An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- For Health Professionals
- Clinical Tools & Patient Management
- Kidney Disease for Health Professionals
- Identify & Manage Patients
- Identify & Evaluate Patients with CKD

Identify & Evaluate Patients with Chronic Kidney Disease
Urine and blood tests are used to detect and monitor kidney disease. Currently, the key markers used include abnormal urine albumin levels and a persistent reduction in the estimated glomerular filtration rate (eGFR) . Identification of the etiology may help guide management. Diabetes and hypertension are the leading causes of CKD in adults. Many diseases that cause kidney failure may have their origins in childhood . Early detection and appropriate treatment may improve prognosis in all age groups.
Identify Patients with CKD
Screen people at risk for CKD, including those with
- diabetes mellitus type 1 or type 2
- hypertension
- cardiovascular disease (CVD)
- family history of kidney failure
The benefit of CKD screening in the general population is unclear.
The two key markers for CKD are urine albumin and eGFR. To screen for CKD:
- assess urine albumin excretion to diagnose and monitor kidney damage . Screen using a spot urine albumin-to-creatinine ratio.
- calculate eGFR from stable serum creatinine levels to assess kidney function . Use the Modification of Diet in Renal Disease (MDRD) Study Equation or the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
CKD is generally diagnosed when there is evidence, for more than 3 months, of
- kidney damage (usually urine albumin > 30 mg/g creatinine, but includes other clinical findings such as hematuria, congenital malformations, etc.) and/or
- decreased kidney function (eGFR < 60 mL/min/1.73 m 2 )
Staging systems for chronic disease should identify risk for progression and complications. The current staging system for CKD, based exclusively on eGFR, does not appear to reliably identify those people at greatest risk for progression. Emerging research suggests an approach that includes multiple factors, such as urine albumin, age, and diabetes status may better predict progression.
In addition, the current staging requires accuracy of eGFR above 60 mL/min/1.73 m 2 . However, values above 60 calculated using the MDRD Study equation are not accurate. When using the MDRD Study equation, NIDDK encourages laboratories to report eGFR above 60 as age "≥ 60" rather than as numerical values . While the CKD-EPI equation has increased accuracy for eGFR values above 60 mL/min/1.73 m 2 compared to the MDRD Study equation, the influence of imprecision of creatinine assays on the uncertainty of an eGFR value is greater at higher eGFR values.
Although kidney function tends to decrease with age, this process has not been well investigated. Many people with age-related kidney function decline may not progress to kidney failure. Thus, the prognosis for a 75-year-old patient with an eGFR of 55 may be different than that for a 45-year-old patient with the same eGFR.
In addition, GFR may be too narrow a basis on which to assess risk for progression. The approach to staging is likely to evolve as it is informed by ongoing longitudinal research, e.g., the Chronic Renal Insufficiency Cohort Study .
Establish Cause of CKD
Because kidney damage is generally irreversible, it is important to identify the etiology as early as possible. Specific treatments are available in many cases (e.g., membraneous nephropathy, lupus nephropathy) and a diagnosis will guide management.
Although diabetes is the most common cause of CKD, it is important not to assume that a patient with diabetes and CKD has diabetic kidney disease. However, non-diabetic kidney disease is unlikely in a person with diabetes of long duration with other diabetic complications, physical findings of end-organ diabetic damage, and negative screening laboratory studies.
Suggested initial evaluation:
- complete urinalysis (U/A)
- urine albumin-to-creatinine ratio (UACR)
- creatinine with estimated GFR, blood urea nitrogen (BUN), electrolytes, glucose, calcium, phosphorus, albumin
- complete blood count (CBC)
For further evaluation, the following tests are often ordered, depending on clinical presentation:
- hepatitis B serology
- hepatitis C serology
- antinuclear antibody test (ANA)
- rheumatoid factor (RF)
- complement 3 (C3)
- complement 4 (C4)
- serum protein electrophoresis (SPEP) and urine protein electrophoresis (UPEP) (in patients over the age of 40)
- renal ultrasound to measure kidney size and to check for echogenicity and hydronephrosis
- dilated retinal exam
If a patient with diabetes has retinopathy, albuminuria, and negative screening tests listed above, it is reasonable to assume the diagnosis is diabetic kidney disease. Patients who do not conform to these criteria should be discussed with a nephrologist .
Additional Links
- Quick Reference on UACR and GFR (PDF, 150.98 KB)
- Making Sense of CKD—A Concise Guide for Managing Chronic Kidney Disease in the Primary Care Setting (PDF, 3.66 MB)
- NIDDK’s Diabetes Discoveries and Practice Blog for health care professionals
Collaborate with the Health Care Team
Patients with CKD and other chronic illnesses benefit from interdisciplinary care. A cohesive, interdisciplinary care approach that begins in the primary care setting and includes referrals to appropriate health care professionals—including nephrologists, registered dietitians , and mental health professionals—is essential to CKD patients’ overall health. They also play an important role in improved patient outcomes.
Nephrologist
Due to the complex nature of CKD, a referral to a nephrologist is often beneficial to the patient. Consult with a nephrologist to
- assist with a diagnostic challenge (e.g., decision to biopsy)
- assist with a therapeutic challenge (e.g., blood pressure, anemia, hyperphosphatemia, secondary hyperparathyroidism, hyperkalemia, metabolic acidosis)
- assess rapid decrease of eGFR
- treat most primary kidney diseases (e.g., glomerulonephritis)
- prepare for RRT, especially when eGFR < 30 mL/min/1.73 m2
Use the NIDDK Nephrology Referral Form (PDF, 746 KB) to share important patient data with the consulting nephrologist.
explain to patients how what they eat and drink affects their health
- work with patients to create eating plans with the right foods and nutrients in the right amounts
- suggest adjustments to the amount and types of food CKD patients consume as their kidney disease progresses
- identify possible nutritional deficiencies caused by kidney disease
- advise patients on regulating fluid intake
To find an RD who specializes in kidney disease, visit the Academy of Nutrition and Dietetics. Use the NIDDK CKD Diet Counseling (MNT) Referral Form (PDF, 452 KB) to share important patient data with the consulting dietitian.
Mental Health Professional
Depression is common in any chronic disease, including CKD. A mental health professional, such as a psychologist, can help patients find healthy ways to cope with the anxiety and stress of having CKD.
Community Resources
Community support programs are a valuable resource to help patients overcome barriers to managing their kidney disease, such as lack of access to transportation, childcare, medicines, and healthy food. A social worker can help locate services such as transportation and counseling, recommend support groups, and help submit applications for Medicare and Medicaid.
- Patient Care & Health Information
- Diseases & Conditions
- Chronic kidney disease
- What is kidney disease? An expert explains
Learn more from kidney doctor Andrew Bentall, M.D.
I'm Dr. Andrew Bentall, a kidney doctor at Mayo Clinic. I look after patients with kidney disease, either in the early stages, or with more advanced kidney disease considering dialysis and transplantation as treatment options. In this video, we'll cover the basics of chronic kidney disease. What is it? Who gets it? The symptoms, diagnosis and treatment. Whether you are looking for answers for yourself or for someone you love, we're here to give you the best information available.
Chronic kidney disease is a disease characterized by progressive damage and loss of function in the kidneys. It's estimated that chronic kidney disease affects about one in seven American adults. And most of those don't know they have it. Before we get into the disease itself, let's talk a little bit about the kidneys and what they do. Our kidneys play many important roles keeping our bodies in balance. They remove waste and toxins, excess water from the bloodstream, which is carried out of the body in urine. They helped to make hormones to produce red blood cells, and they turn vitamin D into its active form, so it's usable in the body.
There are quite a few things that can cause or put you at higher risk for chronic kidney disease. Some of them are not things that can be avoided. Your risk is simply higher if you have a family history of certain genetic conditions like polycystic kidney disease or some autoimmune diseases like lupus or IgA nephropathy. Defects in the kidney structure can also cause your kidneys to fail, and you have an increased risk as you get older. Sometimes, other common medical conditions can increase your risk. Diabetes is the most common cause of kidney disease. Both type 1 and type 2 diabetes. But also heart disease and obesity can contribute to the damage that causes kidneys to fail. Urinary tract issues and inflammation in different parts of the kidney can also lead to long-term functional decline. There are things that are more under our control: Heavy or long-term use of certain medications, even those that are common over-the-counter. Smoking can also be a contributing factor to chronic kidney disease.
Often there are no outward signs in the earlier stages of chronic kidney disease, which is grouped into stages 1 through 5. Generally, earlier stages are known as 1 to 3. And as kidney disease progresses, you may notice the following symptoms. Nausea and vomiting, muscle cramps, loss of appetite, swelling via feet and ankles, dry, itchy skin, shortness of breath, trouble sleeping, urinating either too much or too little. However, these are usually in the later stages, but they can also happen in other disorders. So don't automatically interpret this as having kidney disease. But if you're experiencing anything that concerns you, you should make an appointment with your doctor.
Even before any symptoms appear, routine blood work can indicate that you might be in the early stages of chronic kidney disease. And the earlier it's detected, the easier it is to treat. This is why regular checkups with your doctor are important. If your doctor suspects the onset of chronic kidney disease, they may schedule a variety of other tests. They may also refer you to a kidney specialist, a nephrologist like myself. Urine tests can reveal abnormalities and give clues to the underlying cause of the chronic kidney disease. And this can also help to determine the underlying issues. Various imaging tests like ultrasounds or CT scans can be done to help your doctor assess the size, the structure, as well as evaluate the visible damage, inflammation or stones of your kidneys. And in some cases, a kidney biopsy may be necessary. And a small amount of tissue is taken with a needle and sent to the pathologist for further analysis.
Treatment is determined by what is causing your kidneys to not function normally. Treating the cause is key, leading to reduced complications and slowing progression of kidney disease. For example, getting better blood pressure control, improved sugar control and diabetes, and reducing weight are often key interventions. However, existing damage is not usually reversible. In some conditions, treatment can reverse the cause of the disease. So seeking medical review is really important. Individual complications vary, but treatment might include high blood pressure medication, diuretics to reduce fluid and swelling, supplements to relieve anemia, statins to lower cholesterol, or medications to protect your bones and prevent blood vessel calcification. A lower-protein diet may also be recommended. It reduces the amount of waste your kidneys need to filter from your blood. These can not only slow the damage of kidney disease, but make you feel better as well. When the damage has progressed to the point that 85 to 90 percent of your kidney function is gone, and they no longer work well enough to keep you alive, it's called end-stage kidney failure. But there are still options. There's dialysis, which uses a machine to filter the toxins and remove water from your body as your kidneys are no longer able to do this. Where possible, the preferred therapy is a kidney transplant. While an organ transplant can sound daunting, it's actually often the better alternative, and the closest thing to a cure, if you qualify for a kidney transplant.
If you have kidney disease, there are lifestyle choices. Namely quit smoking. Consuming alcohol in moderation. If you're overweight or obese, then try to lose weight. Staying active and getting exercise can help not only with your weight, but fatigue and stress. If your condition allows, keep up with your routine, whether that's working, hobbies, social activities, or other things you enjoy. It can be helpful to talk to someone you trust, a friend or relative who's good at listening. Or your doctor could also refer you to a therapist or social worker. It can also be helpful to find a support group and connect with people going through the same thing. Learning you have chronic kidney disease and learning how to live with it can be a challenge. But there are lots of ways to help you to be more comfortable for longer before more drastic measures are needed. And even then, there is plenty of hope. If you'd like to learn even more about chronic kidney disease, watch our other related videos or visit mayoclinic.org. We wish you well.
Chronic kidney disease, also called chronic kidney failure, involves a gradual loss of kidney function. Your kidneys filter wastes and excess fluids from your blood, which are then removed in your urine. Advanced chronic kidney disease can cause dangerous levels of fluid, electrolytes and wastes to build up in your body.
In the early stages of chronic kidney disease, you might have few signs or symptoms. You might not realize that you have kidney disease until the condition is advanced.
Treatment for chronic kidney disease focuses on slowing the progression of kidney damage, usually by controlling the cause. But, even controlling the cause might not keep kidney damage from progressing. Chronic kidney disease can progress to end-stage kidney failure, which is fatal without artificial filtering (dialysis) or a kidney transplant.
- How kidneys work
One of the important jobs of the kidneys is to clean the blood. As blood moves through the body, it picks up extra fluid, chemicals and waste. The kidneys separate this material from the blood. It's carried out of the body in urine. If the kidneys are unable to do this and the condition is untreated, serious health problems result, with eventual loss of life.
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- A Book: The Body's Keepers
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Signs and symptoms of chronic kidney disease develop over time if kidney damage progresses slowly. Loss of kidney function can cause a buildup of fluid or body waste or electrolyte problems. Depending on how severe it is, loss of kidney function can cause:
- Loss of appetite
- Fatigue and weakness
- Sleep problems
- Urinating more or less
- Decreased mental sharpness
- Muscle cramps
- Swelling of feet and ankles
- Dry, itchy skin
- High blood pressure (hypertension) that's difficult to control
- Shortness of breath, if fluid builds up in the lungs
- Chest pain, if fluid builds up around the lining of the heart
Signs and symptoms of kidney disease are often nonspecific. This means they can also be caused by other illnesses. Because your kidneys are able to make up for lost function, you might not develop signs and symptoms until irreversible damage has occurred.
When to see a doctor
Make an appointment with your doctor if you have signs or symptoms of kidney disease. Early detection might help prevent kidney disease from progressing to kidney failure.
If you have a medical condition that increases your risk of kidney disease, your doctor may monitor your blood pressure and kidney function with urine and blood tests during office visits. Ask your doctor whether these tests are necessary for you.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
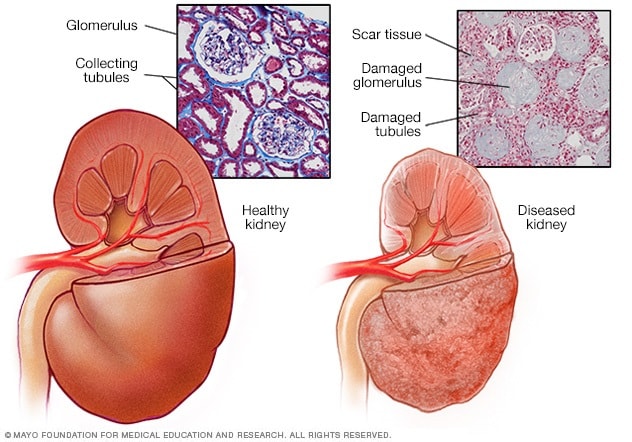
- Healthy kidney vs. diseased kidney
A typical kidney has about 1 million filtering units. Each unit, called a glomerulus, joins a tubule. The tubule collects urine. Conditions such as high blood pressure and diabetes harm kidney function by damaging these filtering units and tubules. The damage causes scarring.
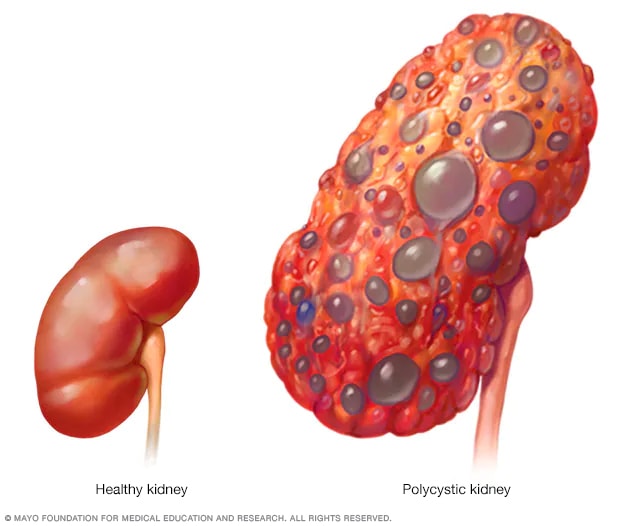
- Polycystic kidney
A healthy kidney (left) eliminates waste from the blood and maintains the body's chemical balance. With polycystic kidney disease (right), fluid-filled sacs called cysts develop in the kidneys. The kidneys grow larger and gradually lose the ability to function as they should.
Chronic kidney disease occurs when a disease or condition impairs kidney function, causing kidney damage to worsen over several months or years.
Diseases and conditions that cause chronic kidney disease include:
- Type 1 or type 2 diabetes
- High blood pressure
- Glomerulonephritis (gloe-mer-u-low-nuh-FRY-tis), an inflammation of the kidney's filtering units (glomeruli)
- Interstitial nephritis (in-tur-STISH-ul nuh-FRY-tis), an inflammation of the kidney's tubules and surrounding structures
- Polycystic kidney disease or other inherited kidney diseases
- Prolonged obstruction of the urinary tract, from conditions such as enlarged prostate, kidney stones and some cancers
- Vesicoureteral (ves-ih-koe-yoo-REE-tur-ul) reflux, a condition that causes urine to back up into your kidneys
- Recurrent kidney infection, also called pyelonephritis (pie-uh-low-nuh-FRY-tis)
Risk factors
Factors that can increase your risk of chronic kidney disease include:
- Heart (cardiovascular) disease
- Being Black, Native American or Asian American
- Family history of kidney disease
- Abnormal kidney structure
- Frequent use of medications that can damage the kidneys
Complications
Chronic kidney disease can affect almost every part of your body. Potential complications include:
- Fluid retention, which could lead to swelling in your arms and legs, high blood pressure, or fluid in your lungs (pulmonary edema)
- A sudden rise in potassium levels in your blood (hyperkalemia), which could impair your heart's function and can be life-threatening
- Heart disease
- Weak bones and an increased risk of bone fractures
- Decreased sex drive, erectile dysfunction or reduced fertility
- Damage to your central nervous system, which can cause difficulty concentrating, personality changes or seizures
- Decreased immune response, which makes you more vulnerable to infection
- Pericarditis, an inflammation of the saclike membrane that envelops your heart (pericardium)
- Pregnancy complications that carry risks for the mother and the developing fetus
- Irreversible damage to your kidneys (end-stage kidney disease), eventually requiring either dialysis or a kidney transplant for survival
To reduce your risk of developing kidney disease:
- Follow instructions on over-the-counter medications. When using nonprescription pain relievers, such as aspirin, ibuprofen (Advil, Motrin IB, others) and acetaminophen (Tylenol, others), follow the instructions on the package. Taking too many pain relievers for a long time could lead to kidney damage.
- Maintain a healthy weight. If you're at a healthy weight, maintain it by being physically active most days of the week. If you need to lose weight, talk with your doctor about strategies for healthy weight loss.
- Don't smoke. Cigarette smoking can damage your kidneys and make existing kidney damage worse. If you're a smoker, talk to your doctor about strategies for quitting. Support groups, counseling and medications can all help you to stop.
- Manage your medical conditions with your doctor's help. If you have diseases or conditions that increase your risk of kidney disease, work with your doctor to control them. Ask your doctor about tests to look for signs of kidney damage.
Chronic kidney disease care at Mayo Clinic
- Goldman L, et al., eds. Chronic kidney disease. In: Goldman-Cecil Medicine. 26th ed. Elsevier; 2020. http://www.clinicalkey.com. Accessed April 27, 2021.
- Chronic kidney disease (CKD). National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/kidney-disease/chronic-kidney-disease-ckd#:~:text=Chronic kidney disease (CKD) means,family history of kidney failure. Accessed April 26, 2021.
- Rosenberg M. Overview of the management of chronic kidney disease in adults. https://www.uptodate.com/contents/search. Accessed April 26, 2021.
- Chronic kidney disease (CKD) symptoms and causes. National Kidney Foundation. https://www.kidney.org/atoz/content/about-chronic-kidney-disease. Accessed April 26, 2021.
- Chronic kidney disease. Merck Manual Professional Version. https://www.merckmanuals.com/professional/genitourinary-disorders/chronic-kidney-disease/chronic-kidney-disease?query=Chronic kidney disease. Accessed April 26, 2021.
- Ammirati AL. Chronic kidney disease. Revista da Associação Médica Brasileira. 2020; doi:10.1590/1806-9282.66.S1.3.
- Chronic kidney disease basics. Centers for Disease Control and Prevention. https://www.cdc.gov/kidneydisease/basics.html. Accessed April 26, 2021.
- Warner KJ. Allscripts EPSi. Mayo Clinic; April 21, 2021.
- Office of Patient Education. Chronic kidney disease treatment options. Mayo Clinic; 2020.
- Chronic kidney disease: Is a clinical trial right for me?
- Eating right for chronic kidney disease
- Effectively managing chronic kidney disease
- Kidney biopsy
- Kidney disease FAQs
- Low-phosphorus diet: Helpful for kidney disease?
- MRI: Is gadolinium safe for people with kidney problems?
- Renal diet for vegetarians
Associated Procedures
- Deceased-donor kidney transplant
- Hemodialysis
- Kidney transplant
- Living-donor kidney transplant
- Nondirected living-donor transplant
- Peritoneal dialysis
- Preemptive kidney transplant
News from Mayo Clinic
- Mayo Clinic Minute: Why Black Americans are at higher risk of chronic kidney disease March 05, 2024, 05:00 p.m. CDT
- Mayo Clinic Minute: Can extra salt hurt your kidneys? Feb. 16, 2024, 04:00 p.m. CDT
- Mayo Clinic Minute: Using AI to predict kidney failure in patients with polycystic kidney disease April 06, 2023, 04:00 p.m. CDT
- Mayo Clinic Q and A: Understanding chronic kidney disease March 23, 2023, 12:35 p.m. CDT
- Mayo Clinic Minute: Game-changing treatment for chronic kidney disease could slow down progression of the disease March 06, 2023, 04:01 p.m. CDT
- Science Saturday: Seeking a cellular therapy for chronic kidney disease Nov. 12, 2022, 12:00 p.m. CDT
- Science Saturday: Mayo Clinic researchers integrate genomics into kidney disease diagnosis, care Sept. 17, 2022, 11:00 a.m. CDT
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Let’s celebrate our doctors!
Join us in celebrating and honoring Mayo Clinic physicians on March 30th for National Doctor’s Day.
- Introduction
- Conclusions
- Article Information
eGFR indicates estimated glomerular filtration rate; SCr, serum creatinine.
RCT indicates randomized clinical trial.
ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; CCB, calcium channel blocker; DAPT, double antiplatelet therapy; DOAC, direct oral anticoagulant; DPP-4 inhibitor, dipeptidyl peptidase 4; eGFR, estimated glomerular filtration rate; GLP-1 receptor agonist, glucagon-like peptide 1; MRA, mineralocorticoid receptor antagonist; PCSK9 inhibitor, proprotein convertase subtilisin/kexin type 9; RCT, randomized clinical trial; SAPT, single antiplatelet therapy; SGLT2 inhibitor, sodium-glucose cotransporter 2; TAPT, triple antiplatelet therapy.
eFigure 1. Flowchart of Literature Search
eFigure 2. The Exclusion of Patients With CKD for Different Types of Cardiovascular Medications
eFigure 3. The Exclusion of Patients With CKD Stratified by Prescribing Recommendations for Exclusion of Patients With CKD
eFigure 4. The Number of Analyses for Patients With CKD for Different Cardiovascular Outcomes
eFigure 5. Overview of Individual Studies With Analysis on eGFR or Kidney Replacement Therapy for Major Adverse Cardiovascular Events in Patients With CKD
eFigure 6. Heat Map of (Subgroup) Analyses for All-Cause Mortality for People With Different Stages of CKD
eFigure 7. Heat Map of (Subgroup) Analyses for Cardiovascular Mortality for People With Different Stages of CKD
eFigure 8. Heat Map of (Subgroup) Analyses for Coronary Artery Disease for People With Different Stages of CKD
eFigure 9. Heat Map of (Subgroup) Analyses for Heart Failure for People With Different Stages of CKD
eFigure 10. Heat Map of (Subgroup) Analyses for Cerebrovascular Disease for People With Different Stages of CKD
eFigure 11. Heat Map of (Subgroup) Analyses for Peripheral Arterial Disease for People With Different Stages of CKD
eFigure 12. Heat Map of (Subgroup) Analyses Kidney Failure for People With Different Stages of CKD
eTable 1. Antihypertensives (Mono)
eTable 2. Antihypertensives (Multiple)
eTable 3. Antihypertensives (Other)
eTable 4. Lipid-Lowering (Mono)
eTable 5. Lipid-Lowering (Multiple)
eTable 6. Lipid-Lowering (Other)
eTable 7. Antiplatelet (Mono)
eTable 8. Antiplatelet (Multiple)
eTable 9. Glucose-Lowering (Mono)
eTable 10. Glucose-Lowering (Multiple)
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Colombijn JMT , Idema DL , van Beem S, et al. Representation of Patients With Chronic Kidney Disease in Clinical Trials of Cardiovascular Disease Medications : A Systematic Review . JAMA Netw Open. 2024;7(3):e240427. doi:10.1001/jamanetworkopen.2024.0427
Manage citations:
© 2024
- Permissions
Representation of Patients With Chronic Kidney Disease in Clinical Trials of Cardiovascular Disease Medications : A Systematic Review
- 1 Department of Nephrology and Hypertension, University Medical Center Utrecht, Utrecht, the Netherlands
- 2 Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands
- 3 Cochrane Netherlands, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands
- 4 Department of Cardiology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam Cardiovascular Sciences, Amsterdam, the Netherlands
- 5 Amsterdam Cardiovascular Sciences (Heart Failure and Arrhythmias), Amsterdam, the Netherlands
- 6 Medical Library, Amsterdam UMC, University of Amsterdam, Amsterdam Public Health, Amsterdam, the Netherlands
Question How often are patients with chronic kidney disease (CKD) excluded in cardiovascular randomized clinical trials (RCTs), and what are the evidence gaps in cardiovascular medications for these patients?
Findings In this systematic review of 1194 RCTs involving over 2 million patients, the proportion of RCTs that excluded patients with CKD increased in the past 20 years. Such RCTs typically excluded more patients than expected on safety grounds.
Meaning Findings of this study suggest that lack of RCTs reporting results for patients with CKD plays a role in the significant evidence gaps in the effectiveness of cardiovascular disease medications for patients with all stages of CKD, especially stages 4 to 5.
Importance Patients with chronic kidney disease (CKD) are at high risk for cardiovascular disease, but their systematic underrepresentation in cardiovascular randomized clinical trials (RCTs) limits the generation of appropriate evidence to guide cardiovascular risk management (CVRM).
Objective To evaluate the underrepresentation of patients with CKD in cardiovascular RCTs, and to highlight evidence gaps in CVRM medications in this population.
Evidence Review A systematic search was conducted in ClinicalTrials.gov from February 2000 through October 2021 for RCTs with full-text publications. If no full-text publications were found in ClinicalTrials.gov, MEDLINE, Embase, and Google Scholar were also searched. Eligible RCTs were those evaluating the effectiveness of antiplatelets, anticoagulants, blood pressure–lowering drugs, glucose-lowering drugs, or cholesterol-lowering drugs in adults with cardiovascular disease or cardiovascular risk factors. Trials with a sample size of fewer than 100 patients were excluded.
Findings In total, 1194 RCTs involving 2 207 677 participants (mean [SD] age, 63 [6] years; 1 343 970 males [64%]) were included. Since 2000, the percentage of cardiovascular RCTs excluding patients with CKD has increased from 66% to 79% (74% overall [884 RCTs]). In 864 RCTs (72%), more patients were excluded than anticipated on safety grounds (63% [306] of trials required no dose adjustment, and 79% [561] required dose adjustment). In total, 158 RCTs (13%) reported results for patients with CKD separately (eg, in subgroup analyses). Significant evidence gaps exist in most CVRM interventions for patients with CKD, particularly for those with CKD stages 4 to 5. Twenty-three RCTs (2%) reported results for patients with an estimated glomerular filtration rate less than 30 mL/min/1.73 m 2 , 15 RCTs (1%) reported for patients receiving dialysis, and 1 RCT (0.1%) reported for recipients of kidney transplant.
Conclusions and Relevance Results of this systematic review suggest that representation of patients with CKD in cardiovascular RCTs has not improved in the past 2 decades and that these RCTs excluded more patients with CKD than expected on safety grounds. Lack of reporting or underreporting of results for this patient population is associated with evidence gaps in the effectiveness of most CVRM medications in patients with all stages of CKD, particularly CKD stages 4 to 5.
Chronic kidney disease (CKD) affects almost 700 million people worldwide and is the cause of 1.9 million cardiovascular deaths annually. 1 , 2 Over 60% of patients with CKD have a history of cardiovascular disease (CVD), which is also the main cause of death in this population. 3 , 4 Almost all patients with CKD have a much higher risk for CVD than kidney failure. 5 , 6 This elevated CVD risk is already observed for patients with an estimated glomerular filtration rate (eGFR) less than 75 mL/min/1.73 m 2 and increases as CKD progresses independent of other risk factors, such as hypertension and diabetes. 3 , 7
The high cardiovascular risk in patients with CKD underscores the importance of effective cardiovascular risk management (CVRM) for these patients. Nevertheless, even though over 90% of patients with CKD are prescribed CVRM medications, evidence is limited on the safety and effectiveness of these medications in this population. 8 , 9 Historically, patients with CKD largely have been underrepresented in cardiovascular randomized clinical trials (RCTs). They are frequently excluded due to concerns about the safety and effectiveness of interventions. Even the RCTs without explicit CKD exclusion criteria often do not include these patients nor assess treatment effects for them. 10 - 13
Lack of information about the effectiveness of CVRM medications in patients with CKD undermines effective CVRM. Effectiveness estimates about CVRM medications from RCTs that excluded patients with CKD cannot be extrapolated carelessly since the increased CVD risk in patients with CKD and altered pathophysiological processes of CVD can modify the effectiveness of treatments. 14 As CKD progresses to kidney failure, patients’ CVD burden shifts from atherosclerotic CVD to medial arterial calcification, cardiac arrhythmias, left-ventricular hypertrophy, and sudden cardiac death. 14 A higher cardiovascular risk could enhance the effectiveness of CVRM for patients with CKD because a greater absolute risk reduction can be achieved. However, lower life expectancy and the induction of additional pathways in CVD pathophysiological processes, which are not inhibited by traditional CVRM medications, could offset these benefits and render treatment futile. 15 , 16
Several systematic reviews, which included RCTs published up to 2014, have reported on the underrepresentation of patients with CKD in cardiovascular RCTs. 10 - 13 However, it is unclear whether the representation of patients with CKD in cardiovascular RCTs has improved over the past years and whether this population has been included in RCTs evaluating the effectiveness of new treatments, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors and direct oral anticoagulants (DOACs). Furthermore, the systematic exclusion of patients with CKD makes it difficult to ascertain which CVRM medications have available evidence on their effectiveness and safety, specifically for people with CKD. An overview of the RCTs evaluating the effectiveness of CVRM medications for patients with different stages of CKD is currently lacking. Therefore, this systematic review aimed to evaluate the underrepresentation of patients with CKD in cardiovascular RCTs in the past 20 years and to highlight evidence gaps in CVRM medications in this population.
This systematic review is registered prospectively in the PROSPERO International Prospective Register of Systematic Reviews ( CRD42022296746 ). The full protocol has been published previously. 17 We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses ( PRISMA ) reporting guideline.
ClinicalTrials.gov was searched through the Cochrane Central Register of Controlled Trials from inception (February 2000) through October 2021 using a combination of keywords for CVD, cardiovascular risk factors, and included interventions to identify planned, ongoing, terminated, and completed RCTs. Full-text publications were retrieved up to May 2023 from ClinicalTrials.gov. If no full-text publications were found in ClinicalTrials.gov, MEDLINE, Embase, and Google Scholar were also searched to retrieve full texts. Trial records were excluded if no publications could be found. Landmark RCTs that were not identified in the search were added manually.
Two reviewers (including J.M.T.C., D.L.I., S.V.B., A.M.B., K.V.D.B., N.K.A., I.J.O., R.W.M.V.) screened clinical trial records and publications independently based on the eligibility criteria. Disagreements were resolved by discussion.
Eligible RCTs were those that evaluated the association of antiplatelets, anticoagulants, blood pressure–lowering drugs, glucose-lowering drugs, or cholesterol-lowering drugs, which are recommended by the European Society of Cardiology, the American Heart Association, the American Stroke Association, the American College of Cardiology, and the American Diabetes Association for the prevention of CVD, 18 - 39 with all-cause or cardiovascular mortality, CVD (as composite end points and individual events), peripheral arterial disease, or kidney failure in adults with a history of CVD or 1 or more CVD risk factors. In these RCTs, interventions were compared with placebo, usual care, another therapy, or a different treatment dose or duration. Trials with a sample size of fewer than 100 patients were excluded.
Data extraction was performed by 1 reviewer (including D.L.I., S.V.B., A.M.B., K.V.D.B., L.F.H.I.V., T.K., N.K.A., M.P.T.K., I.J.O.) using a standardized form and was verified by another reviewer (J.M.T.C.). A list of extracted variables is described in the protocol. 17 Risk of bias was not assessed since bias in study design is unlikely to affect whether patients with CKD are excluded from RCTs or whether authors report results for these patients (as a subgroup analysis or by restriction of the study population).
Outcomes of interest were the frequency of excluding patients with CKD and reporting results for patients with CKD through subgroup analyses or restriction of the study population. Exclusion of patients with CKD was defined as the exclusion of patients meeting kidney-related eligibility criteria. If RCTs did not specify kidney-related eligibility criteria, we presumed these patients were not excluded.
Categorical variables were described as frequency (percentage), and continuous variables were described as mean (SD) if they followed a normal distribution or as median (IQR) otherwise. The frequency of excluding patients with CKD was evaluated for different periods, medications, and dose recommendations for patients with CKD. Dose recommendations were categorized based on The Renal Drug Handbook as follows: no dose adjustment, dose adjustment in CKD stage 3 or stages 4 to 5, and contraindication in CKD stages 4 or 5. 40 An overview of RCTs published for patients with different stages of CKD was visualized in an evidence map. Data analysis was performed with R, version 4.3 (R Project for Statistical Computing).
Overall, 1194 RCTs involving 2 207 677 participants were included (eFigure 1 in Supplement 1 ). The search identified 13 017 RCTs, of which 8780 were excluded. Of 1419 RCTs, no full text could be retrieved. The remaining 2818 records were screened on full text. The main reasons for exclusion were no outcomes of interest (n = 884), wrong intervention (n = 304), and insufficient sample size (n = 77). Included RCTs (n = 1194) had a median (IQR) follow-up of 24.0 (12.0-39.6) months, and 81 trials (7%) had published a protocol only. Glucose-lowering drugs were evaluated in 552 RCTs (46%), antiplatelets and anticoagulants in 229 RCTs (19%), blood pressure–lowering drugs in 221 RCTs (19%), and a combination of these interventions in 30 RCTs (3%) ( Table ).
Participants had a mean (SD) age of 63 (6) years and included 747 390 females (36%) and 1 343 970 males (64%); 80 trials had missing data on sex (n = 116 317). The mean (SD) eGFR was 73 (13) mL/min/1.73 m 2 and the median (IQR) serum creatinine level was 1.00 (0.96-1.04) mg/dL, but these variables were reported in only 295 (25%) and 154 (13%) RCTs, respectively. Patients receiving dialysis were included in 17 RCTs (<1%), and recipients of a kidney transplant were included in 1 RCT (<1%). An overview of included RCTs and their characteristics are provided in eTables 1 to 10 in Supplement 2 .
Since 2000, the percentage of RCTs excluding subgroups of patients with CKD has increased from 66% to 79% (74% overall [884 RCTs]) ( Figure 1 A). Patients with an eGFR greater than 30 mL/min/1.73 m 2 , serum creatinine level less than 2 mg/dL, or a history of CKD (hereafter, CKD stages 1-3) were excluded from 458 RCTs (38% of all included RCTs, and 52% of RCTs that excluded patients with CKD) ( Figure 1 A). In the past 20 years, patients with CKD stages 4 to 5 have been excluded from cardiovascular RCTs more frequently, whereas the exclusion of patients with CKD stages 1 to 3 has remained stable ( Figure 1 A). The proportion of RCTs in which dose adjustment based on kidney function was required or medication was contraindicated based on kidney function remained consistent across different periods (eg, 2000-2005 to 2021-2023: 38% to 35% for CKD stages 1-3; 58% to 71% for CKD stages 4-5) ( Figure 2 A; eFigure 3 in Supplement 1 ). The kidney exclusion criteria applied were heterogeneous but generally based on eGFR (442 RCTs [50%]) or serum creatinine level (324 RCTs [37%]) ( Figure 1 B). The exclusion of patients with CKD for individual drug groups is illustrated in eFigure 2 in Supplement 1 .
In 864 RCTs (72%), more patients with CKD were excluded than expected on safety grounds. Patients with CKD were excluded in 306 of 488 RCTs (63%) in which no dose adjustment for the interventions on kidney function was required. The rate of exclusion of patients with CKD was over 80% in RCTs in which dose adjustments based on kidney function were necessary or interventions were contraindicated based on kidney function. However, 561 of 706 RCTs (79%) also excluded more patients with CKD than necessary on safety grounds ( Figure 2 B; eFigure 3 in Supplement 1 ).
In total, 158 RCTs (13%) reported results for patients with CKD. Of these RCTs, 34 (3%) included patients with CKD only (4 cholesterol-lowering drugs, 13 blood pressure–lowering drugs, 15 glucose-lowering drugs, and 2 antithrombotic drugs). Twenty-three RCTs (2%) reported results for patients with an eGFR less than 30 mL/min/1.73 m 2 , 15 RCTs (1%) reported for patients receiving dialysis, and 1 RCT (0.1%) reported for recipients of kidney transplant. The percentage of RCTs that reported results for patients with CKD has not increased in the past 20 years, from 30% in 2000 to 2005 to 25% in 2021 to 2023 ( Figure 3 ). Analyses for patients with CKD were predominantly performed for composite cardiovascular end points (112 RCTs [66%]) in heterogeneous strata ( Figure 4 ; eFigures 4 and 5 in Supplement 1 ). Few RCTs conducted analyses for individual cardiovascular end points, particularly for heart failure, peripheral arterial disease, and kidney failure (eFigures 4 and 6-12 in Supplement 1 ). The mean (SD) eGFR in RCTs that conducted subgroup analyses was 71 (12) mL/min/1.73 m 2 , but this parameter was reported in 102 of 171 RCTs (60%).
We identified significant evidence gaps in CVRM medications for all patients with CKD. Evidence gaps were most notable for patients with CKD stages 4 to 5. An overview of analyses for end points other than major adverse cardiovascular events (MACE) is provided in eFigures 6 to 11 in Supplement 1 .
Most RCTs (26 of 52 [50%]) that evaluated blood pressure–lowering drugs preventing MACE for patients with CKD focused on angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and mineralocorticoid receptor antagonists ( Figure 4 ; eFigure 5 in Supplement 1 ). Each antihypertensive drug was evaluated for patients with an eGFR less than 60 mL/min/1.73 m 2 , except for α-blockers. Angiotensin receptor blockers, β-blockers, and mineralocorticoid receptor antagonists were assessed for patients with an eGFR less than 30 mL/min/1.73 m 2 and patients receiving dialysis. Angiotensin-converting enzyme inhibitors and calcium channel blockers were also evaluated for patients receiving dialysis and thiazides for patients with an eGFR less than 30 mL/min/1.73 m 2 . Other antihypertensives were not evaluated in these populations. None of the antihypertensives were evaluated for recipients of a kidney transplant ( Figure 4 ; eFigure 5 in Supplement 1 ).
The effectiveness of cholesterol-lowering drugs for preventing MACE in patients with CKD was evaluated almost exclusively for statins ( Figure 4 ; eFigure 5 in Supplement 1 ). Statins were evaluated for patients with an eGFR less than 60 mL/min/1.73 m 2 as monotherapy or in combination with ezetimibe. Proprotein convertase subtilisin/kexin type 9 inhibitors, niacin, and icosapent ethyl were also evaluated for this population. For patients with an eGFR less than 30 mL/min/1.73 m 2 , only the combination of statins and ezetimibe was evaluated. For patients receiving dialysis, statins were evaluated as monotherapy and in combination with ezetimibe. For kidney transplant recipients, only statin monotherapy was evaluated ( Figure 4 ; eFigure 5 in Supplement 1 ).
Of all CVRM medications, antiplatelets and anticoagulants were studied most frequently for patients with CKD ( Figure 4 ; eFigure 5 in Supplement 1 ). The effectiveness of single antiplatelet therapy (SAPT; 17 of 55 RCTs [31%]), double antiplatelet therapy (DAPT; 13 [24%]), and DOACs (12 [22%]) was evaluated for patients with CKD in multiple RCTs. However, few of these RCTs reported results for patients with an eGFR less than 45 mL/min/1.73 m 2 ( Figure 4 ; eFigure 5 in Supplement 1 ). For patients with an eGFR less than 30 mL/min/1.73 m 2 , the effectiveness of SAPT, DAPT, DOACs, and DOACs plus SAPT was evaluated. For patients receiving dialysis, evidence was limited to the comparison of DOACs with vitamin K antagonists, and the effectiveness of antiplatelets was not evaluated at all. None of the RCTs evaluated the effectiveness of antiplatelets and anticoagulants for recipients of a kidney transplant ( Figure 4 ; eFigure 5 in Supplement 1 ).
The effectiveness of SGLT2 inhibitors (13 of 51 RCTs [25%]), glucagon-like peptide 1 (GLP-1) receptor agonists (10 [20%]), and dipeptidyl peptidase 4 (DPP-4) inhibitors (6 [12%]) for preventing MACE for patients with CKD was evaluated in multiple RCTs. However, hardly any evidence was available for older glucose-lowering drugs, such as metformin, sulphonylureas, and insulin ( Figure 4 ; eFigure 5 in Supplement 1 ). Similar to antiplatelets and anticoagulants, there were little data for patients with an eGFR less than 30 mL/min/1.73 m 2 . For these patients, the effectiveness of SGLT2 inhibitors, DPP-4 inhibitors, GLP-1 receptor agonists, and insulin was evaluated. For patients receiving dialysis, DPP-4 inhibitors and sulphonylureas were compared. None of the glucose-lowering drugs were assessed in recipients of a kidney transplant ( Figure 4 ; eFigure 5 in Supplement 1 ).
In this systematic review, we found no improvement in the representation in RCTs of patients with CKD over the past 2 decades. On the contrary, since 2000, the number of cardiovascular RCTs that excluded subgroups of patients with CKD has increased. Exclusion criteria were heterogeneous and cardiovascular RCTs consistently excluded a larger number of patients with CKD than would be anticipated on safety grounds. In addition, only 13% of included cardiovascular RCTs evaluated the effectiveness of CVRM medications for patients with CKD, mostly in subgroup analyses. Although for almost all medications some data were published for patients with CKD stage 3, there were evidence gaps across all CVRM medications and patients with all stages of CKD, particularly stages 4 to 5.
The persistently high proportion of RCTs that excluded patients with CKD in the past 20 years cannot be attributed solely to safety concerns. Although the absolute number of RCTs requiring dose adjustment on kidney function for CVRM medications increased in this period, the proportion of RCTs requiring such adjustment remained stable. While excluding patients with CKD from RCTs due to safety concerns can be justifiable, the substantially more stringent kidney exclusion criteria compared with prescription thresholds in clinical practice suggest there were additional reasons for excluding patients with CKD. Practical issues, such as the necessity for dose adjustments, concerns about heterogeneity in treatment effects, or limited life expectancy, could also discourage investigators from including patients with CKD in their RCTs.
The evidence gaps for patients with CKD in cardiovascular RCTs can be traced back to the ongoing widespread exclusion of this population. Between 1980 and 2005, 56% to 76% of cardiovascular RCTs excluded patients with CKD. 10 , 11 The representation of patients with CKD has not improved after this period with reported rates of exclusion, including this study, ranging from 46% to 79%. 12 , 13 These rates likely underestimate the underrepresentation of patients with CKD because RCTs without explicit exclusion criteria may not enroll an adequate proportion of participants with CKD. Excluding patients with CKD due to possible treatment heterogeneity or initial safety concerns does not necessarily lead to evidence gaps, provided that separate RCTs are conducted to assess the effectiveness of medications for patients with CKD. However, in practice, just 3% of included cardiovascular RCTs were conducted specifically for patients with CKD. Although the proportion of RCTs that reported results for patients with CKD has increased from 8% of the RCTs published between 1980 and 2005, 11 only 25% of RCTs published after 2020 reported results of patients with CKD.
Currently, most cardiovascular RCTs that reported results for patients with CKD focused on those with CKD stage 3. For patients with CKD stages 4 to 5, which compose 10% of patients with CKD (ie, 85 million patients), 41 analyses were often absent, particularly for recipients of a kidney transplant. The lack of RCTs assessing the effectiveness of CVRM medications for patients with CKD means that, in practice, practitioners must resort to extrapolating results from RCTs conducted in other populations, assuming that the treatment effects are comparable. However, this assumption is increasingly less likely to hold for patients with more advanced CKD stages where CKD-specific risk factors like vascular calcification, uremia, chronic inflammation, and immunosuppressive therapy to prevent graft rejection, combined with high risk and reduced life expectancy, can modify the treatment effect. 42 , 43
The complexity of extrapolating results to patients with CKD was illustrated by statins. Although these drugs reduced cardiovascular risk in patients with an eGFR less than 60 mL/min/1.73 m 2 , their effectiveness has not been demonstrated in individuals with kidney failure. 44 The lack of RCTs conducted in patients between these ends of the CKD spectrum makes it impossible to determine the tipping point at which statins lose their benefits. Consequently, patients may unintentionally be overtreated or undertreated since the balance between benefits and adverse effects remains unknown.
In addition to an absolute lack of RCTs, limitations in the analyses further hamper CVRM treatment for patients with CKD. Heterogeneity in exclusion criteria and inadequate reporting of baseline kidney function are associated with reduced comparability of RCTs, whereas the small sample size of strata plays a role in underpowered analyses and imprecision in effect size estimates. Furthermore, the lack of RCTs evaluating the association of CVRM medications with individual cardiovascular end points and kidney end points in patients with CKD means that the effectiveness of these drugs for these individual end points remains unknown. Stratifying RCT cohorts breaks randomization and can introduce confounding due to clustering of other CVD risk factors in patients with CKD. 45 , 46 These limitations are likely to amount in a GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) of low or very low certainty of evidence for most CVRM medications in patients with CKD, meaning that their effectiveness might be markedly different from the estimated treatment effect. 47
The increasing prevalence of CKD (including dialysis and transplant), widespread prescription of CVRM medications, and uncertainty about the effectiveness of various CVRM medications in patients with CKD underscore the urgency of adequate representation of this population in cardiovascular RCTs. 48 , 49 Despite efforts of the US Food and Drug Administration and European Medicines Agency to promote the enrollment of patients with CKD and numerous reviews and editorials addressing this issue, the representation of patients with CKD in cardiovascular RCTs has not increased in the past 40 years. 12 , 13 , 50 , 51 Moreover, although we found a significant underrepresentation of patients with CKD in cardiovascular RCTs, the results likely underestimated the actual underrepresentation of patients with CKD because RCTs without explicit exclusion criteria may not enroll an adequate proportion of patients with CKD, only aggravating the problem.
Bridging the evidence gap for treatment of cardiovascular risk in patients with CKD requires the collaboration among different stakeholders, including pharmaceutical companies, medication regulatory authorities, scientific societies, funding bodies, and clinical steering committees, and starts with the adequate documentation of kidney function and disease as well as proportional inclusion of patients with CKD to enable separate analyses for patients with vs without kidney disease or patients with different stages of kidney disease. New evidence for patients with CKD stages 4 to 5 (including those receiving kidney replacement therapy) should be prioritized, considering that the evidence gaps are largest for this population. Despite the challenges of including patients with CKD stages 4 to 5 or conducting separate RCTs for them, analyses to obtain reliable estimates on the effectiveness of cardiovascular medications in patients with CKD are only feasible if a sufficient number of these patients are included in RCTs. Additionally, more evidence is needed on the effectiveness of CVRM medications for individual cardiovascular and kidney end points. Innovative RCT designs, such as adaptive platform trials based on a master protocol, might be a means to rapidly generate evidence for a range of treatment strategies for different groups of patients with CKD. Furthermore, emulated target trials with clinical data present another opportunity to fill evidence gaps in the effectiveness of CVRM medications in patients with CKD, especially for drugs regularly prescribed in practice where conducting new RCTs is prohibitively expensive and time consuming. 52
This study has several limitations. We might have underestimated the exclusion of patients with CKD in RCTs with ambiguous exclusion criteria, such as chronic disease or life-limiting disease, and might have missed RCTs that were not registered in ClinicalTrials.gov. However, we are confident that the number of missed RCTs was small since the sensitivity of searches in trial registries and electronic databases has been demonstrated to be comparable. 53 Moreover, the validation study we conducted showed that the search strategy identified almost all eligible RCTs from a bibliographic database search. Searching for RCTs registered in or after 2000 may appear to be a limitation because we did not include most RCTs published before this date without retrospective registration. However, these older RCTs are less relevant for contemporary clinical practice and guideline recommendations given that more comprehensive CVRM care and new therapies have vastly improved patients’ outcomes. 54
This systematic review found that representation of patients with CKD in cardiovascular RCTs has not increased in the past 20 years. Cardiovascular RCTs systematically excluded more patients with CKD than expected on safety grounds. Lack of cardiovascular RCTs that reported results for patients with CKD has played a role in the significant evidence gaps in the effectiveness of most CVRM medications for patients with CKD, particularly CKD stages 4 to 5.
Accepted for Publication: December 27, 2023.
Published: March 7, 2024. doi:10.1001/jamanetworkopen.2024.0427
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Colombijn JMT et al. JAMA Network Open .
Corresponding Author: Robin W. M. Vernooij, PhD, Heidelberglaan 100, 3584 CX, Utrecht, the Netherlands ( [email protected] ).
Author Contributions: Ms Colombijn and Dr Vernooij had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Colombijn, Kolagasigil-Akdemir, Meijvis, Spijker, Bots, Hooft, Verhaar, Vernooij.
Acquisition, analysis, or interpretation of data: Colombijn, Idema, van Beem, Blokland, van der Braak, Handoko, Huis in 't Veld, Kaul, Kolagasigil-Akdemir, Kusters, Oosting, Spijker, Hooft, Verhaar, Vernooij.
Drafting of the manuscript: Colombijn, Verhaar, Vernooij.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: Colombijn, Vernooij.
Obtained funding: Verhaar, Vernooij.
Administrative, technical, or material support: Colombijn, van Beem, Blokland, Handoko, Kolagasigil-Akdemir, Kusters, Spijker, Verhaar, Vernooij.
Supervision: Meijvis, Bots, Hooft, Verhaar, Vernooij.
Conflict of Interest Disclosures: Dr Handoko reported receiving personal fees from Novartis, AstraZeneca, Boehringer Ingelheim, Vifor Pharma, Abbott, and Bayer; grants from Vifor Pharma, Novartis, and Boehringer Ingelheim; and the E. Dekker Senior Clinical Scientist stipend from the Dutch Heart Foundation outside the submitted work. Mr Spijker reported receiving grants from Dutch Heart Foundation during the conduct of the study. No other disclosures were reported.
Funding/Support: This study was supported by grant 2020B008 RECONNEXT from the Dutch CardioVascular Alliance, an initiative with support of the Dutch Heart Foundation (Dr Verhaar).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 3 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Question and Answer
- Open access
- Published: 17 August 2021
Recent advances in diabetic kidney disease
- Mohamad Hanouneh 1 , 2 ,
- Justin B. Echouffo Tcheugui 1 , 3 , 4 &
- Bernard G. Jaar 1 , 2 , 3 , 4
BMC Medicine volume 19 , Article number: 180 ( 2021 ) Cite this article
4961 Accesses
14 Citations
1 Altmetric
Metrics details
What is diabetic kidney disease and what do we know so far about its clinical presentation?
Diabetes mellitus is the leading cause of chronic kidney disease (CKD) in the USA and worldwide. An estimated 422 million adults are living with diabetes globally, and up to 40% of them may develop CKD during their lifetime [ 1 ]. Diabetic kidney disease (DKD) does not reflect a specific pathological phenotype. In fact, it can be diagnosed clinically based on the presence of persistent albuminuria, sustained reduction in the estimated glomerular filtration rate (eGFR), or both in patients with diabetes [ 2 ]. DKD is usually identified after five years of the diagnosis of type 1 diabetes, while it can be recognized at the time of diagnosis of type 2 diabetes. The presence of proliferative diabetic retinopathy typically correlates with ongoing DKD in patients with albuminuria. Even though a kidney biopsy can confirm the diagnosis of DKD, this procedure is usually considered when an alternative diagnosis suspected.
Albuminuria has more recently been classified into moderate (30 to 300 mg/g) or severe (> 300 mg/g). Nonetheless, any degree of albuminuria has been associated with an increased risk for CKD progression, end-stage kidney disease (ESKD), adverse cardiovascular disease outcomes, and mortality in patients with diabetes [ 3 ]. A reduced eGFR in diabetic patients has been observed in the absence of albuminuria; however, the progression of DKD appears to be slower in these individuals [ 3 ]. Furthermore, the combined presence of albuminuria and lower eGFR independently increases the risks for cardiovascular events and mortality in individuals with diabetes [ 3 ]. The Kidney Disease: Improving Global Outcomes (KDIGO) and the American Diabetes Association (ADA) guidelines recommend that all diabetic patients undergo annual screening by checking serum creatinine-based eGFR and urine tests to evaluate for albuminuria [ 2 ].
What is unique about the challenges with DKD compared with other types of kidney disease?
Individuals with type 2 diabetes may develop DKD before a clear diagnosis of diabetes is established. This has the consequence of delaying the diagnosis and appropriate treatment of DKD. More recently, we have witnessed significant progress in the treatment options for slowing DKD, but no real advance in reversing DKD. To date, available therapies are targeting DKD progression. Furthermore, not all DKD patients are eligible for these therapies because of variable side effects such as hyperkalemia, acute kidney injury (AKI), and extent of the DKD. Indeed, because of safety concerns, many of these newer medications are not approved for patients with eGFR below 30 mL/min/1.73 m 2 .
What is known about the causes of DKD?
Hyperaminoacidemia, glomerular hyperfiltration and hyperperfusion, and hyperglycemia are the major metabolic abnormalities that affect the kidneys and are associated with inflammation and eventually fibrosis in diabetic patients [ 4 ]. The classic sequence of events in the natural history of DKD is driven by hyperglycemia in conjunction with hypertension and is characterized by glomerular hyperfiltration progressing to albuminuria, and then leading to a decline in kidney function. One mechanistic hypothesis suggests that a decrease in distal delivery of sodium chloride to the macula densa results from an increased proximal tubular reabsorption of glucose via sodium–glucose cotransporter 2 leading to a decrease in tubulo-glomerular feedback. This results in dilation of the afferent arteriole and increased glomerular perfusion [ 5 ]. On the other hand, increased production of angiotensin II leads to vasoconstriction in the efferent arteriole. The net effect is an elevated intraglomerular pressure leading to glomerular hyperfiltration [ 5 ]. Additionally, systemic hypertension and obesity can also contribute to glomerular hyperfiltration via glomerular enlargement [ 4 ].
A number of other factors can play a significant role in the pathogenesis of DKD. These include, for example, oxidative stress. Activation of advanced glycation end-products (AGE) receptors, which are represented on multiple cell types in the kidneys, induces the production of numerous cytokines. Hyperglycemia causes increased formation of AGE and activates protein kinase C, resulting in decreased production of endothelial nitric oxide synthase and increased levels of the endothelin 1, Angiopoietins 2, and vascular endothelial growth factor. Furthermore, hyperglycemia, angiotensin II, and AGE can activate macrophages, which are rich in cytokines and tumor necrosis factor. The net effect of these different pathways leads to endothelial instability, increased vascular proliferation, renal hypertrophy, podocyte injury, tubular epithelial cell injury, and increased cytokine production [ 6 ].
The structural changes of DKD start with thickening of the glomerular basement membrane followed by mesangial matrix expansion and foot process effacement [ 7 ]. Segmental mesangiolysis and Kimmelstiel–Wilson nodules are signs of DKD progression [ 8 ]. Interstitial fibrosis and global sclerosis develop in later DKD stages.
What are the general treatment options for DKD?
Intensive glycemic control is critical in the prevention of DKD in the early course of the disease. However, a number of studies have shown that intensive glucose control may not reduce the risk of CKD progression or cardiovascular mortality in advanced stages of DKD [ 9 ]. The KDIGO guidelines recommend a target HbA1c ranging from < 6.5 to < 8.0%, with the choice of an exact target guided by the extent of hypoglycemia risk in each patient [ 10 ].
For glycemic control, current guidelines suggest using both metformin and sodium-glucose cotransporter 2 inhibitors for patients with DKD and GFR > 30 ml/min per 1.73 m 2 [ 10 ]. Glucagon-like peptide-1 receptor agonists can be added to manage hyperglycemia if needed [ 10 ]. Uncontrolled hypertension can worsen DKD and increase the risk of progression to ESKD. The KDIGO guidelines recommend using an angiotensin-converting enzyme inhibitor (ACEi) or an angiotensin receptor blocker (ARB) to maintain blood pressure below 130/80 mmHg in all patients with CKD and albuminuria regardless of their diabetic status. Prior studies showed that ACEis and ARBs offer kidney protection by lowering proteinuria and slowing the rate of CKD progression [ 10 ]. Combination regimens with ACEis and ARBs are not recommended due to an increased risk of acute kidney injury and hyperkalemia.
Regarding the non-pharmacological therapies, KDIGO guidelines recommend the implementation of lifestyle modification among DKD patients, including low sodium intake (< 2 g/day), maintaining a protein intake of 0.8 g/kg/day for patients who are not on dialysis, and moderate-intensity physical activity for a cumulative duration of at least 150 min per week as tolerated [ 10 ].
Availability of data and materials
Not applicable.
Abbreviations
Chronic kidney disease
Estimated glomerular filtration rate
Diabetic kidney disease
End-stage kidney disease
Kidney Disease: Improving Global Outcomes
American Diabetes Association
Acute kidney injury
Advanced glycation end-products
Angiotensin-converting enzyme inhibitor
Angiotensin receptor blocker
Jager KJ, Kovesdy C, Langham R, Rosenberg M, Jha V, Zoccali C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant. 2019;34(11):1803–5. https://doi.org/10.1093/ndt/gfz174 .
Article PubMed Google Scholar
Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64(4):510–33. https://doi.org/10.1053/j.ajkd.2014.08.001 .
de Boer IH, Gao X, Cleary PA, Bebu I, Lachin JM, Molitch ME, et al. Albuminuria changes and cardiovascular and renal outcomes in type 1 diabetes: the DCCT/EDIC study. Clin J Am Soc Nephrol. 2016;11(11):1969–77. https://doi.org/10.2215/CJN.02870316 .
Article PubMed PubMed Central Google Scholar
Grabias BM, Konstantopoulos K. The physical basis of renal fibrosis: effects of altered hydrodynamic forces on kidney homeostasis. Am J Physiol Renal Physiol. 2014;306(5):F473–85. https://doi.org/10.1152/ajprenal.00503.2013 .
Article CAS PubMed Google Scholar
Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752–72. https://doi.org/10.1161/CIRCULATIONAHA.116.021887 .
Gnudi L. Angiopoietins and diabetic nephropathy. Diabetologia. 2016;59(8):1616–20. https://doi.org/10.1007/s00125-016-3995-3 .
Article CAS PubMed PubMed Central Google Scholar
Fioretto P, Mauer M. Histopathology of diabetic nephropathy. Semin Nephrol. 2007;27(2):195–207. https://doi.org/10.1016/j.semnephrol.2007.01.012 .
Stout LC, Kumar S, Whorton EB. Focal mesangiolysis and the pathogenesis of the Kimmelstiel-Wilson nodule. Hum Pathol. 1993;24(1):77–89. https://doi.org/10.1016/0046-8177(93)90066-P .
Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72. https://doi.org/10.1056/NEJMoa0802987 .
KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int 2020, 98 (4 s), S1-s115.
Download references
Acknowledgements
No applicable.
Dr. Echouffo Tcheugui was supported by the National Heart, Lung, and Blood Institute (NHLBI) Grant K23HL153774.
Author information
Authors and affiliations.
Department of Medicine, Johns Hopkins University School of Medicine, 5601 Loch Raven Boulevard, Suite 3 North, Baltimore, MD, 21239, USA
Mohamad Hanouneh, Justin B. Echouffo Tcheugui & Bernard G. Jaar
Nephrology Center of Maryland, Baltimore, MD, USA
Mohamad Hanouneh & Bernard G. Jaar
Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Justin B. Echouffo Tcheugui & Bernard G. Jaar
Welch Center for Prevention, Epidemiology, and Clinical Research, Baltimore, MD, USA
You can also search for this author in PubMed Google Scholar
Contributions
Drafting of manuscript or critical revision of manuscript (all authors). All authors read and approved the final manuscript.
Corresponding author
Correspondence to Bernard G. Jaar .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
BGJ is a member of the Editorial Board of BMC Medicine.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hanouneh, M., Echouffo Tcheugui, J.B. & Jaar, B.G. Recent advances in diabetic kidney disease. BMC Med 19 , 180 (2021). https://doi.org/10.1186/s12916-021-02050-0
Download citation
Received : 30 June 2021
Accepted : 30 June 2021
Published : 17 August 2021
DOI : https://doi.org/10.1186/s12916-021-02050-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Kidney disease in diabetes: From mechanisms to clinical presentation and treatment strategies
Affiliations.
- 1 School of Cardiovascular Medicine & Science, King's College London, London, UK.
- 2 School of Cardiovascular Medicine & Science, King's College London, London, UK. Electronic address: [email protected].
- PMID: 34560098
- DOI: 10.1016/j.metabol.2021.154890
Metabolic and haemodynamic perturbations and their interaction drive the development of diabetic kidney disease (DKD) and its progression towards end stage renal disease (ESRD). Increased mitochondrial oxidative stress has been proposed as the central mechanism in the pathophysiology of DKD, but other mechanisms have been implicated. In parallel to increased oxidative stress, inflammation, cell apoptosis and tissue fibrosis drive the relentless progressive loss of kidney function affecting both the glomerular filtration barrier and the renal tubulointerstitium. Alteration of glomerular capillary autoregulation is at the basis of glomerular hypertension, an important pathogenetic mechanism for DKD. Clinical presentation of DKD can vary. Its classical presentation, often seen in patients with type 1 diabetes (T1DM), features hyperfiltration and albuminuria followed by progressive fall in renal function. Patients can often also present with atypical features characterised by progressive reduction in renal function without albuminuria, others in conjunction with non-diabetes related pathologies making the diagnosis, at times, challenging. Metabolic, lipid and blood pressure control with lifestyle interventions are crucial in reducing the progressive renal function decline seen in DKD. The prevention and management of DKD (and parallel cardiovascular disease) is a huge global challenge and therapies that target haemodynamic perturbations, such as inhibitors of the renin-angiotensin-aldosterone system (RAAS) and SGLT2 inhibitors, have been most successful.
Keywords: Albuminuria; Diabetes; End-stage renal disease; Hypertension; Kidney.
Copyright © 2021 Elsevier Inc. All rights reserved.
Publication types
- Research Support, Non-U.S. Gov't
- Apoptosis / physiology
- Diabetes Mellitus, Type 1 / physiopathology*
- Diabetic Nephropathies / diagnosis*
- Diabetic Nephropathies / physiopathology
- Hemodynamics / physiology
- Inflammation / physiopathology
- Oxidative Stress / physiology*
Grants and funding
- MR/T032251/1/MRC_/Medical Research Council/United Kingdom
- PG/16/41/32138/BHF_/British Heart Foundation/United Kingdom
- DH_/Department of Health/United Kingdom
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
INTRODUCTION
The pathogenesis, etiology, presentation, and diagnosis of neonatal AKI are presented in this topic review. The diagnostic evaluation, management, and prognosis of neonatal AKI in children are presented separately. (See "Neonatal acute kidney injury: Evaluation, management, and prognosis" .)
● SCr-based definitions – For clinical care, neonatal AKI has been most commonly defined as:
• SCr ≥1.5 mg/dL (133 micromol/L), or
A Medical Information Site. Great Information. Great Health.
Clinical presentation of kidney disease.
The clinical presentation of kidney disease.
Renal disease may present in many ways, including: (1) the screening of asymptomatic individuals; (2) with symptoms and signs resulting from renal dysfunction; and (3) with symptoms and signs of an underlying disease, often systemic, which has resulted in renal dysfunction.
History and clinical signs—in many cases these are nonspecific or not apparent, and detection of renal disease relies on a combination of clinical suspicion and simple investigations, including urinalysis (by dipstick for proteinuria and haematuria, with quantification of proteinuria most conveniently performed by estimation of the albumin:creatinine ratio, ACR, or protein:creatinine ratio, PCR) and estimation of renal function (by measurement of serum creatinine, expressed as estimated glomerular filtration rate, eGFR).
Asymptomatic renal disease—this is common and most often detected as chronic depression of glomerular filtration rate (known as chronic kidney disease, CKD), proteinuria, or haematuria, either as isolated features or in combination.
Symptomatic renal disease—may present in many ways, including: (1) with features of severe chronic depression of glomerular filtration rate—‘uraemia’, manifesting with some or all of anorexia, nausea, vomiting, fatigue, weakness, pruritus, breathlessness, bleeding tendency, apathy and loss of mental concentration, and muscle twitching and cramps; (2) acute kidney injury—also known as acute renal failure; (3) with urinary symptoms—frequency, polyuria, nocturia, oliguria, anuria, and macroscopic haematuria; and (4) loin pain.
Specific renal syndromes—these include: (1) nephrotic syndrome—comprising oedema, proteinuria, and hypoalbuminaemia—caused by primary or secondary glomerular disease; and (2) rapidly progressive glomerulonephritis with acute renal failure.
Other conditions—renal disease may be associated with and present in the context of many underlying conditions, including: (1) diabetes mellitus; (2) renovascular disease; (3) myeloma and other malignancies; (4) infectious diseases, either as a nonspecific manifestation of the sepsis syndrome or as a specific complication of the particular infection, e.g. haemolytic uraemic syndrome, poststreptococcal glomerulonephritis, hantavirus infection, leptospirosis, HIV nephropathy; (5) systemic inflammatory diseases, e.g. systemic vasculitides, rheumatological disorders, sarcoidosis, amyloidosis; (6) drug-induced renal disease; and (7) pregnancy.
Clinical Presentation of Kidney Disease in detail - technical
Introduction.
Renal disease may present in a multitude of ways. In practice it is usually detected as a result of:
- ◆ screening of asymptomatic individuals
- ◆ symptoms and signs resulting from renal dysfunction
- ◆ symptoms and signs of an underlying disease, often systemic, which has resulted in renal dysfunction
Symptoms and signs of renal pathology are often absent or subtle, even in the presence of significant disease, hence the detection of renal problems requires careful evaluation of the history and clinical findings to assess the potential risk of underlying renal disease. This evaluation should focus on features of systemic and inflammatory disease as well as those relating directly to the renal tract, and similarly a drug and obstetric history may help elucidate the cause of renal disease. However, in many cases the history and clinical signs are nonspecific or not apparent, with detection of renal disease relying on a combination of clinical suspicion and simple investigations, including urinalysis and estimation of renal function.
Presentation of aymptomatic renal disease
Asymptomatic renal disease is common and may often remain stable and undetected. However, some patients with asymptomatic disease are at increased risk of developing renal failure with the passage of time or in the event of intercurrent illnesses. Active screening for subclinical disease is thus carried out in certain subpopulations with the result that patients may be identified with abnormal renal function or with abnormalities on urinalysis that may indicate significant renal pathology. Examples of such screening include:
- ◆ screening patients in primary care—general population screening via eGFR reporting (see below); monitoring of patients at ‘high risk’ of developing renal disease (e.g. hypertension, diabetes, multisystem disease); occupational and insurance medicals
- ◆ screening patients admitted to hospital with acute illnesses—as an incidental finding; as part of renal and electrolyte surveillance in patients at risk (e.g. in the presence of sepsis, hypovolaemia, and usage of nephrotoxic drugs)
- ◆ incidental finding on abdominal imaging—stones, cysts and tumours, reduced renal size
- ◆ screening of the family members of patients with inherited renal disease
Asymptomatic renal dysfunction and screening for chronic kidney disease
Traditionally, the basic means of assessing renal function has been estimation of the serum creatinine, and this can be used—with or without estimation of urinary creatinine excretion—to estimate the GFR. The Cockcoft–Gault equation, which estimates creatinine clearance from serum creatinine, weight, age, and sex, has largely given way as a screening tool to the Modification of Diet in Renal Disease (MDRD) formula, which in its simplest form generates an estimated glomerular filtration rate (eGFR) normalized to body surface area from serum creatinine, age, sex, and race. Using this method, population studies in the Western world have estimated that between 4 and 6% of the adult population have moderate to severe renal failure, with an eGFR of less than 60 ml/min per 1.73 m 2 body surface area (stage 3 to 5 chronic kidney disease (CKD). Most of these are elderly, and only very few of them (1–4%) progress to endstage renal failure when followed over a 5-year period, while over the same time up to 69% die owing to cardiovascular disease.
Population and ‘risk group’ screening for renal dysfunction
Population data suggest that most renal disease identified by screening is not progressive, but there are subpopulations in which progressive renal disease is more likely and in whom early intervention and optimal management may delay or prevent the need for dialysis. Hence in the United States of America and the United Kingdom guidelines have been drafted in which ‘risk groups’ are screened for renal dysfunction/CKD (Bullet list 1). It is not yet known whether screening for renal dysfunction has any effect on outcome, but in the United Kingdom, data on the prevalence of CKD and associated information such as blood pressure measurement, its control, and the use of angiotensin converting enzyme (ACE) inhibitors in the CKD population, are now incorporated into the Primary Care Quality and Outcomes Framework, by which means funding is related to achievement of targets. As a consequence, there has been a substantial rise in the number of patients identified with asymptomatic renal dysfunction, and an increased rate of referral to secondary care, especially of elderly patients. There is still considerable doubt about the validity and value of labelling many very elderly people as having moderate to severe renal failure, especially since in many patients an eGFR in this range seems to confer a very much higher risk of cardiovascular demise than of endstage renal failure.
Bullet list 1 Summary of United Kingdom guidelines for serum creatinine measurement and estimation of GFR
Serum creatinine concentration should be measured at initial assessment and then at least annually in all adult patients with the following conditions:
- • Persistent proteinuria
- • Unexplained haematuria
- • Identified renal pathology
- • Bladder voiding dysfunction (outflow obstruction, neurogenic bladder)
- • Urinary diversion surgery
- • Urinary stones
- • Hypertension
- • Cardiovascular disease—ischaemic heart disease, chronic heart failure, peripheral vascular disease and cerebrovascular disease.
- • ACE inhibitors, angiotensin receptor blockers, NSAIDs, lithium, mesalazine, ciclosporin, tacrolimus
- • Systemic lupus erythematosus
- • Systemic vasculitides
- • Rheumatoid arthritis
- • Individuals with a family history of stage 5 CKD or hereditary renal disease
Employment or insurance health screening
As well as targeted screening of ‘at-risk’ populations, asymptomatic renal disease may also be identified as a result of employment or insurance health screening. Common abnormalities identified are hypertension or abnormal urinalysis, such as proteinuria and microscopic haematuria. Patients identified in this way will often be referred for subsequent investigation.
Screening for renal dysfunction in secondary care
In the secondary care setting, patients in specialist clinics who are at risk of renal disease, such as patients with diabetes, are periodically screened for the development of hypertension, proteinuria, and renal dysfunction. Renal disease also often presents in acute medical and surgical patients. Up to 5% of acute patients have some acute deterioration in renal function during a hospital admission, mostly owing to hypotension, sepsis, or the use of nephrotoxic drugs. Monitoring renal function in such patients may help in the acute management of their illness and may also identify those with underlying chronic renal impairment who require long-term management.
Screening for drug-induced renal disease
Renal disease resulting from the use of nephrotoxic drugs is often asymptomatic, and CKD may develop as a result of long-term use of agents such as nonsteroidal anti-inflammatory drugs (NSAIDs), lithium, and calcineurin inhibitors. Often the only evidence for this is a progressive rise in serum creatinine and fall in eGFR, which may be progressive and—if not detected by routine screening—may present with advanced renal failure. Other drugs such as ACE inhibitors may cause an acute deterioration in renal function, screening for which is required, especially in high-risk groups.
Other incidental findings of renal disease
Subclinical renal disease may also present as an incidental finding on biochemical testing, e.g. abnormalities of potassium and acid–base homeostasis identified on a ‘routine’ sample may indicate a renal tubular acidosis and prompt further investigation for an underlying cause.
Renal disease identified incidentally with imaging
Advances in imaging technology combined with their widespread use have increased the number of incidental renal abnormalities identified. Many of these are anatomical abnormalities which are of little consequence, such as duplex ureters and isolated renal cysts, but significant pathology is sometimes found incidentally, such as polycystic kidneys, renal tumours, and asymmetrical kidneys.
Family screening for renal disease
Patients with a family history of inherited renal disease may also be identified with early, asymptomatic renal disease as a result of screening. The most common example is autosomal dominant polycystic kidney disease, which may be reliably identified by ultrasonography from the third decade onwards. The identification of disease genes for inherited renal diseases such as autosomal dominant polycystic kidney disease, tuberous sclerosis, von Hippel–Lindau disease, Alport’s syndrome, and congenital nephrotic syndrome raises the possibility of future antenatal screening and earlydetection of these diseases long before they become clinically manifest.
Screening and management of asymptomatic proteinuria
The availability of reliable and cheap urine dipstick reagent strips has led to their widespread use to screen for and monitor renal disease in primary and secondary health care. Within the general population, up to 5% of apparently healthy adults and 16% of those aged over 80 years have either a ‘trace’ or ‘+’ of protein, but most of these do not have significant treatable disease, making routine population screening uneconomic and unnecessary. Guidelines aimed at identifying subclinical renal disease therefore suggest proteinuria screening only for patients at increased risk of renal disease. The 2005 United Kingdom guidelines are summarized in Bullet list 2.
Bullet list 2 United Kingdom guidelines for proteinuria screening
Dipstick urinalysis for protein should be undertaken:
- • Newly discovered hypertension, haematuria, or reduced GFR
- • Unexplained oedema or suspected heart failure
- • Suspected multisystem disease, e.g. lupus, vasculitis, and myeloma
- • Diabetes mellitus
- • Urologically unexplained haematuria or persistent proteinuria
- ◆ As part of routine monitoring for patients receiving nephrotoxic drugs, e.g. gold and penicillamine
Detection of proteinuria
Most multireagent strips are sensitive to 100 to 200 mg/litre of protein, giving either a ‘trace’ or ‘+’, although some designed to screen for microalbuminuria are more sensitive. They do not detect low-molecular-weight proteins such as immunoglobulin light chains, and thus assay of light chains using urine immunoelectrophoresis is essential as part of the investigations for myeloma, primary amyloidosis, and light-chain glomerulopathy.
The kidney normally excretes less than 150 mg of protein in 24 h, mainly owing to failed tubular reabsorption of albumin. Urinary protein excretion also reduces overnight while recumbent, but increases during the day owing to posture and exercise. Urinary protein concentration also depends on urine flow rate. To overcome the diurnal variation, proteinuria has been traditionally evaluated from a 24-h urine collection, but these have been largely superseded by measuring the ratio of albumin or protein to creatinine in the urine (albumin:creatinine ratio, ACR; protein:creatinine ratio, PCR). This method has been validated against 24-h urinary collections and—as a rule of thumb—a urinary ACR of 70 mg/mmol or PCR of 100 mg/mmol equates approximately to a 24-h protein excretion of 1 g/24 h.
Management of asymptomatic proteinuria without haematuria
Proteinuria may be an early presentation of renal disease, but transient proteinuria is not associated with significant renal disease. A finding of proteinuria should lead the physician to take a history focusing on risk factors for renal disease (e.g. diabetes, drugs, multisystem disease, and family history), measure the blood pressure, and examine for oedema (Bullet list 3
Bullet list 3 Approach to the patient with dipstick-positive proteinuria
- ◆ Is there any evidence of diabetes or urinary infection?
- ◆ Are there any risk factors for or signs of renal disease?
- ◆ Is proteinuria transient or persistent?
Causes include:
- ◆ Urinary tract infection
- ◆ Orthostatic proteinuria
- ◆ Send urine for spot ACR (or PCR)
- • Diabetes, hypertension, systemic inflammatory disease, myeloma, family history of renal disease
- • Are there any features of nephrotic syndrome (heavy proteinuria with oedema and low serum albumin)?
In the absence of risk factors or signs of renal disease, transient proteinuria is not likely to indicate underlying renal disorder, hence an initial finding of proteinuria on dipstick testing should be repeated a week or so later, and any positive result confirmed and quantitated by estimation of ACR (or PCR). If postural or orthostatic proteinuria is suspected, an early-morning urine specimen should be sent for ACR (or PCR), in which case the diagnosis is substantiated by the finding of normal urinary protein excretion in this specimen.
Persistent proteinuria (ACR >70 mg/mmol, PCR >100 mg/mmol) on two or more occasions requires further investigation with:
- ◆ renal function (eGFR)
- ◆ serum albumin, for diagnosis of nephrotic syndrome
- ◆ serum paraprotein electrophoresis and urinary Bence Jones protein for myeloma
- ◆ immunological screen (antinuclear antibodies, complement, antineutrophil cytoplasmic antibodies (ANCA))
- ◆ renal ultrasonography
- ◆ consideration of renal biopsy if heavy proteinuria (ACR >150–200 mg/mmol, PCR >200–300 mg/mmol) or renal dysfunction
Management of asymptomatic proteinuria with microscopic haematuria
Proteinuria with haematuria on urinalysis indicates intrinsic renal disease. It may be the first sign of a severe glomerulonephritis and acute renal failure, hence this presentation must be considered seriously (see later). Patients with abnormal renal function, haematuria, and proteinuria require urgent referral to a nephrologist for investigation.
Apparently asymptomatic patients with normal renal function but persistent proteinuria and haematuria may describe subtle symptoms of multisystem disease on close questioning (e.g. myalgia, arthralgia, ‘sinusitis’, rash, or fever). These may be clues to an underlying disease, hence patients with such symptoms require screening for multisystem disease with urine microscopy for red cell casts, serum ANCA, antiglomerular basement membrane antibodies, antinuclear and anti-double-stranded DNA antibodies and complement levels, and referral to a nephrologist for further evaluation and consideration of renal biopsy.
Asymptomatic microscopic (non visible) haematuria
Microscopic haematuria may potentially arise from anywhere in the urinary tract. As with renal dysfunction and proteinuria, isolated microscopic haematuria is common. Population studies indicate a prevalence between 0.2 and 16%, with a higher prevalence of 18% in men aged over 50 years. Studies of male army recruits screened and followed up for 12 years showed that 39% had microscopic haematuria on one occasion, and 16% had microscopic haematuria on two or more occasions. Although isolated microscopic haematuria may be associated with benign glomerular disease, in practice the main concern is the possibility of renal and urinary tract malignancy.
Urothelial and bladder carcinomas account for approximately 5% of microscopic haematuria. This risk increases with age, particularly in men over the age of 65 years. In contrast, underlying malignancy in those younger than 40 years is very rare, particularly in the absence of risk factors such as smoking and exposure to azo dyes.
Causes of microscopic (non visible) haematuria
The causes of microscopic haematuria are summarized in Bullet list 4. The true prevalence of intrinsic renal disease is unknown because renal biopsies are not routinely performed in the absence of proteinuria or abnormal renal function. However, small biopsy studies of patients with no other cause for haematuria identified a glomerular cause in 16 to 30%. Within this group, IgA nephropathy and thin basement membrane disease are most common.
Bullet list 4 Causes of microscopic haematuria without proteinuria
- ◆ IgA nephropathy
- ◆ Thin basement membrane disease
- ◆ Hereditary nephritis (Alport’s syndrome)
- ◆ Other glomerulonephritides (mesangiocapillary glomerulonephritis, vasculitis, lupus, etc.)
- ◆ Nephrolithiasis
- ◆ Pyelonephritis
- ◆ Renal cell carcinoma
- ◆ Cystic kidney disease (polycystic and medullary sponge)
- ◆ Papillary necrosis
- ◆ Ureteric strictures
- ◆ Hydronephrosis
- ◆ Sickle cell disease
- ◆ Renal infarcts and arteriovenous malformations
- ◆ Renal tuberculosis
- ◆ Cystitis, prostatitis
- ◆ Bladder carcinoma
- ◆ Benign bladder and ureteral tumours and polyps
- ◆ Urethral strictures
- ◆ Overanticoagulation
- ◆ Factitious
Management strategy for microscopic (non visible) haematuria
The key to managing patients with asymptomatic microscopic haematuria is identifying risk factors for malignancy. In routine practice, patients older than 50 years, smokers, or those with an occupational history of dye exposure should be investigated for malignancy and referred to a urologist for cystoscopy.
Numerous different approaches to the management of patients with microscopic haematuria have been published, reflecting a lack of consensus and an insufficient evidence base. There are no indications for screening for microscopic haematuria as the positive predictive value for malignancy is as low as 5% in an elderly population, and early detection of disease has not been shown to improve prognosis.
Following the detection of microscopic haematuria without proteinuria on urine dipstick, menstruation, recent exercise, or sexual activity should be excluded, and the urine sent for microscopy and culture. Urinalysis should then be repeated after 7 days: if this remains positive, urinary tract infection must be excluded, and blood pressure, urine protein-creatinine ratio (PCR) or albumin creatinine ratio (ACR), and serum creatinine/eGFR measured.
Patients should be referred to urological services to exclude urinary tract malignancy and disease if they are over 40 years of age with:
- (1) persistent asymptomatic haematuria (defined as 2 out of 3 positive dipsticks), or
- (2) symptomatic non-visible (microscopic) haematuria, or
- (3) visible (macroscopic) haematuria.
Patients should be referred to a nephrologist if a urological cause has been excluded, or the criteria for urological assessment are not met, and the patient has:
- (1) declining GFR, or
- (2) CKD stage 4 or 5, or
- (3) proteinuria with urinary PCR >50mg/mmol or ACR >30mg/mmol, or
- (4) age <40 years and hypertension
Patients should be referred to a nephrologist if:
Patients not meeting criteria for referral to urological or renal services, or who have had negative urological or nephrological investigations, need long term monitoring in primary care due to the uncertainty of the underlying diagnosis, with appropriate referral should they develop:
- (1) voiding lower urinary tract symptoms (LUTS), or
- (2) visible haematuria, or
- (3) significant or increasing proteinuria, or
- (4) progressive renal impairment (falling eGFR), or
- (5) hypertension.
Symptomatic renal disease
Many patients with renal disease remain asymptomatic, but others develop symptoms that may be nonspecific, e.g. due to the gradual onset of uraemia in patients with progressive CKD, renal-specific, e.g. loin pain or polyuria, or unrelated to the kidney and manifest as isolated ‘nonrenal’ symptoms or as a constellation of symptoms suggestive of a particular systemic condition. Key features to establish are the duration of symptoms, the presence of nonspecific symptoms possibly related to uraemia, the presence of specific renal symptoms, and the presence of symptoms possibly indicative of systemic disease.
Chronic kidney disease
The symptoms of CKD are attributed to the gradual onset of uraemia, anaemia, and salt and water retention. Patients often develop these slowly and may not report them until renal function is severely impaired, perhaps even an eGFR as low as lesss than 10 ml/min per 1.73 m 2 body surface area. The number of symptoms and their severity tend to increase as renal function declines, forming a spectrum from asymptomatic to overtly symptomatic uraemia. Symptoms and the level of eGFR may not correlate well: some patients with an eGFR of 15 to 20 ml/min per 1.73 m 2 may be symptomatic, whereas a few with an eGFR of less than 5 ml/min per 1.73 m 2 may be remarkably symptom free.
Most patients have some symptoms by the time that they require dialysis (CKD stage 5, eGFR <15 ml/min per 1.73 m 2 ) (Bullet list 5). These include anorexia, nausea, and vomiting (in 76% of patients), fatigue and weakness (72%), pruritus (40%), breathlessness and orthopnoea (26%), bleeding tendency (14%), apathy and loss of mental concentration (12%), and muscle twitching and cramps (11%).
Bullet list 5 Features of uraemia and an eGFR less than 15 ml/min per 1.73 m 2 body surface area
- ◆ Anorexia and malnutrition
- ◆ Nausea and vomiting
- ◆ Tiredness
- ◆ Fluid overload with oedema, breathlessness, and orthopnoea
- ◆ Mental apathy and depression
- ◆ Muscle twitching, restless legs, and cramps
- ◆ Bleeding tendency—haematemesis, epistaxis
- ◆ Sexual dysfunction—loss of libido and impotence
- ◆ Cardiac—pericarditis
Factors contributing to the development of ‘uraemia’ and other symptoms include small-molecule nitrogenous substances, endproducts of protein metabolism, metabolic acidosis, salt and water retention, electrolyte disturbances (e.g. phosphate retention), malnutrition, and anaemia.
Some of these symptoms may be improved by treatment with agents such as erythropoietin, diuretics, and oral sodium bicarbonate, and dietary advice to improve malnutrition and phosphate control. Others respond to the initiation of dialysis. Some symptoms may persist in spite of all these measures.
It is unfortunately not uncommon for patients to present for the first time very late in the course of progressive CKD, with profound and symptomatic uraemia. This is the initial mode of presentation in 20 to 40% of patients entering dialysis programmes in the United Kingdom, who tend to be older, more dependent, and with greater comorbidities than those presenting earlier. Late presentation presents major problems: it is not possible to plan dialysis initiation, and patient choice of modality is limited, with haemodialysis being the default mode. Furthermore, it is often not possible to create definitive vascular access, hence patients often need to begin dialysis with temporary or semipermanent central venous lines. These and other features increase morbidity and mortality after late presentation.
It can be difficult and sometimes impossible to distinguish patients presenting late with advanced chronic renal failure (‘crash-landers’) from those with acute renal failure due to potentially reversible disease. Failure to become dialysis independent by 90 days after initiation is often taken as proof that the acute presentation was with endstage rather than acute renal failure. Patients who ‘crash-land’ are often extremely unwell and may be obtunded with uraemic encephalopathy. Fluid overload is common, with pulmonary and peripheral oedema. Metabolic acidosis is often present and if severe may cause Kussmaul’s respiration as well as cerebral and cardiac depression. Patients may also show signs of muscle twitching, which may be a sign of hyperkalaemia or hypocalcaemia. A pericardial friction rub indicates uraemic pericarditis, which if unrecognized may lead to pericardial tamponade and occasionally to fatal pericardial haemorrhage.
Acute renal failure (acute kidney injury)
Acute kidney injury (in brief) causes can be classified as being prerenal, renal (due to intrinsic renal disease) or postrenal (obstruction). In the general hospital setting, most cases are prerenal and occur as the result of reduced renal perfusion due to volume depletion (30%), cardiac failure (12%), and sepsis (12%). Drug-induced kidney injury accounts for 30%, urinary tract obstruction 10%, and acute glomerular disease and interstitial nephritis cause 5 to 10%.
Key features to establish sequentially when managing a patient with acute renal failure are as follows:
- 1 How ill are they? The condition of patients with similar biochemical abnormalities can range from the asymptomatic to the moribund: those with cardiorespiratory compromise need critical care support.
- 2 Does the patient need emergency haemodialysis or haemofiltration? The major indications are severe hyperkalaemia, pulmonary oedema, profound acidosis, and severe uraemia—the latter being defined more on clinical than biochemical grounds.
- 3 Is there a prerenal element that may respond to volume repletion or inotopic support? Clinical examination, perhaps supplemented by central venous pressure measurement, facilitates this decision.
- 4 Is the patient obstructed? Clinical features can be helpful, and a urinary tract ultrasound is usually diagnostic.
- 5 Is this acute or chronic renal failure? Sometimes this is difficult or impossible to determine on clinical grounds, but small kidneys on ultrasound signify chronic disease.
- 6 Is this intrinsic renal disease? Clinical features of systemic disease and relevant immunological tests (including ANCA and antiglomerular basement membrane antibody) must be pursued, and renal biopsy will usually be required to establish the diagnosis.
As with chronic kidney disease, many of the symptoms and signs attributed to loss of renal function are nonspecific and occur with advanced acute renal failure (GFR <15 ml/min per 1.73 m 2 ). However, in contrast to CKD, the acute metabolic changes are often less well tolerated. The greatest danger is hyperkalaemia, which may develop quickly and is almost always asymptomatic until the onset of cardiac arrhythmias and cardiac arrest. Other potentially life-threatening features include pulmonary oedema, metabolic acidosis, and uraemic pericarditis.
The clinical context and history are of overriding importance in establishing the likely aetiology of acute renal failure. A patient developing acute renal failure postsurgery is likely to have prerenal and acute tubular injury due to a combination of hypovolaemia, sepsis, and analgesia with an NSAID. A patient presenting acutely after a prolonged period of unconsciousness following a drug overdose is likely to have rhabdomyolysis. A patient with a past history of lupus presenting with a recent fever, myalgia, and rash is likely to have rapidly progressive lupus nephritis. A patient with a history of lower urinary tract symptoms or of urinary stones is likely to have obstruction.
It is always important to consider the possibility of urinary tract obstruction as it may be readily reversible. Complete anuria is highly suggestive of total obstruction, although it may also occur in patients with rapidly progressive glomerulonephritis and those with acute obstruction of the renal arterial supply. However, urinary output is generally a poor guide to the presence of urinary tract obstruction, and a normal or even increased output does not exclude the diagnosis. All patients with unexplained acute renal failure should undergo ultrasound imaging of the kidneys and urinary tract. This permits the diagnosis or exclusion of obstruction in most cases, and also allows renal size to be assessed: small kidneys indicate chronic renal failure.
It is important to emphasize that, after stabilization, patients in whom the clinical features and initial investigations do not give sufficient clues to allow a diagnosis to be established will require a renal biopsy to avoid missing potentially reversible intrinsic renal disease.
Urinary symptoms
Micturition.
Most symptoms related to micturition relate to problems arising in the lower urinary tract. Bladder outflow obstruction is commonly associated with symptoms such as urgency, hesitancy, poor urinary stream, nocturia, dysuria, and dribbling. Recognition of these symptoms is important as outflow obstruction may result in complete obstruction with acute renal failure or chronic obstructive uropathy with CKD.
Patients may also describe discomfort or pain on micturition. This symptom of dysuria may also be associated with burning within the urethra or suprapubic pain during or after micturition. When associated with urinary frequency or fevers in young women, dysuria is likely to be caused by a urinary tract infection. However, dysuria occurring in isolation in men of any age suggests structural lesions within the prostate or bladder and warrants further investigation. Perineal or rectal pain associated with micturition suggests prostatic inflammation, such as prostatitis or malignancy.
Patients may present with symptoms of increased frequency of micturition. In this situation, it is important to distinguish between frequent voiding of small volumes of urine and an overall increase in urinary volume with more frequent emptying of a full bladder. Charting urinary frequency and voided volumes over a number of days can allow these to be distinguished. The frequent passage of small volumes of urine suggests bladder irritation (from inflammation, stone, or tumour) or reduced volume from extrinsic compression or contraction (e.g. following radiotherapy). Increased frequency of emptying a full bladder is suggestive of polyuria, especially if the volume and frequency is unaffected during the night.
Polyuria (defined as a urinary output>3 litre/24 h) may result from solute diuresis, water diuresis, or a combination of both. Solute diuresis occurs in conditions such as hyperglycaemia and salt-losing states, e.g. overuse of diuretics and salt-losing nephropathies. Water diuresis may result from primary polydipsia, failure to synthesize or secrete ADH normally (congenital and acquired cranial diabetes insipidus), or failure of cortical and medullary collecting ducts to respond to ADH (congenital and acquired nephrogenic diabetes insipidus).
There are numerous causes of acquired nephrogenic diabetes insipidus, including chronic kidney disease (especially associated with ureteric obstruction, postobstructive states, and chronic interstitial nephritis), electrolyte abnormalities (hypercalcaemia and hyperkalaemia), nephrotoxic drugs (such as lithium and amphotericin), and many other miscellaneous conditions including sickle cell disease, Sjögren’s syndrome, and sarcoidosis. Most patients with polyuria have associated thirst, polydipsia, and nocturia. Polyuria needs confirmation by 24-h urinary collection as most patients are unclear as to their true daily urine output. Once it is established that the patient is polyuric, common causes such as hyperglycaemia and excessive diuretic use need to be excluded, after which investigations should focus on excluding primary polydipsia and distinguishing between cranial and nephrogenic diabetes insipidus.
Nocturia is defined as the need to get up once or more times for nocturnal voids. It may have a considerable negative impact on quality of life and in older people predisposes to falls. Three types of nocturia have been identified: low voided volume, nocturnal polyuria, and mixed origin. Nocturia due to low voided volumes occurs in patients with bladder outflow obstruction and those with hyperactive bladders from any cause. Nocturnal polyuria occurs when there is a reversal of the normal circadian pattern of voiding such that there is an increased nocturnal urine output. These types of nocturia are distinguishable by the use of voiding diaries. Elderly patients who void in excess of 33% of their total 24-h output between 11 p.m. and 7 a.m. are said to have nocturnal polyuria, the corresponding fraction in young adults being 20%. Factors predisposing to nocturnal polyuria include renal impairment, diabetes mellitus, congestive cardiac failure, sleep apnoea, and the mobilization of peripheral oedema due to any cause. In patients without predisposing causes, usually elderly, low nocturnal levels of ADH have been described.
Oliguria and anuria
Oliguria is arbitrarily defined as a urinary output of less than 400 ml/24 h or 0.5 ml/kg body weight per hour. Oliguria is the normal renal physiological response to reduced renal perfusion from any cause and is common in hospital inpatients, particularly those with acute illnesses associated with hypotension and reduced effective circulating volume. Monitoring of fluid balance and urinary output in such patients allows its early detection and treatment, which may help prevent progression to established acute renal failure. The recognition of oliguria should prompt an evaluation of the patient with attention to volume status, blood pressure and the detection/exclusion of sepsis, followed by appropriate management to optimize blood pressure and circulating volume.
Oliguria may also be a feature of intrinsic renal failure due to nephrotoxic drugs, acute glomerulonephritis, or interstitial nephritis, but it is a poor marker of intrinsic renal disease as urinary output often remains normal despite significantly impaired renal function.
The development of anuria, meaning the total absence of urine, is strongly suggestive of urinary tract obstruction, which may occur at any level in the urinary tract. A careful history, examination for an enlarged bladder and digital rectal examination for a prostatic or pelvic mass, should be followed by an urgent ultrasound of the kidneys and bladder. Very occasionally, anuria may be a manifestation of severe intrinsic renal disease, such as a rapidly progressive glomerulonephritis, cortical necrosis, or renal infarction.
Urine appearance and macroscopic haematuria
Macroscopic haematuria is the most common abnormality of the urine noted by patients. As little as 5 ml of blood in a litre of urine will lead to a visible change in urinary colour. Haematuria may arise from anywhere within the urinary tract, but bright red haematuria (with or without clots) is suggestive of lower urinary tract bleeding, whereas dark, smoky brown–black urine is more suggestive of renal pathology. Haematuria at the beginning of micturition, which then clears, suggests urethral pathology, whereas endstream haematuria is consistent with bladder pathology. Although the causes of haematuria are numerous (Bullet list 6), infection, stones, and malignancy are the most common. Macroscopic haematuria warrants investigation in all patients.
Bullet list 6 Causes of macroscopic haematuria
- • Cystitis and pyelonephritis
- • Prostatitis
- • Urethritis
- • Schistosomiasis
- ◆ Urinary stones
- • Renal cell
- • Transitional cell
- • Prostatic
- • IgA nephropathy
- • Alport’s syndrome
- • Crescentic glomerulonephritis
- • Polycystic kidneys
- • Interstitial nephritis
- • Papillary necrosis
- • Tuberculosis
- • Release of urinary obstruction
- • Loin pain–haematuria syndrome
- • Arteriovenous malformations
- • Anticoagulation
- • Factitious
Frank haematuria is uncommon in glomerular disease, with the notable exception of IgA nephropathy in which macroscopic haematuria classically occurs immediately following mucosal inflammation, typically an upper respiratory tract infection. In patients with polycystic disease, cysts may haemorrhage to cause loin pain and haematuria. This may be associated with infection of the cysts and usually resolves with conservative management, with antibiotics if there are signs of infection.
Red–brown–black urine is occasionally caused by haemoglobinuria due to haemolysis or myoglobinuria precipitated by rhabdomyolysis. Beetroot and food colouring may turn the urine pink, whereas drugs such as rifampicin may discolour the urine orange–red. Rarely, urine is found to darken following exposure to light, suggesting a diagnosis of porphyria or alkaptonuria.
The presence of pain in the renal angle (loin pain) is consistent with inflammation, obstruction, or stretching of the renal capsule by a mass lesion. Pain arising from acute obstruction is common and typically colicky in nature, with radiation into the groin and scrotum. The pain may be exacerbated by oral fluids, which increase urinary volume and pressure within the renal pelvis. Pyelonephritis typically causes renal angle pain on the affected side and is often associated with pyrexia and leucocytes in the urine. Similarly, a renal abscess extending into the renal capsule may present with loin pain or with isolated symptoms of diaphragmatic irritation or involvement of the psoas muscle, with pain on leg extension. Patients with polycystic kidneys may also develop loin pain as a result of infection or haemorrhage of single or multiple cysts.
Renal pain is an uncommon feature of glomerulonephritis and other intrinsic renal diseases: IgA nephropathy is very occasionally associated with renal pain, but active destructive glomerulonephritis and interstitial nephritis are invariably pain free.

Loin pain–haematuria syndrome
Rarely, patients may present with recurrent intermittent loin pain, haematuria (microscopic or macroscopic), and normal renal function, with no relevant structural abnormality of the renal tract. The cause of this condition, termed the loin pain–haematuria syndrome, is unknown: it is a diagnosis of exclusion which is most often seen in young women.
The pain—often described as ‘deep’, ‘burning’, or ‘throbbing’—is usually felt in the loin, but can radiate in a typical renal pattern to the groin, genital area, and medial thigh. Some will describe a psychologically traumatic event before the onset of pain. The pain can sometimes be induced or exacerbated by exercise and affected by posture, e.g. sitting for a prolonged length of time can be uncomfortable, and in some cases there is associated nausea and vomiting. Some patients report continuous pain that never goes away, whereas others describe episodic pain that lasts more or less continuously for days or (more typically) weeks, interspersed with periods of remission. The pain is usually unilateral at presentation, but many patients eventually develop pain bilaterally. Many patients are taking large quantities of opioids and other analgaesics (e.g. amitriptyline, gabapentin) by the time they are referred to specialist services.
Urological investigation is unremarkable, or shows incidental abnormalities only. If renal biopsy is performed, the appearances may be normal, but thinning or thickening of the glomerular basement membrane has been reported in about 60% of cases in some series, and appearances of IgA nephropathy are sometime seem, but the relationship—if any—between these findings and symptomatology remains obscure.
Aside from loin pain, many patients will have other medically unexplained somatic symptoms, raising the possibility that this symptom is also a somatoform disorder. They may request nephrectomy and/or renal autotransplantation, which the wise physician will not accede to, preferring to help the patient by sympathetic discussion and referral to pain management services.
Specific renal syndromes
Nephrotic syndrome.
See this link for very detailed technical article about nephrotic syndrome: Nephrotic Syndrome
Nephrotic syndrome is the triad of oedema, proteinuria, and hypoalbuminaemia (see Bullet list 7 for an example). Proteinuria is usually greater than 3.5 g in 24 h, which equates approximately to an ACR of 250 mg/mmol or PCR of 350 mg/mmol. When patients have clinically apparent oedema, serum albumin is usually less than 25 g/litre. However, in practice the definition is somewhat arbitrary, and the correlation between the degree of proteinuria, serum albumin, and presence of oedema is poor. Some patients (particularly older people) may develop oedema with proteinuria less than 3.5 g, whereas others remain free of oedema despite having a serum albumin considerably less than 25 g/litre. Other patients may have heavy proteinuria but maintain a normal serum albumin and remain free of oedema.
Bullet list 7 Case illustration—proteinuria and oedema
A 54-year-old woman presents with worsening peripheral oedema. She had been diagnosed with type 2 diabetes 6 months earlier, but remained well until 4 weeks ago, when she suddenly noted frothy urine and mild peripheral oedema. Over the following weeks the oedema had worsened and she noted some abdominal distension. Her only regular medication is gliclazide.
- ◆ Pitting oedema to her lumbar spine, with bilateral small pleural effusions
- ◆ Jugular venous pressure not elevated and heart sounds normal
- ◆ Mild erythema over right ankle and lower leg
- ◆ Urine dipstick test: protein 4+, no haematuria
- ◆ Urine albumin:creatinine ratio (ACR): 4520 mg/mmol
- ◆ 24-h urinary collection: 6.8 g proteinuria
- ◆ Serum albumin: 13 g/litre
- ◆ Serum creatinine: 82 µmol/litre
- ◆ Autoimmune and hepatitis serology: negative
- ◆ Renal ultrasonography and venous Doppler: normal
- ◆ Doppler ultra sonography of right leg: normal
- ◆ Renal biopsy: membranous nephropathy with subepithelial spikes on silver stain
- ◆ Membranous nephropathy with nephrotic syndrome
Frothy urine, oedema, and hypoalbuminaemia indicate the onset of heavy proteinuria and nephrotic syndrome. The rapid onset of symptoms suggests a primary glomerular lesion rather than long-standing diabetic nephropathy. The presence of leg erythema may be due to infection or deep venous thrombosis, hence a Doppler ultrasound scan was requested. To make the diagnosis, a renal biopsy was performed, which showed membranous nephropathy. The patient was initially managed conservatively with diuretics and low-molecular-weight heparin as thromboembolic prophylaxis.
Nephrotic syndrome indicates the presence of glomerular disease. Causes can usefully be divided into primary glomerular diseases and those arising secondary to systemic disease (Bullet list 8), with the geographical context important in determining the most likely cause in any particular case. The most common cause of nephrotic syndrome in Western countries is diabetes mellitus, whereas in developing countries it is most commonly associated with infection. Nephrotic syndrome due to malaria and hepatitis are particularly common in sub-Saharan Africa, and poststreptococcal glomerulonephritis is also an important cause.
Bullet list 8 Causes of nephrotic syndrome
- • Minimal change
- • Focal segmental glomerulosclerosis (FSGS)
- • Membranous
- • Mesangiocapillary glomerulonephritis (MCGN)
- • Gold, penicillamine, NSAIDs, captopril, heroin
- • Poststreptococcal glomerulonephritis
- • Hepatitis B and C
- ◆ Pre-eclampsia
- • Nail–patella syndrome
Clinical features
One of the earliest symptoms patients may report is that of frothy urine. This often occurs before the onset of oedema and may be a useful indicator of the onset of heavy proteinuria. As proteinuria develops and serum albumin falls, patients gradually develop oedema. This may be noticed first as periorbital swelling and ‘puffiness’ in the morning, or as ankle swelling in the evening due to the effects of gravity. Worsening leg oedema develops as salt and water retention increases, followed by abdominal distension from ascites. In men, scrotal oedema may be marked and very uncomfortable. Further fluid retention leads to pleural effusions, which are often bilateral but may be unilateral. Patients often feel lethargic, with a loss of appetite and nausea due to associated gut oedema.
Clinical examination of the patient’s volume status may reveal a normal or low jugular venous pressure despite marked oedema. Although rare in untreated adult patients, it is important to identify intravascular volume depletion because the use of high-dose diuretic therapy in this setting may provoke circulatory collapse from hypovolaemia, or less dramatically may further reduce renal perfusion and exacerbate renal dysfunction. Conversely, a raised jugular venous pressure with a low blood pressure may suggest a significant pericardial effusion or underlying amyloid with cardiac involvement.
Patients may also present with complications associated with nephrotic syndrome. Thromboembolism may be difficult to detect clinically. Patients with marked peripheral oedema often have swollen legs of unequal size and associated erythema due to an increased susceptibility to cellulitis. These may mask the signs of deep venous thrombosis. Similarly, subtle symptoms of breathlessness, perhaps suggesting pulmonary embolism, or headache, perhaps suggesting cerebral venous sinus thrombosis, may be overlooked. In practice, a low threshold is required for investigation and treatment of suspected thromboembolism.
The combination of severe peripheral oedema and susceptibility to infection following skin breakdown often leads to cellulitis. Long-standing hypoalbuminaemia may lead to leuconychia. Severe hyperlipidaemia, which is a feature of nephrotic syndrome, may lead to cutaneous xanthomas.
Establishing a clinical diagnosis of nephrotic syndrome is often straightforward. The clinical history and examination may also provide clues to an underlying cause, which may be clear, such as in a patient with long-standing diabetes and progressive diabetic nephropathy. Alternatively, the immediate cause may only become apparent after a detailed history revealing long-standing use of drugs that may precipitate the condition (e.g. ACE inhibitors, NSAIDs, gold, or penicillamine). A history of chronic infections (such as hepatitis) may suggest an underlying membranous or mesangiocapillary glomerulonephritis, whereas a rash and arthralgia may lead to a diagnosis of an autoimmune condition such as systemic lupus erythematosus or cryoglobulinaemia. The presence of other long-standing inflammatory conditions, such as rheumatoid arthritis, raises the possibility of systemic amyloidosis. In older patients, an associated malignancy remains a possibility and should be sought in the history and examination, but does not warrant further investigation apart from a chest radiograph in the absence of clinical clues, e.g. disturbance of bowel habit would merit imaging of the colon. Very occasionally, a family history may reveal an inherited nephrotic syndrome such as familial focal segmental glomerulosclerosis.
Rapidly progressive glomerulonephritis with acute renal failure
Around 5% of cases of acute renal failure are caused by a rapidly progressive glomerulonephritis (RPGN). Recognizing this relatively small group of patients is important because many of the causes respond well to treatment, provided the diagnosis is made early and treatment started promptly. The key to making a diagnosis is having a high index of clinical suspicion such that important features of the syndrome are identified (see Bullet list 9for an example).
Bullet list .9 Case illustration—ANCA-associated vasculitis
An 80-year-old woman presents with a 2-week history of increasing malaise and lethargy. On close questioning she also reported arthralgia in the small joints of her hands, and numbness in her hands and feet in the last few months.
Examination
- ◆ Subtle purpuric rash on both legs
- ◆ Bibasal crepitations
- ◆ Reduced pinprick sensation in a glove and stocking distribution
- ◆ Creatinine: 854 µmol/litre (56 µmol/litre 10 months before)
- ◆ Urea: 45 mmol/litre
- ◆ Hb: 8.3 g/dl
- ◆ Urine dipstick test: blood 3+, protein 2+
- ◆ Urine microscopy: red cell casts
- ◆ Serological testing: p-ANCA positive, with myeloperoxidase titre 78%
- ◆ Renal biopsy: focal necrotizing glomerulonephritis
- ◆ Acute renal failure due to microscopic polyangiitis (an ANCA-associated vasculitis) with associated peripheral neuropathy
The history is nonspecific, except that the onset of symptoms is recent and suggestive of a systemic disorder. The presence of a purpuric rash makes the diagnosis of vasculitis a possibility. Dipstick testing of the urine and checking the renal function are critical in making the diagnosis of acute renal failure due to an inflammatory condition. Confirmation of a systemic vasculitis is made with a positive p-ANCA and renal biopsy.
The hallmarks of an RPGN are rapidly declining renal function, haematuria and proteinuria on urine dipstick testing, dysmorphic red cells or red cell casts on urine microscopy, and crescentic and focal necrotizing glomerulonephritis on renal biopsy.
An RPGN may present either de novo in a previously well patient or as a complication in a patient known to have a systemic disease (Bullet list 10). Their clinical features may be diverse. Occasionally, patients may present with very few symptoms and signs, except for proteinuria and haematuria with a recent decline in renal function, and at the other end of the spectrum patients may present with severe acute renal failure associated with features of uraemia. Importantly, patients may also present with clinical features of systemic inflammation that indicate an underlying cause for glomerulonephritis. These range from the subtle, such as arthralgia or myalgia, to the florid, such as a purpuric rash, haemoptysis and peripheral neuropathy. Clinical features of specific inflammatory diseases associated with an RPGN are detailed in Table 1 below.
Bullet list 10 Causes of a rapidly progressive glomerulonephritis
- • Wegener’s granulomatosis
- • Microscopic polyangiitis
- • Churg–Strauss syndrome
- ◆ Other primary systemic vasculitides (ANCA-negative)
- • Cryoglobulinaemia
- • Henoch–Schönlein purpura
- • Postinfectious glomerulonephritis
- • Infective endocarditis
- ◆ Antiglomerular basement membrane disease (Goodpasture’s syndrome)
- • Mesangiocapillary glomerulonephritis
In practice, specific features to elicit in patients presenting with an acute decline in renal function include arthralgia and arthritis, myalgia and muscle tenderness, rashes, eye symptoms (pain and redness), ear, nose, and throat symptoms (epistaxis, nasal crusting, and new deafness), haemoptysis (important, as may be life-threatening if severe), and neuropathic symptoms and signs. Conversely, the clinician should have a high index of suspicion for an RPGN in patients presenting with any of the above features, and in this context suspicions are heightened by the presence of dysmorphic red cells and red cell casts on urinary microscopy.
If an RPGN is suspected, then investigations should include ANCA, antiglomerular basement membrane (anti-GBM) antibodies, antinuclear and anti-double-stranded DNA antibodies, serum complement, antistreptolysin-O titre, and immunoglobulins and serum electrophoresis (including tests for cryoglobulins). It is almost certain that a patient with an RPGN will require a renal biopsy to confirm the diagnosis and to guide management, and thus all patients with suspected RPGN should be referred urgently to a nephrologist.
Presentation of renal disease associated with other underlying diseases
Renal disease is capable of presenting in many complex and diverse ways, and many renal problems arise as either direct or indirect complications of other disease. Examples include acute renal failure caused by sepsis, and progressive chronic kidney disease due to diabetes (Bullet list 11. This section illustrates some common and important presentations of renal disease.
Bullet list 11 Important and common presentations of renal disease
- ◆ Diabetic nephropathy with progressive chronic kidney disease
- • Renal atheroemboli
- • Renal artery stenosis
- • Urinary tract obstruction
- • Hypercalcaemia
- • Acute presentation with renal failure: (1) general syndromes—sepsis, rhabdomyolysis, haemolytic uraemic syndrome, postinfectious glomerulonephritis, tubulointerstitial nephritis; (2) specific syndromes—hantavirus, leptospirosis, malaria
- • Chronic infections associated with renal disease: hepatitis B, hepatitis C, filaria, schistosomiasis, HIV
- • Sarcoidosis
- ◆ Drug-induced renal disease
- ◆ Pregnancy
Diabetic nephropathy
In the Western world, diabetes is the most common cause of renal disease, accounting for 43% of endstage renal failure in the United States of America and 19% in the United Kingdom (Bullet list 12). Diabetic nephropathy develops over the course of years and is preceded by a clinically silent phase of microalbuminuria, which is often detected as a result of diabetic screening programmes, enabling a targeted approach to management in which tight glycaemic control and blood pressure control with the use of agents to block the renin–angiotensin system aim to reduce the rate of progression of the nephropathy. As with other causes of progressive CKD, patients with diabetic nephropathy often only develop symptoms of kidney disease late in the course of their disease, but there is a tendency for those with this condition to become symptomatic, particularly in relation to anaemia and fluid retention, with lesser impairment of renal function than their nondiabetic counterparts. This leads to an earlier requirement for initiation of dialysis in patients with diabetic nephropathy.
Bullet list 12 Case illustration—progressive chronic kidney disease due to diabetic nephropathy
A 66-year-old Asian man presents with nausea, anorexia, ankle swelling and breathlessness. He has a 25-year history of type 2 diabetes mellitus, a 14-year history of hypertension, and had coronary artery bypass grafts 3 years ago. Insulin, furosemide, and ramipril are his only regular medications.
- ◆ Cardiovascular—blood pressure 167/88 mmHg, jugular venous pressure +3 cm, cardiomegaly, bibasal crepitations, and peripheral oedema to sacrum
- ◆ Fundi—treated diabetic retinopathy
- ◆ Neurological—reduced pinprick sensation in stocking distribution to knees, with absent ankle reflexes, proprioception, and vibration sensation
- ◆ Urine albumin:creatinine ratio (ACR): 1720 mg/mmol
- ◆ Serum creatinine: 568 µmol/litre (eGFR 9 ml/min per 1.73 m 2 body surface area)
- ◆ Serum bicarbonate: 15 mmol/litre
- ◆ Full blood count: Hb 9.8 g/dl
- ◆ Hb A1C : 10.2%
Five years previously his blood pressure was 189/92 mmHg with creatinine of 154 µmol/litre and protein+ on urine dipstick. At the time of his coronary surgery, blood pressure was 165/86 mmHg with creatinine 210 µmol/litre. Over the last year he had felt well until the last 2 months, since when he had developed increasing lethargy, anorexia, and breathlessness on exertion, and noted increasing ankle swelling.
This man with long-standing diabetes presents with nonspecific symptoms and oedema. He also has evidence of end-organ damage, with cardiovascular disease, retinopathy, and neuropathy. Five years ago he had evidence of nephropathy with proteinuria and an eGFR of 43 ml/min (CKD stage 3). Since then his blood pressure and glycaemic control have been poor, which contributed to the progression of nephropathy to eGFR of 30 ml/min (CKD stage 3/4) 3 years ago, and now to CKD stage 5 with symptoms of uraemia.
Screening patients with diabetes for microalbuminuria and hypertension enables early diagnosis of complications and intensive management of glucose and blood pressure. As eGFR falls to 30 ml/min per 1.73 m 2 body surface area, patients should be referred to a nephrologist to plan for renal replacement therapy.
Patients with diabetes are also subject to develop other microvascular and macrovascular complications, which may lead to superimposed renal atheroembolic disease and renal artery stenosis. These may present as an abrupt decline in renal function following the introduction of an ACE inhibitor or angiotensin receptor antagonist. Patients with diabetic nephropathy are also at increased risk of acute or chronic renal failure, with common causes for this including use of radiocontrast media for investigations such as coronary or peripheral angiography, surgery (especially cardiac surgery), and, in the context of diabetic emergencies, particularly diabetic ketoacidosis.
See here for a detailed article about diabetic renal disease .
Many patients with diffuse atherosclerosis have evidence of renovascular disease, and a history of cerebrovascular, coronary, or peripheral vascular disease makes a diagnosis of renovascular disease likely. Up to 24% of patients presenting with peripheral vascular disease have stenoses in both renal arteries, and up to 50% have more than 50% stenosis in at least one renal artery. The absence of peripheral pulses and the presence of a femoral bruit make the diagnosis of renovascular disease extremely likely, although most of these patients remain asymptomatic from the renal point of view. Common presentations of renovascular disease are outlined in Bullet list 13
Bullet list 13 Common presentations of renovascular disease
- ◆ As part of the investigation for acute, severe, or refractory hypertension
- ◆ An acute rise (>20%) in creatinine following introduction of an ACE inhibitor or angiotensin receptor antagonist
- ◆ Incidental finding of asymmetric kidney size on renal ultrasound
- ◆ As part of the investigation for progressive CKD.
- ◆ Symptomatically as acute (‘flash’) pulmonary oedema in the absence of cardiac failure or fluid overload
- ◆ Postoperative acute renal failure, especially following coronary artery bypass or aortic aneurysm surgery
Myeloma and other malignancies
Myeloma can cause acute renal failure in several ways. Features suggestive of underlying myeloma in a patient presenting with unexplained renal failure are older age, bone pain (often nonspecific), hypercalcaemia (sometimes mild, and sometimes ‘relative’ considering the degree of renal impairment), anaemia (often inappropriately severe for the degree of renal impairment), abrupt decline in renal function after relatively minor prerenal ‘insult’, and unremarkable urine dipstick.
Up to 50% of patients presenting with myeloma have impaired renal function at the time of diagnosis. This may be reversible and due to hypercalcaemia, dehydration, hyperuricaemia, or infection. Cast nephropathy accounts for 10% of all renal dysfunction in patients with myeloma and is characterized by the formation of tubular casts of excreted light chains and Tamm–Horsfall protein: these are thought to cause renal failure by obstructing the tubule and by direct tubular toxicity.
The key to the diagnosis is to maintain a high index of suspicion, particularly in elderly patients presenting with renal failure and hypercalcaemia. Serum electrophoresis and urinary Bence Jones proteins are the required investigations, followed—if either is positive—by bone marrow examination.
Other malignancies may present with renal involvement due to a number of mechanisms, including acute renal failure due to urinary tract obstruction by pelvic or retroperitoneal tumour. Other possible causes are outlined in Bullet list 14
Bullet list 14 Renal presentations associated with malignancy
- • Tumour lysis with urate nephropathy
- • Chemotherapy (e.g. cisplatin, ifosfamide)
- • Leukaemic infiltration
- • Microangiopathy
Renal presentation of infectious diseases
A wide range of systemic infections, resulting in either acute renal failure or chronic kidney disease, can affect the kidney. The presentation of infection-related kidney disease varies worldwide, and in the developing world—in contrast to the developed world—infectious diseases are the leading cause of acute and chronic kidney disease.
Acute renal failure may occur as part of a general systemic syndrome induced by infection, such as sepsis and septic shock, haemolytic uraemic syndrome (HUS), rhabdomyolysis, postinfectious glomerulonephritis, and tubulointerstitial nephritis. Alternatively, an infectious agent may cause specific nephrotoxicity, e.g. hantavirus, leptospirosis, or malaria.
General systemic syndromes caused by infection
In Western countries, the most common infectious cause for renal disease is sepsis, which accounts for 10% of all hospital-acquired renal failure and, if severe, may lead to acute renal failure in the context of multiorgan failure.
Other general syndromes that may be induced by infection include HUS and rhabdomyolysis. For example, the verotoxin of Escherichia coli O157:H7 causes (D+) HUS, which is a thrombotic microangiopathy characterized by diarrhoea, acute renal failure, and thrombocytopenia. Patients with influenza, legionella, or streptococcal infection may present with fever, severe myalgia, and dark urine in the context of acute renal failure due to rhabdomyolysis.
Poststreptocccal glomerulonephritis is still one of the most common causes of acute renal failure in the developing world, although now seen rarely in the United Kingdom and developed countries. Typical presentation is 10 days to a few weeks following a streptococcal infection of the throat or skin with a ‘nephritic’ syndrome characterized by hypertension, oedema, haematuria and proteinuria, and acute renal failure.
Specific nephrotoxicity caused by infection
Hantavirus and leptospirosis.
Hantaviruses are endemic in specific rodent reservoirs and are transmitted to man by inhalation of infectious aerosols or rodent excreta. In Europe, the main pattern of disease is haemorrhagic fever with renal syndrome (HFRS). The disease presents in four stages: (1) an abrupt febrile stage characterized by fever, loin or abdominal pain, nausea, vomiting, and periorbital oedema, lasting for 3 to 7 days; (2) a hypotensive phase associated with haemorrhages and ecchymoses, lasting hours to 2 days; (3) an oliguric phase for 3 to 14 days, with worsening acute renal failure due to a tubulointerstitial nephritis and haemorrhage; and (4) a polyuric phase as renal function returns to normal.
Leptospirosis may present with similar features to hantavirus. However, leptospirosis is endemic worldwide and is typically associated with jaundice and hepatomegaly. Acute renal failure occurs in 20 to 85% of patients owing to tubulointerstitial nephritis.
Severe infection with Plasmodium falciparum occurs in nonimmune adults. Acute renal failure may occur either in the acute phase of the disease or in the recovery phase. Sequestration of parasitized erythrocytes in the renal vasculature and proinflammatory cytokine release cause tubular cell ischaemia and injury. Rarely, patients with falciparum malaria present with ‘blackwater fever’ due to massive intravascular haemolysis, which often occurs following quinine administration in association with glucose-6-phosphate dehydrogenase deficiency.
Infections and chronic kidney disease
In the developing world, CKD is commonly secondary to infectious disease, with the underlying infection often remaining subclinical until the presentation with renal manifestations. Examples of CKD secondary to infective agents include hepatitis B, hepatitis C, Plasmodium malariae , filaria, schistosomiasis, and HIV.
Hepatitis B is classically associated with nephrotic syndrome due to membranous nephropathy, but occasionally it may result in a mesangiocapillary glomerulonephritis. Hepatitis B virus infection is associated with the development of polyarteritis nodosa, although the reported frequency of this appears to be falling. Patients who develop such complications usually have chronic hepatitis, having contracted the virus in childhood.
Hepatitis C is increasingly recognized as a common cause for cryoglobulinaemia, but this remains asymptomatic in most patients, with only a few developing clinical evidence of vasculitis. The associated mesangiocapillary glomerulonephritis can present as nephrotic syndrome or chronic kidney disease.
Many infectious agents endemic in sub-Saharan Africa may also cause a mesangiocapillary glomerulonephritis presenting as nephrotic syndrome: most common is P. malariae , but filaria and schistosomiasis remain in the differential diagnosis.
HIV-associated nephropathy (HIVAN) is an increasingly recognized complication of HIV infection, and it now accounts for 1% of the dialysis population in the United States of America. Patients usually present with heavy proteinuria and nephrotic syndrome due a collapsing form of focal segmental glomerulosclerosis. HIVAN predominates in young African-American men and is rare in areas endemic for HIV.
Systemic inflammatory diseases
Patients with systemic inflammatory disease are at risk of developing renal disease. Sometimes this may be the presenting feature of the condition, such as systemic vasculitis or systemic lupus, and on other occasions renal disease may develop as a complication later in the course of disease. Examples of systemic inflammatory diseases associated with renal involvement are detailed in Bullet list 15. The presentation of an acute glomerulonephritis and progressive renal failure due to systemic inflammatory diseases such as the vasculitides and systemic lupus erythematosus has been discussed earlier in the chapter, and further details can be found in Chapters 21.10.2 (vasculitides) and 21.10.3 (renal involvement in rheumatological disorders). Other inflammatory diseases may present in different ways.
Bullet list 15 Systemic inflammatory diseases associated with renal involvement
- • Systemic sclerosis
- • Relapsing polychondritis
- • Ankylosing spondylitis
- • Behçet’s disease
- • Amyloidosis (of AA type)
Sarcoidosis
Renal disease is common in sarcoidosis and characterized histologically by granulomatous tubulointerstitial nephritis. The mean prevalence from biopsy studies is 35%, but this is likely to be an overestimate. Most renal disease is subclinical, but may be identified by the presence of proteinuria or tubular dysfunction with a renal tubular acidosis. However, sarcoidosis may present with acute renal failure, which may be caused by an acute tubulointerstitial nephritis associated with an eosinophilia and eosinophiluria, or be precipitated by hypercalcaemia, which may be more common in summer months owing to ultraviolet light exposure. The presence of extrarenal features of sarcoidosis (including bilateral hilar lymphadenopathy and erythema nodosum) helps to establish the diagnosis, but sometimes the diagnosis may only become apparent following a renal biopsy for unexplained renal impairment and measurement of serum ACE.
Systemic sclerosis
Systemic sclerosis may present with an acute crisis characterized by an abrupt rise in blood pressure (>160/90 mmHg) with hypertensive encephalopathy, acute renal failure, and a microangiopathic haemolytic anaemia. This may occur before the onset of the cutaneous features of the disease. Patients are typically tachycardic, with evidence of heart failure and a high systemic vascular resistance. This diagnosis should be suspected in any patient presenting with malignant-phase hypertension and acute renal failure.
Systemic amyloidosis
Systemic amyloidosis is characterized by extracellular deposition of insoluble fibrillar proteins that lead to organ dysfunction. In AL amyloidosis this arises from light chains produced by a malignant plasma cell clone. AA amyloidosis occurs in the setting of long-standing inflammation, the amyloidogenic protein being an N-terminal fragment of serum amyloid A (SAA), an acute phase reactant. Patients may present with heavy proteinuria or nephrotic syndrome. Renal involvement can be demonstrated by serum amyloid P (SAP) scanning or by renal biopsy.
Drug-induced renal disease
Numerous drugs have the potential for causing both acute renal failure and chronic kidney disease (Bullet list 16). As well as prescribed and over-the-counter medication, renal disease may also arise from herbal and traditional Chinese medicines or illicit drugs. Mechanisms by which drugs cause renal disease include salt and water depletion, effects on renal perfusion, direct nephrotoxicity, and intrarenal obstruction.
Bullet list 16 Adverse effects of drugs on the kidney
- • Aminoglycosides
- • Amphotericin
- • Cisplatin
- • Radiocontrast media
- • Paracetamol poisoning
- • Statins (by inducing rhabdomyolysis)
- • β-Lactam antibiotics (penicillins, cephalosporins)
- • Furosemide
- • Allopurinol
- • Azathioprine
- • Sulphonamides
- • Aristolochic acid (‘Chinese herb nephropathy’)
- • High-dose captopril
- • Penicillamine
- • Phenytoin
- • Pamidronate
- • Acetazolamide
- • Demeclocycline
- • Aspirin with phenacetin
- • Aciclovir
- • Indinavir
- • Methotrexate
Salt and water depletion
Acute renal failure may follow hypotension and reduced renal perfusion due to sodium and water depletion. This may be caused by excess diuretic use or diarrhoea and vomiting as a drug side effect.
Effect on renal perfusion and the regulation of intrarenal haemodynamics
Overdose of any hypotensive agent may compromise renal perfusion and interfere with renal function. Agents which block the renin–angiotensin system, the ACE inhibitors and angiotensin receptor blockers, require special consideration. These agents abrogate the effect of angiotensin II on efferent arteriole constriction, which is the normal adaptive response to any reduction in renal perfusion. A small and acceptable deterioration in renal function (up to a 20% increase in serum creatinine) often occurs following the introduction of an ACE inhibitor. A greater increase in serum creatinine may indicate underlying renal artery stenosis, for which further investigation may need to be carefully considered, and these agents are implicated in a significant proportion of cases of acute renal failure in patients with sepsis and dehydration. This is because renal perfusion is frequently compromised in these settings and the kidney is unable to autoregulate its blood flow in the presence of renin–angiotensin system blockade.
NSAIDs have numerous renal effects, including disturbances of autoregulation of intrinsic renal haemodynamics. These effects are mediated by inhibition of prostaglandin synthesis from arachidonic acid by nonspecific blocking of the enzyme cyclooxygenase. This may lead to vasoconstriction and reversible renal impairment in volume-contracted states. Long-term use of NSAIDs may cause chronic renal impairment, with selective COX-2 inhibitors seeming to confer no renal advantage.
Direct nephrotoxicity
The mechanisms of drug toxicity on the kidney include direct tubulotoxicity, drug-induced tubulointerstitial nephritis, tubular dysfunction, and glomerular disease. Common nephrotoxic drugs and mechanism are outlined in Bullet list 16.
Tubulointerstitial nephritis is classically caused by penicillins and NSAIDs, but most drugs have the potential to cause the condition. Drug-induced tubulointerstitial nephritis usually occurs days to weeks after starting the drug. Symptoms may be nonspecific, with malaise, fatigue, and anorexia. A low-grade fever, fleeting rash, and arthralgia may also be reported. Investigations show a variable degree of renal dysfunction along with proteinuria and microscopic haematuria. Urine microscopy may show white and red cell casts, and there may be a blood eosinophilia. However, in practice the key is to suspect the diagnosis, stop the potentially offending drug (or drugs), and proceed with a renal biopsy if renal function does not improve.
Obstruction
Specific drugs, such as aciclovir and the protease inhibitor indinavir, may precipitate as crystals within the tubule, causing obstruction and sometimes renal failure. This is more likely to occur if the patient is dehydrated, hence adequate fluid input to achieve a high urinary volume is advised before these drugs are taken.
Pregnancy provides a unique set of circumstances in which renal disease may present. Renal disease may be present before pregnancy and be detected as part of screening for proteinuria and hypertension during the first trimester. Alternatively, de novo renal disease may be precipitated by pregnancy and present with specific syndromes, such as nephrotic syndrome or acute renal failure due to pre-eclampsia. A summary of the presentation of renal disease in pregnancy is detailed in Bullet list 17.
The clinical presentation of renal disease during pregnancy is often varied and nonspecific, but certain features may guide the diagnosis. Key points to note are as follows:
- ◆ Is there evidence of pre-existing renal disease?
- ◆ Were previous pregnancies complicated by hypertension or pre-eclampsia (PET)?
- ◆ What was the time of onset of renal disease during pregnancy? Onset in late pregnancy implies that the renal disease is likely to be pregnancy induced.
- ◆ Hypertension with proteinuria and oedema suggest PET or underlying renal disease (e.g. systemic lupus erythematosus).
- ◆ Hypotension and hypovolaemia suggest sepsis or haemorrhage.
Bullet list 17 Renal disease in pregnancy
- • Hyperemesis
- • Haemorrhage
- • Abruption
- • Pyelonephritis
- • Septic abortion
- • Puerperal sepsis
- • Gravid uterus
- • Pre-eclampsia
- • Acute fatty liver of pregnancy
- • Syndrome of haemolysis, elevated liver enzymes and low platelets (HELLP)
- • Haemolytic uraemic syndrome (HUS)
- ◆ Any chronic kidney disease with deterioration of function, proteinuria, and pre-eclampsia
- ◆ Flare of systemic and renal disease (systemic lupus erythematosus and systemic sclerosis)
- ◆ Systemic lupus erythematosus
- ◆ Minimal change disease
Pre-eclampsia and HELLP
Pre-eclampsia classically presents with hypertension, oedema, and proteinuria. Other recognized features include elevation of serum urate, liver transaminases, and haematocrit, along with thrombocytopenia. However, patients may not be hypertensive or demonstrate other features, hence distinguishing pre-eclampsia from pre-existing renal disease may be difficult, and the condition may occasionally present with heavy proteinuria and nephrotic syndrome. The HELLP syndrome (haemolysis, elevated liver enzymes, and low platelets) is a severe variant of pre-eclampsia that is commonly associated with renal failure, severe haemolysis, and coagulopathy, and may progress to multiorgan failure. See Chapters 14.4 (hypertension in pregnancy) and 14.9 (liver and gastrointestinal disease in pregnancy) for further discussion.
Haemolytic uraemic syndrome and thrombotic thrombocytopenic purpura (HUS/TTP) are related disorders that are occasionally associated with pregnancy and can both cause acute renal failure. HUS usually occurs 2 days to 10 weeks postpartum and may cause severe renal failure. By contrast, TTP usually presents in the first or second trimester with predominant neurological features and only mild proteinuria and haematuria.
Conditions and Diseases
Highlighted article.
Preparing for pregnancy
Why is being ready for pregnancy so important?
Conception occurs about 2 weeks before your period is due. That means you may not even know you're pregnant until you're more than 3 weeks pregnant. Yet your baby is most sensitive to harm 2 to 8 weeks after conception. This is when your baby's organs (such as the heart) begin to form. Anything you eat, drink, smoke or are exposed to can affect your baby. That's why it's best to start acting as if you're pregnant before you actually are.
Articles about various types of diet:
- A balanced diet
- Acid reflux diet
- Anti-aging diet
- Anti-cancer diet
- Anti-candida diet
- Diet for constipation
- Diet for stress
- A blood pressure diet
- Diet to reduce cancer risk
- Diets: a general overview
- GERD diet - in detail
- GERD diet - top ten tips
- GERD diet plan and table
- Gluten-free diet -labelling and shopping - with tables
- Gluten-free diet - eating and dining
- Gluten-free diet - eating healthily - about nutrition and diet
- Nutrition in pregnancy
- Peptic ulcer diet in brief
- Table of anti-cancer foods
- The eatwell plate
- Tips and guidelines for healthy eating
- Weight loss and heartburn
- Keeping salt out of your diet
Herbal Treatments
Complementary medicine - commonly used herbal therapies and herbal substances - links to article:
- St Johns Wort
- St Johns Wort for Depression and Anxiety
- Treat anxiety and insomnia with Californian poppy, hops and passion flower
Miscellaneous Detailed Articles
- Atherosclerosis biology and pathology
- Bioterrorism
- Cardiac involvement in genetic disease (e.g. Marfan's Syndrome)
- Coronary heart disease - influences acting in utero and early childhood
- Iron metabolism
- Chest pain, breathlessness and fatigue
- Hormones and the gastrointestinal tract
- Vitamins and trace elements
Alzheimer's Disease
- Alzheimer's Disease - main section and non-technical article
- Detailed technical article about dementia - includes Alzheimer's disease technical section
- Alzheimer's disease in detail -technical
- Alzheimer's detected decades before symptoms
- Acute Abdominal Pain
- Chest Pain, Breathlessness and Fatigue
- Chronic pain
- Dysmenorrhoea
- Fibroid Pain
- Pelvic Pain
- Vaginal Discharge
Section about medical treatments - links to articles:
- Coronary artery bypass surgery
- Lipid lowering drugs
- Pain management
- Percutaneous interventional cardiac procedures (PCI and others)
- Psychopharmacology
- Robotic surgery
- Stable angina management and treatment
- Tinnitus treatment
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Nephrol
- v.2021; 2021

Clinical Presentation, Renal Histopathological Findings, and Outcome in Patients with Monoclonal Gammopathy and Kidney Disease
Gaetano alfano.
1 Surgical, Medical and Dental Department of Morphological Sciences, Section of Nephrology, University of Modena and Reggio Emilia, Modena, Italy
2 Nephrology Dialysis and Transplant Unit, University Hospital of Modena, Modena, Italy
Alice Delrio
3 General Medicine, Azienda Unità Sanitaria Locale, Modena, Italy
Francesco Fontana
Giacomo mori, silvia cazzato, annachiara ferrari, rossella perrone, silvia giovanella, giulia ligabue, riccardo magistroni, gianni cappelli, associated data.
The data will be shared on request to the corresponding author. Please contact Dr. Gaetano Alfano (e-mail: [email protected] ) to request them.
Monoclonal gammopathies are associated with acute and chronic kidney injury. Nephrotoxicity of the secreted monoclonal (M)-protein is related to its biological properties and blood concentration. Little is known about epidemiology, clinical manifestations, and outcome of monoclonal gammopathies in patients with kidney disease. We retrospectively collected data about demographics, clinical manifestations, and renal histological lesions of all patients ( n = 1334) who underwent kidney biopsy between January 2000 and March 2017. Monoclonal gammopathy was detected in 174 (13%) patients with a mean age of 66.4 ± 13.1 years. The spectrum of monoclonal gammopathies comprised monoclonal gammopathy of undetermined significate (MGUS) (52.8%), multiple myeloma (MM) (25.2%), primary amyloidosis (AL) (9.1%), smoldering MM (SMM) (4%), non-Hodgkin lymphoma (NHL) (6.8%), and Hodgkin lymphoma (HL) (1.7%). Monoclonal gammopathy of renal significance (MGRS) accounted for 6.5% in patients with MGUS and 14.2% in patients with SMM. Evaluation of kidney biopsy revealed that M-protein was directly involved in causing kidney injury in MM (93.1%). MM was the only gammopathy significantly associated with an increased risk of kidney injury (odds ratio [OR] = 47.5, CI 95%, 13.7–164.9; P ≤ 0.001). While there were no significant differences in the progression toward end-stage renal disease or dialysis ( P = 0.776), monoclonal gammopathies were associated with a different risk of death ( P = 0.047) at the end of the follow-up. In conclusion, monoclonal gammopathy was a frequent finding (13%) in patients who underwent kidney biopsy. M-protein was secreted by both premalignant (56.8%) and malignant (43.2%) lymphoproliferative clones. Kidney biopsy had a key role in identifying MGRS in patients with MGUS (6.5%) and SMM (14.2%). Among monoclonal gammopathies, only MM was significantly associated with biopsy-proven kidney injury. The rate of end-stage renal disease or dialysis was similar among monoclonal gammopathies, whereas NHL, MM, and SMM showed a higher rate of deaths.
1. Introduction
Monoclonal gammopathy is a clinical condition characterized by the presence of an abnormal protein—known as monoclonal (M)-protein or paraprotein—in the blood [ 1 ]. M-protein is an intact antibody, or any chain fragment produced and secreted by a pathological lymphoproliferative clone. The ability of M-protein to disrupt cellular homeostasis is unpredictable. It is principally related to its physicochemical properties and blood concentration [ 2 ].
Historically, renal toxicity of the M-protein has been associated with the malignancy of the underlying lymphoproliferative disorder. Multiple myeloma (MM), one of the most common hematologic malignancies [ 3 ], has been widely associated with kidney disease [ 4 , 5 ]. Renal injury in this disease relies principally on the overproduction of M-protein. This, freely filtered by the glomerulus, overwhelms tubular reabsorption capacity leading to intraluminal precipitation with tubular obstruction and activation of inflammatory pathways [ 6 ].
Other malignant lymphoproliferative diseases, such as Waldenström macroglobulinemia (WM) [ 7 ] and lymphomas [ 8 , 9 ], have been frequently associated with renal injury and can present with a wide range of glomerular lesions including M-immunoglobulin deposition disease, proliferative glomerulonephritis with M-immunoglobulin deposit, and cryoglobulinemic vasculitis. Even clones, which have a low propensity to progress toward malignancy and secrete a low amount of M-protein, can be involved in tissue damage, including neuropathy and autoimmune diseases as well as kidney diseases [ 10 ]. In this setting, tissue deposition of M-protein (direct mechanism) or activation of the complement system without tissue deposition M-protein (indirect mechanism) may lead to kidney injury [ 11 ]. Recently, the nephrological community has coined a new term: monoclonal gammopathy of renal significance (MGRS). The definition of MGRS includes all small B-cell or plasma cell clones that, per se, do not meet strict hematological criteria for cytoreductive therapy but are implied in kidney injury through the production of M-protein [ 12 ]. The most glaring example is monoclonal gammopathy of undetermined significate (MGUS), which may be associated with renal lesions despite the low measureable levels of secreted paraprotein and the rare progression to MM [ 13 ]. The growing interest in this new pathological entity has spurred nephrologists and hematologists to reevaluate the pathogenicity of these small and apparently indolent clones. The enthusiasm to understand the causative role of M-protein in patients with renal impairment was tempered by the rarity of the phenomena. Considering these limits and the fragmentation of the data published in the literature, we explored the association between monoclonal gammopathies and kidney disease with the aim to define prevalence and clinical manifestations as well as outcomes of these disorders in a cohort of patients who underwent kidney biopsy for renal impairment.
2. Material and Methods
We conducted a retrospective study at the Nephrology Unit of the University Hospital of Modena. The medical charts of all patients with biopsy-proven kidney disease were evaluated from January 2000 to March 2017. Among all patients who underwent kidney biopsy, we enrolled only those with a diagnosis of serum M-protein that was detected by protein electrophoresis (SPEP) and subsequently characterized by serum or urine immunofixation. Hence, patients with an unconfirmed diagnosis of monoclonal gammopathies were excluded from the study. The study protocol was approved by the Provincial Ethics Committee of the University Hospital of Modena (CE/1476).
2.1. Renal Biopsy
Biopsy specimens were examined using light microscopy (LM) and immunofluorescence (IF). Kidney tissue sections have been evaluated by hematoxylin and eosin, periodic acid-Schiff (PAS), periodic acid-methenamine silver (Jones), and Masson's trichrome stains. For immunofluorescence, cryostat sections were stained with fluorescein isothiocyanate-conjugated rabbit anti-human IgG, IgM, IgA, C3, C1q, kappa ( k ), and lambda ( λ ) light chain. Staining with Congo red was used to confirm amyloid deposits. Primary amyloidosis or light chain amyloidosis (AL) was identified by light chain restriction using IF or immunohistochemistry performed in another center. Electron microscopy examination was performed only on pathologists' indication. Monoclonal gammopathy was considered directly involved in the pathogenesis of renal lesions if histological examination revealed immunoglobulin-associated kidney lesions with heavy or light chain restriction on IF (restriction for the same immunoglobulin isotype detected in the blood). Cast nephropathy, M-immunoglobulin deposition disease, membranoproliferative glomerulonephritis with M-immunoglobulin deposit, and primary amyloidosis or light chain amyloidosis (AL) were part of the group of M-protein-associated kidney diseases [ 11 ].
2.2. Data Collection and Definition
Demographics and laboratory data were collected at the time of kidney biopsy. Data about complete blood count (leukocytes, erythrocytes, hemoglobin, and platelets), serum calcium, serum creatinine (sCr), estimated glomerular filtration (eGFR) (calculated using CKD-EPI equation) [ 14 ], proteinuria, serum and urine M-protein, serum M-protein concentration, and serum-free light chain (FLC) were extracted from medical records.
Proteinuria was principally estimated through the urine protein-to-creatinine ratio.
Nephrotic syndrome was defined as urine protein-to-creatinine ratio greater than 3 mg/mg and serum albumin less than 3.5 gr/dl. We considered AL amyloidosis and light chain deposition disease associated with MM in the presence of lytic bone lesions and other signs of over MM or plasma cells count greater than 30% in the bone marrow [ 15 ].
Acute kidney injury (AKI) referred to an abrupt decrease in kidney function. The definition is based on the following criteria: increase in sCr by ≥0.3 mg/dL within 48 hours, or increase in sCr level to ≥1.5 times from baseline [ 16 ]. The urinary criterium was not used for the diagnosis of AKI. Baseline sCr corresponded to the last sCr before kidney biopsy. When it was not available, we considered sCr measured at hospital admission.
Severe impairment of renal function referred to acute worsening of renal function with a serum sCr level ≥3 mg/dL.
Bone marrow biopsy was performed according to the hematologist's indications after identification and characterization of the serum M-protein, radiologic workup, and flow cytometry.
2.3. Statistical Analysis
Continuous variables with normal distribution were presented as mean and standard deviation (SD). The difference between the means of two groups was performed with a two-sample t -test or Mann–Whitney test.
One-way ANOVA and Kruskal–Wallis test (or one-way ANOVA on ranks) were used to perform multiple comparisons between groups. Tukey's test and χ2 test or Fisher's exact test were used to determine the statistical difference between the mean of all possible pair and categorical data, respectively. Logistic regression analysis was done to compute odds ratios (OR), 95% confidence intervals (95% CI), and P values of lymphoproliferative disorders in predicting direct kidney injury. Logistic regression was also used to evaluate the association between histological findings in MM patients and severe renal impairment. P value <0.05 was considered to be statistically significant. All analyses were performed using SPSS version 23 (SPSS, Inc., Chicago, IL).
3.1. Patient's Characteristics
We reviewed the charts of 1334 patients who underwent native kidney biopsy for renal dysfunction. Monoclonal gammopathy was found in 174 patients (13%) with a mean age of 66.4 ± 13.1 years. Most of them were of Caucasian origin (96%), and males were predominant over females (67.2 vs. 32.8%) ( Table 1 ). Compared to the entire study population, monoclonal gammopathy was more frequent in patients aged 50–79 years ( Table 2 ).
Demographics and clinical characteristics of patients with monoclonal gammopathy.
HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; MGUS, monoclonal gammopathy of indeterminate significance; MM, multiple myeloma; SD, standard deviation; SMM, smoldering multiple myeloma.
Range of age in patients with monoclonal gammopathy.
HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; MGUS, monoclonal gammopathy of indeterminate significance; MM, multiple myeloma; SMM, smoldering multiple myeloma.
The hematologic disorders presenting with M-protein in peripheral blood were MGUS (52.8%), MM (25.2%), AL amyloidosis (9.1%), non-Hodgkin lymphoma (NHL) (6.8%), smoldering MM (SMM) (4%), and Hodgkin lymphoma (HL) (1.7%) ( Table 1 and Figure 1 ). The detection of M-protein was associated with 56.8% of premalignant lymphoproliferative diseases and 43.2% of malignant diseases. There were no differences ( P = 0.16) between the age of patients with benign (5.3 ± 13.7 years) and malignant lymphoproliferative diseases (67.5 ± 11.9 years).

Distribution of monoclonal gammopathy in our cohort of patients.
MGUS was the most common monoclonal gammopathy. Its prevalence was estimated to be 52.8% among patients with serum M-protein.
The mean age of patients was 64.9 ± 13.9 years with predominance of males of 62% ( Table 3 ).
Clinical characteristics and lab examinations of patients with monoclonal gammopathy.
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; FLC, free light chain; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; MGUS, monoclonal gammopathy of indeterminate significance; MM, multiple myeloma; M-protein, monoclonal protein; sCr, serum creatinine; SD, standard deviation; SMM, smoldering multiple myeloma. The symbols ∗,#,† , and ‡ indicate statistical significance ( P ≤ 0.05) among the single variables.
In 16 (17.3%) patients, the histological evaluation revealed glomerular lesions compatible with a membranoproliferative glomerulonephritis pattern. It was secondary to viral hepatitis in 4 subjects. Further kidney diseases included membranous glomerulopathy (16.3%), end-stage kidney disease (ESRD) (16.3%), ANCA-associated vasculitis (8.6%), postinfectious glomerulonephritis (7.6%), focal segmental glomerular sclerosis (5.4), and interstitial nephritis (4.3%). Surprisingly, IgA glomerulonephritis was underrepresented in this group (1%).
In four patients (4.3%), MGUS was directly involved in the development of kidney injury through the deposition of light chains. In two cases, IF analysis showed intact immunoglobulin restriction in a setting of membranoproliferative pattern of glomerular injury. Overall, parenchymal lesions compatible with MGRS criteria accounted for 6.5% of all cases of MGUS.
At the end of the observational period of 5.4 years, 37% of patients died ( Table 4 ) and 32% were on replacement renal therapy ( Table 5 ).
Case fatality rate in patients with monoclonal gammopathy.
Rate of end-stage renal disease or dialysis in patients with monoclonal gammopathy.
∗ Missing data = 13%. CKD, chronic kidney disease; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; MGUS, monoclonal gammopathy of indeterminate significance; MM, multiple myeloma; SMM, smoldering multiple myeloma.
3.3. Multiple Myeloma
MM was the second most common gammopathy in our study population. The disorder was detected in 44 subjects with a mean age of 66.9 ± 12.9 years. MM was more frequent in males than in females (72.7 vs. 27.3%). According to the criteria CRAB (hypercalcemia, renal disease, anemia, and bone disease), laboratory tests at presentation revealed serum calcium of 9 ± 1.1 mg/dl, hemoglobin of 10.2 ± 1.6 gr/dl, myeloma bone lesions in 75% of patients, and average sCr of 4.3 ± 2.9 mg/dl, corresponding to an eGFR of 28.4.7 ± 28.9 ml/min ( Table 3 ). Plasma cells infiltrate in bone marrow biopsy accounted for 50% of the cells. The majority of the patients (81.8%) was admitted with severe impairment of renal function and 27.2% needed urgent kidney replacement therapy. In seven patients (15.9%), clinical manifestation of kidney disease was nephrotic syndrome with average urine protein-to-creatinine ratio ranging from 4.2 to 18.5 mg/mg associated with a wide variability of renal function (sCr ranged from 1.01 to 6 mg/dl).
Light chain MM accounted for 34.1% of the cases and, as expected, k light chain MM was more prevalent compared to λ light chain MM (60% vs. 40%). M-protein isotypes were IgGk (22.7%), IgG λ (15.9%), IgAk (6.8%), IgA λ (13.6%), and IgMk (6.8%). Bence Jones protein was detected in 93.1% of the tested patients.
Histological evaluation of kidney biopsy specimens showed cast nephropathy (68.1%), AL amyloidosis (15.9%), light chain deposition disease (6.8%), and interstitial nephritis (9.1%). Cast nephropathy was the only histological lesion associated with severe renal impairment (OR = 26.2, 95% CI, 2.8–245.5; P = 0.004) (Supplemental Table 1 ). Lastly, all patients with histological diagnosis, different from cast nephropathy, had bone osteolytic lesions compatible with myeloma bone disease. The case fatality rate was high (52.3%) and more than one-third of the patients were on ESRD (38.2%) at the end of the follow-up period (Tables (Tables4 4 and and5 5 ).
3.4. Smoldering Multiple Myeloma
Seven (4%) patients had a diagnosis of SMM. The disorder manifested at a mean age of 71.8 ± 11.9 years and showed a slightly higher prevalence in men than women (72.7% vs. 27.3%).
According to the definition of SMM, bone lytic lesions were absent in all patients. Hemoglobin and serum calcium were in the normal range, 12.3 ± 2.3 gr/dl and 8.6 ± 0.8 mg/dl, respectively ( Table 3 ). At presentation, mean sCr was 2.7 ± 2.9 mg/dl (eGFR of 43.5 ± 30.3 ml/min) with proteinuria of 1.4 ± 1.1 mg/mg.
Immunofixation of the serum M-protein detected the following isotypes: IgG λ (28.2%), IgGk (28.2%), IgMk (14.1%), IgA λ (14.1%), and k light chain (14.1%). Bence Jones protein was found in 71.4% of the patients. Bone marrow biopsy revealed a mean plasma cell count of 18%.
Evaluation of renal biopsies showed different patterns of glomerular diseases including membranoproliferative glomerulonephritis (28.5%), interstitial nephritis (14.2%), light chain deposition disease (14.2%), acute tubular necrosis (ATN) (14.2%), ANCA-negative vasculitis (14.2%), and membranous glomerulonephritis (14.2%). Light chain restriction was diagnosed only in one patient (14.2%) affected by membranoproliferative glomerulonephritis.
3.5. Hodgkin Lymphoma
Three patients (1.12%) had a diagnosis of HL at an average age of 69.04 ± 5.3 years. All patients had a normal renal function manifesting with a mean sCr of 0.93 ± 0.07 mg/dl corresponding to an eGFR of 62.7.3 ± 7.4 ml/min. Mean urine protein-to-creatinine ratio was 0.3 ± 0.2 mg/mg ( Table 3 ). Mild proteinuria was present in only one patient (urine protein-to-creatinine ratio of 0.5 mg/mg). Bence Jones protein was present in only one patient. Cryoglobulinemic glomerulonephritis was found in two-thirds of the patients (66.6%) and hypertensive nephrosclerosis in one (33.3%).
3.6. AL Amyloidosis
Amyloidosis, defined as primary amyloidosis or AL amyloidosis, was diagnosed in 16 patients (9.1%). Amyloidosis was secondary to MGUS (75%) and SMM (25%). The mean age of the affected subjects was 66.34 ± 11.38 years and females were slightly more prevalent than males (53 vs. 46%).
sCr ranged from 0.5 to 4.5 mg/dl with a mean level of 1.4 gr/dl, corresponding to 56.5 ml/min of eGFR. Nephrotic syndrome was the most common presentation (75%). Overall, patients presented with high proteinuria (8.33 ± 3.2 mg/mg) associated with hypoalbuminemia (2.74 ± 0.84 gr/dl) ( Table 3 ).
The diagnosis of amyloidosis was performed by the detection of deposit of amorphous material in the mesangium and capillary loops of glomeruli. Congo red stain confirmed the diagnosis of amyloidosis and immunohistochemical analysis identified the corresponding serum light chain.
3.7. Non-Hodgkin's Lymphoma
Twelve patients (6.8%) with monoclonal gammopathy had a diagnosis of NHL, whose term includes several heterogeneous lymphoproliferative disorders. According to the WHO classification [ 17 ], lymphoplasmacytic lymphoma accounted for 41.6%, Waldenstrom's macroglobulinemia for 30.7%, marginal zone lymphoma for 15.2%, diffuse large B-cell lymphoma for 7.6%, and anaplastic large cell lymphoma for 7.6%. Male gender was fully associated (100%) with NHL in our cohort of patients. The average age of subjects was 72.6 ± 9.6 years. Renal function was extremely variable at presentation, with sCr ranging between 0.85 and 6.35 mg/dl; mean sCr was 2.4 ± 1.6 mg/dl corresponding at an eGFR of 30.4 ± 22.7 ml/min. Five out of 12 patients had nephrotic syndrome at hospital admission. Daily proteinuria ranged from 0.7 to 8.2 mg/mg, and mean proteinuria was 4.36 ± 3.36 mg/mg/24 hours.
IgMk(66.6%) was the most common monoclonal protein, whereas IgAλ, IgGk, and IgM λ accounted for 8.33%. A patient with marginal zone lymphoma had a circulating biclonal M-protein.
NHL subjects had a circulating M-protein of 0.6 ± 0.4 gr/dl. Urine monoclonal component was found in 68.7% of patients.
Histological evaluation of biopsy specimen revealed amyloidosis (25%), glomerular injury with membranoproliferative pattern (16.6%), LCDD (25%), ANCA-associated vasculitis (8.3%), cast nephropathy (8.3%), and hypertensive nephrosclerosis (8.3%). In one case (8.3%) Ig deposits were restricted for the same serum M-protein in a context of a membranoproliferative pattern of glomerular injury.
3.8. Comparison between Groups
Kruskal–Wallis test showed that mean serum sCr levels ( P ≤ 0.0001), eGFR ( P =0.004), proteinuria ( P ≤ 0.042), serum calcium ( P ≤ 0.0001), serum albumin ( P ≤ 0.0001), white blood count ( P ≤ 0.006), and hemoglobin ( P ≤ 0.002) were statistically different between the groups ( Table 3 ).
Lymphoproliferative diseases were variably associated with renal lesions due to M-protein. Documented M-protein-associated kidney injury accounted for 58.3%, 6.5%, 91.3%, 14.1%, and 100% in patients with NHL, MGUS, MM, SMM, and AL amyloidosis, respectively ( Table 6 ). Excluding AL amyloidosis, regression analysis showed that MM was significantly associated with a 47.5-fold increased risk of renal lesions (95% CI, 13.7–164.9; P ≤ 0.001) .
Association between monoclonal gammopathies and direct kidney injury.
HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; MGUS, monoclonal gammopathy of indeterminate significance; MM, multiple myeloma; SMM, smoldering multiple myeloma. AL amyloidosis was not tested because the pathogenesis of amyloidosis depends on a proven direct kidney injury due to the deposition of light chain within the renal parenchyma. P value for MGUS was statistically but not clinically significant. The protective effect of MGUS in developing kidney disease is not applicable in this context.
The rate of ESRD or dialysis ( P = 0.74) and death ( P = 0.11) was not statistically significant in patients with monoclonal gammopathies at the end of follow-up. There was a trend toward high mortality in patients with SMM (71.4%), NHL (66.7%), and MM (52.3%).
There were no statistically significant differences in crude case-rate fatality ( P = 0.113) and incidence of ESRD or dialysis ( P = 0.751) among groups with monoclonal gammopathies. Kaplan–Meier survival analysis revealed a statistically significant difference in the survival of patients with monoclonal gammopathy ( P = 0.047) ( Figure 2 ) and confirmed that there were no differences in the incidence of ESRD or dialysis ( Figure 3 ).

Overall survival according to the diagnosis of monoclonal gammopathy.

Death-censored kidney survival (CKD 5 or dialysis) according to the diagnosis of monoclonal gammopathy.
4. Discussion
The recent literature has placed great emphasis on the pathogenic role of monoclonal gammopathy as a potential cause of kidney disease. Our study showed that monoclonal gammopathy was a frequent diagnosis (13%) in patients with renal impairment who underwent kidney biopsy. Monoclonal gammopathy occurred predominantly in subjects aged more than 50 years with a peak over 70 years. In our cohort of patients, we diagnosed MGUS, SMM, NHL, LH, MM, and AL amyloidosis. MGUS was the most common disorder. It accounted for more than half (52.8%) of all monoclonal gammopathies. Nevertheless, the prevalence rate of malignant lymphoproliferative disorders was surprisingly high in our cohort of patients since MM, HL, and NHL accounted for 42.8% of all gammopathies.
Besides the malignancy of these disorders, the nephrotoxicity of M-protein should be considered when evaluating monoclonal gammopathy. M-protein, may be extremely harmful to renal parenchyma, even though it is secreted by an indolent clone. Etiological mechanisms of M-protein nephrotoxicity are strictly dependent on the idiosyncratic properties of the secreted paraprotein. Deposition of M-protein [ 18 ] and activation of complement [ 19 ] are the leading pathological processes underlying the onset of monoclonal gammopathy-associated renal lesions [ 2 ].
According to the recent definition of MGRS [ 12 ], renal lesions due to the interplay with circulating M-protein were detected in 6.5% and 14.1% of MGUS and SMM patients, respectively. Histological lesions compatible with MGRS included light chain deposition diseases and proliferative glomerulonephritis with M-immunoglobulin deposits. While light chain deposition disease is known to be associated with the deposition of circulation of M-protein [ 20 ], little is known about the role of M-protein in promoting membranoproliferative glomerulonephritis [ 21 ]. This latter histologic pattern has been frequently encountered in patients with MGUS, but it is not uncommon in chronic lymphocytic leukemia, lymphomas, and MM [ 21 ]. Deposition of secreted monotypic immunoglobulin protein along the capillary walls and the activation of the complement system (both classical and alternative pathway) are believed to be the main triggers for the development of the membranoproliferative pattern of glomerular damage [ 21 ].
It is worth noting that the histological detection of glomerular lesions with membranoproliferative patter is not sufficient to meet the diagnosis of MGRS. The identification of the restricted circulating immunoglobulin in renal parenchyma by immunofluorescence (or immunoperoxidase) and transmission electron microscopy is a practical and effective way to demonstrate direct M-protein nephrotoxicity [ 12 ]. Although the membranoproliferative pattern of glomerular injury was the predominant histopathological finding in MGUS and SMM patients, we found a few cases with Ig-restriction, corresponding to about one-tenth of all patients with these lesions.
Among all lymphoproliferative disorders, MM was significantly associated with direct kidney injury ( P ≤ 0.0001). The etiological mechanism underlying kidney dysfunction was the production of a great amount of M-protein directly involved in the pathogenesis of myeloma-associated kidney disease. The majority of the patients with MM (81.8%) were admitted with AKI requiring renal replacement treatment in about a third of cases. In line with previous native renal biopsy studies [ 22 , 23 ], cast nephropathy was the most prevalent histopathological finding (68.1%), and as expected, it was also significantly associated with severe renal impairment. Interestingly, tubulointerstitial nephritis, a rare renal manifestation of MM, was found to be 9% of all cases [ 24 ].
Lymphoma can be associated with kidney involvement presenting with a wide spectrum of manifestations. Lymphocytic infiltration of the parenchyma is the most prevalent finding in the largest case series of autopsies [ 25 ]. Further kidney manifestations rely on several distinct malignancy-related mechanisms and include minimal change disease, amyloidosis, membranoproliferative glomerulonephritis, immunotactoid glomerulopathy, and M-protein deposition disease. In the setting of HL, presentation of kidney involvement in our series was mild proteinuria with normal renal function, but the limited number of cases does not allow us to generalize thesedata. On the other hand, renal function was extremely variable in NHL patients, ranging from normal renal function to acute kidney failure. Glomerulonephritis with membranoproliferative-like patterns and M-immunoglobulin deposition disease were the most common histological findings in this group of patients. Similar to the literature, glomerular involvement with membranoproliferative glomerulonephritis [ 26 , 27 ] and M-protein deposition disease [ 9 , 28 , 29 ] was common in patients with NHL [ 9 , 30 ].
AL amyloidosis represented only a small percentage (8.9%) of all monoclonal gammopathies. AL amyloidosis was characterized by the deposition of light chain deposition in the renal parenchyma of all renal biopsy specimens [ 31 ]. Our results confirmed the high prevalence of λ light chain isotype. AL amyloidosis manifested with a nephrotic syndrome characterized by a significantly higher level of proteinuria than other gammopathies. Renal function was not severely impaired and showed only a slight increase in sCr.
At the end of the follow-up, the evolution of renal function was extremely heterogeneous. The rate of ESRD or dialysis ranged from 0% (HL) to 38.2% (MGUS) without statistically significant differences among the groups of patients. In particular, renal outcome of MM patients was less dramatic than the initial stage of the disease. Recovery of renal function occurred in many of them, and the prevalence of ESRD or dialysis did not increase after 4.4 ± 5 years of follow-up. Seven subjects with MGRS had a different renal prognosis at the end of the observation period; indeed in only two cases, CKD progressed to renal failure.
Survival of patients with malignant gammopathies was poorer than patients with a premalignant clone. However, multiple factors (underlying kidney disease, disease-specific therapies, and supportive care) may have influenced the outcomes of these patients. In particular, the prognosis of patients with malignant disorders is changed in the past 10–15 years with the administration of promising therapeutic strategies such as proteasome inhibitor bortezomib, monoclonal antibodies, and the immunomodulatory drugs such as thalidomide and lenalidomide [ 32 , 33 ].
In clinical practice, the workflow process for the assessment of monoclonal gammopathy-associated renal lesions is based on the identification of the hematological disorder and underlying nephropathy. Once M-protein has been identified and characterized, exclusion of a malignant disorder should remain a high priority among nephrologists, as the outcome of the patient is associated with a poor prognosis if left untreated. Diagnostic tests such as flow cytometry, bone marrow biopsy, and radiological examinations should have a low threshold if there is a high suspicion for lymphoproliferative disease. Evaluation of renal function trajectory and urinary abnormalities is essential to assess renal function. Kidney biopsy has a crucial role in the diagnosis of the underlying hematological disorder and renal injuries driven by M-protein. Lastly, kidney biopsy carries important therapeutic and prognostic implications in subjects with MGRS, as this condition is associated with a concerning poor renal outcome and with a high rate of recurrence after renal transplantation [ 34 ].
The main limitations of the study are the retrospective analysis, the different follow-up duration, and the small sample size of certain groups of patients with rare monoclonal gammopathies (especially HL and SMM) that do not allow us to generalize our results. The not routine use of electron microscopy has potentially led to the underestimation of some cases of MGRS and points out an unintentional bias frequently present in the current literature. The data collected over 17 years underlines the difficulty to categorize some kidney biopsies reporting a diagnosis of “membranoproliferative glomerulonephritis.” This term now refers to a histological pattern of glomerular lesions rather than a diagnosis of kidney disease. To avoid misclassification, we classified all the diagnosis of “membranoproliferative glomerulonephritis” with the term “glomerular injury with membranoproliferative pattern.”
5. Conclusion
Lymphoproliferative disorders secreting M-protein carry a different potential for kidney injury. MGUS is the most frequent monoclonal gammopathy (52,8%) among patients who undergo kidney biopsy. Although MGUS has a low propensity to progress toward malignant disease, it is related to the development of MGRS (6.5%). MM is significantly associated with renal impairment and commonly manifesting with severe impairment of renal function. Patients diagnosed with AL amyloidosis had a higher level of proteinuria compared to the other monoclonal gammopathies. Careful evaluation is mandatory to identify malignant monoclonal disorders and MGRS because both conditions require specific chemotherapy treatment and have a different prognosis compared to other monoclonal gammopathies.
Acknowledgments
The authors thank Dr. Luciana Furci and Marco Leonelli for their remarkable expertise in the field of kidney pathology and all healthcare workers that have delivered their support for cancer patients and their families.
Data Availability
Ethical approval.
The study has been approved by the Ethical Committee of Emilia Romagna and has been conducted in accordance with the Declaration of Helsinki.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Supplementary Materials
Supplemental Table 1: prediction of severe renal impairment (serum creatinine ≥ 3 mg/dl) according to renal histological findings found in kidney biopsy of MM patients.
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Health and medicine
Course: health and medicine > unit 7.
- What is diabetes mellitus?
- Breaking down diabetes
- Types of diabetes
- Pathophysiology - Type I diabetes
- Pathophysiology - Type II diabetes
- Diagnosing diabetes
- Treating type I diabetes
- Treating type II diabetes - Pharmacology
- Treating type II diabetes - A practical approach
- Acute complications of diabetes - Diabetic ketoacidosis
- Acute complications of diabetes - Hyperosmolar hyperglycemic nonketotic state
- Diabetic nephropathy - Mechanisms
Diabetic nephropathy - Clinical presentation & treatment
- Diabetic retinopathy
Want to join the conversation?
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page
Video transcript
Kidney damage relates to agonal bacteremia: a single-center retrospective study
- Original article
- Published: 20 March 2024
Cite this article
- Yumiko Mikami 1 ,
- Meiko Ogawa 2 ,
- Yuuki Hayasaka 1 ,
- Asuka Yamakami 1 ,
- Kanako Hattori 1 ,
- Chizumi Fukazawa 1 ,
- Takafumi Ito 3 ,
- Naoki Kanomata 2 &
- Hiroyuki Terawaki ORCID: orcid.org/0000-0001-9165-551X 1 , 3
24 Accesses
Explore all metrics
Agonal bacteremia, diagnosed with postmortem positive blood culture results, is considered a possible contributing factor to death. We hypothesized that some premortem organ damage, such as kidney damage, can enhance agonal bacteremia.
We performed a postmortem blood and alveolar fluid culture study in 30 cadavers and evaluated the relationship between blood culture results and clinical parameters, including organ damage (brain, heart, lung, kidney, liver and gastrointestinal tract).
A total of 23 cases (76.7%) were positive for blood culture; the number of cultured species was one in 12 cases, two in 7 cases, and three in 4 cases. The ratio of agonal bacteremia was significantly higher in patients with heart damage (100%, n = 13) and those with kidney damage (end-stage kidney damage, acute kidney injury, obstructive kidney failure, or metastatic kidney tumours) (100%, n = 13). The mean number of cultured species was 0.67 ± 0.98 in heart or kidney damage, 1.40 ± 0.55 in heart damage only, 1.40 ± 0.55 in kidney damage only, and 2.00 ± 0.93 in heart and kidney damage. As the number of damaged organs increased (0 organs, no heart/kidney damage; 1 organ, heart or kidney damage; and 2 organs, heart and kidney damage), the mean number of cultured species increased significantly ( p for trend = 0.001964).
Premortem kidney damage relates to agonal bacteremia.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Data availability
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding authors.
Morris JA, Harrison LM, Partridge SM. Postmortem bacteriology: a re-evaluation. J Clin Pathol. 2006;59:1–9. https://doi.org/10.1136/jcp.2005.028183 .
Article CAS PubMed PubMed Central Google Scholar
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8. https://doi.org/10.1038/bmt.2012.244 .
Article CAS PubMed Google Scholar
Koneman EW, Mincker TM, Shires DB, De Jonch DS. Postmortem bacteriology: II. Selection of cases for culture. Am J Clin Pathol. 1971; 55(1):17–23. https://doi.org/10.1093/ajcp/55.1.17 .
Wood WH, Oldstone M, Schultz RB. A re-evaluation of blood culture as an autopsy procedure. Am J Clin Pathol. 1965;43:241–7. https://doi.org/10.1093/ajcp/43.3.241 .
Li J, Jia H, Cai X, et al. An integrated catalog of reference genes in the human gut microbiome. Nature Biotechnol. 2014;32:834–41. https://doi.org/10.1038/nbt.2942 .
Article CAS Google Scholar
Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979;23(2):403–11. https://doi.org/10.1128/iai.23.2.403-411.1979 .
Marshall JC, Christou NV, Meakins JL. The gastrointestinal tract; the undrained abscess of multiple organ failure. Ann Surg. 1993;218:111–9. https://doi.org/10.1097/00000658-199308000-00001 .
Sedman PC. The prevalence of gut translocation in humans. Gastroenterology. 1994;107:643–9. https://doi.org/10.1016/0016-5085(94)90110-4 .
Burn CG. Postmortem bacteriology. J Infect Dis. 1934;54(3):395–403. https://doi.org/10.1093/infdis/54.3.395 .
Article Google Scholar
Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol. 2019;16(3):137–54. https://doi.org/10.1038/s41569-018-0108-7 .
Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270(5239):1203–7. https://doi.org/10.1126/science.270.5239.1203 .
Article ADS CAS PubMed Google Scholar
Szeto CC, Kwan BC, Chow K, et al. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2008;3(2):431–6. https://doi.org/10.2215/CJN.03600807 .
Terawaki H, Yokoyama K, Yamada Y, et al. Low-grade endotoxemia contributes to chronic inflammation in hemodialysis patients: examination with a novel lipopolysaccharide detection method. Ther Apher Dial. 2010;14(5):477–82. https://doi.org/10.1111/j.1744-9987.2010.00815.x .
Article PubMed Google Scholar
de Almeida Duarte JB, de Aguilar-Nascimento JE, Nascimento M, Nochi RJ Jr. Bacterial translocation in experimental uremia. Urol Res. 2004;32(4):266–70. https://doi.org/10.1007/s00240-003-0381-7 .
Okumura R, Kurakawa T, Nakano T, et al. Lypd8 promotes the segregation of flagellated microbiota and colonic epithelia. Nature. 2016;532(7597):117–21. https://doi.org/10.1038/nature17406 .
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. https://doi.org/10.1056/NEJMoa041031 .
Nakayama M, Metoki H, Terawaki H, et al. Kidney dysfunction as a risk factor for first symptomatic stroke events in a general Japanese population–the Ohasama study. Nephrol Dial Transplant. 2007;22(7):1910–5. https://doi.org/10.1093/ndt/gfm051 .
Yoon JW, Pahl MV, Vaziri ND. Spontaneous leukocyte activation and oxygen-free radical generation in end-stage renal disease. Kidney Int. 2007;71:167–72. https://doi.org/10.1038/sj.ki.5002019 .
Terawaki H, Yoshimura K, Hasegawa T, et al. Oxidative stress is enhanced in correlation with renal dysfunction: examination with the redox state of albumin. Kidney Int. 2004;66(5):1988–93. https://doi.org/10.1111/j.1523-1755.2004.00969.x .
Terawaki H, Hayashi T, Murase M, et al. Relationship between xanthine oxidoreductase redox and oxidative stress among chronic kidney disease patients. Oxid Med Cell Longev. 2018;2018:9714710. https://doi.org/10.1155/2018/9714710 .
Terawaki H, Takada Y, Era S, et al. The redox state of albumin and serious cardiovascular incidence in hemodialysis patients. Ther Apher Dial. 2010;14(5):465–71. https://doi.org/10.1111/j.1744-9987.2010.00841.x .
Terawaki H, Matsuyama Y, Matsuo N, et al. A lower level of reduced albumin induces serious cardiovascular incidence among peritoneal dialysis patients. Clin Exp Nephrol. 2012;16(4):629–35. https://doi.org/10.1007/s10157-012-0610-x .
Higuchi C, Ito M, Masakane I, Sakura H. Peritonitis in peritoneal dialysis patients in Japan: a 2013 retrospective questionnaire survey of Japanese Society for Peritoneal Dialysis member institutions. Ren Rep Ther. 2016;2:2. https://doi.org/10.1186/s41100-016-0014-6 .
Wei M, Wang Z, Liu H, et al. Probiotic Bifidobacterium animalis subsp. lactis Bi-07 alleviates bacterial translocation and ameliorates microinflammation in experimental uraemia. Nephrology (Carlton). 2014;19(8):500–6. https://doi.org/10.1111/nep.12272 .
Sun L, Liu H, Jiang H, et al. Macrophages are involved in gut bacterial translocation and reversed by Lactobacillus in experimental uremia. Dig Dis Sci. 2016;61(6):1534–44. https://doi.org/10.1007/s10620-015-3950-z .
Download references
Acknowledgements
The authors thank Dr. Genji Izumi for critical review and advice.
Author information
Authors and affiliations.
Clinical Laboratory Department, St. Luke’s International University, 9-1 Akashi-Cho, Chuo-City, Tokyo, Japan
Yumiko Mikami, Yuuki Hayasaka, Asuka Yamakami, Kanako Hattori, Chizumi Fukazawa & Hiroyuki Terawaki
Department of Pathology, St. Luke’s International Hospital, Tokyo, Japan
Meiko Ogawa & Naoki Kanomata
Department of Nephrology, Teikyo University Chiba Medical Hospital, Chiba, Japan
Takafumi Ito & Hiroyuki Terawaki
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the conception of the study. YM, NK, and HT designed the study. MO and NK collected samples for culture, and YM, YH, and AY performed the culture procedure under the supervision of KH and CF. YM created the initial database, and HT performed the statistical analyses. TI reviewed the manuscript thoroughly and gave critical suggestions. All authors have read and approved the final manuscript, which was mainly modified by HT.
Corresponding author
Correspondence to Hiroyuki Terawaki .
Ethics declarations
Conflict of interest.
The authors have declared that no conflict of interest exists.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (IRB approval number 23-R061, the institutional review board of St. Luke’s International University) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent (Consent for publication)
Informed consent was not obtained from individual patients because the laboratory data used in this study were extracted from routine examination files and analyzed retrospectively. However, we posted the research content at the hospital and gave all participants the opportunity to refuse to participate.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (XLSX 17 KB)
About this article.
Mikami, Y., Ogawa, M., Hayasaka, Y. et al. Kidney damage relates to agonal bacteremia: a single-center retrospective study. Clin Exp Nephrol (2024). https://doi.org/10.1007/s10157-024-02485-8
Download citation
Received : 15 November 2023
Accepted : 04 March 2024
Published : 20 March 2024
DOI : https://doi.org/10.1007/s10157-024-02485-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Agonal bacteremia
- Heart failure
- Chronic kidney disease
- Find a journal
- Publish with us
- Track your research
NKF Launches KidneyCARE™ Study To Empower Patients, Advance Kidney Disease Research

Groundbreaking interactive patient registry will help empower individuals with kidney disease to help pave the way for improved outcomes in kidney care
(March 20, 2024, New York, NY) — The National Kidney Foundation (NKF) proudly announces the launch of the KidneyCARE (Community Access to Research Equity)™ Study, a cutting-edge online research initiative combining patients’ insights on the impact of living with kidney disease and patient health data to improve and advance kidney disease research.
With more than one in 7 Americans affected by kidney disease and nearly one in 3 at risk of developing it, the need for innovative solutions and comprehensive support is critical. The KidneyCARE Study provides hope for those navigating the challenges of kidney disease, offering a transformative platform to enhance kidney care and advance research efforts. The Study will collect both rigorous clinical and laboratory data from electronic health records (EHR), in addition to patient-entered data, which together allow for a complete picture of the patient experience. This model is innovative in that most research initiatives follow one path or the other—EHR or patient self-reporting. The Study will compile data on demographics, medical history, lifestyle, medications, blood and urine test results, in addition to extensive data on patient perceptions, challenges, and priorities.
People with all types of kidney disease can join the KidneyCARE Study to share how kidney disease impacts their overall health and daily living. Participants in the Study will benefit by receiving kidney health education, access to clinical trial opportunities, and peer support information.
"This type of research is key to the future of patient-centered kidney care," said Kevin Longino, CEO of the National Kidney Foundation and a kidney transplant recipient. "In addition to collecting data on patient outcomes over time, the study aims to provide a lifeline of support and resources tailored to the unique needs of each participant, paving the way for improved outcomes."
NKF partnered with HHS Technology Group , (HTG) to utilize HTG’s proven data analytics platform, Discover Your Data (DyD®), as the foundation of the online patient registry, alongside the expertise of HTG’s subcontractor partners, Datavant, Inc. and Mathematica, Inc. DyD is an end-to-end platform solution that allows healthcare payers, nonprofit and public sector organizations, pharma, researchers, academic medical centers, and providers to translate big questions into deep understanding by seamlessly connecting to the nation’s largest health data ecosystem and allowing for organizations to bring their own data.
“We are proud to collaborate with NKF on this important initiative to improve kidney disease education, support, and treatment,” said Brett Furst, President, HTG. “Notably, the registry will include patient-reported outcomes, which are among the most difficult types of data to obtain but provide immense value as an indicator of the efficacy of various kidney disease treatments.”
“We invite individuals living with kidney disease to join us in this groundbreaking initiative," added Longino. "Together, we can drive meaningful change, transform kidney care, and ultimately work towards a future free from the burden of kidney disease."
For more information about the KidneyCARE Study and how to participate, visit kidneycarestudy.org.
About Kidney Disease In the United States, 37 million adults are estimated to have kidney disease , also known as chronic kidney disease (CKD)—and approximately 90 percent don’t know they have it. About 1 in 3 adults in the U.S. are at risk for kidney disease. Risk factors for kidney disease include: diabetes , high blood pressure , heart disease , obesity ,and family history. People of Black/African American, Hispanic/Latino, American Indian/Alaska Native, Asian American, or Native Hawaiian/Other Pacific Islander descent are at increased risk for developing the disease. Black/African American people are more than 3 times as likely as White people to have kidney failure. Hispanics/Latinos are 1.3 times more likely than non-Hispanics to have kidney failure.
About the National Kidney Foundation The National Kidney Foundation is revolutionizing the fight to save lives by eliminating preventable kidney disease, accelerating innovation for the dignity of the patient experience, and dismantling structural inequities in kidney care, dialysis, and transplantation.
Novo kidney trial finds Ozempic cuts cardiac deaths in diabetics
- Novo Nordisk A/S Follow
- Baxter International Inc Follow
- DaVita Inc Follow
Keep up with the latest medical breakthroughs and healthcare trends with the Reuters Health Rounds newsletter. Sign up here.
Reporting by Stine Jacobsen and Maggie Fick, additional reporting by Bhanvi Satija in Bengaluru and Ludwig Burger in Frankfurt; Editing by Josephine Mason, Terje Solsvik, Alexander Smith and Susan Fenton
Our Standards: The Thomson Reuters Trust Principles. , opens new tab
An underground gas storage site in Ukraine was attacked on Sunday in the latest wave of Russian missile strikes on power facilities, while officials restored power in cities, ordered imports and imposed rolling blackouts to deal with shortfalls.

Woodside seeks better terms to develop Trinidad's Calypso gas project
Australian oil and gas producer Woodside Energy would like to see improved fiscal terms from the Trinidad and Tobago government before committing to develop a deepwater natural gas project there, CEO Meg O'Neill told Reuters this week.


Vertex expands kidney disease research as FDA clears clinical trial
V ertex Pharmaceuticals ( NASDAQ: VRTX ) said Thursday the U.S. Food and Drug Administration (FDA) greenlighted a clinical trial for its kidney disease candidate VX-407, further expanding its R&D work related to renal diseases.
Its investigational new drug (IND) application is related to investigations into the potential of VX-407 in autosomal dominant polycystic kidney disease (ADPKD), a hereditary form of kidney disease that can require dialysis and transplantation and can ultimately lead to death.
The company plans to start a Phase 1 trial for the small-molecule candidate in healthy volunteers this month.
Vertex ( VRTX ), a company known for its cystic fibrosis therapies, brings its 10th disease area into the clinic with the IND clearance.
Boston, Massachusetts-based biotech’s pipeline includes oral experimental therapy inaxaplin targeted at APOL1-mediated kidney disease, a chronic kidney disease caused by mutations in the APOL1 gene.
More on Vertex Pharmaceuticals
- Vertex Pharmaceuticals: Diversifying Its Drug Portfolio For Future Growth
- Vertex Pharmaceuticals Incorporated (VRTX) Leerink Partners Global Biopharma Conference (Transcript)
- Vertex Pharmaceuticals Incorporated (VRTX) TD Cowen 44th Annual Health Care Conference (Transcript)
- Merck, Vertex cut from Goldman Sachs conviction list; Amgen added
- Vertex gets EU backing for label expansion of Kalydeco in cystic fibrosis


IMAGES
VIDEO
COMMENTS
Clinical presentation - Patients with CKD may present with symptoms and signs resulting directly from diminished kidney function, such as edema or hypertension. However, many have no clinical symptoms, and kidney disease is often detected in these patients when an elevated serum creatinine, reduced estimated GFR (eGFR), or an abnormal ...
Gastrointestinal symptoms: Anorexia, nausea, vomiting, diarrhea. Chronic kidney disease (CKD)—or chronic renal failure (CRF), as it was historically termed—is a term that encompasses all degrees of decreased renal function, from damaged-at risk through mild, moderate, and severe chronic kidney failure. CKD is a worldwide public health ...
Chronic kidney disease (CKD) is defined as the presence of kidney damage or an estimated glomerular filtration rate (eGFR) less than 60 ml/min/1.73 mt2, persisting for 3 months or more, irrespective of the cause.[1] It is a state of progressive loss of kidney function, ultimately resulting in the need for renal replacement therapy (dialysis or transplantation).
Clinical Presentation. Chronic kidney disease is typically identified through routine screening with serum chemistry profile and urine studies or as an incidental finding. Less commonly, patients may present with symptoms such as gross hematuria, "foamy urine" (a sign of albuminuria), nocturia, flank pain, or decreased urine output. ...
Identify Patients with CKD. Screen people at risk for CKD, including those with. diabetes mellitus type 1 or type 2. hypertension. cardiovascular disease (CVD) family history of kidney failure. The benefit of CKD screening in the general population is unclear. The two key markers for CKD are urine albumin and eGFR.
Signs and symptoms of chronic kidney disease develop over time if kidney damage progresses slowly. Loss of kidney function can cause a buildup of fluid or body waste or electrolyte problems. Depending on how severe it is, loss of kidney function can cause: Nausea. Vomiting.
Chronic kidney disease (newly identified): Clinical presentation and diagnostic approach in adults; Chronic kidney disease and coronary heart disease; Clinical manifestations and diagnosis of urinary tract obstruction and hydronephrosis; Contrast-associated and contrast-induced acute kidney injury: Clinical features, diagnosis, and management
Practice Point 2.2.2: A 2-year kidney failure risk of >10% can be used to determine the timing of multidisciplinary care in addition to eGFR-based criteria and other clinical considerations. Practice Point 2.2.3: A 2-year kidney failure risk threshold of >40% can be used to determine the modality education, timing of preparation for kidney
The first task in evaluating the patient with an elevated creatinine level is to categorize the patient's clinical presentation as 1 of 3 possible types of renal failure: postrenal failure, prerenal azotemia, or intrinsic renal failure. ... Renal vein thrombosis can present as acute kidney failure with flank pain and gross hematuria. Renal vein ...
KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease Kidney Int . 2024 Apr;105(4S):S117-S314. doi: 10.1016/j.kint.2023.10.018.
More than 500,000 people in the United States live with end-stage renal disease (ESRD). The development of chronic kidney disease (CKD) and its progression to this terminal disease remains a significant cause of reduced quality of life and premature mortality.[1] Chronic kidney disease (CKD) is a debilitating disease, and standards of medical care involve aggressive monitoring for signs of ...
Chronic kidney disease (CKD) affects almost 700 million people worldwide and is the cause of 1.9 million cardiovascular deaths annually. 1,2 Over 60% of patients with CKD have a history of cardiovascular disease (CVD), which is also the main cause of death in this population. 3,4 Almost all patients with CKD have a much higher risk for CVD than ...
Chronic kidney disease (CKD) is asymptomatic in its earliest stages (stage I and stage II), although urinalysis findings or blood pressure may be abnormal. As chronic kidney disease progresses to more advanced stages, signs and symptoms greatly increase. Polydipsia and nocturia (secondary to a reduced capacity to concentrate the urine) may be ...
Clinical presentation and outcomes of chronic kidney disease patients with COVID-19 admitted to the intensive care unit of a teaching hospital of Northern India during the third wave of the pandemic: A retrospective study : Journal of Family Medicine and Primary Care ... Chronic kidney disease (CKD) patients have impaired immune status; that ...
Next: Autosomal dominant polycystic kidney disease (ADPKD) is a multisystemic and progressive disorder characterized by cyst formation and enlargement in the kidney (see the image below) and other organs (eg, liver, pancreas, spleen). Up to 50% of patients with ADPKD require renal replacement therapy by 60 years of age.
What is diabetic kidney disease and what do we know so far about its clinical presentation? Diabetes mellitus is the leading cause of chronic kidney disease (CKD) in the USA and worldwide. An estimated 422 million adults are living with diabetes globally, and up to 40% of them may develop CKD during their lifetime [ 1 ].
Alteration of glomerular capillary autoregulation is at the basis of glomerular hypertension, an important pathogenetic mechanism for DKD. Clinical presentation of DKD can vary. Its classical presentation, often seen in patients with type 1 diabetes (T1DM), features hyperfiltration and albuminuria followed by progressive fall in renal function.
Autosomal recessive polycystic kidney disease in children; Clinical presentation and diagnosis of posterior urethral valves; Congenital nephrotic syndrome; Etiology, clinical features, and diagnosis of neonatal hypertension; Identifying newborns with critical congenital heart disease; Investigational biomarkers and the evaluation of acute ...
The clinical presentation of kidney disease. Essentials. Renal disease may present in many ways, including: (1) the screening of asymptomatic individuals; (2) with symptoms and signs resulting from renal dysfunction; and (3) with symptoms and signs of an underlying disease, often systemic, which has resulted in renal dysfunction. ...
This article will review the clinical presentation and pathologic hallmarks of TMA involving the kidney, and the disease-specific mechanisms that contribute to the endothelial injury that characterizes TMA lesions. Diagnostic approach and both empirical and disease-specific treatment strategies will be discussed, along with the potential role ...
Little is known about epidemiology, clinical manifestations, and outcome of monoclonal gammopathies in patients with kidney disease. We retrospectively collected data about demographics, clinical manifestations, and renal histological lesions of all patients ( n = 1334) who underwent kidney biopsy between January 2000 and March 2017.
Presentation of Case. Dr. Helen I. Healy (Pediatrics): A 15-year-old girl was admitted to this hospital during the summer because of acute kidney injury. The patient had been well until 8 days ...
Now fortunately the mechanisms underlying diabetic nephropathy, directly correlate with the clinical presentation. And the first clinical finding of the disease is somewhat paradoxically an increased kidney filtration rate or glomerular filtration rate. So, diabetic nephropathy, if you break down the term into nephro and pathy literally means ...
The results from a recent clinical trial show promise for semaglutide as a treatment to help slow progression of kidney disease and reduce the risk of death from kidney disease or heart disease for people living with diabetic kidney disease.. Semaglutide is a type of glucagon-like peptide-1 (GLP-1) drug that is taken as an injection once a week or as a daily oral medicine (depending on the ...
Acquired cystic kidney disease (ACKD) has been described in nearly every type of kidney disease that causes progressive kidney insufficiency, with the exception of hereditary cystic disorders. The incidence and the number and size of cysts correlate with the number of years the patient is on dialysis. In its early stages, ACKD does not produce ...
Background Agonal bacteremia, diagnosed with postmortem positive blood culture results, is considered a possible contributing factor to death. We hypothesized that some premortem organ damage, such as kidney damage, can enhance agonal bacteremia. Methods We performed a postmortem blood and alveolar fluid culture study in 30 cadavers and evaluated the relationship between blood culture results ...
Groundbreaking interactive patient registry will help empower individuals with kidney disease to help pave the way for improved outcomes in kidney care (March 20, 2024, New York, NY) — The National Kidney Foundation (NKF) proudly announces the launch of the KidneyCARE (Community Access to Research Equity)™ Study, a cutting-edge online research initiative combining patients' insights on ...
Novo Nordisk's widely used diabetes drug Ozempic delayed progression of chronic kidney disease in diabetes patients, a large late-stage study found, cutting the risk of death from that and major ...
In addition to clinical observation, rats were evaluated for markers of kidney function at 24 and/or 72 hours post-surgery, and markers of kidney damage at 72 hours post-surgery.
Vertex Pharmaceuticals (NASDAQ:VRTX) said Thursday the U.S. Food and Drug Administration (FDA) greenlighted a clinical trial for its kidney disease candidate VX-407, further expanding its R&D work ...