- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español

You are here
Research for the people.
The NIH invests most of its nearly $48 billion budget 1 in medical research for the American people.
Nearly 83 percent 2 of NIH’s funding is awarded for extramural research , largely through almost 50,000 competitive grants to more than 300,000 researchers at more than 2,500 universities, medical schools, and other research institutions in every state.
In addition, approximately 11 percent 2 of the NIH's budget supports projects conducted by nearly 6,000 scientists in its own laboratories , most of which are on the NIH campus in Bethesda, Maryland . The remaining 6 percent 2 covers research support, administrative, and facility construction, maintenance, or operational costs.
Justifications, Testimonies, and Appropriations
- Budget Requests — congressional justifications
- Congressional Testimony — budget requests & testimony by the NIH Director
- History of NIH Appropriations — from 1938
Funding for Diseases, Conditions, Research Areas
- Funding levels for diseases , conditions, and research areas, based on actual grants, contracts, research conducted at NIH, and other mechanisms of support.
Grants Awarded
- Funded Organization — universities and research organizations around the nation receiving research grants and contracts
- Budget and Spending — funding for grants and contracts
- Success Rates — annual percentage of research grant applications that are funded
- RePORTER — a searchable database of NIH-funded research projects
1 Based on historical distribution of actual FY 2022 obligations across extramural and intramural mechanisms that comprise the annual NIH budget.
2 Reflects the sum of enacted discretionary budget authority of slightly over $46.1 billion received under the Consolidated Appropriations Act, 2023 (P.L. 117-328). The budget total of $47.7 billion also includes $1.412 billion derived from PHS Evaluation financing, $141.5 million mandatory funding for the Special type 1 diabetes account, and $1.085 billion received from 21 st Century Cures Act allocations. Appropriations received by the recently established Advanced Research Projects Agency for Health (ARPA-H) are excluded as is unobligated carryover related to emergency pandemic supplemental appropriations enacted prior to FY 2022 and resources from the HHS Nonrecurring Expenses Fund (NEF).
This page last reviewed on October 24, 2023
Connect with Us
- More Social Media from NIH
- Economics & Finance
- Foreign Policy
- Domestic Policy
- Health & Science
- All Publications
U.S. Federal Scientific Research and Development: Budget Overview and Outlook

Table of Contents
Kenneth m. evans, kirstin r.w. matthews, gabriella hazan, spoorthi kamepalli, share this publication.
- Download PDF
- Print This Publication
Evans, Kenneth M., Gabriella Hazan, Spoorthi Kamepalli, and Kirstin R.W. Matthews. 2021. U.S. Federal Scientific Research and Development: Budget Overview and Outlook . Baker Institute Report no. 07.23.21. Rice University’s Baker Institute for Public Policy, Houston, Texas.
The Biden administration has made science and technology (S&T) a centerpiece of its early policy agenda, which includes ambitious targets for federal investments in research and development (R&D). In parallel, growing concerns among some members in Congress about U.S. global leadership in S&T-focused industries, especially in relation to China, have inspired a number of recent legislative efforts to strengthen the national innovation ecosystem. While President Biden’s first budget proposal works to build on this momentum in an effort to authorize historic increases to federal R&D agencies, challenges remain to ensure long-term, international competitiveness across scientific disciplines and advanced technologies. In this paper, we describe the U.S. federal budget process for R&D, discuss trends in federal R&D funding, and provide an outlook for the future of federal R&D expenditures during the Biden administration.
The Role and Value of Federal S&T R&D
Scientific R&D is essential to creating new knowledge and tools, which in turn spur the development of new products and technologies that drive the domestic and global economies. 1 Although difficult to quantify, economists cite “strongly positive” private returns on investments in R&D and estimate that innovations stemming from S&T account for more than 60% of economic growth over the last century. 2
U.S. R&D involves a complex system of actors: individuals and institutions that perform R&D, federal and state agencies that regulate R&D, and funders, both public and private, who support R&D activities. In fiscal year (FY) 2019, the U.S. spent more than $656 billion on R&D, equivalent to 3.06% of the U.S. gross domestic product (GDP). 3 This money sponsors hundreds of thousands of S&T research projects across R&D sectors—colleges and universities, research hospitals, private industry, national laboratories, and other federally funded research and development centers (FFRDCs) (e.g., the RAND Corporation), as well as other independent organizations who participate in or manage research activities.
More than two-thirds of U.S. R&D is funded by private corporations, approximately 20% is funded by the federal government, and the remaining share is sponsored by nonprofit organizations, state governments, and universities. The nature of R&D varies between federally funded and industry-sponsored R&D. Industry focuses primarily on the development of new products (Figure 1). It accounts for 85% of the U.S. development total. In contrast, federal spending favors research—both “basic” research, which is conducted without a targeted outcome, and “applied” research, which seeks to advance knowledge toward a predetermined goal. 4
Figure 1 — U.S. R&D by Sector and Character, FY 2019

Traditionally, federal funding for R&D receives bipartisan support in Congress, particularly for health- and defense-related research activities. However, since the mid-1990s, government spending on basic research has declined or stagnated as a share of the U.S. GDP, in part due to the intrinsic uncertainties about the ultimate impacts of basic research. In addition, certain areas of S&T R&D have become increasingly politicized, including climate research and research using human embryos. Moreover, academic scientists historically do not participate in advocacy for increases to federal R&D funding. Relatively little value has been placed on evaluating and communicating the broader societal impacts of basic research to the public and especially to policymakers. 5 As the high technology sector (e.g., advanced computing and communications, social media platforms, and other web-based services) becomes an increasingly large part of the overall U.S. economy, federal funding for early stage R&D, which has been at the root of much of the technological progress of this past century, is more important than ever. 6 The emergence of the COVID-19 pandemic in 2020, its spread in the United States, and the record-setting development of effective vaccines reinforces the need for a robust R&D infrastructure. This infrastructure requires sustained investment in basic research to increase our fundamental understanding of infectious diseases, as well as the ability to respond to and manage future global crises. 7
The U.S. Federal Budget Process for S&T R&D
The total U.S. annual federal budget is more than $4.8 trillion. There are three major funding categories in the budget: 1) the interest on the national debt ($378 billion in FY 2021, 8% of the total budget); 2) nondiscretionary or mandatory spending, which includes Social Security, Medicare, and Medicaid ($2.97 trillion in FY 2021, 62% of the total budget); and 3) discretionary spending. Mandatory spending and interest on the debt are non-negotiable expenses. Discretionary spending encompasses all other government spending, from transportation to military operations ($1.49 trillion in FY 2021, 31% of the total budget), including the entire federal R&D budget. 8 As a result, discretionary spending—less than a third of total federal expenditures— is the only part of the budget that is publicly debated each year between the two houses of Congress and the president.
S&T is just one of many policy areas vying for public funding. Federal funding for S&T is also complicated not just by the political nature of the U.S. budget process, but by the highly decentralized organization of federal R&D activities—over a dozen federal agencies have an annual R&D budget of more than $1 billion. In Congress, these activities are managed and funded across numerous congressional oversight and appropriations committees without a central mechanism to coordinate related federal R&D programs. This pluralism results in a complex, often contentious, multi-year process characterized by a series of lengthy negotiations between Congress, the White House, and R&D granting and regulatory agencies and cabinet departments, all of which are responding to conflicting expectations and demands (Figure 2). Shifting priorities between presidential administrations, changes to the makeup and ideologies of Congress, and broader economic conditions in the United States at large have resulted in the inconsistent funding for R&D, especially for basic research, despite strong and consistent support from the American public. 9
Figure 2 — U.S. Federal Budget Process

The annual budget funds government operations for the U.S. fiscal year, which begins October 1 and ends September 30 the following year (i.e., the FY 2022 budget will start October 1, 2021 and end September 30, 2022). However, the entire budget process is a multi-year endeavor that starts two years prior to funds being released. The process has four phases (Figure 2):
- Budget Plan: Initial internal planning within cabinet departments and agencies
- White House Review: White House Office of Management and Budget (OMB) review of each department and agency’s budget and subsequent negotiations between agencies and OMB to finalize the president’s budget request to Congress
- Congressional Appropriation: Submission of the president’s budget to Congress, followed by negotiations in Congress and between Congress and the president, resulting in the passage of the full public budget into federal law
- Budget Implementation: Appropriated funds are distributed
The first phase of the budget process consists of internal, nonpublic planning of budget proposals within the cabinet departments and independent agencies beginning up to two years prior to the start of the fiscal year. In parallel, the president develops government-wide priorities for federal departments and agencies. For agencies involved with R&D funding and regulation, the president’s S&T priorities are detailed in a joint memorandum co-signed by the directors of OMB and the Office of Science and Technology Policy (OSTP), an agency within the White House that works to coordinate the expansive federal R&D system, including budgets and interagency R&D programs and activities. 10 Agencies and departments are expected to take into consideration the president’s priorities, including the OMB-OSTP budget memo, when planning their budgets for their activities for a given fiscal year.
During the second phase, agencies send their preliminary budgets to OMB, initiating a series of negotiations between OMB and each agency that are often called “passbacks.” The result of these negotiations becomes the president’s budget request to Congress, typically submitted in mid-February. While the Budget and Accounting Act of 1921 requires a submission to Congress by the first Monday in February, the budget can be delayed until later in the spring, which is often the case during presidential transitions.
Figure 3 — Federal R&D as a Percent of Discretionary Spending, 1962–2020

In the third phase, Congress reviews the presidential budget proposal and determines the final budget. The House and Senate appropriations committees divide the total discretionary budget across 12 appropriations subcommittees in their respective chambers. Each subcommittee prepares a funding bill covering the agencies under its jurisdiction. 11 In early April, the House and Senate typically agree on a “budget resolution,” which provides nonbinding guidance to the appropriations subcommittees on national budget priorities. From FY 2011 to FY 2021, the budget resolution also addressed top-line funding levels for defense and nondefense discretionary budgets, or “caps,” as set and later amended by the Budget Control Act of 2011 (S. 365) in a response to the 2011 debt-ceiling crisis. However, over the past decade Congress has raised the caps on several occasions through new legislation to increase the available pool of funds for discretionary programs and avoid budget cuts across the government.
The size of these caps are incredibly important to the future of federal R&D, as the overall federal R&D budget has been proportional to total discretionary spending since the late 1970s. Over the past 40 years, federal R&D has accounted for approximately 12% of the total discretionary budget irrespective of broader national policy priorities, the makeup of Congress or the president’s political party, the state of the national economy, wars, crises, or any other historical context (Figure 3). 12
Defense spending has always been the largest portion of the R&D budget (Figure 4). However, the balance of funding between nondefense S&T disciplines (space, engineering, physical sciences, health and medicine, etc.) has shifted considerably with time. For example, in the 1960s a large portion of R&D funding was allocated to the Apollo program and space-related R&D. In contrast, since the early 2000s, almost half of nondefense spending has been focused on health and biomedical research.
The United States, in contrast to many other countries, has no central mechanism to coordinate annual R&D budgets across the federal government or assess the nation’s overall progress in S&T. OSTP is the only federal agency that works to coordinate the nation’s overall S&T enterprise and national R&D programs. 13 However, OSTP’s role in the funding process is, by statute, purely advisory, and it does not have funding authority for federal R&D activities. Large interagency R&D programs (e.g., the National Nanotechnology Initiative) are coordinated by the National Science and Technology Council (NSTC), a cabinet-level committee managed by OSTP and chaired by the president that works to harmonize policy, including budgets, across the many agencies involved in S&T. However, funding for individual agency R&D budget requests are set by the agencies according to their mission priorities and subsequent negotiations in Congress.
Figure 4 — Federal R&D Funding by Function, 1955–2020 (in Billions of Constant FY 2020 USD)

This disaggregated system of appropriations means that most R&D agencies do not compete directly with one another for funds, but rather with nonscience programs, many of which are popular with the public and special interest groups. Out of the 12 appropriations bills, budgets for R&D granting or regulatory agencies are dispersed across nine separate appropriation subcommittees. For example, the National Science Foundation (NSF) and National Aeronautics and Space Administration (NASA) are in the same appropriations bill as the Departments of Commerce and Justice. The Department of Energy (DOE) is appropriated through a bill that includes energy and water development projects. The National Institutes of Health (NIH), which resides in the Department of Health and Human Services (DHHS), is included on a bill with the Departments of Education and Labor. These congressional subcommittees wield considerable power over the operations of the agencies. If, at any time during budget negotiations, an agency wishes to deviate from the original budget, even to move relatively small amounts of money from one activity to another, it must obtain approvals from OMB and the relevant subcommittees in both the House and Senate before proceeding.
The Senate and House must agree on the final funding bills and send them to the president to be signed into law before the start of the following fiscal year on October 1. Unless an agency “has an appropriation” (i.e., the bill including its funding has been signed into law), by the end of a fiscal year, it cannot spend money and must cease operations, except for a small number of specified essential services. For example, during the FY 2019 budget cycle, Congress and the president failed to pass an appropriation bill for the majority of agencies and departments, which resulted in a government shutdown for 35 days from December 22, 2018 to January 25, 2019.
Since the late 1970s, only on a few occasions has the complete federal budget been approved in time for the start of the fiscal year. Delays typically occur due to partisan discord within Congress or between Congress and the president, such as the fight between President Trump and the House Democrats in FY 2019 over border wall funding. If Congress is unable to pass all 12 appropriation bills by the deadline, it must enact one or several consecutive continuing resolutions that extend the deadline for negotiations into the start of the new fiscal year. In order to avoid a chain of continuing resolutions, Congress will often bundle the unresolved budget requests together as a single piece of legislation, known as an omnibus appropriations bill. Omnibus bills, which are becoming progressively more common, tend to contain a diverse set of unrelated legislative items. In FY 2020, for example, the federal budget was passed in December 2019 in two omnibus bills after a continuing resolution was signed by President Trump an hour before the October 1 deadline.
Delayed budget approvals can severely disrupt agency operations, with agencies forced to continue to work under the guidelines of their previous budgets with no way of knowing when their budget will be approved or what it will look like. Programs that a new fiscal budget would end or substantially alter still have funding for this period, while new projects cannot be started until the budget is approved. This system is particularly detrimental to R&D agencies, which need predictable budgets to ensure the continuity of data collection for long-term research programs, as well as adequate staffing of research laboratories with scientists at all stages of their careers. 14 Graduate students and postdocs in training are particularly sensitive to gaps in access to research facilities or abrupt changes to career trajectories due to loss of federal funding. 15 Additionally, the construction of large-scale research facilities, such as telescopes, satellites, and particle accelerators, can span a decade or more, and relies on consistent funding to make predictable progress to meet program goals.
After the president has signed each agency’s appropriation bill into law, its legislatively mandated funds, or “obligations,” are spent over the course of the following fiscal year—the fourth and final stage of the budget process. The agencies report expenditures back to the government to get the final totals for the budget spent, or “outlays,” which typically fall within several percent of an agency’s allotted obligations. By the time agencies are spending their annual appropriations, they are already well into negotiating the following fiscal year and setting priorities for the year after.
R&D Funding During the Trump Administration
President Trump campaigned on increased defense spending and decreased nondefense discretionary spending, including many areas of S&T R&D. 16 Each of President Trump’s four annual budget requests to Congress reflected these overall priorities, and called for cuts in spending across R&D funding and regulatory agencies (Figure 5). For example, in FY 2020, President Trump requested an 8% reduction to federal R&D programs from the appropriated FY 2018 total—from roughly $164 billion to $151 billion. 17 In addition, discretionary budget caps to limit deficit spending would have required large budget cuts for all discretionary programs, which if enacted would have had a devastating impact on federal R&D.
Fortunately, Congress largely ignored President Trump’s budget proposals, and, in parallel, raised the discretionary budget caps in favor of more generous funding for R&D. Both Democrats and Republicans in Congress criticized the Trump administration’s requests, taking issue with the president’s proposed cuts to R&D programs—especially to energy research and demonstration programs at the DOE, including his proposal to outright abolish the DOE Advanced Research Projects Agency-Energy (ARPA-E), an agency which has had bipartisan support since its founding in 2009. 18
President Trump’s final budget proposal, released on February 10, 2020 and passed on December 27, 2020, followed his previous requests. His plan once again called for sharp reductions to basic and applied research expenditures—8.4%, or $13.2 billion, from FY 2020 levels. However, Congress appropriated modest increases roughly in line with discretionary budget caps, including a 3% increase to NIH, 2.5% for NSF, and 0.4% for the DOE Office of Science. 19
Figure 5 — Change in R&D Budget Request an Appropriation from Previous Fiscal Year

In addition to these annual increases, federal R&D received short-term boosts in five of the six COVID-19 emergency relief bills passed over the past two fiscal years. The first such package, The Coronavirus Preparedness and Response Supplemental Appropriations Act (H.R. 6074), was passed on March 5, 2020. It allocated $8.3 billion to “prevent, prepare for, and respond to” the COVID-19 pandemic, with approximately $6.5 billion directed to agencies under the Department of Health and Human Services (HHS), including NIH, the Centers for Disease Control and Prevention (CDC), and the Biomedical Advance Research and Development Authority (BARDA). Passed on March 27, 2020, the $2.2 trillion stimulus package titled the Coronavirus Aid, Relief, and Economic Security (CARES) Act (H.R. 748) included $1.25 billion to support R&D. 20 The latest pandemic stimulus package, signed into law by President Joe Biden on March 11, 2021 and titled The American Rescue Plan of 2021 (H.R. 1319), added another significant one-time increase to select R&D agencies, including $600 million to NSF and $150 million to the National Institute of Standards and Technology (NIST). 21 Overall, BARDA and the CDC have each received more than $25 billion in stimulus funding so far, and NIH and NSF have received $5 billion and $676 million respectively (Figure 6). 22
The Future of Federal R&D Funding
On April 28, 2021, President Biden, in his first address to Congress, highlighted S&T as part of his broader policy approach toward maintaining U.S. economic and national security:
“We will see more technological change in the next 10 years than we saw in the last 50 years. And we’re falling behind in that competition. Decades ago we used to invest 2% of our GDP on research and development. Today, we spend less than 1%. China and other countries are closing in fast. We have to develop and dominate the products and technologies of the future: advanced batteries, biotechnology, computer chips, and clean energy.” 23
In line with these remarks, President Biden’s FY 2022 budget request to Congress, released at the end of May 2021, recommends historic increases across all civilian federal R&D granting agencies—an overall 9% percent increase to federal R&D, including a 10% increase to basic research and 14% increase to applied research (Figure 5). The proposal favors civilian defense, requesting a 21% increase to NIH and a 20% boost to NSF, while cutting basic research and applied research at DOD by 11% and 16%, respectively. 24 These totals align with the Biden administration’s budget outline released in April 2021, as well as the president’s infrastructure and jobs proposals announced earlier in spring 2021, which called for $250 billion for R&D activities and research infrastructure over the next four years. 25
Figure 6 — COVID-19 Emergency Funds to R&D Agencies

In parallel, congressional leaders in both chambers have introduced legislation addressing scientific and industrial competitiveness with China. In particular, two bills—Senate Majority Leader Chuck Schumer’s (D-NY) Endless Frontier Act 26 and The NSF for the Future Act (H.R. 2225), 27 sponsored by Representative Eddie Bernice Johnson (D-TX-30), who chairs the House Science Committee— offer two distinct visions for augmenting NSF’s research portfolio to meet current and future challenges to the U.S. research and innovation ecosystem. The Senate bill significantly increases NSF’s budget and creates a new technology directorate that would work to translate basic research discoveries to broader commercial use. The House bill offers more modest funding and would also work to improve R&D’s broader public impact, including the creation of a new directorate to address societal challenges. 28 A heavily amended version of the Endless Frontier Act was passed by the Senate on June 8, 2021, as part of a larger legislative package titled the U.S. Innovation and Competition Act (USICA, S.1260). This bill would appropriate $52 billion in emergency funding for domestic semiconductor manufacturing and R&D, authorize $29 billion to NSF’s new technology directorate over five years, and increase the rest of NSF’s annual budget from its current level of $8.5 billion to $12 billion over the same time period. The NSF for the Future Act, passed by the House on June 28, 2021, would authorize funding for its proposed directorate, starting at $1 billion annually and growing to $3.4 billion over five years, as well as roughly double the total NSF budget to $18.3 billion by FY 2026. Over the course of the next several months, the two chambers will work to reconcile these two bills into a final NSF reauthorization, in parallel with their annua appropriations negotiations before the October 1, 2021 deadline for FY 2022.
These ambitious proposals to vastly increase federal R&D spending face significant hurdles, including record federal deficit spending and an uncertain future for the discretionary budget, which could limit appropriations over the next several years even if a new authorization bill for NSF’s budget or other key R&D agencies is passed. If Congress limits discretionary spending through new caps, federal funding for R&D across departments and agencies will likely suffer, unless it is prioritized over other discretionary programs. However, Democratic control of both the White House and Congress could provide short-term increases to funding for prioritized S&T research areas through the budget reconciliation process, where only a simple majority vote on fiscal legislation is needed and is not subject to filibuster. 29 This maneuver was used to pass President Biden’s COVID-19 relief package in March and has been ruled by the Senate parliamentarian to be available for use once more during the coming budget cycle.
The Biden presidency arrives at a time when policy challenges reliant on S&T data and analysis have come into sharp public focus—from the COVID-19 pandemic, to record numbers of and intensity in U.S. wildfires and tropical storms in 2020, to mounting challenges relating to the tech sector’s role in the U.S.’ democracy, domestic economy, and foreign policy. While President Biden has made clear he will “listen to scientists,” not all members of Congress and the public are willing to heed to scientists’ voices on contentious policy issues, such as COVID-19 vaccine policy and climate change. Building public support for R&D, strengthening trust in scientific institutions and expertise, and increasing scientists’ participation in decision-making related to S&T issues are critical to ensuring that scientific discoveries and innovation benefit the broader public and that increased investment in R&D serves the public interest. Scientists driven to action during the past four years of shorton- science budget proposals—by the Trump administration and public statements from President Trump and other federal officials that sometimes fell outside of scientific consensus—should continue their public outreach efforts to let state and federal policymakers know that advancing U.S. S&T is vital to the lives of all Americans and deserves special attention and support.
Academic scientists in particular need to effectively communicate the value of their research to the public and to policymakers to ensure their work addresses broader societal needs. This outreach can be accomplished through public lectures, meetings, and other events with civic groups, churches, K-12 schools, professional societies, and meetings with state and federal legislators. Universities and other research institutions should encourage and incentivize these avenues for public engagement through increased support of existing programs or funding new activities for interested faculty, postdocs, graduate students, and research staff. Scientists also need to listen to the concerns of the public and help address misconceptions. These “civic scientists” and their outreach are vital to helping promote science as a public good worthy of federal support. By better communicating the progress and importance of their research, civic scientists can increase the transparency of the scientific process—from vaccine development to tech sector innovation to climate research and resilience—and serve as a force to promote ethical and equitable research and innovation policy as Congress deliberates on the future of research, innovation, and STEM education policy and funding during the first fiscal year of the Biden administration.
1. National Research Council, Measuring the Impacts of Federal Investments in Research: A Workshop Summary , (Washington, D.C.: The National Academies Press, 2011), https://www.nap.edu/ catalog/13208
2. Benjamin Jones and Lawrence Summers, “A Calculation of the Social Returns to Innovation,” National Bureau of Economic Research , September 2020, https://doi.org/10.3386/w27863; Bronwyn H. Hall, Jacques Mairesse, and Pierra Mohnen, “Measuring the Returns to R&D,” National Bureau of Economic Research , December 2009, https://doi.org/10.3386/ w15622; Peter L. Singer, Federally Supported Innovations: 22 Examples of Major Technology Advances that Stem from Federal Research Support (Washington, D.C.: Information Technology and Innovation Foundation, 2014), https://dc.mit.edu/sites/default/files/pdf/2014-federally-supportedinnovations.pdf.
3. Mark Boroush, “U.S. R&D Increased by $51 Billion, to $606 Billion, in 2018; Estimate for 2019 Indicates a Further Rise to $656 Billion,” National Science Foundation , April 13, 2021, https://ncses.nsf.gov/pubs/nsf21324.
4. OMB, Circular A-11, Sec. 84 (Washington, DC: Executive Office of the President, 2020).
5. Barry Bozeman, “Public Value Science,” Issues in Science and Technology XXXVI , no. 4 (2020), https://issues.org/public-value-science-innovation-equitybozeman/.
6. Paul Alivisatos et. al., eds, The Future Postponed 2.0: Why Declining Investment in Basic Research Threatens a U.S. Innovation Deficit (Washington, D.C.: MIT Washington Office, 2016), http://www.futurepostponed.org/.
7. American Academy of Arts and Sciences, The Perils of Complacency: America at a Tipping Point in Science and Engineering (Cambridge, MA: American Academy of Arts and Sciences, 2020). https://www.amacad.org/publication/perils-of-complacency.
8. OMB, “A Budget for America’s Future: Budget of the U.S. Government,” (Washington, D.C.: Government Printing Office, 2020). https://www.govinfo.gov/app/details/BUDGET-2021-BUD/.
9. Research!America, “America Speaks: Survey Data Reflecting the Views of Americans on Medical, Health, and Scientific Research,” America Speaks! 20 (Washington, D.C.: Research!America, 2021), https://www.researchamerica.org/sites/default/files/Publications/RA_PollDataSummary_Booklet_screenRes.pdf.
10. OMB and OSTP, “Fiscal Year (FY) 2022 Administration Research and Development Budget Priorities and Crosscutting Actions,” August 12, 2020, https://www.whitehouse.gov/wp-content/uploads/2020/08/M-20-29.pdf.
11. Matt Hourihan, “The Federal Budget Process 101,” American Association for the Advancement of Science , July 15, 2014, https://www.aaas.org/news/federalbudget- process-101.
12. Matt Hourihan and David Parkes, “Federal R&D Budget Trends: A Short Summary,” American Association for the Advancement of Science , January 2019, https://www.aaas.org/sites/default/files/2019-01/AAAS%20RD%20Primer%202019_2.pdf.
13. Neal F. Lane, Kenneth M. Evans, and Kirstin R.W. Matthews, “The Vital Role of the White House Office of Science and Technology Policy in the New Administration,” Rice University’s Baker Institute for Public Policy (Houston, TX), September 14, 2016, https:// www.bakerinstitute.org/media/files/files/2754ab9e/ST-pub-OSTPRecs-Report-091416.pdf.
14. Alan Blinder, “Toll on Science and Research Mounts as Government Shutdown Continues,” New York Times , January 5, 2019, https://www.nytimes.com/2019/01/05/us/governmentshutdown-science.html.
15. Jamie Ducharme, “Scientists Face Delays and Uncertainty as Government Shutdown Continues,” Time , January 3, 2019, https://time.com/549301/scientistsgovernment-shutdown/.
16. Committee for a Responsible Federal Budget, “Promises and Price Tags: An Update,” http://www.crfb.org/sites/default/files/Promises_and_Price_Tags_Preliminary_Update.pdf.
17. Matt Hourihan and David Parkes, “R&D in the FY 2020 White House Budget: An Overview,” AAAS, March 25, 2019, https://www.aaas.org/news/rd-fy-2020- white-house-budget-overview.
18. Matt Hourihan, “Update: In the Age of Trump, Congress Keeps Boosting Science Funding,” AAAS , December 17, 2019, https://www.aaas.org/news/update-age-trumpcongress-keeps-boosting-science-funding.
19. Committee for a Responsible Federal Budget, “Appropriations Watch: FY 2021,” May 21, 2021, http://www.crfb.org/blogs/appropriations-watch-fy-2021; “Massive 2021 U.S. spending bill leaves research advocates hoping for more,” Science Mag , December 22, 2020, https://www.sciencemag.org/news/2020/12/massive-2021-us-spending-bill-leaves-researchadvocates-hoping-more.
20. Andrea Widener, “US stimulus bill includes funding for coronavirus research,” Chemical & Engineering News, March 27, 2020, https://cen.acs.org/policy/researchfunding/US-stimulus-bill-includesfunding/98/web/2020/03.
21. David Malakoff, “What’s in the huge pandemic relief bill for science?,” Science Mag , March 10, 2021, https://doi.org/10.1126/science.abi4558.
22. AAAS , “FY 2021 R&D Appropriations Dashboard,” accessed March 17, 2021, https://www.aaas.org/page/fy-2021-rdappropriations-dashboard.
23. White House, “Remarks as Prepared for Delivery by President Biden — Address to a Joint Session of Congress,” April 8, 2021, https://www.whitehouse.gov/briefingroom/ speeches-remarks/2021/04/28/remarks-as-prepared-for-delivery-bypresident- biden-address-to-a-jointsession-of-congress/.
24. “Biden Seeks Big Increases for Science Budgets,” Science Mag , May 28, 2021, https://www.sciencemag.org/news/2021/05/biden-seeks-big-increasesscience-budgets.
25. Jeffrey Mervis, “Biden Proposes a Funding Surge—and New Agencies to Manage It,” Science 372, no. 6539 (April 16, 2021): 221–22. https://science.sciencemag. org/content/372/6539/221.
26. United States Innovation and Competition Act of 2021, S. 1260, 117th Congress (2021), https://www.congress.gov/bill/117th-congress/senate-bill/1260/text.
27. National Science Foundation for the Future Act, H.R. 2225, 117th Congress (2021), https://www.congress.gov/bill/117thcongress/house-bill/2225/text.
28. A Bipartisan Vision for the Future of American Science, Issues in Science and Technology , April 27, 2021, https://issues. org/bipartisan-vision-future-americanscience- policy-eddie-bernice-johnson/.
29. David Reich and Richard Kogan, “Introduction to Budget ‘Reconciliation,’” Center on Budget and Policy Priorities, January 21, 2021, https://www.cbpp.org/research/federal-budget/introduction-tobudget-reconciliation.
This material may be quoted or reproduced without prior permission, provided appropriate credit is given to the author and Rice University’s Baker Institute for Public Policy. The views expressed herein are those of the individual author(s), and do not necessarily represent the views of Rice University’s Baker Institute for Public Policy.
Related Research

One Easy Way to Increase Transparency in Human Embryo Research

What is Synthetic Biology?

Using Engineered Bacteria to Detect Viruses in Wastewater
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Health Serv Res
- v.53(Suppl Suppl 2); 2018 Oct
Show Me the Money! Trends in Funding for Health Services Research
Lisa a. simpson.
1 AcademyHealth, Washington, DC
Liz Koechlein
2 Food and Agriculture Organization, United Nations, Viale delle Terme di Caracalla, Rome, Italy
Nir Menachemi
3 Department of Health Policy and Management, Richard M. Fairbanks School of Public Health, Indiana University, Bloomington, IN
Meghan J. Wolfe
4 Department of Health Policy and Management, Milken Institute School of Public Health, The George Washington University, Washington, DC
Associated Data
This paper presents longitudinal data representing federal funding for health services research and discusses the observed trends in the larger context of overall funding for research and development in the United States. By putting into context public and private funding trends, the authors examine how these trends effect the supply and demand of the health services research workforce.
Discussion pertaining to the future of the health services research workforce must take into account the context of public and private financial support for work in the field. As noted by other authors in the current special issue, the demand for health services research is diversifying, while estimates of the current workforce point to continued growth. In the present brief report, we present longitudinal data representing federal funding for health services research and place the observed trends in the larger context of overall funding for research and development.
Trends in Federal Health Services Research Funding
Two data sources were used to summarize trends in federal support for health services research. Total funding for health services research is summarized by AcademyHealth annually using official federal agency budgets and the NIH RePORTER tool for NIH support. The RePORTER system is an electronic tool made available by the National Institutes of Health (NIH) that supports searches for NIH‐funded research projects (both intramural and extramural) from the past 25 years resulting from NIH funding. The reporter system also provides the annual support level for various research, condition, and disease categories based on grants, contracts, and other funding mechanisms used across the National Institutes of Health (NIH). This list contains the category of health services research ( NIH Reporter ). In addition, trends in the number and distribution of health services research projects funded are tracked by HSRProj, the most comprehensive repository for health services research projects in the United States with data from 360 public and private funding organizations. HSRProj is managed by AcademyHealth and the Cecil G. Sheps Center for Health Services Research at the University of North Carolina, Chapel Hill, on behalf of the US National Library of Medicine. HSRProj contains information on health services research projects spanning decades, including information on ongoing projects before results are available in a published format. The number of projects in HSRProj has increased over time, currently including information on 32,244 research projects, approximately half of which are ongoing or have been completed within the last 5 years. While discussing the methodologies used to categorize health services research projects is outside the scope of this article, we recognize that these data sources may be subject to classification bias in which some health services research‐funded projects are omitted and/or some nonhealth services research projects are erroneously included.
Table 1 displays funding for health services research from US federal agencies during the FY 2010–FY 2017 time frame. In FY 2017, 1 the National Institutes of Health (NIH) reported spending nearly $1.8 billion on health services research, accounting for 59.5 percent of all federal health services research funding; however, this represents only 5.3 percent of the total budget ($33.1 billion) for NIH in that year. The Affordable Care Act (ACA) established the Patient‐Centered Outcomes Research (PCOR) Trust Fund with a new tax on health plans based on a formula using the number of covered lives and transfers from Treasury. This new fund has provided nearly $1.9 billion since 2010 in support of PCOR, a subset of health services research. However, this new funding source has also been used to supplant base funding for the Agency for HealthCare Research and Quality (AHRQ) since 2013. This budget strategy was a direct result of the intense pressure on discretionary funding created by the Budget Control Act of 2011. However, if the Trust Fund is not reauthorized in 2019, not only would funding for the Patient‐Centered Outcomes Research Institute (PCORI) disappear, AHRQ would lose nearly $100 million, or 22 percent of its budget. Another way to examine trends in AHRQ funding is to examine its budget levels in inflation‐adjusted dollars. In doing so, it reveals that the amount requested by the administration for FY 2019 is $120 million below FY 2010 levels when adjusting for inflation. A final notable comparison is total federal support for health services research compared to national health care expenditures. In FY 2016, the $2.9 billion spent on health services research was less than one‐tenth of one percent of the $3.3 trillion spent on health care overall; and less than a quarter of one percent of the $1.24 trillion spent on Medicare and Medicaid (Centers for Medicare and Medicaid Services 2018 ).
Federal Funding for Health Services Research 2010–2017
Source: Official federal agency budgets.
Turning from the total funding estimates for health services research using agency reported publicly available data, HSRProj enables comparison of the number of projects (as opposed to their dollar amount) by funding agency (HSRProj). Looking across all public and private sector sources of health services research support between 2005 and 2016, NIH has consistently funded the largest number of projects (between 43 percent and 56 percent). In 2016, AHRQ funded the second highest number of projects (245) followed by PCORI and the Department of Veterans Affairs (VA) (Table 2 ). Looking only at the top eight funders listed in Table 2 , there has been a 20 percent decline in the number of projects over the 11‐year period. This may reflect a real overall decrease in support or a trend toward a smaller number of larger funded projects. There was a noticeable increase in the number of projects at NIH and the VA in 2009 after the infusion of $1.1 billion to support comparative effectiveness research (CER), but this increase was not sustained. Of note is the sharp decrease in the number of projects supported by the Robert Wood Johnson Foundation after the 2008 recession, a trend that continued as the Foundation's vision shifted to broader concerns of a culture of health resulting in the elimination of numerous long‐standing programs.
Number of projects supported by top health services research funders, 2005–2016
Source: HSRProj, 2017 .
Turning to NIH‐supported health services research across the Institutes and Centers, there has been an overall 7 percent decrease in the number of projects supported between 2005 and 2016 (Table 2 ). The largest reductions were at the National Institute of Mental Health and National Cancer Institute (65 percent and 46 percent, respectively; Table 3 ), followed by the National Institute of Drug Abuse (29 percent) and the National Institute for Child Health and Human Development (18 percent). In 2016, the National Institute of Mental Health and the National Institute on Aging supported the greatest number of health services research projects (66 projects each). The concentration of health services research projects funded by the top funding NIH institutes decreased across the decade represented in these data.
Number of Health Services Research Projects Supported by NIH Institutes, 2005–2016
In a reversal of these trends of reductions and/or stagnation in federal support for health services research, the final FY 2018 budget provided significant increases for research funding across multiple agencies, including a $3 billion more for NIH, an 8.3 percent increase to $37 billion (Science Magazine). Of note, in this budget deal, AHRQ received a small increase—$10 million—but one that is notable as the first increase in 9 years. However, it is not clear at this time whether the FY 2018 increases are auguring in a new trend of enhanced support for health services research. In fact, the FY 2019 proposed budget by the Trump administration continues to include proposed cuts to AHRQ and other HHS entities and for the second time eliminates AHRQ and creates instead a National Institute for Research on Safety and Quality, a structural change that raises numerous questions for all health services research stakeholders.
What the above data do not shed light on is the degree to which the private sector is supporting health services research. A recent study estimated overall funding for health services research to be between 0.2 and 0.3 percent of national health care expenditures between 2003 and 2011 (Moses et al. 2015 ). This higher estimates stem from the fact that the authors estimated total health services research funding at $5 billion in 2011, an estimate significantly higher because of two factors. First, 2011 was a year that AHRQ's budget was enhanced by the one‐time investment in CER, and second, their total health services research funding includes $1.4 billion in estimated health services research funding from the health services industry, including hospitals and other health care provider organizations. Moses et al. noted that while the estimate of health industry support may be an underestimate, it is still very low compared to other industrial sectors. Health services companies invest just 0.1 percent of revenue in health services research compared to 1.7–2.5 percent of revenue invested in research and development in other sectors of the economy.
The future size, scope, and focus of federal support for health services research remain uncertain given recent trends including continued pressures on federal discretionary spending. While the administration's proposal to move AHRQ into the NIH as part of the FY 2018 budget and again in FY 2019 was roundly rejected by Congress, the Congress did include in the Agency's budget a requirement to conduct a study to “identify research gaps and areas for consolidation, as well as propose strategies for better coordination of the Federal health services research enterprise,” signaling their willingness to consider other structural options for funding and coordination of health services research. This could prove to be an opportunity for the health services research community to step back and assess the changing nature, purpose and impact of health services research, and the priorities for federal support, including support for research, data, and training.
Supporting information
Appendix SA1: Author Matrix.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: The authors thank the Agency for Healthcare Research and Quality (HHSP233201600155P), the Robert Wood Johnson Foundation, and the Patient‐Centered Outcomes Research Institute for generous funding and guidance. The views expressed here do not necessarily reflect the views of these organizations.
Disclosures: None.
Disclaimer: None.
[The copyright line in this article was changed on 22 October 2018 after online publication.]
1 This is the latest year with data available for the Patient Centered Outcomes Research Institute (PCORI).
- Centers for Medicare and Medicaid Services, National Health Expenditures Fact Sheet . 2018. Available at https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/nationalhealthexpenddata/nhe-fact-sheet.html [accessed on March 4, 2018].
- HSRProj . 2017. HSRProj Research Includes Studies and Papers on a Variety of Topics . Inclusion and exclusion criteria can be found here: http://www.academyhealth.org/hsrprojsubmit . Interactive charts are available here: http://www.academyhealth.org/hsrprojcharts [accessed on April 22, 2018].
- Moses, H. , Matheson D. H., Cairns‐Smith S., George B. P., Palisch C., and Dorsey E. R.. 2015. “ The Anatomy of Medical Research: US and International Comparisons .” Journal of the American Medical Association 313 ( 2 ): 174–89. [ PubMed ] [ Google Scholar ]
- NIH Reporter . Available at https://report.nih.gov/categorical_spending.aspx [accessed on July 26, 2018].
- Science Magazine . “ Trump, Congress Approve Largest U.S. Research Spending Increase in a Decade ” [accessed on March 26, 2018]. Available at http://www.sciencemag.org/news/2018/03/updated-us-spending-deal-contains-largest-research-spending-increase-decade
Federal Funding for Key Medical Programs in FY 2024: A Closer Look

- Congress recently finalized an appropriations package of nearly $2 trillion for fiscal year 2024, funding critical medical research and public health programs through September 30, 2024.
- Many programs received flat funding, which failed to keep pace with inflation.
- STS will continue to advocate for robust funding levels that exceed the rate of inflation for fiscal year 2025.
In March, Congress passed its annual appropriations package for fiscal year (FY) 2024, allocating nearly $2 trillion to fund the government through September 30, 2024. This package includes resources for essential government agencies and research programs that drive medical advances, enhance care quality, and disseminate best practices. Below is a breakdown of the key programs that the STS supported and their funding outcomes.
National Institutes of Health (NIH)
NIH is the primary agency responsible for biomedical and public health research in the United States. This work is crucial for understanding, treating, and preventing diseases. Strong funding for NIH helps ensure appropriate funding levels for key institutes that directly impact cardiothoracic conditions.
Funding for FY24: $47.08 billion (a $300 million increase)
National Cancer Institute (NCI)
NCI leads the national effort to eliminate suffering and death caused by cancer. The agency supports research, training, and dissemination of information on cancer prevention, diagnosis, and treatment.
Funding for FY24: $7.22 billion (a $120 million increase)
National Heart, Lung, and Blood Institute (NHLBI)
NHLBI provides global leadership in research, training, and education to prevent and treat heart, lung, blood, and sleep disorders. STS participates in coalitions to promote NHLBI funding and meets annually with its leadership to better understand current activities and funding needs.
Funding for FY24: $3.98 billion (no change)
Agency for Healthcare Research and Quality (AHRQ)
AHRQ aims to produce evidence to make healthcare safer, higher quality, more accessible, equitable, and affordable. STS actively opposes efforts in Congress to terminate AHRQ funding.
Funding for FY24: $370.5 million (a $3 million decrease)
Centers for Disease Control and Prevention's (CDC) Office on Smoking and Health (OSH)
OSH leads national efforts to reduce tobacco-related death and disease.
Funding for FY24: $125.85 million (no change)
Lung Cancer Program at Congressionally Directed Medical Research Programs (CDMRP)
Administered by the Department of Defense, the CDMRP finances high-impact, high-risk, and high-gain projects that accelerate medical research in specific areas. One of these programs, the Lung Cancer Research Program, has received $194.5 million in funding over the past ten years (FY09-FY22), making it the largest lung cancer research program outside of the NCI. This program specifically researches topics that include biomarkers of disease recurrence, cutting edge immunotherapies, targeted tests, and treatments with added emphasis on under-researched small cell lung cancer.
Funding for FY24: $25 million for lung cancer research (no change)
Research into Firearm Morbidity and Mortality Prevention
Administered by the CDC, NIH, and National Institute of Justice (NIJ), this research is critical for understanding and preventing firearm-related injuries and deaths.
Funding for FY24: $12.5 million (no change)
Pediatric Specialty Loan Repayment Program
This program provides up to $100,000 in loan forgiveness to pediatric medical specialists to encourage them to pursue careers in underserved areas. Last year was the first time this program was funded after STS advocated for its creation for more than 10 years. Learn more .
Funding for FY24: $10 million (no change)
Looking Forward
As Congress begins to determine appropriations levels for FY25, STS will continue to advocate for robust funding that exceeds inflation for these critical government programs, which are vital for advancing patient care and medical research.
The independent source for health policy research, polling, and news.
FAQs on Health Spending, the Federal Budget, and Budget Enforcement Tools
Juliette Cubanski , Jeannie Fuglesten Biniek , and Tricia Neuman Published: Mar 20, 2023
Note: This brief was updated on March 20, 2023, to include details on fiscal year 2023 spending from the fiscal year 2024 budget released by the Biden Administration on March 9, 2023.
In January 2023, Treasury Secretary Yellen announced that the U.S. had reached the $31.381 trillion debt limit, prompting the Treasury Department to begin taking so-called “extraordinary measures” that are expected to help the government avoid defaulting on its debt until the summer of 2023. The debt limit, also known as the debt ceiling, is the maximum amount of money that the federal government is legally authorized to borrow to cover federal spending, including Social Security, Medicare, defense, and other federal government programs and obligations. The amount of the debt limit is established by law and increasing or suspending it requires legislative action. Congress has passed legislation 20 times since 2001 to increase or suspend the debt limit to avoid the federal government defaulting on its obligations.
In current discussions around the debt limit, some Republican lawmakers have pushed for reductions in future federal spending as part of a deal to raise the debt limit. The Biden Administration has said it will not negotiate spending reductions as part of debt limit talks but is open to separate discussions about approaches to debt and deficit reduction. House Speaker McCarthy has agreed that cuts to Social Security and Medicare are “off the table” in these discussions but has not ruled out seeking other spending cuts. This leaves open the question of whether Medicaid , the Affordable Care Act (ACA) premium tax credits, and possibly other health programs and services could be targeted for spending reductions in the near future.
These FAQs answer basic questions about health spending and the federal budget and budget enforcement tools, including the debt limit and sequestration. Health spending includes mandatory spending on health insurance programs like Medicare, Medicaid, the Children’s Health Insurance Program (CHIP), and the ACA Marketplaces; and discretionary spending on federal agencies such as the Centers for Disease Control and Prevention (CDC), the National Institutes of Health (NIH), the Food and Drug Administration (FDA), and the Health Resources and Services Administration (HRSA. Discretionary spending also includes domestic health programs and services, such as hospital and medical care for veterans, and the Indian Health Service; and spending for global health programs and services, such as the U.S. President’s Plan for AIDS Relief (PEPFAR). (See the Methods box for details on the data used for this analysis.)
How much support does the federal government provide for health programs and services?
The federal government provides support for health programs and services both through spending on programs and services and through tax expenditures. Federal spending on domestic and global health programs and services accounted for 29% of net federal outlays in fiscal year (FY) 2023 (taking into account offsetting receipts), or $1.9 trillion out of $6.4 trillion (Figure 1). Specifically, Medicare accounted for 13% of the total, Medicaid and CHIP accounted for 10%, other domestic health spending accounted for 4%, hospital and medical care for veterans was 2%, and global health was 0.1%. By comparison, Social Security accounted for 21% of federal outlays in FY 2023, while defense accounted for 13%.
Mandatory spending comprises the majority (88% or $1.6 trillion) of federal spending on health programs and services. Mandatory spending is not subject to annual appropriations votes by Congress but instead mandated by existing laws. Mandatory health spending includes nearly all Medicare spending, federal spending on Medicaid and CHIP (which are jointly funded by states and the federal government), and the refundable portion of the health insurance premium tax credit for coverage through the ACA Marketplaces, along with other mandatory health spending, which is detailed in Table 1. Medicare alone, which covers 65 million older adults and younger people with long-term disabilities, accounts for half of mandatory spending on federal health programs and services, while Medicaid, which covers 84 million individuals , accounts for another 37% (Figure 2). ACA premium tax credits—which include a refundable portion that counts as outlays and a non-refundable portion that counts as lost revenue—represent a much smaller portion (5% of mandatory outlays).
The remaining 12% of federal health spending ($231 billion) is discretionary spending, which is subject to votes by Congress during the annual appropriations process. Discretionary health spending includes nearly all spending on veterans’ hospital and medical care, estimated to provide services to more than 7 million veteran patients in FY 2022 ; spending on agencies such as the CDC, NIH, FDA, and HRSA; global health spending; and certain other health programs and services (Figure 3, Table 2).
In addition to federal spending on health programs and services, the federal government provides several tax benefits that support health-related activities, known as tax expenditures. These tax provisions are similar to federal spending in that they provide benefits from the federal government to employers, individuals, and other entities. Tax expenditures are revenue losses to the federal government because they allow for certain exclusions, exemptions, or deductions from income for the purpose of determining the amount of income taxes owed; provide preferential tax rates for certain programs; or reduce tax liability through tax credits.
Based on data from the Treasury Department , the three largest health-related tax expenditures in FY 2022 were:
- the tax exemption of employer contributions for medical insurance premiums and medical care: $224.5 billion (not including additional lost revenue from exempting employer contributions from payroll taxes for Social Security and Medicare);
- the premium tax credit for ACA Marketplace coverage: $14.7 billion (excluding the value of the refundable portion of the tax credit, which is classified as a mandatory outlay); and
- tax deductions for contributions to Medical Savings Accounts and Health Savings Accounts: $13 billion.
How does the debt limit affect federal health spending?
The debt limit itself does not directly affect levels of spending by the federal government, including mandatory and discretionary health spending. Government spending and revenues are, however, directly affected by legislation passed by Congress and signed by the President, which can add to the federal deficit and debt if, on balance, spending exceeds revenue. Over time, budget deficits have added to the government’s borrowing needs. Based on the latest projections from the Congressional Budget Office , annual federal budget deficits, amounting to a cumulative $20.2 trillion over the coming decade, will result in a substantial increase in federal debt.
Raising the debt limit does not mean the federal government is allowed to spend more money than Congress has previously authorized it to spend. But in a scenario where Congress failed to raise the debt limit , the government would be unable to borrow more money and would have insufficient funds to meet its current obligations. The amount of allowable spending would be limited to cash on hand and incoming revenues.
If the debt limit were reached and the Treasury Department had taken all “extraordinary measures” to avoid default, it is not clear what steps the government would or could take in response to meet the government’s obligations, but could include payment delays, prioritizing some payments over others, and automatic across-the-board payment reductions. Any of these options could affect payments for health and retirement benefits , including Social Security, Medicare, Medicaid and CHIP, veterans’ benefits, and other programs.

How does the regular congressional budget process affect federal health spending?
The regular Congressional budget process begins with a concurrent resolution that sets the overall federal spending and revenue levels. The budget resolution may also include reconciliation instructions to allow for a fast-track process that requires only a majority vote in the Senate for changes to spending on mandatory programs and federal revenues. The reconciliation process has recently been used to pass the Inflation Reduction Act of 2022 and the Tax Cuts and Jobs Act of 2017 , as well as portions of the Affordable Care Act of 2010 .
In most years over the last two decades, however, Congress has failed to agree to a budget resolution. When a resolution is not adopted, each chamber adopts its own (and usually different) targets for discretionary spending, and changes to mandatory spending and revenues may only be done through regular order (rather than reconciliation), and thus typically require a three-fifths vote in the Senate.
The Appropriations Committees develop 12 separate appropriates measures, including the Labor, Health and Human Services, Education and Related Agencies appropriations bill, which includes most domestic discretionary health spending. These bills are often combined for consideration by the full House and Senate into an omnibus bill. Congress is required to approve appropriations and the President is required to sign them into law before September 30 (the end of the federal fiscal year). If this deadline isn’t met, Congress can pass a continuing resolution to fund the government based on the preceding fiscal year amounts. When neither full year appropriations nor a continuing resolution is passed, the government “shuts down.” Continuing resolutions are more common than government shutdowns. For example, before approving the Consolidated Appropriations Act, 2023 , which funds the government through September 30, 2023, Congress passed two continuing resolutions as lawmakers negotiated.
In years that a budget resolution is agreed to, it may also include rules designed to impose fiscal discipline on the legislative process. These rules remain in effect until removed or revised by a future resolution. Two examples include the House and Senate PAYGO rules, which require any legislative provisions that are projected to increase the deficit over various time periods (different under House and Senate PAYGO rules) to be offset by spending reductions or tax increases. The rules are enforced through a “point-of-order” against legislation that violates the rule, though these are routinely waived when the legislation has sufficient support in the chamber.
What is sequestration and how does it affect federal health spending?
Sequestration is a budget enforcement tool that requires automatic, across-the-board reductions in federal spending, typically by a specified percentage. The sequestration process was established by Congress in 1985 to encourage Congress to meet specific budgetary goals. It is of recent interest because of the budget sequesters called for under the Budget Control Act of 2011 (BCA) and the Statutory Pay-As-You-Go Act of 2010 (PAYGO).
The BCA was enacted over a decade ago during a time when many lawmakers were expressing concern over federal budget deficits. The law included limits on annual discretionary spending , established a committee to develop proposals to reduce the deficit , and required sequestration of mandatory spending and further reductions in discretionary spending if the committee failed to report deficit reduction legislation. Because the committee did not come to agreement, sequestration was triggered. Under the BCA, a sequestration of mandatory federal spending was established for FY 2013 to FY 2021 but has been extended several times and is currently in effect through FY 2031 . (The separate BCA requirement for limits on discretionary spending expired at the end of FY 2021 .)
Under the Statutory PAYGO Act, sequestration is triggered when legislation enacted by Congress during a session is projected to increase the deficit on average over a five- or ten-year period, as determined by the Office of Management and Budget (OMB). However, sequestration under Statutory PAYGO has not yet occurred since it has been waived by Congress each time it would otherwise have been required.
Certain programs and types of spending are exempt from sequestration . Most of these exemptions relate to mandatory spending. Health spending exempt from sequestration includes certain health programs such as Medicaid, CHIP, ACA tax credits, Medicare Part D Low-Income Subsidies, Medicare Part D reinsurance spending, and veterans’ medical care. Social Security is also exempted.
Notably, most Medicare spending is not exempt from sequestration, but for Medicare (as well as certain other programs), special rules apply that limit the percentage reduction in spending. Under the BCA, reductions in Medicare benefits spending—including payments to providers under Part A and Part B and payments to plans under Part C (Medicare Advantage) and Part D—are limited to 2% rather than the uniform percentage reduction that would be applied to other nonexempt mandatory spending. Under a Statutory PAYGO sequester, Medicare benefit payment reductions are limited to 4%.
Sequestration of Medicare spending is currently in effect under the BCA’s mandatory spending sequester, although during the COVID-19 pandemic, it was suspended from May 2021 to March 2022 and reduced from 2% to 1% from April 2022 through June 2022. The Consolidated Appropriations Act (CAA), 2023 extended the BCA’s 2% sequestration of Medicare spending specifically partway into FY 2032 rather than expiring at the end of FY 2031, as it does for other nonexempt mandatory spending. In addition, while enactment of the American Rescue Plan Act of 2021 triggered a 4% Medicare sequester under Statutory PAYGO for FY 2022 (along with across-the-board cuts in certain other mandatory spending), Congress delayed these cuts to January 1, 2023 and then waived them for 2023 and 2024 .
Health Spending and Budget Issues to Watch
The Biden Administration has released the President’s budget for FY 2024 , which includes many savings and revenue proposals to reduce the federal deficit , along with many health-related proposals to lower prescription drug costs, extend the solvency of the Medicare Part A trust fund, and make permanent the ACA’s enhanced premiums tax credits, among other proposals. Under regular order, release of the President’s budget would be followed by the passage of a budget resolution by Congress in April. These actions could set the stage for discussions over the federal budget deficit and debt between the Administration and members of Congress in subsequent months. House Republicans have expressed support for a plan to balance the budget within 10 years by cutting government spending (and not increasing taxes). But without new revenues and without reducing spending on Social Security, Medicare, or defense, steep spending reductions in other areas would be needed to balance the budget . This could mean large spending cuts to health programs and services such as Medicaid, CHIP, and ACA subsidies, as well as veterans’ hospital and medical care, the NIH, FDA, and other health agencies, and other health programs and services.
The Treasury Department estimates that it will have exhausted the extraordinary measures being taken to avoid a default on the debt by early June. The Biden Administration supports a so-called ‘clean’ vote on raising the debt limit, separate from any discussions about approaches to reduce the federal deficit and debt. Although President Biden and Speaker McCarthy have ruled out changes to Medicare spending in discussions over the debt limit, it is possible that cuts to other federal health spending programs and services could be considered. Policymakers who are seeking to negotiate reductions in federal spending in exchange for a vote to raise the debt limit have proposed limiting new discretionary spending , which would affect many federally funded health programs and services, and imposing work requirements for Medicaid , among other options.
While most policymakers have not expressed support for cuts to Medicare in current budget discussions, CBO projects that higher spending on both Medicare and Social Security will put growing pressure on the federal budget . Federal law requires annual reports on the financial status of the Medicare and Social Security trust funds to be issued by April 1 each year, and typically these reports draw attention to this issue. Based on the 2022 report , the Medicare Trustees projected that the Medicare Part A trust fund, which pays for inpatient hospital, skilled nursing facility, home health and other Part A services, would be depleted in 2028. The projected shortfall is attributable to both the rise in spending, due to rising health care costs, growing enrollment, and an aging population, combined with insufficient revenues to cover all expenditures.
Some policymakers in Congress have proposed the creation of bipartisan, bicameral committees to address the solvency of government programs with trust funds, including Medicare, Social Security, and the highway trust fund. The President’s FY 2024 budget proposes to generate new tax revenue for the Part A trust fund through tax increases on high-income taxpayers (households earning more than $400,000 per year). These additional revenues, together with savings from prescription drug proposals, which would be credited to the Part A trust fund, are estimated to extend the solvency of the Medicare Part A trust fund by at least 25 years .
In the near term, the question of how lawmakers will respond to the debt limit looms large in current discussions about the federal budget and federal spending on government programs such as Social Security, Medicare, and Medicaid. The government has been funded through the end of the current fiscal year (September 30, 2023), but action will be needed by then to avoid funding gaps and avert a government shutdown , which could have the effect of curtailing the operations of government agencies like the FDA, NIH, and CDC and the provision of certain veterans’ services. Over the longer term, public and private payers and individuals face financial pressures associated with higher health care costs. For the federal government, rising health care costs affect spending on the government’s major health care programs, Medicare and Medicaid, along with spending on certain other health programs and services, such as veterans’ medical care. Providing funding for both mandatory and discretionary health programs and services that millions of people rely on while taking steps to address the growing federal budget deficit and debt could pose a challenge for policymakers in the months and years ahead.
This work was supported in part by Arnold Ventures. KFF maintains full editorial control over all of its policy analysis, polling, and journalism activities.
- Medicare's Future
- Federal Budget
- Medicaid's Future
- Children's Health Insurance Program (CHIP)
news release
- As Debate Heats Up in Washington Over Possible Entitlement Cuts, A New KFF Analysis Details the 30% of Federal Spending That Goes to Health Care Programs
Also of Interest
- What to Know about Medicare Spending and Financing
- FAQs on Medicare Financing and Trust Fund Solvency
- Medicaid: What to Watch in 2023
- Is the Biden Administration Proposing Cuts to Medicare Advantage?

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- About the Director
- Advisory Boards and Groups
- Strategic Plan
- Offices and Divisions
- Careers at NIMH
- Staff Directories
- Getting to NIMH
FY 2022 Budget - Congressional Justification
National institute of mental health, congressional justification fy 2022, national institutes of health, department of health and human services, director’s overview, ic fact sheet.
- Major Changes in Budget Request
- Budget Mechanism Table
Appropriations Language
Summary of changes, budget graphs, organization chart.
- Budget Authority by Activity
Justification of Budget Request
Appropriations history, authorizing legislation, amounts available for obligation, budget authority by object class, salaries and expenses, detail of full-time equivalent employment (fte), detail of positions.

The National Institute of Mental Health (NIMH) is the lead federal agency for research on mental illnesses, with a mission to transform the understanding and treatment of mental illnesses through basic and clinical research, paving the way for prevention, recovery, and cure.
In the United States, an estimated 51.5 million adults suffer from a mental illness, which may be significantly impairing and life-threatening. 1 Mental illnesses are the fifth leading cause of disability in the United States, accounting for 6.6 percent of all disability-adjusted life years. 2 One of the most tragic outcomes of untreated mental illness is suicide. Suicide accounted for the loss of over 47,000 American lives in 2019 alone; it is the second leading cause of death in youth and young adults aged 10-34, and the tenth leading cause of death overall. 3
NIMH supports a diverse portfolio of basic, translational, and clinical research, with the potential to improve clinical care over the short, medium, and long-term. On May 19, 2020, we published the new NIMH Strategic Plan for Research to optimize our scientific investments across the long arc of mental health research. 4 We use the Strategic Plan to communicate our priorities and help guide mental health research efforts funded by the Institute. We developed the Plan with input from a variety of stakeholders, including researchers, mental health advocates, and individuals with lived experience. 5 The Plan will be updated regularly to keep pace with ever-evolving scientific approaches and research priorities. Key research projects and findings from NIMH and NIMH-funded investigators that advance the Institute’s mission will be highlighted on our Research Progress webpage for each research Goal. 6
Answering the call
Impacts of the COVID-19 Pandemic on Mental Health. As the coronavirus disease 2019 (COVID-19) pandemic continues to affect us all, NIMH is providing guidance to researchers and resources for individuals managing stress and mental illness. In April 2020, we partnered with three other Institutes to issue a Notice of Special Interest to encourage research proposals to strengthen the mental health response to COVID-19 and future public health emergencies. 7 In addition, we are participating in trans-NIH initiatives supporting research to determine the role and impact of digital health interventions to address secondary health effects of COVID-19 8 and to evaluate the role and impact of community interventions to address the consequences of COVID-19 among health disparity populations and other vulnerable groups. 9 Within these trans-NIH initiatives, NIMH has awarded supplemental funding to numerous existing projects to understand and mitigate the pandemic’s impact on suicide, depression, and eating disorders, among other issues. We have also provided strategies and resources for managing fear, anxiety, and stress during this time, through multiple NIMH Director’s Messages, press releases, and shareable media. 10 , 11 , 12
Suicide Prevention Research Priorities. Suicide prevention research remains a top priority for NIMH, with approximately one suicide death every 11 minutes in 2019. 13 , 14 We are committed to bending the curve of suicide in the United States, and together with the National Action Alliance for Suicide Prevention (NAASP), we pledged to reduce the suicide rate by 20 percent by 2025. 15 This aspirational goal has helped guide our suicide prevention research agenda for the past seven years, emphasizing risk detection, screening, and intervention in health care settings. For example, NIMH-supported researchers aim to improve screening for suicide among vulnerable populations by validating, adapting, and extending the utility of the NIMH-developed Ask Suicide Screening Questions (ASQ) tool. 16 Our suicide prevention research priorities in the next five years are aligned with the National Strategy for Suicide Prevention developed by the NAASP that, among its other objectives, seeks to transform health care systems to reduce suicide. 17 , 18
The Opioid Crisis and Mental Health. NIMH recognizes the urgent need to identify effective approaches to treat people who have an opioid use disorder (OUD) and co-occurring mental health conditions, especially in primary care settings. The Collaborative Care Model is a promising approach to meeting the needs of people in primary care settings who have both OUD and mental illnesses. 19 In the Collaborative Care Model, a team of primary health providers and mental health specialists monitor each individual’s progress toward personal treatment goals, and treatments are actively changed if the individual is not improving as expected. As part of the NIH Helping to End Addiction Long-termSM Initiative, or NIH HEAL Initiative SM , we led an effort to solicit research proposals for effectiveness trials to optimize, implement, scale, and sustain the Collaborative Care Model. 20 To date, NIH has awarded four such projects for a total of $50 million.
Closing the gap in health disparities
Addressing Black Youth Suicide Suicide rates among Black youth more than doubled between 1999 and 2017, and Black youth under 13 years of age are now approximately twice as likely to die by suicide as their white counterparts. 21 , 22 We are funding a number of studies aimed at optimizing suicide risk detection and interventions among Black youth throughout the country. 23 , 24 We are also acting on a recent report by the Congressional Black Caucus (CBC) on the alarming rise in suicide and suicide-related behaviors among Black youth. 25 On April 21, 2020, the NIMH Office for Disparities Research and Workforce Diversity and the Office of Behavioral Health Equity at the Substance Abuse and Mental Health Services Administration co-hosted a virtual panel to discuss the CBC’s report and formulate strategies to engage and care for these vulnerable youth. 26 Additionally, NIMH is interested in supporting research focused on suicide risk and prevention among Black youth and is currently seeking guidance and input from stakeholders to address to crisis of Black youth suicide. 27 , 28
Suicide Prevention in Native American Communities. American Indian/Alaska Native (AI/AN) communities have the highest rates of suicide of any racial/ethnic group in the United States. 29 NIMH continues to partner with the National Institute on Minority Health and Health Disparities to support three collaborative research hubs which aim to develop and increase the reach of effective, culturally relevant preventive interventions to reduce the burden of suicide and promote resilience among AI/AN youth. 30 We are also supporting a number of intervention studies that are developing, adapting, and testing the effectiveness of health promotion and disease prevention interventions among AI/AN communities. 31 , 32 Further, NIMH recently announced a funding opportunity that will enable transdisciplinary teams to establish Suicide Prevention Research Centers; these Centers will be dedicated to the rapid development of scalable approaches to identify high-risk individuals and improve continuity of care across healthcare settings. 33 NIMH is encouraging applicants to consider how these Centers could best serve differentially affected groups such as sexual and gender minorities, Black youth, and AI/AN communities.
Mental Illnesses among People who are Incarcerated. To address the high rates of mental illnesses among incarcerated individuals, 34 NIMH has awarded more than $3.5 million in research funding to assess the effectiveness of the Stepping Up Initiative, which aims to reduce the number of people with mental illnesses who are incarcerated by determining common treatment and jail reduction priorities across mental health, jail, probation, parole, and county administration agencies. 35 We also support efforts to reduce suicide among individuals in the juvenile justice system, who are at particularly high risk for suicide. 36 , 37 Additionally we have partnered with the NIH Office of Behavioral and Social Science Research and the National Institute of Justice to fund the Suicide Prevention for at-Risk Individuals in Transition (SPIRIT) study, a randomized control trial to evaluate the effectiveness of an evidence-based Safety Planning Intervention for reducing suicide events in the year following incarceration. 38 , 39
Children with Autism Spectrum Disorder. An estimated 1 in 54 eight year old children in the United States have autism spectrum disorder (ASD), a developmental disorder that affects social communication and behavior. 40 , 41 Reliably detecting ASD in young children is difficult, and delays in diagnosis can have profound and long-lasting effects on children, while early intervention can improve cognitive and behavioral outcomes. 42 Race, culture, socioeconomic status, and the lack of trained providers in the community may also affect the age of ASD diagnosis. NIMH is committed to identifying and addressing disparities in access to screening, diagnosis, and treatment services among ethnic and racial minority children at risk for ASD. Recognizing the need for effective and widely adoptable tools for early screening and diagnosis of ASD, we have partnered with the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Neurological Disorders and Stroke (NINDS), and the National Institute on Deafness and Other Communication Disorders to support seven projects that seek to develop screening tools that can be implemented in community settings to detect signs of ASD in the first year of life. 43
Capitalizing on foundational investments and beyond
Piecing Together the Genetic Puzzle of Schizophrenia. NIMH is committed to supporting research focused on understanding the complex mechanisms linking genetic risk factors to the development of schizophrenia, in order to develop more effective preventions and interventions for this illness. Between 2014 and 2020, NIMH-funded investigators and collaborators compiled large genetic datasets, representing nearly 70,000 individuals with schizophrenia and more than 235,000 individuals without the disorder. By comparing data from these two groups, researchers have identified over 270 places in the genome where common DNA changes with small effects contribute to overall risk for schizophrenia. 44 , 45 In another NIMH-funded study involving sequencing nearly 25,000 individuals with and 100,000 without schizophrenia, researchers discovered 10 specific genes with large effects on schizophrenia risk. 46 These big data efforts in genetics, combined with changes observed in an NIMH-supported collection of over 2,000 donated brains, are pointing to novel biological mechanisms of this illness. 47 Our investments in genetics have produced advances such as these that may lead to new ways to identify at-risk individuals and to develop treatments for individuals.
Brain Research through Advancing Innovative Neurotechnologies Initiative® Highlights. To support the development of new tools and technologies to revolutionize our understanding of the brain, NIMH partners with NINDS to co-lead the NIH Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative®. 48 Since the launch of the BRAIN Initiative in 2013, we have supported the development of incredible technologies to characterize all cell types in the brain, map connected neurons in circuits and systems, and measure and modulate the activity of specific circuits. Multiple BRAIN Initiative reports have called for a data infrastructure to enable the research community to access tools to analyze and visualize these rich data. 49 , 50 Therefore, we have invested in a data infrastructure with three components: data archives, data standards, and software for data integration and analysis. We will continue to support this infrastructure, which provides a basis for implementing the BRAIN Initiative data sharing policy and for enabling secondary analyses of these massive data sets. 51 , 52
Machine Learning to Predict Individual Responses to Depression Treatments. Advances in new computational modelling tools like machine learning, coupled with the ever-increasing availability of aggregated, harmonized data sets, are revolutionizing the efficiency with which researchers turn data into knowledge. NIMH invests in research to translate this knowledge into improvements in mental health outcomes. For example, NIMH-supported researchers developed a machine learning algorithm to identify patterns in electroencephalogram recordings made available through the NIMH Data Archive, 53 which enabled them to reliably predict how individuals with major depression responded to the antidepressant sertraline. 54 Advances like this may optimize the treatment of mental illnesses by helping healthcare providers select the most effective treatments for their patients.
Mobile Health Technology. The use of mobile, wireless, and sensor technologies for health, collectively referred to as mobile health (mHealth), offers unprecedented opportunities to help consumers, clinicians, and researchers measure, manage, and improve health. mHealth also has the potential to reduce health disparities by increasing access to care and medical monitoring and enhancing population-based health research. NIMH currently supports several initiatives to further the development and evaluation of mHealth interventions for mental health, with an emphasis on using minimally intrusive digital technologies to enhance assessment, detection, and prevention of mental illnesses, accessibility and deliverability of mental health services, adherence to treatment, and efficiency and clinical impact of existing mental health services. 55 , 56 , 57 , 58
Overall Budget Policy : The FY 2022 President’s Budget request is $2,213.6 million, an increase of $107.7 million or 5.1 percent compared to the FY 2021 Enacted level. The request includes an increase of $26.0 million for the BRAIN Initiative, as authorized by the 21st Century Cures Act. It also includes $25.0 million in funding to increase research on the impact of the COVID-19 pandemic on mental health. This will be done in part by utilizing participants in existing cohort studies, who will be surveyed on the effect of the pandemic and various mitigation measures on their physical and mental health.

The National Institute of Mental Health (NIMH) is the lead federal agency for research on mental disorders. NIMH’s mission is to transform the understanding and treatment of mental illnesses through basic and clinical research, paving the way for prevention, recovery, and cure. NIMH conducts and supports biomedical and behavioral research, health services research, research training, and health information dissemination with respect to the causes, diagnosis, treatment, management, and prevention of mental illnesses.
The NIMH Strategic Plan forms a roadmap for the Institute's research priorities, spanning fundamental science to public health impact.

NIMH Quick Facts
- NIMH annually supports more than 3,000 research grants and contracts at universities, academic health centers, and other research institutions across the country and around the world.
- NIMH awarded 607 new and competing research project grants in FY 2020, with an application success rate of approximately 24 percent.
- In FY 2020, NIMH awarded grants to 99 unique early-stage investigators and 183 investigators with no other National Institute of Health (NIH) research funding.
- The NIMH Intramural Research Program (IRP) supports approximately 600 scientists, the majority of whom work on the NIH campus in Bethesda, Maryland.
Achievements: New Treatments and Interventions Based on NIMH Research
While typical antidepressants may take weeks to work, ketamine drmatically reduces depressive symptoms within hours. Based on technology developed by NIMH's intramural research patent portfolio, esketamine nasal spray medication for treatment-resistant depression received FDA approval in 2019.
Postpartum depression impacts 1 in 9 women in the United States. In 2019, the FDA approved brexalone intravenous infusion, the first-ever drug specifically designed to treat postpartum depression. The formulation of this breakthrough therapy was made possible by decades of NIMH-supported basic, translational, and clinical research.
NIMH's Recovery After an Initial Schizophrenia Episode (RAISE) project demonstrated that early intervention improves clinical outcomes among youth with first episode psychosis and that coordinated specialty care (CSC) is a feaible and cost-effective approach. Through collaborations with other federal agencies, CSC is now the standard of care for early psychosis, with over 280 CSC programs across the country.
Future Directions: Accelerating Medicines Partnership for Schizophrenia
To generate tools that will improve success in developing early-stage interventions for patients who are at risk of developing schizophrenia, the Foundation for NIH and NIMH launched the Accelerating Medicines Partnership for Schizophrenia (AMP SCZ), a public-private partnership between the NIH, the Food and Drug Administration, and public and private organizations. Core components of AMP SCZ include establishing a research network with U.S. and international sites and a data processing, analysis, and coordination center. The research network will focus on individuals at clinical high risk (CHR) for schizophrenia, identifying biological markers, clinical endpoints, and other measures that predict disease trajectory and outcomes. AMP SCZ data and analyses will be made available to the broad biomedical community through the NIMH Data Archive. Findings from AMP SCZ studies will enable researchers to develop algorithms that predict the course of illness for CHR individuals, allowing for early intervention and testing of treatments that may prevent the development of schizophrenia and reduce the impact of CHR.

Major Changes in the Fiscal Year 2022 President’s Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity details and these highlights will not sum to the total change for the FY 2022 President’s Budget request for NIMH. The FY 2022 President’s Budget request is $2,213.6 million, an increase of $107.7 million compared to the FY 2021 Enacted level.
Research Project Grants ($83.5 million; total $1,545.6 million) NIMH expects to increase funding for non-competing Research Project Grants by $31.4 million to fund projects receiving competing awards in prior years. Competing Research Project Grants are expected to increase by 29 grants or $47.4 million. This increase is distributed across all programmatic areas and basic, translational or clinical research.
Research Centers ($10.0 million; total $88.2 million) NIMH expects to increase funding for Research Centers by $10.0 million or two Research Center grants.
Intramural Research Programs ($5.6 million; total $218.9 million) NIMH expects to increase Intramural Research by $5.6 million, funding pay raises and inflation, and will continue to fund innovative research studies conducted by the Institute’s intramural scientists.
Research Management and Support ($2.3 million; total $99.0 million) NIMH expects to increase funding for Research Management and Support by $2.3 million and will continue to support the oversight and management of scientific programs critical to fulfilling the Institute’s mission.
Budget Mechanism - Total
1 All items in italics and brackets are non-add entries
2 Of which $70 million in FY 2020, $50.0 million in FY 2021, and $76.0 million in FY 2022 is derived by transfer from the NIH Innovation Account under the 21st Century Cures Act
3 Reflects 21st Century Cures Act funding not obliged in FY2020, and carried over into FY 2021
NATIONAL INSTITUTES OF HEALTH
For carrying out section 301 and title IV of the PHS Act with respect to mental health, [$2,053,708,000] $2,137,574,000 .
NIH INNOVATION ACCOUNT, CURES ACT
(including transfer of funds).
For necessary expenses to carry out the purposes described in section 1001(b)(4) of the 21st Century Cures Act, in addition to amounts available for such purposes in the appropriations provided to the NIH in this Act, [$404,000,000] $496,000,000 , to remain available until expended: Provided, That such amounts are appropriated pursuant to section 1001(b)(3) of such Act, are to be derived from amounts transferred under section 1001(b)(2)(A) of such Act, and may be transferred by the Director of the National Institutes of Health to other accounts of the National Institutes of Health solely for the purposes provided in such Act: Provided further, That upon a determination by the Director that funds transferred pursuant to the previous proviso are not necessary for the purposes provided, such amounts may be transferred back to the Account: Provided further, That the transfer authority provided under this heading is in addition to any other transfer authority provided by law.
Fiscal Year 2022 Budget Graphs
History of Budget Authority and FTEs:
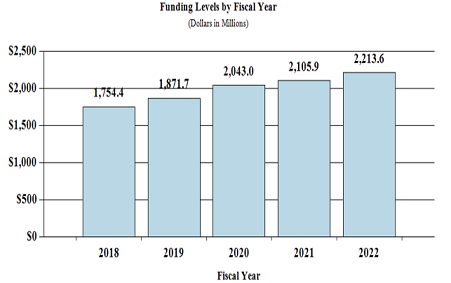
Distribution by Mechanism:
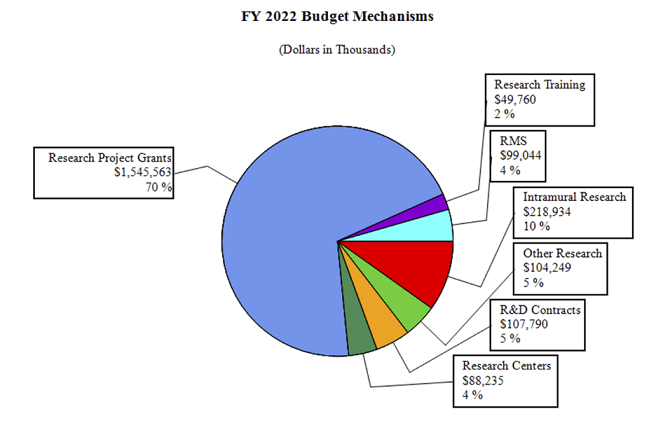
Change by Selected Mechanism:
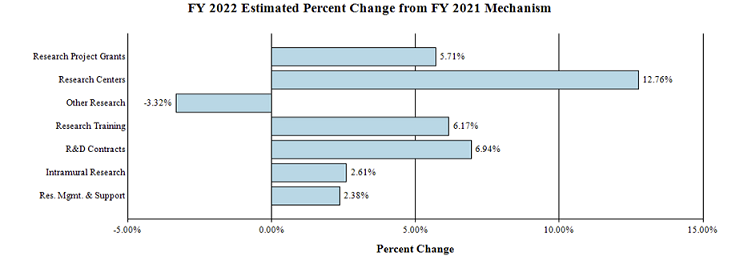
Budget Authority by Activity 1
1 Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
Authorizing Legislation: Section 301 and title IV of the Public Health Service Act, as amended.
Budget Authority (BA):
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Program Descriptions
Office of the director.
The NIMH Office of the Director (OD) leads the Institute in carrying out the NIMH mission to transform the understanding and treatment of mental illnesses. The OD provides scientific leadership, sets programmatic priorities, coordinates cross-cutting programs, determines Institute policies, directly funds several research projects, and provides overall administrative and operational coordination for the Institute. The OD houses nine offices: Office on AIDS; Office of Autism Research Coordination; Office of Clinical Research; Office of Genomics Research Coordination; Office for Disparities Research and Workforce Diversity; Office of Management; Office of Rural Mental Health Research; Office of Science Policy, Planning, and Communications; and, Office of Technology Development and Coordination. Each of the offices within the OD play an important role in supporting the Institute.
As an example of research that OD funds, NIMH is planning to launch an initiative supporting projects aimed at implementing and sustaining evidence-based mental health practices in low-resource settings to achieve equity in outcomes for underserved communities. 60 Limited capital and human resources can create barriers to care delivery. The initiative would encourage innovative approaches to remediate barriers to provision, receipt, and/or benefit from evidence-based practices. Studies may also generate new information about factors integral to achieving equity in mental health outcomes, with due consideration for the needs of individuals across the life span. The ultimate goal of the initiative will be to improve mental health outcomes for underserved populations and reduce or eliminate health disparities.
Budget Policy : The FY 2022 President’s Budget request is $44.5 million, an increase of $2.0 million or 4.7 percent compared with the FY 2021 Enacted level.
OD Program Portrait: Mental Health Research Awards for Innovative New Scientists in Low- and Middle-Income Countries
Investigators from low- and middle-income countries (LMICs) are underrepresented in mental health research. This limits NIMH’s ability to support mental health research that is relevant across diverse populations and settings, and to partner with other countries in research on shared priorities. The NIMH Research Awards for Innovative New Scientists in LMICs program will support basic, translational, clinical, or services research by outstanding scientists who are in the early stages of a career in mental health research. 59 Through these awards, NIMH aims to assist such individuals in launching innovative, high impact, independent research programs. In addition, these awards will encourage collaborative science while preserving the leadership role of new or early stage investigators in LMICs. This award program aims to support the next generation of scientists dedicated to producing the scientific knowledge that will help to prevent, treat, and ultimately cure mental illnesses across diverse populations.
Neuroscience and Basic Behavioral Science
The Division of Neuroscience and Basic Behavioral Science (DNBBS) supports research in the areas of basic neuroscience, genetics, integrative neuroscience, research training, resource development, and drug discovery. In cooperation with other NIMH programs and the wider research community, this Division ensures that relevant basic scientific knowledge is generated and used in pursuit of improved methods that, in the long term, could be used to diagnose, treat, and prevent mental illnesses.
DNBBS funds grants across a range of research topics to enhance understanding of the basic neurobiology underlying mental illnesses. In FY 2022, DNBBS plans to expand research into the brain subsystems that mediate anhedonia, the inability to feel pleasure – a core symptom of major depression as well as other major mental illnesses. In addition, DNBBS will expand research using artificial intelligence to understand neural circuitry underlying cognitive and social function with a focus on techniques to clarify the critical factors that drive the computer models and might ultimately be used to modulate brain function.
Budget Policy : The FY 2022 President’s Budget request is $910.0 million, an increase of $63.1 million or 7.4 percent compared with the FY 2021 Enacted level.
DNBBS Program Portrait: Discovery of Chemical Probes for Novel Brain Targets
Recognizing that the psychiatric drug development pipeline has slowed, NIMH seeks to accelerate the discovery and validation of new biological targets for treating brain disorders. NIMH recently announced its intent to support initiatives focused on the discovery of chemical probes for the nervous system – that is, small molecules that bind to and impact the function of biological targets in the nervous system, such as ion channels. Research projects will aim to discover novel chemical probes for use in studying biological processes relevant to the mission of NIMH and to identify novel biological targets that will inform studies of brain disease mechanisms. These initiatives will complement other NIMH-supported activities to support research at multiple stages in the drug development pipeline, including assay development and screening, optimization and early preclinical discovery,and the transition from early discovery to Phase I Clinical Trials. Newly discovered chemical probes could also be used as starting points for further development as potential therapeutic drugs through the Blueprint Neurotherapeutics program or small business development initiatives. Results from these research initiatives may provide new insight into important disease-related biological targets and biological processes.
Translational Research
The Division of Translational Research (DTR) supports integrative, multidisciplinary research and training programs that translate findings from basic science to discover the causes, mechanisms, and trajectories of mental illnesses, and to develop effective interventions for individuals across the lifespan. DTR supports research using innovative forms of scientific analysis, including computational psychiatry and machine learning, to elucidate the characteristics of, and risk factors for, mental illnesses; the neurobehavioral mechanisms of psychopathology; the trajectories of risk and resilience based on the interactive influences of genetics, brain development, environment, and experience; and, the design and testing of innovative treatments and interventions. As such, DTR-supported research efforts may have intermediate-term impact and pave the way towards effective treatment and prevention for mental illnesses.
One area of high priority for DTR is to improve outcomes for individuals at clinical high risk (CHR) for psychosis. A major gap in knowledge has been the lack of a means to reliably predict which individuals with CHR will develop schizophrenia or other adverse outcomes, which would enable the implementation of effective prevention strategies. To address this gap, NIMH and the Foundation for NIH launched a major public-private partnership to develop the tools that are needed to develop early therapeutic interventions for people at risk of developing schizophrenia. The Accelerating Medicines Partnership for Schizophrenia (AMP SCZ) 61 brings together NIH, the Food and Drug Administration, and numerous private and non-profit organizations to join forces in this unprecedented effort to prevent schizophrenia and other outcomes of CHR. AMP SCZ seeks to achieve this goal by developing a set of validated biomarkers that can identify individuals at risk for schizophrenia, and identify novel targets for treatment development. In addition, DTR, in collaboration with the Department of Defense, private foundations, and industry, supports the Advancing Understanding of RecOvery afteR traumA (AURORA) Study, 62 a landmark study to understand the consequences of trauma, as well as research using advanced digital techniques, including natural language processing, 63 machine learning, and predictive coding, 64 that aim to predict mental health outcomes, such as risk for suicide. DTR also supports work harnessing the latest advances in artificial intelligence to advance mechanistic understanding of how circuits in the brain shape behavior. 65
Budget Policy : The FY 2022 President’s Budget request is $573.7 million, an increase of $26.0 million or 4.8 percent increase compared with the FY 2021 Enacted level.
DTR and DSIR Program Portrait: Women’s Mental Health
Perinatal depression, or depression that develops during pregnancy or after childbirth, is one of the most common complications of pregnancy and the postpartum period. It affects as many as one in seven pregnant women and can result in negative short- and long-term consequences for mother and baby. NIMH is committed to identifying women at increased risk for perinatal depression and determining ways to improve intervention delivery, particularly for underserved populations. NIMH encourages research that includes: strategies for identifying women at risk for perinatal depression; evidence-based, service-ready, and scalable treatments and preventive interventions; and strategies that support the delivery of interventions with fidelity in the healthcare setting or other settings where women receive mental health services in the community. Further, NIMH is interested in studies conducted in real-world settings that leverage patient information from electronic health record data to determine which interventions are predicted to work best for which individuals. Identifying risk factors and developing appropriate screening, treatment, and preventive interventions has the potential to optimize care and improve public health outcomes.
The menopause transition (MT) is also a window of vulnerability for the development of mood and psychotic symptoms, and the mechanisms underlying this vulnerability are largely unknown. NIMH encourages comprehensive interdisciplinary research to identify biological, genetic, and environmental factors that could be used to identify women at risk of new or recurring mood and psychotic disorders during the MT, and to better understand the mechanistic links between the MT and these disorders. By supporting such research, NIMH ultimately aims to improve women’s health outcomes by identifying therapeutic targets for future development of novel treatment interventions.
Services and Intervention Research
The Division of Services and Intervention Research (DSIR) supports research that evaluates the effectiveness of psychosocial, pharmacological, somatic, rehabilitative, and combined interventions to prevent or treat mental illnesses. DSIR refines and evaluates treatment and preventive interventions for children, adolescents, and adults, focusing on acute and long-term symptom reduction, remission, and improved community functioning. DSIR also supports mental health services research, including interventions to improve the quality and outcomes of care; organization- and system-level interventions to enhance service delivery; and, strategies for widespread dissemination and implementation of evidence-based treatments into routine care settings. DSIR funds studies that are designed to have near-term impact, targeted at improving care for individuals currently suffering from mental illnesses.
DSIR initiatives encourage practice-based research with near-term potential for improving intervention effectiveness and service delivery, as illustrated by the Advanced Laboratories for Accelerating the Reach and Impact of Treatments for Youth and Adults with Mental Illness (ALACRITY) Research Centers program. 68 , 69 These centers incorporate a variety of transdisciplinary collaborations and prioritize a deployment-focused approach to yield interventions and service strategies that are relevant and can be rapidly integrated into practice. Many of the centers capitalize digital health platforms and data science methods to learn about mental illness onset and progression in clinical populations, improve diagnosis, and deliver targeted interventions via smart communication technologies. The centers also account for the perspectives of a variety of stakeholders, including patients, families, and providers. The currently funded ALACRITY Centers span a variety of key populations and practice settings and cover a range of science, spanning intervention refinement and optimization through implementation and services research.
Budget Policy : The FY 2022 President’s Budget request is $193.8 million, an increase of $8.7 million or 4.7 percent compared with the FY 2021 Enacted level.
AIDS Research
The Division of AIDS Research (DAR) supports research and research training that addresses the priority areas outlined in the NIH Strategic Plan for HIV and HIV-Related Research 70 and the HHS National HIV/AIDS Strategy. 71 DAR-supported research includes behavioral and social science studies aimed at reducing HIV/AIDS incidence through the development, testing, and implementation of new and improved prevention strategies, improving health outcomes of those living with HIV through improved linkage to care, and adherence to effective treatments. DAR also supports research to understand, prevent, and treat the neurological and mental health conditions associated with living with HIV. DAR is participating in cure research by supporting studies to eradicate or silence HIV from biological reservoirs in the central nervous system (CNS), where the virus may evade detection and treatment. HIV latency in the CNS is critically important to consider in studies of eradication and reactivation. Many drugs designed to eradicate the virus are unable to penetrate the CNS, because the CNS acts as a protective reservoir for HIV. This work may also inform methods to prevent or treat the neurological comorbidities of HIV, such as cognitive and behavioral impairments, with targeted research to understand HIV-induced neurological pathology, and emphasis on long-term antiretroviral therapy.
DAR research also places special emphasis on World Health Organization-defined key populations, health disparities, and the impact of mental illnesses that may increase the risk for contracting HIV or negatively impact the health outcomes of those living with HIV. Additionally, DAR ensures effective integration of biomedical approaches and multidisciplinary expertise are considered in NIH-wide planning efforts, to help achieve an AIDS-free generation.
Budget Policy : The FY 2022 President’s Budget request is $173.6 million, unchanged from the FY 2021 Enacted level.
Intramural Research Programs
The Division of Intramural Research Programs (IRP) is the internal research component of NIMH, complementing the Institute’s extramural grant funding program. IRP scientists investigate basic, translational, and clinical aspects of brain function and behavior, conducting state-of-the-art research using unique NIH resources. In addition, the IRP provides an excellent environment for training the next generation of basic and clinical scientists.
IRP researchers are developing new and improved methods in functional magnetic resonance imaging (fMRI) and exploring advanced computational methods to evaluate brain function and mental illnesses. Many IRP researchers use fMRI and behavioral tasks to investigate differences in brain circuitry underlying key brain functions such as learning, perception, and attention, which are affected in mental illnesses. IRP scientists are also exploring novel medications and other treatments for depression in adults, including ketamine and other experimental fast-acting antidepressant medications, transcranial magnetic stimulation (TMS), and next generation seizure therapy. Using clinical assessments, brain imaging, and sleep studies, they aim to better understand suicide. 72 IRP researchers have also developed the Ask Suicide-Screening Questions (ASQ) tool 73 for use among both youth and adults in various medical settings, and they are now collaborating with the Indian Health Service (IHS) to implement the ASQ in all 170 IHS medical facilities. Physician scientists are working to identify causes, treatments for, and predictors of risk for reproductive endocrine-related mood disorders, recently creating an in vitro model of perimenopausal depression (PMD), 74 which pointed to expression of genes that could contribute to vulnerability to PMD. IRP researchers are currently investigating how the circadian clock and light affect mood and behavior. For example, one study found a link between levels of outdoor light exposure and sleep and mental health in teens. 75 To understand the biology of childhood-onset mental illnesses, IRP scientists are collecting extensive clinical, genetic, and anatomical data to investigate the effects of gene expression and sex differences on brain structure. 76
Budget Policy : The FY 2022 President’s Budget request is $218.9 million, an increase of $5.6 million or 2.6 percent compared with the FY 2021 Enacted level.
Research Management and Support
Research Management and Support (RMS) activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research and training grants, and research and development contracts. RMS functions include strategic planning, coordination, and evaluation of NIMH programs, regulatory compliance, coordination of global mental health efforts, and liaising with other Federal agencies, Congress, and the public. Through RMS activities, NIMH continues to provide accountability and administrative support for meritorious basic, clinical, and translational research and continues to promote health information dissemination, education, and outreach activities.
Budget Policy : The FY 2022 President’s Budget request is $99.0 million, an increase of $2.3 million or 2.4 percent compared with the FY 2021 Enacted level.
1 Budget Estimate to Congress includes mandatory financing.
2 Includes funds derived by transfer from the NIH Innovation Account under the 21st Century Cures Act
1 Excludes the following amounts for reimbursable activities carried out by this account: FY 2020 - $8,775 FY 2021 - $10,050 FY 2022 - $10,050
2 Of which $70 million in FY 2020, $50.0 million in FY 2021, and $76.0 million in FY 2022 is derived by transfer from the NIH Innovation Account under the 21st Century Cures Act.
3 Reflects 21st Century Cures Act funding not obligated in previous years and carried over into FY 2021
(Dollars in Thousands)
1 Substance Abuse and Mental Health Services Administration. (2020). Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health (HHS Publication No. PEP20-07-01-001, NSDUH Series H-55). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Retrieved from samhsa.gov/data/
2 Institute of Health Metrics and Evaluation. ghdx.healthdata.org/gbd-results-tool accessed March 2021.
3 CDC, NCIPC. WISQARS: cdc.gov/injury/wisqars/index.html accessed March 2021.
4 https://nimh.nih.gov/about/strategic-planning-reports/index.shtml
5 https://www.nimh.nih.gov/about/strategic-planning-reports/strategic-planning-process.shtml
6 https://www.nimh.nih.gov/about/strategic-planning-reports/index.shtml#progress
7 https://grants.nih.gov/grants/guide/notice-files/NOT-MH-20-047.html
8 http://grants.nih.gov/grants/guide/pa-files/PAR-20-243.html
9 https://grants.nih.gov/grants/guide/pa-files/PAR-20-237.html
10 https://www.nimh.nih.gov/about/director/messages/2020/coping-with-coronavirus-managing-stress-fear-and-anxiety.shtml
11 https://www.nimh.nih.gov/news/science-news/2020/supporting-mental-health-during-the-covid-19-pandemic.shtml
12 https://www.nimh.nih.gov/health/education-awareness/shareable-resources-on-coping-with-covid-19.shtml
13 h ttps://cdc.gov/nchs/data/databriefs/db398-H.pdf
14 https://www.cdc.gov/violenceprevention/suicide/fastfact.html
15 https://www.nimh.nih.gov/news/science-news/2020/nimh-leadership-describes-suicide-prevention-research-priorities.shtml
16 https://www.nimh.nih.gov/research/research-conducted-at-nimh/asq-toolkit-materials/index.shtml
17 https://www.hhs.gov/surgeongeneral/reports-and-publications/suicide-prevention/index.html
18 https://pubmed.ncbi.nlm.nih.gov/32432690/
19 https://heal.nih.gov/news/stories/collaborative-care
20 https://grants.nih.gov/grants/guide/rfa-files/RFA-MH-19-525.html
21 https://www.cdc.gov/nchs/data/hestat/suicide/rates_1999_2017.htm
22 https://www.ncbi.nlm.nih.gov/pubmed/29799931
23 https://reporter.nih.gov/project-details/9807076
24 https://reporter.nih.gov/project-details/9725522
25 https://www.stevefund.org/wp-content/uploads/2019/12/FULL-TASKFORCE-REPORT.pdf
26 https://www.nimh.nih.gov/news/events/announcements/webinar-responding-to-the-alarm-addressing-black-youth-suicide.shtml
27 https://grants.nih.gov/grants/guide/notice-files/NOT-MH-20-055.html
28 http://grants.nih.gov/grants/guide/notice-files/NOT-MH-21-035.html
29 https://www.cdc.gov/mmwr/volumes/67/wr/mm6708a1.htm
30 https://grants.nih.gov/grants/guide/rfa-files/RFA-MH-17-350.html
31 https://reporter.nih.gov/project-details/9899318
32 https://reporter.nih.gov/project-details/9706932
33 http://grants.nih.gov/grants/guide/pa-files/PAR-20-286.html
34 https://bjs.ojp.gov/content/pub/pdf/imhprpji1112.pdf
35 http://nimh.nih.gov/news/research-highlights/2020/identifying-practices-for-reducing-incarceration-of-those-with-mental-illnesses-a-study-of-stepping-up.shtml
36 https://reporter.nih.gov/project-details/9768575
37 https://ojjdp.ojp.gov/sites/g/files/xyckuh176/files/pubs/243891.pdf
38 http://www.nimh.nih.gov/news/science-news/2015/embracing-the-spirit-of-reducing-suicide.shtml
39 https://reporter.nih.gov/project-details/9312313
40 https://www.nimh.nih.gov/health/statistics/autism-spectrum-disorder-asd.shtml
41 https://www.nimh.nih.gov/health/topics/autism-spectrum-disorders-asd/index.shtml
42 https://pubmed.ncbi.nlm.nih.gov/18349708/
43 https://www.nimh.nih.gov/news/science-news/2019/nih-awards-funding-for-early-autism-screening.shtml
44 https://www.nimh.nih.gov/about/director/messages/2020/piecing-together-the-genetic-puzzle-of-schizophrenia.shtml
45 https://www.medrxiv.org/content/10.1101/2020.09.12.20192922v1
46 https://www.medrxiv.org/content/10.1101/2020.09.18.20192815v1
47 http://www.nimh.nih.gov/news/science-news/2018/2-000-human-brains-yield-clues-to-how-genes-raise-risk-for-mental-illnesses.shtml
48 https://braininitiative.nih.gov/
49 https://braininitiative.nih.gov/strategic-planning/brain-2025-report
50 https://braininitiative.nih.gov/strategic-planning/acd-working-groups/brain-initiative-20-cells-circuits-toward-cures
51 https://grants.nih.gov/grants/guide/notice-files/NOT-MH-19-010.html
52 https://grants.nih.gov/grants/guide/rfa-files/rfa-mh-20-120.html
53 https://nda.nih.gov/edit_collection.html?id=2199
54 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7145761/
55 https://grants.nih.gov/grants/guide/pa-files/PAR-19-376.html
56 https://grants.nih.gov/grants/guide/pa-files/PA-18-579.html
57 https://grants.nih.gov/grants/guide/pa-files/PA-18-566.html
58 https://grants.nih.gov/grants/guide/notice-files/not-mh-18-031.html
59 https://www.nimh.nih.gov/funding/grant-writing-and-application-process/concept-clearances/2020/mental-health-research-awards-for-innovative-new-scientists-in-low-and-middle-income-countries-lmics.shtml
60 https://www.nimh.nih.gov/funding/grant-writing-and-application-process/concept-clearances/2019/effectiveness-of-implementing-sustainable-evidence-based-mental-health-practices-in-low-resource-settings-to-achieve-equity-in-outcomes-for-traditionally-underserved-populations.shtml
61 https://www.nih.gov/news-events/news-releases/nih-public-private-partnership-advance-early-interventions-schizophrenia
62 https://reporter.nih.gov/project-details/9756462
63 https://pubmed.ncbi.nlm.nih.gov/30710497/
64 https://www.ncbi.nlm.nih.gov/pubmed/30389840
65 https://grants.nih.gov/grants/guide/pa-files/PAR-19-344.html
66 https://www.nimh.nih.gov/funding/grant-writing-and-application-process/concept-clearances/2020/prevention-of-perinatal-depression-improving-intervention-delivery-for-at-risk-individuals.shtml
67 https://www.nimh.nih.gov/funding/grant-writing-and-application-process/concept-clearances/2020/mood-and-psychosis-symptoms-during-the-menopause-transition.shtml
68 https://grants.nih.gov/grants/guide/pa-files/PAR-16-354.html
69 https://grants.nih.gov/grants/guide/pa-files/PAR-18-701.html
70 https://oar.nih.gov/hiv-policy-and-research/strategic-plan
71 https://www.hiv.gov/blog/hhs-and-the-national-hivaids-strategy
72 https://clinicaltrials.gov/ct2/show/NCT02543983?term=Neurobiology+of+Suicide&draw=2&rank=1
73 https://www.nimh.nih.gov/research/research-conducted-at-nimh/asq-toolkit-materials/index.shtml
74 https://pubmed.ncbi.nlm.nih.gov/32788687/
75 https://www.nimh.nih.gov/news/science-news/2020/outdoor-light-linked-with-teens-sleep-and-mental-health.shtml
76 https://www.nimh.nih.gov/news/science-news/2020/study-shows-highly-reproducible-sex-differences-in-aspects-of-human-brain-anatomy.shtml
Mobile Menu Overlay
The White House 1600 Pennsylvania Ave NW Washington, DC 20500
FACT SHEET: President Biden Issues Executive Order and Announces New Actions to Advance Women’s Health Research and Innovation
In his State of the Union address, President Biden laid out his vision for transforming women’s health research and improving women’s lives all across America. The President called on Congress to make a bold, transformative investment of $12 billion in new funding for women’s health research. This investment would be used to create a Fund for Women’s Health Research at the National Institutes of Health (NIH) to advance a cutting-edge, interdisciplinary research agenda and to establish a new nationwide network of research centers of excellence and innovation in women’s health—which would serve as a national gold standard for women’s health research across the lifespan.
It is long past time to ensure women get the answers they need when it comes to their health—from cardiovascular disease to autoimmune diseases to menopause-related conditions. To pioneer the next generation of discoveries, the President and the First Lady launched the first-ever White House Initiative on Women’s Health Research , which aims to fundamentally change how we approach and fund women’s health research in the United States.
Today, President Biden is signing a new Executive Order that will direct the most comprehensive set of executive actions ever taken to expand and improve research on women’s health. These directives will ensure women’s health is integrated and prioritized across the federal research portfolio and budget, and will galvanize new research on a wide range of topics, including women’s midlife health.
The President and First Lady are also announcing more than twenty new actions and commitments by federal agencies, including through the U.S. Department of Health and Human Services (HHS), the Department of Defense (DoD), the Department of Veterans Affairs (VA), and the National Science Foundation (NSF). This includes the launch of a new NIH-wide effort that will direct key investments of $200 million in Fiscal Year 2025 to fund new, interdisciplinary women’s health research—a first step towards the transformative central Fund on Women’s Health that the President has called on Congress to invest in. These actions also build on the First Lady’s announcement last month of the Advanced Research Projects Agency for Health (ARPA-H) Sprint for Women’s Health , which committed $100 million towards transformative research and development in women’s health.
Today, the President is issuing an Executive Order that will:
- Integrate Women’s Health Across the Federal Research Portfolio . The Executive Order directs the Initiative’s constituent agencies to develop and strengthen research and data standards on women’s health across all relevant research and funding opportunities, with the goal of helping ensure that the Administration is better leveraging every dollar of federal funding for health research to improve women’s health. These actions will build on the NIH’s current policy to ensure that research it funds considers women’s health in the development of study design and in data collection and analysis. Agencies will take action to ensure women’s health is being considered at every step in the research process—from the applications that prospective grantees submit to the way that they report on grant implementation.
- Prioritize Investments in Women’s Health Research . The Executive Order directs the Initiative’s constituent agencies to prioritize funding for women’s health research and encourage innovation in women’s health, including through ARPA-H and multi-agency initiatives such as the Small Business Innovation Research Program and the Small Business Technology Transfer Program. These entities are dedicated to high-impact research and innovation, including through the support of early-stage small businesses and entrepreneurs engaged in research and innovation. The Executive Order further directs HHS and NSF to study ways to leverage artificial intelligence to advance women’s health research. These additional investments—across a wide range of agencies—will support innovation and open new doors to breakthroughs in women’s health.
- Galvanize New Research on Women’s Midlife Health . To narrow research gaps on diseases and conditions associated with women’s midlife health or that are more likely to occur after menopause, such as rheumatoid arthritis, heart attack, and osteoporosis, the President is directing HHS to: expand data collection efforts related to women’s midlife health; launch a comprehensive research agenda that will guide future investments in menopause-related research; identify ways to improve management of menopause-related issues and the clinical care that women receive; and develop new resources to help women better understand their options for menopause-related symptoms prevention and treatment. The Executive Order also directs the DoD and VA to study and take steps to improve the treatment of, and research related to, menopause for Service women and women veterans.
- Assess Unmet Needs to Support Women’s Health Research . The Executive Order directs the Office of Management and Budget and the Gender Policy Council to lead a robust effort to assess gaps in federal funding for women’s health research and identify changes—whether statutory, regulatory, or budgetary—that are needed to maximally support the broad scope of women’s health research across the federal government. Agencies will also be required to report annually on their investments in women’s health research, as well as progress towards their efforts to improve women’s health.
Today, agencies are also announcing new actions they are taking to promote women’s health research , as part of their ongoing efforts through the White House Initiative on Women’s Health Research. Agencies are announcing actions to:
Prioritize and Increase Investments in Women’s Health Research
- Launch an NIH-Cross Cutting Effort to Transform Women’s Health Throughout the Lifespan. NIH is launching an NIH-wide effort to close gaps in women’s health research across the lifespan. This effort—which will initially be supported by $200 million from NIH beginning in FY 2025—will allow NIH to catalyze interdisciplinary research, particularly on issues that cut across the traditional mandates of the institutes and centers at NIH. It will also allow NIH to launch ambitious, multi-faceted research projects such as research on the impact of perimenopause and menopause on heart health, brain health and bone health. In addition, the President’s FY25 Budget Request would double current funding for the NIH Office of Research on Women’s Health to support new and existing initiatives that emphasize women’s health research.
This coordinated, NIH-wide effort will be co-chaired by the NIH Office of the Director, the Office of Research on Women’s Health, and the institute directors from the National Institute on Aging; the National Heart, Lung, and Blood Institute; the National Institute on Drug Abuse; the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the National Institute on Arthritis, Musculoskeletal and Skin Diseases.
- Invest in Research on a Wide Range of Women’s Health Issues. The bipartisan Congressionally Directed Medical Research Program (CDMRP), led out of DoD, funds research on women’s health encompassing a range of diseases and conditions that affect women uniquely, disproportionately, or differently from men. While the programs and topic areas directed by Congress differ each year, CDMRP has consistently funded research to advance women’s health since its creation in 1993. In Fiscal Year 2022, DoD implemented nearly $490 million in CDMRP investments towards women’s health research projects ranging from breast and ovarian cancer to lupus to orthotics and prosthetics in women. In Fiscal Year 2023, DoD anticipates implementing approximately $500 million in CDMRP funding for women’s health research, including in endometriosis, rheumatoid arthritis, and chronic fatigue.
- Call for New Proposals on Emerging Women’s Health Issues . Today, NSF is calling for new research and education proposals to advance discoveries and innovations related to women’s health. To promote multidisciplinary solutions to women’s health disparities, NSF invites applications that would improve women’s health through a wide range of disciplines—from computational research to engineering biomechanics. This is the first time that NSF has broadly called for novel and transformative research that is focused entirely on women’s health topics, and proposals will be considered on an ongoing basis.
- Increase Research on How Environmental Factors Affect Women’s Health. The Environmental Protection Agency (EPA) is updating its grant solicitations and contracts to ensure that applicants prioritize, as appropriate, the consideration of women’s exposures and health outcomes. These changes will help ensure that women’s health is better accounted for across EPA’s research portfolio and increase our knowledge of women’s environmental health—from endocrine disruption to toxic exposure.
- Create a Dedicated, One-Stop Shop for NIH Funding Opportunities on Women’s Health. Researchers are often unaware of existing opportunities to apply for federal funding. To help close this gap, NIH is issuing a new Notice of Special Interest that identifies current, open funding opportunities related to women’s health research across a wide range of health conditions and all Institutes, Centers, and Offices. The NIH Office of Research on Women’s Health will build on this new Notice by creating a dedicated one-stop shop on open funding opportunities related to women’s health research. This will make it easier for researchers and institutions to find and apply for funding—instead of having to search across each of NIH’s 27 institutes for funding opportunities.
Foster Innovation and Discovery in Women’s Health
- Accelerate Transformative Research and Development in Women’s Health. ARPA-H’s Sprint for Women’s Health launched in February 2024 commits $100 million to transformative research and development in women’s health. ARPA-H is soliciting ideas for novel groundbreaking research and development to address women’s health, as well as opportunities to accelerate and scale tools, products, and platforms with the potential for commercialization to improve women’s health outcomes.
- Support Private Sector Innovation Through Additional Federal Investments in Women’s Health Research. The NIH’s competitive Small Business Innovation Research Program and the Small Business Technology Transfer Program is committing to further increasing—by 50 percent—its investments in supporting innovators and early-stage small businesses engaged in research and development on women’s health. These programs will solicit new proposals on promising women’s health innovation and make evidence-based investments that bridge the gap between performance of basic science and commercialization of resulting innovations. This commitment for additional funds builds on the investments the Administration has already made to increase innovation in women’s health through small businesses, including by increasing investments by sevenfold between Fiscal Year 2021 and Fiscal Year 2023.
- Advance Initiatives to Protect and Promote the Health of Women. The Food and Drug Administration (FDA) seeks to advance efforts to help address gaps in research and availability of products for diseases and conditions that primarily impact women, or for which scientific considerations may be different for women, and is committed to research and regulatory initiatives that facilitate the development of safe and effective medical products for women. FDA also plans to issue guidance for industry that relates to the inclusion of women in clinical trials and conduct outreach to stakeholders to discuss opportunities to advance women’s health across the lifespan. And FDA’s Office of Women’s Health will update FDA’s framework for women’s health research and seek to fund research with an emphasis on bridging gaps in knowledge on important women’s health topics, including sex differences and conditions that uniquely or disproportionately impact women.
- Use Biomarkers to Improve the Health of Women Through Early Detection and Treatment of Conditions, such as Endometriosis. NIH will launch a new initiative dedicated to research on biomarker discovery and validation to help improve our ability to prevent, diagnose, and treat conditions that affect women uniquely, including endometriosis. This NIH initiative will accelerate our ability to identify new pathways for diagnosis and treatment by encouraging multi-sector collaboration and synergistic research that will speed the transfer of knowledge from bench to bedside.
- Leverage Engineering Research to Improve Women’s Health . The NSF Engineering Research Visioning Alliance (ERVA) is convening national experts to identify high-impact research opportunities in engineering that can improve women’s health. ERVA’s Transforming Women’s Health Outcomes Through Engineering visioning event will be held in June 2024, and will bring together experts from across engineering—including those in microfluidics, computational modeling, artificial intelligence/imaging, and diagnostic technologies and devices—to evaluate the landscape for new applications in women’s health. Following this event, ERVA will issue a report and roadmap on critical areas where engineering research can impact women’s health across the lifespan.
- Drive Engineering Innovations in Women’s Health Discovery . NSF awardees at Texas A&M University will hold a conference in summer 2024 to collectively identify challenges and opportunities in improving women’s health through engineering. Biomedical engineers and scientists will explore and identify how various types of engineering tools, including biomechanics and immuno-engineering, can be applied to women’s health and spark promising new research directions.
Expand and Leverage Data Collection and Analysis Related to Women’s Health
- Help Standardize Data to Support Research on Women’s Health. NIH is launching an effort to identify and develop new common data elements related to women’s health that will help researchers share and combine datasets, promote interoperability, and improve the accuracy of datasets when it comes to women’s health. NIH will initiate this process by convening data and scientific experts across the federal government to solicit feedback on the need to develop new NIH-endorsed common data elements—which are widely used in both research and clinical settings. By advancing new tools to capture more data about women’s health, NIH will give researchers and clinicians the tools they need to enable more meaningful data collection, analysis, and reporting and comprehensively improve our knowledge of women’s health.
- Reflect Women’s Health Needs in National Coverage Determinations. The Centers for Medicare & Medicaid Services (CMS) will strengthen its review process, including through Coverage with Evidence Development guidance, to ensure that new medical services and technologies work well in women, as applicable, before being covered nationally through the Medicare program. This will help ensure that Medicare funds are used for treatments with a sufficient evidence base to show that they actually work in women, who make up more than half of the Medicare population.
- Leverage Data and Quality Measures to Advance Women’s Health Research. The Centers for Disease Control and Prevention (CDC) and the Health Resources and Services Administration (HRSA) are building on existing datasets to improve the collection, analysis, and reporting of information on women’s health. The CDC is expanding the collection of key quality measures across a woman’s lifespan, including to understand the link between pregnancy and post-partum hypertension and heart disease, and plans to release the Million Hearts Hypertension in Pregnancy Change Package. This resource will feature a menu of evidence-informed strategies by which clinicians can change care processes. Each strategy includes tested tools and resources to support related clinical quality improvement. HRSA is modernizing its Uniform Data System in ways that will improve the ability to assess how women are being served through HRSA-funded health centers. By improving the ability to analyze data on key clinical quality measures, CDC and HRSA can help close gaps in women’s health care access and identify new opportunities for high-impact research.
Strengthen Coordination, Infrastructure, and Training to Support Women’s Health Research
- Launch New Joint Collaborative to Improve Women’s Health Research for Service Members and Veterans. DoD and VA are launching a new Women’s Health Research collaborative to explore opportunities that further promote joint efforts to advance women’s health research and improve evidence-based care for Service members and veterans. The collaborative will increase coordination with the goal of helping improve care across the lifespan for women in the military and women veterans. The Departments will further advance research on key women’s health issues and develop a roadmap to close pressing research gaps, including those specifically affecting Service women and women veterans.
- Coordinate Research to Advance the Health of Women in the Military. DoD will invest $10 million, contingent on available funds, in the Military Women’s Health Research Partnership. This Partnership is led by the Uniformed Services University and advances and coordinates women’s health research across the Department. The Partnership is supporting research in a wide range of health issues affecting women in the military, including cancers, mental and behavioral health, and the unique health care needs of Active Duty Service Women. In addition, the Uniformed Services University established a dedicated Director of Military Women’s Health Research Program, a role that is responsible for identifying research gaps, fostering collaboration, and coordinating and aligning a unified approach to address the evolving needs of Active Duty Service Women.
- Support EPA-Wide Research and Dissemination of Data on Women’s Health. EPA is establishing a Women’s Health Community of Practice to coordinate research and data dissemination. EPA also plans to direct the Board of Scientific Counselors to identify ways to advance EPA’s research with specific consideration of the intersection of environmental factors and women’s health, including maternal health.
- Expand Fellowship Training in Women’s Health Research. CDC, in collaboration with the CDC Foundation and American Board of Obstetrics and Gynecology, is expanding training in women’s health research and public health surveillance to OBGYNs, nurses and advanced practice nurses. Through fellowships and public health experiences with CDC, these clinicians will gain public health research skills to improve the health of women and children exposed to or affected by infectious diseases, mental health and substance use disorders. CDC will invite early career clinicians to train in public health and policy to become future leaders in women’s health research.
Improve Women’s Health Across the Lifespan
- Create a Comprehensive Research Agenda on Menopause. To help women get the answers they need about menopause, NIH will launch its first-ever Pathways to Prevention series on menopause and the treatment of menopausal symptoms. Pathways to Prevention is an independent, evidence-based process to synthesize the current state of the evidence, identify gaps in existing research, and develop a roadmap that can be used to help guide the field forward. The report, once completed, will help guide innovation and investments in menopause-related research and care across the federal government and research community.
- Improve Primary Care and Preventive Services for Women . The Agency for Healthcare Research and Quality (AHRQ) will issue a Notice of Intent to publish a funding opportunity announcement for research to advance the science of primary care, which will include a focus on women’s health. Through this funding opportunity, AHRQ will build evidence about key elements of primary care that influence patient outcomes and advance health equity—focusing on women of color—such as care coordination, continuity of care, comprehensiveness of care, person-centered care, and trust. The results from the funding opportunity will shed light on vital targets for improvements in the delivery of primary healthcare across a woman’s lifespan, including women’s health preventive services, prevention and management of multiple chronic diseases, perinatal care, transition from pediatric to adult care, sexual and reproductive health, and care of older adults.
- Promote the Health of American Indian and Alaska Native Women. The Indian Health Service is launching a series of engagements, including focus groups, to better understand tribal beliefs related to menopause in American Indian and Alaska Native Women. This series will inform new opportunities to expand culturally informed patient care and research as well as the development of new resources and educational materials.
- Connect Research to Real-World Outcomes to Improve Women’s Mental and Behavioral Health. The Substance Abuse and Mental Health Services Administration (SAMHSA) is supporting a range of health care providers to address the unique needs of women with or at risk for mental health and substance use disorders. Building on its current efforts to provide technical assistance through various initiatives , SAMHSA intends, contingent on available funds, to launch a new comprehensive Women’s Behavioral Health Technical Assistance Center. This center will identify and improve the implementation of best practices in women’s behavioral health across the life span; identify and fill critical gaps in knowledge of and resources for women’s behavioral health; and provide learning opportunities, training, and technical assistance for healthcare providers.
- Support Research on Maternal Health Outcomes. USDA will fund research to help recognize early warning signs of maternal morbidity and mortality in recipients of Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), and anticipates awarding up to $5 million in Fiscal Year 2023 to support maternal health research through WIC. In addition, research being conducted through the Agricultural Research Service’s Human Nutrition Research Centers is focusing on women’s health across the lifespan, including the nutritional needs of pregnant and breastfeeding women and older adults.
Stay Connected
We'll be in touch with the latest information on how President Biden and his administration are working for the American people, as well as ways you can get involved and help our country build back better.
Opt in to send and receive text messages from President Biden.

- Diversity, equity and inclusion
- Financial Decision Making
- Patient Experience
- Point of Care Tools
- Product Solutions
- Healthcare Transformation
- Data + Technology
- Safer Hospitals
- Providers in Practice
- Mergers and Acquisitions
- AI & Data Analytics
- Cybersecurity
- Interoperability & EHRs
- Medical Devices
- Pop Health Tech
- Precision Medicine
- Virtual Care
- Health equity
Healthcare, Research Could See Big Increases in 2022 Federal Budget
Lawmakers are proposing to spend billions more on some key health and science programs, but it’s a long way to the finish line.
While Congress works to approve President Biden’s “Build Back Better” plan, lawmakers are also working on a federal budget for 2022 with major implications for healthcare and hospitals.
Lawmakers are crafting spending bills that could offer big increases for medical research and healthcare programs. Both House and Senate Democrats are proposing substantial increases for the National Institutes of Health, the federal government’s primary source of funding for biomedical research.
The 2022 spending plan could also include more money for public health programs.
Overall, the federal budget could be a bonanza for research programs, said Karen Fisher, chief public policy officer for the Association of American Medical Colleges.
“We know President Biden is a very, very strong proponent of research, particularly cancer research,” Fisher said at a Nov. 10 session on politics at AAMC’s annual conference.
Democrats are working largely on their own to pass the $1.7 trillion “Build Back Better” package, which could offer hefty increases for healthcare . But the federal budget process will require some bipartisan support, notably in the Senate.
The Senate is evenly split between Democrats and Republicans. Democrats control the chamber - Vice President Kamala Harris can cast a tie-breaking vote. But a budget bill is going to need 60 votes in the Senate, Fisher noted. Federal research programs have enjoyed bipartisan support in the past.
Fisher pointed to a looming deadline for lawmakers: the federal government is only funded for a few more weeks. In September, Congress approved a short-term spending bill to fund the federal government through Dec. 3. It’s conceivable Biden and lawmakers could approve another short-term funding measure.
Fisher said she hopes lawmakers can come to an agreement on the 2022 budget before the end of the year or early next year. If the budget talks go too far into the spring, it raises the possibility lawmakers may simply vote to keep federal programs funded at their current levels, she said.
Some of the healthcare and research proposals from House Democrats typically have bigger numbers. But if the Senate Democratic proposals ultimately were approved, healthcare and research programs would still see hefty increases.
Here’s a look at what the 2022 spending proposals offer for healthcare and research, including some of the current funding levels being discussed. As lawmakers move the budget legislation forward, the numbers could change.
The National Institutes of Health would get billions more in either the Senate or House plans.
The NIH’s current budget is $42.9 billion. The Senate draft plan would direct $47.9 billion to the NIH. Under the House Democrats’ proposal, the NIH would get $49.4B, the AAMC said. Some of the money in the House and Democratic proposals would go to a new research agency.
Biden has proposed the creation of a new health research agency in the NIH: the Advanced Research Projects Agency for Health. The White House said the new agency would finance “high risk, high reward” projects aimed at biomedical breakthroughs. For health research, It’s envisioned to serve in a similar fashion as the Defense Advanced Research Projects Agency, which helped lead the development of the Internet and stealth technology
House Democrats are proposing $3 billion for the new health research agency; Senate Democrats are looking at $2.4 billion.
The U.S. Centers for Disease Control and Prevention would see major increases under the proposals. The CDC’s current budget is $7.9 billion. The Senate Democrats would direct $9.7 billion to the CDC (an increase of 23.6%); the House Democrats would offer $10.6 billion (an increase of 34.2%).
National Science Foundation
The NSF, which finances basic research, receives $8.5 billion. Both House and Senate Democrats propose boosting the agency’s budget to $9.5 billion, an increase of 12%.
Hospital preparedness
The federal budget currently directs $280.6 million for the hospital preparedness program. Senate Democrats are looking to spend $296.8 million on the program; House Democrats would move it up to $320 million.
The Agency for Health Research and Quality, which is charged with improving the nation’s healthcare system, currently gets $338 million. The AHRQ would see an increase of 12% under both the House and Senate Democratic plans, lifting the agency’s budget to $380 million.
VA Research
Both House and Senate Democratic plans offer increases in research programs for the U.S. Department of Veterans Affairs. VA research currently receives $815 million. A Senate Democrat draft would direct $882 million for VA research, while House Democrats would offer $904 million.

The rise of third party cyberattacks in healthcare | Data Book podcast
Dan Dodson, the CEO of Fortified Health Security, talks about the risks to hospitals, AI in attacks and defense, and what health systems can do to protect themselves.

American Hospital Association CEO talks about Change Healthcare ransomware attack and cybersecurity
Rick Pollack discussed the cyberattack and its ramifications at the Hospital + Healthcare Association of Pennsylvania Leadership Summit.

Assessing progress toward interoperability in healthcare | Data Book podcast
John Blair, CEO of MedAllies, talks about the strides that have been made, the work that must be done, and the hopes for TEFCA to make interoperability a reality.

How Providence is making its hospitals more sustainable
The health system has worked to reduce emissions and waste for years. Beth Schenk of Providence talks about those efforts.

Hospitals, medical schools warned to get consent for pelvic, breast, and other sensitive exams
Citing increasing concerns, the federal government advises teaching hospitals that they must obtain informed consent as part of their participation in Medicare and Medicaid.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

OMB Provides Guidance to Federal Agencies About AI Procurement, Governance
The page you recommended will be added to the "what others are reading" feed on "My ACR".
The page you bookmarked will be added to the "my reading list" feed on "My ACR".
- Yale Directories
Institution for Social and Policy Studies
Advancing research • shaping policy • developing leaders, yale-led study spurs federal action: hhs requires consent for intimate medical procedures.
The U.S. Department of Health and Human Services issued new guidance to teaching hospitals and medical schools April 1, requiring that medical providers obtain written consent before performing intimate examinations, particularly on patients under anesthesia.
The national directive marks a major milestone in a yearslong effort driven by the research and advocacy of Lori Bruce , associate director of Yale’s Interdisciplinary Center for Bioethics, supported by the Institution for Social and Policy Studies.
“This landmark achievement for patient consent was attained because countless advocates — including patients, community members, medical students, ethicists, and some physicians — spoke up despite pressure to remain silent,” Bruce said. “Our work isn’t done. We still need to ensure the careful implementation of the updated guidelines. But HHS’s commitment to patient rights is profoundly redeeming.”
In a letter signed by HHS Secretary Xavier Becerra, the department acknowledged media reports and scientific literature demonstrating how hospitals often perform pelvic, breast, and rectal examinations on unconscious patients without their informed consent. Such examinations are often medically unnecessary, Bruce said, serving as an opportunity for trainees and new clinicians to gain practical experience. But unconsented procedures can be traumatic for patients, particularly those who have experienced previous sexual trauma.
“It is critically important that hospitals set clear guidelines to ensure providers and trainees performing these examinations first obtain and document informed consent from patients before performing sensitive examinations in all circumstances,” the HHS letter said, linking to guidelines hospitals must follow to receive Medicare and Medicaid funds . “Informed consent includes the right to refuse consent for sensitive examinations conducted for teaching purposes and the right to refuse to consent to any previously unagreed examinations to treatment while under anesthesia.”
In 2020, Connecticut elected officials invited Bruce to shine a light on the ethics of consent for intimate medical examinations. She gave public lectures explaining the ethical tensions, served on the state’s strategic task force, solicited community insights through Yale’s Community Bioethics Forum , and conducted a national survey — the first of its kind — that helped instigate the passage of Connecticut’s 2022 bill requiring explicit consent for these examinations.
Bruce and her co-authors uncovered how potentially 3.6 million American women and men are likely to have received unconsented pelvic or prostate exams and that Black patients are four times more likely than white patients to report having received such unconsented exams.
In September, the national NBC Nightly News ran a story citing Bruce’s report , drawing the attention of RAINN (Rape, Abuse & Incest National Network), who sought out federal legislators who might help end the practice, Bruce said. About six weeks ago, an aide for U.S. Rep. Nancy Mace (R-South Carolina) reached out to Bruce and collaborated with her on a letter to HHS.
“It’s a step in the right direction that HHS has finally mandated hospitals obtain written consent for these invasive examinations,” Mace said. “The fact that doctors were conducting these exams on women under anesthesia without their consent or medical need is simply unacceptable.”
Bruce said that while medical professionals do not intend any harm in conducting intimate examinations on unconscious patients, research has uncovered how these practices can have a negative impact on medical students as well.

In addition, several research studies have shown that if asked for permission to conduct an extra training exam, about 90% of patients will agree, Bruce said.
“We have been risking the mental health of patients and putting students and everyone in the room watching through this moral angst for no reason,” Bruce said. “Recent studies demonstrate that obtaining explicit consent improves clinicians’ relationship with their patients.”
Connecticut State Rep. Josh Elliot (D-Hamden) recruited Bruce to help inform the Public Health Committee and overcome what he called inertia over the informed consent bill in the years before it eventually passed.
“The biggest problem is that doctors in the state were coming out and saying this was not a problem and this law would cause more problems than it would solve,” Elliot said. “Being able to undercut that argument with specific figures and surveys was necessary. This wouldn’t have happened without Lori’s help.”
Other states followed with similar laws, Bruce said, and she is unaware of any institution reporting significant harm in their ability to train the next generation of medical providers.
ISPS Director Alan Gerber , Sterling Professor of Political Science, praised Bruce for demonstrating how science can inform effective, ethical policy.
“Lori and Interdisciplinary Center for Bioethics Director Stephen Latham exemplify the ideals we strive to uphold at ISPS,” Gerber said. “The Connecticut law and new HHS guidance on consent for intimate medical exams show how expertise, tenacity, and collaboration can culminate in more sensitive and thoughtful practices for everyone.”
Bruce agreed and hopes that the lessons of this overdue correction will reverberate to other areas of medical research and practice.
“Ethical medicine is reliant on good data,” she said. “When we don’t know exactly what is happening, it is challenging to write an ethical law.”
Levamisole-associated multifocal inflammatory encephalopathy: clinical and MRI characteristics, and diagnostic algorithm
Affiliations.
- 1 Institute of Higher Nervous Activity and Neurophysiology, Butlerova street 5a, Moscow 117485, Russia. Electronic address: [email protected].
- 2 Bujanov Moscow City Clinical Hospital, Moscow, Russia.
- 3 Institute of Higher Nervous Activity and Neurophysiology, Butlerova street 5a, Moscow 117485, Russia; Federal State Budget Educational Institution of Higher Education M.V.Lomonosov Moscow State University, Moscow, Russia.
- 4 City Clinical Hospital № 24, Moscow, Russia.
- 5 Research Center of Neurology, Moscow, Russia.
- 6 Research Center of Neurology, Moscow, Russia; Pavlov First Saint Petersburg Medical University, Saint Petersburg, Russia.
- 7 Pavlov First Saint Petersburg Medical University, Saint Petersburg, Russia.
- 8 Institute of Higher Nervous Activity and Neurophysiology, Butlerova street 5a, Moscow 117485, Russia; Bujanov Moscow City Clinical Hospital, Moscow, Russia; Moscow Research and Clinical Center for Neuropsychiatry, Moscow Healthcare Department, Russia.
- 9 Moscow Research and Clinical Center for Neuropsychiatry, Moscow Healthcare Department, Russia; Pirogov Russian National Research Medical University, Moscow, Russia.
- PMID: 36450175
- DOI: 10.1016/j.msard.2022.104418
Levamisole-associated multifocal inflammatory encephalopathy (LAMIE) is a devastating adverse effect of levamisole (LEV) treatment. In Russia, people often use LEV without a doctor's prescription for anthelmintic prophylaxis. LAMIE often misdiagnosed as the first episode of MS or acute disseminated encephalomyelitis (ADEM). The aim of our study was to describe clinical, laboratory and morphological characteristics of LAMIE, magnetic resonance imaging (MRI) patterns and create an algorithm for the differential diagnosis. This study was a prospective observational study with retrospective analysis of cases. It was performed at two hospitals with ambulatory service for MS. We included 43 patients with LAMIE with follow-up was from 1 year to 5 years. Age was 19-68 y.o. with female predominance. The most typical manifestations of LAMIE were cerebellar, pyramidal and cognitive symptoms, and majority of patients had biphasic course of the disease. Three main types of MRI patterns were described: ADEM-like, MS-like, atypical demyelination. About 40% of patients had CSF specific oligoclonal bands synthesis, but only 20 % of them converted to MS during the period from 1 month until 2 years. The CSF albumin levels and immunoglobulin G index were elevated in LAMIE patients compared to reference values. We described results of brain biopsy in two cases. Therefore LAMIE should be considered in patients with demyelinating or inflammatory conditions with biphasic onset of the disease and variable MRI presentation.
Keywords: ADEM; Demyelinating disorders; Levamisole; Levamisole-associated multifocal inflammatory encephalopathy; Multiple sclerosis; Neuroinflammation.
Copyright © 2022 Elsevier B.V. All rights reserved.
Publication types
- Observational Study
- Brain / diagnostic imaging
- Brain / pathology
- Encephalomyelitis, Acute Disseminated* / pathology
- Levamisole / adverse effects
- Magnetic Resonance Imaging
- Multiple Sclerosis* / diagnostic imaging
- Multiple Sclerosis* / drug therapy
- Multiple Sclerosis* / pathology
- Retrospective Studies

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
CMS Finalizes Payment Updates for 2025 Medicare Advantage and Medicare Part D Programs
Finalized policies will continue to ensure the strength and stability of Medicare Advantage and Medicare Part D programs
Today, the Centers for Medicare & Medicaid Services (CMS) finalized the Calendar Year (CY) 2025 Rate Announcement for the Medicare Advantage (MA) and Medicare Part D Prescription Drug (Part D) Programs that updates payment policies for these programs and ensures payment accuracy. The Rate Announcement complements policies in the CY 2025 MA and Part D proposed rule that would strengthen protections for the millions of people who rely on MA and Medicare Part D prescription drug coverage, which will be finalized in the coming days. Under this CY 2025 Rate Announcement, payments from the government to MA plans are expected to increase on average by 3.70 percent, or over $16 billion, from 2024 to 2025. The federal government is projected to pay between $500 and $600 billion in Medicare Advantage payments to private health plans in 2025.
CMS is also finalizing improvements to the structure of the Medicare Part D drug benefit for CY 2025 that will result in lower drug costs for millions of people with Medicare through the concurrent release of the Final CY 2025 Part D Redesign Program Instructions. Thanks to the Inflation Reduction Act, President Biden’s lower-cost prescription drug law, annual out-of-pocket costs will be capped at $2,000 for people with Medicare Part D in 2025, leading to even more savings for people with Medicare Part D in CY 2025.
“Thanks to the President's lower cost prescription drug law, the Inflation Reduction Act, millions of people with Medicare Part D will see even lower costs next year. Your out-of-pocket costs for prescription drugs will be limited to no more than $2,000, keeping more of your money in your pocket,” said HHS Secretary Xavier Becerra. “In addition to improving the Part D drug benefit, we are updating payments to Medicare managed care plans for people who rely on these plans. The Biden-Harris Administration will continue to work on lowering health care costs for all Americans, fulfilling a promise the President made.”
“CMS continues to take steps to maintain the stability of the Medicare Advantage and Part D prescription drug programs,” said CMS Administrator Chiquita Brooks-LaSure. “The finalized policies in the Rate Announcement and the Part D Redesign Program Instructions will make improvements to keep Medicare Advantage payments up-to-date and accurate, lower prescription drug costs, and ensure that people with Medicare have access to robust and affordable health care options.”
The Rate Announcement finalizes annual updates to MA payment growth rates and changes to the MA and Part D payment methodologies to improve payment accuracy. The finalized CY 2025 Rate Announcement incorporates the most recent available fee-for-service payment data through quarter 4 of 2023 and includes the continued phase-in of the updated MA risk adjustment model that was first implemented in 2024 and continued phase-in of updates to the calculation of growth rates related to medical education costs, as well as other technical improvements. Last year, CMS finalized CY 2024 technical and clinical updates to the MA risk adjustment model to keep it up-to-date and improve payment accuracy, as well as updates to the calculation of growth rates to better account for medical education costs. For 2024, MA offerings for people with Medicare remained stable—including premiums, supplemental benefits, and choice.
“We are also pleased to finalize guidance on the new $2,000 out-of-pocket cap for prescription drugs under Medicare Part D in 2025, which was enacted in the President’s prescription drug law. This new provision will provide meaningful additional cost savings and relief to enrollees who have been facing high and rising drug costs,” said CMS Deputy Administrator and Director of the Center for Medicare Meena Seshamani, MD, Ph.D.
The finalized CY 2025 Rate Announcement may be viewed by going to: https://www.cms.gov/medicare/payment/medicare-advantage-rates-statistics/announcements-and-documents/2025
A fact sheet discussing the provisions of the finalized CY 2025 Rate Announcement, as well as frequently asked questions, can be viewed here: https://www.cms.gov/newsroom/fact-sheets/2025-medicare-advantage-and-part-d-rate-announcement
The Final CY 2025 Part D Redesign Program Instructions can be found at: https://www.cms.gov/files/document/fact-sheet-final-cy-2025-part-d-redesign-program-instructions.pdf
A fact sheet discussing the provisions of the Final CY 2025 Part D Redesign Program Instructions can be viewed here: https://www.cms.gov/files/document/fact-sheet-final-cy-2025-part-d-redesign-program-instructions.pdf
Sign Up for Email Updates
Receive the latest updates from the Secretary, Blogs, and News Releases
Subscribe to RSS
Receive latest updates

Related News Releases
Biden-harris administration finalizes rule expanding access to care and increasing protections for people with medicare advantage and medicare part d, hhs finalizes policies to make marketplace coverage more accessible and expand essential health benefits, hhs releases white paper focused on preventing drug shortages, media inquiries.
For general media inquiries, please contact [email protected] .

IMAGES
COMMENTS
Research for the People. The NIH invests most of its nearly $48 billion budget 1 in medical research for the American people.. Nearly 83 percent 2 of NIH's funding is awarded for extramural research, largely through almost 50,000 competitive grants to more than 300,000 researchers at more than 2,500 universities, medical schools, and other research institutions in every state.
This report details the National Institutes of Health (NIH) budget and appropriations process with a focus on FY2022 and FY2023. NIH is the primary federal agency charged with conducting and supporting medical, health, and behavioral research. It is made up of 27 Institutes and Centers and the Office of the Director (OD).
Federal funding for research and development (R&D) is ... (VA), the medical and prosthetic re-search budget includes $916 million encompassing direct
The annual budget funds government operations for the U.S. fiscal year, which begins October 1 and ends September 30 the following year (i.e., the FY 2022 budget will start October 1, 2021 and end September 30, 2022). However, the entire budget process is a multi-year endeavor that starts two years prior to funds being released.
On March 11, 2024, President Biden submitted to Congress his FY 2025 Budget request encompassing all Federal agencies - including a proposed budget of $50.1 billion for the NIH, excluding the Advanced Research Projects Agency for Health (ARPA-H). ... (NIH) on budget policy issues affecting the NIH, the medical research community and the public ...
topped $1.1 trillion in 2018, more than 26 times federal medical and health research and development spending that year.3,4,5 6,7,8 As the economic and human costs of deadly and debilitating diseases mount, the obvious question is: ... representing about 1% of the federal budget; spending on national defense, at about $629 billion, represents ...
Budget requests a total of $51.1 billion in funding for NIH and the Advanced Research Projects Agency for Health (ARPA-H). The Budget requests funding for ARPA-H as a separate ... research, advancing nutrition science to promote health, and to reduce the burden of diet-related diseases and nutrition health disparities, and drastically reduce ...
All of Us. In FY 2021, the All of Us Research Program continued its mission to accelerate health research and medical breakthroughs to enable individualized prevention, treatment, and care. All of Us is on its way to enrolling one million or more participants, and as of February 2022, nearly 466,000 participants have consented to join the program
In FY 2017, 1 the National Institutes of Health (NIH) reported spending nearly $1.8 billion on health services research, accounting for 59.5 percent of all federal health services research funding; however, this represents only 5.3 percent of the total budget ($33.1 billion) for NIH in that year. The Affordable Care Act (ACA) established the ...
Key Points. Congress recently finalized an appropriations package of nearly $2 trillion for fiscal year 2024, funding critical medical research and public health programs through September 30, 2024. Many programs received flat funding, which failed to keep pace with inflation. STS will continue to advocate for robust funding levels that exceed ...
We identified 2.2 million published research papers related to these drugs or targets, of which 21% acknowledged funding from the NIH totaling 332 thousand fiscal years of research funding amassing more than $230 billion. This research was also cited in 22 thousand issued US patents.
Federal spending on domestic and global health programs and services accounted for 29% of net federal outlays in fiscal year (FY) 2023 (taking into account offsetting receipts), or $1.9 trillion ...
The FY 2022 President's Budget request is $2,213.6 million, an increase of $107.7 million compared to the FY 2021 Enacted level. NIMH expects to increase funding for non-competing Research Project Grants by $31.4 million to fund projects receiving competing awards in prior years.
A primary source of federal funding for tomorrow's cures comes from the National Institutes of Health (NIH). AAMC-member institutions conduct over 50 percent of the extramural research the NIH funds, which in turn creates hope for millions of Americans affected by serious diseases. NIH-funded research has contributed to a 60 percent reduction ...
The Executive Order directs the Office of Management and Budget and the Gender Policy Council to lead a robust effort to assess gaps in federal funding for women's health research and identify ...
By Doug Lederman. Getty Images. Congress voted late last week to approve a 2024 fiscal year budget for agencies covered by six of its 12 annual appropriations bills, belatedly completing its work nearly halfway into the fiscal year. Members of Congress had approved legislation covering the other six appropriations bills earlier in March.
A version of this story appeared in Science, Vol 383, Issue 6690. Congress has given the National Institutes of Health (NIH) an essentially flat budget of $47.1 billion, in a final 2024 spending bill that lawmakers are expected to approve in time to avert a partial government shutdown this weekend. And several policy directives opposed by ...
Overall, the federal budget could be a bonanza for research programs, said Karen Fisher, chief public policy officer for the Association of American Medical Colleges. "We know President Biden is a very, very strong proponent of research, particularly cancer research," Fisher said at a Nov. 10 session on politics at AAMC's annual conference.
The Government will provide $3.9 million over 3 years from 2022-23 for innovative, evidence based mental health and suicide prevention research activities. In a separate line item in the budget, the Government will also provide $4.0 million over 2 years from 2022-23 for suicide prevention research.
Affiliations 1 Clinical Research Department, The Federal Budget Institute of Science "Central Research Institute for Epidemiology" of The Federal Service on Customers' Rights Protection and Human Well-being Surveillance Moscow, Russia.; 2 Medical Center "Eco-safety" Saint-Petersburg, Russia.; 3 Medical Center "Group of Companies "MEDSI" JSC Moscow, Russia.
The federal Office of Research Integrity (ORI) is proposing changes that would give the government more oversight of investigations of research misconduct at colleges and universities.. But scores of university and research hospital leaders and the organizations representing them are opposed and say the proposed rules would be burdensome to institutions and could potentially deter people from ...
The White House Office of Management and Budget (OMB) released a memorandum March 28, providing guidance on artificial intelligence (AI) implementation, governance and use by federal agencies. The memorandum applies broadly across the Executive Branch, and therefore will influence the digital infrastructure within agencies that regulate or research aspects of healthcare (e.g., agencies within ...
The U.S. Department of Health and Human Services issued new guidance to teaching hospitals and medical schools April 1, requiring that medical providers obtain written consent before performing intimate examinations, particularly on patients under anesthesia.. The national directive marks a major milestone in a yearslong effort driven by the research and advocacy of Lori Bruce, associate ...
"The public deserves confidence that the federal government will use the technology responsibly," said Shalanda Young, the director of the Office of Management and Budget (OMB). Agencies have ...
3 Institute of Higher Nervous Activity and Neurophysiology, Butlerova street 5a, Moscow 117485, Russia; Federal State Budget Educational Institution of Higher Education M.V.Lomonosov Moscow State University, Moscow, Russia. 4 City Clinical Hospital № 24, Moscow, Russia. 5 Research Center of Neurology, Moscow, Russia.
Government of Russia. Government Phase 1 Phase 2 Phase 3 Phase 4. Founded: Moscow Russian Federation (1993) Organization Overview. First Clinical Trial. 2001. NCT04180774. First Marketed Drug. None.
Under this CY 2025 Rate Announcement, payments from the government to MA plans are expected to increase on average by 3.70 percent, or over $16 billion, from 2024 to 2025. The federal government is projected to pay between $500 and $600 billion in Medicare Advantage payments to private health plans in 2025. CMS is also finalizing improvements ...