- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- COVID-19 Vaccines
- Occupational Therapy
- Healthy Aging
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board

Is a Cure for MS on the Horizon?
- New Treatments
- FDA Approvals
- Latest Research
Lifestyle Changes
Frequently asked questions.
There is not yet a cure for multiple sclerosis (MS) , and the condition isn't fully understood. But there have been significant advancements in treatment , some of which successfully slow the progression of the disease in many people.
Several new medications have been approved in recent years that slow the course of the disease and reduce symptoms and relapses. Other potential treatments are in trials, including stem cell therapies. And researchers are making progress in understanding the risk factors and causes of MS.
Read on to find out more about the latest research on MS, including the efforts to find a cure for the condition.
sinology / Getty Images
Latest Treatments
Experimental therapies are being explored, as MS treatments and various clinical trials have shown promise. One medication, ibudilast, completed a phase 2 clinical trial in 2018 that showed it can slow the progression of the disease.
Ibudilast is an anti-inflammatory medication that works by reducing inflammation in the body and decreasing the action of a specific enzyme known as phosphodiesterase. Phosphodiesterase breaks down certain organic molecules and, in the process, relaxes muscles and enhances blood flow.
Studies found that while not able to prevent the development of new MS lesions, ibudilast was able to reduce brain atrophy over time compared to a placebo.
The medication can also inhibit certain actions of the immune system that are believed to be behind the nerve cell damage that occurs in the brains of people with MS.
There have also been recent advancements in the use of stem cell therapy for MS. Stem cells are the cells that all other cells in the body are generated from. These cells help the body essentially repair itself.
In 2020, a clinical trial followed patients for one year. In that time:
- About 60% of the patients treated with intrathecal (injected into their spinal fluid) mesenchymal stem cell therapy had no evidence of disease.
- About 40% of the patients treated with intravenous (given in a vein) mesenchymal stem cell therapy had no evidence of disease.
- About 10% of patients in the control group (that did not get a real treatment) had no evidence of disease.
New FDA Approvals
One of the main treatments used to help manage the symptoms and progression of MS is disease-modifying therapies (DMTs). These medications are designed to change the course of MS progression, which ultimately helps reduce its symptoms.
Several new DMT therapies have been approved by the Food and Drug Administration (FDA) to treat and manage MS, including:
- Fingolimod (Gilenya) : First used to treat MS in adults, Gilenya became the first DMT therapy approved by the FDA for use in children with pediatric MS in 2018.
- Diroximel fumarate (Vumerity) : This medication is similar to an older type of DMT known as Tecfidera. It was approved for use in 2019 after it was shown to possess the same medicinal benefits with fewer side effects.
- Ozanimod (Zeposia) : This medication has been approved to treat three types of MS: clinically isolated syndrome , relapsing-remitting MS , and active secondary progression MS . It received FDA approval in March 2020.
- Ofatumumab, Novartis (Kesimpta) : This injectable medication was approved in 2020 after it demonstrated the ability to reduce MS symptom relapses more effectively than previously used DMTs. It was also shown to reduce disease activity in the brains of people with MS, as seen with scans taken by an MRI machine.
- Ponesimod (Ponvory) : In March 2021, the FDA approved this medication after it was shown to help reduce MS symptom relapses by more than 30%.
Two other oral DMTs were approved in 2019: siponimod (Mayzent) and cladribine (Mavenclad). Both of these treatments were shown to reduce the relapse rate of people with MS.
Cladribine was the first oral medication approved for use as a short-course oral DMT, which means that it is taken for a shorter duration of time. Specifically, people with MS take cladribine in two short-term courses that are one year apart.
Recent Research
Another type of stem cell therapy that is being investigated for MS is called hematopoietic stem cell transplantation (AHSCT). The main goal of this type of therapy is to reset the immune system by using chemotherapy to get rid of harmful immune cells that are causing damage and replace them with healthy immune cells (that were harvested prior to chemotherapy) that can reconstitute the immune system.
This method of treating MS is being explored in clinical trials. According to the National Multiple Sclerosis Society, a call for participants in a new trial was sent out in May 2021.
BEAT-MS Trial
The study is referred to as BEAT-MS, and the participants chosen for the trial will be assigned a specific treatment plan—either AHSCT or another effective treatment called best available therapy (BAT). Once the study begins, each participant will be treated and monitored for six years.
Risk Factors
Research on the risk factors associated with the development of the disease is also underway. While some risk factors are known, others have yet to be discovered.
Some unproven theories that medical researchers have theorized might play a role in the onset of MS include:
- Environmental allergies
- Exposure to house pets
- Heavy metal toxicity
- Exposure to organic chemicals
Viruses and MS
According to the National Multiple Sclerosis Society, researchers are also looking at the possible role of viruses in a person’s risk of developing MS. Several viruses are being investigated, including:
- Epstein-Barr virus
- Human herpes virus 6
- Varicella-zoster virus
- Cytomegalovirus
- John Cunningham virus
- Human endogenous retroviruses
Sex Differences
Research has shown that women are more likely than men to develop MS. However, studies have also found that the type of MS that is more common also varies between the sexes.
While women are more at risk for the disease overall, men are more often diagnosed with a specific type of MS known as primary progressive MS. Men with MS are also more likely to experience a faster disease progression and cognitive impairment than women.
Finding out why these sex-related disparities exist would help medical researchers develop an optimal treatment for everyone with MS.
Genetic Research
Genetics may play a role in why some people develop MS but others do not. The role of genetic variants in MS is another key research area. A study published in 2018 added four new genes to the more than 200 genetic variants already associated with MS.
Genetic Research and MS
Understanding which genes might increase a person’s risk of developing MS would give medical researchers the information they need to create clinical tools that could help providers treat and possibly prevent MS.
Research has shown that there are several lifestyle factors associated with developing MS. For example, smoking cigarettes, being overweight as a child, and having low levels of vitamin D have all been identified as potential triggers for the disease.
Understanding how other lifestyle influences might affect MS risk could assist researchers in identifying new ways to treat and prevent the disease.
Diet and Gut Health
Diet and chronic disease often go hand in hand. “Gut microbiome” is the term used to describe the collection of living organisms that inhabit the intestines.
The gut microbiome has been a main area of interest for MS researchers. Studies have found that there might be a connection between the state of a person’s gut microbiome and their risk for developing MS.
A study published in 2020 showed that the diversity of the organisms in the guts of people with MS and people without MS were not significantly different. However, there were marked dissimilarities which the researchers said mean that a more long-term and extensive review of MS and the gut microbiome’s possible role in its development is needed.
MS treatments and management techniques have come a long way. The latest advancements in DMTs have given people with MS more options than ever, some providing even fewer side effects than older treatments.
Aside from oral and injectable DMTs—typically the first-line therapies for MS—other experimental treatments such as stem cell therapy have been showing great promise in helping people with MS manage the disease.
The more educated medical researchers become about the potential genetic risk factors and lifestyle choices that may play a role in the development of MS, as well as what causes the disease in the first place, the more equipped they will be to find better treatments.
A Word From Verywell
As of yet, no cure for MS has been found. However, the major advancements in treatments and the new information that has been learned about the potential causes and risk factors are showing great promise at helping slow or completely halt disease progression in people who do develop MS.
For people with MS experiencing disease progression and worsening of symptoms, the latest FDA-approved treatments might help reduce relapses, which in turn can improve their quality of life.
It’s hard to give an exact timeline for when scientists will find a cure for MS, but new treatments and potential causes (like genetic links) are being explored right now.
Research on MS is exciting and covers a lot of ground. New medications and experimental treatments such as stem cell therapy are being thoroughly investigated. Researchers are also looking at why the disease develops in the first place, which could help them find a way to prevent it.
There is no way to completely halt MS progression, but there are treatments that have been shown to significantly slow it. A type of stem cell therapy known as mesenchymal stem cell therapy is getting close to becoming a treatment that could completely halt MS progression, but more research is needed.
Fox RJ, Coffey CS, Conwit R, et al. Phase 2 trial of ibudilast in progressive multiple sclerosis . N Engl J Med. 2018;379(9):846-855. doi:10.1056/NEJMoa1803583
Petrou P, Kassis I, Levin N, et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis . Brain. 2020;143(12):3574-3588. doi:10.1093/brain/awaa333
Food and Drug Administration. FDA expands approval of Gilenya to treat multiple sclerosis in pediatric patients .
National Multiple Sclerosis Society. FDA approves oral Vumerity™️ (diroximel, fumarate), similar to Tecfidera®, for relapsing MS .
National Multiple Sclerosis Society. UPDATE: FDA-approved oral Zeposia® (ozanimod) for relapsing forms of MS now available for prescription .
National Multiple Sclerosis Society. FDA approves Kesimpta® (ofatumumab), similar to Ocrevus®, for relapsing MS .
Kappos L, Fox RJ, Burcklen M, et al. Ponesimod compared with teriflunomide in patients with relapsing multiple sclerosis in the active-comparator phase 3 OPTIMUM study: a randomized clinical trial . JAMA Neurol. 2021;78(5):558-567. doi:10.1001/jamaneurol.2021.0405
Kappos L, Bar-Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study . Lancet. 2018;391(10127):1263-1273. doi:10.1016/S0140-6736(18)30475-6
Deeks ED. Cladribine tablets: a review in relapsing MS . CNS Drugs. 2018;32(8):785-796. doi:10.1007/s40263-018-0562-0
National Multiple Sclerosis Society. MS trial alert: investigators recruiting nationwide study comparing AHSCT to other therapies in active relapsing MS .
Immune Tolerance Network. About BEAT-MS .
National Multiple Sclerosis Society. What causes MS?
Tarlinton RE, Martynova E, Rizvanov AA, Khaiboullina S, Verma S. Role of viruses in the pathogenesis of multiple sclerosis . Viruses. 2020;12(6):643. doi:10.3390/v12060643
Eccles A. Delayed diagnosis of multiple sclerosis in males: may account for and dispel common understandings of different MS ‘types’ . Br J Gen Pract. 2019;69(680):148-149. doi:10.3399/bjgp19X701729
Airas L. Hormonal and gender-related immune changes in multiple sclerosis . Acta Neurol Scand. 2015;132(199):62-70. doi:10.1111/ane.12433
International Multiple Sclerosis Genetics Consortium. Low-frequency and rare-coding variation contributes to multiple sclerosis risk . Cell. 2018;175(6):1679-1687.e7. doi:10.1016/j.cell.2018.09.049
Jakimovski D, Guan Y, Ramanathan M, et al. Lifestyle-based modifiable risk factors in multiple sclerosis: review of experimental and clinical findings . Neurodegener Dis Manag. 2019;9(3):149-172. doi:10.2217/nmt-2018-0046
Mirza A, Forbes JD, Zhu F, et al. The multiple sclerosis gut microbiota: a systematic review . Mult Scler Relat Disord. 2020;37:101427. doi:10.1016/j.msard.2019.101427
By Angelica Bottaro Angelica Bottaro is a professional freelance writer with over 5 years of experience. She has been educated in both psychology and journalism, and her dual education has given her the research and writing skills needed to deliver sound and engaging content in the health space.

FDA OKs Phase 2 clinical trial of KYV-101 for progressive MS
Cell therapy to be tested in treatment-resistant patients in small study

by Lindsey Shapiro, PhD | January 5, 2024
Share this article:

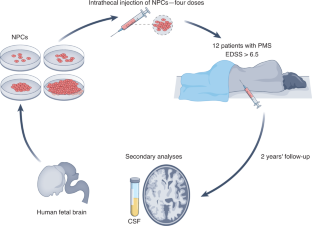
The U.S. Food and Drug Administration (FDA) has cleared a Phase 2 clinical trial to test Kyverna Therapeutics ‘ cell-based therapy candidate KYV-101 in people with treatment-resistant progressive multiple sclerosis (MS).
Called KYSA-7 (NCT06138132) , the open-label trial will enroll an estimated 12 patients with either primary progressive MS (PPMS) or secondary progressive MS (SPMS). Both of these forms of MS are characterized by symptoms that gradually worsen over time — unlike the more common disease type that’s marked by exacerbations, known as relapses.
Eligible participants must be adults, ages 18-55, and have not experienced any disease relapses or MRI activity in the last two years. As the trial is open-label, both researchers and participants will know the treatment being given.
“This approval is a critically necessary step that paves the way to enroll patients with treatment-refractory [resistant] progressive MS for whom there are no currently available treatment options in the KYSA-7 trial,” Bruce Cree , MD, PhD, clinical research director and professor of clinical neurology at the University of California, San Francisco , said in a company press release .
“This study offers participants a new hope for arresting relentless disability worsening and a potentially durable, treatment-free remission,” Cree added.
Anti-inflammatory diet, synbiotics ease progressive MS symptoms
Phase 2 clinical trial to test therapy for dose-limiting toxicities.
The trial page lists a single study site at the Stanford Multiple Sclerosis Center, in California. The KYSA-7 study is expected to run through mid-2027.
MS is an autoimmune disease in which the immune system mistakenly attacks the body’s own cells in the brain and spinal cord. In progressive forms of the disease , symptoms steadily worsen and disability accumulates even in the absence of relapses or evidence of active inflammation on MRI scans.
There are few therapeutic options for progressive MS patients who aren’t experiencing relapses — known as non-active disease. Ocrevus (ocrelizumab) is the only approved treatment for people with PPMS, while mitoxantrone is the only cleared therapy for non-active SPMS in the U.S. Patients who are not responsive, or are refractory, to these treatments don’t have any other options, according to Kyverna.
KYV-101 aims to equip the immune system with the ability to target and eliminate antibody-producing B-cells, which are implicated in MS. Kyverna is developing it for a range of B-cell mediated conditions, including autoimmune diseases and certain types of cancer.
This type of treatment is called a CAR T-cell therapy, where a person’s own immune T-cells are isolated and engineered in the lab to contain a man-made receptor, or chimeric antigen receptor (CAR), designed to recognize the CD19 protein found on B-cells’ surfaces.
When the engineered T-cells are returned to the body, they’re equipped to specifically target and attack problematic B-cells, thereby resetting the immune system. It is believed that this will offer sustained disease remission for progressive MS patients with a single treatment.
KYV-101 also is designed to reduce certain side effects and improve tolerability relative to previously designed CAR T-cell therapies.
This very important study will answer whether CAR T-cell therapy offers a new treatment option for patients living with MS.
In a previous Phase 1/2 study (NCT02659943) , KYV-101 was found to have anti-cancer effects in people with B-cell cancers, and was associated with a lower release of inflammatory molecules that drive certain side effects typically linked to CAR T-cell therapies.
The upcoming trial will involve progressive multiple sclerosis patients who have not experienced any MS relapses in the last two years and who are refractory to other MS treatments. Its main goal is to evaluate patients for any dose-limiting toxicities over a one-year period. Secondary goals relate to safety and clinical responses to treatment.
“This very important study will answer whether CAR T-cell therapy offers a new treatment option for patients living with MS,” said Manuel Friese , MD, professor of neurology at the University Medical Center Hamburg-Eppendorf, in Germany.
“This therapy holds the promise to alter the treatment paradigm of MS by fundamentally readjusting the immune system,” Friese added.
KYV-101 also is being evaluated in a Phase 2 study involving people with lupus nephritis, another type of autoimmune disease. Trials also are being planned for indications in systemic sclerosis and myasthenia gravis .
About the Author

Recent Posts
- Shorter time to 1st MS DMT found for more recent diagnoses: Study
- How to prepare for an MRI: What they don’t tell you
- Early high-efficacy MS treatment recommended for young patients
Recommended reading

Cell therapy using Tregs in MS headed to Phase 2 trials in Poland
FDA clears BrainSpec’s tool to measure metabolites on brain scans

FDA clears AI tool for detecting disease activity on MRI scans
Subscribe to our newsletter.
Get regular updates to your inbox.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- News & Views
- Published: 13 January 2023
Neurodegenerative disease
A neural stem-cell treatment for progressive multiple sclerosis
- Valentina Fossati ORCID: orcid.org/0000-0003-1825-9371 1 ,
- Luca Peruzzotti-Jametti ORCID: orcid.org/0000-0002-9396-5607 2 &
- Stefano Pluchino ORCID: orcid.org/0000-0002-6267-9472 2
Nature Medicine volume 29 , pages 27–28 ( 2023 ) Cite this article
2721 Accesses
1 Citations
31 Altmetric
Metrics details
- Multiple sclerosis
- Stem cells in the nervous system
A phase 1 trial using an allogeneic stem-cell-based therapy in people with progressive multiple sclerosis (MS) shows the feasibility and tolerability of the approach; rigorous evaluation of this and other regenerative strategies for MS is now urgently needed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Pluchino, S., Smith, J. A. & Peruzzotti-Jametti, L. Trends Mol. Med. 26 , 898–912 (2020).
Article CAS Google Scholar
Genchi, E., Brambilla, E., Sangalli, F. & Martino, G. Nat. Med. https://doi.org/10.1038/s41591-022-02097-3 (2023).
Lindvall, O. et al. Lancet 332 , 1483–1484 (1988).
Article Google Scholar
Mazzini, L. et al. Stem Cells Transl. Med. 8 , 887–897 (2019).
Baloh, R. H. et al. Nat. Med. 28 , 1813–1822 (2022).
Leone, M. et al. Preprint at http://medrxiv.org/lookup/doi/10.1101/2022.11.14.22282124 (2022).
Harris, V. K. et al. Neurol. Neuroimmunol. Neuroinflamm. 8 , e928 (2021).
Petrou, P. et al. Brain 143 , 3574–3588 (2020).
Uccelli, A. et al. Lancet Neurol. 20 , 917–929 (2021).
Yamanaka, S. Cell Stem Cell 27 , 523–531 (2020).
Deinsberger, J., Reisinger, D. & Weber, B. NPJ Regen. Med. 5 , 15 (2020).
Download references
Author information
Authors and affiliations.
The New York Stem Cell Foundation Research Institute, New York, NY, USA
Valentina Fossati
Department of Clinical Neurosciences and NIHR Biomedical Research Centre, University of Cambridge, Cambridge, UK
Luca Peruzzotti-Jametti & Stefano Pluchino
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Valentina Fossati .
Ethics declarations
Competing interests.
S.P. is founder, chief scientific officer and shareholder (>5%) of CITC Ltd and chair of the scientific advisory board at ReNeuron plc. The other authors have no conflicts to declare.
Rights and permissions
Reprints and permissions

About this article
Cite this article.
Fossati, V., Peruzzotti-Jametti, L. & Pluchino, S. A neural stem-cell treatment for progressive multiple sclerosis. Nat Med 29 , 27–28 (2023). https://doi.org/10.1038/s41591-022-02164-9
Download citation
Published : 13 January 2023
Issue Date : January 2023
DOI : https://doi.org/10.1038/s41591-022-02164-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Featured Links : >
- Newly Diagnosed
- Ask an MS Expert
- MS Navigator
- Ways to Give
Our Vision is a World Free of MS. We are a driving force of MS research and treatment to stop disease progression, restore function, and end MS forever.
How & Why We Fund Research Initiatives
STOP Disease Progression
RESTORE What's Been Lost
END MS Forever
The complexity of MS necessitates a comprehensive approach that will uncover solutions for everyone –
A comprehensive strategy that can propel knowledge, better treatments, health care policies, and new disease management therapies forward, faster.
A research strategy that can fuel knowledge and speed better treatments, health care policies, and new disease and symptom management therapies.
Participate in Research Studies
Research volunteers are critical for progress.
Research News & Progress
Research updates from around the globe, and hot topics including research on stem cell therapy, progressive MS, vitamin D, and diet.
Research We Fund
We pursue all promising paths to uncover solutions.
For Researchers
Funding, tools and resources to support MS research.
Clinical Trials In Your Area
People with MS can help free the world of this disease by volunteering to participate in studies testing new therapies.
Become a Research Champion
Support MS Research
Get Email Updates
Common household chemicals pose new threat to brain health, study finds
Research shows chemicals in countless household items harm specialized cells in the brain.
A team of researchers from the Case Western Reserve University School of Medicine has provided fresh insight into the dangers some common household chemicals pose to brain health. They suggest that chemicals found in a wide range of items, from furniture to hair products, may be linked to neurological conditions like multiple sclerosis and autism spectrum disorders.
Neurological problems impact millions of people, but only a fraction of cases can be attributed to genetics alone, indicating that unknown environmental factors are important contributors.
The new study published today in the journal Nature Neuroscience , discovered that some common home chemicals specifically affect the brain's oligodendrocytes, a specialized cell type that generates the protective insulation around nerve cells.
"Loss of oligodendrocytes underlies multiple sclerosis and other neurological diseases," said the study's principal investigator, Paul Tesar, the Dr. Donald and Ruth Weber Goodman Professor of Innovative Therapeutics and director of the Institute for Glial Sciences at the School of Medicine. "We now show that specific chemicals in consumer products can directly harm oligodendrocytes, representing a previously unrecognized risk factor for neurological disease."
On the premise that not enough thorough research has been done on the impact of chemicals on brain health, the researchers analyzed over 1,800 chemicals that may be exposed to humans. They identified chemicals that selectively damaged oligodendrocytes belong to two classes: organophosphate flame retardants and quaternary ammonium compounds. Since quaternary ammonium compounds are present in many personal-care products and disinfectants, which are being used more frequently since the COVID-19 pandemic began, humans are regularly exposed to these chemicals. And many electronics and furniture include organophosphate flame retardants.
The researchers used cellular and organoid systems in the laboratory to show that quaternary ammonium compounds cause oligodendrocytes to die, while organophosphate flame retardants prevented the maturation of oligodendrocytes.
They demonstrated how the same chemicals damage oligodendrocytes in the developing brains of mice. The researchers also linked exposure to one of the chemicals to poor neurological outcomes in children nationally.
"We found that oligodendrocytes -- but not other brain cells -- are surprisingly vulnerable to quaternary ammonium compounds and organophosphate flame retardants," said Erin Cohn, lead author and graduate student in the School of Medicine's Medical Scientist Training Program. "Understanding human exposure to these chemicals may help explain a missing link in how some neurological diseases arise."
The association between human exposure to these chemicals and effects on brain health requires further investigation, the experts warned. Future research must track the chemical levels in the brains of adults and children to determine the amount and length of exposure needed to cause or worsen disease.
"Our findings suggest that more comprehensive scrutiny of the impacts of these common household chemicals on brain health is necessary," Tesar said. "We hope our work will contribute to informed decisions regarding regulatory measures or behavioral interventions to minimize chemical exposure and protect human health."
Additional contributing researchers from Case Western Reserve School of Medicine and from the U.S. Environmental Protection Agency included Benjamin Clayton, Mayur Madhavan, Kristin Lee, Sara Yacoub, Yuriy Fedorov, Marissa Scavuzzo, Katie Paul Friedman and Timothy Shafer.
The research was supported by grants from the National Institutes of Health, National Multiple Sclerosis Society, Howard Hughes Medical Institute and New York Stem Cell Foundation, and philanthropic support by sTF5 Care and the Long, Walter, Peterson, Goodman and Geller families.
- Diseases and Conditions
- Medical Topics
- Multiple Sclerosis Research
- Disorders and Syndromes
- Multiple Sclerosis
- Schizophrenia
- Environmental Policy
- Environmental Issues
- Geochemistry
- Brain damage
- Ketone bodies
- Indoor air quality
- Hair follicle
- Color blindness
- Water resources
Story Source:
Materials provided by Case Western Reserve University . Note: Content may be edited for style and length.
Journal Reference :
- Erin F. Cohn, Benjamin L. L. Clayton, Mayur Madhavan, Kristin A. Lee, Sara Yacoub, Yuriy Fedorov, Marissa A. Scavuzzo, Katie Paul Friedman, Timothy J. Shafer, Paul J. Tesar. Pervasive environmental chemicals impair oligodendrocyte development . Nature Neuroscience , 2024; DOI: 10.1038/s41593-024-01599-2
Cite This Page :
Explore More
- Simple Brain-Computer Link: Gaming With Thoughts
- Clinical Reasoning: Chatbot Vs Physicians
- Understanding People Who Can't Visualize
- Illuminating Oxygen's Journey in the Brain
- DNA Study IDs Descendants of George Washington
- Heart Disease Risk: More Than One Drink a Day
- Unlocking Supernova Stardust Secrets
- Why Do Some Memories Become Longterm?
- Cell Division Quality Control 'Stopwatch'
- What Controls Sun's Differential Rotation?
Trending Topics
Strange & offbeat.

Protecting the data of our commercial and public sector customers in the AI era
Mar 28, 2024 | Julie Brill - Corporate Vice President and Chief Privacy Officer
- Share on Facebook (opens new window)
- Share on LinkedIn (opens new window)
- Share on Twitter (opens new window)

Organizations across industries are leveraging Microsoft Azure OpenAI Service and Copilot services and capabilities to drive growth, increase productivity, and create value-added experiences. From advancing medical breakthroughs to streamlining manufacturing operations, our customers trust that their data is protected by robust privacy protections and data governance practices. As our customers continue to expand their use of our AI solutions, they can be confident that their valuable data is safeguarded by industry-leading data governance and privacy practices in the most trusted cloud on the market today.
At Microsoft, we have a long-standing practice of protecting our customers’ information. Our approach to Responsible AI is built on a foundation of privacy, and we remain dedicated to upholding core values of privacy, security, and safety in all our generative AI products and solutions.
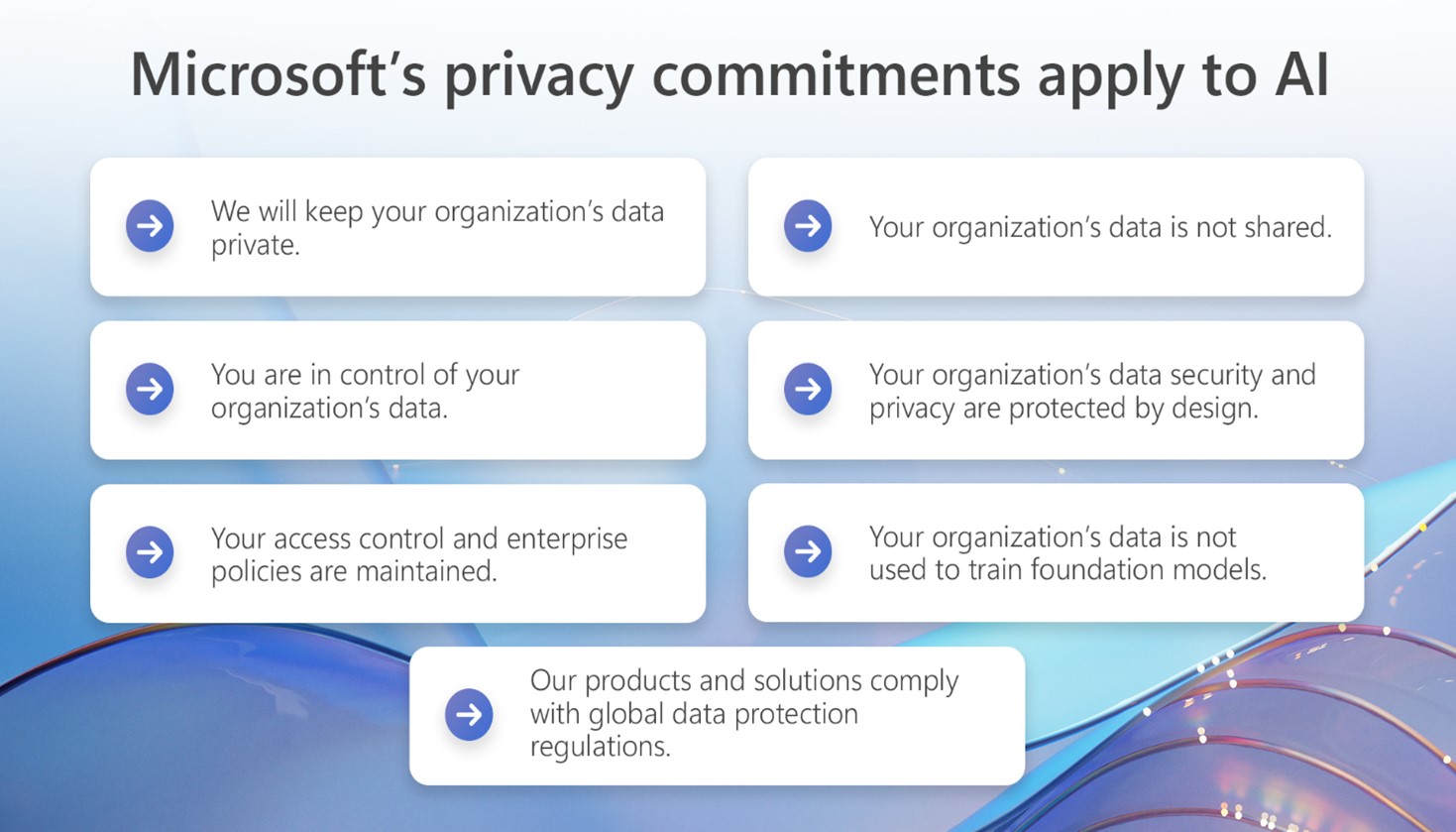
Commercial and public sector customers can rest assured that the privacy commitments they have long relied on for our enterprise cloud products also apply to our enterprise generative AI solutions, including Azure OpenAI Service and our Copilots.
- We will keep your organization’s data private. Your data remains private when using Azure OpenAI Service and Copilots and is governed by our applicable privacy and contractual commitments, including the commitments we make in the Microsoft’s Data Protection Addendum , Microsoft’s Product Terms , and the Microsoft Privacy Statement .
- You are in control of your organization’s data. Your data is not used in undisclosed ways or without your permission. You may choose to customize your use of Azure OpenAI Service by opting to use your data to fine tune models for your organization’s own use. If you do use your organization’s data to fine tune, any fine-tuned AI solutions created with your data will be available only to you.
- Your access control and enterprise policies are maintained. To protect privacy within your organization when using enterprise products with generative AI capabilities, your existing permissions and access controls will continue to apply to ensure that your organization’s data is displayed only to those users to whom you have given appropriate permissions.
- Your organization’s data is not shared. Microsoft does not share your data with third parties without your permission. Your data, including the data generated through your organization’s use of Azure OpenAI Service or Copilots – such as prompts and responses – are kept private and are not disclosed to third parties.
- Your organization’s data privacy and security are protected by design. Security and privacy are incorporated through all phases of design and implementation of Azure OpenAI Service and Copilots. As with all our products, we provide a strong privacy and security baseline and make available additional protections that you can choose to enable. As external threats evolve, we will continue to advance our solutions and offerings to ensure world-class privacy and security in Azure OpenAI Service and Copilots, and we will continue to be transparent about our approach.
- Your organization’s data is not used to train foundation models . Microsoft’s generative AI solutions, including Azure OpenAI Service and Copilot services and capabilities, do not use your organization’s data to train foundation models without your permission. Your data is not available to OpenAI or used to train OpenAI models.
- Our products and solutions continue to comply with global data protection regulations . The Microsoft AI products and solutions you deploy continue to be compliant with today’s global data protection and privacy regulations. As we continue to navigate the future of AI together, including the implementation of the EU AI Act and other laws globally, organizations can be certain that Microsoft will be transparent about our privacy, safety, and security practices. We will comply with laws globally that govern AI, and back up our promises with clear contractual commitments.
You can find additional details about how Microsoft’s privacy commitments apply to Azure OpenAI and Copilots here .
We provide programs, transparency documentation, and tools to assist your AI deployment
To support our customers and empower their use of AI, Microsoft offers a range of solutions, tooling, and resources to assist in their AI deployment, from comprehensive transparency documentation to a suite of tools for data governance, risk, and compliance. Dedicated programs such as our industry-leading AI Assurance program and Customer Copyright Commitment further broaden the support we offer commercial customers in addressing their needs.
Microsoft’s AI Assurance Program helps customers ensure that the AI applications they deploy on our platforms meet the legal and regulatory requirements for responsible AI. The program includes support for regulatory engagement and advocacy, risk framework implementation and the creation of a customer council.
For decades we’ve defended our customers against intellectual property claims relating to our products. Building on our previous AI customer commitments , Microsoft announced our Customer Copyright Commitment , which extends our intellectual property indemnity support to both our commercial Copilot services and our Azure OpenAI Service. Now, if a third party sues a commercial customer for copyright infringement for using Microsoft’s Copilots or Azure OpenAI Service, or for the output they generate, we will defend the customer and pay the amount of any adverse judgments or settlements that result from the lawsuit, as long as the customer has used the guardrails and content filters we have built into our products.
Our comprehensive transparency documentation about Azure OpenAI Service and Copilot and the customer tools we provide help organizations understand how our AI products work and provide choices our customers can use to influence system performance and behavior.
- Copilot: Customers can find detailed information about Copilots in our Learn portal , adoption resources , and on our new Copilot Lab resource page.
- Azure OpenAI Services: Customers can find detailed information about Azure Open AI Services through the Azure OpenAI Service – Documentation, quickstarts, and API reference guides.
Azure’s enterprise-grade protections provide a strong foundation upon which customers can build their data privacy, security, and compliance systems to confidently scale AI while managing risk and ensuring compliance. With a range of solutions in the Microsoft Purview family of products, organizations can further discover, protect, and govern their data when using Copilot for Microsoft 365 within their organizations.
With Microsoft Purview, customers can discover risks associated with data and users, such as which prompts include sensitive data. They can protect that sensitive data with sensitivity labels and classifications, which means Copilot will only summarize content for users when they have the right permissions to the content. And when sensitive data is included in a Copilot prompt, the Copilot generated output automatically inherits the label from the reference file. Similarly, if a user asks Copilot to create new content based on a labeled document, the Copilot generated output automatically inherits the sensitivity label along with all its protection, like data loss prevention policies.
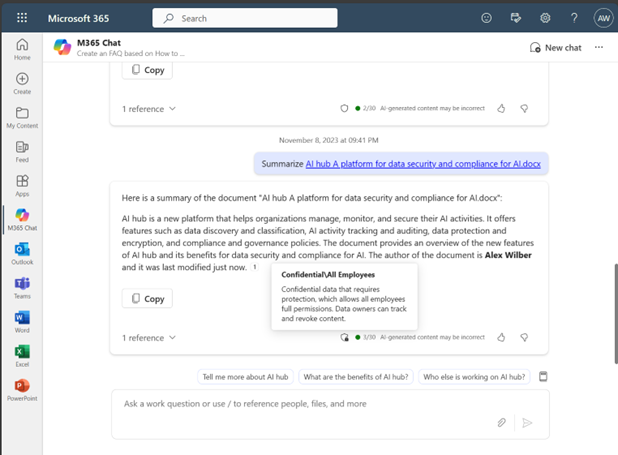
Copilot conversation inherits sensitivity label
Finally, our customers can govern their Copilot usage to comply with regulatory and code of conduct policies through audit logging, eDiscovery, data lifecycle management, and machine-learning based detection of policy violations.
As we continue to innovate and provide new kinds of AI solutions, Microsoft will continue to offer industry-leading tools, transparency resources, and support for our customers in their AI journey, and remain steadfast in protecting our customers’ data.
Tags: AI , Azure , Azure OpenAI Service , Copilot , copilot copyright commitment , generative ai , Responsible AI
- Check us out on RSS
Machine Learning and Optimization
News & features.
- Follow on Twitter
- Like on Facebook
- Follow on LinkedIn
- Subscribe on Youtube
- Follow on Instagram
- Subscribe to our RSS feed
Share this page:
- Share on Twitter
- Share on Facebook
- Share on LinkedIn
- Share on Reddit
What’s New in MS Research – November 2023
Reviewed by MSAA Chief Medical Officer Barry A. Hendin, MD
In this Article
Study supports using high-efficacy medications from the start in relapsing ms, are older people with highly active ms being under-treated, ocrevus ® trial exclusively enrolling black and hispanic people yields encouraging results, australian study finds evidence of enduring benefit from mavenclad ®, briumvi ® shows reduction in brain volume loss and new lesion formation, examining the long-term efficacy and safety of evobrutinib, tolebrutinib and lesion formation in ms: 144-week data from long-term study, assessing impact of frexalimab on lesion formation in open-label study, study finds that injected ocrevus ® is as effective as iv ocrevus in suppressing brain lesions, is the average age at the time of ms onset increasing more than 100 years of data from norway suggest so, welcome news on menopause and disability worsening in ms, study finds that better eating equates with fewer ms symptoms, for more information.
Milan, Italy served as the setting for cutting-edge science and high hopes last month, when multiple sclerosis (MS) researchers and clinicians from around the world gathered there in October for MSMilan2023, the 9th joint meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS).
The results from more than 1,900 studies were published at the session, which organizers note is the world’s largest MS research meeting. This edition of “What’s New in MS Research” provides a sense of the breadth and depth of findings presented in Milan. The studies highlighted below include encouraging study results on investigational medications that rely on new mechanisms of action to treat MS, ongoing evaluations of approved medications – including the first trial of a disease-modifying therapy that exclusively enrolled Black and Hispanic people – and research looking at the impact of menopause and diet on MS.
As always, we are excited to present this research to the MS community.
Is it better to begin treatment with a disease-modifying therapy (DMT) that offers high efficacy but may be associated with relatively frequent or significant side effects, or to start with a low- or moderate-efficacy medication with better tolerability and see if that treatment is sufficient, at least for the present?
It’s a question that people newly diagnosed with relapsing-remitting multiple sclerosis (RRMS) and their clinicians must consider carefully when weighing the choice of initial therapy. Fortunately, it also is a question receiving intense scrutiny from researchers, including investigators who reviewed data on 762 people with RRMS who began treatment in London, England between 2002 and 2022. 1
The people studied had an average age of 38.3 years when they started treatment, and had experienced an average of 1.1 relapses in the year before initiating a DMT. Their average Expanded Disability Status Scale (EDSS) was 2.1 at baseline, indicating mild impairment from MS. Seventy percent were females.
The study compared outcomes between 150 people who began treatment with a high-efficacy DMT, such as Ocrevus ® (ocrelizumab) or Tysabri ® (natalizumab), and 612 people who were prescribed low- or moderate-efficacy medications, including Tecfidera ® (dimethyl fumarate), Gilenya ® (fingolimod), and Copaxone ® (glatiramer acetate). In conducting their comparison, researchers matched study participants based on factors such as age, gender, number of relapses in the past year, and EDSS score. The main outcome measures were having no evidence of disease activity – defined by no relapses, no EDSS progression, and no new lesions as seen on magnetic resonance imaging (MRI) – at one year and two years after starting a medication.
At one year, people taking high-efficacy DMTs were 64% more likely than those on low- or moderate-efficacy DMTs to be without evidence of disease activity. At the two-year mark, they were 52% less likely to have no evidence of disease activity. Both results were statistically significant.
The investigators concluded that their findings suggest “that high-efficacy DMTs should be considered as [a] first option when discussing DMTs with treatment-naïve patients.” (“Treatment-naïve” refers to individuals who have not received any type of DMT previously.)
With more than two dozen DMTs approved by the Food and Drug Administration (FDA) for use in relapsing forms of multiple sclerosis, people with MS and their clinicians have an abundance of options to choose from when deciding which medication is best for them. Along with factors such as route of administration (e.g., a pill taken by mouth vs. a medication given by injection or infusion) and frequency of dosing, the benefit/risk profile of various treatments is a major consideration. This study and others examining the same question can facilitate more-informed discussions and decision-making on this important issue.
Argentinian researchers analyzing data from a national patient registry found that less than one-quarter of people aged 50 years or older who had highly active relapsing-remitting multiple sclerosis (RRMS) were receiving high-efficacy disease-modifying therapies (DMTs). 2
Of 950 people with RRMS who were 50 or older, 189 met the criteria for highly active MS, as defined by two or more relapses in the prior year and/or two or more new T2 gadolinium-enhancing lesions on MRI (gadolinium enhancement can indicate inflammatory activity).
While clinicians often prescribe high-efficacy DMTs to try to control highly active MS, only 23.2% of the 189 people received a high-efficacy therapy. The researchers found that the more relapses a person had experienced in the previous year, the more likely they were to be prescribed such a therapy. Conversely, the more comorbid conditions – such as hypertension or diabetes – people had, the less likely they were to receive a more-efficacious medication. Further, the likelihood of being prescribed a high-efficacy DMT declined steadily with age.
The researchers concluded, “It is essential to improve therapeutic benefit/risk ratios in the older population with MS to better prevent disease progression in this age group.”
While this study shines a spotlight on an important issue, two caveats should be noted. First, there are significant differences between the Argentine and American healthcare systems, so practice patterns in Argentina may not reflect the situation in the United States. Second, the presence of comorbidities can affect the chances of experiencing side effects from DMTs, and advancing age can affect how well a person can tolerate those side effects, so the fact that use of high-efficacy therapies declines with age and with worse overall health is not surprising and likely reflects an effort to minimize risk.
At the same time, highly active MS has both an immediate and a long-term impact on health and quality of life, making it important to provide effective treatment. As the study’s authors noted, better understanding the benefit/risk ratio faced by people of various ages and with different medical conditions is essential to help people with MS and their clinicians avoid both under-treatment and undue exposure to side effects.
More than 94% of Black and Hispanic people with relapsing MS had no relapses during 48 weeks of treatment with the disease-modifying therapy (DMT) Ocrevus ® (ocrelizumab). 3 , 4
That finding emerged from the CHIMES study, the first clinical trial of a DMT conducted exclusively in Black and Hispanic people with MS. CHIMES – which stands for characterization of ocrelizumab in minorities with multiple sclerosis – was a Phase IV trial, meaning that it was conducted following the Phase III studies that companies conduct to secure Food and Drug Administration (FDA) approval of a medication. The study was sponsored by Genentech, a member of the Roche Group and the pharmaceutical company that markets Ocrevus.
The prospective, single-arm trial enrolled 182 Black or Hispanic people with relapsing MS who were 18 to 65 years old and who had an Expanded Disability Status Scale (EDSS) score of 0-5.5, indicating minimal to moderate MS disease burden. Sixty-two percent of the patients were Black, while 38% were Hispanic. The average age of study participants was 35.5 years, and 72.5% of the people in the trial were female. On average, study participants had begun experiencing symptoms 4.9 years before entering the trial and had been diagnosed with relapsing MS 2.9 years before starting the study.
The trial’s primary endpoint was no evidence of disease activity (NEDA) at Week 48. NEDA was defined as no relapse, no confirmed disability progression at Week 24, and no T1 gadolinium-enhancing lesion or new/enlarging T2 lesions on MRI – at Week 48. Forty-six percent of Black patients and 58% of Hispanic patients achieved NEDA at Week 48. Further, more than 94% of patients had no relapses over 48 weeks and no 24-week confirmed disability progression or T1 gadolinium-enhancing lesions. Forty-six percent of Black patients and 63.8% of Hispanic patients had no new or enlarging T2 lesions. Adverse events occurred in 80.2% of people, with 29.1% having an infusion-related reaction. While 5.5% of people had a serious adverse event, no deaths occurred. Researchers noted that the safety results of CHIMES were consistent with the findings of other trials of Ocrevus, with no new safety signals emerging.
“We know that Black and Hispanic/Latinx people with MS often experience more severe disease and greater disability compared with their white counterparts. But until now, there has been limited research conducted in these populations,” said Mitzi Joi Williams, MD, lead trial investigator, founding medical director at Joi Life Wellness MS Center in Atlanta, GA, and chairperson of MSAA’s African American Advisory Board. Dr. Williams added, “The CHIMES trial is a critical step in breaking the cycle of health inequity. The results, for the first time, provide evidence on the benefit of treatment in Black and Hispanic/Latinx people with MS.” The CHIMES trial took several steps to overcome barriers to recruitment and promote retention among Black and Hispanic people with MS. Trial sites, which were located across the United States, Kenya, and Puerto Rico, included academic institutions, hospitals, outpatient clinics, community centers, and healthcare provider practices. To facilitate enrollment, trial-related materials were available in multiple languages (English, Spanish, and Swahili) and reviewed by an advisory panel to ensure understanding and cultural appropriateness. Trial participants were offered flexible scheduling options; appropriate compensation or reimbursement for loss of earnings, childcare, accommodation, travel, and meals; and utilization of ride-sharing companies for transportation. The CHIMES trial has been extended to three years to gather long-term data on MS progression among Black and Hispanic populations, with further results anticipated to be available in 2024.
Does Mavenclad ® (cladribine) continue to provide benefit after people with MS have completed the treatment course of four doses administered over roughly two years, or should subsequent re-dosing be considered?
The question is an important one because the oral disease-modifying therapy (DMT) has proven efficacy in treating relapsing forms of MS but also may be associated with increased risk for cancer. Because of the medication’s safety profile, its FDA-approved prescribing information notes that “use of Mavenclad is generally recommended for patients who have had an inadequate response to, or are unable to tolerate, an alternate drug indicated for the treatment of MS.” 5
To understand the long-term efficacy of Mavenclad and assess the potential need for additional dosing, Australian investigators are following 156 people with MS who are taking the medication. 6 The researchers are tracking the patients’ levels of serum neurofilament light chain (sNfL) and serum Glial Fibrillary Acidic Protein (sGFAP), which are thought to be markers of inflammation and neurodegeneration. They also are assessing changes in Expanded Disability Status Scale (EDSS) scores and cognitive assessments.
At the MSMilan2023 meeting in Milan last month, the researchers reported findings on 56 study participants for whom they have 30 months’ worth of data. Those patients had statistically significant decreases in both sNfL and sGFAP from baseline through 30 months, indicating a positive effect on inflammation and neurodegeneration. Meanwhile, there was no change in average EDSS score or measures of cognitive function over the 30-month period, suggesting an enduring stabilizing impact on disability and cognition. While the study is ongoing and will be reporting data on more people over more time, the results available thus far point toward ongoing benefit and have not identified a waning of effect that might warrant consideration of re-dosing.
People with relapsing MS who received the infused disease-modifying therapy (DMT) Briumvi ® (ublituximab-xiiy) in two clinical trials had significantly less thalamic volume loss over two years than study participants who received the oral DMT Aubagio ® (teriflunomide), researchers reported.
The findings emerged from an analysis of the ULTIMATE 1 and ULTIMATE 2 Phase III trials. Based on other findings from those studies, the Food and Drug Administration approved Briumvi in December 2022 for the treatment of relapsing forms of MS. In an analysis presented at the MSMilan2023 meeting, researchers focused on changes in volume of the thalamus, the section of the brain that is responsible for relaying motor and sensory signals to the cerebral cortex.
In examining magnetic resonance imaging (MRI) data on almost 1,100 patients participating in the two studies, investigators found that people receiving Briumvi had 22% less loss in thalamic volume from baseline to Week 96 than study participants receiving Aubagio, with the difference being statistically significant. Meanwhile, people taking Aubagio had roughly a four-times greater increase in T1 hypointense lesion volume, a marker of brain tissue loss, over 96 weeks than people receiving Briumvi. That difference also was statistically significant. Investigators added that in people receiving Briumvi, “New T1 and T2 lesion formation was almost completely suppressed after the first year of treatment.” 7
Eighty-seven percent of people with relapsing forms of MS who took the investigational medication evobrutinib had no evidence of clinical worsening (NEcW) after five years on the oral therapy, researchers reported. 8
Evobrutinib belongs to a class of medications called Bruton’s tyrosine kinase inhibitors, or BTKis. Bruton tyrosine kinase (BTK) is an enzyme that plays a key role in the activation and inflammatory activity of B cells, immune-system cells that have been implicated in multiple sclerosis. Scientists hope that BTK inhibition will provide clinicians with a new means of combating MS. Several BTK inhibitors are in late stages of evaluation in MS, while some BTK inhibitors already are approved for treatment of leukemia and other cancers.
Researchers recently assessed efficacy and safety outcomes in 124 people who had participated in a 48-week, Phase II trial of evobrutinib and then continued to receive the medication for up to 240 weeks in a long-term, open-label study. In the 48-week trial, patients were randomly assigned to receive one of the following treatment regimens:
- placebo for the first 24 weeks followed by 25 mg of evobrutinib once-daily for the remaining 24 weeks
- 25 mg of evobrutinib daily
- 75 mg of evobrutinib once daily
- 75 mg of evobrutinib twice daily
- another disease-modifying therapy (DMT), dimethyl fumarate
On entering the long-term, open-label study, patients received 75 mg of evobrutinib once a day before transitioning to 75 mg twice daily.
The 87.1% overall proportion of patients having NeCW at Week 240 reflected strong performance across these dose groups. For example, at the end of the earlier, 48-week trial, 70.8% of people who started on placebo and then switched to 25 mg of evobrutinib daily had no evidence of clinical worsening. After switching to 75 mg twice daily in the long-term, open-label trial, 90% of those patients had no evidence of clinical worsening at Week 240.
Looking at the 164 evobrutinib-treated patients in the long-term study (including the 124 who had reached Week 240, and others who had not reached Week 240 at the time the data were analyzed), 26.2% reported treatment-related adverse events (TEAEs), with 2.4% reporting serious TEAEs. Researchers noted, “The safety profile [of evobrutinib] remains consistent with that previously reported.”
Tolebrutinib is a Bruton’s tyrosine kinase inhibitor (BTKi) being evaluated for a potential role in treating relapsing MS. A Phase II trial found an association between use of the investigational medication and a reduction in new gadolinium-enhancing lesions on Tesla 1 (T1) magnetic resonance imaging (MRI) and in new or enlarging lesions on Tesla 2 (T2) MRI. That trial evaluated four different doses of tolebrutinib – ranging from 5 mg per day to 60 mg each day – and investigators saw a dose-dependent relationship between the oral medication and lesion reduction, with higher doses generally associated with fewer lesions. 9
Based on those encouraging findings, researchers went on to assess the ongoing impact of tolebrutinib on MS lesions in a long-term safety [LTS] study. In Part A of the study, participants continued on whatever dose of tolebrutinib they had received in the earlier trial. In Part B of the study, all patients received 60 mg per day. At the MSMilan2023 meeting in Milan, investigators shared data on 103 patients for whom they had 144 weeks’ worth of MRI data. All the patients had relapsing MS. Sixty-nine percent were women, and their average age was 37.7 years.
At Week 144, the average number of new, gadolinium-enhancing T1 lesions appearing since the prior scan at Week 96 (approximately one year earlier) were:
- 0.5 for people receiving 5 mg per day of tolebrutinib initially before moving to 60 mg per day (the “5mg/60mg” group”)
- 0.48 for the 15mg/60mg group
- 0.49 for the 30mg/60mg group
- 0.48 for the 60mg/60mg group
The average number of new or enlarging T2 lesions at Week 144 was even lower for each group. Further, the great majority of people in all groups had no new gadolinium-enhancing lesions on T1 MRI, with no additional lesions seen in 79.2% of people in the 5mg/60mg group, 72% in the 15mg/60mg cohort, 87% in the 30mg/60mg group, and 79.3% in the 60mg/60mg group.
The researchers concluded, “Participants treated with tolebrutinib show a stable, low rate of formation of new [gadolinium-] enhancing T1 lesions and new/enlarging T2 lesions between LTS [Week] 96 and [Week] 144, supporting the efficacy of tolebrutinib in reducing acute inflammation in people with relapsing multiple sclerosis.”
Ninety-six percent of people with relapsing MS who took a high dose of the investigational medication frexalimab developed no new gadolinium-enhancing lesions on Tesla 1 (T1) magnetic resonance imaging (MRI) over a 24-week period, and 91% had no new or enlarging lesion on Tesla 2 (T2) MRI. Meanwhile, 80% of people with relapsing MS taking a lower dose of the medication had no new T1 gadolinium-enhancing lesions and 74% had no new or enlarging T2 lesions after 24 weeks. 10
Those findings emerged from the open-label portion of a Phase II study of frexalimab, a monoclonal antibody that may modify B-cell and T-cell activation and innate immune cell function, potentially reducing inflammation without depleting those immune-system cells. Frexalimab targets the CD40/CD40L pathway that has been implicated in the development of MS.
In the initial, 12-week portion of the study, participants received either high-dose frexalimab, low-dose frexalimab, or placebo. In the open-label portion that followed, the people who had received frexalimab initially remained on the medication, while the people who had been on placebo were switched to either high-dose or low-dose frexalimab. Among the people who had started the study on placebo and then went on to receive a high dose of the study medication, the average monthly count of new gadolinium-enhancing T1 lesions declined from 2.3 before they received frexalimab to 0.4 after 12 weeks on the medication. For people who moved from placebo to low-dose frexalimab, that average count declined from 3.7 to 0.6 over 12 weeks of treatment.
Researchers added that no new safety concerns emerged during the open-label portion of the trial. They noted that the most common adverse events were uncomplicated cases of mild or moderate COVID-19, nasopharyngitis (“the common cold”), and headache.
The investigators concluded, “These findings provide proof of concept for targeting CD40L in MS and support development of frexalimab as a potential high-efficacy, non-depleting MS therapy.”
Administering Ocrevus ® (ocrelizumab) twice a year by 10-minute subcutaneous injection is as effective as delivering the monoclonal antibody via longer twice-yearly intravenous infusions in suppressing brain lesions in people with relapsing or primary-progressive forms of MS, according to the medication’s parent company (Genentech). 11 This comparable impact on brain lesions was one of the key findings of the OCARINA II study reported at the MSMilan2023 meeting last month. The Phase III, global, randomized study is evaluating the pharmacokinetics, safety, and radiological and clinical effects of the subcutaneous formulation of Ocrevus compared with Ocrevus intravenous (IV). It has enrolled 236 patients with relapsing MS (RMS) or primary-progressive MS (PPMS). The study’s primary endpoint is non-inferiority in a measure of the serum concentration of subcutaneously injected Ocrevus from Day One to 12 weeks after injection compared to IV infusion. Secondary endpoints include the total number of active, gadolinium-enhancing T1 lesions at eight and 12 weeks, and new or enlarging T2 lesions at 12 and 24 weeks, as well as safety outcomes.
Genentech reported that Ocrevus subcutaneous injection was non-inferior to Ocrevus IV infusion as measured by blood levels of the medication from Day One to 12 weeks (3,500 dayµg/mL for subcutaneous injection vs. 2,750 dayµg/mL for IV infusion). Similarly, the company said, peak Ocrevus blood concentrations were similar for subcutaneous injection and IV infusion, at 132 µg/mL and 137 µg/mL, respectively.
In terms of impact on brain lesions, people in the Ocrevus subcutaneous injection arm of OCARINA II had an average 0.54 T1 gadolinium-enhancing lesions at baseline and an adjusted lesion rate of 0.00 at 24 weeks. Their counterparts in the Ocrevus IV arm of the trial had an average 0.98 T1 gadolinium-enhancing lesions at baseline and a 0.00 adjusted lesion rate at the 24-week mark. Both groups also saw the adjusted lesion rate for new or enlarging T2 lesions reach 0.00 at the 24-week mark.
Genentech reported that the safety profile of Ocrevus subcutaneous injection was consistent with the established safety profile of Ocrevus IV infusion. No new safety signals were identified for Ocrevus subcutaneous injection. The most common adverse events (AEs) in the Ocrevus subcutaneous injection group were injection reactions (48% of all exposed patients), all of which were either mild or moderate. The most common AEs in the Ocrevus IV infusion group were infusion-related reactions (17%). A total of four and seven serious AEs were experienced by 2.5% and 3.4% patients in the Ocrevus subcutaneous and IV infusion groups, respectively.
The company added that OCARINA II data will be submitted to health authorities around the world in the months ahead. Ocrevus IV is the only therapy approved in the United States for both relapsing forms of MS and PPMS. The investigational subcutaneous formulation combines Ocrevus with Halozyme Therapeutics’ Enhanze ® drug delivery technology.
From 1920 to 1969, only 2.6% of people diagnosed with relapsing-remitting multiple sclerosis (RRMS) in two Norwegian counties experienced their first MS symptoms after age 40. From 2011 to 2022, that proportion more than quadrupled to 11.9%. 12
The researchers presenting those results say their findings are in keeping with other reports from elsewhere in the world. They added that, in step with other reports, their analysis showed that the trend toward an increase in later-onset MS was more evident in women than in men. Further, the researchers found a bimodal pattern of MS-symptom onset, with one peak at around 30 years of age and a second peak between ages 40 and 45.
The findings emerged from an analysis of data on 3,364 people diagnosed with MS in the Norwegian counties of Hordaland and Møre og Romsdal between 1920 and 2022. Norway, like many other northern countries, has a relatively high incidence of MS and, as a result, a long history of tracking the disease. The researchers noted, “The mean age at onset significantly increased throughout the study period, despite a decrease in time from symptom onset to diagnosis,” noting that those findings were statistically significant.
An Australian study of more than 200 women with clinically isolated syndrome or relapsing MS found that the onset of menopause was not associated with significant worsening of disability. 13
Researchers explained that they looked for an association because, “There is emerging evidence that the post-menopausal period may represent a time of more-rapid disease progression for women with multiple sclerosis.” They added, “Following menopause, concentrations of protective sex hormones are at their lowest, which may have a deleterious effect on neurodegeneration in MS.”
The investigators conducted a retrospective study that analyzed data on 205 women recruited from eight MS centers across Australia in 2018 and 2019. They tracked the participants’ Expanded Disability Status Scale (EDSS) scores over 10 years spanning the pre-menopausal and post-menopausal periods. Using a statistical technique called inflection point analysis, they looked at whether the slope of change in EDSS scores became steeper following menopause, which would indicate that the “change of life” also represented a change in the pace of disability worsening.
While the researchers saw a trend toward post-menopausal worsening of MS disability, they said that menopause did not constitute a significant inflection point in terms of changes in EDSS scores. They added, however, that further studies are needed to explore potential links between menopause and other aspects of clinical progression, such as the risk of transition from relapsing forms of MS to secondary-progressive multiple sclerosis.
A study involving more than 5,000 people with MS in the United Kingdom has found that a diet rich in fruits, legumes, and vegetables is associated with a lower burden of several MS symptoms, while a traditional Western diet is linked to more symptoms. 14
Researchers drew on the UK MS Register, a national database that records information of clinical status, lifestyle factors, and health outcomes in people with MS to examine data from 2016 on 2,278 people and data from 2022 on 2,887 people. Most people included in the analysis were female, White, and had relapsing forms of MS. The average age of study subjects was 54 years. Based on participants’ reported eating habits, they identified people who followed what the investigators termed a “prudent” eating pattern, meaning that fruits, legumes, and vegetables were major components of the people’s diets. They also identified people who followed a “Western” diet, which researchers defined as consisting heavily of biscuits, cakes and pies, chips, and takeaway (“take-away” being British English for “take-out,” or “fast food”).
The researchers found that following the “prudent” eating pattern was associated with lower scores (meaning less disability) on the MS Walking Scale (MSWS) and MS Impact Scale-Physical (MSIS). They also had a lower frequency of severe depression compared to other people in the study. By contrast, following the Western dietary pattern was associated with higher scores – indicating greater disability – on the MSWS and MSIS. There also was a higher frequency of fatigue, severe depression, and severe anxiety among people following the Western diet relative to other people studied.
While citing a need for prospective studies to confirm the results of their study, the researchers concluded, “These findings suggest a role for diet in symptom management” in people with MS.
1 Al-Araji S, Moccia M, Jha A, et al. Real-world comparison of high efficacy versus low/moderate efficacy DMTs in treatment naïve relapsing-remitting multiple sclerosis patients using propensity score matching. MSMilan2023. P724/1420. Mult Scler J. 2023; 29:(3S) 623.
2 Ysrraelit MC, Piedrabuena MA, Fiol M, et al. Selection of high efficacy disease modifying therapies in multiple sclerosis patients older than 50 years. MSMilan2023. P734/1544. Mult Scler J . 2023; 29:(3S) 633.
3 Williams MJ, Vartanian T, Reders A, et al. One-year analysis of efficacy and safety in Black and Hispanic patients with relapsing multiple sclerosis receiving ocrelizumab treatment in the CHIMES trial. MSMilan2023. P691/711. Mult Scler J. 2023; 29:(3S) 597-8.
4 Genentech. First-ever clinical trial exclusively in Black and Hispanic/Latinx people living with multiple sclerosis shows Genentech’s Ocrevus effectively manages disease activity. October 10, 2023. South San Francsico, CA. Available at https://www.gene.com/media/press-releases/15005/2023-10-10/first-ever-clinical-trial-exclusively-in . Accessed November 12, 2023.
5 MAVENCLAD ® (cladribine) tablets, for oral use. [Prescribing information.] EMD Serono, Inc.: Rockland, MA. September 2022.
6 Maltby V, Leaz R, Xavier A, et al. Cladribine: a multicentre, long-term efficacy and Biomarker Australian Study (CLOBAS) – results from the first 30 months. MSMilan2023. P656/1564. Mult Scler J. 2023; 29:(3S) 572.
7 Arnold DL, Steinman L, Hartung HP, et al. Ublituximab reduces thalamic volume loss and new lesion formation in participants in the ULTIMATE I and II Phase 3 studies. MSMilan2023. P162/1262. Mult Scler J. 2023; 29:(3S) 246-7.
8 Montalban X, Wollinsky J, Arnold DL, et al. Update on long-term safety and efficacy of evobrutinib, a Bruton’s tyrosine kinase inhibitor, over 5 years from an ongoing Phase 2 open-label extension. MSMilan2023. P706/1280. Mult Scler J. 2023; 29:(3S) 610.
9 Reich D, Traboulsee A, Oh J, et al. MRI outcomes from the long-term extension study of tolebrutinib in participants with relapsing multiple sclerosis: 3-year results. MSMilan2023. P684/1478. Mult Scler J . 2023; 29:(3S) 592.
10 Vermersch P, Granziera C, Mao-Draayer Y, et al. Phase 2 efficacy and safety of frexalimab: 6-month results of a novel CD40L inhibitor in relapsing multiple sclerosis. MSMilan2023. P275/134. Mult Scler J. 2023; 29:(3S) 332.
11 Genentech. Genentech’s Ocrevus twice-yearly, 10-minute subcutaneous injection was non-inferior to intravenous infusion and provided near-complete suppression of brain lesions. October 11, 2023. South San Francsico, CA. Available at https://www.businesswire.com/news/home/20231010331768/en/Genentech%E2%80%99s-Ocrevus-Twice-Yearly-10-Minute-Subcutaneous-Injection-Was-Non-Inferior-to-Intravenous-Infusion-and-Provided-Near-Complete-Suppression-of-Brain-Lesions . Accessed November 17, 2023.
12 Habbestad A, Willumsen JS, Aarseth JH, et al. Changing age of multiple sclerosis onset from 1920 to 2022: a population-based study in western Norway. MSMilan2023. P449/2058. Mult Scler J. 2023; 29:(3S) 492.
13 Bridge F, Sanfilippo P, Skibinaz O, et al. The impact of menopause on the clinical trajectory of multiple sclerosis. MSMilan2023. P460/885. Mult Scler J. 2023; 29:(3S) 436-7.
14 Simpson-Yap S, Coe S, Neate S, et al. Healthier dietary intake is associated with less clinical severity in people with multiple sclerosis: a cross-sectional study within the UK MS Register. MSMilan2023. P380/3122. Mult Scler J . 2023; 29:(3S) 1068.
For general information or to speak with a trained Client Services Specialist, please call MSAA’s Helpline at (800) 532-7667, extension 154 . Questions to MSAA’s Client Services department may also be emailed to [email protected] .
Written by Tom Garry, Medical Writer Reviewed by Dr. Barry Hendin, MSAA Chief Medical Officer Edited by Susan Wells Courtney, MSAA Senior Writer
About Hoff Communications
We use cookies to understand how you use our site and to improve your experience. This includes personalizing content and advertising. To learn more, click here . By continuing to use our site, you accept our use of cookies, revised Privacy Policy and Terms of Service .

New to Zacks? Get started here.
Member Sign In
Don't Know Your Password?

- Zacks #1 Rank
- Zacks Industry Rank
- Zacks Sector Rank
- Equity Research
- Mutual Funds
- Mutual Fund Screener
- ETF Screener
- Earnings Calendar
- Earnings Releases
- Earnings ESP
- Earnings ESP Filter
- Stock Screener
- Premium Screens
- Basic Screens
- Research Wizard
- Personal Finance
- Money Managing
- Real Estate
- Retirement Planning
- Tax Information
- My Portfolio
- Create Portfolio
- Style Scores
- Testimonials
- Zacks.com Tutorial
Services Overview
- Zacks Ultimate
- Zacks Investor Collection
- Zacks Premium
Investor Services
- ETF Investor
- Home Run Investor
- Income Investor
- Stocks Under $10
- Value Investor
- Top 10 Stocks
Other Services
- Method for Trading
- Zacks Confidential
Trading Services
- Black Box Trader
- Counterstrike
- Headline Trader
- Insider Trader
- Large-Cap Trader
- Options Trader
- Short Sell List
- Surprise Trader
- Alternative Energy

You are being directed to ZacksTrade, a division of LBMZ Securities and licensed broker-dealer. ZacksTrade and Zacks.com are separate companies. The web link between the two companies is not a solicitation or offer to invest in a particular security or type of security. ZacksTrade does not endorse or adopt any particular investment strategy, any analyst opinion/rating/report or any approach to evaluating individual securities.
If you wish to go to ZacksTrade, click OK . If you do not, click Cancel.
Image: Bigstock
Microsoft (MSFT) Gains As Market Dips: What You Should Know
Microsoft ( MSFT Quick Quote MSFT - Free Report ) closed the most recent trading day at $424.57, moving +0.92% from the previous trading session. The stock's change was more than the S&P 500's daily loss of 0.2%. On the other hand, the Dow registered a loss of 0.6%, and the technology-centric Nasdaq increased by 0.11%.
Coming into today, shares of the software maker had gained 1.26% in the past month. In that same time, the Computer and Technology sector gained 2.99%, while the S&P 500 gained 3.32%.
Investors will be eagerly watching for the performance of Microsoft in its upcoming earnings disclosure. The company is expected to report EPS of $2.84, up 15.92% from the prior-year quarter. Simultaneously, our latest consensus estimate expects the revenue to be $60.63 billion, showing a 14.71% escalation compared to the year-ago quarter.
For the full year, the Zacks Consensus Estimates are projecting earnings of $11.63 per share and revenue of $243.6 billion, which would represent changes of +18.55% and +14.95%, respectively, from the prior year.
Additionally, investors should keep an eye on any recent revisions to analyst forecasts for Microsoft. These revisions typically reflect the latest short-term business trends, which can change frequently. As such, positive estimate revisions reflect analyst optimism about the company's business and profitability.
Research indicates that these estimate revisions are directly correlated with near-term share price momentum. To take advantage of this, we've established the Zacks Rank, an exclusive model that considers these estimated changes and delivers an operational rating system.
The Zacks Rank system, running from #1 (Strong Buy) to #5 (Strong Sell), holds an admirable track record of superior performance, independently audited, with #1 stocks contributing an average annual return of +25% since 1988. Over the past month, the Zacks Consensus EPS estimate remained stagnant. Microsoft is holding a Zacks Rank of #2 (Buy) right now.
Looking at valuation, Microsoft is presently trading at a Forward P/E ratio of 36.17. Its industry sports an average Forward P/E of 30.66, so one might conclude that Microsoft is trading at a premium comparatively.
It's also important to note that MSFT currently trades at a PEG ratio of 2.24. The PEG ratio is similar to the widely-used P/E ratio, but this metric also takes the company's expected earnings growth rate into account. The average PEG ratio for the Computer - Software industry stood at 2.4 at the close of the market yesterday.
The Computer - Software industry is part of the Computer and Technology sector. With its current Zacks Industry Rank of 51, this industry ranks in the top 21% of all industries, numbering over 250.
The Zacks Industry Rank gauges the strength of our industry groups by measuring the average Zacks Rank of the individual stocks within the groups. Our research shows that the top 50% rated industries outperform the bottom half by a factor of 2 to 1.
Ensure to harness Zacks.com to stay updated with all these stock-shifting metrics, among others, in the next trading sessions.
See More Zacks Research for These Tickers
Normally $25 each - click below to receive one report free:.
Microsoft Corporation (MSFT) - free report >>
Published in
This file is used for Yahoo remarketing pixel add
Due to inactivity, you will be signed out in approximately:

IMAGES
VIDEO
COMMENTS
Latest Treatments. Experimental therapies are being explored, as MS treatments and various clinical trials have shown promise. One medication, ibudilast, completed a phase 2 clinical trial in 2018 that showed it can slow the progression of the disease. Ibudilast is an anti-inflammatory medication that works by reducing inflammation in the body ...
Pathways to Cures is the biggest, most collaborative MS research effort of our time with MS organizations and scientific leaders from across the globe agreeing that this is the way forward. Research is key to accelerating cures for people with MS. And we have come a long way. Research has shown us new and exciting pathways to stopping MS in its tracks, restoring function, and ending MS forever.
Experts Review Recent Clinical Trials in Progressive MS and Outline Steps to Speed New Treatments ... It's easy to be a champion for MS Research - join us and proudly let everyone know that you're helping to lead the MS Research Revolution. Become a Research Champion. Become a Research Champion.
By Levi Gadye. A study of more than 22,000 people with multiple sclerosis has discovered the first genetic variant associated with faster disease progression that can rob patients of their mobility and independence over time. Multiple sclerosis (MS) is the result of the immune system mistakenly attacking the brain and the spinal cord, resulting ...
S.S. Zamvil and S.L. Hauser. N Engl J Med 2021;384:378-381. A recent study of a class of HLA molecules on the surface of B cells in persons with multiple sclerosis revealed that many of these HLA ...
In 2023, three prominent themes have dominated multiple sclerosis research: (1) the view of the disease as a continuum, whereby inflammation and neurodegeneration coexist from onset and evolve over time in a seamless manner; (2) the search for new biomarkers to help define, monitor, or predict the disease course over time; and (3) the early identification of the disease, with implications for ...
The U.S. Food and Drug Administration (FDA) has cleared a Phase 2 clinical trial to test Kyverna Therapeutics ' cell-based therapy candidate KYV-101 in people with treatment-resistant ...
Multiple sclerosis went from an untreatable disease to a known, druggable condition in less than 40 years. Now a study finds subtypes that may benefit from different courses of treatment.
Multiple sclerosis is an autoimmune disease in which immune cells attack and destroy the protective myelin sheaths that surround nerve fibres, leading to neurological disturbances. Latest Research ...
A phase 1 trial using an allogeneic stem-cell-based therapy in people with progressive multiple sclerosis (MS) shows the feasibility and tolerability of the approach; rigorous evaluation of this ...
Multiple sclerosis: New biomarker confirmed for early diagnosis. ScienceDaily . Retrieved March 27, 2024 from www.sciencedaily.com / releases / 2023 / 07 / 230706124618.htm
In this report, we share the Society's Pathways to MS Cures Research Roadmap. The Roadmap was developed with input from scientific experts, health care providers, and people affected by MS from the United States, Canada, and the United Kingdom ().The Roadmap has also been endorsed by leading MS patient and professional organizations ().We hope the Roadmap will inspire greater collaboration ...
Advances in understanding and managing multiple sclerosis (MS) were front and center when the nation's neurologists convened in Seattle in April to share their latest research at the 2022 Annual Meeting of the American Academy of Neurology (AAN). Almost 300 of the 2,400+ abstracts presented at the gathering focused on MS.
Read current research articles and learn about multiple sclerosis diagnosis, symptoms as well as the latest MS treatment options. ... Sep. 25, 2023 — New research is painting a clearer picture ...
Latest News, What's New in MS Research September 25, 2023. Reviewed by MSAA Chief Medical Officer Barry A. Hendin, MD. This edition of "What's New in MS Research" opens with a pair of "firsts:" the first listing of multiple sclerosis (MS) therapies on the World Health Organization's List of Essential Medicines and the first United ...
Another study, MS-STAT2, is looking at whether simvastatin, a drug commonly used to treat high cholesterol, could also be used to slow secondary progressive MS. This phase 3 trial completed its ...
Topics include racial and ethnic differences in MS presentation and treatment response, an app for dealing with MS-related anxiety, and promising results from a Phase II trial of the investigational agent IMU-838. Factors predicting the persistence of COVID-19 symptoms in people with MS, as well as the links between MS severity and the prevalence of other health conditions, also are examined.
Today there have been reports in the news that a common anti-histamine might be able to repair myelin in MS. We look at the evidence. Our Research Network make sure all the research we fund reflects the needs and interests of people living with MS. And they help us talk about it in a way that's accessible to everyone.
Read about the latest MS research breakthroughs. Read the latest developments. Browse our research blog Go behind the headlines with insights from our scientists and research team. ... Multiple Sclerosis Society (MS Society UK). Registered charity nos 1139257 / SC041990. Registered as a limited company in England and Wales 07451571.
The complexity of MS necessitates a comprehensive approach that will uncover solutions for everyone -. A research strategy that can fuel knowledge and speed better treatments, health care policies, and new disease and symptom management therapies. $1.06 Billion. In Research Funding To Date.
The research was supported by grants from the National Institutes of Health, National Multiple Sclerosis Society, Howard Hughes Medical Institute and New York Stem Cell Foundation, and ...
Examining data on 1,622 people who experienced their first symptoms of MS between January 1970 and December 2019, the investigators found that the average age of onset increased from 23.8 years in the 1970s to 34.3 years in the 2010s. The average ages by decade were: 1970 to 1979: 23.79 ±10.19 years.
With a range of solutions in the Microsoft Purview family of products, organizations can further discover, protect, and govern their data when using Copilot for Microsoft 365 within their organizations. With Microsoft Purview, customers can discover risks associated with data and users, such as which prompts include sensitive data.
The CORE group conducts research in the intersection of algorithms, operations research and computer systems. Our focus is on designing large-scale optimization solutions for cloud systems and applications.
Latest News, What's New in MS Research November 28, 2023. Reviewed by MSAA Chief Medical Officer Barry A. Hendin, MD. Study supports using high-efficacy medications from the start in relapsing MS. Milan, Italy served as the setting for cutting-edge science and high hopes last month, when multiple sclerosis (MS) researchers and clinicians from ...
By harnessing the power of Microsoft Azure alongside NVIDIA DGX™ Cloud and the NVIDIA Clara™ suite of microservices, healthcare providers, pharmaceutical and biotechnology companies, and medical device developers will soon be able to innovate rapidly across clinical research and care delivery with improved efficiency.
Microsoft (MSFT Quick Quote MSFT - Free Report) closed the most recent trading day at $424.57, moving +0.92% from the previous trading session. The stock's change was more than the S&P 500's daily ...