Covidence website will be inaccessible as we upgrading our platform on Monday 23rd August at 10am AEST, / 2am CEST/1am BST (Sunday, 15th August 8pm EDT/5pm PDT)

The difference between a systematic review and a literature review
- Best Practice
Home | Blog | Best Practice | The difference between a systematic review and a literature review
Covidence takes a look at the difference between the two
Most of us are familiar with the terms systematic review and literature review. Both review types synthesise evidence and provide summary information. So what are the differences? What does systematic mean? And which approach is best 🤔 ?
‘ Systematic ‘ describes the review’s methods. It means that they are transparent, reproducible and defined before the search gets underway. That’s important because it helps to minimise the bias that would result from cherry-picking studies in a non-systematic way.
This brings us to literature reviews. Literature reviews don’t usually apply the same rigour in their methods. That’s because, unlike systematic reviews, they don’t aim to produce an answer to a clinical question. Literature reviews can provide context or background information for a new piece of research. They can also stand alone as a general guide to what is already known about a particular topic.
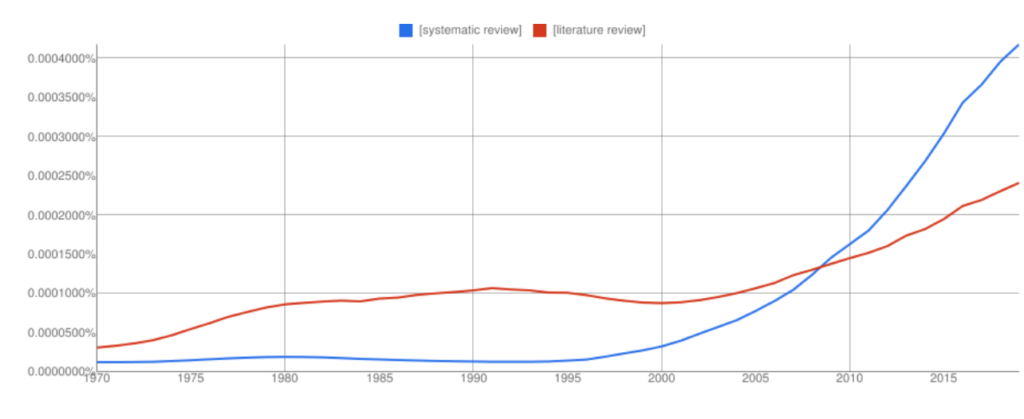
Interest in systematic reviews has grown in recent years and the frequency of ‘systematic reviews’ in Google books has overtaken ‘literature reviews’ (with all the usual Ngram Viewer warnings – it searches around 6% of all books, no journals).
Let’s take a look at the two review types in more detail to highlight some key similarities and differences 👀.
🙋🏾♂️ What is a systematic review?
Systematic reviews ask a specific question about the effectiveness of a treatment and answer it by summarising evidence that meets a set of pre-specified criteria.
The process starts with a research question and a protocol or research plan. A review team searches for studies to answer the question using a highly sensitive search strategy. The retrieved studies are then screened for eligibility using the inclusion and exclusion criteria (this is done by at least two people working independently). Next, the reviewers extract the relevant data and assess the quality of the included studies. Finally, the review team synthesises the extracted study data and presents the results. The process is shown in figure 2 .

The results of a systematic review can be presented in many ways and the choice will depend on factors such as the type of data. Some reviews use meta-analysis to produce a statistical summary of effect estimates. Other reviews use narrative synthesis to present a textual summary.
Covidence accelerates the screening, data extraction, and quality assessment stages of your systematic review. It provides simple workflows and easy collaboration with colleagues around the world.
When is it appropriate to do a systematic review?
If you have a clinical question about the effectiveness of a particular treatment or treatments, you could answer it by conducting a systematic review. Systematic reviews in clinical medicine often follow the PICO framework, which stands for:
👦 Population (or patients)
💊 Intervention
💊 Comparison
Here’s a typical example of a systematic review title that uses the PICO framework: Alarms [intervention] versus drug treatments [comparison] for the prevention of nocturnal enuresis [outcome] in children [population]
Key attributes
- Systematic reviews follow prespecified methods
- The methods are explicit and replicable
- The review team assesses the quality of the evidence and attempts to minimise bias
- Results and conclusions are based on the evidence
🙋🏻♀️ What is a literature review?
Literature reviews provide an overview of what is known about a particular topic. They evaluate the material, rather than simply restating it, but the methods used to do this are not usually prespecified and they are not described in detail in the review. The search might be comprehensive but it does not aim to be exhaustive. Literature reviews are also referred to as narrative reviews.
Literature reviews use a topical approach and often take the form of a discussion. Precision and replicability are not the focus, rather the author seeks to demonstrate their understanding and perhaps also present their work in the context of what has come before. Often, this sort of synthesis does not attempt to control for the author’s own bias. The results or conclusion of a literature review is likely to be presented using words rather than statistical methods.
When is it appropriate to do a literature review?
We’ve all written some form of literature review: they are a central part of academic research ✍🏾. Literature reviews often form the introduction to a piece of writing, to provide the context. They can also be used to identify gaps in the literature and the need to fill them with new research 📚.
- Literature reviews take a thematic approach
- They do not specify inclusion or exclusion criteria
- They do not answer a clinical question
- The conclusions might be influenced by the author’s own views
🙋🏽 Ok, but what is a systematic literature review?
A quick internet search retrieves a cool 200 million hits for ‘systematic literature review’. What strange hybrid is this 🤯🤯 ?
Systematic review methodology has its roots in evidence-based medicine but it quickly gained traction in other areas – the social sciences for example – where researchers recognise the value of being methodical and minimising bias. Systematic review methods are increasingly applied to the more traditional types of review, including literature reviews, hence the proliferation of terms like ‘systematic literature review’ and many more.
Beware of the labels 🚨. The terminology used to describe review types can vary by discipline and changes over time. To really understand how any review was done you will need to examine the methods critically and make your own assessment of the quality and reliability of each synthesis 🤓.
Review methods are evolving constantly as researchers find new ways to meet the challenge of synthesising the evidence. Systematic review methods have influenced many other review types, including the traditional literature review.
Covidence is a web-based tool that saves you time at the screening, selection, data extraction and quality assessment stages of your systematic review. It supports easy collaboration across teams and provides a clear overview of task status.
Get a glimpse inside Covidence and how it works

Laura Mellor. Portsmouth, UK
Perhaps you'd also like....

Data Extraction Tip 5: Communicate Regularly
The Covidence Global Scholarship recipients are putting evidence-based research into practice. We caught up with some of the winners to discover the impact of their work and find out more about their experiences.

Data Extraction Tip 4: Extract the Right Amount of Data

Data Extraction Tip 3: Pilot the Template
Better systematic review management, head office, working for an institution or organisation.
Find out why over 350 of the world’s leading institutions are seeing a surge in publications since using Covidence!
Request a consultation with one of our team members and start empowering your researchers:
By using our site you consent to our use of cookies to measure and improve our site’s performance. Please see our Privacy Policy for more information.

- Research Process
Systematic Literature Review or Literature Review?
- 3 minute read
Table of Contents
As a researcher, you may be required to conduct a literature review. But what kind of review do you need to complete? Is it a systematic literature review or a standard literature review? In this article, we’ll outline the purpose of a systematic literature review, the difference between literature review and systematic review, and other important aspects of systematic literature reviews.
What is a Systematic Literature Review?
The purpose of systematic literature reviews is simple. Essentially, it is to provide a high-level of a particular research question. This question, in and of itself, is highly focused to match the review of the literature related to the topic at hand. For example, a focused question related to medical or clinical outcomes.
The components of a systematic literature review are quite different from the standard literature review research theses that most of us are used to (more on this below). And because of the specificity of the research question, typically a systematic literature review involves more than one primary author. There’s more work related to a systematic literature review, so it makes sense to divide the work among two or three (or even more) researchers.
Your systematic literature review will follow very clear and defined protocols that are decided on prior to any review. This involves extensive planning, and a deliberately designed search strategy that is in tune with the specific research question. Every aspect of a systematic literature review, including the research protocols, which databases are used, and dates of each search, must be transparent so that other researchers can be assured that the systematic literature review is comprehensive and focused.
Most systematic literature reviews originated in the world of medicine science. Now, they also include any evidence-based research questions. In addition to the focus and transparency of these types of reviews, additional aspects of a quality systematic literature review includes:
- Clear and concise review and summary
- Comprehensive coverage of the topic
- Accessibility and equality of the research reviewed
Systematic Review vs Literature Review
The difference between literature review and systematic review comes back to the initial research question. Whereas the systematic review is very specific and focused, the standard literature review is much more general. The components of a literature review, for example, are similar to any other research paper. That is, it includes an introduction, description of the methods used, a discussion and conclusion, as well as a reference list or bibliography.
A systematic review, however, includes entirely different components that reflect the specificity of its research question, and the requirement for transparency and inclusion. For instance, the systematic review will include:
- Eligibility criteria for included research
- A description of the systematic research search strategy
- An assessment of the validity of reviewed research
- Interpretations of the results of research included in the review
As you can see, contrary to the general overview or summary of a topic, the systematic literature review includes much more detail and work to compile than a standard literature review. Indeed, it can take years to conduct and write a systematic literature review. But the information that practitioners and other researchers can glean from a systematic literature review is, by its very nature, exceptionally valuable.
This is not to diminish the value of the standard literature review. The importance of literature reviews in research writing is discussed in this article . It’s just that the two types of research reviews answer different questions, and, therefore, have different purposes and roles in the world of research and evidence-based writing.
Systematic Literature Review vs Meta Analysis
It would be understandable to think that a systematic literature review is similar to a meta analysis. But, whereas a systematic review can include several research studies to answer a specific question, typically a meta analysis includes a comparison of different studies to suss out any inconsistencies or discrepancies. For more about this topic, check out Systematic Review VS Meta-Analysis article.
Language Editing Plus
With Elsevier’s Language Editing Plus services , you can relax with our complete language review of your systematic literature review or literature review, or any other type of manuscript or scientific presentation. Our editors are PhD or PhD candidates, who are native-English speakers. Language Editing Plus includes checking the logic and flow of your manuscript, reference checks, formatting in accordance to your chosen journal and even a custom cover letter. Our most comprehensive editing package, Language Editing Plus also includes any English-editing needs for up to 180 days.

- Publication Recognition
How to Make a PowerPoint Presentation of Your Research Paper

- Manuscript Preparation
What is and How to Write a Good Hypothesis in Research?
You may also like.

Descriptive Research Design and Its Myriad Uses

Five Common Mistakes to Avoid When Writing a Biomedical Research Paper

Making Technical Writing in Environmental Engineering Accessible

To Err is Not Human: The Dangers of AI-assisted Academic Writing

When Data Speak, Listen: Importance of Data Collection and Analysis Methods

Choosing the Right Research Methodology: A Guide for Researchers

Why is data validation important in research?

Writing a good review article
Input your search keywords and press Enter.
Penn State University Libraries
- Home-Articles and Databases
- Asking the clinical question
- PICO & Finding Evidence
- Evaluating the Evidence
- Systematic Review vs. Literature Review
- Ethical & Legal Issues for Nurses
- Nursing Library Instruction Course
- Data Management Toolkit This link opens in a new window
- Useful Nursing Resources
- Writing Resources
- LionSearch and Finding Articles
- The Catalog and Finding Books
Know the Difference! Systematic Review vs. Literature Review
It is common to confuse systematic and literature reviews as both are used to provide a summary of the existent literature or research on a specific topic. Even with this common ground, both types vary significantly. Please review the following chart (and its corresponding poster linked below) for the detailed explanation of each as well as the differences between each type of review.
- What's in a name? The difference between a Systematic Review and a Literature Review, and why it matters by Lynn Kysh, MLIS, University of Southern California - Norris Medical Library
- << Previous: Evaluating the Evidence
- Next: Ethical & Legal Issues for Nurses >>
- Last Updated: Mar 1, 2024 11:54 AM
- URL: https://guides.libraries.psu.edu/nursing

Literature Reviews
- Overview of Literature Reviews and Systematic Reviews
- How to Get Started and Developing a Research Question
- Finding and Evaluating Sources
- Citations This link opens in a new window
- Synthesizing Sources
- Writing the Literature Review
- Systematic Reviews This link opens in a new window
- Suggested Readings
Literature Reviews and Systematic Reviews
- What is a Literature Review
- What is a Systematic Review
- Literature Review vs. Systematic Review
A literature review summarizes and analyzes the relevant publications on a topic. It demonstrates to your readers that you are knowledgeable of the ongoing scholarly conversation and how your research fits within the broader field of study. An effective literature review will lay the foundation for the importance of your stated problem and research question.
Literature Reviews: An Overview for Graduate Students from NC State University Libraries on Vimeo .
A systematic review attempts to identify, appraise and synthesize all available relevant evidence to answer a specific, focused research question. Researchers conducting systematic reviews use standardized, systematic methods and pre-selected eligibility criteria to reduce the risk of bias in identifying, selecting, and analyzing relevant studies.
Prepared by the Cochrane Consumers and Communication Group, La Trobe University and generously support by Cochrane Australia. Written by Jack Nunn and Sophie Hill.
Robinson, P. and Lowe, J. (2015), Literature reviews vs systematic reviews. Australian and New Zealand Journal of Public Health, 39: 103-103. https://doi.org/10.1111/1753-6405.12393
Why Do a Literature Review
Why do a literature review?
- To increase your knowledge of this topic
- To identify other researchers and seminal works in this field of study
- To provide context for your work
- To locate gaps in the literature
- To demonstrate the credibility of your research
What is 'The Literature'
What is 'the literature' that is reviewed in a literature review?
‘The literature’ consists of the published works that document a scholarly conversation in a field of study, including:
- scholarly articles
- conference proceedings
- dissertations
The literature can also include newspapers, encyclopedias, textbooks, as well as websites and reports written by government agencies and professional organizations ("grey literature").
Attribution
The content of this page was developed from Chapter 1, "Introduction", and Chapter 2, "What is a Literature Review?", in:
Frederiksen, L., & Phelps, S. F. (2017). Literature reviews for education and nursing graduate students. Rebus Community. https://open.umn.edu/opentextbooks/textbooks/literature-reviews-for-education-and-nursing-graduate-students
- Next: How to Get Started and Developing a Research Question >>
- Last Updated: Nov 30, 2023 1:07 PM
- URL: https://libguides.chapman.edu/literature_reviews

Start your free trial
Arrange a trial for your organisation and discover why FSTA is the leading database for reliable research on the sciences of food and health.
REQUEST A FREE TRIAL
- Thought for Food Blog
What is the difference between a systematic review and a systematic literature review?
By Carol Hollier on 07-Jan-2020 12:42:03

For those not immersed in systematic reviews, understanding the difference between a systematic review and a systematic literature review can be confusing. It helps to realise that a “systematic review” is a clearly defined thing, but ambiguity creeps in around the phrase “systematic literature review” because people can and do use it in a variety of ways.
A systematic review is a research study of research studies. To qualify as a systematic review, a review needs to adhere to standards of transparency and reproducibility. It will use explicit methods to identify, select, appraise, and synthesise empirical results from different but similar studies. The study will be done in stages:
- In stage one, the question, which must be answerable, is framed
- Stage two is a comprehensive literature search to identify relevant studies
- In stage three the identified literature’s quality is scrutinised and decisions made on whether or not to include each article in the review
- In stage four the evidence is summarised and, if the review includes a meta-analysis, the data extracted; in the final stage, findings are interpreted. [1]
Some reviews also state what degree of confidence can be placed on that answer, using the GRADE scale. By going through these steps, a systematic review provides a broad evidence base on which to make decisions about medical interventions, regulatory policy, safety, or whatever question is analysed. By documenting each step explicitly, the review is not only reproducible, but can be updated as more evidence on the question is generated.
Sometimes when people talk about a “systematic literature review”, they are using the phrase interchangeably with “systematic review”. However, people can also use the phrase systematic literature review to refer to a literature review that is done in a fairly systematic way, but without the full rigor of a systematic review.
For instance, for a systematic review, reviewers would strive to locate relevant unpublished studies in grey literature and possibly by contacting researchers directly. Doing this is important for combatting publication bias, which is the tendency for studies with positive results to be published at a higher rate than studies with null results. It is easy to understand how this well-documented tendency can skew a review’s findings, but someone conducting a systematic literature review in the loose sense of the phrase might, for lack of resource or capacity, forgo that step.
Another difference might be in who is doing the research for the review. A systematic review is generally conducted by a team including an information professional for searches and a statistician for meta-analysis, along with subject experts. Team members independently evaluate the studies being considered for inclusion in the review and compare results, adjudicating any differences of opinion. In contrast, a systematic literature review might be conducted by one person.
Overall, while a systematic review must comply with set standards, you would expect any review called a systematic literature review to strive to be quite comprehensive. A systematic literature review would contrast with what is sometimes called a narrative or journalistic literature review, where the reviewer’s search strategy is not made explicit, and evidence may be cherry-picked to support an argument.
FSTA is a key tool for systematic reviews and systematic literature reviews in the sciences of food and health.

The patents indexed help find results of research not otherwise publicly available because it has been done for commercial purposes.
The FSTA thesaurus will surface results that would be missed with keyword searching alone. Since the thesaurus is designed for the sciences of food and health, it is the most comprehensive for the field.
All indexing and abstracting in FSTA is in English, so you can do your searching in English yet pick up non-English language results, and get those results translated if they meet the criteria for inclusion in a systematic review.
FSTA includes grey literature (conference proceedings) which can be difficult to find, but is important to include in comprehensive searches.
FSTA content has a deep archive. It goes back to 1969 for farm to fork research, and back to the late 1990s for food-related human nutrition literature—systematic reviews (and any literature review) should include not just the latest research but all relevant research on a question.
You can also use FSTA to find literature reviews.
FSTA allows you to easily search for review articles (both narrative and systematic reviews) by using the subject heading or thesaurus term “REVIEWS" and an appropriate free-text keyword.
On the Web of Science or EBSCO platform, an FSTA search for reviews about cassava would look like this: DE "REVIEWS" AND cassava.
On the Ovid platform using the multi-field search option, the search would look like this: reviews.sh. AND cassava.af.
In 2011 FSTA introduced the descriptor META-ANALYSIS, making it easy to search specifically for systematic reviews that include a meta-analysis published from that year onwards.
On the EBSCO or Web of Science platform, an FSTA search for systematic reviews with meta-analyses about staphylococcus aureus would look like this: DE "META-ANALYSIS" AND staphylococcus aureus.
On the Ovid platform using the multi-field search option, the search would look like this: meta-analysis.sh. AND staphylococcus aureus.af.
Systematic reviews with meta-analyses published before 2011 are included in the REVIEWS controlled vocabulary term in the thesaurus.
An easy way to locate pre-2011 systematic reviews with meta-analyses is to search the subject heading or thesaurus term "REVIEWS" AND meta-analysis as a free-text keyword AND another appropriate free-text keyword.
On the Web of Science or EBSCO platform, the FSTA search would look like this: DE "REVIEWS" AND meta-analysis AND carbohydrate*
On the Ovid platform using the multi-field search option, the search would look like this: reviews .s h. AND meta-analysis.af. AND carbohydrate*.af.
Related resources:
- Literature Searching Best Practise Guide
- Predatory publishing: Investigating researchers’ knowledge & attitudes
- The IFIS Expert Guide to Journal Publishing
Library image by Paul Schafer , microscope image by Matthew Waring , via Unsplash.

- FSTA - Food Science & Technology Abstracts
- IFIS Collections
- Resources Hub
- Diversity Statement
- Sustainability Commitment
- Company news
- Frequently Asked Questions
- Privacy Policy
- Terms of Use for IFIS Collections
Ground Floor, 115 Wharfedale Road, Winnersh Triangle, Wokingham, Berkshire RG41 5RB
Get in touch with IFIS
© International Food Information Service (IFIS Publishing) operating as IFIS – All Rights Reserved | Charity Reg. No. 1068176 | Limited Company No. 3507902 | Designed by Blend

Literature Reviews vs Systematic Reviews: What’s the Difference?

A lot of times, we compare literature reviews vs systematic reviews. It is like comparing an orange with a tangerine – the same group but different fruits! A literature review and a systematic review are both research methods used to analyze the existing literature on a topic. But what exactly is the difference between these two? A literature review focuses on specific works on a given subject and analyzes them in-depth. A systematic review, on the other hand, examines the scope of available data. It looks at it from a neutral perspective.
However, there is also some overlap between the two. In some cases, you may need to use elements of one method as part of another one. Here’s more about this pair of research methods. Keep reading to find out more.
In This Article – Literature reviews vs systematic reviews

What is a Literature Review?
What is a systematic review.
- How Are They Different?
- How are they the same?
When You’d Use a Literature Review
When you’d use a systematic review, in previous articles.
- White Paper in Marketing: What, Why & How
- Reviewing a Document & Giving Feedback – A Quick Guide
- 5 Ways to Turn Your Job Interview “Weaknesses” Into Strengths
- How to Write a Reference List
- How To Become a Medical Writer: A Guide for Beginners
A literature review is a research method used to analyze the existing literature on a given topic. It’s certainly not the only method you can use to conduct research, but it’s a very common one. It’s particularly useful when you’re doing a research project that builds on or critiques existing research. A literature review can help you understand the current state of knowledge on a given subject. It can also direct your research toward areas that need more exploration.
There are different types of literature reviews. A descriptive literature review simply describes what has been written on a given topic. A conceptual literature review aims to make sense of the existing literature on a topic. A critical literature review examines the strengths and weaknesses of existing research.
A systematic review is a research method that aims to summarise existing literature on a given topic and make it more accessible to readers. It is often used in place of a literature review. It can be particularly useful when the research being reviewed is limited in some way. For example, if it’s outdated or if it only covers a specific aspect of the topic in question. Systematic reviews are generally more thorough than literature reviews.
They look at the scope of available data and draw conclusions based on that, rather than just analyzing a specific set of studies. In many cases, you can’t use one method in place of another. However, there are some situations in which you can use elements of one method in another. For example, you may need to use a literature review to gather information for a systematic review.
Literature Reviews vs Systematic Reviews – How Are They Different?
A literature review is a more in-depth analysis of the existing literature than a systematic review is. A systematic review looks at the overall scope of existing data and makes conclusions based on that. On the other hand, a literature review focuses on specific works and analyzes them in-depth. A systematic review can be used to understand the current state of knowledge on a topic, while a literature review focuses on specific works. However, a systematic review can certainly be in-depth. The amount of research that goes into it will impact the level of detail it discusses.
The main difference is that a literature review is a critical analysis of specific works, while a systematic review is a general analysis of the available data. A literature review is often qualitative and focused on a specific topic. A systematic review can be quantitative and cover a broader topic.
Literature Reviews vs Systematic Reviews – How Are They The Same?
A literature review and a systematic review are both research methods that are used to analyze the existing literature on a topic. They’re both very similar in that they examine the existing data and attempt to make sense of it. These methods are often used together in order to create more in-depth research. For example, you might conduct a literature review to examine the current research on a topic and then use that to inform the design of a systematic review.
Literature reviews are often used as a preliminary step before more in-depth research. For example, if you’re writing a paper that critiques an existing theory or paper, you may want to first examine the literature on that topic. You can then use what you find in your literature review to frame your critique and support your arguments with evidence. Literature reviews can be useful in a wide variety of subjects, though some are more common than others.
For instance, if you’re studying a specific author, you may want to conduct a literature review of their works. If you’re studying a specific theory or concept, you might want to examine the literature related to it. If you’re studying a specific field, like psychology or sociology, you’re likely to come across a lot of literature related to that field.
Systematic reviews are often used as a way to gather data and create a more thorough overview of the current state of knowledge on a given topic. They can also be used as a way to create a foundation for further research on a topic. You may choose to conduct a systematic review if you need to access data that is currently inaccessible because it is outdated or if you need to access data that only covers a specific aspect of the topic you’re interested in.
For example, if you’re studying a specific topic in the field of public health, you may need to examine the current data on hygiene practices in the developing world. You may find that there are very few studies on this topic, but you can use a systematic review to examine existing data on hygiene practices in first-world countries and use that as a starting point for your research.
Literature reviews vs systematic reviews- Key takeaways
Literature reviews and systematic reviews are both methods used to analyze the existing literature on a given topic. A literature review examines specific works on a topic, while a systematic review examines the overall scope of existing data and makes conclusions based on that. These methods are often used together in order to create more in-depth research. For example, you might conduct a literature review to examine the current research on a topic and then use that to inform the design of a systematic review.
When comparing literature reviews vs systematic reviews, remember that these methods are very similar in that they examine the existing data and attempt to make sense of it. These methods are often used together in order to create more in-depth research. For example, you might conduct a literature review to examine the current research on a topic and then use that to inform the design of a systematic review.
Similar Posts

Finding Topics for Literature Review: The Pragmatic Guide
A literature review is a crucial part of any essay or research paper. It’s essentially a summary of other people’s work in your chosen field. Choosing topics for literature review is often hard. Topics should be broad and general, and should not focus on any specific piece of research or an individual author. Instead, it…

How to Write a Clinical Evaluation Report in Pharma
A clinical evaluation report (CER) is an essential document that records the findings of a clinical trial. It plays an important role in determining the safety and efficacy of a drug. A CER is prepared by a medical researcher after concluding the evaluation process of participants from clinical trials. The function of a CER is…

How To Record Audio on PowerPoint: A Guide to Make the Most of Your Presentation
Do you find yourself making the same PPT presentation over and over again? Are you tired of giving the same old presentations? If this sounds like you, it’s time to take your PPT game up. But, how do you record audio on Powerpoint? Instead of using the same old presentation which can get monotonous for…

10 Proven Tips to Improve Your Medical Writing
What are the best tips to improve your medical writing? Well, good medical writing is essential for the medical industry to operate efficiently. If the medical industry produces bad medical writing it will negatively affect the people it is trying to help. However, writing good, engaging content can be hard for writers. Everyone’s writing skills…

5 Steps to Perform Regression Analysis in Excel: A Beginner’s Guide to Data Analysis
Regression analysis is a common technique used in data analysis to understand and predict the relationship between variables. If you’re new to data analysis, the concept of regression analysis may seem intimidating. But don’t worry, it’s not as complex as it sounds! In this article, we’ll take you through the five steps to perform a…

What is an Investigator’s Brochure in Pharma?
The investigator’s brochure (popularly referred to as IB) is an important tool for the pharmaceutical company to share information about the new drug and its indications with healthcare professionals. An investigator’s brochure keeps all clinical and nonclinical data on an investigational product (drug, supplement, device, or other product) under investigation. Staff constantly update the IB…
Privacy Overview
The Library Life Pulse Survey has been sent to all Manchester Met students to hear their views and feedback. Find out more about the survey and how you can help to improve the service we provide.
- Manchester Met Library
- Special Collections Museum
- North West Film Archive
- Poetry Library
- Researcher Profiles
- Research Metrics
What is a Systematic Review?
- Review Types
- Preparation and Planning
- Search Methods
- Grey Literature
- Documentation and Data Management
- Further Information and Resources
- Cultural Services
- Open Research
- What is Research Data Management
- Legal and Governance
- Data Management Plans
- Collecting and Organising Data
- Storing Data
- Sharing Data
- Sensitive Data
- Publishing Open Access
- What to Consider
- Funding for Open Access
- Funder Requirements
- Deposit in e-space
- Search e-space
- Rights Retention
- Workshops and Development
- Research Support
- Systematic Reviews
- Research Data Management
- Open Access Publishing
- email us at [email protected]
What is a systematic review?
A systematic review is a firmly structured literature review, undertaken according to a fixed plan, system or method. As such, it is highly focused on a particular and explicit topic area with strict research parameters. Systematic reviews will often have a detailed plan known as a protocol, which is a statement of the approach and methods to be used in the review prior to undertaking it.
Systematic review methodology is explicit and precise because it aims to minimise bias, thereby enhancing the reliability of any conclusions. It is therefore considered an evidence-based approach. Systematic reviews are commonly used by health professionals, but also policy makers and researchers.
There is information about the difference between a systematic review and a literature review on this page. If you are undertaking systematic approach to a literature review, however, you might find certain aspects of this guide useful.
LITERATURE REVIEW VS SYSTEMATIC REVIEW
You can find further information on literature reviews on our literature reviews page .
How we can help
What we need you to do: .
- Have a firm idea of your research question or area
- List your main keywords and alternatives. You may want to use a table to organise your keywords.
- Think about how you will use your keywords to search using connectors such as AND/OR
- Define what you want to include and exclude from your search
- Consider where you want to search
- Run some initial searches and identify any problems or issues you want to discuss
What your Librarian can help you with:
- Identifying relevant databases and other subject resources that could be used to supplement your review
- Demonstrating library resources for use in the review
- Replicating searches on other databases and resources
- Reviewing your search strategy/approach
- Directing you to referencing software support
- Suggesting ways to save and document your search results
- Helping to locate difficult to find material, using the Request It! service
- PREPARATION AND PLANNING
- review types
- SEARCH METHODS
- GREY LITERATURE
- DOCUMENTATION AND DATA MANAGEMENT
- FURTHER INFORMATION AND RESOURCES

Library Services
UCL LIBRARY SERVICES
- Guides and databases
- Library skills
- Systematic reviews
What are systematic reviews?
- Types of systematic reviews
- Formulating a research question
- Identifying studies
- Searching databases
- Describing and appraising studies
- Synthesis and systematic maps
- Software for systematic reviews
- Online training and support
- Live and face to face training
- Individual support
- Further help

Systematic reviews are a type of literature review of research which require equivalent standards of rigour as primary research. They have a clear, logical rationale that is reported to the reader of the review. They are used in research and policymaking to inform evidence-based decisions and practice. They differ from traditional literature reviews particularly in the following elements of conduct and reporting.
Systematic reviews:
- use explicit and transparent methods
- are a piece of research following a standard set of stages
- are accountable, replicable and updateable
- involve users to ensure a review is relevant and useful.
For example, systematic reviews (like all research) should have a clear research question, and the perspective of the authors in their approach to addressing the question is described. There are clearly described methods on how each study in a review was identified, how that study was appraised for quality and relevance and how it is combined with other studies in order to address the review question. A systematic review usually involves more than one person in order to increase the objectivity and trustworthiness of the reviews methods and findings.
Research protocols for systematic reviews may be peer-reviewed and published or registered in a suitable repository to help avoid duplication of reviews and for comparisons to be made with the final review and the planned review.
- History of systematic reviews to inform policy (EPPI-Centre)
- Six reasons why it is important to be systematic (EPPI-Centre)
- Evidence Synthesis International (ESI): Position Statement Describes the issues, principles and goals in synthesising research evidence to inform policy, practice and decisions
On this page
Should all literature reviews be 'systematic reviews', different methods for systematic reviews, reporting standards for systematic reviews.
Literature reviews provide a more complete picture of research knowledge than is possible from individual pieces of research. This can be used to: clarify what is known from research, provide new perspectives, build theory, test theory, identify research gaps or inform research agendas.
A systematic review requires a considerable amount of time and resources, and is one type of literature review.
If the purpose of a review is to make justifiable evidence claims, then it should be systematic, as a systematic review uses rigorous explicit methods. The methods used can depend on the purpose of the review, and the time and resources available.
A 'non-systematic review' might use some of the same methods as systematic reviews, such as systematic approaches to identify studies or quality appraise the literature. There may be times when this approach can be useful. In a student dissertation, for example, there may not be the time to be fully systematic in a review of the literature if this is only one small part of the thesis. In other types of research, there may also be a need to obtain a quick and not necessarily thorough overview of a literature to inform some other work (including a systematic review). Another example, is where policymakers, or other people using research findings, want to make quick decisions and there is no systematic review available to help them. They have a choice of gaining a rapid overview of the research literature or not having any research evidence to help their decision-making.
Just like any other piece of research, the methods used to undertake any literature review should be carefully planned to justify the conclusions made.
Finding out about different types of systematic reviews and the methods used for systematic reviews, and reading both systematic and other types of review will help to understand some of the differences.
Typically, a systematic review addresses a focussed, structured research question in order to inform understanding and decisions on an area. (see the Formulating a research question section for examples).
Sometimes systematic reviews ask a broad research question, and one strategy to achieve this is the use of several focussed sub-questions each addressed by sub-components of the review.
Another strategy is to develop a map to describe the type of research that has been undertaken in relation to a research question. Some maps even describe over 2,000 papers, while others are much smaller. One purpose of a map is to help choose a sub-set of studies to explore more fully in a synthesis. There are also other purposes of maps: see the box on systematic evidence maps for further information.
Reporting standards specify minimum elements that need to go into the reporting of a review. The reporting standards refer mainly to methodological issues but they are not as detailed or specific as critical appraisal for the methodological standards of conduct of a review.
A number of organisations have developed specific guidelines and standards for both the conducting and reporting on systematic reviews in different topic areas.
- PRISMA PRISMA is a reporting standard and is an acronym for Preferred Reporting Items for Systematic Reviews and Meta-Analyses. The Key Documents section of the PRISMA website links to a checklist, flow diagram and explanatory notes. PRISMA is less useful for certain types of reviews, including those that are iterative.
- eMERGe eMERGe is a reporting standard that has been developed for meta-ethnographies, a qualitative synthesis method.
- ROSES: RepOrting standards for Systematic Evidence Syntheses Reporting standards, including forms and flow diagram, designed specifically for systematic reviews and maps in the field of conservation and environmental management.
Useful books about systematic reviews
Systematic approaches to a successful literature review
An introduction to systematic reviews
Cochrane handbook for systematic reviews of interventions
Systematic reviews: crd's guidance for undertaking reviews in health care.
Finding what works in health care: Standards for systematic reviews
Systematic Reviews in the Social Sciences
Meta-analysis and research synthesis.
Research Synthesis and Meta-Analysis
Doing a Systematic Review
Literature reviews.
- What is a literature review?
- Why are literature reviews important?
- << Previous: Systematic reviews
- Next: Types of systematic reviews >>
- Last Updated: Apr 4, 2024 10:09 AM
- URL: https://library-guides.ucl.ac.uk/systematic-reviews
Literature Review vs Systematic Review
Literature review vs. systematic review.
- Primary vs. Secondary Sources
- Databases and Articles
- Specific Journal or Article
Your Librarian

Additional Resources
Be sure to check out these related LibGuides when performing your research.
- Culture & Health
- Health Literacy
- Nutrition & Food Science
- Biological Sciences
“In evidence-based practice, systematic reviews are considered one of the highest levels of information.” - Kysh, Lynn (2013): Difference between a systematic review and a literature review. [figshare]. Available at: http://dx.doi.org/10.6084/m9.figshare.766364
Library Services
Your Library Account is the same as your SJSU One Account (Student/Faculty ID # and your SJSUOne Password)
You will need to set up the following accounts in order to help you with your research:
1) Citation managers , such as Zotero, and Paperpile, are programs that allow the users to collect, organize, and cite sources. They also allow for collaboration, and some provide the ability to store and annotate PDFs within the system.
2) Your SJSUOne credentials work for Interlibrary Loan (ILLiad) . Request non-SJSU Library owned or subscribed content through this free service. Allow 3-10 days for articles and up to two weeks for books.
3) Customize Google Scholar to search SJSU holdings (for full-text articles) and to export citations to a citation manager such as Paperpile. You can also access the Google Scholar tab on the home page of the library's website, which will automatically connect your search results with links to the full-text articles in the library's databases.
- Next: Literature Review vs. Systematic Review >>
- Last Updated: Dec 15, 2023 10:19 AM
- URL: https://libguides.sjsu.edu/LitRevVSSysRev
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Family Med Prim Care
- v.2(1); Jan-Mar 2013
Systematic Reviews and Meta-analysis: Understanding the Best Evidence in Primary Healthcare
S. gopalakrishnan.
Department of Community Medicine, SRM Medical College, Hospital and Research Centre, Kattankulathur, Tamil Nadu, India
P. Ganeshkumar
Healthcare decisions for individual patients and for public health policies should be informed by the best available research evidence. The practice of evidence-based medicine is the integration of individual clinical expertise with the best available external clinical evidence from systematic research and patient's values and expectations. Primary care physicians need evidence for both clinical practice and for public health decision making. The evidence comes from good reviews which is a state-of-the-art synthesis of current evidence on a given research question. Given the explosion of medical literature, and the fact that time is always scarce, review articles play a vital role in decision making in evidence-based medical practice. Given that most clinicians and public health professionals do not have the time to track down all the original articles, critically read them, and obtain the evidence they need for their questions, systematic reviews and clinical practice guidelines may be their best source of evidence. Systematic reviews aim to identify, evaluate, and summarize the findings of all relevant individual studies over a health-related issue, thereby making the available evidence more accessible to decision makers. The objective of this article is to introduce the primary care physicians about the concept of systematic reviews and meta-analysis, outlining why they are important, describing their methods and terminologies used, and thereby helping them with the skills to recognize and understand a reliable review which will be helpful for their day-to-day clinical practice and research activities.
Introduction
Evidence-based healthcare is the integration of best research evidence with clinical expertise and patient values. Green denotes, “Using evidence from reliable research, to inform healthcare decisions, has the potential to ensure best practice and reduce variations in healthcare delivery.” However, incorporating research into practice is time consuming, and so we need methods of facilitating easy access to evidence for busy clinicians.[ 1 ] Ganeshkumar et al . mentioned that nearly half of the private practitioners in India were consulting more than 4 h per day in a locality,[ 2 ] which explains the difficulty of them in spending time in searching evidence during consultation. Ideally, clinical decision making ought to be based on the latest evidence available. However, to keep abreast with the continuously increasing number of publications in health research, a primary healthcare professional would need to read an insurmountable number of articles every day, covered in more than 13 million references and over 4800 biomedical and health journals in Medline alone. With the view to address this challenge, the systematic review method was developed. Systematic reviews aim to inform and facilitate this process through research synthesis of multiple studies, enabling increased and efficient access to evidence.[ 1 , 3 , 4 ]
Systematic reviews and meta-analyses have become increasingly important in healthcare settings. Clinicians read them to keep up-to-date with their field and they are often used as a starting point for developing clinical practice guidelines. Granting agencies may require a systematic review to ensure there is justification for further research and some healthcare journals are moving in this direction.[ 5 ]
This article is intended to provide an easy guide to understand the concept of systematic reviews and meta-analysis, which has been prepared with the aim of capacity building for general practitioners and other primary healthcare professionals in research methodology and day-to-day clinical practice.
The purpose of this article is to introduce readers to:
- The two approaches of evaluating all the available evidence on an issue i.e., systematic reviews and meta-analysis,
- Discuss the steps in doing a systematic review,
- Introduce the terms used in systematic reviews and meta-analysis,
- Interpret results of a meta-analysis, and
- The advantages and disadvantages of systematic review and meta-analysis.
Application
What is the effect of antiviral treatment in dengue fever? Most often a primary care physician needs to know convincing answers to questions like this in a primary care setting.
To find out the solutions or answers to a clinical question like this, one has to refer textbooks, ask a colleague, or search electronic database for reports of clinical trials. Doctors need reliable information on such problems and on the effectiveness of large number of therapeutic interventions, but the information sources are too many, i.e., nearly 20,000 journals publishing 2 million articles per year with unclear or confusing results. Because no study, regardless of its type, should be interpreted in isolation, a systematic review is generally the best form of evidence.[ 6 ] So, the preferred method is a good summary of research reports, i.e., systematic reviews and meta-analysis, which will give evidence-based answers to clinical situations.
There are two fundamental categories of research: Primary research and secondary research. Primary research is collecting data directly from patients or population, while secondary research is the analysis of data already collected through primary research. A review is an article that summarizes a number of primary studies and may draw conclusions on the topic of interest which can be traditional (unsystematic) or systematic.
Terminologies
Systematic review.
A systematic review is a summary of the medical literature that uses explicit and reproducible methods to systematically search, critically appraise, and synthesize on a specific issue. It synthesizes the results of multiple primary studies related to each other by using strategies that reduce biases and random errors.[ 7 ] To this end, systematic reviews may or may not include a statistical synthesis called meta-analysis, depending on whether the studies are similar enough so that combining their results is meaningful.[ 8 ] Systematic reviews are often called overviews.
The evidence-based practitioner, David Sackett, defines the following terminologies.[ 3 ]
- Review: The general term for all attempts to synthesize the results and conclusions of two or more publications on a given topic.
- Overview: When a review strives to comprehensively identify and track down all the literature on a given topic (also called “systematic literature review”).
- Meta-analysis: A specific statistical strategy for assembling the results of several studies into a single estimate.
Systematic reviews adhere to a strict scientific design based on explicit, pre-specified, and reproducible methods. Because of this, when carried out well, they provide reliable estimates about the effects of interventions so that conclusions are defensible. Systematic reviews can also demonstrate where knowledge is lacking. This can then be used to guide future research. Systematic reviews are usually carried out in the areas of clinical tests (diagnostic, screening, and prognostic), public health interventions, adverse (harm) effects, economic (cost) evaluations, and how and why interventions work.[ 9 ]
Cochrane reviews
Cochrane reviews are systematic reviews undertaken by members of the Cochrane Collaboration which is an international not-for-profit organization that aims to help people to make well-informed decisions about healthcare by preparing, maintaining, and promoting the accessibility of systematic reviews of the effects of healthcare interventions.
Cochrane Primary Health Care Field is a systematic review of primary healthcare research on prevention, treatment, rehabilitation, and diagnostic test accuracy. The overall aim and mission of the Primary Health Care Field is to promote the quality, quantity, dissemination, accessibility, applicability, and impact of Cochrane systematic reviews relevant to people who work in primary care and to ensure proper representation in the interests of primary care clinicians and consumers in Cochrane reviews and review groups, and in other entities. This field would serve to coordinate and promote the mission of the Cochrane Collaboration within the primary healthcare disciplines, as well as ensuring that primary care perspectives are adequately represented within the Collaboration.[ 10 ]
Meta-analysis
A meta-analysis is the combination of data from several independent primary studies that address the same question to produce a single estimate like the effect of treatment or risk factor. It is the statistical analysis of a large collection of analysis and results from individual studies for the purpose of integrating the findings.[ 11 ] The term meta-analysis has been used to denote the full range of quantitative methods for research reviews.[ 12 ] Meta-analyses are studies of studies.[ 13 ] Meta-analysis provides a logical framework to a research review where similar measures from comparable studies are listed systematically and the available effect measures are combined wherever possible.[ 14 ]
The fundamental rationale of meta-analysis is that it reduces the quantity of data by summarizing data from multiple resources and helps to plan research as well as to frame guidelines. It also helps to make efficient use of existing data, ensuring generalizability, helping to check consistency of relationships, explaining data inconsistency, and quantifies the data. It helps to improve the precision in estimating the risk by using explicit methods.
Therefore, “systematic review” will refer to the entire process of collecting, reviewing, and presenting all available evidence, while the term “meta-analysis” will refer to the statistical technique involved in extracting and combining data to produce a summary result.[ 15 ]
Steps in doing systematic reviews/meta-analysis
Following are the six fundamental essential steps while doing systematic review and meta-analysis.[ 16 ]
Define the question
This is the most important part of systematic reviews/meta-analysis. The research question for the systematic reviews may be related to a major public health problem or a controversial clinical situation which requires acceptable intervention as a possible solution to the present healthcare need of the community. This step is most important since the remaining steps will be based on this.
Reviewing the literature
This can be done by going through scientific resources such as electronic database, controlled clinical trials registers, other biomedical databases, non-English literatures, “gray literatures” (thesis, internal reports, non–peer-reviewed journals, pharmaceutical industry files), references listed in primary sources, raw data from published trials and other unpublished sources known to experts in the field. Among the available electronic scientific database, the popular ones are PUBMED, MEDLINE, and EMBASE.
Sift the studies to select relevant ones
To select the relevant studies from the searches, we need to sift through the studies thus identified. The first sift is pre-screening, i.e., to decide which studies to retrieve in full, and the second sift is selection which is to look again at these studies and decide which are to be included in the review. The next step is selecting the eligible studies based on similar study designs, year of publication, language, choice among multiple articles, sample size or follow-up issues, similarity of exposure, and or treatment and completeness of information.
It is necessary to ensure that the sifting includes all relevant studies like the unpublished studies (desk drawer problem), studies which came with negative conclusions or were published in non-English journals, and studies with small sample size.
Assess the quality of studies
The steps undertaken in evaluating the study quality are early definition of study quality and criteria, setting up a good scoring system, developing a standard form for assessment, calculating quality for each study, and finally using this for sensitivity analysis.
For example, the quality of a randomized controlled trial can be assessed by finding out the answers to the following questions:
- Was the assignment to the treatment groups really random?
- Was the treatment allocation concealed?
- Were the groups similar at baseline in terms of prognostic factors?
- Were the eligibility criteria specified?
- Were the assessors, the care provider, and the patient blinded?
- Were the point estimates and measure of variability presented for the primary outcome measure?
- Did the analyses include intention-to-treat analysis?
Calculate the outcome measures of each study and combine them
We need a standard measure of outcome which can be applied to each study on the basis of its effect size. Based on their type of outcome, following are the measures of outcome: Studies with binary outcomes (cured/not cured) have odds ratio, risk ratio; studies with continuous outcomes (blood pressure) have means, difference in means, standardized difference in means (effect sizes); and survival or time-to-event data have hazard ratios.
Combining studies
Homogeneity of different studies can be estimated at a glance from a forest plot (explained below). For example, if the lower confidence interval of every trial is below the upper of all the others, i.e., the lines all overlap to some extent, then the trials are homogeneous. If some lines do not overlap at all, these trials may be said to be heterogeneous.
The definitive test for assessing the heterogeneity of studies is a variant of Chi-square test (Mantel–Haenszel test). The final step is calculating the common estimate and its confidence interval with the original data or with the summary statistics from all the studies. The best estimate of treatment effect can be derived from the weighted summary statistics of all studies which will be based on weighting to sample size, standard errors, and other summary statistics. Log scale is used to combine the data to estimate the weighting.
Interpret results: Graph
The results of a meta-analysis are usually presented as a graph called forest plot because the typical forest plots appear as forest of lines. It provides a simple visual presentation of individual studies that went into the meta-analysis at a glance. It shows the variation between the studies and an estimate of the overall result of all the studies together.
Forest plot
Meta-analysis graphs can principally be divided into six columns [ Figure 1 ]. Individual study results are displayed in rows. The first column (“study”) lists the individual study IDs included in the meta-analysis; usually the first author and year are displayed. The second column relates to the intervention groups and the third column to the control groups. The fourth column visually displays the study results. The line in the middle is called “the line of no effect.” The weight (in %) in the fifth column indicates the weighting or influence of the study on the overall results of the meta-analysis of all included studies. The higher the percentage weight, the bigger the box, the more influence the study has on the overall results. The sixth column gives the numerical results for each study (e.g., odds ratio or relative risk and 95% confidence interval), which are identical to the graphical display in the fourth column. The diamond in the last row of the graph illustrates the overall result of the meta-analysis.[ 4 ]

Interpretation of meta-analysis[ 4 ]
Thus, the horizontal lines represent individual studies. Length of line is the confidence interval (usually 95%), squares on the line represent effect size (risk ratio) for the study, with area of the square being the study size (proportional to weight given) and position as point estimate (relative risk) of the study.[ 7 ]
For example, the forest plot of the effectiveness of dexamethasone compared with placebo in preventing the recurrence of acute severe migraine headache in adults is shown in Figure 2 .[ 17 ]

Forest plot of the effectiveness of dexamethasone compared with placebo in preventing the recurrence of acute severe migraine headache in adults[ 17 ]
The overall effect is shown as diamond where the position toward the center represents pooled point estimate, the width represents estimated 95% confidence interval for all studies, and the black plain line vertically in the middle of plot is the “line of no effect” (e.g., relative risk = 1).
Therefore, when examining the results of a systematic reviews/meta-analysis, the following questions should be kept in mind:
- Heterogeneity among studies may make any pooled estimate meaningless.
- The quality of a meta-analysis cannot be any better than the quality of the studies it is summarizing.
- An incomplete search of the literature can bias the findings of a meta-analysis.
- Make sure that the meta-analysis quantifies the size of the effect in units that you can understand.
Subgroup analysis and sensitivity analysis
Subgroup analysis looks at the results of different subgroups of trials, e.g., by considering trials on adults and children separately. This should be planned at the protocol stage itself which is based on good scientific reasoning and is to be kept to a minimum.
Sensitivity analysis is used to determine how results of a systematic review/meta-analysis change by fiddling with data, for example, what is the implication if the exclusion criteria or excluded unpublished studies or weightings are assigned differently. Thus, after the analysis, if changing makes little or no difference to the overall results, the reviewer's conclusions are robust. If the key findings disappear, then the conclusions need to be expressed more cautiously.
Advantages of Systematic Reviews
Systematic reviews have specific advantages because of using explicit methods which limit bias, draw reliable and accurate conclusions, easily deliver required information to healthcare providers, researchers, and policymakers, help to reduce the time delay in the research discoveries to implementation, improve the generalizability and consistency of results, generation of new hypotheses about subgroups of the study population, and overall they increase precision of the results.[ 18 ]
Limitations in Systematic Reviews/Meta-analysis
As with all research, the value of a systematic review depends on what was done, what was found, and the clarity of reporting. As with other publications, the reporting quality of systematic reviews varies, limiting readers’ ability to assess the strengths and weaknesses of those reviews.[ 5 ]
Even though systematic review and meta-analysis are considered the best evidence for getting a definitive answer to a research question, there are certain inherent flaws associated with it, such as the location and selection of studies, heterogeneity, loss of information on important outcomes, inappropriate subgroup analyses, conflict with new experimental data, and duplication of publication.
Publication Bias
Publication bias results in it being easier to find studies with a “positive” result.[ 19 ] This occurs particularly due to inappropriate sifting of the studies where there is always a tendency towards the studies with positive (significant) outcomes. This effect occurs more commonly in systematic reviews/meta-analysis which need to be eliminated.
The quality of reporting of systematic reviews is still not optimal. In a recent review of 300 systematic reviews, few authors reported assessing possible publication bias even though there is overwhelming evidence both for its existence and its impact on the results of systematic reviews. Even when the possibility of publication bias is assessed, there is no guarantee that systematic reviewers have assessed or interpreted it appropriately.[ 20 ]
To overcome certain limitations mentioned above, the Cochrane reviews are currently reported in a format where at the end of every review, findings are summarized in the author's point of view and also give an overall picture of the outcome by means of plain language summary. This is found to be much helpful to understand the existing evidence about the topic more easily by the reader.
A systematic review is an overview of primary studies which contains an explicit statement of objectives, materials, and methods, and has been conducted according to explicit and reproducible methodology. A meta-analysis is a mathematical synthesis of the results of two or more primary studies that addressed the same hypothesis in the same way. Although meta-analysis can increase the precision of a result, it is important to ensure that the methods used for the reviews were valid and reliable.
High-quality systematic reviews and meta-analyses take great care to find all relevant studies, critically assess each study, synthesize the findings from individual studies in an unbiased manner, and present balanced important summary of findings with due consideration of any flaws in the evidence. Systematic review and meta-analysis is a way of summarizing research evidence, which is generally the best form of evidence, and hence positioned at the top of the hierarchy of evidence.
Systematic reviews can be very useful decision-making tools for primary care/family physicians. They objectively summarize large amounts of information, identifying gaps in medical research, and identifying beneficial or harmful interventions which will be useful for clinicians, researchers, and even for public and policymakers.
Source of Support: Nil
Conflict of Interest: None declared.
- Chester Fritz Library
- Library of the Health Sciences
- Thormodsgard Law Library
Literature Reviews
- Get started
Literature Reviews within a Scholarly Work
Literature reviews as a scholarly work.
- Finding Literature Reviews
- Your Literature Search
- Library Books
- How to Videos
- Communicating & Citing Research
- Bibliography
Literature reviews summarize and analyze what has been written on a particular topic and identify gaps or disagreements in the scholarly work on that topic.
Within a scholarly work, the literature review situates the current work within the larger scholarly conversation and emphasizes how that particular scholarly work contributes to the conversation on the topic. The literature review portion may be as brief as a few paragraphs focusing on a narrow topic area.
When writing this type of literature review, it's helpful to start by identifying sources most relevant to your research question. A citation tracking database such as Web of Science can also help you locate seminal articles on a topic and find out who has more recently cited them. See "Your Literature Search" for more details.
A literature review may itself be a scholarly publication and provide an analysis of what has been written on a particular topic without contributing original research. These types of literature reviews can serve to help keep people updated on a field as well as helping scholars choose a research topic to fill gaps in the knowledge on that topic. Common types include:
Systematic Review
Systematic literature reviews follow specific procedures in some ways similar to setting up an experiment to ensure that future scholars can replicate the same steps. They are also helpful for evaluating data published over multiple studies. Thus, these are common in the medical field and may be used by healthcare providers to help guide diagnosis and treatment decisions. Cochrane Reviews are one example of this type of literature review.
Semi-Systematic Review
When a systematic review is not feasible, a semi-systematic review can help synthesize research on a topic or how a topic has been studied in different fields (Snyder 2019). Rather than focusing on quantitative data, this review type identifies themes, theoretical perspectives, and other qualitative information related to the topic. These types of reviews can be particularly helpful for a historical topic overview, for developing a theoretical model, and for creating a research agenda for a field (Snyder 2019). As with systematic reviews, a search strategy must be developed before conducting the review.
Integrative Review
An integrative review is less systematic and can be helpful for developing a theoretical model or to reconceptualize a topic. As Synder (2019) notes, " This type of review often re quires a more creative collection of data, as the purpose is usually not to cover all articles ever published on the topic but rather to combine perspectives and insights from di ff erent fi elds or research traditions" (p. 336).
Source: Snyder, H. (2019). Literature review as a research methodology: An overview and guidelines. Journal of Business Research. 104. 333-339. doi: 10.1016/j.jbusres.2019.07.039
- << Previous: Get started
- Next: Finding Literature Reviews >>
- Last Updated: Dec 5, 2023 8:31 PM
- URL: https://libguides.und.edu/literature-reviews

About Systematic Reviews
The Difference Between Narrative Review and Systematic Review

Automate every stage of your literature review to produce evidence-based research faster and more accurately.
Reviews in scientific research are tools that help synthesize literature on a topic of interest and describe its current state. Different types of reviews are conducted depending on the research question and the scope of the review. A systematic review is one such review that is robust, reproducible, and transparent. It involves collating evidence by using all of the eligible and critically appraised literature available on a certain topic. To know more about how to do a systematic review , you can check out our article at the link. The primary aim of a systematic review is to recommend best practices and inform policy development. Hence, there is a need for high-quality, focused, and precise methods and reporting. For more exploratory research questions, methods such as a scoping review are employed. Be sure you understand the difference between a systematic review and a scoping review , if you don’t, check out the link to learn more.
When the word “review” alone is used to describe a research paper, the first thing that should come to mind is that it is a literature review. Almost every researcher starts off their career with literature reviews. To know the difference between a systematic review and a literature review , read on here. Traditional literature reviews are also sometimes referred to as narrative reviews since they use narrative analysis to synthesize data. In this article, we will explore the differences between a systematic review and a narrative review, in further detail.
Learn More About DistillerSR
(Article continues below)
Narrative Review vs Systematic Review
Both systematic and narrative reviews are classified as secondary research studies since they both use existing primary research studies e.g. case studies. Despite this similarity, there are key differences in their methodology and scope. The major differences between them lie in their objectives, methodology, and application areas.
Differences In Objective
The main objective of a systematic review is to formulate a well-defined research question and use qualitative and quantitative methods to analyze all the available evidence attempting to answer the question. In contrast, narrative reviews can address one or more questions with a much broader scope. The efficacy of narrative reviews is irreplaceable in tracking the development of a scientific principle, or a clinical concept. This ability to conduct a wider exploration could be lost in the restrictive framework of a systematic review.
Differences in Methodology
For systematic reviews, there are guidelines provided by the Cochrane Handbook, ROSES, and the PRISMA statement that can help determine the protocol, and methodology to be used. However, for narrative reviews, such standard guidelines do not exist. Although, there are recommendations available.
Systematic reviews comprise an explicit, transparent, and pre-specified methodology. The methodology followed in a systematic review is as follows,
- Formulating the clinical research question to answer (PICO approach)
- Developing a protocol (with strict inclusion and exclusion criteria for the selection of primary studies)
- Performing a detailed and broad literature search
- Critical appraisal of the selected studies
- Data extraction from the primary studies included in the review
- Data synthesis and analysis using qualitative or quantitative methods [3].
- Reporting and discussing results of data synthesis.
- Developing conclusions based on the findings.
A narrative review on the other hand does not have a strict protocol to be followed. The design of the review depends on its author and the objectives of the review. As yet, there is no consensus on the standard structure of a narrative review. The preferred approach is the IMRAD (Introduction, Methods, Results, and Discussion) [2]. Apart from the author’s preferences, a narrative review structure must respect the journal style and conventions followed in the respective field.
Differences in Application areas
Narrative reviews are aimed at identifying and summarizing what has previously been published. Their general applications include exploring existing debates, the appraisal of previous studies conducted on a certain topic, identifying knowledge gaps, and speculating on the latest interventions available. They are also used to track and report on changes that have occurred in an existing field of research. The main purpose is to deepen the understanding in a certain research area. The results of a systematic review provide the most valid evidence to guide clinical decision-making and inform policy development [1]. They have now become the gold standard in evidence-based medicine [1].
Although both types of reviews come with their own benefits and limitations, researchers should carefully consider the differences between them before making a decision on which review type to use.
- Aromataris E, Pearson A. The systematic review: an overview. AJN. Am J Nurs. 2014;114(3):53–8.
- Green BN, Johnson CD, Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropratic Medicine 2006;5:101–117.
- Linares-Espinós E, Hernández V, Domínguez-Escrig JL, Fernández-Pello S, Hevia V, Mayor J, et al. Metodología de una revisión sistemática. Actas Urol Esp. 2018;42:499–506.
3 Reasons to Connect

- Search Menu
- Aesthetic Breast Surgery
- Aesthetic Breast Reconstruction
- Aesthetic Genital Plastic Surgery
- Body Contouring
- Breast Surgery
- Cosmetic Medicine
- Facial Surgery
- Oculoplastic Surgery
- Rhinoplasty
- SoMe and Behavioral Science
- Advance articles
- Thematic Issues
- Residents Publishing Program
- Video Content
- Author Guidelines
- Author Guidelines in Chinese
- Instructions for Reviewers
- Submission Site
- Permissions
- Self-Archiving Policy
- Open Access
- Advertising & Corporate Services
- Advertising
- Reprints & ePrints
- About Aesthetic Surgery Journal Open Forum
- The Aesthetic Society
- Editorial Board
- Journals Career Network
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Conclusions, disclosures.
- < Previous
The Innovative and Evolving Landscape of Topical Exosome and Peptide Therapies: A Systematic Review of the Available Literature
Ms Ash and Ms Menon are medical students, Dr Shauly is a resident, and Dr Losken is a plastic surgeon, Division of Plastic and Reconstructive Surgery, Emory University, Atlanta, GA, USA.
Ms Zibitt is a medical student at Medical College of Georgia, School of Medicine, Augusta, GA, USA.
Dr Gould is a plastic surgeon in private practice, Beverly Hills, CA, USA.
- Article contents
- Figures & tables
- Supplementary Data
Makenna Ash, Meira Zibitt, Orr Shauly, Ambika Menon, Albert Losken, Daniel Gould, The Innovative and Evolving Landscape of Topical Exosome and Peptide Therapies: A Systematic Review of the Available Literature, Aesthetic Surgery Journal Open Forum , Volume 6, 2024, ojae017, https://doi.org/10.1093/asjof/ojae017
- Permissions Icon Permissions
Topical antiaging therapies provide noninvasive delivery of active therapeutics. Exosomes, or extracellular nanovesicles, and peptides, small strings of amino acids, have shown promise as topical therapies in early trials, but neither is FDA approved. This review aims to elucidate the current and future landscape of topical exosomes and peptides as therapeutics for skin rejuvenation. A literature search was conducted using the keywords “peptides” OR “exosomes” AND “skin” OR “rejuvenation.” Primary endpoints included mechanisms of action in humans or live animals as well as clinical data supporting the use of exosomes or peptides topically for skin rejuvenation or wound healing. Secondary endpoints were safety, side effects, and efficacy. The articles were collected, organized, and sorted using the Covidence software (Melbourne, Australia) for systematic review. Nine articles evaluating topical application of exosomes and 9 of peptides met inclusion criteria. Topical exosomes were found to increase collagen deposition, accelerate wound healing, and improve overall cosmesis. Several clinical trials are currently underway. Topical peptides were found to improve appearance of fine lines and wrinkles, elasticity and viscoelasticity, skin texture, skin thickness, and the potential for accelerated wound healing. Peptides are quite common in “cosmeceutical” products, and several patents have been filed for topical peptide products aimed at increasing skin rejuvenation. This could indicate a movement toward pursuing FDA approval. The future of topical exosome and peptide products for the purpose of skin rejuvenation appears promising. Preliminary data from the studies reviewed here indicates that these products have the potential to be safe and effective.

The United States population is aging. In 2020, 1 in 6 US Americans was over 65, representing a 38.1% increase over the previous 10 years. 1 As the population continues to age, there is an increased effort to identify products that can be used to prevent signs of aging, such as wrinkles or solar spots, and rejuvenate skin through increased elasticity and hydration. Products that can be applied topically are preferred due to easier consumer accessibility and use. Topical products are less invasive than those that are injected or consumed. 2 However, use of topical products requires unique considerations for methods of drug delivery due to difficulty penetrating the skin barrier. Therefore, active ingredients in topical medications can be less effective than in other methods of drug delivery.
As the interest in topical antiaging therapies has grown, exosomes and peptides have shown some promise in early trials. However, there are no FDA-approved topical therapies as of yet, using either peptides or exosomes as the primary ingredient. In this article, we investigate the current landscape around topical exosomes and peptides for skin rejuvenation, the available data on effectiveness, and the outlook for the future of these therapies.
Exosomes, extracellular nanovesicles, emerged into scientific literature in the 1960s by Bonucci and Anderson, described as extracellular vesicles produced by cell membrane budding. They may contain anything from lipids to proteins or nucleic acids, with the function of cellular signaling as well as waste management. 3 , 4 Since these early descriptions, exosomes have been investigated for their roles in everything from cancer biology to immunology, atherosclerosis, and neurology. Their molecular roles are vast due to the potential for communication between adjacent cells, modulation of protein expression, control of cellular life cycles, influence on cell behaviors, establishment of cellular polarity, and remodeling of the extracellular matrix. 5
Exosomes became of interest in the skincare industry due to their ability to alter the extracellular matrix, induce cell regeneration, influence the cell cycle, and target drug delivery. They have been found to have numerous cosmetic benefits, including wound healing, hydration, texture improvement, antiaging effects, and improvements in discoloration 6 ( Figure 1 ). Exosomes can be generated from any cell line, but it has been established that stem cell exosomes are better able to induce cell proliferation, regeneration, and wound healing and, therefore, are better fit for improvements in skin aging. 7 Many of these benefits are seen in the injection of exosomes derived from stem cells. Less research has evaluated the cosmetic outcomes of topical application of exosomes, which is the focus of this review. Exosomes are currently not FDA approved for cosmetic use and are, therefore, unregulated as of now. Research into the use of exosomes is at the forefront of cosmetic medicine, in addition to the numerous uses of exosomes in general medicine.
Diagram of the potential uses and benefits of topical exosome therapy in patients.
Topical peptides have gained popularity in the skincare industry for their potential benefits in promoting skin health and rejuvenation. Peptides are strings of amino acids that are used in the body to build proteins, such as collagen, which are essential for skin structure and elasticity. Peptides, as injectables, have demonstrated health benefits in inflammation, wound healing, and antimicrobial defense, and substantial research is being done evaluating the therapeutic potential of peptides for skin rejuvenation. 8 Peptides may have the ability to stimulate collagen production, improve skin texture, and reduce the appearance of fine lines and wrinkles. Topical peptides have been used in commercial dermatology and cosmetic formulations for some years; however, there is not currently a medical-grade FDA-approved topical peptide on the dermatology market.
A hurdle to the use of topical peptides is their questionable efficacy in penetrating the skin barrier. For active ingredients to be able to affect skin texture and appearance, they must be absorbed into the dermis in a stable form. As of the time of this review, there are hundreds, if not thousands, of cosmeceuticals on the market containing peptides for the stated purpose of rejuvenating skin. Although there is substantial evidence investigating oral consumption or injection of peptides for skin rejuvenation and wound healing, there is a paucity of data, especially studies published since 2010, supporting the efficacy of peptides applied topically for the purpose of rejuvenating skin.
This review aims to elucidate the current landscape and future of topical exosomes and peptides as therapeutics for skin rejuvenation, including future FDA approval. A formal systematic review was done following the PRISMA guidelines, and discussion was supplemented using additional sources identified as being pertinent that did not fall into the inclusion/exclusion criteria of the formal review.
An initial search of the literature was conducted on PubMed using the keywords “peptides” OR “exosomes” AND “skin” OR “rejuvenation.” The initial search found 662 distinct articles. The parameters for the search were articles that had been published since 2010 and English. The search was run on August 15, 2023. The primary endpoints of the review were investigating mechanisms of action in humans or live animals, as well as clinical data supporting the use of exosomes or peptides topically for skin rejuvenation or wound healing. Secondary endpoints of the review were safety, side effects, and efficacy. The articles were collected, organized, and sorted using the Covidence software (Melbourne, Australia) for systematic review ( Figure 2 ).
Systematic review of the literature flowchart and selected studies.
Inclusion criteria were: studies conducted on humans or nonhuman animals, topical application of therapy, articles published between 2010 and 2023, originally published in English, skin rejuvenation or wound healing as a primary outcome, and measureable or quantitative outcomes. Studies were excluded if they met any of the following criteria: nontopical method of delivery, in vitro study, case studies/case reports/editorials/commentaries/literature reviews/systematic reviews, full text not available, or duplicates. If a literature review or systematic review was found to be relevant, then the cited articles were included in the original data pull.
This systematic review is evaluating the clinical data supporting products that have not yet been approved by the FDA. Therefore, the results of this review alone should not be used to inform clinical judgment pending FDA approval of the products discussed herein. It is the authors' goal to evaluate the available data and the current clinical landscape in order to provide insight into the future of these therapies, and to assist the reader in their own clinical decision-making process.
Exosome Outcomes
The primary outcomes of the articles included in this literature review were wound healing and re-epithelialization, collagen deposition, inflammatory markers, facial aging (including wrinkles, radiance, and firmness), and photoaging. Exosomes were derived from various cell lines, including human fetal fibroblasts, acellular Wharton's Jelly, adipose stem cells, mesenchymal stem cells, and human platelet extract. Exosomes were all administered topically, as per the inclusion criteria, but a single study topically administered exosomes with sponge spicules 9 and 1 study applied a topical gel matrix seeded with exosomes. 10 Of note, 1 study also assessed exosome treatment with or without the coadministration of botulinum toxin A. 11 One study also enriched exosomes with siRNA used to knockdown molecules, such as NF-kB. 12
Exosomes were generally isolated by harvesting the supernatant from the cell culture growth medium, centrifuging the medium at sequentially increasing forces, filtering, and resuspending the resultant pellet. Nanoparticle tracking analysis, transmission electron microscopy, and western blots are commonly used to determine the character, morphology, and protein content of the exosomes.
Exosome Product Availability
The studies included in this systematic literature review did not include any commercially available products. Some studies did utilize topical exosome application in conjunction with commercially available skincare products, most commonly moisturizers or SPF.

Exosome Safety and Efficacy
Five studies determined that exosome-treated wounds showed accelerated wound healing and re-epithelialization rates or a smaller wound at some interval after treatment compared with controls. 10-14 Three studies found that exosome-treated wounds had increased collagen deposition, with 1 study finding that, in addition to increased collagen deposition, the exosome-treated group had improved collagen alignment. 10 , 11 , 14 Three studies found increases in inflammation in exosome-treated skin, although 1 study noted this was transient and resolved within 72 h of topical application with sponge spicules. 9 , 11 , 13 One study found decreased inflammatory cytokines, although the exosomes contained siRNAs targeting inflammatory regulators. 12 One study found increased polymorphonuclear leukocytes in the exosome-treated group, 13 and Lu et al found that the exosome-treated group had decreased macrophages at the wound site. 12 Two studies found that treatment with exosomes caused increased angiogenesis. 10 , 11 Lastly, 2 studies found improvement in granulation in exosome-treated groups. 10 , 13
In the discussion of cosmesis improvement, Ye et al found that exosome treatment improved sensitive skin scoring of roughness, scaling, erythema, as well as burning, tension, itching, and dryness, as assessed by 1 professional dermatologist on an objective and subjective sensitive skin index. 16 Proffer et al found that exosome treatment produced improvements in facial aging, such as erythema, color evenness, luminosity, wrinkling, and firmness, as measured by computer analysis of photo documentation. 15 Both of these studies excluded anyone who had prior dermatologic or cosmetic procedures, excluding a large population that would potentially be interested in this type of product. Proffer et al also utilized a twice-daily skin regimen in addition to the use of exosomes, including products such as a repair serum, SPF, and specific moisturizers which may further improve skin outcomes. 15
Federal Drug Administration: Exosomes
No studies of exosome therapies included in this systematic literature review are currently FDA approved or undergoing an FDA-approved clinical trial. Please see the Discussion section for further elaboration.
Peptides Outcomes
The primary outcomes of the studies in the reviewed outcomes were wound healing, skin rejuvenation, and resolution of photoaging. Of the peptides investigated in the reviewed articles, 4 were derived from rice proteins, 3 were derived from marine collagen extracted from sea life such as fish or oysters, and 3 referred only to the product as “peptides” with no source specification. Methods for deriving the peptides included using acid to solubilize and extract the collagen proteins from tilapia skin, fermenting rice grains with Lactobacillus and extracting peptides from the remaining protein, and hydrolyzing oyster protein. The peptides were then prepared in a lipophilic suspension or a hydrogel to facilitate penetration of hydrophilic peptides into the lipophilic external barrier of the skin ( Figure 3 ). In 1 study, the peptides were combined into a sunscreen formulation also containing ascorbyl tetraisopalmitate. 17-26
Diagram of the proposed benefits of topical peptide therapy in patients.
Peptide Products
Of the assessed studies, 1 investigated a commercially available peptide product. This product was an ampoule containing peptides sourced from rice and lupin, Vitamin C, hyaluronic acid, and volcanic mineralizing water. 17 Formulations of other peptide products investigated were varied. Some trials added peptides to an existing moisturizing solution. Others combined these peptides into a water-based solution, or even hydrogels. 18-26
Peptide Safety and Efficacy
Three of the assessed studies found topical peptide interventions to improve the appearance of fine lines and wrinkles. Two found these interventions to improve elasticity and viscoelasticity, although 1 found no difference in elasticity and viscoelasticity. Three found improvement in skin texture, and 2 found potential for increased wound healing. One study found an increase in skin thickness, and 3 found improved skin appearance and decreased markers of photoaging. None of the assessed studies observed any negative side effects in human participants.
Federal Drug Administration: Peptides
There are no FDA-approved topical peptide products currently available. Since 2017, several patents have been filed in the United States and internationally for various cosmetic peptides for improving skin rejuvenation and use of the same. 27-30 This could mark a growing interest in the field of topical bioactive peptide application, specifically for skin rejuvenation.
Topical Exosomes: Proposed Mechanisms of Action
As previously noted, exosomes have many potential mechanisms of actions, varying based on the cell that secretes them as well as their target and the content of the exosome itself, which can be highly variable. The various biologic materials present in the exosome, ranging from nucleic acids, to proteins or lipids and more, play a large role in determining these interactions and cellular communication. This is further complicated by the various extravesicular proteins that may be present on the exosomal surface.
The biggest challenge for topical treatments to be effective is whether or not they are able to permeate the lipophilic skin barrier. As exosomes are derived from cell membrane budding, the hypothesis is that these extracellular vesicles are able to merge with the cellular membrane of dermatocytes, and in doing so, deliver their active product into the interior of the skin cell.
Topical exosomes may affect skin rejuvenation through modulation of transforming growth factor beta (TGF-B), mitogen-activated protein kinase, and extracellular signal-regulated kinase, which all play roles in cell differentiation, cellular proliferation, and apoptosis regulation. 31 , 32 Modulation of cytokines, such as TGF-B, leads to differences in extracellular matrix makeup, production of collagen, and collagenases, as well as mediates the inflammatory response to tissue damage. Increasing collagen production and modulating collagenase production can result in improved wound healing and scar formation as well as skin plumpness and elasticity. Exosomes also may mediate the inflammatory response to skin damage, improving wound healing, while promoting angiogenesis, tissue remodeling, and extracellular matrix deposition. 33 Additionally, these cytokines play an integral role in many dermatologic processes, leading to potential for exosome use in treatment of dermatologic pathologies such as dermatitis, psoriasis, and wound healing, among others.
Topical Exosomes: Quality and Safety Considerations
There are currently a wide range of exosome therapies under investigation. According to clinicaltrials.gov , there are hundreds of ongoing exosome-related clinical trials across a wide range of medical specialties, indicating a recent increase in interest around exosomes. The primary outcomes of current trials range from the use of exosomes as biomarkers and drug delivery systems to therapeutics and vaccines. 34 Currently, there are FDA-approved clinical trials investigating exosome-based products for conditions such as atopic dermatitis, skin rejuvenation, psoriasis, alopecia, epidermolysis bullosa, and diabetic wound healing.
One consideration, when looking to the future of exosome-based medical treatments, is the significant overlap with stem-cell-based treatments, as many exosome therapies are derived from stem cell lines. The FDA released a warning against the use of stem-cell-derived therapies as well as a specific Public Safety Notice addressing exosome products in 2019. 35 This warning addressed an increase in false claims that exosomes are not held to the same rigorous standards as other drugs and biologics. 36
The exosomes examined in this systematic review were harvested through many different methods, and exosomes under investigation for other therapeutic reasons may be harvested in different manners still. 6-16 Isolation and purification is typically done by ultracentrifugation by either differential or density gradient. Immunoaffinity is occasionally used by utilizing exosome markers, but these markers are not present on all exosomes and, therefore, are not a perfect solution. Concern for altering exosomes due to chemical interactions with the reagents must also be considered. Because of these production and quality control issues, mass production is a significant consideration when aiming to produce commercially available cosmetics. When considering dosing, many factors unique to biologics must be considered, including cellular uptake, off-target interactions, biodistribution, and exosome half-life, in addition to other pharmacodynamics and pharmacokinetic properties to consider. 36 Many current studies are working to address these issues.
The future of exosome-based medical treatments is promising, although the FDA approval process is lengthy and some aspects of exosome-based therapies raise unique challenges. Important considerations include purification and reliability issues, production optimization and tissue-specific delivery, standardization of dosing and potency, and long-term safety questions. The heavy reliance on cell culture and the ability to reliably extract and purify the exosome product is a relatively low-yield process. Additionally, different cell lines have different culture requirements and have cell-line-specific senescence rates. Because of this, techniques to extract exosomes from 1 cell type are not likely to be the solution for others. 37
Topical Exosomes: Implications in Practice
Topical exosome products could be used both short and long terms for skin rejuvenation, scar improvement, hyperpigmentation, and other dermatologic or aesthetic processes. They would provide an alternative for some other popular autologous treatments, such as platelet-rich-plasma (PRP) injections, which are also thought to improve skin appearance through the introduction of growth factors into the interior of the cell. Current studies, such as those included in this systematic literature review, focused on skin outcomes over a period of weeks. 16-25 There are currently no studies assessing the long-term implications of topical exosome products.
There are currently no studies comparing the effectiveness of topical exosome application to other treatments, such as PRP or laser. Future studies should investigate this in order to establish where this treatment would fall in the lineup of treatments available for skin rejuvenation, in both efficacy and invasiveness.
There are many possible clinical implications of an FDA-approved topical exosome product. These products could be an additional service that plastic surgeons and other providers focusing on aesthetics could add to their arsenal of treatments. These products could be used as a less invasive option for people who are not interested in undergoing procedures, lasers, or injections. Additionally, these products could be offered as a treatment to take in between more invasive treatments, as a maintenance treatment. Finally, exosome availability as a commercial product would be a significant paradigm shift toward the use of autologous products in cosmetics ( Table 1 ).
Studies Investigating the Use of Topical Exosomes With or Without Combination Treatments
Topical Peptides: Proposed Mechanisms of Action
There are 3 main types of peptides currently used in cosmeceuticals—these include signal peptides, neurotransmitter-affecting peptides, and carrier peptides. 38 Signal peptides are peptides that have the ability to signal fibroblasts to either increase collagen production or decrease collagenase production to slow collagen breakdown. In several studies, which were conducted prior to 2010 and, therefore, excluded from our review, topical application of signal peptides was shown to decrease fine lines and wrinkles significantly compared to placebo alone, and similarly to the application of retinol. 40-42
Neurotransmitter-affecting peptides include botulinum neurotoxin Type A and peptides that have been developed to imitate this structure. These peptides inhibit the contraction of facial muscles when injected, preventing the formation of fine lines and wrinkles.
Carrier peptides are peptides that are used to deliver and stabilize trace elements that support wound healing and enzymatic function. The most common element delivered by these peptides is copper, which enhances wound healing and angiogenesis. Several of the enzymes essential for collagen production, such as lysyl oxidase, are dependent on copper. 39 The most common carrier peptide is glycil- L- histidyl- L- lysine, used to transport copper to Collagen I. It has been studied in both wound healing and skin rejuvenation, with the majority of available evidence supporting wound healing. 43-45
The theory behind topical application of peptides is that when they are combined with a lipophilic carrier, they will be able to permeate the skin barrier. Once past the skin barrier, these peptides will perform their respective modulations to the dermis, through increasing collagen production, inhibiting collagenase, promoting angiogenesis, and wound healing.
Topical Peptides: Quality and Safety Considerations
Peptides are considered to be very safe therapeutics. They are easily degraded through physiologic enzymatic pathways, giving them a short half-life and little potential for long-lasting negative effects. 8 Randomized controlled trials are few and far between. Search conducted on October 2, 2023, on Clinicaltrials.gov for “topical peptide” yielded 2 clinical trials investigating topical application of various peptide formulations for cosmetic purposes. 46 , 47
Currently, topical peptide therapeutics tend to be designed for frequent use with shorter effect times. This may be due, in part, to the relatively quick degradation of peptide products in the human body, as well as the fact that topical formulations are exposed to weather, sweat, sun, and soap, decreasing their ability to remain on the surface of the skin for a lengthy period. In order for a topical peptide formulation to have longer lasting effects, it would likely have to be in a slow-release formula that is able to penetrate the skin barrier effectively without immediately diluting in the bloodstream.
Implications of Topical Peptides in Practice
The introduction of topical peptide products into the plastic surgeon's practice, perhaps as an adjunct to other aesthetic procedures, such as injectables or cosmetic surgery, could help improve patient experience and preserve cosmetic outcomes for much longer. Topical peptide products applied after botox could hypothetically extend the lifespan of the injection and provide further cosmetic benefit by improving skin plumpness and texture. The use of these products in conjunction with available aesthetic interventions could improve patient satisfaction and potentially lead to overall better outcomes.
The advent of topical peptides as a therapy for skin rejuvenation would have the greatest impact on dermatology, aestheticians, and plastic surgery. However, although many products have the capability to reverse the visual signs of skin aging, they often work for only brief intervals at a time and must be applied up to twice daily to maintain effect. Although topical application is easier to access and use, the high burden of use frequency may lead to many people choosing interventions to improve skin rejuvenation that have a longer lasting effect, such as laser therapy or fat grafting ( Table 2 ).
Studies Investigating the Use of Topical Peptide Therapy With or Without Combination Treatments
Landscape of FDA Approval for Topical Exosome and Topical Peptide Products
The pathway to FDA approval has many steps. Generally, new therapeutics begin with preclinical testing, to assess safety, then move on to investigational new drug applications. Once these are approved, clinical trials may begin. Once clinical trials confirm safety and effectiveness, the manufacturer may submit a new drug application; after the new drug application has been received, all of the available data are reviewed by the FDA and they pass a verdict on whether the drug will be FDA approved or not. The average length of time from submission of a new drug application to FDA approval is about 6 to 10 months.
There are currently no FDA-approved topical peptide or exosome products commercially available. One of the difficulties of investigating skin rejuvenation products is the multitude and variability of different primary outcomes to be investigated, such as overall skin appearance, skin elasticity, photoaging, etc. Additionally, many of these endpoints are subjective and dependent on the rating of the study team. Ideally, future clinical trials investigating the efficacy of topical peptides and topical exosomes for skin rejuvenation would identify at least 1 primary objective endpoint to assess outcomes.
There are many current clinical trials investigating the effectiveness of topical exosomes in treating a myriad of dermatologic conditions as well as the effects of exosomes on skin rejuvenation. In addition to proving efficacy of topical exosomes for skin rejuvenation and elucidating possible adverse effects, exosome manufacturers will likely have to address the unique concerns for exosome production, including quality control, dosing, and production concerns. Additionally, because exosomes may be derived from stem cells, extra considerations and approval may be required.
Many topical peptide formulations currently on the market contain small di- or tri-peptides suspended in a moisturizing solution and packaged with other therapeutic ingredients, such as vitamin C, hyaluronic acid, or retinoids. 17 , 18 , 22 As topical peptides have been used in cosmeceuticals for some time, that are not FDA regulated, manufacturers will likely be able to begin with randomized controlled clinical trials in order to establish effectiveness before applying for FDA approval. There will likely not be a need to establish safety as these products are already proven safe for consumers. This provides the groundwork for a relatively quick and straightforward path to FDA approval, as long as the investigational products are proven to be effective in a randomized, controlled trial setting.
The paucity of evidence demonstrating the effectiveness of topical peptides in a controlled setting is a potential barrier to FDA approval. This gap in research may be due to the ability of cosmeceuticals to bypass FDA approval, reducing the incentive for pharmaceutical companies to fund clinical trials investigating these products.
FDA approval of topical products for skin rejuvenation would lend another tool for plastic surgeons, dermatologists, and aestheticians to offer to patients who desire skin rejuvenation but may be opposed to or have clinical contraindications to current therapies, such as injectables or laser therapy. Additionally, as no FDA-approved topicals are currently on the market, the first drug to achieve this designation would be uncontested in the marketplace.
Limitations of this Review
The major limitation of this systematic review is that it investigates experimental products that are not currently FDA approved, and therefore, there are few available articles and trials. If FDA approval for either topical exosomes or peptides was to be achieved, this would open the door for future studies further investigating effectiveness.
The future of topical exosome and topical peptide products for the purpose of skin rejuvenation appears promising. There are a plethora of clinical trials currently in progress to evaluate the safety and effectiveness of exosomes for various dermatologic treatments, in addition to the significant research exploring exosomes' role in wound healing. The biggest challenge that these products must overcome in order to gain FDA approval is standardizing exosome production and addressing in vivo stability, as well as continuing to deepen our understanding of the mechanism of action of exosomes at a biochemical level. Once this is achieved, we may see topical exosome products on the market in the near future.
It is difficult to be specific about the future potential of topical peptide therapeutics for skin rejuvenation because the array of peptides available or able to be formulated in a lab is quite broad. The topical peptide formulations that have been formally investigated are not dangerous to human health and have few to no negative side effects. There have been few trials conducted to assess the efficacy of topical peptides; it seems that many trials have focused mainly on safety. Although there is not yet an FDA-approved formulation for topical exosomes or topical peptides, peptides are plentiful in so-called “cosmeceuticals” and may be purchased at any drugstore or online. For a topical peptide product to truly pave the way for FDA approval and regulation, the authors postulate that it would have to have quite a significant impact that is not currently available from commercial cosmeceutical topical peptide preparations.
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
The authors received no financial support for the research, authorship, and publication of this article, including payment of the article processing charge.
Bureau UC. Older population and aging data. Census.gov. December 3, 2021. Accessed January 4, 2024. https://www.census.gov/topics/population/older-aging/data.html
Jiménez-Rodríguez A , Guardado-Félix D , Antunes-Ricardo M . Challenges and strategies for topical and transdermal delivery of bioactive peptides . Crit Rev Ther Drug Carrier Syst . 2022 ; 39 ( 1 ): 1 – 31 . doi: 10.1615/CritRevTherDrugCarrierSyst.2021038141
Google Scholar
Bonucci E . Fine structure of early cartilage calcification . J Ultrastruct Res . 1967 ; 20 ( 1-2 ): 33 – 50 . doi: 10.1016/S0022-5320(67)80034-0
Anderson HC . Vesicles associated with calcification in the matrix of epiphyseal cartilage . J Cell Biol . 1969 ; 41 ( 1 ): 59 – 72 . doi: 10.1083/jcb.41.1.59
Kalluri R , LeBleu VS . The biology, function, and biomedical applications of exosomes . Science . 2020 ; 367 ( 6478 ): eaau6977 . doi: 10.1126/science.aau6977
Thakur A , Shah D , Rai D , et al. Therapeutic values of exosomes in cosmetics, skin care, tissue regeneration, and dermatological diseases . Cosmetics . 2023 ; 10 ( 2 ): 65 . doi: 10.3390/cosmetics10020065
Oh M , Lee J , Kim Y , Rhee W , Park J . Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts . Int J Mol Sci . 2018 ; 19 ( 6 ): 1715 . doi: 10.3390/ijms19061715
Reddy B , Jow T , Hantash BM . Bioactive oligopeptides in dermatology: part I . Exp Dermatol . 2012 ; 21 ( 8 ): 563 – 568 . doi: 10.1111/j.1600-0625.2012.01528.x
Zhang K , Yu L , Li FR , et al. Topical application of exosomes derived from human umbilical cord mesenchymal stem cells in combination with sponge spicules for treatment of photoaging . Int J Nanomedicine . 2020 ; 15 : 2859 – 2872 . doi: 10.2147/IJN.S249751
Zhao D , Yu Z , Li Y , Wang Y , Li Q , Han D . GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration . J Mol Histol . 2020 ; 51 ( 3 ): 251 – 263 . doi: 10.1007/s10735-020-09877-6
Zheng HH , Ben XY , Wang YR , et al. Experimental study on the effect and mechanism of adipose stem cell-derived exosomes combined with botulinum toxin A on skin trauma in rats . J Cosmet Dermatol . 2024 ; 23 ( 1 ): 271 – 283 . doi: 10.1111/jocd.15922
Lu W , Zhang J , Wu Y , Sun W , Jiang Z , Luo X . Engineered NF-κB siRNA-encapsulating exosomes as a modality for therapy of skin lesions . Front Immunol . 2023 ; 14 : 1109381 . doi: 10.3389/fimmu.2023.1109381
Ahmadpour F , Rasouli HR , Talebi S , Golchin D , Esmailinejad MR , Razie A . Effects of exosomes derived from fibroblast cells on skin wound healing in Wistar rats . Burns . 2023 ; 49 ( 6 ): 1372 – 1381 . doi: 10.1016/j.burns.2023.02.003
Bakhtyar N , Jeschke MG , Herer E , Sheikholeslam M , Amini-Nik S . Exosomes from acellular Wharton’s jelly of the human umbilical cord promotes skin wound healing . Stem Cell Res Ther . 2018 ; 9 ( 1 ): 193 . doi: 10.1186/s13287-018-0921-2
Proffer SL , Paradise CR , DeGrazia E , et al. Efficacy and tolerability of topical platelet exosomes for skin rejuvenation: six-week results . Aesthet Surg J . 2022 ; 42 ( 10 ): 1185 – 1193 . doi: 10.1093/asj/sjac149
Ye C , Zhang Y , Su Z , et al. hMSC exosomes as a novel treatment for female sensitive skin: an in vivo study . Front Bioeng Biotechnol . 2022 ; 10 : 1053679 . doi: 10.3389/fbioe.2022.1053679
Akulinina I , Stefanaki I , Pavlíčková E , et al. Topical formulation containing peptides and vitamin C in ampoules improves skin aging signs: results of a large, international, observational study . J Cosmet Dermatol . 2022 ; 21 ( 9 ): 3910 – 3916 . doi: 10.1111/jocd.14733
Fossa Shirata MM , Maia Campos PMBG . Sunscreens and cosmetic formulations containing ascorbyl tetraisopalmitate and rice peptides for the improvement of skin photoaging: a double-blind, randomized placebo-controlled clinical study . Photochem Photobiol . 2021 ; 97 ( 4 ): 805 – 815 . doi: 10.1111/php.13390
Hu Z , Yang P , Zhou C , Li S , Hong P . Marine collagen peptides from the skin of Nile tilapia ( Oreochromis niloticus ): characterization and wound healing evaluation . Mar Drugs . 2017 ; 15 ( 4 ): 102 . doi: 10.3390/md15040102
Jeong S , Yoon S , Kim S , et al. Anti-wrinkle benefits of peptides complex stimulating skin basement membrane proteins expression . Int J Mol Sci . 2019 ; 21 ( 1 ): 73 . doi: 10.3390/ijms21010073
Kong S , Lv L , Guo J , et al. Preparation of cod skin collagen peptides/chitosan-based temperature-sensitive gel and its anti-photoaging effect in skin . Drug Des Devel Ther . 2023 ; 17 : 419 – 437 . doi: 10.2147/DDDT.S391812
Maia Campos PMBG , Melo MO , César FCS . Topical application and oral supplementation of peptides in the improvement of skin viscoelasticity and density . J Cosmet Dermatol . 2019 ; 18 ( 6 ): 1693 – 1699 . doi: 10.1111/jocd.12893
Mo Q , You S , Fu H , et al. Purification and identification of antioxidant peptides from rice fermentation of Lactobacillus plantarum and their protective effects on UVA-induced oxidative stress in skin . Antioxidants (Basel) . 2022 ; 11 ( 12 ): 2333 . doi: 10.3390/antiox11122333
Park KC , Kim SY , Khan G , Park ES . Ultrasonographic assessment of the cutaneous changes induced by topical use of novel peptides comprising laminin 5 . Arch Plast Surg . 2022 ; 49 ( 3 ): 304 – 309 . doi: 10.1055/s-0042-1748642
Peng Z , Chen B , Zheng Q , et al. Ameliorative effects of peptides from the oyster ( Crassostrea hongkongensis ) protein hydrolysates against UVB-induced skin photodamage in mice . Mar Drugs . 2020 ; 18 ( 6 ): 288 . doi: 10.3390/md18060288
Qin P , Tang J , Sun D , et al. Zn2+ cross-linked alginate carrying hollow silica nanoparticles loaded with RL-QN15 peptides provides promising treatment for chronic skin wounds . ACS Appl Mater Interfaces . 2022 ; 14 ( 26 ): 29491 – 29505 . doi: 10.1021/acsami.2c03583
United States Patent, Patent No.: WO2023014375A1. 2023 .
United States Patent, Patent No.: US 11,331,305 B2. 2022 .
United States Patent, Patent No.: US 10, 543,195 B2. 2020 .
United States Patent, Patent No.: US 9,375,398 B2. 2017 .
Gartz M , Darlington A , Afzal MZ , Strande JL . Exosomes exert cardioprotection in dystrophin-deficient cardiomyocytes via ERK1/2-p38/MAPK signaling . Sci Rep . 2018 ; 8 ( 1 ): 16519 . doi: 10.1038/s41598-018-34879-6
Geng HY , Feng ZJ , Zhang JJ , Li GY . Exosomal CLIC1 released by CLL promotes HUVECs angiogenesis by regulating ITGβ1-MAPK/ERK axis . Kaohsiung J Med Sci . 2021 ; 37 ( 3 ): 226 – 235 . doi: 10.1002/kjm2.12287
Zhang B , Gong J , He L , et al. Exosomes based advancements for application in medical aesthetics . Front Bioeng Biotechnol . 2022 ; 10 : 1083640 . doi: 10.3389/fbioe.2022.1083640
Clinicaltrials.gov. ClinicalTrials.gov. Accessed January 4, 2024. https://clinicaltrials.gov/
Office of the Commissioner . FDA warns about stem cell therapies. U.S. Food and Drug Administration. (available at https://www.fda.gov/consumers/consumer-updates/fda-warns-about-stem-cell-therapies )
CTG Labs—NCBI. https://clinicaltrials.gov/study/NCT05243368? intr=exosome&term=skin&page=2&rank=13 .
Popowski K , Lutz H , Hu S , George A , Dinh PU , Cheng K . Exosome therapeutics for lung regenerative medicine . J Extracell Vesicles . 2020 ; 9 ( 1 ): 1785161 . doi: 10.1080/20013078.2020.1785161
Lupo MP , Cole AL . Cosmeceutical peptides . Dermatol Ther . 2007 ; 20 ( 5 ): 343 – 349 . doi: 10.1111/j.1529-8019.2007.00148.x
Imhof L , Leuthard D . Topical over-the-counter antiaging agents: an update and systematic review . Dermatology . 2021 ; 237 ( 2 ): 217 – 229 . doi: 10.1159/000509296
Robinson F , Doughty NC , Dawes DG , Berge NC , Bissett CA , L D . Topical palmitoyl pentapeptide provides improvement in photoaged human facial skin . Int J Cosmet Sci . 2005 ; 27 ( 3 ): 155 – 160 . doi: 10.1111/j.1467-2494.2005.00261
Lintner K . Promoting production in the extracellular matrix without compromising barrier . Cutis (New York, NY) . 2002 ; 70 ( 6S ): 13 – 16 .
Fu JJJ , Hillebrand GG , Raleigh P , et al. A randomized, controlled comparative study of the wrinkle reduction benefits of a cosmetic niacinamide/peptide/retinyl propionate product regimen vs. a prescription 0·02% tretinoin product regimen . Br J Dermatol . 2010 ; 162 ( 3 ): 647 – 654 . doi: 10.1111/j.1365-2133.2009.09436
Maquart FX , Bellon G , Chaqour B , et al. In vivo stimulation of connective tissue accumulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine Cu2+ in rat experimental wounds . J Clin Invest . 1993 ; 92 ( 5 ): 2368 – 2376 . doi: 10.1172/JCI116842
Maquart FX , Pickart L , Laurent M , Gillery P , Monboisse J-C , Borel J-P . Stimulation of collagen synthesis in fibroblast cultures by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu 2 . FEBS Lett . 1988 ; 238 ( 2 ): 343 – 346 . doi: 10.1016/0014-5793(88)80509-X
Pollard JD , Quan S , Kang T , Koch RJ . Effects of copper tripeptide on the growth and expression of growth factors by normal and irradiated fibroblasts . Arch Facial Plast Surg . 2005 ; 7 ( 1 ): 27 – 31 . doi: 10.1001/archfaci.7.1.27
Shi VY , Burney W , Shakhbazova A , et al. The effect of synthetic acetylhexapeptide-8 (AH8) on sebaceous function . Int J Cosmet Sci . 2022 ; 44 ( 4 ): 477 – 483 . doi: 10.1111/ics.12795
Kavali CM , Nguyen TQ , Zahr AS , Jiang LI , Kononov T. A randomized, double-blind, split-body, placebo-controlled clinical study to evaluate the efficacy and tolerability of a topical body firming moisturizer for upper arm rejuvenation . Aesthet Surg J. 2021 ; 41 ( 6 ): NP472 – NP483 . doi: 10.1093/asj/sjaa134
Author notes
Email alerts, more on this topic, citing articles via.
- Recommend to Your Librarian
- Advertising and Corporate Services
Affiliations
- Online ISSN 2631-4797
- Copyright © 2024 The Aesthetic Society
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Open access
- Published: 13 December 2023
Arts and creativity interventions for improving health and wellbeing in older adults: a systematic literature review of economic evaluation studies
- Grainne Crealey 1 ,
- Laura McQuade 2 ,
- Roger O’Sullivan 2 &
- Ciaran O’Neill 3
BMC Public Health volume 23 , Article number: 2496 ( 2023 ) Cite this article
1173 Accesses
22 Altmetric
Metrics details
As the population ages, older people account for a larger proportion of the health and social care budget. A significant body of evidence suggests that arts and creativity interventions can improve the physical, mental and social wellbeing of older adults, however the value and/or cost-effectiveness of such interventions remains unclear.
We systematically reviewed the economic evidence relating to such interventions, reporting our findings according to PRISMA guidelines. We searched bibliographic databases (MEDLINE, EMBASE, Econlit and Web of Science and NHSEED), trial registries and grey literature. No language or temporal restrictions were applied. Two screening rounds were conducted independently by health economists experienced in systematic literature review. Methodological quality was assessed, and key information extracted and tabulated to provide an overview of the published literature. A narrative synthesis without meta-analysis was conducted.
Only six studies were identified which provided evidence relating to the value or cost-effectiveness of arts and creativity interventions to improve health and wellbeing in older adults. The evidence which was identified was encouraging, with five out of the six studies reporting an acceptable probability of cost-effectiveness or positive return on investment (ranging from £1.20 to over £8 for every £1 of expenditure). However, considerable heterogeneity was observed with respect to study participants, design, and outcomes assessed. Of particular concern were potential biases inherent in social value analyses.
Conclusions
Despite many studies reporting positive health and wellbeing benefits of arts and creativity interventions in this population, we found meagre evidence on their value or cost-effectiveness. Such evidence is costly and time-consuming to generate, but essential if innovative non-pharmacological interventions are to be introduced to minimise the burden of illness in this population and ensure efficient use of public funds. The findings from this review suggests that capturing data on the value and/or cost-effectiveness of such interventions should be prioritised; furthermore, research effort should be directed to developing evaluative methods which move beyond the confines of current health technology assessment frameworks, to capture a broader picture of ‘value’ more applicable to arts and creativity interventions and public health interventions more generally.
PROSPERO registration
CRD42021267944 (14/07/2021).
Peer Review reports
The number and proportion of older adults in the population has increased in virtually every country in the world over past decades [ 1 ]. In 2015, there were around 901 million people aged 60 years and over worldwide, by 2030, this will have increased to 1.4 billion [ 2 ]. An ageing population is one of the greatest successes of public health but it has implications for economies in numerous ways: slower labour force growth; working-age people will have to make greater provisions in welfare payments for older people who are no longer economically active; provisions for increased long-term care; and, society must adjust to the changing needs, expectations and capabilities of an expanding group of its citizens.
The Covid-19 pandemic shone an uncompromising light on the health and social care sector, highlighting the seriousness of gaps in policies, systems and services. It also focused attention on the physical and mental health consequences of loneliness and social isolation. To foster healthy ageing and improve the lives of older people, their families and communities, sustained and equitable investment in health and wellbeing is required [ 3 ]. The prevailing model of health and social care which is based ostensibly on formal care provision is unlikely to be sustainable over the longer term. New models, which promote healthy ageing and recognise the need for increasing reliance on self-care are required, as will be evidence of their effectiveness, cost-effectiveness and scalability.
Arts and creativity interventions (ACIs) can have positive effects on health and well-being, as several reviews have shown [ 4 , 5 ]. For older people, ACI’s can enhance wellbeing [ 6 , 7 , 8 , 9 ], quality of life [ 10 , 11 ] and cognitive function [ 12 , 13 , 14 , 15 , 16 ]. They can also foster social cohesion [ 17 , 18 , 19 ] and reduce social disparities and injustices [ 20 ]; promote healthy behaviour; prevent ill health (including enhancing well-being and mental health) [ 21 , 22 , 23 , 24 , 25 ], reducing cognitive decline [ 26 , 27 ], frailty [ 28 , 29 , 30 , 31 , 32 , 33 ] and premature mortality [ 34 , 35 , 36 , 37 , 38 ]); support people with stroke [ 39 , 40 , 41 , 42 ]; degenerative neurological disorders and dementias and support end of life care [ 43 , 44 ]. Moreover, ACIs can benefit not only individuals, but also others, such as supporting the well-being of formal and informal carers, enriching our knowledge of health, and improving clinical skills [ 4 , 5 ].
The benefits of ACIs have also been acknowledged at a governmental level by those responsible for delivering health and care services: The UK All-Party Parliamentary Special Interest group on Arts, Health and Wellbeing produced a comprehensive review of creative intervention for health and wellbeing [ 45 ]. This report contained three key messages: that the arts can keep us well, aid recovery and support longer better lived lives; they can help meet major challenges facing health and social care; and that the arts can save money for the health service and social care.
Despite robust scientific evidence and governmental support, no systematic literature review has collated the evidence with respect to the value, cost or cost-effectiveness of such interventions. Our objective was to assess the economic impact of ACIs aimed at improving the health and wellbeing of older adults; to determine the range and quality of available studies; identify gaps in the evidence-base; and guide future research, practice and policy.
A protocol for this review was registered at PROSPERO, an international prospective register of systematic reviews (Registration ID CRD42021267944). We used pre-determined criteria for considering studies to include in the review, in terms of types of studies, participant and intervention characteristics.
The review followed the five-step approach on how to prepare a Systematic Review of Economic Evaluations (SR-EE) for informing evidence-based healthcare decisions [ 46 , 47 , 48 ]. Subsequent to developing and registering the protocol, the International Society for Pharmacoeconomic Outcomes and Research (ISPOR) published a good practice task force report for the critical appraisal of systematic reviews with costs and cost-effectiveness outcomes (SR-CCEOs) [ 49 ]. This was also used to inform the conduct of this review.
Eligibility criteria
Full economic evaluations are regarded as the optimal type of evidence for inclusion in a SR-EE [ 46 ], hence cost-minimisation analyses (CMA), cost-effectiveness analyses (CEA), cost-utility analyses (CUA) and cost–benefit analyses (CBA) were included. Social value analyses were also included as they are frequently used to inform decision-making and commissioning of services within local government. Additionally, they represent an important intermediate stage in our understanding of the costs and consequences of public health interventions, where significant challenges exist with regard to performing full evaluations [ 50 , 51 , 52 , 53 ].
Development of search strategies
The population (P), intervention (I), comparator (C) and outcomes (O) (PICO) tool provided a framework for development of the search strategy. Studies were included if participants were aged 50 years or older (or if the average age of the study population was 50 years or over). Interventions could relate to performance art (dance, singing, theatre, drama etc.), creative and visual arts (painting, sculpture, art making and design), or creative writing (writing narratives, poetry, storytelling). The intervention had to be active (for example, creating art as opposed to viewing art; playing an instrument as opposed to listening to music). The objective of the intervention had to be to improve health and wellbeing; it had to be delivered under the guidance of a professional; delivered in a group setting and delivered on more than one occasion. No restrictions were placed on the type of comparator(s) or the type of outcomes captured in the study. We deliberately limited the study to professionally led activities to provide a sharper distinction between social events where arts and creativity may occur and arts and creativity interventions per se. We set no language restriction nor a restriction on the date from which studies were reported.
Search methods
PRESS (peer-review electronic search strategies) guidelines informed the design our search strategy [ 54 , 55 ] and an information specialist adapted the search terms (outlined in Table S 1 ) for the following electronic bibliographic databases: MEDLINE, PubMed, EMBASE, Econlit and Web of Science and NHSEED. We also inspected references of all relevant studies; and searched trials registers (ClinicalTrials.gov). Search terms used included cost, return on investment, economic, arts, music, storytelling, dancing, writing and older adult as well as social return on investment (SROI). The last search was performed on 09/11/2022. As many economic evaluations of ACIs (especially SROIs) are commissioned by government bodies or charitable organisations, a search of the grey literature was undertaken.
Handling searches
A PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart was used to document study selection, illustrating the numbers of records retrieved and selection flow through the screening rounds [ 56 , 57 , 58 ]; all excluded records (with rationale for exclusion) were documented.
Selection of studies
Two screening rounds were conducted independently by two health economists experienced in undertaking reviews (GC, CO’N). The first round screened the title and abstract of articles based on the eligibility criteria; those selected at this stage entered a second round of full text screening with eligibility based on the inclusion and exclusion criteria. Any disagreements were discussed among the two reviewers, with access to a third reviewer available to resolve disagreements, though this proved unnecessary.
Data extraction and management
Two reviewers extracted relevant information independently using an proforma developed specifically for the purposes of this study, which included all 35 items suggested by Wijnen et al. (2016) [ 48 ]. Information was extracted in relation to the following factors: (1) general information including study title, author, year, funding source, country, setting and study design; (2) recruitment details, sample size, demographic characteristics (age, gender) and baseline health data (diagnosis, comorbidities); (3) interventions, effectiveness and cost data; (4) type of economic evaluation, perspective, payer, beneficiary, time horizon, measure of benefit and scale of intervention; (5) quality assessment, strength of evidence, any other important information; (6) results; (7) analysis of uncertainty and (8) conclusions. The quality assessment/risk of bias checklists were included in the data extraction proforma, and picklists were used to enhance uniformity of responses. The data extraction form was piloted by two reviewers (GC and CON) on one paper and discussion used to ensure consistent application thereafter.
Assessment of study quality
Two reviewers (GC & CON) independently assessed study quality, with recourse to a third reviewer for resolution of differences though this proved unnecessary. Quality assessment was based on the type of economic evaluation undertaken. Full and partial trial-based economic evaluations were assessed using the CHEC-extended checklist [ 59 ]. SROI analyses were assessed using a SROI-specific quality framework developed for the purpose of systematic review [ 60 ].
Data analysis methods
Due to the small number of evaluations detected, possible sources of heterogeneity and a lack of consensus on appropriate methods for pooling cost-effectiveness estimates [ 61 ] a narrative synthesis analysis was undertaken.
Database searches returned 11,619 records; from this, 402 duplicates were removed leaving 11,214 reports. From these 113 reports were assessment against the inclusion and exclusion criteria resulting in 4 studies for inclusion in the review. Over 40 websites were searched for relevant content returning 2 further studies for inclusion. The PRISMA 2020 diagram is presented in Fig. 1 . A high sensitivity search strategy was adopted to ensure all relevant studies were identified, resulting in a large number of studies being excluded at the first stage of screening.
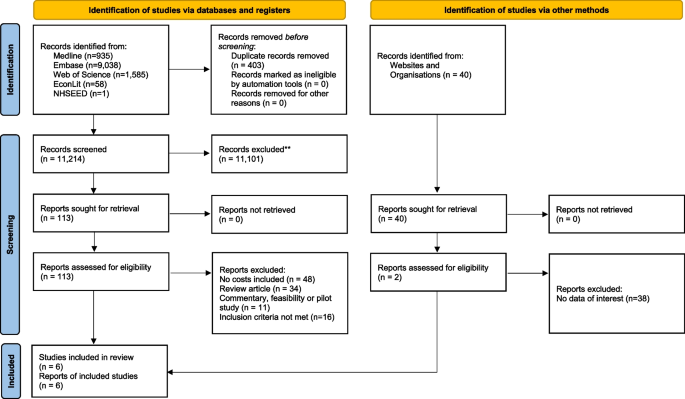
PRISMA 2020 flow diagram for new systematic reviews which include searches of databases, registers and other sources
A total of six studies were identified; key characteristics are presented in Table 1 . Identified studies were published between 2011 and 2020. Two studies used a health technology assessment (HTA) framework alongside clinical trials [ 62 , 63 ] to assess the cost-effectiveness of community singing interventions. Both evaluations scored highly on the CHEC-extended checklist (Table 2 ), with findings reported in line with the CHEERS (Consolidated Health Economic Estimation Reporting Standards) checklist 2022 [ 64 ].
Four further studies employed an SROI framework to assess art and/or craft interventions: two studies were published in the peer-reviewed literature [ 65 , 66 ] and a further two in the grey literature [ 67 , 68 ]. All four adhered closely to the suggested steps for performing an SROI and consequently secured high scores (Table 3 ). No quality differential was discerned between those studies published in the academic literature when compared with those from the grey literature.
Five of the studies were undertaken in the UK [ 63 , 66 , 67 , 68 , 69 ] and one in the US [ 63 ]. Four of the studies were designed for older adults with no cognitive impairment [ 62 , 63 , 67 , 68 ]; one was designed for participants with or without dementia [ 65 ], and another was specifically for older adults with dementia and their caregivers [ 66 ]. Three of the studies were delivered in a community setting [ 62 , 63 , 67 ], two in care homes [ 65 , 68 ] and one across a range of settings (hospital, community and residential) [ 66 ]. The length and duration of the ACIs varied; some lasted 1–2 h (with multiple classes available to participants) [ 65 ], whereas others were structured programmes with sessions lasting 90 min over a 14-week period [ 62 ]. The number of participants included in studies varied; the largest study contained data from 390 participants [ 63 ], whereas other studies measured engagement using numbers of care homes or housing associations included [ 67 , 68 ].
Costs were captured from a narrower perspective (i.e., the payer—health service) for those economic evaluations which followed a health technology assessment (HTA) framework [ 62 , 63 ]. Costs associated with providing the programme and health and social care utilisation costs were captured using cost diaries. Valuation of resource usage was in line with the reference case specified for each jurisdiction.
Social value analyses included in the review [ 65 , 66 , 67 , 68 ] captured a broader picture of cost; programme provision costs included were similar in nature to those identified using an HTA framework, however, the benefits captured went beyond the individual to capture costs to a wide range of stakeholders such as family members, activity co-ordinations and care home personnel. Costs were apportioned using financial proxies from a range of sources including HACT Social Value Bank [ 69 ] and market-based valuation methods.
The range of outcomes captured and valued across HTAs and SROIs was extensive: including, but not limited to, wellbeing, quality of life, physical health, cognitive functioning, communication, control over daily life choices, engagement and empowerment, social isolation, mobility, community inclusion, depressive symptoms, sadness, anxiety, loneliness, positive affect and interest in daily life. In the programmes assessed using an HTA framework, outcomes were captured using standardised and validated instruments, for both control and intervention groups across multiple time points. Statistical methods were used to assess changes in outcomes over time. Programmes assessed using SROI relied primarily on qualitative methods (such as reflective diaries and in-depth interviews) combined with routinely collected administrative data.
The evidence from the singing interventions was encouraging but not conclusive. The ‘Silver Song Club’ programme [ 62 ] reported a 64% probability of being cost-effective at a willingness-to-pay threshold of £30,000. This study was also included in the Public Health England (PHE) decision tool to support local commissioners in designing and implementing services to support older people’s healthy ageing, reporting a positive societal return on investment [ 70 ]. Evidence from the ‘Community of Voices’ trial [ 63 ] suggested that although intervention group members experienced statistically significant improvements in loneliness and interest in life compared to control participants, no significant group differences were observed for cognitive or physical outcomes or for healthcare costs.
A positive return on investment was reported by all social value analyses undertaken. The ‘Imagine Arts’ programme, reported a positive SROI of £1.20 for every £1 of expenditure [ 65 ]. A higher yield of between £3.20-£6.62 for each £1 invested was reported in the ‘Dementia and Imagination’ programme [ 66 ]. The ‘Craft Café’ programme, reported an SROI of £8.27 per £1 invested [ 68 ], and the ‘Creative Caring’ programme predicted a SROI of between £3 to £4 for every £1 spent [ 67 ]. The time period over which return on investment was calculated differed for each evaluation from less than one year to 4 years.
The primary finding from our review concerns the paucity of evidence relating to the value, cost and/or cost-effectiveness of ACIs aimed at improving health and wellbeing in this population. Despite few restrictions being applied to our search, only six studies were found which met our inclusion criteria. This is not indicative of research into ACIs in this population, as evidenced by the identification of ninety-three studies where arts and creativity interventions were found to support better health and wellbeing outcomes in another recent review [ 5 ]. An alternative explanation is that funders do not see the added value of undertaking such evaluations in this area. That is, for funders, the cost of evaluating an ACIs is likely to be deemed unjustified given the relatively small welfare loss a misallocation of resources to them might produce. While at first glance this may seem reasonable, it disadvantages ACIs in competing with other interventions for funding and arguably exposes an implicit prejudice in the treatment of interventions from which it may be difficult to extract profit in general. That is, the paucity of evidence, may reflect inherent biases within our political economy that favour the generation of marketable solutions to health issues from which value can be appropriated as profit. Pharmaceuticals are an obvious example of such solutions, where the literature is replete with examples of evaluations sponsored by pharmaceutical companies or where public funds are used to test the claims made by pharmaceutical companies in respect of the value of their products. If the potential of ACIs to improve health and well-being is to be robustly established, ACIs must effectively compete for funding with other interventions including those from pharma. This requires a larger, more robust evidence base than is currently available and investment in the creation of such an evidence base. As there is currently no ‘for-profit’ industry to generate such an evidence base, public funding of evaluations will be central to its creation.
Our second finding concerns the values reported in the meagre evidence we did find. In five of the six studies we identified, evidence indicated that ACIs targeted at older people offered value for money [ 62 , 65 , 66 , 67 , 68 ]. One study provided mixed evidence [ 63 ], however, in this study a ‘payer’ perspective was adopted when applying an HTA framework which, by virtue of the perspective adopted, excluded a range of benefits attributable to ACIs and public health interventions more generally. Among the four studies that adopted a SROI approach, estimated returns per £1 invested ranged from £1.20 to £8.27. Given the evident heterogeneity among studies in terms of context and methods, care is warranted in comparing estimates with each other or with other SROIs. Care is also required in accepting at face value the estimates reported given methodological issues that pertain to the current state of the art with respect to SROI. With these caveats in mind noted, the values reported for ACIs using the SROI approach are comparable with those from other SROI studies in other contexts including those as diverse as a first aid intervention [ 71 ], investment in urban greenways [ 72 ] and the provision of refuge services to those experiencing domestic violence [ 73 ] (a return on investment of £3.50-£4, £2.88-£5.81 and £4.94 respectively). Similarly, with respect to the study that adopted a cost-effectiveness approach, Coulton and colleagues (2015) reported a 64% probability of the intervention being cost-effective at a threshold of £30,000 [ 62 ]. Again, it is difficult to compare studies directly, but this is similar to that reported for interventions as diverse as a falls prevention initiative [ 74 ] and the treatment of depression using a collaborative approach [ 75 ] both in the UK. That the evidence base is meagre notwithstanding, there is, in other words, a prima facie case that ACIs are capable of offering value for money when targeted at older persons.
Our third finding relates to the state of the art with respect to SROIs in this area. Over the past 40 years, considerable time, effort and resources have been expended in the development of cost-effectiveness techniques in health and social care. While considerable heterogeneity can exist around their conduct, national guidance exists in many jurisdictions on the conduct of cost-effectiveness analyses (CEA) – such as the NICE reference case in the UK [ 76 ]– as well as in the reporting of these as set out in the CHEERS 2022 guidance [ 64 ]. This has helped raise the quality of published evaluations and the consistency with which they are reported. Despite the existence of a step-by-step guidance document on how to perform SROIs [ 77 ] which outlines how displacement effects, double counting, effect attribution and drop-off should be addressed, a significant body of work still remains to ensure that the methodology addresses a range of known biases in a robust manner. Where there is no comparator to the intervention being evaluated (as was the case in the SROIs reported here) it may be difficult to convince funders that the implicit incremental costs and benefits reported are indeed incremental and attributable to the intervention. Equally, where a comparator is present, greater consensus and standardisation is required regarding the identification, generation and application of, for example, financial proxies. Currently, SROI ratios combine value across a wide range of stakeholders, which is understandable if the objective is to capture all aspects of social benefit generated. This ratio, however, may not reflect the priorities and statutory responsibilities of healthcare funders. Whist all of the aforementioned issues can be addressed, investment is required to develop the SROI methodology further to more closely meet the needs of commissioning bodies.
Notwithstanding these challenges, social value analyses play a pivotal role within the procurement processes employed by government, local authorities and other non-departmental public bodies and should not be dismissed simply because the ‘burden of proof’ falls short of that required to secure remuneration within the health sector. As most SROIs are published in the grey literature, this means they often avoid peer scrutiny prior to publication and the potential quality assurance this can offer. It is noteworthy however that two of the SROIs included in this review [ 65 , 66 ] were published in the academic literature, suggesting that the academic community are engaging with this method which is to be applauded.
Moving forward, it is unlikely we will be able to meet all of the health and wellbeing needs of our ageing population solely in a primary or secondary care setting. New models of care are required, as are new models of funding to support interventions which can be delivered in non-healthcare settings. New hybrid models of evaluation will be required to provide robust economic evidence to assist in the allocation of scarce resources across health and non-healthcare settings; such evaluative frameworks must have robust theoretical underpinnings and be capable of delivering evidence from a non-clinical setting in a timely and cost-effective manner.
In the absence of a definitive evaluation framework for ACIs being currently available, we have a number of recommendations. First, and most importantly, all impact assessments should have a control group or credible counterfactual. This is currently not required when performing an SROI making it difficult to determine if all of the benefits ascribed to an intervention are in fact attributable. This recommendation is in line with the conclusion of a report by the London School of Economics [ 78 ] for the National Audit Office (NAO) which concluded that ‘any impact evaluation (and subsequent value for money calculation) requires construction of a counterfactual’. Second, a detailed technical appendix should accompany all impact assessments to allow independent review by a subject specialist. While this would assist peer review, it would allow providing greater transparency where peer review was not undertaken prior to publication. Furthermore, it would enable recalculation of SROI ratios to exclude ‘value’ attributable to stakeholders which are not relevant to a particular funder. Third, equity considerations should be addressed explicitly in all evaluations (this is currently not required in HTAs). Fourth, both costs and outcomes should be captured from a ‘broad’ perspective (adopting a ‘narrow’ healthcare perspective may underestimate the full economic impact), with non-healthcare sector costs being detailed as part of the analysis. Finally, data should be collected post-implementation to ensure that resources continue to be allocated efficiently.
As with any review, there are limitations which should be noted. A search of the grey literature was included as evaluations of applied public health interventions are not always reported in the academic literature. Systematically identifying grey literature and grey data can be problematic [ 79 , 80 , 81 , 82 , 83 ] as it is not collected, organised or stored in a consistent manner. Hence it is possible that we have not identified all relevant studies. Furthermore, as applied public health interventions can be performed in a non-healthcare setting we included SROIs in our review of economic evaluations. Current guidance on the systematic review of economic evaluations has been developed primarily for review of HTA as opposed to public health interventions and hence SROIs would be excluded, or if included would score poorly due to the inherent biases arising from no comparator or counterfactual being included.
This systematic review found that participation in group-based arts and creativity programmes was generally cost-effective and/or produced a positive return on investment whilst having a positive impact on older people’s physical, psychological, and social health and wellbeing outcomes. Unfortunately, the small number of studies identified, coupled with differences in methods used to assess economic impact hinders our ability to conclusively determine which types of art and creativity-based activities are more cost-effective or represent best value for money.
As well as the need for a greater focus on prevention of poor health as we age, new hybrid models of healthcare delivery are necessary to meet the needs of our ageing population. These models will integrate traditional medical care with other services such as home health aides (some of which may include artificial intelligence), telemedicine and social support networks. Alongside these, ACIs have the potential to provide a low cost, scalable, easily implementable and cost-effective solution to reduce the burden of illness in this age group and support healthy ageing.
Evidence on the cost-effectiveness of a range of ACIs is of utmost importance for policy and decision makers as it can both inform the development of policies that support the provision of ACIs in the context of ageing, but also identify the most cost-effective approaches for delivering such interventions. The development of hybrid models of evaluation, capable of capturing cost-effectiveness and social value, is becoming increasingly necessary as healthcare delivery for this age group moves beyond the realms of primary and secondary care and into the community. The development and refinement of such models will ensure a more comprehensive assessment of the impact of a diverse range of interventions providing a more nuanced understanding of the impact of an intervention. This will help inform decision making and ensure interventions are implemented in a cost-effective and socially beneficial manner.
Availability of data and materials
All data generated or analysed during this study are included in the published article and its supplementary information files.
United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2015 Revision, Key Findings and Advance Tables. Working Paper No. ESA/P/WP.241. 2015.
Office for National Statistics. Living longer: how our population is changing and why it matters. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/ageing/articles/livinglongerhowourpopulationischangingandwhyitmatters/2018-08-13#how-do-incomes-of-older-people-compare-with-younger-ages . 2018. Accessed 07/12/2022
Dyakova M, Hamelmann C, Bellis MA, Besnier E, Grey CNB, Ashton K, Schwappach A, Clar C. Investment for health and well-being: a review of the social return on investment from public health policies to support implementing the Sustainable Development Goals by building on Health 2020 [Internet]. Copenhagen: WHO Regional Office for Europe; 2017.
Google Scholar
Fancourt D, Finn S. What is the evidence on the role of the arts in improving health and well-being? A scoping review. Copenhagen: WHO Regional Office for Europe; 2019.
McQuade L, O’Sullivan R. Examining arts and creativity in later life and its impact on older people’s health and wellbeing: a systematic review of the evidence. Perspect Publ Health. 2023;0(0). https://doi.org/10.1177/17579139231157533
Skingley A, De’Ath S, Napleton L. Evaluation of Edna: arts and dance for older people. Work Older People. 2016;20(1):46–56.
Article Google Scholar
Brustio PR, Liubicich ME, Chiabrero M, et al. Dancing in the golden age: a study on physical function, quality of life, and social engagement. Geriatr Nurs. 2018;39(6):635–9.
Article PubMed Google Scholar
Beauchet O, Bastien T, Mittelman M, et al. Participatory art-based activity, community-dwelling older adults and changes in health condition: results from a pre-post intervention, single-arm, prospective and longitudinal study. Maturitas. 2020;134:8–14.
Article CAS PubMed Google Scholar
Roswiyani R, Hiew CH, Witteman CLM, et al. Art activities and qigong exercise for the well-being of older adults in nursing homes in Indonesia: a randomized controlled trial. Aging Ment Health. 2020;24(10):1569–78.
Shanahan J, Bhriain ON, Morris ME, et al. Irish set dancing classes for people with Parkinson’s disease: the needs of participants and dance teachers. Complement Ther Med. 2016;27:12–7.
Garcia Gouvêa JA, Antunes MD, Bortolozzi F, et al. Impact of senior dance on emotional and motor parameters and quality of life of the elderly. Rev Rene. 2017;18(1):51–8.
Sun J, Zhang N, Buys N, et al. The role of Tai Chi, cultural dancing, playing a musical instrument and singing in the prevention of chronic disease in Chinese older adults: a mind–body meditative approach. Int J Ment Health Pr. 2013;15:227–39.
Fu MC, Belza B, Nguyen H, et al. Impact of group-singing on older adult health in senior living communities: a pilot study. Arch Gerontol Geriatr. 2018;76:138–46.
Feng L, Romero-Garcia R, Suckling J, et al. Effects of choral singing versus health education on cognitive decline and aging: a randomized controlled trial. Aging-us. 2020;12(24):24798–816.
Seinfeld S, Figueroa H, Ortiz-Gil J, et al. Effects of music learning and piano practice on cognitive function, mood and quality of life in older adults. Front Psychol. 2013;4:810.
Article PubMed PubMed Central Google Scholar
MacRitchie J, Breaden M, Milne AJ, et al. Cognitive, motor and social factors of music instrument training programs for older adults’ improved wellbeing. Front Psychol. 2020;10:2868.
Freeman WJI. A neurobiological role of music in social bonding. In: Wallin N, Merkur B, Brown S, editors. The origins of music. Cambridge: MIT Press; 2000. http://escholarship.org/uc/item/9025x8rt .
Huron D. Is music an evolutionary adaptation? Ann N Y Acad Sci. 2001;930(1):43–61. https://doi.org/10.1111/j.1749-6632.2001.tb05724.x .
Tarr B, Launay J, Dunbar RIM. Music and social bonding: “self–other” merging and neurohormonal mechanisms. Front Psychol. 2014;5:1096. https://doi.org/10.3389/fpsyg.2014.01096 .
Cain M, Lakhani A, Istvandity L. Short and long term outcomes for culturally and linguistically diverse (cald) and at-risk communities in participatory music programs: a systematic review. Arts Health. 2016;8(2):105–24. https://doi.org/10.1080/17533015.2015.1027934 .
Martin L, Oepen R, Bauer K, Nottensteiner A, Mergheim K, Gruber H, et al. Creative arts interventions for stress management and prevention – a systematic review. Behav Sci (Basel). 2018;8(2):pii:E28. https://doi.org/10.3390/bs8020028 .
Linnemann A, Wenzel M, Grammes J, Kubiak T, Nater UM. Music listening and stress in daily life: a matter of timing. Int J Behav Med. 2018;25(2):223–30. https://doi.org/10.1007/s12529-017-9697-5 .
Linnemann A, Strahler J, Nater UM. The stress-reducing effect of music listening varies depending on the social context. Psychoneuroendocrinology. 2016;72:97–105. https://doi.org/10.1016/j.psyneuen.2016.06.003 .
Panteleeva Y, Ceschi G, Glowinski D, Courvoisier DS, Grandjean DM. Music for anxiety? meta-analysis of anxiety reduction in non-clinical samples. Psychol Music. 2017;46(4):473–87. https://doi.org/10.1177/0305735617712424 .
Fancourt D, Tymoszuk U. Cultural engagement and incident depression in older adults: evidence from the English longitudinal study of ageing. Br J Psychiatry. 2018;214(4):225–9. https://doi.org/10.1192/bjp.2018.267 .
Balbag MA, Pedersen NL, Gatz M. Playing a musical instrument as a protective factor against dementia and cognitive impairment: a population-based twin study. Int J Alzheimer’s Dis. 2014;2014:836748. https://doi.org/10.1155/2014/836748 .
Porat S, Goukasian N, Hwang KS, Zanto T, Do T, Pierce J, et al. Dance experience and associations with cortical gray matter thickness in the aging population. Dement Geriatr Cogn Dis Extra. 2016;6(3):508–17. https://doi.org/10.1159/000449130 .
Federici A, Bellagamba S, Rocchi MBL. Does dance-based training improve balance in adult and young old subjects? a pilot randomized controlled trial. Aging Clin Exp Res. 2005;17(5):385–9 PMID: 16392413.
Alpert PT, Miller SK, Wallmann H, Havey R, Cross C, Chevalia T, et al. The effect of modified jazz dance on balance, cognition, and mood in older adults. J Am Acad Nurse Pract. 2009;21(2):108–15. https://doi.org/10.1111/j.1745-7599.2008.00392.x .
Jeon MY, Bark ES, Lee EG, Im JS, Jeong BS, Choe ES. The effects of a Korean traditional dance movement program in elderly women. Taehan Kanho Hakhoe Chi. 2005;35(7):126876 (in Korean). PMID: 16418553.
Eyigor S, Karapolat H, Durmaz B, Ibisoglu U, Cakir S. A randomized controlled trial of Turkish folklore dance on the physical performance, balance, depression and quality of life in older women. Arch Gerontol Geriatr. 2009;48(1):84–8. https://doi.org/10.1016/j.archger.2007.10.008 .
Noopud P, Suputtitada A, Khongprasert S, Kanungsukkasem V. Effects of Thai traditional dance on balance performance in daily life among older women. Aging Clin Exp Res. 2018;31(7):961–7. https://doi.org/10.1007/s40520-018-1040-8 .
Trombetti A, Hars M, Herrmann FR, Kressig RW, Ferrari S, Rizzoli R. Effect of musicbased multitask training on gait, balance, and fall risk in elderly people: a randomized controlled trial. Arch Intern Med. 2011;171(6):525–33. https://doi.org/10.1001/archinternmed.2010.446 .
Hyyppä MT, Mäki J, Impivaara O, Aromaa A. Individual-level measures of social capital as predictors of all-cause and cardiovascular mortality: a population-based prospective study of men and women in Finland. Eur J Epidemiol. 2007;22(9):589–97. https://doi.org/10.1007/s10654-007-9153-y .
Hyyppä MT, Mäki J, Impivaara O, Aromaa A. Leisure participation predicts survival: a population-based study in Finland. Health Promot Int. 2006;21(1):5–12. https://doi.org/10.1093/heapro/dai027 .
Lennartsson C, Silverstein M. Does engagement with life enhance survival of elderly people in Sweden? the role of social and leisure activities. J Gerontol B Psychol Sci Soc Sci. 2001;56(6):S335–42. https://doi.org/10.1093/geronb/56.6.s335 .
Sundquist K, Lindström M, Malmström M, Johansson SE, Sundquist J. Social participation and coronary heart disease: a follow-up study of 6900 women and men in Sweden. Soc Sci Med. 1982;58(3):615–22. https://doi.org/10.1016/s0277-9536(03)00229-6 .
Väänänen A, Murray M, Koskinen A, Vahtera J, Kouvonen A, Kivimäki M. Engagement in cultural activities and cause-specific mortality: prospective cohort study. Prev Med. 2009;49(2–3):142–7. https://doi.org/10.1016/j.ypmed.2009.06.026 .
Särkämö T, Soto D. Music listening after stroke: beneficial effects and potential neural mechanisms. Ann N Y Acad Sci. 2012;1252(1):266–81. https://doi.org/10.1111/j.1749-6632.2011.06405.x .
Särkämö T, Pihko E, Laitinen S, Forsblom A, Soinila S, Mikkonen M, et al. Music and speech listening enhance the recovery of early sensory processing after stroke. J Cogn Neurosci. 2010;22(12):2716–27. https://doi.org/10.1162/jocn.2009.21376 .
Särkämö T, Ripollés P, Vepsäläinen H, Autti T, Silvenno HM, Salli E, et al. Structural changes induced by daily music listening in the recovering brain after middle cerebral artery stroke: a voxel-based morphometry study. Front Hum Neurosci. 2014;8:245. https://doi.org/10.3389/fnhum.2014.00245 .
Särkämö T, Tervaniemi M, Laitinen S, Forsblom A, Soinila S, Mikkonen M, et al. Music listening enhances cognitive recovery and mood after middle cerebral artery stroke. Brain. 2008;131(3):866–76. https://doi.org/10.1093/brain/awn013 .
Fancourt D, Steptoe A, Cadar D. Cultural engagement and cognitive reserve: museum attendance and dementia incidence over a 10-year period. Br J Psychiatry. 2018;213(5):661–3. https://doi.org/10.1192/bjp.2018.129 .
Fancourt D, Steptoe A, Cadar D. Cultural engagement predicts changes in cognitive function in older adults over a 10 year period: findings from the English longitudinal study of ageing. Sci Rep. 2018;8(1):10226. https://doi.org/10.1192/bjp.2018.129 .
All Party Parliamentary group on arts, health and wellbeing. Creative health: the arts for health and wellbeing. 2017.
van Mastrigt GA, Hiligsmann M, Arts JJ, Broos PH, Kleijnen J, Evers SM, Majoie MH. How to prepare a systematic review of economic evaluations for informing evidence-based healthcare decisions: a five-step approach (part 1/3). Expert Rev Pharmacoecon Outcomes Res. 2016;16(6):689–704. https://doi.org/10.1080/14737167.2016.1246960 . Epub 2016 Nov 2 PMID: 27805469.
Thielen FW, Van Mastrigt G, Burgers LT, Bramer WM, Majoie H, Evers S, Kleijnen J. How to prepare a systematic review of economic evaluations for clinical practice guidelines: database selection and search strategy development (part 2/3). Expert Rev Pharmacoecon Outcomes Res. 2016;16(6):705–21. https://doi.org/10.1080/14737167.2016.1246962 . Epub 2016 Nov 2 PMID: 27805466.
Wijnen B, Van Mastrigt G, Redekop WK, Majoie H, De Kinderen R, Evers S. How to prepare a systematic review of economic evaluations for informing evidence-based healthcare decisions: data extraction, risk of bias, and transferability (part 3/3). Expert Rev Pharmacoecon Outcomes Res. 2016;16(6):723–32. https://doi.org/10.1080/14737167.2016.1246961 . Epub 2016 Oct 21 PMID: 27762640.
Mandrik OL, Severens JLH, Bardach A, Ghabri S, Hamel C, Mathes T, Vale L, Wisløff T, Goldhaber-Fiebert JD. Critical appraisal of systematic reviews with costs and cost-effectiveness outcomes: an ISPOR good practices task force report. Value Health. 2021;24(4):463–72. https://doi.org/10.1016/j.jval.2021.01.002 . PMID: 33840423.
Kelly MP, McDaid D, Ludbrook A, Powell J: Economic appraisal of public health interventions. http://www.cawt.com/Site/11/Documents/Publications/Population%20Health/Economics%20of%20Health%20Improvement/Economic_appraisal_of_public_health_interventions.pdf
Weatherly H, Drummond M, Claxton K, Cookson R, Ferguson B, Godfrey C, Rice N, Sculpher M, Sowden A. Methods for assessing the cost-effectiveness of public health interventions: key challenges and recommendations. Health Policy. 2009;93(2–3):85–92. https://doi.org/10.1016/j.healthpol.2009.07.012 . Epub 2009 Aug 25 PMID: 19709773.
Payne K, McAllister M, Davies LM. Valuing the economic benefits of complex interventions: when maximising health is not sufficient. Health Econ. 2012. https://doi.org/10.1002/hec.2795 .
Edwards RT, Charles JM, Lloyd-Williams H. Public health economics: a systematic review of guidance for the economic evaluation of public health interventions and discussion of key methodological issues. BMC Public Health. 2013;24(13):1001. https://doi.org/10.1186/1471-2458-13-1001.PMID:24153037;PMCID:PMC4015185 .
Rethlefsen ML, Farrell AM, Osterhaus Trzasko LC, Brigham TJ. Librarian co-authors correlated with higher quality reported search strategies in general internal medicine systematic reviews. J Clin Epidemiol. 2015;68(6):617–26. https://doi.org/10.1016/j.jclinepi.2014.11.025 . Epub 2015 Feb 7 PMID: 25766056.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. https://doi.org/10.1016/j.jclinepi.2016.01.021 . Epub 2016 Mar 19 PMID: 27005575.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2008;6:e1000097. https://doi.org/10.1371/journal.pmed.1000097 .
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;21(339):b2700. https://doi.org/10.1136/bmj.b2700.PMID:19622552;PMCID:PMC2714672 .
Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. PLoS Med. 2009;6(7):e1000097 Evers S, Goossens M, De Vet H, et al. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care. 2005;21(02):240–245.
Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care. 2005;21(2):240–5 PMID: 15921065.
Hutchinson CL, Berndt A, Gilbert-Hunt S, George S, Ratcliffe J. Valuing the impact of health and social care programmes using social return on investment analysis: how have academics advanced the methodology? A protocol for a systematic review of peer-reviewed literature. BMJ Open. 2018;8(12):e022534. https://doi.org/10.1136/bmjopen-2018-022534 . PMID:30530579;PMCID:PMC6303612.
Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.1. 0. Chichester: The Cochrane Collaboration; 2013.
Coulton S, Clift S, Skingley A, Rodriguez J. Effectiveness and cost-effectiveness of community singing on mental health-related quality of life of older people: randomised controlled trial. Br J Psychiatry. 2015;207(3):250–5. https://doi.org/10.1192/bjp.bp.113.129908 . Epub 2015 Jun 18 PMID: 26089304.
Johnson JK, Stewart AL, Acree M, Nápoles AM, Flatt JD, Max WB, Gregorich SE. A community choir intervention to promote well-being among diverse older adults: results from the community of voices trial. J Gerontol B Psychol Sci Soc Sci. 2020;75(3):549–59. https://doi.org/10.1093/geronb/gby132 . PMID:30412233;PMCID:PMC7328053.
Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, Caulley L, Chaiyakunapruk N, Greenberg D, Loder E, Mauskopf J, Mullins CD, Petrou S, Pwu RF, Staniszewska S, CHEERS 2022 ISPOR Good Research Practices Task Force. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25(1):3–9. https://doi.org/10.1016/j.jval.2021.11.1351 . PMID: 35031096.
Bosco A, Schneider J, Broome E. The social value of the arts for care home residents in England: a social return on investment (SROI) analysis of the imagine arts programme. Maturitas. 2019;124:15–24. https://doi.org/10.1016/j.maturitas.2019.02.005 . Epub 2019 Mar 13 PMID: 31097173.
Jones C, Windle G, Edwards RT. Dementia and imagination: a social return on investment analysis framework for art activities for people living with dementia. Gerontologist. 2020;60(1):112–23. https://doi.org/10.1093/geront/gny147 . PMID: 30476114.
Social Value Lab and Impact Arts Craft Café: creative solutions to isolation and loneliness; Social return on investment. 2011. http://www.socialvaluelab.org.uk/wp-content/uploads/2013/05/CraftCafeSROI.pdf
MB associates. Make my day: the impact of Creative Caring in older people’s care homes. 2013. https://www.suffolkartlink.org.uk/wp-content/uploads/2014/10/CreativeCarersSROIReport_Nov2013.pdf
HACT. n.d. UK Social Value Bank. Retrieved December 11, 2023. from https://hact.org.uk/tools-and-services/uk-social-value-bank/ .
The Older Adults’ NHS and social care return on investment tool. Project report. Public health England. December 2019. Last accessed 27/03/2023.
British Red Cross – Valuing First Aid Education. 2018. https://socialvalueuk.org/wp-content/uploads/2018/12/Valuing-First-Aid-Education-Social-Return-on-Investment-Report-on-the-value-of-First-Aid-Education-Assured-Report.pdf . Accessed 17/02/2023
Hunter R, Dallat M, Tully M, O’Neill C, Heron L, Kee F. Social return on investment analysis of an urban greenway. Cities and Health. 2020. https://doi.org/10.1080/23748834.2020.1766783 .
NEF Consulting. Refuge: A social return on investment evaluation. 2016. https://socialvalueuk.org/wp-content/uploads/2017/04/Refuge-SROI-2016.pdf Accessed 17/02/2022
Corbacho B, Cockayne S, Fairhurst C, Hewitt CE, Hicks K, Kenan AM, Lamb SE, MacIntosh C, Menz HB, Redmond AC, Rodgers S, Scantlebury A, Watson J, Torgerson DJ, on behalf of the REFORM study. Cost-Effectiveness of a Multifaceted Podiatry Intervention for the Prevention of Falls in Older People: The REducing Falls with Orthoses and a Multifaceted Podiatry Intervention Trial Findings. Gerontology. 2018;64(5):503–12. https://doi.org/10.1159/000489171 . Epub 2018 Jun 26 PMID: 29945150.
Green C, Richards DA, Hill JJ, Gask L, Lovell K, Chew-Graham C, Bower P, Cape J, Pilling S, Araya R, Kessler D, Bland JM, Gilbody S, Lewis G, Manning C, Hughes-Morley A, Barkham M. Cost-effectiveness of collaborative care for depression in UK primary care: economic evaluation of a randomised controlled trial (CADET). PLoS ONE. 2014;9(8):e104225. https://doi.org/10.1371/journal.pone.0104225.PMID:25121991;PMCID:PMC4133193 .
National Institute for Health and Care Excellence (NICE). NICE health technology evaluations: the manual. 2022. Retrieved 27 March, 2023 from https://www.nice.org.uk/process/pmg36/chapter/introduction-to-health-technology-evaluation
NEF Consulting. SSE – Beatrice SROI framework – guidance document. https://www.sse.com/media/svnn5jpk/sroi-methodology-guidance-nef-consulting.pdf . Accessed 17/02/2022
Gibbons S, McNally S, Overman H. Review of Government Evaluations: A report for the NAO. London: National Audit Office; 2013.
Turner AM, Liddy ED, Bradley J, Wheatley JA. Modeling public health interventions for improved access to the gray literature. J Med Libr Assoc. 2005;93(4):487–94 PMID: 16239945; PMCID: PMC1250325.
PubMed PubMed Central Google Scholar
Benzies KM, Premji S, Hayden KA, Serrett K. State-of-the-evidence reviews: advantages and challenges of including grey literature. Worldviews Evid Based Nurs. 2006;3(2):55–61. https://doi.org/10.1111/j.1741-6787.2006.00051.x . PMID: 17040510.
Franks H, Hardiker NR, McGrath M, McQuarrie C. Public health interventions and behaviour change: reviewing the grey literature. Public Health. 2012;126(1):12–7. https://doi.org/10.1016/j.puhe.2011.09.023 . Epub 2011 Nov 29 PMID: 22130477.
Mahood Q, Van Eerd D, Irvin E. Searching for grey literature for systematic reviews: challenges and benefits. Res Synth Methods. 2014;5(3):221–34. https://doi.org/10.1002/jrsm.1106 . Epub 2013 Dec 6 PMID: 26052848.
Godin K, Stapleton J, Kirkpatrick SI, Hanning RM, Leatherdale ST. Applying systematic review search methods to the grey literature: a case study examining guidelines for school-based breakfast programs in Canada. Syst Rev. 2015;22(4):138. https://doi.org/10.1186/s13643-015-0125-0 . PMID:26494010;PMCID:PMC4619264.
Download references
Acknowledgements
We would like to thank Ms. Louise Bradley (Information Resource Officer, Institute of Public Health) for her assistance in refining search strategies and literature search.
This study was supported by the Institute of Public Health (IPH), 200 South Circular Road, Dublin 8, Ireland, D08 NH90. This study was a collaboration between two health economists (GC, CO’N) and two members of staff from the funding organisation (LM, RO’S). Input from IPH staff was fundamental in defining the scope of work and research question, refining search terms and review and editing of the manuscript. Staff from IPH were not involved in quality assurance or review of papers included in the manuscript.
Author information
Authors and affiliations.
Clinical Costing Solutions, Belfast, BT15 4EB, UK
Grainne Crealey
Institute of Public Health, 200 South Circular Road, Dublin 8, D08 NH90, Ireland
Laura McQuade & Roger O’Sullivan
Centre for Public Health, Institute of Clinical Sciences, Royal Victoria Hospital, Belfast, BT12 6BA, UK
Ciaran O’Neill
You can also search for this author in PubMed Google Scholar
Contributions
LMcQ and ROS were involved in defining the scope of work, refining the research question, provision of subject specific (public health) context, review of search strategy, review & editing of manuscript. CON and GC were involved in refining the research question and search strategy, provision of health economics and systematic reviewing expertise, review of returned reports, original draft preparation, review, editing and submission of manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Ciaran O’Neill .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
: Table S1. Search strategy for electronic databases and grey literature.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Crealey, G., McQuade, L., O’Sullivan, R. et al. Arts and creativity interventions for improving health and wellbeing in older adults: a systematic literature review of economic evaluation studies. BMC Public Health 23 , 2496 (2023). https://doi.org/10.1186/s12889-023-17369-x
Download citation
Received : 23 April 2023
Accepted : 28 November 2023
Published : 13 December 2023
DOI : https://doi.org/10.1186/s12889-023-17369-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Economic evaluation
- Older adults
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
SYSTEMATIC REVIEW article
Evaluating the efficacy of mesenchymal stem cells for diabetic neuropathy: a systematic review and meta-analysis of preclinical studies provisionally accepted.

- 1 Department of Plastic Surgery, First Affiliated Hospital of Zhengzhou University, China
- 2 Key Laboratory of Tissue Engineering Research, Shanghai Ninth People’s Hospital, School of Medicine, Shanghai Jiao Tong University, China
The final, formatted version of the article will be published soon.
Diabetic neuropathy affects nearly half of all diabetics and poses a significant threat to public health. Recent preclinical studies suggest that mesenchymal stem cells (MSCs) may represent a promising solution for the treatment of diabetic neuropathy. However, an objective assessment of the preclinical effectiveness of MSCs is still pending. We conducted a comprehensive search of PubMed, Web of Science, Embase, and Cochrane library to identify preclinical studies that investigate the effects of MSCs on diabetic neuropathy up until 15 September 2023. Outcome indicators consisted of motor and sensory nerve conduction velocities, intra-epidermal nerve fiber density, sciatic nerve blood flow, capillary-to-muscle fiber ratio, neurotrophic factors, angiogenic factors and inflammatory cytokines. The literature review and meta-analysis were conducted independently by two researchers. 23 studies that met the inclusion criteria were included in this system review for qualitative and quantitative analysis. Pooled analyses indicated that MSCs exhibited an evident benefit in diabetic neuropathy in terms of motor (SMD=2.16, 95% CI: 1.71 to 2.61) and sensory nerve conduction velocities (SMD=2.93, 95% CI: 1.78 to 4.07), intra-epidermal nerve fiber density (SMD=3.17, 95% CI: 2.28 to 4.07), sciatic nerve blood flow (SMD=2.02, 95% CI: 1.37 to 2.66), and capillary-tomuscle fiber ratio (SMD=2.28, 95% CI: 1.55 to 3.01, P<0.00001). Furthermore, after MSC therapy, the expressions of neurotrophic and angiogenic factors increased significantly in most studies, while the levels of inflammatory cytokines were significantly reduced. The relevance of this review relies on the fact that summarizes an extensive body of work entailing substantial preclinical evidence that supports the efficacy of MSCs in mitigating diabetic neuropathy. While MSCs emerge as a promising potential treatment for diabetic neuropathy, further research is essential to elucidate the underlying mechanisms and the best administration strategy for MSCs.
Keywords: Diabetic neuropathy, Neurological Disorder, Stem Cell Therapy, Mesenchymal Stem Cells, Meta-analysis
Received: 04 Dec 2023; Accepted: 17 Apr 2024.
Copyright: © 2024 Li, Yue, Yu, Cheng, Cao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: MD, PhD. Ximei Wang, Department of Plastic Surgery, First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China
People also looked at
Unpaid caregiving and mental health during the COVID-19 pandemic-A systematic review of the quantitative literature
Affiliation.
- 1 Centre for Health Policy, Melbourne School of Population and Global Health, The University of Melbourne, Parkville, Victoria, Australia.
- PMID: 38635604
- PMCID: PMC11025839
- DOI: 10.1371/journal.pone.0297097
The COVID-19 pandemic imposed additional and specific challenges on the lives and wellbeing of informal unpaid carers. Addressing an important gap in the existing literature, this systematic review (prospectively registered with PROSPERO CRD42022376012) synthesises and evaluates the quantitative evidence examining the association between unpaid caregiving and mental health (compared to non-caring), during the pandemic. Five databases were searched (Medline, PsycInfo, EMBASE, Scopus, Web of Science) from Jan 1, 2020, to March 1, 2023. Population-based, peer-reviewed quantitative studies using any observational design were included, with screening, data extraction and quality assessment (amended NOS) independently conducted by two reviewers. Of the 3,073 records screened, 20 eligible studies (113,151 participants) were included. Overall quality of evidence was moderate. Narrative synthesis was complemented by Effect-direction and Albatross plots (given significant between-study heterogeneity precluded meta-analysis). Results indicate that the mental health of informal carers, already poorer pre-COVID compared to non-caregivers, was disproportionally impacted as a result of the pandemic and its associated public health containment measures. This review highlights the vulnerability of this group and should motivate political will and commensurate policies to ensure unpaid caregivers are better supported now, in the medium term, and crucially if, and when, another global public health emergency emerges.
Copyright: © 2024 Ervin et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication types
- Systematic Review
- COVID-19* / epidemiology
- Caregivers / psychology
- Mental Health*
- Quality of Life / psychology
Grants and funding
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 22 April 2024
Folic acid supplementation on inflammation and homocysteine in type 2 diabetes mellitus: systematic review and meta-analysis of randomized controlled trials
- Kabelo Mokgalaboni ORCID: orcid.org/0000-0002-3224-7433 1 ,
- Given. R. Mashaba 1 ,
- Wendy N. Phoswa 1 &
- Sogolo. L. Lebelo 1
Nutrition & Diabetes volume 14 , Article number: 22 ( 2024 ) Cite this article
Metrics details
- Cardiovascular diseases
- Type 2 diabetes
The beneficial effects of folate have been observed under different conditions, but the available evidence on inflammation and reduction of cardiovascular disease (CVD) in type 2 diabetes mellitus (T2DM) is limited. The study aimed to explore the effects of folate on inflammation and homocysteine amongst individuals with T2DM.
PubMed, Scopus, and Cochrane Library were used to search for evidence. A random-effect model meta-analysis through Review Manager (version 5.4) and metaHun was performed. Results were reported as standardized mean differences (SMD) and 95% confidence intervals graphically using forest and funnel plots.
Data from 9 trials with 426 patients living with T2DM were analyzed. Folic acid supplementation significantly revealed a large effect size on homocysteine levels compared to placebo, SMD = −1.53, 95%CI (−2.14,−0.93), p < 0.05. Additionally, we observed a medium marginal effect size on C-reactive protein (SMD = −0.68, 95%CI (−1.34, −0.01), p = 0.05). However, no significant effect on tumor necrosis factor-α (SMD = −0.86, 95%CI (−2.65, 0.93), p = 0.34), and interleukin-6 (SMD = −0.04, 95%CI (−1.08, 1.01), p = 0.95) was observed.
Evidence analyzed in this study suggests that folic acid supplementation in T2DM reduces homocysteine and may mitigate CVDs. However, its effect on inflammation is inconclusive.
Introduction
Type 2 diabetes mellitus (T2DM) is a condition that elevates blood glucose, also known as hyperglycemia [ 1 ]. Patients living with T2DM are at higher risk of developing cardiovascular diseases (CVD) than healthy individuals [ 2 ]. The prevalence of diabetes worldwide in 2021 was estimated to be 10.3% and this is continually rising with estimated projections of around 12.2% in 2045 [ 3 ]. The main factors that exacerbate the development of CVD in T2DM include but are not limited to hyperinsulinemia, obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, and homocysteinemia [ 4 ]. Inflammation is a central feature in T2DM, contributing to CVD among patients with T2DM. For instance, an increased circulating plasma C-reactive protein (CRP) [ 5 , 6 ] is noted in patients with T2DM, which may further increase the risk of CVD. Moreover, T2DM are more likely to develop hyperhomocysteinemia primarily due to folate and vitamin B 12 deficiency [ 7 , 8 ]. It is noteworthy to indicate that patients with T2DM who rely on metformin to control hyperglycemia often develop hyperhomocysteinemia, which further makes them susceptible to CVDs [ 9 ].
Previous studies have reported a link between homocysteine and inflammation [ 10 , 11 , 12 ]. For instance, evidence from preclinical and clinical studies demonstrated an association between homocysteine and proinflammatory responses [ 10 , 13 ]. Homocysteine is an amino acid associated with the risk of CVD if its level is elevated in the body [ 14 ]. Although homocysteine and inflammation markers are frequently detected at the same time, they are not correlated as they have revealed an inverse relationship in previous scientific evidence [ 8 , 15 ]. We anticipate that measuring them simultaneously will improve the overall interpretation and understanding of their correlation in T2DM. Due to their contribution to the development of CVDs in T2DM, an approach that can reduce the circulating levels of inflammatory markers and homocysteine in T2DM can be important to ameliorate inflammation, halt cardiovascular-related complications amongst T2DM and further reduce morbidity and mortality. It is important to note that T2DM medications widely used to control hyperglycemia in T2DM are available; however, their long-term may result in vitamin B 12 and folate deficiency [ 16 , 17 ]. Recent evidence has shown that folate deficiency is associated with an increased level of homocysteine, increasing the risk of CVD in T2DM [ 18 , 19 ]. The above shortfalls for drugs and related side effects have prompted an exploration of dietary supplements and macronutrients in alleviating cardiovascular-related complications in T2DM. Other examples include vitamin D [ 20 ], and folate, a natural form of vitamin B 9 due to its pleiotropic effects in diabetes and fewer side effects than other expensive and toxic therapies [ 21 ]. Folate is primarily found in green leafy vegetables and plays a role in cell division and synthesis of nucleic acids [ 22 ].
Folate has been explored by previous studies on inflammation, focusing on CRP levels in patients with T2DM. However, the findings reported by various studies are contradictory [ 23 , 24 ]. While Fatahi et al. [ 25 ] through meta-analysis, demonstrated a positive effect of folate on inflammation, the results must be treated with caution as only CRP as a marker of inflammation was assessed, and this might introduce bias, as it is difficult to conclude on the severity of inflammation based on one biomarker. Sato has reported no effect of 20 mg of folic acid on CRP and IL-6 as markers of inflammation in T2DM [ 15 ]. Additionally, the findings by Kaye et al., [ 26 ] suggest that folic acid may reduce the homocysteine levels in T2DM. However, completely different results were reported in a randomized, placebo-controlled, cross-over trial where there was no significant difference between folic acid and placebo groups on homocysteine [ 27 ]. Thus, the current study aims to systematically review and meta-analyze data from RCT to evaluate the effect of folic acid/folate on the level of homocysteine and inflammation in T2DM adult patients to rule out any inconsistencies observed in previous evidence. Furthermore, this review and meta-analysis also indicate the effective doses of folic acid/folate therapy that can alleviate inflammation among patients living with T2DM.
Methodology
This systematic review and meta-analysis are prepared and reported using an updated Preferred Reporting Items for Systematic Review and Meta-Analysis guideline [ 28 ] and checklist (Appendix 1). The protocol for this study has been registered with PROSPERO (the International Prospective Register of Systematic Reviews), CRD2023476986, for transparency.
Aim of the study
To evaluate if folic acid supplementation can ameliorate inflammation. In addition, we sought to determine the overall effect of folic acid on homocysteine in T2DM.
Information source and search strategy
A comprehensive literature search was conducted using online databases, including PubMed, Scopus, and Cochrane Library. The following medical subject headings (MeSH) and keywords were used: “Folate” OR “folic acid” OR “Folacin” OR “Vitamin B 9 ” AND “type 2 diabetes mellitus” OR “type 2 diabetes” OR “hyperglycemia.” These search terms were adjusted to suit each database used. The search was restricted to randomized controlled trials published from inception until 15 October 2023. The search was also restricted to RCT published in English. Furthermore, the reference lists of retrieved studies were also screened for additional relevant studies. Two researchers (KM and GRM) independently screened titles and abstracts of all retrieved studies, and discrepancies were resolved through discussion and re-evaluation of the study.
Eligibility criteria
Inclusion criteria.
All trials that satisfied our PICOS criteria were included (Table 1 ); for instance, all participants were adult patients living with T2DM, folic acid or folate treatment as an intervention, placebo as a control group, and inflammation and homocysteine levels as the outcomes of interest. Therefore, all randomized controlled trials that evaluated the effect of folic acid on concentrations of homocysteine, CRP, IL-6, and TNF-α were included.
Exclusion criteria
In case of any unavailable full-text article, we contacted the corresponding author, and if there was no response, the paper was excluded. Clinical trials using other interventions were excluded: no information about inflammation, no control groups, pregnant women, animal models of T2DM, gray literature, or other supplements were excluded.
Data items and extraction
The main researcher (KM) designed an Excel extraction sheet, and this was shared with the secondary researcher GRM prior to extraction. This Excel sheet included data items such as leading author surname, year of publication, study location, study design, population, gender distribution, mean age, mean body mass index, glycated hemoglobin, method of determining homocysteine, study duration, dosage of folic acid supplements, as the means ± standard deviation (SD) of the outcome measures in the folate/folic acid and placebo groups at baseline and post-intervention (change in values) for inflammatory markers (CRP, IL-6, and TNF-α) and homocysteine levels. The data were extracted independently by KM and GRM to minimize the risk of bias and extraction errors. Any disagreement was resolved by a third independent researcher WNP, who re-evaluated the study or data items in question.
Risk of bias in individual studies
The risk of bias (ROB) across all eligible studies was assessed following the Cochrane risk of bias tool [ 29 ]. Each included trial was evaluated based on five domains: bias arising from the randomization process, bias due to deviation from intended intervention, bias due to missing outcome data, bias due to missing outcome data, bias in the measurement of the outcome, bias in the selection of the reported results. A study was judged as low risk if all domains were at low risk of bias and judged as some concern if one domain was judged as some concern. Two independent researchers (KM and GRM) made the overall judgment. In the event of disagreement, a third researcher (WNP) made a judgment of the domain in question.
Data synthesis and statistical analysis
The meta-analysis was carried out using Review Manager (RevMan Version 5.4) and Meta-Hun http://softmed.hacettepe.edu.tr/metaHUN/ (accessed on 28/10/23). Means and SD of the outcome measures (CRP, IL-6, TNF-α, and homocysteine) reported for the folate and placebo groups were used to obtain the overall estimates. Where the SD of the mean difference was not reported in the studies, we estimated it using the following formula: change in SD = √[(SD pre-treatment) 2 + (SD post-treatment) 2 – (2 R × SD pre-treatment × SD post-treatment)]. The correlation coefficient (R) of 0.8 was considered based on previous reports [ 30 ]. Additionally, the mean was estimated using formulae \({\rm{X}}{\rm{\bar{} }}=\frac{{\rm{a}}+2{\rm{m}}+{\rm{b}}}{4}\) , and SD was estimated using \({\rm{SD}}=\frac{{\rm{range}}}{4}\) when the sample size is smaller than 70 if the trial reported median and interquartile range (IQR) [ 31 ]. In contrast, SD was estimated using the standard error of the mean (SEM) \(={\rm{SD}}\div\surd {\rm{n}}\) when the trial reported a standard error of the mean. The overall effect sizes for all effect measures were reported as standardized mean differences (SMD) and 95% confidence intervals (CI). SMD was preferred due to variables being reported in different units of measure. Random-effect model was used due to moderate heterogeneity, respectively. We further assessed heterogeneity between studies through the I 2 statics test ( I 2 > 50%), considered as moderate heterogeneity [ 32 , 33 ]. A p -value of < 0.05 was considered statistically significant, and the Cohens d was used to interpret the magnitude of the effect size. A Cohens d of 0.1, 05, and 0.8 was classified as a small, medium, and large effect, respectively. Publication bias was evaluated graphically through funnel plot inspection and statistically using Egger’s regression test. A sensitivity analysis was conducted using a leave-one-out analysis to determine each study’s effect on the overall effect size [ 34 , 35 ]. The overall certainty of evidence across the studies was evaluated in accordance with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guideline [ 36 ]. The quality of evidence was classified into four categories based on the corresponding evaluation criteria: high, moderate, low, and very low.
Characteristics of included studies
Nine trials [ 23 , 24 , 27 , 37 , 38 , 39 , 40 , 41 , 42 ] with 426 T2DM on folate/folic acid compared to placebo were included. The intervention group had 226 patients in the T2DM compared to 201 in the placebo group. These trials were conducted in Australia [ 40 ], Canada [ 27 ], India [ 38 ], Iran [ 24 , 38 , 39 , 41 ], Egypt [ 37 ], Norway [ 42 ] and the Netherlands [ 23 ] between 1998 and 2022. All included trials were randomized controlled trials, with one open-labeled [ 38 ], one double-blinded cross-over [ 27 ], and the rest were double-blinded trials. The sample size varied from smallest (26) to largest (100). The treatment was administered as either folate/folic acid in all trials between doses of 0.25 mg to 10 mg for a period ranging from 2 weeks to 28 weeks. At least seven trials [ 23 , 24 , 37 , 38 , 39 , 40 , 41 ] used folate at 5 mg for 4–26 weeks. The mean age of participants in the folate group was 59.57 ± 4.68 years, with a body mass index of 27.39 ± 3.05 kg/m 2 . The baseline glycated hemoglobin in the folate group was 7.67 ± 0.40%. The gender distribution across all trials was 134 males in folate and 130 in the placebo group, respectively. Different techniques were used to measure homocysteine in these trials, with the common one being an Enzyme immunoassay by homocysteine kit followed by an enzyme-linked immunosorbent assay (ELISA) kit. A detailed overview of the included trials is presented in Table 2 .
Literature search
A comprehensive overview of the study selection process is presented in the PRISMA flow diagram in Fig. 1 . The electronic search across three main databases, namely PubMed, Scopus, and the Cochrane Library, identified forty-eight records. PubMed contributed two records, Scopus contributed twenty, and the Cochrane Library contributed twenty-six (Table 1S ). All forty-eight records were stored in the Mendeley Reference Manager (version 2.104.0). Duplicate identification revealed seven records replicated in the databases and were excluded. Consequently, forty-one unique records underwent screening by independent researchers (KM and GRM). During the initial screening based on eligibility criteria, nineteen records were deemed irrelevant due to unrelated titles, abstracts, keywords, and focus.
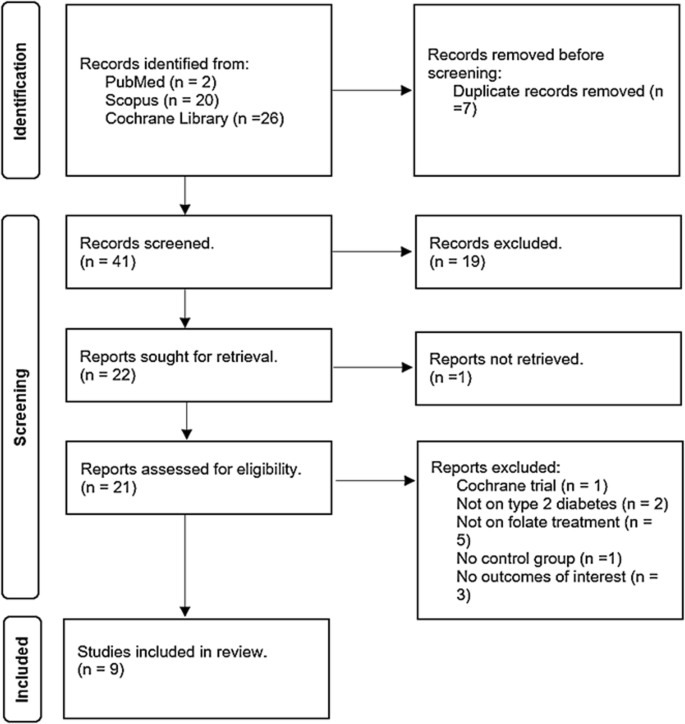
Flow diagram showing study selection.
Among the remaining twenty-two records subjected to full screening, one was published in Chinese and was excluded to prevent potential misinterpretation upon translation to English. Of the remaining twenty-one records, five employed interventions other than folate; three did not address our specified outcomes of interest, two involved irrelevant populations as they were not T2DM, one did not have a control group, and one represented a registration for an ongoing Cochrane trial. Consequently, only nine trials met the eligibility criteria for relevance to our study.
Effect of folic acid supplementation on homocysteine
The effect of folic acid supplementation on homocysteine was analyzed from nine trials [ 23 , 24 , 27 , 37 , 38 , 39 , 40 , 41 , 42 ] with a sample size of 426 T2DM on folic acid versus placebo. The overall effect from the random effect model showed a large effect size demonstrated by a significant reduction in the level of homocysteine in T2DM on folic acid compared to placebo, SMD = −1.53, 95%CI (−2.14,−0.93), p < 0.05 (Fig. 2 ). Of concern was an observed level of heterogeneity ( I 2 = 86%) across the included studies.
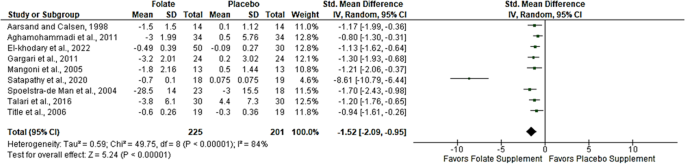
Effect of folic acid supplementation on homocysteine in T2DM.
Effect of folic acid supplementation on inflammation
Three markers of inflammation were evaluated: CRP, TNF-α, and IL-6; however, only five trials [ 21 , 25 , 35 , 36 , 37 ] evaluated CRP in T2DM following folic acid supplementation compared to placebo. The results from the random-effect meta-analysis revealed a medium effect size demonstrated by a marginal reduction in CRP; SMD = −0.68, 95%CI (−1.34, −0.01), p = 0.05 (Fig. 3A ). This was accompanied by a high level of heterogeneity ( I 2 = 85%). Likewise, the results from 3 trials [ 23 , 27 , 38 ] that evaluated TNF-α showed a large effect size; however, this was statistically not significant, SMD = −0.86, 95%CI (−2.65, 0.93), p = 0.34 (Fig. 3B ). These trials revealed moderate evidence of heterogeneity ( I 2 = 94%). Only two trials [ 23 , 38 ] assessed the effect of folate on IL-6; the evidence from the random-effect meta-analysis showed a small effect size, and this also not significant, SMD = −0.04, 95%CI (−1.08, 1.01), p = 0.95 (Fig. 3C ). Similarly, there was moderate evidence of heterogeneity across these trials ( I 2 = 81%).
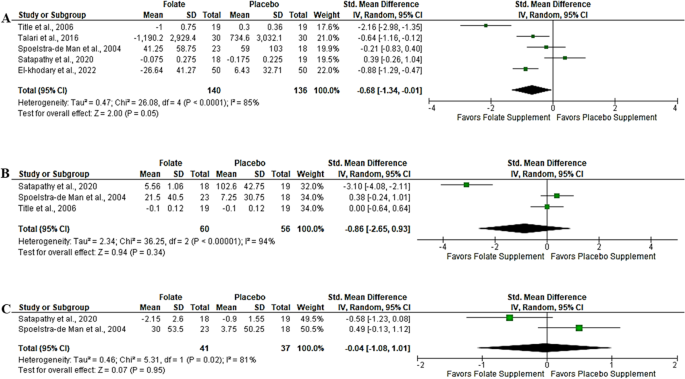
A high sensitive-C-reactive protein (hs-CRP); B Tumor necrosis factor alpha (TNF-α); C Interleukin-6 (IL-6).
Heterogeneity and subgroup analysis
Due to observed heterogeneity in homocysteine results, we performed subgroup analyses based on both sample size (< or > 50 T2DM patients) and folate dosage (0.25, 5, or 10 mg). The subgroup analysis of the sample size revealed evidence of heterogeneity ( I 2 = 70.5%) (Fig. 1SA ). Notably, studies with a sample size below 50 exhibited high heterogeneity ( I 2 = 89%), contrasting with those above 50, which displayed no heterogeneity ( I 2 = 0%) (Fig. 1SA ).
Additionally, the subgroup analysis on the dosage of folic acid supplementation showed a decrease in heterogeneity ( I 2 = 20.1%); however, a 5 mg administration led to a 2% decrease in heterogeneity ( I 2 = 86%) (Fig. 1SB ). Subgroup analysis on duration of intervention revealed reduced heterogeneity (I 2 = 35.8%). Interestingly, medium duration demonstrated no evidence of heterogeneity ( I 2 = 0%) (Fig. 2SA ). Finally, a subgroup analysis based on gender distribution for homocysteine revealed an overall test difference indicating heterogeneity ( I 2 = 95.7%). However, studies conducted in males showed no heterogeneity( I 2 = 0%), whereas those involving both genders resulted in high heterogeneity ( I 2 = 89%) (Fig. 2SB ). The results of a meta-analysis on CRP revealed a moderate heterogeneity( I 2 = 73%). This warranted subgroup to find the source of this variation; firstly, we subgrouped studies according to sample size and dosage of folic acid supplementation. We found that the evidence from trials with a sample size below 50 slightly changed heterogeneity ( I 2 = 50%), while more than 50 had I 2 = 0% (Fig. 3SA ). Interestingly, the overall test for subgroup differences also revealed no heterogeneity ( I 2 = 0). On the other hand, subgroups according to dosage showed that trials that used 5 mg of folate/folic acid had I 2 = 74%, while those that used 10 mg had I 2 = 85%. Moreover, the test for subgroup difference showed I 2 = 92.3% (Fig. 3SB ).
Publication bias (Funnel plots and Egger’s regression test)
Publication bias was assessed by visualization of the funnel plot, and we noted evidence of bias on homocysteine (Fig. 4A ), and this was consistent with the findings of the Eggers regression test (Z score = −6.06, p < 0.05). This suggests that trials with positive significant results were more likely to be published than negative trials. On the contrary, the funnel plot revealed no evidence of bias on hs-CRP- (Fig. 4B ), which was supported by the Eggers regression test (Z score = −0.75, p = 0.46). For TNF-α, Eggers regression showed some potential level of bias (Z score = −6.15, p < 0.05); this was supported by a funnel plot (Fig. 4C ). Additionally, IL-6 revealed no bias graphically (Fig. 4D ); however, due to few trials, no regression was assessed.
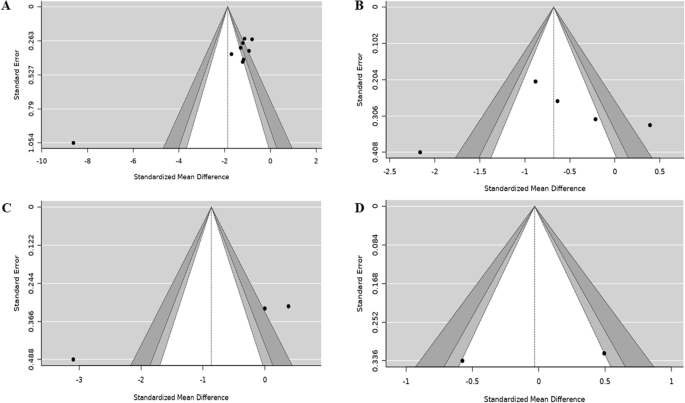
A Homocysteine; B C-reactive protein (CRP); C Tumor necrosis factor (TNF)-α; D Interleukin (IL)-6.
Sensitivity analysis across the trials
Sensitivity results for homocysteine showed that the exclusion of the study by Satapathy et al. [ 38 ], due to small weight, resulted in the change in overall effect size, SMD = −1.14, 95%CI (−1.36, −0.92), p = 0.0000 (Table 2S ). The exclusion of El-khodary et al. [ 37 ] in CRP results changed the overall effect size to SMD = −0.63, 95%CI (−1.56, 0.30), p = 0.16 (Table 3S ). When Satapathy et al., [ 38 ] was excluded from TNF-α analysis, the effect size changed to SMD = 0.20, 95%CI (−0.27, 0.66), p = 0.0000 (Table 4S ). For IL-6, exclusion of [ 38 ] led to SMD = 0.49, 95%CI (−0.13, 1.12), p = 0.0000 (Table 5S ).
Assessment of risk of bias across and certainty of evidence across the included trials
Among nine trials, two trials [ 39 , 41 ] were judged as having some concerns of bias as the process of randomization was not clear in their methodology. Only one trial was judged as high risk [ 42 ], due to lack of information about the randomization method used. Interestingly, 67% of trials [ 23 , 24 , 27 , 37 , 38 , 40 ] were judged as low risk of bias as they scored low risk across all domains (Fig. 5 ). GRADING of trials revealed moderate certainty of evidence on homocysteine, CRP, and TNF-α, while IL-6 evidence was found to be of low certainty (Table 6S ). The downgrade was due to heterogeneity, risk of bias, or imprecision due to the small sample size (<400).
Risk of bias according to ROB tool 2.0.
To the best of our knowledge, this is the first comprehensive meta-analysis of RCT to evaluate the effect of folic acid supplementation on homocysteine and inflammation in adult patients with T2DM. We found that folic acid supplementation was associated with a reduction in homocysteine levels. The observed SMD (1.52) was large effect, suggesting anti-homocysteine properties. Additionally, there was a marginal effect of folic acid on CRP without a significant effect on TNF-α and IL-6 in patients with T2DM. Subgroup analysis showed that the folate effect on homocysteine was more pronounced at a higher dose (10 mg) than 5 mg supplementation. However, it is important to note that only one trial used 10 mg compared to 5 mg that used 5 mg of folic acid. It was evident that studies with a sufficient sample size (50 and above patients) had a more pronounced effect than those with a smaller sample size. Although folic acid supplementation at both short and medium periods reduced homocysteine, the reduction was more pronounced at short periods (0–4 weeks) compared to medium periods (8–12 weeks). Our research reveals lower homocysteine levels in individuals living with T2DM receiving folate supplements, indicating a potential decrease in the risk of CVD. We are confident with the evidence synthesized in this study as the evidence showed moderate certainty in homocysteine. In T2DM, insulin resistance and associated impaired kidney function results in an elevated homocysteine level [ 43 , 44 , 45 , 46 , 47 ]. This elevation promotes the development of CVD complications associated with T2DM.
Interestingly, the evidence from this study shows that folic acid supplementation can reduce homocysteine by converting it into methionine, lowering the risk of cardiovascular complications in T2DM patients [ 26 ]. Notably, previous evidence has shown that folic acid supplementation can reduce homocysteine levels in patients living with T2DM by increasing the 5-methyltetrahydrofolate intracellular pool [ 37 ]. This effect is crucial as an elevated level of homocysteine can damage blood vessels and contribute to the development of CVD [ 45 ]. Therefore, any strategies therapeutically that reduce homocysteine may assist in alleviating CVD among T2DM. In obese children, similar trends have been observed when administering a minimum of 1 g of folic acid, leading to a substantial reduction in homocysteine levels [ 48 ]. Similar findings are observed in gestational diabetes, as 1 mg and 5 mg of folic acid supplementation significantly reduced homocysteine levels [ 49 ]. Although the pathological and physiological mechanisms of these conditions differ, these findings demonstrate the efficacy of folate supplementation across diverse conditions and age groups. A non-randomized trial in menopausal T2DM women also showed that 800 µg of folate significantly reduced homocysteine levels. However, this study revealed an inverse correlation between folic acid and homocysteine (r = −0.4876, p -value = 0.0134) [ 50 ]. Although the mechanism by which folate reduces homocysteine in T2DM is poorly documented, it is assumed that this is associated with the role of folate in one-carbon metabolism. Folic acid supplementation increases the availability of one-carbon units, which then promotes the remethylation of homocysteine to methionine [ 51 ]. This subsequently results in a decrease in homocysteine levels in the body. While such benefits are acknowledged, contrasting findings from other studies suggest a possible risk of CVD in T2DM, even with folic acid supplementation [ 52 ]. These findings suggest a limitation in the beneficial effect of folate, especially in T2DM. It is assumed that folate deficiency impairs the conversion of homocysteine to methionine, resulting in homocysteine accumulation in the blood [ 53 ]. High homocysteine levels are associated with an increased risk of CVDs and other health problems.
Although there was a marginal effect on hs-CRP ( p = 0.05), no significant effect of folic acid supplementation on other markers of inflammation was observed. This was shown by no significant effect on TNF-α and IL-6 following supplementation with folic acid compared to placebo. A reduction in hs-CRP following folic acid supplementation reveals, to some extent, the beneficial effect of folic acid as an anti-inflammatory agent. However, as not all inflammatory markers were reduced, the findings are thus inconclusive. Among some factors contributing to the challenge in elucidating conflicting findings regarding the impact of folic acid on inflammation is the limited number of trials conducted. The inability of folic acid to improve some markers of inflammation indicates that it does not exhibit anti-inflammatory properties. For instance, Spoelstra-de Man et al., [ 23 ] reported no effect of folic acid on hs-CRP, IL-6, and TNF-α. The same findings were observed by Title et al. [ 27 ], however, only TNF-α and hs-CRP were investigated, and no effect was observed. Despite these null findings, another trial observed a significant effect of folic acid on hs-CRP in T2DM, as demonstrated by a significant decrease in hs-CRP within the folic acid group before and after folic acid supplementation. This same trend was also observed when folic acid supplementation was compared to placebo [ 24 ]. The latter supports our findings as we observed a reduction in hs-CRP with a medium to large effect size (Cohen d = 0.68). Other researchers reported a significant decrease in IL-6 and TNF-α following folic acid supplementation compared to placebo, suggesting the anti-inflammatory effects of folic acid in T2DM [ 38 ]. These findings differ from our overall findings in this study as we reported no significant effect of folic acid on TNF-α and IL-6. Another study showed a significant change between baseline and post-treatment on hs-CRP, however, there were no significant changes between the folic acid and placebo groups [ 38 ]. In obese children, 1 mg of folic acid has proven to offer an anti-inflammatory effect, as demonstrated by a significant decrease in IL-6, TNF-α, and IL-8 [ 48 ].
Similarly, El-khodary et al. [ 37 ] also showed a significant decrease in hs-CRP between baseline and post-treatment ( p = 0.008). The same trial reported a significant decrease in hs-CRP following three months of folic acid supplementation compared to placebo ( p = 0.005). This study also showed a positive correlation between homocysteine and hs-CRP (r = 0.308, p = 0.002). Due to these contradicting results on inflammation, the effect of folic acid on inflammation is not clear, other researchers have suggested that folic acid may be involved in the reduction of hs-CRP by reducing homocysteine and oxidative stress. For instance, Talari et al., [ 24 ] reported an increased adjusted glutathione (GSH) following folic acid supplementation in T2DM compared to placebo.
Additionally, folic acid exhibits anti-insulinemic activities [ 54 , 55 ] may further alleviate inflammation by suppressing the synthesis of inflammatory cytokines. It is important to note that while the benefits were not observed in this study, this might be attributable to the number of trials analyzed primarily because observational differences were noted. Previous evidence suggests that homocysteine promotes the expression of inflammatory markers by increasing the activation of nuclear factor kappa β (NF-kβ) and poly-adenosine diphosphate (ADP) ribose polymerase. Therefore, we speculate that folic acid anti-homocysteine properties may alleviate inflammation by inhibiting the activation of NF-κβ and ADP and thus suppressing the expression of inflammatory markers [ 56 , 57 ]. Evidence from in vitro studies has also shown that folic acid may reduce inflammation by inhibiting the phosphoinositide 3-kinases (PI3K)/hypoxia-inducible factor 1-alpha (HIF-1α) pathway [ 58 ]. Even though the reduction in homocysteine following folate supplementation is normally accompanied by a reduction in CRP and subsequent deactivation of NF-κβ and low IL-6 and TNF-α, the contradictory findings observed in our study may be due to few trials and sample size across the trials analyzed in this study.
Strength and limitation
The present analyses exclusively examined evidence from randomized trials, considered to provide high clinical evidence. Notably, there was a low risk of bias observed across various domains in the risk of bias assessment, indicating that the quality of the studies was satisfactory. The GRADE tool was also employed to evaluate the overall quality of the analyzed evidence, and it was categorized as either moderate or very low in one outcome due to the small sample size.
Furthermore, a comprehensive subgroup analysis was performed, considering various confounding factors. The I 2 statistics revealed moderate heterogeneity. For transparency, the study was registered with PROSPERO, and the experienced researchers adhered to PRISMA guidelines, boosting confidence in the reliability of the current findings. However, it is crucial to acknowledge certain limitations in our study, such as few relevant trials, indicating a minimal sample size of only 426 patients living with T2DM. Moreover, existing trials have employed varying quantitative methodologies, introducing potential differences in sensitivity and specificity, especially with the use of ELIZA AND HPLC for the determination of homocysteine.
Conclusion and future recommendations
The findings from nine trials involving a sample of 426 participants in this study indicate that folic acid supplementation in T2DM may reduce homocysteine levels, a potential biomarker for CVDs. However, due to the limited number of trials analyzed, null effects were observed concerning some of the inflammatory markers. It is crucial to interpret the conclusions of our study with caution, emphasizing the need for further trials with adequate sample sizes.
Considering the limitations acknowledged in this study, we propose recommendations for future investigations into folic acid in T2DM, particularly focusing on inflammation. We suggest that forthcoming RCTs use sufficient sample sizes and adhere to the reporting guidelines outlined in the consolidated standards of reporting trials (CONSORT). Additionally, these trials should adhere to standardized methodologies, implementing an accurate randomization process, blinding of personnel and participants. Furthermore, we emphasize the necessity for high-quality meta-analyses to comprehensively elucidate the benefits of folic acid supplementation in managing T2DM.
Data availability
The data used in this review are available from the corresponding author upon reasonable request.
American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2022;45:S17–S38.
Article Google Scholar
Dal Canto E, Ceriello A, Rydén L, Ferrini M, Hansen TB, Schnell O, et al. Diabetes as a cardiovascular risk factor: an overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. 2019;26:25–32.
Article PubMed Google Scholar
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pr. 2022;183:109119.
Giorgino F, Leonardini A, Laviola L. Cardiovascular disease and glycemic control in type 2 diabetes: Now that the dust is settling from large clinical trials. Ann N. Y Acad Sci. 2013;1281:36–50.
Article CAS PubMed PubMed Central Google Scholar
Hayfron-Benjamin CF, Maitland-Van Der Zee AH, van den Born BJ, Amoah AGB, Meeks KAC, Klipstein-Grobusch K, et al. Association between C reactive protein and microvascular and macrovascular dysfunction in sub-Saharan Africans with and without diabetes: The RODAM study. BMJ Open Diabetes Res Care 2020;8. https://doi.org/10.1136/bmjdrc-2020-001235 .
Pfützner A, Schöndorf T, Hanefeld M, Forst T. High-sensitivity C-reactive protein predicts cardiovascular risk in diabetic and nondiabetic patients: effects of insulin-sensitizing treatment with pioglitazon. J Diabetes Sci Technol. 2010;4:706–16.
Article PubMed PubMed Central Google Scholar
Abdulhameed LQ, Sultan AA, AL-Mahdawi ZMM. Homocysteine: A recent potential risk factor for type 2 diabetes mellitus patients in Diyala Province. AIP Conf Proc. 2023;2593:050004.
Article CAS Google Scholar
Wu JT. Circulating homocysteine is an inflammation marker and a risk factor of life-threatening inflammatory diseases. J Biomed Lab Sci. 2007;19:107–12.
Google Scholar
Lala K, Lala D, Duggad S. Serum vitamin B12 and homocysteine levels in type 2 diabetes patients on metformin. Int J Adv Med. 2020;7:1376.
Durga J, Van Tits LJH, Schouten EG, Kok FJ, Verhoef P. Effect of lowering of homocysteine levels on inflammatory markers a randomized controlled trial. Arch Intern Med. 2005;165:1388–94.
Article CAS PubMed Google Scholar
Li T, Chen Y, Li J, Yang X, Zhang H, Qin X, et al. Serum homocysteine concentration is significantly associated with inflammatory/immune factors. PLoS One. 2015;10:e0138099.
El Oudi M, Aouni Z, Mazigh C, Khochkar Phd R, Gazoueni Phd E, Haouela H, et al. Homocysteine and markers of inflammation in acute coronary syndrome. Exp Clin Cardiol. 2010;15:e25–e28.
Elsherbiny NM, Sharma I, Kira D, Alhusban S, Samra YA, Jadeja R, et al. Homocysteine induces inflammation in retina and brain. Biomolecules. 2020;10:393.
Zhang Z, Gu X, Fang X, Tang Z, Guan S, Liu H, et al. Homocysteine and the risk of cardiovascular events and all-cause death in elderly population: a community-based prospective cohort study. Ther Clin Risk Manag. 2020;16:471–81.
Sato S, Tajiri Y, Nakayama H, Yamada K. Folic acid supplementation of aspirin therapy further improves vascular endothelial function among patients with type 2 diabetes: a short-term crossover study. Diabetol Int. 2015;6:284–9.
Miyan Z, Waris N. Association of vitamin B 12 deficiency in people with type 2 diabetes on metformin and without metformin: a multicenter study, Karachi, Pakistan. BMJ Open Diabetes Res Care. 2020;8:e001151.
Awad NA, Alsaady ZA, Albayati MAM, Mutlak SS. Correlation study of metformin drug with vit.B12 and folic acid in women suffer of type 2 diabetic disease. J Pharm Negat Results. 2022;13:308–12.
CAS Google Scholar
Song S, Song BM, Park HY. Associations of serum folate and homocysteine concentrations with all-cause, cardiovascular disease, and cancer mortality in men and women in Korea: the Cardiovascular Disease Association Study. J Nutr. 2023;153:760–70.
Ebesunun MO, Obajobi EO. Elevated plasma homocysteine in type 2 diabetes mellitus: a risk factor for cardiovascular diseases. JAMA Netw Open. 2022;5:e2146124.
MacGirlley R, Phoswa WN, Mokgalaboni K. Modulatory Properties of Vitamin D in Type 2 Diabetic Patients: A Focus on Inflammation and Dyslipidemia. Nutrients 2023;15. https://doi.org/10.3390/nu15214575 .
Qudsia F, Matti HA, Riaz S. Pleiotropic effect of folate-cobalamin combinational therapy on diabetes mellitus. Ann Nutritional Disord Ther. 2020;7:1–8.
Fact Sheet for Health Professionals. Folate - Health Professional Fact Sheet https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/ (accessed 28 Oct 2023).
Spoelstra-de MA, Brouwer CB, Terheggen F, Bollen JM, Stehouwer CD, Smulders YM. No effect of folic acid on markers of endothelial dysfunction or inflammation in patientswith type 2diabetes mellitus and mild hyperhomocysteinaemia. J Med. 2004;62:246–53.
Talari HR, Rafiee M, Farrokhian A, Raygan F, Bahmani F, Darooghegi Mofrad M, et al. The effects of folate supplementation on carotid intima-media thickness and metabolic status in patients with metabolic syndrome. Ann Nutr Metab. 2016;69:41–50.
Fatahi S, Pezeshki M, Mousavi SM, Teymouri A, Rahmani J, Kord Varkaneh H, et al. Effects of folic acid supplementation on C-reactive protein: A systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovascular Dis. 2019;29:432–9.
Kaye AD, Jeha GM, Pham AD, Fuller MC, Lerner ZI, Sibley GT, et al. Folic Acid Supplementation in Patients with Elevated Homocysteine Levels. Adv Ther. 2020;37:4149–64.
Title LM, Ur E, Giddens K, McQueen MJ, Nassar BA. Folic acid improves endothelial dysfunction in type 2 diabetes - An effect independent of homocysteine-lowering. Vasc Med. 2006;11:101–9.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:1–9.
McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55–61.
Giuliano C, Karahalios A, Neil C, Allen J, Levinger I. The effects of resistance training on muscle strength, quality of life and aerobic capacity in patients with chronic heart failure — A meta-analysis. Int J Cardiol. 2017;227:413–23.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:1–10.
Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med. Res. Methodol. 2011;11. https://doi.org/10.1186/1471-2288-11-41 .
Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I 2 Index? Psychol Methods. 2006;11:193–206.
Moreno SG, Sutton AJ, Ades A, Stanley TD, Abrams KR, Peters JL, et al. Assessment of regression-based methods to adjust for publication bias through a comprehensive simulation study. BMC Med. Res. Methodol. 2009;9. https://doi.org/10.1186/1471-2288-9-2 .
Doleman B, Freeman SC, Lund JN, Williams JP, Sutton AJ. Funnel plots may show asymmetry in the absence of publication bias with continuous outcomes dependent on baseline risk: presentation of a new publication bias test. Res Synth Methods. 2020;11:522–34.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94.
El-khodary NM, Dabees H, Werida RH. Folic acid effect on homocysteine, sortilin levels and glycemic control in type 2 diabetes mellitus patients. Nutr Diabetes. 2022;12:1–8.
Satapathy S, Bandyopadhyay D, Patro BK, Khan S, Naik S. Folic acid and vitamin B12 supplementation in subjects with type 2 diabetes mellitus: A multi-arm randomized controlled clinical trial. Complement Ther Med. 2020;53:102526.
Gargari BP, Aghamohammadi V, Aliasgharzadeh A. Effect of folic acid supplementation on biochemical indices in overweight and obese men with type 2 diabetes. Diabetes Res Clin Pr. 2011;94:33–38.
Mangoni AA, Sherwood RA, Asonganyi B, Swift CG, Thomas S, Jackson SHD. Short-term oral folic acid supplementation enhances endothelial function in patients with type 2 diabetes. Am J Hypertens. 2005;18:220–6.
Aghamohammadi V, Gargari P, Aliasgharzadeh A. Effect of folic acid supplementation on homocysteine, serum total antioxidant capacity, and malondialdehyde in patients with type 2 diabetes mellitus. J Am Coll Nutr. 2011;30:210–5.
Aarsand AK, Carlsen SM. Folate administration reduces circulating homocysteine levels in NIDDM patients on long-term metformin treatment. J Intern Med. 1998;244:169–74.
Zhang X, Qu YY, Liu L, Qiao YN, Geng HR, Lin Y, et al. Homocysteine inhibits pro-insulin receptor cleavage and causes insulin resistance via protein cysteine-homocysteinylation. Cell Rep. 2021;37. https://doi.org/10.1016/j.celrep.2021.109821 .
Shen Z, Zhang Z, Zhao W Relationship between plasma homocysteine and chronic kidney disease in US patients with type 2 diabetes mellitus: a cross-sectional study. BMC Nephrol. 2022;23. https://doi.org/10.1186/s12882-022-03045-6 .
Lei X, Zeng G, Zhang Y, Li Q, Zhang J, Bai Z, et al. Association between homocysteine level and the risk of diabetic retinopathy: a systematic review and meta-analysis. Diabetol. Metab. Syndr. 2018;10. https://doi.org/10.1186/s13098-018-0362-1 .
Zhao W, Chen L, Lin Y, He H, Ma H, Hu Q, et al. Association of homocysteine and insulin resistance with increased risk of mortality in a nondiabetic population: third national health and nutrition examination survey. Metab Syndr Relat Disord. 2022;20:255–63.
CAS PubMed Google Scholar
Meigs JB, Jacques PF, Selhub J, Singer DE, Nathan DM, Rifai N, et al. Fasting plasma homocysteine levels in the insulin resistance syndrome the Framingham Offspring Study. Diabetes Care. 2001;24:1403–10.
Dehkordi E, Sedehi M, Shahraki Z, Najafi R. Effect of folic acid on homocysteine and insulin resistance of overweight and obese children and adolescents. Adv Biomed Res. 2016;5:88.
Davari Tanha F, Kaveh M, Shariat M. Homocysteine in gestational diabetes and normal pregnancy plus effects of folic acid. Iran J Public Health. 2008;37:118–26.
Vijayakumar A, Kim EK, Kim H, Choi YJ, Huh KB, Chang N. Effects of folic acid supplementation on serum homocysteine levels, lipid profiles, and vascular parameters in post-menopausal Korean women with type 2 diabetes mellitus. Nutr Res Pr. 2017;11:327–33.
Williamson JM, Arthurs AL, Smith MD, Roberts CT, Jankovic-Karasoulos T. High folate, perturbed one-carbon metabolism and gestational diabetes mellitus. Nutrients 2022;14. https://doi.org/10.3390/nu14193930 .
Song Y, Cook NR, Albert CM, Van Denburgh M, Manson JAE. Effect of homocysteine-lowering treatment with folic acid and B vitamins on risk of type 2 diabetes in women: A randomized, controlled trial. Diabetes. 2009;58:1921–8.
Varela-Moreiras A, Martínez-Vega G, Partearroyo R, Pajares T, Varela-Nieto MA. Folic acid deficiency impairs homocysteine metabolism in the inner ear inducing premature hearing loss in C57BL/6J mice. 2013. http://hdl.handle.net/10261/125351 .
Lind MV, Lauritzen L, Kristensen M, Ross AB, Eriksen JN. Effect of folate supplementation on insulin sensitivity and type 2 diabetes: A meta-analysis of randomized controlled trials. Am J Clin Nutr. 2019;109:29–42.
Asbaghi O, Ashtary-Larky D, Bagheri R, Moosavian SP, Olyaei HP, Nazarian B et al. Folic acid supplementation improves glycemic control for diabetes prevention and management: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutrients 2021;13. https://doi.org/10.3390/nu13072355 .
Zhang X, Chen S, Li L, Wang Q, Le W. Folic acid protects motor neurons against the increased homocysteine, inflammation and apoptosis in SOD1G93A transgenic mice. Neuropharmacology. 2008;54:1112–9.
Zhang L, Li Z, Xing C, Gao N, Xu R. Folate Reverses NF-κB p65/Rela/IL-6 level induced by hyperhomocysteinemia in spontaneously hypertensive rats. Front Pharmacol. 2021;12. https://doi.org/10.3389/fphar.2021.651582 .
Huang X, He Z, Jiang X, Hou M, Tang Z, Zhen X et al. Folic acid represses hypoxia-induced inflammation in THP-1 cells through inhibition of the PI3K/Akt/HIF-1α pathway. PLoS ONE 2016;11. https://doi.org/10.1371/journal.pone.0151553 .
Download references
Acknowledgements
We acknowledge University of South Africa research office for payment of article processing charges.
This study is partially funded by the South African Research Excellence Award for Next Generation Researchers (NONF230515106418), Research Development Grants for nGAP Scholars (NGAP23022780506) and South African National Research Foundation Special Transformative Awards (NSTA231114163486). Funders have no role in the conceptualization, preparation, data analysis, and conclusion reached in this manuscript.
Author information
Authors and affiliations.
Department of Life and Consumer Science, College of Agriculture and Environmental Sciences, University of South Africa, Florida Campus, Roodepoort, South Africa
Kabelo Mokgalaboni, Given. R. Mashaba, Wendy N. Phoswa & Sogolo. L. Lebelo
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization, KM.; methodology, KM, GRM, and WNP.; software, KM.; validation, KM, WNP, and SLL.; formal analysis, K.M.; investigation, K.M.; resources, KM.; data curation, KM and WNP; funding, KM; writing—original draft preparation, KM; writing—review and editing, KM, GRM, WNP and SLL; supervision, WNP and SLL. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Kabelo Mokgalaboni .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Mokgalaboni, K., Mashaba, G.R., Phoswa, W.N. et al. Folic acid supplementation on inflammation and homocysteine in type 2 diabetes mellitus: systematic review and meta-analysis of randomized controlled trials. Nutr. Diabetes 14 , 22 (2024). https://doi.org/10.1038/s41387-024-00282-6
Download citation
Received : 21 November 2023
Revised : 09 April 2024
Accepted : 10 April 2024
Published : 22 April 2024
DOI : https://doi.org/10.1038/s41387-024-00282-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- For authors
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Online First
- Interventions to suppress puberty in adolescents experiencing gender dysphoria or incongruence: a systematic review
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0001-5898-0900 Jo Taylor ,
- Alex Mitchell ,
- Ruth Hall ,
- Claire Heathcote ,
- Trilby Langton ,
- Lorna Fraser ,
- http://orcid.org/0000-0002-0415-3536 Catherine Elizabeth Hewitt
- Department of Health Sciences , University of York , York , UK
- Correspondence to Dr Jo Taylor, Health Sciences, University of York, York, North Yorkshire, UK; dohs-gender-research{at}york.ac.uk
Background Treatment to suppress or lessen effects of puberty are outlined in clinical guidelines for adolescents experiencing gender dysphoria/incongruence. Robust evidence concerning risks and benefits is lacking and there is a need to aggregate evidence as new studies are published.
Aim To identify and synthesise studies assessing the outcomes of puberty suppression in adolescents experiencing gender dysphoria/incongruence.
Methods A systematic review and narrative synthesis. Database searches (Medline, Embase, CINAHL, PsycINFO, Web of Science) were performed in April 2022, with results assessed independently by two reviewers. An adapted version of the Newcastle-Ottawa Scale for cohort studies was used to appraise study quality. Only moderate-quality and high-quality studies were synthesised. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guidelines were used.
Results 11 cohort, 8 cross-sectional and 31 pre-post studies were included (n=50). One cross-sectional study was high quality, 25 studies were moderate quality (including 5 cohort studies) and 24 were low quality. Synthesis of moderate-quality and high-quality studies showed consistent evidence demonstrating efficacy for suppressing puberty. Height increased in multiple studies, although not in line with expected growth. Multiple studies reported reductions in bone density during treatment. Limited and/or inconsistent evidence was found in relation to gender dysphoria, psychological and psychosocial health, body satisfaction, cardiometabolic risk, cognitive development and fertility.
Conclusions There is a lack of high-quality research assessing puberty suppression in adolescents experiencing gender dysphoria/incongruence. No conclusions can be drawn about the impact on gender dysphoria, mental and psychosocial health or cognitive development. Bone health and height may be compromised during treatment. More recent studies published since April 2022 until January 2024 also support the conclusions of this review.
PROSPERO registration number CRD42021289659.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/archdischild-2023-326669
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Increasing numbers of children and adolescents experiencing gender dysphoria/incongruence are being referred to specialist gender services.
National and international guidelines have changed over time and outline that medications to suppress puberty can be considered for adolescents experiencing gender dysphoria/incongruence.
Several systematic reviews report a limited evidence base for these treatments, and uncertainty about the benefits, risks and long-term effects.
WHAT THIS STUDY ADDS
No high-quality studies were identified that used an appropriate study design to assess the outcomes of puberty suppression in adolescents experiencing gender dysphoria/incongruence.
There is insufficient and/or inconsistent evidence about the effects of puberty suppression on gender-related outcomes, mental and psychosocial health, cognitive development, cardiometabolic risk, and fertility.
There is consistent moderate-quality evidence, although from mainly pre-post studies, that bone density and height may be compromised during treatment.
HOW THIS STUDY MIGHT AFFECT RESEARCH, POLICY OR PRACTICE
There is a lack of high-quality evidence to support the use of puberty suppression in adolescents experiencing gender dysphoria/incongruence, and large well-designed research is needed.
Introduction
Over the last 10-15 years, increasing numbers of children and adolescents experiencing gender dysphoria/incongruence are being referred to specialist paediatric gender services. 1 2
Gender dysphoria/incongruence in childhood is associated with high rates of co-occurring mental health and psychosocial difficulties, which can affect health and well-being. 3 Clinical guidelines recommend psychosocial care to alleviate gender-related distress and any co-occurring difficulties. For pubertal adolescents, medications to suppress or lessen effects of puberty are also outlined. Gonadotropin-releasing hormone analogues (GnRH-a) are used as first-line treatment, although other drugs with anti-androgenic properties including progestins and spironolactone are used in this population. 4 5 The effects differ depending on whether they are initiated in early puberty or mid-puberty, as well as the type of intervention used, with GnRH-a suppressing puberty when started early or suspending further progression when initiated in mid-puberty, and anti-androgens instead blocking specific downstream effects of sex hormones. 4
Rationales for puberty suppression in the Dutch treatment protocol, which has informed practice internationally, were to alleviate worsening gender dysphoria, allow time for gender exploration, and pause development of secondary sex characteristics to make passing in the desired gender role easier. 6 Practice guidelines propose other indications for puberty suppression, including allowing time and/or capacity for decision-making about masculinising or feminising hormone interventions, and improving quality of life. 4 7 8
Criteria in early treatment protocols for puberty suppression specified adolescents be at least age 12 years, at Tanner stage 2 in puberty, experienced gender dysphoria in childhood which persisted and intensified during puberty and met criteria for diagnosis of gender dysphoria. 6 It was also expected that any psychosocial difficulties that could interfere with treatment were managed. 6 The World Professional Association for Transgender Health standards of care 4 and other practice guidelines 5 8 9 have broadened these criteria, for example, removing minimum age. However, other recent guidelines have taken a more cautious approach and restricted inclusion criteria in response to uncertainties in the evidence base. 7 10
Systematic reviews have consistently found mainly low-quality evidence, limited data on key outcomes or long-term follow-up. 11–16 These reviews report that while puberty suppression may offer some benefit, there are concerns about the impact on bone health, and uncertainty regarding cognitive development, psychosocial outcomes and cardiometabolic health. They conclude there is insufficient evidence to support clinical recommendations.
The proliferation of research in this area and lack of evidence to support practice means there is an ongoing need to aggregate evidence. This systematic review aims to synthesise evidence published to April 2022 that reports outcomes of puberty suppression in adolescents experiencing gender dysphoria/incongruence.
The review forms part of a linked series examining the epidemiology, care pathways, outcomes and experiences for children and adolescents experiencing gender dysphoria/incongruence and is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 17 The protocol was registered on PROSPERO (CRD42021289659. 18
Search strategy
A single search strategy was used to identify studies comprising two combined concepts: ‘children’, which included all terms for children and adolescents and ‘gender dysphoria’, which included associated terms such as gender-related distress and gender incongruence, and gender identity terms including transgender, gender diverse and non-binary.
MEDLINE ( online supplemental table S1 ), EMBASE and PsycINFO through OVID, CINAHL Complete through EBSCO, and Web of Science (Social Science Citation Index) were searched (13–23 May 2021 and updated on 27 April 2022).
Supplemental material
Reference lists of included studies and relevant systematic reviews were assessed for inclusion. 11–16 19 20
Inclusion criteria
The review included published research that reported outcomes of interventions used to suppress puberty for children and/or adolescents experiencing gender dysphoria/incongruence ( table 1 ).
- View inline
Inclusion and exclusion criteria
Selection process
The results of database and other searches were uploaded to Covidence 21 and screened independently by two reviewers. Full texts of potentially relevant articles were retrieved and reviewed against inclusion criteria by two reviewers independently. Disagreements were resolved through discussion and inclusion of a third reviewer.
Data extraction
Data on study characteristics, methods and reported outcomes were extracted into prepiloted data extraction templates by one reviewer and second-checked by another.
Study quality
Critical appraisal was undertaken by two reviewers independently, with consensus reached through discussion and involvement of a third reviewer where necessary.
Quality was assessed using a modified version ( online supplemental file 1 ) of the Newcastle-Ottawa Scale for cohort studies, a validated scale of eight items covering three domains: selection, comparability and outcome. 22 Scale modification included not scoring certain question(s) for cross-sectional and single-group designs, or particular outcomes; specification of key confounders to assess comparability of cohorts; guidance regarding sufficiency of follow-up and use of numerical scores for items and overall (maximum score 9 for cohorts, 8 for pre-post and cross-sectional studies with comparator). Total scores were calculated as percentages to account for different total scores (≤50% low quality, >50%–75% moderate quality, >75% high quality).
Narrative synthesis methods were used because of heterogeneity in study design, intervention, comparator, outcome and measurement. Due to high risk of bias in low-quality studies, these were excluded from the synthesis.
When synthesising results by outcome domains, care was taken to differentiate between different study designs, comparators and interventions. Where possible, potential differences in effects by birth-registered sex, treatment duration or treatment in early puberty versus late puberty were examined.
The database search yielded 28 147 records, 3181 of which were identified as potentially relevant for the linked systematic reviews and full texts reviewed. From these, 50 studies met inclusion criteria for this review ( figure 1 ).
- Download figure
- Open in new tab
- Download powerpoint
Study flow diagram.
Study characteristics
Studies were published from 2006 to 2022 with the majority published in 2020–2022 (n=29). Studies were conducted in the Netherlands (n=17), 23–39 the US (n=15), 40–54 the UK (n=6), 55–60 Canada (n=4), 61–64 three in Belgium 65–67 and Israel 68–70 and one in Brazil 71 and Germany 72 ( online supplemental table S2 ).
The 50 studies included 11 cohorts comparing adolescents experiencing gender dysphoria/incongruence receiving puberty suppression with a comparator, 35 39–42 45 49 50 52 56 72 8 cross-sectional with a comparator 23 33 37 47 51 53 60 71 and 31 pre-post single group studies. 24–32 34 36 38 43 44 46 48 54 55 57–59 61–70 More than half of studies (n=29) used retrospective chart review.
All but 4 studies selected adolescents experiencing gender dysphoria/incongruence from specialist gender or endocrinology services: 43 from single services (in Belgium, Israel, the Netherlands and the UK these were large regional or national services) and 3 from multiple US services. 48–50 The other four included three US studies (national survey recruiting via community settings, 53 clinical and community settings, 51 US Military Healthcare Data Repository 54 ) and a study from Brazil recruiting via Facebook. 71
Overall, studies included 10 673 participants: 9404 were adolescents experiencing gender dysphoria/incongruence (4702 received puberty suppression, 4702 did not) and 1269 other comparators. Comparator groups included adolescents or adults experiencing gender dysphoria/incongruence who had not received puberty suppression, 35 39 40 42 51–53 60 71 72 untreated adolescents not experiencing gender dysphoria/incongruence, 36 47 50 both of these comparators 23 33 37 56 or adolescents receiving treatment for a different medical reason. 41 45 49
Most studies (n=39) assessed GnRH-a. In one, some participants received GnRH-a and some (birth-registered males) spironolactone. 62 In another, GnRH-a or progestins/anti-androgens were used but numbers taking each were not reported. 40 Among the other 11 studies, 5 assessed effects of progestins (cyproterone acetate, 66 67 lynestrenol, 65 66 medroxyprogesterone 44 and levonorgestrel-releasing intrauterine system 41 ) as alternatives to GnRH-a, 41 44 65–67 1 assessed bicalutamide 46 and 5 did not specify. 43 52–54 71
Of the 50 studies, 29 reported outcomes for feminising or masculinising hormones as well as for puberty suppression, either by including a mixed sample of those receiving the two different interventions or by assessing those who progressed to hormones following puberty suppression.
The most frequently measured outcomes were puberty suppression (n=30) and physical health outcomes (n=27) ( figure 2 , online supplemental table S3 ). Gender-related outcomes and body image were measured in five and four studies, respectively. Psychological health was measured in 13 studies, psychosocial in 9 studies and cognitive/neurodevelopmental outcomes in 3 studies. Side effects were reported in six, bone health in nine, and one study measured fertility.
Outcome categories by study quality and design.
One cross-sectional study was rated high quality, 37 25 moderate quality 23 24 29–32 34–36 39 48–51 54–59 64 65 67–69 and 24 low quality. 25–28 33 38 40–47 52 53 60–63 66 70–72 Of the 11 cohort studies, which were the only studies to include a comparator and assess outcomes over time, only 5 were rated moderate quality ( figure 2 , online supplemental table S4 ). 35 39 49 50 56
In most studies, there were concerns about sample representativeness due to single site recruitment, inclusion of a selected group and/or poor reporting of the eligible population. In studies including a comparator, most did not report or control for key differences between groups and only four used matched controls. 23 33 41 47 Most studies presented results for birth-registered males and females separately or controlled for this. Few studies controlled for age or Tanner stage or co-interventions that could influence outcomes.
Overall, studies used appropriate methods to ascertain exposure and assess outcomes. Adequacy of follow-up was evident in 18 studies, with multiple studies not reporting treatment duration, including participants receiving treatment at baseline, and not aligning follow-up with treatment initiation. Missing data at follow-up/analysis or poor reporting of this affected many studies.
Four studies did not report separate outcome data for adolescents receiving puberty suppression or masculinising/feminising hormones. 39 54 60 71 Two of these were of moderate quality and not included in the synthesis, 39 54 one of which was the only study to assess fertility outcomes. 39 One moderate-quality study assessed amplitude of click-evoked otoacoustic emissions. 23 This was excluded from the synthesis on the basis of not being clinically relevant.
Synthesis of outcomes
Gender dysphoria and body satisfaction.
Two pre-post studies measured gender dysphoria and body satisfaction (with primary and secondary sex or neutral body characteristics) and reported no change before and after receiving treatment 24 55 ( table 2 ).
Gender-related, body image, psychological, psychosocial, and cognitive/neurodevelopmental outcomes
Psychological health
One cross-sectional 37 and two pre-post studies 24 55 measured symptoms of depression (n=1), anxiety (n=1), anger (n=1), internalising and externalising symptoms (n=3), suicide and/or self-harm (n=2) and psychological functioning (n=2).
Three studies assessed internalising and externalising symptoms with one reporting improvements in both (pre-post 24 ), one improvement in internalising but not externalising symptoms when compared with adolescents under assessment by a gender service (cross-sectional 37 ) and one observed no change in either (pre-post). 55
For other psychological outcomes, there was either a single study, or two studies showing inconsistent results, with studies reporting either a small to moderate significant improvement or no change ( table 2 ).
Psychosocial outcomes
One cohort 56 and two pre-post 24 55 studies measured psychosocial functioning, one pre-post study assessed quality of life 55 and one cross-sectional study measured peer-relations ( table 2 ). 37
For psychosocial functioning, both pre-post studies reported no clinically significant change at follow-up. 24 55 The cohort study compared adolescents who were not immediately eligible for puberty suppression and received psychological support only, and adolescents who additionally received GnRH-a after 6 months. 56 Improvements were seen in both groups after 6 months of psychological support. This improvement was maintained over time for those receiving psychological support only. For those receiving GnRH-a, further improvements were observed at 12 and 18 months. At 18 months, psychosocial functioning in this group was considerably higher than in those still waiting for puberty suppression, and similar to adolescents not experiencing gender dysphoria/incongruence. However, there were considerably fewer participants included at final follow-up.
There was no change in quality of life pre-post, 55 and treated adolescents had better peer-relations compared with adolescents under assessment at a gender service but poorer peer-relations than adolescents not experiencing gender dysphoria/incongruence. 37
Cognitive/neurodevelopmental outcomes
One cross-sectional study measured executive functioning and found no difference between adolescents who were treated for <1 year compared with those not treated, but worse executive functioning in those treated for >1 year compared with those not treated. 51 A pre-post study found no differences in features typically associated with autism spectrum condition after treatment ( table 2 ). 59
Physical health outcomes
Bone health.
Five studies found decreases in bone mineral apparent density and z-scores pre-post treatment; however, absolute measures generally remained stable or increased/decreased slightly. 29 32 34 55 58 Results were similar across birth-registered males and females. 29 32 55 58 One study considered timing of treatment, and found similar decreases among those starting GnRH-a in early or late puberty ( table 3 ). 32
Physical health outcomes and side effects
Cardiometabolic health
Twelve pre-post studies measured body mass index (BMI), and in 10 studies there was no evidence of a clinically significant change in BMI and/or BMI SD score. 29 30 32 34 55 57 65 67–69 In one study, BMI increased for birth-registered males but not females. 58 Another study found BMI increased for birth-registered females who started GnRH-a in early puberty or mid-puberty, and birth-registered males in early puberty. 36
Three studies assessed cholesterol markers, one after GnRH-a (no changes), 34 one after cyproterone acetate (decrease in high-density lipoprotein (HDL) and triglycerides) 67 and one after lynestrenol (decrease in HDL, increase in low-density lipoprotein). 65 Three studies assessing GnRH-a reported blood pressure: two found similar systolic and diastolic blood pressure before and after treatment, 34 68 and one found a non-clinically significant increase in diastolic but not systolic blood pressure. 69 Two studies measured markers of diabetes (fasting glucose, HbA1c and/or insulin) and noted no changes. 65 67
Other physiological parameters
Five pre-post studies assessed other parameters from blood tests undertaken at baseline and follow-up, 30 31 34 65 67 three in those treated with GnRH-a, 30 31 34 one lynestrenol 65 and one cyproterone acetate. 67 Measurements included haemoglobin count (n=3), haematocrit percentage (n=3), creatinine (n=4), aspartate aminotransferase (n=3), alanine aminotransferase (n=3), γ-glutamyl transferase (n=1), alkaline phosphatase (n=2), prolactin (n=2), free thyroxin (n=3), thyroid-stimulating hormone (n=3), sex hormone binding globulin (n=3), vitamin D levels (n=1), dehydroepiandrosterone sulfate (n=3) and androstenedione (n=2). For most outcomes, no changes were reported. Where there were changes, these were not consistent in direction across studies.
One pre-post study assessing GnRH-a reported QTc prolongation, 64 and found no change in mean QTc, with no participants outside normal range.
Side effects
A cohort study of GnRH-a reported side effects including mild headaches or hot flushes (~20%) and moderate/severe headaches or hot flushes, mild fatigue, mood swings, weight gain and sleep problems (<10%) ( table 3 ). 55
Two studies assessed other medications and reported headaches and hot flushes as common and an increase in acne in a sample of birth-registered females receiving lynestrenol, 65 and complaints of fatigue in birth-registered males receiving cyproterone acetate. 67
Puberty suppression
Hormone levels.
Hormone levels were reported in nine studies of GnRH-a (two cohort, 49 50 seven pre-post 30 34 36 48 55 68 69 ), two in birth-registered females, 34 69 one in birth-registered males 68 and six including both ( table 4 ). 30 36 48–50 55
Puberty suppression outcomes
Five studies reported decreases in luteinising hormone, follicle-stimulating hormone, oestradiol and testosterone after receiving GnRH-a. 30 34 48 68 69 Another study, which reported luteinising and follicle-stimulating hormones, also found decreases in both pre-post. 55 One study reported that where baseline levels were high due to puberty starting, decreases were reported in testosterone and oestradiol. 36 One cohort study reporting pre-post data found smaller decreases in luteinising hormone, follicle-stimulating hormone, oestradiol and testosterone compared with other studies; however, it included a younger population, some of who were likely prepubertal. 50 The other cohort study included a comparator of adolescents with precocious puberty and found similar decreases in luteinising hormone and oestradiol. 49
One pre-post study of lynestrenol (birth-registered females) found a decrease in luteinising hormones but not follicle-stimulating hormone, oestradiol or testosterone. 65 One study of cyproterone acetate (birth-registered males) found no changes in luteinising hormone, follicle-stimulating hormone or oestradiol, but a decrease in total testosterone. 67
Pubertal progression
Puberty development was reported in four studies (two cohort, two pre-post). 30 35 49 67 One only included birth-registered males, 67 and three included both birth-registered males and females. 30 35 49
A cohort study assessing GnRH-a reported clinical pubertal escape in 2/21 adolescents treated for gender dysphoria/incongruence, in the form of breast enlargement or testicular enlargement together with deepening of voice, compared with no children treated for precocious puberty. 49 A pre-post study reported a decrease in testicular volume in birth-registered males, but unclear results with regard to breast development in birth-registered females (most started treatment at Tanner stage 4–5). 30 A pre-post study of birth-registered males using cyproterone acetate reported decreases in facial shaving and spontaneous erections. 67
A cohort study assessed whether secondary sex characteristics differed depending on receipt or timing of GnRH-a, and whether this affected which surgical interventions/techniques were later used. 35 The study found breast size was smallest in birth-registered females who received GnRH-a in Tanner stage 2/3 and largest in untreated participants. Those treated early in puberty were less likely to require a mastectomy and when surgery was required it was less burdensome. In birth-registered males, penile length was greater in those who received GnRH-a at Tanner stage 4/5 compared with Tanner stage 2/3, and greatest in untreated participants. 35 Those who received GnRH-a early required more invasive vaginoplasty techniques than those who received it later or not at all.
Menstrual suppression
Three studies (one cohort, two pre-post) measured menstrual suppression in birth-registered females, and found full suppression at follow-up, 30 49 55 which was similar to the effect seen in those with precocious puberty in the cohort study. 49
Height/Growth
Eleven studies (1 cohort, 50 10 pre-post 29 30 32 34 36 55 57 58 65 67 ) reported height, nine after GnRH-a, 29 30 32 34 36 50 55 57 58 one lynestrenol 65 and one cyproterone acetate. 67 The cohort study found a similar height velocity between the GnRH-a group and adolescent controls. 50 Six studies reported height Z or SD score 29 30 34 55 57 67 with two studies finding no change, 34 55 two a decrease for birth-registered males but not females, 29 57 one a decrease across birth-registered males and females 30 and one a decrease in birth-registered males with cyproterone acetate. 67 Absolute measures of height generally increased slightly or remained the same. 29 30 32 34 36 58 65 67
Body composition
Two studies reported changes in body composition pre-post, 30 57 reporting a significant decrease in lean mass SD score 57 and percentage 30 in males and females. One also measured body fat percentage and reported significant increases in both groups. 30
Bone geometry
One pre-post study measured the subperiosteal width and endocortical diameter of the hip bone and found that in birth-registered males these increased in those starting GnRH-a in early puberty and mid-puberty, but only in the early puberty group for birth-registered females. 36
This systematic review identified 50 studies reporting outcomes relating to puberty suppression in adolescents experiencing gender dysphoria/incongruence. No high-quality studies using an appropriate design were identified, and only four measured gender dysphoria as an outcome. Only 5 of the 11 cohort studies, which were the only studies to compare groups over time, were rated as moderate quality. 35 40 49 50 56
There was evidence from multiple mainly pre-post studies that puberty suppression exerts its expected physiological effect, as previously demonstrated in children with precocious puberty. 73 In adolescents experiencing gender dysphoria/incongruence, puberty suppression is initiated at different stages of puberty, 74 and two studies found that the effects on secondary sex characteristics may vary depending on whether treatment is initiated in early puberty versus mid-puberty, with potentially different outcomes for birth-registered males and females. 30 35 Multiple studies also found that bone density is compromised during puberty suppression, and gains in height may lag behind that seen in other adolescents. High-quality research is needed to confirm these findings; however, these potential risks should be explained to adolescents considering puberty suppression.
These findings add to other systematic reviews in concluding there is insufficient and/or inconsistent evidence about the effects of puberty suppression on gender dysphoria, body satisfaction, psychological and psychosocial health, cognitive development, cardiometabolic risk and fertility. 11–16 Regarding psychological health, one recent systematic review 14 reported some evidence of benefit while others have not. The results in this review found no consistent evidence of benefit. Inclusion of only moderate-quality to high-quality studies may explain this difference, as 8 of the 12 studies reporting psychological outcomes were rated as low-quality.
The lack of representativeness of samples and comparability of selected control groups were key concerns across studies. Only one study attempted to compare puberty suppression with psychosocial care, which is the only other treatment offered for gender dysphoria/incongruence in childhood, and this included a small sample, limited analyses, and little detail about the intervention. 56 Other studies lacked information about any psychological care provided to participants, and in studies that included a comparator there was limited information about any differences between groups. Large, well-designed studies with appropriate comparators that enable long-term outcomes of puberty suppression to be measured are needed.
Many studies reported effects of both puberty suppressants and hormones used in later adolescence for feminisation/masculinisation. In adolescents, GnRH-a often continues during hormone treatment, 74 or for adolescents who do not receive puberty suppression, GnRH-a or other anti-androgens may be offered at initiation of hormones. 66 This makes long-term follow-up of puberty suppression difficult to assess, including any differences between the types of interventions that are offered and when these are initiated, and the few studies reporting long-term outcomes either did not control for this or reported overall effects for both interventions. Although recent studies suggest nearly all adolescents who receive puberty suppression go on to feminising/masculinising hormones, 74–76 research is still needed to assess whether suppression will have any lasting effects for those who do not. Aggregation of studies reporting proportions of adolescents who progress to hormones and reasons for discontinuation would also offer useful insights.
Included studies assessed different outcomes across various outcome domains and employed multiple different measures. Agreement about the primary aim and related core outcomes of puberty suppression in this population would help to ensure studies measure key outcomes and facilitate future aggregation of evidence. Expert consensus recommendations to guide the methods and domains for assessing the neurodevelopmental effects of puberty suppression have been developed 77 ; however, there is currently no agreement across other outcome domains.
Strengths and limitations
Strengths include a published protocol with robust search strategies, use of PRISMA guidelines and comprehensive synthesis of moderate and high quality studies. Poor reporting across studies may have resulted in moderate-quality studies being rated low-quality and excluded from synthesis. As searches were conducted up to April 2022, this review does not include more recently published studies. However, the findings are in line with previous reviews despite the inclusion of numerous additional studies. In an update of the National Institute for Health and Care Excellence evidence review of GnRH-a performed in April 2023, 78 nine additional studies were identified, two of which they felt might materially affect their conclusions. 72 74 One was already included in this review, 72 and the other examined treatment trajectories which was not an outcome of interest. 74
Of other studies that we are aware have been published since April 2022 until January 2024, very few used a cohort design or an appropriate comparator and were of a similar low quality to moderate quality as the studies summarised in this review. Of those likely to contribute new data for synthesis, five examined physical growth and development, 79–83 one cardiometabolic health 84 and one psychological health. 85 The latter, a study from the US, found that adolescents who received puberty suppression before assessment for masculinising or feminising hormones had fewer symptoms of depression, anxiety, stress and suicidal thoughts compared with those who had not received puberty suppression. A sensitivity analysis found similar results, although no difference in suicidal thoughts. 85 Adding this study would provide no further clarity about whether puberty suppression improves psychological health due to the inconsistency of results between studies, and the limited high-quality research measuring these outcomes.
Two studies from the Netherlands found that height growth and bone maturation both decelerated during GnRH-a treatment, 80 81 and a third assessing only bone health found the same. 83 A Belgian study found stable height growth in birth-registered females but deceleration in birth-registered males. 82 These studies add strength to the conclusion that bone health and adult height may be compromised during GnRH-a, although like in previous studies the participants went on to receive masculinising or feminising hormones, and therefore the long-term outcomes of puberty suppression alone were not possible to determine.
Another new study, also from the Netherlands, assessed changes in body composition. 79 This found that in both birth-registered males and females lean mass z-scores decreased during puberty suppression and fat mass z-scores increased, although the rate and duration of change differed by birth-registered sex. These changes were also found in the two studies synthesised, 30 57 but as all three included no comparator uncertainty continues about the effect of puberty suppression on body composition.
A large study of adults in the US examined whether receipt of hormone interventions during adolescence was associated with cardiometabolic-related diagnoses, and for GnRH-a found no statistically significant differences for any diagnosis. 84 However, the study uses a retrospective cross-sectional design and is the only study to have examined cardiometabolic diagnoses, so no conclusions can be drawn about these outcomes.
To our knowledge, there are no additional moderate-quality or high-quality studies that have measured psychosocial or fertility outcomes, and only a single study assessing cognitive effects which measured a different outcome (white matter microstructure) to those included in this review. 86
Conclusions
There are no high-quality studies using an appropriate study design that assess outcomes of puberty suppression in adolescents experiencing gender dysphoria/incongruence. No conclusions can be drawn about the effect on gender-related outcomes, psychological and psychosocial health, cognitive development or fertility. Bone health and height may be compromised during treatment. High-quality research and agreement on the core outcomes of puberty suppression are needed.
Ethics statements
Patient consent for publication.
Not applicable.
- Thompson L ,
- Sarovic D ,
- Wilson P , et al
- Kaltiala R ,
- Bergman H ,
- Carmichael P , et al
- Coleman E ,
- Bouman WP , et al
- Hembree WC ,
- Cohen-Kettenis PT ,
- Gooren L , et al
- de Vries ALC ,
- Cohen-Kettenis PT
- The Swedish National Board of Health and Welfare
- Telfer MM ,
- Tollit MA ,
- Pace CC , et al
- University of California San Francisco Gender Affirming Health Program
- ↵ Medical treatment methods for Dysphoria associated with variations in gender identity in minors – recommendation . 2020 . Available : https://palveluvalikoima.fi/en/recommendations#genderidentity
- National Institute for Health and Care Excellence (NICE)
- Pasternack I ,
- Söderström I ,
- Saijonkari M , et al
- Ludvigsson JF ,
- Adolfsson J ,
- Höistad M , et al
- Wilson LM ,
- Sharma R , et al
- Anderson J ,
- Williams K , et al
- McKenzie JE ,
- Bossuyt PM , et al
- Taylor J , et al
- Ramos GGF ,
- Mengai ACS ,
- Daltro CAT , et al
- Veritas Health Innovation
- O’Connell D , et al
- van Heesewijk JO ,
- Menks WM , et al
- Steensma TD ,
- Doreleijers TAH , et al
- McGuire JK ,
- Steensma TD , et al
- Delemarre-van de Waal HA ,
- de Mutsert R ,
- Wiepjes CM , et al
- van der Loos MATC , et al
- Heijboer A , et al
- Schagen SEE ,
- Delemarre-van de Waal HA , et al
- Lustenhouwer P ,
- Cohen-Kettenis PT , et al
- Wouters FM ,
- Staphorsius AS ,
- Kreukels BPC ,
- Stoffers IE ,
- de Vries MC ,
- van de Grift TC ,
- van Gelder ZJ ,
- Mullender MG , et al
- van der Loos MA ,
- Hellinga I ,
- Vlot MC , et al
- van der Miesen AIR ,
- de Vries ALC , et al
- den Heijer M , et al
- Mulder CL ,
- Meißner A , et al
- Achille C ,
- Taggart T ,
- Eaton NR , et al
- Ford N , et al
- Grimstad FW ,
- Jacobson JD
- Stewart S ,
- Preston S , et al
- Khandheria MM ,
- Mejia-Otero JD ,
- Nokoff NJ ,
- Scarbro SL ,
- Moreau KL , et al
- Olson-Kennedy J ,
- Streeter LH ,
- Garofalo R , et al
- Pine-Twaddell E ,
- Newfield RS ,
- Marinkovic M
- Schulmeister C ,
- Millington K ,
- Kaufman M , et al
- Strang JF ,
- Nelson E , et al
- Tordoff DM ,
- Collin A , et al
- Turban JL ,
- Carswell JM , et al
- Hisle-Gorman E ,
- Schvey NA ,
- Adirim TA , et al
- Carmichael P ,
- Masic U , et al
- Dunsford M ,
- Skagerberg E , et al
- Ghelani R ,
- Brain C , et al
- Russell I ,
- Pearson B ,
- Arcelus J ,
- Witcomb GL , et al
- Chiniara LN ,
- Bonifacio HJ ,
- Khatchadourian K ,
- Khatchadourian K , et al
- Waldner RC ,
- Atallah J , et al
- Dhondt K , et al
- Lapauw B , et al
- Craen M , et al
- Elkon-Tamir E ,
- Segev-Becker A , et al
- Segev-Becker A ,
- Israeli G , et al
- Israeli G ,
- Elkon-Tamir E , et al
- Fontanari AMV ,
- Vilanova F ,
- Schneider MA , et al
- Becker-Hebly I ,
- Fahrenkrug S ,
- Campion F , et al
- Hou L , et al
- van der Loos MATC ,
- Hannema SE , et al
- Carruthers P , et al
- Hannema SE ,
- Klink DT , et al
- Kolbuck VD , et al
- NHS England
- Boogers LS ,
- Reijtenbagh SJP ,
- Wiepjes CM ,
- Willemsen LA ,
- Ciancia S ,
- Valentine A ,
- Furniss A , et al
- McGregor K ,
- McKenna JL ,
- Williams CR , et al
- van Heesewijk J ,
- Steenwijk MD ,
- Kreukels BPC , et al
Supplementary materials
Contributors LF, CEH, RH, TL and JT contributed to the conception of this review. RH, CEH, CH, AM and JT contributed to screening and selection. AM and JT completed data extraction. CEH, RH, AM and JT contributed to critical appraisal. CEH, AM and JT completed the synthesis and drafted the manuscript. All authors contributed to interpretation and reviewed and approved the manuscript prior to submission. CEH accepts full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding This work was funded by NHS England to inform the Cass Review (Independent review of gender identity services for children and young people). The funder and Cass Review team had a role in commissioning the research programme but no role in the study conduct, interpretation or conclusion.
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Linked Articles
- Original research Clinical guidelines for children and adolescents experiencing gender dysphoria or incongruence: a systematic review of guideline quality (part 1) Jo Taylor Ruth Hall Claire Heathcote Catherine Elizabeth Hewitt Trilby Langton Lorna Fraser Archives of Disease in Childhood 2024; - Published Online First: 09 Apr 2024. doi: 10.1136/archdischild-2023-326499
- Original research Care pathways of children and adolescents referred to specialist gender services: a systematic review Jo Taylor Ruth Hall Trilby Langton Lorna Fraser Catherine Elizabeth Hewitt Archives of Disease in Childhood 2024; - Published Online First: 09 Apr 2024. doi: 10.1136/archdischild-2023-326760
- Original research Psychosocial support interventions for children and adolescents experiencing gender dysphoria or incongruence: a systematic review Claire Heathcote Jo Taylor Ruth Hall Stuart William Jarvis Trilby Langton Catherine Elizabeth Hewitt Lorna Fraser Archives of Disease in Childhood 2024; - Published Online First: 09 Apr 2024. doi: 10.1136/archdischild-2023-326347
- Original research Gender services for children and adolescents across the EU-15+ countries: an online survey Ruth Hall Jo Taylor Claire Heathcote Trilby Langton Catherine Elizabeth Hewitt Lorna Fraser Archives of Disease in Childhood 2024; - Published Online First: 09 Apr 2024. doi: 10.1136/archdischild-2023-326348
- Original research Impact of social transition in relation to gender for children and adolescents: a systematic review Ruth Hall Jo Taylor Catherine Elizabeth Hewitt Claire Heathcote Stuart William Jarvis Trilby Langton Lorna Fraser Archives of Disease in Childhood 2024; - Published Online First: 09 Apr 2024. doi: 10.1136/archdischild-2023-326112
- Original research Characteristics of children and adolescents referred to specialist gender services: a systematic review Jo Taylor Ruth Hall Trilby Langton Lorna Fraser Catherine Elizabeth Hewitt Archives of Disease in Childhood 2024; - Published Online First: 09 Apr 2024. doi: 10.1136/archdischild-2023-326681
- Original research Clinical guidelines for children and adolescents experiencing gender dysphoria or incongruence: a systematic review of recommendations (part 2) Jo Taylor Ruth Hall Claire Heathcote Catherine Elizabeth Hewitt Trilby Langton Lorna Fraser Archives of Disease in Childhood 2024; - Published Online First: 09 Apr 2024. doi: 10.1136/archdischild-2023-326500
- Original research Masculinising and feminising hormone interventions for adolescents experiencing gender dysphoria or incongruence: a systematic review Jo Taylor Alex Mitchell Ruth Hall Trilby Langton Lorna Fraser Catherine Elizabeth Hewitt Archives of Disease in Childhood 2024; - Published Online First: 09 Apr 2024. doi: 10.1136/archdischild-2023-326670
- Editorial Holistic approach to gender questioning children and young people Camilla C Kingdon Archives of Disease in Childhood 2024; - Published Online First: 09 Apr 2024. doi: 10.1136/archdischild-2024-327100
Read the full text or download the PDF:

IMAGES
VIDEO
COMMENTS
Regardless of this commonality, both types of review vary significantly. The following table provides a detailed explanation as well as the differences between systematic and literature reviews. Kysh, Lynn (2013): Difference between a systematic review and a literature review.
Systematic review methods have influenced many other review types, including the traditional literature review. Covidence is a web-based tool that saves you time at the screening, selection, data extraction and quality assessment stages of your systematic review. It supports easy collaboration across teams and provides a clear overview of task ...
The difference between literature review and systematic review comes back to the initial research question. Whereas the systematic review is very specific and focused, the standard literature review is much more general. The components of a literature review, for example, are similar to any other research paper.
A systematic review is a type of review that uses repeatable methods to find, select, and synthesize all available evidence. ... Systematic review vs. literature review. A literature review is a type of review that uses a less systematic and formal approach than a systematic review. Typically, an expert in a topic will qualitatively summarize ...
Introduction. A systematic review collects secondary data, and is a synthesis of all available, relevant evidence which brings together all existing primary studies for review (Cochrane 2016).A systematic review differs from other types of literature review in several major ways.
Systematic Review vs. Literature Review. It is common to confuse systematic and literature reviews as both are used to provide a summary of the existent literature or research on a specific topic. Even with this common ground, both types vary significantly. Please review the following chart (and its corresponding poster linked below) for the ...
The methodology involved in a literature review is less complicated and requires a lower degree of planning. For a systematic review, the planning is extensive and requires defining robust pre-specified protocols. It first starts with formulating the research question and scope of the research. The PICO's approach (population, intervention ...
Literature reviews establish the foundation of academic inquires. However, in the planning field, we lack rigorous systematic reviews. In this article, through a systematic search on the methodology of literature review, we categorize a typology of literature reviews, discuss steps in conducting a systematic literature review, and provide suggestions on how to enhance rigor in literature ...
A literature review summarizes and analyzes the relevant publications on a topic. It demonstrates to your readers that you are knowledgeable of the ongoing scholarly conversation and how your research fits within the broader field of study. ... A systematic review attempts to identify, appraise and synthesize all available relevant evidence to ...
Acommon type of submission at any Journal is a review of the published information related to a topic.These are often returned to their authors without review, usually because they are literature reviews rather than systematic reviews. There is a big difference between the two (Table 1).Here, we summarise the differences, how they are used in academic work, and why a general literature review ...
Apart from systematic literature review, some other common types of literature review are1: The most commonly used form of review, however, is the systematic literature review. Compared to the other types of literature reviews described above, this one requires a more rigorous and well-defined approach. The systematic literature review can be ...
In contrast, a systematic literature review might be conducted by one person. Overall, while a systematic review must comply with set standards, you would expect any review called a systematic literature review to strive to be quite comprehensive. A systematic literature review would contrast with what is sometimes called a narrative or ...
A literature review is a systematic way of collecting and synthesizing previous research (Snyder, 2019).An integrative literature review provides an integration of the current state of knowledge as a way of generating new knowledge (Holton, 2002).HRDR is labeling Integrative Literature Review as one of the journal's four non-empirical research article types as in theory and conceptual ...
A systematic review can be used to understand the current state of knowledge on a topic, while a literature review focuses on specific works. However, a systematic review can certainly be in-depth. The amount of research that goes into it will impact the level of detail it discusses. The main difference is that a literature review is a critical ...
A systematic review is a firmly structured literature review, undertaken according to a fixed plan, system or method. As such, it is highly focused on a particular and explicit topic area with strict research parameters. Systematic reviews will often have a detailed plan known as a protocol, which is a statement of the approach and methods to ...
A 'non-systematic review' might use some of the same methods as systematic reviews, such as systematic approaches to identify studies or quality appraise the literature. ... In a student dissertation, for example, there may not be the time to be fully systematic in a review of the literature if this is only one small part of the thesis. In ...
Among the various types of reviews, the systematic review of the literature is ranked as the most rigorous since it is a high-level summary of existing evidence focused on answering a precise question. Systematic reviews employ a pre-defined protocol to identify relevant and trustworthy literature. Such reviews can accomplish several critical ...
Literature Review vs. Systematic Review "In evidence-based practice, systematic reviews are considered one of the highest levels of information." - Kysh, Lynn (2013): Difference between a systematic review and a literature review.
A systematic review collects all possible studies related to a given topic and design, and reviews and analyzes their results [ 1 ]. During the systematic review process, the quality of studies is evaluated, and a statistical meta-analysis of the study results is conducted on the basis of their quality. A meta-analysis is a valid, objective ...
Systematic review. A systematic review is a summary of the medical literature that uses explicit and reproducible methods to systematically search, critically appraise, and synthesize on a specific issue. It synthesizes the results of multiple primary studies related to each other by using strategies that reduce biases and random errors.
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
Systematic Review. Systematic literature reviews follow specific procedures in some ways similar to setting up an experiment to ensure that future scholars can replicate the same steps. They are also helpful for evaluating data published over multiple studies.
When the word "review" alone is used to describe a research paper, the first thing that should come to mind is that it is a literature review. Almost every researcher starts off their career with literature reviews. To know the difference between a systematic review and a literature review, read on here. Traditional literature reviews are ...
A systematic review was performed following PRISMA guidelines using Pubmed (MEDLINE), EMBASE, and Cochrane databases to search for relevant studies published in the last twenty years. Results Thirty-one studies were included with a total of 39,505 implant patients and mean age of 44.2 ± 9.30 years.
If a literature review or systematic review was found to be relevant, then the cited articles were included in the original data pull. This systematic review is evaluating the clinical data supporting products that have not yet been approved by the FDA.
Despite robust scientific evidence and governmental support, no systematic literature review has collated the evidence with respect to the value, cost or cost-effectiveness of such interventions. Our objective was to assess the economic impact of ACIs aimed at improving the health and wellbeing of older adults; to determine the range and ...
The literature review and meta-analysis were conducted independently by two researchers. 23 studies that met the inclusion criteria were included in this system review for qualitative and quantitative analysis. ... A Systematic Review and Meta-Analysis of Preclinical Studies Provisionally Accepted. Yu Li 1 Guangren Yue 1 Shuying Yu 1 Xinhao ...
The COVID-19 pandemic imposed additional and specific challenges on the lives and wellbeing of informal unpaid carers. Addressing an important gap in the existing literature, this systematic review (prospectively registered with PROSPERO CRD42022376012) synthesises and evaluates the quantitative evidence examining the association between unpaid caregiving and mental health (compared to non ...
This systematic review and meta-analysis are prepared and reported using an updated Preferred Reporting Items for Systematic Review and Meta-Analysis guideline and checklist (Appendix 1). The ...
Robust evidence concerning risks and benefits is lacking and there is a need to aggregate evidence as new studies are published. Aim To identify and synthesise studies assessing the outcomes of puberty suppression in adolescents experiencing gender dysphoria/incongruence. Methods A systematic review and narrative synthesis.