- Privacy Policy
Buy Me a Coffee

Home » Informed Consent in Research – Types, Templates and Examples

Informed Consent in Research – Types, Templates and Examples
Table of Contents

Informed Consent in Research
Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of participants.
Types of Informed Consent in Research
There are different types of informed consent in research , which may vary depending on the nature of the study, the type of participants, and the context. Some of the common types of informed consent in research include:
Written Consent
This is the most common type of informed consent, where participants are provided with a written document that explains the study and its requirements. The document typically includes information about the purpose of the study, procedures involved, risks and benefits, confidentiality, and participant rights. Participants are asked to sign the document as an indication of their willingness to participate.
Oral Consent
In some cases, oral consent may be used when a written document is not practical or feasible. Oral consent involves explaining the study and its requirements to participants verbally and obtaining their consent. This method may be used for studies with illiterate or visually impaired participants or when conducting research remotely.
Implied Consent
Implied consent is used in studies where participants’ actions are taken as an indication of their willingness to participate. For example, a participant may be considered to have given implied consent if they show up for a scheduled appointment for the study.
Opt-out Consent
This method is used when participants are given the opportunity to decline participation in a study. Participants are provided with information about the study and are given the option to opt-out if they do not wish to participate. This method is commonly used in population-based studies or surveys.
Assent is used in studies involving minors or participants who are unable to provide informed consent due to cognitive impairment or disability. Assent involves obtaining the agreement of the participant to participate in the study, along with the consent of a legally authorized representative.
Informed Consent Format in Research
Here’s a basic format for informed consent that can be customized for specific research studies:
- Introduction : Begin by introducing yourself and the purpose of the study. Clearly state that participation is voluntary and that participants can withdraw at any time without penalty.
- Study Overview : Provide a brief overview of the study, including its purpose, methods, and expected outcomes.
- Procedures : Describe the procedures involved in the study in clear, concise language. Include information about the types of data that will be collected, how they will be collected, and how long the study will take.
- Risks and Benefits : Outline the potential risks and benefits of participating in the study. Be honest and upfront about any discomfort, inconvenience, or potential harm that may be involved, as well as any potential benefits.
- Confidentiality and Privacy : Explain how participant data will be collected, stored, and used, and what measures will be taken to ensure confidentiality and privacy.
- Voluntary Participation: Emphasize that participation is voluntary and that participants can withdraw at any time without penalty. Explain how to withdraw from the study and who to contact if participants have questions or concerns.
- Compensation and Incentives: If applicable, explain any compensation or incentives that will be offered to participants for their participation.
- Contact Information: Provide contact information for the researcher or a representative from the research team who can answer questions and address concerns.
- Signature : Ask participants to sign and date the consent form to indicate their voluntary agreement to participate in the study.
Informed Consent Templates in Research
Here is an example of an informed consent template that can be used in research studies:
Introduction
You are being invited to participate in a research study. Before you decide whether or not to participate, it is important for you to understand why the research is being done, what your participation will involve, and what risks and benefits may be associated with your participation.
Purpose of the Study
The purpose of this study is [insert purpose of study].
If you agree to participate, you will be asked to [insert procedures involved in the study].
Risks and Benefits
There are several potential risks and benefits associated with participation in this study. Some of the risks include [insert potential risks of participation]. Some of the benefits include [insert potential benefits of participation].
Confidentiality
Your participation in this study will be kept confidential to the extent allowed by law. All data collected during the study will be stored in a secure location and only accessed by authorized personnel. Your name and other identifying information will not be included in any reports or publications resulting from this study.
Voluntary Participation
Your participation in this study is completely voluntary. You have the right to withdraw from the study at any time without penalty. If you choose not to participate or if you withdraw from the study, there will be no negative consequences.
Contact Information
If you have any questions or concerns about the study, you can contact the investigator(s) at [insert contact information]. If you have questions about your rights as a research participant, you may contact [insert name of institutional review board and contact information].
Statement of Consent
By signing below, you acknowledge that you have read and understood the information provided in this consent form and that you freely and voluntarily consent to participate in this study.
Participant Signature: _____________________________________ Date: _____________
Investigator Signature: ____________________________________ Date: _____________
Examples of Informed Consent in Research
Here’s an example of informed consent in research:
Title : The Effects of Yoga on Stress and anxiety levels in college students
Introduction :
We are conducting a research study to investigate the effects of yoga on stress and anxiety levels in college students. We are inviting you to participate in this study.
If you agree to participate, you will be asked to attend four yoga classes per week for six weeks. Before and after the six-week period, you will be asked to complete surveys about your stress and anxiety levels. Additionally, we will measure your heart rate variability at the beginning and end of the six-week period.
Risks and Benefits:
There are no known risks associated with participating in this study. However, the benefits of practicing yoga may include decreased stress and anxiety levels, increased flexibility and strength, and improved overall well-being.
Confidentiality:
All information collected during this study will be kept strictly confidential. Your name will not be used in any reports or publications resulting from this study.
Voluntary Participation:
Participation in this study is completely voluntary. You are free to withdraw from the study at any time without penalty.
Contact Information:
If you have any questions or concerns about this study, you may contact the principal investigator at (phone number/email address).
By signing this form, I acknowledge that I have read and understood the above information and agree to participate in this study.
Participant Signature: ___________________________
Date: ___________________________
Researcher Signature: ___________________________
Importance of Informed Consent in Research
Here are some reasons why informed consent is important in research:
- Protection of participants’ rights : Informed consent ensures that participants understand the nature and purpose of the research, the risks and benefits of participating, and their rights as participants. It empowers them to make an informed decision about whether to participate or not.
- Ethical responsibility : Researchers have an ethical responsibility to respect the autonomy of participants and to protect them from harm. Informed consent is a crucial way to uphold these principles.
- Legality : Informed consent is a legal requirement in most countries. It is necessary to protect researchers from legal liability and to ensure that research is conducted in accordance with ethical standards.
- Trust : Informed consent helps build trust between researchers and participants. When participants understand the research process and their role in it, they are more likely to trust the researchers and the study.
- Quality of research : Informed consent ensures that participants are fully informed about the research and its purpose, which can lead to more accurate and reliable data. This, in turn, can improve the quality of research outcomes.
Purpose of Informed Consent in Research
Informed consent is a critical component of research ethics, and it serves several important purposes, including:
- Respect for autonomy: Informed consent respects an individual’s right to make decisions about their own health and well-being. It recognizes that individuals have the right to choose whether or not to participate in research, based on their own values, beliefs, and preferences.
- Protection of participants : Informed consent helps protect research participants from potential harm or risks that may arise from their involvement in a study. By providing participants with information about the study, its risks and benefits, and their rights, they are able to make an informed decision about whether to participate.
- Transparency: Informed consent promotes transparency in the research process. It ensures that participants are fully informed about the research, including its purpose, methods, and potential outcomes, which helps to build trust between researchers and participants.
- Legal and ethical requirements: Informed consent is a legal and ethical requirement in most research studies. It ensures that researchers obtain voluntary and informed agreement from participants to participate in the study, which helps to protect the rights and welfare of research participants.
Advantages of Informed Consent in Research
The advantages of informed consent in research are numerous, and some of the most significant benefits include:
- Protecting participants’ autonomy: Informed consent allows participants to exercise their right to self-determination and make decisions about whether to participate in a study or not. It also ensures that participants are fully informed about the risks, benefits, and implications of participating in the study.
- Promoting transparency and trust: Informed consent helps build trust between researchers and participants by providing clear and accurate information about the study’s purpose, procedures, and potential outcomes. This transparency promotes open communication and a positive research experience for all parties involved.
- Reducing the risk of harm: Informed consent ensures that participants are fully aware of any potential risks or side effects associated with the study. This knowledge enables them to make informed decisions about their participation and reduces the likelihood of harm or negative consequences.
- Ensuring ethical standards are met : Informed consent is a fundamental ethical requirement for conducting research involving human participants. By obtaining informed consent, researchers demonstrate their commitment to upholding ethical principles and standards in their research practices.
- Facilitating future research : Informed consent enables researchers to collect high-quality data that can be used for future research purposes. It also allows participants to make an informed decision about whether they are willing to participate in future studies.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

How to Cite Research Paper – All Formats and...

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Paper Format – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples
As the nation’s largest public research university, the Office of the Vice President for Research (OVPR) aims to catalyze, support and safeguard U-M research and scholarship activity.
The Office of the Vice President for Research oversees a variety of interdisciplinary units that collaborate with faculty, staff, students and external partners to catalyze, support and safeguard research and scholarship activity.
ORSP manages pre-award and some post-award research activity for U-M. We review contracts for sponsored projects applying regulatory, statutory and organizational knowledge to balance the university's mission, the sponsor's objectives, and the investigator's intellectual pursuits.
Ethics and compliance in research covers a broad range of activity from general guidelines about conducting research responsibly to specific regulations governing a type of research (e.g., human subjects research, export controls, conflict of interest).
eResearch is U-M's site for electronic research administration. Access: Regulatory Management (for IRB or IBC rDNA applications); Proposal Management (eRPM) for the e-routing, approval, and submission of proposals (PAFs) and Unfunded Agreements (UFAs) to external entities); and Animal Management (for IACUC protocols and ULAM).
Sponsored Programs manages the post-award financial activities of U-M's research enterprise and other sponsored activities to ensure compliance with applicable federal, state, and local laws as well as sponsor regulations. The Office of Contract Administration (OCA) is also part of the Office of Finance - Sponsored Programs.
Ethics & Compliance
- eResearch IRB NextGen Project
- Class Assignments & IRB Approval
- Operations Manual (OM)
- Authorization Agreement Process
- ORCR Policies and Procedures
- Self-Assessment Tools
- Resources and Web Links
- Single IRB-of-Record (sIRB) Process
- Certificate of Confidentiality Process
- HRPP Education Resources
- How to Register a Clinical Trial
- Maintaining and Updating ClinicalTrial.gov Records
- How to Report Clinical Trial Results
- Research Study Participation - FAQ
- International Research
- Coordinated Services & Practices (CSP)
- Collaborative Research: IRB-HSBS sIRB Process
- Data Security Guidelines
- Research Incentive Guidelines
- Routine fMRI Study Guidelines
- IRB-HSBS Website Directory and Guidance
- Waivers of Informed Consent Guidelines
- IRB Review Process
- IRB Amendment Process
- Continuing Review Process
- Incident Reporting (AE/ORIO)
- IRB Repository Application
- IRB-HSBS Education
- Newsletter Archive
You are here
- Human Subjects
- IRB Health Sciences and Behavioral Sciences (HSBS)
Informed Consent Guidelines & Templates
U-m hrpp informed consent information.
See the HRPP Operations Manual, Part 3, Section III, 6 e .
The human subjects in your project must participate willingly , having been adequately informed about the research.
- If the human subjects are part of a vulnerable population (e.g., prisoners, cognitively impaired individuals, or children), special protections are required.
- If the human subjects are children , in most cases you must first obtain the permission of parents in addition to the consent of the children.
Contact the IRB Office for more information .
See the Waiver Guidelines for information about, and policies regarding, waivers for informed consent or informed consent documentation.

See the updated Basic Informed Consent Elements document for a list of 2018 Common Rule basic and additional elements.
Informed Consent Process
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent document containing the required information (i.e., elements of informed consent) and the presentation of that information to prospective participants.
In most cases, investigators are expected to obtain a signature from the participant on a written informed consent document (i.e., to document the consent to participate) unless the IRB has waived the consent requirement or documentation (signature) requirement .
- Projects which collect biospecimens for genetic analysis must obtain documented (signed) informed consent.
- It is an ethical best practice to include an informed consent process for most exempt research . IRB-HSBS reviews, as applicable, the IRB application for exempt research, but not the informed consent document itself. A suggested consent template for exempt research can be found below under the References and Resources section. A companion protocol template for exempt research may be found in the feature box, Related Information (top right).
Informed consent documents
An informed consent document is typically used to provide subjects with the information they need to make a decision to volunteer for a research study. Federal regulations ( 45 CFR 46.116 ) provide the framework for the type of information (i.e., the "elements") that must be included as part of the consent process. New with the revised 2018 Common Rule is the requirement that the consent document begin with a "concise and focused" presentation of key information that will help potential participants understand why they might or might not want to be a part of a research study.
Key Information Elements
The image below displays the five elements identified in the preamble to the revised Final Rule as suggested key information.
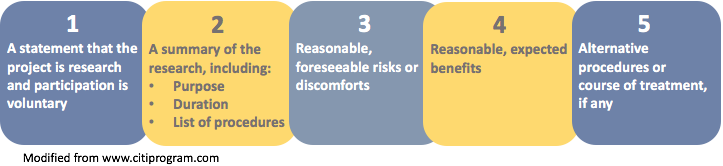
Note: Element number 5 (alternative procedures) applies primarily to clinical research.
General Information & Tips for Preparing a Consent Document
Reading level.
Informed consent documents should be written in plain language at a level appropriate to the subject population, generally at an 8th grade reading level . A best practice is to have a colleague or friend read the informed consent document for comprehension before submission with the IRB application. Always:
For guidance on using plain language, examples, and more, visit: http://www.plainlanguage.gov/
- Tailor the document to the subject population.
- Avoid technical jargon or overly complex terms.
- Use straightforward language that is understandable.
Writing tips
The informed consent document should succinctly describe the research as it has been presented in the IRB application.
- Use the second (you) or third person (he/she) to present the study details. Avoid use of the first person (I).
- Include a statement of agreement at the conclusion of the informed consent document.
- The consent doucment must be consistent with what is described in the IRB application.
Document Formating for Uploading into eResearch
- Remove "track changes" or inserted comments from the consent documentation prior to uploading the document into the IRB application (Section 10-1) for review.
- Use a consistent, clearly identified file naming convention for multiple consent/assent documents.
Informed Consent Templates
IRB-HSBS strongly recommends that investigators use one of the informed consent templates developed to include the required consent elements (per 45 CFR 46.116 ), as well as other required regulatory and institutional language. The templates listed below include the new consent elements outlined in the 2018 Common Rule.
References and Resources
Informed consent guidance.
PDF. Lists the basic and additional elements required for inclusion or to be included, as appropriate to the research, in the informed consent documentation, along with the citiation number [e.g., _0116(b)(1)] within the revised Common Rule. New elements associated with the 2018 Common Rule are indicated in bold text.
Informed Consent Templates (2018 Common Rule)
Strongly recommended for studies that involve the collection of biospecimens and/or genetic or genomic analysis, particularly federally sponsored clinical trials that are required to post a consent document on a public website. Last updated: 04/10/2024.
(Word) Blank template with 2018 revised Common Rule key information and other required informed consent elements represented as section headers; includes instructions and recommended language. It is strongly advised that you modify this template to draft a project-specific informed consent document for your study for IRB review and approval. Last updated: 04/10/2024
Other Templates
Informed Consent documents are not reviewed by the IRB for Exempt projects. However, researchers are ethically bound to conduct a consent process with subjects. This template is suggested for use with Exempt projects. Last updated 4/10/24
(Word) General outline to create and post a flyer seeking participation in a human subjects study. Includes instructions.
(Word) Two sample letters for site approval cooperation between U-M and other institutions, organizations, etc. Letters of cooperation must be on U-M letterhead and signed by an appropriate official. These letters are uploaded into the Performance Site section of the eResearch IRB application.
For use by U-M Dearborn faculty, staff, and students conducting non-exempt human subjects research using subject pools. Last updated 4/10/24
For use by U-M Dearborn faculty, staff, and students conducting exempt human subjects research using subject pools
Researchers who will conduct data collection that is subject to the General Data Protection Regulation (GDPR) must use this template in tandem with a general consent for participation template/document.
- Brief protocol for exempt research including data management and security questionnaire
Child Assent and Parental Permission
- Child assent ages 3-6
- Child assent 7-11
- Parent permission
- Child assent 12-14
IRB-Health Sciences and Behavioral Sciences (IRB-HSBS)
Phone: (734) 936-0933 Fax: (734) 936-1852 [email protected]
- Human Subjects Protections

Sample Consent Forms
Consent form templates.
These consent form templates have been posted for your reference. When completing and IRB submission in IRBIS, please fill in the application and use the consent form builder specific to your project. For more information, please find instructions here .
Summary of Changes to the Regulations for Informed Consent: Revised Common Rule Changes to Informed Consent and Waiver Requirements
Summary of Changes to Consent Documents:
- Informed Consent Documents – Version 2.0 Summary of Changes
- Informed Consent Documents – Version 2.1 Summary of Changes
- Informed Consent Documents – 10/26/2020 Summary of Changes
- Informed Consent Documents – 4/10/2023 Summary of Changes
Concise Summary examples can be found here .
Guidance on the use of plain language in consent forms:
- Clinical Research Glossary
- Webinar: The Promise of Plain Language: Launching a Glossary to Support Participant Understanding of Clinical Research – Recording & Slides
There are a few additional forms that are not provided online and may be accessed below. As needed, these should be completed and uploaded to your IRB application.
Foreign Language Consent Forms
COVID-19 Related Forms:
- Spanish-IRB-COVID Information Sheet
- Spanish COVID Consent Letter v2
- Spanish COVID Informational Sheet Translation Certificate
Informed Consent Short Form (for a single subject who may be illiterate, or otherwise unable to read the consent form — used when full consent form has to be read or translated for subject).
- Informed Consent Short Form Guidance
- Simplified Chinese
HIPAA Templates
- Sample HIPAA Authorization Template
- Sample HIPAA Authorization Template in Spanish ( Certification )
Human Subjects Division
- [email protected]
- 206.543.0098
QUICK GUIDE
While all stakeholders are encouraged to read and become familiar with this entire guidance, the following resources provide a summary of the most vital aspects of consent:
- Consent Overview [Five high-level consent considerations which must be a part of a meaningful informed consent process and form]
- TIPSHEET Consent [Researcher summary of the main points in this guidance]
- GUIDANCE Consent Elements for Externally Reviewed Studies [Consent requirements for studies reviewed by a non-UW IRB.]
- Consent Templates [Webpage with links to all current HSD consent templates.]
- Guidance on Designing the Consent Process [Use with or without a UW template to build the consent process and form.]
GUIDANCE Contents
Purpose and applicability, definitions, regulatory oversight, exempt research, consent overview, the key information requirement, identifying and describing reasonably foreseeable risks in research, undue influence and coercion.
- Diminished or Fluctuating Consent Capacity and Use of a Legally Authorized Representative (LAR)
- Interpretation
- Translation
- Short form consent requirements
Subjects with low literacy
Subjects with visual impairments, subjects who cannot write a signature on a consent form, electronic consent.
- Required signatories
Verification of identity
Signature format, approvable methods for obtaining handwritten signatures, approvable methods for obtaining electronic signatures, approval watermark, records retention and access, reconsent and ongoing subject communication, washington state law and consent.
- Audio recording
Related Materials
- Version Table
This guidance provides researchers, the Human Subjects Division (HSD), and the UW Institutional Review Boards (IRBs) with an overview of the requirements and best practices for obtaining regulatorily compliant and meaningful consent, parental permission, and assent for participation in research. This information applies to:
- All research reviewed by the UW IRB, including non-UW institutions and sites for which the UW IRB is providing review, unless otherwise indicated.
- UW research reviewed by an external (non-UW) IRB.
The following specialized consent-related topics are covered elsewhere:
- UW research determined to be exempt or reviewed by the Limited IRB process. Details can be found in the guidance on Exempt Research .
- Exception from Informed Consent ( EFIC supplement ), which may be appropriate when the time frame between recruiting and study procedures is too short for obtaining consent (e.g., emergency medicine studies).
- Emergency or Compassionate Use of investigational drugs or devices for clinical care (not research) ( Single Patient Emergency or Compassionate Use ).
- Clinical use of Humanitarian Use Devices .
Informed consent for research must be legally effective and obtained before the subject can participate in any study-related activities. It should include an active process of sharing information between the researcher and potential subject and an affirmative agreement by the subject that they want to participate. The concept of “implied” or “passive” consent (e.g., parental permission is assumed unless the parent “opts out” of their child’s participation in the research) does not meet the requirements for informed consent for research. Informed consent serves to:
- provide sufficient details about the study so prospective subjects can make an informed decision about whether to participate;
- facilitate understanding of what has been disclosed; and
- promote voluntariness about whether to participate.
Consent method . The method by which consent is obtained depends on the specifics of the study and the regulations that govern the research. When documentation of consent can be waived, researchers have greater flexibility in the ways in which they can design the consent process. For example, it may be appropriate to conduct an oral consent process without providing the subjects with printed consent materials if the study is a low risk, one-time interview with adults. If subjects will participate in the study remotely (e.g., low risk computer surveys) then providing the subjects with an electronic information statement would be appropriate. Regardless of the method used (e.g., oral, electronic, in-person), consent processes must include all the applicable regulatory requirements ( Regulatory Oversight ) unless the IRB determines any requirements can be waived.
Assent is obtained from subjects who are unable to provide legally-effective informed consent on their own behalf because they are minors or have diminished decision-making capacity. Subjects who have the cognitive capability may provide their assent to participate and a parent/guardian or legally-authorized representative (LAR) may provide consent on behalf of the subject. Alternatively, assent, LAR consent, and/or parental permission may be waived by the IRB. Failure to object should not be equated with an active willingness to participate. Review the section on Assent for more information.
Subject . Unless otherwise indicated, in this guidance the term “subject” refers to: the subject, the parent(s) or guardian of a minor subject, and the LAR for a decisionally-impaired adult subject.
Recruitment . Advertisements, announcements, social media (e.g., Facebook, Twitter) postings, and other recruitment processes and materials are generally considered to be part of the consent process.
Which regulations apply?
Consent requirements may vary depending on the subject population, federal regulations, state laws, international laws, and institutional polices that apply to the research.
Federal agency consent requirements
It is HSD policy to voluntarily apply the Common Rule (45 CFR 46) consent regulations to all research reviewed by the UW IRB except as described in the HSD Flexibility Policy ( GUIDANCE Authority and Responsibilities of HSD and UW IRB ), in addition to other applicable requirements. The guidance, Designing the Consent Process provides information about the general requirements and elements of consent as well as the criteria for waiving elements and documentation of consent for the Common Rule, FDA, and other federal regulatory agencies. That guidance also lists the consent requirements for federally-designated protected populations (i.e., pregnant women and neonates, prisoners , children).
State or other local law
State laws in the jurisdiction where the research is being conducted may affect the consent process (e.g., mandatory reporting; age of majority). Refer to the guidance on Human Subjects Regulations , Mandatory State Reporting , and the sections in this guidance on consent using a legally authorized representative and the Washington State law on audio recording for details. Researchers are responsible for identifying applicable state or other local laws when the research is conducted outside of Washington State, including internationally ( OHRP webpage for international research ).
HSD/UW IRB policy
HSD/UW IRB policies related to consent can be found in this guidance, the guidance on Designing the Consent Process , and the worksheet Consent Requirements and Waivers .
HSD does not apply consent regulations to research that is determined to be exempt from IRB review. Nor does HSD review and approve consent plans and consent materials for exempt research. Researchers are still responsible for protecting the rights and the welfare of subjects in their research and for providing subjects with information about the research prior to their agreement to participate. Review the section on Information for subjects in the guidance on Exempt Research for full details.
More on The Purpose of Consent
An effective consent process provides the information that a reasonable person would want to have, in a transparent way , so that the prospective subjects are in control of their decision to provide authorization (or not) to participate, based on whether their own values and opinions align with those of the research, and considering the risks and benefits from their individual perspectives.
Most research generates knowledge to promote a common good. It is often funded by public sources and is increasingly integrated into health care delivery systems. In addition to the value of consent to the individual, there is a social value to establishing effective and ethical consent processes and norms to ensure accountability of the research enterprise.
More on Facilitating Informed Decision-Making
Information provided during consent should emphasize the Key Information that is most likely to assist the particular subject population with making a decision about whether to participate in research. This means that the consent process and/or form does not necessarily need to include a detailed description of every procedure the enrolled subject would undergo. Rather, it should emphasize the information that will be most influential for enrollment decisions. Subjects can be provided with tables or supplemental information sheets with additional study details after the enrollment decision has been made.
Consent information must be presented in a way that facilitates comprehension. This means providing consent information in a logical sequence and in a way that allows for real-time review during discussion between study staff and the prospective subject. Providing context that is meaningful and language that is familiar and at an appropriate reading level for the particular subject population will make the content easier for subjects to understand.
More on Written Consent Materials
Written consent materials should be presented from the perspective of the subject and what it would mean to them to participate (e.g., the pros and cons, and whys and why nots of the many aspects of the study). When consent information is provided in writing, understanding may be facilitated by breaking up dense text with sectioning, pictures, icons, schematic diagrams of study design, or putting information in side-by-side comparison tables. Headings should be subject-focused rather than regulations-focused. For example, depending on the study and population-specifics, information about risks and benefits, time commitments, and certain procedures may be appropriate under the headings, “Why I may want to participate” and, “Why I may not want to participate”. As described in Consideration 2, the consent form does not need to include every procedure the subject would undergo and should instead focus on the procedures and other information that would be most likely to influence the subject’s decision about whether to enroll in the study .
More on The Consent Process
The presentation and discussion of consent information, as well as the consent form itself, are single elements of the overall consent process. Decision-making by prospective subjects typically begins with the information presented in recruitment materials and in initial discussions with study staff, well before the consent form is presented. This information may be equally or more influential in final decision-making as the consent form. Post-enrollment communication, such as answering subject questions and providing them with relevant new information, is also part of the consent process, because subjects consider throughout a study whether they wish to continue their participation. The continued education and engagement of subjects throughout the research process is vital. In addition, it is a way to demonstrate respect and gratitude for their contribution and to maintain trust. Finally, the initial consent procedure may need to be repeated or supplemented if relevant new information becomes available or if the study involves a lengthy commitment from subjects.
More on Coercion, Undue Influence, Diminished Consent Capacity, and Vulnerable Populations
Federal regulations identify pregnant women*, prisoners, and children as Protected Populations and specify additional protections and consent requirements for them. However, these additional required protections may not be enough to ensure consent comprehension and voluntary participation for these groups. Other populations are also vulnerable to undue influence or coercion. Similar protections may be appropriate for them. These additional safeguards must be considered throughout the vulnerable subject’s participation in the study (i.e., recruitment, obtaining consent, and after enrollment). *Pregnant women are not designated as a vulnerable population in the Common Rule or FDA regulations. However, there are additional regulatory requirements for enrolling this population for research funded or supported by the agencies that signed Subpart B. Review Protected and Vulnerable Populations for additional discussion.
The Key Information requirement and its purpose
The Common Rule requires that informed consent must begin with a concise and focused presentation of Key Information that is most likely to assist prospective subjects or their representatives in understanding the reasons why they might or might not want to participate in the research . The Key Information must be organized and presented in a way that facilitates comprehension. Although a Key Information section may serve as an abstract or executive summary for a longer consent form, that is not its primary function.
When is Key Information required?
All consent-related materials must include Key Information if the study meets these criteria:
- The study is non-exempt human subjects research reviewed by the UW IRB (per HSD policy , it does not matter whether the Common Rule governs the study); and
- The IRB has not fully waived the requirement to obtain consent; and
- The study is fully or conditionally approved by the IRB on or after January 21, 2019 (the date when this regulatory requirement was enacted).
- This includes consent forms, online or paper information statements, e-consent information, the short form consent process, oral consent with no written component, parental permission, and LAR consent.
The Common Rule does not require Key Information in assent forms for children and decisionally-impaired adults, however, it is HSD policy to require Key Information when assent forms have more than 2000 words.
Consent materials must have a distinct Key Information section when they are more than 2,000 words (not counting any signatures sections; approximately 5 pages; single-spaced; 1-inch margins). It is HSD policy to consider shorter documents (less than 2000 words) to have met the requirement to present Key Information in a concise and focused manner because of their short length. However, the IRB has the authority to require a separate Key Information section if appropriate.
There is no specific information that must be included in the Key Information. The appropriate information depends on the nature of the study, nature of the subject population, and on the other information presented as part of the consent process and/or form. Federal guidance stresses that the Key Information should be meaningful within the context of the study and has therefore avoided strictly defining what information should be included. However, the guidance does generally expect that Key Information include a concise explanation of the following elements:
Key Information elements
- voluntary consent is being sought for research;
- research purpose, expected duration of participation, and procedures;
- the most important, reasonably foreseeable risks or discomforts * ;
- reasonably expected benefits to the subjects or others; and
- appropriate alternative procedures or courses of treatment, if any.
*Risks and discomforts in Key Information should be described with the study context and subject perspective in mind. For example, if the most important risks associated with the study are from a blood draw, these risks should be described in Key Information. However, if a blood draw is only one of many procedures and the other study procedures are associated with more significant risks to subjects, then information about the blood draw may be left out of Key Information and instead described in a more detailed Risks section later in the consent process or form.
HSD tip . For most biomedical studies, information about compensation for injury, specific protections for privacy and confidentiality, and how data and specimens will be shared and stored does not need to be in the Key Information. Such information can be described elsewhere in the consent form or process.
Primary factor: the subject population . Key information is intended to be the information that is most likely to assist the specific subject population (e.g., end-stage cancer patients; first-year college students; parents of toddlers with autism; prisoners). Identifying this information is the responsibility of the researcher . This may mean that the researcher needs to consult with publications about research subjects’ preferences, disease-focused nonprofit groups, patient interest groups, or other researchers/study staff with experience with the specific population. It may also involve directly consulting selected members of the study population.
When writing Key Information, consider the following questions :
Questions to consider
- What are the main reasons a subject will want to join, or not join, this study?
- What is the research question the study is trying to answer and why is it relevant to the prospective subject?
- What aspects of research participation in this study are likely to be unfamiliar to a prospective subject, diverge from a subject’s expectations, or require special attention?
- What information about the subject is being collected as part of this research?
- What are the types of activities (procedures) that subjects will do in the research?
- What impact will participating in this research have on the subject outside of the research? For example, will it reduce options for standard treatments?
- How will their experience as a research subject in this study differ from treatment they might receive as a patient outside of the study?
- In what ways is this research novel?
- What is the anticipated time commitment for the subject?
Relationship to the rest of the consent form/materials . A Key Information section may appropriately include a summary of relevant pieces of information that are then explained in greater detail later in the consent form or process. However, information that is fully described in the Key Information section does not need to be repeated later in the form or process. Similarly, if the Key Information section includes any of the elements of consent described in the worksheet Consent Requirements and Waivers , those elements do not need to be repeated later in the form or consent process. Review our Key Information examples .
Structure and presentation
The Key Information may be in a separate document or in the beginning of the consent form, but it must be presented to subjects at the beginning of the consent process. The Key Information requirement applies to the consent process as a whole – not simply to consent documents. In other words, there is flexibility in how the presentation of Key Information is structured if it is organized and presented in a way that facilitates comprehension for prospective subjects.
In most cases, when there is a separate Key Information section, it will be relatively short compared to the rest of the consent document or process. For example, complicated clinical trials involving high risk procedures typically involve consent forms of more than 20 pages. In these cases, the federal agencies expect that the Key Information section would be no more than a few pages. However, those agencies have also stressed that the section should be meaningful within the context of the study and have therefore purposely avoided specifying length requirements. Review our Key Information examples .
The UW IRB is defining the regulatory term reasonably foreseeable as those risks or discomforts that must be included in the informed consent process because they are both reasonably foreseeable and meet any of several additional criteria. A workable definition of reasonably foreseeable is required to ensure that a description of any reasonably foreseeable risks or discomforts to the subject are presented to a potential research participant as part of the informed consent process ( 45 CFR 46. 116 (b) (2) ; 21 CFR 50.25(a)(2) ). This is particularly relevant for risks associated with drugs, devices, or complex procedures where the number of risks may be large, and inclusion of all possible risks may detract from an individual’s ability to consider those risks that are relevant to their decision to participate in the research.
The following definition, built upon FDA guidance and SACHRP guidance , should be used to help evaluate which risks the UW IRB considers reasonably foreseeable and, therefore, should be included in the consent form or included as part of the informed consent process.
Reasonably Foreseeable Risks Reasonably foreseeable risks are those risks that a reasonable person in the target population would find meaningful to their decision to participate in the research. This should include (but not be limited to) any of the following: (a) risks that are more likely to occur ; (b) risks that are serious ; OR (c) risks that the research is evaluating and that an individual would not otherwise be exposed to if they were to decide not to participate in the research.
(a) “More likely to occur” are risks that are frequent/common or very frequent/very common as described in the table below. Risks expected or known to be very frequent/very common or frequent/common should generally be included in the consent form and/or consent process. The IRB can always exclude frequent/very frequent risks that don’t apply to the target population (for example the study includes an adverse drug reaction with acetaminophen but subjects taking acetaminophen are excluded from participating). Conversely, the IRB can require the inclusion of infrequent, rare, or very rare risks that don’t otherwise meet the overall definition above if they determine the target population would find them meaningful to their decision to participate in the research (e.g., rare permanent teeth discoloration).
We recognize that in some cases, there is no written source or data (e.g., drug Investigator Brochure or package insert) to rely on when determining frequency of risks. In those instances, the expert judgement of the researcher, or relevant published literature, would need to be applied when determining frequency. The IRB, in their review, would have the opportunity to check these assumptions.
(b) “Serious” are risks that fall under the FDA’s definition of a Serious Adverse Event (i.e., anything that would result in death, be life-threatening, would require hospitalization to treat, could cause disability/permanent damage, cause birth defects, require an intervention to prevent permanent impairment or damage or other important medical events). For a full description of the definition, visit this FDA webpage . These risks should generally be included regardless of the potential frequency of occurrence. As with ‘more likely to occur’ the IRB has discretion to leave out serious risks that are not relevant to a particular population, may be theoretical or unsupported by the data, or would detract from a participant’s understanding of the more significant risks associated with the primary aim(s) of the research. For example:
- A drug can be life threatening to children under 5 years of age, but all children are excluded from participating in the study
- A researcher proposes including Stevens-Johnson syndrome (SJS) as a possible risk of the study but there has been no incident of SJS to date
- A study of a novel diabetes drug involves routine blood draws with use of topical lidocaine to numb the area of the needlestick but the very rare risk of anaphylaxis from the lidocaine is excluded (i.e., inclusion is not relevant to a reasonable person’s meaningful decision to participate in the research).
(c) “Risks that the research is evaluating.” If researchers design and conduct a study for the purpose of evaluating a particular risk then the research risk being evaluated has been recognized as a sufficiently possible outcome to be considered “reasonably foreseeable” and should be disclosed to prospective subjects (adapted from OHRP Comparative Effectiveness Guidance ).
A researcher may suspect a new study drug might cause slightly increased blood sugar levels. There is no prior evidence of this, and they think it’s very unlikely (so it doesn’t trigger inclusion based on frequency), and it doesn’t meet the ‘serious’ criteria for inclusion in the consent. However, they are planning to actively monitor subject blood glucose levels throughout the study and intervene when appropriate. This would be an example where we would expect the researcher to include risks of increased blood sugar levels in the consent form.
Additional Considerations Risk statements in consent forms should be simplified such that the information included is understandable and relevant to the subject population. Detailed descriptions of all potential risks are counterproductive if they do not provide potential subjects with useful information and may inadvertently distract subjects from relevant data. ( SACHRP recommendations )
For minimal risk procedures , risks or burdens that are immaterial or obvious to potential participants need not be explicitly addressed in the consent form or dialogue. For example, participants need not be told that needle sticks can cause minor pain or that surveys can be boring. There is also no need to specifically state the absence of risk where none exists. ( SACHRP recommendations )
Applying the Guidance – Examples
Study Summary A particular condition has several treatment options, but an individual’s response to the treatment can be highly variable and unpredictable. Many patients undergo a trial-and-error period until they find a treatment plan that is effective for them. The purpose of this study is to identify biomarkers that may help predict a response to a specific drug in individuals diagnosed with the condition. The drug is an FDA-approved drug for the treatment of this condition and is frequently used in clinical care. A revised package insert includes three new post-market risks.
- risk of serious infection (12%)
- diarrhea (5.3%)
- mild back pain (0.5%)
Should these risks be added to the consent form/process as reasonably foreseeable risks?
Analysis Serious infections are very frequent according to the investigator’s brochure. Diarrhea is a frequent risk according to the investigator’s brochure. Therefore, both serious infections and diarrhea meet the definition of a reasonably foreseeable risk as they are risks that are more likely to occur .
Risk of mild back pain does not meet the definition of a reasonably foreseeable risk because it is not more likely to occur , nor is it serious or being evaluated by the study .
Answer The risks of serious infection and diarrhea need to be added to the consent form/process.
Study Summary The study aims to optimize the imaging of abdominal organs with a contrast enhanced ultrasound comparing clinical ultrasounds with research ultrasounds. Study procedures include a contrast-enhanced ultrasound using a contrast agent (FDA approved). This procedure adds approximately 15 additional minutes to the patient’s standard of care ultrasound. Although rare, the contrast agent does have a risk of severe allergic reaction.
Should this risk be added to the consent form/process as a reasonably foreseeable risk?
Analysis Severe allergic reaction is a rare risk and is therefore not more likely to occur . However, severe allergic reaction meets the definition of a serious risk as it could be life threatening.
Answer Yes, the risk of severe (life-threatening) allergic reaction should be added to the consent form.
Study Summary The purpose of this study is to investigate the safety and effectiveness of long-term treatment using either a combination of drugs or a single drug to prevent subjects acquiring HIV in subjects who are HIV negative.The investigator brochure lists three rare but serious risks for the drug combination that are not currently in the consent form (viral resistant mutations, interstitial nephritis, and immune reconstitution syndrome) that exclusively affect HIV positive individuals.
Analysis These are risks associated with taking the drug if HIV positive, however, the study is excluding HIV positive subjects and regularly testing subjects for HIV transmission.
Answer No, these risks do not need to be added to the consent form.
When to Describe Risks for Studies Evaluating Medically Recognized Standards of Care It can be difficult to determine which risks to include in the consent form when the research involves medically recognized standards of care procedures or treatments in addition to, or instead of, any investigational procedures or treatments.
In general, the reasonably foreseeable risks associated with a standard of care procedure or treatment should be described in the consent form when:
- the standard care procedure or treatment is required solely for research purposes ;
- the research design dictates the procedure or treatment that will be provided to the subject;
- the research design restricts the ability of the subject or the subject’s care provider to choose the procedure or treatment that will be provided to the subject.
The examples below illustrate how to identify: (1) which risks are research risks and should be described in the consent process/form; and (2) which risks are not research risks and should not be described in the consent process/form. Additional information can be found in the OHRP draft Guidance, “Disclosing Reasonably Foreseeable Risks in Research Evaluating Standards of Care” .
Example 1 - biomedical
Example 2 - biomedical.
Study Summary A physician researcher at a local clinic plans to do a research study with patients who have been recently diagnosed with osteoporosis and who require treatment. The physician wants to compare the effects of two different FDA-approved estrogens on the osteoporosis. As part of clinical care, the physician normally does a DEXA scan to measure bone density before beginning any osteoporosis treatment, and then repeats the DEXA scan after one year to see whether the treatment is having any beneficial effects.
The physician will randomly assign each subject to one of two FDA-approved estrogen treatments for osteoporosis.
Research Risks
The risks associated with the two estrogen treatments are research risks and must be included in the consent process/form. The choice of how the osteoporosis will be treated has been restricted by the research design (to the two estrogens).
Not Research Risks
The risks associated with the two DEXA scans are not research risks because the physician obtains DEXA scans for all of their patients being treated for osteoporosis, not just those who are in the research study.
The physician is interested in the effects of the two FDA-approved estrogens. However, they will prescribe osteoporosis treatment to all of their patients based solely on clinical factors. The treatment could be one of many estrogens (including the two they are interested in), or one of many bisphosphonate drugs. For example, patients who are breast cancer survivors would receive a bisphosphonate drug instead of estrogen because of the effects of estrogens on the growth of some types of cancer cells. Per the physician’s normal clinical procedures, they will do DEXA scans on all their patients before they begin any treatment and after one year on the treatment.
None of the risks associated with the two estrogen treatments and none of the risks associated with the DEXA scans are research risks because they are not dictated by the research protocol.
Example 3 - behavioral
Study Summary A university has counseling services available for students who engage in binge alcohol drinking. The counseling always includes two educational sessions, followed by sessions based on one of several widely accepted approaches to reducing binge drinking. All students fill out a series of standard validated questionnaires about drinking behavior and attitudes before and after they receive counseling. A psychologist at the counseling center is interested in comparing the effectiveness of two of these approaches: a short series of motivational interviews versus participation in a weekly cognitive-behavioral group session.
Each student who agrees to participate in the research will have the two educational sessions and will then be randomly assigned to either the motivational interviews or the cognitive-behavioral group. The psychologist will use the results of the clinic’s pre/post questionnaires to assess the two approaches.
The risks associated with motivational interview and the cognitive-behavioral group are research risks and must be described in the consent process/form. the choice of counseling techniques is being dictated by the research design. The confidentiality risks associated with the questionnaire for research purposes are research risks and must be described in the consent form.
The risks associated with the educational session are not research risks because all students who come into the counseling center participate in those sessions whether or not they are in the research study.
Each psychologist at the counseling center works with their student clients to decide which approach is best suited to the student’s circumstances. Students who join the cognitive-behavioral group or who undergo the motivational interview approach are referred to the psychologist researcher before they begin counseling. The psychologist researcher gives those students a series of questionnaires about depression, social anxiety, and stress coping strategies before and after they receive the counseling. The psychologist researcher also obtains the results of their standard clinic questionnaires.
The risks associated with the research questionnaires (depression, social anxiety, stress coping strategies) and with research use of the standard clinic questionnaires are research risks and must be described in the consent process/form.
None of the risks associated with the two counseling approaches or with administration of the standard clinic questionnaires are research risks.
Protected and Vulnerable Populations
Human subjects regulations require that the consent process minimizes the possibility of undue influence or coercion and notes that some populations of subjects are considered especially vulnerable. The regulations designate three “protected populations” (pregnant women, prisoners, children) that each have additional required protections. Other vulnerable groups may also require additional protections against the potential for coercion or undue influence.
Undue influence may occur through an excessive offer of something valuable or desirable that influences decision-making in inappropriate ways. Undue influence is often scrutinized by the IRB when subjects will receive significant payment for participation ( Subject Payment guidance ). There are other situations when concerns about undue influence may arise. Federal regulatory guidance concedes that there is no bright line between mere influence and undue influence and so it is up to the IRB to make that distinction.
A study benefit might raise concerns about undue influence when it is likely to: 1) inhibit a potential subject’s adequate consideration of, and reflection about, important study features such as risks, burdens, and discomforts; or 2) impair a potential subject’s understanding of the research and their participation in it.
Coercion occurs when an overt or implicit threat of harm is intentionally presented by one person to another to obtain compliance. Prior to implementation of human subjects research regulations, there were many instances of subjects being coerced into research. Regulatory protections and IRB oversight have reduced the likelihood of coercion in research, but it is still something researchers and the IRB should be cautious about, particularly when researchers are in a position of power over subjects (e.g., physician and patient or professor and student).
Minimizing the potential for undue influence or coercion . It is important to remember that the IRB is tasked with minimizing , not eliminating the possibility of undue influence or coercion. Whether a subject may be vulnerable to being unduly influenced or coerced to participate in research is contextual and dependent upon the individual subject’s situation, yet the IRB approves research on a population-level.
There will be some situations where identification of the potential for unduly influencing a subject group or groups will be clear, as will the steps for minimizing that potential. For example, when there are power dynamics involved (e.g., professor/student; supervisor/employee), it may be appropriate to ensure the consent process is conducted by someone outside the power dynamic. It may also be important to ensure that the person in power is not aware of which of their students, supervisees, etc. participated and which did not. For many other situations, rather than trying to anticipate what might be unduly influential for every individual subject, the IRB should make use of existing minimization tools including some of the other regulatory consent requirements and protections that have been described in this guidance. These include ensuring that:
- subjects have adequate time to consider and discuss participation prior to giving consent; and
- consent processes and materials are understandable and include Key Information in sufficient detail for the specific subject population to be able to make an informed decision about participation.
The IRB should pay particular attention to the way a study’s benefits are described. Known benefits should be accurately described and not exaggerated. Potential or uncertain benefits should be described clearly as to what is known about the uncertainty or likelihood of the potential benefits. A meaningful consent process enables prospective subjects to decline participation in research that they judge to be harmful or inappropriate according to their own interests, values, and obligations.
Examples of undue influence and coercion adapted from an Advarra IRB blog post :
Examples of Undue Influence and Coercion
Children are a federally designated “protected population” with additional regulatory protections and requirements described in Subpart D of the Common Rule. When children participate in research, parent/guardian permission and child assent are sought rather than consent.
Review our guidance on Involvement of Children in Research for a discussion of who qualifies as a child. Review the assent section of this guidance for details about when assent must be obtained and for guidance about designing the assent process and form.
Parent/guardian permission
Definitions . Permission is the agreement of parent(s) or guardian(s) to a child’s participation in research. A parent is defined as the child’s biological or adoptive parent and a guardian is an individual who is authorized under applicable State or local law to consent on behalf of the child to general medical care. In general, one or both parents or guardians must be provided with the same information that is provided during an adult consent process, unless the regulatory criteria are met for waiving permission or waiving or altering elements of permission ( Children worksheet ).
One or two parent permission . The permission of one or both parents may be required depending on the children’s risk level category as determined by the IRB ( Involvement of Children in Research guidance ; Children worksheet ).
Waivers and alterations . Parental permission can be waived or altered, and documentation of permission can be waived, under the same criteria for waiving or altering consent and consent documentation. [ For FDA-regulated research, parental permission can only be waived under the minimal risk criteria .] Review the Children worksheet for a full description of waiver criteria.
Pregnant women and neonates
Pregnant women and neonates are a federally designated “protected population” with additional regulatory requirements and protections described in Subpart B of the Common Rule. However, bioethicists now tend to view pregnant subjects as a complex or special rather than vulnerable population, because pregnancy itself does not impair a subject’s ability to comprehend an informed consent process. Some research with pregnant women may have additional complexities such as weighing the risks and benefits of both the pregnant woman and the fetus or mitigating risk of exploitation in some specific contexts. However, these potential complexities are dependent upon the specific research design and are not necessarily applicable to all studies enrolling pregnant women. It is noteworthy that, in the 2018 revision to the Common Rule, pregnant women were removed as an example of a population that is potentially vulnerable to coercion or undue influence. Subpart B consent requirements for pregnant women and neonates are listed in the worksheets on Pregnant Women and Neonates .
Prisoners are a federally designated “protected population” with additional regulatory requirements and protections described in Subpart C of the Common Rule ( Prisoners guidance ). These protections, and restrictions about the types of research in which prisoners may participate, were put into place to prevent a return to past research practices that exploited prisoners by exposing them to an unfair burden of risks, coercive and unduly influential enrollment practices, and an absence of informed consent procedures. However, these same protections can unfortunately restrict prisoners’ autonomy to independently weigh what risks they are willing to assume and possibly limit their access to potentially beneficial research.
Designing consent with prisoners . Poor reading and communication skills tend to be more common in prison populations ( Committee on Ethical Considerations for Revisions to DHHS Regulations for Protecting Prisoners in Research, 2006 ). This creates challenges for obtaining informed consent because the process typically relies heavily on written materials. For example, it has been shown that undue influence was significantly higher among prisoners with lower educational attainment and those who had spent a longer time in prison ( Ravi et al., 2018 ). This makes it particularly important that the Key Information about a study be tailored to the prisoner population and communicated in language that is understandable to them. Allowing adequate time during the consent process for discussion and opportunities for the subject to ask questions becomes extremely important when enrolling prisoners. Providing a clear and accurate description of confidentiality measures should also be discussed in detail, given the confidentiality limits that may exist in prisons because of institutional policies. As is noted in Consent Consideration # 5 , with the appropriate protections in place, prisoners may still be able to take advantage of opportunities to share in the risks and benefits of research.
Assent is a subject’s affirmative agreement to participate in research. It must be accompanied by parent/guardian permission or the consent of a legally authorized representative ( Use of a LAR ). Alternatively, assent, LAR consent, and/or parental permission may be waived by the IRB. It is HSD policy that researchers must obtain assent from subjects who are minors or decisionally-impaired adults, when the individuals are capable of providing assent and when it is appropriate to do so. Researchers who do not plan to obtain assent from these subjects must provide the IRB with justification about why obtaining assent is not appropriate.
Assent determinations . The IRB must determine whether the prospective subjects are capable of providing assent and, if they are, whether the proposed assent process is adequate for helping subjects decide whether they want to participate in the research ( Consent Requirements and Waivers worksheet ). If the IRB determines that assent is possible, the IRB may nonetheless waive the assent requirement under the same criteria for waiving consent ( Consent Requirements and Waivers worksheet ). These determinations will depend on several factors including the age, maturity of a minor, psychological state, and/or cognitive capabilities of the prospective subjects. The decision may apply to all subjects in the study or may differ by individual subject or group of subjects.
Assent requirements . Researchers are asked to describe their plans (if any) for obtaining and documenting assent in the IRB application. The assent process and any materials should provide information that is relevant for the subject population considering their capacity for comprehension and the research procedures . There is no regulatory requirement to provide all the standard elements of consent during the assent process. There is also no requirement to document assent with an assent form, although researchers should have some way of recording that they obtained assent.
Assent outcomes . Potential subjects may express an active willingness to participate, may simply fail to object, or may dissent (express unwillingness to participate). Failure to object should not be equated with an active willingness to participate. In general, dissent should be respected. A disagreement between a parent(s)/LAR and the potential participant may arise for many reasons and every effort should be made to reach consensus between the parent(s)/LAR and potential participant. However, the IRB may allow the parent(s)/LAR wishes to prevail over the potential participant’s dissent when the potential participant may directly benefit from the research. In these cases, it may be more appropriate to waive assent, rather than ask the subject and then not take their wishes into account. A potential participant’s assent cannot override a “no” from a parent or LAR unless the IRB has waived the requirement for parental permission or completely waived consent.
Child assent
Design . When designing the assent process and, if applicable, assent form, researchers should consider the nature of the research as well as the age, maturity, and psychological state of the children. When the research involves adolescents whose capacity to understand resembles that of adults, the assent procedure and form would be similar to one designed for adults. In some cases it may be appropriate to use a single form to obtain both parental permission and child assent. For younger children, researchers should focus the assent process and information on the aspects of the research that the children would be mostly likely to understand and be interested in. This would likely be a short description of what the experience would be like, how long it would take, and whether there would be any pain or discomfort as a result of participating. For studies that span several age groups, it may be necessary to design multiple assent procedures and/or forms based on the comprehension capability of the different groups.
Longitudinal research and children who reach the age of majority . The assent process should be viewed as ongoing throughout the duration of the research. If a subject enrolls in research at a young age and remains enrolled for many years, it may be necessary to re-assent as the child’s capacity to understand increases. If the child reaches the legal age of consent while enrolled, the regulations about child participants no longer apply. In these cases, unless the IRB has waived the requirement, the researcher must obtain legally effective research consent from the now-adult subject for any ongoing interactions or interventions or continued analysis of identifiable specimens or data.
Diminished or Fluctuating Consent Capacity and use of a Legally Authorized Representative (LAR)
Presumption of capacity to consent.
Individuals who have reached the age of legal consent in the jurisdiction in which the research is being conducted are presumed to have capacity to give informed consent for research. In the absence of indications to the contrary (e.g., a diagnosis of advanced dementia; impaired decision-making ability due to a stroke in specific brain areas), such capacity can be assumed without further evaluation of documentation unless required by the IRB.
Nature of decision-making impairments
Decision-making impairments may be permanent, temporary, progressive, or fluctuating. Also, the capacity to consent is protocol-specific and situation-specific. For example, an individual may have the capacity to make the research participation decision for a low-risk study in usual circumstances but not have the capacity in a stressful situation to understand and evaluate a high-risk study. In other words, the presence of a cognitive impairment or cognitive-state-altering circumstances does not necessarily mean that the person is incapable of an informed consent decision. However, the IRB will expect the researcher to assess the impact of the circumstances on the person’s decision-making ability about the specific participation decision.
Legally-authorized representative
If an adult’s capacity to consent is reduced, then they can participate in the research only if a legally-authorized representative (LAR) provides consent on their behalf. In addition, researchers must obtain some type of assent from the subject, when the individuals are capable of providing assent ( assent ). A careful balance of the Belmont Ethical Principles is vital to enrolling subjects with diminished consent capacity. So long as the additional protections afforded by LAR consent are in place to offset the subject’s diminished autonomy, it is possible that individuals with reduced consent capacity can still participate in research.
Who can be a LAR is determined by the laws of the jurisdiction in which the research is conducted. There are no Washington State laws that directly address the use of LARs in research. However, Washington State law RCW 7.70.65 defines who may serve as a LAR for providing informed consent for health care. Established legal opinion has determined that it is appropriate to apply this definition of LAR for research consent as well.
For information about who can be LAR for a minor , review the section on Parent/Guardian Permission in Protected and Vulnerable Populations and RCW 7.70.65(2) which describes the somewhat different requirements for an LAR for minors in Washington State, when the parents are not available.
Washington State Law about who can be a LAR for an adult
A LAR for an adult must be a member of one of the following classes of persons in the following order or priority:
- The appointed guardian
- An individual, if any, to whom the person has given a durable power of attorney that encompasses the authority to make health care decisions
- Spouse or state-registered domestic partner
- Children, if they are at least 18 years old
- Adult siblings
- Adult grandchildren, who are familiar with the person
- Adult nieces and nephews, who are familiar with the person
- Has exhibited special care and concern for the person
- Is familiar with the person’s personal values
- Is reasonably available to make health care (and research) decisions
- Is not any of the following: a physician for the person; an employee of the person; the owner, administrator, or employee of a health care facility, nursing home, or long-term care facility where the person resides or receives care; or a person who receives compensation to provide care to the person
- Meet the requirements described above
- Are a close friend of the person
- Are willing and able to become involved in the person’s healthcare (or research participation)
- Have maintained such regular contact with the person as to be familiar with the person’s activities, health, personal values and morals, and
- Are not aware of a person in a higher priority class willing and able to provide informed consent on behalf of the person.
Availability . If reasonable efforts to locate and secure consent from a competent person in the first of succeeding class are unsuccessful, then consent may be given by any person in the next class in order of descending priority.
Disagreement among possible LARs . No LAR may provide consent on behalf of the person if:
- A person of higher priority has refused to give consent, or
- There are two or more individuals in the same class and the decision is not unanimous among all available members of that class.
Decision-making standard . The LAR must decide in good faith whether the person would consent to the research. If this is not possible, the LAR should consider the person’s best interests.
IRB approval and authority
Prior IRB approval of using LARs to obtain consent is not required by federal regulations. However, the IRB has the authority to limit, or explicitly not allow the use of LARs to obtain consent when such limits or prohibition is appropriate for a specific study.
Subjects with Comprehension Barriers (e.g., language, literacy, visual impairment) or Who Cannot Write or Speak
Researchers may encounter individuals who are interested in participating in research and have the cognitive ability to consent on their own behalf, but who have limited ability to understand or read consent information presented in English, or to sign a consent form .
The consent process for these individuals must meet the same regulatory requirements as for any other consent process . This includes the requirement for consent information to be presented in a language that is understandable to the subject. The researcher may need to take additional steps to ensure the subject comprehends the consent process, has adequate opportunity to ask questions, and voluntarily agrees to participate. Accommodations for the consent form or process will be specific to the needs of the particular subject(s).
The IRB must approve the consent plan , including the process that will be used to ensure that oral and written information will be in a language understandable to subjects throughout the study and at an appropriate reading and comprehension level. The IRB has the authority to require revisions or additions to the consent process to ensure that all subjects are adequately informed and are providing truly voluntary consent.
In keeping with the Belmont Principle of Justice that selection of subjects should be equitable in terms of fairly distributing the risks and benefits of research, researchers should carefully consider the purpose of the research and the scientific question when considering the inclusion and exclusion of these subject populations. This is particularly important when the study may offer significant benefit to the individual subjects or subject population.
Anticipated involvement of subjects with limited English proficiency
For research intended, or likely, to involve subjects who are not fluent in English, researchers are responsible for ensuring all consent information and materials are presented in a language understandable by the subjects and in a way that accurately conveys the information. Remembering that consent is a process, researchers must have a plan for ongoing communication between the research team and the subjects throughout their participation in the research.
Interpretation . Researchers must describe in their IRB application how and when interpretation will be provided and the qualifications of the interpreter(s) (e.g., certifications, experience, familiarity with research-related vocabulary in English and the target language). If no member of the research team is fluent in the subjects’ language, interpretation services should be made available throughout the course of the research.
When working with interpreters, consider the following:
- If the subject’s family members or friends will be asked to serve as interpreter, the researcher should think carefully about privacy and confidentiality issues, particularly for research that involves health or other sensitive topics. They should also ensure that the interpreter will accurately convey the information (e.g., Are they capable of interpreting complicated biomedical information?).
- It is almost never appropriate to use children as interpreters. Exceptions may rarely be allowed when the children are considered adults in the local setting, the risks are low, there are no alternatives, and there are no potential conflicts of interest.
- Researchers should discuss the consent process, including the HSD Consent Considerations , with interpreters before conducting consent. For example, how will the researcher and interpreter determine whether the subject truly understands the consent information?
Translation . Researchers must describe in the IRB application how translations will be obtained for consent and all study materials that will be presented to subjects (e.g., surveys). The qualifications of the translator must also be described. Translated documents must be: 1) linguistically accurate; 2) at an appropriate reading level for the participant population; and 3) culturally sensitive for the locale in which they will be used. The IRB review focuses on whether the translation method is appropriate rather than approving the translated text.
- Translated materials that must be provided to the IRB . It is HSD policy that researchers obtain and provide the IRB with translations for all consent materials that will be provided to the subject in written or electronic form. The IRB may request translated versions of any other materials.
- The IRB will request that researchers fill out the, Translation Attestation for studies that are greater than minimal risk or if they otherwise deem it necessary. Researchers should only submit this form in response to a request from the IRB.
Possible translation services
- The American Translators Association maintains two online directories: Directory of Translation and Interpreting Services (by individual); Directory of Translation and Interpretation companies. http://www.atanet.org/onlinedirectories/
- Dynamic Language . UW Medicine uses and recommends this local translation service, which is experienced in working with UW researchers (including biomedical researchers) and with invoicing to UW budgets.
- Native speakers who have demonstrated proficiency in English, including knowledgeable members of the research team, academics at other institutions, bi-lingual tribal leaders, etc.
- Graduate students or instructors in foreign language programs.
Unexpected involvement of subjects with limited English proficiency
The regulations allow an alternative method of obtaining and documenting consent called short form consent . This method is intended to be used only for the infrequent and unanticipated enrollment of an individual with limited English proficiency in a study for which no consent form in the subject’s language has been prepared and there is insufficient time and opportunity to obtain an appropriate written translation of the IRB-approved English consent forms. Because this method is only intended for unanticipated enrollment of an individual with limited English proficiency, the IRB does not need to approve the use of the short form method in advance. Researchers who use the short form method should assess whether they are likely to encounter additional subjects who require languages other than English. If so, translated materials should be prepared and submitted for review. It is HSD policy that:
- The short form method may only be used when an appropriately qualified interpreter will be available to the subject throughout their participation in the research.
- For research that involves more than minimal risk, the short form method may only be used for studies that provide the prospect of direct benefit that is not available outside the research context .
- Ancillary benefits of research participation, such as additional follow-up visits or free diagnostic tests are not considered direct benefits.
- Randomized clinical trials involving an investigational arm compared with standard of care or placebo are eligible for use of short form consent provided that the investigational arm provides prospect of direct benefit that is not available outside the research context.
- Comparative effectiveness trials are not eligible to use the short form because they do not generally provide a benefit that is unavailable outside of the research context.
Short Form Requirements
- Short form written consent form . This generic document states that the required elements of consent (unless waived by the IRB) have been presented to the subject in the subject’s language, with Key Information being presented first. The short form written consent form must be translated into the subject’s language so that literate subjects have the opportunity to read it. A pre-approved English version of the short form and translations in common languages spoken in King County are posted on the HSD website [ Consent Templates ]. Contact [email protected] if the language you need is not posted. The short form written consent form is signed by the subject and a witness. A copy of the form is provided to the subject.
- Summary document . The IRB must approve a written summary of the information that will be presented orally to the subject during the consent process. This summary is almost always the English version of the consent form that is presented by a member of the research team through use of a qualified interpreter. The summary document is signed by the witness and the person obtaining consent, but it is not signed by the subject. A copy of the form is provided to the subject.
- Witness . The short form process requires a witness to the oral presentation of the consent information and the subject’s voluntary participation. The witness must be impartial (i.e., not a member of the research team), an adult, and fluent in both English and the subject’s primary language. The interpreter may serve as the witness provided that they are not a member of the research team.
It is HSD policy to generally apply the guidelines described in the FDA Guidance on Informed Consent when enrolling subjects who are illiterate or who have low literacy.
FDA guidelines
- Consent materials are read to the subject in the presence of an impartial witness who observes the entire consent process.
- Sufficient time is allowed for questions to ensure subject comprehension.
- Researchers may consider using a video or audio recording of the consent process as part of documenting consent. If the consent process is not captured by audio or video, the researcher should create a written description of how the consent information was communicated to the subject and how the researcher ensured the subject’s questions were answered.
- If the IRB did not waive documentation of consent, the subject documents their willingness to participate by signing, or marking an X, on the consent form. The witness and the researcher should also sign and date the form. On rare occasions, the IRB may approve a process that involves reading the consent form to the subject and noting the consent in some official record that is not part of the research records (e.g., the subject’s medical record).
The consenting process described above for illiterate individuals may be used, but it is preferable to provide the subjects with an electronic copy of the materials which can then be examined by using an electronic device (e.g., computer) with a screen reader. In general, the use of Braille-based materials is discouraged.
A subject may be unable to physically sign a consent form due to some impairment (e.g., injury to hands). in these cases, the subject may “sign” the form by marking an X on the signature line. An impartial witness should witness the mark and sign the form. If the subject cannot use their hands at all, the IRB will consider alternative methods of documenting consent.
Electronic informed consent (“e-consent”) refers to the use of electronic systems and processes that employ some type of electronic media (including text, graphics, audio, video, podcasts, passive and interactive websites, biological recognition devices, card readers, etc.) to convey consent information and/or to document informed consent. These may be used in place of, or in combination with, paper-based consent methods.
Paper vs. e-consent – choosing the appropriate method
E-consent processes are invaluable when it is not possible to have an in-person interaction with a subject. E-consent can take many different forms including, consent information or documents that are delivered electronically (e.g., email, text message); passive or interactive websites; social media platforms; audio; video; podcasts; or any combination of these. E-consent may also be useful and appropriate for in-person consent interactions. For example, a consent form may be emailed in advance to a potential subject, followed by an in-person meeting in which the study is discussed, after which an electronic signature is obtained.
E-consent allows for using images, animations, embedded comprehension checks, and other technological tools that can improve consent comprehension. However, there are also potential limitations to using e-consent. The subject population will require infrastructure (e.g., reliable internet connection), hardware (e.g., smartphone, computer), and technological experience in order to benefit from the potential advantages of e-consent.
When choosing whether to employ paper-based consent or e-consent, it is important to understand the needs and capabilities of the subject population(s) . To ensure subject comprehension, it might be necessary to provide consent using a combination of both paper-based and electronic methods.
Facilitating comprehension and the opportunity to consider participation
When consent is not an “in-person” process, the researcher and the IRB must consider how subjects will be provided with an opportunity to ask questions and have the research team answer them (if appropriate) and/or whether alternative ways of facilitating comprehension may be appropriate. For example, this may be accomplished through telephone calls, electronic messaging (examples: email, text messages), video conferencing, live chat, or other methods. Regardless of the subject’s location, there may also be optional information (for example, hyperlinks or help text) embedded in the electronically delivered material to aid in comprehension of key study elements. Similarly, subjects may be asked questions embedded during the electronic process to gauge their comprehension.
Regulatory requirements for e-consent
Electronic delivery of consent information must meet the same human subjects regulatory requirements as paper-based delivery of the information. This includes ensuring the information is accessible by subjects for the duration of the research and is easily retrievable for auditors and monitors. FDA-regulated research . Use of electronic systems, archiving, and retention of consent materials must meet the FDA “Part 11” requirements . The Part 11 regulations are separate from the FDA’s human subject regulations and have nothing to do with IRB review and approval. Part 11 compliance is the responsibility of the researcher.
- The Part 11 requirements are outlined in the FDA Guidance for Industry: Part 11, Electronic Records; Electronic Signatures – Scope and Application (September 2003) .
- The UW-ITHS-supported non-mobile version of REDCap meets the FDA’s Part 11 electronic system requirements. However, to fully comply with Part 11, researchers must also implement all other procedural requirements for Part 11 (e.g., limiting system access to only authorized users).
Electronic Documentation of Consent (e-signature)
Review Documentation of Consent .
Documentation of Consent
Documentation of Consent . Prior to initiating any research activities, including screening procedures or extracting information from records, federal regulations require that the subject sign the IRB-approved consent form and that a copy of the consent form be provided to the subject. HSD considers it best practice for the subject to receive a copy of the consent form that they have signed and dated, but it is not a regulatory requirement.
Waiver of documentation of consent . Alternatively, the IRB may grant a waiver of documentation of consent if the criteria are met ( Conditions for waiving documentation of consent ). Most minimal risk studies will qualify for a waiver of documentation of consent. If a waiver is granted, none of the requirements listed below in this section apply to the study.
Researchers must provide the IRB with a written description of what subjects will be told during the consent process , even if consent will not be documented (i.e., no consent form). The process and information presented must include the general requirements and elements of consent as described in the guidance on Designing the Consent Process . HSD recommends, and the IRB may require, that researchers provide subjects with a written description of the consent information (e.g., information statement) even when a waiver of consent documentation has been granted (i.e., no consent form).
Required Signatories
Standard consent.
Subject . The subject is required to sign and date the consent form to document that all their questions have been answered and they agree to participate in the research.
When a legally authorized representative (LAR) or parent/guardian provides consent, the subject’s name should be printed on the subject’s signature line. Ideally there should be a designated line for the LAR or parent/guardian signature and date, but this information can be added anywhere in the signature area of the consent form. The LAR or parent/guardian name and relationship to the subject should also be recorded in the signature area.
Researcher . It is HSD policy that for greater than minimal risk research, the consent form must contain the legible name of the person who obtained consent from the subject. The person’s signature is not required. The name may be placed on the consent form in advance of the consenting interaction. It is best practice to date the form at the time when consent is obtained. By placing their name on the consent form, the researcher is confirming that they provided the subject with information about the study, that the subject was given sufficient time to consider participation, that the researcher answered all the subject’s questions, and that the subject indicated they understood the nature of the study, including the risks and benefits of participating.
Witness . Witness signatures are required by federal regulations in limited circumstances (e.g., when using short form consent) or may be required by the IRB to ensure an adequate informed consent process. A witness signature documents that the requirements for consent have been satisfied and that consent was voluntarily given by the subject.
Short Form Consent
Review HSD policy before using the short form process. When it is allowed, the short form process includes the short form itself and a summary document and must involve an impartial (not a member of the research team) adult witness who is fluent in both English and the subject’s primary language.
Short form written consent form. Use one of the HSD provided translations or contact HSD if the language you need is not provided. The short form is signed by the subject and the witness. A copy is provided to the subject.
Summary document. This is a written summary of the information that will be presented orally to the subject during the consent process and is usually the English version of the full consent form. The summary document is signed by the person obtaining consent and the witness. A copy is provided to the subject, but the subject should not sign it.
This refers to the process for confirming that the individual who provided the signature is the subject. This can be accomplished in a number of ways such as: the research team visually witnesses the signature; subjects share some form of official identification with the research team (e.g., scanned copy or digital photo); the subject answers security questions (similar to questions sometimes used by banks).
- Examples where more formal verification might be appropriate include: 1) a study that involves giving alcohol to subjects (verify age); 2) a longitudinal study where it is necessary to ensure that the same person is providing information over time; and 3) a study involving access to sensitive data such as a person’s mental health or sexually-transmitted infection (STI) records.
- UW eSignature (DocuSign) is not valid for FDA-regulated research; and
- FDA considered the UW ITHS REDCap signature tool a “hand-written” signature so the identity verification requirements do not apply.
- Handwritten signature using a permanent method (e.g., ballpoint pen).
- Electronic signature (e.g., REDCap or DocuSign).
- Other formats . Review Subjects With Comprehension Barriers or Who Cannot Write or Speak for alternative formats.
- The subject signs the consent form in the presence of the researcher. The research may begin immediately.
- The subject receives the consent form by mail or email, the consent discussion occurs by phone or video, the subject signs the form and texts or emails a photo of the signature to the researcher, and the subject mails the signed consent form to the researcher. The research may begin as soon as the researcher receives the photo of the signature.
- The IRB may approve other methods so long as they meet regulatory requirements and are consistent with any applicable local law.
There are many electronic alternatives to a handwritten signature, including: electronic signatures using tools such as DocuSign or REDCap; digital signatures; computer-readable ID cards; biometrics; or username and password combinations. Watch HSD’s tutorial, Electronic Consent: What You Need to Know for an overview.
There are two electronic signature tools that have been vetted by the UW and its legal counsel as meeting the federal and Washington State definitions of a “legally valid” electronic signature. Review the instructions for using UW E-Signature Tools for more information.
- NOTE: The ITHS REDCap Mobile software application does not support a “legally valid” electronic signature under federal and Washington State law.
- DocuSign is not valid for FDA-regulated research (details in Requirements specific to electronic consent documentation ).
Using a non-UW installation of REDCap may be permitted, so long as it meets all of the requirements outlined in the supplement, Other REDCap Installation and a copy is provided with the IRB application.
Using an e-signature method not vetted by the UW is permitted on a case-by-case basis. The researcher must provide (as part of their Zipline application) a signed Other E-signature Attestation Letter confirming the signature system meets all the applicable e-signature laws in the jurisdiction where the signature will be obtained and follows best practices for technical security. This letter must be signed by the Chief Information Officer, Chief Information Security Officer, or other individual at the company/institution with sufficient authority and subject matter expertise to make the above attestation. HSD and the UW will not vet other e-signature methods.
Regulatory requirements . Electronic documentation of consent must meet the same requirements as for handwritten signatures in addition to the following requirements that are specific to electronic consent documentation:
Requirements that are specific to electronic consent documentation
- Legally effective electronic signature . The signature method must be consistent with general laws about electronic signatures in the jurisdiction in which the research will be conducted. Only UW eSignatures (DocuSign) and UW ITHS REDCap have been vetted as meeting U.S. federal and Washington State requirements. HSD cannot advise researchers about the legality of other e-signature methods.
It is HSD’s general expectation that this additional required information is provided in the consent form(s) and HIPAA authorization (if applicable). In rare instances, HSD may permit the required information to be communicated to study participants via an alternative method. For example, a UW researcher participates in a multicenter trial using an external IRB and there may be no UW-site-specific form that can include this information. Any alternative method of providing this additional required information must still provide the information: 1) in writing; 2) prior to obtaining documentation of informed consent or authorization (e-signature); 3) in a manner that allows the participant to print, access it at a later date, or otherwise retain a copy in some fashion.
- UW e-Signature (DocuSign) is not allowed because DocuSign at the UW is not compliant with the FDA Part 11 regulations.
- UW ITHS REDCap is allowed because it is meets the FDA’s Part 11 electronic system requirements. FDA considers documentation of consent using REDCap to be a handwritten signature captured as an electronic record rather than a true electronic signature. While the FDA e-signature subject identity verification requirements do not apply, researchers are encouraged to document how identity is confirmed in case of an FDA audit.
- Other e-signature tools must be considered against the three key issues named above.
HSD and/or IRB approval . It is HSD policy that that IRB approval must be obtained in advance for the use of any e-signature or electronic signature capture system (e.g., DocuSign, UW ITHS REDCap).
Special considerations
- HIPAA authorization . Both DocuSign and the UW ITHS REDCap system (and equivalent, IRB-approved non-UW REDCap systems) may be used to obtain HIPAA authorization for access to PHI at UW Medicine and Seattle Children’s. E-signatures captured in any other systems are not acceptable. The authorization form must contain the electronic signature-specific consent elements as listed in the guidance on Designing the Consent Process . For authorization to obtain/access PHI at any other HIPAA-covered institution, consult that institution’s privacy and/or health records information office.
- Review by an external IRB . It is HSD policy that UW research that is being reviewed by an external IRB instead of the UW IRB must obtain HSD concurrence before implementing the use of e-signatures for consent and/or HIPAA authorization. This is accomplished by answering the e-signature questions in the External IRB Review request form and, if applicable, uploading the Other REDCap Installation supplement or Other E-signature Attestation Letter to the Local Site Documents SmartForm in Zipline.
- International research . Other countries may have their own laws regarding the legal validity of an e-signature and what methods of capture are acceptable. It is the researcher’s responsibility to be aware of and comply with these laws. Review online resources such as DocuSign eSignature Legality Guide . This resource is published by DocuSign and is informational only. HSD cannot guarantee that it remains current and accurate. Collaborating partners and experts in the target country(ies) may also be consulted.
The UW IRB system (“Zipline”) automatically places a watermark on consent forms when IRB approval is granted. It is best practice (but not required) for researchers to use the Zipline watermarked version of the consent form. Any non-watermarked version used must match exactly the content and format of the IRB-approved watermarked version.
The original signed consent form, regardless of format (e.g., paper, electronic) is considered a research record and federal regulations require researchers to retain consent forms for a minimum of three years after completion of the research. Washington State records retention periods are much longer ( UW Records Management website ).
Signed consent materials must be easily retrievable for auditors and monitors.
Throughout the course of a study, subjects may need to be informed about new information or consent may need to be revisited due to fluctuating decision-making capacity [ Diminished or Fluctuating Consent Capacity and use of a Legally Authorized Representative (LAR) ] or because a child subject has reached the age of majority (review the section on Children under Protected and Vulnerable Populations ). In these situations, it is important for subjects to be able to reaffirm their willingness to participate in research.
The regulations do not provide details about how new information should be provided and do not specifically mention “reconsent”, although the Secretary’s Advisory Committee on Human Research Protections (SACHRP) provides recommendations on reconsent . Ultimately, it is up to the IRB to determine whether reconsent is required and to identify the most appropriate method for communicating new or revised information. In making this determination, the IRB should consider:
- Study status (procedures: not yet begun; ongoing; limited to long-term follow-up; complete)
- Time sensitivity of the information
- Magnitude of the changes and their likelihood to influence a subject’s decision to continue participation
Methods for providing new information to subjects. These methods are based on the SACHRP recommendations and an article from WCG IRB. The UW IRB may consider alternative methods.
Verbal discussion. This method may be appropriate for communicating information that: (1) is simple; (2) does not change the individual subject’s anticipated risks or benefits; (3) is not likely to affect a subject’s willingness to continue participation; and (4) does not require documentation that subjects are willing to remain in the study.
- Eliminating certain procedures from a study visit
- Payment method being changed from cash to a gift card
- A verbal discussion may be the first step for time sensitive information that should be shared immediately with subjects while a written notification (e.g., consent addendum; revised consent form) is being prepared
Letter or email. Sending the new information in a letter or by email may be appropriate when: (1) the information is easy to understand; (2) the information is not likely to affect a subject’s willingness to participate; and (3) it is important for subjects to have the information in writing for future reference.
- A new questionnaire is being added to the weekly battery of measures that subjects complete that does not change the type of scope of questions that were already being asked and is estimated to increase participation time by about 5-7 minutes each week
- A confidentiality breach is described in a Report of New Information (RNI). This risk is already described in the consent form but as part of the corrective action plan, the IRB determines that subjects should be informed of the breach.
- Informing subjects that there is a new principal investigator
- Informing subjects that they can use a commercial lab for blood draws
Consent addendum. This method may be appropriate for complicated or important information that requires consent because it (1) may impact a subject’s willingness to continue participation; but (2) does not require a full reconsent and review of the entire study. An addendum tends to be the least burdensome for subjects as it allows them to focus on the new information as they consider whether they want to continue participation. For this reason, HSD recommends the use of a consent addendum, rather than a re-review of the entire consent form, for most situations.
- A modification to add a new battery of measures is submitted; the new measures include sensitive mental health topics that subjects did not previously consent to
- The researchers want to add a new genetic analysis to the samples already being collected for the study
- New research-related risks are identified or there is an increase in the frequency or magnitude of previously described risks
- A decrease in anticipated benefits to subjects is identified
- New or alternative therapies are identified (e.g., FDA approval of a new drug or device for the condition under study) or there is a change on the impact of participation on alternative therapies [e.g., the investigational drug or device reduces the effectiveness of alternative treatment(s)]
- When a study includes online or in-app check-ins, a consent refresher or informing subjects about changes to the research can occur electronically with a checkbox or other method for the subject to confirm they wish to continue participation
Reconsent. This method requires the most time and effort and should be used when (1) there is no time sensitivity; and (2) the new information is complicated and/or affects many aspects of the study. A full reconsent is required when a LAR consent or parental permission is being replaced by the subject consenting on their own behalf.
- Subjects are moving into a new phase of the study with very different procedures
- Many changes are being made to the study
- A subject enrolled with parental permission as a minor reaches the age of majority and must provide adult consent
- Changes in the cognitive functioning, mental health, or physical health of the subject have occurred over the course of the research and the subject can replace LAR consent with consent on their own behalf
- There is a significant delay between providing consent and beginning the study
- Subjects are enrolled in a longitudinal study and a consent refresher may be appropriate even if procedures have not changed
- There are concerns about the way in which consent has been obtained (e.g., the wrong version of the consent form was used; study staff have not been appropriately trained in obtaining consent)
Additional considerations.
Study status. In many cases, multiple approaches will be required depending on where subjects are in the course of the study. For example, if new risks are identified for a study in which enrollment is ongoing, it might be appropriate to provide subjects who have already been enrolled with an addendum while also updating the consent form for subjects who have not yet been enrolled.
Revised consent form. Changes to the consent form do not necessarily require researchers to inform all enrolled subjects. Researchers should use the information in this guidance to assess the new/revised information and determine what, if any, method should be used to inform subjects of new information and/or obtain reconsent. Note that some sponsors or funders may require a full reconsent for any change to the consent form. In these cases, HSD defers to the sponsor/funder.
Waiver of documentation of consent. The IRB should assess the criteria for waiving documentation of consent when reconsent is being obtained whether it is an addendum, or full consent, or some alternative method.
- New risk information alters the IRB’s previous determination that the study is no greater than minimal risk and waiver of documented consent. The IRB requires a full reconsent for all enrolled subjects including obtaining documentation.
- A new genetic analysis is presented to subjects in the form of an addendum. Although the overall study is greater than minimal risk and requires documentation of consent, the new component is minimal risk, so the IRB waives documentation of consent for the addendum.
Washington State Law on Consent for Audio Recording (RCW 9.73.030)
When an audio recording will capture a private communication or conversation between two or more individuals, Washington State law requires that consent must be obtained from all individuals who will be recorded. The law applies if any participant in the conversation or communication is in Washington State at the time of recording.
Private refers to situations in which an individual can reasonably expect privacy, such as a confidential interview with a research subject or a conversation between doctor and patient. In some circumstances, it can be challenging to determine whether a communication or conversation is private. This will be assessed by HSD staff as part of the HSD/IRB review process.
Communication or conversation means audio only and does not refer to visual recording. However, the State law applies to video recordings that include audio.
Consent may be obtained from a legally authorized representative when appropriate. If a child will be recorded, permission should be obtained from a parent or guardian .
Consent methods. The Washington State law consent requirement for audio recording may be satisfied using various methods:
- Written consent. This can be a short statement signed by participants (included with research consent or separately). This option is generally appropriate when the research plan is to obtain documented consent from research participants.
- Recording verbal consent with the communication or recording an announcement that the conversation is being recorded. This option is generally appropriate when the research plan is to obtain verbal consent and the IRB approves a waiver of the requirement for documented consent under human subjects regulations.
Consent should consider the individuals’ preferred language(s), literacy, and whether individuals may be subject to coercion if they decline to be recorded (e.g., an employee unable to perform their job duties in an area being recorded).
Recording in a healthcare setting.
- HIPAA requirements: When in a healthcare setting, recordings are likely to capture Protected Health Information (PHI). If PHI will be captured on the recording, HIPAA Authorization must be obtained from the subject unless the IRB approves a Waiver of HIPAA Authorization. Refer to HSD’s HIPAA Guidance for more information about HIPAA requirements.
- UW Medicine approval: UW Medicine policy requires approval from the applicable UW Medicine entity’s Chief Executive Officer or Executive Director when audio recordings will be made for research purposes in a UW Medicine clinical setting. Refer to the Audio Recordings in a Clinical Setting policy and FAQ on the UW Medicine Compliance website for more information about this requirement. The IRB may request documentation of UW Medicine approval.
Other Consent Materials
Consent Examples Consent Templates EXAMPLE Key Information GUIDANCE Consent Elements for Externally Reviewed Studies GUIDANCE Designing the Consent Process INSTRUCTIONS UW E-Signature Tools TEMPLATE Translation Attestation TIPSHEET Consent TUTORIAL Electronic Consent: What You Need to Know WORKSHEET Consent Requirements and Waivers
Help Materials for HSD Staff and IRB Members
SOP Consent [HSD staff and IRB member access only] WORKSHEET Consent Review for IRB Members [HSD staff and IRB member access only]
Other Materials Referenced in this Guidance
CHECKLIST Exception from Informed Consent GLOSSARY Capacity to Consent GLOSSARY Exempt Research GLOSSARY Legally Authorized Representative GLOSSARY Legally Effective Research Consent GUIDANCE Authority and Responsibilities of HSD and UW IRB GUIDANCE The Belmont Report GUIDANCE Exempt Research GUIDANCE HIPAA GUIDANCE Humanitarian Use Devices (HUDs) GUIDANCE Human Subjects Regulations GUIDANCE Involvement of Children in Research GUIDANCE Mandatory State Reporting GUIDANCE Prisoners GUIDANCE Subject Payment REQUEST External IRB Review SUPPLEMENT Other REDCap Installation TEMPLATE Other E-signature Attestation Letter WEBPAGE Reliance on a Non-UW IRB WEBPAGE Single Patient Emergency or Compassionate Use WORKSHEET Children WORKSHEET Neonates WORKSHEET Pregnant Women WORKSHEET Prisoners
- 45 CFR 46.116 [pre-2018 requirements and 2018 requirements]
- 45 CFR 46.117 [pre-2018 requirements and 2018 requirements]
- 45 CFR 46.204 – .205
- 45 CFR 46.305
- 45 CFR 46.408
- 21 CFR 50.20
- 21 CFR 50.27
- 21 CFR 56.109
- 21 CFR 50, Part D
- OHRP Draft Guidance on Disclosing Reasonably Foreseeble Risks in Research Evaluating Standards of Care, 2014
- OHRP, Guidance on Institutional Review Board Review of Clinical Trial Websites; September 20, 2005.
- OHRP Guidance Documents on Informed Consent, from the OHRP website https://www.hhs.gov/ohrp/regulations-and-policy/guidance/informed-consent/index.html
- OHRP, “Obtaining and Documenting Informed Consent of Subjects Who Do Not Speak English”, memorandum; November 9, 1995.
- OHRP, “Simplifying Informed Consent”, webinar https://www.hhs.gov/ohrp/education-and-outreach/online-education/videos/simplifying-informed-consent-ohrp
- FDA, Guide to Informed Consent Information Sheet, July, 2014
- FDA and OHRP Guidance, “Use of Electronic Informed Consent: Questions and Answers”, December, 2016
- FDA Guidance, “Adverse Reactions Section of Labeling for Human Prescription Drug and Biological Products – Content and Format” January 2006
- FDA Guidance, “Part 11, Electronic Records: Electronic Signatures – Scope and Application”, August 2003
- FDA Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Public Health Emergency (updated July 2, 2020)
- FDA. “Guidance for Industry. E6 Good Clinical Practice: Consolidated Guidance”, April 1996, section 4.8.9.
- FDA – Reporting Serious Problems to FDA, “What is a Serious Adverse Event?”, 2016
- Meeting of the Secretary’s Advisory Committee on Human Research Protections, Key Information videotape, July 10-11, 2018
- SACHRP Recommendations, “Attachment A – Addressing Ethical Concerns Regarding Offers of Payment to Research Participants”, October 18, 2019
- SACHRP Recommendations, “Attachment A – Guidance on Applying the Regulatory Requirements for Research Consent Forms: What Should and Should Not be Included?”, July 2011
- SACHRP Recommendations, “Attachment A – Recommended Guidance on Minimal Risk Research and Informed Consent”, 2015
- SACHRP Recommendations, “Attachment A1 – Reconsent Appendix 1. New Information Provided to Previously Enrolled Subjects”, May 4, 2020
- SACHRP Recommendations, “Attachment A2 – Reconsent Appendix 2. Additional Information Scenarios and Suggested Options”, May 4, 2020
- SACHRP Recommendations, “Attachment A – SACHRP Commentary on the FDA Draft Guidance Entitled, ‘Informed Consent Information Sheet; Guidance for IRBs, Clinical Investigators and Sponsors’”, February 11, 2015
- CIOMS III – Core Clinical Safety Information, “Guidelines for Preparing Core Clinical-Safety Information on Drugs”, 1995
- 15 U.W. Code Chapter 96 – The Electronic Signatures in Global and National Commerce Act (E-Sign Act)
- National Conference of Commissioners of Uniform State Laws, Uniform Electronic Transactions Act (UETA), (1999)
- Revised Code of Washington (RCW) Chapter 19.360, Electronic Signatures and Records
- Chapter 1.80 Revised Code of Washington (RCW) Uniform Electronic Transactions Act (Washington’s adoption of UETA)
- Committee On Ethical Considerations for Revisions to DHHS Regulations for Protecting Prisoners in Research, “Ethical Considerations for Research Involving Prisoners” (2006)
- Dickert et al., “Reframing Consent for Clinical Research: A Function-Based Approach”. American Journal of Bioethics, 17:12, 3-11 (2017)
- Dickert et al., “Partnering with Patients to Bridge Gaps in Consent for Acute Care Research”. The American Journal of Bioethics, 20(5), 7-17 (2020)
- Gelinas, L. “The Many Faces of ‘Coercion’ and ‘Undue Influence'”. Advarra Blog, Dec 2, 2020 https://www.advarra.com/blog/the-many-faces-of-coercion-and-undue-influence/
- Kraft et al., “Comprehension and Choice Under the Revised Common Rule: Improving Informed Consent by Offering Reasons Why Some Enroll in Research and Others Do Not”. The American Journal of Bioethics, 17:7, 53-55 (2017)
- Ravi et al., “Financial Payments for Participating in Research while Incarcerated: Attitudes of Prisoners”. IRB Ethics & Human Research, 40:6, 1-6 (2018)
- The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, April 18, 1979
- The PHASES Working Group, Pregnancy and HIV/AIDS: Seeking Equitable Study, “Ending the evidence gap for pregnant women around HIV and co-infections: A call to action” (2020)
- WCG IRB Insights, “Providing Research Participants with New Information: Is ‘Re-Consent’ Always Necessary?” https://www.wcgirb.com/insights/providing-research-participants-with-new-information-is-re-consent-always-necessary/
- Wilfond and Kraft, “Attending to the Interrelatedness of the Functions of Consent”. The American Journal of Bioethics, 17:12, 12-13 (2017)
Version Information
Open the accordion below for version changes to this guidance.
Version History
Keywords : Consent
OR Support Offices
- Human Subjects Division (HSD)
- Office of Animal Welfare (OAW)
- Office of Research (OR)
- Office of Research Information Services (ORIS)
- Office of Sponsored Programs (OSP)
OR Research Units
- Applied Physics Laboratory (APL-UW)
- WA National Primate Research Center (WaNPRC)
Research Partner Offices
- Corporate and Foundation Relations (CFR)
- Enivronmental Health and Safety (EH&S)
- Grant and Contract Accounting (GCA)
- Institute of Translational Health Sciences (ITHS)
- Management Accounting and Analysis (MAA)
- Post Award Fiscal Compliance (PAFC)
Collaboration
- Centers and Institutes
- Collaborative Proposal Development Resources
- Research Fact Sheet
- Research Annual Report
- Stats and Rankings
- Honors and Awards
- Office of Research
© 2024 University of Washington | Seattle, WA

Informed Consent and Consent Forms for Research Participants
Informed consent is a communication process by which researchers reach agreement with people about whether they wish to participate in research. Confusing informed consent with a signed consent form may violate the ethical intent of informed consent, which is to communicate clearly and respectfully, to foster trust, comprehension, and good decision making, and to ensure that participation is voluntary.
Consent forms are often written in “legalese” and are long, complex, and often inappropriate to the culture or language of the potential subject, insulting, and virtually impossible for most people to comprehend. They convey to some the impression that signing such a formal-looking document commits them to participation. Among subjects who willingly sign documents, most sign the consent form without reading it.
How has this come to pass? Early concern with ethics of human research was about biomedical research and focused on the necessity of obtaining informed consent. Over the decades, the elements of informed consent have grown in number, as has the idea that informed consent is a form that is to be signed by the subject. According to the Federal Regulation of Human Research 46.117(a):
Except as provided in paragraph (c) of this section, informed consent shall be documented by the use of a written consent form approved by the IRB and signed by the subject or the subject’s legally authorized representative. A copy shall be given to the person signing the form.
However, many researchers and the Institutional Review Boards that govern their research fail to recognize that 46.117(c) provides for a waiver of signed consent forms:
(c) An IRB may waive the requirement for the investigator to obtain a signed consent form for some or all subjects if it finds either: (1) That the only record linking the subject and the research would be the consent document and the principal risk would be potential harm resulting from a breach of confidentiality. Each subject will be asked whether the subject wants documentation linking the subject with the research, and the subject’s wishes will govern, or (2) That the research presents no more than minimal risk of harm to subjects and involves no procedures for which written consent is normally required outside of the research context.
The reason for obtaining a signed consent form has always been much more to protect the researcher and the institution than to serve the interests of the research subject. In case the subject claims later that consent was inadequate or omitted, the researcher can counter by showing the form. Recently, the Office of Human Research Protection has imposed highly publicized and costly sanctions against a few research institutions. Understandably, IRBs and research administrators consider it in their self-interest to make highly conservative decisions. Since IRBs must take steps to justify waiving documentation of informed consent by deeming the research to be minimal risk, many consider it safer not to do so, fearing that such an action might leave them open to questions by the OHRP. Thus, the reason for obtaining a signed consent form is typically to protect the institution, not the subject. Researchers, science, institutions, subjects, and IRBs would all be better off if they made intelligent interpretations of the requirements of the Common Rule.
The Social and Behavioral Sciences Working Group has made various recommendations based on the Common Rule, designed to guide social and behavioral researchers and IRBs out of such conundrums. The authors, both members of the Working Group, developed recommendations concerning informed consent, some of which are summarized here:
1. Informed consent should take the form of an open, easily understood communication process. Typically, this means a friendly verbal exchange between researcher and subject, with a written summary of the information for the subject to keep, as appropriate. (The copy for the subject to keep would be inappropriate if the written record of the subject’s participation could be damaging to the subject, as when the research is about domestic violence, or illegal behavior). Both the verbal and written discussion should be brief, and simply phrased at such a level that all of the subjects can understand it.
2. Subjects must receive enough easily understood, accurate information to judge whether the risk or inconvenience involved is at a level they can accept. The responsibility rests with the investigator to describe any risks accurately and understandably. There are many kinds of minor or everyday risks or inconveniences that most persons would gladly undertake if it were their choice to do so, but which they would not wish to have imposed upon them unilaterally. However, some may make a rational decision that the experience would be too stressful, risky, or unpleasant for some idiosyncratic reason that applies to them and not to other subjects.
3. Especially when the research procedure is long and complex, the researcher must make it quite clear that the subject is free to ask questions at any time. Informed consent, as a conversation (not a form), needs to be available throughout the research, as subjects do not necessarily develop questions or concerns about their participation until they are well into the research experience. For example, a discussion of confidentiality may not capture subjects’ attention or comprehension until they are asked some quite personal questions in the ensuing research experience.
4. When subjects can readily refuse to participate by hanging up the phone or tossing out a mailed survey, the informed consent can be extremely brief (a sentence or two). Courtesy and professionalism require that the identity of the researcher and research institution be mentioned, along with the nature and purpose of the research. However, if there are no risks, benefits, or confidentiality issues involved, these topics and the right to refuse to participate need not be mentioned, as such details would be gratuitous and might decrease participation by implying greater risk that actually exists. If the researcher has any connection with the institution at which the subjects receive health care or other essential services, it is necessary to mention the right of the research subject to refuse or withdraw without prejudice. Such rights may be honored implicitly by making it clear that you are asking their permission to involve them as research subjects.
5. Verbal informed consent need not be detailed and written consent is not appropriate when the research is not concerned with sensitive personal information and when subjects are peers or superiors of the researcher.
6. The cultural norms and life-styles of subjects should be considered when deciding how to approach informed consent. For example, research on homeless injection drug users should probably be preceded by a several week-long process of “hanging out” and talking with them. The resulting informal communication will raise issues they wish to discuss with the researcher. The conditions under which the research is conducted can then be negotiated orally between the researcher and the community members, as appropriate. Written documents and signed forms would expose subjects to risk of arrest and serve no redeeming purpose.
7. A wide range of media are appropriate for administering informed consent. Video tapes, brochures, group discussions, web sites, community newsletters, and the “grape vine” can be more appropriate ways of communicating with potential subjects than the potentially confusing formal consent forms that often are used.
8. When written or signed consent places subjects at risk, it must be waived. There are times when the written record is the only evidence that the subject has participated in a study in which there is acknowledgement or appearance of situations that would place the subject at risk.
9. When it is important to have some record of the informed consent but when written or signed consent would place the subject at risk or be difficult for the subject to read, one useful procedure is to have a trusted colleague witness the verbal consent.
10. Community consultation, or meeting with community leaders of the potential subjects, is a useful way to plan research that is likely to raise sensitive questions among those to be studied and members of their community. This is not a substitute for individual informed consent, but often clears the way for potential subjects to be ready to decide whether to participate.
11. In certain circumstances, persons are not in a position to decide whether to consent until immediately after their participation, e.g., in brief sidewalk interviews, which persons are likely to welcome.
12. Some research cannot validly be conducted if all details are disclosed at the outset. The alternatives to outright deception of subjects are to a) obtain permission to provide only a description of what the subject will experience, with an agreement that the full details of the study will be disclosed afterward; b) obtain permission to engage in concealment or deception with the understanding that pilot research has shown that peers of the subject do not find such concealment or deception objectionable and that a full explanation will follow their participation, c) explain that the subject might be enrolled in one of several possible conditions and to gain permission to disclose in which of these the subject was actually enrolled after his or her participation is completed.
Author’s Note: The Social and Behavioral Sciences Working Group (formerly a part of the National Human Research Protections Advisory Council but now an independent body) chaired by Felice Levine helped to develop these ideas.
Reference Melton, G., Levine, R. J., Koocher, G., Rosethal, R., & Thompson, W. (1988). Community Consultation in Socially Sensitive Research: Lessons from Clinical Trials on Treatments for AIDS. American Psychologist , 43, 573-581.
APS regularly opens certain online articles for discussion on our website. Effective February 2021, you must be a logged-in APS member to post comments. By posting a comment, you agree to our Community Guidelines and the display of your profile information, including your name and affiliation. Any opinions, findings, conclusions, or recommendations present in article comments are those of the writers and do not necessarily reflect the views of APS or the article’s author. For more information, please see our Community Guidelines .
Please login with your APS account to comment.
About the Authors
Joan Sieber is professor of psychology at California State University, Hayward. She received her bachelor's, master's, and doctorate from the University of Delaware. Robert J. Levine is professor of medicine and co-chair of the interdisciplinary bioethics project at Yale University. He is also the founding editor of IRB: A Review of Human Subjects Research.

Student Notebook: Finding Your Path in Psychology
Feeling unsure or overwhelmed as an early-career psychology student? Second-year graduate student Mariel Barnett shares advice to quell uncertainties.

Matching Psychology Training to Job Market Realities
APS President Wendy Wood discusses how graduate programs can change the habit of focusing on academic-career preparation.

Scientists Propose Upgrades to Research-Methods Education for Psychology Students
Many undergraduate psychology courses fail to ensure students fully understand research design and analysis. An international team of psychological scientists have recommended some systemic steps to remedy that shortcoming.
Privacy Overview

- Find My GCO
- IACUC applications (Cayuse Animal Management System)
- IBC Applications (eMUA)
- IRB Applications (RASS-IRB) External
- Institutional Profile & DUNS
- Rates and budgets
- Report external interests (COI)
- Join List Servs
- Ask EHS External
- Research Development Services
- Cornell Data Services External
- Find Your Next Funding Opportunity
- Travel Registry External
- RASS (Formerly Form 10 and NFA) External
- International research activities External
- Register for Federal and Non-Federal Systems
- Disclose Foreign Collaborations and Support
- Web Financials (WebFin2) External
- PI Dashboard External
- Research metrics & executive dashboards
- Research Financials (formerly RA Dashboard) External
- Subawards in a Proposal
- Proposal Development, Review, and Submission
- Planning for Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Budgets, Costs, and Rates
- Collaborate with Weill Cornell Medicine
- Award Negotiation and Finalization
- Travel and International Activities
- Project Finances
- Project Modifications
- Research Project Staffing
- Get Confidential Info, Data, Equipment, or Materials
- Managing Subawards
- Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Project Closeout Financials
- Project Closeout
- End a Project Early
- Protecting an Invention, Creation, Discovery
- Entrepreneurial and Startup Company Resources
- Gateway to Partnership Program
- Engaging with Industry
- Responsible Conduct of Research (RCR)
- Export Controls
- Research with Human Participants
- Research Security
- Work with Live Vertebrate Animals
- Research Safety
- Regulated Biological Materials in Research
- Financial Management
- Conflicts of Interest
- Search
IRB Consent Form Templates
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study.
General Consent Form Templates
Social and Behavioral Research Projects (last updated 03/16/2023)
Biomedical Research Projects (last updated 07/18/2022)

Consent Form Templates for Specific Biomedical Procedures
MRI and fMRI
Blood Collection by Finger Stick
Blood Collection by Venipuncture
Oral Consent Template
Guidance for Protocols Involving Oral Consent
Debriefing Template
Guidance and Template for Debriefing Participants
Studies Involving Children (Assent/Permission Forms)
Parent-Guardian Permission for Studies Involving Children
Sample Parental Notification Form
Sample Child Assent Form
Performance Release for Minors
Performance Releases
Performance Release for Adults
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Clin Pediatr Dent
- v.10(1); Jan-Mar 2017
Comprehensive Format of Informed Consent in Research and Practice: A Tool to uphold the Ethical and Moral Standards
P arun bhupathi.
1 Assistant Professor, Department of Orthodontics, Vishnu Dental College, Kovvada Andhra Pradesh, India
2 Reader, Department of Pedodontics and Preventive Dentistry, Drs Sudha and Nageswara Rao Siddhartha Institute of Dental Sciences, Allapuram, Andhra Pradesh, India
Informed consent in research, clinical trial, and practice is a process in which a patient/participant consents to participate or undergo the proposed procedures after being informed of its procedures, risks, and benefits. Ideally, the patient/participant is expected to give his consent only after fully comprehending the information about the procedures, benefits, and risks involved in research/clinical trial/practice. Thus, many ethical issues are entwined in the process of obtaining a proper informed consent. Certain untoward events in the past led to propose guidelines to prevent exploitations and unhealthy practices in the field of life science. Eventually, the practice of obtaining informed consent was emphasized to make sure that a participant’s rights were not in jeopardy. Yet, there are flaws in the practical application of obtaining consent due to lack of understanding, barriers in communication, culture, custom, and various other factors. The present article highlights the need for a complete and comprehensive format of recording informed consent without compromising the rights of an individual and the standards of research or practice on ethical and moral grounds.
How to cite this article
Bhupathi PA, Ravi GR. Comprehensive Format of Informed Consent in Research and Practice: A Tool to uphold the Ethical and Moral Standards. Int J Clin Pediatr Dent 2017;10(1):73-81.
INTRODUCTION
New advents in science and technology have expanded the horizons of every field including the field of medicine. Concomitantly expensive health care and scarcity of the required resources and demanding expectations of the public have led to a paradigm shift in the concepts of certain old ethical practices. Thus, new questions concerning the ethical principles are being raised time and again to adapt to the changing scenario.
Most of the biomedical research is conducted in the developing countries, which are known to have limited resources and the populations live in high-risk health conditions. Further, social and cultural factors and beliefs vary, raising the ethical concerns, such as standard of care and posttrial obligations. Henceforth, the assurance for conducting research in these countries is being discussed very often. 1
For centuries, general medical practice has been guided by ethical principles and the basics can be dated to the Hippocratic code of conduct, which specifies that the physician will use the treatment to help the sick according to his ability and judgment, but never with the view to injury and wrongdoing. However, there was relative paucity of universally agreed guidelines or a framework for the ethical conduct of research, including medical experimentation. The Nuremberg Code of conducting research on human subjects was put forth following the atrocities post-World War II and, in 1964, the Helsinki Declaration was drafted by the World Medical Association. This was the first of its kind, a move toward developing guidelines for ethical regulation globally. 2 , 3 An important component of conducting research in any setting is obtaining informed consent, as it has been the cornerstone for ethical conduct and regulation of research. It is the focus of attention in the guidelines of conducting research and the ethical oversight of research. 3
The basic rights of a person cannot be ignored since the autonomy and responsibility of every person to decline or take part in the research is of extreme importance. The decisions concerning one’s own body or health is universally recognized as a right. Hence, emphasis is placed on the importance of informed consent in research as well as clinical practice settings, and the need of it to be enterprising and innovative in obtaining it is justified. 1
The purpose of obtaining informed consent as a protocol for planned treatment differs from that obtained for research context. This is because level of protection for the patients varies when compared with the research subject. As the levels of protection differ, exceptions to the policy have been allowed for situations when obtaining consent is impossible or not feasible. As the consent should be suitable to varying circumstances, they may be broadly categorized into implied consent, written consent, expressed consent, informed consent, proxy consent, loco parentis, blanket consent, and oral consent. 4
The purpose of this article is to highlight the importance of a complete, comprehensive format of consent, which upholds the rights of the individuals without compromising the standards of the research on ethical and moral grounds.
INFORMED CONSENT
Consent has been defined by Webster’s Dictionary as “to give assent or approval.” This statement needs to be changed when applied to various fields, dentistry being no exception. The European Commission on ethical research has defined it as “Informed Consent is the decision, which must be written, dated and signed, to take part in a clinical trial, taken freely after being duly informed of its nature, significance, implications and risks and appropriately documented, by any person capable of giving consent or, where the person is not capable of giving consent, by his or her legal representative; if the person concerned is unable to write, oral consent in the presence of at least one witness may be given in exceptional cases, as provided for in national legislation.” 5
The British Dental Association’s “ethics in dentistry” advice sheet has defined the process of expressing consent as “A patient gives express consent when he or she indicates orally or in writing, consent to undergo examination or treatment or for personal information to be processed.” 5
The Health Care Consent Act, 1996 Ontario has highlighted the salient features for informed consent, which include: (1) nature of proposed treatment, (2) expected benefits, (3) material risks and side effects, (4) alternative courses of action, (5) consequences of not having the proposed treatment, (6) answers to any questions the patient has regarding the proposed treatment, and (7) cost of the treatment. 6
An informed consent form is mandatory when the research/clinical trial involves any human volunteer like children, differently-abled individuals, immigrants, or healthy individuals. It is also required whenever the personal data, biological samples or specimens, or human genetic material are used or collected. 5
General Format for Consent—Practice and/or Research
Commonly used formats of the consent include a statement that confirms that the participant has been explained about the proposed treatment plan/clinical trial/research and his/her participation is voluntary ( Fig. 1 ). There is a provision for the witness to sign in the document to authenticate that the above-said protocol was followed in his/her presence. In addition, there will be the details of the investigator. The informed consent is considered to be valid only when the participant, investigator, and the witness sign the document at their designated areas.

Consent form
Limitations
In this format, the content of the informed consent can be considered to be inadequate for the following reasons. This does not provide any written evidence explaining the role of the participant, investigator, and translator. Further, it lacks the structured format of explanation, which enables the participant to read about the proposed study design/treatment plan; risks involved; and assurance about the confidentiality of the identity. There is no separate declaration for participant, investigator, and translator, which commits each of them to their duties.
Thus, an informed consent that upholds the rights of the individuals without compromising the standards of the research on ethical and moral grounds is needed. This can be formulated by adapting the guidelines of the Helsinki Declaration.
Importance of having the Consent as per Helsinki Declaration
If the informed consent is designed as per the norms of the International Declaration of Helsinki, it upholds the safety of those participating in research as well as seeking treatment in the practice. All the details shown in the template have to be filled for proper documentation. For better understanding, the entire format can be categorized into three parts. The initial part of the document should have the details of the title of the research/study along with the name, address, and contact details of the principal investigator, and the ethical committee reference number. The second part should consist of patient information sheet ( Fig. 2A ), and the consent certificate or the declaration should be the last part ( Figs 2B to toD). D ). The entire informed consent should be printed on the letter head of the institution or the organization, which is carrying out the proposed research or clinical trial. 7 , 8 The header of the document should have logo, name, and the complete postal address of the organization or the institution, which is carrying out the research/study/ clinical trial, while the footer should have the research title and date.

Consent form in Helsinki declaration format—patient information sheet

Consent form in Helsinki declaration format—statement or declaration by participant

Consent form in Helsinki declaration format—statement or declaration by principal investigator and translator
PATIENT INFORMATION SHEET
Invitation to the subjects to participate in the proposed study.
The participants should be invited to take part in the proposed research/study/clinical trial. The participant must be instructed to take some time to read the information presented here, which will explain the details of this project. Then, assure them that they are free to ask the study staff/doctor/investigator any questions about any part of this project and clarify their doubts, as it is very important for them clearly understand and be fully satisfied with the details of the proposed research. This will further help the participants in knowing their involvement in the study. It should be clearly stated that their participation is “entirely voluntary” and the individual is free to decline to participate. If declined, this will not affect them by any means. It should also be mentioned that participant is free to withdraw from the study at any point, even if after agreeing to take part.
Prior approval from the Committee for Human Research/Institutional Ethical Committee of the concerned dental or medical college or the hospital has to be obtained. Further, it has to be declared that the proposed study will be conducted according to the ethical guidelines and principles of the International Declaration of Helsinki, guidelines of the statutory body involved, and the Medical Research Council—Ethical Guidelines for Research of the country.
Questionnaire-based patient information sheet is usually designed as it enables the participant to understand better. The proficiency of the language used should be simple. 8 The description of the details to be covered in the questionnaire is explained below.
What is the Purpose of the Proposed Research/Study/Clinical Trial?
Describe the details of the study in terms of:
- Aims and objectives of the study.
- Why this study has to be done?
- How this study is intended to be done?
- How are the observations of the study going to be useful to the individual/community?
Why have I been asked to participate?
Inform the participant that he or she has been chosen to participate because he/she would fulfill the selection criteria. Explain briefly the aims and objectives of the studies based on which selection is made.
What is the Duration of the Proposed Research/Study/Clinical Trial?
The duration required for the completion has to be mentioned clearly to all of the participants. This is beneficial to both the participant and the investigating team as it prevents bias due to sample attrition
What are My Responsibilities as a Participant?
The participants should provide the required information/samples/specimens whichever is required as per the study/research/clinical trial.
Are there any Benefits for participating in the Proposed Research/Study/Clinical Trial?
The participants have to be explained that he/she will not benefit from this research directly by themselves. Their participation would, however, be very valuable, as this contributes to medical/dental knowledge, in general. Further, it might lead to develop new diagnostic or preventive measures and better treatment modalities.
Will I be at Risk during and after the Completion of the Proposed Research/Study/Clinical Trial?
If any risks are involved in the research, they should be clearly explained and how it could affect the individual in future.
Are there any Chances of Me getting injured during or after the Completion of the Proposed Research/Study/Clinical Trial (As a Consequence)?
If applicable, it has to be clearly explained, as how this would affect the individual’s life. If not applicable, assurance has to be given about the same.
Is It Compulsory for all the Invitees to accept and participate?
No, it is never a compulsion to the invitees to accept and participate. It is absolutely voluntary. Further, every individual can withdraw from participation at any given point of time.
Will I be penalized for declining, withdrawing from Participation?
None of the invitees or participants will be penalized for declining or withdrawing from participation.
If You disagree to participate, do I have any Alternatives or Options?
Assurance should be given to every individual who declines to participate that it is never going affect them in seeking the heath care.
Who will have access to My Clinical Records or Data?
A statement should be issued assuring that all personal information collected will be treated as confidential and access to it will be strictly controlled and limited to the team of investigators. All identifying information will be made anonymous at the earliest possible time point and all the specimens will be designated by numbers for identification purposes when used in a publication or thesis.
Will I get to know My Results? Or will I be able to have a Copy of My Data?
Yes, every participant is entitled to know their results and to receive a copy of it.
Will I be paid for participating? To participate in the Proposed Research/Study/Clinical Trial, should I bear the Expenses?
It has to be clearly mentioned that no one will be paid for taking part in the study, and the participant will not incur any costs either.
Is there any Additional Information that I should know?
Assurance should be given that:
- Participants can contact the principal investigator at the given telephone number if they have any further queries or encounter any problems.
- Participants can contact the committee for human research/institutional ethical committee at the given contact number if they have any concerns or complaints that have not been adequately addressed by their study doctor.
- Participants will receive a copy of this information and consent form for their own records.
CONSENT CERTIFICATE AND DECLARATION
Separate declaration should be signed by the participant ( Fig. 2C ), investigator, and translator as shown in the template ( Fig. 2D ). This ensures that all the signatories are aware of their duties and are committed to the process by giving their consent.

Consent form in Helsinki declaration format—statement or declaration by parent or guardian (if the participant is minor)
It has been 50 years since the International Declaration of Helsinki was drafted for protecting the human rights in the field of research. Yet, it is not being well acclaimed, especially in the developing countries. Hence, a thorough knowledge is required to understand and implement the doctrine of consent process in populations with different ethnic, race, and cultural backgrounds. This would not only uplift the ethical and moral values of the profession, but also ensures the practice of obtaining genuine and voluntary informed consent. This can be achieved by strictly adhering to the principles of the International Declaration of Helsinki as every health professional has the mission of safeguarding the health of the people.
Source of support : Nil
Conflict of interest : None

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
Informed Consent FAQs
What is informed consent and when, why, and how must it be obtained.
The HHS regulations at 45 CFR part 46 for the protection of human subjects in research require that an investigator obtain the legally effective informed consent of the subject or the subject’s legally authorized representative, unless (1) the research is exempt under 45 CFR 46.101(b) ; (2) the IRB finds and documents that informed consent can be waived ( 45 CFR 46.116(c) or (d) ); or (3) the IRB finds and documents that the research meets the requirements of the HHS Secretarial waiver under 45 CFR 46.101(i) that permits a waiver of the general requirements for obtaining informed consent in a limited class of research in emergency settings. When informed consent is required, it must be sought prospectively, and documented to the extent required under HHS regulations at 45 CFR 46.117 . [Food and Drug Administration (FDA) regulations at 21 CFR part 50 may also apply if the research involves a clinical investigation regulated by FDA.]
The requirement to obtain the legally effective informed consent of individuals before involving them in research is one of the central protections provided for under the HHS regulations at 45 CFR part 46 . This requirement is founded on the principle of respect for persons, one of the three ethical principles governing human subjects research described in the Belmont Report. The principle of respect for persons requires that individuals be treated as autonomous agents and that the rights and welfare of persons with diminished autonomy be appropriately protected. The Belmont Report states that an autonomous agent is “an individual capable of deliberation about personal goals and of acting under the direction of such deliberation.” Respect for persons requires that prospective research subjects “be given the opportunity to choose what shall or shall not happen to them” and thus necessitates adequate standards for informed consent.
The informed consent process involves three key features: (1) disclosing to potential research subjects information needed to make an informed decision; (2) facilitating the understanding of what has been disclosed; and (3) promoting the voluntariness of the decision about whether or not to participate in the research. Informed consent must be legally effective and prospectively obtained. HHS regulations at 45 CFR 46.116 and 45 CFR 46.117 describe the informed consent requirements.
The informed consent process is the critical communication link between the prospective human subject and an investigator, beginning with the initial approach of an investigator to the potential subject (e.g., through a flyer, brochure, or any advertisement regarding the research study) and continuing until the completion of the research study. For the purposes of the HHS regulations at 45 CFR part 46, “investigators” are individuals who conduct human subjects research projects, including individuals directly involved in seeking the voluntary informed consent of potential subjects. Investigators can include physicians, scientists, nurses, administrative staff, teachers, and students, among others.
The informed consent process should be an active process of sharing information between the investigator and the prospective subject. The exchange of information between the investigator and prospective subjects can occur via one or more of the following modes of communication, among others: face-to-face contact; mail; telephone; video; or fax. Prospective subjects should be provided with ample opportunity to ask questions and seek clarification from the investigator. The prospective subjects should be in a position to freely decide whether to initially enroll in the research, or later, to withdraw or continue participating in the research. The informed consent process should ensure that all critical information about a study is completely disclosed, and that prospective subjects or their legally authorized representatives adequately understand the research so that they can make informed choices.
The procedures used in seeking and obtaining informed consent should be designed to communicate with the subject population in terms that they can understand. Information about a research project must be presented in such a way that enables each person to voluntarily decide whether or not to participate as a research subject. Thus, the information must be conveyed in language understandable to those being asked to participate as subjects in the research ( 45 CFR 46.116 ).
For most research, informed consent is documented using a written document that provides key information regarding the research. The consent form is intended, in part, to provide information for the potential subject’s current and future reference and to document the interaction between the subject and the investigator. However, even if a signed consent form is required, it alone does not constitute an adequate consent process. The informed consent process is an ongoing exchange of information between the investigator and the subject and could include, for example, use of question and answer sessions, community meetings, and videotape presentations. In all circumstances, however, individuals should be provided with an opportunity to have their questions and concerns addressed on an individual basis.
The consent process and its documentation should be revised when deficiencies in its accuracy or completeness are noted, when new information about reasonably foreseeable risks and potential benefits becomes available, or when other additional information becomes known that will improve the consent process. Such revisions must be reviewed and approved by an IRB prior to the revised consent being utilized except when necessary to eliminate apparent immediate hazards to subjects ( 45 CFR 46.103(b)(4) ).
Is it possible to obtain legally effective informed consent to research in an urgent or emergency care setting?
Yes, in certain circumstances it is possible to obtain legally effective informed consent in an urgent or emergency care setting. For a particular research study, the answer depends on (1) the expected medical condition of the prospective subject population; (2) the nature of the research; (3) whether there is sufficient time for the potential subjects or their legally authorized representatives to consider participation; and (4) whether the circumstances for obtaining informed consent appropriately minimize the possibility of coercion or undue influence. The Institutional Review Board (IRB) and investigator(s) would have to consider several variables. For example, what is the likely health and emotional condition of the patient population being considered for the proposed research (e.g., conscious but receiving emergency care, undergoing preparation prior to surgery)? What is the likely ability of this population during the consent process to process information, ask questions, and consider the risk involved? What is the timing of the consent process and is it so close to the receipt of care that the patient might blur the distinction between treatment and research?
Because individuals receiving urgent or emergent medical care frequently may be vulnerable to coercion or undue influence, even if temporarily, additional protections may be required to ensure the subject's consent to participate in research is truly voluntary and sought under circumstances that minimize the possibility of coercion or undue influence ( 45 CFR 46.111(b) , ( 45 CFR 46.116 ). In addition, in some cases, it might be possible to obtain consent from a legally authorized representative (e.g., in the case of decisionally incapacitated individuals). In certain emergency circumstances, the Secretarial waiver of informed consent under 45 CFR 46.101(i) may be applicable. It should be noted that if the research is regulated by FDA, the Secretarial waiver permits the research to be conducted under a comparable provision.
See the Office for Human Research Protections' (OHRP) guidance ; and HHS policy (PDF - 22KB) .
What are the basic elements of informed consent?
The basic required elements of informed consent can be found in the HHS regulations at 45 CFR 46.116(a) . Also see OHRP Informed Consent Tips .
The regulations require that the following information must be conveyed to each subject:
- a statement that the study involves research, an explanation of the purposes of the research and the expected duration of the subject’s participation, a description of the procedures to be followed, and identification of any procedures which are experimental;
- a description of any reasonably foreseeable risks or discomforts to the subject;
- a description of any benefits to the subject or to others which may reasonably be expected from the research;
- a disclosure of appropriate alternative procedures or courses of treatment, if any, that might be advantageous to the subject;
- a statement describing the extent, if any, to which confidentiality of records identifying the subject will be maintained;
- for research involving more than minimal risk, an explanation as to whether any compensation and an explanation as to whether any medical treatments are available if injury occurs and, if so, what they consist of, or where further information may be obtained;
- an explanation of whom to contact for answers to pertinent questions about the research and research subjects’ rights, and whom to contact in the event of a research-related injury to the subject; and
- a statement that participation is voluntary, refusal to participate will involve no penalty or loss of benefits to which the subject is otherwise entitled, and the subject may discontinue participation at any time without penalty or loss of benefits to which the subject is otherwise entitled.
Additional elements are described at 45 CFR 46.116(b)
What additional information might be appropriate to provide during the consent process?
When determined to be appropriate by the Institutional Review Board (IRB), subjects must be provided with one or more of the following additional elements of information during the informed consent process (see 45 CFR 46.116(b) ):
- a statement that the particular treatment or procedure may involve risks to the subject (or to the embryo or fetus, if the subject is or may become pregnant) which are currently unforeseeable;
- anticipated circumstances under which the subject’s participation may be terminated by the investigator without regard to the subject’s consent;
- any additional costs to the subject that may result from participation in the research;
- the consequences of a subject’s decision to withdraw from the research and procedures for orderly termination of participation by the subject;
- a statement that significant new findings developed during the course of the research which may relate to the subject’s willingness to continue participation will be provided to the subject; and
- the approximate number of subjects involved in the study.
It is up to the IRB to determine in a particular instance whether some or all of the above additional elements must be included as part of the informed consent process for a particular study. The IRB should make this determination based on the nature of the research and its knowledge of the local research context. If the IRB determines that additional elements are appropriate to the research study, this additional information should be considered just as essential as the eight basic elements of informed consent described in the HHS regulations at 45 CFR 46.116(a) .
Furthermore, an IRB may require that additional information beyond the basic and additional elements be given to subjects during the informed consent process, when in the IRB’s judgment the additional information would meaningfully add to the protection of the rights and welfare of the subjects 45 CFR 46.109(b) .
Can consent or parental permission ever be "passive" or "implied?"
Terms such as “passive” or “implied” consent are not referenced in the HHS regulations. However, OHRP is aware that these terms are sometimes used by investigators or IRBs to describe a process in which consent or parental permission requirements have been altered or waived, or for which the requirement to document consent or parental permission has been waived.
HHS regulations at 45 CFR 46.116 state that no investigator may involve a human being as a subject unless the investigator has obtained the legally effective informed consent of the subject or the subject’s legally authorized representative. However, under conditions specified in the regulations at 45 CFR 46.116(c) or (d) an IRB may approve a consent procedure that does not include, or that alters some or all of the elements of informed consent set forth in 45 CFR 46.116 . In some cases, an IRB also can waive the requirement to obtain consent ( 45 CFR 46.116(c) and (d) ). In addition, under conditions specified in the regulations at 45 CFR 46.117 , an IRB may also waive the requirement for documentation of informed consent. (Note that the regulations at 45 CFR 46.408(c) also permit an IRB to waive parental permission.)
For example, a researcher conducting a survey (that does not qualify for an exemption under 45 CFR 46.101(b) mails a survey questionnaire to a random sample of adults. The survey materials clearly state that by responding to the questions and mailing the survey back, the recipients have agreed to participate in the research. However, the materials accompanying the questionnaire do not include all of the elements of consent listed at 45 CFR 46.116(a) and do not require that the subject sign a consent form. If the IRB has approved this alteration of the consent process and has waived the need for documentation of consent, then such procedures are permissible under the regulations. By sending back a completed survey the recipient has implied that he or she consents to participate but has not signed an informed consent document. Although some might call this “implied informed consent,” OHRP would consider this to be a permissible informed consent process if the IRB has approved the informed consent alteration and waived the requirement for documentation of informed consent.
The term “passive consent” is sometimes used in research with children to describe situations in which the investigator can assume that a parent is permitting a child to participate. For example, researchers collecting survey and behavioral data from children at school provide parents with information regarding the study by mail and ask the parent(s) to return a form if they do not want their child to participate. Sometimes this practice is referred to as an opt out procedure, which is not consistent with the regulatory requirement for seeking and obtaining parental permission. If the IRB determines that the conditions for waiver of parental permission can be met, then the IRB could waive the requirement for parental permission under 45 CFR 46.408(c) or 45 CFR 46.116(c) or (d) . Even though not required by the regulations, an IRB may require that parents be given the opportunity to refuse permission even when the IRB has waived the regulatory requirement to obtain parental permission.
What does it mean to minimize the possibility of coercion or undue influence?
The HHS regulations state that “An investigator shall seek such consent only under circumstances that provide the prospective subject or the representative sufficient opportunity to consider whether or not to participate and that minimize the possibility of coercion or undue influence” ( 45 CFR 46.116 ). This requirement applies to all nonexempt human subjects research not eligible for a waiver of the consent requirements.
Coercion occurs when an overt or implicit threat of harm is intentionally presented by one person to another in order to obtain compliance. For example, an investigator might tell a prospective subject that he or she will lose access to needed health services if he or she does not participate in the research.
Undue influence , by contrast, often occurs through an offer of an excessive or inappropriate reward or other overture in order to obtain compliance. For example, an investigator might promise psychology students extra credit if they participate in the research. If that is the only way a student can earn extra credit, then the investigator is unduly influencing potential subjects. If, however, she offers comparable non-research alternatives for earning extra credit, the possibility of undue influence is minimized.
In addition to undue influence that can arise with the offering of rewards, undue influence also can be subtle. For example, patients might feel obligated to participate in research if their physician is also the investigator, or students might feel pressure to participate in research if everyone else in the class is doing so. Because influence is contextual, and undue influence is likely to depend on an individual’s situation, it is often difficult for IRBs to draw a bright line delimiting undue influence. It is up to the IRB to use its discretion in determining which circumstances give rise to undue influence. For example, an IRB might consider whether the informed consent process will take place at an appropriate time and in an appropriate setting, and whether the prospective subject may feel pressured into acting quickly or be discouraged from seeking advice from others.
Because of their relative nature and lack of clear-cut standards on the boundaries of inappropriate and appropriate forms of influence, investigators and IRBs must be vigilant about minimizing the possibility for coercion and undue influence. Reasonable assessments can be made to minimize the likelihood of undue influence or coercion occurring. For example, IRBs may restrict levels of financial or nonfinancial incentives for participation and should carefully review the information to be disclosed to potential subjects to ensure that the incentives and how they will be provided are clearly described. Known benefits should be stated accurately but not exaggerated, and potential or uncertain benefits should be stated as such, with clear language indicating how much is known about the uncertainty or likelihood of these potential benefits.
The regulatory requirements for IRB review and approval also specify the need for the IRB -- in order to approve research covered by the HHS regulations -- to ensure that “When some or all of the subjects are likely to be vulnerable to coercion or undue influence, such as children , prisoners , pregnant women , mentally disabled persons, or economically or educationally disadvantaged persons, additional safeguards have been included in the study to protect the rights and welfare of these subjects” ( 45 CFR 46.111(b) ). Thus, inducements that would ordinarily be acceptable in some populations may become undue influences for these vulnerable subject groups.
When does compensating subjects undermine informed consent or parental permission?
The HHS regulations require that “An investigator shall seek such consent only under circumstances that provide the prospective subject or the representative sufficient opportunity to consider whether or not to participate and that minimize the possibility of coercion or undue influence” ( 45 CFR 46.116 ).
Paying research subjects in exchange for their participation is a common and, in general, acceptable practice. However, difficult questions must be addressed by the IRB. For example, how much money should research subjects receive, and for what should subjects receive payment -- their time, inconvenience, discomfort, or some other consideration -- IRBs must be sensitive to whether any aspect of the proposed remuneration will be an undue influence, thus interfering with the potential subjects’ ability to give voluntary informed consent.
Remuneration for participation in research should be just and fair. However, the specifics of each protocol will influence how those determinations are made. Both researchers and IRBs need to be familiar with the study population and the context of the research in order to make reasonable judgments about how compensation might affect participation. Wherever the remuneration is set, it will influence the decisions of some more than others. In particular, it will be more important to those for whom it will make a significant financial difference. Thus, IRBs should be cautious that payments are not so high that they create an “undue influence” or offer undue inducement that could compromise a prospective subject’s examination and evaluation of the risks or affect the voluntariness of his or her choices.
Information submitted to IRBs should indicate and justify proposed levels and purposes of remuneration, which also should be clearly stated in the accompanying consent forms.
Some institutions have adopted policies regarding the recruitment and payment of volunteers. IRBs and investigators should ensure that the consent process includes a detailed account of the terms of payment, including a description of the conditions under which a subject would receive partial or no payment (e.g., what will happen if he or she withdraws part way through the research or the investigator removes a subject from the study for medical or noncompliance reasons).
Finally, in studies of considerable duration or that involve multiple interactions or interventions, OHRP recommends that payment be prorated for the time of participation in the study rather than delayed until study completion, because the latter could unduly influence a subject’s decision to exercise his or her right to withdraw at any time. For example, if the study is conducted over a period of 6 months, there might be a monthly or bi-monthly payment. Or, if the study involves 12 sessions, there might be payment after every two sessions.
The above principles would apply to remuneration offered to parents whose children are prospective subjects.
[ Note: The previous version of the response to this FAQ included the following sentences. “In no case should remuneration be viewed as a way of offsetting risks; that is, it should not be considered a benefit to be weighed against study risks. The level of remuneration should not be so high as to cause a prospective subject to accept risks that he or she would not accept in the absence of the remuneration.” The first sentence has been struck because this FAQ focuses on potential undue influence in the consent process (45 CFR 46.116) rather than on IRB considerations under 45 CFR 46.111. However, OHRP continues to assert that IRBs should not consider remuneration as a way of offsetting risks. The second sentence has been deleted to clarify that remuneration to subjects may include compensation for risks associated with their participation in research and that compensation may be an acceptable motive for agreeing to participate in research. In addition, the previous version contained the following sentence, which has been struck because it is focused on IRB considerations under 45 CFR 46. 111 rather than informed consent, and was misplaced in this FAQ: “IRBs may need to request of the investigator some plan for monitoring subject recruitment to ensure that such inducements do not result in inequitable subject recruitment (e.g., recruiting only economically disadvantaged individuals).” ]
Can non-financial enrollment incentives constitute undue influence?
Yes, in certain circumstances. Non-monetary incentives (e.g., extra credit for students, access to services or programs) also can create undue influence on a potential subject’s decision about research participation. Informed consent always must be voluntary ( 45 CFR 46.116 ).
IRBs should ensure that non-financial incentives are not so great as to diminish the voluntariness of consent or cloud someone’s appreciation of risks or potential benefits that might be gained from participating in a study ( 45 CFR 46.116 ). Moreover, it must be clear that choosing to not participate will not adversely affect an individual’s relationship with the institution or its staff or the provision of services in any way (e.g., loss of credits or access to programs) ( 45 CFR 46.116(a)(8) ).
Overt coercion (e.g., threatening loss of services or access to programs to which the potential subjects are otherwise entitled) is never appropriate. However, it might be permissible to provide incentives to participate that do not constitute undue influence. Using enrollment incentives to recruit subjects may be ethically permissible as long as the IRB has determined that, although they may be a factor in a subject’s decision to participate, they have not served to unduly influence the subject to participate. To make this determination, IRBs should know who the subject population will be, what incentives are being offered, and the conditions under which the offer will be made.
What constitutes coercion or undue influence when students are involved in research in a college or university setting?
The regulations require that the investigator seek consent only under circumstances that minimize the possibility of coercion or undue influence ( 45 CFR 46.116 ). The Office for Human Research Protections (OHRP) recommends that institutions have policies in place that clarify for students and faculty that any participation of students in research must be voluntary. Reasonable levels of extra credit or rewards may be offered for participating in research. If extra credit or rewards are offered for participation, students must be provided with and informed of non-research alternatives involving comparable time and effort to obtain the extra credit in order for the possibility of undue influence to be minimized. However, if participation in research is a course requirement, students must be informed of non-research alternatives involving comparable time and effort to fulfill those requirements in order for the possibility of undue influence to be minimized. Moreover, students must not be penalized for refusing to participate in research ( 45 CFR 46.116(a)(8) ).
In addition, some research institutions use a so-called “student subject pool” to identify students who might be willing to participate in research, even when the exact nature of the research to be conducted has not yet been determined. Extra credits or other rewards are often offered as an incentive to encourage participation. Students who sign up for such pools have not legally consented to participate in a research study since they have not been provided with sufficient information concerning the exact study in which they would participate. Thus, signing up to be in a subject pool is only a first and preliminary step by which individuals can indicate their willingness to be considered for research participation. The student must also provide informed consent, unless the consent requirement is waived by an IRB once he or she is being considered for a specific study ( 45 CFR 46.116 ). Furthermore, individuals in the pool must be free to decline participation in any available research projects without penalty ( 45 CFR 46.116(a)(8) ).
What constitutes coercion or undue influence when employees are the subjects of research?
The issues involving employees as research subjects are essentially identical to those involving students as research subjects: that is, investigators and IRBs must be cautious about the potential for coercion or undue influence and the need to protect confidentiality.
Employee participation raises questions about the ability of employees to exercise free choice, for example, because of the possibility that a decision to participate could affect performance evaluations or job advancement, even if it is only the employee’s perception that this is the case. In the case of coercion, refusal to participate might result in a loss of benefits (e.g., salary increases, time off). In the case of undue influence, a decision to participate could result in a job promotion. Employees are likely to view their employers as authority figures to whom they must show deference, which could undermine the freedom of their choice.
Should the initial consent or parental permission procedure ever be repeated or supplemented?
Yes, in some circumstances. The HHS regulations require that an investigator obtain legally effective informed consent from subjects or a legally authorized representative before the subjects may be involved in research ( 45 CFR 46.116 ), unless this requirement has been waived by an IRB . Likewise, for research involving children, permission of the potential subjects' parents or guardians must be obtained ( 45 CFR46.408(c) ), unless an IRB has waived this requirement . Ensuring an adequate consent or parental permission process may require repeating or supplementing the initial consent procedure. The regulations also stipulate that “An investigator shall seek such consent only under circumstances that provide the prospective subject or the representative sufficient opportunity to consider whether or not to participate and that minimizes the possibility of coercion or undue influence” ( 45 CFR 46.116 ). This requirement also might necessitate repeating or supplementing the initial consent procedure.
Informed consent and parental permission should be viewed as an ongoing process. The regulations do not explicitly describe all of the circumstances that might require repeating or supplementing the informed consent process. However, they do require that potential subjects be provided, when appropriate, with a “statement that significant new findings developed during the course of the research which may relate to the subject’s willingness to continue participation will be provided to the subject” ( 45 CFR 46.116(b)(5) ). Thus, to ensure that consent remains legally effective -- for example, if the protocol design or risks have changed, or if a substantial period of time has elapsed between the time consent was obtained and the study begins -- it might be necessary to ensure that subjects still want to participate in the research. For example, the prospective subject may no longer be interested in participating, may no longer meet the eligibility criteria, may no longer find the risks acceptable, or may no longer have the time to complete all study-related activities.
The IRB must review and approve any changes in the approved consent procedure, including alterations of the content, as described in the elements listed at 45 CFR 46.116 , or in its timing, and may consider whether there is a need to reiterate the process ( 45 CFR 46.103(b)(4) ). The IRB should take into account whether the changes could potentially affect a subject’s understanding of the nature of the study or potentially affect a subject’s willingness to participate. If so, such changes need to be made in the informed consent document. Even without significant changes to a protocol or informed consent document, periodic reiteration or affirmation of consent is often a good idea, especially if the study takes place over a long period of time or is particularly complex. Minor changes, such as correcting nonsubstantive typographical errors in the consent document, would not generally rise to a level requiring repeating the consent process.
How far in advance of research participation can consent be obtained?
The HHS regulations at 45 CFR part 46 do not specify how far in advance of study entry a subject can provide consent. The amount of time required by a subject to make a decision would presumably depend on the nature of the study, taking into account, among other factors, the degree of risk, potential benefits, alternatives, and desire to consult with family members or others. However, if a prolonged period of time elapses from the date of consent to the date of entry into the study even if there have been no changes in the study design or no new significant findings affecting the study it might be prudent to review the information contained in the consent form with the subject prior to initiating any research procedures with the subject.
Can records or databases be reviewed to identify potential subjects without obtaining informed consent or parental permission?
Yes, under certain circumstances. Although the HHS regulations do not specifically reference this type of activity, sometimes referred to as “preparatory to research,” such an activity must be reviewed and approved by an IRB in accordance with HHS regulations at 45 CFR 46.109(a) when:
- The activity involves human subjects research, as defined by the regulations at 45 CFR 46.102(f) ;
- The research does not meet the criteria for exemption under HHS regulations at 45 CFR 46.101(b) .
In general, informed consent of the subjects, or parental permission for children involved in research, must be sought and documented in accordance with, and to the extent required by, HHS regulations at 45 CFR 46.116 and 45 CFR 46.117 respectively.
However, an IRB may approve a consent or parental permission procedure that does not include, or that alters, some or all of the elements of informed consent, or may waive the requirements to obtain informed consent ( 45 CFR 46.116(c) or (d) ). In order to permit investigators to obtain and record identifiable private information for the purposes of identifying potential subjects, OHRP expects that IRBs routinely will waive the requirement for informed consent for such activities. In assessing the level of risk to determine whether a waiver of informed consent or parental permission is permissible for the identification of potential subjects, the IRB need only consider the risk of investigators accessing the subjects’ identifiable private information, not the risks of the research in toto.
How can the consent and parental permission processes be designed to facilitate understanding?
The procedures used in obtaining informed consent and parental permission should be designed to inform the subject population or the parents of the subject population about the research in terms that they can understand. Therefore, informed consent and parental permission language and its documentation in the accompanying forms (especially explanation of the study’s purpose, duration, experimental procedures, alternatives, risks, and benefits) should be provided in language that is understandable and culturally sensitive to those being asked to participate or provide permission for their child’s participation.
If the prospective subjects include, for example, persons whose primary language is not English, or populations with low literacy levels, the IRB should take special care to ensure that both oral presentations and consent or permission forms are comprehensible to all subjects or the parents of subjects who are children. Subjects who do not speak English should be presented with a consent or permission document written in a language understandable to them. OHRP strongly encourages the use of such a document whenever possible. (See OHRP guidance on this topic at http://www.hhs.gov/ohrp/regulations-and-policy/guidance/obtaining-and-documenting-infomed-consent-non-english-speakers/index.html ; for information about requirements for child assent, see FAQs regarding research with children .)
In general, ordinary language should replace technical terms (e.g., upper extremities are better referred to as arms, venipuncture as taking blood from your arm with a needle, and so forth).
Some IRBs find that their lay members (e.g., community or non-scientist members) are particularly helpful in suggesting necessary modifications to language. Others ask members of the proposed subject population (e.g., clinic patients) to review consent or permission forms and indicate which parts they do not understand.
Can an electronic signature be used to document consent or parental permission?
Yes, under certain circumstances. First, the investigator and the IRB need to be aware of relevant laws pertaining to electronic signatures in the jurisdiction where the research is going to be conducted.
Unless the IRB waives the requirement for the investigator to obtain a signed consent or permission form based on the HHS regulations at 45 CFR 46.117(c) , a written consent or permission form, which may be an electronic version, must be given to and signed by the subjects or the subjects' legally authorized representatives or the parents of subjects who are children. Some form of the consent document must be made available to the subjects or the parents of subjects who are children in a format they can retain. OHRP would allow electronic signature of the document if such signatures are legally valid within the jurisdiction where the research is to be conducted.
OHRP does not mandate a specific method of electronic signature. Rather, OHRP permits IRBs to adopt such technologies for use as long as the IRB has considered applicable issues such as how the electronic signature is being created, if the signature can be shown to be legitimate, and if the consent or permission document can be produced in hard copy for review by the potential subject. One method of allowable electronic signatures in some jurisdictions is the use of a secure system for electronic or digital signature that provides an encrypted identifiable “signature.” If properly obtained, an electronic signature can be considered an “original” for the purposes of recordkeeping.
Is a faxed copy of the signed consent or parental permission form acceptable to document informed consent?
Yes, if it is more convenient for the subjects or parents of children who are subjects to fax a signed copy of the consent or permission form to the investigator, the research subjects or parents may fax the signed form. The subjects or parents need not provide the investigator with the original signed consent or parental permission documents.
Who must sign the informed consent or parental permission document?
When a written consent or parental permission form is used that embodies some or all of the elements of informed consent required by the regulations at 45 CFR 46.116 , the regulations only require that the informed consent or parental permission document be signed by the subjects or the subjects' legally authorized representatives or by the parents of children who are subjects ( 45 CFR 46.117(a) ) and 45 CFR 46.408(d) ). Only in situations where a short form is used, stating that the elements of informed consent required by 45 CFR 46.116 have been presented orally to the subject or the subject’s legally authorized representative or to the parent(s) of a child who is a subject, are there additional requirements for signatures ( 45 CFR 46.117(b)(2) ).
For the consent or parental permission process using the short form, the regulations state that there must be a witness to the oral presentation, who then signs both the short form and a copy of the IRB-approved written summary of what is to be said to the subject or the subject's legally authorized representative or to the parent(s) of a child who is a subject. The subject or the subject’s legally authorized representative or the parent(s) must sign the short form, and the person actually obtaining the consent must sign the copy of the summary ( 45 CFR 46.117(b)(2) ). Thus, three types of persons are involved in this specific consent process -- the subject or legally authorized representative or parent(s) of a child who is a subject, the person obtaining consent, and the witness.
Do signatures on consent forms have to be dated?
Although the HHS regulations at 45 CFR 46.117 do not require the consent form to be dated at the time it is signed, OHRP recommends that it be dated so that the IRB and others can document that informed consent was obtained prior to a subject’s participation in the research.
Who can be a legally authorized representative (LAR) for the purpose of providing consent on behalf of a prospective subject?
Legally authorized representative (LAR) means an individual or judicial or other body authorized under applicable law to consent on behalf of a prospective subject to the subject’s participation in the procedure(s) involved in the research ( 45 CFR 46.102(c) ). The regulations state that “no investigator may involve a human being as a subject in research covered by this policy unless the investigator has obtained the legally effective informed consent of the subject or the subject’s legally authorized representative” ( 45 CFR 46.116 ). The issue as to who can be an LAR is determined by the laws of the jurisdiction in which the research is conducted (e.g., local or state law). Some states have statutes, regulations, or common law that specifically address consent by someone other than the subject for participation in research. Most states have no law specifically addressing the issue of consent in the research context. In these states, law that addresses who is authorized to give consent on behalf of another person to specific medical procedures or generally to medical treatment may be relevant if the research involves those medical procedures or medical treatment.
When the laws of the jurisdiction in which the research is being conducted provide a reasonable basis for authorizing an individual to consent on behalf of a prospective subject to their participation in the research procedure(s), OHRP would consider such an individual to be an LAR as defined by HHS regulations at 45 CFR 46.102(c) . IRBs may wish to consult with legal counsel when deciding who can serve as an LAR for subjects of proposed research.
When may a legally authorized representative provide consent on behalf of an adult with diminished decision-making capacity?
In answering this question, the HHS regulations at 45 CFR part 46 should be consulted in addition to the laws of the jurisdiction in which the research is conducted. As a general matter, if an adult lacks capacity to consent, for example, as a result of trauma, mental retardation, some forms of mental illness, or dementia - whether temporary, progressive, or permanent - only a legally authorized representative for that adult can give consent for participation in the research, unless the requirement to obtain informed consent is waived by the IRB in accordance with the requirements at 45 CFR 46.116(c)(d) , or in accordance with the provisions for emergency waiver, which are permitted under the authority of the HHS Secretary at 45 CFR 46.101(i) .
( See the Federal Register notice of this waiver .) Should the subject regain or develop the capacity to consent, then his or her consent must be obtained for any further research, as the consent of the legally authorized representative is no longer valid.
What should be considered in seeking informed consent from individuals with diminished decision-making capacity?
The HHS regulations are silent on the consent procedures specific to subjects with impaired decision-making capacity, for example, as a result of trauma, mental retardation, some forms of mental illness, or dementia, whether temporary, progressive, or permanent. The regulations do require that the IRB ensure that “additional safeguards have been included in the study to protect the rights and welfare” of all subjects that are “likely to be vulnerable to coercion or undue influence.” The regulations include “mentally disabled persons” in this category ( 45 CFR 46.111(b) ).
In research involving adult subjects with mental illnesses or cognitive impairments, the IRB and investigator(s) must be knowledgeable about the condition and any level of impairment that is likely to be present in the subject population. The regulations do speak to the fact that the IRB must possess “the professional competence necessary to review specific research activities” ( 45 CFR 46.107(a) ). This is achieved either by having members with the appropriate experience and expertise or inviting consultants with competence in the special area to assist in the review of issues that require expertise beyond or in addition to that available on the IRB ( 45 CFR 46.107(a) and (f) ). Ensuring such expertise on the IRB improves its ability to make determinations about subject recruitment, enrollment, and informed consent requirements that best match the needs of the subjects.
In some research, such as longitudinal studies involving progressive disorders or aging populations, enrolled subjects may be competent to consent on their own behalf at the outset, yet may experience effects of progressive or intermittent disorders that lead to decisional impairment during the course of the study. In these situations IRBs and investigators should consider the need to discuss with the prospective subjects whether they should designate someone to serve as a legally authorized representative at the outset of the study, consistent with all applicable laws. Even if a subject has consented on his or her own accord, a designated representative would be ready to step in as the legally authorized representative if the subject’s ability to assess his or her own needs and interests becomes compromised during the study.
What are the requirements for assent and parental permission in research with children?
The IRB must determine, to the extent required by 45 CFR 46.116 , that adequate provisions are made for soliciting the assent of the children -- when in the judgment of the IRB the children are capable of providing assent -- as well as the permission of the parents ( 45 CFR 46.408 ). Permission means the agreement of parent(s) or guardian to the participation of their child or ward in research ( 45 CFR 46.402(c) ).
By regulatory definition, children are “persons who have not attained the legal age for consent to treatments or procedures involved in the research, under the applicable law of the jurisdiction in which the research will be conducted” ( 45 CFR 46.402(a) ). In the United States the legal age of adulthood is a matter of state and local law. This means that who is legally considered a child may vary from state to state; in a large majority of states 18 years of age is the legal age of adulthood, but this is not true in every state, locality, or territory. State law also may address specific circumstances in which a person younger than the age of adulthood is legally authorized to consent to medical procedures: for example, some states allow children younger than the legal age of adulthood to consent to the provision of contraceptive services. Certain states provide a mechanism for the emancipation of minors, through which a child younger than the legal age of adulthood may gain certain civil rights, which might include the legal ability to consent to research participation.
The definition of children also takes into account the particular interventions or interactions involved in the proposed research (e.g., surveys, blood tests). For example, in some places individuals who are 16 years of age may legally consent to certain clinical interventions or interactions. If the involvement of human subjects in a proposed research activity consists of these interventions or interactions, then those individuals may be considered as adults for that purpose. If a proposed activity includes an intervention or interaction for which the subject has not yet reached the legal age of consent, however, that person must be considered a child.
Under 45 CFR 408(b) the IRB may find that the permission of one parent is sufficient for research to be conducted under 45 CFR 46.404 or 45 CFR 46.405 . Where research is conducted under 45 CFR 46.406 or 45 CFR 46.407 , permission must be obtained from both parents unless one parent is deceased, unknown, incompetent, or not reasonably available, or when only one parent has legal responsibility for the care and custody of the child.
Although the regulations state that children are unable to provide legally effective informed consent to participate in research, some might be able to give their assent. Assent means a child’s affirmative agreement to participate in research. Mere failure to object should not, absent affirmative agreement, be construed as assent ( 45 CFR 46.402(b) ).
If the IRB determines that the capability of some or all of the children is so limited that they cannot reasonably be consulted regarding assent, or that the intervention or procedure involved in the research holds out a prospect of direct benefit that is important to the health or well-being of the children, and is available only in the context of the research, the assent of the children is not a necessary condition for proceeding with the research. Even where the IRB determines that the subjects are capable of assenting, the IRB may still waive the assent requirement under certain circumstances in accord with 45 CFR 46.116 and 45 CFR 46.408(a) .
May the requirement for obtaining informed consent or parental permission be altered or waived?
Waiver or alteration of the requirements for obtaining informed consent from adult subjects can occur under any of the following three provisions:
- the research could not practicably be carried out without the waiver or alteration; and
- public benefit or service programs;
- procedures for obtaining benefits or services under those programs;
- possible changes in or alternatives to those programs or procedures; or
- possible changes in methods or levels of payment for benefits or services under those programs.
- the research involves no more than minimal risk to the subjects;
- the waiver or alteration will not adversely affect the rights and welfare of the subjects;
- the research could not practicably be carried out without the waiver or alteration; and
- whenever appropriate, the subjects will be provided with additional pertinent information after participation.
- Research in emergency settings: an IRB may also waive the requirement for obtaining informed consent if it finds and documents that the research meets the requirements of the HHS Secretarial waiver under 45 CFR 46.101(i) that permits a waiver of the general requirements for obtaining informed consent in a limited class of research in emergency settings (PDF) (23KB).
For research involving children, an IRB may waive the requirements for obtaining parental or guardian permission under any of the following four provisions:
- The IRB makes and documents the required findings under 45 CFR 46.116(c) as described above.
- The IRB makes and documents the required findings under 45 CFR 46.116(d) as described above.
- an appropriate mechanism is in place to protect the children, and
- the waiver is not inconsistent with federal, state, or local law ( 45 CFR 46.408(c) ). The choice of an appropriate substitute mechanism (for example, appointing a child advocate or an assent monitor) for protecting children participating in research would depend on the nature and purpose of the activities described in the protocol, the risk and anticipated benefit to the research subjects, and the child’s age, maturity, status, and condition ( 45 CFR 46.408(c) ). Note that an IRB may waive the requirement for obtaining parental or guardian permission under 45 CFR 46.408(c) even if the research involves more than minimal risk to the child subjects.
- The IRB finds and documents that the research meets the requirements of the HHS Secretarial waiver under 45 CFR 46.101(i) that permits a waiver of the general requirements for obtaining informed consent in a limited class of research in emergency settings (PDF) (23KB).
What is the definition of guardian in the context of obtaining consent for research involving children?
The term guardian means “an individual who is authorized under applicable State or local law to consent on behalf of a child to general medical care” ( 45 CFR 46.402(e) ) The role of a guardian in the context of research involving a child who is a ward is to provide permission, in lieu of a child’s biological or adoptive parents, for the ward to participate in the research ( 45 CFR 46.402(c) ). For a more extensive discussion see FAQs on Research with Children .
What happens if a child reaches the legal age of consent while enrolled in a study?
The Office for Human Research Protections (OHRP) notes that informed consent should be viewed as an ongoing process throughout the duration of a research project. When a child who was enrolled in research with parental or guardian permission subsequently reaches the legal age of consent to the procedures involved in ongoing research, the subject’s participation in the research is no longer regulated by the requirements of 45 CFR part 46.408 regarding parental or guardian permission and subject assent.
Unless the Institutional Review Board (IRB) determines that the requirements for obtaining informed consent can be waived, the investigators should seek and obtain the legally effective informed consent, as described in 45 CFR 46.116 , for the now-adult subject for any ongoing interactions or interventions with the subjects. This is because the prior parental permission and child assent are not equivalent to legally effective informed consent for the now-adult subject. However, the IRB could approve a waiver of informed consent under 45 CFR 46.116(d) , if the IRB finds and documents that the required conditions are met.
Similarly, if the research does not involve any ongoing interactions or interventions with the subjects, but continues to meet the regulatory definition of “human subjects research” (for example, it involves the continued analysis of specimens or data for which the subject’s identity is readily identifiable to the investigator(s)), then it would be necessary for the investigator(s) to seek and obtain the legally effective informed consent of the now-adult subjects. The IRB may consider, if appropriate, a waiver under 45 CFR 46.116(d) of the requirements for obtaining informed consent in order for the subjects to continue their participation in the research.
What is a waiver or alteration of informed consent or parental permission?
The HHS regulations allow the IRB to waive the requirement for obtaining informed consent or parental permission or to approve a consent procedure that leaves out or alters some or all of the elements of informed consent otherwise required under 45 CFR 46.116(a) and (b) .
Waiving the requirement for obtaining informed consent or parental permission means that the IRB has determined that investigators need not obtain the subjects’ informed consent to participate in research. For example, some research about natural behavior may require that subjects be unaware that the research is taking place. Such research can only be approved by the IRB if the research meets the criteria for a waiver of informed consent under HHS regulations and for approving research according to 45 CFR 46.111 .
An IRB may approve research for which some or all of the elements of informed consent at 45 CFR 46.116 (a) and (b) have been altered, or for which some elements have been left out. For example, some research designs require that subjects be left unaware of the particular purpose of the research, because the subjects’ responses might be biased if they know in advance what the investigators are seeking. Such research designs do not preclude offering potential subjects some information about the research and giving them the opportunity to decide whether to participate. The IRB may approve such research in which investigators will leave out or alter elements of informed consent, so long as the research meets the criteria for approving research in 45 CFR 46.111 , and the research meets the criteria specified in the HHS regulations for leaving out or altering those elements.
What are the regulatory bases for waiving or altering some or all of the required elements of informed consent or parental permission?
The conditions under which an IRB may waive the requirement for obtaining informed consent or parental permission or may approve a consent procedure that leaves out or alters some or all of the elements of informed consent derive from four sources in the HHS regulations.
- At 45 CFR 46.116(c) , the regulations identify when IRBs may waive or approve an alteration of informed consent in some research examining state or local public benefit or service programs , or certain features of those programs.
- At 45 CFR 46.116(d) the regulations identify when IRBs may waive or approve an alteration of informed consent in research that meets four specified criteria .
- At 45 CFR 46.408(c) , the regulations identify when IRBs may approve waiver of parental permission in certain research involving children.
- Under the provisions of 45 CFR 46.101(i) , the Secretary, HHS, has waived the general requirements for obtaining informed consent in a limited class of research in emergency settings .
What are the criteria under 45 CFR 46.116(c) for waiving or altering some or all of the required elements of informed consent or parental permission?
Under 45 CFR 46.116(c) , an IRB may waive the requirement for obtaining informed consent or parental permission or approve a consent or parental permission procedure that leaves out or alters some or all of the elements of informed consent, provided that the IRB finds and documents that the following two criteria are satisfied:
the research or demonstration project is to be conducted by or subject to the approval of state or local government officials and is designed to study, evaluate, or otherwise examine:
- possible changes in methods or levels of payment for benefits or services under those programs; 45 CFR 46.116(c)(1) .
Note that this criterion means that only public benefit or service program research activities that are under state or local authority meet this criterion; similar research conducted under federal authority would not qualify here and is treated elsewhere in the regulations. Research conducted by or subject to the approval of only a private entity also would not qualify.
- the research could not practicably be carried out without the waiver or alteration ( 45 CFR 46.116(c)(2) ).
This criterion means that the practical circumstances of the research are such that the research is not feasible if the informed consent of the subjects must be obtained. For example, a study of identifiable private information about program benefit recipients using 20-year-old records might meet this criterion, if current contact information for those recipients is not available.
What are the criteria under 45 CFR 46.116(d) for waiving or altering some or all of the required elements of informed consent or parental permission?
Under 45 CFR 46.116(d) the IRB may waive the requirement for obtaining informed consent or approve a consent procedure that leaves out or alters some or all of the elements of informed consent, provided that the IRB finds and documents that all of the following four criteria are met:
- the research could not practicably be carried out without the waiver or alteration; and,
Is it possible to waive the informed consent requirement when conducting research in an emergency setting?
In 1996, the HHS Secretary announced, under 45 CFR 46.101(i) , a waiver of the applicability of the regulatory requirement for obtaining and documenting informed consent for a strictly limited class of research, that is, research that may be carried out in human subjects who are in need of emergency therapy and for whom, because of the subjects’ medical condition and the unavailability of legally authorized representatives of the subjects, no legally effective informed consent can be obtained. This waiver applies to research involving adults or children, but does not apply to research involving pregnant women, human fetuses, neonates of uncertain viability, and nonviable neonates, or prisoners.
For more detailed information, see OHRP’s guidance on Emergency Research Consent Waiver . It should be noted that FDA also has a comparable provision for a waiver of informed consent for emergency research at 21 CFR 50.24 .
When may the requirement for documentation of informed consent or parental permission be waived or altered?
When an Institutional Review Board (IRB) has not waived the requirement for seeking prospective informed consent of the subjects or the parental permission of children who are subjects, under the HHS regulations at 45 CFR 46.117(c) , it may waive the requirement for the investigator to obtain a signed consent form for some or all subjects if it finds either:
- That the only record linking the subject and the research would be the consent document and the principal risk would be potential harm resulting from a breach of confidentiality. Each subject will be asked whether the subject wants documentation linking the subject with the research and the subject’s wishes will govern; or
- That the research presents no more than minimal risk of harm to subjects and involves no procedures for which written consent is normally required outside of the research context (e.g., drawing a blood sample, or asking shoppers in a mall about the ambient lighting or temperature).
Some subjects might refuse a copy of the consent form once signed out of concern that their possession of the form could compromise their privacy. This is fully consistent with the idea behind one of the bases for a waiver of the requirements for documentation of informed consent - that harm would result to the subject if his/her identity were compromised by the documentation itself. The investigator may document that the subject refused a copy of the informed consent document and still include the subject in the study.
In cases in which the documentation requirement is waived, the IRB may require the investigator to provide subjects or the parents of children who are subjects with a written statement regarding the research.
Can parental or guardian permission for research involving children be waived?
Yes, under certain circumstances. An IRB may waive the requirements for obtaining parental or guardian permission if either of the following two conditions is met:
- The IRB makes and documents the required findings under either 45 CFR 46.116(c) or (d) ; or
- An appropriate mechanism is in place to protect the children, and
- The waiver is not inconsistent with federal, state, or local law ( 45 CFR 46.408(c) ).
The choice of an appropriate substitute mechanism (for example, appointing a child advocate or an assent monitor) for protecting children participating in research would depend on the nature and purpose of the activities described in the protocol, the risk and anticipated benefit to the research subjects, and the child’s age, maturity, status, and condition ( 45 CFR 46.408(c) ).
Note that an IRB may waive the requirement for obtaining parental or guardian permission under 45 CFR 46.408(c) even if the research involves more than minimal risk to the child subjects.
Is child assent always required when research involves children?
No, the IRB is responsible for deciding whether child assent is required in proposed research activities. Assent means a child’s affirmative agreement to participate in research. Mere failure to object should not, absent affirmative agreement, be construed as assent ( 45 CFR 46.402(b) ). Child assent is required, except in the following three circumstances described at 45 CFR 46.408(a) :
- the capability of some or all of the children is so limited that they cannot reasonably be consulted;
- the intervention or procedure involved in the research holds out the prospect of direct benefit to the health or well-being of the children and is available only in the context of the research;
- the research meets the same conditions as those for waiver or alteration of informed consent in research involving adults, as specified in the regulations at either 45 CFR 46.116(c) or 45 CFR 46.116(d) .
How should child assent be documented?
The HHS regulations do not require documentation of assent. The IRB has the discretion to determine the appropriate manner, if any, of documenting child assent. Based on such considerations as the child’s age, maturity, and degree of literacy, the IRB should decide what form of documentation, if any, is most appropriate. If adolescents are involved in research where a consent form would have been used if the subjects were adults, it would generally be appropriate to use a similar form to document an adolescent’s assent.
If young children are involved who are as yet unable to read, documentation should take a form that is appropriate for the purpose of recording that assent took place. The IRB may also decide that documentation of assent is not warranted.
What is the meaning of "legally effective informed consent?"
Informed consent is legally effective if it is both obtained from the subject or the subject’s legally authorized representative and documented in a manner that is consistent with the HHS protection of human subjects regulations and with applicable laws of the jurisdiction in which the research is conducted. In general terms, the regulations stipulate that an investigator should seek consent only under circumstances that provide the prospective subject or the legally authorized representative sufficient opportunity to consider whether to participate and that minimize the possibility of coercion or undue influence. The information provided should be in language that is understandable to the subject or the representative. No informed consent, whether oral or written, may include any exculpatory language .
It is important to note that the informed consent requirements in the regulations are not intended to preempt any applicable federal, state, or local laws that require additional information to be disclosed for consent to be legally effective ( 45 CFR 46.116(e) ).
Sample consent and permission forms
General consent form to participate in research (DOC)
Two stage project consent form (DOC)
Parent permission form for research with child (DOC)
Child assent form (DOC)
Multiple consent form including audio-recording and quotations (DOC)
Photo and video consent form (DOC)
Video-recording consent form (DOC)
Re-contact agreement form (DOC)
Post-debriefing consent form (DOC)
Our websites may use cookies to personalize and enhance your experience. By continuing without changing your cookie settings, you agree to this collection. For more information, please see our University Websites Privacy Notice .
Neag School of Education
Educational Research Basics by Del Siegle
Research ethics and informed consent.
As researchers, we are bound by rules of ethics. For example, we usually cannot collect data from minors without parental or guardian permission. All research participants must give their permission to be part of a study and they must be given pertinent information to make an “informed” consent to participate. This means you have provided your research participants with everything they need to know about the study to make an “informed” decision about participating in your research. Researchers must obtain a participant’s (and parents’ if the participant is a minor) permission before interacting with the participant or if the participant is the focus of the study. Generally, this permission is given in writing; however, there are cases where the research participant’s completion of a task (such as a survey) constitutes giving informed consent. Research participants have the right to refuse to participate without penalty if they wish. Each university that receives federal funds (and most do) must have an Institutional Review Board (IRB) that reviews all research conducted at the university. Therefore, anyone doing research associated with the university must submit and receive IRB approval before beginning research. Even if the research is exempt from a full review by the IRB, an Exemption Form must be filed and approved by the Department chair and submitted and reviewed by the IRB.
Researchers are bound by a code of ethics that includes the following protections for subjects
- Protected from physical or psychological harm (including loss of dignity, loss of autonomy, and loss of self-esteem)
- Protection of privacy and confidentiality
- Protection against unjustifiable deception
- The research participant must give voluntary informed consent to participate in research. Guardians must give consent for minors to participate. In addition to guardian consent, minors over age 7 (the age may vary) must also give their consent to participate.
NOTE: Voluntary informed consent means that the person involved should have legal capacity to give consent; should be so situated as to be able to exercise free power of choice, without the intervention of any element of force, fraud, deceit, duress, over reaching, or other ulterior form of constraints or coercion; and should have sufficient knowledge and comprehension of the elements of the subject matter involved as to enable them to make an understanding and enlightened decision. This latter element requires that before the acceptance of an affirmation decision by the participant there should be made known to them the nature, duration, and purpose of the experiment; the method and means by which it is to be conducted; all inconveniences and hazards reasonably to be expected; and the effects upon their health or person which may possibly come from their participation in the study.
The consent form that study participants sign should cover the following main points:
- It should tell the participants what they are being asked to do, by whom, and for what purpose. Participants must know the identity of the researcher, his or her affiliations if any, and whom to contact for information if they have problems with the research process. This not only includes contact information for the researcher, but also contact information for the university IRB.
- It should inform the participants of any risks they might be taking by participating in the research.
- It should inform the participants what rights they have in the process, particularly the right of review of material and the right to withdraw from the process.
- It should indicate whether or not participants’ names will be used in the study, whether any other names will be used, or whether pseudonyms will be substituted.
- It should indicate how the results of the study will be disseminated and whether participants can expect to benefit in any way, monetarily or otherwise, from participating in the study.
- It should indicate that participants are free to participate or not participate in the research without prejudice to them.
- In the case of children, it must be signed by the child’s legal guardian. Children cannot be expected to give total informed consent.
- The consent form should be written in the second person (e.g., “You have the right to …”) and in easy to understand language.
THE UNIVERSITY OF CONNECTICUT gives assurance that it will comply with the Department of Health and Human Services (DHHS) regulations for the protection of human research subjects and has set up an Institutional Review Board to review all research associated with the University. Researchers (including student researchers) are required to file a IRB prior to conducting research. Certain types of studies qualify for exempt or expedited review. Research involving minors SELDOM qualifies for exempt status . Even if a study qualifies for exempt status, the researcher must still file a IRB with the Department Head and submit it to the IRB.
Note: Exempt and expedited studies that are not DoJ-funded or subject to FDA regulations must complete a short study status report every year. Full board review and expedited studies that are DoJ-funded or subject to FDA regulations must complete the continuing review process. Therefore, if a research project extends beyond one year, the project must be reviewed each year by the institutional review board as long as data are being collected. If a researcher changes any aspect of a study (including adding or changing questions on a survey) an amendment must be filed and approved by the IRB before using the survey. If a researcher plans to enroll more participants than he or she indicated in the initial IRB, an amendment must be filed and approved by the IRB before enrolling the additional participants. If a researcher wishes to change any of the research procedures that were approved in the approved IRB, and amendment must be filed and approved by the IRB before those changes are made. If a researcher has completed data collection and is only analyzing data and writing the research results, then IRB renewals are no longer required. Informed consent must also be given for interviews. Informed consent can be given verbally, provided there is a witness.
The following criteria are often considered by Institutional Review Boards for the Protection of Human Subjects:
- Are risks greater than “minimal risk”*?
- Are risks minimized?
- Are risks reasonable in relation to the benefits?
- Is subject selection equitable (e.g., subject population included or excluded; risk of coercion in recruitment, etc.)
- Is the process for obtaining consent appropriate?
- Is informed consent appropriately documented?
- Is there adequate provision for monitoring the data collection to insure safety of the subjects?
- Are the provisions for protecting privacy adequate?
- Are the provisions for maintaining confidentiality adequate?
- Have additional safeguards for subjects vulnerable to coercion or undue influence been included?
- Is “annual” continuing review sufficient?
- Do the research staff/investigators have appropriate expertise to perform their responsibilities in the study?
*”minimal risk” means that the probability and magnitude of harm or discomfort anticipated in the research are no greater in and of themselves from those ordinarily encountered in daily life or during the performance of routine physical or psychological examination or tests.
Heightened Awareness of Problems with Unethical Research
In 1966 Dr. Henry Beecher, an anesthesiologist, wrote an article for the June 16, 1966 New England Journal of Medicine called “Ethics and Clinical Research”. In it he described 22 examples of research studies with controversial ethics that had been conducted by reputable researchers and published in major journals. He noted that “unethical or questionable ethical procedures are not uncommon.” Beecher’s article played an important role in heightening the awareness of researchers, the public, and the press to the problem of unethical human subjects research.
Establishment of the National Research Act The publicizing of the Public Health Service Syphilis Study at Tuskeegee (1932-1971) led to the establishment of the National Research Act of 1974 which created a national commission that ultimately issued the Belmont Report (1979). The Belmont Report outlined three basic ethical principles. The TUSKEGEE SYPHILIS STUDY involved 399 African-American men with latent syphilis who were not told by researchers there was a cure for the disease. This was done so the researchers could study the long-term effects of the disease. The Tuskegee syphilis study, coupled with abuses reported in the NUREMBERG TRIALS indicated that researchers and the research they conduct needed to be monitored.
Three Basic Ethical Principles Outlined in the Belmont Report
Respect for Persons (Treat individuals as autonomous human beings, capable of making their own decisions and choices, and do not use people as a means to an end) Three Requirements Based on Respect for Persons
– obtain and document informed consent – respect the privacy interests of research participants – consider additional protection when conducting research on individuals with limited autonomy
Beneficence (Minimize the risks of harm and maximize the potential benefits) Five Requirements Based on Beneficence
– use procedures that present the least risk to participants consistent with answering the scientific question – gather data from procedures or activities that are already being performed for non-research reasons – risks to subjects should be reasonable in relation to both the potential benefits to the participants and the importance of the knowledge expected to result – maintain promises of confidentiality – monitor the data to ensure the safety of participants
Justice (Treat people fairly and design research so that its burdens and benefits are shared equitably) Two Requirements Based on Justice
– select participants equitably – avoid exploitation of vulnerable populations or populations of convenience
Rationale for an Institutional Review Board (IRB) The ethical principles and federal regulation generated by the Belmont Report provide a framework for IRBs to evaluate research involving human subjects. An objective review of research is necessary because
- highly motivated people tend to focus on their goals and may unintentionally overlook other implications or aspects of their work and
- no one can be totally objective about his or her work.
The IRB review system is designed to provide an independent, objective review of research involving human subjects so that the privilege of conducting human subjects research may be maintained.
Only activities that meet the definition of research with human subjects need review by an Institutional Review Board (IRB).
Research is a
- systematic investigation (this might range from applying scientific methodology involving independent and dependent variables to an ethnographic study of a community)
- including research development, testing, and evaluation (this also includes pilot studies, feasibility studies, and other preliminary studies)
- designed to develop or contribute to generalizable knowledge (An essential consideration is whether it is the intention of the investigator to contribute to generalizable knowledge).
Some activities that involve interactions with humans and data gathering may not fit the definition of research with human subjects, since they are designed to accomplish something else, such as in-house quality improvement. For example, a survey of college students about their university’s counseling services may be designed to improve the service delivery for students on campus. Publication of the results is sometimes used as a measure of whether research is generalizable, but this is too narrow a measure for two reasons. First, not every study will produce results worthy of publication. Second, there are other ways that results can be made available to others. They may be presented at a conference. They may be shared with colleagues through the Internet, appear in a dissertation, provided to Board members in a project report, or archived for future research).
A human subject is a “living individual about whom an investigator (whether professional or student) conducting research obtains:
- Data through intervention or interaction (does not need to be face-to-face, could be via email or a participant observation) with the individual or
- Identifiable private information” (a) information about behaviors that occur in a context where the individual can reasonably expect that no observations or recording is taking place or b) information that is provided for a specific purpose and for which the individual can reasonably expect will not be made public).
Once the IRB approves a protocol, it must be reviewed at least annually (every 12 months) until data collection is complete, although IRBs may specify a shorter review period. The extent of the yearly review will vary depending on the research. Amendments and changes to approved protocols must be approved prior to their implementation. An exception could occur if a revision to approved procedures was in response to an unanticipated risk and had to be implemented immediately for the health or well-being of the subjects. Such revisions must be reported promptly to the IRB, not when the research is completed.
The federal regulations governing research with human subjects, which have been adopted by numerous federal departments and agencies, are often referred to as the Common Rule, which is modified from time to time. They were first written by the Department of Health and Human Services (DHHS). The DHHS regulations are often referred to as 45 CFR 46. Pregnant women, fetuses, neonates, children, and prisoners are considered vulnerable populations and are provided additional protection in the DHHS regulations . The DHHS regulations do not have specific additional protections for the elderly, for mentally disabled persons, or for persons whose decision-making capabilities are impaired. Investigators may consider and the IRB may require additional safeguards for these populations. The Common Rule does not include requirements for formatting protocols for IRB review. Formatting requirements are institution specific. Most institutions decide to apply the Common Rule to all research with human subjects, regardless of the funding source.
Research is eligible for expedited review when it poses no more than minimal risk (minimal risk means that “the probability and magnitude of harm or discomfort anticipated in the research are not greater…than those ordinarily encountered in daily life…”) to the participants and when all the activities fall within the categories identified as eligible.
Research is only eligible for exemption if all the activity associated with the research fall into one of six categories of activities described in federal regulations. Three of these are frequently used by social and behavioral scientists:
- Research conducted in established or commonly accepted educational settings, involving normal educational practices
- Research involving survey procedures, interview procedures, or observations of public behavior providing that any disclosure of identifiable information outside the research setting would not place the subjects at risk of criminal or civil liability or be damaging to the subjects’ financial standing, employability, or reputation (Note: By institution choice, interviews with children and participant observation with children may not be exempt).
- Research involving the collection or study of existing data (collected prior to the research for purposes other than the research) if the data is publicly available or recorded by the investigator in such a manner that the subjects cannot be identified.
The regulations do allow some research with children to be exempt (although institutional policy may not). The duration of the study and the experience of the investigator are not criteria for determining eligibility for exemption.
IRBs must either have sufficient expertise among their members or seek expertise through consultation if the members are not familiar with a methodology or population under consideration. They must have the expertise and professional competence to evaluate research activities commonly conducted by their institution. The primary purpose of the IRB is to protect the rights and welfare of research subjects. An IRB must exercise all of its authorities in order to do so, including monitoring research when appropriate. Although IRBs serve their institutions, they do not represent the interests of their institutions. According to federal regulations, institutional officials may not override an IRB disapproval of a protocol.
While physical risks are minimal in social and behavioral science research, risks associated with participation in social and behavioral science research are often more elusive and less predictable. Every interaction in a research context is a communication of some sort, and communications can go awry. For example, notification by mail to set up a follow-up appointment for a participant in a research study may result in an inadvertent breach of confidentiality.
Risks in social and behavioral sciences generally fall into three categories (in rare circumstances the risk may be physical such as a study of victims of domestic violence who may become the victims of retaliatory violence):
- Invasion of Privacy – This can occur if personal information is accessed or collected without the subjects’ knowledge or consent. The subjects’ participation may be revealed without their knowledge (e.g., e-mail communications with a subject about recovering from sexual assault might be read by family members).
- Breach of Confidentiality — The primary source of risk in the social and behavioral sciences is that information obtained by researchers could harm subjects if disclosed outside the research setting. This could include unintended disclosure of subject’s HIV status resulting in loss of health insurance coverage; revelation of sexual preferences that results in discrimination; disclosure of employee attitudes about their employer resulting in job lose; revealing information about illegal activities or status (drug use or immigrant status) resulting in legal consequences.
- Study Procedures — In some cases, simply participating in the research can cause social or psychological harm. Subjects who experienced abuse as children may experience emotional or psychological distress by participating in a study.
When assessing risk associated with participation in a research study, there are two distinct elements of risk that need to be considered.
- The Probability of Harm — The likelihood that a specific harm might occur
- The Magnitude of Such Harm
Risks in research participation are specific to time, situation, and culture. Thus, what may be a socially sensitive issue or topic at one time or place may not be so at another time or place. Risks in social and behavioral science research are mostly culturally determined. For example, asking women if they have had abortions would carry very different risk in cultures where abortion is a routine medical practice, a country where it is illegal, and a country where it is legal but fraught with religious and political controversy. Risks will differ according to the subject population. A survey about sexually transmitted disease would carry different risks for middle class suburban men, Catholic clergy, and gang members (who in one study claimed to have STD’s when they did not). The risk of emotional distress cannot be managed by anonymizing data, but rather by developing a plan to respond to the distress should it occur.
Risks and Benefits — Researchers tend to underestimate risks involved in activities with which they are familiar and to overestimate the benefits of things that are important to them. Thus, an independent assessment of risk is critical. One function of Institutional Review Boards is to provide this independent assessment. When potential outcomes are severe, people tend to overestimate their probability, regardless of the true probability. And when potential outcomes are less severe, such as embarrassment, people tend to underestimate their probability. Federal regulations, based on the ethical principle of beneficence, require that risks associated with research be reasonable in relation to the anticipated benefits. A great deal of research in the social and behavioral sciences offers little potential for direct benefits to the subjects themselves. The benefits of the research often lie in the importance of the knowledge to be gained. Most research in the social and behavioral sciences poses little or no risk to the subject.
A Certificate of Confidentiality protects sensitive information provided by research subjects from civil, criminal, or administrative subpoena. This protects identifiable research information from forced disclosure. The Certificates are issued by the National Institute of Health (NIH) and may be secured for any research (even non-NIH research), regardless of the source of funding or even for un-funded research. Certificates of Confidentiality may be granted for studies collecting information that, if disclosed, could have adverse consequences for subjects or damage their financial standing, employability, insurability, or reputation. Substance abuse or other illegal behaviors; sexual attitudes, preferences, or practices; genetic information; and psychological well-being are kinds of information that can be protected.
If the only identifier collected in the course of a study would be the signature on the consent document and the principal source of harm would be a breach of confidentiality, a waiver of documentation of informed consent should be sought. A waiver of documentation of informed consent is helpful when the consent form is the only document that links the subject to the study.
Note: Some of the material provided here was adapted from material available in CITI (Course in The Protection of Human Research Subjects).
Last updated on January 16, 2023
Informed consent
Information and guidance for researchers, what is informed consent.
Informed consent is one of the founding principles of research ethics. Its intent is that human participants can enter research freely (voluntarily) with full information about what it means for them to take part, and that they give consent before they enter the research.
Consent should be obtained before the participant enters the research (prospectively), and there must be no undue influence on participants to consent. The minimum requirements for consent to be informed are that the participant understands what the research is and what they are consenting to.
There are two distinct stages to a standard consent process for competent adults:
- Stage 1 (giving information) : the person reflects on the information given; they are under no pressure to respond to the researcher immediately.
- Stage 2 (obtaining consent): the researcher reiterates the terms of the research, often as separate bullet points or clauses; the person agrees to each term (giving explicit consent) before agreeing to take part in the project as a whole. Consent has been obtained.
Researchers should ensure that they comply with the General Data Protection Regulation (GDPR) during and after the consent process, especially if they will be collecting 'special category' (ie sensitive) data or personal data in the course of their research (also refer to the advice on consent in research involving children ). See also the guidance on data protection and research and the data protection checklist for use when preparing an application for ethical review.
Where your research includes filming or photography, you should refer to specific guidance in the Photography and GDPR toolkit .
Written or oral consent – which process suits your project?
Which process to use depends on the research project (its context, design and participants), though an oral process is usually only appropriate where a written process is not feasible. Any consent process must be understandable to the participants concerned. Please see the sections below to find out about different processes which may be used depending on the context, as well as informed consent templates for each process.
Written informed consent process
A written process is used where:
- Reading and signing forms is not problematic.
- The research is complex or has multiple stages.
- First access to the research participants is by providing written information.
Though opinions differ about the legal force of signed consent forms, they provide extra proof that the terms of consent have been understood. This can be especially important when seeking consent for copyright over data, or for future uses of data. Also, future funders or regulators may want written proof of the terms of original consent.
For literate participants who are not put off by written information, a written process is often a straightforward way of communicating the 'research contract'.
Between the provision of information and obtaining consent, the participant should be given a reasonable amount of time to consider whether to consent and to ask questions, though the time given depends on the project design, the context of the research and the participants.
The written consent templates below can be adapted to suit your study.
Oral informed consent process
An oral consent process is where researcher and participant have a conversation to give information and obtain consent. There is no paper form to sign. It is normally used:
- where literacy is a problem
- where there are cultural or political concerns with signing contract-like documents
- where either the researcher and/or the participant could be put at risk by existence of a paper record
- where time for consent is limited, eg a chance interaction between researcher and participant (although you should not use an oral process merely to correct poor planning of research)
- for research conducted via remote video conferencing software
It may also be more appropriate when interviewing elite participants as part of the research.
For all other research, how you arrange the oral process depends on how you will encounter your participants (for example email, phone, an on-the-street-meeting by chance). Between the information-giving and consent stage the participant should be given a reasonable amount of time to consider whether to consent, though this depends on the project design, the type of participants and the context of the research.
When obtaining oral consent, please ensure you are recording the consent process either using a recording device (for example audio recorder if you are conducting an interview that needs to be recorded) or, if participants do not agree to audio recording or if using or keeping audio records is unsafe, by using a researcher record of oral consent template or completing a written consent form on their behalf.
The oral consent templates below can be adapted to suit your study, but careful consideration is required to ensure that these are appropriate for the research and the participants.
Informed consent templates
Written informed consent process (including online surveys), template agreements, cases where 'implied consent' may be acceptable (for example online surveys).
Researchers should always aim to inform people fully and obtain appropriate consent. However, in some cases the research may be straightforward enough that a separate, deliberate process for obtaining consent is not needed. In these cases participants, by their actions, imply consent. This is seen most often in research:
BPG 06 Internet-mediated research
Template information sheet for online research
- where no participant personal details are obtained
- where the topic of research is very low risk and no special category data will be collected
- where participation is confined to one small task, eg completing a survey or simple pencil or computer task
Please note: consent cannot be inferred from inaction (eg failure to move away from a camera).
When consent works against the aims of research
If your research employs deception, you will not be able to inform participants fully about your project’s true aims. In this case please check if you can fully apply the CUREC approved procedure on research involving the deception of participants . If the deception raises ethical concerns such that the application is not covered by this procedure please complete a CUREC 2 application form.
Some research settings evolve very rapidly (for example in conflict studies). Similarly, some research participants may only be revealed in time-poor or emergency settings (eg heart attack patients). This infringes on the standard information-giving stage of research. The vulnerability of participants in those settings may justify an expedited or fully waived consent process. Again it is important to describe the research setting clearly. You may need to complete a CUREC 2 application to the relevant committee in these cases: please check with your DREC or your IDREC .
Last updated Thursday 2 December 2021
Related links
- Where and how to apply for ethical review
- Committee information
- Research ethics FAQs and glossary
- Research integrity and ethics policy
- Ethics committee contacts

My Timetable
LOG IN to show content
My Quick Links
Informed consent guidelines.
If you are conducting any research involving human participants , you must normally give them, and have them sign, an informed consent statement . Detailed information about the research may be provided as part of the informed consent statement or in an additional information letter .
Informed Consent Template
Child Assent Template
Debriefing Form Template
An informed consent statement/information letter has two purposes:
- To enable potential research participants to make an informed choice as to their whether they wish to participate in a study; and
- To document their decision to participate.
- oral consent documented in some form of recording (e.g., field notes, consent log, audio recording)
- building a consent form into your online survey and utilizing checkboxes to document consent (e.g., Qualtrics )
Guidelines for Completing the Informed Consent Statement
The Research Ethics Board provides a downloadable informed consent statement template . We strongly suggest you use the wording provided in the template and add additional information as it applies to your study. Guidelines for the additional information are provided here.
The informed consent statement should be written in second person ("You are invited..."). Use of first person ("I") can be interpreted as suggestive and coercive. If the informed consent statement or information letter is to be in a foreign language, submit the foreign language version and an English translation when seeking Research Ethics Board (REB) review .
Depending on the research method used in the project, each participant must be given a copy of the informed consent statement (and information letter if applicable) to read prior to participation in the study, in paper or electronic format.
- List the title of the project and state the researcher's/researchers' affiliation to Laurier or to another institution as the case may be. Be sure to list the names and affiliations of all the project’s researchers in the informed consent statement/information letter.
- In the case of a student’s project, it is customary to include the name, affiliation, and office telephone number/email address of all faculty advisors or supervisors. Use the faculty member's title (i.e. "Professor," "Associate Professor," etc.) or add "PhD" to the end of the researcher’s name rather than using “Dr” as this may not be appropriate in some cases, particularly in the case of clinical research as it may lead some participants to conclude incorrectly that the researcher is a medical doctor.
- In describing a researcher’s academic qualifications please list only those degrees or qualifications that have been fully completed and awarded. Researchers may indicate that they are presently enrolled in a specific academic program (e.g., I am a doctoral student in the sociology department at Laurier).
- If the project is being done for a client or sponsor, include the name of that client or sponsor in the informed consent statement/information letter.
Information
- Invite the participants to participate and state the purpose of the research project.
- Describe the procedures – i.e. what participants will be asked to do. If a survey, questionnaire, focus group, or interview is used, mention briefly the topics that will be covered during the procedure.
- Since some people relate confidentiality and anonymity to the total number of participants, the approximate number of participants involved in the study should be indicated.
- Provide a brief and general description of the participants (e.g., five to 10 educational policy analysts from Ontario will be interviewed; approximately 200 undergraduate students 17-25 years of age). For some projects, it may be appropriate to tell participants why and how they were selected.
- Indicate the expected duration of participation. This includes the time required for each aspect of participation and, in projects where participation extends through time, the participant’s total time commitment.
- If you plan to record the participants through video or audio, request permission to do so in writing and indicate how you will be using this material, who will have access to the material, who will view/hear the material, what will happen to the multimedia material and transcripts at the end of the study, and what will happen to the material if the participant withdraws. All possible uses of the digital recordings/tapes/films/photos (current and future) must be described. Indicate how recordings will be transcribed and who has access to the transcripts. Inform the participants if the transcriber is someone other than the researcher and indicate that the transcriber will keep all information on the recordings confidential. If the researcher keeps the recordings beyond the end of the study and/or they are archived, then the researchers must notify participants that the recordings/photos will not be used for any additional purposes without their additional permission.
- If deception or concealment is used, include a statement explaining that the research cannot be fully described at this time, but that an explanation will be provided at the conclusion of participation. Provide a copy of the debriefing statement/script for REB review.
- Any costs to the participants (e.g., paying for transportation to or parking at the research site) that may result from participation in the research need to be mentioned in the informed consent statement/information letter.
- Outline any reasonably foreseeable risks and/or discomforts to participants that could result from participating in the research.
- Describe the safeguards to be used to minimize those risks and/or discomforts.
- In some situations, a statement should be included in the informed consent statement/information letter that the particular treatment or procedure may involve currently unforeseeable risks to the participant.
Confidentiality
- Inform participants of the extent to which the information that they provide will be kept confidential and/or anonymous. Describe the extent, if any, to which confidentiality and/or anonymity of records identifying the participant will be maintained. Describe how confidentiality and anonymity will be maintained.
- Explain when and how confidentiality will be broken (i.e., when required by professional codes of conduct or laws e.g. requirement to report children in need of protection).
- Provide a statement about the ways in which the research results will be published or distributed (e.g. only aggregate results will be published or distributed).
- If participants might be identifiable in reports because individual responses will be described, a statement to this effect must be included in the informed consent statement/information letter.
- The consequences of a participant's decision to withdraw from the research and procedures for orderly termination of participation by the participant needs to be described in the informed consent statement/information letter. Explain what will happen to data if a participant withdraws. If data are gathered that contain participant identifiers, the disposition of the data must be stated.
- Indicate to participants in the informed consent statement/information letter how long the information that they provide will be kept and how that information will be disposed of, if that will occur. You may keep the data indefinitely.
Compensation
- State the terms of any compensation for study participation. If participants will receive compensation for participating, indicate how and when they will receive that compensation (e.g., compensation of cash, donation, toys, books, gifts). Indicate the value of the compensation where appropriate.
- Explain if there will be any partial payment if the participant withdraws prior to completion of the study.
- If class credit will be given, indicate the amount of credit to be earned by participating and the conditions for earning credit toward the final grade. Mention any alternative ways to earn the same amount of credit.
- If participants are compensated through a draw or lottery, provide the details of the draw in the informed consent statement/information letter: who is eligible, the odds of winning, the method for determining the winner(s), the prize(s) to be won, when and how the winner(s) will be notified.
- Money (e.g., cash, cheque, or direct deposit) must be reported.
- Near-cash (e.g., gift certificates) must be reported.
- Tangible items do not need to be reported provided the value is nominal (e.g., t-shirts, food item).
- Gift certificates or other tangible items given as a prize for a draw where all parties have equal opportunity to win the prize do not need to be reported.
- Include an invitation for participants to ask any questions about the study, its procedures, or their rights as participants; include a means to contact the researcher (usually a telephone number and/or email address). Only provide institutional contact information and do not provide home addresses or personal phone numbers or emails.
- Indicate that the project has been reviewed and approved by the Research Ethics Board and include REB Chair contact information.
Participation
- Inform participants that participation is voluntary and that they may decline to participate without penalty or withdraw from the study at any time without penalty.
- Tell participants that they have the right to refuse to answer any question or participate in any activity.
- Tell participants what will happen to their data if they withdraw from the study. Explain what will happen to data if a participant withdraws. If data are gathered that contain participant identifiers, the disposition of the data must be stated.
- Outline any anticipated circumstances under which the investigator may terminate the participant's participation without regard to the participant's consent.
Feedback and Publication
- Provide a statement about the ways in which the research results will be written up or presented (for example, a thesis, course project report, book, journal article, conference presentation, class presentation).
- If the project is being conducted on behalf of an agency, company, or client, indicate who will receive a report upon completion of the project.
- Tell participants how and when they will be informed of the results of the research. This could be replaced by a question asking the participants whether they would like a written summary of the results at the conclusion of the study.
- Laurier’s REB does not allow the use of negative option or passive consent, where participants are required to contact the principal investigator (PI) to indicate that they do not wish to be a participant.
- Provide two copies of the informed consent statement, one to be retained by the participant and one to be signed by the participant and, if applicable, the participant's parent(s)/guardian(s)/legal representative(s) and returned to you. For online surveys, participants should be instructed to print or save a copy of the form.
- If your form is more than one page, there should be a line at the bottom of each page for the participant’s initials, except for the last page where the signature is obtained.
- Include a statement that says the participant has read and understands the informed consent statement, acknowledges receiving a copy of the statement, and agrees to participate in the study. Provide lines for signature of the participant and the researcher, and the date. In some cases the participant's parent(s)/guardian(s)/legal representative(s) will also sign the/an informed consent statement.
- If the proposed participants include persons under the age of 16 a separate informed consent statement or, in the case of very young children, oral assent must be obtained both from them and from their parent(s) or guardian(s), and, if applicable, from a school authority, agency director, etc. Normally, persons age 16 and over may give free and informed consent on their own behalf.
- For younger children, prepare a script for REB review of what will be said to them to verbally recruit their participation, using age-appropriate language. Despite the informed consent of parent(s)/guardian(s), children must be able to refuse to participate in research or to withdraw their participation at any time.
- In research involving participants who are not competent to give free and informed consent on their own behalf, free and informed consent must be sought from their authorized representative(s). In such situations where third-party consent has been obtained, but participants understand the nature and consequence of the research, their assent must be obtained; a potential participant's dissent will preclude his or her participation.
Contact Us:
REB General Inquiries
E: [email protected] T: 548.889.3518 Office Location: Alumni Hall
Office Hours:
Search for academic programs , residence , tours and events and more.
Permission Letter To Conduct Research: How To Draft It Right!
In this article, I’ll share my insights and provide you with a step-by-step guide, including customizable templates , to craft your own effective permission letter for research.
Key Takeaways Understand the purpose and importance of a permission letter for research. Learn the essential components to include in your letter. Get a step-by-step guide to writing a compelling permission letter. Benefit from a customizable template to streamline your writing process. Discover practical tips from my personal experience to enhance your letter.
Understanding the Importance of a Permission Letter for Research
A permission letter for research is a crucial document that formally requests authorization to conduct a study in specific locations or collect data from a particular group.

It serves as a formal agreement between the researcher and the authority or individuals involved, ensuring that the research is conducted ethically and legally.
Step-by-Step Guide to Writing Your Permission Letter
Step 1: start with contact information and date.
Always begin your letter by stating your contact information at the top, followed by the date. This should include your name, address, phone number, and email address.
Step 2: Address the Recipient Properly
Address the recipient by their proper title and name. If you’re unsure, a general “To Whom It May Concern” can suffice, but personalized greetings are always more impactful.
Step 3: Introduce Yourself and Your Affiliation
Introduce yourself, your position, and your affiliation. This sets the context and establishes your credibility.
Step 4: Clearly State the Purpose of Your Letter
Be clear and concise about your intent to seek permission for research. Mention the research topic and why the specific site or group is essential for your study.
Step 5: Provide Details of Your Research
Explain the scope of your research, the methodology you’ll use, and the expected duration. Transparency is key to gaining trust and approval.
Step 6: Assure Ethical Compliance
Highlight your commitment to ethical standards, including how you’ll ensure participant confidentiality and data protection.
Step 7: Request for Approval
Politely request permission to proceed with your research, expressing your willingness to comply with any required protocols or guidelines.
Step 8: Include Contact Information for Follow-up
Offer your contact information again, encouraging the recipient to reach out with any questions or requests for further details.
Step 9: Close with a Professional Salutation
End your letter with a professional closing, such as “Sincerely,” followed by your name and signature.
Template for a Permission Letter To Conduct Research
[Your Name] [Your Address] [City, State, Zip Code] [Phone Number] [Email Address] [Date]
[Recipient’s Name or Title] [Organization’s Name] [Address] [City, State, Zip Code]
Dear [Recipient’s Name or Title],
I am writing to request permission to conduct research at [location/site/group], as part of my [research project/study] on [topic]. My name is [Your Name], and I am a [Your Position] at [Your Institution or Organization].
The purpose of my research is to [briefly state the objective]. I believe that [location/site/group] is essential for my study because [reason]. The research will involve [describe the methodology], and I anticipate it will take approximately [duration] to complete.
I assure you that all research activities will adhere to the highest ethical standards. Participant confidentiality and data protection will be strictly maintained throughout the research process.
Your approval to conduct this research would be greatly appreciated. I am more than willing to adhere to any specific protocols or requirements you may have. Please feel free to contact me at [Your Phone Number] or [Your Email Address] if you have any questions or need further information.
Thank you for considering my request. I look forward to your positive response.
[Your Name] [Your Signature, if sending a hard copy]
Personal Tips from My Experience
- Personalize Your Letter: Tailoring the letter to the recipient shows respect and attention to detail.
- Be Concise but Thorough: Provide enough detail to inform but not so much that it overwhelms the reader.
- Follow-Up: Don’t hesitate to follow up if you haven’t received a response within a reasonable time frame.
- Show Appreciation: Always express gratitude for the recipient’s time and consideration.
I hope this guide helps you craft an effective permission letter for your research. I’d love to hear about your experiences or any additional tips you might have. Please share your thoughts and questions in the comments below!
Related Posts
- Free Templates for Research Permission Letters
- 3 Must-Have Templates for Requesting Permission Easily
- Sample Letter To Request To Attend A Conference: Free & Effective
Frequently Asked Questions (FAQs)

Q: What is a permission letter to conduct research?
Answer : A permission letter to conduct research is a formal request to obtain permission from an organization or individual to conduct research on a particular topic. This type of letter is commonly used by students, researchers, and scholars who require permission to carry out their research.
Q: Why is a permission letter to conduct research important?
Answer : A permission letter to conduct research is important because it shows that the researcher has obtained the necessary permissions to conduct their research. It also provides a clear understanding of the scope and nature of the research and how it will be conducted, which can help to prevent misunderstandings or legal issues.
Q: Who should I address my permission letter to?
Answer : You should address your permission letter to the individual or organization that has the authority to grant permission for your research. This could be the head of the organization, a department manager, or an individual who is responsible for the area that you wish to conduct research in.
Q: What should I include in my permission letter to conduct research?
Answer : Your permission letter to conduct research should include an introduction that outlines your research topic and objectives, an explanation of why you need permission, an overview of your research methodology, details on the timeline and logistics of your research, and a formal closing that thanks the recipient for their time and consideration.
Q: How do I ensure that my permission letter to conduct research is effective?
Answer : To ensure that your permission letter to conduct research is effective, make sure that it is clear, concise, and polite. Provide detailed information about your research and the nature of your request, and address any potential concerns or objections that the recipient may have. Finally, proofread your letter carefully to ensure that it is free from errors and typos.
Related Articles
Sample request letter for air conditioner replacement: free & effective, goodbye email to coworkers after resignation: the simple way, sample absence excuse letter for work: free & effective, salary negotiation counter offer letter sample: free & effective, formal complaint letter sample against a person: free & effective, medical reimbursement letter to employer sample: free & effective, leave a comment cancel reply.
Your email address will not be published. Required fields are marked *
- Yale Directories
Institution for Social and Policy Studies
Advancing research • shaping policy • developing leaders, yale-led study spurs federal action: hhs requires consent for intimate medical procedures.
The U.S. Department of Health and Human Services issued new guidance to teaching hospitals and medical schools April 1, requiring that medical providers obtain written consent before performing intimate examinations, particularly on patients under anesthesia.
The national directive marks a major milestone in a yearslong effort driven by the research and advocacy of Lori Bruce , associate director of Yale’s Interdisciplinary Center for Bioethics, supported by the Institution for Social and Policy Studies.
“This landmark achievement for patient consent was attained because countless advocates — including patients, community members, medical students, ethicists, and some physicians — spoke up despite pressure to remain silent,” Bruce said. “Our work isn’t done. We still need to ensure the careful implementation of the updated guidelines. But HHS’s commitment to patient rights is profoundly redeeming.”
In a letter signed by HHS Secretary Xavier Becerra, the department acknowledged media reports and scientific literature demonstrating how hospitals often perform pelvic, breast, and rectal examinations on unconscious patients without their informed consent. Such examinations are often medically unnecessary, Bruce said, serving as an opportunity for trainees and new clinicians to gain practical experience. But unconsented procedures can be traumatic for patients, particularly those who have experienced previous sexual trauma.
“It is critically important that hospitals set clear guidelines to ensure providers and trainees performing these examinations first obtain and document informed consent from patients before performing sensitive examinations in all circumstances,” the HHS letter said, linking to guidelines hospitals must follow to receive Medicare and Medicaid funds . “Informed consent includes the right to refuse consent for sensitive examinations conducted for teaching purposes and the right to refuse to consent to any previously unagreed examinations to treatment while under anesthesia.”
In 2020, Connecticut elected officials invited Bruce to shine a light on the ethics of consent for intimate medical examinations. She gave public lectures explaining the ethical tensions, served on the state’s strategic task force, solicited community insights through Yale’s Community Bioethics Forum , and conducted a national survey — the first of its kind — that helped instigate the passage of Connecticut’s 2022 bill requiring explicit consent for these examinations.
Bruce and her co-authors uncovered how potentially 3.6 million American women and men are likely to have received unconsented pelvic or prostate exams and that Black patients are four times more likely than white patients to report having received such unconsented exams.
In September, the national NBC Nightly News ran a story citing Bruce’s report , drawing the attention of RAINN (Rape, Abuse & Incest National Network), who sought out federal legislators who might help end the practice, Bruce said. About six weeks ago, an aide for U.S. Rep. Nancy Mace (R-South Carolina) reached out to Bruce and collaborated with her on a letter to HHS.
“It’s a step in the right direction that HHS has finally mandated hospitals obtain written consent for these invasive examinations,” Mace said. “The fact that doctors were conducting these exams on women under anesthesia without their consent or medical need is simply unacceptable.”
Bruce said that while medical professionals do not intend any harm in conducting intimate examinations on unconscious patients, research has uncovered how these practices can have a negative impact on medical students as well.

In addition, several research studies have shown that if asked for permission to conduct an extra training exam, about 90% of patients will agree, Bruce said.
“We have been risking the mental health of patients and putting students and everyone in the room watching through this moral angst for no reason,” Bruce said. “Recent studies demonstrate that obtaining explicit consent improves clinicians’ relationship with their patients.”
Connecticut State Rep. Josh Elliot (D-Hamden) recruited Bruce to help inform the Public Health Committee and overcome what he called inertia over the informed consent bill in the years before it eventually passed.
“The biggest problem is that doctors in the state were coming out and saying this was not a problem and this law would cause more problems than it would solve,” Elliot said. “Being able to undercut that argument with specific figures and surveys was necessary. This wouldn’t have happened without Lori’s help.”
Other states followed with similar laws, Bruce said, and she is unaware of any institution reporting significant harm in their ability to train the next generation of medical providers.
ISPS Director Alan Gerber , Sterling Professor of Political Science, praised Bruce for demonstrating how science can inform effective, ethical policy.
“Lori and Interdisciplinary Center for Bioethics Director Stephen Latham exemplify the ideals we strive to uphold at ISPS,” Gerber said. “The Connecticut law and new HHS guidance on consent for intimate medical exams show how expertise, tenacity, and collaboration can culminate in more sensitive and thoughtful practices for everyone.”
Bruce agreed and hopes that the lessons of this overdue correction will reverberate to other areas of medical research and practice.
“Ethical medicine is reliant on good data,” she said. “When we don’t know exactly what is happening, it is challenging to write an ethical law.”

IMAGES
VIDEO
COMMENTS
Informed Consent in Research. Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of ...
permission, adult consent, teacher consent, screening consent, etc.). • In this template, "we" refers to the researchers. If there is only one researcher, edit as appropriate. If the PI is a student, always use "we" to include the faculty advisor. • Submit consent documents in MS Word whenever possible. The iMedRIS comparison tool for
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent ...
2023-04-10. Assent Form Ages 7-14. 2023-06-27. Consent Addendum for Unencrypted Communication. 2020-10-26. Information or Fact Sheet. 2023-04-10. The following documents are samples. IRBIS does NOT generate these documents with application-specific information.
The informed consent process. The voluntary expression of the consent by a competent subject and the adequate information disclosure about the research are critical and essential elements of the informed consent process [].Competent subjects able to comprehend the research-related information should personally decide and provide the consent on research participation.
Documenting informed consent occurs after explaining the research and assessing participant comprehension. At minimum, it involves obtaining the signature of the participant (or the legally-authorized representative or parent (s), when approved) as well as the person obtaining consent.
Definitions. Informed consent for research must be legally effective and obtained before the subject can participate in any study-related activities. It should include an active process of sharing information between the researcher and potential subject and an affirmative agreement by the subject that they want to participate. The concept of "implied" or "passive" consent (e.g ...
Informed consent is a communication process by which researchers reach agreement with people about whether they wish to participate in research. Confusing informed consent with a signed consent form may violate the ethical intent of informed consent, which is to communicate clearly and respectfully, to foster trust, comprehension, and good ...
Consent for participation in research still must be obtained according to appropriate ethical standards. The best way to ensure you have obtained appropriate consent for publication in one of BMJ's journals and to prevent delays in the editorial process is to use our B MJ consent form . This includes a comprehensive description of what is ...
An information-consent letter is used most often to inform a potential participant about a research study and to document a participant's agreement to take part in the study. Guide to Creating an Information Letter and Consent Form. The (docx) is intended to provide researchers with the information needed to develop their information letters ...
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. General Consent Form Templates Social and Behavioral Research Projects (last updated 03/16/2023)
The following is a sample consent form for a research project. It is a research project on faculty life on campus, carried out by the principle investigator (PI) of this project from the fake-named Century University. The interviewer (the investigator) should have the interviewee read this form carefully and ask any questions the interviewee ...
The entire informed consent should be printed on the letter head of the institution or the organization, which is carrying out the proposed research or clinical trial. 7,8 The header of the document should have logo, name, and the complete postal address of the organization or the institution, which is carrying out the research/study/ clinical ...
The HHS regulations at 45 CFR part 46 for the protection of human subjects in research require that an investigator obtain the legally effective informed consent of the subject or the subject's legally authorized representative, unless (1) the research is exempt under 45 CFR 46.101(b); (2) the IRB finds and documents that informed consent can be waived (45 CFR 46.116(c) or (d)); or (3) the ...
How to Write. Step 1 - Download in PDF, Microsoft Word (.docx), or Open Document Text (.odt). Step 2 - The title of the research study being conducted must be included at the top of the consent form. Step 3 - Enter the following information related to the primary researcher in the fields provided: Step 4 - The purpose of the study, the ...
Sample consent and permission forms. General consent form to participate in research (DOC) Two stage project consent form (DOC) Parent permission form for research with child (DOC) Child assent form (DOC) Multiple consent form including audio-recording and quotations (DOC) Photo and video consent form (DOC)
Informed consent is the agreement by a subject (or their Legally Authorized Representative) to participate, or continue participation, in human subjects research. It includes the ongoing process of information exchange that takes place between the subject and the investigator throughout research participation. The purpose of informed consent is ...
SAMPLE LETTER OF CONSENT. (Place on Department or Faculty Letterhead) (Insert Date) Dear (Insert Research Participant's Name): You are being invited to participate in a research study on motor development in infants. In particular, we are interested in the motor development of skilled limb movements and corresponding neural development.
Research Ethics and Informed Consent. As researchers, we are bound by rules of ethics. For example, we usually cannot collect data from minors without parental or guardian permission. All research participants must give their permission to be part of a study and they must be given pertinent information to make an "informed" consent to ...
Informed consent is one of the founding principles of research ethics. Its intent is that human participants can enter research freely (voluntarily) with full information about what it means for them to take part, and that they give consent before they enter the research. Consent should be obtained before the participant enters the research ...
Letter of Informed Consent. This research is being conducted by (Insert your full name) who is a student in the College of Business at Westcliff University, Irvine working on a dissertation. This study is a requirement to fulfill my degree and will not be used for decision-making by any organization. This study is for research purposes only.
TCPS 2 (2022) Consent Guidelines. An informed consent statement/information letter has two purposes: To enable potential research participants to make an informed choice as to their whether they wish to participate in a study; and. To document their decision to participate. In order to make an informed choice, potential participants must ...
A permission letter for research is a crucial document that formally requests authorization to conduct a study in specific locations or collect data from a particular group. It serves as a formal agreement between the researcher and the authority or individuals involved, ensuring that the research is conducted ethically and legally. ...
The U.S. Department of Health and Human Services issued new guidance to teaching hospitals and medical schools April 1, requiring that medical providers obtain written consent before performing intimate examinations, particularly on patients under anesthesia.. The national directive marks a major milestone in a yearslong effort driven by the research and advocacy of Lori Bruce, associate ...