) and those of others to illustrate our discussion[ 3 – 7 ].
What is a case study?
A case study is a research approach that is used to generate an in-depth, multi-faceted understanding of a complex issue in its real-life context. It is an established research design that is used extensively in a wide variety of disciplines, particularly in the social sciences. A case study can be defined in a variety of ways (Table 5 ), the central tenet being the need to explore an event or phenomenon in depth and in its natural context. It is for this reason sometimes referred to as a "naturalistic" design; this is in contrast to an "experimental" design (such as a randomised controlled trial) in which the investigator seeks to exert control over and manipulate the variable(s) of interest.
Stake's work has been particularly influential in defining the case study approach to scientific enquiry. He has helpfully characterised three main types of case study: intrinsic , instrumental and collective [ 8 ]. An intrinsic case study is typically undertaken to learn about a unique phenomenon. The researcher should define the uniqueness of the phenomenon, which distinguishes it from all others. In contrast, the instrumental case study uses a particular case (some of which may be better than others) to gain a broader appreciation of an issue or phenomenon. The collective case study involves studying multiple cases simultaneously or sequentially in an attempt to generate a still broader appreciation of a particular issue.
These are however not necessarily mutually exclusive categories. In the first of our examples (Table 1 ), we undertook an intrinsic case study to investigate the issue of recruitment of minority ethnic people into the specific context of asthma research studies, but it developed into a instrumental case study through seeking to understand the issue of recruitment of these marginalised populations more generally, generating a number of the findings that are potentially transferable to other disease contexts[ 3 ]. In contrast, the other three examples (see Tables 2 , 3 and 4 ) employed collective case study designs to study the introduction of workforce reconfiguration in primary care, the implementation of electronic health records into hospitals, and to understand the ways in which healthcare students learn about patient safety considerations[ 4 – 6 ]. Although our study focusing on the introduction of General Practitioners with Specialist Interests (Table 2 ) was explicitly collective in design (four contrasting primary care organisations were studied), is was also instrumental in that this particular professional group was studied as an exemplar of the more general phenomenon of workforce redesign[ 4 ].
What are case studies used for?
According to Yin, case studies can be used to explain, describe or explore events or phenomena in the everyday contexts in which they occur[ 1 ]. These can, for example, help to understand and explain causal links and pathways resulting from a new policy initiative or service development (see Tables 2 and 3 , for example)[ 1 ]. In contrast to experimental designs, which seek to test a specific hypothesis through deliberately manipulating the environment (like, for example, in a randomised controlled trial giving a new drug to randomly selected individuals and then comparing outcomes with controls),[ 9 ] the case study approach lends itself well to capturing information on more explanatory ' how ', 'what' and ' why ' questions, such as ' how is the intervention being implemented and received on the ground?'. The case study approach can offer additional insights into what gaps exist in its delivery or why one implementation strategy might be chosen over another. This in turn can help develop or refine theory, as shown in our study of the teaching of patient safety in undergraduate curricula (Table 4 )[ 6 , 10 ]. Key questions to consider when selecting the most appropriate study design are whether it is desirable or indeed possible to undertake a formal experimental investigation in which individuals and/or organisations are allocated to an intervention or control arm? Or whether the wish is to obtain a more naturalistic understanding of an issue? The former is ideally studied using a controlled experimental design, whereas the latter is more appropriately studied using a case study design.
Case studies may be approached in different ways depending on the epistemological standpoint of the researcher, that is, whether they take a critical (questioning one's own and others' assumptions), interpretivist (trying to understand individual and shared social meanings) or positivist approach (orientating towards the criteria of natural sciences, such as focusing on generalisability considerations) (Table 6 ). Whilst such a schema can be conceptually helpful, it may be appropriate to draw on more than one approach in any case study, particularly in the context of conducting health services research. Doolin has, for example, noted that in the context of undertaking interpretative case studies, researchers can usefully draw on a critical, reflective perspective which seeks to take into account the wider social and political environment that has shaped the case[ 11 ].
How are case studies conducted?
Here, we focus on the main stages of research activity when planning and undertaking a case study; the crucial stages are: defining the case; selecting the case(s); collecting and analysing the data; interpreting data; and reporting the findings.
Defining the case
Carefully formulated research question(s), informed by the existing literature and a prior appreciation of the theoretical issues and setting(s), are all important in appropriately and succinctly defining the case[ 8 , 12 ]. Crucially, each case should have a pre-defined boundary which clarifies the nature and time period covered by the case study (i.e. its scope, beginning and end), the relevant social group, organisation or geographical area of interest to the investigator, the types of evidence to be collected, and the priorities for data collection and analysis (see Table 7 )[ 1 ]. A theory driven approach to defining the case may help generate knowledge that is potentially transferable to a range of clinical contexts and behaviours; using theory is also likely to result in a more informed appreciation of, for example, how and why interventions have succeeded or failed[ 13 ].
For example, in our evaluation of the introduction of electronic health records in English hospitals (Table 3 ), we defined our cases as the NHS Trusts that were receiving the new technology[ 5 ]. Our focus was on how the technology was being implemented. However, if the primary research interest had been on the social and organisational dimensions of implementation, we might have defined our case differently as a grouping of healthcare professionals (e.g. doctors and/or nurses). The precise beginning and end of the case may however prove difficult to define. Pursuing this same example, when does the process of implementation and adoption of an electronic health record system really begin or end? Such judgements will inevitably be influenced by a range of factors, including the research question, theory of interest, the scope and richness of the gathered data and the resources available to the research team.
Selecting the case(s)
The decision on how to select the case(s) to study is a very important one that merits some reflection. In an intrinsic case study, the case is selected on its own merits[ 8 ]. The case is selected not because it is representative of other cases, but because of its uniqueness, which is of genuine interest to the researchers. This was, for example, the case in our study of the recruitment of minority ethnic participants into asthma research (Table 1 ) as our earlier work had demonstrated the marginalisation of minority ethnic people with asthma, despite evidence of disproportionate asthma morbidity[ 14 , 15 ]. In another example of an intrinsic case study, Hellstrom et al.[ 16 ] studied an elderly married couple living with dementia to explore how dementia had impacted on their understanding of home, their everyday life and their relationships.
For an instrumental case study, selecting a "typical" case can work well[ 8 ]. In contrast to the intrinsic case study, the particular case which is chosen is of less importance than selecting a case that allows the researcher to investigate an issue or phenomenon. For example, in order to gain an understanding of doctors' responses to health policy initiatives, Som undertook an instrumental case study interviewing clinicians who had a range of responsibilities for clinical governance in one NHS acute hospital trust[ 17 ]. Sampling a "deviant" or "atypical" case may however prove even more informative, potentially enabling the researcher to identify causal processes, generate hypotheses and develop theory.
In collective or multiple case studies, a number of cases are carefully selected. This offers the advantage of allowing comparisons to be made across several cases and/or replication. Choosing a "typical" case may enable the findings to be generalised to theory (i.e. analytical generalisation) or to test theory by replicating the findings in a second or even a third case (i.e. replication logic)[ 1 ]. Yin suggests two or three literal replications (i.e. predicting similar results) if the theory is straightforward and five or more if the theory is more subtle. However, critics might argue that selecting 'cases' in this way is insufficiently reflexive and ill-suited to the complexities of contemporary healthcare organisations.
The selected case study site(s) should allow the research team access to the group of individuals, the organisation, the processes or whatever else constitutes the chosen unit of analysis for the study. Access is therefore a central consideration; the researcher needs to come to know the case study site(s) well and to work cooperatively with them. Selected cases need to be not only interesting but also hospitable to the inquiry [ 8 ] if they are to be informative and answer the research question(s). Case study sites may also be pre-selected for the researcher, with decisions being influenced by key stakeholders. For example, our selection of case study sites in the evaluation of the implementation and adoption of electronic health record systems (see Table 3 ) was heavily influenced by NHS Connecting for Health, the government agency that was responsible for overseeing the National Programme for Information Technology (NPfIT)[ 5 ]. This prominent stakeholder had already selected the NHS sites (through a competitive bidding process) to be early adopters of the electronic health record systems and had negotiated contracts that detailed the deployment timelines.
It is also important to consider in advance the likely burden and risks associated with participation for those who (or the site(s) which) comprise the case study. Of particular importance is the obligation for the researcher to think through the ethical implications of the study (e.g. the risk of inadvertently breaching anonymity or confidentiality) and to ensure that potential participants/participating sites are provided with sufficient information to make an informed choice about joining the study. The outcome of providing this information might be that the emotive burden associated with participation, or the organisational disruption associated with supporting the fieldwork, is considered so high that the individuals or sites decide against participation.
In our example of evaluating implementations of electronic health record systems, given the restricted number of early adopter sites available to us, we sought purposively to select a diverse range of implementation cases among those that were available[ 5 ]. We chose a mixture of teaching, non-teaching and Foundation Trust hospitals, and examples of each of the three electronic health record systems procured centrally by the NPfIT. At one recruited site, it quickly became apparent that access was problematic because of competing demands on that organisation. Recognising the importance of full access and co-operative working for generating rich data, the research team decided not to pursue work at that site and instead to focus on other recruited sites.
Collecting the data
In order to develop a thorough understanding of the case, the case study approach usually involves the collection of multiple sources of evidence, using a range of quantitative (e.g. questionnaires, audits and analysis of routinely collected healthcare data) and more commonly qualitative techniques (e.g. interviews, focus groups and observations). The use of multiple sources of data (data triangulation) has been advocated as a way of increasing the internal validity of a study (i.e. the extent to which the method is appropriate to answer the research question)[ 8 , 18 – 21 ]. An underlying assumption is that data collected in different ways should lead to similar conclusions, and approaching the same issue from different angles can help develop a holistic picture of the phenomenon (Table 2 )[ 4 ].
Brazier and colleagues used a mixed-methods case study approach to investigate the impact of a cancer care programme[ 22 ]. Here, quantitative measures were collected with questionnaires before, and five months after, the start of the intervention which did not yield any statistically significant results. Qualitative interviews with patients however helped provide an insight into potentially beneficial process-related aspects of the programme, such as greater, perceived patient involvement in care. The authors reported how this case study approach provided a number of contextual factors likely to influence the effectiveness of the intervention and which were not likely to have been obtained from quantitative methods alone.
In collective or multiple case studies, data collection needs to be flexible enough to allow a detailed description of each individual case to be developed (e.g. the nature of different cancer care programmes), before considering the emerging similarities and differences in cross-case comparisons (e.g. to explore why one programme is more effective than another). It is important that data sources from different cases are, where possible, broadly comparable for this purpose even though they may vary in nature and depth.
Analysing, interpreting and reporting case studies
Making sense and offering a coherent interpretation of the typically disparate sources of data (whether qualitative alone or together with quantitative) is far from straightforward. Repeated reviewing and sorting of the voluminous and detail-rich data are integral to the process of analysis. In collective case studies, it is helpful to analyse data relating to the individual component cases first, before making comparisons across cases. Attention needs to be paid to variations within each case and, where relevant, the relationship between different causes, effects and outcomes[ 23 ]. Data will need to be organised and coded to allow the key issues, both derived from the literature and emerging from the dataset, to be easily retrieved at a later stage. An initial coding frame can help capture these issues and can be applied systematically to the whole dataset with the aid of a qualitative data analysis software package.
The Framework approach is a practical approach, comprising of five stages (familiarisation; identifying a thematic framework; indexing; charting; mapping and interpretation) , to managing and analysing large datasets particularly if time is limited, as was the case in our study of recruitment of South Asians into asthma research (Table 1 )[ 3 , 24 ]. Theoretical frameworks may also play an important role in integrating different sources of data and examining emerging themes. For example, we drew on a socio-technical framework to help explain the connections between different elements - technology; people; and the organisational settings within which they worked - in our study of the introduction of electronic health record systems (Table 3 )[ 5 ]. Our study of patient safety in undergraduate curricula drew on an evaluation-based approach to design and analysis, which emphasised the importance of the academic, organisational and practice contexts through which students learn (Table 4 )[ 6 ].
Case study findings can have implications both for theory development and theory testing. They may establish, strengthen or weaken historical explanations of a case and, in certain circumstances, allow theoretical (as opposed to statistical) generalisation beyond the particular cases studied[ 12 ]. These theoretical lenses should not, however, constitute a strait-jacket and the cases should not be "forced to fit" the particular theoretical framework that is being employed.
When reporting findings, it is important to provide the reader with enough contextual information to understand the processes that were followed and how the conclusions were reached. In a collective case study, researchers may choose to present the findings from individual cases separately before amalgamating across cases. Care must be taken to ensure the anonymity of both case sites and individual participants (if agreed in advance) by allocating appropriate codes or withholding descriptors. In the example given in Table 3 , we decided against providing detailed information on the NHS sites and individual participants in order to avoid the risk of inadvertent disclosure of identities[ 5 , 25 ].
What are the potential pitfalls and how can these be avoided?
The case study approach is, as with all research, not without its limitations. When investigating the formal and informal ways undergraduate students learn about patient safety (Table 4 ), for example, we rapidly accumulated a large quantity of data. The volume of data, together with the time restrictions in place, impacted on the depth of analysis that was possible within the available resources. This highlights a more general point of the importance of avoiding the temptation to collect as much data as possible; adequate time also needs to be set aside for data analysis and interpretation of what are often highly complex datasets.
Case study research has sometimes been criticised for lacking scientific rigour and providing little basis for generalisation (i.e. producing findings that may be transferable to other settings)[ 1 ]. There are several ways to address these concerns, including: the use of theoretical sampling (i.e. drawing on a particular conceptual framework); respondent validation (i.e. participants checking emerging findings and the researcher's interpretation, and providing an opinion as to whether they feel these are accurate); and transparency throughout the research process (see Table 8 )[ 8 , 18 – 21 , 23 , 26 ]. Transparency can be achieved by describing in detail the steps involved in case selection, data collection, the reasons for the particular methods chosen, and the researcher's background and level of involvement (i.e. being explicit about how the researcher has influenced data collection and interpretation). Seeking potential, alternative explanations, and being explicit about how interpretations and conclusions were reached, help readers to judge the trustworthiness of the case study report. Stake provides a critique checklist for a case study report (Table 9 )[ 8 ].
Conclusions
The case study approach allows, amongst other things, critical events, interventions, policy developments and programme-based service reforms to be studied in detail in a real-life context. It should therefore be considered when an experimental design is either inappropriate to answer the research questions posed or impossible to undertake. Considering the frequency with which implementations of innovations are now taking place in healthcare settings and how well the case study approach lends itself to in-depth, complex health service research, we believe this approach should be more widely considered by researchers. Though inherently challenging, the research case study can, if carefully conceptualised and thoughtfully undertaken and reported, yield powerful insights into many important aspects of health and healthcare delivery.
Yin RK: Case study research, design and method. 2009, London: Sage Publications Ltd., 4
Google Scholar
Keen J, Packwood T: Qualitative research; case study evaluation. BMJ. 1995, 311: 444-446.
Article CAS PubMed PubMed Central Google Scholar
Sheikh A, Halani L, Bhopal R, Netuveli G, Partridge M, Car J, et al: Facilitating the Recruitment of Minority Ethnic People into Research: Qualitative Case Study of South Asians and Asthma. PLoS Med. 2009, 6 (10): 1-11.
Article Google Scholar
Pinnock H, Huby G, Powell A, Kielmann T, Price D, Williams S, et al: The process of planning, development and implementation of a General Practitioner with a Special Interest service in Primary Care Organisations in England and Wales: a comparative prospective case study. Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation R&D (NCCSDO). 2008, [ http://www.sdo.nihr.ac.uk/files/project/99-final-report.pdf ]
Robertson A, Cresswell K, Takian A, Petrakaki D, Crowe S, Cornford T, et al: Prospective evaluation of the implementation and adoption of NHS Connecting for Health's national electronic health record in secondary care in England: interim findings. BMJ. 2010, 41: c4564-
Pearson P, Steven A, Howe A, Sheikh A, Ashcroft D, Smith P, the Patient Safety Education Study Group: Learning about patient safety: organisational context and culture in the education of healthcare professionals. J Health Serv Res Policy. 2010, 15: 4-10. 10.1258/jhsrp.2009.009052.
Article PubMed Google Scholar
van Harten WH, Casparie TF, Fisscher OA: The evaluation of the introduction of a quality management system: a process-oriented case study in a large rehabilitation hospital. Health Policy. 2002, 60 (1): 17-37. 10.1016/S0168-8510(01)00187-7.
Stake RE: The art of case study research. 1995, London: Sage Publications Ltd.
Sheikh A, Smeeth L, Ashcroft R: Randomised controlled trials in primary care: scope and application. Br J Gen Pract. 2002, 52 (482): 746-51.
PubMed PubMed Central Google Scholar
King G, Keohane R, Verba S: Designing Social Inquiry. 1996, Princeton: Princeton University Press
Doolin B: Information technology as disciplinary technology: being critical in interpretative research on information systems. Journal of Information Technology. 1998, 13: 301-311. 10.1057/jit.1998.8.
George AL, Bennett A: Case studies and theory development in the social sciences. 2005, Cambridge, MA: MIT Press
Eccles M, the Improved Clinical Effectiveness through Behavioural Research Group (ICEBeRG): Designing theoretically-informed implementation interventions. Implementation Science. 2006, 1: 1-8. 10.1186/1748-5908-1-1.
Article PubMed Central Google Scholar
Netuveli G, Hurwitz B, Levy M, Fletcher M, Barnes G, Durham SR, Sheikh A: Ethnic variations in UK asthma frequency, morbidity, and health-service use: a systematic review and meta-analysis. Lancet. 2005, 365 (9456): 312-7.
Sheikh A, Panesar SS, Lasserson T, Netuveli G: Recruitment of ethnic minorities to asthma studies. Thorax. 2004, 59 (7): 634-
CAS PubMed PubMed Central Google Scholar
Hellström I, Nolan M, Lundh U: 'We do things together': A case study of 'couplehood' in dementia. Dementia. 2005, 4: 7-22. 10.1177/1471301205049188.
Som CV: Nothing seems to have changed, nothing seems to be changing and perhaps nothing will change in the NHS: doctors' response to clinical governance. International Journal of Public Sector Management. 2005, 18: 463-477. 10.1108/09513550510608903.
Lincoln Y, Guba E: Naturalistic inquiry. 1985, Newbury Park: Sage Publications
Barbour RS: Checklists for improving rigour in qualitative research: a case of the tail wagging the dog?. BMJ. 2001, 322: 1115-1117. 10.1136/bmj.322.7294.1115.
Mays N, Pope C: Qualitative research in health care: Assessing quality in qualitative research. BMJ. 2000, 320: 50-52. 10.1136/bmj.320.7226.50.
Mason J: Qualitative researching. 2002, London: Sage
Brazier A, Cooke K, Moravan V: Using Mixed Methods for Evaluating an Integrative Approach to Cancer Care: A Case Study. Integr Cancer Ther. 2008, 7: 5-17. 10.1177/1534735407313395.
Miles MB, Huberman M: Qualitative data analysis: an expanded sourcebook. 1994, CA: Sage Publications Inc., 2
Pope C, Ziebland S, Mays N: Analysing qualitative data. Qualitative research in health care. BMJ. 2000, 320: 114-116. 10.1136/bmj.320.7227.114.
Cresswell KM, Worth A, Sheikh A: Actor-Network Theory and its role in understanding the implementation of information technology developments in healthcare. BMC Med Inform Decis Mak. 2010, 10 (1): 67-10.1186/1472-6947-10-67.
Article PubMed PubMed Central Google Scholar
Malterud K: Qualitative research: standards, challenges, and guidelines. Lancet. 2001, 358: 483-488. 10.1016/S0140-6736(01)05627-6.
Article CAS PubMed Google Scholar
Yin R: Case study research: design and methods. 1994, Thousand Oaks, CA: Sage Publishing, 2
Yin R: Enhancing the quality of case studies in health services research. Health Serv Res. 1999, 34: 1209-1224.
Green J, Thorogood N: Qualitative methods for health research. 2009, Los Angeles: Sage, 2
Howcroft D, Trauth E: Handbook of Critical Information Systems Research, Theory and Application. 2005, Cheltenham, UK: Northampton, MA, USA: Edward Elgar
Book Google Scholar
Blakie N: Approaches to Social Enquiry. 1993, Cambridge: Polity Press
Doolin B: Power and resistance in the implementation of a medical management information system. Info Systems J. 2004, 14: 343-362. 10.1111/j.1365-2575.2004.00176.x.
Bloomfield BP, Best A: Management consultants: systems development, power and the translation of problems. Sociological Review. 1992, 40: 533-560.
Shanks G, Parr A: Positivist, single case study research in information systems: A critical analysis. Proceedings of the European Conference on Information Systems. 2003, Naples
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2288/11/100/prepub
Download references
Acknowledgements
We are grateful to the participants and colleagues who contributed to the individual case studies that we have drawn on. This work received no direct funding, but it has been informed by projects funded by Asthma UK, the NHS Service Delivery Organisation, NHS Connecting for Health Evaluation Programme, and Patient Safety Research Portfolio. We would also like to thank the expert reviewers for their insightful and constructive feedback. Our thanks are also due to Dr. Allison Worth who commented on an earlier draft of this manuscript.
Author information
Authors and affiliations.
Division of Primary Care, The University of Nottingham, Nottingham, UK
Sarah Crowe & Anthony Avery
Centre for Population Health Sciences, The University of Edinburgh, Edinburgh, UK
Kathrin Cresswell, Ann Robertson & Aziz Sheikh
School of Health in Social Science, The University of Edinburgh, Edinburgh, UK
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Sarah Crowe .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contributions
AS conceived this article. SC, KC and AR wrote this paper with GH, AA and AS all commenting on various drafts. SC and AS are guarantors.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Crowe, S., Cresswell, K., Robertson, A. et al. The case study approach. BMC Med Res Methodol 11 , 100 (2011). https://doi.org/10.1186/1471-2288-11-100
Download citation
Received : 29 November 2010
Accepted : 27 June 2011
Published : 27 June 2011
DOI : https://doi.org/10.1186/1471-2288-11-100
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Case Study Approach
- Electronic Health Record System
- Case Study Design
- Case Study Site
- Case Study Report
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]
What is Clinical Data Management?

Learn about clinical data management's key role in clinical trials and how technology can improve how that data is connected, prepared, reviewed, and analyzed.

Sharmin Nasrullah
Share article.
Clinical data management (CDM) manages the data protocols for clinical trials, including establishing the right systems, processes, procedures, and training to extract meaningful results and comply with local and federal regulations around the world.
Clinical data management ensures that data collected throughout — and resulting from — a clinical trial is accurate, reliable, and compliant with regulatory standards. It protects the integrity of the trial and lays a foundation on which innovation can begin to take place.
Managing data that arises out of a clinical trial is a big job. The number of data points for just Phase III trials tripled to 3.6 million data points over the course of 10 years. Connecting the dots between all this information and setting up structures that enable researchers to turn them into insights and innovation is at the heart of clinical data management.
You’ll learn how to
Lay a foundation for effective clinical data management with collaboration and coordination Understand the key skills required for clinical data management Optimize clinical data management with a dedicated technology platform Prepare for new efficiencies in clinical data management with emerging technologies
Meet Life Sciences Cloud
Get more insights into how Salesforce is helping pharmaceutical companies use artificial intelligence to recruit patients, keep them, and assess results.

Lay a foundation for effective data management with collaboration and coordination
The key players in clinical data management need to work together closely to ensure successful outcomes. This includes the pharmaceutical companies and medical device makers who sponsor trials, the clinical research organizations who often run the trials, and the site personnel who collect and enter data into systems.
The primary objective of clinical data management processes is to provide high-quality data . Effective clinical data management keeps the number of errors and amount of missing data to a minimum. Accessible, high-quality data enables teams, researchers, and organizations to garner insights and draw important analyses. To do this properly, a variety of processes are put in place, namely clinical data management systems with embedded audit trails.
Though the scope of a clinical trial may be broad, the scope of clinical data management is highly defined to a few key tasks. These include:
- Data governance
- Data analytics
- Data collection
- Patient safety
- Regulatory compliance
When done correctly, these tasks set standards for how data is collected, stored, used, and shared. They make sure trials adhere to regulatory statutes and while keeping data and information secure.
Implementing advanced analytic tools enables teams to turn complex data sets into meaningful insights with the potential to greatly improve patient outcomes. Organizing data and making it accessible also enables organizations and trial teams to provide patients with easy access to their personal health data. This can help keep them engaged and encourage continued participation in the trial.
This is valuable, especially when you consider that recruiting patients can be one of the biggest challenges . Retention efforts claim 30% of the trial timeline and costs approximately $1.2 billion annually. In fact, 80% of trials fail to enroll on time, and worse, 11% of clinical sites fail to enroll any participants at all. These expensive delays cost pharmaceutical companies $600,000 to $8 million for every day of delay . ( Back to top. )
The key skills required for clinical data management
Clinical data managers have a long list of responsibilities, many of them supervisory. One of their primary responsibilities revolves around designing a data management plan. This plan describes the activities around data acquisition, data review, data cleaning and preparation for data analysis, and submission. These processes make certain that the right data is being collected in the right way, ensuring the study questions can be answered.
Solutions that can adequately connect data and streamline the preparation, review, and transformation of that data will be a game-changer.” Sharmin Nasrullah
Clinical data managers also oversee the creation of a clinical database (alongside database developers and programmers). They have an important role in developing the case report form (CRF) or electronic case report form (eCRF). Sponsors create these forms in conjunction with medical experts, statisticians, and regulatory authorities. Clinical data managers then implement them. The forms define the data fields, specify data types, outline the units of measurement. They also set the guidelines for form completion. Though CRFs can be paper or electronic the digital age has pushed heavily toward electronic data collection.
Other responsibilities include managing electronic data, standardizing that data, and locking the database at the right moment. They also review data and perform any necessary data cleaning activities. ( Back to top. )
Optimize clinical data management with a dedicated technology platform
The pharmaceutical industry, and clinical trials in particular, notoriously struggle to manage data. This makes it hard to connect the dots and requires dedicated staff with the right expertise to manage it. However, even with such expertise, manual data management can present a huge challenge.
With so many moving parts, sponsors need a way to centralize data in one place. CDMs would allow them to collect, monitor, review, and clean data more easily, making it ready to analyze and submit. It would also enable them to automate data collection, organize that data , and gain insights in real-time. These capabilities will become even more important as clinical trials become more complex and the industry moves toward decentralized trials, which require more sophisticated monitoring.
Organizations understand the inherent data management challenges, with many turning to clinical data management tools to help streamline processes, minimize errors, and centralize data collection and related activities. These data management systems not only create the single source of truth, but they also provide an audit trail. When it comes time to submitting the data and results to the Food and Drug Administration for approval (in what can be a labor-intensive process), the audit trail can save time and resources.
While powerful on their own, integrating data management systems with other systems can deliver a variety of benefits across a pharmaceutical organization. It makes it possible for teams to benefit from associated technologies like artificial intelligence (AI) and automation that can deliver insights and flag things like quality issues that may occur with the data. ( Back to top. )
Prepare for new efficiencies in clinical data management with emerging technologies
Data growth is explosive across the health and life sciences industry, as evidenced by the fact that health data is expected to account for 36% of the world’s data by 2025 —the most of any industry. Clinical trials are producing a substantial part of that data. In fact, between 2024 and 2030, clinical trials are expected to grow at an impressive compound annual rate of nearly 6.5% .
As with many other industries, professionals in clinical data management are looking to use AI to gain better insights into trials. This will require careful data governance to assure that data is HIPAA-compliant, secure, and in the right format for AI. This convergence of data and AI holds great potential for the health and life sciences industries. It spurs innovation, leads to better outcomes, and improves patient care.
Just For You

Your Patients Want Holistic Care — Here’s How Data Helps You Treat the Whole Person

Bots Coming For Your Job? Don’t Fret – Your Humanity Is Your Strength

Explore related content by topic
- Healthcare and Life Sciences

Sharmin is the GM of Life Sciences, focusing on Clinical. She has spent the majority of her 20 year career in clinical trial delivery, digital health, and technology development in academia, pharmaceutical, and software product companies.
Get the latest articles in your inbox.
Learn How These Health Companies Improved Employee Experiences During Massive Disruption

Connections 2022: What the New Marketing and Commerce Cloud Launches Mean for You

Only 23% Of Consumers Trust the Health Industry — Here’s How Ecommerce Can Help

Prioritizing Safety in Field Service Can Boost Your Bottom Line, Too

3 Communication Strategies To Increase Trust in Healthcare Providers

How Do You Create a Better Customer Experience? Here’s What Our Research Shows

Virtual Care Can Reach More Pediatric Patients and Drive Better Health Outcomes

7 Ways to Support Ukraine Now

360 Highlights
Yes, I would like to receive the Salesforce 360 Highlights newsletter as well as marketing emails regarding Salesforce products, services, and events. I can unsubscribe at any time.
By registering, you confirm that you agree to the processing of your personal data by Salesforce as described in the Privacy Statement .

Thanks, you're subscribed!

New to Salesforce?
- What is Salesforce?
- Best CRM software
- Explore all products
- What is cloud computing
- Customer success
- Product pricing
About Salesforce
- Salesforce.org
- Sustainability
Popular Links
- Salesforce Mobile
- AppExchange
- CRM software
- Salesforce LIVE
- Salesforce for startups
- América Latina (Español)
- Brasil (Português)
- Canada (English)
- Canada (Français)
- United States (English)
Europe, Middle East, and Africa
- España (Español)
- Deutschland (Deutsch)
- France (Français)
- Italia (Italiano)
- Nederland (Nederlands)
- Sverige (Svenska)
- United Kingdom (English)
- All other countries (English)
Asia Pacific
- Australia (English)
- India (English)
- Malaysia (English)
- ประเทศไทย (ไทย)
© Copyright 2024 Salesforce, Inc. All rights reserved. Various trademarks held by their respective owners. Salesforce, Inc. Salesforce Tower, 415 Mission Street, 3rd Floor, San Francisco, CA 94105, United States
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Pediatr Investig
- v.3(4); 2019 Dec

Clinical research study designs: The essentials
Ambika g. chidambaram.
1 Children's Hospital of Philadelphia, Philadelphia Pennsylvania, USA
Maureen Josephson
In clinical research, our aim is to design a study which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods. The conclusions derived from a research study can either improve health care or result in inadvertent harm to patients. Hence, this requires a well‐designed clinical research study that rests on a strong foundation of a detailed methodology and governed by ethical clinical principles. The purpose of this review is to provide the readers an overview of the basic study designs and its applicability in clinical research.
Introduction
In clinical research, our aim is to design a study, which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods that can be translated to the “real world” setting. 1 Before choosing a study design, one must establish aims and objectives of the study, and choose an appropriate target population that is most representative of the population being studied. The conclusions derived from a research study can either improve health care or result in inadvertent harm to patients. Hence, this requires a well‐designed clinical research study that rests on a strong foundation of a detailed methodology and is governed by ethical principles. 2
From an epidemiological standpoint, there are two major types of clinical study designs, observational and experimental. 3 Observational studies are hypothesis‐generating studies, and they can be further divided into descriptive and analytic. Descriptive observational studies provide a description of the exposure and/or the outcome, and analytic observational studies provide a measurement of the association between the exposure and the outcome. Experimental studies, on the other hand, are hypothesis testing studies. It involves an intervention that tests the association between the exposure and outcome. Each study design is different, and so it would be important to choose a design that would most appropriately answer the question in mind and provide the most valuable information. We will be reviewing each study design in detail (Figure 1 ).

Overview of clinical research study designs
Observational study designs
Observational studies ask the following questions: what, who, where and when. There are many study designs that fall under the umbrella of descriptive study designs, and they include, case reports, case series, ecologic study, cross‐sectional study, cohort study and case‐control study (Figure 2 ).

Classification of observational study designs
Case reports and case series
Every now and then during clinical practice, we come across a case that is atypical or ‘out of the norm’ type of clinical presentation. This atypical presentation is usually described as case reports which provides a detailed and comprehensive description of the case. 4 It is one of the earliest forms of research and provides an opportunity for the investigator to describe the observations that make a case unique. There are no inferences obtained and therefore cannot be generalized to the population which is a limitation. Most often than not, a series of case reports make a case series which is an atypical presentation found in a group of patients. This in turn poses the question for a new disease entity and further queries the investigator to look into mechanistic investigative opportunities to further explore. However, in a case series, the cases are not compared to subjects without the manifestations and therefore it cannot determine which factors in the description are unique to the new disease entity.
Ecologic study
Ecological studies are observational studies that provide a description of population group characteristics. That is, it describes characteristics to all individuals within a group. For example, Prentice et al 5 measured incidence of breast cancer and per capita intake of dietary fat, and found a correlation that higher per capita intake of dietary fat was associated with an increased incidence of breast cancer. But the study does not conclude specifically which subjects with breast cancer had a higher dietary intake of fat. Thus, one of the limitations with ecologic study designs is that the characteristics are attributed to the whole group and so the individual characteristics are unknown.
Cross‐sectional study
Cross‐sectional studies are study designs used to evaluate an association between an exposure and outcome at the same time. It can be classified under either descriptive or analytic, and therefore depends on the question being answered by the investigator. Since, cross‐sectional studies are designed to collect information at the same point of time, this provides an opportunity to measure prevalence of the exposure or the outcome. For example, a cross‐sectional study design was adopted to estimate the global need for palliative care for children based on representative sample of countries from all regions of the world and all World Bank income groups. 6 The limitation of cross‐sectional study design is that temporal association cannot be established as the information is collected at the same point of time. If a study involves a questionnaire, then the investigator can ask questions to onset of symptoms or risk factors in relation to onset of disease. This would help in obtaining a temporal sequence between the exposure and outcome. 7
Case‐control study
Case‐control studies are study designs that compare two groups, such as the subjects with disease (cases) to the subjects without disease (controls), and to look for differences in risk factors. 8 This study is used to study risk factors or etiologies for a disease, especially if the disease is rare. Thus, case‐control studies can also be hypothesis testing studies and therefore can suggest a causal relationship but cannot prove. It is less expensive and less time‐consuming than cohort studies (described in section “Cohort study”). An example of a case‐control study was performed in Pakistan evaluating the risk factors for neonatal tetanus. They retrospectively reviewed a defined cohort for cases with and without neonatal tetanus. 9 They found a strong association of the application of ghee (clarified butter) as a risk factor for neonatal tetanus. Although this suggests a causal relationship, cause cannot be proven by this methodology (Figure 3 ).

Case‐control study design
One of the limitations of case‐control studies is that they cannot estimate prevalence of a disease accurately as a proportion of cases and controls are studied at a time. Case‐control studies are also prone to biases such as recall bias, as the subjects are providing information based on their memory. Hence, the subjects with disease are likely to remember the presence of risk factors compared to the subjects without disease.
One of the aspects that is often overlooked is the selection of cases and controls. It is important to select the cases and controls appropriately to obtain a meaningful and scientifically sound conclusion and this can be achieved by implementing matching. Matching is defined by Gordis et al as ‘the process of selecting the controls so that they are similar to the cases in certain characteristics such as age, race, sex, socioeconomic status and occupation’ 7 This would help identify risk factors or probable etiologies that are not due to differences between the cases and controls.
Cohort study
Cohort studies are study designs that compare two groups, such as the subjects with exposure/risk factor to the subjects without exposure/risk factor, for differences in incidence of outcome/disease. Most often, cohort study designs are used to study outcome(s) from a single exposure/risk factor. Thus, cohort studies can also be hypothesis testing studies and can infer and interpret a causal relationship between an exposure and a proposed outcome, but cannot establish it (Figure 4 ).

Cohort study design
Cohort studies can be classified as prospective and retrospective. 7 Prospective cohort studies follow subjects from presence of risk factors/exposure to development of disease/outcome. This could take up to years before development of disease/outcome, and therefore is time consuming and expensive. On the other hand, retrospective cohort studies identify a population with and without the risk factor/exposure based on past records and then assess if they had developed the disease/outcome at the time of study. Thus, the study design for prospective and retrospective cohort studies are similar as we are comparing populations with and without exposure/risk factor to development of outcome/disease.
Cohort studies are typically chosen as a study design when the suspected exposure is known and rare, and the incidence of disease/outcome in the exposure group is suspected to be high. The choice between prospective and retrospective cohort study design would depend on the accuracy and reliability of the past records regarding the exposure/risk factor.
Some of the biases observed with cohort studies include selection bias and information bias. Some individuals who have the exposure may refuse to participate in the study or would be lost to follow‐up, and in those instances, it becomes difficult to interpret the association between an exposure and outcome. Also, if the information is inaccurate when past records are used to evaluate for exposure status, then again, the association between the exposure and outcome becomes difficult to interpret.
Case‐control studies based within a defined cohort
Case‐control studies based within a defined cohort is a form of study design that combines some of the features of a cohort study design and a case‐control study design. When a defined cohort is embedded in a case‐control study design, all the baseline information collected before the onset of disease like interviews, surveys, blood or urine specimens, then the cohort is followed onset of disease. One of the advantages of following the above design is that it eliminates recall bias as the information regarding risk factors is collected before onset of disease. Case‐control studies based within a defined cohort can be further classified into two types: Nested case‐control study and Case‐cohort study.
Nested case‐control study
A nested case‐control study consists of defining a cohort with suspected risk factors and assigning a control within a cohort to the subject who develops the disease. 10 Over a period, cases and controls are identified and followed as per the investigator's protocol. Hence, the case and control are matched on calendar time and length of follow‐up. When this study design is implemented, it is possible for the control that was selected early in the study to develop the disease and become a case in the latter part of the study.
Case‐cohort Study
A case‐cohort study is similar to a nested case‐control study except that there is a defined sub‐cohort which forms the groups of individuals without the disease (control), and the cases are not matched on calendar time or length of follow‐up with the control. 11 With these modifications, it is possible to compare different disease groups with the same sub‐cohort group of controls and eliminates matching between the case and control. However, these differences will need to be accounted during analysis of results.
Experimental study design
The basic concept of experimental study design is to study the effect of an intervention. In this study design, the risk factor/exposure of interest/treatment is controlled by the investigator. Therefore, these are hypothesis testing studies and can provide the most convincing demonstration of evidence for causality. As a result, the design of the study requires meticulous planning and resources to provide an accurate result.
The experimental study design can be classified into 2 groups, that is, controlled (with comparison) and uncontrolled (without comparison). 1 In the group without controls, the outcome is directly attributed to the treatment received in one group. This fails to prove if the outcome was truly due to the intervention implemented or due to chance. This can be avoided if a controlled study design is chosen which includes a group that does not receive the intervention (control group) and a group that receives the intervention (intervention/experiment group), and therefore provide a more accurate and valid conclusion.
Experimental study designs can be divided into 3 broad categories: clinical trial, community trial, field trial. The specifics of each study design are explained below (Figure 5 ).

Experimental study designs
Clinical trial
Clinical trials are also known as therapeutic trials, which involve subjects with disease and are placed in different treatment groups. It is considered a gold standard approach for epidemiological research. One of the earliest clinical trial studies was performed by James Lind et al in 1747 on sailors with scurvy. 12 Lind divided twelve scorbutic sailors into six groups of two. Each group received the same diet, in addition to a quart of cider (group 1), twenty‐five drops of elixir of vitriol which is sulfuric acid (group 2), two spoonfuls of vinegar (group 3), half a pint of seawater (group 4), two oranges and one lemon (group 5), and a spicy paste plus a drink of barley water (group 6). The group who ate two oranges and one lemon had shown the most sudden and visible clinical effects and were taken back at the end of 6 days as being fit for duty. During Lind's time, this was not accepted but was shown to have similar results when repeated 47 years later in an entire fleet of ships. Based on the above results, in 1795 lemon juice was made a required part of the diet of sailors. Thus, clinical trials can be used to evaluate new therapies, such as new drug or new indication, new drug combination, new surgical procedure or device, new dosing schedule or mode of administration, or a new prevention therapy.
While designing a clinical trial, it is important to select the population that is best representative of the general population. Therefore, the results obtained from the study can be generalized to the population from which the sample population was selected. It is also as important to select appropriate endpoints while designing a trial. Endpoints need to be well‐defined, reproducible, clinically relevant and achievable. The types of endpoints include continuous, ordinal, rates and time‐to‐event, and it is typically classified as primary, secondary or tertiary. 2 An ideal endpoint is a purely clinical outcome, for example, cure/survival, and thus, the clinical trials will become very long and expensive trials. Therefore, surrogate endpoints are used that are biologically related to the ideal endpoint. Surrogate endpoints need to be reproducible, easily measured, related to the clinical outcome, affected by treatment and occurring earlier than clinical outcome. 2
Clinical trials are further divided into randomized clinical trial, non‐randomized clinical trial, cross‐over clinical trial and factorial clinical trial.
Randomized clinical trial
A randomized clinical trial is also known as parallel group randomized trials or randomized controlled trials. Randomized clinical trials involve randomizing subjects with similar characteristics to two groups (or multiple groups): the group that receives the intervention/experimental therapy and the other group that received the placebo (or standard of care). 13 This is typically performed by using a computer software, manually or by other methods. Hence, we can measure the outcomes and efficacy of the intervention/experimental therapy being studied without bias as subjects have been randomized to their respective groups with similar baseline characteristics. This type of study design is considered gold standard for epidemiological research. However, this study design is generally not applicable to rare and serious disease process as it would unethical to treat that group with a placebo. Please see section “Randomization” for detailed explanation regarding randomization and placebo.
Non‐randomized clinical trial
A non‐randomized clinical trial involves an approach to selecting controls without randomization. With this type of study design a pattern is usually adopted, such as, selection of subjects and controls on certain days of the week. Depending on the approach adopted, the selection of subjects becomes predictable and therefore, there is bias with regards to selection of subjects and controls that would question the validity of the results obtained.
Historically controlled studies can be considered as a subtype of non‐randomized clinical trial. In this study design subtype, the source of controls is usually adopted from the past, such as from medical records and published literature. 1 The advantages of this study design include being cost‐effective, time saving and easily accessible. However, since this design depends on already collected data from different sources, the information obtained may not be accurate, reliable, lack uniformity and/or completeness as well. Though historically controlled studies maybe easier to conduct, the disadvantages will need to be taken into account while designing a study.
Cross‐over clinical trial
In cross‐over clinical trial study design, there are two groups who undergoes the same intervention/experiment at different time periods of the study. That is, each group serves as a control while the other group is undergoing the intervention/experiment. 14 Depending on the intervention/experiment, a ‘washout’ period is recommended. This would help eliminate residuals effects of the intervention/experiment when the experiment group transitions to be the control group. Hence, the outcomes of the intervention/experiment will need to be reversible as this type of study design would not be possible if the subject is undergoing a surgical procedure.
Factorial trial
A factorial trial study design is adopted when the researcher wishes to test two different drugs with independent effects on the same population. Typically, the population is divided into 4 groups, the first with drug A, the second with drug B, the third with drug A and B, and the fourth with neither drug A nor drug B. The outcomes for drug A are compared to those on drug A, drug A and B and to those who were on drug B and neither drug A nor drug B. 15 The advantages of this study design that it saves time and helps to study two different drugs on the same study population at the same time. However, this study design would not be applicable if either of the drugs or interventions overlaps with each other on modes of action or effects, as the results obtained would not attribute to a particular drug or intervention.
Community trial
Community trials are also known as cluster‐randomized trials, involve groups of individuals with and without disease who are assigned to different intervention/experiment groups. Hence, groups of individuals from a certain area, such as a town or city, or a certain group such as school or college, will undergo the same intervention/experiment. 16 Hence, the results will be obtained at a larger scale; however, will not be able to account for inter‐individual and intra‐individual variability.
Field trial
Field trials are also known as preventive or prophylactic trials, and the subjects without the disease are placed in different preventive intervention groups. 16 One of the hypothetical examples for a field trial would be to randomly assign to groups of a healthy population and to provide an intervention to a group such as a vitamin and following through to measure certain outcomes. Hence, the subjects are monitored over a period of time for occurrence of a particular disease process.
Overview of methodologies used within a study design
Randomization.
Randomization is a well‐established methodology adopted in research to prevent bias due to subject selection, which may impact the result of the intervention/experiment being studied. It is one of the fundamental principles of an experimental study designs and ensures scientific validity. It provides a way to avoid predicting which subjects are assigned to a certain group and therefore, prevent bias on the final results due to subject selection. This also ensures comparability between groups as most baseline characteristics are similar prior to randomization and therefore helps to interpret the results regarding the intervention/experiment group without bias.
There are various ways to randomize and it can be as simple as a ‘flip of a coin’ to use computer software and statistical methods. To better describe randomization, there are three types of randomization: simple randomization, block randomization and stratified randomization.
Simple randomization
In simple randomization, the subjects are randomly allocated to experiment/intervention groups based on a constant probability. That is, if there are two groups A and B, the subject has a 0.5 probability of being allocated to either group. This can be performed in multiple ways, and one of which being as simple as a ‘flip of a coin’ to using random tables or numbers. 17 The advantage of using this methodology is that it eliminates selection bias. However, the disadvantage with this methodology is that an imbalance in the number allocated to each group as well as the prognostic factors between groups. Hence, it is more challenging in studies with a small sample size.
Block randomization
In block randomization, the subjects of similar characteristics are classified into blocks. The aim of block randomization is to balance the number of subjects allocated to each experiment/intervention group. For example, let's assume that there are four subjects in each block, and two of the four subjects in each block will be randomly allotted to each group. Therefore, there will be two subjects in one group and two subjects in the other group. 17 The disadvantage with this methodology is that there is still a component of predictability in the selection of subjects and the randomization of prognostic factors is not performed. However, it helps to control the balance between the experiment/intervention groups.
Stratified randomization
In stratified randomization, the subjects are defined based on certain strata, which are covariates. 18 For example, prognostic factors like age can be considered as a covariate, and then the specified population can be randomized within each age group related to an experiment/intervention group. The advantage with this methodology is that it enables comparability between experiment/intervention groups and thus makes result analysis more efficient. But, with this methodology the covariates will need to be measured and determined before the randomization process. The sample size will help determine the number of strata that would need to be chosen for a study.
Blinding is a methodology adopted in a study design to intentionally not provide information related to the allocation of the groups to the subject participants, investigators and/or data analysts. 19 The purpose of blinding is to decrease influence associated with the knowledge of being in a particular group on the study result. There are 3 forms of blinding: single‐blinded, double‐blinded and triple‐blinded. 1 In single‐blinded studies, otherwise called as open‐label studies, the subject participants are not revealed which group that they have been allocated to. However, the investigator and data analyst will be aware of the allocation of the groups. In double‐blinded studies, both the study participants and the investigator will be unaware of the group to which they were allocated to. Double‐blinded studies are typically used in clinical trials to test the safety and efficacy of the drugs. In triple‐blinded studies, the subject participants, investigators and data analysts will not be aware of the group allocation. Thus, triple‐blinded studies are more difficult and expensive to design but the results obtained will exclude confounding effects from knowledge of group allocation.
Blinding is especially important in studies where subjective response are considered as outcomes. This is because certain responses can be modified based on the knowledge of the experiment group that they are in. For example, a group allocated in the non‐intervention group may not feel better as they are not getting the treatment, or an investigator may pay more attention to the group receiving treatment, and thereby potentially affecting the final results. However, certain treatments cannot be blinded such as surgeries or if the treatment group requires an assessment of the effect of intervention such as quitting smoking.
Placebo is defined in the Merriam‐Webster dictionary as ‘an inert or innocuous substance used especially in controlled experiments testing the efficacy of another substance (such as drug)’. 20 A placebo is typically used in a clinical research study to evaluate the safety and efficacy of a drug/intervention. This is especially useful if the outcome measured is subjective. In clinical drug trials, a placebo is typically a drug that resembles the drug to be tested in certain characteristics such as color, size, shape and taste, but without the active substance. This helps to measure effects of just taking the drug, such as pain relief, compared to the drug with the active substance. If the effect is positive, for example, improvement in mood/pain, then it is called placebo effect. If the effect is negative, for example, worsening of mood/pain, then it is called nocebo effect. 21
The ethics of placebo‐controlled studies is complex and remains a debate in the medical research community. According to the Declaration of Helsinki on the use of placebo released in October 2013, “The benefits, risks, burdens and effectiveness of a new intervention must be tested against those of the best proven intervention(s), except in the following circumstances:
Where no proven intervention exists, the use of placebo, or no intervention, is acceptable; or
Where for compelling and scientifically sound methodological reasons the use of any intervention less effective than the best proven one, the use of placebo, or no intervention is necessary to determine the efficacy or safety of an intervention and the patients who receive any intervention less effective than the best proven one, placebo, or no intervention will not be subject to additional risks of serious or irreversible harm as a result of not receiving the best proven intervention.
Extreme care must be taken to avoid abuse of this option”. 22
Hence, while designing a research study, both the scientific validity and ethical aspects of the study will need to be thoroughly evaluated.
Bias has been defined as “any systematic error in the design, conduct or analysis of a study that results in a mistaken estimate of an exposure's effect on the risk of disease”. 23 There are multiple types of biases and so, in this review we will focus on the following types: selection bias, information bias and observer bias. Selection bias is when a systematic error is committed while selecting subjects for the study. Selection bias will affect the external validity of the study if the study subjects are not representative of the population being studied and therefore, the results of the study will not be generalizable. Selection bias will affect the internal validity of the study if the selection of study subjects in each group is influenced by certain factors, such as, based on the treatment of the group assigned. One of the ways to decrease selection bias is to select the study population that would representative of the population being studied, or to randomize (discussed in section “Randomization”).
Information bias is when a systematic error is committed while obtaining data from the study subjects. This can be in the form of recall bias when subject is required to remember certain events from the past. Typically, subjects with the disease tend to remember certain events compared to subjects without the disease. Observer bias is a systematic error when the study investigator is influenced by the certain characteristics of the group, that is, an investigator may pay closer attention to the group receiving the treatment versus the group not receiving the treatment. This may influence the results of the study. One of the ways to decrease observer bias is to use blinding (discussed in section “Blinding”).
Thus, while designing a study it is important to take measure to limit bias as much as possible so that the scientific validity of the study results is preserved to its maximum.
Overview of drug development in the United States of America
Now that we have reviewed the various clinical designs, clinical trials form a major part in development of a drug. In the United States, the Food and Drug Administration (FDA) plays an important role in getting a drug approved for clinical use. It includes a robust process that involves four different phases before a drug can be made available to the public. Phase I is conducted to determine a safe dose. The study subjects consist of normal volunteers and/or subjects with disease of interest, and the sample size is typically small and not more than 30 subjects. The primary endpoint consists of toxicity and adverse events. Phase II is conducted to evaluate of safety of dose selected in Phase I, to collect preliminary information on efficacy and to determine factors to plan a randomized controlled trial. The study subjects consist of subjects with disease of interest and the sample size is also small but more that Phase I (40–100 subjects). The primary endpoint is the measure of response. Phase III is conducted as a definitive trial to prove efficacy and establish safety of a drug. Phase III studies are randomized controlled trials and depending on the drug being studied, it can be placebo‐controlled, equivalence, superiority or non‐inferiority trials. The study subjects consist of subjects with disease of interest, and the sample size is typically large but no larger than 300 to 3000. Phase IV is performed after a drug is approved by the FDA and it is also called the post‐marketing clinical trial. This phase is conducted to evaluate new indications, to determine safety and efficacy in long‐term follow‐up and new dosing regimens. This phase helps to detect rare adverse events that would not be picked up during phase III studies and decrease in the delay in the release of the drug in the market. Hence, this phase depends heavily on voluntary reporting of side effects and/or adverse events by physicians, non‐physicians or drug companies. 2
We have discussed various clinical research study designs in this comprehensive review. Though there are various designs available, one must consider various ethical aspects of the study. Hence, each study will require thorough review of the protocol by the institutional review board before approval and implementation.
CONFLICT OF INTEREST
Chidambaram AG, Josephson M. Clinical research study designs: The essentials . Pediatr Invest . 2019; 3 :245‐252. 10.1002/ped4.12166 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
More than 500,000 people from around the world have participated in clinical research since the hospital opened in 1953. We do not charge patients for participation and treatment in clinical studies at NIH. In certain emergency circumstances, you may qualify for help with travel and other expenses Read more, to see if clinical studies are for you.
Challenges Faced and Lessons Learned from Our Trial of VTE Prophylaxis. G. Le Gal and D. MottierNEJM Evid 2023;2 (9) In this Clinical Trials Case Study, the authors describe the challenges faced and lessons learned conducting a trial of venous thromboembolism prophylaxis among hospitalized older adults. Clinical Trials Case Study.
Search NIH Clinical Research Studies The NIH maintains an online database of clinical research studies taking place at its Clinical Center, which is located on the NIH campus in Bethesda, Maryland. Studies are conducted by most of the institutes and centers across the NIH. The Clinical Center hosts a wide range of studies from rare diseases to ...
The simplified case studies apply the following four questions to determine whether NIH would consider the research study to be a clinical trial: ... An internal medicine fellow designs an independent ancillary trial where a subset of patients from the parent trial in Case Study #42a will also receive drug B, based on the assumption that a two ...
A case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine. Some reports contain an extensive review of the relevant ...
Observational study. A type of study in which people are observed or certain outcomes are measured. No attempt is made by the researcher to affect the outcome — for example, no treatment is given by the researcher. Clinical trial (interventional study). During clinical trials, researchers learn if a new test or treatment works and is safe.
At baseline, the mean AHI was 51.5 events per hour in trial 1 and 49.5 events per hour in trial 2, and the mean body-mass index (BMI, the weight in kilograms divided by the square of the height in ...
Clinical Case Studies (CCS), peer-reviewed & published bi-monthly electronic only, is the only journal devoted entirely to innovative psychotherapy case studies & presents cases involving individual, couples, & family therapy.The easy-to-follow case presentation format allows you to learn how interesting & challenging cases were assessed & conceptualized, & how treatment followed such ...
Editorial. Introduction. Case reports and case series or case study research are descriptive studies to present patients in their natural clinical setting. Case reports, which generally consist of three or fewer patients, are prepared to illustrate features in the practice of medicine and potentially create new research questions that may contribute to the acquisition of additional knowledge ...
Clinical trials are research studies that explore whether a medical strategy, treatment or device is safe and effective for humans. These studies may also show which medical approaches work best for certain illnesses or groups of people. Clinical trials produce information that helps patients and their health-care providers make better health ...
Clinical research in this review refers to scientific research related to clinical practices. There are many ways a clinical research's findings can become invalid or less impactful including ignorance of previous similar studies, a paucity of similar studies, poor study design and implementation, low test agent efficacy, no predetermined ...
ClinicalTrials.gov. Glossary. Study record managers: refer to the Data Element Definitions if submitting registration or results information. Search for terms.
Many clinical research studies are also supervised by a data and safety monitoring committee. This is a group made up of experts in the area being studied. These biomedical professionals regularly monitor clinical studies as they progress. If they discover or suspect any problems with a study, they immediately stop the trial.
As the most common cause of dementia in later life, AD is projected to affect 152.8 million people by 2050 worldwide 1.Historically, AD has been diagnosed by clinical symptoms based on impaired ...
A second example is the U.S. Department of Veterans Affairs' National Center for PTSD, which seeks to improve clinical care for military veterans through interdisciplinary research on stress ...
Clinical research is the study of health and illness in people. There are two main types of clinical research: observational studies and clinical trials. Read and share this infographic (PDF, 317K) to learn why researchers do different kinds of clinical studies. Observational studies monitor people in normal settings.
Clinical trials are research studies in which people volunteer to help find answers to specific health questions. When carefully conducted, they are the safest and fastest way to find new ...
Case studies have acquired an unmerited reputation as being anecdotal, unscientific and intrinsically inferior to group studies. The subsequent disregarding of individual patients as the focus of investigation has led to the neglect of an extremely useful clinical research method, and has probably impaired the pace of therapeutic innovation.
A case study is a research approach that is used to generate an in-depth, multi-faceted understanding of a complex issue in its real-life context. It is an established research design that is used extensively in a wide variety of disciplines, particularly in the social sciences. A case study can be defined in a variety of ways (Table.
A clinical trial is a research study that involves people like you. Researchers conduct clinical trials to find new or better ways to prevent, detect, or treat health conditions. Often, researchers want to find out if a new test, treatment, or preventive measure is safe and effective. Tests can include ways to screen for, diagnose, or prevent a ...
Below are descriptions of some different kinds of clinical research. Treatment Research generally involves an intervention such as medication, psychotherapy, new devices, or new approaches to ...
A case study is a research approach that is used to generate an in-depth, multi-faceted understanding of a complex issue in its real-life context. It is an established research design that is used extensively in a wide variety of disciplines, particularly in the social sciences. A case study can be defined in a variety of ways (Table 5 ), the ...
A clinical trial is a type of clinical research study which uses human volunteers, also called participants, to increase medical knowledge. The two main types are clinical trials (also called interventional studies) and observational studies. The Food and Drug Administration tests medicine and medical devices to ensure safety and effectiveness ...
A clinical trial is a type of clinical research that evaluates the effects of intervention(s), including drugs, devices, surgeries, diets, behavioral approaches, and lifestyle interventions, on health-related biomedical or behavioral outcomes.. To account for the diverse lived experiences and exposures of various populations, clinical research should be appropriately inclusive of racial and ...
Additionally, more diverse samples are needed, said Alpert, who wasn't involved in the study and is a professor of psychiatry, neuroscience and pediatrics at the Albert Einstein College of Medicine.
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management. ... The BE studies may be undertaken in vitro (fasting, non ...
Clinical research is the study of health and illness in people. Scientists may have many reasons for doing a clinical study, such as: • To explore the cause of a disease or a set of symptoms. • To test if a treatment will help with a symptom or condition. • To learn how a certain behavior affects people's health.
RESEARCH TRIANGLE PARK, N.C., June 27, 2024 (GLOBE NEWSWIRE) -- Science 37, a leader in enhancing patient access to clinical trials, has been added to over 20 studies that were lagging in ...
Clinical data management ensures that data collected throughout — and resulting from — a clinical trial is accurate, reliable, and compliant with regulatory standards. It protects the integrity of the trial and lays a foundation on which innovation can begin to take place. Managing data that arises out of a clinical trial is a big job.
Introduction. In clinical research, our aim is to design a study, which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods that can be translated to the "real world" setting. 1 Before choosing a study design, one must establish aims and objectives of the study, and choose an appropriate target population that is most representative of ...