- Open access
- Published: 14 March 2024

Systematic review and pooled analysis of the impact of treatment-induced lymphopenia on survival of glioblastoma patients
- A. M. Saeed 1 , 2 ,
- S. M. Bentzen 1 , 3 ,
- H. Ahmad 4 ,
- L. Pham 4 ,
- G. F. Woodworth 5 &
- M. V. Mishra 1 , 2
Radiation Oncology volume 19 , Article number: 36 ( 2024 ) Cite this article
520 Accesses
Metrics details
Purpose/objective(s)
Treatment related lymphopenia is a known toxicity for glioblastoma (GBM) patients and several single-institution studies have linked lymphopenia with poor survival outcomes. We performed a systematic review and pooled analysis to evaluate the association between lymphopenia and overall survival (OS) for GBM patients undergoing chemotherapy and radiation therapy (RT).
Materials/methods
Following PRISMA guidelines, a systematic literature review of the MEDLINE database and abstracts from ASTRO, ASCO, and SNO annual meetings was conducted. A pooled analysis was performed using inverse variance-weighted random effects to generate a pooled estimate of the hazard ratio of association between lymphopenia and OS.
Ten of 104 identified studies met inclusion criteria, representing 1,718 patients. The lymphopenia cutoff value varied (400–1100 cells/uL) and as well as the timing of its onset. Studies were grouped as time-point (i.e., lymphopenia at approximately 2-months post-RT) or time-range (any lymphopenia occurrence from treatment-start to approximately 2-months post-RT. The mean overall pooled incidence of lymphopenia for all studies was 31.8%, and 11.8% vs. 39.9% for time-point vs. time-range studies, respectively. Lymphopenia was associated with increased risk of death, with a pooled HR of 1.78 (95% CI 1.46–2.17, P < 0.00001) for the time-point studies, and a pooled HR of 1.38 (95% CI 1.24–1.55, P < 0.00001) for the time-point studies. There was no significant heterogeneity between studies.
These results strengthen observations from previous individual single-institution studies and better defines the magnitude of the association between lymphopenia with OS in GBM patients, highlighting lymphopenia as a poor prognostic factor.
Introduction
Survival rates for glioblastoma remain poor despite advances in treatments that have iteratively extended survival times. The current standard of care involves maximal safe resection in combination with chemotherapy and radiation therapy (RT), followed by chemotherapy alone. Though these treatments have level I evidence for extending survival, they are associated with toxicities, some of which can be severe. An increasingly recognized treatment-related toxicity for various cancers including glioblastoma (GBM) is lymphopenia. The significance of this problem is highlighted by the well documented association between poorer survival outcomes and lymphopenia across various cancers, including GBM, esophageal, breast, cervical, lung, and pancreatic cancers [ 1 , 2 , 3 , 4 ].
The three main GBM treatments that contribute toward treatment-related lymphopenia are RT, chemotherapy, and corticosteroids – all of which are typically utilized during the course of GBM treatment, and independently contribute towards lymphopenia [ 4 ]. In addition, even prior to treatment GBM patients exhibit lymphopenia due to bone marrow sequestration of T cells [ 5 ]. The most widely used chemotherapy agent for GBM is temozolomide (TMZ) and it has myelosuppressive activity that may lead to lymphopenia [ 6 , 7 ]. In the setting of GBM, RT may induce lymphopenia due to irradiation of circulating lymphocytes, which are among the most radiosensitive cell types [ 8 , 9 ]. As modeled by Yovino et al., over the course of a typical GBM radiation therapy plan, about 99% of the circulating lymphocyte pool receives a lethal dose of radiation [ 9 ]. Combining these treatments (i.e., TMZ and RT) can lead to at least additive lymphocyte suppression. In the pre-TMZ-era, Hughes et al. demonstrated that RT-alone led to lymphopenia in about 24% of high-grade glioma patients [ 10 ]. A subsequent prospective observational study of high-grade glioma patients undergoing combination TMZ + RT found a much higher incidence of lymphopenia (40%) and lymphocytes remained suppressed for up to a year [ 10 ]. Moreover, patients with lymphopenia from Grossman et al.’s cohort had worse survival outcomes when compared with those that did not develop lymphopenia (median survival of 13.1 months vs. 19.7 months, respectively) [ 11 ].
Following this seminal publication, a number of studies have examined the association between lymphopenia and survival outcomes of GBM patients undergoing chemo-radiation therapy (CRT). In the present study we review and identify the available literature examining the association of treatment-related lymphopenia on the survival of GBM patients. In addition, we conducted a pooled analysis to better quantify and measure the magnitude of the association between treatment related lymphopenia and survival outcomes.
Literature search
This systematic review and pooled-analysis followed the PRISMA guidelines [ 12 ]. Primary clinical studies were identified by querying the PubMed MEDLINE database. The search was conducted using the following keywords: “lymphopenia”, “glioma OR glioblastoma”, “radiation OR radiotherapy”, with additional search employing MeSH terms “Lymphopenia[Mesh]”, “Glioma[Mesh]”, and “Radiotherapy[Mesh]”. Additionally, abstracts were also identified from the annual meetings of American Society for Radiation Oncology (ASTRO), American Society of Clinical Oncology (ASCO), and Society of Neuro-Oncology (SNO) using the keywords “lymphopenia”, “glioma OR glioblastoma”, “radiation OR radiotherapy”. Articles were last collected on September 2022. Inclusion criteria included the following: (1) retrospective or prospective clinical studies of human subjects (2) included high-grade glioma (HGG) patient, grade III or grade IV (where the majority of the entire cohort was HGG, and of the HGG cohort, majority were GBM/grade IV) (3) treatment involved combination chemotherapy and radiation therapy (4) reported lymphopenia outcomes (5) reported survival outcome (6) analyzed association between lymphopenia and survival. Exclusion criteria included the following: (1) low-grade glioma only patients (2) non-human studies (3) non-English language manuscripts or abstracts (4) patients treated with either chemotherapy alone/radiation therapy alone/surgery alone. In the case of manuscripts from the same institution covering overlapping inclusion times, only the most recent manuscript was included to prevent analysis of overlapping patient populations.
Statistical analysis
The primary outcome of interest from the collected studies was a determination of the hazard ratio associated between the incidence of lymphopenia and overall survival (OS). To quantify this, a fixed-effect, inverse variance-weighted analysis of the logarithm of the hazard ratio (HR) of association between lymphopenia and OS was conducted using Review Manager 5.3. The pooled HR is reported with 95% CI, and p-values are 2-sided. A chi-square test for heterogeneity, the fraction of variance due to heterogeneity (I 2 ), and a Z-test for overall effect were all estimated using RevMan.
Based on the parameters delineated in the materials and Methods sections, after screening an initial 104 potential studies (Fig. 1 ), 10 studies met the inclusion criteria and were included for analysis (Table 1 ) [ 11 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 ]. Briefly, these were studies including majority GBM patient cohorts undergoing combination chemotherapy and RT, with analysis of an association between lymphopenia and OS. Eight of the studies were single-institution retrospective series, one was a multi-center prospective observational study, and one was a single-institution phase II randomized control trial. Eight of the studies were exclusively GBM/grade 4 glioma cohorts, while one study (Grossman et al.) was entirely HGG (i.e., grade 3 and 4 with majority [85%] GBM) [ 21 ]. Another study included grade 2 gliomas (Ahn et al.), but HGG represented the majority of patients (66%), and GBM patients were the largest fraction of patients of the HGGs [ 18 ]. Collectively, the 10 included studies represent 1,718 unique patients.
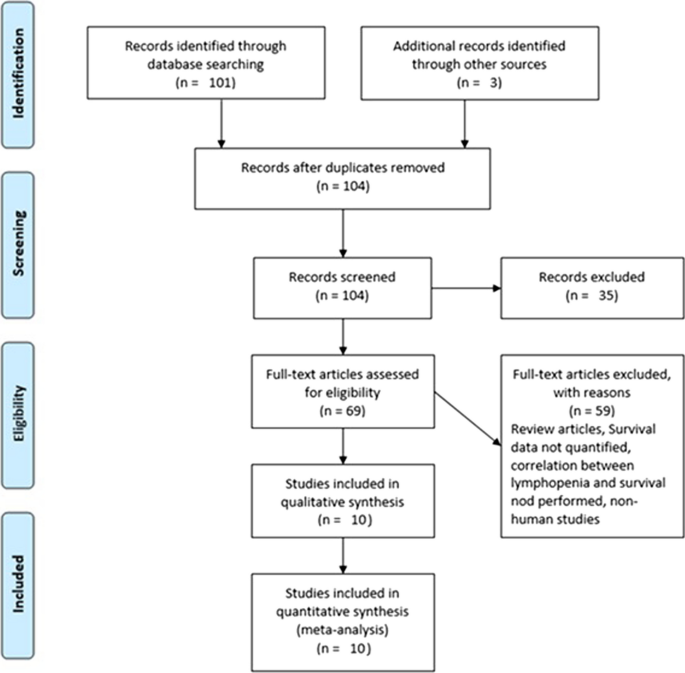
PRISMA literature review scheme
In reviewing the 10 primary studies, there was significant heterogeneity in defining lymphopenia, ranging from 200 to 1,000 total lymphocyte count per cubic centimeter. Similarly, there was not a defined time at which the incidence of lymphopenia was tabulated (e.g., during active CRT versus after CRT). Also, there was a key distinction in how lymphopenia in relation to time was reported. Five of the studies tabulated the incidence of lymphopenia defined at a fixed time-point after CRT (e.g., at 1- or 2-months post-CRT) and these series were labeled ‘time-point’ studies for the purpose of our analysis (Table 1 ). Another four studies tabulated the incidence of lymphopenia as any occurrence of lymphopenia from the start of CRT to some defined time (e.g., end of CRT, 1-month post-CRT, or 2-month post-CRT, etc.). These series were labeled ‘time-range’ studies (Table 1 ). One study (Byun et al.) reported and analyzed lymphopenia separately both as a ‘time-point’ and ‘time-range’ [ 15 ]. The overall incidence of lymphopenia for the studies ranged from 2.9 to 46.6%, with a combined average of 31.8% (utilizing the criteria of lymphopenia defined by each individual study in terms of timing and cutoff value). The average incidence of lymphopenia differed between time-point and time-range studies, measuring 11.8% and 39.9%, respectively. For each of these studies, all but one (Mohan et al.) demonstrated a statistically significant lower median OS associated with lymphopenia patients versus non-lymphopenia patients (Table 1 ) [ 19 ].
The primary objective of our analysis was to estimate the magnitude of association between lymphopenia development and OS in the published literature. Given the inherit different nature by which lymphopenia was defined between time-point and time-range studies, the analysis was dichotomized with separate pooled HR analysis performed for the time-point and time-range studies. Lymphopenia was associated with increased risk of death for both study types, with a pooled HR of 1.78 (95% CI 1.46–2.17, P < 0.00001) for the time-range studies, and a pooled HR of 1.38 (95% CI 1.24–1.55, P < 0.00001) for the time-point studies (Figs. 2 and 3 ). There was minimal overall HR heterogeneity among the studies (either for time-point or time-range) with I 2 = 0% for both.
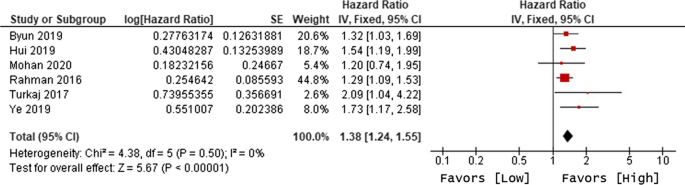
Forest plot for lymphopenia and overall survival of time-point studies
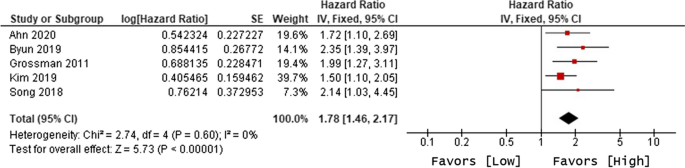
Forest plot for lymphopenia and overall survival of time-range studies
Given the increasingly recognized link between treatment-related lymphopenia and poor survival outcomes for GBM patients, we performed a systematic literature review and pooled analysis to better quantify this association. After identifying 10 studies that met our inclusion criteria, our pooled analysis confirms that GBM patients who experience lymphopenia have an inferior OS with a pooled HR of 1.78 for the time-range studies and a HR of 1.38 for the time-point studies. This significant association highlights the importance of the immune system and lymphocytes in particular for the survival of GBM patients.
Lymphocytes play a crucial role in host defense against pathogens and elimination of tumor cells. The latter mechanism may be responsible for the inferior survival of GBM patients. Grossman et al.’s seminal study was the only study that examined cause of death and demonstrated that lymphopenic patients did not develop higher rates of infection and their cause of death was almost entirely due to early tumor progression [ 11 ]. Lymphopenia is particularly important for GBM given it is classified to be an immunologically ‘cold’ tumor due to its poor response to immunotherapy [ 22 , 23 , 24 ]. Mechanistically, this is thought to be due to its immune privileged site of origin, low antigen burden, and inhibitory tumor microenvironment [ 25 , 26 ]. As highlighted by our review and analysis, another possible contributor to poor immunotherapy response may be iatrogenic lymphopenia from the current standard-of-care treatments (i.e., TMZ and RT). Checkmate-548 failed to show a benefit to the addition of nivolumab (compared to placebo) to standard TMZ + RT for newly diagnosed GBM patients [ 22 ]. The lack of efficacy may in part be due to impairment and depletion of lymphocytes, whose activity is critical to the efficacy of Nivolumab.
Efforts to mitigate treatment-related lymphopenia may improve outcomes of GBM patients. From a radiation therapy standpoint this can be accomplished by hypofractionation, reducing treatment volumes, and possibly with the use of proton therapy. Regarding reduced treatment volumes, Rudra et al. examined the impact of limiting the radiation treatment volume from targeting the MRI T2 abnormality (the standard United States volume) to targeting just the T1 contrast enhancement [ 27 ]. The T1-based planning treatment volumes (PTVs) were smaller compared to the T2-based PTVs (375 cc vs. 245.7 cc, p < 0.001), and interestingly patients treated with the smaller T2-based PTVs had a trend toward decreased lymphopenia at 3-months after the start of CRT (15.5% vs. 33.8%, p = 0.12). Their analysis also uncovered brain V25 Gy as independent predictor of developing lymphopenia, highlighting the importance of sparing radiation to uninvolved brain, which presumably spares dose to circulating lymphocytes. Another means of limiting radiation to the brain is with the use of proton therapy, due to its inherent property of lacking an exit dose. Mohan et al. examined lymphopenia (a study included in our pooled analysis) in patients on a randomized prospective phase II trial comparing proton therapy and conventional photon-based RT [ 19 ]. Their analysis found that patients undergoing proton therapy (compared with photon-based RT) had lower whole brain V25 Gy (35.3 cc vs. 43.8 cc) and had lower rates of developing lymphopenia (15% vs. 39%, P = 0.024).
Other means of mitigating lymphopenia involve directly boosting lymphocyte counts via lymphocyte re-infusion, recombinant IL-7, and transient sequestration of lymphocytes using fingolimod. Early-stage trials testing these interventions for GBM patients are already underway with the intention to improve survival outcomes [ 28 , 29 , 30 ].
Another important possible treatment-related lymphopenia contributor (other than TMZ and RT) for GBM patients is corticosteroids, which are commonly used for GBM patients to control vasogenic brain edema and associated symptoms. Corticosteroids have a well-documented lymphotoxic effect and cause lymphopenia [ 31 , 32 ]. One of our included studies, Hui et al., analyzed the impact of corticosteroid use on survival outcomes of GBM patients [ 16 ]. Results demonstrated that patients who received higher doses of corticosteroids (> 2 mg/day) had significantly higher rates of developing lymphopenia and also had decreased OS. In a meta-analysis of 22 studies by Petrelli et al., steroid use in GBM patients was also associated with decreased OS [ 33 ]. The interpretation of corticosteroid-inducted lymphopenia association with decreased OS is challenging given the known morbidity of additional corticosteroid-induced side effects (hyperglycemia, hypertension, electrolyte abnormalities, etc.). Additionally confounding the association between corticosteroid use and OS is the fact that steroids are typically only used in symptomatic patients, thus corticosteroids may be functioning as a marker of larger tumor burden and/or progressing disease.
Our review of the literature uncovered a lack of uniformity in defining lymphopenia across the various studies in terms of the cutoff value and the timing of when lymphopenia is recorded as an event. The cutoff values ranged from 400 to 1000 TLC per cc, though the majority of studies used a cutoff of < 500 TLC per cc, which is the CTCAE grade 3 lymphopenia classification [ 34 ]. This could bias the effect size estimates if the cutoff was chosen to maximize the contrast between patients with and without lymphopenia. Clearly, this bias would be inherited in our meta-analysis. Regarding the timing of lymphopenia, about half of the included studies defined lymphopenia at a time-point and the other half over a time-range. Though our separate analyses of the time-point and time-range studies both demonstrated a significant association between lymphopenia and OS, differences in these definitions should be noted. There is a greater likelihood of classifying a patient as lymphopenic with the time-range definition (compared to time-point definition) since this captures any lymphopenia event over typically a 3-month window (from start of CRT to 1-month post-CRT, for instance). In contrast, there is presumably a lesser likelihood of classifying a patient as lymphopenic using the specific time-point definition (e.g., at 1-month post-CRT) given the singularity of the allowable time. Thus, the time-range definition may inflate the incidence of lymphopenia, which is supported by our analysis demonstrating a higher incidence of lymphopenia for time-range studies when compared with time-point studies (about 40% vs. 12%, respectively). Also, the time-point versus time-range may have differing biological implications. The time-range criteria may include patients who experience lymphopenia early in the course of CRT, but ultimately recover some time after. However, the time-point definition captures patients who may have experienced lymphopenia at some point during CRT, but fail to recover or who experience persistent lymphopenia. The persistence of lymphopenia may lead to worse tumor control and other sequalae. Interestingly, Byun et al. was the only study to conduct analysis using both time-point and time-range studies [ 15 ]. They found that using either definition, lymphopenia was significantly associated with worse OS on univariate analysis; however, only the time-point definition showed significance on multivariate analysis.
Limitations of our analysis should be noted. First, the majority of the included studies were retrospective single-institution experiences, with the exception of Grossman et al. (a multicenter prospective study) and Mohan et al. (a prospective single-instruction randomized phase II clinical trial) [ 11 , 19 ]. There are inherent biases to such single-institution retrospective studies which can introduce confounding factors such as inclusion of varied patient populations (e.g., inclusion of grade III gliomas, low performance status patients, unknown MGMT status), differences in treatment (e.g., RT above and below standard 60 Gy). Second, with the exception of Mohan et al., all of the included studies were positive (i.e., found that lymphopenia correlated with worse survival) [ 19 ]. This naturally raises the possibility of positive publication bias, where analyses that did not demonstrate a correlation between treatment-related lymphopenia and survival were not published. Of note, Mohan et al. was one of the only studies in our pooled analysis that did not show lymphopenia associated with worse OS [ 19 ]. Notably, this was also the study with the fewest patients (N = 84) which affected the power to detect a given effect size. Third, given this was a pooled analysis from existing literature, patient-level data was not available and therefore patient/treatment factors could not be controlled. Further, even though individual studies created adjusted hazard ratios accounting for covariates, given each study used different prognostic factors in these models, we could not construct pooled adjusted model of the hazard ratios.
These limitations highlight the need for prospectively collected data from large well defined patient populations homogenously treated and with a predefined statistical analysis plan for testing the prognostic effect of lymphopenia. As an example of potential differences between the retrospective studies and prospective RCT data, RTOG 0825’s standard arm of patients (N = 300) undergoing standard CRT (experimental arm was standard CRT + bevacizumab) had an 7.3% incidence of lymphopenia (defined as TLC < 500 per cc, over the time-range of CRT), which is much lower than the average incidence of 40% for the time-range studies included in our analysis [ 35 ]. Encouragingly, lymphopenia is a pre-specified exploratory endpoint in the open NRG BN-001 radiation dose-escalation trial which is also evaluating outcomes in patients treated with protons vs. photons [ 36 ].
This pooled analysis shows a significant association between treatment-related lymphopenia and decreased OS in GBM patients undergoing CRT, consistent with the majority of the published studies. Future and ongoing prospective data will help confirm these findings. From a clinical standpoint, efforts to minimize lymphopenia (when feasible) would be prudent but it remains to be clarified if such measures will lead to improved outcomes for GBM patients. Further, in the expanding era of immunotherapy, it remains to be tested whether limiting or reversing lymphopenia (e.g., lymphocyte re-infusion, lymphocyte expansion with IL-7) may help uncover the yet to be realized potential of immunotherapy for the treatment of GBM.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
American Society of Clinical Oncology
American Society for Radiation Oncology
Confidence interval
Chemo-radiation therapy
Clinical treatment volume
Common terminology criteria for adverse events
Glioblastoma multiforme
High grade glioma
Hazard ratio
O6-methylguanine-DNA-methyltransferase
Magnetic resonance imaging
Overall Survival
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Planning treatment volume
Randomized control trial
- Radiation therapy
Radiation therapy oncology group
Society of Neuro-Oncology
Total lymphocyte count
- Temozolomide
Grossman SA, Ellsworth S, Campian J, Wild AT, Herman JM, Laheru D, et al. Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. JNCCN J Natl Compr Cancer Netw. 2015;13:1225–31.
Article CAS Google Scholar
Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol. 2018;123:42–51.
Article PubMed Google Scholar
Shiraishi Y, Fang P, Xu C, Song J, Krishnan S, Koay EJ, et al. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: A propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol. 2018;128:154–60. https://doi.org/10.1016/j.radonc.2017.11.028 .
Kleinberg L, Sloan L, Grossman S, Lim M. Radiotherapy. Lymphopenia, and Host Immune Capacity in Glioblastoma: a potentially actionable toxicity Associated with reduced efficacy of Radiotherapy. Clin Neurosurg. 2019;85:441–53.
Article Google Scholar
Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, Farber SH, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24:1459–68. https://doi.org/10.1038/s41591-018-0135-2 .
Article CAS PubMed PubMed Central Google Scholar
Khan RB, Raizer JJ, Malkin MG, Bazylewicz KA, Abrey LE. A phase II study of extended low-dose temozolomide in recurrent malignant gliomas. Neuro Oncol. 2002;4:39–43.
Brock CS, Newlands ES, Wedge SR, Bower M, Evans H, Colquhoun I, et al. Phase I trial of temozolomide using an extended continuous oral schedule. Cancer Res. 1998;58:4363–7.
CAS PubMed Google Scholar
Nakamura N, Kusunoki Y, Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res. 1990;123:224–7.
Article CAS PubMed ADS Google Scholar
Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31:140–4.
Hughes MA, Parisi M, Grossman S, Kleinberg L. Primary brain tumors treated with steroids and radiotherapy: low CD4 counts and risk of Infection. Int J Radiat Oncol Biol Phys. 2005;62:1423–6.
Article CAS PubMed Google Scholar
Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–80.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Article PubMed PubMed Central Google Scholar
Rahman R, Catalano PJ, Arvold ND, Aizer AA, Weiss SE, Pinnell N, et al. Chemoradiation-Related Lymphopenia Is Common Among Glioblastoma Patients and Is Associated With Worse Progression-Free and Overall Survival. Int J Radiat Oncol. 2016;96:E123.
Song AJ, Gowda S, Kim L, Werner-Wasik M, Andrews DW, Glass J, et al. Severe Lymphopenia after Chemoradiation Treatment is Associated with Worse Survival in Patients with Newly Diagnosed GBM. Int J Radiat Oncol. 2018;102.
Byun HK, Kim N, Yoon HI, Kang SG, Kim SH, Cho J, et al. Clinical predictors of radiation-induced lymphopenia in patients receiving chemoradiation for glioblastoma: clinical usefulness of intensity-modulated radiotherapy in the immuno-oncology era. Radiat Oncol. 2019;14:1–10.
Hui CY, Rudra S, Ma S, Campian JL, Huang J. Impact of overall corticosteroid exposure during chemoradiotherapy on lymphopenia and survival of glioblastoma patients. J Neurooncol. 2019;129–36.
Ye LL, Fan XW, Hu CS, He XY, Wang XS, Shen CY, et al. Dosimetry of the brain and hypothalamus predicting acute lymphopenia and the survival of glioma patients with postoperative radiotherapy. Cancer Med. 2019;8:2759–68.
Ahn S, Park JS, Jang J, Ahn KJ, Hong YK, Yang SH, et al. The association between total lymphocyte count after concomitant chemoradiation and overall survival in patients with newly diagnosed glioblastoma. J Clin Neurosci. 2020;71:21–5.
Mohan R, Liu AY, Brown PD, Mahajan A, Dinh J, Chung C et al. Proton therapy reduces the likelihood of high-grade radiation–induced lymphopenia in glioblastoma patients: phase II randomized study of protons vs photons. Neuro Oncol. 2020;1–11.
Kim WJ, Dho YS, Ock CY, Kim JW, Choi SH, Lee ST, et al. Clinical observation of lymphopenia in patients with newly diagnosed glioblastoma. J Neurooncol. 2019;143:321–8. https://doi.org/10.1007/s11060-019-03167-2 .
Campian JL, Ye X, Gladstone DE, Ambady P, Borrello I, Golightly M, et al. Feasibility of lymphocyte harvesting and reinfusion in patients with newly diagnosed high-grade gliomas. J Clin Oncol. 2014;32:2094–2094. https://doi.org/10.1200/jco.2014.32.15_suppl.2094 .
Weller M, Lim M, Idbaih A, Steinbach J, Finocchiaro G, Raval R. Randomized phase 3 study of nivolumab or placebo combined with radiotherapy plus temozolomide in patients with newly diagnosed glioblastoma with methylated MGMT promoter: checkmate 548. CTIM-25. A. Neuro Oncol. 2021;23:vi55-6.
Article PubMed Central Google Scholar
Sampson JH, Omuro AMP, Preusser M, Lim M, Butowski NA, Cloughesy TF et al. A randomized, phase 3, open-label study of nivolumab versus temozolomide (TMZ) in combination with radiotherapy (RT) in adult patients (pts) with newly diagnosed, O-6-methylguanine DNA methyltransferase (MGMT)-unmethylated glioblastoma (GBM): CheckMate-49. J Clin Oncol. 2016;34:TPS2079–TPS2079. https://doi.org/10.1200/JCO.2016.34.15_suppl.TPS2079 .
Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the checkmate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1003–10.
Lim M, Xia Y, Bettegowda C, Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol. 2018;. https://doi.org/10.1038/s41571-018-0003-5 .
Yu MW, Quail DF. Immunotherapy for glioblastoma: current progress and challenges. Front Immunol. 2021;12.
Rudra S, Hui C, Rao YJ, Samson P, Lin AJ, Chang X, et al. Effect of radiation treatment volume reduction on lymphopenia in patients receiving chemoradiotherapy for glioblastoma. Int J Radiat Oncol Biol Phys. 2018;101:217–25.
NCT02490930, A safety study of fingolimod with radiation and temozolomide. in Newly Diagnosed High Grade Glioma. https://www.clinicaltrials.gov/ct2/show/NCT02490930 .
NCT03619239, Dose-escalation study to evaluate the safety and tolerability of GX-I7 in patients with glioblastoma [Internet]. https://clinicaltrials.gov/ct2/show/NCT03619239 .
Campian JL, Ye X, Gladstone DE, Ambady P, Nirschl TR, Borrello I, et al. Pre-radiation lymphocyte harvesting and post-radiation reinfusion in patients with newly diagnosed high grade gliomas. J Neurooncol. 2015;124:307–16.
Craddock CG. Corticosteroid-induced lymphopenia, immunosuppression, and body defense. Ann Intern Med. 1978;88:564–6.
Chitadze G, Flüh C, Quabius ES, Freitag-Wolf S, Peters C, Lettau M et al. In-depth immunophenotyping of patients with glioblastoma multiforme: Impact of steroid treatment. Oncoimmunology. 6:e1358839
Petrelli F, De Stefani A, Ghidini A, Bruschieri L, Riboldi V, Dottorini L, et al. Steroids use and survival in patients with glioblastoma multiforme: a pooled analysis. J Neurol. 2020. https://doi.org/10.1007/s00415-020-09731-5 .
NCI Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. U.S. Department of Health and Human Services; 2017.
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708.
NCT02179086. Dose-escalated photon IMRT or proton beam radiation therapy versus standard-dose radiation therapy and temozolomide in treating patients with newly diagnosed glioblastoma [Internet]. https://clinicaltrials.gov/ct2/show/NCT02179086 .
Download references
MM reports research support from NCI Grant 3P30CA134274-15S1.
Author information
Authors and affiliations.
Department of Radiation Oncology, University of Maryland Greenebaum Comprehensive Cancer Center, University of Maryland School of Medicine, Baltimore, USA
A. M. Saeed, S. M. Bentzen & M. V. Mishra
Maryland Proton Treatment Center, Baltimore, MD, USA
A. M. Saeed & M. V. Mishra
Department of Epidemiology and Public Health, Division of Biostatistics and Bioinformatics, University of Maryland School of Medicine, Baltimore, USA
S. M. Bentzen
Department of Medical Oncology, University of Maryland Greenebaum Comprehensive Cancer Center, University of Maryland School of Medicine, Baltimore, USA
H. Ahmad & L. Pham
Department of Neurosurgery, University of Maryland School of Medicine, Baltimore, MD, USA
G. F. Woodworth
You can also search for this author in PubMed Google Scholar
Contributions
All authors were involved with the conception of the study. AS and MM were responsible for literature review and classification of articles. AS was responsible for all data collection. SB was responsible for data and statistical analysis. AS and MM wrote the manuscript primarily. HA, LP, and GM contributed to the manuscript writing and editing. All authors read and approved the final manuscript.
Corresponding author
Correspondence to M. V. Mishra .
Ethics declarations
Ethical approval and consent to participate.
Not applicable.
Consent for publication
All authors consent to the publication of this article.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Saeed, A.M., Bentzen, S.M., Ahmad, H. et al. Systematic review and pooled analysis of the impact of treatment-induced lymphopenia on survival of glioblastoma patients. Radiat Oncol 19 , 36 (2024). https://doi.org/10.1186/s13014-023-02393-3
Download citation
Received : 10 August 2023
Accepted : 17 December 2023
Published : 14 March 2024
DOI : https://doi.org/10.1186/s13014-023-02393-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Lymphopenia
- Glioblastoma
- Proton beam therapy
Radiation Oncology
ISSN: 1748-717X
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 05 January 2022
The 5 min meta-analysis: understanding how to read and interpret a forest plot
- Yaping Chang ORCID: orcid.org/0000-0002-0549-5087 1 , 2 ,
- Mark R. Phillips ORCID: orcid.org/0000-0003-0923-261X 1 , 3 ,
- Robyn H. Guymer ORCID: orcid.org/0000-0002-9441-4356 4 , 5 ,
- Lehana Thabane ORCID: orcid.org/0000-0003-0355-9734 1 , 6 ,
- Mohit Bhandari ORCID: orcid.org/0000-0001-9608-4808 1 , 2 , 3 &
- Varun Chaudhary ORCID: orcid.org/0000-0002-9988-4146 1 , 3
on behalf of the R.E.T.I.N.A. study group
Eye volume 36 , pages 673–675 ( 2022 ) Cite this article
79k Accesses
22 Citations
250 Altmetric
Metrics details
- Outcomes research
A Correction to this article was published on 08 May 2023
This article has been updated
Introduction
In the evidence-based practice of ophthalmology, we often read systematic reviews. Why do we bother about systematic reviews? In science, new findings are built cumulatively on multiple and repeatable experiments [ 1 ]. In clinical research, rarely is one study definitive. Using a comprehensive and cumulative approach, systematic reviews synthesize results of individual studies to address a focused question that can guide important decisions, when well-conducted and current [ 2 , 3 , 4 , 5 ].
A systematic review may or may not include a meta-analysis, which provides a statistical approach to quantitatively combine results of studies eligible for a systematic review topic [ 2 , 3 , 4 , 5 ]. Such pooling also improves precision [ 2 , 4 , 5 ]. A “forest plot” is a form of graphical result presentation [ 2 , 4 ]. In this editorial, we start with introducing the anatomy of a forest plot and present 5 tips for understanding the results of a meta-analysis.
Anatomy of a forest plot
We demonstrate the components of a typical forest plot in Fig. 1 , using a topic from a recently published systematic review [ 6 ] but replaced with mockup numbers in analysis. In this example, four randomized trials (Studies #1 to #4) are included to compare a new surgical approach with the conventional surgery for patients with pseudoexfoliation glaucoma. Outcomes of intraocular pressure (IOP) and incidence of minor zonulolysis are evaluated at 1-year follow-up after surgery.
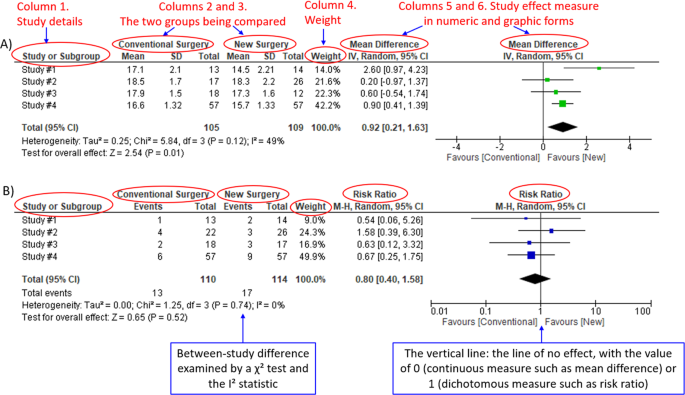
A Example of a continuous outcome measure: Intraocular pressure assessed with mean difference; B Example of a dichotomous outcome measure: Incidence of minor zonulolysis, at 1 year after surgery. Tau, the estimated standard deviation of underlying effects across studies (Tau 2 is only displayed in the random model). Chi 2 , the value of Chi-square test for heterogeneity. Random, random model (an analysis model in meta-analysis).
In a forest plot, the box in the middle of each horizontal line (confidence interval, CI) represents the point estimate of the effect for a single study. The size of the box is proportional to the weight of the study in relation to the pooled estimate. The diamond represents the overall effect estimate of the meta-analysis. The placement of the center of the diamond on the x-axis represents the point estimate, and the width of the diamond represents the 95% CI around the point estimate of the pooled effect.
Tip 1: Know the type of outcome than
There are differences in a forest plot depending on the type of outcomes. For a continuous outcome, the mean, standard deviation and number of patients are provided in Columns 2 and 3. A mean difference (MD, the absolute difference between the mean scores in the two groups) with its 95% CI is presented in Column 5 (Fig. 1A ). Some examples of continuous outcomes include IOP (mmHg), visual acuity in rank values, subfoveal choroidal thickness (μm) and cost.
For a dichotomous outcome, the number of events and number of patients, and a risk ratio (RR), also called relative risk, along with its 95% CI are presented in Columns 2,3 and 5 (Fig. 1B ). Examples of dichotomous outcomes include incidence of any adverse events, zonulolysis, capsulotomy and patients’ needing of medication (yes or no).
Tip 2: Understand the weight in a forest plot
Weights (Column 4) are assigned to individual studies according to their contributions to the pooled estimate, by calculating the inverse of the variance of the treatment effect, i.e., one over the square of the standard error. The weight is closely related to a study’s sample size [ 2 ]. In our example, Study #4 consisting of the largest sample size of 114 patients (57 in each group) has the greatest weight, 42.2% in IOP result (Figs. 1A ) and 49.9% in zonulolysis result (Fig. 1B ).
Tip 3: Pay attention to heterogeneity
Heterogeneity represents variation in results that might relate to population, intervention, comparator, outcome measure, risk of bias, study method, healthcare systems and other factors of the individual studies in a meta-analysis [ 2 , 7 ]. If no important heterogeneity is observed, we can trust the pooled estimate more because most or all the individual studies are telling the same answer [ 7 ].
We can identify heterogeneity by visual inspection of similarity of point estimates, overlapping of confidence intervals, and looking at the results of statistical heterogeneity tests outlined at near the bottom of a forest plot [ 2 , 7 ]. When more similarity of point estimates and more overlapping of confidence intervals are observed, it means less heterogeneity [ 2 , 7 ]. The P value generated by the Chi-squared test is the probability of the null hypothesis that there is no heterogeneity between studies. When P < 0.10 is shown, we reject this null hypothesis and consider that there is heterogeneity across the studies [ 2 ]. P value of 0.10 is typically used for the test of heterogeneity because of the lack of power for the test [ 2 ]. The I 2 statistic ranging from 0 to 100%, indicates the magnitude of heterogeneity. Greater I 2 indicates more heterogeneity. The I 2 below 40% may suggest not important heterogeneity; while the I 2 over 75% may suggest considerable heterogeneity [ 2 ].
For example in Fig. 1A , the point estimate of Study #1 (i.e., the between-group difference of mean IOP, 2.60 mmHg) is different from the point estimates of Studies #2 to #4 (0.20, 0.60 and 0.90 mmHg, respectively). By virtual observation of 95% CI (the horizontal lines), the 95% of Study #1 just partly overlaps with the other studies’. P -value for heterogeneity of 0.12 is relatively small but still >0.05. The I 2 of 49% indicates that a moderate heterogeneity may present [ 2 ]. In Fig. 1B , the 95% CIs of all the four studies largely overlap. The large P value for heterogeneity of 0.74 and the I 2 of 0% both indicate that no important heterogeneity is detected.
Tip 4: Understand subgroups
When heterogeneity is detected, which may indicate the unexplained differences between study estimates, using a subgroup analysis is one of the approaches to explain heterogeneity [ 2 ]. In our example, Study #3 only studied patients who were equal and below 65 years; Studies #1, 2, and 4 also reported IOP for patients of the two different age groups separately (Fig. 2 ). We can find the pooled effects of the two subgroups respectively in the forest plot: 1.1.1 over 65 years, the overall effect favours the new surgery (Section A in Fig. 2 , subtotal MD and 95% CI does not include the line of no effect, P value for overall effect <0.00001, I 2 = 0); and 1.1.2 equal and below 65 years, there is no difference between the conventional and new surgeries (Section B in Fig. 2 , subtotal MD and 95% CI includes the line of no effect, P value for overall effect is 0.10, I 2 = 0%).
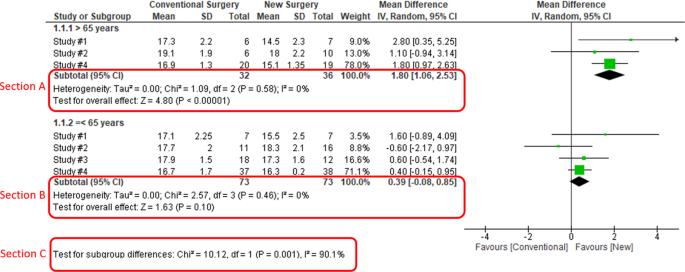
Subgroup results of IOP by age groups.
There is a subgroup effect by patients' age groups. We can find the result of test for subgroup difference in the last row of the forest plot (Section C in Fig. 2 ): P value of 0.001 and I 2 of 90.1% indicate a significant difference in treatment effects between the subgroups of patients of older or younger age.
Tip 5: Interpret the results in plain language
In our example, lower IOP and fewer zonulolysis are favoured outcomes. The statistical significance of a pooled estimate can be detected by visual inspection of the diamond (if the diamond width includes the line of no effect, there is no statistical difference between the two groups) or checking the p-value in the last row of a forest plot, “Test for overall effect” ( P < 0.05 indicates a significant difference).
In plain language, for patients with pseudoexfoliation glaucoma, the overall effect for IOP is in favour of the new surgery. More specifically, the new surgery is associated with the lower IOP compared to the conventional surgery 1 year after surgery (mean difference, 0.92 mmHg; 95% CI, 0.21 to 1.63 mmHg) with some concerns of heterogeneity and risk of bias. There is no difference in the incidence of minor zonulolysis between new and conventional surgeries.
In summary, knowing the structure of a forest plot, types of outcome measures, heterogeneity and risk of bias assessments will help us to understand the results of a systematic review. With more practice, the readers will gain more confidence in interpreting a forest plot and making application of systematic reviews’ results in your clinical practice.
Change history
08 may 2023.
A Correction to this paper has been published: https://doi.org/10.1038/s41433-023-02493-0
Zeigler D. Evolution and the cumulative nature of science. Evolution: Education Outreach. 2012;5:585–8. https://doi.org/10.1007/s12052-012-0454-6 .
Article Google Scholar
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2019.
Haynes RB. Clinical epidemiology: how to do clinical practice research. Lippincott williams & wilkins; 2012.
Murad MH, Montori VM, Ioannidis JP, Neumann I, Hatala R, Meade MO, et al. Understanding and applying the results of a systematic review and meta-analysis. User’s guides to the medical literature: a manual for evidence-based clinical practice. 3rd edn. New York: JAMA/McGraw-Hill Global. 2015.
Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64:1283–93. https://doi.org/10.1016/j.jclinepi.2011.01.012 .
Article PubMed Google Scholar
Pose-Bazarra S, López-Valladares MJ, López-de-Ullibarri I, Azuara-Blanco A. Surgical and laser interventions for pseudoexfoliation glaucoma systematic review of randomized controlled trials. Eye. 2021;35:1551–61. https://doi.org/10.1038/s41433-021-01424-1 .
Article PubMed PubMed Central Google Scholar
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clinl Epidemiol. 2011;64:1294–302. https://doi.org/10.1016/j.jclinepi.2011.03.017 .
Download references
Author information
Authors and affiliations.
Department of Health Research Methods, Evidence & Impact, McMaster University, Hamilton, ON, Canada
Yaping Chang, Mark R. Phillips, Lehana Thabane, Mohit Bhandari & Varun Chaudhary
OrthoEvidence Inc., Burlington, ON, Canada
Yaping Chang & Mohit Bhandari
Department of Surgery, McMaster University, Hamilton, ON, Canada
Mark R. Phillips, Mohit Bhandari & Varun Chaudhary
Centre for Eye Research Australia, Royal Victorian Eye and Ear Hospital, East Melbourne, Australia
Robyn H. Guymer
Department of Surgery, (Ophthalmology), The University of Melbourne, Melbourne, Australia
Biostatistics Unit, St. Joseph’s Healthcare Hamilton, Hamilton, ON, Canada
Lehana Thabane
Retina Consultants of Texas (Retina Consultants of America), Houston, TX, USA
Charles C. Wykoff
Blanton Eye Institute, Houston Methodist Hospital, Houston, TX, USA
NIHR Moorfields Biomedical Research Centre, Moorfields Eye Hospital, London, UK
Sobha Sivaprasad
Cole Eye Institute, Cleveland Clinic, Cleveland, OH, USA
Peter Kaiser
Retinal Disorders and Ophthalmic Genetics, Stein Eye Institute, University of California, Los Angeles, CA, USA
David Sarraf
Department of Ophthalmology, Mayo Clinic, Rochester, MN, USA
Sophie Bakri
The Retina Service at Wills Eye Hospital, Philadelphia, PA, USA
Sunir J. Garg
Center for Ophthalmic Bioinformatics, Cole Eye Institute, Cleveland Clinic, Cleveland, OH, USA
Rishi P. Singh
Cleveland Clinic Lerner College of Medicine, Cleveland, OH, USA
Department of Ophthalmology, University of Bonn, Boon, Germany
Frank G. Holz
Singapore Eye Research Institute, Singapore, Singapore
Tien Y. Wong
Singapore National Eye Centre, Duke-NUD Medical School, Singapore, Singapore
You can also search for this author in PubMed Google Scholar
- Varun Chaudhary
- , Mohit Bhandari
- , Charles C. Wykoff
- , Sobha Sivaprasad
- , Lehana Thabane
- , Peter Kaiser
- , David Sarraf
- , Sophie Bakri
- , Sunir J. Garg
- , Rishi P. Singh
- , Frank G. Holz
- , Tien Y. Wong
- & Robyn H. Guymer
Contributions
YC was responsible for the conception of idea, writing of manuscript and review of manuscript. MRP was responsible for the conception of idea, and review of the manuscript. VC was responsible for conception of idea, and review of manuscript. MB was responsible for conception of idea, and review of manuscript. RHG was responsible for critical review and feedback on manuscript. LT was responsible for critical review and feedback on manuscript.
Corresponding author
Correspondence to Varun Chaudhary .
Ethics declarations
Competing interests.
YC: Nothing to disclose. MRP: Nothing to disclose. RHG: Advisory boards: Bayer, Novartis, Apellis, Roche, Genentech Inc. LT: Nothing to disclose. MB: Research funds: Pendopharm, Bioventus, Acumed – unrelated to this study. VC: Advisory Board Member: Alcon, Roche, Bayer, Novartis; Grants: Bayer, Novartis – unrelated to this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: in part 'TIP 4: UNDERSTAND SUBGROUPS', the phrase "In our example, Study #3 only studied patients over 65 years" was corrected to read "In our example, Study #3 only studied patients who were equal and below 65 years".
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Chang, Y., Phillips, M.R., Guymer, R.H. et al. The 5 min meta-analysis: understanding how to read and interpret a forest plot. Eye 36 , 673–675 (2022). https://doi.org/10.1038/s41433-021-01867-6
Download citation
Received : 11 November 2021
Revised : 12 November 2021
Accepted : 16 November 2021
Published : 05 January 2022
Issue Date : April 2022
DOI : https://doi.org/10.1038/s41433-021-01867-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Estimate the burden of malnutrition among children with cerebral palsy in sub-saharan africa: a systematic review with meta-analysis.
- Ermias Sisay Chanie
- Natnael Moges
- Sewunt Sisay Chanie
Scientific Reports (2024)
Surrogate markers of metabolic syndrome and insulin resistance in children and young adults with type 1 diabetes: a systematic review & meta-analysis (MetS and IR in T1DM)
- Sukeshini B. Khandagale
- Vinesh S. Kamble
- Satyajeet P. Khare
International Journal of Diabetes in Developing Countries (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Peak oxygen uptake in Paralympic sitting sports: A systematic literature review, meta- and pooled-data analysis
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Centre for Elite Sports Research, Department of Neuromedicine and Movement Science, Faculty of Medicine and Health Science, Norwegian University of Science and Technology, Trondheim, Norway
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing
Affiliations Centre for Elite Sports Research, Department of Neuromedicine and Movement Science, Faculty of Medicine and Health Science, Norwegian University of Science and Technology, Trondheim, Norway, Department of Physical Medicine and Rehabilitation, St. Olav’s University Hospital, Trondheim, Norway
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing
- Julia Kathrin Baumgart,
- Berit Brurok,
- Øyvind Sandbakk

- Published: February 23, 2018
- https://doi.org/10.1371/journal.pone.0192903
- Reader Comments
3 Jul 2018: The PLOS ONE Staff (2018) Correction: Peak oxygen uptake in Paralympic sitting sports: A systematic literature review, meta- and pooled-data analysis. PLOS ONE 13(7): e0200326. https://doi.org/10.1371/journal.pone.0200326 View correction
Peak oxygen uptake (VO 2peak ) in Paralympic sitting sports athletes represents their maximal ability to deliver energy aerobically in an upper-body mode, with values being influenced by sex, disability-related physiological limitations, sport-specific demands, training status and how they are tested.
To identify VO 2peak values in Paralympic sitting sports, examine between-sports differences and within-sports variations in VO 2peak and determine the influence of sex, age, body-mass, disability and test-mode on VO 2peak .
Systematic literature review and meta-analysis.
Data sources
PubMed, CINAHL, SPORTDiscus TM and EMBASE were systematically searched in October 2016 using relevant medical subject headings, keywords and a Boolean.
Eligibility criteria
Studies that assessed VO 2peak values in sitting sports athletes with a disability in a laboratory setting were included.
Data synthesis
Data was extracted and pooled in the different sports disciplines, weighted by the Dersimonian and Laird random effects approach. Quality of the included studies was assessed with a modified version of the Downs and Black checklist by two independent reviewers. Meta-regression and pooled-data multiple regression analyses were performed to assess the influence of sex, age, body-mass, disability, test mode and study quality on VO 2peak .
Of 6542 retrieved articles, 57 studies reporting VO 2peak values in 14 different sitting sports were included in this review. VO 2peak values from 771 athletes were used in the data analysis, of which 30% participated in wheelchair basketball, 27% in wheelchair racing, 15% in wheelchair rugby and the remaining 28% in the 11 other disciplines. Fifty-six percent of the athletes had a spinal cord injury and 87% were men. Sports-discipline-averaged VO 2peak values ranged from 2.9 L∙min -1 and 45.6 mL∙kg -1 ∙min -1 in Nordic sit skiing to 1.4 L∙min -1 and 17.3 mL∙kg -1 ∙min -1 in shooting and 1.3 L∙min -1 and 18.9 mL∙kg -1 ∙min -1 in wheelchair rugby. Large within-sports variation was found in sports with few included studies and corresponding low sample sizes. The meta-regression and pooled-data multiple regression analyses showed that being a man, having an amputation, not being tetraplegic, testing in a wheelchair ergometer and treadmill mode, were found to be favorable for high absolute and body-mass normalized VO 2peak values. Furthermore, high body mass was favourable for high absolute VO 2peak values and low body mass for high body-mass normalized VO 2peak values.
The highest VO 2peak values were found in Nordic sit skiing, an endurance sport with continuously high physical efforts, and the lowest values in shooting, a sport with low levels of displacement, and in wheelchair rugby where mainly athletes with tetraplegia compete. However, VO 2peak values need to be interpreted carefully in sports-disciplines with few included studies and large within-sports variation. Future studies should include detailed information on training status, sex, age, test mode, as well as the type and extent of disability in order to more precisely evaluate the effect of these factors on VO 2peak .
Citation: Baumgart JK, Brurok B, Sandbakk Ø (2018) Peak oxygen uptake in Paralympic sitting sports: A systematic literature review, meta- and pooled-data analysis. PLoS ONE 13(2): e0192903. https://doi.org/10.1371/journal.pone.0192903
Editor: Nicola Bragazzi, University of Genoa, School of Public Health, ITALY
Received: October 18, 2016; Accepted: January 12, 2018; Published: February 23, 2018
Copyright: © 2018 Baumgart et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files.
Funding: The review was funded by the Centre for Elite Sports Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
1. Introduction
The Paralympic Games are the world’s second largest sporting event, and athletes with 10 different eligible physical impairments [ 1 ] participated in 23 summer disciplines in Rio 2016 and will participate in 6 winter disciplines in Pyoengchang 2018 ( https://www.paralympic.org/sports ). Of these, 16 of the summer sports and 5 of the winter sports disciplines have at least one sitting class. Depending on the eligibility criteria of each sitting sports discipline, athletes with impaired muscle power, impaired passive range of movement, limb deficiency, leg length difference, hypertonia, ataxia and athetosis are allowed to compete ( https://www.paralympic.org/sports ). Even though performance in all Paralympic sitting sports disciplines is mainly dependent on the work done by the upper body, the physical demands vary within a spectrum from typical endurance sports requiring high aerobic energy delivery over sustained periods to those performed with relatively low levels of displacement and corresponding low aerobic demands [ 2 ].
As an indicator of the humans’ maximal ability to deliver energy aerobically, the measurement of maximal oxygen uptake (VO 2max ) is regarded as the “gold standard” [ 3 ]. However, during exercise employing relatively low muscle mass, like in upper-body modes, the cardiorespiratory system is not fully taxed and VO 2max is rarely reached even in able-bodied participants [ 4 , 5 ]. In such cases, peak oxygen uptake (VO 2peak ) denotes the highest oxygen uptake reached during exercise to voluntary exhaustion [ 3 ] and is a common indicator of peak aerobic energy delivery capacity during upper-body exercise.
In sitting endurance sports with a continuously high physical effort, VO 2peak is suggested to be a paramount determinant of performance [ 6 ]. Whereas VO 2max values are available for elite athletes in a wide range of Olympic sports disciplines [ 7 – 10 ], only one study by Bhambhani et al. [ 11 ] provides a general overview of VO 2peak values in trained male wheelchair athletes. However, the latter study does not systematically report VO 2peak values for the individual Paralympic sitting sports disciplines. A systematic literature review on VO 2peak in Paralympic sports disciplines may, therefore, improve the scientific understanding of sport-specific aerobic demands, which is of importance for scientists as well as coaches and athletes. Furthermore, VO 2peak values of sitting sport athletes provide clinicians with a framework of what is possible to achieve in terms of peak aerobic capacity when exercising with a given modality and disability. This might be of relevance for providing feedback to their patients once they start engaging in a particular sitting sport activity.
In addition to the sport-specific demands, disability-related physiological limitations also influence VO 2peak in athletes with a disability. One study provided absolute VO 2peak in well-trained spinal cord injured (SCI) individuals (1.0–1.2 vs. 2.0–2.3 L∙min -1 for tetraplegic (TETRA) vs. paraplegic (PARA), respectively) [ 12 ]. In the latter study, large differences in VO 2peak were found even within the well-trained individuals with different levels of SCI [ 12 ]. Whereas the focus in the few previous studies is on the influence of the different levels of SCI on VO 2peak [ 12 , 13 ], there is lack of knowledge on how VO 2peak is influenced in Paralympic sitting sports athletes with other common disabilities, such as amputations, spina bifida and poliomyelitis. Furthermore, in the studies that focus on individuals with SCI, an inverse relationship between level of SCI and VO 2peak has been shown [ 14 ]. One may therefore expect high within-sports variation in VO 2peak in Paralympic sitting sports, since they include athletes with a large heterogeneity in disabilities.
Therefore, the purpose of this systematic literature review and meta-analysis was to (i) identify VO 2peak values for Paralympic sitting sports, (ii) examine between-sports differences and within-sports variations in VO 2peak and (iii) determine the influence of sex, age, body-mass, disability, test-mode and study-quality on VO 2peak . We hypothesized that VO 2peak values would be highest in Paralympic endurance sports with continuously high physical efforts over sustained periods. The lowest VO 2peak values were expected in sports with low levels of displacement and sports where athletes with large disability-related physiological limitations, such as athletes with tetraplegia, participate. Furthermore we expected that within-sports variation would be highest in sitting sports disciplines where athletes with a wide range of disabilities are included.
We conducted a systematic literature review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [ 15 ]. Additionally, we registered the study protocol a priori in the International Prospective Register of Systematic Literature Reviews (PROSPERO) under the following registration number: CRD42015025134.
2.1 Eligibility criteria
Athletes with a physical disability above the age of 15, who were participating in sitting sports, were eligible for inclusion. An athlete was defined as a person who participates “[…] in an organized team or individual sport requiring systematic training and regular competition against others […]”[ 16 ] at least on a national level. This rather broad definition may have resulted in the inclusion of some athletes that cannot be considered “elite”. Athletes with a cognitive impairment were not included, since we would have not been able the separate the influence of the cognitive versus the physical disability on VO 2peak . Studies were included if absolute or body-mass normalized VO 2peak values were directly measured in a standardized laboratory setting. Studies that measured VO 2peak in a field setting were excluded due to lack of standardization. Only full-text, cross-sectional and intervention studies published in peer-reviewed journals in English, German or French were considered. Abstracts and conference proceedings were not eligible due to lack of detailed reporting of methods and results.
2.2 Data sources and search strategy
PubMed, CINAHL (through EBSCOhost), SPORTDiscus TM (through EBSCOhost) and EMBASE were systematically and independently searched by JKB and BB in October 2016 using relevant medical subject headings, keywords and a Boolean search string. The search string combined synonyms and MeSH terms (the latter only relevant for our search in PubMed) of the two parts of the research question: peak oxygen uptake (outcome measure) and sitting athletes with a disability (population) (see S1 Fig ). We decided to construct a broad search string to limit the potential of missing out on studies meeting our inclusion criteria. References of the included studies were searched manually and main research groups in the field were contacted for further identification of studies relevant to the research question.
2.3 Study selection
After eliminating duplicate s articles, the titles were screened by JKB and BB. We only excluded titles that we were certain not to fit in the area of our review topic (e.g. the title being off topic, the title clearly stating that patients/able-bodied participants were investigated, etc.). Studies that did not directly mention VO 2peak in their title but were likely to have included it as a secondary outcome measure, were also included. In a second step, the abstracts of studies deemed relevant by title were read. Articles considered relevant by abstract, were then read in full-text. Details on the studies that were included or excluded based on abstract and full-text, and reasons for the excluded studies can be found in attachment S1 Excel file , sheet “study selection”. All disagreements in the selection process were resolved by discussion between JKB and BB. The two reviewers were not blinded to the names of the authors of the included studies. If multiple studies from the same research group included the same data, only the first published study or the study with the most comprehensive information was included.
2.4 Data extraction
Data on the sports discipline competed in, the characteristics of the participants (number of participants, sex, age, body mass, type of disability and training status), test mode and peak oxygen uptake (absolute and body-mass normalized VO 2peak values) was extracted from the included studies by JKB with BB cross-checking all the data. Where necessary the unit of the training data was converted from minutes to hours and from miles to kilometers.
In the absence of a valid allometric scaling method that is generalizable to athletes with different disabilities [ 17 ], we chose to extract and report absolute and body-mass normalized VO 2peak values. When studies did not report absolute VO 2peak values (L∙min -1 ), these were calculated by multiplying the individual body-mass normalized VO 2peak values (converted from mL to L) by the respective participants’ body mass. When body-mass normalized VO 2peak values (mL∙kg -1 ∙min -1 ) values were not reported, these were calculated by dividing the individual absolute VO 2peak values (converted from L to mL) by the individual body mass (in kg -1 ). When body-mass was not provided, this was calculated by dividing the individual absolute VO 2peak values (converted from L to mL) by the individual body-mass normalized VO 2peak values. In case of missing individual data, these calculations were not possible and data are not reported accordingly.
2.5 Assessment of methodological quality
The quality of the included studies was assessed by JKB and BB with a modified version of the Downs and Black checklist [ 18 ]. Modified versions of this checklist have been employed in several reviews in the field of sports science, which also mainly used cross-sectional studies for data retrieval [ 19 – 21 ]. The original checklist comprises 27 items, which are distributed over five sub-scales: reporting (item 1–10), external validity (item 11–13), bias (item 14–20), confounding (items 21–26) and power (item 27) [ 18 ]. For the purpose of the present review the following 12 items were included: 1–3, 5–7, 11, 12, 20–22 and 25. The other items were excluded since our review did not focus on interventions or differences between groups, where statistical considerations needed to be made and significance values or power would have been important. The term ‘patient’ was replaced by participant and ‘treatment’ was interpreted in the context of testing as described by Hebert-Losier et al [ 21 ]. The ‘source population’ was defined as all athletes with a disability within the respective sports discipline. All items, except item number 5, were rated as ‘Yes’ (1 point), ‘No’ (0 points) or ‘Unknown’ (0 points). For item 5, sex, age, weight, type of disability and training status were considered to be core confounders [ 17 ]. Test mode as well as the time of testing within the season were determined to be secondary confounders. Item 5 was scored with 2 points if all core confounders were mentioned. 1 point was scored if 4 out of the 5 core confounders and 1 secondary confounder were explained. ‘No’ or ‘Unknown’ were scored with 0, as described above. As we regarded the core confounders to be sufficiently assessed in item 5, we chose to in more detail address the determination criteria for VO 2peak in item 25. As no uniform criteria for the determination of maximal effort exist in a VO 2peak test in an upper-body mode, we defined our own minimum criteria. In accordance with Leicht et al. [ 22 ], these criteria should be viewed as a way to exclude studies in which maximal effort was clearly not reached rather than to confirm that VO 2peak was reached. In case studies ‘Not applicable’ (N/A) was added as a fourth option for items 7, 11, 12, 21 and 22; and items rated as such were excluded from the analysis. The modified version of the Downs and Black checklist used in this literature review can be found in the S1 Table . Quality cut-off points were decided on retrospectively and studies were ranked to be of low (0–5 points), moderate (6–8 points) or good (9–13 points) methodological quality. The level of evidence for each sports discipline was ranked from unknown to strong by combining the quality scores of each of the studies included in the respective discipline (see Table 1 ). The case studies were excluded from the analysis on level of evidence.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0192903.t001
2.6 Statistics
All data are presented as means ± standard error (SE) and 95% confidence intervals (CI) unless specified otherwise. A meta-analysis, which is defined as “[…] the use of statistical techniques to integrate and summarize the results of included studies.”[ 15 ], was performed by grouping together studies that determined VO 2peak in the same sports discipline. Sports discipline means were calculated in Microsoft Excel 2016 (Microsoft Cooperation, Washington, USA) by pooling study means by the random effects approach described more in detail by DerSimonian and Laird [ 24 ]. In connection to this, TETRA athletes were previously shown to display significantly lower VO 2peak values compared to athletes with other disabilities [ 25 , 26 ]. Therefore, to lower the variation around the mean and to increase the sensitivity of the statistical tests, only the studies where it was possible to remove the VO 2peak data from TETRA athletes were included in the pooling procedure. The only exception was wheelchair rugby where all athletes included had TETRA and all studies in this sports discipline were pooled.
Between-sports differences were analyzed in Microsoft Excel by a one-way ANOVA with Tukey-Kramer Q tests to localize pair-wise differences based on study means and pooled study variances. An α level of 0.05 was employed to indicate statistical significance. To investigate the influence of each of the included studies on the VO 2peak values presented for the different sports disciplines, leave-one-out sensitivity analyses were performed in Stata 14.2 (StataCorp LLC, Texas, USA). Furthermore, cumulative meta-analyses were conducted to investigate possible VO 2peak changes as a function of time for each of the sport disciplines.
A meta-regression was performed in Stata 14.2 to investigate the relationship between absolute and body-mass normalized VO 2peak values, respectively, and the following 11 factors (levels of categorical factors are presented in brackets): age, body mass, percentage of men in each study (%Men), percentage of athletes with tetraplegia (%TETRA), paraplegia (%PARA), an amputation (%AMP), spina bifida (%SB), poliomyelitis (%PM) and athletes with other disabilities (%LA), test mode (arm crank ergometry (ACE), wheelchair ergometry (WERG) and wheelchair treadmill (treadmill) and study quality (moderate, good). Studies that provided information on all factors either as group or individual athlete data were used in the meta-regression. Because of too few studies with complete information, individual athlete data was included where the standard error was replaced by the standard deviation of all participants within each respective study. The levels “poling” and “handbiking” for the factor test mode and the level “low” for the factor study quality were excluded from the meta-regression. This is due to these levels providing only few data points for each factor. Baseline levels for dummy coding the two categorical factors test mode and study quality were “ACE” and “good”, respectively. Only factors that significantly contributed to the model and decreased the Tau 2 estimate were included in the final meta-regression model. Before performing the meta-regression analyses, the variables were checked for multicollinearity.
A pooled-data multiple regression analysis was performed in IBM SPSS Statistics 24.0 (SPSS Inc., Chicago, USA) to investigate the relationship between absolute and body-mass normalized VO 2peak values, respectively, and the following six factors (levels of the categorical factors are presented in brackets): age, body mass, sex (male, female), disability (TETRA, PARA, amputation (AMP), spina bifida), test mode (ACE, WERG, treadmill) and study quality (low, moderate, good). Pooled data of studies that provided individual athlete data on all factors was used in the multiple regression analysis. Excluded from the regression analysis were the levels Les Autres and poliomyelitis for the factor disability, and poling and handcycling for the factor test mode. This is due to these levels comprising less than five percent of the data points of these two factors. Study quality was not entered in the multiple regression analysis as a factor due to too few data points with the level “low” and “good”. Baseline levels for dummy coding the three categorical factors disability, test mode and study quality were “PARA”, “ACE” and “good”, respectively [ 27 ]. Only factors that significantly contributed to the model and increased the adjusted R 2 were included in the final regression model. Before performing the regression analyses, the data set was checked for outliers and multicollinearity, and each variable was tested for normality and homoscedasticity of residuals.
Sports discipline was not included in the meta-regression and multiple regression analyses due to multicollinearity with several of the other included factors. Furthermore, only data in the sports disciplines wheelchair basketball, wheelchair tennis, wheelchair racing and wheelchair rugby was included due to too few data points in other sports disciplines.
All figures and tables including information on VO 2peak values are arranged according to absolute VO 2peak values from highest to lowest values.
3.1 Study selection and characteristics of included athletes
The systematic search resulted in 6542 studies. After removal of duplicate articles and the subsequent screening process, 57 full text studies were included. These 57 studies reported VO 2peak values in 771 athletes from 14 different Paralympic sitting sports disciplines ( Fig 1 ). Athletics was divided into its two sub-disciplines, throwing disciplines and wheelchair racing due to the distinct differences in movement demands. No VO 2peak values were reported for wheelchair boccia, para-canoeing, para-equestrian, para-rowing, para-sailing, sitting volleyball, para-triathlon, and para-biathlon.
The sports disciplines presented in the box at the bottom are ranked according to their absolute peak oxygen uptake (VO 2peak ) values, from highest to lowest. * Note that 1) some of the studies provide values for more than one sports discipline and 2) athletics was divided into throwing events and wheelchair racing due to the distinct differences in movement demands between these two sub-disciplines.
https://doi.org/10.1371/journal.pone.0192903.g001
3.2 Methodological quality
Agreement on all assessed quality items was reached by JKB and BB. Four studies were ranked as having low and 6 studies as having good methodological quality ( S2 Table ). No quality label was attached to the 2 included case-studies. The remaining 45 studies were regarded to have moderate methodological quality. The quality of the studies that are included in each sports discipline determines the level of evidence of the VO 2peak values.
3.3 Between-sports differences
Mean absolute and body-mass normalized VO 2peak ± standard error (SE) of the sports disciplines ranged from 2.9 ± 0.3 L∙min -1 and 45.6 ± 5.1 ml∙kg -1 ∙min -1 in Nordic sit skiing to 1.4 ± 0.2 L∙min -1 and 17.3 ± 3.5 ml∙kg -1 ∙min -1 in shooting and 1.3 ± 0.1 and 18.9 ± 1.6 in wheelchair rugby. In Table 2 an overview of absolute and body-mass normalized VO 2peak values of all sports disciplines with more than one study with at least 3 participants is provided. In this overview, several factors, such as sex, age, body mass, type of disability, training status and test modes are grouped together. Table 3 and the regression analyses provide details on the influence of these factors on absolute and body-mass normalized VO 2peak . In the sports with a strong level of evidence and a large number of included studies (wheelchair basketball, wheelchair racing and wheelchair rugby), leave-one-out analyses, examining the effect of each of the included studies, did not have a great impact on neither absolute nor body-mass normalized VO 2peak values ( S1 Excel file , sheet “MetaInf Output”). However, in sports with a low level of evidence and few included studies, omitting some of the studies had a larger impact on the VO 2peak values. With regards to the cumulative meta-analysis, wheelchair basketball and wheelchair racing showed a relatively stable VO 2peak over time, whereas wheelchair rugby showed a trend towards an increase in VO 2peak ( S1 Excel file , sheet “MetaCum Output”). For all other sports, changes over time could not be investigated due to the few number of included studies.
Sports disciplines are presented in order of absolute VO 2peak values, from high to low.
https://doi.org/10.1371/journal.pone.0192903.t002
Mean age and body mass ± SE are presented of each sports discipline are presented in the grey lines.
https://doi.org/10.1371/journal.pone.0192903.t003
3.4 Within-sports variations
Within-sports variations in absolute and body-mass normalized VO 2peak values, based on CI ranges ( Table 2 ), were relatively small in wheelchair basketball (0.4 L∙min -1 and 7.2 mL∙kg -1 ∙min -1 ), wheelchair racing (0.6 L∙min -1 and 7.4 mL∙kg -1 ∙min -1 ) and wheelchair rugby (0.4 L∙min -1 and 6.1 mL∙kg -1 ∙min -1 ), but above 0.6 L∙min -1 and 7.5 mL∙kg -1 ∙min -1 for the remaining sport disciplines. CI’s for absolute and body-mass normalized VO 2peak values could not be reported for throwing, wheelchair curling and archery, and for body-mass normalized values in Para ice hockey, as only one study with a sample size of more than two athletes was included for each of these sports disciplines.
3.5 Meta-regression analyses
The meta-regression analyses, based on 35 studies that provided data of 26 sub-groups and 171 individual athletes in 4 different sports disciplines (wheelchair basketball, wheelchair racing, wheelchair tennis and wheelchair rugby), resulted in the following two equations as the best predictions of absolute (1) and body-mass normalized (2) VO 2peak values.
The factors included in Eq ( 1 ) all significantly contribute to the model (all p < 0.001) and explain 77% of the variance in absolute VO 2peak . The coefficients presented in the model are unstandardized.
The factors included in Eq ( 2 ) all significantly contribute to the model (all p < 0.001) and explain 82% of the variance in body-mass normalized VO 2peak . The coefficients presented in the model are unstandardized
3.6 Pooled-data multiple regression analyses
The multiple regression analyses, based on 22 studies which provided individual data of 169 athletes in 4 different sports disciplines (wheelchair basketball, wheelchair racing, wheelchair tennis and wheelchair rugby), resulted in the following two equations as the best predictions of absolute (3) and body-mass normalized (4) VO 2peak values.
The factors included in Eq ( 3 ) all significantly contribute to the model (all p < 0.01) and explain 65% of the variance in absolute VO 2peak . The coefficients presented in the model are unstandardized [and standardized].
The factors included in Eq ( 4 ) all significantly contribute to the model (all p < 0.01) and the model explain 65% of the variance in body-mass normalized VO 2peak . The coefficients presented in the model are unstandardized [and standardized].
4. Discussion
This systematic literature review aimed to (i) identify VO 2peak for Paralympic sitting sports, (ii) examine between-sports differences and within-sport variations in VO 2peak and iii) determine the influence of other factors on VO 2peak . The main finding is that VO 2peak values in general reflect the sport-specific demands and the type of disability of the athletes who compete in the respective sitting sports disciplines. VO 2peak values range from 2.9 L∙min -1 and 45.6 ml∙kg -1 ∙min -1 in Nordic sit skiing, an endurance sport with a continuously high physical effort over sustained periods, to 1.4 L∙min -1 and 17.3 ml∙kg -1 ∙min -1 in shooting, a sport with low levels of displacement, to 1.3 L∙min -1 and 18.9 ml∙kg -1 ∙min -1 in wheelchair rugby, a sport that includes athletes with TETRA. Within-sports variation in absolute and body-mass normalized VO 2peak was relatively small in the sports with high sample sizes and a strong level of evidence, i.e. wheelchair basketball, wheelchair racing and wheelchair rugby, but above 0.5 L∙min -1 and 8 mL∙kg -1 ∙min -1 in all other sports. Since the VO 2peak values presented for each of the sports disciplines include data of athletes that differ in their sex, age, body mass, type of disability, training status and the mode they were tested in, we additionally conducted regression analyses. These analyses show that being a man, having an amputation, not being tetraplegic, testing in a wheelchair ergometer and treadmill mode, were favorable for high absolute and body-mass normalized VO 2peak values. Furthermore, high body mass was favourable for high absolute VO 2peak values and low body mass for high body-mass normalized VO 2peak values.
In line with our hypothesis, Nordic sit skiing, an endurance sport with continuously high physical efforts, was the Paralympic sitting sport with the highest observed absolute and body-mass normalized VO 2peak values. Although endurance disciplines by nature require high aerobic energy delivery, VO 2peak values may be particularly high in Nordic sit skiers since they compete in varying terrain, which requires both high absolute VO 2peak values to accompany the relatively large upper-body muscle mass required to produce sufficient power on flat terrain, as well as high body-mass normalized VO 2peak values to carry their body mass up inclines. The same applies to their able-bodied counterparts, standing cross-country skiers, who have shown some of the highest VO 2max values among Olympic athletes [ 9 , 81 , 82 ], although VO 2peak values in elite Nordic sit skiers are lower due to less active muscle mass while being tested in an upper-body mode and the adverse influence of having a disability. For example, athletes with a SCI display lower VO 2peak values, which is mainly related to loss of motor- and sympathetic nervous system control below the level of injury. Depending on the level and extent of injury, a SCI is associated with a range of autonomic dysregulations, which amongst other things attenuates exercise performance [ 83 ]. In fact, an inverse relationship between the level of SCI and VO 2peak has been found [ 14 ]. There is, however, lack of knowledge in terms of the difference in VO 2peak between the different disabilities represented in Paralympic sitting sports. Hutzler et al.[ 84 ] examined the aerobic power of fifty well-trained individuals with lower limb impairments including SCI, polio and amputations during arm-cranking tests in a standardized laboratory setting. It was found that individuals with high and low SCI (above and below T5, respectively) displayed lower aerobic power compared to individuals with lower limb amputations [ 84 ], which may also reflect a difference in VO 2peak between the SCI and other types of disability [ 85 ].
Even though not significantly different from some of the other sports disciplines, we also observed relatively high absolute VO 2peak values for Para ice hockey. Although this sport is characterized by short, repeated sprints requiring maximal power and speed production, aerobic capacity was shown to be highly correlated to the maintenance of sprint ability [ 86 ]. Furthermore, the high absolute VO 2peak values in Para ice hockey players may also be related to their large amount of upper-body muscle mass, which is required to produce power in sport-specific situations. In addition, the lack of a classification system in Para ice hockey allows athletes to perform on a high international level only if they possess good trunk control and the influence of disability is minimal, such as in athletes with a low level SCI or a lower limb amputation. Accordingly, we would have expected a low within-sports variation in the VO 2peak values of Para ice hockey players who are a homogenous group of Paralympic athletes with respect to gender and disabilities. However, the low number of studies and participants included in the present review in this sports discipline resulted in a limited level of evidence and wider confidence intervals for both absolute and body-mass normalized VO 2peak as compared to sports with a larger number of studies and participants. Therefore, the values presented might not be representative for the population of Para ice hockey players and we need to be cautious in our interpretation of VO 2peak values in this sport.
Wheelchair racing and handcycling are endurance sports where athletes need to sustain power over longer periods and, therefore, display relatively high body-mass normalized as well as absolute VO 2peak values. In fact, wheelchair racers in the present meta-analysis were found to display the second highest body-mass normalized VO 2peak values, which further highlights the high aerobic demands in this sports. Even though there is some variation within wheelchair racing for both absolute and body-mass normalized VO 2peak values, the relatively large amount of studies and participants in this sport resulted in a strong level of evidence and in narrower confidence intervals as compared to other sports disciplines with fewer studies and participants. The variance that remains can partially be explained by the classification system that allows athletes with a broad spectrum of disabilities to compete against each other in separate classes. For example, in athletes with a SCI, VO 2peak may be lower compared to athletes with other disabilities due to lack of sympathetic control to the paralyzed trunk and lower limbs [ 83 ] and even lower in individuals with a complete SCI above T6, who additionally lack innervation to the splanchnic area [ 87 ]. Moreover, individuals with TETRA and autonomic completeness of the injury lack sympathetic innervation to the heart and display considerably lower heart rates than athletes with other disabilities [ 88 ]. We, therefore, decided to exclude studies that included athletes with TETRA from the overview table to limit the variability in VO 2peak . Since all these factors may influence VO 2peak , the extent to which disability affects VO 2peak largely differs between Paralympic athletes, and may explain at least part of the variation in Paralympic sitting disciplines with different disability classes as compared to disciplines without. However, very small sample sizes in each of the disability classes, lack of detailed reporting on disability classes in most of the studies, and a change in the division of classes over time, prevented us from investigating the effect of disability classes on variation in VO 2peak . Concluding from the above, differences in VO 2peak values between sports are fairly well reflected by the sport-specific demands and, therefore, highest in sports with continuously high physical efforts. However, they are also influenced by the heterogeneity in disabilities between athletes and the number of studies and athletes within each sports discipline, which in turn lead to differences in the magnitude of within-sport variations.
Shooting is a sport with low levels of displacement and consequently low aerobic demands. This was also reflected by the low VO 2peak values revealed in this sport. However, caution is warranted in the interpretation of the VO 2peak values in shooting despite a moderate level of evidence due to wide confidence intervals, as a result of the few studies with small sample sizes in this sports discipline. In contrast to shooting, the within-sports variation is lower and the level of evidence strong in wheelchair rugby, which increases our ability to interpret the VO 2peak values in this sport with more accuracy. In wheelchair rugby, a sport only eligible to athletes with impairments in both the upper and the lower limbs, the low VO 2peak values can be explained by the extent of the impairment. A study by West et al. [ 88 ] found that in athletes with TETRA physiological responses, including VO 2peak , were lower as compared to other disabilities and varied based on autonomic completeness of the SCI. However, the competitiveness has increased in wheelchair rugby and athletes are training more today than previously. This is likely reflected by the increase of VO 2peak over time in this sport. Maybe the care (e.g. catheterization) and the access to better-adapted training facilities (e.g. endurance training equipment such as lying handbikes with supportive handles) and modalities (e.g. electrostimulation while exercising) have improved more over the last years in tetraplegic athletes than in athletes with other disabilities. Overall, VO 2peak values are lowest in sports with low levels of displacement or sports which include athletes with TETRA. However, the certainty in the interpretation of these values depends on the level of evidence and the within-sports variation, which are dependent on the amount of studies and sample sizes included in each sports discipline.
The VO 2peak values presented for each of the sports disciplines include data of athletes that differ in their sex, age, body mass, type of disability, training status and the mode they were tested in. Therefore, the effect of VO 2peak was considered in the meta-regression and pooled-data multiple regression analyses. These analyses indicate that being a man, having an amputation, not being tetraplegic, testing in a WERG or treadmill mode, as well as having higher or lower body mass, respectively, is favorable for high absolute and body-mass normalized VO 2peak . The finding that being a man is beneficial for VO 2peak is also in line with previous studies [ 89 , 90 ]. Tetraplegia may negatively influence VO 2peak due to a small amount of innervated muscle mass and a lack of autonomic innervation as previously discussed. In addition, that a higher body mass is beneficial for high absolute VO 2peak and lower body mass for high body-mass normalized VO 2peak was shown previously [ 91 ]. Furthermore, the finding that the WERG mode resulted in higher VO 2peak values compared to ACE is in line with two previous studies [ 92 , 93 ], although several studies also report no differences between modes [ 94 – 97 ]. The reason for the clear differences between employing WERG or wheelchair treadmill testing as compared to ACE might be related to the former two modes being more sports-specific for the athletes included in the regression analyses, who all participated in wheelchair sports. Smaller coefficients for the WERG and the wheelchair treadmill mode might be expected if sitting athletes of the non-wheelchair sports are tested. The extent to which these coefficients would decrease is speculative though, as most of the latter athletes are likely using a wheelchair in at least some parts of daily life. In this context the influence of the test protocol on VO 2peak should also be taken into consideration. During ACE, an increased crank rate led to increases in test time and VO 2peak [ 98 ], whereas similar values were found in stepwise as compared to ramp type protocols [ 99 ]. Even though caution is required when drawing conclusions from the meta-regression and pooled-data multiple regression analysis, our findings provide a point of departure for understanding the influence of the above-mentioned factors on VO 2peak in Paralympic sitting sports athletes.
Methodological considerations
VO 2peak values were provided for only 14 out of 21 Paralympic sitting sports disciplines. This is partly due to new sports disciplines being added to the Paralympic games. For example, athletes competed in para-triathlon and para-canoeing for the first time in the Paralympic games in Rio 2016, and VO 2peak values in these disciplines are hence missing. So far, the only sports where a considerable number of VO 2peak values was provided with a strong level of evidence and we can hence conclude with more certainty are wheelchair basketball, wheelchair racing and wheelchair rugby with 234, 205 and 114 included athletes, respectively. Thus, more studies and bigger data pools established through international collaborations are required. Alternatively, systematically combining the results of multiple studies in a literature review and meta-analysis can compensate for the small sample sizes in original studies in the Paralympic field. However, to allow for more valid analyses, future studies are encouraged to provide sufficient detail on outcome measures in their abstracts and to provide individual, more detailed anthropometric and training data of their athletes. Furthermore, possible changes in the demands of the sports and improvements in performance and physiological capacity over the years should further be elucidated in future studies.
The studies included in the current review vary widely in terms of test equipment, such as ergospirometers and weighing scales, as well as the test mode, warm-up procedure and test protocol (stepwise increments in resistance, speed, incline or a combination of speed and incline). The effect of variations in these factors on upper-body VO 2peak in athletes with a disability remains unclear. To enable a more valid comparison of findings between studies, future studies should aim at providing enough details on the above mentioned factors and on finding standardized criteria for determination of VO 2peak in upper-body exercise modes.
In case of the present literature review, the consequences of publication bias are not only related to being able to publish data with significant findings and/or positive findings. It may also be related to the nature of elite sports where many countries test their best athletes without publishing this information. The reason may be two-fold, since giving away interesting information may help competitors and/or simply because publishing would be too resource demanding. Furthermore, data of Paralympic athletes might not be published because of a too low number of participants included to run statistical analyses on the data or due to the tested athletes not being considered elite, which was especially the case a few decades ago. Therefore, the average VO 2peak values presented here might not fully reflect the VO 2peak of medal-winning elite athletes in many of the sports. However, we are confident that in the sports with a strong level of evidence (wheelchair basketball, wheelchair racing and wheelchair rugby), the ranges provided for the VO 2peak values reflect the aerobic capacity of athletes in the respective sports. Although we limited the effect of publication bias by excluding articles with a complete overlap of data, we cannot exclude that duplicate data of individual athletes is included in this review. The likelihood of publication bias is illustrated by the fact that 15 of the articles included are from the research-group of Goosey-Tolfrey et al.[ 36 , 39 – 43 , 45 , 46 , 53 – 55 , 64 , 65 , 72 , 79 ]. Additionally, VO 2peak was a secondary measure in many of the reviewed studies. We, therefore, screened a large amount of abstracts of studies that did not directly mention VO 2peak in their title in order to reduce the possibility to miss articles that could have fit our inclusion criteria.
A limitation in the present review is that information on training status, which is known to influence VO 2peak , was missing in a considerable amount of studies.
In the absence of a valid allometric scaling method for athletes with different disabilities and across various sports, we provided only absolute and body-mass normalized values in this review. However, we refer to the studies of Batterham et al. [ 100 ] for the challenges surrounding the units in which VO 2peak is presented and to Goosey-Tolfrey et al. [ 17 ] for the only study on scaling of VO 2peak values in athletes with a disability.
5. Conclusion
In the current review, VO 2peak values for Paralympic sitting sports were systematically reported in 14 out of 21 possible sitting sports disciplines. Of these, VO 2peak was highest in the typical endurance sports and lowest in sports with low levels of displacement and in those including athletes with TETRA. However, the only sports where a sufficient number of VO 2peak values are combined with a strong level of evidence, thereby allowing us to conclude with more certainty, are wheelchair basketball, wheelchair racing and wheelchair rugby. In contrast, VO 2peak values should be interpreted carefully in disciplines with limited level of evidence or with only one study mean and in disciplines with large within-sports variations. Large within-sports variation was found in sports with few included studies and corresponding low sample sizes. The VO 2peak values presented for each of the sports disciplines include data of athletes that differ in their sex, age, body mass, type of disability, training status and the mode they were tested in. The influence of these factors on VO 2peak was investigated in regression analyses, which indicated that–in wheelchair basketball, wheelchair racing, wheelchair tennis and wheelchair rugby athletes–being a man, having an amputation, not being tetraplegic, testing in a wheelchair ergometry or treadmill mode, was beneficial for attaining high absolute or body-mass normalized VO 2peak values. Furthermore, high body mass was favourable for high absolute VO 2peak values and low body mass for high body-mass normalized VO 2peak values. In general, the practical applications of this review are limited due to most sports disciplines having large within-sports variations in VO 2peak , a limited level of evidence or including only one study mean. Based on the findings of this study and as a take-home message for future studies, we encourage the use of standardized determination criteria for reaching VO 2peak , and the inclusion of more detailed information on training status, sex, age, body mass, type of disability and testing mode, as well as larger study samples from international collaborations.
Supporting information
S1 fig. boolean search string..
Boolean search string with combined synonyms and MeSH terms (the latter only for the search in PubMed), which was entered into the data bases.
https://doi.org/10.1371/journal.pone.0192903.s001
S1 Table. Adjusted black and downs checklist.
https://doi.org/10.1371/journal.pone.0192903.s002
S2 Table. Quality scores of the 57 included studies.
Scoring of quality items for each of the studies included in this systematic literature review on peak oxygen uptake in Paralympic sitting sports.
https://doi.org/10.1371/journal.pone.0192903.s003
S3 Table. PRISMA 2009 checklist.
https://doi.org/10.1371/journal.pone.0192903.s004
S1 Excel file. Extracted and analyzed data of this systematic literature review on peak oxygen uptake in Paralympic sitting sports.
https://doi.org/10.1371/journal.pone.0192903.s005
Acknowledgments
The support of the staff at the medical and health library at the St.Olav’s University hospital in checking our search strategy and providing us with missing articles, as well as the help of Øyvind Salvesen with the statistical analysis, is highly appreciated.
- View Article
- PubMed/NCBI
- Google Scholar
- 27. Field A. Discovering statistics using IBM SPSS statistics: Sage; 2013.
- 90. Zinner C, Sperlich B, Wahl P, Mester J. Classification of selected cardiopulmonary variables of elite athletes of different age, gender, and disciplines during incremental exercise testing. Springerplus. 2015;4:544.
- Open access
- Published: 11 December 2023
Neuromuscular diseases associated with COVID-19 vaccines: a systematic review and pooled analysis of 258 patients
- Amirhossein Tayebi 1 na1 ,
- Parham Samimisedeh 1 na1 ,
- Elmira Jafari Afshar 1 ,
- Saeideh Mahmoudnia 2 ,
- Nesa Milan 3 ,
- Aryan Ayati 4 ,
- Aryan Madady 2 &
- Hadith Rastad 1
BMC Neurology volume 23 , Article number: 437 ( 2023 ) Cite this article
1648 Accesses
33 Altmetric
Metrics details
Neuromuscular diseases (NMD) emerged as one of the main side effects of the COVID-19 vaccination. We pooled and summarized the evidence on the clinical features and outcomes of NMD associated with COVID-19 vaccination.
We comprehensively searched three databases, Medline, Embase, and Scopus, using the key terms covering “Neuromuscular disease” AND “COVID-19 vaccine”, and pooled the individual patient data extracted from the included studies.
A total of 258 NMD cases following COVID-19 have been reported globally, of which 171 cases were Guillain-Barré syndrome (GBS), 40 Parsonage-Turner syndrome (PTS), 22 Myasthenia Gravis (MG), 19 facial nerve palsy (FNP), 5 single fiber neuropathy, and 1 Tolosa-Hunt syndrome. All (100%) SFN patients and 58% of FNP patients were female; in the remaining NMDs, patients were predominantly male, including MG (82%), GBS (63%), and PTS (62.5%).
The median time from vaccine to symptom was less than 2 weeks in all groups. Symptoms mainly appeared following the first dose of vector vaccine, but there was no specific pattern for mRNA-based.
COVID-19 vaccines might induce some NMDs, mainly in adults. The age distribution and gender characteristics of affected patients may differ based on the NMD type. About two-thirds of the cases probably occur less than 2 weeks after vaccination.
• Neuromuscular diseases (NMD) have emerged as a significant side effect of COVID-19 vaccination.
• The study provides a comprehensive summary of the clinical features and outcomes of NMD associated with COVID-19 vaccination.
• A total of 258 cases of NMD following COVID-19 vaccination were reported globally.
• The most commonly reported NMDs were Guillain-Barré syndrome (GBS) and Parsonage-Turner syndrome (PTS).
• Symptoms typically appeared within 2 weeks after vaccination, with a higher likelihood following the first dose of vector vaccine.
Peer Review reports
Introduction
In the long term, vaccination remains vital in the COVID-19 pandemic control strategy [ 1 ]. More than 13.3 billion COVID-19 vaccines have been administered globally; however, the fading of immunity over time necessitates booster doses; hence, about 1 million shots are still administered daily worldwide [ 2 ].
However, the extraordinary speed of vaccine production and the waiver of essential testing steps raised hesitancy regarding the vaccines’ safety [ 3 , 4 , 5 ]. A wide range of side effects are reported worldwide shortly after vaccination. While most side effects of vaccines are mild, their impact on the central and peripheral nervous systems often necessitates medical interventions [ 6 , 7 , 8 , 9 , 10 ].
Recently, increasing case series/reports have characterized the clinical course in patients with some types of peripheral neuropathy (Guillain-Barré syndrome, Parsonage-Turner syndrome, Small fiber neuropathy, and Tolosa-Hunt syndrome), cranial neuropathy (Facial nerve palsy), and neuromuscular junction disorder (Myasthenia gravis) [ 11 ] following COVID-19 vaccination. In this study, we pooled and summarized available evidence to enhance the knowledge of the course and prognosis of the neuromuscular diseases associated with COVID-19 vaccines, which can lead to a well-timed diagnosis and management of these patients.
We conducted this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Patient consent or ethical approval was not required due to the review nature of the study. All studies reporting the symptoms and clinical course of patients with neuromuscular disease (NMD) associated with the COVID-19 vaccine were included.
We comprehensively searched three databases, Scopus, Medline, and Embase, on 25 January 2023 and updated on 20 February 2023, using a combination of the key terms in multiple domains, including “COVID-19 vaccine”, “Peripheral neuropathies”, “Cranial neuropathy”, “neuromuscular disease”. Also, we checked the reference lists and citing publications of the included articles for any additional eligible studies. We imported all retrieved citations into Zotero 6.0.20 and removed the duplicate items.
Inclusion process and criteria
Two researchers [AT and PS] independently screened the titles, abstracts, and full texts of the studies to include the eligible articles. All studies meeting our eligibility criteria were included:
Reporting new-onset case (s) of any NMD following COVID-19 vaccination
An observational design: case series, case reports, and cohort studies
Written in English
Data extraction
Three researchers [EJ, SM, NM] extracted the data from the retrieved articles into a predefined form in Microsoft Excel (version 2019, Microsoft Corp., Redmond, WA, USA). The extracted data included the first author’s name, year, country, age, gender, clinical data, type of the NMD, subtype of the disease, vaccine type, vaccine dose number (first, second, booster), the time between injection and symptom onset, presenting signs and symptoms, electrophysiological findings, laboratory data, treatment, and patients follow up.
Quality assessment
The quality of the included case series was assessed independently by two trained researchers (AA and SM) using the Joanna Briggs Institute (JBI) appraisal tool adapted for case series [ 12 ]; a third researcher (AT or HR) resolved any disagreement. JBI appraisal tool included 10 items, each receiving one score (Supplementary Table 1 ).
Statistical analyses
To organize the extracted individual patient data (IPD) and to produce tables, we used Microsoft Excel (version 2019, Microsoft Corp., Redmond, WA, USA). Based on the disease, we categorized integrated IPD case series and case reports into a relevant case series. After excluding cases without data for the characteristic of interest, we calculated a valid percent for each categorical and a median (Interquartile range) or mean and standard deviation (SD) for continuous variables. Data analysis was conducted using Stata/MP Version 16 (Stata Corp. LP, USA/ METAN package).
After excluding duplicates ( n = 244) and ineligible items from the 879 publications identified in the initial search, 133 case reports/case series met our eligibility criteria. All included studies had a quality score greater than 7/10 (Supplementary Table 1 ).
Based on our pooled analyses, a total of 258 cases of NMD associated with COVID-19 vaccines have been reported globally. In order by frequency, the type of NMD was Guillain-Barré syndrome (GBS, n = 171), Parsonage-Turner syndrome (PTS, n = 40), Myasthenia gravis (MG, n = 22), Facial nerve palsy (FNP, n = 19), Small fiber neuropathy (SFN, n = 5), and Tolosa-Hunt syndrome ( n = 1) (Fig. 1 ). Reported cases were mainly adults (98%) aged 18 years or older, and in more than two-thirds of them, the symptoms appeared less than 2 weeks after the vaccination.
PRISMA flow chart of the eligible study selection
- Guillain-Barré syndrome
Seventy-eight articles (57 case reports [ 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 ] and 21 case series [ 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 ]) containing 171 COVID-19 vaccine-associated GBS patients were reported. About half of the cases were reported from South Korea ( n = 21), Italy ( n = 20), The USA ( n = 18), and Australia ( n = 18) (Supplementary Tables 2 and 3 ).
The reported cases were aged between 14 and 90 years old, with a median (IQR) of 58 [ 43 , 70 ] years, and 108 (63%) of cases were male. Regarding vaccine type, 31% (52/168) of patients received an mRNA and 69% (116/168) a vector-based vaccine. In most cases vaccinated with vector-based vaccines, symptoms appeared after the first dose (First/ Second doses (n): 105/ 11); however, such a pattern was not observed in cases vaccinated with mRNA-based vaccines (First/ Second/ Booster doses (n): 34/ 16/ 2). The median (IQR) time from vaccine to symptoms was 13 [ 8 , 17 ] days, ranging from 1 to 44 days. Regarding GBS pathogenic subtypes, 81% (77/95) of patients had acute inflammatory demyelinating polyneuropathy, 12% (11/95) acute motor axonal neuropathy, and 7% (7/95) acute motor and sensory axonal neuropathy. GBS clinical variants and pathogenic subtypes are summarized in Table 1 .
Brain MRI was diagnostic for GBS in only 20 out of 69 patients (sensitivity: 29%); however, Electromyogram / Nerve conduction study (EMG / NCS) was diagnostic in almost all cases (131/135, 97%). Regarding laboratory tests, Albuminocytological dissociation and Anti-ganglioside antibodies were positive in 85% (105/123) and 24% (17/70) of patients, respectively. Based on the reported data, most patients were treated with IVIG only (107/142, 75%); 7% (10/142) underwent IVIG plus plasma exchange treatment, and 6% (9/160) received plasma exchange alone (Table 1 ). At the follow-up, complete or partial recovery was noted for most patients (65/123 (53%) and 46/123 (37%), respectively). However, death occurred in 3 patients (2%), and 11 out of 123 patients (9%) had a poor recovery.
- Parsonage-turner syndrome
A total of 40 patients with PTS associated with COVID-19 vaccines [ 77 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 ]. Most cases were reported from The USA ( n = 15) and South Korea ( n = 13) (Table 1 , Supplementary Table 4 ).
The median (IQR) age was 50 [ 38 , 63 ] years, ranging from 14 to 84 years, and 25 (62.5%) patients were male. Regarding vaccine type, 70% of cases ( n = 28) received mRNA-based vaccines. While in most of the patients with vector vaccine-associated PTS, the symptoms appeared on the first dose of the vaccine (10/12), such a pattern was not observed in mRNA vaccine-related PTS (First/Second doses (n): 14/14).
Of the 38 cases reporting the symptoms localizations, 30 (79%) experienced symptoms ipsilaterally on the injection side, 5 contralateral to the injection side, and 3 bilateral. The presenting symptoms were shoulder or arm pain (20/28), muscle weakness (17/28), and paresthesia (8/28). The median (IQR) interval from vaccine injection to symptoms was 8 [ 5 , 15 ] days, ranging from 1 to 56 days. EMG / NCS was diagnostic in 85% (22/26) of the patients, and brachial plexus MRI in 31% (11/35). The follow-up duration varied among the studies. However, a complete recovery was noted in 41% (15/37) of patients, a partial recovery in 41% (15/37), and a poor improvement in symptoms in 18% (7/37) (Table 1 , Supplementary Table 5 ).
- Facial nerve palsy
Nineteen FNP cases were reported following COVID-19 vaccination [ 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 ] across the world. The patients’ ages ranged from 17 to 62 years (median (IQR): 38 [ 34 , 57 ] years), 11 out of 19 patients were female (58%), and 4 patients had a prior history of FNP. In 14 patients, the symptoms occurred after receiving mRNA-based (Pfizer or Spikevax) and 5 after vector-based vaccines (AstraZeneca, Covaxin, Sputnik V, or Johnson and Johnson). Symptoms appeared mainly after the first dose in mRNA- and vector-based vaccine-associated FNP patients. Almost all patients had an ipsilateral FNP (17/19). The time interval from vaccine injection to symptoms ranged from 1 to 18 days, with a median (IQR) of 3 [ 2 , 6 ] days (Table 1 , Supplementary Table 6 ).
Regarding medications, 10 (56%) patients were treated with corticosteroids alone, 6 (33%) with both corticosteroids and a viral agent. Corticosteroids were combined with Fluorometholone eye drops in one case and with a topical antibiotic in another patient. At the follow-up, the symptoms had resolved in all patients without any complications.
- Small fiber neuropathy
We included three articles (two case reports [ 128 , 129 ] and one case series [ 130 ]) reporting five COVID-19-associated small fiber neuropathy (SFN) in our study (Table 1 , Supplementary Table 7 ). These patients were reported from Austria ( n = 3) and the USA ( n = 2). All five patients were female, aged between 32 and 64, and had received mRNA vaccines (Pfizer and Spikevax). In 4 out of 5 patients, symptoms appeared after receiving the second dose.
The presenting symptoms were weakness (2/5), gait disturbances (2/5), dysesthesia (2/5), paresthesia (1/5), and dysphagia (1/5). Skin punch biopsy helped the diagnosis in all cases (4/4). Treatment was reported in 4 patients. Except for patient #1, who was treated with corticosteroid and IVIG, the other patients only received symptomatic therapies. The outcome was reported in 3 of the patients; symptoms resolved in all three in less than 2weeks (Table 1 ).
Tolosa-hunt syndrome
One Tolosa-Hunt patient from the USA was included in our study [ 131 ] (Supplementary Table 7 ). The patient was a 45-year-old male who received a vector-based vaccine. The patient presented with left-sided headache, periorbital pain, ptosis, and decreased visual acuity. The brain CT indicated a sinus thrombosis, while the brain MRI reported a perineural enhancement surrounding the optic nerve sheath. The patient underwent steroid treatment, and the symptoms improved in 2 months.
- Myasthenia gravis
Twenty-two Myasthenia Gravis (MG) patients associated with COVID-19 vaccines were reported from eight countries [ 8 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 ], more frequently from the UK ( n = 7) and Italy ( n = 5) (Supplementary Tables 8 and 9 ).
Patients age ranged from 13 to 90 years (median (IQR): 64 [ 50 , 74 ] years) and 18 of them were male (18/22). Most patients (14/22) received an mRNA-based vaccine (Pfizer-BioNTech or Spikevax). Vector-based vaccine receivers developed symptoms mainly after the first dose (7/8); however, such a pattern was not observed in patients who received mRNA-based vaccines (First/ second/ third doses (n): 5/ 5/ 4, Table 2 ).
The presenting symptoms were binocular diplopia (10/13), ptosis (6/13), dysarthria (3/13), dysphagia (3/13), and/or weakness of the lower or upper limbs (1/13). The median (IQR) time interval from vaccine injection to symptoms was 6 (2, 8.5) days. In terms of MG type, 10 patients had an ocular or bulbar MG, and the rest generalized MG (Table 2 ).
Imaging evaluations were performed for some patients to rule out other causes, including brain CT scan (abnormal findings: 0/5), brain MRI (abnormal findings: 1/6), chest CT scan (abnormal findings: 2/8, Thymoma or thymic hyperplasia) (Table 2 , Supplementary Table 9 ).
Repetitive nerve stimulation (RNS) was positive in 13 out of 15, and Single fiber electromyography (SFEMG) in all 4 patients who received the test. Acetylcholine receptor (AChR) antibody was positive in 16 out of 20 patients and Muscle-specific kinase (MuSK) negative in all 4 tested patients. Complete improvement at discharge/1-month follow-up was reported in 79% (11/14), a myasthenic crisis in 14% (2/14), and unchanged symptoms at 3 months in 7% (1/14) of the cases.
Based on our comprehensive literature review, a total of 171 GBS, 40 PTS, 22 MG, 19 FNP, 5 SFN, and 1 Tolosa-Hunt syndrome cases following COVID-19 vaccination have been reported so far across the world. Overall, reported cases were mainly adults; FNP patients (median age: 38 years) were the youngest, and MG patients (64 years) were the oldest group. While all SNF and most FNP cases were female, in other groups, the male gender was predominant. The median time from vaccine to symptom was less than 2 weeks in all groups, ranging from 3 days in FNP patients to 13 days in GBS cases. Symptoms mainly appeared following the first dose of vector-based vaccine, but there was no specific pattern for mRNA-based associated NMD. While most reported cases experienced a benign course of the disease, death occurred in 3 out of 123 GBS patients, and 2 out of 14 MG patients developed a myasthenic crisis.
The occurrence of NMD has been reported following influenza and other vaccines [ 133 , 144 , 145 , 146 , 147 , 148 ]. A pooled analysis of GBS following mass immunizations with Influenza, Human papillomavirus, and Measles-Rubella vaccines, including 58 studies (2,110,441,600 participants), revealed an incidence rate of 3.09 per million in 6 weeks of vaccination, without a sex difference. Contrary to GBS induced by COVID-19 vaccines, most GBS cases associated with other vaccines were younger than 18 or older than 60 years old; their different target population could explain this dissimilarity. Furthermore, based on the CDC’s vaccine adverse events reporting system (VAERS) database, 944 individuals developed FNP after other vaccines in the United States between 2009 and 2018 [ 149 ]. A little more than half of the patients were female (55.7%), and only 2.2% had a prior FNP history. A similar gender pattern was observed in FNP following COVID-19 vaccines. However, a prior history of FNP was reported in 21% of patients.
Also, a total of 42 definite incident cases of MG occurred following Influenza and Hepatitis B vaccines in American adults between 1990 and 2017 [ 150 ]; these cases had a higher mean (SD) time from vaccine to symptoms compared to MG cases associated with COVID-19 vaccines (10 (10.7) vs 7.1(6.6), respectively). Overall, MG is mainly diagnosed between 10 and 70 years old [ 23 , 24 ], and affected female patients are younger than their male counterparts (mean age: 28 vs. 42 years, respectively) [ 25 ]. Gender ratio differs by MG type; contrary to pure ocular or bulbar MG, cases with generalized MG are predominantly female (female/male ratio: 3:2 or higher) [ 26 ]. Less than 40% of MG cases following other vaccines were older than 60 years (Mean age (SD): 49.0 (18.9) years, Range: 18 to 84 years), without a gender predominance [ 22 ]. However, COVID-19 vaccine-associated MG cases were predominantly male, overall, and by MG type, and half of them were aged 70 years or older (Mean age (SD): 61.0 (19.3) years).
In this study, we summarized data on COVID-19 vaccine-associated NMD provided by case reports/series. The exact mechanism by which vaccines could induce neuromuscular diseases remains unknown. However, the immunization processes following the vaccines could provoke an autoimmune response by establishing an inflammatory environment. If vaccine antigens mimic self-antigens, the immune response could cross-react with self-antigens, leading to an autoimmune reaction (Molecular mimicry mechanism). Furthermore, during immune responses to vaccine antigens, inflammatory signals can activate self-reactive T cells involved in the autoimmune processes (Bystander effect mechanism). Also, the activation of self-reactive T cells could be triggered via self-antigens released upon self-tissue damage following inflammatory cascade (Epitope spreading mechanism). Vaccines containing dsRNA or its analogs can also overexpress IFN-b, a key factor in thymic events leading to MG [ 3 ].
The temporal association of neuromuscular disorders and COVID-19 vaccination cannot easily be translated into a causal relationship, and the evidence in this regard is inadequate [ 151 ]. Thus, further studies are required to clarify this association.
SFN is categorized as primary and secondary to vaccination or diseases such as kidney failure, diabetes, infections, or autoimmune. As Finsterer et al.129 noted, the clinical presentation of COVID-19 vaccine-associated SFN is similar to other secondary SFNs. Also, evidence on the beneficial effects of IVIG supports the autoimmune nature of the SFN associated with COVID-19 vaccines.
The median time from vaccine to symptom was less than 2 weeks in all NMD, mainly after the first dose; a case can be made that vaccination may have exacerbated an already existing but asymptomatic form in the vaccinated individuals instead of triggering a new onset of the disease.
Limitation and strengths
This review acknowledges certain limitations inherent in our study design. Firstly, due to the nature of the collected data, We couldn’t analyze the link between the number of COVID-19 vaccine doses and neuromuscular disorders. Furthermore, we couldn’t assess the effects of mixing different COVID-19 vaccines for primary and booster doses. Our study can’t conclusively establish a causal relationship between COVID-19 vaccination and neuromuscular events. Few cases of neuromuscular disorders like Tolosa-Hunt syndrome and SFN were reported.
Furthermore, our pooled data sets were incomplete for some variables, such as laboratory tests or imaging. However, we pooled data from all reported cases of NMD following COVID-19 vaccines using a comprehensive search strategy to provide a better understanding of this issue. To our knowledge, this is the first systematic review of reported cases of COVID-19 vaccine-associated NMD.
Based on our comprehensive literature review, COVID-19 vaccines might induce some neuromuscular diseases. These cases mainly occurred after administering the most frequently used COVID-19 vaccines. The age distribution and gender characteristics of affected patients may differ based on the NMD type. About two-thirds of the cases occurred in less than 2 weeks after vaccination. Most cases developed the symptoms following the first dose of vector-based vaccines. The majority of these patients experienced a benign disease course.
Availability of data and materials
All data and materials used in this study are accessible upon request. For inquiries regarding data and materials, please contact the corresponding author.
Mariatulqabtiah AR, Buttigieg KR. COVID-19 vaccinations for children. Lancet Infect Dis. 2022;22:1255–6.
Article PubMed PubMed Central CAS Google Scholar
Mathieu E, et al. Coronavirus pandemic (COVID-19). Our World Data; 2020.
Google Scholar
Zhou Q, Zhou R, Yang H, Yang H. To be or not to be vaccinated: that is a question in myasthenia gravis. Front Immunol. 2021;12:733418.
Samimisedeh P, Jafari Afshar E, Shafiabadi Hassani N, Rastad H. Cardiac MRI findings in COVID-19 vaccine-related myocarditis: A pooled analysis of 468 patients. J Magn Reson Imaging. 2022;56:971–82.
Article PubMed PubMed Central Google Scholar
Haj Mohamad Ebrahim Ketabforoush A, Molaverdi G, Nirouei M, Abbasi Khoshsirat N. Cerebral venous sinus thrombosis following intracerebral hemorrhage after COVID-19 AstraZeneca vaccination: A case report. Clin Case Rep. 2022;10:e6505.
Taga A, Lauria G. COVID-19 and the peripheral nervous system. A 2-year review from the pandemic to the vaccine era. J Peripher Nerv Syst JPNS. 2022;27:4–30.
Article PubMed CAS Google Scholar
Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43:3–40.
Article PubMed Google Scholar
Watad A, et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines. 2021;9:435.
Mirmosayyeb O, et al. Is myasthenia gravis a real complication of the COVID-19 vaccine? A case report-based systematic review. Can J Infect Dis Med Microbiol J Can Mal Infect Microbiol Medicale. 2022;2022:5009450.
Zargarbashi R, Vosoughi F, Milan N. Wide resection as a solution to excruciating pain in intraneural hemangioma: follow-up of a previously published case report. Int J Surg Case Rep. 2022;98:107562.
Tayebi AH, et al. Clinical features and outcomes of myasthenia gravis associated with COVID-19 vaccines: A systematic review and pooled analysis. Medicine (Baltimore). 2023;102:e34890.
Munn Z, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synthesis. 2019;18(10):2127–33.
Siddiqi AR, et al. Miller fisher syndrome after COVID-19 vaccination: case report and review of literature. Medicine (Baltimore). 2022;101:e29333.
Thant HL, et al. Guillain-Barré syndrome after Ad26.COV2.S vaccination. Am J Case Rep. 2022;23:e935275.
Masuccio FG, Comi C, Solaro C. Guillain-Barrè syndrome following COVID-19 vaccine mRNA-1273: a case report. Acta Neurol Belg. 2022;122:1369–71.
Finsterer J. Exacerbating Guillain–Barré Syndrome Eight Days after Vector-Based COVID-19 Vaccination. Case Rep Infect Dis. 2021;2021:1–3.
CAS Google Scholar
Abičić A, Adamec I, Habek M. Miller fisher syndrome following Pfizer COVID-19 vaccine. Neurol Sci. 2022;43:1495–7.
Matarneh AS, Al-battah AH, Farooqui K, Ghamoodi M, Alhatou M. COVID-19 vaccine causing Guillain-Barre syndrome, a rare potential side effect. Clin Case Rep. 2021;9:e04756.
Khadka B, Khanal K. Post COVID-19 vaccine Guillain-Barré syndrome. J Nepal Health Res Counc. 2022;19:852–4.
PubMed Google Scholar
McKean N, Chircop C. Guillain-Barré syndrome after COVID-19 vaccination. BMJ Case Rep. 2021;14:e244125.
Loza AM, Holroyd KB, Johnson SA, Pilgrim DM, Amato AA. Guillain-Barré syndrome in the placebo and active arms of a COVID-19 vaccine clinical trial: Temporal Associations Do Not Imply Causality. Neurology. 2021;96:1052–4.
Article Google Scholar
Azam S, Khalil A, Taha A. Guillain-Barré syndrome in a 67-year-old male post COVID-19 vaccination (Astra Zeneca). Am J Med Case Rep. 2021;9:424–7.
Zubair AS, Bae JY, Desai K. Facial Diplegia variant of Guillain-Barré syndrome in pregnancy following COVID-19 vaccination: A case report. Cureus. 2022;14:e22341.
PubMed PubMed Central Google Scholar
Michaelson NM, Lam T, Malhotra A, Schiff ND, MacGowan DJL. Miller fisher syndrome presenting after a second dose of Pfizer-BioNTech vaccination in a patient with resolved COVID-19: A case report. J Clin Neuromuscul Dis. 2021;23:113–5.
Hwang BW, Bong JB. Two possible etiologies of Guillain-Barré syndrome: mRNA-1273 (Moderna) vaccination and scrub typhus: A case report. Medicine (Baltimore). 2022;101:e32140.
Bouattour N, et al. Guillain-Barré syndrome following the first dose of Pfizer-BioNTech COVID-19 vaccine: case report and review of reported cases. Neurol Sci. 2022;43:755–61.
Prasad A, et al. A novel case of bifacial Diplegia variant of Guillain-Barré syndrome following Janssen COVID-19 vaccination. Neurol Int. 2021;13:404–9.
Morehouse ZP, Paulus A, Jasti SA, Bing X. A rare variant of Guillain-Barre syndrome following Ad26.COV2.S vaccination. Cureus. 2021;13:e18153.
Ilyas U, Umar Z, Bhangal R, Shah D, Fayman B. Guillain-Barré syndrome: A sequela of the original COVID-19 infection or vaccination. Cureus. 2022;14:e28044.
Dang YL, Bryson A. Miller-fisher syndrome and Guillain-Barre syndrome overlap syndrome in a patient post Oxford-AstraZeneca SARS-CoV-2 vaccination. BMJ Case Rep. 2021;14:e246701.
Nasuelli NA, et al. A case of acute demyelinating polyradiculoneuropathy with bilateral facial palsy after ChAdOx1 nCoV-19 vaccine. Neurol Sci. 2021;42:4747–9.
da Silva GF, et al. Guillain-Barré syndrome after coronavirus disease 2019 vaccine: A temporal association. Clin Exp Neuroimmunol. 2022;13:92–4.
Rossetti A, Gheihman G, O’Hare M, Kosowsky JM. Guillain-Barré syndrome presenting as facial Diplegia after COVID-19 vaccination: A case report. J Emerg Med. 2021;61:e141–5.
Nishiguchi Y, Matsuyama H, Maeda K, Shindo A, Tomimoto H. Miller fisher syndrome following BNT162b2 mRNA coronavirus 2019 vaccination. BMC Neurol. 2021;21:452.
Scendoni R, Petrelli C, Scaloni G, Logullo FO. Electromyoneurography and laboratory findings in a case of Guillain-Barré syndrome after second dose of Pfizer COVID-19 vaccine. Hum Vaccines Immunother. 2021;17:4093–6.
Article CAS Google Scholar
Ogbebor O, Seth H, Min Z, Bhanot N. Guillain-Barré syndrome following the first dose of SARS-CoV-2 vaccine: A temporal occurrence, not a causal association. IDCases. 2021;24:e01143.
Kripalani Y, et al. A rare case of Guillain-Barré syndrome following COVID-19 vaccination. Eur J Case RepIntern Med. 2021;8:002707.
Hasan T, Khan M, Khan F, Hamza G. Case of Guillain-Barré syndrome following COVID-19 vaccine. BMJ Case Rep. 2021;14:e243629.
Trimboli M, Zoleo P, Arabia G, Gambardella A. Guillain-Barré syndrome following BNT162b2 COVID-19 vaccine. Neurol Sci. 2021;42:4401–2.
Patel SU, Khurram R, Lakhani A, Quirk B. Guillain-Barre syndrome following the first dose of the chimpanzee adenovirus-vectored COVID-19 vaccine, ChAdOx1. BMJ Case Rep. 2021;14:e242956.
Lanman TA, Wu C, Cheung H, Goyal N, Greene M. Guillain-Barré syndrome with rapid onset and autonomic dysfunction following first dose of Pfizer-BioNTech COVID-19 vaccine: A case report. The Neurohospitalist. 2022;12:388–90.
Hughes DL, et al. Guillain-Barré syndrome after COVID-19 mRNA vaccination in a liver transplantation recipient with favorable treatment response. Liver Transpl. 2022;28:134–7.
Tutar NK, Eyigürbüz T, Yildirim Z, Kale N. A variant of Guillain-Barre syndrome after SARS-CoV-2 vaccination: AMSAN. Ideggyogyaszati Szle. 2021;74:286–8.
Nagalli S, Shankar Kikkeri N. Sub-acute onset of Guillain-Barré syndrome post-mRNA-1273 vaccination: a case report. SN Compr Clin Med. 2022;4:41.
Introna A, et al. Guillain-Barré syndrome after AstraZeneca COVID-19-vaccination: A causal or casual association? Clin Neurol Neurosurg. 2021;208:106887.
Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Neurological complications of COVID-19: Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Cureus. 2021;13:e13426.
Donaldson L, Margolin E. Variant Guillain-Barre syndrome following SARS-CoV-2 vaccination: case report and review of the literature. Can J Neurol Sci J Can Sci Neurol. 2022;1–3 https://doi.org/10.1017/cjn.2021.492 .
Nanatsue K, Takahashi M, Itaya S, Abe K, Inaba A. A case of miller fisher syndrome with delayed onset peripheral facial nerve palsy after COVID-19 vaccination: a case report. BMC Neurol. 2022;22:309.
Jain E, Pandav K, Regmi P, Michel G, Altshuler I. Facial Diplegia: A rare, atypical variant of Guillain-Barré syndrome and Ad26.COV2.S vaccine. Cureus. 2021; https://doi.org/10.7759/cureus.16612 .
Anjum Z, et al. Guillain-Barré syndrome after mRNA-1273 (Moderna) COVID-19 vaccination: A case report. Clin Case Rep. 2022;10:e05733.
Gunawan PY, Tiffani P, Lalisang L. Guillain-Barre syndrome following SARS-CoV-2 vaccination: A case report. Clin Psychopharmacol Neurosci. 2022;20:777–80.
Ogata S, et al. Sensory ataxic Guillain-Barré syndrome with Dysgeusia after mRNA COVID-19 vaccination. Intern Med Tokyo Jpn. 2022;61:1757–60.
Ling L, Bagshaw SM, Villeneuve P-M. Guillain-Barré syndrome after SARS-CoV-2 vaccination in a patient with previous vaccine-associated Guillain-Barré syndrome. CMAJ Can Med Assoc J J Assoc Medicale Can. 2021;193:E1766–9.
Bazrafshan H, Mohamadi Jahromi LS, Parvin R, Ashraf A. A case of Guillain-Barre syndrome after the second dose of AstraZeneca COVID-19 vaccination. Turk J Phys Med Rehabil. 2022;68:295–9.
Hilts A, Schreiber A, Singh A. A clinical case of COVID-19 vaccine-associated Guillain-Barré syndrome. Am J Case Rep. 2022;23:e936896.
Pirola FJC, et al. Miller-fisher syndrome after first dose of Oxford/AstraZeneca coronavirus disease 2019 vaccine: a case report. J Med Case Rep. 2022;16:437.
Bellucci M, et al. Case report: post-COVID-19 vaccine recurrence of Guillain-Barré syndrome following an antecedent Parainfectious COVID-19-related GBS. Front Immunol. 2022;13:894872.
Rao SJ, et al. A case of Guillain-Barre syndrome following Pfizer COVID-19 vaccine. J Community Hosp Intern Med Perspect. 2021;11:597–600.
Malamud E, Otallah SI, Caress JB, Lapid DJ. Guillain-Barré syndrome after COVID-19 vaccination in an adolescent. Pediatr Neurol. 2022;126:9–10.
Prado MB, Adiao KJB. Facial Diplegia as the sole manifestation of post-vaccination Guillain-Barre syndrome: A case report and literature review. The Neurohospitalist. 2022;12:508–11.
Richardson-May J, Purcaru E, Campbell C, Hillier C, Parkin B. Guillain-Barré syndrome and unilateral optic neuritis following vaccination for COVID-19: A case report and literature review. Neuro-Ophthalmol Aeolus Press. 2022;46:413–9.
Aomar-Millán IF, Martínez de Victoria-Carazo J, Peregrina-Rivas JA, Villegas-Rodríguez I. COVID-19, Guillain-Barré y vacuna. Una mezcla peligrosa. Rev Clínica Esp. 2021;221:555–7.
Čenščák D, Ungermann L, Štětkářová I, Ehler E. Guillan-Barré syndrome after first vaccination dose against COVID-19: case report. Acta Med (Hradec Kralove). 2021;64:183–6.
Kim N, Kim J-H, Park J-S. Guillain-Barré syndrome associated with BNT162b2 COVID vaccination: a first case report from South Korea. Neurol Sci. 2022;43:1491–3.
Kim Y, et al. A pediatric case of sensory predominant Guillain-Barré syndrome following COVID-19 vaccination. Child Neurol Open. 2022;9:2329048X221074549.
Finsterer J. Guillain-Barre syndrome 15 days after COVID-19 despite SARS-CoV-2 vaccination. IDCases. 2021;25:e01226.
Theuriet J, et al. Guillain-Barré syndrome following first injection of ChAdOx1 nCoV-19 vaccine: first report. Rev Neurol (Paris). 2021;177:1305–7.
Liang H, et al. Miller-fisher syndrome and Guillain-Barre syndrome overlap syndrome following inactivated COVID-19 vaccine: case report and scope review. Hum Vaccines Immunother. 2022;18:2125753.
Razok A, Shams A, Almeer A, Zahid M. Post-COVID-19 vaccine Guillain-Barré syndrome; first reported case from Qatar. Ann Med Surg. 2021;67:102540.
Allen CM, et al. Guillain-Barré syndrome variant occurring after SARS-CoV-2 vaccination. Ann Neurol. 2021;90:315–8.
Bax F, Gigli GL, Belgrado E, Brunelli L, Valente M. Guillain-Barré syndrome following Covid-19 immunization: a report of two cases. Acta Neurol Belg. 2022;122:1365–7.
Bonifacio GB, et al. Bilateral facial weakness with paraesthesia variant of Guillain-Barré syndrome following Vaxzevria COVID-19 vaccine. J Neurol Neurosurg Psychiatry. 2022;93:341–2.
Min YG, et al. Sensory Guillain-Barre syndrome following the ChAdOx1 nCov-19 vaccine: report of two cases and review of literature. J Neuroimmunol. 2021;359:577691.
Germano F, et al. COVID-19 vaccine-related Guillain-Barré syndrome in the Liguria region of Italy: A multicenter case series. J Neurol Sci. 2022;440:120330.
Hai PD, et al. Guillain-Barré syndrome after COVID-19 vaccination: report of two cases from Vietnam. J Infect Dev Ctries. 2022;16:1703–5.
Berrim K, et al. Guillain-Barré syndrome after COVID-19 vaccines: A Tunisian case series. Br J Clin Pharmacol. 2023;89:574–8.
James J, Johnson J, Jose J. Neuralgic Amyotrophy after ChAdOx1 nCoV-19 COVID-19 vaccination. J Clin Neuromuscul Dis. 2022;24:112–3.
Kanabar G, Wilkinson P. Guillain-Barré syndrome presenting with facial diplegia following COVID-19 vaccination in two patients. BMJ Case Rep. 2021;14:e244527.
Karimi N, et al. Guillain-Barre syndrome and COVID-19 vaccine: A report of nine patients. Basic Clin Neurosci. 2021;12:703–10.
Chun JY, et al. Guillain-Barré syndrome after vaccination against COVID-19. Lancet Neurol. 2022;21:117–9.
Castiglione JI, et al. Bilateral facial palsy with paresthesias, variant of Guillain-Barré syndrome following COVID-19 vaccine: A case series of 9 patients. Neuromuscul Disord NMD. 2022;32:572–4.
Nagdev G, Chavan G, Sahu G, Devasilpa Raju PD. COVID-19 vaccination a cause of Guillain-Barré syndrome? A Case Series. Cureus. 2022;14:e30888.
Tabatabaee S, et al. Post COVID-19 vaccination Guillain-Barre syndrome: three cases. Hum Vaccines Immunother. 2022;18:2045153.
Wan MM, Lee A, Kapadia R, Hahn C. Case series of Guillain-Barré syndrome after the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine. Neurol Clin Pract. 2022;12:149–53.
Oo WM, Giri P, de Souza A. AstraZeneca COVID-19 vaccine and Guillain- Barré syndrome in Tasmania: A causal link? J Neuroimmunol. 2021;360:577719.
García-Grimshaw M, et al. Guillain-Barré syndrome is infrequent among recipients of the BNT162b2 mRNA COVID-19 vaccine. Clin Immunol. 2021;230:108818.
Kim J-E, et al. Guillain-Barré syndrome and variants following COVID-19 vaccination: report of 13 cases. Front Neurol. 2021;12:820723.
Kim JW, et al. Guillain-Barre syndrome after two COVID-19 vaccinations: two case reports with follow-up Electrodiagnostic study. J Korean Med Sci. 2022;37:e58.
Osowicki J, et al. G uillain -B arré syndrome in an Australian state using both m RNA and adenovirus-vector SARS-C o V −2 vaccines. Ann Neurol. 2021;90:856–8.
Maramattom BV, et al. G uillain -B arré syndrome following C h A d O x 1-S / n C o V −19 vaccine. Ann Neurol. 2021;90:312–4.
Shields LBE, Iyer VG, Zhang YP, Burger JT, Shields CB. Parsonage-turner syndrome following COVID-19 vaccination: clinical and Electromyographic findings in 6 patients. Case Rep Neurol. 2022;14:58–67.
Queler SC, Towbin AJ, Milani C, Whang J, Sneag DB. Parsonage-turner syndrome following COVID-19 vaccination: MR Neurography. Radiology. 2022;302:84–7.
Min YG, et al. Parsonage-turner syndrome following COVID-19 vaccination. J Neurol Neurosurg Psychiatry. 2022;93:1231–2.
Koh JS, et al. Neuralgic amyotrophy following COVID-19 mRNA vaccination. QJM Int J Med. 2021;114:503–5.
Vitturi BK, et al. Parsonage-turner syndrome following coronavirus disease 2019 immunization with ChAdOx1-S vaccine: a case report and review of the literature. J Med Case Rep. 2021;15:589.
Sharma R, Dua B, Goyal S, Tiwari T. Parsonage–Turner Syndrome Following COVID-19 Vaccine. Ann Indian Acad Neurol. 2022;25:973–5.
Öncel A, Coşkun E. Parsonage-turner syndrome after SARS-CoV-2 vaccination: A case report. Turk J Phys Med Rehabil. 2022;68:418–21.
Mejri I, et al. Parsonage-turner syndrome of the brachial plexus secondary to COVID-19 vaccine: A case report. Clin Case Rep. 2022;10:e6483.
Mahajan S, Zhang F, Mahajan A, Zimnowodzki S. Parsonage turner syndrome after COVID-19 vaccination. Muscle Nerve. 2021;64:E3–4.
Lakkireddy M, Sathu S, Kumar R, Madhu Latha K, Maley DK. Parsonage-turner syndrome following Covishield (AstraZeneca ChAdOx1 nCoV-19) vaccination: A case report. Cureus. 2022;14:e27867.
Fukahori K, et al. Neuralgic Amyotrophy after COVID-19 vaccination in an adolescent: successful intravenous immunoglobulin treatment. Pediatr Neurol. 2022;140:50–1.
Amjad MA, et al. COVID-19 vaccine-induced parsonage-turner syndrome: A case report and literature review. Cureus. 2022;14:e25493.
Bernheimer JH, Gasbarro G. Parsonage turner syndrome following vaccination with mRNA-1273 SARS-CoV-2 vaccine. J Clin Neuromuscul Dis. 2022;23:229–30.
Civardi C, Delconte C, Pisano F, Collini A, Geda C. Isolated musculocutaneous involvement in neuralgic amyotrophy associated with SARS-CoV2 vaccination. Neurol Sci. 2022;43:3515–7.
Diaz-Segarra N, et al. Painless idiopathic neuralgic amyotrophy after COVID-19 vaccination: A case report. PM R. 2022;14:889–91.
Crespo Burillo JA, Loriente Martínez C, García Arguedas C, Mora Pueyo FJ. Amyotrophic neuralgia secondary to Vaxzevri (AstraZeneca) COVID-19 vaccine. Neurol Engl Ed. 2021;36:571–2.
Coffman JR, Randolph AC, Somerson JS. Parsonage-Turner Syndrome After SARS-CoV-2 BNT162b2 Vaccine: A Case Report. JBJS Case Connector. 2021;11(3):e21.00370. https://doi.org/10.2106/JBJS.CC.21.00370 .
Flikkema K, Brossy K. Parsonage-Turner Syndrome After COVID-19 Vaccination: A Case Report. JBJS Case Connect. 2021;11(4):e21.00577. https://doi.org/10.2106/JBJS.CC.21.00577 .
Kim SI, Seok HY, Yi J, Cho JH. Leg paralysis after AstraZeneca COVID-19 vaccination diagnosed as neuralgic amyotrophy of the lumbosacral plexus: a case report. J Int Med Res. 2021;49:030006052110567.
Chua MMJ, Hayes MT, Cosgrove R. Parsonage-turner syndrome following COVID-19 vaccination and review of the literature. Surg Neurol Int. 2022;13:152.
Mirmosayyeb O, Barzegar M, Rezaei M, Baharlouie N, Shaygannejad V. Bell’s palsy after sputnik V COVID-19 (gam-COVID-Vac) vaccination. Clin Case Rep. 2022;10:e05468.
SALBAŞ E, ÖCEK Ö, ÖCEK L, KARAHAN AY. Letter to the Editor: Facial paralysis following messenger RNA COVID-19 Vaccines: The report of two Cases. Ege Tıp Bilimleri Dergisi. 2021;4(2):73–7.
Repajic M, Lai XL, Xu P, Liu A. Bell’s palsy after second dose of Pfizer COVID-19 vaccination in a patient with history of recurrent Bell’s palsy. Brain Behav Immun – Health. 2021;13:100217.
Poudel S, Nepali P, Baniya S, Shah S, Bogati S, Nepal G, Ojha R, Edaki O, Lazovic G, Kara S. Bell's palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. https://doi.org/10.1016/j.amsu.2022.103897 .
Colella G, Orlandi M, Cirillo N. Bell’s palsy following COVID-19 vaccination. J Neurol. 2021;268:3589–91.
Pothiawala S. Bell’s palsy after second dose of Moderna COVID-19 vaccine: coincidence or causation? Acta Medica Litu. 2021;28:298–301.
Iftikhar H, Noor SMU, Masood M, Bashir K. Bell’s palsy after 24 hours of mRNA-1273 SARS-CoV-2 vaccine. Cureus. 2021;13:e15935.
Zhang H, Sanchez Gomez D, Repajic M, Liu AK. Another case of Bell’s palsy recurrence after Pfizer-BioNTech COVID-19 vaccination. Cureus. 2022;14:e27422.
Nishizawa Y, Hoshina Y, Baker V. Bell’s palsy following the Ad26.COV2.S COVID-19 vaccination. QJM Int J Med. 2021;114(9):657–8. https://doi.org/10.1093/qjmed/hcab143 .
Cellina M, D’Arrigo A, Floridi C, Oliva G, Carrafiello G. Left Bell’s palsy following the first dose of mRNA-1273 SARS-CoV-2 vaccine: A case report. Clin Imaging. 2022;82:1–4.
Martin-Villares C, Vazquez-Feito A, Gonzalez-Gimeno MJ, de la Nogal-Fernandez B. Bell’s palsy following a single dose of mRNA SARS-CoV-2 vaccine: a case report. J Neurol. 2022;269:47–8.
Obermann M, Krasniqi M, Ewers N, Fayad J, Haeberle U. Bell’s palsy following COVID-19 vaccination with high CSF antibody response. Neurol Sci. 2021;42:4397–9.
Yu B-Y, Cen L-S, Chen T, Yang T-H. Bell’s palsy after inactivated COVID-19 vaccination in a patient with history of recurrent Bell’s palsy: A case report. World J Clin Cases. 2021;9:8274–9.
Burrows A, Bartholomew T, Rudd J, Walker D. Sequential contralateral facial nerve palsies following COVID-19 vaccination first and second doses. BMJ Case Rep. 2021;14:e243829.
Ish S, Ish P. Isolated peripheral facial nerve palsy post COVID-19 vaccination with complete clinical recovery. Indian J Ophthalmol. 2022;70:347.
Galimi R, Galimi M. Adverse Event Reporting after mRNA COVID-19 Vaccination: A Bell’s Palsy Case the Day after. Neurol Case Rep. 2021;4(2):1026.
Mussatto CC, Sokol J, Alapati N. Bell’s palsy following COVID-19 vaccine administration in HIV+ patient. Am J Ophthalmol Case Rep. 2022;25:101259.
Khokhar F, Khan A, Hussain Z, Yu J. Small Fiber neuropathy associated with the Moderna SARS-CoV-2 vaccine. Cureus. 2022;14:e25969.
Waheed W, Carey ME, Tandan SR, Tandan R. Post COVID-19 vaccine small fiber neuropathy. Muscle Nerve. 2021;64:E1–2.
Finsterer J. Small fiber neuropathy as a complication of SARS-CoV-2 vaccinations. J Fam Med Prim Care. 2022;11:4071.
Chuang TY, Burda K, Teklemariam E, Athar K. Tolosa-hunt syndrome presenting after COVID-19 vaccination. Cureus. 2021;13:e16791.
Fanella G, et al. New-onset myasthenia gravis after mRNA SARS-CoV-2 vaccination: a case series. Neurol Sci. 2022;43:5799–802.
Ramdas S, et al. SARS-CoV-2 vaccination and new-onset myasthenia gravis: A report of 7 cases and review of the literature. Neuromuscul Disord. 2022;S0960896622006502 https://doi.org/10.1016/j.nmd.2022.09.001 .
Devaraj R, Shafi P, Nagesh C, Naidu A, Satishchandra P. Spectrum of neurological complications following COVID-19 vaccination in India. J Clin Neurol Seoul Korea. 2022;18:681–91.
Abicic A, Sitas B, Adamec I, Bilic E, Habek M. New-onset ocular myasthenia gravis after booster dose of COVID-19 vaccine. Cureus. 2022; https://doi.org/10.7759/cureus.27213 .
Chavez A, Pougnier C. A case of COVID-19 vaccine associated new diagnosis myasthenia gravis. J Prim Care Community Health. 2021;12:215013272110519.
Hoshina Y, Sowers C, Baker V. Myasthenia gravis presenting after administration of the mRNA-1273 vaccine. Eur J Case Rep Intern Med. 2022;1 https://doi.org/10.12890/2022_003439 .
Kang MC, Park K-A, Min J-H, Oh SY. Myasthenia gravis with ocular symptoms following a ChAdOx1 nCoV-19 vaccination: A case report. Am J Ophthalmol Case Rep. 2022;27:101620.
Lee MA, Lee C, Park JH, Lee JH. Early-onset myasthenia gravis following COVID-19 vaccination. J Korean Med Sci. 2022;37:e50.
Slavin E, Fitzig J, Neubert C, Garcia-Lopez F, Cuevas-Trisan R. New-Onset Myasthenia Gravis Confirmed by Electrodiagnostic Studies After a Third Dose of SARS-CoV-2 mRNA-1273 Vaccine. Am J Phys Med Rehabil. 2022;101(12):e176-9. https://doi.org/10.1097/PHM.0000000000002076 .
Maher DI, Hogarty D, Ben Artsi E. Acute onset ocular myasthenia gravis after vaccination with the Oxford-AstraZeneca COVID-19 vaccine. Orbit. 2022;1–5 https://doi.org/10.1080/01676830.2022.2062777 .
Galassi G, Marchioni A. Myasthenia gravis at the crossroad of COVID-19: focus on immunological and respiratory interplay. Acta Neurol Belg. 2021;121:633–42.
Virgilio E, Tondo G, Montabone C, Comi C. COVID-19 vaccination and late-onset myasthenia gravis: A new case report and review of the literature. Int J Environ Res Public Health. 2022;20:467.
Davalos L, Kushlaf H. New onset of seropositive generalized myasthenia gravis following intravesical bacille Calmette-Guerin treatment for bladder cancer: A case study. Muscle Nerve. 2019;59:E1–2.
Chung JY, Lee SJ, Shin B-S, Kang HG. Myasthenia gravis following human papillomavirus vaccination: a case report. BMC Neurol. 2018;18:222.
Takizawa T, et al. New onset of myasthenia gravis after intravesical Bacillus Calmette-Guerin: A case report and literature review. Medicine (Baltimore). 2017;96:e8757.
Stübgen J-P. Neuromuscular disorders associated with hepatitis B vaccination. J Neurol Sci. 2010;292:1–4.
Wang F, et al. Population-based incidence of Guillain-Barré syndrome during mass immunization with viral vaccines: A pooled analysis. Front Immunol. 2022;13:782198.
Ahsanuddin S, Nasser W, Roy SC, Povolotskiy R, Paskhover B. Facial paralysis and vaccinations: a vaccine adverse event reporting system review. Fam Pract. 2022;39:80–4.
Sanghani N, Rajanigandhi H, Shreya S, Nizar S. “Myasthenia Gravis after Vaccination in Adults the United States: A Report from the CDC/FDA Vaccine Adverse Event Reporting System (1990–2017) (P6.437).” Neurology. 2018;90.
Abolmaali M, et al. Guillain-Barré syndrome in association with COVID-19 vaccination: a systematic review. Immunol Res. 2022;70:752–64.
Download references
Acknowledgments
Researchers appreciated the Clinical Research Development Units of Kamali and Rajaei Hospitals at Alborz University of Medical Sciences.
There is no funding source with authors to declare.
Author information
Amirhossein Tayebi and Parham Samimisedeh share the first authorship.
Authors and Affiliations
Cardiovascular Research Center, Alborz University of Medical Sciences, Karaj, Iran
Amirhossein Tayebi, Parham Samimisedeh, Elmira Jafari Afshar & Hadith Rastad
Department of Neurology, Shahid Rajaei Hospital, Alborz University of Medical Sciences, Karaj, Iran
Saeideh Mahmoudnia & Aryan Madady
Center of Orthopedic Trans-Disciplinary Applied Research (COTAR), Department of Orthopedics, Tehran university of medical sciences, Tehran, Iran
Tehran University of Medical Sciences (TUMS), Tehran, Iran
Aryan Ayati
You can also search for this author in PubMed Google Scholar
Contributions
A.T, P.S, and H.R contributed to the conception and design of the study. E.J organized the database. P.S performed the statistical analysis. A.T wrote the first draft of the manuscript. S.M, A.A, N.M, and A.M wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding author
Correspondence to Hadith Rastad .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: supplementary table 1..
Quality assessment of the included case series based on the JBI checklist for case series. Supplementary Table 2. Guillain barre syndrome studies and patients’ characteristics by case. Supplementary Table 3. Guillain Barre patients’ findings, treatments, and outcomes by case. Supplementary Table 4. Parsonage turner studies and patients’ characteristics by case. Supplementary Table 5. Parsonage turner patients’ findings, treatments, and outcomes by case. Supplementary table 6. Facial nerve palsy patients’ findings, treatments, and clinical outcomes by case. Supplementary table 7. Small fiber neuropathy and tolosa-hunt patients’ findings, treatments, and clinical outcomes by case. Supplementary Table 8. Myasthenia gravis studies and patients’ characteristics by case. Supplementary Table 9. Myasthenia gravis patients’ findings, treatments, and clinical outcomes by case.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Tayebi, A., Samimisedeh, P., Jafari Afshar, E. et al. Neuromuscular diseases associated with COVID-19 vaccines: a systematic review and pooled analysis of 258 patients. BMC Neurol 23 , 437 (2023). https://doi.org/10.1186/s12883-023-03486-y
Download citation
Received : 19 August 2023
Accepted : 04 December 2023
Published : 11 December 2023
DOI : https://doi.org/10.1186/s12883-023-03486-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- COVID-19 vaccine
- Peripheral neuropathy
- Neuromuscular diseases
- Tolosa-Hunt syndrome
BMC Neurology
ISSN: 1471-2377
- General enquiries: [email protected]
ORIGINAL RESEARCH article
Ovarian strumal carcinoid: case report, systematic literature review and pooled analysis.

- 1 Department of Medical and Surgical Specialties, Radiological Sciences, and Public Health, University of Brescia, Medical Oncology, ASST Spedali Civili, Brescia, Italy
- 2 1st Pathology Division, Department of Pathology and Laboratory Medicine, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy
Background: Ovarian strumal carcinoid is a rare tumor in which thyroid (struma) and carcinoid components coexist. The disease is generally considered to be a borderline malignancy, however, cases with metastatic disease have been described. No data in the literature are available to guide diagnosis and therapy.
Methods: We performed a pooled analysis and a systematic review of histopathological-confirmed strumal carcinoid cases published in the literature using the following keywords: “strumal carcinoid of the ovary”, “strumal carcinoid case report”. A case of strumal carcinoid tumor diagnosed and followed-up at the Medical Oncology Unit of Spedali Civili (Brescia, Italy) was also described and included.
Results: Sixty-six eligible publications were identified, providing data from one hundred and seventeen patients, plus a case diagnosed at our institution. At presentation, among the eighty-eight patients with symptomatic disease, 37% of patients suffered from abdominal distention and 49% from pain due to a growing abdominal tumor mass, 37% from constipation (peptide YY was analyzed in only nine of them, resulting above the physiologic range). Surgery was the primary therapy in 99% of the patients. Three patients had metastatic disease at diagnosis and five patients underwent recurrence after radical surgery. Histology at disease recurrence concerned the thyroid component in two patients, the carcinoid component in two patients, both histologies in one patient. Median disease-free survival and overall survival in this series were not attained.
Conclusion: Strumal carcinoid of the ovary generally presents a benign behavior and surgery is curative in most cases. However, a small group of patients with this disease can undergo disease recurrence due to both the thyroid and the neuroendocrine (carcinoid) components. A follow-up in radically operated patients is therefore needed, particularly in those with a voluminous disease at diagnosis.
Introduction
Ovarian carcinoid tumors are the most common primary neuroendocrine neoplasms in the female genital tract, and almost all arise among mature teratomas ( 1 ). Two main architectural patterns of primary ovarian carcinoids were described: insular and trabecular ( 2 ). Strumal carcinoid is a distinctive form of ovarian teratoma consisting of thyroid tissue intermixed with a neuroendocrine tumor (carcinoid) component ( 3 ), usually either insular or trabecular in type ( 2 ). It is classified as a monodermal teratoma (struma) with a secondary somatic tumor (carcinoid). The thyroid component can be normal thyroid, containing colloidal material, microfollicular or macrofollicular adenoma or papillary and follicular carcinoma. Other teratomatous elements are noted in over 80% of cases ( 3 ). The disease is extremely rare accounting for 0.1% of all ovarian malignancies ( 4 ). Strumal carcinoid is a borderline malignancy and a conservative surgical treatment, such as salpingo-oophorectomy or a simple oophorectomy, can be resolutive. However, metastases have been rarely described. The neoplastic neuroendocrine cells acquire an aggressive behavior, replacing the follicular lining cells. Rarely the thyroid component can be manifest as papillary or follicular thyroid carcinoma. Most strumal carcinoids are incidental findings; frequently they can cause symptoms due to mass enlargement and/or releasing of specific peptides and hormones. Serotonin-like substances are released directly into systemic circulation through the ovarian venous system bypassing hepatic deactivation, therefore, like all primary ovarian carcinoids, carcinoid syndrome (facial flushing, diarrhea, bronchospasm, and edema) is reported relatively frequent and has been estimated to occur in one-third of patients with insular patterns ( 5 ). Ovarian carcinoids may also typically produce and release the gut hormone peptide YY, which function is to inhibit gut motility and can cause severe constipation and painful defecation. Other hormone-related symptoms, such as hirsutism and sexual pilosity, are also described due to stimulated ovarian stroma adjacent to the tumor. Strumal carcinoid is a neoplastic pathology whose management can be difficult because of its extreme rarity and the dual thyroid and neuroendocrine component, both potentially responsible for a malignant behavior and endocrine associated syndromes. Published literature is essentially represented by case reports, small series and pathology reviews and does not provide useful information regarding the appropriate diagnostic workup and treatment and no internationally agreed guidelines are currently available.
This study was undertaken to systematically review all strumal carcinoid cases published in literature with the description of an additional patient observed in our Institution. We performed a pooled analysis to obtain information on clinicopathological features, treatment strategies and prognostic factors of this rare disease. On these bases, we provided some recommendations about the possible clinical approach.
Patients And Methods
Search strategy.
We performed a systematic literature search on PubMed/Embase using the following keywords: “strumal carcinoid of the ovary”, “strumal carcinoid case report”, “ovarian strumal carcinoid”; an addition search based on the main publication references was carried out. Only papers reporting individual clinicopathological data were eligible. Case reports were selected if the histological diagnosis of described cases reported the coexistence of both carcinoid tumor and strumal tissue. A restriction for language and duplicated publications was applied. A case of strumal carcinoid tumor diagnosed and followed-up at the Medical Oncology Unit of Spedali Civili (Brescia, Italy) was also included in this study.
Statistical Analysis
Data concerning demographic, tumor sizes, histopathological features, treatments and recurrence of the disease were collected into a database; the resulted population was analyzed as a single cohort. Survival curves were obtained using the Kaplan-Meier method and compared with the log-rank test. Exploratory analyses were performed using Cox proportional hazards regression models to test the prognostic value of clinical features and treatment approaches [hazard ratios (HRs) and 95% confidence intervals (CIs)] for overall survival (OS), which is defined as the time from diagnosis to patient death or the date of the last follow-up used for censoring, and disease-free survival (DFS), which is defined as the time to relapse, second cancer, or death, whichever event came first. Patients who did not experience any of these events were censored at the reported last follow-up. P values <0.05 (two-sided) were considered statistically significant. All statistical analyses were obtained using SPSS version 23.0 (SPSS, Chicago, IL).
Case Report
A 55-years-old white woman with no significant co-morbidities underwent an ultrasound in September 2019 for persistent constipation for several weeks associated with abdominal pain. Ultrasonography detected a cystic lesion (41 x 32 mm) on the left ovary. Transvaginal ultrasonography confirmed a dishomogeneous mass (hypoechogenic in the middle) in the left ovary while no abnormalities were found in the right ovary and the uterus. Serum CA 125 was in the normal range. A laparoscopic bilateral salpingo-oophorectomy was performed in December 2019. Pelvic and para-aortic lymph node metastases were not observed.
Histology revealed a strumal carcinoid: the first component was carcinoid tumor; the other was constituted by thyroid follicle-like tissue. At immunohistochemistry the carcinoid component was positive for chromogranin A, synaptophysin, CD56 and cytokeratin 7, while the strumal component was positive for TTF1. AFP (Alpha-Fetoprotein) expression was negative. Ki67 immunostaining was detected in about 2% of cells. The tumor was limited to the ovarian parenchyma and did not involve the capsule. Cytological examination of the ascites fluid was negative. A post-operative Gallium-68 PET/TC was negative.
Constipation resolved some weeks after the surgical intervention and at the last follow-up evaluation in October 2021 the patient was alive and disease-free.
Search Results
Data from one hundred and eighteen patients were analyzed: one hundred and seventeen were obtained from sixty-six eligible publications, while the one hundred and eighteenth case is represented by the patient followed at our Institution. Thirteen publications were excluded because they were published as abstracts or were written in a language other than English (or the English translation was not available). Among the seventy-five records analyzed for the eligibility process, nine publications were removed for lack of individual clinical or histological data.
Patients’ Characteristics
Patients’ characteristics are reported in Table 1 .

Table 1 Patients’ characteristics and disease-related symptoms and signs.
Median age was 50 years (range 14-78). Sixty-two (53%) were post-menopausal. Among the one hundred and four patients in which this information was available, sixty-one (58%) patients were Caucasian, thirty-six (35%) Asian and seven (7%) Afro-American.
Median tumor size was 8 cm (range 1-26). Six patients (5%) presented a mass in the contralateral ovary, consisting of teratoma in four patients ( 6 – 9 ), clear cell adenocarcinoma in one patient ( 10 ) and endometrial cyst in the remaining one ( 11 ).
Three patients had metastatic disease at diagnosis: one patient presented with peritoneal implants ( 12 ); one patient had a disease involving lymph nodes, peritoneum and abdominal organs (contralateral ovary, bladder and pelvic floor) ( 13 ); in one case pulmonary lesions and metastases in contralateral ovary and myometrium were detected ( 14 ). In this last record the metastatic lesions in the contralateral ovary and myometrium turned out to be deposits of carcinoid ( 14 ), while in the second case the peritoneal nodules presented thyroidal compound ( 13 ). Quiñonez et al. described a peculiar case of pseudomyxoma peritonei with multiple mucinous peritoneal implants, in the absence of appendiceal lesion, associated with ovarian strumal carcinoid ( 12 ).
Disease-Related Symptoms and Signs
As shown in Table 1 , tumor presentation was heterogeneous. In two patients this information was not available. Twenty-eight out of the one hundred and sixteen patients in which this information was available had no symptoms (24%), while in the remaining eighty-eight patients (76%) the diagnosis of the disease was associated with the occurrence of symptoms and signs such as abdominal distention (37%), pain (49%), constipation (37%). Less often patients presented gynecological features, such as heavy menstrual bleeding (16%), amenorrhea (7%) and vaginal discharge (4%). A pelvic mass was appreciable at the physical examination in thirty-seven patients (42%). Rarely, symptoms at presentation were generalized edema (1%); nausea (4%) and vomiting (4%); hirsutism (8%); alopecia (2%); weight loss (7%); ulcerated skin lesions (2%); hyperthyroidism (2%); hypoglycaemia (1%); tricuspid regurgitation (1%). Three patients had glucagonoma syndrome, such as necrolytic migratory erythema, weight loss, anemia, and stomatitis ( 15 – 17 ). One patient, suffering from hypoglycemic attacks, presented concomitantly raised levels of both glucagon and insulin ( 18 ).
Peptide YY was measured in nine patients suffering from severe constipation and resulted above the reference range in all of them.
Secretin serum levels were measured in one patient, showing elevated levels ( 15 ). Circulating serotonin was analyzed in thirteen patients and resulted to be above the normal range in nine of them ( 5 , 17 – 24 ). In one case serotonin hypersecretion caused flushing, diarrhea, hypertension (carcinoid syndrome) ( 5 ); in another case ( 20 ) serotonin hypersecretion was associated with tricuspid regurgitation, due to fibrous deposits, which required valve bioprosthesis placement. In the remaining seven cases, serotonin hypersecretion was not associated with a typical carcinoid syndrome.
Data on imaging techniques prior to surgery are available in only forty-seven patients, thirty-four were evaluated with ultrasound, twenty-six with Computed Tomography (CT) scan and ten with Magnetic Resonance Imaging (MRI). In most cases the pelvic ultrasound was the first-step imaging, followed by a CT scan and/or MRI. No patient was suspected of strumal carcinoid before surgery, including two patients who underwent biopsies to exclude neoplastic ascites ( 10 ) and lymph node ( 25 ) metastases, respectively; both were negative for neoplastic cells.
68Ga-PET was prescribed after surgery in only one patient ( 26 ); one patient performed a post-surgery 131I whole-body scan ( 13 ); in one case thyroid ultrasound and scintigraphy imaging were evaluated after the intervention ( 27 ).
Histologic and Immunohistochemical Features
As depicted in Table 2 , among one hundred and twelve patients in which histology was described, the strumal component was normal thyroid-like tissue in one hundred and three patients (91%), however in nine cases (9%) it was constituted by malignant tumor tissue which consisted in papillary, follicular variant of papillary or follicular carcinomas in four, three, and two patients, respectively. Among the one hundred patients in which the information was available, the carcinoid component was described as trabecular in fifty-five (55%), insular in five (5%), or a mixture of the two histotypes in forty (40%) of them. Various differentiated cells and tissues, like hair, fat, bony elements were observed in fifty patients (42%), while mucinous component, both endocervical and intestinal, was observed in twenty-four cases (20%). Concomitant adenomyosis was reported in six patients (5%). The strumal component presented focal amyloid deposits in twenty-three patients (20%) and birefringent crystals in three patients (3%), respectively.

Table 2 Tumor characteristics at histopathological examination.
Immunohistochemical features confirmed the dual nature of the disease. As shown in Table 3 , the neuroendocrine markers: Neuron Specific Enolase (NSE), chromogranin A, synaptophysin and CD56 were expressed in 100% of the tumor specimens in which they were tested. As regards as thyroid-tissue markers, thyroglobulin, thyroid transcription factor 1 (TTF1), were expressed in 91% and 88% of tumors, respectively, and calcitonin, a neuroendocrine marker of the medullary thyroid component, was found to be expressed in 52% of tumors. As expected, the immunohistochemical evaluation also documented immunostaining positivity to peptide YY in 100% of the tested tumors and serotonin in 57%. Interestingly, peptides typical of the neuroendocrine differentiation of pancreatic tumors such as glucagon and pancreatic polypeptide were expressed in 80% and 100% of the strumal carcinoids tested. Moreover, CDX2, which is expressed in the nuclei of intestinal epithelial cells, was detected in three out of five strumal carcinoids in which it was assessed (60%).

Table 3 Immunohistochemical expression of tumor markers.
Proliferative activity, as measured by Ki67 expression, was tested in fourteen patients and ranged between <1% to <5%.
Treatment Strategies Adopted After Diagnosis
Surgery was the primary therapeutic approach in all patients but one (99%), even in case of metastatic disease ( 12 – 14 ). One patient did not undergo a surgical approach due to an early death for cardiorespiratory arrest during hospitalization due to a hypoglycaemic attack; the diagnosis of strumal carcinoid was performed at autopsy ( 18 ). Surgery consisted of salpingo-oophorectomy, which was bilateral in sixty-seven patients (57%) and unilateral in the remaining fifty (43%). Hysterectomy was performed in fifty-nine cases (50%), while lymph nodes dissection and omentectomy were performed in seven (6%) and ten patients (8%), respectively; appendectomy was reported in fourteen (12%). One patient received two courses of radiation therapy with implants before surgery ( 5 ), while another patient with pulmonary metastatic lesions was administered three cycles of chemotherapy (the regimen was not specified) before ovarian surgery ( 14 ).
Seven patients received additional therapies after surgery. Post-operative radiotherapy was performed in three cases with bilateral disease or huge masses (one case associated with invasive cervical carcinoma). The dose delivered in the pelvic region was 30 Gy in one patient (combined with chemotherapy) ( 28 ) and 70 Gy in another one ( 6 ). In one patient, due to persistent disease after surgery (multiple nodules on the liver surface, bowel and the parietal peritoneum), I3lAu was instilled into the peritoneal cavity followed by radiotherapy ( 29 ); the radiotherapy dose delivered in this third case is missing.
Three patients were addressed to adjuvant chemotherapy due to bilateral disease, voluminous lesion (median size 12cm) or concomitant invasive cervical carcinoma. Chemotherapy consisted of three or four cycles of paclitaxel associated with cisplatin ( 6 ) or carboplatin ( 10 ) or cisplatin, adriamycin and cyclophosphamide ( 16 ).
In one case chemotherapy according to the PEB scheme (cisplatin, etoposide and bleomycin) was administered after surgery due to the persistence of residual metastatic disease ( 13 ).
Disease Recurrence and Survival Outcome
Follow-up data were not available in seven patients. In the one hundred and eleven patients fully assessable for prognosis, the median follow-up duration was 24 months (range 1-384 months). Three patients (3%) had metastatic disease at diagnosis, one of these was radically operated for peritoneal metastases, the remaining two did not receive radical treatment. Five patients (4.5%), radically resected, underwent disease recurrence in the follow-up. Median overall survival (OS) and median disease-free survival (DFS) were not reached ( Figure 1 ). Five-year DFS was 89%, 10-year 78%; while 5-year OS was 91% and 10-year OS was 80%. Disease-related deaths were reported in two out of the nine cases (22%), both with distant metastases ( 11 , 29 ), while seven patients (88%) died due to other independent causes (one patient for car accident, one for cirrhosis, one for cardiorespiratory arrest, one for rheumatic heart disease, one for cerebrovascular accident, one for intestinal necrosis, one for not specified causes).
Figure 1 Overall Survival (OS) and Disease-Free Survival (DFS) in global population and in patients with recurrent or metastatic disease (Met.).
In the five patients showing disease recurrence, this event occurred after 2 ( 13 ), 10 ( 17 ), 18 ( 29 ), 42 months ( 11 ) and 7 years ( 26 ), respectively. Four patients developed distant metastases involving peritoneum (carcinosis), breast, bone and liver; one patient underwent recurrence in the homolateral ovary 7 years after surgery and subsequently a second strumal carcinoid in the contralateral ovary almost 30 years later ( 26 ). Disease recurrence was asymptomatic (detected by follow-up imaging) in one patient ( 26 ) and symptomatic in the other four patients. The symptoms associated with the disease recurrence were: lumbago; recrudescence of constipation; amenorrhea; abdominal pain and weight loss ( Table 4 ).
Table 4 Characteristics of patients with recurrent disease.
Due to the low number of patients who have had the event, none of the following factors, age, mucinous phenotype, tumor size, symptoms, abdominal distension, lymph node dissection + omentectomy, were associated with a significantly higher risk of disease recurrence at univariate Cox analysis. However, patients who experienced disease recurrence presented large tumor size (median size 11 cm), and, histologically, primary tumors had thyroid follicle-like structure as strumal component, while the carcinoid component was trabecular carcinoid in three patients and a mixture of trabecular and insular carcinoid in the remaining two patients. Histology at disease recurrence revealed recurrence of the thyroid component in two cases, one of them depicting poorly differentiated adenocarcinoma with areas of carcinoid ( 29 ), the second one showing well-differentiated thyroid tissue-forming follicles, similarly to the histology of primary disease ( 13 ). In two patients it was the carcinoid component that relapsed: one patient developed metastatic carcinoid in the breast ( 11 ), another patient had carcinoid recurrence in the liver associated with recrudescence of peptide YY-related constipation ( 17 ). The 5th had a peculiar disease history characterized by an initial recurrence of struma component and a second recurrence of carcinoid histology after 7 and 30 years from primary surgery, respectively ( 26 ).
As regard as treatment for metastatic disease, in the last case the double recurrence of the disease was radically removed (left adnexectomy for the first recurrence, right adnexectomy and lymphadenectomy for the second one) ( 26 ). In the remaining four cases not amenable to surgery, two of them, whose disease recurrence was thyroid histology, received thiotepa chemotherapy ( 29 ) and radioiodine therapy ( 13 ), respectively. The patient submitted to radionuclide therapy, who previously had undergone chemotherapy according to the PEB scheme for the persistence of multiple peritoneal nodules, achieved a partial remission whose duration was not specified. The patient who received thiotepa underwent disease progression at first restaging. Of the two patients, whose disease recurrence was a carcinoid tumor, one ( 11 ) had metastatic disease in breast and bone and received chemotherapy, which consisted of paclitaxel and carboplatin (three cycles) followed by etoposide and cisplatin (four cycles); both schemes administered in association with zoledronic acid. No systemic treatment was prescribed in the second case bearing liver metastases ( 17 ). This patient refused surgery and was kept in follow-up only. She was alive with progressive hepatic disease after 36 months after diagnosis suffering from drug-refractory constipation.
In the seven patients showing metastatic disease at diagnosis or experiencing disease recurrence after surgery, median OS and DFS were 42 (range 24-60) and 10 (range 0-24) months, respectively ( Figure 1 ).
Due to its extreme rarity, little is known about the natural history of ovarian carcinoid. Therefore, no data in the literature are available to guide diagnosis and therapy.
The present series of published cases confirms that this rare disease is detected either in pre-or post-menopausal women with a slight prevalence in the latter condition. Symptoms and signs leading to the diagnosis were those related to the presence of a growing tumor mass, such as abdominal distention and pain, and those related to hormonal hypersecretion. As mentioned in the introduction, the frequent hormone-related syndromes in non-metastatic cases notoriously distinguish ovarian carcinoids from lung and gastroenteropancreatic NET ( 30 , 31 ) and it is due to the hormone release directly into systemic circulation through the ovarian venous system, thus bypassing the hepatic deactivation. Among the hormone-related symptoms, constipation, as a consequence of hormone YY hyperproduction, prevailed. This is another peculiarity of ovarian carcinoids since GEP NETs do not produce YY. Noteworthy, typical carcinoid syndrome was observed in only two patients, one of them developing a carcinoid heart disease, whereas three patients developed a glucagonoma syndrome and one insulinoma syndrome; this last case was peculiar since the patient presented hyperinsulinaemic hypoglycemia and concomitant raised levels of glucagon. These data suggest that ovarian carcinoids can assume biological and clinical characteristics of both intestinal and pancreatic NETs. Amenorrhea and hirsutism, due to stimulated ovarian stroma adjacent to the tumor, were other frequent hormone-related symptoms. The thyroid component remained mostly silent in terms of hormonal-related symptoms, although two patients experienced hyperthyroidism.
Regarding the histological features, the carcinoid component had the trabecular pattern in most patients, and this is in contrast with literature data reporting insular as the most prevalent architecture in ovary carcinoids ( 2 ). These data confirm the findings of Robboy et al. which showed, in a small series of patients with strumal carcinoids, the trabecular phenotype in half of the patients ( 29 ). The strumal component in this series was mainly constituted by normal thyroid-like tissue, although malignant tumor tissue was observed in nearly 10% of cases. As expected, immunohistochemical evaluation confirms the duality of the disease, with expression of specific markers both of thyroid (thyroglobulin, TTF1, calcitonin) and carcinoid (chromogranin A, synaptophysin, NSE and CD56). The expression of peptide YY, which a typical characteristic of ovarian carcinoids, is also an expected finding. However, the positivity at immunohistochemical staining for serotonin (57% of patients), CDX-2 (60% of patients) which are markers of neuroendocrine tumors of intestinal origin, and glucagon (80%) and pancreatic polypeptide (100%) which are more typical of pancreatic NETs, was somewhat unexpected and underlines the heterogeneity of the disease and its differences from carcinoids of other districts.
The present data confirm that patients with strumal carcinoid have a favorable disease outcome, medians DFS and OS were not attained. The follow-up of this series, however, was relatively short and this is a limitation of all pooled analyses of published cases ( 32 , 33 ). In most cases, the disease was diagnosed in an early stage and most patients received radical surgery. The surgical approach, however, was quite heterogeneous: all patients underwent salpingo-oophorectomy, which was bilateral in the majority of them. In about 15% of patients the operation extended to the regional lymph nodes and omentectomy, as is done in ovarian cancer. In addition, hysterectomy was performed in half of the patients. The good prognosis of this disease suggests that a conservative surgical approach can be resolutive. This issue is relevant since in many patients the disease is diagnosed in pre-menopause where the maintenance of fertility is crucial. The probable reason why extensive surgery was performed in many cases is that it was not possible to have a diagnosis of the disease pre-operatively. The assessment of hormonal production (such as peptide YY, secretin, serotonin and insulin) has to be considered when the detection of an ovarian lesion is accompanied by peculiar symptoms (constipation, flushing, diarrhea, hyper or hypoglycaemic attacks), for a pre-operative diagnosis. This is crucial since no pathognomonic imaging findings were shown. On ultrasound imaging, in fact, strumal carcinoid usually presents as a unilateral echo-mixed solid cystic mass, which often mimics a malignant tumor ( 34 ). To discriminate between benign and malignant lesions, ultrasound could be implemented with the IOTA Assessment of Different NEoplasias in the adneXa (ADNEX) RISK MODEL, which was found to be an effective diagnostic tool for preoperative evaluation of ovarian masses, resulting to be particularly useful in presence of borderline ovarian tumors ( 35 – 37 ). Borghese et al. applied this model showing a malignancy risk of 19.8%, above the 10% assigned as cut-off risk for malignancy ( 26 ).
It should be noted, however, that not in all cases the disease had a benign behavior. Lymph node metastases were found in two patients and the disease presented peritoneal spread in an additional one. These data underline the importance of accurate abdominal staging before surgery; the staging process should comprehend gynecologic examination with transvaginal ultrasonography, CT scan of the thorax and the abdomen and MRI in presence of suspicious liver or peritoneal metastases. More importantly, disease recurrence occurred in five strumal carcinoid patients after surgery, four of them with metastatic disease. These data suggest that a follow-up should be implemented in these patients, with periodic clinical examination and ultrasound. Second level imaging exams, such as CT scan, MRI, 68Ga PET may be prescribed as needed. Follow-up should be prosecuted for at least 5 years since most of the recurrence observed in this series occurred within 60 months. It should be noted that one patient in this series developed metastases after 7 and 30 years, suggesting a possible extension of follow-up beyond 5 years in selected cases. Due to the limited number of cases with disease recurrence, we were unable to identify baseline prognostic factors for disease recurrence.
Since there are potentially two neoplastic diseases in one, an obvious question is which of the two (thyroid carcinoma or ovarian carcinoid) drive disease recurrence. Of the five patients who relapsed, two had a carcinoid recurrence, two thyroid recurrences, while one both histologies. This observation could have a potential impact on the follow-up which should also concern the thyroid component. As pointed out in ESMO guidelines ( 38 ), isolated measurements of serum thyroglobulin cannot be reliably interpreted in the presence of normal thyroid tissue, however, the trend over time of basal thyroglobulin should be used to detect recurrent disease, and the same may be true for rising thyroglobulin Ab levels.
Noteworthy, only one of the five patients with disease recurrence regained a disease-free status after rescue surgery, all the others received systemic therapies, such as chemotherapy and 131-iodine ablation, which were scarcely effective since the only patient with recurrent thyroid component obtained a partial response after radionuclide therapy. It should be noted that patients, in which the carcinoid component drove the recurrence, received systemic chemotherapy regimens commonly used in ovarian cancer and none of them was addressed to specific therapies, such as somatostatin analogues, as recommended by international guidelines for advanced NETs ( 30 , 31 , 39 , 40 ). Chemotherapy, in fact, is not indicated in patients with NET since they are low proliferating tumors with an indolent disease course. As a matter of fact, one of the patients in this series with metastatic disease exhibited a long survival without receiving any systemic treatment.
Strumal carcinoid of the ovary represents a rare form of primary ovarian carcinoid; it can be asymptomatic, or cause symptoms related to peptide-producing components. Since most of the cases presented a benign behavior, surgical intervention is usually the only required treatment. However, a small group of patients with this disease can undergo disease recurrence due to both the thyroid and the neuroendocrine (carcinoid) components. A follow-up in radically operated patients is therefore needed, particularly in those with a voluminous disease at diagnosis. Based on the results of this pooled analysis, we propose a list of suggestions for the management of patients affected by this rare pathology ( Table 5 ).

Table 5 Suggestions for the clinical management of patients with strumal carcinoids.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
AT and AB conceived the idea of this manuscript. SG, VA, RP, and DC clinically followed the patient. AT, MM, and MZ collected, interpreted the literature data and wrote the manuscript. All authors read and approved the final manuscript.
This manuscript was supported in part by FIRM Onlus, Cremona, Italy.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Howitt BE, Kelly P, McCluggage WG. Pathology of Neuroendocrine Tumours of the Female Genital Tract. Curr Oncol Rep (2017) 19(9):59. doi: 10.1007/s11912-017-0617-2
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Berney DM, Stoneham S, Arora R, Shamash J, Lockley M. Ovarian Germ Cell Tumour Classification: Views From the Testis. Histopathol (2020) 76(1):25–36. doi: 10.1111/his.14016
CrossRef Full Text | Google Scholar
3. Roth LM, Talerman A. The Enigma of Struma Ovarii. Pathol (2007) 39(1):139–46. doi: 10.1080/00313020601123979
4. Selvaggi SM. Tumors of the Ovary, Maldeveloped Gonads, Fallopian Tube, and Broad Ligament. Arch Pathol Lab Med (2000) 124(3):477. doi: 10.5858/2000-124-474-TOTOMG
5. Ulbright TM, Roth LM, Ehrlich CE. Ovarian Strumal Carcinoid. An Immunocytochemical and Ultrastructural Study of Two Cases. Am J Clin Pathol (1982) 77(5):622–31. doi: 10.1093/ajcp/77.5.622
6. Kim SM, Choi HS, Byun JS, Kim YH, Kim KS, Rim SY, et al. Mucinous Adenocarcinoma and Strumal Carcinoid Tumor Arising in One Mature Cystic Teratoma of the Ovary With Synchronous Cervical Cancer. J Obstet Gynaecol Res (2003) 29(1):28–32. doi: 10.1046/j.1341-8076.2003.00073.x
7. Matsunami K, Takagi H, Ichigo S, Murase T, Ikeda T, Imai A. Peptide YY Producing Strumal Carcinoid Tumor of the Ovary. Eur J Gynaecol Oncol (2011) 32(2):201–2.
PubMed Abstract | Google Scholar
8. Kimura N, Sasano N, Namiki T. Evidence of Hybrid Cell of Thyroid Follicular Cell and Carcinoid Cell in Strumal Carcinoid. Int J Gynecol Pathol (1986) 5(3):269–77. doi: 10.1097/00004347-198609000-00009
9. González B, Muniesa M, Hernandez Saborit A, Gonzalez Nuñez S, Suño M. Strumal Carcinoid Focus in Mature Cystic Teratoma in a Patient With Breast Cancer and Desire for Fertility Preservation. Surg Case Rep (2020) 3(7):2–3. doi: 10.31487/j.SCR.2020.07.08
10. Kawano K, Ushijima K, Fujimoto T, Komai K, Kamura T. Peptide YY Producing Strumal Carcinoid of the Ovary as the Cause of Severe Constipation With Contralateral Epithelial Ovarian Cancer. J Obstet Gynaecol Res (2007) 33(3):392–6. doi: 10.1111/j.1447-0756.2007.00544.x
11. Kurabayashi T, Minamikawa T, Nishijima S, Tsuneki I, Tamura M, Yanase T, et al. Primary Strumal Carcinoid Tumor of the Ovary With Multiple Bone and Breast Metastases. J Obstet Gynaecol Res (2010) 36(3):567–71. doi: 10.1111/j.1447-0756.2010.01231.x
12. Quiñonez E, Schuldt M, Retamero JA, Nogales FF. Ovarian Strumal Carcinoid Containing Appendiceal-Type Mucinous Tumor Patterns Presenting as Pseudomyxoma Peritonei. Int J Gynecol Pathol (2015) 34(3):293–7. doi: 10.1097/PGP.0000000000000138
13. Armes JE, Ostör AG. A case of malignant strumal carcinoid. Gynecol Oncol (1993) 51(3):419–23. doi: 10.1006/gyno.1993.1316
14. Khadilkar UN, Pai RR, Lahiri R, Kumar P. Ovarian Strumal Carcinoid–Report of a Case That Matastasized. Indian J Pathol Microbiol (2000) 43(4):459–61.
15. Sakura H, Hamada Y, Tsuruta S, Okamoto K, Nakamura S. Large Glucagon-Like Immunoreactivity in a Primary Ovarian Carcinoid. Cancer (1985) 55(5):1001–6. doi: 10.1002/1097-0142(19850301)55:5<1001::aid-cncr2820550514>3.0.co;2-9
16. Shigeta H, Taga M, Kurogi K, Kitamura H, Motoyama T, Gorai I. Ovarian Strumal Carcinoid With Severe Constipation: Immunohistochemical and mRNA Analyses of Peptide YY. Hum Pathol (1999) 30(2):242–6. doi: 10.1016/s0046-8177(99)90284-8
17. Matsuda K, Maehama T, Kanazawa K. Strumal Carcinoid Tumor of the Ovary: A Case Exhibiting Severe Constipation Associated With PYY. Gynecol Oncol (2002) 87(1):143–5. doi: 10.1006/gyno.2002.6785
18. Ashton MA. Strumal Carcinoid of the Ovary Associated With Hyperinsulinaemic Hypoglycaemia and Cutaneous Melanosis. Histopathol (1995) 27(5):463–7. doi: 10.1111/j.1365-2559.1995.tb00311.x
19. Takemori M, Nishimura R, Sugimura K, Obayashi C, Yasuda D. Ovarian Strumal Carcinoid With Markedly High Serum Levels of Tumor Markers. Gynecol Oncol (1995) 58(2):266–9. doi: 10.1006/gyno.1995.1224
20. Brunaud L, Antunes L, Sebbag H, Bresler L, Villemot JP, Boissel P. Ovarian Strumal Carcinoid Tumor Responsible for Carcinoid Heart Disease. Eur J Obstet Gynecol Reprod Biol (2001) 98(1):124–6. doi: 10.1016/s0301-2115(00)00563-7
21. Muller KE, Tafe LJ, Gonzalez JL, West LA, Schned AR. Ovarian Strumal Carcinoid Producing Peptide YY Associated With Severe Constipation: A Case Report and Review of the Literature. Int J Gynecol Pathol (2015) 34(1):30–5. doi: 10.1097/PGP.0000000000000117
22. Senterman MK, Cassidy PN, Fenoglio CM, Ferenczy A. Histology, Ultrastructure, and Immunohistochemistry of Strumal Carcinoid: A Case Report. Int J Gynecol Pathol (1984) 3(2):232–40. doi: 10.1097/00004347-198402000-00011
23. Matías-Guiu X, Forteza J, Prat J. Mixed Strumal and Mucinous Carcinoid Tumor of the Ovary. Int J Gynecol Pathol (1995) 14(2):179–83. doi: 10.1097/00004347-199504000-00013
24. Rizk DEE, Danial MF. Strumal Carcinoid in a Mucinous Cystadenoma of the Ovary: A Case Report. J Gynecol Surg (1998) 14(2):95–9. doi: 10.1089/gyn.1998.14.95
25. Hinshaw HD, Smith AL, Desouki MM, Olawaiye AB. Malignant Transformation of a Mature Cystic Ovarian Teratoma Into Thyroid Carcinoma, Mucinous Adenocarcinoma, and Strumal Carcinoid: A Case Report and Literature Review. Case Rep Obstet Gynecol (2012) 2012:269489. doi: 10.1155/2012/269489
26. Borghese M, Razzore P, Ferrero A, Daniele L, Mariani LL, Sgro LG, et al. Metastatic Bilateral Strumal Carcinoid: A Case Report and Review of the Literature. Anticancer Res (2019) 39(9):5053–56. doi: 10.21873/anticanres.13697
27. Percicote AP, Hatschbach SBB, Ioshii SO, Coelho de Castilho TJ, Bahr JA, Zamboni CG. Papillary Thyroid Carcinoma in Ovarian Strumal Carcinoid Tumor: Case Report. J Bras Patol Med Lab (2013) 49(2):126–9. doi: 10.1590/S1676-24442013000200008
28. Hart WR, Regezi JA. Strumal Carcinoid of the Ovary: Ultrastructural Observations and Long-Term Follow-Up Study. Am J Clin Pathol (1978) 69(3):356–59. doi: 10.1093/ajcp/69.1.356
29. Robboy SJ, Scully RE. Strumal Carcinoid of the Ovary: An Analysis of 50 Cases of a Distinctive Tumor Composed of Thyroid Tissue and Carcinoid. Cancer (1980) 46(9):2019–34. doi: 10.1002/1097-0142(19801101)46:9<2019::aid-cncr2820460921>3.0.co;2-w
30. Baudin E, Caplin M, Garcia-Carbonero R, Fazio N, Ferolla P, Filosso PL, et al. ESMO Guidelines Committee. Electronic Address: [email protected]. Lung and Thymic Carcinoids: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2021) 32(4):439–51. doi: 10.1016/j.annonc.2021.01.003
31. Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, et al. ESMO Guidelines Committee. Electronic Address: [email protected]. Gastroenteropancreatic Neuroendocrine Neoplasms: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2020) 31(7):844–60. doi: 10.1016/j.annonc.2020.03.304
32. Roca E, Gurizzan C, Amoroso V, Vermi W, Ferrari V, Berruti A. Outcome of Patients With Lung Adenocarcinoma With Transformation to Small-Cell Lung Cancer Following Tyrosine Kinase Inhibitors Treatment: A Systematic Review and Pooled Analysis. Cancer Treat Rev (2017) 59:117–22. doi: 10.1016/j.ctrv.2017.07.007
33. Gurizzan C, Zamparini M, Volante M, Tovazzi V, Amoroso V, Consoli F, et al. Outcome of Patients With Intrathyroidal Thymic Carcinoma: A Pooled Analysis. Endocr Relat Cancer (2021) 28(8):593–604. doi: 10.1530/ERC-21-0123
34. Li X, Wang JG, Zhao J, Song X, Wang HQ. Ultrasound Imaging and Clinical Pathologic Analysis of Ovarian Strumal Carcinoid. Int J Clin Exp Pathol (2017) 10(3):3691–96.
Google Scholar
35. Van Calster B, Van Hoorde K, Valentin L, Testa AC, Fischerova D, Van Holsbeke C, et al. International Ovarian Tumour Analysis Group. Evaluating the Risk of Ovarian Cancer Before Surgery Using the ADNEX Model to Differentiate Between Benign, Borderline, Early and Advanced Stage Invasive, and Secondary Metastatic Tumours: Prospective Multicentre Diagnostic Study. BMJ (2014) 349:g5920. doi: 10.1136/bmj.g5920
36. Van Calster B, Van Hoorde K, Froyman W, Kaijser J, Wynants L, Landolfo C, et al. Practical Guidance for Applying the ADNEX Model From the IOTA Group to Discriminate Between Different Subtypes of Adnexal Tumors. Facts Views Vis Obgyn (2015) 7(1):32–41.
37. Gaurilcikas A, Gedgaudaite M, Cizauskas A, Atstupenaite V, Paskauskas S, Gaurilcikiene D, et al. Performance of the IOTA ADNEX Model on Selected Group of Patients With Borderline Ovarian Tumours. Med (Kaunas) (2020) 56(12):690. doi: 10.3390/medicina56120690
38. Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. ESMO Guidelines Committee. Electronic Address: [email protected]. Thyroid Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2019) 30(12):1856–83. doi: 10.1093/annonc/mdz400
39. Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, et al. Vienna Consensus Conference Participants. ENETS Consensus Guidelines Update for the Management of Patients With Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinol (2016) 103(2):153–71. doi: 10.1159/000443171
40. Falconi M, Bartsch DK, Eriksson B, Klöppel G, Lopes JM, O'Connor JM, et al. Barcelona Consensus Conference Participants. ENETS Consensus Guidelines for the Management of Patients With Digestive Neuroendocrine Neoplasms of the Digestive System: Well-Differentiated Pancreatic non-Functioning Tumors. Neuroendocrinol (2012) 95(2):120–34. doi: 10.1159/000335587
Keywords: ovarian strumal carcinoid, neuroendocrine tumors, teratomas, peptide YY, constipation
Citation: Turla A, Zamparini M, Milione M, Grisanti S, Amoroso V, Pedersini R, Cosentini D and Berruti A (2022) Ovarian Strumal Carcinoid: Case Report, Systematic Literature Review and Pooled Analysis. Front. Endocrinol. 13:871210. doi: 10.3389/fendo.2022.871210
Received: 07 February 2022; Accepted: 24 March 2022; Published: 21 April 2022.
Reviewed by:
Copyright © 2022 Turla, Zamparini, Milione, Grisanti, Amoroso, Pedersini, Cosentini and Berruti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alfredo Berruti, [email protected]
† These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Open access
- Published: 13 April 2024
Associations between transport modes and site-specific cancers: a systematic review and meta-analysis
- Win Thu 1 ,
- Alistair Woodward 1 ,
- Alana Cavadino 1 &
- Sandar Tin Tin 1 , 2
Environmental Health volume 23 , Article number: 39 ( 2024 ) Cite this article
90 Accesses
Metrics details
Physical inactivity is a global public health problem. A practical solution would be to build physical activity into the daily routine by using active modes of transport. Choice of transport mode can influence cancer risk through their effects on levels of physical activity, sedentary time, and environmental pollution. This review synthesizes existing evidence on the associations of specific transport modes with risks of site-specific cancers.
Relevant literature was searched in PubMed, Embase, and Scopus from 1914 to 17th February 2023. For cancer sites with effect measures available for a specific transport mode from two or more studies, random effects meta-analyses were performed to pool relative risks (RR) comparing the highest vs. lowest activity group as well as per 10 Metabolic Equivalent of Task (MET) hour increment in transport-related physical activity per week ( ∼ 150 min of walking or 90 min of cycling).
27 eligible studies (11 cohort, 15 case-control, and 1 case-cohort) were identified, which reported the associations of transport modes with 10 site-specific cancers. In the meta-analysis, 10 MET hour increment in transport-related physical activity per week was associated with a reduction in risk for endometrial cancer (RR: 0.91, 95% CI: 0.83–0.997), colorectal cancer (RR: 0.95, 95% CI: 0.91–0.99) and breast cancer (RR: 0.99, 95% CI: 0.89–0.996). The highest level of walking only or walking and cycling combined modes, compared to the lowest level, were significantly associated with a 12% and 30% reduced risk of breast and endometrial cancers respectively. Cycling, compared to motorized modes, was associated with a lower risk of overall cancer incidence and mortality.
Active transport appears to reduce cancer risk, but evidence for cancer sites other than colorectum, breast, and endometrium is currently limited.
Peer Review reports
Introduction
Physical inactivity is a global public health problem, contributing to substantial disease and economic burden worldwide [ 1 , 2 ]. With rapid changes in technology, lifestyle, and habitual environment, people have been less active and more sedentary over the past few decades. Globally, about 1 in 4 adults were not active, i.e., did not meet the World Health Organization (WHO) recommendation of engaging at least 150–300 min of moderate-intensity or 75–150 min of vigorous-intensity aerobic physical activity per week [ 3 ], but the prevalence varied widely within and across countries [ 4 ]. If the current trends continue, it is unlikely that the WHO’s target to reduce physical inactivity by 10% in 2025 will be met.
One practical solution would be to build physical activity into the daily routine by using active modes of transport [ 5 ]. Walking and cycling have been shown to improve health (mainly all-cause mortality, cardiovascular disease, diabetes, and cancer) [ 6 ] and also provide social, economic and environmental benefits [ 7 , 8 ]. Car use, on the other hand, contributes to a significant proportion of daily sedentary time, and the situation is worsening with increasing traffic congestion/delays [ 9 ]; it has been associated with an increased risk of obesity and related outcomes [ 10 ]. Further, exposure to environmental pollutants such as nitrogen dioxide and/or particulate matter could differ across different road users [ 11 ], while it has been shown to increase the risk of certain cancers, particularly lung cancer [ 12 ].
While there is ample evidence linking leisure time physical activity or physical activity in general with a reduced risk for a number of cancer sites [ 13 , 14 ], and sedentary behavior in general with an increased risk [ 15 ], the findings may not be directly applicable to transport-related activity because the context and correlates of activity as well as its frequency, duration and intensity are likely to be different across different domains. We therefore reviewed the existing literature that reported the associations between transport modes and risks of site-specific cancers.
A systematic literature review and meta-analysis was conducted and reported according to the PRISMA guideline (Supplementary file S1 ). The review was not registered.
Search strategy and study selection
Relevant literature was searched from 1914 to 17th February 2023 in PubMed, Scopus, and Embase databases using the relevant search terms such as walking, cycling, car, public transport, commute and cancers. Site-specific cancers known to be associated with physical activity and body weight such as breast, colon, liver, esophageal adenocarcinoma and those associated with environmental factor such as lung and melanoma of skin were also searched (Supplementary file S2 ). The reference lists of systematic reviews on physical activity and cancers were also reviewed. Studies were included if they (1) used cohort, case-control, case-cohort or experimental design, (2) assessed transport modes such as walking, cycling, public transport or car use as the exposures of interest, (3) investigated one or more site-specific cancers, overall cancer incidence and/or mortality as the outcome(s), (4) reported effect measures associated with transport modes, and (5) published the full article in English. Studies that used cross-sectional design or mathematical modeling to estimate health impacts at the population level were excluded. Details of excluded studies after full text review, together with the reasons for exclusion, were provided in the Supplementary file S3 . WT conducted the search and selection, and STT oversaw the process.
Data extraction and study quality assessment
Information about title, first author, year of publication, study name (if available), country, study design, sample size, age range of the participants, follow-up duration (for cohort and case-cohort studies), data collection tool, measurement units for exposure(s), data sources for outcome(s), site-specific cancer assessed, effect measures, and confounders adjusted were extracted in a standardized data collection spreadsheet. The study quality was evaluated using the Newcastle-Ottawa Scale (NOS) [ 16 ], which scores the cohort and case-control studies based on three domains: selection of study groups, comparability of the groups and ascertainment of exposure (case-control studies) or outcome (cohort studies). For the second domain, a point was awarded for adjustment of Body Mass Index (BMI) - to evaluate the direct vs. indirect (through BMI) effect of physical activity on cancer risk, and another point for adjustment of physical activities from other domains - to isolate the effects of transport-related physical activity from other activities. A maximum of nine points were awarded, with a higher score indicating better quality [ 16 ]. For case-cohort studies, the NOS scale for cohort studies was used. WT conducted the data extraction and quality assessment, and STT oversaw the process.
Data analysis
For cancer sites with effect measures available for a specific transport mode from two or more studies, meta-analyses were performed using random effects models. The analyses compared the highest level of active transport such as walking, cycling or mixed mode with the lowest level as reported in the individual studies. Where necessary, the reference category for exposure was changed to the lowest group to facilitate pooling of the risks [ 17 ]. The pooled relative risks (RRs) and 95% CI were presented for breast, endometrial, colorectal and testicular cancers, and overall cancer mortality.
For studies that reported time or MET as measurement units, the dose-response effects were estimated using the trend estimation method proposed by Greenland and Longnecker [ 18 ]. The reported time spent for each mode/category was converted to MET hours (see Supplementary file S4 for conversion values and formulas used). For studies that only reported estimates for categorical exposures, study-specific slopes were calculated from the natural logs of the reported risk estimates across categories and risk estimates per unit change were then estimated. The pooled results were presented per 10 Metabolic Equivalent of Task (MET) hour increment in transport-related physical activity per week ( ∼ 150 min of walking or 90 min of cycling) to align with the WHO’s physical activity recommendation [ 3 ]. This approach enabled us to pool risk estimates from a large number of studies irrespective of how the exposures were assessed (e.g., walking and cycling separately or combined) or categorised. The results were presented for breast, endometrial, colorectal, prostate cancers, and overall cancer mortality.
Meta-analysis was not conducted for the studies that compared active and non-active modes in relation to overall cancer incidence and mortality due to the potential overlap of the study samples.
For meta-analyses involving four or more studies, publication bias was assessed through the visual inspection of funnel plots, Begg’s rank correlation test, and Egger’s regression test for asymmetry. If significant associations were observed, sensitivity analyses were conducted by removing one study at a time from the initial meta-analysis to test the robustness of the results. Where possible, sub-group analyses were performed to assess variability of summary effects across population groups (Western vs. Asian), study design (cohort vs. case-control), measurement units (time vs. MET) and adjustment for BMI (yes vs. no). Metafor [ 19 ] and dosresmeta [ 20 ] R packages were used for meta-analysis and trend estimation. All authors have access to the data.
Of the 11,829 records identified, 27 unique studies (total 34 publications) were included, of which 22 studies (28 records) contributed to the meta-analyses (Fig. 1 ). There were four publications from the Netherlands Cohort Study which reported endometrial [ 21 ], ovarian [ 22 ], prostate [ 23 ], and colorectal [ 24 ] cancers, three publications from United Kingdom Biobank which reported lung [ 25 ], breast and colon [ 26 ], and overall cancer incidence and mortality [ 27 ], two publications from Shanghai Women’s Health Study which reported breast [ 28 ] and overall cancer mortality [ 29 ], and two publications from National Institutes of Health - American Association of Retired Persons Diet and Health Study which reported breast [ 30 ] and endometrial [ 31 ] cancers. Of the included studies, 20 compared the risks between the highest and lowest levels of active transport (e.g., walking, cycling, walking and cycling) and two compared the risk between active and non-active commuting modes. The majority used case-control design ( n = 15), followed by cohort ( n = 11) and case-cohort ( n = 1) designs. Most of the studies were conducted in North America, mainly in the United States (US) ( n = 7), followed by Europe ( n = 5), China ( n = 5), United Kingdom ( n = 4), Australia ( n = 2) and the remaining four studies were from India, Iran, Brazil and Nigeria. (Table 1 )
Almost half of the studies assessed walking and cycling combined, i.e., did not provide the risk estimates for each mode ( n = 13), while others assessed walking and cycling separately ( n = 8), or assessed only one mode (walking: n = 3 and cycling: n = 3). Most studies quantified active transport in terms of time spent (e.g., minutes per day, hours per week) ( n = 14) or MET ( n = 7), but others assessed it in terms of activity status (e.g., yes, no) ( n = 3), or in comparison to car or motorized mode ( n = 2), and distance ( n = 1) (Supplementary file S5 ). The studies reported the risks associated with ten site-specific cancers, most commonly breast ( n = 12), endometrial ( n = 5), and colorectal ( n = 4) cancers (Fig. 2 ). Cancer cases were identified through cancer registries, death registries, pathological reports, or hospital or medical records (Supplementary file S5 ). The NOS score for cohort studies ranged from 5 to 9, with an average score of 6.5, and the score for case-control studies ranged from 4 to 7, with an average score of 5.6 (Table 1 , detailed scoring in Supplementary file S6 , S7 ).
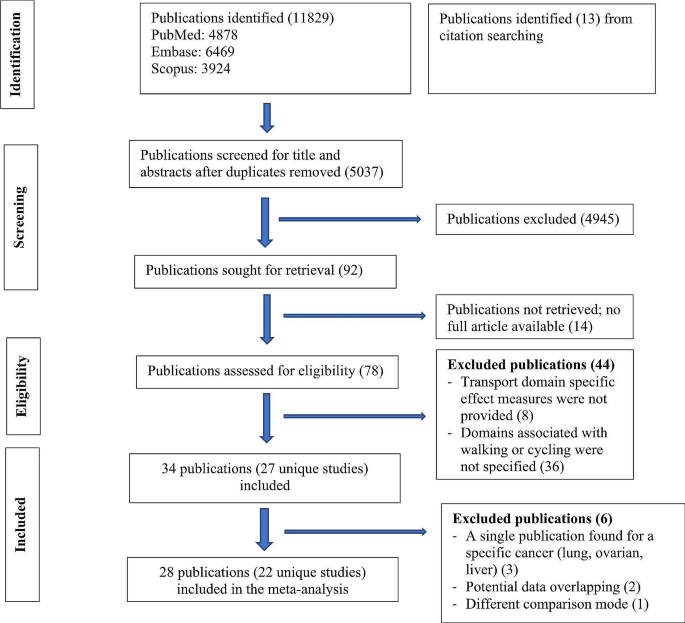
Flow diagram for study selection
Cancers reported in the studies
Active transport studies
The pooled results were presented for breast, endometrial, colorectal, testicular and prostate cancers, and overall cancer mortality (Fig. 3 ). For other cancers where only one study was identified, the results from the individual study were presented.
Breast cancer
In the meta-analysis of six studies comparing the highest vs. lowest activity group, an inverse association was observed for walking (RR: 0.88, 95% CI: 0.78–0.98), a borderline inverse association for cycling (RR: 0.90, 95% CI: 0.77–1.05) and no significant association for walking and cycling combined (RR: 0.97, 95% CI: 0.84–1.12). 10 MET hour increment in transport-related physical activity per week ( ∼ 150 min of walking or 90 min of cycling) was associated with a marginally reduced risk (RR: 0.99, 95% CI: 0.97–0.996). (Fig. 3 , detailed forest plots in the supplementary file S9 )
Endometrial cancer
The meta-analysis of four studies indicated that walking and cycling combined was associated with a reduced risk of endometrial cancer (RR comparing highest vs. lowest: 0.70, 95% CI: 0.56–0.87; RR per 10 MET hour increment in activity per week: 0.91, 95% CI: 0.83–0.997). (Fig. 3 , detailed forest plots in S9 )
Colorectal cancer
In the meta-analysis of two studies, walking and cycling combined was associated with a reduced risk of colorectal cancer (RR comparing highest vs. lowest: 0.89, 95% CI: 0.78–1.01; RR per 10 MET hour increment in activity per week: 0.95, 95% CI: 0.91–0.99) (Fig. 3 , detailed forest plot in S9 ).
Testicular cancer
In the meta-analysis of two studies, there was no significant association between cycle commuting in adolescence and testicular germ cell cancer (RR comparing highest vs. lowest: 1.23, 95% CI: 0.71–2.13). (Fig. 3 , detailed forest plot in S9 )
Prostate cancer
10 MET hour increment per week for transport related physical activity was associated with a reduced risk of prostate cancer (RR: 0.96, 95% CI: 0.88–1.04) (Fig. 3 , detailed forest plot in S9 ).
Ovarian cancer
Only a case-cohort study assessed the relationship of walking and cycling combined mode with ovarian cancer risk, and reported no significant association (Supplementary file S5 ) [ 22 ].
Liver, gallbladder and biliary tract cancers
A cohort study reported a significant association of commuting physical activity with a reduced risk of gallbladder and biliary tract cancers in women (HR: 0.51, 95% CI: 0.28–0.94) but not in men (HR: 0.92, 95% CI: 0.61–1.37); there was no significant association with liver cancer in both sexes (supplementary file S5 ) [ 49 ].
Renal cancer
A case-control study assessed the association of walking and cycling with risk of renal cell carcinoma in white and black participants in the ages of 20s and 50s, and reported a significant association in the white participants in their 20s (OR comparing lowest vs. highest: 1.42, 95% CI: 1.10–1.83) but not in the black counterparts; the associations were also not significant in both groups in their 50s. (Supplementary file S5 ) [ 50 ].
Overall cancer mortality
In the meta-analyses of two studies, there was an inverse association for cycling only (RR comparing highest vs. lowest: 0.60, 95% CI: 0.34–1.04) and walking and cycling combined (RR: 0.98, 95% CI: 0.86–1.12), and also per 10 MET hour increment in activity per week (RR: 0.97, 95% CI: 0.92–1.01). (Fig. 3 , detailed forest plots in S9 )
In sub-group analyses, similar associations were observed between walking and breast cancer risk in terms of study design (cohort, case-control), population (western vs. Asian), measurement unit (time vs. MET), menopausal status (premenopausal and postmenopausal) and adjustment of BMI (yes vs. no); however, the associations were stronger in studies that adjusted for physical activity from other domains (Supplementary file S10 ). In the leave-one-out analyses assessing walking and breast cancer risk, the results were sensitive to effect sizes from some studies, but this was not the case for walking and cycling combined mode and endometrial cancer (Supplementary file S11 ). There was no evidence for funnel plot asymmetry; Egger’s regression tests and Begg’s ranks correlation tests were not significant (Supplementary file S12 ).
Results of meta-analysis for active transport studies. RE = a random-effects model, MET = Metabolic Equivalent of Task, I 2 = I 2 statistics for heterogeneity, RR = Summary relative risk
Studies comparing active vs. non-active modes for commuting
Four eligible publications were identified, of which three used the data from UK Biobank [ 25 , 26 , 27 ], one used the UK census data [ 51 ]. Three reported the associations for overall cancer incidence and mortality, and one reported the risk associated with lung cancer (Fig. 4 ). In the study that assessed lung cancer using the data from UK Biobank, when compared to automobile only mode, active modes did not show a significant association whereas frequent use of public transport (≥ 5 trips per week) was associated with an increased risk of lung cancer (HR: 1.58, 95% CI: 1.08–2.33) [ 25 ] (Fig. 4 ). In another UK Biobank study, no significant associations were observed for breast and colon cancers, and overall cancer incidence and mortality when more active patterns of commuting (walking, cycling, public transport, either alone or in combination with car) were compared to car only mode [ 26 ].
The results of two studies [ 27 , 51 ] that assessed overall cancer incidence and mortality were not combined as the outcome data was extracted from the same national cancer registry with an overlapped time frame (1991–2011 and 2007–2014), although the exposure information came from different sources (census and UK Biobank). In these studies, compared to private motorized mode or non-active mode, cycling was inversely associated with overall cancer incidence and mortality. Walking and public transport were also inversely associated with overall cancer incidence in the study that used the census data [ 51 ].
Results of the individual studies comparing active vs. non-active modes for commuting. Private motorized mode = car or motorcycle, Non-active = car or public transport, Active patterns of commuting = any other patterns including walking, cycling, public transport, either alone or in combination with car, HR = Hazard Ratio, regular:1–4, often: ≥5 work-bound trips/week
This review identified 27 studies (34 publications) that reported the associations of specific transport modes, mainly active transport modes, with risks of ten site-specific cancers along with overall cancer incidence and mortality. The most frequently studied cancer sites were breast, endometrium, and colorectum; our meta-analysis showed a reduction in risk of these cancers (1%, 9% and 5%, respectively) per 10 MET hour per week increment in transport-related physical activity ( ∼ 150 min of walking or 90 min of cycling).
We found an inverse association between active transport and risks of breast and endometrial cancers, with similar magnitude of risk reduction observed in previous systematic reviews on physical activity in general [ 55 , 56 ]. While obesity is known to increase post-menopausal but not pre-menopausal breast cancer risk [ 57 ], we found similar results by menopausal status. In contrast, an earlier review did not find any significant association between walking in general and risk of pre- or post-menopausal breast cancer [ 58 ], possibly because compared to walking for transport, walking for leisure or at home generally uses lower energy [ 59 ], and therefore may have less effect on body weight.
The inverse association of active transport with colorectal cancer risk observed in this review is also consistent with the findings from existing reviews on transport-related physical activity [ 60 ] as well as physical activity in general [ 61 ]. While physical activity in general or for leisure has also been associated with a reduced risk of many other cancer sites including liver, gastric, renal and lung [ 13 , 14 ], the evidence related to transport-related physical activity is currently limited.
Mechanisms linking physical activity with specific cancer sites have been proposed, including its effects on sex hormones (breast, endometrial and prostate cancers), insulin sensitivity, glucose metabolism and adipokines (obesity-related cancers), and inflammation and immune function (most cancers) [ 62 ]. For colorectal cancer, another potential mechanism is reduced contact time between carcinogens and bowel mucosa cells due to exercise-induced intestinal mobility [ 63 ].
The overall quality of the included studies, evaluated by NOS score, ranged from 4 to 9, and in general, cohort studies tend to have higher scores compared to case-control studies. The common criteria the studies did not meet include: inadequate exposure assessment, loss to follow-up (cohort studies) and low response rates (case-control studies). While we were not able to undertake subgroup analyses by NOS score due to the limited number of studies available, our subgroup analyses by study design showed similar associations between walking and breast cancer in cohort vs. case-control studies.
To our knowledge, this review represents the first systematic attempt to synthesize the existing evidence on specific transport modes and site-specific cancers. We provided mode-specific summary effects where possible and calculated the dose-response effects for transport-related physical activity, in line with WHO physical activity recommendation. When interpreting the findings, some limitations need to be considered. First, the review may not have included some eligible studies published in languages other than English. Second, due to the limited number of available studies, we were not able to pool the results separately for cohort and case-control studies; however, we conducted sub-group analyses by study design where possible. We were not able to evaluate the non-linear relationship between transport-related physical activity and the risks of site-specific cancers. While a recent systematic review on breast and colon cancers reported a linear relationship with physical activity [ 64 ], others suggested a non-linear relationship between physical activity and cancer risk [ 65 , 66 ]. Further, variations in measurement and categorization of the exposure across the studies make direct comparison of the results between different modes (e.g., walking vs. cycling) difficult. Finally, the majority of the studies included were conducted in high income countries in Europe, UK, and North America, limiting the generalizability of the findings to other populations and low and middle income countries where urbanization and motorization are mainly taking place [ 67 ].
Our findings suggest that transport choices may influence cancer risk, particularly of obesity-related cancers such as breast, colon and endometrial cancers. Breast cancer is the most common cancer in women globally, with an estimated over 2 million new cases (11.7% of all new cases) in 2020, while colon cancer stood at fourth place (over 1 million cases, 6% of total cases) [ 68 ]. The incidence of endometrial cancer also seems to be increasing in many countries particularly in younger women. Our findings indicate that the risks of these cancers can be reduced by meeting the WHO physical activity recommendation through active commuting ( ∼ 150 min of walking or 90 min of cycling per week). Yet, the current evidence is limited in relation to other cancer sites, underlying mechanisms, and potential environmental influences, requiring further exploration.
Given heterogeneity in exposure measurements in the existing studies, harmonizing choice of the assessment tool (e.g., using International Physical Activity Questionnaires that can capture information about all four physical activity domains including transport modes), and reporting the dose-response estimates for each transport mode such as walking and cycling separately rather than a combined mode would enhance comparability of results and provide mode-specific effects. Repeated or regular assessments of exposures/transport modes used throughout the study duration would capture changes and their potential impact on outcomes in cohort studies. Importantly, more research is needed in low and middle-income settings to generate context-specific evidence.
In conclusion, active transport modes appear to reduce cancer risk, but evidence for cancer sites other than colorectum, breast and endometrium is currently limited.
Data availability
No datasets were generated or analysed during the current study.
Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–29. https://doi.org/10.1016/S0140-6736(12)61031-9 .
Article Google Scholar
Ding D, Lawson KD, Kolbe-Alexander TL, et al. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet. 2016;388(10051):1311–24. https://doi.org/10.1016/S0140-6736(16)30383-X .
World Health Organization. Global status report on physical activity 2022.; 2022.
Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Global Health. 2018;6(10):e1077–86. https://doi.org/10.1016/S2214-109X(18)30357-7 .
Berrigan D, Troiano RP, McNeel T, DiSogra C, Ballard-Barbash R. Active transportation increases adherence to activity recommendations. Am J Prev Med. 2006;31(3):210–6. https://doi.org/10.1016/j.amepre.2006.04.007 .
Dinu M, Pagliai G, Macchi C, Sofi F. Active commuting and multiple Health outcomes: a systematic review and Meta-analysis. Sports Med. 2019;49(3):437–52. https://doi.org/10.1007/s40279-018-1023-0 .
Boniface S, Scantlebury R, Watkins SJ, Mindell JS. Health implications of transport: evidence of effects of transport on social interactions. J Transp Health. 2015;2(3):441–6. https://doi.org/10.1016/j.jth.2015.05.005 .
Higgins PAT. Exercise-based transportation reduces oil dependence, carbon emissions and obesity. Envir Conserv. 2005;32(3):197–202. https://doi.org/10.1017/S037689290500247X .
Article CAS Google Scholar
World Health Organization. Global Status Report on Road Safety 2018: Summary.; 2018.
Sugiyama T, Chandrabose M, Homer AR, Sugiyama M, Dunstan DW, Owen N. Car use and cardiovascular disease risk: systematic review and implications for transport research. J Transp Health. 2020;19:100930. https://doi.org/10.1016/j.jth.2020.100930 .
Panchal R, Panagi M, May HR, et al. Personal air pollution exposure during morning commute car and active transport journeys. J Transp Health. 2022;26:101365. https://doi.org/10.1016/j.jth.2022.101365 .
Turner MC, Andersen ZJ, Baccarelli A, et al. Outdoor air pollution and cancer: an overview of the current evidence and public health recommendations. CA Cancer J Clin. 2020;70(6):460–79. https://doi.org/10.3322/caac.21632 .
Moore SC, Lee IM, Weiderpass E, et al. Association of leisure-time physical activity with risk of 26 types of Cancer in 1.44 million adults. JAMA Intern Med. 2016;176(6):816. https://doi.org/10.1001/jamainternmed.2016.1548 .
Rezende LFMD, Sá THD, Markozannes G, et al. Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases. Br J Sports Med. 2018;52(13):826–33. https://doi.org/10.1136/bjsports-2017-098391 .
Hermelink R, Leitzmann MF, Markozannes G, et al. Sedentary behavior and cancer–an umbrella review and meta-analysis. Eur J Epidemiol. 2022;37(5):447–60. https://doi.org/10.1007/s10654-022-00873-6 .
Wells G, Shea B, O’Connell D et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
Taylor K. Wanting a particular reference category in categorical risk data. Data extraction tips for meta-analysis. https://www.cebm.ox.ac.uk/resources/data-extraction-tips-meta-analysis/reference-category-risk-data .
Greenland S, Longnecker MP. Methods for Trend Estimation from Summarized Dose-Response Data, with applications to Meta-Analysis. Am J Epidemiol. 1992;135(11):1301–9. https://doi.org/10.1093/oxfordjournals.aje.a116237 .
Viechtbauer W. Conducting Meta-analyses in R with the metafor Package. J Stat Soft. 2010;36(3). https://doi.org/10.18637/jss.v036.i03 .
Crippa A, Orsini N. Multivariate Dose-Response Meta-Analysis: The dosresmeta R Package. J Stat Soft. 2016;72(Code Snippet 1). https://doi.org/10.18637/jss.v072.c01 .
Schouten LJ, Goldbohm RA, van den Brandt PA, Anthropometry. Physical activity, and Endometrial Cancer Risk: results from the Netherlands Cohort Study. JNCI J Natl Cancer Inst. 2004;96(21):1635–8. https://doi.org/10.1093/jnci/djh291 .
Biesma RG, Schouten LJ, Dirx MJM, Goldbohm RA, van den Brandt PA. Physical activity and risk of ovarian Cancer: results from the Netherlands Cohort Study (the Netherlands). Cancer Causes Control. 2006;17(1):109–15. https://doi.org/10.1007/s10552-005-0422-3 .
Zeegers MPA, Dirx MJM, van den Brandt PA. Physical activity and the risk of prostate Cancer in the Netherlands Cohort Study, results after 9.3 years of follow-up. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1490–5. https://doi.org/10.1158/1055-9965.EPI-04-0771 .
Simons CCJM, Hughes LAE, van Engeland M, Goldbohm RA, van den Brandt PA, Weijenberg MP. Physical activity, occupational sitting time, and Colorectal Cancer Risk in the Netherlands Cohort Study. Am J Epidemiol. 2013;177(6):514–30. https://doi.org/10.1093/aje/kws280 .
Wong JYY, Jones RR, Breeze C, et al. Commute patterns, residential traffic-related air pollution, and lung cancer risk in the prospective UK Biobank cohort study. Environ Int. 2021;155:106698. https://doi.org/10.1016/j.envint.2021.106698 .
Panter J, Mytton O, Sharp S, et al. Using alternatives to the car and risk of all-cause, cardiovascular and cancer mortality. Heart. 2018;104(21):1749–55. https://doi.org/10.1136/heartjnl-2017-312699 .
Celis-Morales CA, Lyall DM, Welsh P, et al. Association between active commuting and incident cardiovascular disease, cancer, and mortality: prospective cohort study. BMJ Published Online April. 2017;19:j1456. https://doi.org/10.1136/bmj.j1456 .
Pronk A, Ji BT, Shu XO, et al. Physical activity and breast cancer risk in Chinese women. Br J Cancer. 2011;105(9):1443–50. https://doi.org/10.1038/bjc.2011.370 .
Matthews CE, Jurj AL, Shu Xo, et al. Influence of Exercise, walking, Cycling, and overall nonexercise physical activity on Mortality in Chinese women. Am J Epidemiol. 2007;165(12):1343–50. https://doi.org/10.1093/aje/kwm088 .
George SM, Irwin ML, Matthews CE, et al. Beyond recreational physical activity: examining Occupational and Household Activity, Transportation Activity, and sedentary behavior in relation to postmenopausal breast Cancer Risk. Am J Public Health. 2010;100(11):2288–95. https://doi.org/10.2105/AJPH.2009.180828 .
Gierach GL, Chang SC, Brinton LA, et al. Physical activity, sedentary behavior, and endometrial cancer risk in the NIH-AARP Diet and Health Study. Int J Cancer. 2009;124(9):2139–47. https://doi.org/10.1002/ijc.24059 .
Luoto R, Latikka P, Pukkala E, Hakulinen T, Vihko V. The effect of physical activity on breast cancer risk: a cohort study of 30,548 women. Eur J Epidemiol. 2000;16(10):973–80. https://doi.org/10.1023/A:1010847311422 .
Gomes MLB, Pinto SS, Domingues MR. Physical activity and breast Cancer: a case-control study in Southern Brazil. Nutr Cancer. 2022;74(1):149–57. https://doi.org/10.1080/01635581.2021.1880607 .
Azubuike SO, Hayes L, Sharp L, Alabi A, Oyesegun RA, McNally R. Physical activity and the risk of breast cancer among Nigerian women. Cancer Epidemiol. 2022;78:102163. https://doi.org/10.1016/j.canep.2022.102163 .
Si S, Boyle T, Heyworth J, Glass DC, Saunders C, Fritschi L. Lifetime physical activity and risk of breast cancer in pre-and post-menopausal women. Breast Cancer Res Treat. 2015;152(2):449–62. https://doi.org/10.1007/s10549-015-3489-x .
Mathew A, Gajalakshmi V, Rajan B, et al. Physical activity levels among urban and rural women in south India and the risk of breast cancer: a case–control study. Eur J Cancer Prev. 2009;18(5):368–76. https://doi.org/10.1097/CEJ.0b013e32832e1c46 .
Steindorf K. Case-control study of physical activity and breast Cancer risk among Premenopausal women in Germany. Am J Epidemiol. 2003;157(2):121–30. https://doi.org/10.1093/aje/kwf181 .
John EM, Horn-Ross PL, Koo J. Lifetime physical activity and breast cancer risk in a multiethnic population: the San Francisco Bay area breast cancer study. Cancer Epidemiol Biomarkers Prev. 2003;12(11 Pt 1):1143–52.
Google Scholar
Matthews CE, Shu XO, Jin F, et al. Lifetime physical activity and breast cancer risk in the Shanghai breast Cancer Study. Br J Cancer. 2001;84(7):994–1001. https://doi.org/10.1054/bjoc.2000.1671 .
Marcus PM, Newman B, Moorman PG, et al. Physical activity at age 12 and adult breast cancer risk (United States). Cancer Causes Control. 1999;10(4):293–302. https://doi.org/10.1023/A:1008971417282 .
Friberg E, Mantzoros CS, Wolk A. Physical activity and risk of Endometrial Cancer: a Population-based prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2136–40. https://doi.org/10.1158/1055-9965.EPI-06-0465 .
John EM, Koo J, Horn-Ross PL. Lifetime physical activity and risk of Endometrial Cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1276–83. https://doi.org/10.1158/1055-9965.EPI-09-1316 .
Matthews CE, Xu WH, Zheng W, et al. Physical activity and risk of Endometrial Cancer: a report from the Shanghai Endometrial Cancer Study. Cancer Epidemiol Biomarkers Prev. 2005;14(4):779–85. https://doi.org/10.1158/1055-9965.EPI-04-0665 .
Mahmood S, English DR, MacInnis RJ, et al. Domain-specific physical activity and the risk of colorectal cancer: results from the Melbourne Collaborative Cohort Study. BMC Cancer. 2018;18(1):1063. https://doi.org/10.1186/s12885-018-4961-x .
Hou L. Commuting physical activity and risk of Colon cancer in Shanghai, China. Am J Epidemiol. 2004;160(9):860–7. https://doi.org/10.1093/aje/kwh301 .
Littman AJ, Doody DR, Biggs ML, Weiss NS, Starr JR, Schwartz SM. Physical activity in adolescence and testicular germ cell cancer risk. Cancer Causes Control. 2009;20(8):1281–90. https://doi.org/10.1007/s10552-009-9347-6 .
Coldman AJ, Elwood JM, Gallagher RP. Sports activities and risk of testicular cancer. Br J Cancer. 1982;46(5):749–56. https://doi.org/10.1038/bjc.1982.267 .
Hosseini M, SeyedAlinaghi S, Mahmoudi M, McFarland W. A case-control study of risk factors for prostate cancer in Iran. Acta Med Iran. 2010;48(1):61–6.
Pang Y, Lv J, Kartsonaki C, et al. Association of physical activity with risk of hepatobiliary diseases in China: a prospective cohort study of 0.5 million people. Br J Sports Med. 2021;55(18):1024–33. https://doi.org/10.1136/bjsports-2020-102174 .
Xiao Q, Liao L, Matthews CE, et al. Physical activity and renal cell carcinoma among black and white americans: a case-control study. BMC Cancer. 2014;14(1):707. https://doi.org/10.1186/1471-2407-14-707 .
Patterson R, Panter J, Vamos EP, Cummins S, Millett C, Laverty AA. Associations between commute mode and cardiovascular disease, cancer, and all-cause mortality, and cancer incidence, using linked Census data over 25 years in England and Wales: a cohort study. Lancet Planet Health. 2020;4(5):e186–94. https://doi.org/10.1016/S2542-5196(20)30079-6 .
Sahlqvist S, Goodman A, Simmons RK, et al. The association of cycling with all-cause, cardiovascular and cancer mortality: findings from the population-based EPIC-Norfolk cohort. BMJ Open. 2013;3(11):e003797. https://doi.org/10.1136/bmjopen-2013-003797 .
Autenrieth CS, Baumert J, Baumeister SE, et al. Association between domains of physical activity and all-cause, cardiovascular and cancer mortality. Eur J Epidemiol. 2011;26(2):91–9. https://doi.org/10.1007/s10654-010-9517-6 .
Batty GD, Shipley MJ, Marmot M, Smith GD. Physical activity and cause-specific mortality in men: further evidence from the Whitehall study. Eur J Epidemiol. 2001;17(9):863–9. https://doi.org/10.1023/A:1015609909969 .
Wu Y, Zhang D, Kang S. Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2013;137(3):869–82. https://doi.org/10.1007/s10549-012-2396-7 .
Moore SC, Gierach GL, Schatzkin A, Matthews CE. Physical activity, sedentary behaviours, and the prevention of endometrial cancer. Br J Cancer. 2010;103(7):933–8. https://doi.org/10.1038/sj.bjc.6605902 .
García-Estévez L, Cortés J, Pérez S, Calvo I, Gallegos I, Moreno-Bueno G. Obesity and breast Cancer: a paradoxical and controversial relationship influenced by Menopausal Status. Front Oncol. 2021;11:705911. https://doi.org/10.3389/fonc.2021.705911 .
Chan DSM, Abar L, Cariolou M, et al. World Cancer Research Fund International: continuous update project—systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control. 2019;30(11):1183–200. https://doi.org/10.1007/s10552-019-01223-w .
Compedium of Physical Activities. 17-Walking. Accessed August 3. 2023. https://sites.google.com/site/compendiumofphysicalactivities/Activity-Categories/walking?authuser=0 .
Mahmood S, MacInnis RJ, English DR, Karahalios A, Lynch BM. Domain-specific physical activity and sedentary behaviour in relation to colon and rectal cancer risk: a systematic review and meta-analysis. Int J Epidemiol. 2017;46(6):1797–813. https://doi.org/10.1093/ije/dyx137 .
Samad AKA, Taylor RS, Marshall T, Chapman MAS. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorect Dis. 2005;7(3):204–13. https://doi.org/10.1111/j.1463-1318.2005.00747.x .
McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8(3):205–11. https://doi.org/10.1038/nrc2325 .
Peters HPF. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut. 2001;48(3):435–9. https://doi.org/10.1136/gut.48.3.435 .
Diao X, Ling Y, Zeng Y, et al. Physical activity and cancer risk: a dose-response analysis for the global burden of Disease Study 2019. Cancer Commun. 2023;43(11):1229–43. https://doi.org/10.1002/cac2.12488 .
Garcia L, Pearce M, Abbas A, et al. Non-occupational physical activity and risk of cardiovascular disease, cancer and mortality outcomes: a dose–response meta-analysis of large prospective studies. Br J Sports Med. 2023;57(15):979–89. https://doi.org/10.1136/bjsports-2022-105669 .
Li T, Wei S, Shi Y, et al. The dose–response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med. 2016;50(6):339–45. https://doi.org/10.1136/bjsports-2015-094927 .
Unite, Nations. Department of Economic and Social Affair. World urbanization prospects: the 2018 revision. United Nations; 2019.
Sung H, Ferlay J, Siegel RL, Cancer, et al. J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660 .
Download references
Acknowledgements
STT is supported by Sir Charles Hercus Health Research Fellowship from the Health Research Council of New Zealand (Ref: 23/051).
STT is supported by Sir Charles Hercus Health Research Fellowship from the Health Research Council of New Zealand (Ref: 23/051). The funder had no role in the study design, data collection, data analysis, data interpretation, writing of the report, approval of the manuscript, or decision to submit the manuscript for publication.
Author information
Authors and affiliations.
Epidemiology and Biostatistics, School of Population Health, University of Auckland, Auckland, New Zealand
Win Thu, Alistair Woodward, Alana Cavadino & Sandar Tin Tin
Cancer Epidemiology Unit, Oxford Population Health, University of Oxford, Oxford, UK
Sandar Tin Tin
You can also search for this author in PubMed Google Scholar
Contributions
STT, AW, WT designed the study. STT supervised the study. WT conducted literature search, data analysis and wrote the original draft with critical inputs from STT, AC, and AW. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. More than one author has directly accessed and verified the underlying data reported in the manuscript.
Corresponding author
Correspondence to Sandar Tin Tin .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
: Supplementary file S1 PRISMA checklist. Supplementary file S2 Literature search strategy. Supplementary file S3 List of excluded full texts with reasons. Supplementary file S4 Metabolic Equivalent of Task (MET) values used and MET hour per week conversion formulas. Supplementary file S5 Measurement units, effect measures and covariates included in the studies. Supplementary file S6 Newcastle-Ottawa Score of the studies (cohort studies). Supplementary file S7 Newcastle-Ottawa Score of the studies (case control studies). Supplementary file S8 Risks estimates used in the meta-analyses (separate excel sheet). Supplementary file S9 Forest plots. Supplementary file S10 Sub-group and covariates adjustment analyses. Supplementary file S11 Sensitivity analysis. Supplementary file S12 Funnel plots
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Thu, W., Woodward, A., Cavadino, A. et al. Associations between transport modes and site-specific cancers: a systematic review and meta-analysis. Environ Health 23 , 39 (2024). https://doi.org/10.1186/s12940-024-01081-3
Download citation
Received : 20 November 2023
Accepted : 08 April 2024
Published : 13 April 2024
DOI : https://doi.org/10.1186/s12940-024-01081-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Transport modes
- Active transport
- Site-specific cancers
- Systematic review
- Meta-analysis

Environmental Health
ISSN: 1476-069X
- General enquiries: [email protected]
- Systematic Review
- Open access
- Published: 09 April 2024
Timing of early water intake post-general anaesthesia: a systematic review and meta-analysis
- Suwan Dai 1 ,
- Lingyan Chen 1 ,
- Liangyou Guo 1 &
- Rong Wang 2
BMC Anesthesiology volume 24 , Article number: 135 ( 2024 ) Cite this article
139 Accesses
Metrics details
Early water intake has gained widespread attention considering enhanced recovery after surgery (ERAS). In the present systematic evaluation and meta-analysis, we assessed the effects of early water intake on the incidence of vomiting and aspiration in adult patients who received general anaesthesia on regaining consciousness during the resuscitation period.
To systematically analyse the results of randomised controlled trials on early postoperative water intake in patients who underwent different types of surgery under general anaesthesia, both at home and abroad, to further explore the safety and application of early water intake and provide an evidence-based foundation for clinical application.
Systematic review and meta-analysis.
To perform the systematic evaluation and meta-analysis, we searched the Web of Science, CINAHL, Embase, PubMed, Cochrane Library, Sinomed, China National Knowledge Infrastructure (CNKI), Wanfang, and Vipshop databases to identify randomised controlled trial studies on early water intake in adult patients who received general anaesthesia.
Herein, we included 10 publications with a total sample size of 5131 patients. Based on statistical analysis, there was no statistically significant difference in the incidence of vomiting (odds ratio [OR] = 0.81; 95% confidence interval [CI] [0.58–1.12]; p = 0.20; I-squared [I 2 ] = 0%) and aspiration (OR = 0.78; 95%CI [0.45–1.37]; p = 0.40; I 2 = 0%) between the two groups of patients on regaining consciousness post-general anaesthesia.
Based on the available evidence, early water intake after regaining consciousness post-anaesthesia did not increase the incidence of adverse complications when compared with traditional postoperative water abstinence. Early water intake could effectively improve patient thirst and facilitate the recovery of gastrointestinal function.
Peer Review reports
Introduction
Enhanced recovery after surgery (ERAS) was first introduced in 1997 by Kehlet [ 1 ], who mentioned that despite advances in anaesthesia, surgery, and perioperative care, several major surgical procedures were persistently impacted by adverse stress reactions, leading to poor outcomes such as nausea and vomiting, gastrointestinal paralysis, pain, and prolonged recovery time. However, accelerated multidisciplinary and multimodal interventions in rehabilitation surgery with a series of evidence-based optimisation measures can reduce patient stress, substantially reduce postoperative complication rates and lengths of stay, improve postoperative quality of life, and reduce overall healthcare costs [ 2 ]. With advances in society and economic growth, patient demand for comfort care is becoming more urgent. A comfortable medical experience can reduce postoperative stress, enhance patient cooperation, and facilitate postoperative recovery, aligning with the ERAS concept.
Fluid management is an important component of ERAS, and the implementation of early postoperative water intake is a critical initiative to promote fluid management and a favourable condition that facilitates gastrointestinal function recovery and reduces adverse stress reactions [ 3 , 4 ]. According to traditional concepts, patients are required to abstain from routine food and fluid intake In the early postoperative stage, and considering patient safety during the postoperative period, to avoid complications such as aspiration and vomiting due to incomplete water intake on regaining consciousness [ 5 ]. The incidence of thirst in post-surgery patients reportedly exceeds 70%, and it the most urgent and intense sensations experienced during the perioperative period [ 6 , 7 ]. Prolonged thirst can lead to an inability to concentrate, negative emotions such as anxiety and irritability [ 8 ], even increase the risk of delirium and various complications [ 9 ], and seriously impact the patient’s medical experience [ 10 ], which is in direct conflict with the concept of comfort care.
Previously, water intake immediately after surgery was considered undesirable and influenced by various factors, and adequate fluid post-surgery was maintained intravenously. However, greater or overloaded intravenous fluid volumes administration on the day of surgery was found to be independently associated with delayed recovery of postoperative symptoms and increased risk of postoperative complications, which are detrimental to early postoperative recovery [ 11 , 12 ]. With the emphasis on the ERAS concept, there has been a gradual focus and change in the implementation of early postoperative fluid intake. According to a nationwide survey, healthcare professionals were assessed to determine their understanding and perspectives on early oral fluid intake, and it concluded that patients are suggested to begin oral intake soon after their surgical procedures [ 13 ]. Therefore, patients should be encouraged to drink water as early as possible after regaining complete consciousness and to limit postoperative intravenous fluid therapy post-surgery [ 3 ].
Currently, early postoperative water intake is implemented in adult patients who received general anaesthesia. However, the precise timing for this intervention remains inconsistent across studies, which could be attributed to discrepancies in the type of surgery and outcome indicators of the study population, differences in the study results, and a lack of comprehensive evaluation. Therefore, in the present study, we aimed to systematically analyse the results of randomised controlled trials on early postoperative water intake in patients who underwent different types of surgery under general anaesthesia, both at home and abroad, to further explore the safety and application of early water intake and provide an evidence-based foundation for clinical application.
The present study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the registration ID CRD42023395782.
Search strategy
We searched the Web of Science, Cochrane Library, PubMed, Embase, CINAHL, Sinomed, China National Knowledge Infrastructure (CNKI), WanFang, and VIP databases from initiation to 31 December 2022 to identify relevant reports; we then reviewed the references of the included literature. The search strategy was as follows: (1) early oral hydration, early oral fluid, early oral intake, early drinking water, and drinking water; (2) anaesthesia, general or anaesthesia recovery period, and post-anaesthesia; and (3) adult and adult patients.
Herein, two reviewers independently screened titles and abstracts of identified articles according to inclusion and exclusion criteria, subsequently reviewing the full text to determine eligible studies, as shown in Fig. 1 . No language restrictions were imposed. In case of disagreements, the two reviewers conducted an initial discussion; if necessary, a third reviewer was consulted to reach a consensus. Information from the included studies was extracted and saved in Microsoft Excel. This study adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 statement, an updated guideline for reporting systematic reviews and meta-analyses.
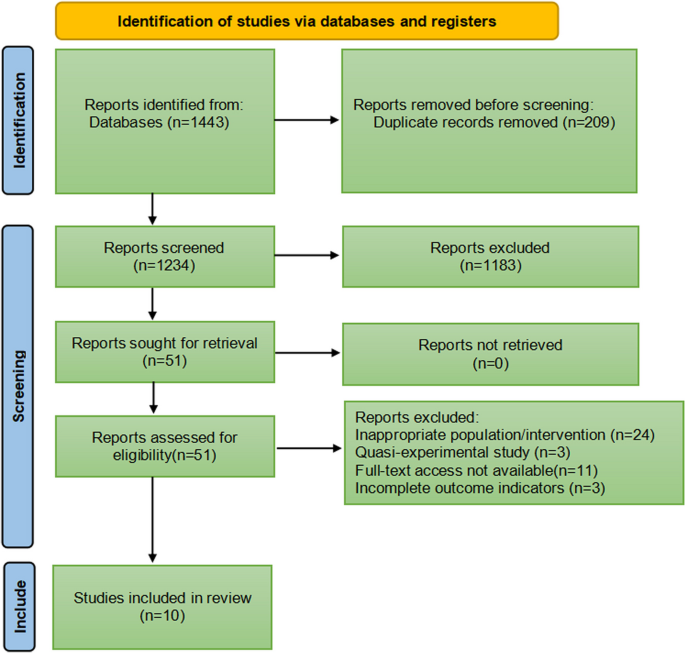
Search strategy flow diagram
Inclusion and exclusion criteria
The inclusion criteria for eligible studies were as follows: (1) patients ≥ 18 years of age who underwent general anaesthesia; (2) patients who had received early oral hydration during recovery after general anaesthesia; (3) randomised controlled trials; (4) non-gastrointestinal surgery.
Exclusion criteria were as follows: (1) studies for which the full text could not be retrieved, or data could not be extracted completely; (2) case reports, reviews, dissertations, quasi-experimental studies, or multiple publications; and (3) studies that did not report relevant outcomes.
Data extraction and study quality
Two reviewers independently extracted data and conducted quality evaluations to avoid bias. Discrepancies in interpretation were resolved by consensus or the involvement of a third reviewer. The following data were extracted using Microsoft Excel: author, year, sample size, type of surgery, intervention, and outcomes.
We assessed the risk of bias in randomised control trials using the Cochrane risk-of-bias tool based on six items: random sequence generation (selection bias); allocation concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); selective reporting (reporting bias) and other bias. Each item was then judged based on three levels: “high risk”, “low risk”, and “unclear”. Moreover, the quality of the results was assessed using the GRADEpro software.
After consultation, we determined that the primary outcomes were vomiting and aspiration rates. The secondary outcomes included nausea rate, thirst score, anal exhaust time, and first defaecation time.
Study analysis
Meta-analysis of included studies was performed using Review Manager Version 5.3 software and Stata (version 16.0), and sensitivity analysis was performed according to the study quality to determine the stability of results. The mean difference (MD) and 95% confidence interval (CI) were used as effect sizes for continuous outcomes, and odds ratio (OR) and 95% CI were used as effect sizes for binary variables. The results of the meta-analysis are depicted as forest plots. The I-squared(I 2 ) values was used to determine the heterogeneity between studies. An I 2 value of < 50% was regarded as homogenous, and a fixed-effects model was selected. An I 2 value of ≥ 50% indicated relatively moderate-to-high heterogeneity between studies, and we used the random-effects model for analysis. The causes of heterogeneity were further analysed, and subgroup analysis was conducted on factors that may lead to heterogeneity. Trial sequential analysis (TSA) was performed using Viewer software (version 0.9.5.10. beta) for the primary outcome to assess the risk of type 1 error caused by repeated testing.
In total, 1443 relevant studies were identified during the initial examination. After eliminating duplicate studies, 1234 studies were obtained. After screening the titles and abstracts, 51 studies were subjected to full-text screening, with 10 studies finally included [ 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 ], as shown in Fig. 1 . The basic features of the included studies are presented in Table 1 . Two studies were published in English [ 14 , 15 ] and eight in Chinese [ 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 ], and all were randomised controlled trials with a total sample size of 5131 patients. Types of surgery included laparoscopic surgery, thoracoscopic surgery, and knee arthroscopy. Figures 2 and 3 present the bias risk assessment. The Cochrane Bias Risk tool reported a potentially ambiguous and high risk of bias, mainly in implementing blinding, given the challenges in double blinding for such procedural trials.
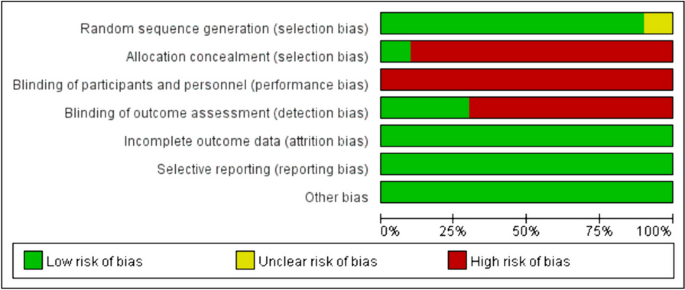
Risk of bias graph
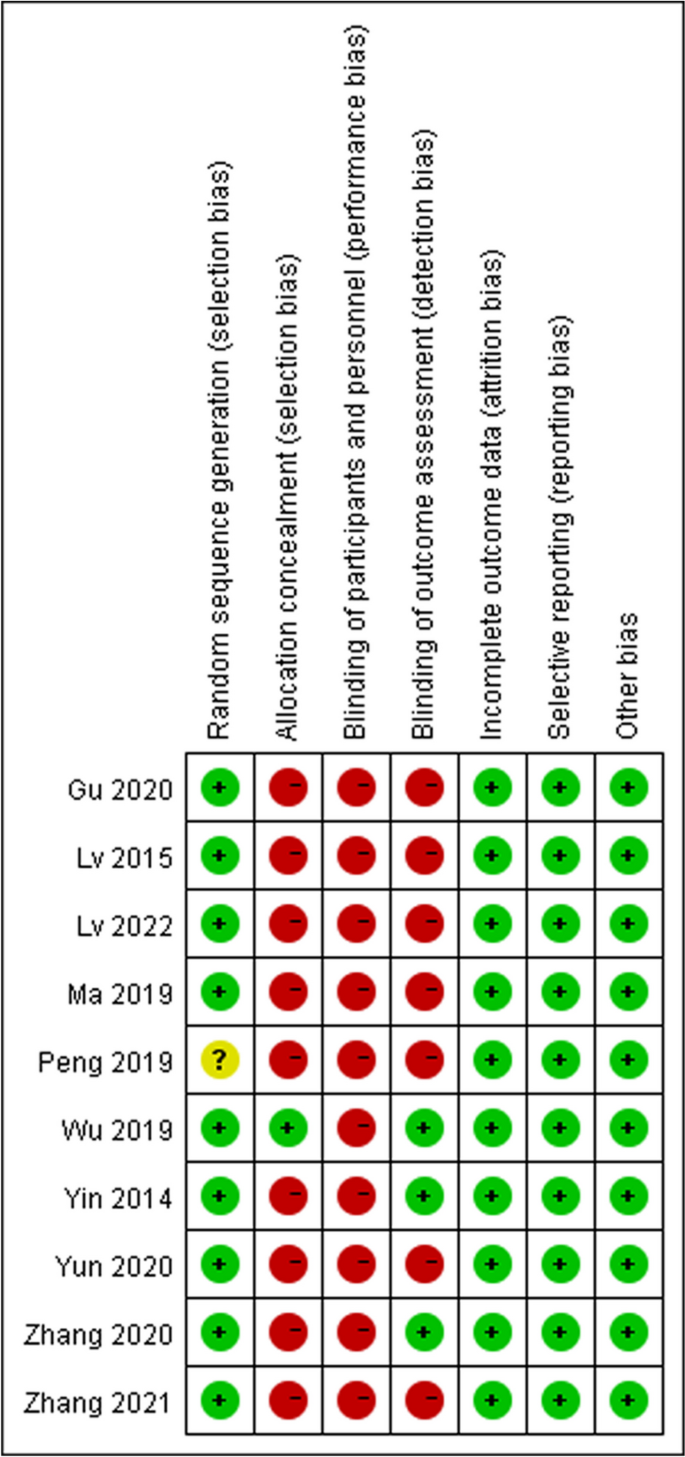
Risk of bias summary
Primary outcomes
The four included studies [ 14 , 15 , 17 , 22 ] showed no heterogeneity (I 2 = 0%, p = 0.77); therefore, a fixed-effects model was used for meta-analysis. According to the results, there was no significant difference in the incidence of postoperative vomiting between the intervention group and the control group (OR = 0.81; 95%CI [0.58–1.12]; p = 0.20) (Fig. 4 ). The experimental and control groups had a sufficient sample size, with both including 664 patients; therefore, the possibility that early drinking water would not increase the incidence of vomiting in patients could be supported to a certain extent. However, owing to the small number of included studies assessing this outcome and the limited involvement of surgical types, further studies are needed.
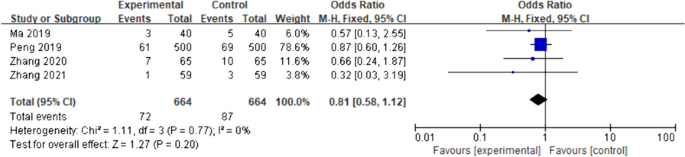
Primary outcome: vomiting. The forest plot of pooled data on changes in the incidence of vomiting in the experimental and control groups using a fixed-effects model
Four studies [ 20 , 21 , 22 , 23 ] mentioned aspiration indicators. Only two studies [ 20 , 21 ] were included, given that the other two studies [ 22 , 23 ] did not include aspiration. I 2 = 0% was used as the fixed-effects model for the meta-analysis. There was no significant difference in the postoperative aspiration rate between the intervention and the control groups (OR = 0.78; 95%CI [0.45–1.37]; p = 0.40), as shown in Fig. 5 .
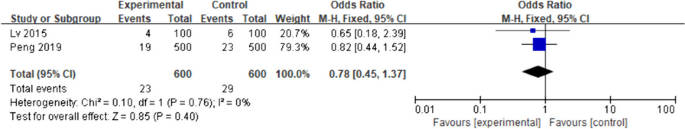
Primary outcome: aspiration. The forest plot of pooled data on changes in the incidence of aspiration in the experimental and control groups using a fixed-effects model. CI, confidence interval
Secondary outcomes
The five included studies [ 14 , 15 , 17 , 20 , 22 ] had low inter-study heterogeneity (I 2 = 27%, p = 0.24); therefore, the fixed-effects model was applied for the meta-analysis. Compared with the control group, the incidence of postoperative nausea (OR) in the intervention group was 0.89 (95%CI [0.69- 1.15]; p = 0.38), although the difference was not statistically significant (Fig. 6 ).
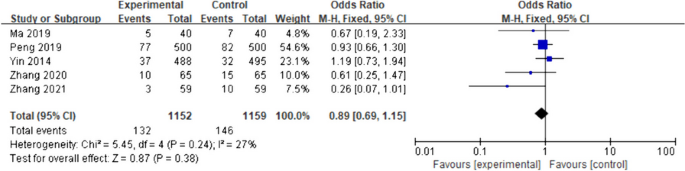
Secondary outcome: nausea. Forest plot of pooled data for nausea across studies with comparator group using a fixed-effects model. CI, confidence interval
Degree of thirst
Statistical heterogeneity among the seven included studies [ 14 , 15 , 16 , 17 , 18 , 21 , 23 ] was large (I 2 = 99%; p < 0.01), with an MD of -9.44 (95%CI [-12.04- -6.83], p < 0.01) for thirst scores in the intervention group when compared with those of the control group. Subgroup analysis was performed according to the different numerical scoring criteria. The results of the subgroup analysis with a score from 0 to 10 showed that early drinking significantly improved thirst (MD = -2.94; 95%CI [-5.43- -0.45]; p = 0.02; I 2 = 99%), while the results of the other subgroup with a score from 0 to 100 also revealed that early drinking significantly improved thirst (MD = -23.38; 95%CI [-32.06- -14.71]; p < 0.01; I 2 = 94%), as shown in Fig. 7 . Sensitivity analyses were performed for each subgroup, excluding the studies individually. No sources of heterogeneity were detected; this could be attributed to the individualisation of numbers expressing the degree of thirst for each patient according to their own comprehension, resulting in a wide variation in scores.
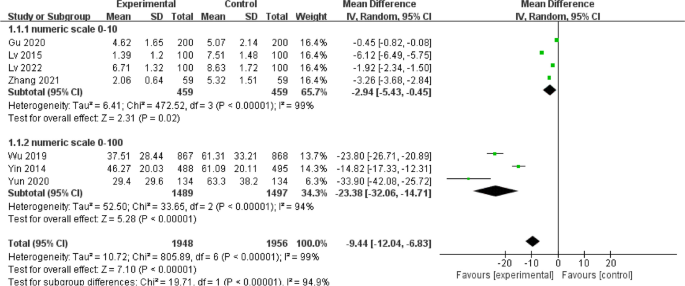
Secondary outcome: degree of thirst. Forest plot of pooled data for the degree of thirst across studies with comparator group using a random-effects model. CI, confidence interval; SD, standard deviation
Anal exhaust time
Five studies [ 16 , 19 , 20 , 22 , 23 ] reported the effect of early water intake on anal exhaust time in postoperative patients. The heterogeneity between the studies was high (I 2 = 95%, p < 0.01); therefore, a random-effects model was used. Patients in the intervention group had a shorter anal exhaust time than patients in the control group, with a statistically significant difference (MD = -5.48; 95%CI [-7.74- -3.22]; p < 0.01), as shown in Fig. 8 . Sensitivity analysis was performed, with the effect estimates held constant, indicating the robustness of the pooled results.
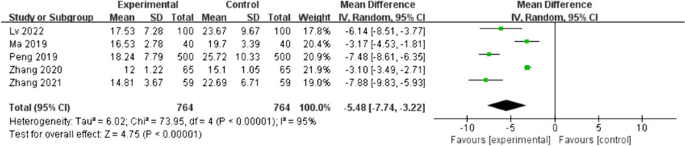
Secondary outcome: anal exhaust time. Forest plot of pooled data on anal exhaust time across studies with comparator group using a random-effects model
First defecation time
Three studies [ 16 , 20 , 23 ] included postoperative patient defaecation time as an outcome indicator; there was high heterogeneity between these studies (I 2 = 59%; p = 0.09); therefore, a random-effects model was employed. Patients in the intervention group had significantly shorter postoperative defaecation times than those in the control group (MD = -6.34, 95%CI [-8.90- -3.79], p < 0.01), as shown in Fig. 9 . A sensitivity analysis was conducted, where the effect estimates were kept constant, revealing the reliability and stability of the combined outcomes.
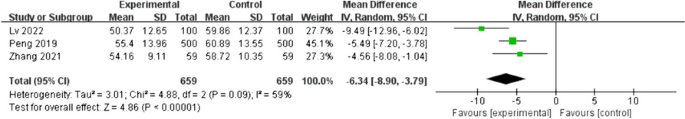
Secondary outcome: first defecation time. Forest plot of pooled data on first defecation time across studies with comparator group using a random-effects model
Trial sequential analysis(TSA)
The TSA results of both primary outcomes indicated that the curve was not crossed the traditional and TSA thresholds, and the cumulative sample size was not reached the expected value, requiring further research, as shown in Supplementary Figs. 1 and 2 .
GRADE assessment
We assessed the quality of the outcome using the GRADE evaluation. The quality of the studies that were included was generally low, which led to a downgrading of the risk of bias to “serious”. Moreover, the I 2 values for both the degree of thirst, anal exhaust time and first defecation time displayed a significant level of inconsistency, resulting in a downgrade of the inconsistency rating to “serious”. In the GRADE evaluation, except for the quality assessment of degree of thirst, anal exhaust time and first defecation time, the quality of other outcomes was moderate. Supplementary Table 1 summarized the overall results of the GRADE assessment.
In the present systematic review and meta-analysis, we pooled data from 10 randomised controlled studies. We found that contrary to conventional beliefs, early water intake did not increase the incidence of nausea and vomiting or the risk of aspiration in patients. Moreover, implementing early water intake in fully conscious patients post-general anaesthesia could significantly improve thirst and shorten the time to defaecation and bowel movement in accordance with ERAS.
According to conventional wisdom, early postoperative water intake can lead to nausea and vomiting, and even aspiration. Postoperative nausea and vomiting (PONV) is influenced by factors such as inhaled anaesthesia and opioid analgesics, as well as the type of surgery [ 24 ], and is not caused by fluid consumption. The main mechanism of aspiration is the relaxation of the cardiac sphincter due to general anaesthesia and suppression of the gag reflex, resulting in the regurgitation of gastric contents, causing aspiration [ 25 ]. Considering non-gastrointestinal surgery, gastrointestinal activity in patients who received general anaesthesia can return to baseline levels at an early stage, and multiple small amounts of gradual fluid consumption can allow physiological adaption of gradual gastrointestinal function recovery post-general anaesthesia without increasing the incidence of postoperative complications [ 26 ]. The Guidelines for Perioperative Fasting and Water Fasting in Adults and Children developed by the European Society of Anesthesia also recommends drinking water as early as possible, according to the patient’s subjective desire, to promote gastrointestinal motility and reduce the incidence of nausea and vomiting [ 27 ]. Considering the literature included in the current meta-analysis, four [ 14 , 15 , 17 , 22 ] studies found no statistically significant differences in the incidence of nausea and vomiting during post anaesthesia care unit (PACU) stay and after return to the ward with early water intake between the intervention and control groups. Two studies [ 20 , 21 ] noted that early water intake did not increase the risk of patient aspiration. It should be noted that the present study involved limited types of surgery, and the timing of early water intake and incidence of adverse postoperative complications need to be further explored for other types of surgery, particularly gastrointestinal surgery.
Thirst is defined as the conscious desire to drink water, which is a compensatory mechanism that allows an organism to restore its water balance [ 28 ]. Preoperative fasting and abstinence from food and fluid, anaesthetic medications, tracheal intubation, and intraoperative bleeding can exacerbate thirst [ 29 , 30 ]. Thirst in perioperative patients is a sign and symptom of imbalance and intense discomfort, causing discomfort in patients during recovery from anaesthesia, leading to a series of organismal stress reactions such as negative emotions and increased risk of wound bleeding, markedly prolonging the patient’s postoperative recovery time and failing to achieve comfortable care [ 30 , 31 , 32 ], thereby necessitating the attention and assistance of medical personnel. Lee and others [ 33 ] have shown that moderate to severe postoperative thirst was common in PACU, a finding that is consistent with a cross-sectional observational study conducted at the National Health Service Hospital in the United Kingdom [ 34 ]. The most direct and effective way to alleviate thirst after regaining consciousness from general anaesthesia is to drink water early during the postoperative period [ 35 ]. In one included study, patients who administered water early had significantly lower thirst scores and a correspondingly lower incidence of thirst than patients who routinely abstained from water. Accordingly, early water intake largely reduces postoperative discomfort in patients and improves their satisfaction with medical visits.
Postoperative anal exhaust time and time to defaecation are clinically important indicators of gastrointestinal function recovery; therefore, these two indicators were used as outcome indicators in the present study. Herein, we found that early oral administration could promote the recovery of gastrointestinal function in postoperative patients, which is in line with the ERAS concept and consistent with the results of related studies [ 36 , 37 ]. Water is considered the mildest mechanical stimulus and adequate hydration is crucial for bodily functions [ 38 ]. Early water consumption can stimulate the oral cavity and gastrointestinal tract, promote the secretion of digestive juices through the neurohumoral reflex, increase gastrointestinal tract peristalsis, and promote the recovery of gastrointestinal function [ 39 , 40 ].
Early assessment is the most important prerequisite for implementing drinking water to ensure patient safety. The guidelines for post-anaesthesia care proposed by the American Society of Anesthesiologists state that the safety criteria used to assess patients after anaesthesia include the level of consciousness, airway patency, respiratory rate, and blood oxygen saturation to avoid potential complications [ 41 ]. The literature included in the current study states the assessment measures that were implemented to guarantee patient safety. In two studies [ 14 , 15 ], patients in the intervention group were assessed as fully awake, with stable vital signs, muscle strength grade 5, and good recovery of cough and gag reflexes when water was administered. Two studies [ 17 , 20 ] employed the Steward or Aldrete rating scales to assess whether patients were fully awake and eligible for early water intake. Larger multicentre studies are needed to develop a uniform assessment tool for early water intake during the postoperative awakening period after general anaesthesia that would meet the characteristics of all populations.
Our study had some limitations. The present study did not include additional outcome indicators such as patient comfort, willingness to drink, and abdominal distension. In addition, there were inconsistencies in recording outcome indicators in some studies, with some reports using measures while others using counts; this made it impossible to include the combined literature owing to difficulties in data extraction. Therefore, the number of studies included in the present analysis for certain analysed indicators was insufficient to determine whether publication bias had occurred. The literature not included in the current analysis, owing to data extraction issues, could also have impacted study results, leading to bias. The literature included in the current analysis was mainly focused on adult patients, with no statistical analysis conducted on special populations such as children and patients with acute and critical illnesses. The type of surgery involved was not comprehensive, and additional factors affecting adverse patient outcomes were not explored. Different hospitals in different countries have distinct strategies for implementing early water intake, which could impact the accuracy of the present meta-analysis. Therefore, more randomised controlled studies assessing special populations and different diseases are needed to comprehensively clarify the safety and feasibility of early water intake.
The results of the present meta-analysis revealed that early water intake during the postoperative awakening period in patients who had received general anaesthesia could relieve thirst without increasing the incidence of adverse postoperative complications such as nausea, vomiting, and aspiration, and this could be applied in clinical practice. Owing to the heterogeneity in the included literature, a high-quality, large-sample, multicentre, randomised controlled study is needed. Moreover, the implementation time and amount of early water intake vary across hospitals, and more rigorously designed randomised controlled trials are needed to clarify the timing and amount of early water intake.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Enhanced recovery after surgery
Postoperative nausea and vomiting
Post anaesthesia care unit
China National Knowledge Infrastructure
American Society of Anesthesiology
Confidence interval
Mean difference
Trial sequential analysis
Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606–17.
Article CAS PubMed Google Scholar
Debono B, Wainwright TW, Wang MY, Sigmundsson FG, Yang MMH, Smid-Nanninga H, et al. Consensus statement for perioperative care in lumbar spinal fusion: Enhanced Recovery After Surgery (ERAS®) society recommendations. Spine J. 2021;21(5):729–52.
Article PubMed Google Scholar
Zhu AC, Agarwala A, Bao X. Perioperative fluid management in the Enhanced Recovery after Surgery (ERAS) pathway. Clin Colon Rectal Surg. 2019;32(2):114–20.
Article PubMed PubMed Central Google Scholar
Malbrain MLNG, Caironi P, Hahn RG, Llau JV, McDougall M, Patrão L, et al. Multidisciplinary expert panel report on fluid stewardship: perspectives and practice. Ann Intensive Care. 2023;13(1):89.
Motta NH, Do Nascimento LA, Pierotti I, Conchon MF, Fonseca LF. Evaluation of a safety protocol for the management of thirst in the postoperative period. J Perianesth Nurs. 2020;35(2):193–7.
Do NL, Fonseca LF, Dos SC. Inter-rater reliability testing of the safety protocol for thirst management. J Perianesth Nurs. 2018;33(4):527–36.
Article Google Scholar
Puntillo K, Arai SR, Cooper BA, Stotts NA, Nelson JE. A randomized clinical trial of an intervention to relieve thirst and dry mouth in intensive care unit patients. Intensive Care Med. 2014;40(9):1295–302.
Tsai HY, Chao A, Hsiao WL. The effectiveness of cold oral stimuli in quenching postoperative thirst: a systematic review and meta-analysis. Intensive Crit Care Nurs. 2023;75:103359.
Lin R, Li H, Chen L, He J. Prevalence of and risk factors for thirst in the intensive care unit: an observational study. J Clin Nurs. 2023;32(3–4):465–76.
Alves Do Nascimento L, De Oliveira Lopes MV, Fahl Fonseca L. Development and validation of a new nursing diagnosis: Perioperative thirst. Int J Nurs Knowl. 2021;32(4):253–61.
Aga Z, Machina M, McCluskey SA. Greater intravenous fluid volumes are associated with prolonged recovery after colorectal surgery: a retrospective cohort study. Br J Anaesth. 2016;116(6):804–10.
Malbrain MLNG, Langer T, Annane D, Gattinoni L, Elbers P, Hahn RG, et al. Intravenous fluid therapy in the perioperative and critical care setting: executive summary of the International Fluid Academy (IFA). Ann Intensive Care. 2020;10(1):64.
Huang H, Zhang Y, Shen L, Huang Y. Level of ERAS understanding affects practitioners’ practice and perception of early postoperative resumption of oral intake: a nationwide survey. BMC Anesthesiol. 2021;21(1):279.
Article CAS PubMed PubMed Central Google Scholar
Wu M, Yang L, Zeng X, Wang T, Jia A, Zuo Y, et al. Safety and feasibility of early oral hydration in the postanesthesia care unit after laparoscopic cholecystectomy: a prospective, randomized, and controlled study. J Perianesth Nurs. 2019;34(2):425–30.
Yin X, Ye L, Zhao L, Li L, Song J. Early versus delayed postoperative oral hydration after general anesthesia: a prospective randomized trial. Int J Clin Exp Med. 2014;7(10):3491–6.
PubMed PubMed Central Google Scholar
Lv G, Huang T, Chen Y, Chen Q. Effect of early stepwise small amount of drinking water on digestive tract function in patients undergoing arthroscopic cruciate ligament reconstruction after general anesthesia. Chongqing Medicine. 2022;52(5):792–4.
Google Scholar
Yun L, Pan Y, Liu J, Yin X. Safety of early drinking water after anesthesia resuscitation in patients after thoracoscopic lobectomy. J Nurs Sci. 2020;35(24):55–7.
Gu C, You Y. Safety and feasibility study of small amount of drinking water during anesthesia recovery period in patients undergoing non-gastrointestinal surgery. J Clin Nurs Pract. 2020;6(6):119–21.
Zhang L, Ma J, Wang X, Han Y, Guo M, Zhao Q, et al. Feasibility study of early drinking water after hysteroscopic and laparoscopic salpingoplasty under general anesthesia. Chin J Modern Nurs. 2020;7:914–6.
Peng W, Ke W. Investigation of patients’ willingness to drink water after general anesthesia with tracheal intubation and study on the effect of early drinking water. J Nurses Train. 2019;34(9):791–4.
Lv G, Yao H, Wang L. Safety and feasibility analysis of early postoperative small amount of drinking water in adult patients undergoing general anesthesia. Chin J Modern Nurs. 2015;21(4):445–7.
Ma Z, Jin Y, Feng T. Comparison of nursing effects of different water deprivation time for patients after transsphenoidal pituitary tumor resection. J Nurses Train. 2019;34(6):538–40.
Zhang W, Zhang Y, Shi H. Effect of early postoperative drinking water on postoperative gastrointestinal function recovery in patients with gynecological malignant tumor undergoing laparoscopic surgery under general anesthesia. Oncol Prog. 2021;19(2):194–8.
Stoops S, Kovac A. New insights into the pathophysiology and risk factors for PONV. Best Pract Res Clin Anaesthesiol. 2020;34(4):667–79.
Green SM, Leroy PL, Roback MG, Irwin MG, Andolfatto G, Babl FE, et al. An international multidisciplinary consensus statement on fasting before procedural sedation in adults and children. Anaesthesia. 2020;75(3):374.
Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, et al. ESPEN practical guideline: clinical nutrition in surgery. Clin Nutr. 2021;40(7):4745–61.
Smith I, Kranke P, Murat I, Smith A, O’Sullivan G, Soreide E, et al. Perioperative fasting in adults and children: guidelines from the European society of anaesthesiology. Eur J Anaesthesiol. 2011;28(8):556–69.
Arai S, Stotts N, Puntillo K. Thirst in critically ill patients: from physiology to sensation. Am J Crit Care. 2013;22(4):328–35.
Lin R, Chen H, Chen L, Lin X, He J, Li H. Effects of a spray-based oropharyngeal moisturising programme for patients following endotracheal extubation after cardiac surgery: a randomised, controlled three-arm trial. Int J Nurs Stud. 2022;130:104214.
Lian R, Zhou S, Cui Y, Liang H, Lin J, Li D, et al. The effect of ice-cold water spray following the model for symptom management on postoperative thirst in patients admitted to intensive care unit: a randomized controlled study. Intensive Crit Care Nurs. 2024;81:103571.
Kjeldsen CL, Hansen MS, Jensen K, Holm A, Haahr A, Dreyer P. Patients’ experience of thirst while being conscious and mechanically ventilated in the intensive care unit. Nurs Crit Care. 2018;23(2):75–81.
Nascimento LA, Fonseca LF, Rosseto EG, Santos CB. Development of a safety protocol for management thirst in the immediate postoperative period. Rev Esc Enferm USP. 2014;48(5):834–43.
Lee CW, Liu ST, Cheng YJ, Chiu CT, Hsu YF, Chao A. Prevalence, risk factors, and optimized management of moderate-to-severe thirst in the post-anesthesia care unit. Sci Rep. 2020;10(1):16183.
Walker E, Bell M, Cook TM, Grocott M, Moonesinghe SR. Patient reported outcome of adult perioperative anaesthesia in the United Kingdom: a cross-sectional observational study. Br J Anaesth. 2016;117(6):758–66.
Conchon MF, Fonseca LF. Efficacy of an ice popsicle on thirst management in the immediate postoperative period: a randomized clinical trial. J Perianesth Nurs. 2018;33(2):153–61.
Carmichael L, Rocca R, Laing E, Ashford P, Collins J, Jackson L, et al. Early postoperative feeding following surgery for upper gastrointestinal cancer: a systematic review. J Hum Nutr Diet. 2022;35(1):33–48.
Hao T, Liu Q, Lv X, Qiu J, Zhang HR, Jiang HP. Efficacy and safety of early oral feeding in postoperative patients with upper gastrointestinal tumor: a systematic review and meta-analysis. World J Gastrointest Surg. 2021;13(7):717–33.
Spence C. Encouraging (Nudging) people to increase their fluid intake. Nutrients. 2023;15(12):2702.
Chen X, Wang P, Leng C, Sun H, Liu X, Zhang R, et al. Early oral feeding after esophagectomy accelerated gut function recovery by regulating brain-gut peptide secretion. Surgery. 2022;172(3):919–25.
Li S, Xiao X, Zhang X. Hydration status in older adults: current knowledge and future challenges. Nutrients. 2023;15(11):2609.
Whitaker CD, Booth H, Clyburn P, Harrop-Griffiths W, Hosie H, Kilvington B, et al. Immediate post-anaesthesia recovery 2013: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia. 2013;68(3):288–97.
Download references
Acknowledgements
The authors thank Dr. Shen from The First Hospital of Jiaxing for his writing assistance and proof reading the article.
This work was supported by the Key Discipline of Anesthesiology of Jiaxing City (2023-zc-001) and Key Discipline of Clinical Nursing Innovation of Jiaxing City (2023-zc-007).
Author information
Authors and affiliations.
Zhejiang Chinese Medical University, Hangzhou, China
Suwan Dai, Lingyan Chen, Min Wu & Liangyou Guo
The First Hospital of Jiaxing, Jiaxing, China
You can also search for this author in PubMed Google Scholar
Contributions
All authors have read and approved the manuscript. S.D. conducted a literature review, data collection, and wrote the first draft of the paper. L.C. assisted in statistical analysis and manuscript editing. L.G. and M.W. helped with statistical analysis and made significant contributions to manuscript revision. R.W. helped with manuscript editing and obtained funding.
Corresponding author
Correspondence to Rong Wang .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Dai, S., Chen, L., Wu, M. et al. Timing of early water intake post-general anaesthesia: a systematic review and meta-analysis. BMC Anesthesiol 24 , 135 (2024). https://doi.org/10.1186/s12871-024-02520-x
Download citation
Received : 09 December 2023
Accepted : 02 April 2024
Published : 09 April 2024
DOI : https://doi.org/10.1186/s12871-024-02520-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Early water intake
- General anaesthesia
- Resuscitation period
BMC Anesthesiology
ISSN: 1471-2253
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 12 April 2024
Risk of conversion to mild cognitive impairment or dementia among subjects with amyloid and tau pathology: a systematic review and meta-analysis
- Zsolt Huszár 1 , 2 ,
- Marie Anne Engh 1 ,
- Márk Pavlekovics 1 , 3 ,
- Tomoya Sato 1 ,
- Yalea Steenkamp 1 ,
- Bernard Hanseeuw 4 , 5 ,
- Tamás Terebessy 1 ,
- Zsolt Molnár 1 , 6 , 7 ,
- Péter Hegyi 1 , 8 , 9 , 10 &
- Gábor Csukly 1 , 2
Alzheimer's Research & Therapy volume 16 , Article number: 81 ( 2024 ) Cite this article
65 Accesses
Metrics details
Measurement of beta-amyloid (Aβ) and phosphorylated tau (p-tau) levels offers the potential for early detection of neurocognitive impairment. Still, the probability of developing a clinical syndrome in the presence of these protein changes (A+ and T+) remains unclear. By performing a systematic review and meta-analysis, we investigated the risk of mild cognitive impairment (MCI) or dementia in the non-demented population with A+ and A- alone and in combination with T+ and T- as confirmed by PET or cerebrospinal fluid examination.
A systematic search of prospective and retrospective studies investigating the association of Aβ and p-tau with cognitive decline was performed in three databases (MEDLINE via PubMed, EMBASE, and CENTRAL) on January 9, 2024. The risk of bias was assessed using the Cochrane QUIPS tool. Odds ratios (OR) and Hazard Ratios (HR) were pooled using a random-effects model. The effect of neurodegeneration was not studied due to its non-specific nature.
A total of 18,162 records were found, and at the end of the selection process, data from 36 cohorts were pooled ( n = 7,793). Compared to the unexposed group, the odds ratio (OR) for conversion to dementia in A+ MCI patients was 5.18 [95% CI 3.93; 6.81]. In A+ CU subjects, the OR for conversion to MCI or dementia was 5.79 [95% CI 2.88; 11.64]. Cerebrospinal fluid Aβ42 or Aβ42/40 analysis and amyloid PET imaging showed consistent results. The OR for conversion in A+T+ MCI subjects (11.60 [95% CI 7.96; 16.91]) was significantly higher than in A+T- subjects (2.73 [95% CI 1.65; 4.52]). The OR for A-T+ MCI subjects was non-significant (1.47 [95% CI 0.55; 3.92]). CU subjects with A+T+ status had a significantly higher OR for conversion (13.46 [95% CI 3.69; 49.11]) than A+T- subjects (2.04 [95% CI 0.70; 5.97]). Meta-regression showed that the ORs for Aβ exposure decreased with age in MCI. (beta = -0.04 [95% CI -0.03 to -0.083]).
Conclusions
Identifying Aβ-positive individuals, irrespective of the measurement technique employed (CSF or PET), enables the detection of the most at-risk population before disease onset, or at least at a mild stage. The inclusion of tau status in addition to Aβ, especially in A+T+ cases, further refines the risk assessment. Notably, the higher odds ratio associated with Aβ decreases with age.
Trial registration
The study was registered in PROSPERO (ID: CRD42021288100).
Affecting 55 million people worldwide, dementia is one of the leading causes of years spent with disability and one of the costliest long-term illnesses in society. The most common cause of dementia is Alzheimer's disease (AD), responsible for 60-80% of cases [ 1 , 2 ].
Two specific protein aggregates play a crucial role in the pathophysiology of AD. One is the amyloid plaque formation in the extracellular space, predominantly by Aβ aggregation. These plaques, among other pathological effects, inhibit the signaling function of neurons [ 3 ]. The other protein change is the appearance of neurofibrillary tangles within the neurons, which are formed by the phosphorylation of tau proteins (p-tau) and inhibit the axonal transport inside the cell [ 4 ]. Whereas the specific pathology could only be confirmed by autopsy in the past, in vivo tests are available today. Parallelly to this development, the diagnostic definitions of AD have evolved significantly over time, moving from purely clinical assessments and post-mortem examinations to the integration of in vivo amyloid and later p-tau biomarkers, emphasizing the role of preclinical stages [ 5 , 6 , 7 , 8 ]. Accordingly, researchers are increasingly trying to link the diagnosis of the disease to biological parameters. However, in general, the clinical practice only considers the quality of the symptoms of dementia and the fact of neurodegeneration confirmed by radiology when establishing an AD diagnosis.
The International Working Group (IWG) [ 5 ] emphasizes that diagnosis should align with clinical symptoms. However, for researchers in the field, the U.S. National Institute on Aging – Alzheimer’s Association (NIA-AA) has issued a new framework recommendation [ 6 ]. This recommendation defines AD purely in terms of specific biological changes based on the Aβ (A) and p-tau (T) protein status, while neurodegeneration (N) is considered a non-specific marker that can be used for staging. In the recommendation, the category ‘Alzheimer’s disease continuum’ is proposed for all A+ cases, ‘Alzheimer’s pathological changes’ for A+T- cases, and ‘Alzheimer’s disease’ for A+T+ cases. A-(TN)+ cases are classified as ‘non-Alzheimer pathological changes’.
Aβ and p-tau proteins have long been known to be associated with AD development, and their accumulation can begin up to 15-20 years before the onset of cognitive symptoms [ 9 ]. Pathological amyloid changes are highly prevalent in dementia: 88% of those clinically diagnosed with AD and between 12 and 51% of those with non-AD are A+, according to a meta-analysis [ 10 ]. At the same time, the specificity of the abnormal beta-amyloid level for AD and its central role in its pathomechanism have been questioned [ 11 ]. Their use as a preventive screening target is a subject of ongoing discourse [ 12 ]. Yet it is still unclear to what extent their presence accelerates cognitive decline. What are the predictive prospects for an individual with abnormal protein levels who is otherwise cognitively healthy or with only mild cognitive impairment (MCI), meaning cases where there is a detectable decline in cognitive ability with maintained ability to perform most activities of daily living independently? [ 13 ] Research on non-demented populations shows substantial variation; for example, studies have shown OR values for conversion to dementia ranging from 2.25 [95% CI 0.71; 7.09] [ 14 ] to 137.5 [95% CI 17.8; 1059.6] [ 15 ]. Comparing conversion data systematically is necessary to provide a clearer picture.
In the CU population over 50 years, the prevalence of being A+ ranges from 10 to 44%, while in MCI it ranges from 27 to 71%, depending on age. Taking this into consideration [ 16 ], we aim to investigate the effect of Aβ alone and in combination with p-tau on the conversion to MCI and dementia, through a systematic review and meta-analysis of the available literature. Knowing the prognostic effect can highlight the clinical potential of this current research framework, given that, at present, the therapy of MCI or dementia can only slow down the decline. Prevention starting at an early stage or even before symptoms appear, provides the best chance against the disease.
Study registration
Our study was registered in the PROSPERO database (ID: CRD42021288100), with a pre-defined research plan and detailed objectives, is reported strictly in accordance with the recommendation of the PRISMA 2020 guideline and was performed following the guidance of the Cochrane Handbook [ 17 ].
We aimed to determine the change in odds of progression to MCI or dementia among non-demented subjects based on abnormal Aβ levels alone, or in combination with abnormal p-tau levels.
Search and selection
We included longitudinal prospective and retrospective studies that used the NIA-AA 2018 recommended measurement of Aβ and p-tau (for Aβ: amyloid PET, CSF Aβ42, or Aβ42/40 ratio; for p-tau: tau PET, or CSF p-tau) and investigated the role of Aβ and +/- p-tau in CU and MCI subjects in progression to MCI or dementia. Case reports and case series were excluded. Overlapping populations were taken into account during the data extraction. Our search key was run in the Medline, Embase, and Central databases on 31 October 2021, and the search was updated on 9 January 2024 (see Supplementary Material, Appendix 1 ). After removing duplicates, we screened publications by title and abstract, and in the second round by full text. Two independent reviewers conducted the selection (ZH, MP), and a third reviewer (GC) resolved disagreements. The degree of the agreement was quantified using Cohen’s kappa statistics at each selection stage.
As part of the selection process, articles that only examined the ADNI database [ 18 ] were excluded, as patient-level data were used instead (see Supplementary Material Appendix 2 for details of the patient-level data analysis of the ADNI).
A standardized Excel (Microsoft Corporation, Redmond, Washington, USA) document sheet was used for data extraction (for one special case of data extraction see Supplementary Material Appendix 3 ). Where data were available in graphical form only, we used an online software (Plot Digitizer) [ 19 , 20 ]. The following data were extracted: source of data used in the studies (place of clinical trial or name of database), baseline characteristics of the population (age, gender, APOE status, and education level), type of exposure (Aβ, p-tau, and neurodegeneration), measurement technique of the exposure, data on cognitive impairment separately for the different exposure groups).
Data synthesis
Generally, where several studies used the same population sample or cohort, only data from the study with the largest sample size were used. Conversion to Alzheimer’s dementia and to unspecified dementia was assessed together, as the definition of Alzheimer’s dementia varied between the studies, and the diagnosis was based on neurocognitive tests. If conversion to both types of dementia was given, the value of the conversion to unspecified dementia was used. The population with subjective cognitive symptoms was scored jointly with the CU population, as these subpopulations could not be differentiated objectively.
Odds ratio and hazard ratio values were used or calculated based on the available information (for details on the methodology, see Supplementary Material Appendix 4 ). Considering that studies report their results on different age groups, a meta-regression analysis was performed to investigate how age affects the likelihood of developing dementia based on Aβ levels.
Studies applied different analysis methods to identify Aβ positivity. Where multiple amyloid categories were being considered, the preferred method was amyloid PET. When relying on CSF analysis, the Aβ42/40 ratio was given precedence over Aβ42 since the 42/40 ratio has a higher concordance with amyloid PET [ 21 ]. To estimate the confounding effect caused by different amyloid measurement techniques a subgroup analysis was performed. For the assessment of p-tau, studies measured p-tau181 levels from CSF samples, or employed tau PET. While there is also a limited number of tau PET measurements in the ADNI, in order to ensure consistency in the analyses, we used exclusively the CSF p-tau181 levels from the ADNI database.
For the OR analysis, studies with varying follow-up times were pooled. To estimate the resulting bias, a meta-regression analysis was performed to explore how follow-up time affected the results.
Statistical analysis
Statistical analyses were performed in the R programming environment (version 4.1.2) using the “meta” software package version 5.2-0. To visualize synthesized data, we used forest plots showing ORs or HRs and corresponding confidence intervals for each individual study and pooled effect sizes in terms of ORs and HRs. For dichotomous outcomes, odds ratios and hazard ratios with 95% confidence intervals (CI) were used as effect measures. To calculate odds ratios, the total number of patients in each study and the number of patients with the event of interest in each group were extracted from each study. Raw data from the selected studies were pooled using a random-effects model with the Mantel-Haenszel method [ 22 , 23 , 24 ]. The random-effects model was used as we assumed that the true effect would vary between studies due to differences in demographics and clinical measures, such as age or baseline cognitive impairment.
Heterogeneity was assessed by calculating I 2 , tau 2 , and the prediction interval. I 2 is defined as the percentage of variability in the effect size that is not caused by sampling error, whereas tau 2 is the square root of the standard deviation of the true effect size. As I 2 is heavily dependent on the precision of the studies and tau 2 is sometimes hard to interpret (as it is insensitive to the number of the studies and their precision), the prediction interval has also been calculated. The great advantage of the prediction interval is that this measure is easy to interpret: if the interval does not include zero, further studies are expected to show a similar result.
Sensitivity analysis
We performed outlier detection according to Viechtbauer et al. [ 25 ]. A study is considered an outlier if the confidence interval of the study does not overlap with the confidence interval of the pooled effect. The idea behind is to detect effect sizes that differ significantly from the overall effect. As a sensitivity analysis, we repeated the analyses after removing any outliers and then we compared the pooled effects before and after the exclusion, in order to detect if outliers would have a substiantial impact on the overall effect.
Risk of bias assement
The risk of bias was assessed according to the recommendation of the Cochrane Collaboration; using the QUIPS tool [ 26 ], two investigators (ZH and YS) independently assessed the quality of the studies, and a third author solved disagreements. Publication bias was examined using the Peter’s regression test [ 27 ] and visual inspection of the adjusted Funnel-plots.
Search results
During the systematic search (Fig. 1 ), 18,162 records were found, and finally, 46 eligible articles were obtained (Supplementary Material eTable 1 ); While some of the articles analyzed the same cohorts, we were able to pool data from 36 different cohorts or centres. The Cohens’s kappa was 0.91 for the title and abstract, and 0.86 for the full-text selection. Given the amount of data found, we decided to examine the targeted outcomes separately and focus only on the conversion data in this report.
PRISMA flowchart of selection. Flowchart of the study screening process following the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) 2020 statement
The investigated studies expressed their results in different ways. They calculated unadjusted or adjusted hazard ratios or presented the number of conversions for the different follow-up periods. In the latter case, we calculated odds ratios for the defined time periods. The measured exposures also differed: data were given only for Aβ or in combination with p-tau or neurodegeneration. There were also differences in the techniques used to measure exposure, with CSF sample being used in some cases and PET scan in others.
During data extraction, one [ 28 ] article was excluded because of inconsistently reported conversion data, and four [ 15 , 29 , 30 , 31 ] were excluded from the A/T analysis because the definition of the pathologic Aβ and p-tau was based on Aβ/p-tau ratio, which did not comply with the NIA-AA 2018 recommendation.
The eligible studies investigated three groups: CU, MCI, and mixed - in which the results were collectively expressed for both the MCI and CU groups. The CU group comprised either cognitively healthy subjects or individuals with only subjective cognitive complaints. To define the MCI group, all studies followed the Petersen criteria [ 32 ]. Four studies examined mixed groups. Since all of them studied large samples ( n >180), it was considered more valuable to jointly analyze them with MCI, since the outcome was also the conversion to dementia. As a result of the joint analysis, our findings are based on a substantially larger sample. To support this decision, we performed a subgroup analysis comparing the Aβ positive MCI and mixed population studies. The OR differed significantly from the unexposed group in both the MCI (OR 5.83 [3.80; 8.93]) and the mixed (4.64 [95% CI 1.16; 18.61]) subgroups, and there was no significant difference between the two subgroups ( p =0.55) (Supplementary Material eFigure 1 ).
Conversion from MCI to dementia
Aβ exposition - in or.
Based on a mixed model meta-analysis of 3,576 subjects (Table 1 ), we observed a significant association between Aβ positivity and higher conversion rates. Compared to the unexposed, the OR for conversion to dementia in the amyloid positives were 5.18 [95% CI 3.93; 6.81]; t(21)=12.47; ( p <0.0001). The I 2 - test for heterogeneity revealed that 44.8% of the variance across studies was due to heterogeneity (Fig. 2 A). As a result of the outlier detection we excluded the Balassa study and found a very similar overall effect and a reduced heterogeneity (5.05 [95% CI 3.98; 6.40]; t(20) = 14.2; p < 0.0001; I 2 = 31.4%). Meta-regression analysis of mean age showed a statistically significant decrease in OR values with increasing age (R 2 = 59.05%, beta = -0.04, SE = 0.019, [95% CI = -0.03 to -0.083], df = 18, t = -2.27, p = 0.036) (Fig. 2 B). The Hartunk-Knapp method was applied to adjust test statistics and confidence intervals to reduce the risk of false positives.
Conversion of Aβ exposed MCI groups to dementia in OR. The squares and bars represent the mean values and 95% CIs of the effect sizes, and the squares’ area reflects the weight of the studies. Diamonds represent the combined effects, and the vertical dotted line represents the line of no association. A OR for Aβ exposition; B meta-regression of age and ORs for conversion regarding Aβ exposure. The size of the circle is proportional to the weight of each study in the meta-analysis. The line corresponds to meta-regression with age as covariate, and beta represents the slope of ORs by mean age
Beta-amyloid was determined by CSF Aβ42, CSF Aβ42/40 ratio or amyloid PET. When the three groups were compared in a subgroup analysis, the OR was 5.87 (2.83; 12.19) for CSF Aβ42, 5.00 (3.31; 7.55) for CSF Aβ42/40 ratio, and 5.32 (2.53; 11.18) for amyloid PET. The difference between the subgroups was not significant ( p =0.88) (Supplementary Material eFigure 2 ).
The meta-regression analysis performed to examine the role of follow-up time showed no association with respect to the ORs (R 2 = 0%, beta = -0.002, SE = 0.07, [95% CI = -0.02 - 0.01], df = 11, p = 0.77) (Supplementary Material eFigure 3 A).
We used a funnel plot to examine publication bias (Supplementary Material eFigure 4 A). Most of the studies with large sample sizes lie close to the midline, which confirms that the pooled effect size seems valid. However, the visual inspection of the plot raised the possibility of some publication bias in two ways: (1) Studies in the bottom right corner of the plot have significant results despite having large standard errors (2) The absence of studies in the bottom left corner (blank area in the figure) may indicate that studies with nonsignificant results were not published. In order to quantify funnel plot asymmetry, the Peter’s regression test was applied. The test results were not significant ( t = 1.7, df = 20, p = 0.11) so no asymmetry was proven in the funnel plot.
The effect of Aβ exposition in terms of HR
Several studies reported their results in HRs instead of or in addition to ORs (Supplementary Material eTable 2 ). The advantage of the HR value is that this measure is independent of the length of follow-up times of the studies. For these reasons, we also considered it important to analyze the results expressed in HR. Based on pooled data of patients studied ( n =1,888), the HR for conversion to dementia was 3.16 [95% CI 2.07; 4.83], p < 0.001 (Fig. 3 A).
Conversion of Aβ exposed MCI groups to dementia in HR. The squares and bars represent the mean values and 95% CIs of the effect sizes, and the squares’ area reflects the weight of the studies. Diamonds represent the combined effects, and the vertical dotted line represents the line of no association. A HR for Aβ exposition; B sub-group analysis of studies with adjusted and unadjusted HR values
To investigate the effect of adjustment, we conducted a subgroup analysis between the unadjusted and adjusted measurements. Although there was a trend for higher unadjusted HR values compared to the adjusted HRs, the difference did not reach statistical significance (unadjusted HR : 5.07 [95% CI 2.77 - 9.26], adjusted HR 2.86 [95% CI 1.70 - 4.83] p =0.055) (Fig. 3 B). We could not analyze HR in the A+T-, A+T+, and A-T+ subgroups, due to the low number of available studies.
The effect of Aβ and p-tau exposition in terms of OR
We examined the combined effect of p-tau and Aβ (Table 2 ), and compared A+T+, A+T-, and A-T+ exposures to A-T-. Based on pooled data for patients studied (n=1,327), the OR for conversion to dementia in A+T- was 2.73 [95% CI 1.65; 4.52], and the odds ratio was significantly higher in the presence of both exposures (A+T+) ( p <0.001), with an OR of 11.60 [95% CI 7.96; 16.91]. The effect of A-T+ exposure on conversion was not significant (OR: 1.47 [0.55; 3.92]) (Fig. 4 A).
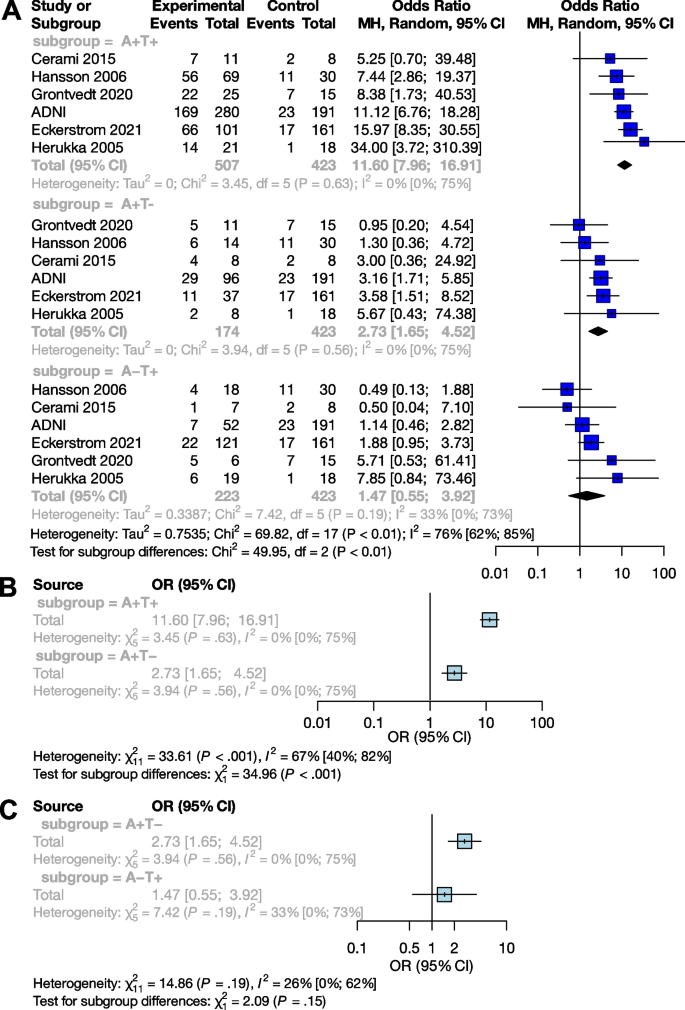
Conversion of Aβ and p-tau exposed MCI groups to dementia in OR. The squares and bars represent the mean values and 95% CIs of the effect sizes, and the squares’ area reflects the weight of the studies. Diamonds represent the combined effects, and the vertical dotted line represents the line of no association. A Aβ and p-tau expositions in OR; B sub-group analysis of comparisons between the A+T+ and A+T- groups; C sub-group analysis of comparisons between the A+T- and A-T+ groups
Subgroup analyses showed that the A+T+ group had a significantly higher odds of conversion compared to the A+T- group ( p <0.001), while the A+T- and A-T+ groups did not differ significantly ( p =0.15) (Fig. 4 B and C).
Conversion from CU to MCI or dementia
The effect of aβ exposition in terms of or.
Analyses on the CU population ( n = 4,217) yielded very similar results to the MCI sample. The OR for conversion to MCI or dementia was 5.79 [95% CI 2.88; 11.64] (t(13) = 5.43; p = 0.0001), the results of the studies did however show a high degree of heterogeneity (I 2 = 73% [55%; 84%]) (Table 3 , Fig. 5 A). As a result of the outlier detection we removed the Aruda study and found a very similar overall effect (6.33 [95% CI 3.42; 11.71]; t(12) = 6.54; p < 0.0001; I 2 = 72.1%).
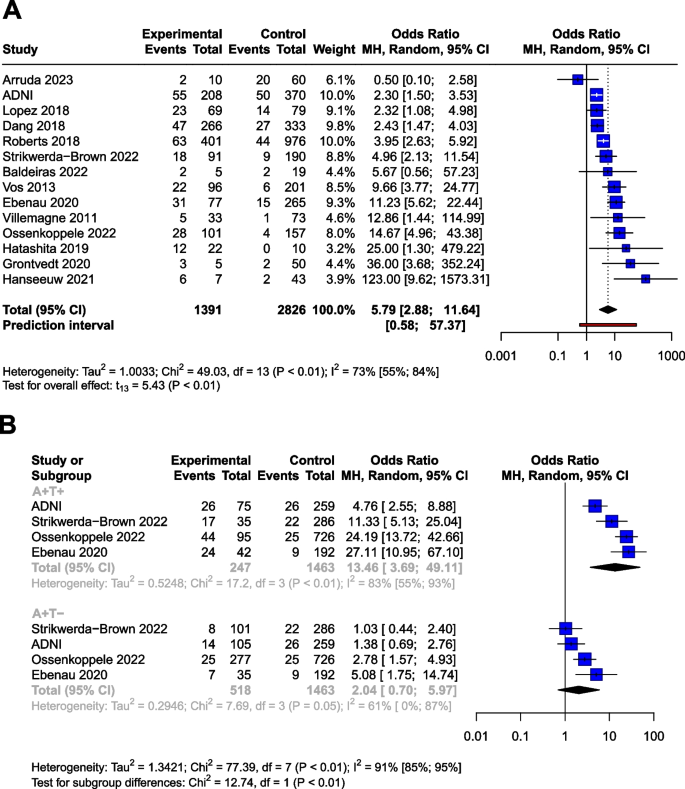
Conversion of Aβ and p-tau exposed CU groups to MCI or dementia in OR. The squares and bars represent the mean values and 95% CIs of the effect sizes, and the squares' area reflects the weight of the studies. Diamonds represent the combined effects, and the vertical dotted line represents the line of no association. A Aβ exposition in OR. B Aβ and p-tau expositions in OR
Meta-regression analysis of mean age did not show a significant association with OR. (R 2 = 8.22%, beta = -0.05, SE = 0.05, [95% CI = -0.17 – 0.7], df = 11, t =, p = 0.37).
Meta-regression analysis also showed no association between follow-up time and ORs (R 2 = 0.35%, beta = -0.014, SE = 0.024, [95% CI = -0.07 - 0.04], df = 8, p = 0.58) (Supplementary Material eFigure 3 B).
We applied a funnel plot to examine publication bias (Supplementary Material eFigure 4 B).Most of the studies with large sample sizes lie close to the midline, which reaffirms the pooled effect size’s validity. In order to quantify funnel plot asymmetry, Peter’s regression test was applied. The test results were not significant ( t = 0.9, df = 12, p = 0.31) indicating that no asymmetry was demonstrated in the funnel plot.
Four cohorts provided HRs for the CU population ( n =2700) with one cohort (ADNI) representing the 55.3% of the total sample (weight: 78.5%) (Supplementary Material eTable 3 ). The pooled HR for conversion was 2.33 [95% CI 1.88; 2.88] ( p =0.001) (Supplementary Material eFigure 5 )
The combined effect of Aβ and p-tau exposition in terms of OR
Using data from a total of 2228 subjects, we investigated the effect of p-tau in combination with Aβ (Table 4 ) in the CU population. The OR for conversion is 2.04 [95% CI 0.70; 5.97] for A+T-, and 13.46 [95% CI 3.69; 49.11] for the A+T+, compared to the A-T- group The OR shows a trend level increased risk (t=2.1, P =0.12) for the A+T- group compared to the A-T- group.
Similarly to the MCI population, subgroup analyses showed that the A+T+ group had significantly higher OR for conversion compared to the A+T- group ( p <0.01). The analysis could not be performed for A-T+ due to the low number of these cases.
Risk of bias assessment
The risk of bias was assessed separately for the analyses discussed above. The overall risk of the studies ranged from low to moderate, except in three cases: twice we found a high risk of bias due to attrition of above 50% [ 59 , 60 ], and once due to a focus on monozygotic twins [ 61 ] (Supplementary Material, eFigure 6 ). These articles ( n =197) were excluded from all analyses.
Summary and context
A pathological Aβ state are strongly correlated with the risk of clinical progression. The odds ratio for conversion is 5.18 in the MCI population and 5.79 in the CU population. Therefore, measuring Aβ levels alone can identify a population at high risk. The OR for conversion to dementia differs significantly between the A+T+ and A+T- groups in both the MCI and CU populations: while the OR is 2.73 [95% CI 1.65; 4.52] for MCI and 2.04 [95% CI 0.70; 5.97] for CU subjects in the A+T- group, it increases to 11.60 [95% CI 7.96; 16.91] for MCI and 14.67 [95% CI 3.69; 49.11] for CU in the A+T+ group. Note that in the case of A+T- at CU population, only a trend-level statistical correlation is visible.
The results of the meta-regression show a decrease in OR with mean age (Fig. 2 B). Based on this result it seems that the impact of Amyloid positivity on conversion is decreasing with age. The fact that age is a risk factor for dementia and vascular and other neurodegenerative damage are more frequent in elderly age is a possible explanation to this finding. Our findings combined with the results of Rodrigue et al. [ 62 ] suggests that amyloid burden increases with age, while its impact on conversion rates slightly decreases with age.
The appearance of Aβ is assumed to be one of the earliest signs of AD [ 63 , 64 ]. Our results fit into this picture by showing that only the A+T+ and A+T- groups showed an increased risk for conversion compared to A-T-, the A-T+ group did not. Thus, Aβ alone is suitable for detecting the population at risk, while p-tau alone is not as effective in the prediction conversion. Our result is in line with previous studies showing that the A-T+ group has a weaker association with cognitive decline compared to the A+T- or A+T+ groups [ 65 , 66 ]. However, it is important to emphasize that previous results showing that T+ status is closely associated with neurodegeneration and the A-T+ group is related to frontotemporal dementia [ 67 ]. More research is needed to fully explain the significance of the A-T+ group.
The PET scan is known to be a more sensitive tool for detecting Amyloid positivity compared to CSF sampling [ 68 ]. However, from a prognostic point of view, our results did not show a significant difference ( p =0.73) between PET measurements (OR: 6.02) and the more cost-effective but invasive CSF Aβ42 measurements (OR: 5.11). It is important to note here that the present meta-analysis is underpowered for detecting prognostic differences between these methods. Due to the heterogeneity among studies, the impact of confounding factors, and standardised studies are required to evaluate the comparative prognostic value of these biomarkers accurately.
Our results based on ORs are further strengthened by the HR analyses giving similar results for Aβ exposure in the MCI (HR: 3.16) and CU (HR: 2.33) populations. It should be noted that in the HR analysis of the CU group, ADNI accounts for 78.5% of the weight, which is a limitation of this meta-analysis. This disproportionate representation may affect the overall result. Regarding the statistical trend-level association with a higher unadjusted HR, it should be noted that in the presence of a random distribution of other risk factors (e.g. baseline MMSE score or educational level), the unadjusted value may overestimate the HR. As in the case of a non-random distribution, the adjusted value underestimates the HR. With this in mind, we recommend reporting both values in the future.
Our analyses were performed on CU and MCI populations. Including mixed populations with the MCI population was a practical simplification, as several studies with a large number of cases gave their results combining MCI subjects with CU subjects, and we aimed to answer the set of questions based on the largest population. To investigate the potential bias of this method, we performed subgroup analysis comparing the mixed and MCI populations, and the result was not significant. The Aβ OR based on the mixed-only group is 4.64 [95% CI 1.16; 18.61], and the OR calculated on the MCI-only studies is 5.83 [95% CI 3.80; 8.93]. Thus, the inclusion of the mixed population in the pool decreases the OR of the main analysis (5.21 [95% CI 3.93; 6.90]) slightly (Supplementary Material eFigure 1 ).
Strengths and limitations
There are several limitations to consider when interpreting our results. The study populations differ in several aspects; for cognitive status, the population ranges from those with no cognitive symptoms through those with subjective cognitive symptoms (these two groups were considered CU) to MCI groups. Therefore, the distance from the cognitive state corresponding to MCI or dementia also varies. Due to the different cut-offs used in the studies, subjects with grey area scores may oscillate between A- and A+ groups, increasing heterogeneity. Our study could not examine the role of other risk factors such as education, cardiovascular status, obesity, diabetes, depression, social and physical activity [ 69 ], or genetic status [ 70 , 71 ], which may also contribute to heterogeneity. Furthermore, there is a considerable heterogeneity by mean age, and our meta-regression analysis of MCI group showed a significant decreasing effect of mean age on ORs.
In the OR analysis of Aβ in the CU group, in the context of the outlier value of the Arruda study, the possibility of a statistical extreme value can be assumed due to the small number of A+ subjects and the much larger A- group. Similarly, in the case of the Grontvedt [ 14 ] and Hanseeuw [ 41 ] studies, which show exceptionally high values, the A+ and A- groups show a similar uneven distribution. Similarly, the outliers in the MCI amyloid OR analysis are also associated with small sample sizes. For the Aβ HR analysis in the CU group, the interpretability of the result is strongly influenced by one specific cohort (ADNI), which accounts for 78% of the overall weight. In the A+T+/A+T-/A-T+ analyses, no outliers were found in either the MCI or CU groups.
Furthermore, we note that although the Aβ OR analyses could be confirmed by also calculating the HRs, the inability to analyze the effect of p-tau on HR due to the low number of studies limits the completeness of the A/T analysis.
We pooled studies reporting AD-type dementia conversion and studies reporting conversion to unspecified dementia. This simplification was necessary because different studies defined Alzheimer’s dementia differently, generally considering the amnestic clinical symptoms rather than biomarkers.
The fact that the studies used different neuropsychology tests to define MCI may contribute to the heterogeneity in the pooled sample. Another contributing factor would be the heterogeneity in the definition of MCI, however among the studies in our pool, only one, by Riemschneider et al. [ 48 ] (sample size = 28), precedes the 2003 ‘Key Symposium’ [ 72 ] that transformed the MCI concept. All other studies were published subsequent to it. While MCI subgroups were deifned after the 2003 Symposium, the definition of MCI (objective cognitive impairment, essentially preserved general cognitive functioning, preserved independence in functional abilities) did not change afterwards. Furthermore, most of the studies pooled in the analyses were published after 2010.
Another source of heterogeneity is the relatively small sample size of some studies, leading to a higher variability of results. However, we thought that including studies with lower sample sizes was also important to get a complete picture.
It is essential to discuss the difference in the follow-up times between studies. The follow-up times ranged from 20 months to more than 10 years. Follow-up times were given in different ways, either as mean, median or up to a certain point. While naturally, the odds of conversion increase over time, our meta-regression analysis suggests that there is no significant difference in the odds ratios over (follow-up) time. The moderate heterogeneity of the studies also points in this direction. We also note here that hazard ratios independent of follow-up time showed similar results to OR analyses. Finally, yet importantly, we would like to point out that pathological protein changes can begin up to 20 years before the appearance of symptoms [ 6 ]. Such an extended follow-up is very difficult to carry out; therefore, all studies were shorter than that.
The results for Aβ are based on 7,793 individuals, and the combined analyses of Aβ and p-tau are based on data of over 3,500 individuals. Studies using CSF sampling or amyloid/tau PET to detect Aβ and p-tau were pooled together, despite using different kits and thresholds for positivity, contributing to the heterogeneity of results. This variation is acknowledged in Tables 1 , 2 , 3 and 4 , where the cut-off values are provided. Previous large population studies have indicated that amyloid and tau PET scans exhibit slightly higher sensitivity compared to CSF sampling techniques [ 73 , 74 , 68 ]. Nonetheless, the concordance between these diagnostic methods remains substantial. Moreover, findings from prior research (Lee et al. [ 75 ], Toledo et al. [ 76 ], Palmqvist et al. [ 77 ]) demonstrating high concordance across different amyloid CSF and amyloid PET measurements suggest that the impact of methodological differences on heterogeneity may be limited, All techniques are recommended by the National Institute on Aging-Alzheimer’s Association (NIA-AA) [ 6 ] for measurement.
Future directions
Conversion to Alzheimer’s disease could not be analyzed specifically, as most of the articles examining conversion either did not define Alzheimer’s disease or the definition was based on neuropsychological testing but not on biomarkers (i.e., Aβ and p-tau status were assessed only at baseline). According to the NIA-AA guideline [ 6 ] and our results, we recommend biomarker-based studies to assess conversion rates to Alzheimer’s disease.
In view of the Aβ and p-tau status, the most endangered population can be identified before the appearance of cognitive symptoms or at least at a mild stage. While the significance of Aβ in conversion is clear, it appears that its ability to predict the onset decreases with age. If we consider the current therapeutic limitations and the importance of early prevention, we believe that the initiation of non-pharmacological and pharmacological treatments should be related to Aβ and p-tau status rather than cognitive status.
Identifying the most endangered population also makes research more effective. The efficacy of different dementia prevention approaches can be more accurately assessed by knowing the Aβ and p-tau status of the patient. As the population targeted by the interventions can be more homogeneous, the effectiveness can be measured more precisely by identifying the population most at risk of conversion.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Non-pathologic levels of beta-amyloid
Pathologic levels of beta-amyloid
- Beta-amyloid
- Alzheimer’s disease
Alzheimer’s Disease Neuroimaging Initiative
Confidance interval
Cognitively unimpaired
Cerebrospinal fluid
Hazard ratio
- Mild cognitive impairment
Absence of neurodegeneration
Presence of neurodegeneration
National Institute on Aging Alzheimer’s Association
Positron emission tomography
- Phosphorylated tau
Non-pathologic levels of phosphorylated tau
Pathologic levels of phosphorylated tau
Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. Geneva: World Health Organization; 2019. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542796/ .
Gauthier S, Rosa-Neto P, Morais JA, & Webster C. 2021. World Alzheimer Report 2021: Journey through the diagnosis of dementia. London: Alzheimer’s Disease International.
De Strooper B. The cellular phase of Alzheimer’s disease. Cell. 2016;164(4):603–15. https://doi.org/10.1016/j.cell.2015.12.056 .
Article CAS PubMed Google Scholar
Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. The Lancet. 2021;397(10284):1577–90. https://doi.org/10.1016/s0140-6736(20)32205-4 .
Article CAS Google Scholar
Dubois B, Villain N, Frisoni GB, et al. Clinical diagnosis of Alzheimer’s disease: recommendations of the international working group. Lancet Neurol. 2021;20(6):484–96. https://doi.org/10.1016/s1474-4422(21)00066-1 .
Article CAS PubMed PubMed Central Google Scholar
Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA Research framework: toward a biological definition of alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–62. https://doi.org/10.1016/j.jalz.2018.02.018 .
Article PubMed Google Scholar
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on alzheimer’s disease. Neurology. 1984;34(7):939–44. https://doi.org/10.1212/wnl.34.7.939 .
McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9. https://doi.org/10.1016/j.jalz.2011.03.005 .
Rowe CC, Ellis KA, Rimajova M, et al. Amyloid imaging results from the australian imaging, biomarkers and lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275–83. https://doi.org/10.1016/j.neurobiolaging.2010.04.007 .
Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid PET positivity in dementia syndromes. JAMA. 2015;313(19):1939. https://doi.org/10.1001/jama.2015.4669 .
Article PubMed PubMed Central Google Scholar
Morris GP, Clark IA, Vissel B. Questions concerning the role of amyloid-β in the definition, aetiology and diagnosis of Alzheimer’s disease. Acta Neuropathol. 2018;136(5):663–89. https://doi.org/10.1007/s00401-018-1918-8 .
Van Der Flier WM, Scheltens P. The ATN framework—moving preclinical Alzheimer disease to clinical relevance. JAMA Neurology. 2022;79(10):968. https://doi.org/10.1001/jamaneurol.2022.2967 .
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–8. https://doi.org/10.1001/archneur.56.3.303 .
Grøntvedt GR, Lauridsen C, Berge G, et al. The amyloid, tau, and neurodegeneration (A/T/N) classification applied to a clinical research cohort with long-term follow-up. J Alzheimers Dis. 2020;74(3):829–37. https://doi.org/10.3233/jad-191227 .
Balasa M, Sánchez-Valle R, Antonell A, et al. Usefulness of biomarkers in the diagnosis and prognosis of early-onset cognitive impairment. J Alzheimer’s Di. 2014;40(4):919–27. https://doi.org/10.3233/JAD-132195 .
Article Google Scholar
Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. Jama. 2015;313(19):1924–38. https://doi.org/10.1001/jama.2015.4668 .
Page MJ, McKenzie JE, Bossuyt PM, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021: n71. https://doi.org/10.1136/bmj.n71 .
Weiner MW. Alzheimer’s disease neuroimaging initiative. Available from: https://adni.loni.usc.edu/ .
Aydin O, Yassikaya MY. Validity and reliability analysis of the plotdigitizer software program for data extraction from single-case graphs. Perspect Behav Sci. 2022;45(1):239–57. https://doi.org/10.1007/s40614-021-00284-0 .
Huwaldt, J. A., & Steinhorst, S. (2020). Plot digitizer 2.6.9.PlotDigitizer-Software. http://plotdigitizer.sourceforge.net/ .
Lewczuk P, Matzen A, Blennow K, et al. Cerebrospinal Fluid Aβ42/40 Corresponds better than Aβ42 to amyloid PET in Alzheimer’s disease. J Alzheimers Dis. 2017;55(2):813–22. https://doi.org/10.3233/jad-160722 .
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48.
CAS PubMed Google Scholar
Robins J, Greenland S, Breslow NE. A general estimator for the variance of the Mantel-Haenszel odds ratio. Am J Epidemiol. 1986;124(5):719–23. https://doi.org/10.1093/oxfordjournals.aje.a114447 .
Thompson SG, Turner RM, Warn DE. Multilevel models for meta-analysis, and their application to absolute risk differences. Stat Methods Med Res. 2001;10(6):375–92. https://doi.org/10.1177/096228020101000602 .
Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1(2):112–25. https://doi.org/10.1002/jrsm.11 .
Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–6. https://doi.org/10.7326/0003-4819-158-4-201302190-00009 .
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. Jama. 2006;295(6):676–80. https://doi.org/10.1001/jama.295.6.676 .
Kemppainen NM, Scheinin NM, Koivunen J, et al. Five-year follow-up of 11C-PIB uptake in Alzheimer’s disease and MCI. Eur J Nucl Med Mol Imaging. 2014;41(2):283–9. https://doi.org/10.1007/s00259-013-2562-0 .
Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal fluid levels of β-amyloid 1–42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012;69(1):98–106. https://doi.org/10.1001/archgenpsychiatry.2011.155 .
Forlenza OV, Radanovic M, Talib LL, et al. Cerebrospinal fluid biomarkers in Alzheimer’s disease: diagnostic accuracy and prediction of dementia. Alzheimers Dement (Amst). 2015;1(4):455–63. https://doi.org/10.1016/j.dadm.2015.09.003 .
Hansson O, Buchhave P, Zetterberg H, Blennow K, Minthon L, Warkentin S. Combined rCBF and CSF biomarkers predict progression from mild cognitive impairment to Alzheimer’s disease. Neurobiol Aging. 2009;30(2):165–73. https://doi.org/10.1016/j.neurobiolaging.2007.06.009 .
Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–94. https://doi.org/10.1111/j.1365-2796.2004.01388.x .
Arruda F, Rosselli M, Mejia Kurasz A, et al. Stability in cognitive classification as a function of severity of impairment and ethnicity: a longitudinal analysis. Article in Press. Appl Neuropsychol Adult. 2023:1-14. https://doi.org/10.1080/23279095.2023.2222861 .
Baldeiras I, Silva-Spínola A, Lima M, et al. Alzheimer’s disease diagnosis based on the amyloid, tau, and neurodegeneration scheme (ATN) in a real-life multicenter cohort of general neurological centers. J Alzheimer’s Dis. 2022;90(1):419–32. https://doi.org/10.3233/JAD-220587 .
Bos I, Verhey FR, Ramakers I, et al. Cerebrovascular and amyloid pathology in predementia stages: the relationship with neurodegeneration and cognitive decline. Alzheimers Res Ther. 2017;9(1):101. https://doi.org/10.1186/s13195-017-0328-9 .
Cerami C, Della Rosa PA, Magnani G, et al. Brain metabolic maps in Mild cognitive impairment predict heterogeneity of progression to dementia. Neuroimage Clin. 2015;7:187–94. https://doi.org/10.1016/j.nicl.2014.12.004 .
de Wilde A, Reimand J, Teunissen CE, et al. Discordant amyloid-β PET and CSF biomarkers and its clinical consequences. Alzheimers Res Ther. 2019;11(1):78. https://doi.org/10.1186/s13195-019-0532-x .
Eckerström C, Svensson J, Kettunen P, Jonsson M, Eckerström M. Evaluation of the ATN model in a longitudinal memory clinic sample with different underlying disorders. Alzheimers Dement (Amst). 2021;13(1): e12031. https://doi.org/10.1002/dad2.12031 .
Frölich L, Peters O, Lewczuk P, et al. Incremental value of biomarker combinations to predict progression of mild cognitive impairment to Alzheimer’s dementia. Alzheimers Res Ther. 2017;9(1):84. https://doi.org/10.1186/s13195-017-0301-7 .
Groot C, Cicognola C, Bali D, et al. Diagnostic and prognostic performance to detect alzheimer’s disease and clinical progression of a novel assay for plasma p-tau217. Article Alzheimer’s Res Ther. 2022;14(1):67. https://doi.org/10.1186/s13195-022-01005-8 .
Hanseeuw BJ, Malotaux V, Dricot L, et al. Defining a Centiloid scale threshold predicting long-term progression to dementia in patients attending the memory clinic: an [(18)F] flutemetamol amyloid PET study. Eur J Nucl Med Mol Imaging. 2021;48(1):302–10. https://doi.org/10.1007/s00259-020-04942-4 .
Herukka SK, Hallikainen M, Soininen H, Pirttilä T. CSF Aβ42 and tau or phosphorylated tau and prediction of progressive mild cognitive impairment. Article Neurology. 2005;64(7):1294–7. https://doi.org/10.1212/01.WNL.0000156914.16988.56 .
Jiménez-Bonilla JF, Quirce R, De Arcocha-Torres M, et al. A 5-year longitudinal evaluation in patients with mild cognitive impairment by 11C-PIB PET/CT: a visual analysis. Nucl Med Commun. 2019;40(5):525–31. https://doi.org/10.1097/mnm.0000000000001004 .
Lopez OL, Becker JT, Chang Y, et al. Amyloid deposition and brain structure as long-term predictors of MCI, dementia, and mortality. Neurology. 2018;90(21):E1920–8. https://doi.org/10.1212/WNL.0000000000005549 .
Okello A, Koivunen J, Edison P, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology. 2009;73(10):754–60. https://doi.org/10.1212/WNL.0b013e3181b23564 .
Orellana A, García-González P, Valero S, et al. Establishing in-house cutoffs of CSF Alzheimer’s disease biomarkers for the AT(N) stratification of the Alzheimer center barcelona cohort. Int J Mol Sci. 2022;23(13):6891. https://doi.org/10.3390/ijms23136891 .
Ortega RL, Dakterzada F, Arias A, et al. Usefulness of CSF biomarkers in predicting the progression of amnesic and nonamnesic mild cognitive impairment to Alzheimer’s disease. Curr Aging Sci. 2019;12(1):35–42. https://doi.org/10.2174/1874609812666190112095430 .
Riemenschneider M, Lautenschlager N, Wagenpfeil S, Diehl J, Drzezga A, Kurz A. Cerebrospinal fluid tau and beta-amyloid 42 proteins identify Alzheimer disease in subjects with mild cognitive impairment. Arch Neurol. 2002;59(11):1729–34. https://doi.org/10.1001/archneur.59.11.1729 .
Rizzi L, Missiaggia L, Schwartz IVD, Roriz-Cruz M. Value of CSF biomarkers in predicting risk of progression from aMCI to ADD in a 5-year follow-up cohort. SN Compr Clin Med. 2020;2(9):1543–50. https://doi.org/10.1007/s42399-020-00437-3 .
Roberts RO, Aakre JA, Kremers WK, et al. Prevalence and Outcomes of amyloid positivity among persons without dementia in a longitudinal population-based setting. JAMA Neurol. 2018;75(8):970–9. https://doi.org/10.1001/jamaneurol.2018.0629 .
Villemagne VL, Pike KE, Chételat G, et al. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69(1):181–92. https://doi.org/10.1002/ana.22248 .
Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5(3):228–34. https://doi.org/10.1016/s1474-4422(06)70355-6 .
Dang C, Harrington KD, Lim YY, et al. Relationship Between amyloid-β positivity and progression to mild cognitive impairment or dementia over 8 years in cognitively normal older adults. J Alzheimers Dis. 2018;65(4):1313–25. https://doi.org/10.3233/jad-180507 .
Ebenau JL, Timmers T, Wesselman LMP, et al. ATN classification and clinical progression in subjective cognitive decline: The SCIENCe project. Neurology. 2020;95(1):e46–58. https://doi.org/10.1212/wnl.0000000000009724 .
Hatashita S, Wakebe D. Amyloid β deposition and glucose metabolism on the long-term progression of preclinical Alzheimer’s disease. Future Sci OA. 2019;5(3):Fso356. https://doi.org/10.4155/fsoa-2018-0069 .
Ossenkoppele R, Pichet Binette A, Groot C, et al. Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nature Medicine. 2022;28(11):2381–7. https://doi.org/10.1038/s41591-022-02049-x .
Strikwerda-Brown C, Hobbs DA, Gonneaud J, et al. Association of elevated amyloid and tau positron emission tomography signal with near-term development of alzheimer disease symptoms in older adults without cognitive impairment. JAMA Neurology. 2022;79(10):975. https://doi.org/10.1001/jamaneurol.2022.2379 .
Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12(10):957–65. https://doi.org/10.1016/s1474-4422(13)70194-7 .
Blom ES, Giedraitis V, Zetterberg H, et al. Rapid progression from mild cognitive impairment to Alzheimer’s disease in subjects with elevated levels of tau in cerebrospinal fluid and the APOE epsilon4/epsilon4 genotype. Dement Geriatr Cogn Disord. 2009;27(5):458–64. https://doi.org/10.1159/000216841 .
Hong YJ, Park JW, Lee SB, et al. The influence of amyloid burden on cognitive decline over 2 years in older adults with subjective cognitive decline: a prospective cohort study. Dement Geriatr Cogn Disord. 2021;50(5):437–45. https://doi.org/10.1159/000519766 .
Tomassen J, den Braber A, van der Landen SM, et al. Abnormal cerebrospinal fluid levels of amyloid and tau are associated with cognitive decline over time in cognitively normal older adults: A monozygotic twin study. Alzheimers Dement (N Y). 2022;8(1): e12346. https://doi.org/10.1002/trc2.12346 .
Rodrigue KM, Kennedy KM, Devous MD Sr, et al. β-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012;78(6):387–95. https://doi.org/10.1212/WNL.0b013e318245d295 .
Donohue MC, Jacqmin-Gadda H, Le Goff M, et al. Estimating long-term multivariate progression from short-term data. Alzheimers Dement. 2014;10(5 Suppl):S400–10. https://doi.org/10.1016/j.jalz.2013.10.003 .
Young AL, Oxtoby NP, Daga P, et al. A data-driven model of biomarker changes in sporadic Alzheimer’s disease. Brain. 2014;137(Pt 9):2564–77. https://doi.org/10.1093/brain/awu176 .
Oberstein TJ, Schmidt MA, Florvaag A, et al. Amyloid-β levels and cognitive trajectories in non-demented pTau181-positive subjects without amyloidopathy. Brain. 2022;145(11):4032–41. https://doi.org/10.1093/brain/awac297 .
Wisse LEM, Butala N, Das SR, et al. Suspected non-AD pathology in mild cognitive impairment. Neurobiol Aging. 2015;36(12):3152–62. https://doi.org/10.1016/j.neurobiolaging.2015.08.029 .
Pouclet-Courtemanche H, Nguyen TB, Skrobala E, et al. Frontotemporal dementia is the leading cause of “true” A-/T+ profiles defined with Aβ(42/40) ratio. Alzheimers Dement (Amst). 2019;11:161–9. https://doi.org/10.1016/j.dadm.2019.01.001 .
Vos SJB, Gordon BA, Su Y, et al. NIA-AA staging of preclinical Alzheimer disease: discordance and concordance of CSF and imaging biomarkers. Neurobiol Aging. 2016;44:1–8. https://doi.org/10.1016/j.neurobiolaging.2016.03.025 .
Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46. https://doi.org/10.1016/s0140-6736(20)30367-6 .
Lourida I, Hannon E, Littlejohns TJ, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322(5):430–7. https://doi.org/10.1001/jama.2019.9879 .
Licher S, Ahmad S, Karamujić-Čomić H, et al. Genetic predisposition, modifiable-risk-factor profile and long-term dementia risk in the general population. Nat Med. 2019;25(9):1364–9. https://doi.org/10.1038/s41591-019-0547-7 .
Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the international working group on mild cognitive impairment. J Intern Med. 2004;256(3):240–6. https://doi.org/10.1111/j.1365-2796.2004.01380.x .
La Joie R, Bejanin A, Fagan AM, et al. Associations between [(18)F]AV1451 tau PET and CSF measures of tau pathology in a clinical sample. Neurology. 2018;90(4):e282–90. https://doi.org/10.1212/wnl.0000000000004860 .
Wolters EE, Ossenkoppele R, Verfaillie SCJ, et al. Regional [(18)F]flortaucipir PET is more closely associated with disease severity than CSF p-tau in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2020;47(12):2866–78. https://doi.org/10.1007/s00259-020-04758-2 .
Lee J, Jang H, Kang SH, et al. Cerebrospinal fluid biomarkers for the diagnosis and classification of Alzheimer’s disease spectrum. J Korean Med Sci. 2020;35(44):361. https://doi.org/10.3346/jkms.2020.35.e361 .
Toledo JB, Brettschneider J, Grossman M, et al. CSF biomarkers cutoffs: the importance of coincident neuropathological diseases. Acta Neuropathol. 2012;124(1):23–35. https://doi.org/10.1007/s00401-012-0983-7 .
Palmqvist S, Zetterberg H, Mattsson N, et al. Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology. 2015;85(14):1240–9. https://doi.org/10.1212/wnl.0000000000001991 .
Download references
Acknowledgements
Not applicable.
Open access funding provided by Semmelweis University. 1. Supported by the GINOP-2.3.4-15-2020-00008 project. The project is co-financed by the European Union and the European Regional Development Fund.
2. This is an EU Joint Programme- Neurodegenerative Disease Research (JPND) project. The project is supported through the following funding organization under the aegis of JPND - www.jpnd.eu (National Research, Development and Innovation, Hungary, 2019-2.1.7-ERA-NET-2020-00006).
3. Supported by the National Research, Development and Innovation Office (NKFI/OTKA FK 138385).
Role of funding source: The sponsor(s), did not participate in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and affiliations.
Centre for Translational Medicine, Semmelweis University, Üllői út 26, Budapest, 1085, Hungary
Zsolt Huszár, Marie Anne Engh, Márk Pavlekovics, Tomoya Sato, Yalea Steenkamp, Tamás Terebessy, Zsolt Molnár, Péter Hegyi & Gábor Csukly
Department of Psychiatry and Psychotherapy, Semmelweis University, Balassa utca 6, Budapest, 1083, Hungary
Zsolt Huszár & Gábor Csukly
Department of Neurology, Jahn Ferenc Teaching Hospital, Köves utca 1, Budapest, 1204, Hungary
Márk Pavlekovics
Department of Neurology and Institute of Neuroscience, Cliniques Universitaires Saint-Luc, Université Catholique de Louvain, Brussels, 1200, Belgium
Bernard Hanseeuw
Department of Radiology, Gordon Center for Medical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, 02155, USA
Department of Anesthesiology and Intensive Therapy, Semmelweis University, Üllői út 78/A, Budapest, Hungary
Zsolt Molnár
Department of Anesthesiology and Intensive Therapy, Poznan University of Medical Sciences, 49 Przybyszewskiego St, Poznan, Poland
Institute for Translational Medicine, Medical School, University of Pécs, Pécs, 7624, Hungary
Péter Hegyi
Institute of Pancreatic Diseases, Semmelweis University, Tömő 25-29, Budapest, 1083, Hungary
Translational Pancreatology Research Group, Interdisciplinary Centre of Excellence for Research Development and Innovation University of Szeged, Budapesti 9, Szeged, 6728, Hungary
You can also search for this author in PubMed Google Scholar
Contributions
ZH: conceptualisation, project administration, methodology, formal analysis, writing – original draft; ME: conceptualisation, methodology, formal analysis, writing – review and editing; MP: conceptualisation, formal analysis, writing - review and editing; TS formal analysis, writing – review and editing; YS: formal analysis, writing – review and editing; BH: writing - review and editing; TT: conceptualisation, writing – review and editing; ZM: conceptualisation, supervision, writing - review and editing; PH: conceptualisation, supervision, writing - review and editing; GCs: conceptualization, methodology, formal analysis, supervision, writing – original draft, visualization. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Gábor Csukly .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Huszár, Z., Engh, M., Pavlekovics, M. et al. Risk of conversion to mild cognitive impairment or dementia among subjects with amyloid and tau pathology: a systematic review and meta-analysis. Alz Res Therapy 16 , 81 (2024). https://doi.org/10.1186/s13195-024-01455-2
Download citation
Received : 07 July 2023
Accepted : 08 April 2024
Published : 12 April 2024
DOI : https://doi.org/10.1186/s13195-024-01455-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Alzheimer's Research & Therapy
ISSN: 1758-9193
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Thorac Cancer
- v.13(7); 2022 Apr

Hyperthermic intrathoracic chemotherapy for the treatment of malignant pleural effusion caused by breast and ovarian cancer: A systematic literature review and pooled analysis
Ioannis karampinis.
1 Division of Thoracic Surgery, Academic Thoracic Center, University Medical Center Mainz, Johannes Gutenberg University, Mainz Germany
Anna Dionysopoulou
2 Department of Obstetrics and Gynecology, University Medical Center Mainz, Johannes Gutenberg University, Mainz Germany
Christian Galata
Katrin almstedt, maurizio grilli.
3 Department of Library and Information Sciences, University Medical Center Mannheim, Medical Faculty Mannheim, Heidelberg University, Mannheim Germany
Annette Hasenburg
Eric d. roessner, associated data.
The datasets generated during the current study are not publicly available since the main part is included in this article. The complete database is available from the corresponding author on reasonable request.
Breast and ovarian cancer account for over 30% of malignant pleural effusions (MPEs). Treatment of the metastatic disease requires control of the MPE. Even though primarily symptomatic, the treatment of the MPE can potentially affect the oncological course of the disease. The aim of this review is to analyze the effectiveness of intrathoracic chemotherapy in the treatment of MPE caused by breast and ovarian cancer.
A systematic literature research was conducted up until May 2021. Studies published in English on patients undergoing either surgical or interventional intrapleural chemotherapy were included.
Thirteen studies with a total of 497 patients were included. Analysis was performed on 169 patients with MPE due to breast cancer and eight patients with MPE secondary to ovarian cancer. The pooled success rates of intrathoracic chemotherapy for controlling the MPE were 59.1% and 87.5%, respectively. A survival analysis was not possible with the available data. The overall toxicity of the treatment was low.
Conclusions
Intrathoracic chemotherapy achieves symptomatic control of the MPE in 59.1% of patients with metastatic breast cancer and 87.5% of patients with metastatic ovarian cancer. This is inferior to other forms of surgical pleurodesis. Data from small case series and studies on intraperitoneal chemotherapy show promising results. However, formal oncological studies on the use of intrathoracic chemotherapy for metastatic breast or ovarian cancer are lacking. Further prospective pilot studies are needed to assess the therapeutic oncological effects of this treatment.
A systematic literature research was conducted up until May 2021 to analyze the effectiveness of intrathoracic chemotherapy in the treatment of malignant pleural effusion (MPE) caused by breast and ovarian cancer. Analysis was performed on 169 patients from 13 different studies with MPE due to breast cancer and eight patients with MPE secondary to ovarian cancer. The pooled success rates of intrathoracic chemotherapy for controlling the MPE were 59.1% and 87.5%, respectively. The overall toxicity of the treatment was low.

INTRODUCTION
Every third patient with malignant pleural effusion (MPE) suffers from either metastatic breast or ovarian cancer. 1 The need for treatment for MPE is usually triggered by the patient's symptoms. Simple aspiration, interventional drainage or surgery have all been proposed as treatment options, depending on the symptoms and the recurrence rate of the effusion. 2 There is no clear correlation between the severity of the symptoms and the size of the effusion. 3 However, symptomatic pleural effusions are more likely to be bigger and tend to recur more often.
The diagnosis of MPE has been associated with poor prognosis, which ranges from a few weeks to several months. 4 It is therefore common practice that patients with confirmed MPE only qualify for palliative treatment. However, the current trend in oncology is towards aggressive treatment even in metastatic disease. 5 , 6 Intrathoracic chemotherapy has been proposed as a treatment option for metastatic pleural disease and has been used to treat several malignancies, including mesothelioma, lung cancer, thymic malignancies, and peritoneal surface malignancies. 7 , 8 , 9
A recently published study described the influence of MPE in the survival rate of patients with advanced ovarian cancer and established an algorithm for approaching these cases. 10 According to the proposed algorithm, a thoracoscopy needs to be performed initially to determine the presence or absence of macroscopic pleural disease. Depending on the findings, the authors either proceed with debulking or end the procedure and the patient receives induction chemotherapy followed by thoracic debulking. As a last step the patients undergo abdominal cytoreduction. By following this treatment algorithm, the authors managed to increase the number of patients that qualify for aggressive treatment whilst avoiding overtreating those with advanced disease.
The purpose of our study was to analyze the role of intrathoracic chemotherapy in the treatment of MPE caused by breast and ovarian cancer.
MATERIALS AND METHODS
The objective of this review is to determine the rate of local control of MPE in patients with metastatic breast or ovarian cancer that undergo intrathoracic chemotherapy. Furthermore, we aim to determine the impact of this treatment on the survival rate of these patients.
All studies reporting results on local control of the MPE or reporting survival data were included. Given that the actual recurrence rate and therefore local control rate of the effusion for metastatic breast and ovarian cancer are unknown, studies were included regardless of the number of patients.
Systematic literature research
A computer‐based literature search was performed (MG) up until May 14, 2021 in several databases, including the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Database of Systematic Reviews (CDSR) from The Cochrane Library, MEDLINE (1966 to present), Cinahl (1981 to present), and Web of Science (1945 to present). Reference lists of retrieved articles were scanned for further eligible trials (backward search) and citations of identified trials were checked for inclusion (forward search). Search strategies included proper combinations of the MeSH terms “HITHOC”, “breast cancer”, and “ovarian cancer.” The search was not limited by publication type. Non‐English papers were excluded. The complete search strategy is available as Supporting Information.
Two reviewers (A.D. and I.K.) independently performed the extraction of data from the included studies. The findings of the two reviewers were controlled for concordance and disagreements were resolved with discussion and detailed analysis of the trials and the data.
The success rate of intrathoracic chemotherapy for controlling the MPE was estimated as the proportion of patients with either complete or partial response to the total number of patients that was treated. A quality/bias assessment was not performed due to the low or very low quality of the included studies.
The database search provided 3363 references. After deduplication, 2230 studies were available for screening (Figure 1 ). A total of 2179 records were excluded and 51 abstracts were assessed for eligibility. In addition, 38 were excluded and 13 studies were included in the review. Hand searching of the references of the included studies provided one extra reference, which was eventually excluded.

PRISMA flowchart
Studies on intrathoracic chemotherapy for malignancies other than breast or ovarian cancer, studies on patients undergoing other forms of pleurodesis, as well as studies analyzing the impact of nonchemotherapy associated intrapleural treatment were excluded.
The included studies are listed in Table 1 . Six studies were retrospective, one of which was a multicentre retrospective study, 11 four studies were prospective, two were case reports, and one was a randomized controlled trial. The 13 studies included a total of 497 patients, and 286 of these patients were treated for MPE caused by breast or ovarian cancer.
Included references
Abbreviations: BC, breast cancer; IC, intercostal drain; OC, ovarian cancer; VATS, video‐assisted thoracoscopic surgery.
In 12 studies, the endpoint was local control or recurrence of the MPE. The purpose of the 13th study was to examine the effectiveness of repeated intrapleural chemotherapy through a port system and report only the survival rate of the included patients. 17 Four studies reported no toxicity after the intrapleural chemotherapy. 14 , 17 , 20 , 22 In the complete cohort of 497 patients, 11 cases of myelosuppression and another 18 cases of other grade 3/4 toxicity were described. One study established the levels of intrapleural toxicity of docetaxel. 19 By administering doses between 50 and 125 mg/m 2 the authors achieved a pleural exposure which was 1000 times higher than the systemic exposure, whilst observing a single dose‐limiting toxicity in a patient that had received 50 mg/m 2 . All patients that received doses of 100 mg/m 2 or higher achieved complete resolution of the MPE. 19
The cytotoxic agent, nitrogen mustard or thio‐TEPA, was administered intrapleural in one study. 12 In seven studies a platin‐based chemotherapy was used and further two studies combined platin‐based chemotherapy with paclitaxel. 18 , 20 The other studies used different combinations of chemotherapy. One study that compared intrapleural cisplatin alone and intrapleural cisplatin combined with external application of mirabilite rhubarb in a randomized setting concluded that the second group had a significantly higher success rate for achieving local control of the disease. Six studies excluded patients who were receiving concurrent systemic chemotherapy while in two other studies all patients were receiving systemic chemotherapy.
The median time point that the efficacy of the intrapleural chemotherapy was assessed was 4 weeks (range 3–8 weeks) and was reported in seven studies. The pooled success rate of intrapleural chemotherapy for controlling the MPE was 59.1% for breast cancer patients and 87.5% for ovarian cancer. The success rate in the individual studies is presented on Figure 2 .

Success rate of individual studies in achieving local control of the MPE
MPE is a debilitating diagnosis that is associated with severe symptoms, the need for regular interventions or surgical therapy, and delay in systemic treatment. The standard management of MPE is largely dependent on the symptoms and the prognosis of the patient. Fit patients with an estimated prognosis of a few months or longer usually receive surgical treatment, whereas patients with a poor performance status or with a poor prognosis are usually treated conservatively with pleural taps or indwelling pleural catheters. 2
Intrathoracic chemotherapy for the treatment of MPE was first described in 1955. 24 In advanced abdominal tumors, extensive tumor resection (cytoreduction) followed by intraoperative administration of local chemotherapy with simultaneous warming of the abdominal cavity is used to improve survival in selected patients. This concept of hyperthermic intraperitoneal chemotherapy (HIPEC) was initially developed to treat metastatic disease of the peritoneum and was later transferred to the chest cavity (hyperthermic intrathoracic chemotherapy, HITHOC), mainly to treat malignant mesothelioma. 25 Since then, numerous studies on the use of intrathoracic chemotherapy have been published. However, most of the literature are case series, feasibility studies, and retrospective studies with small numbers of patients. When administered locally, the chemotherapy can be applied in much higher doses than systemically tolerable, leading to both a sclerosing and a cytotoxic effect. 19 The main tumor types that intrathoracic chemotherapy has been implemented on are lung cancer, mesothelioma, and thymic malignancies. Despite the high prevalence of MPE in patients with metastatic breast and ovarian cancer, limited data exist on the use of intrathoracic chemotherapy as a treatment option for these patients.
In this study we reviewed the impact of intrathoracic chemotherapy for the treatment of MPE caused by breast or ovarian cancer. A total of 169 patients with breast cancer and eight patients with ovarian cancer were pooled to calculate a local control rate of 59.1% for breast cancer and 87.5% for ovarian cancer at 4 weeks after treatment. Survival data was not available for analysis.
The pooled success rate of 59.1% is inferior to other forms of surgical pleurodesis, such as talc pleurodesis in patients with metastatic breast cancer. 26 It is therefore reasonable to suggest the use of talc in case only pleurodesis is attempted. For patients with metastatic ovarian cancer the success rate of intrathoracic chemotherapy for achieving pleurodesis was higher than average. However, only eight patients from three different studies were utilized to calculate this percentage, and therefore this may not be representative of the true value.
It is arguably not a worthwhile venture to investigate further trials or studies to research the use of intrathoracic chemotherapy in achieving pleurodesis in patients with breast and ovarian cancer. However, given the low level of toxicity, the incapacitating effects of MPE, and the current trend towards more aggressive treatment of several malignancies, it is certainly worth doing further clinical trials to test its oncological efficacy.
Questions that need to be clarified in upcoming studies should be the selection criteria for this treatment, the chemotherapeutic agents that should be applied as well as the type of surgery that is indicated. The combination of surgical cytoreduction with hyperthermic chemotherapy has been proven to be the most effective from the oncological point of view for other malignancies (thymic tumors, malignant pleural mesothelioma). On the other hand, newer, less invasive procedures like the pressurized intrathoracic chemotherapy are currently gaining approval in this field.
Limitations
This review/pooled analysis has several limitations. First, there is a high level of heterogeneity in the included studies, especially with regards to the oncological status/treatment stadium of the included patients, the protocol, the agents used for intrathoracic chemotherapy, the follow‐up, and the outcome assessment algorithms. Second, this review covers a period of 50 years, which has a significant impact on the interpretation and the comparability of the results of the included studies.
Third, this analysis pools together studies on intrathoracic chemotherapy through an intrapleural catheter and studies on intrathoracic chemotherapy following thoracoscopic cytoreductive surgery. The purpose of the first studies is obviously palliation/pleurodesis while the purpose of the second ones could be potentially curative treatment, although only limited disease can be effectively resected through thoracoscopic cytoreduction. The effect of a pleurectomy/decortication followed by intrapleural chemotherapy is not presented in this review since no proper evidence exists so far. Nevertheless, the pooled results of this analysis can offer a better overview of the available data and direct future studies, rather than being used as absolute numbers, since they may deviate significantly from the truth.
MPE is a common complication of metastatic breast and ovarian cancer. Treating the effusion is often required to ease the symptoms of the patients, but might as well affect the oncological outcome of the disease. Compared to other nonsurgical forms of pleurodesis, intrathoracic chemotherapy has a comparable efficacy in achieving pleurodesis. However, it is inferior to surgical pleurodesis and has a higher rate of adverse events. Results from reports and small case series demonstrate that even thoracoscopic cytoreduction followed by intrathoracic chemotherapy can have a positive impact on survival as part of a multimodality oncological treatment on selected patients and should therefore be evaluated in further prospective pilot studies.
CONFLICT OF INTEREST
The authors have no competing interests to declare.
AUTHOR CONTRIBUTIONS
I.K. was involved in the conception of the project, extraction and analysis of the data, and drafting and revision of the manuscript. A.D. was involved in the conception of the project, performed the extraction, acquisition, and analysis of the data, and drafted the manuscript. C.G. was involved in the processing of the data and the data analysis. K.A. was involved in the processing of the data and the data analysis. M.G. performed the systematic literature research. A.H. was involved in the conception of the project and critically revised the manuscript for important intellectual content. E.R. was involved in the conception of the project and critically revised the manuscript for important intellectual content. I.K., A.D., and C.G. had full access to the data presented in this study.
ACKNOWLEDGMENTS
Open Access funding enabled and organized by Projekt DEAL.
Karampinis I, Dionysopoulou A, Galata C, Almstedt K, Grilli M, Hasenburg A, et al. Hyperthermic intrathoracic chemotherapy for the treatment of malignant pleural effusion caused by breast and ovarian cancer: A systematic literature review and pooled analysis . Thorac Cancer . 2022; 13 :883–888. 10.1111/1759-7714.14361 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
DATA AVAILABILITY STATEMENT
- Scoping Review
- Open access
- Published: 25 May 2023
Global prevalence of drug-resistant tuberculosis: a systematic review and meta-analysis
- Nader Salari 1 , 2 ,
- Amir Hossein Kanjoori 3 ,
- Amin Hosseinian-Far 4 ,
- Razie Hasheminezhad 3 ,
- Kamran Mansouri 5 &
- Masoud Mohammadi ORCID: orcid.org/0000-0002-5722-8300 6
Infectious Diseases of Poverty volume 12 , Article number: 57 ( 2023 ) Cite this article
7393 Accesses
10 Citations
8 Altmetric
Metrics details
Tuberculosis is a bacterial infectious disease, which affects different parts of a human body, mainly lungs and can lead to the patient’s death. The aim of this study is to investigate the global prevalence of drug-resistant tuberculosis using a systematic review and meta-analysis.
In this study, the PubMed, Scopus, Web of Science, Embase, ScienceDirect and Google Scholar repositories were systematically searched to find studies reporting the global prevalence of drug-resistant tuberculosis. The search did not entail a lower time limit, and articles published up until August 2022 were considered. Random effects model was used to perform the analysis. The heterogeneity of the studies was examined with the I 2 test. Data analysis was conducted within the Comprehensive Meta-Analysis software.
In the review of 148 studies with a sample size of 318,430 people, the I 2 index showed high heterogeneity ( I 2 = 99.6), and accordingly random effects method was used to analyze the results. Publication bias was also examined using the Begg and Mazumdar correlation test which indicated the existence of publication bias in the studies ( P = 0.008). According to our meta-analysis, the global pooled prevalence of multi-drug resistant TB is 11.6% (95% CI : 9.1–14.5%).
Conclusions
The global prevalence of drug-resistant tuberculosis was found to be very high, thus health authorities should consider ways to control and manage the disease to prevent a wider spread of tuberculosis and potentially subsequent deaths.
Tuberculosis (TB) is one of the most common infectious diseases, which is the main cause of widespread mortality, especially among people living with HIV (PLHIV) [ 1 , 2 ]. The disease is caused by a type of bacteria called Mycobacterium TB [ 3 ]. Different types of TB are multi drug-resistant (MDR), pre-extensively drug-resistant (Pre-XDR), and extensively drug-resistant (XDR) [ 4 ]. TB usually affects the lungs, however it can also affect other parts of the body, such as the kidneys and the brain [ 4 ].
There were an estimated 450,000 incident cases of MDR in 2021, up 3.1% from 437,000 in 2020, three countries accounted for 42% of global cases in 202: India (26%), the Russian Federation (8.5%), and Pakistan (7.9%) [ 5 ]. A study by Baya et al., reported that the average age of patients was 39.31 ± 14.64 years, whilst 62.6% of patients were less than 40 years old. Patients were predominantly male 76.2%, and 77.1% were married [ 6 ]. Additionally, the prevalence of latent MDR TB has been reported in some countries of Eastern Europe and Central Asia, such as China (6 million people), India (4 million people), and Russia (1.8 million people) [ 4 , 5 , 6 ].
According to the existing literature, risk factors of tuberculosis include demographic characteristics such as gender, age, place of residence, education, marital status, bad habits such as alcohol abuse and smoking, and concomitant infections including diabetes mellitus, HIV, Acid-Fast Bacilli (AFB) smear, pulmonary space, history of tuberculosis, and history of anti-tuberculosis treatment are significant risk factors for MDR TB [ 7 ].
TB often impacts patients with other diseases such as diabetes, HIV, and chronic obstructive pulmonary disease (COPD) [ 7 ]. Cough or fever for > 2 weeks, weight loss, or hemoptysis are among the symptoms of TB which are also associated with lack of health insurance, tuberculin skin test, diagnosis through a process not entailing screening, and ethnicities other than Asian [ 8 ]. Complications of this disease include bronchial stenosis, severe airway obstruction, pneumonia, and hemoptysis, the most common of which is liver damage [ 9 , 10 ]. Vocal cord paralysis, associated with laryngeal TB, can also be found among the patients [ 10 ]. To treat TB, a combination of isoniazid, rifampin, ethambutol, and pyrazinamide, followed by a combination of isoniazid and rifampin are used [ 9 ].
Several studies have been conducted on the prevalence of drug-resistant tuberculosis worldwide. These studies have reported different rates, yet their reported results are heterogeneous and are not aligned. The aim of this systematic review and meta-analysis is to pool the reported results of the existing studies and offer a scientifically consistent prevalence for drug resistant TB. The findings of our study can provide useful insights to health policymakers to devised appropriate interventions, with a view to reducing the subsequent complications from the disease.
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The keywords of prevalence, drug-resistant tuberculosis, burden, outbreak and their combination using the (AND) and (OR) operators, were used to search the PubMed, Google Scholar, Science Direct, Embase, Scopus and Web of Science databases. The search was conducted with no lower time limit and until August 2022. The reference lists within the identified studies were also manually searched to ensure the comprehensive of the collected articles. The information of the identified studies was transferred into the EndNote reference management software, and studies that had reported the prevalence of drug-resistant tuberculosis by continent and were satisfying the inclusion criteria, were selected for final analysis.
Inclusion and exclusion criteria
The following criteria were used to keep an identified study in the systematic review and for meta-analysis: Studies that reported the prevalence of drug-resistant tuberculosis (including cross-sectional, case–control, and cohort studies), Studies with their full-text available, Studies that provided sufficient data (sample size, prevalence), Studies written and published in English. In contrary, the following criteria resulted in excluding identified articles: Case report studies, case series studies, duplicate studies and meta-analysis studies.
Study selection
Similarly, selection of studies was conducted in accordance with the PRISMA guidelines. Initially, articles that were duplicates in different databases were excluded, and only one copy was retained. Subsequently, the initial screening of articles was conducted through reviewing the titles and abstracts, and irrelevant articles were omitted based on the inclusion and exclusion criteria. Then their full text of articles was reviewed in line with the inclusion and exclusion criteria, and at this stage further irrelevant studies were removed. To avoid any potential bias, all the steps of reviews and data extraction were conducted by two reviewers independently. In cases where there was a difference of opinion between two reviewers, the review of the article was finalized by a third reviewer.
Quality evaluation
To evaluate the quality of articles, a checklist appropriate to observational studies was selected. The Strengthening the Reporting of Observational Studies in Epidemiology checklist (STROBE) consists of six scales including: title, abstract, introduction, methods, results, and discussion. In total, this instruction consists of 32 subscales. These 32 subscales denote different methodological aspects of the study, i.e., title, statement of the problem, study objectives, type of study, statistical population of the study, sampling method, determining the appropriate sample size, definition of variables and procedures, study data collection tools, statistical analysis methods and findings. Consider that the fulfilment of each of the subscales award a point, and based on this, articles with scores of 16 and above were considered to be of medium and high methodological quality articles respectively. Articles with a score below 16 were considered to be of poor quality and were therefore excluded from our study.
Data extraction
Data extraction was completed by two researchers using a different pre-prepared checklist. This checklist included: first author's name, year of publication, study location, sample size, age group of men and women, global prevalence of drug-resistant tuberculosis, and research instruments.
Statistical analysis
The extracted information were structured and were inputted into Comprehensive Meta-Analysis software (Version 2, Biostat, Inc., 14 North Dean Street, Englewood, NJ 07631 USA). The heterogeneity of the studies was then assessed using the I 2 test. In order to check the publication bias, the Begg’s test was used at a significance level of 0.1, and associated Funnel plots were drawn.
Following the initial search, 5109 articles were identified from the databases. An additional 60 related articles were also included following manual searches. Information of all identified articles were then transferred into the EndNote reference management software. Throughout the PRISMA’s identification stage, 2491 articles were excluded due to being repeated in various databases, and only one copy was retained. In the screening stage, the title and abstract of the studies were reviewed and 1964 further articles were excluded based on the inclusion and exclusion criteria. In the eligibility evaluation phase, 323 articles were omitted, after examination of the full text of the articles. As part of quality evaluation, and through the evaluation of the full text of the articles and based on the scores obtained from the STROBE checklist, studies with poor methodological quality were removed, and finally 148 studies were kept for analysis. All included studies were cross-sectional and most of the reviewed studies were conducted in Africa (continent). The information related to the 148 included studies is presented in Fig. 1 and Additional file 1 : Tables S1 to S6 [ 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 ].
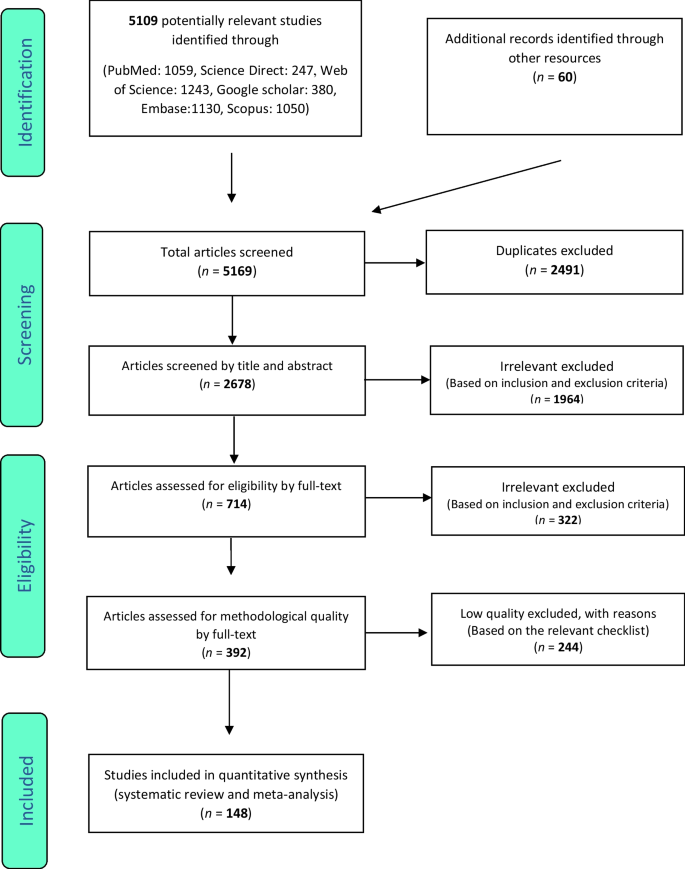
PRISMA flow diagram for study selection
Multi drug-resistant TB
In the review of 148 studies that had studied multi drug resistant TB (sample size of 318,430 people), the I 2 test showed a high heterogeneity ( I 2 = 99.6), and accordingly, random effects method was used to analyze the results. Considering the meta-analysis, the global pooled prevalence of multi-drug resistant TB was found to be 11.6% (95% CI : 9.1–14.5%). Test of publication bias in the studies through the Begg and Mazumdar correlation test showed the existence of publication bias among the studies ( P = 0.008) (Table 1 ) (Figs. S1, S2 in Additional file 2 ).
Isoniazid resistant TB
In 98 studies with a focus on Isoniazid resistant TB (sample size of 102,260 people), the I 2 heterogeneity test showed a high heterogeneity ( I 2 = 99.03), and accordingly, random effects method was used to analyze the results. Considering the meta-analysis, the pooled global prevalence of isoniazid resistant TB was found to be 15.7% (95% CI : 13.7–17.9%). The study of publication bias in the studies through the Begg and Mazumdar correlation test showed the existence of publication bias in the studies ( P = 0.02) (Table 1 ) (Figs. S3, S4 in Additional file 2 ).
Rifampin resistant TB
In the review of 109 studies that had researched rifampin resistant TB (sample size of 215,660 people), the I 2 heterogeneity test showed a high heterogeneity ( I 2 = 98.9), and similarly, random effects method was used to analyze the results. Based on the meta-analysis, the pooled global prevalence of rifampin- resistant TB was found as 9.4% (95% CI : 7.8–11.2%). The study of publication bias in the studies through the Begg and Mazumdar correlation test indicated the existence of publication bias in the studies ( P = 0.00045) (Table 1 ) (Figs. S5, S6 in Additional file 2 ).
Single drug resistant TB
In the review of 35 studies with a focus on single drug resistant TB (sample size of 45,147 people), the I 2 heterogeneity test showed a high heterogeneity ( I 2 = 98.5). Hence, random effects method was used to analyze the results. Considering the meta-analysis results, the pooled global prevalence of single drug resistant TB was found as 11.8% (95% CI : 9.2–15.2%). The study of publication bias in the studies through the Begg and Mazumdar correlation test showed the absence of publication bias in the studies ( P = 0.139) (Table 1 ) (Figs. S7, S8 in Additional file 2 ).
Extensive drug resistant TB
In the review of 56 studies on extensive drug resistant TB (sample size of 350,420 people), the I 2 heterogeneity test showed high heterogeneity ( I 2 = 98.8), and therefore, random effects method was used to analyze the results. Considering the meta-analysis results, the pooled global prevalence of extensive drug resistant TB was found to be 2.5% (95% CI : 2–3%). The study of publication bias using the Begg and Mazumdar correlation test indicated the absence of publication bias in the studies ( P = 0.938) (Table 1 ) (Figs. S9, S10 in Additional file 2 ).
Information in Table 2 outlines the subgroup analysis of the types of tuberculosis resistance among patients by gender, and by TB type. Accordingly, male patients have a higher prevalence in multi-drug resistant TB, Isoniazid resistant TB and Rifampin-resistant TB, compared to female patients, with prevalence of 20% (95% CI : 11.9–31.8%), 17.5% (95% CI : 9.6–29.8%), and 12.7% (95% CI : 5.7–25.9%) respectively. Given that the articles did not report gender-segregated data for single drug-resistant TB and extensively drug-resistant TB, the authors could not include these results in the subgroup analysis.
Tuberculosis is a very common infection with a bacterial agent called Mycobacterium [ 22 , 45 , 180 , 181 , 182 ]. MDR-TB is a strain of Tuberculosis (TB) that is resistant to at least two of the most important anti-tuberculosis drugs (INH and RIF) [ 180 , 181 , 182 , 183 , 184 , 185 ].
This systematic review and meta-analysis was conducted to identify and review existing research works that had examined prevalence of different types of TB. It was also aimed to obtain pooled prevalence of TB types globally. Accordingly, we did not find a specific study on the prevalence of drug-resistant tuberculosis at the global level, despite the fact that there are many articles that have reported the prevalence of this disease at country level, or at most in a continent.
Considering the reported results of an all included studies, the global pooled prevalence of different types of drug-resistant tuberculosis, namely MDR, Isoniazid (INH), Rifampcin (RIF), and XDR were calculated as 11.6%, 15.7%, 9.4%, and 2.5%, respectively.
Eastern European countries, the Russia and Central Asian countries, and parts of China have a high rate of MDR-TB infection [ 184 , 186 ]. In the study by Kindu Alem Mola et al., the authors reported that the level of MDR-TB in East Africa is higher than other regions globally [ 187 ]. In this work, based on the relevant reports from the World Health Organization (WHO) in 2015, the prevalence of global MDR TB in new and previous TB cases were 3.5% and 20.5%, respectively, while countries in southern regions of Africa have greater rates [ 187 , 188 ].
The main reasons for the emergence of MDR TB globally numerous [ 187 ], and they are mostly related to living conditions [ 189 ], lifestyle [ 190 ], previous medical history [ 111 , 191 ], history of diabetes [ 192 , 193 ] and Human Immunodeficiency Viruses (HIV) infection [ 194 ]. A study conducted in Ethiopia shows that HIV infection is one of the most important factors associated with MDR TB [ 187 , 195 ]. In addition, HIV patients, due to the length of hospitalization in hospitals with poorer hygiene and infection control, are more exposed to MDR TB and hence the rate of infection is higher among these patients [ 187 ]. In another study by Al-Derraji et al. [ 187 , 196 ], the incidence of MDR TB among HIV-positive patients was reported to be 20% higher compared to that of HIV-negatives [ 187 ].
In densely populated and poor families, the spread of TB disease is also more prevalent [ 187 ]. According to the literature, unhealthy or poor lifestyles which entail alcohol abuse, smoking, drug use, etc. are the main risk factors related to the spread of MDR TB [ 187 ]. It was also stated that smokers, especially men, are more likely to be infected with MDR TB compared to female smokers [ 187 , 197 , 198 , 199 ].
According to an article by Jilani Talha et al., tuberculosis complications are usually seen more among elderly patients, young children, people with severe respiratory disorders or patients who do not receive proper treatment. Accordingly, patients who do not receive proper treatment are more exposed to tuberculosis complications. Some of these complications are acute respiratory distress syndrome, extensive lung destruction, empyema, pneumothorax, disseminated tuberculosis infection (including tuberculosis meningitis), bronchiectasis, fibrothorax, aspergilloma, and hemoptysis [ 200 ].
According to a study conducted by Jilani et al., with a focus on treating active tuberculosis, a combination of drugs is required during the two intensive and the continuous phases; the first-line drugs that are the most common regimen for tuberculosis treatment include: (1) isoniazid, (2) rifampin, (3) ethambutol, and (4) pyrazinamide [ 200 ].
The intensive phase in the treatment of Tuberculosis includes the combination of the above 4 drugs that are prescribed for 2 months, yet the continuation phase includes the combination of isoniazid and rifampin for an additional 4 months. The second line drugs include: (1) Injectable aminoglycoside: streptomycin, amikacin, kanamycin; (2) Injectable polypeptides: viomycin and capreomycin; (3) Fluoroquinolones: levofloxacin, gatifloxacin, ofloxacin and moxifloxacin, and (4) Para-amino salicylic acid, ethionamide, cycloserine, prothionamide, trazodone, linezolid [ 200 ].
In a study conducted, the side effects of each anti-tuberculosis drug were described as follows: (1) Isoniazid: liver damage (fatigue, nausea, lethargy, abdominal pain, and vomiting), skin rash, numbness, headache and tingling of limbs; (2) Rifampin: jaundice, arthralgia (joint stiffness), and fever; (3) Ethambutol: visual impairment including blurred or reduced vision and blindness, liver damage, headache, and nausea, and (4) Pyrazinamide: nausea, painful or swollen joints, and liver damage [ 200 ]. According to the reported results of the same study, the highest prevalence of multi-drug resistant tuberculosis was reported in males.
Considering the ratio of infections among males vs females, one study reported that the split between males and females with multi-drug resistant tuberculosis was 70.4% and 29.6% respectively [ 201 ], In a study conducted in patients with resistant tuberculosis in Ghana, the ratio of males and females was 69.6% and 30.4% respectively [ 202 ], whilst in another study conducted in Egypt, the ratio of males and females was reported as 67.5% and 32.5%, respectively [ 203 ]. Moreover, in a similar research work conducted in Ethiopia 65.3% male and 34.7% female has multi drug resistant TB [ 204 ].
Our study shows that different strains of Tuberculosis, including drug-resistant TBs, have a high prevalence. On the other hand, these strains can be treated, and there are similar strategies and interventions to control existing and new infections. Considering the complications that this disease may cause, its control and management are vital, since it would be possible to reduce the Tuberculosis induced mortality rate through controlling its different strains.
The main limitation of the present meta-analysis is related to the significant publication bias among the identified studies, and therefore, the results should be considered with caution. Moreover, it is recommended that future meta-analysis studies in this field are conducted using more keywords and databases to potentially eliminate this bias.
According to the results of the present study, the global prevalence of multidrug-resistant, mono drug-resistant, isoniazid, and rifampicin tuberculosis are 11.6%, 11.8%, 15.7%, and 9.4%, respectively. The results of this study can offer some consistency to the heterogeneous results from studies conducted around the world and provide reliable insights to health policymakers. Such insights would be instrumental to devise appropriate preventive, therapeutic and diagnostic measures.
Availability of data and materials
Datasets are available through the corresponding author upon reasonable request.
Abbreviations
Drug susceptibility testing
Whole genome sequencing
Restriction fragment length polymorphism
Decontamination and Ziehl–Neelsen
Chest X-ray
Antimicrobial susceptibility testing
Confidence interval
Tuberculosis
Iacobino A, Fattorini L, Giannoni F. Drug-resistant tuberculosis 2020: where we stand. Appl Sci. 2020;10(6):2153.
Article CAS Google Scholar
Asgedom SW, Teweldemedhin M, Gebreyesus H. Prevalence of multidrug-resistant tuberculosis and associated factors in Ethiopia: a systematic review. J Pathogens. 2018;2018:7104921.
Article Google Scholar
Lange C, Chesov D, Heyckendorf J, Leung CC, Udwadia Z, Dheda K. Drug-resistant tuberculosis: an update on disease burden, diagnosis and treatment. Respirology. 2018;23(7):656–73.
Article PubMed Google Scholar
Mazurek GH. Division of tuberculosis elimination, national center for HIV, STD, and TB prevention, centers for disease control and prevention (CDC). Guidelines for using the QuantiFERON-TB gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49–55.
PubMed Google Scholar
Global Tuberculosis Report 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 .
Baya B, Achenbach CJ, Kone B, Toloba Y, Dabitao DK, Diarra B, et al. Clinical risk factors associated with multidrug-resistant tuberculosis (MDR-TB) in Mali. IJID. 2019;81:149–55.
Xi Y, Zhang W, Qiao R-J, Tang J. Risk factors for multidrug-resistant tuberculosis: a worldwide systematic review and meta-analysis. PLoS ONE. 2022;17(6): e0270003.
Article CAS PubMed PubMed Central Google Scholar
Miller LG, Asch SM, Yu EI, Knowles L, Gelberg L, Davidson P. A population-based survey of tuberculosis symptoms: how atypical are atypical presentations? Clin Infect Dis. 2000;30(2):293–9.
Article CAS PubMed Google Scholar
Suárez I, Fünger SM, Kröger S, Rademacher J, Fätkenheuer G, Rybniker J. The diagnosis and treatment of tuberculosis. DÄ International. 2019;116(43)
Hsu D, Irfan M, Jabeen K, Iqbal N, Hasan R, Migliori GB, et al. Post tuberculosis treatment infectious complications. IJID. 2020;92:S41–5.
Google Scholar
Wang W, Wang J, Zhao Q, Darling ND, Yu M, Zhou B, et al. Contribution of rural-to-urban migration in the prevalence of drug resistant tuberculosis in China. Eur J Clin Microbiol Infect Dis ESCMID. 2011;30(4):581–6.
Timire C, Metcalfe JZ, Chirenda J, Scholten JN, Manyame-Murwira B, Ngwenya M, et al. Prevalence of drug-resistant tuberculosis in Zimbabwe: a health facility-based cross-sectional survey. IJID. 2019;87:119–25.
CAS PubMed Google Scholar
Ahmad N, Javaid A, Sulaiman SA, Ming LC, Ahmad I, Khan AH. Resistance patterns, prevalence, and predictors of fluoroquinolones resistance in multidrug resistant tuberculosis patients. Braz J Infect Dis SBI. 2016;20(1):41–7.
Mohammed KAS, Khudhair GS, Bekheet A-R. Prevalence and drug resistance pattern of Mycobacterium tuberculosis isolated from tuberculosis patients in Basra, Iraq. Pol J Microbiol. 2022;71(2):205–15.
Article PubMed PubMed Central Google Scholar
Hu Y, Mathema B, Zhao Q, Chen L, Lu W, Wang W, et al. Acquisition of second-line drug resistance and extensive drug resistance during recent transmission of Mycobacterium tuberculosis in rural China. Clin Microbiol Infect ESCMID. 2015;21(12):1093.
Mazahir R, Beig FK, Ahmed Z, Alam S. Burden of tuberculosis among household children of adult multi drug resistant patients and their response to first line anti tubercular drugs. Egypt Pediatr Assoc Gazette. 2017;65(4):122–6.
Ayaz A, Hasan Z, Jafri S, Inayat R, Mangi R, Channa AA, et al. Characterizing Mycobacterium tuberculosis isolates from Karachi, Pakistan: drug resistance and genotypes. IJID. 2012;16(4):e303–9.
Al-Dabbagh M, Lapphra K, McGloin R, Inrig K, Schaaf HS, Marais BJ, et al. Drug-resistant tuberculosis: pediatric guidelines. PIDJ. 2011;30(6):501–5.
Yang Y, Zhou C, Shi L, Meng H, Yan H. Prevalence and characterization of drug-resistant tuberculosis in a local hospital of Northeast China. IJID. 2014;22:83–6.
Magula NP, Madala ND, Kriel Y, Bayi V, Duze NP, Manzini TC, et al. Prevalence of drug resistant tuberculosis in patients presenting with a large pericardial effusion at King Edward VIII Hospital. IJID. 2014;21:87.
Mahabeer P, Khan M, Mlisana K. Drug-resistant tuberculosis in children less than 5 years old with culture positive mycobacterium tuberculosis. IJID. 2016;45:212–3.
Berberian G, Gonzalez S, Reijtman V, Miño N, Casimir L, Sarkis C, et al. Seventeen years of drug-resistant tuberculosis in Argentinian children. IJID. 2016;45:387.
Singhal R, Arora J, Sah GC, Bhalla M, Sarin R, Prasad MV. Frequency of multi-drug resistance and mutations in Mycobacterium tuberculosis isolates from Punjab state of India. J Epidemiol Glob Health. 2017;7(3):175–80.
Prakash R, Kumar D, Gupta VK, Jain S, Chauhan DS, Tiwari PK, et al. Status of multidrug resistant tuberculosis (MDR-TB) among the Sahariya tribe of North Central India. J Infect Public Health. 2016;9(3):289–97.
Dujaili JA, Blebil AQ, Dujaili MA, Awaisu A, Hassali MA, Syed Sulaiman SA. Prevelance of pulmonary tuberculosis and multi drug resistant tuberculosis patients in baghdad, Iraq. Value Health. 2013;16(3):A82.
Santos LC, Bousquet Hde M, Pereira AM, Junqueira-Kipnis AP, Kipnis A. A high prevalence of resistance in new tuberculosis cases of midwestern Brazil. Infect Genet Evol. 2010;10(7):1052–7.
Kontsevaya I, Nikolayevskyy V, Kovalyov A, Ignatyeva O, Sadykhova A, Simak T, et al. Tuberculosis cases caused by heterogeneous infection in Eastern Europe and their influence on outcomes. Infect Genet Evol. 2017;48:76–82.
Bhembe NL, Green E. Molecular epidemiological study of multidrug-resistant tuberculosis isolated from sputum samples in Eastern Cape, South Africa. Infect Genet Evol. 2020;80: 104182.
Ghebremichael S, Petersson R, Koivula T, Pennhag A, Romanus V, Berggren I, et al. Molecular epidemiology of drug-resistant tuberculosis in Sweden. Microbes Infect. 2008;10(6):699–705.
Montoro E, Lemus D, Echemendía M, Armas L, González-Ochoa E, Llanes MJ, et al. Drug-resistant tuberculosis in Cuba results of the three global projects. Tuberculosis. 2006;86(3–4):319–23.
Pardini M, Niemann S, Varaine F, Iona E, Meacci F, Orrù G, et al. Characteristics of drug-resistant tuberculosis in Abkhazia (Georgia), a high-prevalence area in Eastern Europe. Tuberculosis. 2009;89(4):317–24.
Jiao W-W, Liu Z-G, Han R, Zhao X-Q, Dong F, Dong H-Y, et al. Prevalence of drug resistant Mycobacterium tuberculosis among children in China. Tuberculosis. 2015;95(3):315–20.
Brandao AP, Pinhata JMW, Simonsen V, Oliveira RS, Ghisi KT, Rabello MCS, et al. Transmission of Mycobacterium tuberculosis presenting unusually high discordance between genotypic and phenotypic resistance to rifampicin in an endemic tuberculosis setting. Tuberculosis. 2020;125: 102004.
Van Rie A, Warren R, Richardson M, Gie RP, Enarson DA, Beyers N, et al. Classification of drug-resistant tuberculosis in an epidemic area. Lancet. 2000;356(9223):22–5.
Chand K, Tewari S, Varghese S. Prevalence of drug resistant tuberculosis in armed forces-study from a tertiary referral chest diseases hospital at Pune. MJAFI. 2000;56(2):130–4.
Ismail NA, Mvusi L, Nanoo A, Dreyer A, Omar SV, Babatunde S, et al. Prevalence of drug-resistant tuberculosis and imputed burden in South Africa: a national and sub-national cross-sectional survey. Lancet Infect Dis. 2018;18(7):779–87.
Daniel O, Osman E. Prevalence and risk factors associated with drug resistant TB in South West, Nigeria. Asian Pac J Trop Med. 2011;4(2):148–51.
Wang G, Peng YL, Zhang G, Zhang L, Xing J, Li D, et al. Sample survey of drug-resistant tuberculosis in Henan, China, 1996. Respirology. 2002;7(1):67–72.
Hannan MM, Peres H, Maltez F, Hayward AC, Machado J, Morgado A, et al. Investigation and control of a large outbreak of multi-drug resistant tuberculosis at a central Lisbon hospital. J Hosp Infect. 2001;47(2):91–7.
Wu B, Zhang L, Liu Z, He H, Pan A, Wang F, et al. Drug-resistant tuberculosis in Zhejiang Province, China: an updated analysis of time trends, 1999–2013. Glob Health Action. 2017;10(1):1293925.
Phyu S, Lwin T, Ti T, Maung W, Mar WW, Shein SS, et al. Drug-resistant tuberculosis in Yangon, Myanmar. Scand J Infect Dis. 2005;37(11–12):846–51.
Hu Y, Mathema B, Wang W, Hoffner S, Kreiswirth B, Xu B. Prevalence of multidrug-resistant pulmonary tuberculosis in counties with different duration of DOTS implementation in rural China. Microb Drug Resist. 2008;14(3):227–32.
Shah MA, Shah I. Increasing prevalence of pediatric drug-resistant tuberculosis in Mumbai, India, and its outcome. PIDJ. 2018;37(12):1261–3.
Ignatova A, Dubiley S, Stepanshina V, Shemyakin I. Predominance of multi-drug-resistant LAM and Beijing family strains among Mycobacterium tuberculosis isolates recovered from prison inmates in Tula Region, Russia. J Med Microbiol. 2006;55(10):1413–8.
Kapata N, Chanda-Kapata P, Bates M, Mwaba P, Cobelens F, Grobusch MP, et al. Multidrug-resistant TB in Zambia: review of national data from 2000 to 2011. TM & IH. 2013;18(11):1386–91.
Kamolwat P, Nateniyom S, Chaiprasert A, Disratthakit A, Mahasirimongkol S, Yamada N, et al. Prevalence and associated risk factors of drug-resistant tuberculosis in Thailand: results from the fifth national anti-tuberculosis drug resistance survey. TM & IH. 2021;26(1):45–53.
Hu Y, Hoffner S, Wu L, Zhao Q, Jiang W, Xu B. Prevalence and genetic characterization of second-line drug-resistant and extensively drug-resistant Mycobacterium tuberculosis in Rural China. Antimicrob Agents Chemother. 2013;57(8):3857–63.
Huo F, Lu J, Zong Z, Jing W, Shi J, Ma Y, et al. Change in prevalence and molecular characteristics of isoniazid-resistant tuberculosis over a 10-year period in China. BMC Infect Dis. 2019;19(1):689.
Zhao LL, Chen Y, Chen ZN, Liu HC, Hu PL, Sun Q, et al. Prevalence and molecular characteristics of drug-resistant Mycobacterium tuberculosis in Hunan, China. Antimicrob Agents Chemother. 2014;58(6):3475–80.
Daum LT, Konstantynovska OS, Solodiankin OS, Liashenko OO, Poteiko PI, Bolotin VI, et al. Next-generation sequencing for characterizing drug resistance-conferring Mycobacterium tuberculosis genes from clinical isolates in the Ukraine. J Clin Microbiol. 2018;56: e00009.
Dinic L, Akande P, Idigbe EO, Ani A, Onwujekwe D, Agbaji O, et al. Genetic determinants of drug-resistant tuberculosis among HIV-infected patients in Nigeria. J Clin Microbiol. 2012;50(9):2905–9.
Agarwal M, Gunal S, Durmaz R, Yang Z. Integration of Mycobacterium tuberculosi s drug susceptibility testing and genotyping with epidemiological data analysis to gain insight into the epidemiology of drug-resistant tuberculosis in Malatya, Turkey. J Clin Microbiol. 2010;48(9):3301–5.
Djuretic T, Herbert J, Drobniewski F, Yates M, Smith EG, Magee JG, et al. Antibiotic resistant tuberculosis in the United Kingdom: 1993–1999. Thorax. 2002;57(6):477–82.
Lv XT, Lu XW, Shi XY, Zhou L. Prevalence and risk factors of multi-drug resistant tuberculosis in Dalian, China. J Int Med Res. 2017;45(6):1779–86.
Fairlie L, Beylis NC, Reubenson G, Moore DP, Madhi SA. High prevalence of childhood multi-drug resistant tuberculosis in Johannesburg, South Africa: a cross sectional study. BMC Infect Dis. 2011;11:28.
Sharaf Eldin GS, Fadl-Elmula I, Ali MS, Ali AB, Salih AL, Mallard K, et al. Tuberculosis in Sudan: a study of Mycobacterium tuberculosis strain genotype and susceptibility to anti-tuberculosis drugs. BMC Infect Dis. 2011;11:219.
Mekonnen F, Tessema B, Moges F, Gelaw A, Eshetie S, Kumera G. Multidrug resistant tuberculosis: prevalence and risk factors in districts of metema and west armachiho, Northwest Ethiopia. BMC Infect Dis. 2015;15:461.
Ba Diallo A, Ossoga GW, Daneau G, Lo S, Ngandolo R, Djaibé CD, et al. Emergence and clonal transmission of multi-drug-resistant tuberculosis among patients in Chad. BMC Infect Dis. 2017;17(1):579.
Juma SP, Maro A, Pholwat S, Mpagama SG, Gratz J, Liyoyo A, et al. Underestimated pyrazinamide resistance may compromise outcomes of pyrazinamide containing regimens for treatment of drug susceptible and multi-drug-resistant tuberculosis in Tanzania. BMC Infect Dis. 2019;19(1):129.
Ogari CO, Nyamache AK, Nonoh J, Amukoye E. Prevalence and detection of drug resistant mutations in Mycobacterium tuberculosis among drug naïve patients in Nairobi, Kenya. BMC Infect Dis. 2019;19(1):279.
Saldanha N, Runwal K, Ghanekar C, Gaikwad S, Sane S, Pujari S. High prevalence of multi drug resistant tuberculosis in people living with HIV in Western India. BMC Infect Dis. 2019;19(1):391.
Gehre F, Otu J, Kendall L, Forson A, Kwara A, Kudzawu S, et al. The emerging threat of pre-extensively drug-resistant tuberculosis in West Africa: preparing for large-scale tuberculosis research and drug resistance surveillance. BMC Med. 2016;14:160.
Diriba G, Kebede A, Tola HH, Alemu A, Tadesse M, Tesfaye E, et al. Surveillance of drug resistance tuberculosis based on reference laboratory data in Ethiopia. Infect Dis Poverty. 2019;8(1):54.
Cox HS, McDermid C, Azevedo V, Muller O, Coetzee D, Simpson J, et al. Epidemic levels of drug resistant tuberculosis (MDR and XDR-TB) in a high HIV prevalence setting in Khayelitsha, South Africa. PLoS ONE. 2010;5(11): e13901.
Hom JK, Wang B, Chetty S, Giddy J, Mazibuko M, Allen J, et al. Drug-resistant tuberculosis among HIV-infected patients starting antiretroviral therapy in Durban, South Africa. PLoS ONE. 2012;7(8): e43281.
Porwal C, Kaushik A, Makkar N, Banavaliker JN, Hanif M, Singla R, et al. Incidence and risk factors for extensively drug-resistant tuberculosis in Delhi region. PLoS ONE. 2013;8(2): e55299.
Isaakidis P, Das M, Kumar AMV, Peskett C, Khetarpal M, Bamne A, et al. Alarming levels of drug-resistant tuberculosis in HIV-infected patients in metropolitan Mumbai, India. PLoS ONE. 2014;9(10): e110461.
Ullah I, Shah AA, Basit A, Ali M, Khan A, Ullah U, et al. Rifampicin resistance mutations in the 81 bp RRDR of rpoB gene in Mycobacterium tuberculosis clinical isolates using Xpert MTB/RIF in Khyber Pakhtunkhwa, Pakistan: a retrospective study. BMC Infect Dis. 2016;16:143.
Mesfin EA, Beyene D, Tesfaye A, Admasu A, Addise D, Amare M, et al. Drug-resistance patterns of Mycobacterium tuberculosis strains and associated risk factors among multi drug-resistant tuberculosis suspected patients from Ethiopia. PLoS ONE. 2018;13(6): e0197737.
Kigozi E, Kasule GW, Musisi K, Lukoye D, Kyobe S, Katabazi FA, et al. Prevalence and patterns of rifampicin and isoniazid resistance conferring mutations in Mycobacterium tuberculosis isolates from Uganda. PLoS ONE. 2018;13(5): e0198091.
Shibabaw A, Gelaw B, Gebreyes W, Robinson R, Wang SH, Tessema B. The burden of pre-extensively and extensively drug-resistant tuberculosis among MDR-TB patients in the Amhara region, Ethiopia. PLoS ONE. 2020;15(2): e0229040.
Gilad J, Borer A, Riesenberg K, Peled N, Schlaeffer F. Epidemiology and ethnic distribution of multidrug-resistant tuberculosis in Southern Israel, 1992–1997. Chest. 2000;117(3):738–43.
Ramzan MM, Sabayev V, Anwar N, Patel A, Asnis D, Avaiya A, et al. Prevalence of drug resistant tuberculosis among asians: a flushing hospital experience. Chest. 2004;126(4, Supplement):753S.
Um S-J, Son C, Roh MS, Lee S-K, Kim KH, Huh J, et al. Prevalence Of Multi Drug Resistant Pulmonary Tuberculosis In Intermediate Endemism Country. A55 Multi-drug resistant and extensively drug-resistant tuberculosis: American Thoracic Society; 2011. p. A1824-A.
Diandé S, Badoum G, Combary A, Zombra I, Saouadogo T, Sawadogo LT, et al. Multidrug-resistant tuberculosis in Burkina Faso from 2006 to 2017: results of national surveys. Eur J Microbiol Immunol. 2019;9(1):23–8.
Becerril-Montes P, Said-Fernández S, Luna-Herrera J, Caballero-Olín G, Enciso-Moreno JA, Martínez-Rodríguez HG, et al. A population-based study of first and second-line drug-resistant tuberculosis in a high-burden area of the Mexico/United States border. Mem Inst Oswaldo Cruz. 2013;108(2):160–6.
Bastos GM, Cezar MC, Mello FC, Conde MB. Prevalence of primary drug resistance in pulmonary tuberculosis patients with no known risk factors for such. J Bras Pneumol. 2012;38(6):733.
Micheletti VCD, Moreira JS, Ribeiro MO, Kritski AL, Braga JU. Drug-resistant tuberculosis in subjects included in the second national survey on antituberculosis drug resistance in Porto Alegre, Brazil. J Bras Pneumol. 2014;40(2):155–63.
Zhao LL, Huang MX, Xiao TY, Liu HC, Li MC, Zhao XQ, et al. Prevalence, risk and genetic characteristics of drug-resistant tuberculosis in a tertiary care tuberculosis hospital in China. Infect Drug Resist. 2019;12:2457–65.
Migliori GB, Ortmann J, Girardi E, Besozzi G, Lange C, Cirillo DM, et al. Extensively drug-resistant tuberculosis, Italy and Germany. Emerg Infect Dis. 2007;13(5):780.
Deng Y, Wang Y, Wang J, Jing H, Yu C, Wang H, et al. Laboratory-based surveillance of extensively drug-resistant tuberculosis, China. Emerg Infect Dis. 2011;17(3):495.
Wallengren K, Scano F, Nunn P, Margot B, Buthelezi SS, Williams B, et al. Drug-resistant tuberculosis, KwaZulu-Natal, South Africa, 2001–2007. Emerg Infect Dis. 2011;17(10):1913.
El Achkar S, Demanche C, Osman M, Rafei R, Ismail MB, Yaacoub H, et al. Drug-resistant tuberculosis, Lebanon, 2016–2017. Emerg Infect Dis. 2019;25(3):564.
Lee SW, Jeon K, Kim KH, Min KH. Multidrug-resistant pulmonary tuberculosis among young Korean soldiers in a communal setting. J Korean Med Sci. 2009;24(4):592–5.
Buyankhishig B, Naranbat N, Mitarai S, Rieder HL. Nationwide survey of anti-tuberculosis drug resistance in Mongolia. Int J Tuberculosis Lung Dis. 2011;15(9):1201–5.
Seddon JA, Hesseling AC, Marais BJ, Jordaan A, Victor T, Schaaf HS. The evolving epidemic of drug-resistant tuberculosis among children in Cape Town, South Africa. IJTLD. 2012;16(7):928–33.
Bojorquez-Chapela I, Bäcker CE, Orejel I, López A, Díaz-Quiñonez A, Hernández-Serrato MI, et al. Drug resistance in Mexico: results from the national survey on drug-resistant tuberculosis. IJTLD. 2013;17(4):514–9.
Ei PW, Aung WW, Nyunt WW, Swe TL, Htwe MM, Win SM, et al. Extensively drug-resistant tuberculosis in Myanmar: burden and mutations causing second-line drug resistance. IJTLD. 2018;22(1):47–53.
Smith CM, Lessells R, Grant AD, Herbst K, Tanser F. Spatial clustering of drug-resistant tuberculosis in Hlabisa subdistrict, KwaZulu-Natal, 2011–2015. IJTLD. 2018;22(3):287–93.
Article CAS PubMed Central Google Scholar
Alikhanova N, Akhundova I, Seyfaddinova M, Mammadbayov E, Mirtskulava V, Rüsch-Gerdes S, et al. First national survey of anti-tuberculosis drug resistance in Azerbaijan and risk factors analysis. Public Health Action. 2014;4(Suppl 2):S17-23.
Tasbiti AH, Yari S, Ghanei M, Shokrgozar MA, Fateh A, Bahrmand A. Low levels of extensively drug-resistant tuberculosis among multidrug resistant tuberculosis isolates and their relationship to risk factors: surveillance in Tehran, Iran; 2006 to 2014. PHRP. 2017;8(2):116–23.
Cox HS, Orozco JD, Male R, Ruesch-Gerdes S, Falzon D, Small I, et al. Multidrug-resistant tuberculosis in central Asia. Emerg Infect Dis. 2004;10(5):865.
Otokunefor K, Otokunefor TV, Omakwele G. Multi-drug resistant Mycobacterium tuberculosis in Port Harcourt, Nigeria. Afr J Lab Med. 2018;7(2):805.
Mehdi RM, Reza MS, Mohammad R. Study prevalence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Tuberculosis in East Azerbaijan province of Iran. HealthMED. 2012;6(9):3091–4.
Israel K, Jean-Baptiste G, Julceus E, Docteur W, Sohler N, editors. Prevalence of Multi-Drug Resistant Tuberculosis in Zanmi Lasante Network among Patients who had a Gene Xpert Testing from October 2014 to September 20152018: 9th Annual CUGH Conference.
Wang SF, Yang Z, Yu P, Hui Wen Z, Yan LZ. Prevalence and risk factors of primary drug-resistant tuberculosis in China. Biomed Environ Sci. 2016;29(2):91–8.
Wang D, Yang C, Kuang T, Lei H, Meng X, Tong A, et al. Prevalence of multidrug and extensively drug-resistant tuberculosis in Beijing, China: a hospital-based retrospective study. Jpn J Infect Dis. 2010;63(5):368–71.
Laghari GS, Hussain Z, Khemani L, Hussain SZM, Yaqoob U. Burden of drug-resistant pulmonary tuberculosis in Pakistani children: a cross-sectional study. F1000Research. 2019;8:344.
Afroz H, Ali MA, Fakruddin M, Kamrunnahar DS. Prevalence and treatment follow-up of drug-resistant extra-pulmonary tuberculosis in rural communities in Narshingdi, Bangladesh. Int J Adv Med. 2014;1:71–7.
Faridi M, Shukla I, Fatima N, Varshney S, Shameem M. Prevalence of primary pulmonary multi-drug resistant tuberculosis in and around Aligarh Region. J Med Microb Diagn. 2018;7(285):2161.
Aguiar F, Vieira M, Staviack A, Buarque C, Marsico A, Fonseca L, et al. Prevalence of anti-tuberculosis drug resistance in an HIV/AIDS reference hospital in Rio de Janeiro, Brazil. IJTLD. 2009;13(1):54–61.
CAS Google Scholar
Rashedi J, Mahdavi Poor B, Rafi A, Asgharzadeh M, Abdolalizadeh J, Moaddab SR. Multidrug-resistant tuberculosis in north-west of Iran and Republic of Azerbaijan: a major public health concern for Iranian people. J Res Health Sci. 2015;15(2):101–3.
Akhtar AM, Arif MA, Kanwal S, Majeed S. Prevalence and drug resistance pattern of MDR TB in retreatment cases of Punjab, Pakistan. JPMA. 2016;66(8):989–93.
Anunnatsiri S, Chetchotisakd P, Wanke C. Factors associated with treatment outcomes in pulmonary tuberculosis in northeastern Thailand. Southeast Asian J Trop Med Public Health. 2005;36(2):324–30.
Surucuoglu S, Ozkutuk N, Celik P, Gazi H, Dinc G, Kurutepe S, et al. Drug-resistant pulmonary tuberculosis in western Turkey: prevalence, clinical characteristics and treatment outcome. Ann Saudi Med. 2005;25(4):313–8.
Bhat J, Rao VG, Yadav R, Muniyandi M, Sharma R, Karfarma C, et al. Situation of drug resistant tuberculosis in Saharia tribe of central India. Indian J Med Res. 2015;141(5):636–9.
CAS PubMed PubMed Central Google Scholar
Kinjal R, Firoz G, Iva C, Gaurang K, Pradeep P. Study On Prevalence Of Drug Resistance And Genetic Mutation Pattern Among Suspected Drug Resistant Pulmonary Tuberculosis Cases In Jamnagar District. National Journal of Integrated Research in Medicine. 2019;10(4)
Jacobs MG, Pinto VL. Characterization of drug-resistant tuberculosis in Brazil, 2014. Epidemiologia e Serviços de Saúd. 2020;28: e2018294.
Dodd PJ, Sismanidis C, Seddon JA. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. Lancet Infect Dis. 2016;16(10):1193–201.
Abouyannis M, Dacombe R, Dambe I, Mpunga J, Faragher B, Gausi F, et al. Drug resistance of Mycobacterium tuberculosis in Malawi: a cross-sectional survey. Bull World Health Organ. 2014;92(11):798–806.
Abdella K, Abdissa K, Kebede W, Abebe G. Drug resistance patterns of Mycobacterium tuberculosis complex and associated factors among retreatment cases around Jimma, Southwest Ethiopia. BMC Public Health. 2015;15:599.
Weyer K, Brand J, Lancaster J, Levin J, Van der Walt M. Determinants of multidrug-resistant tuberculosis in South Africa: results from a national survey. SAMJ. 2007;97(11):1120–8.
Soliman NS. Prevalence of multidrug-resistant tuberculosis using phenotypic drug susceptibility testing and GeneXpert MTB/RIF with characterization of non-tuberculous mycobacteria using MALDI-TOF. EJMM. 2021;30(3):143–51.
Villa-Rosas C, Laniado-Laborín R, Oceguera-Palao L. Primary drug resistance in a region with high burden of tuberculosis. A critical problem. Salud Publica Mex. 2015;57(2):177–9.
Aia P, Kal M, Lavu E, John LN, Johnson K, Coulter C, et al. The burden of drug-resistant tuberculosis in Papua New Guinea: results of a large population-based survey. PLoS ONE. 2016;11(3): e0149806.
Hang NTL, Maeda S, Lien LT, Thuong PH, Hung NV, Thuy TB, et al. Primary drug-resistant tuberculosis in Hanoi, Viet Nam: present status and risk factors. PLoS ONE. 2013;8(8): e71867.
Minion J, Pai M. Assays for drug resistant tuberculosis in high burden countries reply. Lancet Infect Dis. 2011;11(3):162.
Gallo JF, Pinhata JMW, Simonsen V, Galesi VMN, Ferrazoli L, Oliveira RS. Prevalence, associated factors, outcomes and transmission of extensively drug-resistant tuberculosis among multidrug-resistant tuberculosis patients in São Paulo, Brazil: a cross-sectional study. ESCMID. 2018;24(8):889–95.
Bedaso MH, Kalil FS. Trends of Drug Resistance Tuberculosis from 2014 to 2018, Bale Zone, Oromia Region, Ethiopia. Infect Drug Resist. 2021;14:2073–8.
Anwaierjiang A, Wang Q, Liu H, Yin C, Xu M, Li M, et al. Prevalence and molecular characteristics based on whole genome sequencing of Mycobacterium tuberculosis resistant to four anti-tuberculosis drugs from Southern Xinjiang, China. Infect Drug Resist. 2021;14:3379–91.
Patil S, Nakate P, Patil S, Shelke Y. Prevalence of multi-drug resistant (MDR) pulmonary tuberculosis in a tertiary care rural hospital in Western Maharashtra, India. 2019.
Kulkarni GS, Palwe SD, Patil NP, Telkhade AJ, Kadukar J. Prevalence of multidrug-resistant tuberculosis at a regional drug-resistant tuberculosis center of Maharashtra. Indian J Respir Care. 2020;9(1):30.
Adwani S, Desai UD, Joshi JM. Prevalence of pre-extensively drug-resistant tuberculosis (Pre XDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) among pulmonary multidrug resistant tuberculosis (MDR-TB) at a tertiary care center in Mumbai. JKIMSU. 2016;5:13–9.
Dagne B, Desta K, Fekade R, Amare M, Tadesse M, Diriba G, et al. The Epidemiology of first and second-line drug-resistance Mycobacterium tuberculosis complex common species: evidence from selected TB treatment initiating centers in Ethiopia. PLoS ONE. 2021;16(1): e0245687.
Micheni LN, Kassaza K, Kinyi H, Ntulume I, Bazira J. Rifampicin and isoniazid drug resistance among patients diagnosed with pulmonary tuberculosis in southwestern Uganda. PLoS ONE. 2021;16(10): e0259221.
Muhmmad A, Muhammad N, Khan ZU, Jamal T, Ishaq M. Burden of multi-drug resistant and extensive drug resistant of Mycobacterium tuberculosis . KJMS. 2019;12(2):1.
Lai CC, Tan CK, Huang YT, Chou CH, Hung CC, Yang PC, et al. Extensively drug-resistant Mycobacterium tuberculosis during a trend of decreasing drug resistance from 2000 through 2006 at a Medical Center in Taiwan. Clin Infect. 2008;47(7):e57-63.
Lukoye D, Adatu F, Musisi K, Kasule GW, Were W, Odeke R, et al. Anti-tuberculosis drug resistance among new and previously treated sputum smear-positive tuberculosis patients in Uganda: results of the first national survey. PLoS ONE. 2013;8(8): e70763.
Oyedeji GJ, Adeyemo C, Dissou A, Abiodun T, Alli OAT, Onaolapo OJ, et al. Prevalence of multi-drug resistant tuberculosis among tuberculosis patients attending chest clinics in Osun-State, Nigeria. Curr Pharm Biotechnol. 2020;21(10):939–47.
Masood R, Muhammad IN, Siddiqui T, Mushtaque M, Irshad A. High prevalence of DR-TB (drug-resistant tuberculosis): an Indicator of public health negligence. Pak J Pharm Sci. 2019;32(4):1529–36.
Safwat TM, Elmasry AA, Mohamed AKM. Prevalence of multi drug-resistant tuberculosis at Abbassia Chest Hospital from July 2006 to 2009. Egypt J Bronchol. 2011;5(2):124–30.
Abebe G, Abdissa K, Abdissa A, Apers L, Agonafir M, de Jong BC, et al. Relatively low primary drug resistant tuberculosis in southwestern Ethiopia. BMC Res Notes. 2012;5:225.
Yoshiyama T, Supawitkul S, Kunyanone N, Riengthong D, Yanai H, Abe C, et al. Prevalence of drug-resistant tuberculosis in an HIV endemic area in northern Thailand. Int J Tuberc Lung Dis. 2001;5(1):32–9.
Dewan P, Sosnovskaja A, Thomsen V, Cicenaite J, Laserson K, Johansen I, et al. High prevalence of drug-resistant tuberculosis, Republic of Lithuania, 2002. IJTLD. 2005;9(2):170–4.
Pleumpanupat W, Jittimanee S, Akarasewi P, Rienthong S, Jittimanee S, Chiewlian Y, et al. Resistance to anti-tuberculosis drugs among smear-positive cases in Thai prisons 2 years after the implementation of the DOTS strategy. IJTLD. 2003;7(5):472–7.
Sidze LK, Mouafo Tekwu E, Kuaban C, Assam Assam JP, Tedom JC, Eyangoh S, et al. Strong decrease in streptomycin-resistance and absence of XDR 12 years after the Reorganization of the National Tuberculosis Control Program in the Central Region of Cameroon. PLoS ONE. 2014;9(6): e98374.
Nigus DM, Lingerew W, Beyene B, Tamiru A, Lemma M, Melaku MY. Prevalence of multi drug resistant tuberculosis among presumptive multi drug resistant tuberculosis cases in Amhara National Regional State, Ethiopia. J Mycobac Dis. 2014;4(152):2161.
Alberte-Castiñeiras A, Brezmes-Valdivieso MF, Campos-Bueno A, Montes-Martinez I, López-Medrano R, Avellaneda C, et al. Drug-resistant tuberculosis in Castilla-León, Spain, 1996–2000. IJTLD. 2006;10(5):554–8.
Elmi OS, Hasan H, Abdullah S, Jeab MZM, Zilfalil B, Naing NN. Prevalence and associated factors with transmission of latent tuberculosis among household contacts of multi-drug resistant tuberculosis patients in Malaysia. World J Med Sci. 2014;10(3):285–94.
Amala SE, Silas G. The prevalence of tuberculosis (TB) and multiple drug resistant tuberculosis (MDR-TB) in Bayelsa state. NIGERIA AJHR. 2019;5:1–5.
Brito RC, Mello FCQ, Andrade MK, Oliveira H, Costa W, Matos HJ, et al. Drug-resistant tuberculosis in six hospitals in Rio de Janeiro, Brazil. IJTLD. 2010;14(1):24–33.
Wang G, Jiang G, Jing W, Zong Z, Yu X, Chen S, et al. Prevalence and molecular characterizations of seven additional drug resistance among multidrug-resistant tuberculosis in China: a subsequent study of a national survey. J Infect. 2021;82(3):371–7.
Xiang Y, Ying L, Liu J, Su Q, Shen J, Zhan J, et al. An epidemiological study of resistant tuberculosis in Chongqing, China. J Med Colleges of PLA. 2011;26(3):158–73.
Yang X, Yuan Y, Pang Y, Wang B, Bai Y, Wang Y, et al. The burden of MDR/XDR tuberculosis in coastal plains population of China. PLoS ONE. 2015;10(2): e0117361.
Zazueta-Beltran J, Leon-Sicairos N, Muro-Amador S, Flores-Gaxiola A, Velazquez-Roman J, Flores-Villasenor H, et al. Increasing drug resistance of Mycobacterium tuberculosis in Sinaloa, Mexico, 1997–2005. IJID. 2011;15(4):E272–6.
Pang Y, Zhu D, Zheng H, Shen J, Hu Y, Liu J, et al. Prevalence and molecular characterization of pyrazinamide resistance among multidrug-resistant Mycobacterium tuberculosis isolates from Southern China. BMC Infect Dis. 2017;17(1):711.
Lapphra K, Sutthipong C, Vanprapar N, Phongsamart W, Wittawatmongkol O, Udompornwattana S, et al. Drug-resistant tuberculosis in Thai children. IJID. 2012;16: e26.
Liu ZY, Shilkret KI, Finelli L. Epidemiology of drug-resistant tuberculosis in New Jersey from 1991 to 1995. Int J Epidemiol. 1998;27(1):121–6.
Huo F, Luo J, Shi J, Zong Z, Jing W, Dong W, et al. A 10-year comparative analysis shows that increasing prevalence of rifampin-resistant mycobacterium tuberculosis in china is associated with the transmission of strains harboring compensatory mutations. Antimicrob Agents Chemother. 2018;62(4): e02303.
Bruchfeld J, Aderaye G, Palme IB, Bjorvatn B, Ghebremichael S, Hoffner S, et al. Molecular epidemiology and drug resistance of Mycobacterium tuberculosis isolates from Ethiopian pulmonary tuberculosis patients with and without human immunodeficiency virus infection. J Clin Microbiol. 2002;40(5):1636–43.
Amanullah F, Ashfaq M, Khowaja S, Parekh A, Salahuddin N, Lotia-Farrukh I, et al. High tuberculosis prevalence in children exposed at home to drug-resistant tuberculosis. IJTLD. 2014;18(5):520–7.
Ombura IP, Onyango N, Odera S, Mutua F, Nyagol J. Prevalence of drug resistance Mycobacterium tuberculosis among patients seen in coast Provincial General Hospital, Mombasa, Kenya. PLoS ONE. 2016;11(10): e0163994.
Gebeyehu M, Lemma E, Eyob G. Prevalence of drug resistant tuberculosis in Arsi Zone, Ethiopia. The Ethiopian Journal of Health Development. 2001;15(1).
Madukaji L, Okohu I, Usman S, Oyedum U, Enagi A, Usman A, et al. Early detection of Pre-XDR TB with line probe assay in a high TB burden country. Afr Health Sci. 2021;21(3):968–74.
Khunjeli R, Mohsin U, Shrestha S, Adhikari S, Srivastava B, Shrestha B. Prevalence of primary drug resistant tuberculosis in a tertiary care hospital. Nepal JCMC. 2014;4(4):36–8.
Pande JN, Singh UB, Sinha S, Agarwal RC, Singh SPN. Evaluation of risk factors and prevalence of drug resistant tuberculosis in north India. Chest. 2005;128(4):404S.
Pablos-Mendez A, Raviglione MC, Laszlo A, Binkin N, Rieder HL, Bustreo F, et al. Global surveillance for antituberculosis-drug resistance, 1994–1997. N Engl J Med. 1998;338(23):1641–9.
Committee TR. Drug-resistant Mycobacterium tuberculosis in Japan: a nationwide survey, 2002. IJTLD. 2007;11(10):1129–35.
Dorjee K, Sadutshang TD, Rana RS, Topgyal S, Phunkyi D, Choetso T, et al. High prevalence of rifampin-resistant tuberculosis in mountainous districts of India. IJTB. 2020;67(1):59–64.
Mohajeri P, Sadri H, Farahani A, Norozi B, Atashi S. Frequency of mutations associated with rifampicin resistance in Mycobacterium tuberculosis strains isolated from patients in west of Iran. Microb Drug Resist. 2015;21(3):315–9.
Jaleta KN, Gizachew M, Gelaw B, Tesfa H, Getaneh A, Biadgo B. Rifampicin-resistant Mycobacterium tuberculosis among tuberculosis-presumptive cases at University of Gondar Hospital, northwest Ethiopia. Infect Drug Resist. 2017;10:185–92.
Araya S, Negesso AE, Tamir Z. Rifampicin-resistant Mycobacterium tuberculosis among patients with presumptive tuberculosis in Addis Ababa, Ethiopia. Infect Drug Resist. 2020;13:3451–9.
Wang X, Fu Q, Li Z, Chen S, Liu Z, Nelson H, et al. Drug-resistant tuberculosis in Zhejiang province, China, 1999–2008. Emerg Infect Dis. 2012;18(3):496.
El Achkar S. Prevalence of drug-resistant tuberculosis assessed by next-generation sequencing: an 18-month nationwide study in Lebanon: Université de Lille; 2019.
Adejumo OA, Olusola-Faleye B, Adepoju V, Bowale A, Adesola S, Falana A, et al. Prevalence of rifampicin resistant tuberculosis and associated factors among presumptive tuberculosis patients in a secondary referral hospital in Lagos Nigeria. Afr Health Sci. 2018;18(3):472–8.
Gebrehiwet GB, Kahsay AG, Welekidan LN, Hagos AK, Abay GK, Hagos DG. Rifampicin resistant tuberculosis in presumptive pulmonary tuberculosis cases in Dubti Hospital, Afar, Ethiopia. J Infect Dev Ctries. 2019;13(1):21–7.
Bitet DE, Kumurya SA, Joseph L, Bathelomow P. Rifampicin resistant tuberculosis among patients attending General Hospital, Kagarko, Kaduna State, Nigeria. Afr J Clin Exp Microbiol. 2020;21(3):250–4.
Awais M, Ahmad R, Jan F, Anwar B, Rehman R, Mujtaba G, et al. Prevalence and detection of drug-resistant tuberculosis in Hazara Division, Pakistan. Acad J Biotechnol. 2018;6(9):116–23.
Ikuabe PO, Ebuenyi ID. Prevalence of rifampicin resistance by automated Genexpert rifampicin assay in patients with pulmonary tuberculosis in Yenagoa, Nigeria. Pan Afr Med J. 2018;29:204.
Meaza A, Tesfaye E, Mohamed Z, Zerihun B, Seid G, Eshetu K, et al. Diagnostic accuracy of Truenat Tuberculosis and Rifampicin-Resistance assays in Addis Ababa, Ethiopia. PLoS ONE. 2021;16(12): e0261084.
Fadeyi A, Desalu OO, Ugwuoke C, Opanwa OA, Nwabuisi C, Salami AK. Prevalence of rifampicin-resistant tuberculosis among patients previously treated for pulmonary tuberculosis in North-Western, Nigeria. NMJ. 2017;58(6):161–6.
Elion Assiana DO, Abdul J, Linguissi LSG, Epola M, Vouvoungui JC, Mabiala A, et al. Epidemiological profile of multidrug-resistant and extensively drug-resistant Mycobacterium tubrculosis among Congolese patients. Ann Clin Microbiol Antimicrob. 2021;20(1):84.
Huo F, Zhang F, Xue Y, Shang Y, Liang Q, Ma Y, et al. Increased prevalence of levofloxacin-resistant Mycobacterium tuberculosis in China is associated with specific mutations within the gyrA gene. IJID. 2020;92:241–6.
Rivière E, Verboven L, Dippenaar A, Goossens S, De Vos E, Streicher E, et al. Variants in bedaquiline-candidate-resistance genes: prevalence in bedaquiline-naive patients, effect on MIC, and association with Mycobacterium tuberculosis Lineage. Antimicrob Agents Chemother. 2022;66(7): e0032222.
Latrilha FO, Simonsen V, Pinhata JM, Brandao AP, Galesi VMN, Waldman EA, et al. Transmission and prevalence of drug-resistant tuberculosis in a Brazilian setting under a directly observed therapy short-course strategy. Rev Soc Bras Med Trop. 2020;53: e20190404.
Ahmed I, Jabeen K, Inayat R, Hasan R. Susceptibility testing of extensively drug-resistant and pre-extensively drug-resistant Mycobacterium tuberculosis against levofloxacin, linezolid, and amoxicillin-clavulanate. Antimicrob Agents Chemother. 2013;57(6):2522–5.
Kumar RS. Prevalence of pre-extensively drug-resistant tuberculosis and extensively drug-resistant tuberculosis among multidrug-resistant tuberculosis patients in South Tamil Nadu. Int J Sci Stud. 2020;8(9):96–9.
Chuchottaworn C. Extensively drug resistant tuberculosis (XDR-TB) in Chest Disease Institute, 197–205. J Med Assoc Thailand. 2010;93(1):34–7.
Pang Y, Lu J, Huo F, Ma Y, Zhao L, Li Y, et al. Prevalence and treatment outcome of extensively drug-resistant tuberculosis plus additional drug resistance from the National Clinical Center for Tuberculosis in China: a five-year review. J Infect. 2017;75(5):433–40.
Diriba G, Alemu A, Tola HH, Yenew B, Amare M, Eshetu K, et al. Pre-extensively drug-resistant tuberculosis among multidrug-resistant tuberculosis patients in Ethiopia: a laboratory-based surveillance study. IJID Regions. 2022;5:39–43.
Mellor Y, Herron D. Disease management: an introduction to tuberculosis. Aust Pharm. 2020;39(2):52–9.
Agyeman AA, Ofori-Asenso R. Tuberculosis—an overview. J Public Health Emerg. 2017;1(7):1–11.
Onyedum CC, Alobu I, Ukwaja KN. Prevalence of drug-resistant tuberculosis in Nigeria: a systematic review and meta-analysis. PLoS ONE. 2017;12(7): e0180996.
Zager EM, McNerney R. Multidrug-resistant tuberculosis. BMC Infect Dis. 2008;8(1):1–5.
Duan Q, Chen Z, Chen C, Zhang Z, Lu Z, Yang Y, et al. The prevalence of drug-resistant tuberculosis in mainland China: an updated systematic review and meta-analysis. PLoS ONE. 2016;11(2): e0148041.
Organization WH. Anti-tuberculosis drug resistance in the world. Report No. 4: The WHO/IUATLD global project on anti-tuberculosis drug resistance surveillance. Anti-tuberculosis drug resistance in the world Report No 4: The WHO/IUATLD global project on anti-tuberculosis drug resistance surveillance. 2008.
Molla KA, Reta MA, Ayene YY. Prevalence of multidrug-resistant tuberculosis in East Africa: a systematic review and meta-analysis. PLoS ONE. 2022;17(6): e0270272.
Migliori GB, Dheda K, Centis R, Mwaba P, Bates M, O’Grady J, et al. Review of multidrug-resistant and extensively drug-resistant TB: global perspectives with a focus on sub-Saharan Africa. Trop Med Int Health. 2010;15(9):1052–66.
Chen S, Huai P, Wang X, Zhong J, Wang X, Wang K, et al. Risk factors for multidrug resistance among previously treated patients with tuberculosis in eastern China: a case–control study. Int J Infect Dis. 2013;17(12):e1116–20.
Lema NA, Mbelele PM, Majigo M, Abade A, Matee MI. Risk factors associated with multidrug resistant tuberculosis among patients referred to Kibong’oto Infectious Disease Hospital in northern Tanzania. TJHR. 2016;18(4).
Chung-Delgado K, Guillen-Bravo S, Revilla-Montag A, Bernabe-Ortiz A. Mortality among MDR-TB cases: comparison with drug-susceptible tuberculosis and associated factors. PLoS ONE. 2015;10(3): e0119332.
Alkabab YM, Al-Abdely HM, Heysell SK. Diabetes-related tuberculosis in the Middle East: an urgent need for regional research. Int J Infect Dis. 2015;40:64–70.
Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lönnroth K, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9(1):1–15.
Mesfin YM, Hailemariam D, Biadglign S, Kibret KT. Association between HIV/AIDS and multi-drug resistance tuberculosis: a systematic review and meta-analysis. PLoS ONE. 2014;9(1): e82235.
Demile B, Zenebu A, Shewaye H, Xia S, Guadie A. Risk factors associated with multidrug-resistant tuberculosis (MDR-TB) in a tertiary armed force referral and teaching hospital, Ethiopia. BMC Infect Dis. 2018;18(1):1–10.
Al-Darraji HAA, Tan C, Kamarulzaman A, Altice FL. Prevalence and correlates of latent tuberculosis infection among employees of a high security prison in Malaysia. Occup Environ Med. 2015;72(6):442–7.
Gobena D, Ameya G, Haile K, Abreha G, Worku Y, Debela T. Predictor of multidrug resistant tuberculosis in southwestern part of Ethiopia: a case control study. Ann Clin Microbiol Antimicrob. 2018;17(1):1–7.
Dujaili JA, Syed Sulaiman SA, Awaisu A, Muttalif AR, Blebil AQ. Outcomes of tuberculosis treatment: a retrospective cohort analysis of smoking versus non-smoking patients in Penang, Malaysia. J Public Health. 2011;19(2):183–9.
Rajendran M, Zaki RA, Aghamohammadi N. Contributing risk factors towards the prevalence of multidrug-resistant tuberculosis in Malaysia: a systematic review. Tuberculosis. 2020;122: 101925.
Jilani TN, Avula A, Gondal Z, Siddiqui AH. Active tuberculosis. 2018.
Dirie AMH, Çolakoğlu S, Abdulle OM, Abdi BM, Osman MA, Shire AM, Hussein AM. Prevalence of multidrug-resistant TB among smear-positive pulmonary TB patients in Banadir, Somalia: a multicenter study. Infect Drug Resist. 2022;15:7241–8.
Sylverken AA, Kwarteng A, Twumasi-Ankrah S, et al. The burden of drug resistance tuberculosis in Ghana; results of the First National Survey. PLoS ONE. 2021;16(6):1–14.
Emam SA, Kasem EM, Sedhom AE. Characteristics of multidrug resistant tuberculosis in Minia, Egypt. Medico Legal Updat. 2020;20(1):446–52.
Welekidan LN, Skjerve E, Dejene TA, et al. Characteristics of pulmonary multidrug-resistant tuberculosis patients in Tigray Region, Ethiopia: a cross-sectional study. PLoS ONE. 2020;15(8):1–20.
Download references
Acknowledgements
We would like to thank the support provided by the Student Research Committee of Kermanshah University of Medical Sciences.
By Deputy for Research and Technology, Kermanshah University of Medical Sciences (IR) (50002460). This deputy has no role in the study process.
Author information
Authors and affiliations.
Department of Biostatistics, School of Health, Kermanshah University of Medical Sciences, Kermanshah, Iran
Nader Salari
Sleep Disorders Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran
Amir Hossein Kanjoori & Razie Hasheminezhad
Department of Business Systems & Operations, University of Northampton, Northampton, UK
Amin Hosseinian-Far
Medical Biology Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
Kamran Mansouri
Cellular and Molecular Research Center, Gerash University of Medical Sciences, Gerash, Iran
Masoud Mohammadi
You can also search for this author in PubMed Google Scholar
Contributions
NS and RH and AK contributed to the design, MM statistical analysis, participated in most of the study steps. MM and RH and AHF prepared the manuscript. AK and RH and KM assisted in designing the study, and helped in the interpretation of results. All authors have read and approved the content of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Masoud Mohammadi .
Ethics declarations
Ethics approval and consent to participate.
Ethics approval was received from the ethics committee of deputy of research and technology, Kermanshah University of Medical Sciences (50002460).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Supplementary Information
Additional file 1: table s1..
Summary of Characteristics of Included Studies of Prevalence of MDR-TB. TableS2. Summary of Characteristics of Included Studies of Prevalence of Isoniazid Resistant-TB . Table S3. Summary of Characteristics of Included Studies of Prevalence of Rifampcin Resistant-TB. Table S4. Summary of Characteristics of Included Studies of Prevalence of Single Drug Resistant-TB. Table S5. Summary of characteristics of included studies of prevalence of XDR-TB. TableS6. Summary of characteristics of included studies of prevalence of pre-XDR TB.
Additional file 2: Figure S1.
Forest plot of the global prevalence ofmulti-drug resistant TB based on the random effects method. Figure S2. Funnel plotof publication bias in reviewed studies. Figure S3. Forest plot of global prevalence of isoniazid resistant TB based on randomeffects method. Figure S4. Funnel plot of publication biasin reviewed studies. Figure S5. Forest plot ofglobal prevalence of rifampin-resistant TB based on random effects method. FigureS6. Funnel plot of publication bias in reviewed studies. FigureS7. Forest plot of globalprevalence of single drug resistant TB based on random effects method. FigureS8. Funnel plot of publication bias in reviewed studies. Figure S9. Forest plot of global prevalence of extensively drugresistant TB based on random effects method. Figure S10. Funnel Plot of Publication Bias in ReviewedStudies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Salari, N., Kanjoori, A.H., Hosseinian-Far, A. et al. Global prevalence of drug-resistant tuberculosis: a systematic review and meta-analysis. Infect Dis Poverty 12 , 57 (2023). https://doi.org/10.1186/s40249-023-01107-x
Download citation
Received : 09 February 2023
Accepted : 16 May 2023
Published : 25 May 2023
DOI : https://doi.org/10.1186/s40249-023-01107-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Drug-resistant tuberculosis
Infectious Diseases of Poverty
ISSN: 2049-9957
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Ovarian Strumal Carcinoid: Case Report, Systematic Literature Review and Pooled Analysis
Affiliations.
- 1 Department of Medical and Surgical Specialties, Radiological Sciences, and Public Health, University of Brescia, Medical Oncology, ASST Spedali Civili, Brescia, Italy.
- 2 1st Pathology Division, Department of Pathology and Laboratory Medicine, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy.
- PMID: 35528006
- PMCID: PMC9069053
- DOI: 10.3389/fendo.2022.871210
Background: Ovarian strumal carcinoid is a rare tumor in which thyroid (struma) and carcinoid components coexist. The disease is generally considered to be a borderline malignancy, however, cases with metastatic disease have been described. No data in the literature are available to guide diagnosis and therapy.
Methods: We performed a pooled analysis and a systematic review of histopathological-confirmed strumal carcinoid cases published in the literature using the following keywords: "strumal carcinoid of the ovary", "strumal carcinoid case report". A case of strumal carcinoid tumor diagnosed and followed-up at the Medical Oncology Unit of Spedali Civili (Brescia, Italy) was also described and included.
Results: Sixty-six eligible publications were identified, providing data from one hundred and seventeen patients, plus a case diagnosed at our institution. At presentation, among the eighty-eight patients with symptomatic disease, 37% of patients suffered from abdominal distention and 49% from pain due to a growing abdominal tumor mass, 37% from constipation (peptide YY was analyzed in only nine of them, resulting above the physiologic range). Surgery was the primary therapy in 99% of the patients. Three patients had metastatic disease at diagnosis and five patients underwent recurrence after radical surgery. Histology at disease recurrence concerned the thyroid component in two patients, the carcinoid component in two patients, both histologies in one patient. Median disease-free survival and overall survival in this series were not attained.
Conclusion: Strumal carcinoid of the ovary generally presents a benign behavior and surgery is curative in most cases. However, a small group of patients with this disease can undergo disease recurrence due to both the thyroid and the neuroendocrine (carcinoid) components. A follow-up in radically operated patients is therefore needed, particularly in those with a voluminous disease at diagnosis.
Keywords: constipation; neuroendocrine tumors; ovarian strumal carcinoid; peptide YY; teratomas.
Copyright © 2022 Turla, Zamparini, Milione, Grisanti, Amoroso, Pedersini, Cosentini and Berruti.
Publication types
- Case Reports
- Systematic Review
- Research Support, Non-U.S. Gov't
- Carcinoid Tumor* / diagnosis
- Carcinoid Tumor* / surgery
- Neoplasm Recurrence, Local / surgery
- Ovarian Neoplasms* / diagnosis
- Ovarian Neoplasms* / pathology
- Ovarian Neoplasms* / surgery
- Struma Ovarii* / diagnosis
- Struma Ovarii* / pathology
- Struma Ovarii* / surgery
- Open access
- Published: 11 May 2023
Comparative efficacy and safety of PD-1/PD-L1 inhibitors in triple negative breast cancer: a systematic review and network meta-analysis of randomized controlled trials
- Ibrahim Elmakaty 1 ,
- Ruba Abdo 1 ,
- Ahmed Elsabagh 1 ,
- Abdelrahman Elsayed 1 &
- Mohammed Imad Malki ORCID: orcid.org/0000-0002-6801-2126 2
Cancer Cell International volume 23 , Article number: 90 ( 2023 ) Cite this article
2660 Accesses
4 Citations
8 Altmetric
Metrics details
Triple-Negative Breast Cancer (TNBC) is a lethal subtype of breast cancer with limited treatment options. The purpose of this Network Meta-Analysis (NMA) is to compare the efficacy and safety of inhibitors of programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) in treating TNBC.
Our search strategy was used in six databases: PubMed, Cochrane Library, Cumulative Index to Nursing and Allied Health Literature database, Embase, Scopus, and Web of Science up to November 2nd, 2022, as well as a thorough search in the most used trial registries. We included phase II and III randomized controlled trials that looked at the efficacy of PD-1/PD-L1 inhibitors in the treatment of TNBC and reported either Overall Survival (OS), Progression-Free Survival (PFS), or pathological Complete Response (pCR). The risk of bias was assessed utilizing Cochrane's risk of bias 2 tool, and the statistical analysis was performed using a frequentist contrast-based method for NMA by employing standard pairwise meta-analysis applying random effects model.
12 trials (5324 patients) were included in our NMA including seven phase III trials. Pembrolizumab in a neoadjuvant setting achieved a pooled OS of 0.82 (95% Confidence Interval (CI) 0.65 to 1.03), a PFS of 0.82 (95% CI 0.71 to 0.94) and a pCR 2.79 (95% CI 1.07 to 7.24) compared to Atezolizumab’s OS of 0.92 (95% CI 0.74 to 1.15), PFS of 0.82 (95% CI 0.69 to 0.97), and pCR of 1.94 (95% CI 0.86 to 4.37). Atezolizumab had less grade ≥ 3 adverse events (OR 1.48, 95% CI 0.90 to 2.42) than Pembrolizumab (OR 1.90, 95% CI 1.08 to 3.33) in the neoadjuvant setting.
Conclusions
PD-1/PD-L1 inhibitors exhibited varying efficacy in terms of OS, PFS, and pCR. They were associated with an increase in immune-related adverse effects. When used early in the course of TNBC, PD-1/PD-L1 inhibitors exert their maximum benefit. Durvalumab as a maintenance treatment instead of chemotherapy has shown promising outcomes. Future studies should focus on PD-L1 expression status and TNBC subtypes, since these factors may contribute to the design of individualized TNBC therapy regimens.
Systematic review registration PROSPERO Identifier: CRD42022380712.
Breast cancer remains a major health burden, causing considerable morbidity and mortality worldwide [ 1 ]. It has surpassed lung cancer as the most frequently diagnosed malignancy overall and ranks the fifth leading cause of cancer-related mortality, with an estimated 2.3 million new cases (11.7% of all cancers), and 685,000 deaths in 2020 [ 2 ]. The incidence rate has been increasing at an alarming rate over the past years, especially in transitioning countries, and it is predicted that by 2040, this burden will grow further by over 40% to about 3 million new cases and 1 million deaths every year [ 2 , 3 ]. Triple-Negative Breast Cancer (TNBC) is a particularly aggressive subtype that accounts for approximately 15–20% of all cases and is characterized by a lack of expression of both estrogen and progesterone receptors as well as human epidermal growth factor receptor 2 [ 4 ]. The high molecular heterogeneity, great metastatic potential, and limited therapeutic options have all contributed to TNBC having a relatively poor prognosis with a 5-year overall survival rate of 77% [ 5 , 6 ]. Due to the absence of well-defined molecular targets, TNBC therapy predominantly relies on the administration of Taxane and Anthracycline-based regimens in both the neoadjuvant and the adjuvant settings [ 4 , 6 , 7 ]. More favorable response rates are shown to be achieved when using a combination rather than single-agent chemotherapy [ 8 , 9 ]. Although this can be effective initially, chemotherapy is often accompanied by resistance, relapse, and high toxicity [ 10 , 11 ]. Additionally, survival rates in those who develop metastatic disease have not changed over the past 20 years [ 9 ]. The median Overall Survival (OS) for those patients with the current treatment option is 16 months and the median Progression-Free Survival (PFS) is 5.6 months [ 12 ]. These results underscore the urgent need for more effective and less toxic therapies.
The introduction of immunotherapy has revolutionized the field of oncology over the past decade and has been successfully incorporated into the standard treatment paradigm of many malignancies including non-small cell lung cancer and renal cell cancer [ 13 , 14 ]. Whilst breast cancer has traditionally been considered immunogenically quiescent, several lines of evidence have demonstrated TNBC to be highly immunogenic and feature a microenvironment that is enriched with stromal Tumor Infiltrating Lymphocytes (TILs) with a relatively high tumor mutational burden as opposed to other subtypes [ 15 , 16 ]. The high levels of inhibitory checkpoint molecules expressed on the TILs led to the successful implementation of Immune Checkpoint Inhibitors (ICI) in TNBC treatment, particularly inhibitors of the Programmed Cell Death 1 (PD-1) and the Programmed Cell Death Ligand 1 (PD-L1) which have shown great promise in the field’s clinical trials [ 15 ]. The PD‑L1/PD-1 signaling pathway exerts a critical role in forming an adaptive immune resistance mechanism that mediates tumor invasion and metastasis [ 17 ]. Blocking this pathway would therefore restore the antitumor immune responses by reducing the inhibition of innate immunity and reactivating tumor-specific cytotoxic T cells [ 18 ].
Atezolizumab, an anti-PD-L1 antibody was the first Food and Drug Administration (FDA) approved ICI given along with nab-paclitaxel for patients with unresectable locally advanced or metastatic TNBC whose tumors express PD-L1 [ 19 ]. This accelerated approval was based on the results of the Impassion130 trial. Unfortunately, the designated confirmatory trial, IMpassion131 neither met the primary endpoint of PFS superiority nor achieved statistically significant overall OS leading to the withdrawal of this combination as an indication for treatment [ 12 ]. Alternatively, FDA granted approval to pembrolizumab, a PD-1 inhibitor to be used in combination with chemotherapy for patients with high-risk, early-stage TNBC, as well as those with locally recurrent unresectable or metastatic TNBC whose tumors have a PD-L1 Combined Positive Score (CPS) of ≥ 10 [ 12 ]. Nonetheless, there remain several additional clinical trials that have assessed the role of anti‑PD‑L1/PD‑1 agents in TNBC treatment with inconsistent results. The objective of this Network Meta-Analysis (NMA) is to evaluate the efficacy and safety of these agents, as well as compare them in order to determine the optimal therapeutic regimen for patients with TNBC.
Protocol and registration
This systematic review and meta-analysis is reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension for NMA Additional file 1 : (Table S1) [ 20 ]. The NMA protocol was carried in accordance with a protocol that had been registered in the International Prospective Register of Systematic Reviews (PROSPERO) online database (PROSPERO Identifier: CRD42022380712).
Search strategy
We developed our search strategy in the PubMed database using Medical Subject Headings (MeSH) that included the terms (“Immune Checkpoint Inhibitors”[MeSH] OR “programmed cell death 1 receptor/antagonists and inhibitors”[MeSH]) AND “Triple Negative Breast Neoplasms”[MeSH] AND “Randomized Controlled Trial”[Publication Type] with multiple keywords build around them. There was no date or language restriction applied to our strategy. The developed search strategy was then transferred from PubMed to five other databases by the Polyglot translator [ 21 ], namely Cochrane Library, Cumulative Index to Nursing and Allied Health Literature database, Embase, Scopus, and Web of Science. All databases were searched from the inception date until the 2nd of November 2022. The yielded studies were then exported to EndNote X7, where duplicates were identified and excluded. The remaining articles were uploaded to the Rayyan platform for screening [ 22 ]. In addition, we searched popular clinical trial registries such as ClinicalTrials.gov, EU Clinical Trials Register, International Standard Randomised Controlled Trial Number registry, International Clinical Trials Registry Platform, and breastcancertrials.org for Gery literature (unpublished trials) to ensure the comprehensiveness of our search strategy. Additional file 1 contains the complete strategy for each database and trial registries.
Eligibility criteria
We included trials that met the following criteria: (1) usage of FDA-approved PD-1/PD-L1 inhibitors, (2) phase II or III RCTs, (3) for the management of confirmed TNBC, (4) compared against a different Immune Checkpoint Inhibitors (ICIs), multiple agents’ chemotherapy regimen, single agent chemotherapy regimen or placebo (5) reported Hazard Ratios (HR) for OS, PFS or numbers of pathological Complete Response (pCR) in each both arms of the trial. We excluded review articles, non-randomized trials, quasi-randomized trials, meta-analyses and observational studies, as well as studies on animal models. We also excluded trials using non-FDA-approved immune checkpoint inhibitors.
Study selection and screening
The records obtained from applying the search strategy were evaluated on the Rayyan platform [ 22 ]. Titles and abstracts were screened independently by two reviewers either IE/RA or AhE/AbE with any disagreements were resolved by consensus among the entire team (IE, RA, AhE, AbE and MIM). The full texts of studies that were deemed potentially eligible were then retrieved and double-screened independently (IE/RA or AhE/AbE), with discrepancies dealt with through discussion with the whole team (IE, RA, AhE, AbE and MIM).
Data extraction
We extracted information from each eligible study on the first author, publication date, phase, total number of patients included, and number of patients in each arm, as well as patient demographics (median age, cancer stage), treatment given in each arm, duration of treatment, follow-up time and percentage of patients with positive PD-L1 expression at baseline defined by CPS ≥ 1. We also extracted HR values and their 95% Confidence Intervals (CI) for OS and PFS from each study, as well as the number of patients who achieved pCR in both arms. We collected data on the occurrence of common Adverse Events (AEs) in patients from each study arm. When duplicate publications were discovered, only the most recent and complete reports of RCTs were included. Two reviewers extracted all data (IE/RA or AhE/AbE), which was then summarized, discussed by the team, and compiled into an online Microsoft Excel spreadsheet accessible to all authors.
Risk of bias assessment
To assess the risk of bias, version 2 of the Cochrane Risk-Of-Bias (RoB2) assessment tool for randomized trials was used [ 23 ]. This was done independently by the reviewers (IE/RA or AhE/AbE) with disagreement being resolved by discussion and input from a third author (MIM). The RoB2 assessment tool includes five distinct domains with multiple signaling questions to aid in assessing the risk of bias. The five domains in this tool appraise bias arising from the following: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome and selection of the reported result. Accordingly, the signaling questions provided by the ROB2 tool were answered, and the two other reviewers evaluating the trial used those answers to categorize the current domain as “low risk of bias,” “some concerns,” or “high risk of bias.” The reviewer's judgment in each domain resulted in an overall risk-of-bias conclusion for the trial under consideration. The study was deemed to have a “low risk of bias” if it was judged to have a low risk of bias in all domains included in the tool, “some concerns” if it raised some concerns in at least one domain, or “high risk of bias” if it was judged to have a high risk of bias in at least one or some concerns for multiple domains, significantly lowering confidence in the result. This data for all studies was compiled in the tool's template excel sheet, which was made available to all reviewers.
As our aim is to evaluate the efficacy and safety of ICIs, we selected four different outcomes in this NMA. The first two are OS, which is defined as the time from randomization to death from any cause, and PFS, which is defined as the time from randomization to the first documented disease progression per Response Evaluation Criteria in Solid Tumors version 1.1. The HR and its 95% CI comparing the two arms of the trials in Intention-To-Treat (ITT) populations were used to generate our final effect sizes in this NMA. The third outcome is pCR, which is defined as the absence of invasive tumors in the breast and regional nodes at the time of definitive surgery (ypT0/is pN0). Finally, to assess the safety of PD-1/PD-L1 inhibitors, we estimated the likelihood of developing AEs in each arm of the ITT populations by using the number of patients who had AEs in all grades and grade 3 or higher. Both pCR and AEs were calculated using Odds Ratios (OR) and their 95% CI based on the number of reported events in each of the trial arms.
Data analysis
Our NMA used standard pairwise meta-analysis implemented in multivariate meta-analysis models using a frequentist contrast-based approach [ 24 ]. If there is no evidence of importance in transitivity, a random-effects frequentist NMA has to be performed. These models assume that direct and indirect evidence are consistent. The network meta-analysis' net evidence is a weighted average of direct and indirect evidence. For OS and PFS, we calculated the mean log HR and its standard error and entered it into the model [ 25 ], while for pCR and AEs, we entered the number of events in each arm. When the same intervention was used in both arms of an RCT, it was assumed that the effect of that intervention was cancelled out, thus we assumed that all trials used the same comparator chemotherapy, which is necessary because even within the same trial, different chemotherapy regimens were used as controls. The assumption of transitivity was tested by comparing the distribution of study and population characteristics that may act as effect modifiers across the various pairwise comparisons. If transitivity issues were present, we returned to data extraction to verify the stage of TNBC, and the type of chemotherapy regimen used. In the case of indirect evidence, inconsistency between direct and indirect evidence was investigated locally through the use of symmetrical node-splitting [ 26 ]. However, we found no head-to-head comparisons of PD-1/PD-L1 inhibitors. Visual inspection of comparison-adjusted funnel plots for NMA was used to assess publication bias [ 27 ]. Studies were expected to form an inverted funnel centred at zero in the absence of small-study effects. The Surface Under the Cumulative Ranking Curve (SUCRA) value, which represents the re-scaled mean ranking, was also calculated and summarized [ 28 ]. Where quantitative synthesis is deemed invalid due to a small number of studies using the same intervention, narrative synthesis was used to report the findings in the results section, with estimates from the original studies. For all comparisons, we adopted the network suite in Stata to perform analyses and graphs, Stata version 16 (College Station, TX, USA) [ 29 ].
Subgroup analysis
In the event of significant heterogeneity, we conducted a sensitivity analysis, removing each study and comparing its effect. In terms of the outcome of AEs, we investigated the impact of reported symptoms on AEs to check which side effects are likely to produce this effect. We performed a sensitivity analysis for NMA using the Generalized Pairwise Modelling (GPM) framework to investigate the effect of the models used [ 30 ]. The GPM framework was used to generate mixed treatment effects against a common comparator. The common comparator for all outcomes was chemotherapy. Other than transitivity, this framework requires no additional assumptions [ 30 ]. In this sensitivity analysis, the Inverse Variance Heterogeneity model was used to pool the meta-analytical estimates [ 31 ]. The H index was used to assess statistical heterogeneity across pooled direct effects, while the weighted pooled H index ( \(\overline{H }\) ) was used to examine inconsistency across the network and assess transitivity [ 30 ]. The smallest value that H and \(\overline{H }\) can take is 1, and \(\overline{H }\) <3 was thought to represent minimal inconsistency [ 32 ]. MetaXL version 5.3 was used for the GPM framework analyses (EpiGear Int Pty Ltd.; Brisbane, Australia). The results of those sensitivity analyses will be presented in the Additional file 1 .
Study selection
Figure 1 illustrates the PRISMA flow diagram of the study selection process. Our extensive database and trial registry search yielded 1583 results. 397 duplicates were automatically removed through EndNote. A total of 1186 potentially relevant articles were identified, of which 1056 were excluded after the initial review of their titles and abstracts. The full text of the remaining 130 articles was assessed for eligibility. Of those, 71 were found to be duplicate patient records, and only the most recent and inclusive records were kept. Another 31 RCTs were excluded due to a paucity of outcome measures at the time of the search. Other 16 records were similarly removed for a variety of reasons depicted in Fig. 1 . Eventually, 12 studies were eligible for inclusion in our NMA [ 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 ]. Additional file 1 : Table S2 includes all the additional information on the omitted record citations as well as full reasoning.
PRISMA flowchart showing the number of studies at each stage of conducting this NMA
Study characteristics and data collection
Table 1 summarizes the characteristics of the included RCTs. All 12 trials included were two-arm trials that reported results from 5324 patients with median ages ranging from 48 to 59.1 years. There were seven phase III trials and five phase II trials. Six studies looked at the effect of PD-1/PD-L1 inhibitors on unresectable, invasive, or metastatic (advanced) TNBC [ 33 , 35 , 36 , 37 , 40 , 43 ], four looked at non-metastatic/early-stage TNBC [ 39 , 41 , 42 , 44 ], and two looked at treated metastatic TNBC for maintenance therapy [ 34 , 38 ]. Atezolizumab (n = 5 trials) was the most commonly studied ICI [ 33 , 36 , 39 , 40 ], followed by Pembrolizumab (n = 4 trials) [ 34 , 35 , 41 , 42 ], Durvalumab (n = 2 trials) [ 37 , 38 ], and Nivolumab (n = 1 trial) [ 43 ]. Six trials used multiple-agent chemotherapy regimens in combination with PD-1/PD-L1 inhibitors [ 36 , 37 , 39 , 41 , 42 , 44 ], and four used mono-chemotherapy regimens with PD-1/PD-L1 inhibitors, including two Taxane-based [ 33 , 40 ], one Platinum-based [ 43 ], and one Investigator's choice chemotherapy [ 35 ]. The other two trials compared PD-1/PD-L1 inhibitors alone to chemotherapy for maintenance therapy in patients with previously treated metastatic TNBC [ 34 , 38 ]. There were some minor differences in the duration of PD-1/PD-L1 inhibitors used between studies. With the exception of one trial [ 44 ], PD-1/PD-L1 inhibitors were used for four to eight cycles with a follow-up time of more than 12 months. The PD-L1 expression in TNBC tissue samples varied significantly between the included RCTs, ranging from 39 to 87% (see Table 1 ). Table 1 is to be inserted here.
Overall, five RCTs had a low risk of bias [ 33 , 35 , 37 , 40 , 41 ], six had some concerns [ 36 , 38 , 39 , 42 , 43 , 44 ], and only one had a high risk of bias [ 34 ]. When following the intended protocol and performing ITT analysis, all included trials were of high quality. Five of the six trials that raised concerns were due to the trial being non-blinded [ 36 , 38 , 42 , 43 , 44 ], which could affect the assessment of the outcome of interest. One study found a significant difference in one of the baseline parameters [ 39 ], while the high-risk study failed to report one of the secondary outcomes in the main text [ 34 ]. Figure 2 depicts the overall risk of bias across all domains (Fig. 2 A), as well as the reviewers' judgment within each domain for all included trials (Fig. 2 B).
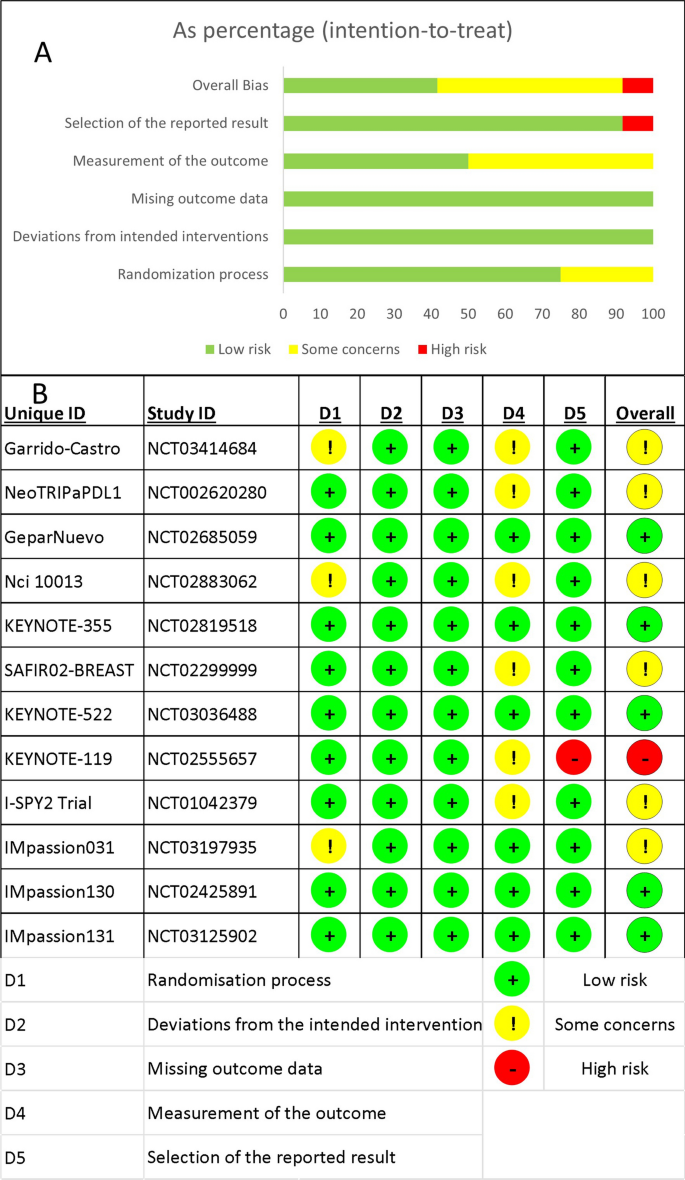
The results of the risk of bias assessment. A Stacked bar chart showing a summary of the risk of bias assessment overall and in each domain. B The detailed answers for all studies in each domain
- Overall survival
The OS was reported in nine RCTs [ 33 , 34 , 35 , 37 , 38 , 39 , 40 , 41 , 43 ], three of which used Atezolizumab [ 33 , 39 , 40 ], two used Pembrolizumab [ 35 , 41 ], and one used either Durvalumab or Nivolumab as a neoadjuvant to chemotherapy (Fig. 3 A) [ 37 , 43 ]. Pembrolizumab in a neoadjuvant setting had a pooled HR of 0.82 (95% CI 0.65 to 1.03, SUCRA = 46%, n = 2 trials, 1449 patients), which was comparable to Atezolizumab’s HR of 0.92 (95% CI 0.74 to 1.15, SUCRA = 28%, n = 3 studies, 1886 patients), demonstrating a prolonged but insignificant OS in PD-1/PD-L1 inhibitors arms (see SUCRA Additional file 1 : Table S3). GeparNuevo using Durvalumab had the only significant reported prolonged OS in PD-1/PD-L1 inhibitors in neoadjuvant settings (HR 0.24, 95% CI 0.08 to 0.72) [ 37 ]. Durvalumab also improved OS when used as a monotherapy for maintenance therapy in patients with metastatic TNBC (SAFIR02-BREAST trial, HR 0.54, 95% CI 0.30 to 0.97) [ 38 ]. This outcome's results were consistent among the studies. The rest of the analysis is shown in Fig. 3 . GPM sensitivity analysis also revealed no significant differences (Additional file 1 : Figure S1).
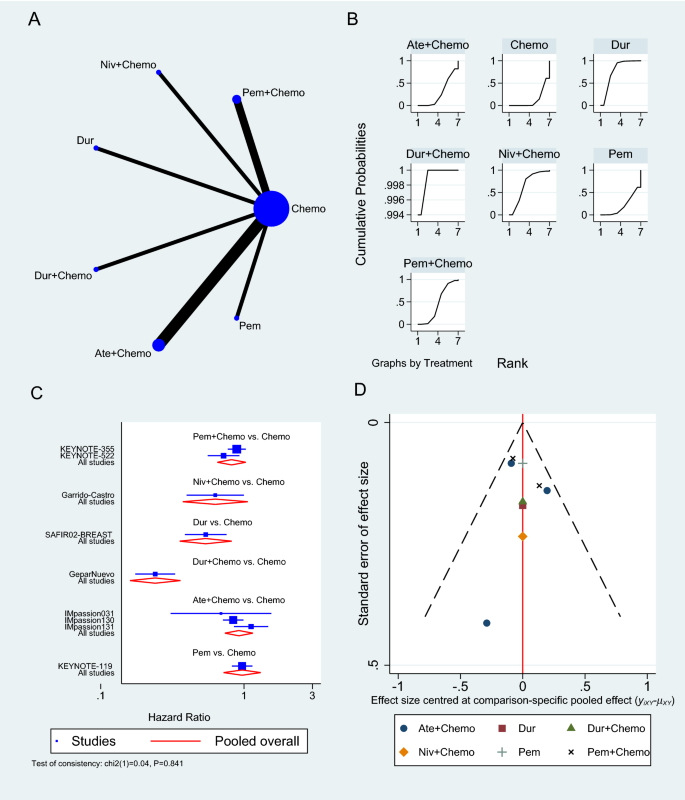
Overall survival network meta-analysis results. A Schematic diagram showing the network map for the treatments included in the analysis. B Rankogram showing the ranking probabilities for the effectiveness of each treatment. C Forest plot showing each trial effect size and confidence interval as well as the pooled effect size. D Bias-adjusted funnel plot showing each treatment separately
- Progression-free survival
Only six RCTs reported PFS [ 33 , 34 , 35 , 38 , 40 , 43 ], two of which used Atezolizumab in neoadjuvant sitting [ 33 , 40 ], as shown in Fig. 4 A. In a neoadjuvant setting along with chemotherapy, Atezolizumab achieved a pooled PFS HR of 0.82 (95% CI 0.69 to 0.97, SUCRA = 76.5%, 1553 patients) (see complete SUCRA values in Additional file 1 : Table S4), whereas Pembrolizumab can also prolong PFS as reported in KEYNOTE-355 trial when combined with chemotherapy (HR 0.82, 95% CI 0.71 to 0.94) [ 35 ]. In the SAFIR02-BREAST trial, Durvalumab had similar PFS to single-agent chemotherapy (HR 0.87, 95% CI 0.54 to 1.42, 82 patients) [ 38 ], whereas Pembrolizumab alone was associated with significantly worse PFS than chemotherapy in KEYNOTE-119 trial (HR 1.60, 95% CI 1.33 to 19.2, 622 patients) [ 34 ]. The rest of the analysis is shown in Fig. 4 , and the GPM sensitivity analysis is illustrated in the Additional file 1 : (Figure S2).
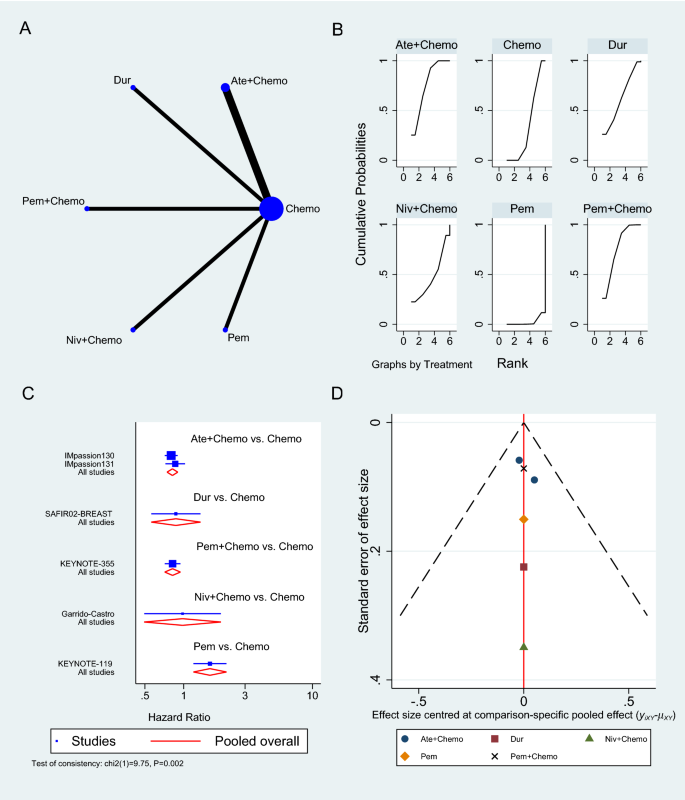
Progression-free survival network meta-analysis results. A Schematic diagram showing the network map for the treatments included in the analysis. B Rankogram showing the ranking probabilities for the effectiveness of each treatment. C Forest plot showing each trial effect size and confidence interval as well as the pooled effect size. D Bias-adjusted funnel plot showing each treatment separately
Pathologic complete response
The number of patients who achieved a complete response was reported in six trials [ 36 , 39 , 41 , 42 , 44 ]: three on Atezolizumab [ 36 , 39 , 44 ], two on Pembrolizumab [ 41 , 42 ], and one on Durvalumab [ 37 ], all in the neoadjuvant setting to chemotherapy. Pembrolizumab in combination with chemotherapy significantly increased the odds of achieving pCR compared to chemotherapy alone (OR 2.79, 95% CI 1.07 to 7.24, SUCRA = 82.1%, 2 studies, 709 patients), whereas Atezolizumab showed an insignificant increase in pCR (OR 1.94, 95% CI 0.86 to 4.37, SUCRA = 62.3, 3 studies, 674 patients) (complete SUCRA values in Additional file 1 : Table S5). In the GeparNuevo trial, the calculated OR of achieving pCR with Durvalumab and chemotherapy was 1.45 (95% CI 0.80 to 2.63) [ 37 ]. Figure 5 summarizes the results of the pCR analysis, and the GPM sensitivity analysis is presented in the Additional file 1 : Figure S3.
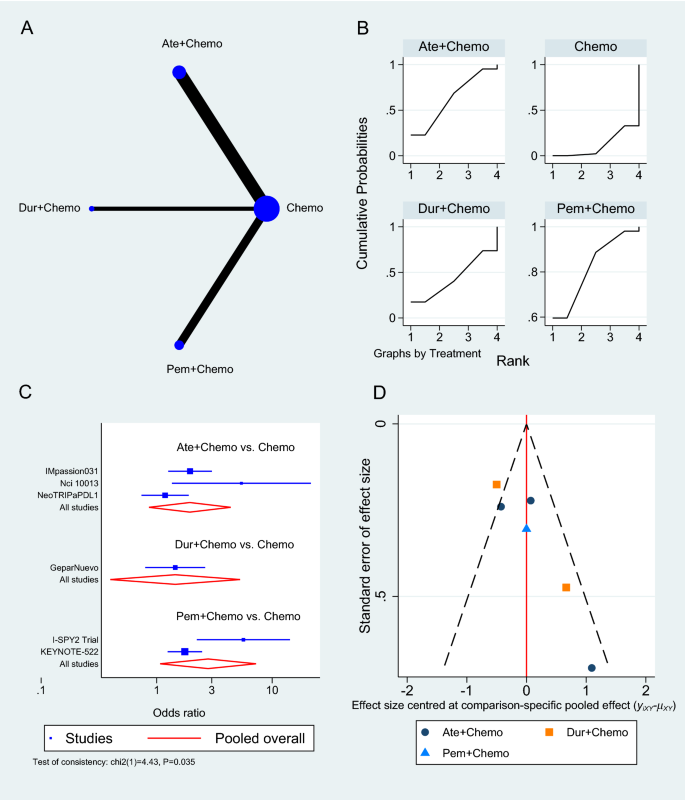
Pathologic complete response network meta-analysis results. A Schematic diagram showing the network map for the treatments included in the analysis. B Rankogram showing the ranking probabilities for the effectiveness of each treatment. C Forest plot showing each trial effect size and confidence interval as well as the pooled effect size. D Bias-adjusted funnel plot showing each treatment separately
- Adverse events
At the time of analysis, nine trials had AEs grade ≥ 3 results reported [ 33 , 34 , 35 , 36 , 39 , 40 , 41 , 42 , 44 ], the majority of which was the effect of Atezolizumab combined with chemotherapy versus chemotherapy alone (n = 5 studies) [ 33 , 36 , 39 , 40 , 44 ], followed by Pembrolizumab with chemotherapy (n = 3 studies) (Fig. 6 A) [ 35 , 41 , 42 ]. The pooled OR of Atezolizumab addition to chemotherapy causing AEs grade 3 or more compared to chemotherapy alone was 1.48 (95% CI 0.90 to 2.42, 5 studies, 2325 patients), whereas Pembrolizumab with chemotherapy showed a slightly greater risk of causing AEs grade ≥ 3 (OR 1.90, 95% CI 1.08 to 3.33, 3 studies, 2263 patients) (Fig. 6 C). Atezolizumab and Pembrolizumab achieved SUCRA values of 26.7% and 9.3% respectively compared to 64.3% for chemotherapy (Additional file 1 : Table S6). When compared to single-agent chemotherapy, the KEYNOTE-119 trial showed a significant reduction in AEs grade ≥ 3 when using Pembrolizumab alone in maintenance therapy (OR 0.29, 95% CI 0.19 to 0.43) [ 34 ].
Grade ≥ 3 adverse events network meta-analysis results. A Schematic diagram showing the network map for the treatments included in the analysis. B Rankogram showing the ranking probabilities for the least odds of causing adverse events for each treatment. C Forest plot showing each trial effect size and confidence interval as well as the pooled effect size. D Bias-adjusted funnel plot showing each treatment separately
In the sensitivity analysis investigating the subtype of the reported AEs, neoadjuvant Pembrolizumab to chemotherapy showed an increase in the odds of developing adrenal insufficiency (OR 26.24, 95% CI 3.50 to 197.86, Additional file 1 : Figure S4), diarrhea (OR 1.47, 95% CI 1.14 to 1.88, Additional file 1 : Figure S5), hyperthyroidism (OR 5.22, 95% CI 2.44 to 11.15, Additional file 1 : Figure S6), hypothyroidism (OR 5.23, 95% CI 3.35 to 8.16, Additional file 1 : Figure S7), infusion reaction (OR 1.64, 95% CI 1.13 to 2.37, Additional file 1 : Figure S8) and pneumonitis (OR 5.94, 95% CI 1.29 to 27.27, Additional file 1 : Figure S9). On the other hand, Atezolizumab in the neoadjuvant settings increased the odds of developing hyperthyroidism (OR 10.91, 95% CI 1.98 to 60.15, Additional file 1 : Figure S6), hypothyroidism (OR 3.77, 95% CI 2.52 to 5.63, Additional file 1 : Figure S7) and pneumonitis (OR 2.73, 95% CI 1.41 to 5.31, Additional file 1 : Figure S9) compared to chemotherapy alone. The remaining results of the sensitivity analysis according to the type of AE developed and GPM are outlined in the Additional file 1 : (Figure S10 to Figure S17).
Principle findings and existing literature
TNBC is an aggressive form of breast cancer that is often associated with poor patient outcomes, largely due to the limited treatment options available [ 6 ]. Intensive research efforts have therefore attempted to improve the efficiency of standard-of-care chemotherapy by incorporating immunotherapeutic agents, particularly ICIs, which have emerged as a novel breakthrough in cancer treatment in the past recent years [ 15 ]. The present network meta-analysis aimed to compare the published data on the efficacy and safety of ICIs in treating TNBC. Our results showed that antiPD-1/PD-L1 therapies can be used as a neoadjuvant to chemotherapy in the first-line treatment or alone in previously treated TNBC. Multiple RCTs that were conducted on this topic have demonstrated a greater benefit of adding ICIs to chemotherapy in terms of OS, PFS, and pCR [ 45 , 46 , 47 , 48 , 49 ]. As a result, existing meta-analyses evaluating those trials were successful in achieving statistical and clinical significance. For example, Zhang et al. group reported that PD-1/PD-L1 inhibitors in combination with chemotherapy improved pCR (OR 1.59, 95% CI 1.28 to 1.98), event-free survival (HR 0.66, 95% CI 0.48 to 0.91, p = 0.01), and overall survival (HR 0.72, 95% CI 0.52 to 0.99) in TNBC patients compared to chemotherapy alone [ 45 ]. Moreover, Li et al. studied the pCR of ICIs in neoadjuvant setting in TNBC and reported that the OR significantly increased in their four included study meta-analysis (OR 2.14, 95% CI 1.37–3.35, P < 0.001) and a better event-free survival (HR 0.66, 95% CI 0.48 to 0.89, P = 0.007) [ 49 ], while similar values for pCR were reported by Rizzo et al. (OR 1.95, 95% CI 1.27 to 2.99) and Xin et al. (OR 1.91, 95% CI 1.32 to 2.78) [ 46 , 48 ]. Villacampa et al. reported that patients with PD-L1-positive tumors had a significantly better PFS with ICIs (HR 0.67, 95% CI 0.58 to 0.79) and a trend towards better OS (HR 0.79, 95% CI 0.60 to 1.03), while no benefit was observed in patients with PD-L1-negative tumors [ 47 ]. This is in contrast to Zhang et al. who found that the pCR rate was almost identical in the PD-L1-positive and negative groups [ 45 ]. However, many have reported high heterogeneity in effect estimates, indicating major systematic differences between the included RCTs [ 45 , 46 , 47 , 48 , 49 ]. Although this heterogeneity has been attributed to many factors including patient population, TNBC stage, PD-L1 levels, randomization process, and type of chemotherapy regimen, these meta-analyses have failed to acknowledge the performance differences and the distinct immunologic mechanisms by which ICIs act. Contrary to our study, they have combined all agents into a large group and regarded them as one entity, assuming they have similar efficacy and safety.
Efficacy of PD-1/PD-L1 inhibitors
In our NMA, only two trials out of nine reported statistical significance in terms of OS, both of which used Durvalumab, one as a neoadjuvant (GeparNuevo phase II trial, HR 0.24, 95% CI 0.08 to 0.72, 174 patients) and the other as maintenance (SAFIR02-BREAST trial, HR 0.54, 95% CI 0.30 to 0.97) [ 37 , 38 ]. Six of the remaining seven trials reported longer, yet statistically insignificant survival. This could be attributed to the small sample size or the lack of follow-up, yet the possibility of Durvalumab having superior efficacy remains, highlighting the need for an additional large phase III RCTs investigating Durvalumab efficacy and safety in TNBC. Five of the seven trials that used neoadjuvant PD-1/PD-L1 inhibitors and reported OS were invasive or metastatic (advanced), with only GeparNuevo achieving a significant reduction in OS HR (0.24, 95% CI 0.08 to 0.72). The remaining two neoadjuvant trials (IMpassion031 trial, HR 0.69, 95% CI 0.25 to 1.87) and (KEYNOTE-522 trial, HR 0.72, 95% CI 0.51 to 1.02) were on non-metastatic or advanced and did not show any improvement in OS.
In general, PFS prolongation followed a positive trend similar to OS when ICIs were used. The IMpassion130 trial demonstrated a significant improvement in PFS with Atezolizumab (HR 0.80, 95% CI 0.69 to 0.92) [ 40 ], as opposed to the confirmatory trial Impassion131which failed to achieve statistical significance with Atezolizumab despite extending PFS (HR 0.86, 95% CI 0.70 to 1.05) [ 33 ]. An FDA review of the discordant findings between these two trials, including chemotherapy regimens, study design, conduct and population found no single component that could be responsible for this discrepancy, as a result, the reason for this is unclear at present. It is also worth mentioning that the only two trials that reported statistical significance, KEYNOTE-355 and IMpassion130, are the ones with the largest population sample, which may have accounted for their outcome.
Alternatively, the KEYNOTE-355 trial found that Pembrolizumab is effective in prolonging PFS in the neoadjuvant setting (HR 0.82, 95% CI 0.69 to 0.97) [ 35 ], while Nivolumab appears to be less effective in improving survival PFS (HR 0.98, 95% CI 0.51 to 1.88). Both KEYNOTE-522 and IMpassion031 trials found that using ICI in the neoadjuvant setting improved disease-free survival [ 39 , 41 ]. ICIs use as maintenance therapy instead of chemotherapy in treated metastatic TNBC has also shown promising results in terms of prolonging survival using Durvalumab in the SAFIR02-BREAST trial, in contrast to Pembrolizumab that showed no significant improvement in the Keynote-119 trial (PFS HR 1.6, 95% CI 1.33 to 1.92) [ 34 , 38 ]. Nonetheless, the Keynote-119 trial demonstrated a significant reduction in AEs grade ≥ 3, negating one of chemotherapy's worst attributes [ 34 ]. Furthermore, ICIs have also been shown to improve the chances of achieving pCR in TNBC patients when compared to chemotherapy alone. According to our NMA, neoadjuvant Pembrolizumab resulted in the highest pCR (OR 2.79, 95% CI 1.07 to 7.24), followed by Atezolizumab (OR 1.94, 95% CI 0.86 to 4.37, 3 studies, 674 patients), and Durvalumab, which had the lowest pCR (1.45, 95% CI 0.80 to 2.63). However, among the six trials that reported pCR, NeoTRIPaPDL1 and GeparNuevo were the only two RCTs that did not report significant improvement in pCR [ 36 , 37 ]. This can be explained by the advanced TNBC stage both studies were conducted upon, implying that using ICIs at an earlier stage of TNBC disease progression will more likely benefit patients and improve their survival. This is supported by the fact that early-stage TNBC has a greater tumor immune microenvironment than advanced TNBC, which increases the effectiveness of ICIs with the additional stimulation to the immune response provided by chemotherapy treatment [ 46 ]. Another possibility for the negative NeoTRIPaPDL1 results could be due to the insufficient immune induction effect of the chemotherapy regimens used in the study design [ 46 ].
Safety of PD-1/PD-L1 inhibitors
In regard to safety, ICIs appear to be associated with a significant toxicity burden, especially in the form of immune-related AEs [ 50 ]. Our NMA showed that Pembrolizumab generally has a worse safety profile than Atezolizumab, causing more grade ≥ 3 AEs (OR 1.90, 95% CI 1.08 to 3.33). Despite the fact that both drugs increased the risk of hyperthyroidism, hypothyroidism, and pneumonitis, Pembrolizumab caused a significant increase in adrenal insufficiency, diarrhea, and infusion reaction, making Atezolizumab a safer option. These AEs are likely to be related to drugs’ mechanism of action. The ability of ICIs to reinvigorate exhausted T-cells in an attempt to kill the tumor may destroy the immune tolerance balance and result in autoimmune and inflammatory responses in normal tissue [ 51 , 52 ]. However, the reason why certain people or specific organs are more susceptible than others is still incompletely understood [ 51 ]. Proposed hypotheses include hereditary predisposition, environmental factors and expression of shared antigens between tumors and affected tissue [ 51 ]. Whilst most of these immune-related AEs are usually manageable and reversible, some may require long-term intervention, such as endocrinopathies [ 50 ]. Of note, close monitoring of patients and early detection of any AEs is of utmost importance to ensure patients can benefit from adding PD-1/PD-L1 inhibitors to their chemotherapy regimen. Careful follow-up care is also warranted to prevent potential later onset immune-related AEs that may present after cessation of ICIs [ 50 ].
Enhancing the benefit of PD-1/PD-L1 inhibitors
It is crucial to note that the response to ICIs as well as to the combination of other agents differs significantly among patients, highlighting the importance of predictive biomarkers [ 53 ]. A multitude of promising novel biomarkers has recently gained considerable attention including the CD274 gene and TILs, but to date, PD-L1 status remains the only biomarker approved to guide patient selection in TNBC [ 53 , 54 , 55 ]. We considered PD-L1 positivity as CPS ≥ 1 in Table 1 , yet the threshold for PD-L1 positivity and at what level ICIs become more effective remains a topic of scientific debate. Analysis of the present NMA showed that IMpassion031, Keynote-522, and GeparNuevo trials have all demonstrated PD-1/PD-L1 inhibitors to improve efficacy regardless of PD-L1 status in patients with early-stage TNBC [ 33 , 41 ]. Conversely, IMpassion130 and Keynote-355 demonstrated improved efficacy in metastatic TNBC but not in early-stage TNBC [ 35 , 40 ]. Following the outcomes of the recently published IMpassion130 and KEYNOTE-355 trials, this biomarker was validated as a predictor of response to PD-1/PD-L1 inhibitors in metastatic breast cancer [ 48 ]. Even though data from a previous meta-analysis found no correlation between pCR rates and PD-L1 expression, further investigation revealed pCR rates to be higher in PD-L1-positive patients [ 46 ]. Notably, the lack of a standardized approach for PD-L1 detection in TNBC has led to inconsistent PD-L1 prevalence, thereby hampering the precise guiding of immunotherapy [ 45 , 54 ]. Another significant challenge is that TNBC is composed of numerous heterogeneous subtypes. Biomarker research on IMpassion130 samples revealed that PD-L1 is expressed higher in basal-like immune-activated subtype (75%) and immune-inflamed tumors (63%) TNBC subtypes [ 56 , 57 ]. Another exploratory study found an improved advantage in PFS in TNBC patients with immune-inflamed tumors, basal-like immune-activated and basal-like immunosuppressed subtypes, in addition to the prolonged OS in inflamed tumors and basal-like immune-activated subtypes [ 47 , 56 , 57 ]. Certainly, the identification of predictive biomarkers of efficacy will greatly aid in optimizing personalized regimens for TNBC patients, as well as predicting the long-term effectiveness of PD-1/PD-L1 inhibitors.
Future RCTs using PD-1/PD-L1 inhibitors in TNBC
Interestingly, the majority of the currently ongoing RCTs are investigating Atezolizumab and Pembrolizumab, both of which were studied the most in nine out of the 12 RCTs included in our NMA. Hoffmann-La Roche, the sponsor of IMpassion130, IMpassion131, and Impassion031, is currently funding three additional phase III RCTs on Atezolizumab. IMpassion132 is a double-blind Phase III RCT on the efficacy and safety of neoadjuvant Atezolizumab for early relapsing TNBC (NCT03371017), while IMpassion030 is planned to be the largest RCT on ICI as it is presently recruiting 2300 patients with operable TNBC to investigate the combination of neoadjuvant Atezolizumab and chemotherapy (NCT03498716). Hoffmann-La Roche’s third RCT is looking into the combination of Atezolizumab, Ipatasertib, and Paclitaxel in patients with advanced or metastatic TNBC (NCT04177108). In another phase III double-blinded RCT, GeparDouze will investigate neoadjuvant Atezolizumab followed by adjuvant Atezolizumab in patients with high-risk TNBC (NCT03281954). The National Cancer Institute (NCI) is also funding a large phase III RCT to assess the efficacy and safety of Pembrolizumab as adjuvant therapy following neoadjuvant chemotherapy (NCT02954874). Additionally, ASCENT-04 and ASCENT-05 are both ongoing phase III RCTs investigating the PFS of Pembrolizumab in combination with Sacituzumab Govitecan versus chemotherapy in either advanced or residual invasive TNBC (NCT05382286, NCT05633654). TROPION-Breast03 is similarly a new phase III RCT looking at Datopotamab Deruxtecan (DatoDXd) with or without Durvalumab in early-stage TNBC (NCT05629585). Finally, Avelumab, another PD-L1 inhibitor, is currently being studied in a phase III RCT on high-risk TNBC patients (A-Brave trial, NCT02926196).
Limitations
There are some limitations that must be addressed in this NMA. Firstly, only 12 studies were included, in addition to the limited number of reported outcomes of interest. This is primarily due to the fact that we only included phase II and phase III RCTs because our goal was to compare the efficacy of PD-1/PD-L1 inhibitors in clinical settings. With the ongoing development of neoadjuvant ICI clinical trials, there will certainly be more comprehensive data to be analyzed in future NMA. Second, the NMA comparisons were solely based on direct evidence, with no head-to-head comparisons of neoadjuvant ICIs in TNBC. Moreover, the small number of studies has caused the limited network connectivity to produce large confidence intervals for some estimates, even when effect sizes were large. It may have also resulted in an immature investigation of heterogeneity and publication bias. We would also like to point out the differences between the included studies in terms of TNBC stage, chemotherapy backbone, ICI duration, follow-up time, and PD-L1 expression status. Different chemotherapy backbone regimens used in different studies may have influenced the interpretation of the results as they could have been added to separate groups in the NMA if the number of included studies allowed. Given this heterogeneity and the limited RCTs number, further subgroup analysis based on PD-L1 expression status and nodal involvement, as well as advanced vs early-stage, was not deemed feasible. Finally, all data in this study were derived from published literature, and no individual patient data were used. Noteworthily, the meta-analysis results could potentially be biased by two of the included RCTs that were published as abstracts, which may have relatively incomplete data, missing safety data, and unclear research methods.
Our NMA found variation in efficacy and safety among PD-1/PD-L1 inhibitors used to treat TNBC, as well as significant systematic differences between the RCTs included. To better assess those variations in efficacy, head-to-head trials between those PD-1/PD-L1 inhibitors are needed. In their use as a neoadjuvant to chemotherapy, ICIs demonstrated comparable efficacy in terms of OS, PFS, and pCR. This benefit is offset by an increase in immune-related adverse events, such as hyperthyroidism, hypothyroidism, pneumonitis, and adrenal insufficiency. We also demonstrated that Atezolizumab is safer than Pembrolizumab in the neoadjuvant setting. Only trials evaluating early-stage TNBC showed a significant improvement in pCR, implying that PD-1/PD-L1 inhibitors may be most effective when started early in the disease course. Durvalumab as a maintenance therapy instead of chemotherapy in patients with metastatic TNBC has also shown promising results in terms of survival extension. Future research should focus on PD-L1 expression status and TNBC subtypes, as these parameters may aid in the optimization of personalized treatment regimens for TNBC patients.
Availability of data and materials
Data used in this study analysis is provided in the Additional file 1 : (Table S7). Further analysis data requests and inquiries can be directed to the corresponding author.
Abbreviations
Confidence interval
Combined positive score
Food and Drug Administration
Generalized pairwise modelling
Hazard ratio
- Immune checkpoint inhibitors
Intention-to-treat
- Network meta-analysis
- Pathological complete response
Programmed cell death protein 1
Programmed cell death ligand 1
Preferred reporting items for systematic reviews and meta-analyses
International prospective register of systematic reviews
Cochrane risk-of-bias tool 2
Surface under the cumulative ranking curve
Tumor infiltrating lymphocytes
Triple-negative breast cancer
Huang J, Chan PS, Lok V, Chen X, Ding H, Jin Y, Yuan J, Lao XQ, Zheng ZJ, Wong MC. Global incidence and mortality of breast cancer: a trend analysis. Aging. 2021;13(4):5748–803.
Article PubMed PubMed Central Google Scholar
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Article PubMed Google Scholar
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022;66:15–23.
Yao H, He G, Yan S, Chen C, Song L, Rosol TJ, Deng X. Triple-negative breast cancer: is there a treatment on the horizon? Oncotarget. 2017;8(1):1913–24.
Hsu JY, Chang CJ, Cheng JS. Survival, treatment regimens and medical costs of women newly diagnosed with metastatic triple-negative breast cancer. Sci Rep. 2022;12(1):729.
Article CAS PubMed PubMed Central Google Scholar
Yin L, Duan JJ, Bian XW, Yu SC. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22(1):61.
Zaheed M, Wilcken N, Willson ML, O’Connell DL, Goodwin A. Sequencing of anthracyclines and taxanes in neoadjuvant and adjuvant therapy for early breast cancer. Cochrane Database Syst Rev. 2019;2(2):Cd012873.
PubMed Google Scholar
de Ruijter TC, Veeck J, de Hoon JP, van Engeland M, Tjan-Heijnen VC. Characteristics of triple-negative breast cancer. J Cancer Res Clin Oncol. 2011;137(2):183–92.
Article CAS PubMed Google Scholar
Zeichner SB, Terawaki H, Gogineni K. A review of systemic treatment in metastatic triple-negative breast cancer. Breast Cancer. 2016;10:25–36.
CAS PubMed PubMed Central Google Scholar
Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460–7.
Manjunath M, Choudhary B. Triple-negative breast cancer: a run-through of features, classification and current therapies. Oncol Lett. 2021;22(1):512.
Abdou Y, Goudarzi A, Yu JX, Upadhaya S, Vincent B, Carey LA. Immunotherapy in triple negative breast cancer: beyond checkpoint inhibitors. NPJ Breast Cancer. 2022;8(1):121.
Pathak N, Chitikela S, Malik PS. Recent advances in lung cancer genomics: application in targeted therapy. Adv Genet. 2021;108:201–75.
Popovic M, Matovina-Brko G, Jovic M, Popovic LS. Immunotherapy: a new standard in the treatment of metastatic clear cell renal cell carcinoma. World J Clin Oncol. 2022;13(1):28–38.
Bai X, Ni J, Beretov J, Graham P, Li Y. Immunotherapy for triple-negative breast cancer: a molecular insight into the microenvironment, treatment, and resistance. J Natl Cancer Cent. 2021;1(3):75–87.
Article Google Scholar
Quinn KM. Innovative approaches to immunotherapy in breast cancer. J Thorac Dis. 2020;12(8):4536–40.
Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10(3):727–42.
Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, Wu X, Ma J, Zhou M, Li X, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18(1):10.
Narayan P, Wahby S, Gao JJ, Amiri-Kordestani L, Ibrahim A, Bloomquist E, Tang S, Xu Y, Liu J, Fu W, et al. FDA approval summary: atezolizumab plus paclitaxel protein-bound for the treatment of patients with advanced or metastatic TNBC whose tumors express PD-L1. Clin Cancer Res. 2020;26(10):2284–9.
Brian H, Georgia S, Deborah MC, Anna C, Christopher HS, Chris C, John PAI, Sharon S, Kristian T, Jeroen PJ, Cynthia M, Ferrán C-L, Peter CG, Kay D, Isabelle B, Douglas GA, David M. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84.
Clark JM, Sanders S, Carter M, Honeyman D, Cleo G, Auld Y, Booth D, Condron P, Dalais C, Bateup S, et al. Improving the translation of search strategies using the polyglot search translator: a randomized controlled trial. J Med Libr Assoc. 2020;108(2):195–207.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
White IR, Barrett JK, Jackson D, Higgins JPT. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3(2):111–25.
Woods BS, Hawkins N, Scott DA. Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: a tutorial. BMC Med Res Methodol. 2010;10(1):54.
Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7–8):932–44.
Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res Synth Methods. 2012;3(2):161–76.
Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–71.
White IR. Network meta-analysis. Stata J. 2015;15(4):951–85.
Doi SAR, Barendregt JJ. A generalized pairwise modelling framework for network meta-analysis. Int J Evid Based Healthc. 2018;16(4):187–94.
Doi SAR, Barendregt JJ, Khan S, Thalib L, Williams GM. Advances in the meta-analysis of heterogeneous clinical trials I: the inverse variance heterogeneity model. Contemp Clin Trial. 2015;45:130–8.
Doi SAR, Barendregt JJ. A generalized pairwise modelling framework for network meta-analysis. JBI Evid Implement. 2018;16(4):187–94.
Google Scholar
Miles D, Gligorov J, André F, Cameron D, Schneeweiss A, Barrios C, Xu B, Wardley A, Kaen D, Andrade L, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021;32(8):994–1004.
Winer EP, Lipatov O, Im SA, Goncalves A, Muñoz-Couselo E, Lee KS, Schmid P, Tamura K, Testa L, Witzel I, et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(4):499–511.
Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396(10265):1817–28.
Gianni L, Huang CS, Egle D, Bermejo B, Zamagni C, Thill M, Anton A, Zambelli S, Bianchini G, Russo S, et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann Oncol. 2022;33(5):534–43.
Loibl S, Schneeweiss A, Huober J, Braun M, Rey J, Blohmer JU, Furlanetto J, Zahm DM, Hanusch C, Thomalla J, et al. Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann Oncol. 2022;33(11):1149–58.
Bachelot T, Filleron T, Bieche I, Arnedos M, Campone M, Dalenc F, Coussy F, Sablin MP, Debled M, Lefeuvre-Plesse C, et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: the randomized phase II SAFIR02-BREAST IMMUNO trial. Nat Med. 2021;27(2):250–5.
Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, Koehler A, Sohn J, Iwata H, Telli ML, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. The Lancet. 2020;396(10257):1090–100.
Article CAS Google Scholar
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Hegg R, Im S-A, Shaw Wright G, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. New Engl J Med. 2018;379(22):2108–21.
Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, et al. Pembrolizumab for early triple-negative breast cancer. New Engl J Med. 2020;382(9):810–21.
Nanda R, Liu MC, Yau C, Shatsky R, Pusztai L, Wallace A, Chien AJ, Forero-Torres A, Ellis E, Han H, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 2020;6(5):676–84.
Garrido-Castro AC, Graham N, Bi K, Park J, Fu J, Keenan T, Richardson ET, Pastorello R, Lange P, Attaya V, et al. Abstract P2–14–18: A randomized phase II trial of carboplatin with or without nivolumab in metastatic triple-negative breast cancer. Cancer Res. 2022;82(4_Supplement):P2-14-18-P12-14–8.
Ademuyiwa FO, Gao F, Chen I, Northfelt DW, Wesolowski R, Arora M, Brufsky A, Dees C, Santa-Maria CA, Connolly RM, et al. Abstract PD14–09: Nci 10013—a randomized phase 2 study of neoadjuvant carboplatin and paclitaxel, with or without atezolizumab in triple negative breast cancer (TNBC). Cancer Res. 2021;81(4_Supplement):PD14-09-PD14-09.
Zhang M, Song J, Yang H, Jin F, Zheng A. Efficacy and safety of PD-1/PD-L1 inhibitors in triple-negative breast cancer: a systematic review and meta-analysis. Acta Oncol. 2022;61(9):1105–15.
Xin Y, Shen G, Zheng Y, Guan Y, Huo X, Li J, Ren D, Zhao F, Liu Z, Li Z, et al. Immune checkpoint inhibitors plus neoadjuvant chemotherapy in early triple-negative breast cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21(1):1261.
Villacampa G, Tolosa P, Salvador F, Sánchez-Bayona R, Villanueva L, Dienstmann R, Ciruelos E, Pascual T. Addition of immune checkpoint inhibitors to chemotherapy versus chemotherapy alone in first-line metastatic triple-negative breast cancer: a systematic review and meta-analysis. Cancer Treat Rev. 2022;104:102352.
Rizzo A, Cusmai A, Massafra R, Bove S, Comes MC, Fanizzi A, Rinaldi L, Acquafredda S, Gadaleta-Caldarola G, Oreste D, et al. Pathological complete response to neoadjuvant chemoimmunotherapy for early triple-negative breast cancer: an updated meta-analysis. Cells. 2022;11(12):1857.
Li Y, Xing L, Li F, Liu H, Gan L, Yang D, Wang M, Yin X, Li H, Ren G. Efficacy and safety of adding immune checkpoint inhibitors to neoadjuvant chemotherapy against triple-negative breast cancer: a meta-analysis of randomized controlled trials. Front Oncol. 2021;11:657634.
Brown LC, Loi S. Immune checkpoint inhibition in the treatment of early stage triple negative breast cancer: 2021 update. Breast. 2022;62 Suppl 1(Suppl 1):S29-s33.
Wang DY, Johnson DB, Davis EJ. Toxicities associated with PD-1/PD-L1 blockade. Cancer J. 2018;24(1):36–40.
Zheng J, Cui T, Gao Y, Li T. Retrospective analysis of immune-related adverse events of the immune checkpoint inhibitors of PD-1/PD-l1 in the Fujian provincial hospital. Eur J Inflam. 2022;20:1721727X221091540.
Carretero-González A, Otero I, Lora D, Carril-Ajuria L, Castellano D, de Velasco G. Efficacy and safety of anti-PD-1/PD-L1 combinations versus standard of care in cancer: a systematic review and meta-analysis. Oncoimmunology. 2021;10(1):1878599.
Chen F, Chen N, Gao Y, Jia L, Lyu Z, Cui J. Clinical progress of PD-1/L1 inhibitors in breast cancer immunotherapy. Front Oncol. 2021;11:724424.
Leite KRM, Barrios CH, Buzaid AC, Gagliato D, Gobbi H, Soares F. Applicability of PD-L1 tests to tailor triple-negative breast cancer treatment in Brazil. Surg Exp Pathol. 2021;4(1):10.
Emens LA, Goldstein LD, Schmid P, Rugo HS, Adams S, Barrios CH, Schneeweiss A, Dieras V, Iwata H, Chang C-W, et al. The tumor microenvironment (TME) and atezolizumab + nab-paclitaxel (A+nP) activity in metastatic triple-negative breast cancer (mTNBC): IMpassion130. J Clin Oncol. 2021;39(15_suppl):1006–1006.
Emens LA, Molinero L, Loi S, Rugo HS, Schneeweiss A, Diéras V, Iwata H, Barrios CH, Nechaeva M, Nguyen-Duc A, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the IMpassion130 study. J Natl Cancer Inst. 2021;113(8):1005–16.
Download references
Acknowledgements
QNL Funded the APC publication of this article.
Open Access funding provided by the Qatar National Library. This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
College of Medicine, QU Health, Qatar University, P. O. Box: 2713, Doha, Qatar
Ibrahim Elmakaty, Ruba Abdo, Ahmed Elsabagh & Abdelrahman Elsayed
Pathology Unit, Department of Basic Medical Sciences, College of Medicine, QU Health, Qatar University, Doha, Qatar
Mohammed Imad Malki
You can also search for this author in PubMed Google Scholar
Contributions
IE: Data curation, Formal analysis, Investigation, Methodology, Writing—original draft, Writing—review and editing, illustration of tables and figures. RA: Data curation, Formal analysis, Writing—original draft, Writing—review and editing. AE: Data curation, Formal analysis, Writing—original draft, Writing—review and editing. AE: Data curation, Formal analysis, Writing—review and editing. MIM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Funding Acquisition, Writing—original draft, Writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Mohammed Imad Malki .
Ethics declarations
Ethics approval and consent to participate.
Not applicable. All the work was developed using published data.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: table s1:.
PRISMA checklist for this network meta-analysis. Table S2: Excluded articles at full-text screening. Table S3: Overall survival treatment ranking and surface under the cumulative ranking curve. Figure S1: Overall survival using generalized pairwise modelling. Table S4: Progression free survival treatment ranking and surface under the cumulative ranking curve. Figure S2: Progression free survival using generalized pairwise modelling. Table S5: Pathologic complete response treatment ranking and surface under the cumulative ranking curve. Figure S3: Pathologic complete response using generalized pairwise modelling. Table S6: Adverse events grade ≥ 3 treatment ranking and surface under the cumulative Table. Figure S4: Adrenal insufficiency odds network meta-analysis results. A Schematic diagram showing the network map for the treatments included in the analysis. B Rankogram showing the ranking probabilities for the least odds of causing this adverse event for each treatment. C Forest plot showing each trial effect size and confidence interval as well as the pooled effect size. D Bias-adjusted funnel plot showing each treatment separately. Figure S5: Diarrhea odds network meta-analysis results. A Schematic diagram showing the network map for the treatments included in the analysis. B Rankogram showing the ranking probabilities for the least odds of causing this adverse event for each treatment. C Forest plot showing each trial effect size and confidence interval as well as the pooled effect size. D Bias-adjusted funnel plot showing each treatment separately. Figure S6: Hyperthyroidism odds network meta-analysis results. A Schematic diagram showing the network map for the treatments included in the analysis. B Rankogram showing the ranking probabilities for the least odds of causing this adverse event for each treatment. C Forest plot showing each trial effect size and confidence interval as well as the pooled effect size. D Bias-adjusted funnel plot showing each treatment separately. Figure S7: Hypothyroidism odds network meta-analysis results. A Schematic diagram showing the network map for the treatments included in the analysis. B Rankogram showing the ranking probabilities for the least odds of causing this adverse event for each treatment. C Forest plot showing each trial effect size and confidence interval as well as the pooled effect size. D Bias-adjusted funnel plot showing each treatment separately. Figure S8: Infusion reaction odds network meta-analysis results. A Schematic diagram showing the network map for the treatments included in the analysis. B Rankogram showing the ranking probabilities for the least odds of causing this adverse event for each treatment. C Forest plot showing each trial effect size and confidence interval as well as the pooled effect size. D Bias-adjusted funnel plot showing each treatment separately. Figure S9: Pneumonitis odds network meta-analysis results. A Schematic diagram showing the network map for the treatments included in the analysis. B Rankogram showing the ranking probabilities for the least odds of causing this adverse event for each treatment. C Forest plot showing each trial effect size and confidence interval as well as the pooled effect size. D Bias-adjusted funnel plot showing each treatment separately. Figure S10: Anemia odds network meta-analysis results. A Schematic diagram showing the network map for the treatments included in the analysis. B Rankogram showing the ranking probabilities for the least odds of causing this adverse event for each treatment. C Forest plot showing each trial effect size and confidence interval as well as the pooled effect size. D Bias-adjusted funnel plot showing each treatment separately. Figure S11: Colitis odds network meta-analysis results. A Schematic diagram showing the network map for the treatments included in the analysis. B Rankogram showing the ranking probabilities for the least odds of causing this adverse event for each treatment. C Forest plot showing each trial effect size and confidence interval as well as the pooled effect size. D Bias-adjusted funnel plot showing each treatment separately. Figure S12: Fatigue odds network meta-analysis results. A Schematic diagram showing the network map for the treatments included in the analysis. B Rankogram showing the ranking probabilities for the least odds of causing this adverse event for each treatment. C Forest plot showing each trial effect size and confidence interval as well as the pooled effect size. D Bias-adjusted funnel plot showing each treatment separately. Figure S13: Nausea odds network meta-analysis results. A Schematic diagram showing the network map for the treatments included in the analysis. B Rankogram showing the ranking probabilities for the least odds of causing this adverse event for each treatment. C Forest plot showing each trial effect size and confidence interval as well as the pooled effect size. D Bias-adjusted funnel plot showing each treatment separately. Figure S14: Neutropenia odds network meta-analysis results. A Schematic diagram showing the network map for the treatments included in the analysis. B Rankogram showing the ranking probabilities for the least odds of causing this adverse event for each treatment. C Forest plot showing each trial effect size and confidence interval as well as the pooled effect size. D Bias-adjusted funnel plot showing each treatment separately. Figure S15: Rash odds network meta-analysis results. A Schematic diagram showing the network map for the treatments included in the analysis. B Rankogram showing the ranking probabilities for the least odds of causing this adverse event for each treatment. C Forest plot showing each trial effect size and confidence interval as well as the pooled effect size. D Bias-adjusted funnel plot showing each treatment separately. Figure S16: Vomiting odds network meta-analysis results. A Schematic diagram showing the network map for the treatments included in the analysis. B Rankogram showing the ranking probabilities for the least odds of causing this adverse event for each treatment. C Forest plot showing each trial effect size and confidence interval as well as the pooled effect size. D Bias-adjusted funnel plot showing each treatment separately. Figure S17: Adverse events grade ≥ 3 using generalized pairwise modelling. Table S7: Extracted data used for the analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Elmakaty, I., Abdo, R., Elsabagh, A. et al. Comparative efficacy and safety of PD-1/PD-L1 inhibitors in triple negative breast cancer: a systematic review and network meta-analysis of randomized controlled trials. Cancer Cell Int 23 , 90 (2023). https://doi.org/10.1186/s12935-023-02941-7
Download citation
Received : 11 January 2023
Accepted : 07 May 2023
Published : 11 May 2023
DOI : https://doi.org/10.1186/s12935-023-02941-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- PD-1/PD-L1 inhibitors
- Systematic review
Cancer Cell International
ISSN: 1475-2867
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
A systematic review collects all possible studies related to a given topic and design, and reviews and analyzes their results [ 1 ]. During the systematic review process, the quality of studies is evaluated, and a statistical meta-analysis of the study results is conducted on the basis of their quality. A meta-analysis is a valid, objective ...
We performed a systematic review and pooled analysis to evaluate the association between lymphopenia and overall survival (OS) for GBM patients undergoing chemotherapy and radiation therapy (RT). Following PRISMA guidelines, a systematic literature review of the MEDLINE database and abstracts from ASTRO, ASCO, and SNO annual meetings was conducted.
We can find the pooled effects of the ... Hatala R, Meade MO, et al. Understanding and applying the results of a systematic review and meta-analysis. User's guides to the medical literature: a ...
Review Manager (RevMan) is a web-based software that manages the entire literature review process and meta-analysis. The meta-analyst uploads all studies to RevMan library, where they can be managed and exanimated for inclusion. Like CMA, RevMan enables authors to conduct overall analysis and moderator analysis. 4.4.6.3 Stata
systematic review. The graphical output of meta-analysis is a forest plot which provides information on individual studies and the pooled effect. Systematic reviews of literature can be undertaken for all types of questions, and all types of study designs. This article
Literature reviews establish the foundation of academic inquires. However, in the planning field, we lack rigorous systematic reviews. In this article, through a systematic search on the methodology of literature review, we categorize a typology of literature reviews, discuss steps in conducting a systematic literature review, and provide suggestions on how to enhance rigor in literature ...
The results of our systematic review of the literature suggest the following: 1) DDS is a common occurrence, affecting up to 60% of neurosurgical patients undergoing RRT, 2) DDS is associated with a high mortality rate of 65% in this patient population, and 3) intermittent dialysis may be associated with an almost twofold increase in the risk ...
Design Systematic literature review and meta-analysis. Data sources PubMed, CINAHL, SPORTDiscusTM and EMBASE were systematically searched in October 2016 using relevant medical subject headings, keywords and a Boolean. ... Peak oxygen uptake in Paralympic sitting sports: A systematic literature review, meta- and pooled-data analysis. PLOS ONE ...
Patients have been diagnosed between 2000 and 2017 at our institution (n = 12) and revealed using a literature review (n = 98). 29 studies were identified by analysing systematic data bases ...
Systematic review. A systematic review is a summary of the medical literature that uses explicit and reproducible methods to systematically search, critically appraise, and synthesize on a specific issue. It synthesizes the results of multiple primary studies related to each other by using strategies that reduce biases and random errors.
Based on our comprehensive literature review, a total of 171 GBS, 40 PTS, 22 MG, 19 FNP, 5 SFN, and 1 Tolosa-Hunt syndrome cases following COVID-19 vaccination have been reported so far across the world. ... Tayebi AH, et al. Clinical features and outcomes of myasthenia gravis associated with COVID-19 vaccines: A systematic review and pooled ...
Meta-analysis is a statistical tool that provides pooled estimates of effect from the data extracted from individual studies in the systematic review. The graphical output of meta-analysis is a forest plot which provides information on individual studies and the pooled effect. Systematic reviews of literature can be undertaken for all types of ...
No data in the literature are available to guide diagnosis and therapy. Methods: We performed a pooled analysis and a systematic review of histopathological-confirmed strumal carcinoid cases published in the literature using the following keywords: "strumal carcinoid of the ovary", "strumal carcinoid case report". A case of strumal ...
A novel systematic review and meta-analysis has been made by our group. Thirty-nine clinical studies have been retrieved ( n = 25,566 unique patients), and the pooled incidence of AKI was 0.154 (95% CI, 0.107; 0.201, p < 0.0001) across the reports; in other words, the frequency of AKI in hospitalized patients with SARS-CoV-2 was 15.4%.
Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. ... Abar L, Cariolou M, et al. World Cancer Research Fund International: continuous update project—systematic literature review and meta-analysis of observational cohort studies on physical ...
Background Early water intake has gained widespread attention considering enhanced recovery after surgery (ERAS). In the present systematic evaluation and meta-analysis, we assessed the effects of early water intake on the incidence of vomiting and aspiration in adult patients who received general anaesthesia on regaining consciousness during the resuscitation period. Objective To ...
Measurement of beta-amyloid (Aβ) and phosphorylated tau (p-tau) levels offers the potential for early detection of neurocognitive impairment. Still, the probability of developing a clinical syndrome in the presence of these protein changes (A+ and T+) remains unclear. By performing a systematic review and meta-analysis, we investigated the risk of mild cognitive impairment (MCI) or dementia ...
This review/pooled analysis has several limitations. First, there is a high level of heterogeneity in the included studies, especially with regards to the oncological status/treatment stadium of the included patients, the protocol, the agents used for intrathoracic chemotherapy, the follow‐up, and the outcome assessment algorithms.
Multi drug-resistant TB. In the review of 148 studies that had studied multi drug resistant TB (sample size of 318,430 people), the I 2 test showed a high heterogeneity (I 2 = 99.6), and accordingly, random effects method was used to analyze the results. Considering the meta-analysis, the global pooled prevalence of multi-drug resistant TB was found to be 11.6% (95% CI: 9.1-14.5%).
No data in the literature are available to guide diagnosis and therapy. Methods: We performed a pooled analysis and a systematic review of histopathological-confirmed strumal carcinoid cases published in the literature using the following keywords: "strumal carcinoid of the ovary", "strumal carcinoid case report". A case of strumal carcinoid ...
Protocol and registration. This systematic review and meta-analysis is reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension for NMA Additional file 1: (Table S1) [].The NMA protocol was carried in accordance with a protocol that had been registered in the International Prospective Register of Systematic Reviews (PROSPERO) online database ...