An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Weight Management
- Understanding Adult Overweight & Obesity
- Clinical Trials
- Español

Clinical Trials for Overweight & Obesity
NIDDK conducts and supports clinical trials in many diseases and conditions, including overweight and obesity. The trials look to find new ways to prevent, detect, or treat disease and improve quality of life.
What are clinical trials for overweight and obesity?
Clinical trials—and other types of clinical studies —are part of medical research and involve people like you. When you volunteer to take part in a clinical study, you help health care professionals and researchers learn more about disease and improve health care for people in the future.
Researchers are studying many aspects of overweight and obesity, such as
- why some people find it harder than other people to maintain weight loss over time
- new medicines that could help people with obesity lose weight and keep it off
- different treatments that may prevent weight regain after weight-loss surgery, also called metabolic and bariatric surgery
- why storing excess fat in some parts of your body—such as in your abdomen, or belly—rather than other parts of your body may increase the risk of developing health problems such as type 2 diabetes
- how weight-loss surgery may lower long-term health costs for adults with obesity
Find out if clinical studies are right for you .
Watch a video of NIDDK Director Dr. Griffin P. Rodgers explaining the importance of participating in clinical trials.
What clinical studies for overweight or obesity are looking for participants?
You can view a filtered list of clinical studies on overweight or obesity that are federally funded, open, and recruiting at www.ClinicalTrials.gov . You can expand or narrow the list to include clinical studies from industry, universities, and individuals; however, the National Institutes of Health does not review these studies and cannot ensure they are safe. Always talk with your health care provider before you participate in a clinical study.
What have we learned about overweight and obesity from NIDDK-funded research?
NIDDK has supported many research projects to learn more about overweight and obesity.
The Look AHEAD: Action for Health in Diabetes study showed that people who had type 2 diabetes and were overweight or had obesity can lose weight and maintain that weight loss through a program of healthy eating and increased physical activity. The study also showed that weight loss provides other health benefits, such as better physical mobility and improved blood glucose, blood pressure, and cholesterol levels. The trial was extended to study the long-term effects in older adults with type 2 diabetes.
The Longitudinal Assessment of Bariatric Surgery (LABS) study looked at the effects of two types of weight-loss surgery in adults, gastric bypass and adjustable gastric band. LABS found that weight-loss surgery is relatively safe when performed by experienced surgeons. It can also lead to significant weight loss and may improve many weight-related health problems. After 7 years, the average weight loss of patients who had gastric bypass surgery was 84 pounds, or about 28% of their starting weight. The average weight loss of patients who had gastric band surgery was 41 pounds, or about 15% of their starting weight. Because gastric band surgery is less effective than other types of weight-loss surgery , it is not often performed.
This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public. Content produced by NIDDK is carefully reviewed by NIDDK scientists and other experts.
Clinical Trials
Displaying 129 studies
The purpose of this research is to establish the normal range of diameter and distensibility of pylorus and the fasting and postprandial antro-pyloric motility in healthy male and female adults.
The purpose of this study is to determine the turnover rate of motor protein isoforms (myosin heavy chain, MHC) and overall muscle protein in skeletal muscle of subjects with obesity and lean controls in the basal state, and to determine stimulation of the turnover rate of motor protein (MHC isoforms) in skeletal muscle of subjects with obesity and lean controls by plasma amino acids and with and without prior exercise.
The reason we are doing this research is to get information about the ORBERA™ Intragastric Balloon to learn if it is safe and if it works. We want to learn if older teenagers who are overweight will lose weight and if their other medical problems will get better. ORBERA™ is a special balloon approved by the FDA for overweight adults, and we would like to try using it for overweight teenagers.
The aim of this study is to assess the quality of life among a sample of obese liver transplant recipients and compare it between those who underwent sleeve gastrectomy and those who did not.
The primary aim is to study the correlation between changes in the gut microbiome of obese subjects undergoing an intragastric balloon procedure.
This research study is being performed to find out if a new device, AspireAssist Aspiration Therapy System, can help people with obesity to lose weight without causing too many side effects.
A prospective, multicenter, open-label, post-approval study of the safety and effectiveness of ORBERA™ as an adjunct to weight reduction for obese adults (22 years of age and older) with a Body Mass Index (BMI) of ≥ 30 kg/m2 and BMI ≤ 40 kg/m2
The purpose of this study is to demonstrate that the safety of the device in the postmarket setting is comparable to what was observed in the US pivotal study and to more accurately determine the rates of certain serious adverse events so that this information can be used to inform patient labeling.
Subjects in the open label, multi-center study will receive dietary/exercise counseling plus the Spatz3 Adjustable Balloon System for 32 weeks.
Eligible subjects will undergo endoscopy and those without endoscopic contraindications will be implanted with the Spatz3 Adjustable Balloon System for 32 weeks. All subjects ...
The purposes of this study are to: evaluate the feasibility of providing a community based referral to Mayo Clinic ECH patients for weight loss by measuring the number of patients who follow through with the referral, to determine the completion rate of patients enrolled through provider referral to a community based weight loss program, and to determine the level of provider and patient satisfaction with the community based weight loss program process.
The purpose of this study is to investigate the change in quality of life after endoscopic bariatric therapies and to correlate the changes in quality of life with changes in weight after endoscopic bariatric Therapies (EBTs).
The purpose of this project is to complete a chart review/ secondary data analysis during the first 6-month program period utilizing data from the 4291 PARTENHEIMER Obesity Registry and interval participant data from the Mayo360 Personalized Support System (Mayo360PSP) questionnaire to examine motivation and behavioral changes in program participants.
The purpose of this research is to assess the experiences of women with high BMI status in accessing and receiving reproductive care which may include services offered and/or denied and prerequisites for treatment, such as weight loss, and to evaluate the effects of social and clinical weight bias and stigma on women with high BMI status in receiving reproductive care.
It is unknown whether the bile acid pathway reacts differently to weight loss resulting from Roux-En-Y Gastric Bypass (RYGB) surgery than weight loss resulting from caloric restriction alone.
The purpose of this study is to test the effectiveness of an online education course for helping obese patients to reach and keep weight loss goals.
The purpose of this study is to establish a registry database of patients that will be utilized for future research studies related to obesity, health, and weight management strategies.
The purpose of this study is to determine whether impaired insulin-induced suppression of lipolysis (as measured by IC50) is related to lipolysis proteins in groups of volunteers known to vary widely with regards to abdominal adipocyte size and regulation of adipose tissue lipolysis, and whether the improved insulin regulation of lipolysis resulting from treatment with the PPARγ agonist pioglitazone, with or without weight loss, can be linked to specific changes in sets of PPARγ-responsive adipocyte lipolysis proteins in UBO adults.
The purpose of this study is is to assess the feasibility and acceptability of telehealth for increasing access to bariatric surgery and compare outcomes to patients who receive F2F visits.
Investigators are doing this research study to find out the effect of T6 dermatomal electrical stimulation (delivered by a Transcutaneous Electrical Nerve Stimulation (TENS) unit) on appetite and weight loss.
The purpose of this study is to assess the effects of Peptamen Intense on body weight in tube-fed adults with obesity.
The purpose of this study is to explore the effectiveness and safety of a modified gastroplication technique for weight loss in five patients with obesity, and includes physiologic appraisal of gastric emptying (via gastric emptying breath test) and post-prandial gastric accommodation (via SPECT).
The purpose of this study is to determine if culturally-relevant interventions will decrease obesity in underserved Hispanic children and help those children learn lifelong healthy habits.
The purpose of this study is to assess changes that occur in the intestine microbe environment of obese people as they perticipate in a structured diet and lifestyle modification.
The purpose of this study is to evaluate a treatment for obesity.
The purpose of this trial is to evaluate the proportion of obese patients who respond to setmelanotide at 52 weeks of treatment. The trial includes multiple independent sub-studies of setmelanotide in patients with obesity and at least one of 6 specific gene defects in the Melanocortin-4 Receptor pathway.
This study aims to examine associations of social support for healthful eating and physical activity with adolescents’ healthful and unhealthful weight management strategies and body mass index (BMI). The study will compare motivators and level of motivation for adolescent weight loss between adolescents and parents and across care sites (i.e., primary care vs. specialty care for obesity). Finally, the study will examine the relations of adolescent- and parent-reported motivators and level of motivation for weight loss with adolescents’ healthful and unhealthful weight management strategies and BMI.
The purpose of this study is to determine how human obesity engages epigenetic mechanisms that impair human mesenchymal stem/stromal cells ( MSC) mitochondrial structure and function and render MSC functionally deficient.
The purpose of this study is to provide the first integrated examination of the interaction between muscle insulin action and exercise-stimulated muscle glucose uptake in obesity from the whole body to the cellular/molecular level.
The purpose of this study is to learn more about the effects of similar weight loss through caloric restriction, gastric reduction, and bypass of the proximal small intestines on gut microbiota and compare them to changes in the microbiome of obese subjects undergoing sham procedures and life-style modification.
The purpose of this study is to compare a ventilation strategy using higher levels of positive end expiratory pressure and recruitment maneuvers to one using lower levels of positive end expiratory pressure without recruitment maneuvers in obese patients at an intermediate-to-high risk for post surgery respiratory complications.
The purpose of this study is to conduct a social network analysis to identify peer interventionists and to assess existing social structures that contribute to obesity-related health behaviors among Somali and Hispanic immigrants.
The purpose of this study is to collect and maintain a clinical and pathology database, and a biobank of blood, stool, and tissue specimens, from patients being treated surgically for obesity. The hope is this will ultimately help identify biological predictors for matching the best bariatric surgery to the patient for maximizing the effectiveness of the weight loss.
The Investigator hypothesizes that the currently used dose of dietary ingredient alpha-cyclodextrin (α-CD) will result in greater loss of dietary fat in the stool compared with placebo. The proposed studies will address the degree to which α-CD increases dietary fat loss. The Investigator will conduct the study and analyze the samples at Mayo Clinic in Rochester, Minnesota.
Obesity is common in adults with complex medical problems with ensuing complications afterwards. Obese patients suffer higher mortality and impaired functional status often as a result of their obesity. One primary goal to reduce both obesity and improve functional status is exercise. The investigators hypothesize that a simple exercise intervention with limited behavioral goal-setting will reduce weight and increase functional status compared to usual care. As a secondary measure, the investigators hypothesize that using this intervention will reduce hospital admissions and ER visits.
To determine whether EOS recruitment in AT is impaired during obesity. Therefore, we hypothesize that chronic, low-grade inflammation of AT initiates epigenetic modifications in EOS and/or in AT, thereby impairing the recruitment of eosinophils.
The purpose of this study is to prospectively evaluate the safety and effectivenes of standard of care, individualized, comprehensive weight loss interventions in carefully selected patients with obesity undergoing cardiac transplant evaluation, including utilization of best lifestyle modifications and guidance to promote a healthy weight, possible medical therapy using FDA-approved weight loss medications and EBMTs with or without concomitant medical therapy.
Our overall goal is to determine the effect of Phentermine and Topiramate on gastric emptying, gastric accommodation, and satiety and satiation in obese participants.
The purpose of this study is to determine if employees will use under desk foot pedal elliptical devices at their workstation to increase their level of physical activity.
The purpose of this study is to determine if providing a vegetarian diet intervention to obese individuals over a twelve-week period can reduce chronic inflammation as well as improve cardio-metabolic parameters that are precursors to the co-morbidities associated with obesity.
The purpose of this study is to understanding the mechanisms of weight gain in patients following liver transplantation, action, and indicate approaches to prevent weight gain.
It is unknown whether the bile acid pathway is altered in obesity. This study is designed to compare obesity and health to determine if the bile acid pathway differs depending on health state.
The purpose of this study is to compare weight loss, improvement of comorbidities, improvement of lipid profile, blood sugar in patients undergoing endoscopic sleeve gastroplasty or intragastric balloon between patients who are in a weight loss program and those who are not.
The primary purpose of this research study is to collect a large enough sample of fat cells (preadipocytes) and blood from patients undergoing surgery to establish a bank of samples for use by researchers to begin to understand what factors regulate the ability of fat tissue to grow better in some people than others.
Obesity is associated with reduced adenosine triphosphate (ATP) turnover in skeletal muscle, a condition that can impair muscle metabolism. The proposed research will discover mechanisms responsible for decreased content in mitochondrial proteins as well as in protein β-F1-ATPase, which is directly responsible for ATP assembly, in the muscle of obese individuals. This research will further examine the effectiveness of interventions, such as increased plasma amino acid availability and exercise, to increase the rate of production of mitochondrial proteins as well as that of β-F1-ATPase in the muscle of obese individuals. The findings will help to develop appropriate interventions to improve ...
The purpose of this study is to define the feasibility and effectiveness of a multi-component intervention aimed at decreasing perception of dyspnea in participants with lung disease who are obese.
The aim of this study is to develop, in close collaboration with stakeholders, an evidence-based decision aid to inform discussions regarding the treatment of obesity (Obesity Choice).
Obesity is associated with differences in stomach function, feeling of fullness after meals, and total calories consumed at a buffet meal. Based upon previous research, our study hypothesis is that weight loss with pharmacological agents may be individualized, based on the abnormality in those gastrointestinal functions. These studies will provide support for the principle that specific obesity medications should be selected according to individual characteristics, and it is anticipated that this approach will enhance the effectiveness of medication treatment of obesity.
This study aims to assess adipose tissue-eosinophil content and adiopose tissue metabolism 3 months after endoscopic gastroplasty weight loss procedure.
The purpose of this study is to create a registry of obese patients who have been phenotyped to understand the heterogeneity of obesity.
This study is being done to understand the short and long term changes in gastric emptying in response to the endoscopic sleeve gastroplasty (ESG).
With, one in three Americans now having a body mass index (BMI) greater than 30, and the fastest growing segment of the population having a BMI greater than 40, it is paramount to conduct validation of approaches to measure body composition. Currently the dual-energy x-ray absorptiometry (iDXA) is the gold standard for measuring the body composition. But it is often difficult to perform since it is limited to specific height, weight and BMI's. InBody is a tool which uses bio electric impedance to measure the body composition. This study will compare both approaches. Validation is necessary as it is clinically ...
This study is being done to better understand the relationship between inflammation and insulin resistance in your Adipose (fat) Tissue .
This research study is being done to evaluate the reliability and reproducibility of an infrared 3D scanner and an iPad app in measuring body measurements in participants.
The purpose of this study is to assess the feasibility of ablation of up to 70% of the gastric mucosa using HybridAPC to induce weight loss.
The purpose of this study is to find out how the naturally occurring bacteria (the microbiome) in your gut can affect your body weight
To evaluate the proportion of obese patients with genetic defects in the melanocortin-4 receptor (MC4R) pathway who achieve a clinically meaningful reduction in body weight in response to setmelanotide after an initial response to open-label treatment
The purpose of this study is to learn if eating a vegetarian diet changes the make up of a person's fatty acids as well as their levels of inflammation.
The Researchers are trying to determine if Costoclavicular brachial plexus block (CCBPB) can be successfully performed in patients with a body mass index greater than thirty.
The purpose of this study is to evaluate the effectiveness of early time-restricted eating versus, late time restricted eating, and daily caloric restriction alone for obesity, in addition to measure difference in A1c, fasting glucose, and lipid panels. Additionally, we hope to assess barriers to compliance and effect on quality of life.
The purpose of this study is to establish a multi-center biobank and outcomes registry of at least 2000 patients with obesity that allows for a better understanding of demographic, clinical, and biological variables that predict the severity of the disease, the development of weight-related complications, and the weight loss and weight-related complications outcomes in response to obesity interventions.
The purpose of this study is to evaluate the safety and effectiveness of the Spatz3 in subjects with a BMI ≥ 30 and < 40 who have failed to achieve and maintain weight-loss with a weight control program.
The purpose of this study is to determine the isotopic composition of the transition metals Cu, Fe and Zn after bariatric surgery.
Leptin is a fat hormone which acts in maintaining energy balance. However, leptin levels are high in obese subjects indicating resistance to the actions of leptin. High leptin levels have been associated with increased cardiovascular and metabolic risks, but it is not clear if increased leptin or leptin resistance contributes to the increased cardiovascular risk. Further, even though leptin receptors are present in fat tissue, leptin's role in fat tissue functions are not completely investigated in humans. Based on preliminary data the investigators hypothesize that resistance to leptin action in obese adipose tissue is responsible for altering the expression of ...
This study aims to examine the role of weight gain in adipose tissue immune cell influx and development of obesity related cardiometabolic disorders. Adipose tissue-mediated chronic systemic inflammation is implicated in the development of cardiometabolic disorders in obesity. Therefore, resolution of adipose tissue inflammation may be key to ameliorating obesity-associated dyslipidemia, insulin-resistance, and cardiovascular disease. Proinflammatory cytokines contribute to the initial influx of immune cells into adipose tissue during weight gain. However, mechanisms regulating these cytokines in the adipose tissue milieu and the effects of weight gain on adipose tissue are not completely understood.
The study proposes to investigate ...
Observational studies suggest that bariatric surgery is the most effective intervention for weight loss. Comparative effectiveness of Roux-en-Y Gastric Bypass (RYGB) and Sleeve Gastrectomy (SG) demonstrate that RYGB is significantly superior to SG in terms of weight loss and glycemic control. Both RYGB and SG increase GLP-1 concentrations which directly affect B-cell function. Data has shown that the postprandial rise in GLP-1 might affect feeding behavior after RYGB and to a lesser extent SG, where the increase in GLP-1 is less marked. In this study the investigators propose to randomize subjects undergoing SG to receive either placebo or Liraglutide, a ...
Currently in the United States about 97 million adults are considered obese, accounting for about 33% of the American adult population (compared to 22.9% in 1988). Obesity, defined as a body mass index of 30.0 or higher, is accountable for 44% of the diabetes, 23% of the ischemic heart disease and between 7% and 41% of certain cancers. The Erchonia® Zerona™ 2.0 Laser (which will be used in this study) has been approved by the FDA (K123237) as a non-invasive dermatological aesthetic treatment which can be used by individuals intending to reduce circumference of hips, waist, and thighs. Lorcaserin is ...
The purpose of this study is to explore the impact of Setmelanotide on obesity in patients with various specific rare genetic mutations.
The proposed study is a prospective, pilot study to assess the feasibility of a novel endoscopic suturing system to reduce gastric volume by changing the shape of the stomach for the primary treatment of obesity. The investigators aim to recruit ten subjects with a body mass index between 30-40 for this study. Vertical sutures will be performed using the endoscopic suturing system to deploy 10-17 interrupted full thickness sutures. Botulinum toxin(approximately 30 units) will be injected around the sutures insertion sites in half of the subjects randomly to slow down muscular grinding of the stomach to see if it improves ...
The purpose of this study is to successfully use physical activity monitoring devices with patients in the LIVE IT weight loss program.
The multi-purpose of this study is to pilot test a social network-informed CBPR-derived health promotion program for feasibility outcomes with overweight or obese adults from two immigrant communities, and to assess the preliminary impact of embedding a social network-informed CBPR-derived intervention within a regional health promotion resource hub on sustainability and uptake outcomes.
The purpose of this study is to define an "individualized diet" approach based on obesity related phenotypes (pathophysiology obesity classification), which would increase weight loss, adherence, and weight loss maintenance.
This study will review and analyze the literature regarding endoscopic revision of Roux-en-Y gastric bypass surgery for the treatment of weight regain using the OverStitch, a minimally invasive endoscopic device. This study will also include information about patients who underwent TORe as part of their clinical evaluation and treatment for weight regain by chart review.
The purpose of this study is comparison of enteroendocrine cells between individuals with obesity and health and storage of tissue samples for future research
In this prospective natural history study, the aim is to identify variables contributing to best outcomes for hematopoietic cell tranplantation (HCT) or other treatment where applicable (enzyme replacement or gene therapy), which is life-saving therapy for children with SCID, leaky SCID, Omenn syndrome and reticular dysgenesis.
The purpose of this study is to assess the impact of participation in a standardized behavioral weight management program on the percentage of excess weight lost and also the psychosocial and medical outcomes after gastric bypass surgery.
The objectives of this study are to assess the safety and durability of combined hybrid APC and ESG compared to traditional ESG, and to assess weight loss and improvement in obesity-related co-morbidities in combined hybrid APC and ESG compared to ESG alone.
Endoscopic Sleeve Gastroplasty (ESG) is an endoscopic minimally invasive weight loss procedure where a commercially available, FDA approved, full-thickness endoscopic suturing device (Overstitch; Apollo Endosurgery, Austin, TX) is used to reduce the stomach volume by 80% through the creation of a restrictive endoscopic sleeve. This is accomplished by a series of endolumenally placed full-thickness sutures through the gastric wall, extending from the antrum to the gastroesophageal junction. Up to 200 participants at 8 locations in the United States will participate in this study. The ESG procedure has been performed clinically for 3 years in the United States. We are completing ...
Body-weight based interval training (IT) performed 3 times per week will lead to reductions in abdominal adiposity and reduce overall body fat percentage in overweight and obese sedentary adults more effectively than moderate intensity continuous training (MICT). Body-weight interval training will improve exercise capacity (peak VO2) in overweight/obese adults.
The researchers are trying to identify the specific characteristics (phenotypes) that may be useful to help select the right medication for weight loss.
Approximately one third of the children and adolescents in the United States are either overweight or obese. Childhood obesity disproportionately affects specific racial and ethnic groups and households with low socioeconomic status and low parental education. The Alternative Learning Center (ALC) within Rochester School District 535 provides viable educational options for students who are experiencing difficulty in regular educational systems. A greater proportion of students at ALC are minorities, qualify for free and reduced lunch and receive special education services. These children are likely to have unique barriers to physical activity and healthy eating.
Specific Aims:
The purpose of this study is to assess the ability of the hyperphagia questionnaires to identify children and young adults with early-onset obesity for genetic testing to identify variants in 36 genes of interest associated with MC4R pathway genetic obesity
The primary purpose of this study is to develop a screening system for factors that impact growth in infants within the newborn nursery, as well as a feeding assessment survey to be completed at well child visits during the first 12 months of life.
The purpose of this extension study of up to an additional 2 years duration beyond the index trial for patients who have completed a previous study of setmelanotide for genetic obesity disorders upstream of the MC4 receptor in the melanocortin-leptin pathway. Since continued assessments of the safety and efficacy of setmelanotide are the same in this extension protocol regardless of the disease studied in the index protocol, all patients can be followed in this single extension protocol. Nevertheless, the analysis of each individual disease will be performed separately with the ability to combine data from the original index protocol and ...
The purpose of this study is to examine whether an education program, designed in partnership with teachers at Moreland Elementary School in West St. Paul and Mayo Clinic InSciEd Out scientists, is able to influence the behavior and health literacy of students. This information will be collected in surveys before and after the students are given the curriculum during the school day.
FIT is grounded in social cognitive, self-determination, and family systems theories and, as such, aims to promote healthy eating and movement habits by facilitating the development of parent-adolescent communication and problem-solving skills to support the adolescents’ and family’s pursuit of health behavior change goals.
Vitamin D deficiency has been linked to endothelial dysfunction in adults. Obese adolescents have a high prevalence of Vitamin D deficiency as well as evidence of endothelial dysfunction. Our hypothesis is that supplementation of Vitamin D deficient adolescents with Vitamin D would lead to improvement in endothelial dysfunction.
Exendin-(9,39) has been shown to have effects on beta-cell function, and after gastric bypass, to accelerate gastrointestinal transit. - infused at rates of 300pmol/kg/min. Given that gastrointestinal transit is typically delayed by Glucagon-Like Peptide-1 (GLP-1) and also that this hormone causes decreased food intake through increased satiation, it is reasonable to expect an effect of Exendin-9,39 on appetite. This may help explain the effects of gastric bypass on food intake. To examine the effect of Exendin on food intake we propose a dose-response study to determine whether the compound has effects in a dose-dependent fashion. We will examine the presence ...
New studies are revealing how a high-fat diet could be making the cells of the intestinal lining more likely to become cancerous. The purpose of this study is to find how the microbe envntironment of the intestines in obesity influences the growth of intestinal stem cells, which could then trigger intestinal tumors.
The purpose of this study is to increase the measurement of BMI and WC in the overweight (BMI at or greater than the 85th percentile) and obese (BMI at or greater than 95th percentile) outpatient pediatric population ages 6-19 at Mayo Clinic Rochester and surrounding satellite clinics. Educate providers and nursing staff on the importance and technique of measuring the WC in the overweight and obese outpatient pediatric population with at least a yearly WC on every child with a BMI at or greater than the 85th percentile. Determine the prevalence of various components of the metabolic syndrome (MS) including ...
This study will evaluate obesity as a comorbidity in a population of patients with irritable bowel syndrome (IBS), and assess this cohort for vitamin D-deficiency. It will also determine whether alterations in the fecal microbiome and metaproteome, associated with vitamin D deficiency or other factors, underpin obesity-IBS comorbidity.
The objective of this study is to leverage existing social networks for health behavior change relevant to obesity and cardiovascular risk among immigrant populations in Southeast MN.
To determine the accuracy of unguided versus ultrasound (US) guided knee joint injections in obese patients with no clinically detectable effusion.
Opioid medications such as morphine, hydrocodone and oxycodone are standard for treating pain after surgery, however there are disadvantages. Because of the way opioids work, gastric bypass patients may have an increased risk of having sedation or problems with breathing. In patients with sleep apnea, opioids may increase the risk of severe apnea. Ketamine is an alternative pain medicine that can be used to treat pain after surgery and may have fewer effects on breathing. Using ketamine as part of the regimen may be a better choice for laparoscopic gastric bypass patients. This study is being done to find out ...
The purpose of this research is to find out if an aggressive intervention to lose weight, will improve symptoms in patients with obesity-related cardiomyopathy, which is also known as the obese phenotype of heart failure with preserved ejection fraction (HFpEF).
The purpose of the study is to conduct qualitative interviews of participants in a sleep restriction study who were allowed free snacking to learn about their self-observations regarding their eating motivations and behaviors.
The purpose of this study is to prospectively evaluate the safety and effectiveness of standard of care, individualized, comprehensive weight loss interventions in carefully selected patients with obesity undergoing liver transplant evaluation, including utilization of best lifestyle modifications and guidance to promote a healthy weight, possible medical therapy using FDA-approved weight loss medications and EBMTs with or without concomitant medical therapy.
This protocol is being conducted to determine the mechanisms responsible for insulin resistance, obesity and type 2 diabetes.
The purpose of this study is to determine through a retrospective cohort if rapid gastric emptying is not only associated with obesity, but if it predicts the future development of the disease.
The purpose of this study is to elicit detailed information from patients diagnosed with a sleep-related breathing disorder with severe obesity and sleep medicine providers regarding attitudes toward and acceptance of a multicomponent weight loss intervention based in health coaching. We anticipate that gaining a deeper understanding of attitudes toward weight loss in this population will provide insight into what may be feasible and acceptable aspects of a weight loss intervention. This information will then help guide the development of a multicomponent weight loss intervention based in health coaching and aimed at decreasing disease burden, increasing PAP adherence, and improving ...
The aim of the study is to investigate the effects of a 3-month resistance exercise program (in people aged 50 to 75) on muscle mass, body composition, muscle strength, brain function and cognition, muscle efficiency processing blood sugar, the body’s ability to build muscle, and fat cells.
Earlier research has shown that exercise has significant benefits in preventing certain diseases and conditions such as diabetes, dementia, heart disease, and more. We also know from other research that resistance exercise (lifting weights) and aerobic exercise (running, biking, walking), improve metabolism through separate ways on the molecular level, also called “molecular pathways.” With ...
The purpose of this study is to test the hypothesis that obesity is associated with impairment of cardiovascular reflex control, and that this impairment is linked to deficient leptin activity.
The primary purpose of this study is to investigate the relationship between a technology-assisted diet and exercise program which is easily implemented in an outpatient setting and the levels of biomarkers that have been associated with breast cancer recurrence risk in overweight women with stage 0, I, or II breast cancer.
Insufficient sleep may be one of the most common, and most preventable, obesity risk factors. The investigators wish to determine whether 14 nights of modest sleep restriction results in increased energy balance, thus potentially increasing the risk of obesity. The investigators hypothesize that sleep restriction will result in increased energy balance.
Muscle insulin resistance is a hallmark of upper body obesity (UBO) and Type 2 diabetes (T2DM). It is unknown whether muscle free fatty acid (FFA) availability or intramyocellular fatty acid trafficking is responsible for the abnormal response to insulin. Likewise, we do not understand to what extent the incorporation of FFA into ceramides or diacylglycerols (DG) affect insulin signaling and muscle glucose uptake. We will measure muscle FFA storage into intramyocellular triglyceride, intramyocellular fatty acid trafficking, activation of the insulin signaling pathway and glucose disposal rates under both saline control (high overnight FFA) and after an overnight infusion of intravenous ...
The purpose of this study is to improve our understanding of why gastrointestinal symptoms occur in diabetes mellitus patients and identify new treatment(s) in the future.
These symptoms are often distressing and may impair glycemic control. We do not understand how diabetes mellitus affects the GI tracy. In 45 patients undergoing sleeve gastrectomy, we plan to compare the cellular composition of circulating peripheral mononuclear cells, stomach immune cells, and interstitial cells of Cajal in the stomach.
Muscle insulin resistance is a hallmark of upper body obesity (UBO) and Type 2 diabetes (T2DM), whereas lower body obesity (LBO) is characterized by near-normal insulin sensitivity. It is unknown whether muscle free fatty acid (FFA) availability or intramyocellular fatty acid trafficking differs between different obesity phenotypes. Likewise, we do not understand to what extent the incorporation of FFA into ceramides or diacylglycerols (DG) affect insulin signaling and muscle glucose uptake. By measuring muscle FFA storage into intramyocellular triglyceride, intramyocellular fatty acid trafficking, activation of the insulin signaling pathway and glucose disposal rates we will provide the first integrated examination ...
To determine if the EndoBarrier safely and effectively improves glycemic control in obese subjects with type 2 diabetes.
The purpose of this study is to evaluate the safety and tolerability of 134 days of daily dosing of HU6, to ealuate the effectiveness of HU6 on weight reduction, and to evaluate the effect of HU6 treatment on exercise capacity.
Using stem cell derived intestinal epithelial cultures (enteroids) derived from obese (BMI> 30) patients and non-obese and metabolically normal patients (either post-bariatric surgery (BS) or BS-naïve with BMI < 25), dietary glucose absorption was measured. We identified that enteroids from obese patients were characterized by glucose hyper-absorption (~ 5 fold) compared to non-obese patients. Significant upregulation of major intestinal sugar transporters, including SGLT1, GLU2 and GLUT5 was responsible for hyper-absorptive phenotype and their pharmacologic inhibition significantly decreased glucose absorption. Importantly, we observed that enteroids from post-BS non-obese patients exhibited low dietary glucose absorption, indicating that altered glucose absorption ...
The purpose of this study is to learn more about how the body stores dietary fat. Medical research has shown that fat stored in different parts of the body can affect the risk for diabetes, heart disease and other major health conditions.
The purpose of this study is to see why the ability of fat cells to respond to insulin is different depending on body shape and how fat tissue inflammation is involved.
The purpose of this study is to determine the mechanism(s) by which common bariatric surgical procedures alter carbohydrate metabolism. Understanding these mechanisms may ultimately lead to the development of new interventions for the prevention and treatment of type 2 diabetes and obesity.
The purpose of this study is to evaluate the usefulness of combining a core liver biopsy guided by endoscopic ultrasound and stomach balloon placement by endoscope for the diagnosis and treatment of fatty liver disease and obesity.
The purpose of this study is to determine the metabolic effects of Colesevelam, particularly for the ability to lower blood sugar after a meal in type 2 diabetics, in order to develop a better understanding of it's potential role in the treatment of obesity.
The purpose of this study is to test whether markers of cellular aging and the SASP are elevated in subjects with obesity and further increased in patients with obesity and Type 2 Diabetes Mellitus (T2DM) and to relate markers of cellular aging (senescence) and the SASP to skeletal parameters (DXA, HRpQCT, bone turnover markers) in each of these groups.
Integration of Diabetes Prevention Program (DPP) and Diabetes Self Management Program (DSMP) into WellConnect.
The purpose of this study is to evaluate if pulsed arterial spin labeling magnetic resonance imaging (PASL MRI) is able to measure a difference in hypothalamic blood flow in patients with anorexia nervosa, opposite than obesity when compared to health.
Endothelial dysfunction, or abnormal functioning of the lining of blood vessels, appears to be a key process in the development of cardiovascular disease. Endothelial dysfunction appears to be caused by both sleep disordered breathing and obesity. As endothelial dysfunction is among the first clinical marker that predicts future cardiovascular events, understanding molecular mechanisms leading to impairment of endothelial function is very important. Endothelial function requires the proper functioning of endothelial nitric oxide synthase (eNOS). eNOS activity is tightly regulated by caveolin-1, a protein important in the formation of cellular structures called caveolae. Low levels of caveolin-1 facilitate optimal nitric oxide ...
Muscle insulin resistance is a hallmark of upper body obesity (UBO) and Type 2 diabetes (T2DM). It is unknown whether muscle free fatty acid (FFA) availability or intramyocellular fatty acid trafficking is responsible for muscle insulin resistance, although it has been shown that raising FFA with Intralipid can cause muscle insulin resistance within 4 hours. We do not understand to what extent the incorporation of FFA into ceramides or diacylglycerols (DG) affect insulin signaling and muscle glucose uptake. We propose to alter the profile and concentrations of FFA of healthy, non-obese adults using an overnight, intra-duodenal palm oil infusion vs. ...
The objectives of this study are to identify circulating extracellular vesicle (EV)-derived protein and RNA signatures associated with Type 2 Diabetes (T2D), and to identify changes in circulating EV cargo in patients whose T2D resolves after sleeve gastrectomy (SG) or Roux-en-Y gastric bypass (RYGB).
The purpose of this study is to learn more about the changes in levels of Spexin, leptin and other biomarkers such as adiponectin and resting energy expenditure before and after hypothalamic surgery.
The purpose of this study is to look at how participants' daily life is affected by their heart failure. The study will also look at the change in participants' body weight. This study will compare the effect of semaglutide (a new medicine) compared to "dummy" medicine on body weight and heart failure symptoms. Participants will either get semaglutide or "dummy" medicine, which treatment participants get is decided by chance. Participants will need to take 1 injection once a week.
This study is being done to better understand the relationship between inflammation in your AT, abnormal deposition of fat around your liver and how this affects its appearance and function and ultimately insulin resistance.
This study aims to evaluate the mechanisms leading to hyperoxaluria and increased risk of kidney stone formation after bariatric surgery.
This randomized phase III trial studies whether weight loss in overweight and obese women may prevent breast cancer from coming back (recurrence). Previous studies have found that women who are overweight or obese when their breast cancer is found (diagnosed) have a greater risk of their breast cancer recurring, as compared to women who were thinner when their cancer was diagnosed. This study aims to test whether overweight or obese women who take part in a weight loss program after being diagnosed with breast cancer have a lower rate of cancer recurrence as compared to women who do not take ...
The purpose of this study is to see if there is a connection between bad experiences in the patient's childhood, either by the patient or the parent, and poor blood sugar control, obesity, poor blood lipid levels, and depression in patients with type 1 diabetes.
The purpose of this study is to evaluate the effect of Aramchol as compared to placebo on NASH resolution, fibrosis improvement and clinical outcomes related to progression of liver disease (fibrosis stages 2-3 who are overweight or obese and have prediabetes or type 2 diabetes).
A variety of liver insults lead to pathological changes in liver architecture that culminate in cirrhosis. While invasive liver biopsy was required to detect cirrhosis, the development of magnetic resonance elastography (MRE) has revolutionized our ability to detect liver fibrosis through non-invasive means that involve measurement of liver stiffness. However, a number of pathological findings occur in liver in response to various insults that precede cirrhosis and are clinically important to identify such as steatosis associated with NASH, inflammation associated with viral hepatitis, and congestion associated with cardiac hepatopathy. Detection of such entities provides essential diagnostic, prognostic, and treatment information ...
The purpose of this study is to determine whether short-term treatment with Fisetin reduces the rate of death and long term complications related to COVID-19.
The purpose of this study is to evaluate the effietiveness of remdesivir (RDV) in reducing the rate of of all-cause medically attended visits (MAVs; medical visits attended in person by the participant and a health care professional) or death in non-hospitalized participants with early stage coronavirus disease 2019 (COVID-19) and to evaluate the safety of RDV administered in an outpatient setting.
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Effectiveness of...
Effectiveness of weight management interventions for adults delivered in primary care: systematic review and meta-analysis of randomised controlled trials
- Related content
- Peer review
- Claire D Madigan , senior research associate 1 ,
- Henrietta E Graham , doctoral candidate 1 ,
- Elizabeth Sturgiss , NHMRC investigator 2 ,
- Victoria E Kettle , research associate 1 ,
- Kajal Gokal , senior research associate 1 ,
- Greg Biddle , research associate 1 ,
- Gemma M J Taylor , reader 3 ,
- Amanda J Daley , professor of behavioural medicine 1
- 1 Centre for Lifestyle Medicine and Behaviour (CLiMB), The School of Sport, Exercise and Health Sciences, Loughborough University, Loughborough LE11 3TU, UK
- 2 School of Primary and Allied Health Care, Monash University, Melbourne, Australia
- 3 Department of Psychology, Addiction and Mental Health Group, University of Bath, Bath, UK
- Correspondence to: C D Madigan c.madigan{at}lboro.ac.uk (or @claire_wm and @lboroclimb on Twitter)
- Accepted 26 April 2022
Objective To examine the effectiveness of behavioural weight management interventions for adults with obesity delivered in primary care.
Design Systematic review and meta-analysis of randomised controlled trials.
Eligibility criteria for selection of studies Randomised controlled trials of behavioural weight management interventions for adults with a body mass index ≥25 delivered in primary care compared with no treatment, attention control, or minimal intervention and weight change at ≥12 months follow-up.
Data sources Trials from a previous systematic review were extracted and the search completed using the Cochrane Central Register of Controlled Trials, Medline, PubMed, and PsychINFO from 1 January 2018 to 19 August 2021.
Data extraction and synthesis Two reviewers independently identified eligible studies, extracted data, and assessed risk of bias using the Cochrane risk of bias tool. Meta-analyses were conducted with random effects models, and a pooled mean difference for both weight (kg) and waist circumference (cm) were calculated.
Main outcome measures Primary outcome was weight change from baseline to 12 months. Secondary outcome was weight change from baseline to ≥24 months. Change in waist circumference was assessed at 12 months.
Results 34 trials were included: 14 were additional, from a previous review. 27 trials (n=8000) were included in the primary outcome of weight change at 12 month follow-up. The mean difference between the intervention and comparator groups at 12 months was −2.3 kg (95% confidence interval −3.0 to −1.6 kg, I 2 =88%, P<0.001), favouring the intervention group. At ≥24 months (13 trials, n=5011) the mean difference in weight change was −1.8 kg (−2.8 to −0.8 kg, I 2 =88%, P<0.001) favouring the intervention. The mean difference in waist circumference (18 trials, n=5288) was −2.5 cm (−3.2 to −1.8 cm, I 2 =69%, P<0.001) in favour of the intervention at 12 months.
Conclusions Behavioural weight management interventions for adults with obesity delivered in primary care are effective for weight loss and could be offered to members of the public.
Systematic review registration PROSPERO CRD42021275529.
Introduction
Obesity is associated with an increased risk of diseases such as cancer, type 2 diabetes, and heart disease, leading to early mortality. 1 2 3 More recently, obesity is a risk factor for worse outcomes with covid-19. 4 5 Because of this increased risk, health agencies and governments worldwide are focused on finding effective ways to help people lose weight. 6
Primary care is an ideal setting for delivering weight management services, and international guidelines recommend that doctors should opportunistically screen and encourage patients to lose weight. 7 8 On average, most people consult a primary care doctor four times yearly, providing opportunities for weight management interventions. 9 10 A systematic review of randomised controlled trials by LeBlanc et al identified behavioural interventions that could potentially be delivered in primary care, or involved referral of patients by primary care professionals, were effective for weight loss at 12-18 months follow-up (−2.4 kg, 95% confidence interval −2.9 to−1.9 kg). 11 However, this review included trials with interventions that the review authors considered directly transferrable to primary care, but not all interventions involved primary care practitioners. The review included interventions that were entirely delivered by university research employees, meaning implementation of these interventions might differ if offered in primary care, as has been the case in other implementation research of weight management interventions, where effects were smaller. 12 As many similar trials have been published after this review, an updated review would be useful to guide health policy.
We examined the effectiveness of weight loss interventions delivered in primary care on measures of body composition (weight and waist circumference). We also identified characteristics of effective weight management programmes for policy makers to consider.
This systematic review was registered on PROSPERO and is reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement. 13 14
Eligibility criteria
We considered studies to be eligible for inclusion if they were randomised controlled trials, comprised adult participants (≥18 years), and evaluated behavioural weight management interventions delivered in primary care that focused on weight loss. A primary care setting was broadly defined as the first point of contact with the healthcare system, providing accessible, continued, comprehensive, and coordinated care, focused on long term health. 15 Delivery in primary care was defined as the majority of the intervention being delivered by medical and non-medical clinicians within the primary care setting. Table 1 lists the inclusion and exclusion criteria.
Study inclusion and exclusion criteria
- View inline
We extracted studies from the systematic review by LeBlanc et al that met our inclusion criteria. 11 We also searched the exclusions in this review because the researchers excluded interventions specifically for diabetes management, low quality trials, and only included studies from an Organisation for Economic Co-operation and Development country, limiting the scope of the findings.
We searched for studies in the Cochrane Central Register of Controlled Trials, Medline, PubMed, and PsychINFO from 1 January 2018 to 19 August 2021 (see supplementary file 1). Reference lists of previous reviews 16 17 18 19 20 21 and included trials were hand searched.
Data extraction
Results were uploaded to Covidence, 22 a software platform used for screening, and duplicates removed. Two independent reviewers screened study titles, abstracts, and full texts. Disagreements were discussed and resolved by a third reviewer. All decisions were recorded in Covidence, and reviewers were blinded to each other’s decisions. Covidence calculates proportionate agreement as a measure of inter-rater reliability, and data are reported separately by title or abstract screening and full text screening. One reviewer extracted data on study characteristics (see supplementary table 1) and two authors independently extracted data on weight outcomes. We contacted the authors of four included trials (from the updated search) for further information. 23 24 25 26
Outcomes, summary measures, and synthesis of results
The primary outcome was weight change from baseline to 12 months. Secondary outcomes were weight change from baseline to ≥24 months and from baseline to last follow-up (to include as many trials as possible), and waist circumference from baseline to 12 months. Supplementary file 2 details the prespecified subgroup analysis that we were unable to complete. The prespecified subgroup analyses that could be completed were type of healthcare professional who delivered the intervention, country, intensity of the intervention, and risk of bias rating.
Healthcare professional delivering intervention —From the data we were able to compare subgroups by type of healthcare professional: nurses, 24 26 27 28 general practitioners, 23 29 30 31 and non-medical practitioners (eg, health coaches). 32 33 34 35 36 37 38 39 Some of the interventions delivered by non-medical practitioners were supported, but not predominantly delivered, by GPs. Other interventions were delivered by a combination of several different practitioners—for example, it was not possible to determine whether a nurse or dietitian delivered the intervention. In the subgroup analysis of practitioner delivery, we refer to this group as “other.”
Country —We explored the effectiveness of interventions by country. Only countries with three or more trials were included in subgroup analyses (United Kingdom, United States, and Spain).
Intensity of interventions —As the median number of contacts was 12, we categorised intervention groups according to whether ≤11 or ≥12 contacts were required.
Risk of bias rating —Studies were classified as being at low, unclear, and high risk of bias. Risk of bias was explored as a potential influence on the results.
Meta-analyses
Meta-analyses were conducted using Review Manager 5.4. 40 As we expected the treatment effects to differ because of the diversity of intervention components and comparator conditions, we used random effects models. A pooled mean difference was calculated for each analysis, and variance in heterogeneity between studies was compared using the I 2 and τ 2 statistics. We generated funnel plots to evaluate small study effects. If more than two intervention groups existed, we divided the number of participants in the comparator group by the number of intervention groups and analysed each individually. Nine trials were cluster randomised controlled trials. The trials had adjusted their results for clustering, or adjustment had been made in the previous systematic review by LeBlanc et al. 11 One trial did not report change in weight by group. 26 We calculated the mean weight change and standard deviation using a standard formula, which imputes a correlation for the baseline and follow-up weights. 41 42 In a non-prespecified analysis, we conducted univariate and multivariable metaregression (in Stata) using a random effects model to examine the association between number of sessions and type of interventionalist on study effect estimates.
Risk of bias
Two authors independently assessed the risk of bias using the Cochrane risk of bias tool v2. 43 For incomplete outcome data we defined a high risk of bias as ≥20% attrition. Disagreements were resolved by discussion or consultation with a third author.
Patient and public involvement
The study idea was discussed with patients and members of the public. They were not, however, included in discussions about the design or conduct of the study.
The search identified 11 609 unique study titles or abstracts after duplicates were removed ( fig 1 ). After screening, 97 full text articles were assessed for eligibility. The proportionate agreement ranged from 0.94 to 1.0 for screening of titles or abstracts and was 0.84 for full text screening. Fourteen new trials met the inclusion criteria. Twenty one studies from the review by LeBlanc et al met our eligibility criteria and one study from another systematic review was considered eligible and included. 44 Some studies had follow-up studies (ie, two publications) that were found in both the second and the first search; hence the total number of trials was 34 and not 36. Of the 34 trials, 27 (n=8000 participants) were included in the primary outcome meta-analysis of weight change from baseline to 12 months, 13 (n=5011) in the secondary outcome from baseline to ≥24 months, and 30 (n=8938) in the secondary outcome for weight change from baseline to last follow-up. Baseline weight was accounted for in 18 of these trials, but in 14 24 26 29 30 31 32 44 45 46 47 48 49 50 51 it was unclear or the trials did not consider baseline weight. Eighteen trials (n=5288) were included in the analysis of change in waist circumference at 12 months.

Studies included in systematic review of effectiveness of behavioural weight management interventions in primary care. *Studies were merged in Covidence if they were from same trial
- Download figure
- Open in new tab
- Download powerpoint
Study characteristics
Included trials (see supplementary table 1) were individual randomised controlled trials (n=25) 24 25 26 27 28 29 32 33 34 35 38 39 41 44 45 46 47 50 51 52 53 54 55 56 59 or cluster randomised controlled trials (n=9). 23 30 31 36 37 48 49 57 58 Most were conducted in the US (n=14), 29 30 31 32 33 34 35 36 37 45 48 51 54 55 UK (n=7), 27 28 38 41 47 57 58 and Spain (n=4). 25 44 46 49 The median number of participants was 276 (range 50-864).
Four trials included only women (average 65.9% of women). 31 48 51 59 The mean BMI at baseline was 35.2 (SD 4.2) and mean age was 48 (SD 9.7) years. The interventions lasted between one session (with participants subsequently following the programme unassisted for three months) and several sessions over three years (median 12 months). The follow-up period ranged from 12 months to three years (median 12 months). Most trials excluded participants who had lost weight in the past six months and were taking drugs that affected weight.
Meta-analysis
Overall, 27 trials were included in the primary meta-analysis of weight change from baseline to 12 months. Three trials could not be included in the primary analysis as data on weight were only available at two and three years and not 12 months follow-up, but we included these trials in the secondary analyses of last follow-up and ≥24 months follow-up. 26 44 50 Four trials could not be included in the meta-analysis as they did not present data in a way that could be synthesised (ie, measures of dispersion). 25 52 53 58 The mean difference was −2.3 kg (95% confidence interval −3.0 to −1.6 kg, I 2 =88%, τ 2 =3.38; P<0.001) in favour of the intervention group ( fig 2 ). We found no evidence of publication bias (see supplementary fig 1). Absolute weight change was −3.7 (SD 6.1) kg in the intervention group and −1.4 (SD 5.5) kg in the comparator group.

Mean difference in weight at 12 months by weight management programme in primary care (intervention) or no treatment, different content, or minimal intervention (control). SD=standard deviation
Supplementary file 2 provides a summary of the main subgroup analyses.
Weight change
The mean difference in weight change at the last follow-up was −1.9 kg (95% confidence interval −2.5 to −1.3 kg, I 2 =81%, τ 2 =2.15; P<0.001). Absolute weight change was −3.2 (SD 6.4) kg in the intervention group and −1.2 (SD 6.0) kg in the comparator group (see supplementary figs 2 and 3).
At the 24 month follow-up the mean difference in weight change was −1.8 kg (−2.8 to −0.8 kg, I 2 =88%, τ 2 =3.13; P<0.001) (see supplementary fig 4). As the weight change data did not differ between the last follow-up and ≥24 months, we used the weight data from the last follow-up in subgroup analyses.
In subgroup analyses of type of interventionalist, differences were significant (P=0.005) between non-medical practitioners, GPs, nurses, and other people who delivered interventions (see supplementary fig 2).
Participants who had ≥12 contacts during interventions lost significantly more weight than those with fewer contacts (see supplementary fig 6). The association remained after adjustment for type of interventionalist.
Waist circumference
The mean difference in waist circumference was −2.5 cm (95% confidence interval −3.2 to −1.8 cm, I 2 =69%, τ 2 =1.73; P<0.001) in favour of the intervention at 12 months ( fig 3 ). Absolute changes were −3.7 cm (SD 7.8 cm) in the intervention group and −1.3 cm (SD 7.3) in the comparator group.

Mean difference in waist circumference at 12 months. SD=standard deviation
Risk of bias was considered to be low in nine trials, 24 33 34 35 39 41 47 55 56 unclear in 12 trials, 25 27 28 29 32 45 46 50 51 52 54 59 and high in 13 trials 23 26 30 31 36 37 38 44 48 49 53 57 58 ( fig 4 ). No significant (P=0.65) differences were found in subgroup analyses according to level of risk of bias from baseline to 12 months (see supplementary fig 7).

Risk of bias in included studies
Worldwide, governments are trying to find the most effective services to help people lose weight to improve the health of populations. We found weight management interventions delivered by primary care practitioners result in effective weight loss and reduction in waist circumference and these interventions should be considered part of the services offered to help people manage their weight. A greater number of contacts between patients and healthcare professionals led to more weight loss, and interventions should be designed to include at least 12 contacts (face-to-face or by telephone, or both). Evidence suggests that interventions delivered by non-medical practitioners were as effective as those delivered by GPs (both showed statistically significant weight loss). It is also possible that more contacts were made with non-medical interventionalists, which might partially explain this result, although the metaregression analysis suggested the effect remained after adjustment for type of interventionalist. Because most comparator groups had fewer contacts than intervention groups, it is not known whether the effects of the interventions are related to contact with interventionalists or to the content of the intervention itself.
Although we did not determine the costs of the programme, it is likely that interventions delivered by non-medical practitioners would be cheaper than GP and nurse led programmes. 41 Most of the interventions delivered by non-medical practitioners involved endorsement and supervision from GPs (ie, a recommendation or checking in to see how patients were progressing), and these should be considered when implementing these types of weight management interventions in primary care settings. Our findings suggest that a combination of practitioners would be most effective because GPs might not have the time for 12 consultations to support weight management.
Although the 2.3 kg greater weight loss in the intervention group may seem modest, just 2-5% in weight loss is associated with improvements in systolic blood pressure and glucose and triglyceride levels. 60 The confidence intervals suggest a potential range of weight loss and that these interventions might not provide as much benefit to those with a higher BMI. Patients might not find an average weight loss of 3.7 kg attractive, as many would prefer to lose more weight; explaining to patients the benefits of small weight losses to health would be important.
Strengths and limitations of this review
Our conclusions are based on a large sample of about 8000 participants, and 12 of these trials were published since 2018. It was occasionally difficult to distinguish who delivered the interventions and how they were implemented. We therefore made some assumptions at the screening stage about whether the interventionalists were primary care practitioners or if most of the interventions were delivered in primary care. These discussions were resolved by consensus. All included trials measured weight, and we excluded those that used self-reported data. Dropout rates are important in weight management interventions as those who do less well are less likely to be followed-up. We found that participants in trials with an attrition rate of 20% or more lost less weight and we are confident that those with high attrition rates have not inflated the results. Trials were mainly conducted in socially economic developed countries, so our findings might not be applicable to all countries. The meta-analyses showed statistically significant heterogeneity, and our prespecified subgroups analysis explained some, but not all, of the variance.
Comparison with other studies
The mean difference of −2.3 kg in favour of the intervention group at 12 months is similar to the findings in the review by LeBlanc et al, who reported a reduction of −2.4 kg in participants who received a weight management intervention in a range of settings, including primary care, universities, and the community. 11 61 This is important because the review by LeBlanc et al included interventions that were not exclusively conducted in primary care or by primary care practitioners. Trials conducted in university or hospital settings are not typically representative of primary care populations and are often more intensive than trials conducted in primary care as a result of less constraints on time. Thus, our review provides encouraging findings for the implementation of weight management interventions delivered in primary care. The findings are of a similar magnitude to those found in a trial by Ahern et al that tested primary care referral to a commercial programme, with a difference of −2.7 kg (95% confidence interval −3.9 to −1.5 kg) reported at 12 month follow-up. 62 The trial by Ahern et al also found a difference in waist circumference of −4.1 cm (95% confidence interval −5.5 to −2.3 cm) in favour of the intervention group at 12 months. Our finding was smaller at −2.5 cm (95% confidence interval −3.2 to −1.8 cm). Some evidence suggests clinical benefits from a reduction of 3 cm in waist circumference, particularly in decreased glucose levels, and the intervention groups showed a 3.7 cm absolute change in waist circumference. 63
Policy implications and conclusions
Weight management interventions delivered in primary care are effective and should be part of services offered to members of the public to help them manage weight. As about 39% of the world’s population is living with obesity, helping people to manage their weight is an enormous task. 64 Primary care offers good reach into the community as the first point of contact in the healthcare system and the remit to provide whole person care across the life course. 65 When developing weight management interventions, it is important to reflect on resource availability within primary care settings to ensure patients’ needs can be met within existing healthcare systems. 66
We did not examine the equity of interventions, but primary care interventions may offer an additional service and potentially help those who would not attend a programme delivered outside of primary care. Interventions should consist of 12 or more contacts, and these findings are based on a mixture of telephone and face-to-face sessions. Previous evidence suggests that GPs find it difficult to raise the issue of weight with patients and are pessimistic about the success of weight loss interventions. 67 Therefore, interventions should be implemented with appropriate training for primary care practitioners so that they feel confident about helping patients to manage their weight. 68
Unanswered questions and future research
A range of effective interventions are available in primary care settings to help people manage their weight, but we found substantial heterogeneity. It was beyond the scope of this systematic review to examine the specific components of the interventions that may be associated with greater weight loss, but this could be investigated by future research. We do not know whether these interventions are universally suitable and will decrease or increase health inequalities. As the data are most likely collected in trials, an individual patient meta-analysis is now needed to explore characteristics or factors that might explain the variance. Most of the interventions excluded people prescribed drugs that affect weight gain, such as antipsychotics, glucocorticoids, and some antidepressants. This population might benefit from help with managing their weight owing to the side effects of these drug classes on weight gain, although we do not know whether the weight management interventions we investigated would be effective in this population. 69
What is already known on this topic
Referral by primary care to behavioural weight management programmes is effective, but the effectiveness of weight management interventions delivered by primary care is not known
Systematic reviews have provided evidence for weight management interventions, but the latest review of primary care delivered interventions was published in 2014
Factors such as intensity and delivery mechanisms have not been investigated and could influence the effectiveness of weight management interventions delivered by primary care
What this study adds
Weight management interventions delivered by primary care are effective and can help patients to better manage their weight
At least 12 contacts (telephone or face to face) are needed to deliver weight management programmes in primary care
Some evidence suggests that weight loss after weight management interventions delivered by non-medical practitioners in primary care (often endorsed and supervised by doctors) is similar to that delivered by clinician led programmes
Ethics statements
Ethical approval.
Not required.
Data availability statement
Additional data are available in the supplementary files.
Contributors: CDM and AJD conceived the study, with support from ES. CDM conducted the search with support from HEG. CDM, AJD, ES, HEG, KG, GB, and VEK completed the screening and full text identification. CDM and VEK completed the risk of bias assessment. CDM extracted data for the primary outcome and study characteristics. HEJ, GB, and KG extracted primary outcome data. CDM completed the analysis in RevMan, and GMJT completed the metaregression analysis in Stata. CDM drafted the paper with AJD. All authors provided comments on the paper. CDM acts as guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: AJD is supported by a National Institute for Health and Care Research (NIHR) research professorship award. This research was supported by the NIHR Leicester Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. ES’s salary is supported by an investigator grant (National Health and Medical Research Council, Australia). GT is supported by a Cancer Research UK fellowship. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: This research was supported by the National Institute for Health and Care Research Leicester Biomedical Research Centre; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work.
The lead author (CDM) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported, and that no important aspects of the study have been omitted.
Dissemination to participants and related patient and public communities: We plan to disseminate these research findings to a wider community through press releases, featuring on the Centre for Lifestyle Medicine and Behaviour website ( www.lboro.ac.uk/research/climb/ ) via our policy networks, through social media platforms, and presentation at conferences.
Provenance and peer review: Not commissioned; externally peer reviewed.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/ .
- Renehan AG ,
- Heller RF ,
- Bansback N ,
- Birmingham CL ,
- Abdullah A ,
- Peeters A ,
- de Courten M ,
- Stoelwinder J
- Aghili SMM ,
- Ebrahimpur M ,
- Arjmand B ,
- KETLAK Study Group
- ↵ Department of Health and Social Care. New specialised support to help those living with obesity to lose weight UK2021. www.gov.uk/government/news/new-specialised-support-to-help-those-living-with-obesity-to-lose-weight [accessed 08/02/2021].
- U.S. Preventive Services Task Force
- ↵ National Institute for Health and Care Excellence. Maintaining a Healthy Weight and Preventing Excess Weight Gain in Children and Adults. Cost Effectiveness Considerations from a Population Modelling Viewpoint. 2014, NICE. www.nice.org.uk/guidance/ng7/evidence/evidence-review-2-qualitative-evidence-review-of-the-most-acceptable-ways-to-communicate-information-about-individually-modifiable-behaviours-to-help-maintain-a-healthy-weight-or-prevent-excess-weigh-8733713.
- ↵ The Health Foundation. Use of primary care during the COVID-19 pandemic. 17/09/2020: The Health Foundation, 2020.
- ↵ Australian Bureau of Statistics. Patient Experiences in Australia: Summary of Findings, 2017-18. 2019 ed. Canberra, Australia, 2018. www.abs.gov.au/AUSSTATS/[email protected]/Lookup/4839.0Main+Features12017-18?OpenDocument.
- LeBlanc ES ,
- Patnode CD ,
- Webber EM ,
- Redmond N ,
- Rushkin M ,
- O’Connor EA
- Damschroder LJ ,
- Liberati A ,
- Tetzlaff J ,
- Altman DG ,
- PRISMA Group
- McKenzie JE ,
- Bossuyt PM ,
- ↵ WHO. Main terminology: World Health Organization; 2004. www.euro.who.int/en/health-topics/Health-systems/primary-health-care/main-terminology [accessed 09.12.21].
- Aceves-Martins M ,
- Robertson C ,
- REBALANCE team
- Glasziou P ,
- Isenring E ,
- Chisholm A ,
- Wakayama LN ,
- Kettle VE ,
- Madigan CD ,
- ↵ Covidence [program]. Melbourne, 2021.
- Welzel FD ,
- Carrington MJ ,
- Fernández-Ruiz VE ,
- Ramos-Morcillo AJ ,
- Solé-Agustí M ,
- Paniagua-Urbano JA ,
- Armero-Barranco D
- Bräutigam-Ewe M ,
- Hildingh C ,
- Yardley L ,
- Christian JG ,
- Bessesen DH ,
- Christian KK ,
- Goldstein MG ,
- Martin PD ,
- Dutton GR ,
- Horswell RL ,
- Brantley PJ
- Wadden TA ,
- Rogers MA ,
- Berkowitz RI ,
- Kumanyika SK ,
- Morales KH ,
- Allison KC ,
- Rozenblum R ,
- De La Cruz BA ,
- Katzmarzyk PT ,
- Martin CK ,
- Newton RL Jr . ,
- Nanchahal K ,
- Holdsworth E ,
- ↵ RevMan [program]. 5.4 version: Copenhagen, 2014.
- Sterne JAC ,
- Savović J ,
- Gomez-Huelgas R ,
- Jansen-Chaparro S ,
- Baca-Osorio AJ ,
- Mancera-Romero J ,
- Tinahones FJ ,
- Bernal-López MR
- Delahanty LM ,
- Tárraga Marcos ML ,
- Panisello Royo JM ,
- Carbayo Herencia JA ,
- Beeken RJ ,
- Leurent B ,
- Vickerstaff V ,
- Hagobian T ,
- Brannen A ,
- Rodriguez-Cristobal JJ ,
- Alonso-Villaverde C ,
- Panisello JM ,
- Conroy MB ,
- Spadaro KC ,
- Takasawa N ,
- Mashiyama Y ,
- Pritchard DA ,
- Hyndman J ,
- Jarjoura D ,
- Smucker W ,
- Baughman K ,
- Bennett GG ,
- Steinberg D ,
- Zaghloul H ,
- Chagoury O ,
- Leslie WS ,
- Barnes AC ,
- Summerbell CD ,
- Greenwood DC ,
- Huseinovic E ,
- Leu Agelii M ,
- Hellebö Johansson E ,
- Winkvist A ,
- Look AHEAD Research Group
- LeBlanc EL ,
- Wheeler GM ,
- Aveyard P ,
- de Koning L ,
- Chiuve SE ,
- Willett WC ,
- ↵ World Health Organization. Obesity and Overweight, 2021, www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- Starfield B ,
- Sturgiss E ,
- Dewhurst A ,
- Devereux-Fitzgerald A ,
- Haesler E ,
- van Weel C ,
- Gulliford MC
- Fassbender JE ,
- Sarwer DB ,
- Brekke HK ,
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 10 October 2022
Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial
- W. Timothy Garvey ORCID: orcid.org/0000-0003-0822-0860 1 ,
- Rachel L. Batterham ORCID: orcid.org/0000-0002-5477-8585 2 , 3 , 4 ,
- Meena Bhatta 5 ,
- Silvio Buscemi ORCID: orcid.org/0000-0003-0730-7649 6 , 7 ,
- Louise N. Christensen 5 ,
- Juan P. Frias 8 ,
- Esteban Jódar ORCID: orcid.org/0000-0002-1234-8560 9 ,
- Kristian Kandler ORCID: orcid.org/0000-0003-0686-0549 5 ,
- Georgia Rigas 10 ,
- Thomas A. Wadden 11 ,
- Sean Wharton 12 &
the STEP 5 Study Group
Nature Medicine volume 28 , pages 2083–2091 ( 2022 ) Cite this article
240k Accesses
151 Citations
899 Altmetric
Metrics details
- Drug therapy
The STEP 5 trial assessed the efficacy and safety of once-weekly subcutaneous semaglutide 2.4 mg versus placebo (both plus behavioral intervention) for long-term treatment of adults with obesity, or overweight with at least one weight-related comorbidity, without diabetes. The co-primary endpoints were the percentage change in body weight and achievement of weight loss of ≥5% at week 104. Efficacy was assessed among all randomized participants regardless of treatment discontinuation or rescue intervention. From 5 October 2018 to 1 February 2019, 304 participants were randomly assigned to semaglutide 2.4 mg ( n = 152) or placebo ( n = 152), 92.8% of whom completed the trial (attended the end-of-trial safety visit). Most participants were female (236 (77.6%)) and white (283 (93.1%)), with a mean (s.d.) age of 47.3 (11.0) years, body mass index of 38.5 (6.9) kg m –2 and weight of 106.0 (22.0) kg. The mean change in body weight from baseline to week 104 was −15.2% in the semaglutide group ( n = 152) versus −2.6% with placebo ( n = 152), for an estimated treatment difference of −12.6 %-points (95% confidence interval, −15.3 to −9.8; P < 0.0001). More participants in the semaglutide group than in the placebo group achieved weight loss ≥5% from baseline at week 104 (77.1% versus 34.4%; P < 0.0001). Gastrointestinal adverse events, mostly mild-to-moderate, were reported more often with semaglutide than with placebo (82.2% versus 53.9%). In summary, in adults with overweight (with at least one weight-related comorbidity) or obesity, semaglutide treatment led to substantial, sustained weight loss over 104 weeks versus placebo. NCT03693430
Similar content being viewed by others
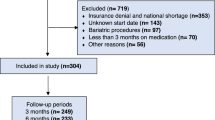
Weight loss and cardiovascular disease risk outcomes of semaglutide: a one-year multicentered study

Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial

Efficacy and safety of the dual GIP and GLP-1 receptor agonist tirzepatide for weight loss: a meta-analysis of randomized controlled trials
Behavioral intervention incorporating modifications in diet and physical activity remains the foundation of treatment for overweight and obesity. However, because behavioral intervention is often not associated with clinically meaningful and sustainable weight loss, pharmacotherapy is recommended as an additional tool for long-term weight management in people with a body mass index (BMI) of at least 30 kg m –2 , or at least 27 kg m –2 in those with weight-related comorbidities 1 .
Semaglutide is a glucagon-like peptide-1 (GLP-1) analog approved for the treatment of type 2 diabetes (oral semaglutide and subcutaneous semaglutide) and for reducing the risk of cardiovascular events in people with type 2 diabetes and cardiovascular disease (subcutaneous semaglutide only) 2 , 3 , 4 , 5 . At a dose of 2.4 mg once-weekly, subcutaneous semaglutide was approved in the United States, Europe, the United Kingdom and Canada for weight management in adults with overweight (BMI ≥ 27 kg m –2 with at least one weight-related comorbidity) or obesity (BMI ≥ 30 kg m –2 ) 2 , 3 , 4 , 5 , based on results from the Semaglutide Treatment Effect in People with Obesity (STEP) clinical trial program. In the STEP 1 and 3 trials in participants without type 2 diabetes, average placebo-subtracted weight losses of 12.4% and 10.3%, respectively, were seen with semaglutide 2.4 mg at week 68 (refs. 6 , 7 ).
Previous studies in the STEP trial program have been limited to treatment durations of up to 68 weeks 6 , 7 , 8 . The 2-year STEP 5 study reported herein was conducted to evaluate the long-term effect of once-weekly subcutaneous semaglutide 2.4 mg compared with placebo, as an adjunct to behavioral intervention, on body weight and cardiometabolic risk factors, in adults with obesity (BMI ≥ 30 kg m –2 ), or with overweight (BMI ≥ 27 kg m –2 ) and at least one weight-related comorbidity, without diabetes (Extended Data Fig. 1 ). This phase 3, randomized, double-blind, placebo-controlled, multinational trial represents the longest study of the use of semaglutide for weight management to date. Co-primary endpoints were percentage change in body weight from baseline to week 104 and achievement of weight loss of at least 5% of baseline weight at week 104.
Participants and treatment
From 5 October 2018 to 1 February 2019, 304 participants were randomly assigned to semaglutide 2.4 mg ( n = 152) or placebo ( n = 152) and included in the full analysis set (all randomized participants according to the intention-to-treat principle). Observation periods included the in-trial period (that is, while in the trial, regardless of treatment discontinuation or rescue intervention) and the on-treatment period (with trial product). Overall, of 304 participants, 282 (92.8%) completed the trial (attended the end-of-trial safety visit), 272 (89.5%) had a body weight assessment at the end-of-treatment visit at week 104, and 243 (79.9%) adhered to treatment (were on-treatment at the end-of-treatment visit) (Fig. 1 ).
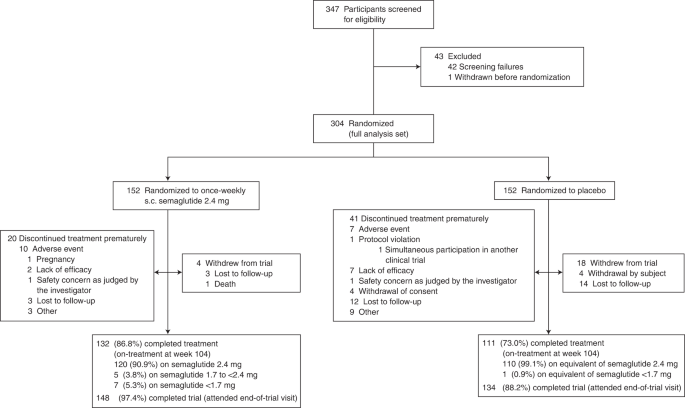
s.c., subcutaneous.
Demographics and baseline characteristics were similar between groups (Table 1 ). Most participants were female (236 (77.6%) of 304) and most were white (283 (93.1%) of 304). Mean age was 47.3 years. Mean body weight was 106.0 kg and mean BMI was 38.5 kg m –2 .
Two estimands were employed for the assessment of efficacy endpoints—estimands assess treatment efficacy from different perspectives and account for intercurrent events (for example, discontinuation of trial product or initiation of other weight loss interventions) and missing data differently. The ‘treatment policy’ estimand quantified the treatment effect for the in-trial period among all randomly assigned participants, regardless of treatment discontinuation or rescue intervention, based on the intention-to-treat principle, and was used as the primary analysis method. The ‘trial product’ estimand quantified the average treatment effect for the on-treatment period in all randomly assigned participants, assuming that the drug or placebo was taken as intended, and was used as the secondary analysis method ( Methods ).
Efficacy endpoint results for the treatment policy estimand
Mean observed change in body weight over time during the in-trial period is shown as percentage change in Fig. 2a and as absolute change (kg) in Extended Data Fig. 2 . Based on the treatment policy estimand, the estimated mean (standard error (s.e.)) change in body weight from baseline to week 104 was –15.2% (0.9) with semaglutide and –2.6% (1.1) with placebo (co-primary endpoint; estimated treatment difference (ETD) –12.6 percentage points, 95% confidence interval (CI) –15.3 to –9.8, P < 0.0001). Semaglutide-treated participants, compared with placebo, were more likely to lose at least 5% of baseline body weight at week 104 (co-primary endpoint; odds ratio (OR) 5.0, 95% CI 3.0 to 8.4; P < 0.0001). At week 104, 111 (77.1%) versus 44 (34.4%) participants in the semaglutide and placebo groups, respectively, were observed to have achieved this endpoint (in-trial period data; among 144 participants for semaglutide and 128 for placebo) (Table 2 and Fig. 2b ). As statistical superiority for both co-primary endpoints was demonstrated for semaglutide versus placebo, the prespecified criteria for a positive trial were met, indicating a significant benefit of semaglutide versus placebo.
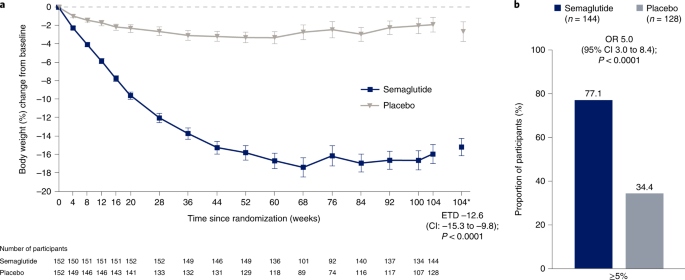
a , Observed mean percentage change from baseline in body weight over time for participants in the full analysis set during the in-trial observation period (error bars are standard error of the mean; numbers below the panels are the number of participants contributing to the mean) and estimated treatment difference for the percentage change from baseline to week 104 in body weight based on the treatment policy estimand. b , Observed proportions of participants and OR for achieving weight loss of at least 5% from baseline at week 104 in the full analysis set during the in-trial observation period, based on the treatment policy estimand. *Estimated means in percent are from the primary analysis. The in-trial observation period was the time from random assignment to last contact with a trial site, regardless of treatment discontinuation or rescue intervention. The treatment policy estimand assesses treatment effect regardless of treatment discontinuation or rescue intervention; see Extended Data Fig. 6 for corresponding data for the trial product estimand (which assesses treatment effect assuming all participants adhered to treatment and did not receive rescue intervention). The change in body weight analysis was conducted with the use of the analysis-of-covariance method, with randomized treatment as a factor and baseline body weight as a covariate. The achievement of at least 5% weight loss analysis was conducted with the use of logistic regression, with the same factor and covariate. A multiple imputation approach was used for missing data. The results were accompanied by two-sided 95% CIs and corresponding P values (significance defined as P < 0.05). As co-primary endpoints, the analyses were controlled for multiple comparisons.
Semaglutide-treated participants, compared with placebo, were also more likely to lose at least 10%, 15% or 20% of baseline body weight at week 104 ( P < 0.0001 for the OR for the 10% and 15% thresholds (both were confirmatory secondary endpoints); the 20% threshold (a supportive secondary endpoint) was not part of statistical testing hierarchy). For the in-trial observation period, these weight loss thresholds were achieved by 89 (61.8%), 75 (52.1%) and 52 (36.1%) of 144 participants in the semaglutide group versus 17 (13.3%), nine (7.0%) and three (2.3%) of 128 participants in the placebo group, respectively (Table 2 and Extended Data Fig. 3 for cumulative distribution of change from baseline).
Semaglutide was associated with greater reductions from baseline to week 104 in waist circumference (–14.4 cm (0.9) with semaglutide versus –5.2 cm (1.2) with placebo; ETD –9.2 cm, 95% CI –12.2 to –6.2, P < 0.0001) and systolic blood pressure (–5.7 mmHg (1.1) with semaglutide versus –1.6 (1.2) with placebo; ETD –4.2 mmHg, 95% CI –7.3 to –1.0; P = 0.01) (both were confirmatory secondary endpoints; Table 2 , Fig. 2 and Extended Data Fig. 4a,b ). Compared with placebo, semaglutide also led to improvements in diastolic blood pressure, glycated hemoglobin (HbA 1c ), fasting plasma glucose, fasting serum insulin, C-reactive protein, total cholesterol, low-density lipoprotein cholesterol, very-low-density lipoprotein cholesterol and triglycerides (all were supportive secondary endpoints; Table 2 and Extended Data Fig. 4c,d ).
Of the participants with prediabetes at baseline who also had a glycemic status assessment at week 104, 59 (79.7%) of 74 treated with semaglutide reverted to normoglycemia at week 104, compared with 20 (37.0%) of 54 participants on placebo (an exploratory endpoint; Table 2 and Extended Data Fig. 5 ). Of the participants with normoglycemia at baseline who also had a glycemic status assessment at week 104, one (1.4%) of 71 treated with semaglutide had prediabetes at week 104, compared with 10 (13.0%) of 77 participants on placebo. Among participants with a week 104 assessment, none in the semaglutide group and three in the placebo group had type 2 diabetes at week 104 (one had normoglycemia at baseline and two had prediabetes at baseline). The proportion of participants with changes in the use of lipid-lowering and antihypertensive medication (among those receiving such medications during the trial) is reported in Table 2 (both were exploratory endpoints).
Efficacy endpoint results for the trial product estimand
Mean observed change in body weight over time during the on-treatment period is shown in Extended Data Fig. 6a . For the trial product estimand, the estimated mean (s.e.) change in body weight from baseline to week 104 was –16.7% (0.9) with semaglutide and –0.6% (0.9) for placebo (ETD –16.0 percentage points, 95% CI –18.6 to –13.5). Semaglutide-treated participants, compared with placebo, were more likely to lose at least 5% of baseline body weight at week 104 (OR 18.1 (95% CI 10.0 to 32.5). At week 104, 110 (83.3%) versus 38 (34.9%) participants in the semaglutide and placebo groups, respectively, were observed to have achieved this endpoint (on-treatment period data; among 132 participants for semaglutide and 109 for placebo) (Supplementary Table 1 and Extended Data Fig. 6b ). Results of analyses of the confirmatory and selected supportive secondary endpoints for the trial product estimand, are provided in Supplementary Table 1 .
Safety and tolerability
Adverse events leading to discontinuation of trial product were reported by nine participants (5.9%) in the semaglutide group and seven participants (4.6%) in the placebo group (Table 3 ).
Gastrointestinal disorders, namely nausea, diarrhea, vomiting and constipation, were the most frequently reported adverse events and occurred in more participants treated with semaglutide than with placebo (125 (82.2%) of 152 versus 82 (53.9%) of 152, respectively) (Table 3 ). Most gastrointestinal adverse events were mild-to-moderate and transient, and such events led to permanent treatment discontinuation in six (3.9%) participants in the semaglutide group and one (0.7%) participant in the placebo group (Table 3 and Extended Data Fig. 7 ).
Serious adverse events were reported by 12 (7.9%) of 152 participants in the semaglutide group and 18 (11.8%) of 152 participants in the placebo group (Table 3 ). One death was reported in the semaglutide group and was considered by the independent external event adjudication committee to be unrelated to the trial product (Table 3 ). In the semaglutide versus placebo groups, gallbladder-related disorders were reported by four (2.6%) versus two (1.3%) participants and malignant neoplasms were reported by two (1.3%) versus four (2.6%), respectively (Table 3 ; details on malignant neoplasms are shown in Supplementary Table 2 ). There were no reports of pancreatitis in either treatment group. Additional safety variables are described in Table 3 and Supplementary Table 3 . COVID-19 infection was reported by 16 (10.5%) of 152 participants in the semaglutide group versus eight (5.3%) of 152 participants in the placebo group, with very few cases in each group classed as serious and none requiring temporary or permanent interruption of semaglutide treatment.
In STEP 5, once-weekly treatment with semaglutide 2.4 mg as an adjunct to behavioral intervention in adults with overweight (with at least one weight-related comorbidity) or obesity led to a substantial initial reduction in weight, which plateaued after approximately week 60 and was maintained for the remainder of the study. At week 104, participants in the semaglutide group had achieved a mean weight loss of 15.2% from baseline—a difference of 12.6 percentage points versus placebo plus behavioral intervention. This weight loss is comparable to the mean reduction of 14.9% (placebo-corrected weight loss of 12.4 percentage points) seen at week 68 in the STEP 1 trial of semaglutide 2.4 mg versus placebo (both plus behavioral intervention) 7 . Thus, our findings indicate that the substantial weight losses reported during 68 weeks’ treatment with semaglutide 2.4 mg in prior STEP trials 6 , 7 , 9 can be maintained with continued semaglutide treatment up to at least 104 weeks. The mean weight loss of ~15% achieved with semaglutide 2.4 mg at week 104 in STEP 5 exceeds weight loss reported at similar time points in trials with other pharmacotherapies for weight management in adults with overweight or obesity 10 , 11 , 12 , 13 , 14 .
Weight loss of ≥5%, a threshold widely used to indicate a clinically meaningful response to therapy 15 , was achieved by >75% of participants in the semaglutide group at week 104. Moreover, 61.8% of participants on semaglutide lost ≥10% of baseline weight, and over a third of participants had achieved at least 20% weight loss at week 104 in the semaglutide group. As was seen in prior studies 6 , 7 , 9 , 16 , while the vast majority of participants receiving semaglutide 2.4 mg had lost weight at the end of the STEP 5 study, a small proportion of participants experienced weight gain. We do not know how weight would have changed in these participants had they not been receiving the drug; notably, the proportion of patients with weight gain during the study was substantially higher in the placebo group. There is marked variability in weight change in patients on weight management treatments; the reason for this is still unclear and likely involves complex biological and societal influences.
Obesity is a chronic, relapsing disease that requires continuous effort to control 6 , 17 . With all nonsurgical interventions and to some extent with bariatric surgery, weight regain after initial weight loss is common 10 , 11 , 12 , 13 , 14 , 18 , 19 , 20 , 21 , 22 . In contrast to findings with behavioral 20 , 21 , 22 and other pharmacological interventions 10 , 12 , 13 , the similar mean weight loss achieved with semaglutide 2.4 mg in STEP 5 at weeks 52 and 104 (–15.6% and –15.2%, respectively) suggests that, on average, there is minimal weight regain over 104 weeks when once-weekly semaglutide therapy is continued. When interpreted together with the findings of the STEP 4 withdrawal trial and STEP 1 off-treatment extension study, which both showed weight regain after semaglutide discontinuation (after 20 weeks’ treatment in STEP 4 and 68 weeks’ treatment in STEP 1) 23 , 24 , these results support the benefit of continued semaglutide treatment for sustained weight loss.
Prior 68-week trials in adults with overweight or obesity have reported cardiometabolic improvements with semaglutide 2.4 mg (refs. 6 , 7 , 9 , 16 ). Consistent with these findings, in STEP 5 semaglutide treatment improved a range of cardiometabolic risk parameters, including waist circumference, systolic and diastolic blood pressure, HbA 1c levels, total cholesterol, low-density lipoprotein cholesterol, very-low-density lipoprotein cholesterol and triglycerides. Collectively, these results indicate a beneficial effect of treatment on overall patient health. In addition, semaglutide treatment reduced C-reactive protein levels, a marker of systemic inflammation that is known to be elevated in patients with obesity 25 , 26 . The reduction in fasting insulin and glucose with semaglutide is indicative of an increase in insulin sensitivity. Similar to the findings of other studies in the STEP trial program 7 , 27 , exploratory outcomes showed that in the semaglutide group 80% of participants with prediabetes at baseline reverted to normoglycemia by the end of the trial (compared with 37% of those receiving placebo), while 99% of participants with normoglycemia at baseline maintained normoglycemia at the end of the trial (compared with 86% with placebo). These findings suggest a potential beneficial effect of semaglutide on glycemic status, but whether semaglutide treatment delays or prevents progression to type 2 diabetes requires confirmation. In the 68-week trials 7 , 9 , reductions in weight, waist circumference, blood pressure and HbA 1c appeared to plateau around week 60 with semaglutide. STEP 5 shows that the changes in these parameters were sustained through 104 weeks’ treatment.
The safety profile of semaglutide 2.4 mg in STEP 5 was consistent with that in other STEP program trials 6 , 7 , 9 , 16 , 23 , and with the GLP-1 receptor agonist class in general 28 . Gastrointestinal disorders were the most common adverse events with semaglutide, typically transient, of mild-to-moderate severity, occurring during dose escalation, and infrequently leading to treatment discontinuation.
Strengths of STEP 5 include the high rates of adherence to treatment and completion of the trial (which contributed to consistency in findings between the two estimands). Limitations include the low proportion of nonwhite participants and the preponderance of female participants. In addition, while the homogenous nature of the prescribed dietary intake deficit, physical activity goal and counseling frequency provided consistency, it may not fully reflect the need for approaches tailored to the health profiles of individuals or to different populations in clinical practice; however, beyond adherence to the stipulated criteria for counseling on diet and physical activity, behavioral intervention was delivered by each study site with no further direction, allowing a degree of local tailoring and aiding real-world applicability.
In conclusion, treatment with once-weekly subcutaneous semaglutide in conjunction with behavioral intervention in adults with overweight (with at least one weight-related comorbidity) or obesity (without diabetes) was associated with clinically impactful and sustained weight loss of 15.2% at week 104, along with improvements in weight-related cardiometabolic risk factors.
Trial design and participants
This phase 3, randomized, double-blind, placebo-controlled study was conducted at 41 sites across five countries (Canada, Italy, Hungary, Spain and the United States), as described in a previous publication 8 and listed in the Supplementary information . Most investigators specialized in endocrinology and internal medicine, with others specializing in family medicine, psychiatry and clinical psychology. The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was approved by independent ethics committees or institutional review boards at each study site (a redacted protocol is provided separately).
Participants were eligible to be included in the trial only if all of the following criteria applied:
Informed consent obtained before any trial-related activities. Trial-related activities were any procedures that were carried out as part of the trial, including activities to determine suitability for the trial.
Male or female, aged ≥18 years at the time of signing informed consent.
BMI ≥ 30.0 kg m – 2 or ≥27.0 kg m –2 with the presence of at least one of the following weight-related comorbidities (treated or untreated): hypertension, dyslipidemia, obstructive sleep apnea or cardiovascular disease.
History of at least one self-reported unsuccessful dietary effort to lose body weight.
Participants were excluded from the trial if any of the following criteria applied:
Glycemia-related
HbA 1c ≥ 48 mmol mol –1 (6.5%) as measured by the central laboratory at screening.
History of type 1 or type 2 diabetes.
Treatment with glucose-lowering agent(s) within 90 days before screening.
Obesity-related
A self-reported change in body weight >5 kg (11 lbs) within 90 days before screening irrespective of medical records.
Treatment with any medication for the indication of obesity within the past 90 days before screening.
Previous or planned (during the trial period) obesity treatment with surgery or a weight loss device. However, the following were allowed: (1) liposuction and/or abdominoplasty, if performed >1 year before screening; (2) lap banding, if the band had been removed >1 year before screening; (3) intragastric balloon, if the balloon had been removed >1 year before screening; or (4) duodenal-jejunal bypass sleeve, if the sleeve had been removed >1 year before screening.
Uncontrolled thyroid disease, defined as thyroid-stimulating hormone >6.0 mIU l –1 or <0.4 mIU l –1 as measured by the central laboratory at screening.
Mental health
History of major depressive disorder within 2 years before screening.
Diagnosis of other severe psychiatric disorder (for example, schizophrenia, bipolar disorder).
A Patient Health Questionnaire-9 score of ≥15 at screening.
A lifetime history of a suicidal attempt.
Suicidal behavior within 30 days before screening.
Suicidal ideation corresponding to type 4 or 5 on the Columbia-Suicide Severity Rating Scale within the past 30 days before screening.
General safety
Presence of acute pancreatitis within the past 180 days before the day of screening.
History or presence of chronic pancreatitis.
Calcitonin ≥100 ng l –1 as measured by the central laboratory at screening.
Personal or first-degree relative(s) history of multiple endocrine neoplasia type 2 or medullary thyroid carcinoma.
Renal impairment measured as estimated glomerular filtration rate value of <15 ml min 1.73 m –2 as defined by KDIGO 2012 (ref. 30 ) by the central laboratory at screening.
History of malignant neoplasms within the past 5 years before screening. Basal and squamous cell skin cancer and any carcinoma in situ were allowed.
Any of the following: myocardial infarction, stroke, hospitalization for unstable angina or transient ischemic attack within the past 60 days before screening.
Participant classified as being in New York Heart Association Class IV.
Surgery scheduled for the duration of the trial, except for minor surgical procedures, in the opinion of the investigator.
Known or suspected abuse of alcohol or recreational drugs.
Known or suspected hypersensitivity to trial product(s) or related products.
Previous participation in the trial. Participation was defined as signed informed consent.
Participation in another clinical trial within 90 days before screening.
Other person(s) from the same household participating in any semaglutide trial.
Female who was pregnant, breast-feeding, or intended to become pregnant, or was of child-bearing potential and not using a highly effective contraceptive method.
Any disorder, unwillingness or inability not covered by any of the other exclusion criteria which, in the investigator’s opinion, might have jeopardized the participant’s safety or compliance with the protocol.
Randomization and masking
Randomization (1:1) to semaglutide 2.4 mg or placebo was done centrally by the clinical research organization (Parexel) in a double-blind manner using an interactive web-based response system (IWRS) with a fixed-size blocking schema, without stratification. The IWRS generated the randomization list and assigned patients to the next available treatment according to the randomization schedule. The IWRS allocated dispensing unit numbers for each patient, with the trial product dispensed by the site investigator or study coordinator at the trial site visits. The active product and corresponding placebo product were visually identical to maintain masking of participants and site staff. The people analyzing the data were blinded to treatment/group assignment until breaking the blinding at database lock.
Participants received subcutaneous semaglutide 2.4 mg or placebo once-weekly for 104 weeks, in addition to standard behavioral intervention, followed by 7 weeks without treatment. Semaglutide was initiated at 0.25 mg per week for the first 4 weeks via a pre-filled pen injector, escalating in a fixed-dose regimen every 4 weeks to reach the maintenance dose of 2.4 mg by week 16 (lower maintenance doses were permitted if participants were unable to tolerate 2.4 mg) (Extended Data Fig. 1 ). Behavioral intervention consisted of counseling by a dietitian or similarly qualified healthcare professional every 4 weeks via in-person visits or telephone on adherence to a reduced-calorie diet (500 kcal deficit a day relative to the energy expenditure estimated at randomization) and increased physical activity (150 minutes a week encouraged, for example, walking), both recorded daily (via a diary, app or other tools, which were reviewed during counseling sessions); beyond these criteria for behavioral intervention, no further standardization of behavioral intervention was applied across study sites. Participants discontinuing treatment prematurely remained in the trial and were encouraged to attend scheduled visits, particularly those at weeks 104 and 111.
Body weight, waist circumference and vital signs (systolic and diastolic blood pressure and pulse) were measured at baseline; these measurements were repeated every 4 weeks until week 20, and every 8 weeks thereafter, until week 100 and week 104 (within 3 days either side of scheduled visit day). These parameters were also measured at the end-of-trial visit at week 111 (within 5 days either side of scheduled visit day). Height was measured at screening. HbA 1c , fasting plasma glucose, lipids and C-reactive protein were measured at baseline and weeks 20, 52, 84, and 104; electrocardiograms were also performed at these time points. Fasting serum insulin was measured at baseline and week 104. Physical examinations were performed at screening and weeks 52 and 104. Hematology and biochemistry laboratory parameters were measured at screening and weeks 20, 52, 84 and 104. Adverse events were recorded at each visit. Control of eating was assessed in a subset of participants from the United States and Canada; these results will be presented in a separate manuscript.
Given the emergence of COVID-19 in the second year of the study, trial visits were permitted to be conducted via telephone, during which counseling was provided and safety-related information was collected; endpoint assessments were not performed during telephone visits. Assessment data were collected at the next possible in-person visit.
Co-primary endpoints were percentage change in body weight from baseline to week 104 and achievement of weight loss of at least 5% of baseline weight at week 104. These were tested first in the statistical testing hierarchy, followed by the confirmatory secondary endpoints, which were tested in the following order: achievement of weight loss of at least 10% or 15% at week 104; and change from baseline to week 104 in waist circumference and systolic blood pressure.
Supportive secondary endpoints were not included in the statistical testing hierarchy and were: achievement of weight loss of ≥20% at week 104; change from baseline to week 104 in body weight (in kg), BMI, HbA 1c , fasting plasma glucose, fasting serum insulin, diastolic blood pressure, lipids (total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, very-low-density lipoprotein cholesterol, free fatty acids and triglycerides) and C-reactive protein; change from baseline to week 52 in body weight (percentage change and kg change), BMI and waist circumference; and achievement of weight loss of ≥5%, ≥10%, ≥15% and ≥20% at week 52.
Exploratory endpoints reported herein include change from baseline to week 104 in glycemic category, antihypertensive medication use and lipid-lowering medication use. Glycemic category (normoglycemia, prediabetes or type 2 diabetes) was determined by investigators on the basis of available information (for example, medical records, concomitant medication, and blood glucose variables) and in accordance with American Diabetes Association criteria 30 , which for prediabetes includes fasting plasma glucose levels of 100 mg dl –1 (5.6 mmol l –1 ) to 125 mg dl –1 (6.9 mmol l –1 ) or HbA 1c levels of 5.7–6.4% (39–47 mmol l –1 ), and for type 2 diabetes includes fasting plasma glucose levels of ≥126 mg dl –1 (7.0 mmol l –1 ) or HbA 1c levels ≥6.5% (48 mmol l –1 ). The allowance for investigators to use all available information (for example, concomitant medication) to assess glycemic category was primarily included to account for scenarios in which glucose-lowering medications were initiated during the trial that would confound glycemic category assessment if based purely on fasting plasma glucose or HbA 1c levels (for example, if a patient developed diabetes during the study and received a glucose-lowering drug that resulted in their glucose level being below the American Diabetes Association threshold for type 2 diabetes diagnosis). Additional exploratory endpoints for which data are not reported were: permanent discontinuation of trial product between baseline and week 104; time to permanent discontinuation of trial product; and Control of Eating Questionnaire scores from the four domains and 19 individual items (applicable for United States and Canada only).
Safety endpoints included the number of treatment-emergent adverse events and serious adverse events, assessed between baseline and week 111; and change from baseline to week 104 in pulse, amylase, lipase and calcitonin. An independent external event adjudication committee reviewed cardiovascular events, acute pancreatitis and deaths.
Statistical analysis
A sample size of 300 participants provided an effective power of at least 96% for the two co-primary endpoints, and at least 43% for all confirmatory secondary endpoints, which were tested in a predefined hierarchical order (Supplementary Table 4 ). The two co-primary endpoints were analyzed independently of each other, and for the trial to be considered to be positive (indicating a significant benefit of semaglutide versus placebo), statistical superiority for both co-primary endpoints was required to be demonstrated.
Efficacy endpoints were analyzed using the full analysis set (all randomized participants according to the intention-to-treat principle). Safety endpoints were analyzed using the safety analysis set of all randomized participants exposed to at least one dose of randomized treatment. Observation periods included the in-trial period (that is, while in the trial, regardless of treatment discontinuation or rescue intervention) and the on-treatment period (with trial product). All results from statistical analyses of confirmatory endpoints were accompanied by two-sided 95% CIs and corresponding P values (significance defined as P < 0.05). Supportive secondary endpoint analyses were not controlled for multiple comparisons and should not be used to infer definitive treatment effects.
Two estimands were employed to assess treatment efficacy from different perspectives and accounted for intercurrent events and missing data differently, as described in a previous publication 31 . The treatment policy estimand quantified the treatment effect among all randomly assigned participants, regardless of treatment discontinuation or rescue intervention (participants in trial; intention to treat). This estimand was used to assess the superiority of semaglutide versus placebo for the co-primary and confirmatory secondary endpoints in a predefined hierarchical order.
For the treatment policy estimand, continuous endpoint analyses were conducted with the use of the analysis-of-covariance method, with randomized treatment as a factor and baseline endpoint value as a covariate. Analyses of categorical endpoints were conducted with the use of logistic regression, with the same factor and covariate. A multiple imputation approach was used to handle missing data 31 , with imputation based on available data from participants in the same treatment arm with the same treatment status (on-treatment or discontinued). Imputation was performed using a linear regression model, with sex, baseline BMI and timing of last observation as factors, and baseline value and last observation value as covariates. One thousand complete datasets were generated for analysis, with results combined using Rubin’s formula.
The trial product estimand addressed the average treatment effect in all randomly assigned participants, assuming that the drug or placebo was taken as intended (participants on treatment). For the trial product estimand, continuous endpoint analyses were conducted using a mixed model for repeated measures with randomized treatment as a factor and baseline endpoint value as a covariate. Analyses of categorical endpoints were conducted with the use of logistic regression, with categorization for missing data based on values predicted from the mixed model for repeated measures. Analyses of endpoints for the trial product estimand were not adjusted for multiplicity.
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc.). Additional details on analytic methods per endpoint are in Supplementary Table 4 . Exploratory endpoints were assessed with descriptive statistics based on observed data.
The trial is closed and completed. The study is registered with ClinicalTrials.gov, NCT03693430 .
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Data will be shared with bona fide researchers submitting a research proposal approved by the independent review board. The research proposal must outline: the scientific rationale and relevance of the proposed research; a short lay summary intended for public disclosure; research methodology and data; statistical analysis plan and publication plan. Data must not be used for commercial purposes. Data will be made available after research completion, and approval of the product and product use in the European Union and the USA. Individual participant data will be shared in datasets in a de-identified and anonymized format. Access request proposals can be found at novonordisk-trials.com.
Jensen, M. D. et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 129 , S102–S138 (2014).
Article Google Scholar
Wegovy prescribing information. Updated June 2021. US Food and Drug Administration https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215256s000lbl.pdf (2022).
Wegovy summary of product characteristics. European Medicines Agency https://www.ema.europa.eu/en/documents/product-information/wegovy-epar-product-information_en.pdf (2022).
Regulatory decision summary – Wegovy. Health Canada https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.php?lang=en&linkID=RDS00896 (2022).
Wegovy summary of product characteristics. Medicines and Healthcare Products Regulatory Agency https://mhraproducts4853.blob.core.windows.net/docs/ee95471f9265e346688fce45e8c9fd8bd5e4e3d3 (2022).
Wadden, T. A. et al. STEP 3 Investigators. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA 325 , 1403–1413 (2021).
Article CAS Google Scholar
Wilding, J. P. H. et al. STEP 1 Study Group. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384 , 989–1002 (2021).
Kushner, R. F. et al. Semaglutide 2.4 mg for the treatment of obesity: key elements of the STEP trials 1 to 5. Obesity (Silver Spring) 28 , 1050–1617 (2020).
Davies, M. et al. STEP 2 Study Group. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 397 , 971–984 (2021).
Sjöström, L. et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet 352 , 167–72. (1998).
Garvey, W. T. et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am. J. Clin. Nutr. 95 , 297–308 (2012).
Torgerson, J. S. et al. XENical in the Prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 27 , 155–161 (2004).
Astrup, A. et al. NN8022-1807 Investigators. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J. Obes. (Lond.) 36 , 843–854 (2012).
le Roux, C. W. et al. SCALE Obesity and Prediabetes NN8022-1839 Study Group. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet 389 , 1399–409. (2017).
PubMed Google Scholar
Williamson, D. A., Bray, G. A. & Ryan, D. H. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity (Silver Spring) 23 , 2319–2320 (2015).
Rubino, D. M. et al. STEP 8 Investigators. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA 327 , 138–150 (2022).
Bray, G. A., Kim, K. K. & Wilding, J. P. H. World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 18 , 715–723 (2017).
Smith, S. R. et al. Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group. Multicenter, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med. 363 , 245–256 (2010).
MacLean, P. S. et al. NIH working group report: innovative research to improve maintenance of weight loss. Obesity (Silver Spring) 23 , 7–15 (2015).
Svetkey, L. P. et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA 299 , 1139–1148 (2008).
Wadden, T. A. et al. Four-year weight losses in the Look AHEAD study: factors associated with success. Obesity (Silver Spring) 19 , 1987–1998 (2011).
Wing, R. R., Tate, D. F., Gorin, A. A., Raynor, H. A. & Fava, J. L. A self-regulation program for maintenance of weight loss. N. Engl. J. Med. 355 , 1563–1571 (2006).
Rubino, D. et al. STEP 4 Investigators. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA 325 , 1414–1425 (2021).
Wilding, J. P. et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: the STEP 1 trial extension. Diabetes Obes. Metab. 24 , 1553–1564 (2022).
Visser, M., Bouter, L. M., McQuillan, G. M., Wener, M. H. & Harris, T. B. Elevated C-reactive protein levels in overweight and obese adults. JAMA 282 , 2131–2135 (1999).
Timpson, N. J. et al. C-reactive protein levels and body mass index: elucidating direction of causation through reciprocal Mendelian randomization. Int J. Obes. (Lond.) 35 , 300–308 (2011).
Perreault, L. et al. Changes in glucose metabolism and glycemic status with once-weekly subcutaneous semaglutide 2.4 mg among participants with prediabetes in the STEP Program. Diabetes Care 45 , 2396–2405 (2022).
Nauck, M. A., Quast, D. R., Wefers, J. & Meier, J. J. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol. Metab. 46 , 101102 (2021).
American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 40 , S11–S24 (2017).
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3 , 1–150 (2013).
Google Scholar
Wharton, S. et al. Estimating and reporting treatment effects in clinical trials for weight management: using estimands to interpret effects of intercurrent events and missing data. Int J. Obes. (Lond.) 45 , 923–933 (2021).
Download references
Acknowledgments
We thank the study participants, and the investigators and study site staff who conducted the study. In addition, we thank N. Beadle of Axis, a division of Spirit Medical Communications Group Limited, for medical writing and editorial assistance (funded by Novo Nordisk A/S, Denmark). The study was funded by Novo Nordisk. The funder designed the trial, oversaw its conduct, monitored trial sites, and collected and analyzed the data; investigators were responsible for trial-related medical decisions and data collection. This article was drafted under the guidance of the authors, with medical writing and editorial support paid for by the funder.
Author information
Authors and affiliations.
Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, AL, USA
- W. Timothy Garvey
University College London Centre for Obesity Research, Division of Medicine, University College London, London, UK
Rachel L. Batterham
National Institute of Health Research, UCLH Biomedical Research Centre, London, UK
Centre for Weight Management and Metabolic Surgery, University College London Hospital, London, UK
Novo Nordisk A/S, Søborg, Denmark
Meena Bhatta, Louise N. Christensen & Kristian Kandler
Unit of Clinical Nutrition, Policlinico University Hospital, Palermo, Italy
Silvio Buscemi
Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties, University of Palermo, Palermo, Italy
National Research Institute, Los Angeles, CA, USA
Juan P. Frias
Department of Endocrinology and Nutrition, Hospital Universitario QuironSalud Madrid, Universidad Europea de Madrid, Madrid, Spain
Esteban Jódar
Department of Bariatric Metabolic Surgery, St George Private Hospital, Kogarah, Sydney, Australia
Georgia Rigas
Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
Thomas A. Wadden
York University, McMaster University and Wharton Weight Management Clinic, Toronto, ON, Canada
Sean Wharton
You can also search for this author in PubMed Google Scholar
- , Rachel L. Batterham
- , Silvio Buscemi
- , Juan P. Frias
- , Esteban Jódar
- , Georgia Rigas
- , Thomas A. Wadden
- & Sean Wharton
Contributions
W.T.G. contributed to acquisition, analysis and interpretation of data; drafting of the manuscript; and critical revision of the manuscript for important intellectual content. R.L.B. contributed to analysis or interpretation of data; drafting of the manuscript; and critical revision of the manuscript for important intellectual content. M.B. contributed to analysis and interpretation of data; drafting of the manuscript; and critical revision of manuscript for important intellectual content. S.B. contributed to concept and design; acquisition, analysis or interpretation of data; drafting of the manuscript; and critical revision of the manuscript for important intellectual content. L.N.C. contributed to analysis and interpretation of data; critical revision of manuscript for important intellectual content; and statistical analysis. J.P.F. contributed to acquisition, analysis or interpretation of data; critical revision of the manuscript for important intellectual content; and supervision. E.J. contributed to acquisition, analysis or interpretation of data; drafting of the manuscript; and critical revision of the manuscript for important intellectual content. K.K. contributed to analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; and administrative, technical or material support. G.R. contributed to interpretation of data; drafting of the manuscript; and critical revision of the manuscript for important intellectual content. T.A.W. contributed to interpretation of data; drafting of the manuscript; and critical revision of the manuscript for important intellectual content. S.W. contributed to acquisition, analysis or interpretation of data; drafting of the manuscript; and critical revision of the manuscript for important intellectual content. Investigators were responsible for data collection, and the sponsor undertook site monitoring, data collation and analysis. All authors had full access to aggregated study data and to unaggregated data on request from the sponsor; participated in the data interpretation, presentation and manuscript drafting (assisted by a sponsor-funded medical writer); approved its submission, and vouched for data accuracy and fidelity to the protocol.
Corresponding author
Correspondence to W. Timothy Garvey .
Ethics declarations
Competing interests.
W.T.G. reports a grant from Novo Nordisk; serving as site principal investigator for the current clinical trial, which was sponsored by his university during the conduct of the study; and receiving grants to serve as site principal investigator for other university-sponsored clinical trials funded by Eli Lilly & Company, Lexicon, Epitomee and Pfizer outside the submitted work. He also served as a compensated consultant on advisory committees for Alnylam, Amgen, Boehringer Ingelheim, Fractyl and Novo Nordisk, and a volunteer uncompensated consultant on advisory committees for Boehringer Ingelheim, Jazz Pharmaceuticals, Novo Nordisk and Pfizer. R.L.B. reports research grant support, on behalf of their institution, from Novo Nordisk and advisory/consultancy fees from Boehringer Ingelheim, Eli Lilly & Company, Gila Therapeutics Inc, GLW-01, International Medical Press, Novo Nordisk, Pfizer and ViiV. M.B. is an employee of Novo Nordisk A/S. S.B. served as site principal investigator for the clinical trial (he received no financial compensation, nor was there a financial relationship) and reports advisory/consulting fees and/or other support from Boehringer Ingelheim, Eli Lilly & Company, Guidotti Laboratories, Menarini Diagnostics, Novo Nordisk and Therascience Lignaform. L.N.C. is an employee of Novo Nordisk A/S. J.P.F. reports research support grants from Akero, AstraZeneca, Boehringer Ingelheim, BMS, 89bio, Eli Lilly & Company, Intercept, IONIS, Janssen, Madrigal, Metacrine, Merck, NorthSea Therapeutics, Novartis, Novo Nordisk, Oramed, Pfizer, Poxel and Sanofi; and advisory/consultancy fees from Akero, Altimmune, Axcella Health, Becton Dickenson, Boehringer Ingelheim, Carmot Therapeutics, Echosens, 89bio, Eli Lilly & Company, Gilead, Intercept, Metacrine, Merck, Novo Nordisk, Pfizer and Sanofi. E.J. reports grants from Amgen, AstraZeneca, Boehringer Ingelheim, FAES, Janssen, Eli Lilly & Company, MSD, Novo Nordisk, Pfizer, Sanofi, Shire and UCB; personal fees from Amgen, AstraZeneca, FAES, Helios-Fresenius, Italfármaco, Eli Lilly & Company, MSD, Mundipharma, Novo Nordisk, UCB and Viatris. K.K. is an employee of Novo Nordisk A/S. G.R. reports personal (advisory/consultancy and lecture) fees and nonfinancial support from iNova Pharmaceuticals, Nestle HealthScience and Novo Nordisk; personal (lecture) fees from Johnson & Johnson, Medtronic (formerly Covidien), Merck Sharpe & Dohme, ReShape Lifesciences (formerly Apollo-Endosurgery and Allergan Australia) and W.L. Gore Device Technologies. T.A.W. serves on advisory boards for Novo Nordisk and WW (formerly Weight Watchers), and has received grant support, on behalf of the University of Pennsylvania, from Novo Nordisk and from Epitomee Medical Ltd (the latter outside of the submitted work). S.W. reports research funding, advisory/consulting fees and/or other support from AstraZeneca, Bausch Health Inc., Boehringer Ingelheim, CIHR, Janssen, Eli Lilly & Company and Novo Nordisk.
Peer review
Peer review information.
Nature Medicine thanks George Bray, Amanda Adler, Victor Volovici and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Jennifer Sargent, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 trial design for step 5 clinical study., extended data fig. 2 body weight (kg) by week..
Observed mean body weight (kg) over time for participants in the full analysis set during the in-trial observation period (from randomization to last contact with trial site, regardless of treatment discontinuation or rescue intervention). Error bars are standard error of the mean. Numbers below the panels are the number of participants contributing to the mean.
Extended Data Fig. 3 Cumulative distribution plot of change from baseline to week 104 in body weight.
( a , b ) Cumulative distribution plot of observed percentage change from baseline over time in body weight for participants in the full analysis set during the in-trial observation period* (a) and on-treatment observation period † (b). *From randomization to last contact with trial site, regardless of treatment discontinuation or rescue intervention. † During treatment with trial product (any dose of trial medication administered within the previous 2 weeks (that is, any period of temporary treatment interruption with trial product was excluded)).
Extended Data Fig. 4 Comparison of change from baseline by week for selected cardiometabolic endpoints for semaglutide versus placebo.
( a - d ) Observed mean percentage change from baseline over time for participants in the full analysis set during the in-trial observation period in waist circumference (a), systolic blood pressure (b), diastolic blood pressure (c), and HbA 1c (d). Error bars are standard error of the mean; numbers below the panels are the number of participants contributing to the mean.
Extended Data Fig. 5 Shift from baseline to week 104 in glycemic status.
( a - d ) Observed data for participants in the full analysis set treated with semaglutide 2.4 mg (a, c) or placebo (b, d) during the in-trial period. As illustrated by the gray shading, the week 104 bars present results at this time point among the subgroups of participants with baseline prediabetes (a and b) or baseline normoglycemia (c and d). Glycemic category was determined by investigators on the basis of available information (for example, medical records, concomitant medication, and blood glucose variables) and in accordance with American Diabetes Association criteria, 30 which for prediabetes includes fasting plasma glucose levels of 100 mg/dL (5.6 mmol/L) to 125 mg/dL (6.9 mmol/L) or HbA 1c levels of 5.7–6.4% (39–47 mmol/L), and for type 2 diabetes includes fasting plasma glucose levels of ≥126 mg/dL (7.0 mmol/L) or HbA 1c levels ≥6.5% (48 mmol/L). *Number of participants in the full analysis set. † Number of participants with prediabetes (a and b) or normoglycemia (c and d) at baseline and evaluable data at week 104.
Extended Data Fig. 6 Comparison of body weight parameters for semaglutide versus placebo (trial product estimand).
( a ) Observed mean percentage change from baseline in body weight over time for participants in the full analysis set during the on-treatment observation period (error bars are standard error of the mean; numbers below the panels are the number of participants contributing to the mean) and estimated treatment difference for the percentage change from baseline to week 104 in body weight based on the trial product estimand. ( b ) Observed proportions of participants and odds ratio for achieving weight loss of at least 5% from baseline at week 104 in the full analysis set during the on-treatment observation period, based on the trial product estimand. *Estimated means in percent. A time point is considered as on treatment if any dose of trial product has been administered within the previous 14 days. The trial product estimand assesses treatment effect assuming all participants adhered to treatment and did not receive rescue intervention. CI, confidence interval; ETD, estimated treatment difference.
Extended Data Fig. 7 Prevalence and duration of gastrointestinal events by severity.
( a - d ) The proportion of participants receiving semaglutide or placebo who reported nausea (a), diarrhea (b), constipation (c), or vomiting (d) events classed as mild, moderate, or severe over the course of the treatment period. Data are from the on-treatment observation period (during treatment with trial product [any dose of trial medication administered within the previous 49 days (that is, any period of temporary treatment interruption with trial product was excluded)). Adverse events were classified by severity as mild (easily tolerated, causing minimal discomfort, and not interfering with everyday activities), moderate (causes sufficient discomfort and interferes with normal everyday activities), or severe (prevents normal everyday activities).
Supplementary information
Supplementary information.
List of investigators in the STEP 5 trial and Supplementary Tables 1–4.
Reporting Summary
Supplementary data 1.
Study protocol.
Supplementary Data 2
ICMJE forms.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Garvey, W.T., Batterham, R.L., Bhatta, M. et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med 28 , 2083–2091 (2022). https://doi.org/10.1038/s41591-022-02026-4
Download citation
Received : 30 March 2022
Accepted : 24 August 2022
Published : 10 October 2022
Issue Date : October 2022
DOI : https://doi.org/10.1038/s41591-022-02026-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Pharmacotherapy for obesity: moving towards efficacy improvement.
- Walmir Coutinho
- Bruno Halpern
Diabetology & Metabolic Syndrome (2024)
Structured lifestyle modification as an adjunct to obesity pharmacotherapy: there is much to learn
- Enda Murphy
- Francis Martin Finucane
International Journal of Obesity (2024)
Targeting the incretin system in obesity and type 2 diabetes mellitus
- Saleem Ansari
- Bernard Khoo
Nature Reviews Endocrinology (2024)
Redefining peptide therapeutics with semaglutide
- Thomas Kruse
- Søren Østergaard
Nature Chemistry (2024)
Cost-effectiveness of weight-management pharmacotherapies in Canada: a societal perspective
- Anamaria-Vera Olivieri
- Sergey Muratov
- David C. W. Lau
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
New insights on how some individuals with obesity can lose weight -- and keep it off
For decades, there's been a persistent one-size-fits-all approach to treating obesity: Embrace a diet that's low in calories. Yet evidence shows that this diet-focused approach simply doesn't work for a subset of adults with obesity who are adherent in a clinical weight management program.
Now, compelling new research published in the journal eBioMedicine challenges the deeply ingrained idea that diet alone should be adequate for everyone seeking to shed pounds.
The important conclusions could significantly improve public health by guiding the advent of personalized treatment plans that will help individuals with difficult-to-treat obesity lose weight -- and keep it off.
"It's exciting and important work. These findings have clinical implications and reveal molecular mechanisms that will drive research for many years to come," says Dr. Mary-Ellen Harper, an award-winning professor and research chair in mitochondrial bioenergetics at the uOttawa Faculty of Medicine who was the study's senior author.
Understanding distinct obesity phenotypes is key to teasing out insights into individual variations in weight loss. And for "diet-resistant" obesity -- patients in the bottom 20% for rate of weight loss following a low-calorie diet -- exercise training should be prioritized, as it decreases fat mass and boosts skeletal muscle metabolism.
The research team mined clinical data from over 5,000 records. Ultimately, 228 files were reviewed and a subset of 20 women with obesity were identified to undergo a closely supervised exercise program made up of 18 progressive sessions using treadmills and weights done three times per week for six weeks.
Using bioinformatics and machine learning approaches to analyze skeletal muscle, the results indicate that exercise preferentially improves skeletal muscle metabolism and enhances weight loss capacity for individuals with obesity who are deemed diet resistant.
These are the type of patients with difficult-to-treat obesity who have often been accused of non-adherence when they have not lost weight with diet restriction.
"For those individuals who have obesity and who've had enormous difficulty losing weight, the message for them is: You are in a group of individuals for whom exercise is particularly important. And that's really going to help you lose weight," says Dr. Ruth McPherson, a leader in cardiovascular genetics who is a professor at the uOttawa Faculty of Medicine and director of the Ruddy Canadian Cardiovascular Genetics Centre, Atherogenomics Laboratory and the Lipid Clinic at the Ottawa Heart Institute.
The stakes are high: The number of people who are overweight or obese has grown to epidemic proportions globally and obesity is a risk factor in a slew of chronic diseases. In Canada, two out of every three adults are overweight or obese, according to Statistics Canada.
Dr. Robert Dent, founder of the Ottawa Hospital's weight management clinic and an endocrinologist at uOttawa , described the findings as the "crowning glory" of the research work done alongside Drs. Harper and McPherson over two decades. The three partners have collaborated on numerous projects over the years, helping to unlock mysteries of mitochondrial energetics and the genetic predictors of weight loss.
"If you look at a large group of people who are overweight and trying to lose weight, they don't respond to exercise very much. But now we've found that people in this [diet-resistant] obesity phenotype really do," Dr. Dent says. "What the findings are telling us is that when we see individuals with obesity who don't respond to dietary restriction, they should be shunted over to physical activity."
The study has the potential to help reshape the science of weight-loss programs so they can be customized for individual patients. And since the study opens up various exciting research possibilities at the molecular level, the team is already recruiting for a study with a larger sample size.
- Diet and Weight Loss
- Personalized medicine
- Anti-obesity drug
- Malnutrition
- Low-carb diets
- Hyperthyroidism
- Atkins Diet
Story Source:
Materials provided by University of Ottawa . Original written by David McFadden. Note: Content may be edited for style and length.
Journal Reference :
- Chantal A. Pileggi, Denis P. Blondin, Breana G. Hooks, Gaganvir Parmar, Irina Alecu, David A. Patten, Alexanne Cuillerier, Conor O'Dwyer, A. Brianne Thrush, Morgan D. Fullerton, Steffany AL Bennett, Éric Doucet, François Haman, Miroslava Cuperlovic-Culf, Ruth McPherson, Robert R.M. Dent, Mary-Ellen Harper. Exercise training enhances muscle mitochondrial metabolism in diet-resistant obesity . eBioMedicine , 2022; 104192 DOI: 10.1016/j.ebiom.2022.104192
Cite This Page :
Explore More
- Mice Given Mouse-Rat Brains Can Smell Again
- New Circuit Boards Can Be Repeatedly Recycled
- Collisions of Neutron Stars and Black Holes
- Advance in Heart Regenerative Therapy
- Bioluminescence in Animals 540 Million Years Ago
- Profound Link Between Diet and Brain Health
- Loneliness Runs Deep Among Parents
- Food in Sight? The Liver Is Ready!
- Acid Reflux Drugs and Risk of Migraine
- Do Cells Have a Hidden Communication System?
Trending Topics
Strange & offbeat.

- April 24, 2024 | Quantum Computing Meets Genomics: The Dawn of Hyper-Fast DNA Analysis
- April 24, 2024 | Scientists Turn to Venus in the Search for Alien Life
- April 24, 2024 | NASA Astronauts Enter Quarantine As Boeing Starliner Test Flight Approaches
- April 23, 2024 | Peeking Inside Protons: Supercomputers Reveal Quark Secrets
- April 23, 2024 | A Cheaper and More Sustainable Lithium Battery: How LiDFOB Could Change Everything
Stanford Study Reveals Secrets to Sustainable Weight Loss: Behaviors and Biomarkers Exposed
By Stanford Medicine January 16, 2023

Stanford Medicine researchers have discovered biomarkers that can predict an individual’s ability to lose weight and maintain weight loss long-term. These biomarkers include signatures from the gut microbiome, proteins made by the human body, and levels of exhaled carbon dioxide. The study found that the bacteria in the gut and the amounts of certain proteins the body produces can impact an individual’s ability to sustain weight loss. Additionally, the research found that some individuals lose more weight on low-fat diets while others have better results on low-carb diets.
A new analysis of data from a yearlong weight-loss study has identified behaviors and biomarkers that contribute to short- and long-term weight loss.
Strictly following a diet— either healthy low-carb or healthy low-fat — was what mattered for short-term weight loss during the first six months. But people who maintained long-term weight loss for a year ate the same number of calories as those who regained weight or who did not lose weight during the second six months.
So what explains this difference?
According to the study, the bacteria living in your gut and the amounts of certain proteins your body makes can affect your ability to sustain weight loss. And some people, it turns out, shed more pounds on low-fat diets while others did better on low-carb diets.
Stanford Medicine researchers have identified several biomarkers that predict how successful an individual will be at losing weight and keeping it off long-term. These biomarkers include signatures from the gut microbiome, proteins made by the human body, and levels of exhaled carbon dioxide. The researchers published their findings on December 13 in the journal Cell Reports Medicine .
“Weight loss is enigmatic and complicated, but we can predict from the outset with microbiome and metabolic biomarkers who will lose the most weight and who will keep it off,” said Michael Snyder, PhD, professor and chair of genetics and co-senior author on the paper.
Willpower does not drive weight loss
The data came from 609 participants who logged everything they ate for a year while following either a low-fat or low-carb diet made up of mostly high-quality, minimally processed foods. The researchers tracked participant exercise, how well they followed their diet, and the number of calories consumed.
The study showed that just cutting calories or exercising were not enough to sustain weight loss over a year. To try and understand why, the team turned their focus to biomarkers of metabolism.
“We found specific microbiome ecologies and amounts of proteins and enzymes at the beginning of the study period — before people started following the diet — that indicated whether they would be successful at losing weight and keeping it off,” said Dalia Perelman, research dietician and co-lead author on the paper.
Throughout the study, the researchers measured the ratio of inhaled oxygen to exhaled carbon dioxide, known as a respiratory quotient, which serves as a proxy for whether carbohydrates or fats are the body’s primary fuel. A lower ratio means the body burns more fat, while a higher ratio means it burns more carbohydrates. So, those who started the diet with a higher respiratory quotient lost more weight on a low-carb diet.
“There are people who can be eating very few calories but still sustain their weight because of how their bodies metabolize fuels. It is not for lack of will: It is just how their bodies work,” Perelman said.
In other words, if your body prefers carbs and you’re predominately eating fat, it will be much harder to metabolize and burn off those calories.
“If you are following a diet that worked for someone you know and it is not working for you, it might be that that specific diet is not as suited for you,” added Xiao Li, PhD, co-lead author of the paper, a former postdoctoral fellow at Stanford Medicine who is now at Case Western University.
For now, focus on nutrients
The predictive information gleaned from the gut microbiome, proteomic analysis, and respiratory quotient signatures is laying the foundation for personalized diets. Snyder said he thinks tracking amounts of certain gut microbe strains will be a way for people to determine which diets are best for weight loss.
We’re not there yet, so until then, according to the researchers, the focus should be on eating high-quality foods that are unprocessed and low in refined flours and sugar.
The research team identified specific nutrients that were correlated with weight loss during the first six months. Low-carb diets should be based on monounsaturated fats — such as those that come from avocados, rather than bacon — and high in vitamins K, C, and E. These vitamins are in vegetables, nuts, olives, and avocados. Low-fat diets should be high in fiber, such as is found in whole grains and beans, and avoid added sugars.
“Your mindset should be on what you can include in your diet instead of what you should exclude,” Perelman said. “Figure out how to eat more fiber, whether it is from beans, whole grains, nuts, or vegetables, instead of thinking you shouldn’t eat ice cream. Learn to cook and rely less on processed foods. If you pay attention to the quality of food in your diet, then you can forget about counting calories.”
Reference: “Distinct factors associated with short-term and long-term weight loss induced by low-fat or low-carbohydrate diet intervention” by Xiao Li, Dalia Perelman, Ariel K. Leong, Gabriela Fragiadakis, Christopher D. Gardner and Michael P. Snyder, 13 December 2022, Cell Reports Medicine . DOI: 10.1016/j.xcrm.2022.100870
Christopher Gardner, professor of medicine and co-senior author on the paper, also contributed to this work.
More on SciTechDaily

The Benefits of a Healthy Gut Microbiome for Weight Loss

Low-Fat, Plant-Based Diet Compared to Low-Carb, Animal-Based Diet in Clinical Trial – Here Are the Results

Not All Calories Are Equal – A Dietitian Explains How the Kinds of Foods You Eat Matter to Your Body

A Low-Glycemic Diet is More Effective at Burning Calories

Low-Carb vs. Low-Fat: Science Reveals Which Diet Is Better for Weight Loss and Diabetes Control

Neuroscientists Discover Beige Fat “Indispensable” in Protecting Brain From Dementia
Researchers find the secret behind maintaining healthy weight loss.

Latest Scientific Findings on Weight Loss Opportunities and Diet Risks
10 comments on "stanford study reveals secrets to sustainable weight loss: behaviors and biomarkers exposed".
How does this tie in with the recent Harvard research that found that where fat ended up was dependent upon the levels of Nitric oxide in the veins, which was also dependent upon the percentage of Brown Adipose fat tissue in the body? According to their research, high carbohydrate diets, especially in childhood determined low levels of brown adipose fat which was not enough to release sufficient quantities of NO from venous Endothelial cells.
How do these 2 sections not contradict each other?
A lower ratio means the body burns more fat, while a higher ratio means it burns more carbohydrates. So, those who started the diet with a higher respiratory quotient lost more weight on a low-carb diet.
In other words, if your body prefers carbs and you’re predominately eating fat, it will be much harder to metabolize and burn off those calories.
Exactly! I read that passage three times trying to break it down in my head to make sense of it! I’m still confused by what they’re trying to say.
Body w/ Lower Ratio = Burns Fats Body w/ Higher Ratio = Burns Carbs
If Body w/ Higher Ratio is in a calorie reduced state with a majority of the fuel as Carbs, it will lose more weight bc it is being given the fuel it is efficiently designed to burn.
If Body w/ Higher Ratio is in a calorie reduced state with a majority of the fuel as Fat, it will struggle to lose weight bc it is NOT being given the fuel it is efficiently designed to burn.
Obviously someone couldn’t implement this without more data and individual testing so here’s my takeaway: If you are dieting and not seeing a response, perhaps change the carb to fat ratio.
It also gives some comfort to know that bc a friend or family member did a particular diet and it was successful and you try it and fail, it doesn’t mean you did something wrong, it just means that diet may not have suited your particular biology. I think that is probably the biggest takeaway, especially for those who continually struggle despite their best efforts.
I am trying to lose 40 lbs. And get slimmer. I also need to fix my legs, they are injured from a motorcycle accident.
This research just supports poor diet and exercise habits. The body will burn the fuel that it has. The easiest fuel comes from sugar followed by other starches. When people diet and limit the easy fuel the body reacts by slowing the metabolism and waiting for you to eat sugar and carbs. Once you break that cycle it will burn stored fat. The most effective way to burn that fat is to exercise after 12 hrs fasting. Its not hard. I went from being overweight with a massive gut to 5% visceral body fat in 4 months.
Ted, Thanks for your comments, but you are a man & I am a woman. For what ever reason, I have observed over the years that men loose twice as fast & loose double the pounds than women do.
I have tried all types of diets, some with better results that others, but eventually the weight has come back. I have never tried your system. I ready to try anything. I’ll your system. If it works for me, I’ll be grateful to you forever. 🙂
I lost 31 pounds last year, and about 27 total inches (from 9 different measurement points) and have, thankfully, kept it off so far! May marks my full one-year anniversary. I’m 52 years old, female, 3 C-sections, hypothyroid with some whacked out hormones, and I still lost weight, fat,and inches. How? 1) Pray 2) Write down your weight and measurements every week or even every few days 3) I did cardio 3-4 times per week and weights once a week 4) My eating plan was low fat, low protein (nuts or seafood only), low-moderate carbs, HIGH nutrition like vitamins, antioxidants, phytonutrients from lots of fruits and vegetables– so basically, pescatarian (vegetarian plus a little seafood here and there). Hope this helps and best of luck to those of you struggling to lose weight! I was finally successful after 2 decades of struggling! If I can do it, so can you! Just keep at it and work with your doctor on what plan is best!
Im keto and Stand still with my weight some weight came back over the holidays 8 pounds and would like to start Incorporating carbs !!carbohydrates !! Good ones of course but I’m so afraid I am insulin resistance 57 and my hormones are changing Very interested in doing a low carb what your thoughts
Carbohydrates aren’t bad for a person. Spinach? Carbs. Strawberries? Carbs. Broccoli? Carbs. Beans and whole grains are mostly carbs too. It’s processed “foods” that are the issue- sugar, flour, and oils. Look up what people in the blue zones eat- the places on the planet where people live the longest
Leave a comment Cancel reply
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Current Evidence to Propose Different Food Supplements for Weight Loss: A Comprehensive Review
Mikiko watanabe.
1 Department of Experimental Medicine, Section of Medical Pathophysiology, Food Science and Endocrinology, Sapienza University of Rome, 00161 Rome, Italy; [email protected] (M.W.); [email protected] (D.M.); [email protected] (A.C.); [email protected] (A.B.); [email protected] (S.M.); ti.oohay@inaicsabanirbas (S.B.); [email protected] (L.G.); [email protected] (C.L.)
Renata Risi
Davide masi, alessandra caputi, angela balena, giovanni rossini.
2 Department of Endocrinology and Diabetes, University Campus Bio-Medico of Rome, 00128 Rome, Italy; [email protected] (G.R.); [email protected] (D.T.); [email protected] (S.M.)
Dario Tuccinardi
Stefania mariani, sabrina basciani, silvia manfrini, lucio gnessi, carla lubrano.
The use of food supplements for weight loss purposes has rapidly gained popularity as the prevalence of obesity increases. Navigating through the vast, often low quality, literature available is challenging, as is providing informed advice to those asking for it. Herein, we provide a comprehensive literature revision focusing on most currently marketed dietary supplements claimed to favor weight loss, classifying them by their purported mechanism of action. We conclude by proposing a combination of supplements most supported by current evidence, that leverages all mechanisms of action possibly leading to a synergistic effect and greater weight loss in the foreseen absence of adverse events. Further studies will be needed to confirm the weight loss and metabolic improvement that may be obtained through the use of the proposed combination.
1. Introduction
The prevalence of obesity has been rising steadily for the past decades all over the world [ 1 , 2 ] leading to an increase in prevalence of many complications of weight excess, some of which are well acknowledged, such as Type 2 Diabetes (T2D), obstructive sleep apnea syndrome (OSAS), non-alcoholic fatty liver disease (NAFLD), and cardiovascular disease [ 3 , 4 , 5 , 6 ], while others are emerging and currently being investigated [ 7 , 8 ].
Several strategies have been proposed for the treatment of weight excess and its detrimental consequences, ranging from dietary regimens [ 9 , 10 , 11 , 12 , 13 , 14 , 15 ], to pharmacological treatments [ 16 , 17 ], physical exercise [ 18 ], and psychological approaches [ 19 ]. Most of these are safe [ 16 , 20 , 21 ], although some have risen concern [ 22 , 23 ]. However, despite leading to improvement in many cases, the major issue is the presence of adverse events and reduced compliance. So-called super foods and food supplements have gained much popularity in recent years, for the generalized perception that natural substances may be synonym of health and balance. Despite this being partially false, given the many adverse events that may derive from natural compounds ingestion, it is indeed true that many of the currently commercialized dietary supplements are virtually devoid of major side effects.
Currently available food supplements feature several purported mechanisms of action, such as improvement of carbohydrate metabolism, increased lipolysis or energy expenditure, and reduced hunger. A vast amount of literature is available, often of low quality, making navigation hard, and adequate recommendation to those who ask for advice remain very challenging. It is also crucial to keep dosage in consideration as it is acknowledged in many fields that dosing can make the difference between beneficial effects and toxicity, and too often commercially available supplements provide highly variable amounts without even properly acknowledging it on labels. Lately, the Italian Ministry of Health has put out an alert recommending that all botanicals should undergo testing similar to actual drugs, and many countries will surely follow. Appropriate evaluation of available evidence is therefore crucial to start with. So, herein, we aimed at summarizing available evidence regarding supplements most likely to be effective in aiding weight loss, classifying them by their purported mechanism of action. We conclude that higher quality evidence is much needed, but many of the investigated products seem to be effective, although of little clinical relevance when taken alone. Therefore, combinations aiming at targeting more than one mechanism of action should be adequately studied both from an efficacy and safety point of view in order to assess synergistic or additive actions in the absence of major adverse events.
Literature was reviewed up until March 2020 to investigate the efficacy and safety of dietary supplements towards weight loss. The research was conducted on MEDLINE, Cochrane Library, EMBASE, and Web of Science databases by using the following keywords: “name of investigated food supplement” and “obesity” or “weight loss”. Studies meeting the following criteria were included: (1) case-control studies, cohort studies, observational prospective and retrospective studies, randomized clinical trials (RCTs); meta-analyses (2) reported body weight or body mass index (BMI) measurements over time; (3) female or male only, or both genders enrolled; (4) sufficient detail reported about dietary supplement studied; (5) studies written in English.
Six independent reviewers (RR, AC, AB, DM, GR) evaluated the title, abstract, and keywords of selected papers, and full articles were retrieved if deemed appropriate. References of retrieved articles, reviews, and meta analyses on the topic were assessed for additional studies. All reports were evaluated by RR, AC, AB, DM, and GR for inclusion, and two reviewers resolved eventual disagreements (MW and DT). Data extraction included year of publication, country where the study was conducted, patient gender, mean age, mean BMI, inclusion and exclusion criteria, sample size, study design, intervention, eventual co-intervention, efficacy (body weight, BMI change), and safety outcomes. The authors of the included studies were contacted for missing values where required. Studies whose supplement dosing could not be obtained neither through study protocol nor manuscript nor direct contact with investigator were excluded.
For each supplement, six independent operators (RR, DM, AB, AC, GR) performed an assessment of the quality of the supporting evidence, based on the GRADE (Grading of Recommendations Assessment, Development and Evaluation) criteria [ 24 ]. The quality of evidence for the specific dietary supplement was defined as:
- - High if based on one or more updated, high-quality systematic reviews based on at least two high quality primary studies with consistent results;
- - Moderate, if based on one or more updated systematic reviews of high or moderate quality based on at least one high-quality primary study or two primary studies of moderate quality with consistent results;
- - Low, if based on a limited number of clinical studies or one or more systematic reviews of variable quality based on primary studies of moderate quality with inconsistent results.
A recommendation for every food supplement was then provided based on the quality of evidence, the strength of preclinical evidence, the clinical relevance, and the safety. Taking into consideration such recommendations, a combination of supplements leveraging all mechanisms of action was then suggested.
Twenty-one dietary supplements were included in the study and classified based on their primary mechanism of action in: food supplements with a primary impact on nutrient absorption ( Table 1 ); food supplements with a primary impact on appetite regulation ( Table 2 ); food supplements with a primary impact on energy expenditure regulation ( Table 3 ); food supplements with a primary impact on fat metabolism ( Table 4 ); food supplements with a primary impact on carbohydrate absorption ( Table 5 ). None of the selected dietary supplements were considered to be supported by high-quality evidence, eight of them (green tea, white kidney bean, caffeine, bitter orange, diacylglycerol, resveratrol, grapefruit, chromium) were considered to be supported by moderate-quality evidence, while the other thirteen were considered of low-quality evidence. In addition, the range of dosages commonly adopted in different studies, the mechanisms of action, and the side effects were reported for each food supplement.
Food supplements with a primary impact on nutrients absorption.
* denotes preclinical evidence, # denotes clinical evidence, A is for possibly recommended, B is for undetermined recommendation.
Food supplements with a primary impact on appetite regulation.
Food supplements with a primary impact on energy expenditure modulation.
* denotes preclinical evidence, # denotes clinical evidence, B is for undetermined recommendation.
Food supplements with a primary impact on fat metabolism.
* denotes preclinical evidence, # denotes clinical evidence, A is for possibly recommended, B is for undetermined recommendation, C is for not recommended.
Food supplements with a primary impact on carbohydrate metabolism.
* denotes preclinical evidence, # denotes clinical evidence, B is for undetermined recommendation, C is for not recommended.
3.1. Reduced Nutrients Absorption as Purported Mechanisms of Action
Commercially available obesity medication orlistat exerts its weight lowering effect through the intestinal inhibition of fat absorption. Many food supplements exert their beneficial action through the same pathway, where lipids or carbohydrates absorption is delayed or limited to some extent. Notably, most of these compounds also recognize other mechanisms of action possibly contributing to their beneficial effect on metabolism, such as gastric emptying delay influencing appetite ( Table 1 ).
3.1.1. Green Tea
Green tea (GT) is an unfermented, popular beverage made from the leaves of the plant Camellia sinensis, historically used for medicinal purposes and, in recent decades, studied for its potentially beneficial health effects. Catechins, such as epigallocatechin-3-gallate (EGCG), and caffeine (CAF), the predominant component of tea, have been confirmed to possess a broad range of biological activities, such as body weight reduction, metabolic syndrome (MetS) improvement, cardiovascular diseases (CVDs) and cancer prevention, and protection against neurodegeneration [ 25 ]. Further, green tea is proven to be safe, with few inconsistent side effects or adverse events [ 26 , 27 ].
One of the most effective ways GT can contrast obesity is through the inhibition of enzymes such as pancreatic lipase [ 28 , 29 , 30 ], amylase, and glucosidase [ 31 ] in the gastrointestinal (GI) tract. Inhibition of lipase in the GI tract, with subsequent reduced fat absorption, is a well-known target for obesity treatment, with orlistat exploiting this very mechanism [ 32 ], whereas inhibition of amylase and glucosidase prevents digestion and absorption of carbohydrates, again reducing energy intake.
The composition of gut microbiota is highly correlated with obesity and related diseases such as T2D [ 33 , 34 , 35 ], as intestinal bacteria have been shown to affect fat storage, blood glucose balance, and appetite hormones [ 36 , 37 ]. GT may influence the gut microbiota through two modes of action. Amylase and glucosidase inhibition increases the presence of undigested carbohydrates in the GI tract, in turn driving the microbiota to produce short-chain fatty acids (SCFA) [ 38 , 39 ], recently found to be capable of activating AMPK, and inducing weight-loss [ 40 ] through lipogenesis and lipolysis down- and up-regulation, respectively [ 41 ]. Moreover, most tea polyphenols (>90%) will pass through the small intestine unabsorbed due to their low bioavailability, eventually coming into direct contact with the gut microbes. These are capable of breaking them down into smaller and more bioavailable phenolic components, and in turn are modulated in terms of bacterial composition [ 42 ]. Although no direct evidence is available to date attributing GT-induced weight loss to gut microbiota modulation, it is reasonable to assume that this might be one of the possible underlying mechanisms.
The effectiveness of GT in reducing body weight and fat is widely discussed in the literature. GT derived EGCG in variable quantities (100–460 mg/day) exhibits measurable weight-loss properties in a large majority of studies according to a recent review and one meta-analysis, especially for trial durations of three or more months [ 43 , 44 ]. In addition, the consumption of caffeine at doses between 80 and 300 mg/day has been shown to be an important factor for these effects, when the participants did not have a high baseline caffeine intake (>300 mg/day). A recent eight-week study investigated the effects of green tea extract (GTE) supplementation on exercise-induced changes in sedentary, overweight women, showing that GTE improves exercise-induced body composition changes by decreasing weight, BMI, waist to hip ratio (WHR), and body fat percentage (BFP). Interestingly, there seems to be an ethnicity-dependent effect, with more important weight loss (mean 1.51 kg) in Asian subjects [ 27 ], compared with one of 0.82 kg in Caucasians [ 45 ]. However, not all evidence suggests a beneficial effect. According to a very recent meta-analysis, there is no evidence that GT or EGCG have a beneficial effect on maintaining weight loss [ 46 ].
In conclusion, GT, alone or in association with other weight loss interventions, seems a possibly useful tool for the treatment of obesity with close to no side effects, and evidence supporting its consumption is of moderate quality. The exact reason behind the presence of controversial results is yet to be elucidated: it has been hypothesized that one possible motivation might be the use of relatively low doses of EGCG (i.e., 200 mg/daily), but some studies investigating the effect of low amounts still reported positive outcomes [ 47 ]. Ethnicity, baseline caffeine intake, duration of obesity, dietary habits, the gut microbiota, and other inter-individual variabilities, as well as trial duration and co-interventions, might explain some of the observed inconsistencies in the data [ 26 , 43 ]. It would therefore be useful to deepen the research and evaluate, in particular, the appropriate dosage and the patient profile possibly benefiting the most from GT consumption.
3.1.2. Ginseng
Ginseng refers to different varieties of a short, slow-growing plant with fleshy roots belonging to the Araliaceae family. The two main types of ginseng are Panax ginseng (Asian ginseng), also known as Korean Ginseng and Panax quinquefolius (American ginseng). Asian ginseng can be red or white depending on the drying method of the root [ 48 ]. Different ginseng extracts are being studied for the treatment of several medical conditions, including body weight management.
Ginseng is supposed to contribute to weight loss through its elevated content of saponins which can delay the intestinal absorption of dietary fat by inhibiting pancreatic lipase activity [ 49 , 50 ]. Moreover, ginseng intake may affect serum levels of leptin, adiponectin, and ghrelin, as demonstrated in obese mice after the administration of Korean ginseng whole extract (8–18 g/kg) for eight weeks [ 51 ].
Although P. ginseng has been shown to exert anti-obesity effects in several animal studies, there have been relatively few studies investigating its effects in humans. In a randomized, double-blind placebo-controlled trial, 24 women with obesity were administered 18 g of Korean red ginseng (KRG) for 8 weeks and showed a decrease in BMI, aspartate aminotransferase, food intake, waist-to-hip ratio, and improved quality of life, but a frank superiority over placebo was not confirmed [ 52 ]. Another trial conducted in patients with T2D confirmed that a much smaller supplementation of KRG (100–200 mg/day) had a positive impact on glucose levels, despite failing to induce significantly more weight loss compared to placebo [ 53 ]. A more recent line of evidence suggested that the administration of 6 g of KRG over 12 weeks had no significant effect on weight, BMI, fat mass, glucose, insulin, and levels of cholesterol when compared to placebo group [ 54 ]. Reeds et al. obtained similar results in a randomized control trial comparing the effect of ginseng and its active component ginsenoside Re in overweight/obese subjects with impaired glucose tolerance or newly diagnosed T2D. However, this study did not use body weight as a primary endpoint [ 55 ].
In conclusion, the clinical relevance of ginseng as a weight loss aid remains uncertain, as the evidence quality supporting its use is low and the dose range very high (100 mg–18 g/day). Further investigation comparing the effects of the two main types of Panax ginseng is also necessary.
3.1.3. White Kidney Bean
The white kidney bean is one species of Phaseolus vulgaris L., also known as common bean, originating from South America. White Kidney Beanis rich in proteins (22–27% of seed weight) and carbohydrates (39–47% of seed weight), with a high content of bioactive compounds, such as peptides, among which are the α-amylase inhibitor named phaseolin, polyphenols, oligosaccharides, and lectins. Notably, the significant amount of lectins also arose some concerns, as, alongside their potential anti-cancer and anti-obesity activities, these peptides may also act as toxins and allergens [ 56 , 57 ]. Several reports are available regarding clinical adverse effects after ingestion of white kidney beans [ 58 ].
Conversely, Phaseolus vulgaris extracts (PVE) have been developed to isolate the action of phaseolin, an α-amylase inhibitor (α-AI) capable of binding to α-amylase non-covalently interfering in the breakdown of complex carbohydrates [ 59 ] with subsequent impaired absorption of these nutrients through the gut wall [ 60 ]. PVE are characterized by antioxidant, anticarcinogenic, anti-inflammatory, glucose lowering, and cardioprotective properties, together with potentially inducing weight loss [ 61 , 62 , 63 ]. Noteworthy, α-AI activity is highly dependent on pH, temperature, incubation time and the presence of specific ions, all of these having been optimized in some PVE commercial products, such as Phase2 ® water extract of Phaseolus vulgaris standardized to alpha amylase (8;12;15;39) inhibiting units (Pharmachem Laboratories, Kearny, NJ, USA) [ 64 ]. Barrett et al. systematically reviewed ten studies conducted between 2000 and 2010 evaluating the effect of Phase 2 ® products on body weight and glycemic control in subjects with overweight and obesity, demonstrating significant weight loss when the product was taken concurrently with carbohydrate containing meals. A recent metanalysis confirmed Phase2 ® PVE efficacy on body weight and fat change [ 65 ], results being confirmed by a recent Chinese RCT involving 120 subjects with obesity, in which the group treated with PVE capsules experienced a mean placebo adjusted weight loss of 1.95 kg after 35 days [ 66 ]. Conversely, another metanalysis conducted by Onakpoya et al. including all kinds of PVE commercial products concluded for a substantial absence of efficacy of these towards weight loss outcomes [ 67 ].
The evidence supporting the use of PVE 1 to 3 g/day for weight loss, and especially that of Phase 2 ® products, is of moderate quality, and its efficacy was demonstrated of sufficient clinical importance. PVE supplementation could be encouraged as a tool for weight loss.
3.1.4. Chitosan
Chitosan is a natural polysaccharide of β-1,4-linked glucosamine residues and derives from deacetylation of chitin, the second most abundant biopolymer on the planet, mostly found in shrimp and crabs [ 68 ]. Thanks to its well-established beneficial effects on health and its favorable safety profile, the European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition, and Allergies (NDA) recommended a maximum intake of 3 g of chitosan per day [ 69 ].
In particular, chitosan has been proposed as dietary supplement for the management of obesity because of its cholesterol-lowering properties. In fact, chitosan forms hydrophobic bonds with dietary cholesterol, therefore interfering with its emulsification and absorption [ 70 ]. Moreover, chitosan has been shown to decrease lipid peroxidation in rats fed a diet enriched with cholesterol, suggesting a possible antioxidant role [ 71 ]. Finally, in vitro studies have demonstrated that chitosan can modulates adipokine secretion and inhibit adipogenesis [ 72 , 73 ].
Chitosan has also been used in human studies. A recent metanalysis evaluated its effect in obese and overweight patients, including 14 studies conducted from 1999 to 2017. A total of 1101 participants were randomized, of whom 570 were allocated to chitosan and 531 allocated to placebo. The mean trial duration was 17 weeks (range 4–52 weeks), mean study size was 79 participants (range 12–250). Chitosan supplementation ranged from 1 to 4.5 g/day and a mean decrease in BMI of −1.27 kg/m 2 in favor of chitosan versus placebo was observed [ 74 ].
Given the low-quality evidence and the poor clinical importance of its consumption, but considering the mild if any adverse events, chitosan consumption cannot be encouraged nor discouraged as obesity treatment at this time.
3.1.5. β-Glucans
β-Glucans are natural bioactive fibers or polysaccharides composed of D-glucose monomers, linked by 1,3, 1,4, or 1,6 β-glycosidic bonds. They are naturally occuring in the cell wall of bacteria, fungi, algae, and higher crops, such as cereals and can be taken orally as a food supplement or as part of a daily diet [ 75 ]. The study of the effects on health of β-glucans is complicated by the variability of their biological activities which depend on the source, the extraction and purification methods.
Numerous pre-clinical and clinical studies have described the antitumor, antimutagenic, immune-modulating, anti-osteoporotic and antioxidant effects of β-Glucans. Moreover, they gained nutritionists attention because of their positive activities on glucose and lipid metabolism. In fact, when glucans are included in a meal, the rate of carbohydrate and lipid absorption slows down, ultimately leading to a decrease in plasma glucose and lipids [ 73 , 75 , 76 , 77 , 78 , 79 , 80 ]. Alongside the well-established anti-diabetic and lipid-lowering properties, preclinical studies also suggest that glucans may exert anti-obesity effects by activating the gut-hypothalamic (Peptide YY- Neuropetide Y) axis, therefore increasing satiety in diet-induced obese mice [ 81 ]. A dose-dependent increase in peptide YY levels has also been demonstrated in overweight adults following oat β-Glucans ingestion, suggesting that glucans may exert anorexigenic effects in humans as well [ 82 ]. At the moment, there are no clinical trials that have evaluated the effect of β-Glucans on weight loss other than one placebo controlled study in which overweight women followed a low-calorie diet plus β-glucans supplementation showing a similar decrease in body mass [ 83 ].
While evidence supporting the use of β-Glucans 5–9 g/day as anti-diabetic and lipid-lowering dietary supplement are promising, data investigating their effects on body weight are lacking and uncertain, so no recommendation can be made regarding their use as weight loss supplements.
3.1.6. Psyllium
Psyllium is a water-soluble fiber derived from the husks of seeds from Plantago ovata , an officinal plant native to western and southern Asia. A soluble fiber has the ability to dissolve in water, forming a viscous gel that may decrease appetite by occupying the stomach and it may interfere with the absorption of carbohydrates [ 84 , 85 ], lipids and bile acids [ 86 , 87 ]. Some evidence suggests that psyllium may be capable of lowering serum lipids, delaying gastric emptying, improving glycemic control, and promoting satiety [ 88 ]. However, little is known relative to the role of psyllium in aiding body weight loss.
Two literature revisions have investigated the effectiveness of psyllium in inducing weight loss, showing that its consumption may exert some beneficial effects in reducing body fat, especially in long-term clinical studies with a duration between 6 and 12 months, when its intake was associated to a dietary program and lifestyle modifications [ 88 , 89 ].
More recently, Xiao et al. conducted a metanalysis on four RCTs evaluating the effects of psyllium on body mass index, showing that BMI and body weight remained unaltered by psyllium intervention, although the results may be partially attributable to the low number of studies included [ 90 ]. Nevertheless, the discordance between the metanalysis conducted by Xiao et al. and the previous revisions may be explained by the different selection criteria adopted. In fact, Xiao et al. included only placebo controlled RCTs evaluating the effect of psyllium without other treatments, whereas the two previous revisions also included non-randomized trials, in which dietary psyllium was associated with other interventions.
Another recent metanalysis investigated the effects of psyllium consumption (whether prescribed through supplements or added to foods) on BMI and/or weight, including RCTs, both parallel and crossover, not necessarily placebo-controlled. From the metanalysis of the eleven studies (involving a total of 632 participants) reporting BMI as outcome, a non-significant reduction in BMI was observed, although subgroup analysis showed that psyllium supplementation at higher doses (≥10 g/d) significantly decreased BMI in studies with a duration ≥10 week [ 91 ].
Overall, the quality of evidence in literature evaluating the effects of psyllium consumption on weight loss is low and limited by the great heterogeneity between different studies in terms of duration, design, type of intervention, and dose utilized. Therefore, the use of psyllium supplementation for inducing weight loss cannot be recommended at the moment.
3.1.7. Glucomannan
Glucomannan is a polysaccharide composed of β-1,4–linked D-mannose and D-glucose monomers which comes from the tuber Amorphophallus konjac . It is a soluble fiber which is present naturally and abundantly in several products, such as softwoods, roots, tubers, and many plant bulbs [ 92 ]. It has been suggested that the ingestion of glucomannan could determine the prolongation of gastric emptying time, thus resulting in increased satiety and decreased post-prandial glucose concentration [ 93 , 94 ]. Moreover, glucomannan is able to absorb up to 50 times its weight in water and cannot be digested by human salivary and pancreatic amylase so that it passes relatively unchanged into the colon, where it is fermented by the gut microbiota [ 95 ].
Two meta-analyses supported the role played by glucomannan in weight loss. According to Keithley et al., glucomannan administration at doses of 2–4 g per day resulted in significant weight loss in overweight and obese individuals [ 95 ]. Moreover, in a metanalysis of 14 RCTs, Sood et al. suggested that patients receiving 1 g glucomannan daily, together with 1 glass of water and an energy-restricted diet, showed statistically significantly lower total cholesterol, LDL cholesterol, triglycerides and body weight when compared to placebo. Of note, paediatric patients, or subjects with impaired glucose metabolism did not benefit from glucomannan administration to the same degree [ 96 , 97 ]. Noteworthy, a prospective, non-randomized controlled trial suggested a possible beneficial role of glucomannan on weight loss in overweight subjects treated with a combination of Garcinia Cambogia and glucomannan at a dosage of 500 mg, twice a day, for six months. However, the administration in association with Garcinia Cambogia made the individual effect of GMM indistinguishable [ 98 ]. Finally, Onakpoya et al., in a systematic review and metanalysis of nine trials, indicated that glucomannan intake does not generate a significant decrease in body weight or BMI when compared to placebo [ 99 ].
In conclusion, the clinical relevance of glucomannan as a weight loss aid remains uncertain and the quality low. Further investigation through larger studies is necessary. At the moment, the consumption of glucomannan for the treatment of obesity should not be encouraged nor recommended against.
3.1.8. Guar Gum
Guar gum, also known as “guaran”, is a fiber derived from the seed of Cyamopsis tetragonolobus , an Indian leguminous plant. From a molecular point of view, guar gum is a complex polysaccharide called galactomannan, which is a polymer of D-galactose and D-mannose. It is widely used as an additive in food, in the form of guar gum powder. Guar gum can be also found in several types of food such as dairy products, cereals, sauces, pudding, kefir, and baked goods [ 100 ].
It has been suggested that guar gum may contribute to lower body weight by increasing the viscosity of the bowel content and the feeling of postprandial fullness, thus reducing appetite and food intake [ 101 ].
Pittler et al., in a systematic review and metanalysis of 11 trials, indicated no statistically-significant difference in patients receiving a supplementation of 9–30g/day of guar gum for up to 6 months, compared with those receiving placebo upon continuation of the usual dietary habits in most studies [ 102 ]. Noteworthy, in a double-blind, placebo-controlled trial included in the previous metanalysis, patients with T2D receiving up to 21 g/day of guar gum reported a placebo-adjusted body weight loss of 3 kg and a total cholesterol decrease of 11% [ 103 ]. These findings suggest a reduction in the risk for cardiovascular complications in diabetic patients after guar gum supplementation. Another more recent line of evidence showed that the intake of guar fiber alone at a dose of >5 g/serving or its combination with protein (2.6 g guar fiber + 8 g protein/serving) led to acute satiety effects in normal weight subjects, but did not affect body mass [ 104 ]. The most frequently reported adverse effect of guar gum supplementation was GI discomfort, such as flatulence, diarrhoea, and abdominal pain, occasionally leading to drop-out from studies [ 102 ].
These results suggest that, although guar gum can lower cholesterol levels and improve insulin sensitivity compared with placebo [ 105 ], it seems not to be effective in lowering body weight and it should therefore be administered together with appropriate dietary manipulation.
3.1.9. Agar
Agar is a gelatinous substance extracted from a type of red algae, agarophytes; it is a fiber-rich mixture of natural polysaccharides, mainly agarose and agaropectin. Agar has the ability to melt to sol at temperatures greater than 85 degrees and form a gel when cooling a hot solution to 30–40 degrees, therefore it falls in the water-soluble fiber category [ 106 ]. Agar has been used in Japan and other Asian countries for many years to make desserts such as jellies, puddings, custards, and bubble tea. It is also used as a thickener for soups and ice creams and as a gelling agent for fish and meat-based products. Moreover, given its resistance to be enzymatically degraded by most bacterial species, agar is also used in microbiology as a substrate for culture media and, because it is not degraded by the human GI tract, it has also been used as a laxative.
Agar has been shown to prolong gastric emptying [ 107 , 108 ], thus increasing fullness sensation [ 107 ]. Surprisingly, the addition of agar to a test meal was shown to increase other appetite parameters such as hunger and desire to eat [ 108 ], questioning its ability to inhibit appetite and caloric intake; no positive effect was also seen on energy expenditure after the test meal.
A 16-week long study by Maeda et al. on 76 patients with T2D or impaired glucose tolerance, showed that the addition of 180 gr of agar to a conventional Japanese diet led to a greater reduction of body weight (4.4% vs. 2.0% in non-agar diet) and BMI (1.5% vs. 1.0%), along with a reduction of total body fat, measured by dual-energy X-ray absorptiometry /DXA) scan, and of visceral and subcutaneous fat areas measured by computed tomography (CT) at the umbilical level; the greater weight reduction in agar diet group was supported by a greater reduction in the total daily calorie intake, especially at the evening meal, suggesting a possible appetite suppressant property despite appetite related parameters not being analyzed in the study. The agar diet also led to an improvement in glycometabolic parameters with a significant reduction in HbA1c, fasting glucose and total cholesterol [ 109 ], and no safety concern emerged.
Overall, the quality of evidence supporting the use of agar for weight loss is low, given the absence of well-designed studies in normoglycaemic obese patients and the paucity of data regarding weight loss in patients with alterations in glucose metabolism. Although current evidence is consistent with other studies that analyzed weight loss with soluble fiber rich diets, suggesting a possible positive effect of agar on weight reduction, there is not enough evidence to recommend its use for this purpose [ 110 ].
3.1.10. Inulin and Inulin Type Fructans
Inulin is a polysaccharide produced by many plants and extracted principally from chicory. It is part of the inulin-type fructans (ITFs) family which covers all β (2←1) linear fructose polymers, such as native inulin, oligofructose and fructo-olysaccharides (FOS). These compounds are resistant to digestion and undergo a selective fermentation, thus acting as dietary fiber and bifidogenic prebiotic [ 111 , 112 ]. Inulin is also used as a fat and sugar replacement and texture modifier in many bakery, dairy and meat products [ 113 ].
ITFs have been shown to be capable of regulating GI hormones release in both animals [ 114 ] and humans [ 115 ]: inulin and FOS supplementation are able to increase Gucagon like petide -1 (GLP-1) and PYY release and suppress ghrelin secretion; furthermore, the prebiotic properties of ITFs may modulate the gut microbiota, favoring the growth of beneficial bacteria such as short-chain fatty-acids (SCFA) producers, thus improving satiety and weight loss and reducing systemic inflammation [ 116 ].
Whether these proposed mechanisms of action are able in turn to suppress appetite and promote weight loss is a matter of debate: the authors of a 2013 review analyzed the effect of different doses of ITFs, ranging from 8 to 21 gr/day, on appetite, energy intake and weight loss in an adult non-diabetic population. Of the 15 RCTs included in the literature revision, none described positive effects regarding appetite suppression and daily energy intake reduction except for one study, and only two showed a significant weight reduction in subjects with obesity, with one of these observing a concomitant reduction in total energy intake [ 117 ]. Pointing in the same direction, two recent RCTs and one crossover double blind study did not report any additional weight loss upon consumption of 9–10 g of inulin alone or with 10 g of maltodextrin in a non-diabetic population with overweight or obesity following a hypocaloric diet, although positive effects on blood pressure and cholesterol were recorded [ 118 , 119 ], suggesting that inulin fails to promote additional weight loss when subjects are following a controlled hypocaloric diet. Interestingly, fat mass loss was also observed in one of these studies [ 119 ]. A recent review analyzing 12 RCTs from 2013 to 2015 described positive effects of 10 gr inulin or FOS-enriched inulin on weight reduction, satiety, and daily energy intake, mostly in patients with T2D, in addition to greater reductions in Hba1c and other metabolic syndrome related parameters [ 116 ]. However, studies on non-diabetic patients with obesity showed, consistent with previous evidence, discordant results, despite positive gut microbiota modulations being observed. These discrepancies could be partly explained by the more marked metabolic alterations in T2D and the potential greater effect of prebiotics on gut microflora in these subjects. Another longer trial conducted on prediabetic subjects and testing higher doses of inulin (30 g) co-administered with a hypocaloric diet, followed by ad libitum food consumption, showed that similar weight loss was observed compared to placebo during the hypocaloric regimen phase, but weight loss became significantly more pronounced during the ad libitum phase in those taking inulin [ 120 ]. These findings suggest that inulin may be able to reduce daily caloric intake possibly promoting fullness and reducing hunger and may therefore be beneficial when food intake is not restricted.
Overall, most evidence shows that inulin and ITFs supplementation has a positive effect in subjects with T2D regarding weight loss, appetite suppression, glucose metabolism and systemic inflammation parameters, while such effects have not been described consistently in those with obesity but no diagnosis of T2D. Noteworthy, the quality of evidence supporting the application of inulin for weight loss is low, considering that most of the analyzed studies are heterogeneous in terms of interventions (inulin and FOS dosages and method of administration, hypocaloric or ad libitum diets), study population (normal weight, patients with obesity or T2D), trial duration, and outcomes analyzed, which may hinder the strength of the results. Better quality studies are therefore needed to fully elucidate the effect of these compounds and to evaluate whether the results described are different based on the type of compound used and if they really are magnified in patients with altered glucose metabolism. Moreover, daily caloric intake should be closely monitored to determine if the weight loss obtained is the result of a reduced daily caloric intake or the effect of changes in gut microbiota and GI hormones milieu.
At this time, inulin should be not recommended for the treatment of obesity, although it may be considered in obese patients suffering from T2D.
3.2. Reduced Appetite as Purported Mechanisms of Action
One of the major obstacles to weight maintenance is the decreased satiety that derives from weight loss and hypocaloric diets. Hence, safe food supplements that may decrease appetite can find a role in the treatment of weight excess. Herein, we provide detail on those that have the strongest evidence in inducing satiety in human studies, those that act on appetite through mechanic distention of the bowels have been included in the previous section as they also inhibit absorption of nutrients in a steric fashion ( Table 2 ).
3.2.1. Caralluma
Caralluma Fimbriata is an edible succulent plant belonging to the Asclepiadaceae family. It is found in Africa, India, Arabia, and Southern Europe [ 121 ]. Pregnane glycosides, the main bioactive compounds, are thought to be the ones responsible for the reported appetite-suppressant and weight loss inducing properties of caralluma. This seems to be obtained through citrate lyase and malonyl coenzyme A inhibition that in turn leads to inhibition of fatty acid synthesis and enhanced fatty acid oxidation [ 122 ]. Furthermore, caralluma glycosides also seem to reduce appetite by acting on the hypothalamus through the amplification of energy sensing function signalling or through the inhibition of ghrelin/neuropeptide Y expression [ 121 ]. A preclinical study investigating the anti-obesogenic effect of caralluma showed that rats supplemented with it reported a significant reduction in food intake, an inhibition of body weight, liver weight and fat mass gain [ 123 ].
Two RCTs conducted on obese and overweight subjects showed a significant reduction in weight and waist circumference after treatment with caralluma 500 mg twice daily for two months [ 124 , 125 ], whereas one other did not find significant changes in body weight and BMI after treatment with the same dosages and longer time (three months) [ 126 ]. No major side effects were reported at the dosages utilized in the human studies.
Given the scarcity of data and the low-quality of the evidence on the anti-obesogenic effect of caralluma in human subjects, it is not possible at this time to recommend its use despite it being reasonably devoid of side effects.

3.2.2. Spirulina
Spirulina is a cyanobacterium, belonging to the Athrospira genus. Some species of spirulina are used as food supplements, as they represent rich sources of high quality and almost all essential amino acids, essential polyunsaturated fatty acids like α-linolenic acid, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), minerals, vitamins, and antioxidants including phycocyanins, carotenoids, tocopherols, and phenolic compounds [ 127 ]. The role of spirulina on body weight control is not fully elucidated yet. In murine models, spirulina extracts administration showed anti-obesity and lipids-lowering effects [ 128 , 129 ], mediated by different mechanisms, such as adipogenesis suppression, browning of white adipose tissue [ 129 ], and modification in brain and liver genes expression [ 128 ]. In humans, it proved effective in decreasing appetite [ 130 ].
Clinical studies evaluating the effects of spirulina consumption on weight reduction are limited. A recent metanalysis investigated the effects of spirulina supplementation on body weight reduction, including only five RCTs, published between 1996 and 2018, and 278 patients overall, consuming doses of spirulina between one and 4.5 g/day, for a duration from six to 12 weeks. An overall significant reduction in body weight (mean −1.56 Kg) body fat percentage and waist circumference were observed, with no change in BMI and waist-to-hip ratio [ 131 ]. However, the analysis was limited by the small number of studies included. A successive placebo controlled RCT evaluating the effects of consumption of 4.5 mg/day of spirulina extract in addition to a physical exercise program for six weeks on 45 overweight and obese men did not find any significant changes in anthropometric parameters, whereas it confirmed the lipid lowering effects [ 132 ].
Overall, despite its promising effects described in animal models, evidence supporting the use of Spirulina for weight loss purposes in humans is scarce, controversial and of low quality. Its supplementation should therefore not be recommended as a treatment for obesity at this time.
3.2.3. Whey Protein
Whey protein (WP) is the water-soluble part of milk and it is considered to be a high biological value protein, including all essential 22 amino acids. WP is usually available in powder form and can be easily added to beverages and some foods. Consumption of WP has been shown to increase circulating concentrations of satiety hormones of the lower gut, including GLP-1 and PYY thus suppressing appetite more than other proteins, such as casein, soy, and egg albumin. Moreover, WP may promote fat mass reduction through its oxidation, with concomitant preservation of lean mass [ 133 ].
A systematic review and metanalysis of 14 RCTs examined the effect of WP, with or without resistance exercise, on body weight and body composition. Five studies analysed the effects of WP as a replacement for other sources of calories (WPR) and the remaining nine studies examined the effects of WP as a supplement without dietary modification, and body weight and fat were significantly decreased from baseline in the WPR within-group analyses [ 134 ]. Similarly, another more recent metanalysis assessed the impact of WP supplementation in its concentrated (WPC), hydrolysed (WPH) and isolated (WPI) forms, comparing it to isocaloric placebo in athletes. WPC was the only form with a statistically significant impact on fat mass loss [ 135 ].
In conclusion, evidence supporting the use of WP 100–600 g/week, either as a supplement combined with resistance exercise or as part of a dietary restriction program in order to improve body composition parameters, is of moderate quality, but further dose/response trials should be performed. Overall, WP supplementation may be recommended for the treatment of obesity.
3.2.4. Coffee, Caffeine and Chlorogenic Acids
Coffee is one of the most popular beverages consumed worldwide, prepared from roasted coffee beans. Scientific studies often lack an adequate differentiation between the effects of its single compounds, whose most important are caffeine, chlorogenic acid (CGA), diterpenes, and trigonelline. Caffeine is a naturally occurring alkaloid with well-established stimulating properties. As a lipid soluble compound, it freely crosses the blood brain barrier [ 136 ], and, therefore, affects neural function. Caffeine appears to suppress hunger and stimulate energy expenditure (EE) through increased excitability of the sympathetic nervous system (SNS), increased fat oxidation and Brown Adipose Tissue activation [ 137 , 138 ]. CGA is an antioxidant and anti-inflammatory phenolic acid playing a role in neuro- and hepatoprotection, and with lipid and glucose lowering properties [ 139 , 140 ].
A recent systematic review and dose-response metanalysis highlighted the positive effects of caffeine consumption on weight and fat loss, taking into account studies published between 1999 and 2014 [ 141 ]. However, among the thirteen studies selected, most had caffeine being administered in association with other compounds, such as ephedrine or green tea, or in the form of coffee beverage, making the effects of caffeine from CGA and other elements indistinguishable. One study conducted on 30 subjects with obesity and lasting 12 weeks showed greater weight loss upon consumption of instant coffee rich in CGA and caffeine compared to decaffeinated with similar amounts of CGA [ 142 ]. Similarly, Davoodi et al. demonstrated greater weight loss in 15 women with obesity taking an oral solution of 5 mg of caffeine/Kg/day for four weeks compared to controls following the same diet without caffeine consumption. The caffeine-treated group demonstrated suppressed hunger and continued to lose weight during the one-month-follow-up period, whereas the controls slightly increased body weight [ 143 ].
Several studies have evaluated how green coffee extracts, rich in both CGA and caffeine, have proven effective in inducing weight loss [ 140 ]. Interestingly, in a cross-over study, 20 volunteers were subjected to the consumption of green coffee (richer in CGA) and black coffee, with a wash-out period in between, showing that BMI fell significantly only upon green coffee intake, whereas both beverages significantly reduced abdominal fat despite constant energy intake and physical activity throughout the intervention.
Overall, moderate-quality evidence suggests that black and green coffee, and both caffeine and CGA, may potentially induce weight loss, and they should be considered as a tool for the treatment of obesity. However, further studies are needed to better evaluate the effects of the single components on body weight.
3.2.5. Bitter Orange
Bitter orange, the fruit of the Citrus aurantium , is rich in p-synephrine, a primary protoalkaloid which has been widely used for weight-loss management as suppressor of appetite and stimulator of energy expenditure and lipolysis [ 144 , 145 ]. P-synephrine has some structural similarity to ephedrine, from which it differs by the presence of a hydroxyl group in the para position on the benzene ring [ 146 ].
Bitter orange extracts’ efficacy in inducing weight loss has been shown in some clinical studies [ 147 , 148 ], and literature revisions including studies conducted on subjects who received products containing p-synephrine alone or in combination with other supplements concluded that the consumption of this dietary supplement is overall safe and may induce modest weight loss [ 149 , 150 ]. The most recent review, involving approximately 30 human studies and over 600 subjects, have confirmed that evidences supporting the anti-obesogenic role of bitter orange extracts are limited and uncertain, as they are often studied in combination with other molecules. P-synephrine does not appear to produce cardiovascular effects at doses up to 100 mg [ 144 ]. Human clinical trials evaluating the effects of bitter orange extracts on weight outcomes have not been published since 2016, although some recent preclinical study showed that Citrus peel extracts attenuated obesity and modulated gut microbiota in a high-fat diet-induced obesity mice [ 151 ] and regulated in vitro adipogenesis and thermogenesis via AMPK activation [ 152 ].
At the moment, bitter orange should not be recommended for the treatment of obesity as the quality of evidence supporting its application for this purpose is low.
3.2.6. Guarana
Guarana is a native plant from the Amazon, where it is traditionally brewed as a drink. Its fruit and seed are rich in catechins and methylxantines, that inhibit adipogenesis [ 153 ], promote browning in animal models [ 154 ], and stimulate energy expenditure in humans [ 155 ]. Their use as nutraceutical supplement for weight loss and as stimulant is widely spread.
Little evidence in human subjects suggests a possible efficacy in inducing weight loss [ 156 ] and in protecting from metabolic syndrome [ 157 ] when used alone, with dosages of 240–285 mg/day. Conversely, its combination with other supplements is reported to delay gastric emptying leading to increased satiety and significant weight loss [ 158 ]. Possible side effects have been reported, generally observed when herbal supplements associations were being used [ 159 ].
Despite the promising preclinical evidence, the paucity of data and the low-quality evidence regarding the effects of guarana consumption on weight loss in human subjects makes its potential application as therapeutic agent to treat obesity uncertain, so its consumption should be not encouraged to induce weight loss.
3.3. Increased Energy Expenditure as Purported Mechanisms of Action
The fascinating hypothesis that natural and harmless substances could increase energy expenditure has been going on for the past several years. In the 1960s, the family of amphetamines was very popular among those willing to lose weight but was later abandoned due to several associated cardiovascular accidents. Thyroid hormones followed the same pathway, and since then, the scientific community has tried to find the “perfect” stimulant. Fibroblast growth factor 21 was recently tested on human beings, as it was shown to induce BAT activation and browning of white adipose tissue in rodents, with a net effect of increased energy expenditure [ 160 , 161 ]. However, studies on primates and human subjects quickly proved that the physiology of energy metabolism is very different across species, and the same effect could not be replicated in men or women. Here, we provide more details concerning natural compounds proven to exert an effect on energy expenditure, although it should be kept in mind that most mechanistic evidence is on rodent models, and the weight loss effect only was proven in human subjects. It is therefore not possible to conclude that the same mechanism of action observed in preclinical studies is the one responsible for the anti-obesogenic effect in human beings ( Table 3 ).
3.3.1. Capsaicin, Capsaicinoids and Capsinoids
Capsaicin is an active compound of chili peppers and a component of the capsaicinoids family [ 162 ]. Besides its use in food preparation, it is used as an analgesic in ointments and patches for neuralgia and neuropathy treatment [ 163 ]. Capsinoids, also present in chili peppers, are structurally similar to capsaicinoids and bind to the same receptor, but they do not have the same characteristic pungency because they cannot reach mouth receptors due to their structural difference [ 164 ].
Capsaicin effects are ascribable to the activation of transient receptor potential channel vanilloid type-1 (TRPV1) [ 165 ], causing an influx of Ca 2+ , and subsequent release of neurotransmitters, such as substance P and catecholamines, and other substances like GLP-1 [ 166 ]. In animal models, capsaicin has also been shown to suppress ghrelin release [ 166 ], increase adiponectin mRNA expression in the adipose tissue and PPARα/PGC-1α mRNA in the liver [ 167 ], enhance AMPK and regulate gluconeogenesis and glycogen synthesis genes [ 168 ], thus reducing obesity-induced insulin resistance. Other studies have demonstrated that capsaicin and capsaicinoids are able to induce thermogenesis, activating UCP-1 and 2 [ 169 ], increase fat oxidation, SNS activity, and energy expenditure via GI TRPV1 activation [ 170 ] and promote SIRT-1 expression, inducing browning of white adipose tissue [ 171 ].
A 2012 review of twenty clinical trials investigated the effects of capsaicinoids and capsinoids on energy expenditure, lipid oxidation and appetite regulation, in both short term (meal tests) and long-term interventions (up to four months). Capsaicinoids doses ranged between 10 and 36 mg/day, while trials assessing the effect of capsinoids used less than 10 mg/day. Most trials showed a beneficial effect of both capsaicinoids and capsinoids on energy expenditure, with a small increase in oxygen consumption, body temperature, and metabolic rate (about 50 kcal/day). Furthermore, capsaicinoids and capsinoids were able to increase satiety and reduce hunger, thus decreasing energy intake, in five of the included studies [ 172 ]. In a 2014, a systematic review and metanalysis analyzing capsaicin and capsaicinoids effect on weight management, capsaicinoids consumption before meals resulted in a significant reduction in ad libitum energy intake (74 kcaL/meal) with significantly higher reduction for dosages over 2 mg [ 173 ]. Subgroup analysis, however, showed high heterogeneity, even when only trials using high capsaicinoids doses were taken into account, suggesting high variability in trial designs. A recent metanalysis showed that ingestion of capsaicin or capsinoids leads to increased energy expenditure (58.56 kcal/day) and decreased respiratory quotient, indicating enhanced fat oxidation. Interestingly, subgroup analysis demonstrated an increase in energy expenditure (69.79 kcal/day) and a decreased respiratory quotient only in subjects with BMI > 25 and a significant effect only in study with short duration (<1 day) [ 174 ]. These results indicate that capsaicinoids and capsinoids consumption may induce a tolerance mechanism long term and may have a BMI dependent enhancement of fat oxidation and energy expenditure. As the authors reported, the latter may be partly a consequence of the depressed SNS activity seen in obese subjects, promoting a positive energy balance [ 175 ].
The body of evidence shows that Capsaicinoids and capsaicin are effective in promoting a negative energy balance short term via thermogenesis enhancement and energy intake reduction, especially in overweight and obese subjects. However, whether this translates in sustained weight loss is still unclear and the quality of evidence supporting its application for obesity treatment is low. Studies conducted in the past showed that the ability to influence energy balance is not always successful in obesity management. Furthermore, long term studies analyzing capsaicinoids effect on energy expenditure failed to demonstrate a beneficial effect consistently, and higher quality studies are therefore needed to elucidate their potential on weight management.
Despite the quality of evidence being classified as low, the strength of preclinical evidence and the promising results from human studies, together with the good safety profile, may support the use of capsaicinoids and capsaicin for the treatment of obesity.
3.3.2. Curcumin
Curcumin is a bioactive polyphenol component derived from turmeric, a rhizomatous herbaceous perennial plant ( Curcuma longa ). It is a popular yellow spice often found in curry powder, claimed to play an important role against several pathological conditions such as atherosclerosis, cancer, and neurodegenerative diseases [ 176 ]. It has also been suggested that curcumin induces the secretion of adiponectin and inhibits adipocyte differentiation, together with possessing insulin-sensitizing and anti-inflammatory properties [ 177 ].
A double-blind, randomized, placebo-controlled trial reported that curcumin administration is well-tolerated and can positively influence weight management in overweight people by increasing weight loss (from 1.88% to 4.91%), and reducing body fat percentage (from 0.70% to 8.43%) [ 178 ]. In addition, a significant reduction of BMI and liver fat was also observed by Rahmani et al. after the administration of 70 mg/day curcumin for eight weeks among people with NAFLD [ 179 ]. Another more recent line of evidence is represented by a systematic review and metanalysis of 21 RCTs which indicated that curcumin intake significantly reduced BMI, weight, and waist-circumference [ 180 ]. However, not all reports point in the same promising direction. For instance, Mohammadi et al. did not find a statistically significant difference in anthropometric parameters such as BMI, weight and total body fat of obese patients receiving a supplementation of curcumin at a dosage of 1 g/day compared to placebo [ 181 ]. Similar findings have been reported by Ghazimoradi et al. after curcumin administration at the same dosage in patients with metabolic syndrome over six weeks [ 182 ].
Even though curcumin administration seems to be associated with significant reduction in body weight and body composition improvement, the majority of the RCTs only included a modest number of participants and were performed with unformulated curcumin which has very low bioavailability because of its poor absorption and rapid metabolism [ 183 ]. Moreover, curcumin supplementation is considered safe and no adverse side effects have been reported at low doses, while mild side effects such as digestive issues, headaches, nausea, or skin rashes have been rarely reported at higher doses (0.5–12g) [ 177 , 184 ].
Overall, evidence supporting the use of curcumin for weight loss are low in quality. Therefore, treatment with curcumin for weight loss purposes should be proposed only when a favorable cost-to-benefit ratio has been found and side effects should be looked for, especially when higher dosages are being proposed.
3.3.3. L-Carnitine
L-Carnitine (L-C) is a naturally occurring amino-acid derivative, found in the majority of mammalian tissues including the brain. The liver and the kidneys produce sufficient amounts from the amino-acids lysine and methionine. However, some individuals (such as preterm infants), do not produce sufficient amounts, making L-C a conditionally essential nutrient. The best sources of L-C are animal products like red meat, fish, poultry, and dairy products [ 185 ].
L-C is reported to contribute to body weight reduction through a variety of different mechanisms. First, it is suggested to improve insulin resistance [ 186 ] and to stimulate energy metabolism in animal models [ 187 ] and fat oxidation in humans [ 188 ]. Moreover, it is also essential in facilitating activated long chain fatty acids transportation into mitochondria, playing an important role in β-oxidation; it seems capable of modulating regulators of lipid catabolism or adipogenesis such as hormone-sensitive lipase, acyl-coenzyme A oxidase, and carnitine palmitoyl transferase I-A [ 189 ]. Finally, it has been suggested that L-C supplementation may induce satiety [ 190 ].
Even though several preclinical studies demonstrate the efficacy of L-carnitine supplementation for weight management, findings in clinical trials are contradictory. On the one hand, two different trials reported no weight loss effect of L-carnitine supplementation in comparison with moderate aerobic training in obese women [ 191 , 192 ]. On the other hand, two different metanalyses showed that L-C supplementation can significantly decrease body weight and fat mass, although a decreased effect over time was reported [ 193 , 194 ]. In addition, a dose–response analysis showed that L-C supplementation changed BMI according to a non-linear function, with higher doses increasing the reduction [ 194 ]. Noteworthy, a specific subgroup analysis revealed that L-C exerts anti-obesity effects in overweight and obese subjects only [ 194 ]. Overall, L-C dosages in clinical studies are highly variable (10 mg–4 g/die), possibly influencing efficacy outcomes.
In conclusion, evidence supporting the use of L-C as drug for weight loss in adults is still of low quality, but given the strength of preclinical evidence and the promising results from human studies, together with the good safety profile, this supplement could be considered as a treatment of obesity. However, dose escalating trials must be performed to improve the evidence in this field thus finding the best and safest daily dose.
3.4. Improved Fat Metabolism as Purported Mechanisms of Action
Obesity is defined by the World Health Organization as a condition of fat excess, rather than as a simple increase in BMI. It goes without explanation that no weight loss intervention is clinically relevant if it does not coincide with a reduction in body fat. The use of supplements claimed to “burn” fat is widespread, but only some of these have been proven to effectively and safely play a beneficial role on fat metabolism in preclinical studies. Of these, even fewer have shown such efficacy in human studies. Herein, we provide detail on those supplements supported by the most clinical evidence ( Table 4 ).
3.4.1. Pyruvate
Pyruvate (a derivative of pyruvic acid) is a physiologic breakdown product of body metabolism. It is a 3-carbon intermediate product of the glycolysis pathway and it can be converted to lactate or to acetyl-CoA in the cytoplasm or mitochondria, respectively. Natural sources of pyruvate are cheese, apples, and red wine. Its mechanism of action is unclear and only partially studied. It has been proposed that pyruvate may induce the shift in substrate utilization from predominantly carbohydrate to predominantly fat following pyruvate consumption, which may in turn contribute to increase fat oxidation, a mechanism to which both lower insulin levels and higher acetylCoA concentrations may contribute to [ 195 ]. The results of Ivy et al. indicated that chronic dietary supplementation with pyruvate reduced weight gain in obese Zucker rats in part by increasing resting metabolic rate and fatty acid oxidation [ 196 ]. Moreover, chronic consumption of pyruvate and dihydroxyacetone led to increased plasma thyroxin and decreased plasma insulin in rodent models [ 197 ].
A recent systematic review of clinical studies investigating the effect of pyruvate on body weight revealed a significant difference in body weight and fat loss favoring pyruvate (5–44 g/day) over placebo. However, the magnitude and the clinical relevance of this effect is small and uncertain, given that only six RCTs were included in the analysis and most had methodological weaknesses, with no study after 2005 being found by the reviewers [ 198 ]. The reason why there has been an abrupt interruption of studies assessing the effect of pyruvate on weight loss is unclear, as no safety concern emerged, and results were modest but promising.
Given the lack of recent data and the low-quality of evidence in literature, pyruvate consumption should not be encouraged as a tool for inducing weight loss.
3.4.2. Diacylglycerol
Diacylglycerol (DAG) is a natural component of several edible oils such as rapeseed and cottonseed oil [ 199 ], and is therefore easily added to foods. It is “generally recognized as safe” by the US FDA and has been allowed as a “food for specific health use” in Japan [ 200 ]. As several studies investigated the beneficial effects of DAG compared to Triacylglycerol (TAG), it is important to underline that DAG and TAG, despite similar energy density (38.9 kJ/g and 39.6 kJ/g, respectively) [ 201 ] and fatty acid composition, play different roles on lipid metabolism thanks to specific structural differences, namely the position of the fatty acid on the glycerol skeleton of DAG [ 202 ]. Indeed, DAG enhances fat oxidation and decreases the re-synthesis of chylomicrons in animal models [ 203 ], and its peculiar structural and metabolic characteristics seem to be responsible for the suppression of body fat accumulation, body weight loss and lower postprandial serum TG levels found upon the consumption of DAG-rich oils [ 204 ].
As highlighted by Rudkowska et al., data from several clinical trials suggest that 1,3-DAG is capable of decreasing body weight, visceral fat, and serum postprandial TAG concentrations, although results are controversial [ 205 ]. Moreover, Li et al. investigated the beneficial effects of DAG consumption on T2D patients, demonstrating an improvement not only in relation to body weight and waist circumference, but also regarding glucose metabolism and blood pressure compared with TAG [ 206 ]. Pointing in the same direction, the consumption of DAG together with alpha linoleic acid (ALA), a fatty acid undergoing easier beta oxidation as opposed to palmitic, stearic, oleic, or linoleic acid [ 207 , 208 , 209 ], resulted in excellent outcomes on body weight and visceral fat loss [ 203 , 210 , 211 ].
Overall, moderate-quality evidence suggests that DAG at a dosage of 1.1–1.2 g/day is effective in reducing body weight in both healthy and diabetic subjects compared to TAG, leading to a reduction in cardiovascular risk, with no adverse events being reported, and its use as a weight loss supplement could therefore be recommended.
3.4.3. Licorice
Licorice is a plant of ancient origin, deriving from the root of Glycyrrhiza glabra L. (Leguminosae). Its major compounds are: Glycyrrhizin (glycyrrhizic acid), used as flavoring agent; Carbenoxolone, a glycyrrhetinic acid derivative with a steroid-like structure which has been used in the treatment of peptic ulcer disease [ 212 ]; and different flavonoids, such as glabridin [ 213 ]. Licorice has been recently shown to exert beneficial effects on health; for example, it seems to be hepatoprotective in case of NAFLD [ 214 ] and contributes to improve the lipid profile in overweight and obese patents [ 215 ]. However, licorice consumption is also associated with the elevation of blood pressure, mediated via the mineralocorticoid receptor [ 216 ]. The way licorice extracts may contribute to body fat loss is still unknown, even if pre-clinical observations suggest that the anti-obesity effects could be mediated by glabridin, which has been capable of improving hepatic steatosis through beta-oxidation induction [ 217 ] and of ameliorating obesity via AMPK activation in high-fat-fed obese mice [ 218 ].
A recent metanalysis evaluated the metabolic changes associated with the consumption of licorice or its derivatives (300–900 mg/day), taking into consideration 26 clinical studies published from 2002 to 2017, with a duration ranging from two to 16 weeks. In particular, they verified that licorice consumption slightly but significantly reduced body weight dependent on the dose and duration of the treatment; the results also confirmed an increase of blood pressure and hypernatremia [ 219 ]. Notably, a recent eight-week long placebo-controlled RCT involving 64 overweight and obese patients did not show significant weight loss in the licorice-extract-treated group [ 220 ].
Overall, evidence supporting the use of licorice or licorice extract for weight loss is conflicting and of low-quality. Given the negative effects of licorice on blood pressure, and being hypertension a common complication in patients with weight excess, the use of this dietary supplement should be discouraged at this time.
3.4.4. Garcinia Cambogia
G. cambogia (GC) is a tree native to the evergreen forests of India, Nepal, and Sri Lanka, where it is most often used for food and medicinal purposes. GC fruit extracts, known to cause watery diarrhea, have been used for constipation. Although numerous chemicals have been isolated from GC fruit, hydroxycitric acid (HCA) is considered the active ingredient for the anti-obesity properties that this dietary supplement has shown.
HCA, extracted from the rind of the fruit, is an organic acid which has been proved to be a potent competitive inhibitor of adenosine triphosphate-citrate lyase [ 221 ], therefore reducing the availability of acetyl-coenzyme A for fatty acids and cholesterol synthesis [ 222 ]. Pre-clinical studies also demonstrated that GC extracts attenuated fat accumulation through regulation of lipolysis genes expression via the adiponectin-AMPK signaling pathway [ 223 ]. Haber et al. reviewed nine randomized, double-blind, placebo-controlled trials, conducted from 1998 to 2014, involving the consumption of GC by individuals with obesity [ 224 ]. The results were controversial, considering that some studies did not show a significant difference in body weight [ 225 , 226 , 227 ] whereas others demonstrated that the supplement was more effective in decreasing body fat compared to placebo [ 228 , 229 , 230 , 231 ]. The controversial results may be explained by the presence of methodological weaknesses, such as short duration (2–12 weeks), variable dosages (400–2400 mg/day), and often lack of information such as sample size calculation. Although in short term trials GC has been proven as generally safe, it is noteworthy to underline that FDA urged consumers to avoid Hydroxycut, a natural product for weight loss containing GC and a variety of other ingredients, because of the report of twenty-three cases of hepatotoxicity associated with its use in 2009 [ 232 ]. Moreover, several hepatoxicity cases have been associated also with the use of pure GC extracts [ 233 , 234 , 235 , 236 , 237 , 238 ].
Overall, the use of GC supplements as treatment for weight excess is to discourage considering the uncertainty of its clinical efficacy and the safety concerns.
3.4.5. Resveratrol
Resveratrol is a natural polyphenol found in a large variety of plant species such as grape, berries, and nuts, and has long been used as food supplement [ 239 ]. It has been reported to exert antioxidant, anti-inflammatory, and anti-carcinogenic effects, and it seems to be cardio- and neuroprotective [ 240 ].
Resveratrol has been found to be one of the strongest activators of SIRT-1 through an AMPK mediated mechanism. This is particularly important as SIRT-1 activation confers protection against aging-associated metabolic diseases, such as glucose metabolism impairment and carcinogenesis, apparently mimicking transcriptional aspects of dietary restriction [ 241 ]. In preclinical studies, resveratrol was found to protect against metabolic disease and weight gain in diet induced obesity models [ 242 ]. Moreover, its supplementation led to decreased adipogenesis and viability in preadipocytes and increased lipolysis and reduced lipogenesis in mature adipocytes [ 243 ].
Some studies conducted in both obese and non-obese subjects supplemented with resveratrol 75–2000 mg daily did not detect any metabolic effect [ 244 , 245 ]. Conversely, a cross-over study conducted on obese men taking 150 mg resveratrol daily for 30 days modestly mimicked the physiological effects of energy restriction as previously reported in rodent models [ 239 ]. A recent systematic review and metanalysis of 36 randomized controlled trials (RCTs) investigating the effect of resveratrol supplementation on weight loss showed that it significantly reduced weight, BMI, waist circumference, and fat mass with a significant increase in lean mass, but with no effect on leptin and adiponectin levels. Notably, despite the statistical significance, reductions were usually very small and not necessarily clinically relevant.
Altogether, studies supporting the use of Resveratrol as an anti-obesity drug are of low-quality, and the clinical relevance of its effects is uncertain. It could be however taken into consideration when associated to other weight loss interventions, given the strong preclinical evidence, the virtual absence of reported side effects and its small effect in weight reduction.
3.4.6. Conjugated Linoleic Acid
Conjugated linoleic acid (CLA) is a group of isomers of linoleic Acid, polyunsaturated fatty acids that are found naturally in the meat and dairy products of ruminant animals. Cis-9, trans-11 CLA and trans-10, cis-12 CLA are some of the main active isomers [ 246 ]. The CLA content of food products is highly dependent on various factors, such as the type of feed [ 247 , 248 ], species and age of the animal [ 249 ], the rumen pH [ 250 ] and the time of the year [ 251 ].
CLA has been reported to exert anti-carcinogenic effects, improve body composition, and aid weight loss [ 252 ], modulate immune and/or inflammatory responses [ 253 ] while reducing cardiovascular risk [ 254 , 255 ]. CLA is GRAS (generally recognized as safe) in the United States since 2008 [ 252 ], with no major side effects reported in several studies except for the appearance of occasional GI complaints [ 256 ].
Several possible mechanisms have been suggested possibly aiding weight loss in animal models and human subjects. First, supplementation with CLA was shown to decrease the size of adipocytes, alter adipocyte differentiation, stimulate apoptotic pathways, and regulate lipid metabolism [ 257 ]. Moreover, some works suggested greater activation of PPAR-γ receptors and pro-inflammatory cytokines [ 246 , 253 ], fatty acids oxidation [ 258 ], and the browning of white adipose tissue as a mechanism of fat mobilization [ 259 , 260 ]. Finally, adding CLA to the diet could also alter the gut microbiota composition and associated gut metabolites [ 261 , 262 ], but more studies are needed to show that these changes play a role in weight loss.
CLA studies in humans are difficult to interpret because of small sample sizes, variable doses, and isomers of CLA, a wide range of supplementation duration, and study population characteristics. A 2018 metanalysis evaluating the effect of CLA reported that CLA 3.4 g/day or more, for a minimum of 12 weeks, in subjects over 44 years of age had the greatest effect on body weight [ 263 ]. However, it should be highlighted that CLA supplementation resulted in only 1.3 kg reduction in body weight, of hardly any clinical relevance [ 263 ]. A recent review has highlighted that CLA, and primarily the 10,12 CLA isomer, consistently gives some degree of adiposity loss with different impact on different species, regardless of the presence of early symptoms of metabolic syndrome, without noticeable lean mass waste [ 264 ]. The same authors also report many studies failing to show any effects of mixed CLA supplementation on body parameters, and they propose several explanations such as the importance of a continuous dosing strategy for human efficacy, the need of higher doses of the active isomer of CLA, and differences in the basal metabolic rate between animals and humans [ 264 ].
In conclusion, the literature to date suggests that CLA, and primarily the 10,12 CLA isomer, promotes weight and fat loss in human subjects. Although its effectiveness seems to be clinically limited and the quality of evidence low, CLA could be considered as a treatment of obesity in addition to a dietary program given the strong preclinical evidence, the minor weight loss effect, and the very good safety profile.
3.4.7. Aloe Vera
Aloe is a plant that belongs to the Liliaceae family; the most popular species is Aloe Vera , which has been used for centuries as a medicinal herb. Among its many applications, skin burns, and wound healing are the most common ones [ 265 ]. Various extracts of A. vera , used in animal and human studies, have also shown positive effects on glucose and lipid metabolism, gut microbiota, and blood pressure [ 266 ].
If its anti-obesity properties have been extensively demonstrated in animal models, only few human studies showed positive results regarding weight reduction [ 266 ]. In rodent models, A. Vera has been shown to reduce visceral fat accumulation, suppress lipogenesis related genes [ 267 ], enhance the expression of UCP-2 [ 268 ] and activate the AMPK pathway, leading to the acceleration of glucose and lipid oxidation in muscle and white adipose tissue [ 267 ].
In human studies, A. Vera supplementation was able to decrease serum triglycerides, total cholesterol, LDL, and fasting blood glucose level, as well as reducing HbA1c and insulin levels [ 265 ]. These effects on glucose metabolism are thought to be ascribable to acemannan, a mucopolysaccharide contained in A. Vera leaves, which is degraded by the intestinal microbiota to form oligosaccharides capable of inhibiting intestinal glucose absorption [ 269 ]; other properties of this plant, seen in animal studies, that may contribute to its positive effect on the lipid and glucose profile are represented by its ability to reduce oxidative stress, activate PPAR transcription, stimulate hepatic lipoprotein activity, and finally also activate hormone-sensitive lipase [ 266 ]. Furthermore, many active compounds present in this plant, such as acemannan and polyphenols, may increase the gut microbiota SCFAs production and stimulate gut anorexigenic hormones release, with the effect of reducing food intake and inducing weight loss [ 265 ].
In a 2013 randomized placebo-controlled trial, 136 obese prediabetic and early non-treated diabetic patients, were given 588 mg of A. Vera gel per day. At the end of the study, the aloe group achieved greater weight loss compared to placebo, showed a significant increase in lean body mass, a significant decrease in body fat mass and lower fasting blood glucose levels. The daily caloric intake, calculated from 24-h food recalls, decreased at four weeks in both groups and increased to baseline levels at week 8, suggesting an effect on weight loss independent of caloric intake. As the authors stated, the use of 24h food recalls to estimate daily caloric intake has several limitations and the estimation of body composition through bioimpedance analysis does not represent the gold standard, all these limitations possibly hindering the results observed [ 270 ].
A. vera has been shown to exert positive effects on glucose and lipid metabolism in both diabetic and nondiabetic patients. Evidence supporting its ability to induce weight loss, however, is limited and of low quality, so its consumption cannot be recommended for this purpose. Further studies are needed to confirm the results seen in animal studies and to elucidate the mechanisms supporting weight loss in humans.
3.4.8. Flaxseed
Flaxseed is a functional food sourced from the flax plant; it is rich in α-linolenic acid (ALA), dietary fiber and lignans (phytoestrogen) [ 271 ]. Flaxseed contains approximately 22% ALA while flaxseed oil contains 50–62% ALA [ 272 ]. Flax and flaxseed have been historically used as a wound-healing and diuretic agent, pain, and cough reliever and to improve skin elasticity and its moisture holding capacity. Nowadays it is mostly used in cloths fabrication, animal feeding and as a functional food ingredient in juices, dairy, bakery, and meat products [ 273 ].
Results from reviews and metanalyses show that flaxseed consumption has beneficial effects on blood pressure [ 274 ], lipid profile [ 275 ] and glucose metabolism [ 276 ]. Evidence regarding its effect on body composition indices, however, is controversial [ 277 ]. Many flaxseed components may have positive effects on weight management: lignans are reported to reduce visceral fat and increase fat oxidation and adiponectin levels in mice [ 278 ], but their supplementation alone was not shown to be effective in improving body composition in humans [ 277 ]; soluble fiber, which represent up to 27% of flaxseed weight, may induce a feeling of fullness, delay gastric emptying and increase SCFA concentration in the gut, inducing satiety and promoting weight loss via GI hormones release [ 279 ]. Furthermore, ALA has been shown to increase adipose leptin expression in animal models [ 280 ] and flaxseed polysaccharides have been able to induce satiety improving leptin resistance together with enhancing lipolysis and suppressing lipogenesis through the AMPK signaling pathway [ 281 ]. Lastly, ALA metabolism products, eicosapentaenoic acid and docosahexaenoic acid, were able to induce adipocytes apoptosis, suppress appetite and enhance fat oxidation and energy expenditure in animal models, while human studies confirmed its potential benefit only in combination with exercise and hypocaloric diets [ 282 ]. A 2017 review and metanalysis including 45 RCTs, highlighted the effect of flaxseed supplementation on body weight and body composition [ 277 ]. Interestingly, only whole flaxseed was able to reduce body weight, BMI, and waist circumference, while flaxseed oil and lignans extracts did not show any benefit. Furthermore, a significant weight loss was observed only in subjects with BMI >27, eating more than 30 gr of flaxseed per day and in trials longer than 12 weeks. These findings suggest that the high fiber content or other compounds present in whole flaxseed are responsible for the higher weight loss. These results may also indicate that flaxseed supplementation has a cumulative and time dependent effect, probably because ALA, lignans or other flaxseed components build up their concentration in tissues over time or because of gut microbiota adaptations happening over a longer period. Among the studies analyzed, only few studies incorporated lifestyle advice or provided hypocaloric diets, and no data were reported regarding daily caloric intake, making it difficult to understand the mechanisms behind the weight reduction. Furthermore, the high heterogeneity of the studies regarding study populations (healthy subjects and subjects with different comorbidities) may hinder the results strength.
Overall, flaxseed showed promising weight reduction properties, backed by numerous health benefits, although the quality of the evidence supporting its application for this purpose is still low; however, considering the absence of side effects, its consumption may be considered for the treatment of obesity. To further understand the mechanisms promoting weight loss, studies analyzing appetite parameters alongside changes in daily caloric intake and gut hormones levels over time are needed.
3.4.9. Grapefruit
Grapefruit is a citrus fruit, known for its bitter taste. It is rich in water, vitamins, minerals, and polyphenols, such as phenolic acids, flavonoids, lignans and stilbenes [ 283 ]. Among its polyphenols, naringin and hesperidin have demonstrated antioxidant, lipid lowering and antihypertensive properties in animal models as well as human clinical trials [ 284 ]. Naringin and hesperidin are also able to improve glycemic control, enhancing insulin secretion and inhibiting gluconeogenesis [ 285 ]; like many other polyphenols, naringin has also been reported to stimulate the AMPK pathway, thus enhancing fatty acid oxidation and inhibiting lipogenesis [ 286 ]. Cells and animal studies demonstrated lipolytic effects of grapefruit extract and polyphenols through the inhibition of cAMP-phosphodiesterase and activation of hepatic peroxisome proliferator-activated receptor γ and α [ 287 , 288 , 289 ].
A 2014 review and metanalysis, taking into account RCTs that analyzed the effect of grapefruit on weight and body composition in humans, showed no benefit regarding weight loss, but a positive effect on waist circumference and body fat percentage reduction [ 283 ]. Of these, only one study reported a significant body weight reduction in 24 obese subjects eating half grapefruit before meals three times per day for 12 weeks, while the group assigned to receive grapefruit juice and grapefruit capsule did not achieve a significant weight loss [ 283 ]. Interestingly, none of the studies included in this literature revision reported a significant reduction in daily caloric intake when subjects following ad libitum diets or hypocaloric diets received grapefruit or grapefruit juice, compared to placebo. These results may indicate that grapefruit effect on adiposity is independent of caloric intake and may be a consequence of the whole fruit consumption. However, the paucity of trials analyzed in the revision and their short duration may limit the results. It should also be highlighted that different results may be seen if grapefruit is consumed in different quantities and modalities, taking into account that the included studies only investigated grapefruit or grapefruit juice as a preload before meals.
Overall, low-quality evidence supports grapefruit or grapefruit juice as a weight loss agent. Moreover, grapefruit may alter the metabolism of several drugs through cytochrome P450 interactions [ 283 ]. This aspect should be therefore be kept in mind in those on a daily pharmacological treatment, and the consumption of high amounts of grapefruit should be therefore discouraged in these subjects. Altogether, its use cannot be recommended at this time.
3.5. Carbohydrate Metabolism Improvement as Purported Mechanisms of Action
As a matter of fact, T2D and insulin resistance are two of the most important complications of obesity. A derangement in glucose metabolism in the obese patient usually has its roots in a vicious circle that includes the onset and progression of NAFLD. Food supplements that exert beneficial effects on glucose metabolism often do so through an improvement in the liver and increased expression of GLUT channels. It is therefore impossible to strictly classify supplements as lipid metabolism and carbohydrate metabolism improving. Here, we provide more detail on the food supplements proven to be more effective on glucose metabolism and insulin resistance improvement rather than in fat mass reduction ( Table 5 ).
3.5.1. Mangosteen
Garcinia mangostana L., commonly known as mangosteen, is a widespread evergreen tree in Southeast Asian countries, and its fruits have found many applications in traditional medicine for centuries. The main compounds are mangostins and isoprenylated xanthones, exerting antioxidant effects [ 290 ]. Recent evidence has also reported a possible role in the treatment of obesity and its comorbidities.
In vitro studies have shown that alpha-mangostin acts as a strong inhibitor of pancreatic lipase, not different from the weight loss medication orlistat [ 291 ]. This compound was also reported to induce apoptosis of preadipocytes and enhance lipolysis through the inhibition of fatty acid synthase [ 292 ]. In rodent models, mangosteen supplementation led to a glucose lowering effect that could likely be due to hyperplasia of pancreatic beta cells and alpha glucosidase activity [ 293 , 294 , 295 ]. Moreover, alpha-mangostin treated diet induced obesity mice experienced weight loss, improved glucose and lipid profile and reduced liver fat accumulation through a Peroxisome proliferator-activated receptor gamma and SIRT-1-AMPK pathway [ 296 , 297 ]. Four to sixteen week-long studies conducted on human subjects and assessing the effect of 200–400 mg of mangosteen extracts reported significant weight loss and waist circumference reduction, and an excellent safety and tolerability profile [ 298 , 299 , 300 , 301 ]. In our hands, a 26-week-long mangosteen extract supplementation led to glucose metabolism improvement in insulin resistant female subjects with obesity, with a frank decrease of HOMA-IR, independent of body weight change [ 302 ].
Considering the promising but scanty and low-quality evidence, mangosteen should not be encouraged nor recommended against as a treatment of obesity and its complications such as insulin resistance, also given its optimal cost-to-benefit ratio.
3.5.2. Chromium
Chromium (Cr) is an essential nutrient widely distributed in the human diet. The main food sources of Cr are meat, nuts, cereal grains, molasses, and brewer’s yeast. The exact mechanism of Cr is not well understood, however, salt forms, such as Cr nicotinate, Cr chloride, and Cr picolinate, are believed to be associated with an increase in the activity of insulin [ 303 ]. Cr may also contribute to weight loss by suppressing appetite and by stimulating human thermogenesis, through sensitization of insulin-sensitive glucoreceptors in the brain, thus increasing energy expenditure [ 304 ].
A recent metanalysis evaluated the efficacy of oral Cr supplementation from randomized controlled trials published from 1996 to 2017 [ 305 ]. Of all selected trials, twelve included subjects who were overweight or obese. Different forms of Cr were used in the included trials, with most administering Cr picolinate, followed by Cr nicotinate and Cr-enriched yeast. The present metanalysis indicates that Cr supplementation for less than 13 weeks is associated with significant overall placebo-controlled weight loss in individuals who were overweight and obese, in terms of BMI and body fat percentage. These findings are partially in contrast with a previous metanalysis by Onakpoya et al., suggesting that Cr should be supplemented for at least 16 weeks in order to reach a maximal weight loss of 1 Kg [ 306 ]. Notably, in three included RCTs Cr was administered in association with other compounds, making its individual effect indistinguishable [ 307 , 308 ]. Subgroup analysis by dosage of Cr supplementation showed significant improvement in the mean change of body weight in trials which administered a dose of Cr ≤ 400 μg/dl. Even though this metanalysis did not outline any evidence of specific adverse effects with Cr supplementation, previous studies have reported concerns regarding the safety of Cr picolinate supplementation which could be responsible for renal and hepatic impairment [ 309 ]. Nevertheless, several studies reported no significant body weight loss after Cr supplementation compared to placebo or even reported significant weight gain. However, it should be noted that in one case no dietary control was performed, possibly hindering results, and in the other subjects were not selected based on BMI, and lean patients may as well have been enrolled [ 310 , 311 ]. Pointing in the same direction, a systematic review investigating weight loss and metabolic and hormonal variables in patients with polycystic ovary syndrome suggested that Cr supplementation has no beneficial effects [ 312 ].
In conclusion, the evidence supporting the use of Cr for weight loss is moderate, although clinical relevance remains uncertain, possibly because of the presence of different forms of supplementation; further investigation through larger studies is necessary. However, given the less favorable safety profile compared to other food supplements, its use cannot be recommended nor discouraged at his time.
3.5.3. Lipoic Acid
Alpha-lipoic acid (LA), or 1,2-dithiolan-3-pentanoic acid is enzymatically synthesized in the mitochondria from octanoic acid. In addition to the synthesis, LA is also absorbed intact from food sources and accumulates transiently in many tissues. LA has been described as a powerful biological antioxidant with glucose lowering effects; it is used to improve cardiovascular, cognitive, and neuromuscular deficits related to aging and is able to modulate various pathways of inflammation [ 313 , 314 , 315 ]. The typical food sources of LA are meat, offal, and to a lesser extent, fruit and vegetables [ 316 ]. Given the limited quantities present in these nutritional sources, LA does not seem to be consumed in an appreciable way in the western diet. Rather, dietary supplements that typically range from 50 to 600 mg are the primary sources of LA and most of the information on its bioavailability comes from studies using supplements.
LA seems to lead to increased GLUT4 expression on the cell membrane of skeletal muscle and adipocyte cells, through a PI3K-dependent mechanism that involves the insulin signaling cascade [ 317 ]. Intraperitoneal administration of LA on Zucker rats led to a skeletal muscle increase in glucose uptake in acute (100 mg/kg body weight for 1 h) and in chronic conditions (50 mg/kg bodyweight for 10 days) [ 318 ]. Combining LA supplementation (30 mg/kg per day for 15 days) with physical exercise in an animal model of insulin resistance, additive effects of improved glucose tolerance and intracellular glucose transport have been observed. A potential mechanism for this additive effect is the upregulation of GLUT4 protein expression in muscle combined with the enhanced translocation of GLUT4 on the LA-induced plasma membrane [ 319 , 320 ]. Improvements in glucose metabolism have also been observed in humans with T2D treated with intravenous or oral LA [ 321 , 322 ]. A systematic review including ten double blind, placebo-controlled RCTs that investigated LA for weight loss purposes showed that its supplementation was associated with a statistically significant 1.27 kg greater mean weight loss compared to placebo [ 323 ].
Several human studies have assessed the efficacy and safety of LA as a food supplement. Clinical trials ALADIN (I, II and III), SYDNEY (I and II), and ORPIL used LA supplements of up to 2400 mg/day with no adverse effects reported compared to placebo. Moreover, oral supplementation of 1800 mg LA for 6 months did not cause significant adverse effects compared to placebo [ 324 ]. LA has also been associated with cases of insulin autoimmune syndrome (IAS). Also known as Hirata disease, IAS is a rare genetic disease that has occurred most often in Japanese subjects. IAS patients develop antibodies to insulin, causing episodes of hypoglycaemia [ 325 ].
Altogether, LA seems to have a clinically relevant glucose lowering effect, but evidence supporting its use towards weight loss are still of low-quality. Its supplementation may be considered for the treatment of obesity, especially when T2D coexists, under the supervision of experienced health professionals, given the possible side effects that have been reported.
4. Conclusions
Given the present literature revision, it is possible to conclude that many of the presented food supplements are likely to exert an anti-obesogenic effect in the absence of significant adverse events. However, none of this is capable of inducing a clinically relevant weight loss, with the most effective ones leading to a mere 2-kg reduction. Given the current coronavirus disease 19 (COVID-19) pandemic, and with obesity being a well-established risk factor for worse prognosis [ 326 , 327 , 328 ], it is now of the utmost importance to find safe and effective ways to tackle weight excess.
Supported by current evidence, we propose a possible combination leveraging all mechanisms of action that could pave the way for future studies investigating the weight loss effect and safety profile of such product.
To inhibit the absorption of nutrients, we suggest using phaseolus vulgaris extract (PVE) at a dosage of 3000 mg (1000 mg per meal) daily, and green tea derived epigallocatechin (EGCG) at a dosage of 500 mg daily. In order to reduce appetite and possibly increase energy expenditure, we propose the use of coffee derived caffeine (300 mg/daily) and chrologenic acid (200 mg/daily). Chili pepper derived capsaicinoids or capsinoids may also be considered, at a dosage of 10 mg and 3 mg, respectively, together with L-C at a dosage of 2 g daily, that can also increase fat mobilization. Similarly enhancing beta oxidation and inhibiting lipogenesis, resveratrol and CLA may be considered, given their proven efficacy and absence of reported adverse events, at a dosage of 200 mg and 4 g daily, respectively. Finally, carbohydrate metabolism may be improved with glucose lowering lipoic acid at a dosage of 600 mg daily, with its long-standing history in the treatment of T2D. Most of the cited dietary supplements were also proven to exert anti-inflammatory and antioxidant effects, possibly aiding the resolution of low-grade chronic inflammation typical of weight excess and metabolic derangements ( Figure 1 ).

Proposed food supplement combination leveraging multiple mechanisms of action to aid weight loss and metabolism improvement based on the current state of the art. Green tea was shown to inhibit pancreatic lipase, amylase, and glucosidase in the gastrointestinal tract reducing the absorption of nutrients and leading to the presence of undigested carbohydrates in the GI tract, in turn driving the microbiota to produce short-chain fatty acids (SCFA). Through an AMPK dependent mechanism, it also inhibits lipogenesis and induces lipolysis. Phaseolus vulgaris extract (PVE) contains phaseolin, an α-amylase inhibitor whose function impairs the absorption of carbohydrates. Caffeine suppresses hunger and stimulates energy expenditure through increased excitability of the sympathetic nervous system (SNS), increased fat oxidation and Brown Adipose Tissue (BAT) activation. Capsaicinoids activate the Transient Receptor Potential Channel Vanilloid type-1 (TRPV1) leading to Glucagon like peptide 1 (GLP-1) release, increased fat oxidation, increased Sirtuin-1 (SIRT-1) expression. They also suppress ghrelin release and increase adiponectin, PPARα and PGC-1α expression. They finally regulate gluconeogenesis and glycogen synthesis genes improving insulin resistance. L-Carnitine was shown to improve insulin resistance, increase acetyl-coenzyme A and glucose supply to the brain leading to increased energy expenditure; it facilitates activated long chain fatty acids transportation into mitochondria, playing an important role in β-oxidation. It also modulates lipid metabolism. Resveratrol increases SIRT-1 expression, decreases adipogenesis and viability in maturing preadipocytes and modulates lipid metabolism in mature adipocytes. Conjugated linoleic acid (CLA) decreases the size of adipocytes, alters adipocyte differentiation, regulates lipid metabolism and activates of PPAR-γ receptors. Lipoic acid increases GLUT4 expression on the cell membrane of skeletal muscle and adipocyte cells leading to increased glucose uptake, hence improved glucose tolerance, chlorogenic acid (CGA).
A specifically designed, placebo-controlled study investigating the proposed combination for weight loss purposes is now needed, in order to confirm the safety profile, the absence of detrimental interactions between the suggested compounds, and the presence of an additive or synergistic effect possibly aiding weight loss in a safe and effective way.
Author Contributions
M.W., D.T., and C.L. conceived and designed the literature revision. M.W., D.T., R.R., A.C., D.M., A.B., and G.R. performed the literature revision and prepared the original draft; S.B., S.M. (Silvia Manfrini), S.M. (Stefania Mariani), L.G., and C.L. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Loading metrics
Open Access
Peer-reviewed
Research Article
Weight loss strategies, weight change, and type 2 diabetes in US health professionals: A cohort study
Contributed equally to this work with: Keyi Si, Yang Hu
Roles Conceptualization, Formal analysis, Validation, Writing – original draft, Writing – review & editing
Affiliation Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, United States of America
Roles Conceptualization, Formal analysis, Writing – review & editing
Roles Formal analysis, Supervision, Writing – review & editing
Affiliations Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, United States of America, Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, United States of America
Roles Writing – review & editing
Affiliation Division of Endocrinology, Diabetes, and Hypertension, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts, United States of America
Roles Formal analysis, Writing – review & editing
Affiliations Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, United States of America, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, United States of America, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts, United States of America
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Current address: Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, United States of America
- Keyi Si,
- Yang Hu,
- Molin Wang,
- Caroline M. Apovian,
- Jorge E. Chavarro,

- Published: September 27, 2022
- https://doi.org/10.1371/journal.pmed.1004094
- Reader Comments
Weight loss is crucial for disease prevention among individuals with overweight or obesity. This study aimed to examine associations of weight loss strategies (WLSs) with weight change and type 2 diabetes (T2D) risk among US health professionals.
Methods and findings
This study included 93,110 participants (24 to 60 years old; 11.6% male) from the Nurses’ Health Study (NHS), NHSII, and Health Professionals Follow-Up Study (HPFS) cohorts who were free of T2D, cardiovascular disease, and cancer at baseline (1988 for NHS/HPFS and 1989 for NHSII) for analyses of weight change and 104,180 (24 to 78 years old; 14.2% male) for T2D risk assessment. WLSs used to achieve an intentional weight loss of 4.5+ kg were collected in 1992 (NHS/HPFS)/1993 (NHSII) and grouped into 7 mutually exclusive categories, including low-calorie diet, exercise, low-calorie diet and exercise, fasting, commercial weight loss program (CWLP), diet pills, and FCP (selected at least 2 methods from fasting, CWLP, and pill). The reference group was participants who did not attempt to lose weight. Generalized estimating equations and Cox regression were applied to estimate up to 10-year weight change trajectory and incident T2D risk through 2016 (NHS/HPFS)/2017 (NHSII), respectively.
The associations of WLSs with weight change and T2D risk were differential by baseline body weight (P interaction < 0.01). Among individuals with obesity, all WLSs tended to associate with less weight gain [ranging from −4.2% (95% confidence interval (CI), −5.1% to −3.2%; P < 0.001) for exercise to −0.3% (−1.2% to 0.7%; P > 0.99) for FCP] and a lower T2D risk [hazard ratios (HRs) ranging from 0.79 (0.66 to 0.95; P = 0.04) for exercise to 0.87 (0.66 to 1.13; P = 0.30) for pill]. Such a pattern was less clear among overweight individuals: the difference of weight change varied from −2.5% (−3.0% to −2.1%; P < 0.001) for exercise to 2.0% (1.3% to 2.7%; P < 0.001) for FCP, and HRs of T2D varied from 0.91 (0.77 to 1.07; P = 0.29) for exercise to 1.42 (1.11 to 1.81; P = 0.02) for pill. The pattern was further inverted among lean individuals in that weight change ranged from −0.4% (−0.6% to −0.1%; P = 0.02) for exercise to 3.7% (3.1% to 4.3%; P < 0.001) for FCP, and the HRs of T2D ranged from 1.09 (0.91 to 1.30; P = 0.33) for exercise to 1.54 (1.13 to 2.10; P = 0.008) for pill. Approximately 15.6% to 46.8% of the association between WLSs and the T2D risk was attributed to weight changes. This study was limited by a single assessment of WLSs, heterogeneity within each WLS, and potential misclassification of the timing of weight loss and weight regain.
Conclusions
The current study showed that individuals with obesity who attempted to lose weight, regardless of the WLSs used, tended to gain less body weight and have a lower diabetes risk. In contrast, lean individuals who intentionally lost weight tended to gain more weight and have a higher diabetes risk. These data support the notion that intentional weight loss may not be beneficial for lean individuals and the use of WLSs for achieving weight loss shall be guided by medical indications only.
Author summary
Why was this study done.
- Weight control is one of the primary and effective strategies for the prevention and management of obesity and related chronic diseases.
- Trying to lose weight is common not only among overweight individuals or those with obesity, but also among lean individuals.
- Long-term weight change and risks of developing type 2 diabetes (T2D) following various weight loss strategies (WLSs) are understudied.
What did the researchers do and find?
- Up to 10-year weight change and 24-year T2D risk were compared between individuals who lost 4.5+ kg (10+ lbs) through various WLSs (low-calorie diet, exercise, low-calorie diet and exercise, fasting, commercial weight loss program (CWLP), diet pills, or a combination of the last 3 strategies collectively named as FCP) and those who did not attempt to lose weight.
- Association of WLSs with weight change and T2D risk varied by baseline body mass index status: individuals with obesity who lost 4.5+ kg intentionally were likely to have less weight gain within 4 years since baseline and lower risk of T2D during 24 years of follow-up, regardless of WLS, but the beneficial associations were attenuated in individuals with overweight and even inverted in lean individuals.
- Of all WLSs, exercise was associated with the least weight gain and the lowest T2D risk among individuals with obesity.
- Approximately 15.6% to 46.8% of the association between WLSs and the T2D risk was attributable to body weight changes after weight loss attempts.
What do these findings mean?
- Individuals with obesity may benefit from intentional weight loss regardless of the methods used to achieve the weight loss, whereas lean individuals may not enjoy the same health benefits from intentionally losing a significant amount of body weight.
- These data suggest that the use of weight loss methods for achieving weight loss should be guided by medical or health indications.
Citation: Si K, Hu Y, Wang M, Apovian CM, Chavarro JE, Sun Q (2022) Weight loss strategies, weight change, and type 2 diabetes in US health professionals: A cohort study. PLoS Med 19(9): e1004094. https://doi.org/10.1371/journal.pmed.1004094
Academic Editor: Barry M. Popkin, Carolina Population Center, UNITED STATES
Received: February 17, 2022; Accepted: August 17, 2022; Published: September 27, 2022
Copyright: © 2022 Si et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: Data can be accessed through a process for which details can be found at https://nurseshealthstudy.org/researchers for accessing data from the Nurses' Health Studies or https://sites.sph.harvard.edu/hpfs/for-collaborators/ for accessing data from the Health Professionals Follow-up Study.
Funding: This study was funded by the National Institutes of Health (CA186107, CA176726, CA167552, DK126698 to QS, DK120870 to QS, DK119268 to QS, ES022981 to QS, and DK046200 to CMA through the Boston Nutrition and Obesity Research Center). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. https://www.nih.gov/grants-funding The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: I have read the journal’s policy and the authors of this manuscript have the following competing interests: CMA has participated on advisory boards for Abbott Nutrition, Allergan, Inc., Altimmune, Inc., Bariatrix Nutrition, Cowen and Company, LLC, Curavit Clinical Research, EnteroMedics, Gelesis, Srl., Janssen, Jazz Pharmaceuticals, Inc., L-Nutra, Inc., Novo Nordisk, Inc., Nutrisystem, Real Appeal, Rhythm Pharmaceuticals, Roman Health Ventures, Inc., SetPoint Health, Scientific Intake Ltd. Co., Tivity Health, Inc., Xeno Biosciences and Zafgen Inc. CMA has received research funding from Novo Nordisk.
Abbreviations: AHEI, Alternative Healthy Eating Index; BMI, body mass index; CI, confidence interval; CWLP, commercial weight loss program; FCP, fasting, commercial weight loss program, or pill; FFM, fat-free mass; %FFML, percentage weight loss as fat-free mass; HPFS, Health Professionals Follow-Up Study; HR, hazard ratio; LCD, low-calorie diet; MET, metabolic equivalent of tasks; NHS, Nurses’ Health Study; T2D, type 2 diabetes; WLS, weight loss strategy
Introduction
Obesity is one of the most common chronic conditions in the United States and globally. In 2017 to 2018, 42.4% of US adults were estimated to have obesity [ 1 ], which predisposed them to numerous chronic diseases, especially type 2 diabetes (T2D) [ 2 ]. As such, weight control is one of the primary and effective strategies for the prevention and management of chronic diseases with obesity-related etiology. On average, the risk of diabetes is estimated to reduce by 16% per kilogram weight loss in individuals with overweight/obesity and prediabetes [ 3 ]. In 2013 to 2016, 49.1% of US adults reported trying to lose weight, mainly through lifestyle modifications, such as exercise (62.9%) and dieting (62.9%) [ 4 ]. However, it is challenging to maintain weight loss, which is often accompanied by weight regain in the long run [ 5 ]. Meanwhile, a systematic review of 8 weight loss trials suggested that the weight regain trajectory in 3 to 5 years after the interventions varied by different weight loss strategies (WLSs) [ 6 ], which may thus exert differential impacts on the risk of developing obesity-related conditions, such as T2D. However, to our knowledge, no study has comprehensively examined multiple commonly practiced WLSs in relation to long-term weight change trajectories or T2D risk in free-living individuals who choose WLSs at will in observational study settings.
To fill the knowledge gaps, the current study aimed to investigate the association of common WLSs with weight change and T2D risk in 3 large-scale prospective cohorts of free-living US men and women. In addition, in light of the evidence that baseline body weight may modulate the benefits of weight loss [ 7 – 9 ], we also evaluated the associations of interest according to baseline body mass index (BMI) before weight loss attempts.
Materials and methods
This study is reported as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline ( S1 Checklist ). The study protocol was drafted prospectively in August 2019 ( S1 Text ).
Study population
This study used data from the Health Professionals Follow-up Study (HPFS), the Nurses’ Health Study (NHS), and the NHSII. The HPFS began in 1986 and enrolled 51,529 male health professionals aged 40 to 76 years from 50 US states. The NHS recruited 121,701 female nurses aged 30 to 55 years from 11 states in 1976. The NHSII, initiated in 1989, included 116,429 female nurses aged 24 to 42 years from 14 states. Follow-up questionnaires were mailed to participants biennially since 1976 (NHS)/1986 (HPFS)/1989 (NHSII) to update lifestyle and medical information. Additional validated semiquantitative food frequency questionnaires were administered every 2 to 4 years thereafter to assess dietary intake. The response rates exceeded 90% in each cycle for 3 cohorts. Weight loss attempts within the last 4 years were self-reported in 1992 (NHS/HPFS)/1993 (NHSII). Since we did not know the exact time when the weight loss began in these 4 years, we considered 1988 for NHS/HPFS and 1989 for NHSII as study baseline.
For the analyses with T2D as the outcome, participants were excluded if they skipped the question of WLSs; if their weight loss was unintentional; if they reported a diagnosis of diabetes, cardiovascular disease, or cancer or deceased by 1992 (NHS/HPFS)/1993 (NHSII); if they only completed the 1992/1993 questionnaire; if they had missing information on the diagnosis date of T2D, age, or baseline BMI; or if they were pregnant at baseline (NHSII only). Other exclusions related to specific WLSs were listed in the next section. In analyses of weight change, the exclusion criteria were the same as those of T2D analyses, except that participants who only answered the 1992/1993 questionnaire remained if they provided valid body weight assessments in that year. Participants who did not report body weight since 1992/1993 or those aged 65+ years in 1992/1993 were further excluded. After the exclusions, 104,180 participants were included in the T2D analyses and 93,110 were considered in the weight change analyses ( S1 Fig ).
This study was approved by the institutional review boards of the Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health, who deemed that the return of a complete self-administered questionnaire implied an informed consent. The last author vouched for the accuracy and completeness of the data and the analyses.
Assessment of weight loss strategies
In the 1992 (NHS/HPFS) and 1993 (NHSII) questionnaires, participants were inquired about the amount (2.3 to 4.1 kg [5 to 9 lbs], 4.5 to 8.6 kg [10 to 19 lbs], 9.1 to 22.2 kg [20 to 49 lbs], 22.7+ kg [50+ lbs]) and frequency (0 times, 1 to 2 times, 3 to 4 times, 5 to 6 times, 7+ times) of intentional weight loss. Participants were asked to mark all the primary methods they had used to achieve the most recent weight loss of 4.5+ kg (10+ lbs) within the last 4 years, including the following possible responses: “did not lose 4.5+ kg,” “weight loss was unintentional (e.g., illness, unusual stress, depression),” “low-calorie diet,” “skipped meals/fasted,” “increased exercise,” “diet pills,” “commercial weight loss program,” “gastric surgery/intestinal bypass,” and “other” in the NHS/HPFS. Three more responses (“low fat diet,” “decreased alcohol intake,” and “resumed/increased smoking”) were included in the NHSII questionnaire only. Based on the responses to these questions, participants were categorized into 3 mutually exclusive groups, including those who did not attempt to lose weight, those who lost less than 4.5 kg at a time, and those who lost 4.5+ kg at a time in the past 4 years. We excluded those who reported losing less than 4.5 kg since the WLS information was not collected for these individuals. Participants who did not attempt to lose weight were treated as the reference group. For participants who lost 4.5+ kg intentionally, we first excluded those who lost weight through surgery or other unspecific methods and then excluded NHSII participants who solely used the 3 methods that were not considered in the NHS and HPFS questionnaires to maintain consistency across 3 cohorts. To facilitate analyses and keep interpretation consistent across 3 cohorts, we grouped the WLSs into 7 mutually exclusive categories, including low-calorie diet (LCD), exercise, LCD and exercise, fasting, commercial weight loss program (CWLP), pill, and a combination (two or more) of fasting, CWLP, and pill (FCP for short). The grouping was largely determined by the distribution of individual WLSs as well as the combinations of WLSs in the study population ( S1 Table ).
Assessment of covariates
In this study, we considered multiple covariates assessed before or in the 1992/1993 questionnaire for multivariate adjustments, including age, ethnicity, height, BMI (weight in kilograms divided by height in meters squared), waist circumference, alcohol intake, smoking status, multivitamin use, physical activity (metabolic equivalents of tasks [METs]), television watching duration, total energy intake, diet quality (Alternative Healthy Eating Index [AHEI] score), family history of diabetes, and history of hypertension or hypercholesterolemia ( S2 Table ).
Assessment of weight change and type 2 diabetes
Body weight and physician-diagnosed diabetes incidence were collected since baseline and updated biennially. Weight change percentage, defined as [(current weight − baseline weight) / baseline weight] * 100%, was used to measure weight change in the current study. A supplementary questionnaire regarding symptoms, diagnostic tests, and hypoglycemic therapy was mailed to participants who self-reported having physician-diagnosed diabetes to confirm the diagnosis ( S2 Text ).
Statistical analysis
Data from the 3 cohorts were pooled to maximize statistical power. For the weight change analyses, follow-up was censored when participants reached aged 65 or older on the incidence of diabetes, cardiovascular disease, cancer, death, or pregnancy (NHSII only). For the T2D analyses, person-time for each participant was counted from the return of the 1992/1993 questionnaires to the date of T2D diagnosis, death, last return of a valid follow-up questionnaire, or the end of follow-up (June 2016 for NHS/HPFS; June 2017 for NHSII), whichever came first.
Generalized linear model and generalized estimating equations with unstructured within-subject correlation matrices were used to assess the association of WLSs with baseline body weight and weight change, respectively. Least squares means of body weight and weight change percentages since baseline were calculated to illustrate the trajectory of weight change over time. All available body weights in 1988/1989, 1992/1993, 1994/1995, 1996/1997, and 1998/1999 and the corresponding weight change percentage since baseline were included as a time-varying dependent variable in these models. Because the biennial weight change percentages were mostly differential among WLSs at year 4 and then largely converged to each other at year 10 (1998 for NHS/HPFS or 1999 for NHSII; S2 Fig ), we focused on weight change by the end of year 4 and year 10, respectively.
Cox proportional hazards model was applied to examine the association of WLSs with the incidence of T2D. The proportional hazards assumption was tested by including the product terms between each exposure indicator and the log-transformed follow-up time. No violation of the assumption was found. Multiple imputation was implemented to minimize the number of missing values in covariates ( S3 Text ). Multiple comparisons were adjusted using Dunnett’s test and false discovery rate when comparing the strength of associations of various WLSs. Given that weight change might be a mediator between WLSs and T2D risks, the extent to which the association might be explained by time-varying BMI was evaluated using a SAS macro %MEDIATE [ 10 ].
Stratified analyses were conducted by baseline BMI (<25 kg/m 2 [lean], 25 to <30 kg/m 2 [overweight], or ≥30 kg/m 2 [obese]). Interactions were tested using a likelihood-ratio test comparing models with and without product terms between WLSs and stratifying variables.
We considered several sensitivity analyses. A cubic spline regression model was fitted to delineate the trajectory of hazard ratios (HRs) over follow-up duration. Given the strong impact of ageing on body weight and composition, we repeated the T2D analysis in participants who were <65 years old in 1992 (NHS/HPFS)/1993 (NHSII). To reduce the possibility of reverse-causation, we excluded participants who were diagnosed with T2D in the first 4 years of follow-up (through 1996 [NHS/HPFS]/1997 [NHSII]). To alleviate the concern that the body weight assessments in 1988 (NHS/HPFS) or 1989 (NHSII) may misclassify the long-term weight status before 1988/1989, we redefined individuals who were consistently lean (BMI was less than 25 kg/m 2 at each biennial follow-up from the initiation of the cohorts to 1988/1989) as the baseline lean group, and the same algorithm was used to define the overweight and obese groups. In another sensitivity analysis, we also used maximum BMI collected before 1992/1993 (1972 to 1992 for HPFS, 1976 to 1992 for NHS, and 1989 to 1993 for NHSII) to define the obesity status. In a sensitivity analysis, we included participants who skipped the WLS question into the reference group. We also stratified the analysis by abdominal obesity (waist circumference ≥102 cm for male and waist circumference ≥88 cm for female). Lastly, in response to peer review comments, we restricted the weight change analyses within participants with complete, valid weight assessments since 1988 (NHS/HPFS)/1989 (NHSII) through 1998 (NHS/HPFS)/1999 (NHSII) to evaluate the impact of missing weight data on associations of interest.
Data were analyzed using SAS software, version 9.4 (SAS Institute). Two-sided multiple comparison adjusted P < 0.05 was considered statistically significant.
Of all participants, including those who lost less than 4.5 kg of body weight, 53.2% (75,201/141,387) reported losing 4.5+ kg intentionally, of whom 13.3% through LCD, 10.7% through exercise, 29.2% through LCD and exercise, 12.6% through fasting, 27.4% through CWLP, 1.9% through pill, and 5.1% through FCP ( S3 Table ). The age-standardized baseline characteristics of the study populations for T2D analyses and weight change analyses are shown in Tables 1 and S4 and S5 , respectively, and those of participants with/without skipping the WLS question were shown in S6 Table .
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pmed.1004094.t001
Weight loss strategies and weight change
The temporal trend of body weight according to WLSs is shown in Fig 1A . Regardless of whether or not participants tried to lose weight or what WLSs they had adopted, their body weight, on average, increased over time. However, the weight gain trajectories were differential among WLS groups. By 10 years of follow-up, all WLS groups were associated with more weight gain than the reference group (ranging from 1.7% for exercise to 6.6% for FCP) ( Tables 2 and S7 ).
( A ) Overall population. ( B ) BMI <25 kg/m 2 . ( C ) BMI 25 to <30 kg/m 2 . ( D ) BMI ≥30 kg/m 2 . All body weights were calculated based on baseline weight and weight change percentage since baseline. For weight change percentage, the multivariable model was adjusted for cohort (HPFS, NHS, or NHSII), age (in month, continuous), ethnicity (white, African American, Asian, or other), baseline body weight (in kilogram, continuous), baseline waist circumference (in centimeter, continuous), physical activity (in quintiles), television watching (0–1, 2–5, 6–10, 11–20, or >20 hour/week), smoking status (never, past, or current smokers), alcohol intake (0, <5.0, 5.0–9.9, 10.0–14.9, 15.0–29.9, or >30.0 gram/day), hypertension (yes or no), hypercholesterolemia (yes or no), family history of diabetes (yes or no), multivitamin use (yes or no), AHEI score (in quintiles), and total energy intake (in quintiles) before weight loss. For baseline body weight, all abovementioned covariates were adjusted for except that body weight and waist circumference were replaced with height (in meter, continuous). AHEI, Alternative Healthy Eating Index; BMI, body mass index; CWLP, commercial weight loss program; FCP, select at least 2 strategies among fasting, CWLP, and pill; HPFS, Health Professionals Follow-Up Study; kg, kilogram; kg/m 2 , kilogram per square meter; LCD, low-calorie diet; NHS, Nurses’ Health Study; WLS, weight loss strategy. 1 kg = 2.2 lbs.
https://doi.org/10.1371/journal.pmed.1004094.g001
https://doi.org/10.1371/journal.pmed.1004094.t002
Weight loss strategies and type 2 diabetes
During 2.14 million person-years of follow-up, 10,149 incident cases of T2D were observed ( Fig 2 ). After multivariate adjustments, all WLSs were significantly associated with a higher risk of developing T2D. In comparison with the reference group, the HR varied from 1.15 (95% confidence interval [CI] 1.05, 1.27; P = 0.005) for exercise to 1.64 (95% CI 1.41, 1.92; P < 0.001) for pill. The proportions of the association between WLSs and T2D risk mediated by time-varying BMI after weight loss ranged from 15.6% (95% CI 7.7%, 29.0%; P < 0.001) for exercise to 46.8% (95% CI 37.7%, 56.1%; P < 0.001) for FCP ( S8 Table ).
( A ) All BMI categories have the same reference (individuals whose BMI was <25 kg/m 2 and who did not attempt to lose weight). ( B ) Each BMI category has its own reference. Multivariable models were adjusted for cohort (HPFS, NHS, or NHSII), age (in month, continuous), ethnicity (white, African American, Asian, or other), baseline BMI (in kg/m 2 , continuous), baseline waist circumference (in centimeter, continuous), physical activity (in quintiles), television watching (0–1, 2–5, 6–10, 11–20, or >20 hour/week), smoking status (never, past, or current smokers), alcohol intake (0, <5.0, 5.0–9.9, 10.0–14.9, 15.0–29.9, or >30.0 gram/day), hypertension (yes or no), hypercholesterolemia (yes or no), family history of diabetes (yes or no), multivitamin use (yes or no), AHEI score (in quintiles), and total energy intake (in quintiles) before weight loss. P for interaction for overall and individual WLSs were less than 0.01. AHEI, Alternative Healthy Eating Index; BMI, body mass index; CI, confidence interval; CWLP, commercial weight loss program; FCP, select at least 2 strategies among fasting, CWLP, and pill; FDR, false discovery rate; HPFS, Health Professionals Follow-Up Study; HR, hazard ratio; kg/m 2 , kilogram per square meter; LCD, low-calorie diet; NHS, Nurses’ Health Study; T2D, type 2 diabetes; WLS, weight loss strategy.
https://doi.org/10.1371/journal.pmed.1004094.g002
Modification by baseline body mass index
Among participants who lost 4.5+ kg, those with higher baseline BMI were less likely to choose exercise, LCD and exercise, or fasting and more likely to choose LCD, CWLP, and FCP than leaner participants ( S3 Table ). We observed significant interactions between overall/individual WLSs and baseline BMI on the associations of interest ( P interaction < 0.001). Among individuals with obesity, all WLSs tended to be associated with less weight gain during the first 4 years of follow-up, whereas among lean individuals, all WLSs except exercise tended to be associated with more weight gain than the reference group ( Table 2 ). Of all WLSs, exercise was associated with the least absolute weight change percentage in all BMI categories: −0.7%, 2.0%, and 2.6% among individuals who were originally obese, overweight, and lean, respectively ( S3 Fig ). During the extended follow-up (10 years), CWLP, pill, and FCP had more weight gain than the other WLSs for all BMI categories. The weight change over time is shown in Fig 1B–1D .
Similar effect modification by baseline BMI on the associations between WLSs and T2D was also observed ( P interaction < 0.01). Compared with the reference group, all WLSs tended to be associated with a lower risk of developing T2D in individuals with obesity (HRs ranging from 0.79 to 0.87), whereas in lean individuals, the opposite pattern of association was observed (HRs ranging from 1.09 to 1.54) ( Fig 2 ) . Of all WLSs, exercise was the only WLS that was not significantly associated with T2D risk among lean participants (HR 1.09 [95% CI 0.91, 1.30; P = 0.33]). The effect modification remained when BMI was treated as a continuous variable. The T2D risk compared with the reference group was significantly lowered by 4.0% (95% CI 2.6%, 5.4%; P < 0.001) for exercise to 8.1% (6.8%, 9.5%; P < 0.001) for FCP for each unit increment of baseline BMI ( S9 Table ).
Secondary and sensitivity analyses
HRs for T2D across different WLSs were generally consistent over time ( S4 Fig ). Results were similar when aged participants or those who were diagnosed T2D in the first 4-year follow-up were excluded from the analyses, when the analysis was based on individuals who were consistently lean/overweight/obese before 1988/1989, when the stratification was based on maximum BMI before 1992/1993, or when participants who skipped the WLS question were included into the reference group ( S5 – S9 Figs and S10 – S12 Tables ). When analyses were stratified by baseline abdominal obesity status, by 10 years of follow-up, all WLSs tended to associate with more weight gain, and the association was stronger in individuals without abdominal obesity ( P interaction < 0.001; S13 Table and S10 and S11 Figs ). Accordingly, all WLSs tended to associate with a higher risk of T2D in participants without abdominal obesity, with HRs varying from 1.02 to 1.69 ( S12 Fig ). Among individuals with abdominal obesity, none of the WLSs was associated with the risk of T2D. The T2D risk compared with the reference group significantly reduced by 1.4% (95% CI 0.8% to 2.1%; P < 0.001) for exercise to 2.9% (95% CI 2.2% to 3.6%; P < 0.001) for FCP for each unit increment of waist circumference ( S14 Table ). Lastly, the results of weight changes among participants with complete weight assessments ( S15 Table ) remained largely unchanged, compared with those in Table 2 .
Principal findings
In 3 cohorts of US men and women, about half of participants reported intentionally losing 4.5+ kg of body weight using various strategies, ranging from lifestyle modifications (e.g., LCD, exercise, and their combinations) to fasting or other commercial interventions (e.g., CWLP and pill). The primary finding is that the associations of various WLSs with weight change and T2D risk are dependent on body weight status before weight loss. Specifically, among individuals with obesity, compared with those who did not attempt to lose weight, those who lost 4.5+ kg gained less weight and had lower T2D risk, regardless of the WLSs used to achieve the weight loss. This pattern of favorable associations was less clear among overweight individuals and even reversed among lean individuals. Of all WLSs, exercise was associated with the least weight gain and the lowest T2D risk among individuals with obesity.
Comparison with other studies
Weight maintenance after weight loss is notoriously challenging [ 5 ]. In our cohorts, we observed universal weight gain from baseline across all groups that underwent weight loss, although different WLSs were associated with differential weight gain trajectories. This is in accordance with previous findings in a systematic review of prospective studies with a minimum 3-year follow-up after weight loss. This review indicated that most individuals who had lost at least 5% of body weight using diet, diet and exercise, or cognitive behavioral treatment regained weight to the preintervention baseline weight without sustained intervention after approximately 4 years [ 6 ]. Nonetheless, of all strategies, exercise appeared to associate with the least weight gain in our study. This was supported by the result of a trial where the exercise group lost 2.9 kg after 1-year intervention and regained 0.2 kg after another year without intervention, whereas the diet group lost 6.8 kg but regained 7.7 kg, and the diet and exercise group lost 8.9 kg and regained 6.7 kg [ 11 ]. It is worth noting that, in addition to the inclusion of a reference group and the use of prospective study design, our study is substantially different from these prior studies in that our focus was long-term weight change since baseline, which is not necessarily equivalent to weight maintenance (weight change since the end of weight loss) in intervention study settings. Nonetheless, the current evidence thus far collectively highlights the role of exercise in long-term weight control after intentional weight loss [ 12 ].
Although all strategies except exercise were associated with more weight gain, we observed a gradient in the weight gain trajectory in that participants who took pills, used CWLPs or their combinations with fasting (FCP) tended to gain more weight than those who followed a LCD or fasted. Evidence for comparisons of long-term weight changes between CWLPs or pills and other WLSs is scarce. Nonetheless, this observation is in line with previous studies showing that individuals who used a self-guided approach were better at maintaining their initial weight loss compared with those who commenced a CWLP [ 13 ]. Regarding LCD versus fasting as weight loss methods, a pilot study suggested that there was no significant difference in weight regain between intermittent fasting and daily caloric restriction, but more % fat mass was lost and more % lean mass was regained by the end of the 24-week follow-up in the fasting group [ 14 ]. In contrast, a recent randomized trial showed that time-restricting eating did not lead to additional weight loss than calorie restriction alone within 12 months [ 15 ]. Apparently, more research is needed to further compare the efficacy of different WLSs on promoting weight loss.
Intriguingly, the pattern of associations with T2D risk clearly mirrors that for long-term weight change in that exercise was associated with the least-elevated T2D risk. We further estimated that a significant proportion of these positive associations might be ascribed to the weight changes following weight loss attempts, highlighting the role of long-term weight control following weight loss in the primary prevention of T2D.
Although it is not entirely clear why exercise may outperform other WLSs, some potential mechanisms may explain the less weight gain and more favorable T2D risk associated with exercise. A series of compensatory physiological adaptations favoring weight regain are triggered by weight loss, such as increases in orexigenic hormones (e.g., ghrelin) and fat accumulation and decreases in anorexigenic hormones (e.g., leptin, cholecystokinin, peptide YY) and energy expenditure [ 16 – 19 ]. Exercise was demonstrated to mitigate weight regain via counteracting some of these adaptations. For example, exercise has been reported to restore the hormone perturbations, increase energy expenditure and fat oxidation, and reduce the adipocyte size, which has not been observed in the context of caloric restriction [ 19 – 21 ]. In addition, exercise was suggested to facilitate weight maintenance by breaking the vicious cycle of stress and obesity [ 22 ]. Importantly, exercise might be more sustainable. In a weight loss trial, 44% of participants in the exercise group reported exercising often after intervention, but only 6.7% in the diet group reported adhering often to previous dietary recommendations [ 11 ]. The better retention of fat-free mass (FFM) and greater fat reduction compared to caloric restriction may explain the minimally increased T2D risk associated with exercise, given the potential protective effect of FFM and the adverse effect of excess body fat on T2D [ 23 , 24 ]. A systematic review summarized that exercise was shown to decrease the percentage weight loss as FFM (%FFML), whereas the degree of caloric restriction was positively associated with %FFML [ 25 ]. Another systematic review revealed that 5% loss in body weight was associated with 21.3% reduction in visceral adiposity after exercise but with 13.4% reduction after a hypocaloric diet, and exercise was related to 6.1% decrease in visceral adiposity even in the absence of weight loss while the corresponding number was only 1.1% for a hypocaloric diet [ 26 ]. Moreover, exercise has been shown to improve insulin sensitivity independent of weight loss [ 27 ].
The effectiveness of CWLP and pills on weight maintenance particularly depends on the duration of use and degree of compliance [ 28 , 29 ]. However, in free-living participants, the retention rate decreased dramatically over time: 73% at 1 month, 42% at 3 months, 22% at 6 months, and 6.6% at 12 months in the Jenny Craig Platinum program [ 30 ]. The greater weight regain in the CWLP group compared with conventional self-directed WLSs might partially be ascribed to their differences regarding to confidence, motivation, and cost [ 13 ], which may result in less sustainable low-calorie dietary habits after weight loss [ 16 ]. In the early 1990s, the most popular diet pills, such as phentermine, fenfluramine, diethylpropion, and others, were restricted to short-term use (a few weeks) because of safety concerns [ 31 , 32 ], such as addiction and side effects of elevation of heart rate and dizziness [ 33 ]. Weight regain is common once the medication is terminated unless the medication is combined with healthy eating habits and increasing physical activity [ 34 ]. Current long-term diet pills (e.g., orlistat, top-dose [15/92 mg] phentermine plus topiramate-extended release), when used in adjunction with lifestyle interventions, have been indicated to increase the likelihood of achieving clinically meaningful 1-year weight loss compared with placebo [ 33 ]. As for fasting, adherence to various regimens was inconclusive [ 35 ]. Some investigators speculated that intermittent fasting might reduce adaptive responses induced by energy restriction by regularly raising energy intake on fed days, but evidence was limited with insufficient power [ 36 ].
Despite the differences among WLSs used to achieve weight loss, we observed universal health benefits associated with all WLSs among individuals with obesity. Another noteworthy point is that among individuals with obesity, although the body weight of LCD, fasting, CWLP, and FCP groups was consistently larger than that of the reference group after weight loss ( Fig 1D ), their T2D risk continued to be lower, suggesting that 4.5+ kg of weight loss, even though transient, can still lead to a decreased T2D risk in individuals with obesity in a long run. This notion was also supported by results from the Diabetes Prevention Program, which suggested that even a one-time weight loss intervention could have lasting effects on reducing T2D risk for at least 10 years [ 37 ]. This is also the case for overweight individuals who lost 4.5+ kg through exercise or LCD and exercise. Similar long-lasting beneficial effects of limited duration lifestyle interventions on T2D incidence were demonstrated in several well-known trials focusing on T2D prevention among those with overweight/obesity [ 38 ].
Weight loss attempts are remarkably prevalent among lean individuals [ 4 ], as was observed in our study population (50.7% [34,987/68,946] of participants who lost 4.5+ kg intentionally were lean at baseline), which might be partly attributed to their weight misperception influenced by the sociocultural pressure of being lean [ 39 ]. Indeed, as a previous study reported, 53.8% of lean women and 22.7% of lean men perceived themselves as being slightly overweight, and 58.1% and 24.7% of them tried to lose weight, respectively [ 40 ]. Our observations of divergent pattern of associations by baseline BMI status were consistent with findings from Finnish cohorts that the risk of having a major weight gain (>10 kg) or increases in BMI or waist circumference in lean dieters versus lean non-dieters was generally higher than that in overweight counterparts [ 7 , 41 ]. Other prospective studies also demonstrated stronger inverse associations between exercise and T2D risk in overweight participants than their lean counterparts [ 8 , 9 ]. For example, the relative risks of diabetes in the exercise group compared with the sedentary group were 1.22, 0.87, 0.69, and 0.61 from the lowest to the highest quartiles of BMI [ 9 ]. As a result, the high proportion of lean individuals in those who lost 4.5+ kg in our population, together with the effect modifications by BMI, may explain our unintuitive finding that participants who lost 4.5+ kg were likely to gain more weight and have higher risk of T2D than those who did not attempt to lose weight. The reason that might explain the modification of baseline BMI was that fat overshooting and FFM loss were more severe in lean individuals than in those with overweight or obesity upon weight loss [ 23 , 24 , 42 ]. A critical mechanism could be that in the process of weight regain, fat is fully regained much earlier than FFM, and such desynchronization results in a state of hyperphagia that persists until FFM is fully recovered, during which fat continues to accumulate, leading to fat (and weight) overshooting [ 42 ]. A reanalysis of data from the Minnesota Semi-Starvation Experiment further showed that the extent of fat overshooting was inversely correlated with the initial percentage of body fat [ 42 ]. Consistently, hyperphagic responses followed by long-term exercise have been reported in lean individuals but not in overweight individuals or those with obesity [ 43 , 44 ]. In addition, the %FFML usually exceeded 35% in normal-weight individuals, while the number was approximately 20% to 30% in the overweight/obese [ 45 ]. Taken together, the current and prior evidence suggests that lean individuals may not benefit from intentional weight loss, possibly due to the physiological process that predisposes lean individuals to fat overshooting or excess weight regain after they lose weight.
Strengths and limitations
To our knowledge, this is the first study that comprehensively examined the long-term associations of multiple WLSs with weight change and T2D risk in a large group of free-living individuals in a real-world setting. Another noteworthy strength is that we only considered intentional weight loss through the strategies and thus minimized the strong impact of chronic diseases and other causes of unintentional weight loss on associations of interest. Several limitations are worth mentioning. First, we could not further distinguish the methods in each broad category of WLSs, which can be rather heterogeneous. As such, what we observed are “average” associations that may not be fully generalizable to a more specific strategy. Second, the homogeneous ethnicity and socioeconomic status, although can help alleviate the confounding by these factors, further limit the generalizability of our observations to other populations with different characteristics. Third, we were unable to evaluate the impact of previous or subsequent WLSs on the associations of interest. Fourth, we did not assess the exact amount nor the exact time of weight loss. We thus cannot assess the role of weight loss amount on weight gain and T2D risk and may pool person-time of heterogeneous scenarios (e.g., weight change right after weight loss versus weight change after several years since weight loss). Lastly, as for any epidemiological studies, we cannot exclude the role of residual/unmeasured confounding or chance in our observations. More studies are needed to further elucidate these important associations.
Conclusion and policy implications
In conclusion, in individuals with obesity, losing 4.5+ kg of body weight intentionally was associated with less weight gain and lower T2D risk, regardless of the methods used to achieve the weight loss. However, for individuals who were lean, losing 4.5+ kg was not associated with these health benefits. Of all WLSs, exercise was optimal for long-term weight control and T2D prevention. Our data support current guidelines for body weight management, such as that issued by the Obesity Society, which recommend a weight loss of 5% to 10% of baseline weight for individuals who are overweight or obese and exercise of 200 to 300 minutes per week to maintain the weight loss [ 46 ].
Supporting information
S1 checklist. strobe statement..
https://doi.org/10.1371/journal.pmed.1004094.s001
S1 Text. Study protocol.
https://doi.org/10.1371/journal.pmed.1004094.s002
S2 Text. The confirmation of self-reported type 2 diabetes.
https://doi.org/10.1371/journal.pmed.1004094.s003
S3 Text. Multiple imputation of the covariates.
https://doi.org/10.1371/journal.pmed.1004094.s004
S1 Table. Components of the weight loss strategies in the type 2 diabetes analyses.
https://doi.org/10.1371/journal.pmed.1004094.s005
S2 Table. Data source of the covariates.
https://doi.org/10.1371/journal.pmed.1004094.s006
S3 Table. Age-standardized proportions of weight loss strategies by baseline body mass index in the type 2 diabetes analyses.
https://doi.org/10.1371/journal.pmed.1004094.s007
S4 Table. Age-standardized characteristics of participants before weight loss in the type 2 diabetes analyses.
https://doi.org/10.1371/journal.pmed.1004094.s008
S5 Table. Age-standardized characteristics of participants before weight loss in the weight change analyses.
https://doi.org/10.1371/journal.pmed.1004094.s009
S6 Table. Comparison of characteristics before weight loss between participants who skipped the weight loss strategy question and those who did not.
https://doi.org/10.1371/journal.pmed.1004094.s010
S7 Table. Baseline weight and weight change percentage since baseline across different weight loss strategies ( P values were unadjusted).
https://doi.org/10.1371/journal.pmed.1004094.s011
S8 Table. Proportions (95% CIs) of the association between weight loss strategies and type 2 diabetes mediated by time-varying body mass index.
https://doi.org/10.1371/journal.pmed.1004094.s012
S9 Table. The hazard ratio of type 2 diabetes reduced by each unit increment of baseline body mass index.
https://doi.org/10.1371/journal.pmed.1004094.s013
S10 Table. Weight loss strategies and weight change percentages (consistently lean/overweight/obese before 1988/1989).
https://doi.org/10.1371/journal.pmed.1004094.s014
S11 Table. Weight loss strategies and weight change percentages (maximum body mass index before 1992/1993).
https://doi.org/10.1371/journal.pmed.1004094.s015
S12 Table. Weight loss strategies and weight change percentages (participants who skipped the weight loss strategy question were included into the reference group).
https://doi.org/10.1371/journal.pmed.1004094.s016
S13 Table. Baseline weight and weight change percentage since baseline across different weight loss strategies stratified by baseline abdominal obesity status.
https://doi.org/10.1371/journal.pmed.1004094.s017
S14 Table. The hazard ratio of type 2 diabetes reduced by each unit increment of baseline waist circumference.
https://doi.org/10.1371/journal.pmed.1004094.s018
S15 Table. Baseline weight and weight change percentage since baseline across different weight loss strategies (complete case analysis).
https://doi.org/10.1371/journal.pmed.1004094.s019
S1 Fig. Flow chart of participants.
https://doi.org/10.1371/journal.pmed.1004094.s020
S2 Fig. Weight change trajectories of different weight loss strategies.
https://doi.org/10.1371/journal.pmed.1004094.s021
S3 Fig. Weight loss strategies and absolute weight change percentages since baseline stratified by baseline body mass index.
https://doi.org/10.1371/journal.pmed.1004094.s022
S4 Fig. Pooled hazard ratios of type 2 diabetes by time according to weight loss strategies.
https://doi.org/10.1371/journal.pmed.1004094.s023
S5 Fig. Pooled hazard ratios for association between weight loss strategies and the incidence of type 2 diabetes in participants <65 years old.
https://doi.org/10.1371/journal.pmed.1004094.s024
S6 Fig. Pooled hazard ratios for association between weight loss strategies and the incidence of type 2 diabetes (participants who were diagnosed type 2 diabetes in the first 4-year follow-up were excluded from the analysis).
https://doi.org/10.1371/journal.pmed.1004094.s025
S7 Fig. Pooled hazard ratios for association between weight loss strategies and the incidence of type 2 diabetes in participants who were consistently lean/overweight/obese before 1988/1989.
https://doi.org/10.1371/journal.pmed.1004094.s026
S8 Fig. Pooled hazard ratios for association between weight loss strategies and the incidence of type 2 diabetes stratified by maximum BMI before 1992/1993.
https://doi.org/10.1371/journal.pmed.1004094.s027
S9 Fig. Pooled hazard ratios for association between weight loss strategies and the incidence of type 2 diabetes (participants who skipped the weight loss strategy question were included into the reference group).
https://doi.org/10.1371/journal.pmed.1004094.s028
S10 Fig. Weight loss strategies and weight change trajectories by baseline abdominal obesity status.
https://doi.org/10.1371/journal.pmed.1004094.s029
S11 Fig. Weight loss strategies and absolute weight change percentages since baseline stratified by baseline abdominal obesity status.
https://doi.org/10.1371/journal.pmed.1004094.s030
S12 Fig. Pooled hazard ratios for association between weight loss strategies and the incidence of type 2 diabetes stratified by baseline abdominal obesity status.
https://doi.org/10.1371/journal.pmed.1004094.s031
Acknowledgments
We thank the participants of the Health Professionals Follow-up Study, the Nurses’ Health Study, and the Nurses’ Health Study II for their contributions and long-term commitment to scientific research.
- View Article
- PubMed/NCBI
- Google Scholar
- 10. Hertzmark E, Pazaris M, Spiegelman D. The SAS MEDIATE Macro 2018 [cited 2022 Jun 10]. Available from: https://cdn1.sph.harvard.edu/wp-content/uploads/sites/271/2012/08/mediate.pdf .
- 34. Prescription Medications to Treat Overweight & Obesity website: National Institute of Diabetes and Digestive and Kidney Diseases; 2021 [updated 2021 Jun; cited 2022 Jun 10]. Available from: https://www.niddk.nih.gov/health-information/weight-management/prescription-medications-treat-overweight-obesity#replace .
Five percent weight loss is a significant 1-year predictor and an optimal 5-year cut-off for reducing the number of obesity-related cardiovascular disease risk components: the Japan Obesity and Metabolic Syndrome Study
Affiliations.
- 1 Department of Endocrinology, Metabolism, and Hypertension Research, Clinical Research Institute, NHO Kyoto Medical Center, Kyoto, Japan.
- 2 Department of General Internal Medicine, Fushimi Momoyama General Hospital, Kyoto, Japan.
- 3 Department of Rehabilitation, Health Science University, Minamitsuru-gun, Japan.
- 4 Department of Endocrinology and Metabolism, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan.
- 5 Division of Translational Research, Clinical Research Institute, NHO Kyoto Medical Center, Kyoto, Japan.
- 6 Diabetes Therapeutics and Research Center, Institute of Advanced Medical Sciences, Tokushima University, Tokushima, Japan.
- 7 Division of Community and Family Medicine, Jichi Medical University, Shimotsuke, Japan.
- 8 Department of Diabetes, Metabolism and Endocrinology, Ichikawa Hospital, International University of Health and Welfare, Ichikawa, Japan.
- 9 Department of Endocrinology and Diabetes, Saitama Medical University, Moroyama, Japan.
- 10 Department of Metabolic Syndrome and Nutritional Science, Research Institute of Environmental Medicine, Nagoya University, Nagoya, Japan.
- PMID: 38601201
- PMCID: PMC11005029
- DOI: 10.3389/fendo.2024.1343153
Objective: This study aimed to identify the amount of weight loss needed in patients with obesity to improve metabolic syndrome (MetS), a risk factor for cardiovascular disease (CVD), over a long period of time.
Methods: A total of 576 patients with obesity were enrolled in this study. Effects of continuous physician-supervised weight loss on the cumulative MetS components excluding abdominal circumference (defined as obesity-related CVD risk score) were investigated during a 5-year follow-up period. The extent of weight loss required to reduce the obesity-related CVD risk components was assessed using receiver operating characteristic (ROC) curve analyses.
Results: Of the 576 participants, 266 completed 5-year follow-up, with 39.1% and 24.1% of them achieving ≥5.0% and ≥7.5% weight loss at the 5-year follow-up, respectively. The area under the ROC curve for reducing the obesity-related CVD risk components was 0.719 [0.662-0.777] at 1 year and 0.694 [0.613-0.775] at 5 years. The optimal cut-off value for weight loss was 5.0% (0.66 sensitivity and 0.69 specificity) and the value with 0.80 specificity was 7.5% (0.45 sensitivity) at 5 years. Greater reductions in weight were associated with greater improvements in the obesity-related CVD risk score at all follow-up periods ( P -trend <0.001). Obesity-related CVD risk score was significantly improved by 5.0-7.5% and ≥7.5% weight loss at 1 year ( P = 0.029 and P < 0.001, respectively) and ≥7.5% weight loss at 5 years ( P = 0.034).
Conclusions: A weight loss of ≥5.0% at 1 year and ≥7.5% at 5 years could reduce the number of obesity-related CVD risk components in patients with obesity.
Keywords: 5-year follow-up; cohort study; metabolic syndrome; obesity; weight loss.
Copyright © 2024 Yamakage, Jo, Tanaka, Kato, Hasegawa, Masuda, Matsuhisa, Kotani, Noda and Satoh-Asahara.
- Cardiovascular Diseases* / epidemiology
- Cardiovascular Diseases* / etiology
- Cardiovascular Diseases* / prevention & control
- Japan / epidemiology
- Metabolic Syndrome* / complications
- Obesity / complications
- Risk Factors
Grants and funding
- See us on twitter
- See us on youtube
- See us on instagram
A Comparative Weight Loss Study of the Atkins, Zone, Ornish, and USDA/LEARN Diets

Obesity is the single most significant nutrition-related health issue of the new millennium. Several "medical experts" have designed and promoted weight loss diets that dramatically differ from one another, and from the USDA Dietary Guidelines. These diets have gained surprisingly widespread and persistent popularity among Americans, despite a lack of scientific evidence supporting their claims. The objective of the A TO Z Study was to examine various health outcomes (e.g. benefits, risks, success) of FOUR popular weight loss strategies representing a spectrum of low to high carbohydrate intake, and compare them.
Study Design
Over 300 free-living pre-menopausal, overweight women were randomly assigned to follow either the Atkins (extremely low carbohydrate), Zone (low-carbohydrate, high protein), Ornish (very low fat), or USDA/Food LEARN (high carbohydrate/moderate-low fat) diet for 1 year.
Conclusions
At the completion of the study, the women assigned to follow the Atkins diet lost more weight (~10 pounds average weight lost in 1 year) and also experienced metabolic effects that were comparable with or more beneficial than the other participants.
To learn more about the details of the study, read:
- Abstract, published in Journal of the American Medical Association
Stanford Prevention Research Center


- Diversity in Clinical Research
- Clinical Research for Older Populations
- Clinical Research for Compensation
- Clinical Trial Information for Parents
- What Is A Clinical Trial?
Weight Loss Clinical Trials
Match to clinical trials, browse by state.
This content is for informational and educational purposes only. It is not intended to provide medical advice or to take the place of such advice or treatment from a personal physician. All readers/viewers of this content are advised to consult their doctors or qualified health professionals regarding specific health questions. Policylab.us does not take responsibility for possible health consequences of any person or persons reading or following the information in this educational content. All viewers of this content, especially those taking prescription or over-the-counter medications, should consult their physicians before beginning any nutrition, supplement or lifestyle program
The independent source for health policy research, polling, and news.
A New Use for Wegovy Opens the Door to Medicare Coverage for Millions of People with Obesity
Juliette Cubanski , Tricia Neuman , Nolan Sroczynski , and Anthony Damico Published: Apr 24, 2024
The FDA recently approved a new use for Wegovy (semaglutide), the blockbuster anti-obesity drug, to reduce the risk of heart attacks and stroke in people with cardiovascular disease who are overweight or obese. Wegovy belongs to a class of medications called GLP-1 (glucagon-like peptide-1) agonists that were initially approved to treat type 2 diabetes but are also highly effective anti-obesity drugs. The new FDA-approved indication for Wegovy paves the way for Medicare coverage of this drug and broader coverage by other insurers. Medicare is currently prohibited by law from covering Wegovy and other medications when used specifically for obesity. However, semaglutide is covered by Medicare as a treatment for diabetes, branded as Ozempic.
What does the FDA’s decision mean for Medicare coverage of Wegovy?
The FDA’s decision opens the door to Medicare coverage of Wegovy, which was first approved by the FDA as an anti-obesity medication. Soon after the FDA’s approval of the new use for Wegovy, the Centers for Medicare & Medicaid Services (CMS) issued a memo indicating that Medicare Part D plans can add Wegovy to their formularies now that it has a medically-accepted indication that is not specifically excluded from Medicare coverage . Because Wegovy is a self-administered injectable drug, coverage will be provided under Part D , Medicare’s outpatient drug benefit offered by private stand-alone drug plans and Medicare Advantage plans, not Part B, which covers physician-administered drugs.
How many Medicare beneficiaries could be eligible for coverage of Wegovy for its new use?
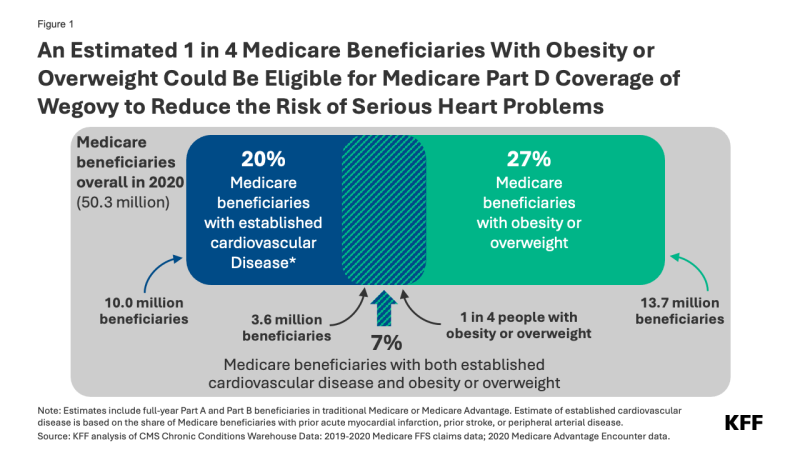
Of these 3.6 million beneficiaries, 1.9 million also had diabetes (other than Type 1) and may already have been eligible for Medicare coverage of GLP-1s as diabetes treatments prior to the FDA’s approval of the new use of Wegovy.
Not all people who are eligible based on the new indication are likely to take Wegovy, however. Some might be dissuaded by the potential side effects and adverse reactions . Out-of-pocket costs could also be a barrier. Based on the list price of $1,300 per month (not including rebates or other discounts negotiated by pharmacy benefit managers), Wegovy could be covered as a specialty tier drug, where Part D plans are allowed to charge coinsurance of 25% to 33%. Because coinsurance amounts are pegged to the list price, Medicare beneficiaries required to pay coinsurance could face monthly costs of $325 to $430 before they reach the new cap on annual out-of-pocket drug spending established by the Inflation Reduction Act – around $3,300 in 2024, based on brand drugs only, and $2,000 in 2025. But even paying $2,000 out of pocket would still be beyond the reach of many people with Medicare who live on modest incomes . Ultimately, how much beneficiaries pay out of pocket will depend on Part D plan coverage and formulary tier placement of Wegovy.
Further, some people may have difficulty accessing Wegovy if Part D plans apply prior authorization and step therapy tools to manage costs and ensure appropriate use. These factors could have a dampening effect on use by Medicare beneficiaries, even among the target population.
When will Medicare Part D plans begin covering Wegovy?
Some Part D plans have already announced that they will begin covering Wegovy this year, although it is not yet clear how widespread coverage will be in 2024. While Medicare drug plans can add new drugs to their formularies during the year to reflect new approvals and expanded indications, plans are not required to cover every new drug that comes to market. Part D plans are required to cover at least two drugs in each category or class and all or substantially all drugs in six protected classes . However, facing a relatively high price and potentially large patient population for Wegovy, many Part D plans might be reluctant to expand coverage now, since they can’t adjust their premiums mid-year to account for higher costs associated with use of this drug. So, broader coverage in 2025 could be more likely.
How might expanded coverage of Wegovy affect Medicare spending?
The impact on Medicare spending associated with expanded coverage of Wegovy will depend in part on how many Part D plans add coverage for it and the extent to which plans apply restrictions on use like prior authorization; how many people who qualify to take the drug use it; and negotiated prices paid by plans. For example, if plans receive a 50% rebate on the list price of $1,300 per month (or $15,600 per year), that could mean annual net costs per person around $7,800. If 10% of the target population (an estimated 360,000 people) uses Wegovy for a full year, that would amount to additional net Medicare Part D spending of $2.8 billion for one year for this one drug alone.
It’s possible that Medicare could select semaglutide for drug price negotiation as early as 2025, based on the earliest FDA approval of Ozempic in late 2017 . For small-molecule drugs like semaglutide, at least seven years must have passed from its FDA approval date to be eligible for selection, and for drugs with multiple FDA approvals, CMS will use the earliest approval date to make this determination. If semaglutide is selected for negotiation next year, a negotiated price would be available beginning in 2027. This could help to lower Medicare and out-of-pocket spending on semaglutide products, including Wegovy as well as Ozempic and Rybelsus, the oral formulation approved for type 2 diabetes. As of 2022, gross Medicare spending on Ozempic alone placed it sixth among the 10 top-selling drugs in Medicare Part D, with annual gross spending of $4.6 billion, based on KFF analysis . This estimate does not include rebates, which Medicare’s actuaries estimated to be 31.5% overall in 2022 but could be as high as 69% for Ozempic, according to one estimate.
What does this mean for Medicare coverage of anti-obesity drugs?
For now, use of GLP-1s specifically for obesity continues to be excluded from Medicare coverage by law. But the FDA’s decision signals a turning point for broader Medicare coverage of GLP-1s since Wegovy can now be used to reduce the risk of heart attack and stroke by people with cardiovascular disease and obesity or overweight, and not only as an anti-obesity drug. And more pathways to Medicare coverage could open up if these drugs gain FDA approval for other uses . For example, Eli Lilly has just reported clinical trial results showing the benefits of its GLP-1, Zepbound (tirzepatide), in reducing the occurrence of sleep apnea events among people with obesity or overweight. Lilly reportedly plans to seek FDA approval for this use and if approved, the drug would be the first pharmaceutical treatment on the market for sleep apnea.
If more Medicare beneficiaries with obesity or overweight gain access to GLP-1s based on other approved uses for these medications, that could reduce the cost of proposed legislation to lift the statutory prohibition on Medicare coverage of anti-obesity drugs. This is because the Congressional Budget Office (CBO), Congress’s official scorekeeper for proposed legislation, would incorporate the cost of coverage for these other uses into its baseline estimates for Medicare spending, which means that the incremental cost of changing the law to allow Medicare coverage for anti-obesity drugs would be lower than it would be without FDA’s approval of these drugs for other uses. Ultimately how widely Medicare Part D coverage of GLP-1s expands could have far-reaching effects on people with obesity and on Medicare spending.
- Medicare Part D
- Chronic Diseases
- Heart Disease
- Medicare Advantage
news release
- An Estimated 1 in 4 Medicare Beneficiaries With Obesity or Overweight Could Be Eligible for Medicare Coverage of Wegovy, an Anti-Obesity Drug, to Reduce Heart Risk
Also of Interest
- An Overview of the Medicare Part D Prescription Drug Benefit
- FAQs about the Inflation Reduction Act’s Medicare Drug Price Negotiation Program
- What Could New Anti-Obesity Drugs Mean for Medicare?
- Medicare Spending on Ozempic and Other GLP-1s Is Skyrocketing
- About the Hub
- Announcements
- Faculty Experts Guide
- Subscribe to the newsletter
Explore by Topic
- Arts+Culture
- Politics+Society
- Science+Technology
- Student Life
- University News
- Voices+Opinion
- About Hub at Work
- Gazette Archive
- Benefits+Perks
- Health+Well-Being
- Current Issue
- About the Magazine
- Past Issues
- Support Johns Hopkins Magazine
- Subscribe to the Magazine
You are using an outdated browser. Please upgrade your browser to improve your experience.

Credit: Getty Images
New Johns Hopkins study challenges benefits of intermittent fasting
Both time-restricted eating and regularly planned meals led to similar weight loss results, suggesting that total calories may be more important than meal timing.
By Hub staff report
When it comes to weight loss, how many calories you consume might be more important than when you consume them, challenging the popularity of intermittent fasting, according to a new study of time-restricted eating by researchers at Johns Hopkins University published April 19 in Annals of Internal Medicine .
The Johns Hopkins researchers randomly assigned 41 adults with obesity and prediabetes to either time-restricted eating (TRE) with a 10-hour eating window or a regular eating pattern for 12 weeks to compare weight loss and other measures of metabolic health, according to a study summary provided by Annals of Internal Medicine . Their randomized, controlled trial found that both TRE and a more traditional daily eating pattern resulted in weight loss when calories were held constant in both groups. It did not seem to matter whether participants consumed most of their calories early in the day or in the evening, suggesting that overall calories may be more important than meal timing when it comes to weight loss.
"It makes us think that people who benefit from time-restricted eating—meaning they lose weight—it's probably from them eating fewer calories because their time window's shorter and not something else," said the study's lead author, Nisa Maruthur , an associate professor of medicine at Johns Hopkins University, in an interview with NBC News . Maruthur presented the findings at the inaugural scientific plenary session "New in Annals of Internal Medicine: Hear it First from the Authors" in Boston last week during the annual American College of Physician's Internal Medicine Meeting.
Image caption: Nisa Maruthur
Similar to intermittent fasting, patients using TRE limit their eating to a window of time during the day and then fast for the remaining hours. During the eating window, patients are not required to count calories or monitor food intake and during the fasting window, patients are limited to water and calorie-free beverages. Evidence shows that when adults with obesity limit their eating window to four to 10 hours, they naturally reduce caloric intake by approximately 200 to 550 calories per day and lose weight over two to 12 months. Whether TRE induces weight loss independent of reductions in calorie intake, as seen in rodent studies, is unknown.
At the beginning of the study, the researchers assessed participants' history and activity level to estimate baseline caloric needs. The participants received prepared meals with identical macronutrient and micronutrient compositions based on their individual calorie estimates and instructions on when to consume the meals. They ate the same number of calories daily throughout the study. The time restricted eating group was instructed to eat only between the hours of 8 a.m. to 6 p.m. and consumed most of their calories before 1 p.m. each day. The usual eating pattern group ate between 8 a.m. and midnight and consumed most of their calories after 5 p.m. each day. After 12 weeks, both groups lost about the same amount of weight and there were no real differences in fasting glucose, waist circumference, blood pressure, or lipid levels. According to the authors, these findings suggest that if or when TRE interventions induce weight loss, it is likely in part due to a reduction in calories, and, therefore, clinicians can counsel patients that TRE may help them lose weight by decreasing their caloric intake.
According to the authors of an accompanying editorial from the University of Illinois Chicago, these results have important clinical implications. While TRE was not found to be more effective for weight loss, it may be easier for patients to follow because it allows them to continue consuming familiar foods. This simplified approach to treating obesity could help patients who don't do well counting calories.
Posted in Health
Tagged nutrition , weight loss , diet
You might also like
News network.
- Johns Hopkins Magazine
- Get Email Updates
- Submit an Announcement
- Submit an Event
- Privacy Statement
- Accessibility
Discover JHU
- About the University
- Schools & Divisions
- Academic Programs
- Plan a Visit
- my.JohnsHopkins.edu
- © 2024 Johns Hopkins University . All rights reserved.
- University Communications
- 3910 Keswick Rd., Suite N2600, Baltimore, MD
- X Facebook LinkedIn YouTube Instagram
- Election 2024
- Entertainment
- Newsletters
- Photography
- Personal Finance
- AP Investigations
- AP Buyline Personal Finance
- AP Buyline Shopping
- Press Releases
- Israel-Hamas War
- Russia-Ukraine War
- Global elections
- Asia Pacific
- Latin America
- Middle East
- Election Results
- Delegate Tracker
- AP & Elections
- Auto Racing
- 2024 Paris Olympic Games
- Movie reviews
- Book reviews
- Personal finance
- Financial Markets
- Business Highlights
- Financial wellness
- Artificial Intelligence
- Social Media
What do weight loss drugs mean for a diet industry built on eating less and exercising more?
Atkins weight loss products are seen on sale at a Kroger supermarket, Friday, April 12, 2024, in Marietta, Ga. (AP Photo/Mike Stewart)
NormaTec athletic recovery systems line the MIORA clinic at the Life Time at Target Center, Wednesday, April 3, 2024, in Minneapolis. Luxury athletic club operator Life Time launched a program that offers comprehensive medical testing, personalized training and a host of alternative therapies like cryotherapy. The Miora program also offers Ozempic and other weight loss drugs through the clinic that opened in Minneapolis last year. (AP Photo/Abbie Parr)
A TheraLight red light therapy bed is pictured at the MIORA clinic at the Life Time at Target Center, Wednesday, April 3, 2024, in Minneapolis. Luxury athletic club operator Life Time launched a program that offers comprehensive medical testing, personalized training and a host of alternative therapies like cryotherapy. The Miora program also offers Ozempic and other weight loss drugs through the clinic that opened in Minneapolis last year. (AP Photo/Abbie Parr)
The TheraLight red light therapy bed is pictured at the MIORA clinic at the Life Time at Target Center, Wednesday, April 3, 2024, in Minneapolis. Luxury athletic club operator Life Time launched a program that offers comprehensive medical testing, personalized training and a host of alternative therapies like cryotherapy. The Miora program also offers Ozempic and other weight loss drugs through the clinic that opened in Minneapolis last year. (AP Photo/Abbie Parr)
Slim Fast weight loss products are seen at a Kroger supermarket, Friday, April 12, 2024, in Marietta, Ga. Sales of SlimFast sold at supermarkets have dropped as people turn to weight loss drugs and retailers cut shelf space for diet products, the brand’s parent company, Glanbia, told investors in February. (AP Photo/Mike Stewart)
Life Time registered nurse and injections specialist Kylie Simko is pictured with the DEXA (Dual-Energy X-ray Absorptiometry) scan at the MIORA clinic at the Life Time at Target Center, Wednesday, April 3, 2024, in Minneapolis. Luxury athletic club operator Life Time launched a program that offers comprehensive medical testing, personalized training and a host of alternative therapies like cryotherapy. The Miora program also offers Ozempic and other weight loss drugs through the clinic that opened in Minneapolis last year. (AP Photo/Abbie Parr)
Actress and singer Lisa Donahey poses for a photo in her backyard in the Sherman Oaks section of Los Angeles on Tuesday, April 16, 2024. Donahey, 54, started Mounjaro under a doctor’s care a year ago to address her Type 2 diabetes. Her weight has since dropped to a little less than 190 pounds. Having used the medication to give her “a kick-start,” Donahey said she plans to wean herself off Mounjaro once she loses another 40 pounds. (AP Photo/Richard Vogel)
Actress and singer Lisa Donahey applies her make-up at her home in the Sherman Oaks section of Los Angeles on Tuesday, April 16, 2024. Her weight has since dropped to a little less than 190 pounds. Having used the medication to give her “a kick-start,” Donahey said she plans to wean herself off Mounjaro once she loses another 40 pounds. (AP Photo/Richard Vogel)
Actress and singer Lisa Donahey displays a shirt she used to wear before her dramatic weightloss at her home in the Sherman Oaks section of Los Angeles on Tuesday, April 16, 2024. Her weight has since dropped to a little less than 190 pounds. Having used the medication to give her “a kick-start,” Donahey said she plans to wean herself off Mounjaro once she loses another 40 pounds. (AP Photo/Richard Vogel)
- Copy Link copied

NEW YORK (AP) — Ever since college, Brad Jobling struggled with his weight, fluctuating between a low of 155 pounds when he was in his 30s to as high as 220. He spent a decade tracking calories on WeightWatchers, but the pounds he dropped always crept back onto his 5-foot-5-inch frame.
A little over a year ago, the 58-year-old Manhattan resident went on a new weight loss drug called Wegovy . He’s lost 30 pounds, and has started eating healthier food and exercising — the habits behind many commercial diet plans and decades of conventional wisdom on sustainable weight loss.
Yet Jobling’s experience also has altered his perspective on dieting. He now sees obesity as a disease that requires medical intervention, not just behavioral changes. In fact, he thinks he will need to stay on a drug like Wegovy for the rest of his life even though it has taken some of the joy out of eating.
“I don’t see how you can maintain (the weight) without medication,” Jobling said. “Obviously, it’s all about self-control. But I think it’s less of a struggle to really maintain healthy eating when you got that assistance.”
Like the lives of the people taking them, recent injected drugs like Wegovy and its predecessor, the diabetes medication Ozempic, are reshaping the U.S. health and fitness industries. They have proven successful in eliminating unwanted pounds more quickly and easily than consuming fewer and burning more calories alone. Such is their disruptive power that even established diet companies like WeightWatchers and brands like Lean Cuisine are getting makeovers.
Although celebrities like Oprah Winfrey have spoken publicly of the drugs as revolutionary, some health experts worry that businesses without any expertise will start dispensing the prescription medications along with bad advice and unproven therapies.
A DEMAND TOO BIG TO IGNORE
At least 3 million prescriptions for the class of medications known as GLP-1 agonists were issued each month in the U.S. during the 12 months that ended in March, according to data from health technology company IQVIA. They include semaglutide, the drug in Ozempic and Wegovy, and tirzepatide, the drug in Mounjaro and Zepbound. Morgan Stanley research analysts have estimated that 24 million people, or 7% of the U.S. population, will be using GLPT-1 drugs by 2035.
The world’s leading diet programs have taken note of such statistics and incorporated the popular drugs into their existing subscription plans.
WeightWatchers, which was founded in 1963, last year acquired telehealth provider Sequence , enabling members to get prescriptions for weight loss drugs. WeightWatchers is sticking with its focus on behavior change as the cornerstone of weight reduction but launched virtual clinics that provide customized exercise and nutrition plans, as well as prescription care, for individuals who want to lose 20% of their body weight on average.
“The weight loss space will be led by the acknowledgement that weight loss is a matter of health care,” WeightWatchers CEO Sima Sistani told analysts earlier this year. “This is a paradigm shift because weight loss has been and, unfortunately, often still is viewed as a vanity issue.”
The Mayo Clinic, which first offered a weight management plan in book form in 1949, has published an updated version of the longtime bestseller, titled “The Mayo Clinic Diet: Weight-Loss Medications Edition.”
The Mayo Clinic Diet program also has expanded to include access to weight loss drugs and advice on managing any side effects, according to Digital Wellness CEO Scott Penn, whose company developed an online platform for the original program.
The new drugs have made being very overweight “feel more medical as a condition,” he said.
GYMS AND DIET FOOD COMPANIES LOOK TO MUSCLE IN
Luxury athletic club operator Life Time launched a membership program last year that offers comprehensive medical testing, personalized training and a host of alternative therapies like cryotherapy. Members of the Miora program also can get Ozempic and other weight loss drugs through the medical staff of a clinic that opened in Minneapolis last year.
Jeff Zwiefel, executive director of Life Time Miora, called the new drugs a “game changer” for the fitness industry.
“We have an opportunity and an obligation and a responsibility to help people achieve results in conjunction with medical providers and make sure that that’s the way to go, " he said.
Fitness chains are banking on the idea people on the drugs will lose enough weight to overcome any self-consciousness or physical limits that kept them from exercising. The gym franchise Equinox started a new personal training program in January for prescription-holders who want to preserve or build muscle mass as they shed unwanted pounds.
The world of drug-assisted weight loss also is altering the ambitions of food companies. Sales of SlimFast, a line of meal replacement shakes and snacks sold at supermarkets, have dropped as people turn to weight loss drugs and retailers cut shelf space for diet products, the brand’s parent company, Glanbia, told investors in February.
Since the drugs suppress the appetites of people taking them, Glanbia and other companies are marketing their products as a source of adequate nutrients for people taking GLP-1s. Swiss multinational Nestle SA thinks it can benefit from the drugs’ popularity and is expanding its Lean Cuisine frozen meals and OPTIFAST protein shakes.
“Diets are cool again,” Nestle SA CEO Ulf Mark Schneider told analysts in February. “It’s something that people used to do quietly on the side, uncertain about their outcomes.”
PROMISING RESULTS AND A WEALTH OF UNKNOWNS
Research has shown that about a third of people lose 5% or more of their body weight with diet and exercise alone, according to Dr. Louis Aronne, director of the Comprehensive Weight Control Center at Weill Cornell Medical school. In comparison, the medicine in the diabetes drug Mounjaro helped people with obesity or who are overweight lose at least a quarter of their weight when combined with restricted calories and exercise, a new study showed .
But some experts worry about businesses marketing the drugs or serving as fitness coaches for patients on the medications. Dr. Cian Wade, a health care consultant for the global strategy and management firm Kearney, said he’s concerned about a proliferation of clinics that don’t have as much experience with obesity and related health conditions.
“There’s a potential worry that for some patients, (the clinics) will not have the right expertise at hand to be able to appropriately manage the side effects, nutrition-related issues,” he said.
Since GLP-1 medications are so new, it’s unclear how many patients will stick with their drug regimens, which produce intolerable side effects for some people. Another reason patients may drop the drugs is cost . A month’s supply of Wegovy costs $1,300, and Zepbound is priced at $1,000.
‘THE NEW VERSION OF ME’
Lisa Donahey, 54, an actress and singer who lives in Los Angeles, started Mounjaro under a doctor’s care a year ago to address her Type 2 diabetes. At the time, Donahey, who is 5-foot-7-inches tall, weighed 260 pounds and was a veteran of diet plans like Jenny Craig, WeightWatchers and Nutrisystem.
Her weight has since dropped to a little less than 190 pounds. She goes to a gym. After always being cast as a character actor, she’s looking for new roles. Having used the medication to give her “a kick-start,” Donahey said she plans to wean herself off Mounjaro once she loses another 40 pounds.
“I had a sense of hopelessness that I was destined to be this way and just could not do it by myself,” she said. “Now, with my weight being managed and the new version of ‘me’ is emerging, I just feel so empowered, excited and hopeful.”
Read more of the AP’s Be Well content: https://apnews.com/hub/be-well

How To Train Your Brain To Truly Enjoy Exercise, According To Science
Yes, you can mold your mind to extract more pleasure from activity.

Truth: They woke up like this. Some people really are more inclined to find joy in exercise. But! You can rewire your brain to join that “love it” group, research shows.
The good news is that you can teach yourself to be more accepting, physically and mentally, of movement—which will help you feel excited about exercise in general and crave it more often. By trying some (or all!) of these tactics, you’ll likely notice benefits immediately, says study lead Marcelo Bigliassi, PhD. To extend the effect, keep efforts ongoing, so subtle changes compound over time. Onward!
Meet the experts: Marcelo Bigliassi, PhD , is an assistant professor of neuroscience and psychophysiology at Florida International University. Diogo Teixeira, PhD , is a professor on the physical education and sport faculty at Lusófona University in Lisbon, Portugal.
1. Add appeal to the flavor of exercise you already like.
Let’s say you don’t mind weight lifting but definitely don’t have the can’t-wait feels leading up to a workout. You can create artificial motivation and enjoyment by listening to music or a podcast while you sweat, using virtual reality, or even just engaging in positive self-talk, Bigliassi says. Or perhaps lifting with a group or a friend is the missing ingredient for you. “You’re creating outside signals that can help you push a little bit harder and a little bit faster.” The goal is to foster positive experiences with your sweat sessions. Gradually, the emotion will become second nature without these external cues.
.css-1cugboc{margin:0rem;font-size:2.125rem;line-height:1.2;font-family:Domaine,Domaine-roboto,Domaine-local,Georgia,Times,Serif;color:#f7623b;font-weight:bold;}.css-1cugboc em,.css-1cugboc i{font-style:italic;font-family:inherit;}.css-1cugboc b,.css-1cugboc strong{font-family:inherit;font-weight:bold;} “You’re creating outside signals that can help you push a little bit harder and a little bit faster.”
Not sure where to start with finding your best-match activity? Think back to your recent past, and even to your childhood, says Bigliassi. “There are usually clues.” For example, if you used to love swimming at your neighborhood pool, maybe that could translate to swimming laps at your local gym. Or perhaps you were a dancer at one point in your life. Taking a virtual or IRL dance fitness class could spark passion.
2. Challenge yourself *just* enough.
No matter what you’re doing, the activity needs to be tough enough that you’ll have a feeling of accomplishment that makes you want to repeat it. But it should also be within your capabilities, in order to protect your sense of self-efficacy (that is, your belief in your abilities), says Bigliassi. When people experience an exercise intensity that’s not aligned with their preference or tolerance, they exercise less in the future, research shows.
Take this thinking a step further: By choosing, say, a running pace you consider pleasurable (read: not all-out), you may find running more enjoyable—and more easily repeatable in the future. This “autonomy promotion” also applies to resistance training, says researcher Diogo Teixeira, PhD. So if resting longer between sets makes you feel better, do it. (It’ll create those positive associations in your brain.) “More is not always better, and a pleasurable activity will be more easily sustained over time,” Teixeira says.
Monitoring with a tracker can also allow you to see the work you’re putting in, which improves mindset around fitness and, therefore, happiness pertaining to exercise, found a study in the Journal of Medical Internet Research .
3. Send your mind a motivational sign.
Humans are wired to save as much energy and store as much fat as possible. So, sometimes—and especially when exercise gets intense—you need to remind yourself why you’re going through this perceived insanity. “It’s difficult for some parts of our brain to make sense of exercise,” Bigliassi says.
For example, recalling that cardio is important for both heart health and cognitive function can act as a motivational signal. That helps you feel more positive in the moment and be more consistent with exercise down the road; you now associate the activity with purpose and appealing health outcomes. Surprisingly, negative thoughts can also act as positive signals (e.g., envisioning your energy and mood tanking from not moving that day can be incredibly powerful). Consider this your sign to go for a walk or gear up for a workout right about…now.

WH Exclusives

Is Carb Cycling Helpful For Weight Loss?

‘My Itchy Rash Turned Out To Be Plaque Psoriasis’

Try This 30-Day Abs Challenge

What Actually Breaks A Fast? Dietitians Weigh In

You’ll Be Dripping After This Shadowboxing Workout

Here’s Exactly What To Eat On The Slow-Carb Diet

‘What I Learned At A Naked Dinner Party’

Your 4-Week Summer Shape-Up Plan

‘How I Lost 41 Pounds With WeightWatchers’

30 Healthy Snacks That Can Help You Lose Weight

Here’s What To Eat On The Mediterranean Diet Plan

IMAGES
COMMENTS
NIDDK has supported many research projects to learn more about overweight and obesity. The Look AHEAD: Action for Health in Diabetes study showed that people who had type 2 diabetes and were overweight or had obesity can lose weight and maintain that weight loss through a program of healthy eating and increased physical activity. The study also ...
Weight loss of 10 to 15% (or more) is recommended in people with many complications of overweight and obesity (e.g., prediabetes, hypertension, and obstructive sleep apnea). 1,20,21,27 In the ...
Ketogenic diet. Consumption of carbohydrates as < 10% of daily calories or < 50 mg/day 41. May decrease appetite, but long-term safety is unknown. High-protein diet. Increase protein intake to 30% of total daily calories or 1-1.2 g/kg of ideal body weight 43. Useful in maintaining weight loss and increasing satiety 47.
This research study is being performed to find out if a new device, AspireAssist Aspiration Therapy System, can help people with obesity to lose weight without causing too many side effects. ... The purpose of this study is to compare weight loss, improvement of comorbidities, improvement of lipid profile, blood sugar in patients undergoing ...
For perspective: the average placebo-adjusted weight reductions with older antiobesity medications that are currently approved by the FDA for the treatment of obesity are approximately 3.0 to 8.6% ...
Objective To examine the effectiveness of behavioural weight management interventions for adults with obesity delivered in primary care. Design Systematic review and meta-analysis of randomised controlled trials. Eligibility criteria for selection of studies Randomised controlled trials of behavioural weight management interventions for adults with a body mass index ≥25 delivered in primary ...
Animal models and human clinical trials have been employed to study changes in body composition and metabolic outcomes to determine the most effective diet. However, the studies present many limitations and should be carefully analyzed. The aim of this review was to discuss the scientific evidence of three categories of diets for weight loss.
Observational studies have suggested that the practice of eating meals later in the day was associated with weight gain and influenced the success of weight-loss therapy. 5,6 Several pilot ...
The mean weight loss of ~15% achieved with semaglutide 2.4 mg at week 104 in STEP 5 exceeds weight loss reported at similar time points in trials with other pharmacotherapies for weight management ...
Current treatments, including lifestyle, diet and exercise, produce a weight loss of 5% to 7% on average. Despite continued attempts to identify superior dietary approaches, most careful comparisons find that low carbohydrate diets are not significantly better than low fat diets for weight loss.
At Lilly, we want to help people living with excess weight access care that is based on research. The first step is to help the public to see obesity as a long-term disease rather than a lifestyle choice. By taking part in a clinical research study focused on chronic weight management, you can help to make a difference. Click here to find a trial.
Although the research is mixed, several studies show improved weight loss outcomes in patients receiving weight maintenance-specific training, compared with those who only receive traditional weight loss training 76-79. Strategies are discussed below for weight maintenance-specific counseling.
The research team mined clinical data from over 5,000 records. Ultimately, 228 files were reviewed and a subset of 20 women with obesity were identified to undergo a closely supervised exercise ...
The study found that the bacteria in the gut and the amounts of certain proteins the body produces can impact an individual's ability to sustain weight loss. Additionally, the research found that some individuals lose more weight on low-fat diets while others have better results on low-carb diets.
The research was conducted on MEDLINE, Cochrane Library, EMBASE, and Web of Science databases by using the following keywords: "name of investigated food supplement" and "obesity" or "weight loss". Studies meeting the following criteria were included: (1) case-control studies, cohort studies, observational prospective and ...
Methods and findings. This study included 93,110 participants (24 to 60 years old; 11.6% male) from the Nurses' Health Study (NHS), NHSII, and Health Professionals Follow-Up Study (HPFS) cohorts who were free of T2D, cardiovascular disease, and cancer at baseline (1988 for NHS/HPFS and 1989 for NHSII) for analyses of weight change and 104,180 (24 to 78 years old; 14.2% male) for T2D risk ...
Objective: This study aimed to identify the amount of weight loss needed in patients with obesity to improve metabolic syndrome (MetS), a risk factor for cardiovascular disease (CVD), over a long period of time. Methods: A total of 576 patients with obesity were enrolled in this study. Effects of continuous physician-supervised weight loss on the cumulative MetS components excluding abdominal ...
where ΔI(t) is the change in energy intake over time relative to the weight-maintenance baseline, P(t) is a parameter that shifts the system away from the baseline equilibrium, and k is a feedback gain parameter relating appetite to weight change, i.e., ΔW(t).In the absence of an intervention, P(t) = 0 and k = 95 kcal/day per kilogram corresponding to the baseline strength of the feedback ...
The extent of weight loss was similar to that achieved in many efficacy studies. 12-14 In contrast with the findings in most weight-loss trials, 19,20 however, participants sustained weight loss ...
A Comparative Weight Loss Study of the Atkins, Zone, Ornish, and USDA/LEARN Diets. Obesity is the single most significant nutrition-related health issue of the new millennium. Several "medical experts" have designed and promoted weight loss diets that dramatically differ from one another, and from the USDA Dietary Guidelines. These diets have ...
Participate in a Clinical Trial or Research Study. The WELL Center provides state-of-the art, evidence-based treatment for weight management, eating disorders and related conditions. ... large National Institutes of Health-funded clinical trials investigating new cognitive and behavioral treatments for weight loss. Most studies provide about 20 ...
The study will evaluate treatment effects on weight and shape concern and explore the impact of intervention on weight loss outcomes. Gender: Female. Ages: Between 25 years and 70 years. Trial Updated: 03/07/2024. Locations: Weight Control and Diabetes Research Center, Providence, Rhode Island.
KFF Headquarters: 185 Berry St., Suite 2000, San Francisco, CA 94107 | Phone 650-854-9400 Washington Offices and Barbara Jordan Conference Center: 1330 G Street, NW, Washington, DC 20005 | Phone ...
The average weight loss reported in the study was 7.5 kilograms, or 16 pounds, which would mean that at their lowest weights, they were feeling the need to eat 622 more calories a day more than ...
The Johns Hopkins researchers randomly assigned 41 adults with obesity and prediabetes to either time-restricted eating (TRE) with a 10-hour eating window or a regular eating pattern for 12 weeks to compare weight loss and other measures of metabolic health, according to a study summary provided by Annals of Internal Medicine.Their randomized, controlled trial found that both TRE and a more ...
The world of drug-assisted weight loss also is altering the ambitions of food companies. Sales of SlimFast, a line of meal replacement shakes and snacks sold at supermarkets, have dropped as people turn to weight loss drugs and retailers cut shelf space for diet products, the brand's parent company, Glanbia, told investors in February.
A ccording to new research, weight loss by intermittent fasting can drastically change brain activity related to addiction and appetite. According to South West News Service, intermittent energy ...
A new study sheds light on why some people are more tolerant of physical activity. ... found a study in the Journal of Medical Internet Research. ... 30 Healthy Snacks That Can Help You Lose Weight .
Karen discusses recent research on the benefits of apple cider vinegar for weight loss. The study found that consuming up to one tablespoon of apple cider vinegar daily for 12 weeks led to an average weight loss of 15 pounds. Listen on for more! Visit our website itchyandbitchy.com to read blog posts on the many topics we have covered on the show.