If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.

Biology library
Course: biology library > unit 2.
- Ionic bonds
- Covalent bonds
- Electronegativity
- Electronegativity and bonding
- Intermolecular forces
- Chemical bonds
- Chemical reactions introduction
Chemical reactions
Introduction, reversibility and equilibrium, attribution:, additional references:, want to join the conversation.
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

3.16: Introduction to Chemical Reactions
Chapter 1: introduction to the human body, chapter 2: diagnostic imaging techniques, chapter 3: fundamentals of chemistry, chapter 4: biochemistry of the cell, chapter 5: cells and their components, chapter 6: cell membrane structure and functions, chapter 7: essential cellular processes, chapter 8: tissues of the human body, chapter 9: the integumentary system, chapter 10: bone tissue and the skeletal system, chapter 11: the axial skeleton, chapter 12: the appendicular skeleton, chapter 13: the joints, chapter 14: muscle tissue, chapter 15: the muscular system, chapter 16: the nervous system and nervous tissue, chapter 17: anatomy of the central and peripheral nervous system, chapter 18: functions of the central and peripheral nervous system, chapter 19: the autonomic nervous system, chapter 20: the special senses, chapter 21: the endocrine system.
The JoVE video player is compatible with HTML5 and Adobe Flash. Older browsers that do not support HTML5 and the H.264 video codec will still use a Flash-based video player. We recommend downloading the newest version of Flash here, but we support all versions 10 and above.
A chemical reaction is a process where atoms in one or more substances, known as reactants, change their arrangement by breaking their chemical bonds and forming new bonds to create the products.
Reactions which result in a net release of energy are exergonic, while endergonic reactions are net absorbers of energy.
The biochemical reactions occurring in the human body are categorized as either synthesis or anabolic, such as protein synthesis, decomposition, or catabolic, such as the breakdown of polysaccharides into simple sugars, or exchange reactions, such as the transfer of ATP's phosphate to glucose to form glucose-phosphate.
Many biochemical reactions use enzymes as catalysts to speed up the reaction.
All biochemical reactions progress towards equilibrium — a state where no net change in the concentrations of reactants and products occurs because the forward and reverse reactions are happening at the same rate.
The biochemical reactions provide energy to maintain homeostasis and perform essential functions such as growth and repair.
All chemical reactions begin with a reactant, the general term for one or more substances entering the reaction. Sodium and chloride ions, for example, are the reactants in the production of table salt. One or more substances produced by a chemical reaction are called the product. Chemical reactions follow the law of conservation of mass, which means that matter cannot be created nor destroyed in a chemical reaction. The components of the reactants—the number of atoms and the elements—are all present in the product(s). Similarly, there is nothing in the products that are not present in the reactants.
Chemical reactions require sufficient energy that causes the matter to collide with enough precision and force to break old chemical bonds and form new ones. In general, kinetic energy is the form of energy powering any type of matter in motion. Potential energy is the energy of position or the energy matter possesses because of the positioning or structure of its components. All atoms have kinetic energy as they are always in motion. The energy needed to break the chemical bonds of the reactants and start a reaction is called the activation energy. The concentration and temperature of the reactants can influence the rate of a chemical reaction.
The convention for writing chemical equations involves placing reactant formulas on the left side of a reaction arrow and product formulas on the right side. By this convention and the definitions of "reactant" and "product," a chemical equation represents the reaction proceeding from left to right. Reversible reactions, however, may proceed in both forward (left to right) and reverse (right to left) directions. When the rates of the forward and reverse reactions are equal, the concentrations of the reactant and product species remain constant over time, and the system is at equilibrium. The relative concentrations of reactants and products in equilibrium systems vary greatly; some systems contain mostly products at equilibrium, some contain mostly reactants, and some contain appreciable amounts of both.
For example, in human blood, excess hydrogen ions (H + ) bind to bicarbonate ions (HCO3 - ), forming an equilibrium state with carbonic acid (H2CO3). If we added carbonic acid to this system, some of it would convert to bicarbonate and hydrogen ions.
However, biological reactions rarely obtain equilibrium because the concentrations of the reactants or products or both are constantly changing, often with one reaction's product a reactant for another. To return to the example of excess hydrogen ions in the blood, forming carbonic acid will be the reaction's major direction. However, the carbonic acid can also leave the body as carbon dioxide gas (via exhalation) instead of converting back to bicarbonate ion; this drives the reaction to the right by the law of mass action. These reactions are important for maintaining homeostasis in our blood.
This text is adapted from Openstax, Anatomy and Physiology 2e, Section 2.3: Chemical Reactions , Openstax, Chemistry 2e, Section 13.1: Chemical equilibria and Openstax, Biology 2e, Section 2.1: Atoms, Isotopes, Ions and Molecules: The Building Blocks
Get cutting-edge science videos from J o VE sent straight to your inbox every month.
mktb-description
We use cookies to enhance your experience on our website.
By continuing to use our website or clicking “Continue”, you are agreeing to accept our cookies.

Chapter 5 Introduction to Chemical Reactions
Opening essay.
Although yeast has been used for thousands of years, its true nature has been known only for the last two centuries. Yeasts are single-celled fungi. About 1,000 species are recognized, but the most common species is Saccharomyces cerevisiae , which is used in bread making. Other species are used for the fermentation of alcoholic beverages. Some species can cause infections in humans.
Yeasts live primarily on sugars, such as glucose (C 6 H 12 O 6 ). They convert glucose into carbon dioxide (CO 2 ) and ethanol (C 2 H 5 OH) in a chemical transformation that is represented as follows:
Bread making depends on the production of carbon dioxide. The gas, which is produced in tiny pockets in bread dough, acts as a leavening agent: it expands during baking and makes the bread rise. Leavened bread is softer, lighter, and easier to eat and chew than unleavened bread. The other major use of yeast, fermentation, depends on the production of ethanol, which results from the same chemical transformation. Some alcoholic beverages, such as champagne, can also be carbonated using the carbon dioxide produced by the yeast.
Yeast is among the simplest life forms on Earth, yet it is absolutely necessary for at least two major food industries. Without yeast to turn dough into bread and juice into wine, these foods and food industries would not exist today.
Chemical change is a central concept in chemistry. The goal of chemists is to know how and why a substance changes in the presence of another substance or even by itself. Because there are tens of millions of known substances, there are a huge number of possible chemical reactions. In this chapter, we will find that many of these reactions can be classified into a small number of categories according to certain shared characteristics.
Introduction to Chemical Reactions
Article objectives.
- The objective of this article is to explain the concept of chemical reactions and the types of chemical reactions.
Introduction
One of the most fundamental concepts of chemistry is something known as the Law of Conservation of Matter , that states that atoms are never introduced or destroyed. This is what allows chemical reactions to work, where one or more chemicals react in such ways that one or more new chemicals are formed, via the moving of electrons between substances. The chemicals present before the reaction are called reactants (for the obvious reason), and the chemicals present after the reaction are called products . No chemical reaction is a chemical reaction without both of these components.
Introduction to Chemical Equations
On paper, a chemical reaction can be written out in the form of a chemical equation , which shows the reaction taking place. Of course there is always a way to describe the reaction in words too.
Example 1: Write a chemical equation for Magnesium Hydroxide and Sulfuric Acid being mixed together in solution to get Magnesium Sulfate and Water.
Solution: The reactants are written on the left, and the products are written on the right, with an arrow in between.
$$Mg(OH)_2 + H_2SO_4 \rightarrow H_2O + MgSO_4$$
States of Matter
Often, it is a good idea to include states of matter in a chemical equation. A state of matter is the phase of the chemical, so either solid, liquid, or gas. In addition, in chemical equations the phrase aqueous is treated as a state of matter. This refers to a chemical being dissolved in a solution with water.
When placing states of matter in chemical equations, use the following symbols:
\((s)\) for a solid
\((l)\) for a liquid (usually water)
\((g)\) for a gas
\((aq)\) for an aqueous substance
Example 2: Write an equation for a reaction between aqueous Gold Perchlorate and solid Titanium which form solid Gold and aqueous Titanium Perchlorate. Include states of matter.
Solution: Use the information given about the chemicals to write the equation:
$$Au(ClO_4)_3 + Ti \rightarrow Ti(ClO_4)_2 + Au$$
Now add the states of matter.
$$Au(ClO_4)_3(aq) + Ti(s) \rightarrow Ti(ClO_4)_2(aq) + Au(s)$$
As a general rule of thumb, always include the states of matter if they are given.
Single Replacement Reactions
There are many different types of chemical reactions, and they are classified based on a combination of the identity of the reactants and products and how many substances/ions are directly involved in the reaction. A single replacement reaction is a reaction where one substance or ion is moved, and nothing else.
Example 3: Based on the following chemical equation, is the reaction a single replacement reaction? How can you tell? The equation is
$$BaCO_3 + Na \rightarrow Na_2CO_3 + Ba$$
Solution: This is a single replacement reaction. The way we can tell is that only the Carbonate ion (\(CO_3^{2-}\)) is moving, and thus influencing the charges of the Sodium and the Barium.
Example 4: Magnesium Chloride and solid Barium react in a single-replacement reaction. What are the products?
Solution: In a single-replacement reaction, one chemical will be moved between substances. Since Magnesium Chloride is an ionic compound, it will likely be an ion. Seeing Magnesium and Barium in a compound together is uncommon because both of these elements are metals, but Barium Chloride is a perfectly plausible ionic compound, so we have the reaction
$$MgCl_2+Ba \rightarrow BaCl_2+Mg$$
So the products are solid Magnesium and Barium Chloride.
Double Replacement Reactions
The counterpart of single replacement reactions, a double replacement reaction is where two substances or ions are moved/inverted to create new substances. Generally double replacement reactions, at least in first-year courses, will have at least two products.
Example 5: Why is the reaction \(HCl + K \rightarrow KCl + H_2\) not a double replacement reaction?
Solution: Only the Chlorine atoms are being moved, so by definition this cannot be a double replacement reaction. It is actually a single replacement reaction.
Example 6: What is one possible double replacement reaction that could theoretically occur between Aluminum Chromate (\(Al(CrO_4)_3\)) and Magnesium Oxide?
Solution: Two components must be inverted to create a double-replacement reaction. It is perfectly valid to allow these substances to be the anions that are present. Therefore we get a chemical equation of
$$Al(CrO_4)_3 + MgO \rightarrow Mg(CrO_4)_2 + Al_2O_3$$
Synthesis Reactions
A synthesis reaction is where two or more chemicals are combined to create one chemical. Generally, if a reaction is known to be a synthesis reaction, predicting the product(s) are easier compared to single or double replacement reactions.
Example 7: The products of a chemical reaction are Sodium Hydroxide and Acetone. Without knowing the products, how do you know that this is not a synthesis reaction?
Solution: There would only be one product, so this must be a different type of reaction.
In the previous example, even though we could tell that it was not a synthesis reaction, we would need to know the reactants in order to determine what type of reaction it actually was.
Example 8: If \(H_2O_2\) and Chromium metal undergo a synthesis reaction, what is one possible product of the reaction?
Solution: Of course there may be multiple answers, but they all must include Chromium, Hydrogen, and Oxygen. This solution creates an ionic compound; the product \(Cr(OH)_3\) appears perfectly valid, as this is indeed a synthesis reaction by definition. For reference, the equation is shown below:
$$H_2O_2 + Cr \rightarrow Cr(OH)_3$$
Decomposition Reaction
A decomposition reaction is a reaction that is in the reverse process of a synthesis reaction; a chemical decomposes into two or more different chemicals. Sometimes the decomposition will be automatic, and other times a trigger such as heat will be necessary.
Example 9: Carbonic acid decomposes naturally. The decomposition reaction is as follows:
$$H_2CO_3 \rightarrow CO_2 + H_2O$$
Oxidation-Reduction Reactions
An oxidation-reduction reaction is a reaction that meets one of two conditions: 1. Elemental oxygen is a reactant. 2. One substance is oxidized , or has electrons taken away from it, and one substance is reduced , where it gets additional electrons.
An oxidation-reduction reaction, or redox reaction, can at the same time be a single replacement reaction, double replacement reaction, or synthesis reaction.
Example 10a: Classify the following chemical reaction in two ways:
$$Sn + O_2 \rightarrow SnO$$
Solution: One of the reactants is elemental oxygen, so it is a redox reaction. In addition, two substances are combined to create one new one, so it is also a synthesis reaction.
The second half of the example generalizes this reaction.
Example 10b: In Example 10a, the Tin metal is being oxidized into Tin cations. All stable metals can undergo basically the same reaction. Here are some specific ones:
$$Li + O_2 \rightarrow Li_2O$$
$$Ca + O_2 \rightarrow CaO$$
$$Al + 3O_2 \rightarrow Al_2O_3$$
In each of these redox reactions, the metal is oxidized and the oxygen is reduced. This property also applies to transition metals (the stable ones):
$$Zr + O_2 \rightarrow ZrO_2$$
Another type of problem is to determine whether a reaction is a redox reaction. As a rule of thumb, check the charges of the ions--redox reactions generally involve ionic compounds.
Example 11: Is this a redox reaction? Explain why.
$$HNO_3 + Cu(OH)_2 \rightarrow Cu(NO_3)_2 + H_2O$$
Solution: There is no elemental oxygen (although substances with oxygen are abundant; don't let this make you think the reaction is redox). Also, there is nothing being oxidized or reduced. Therefore this is not a redox reaction.
Example 12: In the following chemical equation, the element labeled with a question mark is unknown:
$$Mg? + Na \rightarrow Na? + Mg$$
What might this unknown element be if this reaction is a redox reaction?
Solution: There is no room for elemental oxygen in this reaction. However, the "?" can still be oxygen:
$$MgO + Na \rightarrow Na_2O + Mg$$
Here's why: in this reaction, the Magnesium is reduced and the Sodium is Oxidized. Therefore this is a redox reaction.
Basically any anion would work here. For example, if the question mark actually represents Fluorine:
$$MgF_2 + Na \rightarrow NaF + Mg$$
For the same reason, this is still a redox reaction. Even a polyatomic ion would allow this to be a redox reaction (although polyatomic ions are not pure elements). For example, if the ion is Phosphite:
$$Mg_3(PO_3)_2 + Na \rightarrow Na_3PO_3 + Mg$$
Example 13: What type of reaction is this?
$$Mn_2 O_7+Na_2 SO_3 \rightarrow Mn_2 (SO_3)_7+Na_2O$$
Solution: Two different ions, oxide and sulfite, are interchanged. Therefore this is a double-replacement reaction.
Despite being under "Oxidation-Reduction Reactions," the above reaction is not a redox reaction. None of the charges change (nothing is oxidized or reduced). The presence of oxygen may have made you identify the reaction as redox, but that is incorrect because the oxygen is not elemental; it is the anion in the ionic compound Manganese (VII) Oxide.
Combustion Reactions
A combustion reaction is a subtype of redox reactions where a substance is burned with an oxygen flame. Therefore by definition, all combustion reactions are redox reactions, since they always contain elemental oxygen.
Many combustion reactions include elemental oxygen and a hydrocarbon, a chemical comprising only of Carbon and Hydrogen atoms. By default, these reactions always have two products: carbon dioxide and water. The amounts produced are based on the type of hydrocarbon and how much of each reactant are available.
Example 14: Octane is combusted as follows:
$$C_8H_{18} + O_2 \rightarrow CO_2 + H_2O$$
a. Is this reaction a redox reaction?
b. Is this reaction a synthesis reaction?
c. Would the previous answers change if a different hydrocarbon is used?
a. Elemental oxygen is a reactant, so this reaction is a redox reaction.
b. This is not a synthesis reaction, because there are the same number of products as reactants, so two reactants could not combine to form one product.
c. No; regardless, elemental oxygen is still a reactant, and this does nothing to change the products (only the amounts), so this is still not a synthesis reaction.
A carbohydrate is a chemical with only Carbon, Hydrogen, and Oxygen. Combustion of carbohydrates works the same way as the combustion of hydrocarbons.
Example 15: What are the products when \(C_9H_{16}O_2\) is combusted?
Solution: By inspection of the chemical formula \(C_9H_{16}O_2\), it is obvious this is a carbohydrate. As usual, the products will be \(CO_2\) and \(H_2O\). Here is the chemical equation:
$$C_9H_{16}O_2 + O_2 \rightarrow CO_2 + H_2O$$
<div class="article-example" markdown="1">
**Example 16:** Find and balance an equation for the combustion of \(C_7H_{16}O_4\).
**Solution:** "Combustion" implies the addition of elemental oxygen:
$$C_7H_{16}O_4 + O_2 \rightarrow$$
As with any other combustion reaction, the products are carbon dioxide and water:
$$C_7H_{16}O_4 + O_2 \rightarrow CO_2 + H_2O$$
Balance the hydrogen first:
$$C_7H_{16}O_4 + O_2 \rightarrow CO_2 + 8H_2O$$
Now balance carbon:
$$C_7H_{16}O_4 + O_2 \rightarrow 7CO_2 + 8H_2O$$
There are \(22\) carbon atoms on the right side, so we need the same number on the left side. With four oxygen atoms included in the carbohydrate, the remaining \(18\) must come from the elemental oxygen, so
$$C_7H_{16}O_4 + 9O_2 \rightarrow 7CO_2 + 8H_2O$$
</div>
The Law of Conservation of Matter stated that new matter could not be introduced or removed from existence, but many of the equations in this article appeared to violate this concept because the amount of each chemical on both sides of the equation was not the same. However, there is a way to balance equations to make this rule followed yet again; this will be discussed in a different article, as it is too big a concept to include here.
Module 2: Chemistry of Life
Introduction to chemical reactions, understand basic chemical reactions.
In this outcome, we will discuss chemical bonds and reactions.
What You’ll Learn to Do
- Identify the components of simple chemical reactions
- Differentiate between catabolic and anabolic reactions
- Identify enzymes and their role in chemical reactions
Learning Activities
The learning activities for this section include the following:
- Chemical Reactions and Molecules
- Anabolic and Catabolic Pathways
- Self Check: Chemical Reactions
Contribute!
Improve this page Learn More
- Introduction to Chemical Reactions. Authored by : Shelli Carter and Lumen Learning. Provided by : Lumen Learning. License : CC BY: Attribution


- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

5.9: Introduction to Chemical Reactions (Summary)
- Last updated
- Save as PDF
- Page ID 15242
To ensure that you understand the material in this chapter, you should review the meanings of the following bold terms in the following summary and ask yourself how they relate to the topics in the chapter.
Scientific laws are general statements that apply to a wide variety of circumstances. One important law in chemistry is the law of conservation of matter , which states that in any closed system, the amount of matter stays constant.
Chemical equations are used to represent chemical reactions . Reactants change chemically into products . The law of conservation of matter requires that a proper chemical equation be balanced . Coefficients are used to show the relative numbers of reactant and product molecules.
In stoichiometry , quantities of reactants and/or products can be related to each other using the balanced chemical equation. The coefficients in a balanced chemical reaction are used to devise the proper ratios that relate the number of molecules of one substance to the number of molecules of another substance.
Chemical reactions can be classified by type. Combination reactions (also called composition reactions ) make a substance from other substances. Decomposition reactions break one substance down into multiple substances. Combustion reactions combine molecular oxygen with the atoms of another reactant.
Oxidation reactions are reactions in which an atom loses an electron. Reduction reactions are reactions in which an atom gains an electron. These two processes always occur together, so they are collectively referred to as oxidation-reduction (or redox ) reactions . The species being oxidized it called the reducing agent , while the species being reduced is the oxidizing agent . Alternate definitions of oxidation and reduction focus on the gain or loss of oxygen atoms, or the loss or gain of hydrogen atoms. Redox reactions are easily balanced if the overall reaction is first separated into half reactions , which are individually balanced.
Oxidation-reduction reactions are common in organic and biological chemistry. Respiration , the process by which we inhale and metabolize oxygen, is a series of redox reactions. In the absence of oxygen, redox reactions still occur in a process called anaerobic metabolism . Antioxidants such as ascorbic acid also play a part in the human diet, acting as reducing agents in various biochemical reactions. Photosynthesis , the process by which plants convert water and carbon dioxide to glucose, is also based on redox reactions.
Chemically modifying electrodes—a classical tool box
- Review Paper
- Published: 16 January 2024
- Volume 28 , pages 757–827, ( 2024 )
Cite this article
- Ilya Sterin 1 na1 ,
- Anna Tverdokhlebova 1 na1 ,
- Oleh Smutok 1 &
- Evgeny Katz 1
255 Accesses
Explore all metrics
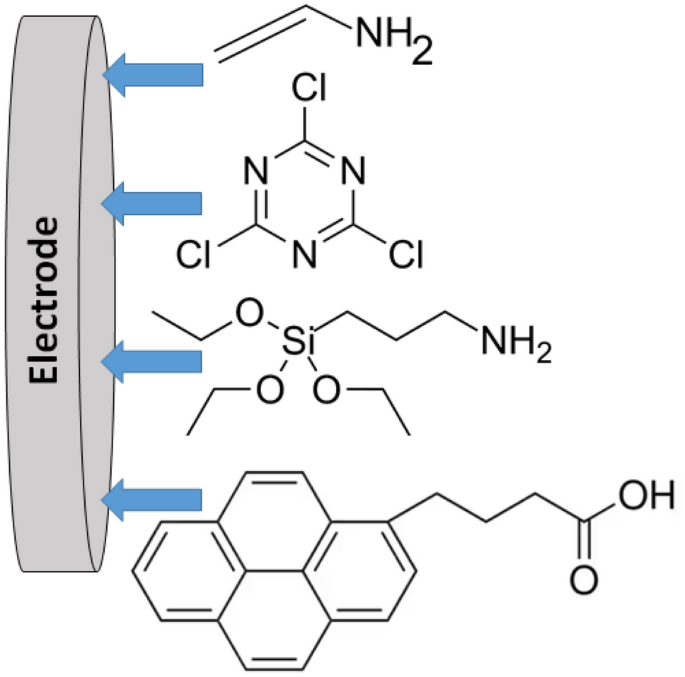
The paper provides a detailed overview of heterogeneous chemical reactions leading to the electrode surface modification and further reactivity of various functionalized surfaces. Notably, the present paper is different from other typical reviews and books about chemically modified electrodes—it is not aimed at highlighting the recent achievements in the research area, but provides a detailed analysis of the area background produced about 30–50 years ago. While there are many reviews on the present state-of-the-art (mostly describing specific applications), the background of the research area is not well remembered, particularly by young researchers and students. Therefore, the paper is mainly aimed at educational aspects, rather than highlighting the modern applications, which are only briefly mentioned in the concluding section. The chemical structures exemplified in the paper represent a comprehensive collection of the systems produced by various modification reactions.

Graphical abstract
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Metal oxide nanoparticles and their applications in nanotechnology
Murthy S. Chavali & Maria P. Nikolova

Electrochemical sensors: basic principles, engineering, and state of the art
Heru Agung Saputra
Acknowledgements
Dr. Oleh Smutok thanks Human Frontier Science Program (HFSP) for the fellowship allowing his work in the USA.
Author information
Ilya Sterin and Anna Tverdokhlebova contributed equally.
Authors and Affiliations
Department of Chemistry and Biomolecular Science, Clarkson University, Potsdam, NY, 13699, USA
Ilya Sterin, Anna Tverdokhlebova, Oleh Smutok & Evgeny Katz
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Oleh Smutok or Evgeny Katz .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Sterin, I., Tverdokhlebova, A., Smutok, O. et al. Chemically modifying electrodes—a classical tool box. J Solid State Electrochem 28 , 757–827 (2024). https://doi.org/10.1007/s10008-023-05743-z
Download citation
Received : 23 October 2023
Revised : 30 October 2023
Accepted : 01 November 2023
Published : 16 January 2024
Issue Date : March 2024
DOI : https://doi.org/10.1007/s10008-023-05743-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Modified electrodes
- Heterogeneous chemical reactions
- Electrode functionalization
- Surface reactivity
- Electrode surface pretreatment
Advertisement
- Find a journal
- Publish with us
- Track your research
Maintenance work is planned for Wednesday 1st May 2024 from 9:00am to 11:00am (BST).
During this time, the performance of our website may be affected - searches may run slowly and some pages may be temporarily unavailable. If this happens, please try refreshing your web browser or try waiting two to three minutes before trying again.
We apologise for any inconvenience this might cause and thank you for your patience.

Chemical Communications
Nitrogen-doped carbon nanosheet composited platinum-cobalt single atom alloy catalyst for effective hydrogen evolution reaction.
An electrocatalyst with ultra-small PtCo single atom alloy species evenly dispersed on nitrogen-doped ultra-thin carbon nanosheets (PtCo SAA/NC) was designed. The introduction of single-atom Pt not only maximizes the atomic utilization efficiency of Pt species, but also synergistically enhances the charge transfer characteristics of Co cluster surfaces, thereby increasing the migration and evolution rate of hydrogen ions. The PtCo SAA/NC catalyst exhibits a Tafel slope of 42 mV·dec-1 and a low overpotential of 45 mV at 10 mA·cm-2 in 0.5 M H2SO4 solution.
Supplementary files
- Supplementary information PDF (4074K)
Article information
Download citation, permissions.
X. Niu, H. Geng, Z. Lv, J. Wei, D. Xu and W. Chen, Chem. Commun. , 2024, Accepted Manuscript , DOI: 10.1039/D4CC00265B
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

4: Experiment 4 - Chemical Reactions
- Last updated
- Save as PDF
- Page ID 214153
Ancillary Documents
- MS Word PDF Google Doc Reactions Worksheet
- MS Word PDF Google Doc Gravimetric Analysis Lab Report
Learning Objectives
- Aqueous solutions
- Precipitation
- Molecular, ionic and net ionic equations
- Mix chemicals, record your observations, write the molecular equation, ionic equation and net ionic equation.
- Watch videos on this page. Before each video try to predict the outcome of the reaction. Write your observations, molecular equation, ionic equation and net ionic equation.
Prior Knowledge:
- 3.1: Introduction to Chemical Equations
- 3.2: Types of Chemical Reactions
- 3.3: Balancing Chemical Equations
- 3.4: Aqueous Reactions
Aqueous solution is any solution where water is present as a solvent. Rain, vinegar, orange juice are all examples of aqueous solutions that you come across in your everyday life. In chemistry aqueous solution indicated by adding "(aq)" to the reactant formula. For example, NaCl(aq) present as individual ions Na + and Cl - dissolved in water. You might have heard that water is the universal solvent, however, water only dissolves substances that are hydrophilic (from the Greek "hydros" - water and "philia" - bonding or friendship). Compounds that do not dissolve in water remain a solid and indicated by "(s)". For example, AgCl(s).
- Magnesium sulfate
- Calcium chloride
- Copper (II) Sulfate
- Sodium bicarbonate (baking soda)
- Vinegar (~5% acidity)
- Scale
- Clean and dry test tubes
- Cell phone with camera
- Laptop or computer with camera, speakers and microphone hooked up to internet
Solubility rules could be useful in our everyday life, but they are also extremely important in medicine. Sometimes doctors prescribe more than one solution to be administered by the intravenous (IV) route. Mixing two solutions that form a precipitate can lead to very serious consequences. For example, magnesium sulfate is used as an electrolyte replenisher or anticonvulsant, calcium chloride is indicated in the immediate treatment of hypocalcemic tetany (abnormally low levels of calcium in the body that cause muscle spasm) and intravenous sodium bicarbonate is a medication primarily used to treat severe metabolic acidosis. But what will happen if you mix them?
- Obtain and wear goggles and gloves!!!
- Do non ingest any chemicals or inhale the vapors.
- Clean up all spills immediately! If contact with skin rinse with water for 15 minutes.
- No Lab Work will be performed outside the stated lab hours.
- All students must report back to their group by 10:40 and all data submitted to the TA by noon.
- Before proceeding with this or any other experiment students must sign the chemical lab safety form.
For each reaction in Part I and Part II record your observations, molecular equation , total ionic equation and net ionic equation . Make sure to write any evidence of any evidence of a chemical reaction with sufficient detail to help you distinguish between similar precipitation reactions. Don't write “became cloudy” or “white solid”. Indicate if a gel is produced or crystals form, if the solid was powdery, etc. Keep in mind that some reactions will not occur and you should write NR (no reaction). You will know that reaction occur if a precipitate, a gas, or stable molecule is formed. Heat (whether it consumed or evolved) can also be an indicator that reaction occurred.
Make sure to write any evidence of any evidence of a chemical reaction with sufficient detail to help you distinguish between similar precipitation reactions.
June 4, 2020
Part I. Kitchen Chemistry Lab
Perform Part I in your Kitchen Chemistry Lab. Mix about 3mL of each solution in a clean and dry test tube. Use pipettes to transfer solutions to test tubes. You must write all equations for a given reaction before you start the next reaction. Keep your goggles on!
You will be given the names of the compounds. You have to use formulas of these compounds for your equations. Mix each pair and record all required information.
- Magnesium sulfate and calcium chloride
- Sodium bicarbonate and vinegar
- Sodium bicarbonate and calcium chloride
- Magnesium sulfate and sodium bicarbonate
- Ammonia and magnesium sulfate
Waste disposal: Do not dispose of any solutions or solids down the drain.
Part II. Video reactions
Watch the videos. Before each video predict the outcome of the reaction. Write down your observations, molecular equation, ionic equation and net ionic equation for each reaction before moving to the next video.
1. Magnesium and hydrochloric acid
2. Copper(II)* sulfate and sodium phosphate
3. Cadmium (II) chloride and sodium sulfide
4. Nickel (II) chloride and sodium carbonate
5. Lead (II) nitrate** and sodium sulfide
6. Nickel(II) chloride and sodium phosphate
7. Silver nitrate and sodium carbonate
Interactive Element
* The video says Cu 2 SO 4 , but the reaction shown in this video is between copper (II) sulfate and sodium phosphate.
** The video says Pb 2 NO 3 , but the reaction shown is between lead (II) nitrate and sodium sulfide
June 5, 2020
Use the Gravimetric Analysis Virtual Lab for this assignment. Read Part I, then work in groups to complete Part II. Part III is your individual assignment and every group member will have their own unknown. You should receive an email from the TA with the unknown solution you need to use for the individual section of this assignment.
NOTE: If virtual lab says "Default Lab Setup" refresh your page
it should say: "Gravimetric Analysis of Unknown Lead Solutions"
Virtual Lab \(\PageIndex{1}\): ChemCollective Virtual Lab developed by Dave Yaron of Carnegie Mellon University, ( http://www.chemcollective.org/ ). NOTE: if the lab loads as "Default Lab Setup" (with a bunch of acids, bases and indicators), refresh the page . You want it to load the "Gravimetric Analysis of Unknown Leas Solutions", which contains sodium chloride, potassium chromate and six unknowns.
Contributors and Attributions
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, [email protected] . You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:
- Elena Lisitsyna

IMAGES
VIDEO
COMMENTS
5.1: Prelude to Introduction to Chemical Reactions. Although yeast has been used for thousands of years, its true nature has been known only for the last two centuries. Yeasts are single-celled fungi. About 1,000 species are recognized, but the most common species is Saccharomyces cerevisiae, which is used in bread making.
Respiration, the process by which we inhale and metabolize oxygen, is a series of redox reactions. In the absence of oxygen, redox reactions still occur in a process called anaerobic metabolism. Antioxidants such as ascorbic acid also play a part in the human diet, acting as reducing agents in various biochemical reactions.
The state of matter of reactants and products is designated with the symbols (s) for solids, (l) for liquids, and (g) for gases. Chemical reaction, a process in which one or more substances, the reactants, are converted to one or more different substances, the products. Substances are either chemical elements or compounds.
Chemical reactions. Chemical reactions occur when chemical bonds between atoms are formed or broken. The substances that go into a chemical reaction are called the reactants, and the substances produced at the end of the reaction are known as the products. An arrow is drawn between the reactants and products to indicate the direction of the ...
Opening Essay. Although yeast has been used for thousands of years, its true nature has been known only for the last two centuries. Yeasts are single-celled fungi. ... This is "Introduction to Chemical Reactions", chapter 5 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0).
3.16: Introduction to Chemical Reactions. All chemical reactions begin with a reactant, the general term for one or more substances entering the reaction. Sodium and chloride ions, for example, are the reactants in the production of table salt. One or more substances produced by a chemical reaction are called the product.
Sulfuric acid is made in a three-step process: (1) the combustion of elemental sulfur to produce sulfur dioxide, (2) the continued reaction of sulfur dioxide with oxygen to produce sulfur trioxide, and (3) the reaction of sulfur trioxide with water to make sulfuric acid (H 2 SO 4 ). Write balanced chemical equations for all three reactions.
Concepts of Chemistry is an adaptation of sections from Introduction to Chemistry and Beginning Chemistry. The source materials and this current work are available under a Creative
Chemical change is a central concept in chemistry. The goal of chemists is to know how and why a substance changes in the presence of another substance or even by itself. Because there are tens of millions of known substances, there are a huge number of possible chemical reactions. In this chapter, we will find that many of these reactions can ...
No chemical reaction is a chemical reaction without both of these components. Introduction to Chemical Equations. On paper, a chemical reaction can be written out in the form of a chemical equation, which shows the reaction taking place. Of course there is always a way to describe the reaction in words too.
Chemical reactions and physical changes. Physical changes, such as melting, boiling and dissolving, do not make new chemicals. They are usually easy to reverse. In a chemical reaction, chemical ...
Introduction to Chemical Reactions. Understand basic chemical reactions. In this outcome, we will discuss chemical bonds and reactions. What You'll Learn to Do. Identify the components of simple chemical reactions; Differentiate between catabolic and anabolic reactions; Identify enzymes and their role in chemical reactions;
Reduction reactions are reactions in which an atom gains an electron. These two processes always occur together, so they are collectively referred to as oxidation-reduction (or redox) reactions. The species being oxidized it called the reducing agent, while the species being reduced is the oxidizing agent.
From the start of a synthetic chemist's training, experiments are conducted based on recipes from textbooks and manuscripts that achieve clean reaction outcomes, allowing the scientist to develop practical skills and some chemical intuition. This procedure is often kept long into a researcher's career, as new recipes are developed based on similar reaction protocols, and intuition-guided ...
a chemical reaction. Reggie and Charlotte are baking oatmeal cookies. They dip the baked cookies in melted chocolate. The chocolate cools to form a hardened coating. Reggie argues the entire chocolate-covered cookie has undergone a chemical change. Charlotte disagrees, saying only the original oatmeal cookie has undergone a chemical change, not ...
A balanced chemical equation gives the ratios in which molecules of substances react and are produced in a chemical reaction. 7.5: Some Types of Chemical Reactions Although there are untold millions of possible chemical reactions, most can be classified into a small number of general reaction types. Classifying reactions has two purposes: it ...
Here you will find material for Experiment 3 - An Introduction to Chemical Reactions. This includes detailed guides for the pre-lab, data sheet, post-lab, and quiz questions. Remember, the lab may change or be modified from semester to semester! Background Knowledge (links): Objective: demonstrate the principle of conservation of mass with ...
Introduction: The goal of lab 4 was to get a better understanding of chemical reactions such as precipitation reactions, acid-base reactions, and oxidation reactions. The objective of the lab was to find out what a given unknown compound is by using the information gathered about known compounds.
If a substance is completely consumed in a chemical reaction, what is that substance? limiting reactant. The following is an example of which type of reaction? combustion. Study with Quizlet and memorize flashcards containing terms like If energy is absorbed because of a chemical reaction, the reaction is________., If a substance is completely ...
5: Introduction to Chemical Reactions. 105856. Chemical change is a central concept in chemistry. The goal of chemists is to know how and why a substance changes in the presence of another substance or even by itself. Because there are tens of millions of known substances, there are a huge number of possible chemical reactions.
6.1: Prelude to Introduction to Chemical Reactions. Although yeast has been used for thousands of years, its true nature has been known only for the last two centuries. Yeasts are single-celled fungi. About 1,000 species are recognized, but the most common species is Saccharomyces cerevisiae, which is used in bread making.
The paper provides a detailed overview of heterogeneous chemical reactions leading to the electrode surface modification and further reactivity of various functionalized surfaces. Notably, the present paper is different from other typical reviews and books about chemically modified electrodes—it is not aimed at highlighting the recent achievements in the research area, but provides a ...
Chemical Communications. Nitrogen-Doped Carbon Nanosheet Composited Platinum-Cobalt Single Atom Alloy Catalyst for Effective Hydrogen Evolution Reaction . Xudong Niu, Huilong Geng, ... was designed. The introduction of single-atom Pt not only maximizes the atomic utilization efficiency of Pt species, but also synergistically enhances the charge ...
3.2: Types of Chemical Reactions. 3.3: Balancing Chemical Equations. 3.4: Aqueous Reactions. Aqueous solution is any solution where water is present as a solvent. Rain, vinegar, orange juice are all examples of aqueous solutions that you come across in your everyday life. In chemistry aqueous solution indicated by adding " (aq)" to the reactant ...