Cookies on this website
We use cookies to ensure that we give you the best experience on our website. If you click 'Accept all cookies' we'll assume that you are happy to receive all cookies and you won't see this message again. If you click 'Reject all non-essential cookies' only necessary cookies providing core functionality such as security, network management, and accessibility will be enabled. Click 'Find out more' for information on how to change your cookie settings.


Critical Appraisal tools
Critical appraisal worksheets to help you appraise the reliability, importance and applicability of clinical evidence.
Critical appraisal is the systematic evaluation of clinical research papers in order to establish:
- Does this study address a clearly focused question ?
- Did the study use valid methods to address this question?
- Are the valid results of this study important?
- Are these valid, important results applicable to my patient or population?
If the answer to any of these questions is “no”, you can save yourself the trouble of reading the rest of it.
This section contains useful tools and downloads for the critical appraisal of different types of medical evidence. Example appraisal sheets are provided together with several helpful examples.
Critical Appraisal Worksheets
- Systematic Reviews Critical Appraisal Sheet
- Diagnostics Critical Appraisal Sheet
- Prognosis Critical Appraisal Sheet
- Randomised Controlled Trials (RCT) Critical Appraisal Sheet
- Critical Appraisal of Qualitative Studies Sheet
- IPD Review Sheet
Chinese - translated by Chung-Han Yang and Shih-Chieh Shao
- Systematic Reviews Critical Appraisal Sheet
- Diagnostic Study Critical Appraisal Sheet
- Prognostic Critical Appraisal Sheet
- RCT Critical Appraisal Sheet
- IPD reviews Critical Appraisal Sheet
- Qualitative Studies Critical Appraisal Sheet
German - translated by Johannes Pohl and Martin Sadilek
- Systematic Review Critical Appraisal Sheet
- Diagnosis Critical Appraisal Sheet
- Prognosis Critical Appraisal Sheet
- Therapy / RCT Critical Appraisal Sheet
Lithuanian - translated by Tumas Beinortas
- Systematic review appraisal Lithuanian (PDF)
- Diagnostic accuracy appraisal Lithuanian (PDF)
- Prognostic study appraisal Lithuanian (PDF)
- RCT appraisal sheets Lithuanian (PDF)
Portugese - translated by Enderson Miranda, Rachel Riera and Luis Eduardo Fontes
- Portuguese – Systematic Review Study Appraisal Worksheet
- Portuguese – Diagnostic Study Appraisal Worksheet
- Portuguese – Prognostic Study Appraisal Worksheet
- Portuguese – RCT Study Appraisal Worksheet
- Portuguese – Systematic Review Evaluation of Individual Participant Data Worksheet
- Portuguese – Qualitative Studies Evaluation Worksheet
Spanish - translated by Ana Cristina Castro
- Systematic Review (PDF)
- Diagnosis (PDF)
- Prognosis Spanish Translation (PDF)
- Therapy / RCT Spanish Translation (PDF)
Persian - translated by Ahmad Sofi Mahmudi
- Prognosis (PDF)
- PICO Critical Appraisal Sheet (PDF)
- PICO Critical Appraisal Sheet (MS-Word)
- Educational Prescription Critical Appraisal Sheet (PDF)
Explanations & Examples
- Pre-test probability
- SpPin and SnNout
- Likelihood Ratios
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 31 January 2022
The fundamentals of critically appraising an article
- Sneha Chotaliya 1
BDJ Student volume 29 , pages 12–13 ( 2022 ) Cite this article
1949 Accesses
Metrics details
Sneha Chotaliya
We are often surrounded by an abundance of research and articles, but the quality and validity can vary massively. Not everything will be of a good quality - or even valid. An important part of reading a paper is first assessing the paper. This is a key skill for all healthcare professionals as anything we read can impact or influence our practice. It is also important to stay up to date with the latest research and findings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Chambers R, 'Clinical Effectiveness Made Easy', Oxford: Radcliffe Medical Press , 1998
Loney P L, Chambers L W, Bennett K J, Roberts J G and Stratford P W. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can 1998; 19 : 170-176.
Brice R. CASP CHECKLISTS - CASP - Critical Appraisal Skills Programme . 2021. Available at: https://casp-uk.net/casp-tools-checklists/ (Accessed 22 July 2021).
White S, Halter M, Hassenkamp A and Mein G. 2021. Critical Appraisal Techniques for Healthcare Literature . St George's, University of London.
Download references
Author information
Authors and affiliations.
Academic Foundation Dentist, London, UK
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Sneha Chotaliya .
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Chotaliya, S. The fundamentals of critically appraising an article. BDJ Student 29 , 12–13 (2022). https://doi.org/10.1038/s41406-021-0275-6
Download citation
Published : 31 January 2022
Issue Date : 31 January 2022
DOI : https://doi.org/10.1038/s41406-021-0275-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

- Critical Appraisal Tools
- Introduction
- Related Guides
- Getting Help
Critical Appraisal of Studies
Critical appraisal is the process of carefully and systematically examining research to judge its trustworthiness, and its value/relevance in a particular context by providing a framework to evaluate the research. During the critical appraisal process, researchers can:
- Decide whether studies have been undertaken in a way that makes their findings reliable as well as valid and unbiased
- Make sense of the results
- Know what these results mean in the context of the decision they are making
- Determine if the results are relevant to their patients/schoolwork/research
Burls, A. (2009). What is critical appraisal? In What Is This Series: Evidence-based medicine. Available online at What is Critical Appraisal?
Critical appraisal is included in the process of writing high quality reviews, like systematic and integrative reviews and for evaluating evidence from RCTs and other study designs. For more information on systematic reviews, check out our Systematic Review guide.
- Next: Critical Appraisal Tools >>
- Last Updated: Nov 16, 2023 1:27 PM
- URL: https://guides.library.duq.edu/critappraise
CASP Checklists
How to use our CASP Checklists
Referencing and Creative Commons
- Online Training Courses
- CASP Workshops
- What is Critical Appraisal
- Study Designs
- Useful Links
- Bibliography
- View all Tools and Resources
- Testimonials
Critical Appraisal Checklists
We offer a number of free downloadable checklists to help you more easily and accurately perform critical appraisal across a number of different study types.
The CASP checklists are easy to understand but in case you need any further guidance on how they are structured, take a look at our guide on how to use our CASP checklists .
CASP Randomised Controlled Trial Checklist
- Print & Fill
CASP Systematic Review Checklist
CASP Qualitative Studies Checklist
CASP Cohort Study Checklist
CASP Diagnostic Study Checklist
CASP Case Control Study Checklist
CASP Economic Evaluation Checklist
CASP Clinical Prediction Rule Checklist
Checklist Archive
- CASP Randomised Controlled Trial Checklist 2018 fillable form
- CASP Randomised Controlled Trial Checklist 2018
CASP Checklist
Need more information?
- Online Learning
- Privacy Policy

Critical Appraisal Skills Programme
Critical Appraisal Skills Programme (CASP) will use the information you provide on this form to be in touch with you and to provide updates and marketing. Please let us know all the ways you would like to hear from us:
We use Mailchimp as our marketing platform. By clicking below to subscribe, you acknowledge that your information will be transferred to Mailchimp for processing. Learn more about Mailchimp's privacy practices here.
Copyright 2024 CASP UK - OAP Ltd. All rights reserved Website by Beyond Your Brand
Critical Appraisal Tools and Reporting Guidelines for Evidence-Based Practice
Affiliations.
- 1 Professor, School of Nursing & Health Professions, University of San Francisco, San Francisco, CA, USA.
- 2 Reference Librarian and Primary Liaison, School of Nursing & Health Professions, Gleeson Library, Geschke Center, University of San Francisco, San Francisco, CA 94117, USA.
- PMID: 28898556
- DOI: 10.1111/wvn.12258
Background: Nurses engaged in evidence-based practice (EBP) have two important sets of tools: Critical appraisal tools and reporting guidelines. Critical appraisal tools facilitate the appraisal process and guide a consumer of evidence through an objective, analytical, evaluation process. Reporting guidelines, checklists of items that should be included in a publication or report, ensure that the project or guidelines are reported on with clarity, completeness, and transparency.
Purpose: The primary purpose of this paper is to help nurses understand the difference between critical appraisal tools and reporting guidelines. A secondary purpose is to help nurses locate the appropriate tool for the appraisal or reporting of evidence.
Methods: A systematic search was conducted to find commonly used critical appraisal tools and reporting guidelines for EBP in nursing.
Rationale: This article serves as a resource to help nurse navigate the often-overwhelming terrain of critical appraisal tools and reporting guidelines, and will help both novice and experienced consumers of evidence more easily select the appropriate tool(s) to use for critical appraisal and reporting of evidence. Having the skills to select the appropriate tool or guideline is an essential part of meeting EBP competencies for both practicing registered nurses and advanced practice nurses (Melnyk & Gallagher-Ford, 2015; Melnyk, Gallagher-Ford, & Fineout-Overholt, 2017).
Results: Nine commonly used critical appraisal tools and eight reporting guidelines were found and are described in this manuscript. Specific steps for selecting an appropriate tool as well as examples of each tool's use in a publication are provided.
Linking evidence to action: Practicing registered nurses and advance practice nurses must be able to critically appraise and disseminate evidence in order to meet EBP competencies. This article is a resource for understanding the difference between critical appraisal tools and reporting guidelines, and identifying and accessing appropriate tools or guidelines.
Keywords: critical appraisal tools; evidence-based nursing; evidence-based practice; reporting guidelines.
© 2017 Sigma Theta Tau International.
Publication types
- Data Collection / methods
- Evidence-Based Practice / methods
- Evidence-Based Practice / standards*
- Nurses / trends*
- Practice Guidelines as Topic / standards*
- Quality of Health Care / standards
- Risk Management / methods
- Risk Management / standards*
- Research article
- Open access
- Published: 16 September 2004
A systematic review of the content of critical appraisal tools
- Persis Katrak 1 ,
- Andrea E Bialocerkowski 2 ,
- Nicola Massy-Westropp 1 ,
- VS Saravana Kumar 1 &
- Karen A Grimmer 1
BMC Medical Research Methodology volume 4 , Article number: 22 ( 2004 ) Cite this article
155k Accesses
207 Citations
15 Altmetric
Metrics details
Consumers of research (researchers, administrators, educators and clinicians) frequently use standard critical appraisal tools to evaluate the quality of published research reports. However, there is no consensus regarding the most appropriate critical appraisal tool for allied health research. We summarized the content, intent, construction and psychometric properties of published, currently available critical appraisal tools to identify common elements and their relevance to allied health research.
A systematic review was undertaken of 121 published critical appraisal tools sourced from 108 papers located on electronic databases and the Internet. The tools were classified according to the study design for which they were intended. Their items were then classified into one of 12 criteria based on their intent. Commonly occurring items were identified. The empirical basis for construction of the tool, the method by which overall quality of the study was established, the psychometric properties of the critical appraisal tools and whether guidelines were provided for their use were also recorded.
Eighty-seven percent of critical appraisal tools were specific to a research design, with most tools having been developed for experimental studies. There was considerable variability in items contained in the critical appraisal tools. Twelve percent of available tools were developed using specified empirical research. Forty-nine percent of the critical appraisal tools summarized the quality appraisal into a numeric summary score. Few critical appraisal tools had documented evidence of validity of their items, or reliability of use. Guidelines regarding administration of the tools were provided in 43% of cases.
Conclusions
There was considerable variability in intent, components, construction and psychometric properties of published critical appraisal tools for research reports. There is no "gold standard' critical appraisal tool for any study design, nor is there any widely accepted generic tool that can be applied equally well across study types. No tool was specific to allied health research requirements. Thus interpretation of critical appraisal of research reports currently needs to be considered in light of the properties and intent of the critical appraisal tool chosen for the task.
Peer Review reports
Consumers of research (clinicians, researchers, educators, administrators) frequently use standard critical appraisal tools to evaluate the quality and utility of published research reports [ 1 ]. Critical appraisal tools provide analytical evaluations of the quality of the study, in particular the methods applied to minimise biases in a research project [ 2 ]. As these factors potentially influence study results, and the way that the study findings are interpreted, this information is vital for consumers of research to ascertain whether the results of the study can be believed, and transferred appropriately into other environments, such as policy, further research studies, education or clinical practice. Hence, choosing an appropriate critical appraisal tool is an important component of evidence-based practice.
Although the importance of critical appraisal tools has been acknowledged [ 1 , 3 – 5 ] there appears to be no consensus regarding the 'gold standard' tool for any medical evidence. In addition, it seems that consumers of research are faced with a large number of critical appraisal tools from which to choose. This is evidenced by the recent report by the Agency for Health Research Quality in which 93 critical appraisal tools for quantitative studies were identified [ 6 ]. Such choice may pose problems for research consumers, as dissimilar findings may well be the result when different critical appraisal tools are used to evaluate the same research report [ 6 ].
Critical appraisal tools can be broadly classified into those that are research design-specific and those that are generic. Design-specific tools contain items that address methodological issues that are unique to the research design [ 5 , 7 ]. This precludes comparison however of the quality of different study designs [ 8 ]. To attempt to overcome this limitation, generic critical appraisal tools have been developed, in an attempt to enhance the ability of research consumers to synthesise evidence from a range of quantitative and or qualitative study designs (for instance [ 9 ]). There is no evidence that generic critical appraisal tools and design-specific tools provide a comparative evaluation of research designs.
Moreover, there appears to be little consensus regarding the most appropriate items that should be contained within any critical appraisal tool. This paper is concerned primarily with critical appraisal tools that address the unique properties of allied health care and research [ 10 ]. This approach was taken because of the unique nature of allied health contacts with patients, and because evidence-based practice is an emerging area in allied health [ 10 ]. The availability of so many critical appraisal tools (for instance [ 6 ]) may well prove daunting for allied health practitioners who are learning to critically appraise research in their area of interest. For the purposes of this evaluation, allied health is defined as encompassing "...all occasions of service to non admitted patients where services are provided at units/clinics providing treatment/counseling to patients. These include units primarily concerned with physiotherapy, speech therapy, family panning, dietary advice, optometry occupational therapy..." [ 11 ].
The unique nature of allied health practice needs to be considered in allied health research. Allied health research thus differs from most medical research, with respect to:
• the paradigm underpinning comprehensive and clinically-reasoned descriptions of diagnosis (including validity and reliability). An example of this is in research into low back pain, where instead of diagnosis being made on location and chronicity of pain (as is common) [ 12 ], it would be made on the spinal structure and the nature of the dysfunction underpinning the symptoms, which is arrived at by a staged and replicable clinical reasoning process [ 10 , 13 ].
• the frequent use of multiple interventions within the one contact with the patient (an occasion of service), each of which requires appropriate description in terms of relationship to the diagnosis, nature, intensity, frequency, type of instruction provided to the patient, and the order in which the interventions were applied [ 13 ]
• the timeframe and frequency of contact with the patient (as many allied health disciplines treat patients in episodes of care that contain multiple occasions of service, and which can span many weeks, or even years in the case of chronic problems [ 14 ])
• measures of outcome, including appropriate methods and timeframes of measuring change in impairment, function, disability and handicap that address the needs of different stakeholders (patients, therapists, funders etc) [ 10 , 12 , 13 ].
Search strategy
In supplementary data [see additional file 1 ].
Data organization and extraction
Two independent researchers (PK, NMW) participated in all aspects of this review, and they compared and discussed their findings with respect to inclusion of critical appraisal tools, their intent, components, data extraction and item classification, construction and psychometric properties. Disagreements were resolved by discussion with a third member of the team (KG).
Data extraction consisted of a four-staged process. First, identical replica critical appraisal tools were identified and removed prior to analysis. The remaining critical appraisal tools were then classified according to the study design for which they were intended to be used [ 1 , 2 ]. The scientific manner in which the tools had been constructed was classified as whether an empirical research approach has been used, and if so, which type of research had been undertaken. Finally, the items contained in each critical appraisal tool were extracted and classified into one of eleven groups, which were based on the criteria described by Clarke and Oxman [ 4 ] as:
• Study aims and justification
• Methodology used , which encompassed method of identification of relevant studies and adherence to study protocol;
• Sample selection , which ranged from inclusion and exclusion criteria, to homogeneity of groups;
• Method of randomization and allocation blinding;
• Attrition : response and drop out rates;
• Blinding of the clinician, assessor, patient and statistician as well as the method of blinding;
• Outcome measure characteristics;
• Intervention or exposure details;
• Method of data analyses ;
• Potential sources of bias ; and
• Issues of external validity , which ranged from application of evidence to other settings to the relationship between benefits, cost and harm.
An additional group, " miscellaneous ", was used to describe items that could not be classified into any of the groups listed above.
Data synthesis
Data was synthesized using MS Excel spread sheets as well as narrative format by describing the number of critical appraisal tools per study design and the type of items they contained. Descriptions were made of the method by which the overall quality of the study was determined, evidence regarding the psychometric properties of the tools (validity and reliability) and whether guidelines were provided for use of the critical appraisal tool.
One hundred and ninety-three research reports that potentially provided a description of a critical appraisal tool (or process) were identified from the search strategy. Fifty-six of these papers were unavailable for review due to outdated Internet links, or inability to source the relevant journal through Australian university and Government library databases. Of the 127 papers retrieved, 19 were excluded from this review, as they did not provide a description of the critical appraisal tool used, or were published in languages other than English. As a result, 108 papers were reviewed, which yielded 121 different critical appraisal tools [ 1 – 5 , 7 , 9 , 15 – 102 , 116 ].
Empirical basis for tool construction
We identified 14 instruments (12% all tools) which were reported as having been constructed using a specified empirical approach [ 20 , 29 , 30 , 32 , 35 , 40 , 49 , 51 , 70 – 72 , 79 , 103 , 116 ]. The empirical research reflected descriptive and/or qualitative approaches, these being critical review of existing tools [ 40 , 72 ], Delphi techniques to identify then refine data items [ 32 , 51 , 71 ], questionnaires and other forms of written surveys to identify and refine data items [ 70 , 79 , 103 ], facilitated structured consensus meetings [ 20 , 29 , 30 , 35 , 40 , 49 , 70 , 72 , 79 , 116 ], and pilot validation testing [ 20 , 40 , 72 , 103 , 116 ]. In all the studies which reported developing critical appraisal tools using a consensus approach, a range of stakeholder input was sought, reflecting researchers and clinicians in a range of health disciplines, students, educators and consumers. There were a further 31 papers which cited other studies as the source of the tool used in the review, but which provided no information on why individual items had been chosen, or whether (or how) they had been modified. Moreover, for 21 of these tools, the cited sources of the critical appraisal tool did not report the empirical basis on which the tool had been constructed.
Critical appraisal tools per study design
Seventy-eight percent (N = 94) of the critical appraisal tools were developed for use on primary research [ 1 – 5 , 7 , 9 , 18 , 19 , 25 – 27 , 34 , 37 – 41 ], while the remainder (N = 26) were for secondary research (systematic reviews and meta-analyses) [ 2 – 5 , 15 – 36 , 116 ]. Eighty-seven percent (N = 104) of all critical appraisal tools were design-specific [ 2 – 5 , 7 , 9 , 15 – 90 ], with over one third (N = 45) developed for experimental studies (randomized controlled trials, clinical trials) [ 2 – 4 , 25 – 27 , 34 , 37 – 73 ]. Sixteen critical appraisal tools were generic. Of these, six were developed for use on both experimental and observational studies [ 9 , 91 – 95 ], whereas 11 were purported to be useful for any qualitative and quantitative research design [ 1 , 18 , 41 , 96 – 102 , 116 ] (see Figure 1 , Table 1 ).

Number of critical appraisal tools per study design [1,2]
Critical appraisal items
One thousand, four hundred and seventy five items were extracted from these critical appraisal tools. After grouping like items together, 173 different item types were identified, with the most frequently reported items being focused towards assessing the external validity of the study (N = 35) and method of data analyses (N = 28) (Table 2 ). The most frequently reported items across all critical appraisal tools were:
Eligibility criteria (inclusion/exclusion criteria) (N = 63)
Appropriate statistical analyses (N = 47)
Random allocation of subjects (N = 43)
Consideration of outcome measures used (N = 43)
Sample size justification/power calculations (N = 39)
Study design reported (N = 36)
Assessor blinding (N = 36)
Design-specific critical appraisal tools
Systematic reviews.
Eighty-seven different items were extracted from the 26 critical appraisal tools, which were designed to evaluate the quality of systematic reviews. These critical appraisal tools frequently contained items regarding data analyses and issues of external validity (Tables 2 and 3 ).
Items assessing data analyses were focused to the methods used to summarize the results, assessment of sensitivity of results and whether heterogeneity was considered, whereas the nature of reporting of the main results, interpretation of them and their generalizability were frequently used to assess the external validity of the study findings. Moreover, systematic review critical appraisal tools tended to contain items such as identification of relevant studies, search strategy used, number of studies included and protocol adherence, that would not be relevant for other study designs. Blinding and randomisation procedures were rarely included in these critical appraisal tools.
Experimental studies
One hundred and twenty thirteen different items were extracted from the 45 experimental critical appraisal tools. These items most frequently assessed aspects of data analyses and blinding (Tables 1 and 2 ). Data analyses items were focused on whether appropriate statistical analysis was performed, whether a sample size justification or power calculation was provided and whether side effects of the intervention were recorded and analysed. Blinding was focused on whether the participant, clinician and assessor were blinded to the intervention.
Diagnostic studies
Forty-seven different items were extracted from the seven diagnostic critical appraisal tools. These items frequently addressed issues involving data analyses, external validity of results and sample selection that were specific to diagnostic studies (whether the diagnostic criteria were defined, definition of the "gold" standard, the calculation of sensitivity and specificity) (Tables 1 and 2 ).
Observational studies
Seventy-four different items were extracted from the 19 critical appraisal tools for observational studies. These items primarily focused on aspects of data analyses (see Tables 1 and 2 , such as whether confounders were considered in the analysis, whether a sample size justification or power calculation was provided and whether appropriate statistical analyses were preformed.
Qualitative studies
Thirty-six different items were extracted from the seven qualitative study critical appraisal tools. The majority of these items assessed issues regarding external validity, methods of data analyses and the aims and justification of the study (Tables 1 and 2 ). Specifically, items were focused to whether the study question was clearly stated, whether data analyses were clearly described and appropriate, and application of the study findings to the clinical setting. Qualitative critical appraisal tools did not contain items regarding sample selection, randomization, blinding, intervention or bias, perhaps because these issues are not relevant to the qualitative paradigm.
Generic critical appraisal tools
Experimental and observational studies.
Forty-two different items were extracted from the six critical appraisal tools that could be used to evaluate experimental and observational studies. These tools most frequently contained items that addressed aspects of sample selection (such as inclusion/exclusion criteria of participants, homogeneity of participants at baseline) and data analyses (such as whether appropriate statistical analyses were performed, whether a justification of the sample size or power calculation were provided).
All study designs
Seventy-eight different items were contained in the ten critical appraisal tools that could be used for all study designs (quantitative and qualitative). The majority of these items focused on whether appropriate data analyses were undertaken (such as whether confounders were considered in the analysis, whether a sample size justification or power calculation was provided and whether appropriate statistical analyses were preformed) and external validity issues (generalization of results to the population, value of the research findings) (see Tables 1 and 2 ).
Allied health critical appraisal tools
We found no critical appraisal instrument specific to allied health research, despite finding at least seven critical appraisal instruments associated with allied health topics (mostly physiotherapy management of orthopedic conditions) [ 37 , 39 , 52 , 58 , 59 , 65 ]. One critical appraisal development group proposed two instruments [ 9 ], specific to quantitative and qualitative research respectively. The core elements of allied health research quality (specific diagnosis criteria, intervention descriptions, nature of patient contact and appropriate outcome measures) were not addressed in any one tool sourced for this evaluation. We identified 152 different ways of considering quality reporting of outcome measures in the 121 critical appraisal tools, and 81 ways of considering description of interventions. Very few tools which were not specifically targeted to diagnostic studies (less than 10% of the remaining tools) addressed diagnostic criteria. The critical appraisal instrument that seemed most related to allied health research quality [ 39 ] sought comprehensive evaluation of elements of intervention and outcome, however this instrument was relevant only to physiotherapeutic orthopedic experimental research.
Overall study quality
Forty-nine percent (N = 58) of critical appraisal tools summarised the results of the quality appraisal into a single numeric summary score [ 5 , 7 , 15 – 25 , 37 – 59 , 74 – 77 , 80 – 83 , 87 , 91 – 93 , 96 , 97 ] (Figure 2 ). This was achieved by one of two methods:
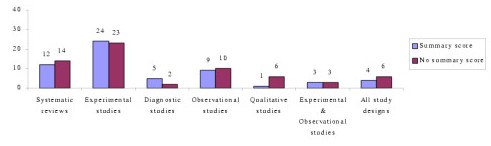
Number of critical appraisal tools with, and without, summary quality scores
An equal weighting system, where one point was allocated to each item fulfilled; or
A weighted system, where fulfilled items were allocated various points depending on their perceived importance.
However, there was no justification provided for any of the scoring systems used. In the remaining critical appraisal tools (N = 62), a single numerical summary score was not provided [ 1 – 4 , 9 , 25 – 36 , 60 – 73 , 78 , 79 , 84 – 90 , 94 , 95 , 98 – 102 ]. This left the research consumer to summarize the results of the appraisal in a narrative manner, without the assistance of a standard approach.
Psychometric properties of critical appraisal tools
Few critical appraisal tools had documented evidence of their validity and reliability. Face validity was established in nine critical appraisal tools, seven of which were developed for use on experimental studies [ 38 , 40 , 45 , 49 , 51 , 63 , 70 ] and two for systematic reviews [ 32 , 103 ]. Intra-rater reliability was established for only one critical appraisal tool as part of its empirical development process [ 40 ], whereas inter-rater reliability was reported for two systematic review tools [ 20 , 36 ] (for one of these as part of the developmental process [ 20 ]) and seven experimental critical appraisal tools [ 38 , 40 , 45 , 51 , 55 , 56 , 63 ] (for two of these as part of the developmental process [ 40 , 51 ]).
Critical appraisal tool guidelines
Forty-three percent (N = 52) of critical appraisal tools had guidelines that informed the user of the interpretation of each item contained within them (Table 2 ). These guidelines were most frequently in the form of a handbook or published paper (N = 31) [ 2 , 4 , 9 , 15 , 20 , 25 , 28 , 29 , 31 , 36 , 37 , 41 , 50 , 64 – 67 , 69 , 80 , 84 – 87 , 89 , 90 , 95 , 100 , 116 ], whereas in 14 critical appraisal tools explanations accompanied each item [ 16 , 26 , 27 , 40 , 49 , 51 , 57 , 59 , 79 , 83 , 91 , 102 ].
Our search strategy identified a large number of published critical appraisal tools that are currently available to critically appraise research reports. There was a distinct lack of information on tool development processes in most cases. Many of the tools were reported to be modifications of other published tools, or reflected specialty concerns in specific clinical or research areas, without attempts to justify inclusion criteria. Less than 10 of these tools were relevant to evaluation of the quality of allied health research, and none of these were based on an empirical research approach. We are concerned that although our search was systematic and extensive [ 104 , 105 ], our broad key words and our lack of ready access to 29% of potentially useful papers (N = 56) potentially constrained us from identifying all published critical appraisal tools. However, consumers of research seeking critical appraisal instruments are not likely to seek instruments from outdated Internet links and unobtainable journals, thus we believe that we identified the most readily available instruments. Thus, despite the limitations on sourcing all possible tools, we believe that this paper presents a useful synthesis of the readily available critical appraisal tools.
The majority of the critical appraisal tools were developed for a specific research design (87%), with most designed for use on experimental studies (38% of all critical appraisal tools sourced). This finding is not surprising as, according to the medical model, experimental studies sit at or near the top of the hierarchy of evidence [ 2 , 8 ]. In recent years, allied health researchers have strived to apply the medical model of research to their own discipline by conducting experimental research, often by using the randomized controlled trial design [ 106 ]. This trend may be the reason for the development of experimental critical appraisal tools reported in allied health-specific research topics [ 37 , 39 , 52 , 58 , 59 , 65 ].
We also found a considerable number of critical appraisal tools for systematic reviews (N = 26), which reflects the trend to synthesize research evidence to make it relevant for clinicians [ 105 , 107 ]. Systematic review critical appraisal tools contained unique items (such as identification of relevant studies, search strategy used, number of studies included, protocol adherence) compared with tools used for primary studies, a reflection of the secondary nature of data synthesis and analysis.
In contrast, we identified very few qualitative study critical appraisal tools, despite the presence of many journal-specific guidelines that outline important methodological aspects required in a manuscript submitted for publication [ 108 – 110 ]. This finding may reflect the more traditional, quantitative focus of allied health research [ 111 ]. Alternatively, qualitative researchers may view the robustness of their research findings in different terms compared with quantitative researchers [ 112 , 113 ]. Hence the use of critical appraisal tools may be less appropriate for the qualitative paradigm. This requires further consideration.
Of the small number of generic critical appraisal tools, we found few that could be usefully applied (to any health research, and specifically to the allied health literature), because of the generalist nature of their items, variable interpretation (and applicability) of items across research designs, and/or lack of summary scores. Whilst these types of tools potentially facilitate the synthesis of evidence across allied health research designs for clinicians, their lack of specificity in asking the 'hard' questions about research quality related to research design also potentially precludes their adoption for allied health evidence-based practice. At present, the gold standard study design when synthesizing evidence is the randomized controlled trial [ 4 ], which underpins our finding that experimental critical appraisal tools predominated in the allied health literature [ 37 , 39 , 52 , 58 , 59 , 65 ]. However, as more systematic literature reviews are undertaken on allied health topics, it may become more accepted that evidence in the form of other research design types requires acknowledgement, evaluation and synthesis. This may result in the development of more appropriate and clinically useful allied health critical appraisal tools.
A major finding of our study was the volume and variation in available critical appraisal tools. We found no gold standard critical appraisal tool for any type of study design. Therefore, consumers of research are faced with frustrating decisions when attempting to select the most appropriate tool for their needs. Variable quality evaluations may be produced when different critical appraisal tools are used on the same literature [ 6 ]. Thus, interpretation of critical analysis must be carefully considered in light of the critical appraisal tool used.
The variability in the content of critical appraisal tools could be accounted for by the lack of any empirical basis of tool construction, established validity of item construction, and the lack of a gold standard against which to compare new critical tools. As such, consumers of research cannot be certain that the content of published critical appraisal tools reflect the most important aspects of the quality of studies that they assess [ 114 ]. Moreover, there was little evidence of intra- or inter-rater reliability of the critical appraisal tools. Coupled with the lack of protocols for use, this may mean that critical appraisers could interpret instrument items in different ways over repeated occasions of use. This may produce variable results [123].
Based on the findings of this evaluation, we recommend that consumers of research should carefully select critical appraisal tools for their needs. The selected tools should have published evidence of the empirical basis for their construction, validity of items and reliability of interpretation, as well as guidelines for use, so that the tools can be applied and interpreted in a standardized manner. Our findings highlight the need for consensus to be reached regarding the important and core items for critical appraisal tools that will produce a more standardized environment for critical appraisal of research evidence. As a consequence, allied health research will specifically benefit from having critical appraisal tools that reflect best practice research approaches which embed specific research requirements of allied health disciplines.
National Health and Medical Research Council: How to Review the Evidence: Systematic Identification and Review of the Scientific Literature. Canberra. 2000
Google Scholar
National Health and Medical Research Council: How to Use the Evidence: Assessment and Application of Scientific Evidence. Canberra. 2000
Joanna Briggs Institute. [ http://www.joannabriggs.edu.au ]
Clarke M, Oxman AD: Cochrane Reviewer's Handbook 4.2.0. 2003, Oxford: The Cochrane Collaboration
Crombie IK: The Pocket Guide to Critical Appraisal: A Handbook for Health Care Professionals. 1996, London: BMJ Publishing Group
Agency for Healthcare Research and Quality: Systems to Rate the Strength of Scientific Evidence. Evidence Report/Technology Assessment No. 47, Publication No. 02-E016. Rockville. 2002
Elwood JM: Critical Appraisal of Epidemiological Studies and Clinical Trials. 1998, Oxford: Oxford University Press, 2
Sackett DL, Richardson WS, Rosenberg W, Haynes RB: Evidence Based Medicine. How to Practice and Teach EBM. 2000, London: Churchill Livingstone
Critical literature reviews. [ http://www.cotfcanada.org/cotf_critical.htm ]
Bialocerkowski AE, Grimmer KA, Milanese SF, Kumar S: Application of current research evidence to clinical physiotherapy practice. J Allied Health Res Dec.
The National Health Data Dictionary – Version 10. http://www.aihw.gov.au/publications/hwi/nhdd12/nhdd12-v1.pdf and http://www.aihw.gov.au/publications/hwi/nhdd12/nhdd12-v2.pdf
Grimmer K, Bowman P, Roper J: Episodes of allied health outpatient care: an investigation of service delivery in acute public hospital settings. Disability and Rehabilitation. 2000, 22 (1/2): 80-87.
CAS PubMed Google Scholar
Grimmer K, Milanese S, Bialocerkowski A: Clinical guidelines for low back pain: A physiotherapy perspective. Physiotherapy Canada. 2003, 55 (4): 1-9.
Grimmer KA, Milanese S, Bialocerkowski AE, Kumar S: Producing and implementing evidence in clinical practice: the therapies' dilemma. Physiotherapy. 2004,
Greenhalgh T: How to read a paper: papers that summarize other papers (systematic reviews and meta-analysis). BMJ. 1997, 315: 672-675.
CAS PubMed PubMed Central Google Scholar
Auperin A, Pignon J, Poynard T: Review article: critical review of meta-analysis of randomised clinical trials in hepatogastroenterology. Alimentary Pharmacol Therapeutics. 1997, 11: 215-225. 10.1046/j.1365-2036.1997.131302000.x.
CAS Google Scholar
Barnes DE, Bero LA: Why review articles on the health effects of passive smoking reach different conclusions. J Am Med Assoc. 1998, 279: 1566-1570. 10.1001/jama.279.19.1566.
Beck CT: Use of meta-analysis as a teaching strategy in nursing research courses. J Nurs Educat. 1997, 36: 87-90.
Carruthers SG, Larochelle P, Haynes RB, Petrasovits A, Schiffrin EL: Report of the Canadian Hypertension Society Consensus Conference: 1. Introduction. Can Med Assoc J. 1993, 149: 289-293.
Oxman AD, Guyatt GH, Singer J, Goldsmith CH, Hutchinson BG, Milner RA, Streiner DL: Agreement among reviewers of review articles. J Clin Epidemiol. 1991, 44: 91-98. 10.1016/0895-4356(91)90205-N.
Sacks HS, Reitman D, Pagano D, Kupelnick B: Meta-analysis: an update. Mount Sinai Journal of Medicine. 1996, 63: 216-224.
Smith AF: An analysis of review articles published in four anaesthesia journals. Can J Anaesth. 1997, 44: 405-409.
L'Abbe KA, Detsky AS, O'Rourke K: Meta-analysis in clinical research. Ann Intern Med. 1987, 107: 224-233.
PubMed Google Scholar
Mulrow CD, Antonio S: The medical review article: state of the science. Ann Intern Med. 1987, 106: 485-488.
Continuing Professional Development: A Manual for SIGN Guideline Developers. [ http://www.sign.ac.uk ]
Learning and Development Public Health Resources Unit. [ http://www.phru.nhs.uk/ ]
FOCUS Critical Appraisal Tool. [ http://www.focusproject.org.uk ]
Cook DJ, Sackett DL, Spitzer WO: Methodologic guidelines for systematic reviews of randomized control trials in health care from the Potsdam Consultation on meta-analysis. J Clin Epidemiol. 1995, 48: 167-171. 10.1016/0895-4356(94)00172-M.
Cranney A, Tugwell P, Shea B, Wells G: Implications of OMERACT outcomes in arthritis and osteoporosis for Cochrane metaanalysis. J Rheumatol. 1997, 24: 1206-1207.
Guyatt GH, Sackett DL, Sinclair JC, Hoyward R, Cook DJ, Cook RJ: User's guide to the medical literature. IX. A method for grading health care recommendations. J Am Med Assoc. 1995, 274: 1800-1804. 10.1001/jama.274.22.1800.
Gyorkos TW, Tannenbaum TN, Abrahamowicz M, Oxman AD, Scott EAF, Milson ME, Rasooli Iris, Frank JW, Riben PD, Mathias RG: An approach to the development of practice guidelines for community health interventions. Can J Public Health. 1994, 85: S8-13.
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF: Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999, 354: 1896-1900. 10.1016/S0140-6736(99)04149-5.
Oxman AD, Cook DJ, Guyatt GH: Users' guides to the medical literature. VI. How to use an overview. Evidence-Based Medicine Working Group. J Am Med Assoc. 1994, 272: 1367-1371. 10.1001/jama.272.17.1367.
Pogue J, Yusuf S: Overcoming the limitations of current meta-analysis of randomised controlled trials. Lancet. 1998, 351: 47-52. 10.1016/S0140-6736(97)08461-4.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB: Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. J Am Med Assoc. 2000, 283: 2008-2012. 10.1001/jama.283.15.2008.
Irwig L, Tosteson AN, Gatsonis C, Lau J, Colditz G, Chalmers TC, Mostellar F: Guidelines for meta-analyses evaluating diagnostic tests. Ann Intern Med. 1994, 120: 667-676.
Moseley AM, Herbert RD, Sherrington C, Maher CG: Evidence for physiotherapy practice: A survey of the Physiotherapy Evidence Database. Physiotherapy Evidence Database (PEDro). Australian Journal of Physiotherapy. 2002, 48: 43-50.
Cho MK, Bero LA: Instruments for assessing the quality of drug studies published in the medical literature. J Am Med Assoc. 1994, 272: 101-104. 10.1001/jama.272.2.101.
De Vet HCW, De Bie RA, Van der Heijden GJ, Verhagen AP, Sijpkes P, Kipschild PG: Systematic reviews on the basis of methodological criteria. Physiotherapy. 1997, 83: 284-289.
Downs SH, Black N: The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998, 52: 377-384.
Evans M, Pollock AV: A score system for evaluating random control clinical trials of prophylaxis of abdominal surgical wound infection. Br J Surg. 1985, 72: 256-260.
Fahey T, Hyde C, Milne R, Thorogood M: The type and quality of randomized controlled trials (RCTs) published in UK public health journals. J Public Health Med. 1995, 17: 469-474.
Gotzsche PC: Methodology and overt and hidden bias in reports of 196 double-blind trials of nonsteroidal antiinflammatory drugs in rheumatoid arthritis. Control Clin Trials. 1989, 10: 31-56. 10.1016/0197-2456(89)90017-2.
Imperiale TF, McCullough AJ: Do corticosteroids reduce mortality from alcoholic hepatitis? A meta-analysis of the randomized trials. Ann Int Med. 1990, 113: 299-307.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ: Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Control Clin Trials. 1996, 17: 1-12. 10.1016/0197-2456(95)00134-4.
Khan KS, Daya S, Collins JA, Walter SD: Empirical evidence of bias in infertility research: overestimation of treatment effect in crossover trials using pregnancy as the outcome measure. Fertil Steril. 1996, 65: 939-945.
Kleijnen J, Knipschild P, ter Riet G: Clinical trials of homoeopathy. BMJ. 1991, 302: 316-323.
Liberati A, Himel HN, Chalmers TC: A quality assessment of randomized control trials of primary treatment of breast cancer. J Clin Oncol. 1986, 4: 942-951.
Moher D, Schulz KF, Altman DG, for the CONSORT Group: The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. J Am Med Assoc. 2001, 285: 1987-1991. 10.1001/jama.285.15.1987.
Reisch JS, Tyson JE, Mize SG: Aid to the evaluation of therapeutic studies. Pediatrics. 1989, 84: 815-827.
Sindhu F, Carpenter L, Seers K: Development of a tool to rate the quality assessment of randomized controlled trials using a Delphi technique. J Advanced Nurs. 1997, 25: 1262-1268. 10.1046/j.1365-2648.1997.19970251262.x.
Van der Heijden GJ, Van der Windt DA, Kleijnen J, Koes BW, Bouter LM: Steroid injections for shoulder disorders: a systematic review of randomized clinical trials. Br J Gen Pract. 1996, 46: 309-316.
Van Tulder MW, Koes BW, Bouter LM: Conservative treatment of acute and chronic nonspecific low back pain. A systematic review of randomized controlled trials of the most common interventions. Spine. 1997, 22: 2128-2156. 10.1097/00007632-199709150-00012.
Garbutt JC, West SL, Carey TS, Lohr KN, Crews FT: Pharmacotherapy for Alcohol Dependence. Evidence Report/Technology Assessment No. 3, AHCPR Publication No. 99-E004. Rockville. 1999
Oremus M, Wolfson C, Perrault A, Demers L, Momoli F, Moride Y: Interarter reliability of the modified Jadad quality scale for systematic reviews of Alzheimer's disease drug trials. Dement Geriatr Cognit Disord. 2001, 12: 232-236. 10.1159/000051263.
Clark O, Castro AA, Filho JV, Djubelgovic B: Interrater agreement of Jadad's scale. Annual Cochrane Colloqium Abstracts. 2001, [ http://www.biomedcentral.com/abstracts/COCHRANE/1/op031 ]October Lyon
Jonas W, Anderson RL, Crawford CC, Lyons JS: A systematic review of the quality of homeopathic clinical trials. BMC Alternative Medicine. 2001, 1: 12-10.1186/1472-6882-1-12.
Van Tulder M, Malmivaara A, Esmail R, Koes B: Exercises therapy for low back pain: a systematic review within the framework of the Cochrane Collaboration back review group. Spine. 2000, 25: 2784-2796. 10.1097/00007632-200011010-00011.
Van Tulder MW, Ostelo R, Vlaeyen JWS, Linton SJ, Morley SJ, Assendelft WJJ: Behavioral treatment for chronic low back pain: a systematic review within the framework of the cochrane back. Spine. 2000, 25: 2688-2699. 10.1097/00007632-200010150-00024.
Aronson N, Seidenfeld J, Samson DJ, Aronson N, Albertson PC, Bayoumi AM, Bennett C, Brown A, Garber ABA, Gere M, Hasselblad V, Wilt T, Ziegler MPHK, Pharm D: Relative Effectiveness and Cost Effectiveness of Methods of Androgen Suppression in the Treatment of Advanced Prostate Cancer. Evidence Report/Technology Assessment No. 4, AHCPR Publication No.99-E0012. Rockville. 1999
Chalmers TC, Smith H, Blackburn B, Silverman B, Schroeder B, Reitman D, Ambroz A: A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981, 2: 31-49. 10.1016/0197-2456(81)90056-8.
der Simonian R, Charette LJ, McPeek B, Mosteller F: Reporting on methods in clinical trials. New Eng J Med. 1982, 306: 1332-1337.
Detsky AS, Naylor CD, O'Rourke K, McGeer AJ, L'Abbe KA: Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol. 1992, 45: 255-265. 10.1016/0895-4356(92)90085-2.
Goudas L, Carr DB, Bloch R, Balk E, Ioannidis JPA, Terrin MN: Management of Cancer Pain. Evidence Report/Technology Assessment No. 35 (Contract 290-97-0019 to the New England Medical Center), AHCPR Publication No. 99-E004. Rockville. 2000
Guyatt GH, Sackett DL, Cook DJ: Users' guides to the medical literature. II. How to use an article about therapy or prevention. A. Are the results of the study valid? Evidence-Based Medicine Working Group. J Am Med Assoc. 1993, 270: 2598-2601. 10.1001/jama.270.21.2598.
Khan KS, Ter Riet G, Glanville J, Sowden AJ, Kleijnen J: Undertaking Systematic Reviews of Research on Effectiveness: Centre of Reviews and Dissemination's Guidance for Carrying Out or Commissioning Reviews: York. 2000
McNamara R, Bass EB, Marlene R, Miller J: Management of New Onset Atrial Fibrillation. Evidence Report/Technology Assessment No.12, AHRQ Publication No. 01-E026. Rockville. 2001
Prendiville W, Elbourne D, Chalmers I: The effects of routine oxytocic administration in the management of the third stage of labour: an overview of the evidence from controlled trials. Br J Obstet Gynae Col. 1988, 95: 3-16.
Schulz KF, Chalmers I, Hayes RJ, Altman DG: Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. J Am Med Assoc. 1995, 273: 408-412. 10.1001/jama.273.5.408.
The Standards of Reporting Trials Group: A proposal for structured reporting of randomized controlled trials. J Am Med Assoc. 1994, 272: 1926-1931. 10.1001/jama.272.24.1926.
Verhagen AP, de Vet HC, de Bie RA, Kessels AGH, Boers M, Bouter LM, Knipschild PG: The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998, 51: 1235-1241. 10.1016/S0895-4356(98)00131-0.
Zaza S, Wright-De Aguero LK, Briss PA, Truman BI, Hopkins DP, Hennessy MH, Sosin DM, Anderson L, Carande-Kullis VG, Teutsch SM, Pappaioanou M: Data collection instrument and procedure for systematic reviews in the guide to community preventive services. Task force on community preventive services. Am J Prevent Med. 2000, 18: 44-74. 10.1016/S0749-3797(99)00122-1.
Haynes BB, Wilczynski N, McKibbon A, Walker CJ, Sinclair J: Developing optimal search strategies for detecting clinically sound studies in MEDLINE. J Am Informatics Assoc. 1994, 1: 447-458.
Greenhalgh T: How to read a paper: papers that report diagnostic or screening tests. BMJ. 1997, 315: 540-543.
Arroll B, Schechter MT, Sheps SB: The assessment of diagnostic tests: a comparison of medical literature in 1982 and 1985. J Gen Int Med. 1988, 3: 443-447.
Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, van der Meulen JH, Bossuyt PM: Empirical evidence of design-related bias in studies of diagnostic tests. J Am Med Assoc. 1999, 282: 1061-1066. 10.1001/jama.282.11.1061.
Sheps SB, Schechter MT: The assessment of diagnostic tests. A survey of current medical research. J Am Med Assoc. 1984, 252: 2418-2422. 10.1001/jama.252.17.2418.
McCrory DC, Matchar DB, Bastian L, Dutta S, Hasselblad V, Hickey J, Myers MSE, Nanda K: Evaluation of Cervical Cytology. Evidence Report/Technology Assessment No. 5, AHCPR Publication No.99-E010. Rockville. 1999
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, DeVet HCW: Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Clin Chem. 2003, 49: 1-6. 10.1373/49.1.1.
Greenhalgh T: How to Read a Paper: Assessing the methodological quality of published papers. BMJ. 1997, 315: 305-308.
Angelillo I, Villari P: Residential exposure to electromagnetic fields and childhood leukaemia: a meta-analysis. Bull World Health Org. 1999, 77: 906-915.
Ariens G, Mechelen W, Bongers P, Bouter L, Van der Wal G: Physical risk factors for neck pain. Scand J Work Environ Health. 2000, 26: 7-19.
Hoogendoorn WE, van Poppel MN, Bongers PM, Koes BW, Bouter LM: Physical load during work and leisure time as risk factors for back pain. Scand J Work Environ Health. 1999, 25: 387-403.
Laupacis A, Wells G, Richardson WS, Tugwell P: Users' guides to the medical literature. V. How to use an article about prognosis. Evidence-Based Medicine Working Group. J Am Med Assoc. 1994, 272: 234-237. 10.1001/jama.272.3.234.
Levine M, Walter S, Lee H, Haines T, Holbrook A, Moyer V: Users' guides to the medical literature. IV. How to use an article about harm. Evidence-Based Medicine Working Group. J Am Med Assoc. 1994, 271: 1615-1619. 10.1001/jama.271.20.1615.
Carey TS, Boden SD: A critical guide to case series reports. Spine. 2003, 28: 1631-1634. 10.1097/00007632-200308010-00001.
Greenhalgh T, Taylor R: How to read a paper: papers that go beyond numbers (qualitative research). BMJ. 1997, 315: 740-743.
Hoddinott P, Pill R: A review of recently published qualitative research in general practice. More methodological questions than answers?. Fam Pract. 1997, 14: 313-319. 10.1093/fampra/14.4.313.
Mays N, Pope C: Quality research in health care: Assessing quality in qualitative research. BMJ. 2000, 320: 50-52. 10.1136/bmj.320.7226.50.
Mays N, Pope C: Rigour and qualitative research. BMJ. 1995, 311: 109-112.
Colditz GA, Miller JN, Mosteller F: How study design affects outcomes in comparisons of therapy. I: Medical. Stats Med. 1989, 8: 441-454.
Turlik MA, Kushner D: Levels of evidence of articles in podiatric medical journals. J Am Pod Med Assoc. 2000, 90: 300-302.
Borghouts JAJ, Koes BW, Bouter LM: The clinical course and prognostic factors of non-specific neck pain: a systematic review. Pain. 1998, 77: 1-13. 10.1016/S0304-3959(98)00058-X.
Spitzer WO, Lawrence V, Dales R, Hill G, Archer MC, Clark P, Abenhaim L, Hardy J, Sampalis J, Pinfold SP, Morgan PP: Links between passive smoking and disease: a best-evidence synthesis. A report of the working group on passive smoking. Clin Invest Med. 1990, 13: 17-46.
Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F: Systematic reviews of trials and other studies. Health Tech Assess. 1998, 2: 1-276.
Chestnut RM, Carney N, Maynard H, Patterson P, Mann NC, Helfand M: Rehabilitation for Traumatic Brain Injury. Evidence Report/Technology Assessment No. 2, Agency for Health Care Research and Quality Publication No. 99-E006. Rockville. 1999
Lohr KN, Carey TS: Assessing best evidence: issues in grading the quality of studies for systematic reviews. Joint Commission J Qual Improvement. 1999, 25: 470-479.
Greer N, Mosser G, Logan G, Halaas GW: A practical approach to evidence grading. Joint Commission J Qual Improvement. 2000, 26: 700-712.
Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, Atkins D: Current methods of the U.S. Preventive Services Task Force: a review of the process. Am J Prevent Med. 2001, 20: 21-35. 10.1016/S0749-3797(01)00261-6.
Anonymous: How to read clinical journals: IV. To determine etiology or causation. Can Med Assoc J. 1981, 124: 985-990.
Whitten PS, Mair FS, Haycox A, May CR, Williams TL, Hellmich S: Systematic review of cost effectiveness studies of telemedicine interventions. BMJ. 2002, 324: 1434-1437. 10.1136/bmj.324.7351.1434.
PubMed PubMed Central Google Scholar
Forrest JL, Miller SA: Evidence-based decision making in action: Part 2-evaluating and applying the clinical evidence. J Contemp Dental Pract. 2002, 4: 42-52.
Oxman AD, Guyatt GH: Validation of an index of the quality of review articles. J Clin Epidemiol. 1991, 44: 1271-1278. 10.1016/0895-4356(91)90160-B.
Jones T, Evans D: Conducting a systematic review. Aust Crit Care. 2000, 13: 66-71.
Papadopoulos M, Rheeder P: How to do a systematic literature review. South African J Physiother. 2000, 56: 3-6.
Selker LG: Clinical research in Allied Health. J Allied Health. 1994, 23: 201-228.
Stevens KR: Systematic reviews: the heart of evidence-based practice. AACN Clin Issues. 2001, 12: 529-538.
Devers KJ, Frankel RM: Getting qualitative research published. Ed Health. 2001, 14: 109-117. 10.1080/13576280010021888.
Canadian Journal of Public Health: Review guidelines for qualitative research papers submitted for consideration to the Canadian Journal of Public Health. Can J Pub Health. 2000, 91: I2-
Malterud K: Shared understanding of the qualitative research process: guidelines for the medical researcher. Fam Pract. 1993, 10: 201-206.
Higgs J, Titchen A: Research and knowledge. Physiotherapy. 1998, 84: 72-80.
Maggs-Rapport F: Best research practice: in pursuit of methodological rigour. J Advan Nurs. 2001, 35: 373-383. 10.1046/j.1365-2648.2001.01853.x.
Cutcliffe JR, McKenna HP: Establishing the credibility of qualitative research findings: the plot thickens. J Advan Nurs. 1999, 30: 374-380. 10.1046/j.1365-2648.1999.01090.x.
Andresen EM: Criteria for assessing the tools of disability outcomes research. Arch Phys Med Rehab. 2000, 81: S15-S20. 10.1053/apmr.2000.20619.
Beatie P: Measurement of health outcomes in the clinical setting: applications to physiotherapy. Phys Theory Pract. 2001, 17: 173-185. 10.1080/095939801317077632.
Charnock DF, (Ed): The DISCERN Handbook: Quality criteria for consumer health information on treatment choices. 1998, Radcliffe Medical Press
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2288/4/22/prepub
Download references
Author information
Authors and affiliations.
Centre for Allied Health Evidence: A Collaborating Centre of the Joanna Briggs Institute, City East Campus, University of South Australia, North Terrace, Adelaide, 5000, Australia
Persis Katrak, Nicola Massy-Westropp, VS Saravana Kumar & Karen A Grimmer
School of Physiotherapy, The University of Melbourne, Melbourne, 3010, Australia
Andrea E Bialocerkowski
You can also search for this author in PubMed Google Scholar

Corresponding author
Correspondence to Karen A Grimmer .
Additional information
Competing interests.
No competing interests.
Authors' contributions
PK Sourced critical appraisal tools
Categorized the content and psychometric properties of critical appraisal tools
AEB Synthesis of findings
Drafted manuscript
NMW Sourced critical appraisal tools
VSK Sourced critical appraisal tools
KAG Study conception and design
Assisted with critiquing critical appraisal tools and categorization of the content and psychometric properties of critical appraisal tools
Drafted and reviewed manuscript
Addressed reviewer's comments and re-submitted the article
Electronic supplementary material
Additional file 1: search strategy. (doc 30 kb), authors’ original submitted files for images.
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, authors’ original file for figure 3, authors’ original file for figure 4, authors’ original file for figure 5, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Katrak, P., Bialocerkowski, A.E., Massy-Westropp, N. et al. A systematic review of the content of critical appraisal tools. BMC Med Res Methodol 4 , 22 (2004). https://doi.org/10.1186/1471-2288-4-22
Download citation
Received : 10 May 2004
Accepted : 16 September 2004
Published : 16 September 2004
DOI : https://doi.org/10.1186/1471-2288-4-22
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Research Consumer
- Empirical Basis
- Ally Health Literature
- Critical Appraisal Tool
- Sample Size Justification
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]

Critical Appraisal : Critical appraisal full list of checklists and tools
- What is critical appraisal?
- Where to start
- Education and childhood studies
- Occupational Therapy
- Physiotherapy
- Interpreting statistics
- Further reading and resources
Which checklist or tool should I use?
There are hundreds of critical appraisal checklists and tools you can choose from, which can be very overwhelming. There are so many because there are many kinds of research, knowledge can be communicated in a wide range of ways, and whether something is appropriate to meet your information needs depends on your specific context.
We have asked for recommendations from lecturers in different academic departments, to give you an idea about which checklists and tools may be the most relevant for you. Please hover over the drop-down menu at the top of the page, underneath 'Critical appraisal checklists and tools' to view the individual subject pages.
Below are lists of as many critical appraisal tools and checklists as we have been able to find. These are split into health sciences and social sciences because the two areas tend to take different approaches to evaluation, for various reasons!
To see a selection of checklists more suitable for your subject, hover over the top tab of this page.
Critical appraisal checklists and tools for Health Sciences
- AACODS Checklist for appraising grey literature
- AMSTAR 2 critical appraisal tool for systematic reviews that include randomised and non-randomised studies of healthcare interventions or both
- AOTA Critically Appraised Papers American Occupational Therapy Association
- Bandolier - "Evidence based thinking about healthcare"
- BestBETS critical appraisal worksheet
- BMJ critical appraisal checklists
- CASP Critical Appraisal Skills Programme includes checklists for case control studies, clinical prediction rule, cohort studies, diagnostic studies, economic evaluation, qualitative studies, RCTs and systematic reviews
- Centre for Evidence Based Medicine (Oxford) Critical Appraisal Tools CEBM's worksheets to assess systematic reviews, diagnostic, prognosis, and RCTs
- Centre for Evidence Based Medicine (Oxford) CATmaker and EBM calculator CEBM's computer assisted critical appraisal tool CATmaker
- CEMB critical appraisal sheets (Centre for Evidence Based Medicine)
- Cochrane Assessing Risk of Bias in a Randomized Trial
- Critical appraisal: a checklist from Students for Best Evidence S4BE (student network with simple explanations of difficult concepts)
- Critical appraisal and statistical skills (Knowledge for Healthcare)
- Critical appraisal of clinical trials from Testing Treatments International
- Critical appraisal of clinical trials (Medicines Learning Portal)
- Critical appraisal of quantitative research
- Critical appraisal of a quantitative paper from Teeside University
- Critical appraisal of a qualitative paper from Teeside University
- Critical appraisal tools from the Centre for Evidence-Based Medicine
- Critical Evaluation of Research Papers – Qualitative Studies from Teeside University
- Critical Evaluation of Research Papers – RCTs/Experimental Studies from Teeside University
- Evaluation tool for mixed methods study designs
- GRADE - The Grading of Recommendations Assessment, Development and Evaluation working group guidelines and publications for grading the quality of evidence in healthcare research and policy
- HCPRDU Evaluation Tool for Mixed Methods Studies - University of Salford Health Care Practice R&D Unit
- HCPRDU Evaluation Tool for Qualitative Studies - University of Salford Health Care Practice R&D Unit
- HCPRDU Evaluation Tool for Quantitative Studies - University of Salford Health Care Practice R&D Unit
- JBI Joanna Briggs Institute critical appraisal tools checklists for Analytical cross sectional studies, case control studies, case reports, case series, cohort studies, diagnostic test accuracy, economic evaluations, prevalence studies, qualitative research, quasi-experimental (non-randomised) studies, RCTs, systematic reviews and for text and opinion
- Knowledge Translation Program - Toronto based KTP critical appraisal worksheets for systematic reviews, prognosis, diagnosis, harm and therapy
- MATT Mixed Methods Appraisal Tool
- McMaster University Evidence Based Practice Research Group quantitative and qualitative review forms
- NHLBI (National Heart, Blood and lung Institute) study quality assessment tools for case control studies, case series, controlled intervention, observational cohort and cross sectional studies, before-after (pre-post) studies with no control group, systematic reviews and meta analyses
- NICE Guidelines, The Manual Appendix H. pp9-24
- QUADAS-2 tool for evaluating risk of bias in systematic reviews from the University of Bristol
- PEDro PEDro (Physiotherapy Evidence Database) Scale - appraisal resources including a tutorial and appraisal tool
- RoB 2 A revised Cochrane risk-of-bias tool for randomized trials
- ROBINS-I Risk Of Bias In Non-Randomized Studies of Interventions
- ROBIS Risk of Bias in Systematic Reviews
- ROB-ME A tool for assessing Risk Of Bias due to Missing Evidence in a synthesis
- SIGN - Critical appraisal notes and checklists for case control studies, cohort studies, diagnostic studies, economic studies, RCTs, meta-analyses and systematic reviews
- Strength of Recommendation Taxonomy - the SORT scale for quality, quantity and consistency of evidence in individual studies or bodies of evidence
- STROBE (Strengthening the Reporting of Observational studies in Epidemiology) for cohort, case-control, and cross-sectional studies (combined), cohort, case-control, cross-sectional studies and conference abstracts
- SURE Case Controlled Studies Critical Appraisal checklist
- SURE Case Series Studies Critical Appraisal checklist
- SURE Cohort Studies Critical Appraisal checklist
- SURE Cross-sectional Studies Critical Appraisal checklist
- SURE Experimental Studies Critical Appraisal checklist
- SURE Qualitative Studies Critical Appraisal checklist
- SURE Systematic Review Critical Appraisal checklist
Critical appraisal checklists and tools for Social Sciences
- AACODS Checklist for appraising grey literature
- CRAAP test to evaluate sources of information
- Critical Appraisal of an Article on an Educational Intervention (variable study design) from the University of Glasgow
- Educational Interventions Critical Appraisal worksheet from BestBETs
- PROMPT from Open University
- PROVEN - tool to evaluate any source of information
SIFT (The Four Moves) to help students distinguish between truth and fake news
Some Guidelines for the Critical Reviewing of Conceptual Papers
- << Previous: Where to start
- Next: Business >>
- Last Updated: Apr 1, 2024 11:00 AM
- URL: https://libguides.qmu.ac.uk/critical-appraisal
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Med Res Methodol

A systematic review of the content of critical appraisal tools
Persis katrak.
1 Centre for Allied Health Evidence: A Collaborating Centre of the Joanna Briggs Institute, City East Campus, University of South Australia, North Terrace, Adelaide, 5000, Australia
Andrea E Bialocerkowski
2 School of Physiotherapy, The University of Melbourne, Melbourne, 3010, Australia
Nicola Massy-Westropp
Vs saravana kumar, karen a grimmer.
This is an open-access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Associated Data
Consumers of research (researchers, administrators, educators and clinicians) frequently use standard critical appraisal tools to evaluate the quality of published research reports. However, there is no consensus regarding the most appropriate critical appraisal tool for allied health research. We summarized the content, intent, construction and psychometric properties of published, currently available critical appraisal tools to identify common elements and their relevance to allied health research.
A systematic review was undertaken of 121 published critical appraisal tools sourced from 108 papers located on electronic databases and the Internet. The tools were classified according to the study design for which they were intended. Their items were then classified into one of 12 criteria based on their intent. Commonly occurring items were identified. The empirical basis for construction of the tool, the method by which overall quality of the study was established, the psychometric properties of the critical appraisal tools and whether guidelines were provided for their use were also recorded.
Eighty-seven percent of critical appraisal tools were specific to a research design, with most tools having been developed for experimental studies. There was considerable variability in items contained in the critical appraisal tools. Twelve percent of available tools were developed using specified empirical research. Forty-nine percent of the critical appraisal tools summarized the quality appraisal into a numeric summary score. Few critical appraisal tools had documented evidence of validity of their items, or reliability of use. Guidelines regarding administration of the tools were provided in 43% of cases.
Conclusions
There was considerable variability in intent, components, construction and psychometric properties of published critical appraisal tools for research reports. There is no "gold standard' critical appraisal tool for any study design, nor is there any widely accepted generic tool that can be applied equally well across study types. No tool was specific to allied health research requirements. Thus interpretation of critical appraisal of research reports currently needs to be considered in light of the properties and intent of the critical appraisal tool chosen for the task.
Consumers of research (clinicians, researchers, educators, administrators) frequently use standard critical appraisal tools to evaluate the quality and utility of published research reports [ 1 ]. Critical appraisal tools provide analytical evaluations of the quality of the study, in particular the methods applied to minimise biases in a research project [ 2 ]. As these factors potentially influence study results, and the way that the study findings are interpreted, this information is vital for consumers of research to ascertain whether the results of the study can be believed, and transferred appropriately into other environments, such as policy, further research studies, education or clinical practice. Hence, choosing an appropriate critical appraisal tool is an important component of evidence-based practice.
Although the importance of critical appraisal tools has been acknowledged [ 1 , 3 - 5 ] there appears to be no consensus regarding the 'gold standard' tool for any medical evidence. In addition, it seems that consumers of research are faced with a large number of critical appraisal tools from which to choose. This is evidenced by the recent report by the Agency for Health Research Quality in which 93 critical appraisal tools for quantitative studies were identified [ 6 ]. Such choice may pose problems for research consumers, as dissimilar findings may well be the result when different critical appraisal tools are used to evaluate the same research report [ 6 ].
Critical appraisal tools can be broadly classified into those that are research design-specific and those that are generic. Design-specific tools contain items that address methodological issues that are unique to the research design [ 5 , 7 ]. This precludes comparison however of the quality of different study designs [ 8 ]. To attempt to overcome this limitation, generic critical appraisal tools have been developed, in an attempt to enhance the ability of research consumers to synthesise evidence from a range of quantitative and or qualitative study designs (for instance [ 9 ]). There is no evidence that generic critical appraisal tools and design-specific tools provide a comparative evaluation of research designs.
Moreover, there appears to be little consensus regarding the most appropriate items that should be contained within any critical appraisal tool. This paper is concerned primarily with critical appraisal tools that address the unique properties of allied health care and research [ 10 ]. This approach was taken because of the unique nature of allied health contacts with patients, and because evidence-based practice is an emerging area in allied health [ 10 ]. The availability of so many critical appraisal tools (for instance [ 6 ]) may well prove daunting for allied health practitioners who are learning to critically appraise research in their area of interest. For the purposes of this evaluation, allied health is defined as encompassing "...all occasions of service to non admitted patients where services are provided at units/clinics providing treatment/counseling to patients. These include units primarily concerned with physiotherapy, speech therapy, family panning, dietary advice, optometry occupational therapy..." [ 11 ].
The unique nature of allied health practice needs to be considered in allied health research. Allied health research thus differs from most medical research, with respect to:
• the paradigm underpinning comprehensive and clinically-reasoned descriptions of diagnosis (including validity and reliability). An example of this is in research into low back pain, where instead of diagnosis being made on location and chronicity of pain (as is common) [ 12 ], it would be made on the spinal structure and the nature of the dysfunction underpinning the symptoms, which is arrived at by a staged and replicable clinical reasoning process [ 10 , 13 ].
• the frequent use of multiple interventions within the one contact with the patient (an occasion of service), each of which requires appropriate description in terms of relationship to the diagnosis, nature, intensity, frequency, type of instruction provided to the patient, and the order in which the interventions were applied [ 13 ]
• the timeframe and frequency of contact with the patient (as many allied health disciplines treat patients in episodes of care that contain multiple occasions of service, and which can span many weeks, or even years in the case of chronic problems [ 14 ])
• measures of outcome, including appropriate methods and timeframes of measuring change in impairment, function, disability and handicap that address the needs of different stakeholders (patients, therapists, funders etc) [ 10 , 12 , 13 ].
Search strategy
In supplementary data [see additional file 1 ].
Data organization and extraction
Two independent researchers (PK, NMW) participated in all aspects of this review, and they compared and discussed their findings with respect to inclusion of critical appraisal tools, their intent, components, data extraction and item classification, construction and psychometric properties. Disagreements were resolved by discussion with a third member of the team (KG).
Data extraction consisted of a four-staged process. First, identical replica critical appraisal tools were identified and removed prior to analysis. The remaining critical appraisal tools were then classified according to the study design for which they were intended to be used [ 1 , 2 ]. The scientific manner in which the tools had been constructed was classified as whether an empirical research approach has been used, and if so, which type of research had been undertaken. Finally, the items contained in each critical appraisal tool were extracted and classified into one of eleven groups, which were based on the criteria described by Clarke and Oxman [ 4 ] as:
• Study aims and justification
• Methodology used , which encompassed method of identification of relevant studies and adherence to study protocol;
• Sample selection , which ranged from inclusion and exclusion criteria, to homogeneity of groups;
• Method of randomization and allocation blinding;
• Attrition : response and drop out rates;
• Blinding of the clinician, assessor, patient and statistician as well as the method of blinding;
• Outcome measure characteristics;
• Intervention or exposure details;
• Method of data analyses ;
• Potential sources of bias ; and
• Issues of external validity , which ranged from application of evidence to other settings to the relationship between benefits, cost and harm.
An additional group, " miscellaneous ", was used to describe items that could not be classified into any of the groups listed above.
Data synthesis
Data was synthesized using MS Excel spread sheets as well as narrative format by describing the number of critical appraisal tools per study design and the type of items they contained. Descriptions were made of the method by which the overall quality of the study was determined, evidence regarding the psychometric properties of the tools (validity and reliability) and whether guidelines were provided for use of the critical appraisal tool.
One hundred and ninety-three research reports that potentially provided a description of a critical appraisal tool (or process) were identified from the search strategy. Fifty-six of these papers were unavailable for review due to outdated Internet links, or inability to source the relevant journal through Australian university and Government library databases. Of the 127 papers retrieved, 19 were excluded from this review, as they did not provide a description of the critical appraisal tool used, or were published in languages other than English. As a result, 108 papers were reviewed, which yielded 121 different critical appraisal tools [ 1 - 5 , 7 , 9 , 15 - 102 , 116 ].
Empirical basis for tool construction
We identified 14 instruments (12% all tools) which were reported as having been constructed using a specified empirical approach [ 20 , 29 , 30 , 32 , 35 , 40 , 49 , 51 , 70 - 72 , 79 , 103 , 116 ]. The empirical research reflected descriptive and/or qualitative approaches, these being critical review of existing tools [ 40 , 72 ], Delphi techniques to identify then refine data items [ 32 , 51 , 71 ], questionnaires and other forms of written surveys to identify and refine data items [ 70 , 79 , 103 ], facilitated structured consensus meetings [ 20 , 29 , 30 , 35 , 40 , 49 , 70 , 72 , 79 , 116 ], and pilot validation testing [ 20 , 40 , 72 , 103 , 116 ]. In all the studies which reported developing critical appraisal tools using a consensus approach, a range of stakeholder input was sought, reflecting researchers and clinicians in a range of health disciplines, students, educators and consumers. There were a further 31 papers which cited other studies as the source of the tool used in the review, but which provided no information on why individual items had been chosen, or whether (or how) they had been modified. Moreover, for 21 of these tools, the cited sources of the critical appraisal tool did not report the empirical basis on which the tool had been constructed.
Critical appraisal tools per study design
Seventy-eight percent (N = 94) of the critical appraisal tools were developed for use on primary research [ 1 - 5 , 7 , 9 , 18 , 19 , 25 - 27 , 34 , 37 - 41 ], while the remainder (N = 26) were for secondary research (systematic reviews and meta-analyses) [ 2 - 5 , 15 - 36 , 116 ]. Eighty-seven percent (N = 104) of all critical appraisal tools were design-specific [ 2 - 5 , 7 , 9 , 15 - 90 ], with over one third (N = 45) developed for experimental studies (randomized controlled trials, clinical trials) [ 2 - 4 , 25 - 27 , 34 , 37 - 73 ]. Sixteen critical appraisal tools were generic. Of these, six were developed for use on both experimental and observational studies [ 9 , 91 - 95 ], whereas 11 were purported to be useful for any qualitative and quantitative research design [ 1 , 18 , 41 , 96 - 102 , 116 ] (see Figure Figure1, 1 , Table Table1 1 ).

Number of critical appraisal tools per study design [1,2]
Summary of tools sourced in this review.
Critical appraisal items
One thousand, four hundred and seventy five items were extracted from these critical appraisal tools. After grouping like items together, 173 different item types were identified, with the most frequently reported items being focused towards assessing the external validity of the study (N = 35) and method of data analyses (N = 28) (Table (Table2). 2 ). The most frequently reported items across all critical appraisal tools were:
The type and number of component items contained in critical appraisal tools per study design.
• Eligibility criteria (inclusion/exclusion criteria) (N = 63)
• Appropriate statistical analyses (N = 47)
• Random allocation of subjects (N = 43)
• Consideration of outcome measures used (N = 43)
• Sample size justification/power calculations (N = 39)
• Study design reported (N = 36)
• Assessor blinding (N = 36)
Design-specific critical appraisal tools
Systematic reviews.
Eighty-seven different items were extracted from the 26 critical appraisal tools, which were designed to evaluate the quality of systematic reviews. These critical appraisal tools frequently contained items regarding data analyses and issues of external validity (Tables (Tables2 2 and and3 3 ).
The type and number of guidelines accompanying critical appraisal tools per study design
Items assessing data analyses were focused to the methods used to summarize the results, assessment of sensitivity of results and whether heterogeneity was considered, whereas the nature of reporting of the main results, interpretation of them and their generalizability were frequently used to assess the external validity of the study findings. Moreover, systematic review critical appraisal tools tended to contain items such as identification of relevant studies, search strategy used, number of studies included and protocol adherence, that would not be relevant for other study designs. Blinding and randomisation procedures were rarely included in these critical appraisal tools.
Experimental studies
One hundred and twenty thirteen different items were extracted from the 45 experimental critical appraisal tools. These items most frequently assessed aspects of data analyses and blinding (Tables (Tables1 1 and and2). 2 ). Data analyses items were focused on whether appropriate statistical analysis was performed, whether a sample size justification or power calculation was provided and whether side effects of the intervention were recorded and analysed. Blinding was focused on whether the participant, clinician and assessor were blinded to the intervention.
Diagnostic studies
Forty-seven different items were extracted from the seven diagnostic critical appraisal tools. These items frequently addressed issues involving data analyses, external validity of results and sample selection that were specific to diagnostic studies (whether the diagnostic criteria were defined, definition of the "gold" standard, the calculation of sensitivity and specificity) (Tables (Tables1 1 and and2 2 ).
Observational studies
Seventy-four different items were extracted from the 19 critical appraisal tools for observational studies. These items primarily focused on aspects of data analyses (see Tables Tables1 1 and and2, 2 , such as whether confounders were considered in the analysis, whether a sample size justification or power calculation was provided and whether appropriate statistical analyses were preformed.
Qualitative studies
Thirty-six different items were extracted from the seven qualitative study critical appraisal tools. The majority of these items assessed issues regarding external validity, methods of data analyses and the aims and justification of the study (Tables (Tables1 1 and and2). 2 ). Specifically, items were focused to whether the study question was clearly stated, whether data analyses were clearly described and appropriate, and application of the study findings to the clinical setting. Qualitative critical appraisal tools did not contain items regarding sample selection, randomization, blinding, intervention or bias, perhaps because these issues are not relevant to the qualitative paradigm.
Generic critical appraisal tools
Experimental and observational studies.
Forty-two different items were extracted from the six critical appraisal tools that could be used to evaluate experimental and observational studies. These tools most frequently contained items that addressed aspects of sample selection (such as inclusion/exclusion criteria of participants, homogeneity of participants at baseline) and data analyses (such as whether appropriate statistical analyses were performed, whether a justification of the sample size or power calculation were provided).
All study designs
Seventy-eight different items were contained in the ten critical appraisal tools that could be used for all study designs (quantitative and qualitative). The majority of these items focused on whether appropriate data analyses were undertaken (such as whether confounders were considered in the analysis, whether a sample size justification or power calculation was provided and whether appropriate statistical analyses were preformed) and external validity issues (generalization of results to the population, value of the research findings) (see Tables Tables1 1 and and2 2 ).
Allied health critical appraisal tools
We found no critical appraisal instrument specific to allied health research, despite finding at least seven critical appraisal instruments associated with allied health topics (mostly physiotherapy management of orthopedic conditions) [ 37 , 39 , 52 , 58 , 59 , 65 ]. One critical appraisal development group proposed two instruments [ 9 ], specific to quantitative and qualitative research respectively. The core elements of allied health research quality (specific diagnosis criteria, intervention descriptions, nature of patient contact and appropriate outcome measures) were not addressed in any one tool sourced for this evaluation. We identified 152 different ways of considering quality reporting of outcome measures in the 121 critical appraisal tools, and 81 ways of considering description of interventions. Very few tools which were not specifically targeted to diagnostic studies (less than 10% of the remaining tools) addressed diagnostic criteria. The critical appraisal instrument that seemed most related to allied health research quality [ 39 ] sought comprehensive evaluation of elements of intervention and outcome, however this instrument was relevant only to physiotherapeutic orthopedic experimental research.
Overall study quality
Forty-nine percent (N = 58) of critical appraisal tools summarised the results of the quality appraisal into a single numeric summary score [ 5 , 7 , 15 - 25 , 37 - 59 , 74 - 77 , 80 - 83 , 87 , 91 - 93 , 96 , 97 ] (Figure (Figure2). 2 ). This was achieved by one of two methods:

Number of critical appraisal tools with, and without, summary quality scores
• An equal weighting system, where one point was allocated to each item fulfilled; or
• A weighted system, where fulfilled items were allocated various points depending on their perceived importance.
However, there was no justification provided for any of the scoring systems used. In the remaining critical appraisal tools (N = 62), a single numerical summary score was not provided [ 1 - 4 , 9 , 25 - 36 , 60 - 73 , 78 , 79 , 84 - 90 , 94 , 95 , 98 - 102 ]. This left the research consumer to summarize the results of the appraisal in a narrative manner, without the assistance of a standard approach.
Psychometric properties of critical appraisal tools
Few critical appraisal tools had documented evidence of their validity and reliability. Face validity was established in nine critical appraisal tools, seven of which were developed for use on experimental studies [ 38 , 40 , 45 , 49 , 51 , 63 , 70 ] and two for systematic reviews [ 32 , 103 ]. Intra-rater reliability was established for only one critical appraisal tool as part of its empirical development process [ 40 ], whereas inter-rater reliability was reported for two systematic review tools [ 20 , 36 ] (for one of these as part of the developmental process [ 20 ]) and seven experimental critical appraisal tools [ 38 , 40 , 45 , 51 , 55 , 56 , 63 ] (for two of these as part of the developmental process [ 40 , 51 ]).
Critical appraisal tool guidelines
Forty-three percent (N = 52) of critical appraisal tools had guidelines that informed the user of the interpretation of each item contained within them (Table (Table2). 2 ). These guidelines were most frequently in the form of a handbook or published paper (N = 31) [ 2 , 4 , 9 , 15 , 20 , 25 , 28 , 29 , 31 , 36 , 37 , 41 , 50 , 64 - 67 , 69 , 80 , 84 - 87 , 89 , 90 , 95 , 100 , 116 ], whereas in 14 critical appraisal tools explanations accompanied each item [ 16 , 26 , 27 , 40 , 49 , 51 , 57 , 59 , 79 , 83 , 91 , 102 ].
Our search strategy identified a large number of published critical appraisal tools that are currently available to critically appraise research reports. There was a distinct lack of information on tool development processes in most cases. Many of the tools were reported to be modifications of other published tools, or reflected specialty concerns in specific clinical or research areas, without attempts to justify inclusion criteria. Less than 10 of these tools were relevant to evaluation of the quality of allied health research, and none of these were based on an empirical research approach. We are concerned that although our search was systematic and extensive [ 104 , 105 ], our broad key words and our lack of ready access to 29% of potentially useful papers (N = 56) potentially constrained us from identifying all published critical appraisal tools. However, consumers of research seeking critical appraisal instruments are not likely to seek instruments from outdated Internet links and unobtainable journals, thus we believe that we identified the most readily available instruments. Thus, despite the limitations on sourcing all possible tools, we believe that this paper presents a useful synthesis of the readily available critical appraisal tools.
The majority of the critical appraisal tools were developed for a specific research design (87%), with most designed for use on experimental studies (38% of all critical appraisal tools sourced). This finding is not surprising as, according to the medical model, experimental studies sit at or near the top of the hierarchy of evidence [ 2 , 8 ]. In recent years, allied health researchers have strived to apply the medical model of research to their own discipline by conducting experimental research, often by using the randomized controlled trial design [ 106 ]. This trend may be the reason for the development of experimental critical appraisal tools reported in allied health-specific research topics [ 37 , 39 , 52 , 58 , 59 , 65 ].
We also found a considerable number of critical appraisal tools for systematic reviews (N = 26), which reflects the trend to synthesize research evidence to make it relevant for clinicians [ 105 , 107 ]. Systematic review critical appraisal tools contained unique items (such as identification of relevant studies, search strategy used, number of studies included, protocol adherence) compared with tools used for primary studies, a reflection of the secondary nature of data synthesis and analysis.
In contrast, we identified very few qualitative study critical appraisal tools, despite the presence of many journal-specific guidelines that outline important methodological aspects required in a manuscript submitted for publication [ 108 - 110 ]. This finding may reflect the more traditional, quantitative focus of allied health research [ 111 ]. Alternatively, qualitative researchers may view the robustness of their research findings in different terms compared with quantitative researchers [ 112 , 113 ]. Hence the use of critical appraisal tools may be less appropriate for the qualitative paradigm. This requires further consideration.
Of the small number of generic critical appraisal tools, we found few that could be usefully applied (to any health research, and specifically to the allied health literature), because of the generalist nature of their items, variable interpretation (and applicability) of items across research designs, and/or lack of summary scores. Whilst these types of tools potentially facilitate the synthesis of evidence across allied health research designs for clinicians, their lack of specificity in asking the 'hard' questions about research quality related to research design also potentially precludes their adoption for allied health evidence-based practice. At present, the gold standard study design when synthesizing evidence is the randomized controlled trial [ 4 ], which underpins our finding that experimental critical appraisal tools predominated in the allied health literature [ 37 , 39 , 52 , 58 , 59 , 65 ]. However, as more systematic literature reviews are undertaken on allied health topics, it may become more accepted that evidence in the form of other research design types requires acknowledgement, evaluation and synthesis. This may result in the development of more appropriate and clinically useful allied health critical appraisal tools.
A major finding of our study was the volume and variation in available critical appraisal tools. We found no gold standard critical appraisal tool for any type of study design. Therefore, consumers of research are faced with frustrating decisions when attempting to select the most appropriate tool for their needs. Variable quality evaluations may be produced when different critical appraisal tools are used on the same literature [ 6 ]. Thus, interpretation of critical analysis must be carefully considered in light of the critical appraisal tool used.
The variability in the content of critical appraisal tools could be accounted for by the lack of any empirical basis of tool construction, established validity of item construction, and the lack of a gold standard against which to compare new critical tools. As such, consumers of research cannot be certain that the content of published critical appraisal tools reflect the most important aspects of the quality of studies that they assess [ 114 ]. Moreover, there was little evidence of intra- or inter-rater reliability of the critical appraisal tools. Coupled with the lack of protocols for use, this may mean that critical appraisers could interpret instrument items in different ways over repeated occasions of use. This may produce variable results [123].
Based on the findings of this evaluation, we recommend that consumers of research should carefully select critical appraisal tools for their needs. The selected tools should have published evidence of the empirical basis for their construction, validity of items and reliability of interpretation, as well as guidelines for use, so that the tools can be applied and interpreted in a standardized manner. Our findings highlight the need for consensus to be reached regarding the important and core items for critical appraisal tools that will produce a more standardized environment for critical appraisal of research evidence. As a consequence, allied health research will specifically benefit from having critical appraisal tools that reflect best practice research approaches which embed specific research requirements of allied health disciplines.
Competing interests
No competing interests.
Authors' contributions
PK Sourced critical appraisal tools
Categorized the content and psychometric properties of critical appraisal tools
AEB Synthesis of findings
Drafted manuscript
NMW Sourced critical appraisal tools
VSK Sourced critical appraisal tools
KAG Study conception and design
Assisted with critiquing critical appraisal tools and categorization of the content and psychometric properties of critical appraisal tools
Drafted and reviewed manuscript
Addressed reviewer's comments and re-submitted the article
Pre-publication history
The pre-publication history for this paper can be accessed here:
http://www.biomedcentral.com/1471-2288/4/22/prepub
Supplementary Material
Search Strategy.
- National Health and Medical Research Council How to Review the Evidence: Systematic Identification and Review of the Scientific Literature Canberra. 2000.
- National Health and Medical Research Council How to Use the Evidence: Assessment and Application of Scientific Evidence Canberra. 2000.
- Joanna Briggs Institute http://www.joannabriggs.edu.au
- Clarke M, Oxman AD. Cochrane Reviewer's Handbook 420. Oxford: The Cochrane Collaboration; 2003. [ Google Scholar ]
- Crombie IK. The Pocket Guide to Critical Appraisal: A Handbook for Health Care Professionals. London: BMJ Publishing Group; 1996. [ Google Scholar ]
- Agency for Healthcare Research and Quality Systems to Rate the Strength of Scientific Evidence Evidence Report/Technology Assessment No 47, Publication No 02-E016 Rockville. 2002.
- Elwood JM. Critical Appraisal of Epidemiological Studies and Clinical Trials. 2. Oxford: Oxford University Press; 1998. [ Google Scholar ]
- Sackett DL, Richardson WS, Rosenberg W, Haynes RB. Evidence Based Medicine How to Practice and Teach EBM. London: Churchill Livingstone; 2000. [ Google Scholar ]
- Critical literature reviews http://www.cotfcanada.org/cotf_critical.htm
- Bialocerkowski AE, Grimmer KA, Milanese SF, Kumar S. Application of current research evidence to clinical physiotherapy practice. J Allied Health Res Dec. [ PubMed ]
- The National Health Data Dictionary – Version 10. http://www.aihw.gov.au/publications/hwi/nhdd12/nhdd12-v1.pdf and http://www.aihw.gov.au/publications/hwi/nhdd12/nhdd12-v2.pdf .
- Grimmer K, Bowman P, Roper J. Episodes of allied health outpatient care: an investigation of service delivery in acute public hospital settings. Disability and Rehabilitation. 2000; 22 :80–87. [ PubMed ] [ Google Scholar ]
- Grimmer K, Milanese S, Bialocerkowski A. Clinical guidelines for low back pain: A physiotherapy perspective. Physiotherapy Canada. 2003; 55 :1–9. [ Google Scholar ]
- Grimmer KA, Milanese S, Bialocerkowski AE, Kumar S. Producing and implementing evidence in clinical practice: the therapies' dilemma. Physiotherapy. 2004.
- Greenhalgh T. How to read a paper: papers that summarize other papers (systematic reviews and meta-analysis) BMJ. 1997; 315 :672–675. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Auperin A, Pignon J, Poynard T. Review article: critical review of meta-analysis of randomised clinical trials in hepatogastroenterology. Alimentary Pharmacol Therapeutics. 1997; 11 :215–225. doi: 10.1046/j.1365-2036.1997.131302000.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barnes DE, Bero LA. Why review articles on the health effects of passive smoking reach different conclusions. J Am Med Assoc. 1998; 279 :1566–1570. doi: 10.1001/jama.279.19.1566. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Beck CT. Use of meta-analysis as a teaching strategy in nursing research courses. J Nurs Educat. 1997; 36 :87–90. [ PubMed ] [ Google Scholar ]
- Carruthers SG, Larochelle P, Haynes RB, Petrasovits A, Schiffrin EL. Report of the Canadian Hypertension Society Consensus Conference: 1. Introduction. Can Med Assoc J. 1993; 149 :289–293. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Oxman AD, Guyatt GH, Singer J, Goldsmith CH, Hutchinson BG, Milner RA, Streiner DL. Agreement among reviewers of review articles. J Clin Epidemiol. 1991; 44 :91–98. doi: 10.1016/0895-4356(91)90205-N. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sacks HS, Reitman D, Pagano D, Kupelnick B. Meta-analysis: an update. Mount Sinai Journal of Medicine. 1996; 63 :216–224. [ PubMed ] [ Google Scholar ]
- Smith AF. An analysis of review articles published in four anaesthesia journals. Can J Anaesth. 1997; 44 :405–409. [ PubMed ] [ Google Scholar ]
- L'Abbe KA, Detsky AS, O'Rourke K. Meta-analysis in clinical research. Ann Intern Med. 1987; 107 :224–233. [ PubMed ] [ Google Scholar ]
- Mulrow CD, Antonio S. The medical review article: state of the science. Ann Intern Med. 1987; 106 :485–488. [ PubMed ] [ Google Scholar ]
- Continuing Professional Development: A Manual for SIGN Guideline Developers http://www.sign.ac.uk
- Learning and Development Public Health Resources Unit http://www.phru.nhs.uk/
- FOCUS Critical Appraisal Tool http://www.focusproject.org.uk
- Cook DJ, Sackett DL, Spitzer WO. Methodologic guidelines for systematic reviews of randomized control trials in health care from the Potsdam Consultation on meta-analysis. J Clin Epidemiol. 1995; 48 :167–171. doi: 10.1016/0895-4356(94)00172-M. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cranney A, Tugwell P, Shea B, Wells G. Implications of OMERACT outcomes in arthritis and osteoporosis for Cochrane metaanalysis. J Rheumatol. 1997; 24 :1206–1207. [ PubMed ] [ Google Scholar ]
- Guyatt GH, Sackett DL, Sinclair JC, Hoyward R, Cook DJ, Cook RJ. User's guide to the medical literature. IX. A method for grading health care recommendations. J Am Med Assoc. 1995; 274 :1800–1804. doi: 10.1001/jama.274.22.1800. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gyorkos TW, Tannenbaum TN, Abrahamowicz M, Oxman AD, Scott EAF, Milson ME, Rasooli Iris, Frank JW, Riben PD, Mathias RG. An approach to the development of practice guidelines for community health interventions. Can J Public Health. 1994; 85 :S8–13. [ PubMed ] [ Google Scholar ]
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999; 354 :1896–1900. doi: 10.1016/S0140-6736(99)04149-5. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oxman AD, Cook DJ, Guyatt GH. Users' guides to the medical literature. VI. How to use an overview. Evidence-Based Medicine Working Group. J Am Med Assoc. 1994; 272 :1367–1371. doi: 10.1001/jama.272.17.1367. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pogue J, Yusuf S. Overcoming the limitations of current meta-analysis of randomised controlled trials. Lancet. 1998; 351 :47–52. doi: 10.1016/S0140-6736(97)08461-4. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. J Am Med Assoc. 2000; 283 :2008–2012. doi: 10.1001/jama.283.15.2008. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Irwig L, Tosteson AN, Gatsonis C, Lau J, Colditz G, Chalmers TC, Mostellar F. Guidelines for meta-analyses evaluating diagnostic tests. Ann Intern Med. 1994; 120 :667–676. [ PubMed ] [ Google Scholar ]
- Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: A survey of the Physiotherapy Evidence Database. Physiotherapy Evidence Database (PEDro) Australian Journal of Physiotherapy. 2002; 48 :43–50. [ PubMed ] [ Google Scholar ]
- Cho MK, Bero LA. Instruments for assessing the quality of drug studies published in the medical literature. J Am Med Assoc. 1994; 272 :101–104. doi: 10.1001/jama.272.2.101. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- De Vet HCW, De Bie RA, Van der Heijden GJ, Verhagen AP, Sijpkes P, Kipschild PG. Systematic reviews on the basis of methodological criteria. Physiotherapy. 1997; 83 :284–289. [ Google Scholar ]
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998; 52 :377–384. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Evans M, Pollock AV. A score system for evaluating random control clinical trials of prophylaxis of abdominal surgical wound infection. Br J Surg. 1985; 72 :256–260. [ PubMed ] [ Google Scholar ]
- Fahey T, Hyde C, Milne R, Thorogood M. The type and quality of randomized controlled trials (RCTs) published in UK public health journals. J Public Health Med. 1995; 17 :469–474. [ PubMed ] [ Google Scholar ]
- Gotzsche PC. Methodology and overt and hidden bias in reports of 196 double-blind trials of nonsteroidal antiinflammatory drugs in rheumatoid arthritis. Control Clin Trials. 1989; 10 :31–56. doi: 10.1016/0197-2456(89)90017-2. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Imperiale TF, McCullough AJ. Do corticosteroids reduce mortality from alcoholic hepatitis? A meta-analysis of the randomized trials. Ann Int Med. 1990; 113 :299–307. [ PubMed ] [ Google Scholar ]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996; 17 :1–12. doi: 10.1016/0197-2456(95)00134-4. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Khan KS, Daya S, Collins JA, Walter SD. Empirical evidence of bias in infertility research: overestimation of treatment effect in crossover trials using pregnancy as the outcome measure. Fertil Steril. 1996; 65 :939–945. [ PubMed ] [ Google Scholar ]
- Kleijnen J, Knipschild P, ter Riet G. Clinical trials of homoeopathy. BMJ. 1991; 302 :316–323. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Liberati A, Himel HN, Chalmers TC. A quality assessment of randomized control trials of primary treatment of breast cancer. J Clin Oncol. 1986; 4 :942–951. [ PubMed ] [ Google Scholar ]
- Moher D, Schulz KF, Altman DG, for the CONSORT Group The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. J Am Med Assoc. 2001; 285 :1987–1991. doi: 10.1001/jama.285.15.1987. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reisch JS, Tyson JE, Mize SG. Aid to the evaluation of therapeutic studies. Pediatrics. 1989; 84 :815–827. [ PubMed ] [ Google Scholar ]
- Sindhu F, Carpenter L, Seers K. Development of a tool to rate the quality assessment of randomized controlled trials using a Delphi technique. J Advanced Nurs. 1997; 25 :1262–1268. doi: 10.1046/j.1365-2648.1997.19970251262.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Van der Heijden GJ, Van der Windt DA, Kleijnen J, Koes BW, Bouter LM. Steroid injections for shoulder disorders: a systematic review of randomized clinical trials. Br J Gen Pract. 1996; 46 :309–316. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Van Tulder MW, Koes BW, Bouter LM. Conservative treatment of acute and chronic nonspecific low back pain. A systematic review of randomized controlled trials of the most common interventions. Spine. 1997; 22 :2128–2156. doi: 10.1097/00007632-199709150-00012. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Garbutt JC, West SL, Carey TS, Lohr KN, Crews FT. Pharmacotherapy for Alcohol Dependence Evidence Report/Technology Assessment No 3, AHCPR Publication No 99-E004 Rockville. 1999. [ PMC free article ] [ PubMed ]
- Oremus M, Wolfson C, Perrault A, Demers L, Momoli F, Moride Y. Interarter reliability of the modified Jadad quality scale for systematic reviews of Alzheimer's disease drug trials. Dement Geriatr Cognit Disord. 2001; 12 :232–236. doi: 10.1159/000051263. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Clark O, Castro AA, Filho JV, Djubelgovic B. Interrater agreement of Jadad's scale. Annual Cochrane Colloqium Abstracts October 2001 Lyon. http://www.biomedcentral.com/abstracts/COCHRANE/1/op031
- Jonas W, Anderson RL, Crawford CC, Lyons JS. A systematic review of the quality of homeopathic clinical trials. BMC Alternative Medicine. 2001; 1 :12. doi: 10.1186/1472-6882-1-12. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Van Tulder M, Malmivaara A, Esmail R, Koes B. Exercises therapy for low back pain: a systematic review within the framework of the Cochrane Collaboration back review group. Spine. 2000; 25 :2784–2796. doi: 10.1097/00007632-200011010-00011. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Van Tulder MW, Ostelo R, Vlaeyen JWS, Linton SJ, Morley SJ, Assendelft WJJ. Behavioral treatment for chronic low back pain: a systematic review within the framework of the cochrane back. Spine. 2000; 25 :2688–2699. doi: 10.1097/00007632-200010150-00024. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Aronson N, Seidenfeld J, Samson DJ, Aronson N, Albertson PC, Bayoumi AM, Bennett C, Brown A, Garber ABA, Gere M, Hasselblad V, Wilt T, Ziegler MPHK, Pharm D. Relative Effectiveness and Cost Effectiveness of Methods of Androgen Suppression in the Treatment of Advanced Prostate Cancer Evidence Report/Technology Assessment No 4, AHCPR Publication No99-E0012 Rockville. 1999. [ PMC free article ] [ PubMed ]
- Chalmers TC, Smith H, Jr, Blackburn B, Silverman B, Schroeder B, Reitman D, Ambroz A. A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981; 2 :31–49. doi: 10.1016/0197-2456(81)90056-8. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- der Simonian R, Charette LJ, McPeek B, Mosteller F. Reporting on methods in clinical trials. New Eng J Med. 1982; 306 :1332–1337. [ PubMed ] [ Google Scholar ]
- Detsky AS, Naylor CD, O'Rourke K, McGeer AJ, L'Abbe KA. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol. 1992; 45 :255–265. doi: 10.1016/0895-4356(92)90085-2. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Goudas L, Carr DB, Bloch R, Balk E, Ioannidis JPA, Terrin MN. Management of Cancer Pain Evidence Report/Technology Assessment No 35 (Contract 290-97-0019 to the New England Medical Center), AHCPR Publication No 99-E004 Rockville. 2000.
- Guyatt GH, Sackett DL, Cook DJ. Users' guides to the medical literature. II. How to use an article about therapy or prevention. A. Are the results of the study valid? Evidence-Based Medicine Working Group. J Am Med Assoc. 1993; 270 :2598–2601. doi: 10.1001/jama.270.21.2598. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Khan KS, Ter Riet G, Glanville J, Sowden AJ, Kleijnen J. Undertaking Systematic Reviews of Research on Effectiveness: Centre of Reviews and Dissemination's Guidance for Carrying Out or Commissioning Reviews: York. 2000.
- McNamara R, Bass EB, Marlene R, Miller J. Management of New Onset Atrial Fibrillation Evidence Report/Technology Assessment No12, AHRQ Publication No 01-E026 Rockville. 2001.
- Prendiville W, Elbourne D, Chalmers I. The effects of routine oxytocic administration in the management of the third stage of labour: an overview of the evidence from controlled trials. Br J Obstet Gynae Col. 1988; 95 :3–16. [ PubMed ] [ Google Scholar ]
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. J Am Med Assoc. 1995; 273 :408–412. doi: 10.1001/jama.273.5.408. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- The Standards of Reporting Trials Group A proposal for structured reporting of randomized controlled trials. J Am Med Assoc. 1994; 272 :1926–1931. doi: 10.1001/jama.272.24.1926. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Verhagen AP, de Vet HC, de Bie RA, Kessels AGH, Boers M, Bouter LM, Knipschild PG. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998; 51 :1235–1241. doi: 10.1016/S0895-4356(98)00131-0. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zaza S, Wright-De Aguero LK, Briss PA, Truman BI, Hopkins DP, Hennessy MH, Sosin DM, Anderson L, Carande-Kullis VG, Teutsch SM, Pappaioanou M. Data collection instrument and procedure for systematic reviews in the guide to community preventive services. Task force on community preventive services. Am J Prevent Med. 2000; 18 :44–74. doi: 10.1016/S0749-3797(99)00122-1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Haynes BB, Wilczynski N, McKibbon A, Walker CJ, Sinclair J. Developing optimal search strategies for detecting clinically sound studies in MEDLINE. J Am Informatics Assoc. 1994; 1 :447–458. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Greenhalgh T. How to read a paper: papers that report diagnostic or screening tests. BMJ. 1997; 315 :540–543. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Arroll B, Schechter MT, Sheps SB. The assessment of diagnostic tests: a comparison of medical literature in 1982 and 1985. J Gen Int Med. 1988; 3 :443–447. [ PubMed ] [ Google Scholar ]
- Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, van der Meulen JH, Bossuyt PM. Empirical evidence of design-related bias in studies of diagnostic tests. J Am Med Assoc. 1999; 282 :1061–1066. doi: 10.1001/jama.282.11.1061. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sheps SB, Schechter MT. The assessment of diagnostic tests. A survey of current medical research. J Am Med Assoc. 1984; 252 :2418–2422. doi: 10.1001/jama.252.17.2418. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McCrory DC, Matchar DB, Bastian L, Dutta S, Hasselblad V, Hickey J, Myers MSE, Nanda K. Evaluation of Cervical Cytology Evidence Report/Technology Assessment No 5, AHCPR Publication No99-E010 Rockville. 1999. [ PMC free article ] [ PubMed ]
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, DeVet HCW. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Clin Chem. 2003; 49 :1–6. doi: 10.1373/49.1.1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Greenhalgh T. How to Read a Paper: Assessing the methodological quality of published papers. BMJ. 1997; 315 :305–308. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Angelillo I, Villari P. Residential exposure to electromagnetic fields and childhood leukaemia: a meta-analysis. Bull World Health Org. 1999; 77 :906–915. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ariens G, Mechelen W, Bongers P, Bouter L, Van der Wal G. Physical risk factors for neck pain. Scand J Work Environ Health. 2000; 26 :7–19. [ PubMed ] [ Google Scholar ]
- Hoogendoorn WE, van Poppel MN, Bongers PM, Koes BW, Bouter LM. Physical load during work and leisure time as risk factors for back pain. Scand J Work Environ Health. 1999; 25 :387–403. [ PubMed ] [ Google Scholar ]
- Laupacis A, Wells G, Richardson WS, Tugwell P. Users' guides to the medical literature. V. How to use an article about prognosis. Evidence-Based Medicine Working Group. J Am Med Assoc. 1994; 272 :234–237. doi: 10.1001/jama.272.3.234. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Levine M, Walter S, Lee H, Haines T, Holbrook A, Moyer V. Users' guides to the medical literature. IV. How to use an article about harm. Evidence-Based Medicine Working Group. J Am Med Assoc. 1994; 271 :1615–1619. doi: 10.1001/jama.271.20.1615. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Carey TS, Boden SD. A critical guide to case series reports. Spine. 2003; 28 :1631–1634. doi: 10.1097/00007632-200308010-00001. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Greenhalgh T, Taylor R. How to read a paper: papers that go beyond numbers (qualitative research) BMJ. 1997; 315 :740–743. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hoddinott P, Pill R. A review of recently published qualitative research in general practice. More methodological questions than answers? Fam Pract. 1997; 14 :313–319. doi: 10.1093/fampra/14.4.313. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mays N, Pope C. Quality research in health care: Assessing quality in qualitative research. BMJ. 2000; 320 :50–52. doi: 10.1136/bmj.320.7226.50. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mays N, Pope C. Rigour and qualitative research. BMJ. 1995; 311 :109–112. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Colditz GA, Miller JN, Mosteller F. How study design affects outcomes in comparisons of therapy. I: Medical. Stats Med. 1989; 8 :441–454. [ PubMed ] [ Google Scholar ]
- Turlik MA, Kushner D. Levels of evidence of articles in podiatric medical journals. J Am Pod Med Assoc. 2000; 90 :300–302. [ PubMed ] [ Google Scholar ]
- Borghouts JAJ, Koes BW, Bouter LM. The clinical course and prognostic factors of non-specific neck pain: a systematic review. Pain. 1998; 77 :1–13. doi: 10.1016/S0304-3959(98)00058-X. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Spitzer WO, Lawrence V, Dales R, Hill G, Archer MC, Clark P, Abenhaim L, Hardy J, Sampalis J, Pinfold SP, Morgan PP. Links between passive smoking and disease: a best-evidence synthesis. A report of the working group on passive smoking. Clin Invest Med. 1990; 13 :17–46. [ PubMed ] [ Google Scholar ]
- Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Systematic reviews of trials and other studies. Health Tech Assess. 1998; 2 :1–276. [ PubMed ] [ Google Scholar ]
- Chestnut RM, Carney N, Maynard H, Patterson P, Mann NC, Helfand M. Rehabilitation for Traumatic Brain Injury Evidence Report/Technology Assessment No 2, Agency for Health Care Research and Quality Publication No 99-E006 Rockville. 1999.
- Lohr KN, Carey TS. Assessing best evidence: issues in grading the quality of studies for systematic reviews. Joint Commission J Qual Improvement. 1999; 25 :470–479. [ PubMed ] [ Google Scholar ]
- Greer N, Mosser G, Logan G, Halaas GW. A practical approach to evidence grading. Joint Commission J Qual Improvement. 2000; 26 :700–712. [ PubMed ] [ Google Scholar ]
- Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, Atkins D. Current methods of the U.S. Preventive Services Task Force: a review of the process. Am J Prevent Med. 2001; 20 :21–35. doi: 10.1016/S0749-3797(01)00261-6. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anonymous How to read clinical journals: IV. To determine etiology or causation. Can Med Assoc J. 1981; 124 :985–990. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Whitten PS, Mair FS, Haycox A, May CR, Williams TL, Hellmich S. Systematic review of cost effectiveness studies of telemedicine interventions. BMJ. 2002; 324 :1434–1437. doi: 10.1136/bmj.324.7351.1434. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Forrest JL, Miller SA. Evidence-based decision making in action: Part 2-evaluating and applying the clinical evidence. J Contemp Dental Pract. 2002; 4 :42–52. [ PubMed ] [ Google Scholar ]
- Oxman AD, Guyatt GH. Validation of an index of the quality of review articles. J Clin Epidemiol. 1991; 44 :1271–1278. doi: 10.1016/0895-4356(91)90160-B. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jones T, Evans D. Conducting a systematic review. Aust Crit Care. 2000; 13 :66–71. [ PubMed ] [ Google Scholar ]
- Papadopoulos M, Rheeder P. How to do a systematic literature review. South African J Physiother. 2000; 56 :3–6. [ Google Scholar ]
- Selker LG. Clinical research in Allied Health. J Allied Health. 1994; 23 :201–228. [ PubMed ] [ Google Scholar ]
- Stevens KR. Systematic reviews: the heart of evidence-based practice. AACN Clin Issues. 2001; 12 :529–538. [ PubMed ] [ Google Scholar ]
- Devers KJ, Frankel RM. Getting qualitative research published. Ed Health. 2001; 14 :109–117. doi: 10.1080/13576280010021888. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Canadian Journal of Public Health Review guidelines for qualitative research papers submitted for consideration to the Canadian Journal of Public Health. Can J Pub Health. 2000; 91 :I2. [ Google Scholar ]
- Malterud K. Shared understanding of the qualitative research process: guidelines for the medical researcher. Fam Pract. 1993; 10 :201–206. [ PubMed ] [ Google Scholar ]
- Higgs J, Titchen A. Research and knowledge. Physiotherapy. 1998; 84 :72–80. [ Google Scholar ]
- Maggs-Rapport F. Best research practice: in pursuit of methodological rigour. J Advan Nurs. 2001; 35 :373–383. doi: 10.1046/j.1365-2648.2001.01853.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cutcliffe JR, McKenna HP. Establishing the credibility of qualitative research findings: the plot thickens. J Advan Nurs. 1999; 30 :374–380. doi: 10.1046/j.1365-2648.1999.01090.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Andresen EM. Criteria for assessing the tools of disability outcomes research. Arch Phys Med Rehab. 2000; 81 :S15–S20. doi: 10.1053/apmr.2000.20619. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Beatie P. Measurement of health outcomes in the clinical setting: applications to physiotherapy. Phys Theory Pract. 2001; 17 :173–185. doi: 10.1080/095939801317077632. [ CrossRef ] [ Google Scholar ]
- Charnock DF, (Ed) The DISCERN Handbook: Quality criteria for consumer health information on treatment choices. Radcliffe Medical Press; 1998. [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
More. Critical appraisal tools and reporting guidelines are the two most important instruments available to researchers and practitioners involved in research, evidence-based practice, and policymaking. Each of these instruments has unique characteristics, and both instruments play an essential role in evidence-based practice and decision-making.
JBI's critical appraisal tools assist in assessing the trustworthiness, relevance and results of published papers. These tools have been revised. Recently published articles detail the revision. "Assessing the risk of bias of quantitative analytical studies: introducing the vision for critical appraisal within JBI systematic reviews".
INTRODUCTION. Critical appraisal of a research paper is defined as "The process of carefully and systematically examining research to judge its trustworthiness, value and relevance in a particular context."[] Since scientific literature is rapidly expanding with more than 12,000 articles being added to the MEDLINE database per week,[] critical appraisal is very important to distinguish ...
Abstract. Healthcare professionals are often expected to critically appraise research evidence in order to make recommendations for practice and policy development. Here we describe the Critical Appraisal Toolkit (CAT) currently used by the Public Health Agency of Canada. The CAT consists of: algorithms to identify the type of study design ...
This section contains useful tools and downloads for the critical appraisal of different types of medical evidence. Example appraisal sheets are provided together with several helpful examples. Critical appraisal worksheets to help you appraise the reliability, importance and applicability of clinical evidence.
Critical appraisal is the assessment of research studies' worth to clinical practice. Critical appraisal—the heart of evidence-based practice—involves four phases: rapid critical appraisal, evaluation, synthesis, and recommendation. This article reviews each phase and provides examples, tips, and caveats to help evidence appraisers ...
Critical appraisal is essential to: Combat information overload; Identify papers that are clinically relevant; Continuing Professional Development (CPD). Carrying out Critical Appraisal: Assessing the research methods used in the study is a prime step in its critical appraisal.
JBI Critical Appraisal Tools All systematic reviews incorporate a process of critique or appraisal of the research evidence. The purpose of this appraisal is to assess the methodological quality of a study and to determine the extent to which a study has addressed the possibility of bias in its design, conduct and analysis. All papers
Clarity on the types of research under scrutiny helps reviewers match suitable critical appraisal criteria and tools to the investigations they are assessing. Steps 2 and 3 warrant separation because different types of primary research are often included in a review, and investigators may need to use multiple critical appraisal criteria and tools.
Here are some of the tools and basic considerations you might find useful when critically appraising an article. In a nutshell when appraising an article, you are assessing: 1. Its relevance ...
Critical Appraisal is the process of carefully and systematically examining research to judge its trustworthiness, and its value and relevance in a particular context. It is an essential skill for evidence-based medicine because it allows people to find and use research evidence reliably and efficiently. Learn more about what critical appraisal ...
Several tools have been developed for the critical appraisal of scientific literature, including grading of evidence to help clinicians in the pursuit of EBM in a systematic manner. In this review, we discuss the broad framework for the critical appraisal of a clinical research paper, along with some of the relevant guidelines and recommendations.
Critical Appraisal of Studies. Critical appraisal is the process of carefully and systematically examining research to judge its trustworthiness, and its value/relevance in a particular context by providing a framework to evaluate the research. During the critical appraisal process, researchers can: Decide whether studies have been undertaken ...
Critical Appraisal Checklists. We offer a number of free downloadable checklists to help you more easily and accurately perform critical appraisal across a number of different study types. The CASP checklists are easy to understand but in case you need any further guidance on how they are structured, take a look at our guide on how to use our ...
Purpose: The primary purpose of this paper is to help nurses understand the difference between critical appraisal tools and reporting guidelines. A secondary purpose is to help nurses locate the appropriate tool for the appraisal or reporting of evidence. Methods: A systematic search was conducted to find commonly used critical appraisal tools ...
A key stage common to all systematic reviews is quality appraisal of the evidence to be synthesized. 1,8 There is broad debate and little consensus among the academic community over what constitutes 'quality' in qualitative research. 'Qualitative' is an umbrella term that encompasses a diverse range of methods, which makes it difficult to have a 'one size fits all' definition of ...
This paper is concerned primarily with critical appraisal tools that address the unique properties of allied health care and research . This approach was taken because of the unique nature of allied health contacts with patients, and because evidence-based practice is an emerging area in allied health [ 10 ].
There are hundreds of critical appraisal checklists and tools you can choose from, which can be very overwhelming. There are so many because there are many kinds of research, knowledge can be communicated in a wide range of ways, and whether something is appropriate to meet your information needs depends on your specific context.
quality. 1 Introduction. Critical appraisal describes the process of analyzing a study in a rigorous and. methodical way. Often, this process involves working through a series of questions. to ...
A qualitative health research study was selected and appraised using the Critical Appraisal Skill Programme (CASP) appraisal tool for qualitative research. Based on the results of the critical appraisal, the study quality is considered, and we discuss whether the qualitative evidence can be applied to practice. Results
The first step is to critique and appraise the research evidence. Through critiquing and appraising the research evidence, dialog with colleagues, and changing practice based on evidence, NPs can improve patient outcomes ( Dale, 2005) and successfully translate research into evidence-based practice in today's ever-changing health care ...
This paper is concerned primarily with critical appraisal tools that address the unique properties of allied health care and research . This approach was taken because of the unique nature of allied health contacts with patients, and because evidence-based practice is an emerging area in allied health [ 10 ].