Information
- Author Services

Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Nanotechnology: a revolution in modern industry.

Graphical Abstract
Share and Cite
Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023 , 28 , 661. https://doi.org/10.3390/molecules28020661
Malik S, Muhammad K, Waheed Y. Nanotechnology: A Revolution in Modern Industry. Molecules . 2023; 28(2):661. https://doi.org/10.3390/molecules28020661
Malik, Shiza, Khalid Muhammad, and Yasir Waheed. 2023. "Nanotechnology: A Revolution in Modern Industry" Molecules 28, no. 2: 661. https://doi.org/10.3390/molecules28020661
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges

First published on 24th February 2021
Nanomaterials have emerged as an amazing class of materials that consists of a broad spectrum of examples with at least one dimension in the range of 1 to 100 nm. Exceptionally high surface areas can be achieved through the rational design of nanomaterials. Nanomaterials can be produced with outstanding magnetic, electrical, optical, mechanical, and catalytic properties that are substantially different from their bulk counterparts. The nanomaterial properties can be tuned as desired via precisely controlling the size, shape, synthesis conditions, and appropriate functionalization. This review discusses a brief history of nanomaterials and their use throughout history to trigger advances in nanotechnology development. In particular, we describe and define various terms relating to nanomaterials. Various nanomaterial synthesis methods, including top-down and bottom-up approaches, are discussed. The unique features of nanomaterials are highlighted throughout the review. This review describes advances in nanomaterials, specifically fullerenes, carbon nanotubes, graphene, carbon quantum dots, nanodiamonds, carbon nanohorns, nanoporous materials, core–shell nanoparticles, silicene, antimonene, MXenes, 2D MOF nanosheets, boron nitride nanosheets, layered double hydroxides, and metal-based nanomaterials. Finally, we conclude by discussing challenges and future perspectives relating to nanomaterials.
| Nadeem Baig |
| Irshad Kammakakam |
| Wail Falath |
1. Introduction
The term nanometer was first used in 1914 by Richard Adolf Zsigmondy. 5 The American physicist and Nobel Prize laureate Richard Feynman introduced the specific concept of nanotechnology in 1959 in his speech during the American Physical Society's annual meeting. This is considered to be the first academic talk about nanotechnology. 5 He presented a lecture that was entitled “There's Plenty of Room at the Bottom”. During this meeting, the following concept was presented: “why can’t we write the entire 24 volumes of the Encyclopedia Britannica on the head of a pin?” The vision was to develop smaller machines, down to the molecular level. 6,7 In this talk, Feynman explained that the laws of nature do not limit our ability to work at the atomic and molecular levels, but rather it is a lack of appropriate equipment and techniques that limit this. 8 Through this, the concept of modern technology was seeded. Due to this, he is often considered the father of modern nanotechnology. Norio Taniguchi might be the first person who used the term nanotechnology, in 1974. Norio Taniguchi stated: “nano-technology mainly consists of the processing of, separation, consolidation, and deformation of materials by one atom or one molecule.” 5,9 Before the 1980s, nanotechnology remained only an area for discussion, but the concept of nanotechnology was seeded in the minds of researchers with the potential for future development.
The invention of various spectroscopic techniques sped up research and innovations in the field of nanotechnology. IBM researchers developed scanning tunneling microscopy (STM) in 1982, and with STM it became feasible to attain images of single atoms on “flat” ( i.e. , not a tip) surfaces. 10 Atomic force microscopy (AFM) was invented in 1986, and it has become the most crucial scanning probe microscope technique. 11 The motivation to develop hard discs with high storage density stimulated the measurement of electrostatic and magnetic forces. This led to the development of Kelvin-probe-, electrostatic-, and magnetic-force microscopy. 12 Currently, nanotechnology is rapidly evolving and becoming part of almost every field related to materials chemistry. The field of nanotechnology is evolving every day, and now powerful characterization and synthesis tools are available for producing nanomaterials with better-controlled dimensions.
Nanotechnology is an excellent example of an emerging technology, offering engineered nanomaterials with the great potential for producing products with substantially improved performances. 13 Currently, nanomaterials find commercial roles in scratch-free paints, surface coatings, electronics, cosmetics, environmental remediation, sports equipment, sensors, and energy-storage devices. 14 This review attempts to provide information in a single platform about the basic concepts, advances, and trends relating to nanomaterials via covering the related information and discussing synthesis methods, properties, and possible opportunities relating to the broad and fascinating area of nanomaterials ( Scheme 1 ). It is not easy to cover all the literature related to nanomaterials, but important papers from history and the current literature are discussed in this review. This review provides fundamental insight for researchers, quickly capturing the advances in and properties of various classes of nanomaterials in one place.
| A schematic representation of nanomaterials and their applications. | ||
2. Descriptions of terms associated with nanomaterials
| Term | Description | Ref. |
|---|---|---|
| Nanotechnology | Nanotechnology refers to technology at the nanoscale level in which materials, devices, or systems are developed via controlling matter at the nanoscale length to stimulate the unique properties of the material at the nano-level. | |
| Nanomanufacturing | Nanomanufacturing refers to manufacturing at the nanoscale level and accomplished via bottom-up or top-down methods. | |
| Nanoscale | A scale covering 1–100 nm. | |
| Nanomaterial | A material is called a nanomaterial if it has at least one dimension in the nanoscale range of 1–100 nm. | |
| Nano-object | A nano-object is a discrete piece of material with one, two, or three external dimensions in the nanoscale range. | |
| Nanoparticle | An object or particle is called a nanoparticle when all of its dimensions are in the nanoscale range. | |
| Aspect ratio | The aspect ratio of a nano-object is defined as the ratio of the length of the major axis to the width of the minor axis. | |
| Nanosphere | A nanosphere is a nanoparticle that has an aspect ratio of 1. | |
| Nanorod | The term nanorod is used when the shortest and longest axes have different lengths. Nanorods have a width in the range of 1 to 100 nm and an aspect ratio greater than 1. | |
| Nanofiber | A nano-object with two dimensions in the nanoscale range and a third dimension that is significantly larger. | |
| Nanowire | Nanowires are analogues to nanorods, but with a higher aspect ratio. | |
| Nanotube | Hollow nanofibers are called nanotubes. | |
| Nanostructured material | This is a term used for materials that have structural elements, molecules, crystallites, or clusters with dimensions in the range of 1–100 nm. | |
| Nanomaterial | A material is called a nanomaterial if it has at least one dimension in the nanoscale range of 1–100 nm. | |
| Engineered nanomaterials | Intentionally produced materials that have one or more dimensions in the order of 100 nm or less are called engineered nanomaterials. | |
| Nanocomposite | Nanocomposites are defined as multicomponent materials with multiple different phase domains, in which at least one of the phases has at least one dimension in the order of nanometers. |
3. Approaches for the synthesis of nanomaterials
| The synthesis of nanomaterials via top-down and bottom-up approaches. Reprinted with permission from . Copyright: ©2019, Elsevier B.V. All rights reserved. | ||
3.1. Top-down approaches
| The principle of the ball milling method. Reprinted with permission from . Copyright: ©2016, John Wiley & Sons, Ltd. | ||
| A schematic diagram of the coaxial electrospinning technique (center), and FESEM (a and c) and TEM (b and d) images of fibers before and after calcination. Reprinted with permission from . Copyright: ©2012, Elsevier Ltd. All rights reserved. | ||
| A schematic diagram of the fabrication of 3D micro-nanostructures with an ion beam through bulk Si structuring. This involves implantation in Si through Ga FIB lithography and mask-writing at nanometer resolution, subsequent anisotropic wet etching in KOH solution, and the fabrication of Si micro-nanostructures via the selective removal of the unimplanted region. Reprinted with permission from . Copyright: ©2020, Elsevier B.V. All rights reserved. | ||
| A schematic diagram of the DC magnetron sputtering process. Reprinted with permission from . Copyright: ©2017, Elsevier Ltd. All rights reserved. | ||
The conditions under which arc discharge takes place play a significant role in achieving new forms of nanomaterials. The conditions under which different carbon-based nanomaterials are formed via the arc discharge method are explained in Fig. 6 . Various carbon-based nanomaterials are collected from different positions during the arc discharge method, as their growth mechanisms differ. 44 MWCNTs, high-purity polyhedral graphite particles, pyrolytic graphite, and nano-graphite particles can be collected from either anode or cathode deposits or deposits at both electrodes. 46–48 Apart from the electrodes, carbon-based nanomaterials can also be collected from the inner chamber. Different morphologies of single-wall carbon nanohorns (SWCNHs) can be obtained under different atmospheres. For example, ‘dahlia-like’ SWCNHs are produced under an ambient atmosphere, whereas ‘bud-like’ SWCNHs are generated under CO and CO 2 atmospheres. 49 The arc discharge method can be used to efficiently achieve graphene nanostructures. The conditions present during the synthesis of graphene can affect its properties. Graphene sheets prepared via a hydrogen arc discharge exfoliation method are found to be superior in terms of electrical conductivity and have good thermal stability compared to those obtained via argon arc discharge. 50
| A schematic illustration of the formation mechanisms of carbon nanomaterials on the inner wall of the chamber using different gases via a DC arc discharge approach. Reprinted with permission from . Copyright: ©2018, Elsevier Ltd. All rights reserved. | ||
| TEM images, corresponding mean sizes, and standard deviations of palladium nanoparticles synthesized via laser ablation in water for 10 min at laser wavelengths and fluences of (a) 532 nm and 8.92 J cm , (b) 532 nm and 19.90 J cm , (c) 1064 nm and 8.92 J cm , (d) 1064 nm and 19.90 J cm , and (e) 355 nm and 0.10 J cm . Reprinted with permission from . Copyright: ©2017, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
3.2. Bottom-up approaches
| A schematic diagram of the growth of in-plane graphene and hBN heterostructures via various techniques: (A) simultaneous in situ CVD growth, (B) sequential in situ CVD growth, (C) lithography-assisted growth, and (D) conversion growth. Reprinted with permission from . Copyright: ©2016, Elsevier B.V. All rights reserved. | ||
| An overview showing two sol–gel method synthesis examples: (a) films from a colloidal sol and (b) powder from a colloidal sol transformed into a gel. Reprinted with permission from . Copyright: ©2010, Elsevier B.V. All rights reserved. | ||
The hard template method is also called nano-casting. Well-designed solid materials are used as templates, and the solid template pores are filled with precursor molecules to achieve nanostructures for required applications ( Fig. 10 ). 78 The selection of the hard template is critical for developing well-ordered mesoporous materials. It is desirable that such hard templates should maintain a mesoporous structure during the precursor conversion process, and they should be easily removable without disrupting the produced nanostructure. A range of materials has been used as hard templates, not limited to carbon black, silica, carbon nanotubes, particles, colloidal crystals, and wood shells. 85 Three main steps are involved in the synthetic pathway for obtaining nanostructures via templating methods. In the first step, the appropriate original template is developed or selected. Then, a targeted precursor is filled into the template mesopores to convert them into an inorganic solid. In the final step, the original template is removed to achieve the mesoporous replica. 86 Via using mesoporous templates, unique nanostructured materials such as nanowires, nanorods, 3D nanostructured materials, nanostructured metal oxides, and many other nanoparticles can be produced. 87 From this brief discussion, it can be seen that a wide range of unique structured nanomaterials can be produced using soft and hard template methods.
| A schematic representation of the synthesis of materials using different types of templates. Published by The Royal Society of Chemistry. | ||
| (a) A schematic diagram showing the synthetic steps to GA-MNPs. (b) The synthesis of L-MNPs through a non-ionic reverse micelle method. | ||
4. Unique nanomaterial features
The electronic properties of semiconductors in the 1–10 nm range are controlled by quantum mechanical considerations. Thus, nanospheres with diameters in the range of 1–10 nm are known as quantum dots. The optical properties of nanomaterials such as quantum dots strongly depend upon their shape and size. 96 A photogenerated electron–hole pair has an exciton diameter on the scale of 1–10 nm. Thus, the absorption and emission of light by semiconductors could be controlled via tuning the nanoparticle size in this range. However, in the case of metals, the mean free path of electrons is ∼10–100 nm and, due to this, electronic and optical effects are expected to be observed in the range of ∼10–100 nm. The colors of aqueous solutions of metal nanoparticles can be changed via changing the aspect ratio. Aqueous solutions of Ag NPs show different colors at different aspect ratios. A red shift in the absorption band appears with an increase in the aspect ratio ( Fig. 12 ). 21
| Aqueous solutions of silver nanoparticles show wide variations in visible color depending on the aspect ratio of the suspended nanoparticles. The far left of the photograph shows silver nanospheres (4 nm in diameter) that are used as seeds for subsequent reactions, while (a–f) show silver nanorods with increasing aspect ratios from 1–10. The corresponding visible absorption spectra for (a)–(f) are also shown in the left panel. Reprinted with permission from . Copyright: ©2002, WILEY-VCH Verlag GmbH, Weinheim, Fed. Rep. of Germany. | ||
Among a range of unique properties, the following key properties can be obtained upon tuning the sizes and morphologies of nanomaterials.
4.1. Surface area
4.2. magnetism, 4.3. quantum effects, 4.4. high thermal and electrical conductivity, 4.5. excellent mechanical properties, 4.6. excellent support for catalysts, 4.7. antimicrobial activity.
Overall, these features have made nanoscale materials valuable for a wide range of applications, substantially boosting the performances of various devices and materials in a number of fields. Details of various nanomaterials, their properties, and applications in various fields will be discussed below.
5. Nanomaterials, characteristics, and applications
5.1. special carbon-based nanomaterials.
In the carbon-based nanomaterial family, fullerenes were the first symmetric material, and they provided new perspectives in the nanomaterials field. This led to the discovery of other carbon-based nanostructured materials, such as carbon nanotubes and graphene. 110 Fullerenes are present in nature and interstellar space. 111 Interestingly, fullerenes were the molecule of the year in 1991 and attracted the most research projects compared to other scientific subjects during that period. 112 Fullerenes possess several unique features that make them attractive for applications in different fields. Fullerenes display solubility to some extent in a range of solvents, and these characteristics make them unique compared to the other allotropes of carbon. 108
The chemical modification of fullerenes is an exciting subject, improving their effectiveness for applications. There are two main ways to modify fullerenes: 113 fullerene inner-space modification, and fullerene outer-surface modification.
Endohedral and exohedral doping examples are shown in Fig. 13 . 114 Fullerenes are hollow cages, and the interior acts as a robust nano-container for host target species when forming endohedral fullerene. 115 Endohedral fullerenes do not always follow the isolated pentagon rule (IPR). 116 To date, fullerene nanocages have received substantial consideration in the materials chemistry field due to their useful potential applications. Neutral and charged single atoms in free space are highly reactive and unstable. In the confined environment of fullerenes, these reactive species can be stabilized; for example, the LaC 60 + ion does not react with the NH 3 , O 2 , H 2 , or NO. Thus, reactive metals can be protected from the surrounding environment by trapping them inside fullerene cages. 117 Another emerging carbon nanomaterial is endohedral fullerene containing lithium (Li@C 60 ). 118 Lithium metal is very reactive, and extreme controlled environmental conditions are required to preserve or use it. In other words, secure structures are required for lithium storage. Li-Based endohedral fullerene shows unique solid-state properties. The encapsulation of lithium atoms in fullerene helps to protect lithium atoms from external agents. Li-Based endohedral fullerenes have the potential to open the door to nanoscale lithium batteries. 119 For the development of endohedral metallofullerenes, larger fullerenes are generally required, as they possess large cages to accommodate lanthanide and transition metal atoms more smoothly. 118 Fullerene nanocages are useful for the storage of gases. Fullerene is under consideration for hydrogen storage. 120,121
| A schematic representation of the two interstitial doping sites in C , leading to exohedral and endohedral doping. Reprinted with permission from . Copyright: ©2009, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Exohedral fullerenes carry more potential for applications as outer surfaces can be more easily modified or functionalized. The exohedral doping of metals into fullerenes strongly affects the electronic properties via shifting electrons from the metal to the fullerene nanocage. 122 The practical application of fullerenes can be achieved with tailor-made fullerene derivatives via chemical functionalization. As fullerene chemistry has matured, a wide range of functionalized fullerenes has been realized through simple synthetic routes. 123 The combination of hydrogen-bonding motifs and fullerenes has allowed the modulation of 1D, 2D, and 3D fullerene-based architectures. 124 The excellent electron affinities of fullerenes have shown great potential for eliminating reactive oxygen species. The presence of excess reactive oxygen species can cause biological dysfunction or other health issues. The surfaces of fullerenes have been functionalized via mussel-inspired chemistry and Michael addition reactions for the fabrication of C 60 –PDA–GSH. The developed C 60 –PDA–GSH nanoparticles demonstrated excellent potential for scavenging reactive oxygen species. 125
Amphiphiles have great importance in industrial processes and daily life applications. Amphiphilic molecules consist of hydrophilic and hydrophobic parts, and they perform functions in water via forming two- and three-dimensional assemblies. Recently, conical fullerene amphiphiles 126 have emerged as a new class of amphiphiles, in which a nonpolar apex is supplied by fullerenes and a hydrophilic part is achieved through functionalization. The selective functionalization of the fullerene on one side helps to achieve a supramolecule due to unique interfacial behavior. The unique supramolecular structure formed via the spontaneous assembly of one-sided selectively functionalized fullerenes through strong hydrophobic interactions between the fullerene apexes and polar functionalized portions is soluble in water. Conical fullerene amphiphiles are mechanically robust. Via upholding the structural integrity, conical fullerene amphiphiles can be readily aggregated with nanomaterials and biomolecules to form multicomponent agglomerates with controllable structural features. 127 Fullerenes, after suitable surface modification, have excellent potential for use in drug delivery, but there have only been limited explorations of their drug delivery applications. 128,129 Fullerene-based nano-vesicles have been developed for the delayed release of drugs. 130 Water-soluble proteins have great potential in the field of nanomedicine. The water-soluble cationic fullerene, tetra(piperazino)[60] fullerene epoxide (TPFE), has been used for the targeted delivery of DNA and siRNA specifically to the lungs. 131 For diseases in lungs or any other organ, efficient treatment requires the targeted delivery of active agents to a targeted place in the organ. The accumulation of micrometer-sized carriers in the lung makes lung-selective delivery difficult, as this may induce embolization and inflammation in the lungs. Size-controlled blood vessel carrier vehicles have been developed using tetra(piperazino)fullerene epoxide (TPFE). TPFE and siRNA agglutinate in the bloodstream with plasma proteins and, as a result, micrometer-sized particles are formed. These particles clog the lung capillaries and release siRNA into lungs cells; after siRNA delivery, they are immediately cleared from the lungs ( Fig. 14 ). 132
| The mechanism of the lung-specific delivery of siRNA mediated by tetra(piperazino)fullerene (TPFE): (1) TPFE aggregates with siRNA to form ca. 100 nm-sized TPFE–siRNA complexes (light blue particles) via electrostatic interactions; (2) the complexes agglutinate with plasma proteins (white particles) in the bloodstream to form >6 μm particles (blue particles); (3) the particles clog and accumulate in the narrow lung capillaries; (4) the TPFE–siRNA complexes were delivered into lung cells and siRNA was released into lung cells. Reprinted with permission from . Copyright: ©2014, Springer Nature. | ||
The supramolecular organization of fullerene (C 60 ) is a unique approach for producing shape-controlled moieties on the nano-, micro-, and macro-scale. Nano-, micro-, and macro-scale supramolecular assemblies can be controlled via manipulating the preparation conditions to achieve unique optoelectronic properties. 133 The development of well-ordered and organized 1D, 2D, and 3D fullerene assemblies is essential for achieving advanced optical and organic-based electronic devices. 134 Fullerene-based nanostructured materials with new dimensions are being developed from zero-dimensional fullerene and tuned to achieve the desired characteristics. 1D C 60 fullerene nanowires have received substantial attention over other crystalline forms due to their excellent features of potential quantum confinement effects, low dimensionality, and large surface areas. 135
Carbon nanomaterials are also used as supports for catalysts, and the main reasons to use them are their high surface areas and electrical conductivities. Carbon supports strongly influence the properties of metal nanoparticles. In fuel cells, the carbon support strongly affects the stability, electronic conductivity, mass transport properties, and electroactive surface area of the supported catalyst. 136 In fuel cells, the degradation of some catalysts, such as platinum-based examples, and carbon is correlated and reinforced as a result of both being present. Carbon support oxidation is catalyzed by platinum and the oxidation of carbon accelerates platinum-catalyst release. Overall, this results in a loss of catalytically active surface area. 137 Fullerenes are considered suitable support materials due to their excellent electrochemical activities and stability during electrochemical reactions. 138 Due to their high stability and good conductivity, fullerenes can replace conventional carbon as catalyst support materials. Fullerenes are also used for the development of efficient solar cells. 139
Apart from the applications mentioned above, fullerenes have a broader spectrum of applications where they can be used to improve outcomes considerably. Fullerenes have the potential to be used in the development of superconductors. 140 The strong covalent bonds in fullerenes make them useful nanomaterials for improving the mechanical properties of composites. 141 The combination of fullerenes with polymers can result in good flame-retardant and thermal properties. 142 Fullerenes and their derivatives are used for the development of advanced lubricants. They are used as modifiers for greases and individual solid lubricants. 138 Fullerenes have tremendous medicinal importance due to their anticancer, antioxidant, anti-bacterial, and anti-viral activities. 104
Fullerenes are vital members of the carbon-based nanomaterial family and they certainly possess exceptional properties. This discussion further emphasizes their importance for advanced applications. However, the discovery of other carbon-based nanomaterials has put fullerenes in the shade, and the pace of their exploration has been reduced. As fullerenes are highly symmetrical molecules with unique properties, they can act as performance boosters, but more attention is needed from researchers for their practical expansion. 110
Single-walled carbon nanotubes consist of a seamless one-atom-thick graphitic layer, in which carbon atoms are connected through strong covalent bonds. 146 Double-walled carbon nanotubes consist of two single-walled carbon nanotubes. One carbon nanotube is nested in another nanotube to construct a double-walled carbon nanotube. 147 In multi-walled carbon nanotubes, multiple sheets of single-layer carbon atom are rolled up. In other words, many single-walled carbon nanotubes are nested within each other. From different types of nanotubes, it can be concluded that the nanotubes may consist of one, tens, or hundreds of concentric carbon shells, and these shells are separated from each other with a distance of ∼0.34 nm. 148 Carbon nanotubes can be synthesized via chemical vapor deposition, 149 laser ablation, 150 arc-discharge, 143 and gas-phase catalytic growth. 151
Single-walled carbon nanotubes display a diameter of 0.4 to 2 nm. The inner wall distance between double-walled carbon nanotubes was found to be in the range of 0.33 to 0.42 nm. MWCNT diameters are usually in the range of 2–100 nm, and the inner wall distance is about 0.34 nm. 147,152 However, it is essential to note that the diameters and lengths of carbon nanotubes are not well defined, and they depend on the synthesis route and many other factors. The electrical conductivities of SWCNTs and MWCNTs are about 10 2 –10 6 S cm −1 and 10 3 –10 5 S cm −1 , respectively. SWCNTs and MWCNTs also display excellent thermal conductivities of ∼6000 W m −1 K −1 and ∼2000 W m −1 K −1 , respectively. CNTs remain stable in air at temperatures higher than 600 °C. 153 These properties indicate that CNTs have obvious advantages over graphite.
Single-walled carbon nanotubes can display metallic or semiconducting behavior. Whether carbon nanotubes show metallic or semiconducting behavior depends on the diameter and helicity of the graphitic rings. 154 The rolling of graphene sheets leads to three different types of CNTs: chiral, armchair, and zigzag ( Fig. 15 ). 155
| The rolling up of a graphene sheet leading to the three different types of CNT. Reprinted with permission from . Copyright: ©2005, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Carbon nanotubes demonstrate some amazing characteristics that make them valuable nanomaterials for possible practical applications. Theoretical and experimental studies of carbon nanotubes have revealed their extraordinary tensile properties. J. R. Xiao et al. used an analytical molecular structural mechanics model to predict SWCNT tensile strengths of 94.5 (zigzag nanotubes) and 126.2 (armchair nanotubes) GPa. 156 In another study, the Young's modulus and average tensile strength of millimeter-long multi-walled carbon nanotubes were analyzed and found to be 34.65 GPa and 0.85 GPa, respectively. 157 Carbon nanotubes possess a high aspect ratio. Due to their high tensile strength, carbon nanotubes are used to enhance the mechanical properties of composites.
Carbon nanotubes have become an important industrial material and hundreds of tonnes are produced for applications. 158 Their high tensile strength and high aspect ratio have made carbon nanotubes an ideal reinforcing agent. 159 Carbon nanotubes are lightweight in nature and are used to produce lightweight and biodegradable nanocomposite foams. 160 The structural parameters of carbon nanotubes define whether they will be semiconducting or metallic in nature. This property of carbon nanotubes is considered to be effective for their use as a central element in the design of electronic devices such as rectifying diodes, 161 single-electron transistors, 162 and field-effect transistors. 163 The chemical stability, nano-size, high electrical conductivity, and amazing structural perfection of carbon nanotubes make them suitable for electron field emitter applications. 164 The unique set of mechanical and electrochemical properties make CNTs a valuable smart candidate for use in lithium-ion batteries. 165 CNTs have the full potential to be used as a binderless free-standing electrode for active lithium-ion storage. CNT-based anodes can have reversible lithium-ion capacities exceeding 1000 mA h g −1 , and this is a substantial improvement compared with conventional graphite anodes. In short, the following factors play a role in controlling and optimizing the performances of CNT-based composites: 166 (i) the volume fraction of carbon nanotubes; (ii) the CNT orientation; (iii) the CNT matrix adhesion; (iv) the CNT aspect ratio; and (iv) the composite homogeneity.
For some applications, a proper stable aqueous dispersion of CNTs at a high concentration is pivotal to allow the system to perform its function efficiently and effectively. 167 One of the major issues associated with carbon nanotubes is their poor dispersion in aqueous media due to their hydrophobic nature. Clusters of CNTs are formed due to van der Waals attraction, π–π stacking, and hydrophobicity. The CNT clusters, due to their strong interactions, hinder solubility or dispersion in water or even organic-solvent-based systems. 168 This challenging dispersion associated with CNTs has limited their use for promising applications, such as in biomedical devices, drug delivery, cell biology, and drug delivery. 167 Carbon nanotube applications and inherent characteristics can be further tuned via suitable functionalization. The functionalization of carbon nanotubes helps scientists to manipulate the properties of carbon nanotubes and, without functionalization, some properties are not attainable. 169 The functionalization of nanotubes can be divided into two main categories: covalent functionalization and non-covalent functionalization.
The heating of CNTs under strongly acidic and oxidative conditions results in the formation of oxygen-containing functionalities. These functional groups, such as carboxylic acid, react further with other functional groups, such as amines or alcohols, to produce amide or ester linkages on the carbon nanotubes. 172 One of the main issues preventing the utilization of CNTs for biomedical applications is their toxicity. The cytotoxicity of pristine carbon nanotubes can be reduced via introducing carbonyl, –COOH, and –OH functional groups. Apart from functionalization through oxidized CNTs, the direct functionalization of CNTs is also possible. However, direct functionalization requires more reactive species to directly react with the CNTs, such as free radicals. Addition reactions to CNTs can cause a transformation from sp 2 hybridization to sp 3 hybridization at the point of addition. At the point where functionalization has taken place, the local bond geometry is changed from trigonal planar to tetrahedral geometry. Some addition reactions to the sidewalls of CNTs are shown in Fig. 16 . 155
| An overview of possible addition reactions for the functionalization of nanotube sidewalls. Reprinted with permission from . Copyright: ©2005, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
It is important to discuss how the covalent functionalization of carbon nanotubes comes at the price of the degradation of the carbon sp 2 network. This substantially affects the electronic, thermal, and optoelectronic properties of the carbon nanotubes. 169 Efforts are being made to introduce a new method of covalent functionalization that can keep the π network of CNTs intact. Antonio Setaro et al. introduced a new [2+1] cycloaddition reaction for the non-destructive, covalent, gram-scale functionalization of single-walled carbon nanotubes. The reaction rebuilds the extended π-network, and the carbon nanotubes retained their outstanding quantum optoelectronic properties ( Fig. 17 ). 173
| (a) A molecular sketch of AuNPs covalently anchored to SH-SWNTs. (b) A TEM micrograph of the Au@SWNTs hybrid; scale bar: 5 nm; few-SWNT bundles can be observed, and AuNPs are assembled along the tubes. (c) The enhancement of the luminescence emission of SWNTs after the covalent attachment of AuNPs onto their surface: a comparison between the emission of Au@SWNTs hybrid (red curve) and that of SH-SWNTs (black curve). Reprinted with permission from . Copyright: ©2017, Springer Nature. | ||
Polymers are frequently combined with CNTs to enhance their dispersion capabilities. Polymers interact with CNTs through CH–π and π–π interactions. 174 Hexanes and cycloalkanes are poor CNT solvents but the good solubility or dispersion of CNTs in these solvents is required for surface coating applications. Poly(dimethylsiloxane) (PDMS) macromer-grafted polymers have been prepared using PDMS macromers and pyrene-containing monomers that strongly adsorb on CNTs, thus improved the solubility of CNTs in chloroform and hexane. 176 The use of head–tail surfactants is another attractive way to achieve a fine dispersion of CNTs in an aqueous medium. In head–tail surfactants, the tail is hydrophobic and interacts with the CNT sidewalls, and the hydrophilic head groups interact with the aqueous environment to provide a fine dispersion. 177
For electrical applications, non-covalently functionalized CNTs are more preferred because the electrical properties of the CNTs are not compromised. CNTs have been non-covalently functionalized with a variety of biomolecules for the fabrication of electrochemical biosensors. 175 Non-covalently functionalized SWCNTs are used for energy applications. Single-walled carbon nanotubes (SWCNTs) have been non-covalently functionalized with 3d transition metal( II ) phthalocyanines, lowering the potential of the oxygen evolution reaction by approximately 120 mV compared with unmodified SWCNTs. 178 The toxicity of pristine CNTs toward living organisms can be lessened via using surfactant-functionalized CNTs. 170 However, in some cases, during polymer non-covalent functionalization, the polymer may wrap CNT bundles and make it difficult to separate the CNTs from each other. Polymers can develop into insulating wrapping that affects the CNT conductivity.
| Term | Pristine graphene | Graphene oxide | Reduced graphene oxide | Ref. |
|---|---|---|---|---|
| Definition | A single layer of 2D carbon atoms | Heavily oxidized graphene | A reduced form of graphene oxide | |
| Composition | Consists of carbon atoms | Consists of C, O, and H | Consists of C, O, and H | |
| C/O ratio | No oxygen | 2–4 | Depends upon the synthesis process; contains less oxygen (8–246) | |
| Hybridization | sp | sp and sp | Predominantly sp and slightly sp | |
| Defects | Defect-free | Defects present | Defects present | |
| Preparation | Relatively tough | Easy | Easy | |
| Production cost | High | Low | Low | |
| Electrical conductivity | Highest | Poor | The electrical properties are partially restored |
In the literature, several graphene-related materials have been reported, such as graphene oxide and reduced graphene oxide. 187 Among graphenoids, graphene oxide is a more reported and explored graphene-related material as a precursor for chemically modified graphene. The synthetic route to graphene oxide is straightforward, and it is synthesized from inexpensive graphite powder that is readily available. 188 Graphene oxide has many oxygen-containing functional groups, such as epoxy, hydroxyl, carboxyl, and carbonyl groups. The basal plane of graphene oxide is generally decorated with epoxide and hydroxyl groups, whereas the edges presumably contain carboxyl- and carbonyl-based functional groups. 189 The presence of active functional groups in graphene oxide allows its further functionalization with different polymers, small organic compounds, or other nanomaterials to realize several applications. 190
Graphene oxide, due to its oxygen functionality, is insulating in nature and displays poor electrochemical performance. The presence of oxygen functionalities in graphene oxide breaks the conjugated structure and localizes the π-electron network, resulting in poor carrier mobility and carrier concentration. 196 Its electrochemical performance is improved substantially after removing the oxygen-containing functional groups. 197 These functional groups can be removed or reduced via thermal, electrochemical, and chemical means. The product obtained after removing or reducing oxygen moieties is called reduced graphene oxide. The properties of reduced graphene oxide depend upon the effective removal of oxygen moieties from graphene oxide. The process used to remove oxygen-containing functionalities from graphene oxide will determine the extent to which reduced the properties of graphene oxide resemble pristine graphene. 198
Reduced graphene oxide is extensively used to improve the performances of various electrochemical devices. 199 It is essential to mention that even after reducing graphene oxide, some residual sp 3 carbon bonded to oxygen still exists, which somehow disturbs the movement of charge through the delocalized electronic cloud of the sp 2 carbon network. 200 Apart from this, the electrochemical activity of reduced graphene oxide is substantially high enough to manufacture electrochemical devices with improved performances. Recently, the demand for super-performance electrochemical devices has increased to overcome modern challenges relating to electronics and energy-storage devices. 201 Graphene-based materials are considered to be excellent electrode materials, and they can be proved to be revolutionary for use in energy-storage devices such as supercapacitors (SC) and batteries. Graphene-based electrodes improve the performances of existing batteries (lithium-ion batteries) and they are considered useful for developing next-generation batteries such as sodium-ion batteries, lithium–O 2 batteries, and lithium–sulfur batteries ( Fig. 18 ). Being flat in nature, each carbon atom of graphene is available, and ions can easily access the surface due to low diffusion resistance, which provides high electrochemical activity. 202
| The applications of graphene in different electrochemical energy storage devices (EESDs). Reprinted with permission from . Copyright: ©2015, Elsevier B.V. All rights reserved. | ||
Graphene and its derivatives are extensively used for the development of electrochemical sensors. 203 The surfaces of bare electrodes are usually not able to sense analytes at trace levels and they cannot differentiate between analytes that have close electrooxidation properties due to their poor surface kinetics. The addition of graphene layers to the surfaces of electrodes can substantially improve the electrocatalytic activity and surface sensitivity towards analytes. 204 Graphene has definite advantages over other materials that are used as electrode materials for sensor applications. Graphene has a substantially high surface-to-volume ratio and atomic thickness, making it extremely sensitive to any changes in its local environment. This is an essential factor in developing advanced sensing tools, as all the carbon atoms are available to interact with target species.
Consequently, graphene exhibits higher sensitivity than its counterparts such as CNTs and silicon nanowires. 205 Graphene has two main advantages over CNTs for the development of electrochemical sensors. First, graphene is mostly produced from graphite, which is a cost-effective route, and second, graphene does not contain metallic impurities like CNTs can. Graphene offers many other advantages when developing sensors and biosensors, such as biocompatibility and π–π stacking interactions with biomolecules. 206 Graphene-based materials are ideal for the construction of nanostructured sensors and biosensors.
The mechanical properties of graphene are used to fabricate highly desired stretchable and flexible sensors. 207 Graphene can be utilized to develop transparent electrodes with excellent optical transmittance. It displays good piezoresistive sensitivity. Researchers are making efforts to replace conventional brittle indium tin oxide (ITO) electrodes with flexible graphene electrodes in optoelectronic devices such as liquid-crystal displays and organic light-emitting diodes. 208 For human–machine interfaces, transparent and flexible tactile sensors with high sensitivity have become essential. Graphene film (GF) and PET have been applied to develop transparent tactile sensors that exhibit outstanding cycling stability, fast response times, and excellent sensitivity ( Fig. 19 ). 209 Similarly, graphene is applied for the fabrication of pressure sensors. 210 Overall, graphene is an excellent material for developing transparent and flexible devices. 211,212
| (a) A schematic diagram of the fabrication procedure of a tactile sensor based on GF and a PET plate. (b) An optical photograph of a bent assembled sensor; the geometrical dimensions of the sensor are shown in the inset. (c) The Raman spectrum of the GF, with typical D (≈1352 cm ), G (≈1583 cm ), and 2D (≈2686 cm ) peaks. (d) Transmittance spectra of pure PET, the GF–PET composite structure, and a multilayer stacked nanofilm sensor in the visible wavelength range from 350 to 700 nm. Published by The Royal Society of Chemistry. | ||
The use of graphene-based materials is an effective way to deal with a broad spectrum of pollutants. 213 There are many ways to deal with environmental pollution; among these, adsorption is an effective and cost-effective method. 214,215 Graphene-based adsorbents are found to be useful in the removal of organic, 216 inorganic, and gaseous contaminants. Graphene-based materials have some obvious advantages over CNT-based adsorbents. For example, graphene sheets offer two basal planes for contaminant adsorption, enhancing their effectiveness as an adsorbent. 192 GO contains several oxygen functional groups that impart hydrophilic features. Due to appropriate hydrophilicity, GO-based adsorbents can efficiently operate in water to remove contaminants. Moreover, graphene-oxide-based materials can be functionalized further through reactive moieties with various organic molecules to enhance their adsorption capacities. 217
In short, extensive research must continue in order to develop graphene-based materials with high performance and bring them to the market. Massive focus on graphene research is also justified due to the extraordinary features described in extensive theoretical and experimental research works.
Nanodiamonds possess a core–shell-like structure and display rich surface chemistry, and numerous functional groups are present on their surface. Several functional groups, such as amide, aldehyde, ketone, carboxylic acid, alkene, hydroperoxide, nitroso, carbonate ester, and alcohol groups, are present on nanodiamond surfaces, assisting in their further functionalization for desired applications ( Fig. 20 ). 226
| The critical surface chemistry of detonation-based NDs. The ND surface is usually covered with several functional groups. Different surface treatments (such as liquid phase purification and ozone oxidation) are needed to replace these functional groups with oxygen-containing species like carboxylic acids and anhydrides. Surface-treated NDs exhibit several attractive properties, such as colloidal stability, drug adsorption, uniform distribution in a polymer matrix, conjugation with biomolecules, and catalytic properties. Reprinted with permission from . Copyright: ©2018, Elsevier Ltd. All rights reserved. | ||
Furthermore, nanodiamond surfaces can be homogenized with a single type of functional group according to the application requirements. 227 The use of nanodiamond particles as a reinforcing material in polymer composites has attracted great attention for improving the performance of polymer composite materials. The superior mechanical properties and rich surface chemistry of nanodiamonds have made them a superior material for tuning and reinforcing polymer composites. Nanodiamonds might operate via changing the interphase properties and forming a robust covalent interface with the matrix. 228 Nanodiamond (ND)-reinforced polymer composites have shown superior thermal stabilities, mechanical properties, and thermal conductivities. Nanodiamonds have shown great potential for energy storage applications. 229 Nanodiamonds and their composites are also used in sensor fabrication, environmental remediation, and wastewater treatment. 230,231 Their stable fluorescence and long fluorescence lifetimes have made nanodiamonds useful for imaging and cancer treatment. For biomedical applications, the rational engineering of nanodiamond particle surfaces has played a crucial role in the carrying of bioactive substances, target ligands, and nucleic acids, resisting their aggregation. 232,233 Nanodiamonds have a great future in nanotechnology due to their amazing surface chemistry and unique characteristics.
Carbon quantum dots can be synthesized through several chemical routes. 241–245 Some methodologies for synthesizing carbon dots are described in Fig. 21 . 246–248 Carbon itself is a black material and displays low solubility in water. In contrast, carbon quantum dots are attractive due to their excellent solubility in water. They contain a plethora of oxygen-containing functional groups on their surface, such as carboxylic acids. These functional moieties allow for further functionalization with biological, inorganic, polymeric, and organic species.
| Methodologies for synthesizing carbon dots (CDs). Reprinted with permission from . Copyright: ©2011, Elsevier Ltd. All rights reserved. | ||
Carbon quantum dots are also called carbon nano-lights due to their strong luminescence. 248 In particular, carbon quantum dots offer enhanced chemiluminescence, 249,250 fluorescent emission, 251 two-photon luminescence under near-infrared pulsed-laser excitation, 252 and tunable excitation-dependent fluorescence. 253 The luminescence characteristics of carbon quantum dots have been used to develop highly sensitive and selective sensors. In most cases, a simple principle is involved in sensing with luminescent carbon quantum dots: their photoluminescence intensity changes upon the addition of an analyte. 254 Based on this principle, several efficient sensors have been developed using carbon quantum dots. 255–257 They can be used as sensitive and selective tools for sensing explosives such as TNT. Recognition molecules on the surfaces of carbon quantum dots can help to sense targeted analytes. Amino-group-functionalized carbon quantum dot fluorescence is quenched in the presence of TNT through a photo-induced electron-transfer effect between TNT and primary amino groups. This quenching phenomenon can help to sense the target analyte ( Fig. 22 ). 258 Chiral carbon quantum dots (cCQDs) can exhibit an enantioselective response. The PL responses of cCQDs were evaluated toward 17 amino acids and it was found that the PL intensity of the cCQDs was only substantially enhanced in the presence of L -Lys ( Fig. 22 ). 254
| (A) A schematic illustration of the fabrication of a CDs@NH nanosensor and its sensing mechanism toward TNT. (B) A schematic diagram of the synthesis and application procedures of cCQDs. Reprinted with permission from . Copyright: ©2018, Elsevier B.V. All rights reserved. | ||
Carbon quantum dots have received significant interest in the fields of biological imaging and nanomedicine ( Fig. 23 ). 239 Direct images of RNA and DNA are essential for understanding cell anatomy. Due to the limitations of current imaging probes, tracking the dynamics of these biological macromolecules is not an easy job. Recently, membrane-penetrating carbon quantum dots have been developed for the imaging of nucleic acids in live organisms. 259 It is important to note that most of the carbon quantum dots utilized to attain cell imaging under UV excitation emit blue radiation. Some biological tissue also emits blue light, specifically that involving carbohydrates, and this interferes with cell imaging carried out with blue-emitting CQDs. This seriously hinders their potential in the field of biomedical imaging. Due to this reason, researchers are focusing on tuning CQDs in a way that their emission peak is red-shifted to avoid interference. 260 Carbon quantum dots with yellow and green fluorescence have been reported for bioimaging purposes. 261,262 The suitable doping of carbon quantum dots can red-shift the emission to enhance the bioimaging effectiveness. 263 Doped carbon quantum dots are capable of biological imaging and display advanced capabilities for scavenging reactive oxygen species. 264
| The applications of CQDs in nanomedicine. Reprinted with permission from . Copyright: ©2019, Elsevier B.V. All rights reserved. | ||
Carbon quantum dots demonstrate photo-induced electron transfer properties 265 that make them valuable for photocatalytic, light-energy conversion, and other related applications. 266 Carbon quantum dots enhance the activities of other photocatalysts to which they are attached. Carbon quantum dots, along with photocatalysts, provide better charge separation and suppress the regeneration of photogenerated electron–hole pairs. Moreover, the proper implantation of carbon quantum dots into photocatalysts can broaden the photo-absorption region. Implanted carbon quantum dots form micro-regional heterostructures that facilitate photo-electron transport. 267 The implantation of carbon quantum dots into g-C 3 N 4 can substantially enhance charge transfer and separation efficiencies, prevent photoexcited carrier recombination, narrow the bandgap, and red shift the absorption edge. 268 The intrinsic catalytic activity of polymeric carbon nitride is improved as a result of the nano-frame heterojunctions formed with the help of CQDs. 269
Carbon quantum dots offer many advantages over conventional semiconductor-based QDs and, thus, they have attracted considerable researcher attention. 244 Due to their remarkable features, they have shown importance in recent years in the fields of light-emitting diodes, nanomedicine, solar cells, sensors, catalysis, and bioimaging. 236
| (a) A TEM micrograph showing a graphitic carbon product that was generated abundantly upon CO laser ablation at room temperature. The product consisted of near-uniform-sized spherical particles with a diameter of 80 nm. (b) A magnified TEM micrograph of the graphitic carbon particles showing aggregations of tubule-like structures sticking out of the particle surface. (c) A highly magnified TEM micrograph of the edge regions of graphitic particles showing conical horn-like protrusions that are up to 20 nm-long on the particle surface with some modified shapes. Each of these carbon nanohorns was made of a single graphene sheet with closed caps, and the diameters were similar to those of fullerene molecules. Reprinted with permission from . Copyright: ©1999, Elsevier Science B.V. All rights reserved. | ||
The production of carbon nanohorns has some obvious advantages over carbon nanotubes, such as the ability for toxic-metal-catalyst-free synthesis and large-scale production at room temperature. Carbon nanotube synthesis involves metal particles, and harsh conditions, such as the use of strong acids, are required to remove metallic catalysts. This process introduces many defects into CNT structures and may cause a loss of carbon material. 270 Carbon nanohorns possess a wide diameter compared to CNTs. CNHs possess good absorption capabilities and their interiors are also available after partial oxidation, which provides direct access to their internal parts. Heat treatment under acidic or oxidative conditions facilitates the facile introduction of holes into carbon nanohorns. Holes in graphene sheets of single-walled carbon nanohorns can be produced with O 2 gas at high temperatures. A large quantity of material can be stored inside CNH tubes. 274 The surface area of CNHs is substantially enhanced upon opening the horns to make their interiors accessible. 275 Carbon nanohorns have great potential for energy storage, 275 electrochemiluminescence, 276 adsorption, 277 catalyst support, 278 electrochemical sensing, 279 and drug delivery system 273 uses. CNHs as catalyst supports can provide a homogeneous dispersion of Pt nanoparticles ( Fig. 25 ). The current density of Pt supported on single-walled carbon nanohorns is double compared to a fuel cell made from Pt supported on carbon black. 280 Thus, carbon nanohorns provide a better uniform dispersion that facilitates a high surface area and better catalyst performance.
| TEM micrographs of a Pt catalyst supported on SWNHs (a) and on carbon black (b). Reprinted with permission from . Copyright: ©2002, Elsevier Science B.V. All rights reserved. | ||
5.2. Nanoporous materials
In nanoporous materials, the size distributions, volumes, and shapes of the pores directly affect the performances of porous materials for particular applications. It has become a hot area of research to develop materials with precisely controlled pores and arrangements. Recent research has focused more on the precise control of the shapes, sizes, and volumes of pores to produce nanoporous materials with high performance. Several state-of-the-art reviews are present in the literature that focus explicitly on the synthesis, properties, advances, and applications of nanoporous materials. 85,287–289 Based on the materials used, nanoporous materials can be divided into three main groups: inorganic nanoporous materials; carbonaceous nanoporous materials; and organic polymeric nanoporous materials.
Inorganic nanoporous materials include porous silicas, clays, porous metal oxides, and zeolites. The generation of pores in the material can introduce striking features into the material that are absent in non-porous materials. Nanoporous materials offer rich surface compositions with versatile characteristics. Nanoporous materials exhibit high surface-to-volume ratios. Their outstanding features and nanoporous framework structures have made these materials valuable in the fields of environmental remediation, adsorption, catalysis, sensing, energy conversion, purification, and medicine. 284,290
Porous silica is a crucial member of the inorganic nanoporous family. Over the decades, it has generated significant research interest for use in fuel cells, chemical engineering, ceramics, and biomedicine. It is essential to note that specific morphologies and pore size diameters are required for each application, and these can be achieved via tuning during the synthesis process. Nanoporous silica offers two functional surfaces: one is the cylindrical pore surfaces, and the second is the exterior surfaces of the nanoporous silica particles. The surfaces of nanoporous silica can be easily functionalized for the desired applications. The nanoporous silica surface is heavily covered with many silanol groups that act as reactive sites for functionalization ( Fig. 26 ). 291,292 For biomedical applications, mesoporous silica has emerged as a new generation of inorganic platform materials compared to other integrated nanostructured materials. Several factors make it a unique material for biomedical applications: 293,294 (a) its ordered porous structure; (b) its tunable particle size; (c) its large pore volume and surface area; (d) its biocompatibility; (e) its biodegradation, biodistribution, and excretion properties; and (f) its two functional surfaces. For instance, ordered MCM-48 nanoporous silica was used for the delivery of the poorly soluble drug indomethacin. It has been found that surface modification can control drug release. 295 Mesoporous silica-based materials have emerged as excellent materials for use in sustained drug delivery systems (SDDSs), immediate drug delivery systems (IDDSs), targeted drug delivery systems (TDDSs), and stimuli-responsive controlled drug delivery systems (CDDSs). The drug release rate from mesoporous silica can also be controlled via introducing appropriate polymers or functional groups, such as CN, SH, NH 2 , and Cl. Researchers are currently focusing on developing MSN-based (MSN = mesoporous silica nanoparticle) multifunctional drug delivery systems that can release antitumor drugs on demand in a targeted fashion via minimizing the premature release of the drug ( Fig. 27 ). 296
| (A) The functionalization of mesoporous silica and the adsorption process of cadmium ions. Reprinted with permission from . Copyright: ©2018, Elsevier B.V. All rights reserved. (B) A schematic illustration of the biofunctionalization of 3D nanoporous SiO film with streptavidin and antibodies. Reprinted with permission from . Copyright: ©2008, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
| (A) The in vivo process of an MSN-based controlled and targeted drug-delivery system. Two approaches for multifunctional MSN-based drug delivery systems are to design (B) a multifunctional targeting molecule that acts both as a targeting and capping agent or (C) a stimuli-responsive gatekeeper that is further modified with a target moiety to achieve multifunctional drug delivery. Reprinted with permission from . Copyright: ©2015, Elsevier Inc. All rights reserved. | ||
Hierarchically nanoporous zeolites are a vital member of the nanoporous material family. They are crystalline aluminosilicate minerals whose structures comprise uniform, regular arrays of nanopores with molecular dimensions. The microporous structures of zeolites contain pores that are usually below 1 nm in diameter. In zeolites, the micropores are uniform in shape and size, and these pores can effectively discriminate between molecules based on shape and size. 297 Currently, based on crystallography, more than 200 zeolites have been classified. 298 Zeolites have been proved to be useful materials in the field of host–guest chemistry. In solid catalysis, about 40% of the entire solid catalyst field is taken up by zeolites in chemical industry. The excellent catalysis success of zeolites is based on their framework stability, shape-selective porosity, solid acidity, and ion-exchange capacity. Oxygen tetrahedrally coordinates with the Al atoms in most zeolite crystalline silicate frameworks, resulting in charge mismatch between the oxide framework and Al. Extra-framework Na + ions compensate for this charge mismatch. The Na + ions are exchangeable for other cations such as H + and K + . 298 The zeolite crystalline networks are remarkable in that they provide high mechanical and hydrothermal stabilities. The most crucial task facing the zeolite community is to find new structures with desired functions and apply them more effectively for different applications.
Apart from these inorganic porous materials, several other metal- and metal-oxide-based nanoporous materials have been introduced that are more prominent for use in electrode material, catalyst, photodegradation, energy storage, and energy conversion applications. 299–302 Nanoporous metal-based materials are famous due to the nanosized crystalline walls, interconnected porous networks, and numerous surface metal sites that provide them with unique physical/chemical properties compared with their bulk counterparts and other nanostructured materials. 303 For example, nanoporous WO 3 films were developed via tuning the anodization conditions for photoelectrochemical water oxidation. It has been observed that the morphology of the film strongly affected the photoelectrochemical performance. 304 Nanoporous alumina is also a unique material in the inorganic nanoporous family due to several aspects. Nanoporous alumina can be prepared in a controlled fashion with any size and shape in polyprotic aqueous media via the anodic oxidation of the aluminum surface. The parallel arrangement of pores on alumina can easily be controlled from 5 nm to 300 nm, and alumina is stable in the range of 1000 °C. The anodizing time plays a significant role in controlling the pore length. Nanoporous alumina membranes offer various unique properties, such as pores of variable widths/lengths, temperature stability, and optical transparency. Nanoporous alumina pores can be filled with magnetically and optically active elements to produce the desired applications at the nanoscale level. Photoluminescent alumina membranes can be produced via introducing cadmium sulfide, gallium nitride, and siloxenes inside nanoporous alumina using appropriate precursors. 305 Porous alumina also acts as an efficient support and template for the designing of other nanomaterials. Palladium nanowires, 306 high aspect ratio cobalt nanowires, 307 and highly aligned Cu nanowires 308 were developed using porous alumina as a template. Ni–Pd as a catalyst was supported on porous alumina for hydrogenation and oxidation reactions. 309 Nanoporous anodic alumina is also considered to be an efficient material for the development of biosensors due to the ease of fabrication, tunable properties, optical/electrochemical properties, and excellent stability in aqueous environments. 310
Nanoporous carbon-based materials are a hot topic in the field of materials chemistry. Nanoporous carbon materials have become ubiquitous choices in the environmental, energy, catalysis, and sensing fields due to their unique morphologies, large pore volumes, controlled porous structures, mechanical, thermal, and chemical stabilities, and high specific surface areas ( Fig. 28A ). 311 Nanoporous materials are found to be useful in the treatment of water. The separation of spilled oil and organic pollutants from water has emerged as a significant challenge. 312–314 The design of materials that can allow the efficient separation of organic, dye, and metal contaminants from water has become a leading environmental research area. 315,316 Nanoporous carbon can be derived from different natural and synthetic sources. 317–319 Nanoporous carbon foam can be derived from natural sources, such as flour, pectin, and agar, via table-salt-assisted pyrolysis. The agar-derived nanoporous carbon foam showed high absorption capacities, a maximum of 202 times its own weight, for oil and organic solvents. Air filtration paper developed from carbon nanoporous materials and non-woven fabrics has shown a filtration efficiency of greater than 99% ( Fig. 28B ). 320 Nanoporous carbon can also be produced from other porous frameworks, such as metal–organic frameworks. MOF- and COF-based materials are promising precursors for nanoporous carbon-based materials. The direct carbonization of amino-functionalized aluminum terephthalate metal–organic frameworks has produced nitrogen-doped nanoporous carbon that shows an adequate removal capacity of 98.5% for methyl orange under the optimum conditions. 321 Fe 3 O 4 /nanoporous carbon was also produced with Fe salts as a magnetic precursor and MOF-5 as a carbon precursor for removing the organic dye methylene blue (MB) from aqueous solutions. 322 The mesoporous carbon removal efficiency could be further enhanced via modifying or functionalizing the surface with various materials. Unmodified mesoporous carbon has shown a mercury removal efficiency of 54.5%. This efficiency can be substantially improved to 81.6% and 94% upon modification with the anionic surfactant sodium dodecyl sulfate and cationic surfactant cetyltrimethyl ammonium bromide (CTAB), respectively. 323
| (A) A schematic illustration of the synthesis, functionalization, and applications of micro- and mesoporous carbon. Reproduced from with permission from The Royal Society of Chemistry. (B) The development of nanoporous carbon foam with high efficiency for oil/organic solvent adsorption. Reprinted with permission from . Copyright: ©2020, American Chemical Society. | ||
Ordered nanoporous carbon, CNTs, and fullerenes are extensively applied for energy and environmental applications. The complicated synthesis routes required for fullerenes and CNTs have slowed down the full exploitation of their potential for highly demanding applications. In comparison, the synthesis of highly ordered nanoporous carbon is facile, and the properties of ordered nanoporous carbon are also appealing for energy and environmental applications. 311 CO 2 is a greenhouse gas, and its sustainable conversion into value-added products has become the subject of extensive research. A nitrogen-doped nanoporous-carbon/carbon-nanotube composite membrane is a high-performance gas-diffusion electrode applied for the electrocatalytic conversion of CO 2 into formate. A faradaic efficiency of 81% was found for the production of formate. 324 Nanoporous carbon materials modified with the non-precious elements P, S, N, and B have emerged as efficient electrode materials for use in the oxygen evolution reaction (OER), hydrogen evolution reaction (HER), oxygen reduction reaction (ORR), batteries, and fuel cells. 311,325–327
Nanoporous polymers, including nanoporous coordination polymers and crystalline nanoporous polymers, have emerged as impressive nanoporous materials. 328 Nanoporous polymers have many applications, and these materials are extensively being evaluated for gas separation and gas storage. The great interest in these applications arises from the presence of pores providing an exceptionally high Brunauer–Emmett–Teller (BET) surface area. Recently, new classes of metal organic framework and covalent organic framework porous materials have been reported that have shown exceptionally high and unprecedented surface areas. For instance, in 2010, a MOF was reported with a surface area of 6143 m 2 g −1 ; 329 in 2012, a MOF was reported with a surface area greater than 7000 m 2 g −1 ; 330 and in 2018, a MOF (DUT-60) was reported with a record surface area of 7836 m 2 g −1 . 331 Mesoporous DUT-60 has also shown a high free volume of 90.3% with a density of 0.187 g cm −3 . 331
Due to their exceptionally high surface areas and porous networks, these MOFs and COFs are ideal for gas storage. Air separation and post-combustion CO 2 capture have become integral parts of mainstream industries related to the energy sector in order to avoid substantial economic penalties. Due to the inefficiencies of available technology and the critical importance of this area, earnest efforts are being made to design gas-selective porous materials for the selective adsorption of desired gases. Nanoporous MOF- and COF-based materials can significantly capture CO 2 and help reach zero or minimum CO 2 emission levels. For instance, nanoporous fluorinated metal–organic frameworks have shown the selective adsorption of CO 2 over H 2 and CH 4 . 332 Hasmukh A. Patel et al. developed N 2 -phobic nanoporous covalent organic polymers for the selective adsorption of CO 2 over N 2 . The azo groups in the framework rejected N 2 , leading to CO 2 selectivity. 333 Nanoporous polymers that are superhydrophobic in nature can also be used for volatile organic compounds and organic contaminants. 334 Nanoporous polymers, due to the presence of a porous network, have been considered as highly suitable materials for catalyst supports. Furthermore, organocatalytic functional groups can be introduced pre-synthetically and post-synthetically into solid catalysts. 335
Nanoporous polymeric materials are amazingly heading towards being extremely lightweight with exceptionally high surface areas. These high surface areas and the fine-tuning of the nanopores has made these nanoporous materials, specifically MOFs and zeolites, ideal support materials for encapsulating ultrasmall metal nanoparticles inside void spaces to produce nanocatalysts with exceptionally high efficiencies. 336 In the coming years, more exponential growth of nanoporous materials is expected in the energy, targeted drug delivery, catalysis, and water treatment fields.
5.3. Ultrathin two-dimensional nanomaterials beyond graphene
However, from a material synthesis standpoint, a graphite-like layered form of Si does not exist in nature and there is no conventional exfoliation process that can generate 2D silicene, although single-walled 351 and multi-walled 352 silicon nanotubes and even monolayers of silicon have been synthesized via exfoliation methods. 353 Forming honeycomb Si nanostructures on substrates like Ag(001) and Ag(110) via molecular beam deposition, so-called “epitaxial growth”, was then proposed as a method for the architectural design of silicene sheets. 354–356 The successful synthesis of a silicene monolayer was first achieved on Ag(111) and ZrB 2 (0001) substrates in 2012; 357,358 later, various demonstrations were made using Ir(111), ZrB 2 (001), ZrC(111), and MoS 2 surfaces as the silicene growth substrates. 359–361 Despite various extensive studies to date involving the “epitaxial growth” of silicene on different substrates and investigations of the electronic properties, 357,362–364 the limited nanometer size, difficulties relating to substrate removal, and air stability issues have substantially impeded the practical applications of silicene. Bearing in mind all these known difficulties, Akinwande and co-workers recently reported a growth–transfer–fabrication process for novel silicene-based field-effect transistor development that involved silicene-encapsulated delamination with native electrodes. 365 An etch-back approach was used to define source/drain contacts in Ag film. Without causing any damage to the silicene, a novel potassium-iodide-based iodine-containing solution was used to etch Ag, avoiding rapid oxidation, unlike other commonly used Ag etchants. The results demonstrated that this was the first proof-of-concept study confirming the Dirac-like ambipolar charge transport predictions made about silicene devices. Comparative studies with a graphene system, the low residual carrier density, and the high gate modulation suggested the opening of a small bandgap in the experimental devices, proving that silicene can be considered a viable 2D nanomaterial beyond graphene.
Nonetheless, the synthesis of silicene on a large-scale is greatly limited, as “epitaxial growth” is the only promising method for obtaining high-quality silicene, and this presents an enduring challenge in relation to silicene research and development. Xu and co-workers recently introduced liquid oxidation and the exfoliation of CaSi 2 as a means for the first scalable preparation of high-quality silicene nanosheets. 366 This new synthetic strategy successfully induced the exfoliation of stacked silicene layers via the mild oxidation of the (Si 2 n ) 2 n layers in CaSi 2 into neutral Si 2 n layers without damage to the pristine silicene structure ( Fig. 29 ). The selective oxidation of pristine CaSi 2 into free-standing silicene sheets without any damage to the original Si framework was carried out via exfoliation in the presence of I 2 in acetonitrile solvent. Furthermore, the obtained silicene sheets yielded ultrathin monolayers or layers with few-layer thickness and exhibited excellent crystallinity. This 2D silicene nanosheet material was extensively explored as a novel anode, which was unlike previously developed silicon-based anodes for lithium-ion batteries. It displayed a theoretical capacity of 721 mA h g −1 at 0.1 A g −1 and superior cycling stability of 1800 cycles. Overall, during the last decade, silicene has been widely accepted as an ideal 2D material with many fascinating properties, suggesting great promise for a future beyond graphene.
| (a) A schematic illustration of the synthesis of silicene from CaSi via liquid oxidation and exfoliation. SEM images of pristine bulk CaSi (b) and the as-reacted product (c), with insets showing photographs. Reprinted with permission from . Copyright: ©2018, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Like other 2D materials, MXenes exhibit crystal geometry with a hexagonal close-packed structure based on the equivalent MAX-phase precursor, and the close-packed structure is formed from M atoms with X atoms occupying octahedral sites. 371 According to the formula, there are three representative structures of MXenes: M 2 XT x , M 3 X2T x , and M 4 X3T x . In these combinations, X atoms are formed with n layers, whereas M atoms have n + 1 layers ( Fig. 30 ). 372 Apart from graphene, MXenes are considered the most dynamic developing material, and they have incredible innovation potential amongst typical 2D nanomaterials because of their remarkable properties, such as hydrophilicity, conductivity, considerable adsorption abilities, and catalytic activity. These vital properties of MXenes suggest their use for various potential applications, including in the photocatalysis, electrocatalysis, 373,374 energy, 375 membrane-based separation, 376,377 and biological therapy 378 fields. In this section, we focus on describing new developments relating to MXenes that are utilized for electrocatalytic and energy storage applications, competing as alternatives to graphene materials.
| The structures of MXenes with different formulas (M XT , M X T , and M X T ) and their compositions (mono-M MXenes and double-M MXenes). Reprinted with permission from . Copyright: ©2019, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Interestingly, due to the presence of abundant terminal groups, mainly –O, –OH, and –F, and their modifying nature, MXenes can exhibit outstanding hydrophilic properties and high conductivity and charge carrier mobility, making them a very attractive material for various electrocatalytic applications, such as the hydrogen evolution reaction, oxygen evolution reaction, oxygen reduction reaction, nitrogen reduction reaction, and CO 2 reduction reaction. To further increase their electrocatalytic activities, recent works involving MXenes have included incorporation with CNTs, 379 g-C 3 N 4 , 380 FeNi-LDH, 381 NiFeCo-LDH, 382 and metal–organic frameworks. 383
Cho and co-workers designed and developed MXene–TiO 2 2D nanosheets via the surface oxidation of MXene with defect-free control. These MXene–TiO 2 2D nanosheets were successfully implemented in nano-floating-gate transistor memory (NFGTM) providing a floating gate ( i.e. , multilayer MXene) and tunneling dielectric ( i.e. , the TiO 2 layer). A process of oxidation in water further represented a cost-effective and environmentally benign method, as depicted in Fig. 31 . The MXene NFGTM with an optimal oxidation process displayed exceptional nonvolatile memory features, having a great memory window, high programming/erasing current ratio, long term retention, and high durability. 384
| (a) A schematic diagram of the synthesis of 2D Ti C T MXene and its controlled oxidation. (b) Cross-sectional STEM images of pristine MXene and oxidized MXene. (c) Raman spectra of pristine MXene and oxidized MXene. (d) Photographic images of MXene solution at different stages of oxidation. (e) A schematic illustration of MXene NFGTM. The right panel shows an optical microscopy (OM) image of the oxidized MXene on the SiO blocking dielectric layer. (f) The transfer characteristics of transistors with different hysteresis behavior. (g) A schematic diagram of the energy bands of MXene NFGTM corresponding to hole trapping and detrapping in the MXene floating gate. Reprinted with permission from . Copyright: ©2020, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
There have been some exciting reports on 2D materials from the pnictogen family, particularly phosphorene. Recently, more attention has also been given to the remaining group 15 elements, 390 with the novel 2D materials arsenene, antimonene, and bismuthene being obtained from the key elements arsenic, antimony, and bismuth, respectively. It is reported that 2D monolayers of group 15 elements, including phosphorene allotropes, have five distinct honeycomb (α, β, γ, δ, and ε) and four distinct non-honeycomb (ζ, η, θ, and ι) structures, as depicted in Fig. 32 . Dissimilar crystal orientations were found for single-layered As, Sb, and Bi. Zeng and co-workers also reported comprehensive density functional theory (DFT) computations that proved the energetic stability and broad-range application of these materials in 2D semiconductors. 391 Previously, following theoretical predictions, Wu and co-workers successfully demonstrated that α-phosphorene showed lowest energy configurations in both honeycomb and non-honeycomb nanosheets. 392 In contrast, Zeng and co-workers proved that the buckled forms of 2D sheets of As, Sb, and Bi allotropes are the most stable structures, particularly their β phases. 391
| Top views of relaxed group 15 monolayer allotropes with five typical honeycomb structures (α, β, γ, δ, and ε) and four typical non-honeycomb structures (ζ, η, θ, and ι). Reprinted with permission from . Copyright ©2016, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Among monolayer group 15 family materials, 2D sheets of arsenic (As) and antimony (Sb) have gained considerable attention from researchers. 393,394 Studies have shown that As and Sb exhibit better stability than black phosphorus; they are highly stable at room temperature and less reactive to air, likely inhibiting the oxidization process. 395–398 Nevertheless, it has been demonstrated that the oxidation process is perhaps favorable for fine-tuning the electronic properties; increases in the indirect band gaps ranging from 0 to a maximum of 2.49 eV are found in free-standing arsenene and antimonene semiconductors. 399–403 Simultaneously, arsenene and antimonene can also be transformed into semiconductors with direct band gaps. These two 2D nanosheets can be used to design mechanical sensors, moving beyond common electronic and optoelectronic applications. These two extraordinary 2D nanosheets have been studied for their structural–property relationships via first-principles methods. 403–405
Continuing the characterization and structural property studies of arsenene carried out by Kamal 404 et al. and Zhang 403 et al. , Anurag Srivastava and co-workers analyzed applications of arsenene to explore the possibility of improving sensor devices that can be utilized to detect ammonia (NH 3 ) and nitrogen dioxide (NO 2 ) molecules. 406,407 They investigated the affinities of NH 3 and NO 2 molecules for pristine arsenene sheets, examining the binding energies, bonding distances, density distributions, and current–voltage features. The results showed that arsenene 2D sheets are highly durable, with significant electronic charge transfer. They also considered germanium-doped arsenene and characterized the 2D lattice based on molecular affinity relationships with respect to the dopant.
However, the incorporation of any dopants into 2D nanomaterials not only results in experimental difficulty but it also lowers the stability of 2D materials. 408 Recently, Dameng Liu and co-workers reported the electronic structures, focusing on band structures, band offsets, and intrinsic defect properties, of few-layer arsenic and antimony. 409 The spontaneous oxide passivation layer that is formed naturally on pristine antimonene provides excellent stability. 410 Very recently, Stefan Wolff and co-workers conducted DFT calculations on various single or few-layer antimony oxide structures to describe the stoichiometry and bonding type. Interestingly, the samples exhibited various structural stabilities and electronic properties with a wide range of direct and indirect band gaps. Showing band gaps between 2.0 and 4.9 eV, these 2D layers of antimonene exhibited the potential to be used as insulators or semiconductors. 411 The same group also analyzed Raman spectra and discussed identifying the predicted antimonene oxide structures experimentally. The enduring task of exploring the utility of antimonene has boosted recent research interest in 2D nanomaterials due to the broad range of potential applications, such as their use in electrochemical sensors, 412,413 stable organic solar cells, 414 and supercapacitors 415 to name a few.
| Electrochemical studies of an as-prepared ultrathin 2D Co-MOF NS//activated carbon aqueous device. (a) The galvanostatic charge–discharge curves at different current densities. (b) The cyclic voltammetry curves at different scan rates. (c) Specific capacitances at different current densities. (d) Charge–discharge cycle testing at a current density of 0.5 A cm in 3.0 M KOH electrolyte (inset: a red LED powered by the aqueous device). Reprinted with permission from . Copyright: ©2019, Elsevier B.V. All rights reserved. | ||
The 2D MOF nanosheets are also evaluated for the development of high-performance power-storage devices. For example, Li et al. 427 recently reported two novel Mn-2D MOFs and Ni-2D MOFs as anode materials for rechargeable lithium batteries. The Mn-based ultrathin metal–organic-framework nanosheets, due to thinner nanosheets, a higher specific surface area, and smaller metal ion radius, had structural advantages over Ni-based ultrathin metal–organic-framework nanosheets. Due to these features, the Mn-based ultrathin metal–organic-framework nanosheets displayed a high reversible capacity of 1187 mA h g −1 at 100 mA g −1 for 100 cycles and a rate capability of 701 mA h g −1 even at 2 A g −1 .
The expensive metal oxides utilized in the catalytic process can be replaced in due course by 2D-MOF-based nanosheets with exposed metal sites that impart an adjustable pore structure, ultrathin thickness, a high surface-to-volume atom ratio, and high design flexibility. As a result, 2D-MOFs have extensively been explored for various electrocatalytic applications, including the hydrogen evolution reaction (HER), oxygen evolution reaction (OER), oxygen reduction reaction (ORR), and carbon dioxide reduction reaction (CO 2 RR). For example, Marinescu et al. 428 combined cobalt dithiolene species with benzenehexathiol (BHT) and yielded 2D-MOFs capable of acting as electrocatalysts for the HER in water ( Fig. 34 ). In the presence of 2D-MOF sheets, a high current density of 41 mA cm −2 , at −0.8 V vs. SHE and a pH value of 1.3, is observed. Similarly, Feng et al. 429 also developed single-layer Ni-based 2D-MOF sheets that are highly effective for electrocatalytic hydrogen evolution. Later, Patra et al. 430 reported similar 2D sheets from covalent organic frameworks (2D-COFs) as metal-free catalysts for HER applications. 2D-MOFs are also being explored as active catalysts for the OER process. For example, Xu et al. 431 reported the preparation of 2D Co-MOF sheets using polyvinylpyrrolidone as a surfactant under mild solvothermal conditions. These novel 2D Co-MOFs displayed ultrathin nanosheets with many surface-based metal active sites, improving the overall OER performance.
| The synthesis of the cobalt dithiolene films 1 and 2 through a liquid–liquid interfacial reaction. The synthesized films are deposited onto desired supports, generating MOS 1 and 2. Reprinted with permission from . Copyright: ©2015, American Chemical Society. | ||
Interestingly, experimental electrochemical measurement data showed that Co-MOF sheets offer a low overpotential ( i.e. , 263 mV at 10 mA cm −2 ). Similarly, Wang et al. 432 also reported that double-metal 2D-sheets (2D NiFe MOFs) consisting of a very ultrathin structure with a thickness of ∼10 nm further offer a low overpotential of 260 mV at 10 mA cm −2 . In other reports, Zhang et al. 433 successfully performed the OER process with ultrathin 2D-MOF sheets prepared via electrochemical and chemical exfoliation strategies.
Recent work on the catalytic activity of 2D-MOFs has also been reported in relation to the ORR and CO 2 RR because of their layered crystal structures and high-volume modifiable porous structures. For example, Dincă et al. 434 demonstrated that ultrathin layered conductive sheets of the 2D-MOF Ni 3 (HITP) 2 (HITP = 2,3,6,7,10,11-hexaiminotriphenylene) could actively be utilized as a catalyst in an alkaline medium for the ORR process. These 2D-MOF sheets show high stability while retaining 88% of the initial current density over 8 h at 0.77 V vs. RHE. In another report, through fabricating Co x Zn 2− x (bim) 4 2D-sheets as precursors, Zhao et al. 435 successfully synthesized cobalt nanodots (Co-NDs) with bimetallic Co x Zn 2− x (bim) 4 nanosheets encapsulating few-layer graphene (Co@FLG). For the CO 2 RR, a cobalt–porphyrin-containing 2D-MOF was achieved for the selective electrochemical reduction of CO 2 to CO with enhanced stability by Peidong Yang and co-workers. 436 The results further proved that these thin-film catalysts have the highest selectivity for CO ( i.e. , 76%) at −0.7 V vs. RHE with the little-to-no substantial decrease in activity over 7 h at −0.7 V vs. RHE, and 16 mL of CO was produced. Besides, like many other porous materials, 2D-MOFs were also shown to be a supporting platform for catalytic nanoparticles because of their high specific surface areas and favorable porosity distributions. To this end, an example can be noted from Wang et al. 437 reporting that fine porous MOF-5 nanosheets can be utilized to immobilize Pd nanoparticles.
| DNA detection with 2D MOFs (Cu–TCPP, Zn-TCPP(Fe), and Co-TCPP). (a) A schematic illustration of the 2D-MOF-nanosheet-based fluorescence assay for DNA. (b) Fluorescence spectra under different experimental conditions: (I) P1; (II) P1 + T1 + Cu–TCPP nanosheets; (III) P1 + Cu–TCPP nanosheets; and (IV) Cu–TCPP nanosheets. The concentrations of P1, T1, and Cu–TCPP nanosheets in the final solutions are 2.5 × 10 M, 20 × 10 M, and 35 μg mL , respectively. Inset: A kinetics study of the fluorescence changes of the P1 and P1/T1 duplexes in the presence of Cu–TCPP nanosheets; the excitation and emission wavelengths are 588 and 609 nm, respectively. (c) Left: The quenching efficiency (η) of Cu–TCPP nanosheets and bulk Cu–TCPP MOFs for P1 and P1/T1; right: the fluorescence intensity ratio (F /F ) values at 609 nm in the presence of Cu–TCPP nanosheets (35 μg mL ) or bulk Cu–TCPP MOFs (35 μg mL ). F is the fluorescence intensity of dsDNA (P1/T1) at 609 nm in the presence of Cu–TCPP nanosheets or bulk Cu–TCPP MOFs. F is the fluorescence intensity of ssDNA (P1) at 609 nm in the presence of Cu–TCPP nanosheets or bulk Cu–TCPP MOFs. The concentrations of P1 and T1 in the final solution are 2.5 × 10 and 20 × 10 M. (d) Fluorescence spectra of P1 (2.5 × 10 M) in the presence of T1 at different concentrations in 2D Cu–TCPP nanosheet solution (35 μg mL ). Reprinted with permission from . Copyright: ©2015, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
| (a) An SEM image of a bare porous AAO support; (b) an image of MOF membranes obtained via filtration; and (c) an SEM top view and (d) cross-sectional view of the Ni (HITP) membrane on an AAO support. (e) Single and binary gas permeance levels through a Ni (HITP) membrane measured at 298 K and 1.2 bar (inset: the separation factors from equimolar mixed gas permeation tests). Reprinted with permission from . Copyright: ©2020, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
5.4. Metal-based nanostructured materials
As discussed, catalysis is one of the main uses of metal-based nanostructured materials. A continuous increase in the demand for energy, the rapid depletion of conventional energy reservoirs, and rising concerns over the emission of CO 2 have increased the challenges and urgency in the energy field. 460 Metal-based nanostructured materials are extensively being explored to produce alternative clean and renewable energy sources. A range of metal-based nanomaterials has been evaluated and is under consideration for developing robust electrodes that can be effectively applied to water splitting, batteries, and solar cells.
High energy demands have led to more pressure to improve the performances of existing highly demanded lithium-ion batteries. Researchers have focused on improving their lifetimes, sizes, and safety. 462 Nanostructured metal-oxide-based materials are promising electrode materials for use in high-performance charge-storage devices. A metal-based nanostructured electrode is evaluated as both the anode and cathode to overcome the challenges of conventional electrodes. 463 In a conventional LIB, LiCoO 2 was used as the cathode material. Controlled morphology plays a crucial role in determining the performance of a material. Powder composed of spherical particles of LiNi 0.8 Co 0.2 O 2 showed a higher tap density compared to irregular particles and the material substantially improved the power density of secondary lithium batteries. 464 Hierarchical nanostructures of metal-based oxides (such as 3D hierarchical ZnCo 2 O 4 nanostructures) have emerged as a new trend for the development of high-capacity electrodes for lithium-ion batteries. 465 Since their commercialization by Sony in the early 1990s, LIBs have achieved tremendous success in bringing portable electronic devices to the market. However, their sustainable development on the grid-scale is hampered due to limited Li resources in nature, and this is causing a continuous increase in cost. 466 Sodium-ion batteries are in the spotlight to replace powerful lithium-ion batteries due to the widespread availability of sodium and its lower cost compared with lithium. 467 It is essential to note that, in terms of energy densities for SIBs, it is difficult to bypass LIBs because of the low standard electrochemical potential and higher weight of Na. SIBs could be proved to be ideal for those applications where cost is a critical factor compared to energy density. 466
SIBs also operate similarly to LIBs, based on an intercalation mechanism. SIBs also consist of cathode and anode electrodes separated through an electrolyte. During the charging process, sodium ions are extracted from the cathode and inserted into the anode via the electrolyte. In the discharging process, the electrons leave the anode through an external circuit to reach the cathode, providing electricity to the load, whereas Na + moves to the cathode during this process. The radius of Na + (1.02 Å) is greater than that of Li + (0.76 Å), making it challenging to intercalate into electrode materials. 468 Thus, appropriate electrode materials are required in which fast Na-ion insertion and extraction is possible. However, SIBs are suffering from a lack of appropriate electrode materials. It is important to develop electrode materials that have enough interstitial space within their crystallographic structures and better electrochemical performance. Among the various proposed electrode materials, Na x MO 2 layered transition-metal oxides (M = V, Fe, Cu, Co, Ni, Cr, Mn, and their combinations) are considered to be promising electrode materials for SIBs. Layered metal oxides are considered to be promising electrode materials due to their facile scalable synthesis, simple structures, appropriate operating potentials, and high capacities. 469,470 Large volume expansion and poor kinetics during the charge–discharge process can severely affect the cyclability and performance of SIBs. One of the effective strategies to deal with the mechanical stress triggered by large volume changes is the design of hollow or porous structures. In response, three-dimensional network-based Sb 2 O 3 @Sb composite anode materials can help to relieve the volume-change-related stress through their uniform porous networks and provide better transportation channels for Na + . 471
The large volume expansion of electrodes can also be buffered via designing 2D metal-oxide materials with large interlayer spacing. The ultrathin nanosheets provide high reversible capacity with enhanced cycling stability and contribute to providing reaction sites for electrons/ions, decreasing the diffusion distance, providing effective diffusion channels, and facilitating fast charge/discharge for sodium and lithium. 2D SnO nanosheet anodes were evaluated for SIBs. The capacity and cyclic stability improved, as the number of atomic SnO layers is decreased in the sheets. 472 Sb is a promising anode material, but during the sodiation/desodiation processes, huge volume expansion of 390% is observed, which hinders its practical use. Nanostructured Sb in the form of nanorod arrays with large interval spacing displays the great capacity to accommodate volume changes during cycling. 473 A comparison of various nanostructured metal-based electrodes for various charge storage purposes is shown in Table 3 . Overall, well-structured metal or metal-based oxide nanomaterials have the capacity to resolve current issues relating to charge storage devices.
| Nanostructured metal oxide | Synthesis route | Charge storage device | Rate capabilities | Retention capacity and cyclability | Ref. |
|---|---|---|---|---|---|
| Mesoporous NiCo O nanowire arrays | A surfactant-assisted hydrothermal method and short post-annealing treatment | LIB, SC | ≈1012 mA h g at 0.5 A g , 1010 F g at 20 A g | Retained 854 mA h g after 100 cycles, negligible specific capacitance decay after 5000 cycles at 8 A g | |
| Zn V O ·nH O nanobelts | A microwave hydrothermal method | ZIB | ∼300 mA h g at 50 mA g | Capacity retention of 80% at 10C after 1000 cycles | |
| Leaf-like CuO nanostructures | In situ precipitation-induced growth and thermal annealing | LIB | 549 mA h g at 0.1 A g | 95.5% after 200 cycles | |
| 3D porous copper skeleton supported zinc anode | Electrodeposition | ZIB | 364 mA h g at 0.1 A g | Retained a capacity of 173 mA h g at 0.4 A g after 300 cycles | |
| ZnO–carbon black nanostructured anode materials | An atomic layer deposition method | LIB | 2096 mA h g at 100 mA g | A specific capacity of 1026 mA h g was maintained after 500 cycles | |
| NiCo O nanoneedle array | A hydrothermal method combined with post-heat treatment | SIB | 400 mA h g at 50 mA g | ∼215 mA h g after 50 cycles | |
| Coral-like nanostructured Sb O @Sb anode | Heating in a furnace | SIB | 497.3 mA h g at 3000 mA g , 724.3 mA h g at 1000 mA g | 574.8 mA h g at 100 mA g after 150 cycles | |
| 3D Fe GeO /N-CNSs | A high-temperature calcination process | SIB | 350 mA h g at 0.1 A g , 180 mA h g at 22.8 A g | ∼86% reversible capacity retention after 6000 cycles | |
| MnO nanoflowers | A hydrothermal method and thermal treatment | SIB | 487.8 mA h g at 50 mA g | 103.3 mA h g at 800 mA g after 100 cycles, 133.6 mA h g at 400 mA g after 1000 cycles | |
| 2D SnO nanosheet anodes | A hydrothermal method | SIB | 1072 mA h g at 0.1 A g (discharge), 848 mA h g at 0.1 A g (charge) | 665 mA h g at 0.1 A g after 100 cycles, 452 mA h g at 1.0 A g after 1000 cycles | |
| TiO /C nanofibers | Electrospinning | SIB | 164.9 mA h g at 2000 mA g | Nearly 100% capacity retention over 1000 cycles | |
| Sb nanorod arrays | A template method | SIB | 557.7 mA h g at 20 A g | 84% at 0.2 A g over 250 cycles | |
| CuCo O -nanodot-inserted N-doped carbon nanofibers | Electrospinning | SIB | 296 mA h g at 5000 mA g | 314 mA h g at 1000 mA g after 1000 cycles |
Recently, an immense focus of research has been to produce H 2 fuel via water-splitting to replace conventional fossil fuels. This will help to eliminate emissions from the use of carbonaceous species. 484 Electrochemical method are considered simple water splitting approaches, as these methods only require an applied voltage and water as inputs to produce hydrogen fuel. 485 The coupling of solar irradiation to electrochemical water splitting has enhanced the performance and reduced the process cost. Due to these reasons, this has become a hot area of research. 486 During water electrolysis, H 2 is produced through the hydrogen evolution reaction at the cathode and O 2 is produced through the oxygen evolution reaction at the anode. However, water splitting is not so straightforward, and it requires an efficient catalyst that can facilitate the splitting of water. Metal- and metal-oxide-based catalysts are extensively being explored for water splitting. For the HER reaction, Pt-based catalysts are found to be suitable, whereas for OER reactions, Ir-/Ru-based compounds are found to be benchmark catalysts. Scarcity and high cost have limited the widespread use of these metals. The barrier of noble-metal cost can be mitigated through developing noble-metal nanostructured surfaces that produce more active sites or via depositing monolayers of noble metals on low-cost materials. The alloying of noble metals with other metals has enhanced site-specific activity. 484 At present, more focus is being placed on developing noble-metal-free catalysts for water splitting. 485 Usually, an efficient electrocatalyst is characterized by: 487 a low overpotential; high stability; low production costs; and high electrocatalytic activity.
The nano-structuring of catalysts is an effective tool to boost their surface areas. The electrolysis of water occurs at the surface of a catalyst, and nanostructured catalysts provide more active sites and the better diffusion of ions and electrolytes. 484 Non-noble metals that are under observation for the development of HER electrocatalysts include nickel (Ni), tungsten (W), iron (Fe), molybdenum (Mo), cobalt (Co), and copper (Cu). 487 For instance, a noble metal-free catalyst, carbon-decorated Co 3 O 4 nanoarrays on carbon paper, required a small overpotential of 370 mV to reach a current density of 10 mA cm −2 . It can maintain a current density of 100 mA cm −2 for 413.8 h and 86.8 h under alkaline and acidic conditions, respectively. 488
Metal-based semiconductor materials play a crucial role in a range of applications. For photoelectrochemical water splitting, the semiconductor material plays a central role in the solar-to-hydrogen conversion efficiency. Some critical features are prerequisites when it comes to selecting the right semiconductor material for the photoelectrochemical splitting of water: 489 an extraordinary capacity to absorb visible light; an appropriate bandgap; suitable valence and conduction band positions; commercial feasibility; and chemical stability.
For an ideal semiconductor for water splitting, the valence band and conduction band edge positions must straddle the oxidation and the reduction potentials of water. Metal oxides have received significant attention among semiconductors due to their wide band gap distributions, remarkable photo-electrochemical stabilities, and favorable band edge positions. 490 Semiconductor-based photoelectrodes become excited upon light irradiation, and electrons from the valence band move to the unoccupied conduction band. Some of the generated electrons at the cathode surface reduce protons to hydrogen gas, whereas holes at the photoanode produce oxygen gas via water splitting. 490 As a result, various nanostructured metal oxides can be used as photoelectrode materials, such as WO 3 , 491 Cu 2 O, 492 TiO 2 , 493 ZnO, 494 SnO 2 , 495 BiVO 4 , 496 and α-Fe 2 O 3 , 490 for the efficient splitting of water. As discussed, the nano-structuring of semiconductors can significantly impact the electrode photoelectrochemical performance during water splitting.
Metal-based nanomaterials have been used for the development of sensitive sensors. These metal-based sensors can replace the complex and expensive instruments that are conventionally used for the sensing of analytes. Metal-oxide-based sensors have the interesting characteristics of low detection limits, low cost, high sensitivity, and facile operation. 497 Mostly, semiconducting metal-oxide-based sensors are used for the sensing of toxic, flammable, and exhaust gases. Semiconductor metal oxides with a size in the range of 1–100 nm have been significantly investigated as gas sensors due to their size-dependent properties. The geometry and size of a nanomaterial can considerably affect the hole and electron movement in semiconductors. 498 The surface-to-volume ratio and surface area are substantially enhanced at the nanoscale level, and this is amazingly beneficial for sensing. Chemiresistive semiconducting metal oxides are potential candidates for gas sensing due to the following features: 499 rapid response times; fast recovery times; low cost; simple electronic interfaces; user-friendliness and low maintenance; and abilities to sense a wide range of gases.
Electrode materials decorated with metal- or metal-oxide-based nanostructured materials have shown better responses and selectivity for determining various analytes over conventional electrode materials. The nano-sized metal structures act as an electrocatalyst and electronic wires to provide rapid electron transfer between the transducers and analyte molecules. 500 The electrochemical redox reaction of H 2 O 2 can be improved via the thermally controlled anchoring of Pt NPs on the electrode surface. 501
Currently, researchers are not just concentrating on the development of randomly shaped nanomaterials; instead, they are very focused on and interested in the rational design of materials with controlled nano-architectures for boosting their performances for specific applications. As a result, extensive research has been carried out to develop metal-based materials with controlled dimensions to achieve better catalytic responses. Particle morphology is a crucial factor in the performance of nanomaterials for specific applications. Laifa Shen et al. rationally designed an electrode architecture via growing mesoporous NiCo 2 O 4 nanowire arrays on carbon textiles, which boosted the electrode performance ( Fig. 37 ). 474
| (a) Low- and (b) high-magnification SEM images of a NiCo-precursor NWA/carbon textile composite, showing the nanowires completely surrounding the carbon microfiber core. (c and d) Low- and (e) high-magnification SEM images of a crystalline NiCo O NWA/carbon textile composite. (f) An SEM image of the urchin-like NiCo O microsphere prepared in the absence of carbon textiles. Reprinted with permission from . Copyright: ©2013, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
The same materials with different morphologies can produce different outcomes. For instance, MnO 2 nanoflowers have provided high initial sodium-ion storage capacity compared with MnO 2 nanorods. 481 Radha Narayanan and Mostafa A. El-Sayed have analyzed various nanoscale morphologies of Pt, such as tetrahedral, cubic, and near-spherical nanoparticles. The highest rate constant is observed with tetrahedral nanoparticles and the lowest rate constant was observed with cubic nanoparticles, whereas spherical nanoparticles exhibited an intermediate rate constant during catalysis. 502 Xiaowei Xie et al. found that Co 3 O 4 nanorods show high activity compared to conventional Co 3 O 4 nanoparticles for the low-temperature oxidation of CO. 503 The catalytic activity of metal-based nanomaterials is strongly affected by their shape. 504 Shape-defined mesoporous materials (TiO 2 ) have shown superior photoanode activities ( Fig. 38 ). 505 As a result, in the literature, several nanostructured morphologies of metal-based materials, such as nanotubes, 506,507 nanorods, 508,509 nanoflowers, 510 nanosheets, 511 nanowires, 512 nanocubes, 513 nanospheres, 514,515 nanocages, 516 and nanoboxes, 517 have been reported for a range of applications.
| A schematic illustration of the controlled chemical growth of TiO nanostructures with desired morphologies. (top row) Path I: the direct growth of nanocubes, rectangular nanorods, and cross-linked nanorods synthesized from seeds in aqueous solutions; (middle row) path II: 3D dendritic structures synthesized from solution with the addition of a non-aqueous co-surfactant; and (bottom row) path III: shape-defined mesoporous TiO nanostructures synthesized from solutions with lyotropic liquid-crystal templates. Reprinted with permission from . Copyright: © 2016, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Hollow nanostructures have surfaced as an amazing class of nanostructured material, and they have received significant attention from researchers. Hollow nanostructures have the unique features of: 518,519 low density; abundant inner void spaces; large surface areas; and the ability to act as nanoscale containers with high loading capacity, nanoreactors, and nanocarriers.
Various metal-based hollow nanostructures, such as hollow SnO 2 , 520 hollow palladium nanocrystals, 521 Co–Mn mixed oxide double-shell hollow spheres, 521 hollow Cu 2 O nanocages, 522 three-dimensional hollow SnO 2 @TiO 2 spheres, 523 hollow ZnO/Co 3 O 4 nano-heterostructure, 524 triple-shell hollow α-Fe 2 O 3 , 525 and hierarchical hollow Mn-doped Ni(OH) 2 nanostructures, 526 have been developed for various applications. The presence of nanoscale hollow interiors and functional shells imparts them with great potential for gas sensing, catalysis, biomedicine, energy storage, and conversion. 519
From this discussion, it can be concluded that metal-based nanostructured materials have great potential compared to their bulk counterparts. The conversion of materials to the nanoscale is not enough to achieve high performance with better selectivity. Now, research is switching from conventional nanomaterials to more advanced and smartly designed nanomaterials. In modern research, nanomaterials are being designed with better-controlled morphologies and regulated features.
5.5. Core–shell nanoparticles
| Different core/shell nanoparticles: (a) spherical core/shell nanoparticles; (b) hexagonal core/shell nanoparticles; (c) multiple small core materials coated with a single shell material; (d) a nanomatryushka material; and (e) a movable core within a hollow shell material. Reprinted with permission from . Copyright: ©2012, American Chemical Society. | ||
A spherical nanoparticle core–shell nanostructure is a practical way to introduce multiple functionalities on the nanoscopic length scale. 528 The properties arising from the core or shell can be different, and these properties can be tuned via controlling the ratio of the constituent materials. The shape, size, and composition play a critical role in tuning the core–shell nanoparticle properties. 529 The shell material can help to improve the chemical and thermal stabilities of the core material. The core–shell design has become effective where an inexpensive material cannot be used directly due to its instability or easily oxidizable nature. The core can consist of an easily oxidizable inexpensive metal, whereas the shell might consist of noble metals, oxides, polymers, or silica. 530 For instance, magnetic nanoparticles when prepared can be sensitive toward air, acids, and bases. Magnetic nanoparticles can be protected via coating with organic or inorganic shells. 528
Core–shell metal nanoparticles are an emerging nanostructured material with great potential in the fields of energy and catalysis. 531 The first report of core–shell nanoparticles (2007) for supercapacitor applications consisted of a polyaniline/multi-walled-carbon-nanotube composite (PANI/MWNTs). 532 Metal-based core–shell structured nanoparticles have shown enhanced catalytic performance due to their shape-controlled properties. 533 Ming-Yu Kuo et al. developed Au@Cu 2 O core–shell particles with controllable shell thicknesses that acted as a dual-functional catalyst. The shell thickness of Cu 2 O increased with an increasing concentration of Cu 2+ precursor. The thicknesses of the shells of Au@Cu 2 O-1.5 (12.2 ± 1.7 nm), Au@Cu 2 O-2 (13.2 ± 1.8 nm), Au@Cu 2 O-3 (18.2 ± 2.2 nm), and Au@Cu 2 O-4 (20.8 ± 2.5 nm) due to various concentrations are shown in Fig. 40 . 534 A NiO@SiO 2 core–shell catalyst provided a higher yield of acrylic acid from acetylene hydroxycarbonylation. 535 Core–shell architecture can be used to prevent active metal nanoparticles from oxidation during operation. For instance, a plasmonic photocatalyst was developed that consisted of silver nanoparticles embedded in titanium dioxide. The direct contact of Ag with TiO 2 could lead to its oxidization; this is prevented via developing core–shell architecture in which Ag is used as the core and SiO 2 is used as a shell to protect it. 536 Another excellent option is to replace an expensive core with a non-noble metal to reduce the core–shell cost while using a thin layer of a noble metal that consumes a small amount of metal as the shell. This will ensure the prolonged stability of the catalyst during operation. 533 Overall, core–shell morphologies provide better catalytic activity due to the synergistic effect of the metallic core–shell components. 152
| TEM images of (a) Au@Cu O-1.5, (b) Au@Cu O-2, (c) Au@Cu O-3, and (d) Au@Cu O-4. Reprinted with permission from . Copyright: ©2018, Elsevier B.V. All rights reserved. | ||
Among the several classes of nanomaterials, core–shell nanoparticles are found to be more promising for different biomedical applications. For instance, magnetic nanoparticles are considered to be useful for biomedical applications due to the following reasons: (a) aggregation is prevented due to superparamagnetism; (b) delivery and separation can be controlled using an external magnetic field; (c) they can be appropriately dispersed; and (d) there is the possibility of functionalization. A range of magnetic nanoparticles is available, such as NiO, Ni, Co, and Mn 3 O 4 . The most famous example is iron oxide, but uncoated iron oxides are unstable under physiological conditions. This may result in controlled drug delivery failure due to improper ligand surface binding and the promotion of the formation of harmful free radicals. Therefore, the formation of shells around magnetic nanoparticles has tremendous significance for biomedical applications. 537 One of the approaches is to use gold shells on magnetic nanoparticles. Au NPs are also called surface plasmons and they substantially enhanced the absorption of light in the visible and near-infrared regions. Thus, coating magnetic nanoparticles with a Au shell can result in a core–shell nanostructure that displays both optical and magnetic functionality in combination. 529
Numerous biocompatible core–shell nanoparticles are being developed for photothermal therapy, as core–shell materials are found to be useful for photothermal therapy. Hui Wang et al. have developed bifunctional core–shell nanoparticles for dual-modal imaging-guided photothermal therapy. The core–shell nanoparticles consist of a magnetic ∼9.1 nm core of Fe 3 O 4 covered by an approximately 3.4 nm fluorescent carbon shell. The Fe 3 O 4 core leads to superparamagnetic behavior, whereas the carbon shell provides near-infrared (NIR) fluorescence properties. The bifunctional nanoparticles have shown dual-modal imaging capacity both in vivo and in vitro . The iron oxide–carbon core–shell nanoparticles absorbed and converted near-infrared light to heat, facilitating photothermal therapy. 538 Au-Based core–shell structures are also being prepared for photothermal therapy. Bulk gold is biocompatible, but Au NPs can accumulate in the spleen and liver, causing severe toxicity. Koo Chul Kwon et al. have developed Au-NP-based core–shell structures that did not result in any gross or histological lesions in the major organs of mice, which revealed that this is a potent and safe agent for photothermal cancer therapy. The core–shell nanoparticles consisted of proteinticle/gold (PGCS-NP) and were developed via proteinticle surface engineering. PGCS-NP was injected intravenously into mice with tumors, and the injected core–shell nanoparticles successfully reached the EGFR-expressing tumor cells. The tumor size was significantly reduced upon exposure to near-infrared laser irradiation ( Fig. 41 ). No accumulation of Au NPs was observed in the mice organs, which indicated that PGCS-NP disassembled into many tiny gold dots, which were easily excreted by the kidneys and liver without causing any toxicity. 539 In another example, multifunctional Au@graphene oxide nanocolloid core@shell nanoparticles were developed, in which the core and shell consisted of gold and a graphene oxide nanocolloid, respectively. The developed core–shell structure showed multifunctional properties, allowing Raman bioimaging and photothermal/photodynamic therapy with low toxicity. 540 Apart from this, numerous other core–shell nanoparticles, such as polydopamine–mesoporous silica core–shell nanoparticles, 541 AuPd@PVP core–shell nanoparticles, 542 Au@Cu 2− x S core–shell nanoparticles, 543 bismuth sulfide@mesoporous silica core–shell nanoparticles, 544 and Ag@S-nitrosothiol core–shell nanoparticles, have been used for photothermal therapy. 545
| (A) PGCS-NPs (40 nm) for the excellent photothermal therapy of cancer. Reprinted with permission from . Copyright: ©2014, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (B) The synthetic procedure (a), multifunctionality (b), and theranosis process (c) of ZnPc–PEG–Au@GON NPs. Reprinted with permission from . Copyright: ©2015, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Due to their unique features and the combination of properties from the shell and core, these core–shell nanoparticles have received considerable interest in many fields, ranging from materials chemistry to the biomedical field. For electrochemical reactions, the core–shell structure conductivity can be enhanced via conducting polymers, carbon materials, and metals. Core–shell nanoparticles as electrode materials showed better performance compared to single components. Most of the core materials are prepared via hydrothermal methods, and shells can be prepared via hydrothermal or electrodeposition methods. 546 Even though significant progress has been made relating to the synthesis methods of core–shell materials, a major challenge is the high-quality production of core–shell materials in more effective ways for required applications, specifically biomedical applications.
6. Challenges and future perspectives
(a) The presence of defects in nanomaterials can affect their performance and their inherent characteristics can be compromised. For instance, carbon nanotubes are one of the strongest materials that are known. However, impurities, discontinuous tube lengths, defects, and random orientations can substantially impair the tensile strength of carbon nanotubes. 547
(b) The synthesis of nanomaterials through cost-effective routes is another major challenge. High-quality nanomaterials are generally produced using sophisticated instrumentation and harsh conditions, limiting their large-scale production. This issue is more critical for the synthesis of 2D nanomaterials. Most of the methods that have been adopted for large-scale production are low cost, and these methods generally produce materials with defects that are of poor quality. The controlled synthesis of nanomaterials is still a challenging job. For example, a crucial challenge associated with carbon nanotube synthesis is to achieve chiral selectivity, conductivity, and precisely controlled diameters. 548,549 Obtaining structurally pure nanomaterials is the only way to achieve the theoretically calculated characteristics described in the literature. More focused efforts are required to develop new synthesis methods that overcome the challenges associated with conventional methods.
(c) The agglomeration of particles at the nanoscale level is an inherent issue that substantially damages performance in relevant fields. Most nanomaterials start to agglomerate when they encounter each other. The process of agglomeration may be due to physical entanglement, electrostatic interactions, or high surface energy. 550 CNTs undergo van der Waals interactions and form bundles, making it difficult to align or properly disperse them in polymer matrices. 159 Similarly, graphene agglomeration is triggered by the basal planes of graphene sheets due to π–π interactions and van der Waals forces. Due to severe agglomeration, the high surface areas and other unique graphene features are compromised. These challenges hinder the practical application of high-throughput electrode materials or composite materials for various applications. 551
(d) The efficiency of nanomaterials can be tuned via developing 3D architectures. 3D architectures have been tried with several nanomaterials, such as graphene, to improve their inherent features. 3D architectures of 2D graphene have provided high specific surface areas and fast mass and electron transport kinetics. This has become possible due to the combination of the exceptional intrinsic properties of graphene and 3D porous structures. 194,552 The combination of graphene and CNT assemblies into 3-D architectures has emerged as the most investigated nanotechnology research area. Porous architectures of other nanomaterials can be developed to enhance their catalysis performance through providing nanomaterial interior availability.
(e) 2D ultrathin materials are an outstanding class of nanomaterial with promising theoretical properties; however, very little experimental evaluation of these materials has been done, apart from the case of graphene. The synthesis and stability of 2D ultrathin materials are some of the major challenges associated with them. In the future, more focus is anticipated to be placed on their synthesis and practical utilization.
(f) Nanomaterial utilization in industry is being increased, and there is also demand for nanoscale material production at higher rates. Moreover, nanotechnology research has vast horizons; the exploration of new nanomaterials with fascinating features will continue and, in the future, more areas will be discovered. One of the significant concerns relating to nanomaterials that cannot be overlooked is their toxicity, which is still poorly understood, and this is a serious concern relating to their environmental, domestic, and industrial use. The extent to which nanoparticle-based materials can contribute to cellular toxicity is unclear. 553 There is a need for the scientific community to put efforts into reducing the knowledge gap between the rapid development of nanomaterials and their possible in vivo toxicity. A proper and systematic understanding of the interaction of nanomaterials with cells, tissues, and proteins is critical for the safe design and commercialization of nanotechnology. 14
The future of advanced technology is linked with advancements in the field of nanotechnology. The dream of clean energy production is becoming possible with the advancement of nanomaterial-based engineering strategies. These materials have shown promising results, leading to new generations of hydrogen fuel cells and solar cells, acting as efficient catalysts for water splitting, and showing excellent capacity for hydrogen storage. Nanomaterials have a great future in the field of nanomedicine. Nanocarriers can be used for the delivery of therapeutic molecules.
7. Conclusions
Conflicts of interest, acknowledgements.
- F. J. Heiligtag and M. Niederberger, Mater. Today , 2013, 16 , 262–271 CrossRef CAS .
- P. Walter, E. Welcomme, P. Hallégot, N. J. Zaluzec, C. Deeb, J. Castaing, P. Veyssière, R. Bréniaux, J.-L. Lévêque and G. Tsoucaris, Nano Lett. , 2006, 6 , 2215–2219 CrossRef CAS PubMed .
- J. Jeevanandam, A. Barhoum, Y. S. Chan, A. Dufresne and M. K. Danquah, Beilstein J. Nanotechnol. , 2018, 9 , 1050–1074 CrossRef CAS PubMed .
- I. Freestone, N. Meeks, M. Sax and C. Higgitt, Gold Bull. , 2007, 40 , 270–277 CrossRef CAS .
- A. Santamaria, Nanotoxicity , Humana Press, Totowa, NJ, 2012, pp. 1–12 Search PubMed .
- R. P. Feynman, Eng. Sci. , 1960, 23 , 22–36 Search PubMed .
- S. Bayda, M. Adeel, T. Tuccinardi, M. Cordani and F. Rizzolio, Molecules , 2019, 25 , 112 CrossRef PubMed .
- M. Nasrollahzadeh, S. M. Sajadi, M. Sajjadi and Z. Issaabadi, Interface Science and Technology , Elsevier B.V., 2019, vol. 28, pp. 1–27 Search PubMed .
- N. Taniguchi, Proc. Int. Conf. Prod. Eng. Issue PART II , 1974, 18–23.
- G. Binnig and H. Rohrer, US Pat. , US4343993A, 1982 Search PubMed .
- G. Binnig, C. F. Quate and C. Gerber, Phys. Rev. Lett. , 1986, 56 , 930–933 CrossRef PubMed .
- H.-J. Butt, B. Cappella and M. Kappl, Surf. Sci. Rep. , 2005, 59 , 1–152 CrossRef CAS .
- A. C. Marques, M. Vale, D. Vicente, M. Schreck, E. Tervoort and M. Niederberger, Global Challenges , 2021, 2000116 CrossRef PubMed .
- S. Sharifi, S. Behzadi, S. Laurent, M. Laird Forrest, P. Stroeve and M. Mahmoudi, Chem. Soc. Rev. , 2012, 41 , 2323–2343 RSC .
- Nat. Nanotechnol. , 2019, 14 , 193 Search PubMed .
- P. N. Sudha, K. Sangeetha, K. Vijayalakshmi and A. Barhoum, Emerging Applications of Nanoparticles and Architecture Nanostructures , Elsevier, 2018, pp. 341–384 Search PubMed .
- W. G. Kreyling, M. Semmler-Behnke and Q. Chaudhry, Nano Today , 2010, 5 , 165–168 CrossRef .
- B. Bhushan, Springer Handbooks , Springer, 2017, pp. 1–19 Search PubMed .
- F. Trotta and A. Mele, Nanosponges: From Fundamentals to Applications , 2019, pp. 1–24 Search PubMed .
- Nanoanalytics , ed. S. Shtykov, De Gruyter, Berlin, Boston, 2018 Search PubMed .
- C. J. Murphy and N. R. Jana, Adv. Mater. , 2002, 14 , 80–82 CrossRef CAS .
- P. Moriarty, Rep. Prog. Phys. , 2001, 64 , 297–381 CrossRef CAS .
- B. Chen, J. R. G. Evans, H. C. Greenwell, P. Boulet, P. V. Coveney, A. A. Bowden and A. Whiting, Chem. Soc. Rev. , 2008, 37 , 568–594 RSC .
- P. Khanna, A. Kaur and D. Goyal, J. Microbiol. Methods , 2019, 163 , 105656 CrossRef CAS PubMed .
- S. Zhuang, E. S. Lee, L. Lei, B. B. Nunna, L. Kuang and W. Zhang, Int. J. Energy Res. , 2016, 40 , 2136–2149 CrossRef CAS .
- T. Prasad Yadav, R. Manohar Yadav and D. Pratap Singh, Nanosci. Nanotechnol. , 2012, 2 , 22–48 CrossRef .
- H. Lyu, B. Gao, F. He, C. Ding, J. Tang and J. C. Crittenden, ACS Sustainable Chem. Eng. , 2017, 5 , 9568–9585 CrossRef CAS .
- R. Ostermann, J. Cravillon, C. Weidmann, M. Wiebcke and B. M. Smarsly, Chem. Commun. , 2011, 47 , 442–444 RSC .
- P. S. Kumar, J. Sundaramurthy, S. Sundarrajan, V. J. Babu, G. Singh, S. I. Allakhverdiev and S. Ramakrishna, Energy Environ. Sci. , 2014, 7 , 3192–3222 RSC .
- P. Du, L. Song, J. Xiong, N. Li, Z. Xi, L. Wang, D. Jin, S. Guo and Y. Yuan, Electrochim. Acta , 2012, 78 , 392–397 CrossRef CAS .
- A. Pimpin and W. Srituravanich, Eng. J. , 2012, 16 , 37–56 CrossRef .
- Z. Szabó, J. Volk, E. Fülöp, A. Deák and I. Bársony, Photonics Nanostruct. , 2013, 11 , 1–7 CrossRef .
- C.-W. Kuo, J.-Y. Shiu, Y.-H. Cho and P. Chen, Adv. Mater. , 2003, 15 , 1065–1068 CrossRef CAS .
- Y. Yin, B. Gates and Y. Xia, Adv. Mater. , 2000, 12 , 1426–1430 CrossRef CAS .
- K. Xu and J. Chen, Appl. Nanosci. , 2020, 10 , 1013–1022 CrossRef CAS .
- R. Matsumoto, S. Adachi, E. H. S. Sadki, S. Yamamoto, H. Tanaka, H. Takeya and Y. Takano, ACS Appl. Electron. Mater. , 2020, 2 , 677–682 CrossRef CAS .
- V. Garg, R. G. Mote and J. Fu, Appl. Surf. Sci. , 2020, 526 , 146644 CrossRef CAS .
- P. Ayyub, R. Chandra, P. Taneja, A. K. Sharma and R. Pinto, Appl. Phys. A: Mater. Sci. Process. , 2001, 73 , 67–73 CrossRef CAS .
- H. H. Son, G. H. Seo, U. Jeong, D. Y. Shin and S. J. Kim, Int. J. Heat Mass Transfer , 2017, 113 , 115–128 CrossRef CAS .
- H. Wender, P. Migowski, A. F. Feil, S. R. Teixeira and J. Dupont, Coord. Chem. Rev. , 2013, 257 , 2468–2483 CrossRef CAS .
- J. Muñoz-García, L. Vázquez, R. Cuerno, J. A. Sánchez-García, M. Castro and R. Gago, Toward Functional Nanomaterials , Springer US, New York, NY, 2009, pp. 323–398 Search PubMed .
- J. H. Nam, M. J. Jang, H. Y. Jang, W. Park, X. Wang, S. M. Choi and B. Cho, J. Energy Chem. , 2020, 47 , 107–111 CrossRef .
- M. Nie, K. Sun and D. D. Meng, J. Appl. Phys. , 2009, 106 , 054314 CrossRef .
- D. Zhang, K. Ye, Y. Yao, F. Liang, T. Qu, W. Ma, B. Yang, Y. Dai and T. Watanabe, Carbon , 2019, 142 , 278–284 CrossRef CAS .
- C. M. Lieber and C.-C. Chen, Solid State Physics – Advances in Research and Applications , Academic Press, 1994, vol. 48, pp. 109–148 Search PubMed .
- J. M. Jones, R. P. Malcolm, K. M. Thomas and S. H. Botrell, Carbon , 1996, 34 , 231–237 CrossRef CAS .
- F. Liang, M. Tanaka, S. Choi and T. Watanabe, Carbon , 2017, 117 , 100–111 CrossRef CAS .
- F. Liang, T. Shimizu, M. Tanaka, S. Choi and T. Watanabe, Diamond Relat. Mater. , 2012, 30 , 70–76 CrossRef CAS .
- N. Li, Z. Wang, K. Zhao, Z. Shi, Z. Gu and S. Xu, Carbon , 2010, 48 , 1580–1585 CrossRef CAS .
- Z.-S. Wu, W. Ren, L. Gao, J. Zhao, Z. Chen, B. Liu, D. Tang, B. Yu, C. Jiang and H.-M. Cheng, ACS Nano , 2009, 3 , 411–417 CrossRef CAS PubMed .
- V. Amendola and M. Meneghetti, Phys. Chem. Chem. Phys. , 2009, 11 , 3805 RSC .
- J. Zhang, M. Chaker and D. Ma, J. Colloid Interface Sci. , 2017, 489 , 138–149 CrossRef CAS PubMed .
- R. A. Ismail, M. H. Mohsin, A. K. Ali, K. I. Hassoon and S. Erten-Ela, Phys. E , 2020, 119 , 113997 CrossRef CAS .
- J. Chrzanowska, J. Hoffman, A. Małolepszy, M. Mazurkiewicz, T. A. Kowalewski, Z. Szymanski and L. Stobinski, Phys. Status Solidi , 2015, 252 , 1860–1867 CrossRef CAS .
- J. Duque, B. Madrigal, H. Riascos and Y. Avila, Colloids Interfaces , 2019, 3 , 25 CrossRef CAS .
- S. S. Su and I. Chang, Commercialization of Nanotechnologies–A Case Study Approach , Springer International Publishing, Cham, 2018, pp. 15–29 Search PubMed .
- H. Park, D. A. Reddy, Y. Kim, S. Lee, R. Ma and T. K. Kim, Chem. – A Eur. J. , 2017, 23 , 13112–13119 CrossRef CAS PubMed .
- A. C. Jones and M. L. Hitchman, in Chemical Vapour Deposition , ed. A. C. Jones and M. L. Hitchman, Royal Society of Chemistry, Cambridge, 2008, pp. 1–36 Search PubMed .
- K. A. Shah and B. A. Tali, Mater. Sci. Semicond. Process. , 2016, 41 , 67–82 CrossRef CAS .
- H. Ago, Frontiers of Graphene and Carbon Nanotubes , Springer, Japan, Tokyo, 2015, pp. 3–20 Search PubMed .
- P. Machac, S. Cichon, L. Lapcak and L. Fekete, Graphene Technol. , 2020, 5 , 9–17 CrossRef .
- Q. Wu, W. Wongwiriyapan, J.-H. Park, S. Park, S. J. Jung, T. Jeong, S. Lee, Y. H. Lee and Y. J. Song, Curr. Appl. Phys. , 2016, 16 , 1175–1191 CrossRef .
- X. Wu, G. Q. (Max) Lu and L. Wang, Energy Environ. Sci. , 2011, 4 , 3565 RSC .
- S. Cao, C. Zhao, T. Han and L. Peng, Mater. Lett. , 2016, 169 , 17–20 CrossRef CAS .
- J. Li, Q. Wu and J. Wu, Handbook of Nanoparticles , Springer International Publishing, Cham, 2015, pp. 1–28 Search PubMed .
- A. Chen and P. Holt-Hindle, Chem. Rev. , 2010, 110 , 3767–3804 CrossRef CAS .
- L.-Y. Meng, B. Wang, M.-G. Ma and K.-L. Lin, Mater. Today Chem. , 2016, 1–2 , 63–83 CrossRef .
- Y. Dong, X. Du, P. Liang and X. Man, Inorg. Chem. Commun. , 2020, 115 , 107883 CrossRef CAS .
- Y. Jiang, Z. Peng, S. Zhang, F. Li, Z. Liu, J. Zhang, Y. Liu and K. Wang, Ceram. Int. , 2018, 44 , 6115–6126 CrossRef CAS .
- B. Chai, M. Xu, J. Yan and Z. Ren, Appl. Surf. Sci. , 2018, 430 , 523–530 CrossRef CAS .
- A. E. Danks, S. R. Hall and Z. Schnepp, Mater. Horiz. , 2016, 3 , 91–112 RSC .
- T. K. Tseng, Y. S. Lin, Y. J. Chen and H. Chu, Int. J. Mol. Sci. , 2010, 11 , 2336–2361 CrossRef CAS .
- M. Parashar, V. K. Shukla and R. Singh, J. Mater. Sci.: Mater. Electron. , 2020, 31 , 3729–3749 CrossRef CAS .
- L. Znaidi, Mater. Sci. Eng., B , 2010, 174 , 18–30 CrossRef CAS .
- C. de Coelho Escobar and J. H. Z. dos Santos, J. Sep. Sci. , 2014, 37 , 868–875 CrossRef PubMed .
- Y. Liu, J. Goebl and Y. Yin, Chem. Soc. Rev. , 2013, 42 , 2610–2653 RSC .
- W. Li and D. Zhao, Chem. Commun. , 2013, 49 , 943–946 RSC .
- R. R. Poolakkandy and M. M. Menamparambath, Nanoscale Adv. , 2020, 2 , 5015–5045 RSC .
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature , 1992, 359 , 710–712 CrossRef CAS .
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. W. Chu, D. H. Olson, E. W. Sheppard, S. B. McCullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc. , 1992, 114 , 10834–10843 CrossRef CAS .
- J. Liu, T. Yang, D.-W. Wang, G. Q. Lu, D. Zhao and S. Z. Qiao, Nat. Commun. , 2013, 4 , 2798 CrossRef .
- R. Lv, C. Cao, H. Zhai, D. Wang, S. Liu and H. Zhu, Solid State Commun. , 2004, 130 , 241–245 CrossRef .
- L. Martins, M. A. Alves Rosa, S. H. Pulcinelli and C. V. Santilli, Microporous Mesoporous Mater. , 2010, 132 , 268–275 CrossRef CAS .
- T. Tang, T. Zhang, W. Li, X. Huang, X. Wang, H. Qiu and Y. Hou, Nanoscale , 2019, 11 , 7440–7446 RSC .
- B. Szczęśniak, J. Choma and M. Jaroniec, Chem. Commun. , 2020, 56 , 7836–7848 RSC .
- Y. Yamauchi and K. Kuroda, Chem. – Asian J. , 2008, 3 , 664–676 CrossRef CAS PubMed .
- S. J. Hurst, E. K. Payne, L. Qin and C. A. Mirkin, Angew. Chem., Int. Ed. , 2006, 45 , 2672–2692 CrossRef CAS PubMed .
- M. A. Malik, M. Y. Wani and M. A. Hashim, Arabian J. Chem. , 2012, 5 , 397–417 CrossRef CAS .
- M. Soleimani Zohr Shiri, W. Henderson and M. R. Mucalo, Materials , 2019, 12 , 1896 CrossRef PubMed .
- T.-D. Nguyen, Nanoscale , 2013, 5 , 9455 RSC .
- S. Yi, F. Dai, C. Zhao and Y. Si, Sci. Rep. , 2017, 7 , 9806 CrossRef .
- R. Jose Varghese, E. H. M. Sakho, S. Parani, S. Thomas, O. S. Oluwafemi and J. Wu, Nanomaterials for Solar Cell Applications , Elsevier, 2019, pp. 75–95 Search PubMed .
- E. Roduner, Chem. Soc. Rev. , 2006, 35 , 583 RSC .
- A. J. Mannix, X.-F. Zhou, B. Kiraly, J. D. Wood, D. Alducin, B. D. Myers, X. Liu, B. L. Fisher, U. Santiago, J. R. Guest, M. J. Yacaman, A. Ponce, A. R. Oganov, M. C. Hersam and N. P. Guisinger, Science , 2015, 350 , 1513–1516 CrossRef CAS PubMed .
- Nanobiotechnology: Concepts and Applications in Health, Agriculture, and Environment , ed. R. S. Tomar, A. Jyoti and S. Kaushik, 1st edn, 2020 Search PubMed .
- A. P. Alivisatos, Science , 1996, 271 , 933–937 CrossRef CAS .
- R. Tomar, A. A. Abdala, R. G. Chaudhary and N. B. Singh, Mater. Today: Proc. , 2020, 29 , 967–973, DOI: 10.1016/j.matpr.2020.04.144 .
- L. D. Geoffrion and G. Guisbiers, J. Phys. Chem. Solids , 2020, 140 , 109320 CrossRef CAS .
- S. K. Krishnan, E. Singh, P. Singh, M. Meyyappan and H. S. Nalwa, RSC Adv. , 2019, 9 , 8778–8881 RSC .
- Q. Wu, W. Miao, Y. Zhang, H. Gao and D. Hui, Nanotechnol. Rev. , 2020, 9 , 259–273 CrossRef CAS .
- W. Zhu, Y. Guo, B. Ma, X. Yang, Y. Li, P. Li, Y. Zhou and M. Shuai, Int. J. Hydrogen Energy , 2020, 45 , 8385–8395 CrossRef CAS .
- X. Liu, M. Xu, L. Wan, H. Zhu, K. Yao, R. Linguerri, G. Chambaud, Y. Han and C. Meng, ACS Catal. , 2020, 10 , 3084–3093 CrossRef CAS .
- P. Makvandi, C. Wang, E. N. Zare, A. Borzacchiello, L. Niu and F. R. Tay, Adv. Funct. Mater. , 2020, 30 , 1910021 CrossRef CAS .
- E. Castro, A. H. Garcia, G. Zavala and L. Echegoyen, J. Mater. Chem. B , 2017, 5 , 6523–6535 RSC .
- M. S. Mauter and M. Elimelech, Environ. Sci. Technol. , 2008, 42 , 5843–5859 CrossRef CAS PubMed .
- L. Chen, S. Zhao, Q. Hasi, X. Luo, C. Zhang, H. Li and A. Li, Global Challenges , 2020, 4 , 1900098 CrossRef PubMed .
- R. G. Mendes, A. Bachmatiuk, B. Büchner, G. Cuniberti and M. H. Rümmeli, J. Mater. Chem. B , 2013, 1 , 401–428 RSC .
- M. T. Beck and G. Mándi, Fullerene Sci. Technol. , 1997, 5 , 291–310 CrossRef CAS .
- X. Fan, N. Soin, H. Li, H. Li, X. Xia and J. Geng, Energy Environ. Mater. , 2020, 3 , 469–491, DOI: 10.1002/eem2.12071 .
- S. Goodarzi, T. Da Ros, J. Conde, F. Sefat and M. Mozafari, Mater. Today , 2017, 20 , 460–480 CrossRef CAS .
- P. Ehrenfreund and B. H. Foing, Science , 2010, 329 , 1159–1160 CrossRef CAS PubMed .
- J. C. Withers, R. O. Loutfy and T. P. Lowe, Fullerene Sci. Technol. , 1997, 5 , 1–31 CrossRef .
- A. Rodríguez-Fortea, A. L. Balch and J. M. Poblet, Chem. Soc. Rev. , 2011, 40 , 3551 RSC .
- N. A. Kaskhedikar and J. Maier, Adv. Mater. , 2009, 21 , 2664–2680 CrossRef CAS .
- A. A. Popov, S. Yang and L. Dunsch, Chem. Rev. , 2013, 113 , 5989–6113 CrossRef CAS .
- B. Q. Mercado, C. M. Beavers, M. M. Olmstead, M. N. Chaur, K. Walker, B. C. Holloway, L. Echegoyen and A. L. Balch, J. Am. Chem. Soc. , 2008, 130 , 7854–7855 CrossRef CAS PubMed .
- S. Yang and C.-R. Wang, Endohedral Fullerenes , World Scientific, 2014 Search PubMed .
- H. Okada, T. Komuro, T. Sakai, Y. Matsuo, Y. Ono, K. Omote, K. Yokoo, K. Kawachi, Y. Kasama, S. Ono, R. Hatakeyama, T. Kaneko and H. Tobita, RSC Adv. , 2012, 2 , 10624 RSC .
- H. Bai, H. Gao, W. Feng, Y. Zhao and Y. Wu, Nanomaterials , 2019, 9 , 630 CrossRef CAS .
- O. V. Pupysheva, A. A. Farajian and B. I. Yakobson, Nano Lett. , 2008, 8 , 767–774 CrossRef CAS .
- M. Gaboardi, N. Sarzi Amadé, M. Aramini, C. Milanese, G. Magnani, S. Sanna, M. Riccò and D. Pontiroli, Carbon , 2017, 120 , 77–82 CrossRef CAS .
- A. Shokuhi Rad and K. Ayub, Comput. Theor. Chem. , 2017, 1121 , 68–75 CrossRef CAS .
- D. Guldi and N. Martin, Comprehensive Nanoscience and Technology , Elsevier, 2011, pp. 379–398 Search PubMed .
- L. Sánchez, N. Martín and D. M. Guldi, Angew. Chem., Int. Ed. , 2005, 44 , 5374–5382 CrossRef PubMed .
- X. Zhang, Y. Ma, S. Fu and A. Zhang, Nanomaterials , 2019, 9 , 1647 CrossRef CAS PubMed .
- N. Martin and G. Bodwell, Acc. Chem. Res. , 2019, 52 , 2757–2759 CrossRef CAS PubMed .
- K. Harano and E. Nakamura, Acc. Chem. Res. , 2019, 52 , 2090–2100 CrossRef CAS PubMed .
- A. Montellano, T. Da Ros, A. Bianco and M. Prato, Nanoscale , 2011, 3 , 4035 RSC .
- E. Alipour, F. Alimohammady, A. Yumashev and A. Maseleno, J. Mol. Model. , 2020, 26 , 7 CrossRef CAS PubMed .
- M.-S. Lin, R.-T. Chen, N.-Y. Yu, L.-C. Sun, Y. Liu, C.-H. Cui, S.-Y. Xie, R.-B. Huang and L.-S. Zheng, Colloids Surf., B , 2017, 159 , 613–619 CrossRef CAS PubMed .
- K. Minami, K. Okamoto, K. Harano, E. Noiri and E. Nakamura, ACS Appl. Mater. Interfaces , 2018, 10 , 19347–19354 CrossRef CAS PubMed .
- K. Minami, K. Okamoto, K. Doi, K. Harano, E. Noiri and E. Nakamura, Sci. Rep. , 2015, 4 , 4916 CrossRef PubMed .
- S. S. Babu, H. Möhwald and T. Nakanishi, Chem. Soc. Rev. , 2010, 39 , 4021 RSC .
- N. Furuuchi, R. Shrestha, Y. Yamashita, T. Hirao, K. Ariga and L. Shrestha, Sensors , 2019, 19 , 267 CrossRef PubMed .
- J. Coro, M. Suárez, L. S. R. Silva, K. I. B. Eguiluz and G. R. Salazar-Banda, Int. J. Hydrogen Energy , 2016, 41 , 17944–17959 CrossRef CAS .
- N. Yousfi-Steiner, P. Moçotéguy, D. Candusso and D. Hissel, J. Power Sources , 2009, 194 , 130–145 CrossRef CAS .
- A. R. Tuktarov, A. A. Khuzin and U. M. Dzhemilev, Pet. Chem. , 2020, 60 , 113–133 CrossRef CAS .
- I. Jeon, A. Shawky, H.-S. Lin, S. Seo, H. Okada, J.-W. Lee, A. Pal, S. Tan, A. Anisimov, E. I. Kauppinen, Y. Yang, S. Manzhos, S. Maruyama and Y. Matsuo, J. Am. Chem. Soc. , 2019, 141 , 16553–16558 CrossRef CAS PubMed .
- Y. Takabayashi and K. Prassides, Philos. Trans. R. Soc., A , 2016, 374 , 20150320 CrossRef PubMed .
- M. A. Rafiee, F. Yavari, J. Rafiee and N. Koratkar, J. Nanopart. Res. , 2011, 13 , 733–737 CrossRef CAS .
- Y. Pan, Z. Guo, S. Ran and Z. Fang, J. Appl. Polym. Sci. , 2020, 137 , 47538 CrossRef .
- S. Iijima, Nature , 1991, 354 , 56–58 CrossRef CAS .
- S. Iijima and T. Ichihashi, Nature , 1993, 363 , 603–605 CrossRef CAS .
- I. V. Zaporotskova, N. P. Boroznina, Y. N. Parkhomenko and L. V. Kozhitov, Mod. Electron. Mater. , 2016, 2 , 95–105 CrossRef .
- X.-Q. Li, P.-X. Hou, C. Liu and H.-M. Cheng, Carbon , 2019, 147 , 187–198 CrossRef CAS .
- C. Shen, A. H. Brozena and Y. Wang, Nanoscale , 2011, 3 , 503–518 RSC .
- V. Popov, Mater. Sci. Eng., R , 2004, 43 , 61–102 CrossRef .
- Z. F. Ren, Science , 1998, 282 , 1105–1107 CrossRef CAS .
- A. G. Rinzler, J. Liu, H. Dai, P. Nikolaev, C. B. Huffman, F. J. Rodríguez-Macías, P. J. Boul, A. H. Lu, D. Heymann, D. T. Colbert, R. S. Lee, J. E. Fischer, A. M. Rao, P. C. Eklund and R. E. Smalley, Appl. Phys. A: Mater. Sci. Process. , 1998, 67 , 29–37 CrossRef CAS .
- P. Nikolaev, M. J. Bronikowski, R. K. Bradley, F. Rohmund, D. T. Colbert, K. Smith and R. E. Smalley, Chem. Phys. Lett. , 1999, 313 , 91–97 CrossRef CAS .
- N. Baig, M. Sajid and T. A. Saleh, TrAC, Trends Anal. Chem. , 2019, 111 , 47–61 CrossRef CAS .
- X.-M. Liu, Z. Dong Huang, S. Woon Oh, B. Zhang, P.-C. Ma, M. M. F. Yuen and J.-K. Kim, Compos. Sci. Technol. , 2012, 72 , 121–144 CrossRef CAS .
- T. W. Odom, J.-L. Huang, P. Kim and C. M. Lieber, Nature , 1998, 391 , 62–64 CrossRef CAS .
- K. Balasubramanian and M. Burghard, Small , 2005, 1 , 180–192 CrossRef CAS PubMed .
- J. R. Xiao, B. A. Gama and J. W. Gillespie, Int. J. Solids Struct. , 2005, 42 , 3075–3092 CrossRef .
- H. Kim, M. Wang, S. K. Lee, J. Kang, J.-D. Nam, L. Ci and J. Suhr, Sci. Rep. , 2017, 7 , 9512 CrossRef PubMed .
- J. H. Lehman, M. Terrones, E. Mansfield, K. E. Hurst and V. Meunier, Carbon , 2011, 49 , 2581–2602 CrossRef CAS .
- N. G. Sahoo, S. Rana, J. W. Cho, L. Li and S. H. Chan, Prog. Polym. Sci. , 2010, 35 , 837–867 CrossRef CAS .
- T. Kuang, L. Chang, F. Chen, Y. Sheng, D. Fu and X. Peng, Carbon , 2016, 105 , 305–313 CrossRef CAS .
- Z. Wei, M. Kondratenko, L. H. Dao and D. F. Perepichka, J. Am. Chem. Soc. , 2006, 128 , 3134–3135 CrossRef CAS PubMed .
- M. Bockrath, Science , 2001, 291 , 283–285 CrossRef CAS PubMed .
- M. Rother, M. Brohmann, S. Yang, S. B. Grimm, S. P. Schießl, A. Graf and J. Zaumseil, Adv. Electron. Mater. , 2017, 3 , 1700080 CrossRef .
- J.-M. Bonard, M. Croci, C. Klinke, R. Kurt, O. Noury and N. Weiss, Carbon , 2002, 40 , 1715–1728 CrossRef CAS .
- B. J. Landi, M. J. Ganter, C. D. Cress, R. A. DiLeo and R. P. Raffaelle, Energy Environ. Sci. , 2009, 2 , 638 RSC .
- S. Mallakpour and E. Khadem, Chem. Eng. J. , 2016, 302 , 344–367 CrossRef CAS .
- M. W. Chik, Z. Hussain, M. Zulkefeli, M. Tripathy, S. Kumar, A. B. A. Majeed and K. Byrappa, Drug Delivery Transl. Res. , 2019, 9 , 578–594 CrossRef CAS PubMed .
- W. Z. Yuan, Y. Mao, H. Zhao, J. Z. Sun, H. P. Xu, J. K. Jin, Q. Zheng and B. Z. Tang, Macromolecules , 2008, 41 , 701–707 CrossRef CAS .
- C. A. Dyke and J. M. Tour, J. Phys. Chem. A , 2004, 108 , 11151–11159 CrossRef CAS .
- S. Singh, S. Vardharajula, P. Tiwari, E. Eroğlu, K. Vig, V. Dennis and S. Z. Ali, Int. J. Nanomed. , 2012, 7 , 5361 CrossRef PubMed .
- K. Zare, V. K. Gupta, O. Moradi, A. S. H. Makhlouf, M. Sillanpää, M. N. Nadagouda, H. Sadegh, R. Shahryari-ghoshekandi, A. Pal, Z. Wang, I. Tyagi and M. Kazemi, J. Nanostruct. Chem. , 2015, 5 , 227–236 CrossRef CAS .
- E. Vázquez and M. Prato, Pure Appl. Chem. , 2010, 82 , 853–861 Search PubMed .
- A. Setaro, M. Adeli, M. Glaeske, D. Przyrembel, T. Bisswanger, G. Gordeev, F. Maschietto, A. Faghani, B. Paulus, M. Weinelt, R. Arenal, R. Haag and S. Reich, Nat. Commun. , 2017, 8 , 14281 CrossRef CAS PubMed .
- P. Bilalis, D. Katsigiannopoulos, A. Avgeropoulos and G. Sakellariou, RSC Adv. , 2014, 4 , 2911–2934 RSC .
- Y. Zhou, Y. Fang and R. Ramasamy, Sensors , 2019, 19 , 392 CrossRef PubMed .
- T. Morishita, M. Matsushita, Y. Katagiri and K. Fukumori, Carbon , 2009, 47 , 2716–2726 CrossRef CAS .
- M. S. Ata, R. Poon, A. M. Syed, J. Milne and I. Zhitomirsky, Carbon , 2018, 130 , 584–598 CrossRef CAS .
- N. Alzate-Carvajal, L. M. Bolivar-Pineda, V. Meza-Laguna, V. A. Basiuk, E. V. Basiuk and E. A. Baranova, ChemElectroChem , 2020, 7 , 428–436 CrossRef CAS .
- G. Liu, W. Jin and N. Xu, Chem. Soc. Rev. , 2015, 44 , 5016–5030 RSC .
- N. N. Anh, N. Van Chuc, B. H. Thang, P. Van Nhat, N. Hao, D. D. Phuong, P. N. Minh, T. Subramani, N. Fukata and P. Van Trinh, Global Challenges , 2020, 2000010 CrossRef PubMed .
- G. K. Dalapati, S. Masudy-Panah, R. S. Moakhar, S. Chakrabortty, S. Ghosh, A. Kushwaha, R. Katal, C. S. Chua, G. Xiao, S. Tripathy and S. Ramakrishna, Global Challenges , 2020, 4 , 1900087 CrossRef PubMed .
- A. K. Geim and K. S. Novoselov, Nat. Mater. , 2007, 6 , 183–191 CrossRef CAS PubMed .
- A. K. Geim, Science , 2009, 324 , 1530–1534 CrossRef CAS PubMed .
- S. Ghosh, I. Calizo, D. Teweldebrhan, E. P. Pokatilov, D. L. Nika, A. A. Balandin, W. Bao, F. Miao and C. N. Lau, Appl. Phys. Lett. , 2008, 92 , 151911 CrossRef .
- A. A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. N. Lau, Nano Lett. , 2008, 8 , 902–907 CrossRef CAS PubMed .
- K. I. Bolotin, K. J. Sikes, Z. Jiang, M. Klima, G. Fudenberg, J. Hone, P. Kim and H. L. Stormer, Solid State Commun. , 2008, 146 , 351–355 CrossRef CAS .
- N. Baig, A.-N. Kawde and M. Ibrahim, Mater. Adv. , 2020, 1 , 783–793, 10.1039/D0MA00322K .
- D. Konios, M. M. Stylianakis, E. Stratakis and E. Kymakis, J. Colloid Interface Sci. , 2014, 430 , 108–112 CrossRef CAS PubMed .
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon , 2007, 45 , 1558–1565 CrossRef CAS .
- J. Chen, Y. Zhang, M. Zhang, B. Yao, Y. Li, L. Huang, C. Li and G. Shi, Chem. Sci. , 2016, 7 , 1874–1881 RSC .
- M. Pumera, Electrochem. Commun. , 2013, 36 , 14–18 CrossRef CAS .
- F. Perreault, A. Fonseca de Faria and M. Elimelech, Chem. Soc. Rev. , 2015, 44 , 5861–5896 RSC .
- A. Pulido, P. Concepción, M. Boronat, C. Botas, P. Alvarez, R. Menendez and A. Corma, J. Mater. Chem. , 2012, 22 , 51–56 RSC .
- N. Baig and T. A. Saleh, Microchim. Acta , 2018, 185 , 283 CrossRef PubMed .
- A. T. Smith, A. M. LaChance, S. Zeng, B. Liu and L. Sun, Nano Mater. Sci. , 2019, 1 , 31–47 CrossRef .
- S. Pei and H.-M. Cheng, Carbon , 2012, 50 , 3210–3228 CrossRef CAS .
- N. Baig and A.-N. Kawde, RSC Adv. , 2016, 6 , 80756–80765 RSC .
- K. K. H. De Silva, H. H. Huang, R. K. Joshi and M. Yoshimura, Carbon , 2017, 119 , 190–199 CrossRef CAS .
- N. Baig, A. Kawde and M. Ibrahim, ChemistrySelect , 2019, 4 , 1640–1649 CrossRef CAS .
- B. Wang, T. Ruan, Y. Chen, F. Jin, L. Peng, Y. Zhou, D. Wang and S. Dou, Energy Storage Mater. , 2020, 24 , 22–51 CrossRef .
- W. Lv, Z. Li, Y. Deng, Q.-H. Yang and F. Kang, Energy Storage Mater. , 2016, 2 , 107–138 CrossRef .
- G. Li, Y. Xia, Y. Tian, Y. Wu, J. Liu, Q. He and D. Chen, J. Electrochem. Soc. , 2019, 166 , B881–B895 CrossRef CAS .
- N. Baig and A.-N. Kawde, Anal. Methods , 2015, 7 , 9535–9541 RSC .
- C. I. L. Justino, A. R. Gomes, A. C. Freitas, A. C. Duarte and T. A. P. Rocha-Santos, TrAC, Trends Anal. Chem. , 2017, 91 , 53–66 CrossRef CAS .
- Q. He, S. Wu, Z. Yin and H. Zhang, Chem. Sci. , 2012, 3 , 1764 RSC .
- H. Yoon, J. Nah, H. Kim, S. Ko, M. Sharifuzzaman, S. C. Barman, X. Xuan, J. Kim and J. Y. Park, Sens. Actuators, B , 2020, 311 , 127866 CrossRef CAS .
- T.-H. Han, Y. Lee, M.-R. Choi, S.-H. Woo, S.-H. Bae, B. H. Hong, J.-H. Ahn and T.-W. Lee, Nat. Photonics , 2012, 6 , 105–110 CrossRef CAS .
- M. Xu, J. Qi, F. Li, X. Liao, S. Liu and Y. Zhang, RSC Adv. , 2017, 7 , 30506–30512 RSC .
- A. Kaidarova, N. Alsharif, B. N. M. Oliveira, M. Marengo, N. R. Geraldi, C. M. Duarte and J. Kosel, Global Challenges , 2020, 4 , 2000001 CrossRef .
- V. H. R. Souza, M. M. Oliveira and A. J. G. Zarbin, J. Power Sources , 2017, 348 , 87–93 CrossRef CAS .
- Y. Zhong, X. Zhang, Y. He, H. Peng, G. Wang and G. Xin, Adv. Funct. Mater. , 2018, 28 , 1801998 CrossRef .
- Mandeep, A. Gulati and R. Kakkar, Chem. Eng. Res. Des. , 2020, 153 , 21–36 CrossRef CAS .
- T. A. Saleh, N. Baig, F. I. Alghunaimi and N. W. Aljuryyed, RSC Adv. , 2020, 10 , 5088–5097 RSC .
- N. Baig, F. I. Alghunaimi, H. S. Dossary and T. A. Saleh, Process Saf. Environ. Prot. , 2019, 123 , 327–334 CrossRef CAS .
- N. Baig, Ihsanullah, M. Sajid and T. A. Saleh, J. Environ. Manage. , 2019, 244 , 370–382 CrossRef CAS PubMed .
- J. Bu, L. Yuan, N. Zhang, D. Liu, Y. Meng and X. Peng, Diamond Relat. Mater. , 2020, 101 , 107604 CrossRef CAS .
- V. V. Danilenko, Phys. Solid State , 2004, 46 , 595–599 CrossRef CAS .
- V. N. Mochalin, O. Shenderova, D. Ho and Y. Gogotsi, Nat. Nanotechnol. , 2012, 7 , 11–23 CrossRef CAS PubMed .
- Y. Zhang, K. Y. Rhee, D. Hui and S.-J. Park, Composites, Part B , 2018, 143 , 19–27 CrossRef CAS .
- T. L. Daulton, M. A. Kirk, R. S. Lewis and L. E. Rehn, Nucl. Instrum. Methods Phys. Res., Sect. B , 2001, 175–177 , 12–20 CrossRef CAS .
- J.-P. Boudou, P. A. Curmi, F. Jelezko, J. Wrachtrup, P. Aubert, M. Sennour, G. Balasubramanian, R. Reuter, A. Thorel and E. Gaffet, Nanotechnology , 2009, 20 , 235602 CrossRef PubMed .
- S. Welz, Y. Gogotsi and M. J. McNallan, J. Appl. Phys. , 2003, 93 , 4207–4214 CrossRef CAS .
- F. Fendrych, A. Taylor, L. Peksa, I. Kratochvilova, J. Vlcek, V. Rezacova, V. Petrak, Z. Kluiber, L. Fekete, M. Liehr and M. Nesladek, J. Phys. D: Appl. Phys. , 2010, 43 , 374018 CrossRef .
- D. Amans, A.-C. Chenus, G. Ledoux, C. Dujardin, C. Reynaud, O. Sublemontier, K. Masenelli-Varlot and O. Guillois, Diamond Relat. Mater. , 2009, 18 , 177–180 CrossRef CAS .
- S. Kumar, M. Nehra, D. Kedia, N. Dilbaghi, K. Tankeshwar and K.-H. Kim, Carbon , 2019, 143 , 678–699 CrossRef CAS .
- D. H. Jariwala, D. Patel and S. Wairkar, Mater. Sci. Eng., C , 2020, 113 , 110996 CrossRef CAS PubMed .
- I. Neitzel, V. N. Mochalin and Y. Gogotsi, Nanodiamonds , Elsevier, 2017, pp. 365–390 Search PubMed .
- H. Wang and Y. Cui, Carbon Energy , 2019, 1 , 13–18 CrossRef .
- H. Lee, H. Kim, S. Weon, W. Choi, Y. S. Hwang, J. Seo, C. Lee and J.-H. Kim, Environ. Sci. Technol. , 2016, 50 , 10134–10142 CrossRef CAS PubMed .
- N. A. Zambianco, T. A. Silva, H. Zanin, O. Fatibello-Filho and B. C. Janegitz, Diamond Relat. Mater. , 2019, 98 , 107512 CrossRef CAS .
- H. Lai, M. H. Stenzel and P. Xiao, Int. Mater. Rev. , 2020, 65 , 189–225 CrossRef CAS .
- U. T. Uthappa, O. R. Arvind, G. Sriram, D. Losic, H.-Y. Jung, M. Kigga and M. D. Kurkuri, J. Drug Delivery Sci. Technol. , 2020, 60 , 101993 CrossRef CAS .
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc. , 2004, 126 , 12736–12737 CrossRef CAS PubMed .
- J. Shen, Y. Zhu, X. Yang and C. Li, Chem. Commun. , 2012, 48 , 3686 RSC .
- X. Wang, Y. Feng, P. Dong and J. Huang, Front. Chem. , 2019, 7 , 671 CrossRef CAS PubMed .
- P. Karfa, S. De, K. C. Majhi, R. Madhuri and P. K. Sharma, Comprehensive Nanoscience and Nanotechnology , Elsevier, 2019, vol. 1–5, pp. 123–144 Search PubMed .
- M. Li, T. Chen, J. J. Gooding and J. Liu, ACS Sens. , 2019, 4 , 1732–1748 CrossRef CAS PubMed .
- P. Devi, S. Saini and K.-H. Kim, Biosens. Bioelectron. , 2019, 141 , 111158 CrossRef CAS PubMed .
- K. J. Mintz, Y. Zhou and R. M. Leblanc, Nanoscale , 2019, 11 , 4634–4652 RSC .
- H. Liu, T. Ye and C. Mao, Angew. Chem., Int. Ed. , 2007, 46 , 6473–6475 CrossRef CAS PubMed .
- D. Pan, J. Zhang, Z. Li, C. Wu, X. Yan and M. Wu, Chem. Commun. , 2010, 46 , 3681 RSC .
- R. Liu, D. Wu, S. Liu, K. Koynov, W. Knoll and Q. Li, Angew. Chem., Int. Ed. , 2009, 48 , 4598–4601 CrossRef CAS PubMed .
- Y. Dong, N. Zhou, X. Lin, J. Lin, Y. Chi and G. Chen, Chem. Mater. , 2010, 22 , 5895–5899 CrossRef CAS .
- J. Zhou, C. Booker, R. Li, X. Zhou, T.-K. Sham, X. Sun and Z. Ding, J. Am. Chem. Soc. , 2007, 129 , 744–745 CrossRef CAS PubMed .
- J. C. G. Esteves da Silva and H. M. R. Gonçalves, TrAC, Trends Anal. Chem. , 2011, 30 , 1327–1336 CrossRef CAS .
- Y. Wang and A. Hu, J. Mater. Chem. C , 2014, 2 , 6921 RSC .
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed. , 2010, 49 , 6726–6744 CrossRef CAS PubMed .
- Y. Li and S. Han, Microchem. J. , 2020, 154 , 104638 CrossRef CAS .
- J. Jiang, Y. He, S. Li and H. Cui, Chem. Commun. , 2012, 48 , 9634 RSC .
- Z.-F. Pu, Q.-L. Wen, Y.-J. Yang, X.-M. Cui, J. Ling, P. Liu and Q.-E. Cao, Spectrochim. Acta, Part A , 2020, 229 , 117944 CrossRef CAS PubMed .
- L. Cao, X. Wang, M. J. Meziani, F. Lu, H. Wang, P. G. Luo, Y. Lin, B. A. Harruff, L. M. Veca, D. Murray, S.-Y. Xie and Y.-P. Sun, J. Am. Chem. Soc. , 2007, 129 , 11318–11319 CrossRef CAS PubMed .
- K. Cheng, R. Qi, S. Lan, H. Wang, X. Zheng, C. Liu, D. Jia, L. Cao and D. Wang, Dyes Pigm. , 2020, 174 , 108106 CrossRef CAS .
- F. Copur, N. Bekar, E. Zor, S. Alpaydin and H. Bingol, Sens. Actuators, B , 2019, 279 , 305–312 CrossRef CAS .
- Z. Nan, C. Hao, X. Zhang, H. Liu and R. Sun, Spectrochim. Acta, Part A , 2020, 228 , 117717 CrossRef CAS PubMed .
- C. Wang, H. Shi, M. Yang, Y. Yan, E. Liu, Z. Ji and J. Fan, J. Photochem. Photobiol., A , 2020, 391 , 112374 CrossRef CAS .
- W. Li, S. Huang, H. Wen, Y. Luo, J. Cheng, Z. Jia, P. Han and W. Xue, Anal. Bioanal. Chem. , 2020, 412 , 993–1002 CrossRef CAS PubMed .
- X. Tian, H. Peng, Y. Li, C. Yang, Z. Zhou and Y. Wang, Sens. Actuators, B , 2017, 243 , 1002–1009 CrossRef CAS .
- G. Han, J. Zhao, R. Zhang, X. Tian, Z. Liu, A. Wang, R. Liu, B. Liu, M. Han, X. Gao and Z. Zhang, Angew. Chem., Int. Ed. , 2019, 58 , 7087–7091 CrossRef CAS PubMed .
- H. Li, F.-Q. Shao, H. Huang, J.-J. Feng and A.-J. Wang, Sens. Actuators, B , 2016, 226 , 506–511 CrossRef CAS .
- Y. Sun, S. Zheng, L. Liu, Y. Kong, A. Zhang, K. Xu and C. Han, Nanoscale Res. Lett. , 2020, 15 , 55 CrossRef CAS PubMed .
- Y. Wei, L. Chen, J. Wang, X. Liu, Y. Yang and S. Yu, Opt. Mater. , 2020, 100 , 109647 CrossRef CAS .
- T. Song, Y. Zhao, T. Wang, J. Li, Z. Jiang and P. Yang, J. Fluoresc. , 2020, 30 , 81–89 CrossRef CAS PubMed .
- G. Huang, Y. Lin, L. Zhang, Z. Yan, Y. Wang and Y. Liu, Sci. Rep. , 2019, 9 , 19651 CrossRef CAS PubMed .
- X. Wang, L. Cao, F. Lu, M. J. Meziani, H. Li, G. Qi, B. Zhou, B. A. Harruff, F. Kermarrec and Y.-P. Sun, Chem. Commun. , 2009, 3774 RSC .
- Y. Li, B.-P. Zhang, J.-X. Zhao, Z.-H. Ge, X.-K. Zhao and L. Zou, Appl. Surf. Sci. , 2013, 279 , 367–373 CrossRef CAS .
- Y. Zhang, Y. Zhao, Z. Xu, H. Su, X. Bian, S. Zhang, X. Dong, L. Zeng, T. Zeng, M. Feng, L. Li and V. K. Sharma, Appl. Catal., B , 2020, 262 , 118306 CrossRef CAS .
- L. Zhang, J. Zhang, Y. Xia, M. Xun, H. Chen, X. Liu and X. Yin, Int. J. Mol. Sci. , 2020, 21 , 1052 CrossRef CAS PubMed .
- Y.-Y. Li, Y. Si, B.-X. Zhou, T. Huang, W.-Q. Huang, W. Hu, A. Pan, X. Fan and G.-F. Huang, Nanoscale , 2020, 12 , 3135–3145 RSC .
- N. Karousis, I. Suarez-Martinez, C. P. Ewels and N. Tagmatarchis, Chem. Rev. , 2016, 116 , 4850–4883 CrossRef CAS PubMed .
- M. Zhang and M. Yudasaka, Carbon Nanostructures , Springer International Publishing, 2016, pp. 77–107 Search PubMed .
- S. Iijima, M. Yudasaka, R. Yamada, S. Bandow, K. Suenaga, F. Kokai and K. Takahashi, Chem. Phys. Lett. , 1999, 309 , 165–170 CrossRef CAS .
- S. Zhu and G. Xu, Nanoscale , 2010, 2 , 2538 RSC .
- J. Fan, M. Yudasaka, J. Miyawaki, K. Ajima, K. Murata and S. Iijima, J. Phys. Chem. B , 2006, 110 , 1587–1591 CrossRef CAS PubMed .
- Z. Zhang, S. Han, C. Wang, J. Li and G. Xu, Nanomaterials , 2015, 5 , 1732–1755 CrossRef CAS PubMed .
- Z. Liu, W. Zhang, W. Qi, W. Gao, S. Hanif, M. Saqib and G. Xu, Chem. Commun. , 2015, 51 , 4256–4258 RSC .
- T. Ohba, K. Murata, K. Kaneko, W. A. Steele, F. Kokai, K. Takahashi, D. Kasuya, M. Yudasaka and S. Iijima, Nano Lett. , 2001, 1 , 371–373 CrossRef CAS .
- D. Ebenezer, M. Jagannatham, M. S. Lahari, P. Bhuwaneshswar and H. Prathap, Diamond Relat. Mater. , 2016, 70 , 26–32 CrossRef CAS .
- H. Wang, L. Pan, Y. Liu, Y. Ye and S. Yao, J. Electroanal. Chem. , 2020, 862 , 113955 CrossRef CAS .
- T. Yoshitake, Y. Shimakawa, S. Kuroshima, H. Kimura, T. Ichihashi, Y. Kubo, D. Kasuya, K. Takahashi, F. Kokai, M. Yudasaka and S. Iijima, Phys. B , 2002, 323 , 124–126 CrossRef CAS .
- A. Thomas, Nat. Commun. , 2020, 11 , 4985 CrossRef CAS .
- R. W. Derlet and T. E. Albertson, West. J. Med. , 1986, 145 , 493–496 CAS .
- R. Mishra, J. Militky and M. Venkataraman, Nanotechnology in Textiles: Theory and Application , 2018, pp. 311–353 Search PubMed .
- N. Pal, Adv. Colloid Interface Sci. , 2020, 280 , 102156 CrossRef CAS PubMed .
- M. E. Davis, Nature , 2002, 417 , 813–821 CrossRef CAS .
- S. Polarz and B. Smarsly, J. Nanosci. Nanotechnol. , 2002, 2 , 581–612 CrossRef CAS .
- B. Szczęśniak, S. Borysiuk, J. Choma and M. Jaroniec, Mater. Horiz. , 2020, 7 , 1457–1473 RSC .
- D. Chen, L. Wei, J. Li and Q. Wu, J. Energy Storage , 2020, 30 , 101525 CrossRef .
- L. Zhang and M. Jaroniec, Chem. Soc. Rev. , 2020, 49 , 6039–6055 RSC .
- Y. Li, H. Cao and J. Yu, ACS Nano , 2018, 12 , 4096–4104 CrossRef CAS .
- Z. Yang, Z. Xie, H. Liu, F. Yan and H. Ju, Adv. Funct. Mater. , 2008, 18 , 3991–3998 CrossRef CAS .
- S. Bagheri, M. M. Amini, M. Behbahani and G. Rabiee, Microchem. J. , 2019, 145 , 460–469 CrossRef CAS .
- Y. Zhou, G. Quan, Q. Wu, X. Zhang, B. Niu, B. Wu, Y. Huang, X. Pan and C. Wu, Acta Pharm. Sin. B , 2018, 8 , 165–177 CrossRef PubMed .
- X. Wang, C. Li, N. Fan, J. Li, Z. He and J. Sun, Mater. Sci. Eng., C , 2017, 78 , 370–375 CrossRef CAS PubMed .
- V. Zeleňák, D. Halamová, M. Almáši, L. Žid, A. Zeleňáková and O. Kapusta, Appl. Surf. Sci. , 2018, 443 , 525–534 CrossRef .
- Y. Wang, Q. Zhao, N. Han, L. Bai, J. Li, J. Liu, E. Che, L. Hu, Q. Zhang, T. Jiang and S. Wang, Nanomedicine , 2015, 11 , 313–327 CrossRef CAS PubMed .
- K. Na and G. A. Somorjai, Catal. Lett. , 2015, 145 , 193–213 CrossRef CAS .
- K. Na, M. Choi and R. Ryoo, Microporous Mesoporous Mater. , 2013, 166 , 3–19 CrossRef CAS .
- S. Zhang, Z. Zhang, H. Li, Z. Yu, Q. Huang, Z. Qiao, L. Zong, L. Yan, J. Li and J. Kang, Chem. Eng. J. , 2020, 383 , 123097 CrossRef CAS .
- A. Mohammadi Zardkhoshoui and S. S. Hosseiny Davarani, Dalton Trans. , 2020, 49 , 10028–10041 RSC .
- S. Ouyang, P. Li, H. Xu, H. Tong, L. Liu and J. Ye, ACS Appl. Mater. Interfaces , 2014, 6 , 22726–22732 CrossRef CAS PubMed .
- T. Xu, H. Zheng and P. Zhang, J. Hazard. Mater. , 2020, 388 , 121746 CrossRef CAS PubMed .
- W. Luc and F. Jiao, Acc. Chem. Res. , 2016, 49 , 1351–1358 CrossRef CAS PubMed .
- T. Zhang, M. Paulose, R. Neupane, L. A. Schaffer, D. B. Rana, J. Su, L. Guo and O. K. Varghese, Sol. Energy Mater. Sol. Cells , 2020, 209 , 110472 CrossRef CAS .
- G. Schmid, J. Mater. Chem. , 2002, 12 , 1231–1238 RSC .
- A. Larsson, G. Abbondanza, W. Linpé, F. Carlà, P. Mousley, C. Hetherington, E. Lundgren and G. S. Harlow, J. Electrochem. Soc. , 2020, 167 , 122514 CrossRef CAS .
- P. G. Schiavi, P. Altimari, A. Rubino and F. Pagnanelli, Electrochim. Acta , 2018, 259 , 711–722 CrossRef CAS .
- A. Yadav, M. S. Bobji and S. J. Bull, J. Nanosci. Nanotechnol. , 2019, 19 , 4254–4259 CrossRef CAS PubMed .
- F. Yang, J. Tang, B. Shao, S. Gao and X. Liu, Nano-Struct. Nano-Objects , 2019, 18 , 100287 CrossRef .
- G. Rajeev, B. Prieto Simon, L. F. Marsal and N. H. Voelcker, Adv. Healthcare Mater. , 2018, 7 , 1700904 CrossRef PubMed .
- M. R. Benzigar, S. N. Talapaneni, S. Joseph, K. Ramadass, G. Singh, J. Scaranto, U. Ravon, K. Al-Bahily and A. Vinu, Chem. Soc. Rev. , 2018, 47 , 2680–2721 RSC .
- N. Baig and T. A. Saleh, Composites, Part B , 2019, 173 , 106805 CrossRef .
- N. Baig and T. A. Saleh, Global Challenges , 2019, 3 , 1800115 CrossRef PubMed .
- N. Baig and T. A. Saleh, Chem. – Asian J. , 2021, 16 , 329–341, DOI: 10.1002/asia.202001368 .
- S. Kundu, I. H. Chowdhury and M. K. Naskar, ACS Omega , 2018, 3 , 9888–9898 CrossRef CAS PubMed .
- T. A. Saleh, N. Baig, H. A. Othman and A. M. Al Harith, Chem. Eng. J. , 2021, 407 , 126216 CrossRef CAS .
- B. Szczęśniak, J. Phuriragpitikhon, J. Choma and M. Jaroniec, J. Mater. Chem. A , 2020, 8 , 18464–18491 RSC .
- Z. Bi, Q. Kong, Y. Cao, G. Sun, F. Su, X. Wei, X. Li, A. Ahmad, L. Xie and C.-M. Chen, J. Mater. Chem. A , 2019, 7 , 16028–16045 RSC .
- Z. Li, D. Guo, Y. Liu, H. Wang and L. Wang, Chem. Eng. J. , 2020, 397 , 125418 CrossRef CAS .
- Z. Li, J. Xu, D. Sun, T. Lin and F. Huang, ACS Appl. Nano Mater. , 2020, 3 , 1564–1570 CrossRef CAS .
- A. O. Abo El Naga, S. A. Shaban and F. Y. A. El Kady, J. Taiwan Inst. Chem. Eng. , 2018, 93 , 363–373 CrossRef CAS .
- C. Jiao, Y. Wang, M. Li, Q. Wu, C. Wang and Z. Wang, J. Magn. Magn. Mater. , 2016, 407 , 24–30 CrossRef CAS .
- M. Anbia and S. Amirmahmoodi, Arabian J. Chem. , 2016, 9 , S319–S325 CrossRef CAS .
- H. Wang, J. Jia, P. Song, Q. Wang, D. Li, S. Min, C. Qian, L. Wang, Y. F. Li, C. Ma, T. Wu, J. Yuan, M. Antonietti and G. A. Ozin, Angew. Chem. , 2017, 129 , 7955–7960 CrossRef .
- Y. Zhang, H. Sun, Y. Qiu, E. Zhang, T. Ma, G. Gao, C. Cao, Z. Ma and P. Hu, Energy , 2018, 165 , 537–548 CrossRef CAS .
- H. Luo, W.-J. Jiang, Y. Zhang, S. Niu, T. Tang, L.-B. Huang, Y.-Y. Chen, Z. Wei and J.-S. Hu, Carbon , 2018, 128 , 97–105 CrossRef CAS .
- J. Tang, J. Liu, N. L. Torad, T. Kimura and Y. Yamauchi, Nano Today , 2014, 9 , 305–323 CrossRef CAS .
- R. Brilmayer, C. Förster, L. Zhao and A. Andrieu-Brunsen, Curr. Opin. Biotechnol. , 2020, 63 , 200–209 CrossRef CAS .
- O. K. Farha, A. Özgür Yazaydın, I. Eryazici, C. D. Malliakas, B. G. Hauser, M. G. Kanatzidis, S. T. Nguyen, R. Q. Snurr and J. T. Hupp, Nat. Chem. , 2010, 2 , 944–948 CrossRef CAS PubMed .
- O. K. Farha, I. Eryazici, N. C. Jeong, B. G. Hauser, C. E. Wilmer, A. A. Sarjeant, R. Q. Snurr, S. T. Nguyen, A. Ö. Yazaydın and J. T. Hupp, J. Am. Chem. Soc. , 2012, 134 , 15016–15021 CrossRef CAS PubMed .
- I. M. Hönicke, I. Senkovska, V. Bon, I. A. Baburin, N. Bönisch, S. Raschke, J. D. Evans and S. Kaskel, Angew. Chem., Int. Ed. , 2018, 57 , 13780–13783 CrossRef PubMed .
- V. Chernikova, O. Shekhah, Y. Belmabkhout and M. Eddaoudi, ACS Appl. Nano Mater. , 2020, 3 , 6432–6439 CrossRef CAS .
- H. A. Patel, S. Hyun Je, J. Park, D. P. Chen, Y. Jung, C. T. Yavuz and A. Coskun, Nat. Commun. , 2013, 4 , 1357 CrossRef PubMed .
- Y. Zhang, S. Wei, F. Liu, Y. Du, S. Liu, Y. Ji, T. Yokoi, T. Tatsumi and F.-S. Xiao, Nano Today , 2009, 4 , 135–142 CrossRef CAS .
- M. Rose, ChemCatChem , 2014, 6 , 1166–1182 CAS .
- N. Wang, Q. Sun and J. Yu, Adv. Mater. , 2019, 31 , 1803966 CrossRef PubMed .
- C. R. Martin, Science , 1994, 266 , 1961–1966 CrossRef CAS PubMed .
- H. Zhao, X. Chen, G. Wang, Y. Qiu and L. Guo, 2D Mater. , 2019, 6 , 032002, DOI: 10.1088/2053-1583/ab1169 .
- C. Tan, X. Cao, X.-J. Wu, Q. He, J. Yang, X. Zhang, J. Chen, W. Zhao, S. Han, G.-H. Nam, M. Sindoro and H. Zhang, Chem. Rev. , 2017, 117 , 6225–6331 CrossRef CAS PubMed .
- A. A. F. K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos and I. V. Grigorieva, Science , 2004, 306 , 666–669 CrossRef PubMed .
- F. Bai, L. Xu, X. Zhai, X. Chen and W. Yang, Adv. Energy Mater. , 2020, 10 , 1–19 Search PubMed .
- K. Takeda and K. Shiraishi, Teorìâ ta Metod. Fìzičnogo Vihovannâ , 1994, 50 , 14916–14922 CAS .
- G. G. Guzmán-Verri and L. C. Lew Yan Voon, Phys. Rev. B: Condens. Matter Mater. Phys. , 2007, 76 , 075131, DOI: 10.1103/PhysRevB.76.075131 .
- S. Cahangirov, M. Topsakal, E. Aktürk, H. Šahin and S. Ciraci, Phys. Rev. Lett. , 2009, 102 , 1–4 CrossRef PubMed .
- N. D. Drummond, V. Zólyomi and V. I. Fal’Ko, Phys. Rev. B: Condens. Matter Mater. Phys. , 2012, 85 , 1–7 CrossRef .
- Z. Ni, Q. Liu, K. Tang, J. Zheng, J. Zhou, R. Qin, Z. Gao, D. Yu and J. Lu, Nano Lett. , 2012, 12 , 113–118 CrossRef CAS PubMed .
- C. C. Liu, W. Feng and Y. Yao, Phys. Rev. Lett. , 2011, 107 , 1–4 Search PubMed .
- F. Liu, C. C. Liu, K. Wu, F. Yang and Y. Yao, Phys. Rev. Lett. , 2013, 111 , 1–5 Search PubMed .
- C. Xu, G. Luo, Q. Liu, J. Zheng, Z. Zhang, S. Nagase, Z. Gao and J. Lu, Nanoscale , 2012, 4 , 3111–3117 RSC .
- J. Zhao, H. Liu, Z. Yu, R. Quhe, S. Zhou, Y. Wang, C. C. Liu, H. Zhong, N. Han, J. Lu, Y. Yao and K. Wu, Prog. Mater. Sci. , 2016, 83 , 24–151 CrossRef CAS .
- M. De Crescenzi, P. Castrucci, M. Scarselli, M. Diociaiuti, P. S. Chaudhari, C. Balasubramanian, T. M. Bhave and S. V. Bhoraskar, Appl. Phys. Lett. , 2005, 86 , 1–3 CrossRef .
- S. Yamada and H. Fujiki, Jpn. J. Appl. Phys., Part 2 , 2006, 45 , 18–21 CrossRef .
- H. Nakano, T. Mitsuoka, M. Harada, K. Horibuchi, H. Nozaki, N. Takahashi, T. Nonaka, Y. Seno and H. Nakamura, Angew. Chem., Int. Ed. , 2006, 45 , 6303–6306 CrossRef CAS PubMed .
- P. De Padova, C. Quaresima, C. Ottaviani, P. M. Sheverdyaeva, P. Moras, C. Carbone, D. Topwal, B. Olivieri, A. Kara, H. Oughaddou, B. Aufray and G. Le Lay, Appl. Phys. Lett. , 2010, 96 , 1–4 CrossRef .
- B. Aufray, A. Kara, Ś. Vizzini, H. Oughaddou, C. Ĺandri, B. Ealet and G. Le-Lay, Appl. Phys. Lett. , 2010, 96 , 1–4 CrossRef .
- C. Léandri, H. Oughaddou, B. Aufray, J. M. Gay, G. Le Lay, A. Ranguis and Y. Garreau, Surf. Sci. , 2007, 601 , 262–267 CrossRef .
- P. Vogt, P. De Padova, C. Quaresima, J. Avila, E. Frantzeskakis, M. C. Asensio, A. Resta, B. Ealet and G. Le Lay, Phys. Rev. Lett. , 2012, 108 , 155501 CrossRef .
- A. Fleurence, R. Friedlein, T. Ozaki, H. Kawai, Y. Wang and Y. Yamada-Takamura, Phys. Rev. Lett. , 2012, 108 , 1–5 CrossRef PubMed .
- L. Meng, Y. Wang, L. Zhang, S. Du, R. Wu, L. Li, Y. Zhang, G. Li, H. Zhou, W. A. Hofer and H. J. Gao, Nano Lett. , 2013, 13 , 685–690 CrossRef CAS PubMed .
- T. Aizawa, S. Suehara and S. Otani, J. Phys. Chem. C , 2014, 118 , 23049–23057 CrossRef CAS .
- D. Chiappe, E. Scalise, E. Cinquanta, C. Grazianetti, B. Van Den Broek, M. Fanciulli, M. Houssa and A. Molle, Adv. Mater. , 2014, 26 , 2096–2101 CrossRef CAS PubMed .
- B. Feng, Z. Ding, S. Meng, Y. Yao, X. He, P. Cheng, L. Chen and K. Wu, Nano Lett. , 2012, 12 , 3507–3511 CrossRef CAS PubMed .
- D. Chiappe, C. Grazianetti, G. Tallarida, M. Fanciulli and A. Molle, Adv. Mater. , 2012, 24 , 5088–5093 CrossRef CAS PubMed .
- P. De Padova, O. Kubo, B. Olivieri, C. Quaresima, T. Nakayama, M. Aono and G. Le Lay, Nano Lett. , 2012, 12 , 5500–5503 CrossRef CAS PubMed .
- L. Tao, E. Cinquanta, D. Chiappe, C. Grazianetti, M. Fanciulli, M. Dubey, A. Molle and D. Akinwande, Nat. Nanotechnol. , 2015, 10 , 227–231 CrossRef CAS PubMed .
- J. Liu, Y. Yang, P. Lyu, P. Nachtigall and Y. Xu, Adv. Mater. , 2018, 30 , 1–7 CAS .
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, Adv. Mater. , 2014, 26 , 992–1005 CrossRef CAS .
- M. Ghidiu, M. R. Lukatskaya, M. Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature , 2014, 516 , 78–81 CrossRef CAS PubMed .
- X. Sang, Y. Xie, M. W. Lin, M. Alhabeb, K. L. Van Aken, Y. Gogotsi, P. R. C. Kent, K. Xiao and R. R. Unocic, ACS Nano , 2016, 10 , 9193–9200 CrossRef CAS PubMed .
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater. , 2011, 23 , 4248–4253 CrossRef CAS PubMed .
- J. Pang, R. G. Mendes, A. Bachmatiuk, L. Zhao, H. Q. Ta, T. Gemming, H. Liu, Z. Liu and M. H. Rummeli, Chem. Soc. Rev. , 2019, 48 , 72–133 RSC .
- Z. Li and Y. Wu, Small , 2019, 15 , 1–10 Search PubMed .
- J. Ran, G. Gao, F. T. Li, T. Y. Ma, A. Du and S. Z. Qiao, Nat. Commun. , 2017, 8 , 13907, DOI: 10.1038/ncomms13907 .
- Z. W. Seh, K. D. Fredrickson, B. Anasori, J. Kibsgaard, A. L. Strickler, M. R. Lukatskaya, Y. Gogotsi, T. F. Jaramillo and A. Vojvodic, ACS Energy Lett. , 2016, 1 , 589–594 CrossRef CAS .
- A. Kumar and A. Bhan, Chem. Eng. Sci. , 2019, 197 , 371–378 CrossRef CAS .
- D. Wang, Z. Wang, L. Wang, L. Hu and J. Jin, Nanoscale , 2015, 7 , 17649–17652 RSC .
- J. Abraham, K. S. Vasu, C. D. Williams, K. Gopinadhan, Y. Su, C. T. Cherian, J. Dix, E. Prestat, S. J. Haigh, I. V. Grigorieva, P. Carbone, A. K. Geim and R. R. Nair, Nat. Nanotechnol. , 2017, 12 , 546–550 CrossRef CAS PubMed .
- G. Reina, J. M. González-Domínguez, A. Criado, E. Vázquez, A. Bianco and M. Prato, Chem. Soc. Rev. , 2017, 46 , 4400–4416 RSC .
- Y. Zhang, H. Jiang, Y. Lin, H. Liu, Q. He, C. Wu, T. Duan and L. Song, Adv. Mater. Interfaces , 2018, 5 , 1–9 Search PubMed .
- T. Y. Ma, J. L. Cao, M. Jaroniec and S. Z. Qiao, Angew. Chem. , 2016, 128 , 1150–1154 CrossRef .
- M. Yu, S. Zhou, Z. Wang, J. Zhao and J. Qiu, Nano Energy , 2018, 44 , 181–190 CrossRef CAS .
- N. Hao, Y. Wei, J. Wang, Z. Wang, Z. Zhu, S. Zhao, M. Han and X. Huang, RSC Adv. , 2018, 8 , 20576–20584 RSC .
- L. Zhao, B. Dong, S. Li, L. Zhou, L. Lai, Z. Wang, S. Zhao, M. Han, K. Gao, M. Lu, X. Xie, B. Chen, Z. Liu, X. Wang, H. Zhang, H. Li, J. Liu, H. Zhang, X. Huang and W. Huang, ACS Nano , 2017, 11 , 5800–5807 CrossRef CAS PubMed .
- B. Lyu, Y. Choi, H. Jing, C. Qian, H. Kang, S. Lee and J. H. Cho, Adv. Mater. , 2020, 32 , 2–9 Search PubMed .
- L. Li, Y. Yu, G. J. Ye, Q. Ge, X. Ou, H. Wu, D. Feng, X. H. Chen and Y. Zhang, Nat. Nanotechnol. , 2014, 9 , 372–377 CrossRef CAS PubMed .
- M. Buscema, D. J. Groenendijk, S. I. Blanter, G. A. Steele, H. S. J. Van Der Zant and A. Castellanos-Gomez, Nano Lett. , 2014, 14 , 3347–3352 CrossRef CAS .
- G. Slotman, A. Rudenko, E. Van Veen, M. I. Katsnelson, R. Roldán and S. Yuan, Phys. Rev. B , 2018, 98 , 1–7 CrossRef .
- C. Hao, B. Yang, F. Wen, J. Xiang, L. Li, W. Wang, Z. Zeng, B. Xu, Z. Zhao, Z. Liu and Y. Tian, Adv. Mater. , 2016, 28 , 3194–3201 CrossRef CAS PubMed .
- C. M. Park and H. J. Sohn, Adv. Mater. , 2007, 19 , 2465–2468 CrossRef CAS .
- M. Pumera and Z. Sofer, Adv. Mater. , 2017, 29 , 1605299, DOI: 10.1002/adma.201605299 .
- S. Zhang, M. Xie, F. Li, Z. Yan, Y. Li, E. Kan, W. Liu, Z. Chen and H. Zeng, Angew. Chem., Int. Ed. , 2016, 55 , 1666–1669 CrossRef CAS PubMed .
- M. Wu, H. Fu, L. Zhou, K. Yao and X. C. Zeng, Nano Lett. , 2015, 15 , 3557–3562 CrossRef CAS PubMed .
- P. Ares, F. Aguilar-Galindo, D. Rodríguez-San-Miguel, D. A. Aldave, S. Díaz-Tendero, M. Alcamí, F. Martín, J. Gómez-Herrero and F. Zamora, Adv. Mater. , 2016, 28 , 6332–6336 CrossRef CAS PubMed .
- Y. Wang and Y. Ding, Nanoscale Res. Lett. , 2015, 10 , 1–10 CrossRef PubMed .
- J. Ji, X. Song, J. Liu, Z. Yan, C. Huo, S. Zhang, M. Su, L. Liao, W. Wang, Z. Ni, Y. Hao and H. Zeng, Nat. Commun. , 2016, 7 , 1–9 Search PubMed .
- H. Liu, A. T. Neal, Z. Zhu, Z. Luo, X. Xu, D. Tománek and P. D. Ye, ACS Nano , 2014, 8 , 4033–4041 CrossRef CAS PubMed .
- A. Favron, E. Gaufrès, F. Fossard, A. L. Phaneuf-Laheureux, N. Y. W. Tang, P. L. Lévesque, A. Loiseau, R. Leonelli, S. Francoeur and R. Martel, Nat. Mater. , 2015, 14 , 826–832 CrossRef CAS PubMed .
- G. Abellán, S. Wild, V. Lloret, N. Scheuschner, R. Gillen, U. Mundloch, J. Maultzsch, M. Varela, F. Hauke and A. Hirsch, J. Am. Chem. Soc. , 2017, 139 , 10432–10440 CrossRef PubMed .
- C. Gibaja, D. Rodriguez-San-Miguel, P. Ares, J. Gómez-Herrero, M. Varela, R. Gillen, J. Maultzsch, F. Hauke, A. Hirsch, G. Abellán and F. Zamora, Angew. Chem. , 2016, 128 , 14557–14561 CrossRef .
- O. Ü. Aktürk, V. O. Özçelik and S. Ciraci, Phys. Rev. B: Condens. Matter Mater. Phys. , 2015, 91 , 1–10 CrossRef .
- S. K. Gupta, Y. Sonvane, G. Wang and R. Pandey, Chem. Phys. Lett. , 2015, 641 , 169–172 CrossRef CAS .
- G. Wang, R. Pandey and S. P. Karna, ACS Appl. Mater. Interfaces , 2015, 7 , 11490–11496 CrossRef CAS PubMed .
- S. Zhang, Z. Yan, Y. Li, Z. Chen and H. Zeng, Angew. Chem. , 2015, 127 , 3155–3158 CrossRef .
- C. Kamal and M. Ezawa, Phys. Rev. B: Condens. Matter Mater. Phys. , 2015, 91 , 1–10 CrossRef .
- Z. Zhang, J. Xie, D. Yang, Y. Wang, M. Si and D. Xue, Appl. Phys. Express , 2015, 8 , 055201, DOI: 10.7567/APEX.8.055201 .
- M. S. Khan, A. Srivastava and R. Pandey, RSC Adv. , 2016, 6 , 72634–72642 RSC .
- M. S. Khan, V. Ranjan and A. Srivastava, Proc. – 2015 IEEE Int. Symp. Nanoelectron. Inf. Syst. iNIS 2015 , 2016, 248–251.
- S. Guo, Y. Zhang, Y. Ge, S. Zhang, H. Zeng and H. Zhang, Adv. Mater. , 2019, 31 , 1–19 Search PubMed .
- Y. Liu, T. Wang, J. Robertson, J. Luo, Y. Guo and D. Liu, J. Phys. Chem. C , 2020, 124 , 7441–7448 CrossRef CAS .
- G. Abellán, P. Ares, S. Wild, E. Nuin, C. Neiss, D. R. S. Miguel, P. Segovia, C. Gibaja, E. G. Michel, A. Görling, F. Hauke, J. Gómez-Herrero, A. Hirsch and F. Zamora, Angew. Chem., Int. Ed. , 2017, 56 , 14389–14394 CrossRef PubMed .
- S. Wolff, R. Gillen, M. Assebban, G. Abellán and J. Maultzsch, Phys. Rev. Lett. , 2020, 124 , 126101 CrossRef CAS PubMed .
- T. García-Mendiola, C. Gutiérrez-Sánchez, C. Gibaja, I. Torres, C. Busó-Rogero, F. Pariente, J. Solera, Z. Razavifar, J. J. Palacios, F. Zamora and E. Lorenzo, ACS Appl. Nano Mater. , 2020, 3 , 3625–3633 CrossRef .
- T. Xue, W. Liang, Y. Li, Y. Sun, Y. Xiang, Y. Zhang, Z. Dai, Y. Duo, L. Wu, K. Qi, B. N. Shivananju, L. Zhang, X. Cui, H. Zhang and Q. Bao, Nat. Commun. , 2019, 10 , 1–9 CrossRef PubMed .
- Z. Wang, Z. Wang, Z. Wang, M. Zhao, Y. Zhou, B. Zhao, Y. Miao, P. Liu, Y. Hao, H. Wang, B. Xu, Y. Wu and S. Yin, 2D Mater. , 2019, 6 , 045017, DOI: 10.1088/2053-1583/ab277d .
- E. Martínez-Periñán, M. P. Down, C. Gibaja, E. Lorenzo, F. Zamora and C. E. Banks, Adv. Energy Mater. , 2018, 8 , 1–8 Search PubMed .
- Y. H. Luo, C. Chen, C. He, Y. Y. Zhu, D. L. Hong, X. T. He, P. J. An, H. S. Wu and B. W. Sun, ACS Appl. Mater. Interfaces , 2018, 10 , 28860–28867 CrossRef CAS PubMed .
- A. Abhervé, S. Mañas-Valero, M. Clemente-León and E. Coronado, Chem. Sci. , 2015, 6 , 4665–4673 RSC .
- H. S. Wang, J. Li, J. Y. Li, K. Wang, Y. Ding and X. H. Xia, NPG Asia Mater. , 2017, 9 , 1–9 Search PubMed .
- W. J. Song, Talanta , 2017, 170 , 74–80 CrossRef CAS PubMed .
- Y. Ding, Y. P. Chen, X. Zhang, L. Chen, Z. Dong, H. L. Jiang, H. Xu and H. C. Zhou, J. Am. Chem. Soc. , 2017, 139 , 9136–9139 CrossRef CAS PubMed .
- T. Kambe, R. Sakamoto, K. Hoshiko, K. Takada, M. Miyachi, J. H. Ryu, S. Sasaki, J. Kim, K. Nakazato, M. Takata and H. Nishihara, J. Am. Chem. Soc. , 2013, 135 , 2462–2465 CrossRef CAS PubMed .
- L. Cao, Z. Lin, F. Peng, W. Wang, R. Huang, C. Wang, J. Yan, J. Liang, Z. Zhang, T. Zhang, L. Long, J. Sun and W. Lin, Angew. Chem. , 2016, 128 , 5046–5050 CrossRef .
- T. Rodenas, I. Luz, G. Prieto, B. Seoane, H. Miro, A. Corma, F. Kapteijn, F. X. Llabrés I Xamena and J. Gascon, Nat. Mater. , 2015, 14 , 48–55 CrossRef CAS PubMed .
- S. C. Junggeburth, L. Diehl, S. Werner, V. Duppel, W. Sigle and B. V. Lotsch, J. Am. Chem. Soc. , 2013, 135 , 6157–6164 CrossRef CAS PubMed .
- Y. Zheng, S. Zheng, Y. Xu, H. Xue, C. Liu and H. Pang, Chem. Eng. J. , 2019, 373 , 1319–1328 CrossRef CAS .
- Y. Wang, Y. Liu, H. Wang, W. Liu, Y. Li, J. Zhang, H. Hou and J. Yang, ACS Appl. Energy Mater. , 2019, 2 , 2063–2071 CrossRef CAS .
- C. Li, X. Hu, W. Tong, W. Yan, X. Lou, M. Shen and B. Hu, ACS Appl. Mater. Interfaces , 2017, 9 , 29829–29838 CrossRef CAS PubMed .
- A. J. Clough, J. W. Yoo, M. H. Mecklenburg and S. C. Marinescu, J. Am. Chem. Soc. , 2015, 137 , 118–121 CrossRef CAS PubMed .
- R. Dong, M. Pfeffermann, H. Liang, Z. Zheng, X. Zhu, J. Zhang and X. Feng, Angew. Chem., Int. Ed. , 2015, 54 , 12058–12063 CrossRef CAS PubMed .
- B. C. Patra, S. Khilari, R. N. Manna, S. Mondal, D. Pradhan, A. Pradhan and A. Bhaumik, ACS Catal. , 2017, 7 , 6120–6127 CrossRef CAS .
- Y. Xu, B. Li, S. Zheng, P. Wu, J. Zhan, H. Xue, Q. Xu and H. Pang, J. Mater. Chem. A , 2018, 6 , 22070–22076 RSC .
- G. Hai, X. Jia, K. Zhang, X. Liu, Z. Wu and G. Wang, Nano Energy , 2018, 44 , 345–352 CrossRef CAS .
- M. Jian, H. Liu, T. Williams, J. Ma, H. Wang and X. Zhang, Chem. Commun. , 2017, 53 , 13161–13164 RSC .
- E. M. Miner, T. Fukushima, D. Sheberla, L. Sun, Y. Surendranath and M. Dinca, Nat. Commun. , 2016, 7 , 1–7 Search PubMed .
- K. Zhao, S. Liu, G. Ye, X. Wei, Y. Su, W. Zhu, Z. Zhou and Z. He, ChemSusChem , 2020, 13 , 1556–1567 CrossRef CAS PubMed .
- N. Kornienko, Y. Zhao, C. S. Kley, C. Zhu, D. Kim, S. Lin, C. J. Chang, O. M. Yaghi and P. Yang, J. Am. Chem. Soc. , 2015, 137 , 14129–14135 CrossRef CAS PubMed .
- S. He, Y. Chen, Z. Zhang, B. Ni, W. He and X. Wang, Chem. Sci. , 2016, 7 , 7101–7105 RSC .
- H. Xu, J. Gao, X. Qian, J. Wang, H. He, Y. Cui, Y. Yang, Z. Wang and G. Qian, J. Mater. Chem. A , 2016, 4 , 10900–10905 RSC .
- W. P. Lustig, S. Mukherjee, N. D. Rudd, A. V. Desai, J. Li and S. K. Ghosh, Chem. Soc. Rev. , 2017, 46 , 3242–3285 RSC .
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev. , 2012, 112 , 1105–1125 CrossRef CAS PubMed .
- M. Zhao, Y. Wang, Q. Ma, Y. Huang, X. Zhang, J. Ping, Z. Zhang, Q. Lu, Y. Yu, H. Xu, Y. Zhao and H. Zhang, Adv. Mater. , 2015, 27 , 7372–7378 CrossRef CAS PubMed .
- H. S. Wang, H. L. Liu, K. Wang, Y. Ding, J. J. Xu, X. H. Xia and H. Y. Chen, Anal. Chem. , 2017, 89 , 11366–11371 CrossRef CAS PubMed .
- Y. Zhang, Y. Luo, J. Tian, A. M. Asiri, A. O. Al-Youbi and X. Sun, PLoS One , 2012, 7 , e30426, DOI: 10.1371/journal.pone.0030426 .
- Y. Y. Fan, Z. L. Mou, M. Wang, J. Li, J. Zhang, F. Q. Dang and Z. Q. Zhang, Anal. Chem. , 2018, 90 , 13708–13713 CrossRef CAS PubMed .
- C. He, K. Lu and W. Lin, J. Am. Chem. Soc. , 2014, 136 , 12253–12256 CrossRef CAS PubMed .
- Y. Peng, Y. Li, Y. Ban, H. Jin, W. Jiao, X. Liu and W. Yang, Science , 2014, 346 , 1356–1359, DOI: 10.1126/science.1254227 .
- Y. Peng, Y. Li, Y. Ban and W. Yang, Angew. Chem. , 2017, 129 , 9889–9893 CrossRef .
- S. Jiang, X. Shi, F. Sun and G. Zhu, Chem. – Asian J. , 2020, 3 , 1–9 Search PubMed .
- Y. Cheng, X. Wang, C. Jia, Y. Wang, L. Zhai, Q. Wang and D. Zhao, J. Membr. Sci. , 2017, 539 , 213–223 CrossRef CAS .
- M. Shete, P. Kumar, J. E. Bachman, X. Ma, Z. P. Smith, W. Xu, K. A. Mkhoyan, J. R. Long and M. Tsapatsis, J. Membr. Sci. , 2018, 549 , 312–320 CrossRef CAS .
- Z. Kang, Y. Peng, Z. Hu, Y. Qian, C. Chi, L. Y. Yeo, L. Tee and D. Zhao, J. Mater. Chem. A , 2015, 3 , 20801–20810 RSC .
- Y. Yang, K. Goh, R. Wang and T. H. Bae, Chem. Commun. , 2017, 53 , 4254–4257 RSC .
- H. L. Liu, Y. J. Chang, T. Fan and Z. Y. Gu, Chem. Commun. , 2016, 52 , 12984–12987 RSC .
- M. H. Pham, G. T. Vuong, F. G. Fontaine and T. O. Do, Cryst. Growth Des. , 2012, 12 , 3091–3095 CrossRef CAS .
- R. Chen, J. Yao, Q. Gu, S. Smeets, C. Baerlocher, H. Gu, D. Zhu, W. Morris, O. M. Yaghi and H. Wang, Chem. Commun. , 2013, 49 , 9500–9502 RSC .
- R. Kumar, K. Jayaramulu, T. K. Maji and C. N. R. Rao, Dalton Trans. , 2014, 43 , 7383–7386 RSC .
- J. Guo, Y. Zhang, Y. Zhu, C. Long, M. Zhao, M. He, X. Zhang, J. Lv, B. Han and Z. Tang, Angew. Chem., Int. Ed. , 2018, 57 , 6873–6877 CrossRef CAS PubMed .
- V. Galstyan, M. Bhandari, V. Sberveglieri, G. Sberveglieri and E. Comini, Chemosensors , 2018, 6 , 16 CrossRef .
- M. F. Baran, H. Acay and C. Keskin, Global Challenges , 2020, 4 , 1900104 CrossRef PubMed .
- G. Maduraiveeran, M. Sasidharan and W. Jin, Prog. Mater. Sci. , 2019, 106 , 100574 CrossRef CAS .
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin and H. Wei, Chem. Soc. Rev. , 2019, 48 , 1004–1076 RSC .
- H. Bin Wu, J. S. Chen, H. H. Hng and X. Wen (David) Lou, Nanoscale , 2012, 4 , 2526 RSC .
- W. Zhang, J. Ai, Y. Lei, Y. Li, C. Lai and J. Xie, Solid State Ionics , 2020, 344 , 115132 CrossRef CAS .
- J. Ying, C. Wan, C. Jiang and Y. Li, J. Power Sources , 2001, 99 , 78–84 CrossRef CAS .
- B. Qu, L. Hu, Q. Li, Y. Wang, L. Chen and T. Wang, ACS Appl. Mater. Interfaces , 2014, 6 , 731–736 CrossRef CAS .
- P.-F. Wang, Y. You, Y.-X. Yin and Y.-G. Guo, Adv. Energy Mater. , 2018, 8 , 1701912 CrossRef .
- Y. Fang, X.-Y. Yu and X. W. (David) Lou, Matter , 2019, 1 , 90–114 CrossRef .
- T. Wang, D. Su, D. Shanmukaraj, T. Rojo, M. Armand and G. Wang, Electrochem. Energy Rev. , 2018, 1 , 200–237 CrossRef CAS .
- Q. Liu, Z. Hu, M. Chen, C. Zou, H. Jin, S. Wang, S. Chou and S. Dou, Small , 2019, 15 , 1805381 CrossRef PubMed .
- Y. Huang, Y. Zheng, X. Li, F. Adams, W. Luo, Y. Huang and L. Hu, ACS Energy Lett. , 2018, 3 , 1604–1612 CrossRef CAS .
- J. Ye, G. Xia, Z. Zheng and C. Hu, Int. J. Hydrogen Energy , 2020, 45 , 9969–9978 CrossRef CAS .
- F. Zhang, J. Zhu, D. Zhang, U. Schwingenschlögl and H. N. Alshareef, Nano Lett. , 2017, 17 , 1302–1311 CrossRef CAS PubMed .
- L. Liang, Y. Xu, C. Wang, L. Wen, Y. Fang, Y. Mi, M. Zhou, H. Zhao and Y. Lei, Energy Environ. Sci. , 2015, 8 , 2954–2962 RSC .
- L. Shen, Q. Che, H. Li and X. Zhang, Adv. Funct. Mater. , 2014, 24 , 2630–2637 CrossRef CAS .
- D. Kundu, B. D. Adams, V. Duffort, S. H. Vajargah and L. F. Nazar, Nat. Energy , 2016, 1 , 16119 CrossRef CAS .
- J. Ha, Y. Kim and J. Choi, ChemSusChem , 2020, 13 , 419–425 CrossRef CAS PubMed .
- Z. Kang, C. Wu, L. Dong, W. Liu, J. Mou, J. Zhang, Z. Chang, B. Jiang, G. Wang, F. Kang and C. Xu, ACS Sustainable Chem. Eng. , 2019, 7 , 3364–3371 CrossRef CAS .
- S. Lu, H. Wang, J. Zhou, X. Wu and W. Qin, Nanoscale , 2017, 9 , 1184–1192 RSC .
- J.-W. Lee, H.-S. Shin, C.-W. Lee and K.-N. Jung, Nanoscale Res. Lett. , 2016, 11 , 45 CrossRef PubMed .
- J. Han, J. Qin, L. Guo, K. Qin, N. Zhao, C. Shi, E. Liu, F. He, L. Ma and C. He, Appl. Surf. Sci. , 2018, 427 , 670–679 CrossRef CAS .
- Z. Zhang, X. Zhao and J. Li, ChemNanoMat , 2016, 2 , 196–200 CrossRef CAS .
- Y. Xiong, J. Qian, Y. Cao, X. Ai and H. Yang, ACS Appl. Mater. Interfaces , 2016, 8 , 16684–16689 CrossRef CAS PubMed .
- X. Wang, K. Cao, Y. Wang and L. Jiao, Small , 2017, 13 , 1700873 CrossRef PubMed .
- X. Li, X. Hao, A. Abudula and G. Guan, J. Mater. Chem. A , 2016, 4 , 11973–12000 RSC .
- Y. Yan, B. Y. Xia, B. Zhao and X. Wang, J. Mater. Chem. A , 2016, 4 , 17587–17603 RSC .
- I. Roger, M. A. Shipman and M. D. Symes, Nat. Rev. Chem. , 2017, 1 , 0003 CrossRef CAS .
- X. Zou and Y. Zhang, Chem. Soc. Rev. , 2015, 44 , 5148–5180 RSC .
- X. Yang, H. Li, A.-Y. Lu, S. Min, Z. Idriss, M. N. Hedhili, K.-W. Huang, H. Idriss and L.-J. Li, Nano Energy , 2016, 25 , 42–50 CrossRef CAS .
- A. G. Tamirat, J. Rick, A. A. Dubale, W.-N. Su and B.-J. Hwang, Nanoscale Horiz. , 2016, 1 , 243–267 RSC .
- Y. Yang, S. Niu, D. Han, T. Liu, G. Wang and Y. Li, Adv. Energy Mater. , 2017, 7 , 1700555 CrossRef .
- N. Nasori, T. Dai, X. Jia, A. Rubiyanto, D. Cao, S. Qu, Z. Wang, Z. Wang and Y. Lei, J. Semicond. , 2019, 40 , 052701 CrossRef CAS .
- Z. Yu, H. Liu, M. Zhu, Y. Li and W. Li, Small , 2019, 1903378 Search PubMed .
- V. Sharma, M. Prasad, P. Ilaiyaraja, C. Sudakar and S. Jadkar, Int. J. Hydrogen Energy , 2019, 44 , 11459–11471 CrossRef CAS .
- X. Li, C. Gao, H. Duan, B. Lu, Y. Wang, L. Chen, Z. Zhang, X. Pan and E. Xie, Small , 2013, 9 , 2005–2011 CrossRef CAS PubMed .
- M. Tayebi and B.-K. Lee, Renewable Sustainable Energy Rev. , 2019, 111 , 332–343 CrossRef CAS .
- J. Guo, Y. Li, B. Jiang, H. Gao, T. Wang, P. Sun, F. Liu, X. Yan, X. Liang, Y. Gao, J. Zhao and G. Lu, Sens. Actuators, B , 2020, 310 , 127780 CrossRef CAS .
- X. Chen and S. S. Mao, Chem. Rev. , 2007, 107 , 2891–2959 CrossRef CAS PubMed .
- K. Wetchakun, T. Samerjai, N. Tamaekong, C. Liewhiran, C. Siriwong, V. Kruefu, A. Wisitsoraat, A. Tuantranont and S. Phanichphant, Sens. Actuators, B , 2011, 160 , 580–591 CrossRef CAS .
- J. M. George, A. Antony and B. Mathew, Microchim. Acta , 2018, 185 , 358 CrossRef PubMed .
- A.-N. Kawde, M. Aziz, N. Baig and Y. Temerk, J. Electroanal. Chem. , 2015, 740 , 68–74 CrossRef CAS .
- R. Narayanan and M. A. El-Sayed, Nano Lett. , 2004, 4 , 1343–1348 CrossRef CAS .
- X. Xie, Y. Li, Z.-Q. Liu, M. Haruta and W. Shen, Nature , 2009, 458 , 746–749 CrossRef CAS PubMed .
- K. M. Bratlie, H. Lee, K. Komvopoulos, P. Yang and G. A. Somorjai, Nano Lett. , 2007, 7 , 3097–3101 CrossRef CAS PubMed .
- Z. Sun, T. Liao, L. Sheng, L. Kou, J. H. Kim and S. X. Dou, Chem. – Eur. J. , 2016, 22 , 11357–11364 CrossRef CAS PubMed .
- Q. Liang, X. Zou, H. Chen, M. Fan and G.-D. Li, Sens. Actuators, B , 2020, 304 , 127241 CrossRef CAS .
- S. Ozkan, N. T. Nguyen, I. Hwang, A. Mazare and P. Schmuki, Small , 2017, 13 , 1603821 CrossRef PubMed .
- S. Cheon, W. W. Lee, W. Il Park, J.-Y. Jung, J.-H. Choi, D.-G. Choi, S. Jeon, J. Jeong and J. Lee, J. Ind. Eng. Chem. , 2020, 81 , 385–392 CrossRef CAS .
- G. A. Cerrón-Calle, A. J. Aranda-Aguirre, C. Luyo, S. Garcia-Segura and H. Alarcón, Chemosphere , 2019, 219 , 296–304 CrossRef PubMed .
- X. Feng, Y. Huang, C. Li, X. Chen, S. Zhou, X. Gao and C. Chen, Chem. Eng. J. , 2019, 368 , 51–60 CrossRef CAS .
- L. Peng, P. Xiong, L. Ma, Y. Yuan, Y. Zhu, D. Chen, X. Luo, J. Lu, K. Amine and G. Yu, Nat. Commun. , 2017, 8 , 15139 CrossRef PubMed .
- K. Xu, S. Ma, Y. Shen, Q. Ren, J. Yang, X. Chen and J. Hu, Chem. Eng. J. , 2019, 369 , 363–369 CrossRef CAS .
- Y. Xie, C. Chen, X. Lu, F. Luo, C. Wang, A. Alsaedi and T. Hayat, J. Hazard. Mater. , 2019, 379 , 120786 CrossRef CAS PubMed .
- S. M. Saleh, Spectrochim. Acta, Part A , 2019, 211 , 141–147 CrossRef CAS PubMed .
- M. Guzman, M. Estrada, S. Miridonov and A. Simakov, Microporous Mesoporous Mater. , 2019, 278 , 241–250 CrossRef CAS .
- X. Zhang, W. Lan, J. Xu, Y. Luo, J. Pan, C. Liao, L. Yang, W. Tan and X. Huang, Sens. Actuators, B , 2019, 289 , 144–152 CrossRef CAS .
- M. Wang, Y. Huang, Y. Zhu, N. Zhang, J. Zhang, X. Qin and H. Zhang, Electrochim. Acta , 2020, 335 , 135694 CrossRef CAS .
- J. Feng and Y. Yin, Adv. Mater. , 2019, 31 , 1802349 CrossRef PubMed .
- Z. Wang, L. Zhou and X. W. David Lou, Adv. Mater. , 2012, 24 , 1903–1911 CrossRef CAS PubMed .
- X. W. Lou, Y. Wang, C. Yuan, J. Y. Lee and L. A. Archer, Adv. Mater. , 2006, 18 , 2325–2329 CrossRef CAS .
- H. Tianou, W. Wang, X. Yang, Z. Cao, Q. Kuang, Z. Wang, Z. Shan, M. Jin and Y. Yin, Nat. Commun. , 2017, 8 , 1261 CrossRef PubMed .
- S. Zhou, M. Chen, Q. Lu, J. Hu, H. Wang, K. Li, K. Li, J. Zhang, Z. Zhu and Q. Liu, Mater. Lett. , 2019, 247 , 15–18 CrossRef CAS .
- W. Xin, T. Gao, W. Zhang, T. Hu, X. Sun and G. Zhou, J. Alloys Compd. , 2019, 784 , 157–164 CrossRef CAS .
- D. Zhang, Z. Yang, Z. Wu and G. Dong, Sens. Actuators, B , 2019, 283 , 42–51 CrossRef CAS .
- T. Zhang, J. Zheng, Z. Liang, B. Zhao, H. Zeng, W. Guo, L. Zhao, Y. Sun, I. Abdulhalim and L. Jiang, Electrochim. Acta , 2019, 306 , 151–158 CrossRef CAS .
- B. Dong, W. Li, X. Huang, Z. Ali, T. Zhang, Z. Yang and Y. Hou, Nano Energy , 2019, 55 , 37–41 CrossRef CAS .
- R. Ghosh Chaudhuri and S. Paria, Chem. Rev. , 2012, 112 , 2373–2433 CrossRef CAS PubMed .
- W. Schärtl, Nanoscale , 2010, 2 , 829 RSC .
- L. León Félix, J. A. H. Coaquira, M. A. R. Martínez, G. F. Goya, J. Mantilla, M. H. Sousa, L. D. L. S. Valladares, C. H. W. Barnes and P. C. Morais, Sci. Rep. , 2017, 7 , 41732 CrossRef PubMed .
- S. Wei, Q. Wang, J. Zhu, L. Sun, H. Lin and Z. Guo, Nanoscale , 2011, 3 , 4474 RSC .
- F. Forato, S. Talebzadeh, N. Rousseau, J.-Y. Mevellec, B. Bujoli, D. A. Knight, C. Queffélec and B. Humbert, Phys. Chem. Chem. Phys. , 2019, 21 , 3066–3072 RSC .
- H. Mi, X. Zhang, S. An, X. Ye and S. Yang, Electrochem. Commun. , 2007, 9 , 2859–2862 CrossRef CAS .
- R. M. Abdel Hameed, New J. Chem. , 2018, 42 , 2658–2668 RSC .
- M.-Y. Kuo, C.-F. Hsiao, Y.-H. Chiu, T.-H. Lai, M.-J. Fang, J.-Y. Wu, J.-W. Chen, C.-L. Wu, K.-H. Wei, H.-C. Lin and Y.-J. Hsu, Appl. Catal., B , 2019, 242 , 499–506 CrossRef CAS .
- H. S. Choi, J. H. Park, J. W. Bae, J. H. Lee and T. S. Chang, Catal. Commun. , 2019, 123 , 86–90 CrossRef CAS .
- K. Awazu, M. Fujimaki, C. Rockstuhl, J. Tominaga, H. Murakami, Y. Ohki, N. Yoshida and T. Watanabe, J. Am. Chem. Soc. , 2008, 130 , 1676–1680 CrossRef CAS PubMed .
- S. V. Salihov, Y. A. Ivanenkov, S. P. Krechetov, M. S. Veselov, N. V. Sviridenkova, A. G. Savchenko, N. L. Klyachko, Y. I. Golovin, N. V. Chufarova, E. K. Beloglazkina and A. G. Majouga, J. Magn. Magn. Mater. , 2015, 394 , 173–178 CrossRef CAS .
- H. Wang, Q. Mu, R. Revia, K. Wang, B. Tian, G. Lin, W. Lee, Y.-K. Hong and M. Zhang, J. Controlled Release , 2018, 289 , 70–78 CrossRef CAS PubMed .
- K. C. Kwon, J. H. Ryu, J.-H. Lee, E. J. Lee, I. C. Kwon, K. Kim and J. Lee, Adv. Mater. , 2014, 26 , 6436–6441 CrossRef CAS PubMed .
- Y.-K. Kim, H.-K. Na, S. Kim, H. Jang, S.-J. Chang and D.-H. Min, Small , 2015, 11 , 2527–2535 CrossRef CAS PubMed .
- A. Seth, H. Gholami Derami, P. Gupta, Z. Wang, P. Rathi, R. Gupta, T. Cao, J. J. Morrissey and S. Singamaneni, ACS Appl. Mater. Interfaces , 2020, 12 , 42499–42510 CrossRef CAS PubMed .
- Y. Xiang, X. Peng, X. Kong, Z. Tang and H. Quan, Colloids Surf., A , 2020, 594 , 124652 CrossRef CAS .
- Q. Lv, H. Min, D. Duan, W. Fang, G. Pan, A. Shen, Q. Wang, G. Nie and J. Hu, Adv. Healthcare Mater. , 2018, 8 , 1801257 CrossRef PubMed .
- L. Li, Y. Lu, C. Jiang, Y. Zhu, X. Yang, X. Hu, Z. Lin, Y. Zhang, M. Peng, H. Xia and C. Mao, Adv. Funct. Mater. , 2018, 28 , 1704623 CrossRef PubMed .
- T. Liu, X. Li, J. Wang, P. Zhang, X. Huang, Z. Zhang, D.-S. Guo and X. Yang, J. Mater. Chem. B , 2020, 8 , 5483–5490 RSC .
- K.-C. Ho and L.-Y. Lin, J. Mater. Chem. A , 2019, 7 , 3516–3530 RSC .
- Y. Bai, R. Zhang, X. Ye, Z. Zhu, H. Xie, B. Shen, D. Cai, B. Liu, C. Zhang, Z. Jia, S. Zhang, X. Li and F. Wei, Nat. Nanotechnol. , 2018, 13 , 589–595 CrossRef CAS PubMed .
- F. Yang, X. Wang, D. Zhang, J. Yang, D. Luo, Z. Xu, J. Wei, J.-Q. Wang, Z. Xu, F. Peng, X. Li, R. Li, Y. Li, M. Li, X. Bai, F. Ding and Y. Li, Nature , 2014, 510 , 522–524 CrossRef CAS PubMed .
- J. Liu, J. Lu, X. Lin, Y. Tang, Y. Liu, T. Wang and H. Zhu, Comput. Mater. Sci. , 2017, 129 , 290–294 CrossRef CAS .
- C. R. Vandenabeele and S. Lucas, Mater. Sci. Eng., R , 2020, 139 , 100521 CrossRef .
- Y. He, W. Chen, X. Li, Z. Zhang, J. Fu, C. Zhao and E. Xie, ACS Nano , 2013, 7 , 174–182 CrossRef CAS PubMed .
- C. Li and G. Shi, Nanoscale , 2012, 4 , 5549 RSC .
- P. Tucci, G. Porta, M. Agostini, D. Dinsdale, I. Iavicoli, K. Cain, A. Finazzi-Agró, G. Melino and A. Willis, Cell Death Dis. , 2013, 4 , e549–e549 CrossRef CAS PubMed .
- T. G. Smijs and S. Pavel, Nanotechnol., Sci. Appl. , 2011, 4 , 95–112 CrossRef CAS PubMed .
- W. J. Stark, P. R. Stoessel, W. Wohlleben and A. Hafner, Chem. Soc. Rev. , 2015, 44 , 5793–5805 RSC .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
After fourteen years of writing for Nature Nanotechnology , Chris Toumey reflects on the role of experts in Science and Technology Studies in his last contribution to the journal.
- Chris Toumey
Social systems for technology
In reviewing a recent book, Chris Toumey reflects on the impact that social networks can have on the development of a number of technologies.
Nanoscale nights of COVID-19
As the spread of SARS-CoV-2 has triggered worldwide closures of research labs and facilities, Kostas Kostarelos shares his views on what may be going wrong in the fight against COVID-19 and how the nanoscience community could and should contribute.
- Kostas Kostarelos
Notes on environmental nanoscience
Although we seem to understand how nanoscience can impact the environment, we seem to be far off using nanotechnology for environmental remediation, says Chris Toumey.
Religious reactions to new technologies
Chris Toumey explores the way in which reactions to nanotechnology from different religious denominations can be translated to other emerging technologies.
Engineering students visit a nano centre
Visiting a research centre specialized in nanoscience and nanotechnology can be an inspiration for students in other disciplines, as Chris Toumey explains.
Later voices on ethics in nanotechnology
Chris Toumey continues his journey through ethics in nanotechnology by examining literature published in the past decade.
Early voices for ethics in nanotechnology
Chris Toumey reflects on the inhomogeneity of the concept of ethics applied to nanoscience and nanotechnology.
Tales of the structures of molecules
Through an overview of James Watson’s recounting of the discovery of the structure of DNA, Chris Toumey illustrates the value to be found in the stories of the interaction among scientists behind great scientific discoveries.
Let there be nano
Some scientific disciplines originate from a single event. This is not the case for nanotechnology, says Chris Toumey.
Chris Toumey explores the potential of sound as a way to investigate nature at the nanoscale.
Denominational interpretations of nanotech
Religious people tend to have a different view of nanotechnology than non-religious people. Chris Toumey explores whether there are also different views between different religious groups.
Reality, fantasy and civility in molecular assemblers
Chris Toumey revisits the 2003 exchange of opinions between Eric Drexler and Richard Smalley, which was one of the most colourful disagreements in the history of nanotechnology.
Science policy in the days of Trump
Chris Toumey explains why the actions on science policy taken so far by the current US administration are cause for concern.
Why do we see history so differently?
Chris Toumey illustrates how different groups of people involved with nanotechnology have different views on the history of the field and feel differently about its importance.

Playing and laughing among the molecules
Chris Toumey reflects on the fun, and not only, provided by nanotechnology in the digital world, as illustrated by Colin Milburn in Mondo Nano .
From nano machines to Nobel prizes
Chris Toumey ponders the nanotechnology behind the 2016 Nobel Prize in Chemistry.
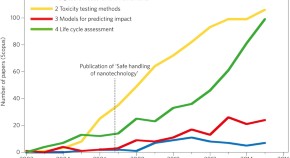
'Safe handling of nanotechnology' ten years on
In 2006, a group of scientists proposed five grand challenges to support the safe handling of nanotechnology. Ten years on, Andrew Maynard and Robert Aitken — two of the original authors — look at where we have come, and where we still need to go.
- Andrew D. Maynard
- Robert J. Aitken
The long way to the market
Nanotechnology is starting to play a role in a number of commercial products, though in an evolutionary, rather than revolutionary way, says Peter Dobson .
- Peter Dobson
Anniversary thoughts
Chris Toumey reflects on his contribution to Nature Nanotechnology since its launch.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
The Library wishes you a nice holiday break. Buildings will be closed from 12/23/22 to 12/31/22. For a full list of closing and opening times, please visit the library hours page.
- Undergraduate Students
- Graduate & Medical Students
- Medical & Clinical Faculty
- Visiting Scholars
- Special Collections Researchers
- Library Staff
Brown University Theses and Dissertations
Brown University Library archives dissertations in accordance with the Brown Graduate School policy .
For dissertations published prior to 2008, please consult the following Dissertation LibGuide

Active filters
Refine your results.
- 5 Biology and Medicine
- 5 Biomedical Engineering
- 4 Chemistry
- 1 Computer Science
- 3 Engineering
- Show More...
- 2 Bachelors Thesis
- 14 Doctoral Dissertation
- 1 Aluminum alloys
- 1 Aluminum oxide
- 1 Aluminum--Anodic oxidation
- 1 Antibacterial agents
- 1 Fluid, Thermal, and Chemical Processes
- 1 Mechanics of Solids
- 1 Artificial intelligence
- 1 Bio-sensors
- 1 Biodegradation
- 1 Bioengineering
- 1 Biomedical engineering
- 1 Biomedical engineering--Research
- 1 Biomimetics
- 1 Biosensing
- 2 Biosensors
- 1 Biotechnology
- 1 Blood-vessels
- 1 Bone Implants
- 1 Cancer cells
- 1 Cell functions
- 1 Chemical engineering
- 1 Coded Computation
- 1 Computational science and engineering
- 1 Contrast media (Diagnostic imaging)
- 1 Deep learning (Machine learning)
- 1 Defective Graphene
- 1 Detection
- 1 Diagnosis
- 1 Diagnostics
- 1 Drug Delivery
- 1 Drug delivery systems
- 1 Drug-delivery
- 1 Electrokinetics
- 1 Emerging Nanomaterials
- 1 Engineering
- 1 Engineering design
- 1 Environment
- 1 Environmental degradation
- 1 Fabry-Perot interferometers
- 1 Fault-Tolerance
- 1 Functionalization
- 1 Gadolinium
- 1 Health risk assessment
- 1 Integrated circuits--Fault tolerance
- 1 Ion Batteries
- 1 Magnetic Nanoparticles
- 1 Magnetic resonance imaging
- 1 Material Science
- 1 Materials science
- 1 Medical instruments and apparatus
- 1 Microelectromechanical systems
- 1 Microelectronics
- 1 Microfluidic devices
- 1 Microfluidics
- 1 Molecular diagnosis
- 1 Molybdenum disulfide
- 1 Nano-Selenium
- 2 Nanomaterials
- 1 Nanomechanics
- 1 Nanomedicine
- 1 Nanoparticle Characterization
- 1 Nanoparticles
- 1 Nanopores
- 1 Nanoscience
- 3 Nanostructured materials
- 16 Nanotechnology
- 1 Nanowire Decoders
- 1 Nanowires
- 1 Orthopedic implants
- 1 Power resources
- 1 Probabilistic Analysis
- 1 Radiographic contrast media
- 1 Remediation
- 1 Self-assembly (Chemistry)
- 1 Single molecular test tube
- 1 Solid Mechanics
- 1 Stochastic Assembly
- 1 Surface chemistry
- 1 Surface properties
- 1 Targeting
- 1 Theranostics
- 1 Thin films--Optical properties
- 1 Toxicology
- 1 Vascular stents
- 1 anodic alumina
- 1 anodization
- 1 anodized aluminum
- 1 antibacterial
- 1 antibiotic resistance
- 1 biomaterial
- 1 bone tissue engineering
- 1 cancer screening
- 1 coloration
- 1 data science
- 1 diagnostic test
- 1 diagnostics
- 1 fabry perot
- 1 filamentation
- 1 metabolic simulation
- 1 nanocrystalline hydroxyapatite
- 2 nanofabrication
- 1 nanomaterials
- 1 nanorough surface
- 1 optical cavity
- 1 optical rectification
- 1 optical resonator
- 1 physical coloration
- 1 resistive switching
- 1 rosette nanotubes
- 1 self-assembly
- 1 structural coloration
- 1 superparamagnetic iron oxide nanoparticles (SPION)
- 1 tissue engineering
Items (1-16) out of 16 results
- title (A-Z)
- title (Z-A)
- date (old to new)
- date (new to old)
- new to the BDR
- 10 per page
- 20 per page
- 50 per page
- 100 per page
Anodic alumina as a scalable platform for structural coloration and optical rectification
Biological Application of Magnetic Nanoparticles in Targeted Therapeutics and Diagnostics
Biologically Inspired Rosette Nanotube Nanocomposites for Bone Tissue Engineering, Orthopedic and Vascular Applications
Biologically Relevant Degradation of 2D Nanomaterials: Kinetics, Hazard Classification and Biomedical Applications
Enhanced Efficacy of Nanotechnology-Driven Approaches against Antibiotic-Resistant Biofilms in the Presence of Metabolites
Evaluating the Human Health and Environmental Impacts of Exposure to Two-Dimensional Manganese Dioxide Nanosheets
Nano-fabrication and Characterization of Novel Titanium Surfaces for Vascular Stent Application
Nano-Selenium: Novel Formulations for Biological and Environmental Applications
Nanopatterned PLGA for Anti-cancer Implant Applications
Novel Devices, Physical Mechanisms, and Analytical Techniques for Use in Next Generation Cellular Diagnostics
Novel Polymers as Phase Transfer Agents for Gadolinium Oxide Nanoplates: Improving Magnetic Resonance Imaging Contrast
Reliable Computing at the Nanoscale
Select Nanofabricated Titanium Materials for Enhancing Bone and Skin Growth of Intraosseous Transcutaneous Amputation Prostheses
Structural and optical characterization of diamond nanowires
The Use of Entropic Cages For Trapping DNA and Controlling its Configurations in Nanopore Studies
Topics in Nanomechanics, Energy Storage Systems, and Emerging Nanomaterials
Philosophy of Science and Nanotechnology
NANOTECNOCIENCIA. NOCIONES PRELIMINARES SOBRE EL UNIVERSO MICROSCOPICO, pp. 69-80, J. Giraldo, E. González & F. Gómez, eds., Buinaima, 2007
13 Pages Posted: 17 Dec 2008
Carlos Eduardo Maldonado
Universidad del Rosario
Date Written: June 15, 2007
This paper focuses on the epistemological status of nanotechnology. It argues a twofold thesis: on the one hand, nanotechnoscience is not just one more chapter in the history of science or technology, but a radical shift that opens new horizons. On the other hand, nanoscience can and must be seen as a new science, namely a cross-disciplinary and a border-science. Thus, the question regarding the philosophy of nanotechnoscience deals with a science defined by border-problems, which can shed new lights into similar sciences, such as the sciences of complex systems, for example. Whilst nanoscale research cannot be seen as reduccionist, from a philosophical point of view the core subject lies on the concept of "scale". The concept of nanotechnoscience allows a real unification of engineering and science, of construction, manipulation and control of nanophenomena and, at the same time, an explanation of what is being ensambled and designed. Throughtout such a unity a quite new understandig of science and technology is possible.
Note: Downloadable document is in Spanish.
Keywords: nanotechnoscience, scale, border-problems, cross-disciplinary research
Suggested Citation: Suggested Citation
Carlos Eduardo Maldonado (Contact Author)
Universidad del rosario ( email ).
Calle 12 No. 6-25 Bogota, DC Colombia
Do you have a job opening that you would like to promote on SSRN?
Paper statistics, related ejournals, entrepreneurship, innovation, & growth ejournal.
Subscribe to this fee journal for more curated articles on this topic
Sustainable Technology eJournal
Social entrepreneurship ejournal, philosophy of science ejournal.
Subscribe to this free journal for more curated articles on this topic
Sociology of Innovation eJournal
Nanomaterials ejournal.
- Bibliography
- More Referencing guides Blog Automated transliteration Relevant bibliographies by topics
- Automated transliteration
- Relevant bibliographies by topics
- Referencing guides
Dissertations / Theses on the topic 'Nanotechnology research'
Create a spot-on reference in apa, mla, chicago, harvard, and other styles.
Consult the top 50 dissertations / theses for your research on the topic 'Nanotechnology research.'
Next to every source in the list of references, there is an 'Add to bibliography' button. Press on it, and we will generate automatically the bibliographic reference to the chosen work in the citation style you need: APA, MLA, Harvard, Chicago, Vancouver, etc.
You can also download the full text of the academic publication as pdf and read online its abstract whenever available in the metadata.
Browse dissertations / theses on a wide variety of disciplines and organise your bibliography correctly.
Houck, Andrew Careaga. "A modular atomic force microscope for nanotechnology research." Thesis, Massachusetts Institute of Technology, 2015. http://hdl.handle.net/1721.1/100129.
Polito, Anthony B. III. "Manipulation of Gold Nanorod Physicochemical Properties to Enhance Biocompatibility, Uptake and Intracellular Preservation of Optical Properties for Bio-Imaging and Plasmonic Photo-Therapeutic Applications." Wright State University / OhioLINK, 2015. http://rave.ohiolink.edu/etdc/view?acc_num=wright1440598544.
Porter, Gregory Thomas. "Science of the Small: Nanotechnology Research Laboratory in Washington, D.C." Thesis, Virginia Tech, 2009. http://hdl.handle.net/10919/30954.
Foschi, Giulia <1985>. "Nanotechnology control of self organized biomolecules and biomaterials for medical research." Doctoral thesis, Alma Mater Studiorum - Università di Bologna, 2013. http://amsdottorato.unibo.it/5336/.
Gastrow, M. "Thinking small : the state of nanotechnology research and development in South Africa." Journal for New Generation Sciences, Vol 7, Issue 1: Central University of Technology, Free State, Bloemfontein, 2009. http://hdl.handle.net/11462/517.
Cortes-Lobos, Rodrigo. "Nanotechnology research in the US agri-food sectoral system of innovation: toward sustainable development." Diss., Georgia Institute of Technology, 2013. http://hdl.handle.net/1853/47541.
Skidmore, Kirsty. "Tight-binding studies of carbon nanotubes." Thesis, University of Oxford, 2002. http://ora.ox.ac.uk/objects/uuid:8291ed37-15c7-40e3-9ef4-a0db1ba763c1.
Czech, Tori. "Fabrication of Osteogenic Protein-loaded Thermoresponsive Hydrogels and Translational Assessment for Osteoporotic Fracture Healing." NEOMED Integrated Pharmaceutical Medicine / OhioLINK, 2021. http://rave.ohiolink.edu/etdc/view?acc_num=ne2mh1614181385140819.
Gollakota, Chaitanya. "Commercial Feasibility of the Multicomponent Nanochain Therapy." Case Western Reserve University School of Graduate Studies / OhioLINK, 2016. http://rave.ohiolink.edu/etdc/view?acc_num=case1467992485.
Altawallbeh, Ghaith. "Endothelial Nitric Oxide Synthase on Nanoscale Phospholipid Bilayers." Cleveland State University / OhioLINK, 2016. http://rave.ohiolink.edu/etdc/view?acc_num=csu1480640206186696.
Tsai, Min-Hua. "Organising the socio-economic relevance of university research : the case of nanomaterials research in Taiwan." Thesis, University of Sussex, 2014. http://sro.sussex.ac.uk/id/eprint/47900/.
Frederick, Armstrong. "Processing and characterisation of nano-enhanced composites this thesis issubmitted in fulfilment of the degree of Master of Engineering by research, February 2008 /." Click here to access this resource online, 2008. http://hdl.handle.net/10292/804.
Cole, James T. "The Synthesis and Characterization of Multifunctional Nanoparticles of Elastin-Like Polypeptides for Theranostic Applications." Cleveland State University / OhioLINK, 2016. http://rave.ohiolink.edu/etdc/view?acc_num=csu1461674813.
Milojevic, Stasa. "Big science, nano science? mapping the evolution and socio-cognitive structure of nanoscience/nanotechnology using fixed methods /." Diss., Restricted to subscribing institutions, 2009. http://proquest.umi.com/pqdweb?did=1930906441&sid=1&Fmt=2&clientId=1564&RQT=309&VName=PQD.
Billade, Nilesh S. "Mechanical Characterization, Computational Modeling and Biological Considerations for Carbon Nanomaterial-Agarose Composites for Tissue Engineering Applications." University of Cincinnati / OhioLINK, 2009. http://rave.ohiolink.edu/etdc/view?acc_num=ucin1250519199.
Akbari, Ehsan. "Studies on Endothelial Mechanotransduction at Branching Vessels Using Biomimetic Microfluidics and DNA-based Nanodevices." The Ohio State University, 2020. http://rave.ohiolink.edu/etdc/view?acc_num=osu1607038628887874.
Shaler, Lisa Marie. "Accelerating Innovation: Assessing Nanotechnologies, Prototypes and Research Teams." Diss., Virginia Tech, 2019. http://hdl.handle.net/10919/89251.
Tang, Li. "The US - China scientific collaboration, knowledge moderation, and China's rise in nanotechnology." Diss., Georgia Institute of Technology, 2011. http://hdl.handle.net/1853/41051.
Shu, Yi. "Assembly of Phi29 pRNA Nanoparticles for Gene or Drug Delivery and for Application in Nanotechnology and Nanomedicine." University of Cincinnati / OhioLINK, 2012. http://rave.ohiolink.edu/etdc/view?acc_num=ucin1336683831.
Gulati, Neetu Mehek. "Characterizing and Manipulating Biological Interactions of Viruses." Case Western Reserve University School of Graduate Studies / OhioLINK, 2018. http://rave.ohiolink.edu/etdc/view?acc_num=case1505991239211632.
Botha, Alwyn Francois. "An investigation into the research and development of nanostructured photovoltaic cells." Thesis, Stellenbosch : University of Stellenbosch, 2010. http://hdl.handle.net/10019.1/4301.
Nicholson, Theodore Roosevelt III. "Interfacial Design and Protein Engineering as Tools of Biomedical Nanotechnology in the Optimization of Protein Detecting Field Effect Transistors." The Ohio State University, 2010. http://rave.ohiolink.edu/etdc/view?acc_num=osu1290575487.
Celik, Hakan. "Time and Temperature Dependent Surface Tension Measurements of Responsive Protein-based Polymer Surfactant Solutions." Cleveland State University / OhioLINK, 2015. http://rave.ohiolink.edu/etdc/view?acc_num=csu1440182119.
Gupta, Cherry. "DNA Translocation and Cell Electroporation in Micro and Nanofluidic Devices." The Ohio State University, 2015. http://rave.ohiolink.edu/etdc/view?acc_num=osu1448318827.
Fang, Huaming. "Structure and Function Study of Phi29 DNA packaging motor." University of Cincinnati / OhioLINK, 2012. http://rave.ohiolink.edu/etdc/view?acc_num=ucin1352488734.
Geng, Jia. "Membrane embedded channel of bacteriophage phi29 DNA packaging motor for single molecule sensing and nanomedicine." University of Cincinnati / OhioLINK, 2012. http://rave.ohiolink.edu/etdc/view?acc_num=ucin1336507840.
Xue, Ruipeng. "Nanofiber Based Optical Sensors for Oxygen Determination." The Ohio State University, 2014. http://rave.ohiolink.edu/etdc/view?acc_num=osu1405508835.
Markopoulos, Marjorie M. "Antimicrobial Activity of Fractionated Borohydride-Capped and Electrochemical Colloidal Silver." Wright State University / OhioLINK, 2017. http://rave.ohiolink.edu/etdc/view?acc_num=wright1515096508634157.
Furchi, Camila Cristina. "A iniciativa brasileira em nanotecnologia : história, debates e impasses (2000 - 2005)." reponame:Repositório Institucional da UFABC, 2015.
Nocera, Tanya Marie. "Magnetic Force Microscopy of Superparamagnetic Nanoparticles for Biomedical Applications." The Ohio State University, 2013. http://rave.ohiolink.edu/etdc/view?acc_num=osu1385914094.
Ellis, David Harold. "Silver nanoparticles: the immediate benefits of low bacterial resistance and the long-term risk of persistent stress in mammalian cells." Wright State University / OhioLINK, 2015. http://rave.ohiolink.edu/etdc/view?acc_num=wright1450827524.
Mani, Rajeswaran. "Preclinical development of a non-immunosuppressive FTY720 derivative OSU-2S forchronic lymphocytic leukemia and other B-cell malignancies." The Ohio State University, 2014. http://rave.ohiolink.edu/etdc/view?acc_num=osu1404067069.
Dinan, Benjamin J. "Growth of Titania Nanowires by Thermal Oxidation." The Ohio State University, 2012. http://rave.ohiolink.edu/etdc/view?acc_num=osu1337650302.
Mabena, Letlhogonolo Fortunate. "Operational review of NCNSM's characterisation facility to determine its delivery on its intended mandate." Thesis, Nelson Mandela Metropolitan University, 2013. http://hdl.handle.net/10948/d1019724.
Parikh, Soham Dipakbhai. "Carbon Nanotube-Coated Scaffolds for Tissue Engineering Applications." Wright State University / OhioLINK, 2021. http://rave.ohiolink.edu/etdc/view?acc_num=wright1622228763428769.
Sharma, Monita. "Simulating hemodynamics in in vitro culture models: Implications on Nano-biointeractions." Wright State University / OhioLINK, 2013. http://rave.ohiolink.edu/etdc/view?acc_num=wright1388681297.
Jung, Hyun Ju. "The generation and flow of knowledge in technology development." Diss., Georgia Institute of Technology, 2013. http://hdl.handle.net/1853/50296.
Manickavasagam, Dharani. "Preparation and Characterization of Polymersomes for Nose-to-Brain Delivery of Combination Therapeutics in Neuroinflammation Treatment." Kent State University / OhioLINK, 2019. http://rave.ohiolink.edu/etdc/view?acc_num=kent1555522694193999.
Santos, Junior Jorge Luiz dos. "Ci?ncia do Futuro: a comunidade de pesquisa e o ciclo da politica de nanoci?ncia no Brasil." Universidade Federal Rural do Rio de Janeiro, 2011. https://tede.ufrrj.br/jspui/handle/jspui/1137.
Meng, Linghui. "Polymer Biomaterial Constructs For Regenerative Medicine and Functional Biological Systems." Case Western Reserve University School of Graduate Studies / OhioLINK, 2012. http://rave.ohiolink.edu/etdc/view?acc_num=case1327682278.
Gallego-Perez, Daniel. "MICRO/NANOSCALE ENGINEERING OF THE CELL MICROENVIRONMENT." The Ohio State University, 2011. http://rave.ohiolink.edu/etdc/view?acc_num=osu1306791490.
Halley, Patrick D. "DNA Origami as a Drug Delivery Vehicle for in vitro and in vivo Applications." The Ohio State University, 2016. http://rave.ohiolink.edu/etdc/view?acc_num=osu1480632777328142.
Johnston, Alyssa N. "A Ternary Drug Delivery Complex to Target CD44 Over Expressing Cancerous Cell Lines." Kent State University Honors College / OhioLINK, 2012. http://rave.ohiolink.edu/etdc/view?acc_num=ksuhonors1335737666.
Hingant, Bénédicte. "Les nanotechnologies dans l'enseignement secondaire : une recherche sur la compréhension des controverses "nanos" par des lycéens." Phd thesis, Université de Grenoble, 2013. http://tel.archives-ouvertes.fr/tel-00949146.
Bukht, Rumana. "Responsibility, regulation and the construction of markets of nanotechnologies in food and food packaging : the cases of Canada and India." Thesis, University of Manchester, 2016. https://www.research.manchester.ac.uk/portal/en/theses/responsibility-regulation-and-the-construction-of-markets-of-nanotechnologies-in-food-and-food-packaging-the-cases-of-canada-and-india(3624dd5f-e9fe-45f8-9225-73de26411bb5).html.
Gulati, Sahil Gulati. "Modulating G Protein-Coupled Receptor Signaling Pathways with Selective Chemical- and Protein-Based Effector Molecules." Case Western Reserve University School of Graduate Studies / OhioLINK, 2018. http://rave.ohiolink.edu/etdc/view?acc_num=case1530642105672697.
Deblock, Michael C. "The Synthesis, In Vitro and In Vivo Testing of Silver N-Heterocyclic Carbenes and Imidazolium Complexes." University of Akron / OhioLINK, 2012. http://rave.ohiolink.edu/etdc/view?acc_num=akron1353951003.
Smith, Leslie TenEyck. "Project NANO: Will Allowing High School Students To Use Research Grade Scanning Electron Microscopes Increase Their Interest in Science?" PDXScholar, 2014. https://pdxscholar.library.pdx.edu/open_access_etds/1549.
McCracken, Christie Joy. "Toxicity of Food-Relevant Nanoparticles in Intestinal Epithelial Models." The Ohio State University, 2015. http://rave.ohiolink.edu/etdc/view?acc_num=osu1437688702.
Knapp, Amanda R. "Antimicrobial and Antitumor Properties of Free and Poly(Ethylene Glycol)-Poly(Lactic Acid) Encapsulated Silver N-Heterocyclic Carbene Complexes." University of Akron / OhioLINK, 2011. http://rave.ohiolink.edu/etdc/view?acc_num=akron1309211795.
DSpace JSPUI
Dspace preserves and enables easy and open access to all types of digital content including text, images, moving images, mpegs and data sets.
- Shodhbhagirathi @ IITR
- NANOTECHNOLOGY
MASTERS' THESES (Nano tech) Collection home page
| Issue Date | Author(s) | Research Supervisor/ Guide | Type | |
|---|---|---|---|---|
| 2011 | ||||
| 2011 | ||||
| 2011 | ||||
| 2011 | ||||
| 2011 | ||||
| 2011 | ||||
| 2011 | ||||
| 2011 | ||||
| 2011 | ||||
| 2011 | ||||
| 2010 | ||||
| 2010 | ||||
| 2010 | ||||
| 2010 | ||||
| 2010 | ||||
| 2010 | ||||
| 2010 | ||||
| 2010 | ||||
| 2010 | ||||
| 2010 |
- 1 Anand, Ankur
- 1 Bind, Umesh Chandra
- 1 Bisht, Anuj
- 1 Chandra, Sushil
- 1 Dass, H. S.
- 1 Devi, Pooja
- 1 Firdoz, Shaik
- 1 Goel, Shreya
- 1 Jain, K. G. S.
- 24 NANOTECHNOLOGY
- 8 CIVIL ENGINEERING
- 1 16 CONICAL
- 1 BEARING CAPACITY
- 1 BUILDING ELEMENTS
- 1 DRAINAGE PROBLEM
- 1 DYE-SENSITIZED SOLAR CELLS
- 1 EARTHQUAKE
- 1 EARTHQUAKE RESISTANT DESIGN
- 1 ELECTRODE MODIFICATION
- 24 2010 - 2012
- 8 1970 - 1979
- 4 Jayaganthan, R.
- 3 Daniel, B. S. S.
- 3 Yadav, K. L.
- 2 Dutta, R. K.
- 2 Kumar, Anil
- 2 Manhas, Sanjeev
- 2 Singh, Dharmendra
- 2 Verma, G. D.
- 1 Agarwal, K. B.
- 1 Agarwal, Vijaya
Nanotechnology: Applications and Implications Research Paper
- To find inspiration for your paper and overcome writer’s block
- As a source of information (ensure proper referencing)
- As a template for you assignment
What is nanotechnology?
Applications of nanotechnology, concerns about nanotechnology.
Nanotechnology is an emerging technology which is developing at an exponential rate. The technology utilizes novel characteristics of materials that are exhibited only at nanoscale level. Although still in early stages, this technology has signaled potential and breakthroughs in many areas such as medicine, computer technology, food industry, building construction, environment protection to mention just a few.
The many exciting products it promises have served to draw a lot of attention to it. Many findings of nanotechnology are quickly being implemented in viable commercial products. This is in spite of insufficient toxicological data about the environmental and biological effects of such nanomaterials.
As nanotechnology gains widespread application in various disciplines, it is imperative to understand its potential effects. This is important for its long terms sustainability. It is also equally critical to set up necessary control legislations and benchmark standards to control research and commercial application of this emerging technology.
The last half of the last century witnessed the technological world going “micro” evidenced by microdevices and microparticles. However, from the start of 21 st century, the “micro” is poised to give way to the “Nano”. Nanotechnology is an emerging technology that is offering promises of breakthroughs cutting across multiple subjects such as medicine, food industry, energy sector and environmental remediation to mention a few.
The Potential of nanotechnology to solve hitherto “unsolvable” problems by conventional technologies has attracted the attention of government and commercial corporations with diverse interests. Billions of dollars for research and development continue to be channeled to nanotechnology projects all over the world. This paper presents the potential applications of nano-inventions in selected areas of medicine, pollution control, energy, construction, computer technology, and food sectors.
While the benefits of this emerging technology appear to be immense, its environmental and social effects also need to be given as much attention. Nanotechnology is a relatively nascent industry and its potential uses and effects need to be exhaustively established researched before mass production and commercialization. Nanotechnology is the most significant emerging technology today and will play a major role in social, economic, and environmental developments in this century.
Nanotechnology is the “creation of functional materials, devices, and systems through the manipulation of matter at a length of ~1-100 nm” (Srinivas, et al., 2010).
At such scale, matter exhibits new properties unlike those observed at larger scales (Wickson, Baun, & Grieger, 2010). This includes enhanced plasticity, change in thermal properties, enhanced reactivity and catalysis, negative refractivity, faster ion/electron transport and novel quantum mechanical properties (Vaddiraju, Tomazos, Burgess, Jain, & Papadimitrakopoulos, 2010).
The novel properties of matter at nanoscale has been explained by the presence of quantum effect, increase in surface area to volume ratio and alterations in atomic configurations (Wickson et al., 2010). The properties of nanomaterials may be characterized in terms of size, shape, crystallinity, light absorption and scattering, chemical composition, surface area, assembly structure, surface structure, as well as surface charge.
Some of the techniques used in nanoscience to study these properties include Scanning Electron Microscopy (SEM), Energy Dispersive X-ray Analysis (EDX), Atomic Force Microscopy (ATM), Transmission Electron Microscopy (TEM), X-ray Diffraction (XRD), UV-Vis-nIR Spectroscopy, Extended X-ray Absorption Fine structure (EXAFS) , Photoluminescence Spectroscopy (XPS), Chemisorption among other new only developed ones.
The applications of nanotechnology are as a result of investigating and utilizing these properties (Wickson et al., 2010). There are a host of substances utilised in nanotechnology, the most researched ones are carbon, silicon dioxide and titanium dioxide (Robinson, 2010). Others are aluminum, zinc, silver, copper and gold (Robinson, 2010).
Nanotechnology projects continue to channel out a wide range of applications at a very high rate (Dang, Zhang, Fan, Chen, & C.Roco, 2010). This exponential growth rate is evident from the number of patent applications. Data by Dang and fellow researchers (2010) shows that patent application for nanotechnology inventions in developed countries increased from zero percent in 1991 to about 27 % in 2008 and that this growth is set to continue for the better part of this century.
Spurred by huge funding from government and commercial players, nanotechnology projects continue to release more and more potential innovations into the market. This may be an indication that nanotechnology will in future play a pivotal role in scientific and economic development (Dang et al., 2010). Nanotechnology may be a critical solution for companies seeking to stay ahead of competitors. The potential of nanotechnology appears limitless as can be shown by the number of areas where it is already being applied.
Nanomedicine
This field encompasses pharmaceutical and medical nanotechnology. It is one of the most active areas of nanotechnology due it promises of novel therapeutic applications in crucial areas such as cancer therapy, drug delivery, imaging, biosensors and diagnosis.
Nanoparticles have been cited as having great potential in vivo imaging applications (Solomon & D’Souza, 2011). Already, a surface functionalized iron oxide nanoparticle is being used in modern imaging technologies such as magnetomotive imaging. This type of imaging is comparatively powerful and is expected to improve disease diagnosis significantly.
Nanoparticles are also being engineered to be used to enhance drug biodistribution and delivery to target sites in the body. This approach seeks to deliver drug agents to affected sites without damaging the healthy cells. This has been promising in the case of solid tumors whereby a transferrin-modified cyclodextrin nanoparticle successfully delivered anti-tumor agents to the target tumor site in human subjects (Solomon & D’Souza, 2011).
Nanoparticles have also displayed the ability to cross the blood-brain barrier, a major impediment to drug delivery to the brain, thus offering hope of improving the efficacy of some drugs. It has also been reported that nanoparticles conjugated to model antigens have been able to stimulate immunity in mice (Solomon & D’Souza, 2011). This indicates potential for application in improving vaccine therapy.
Elsewhere, nanoparticles have been used to engineer self-assembled tissue capable of repairing damaged tissues in rats though this is yet to be replicated in humans. Another area that has generated much interest is in production of microscopic and highly sensitive in vitro and in vivo biosensors. This application holds the promise of increasing portability and lowering the cost of such devices.
Nanoparticles are increasingly gaining application in cancer therapy. Nanoparticles are for this purpose is characterized by surface modifications that enable them interact with receptors of target cells. This makes it possible to develop therapies targeting cancerous cells only while leaving out healthy cells.
Free radical such as superoxide, hydroxides and peroxides has been known to produce disease initiating changes in cells. To counter this adverse effect, neuroprotective compound is being developed using carbon-60 fullerene (Silva, 2010). In terms of detection of biochemical compounds carbon nanotubes have been used for detection DNA and proteins in serum samples.
Nanotechnology has opened up new possibilities in regard to medical application. The technology has potential to alter medical therapy in many ways.
Pollution control
Waste disposal remains a challenging task for many industries. Current waste disposal technologies are expensive and require a lot of time to render the waste less harmful. In addition, current processes such as air stripping, carbon adsorption, biological reactors or chemical precipitation produce highly toxic wastes that require further disposal (Karn, Kuiken, & Otto, 2009).
Nanoremediation is a new form of waste disposal mechanism that utilizes nanoparticles to detoxify pollutants. nZVI, a nanoscale zero-valent iron has gained widespread use in this area and has been applied in remediating polluted in situ groundwater. This technology has been cited as cost-effective and faster compared to traditional pump-and-treat methods (Karn et al., 2009).
Other forms of pollution solutions employ the use of nanocatalysts. Just like biological and chemical catalysts, nanocatalysts speed up chemical reaction leading to decomposition of the reactive species. This is already being used to detoxify harmful vapor in cars and industrial machinery. Notable ongoing projects in pollution control include research on the recycling greenhouse gas emissions using carbon nanotubes (CNT) (Zhao, 2009).
For his effort, the researcher for this “green” solution received an $ 85,000 Foundation Research Excellence Award (Zhao, 2009). Nanoparticles have also been used to treat highly polluted industrial waste (Zhao, 2009). Nanotechnology is also aiding in improving current water purification technologies. The technology has made it possible to decrease the membrane pores to nanoscale levels leading to greater filtration power.
Energy applications
Nanotechnology has offered promises and potential for development of efficient and long-lasting energy devices. Nanofabricated energy storage compounds have been cited as potentially beneficial as they may serve as replacement for traditional environmentally harmful fossil fuels.
It is expected that nanoscience for energy application will transfer the nano-scale effects of energy carriers such as photons, phonons, electrons, and molecules to conventional photovoltaic, photochemical solar cells, thermoelectric, fuel cells and batteries. This is expected to greatly enhance the capacity, life, and efficiency of such energy producers. Laboratory tests have already shown that the nanomaterials-based electrodes enhance the charge storage capacity and reaction rates in fuel cells.
Also, nanomaterials such as carbon nanotubes and carbon nanohorns are proving useful in energy application due to their ability to provide excellent conductivity for charge transport (Yimin, 2011). Some nanomaterials e.g., PbTe-based quantum dot superlattice system, have demonstrated improved energy conversion efficiency. This property has been suggested to be replicated to produce more energy-efficient thermoelectric devices used to convert waste heat energy into electricity (Yimin, 2011).
This is necessary as the energy efficiency of most thermoelectric devices is very low. In terms of energy conservation, semiconductor nanostructures are actively being explored for the development of highly luminous and efficient light-emitting diodes (LED). This can have a significant impact in energy conservation as lighting uses about 20% of the total electric power generated (Yimin, 2011). Nanostructures are also gaining application in solar energy technologies.
Nonastructured photovoltaic materials have been cited as potentially significant in improving the efficiency of solar energy-based devices. To this end, nanomaterials, such as quantum dots and dye-sensitized semiconductors, are being tested for the possible production of next-generation solar devices projects (Yimin, 2011).
Nanotechnology has the potential to revolutionize man-made energy. Although still, in early phases, nanomaterials have the potential to deliver efficient, high capacity, clean and more durable energy solutions. The challenge, perhaps, remains the development of controlled large scale manufacturing approaches that will ensure greater realization of the powers of these promising materials.
Food nanotechnology
Application of nanoscience in food industry has opened up numerous new possibilities for the food sector. Areas that have gained prominence in this area include food packaging and preservation. Attention to this sector has been contributed by projections of enormous economic gains it offers. Data shows that sales of nanotechnology products to food and beverage packaging sector is expected to surpass US $20.4 billion beyond 2010 (Sozer & Kokini, 2008).
Already, bionanocomposites, which are nanostructures with enhanced mechanical, thermal, and porosity properties, are being used in food packaging. Additional benefits of bionanocomposites include being environmentally friendly as are they are biodegradable as well as increasing the food shelf life (Sozer & Kokini, 2008). Bioactive packaging materials made of nanomaterials have been used in controlling oxidation of foodstuffs and formation of undesirable textures and flavors (Sozer & Kokini, 2008).
One of the nanomaterials with high potential here is carbon nanotube. Apart from offering enhanced mechanical properties to food packaging materials, it has been discovered that the same tube could be possessing effective antimicrobial effects.
This is due to the fact that Escherichia coli bacteria have been found to immediately die upon coming in contact with aggregated nanotubes (Sekhon, 2010). Another area being explored is the fortification of food packaging with nano active additives that would allow controlled release of nutrient into the stored food.
Nanomaterials have also been said to have potential application in food preservation. Nanosensors made to fluoresce in different colors when in contact with food spoilage microorganisms, have been selected as a possible solution. This may reduce the time it takes to detect food spoilage and thus lessen cases of food poisoning.
Examples are nanosilica, already used in food packaging and nanoselenium, which has been added into some beverage and said to enhance uptake of selenium. Nano-iron is also available and is used as a health supplement, although it can also be used in the treatment of contaminated water. Said to be still under development, nanosalt has to be cited as having the benefit of enabling reduction in dietary salt intake.
Another nanoagent, nanoemulsion is already being used to add nanoemulfied bioctives and flavors to beverages (Sekhon, 2010). Nanoemulsions have also proved effective against gram-negative bacteria, a major food pathogen (Sekhon, 2010). Elsewhere scientists have also reported improved bioavailability and color changes brought about by iron/zinc-containing nanostructures.
Other areas being explored include probiotics and edible nanocoatings. Probiotics will entail using nanofabrications to deliver beneficial bacterial cells to the gut system while edible nanocoatings will be in the form of edible coatings to provide barrier to moisture, gas exchange, and deliver food enhancement additives.
It is clear that nanotechnology presents unlimited opportunities to the food industry. However, just like the controversy that followed GMOs food, foodstuffs bearing nano components are surely bound to generate a prolonged public debate. This is because the effects of such miniscule particles in the consumer body remain unclear. Nevertheless, given the nascent nature of nanotechnology, such opposition is expected.
Computer technology
Nanotechnology is expected to revolutionize computer architecture technologies. Current processors have an unofficial limit of 4 GHz. This year a synthetic material capable of replacing silicon, the long-standing semiconductor of choice in the 20th century, and attaining a clock speed of 6 GHz was unveiled (Partyka & Mazur, 2012).
This is because nanotechnology presents the possibility of adding even more transistors per a nanometric length than what is possible through current microprocessor development technologies.
What is even more interesting is that this development could not have come at a more opportune time as silicon processors are expected to have attained their maximum performance by 2020 (Partyka & Mazur, 2012). This year scientists have also announced the successful development of a Nano transistor “based on single molecules of a chemical compound” (Partyka & Mazur, 2012, n.p).
Application of nanotechnology in construction
Nanotechnology portends immense benefits for the future of the construction sector. From the amazing self-cleaning window to the “smog-eating” concrete, this technology has the capability of transforming building materials to new levels in terms of energy, light, strength, security, beauty and intelligence (Halicioglu, 2009).
The development of super-strength plastics has a possible application in diverse areas such as in cars, trucks, and planes where it can serve to replace heavy metals leading to significant energy savings (Zhao, 2009). Nanomaterials such as carbon nanotubes have been found to possess strength and flexibility on a much larger scale compared known strong materials such as steel. Nanocoatings have been suggested as possible solutions to insulation, microbial activity, and mildew growth in buildings (Halicioglu, 2009).
Nanotechnology is expected to produce unique bio-products characterized by hyper-performance and superior serviceability (Halicioglu, 2009).
Notable nanoparticles already in use in construction are titanium dioxide (TiO 2 ) and carbon nanotubes (CNT’s). Titanium dioxide is being used in degrading pollutants in buildings while carbon nanotubes have been applied in strengthening and monitoring concrete (Halicioglu, 2009).
Just like other applications of nanotechnology, nanomaterials are used in construction sector yet their environmental, health effect, and other risks remain unclear. However, despite this drawback, nanotechnology has the potential to revolutionize building design and construction in the near future.
Concerns have been raised about nanotechnology. Nanoparticles have been said to be potentially unsafe for the biological system (Vishwakarma, Samal, & N.Manoharan, 2010). Owing to their small size, these particles can gain entry into the body easily through the skin, mucosal membranes of nose or lungs through inhalation. Their catalytic properties are likely to produce dangerous reactive radicals such as hyper-reactive oxygen with much toxic effects.
These reactive radicals have been linked to chronic diseases such as cancer. Once inside the body, nanoparticles may reach the brain or liver. This is because nanoparticles are able to cross the blood-brain barrier. Their effects on these organs are yet to be established. The nature of their toxicity remains a speculation, but the disruption in the body chemistry cannot be ignored.
The Royal Society of UK’s National Science Academy has reported that nanotube can cause lung fibrosis when inhaled in large amount over long periods (Vishwakarma et al., 2010). Early research has also shown that some types of nanoparticles could cause lung damage in rats (Vishwakarma et al., 2010).
Possible environmental effects of nanoparticles have also been documented. Because they are easily airborne, and adhesive, it is claimed nanoparticles may enter the food chain with profound undesirable changes on the ecosystem.
Currently, there are no standard techniques for assessing nanocompounds hazards. This, together with the unique features of nanomaterials – large surface area, multi forms, makes risk assessment difficult (Williams, Kulinowski, White, & Louis, 2010). Quality control for nanomaterials manufacturing, terminology as well as nomenclature standards are also lacking.
Additionally, it is alarming that currently there is no data on potential hazards, dose-response relationships and exposure levels of nanomaterials used in numerous applications (Musee, Brent, & Asthton, 2010). It is also worth stating that much of current funding on nanotechnology is directed toward potentially viable commercial projects while little is channeled towards risk assessment initiatives (Musee et al., 2010). This needs to be reversed.
Nanotechnology has the potential to revolutionize our lives. This is because it presents almost unlimited potential to make remarkable changes in virtually all fields ranging from medicine, computer technology, construction, environmental remediation, food industry, to new energy sources.
Despite presenting many potential benefits in many areas, nanotechnology of today is still in its infancy as just a few projects have been commercialized. Many are yet to undergo full lifecycle assessment. The number of nanotechnology innovations continues to rise. However, the same cannot be said of research about their potential effects on environment and biological systems.
As the world readily adapts to this new technology wave, concomitant effort should be directed to the understanding of their possible impacts. This is essential to ensure that nanomaterials do not become the new hazard of 21 st century. The long-long term sustainability of this new technology may depend on the establishment of its risks.
Dang, Y., Zhang, Y., Fan, L., Chen, H., & C.Roco, M. (2010). Trends in worldwide nanotechnology patent applications: 1991:2008. Journal of Nanoparticles Research, 12 , 687-706.
Halicioglu, FH (2009). The potential benefits of nanotechnology innovative solutions in the construction sector . Web.
Karn, B., Kuiken, T., & Otto, M. (2009). Nanotechnology and in situ remediation: A review of the benefits and potential risks. Environmental Health Perspectives, 117 , 1823-1831.
Misra, R., Acharya, S., & Sahoo, S. K. (2010). Cancer nanotechnology: Application of nanotechnology in cancer therapy. Drug Discovery Today, 15 (19), 843-856.
Musee, N., C.Brent, A., & J.Ashton, P. (2010). South African research agenda to investigate the potential enviromental,health and safety risks of nanotechnology. South African Journal of Science, 106 (3/4), 6 pages.
Partyka, J., & Mazur, M. (2012). Prospects for the appliication of Nanotechnology. Journal of Nano-Electronics Physics, 4 (1).
Robinson, R. (2010). Application of nanotechnology in green building practises . Web.
Sekhon, S.B. (2010). Food nanotechnology-an overview. Nanotechnology, Science and Applications, 3 , 1-15.
Silva, G. A. (2010). Nanotechnology applications and approached for neuroregeneration and drug delivery to the central nervous system. Annals of New York Academy of Science, 1199 , 221-230.
Solomon, M., & D’Souza, G. G. (2011). Recent progress in the therapeutic applications of nanotechnology. Current Opinion in Pediatrics, 23 , 215-220.
Sozer, N., & Kokini, J. L. (2008). Nanotechnology and its applications in the food sector. Trends in Biotechnology, 27 (2), 82-90.
Srinivas, P. R., Philbert, M., Q.Vu, T., Huang, Q., Kokini, J. L., Saos, E., et al. (2010). Nanotechnology research: Applications in nutritional sciences. Journal of Nutrition, 140 (1), 119-124.
Vaddiraju, S., Tomazos, I., Burgess, D. J., Jain, F. C., & Papadimitrakopoulos, F. (2010). Emerging synergy between nanotechnology and implantable biosensors. Biosens Bioelectron, 25 (7), 1553-1565.
Vishwakarma, V., Samal, S. S., & N.Manoharan. (2010). Safety and risk associated with nanoparticles. J or Mineral & Material Characteristics & Engineering, 9 (5), 455-459.
Wickson, F., Baun, A., & Grieger, K. (2010). Nature and nanotechnology: Science,ideology and policy. Int J of Emerging Tech & Society, 8 (1), 5-23.
Williams, R. A., Kulinowski, K. M., White, R., & Louis, G. (2010). Risk characterization for nanotechnology. Risk Analysis, 30 (1), 144-155.
Yimin, Li (2011). Nano scale advances in catalysis and energy applications . Web.
Zhao, J (2009). Turning to nanotechnology for pollution control: Applications of nanoparticles . Web.
- Nanoscale Silver and Stem Cell Research
- Nanotechnology in Regenerative Medicine: Scaffold and Tissue Engineering
- Nanotechnology Risk in NanoBatteries
- Dialysis Water Treatment System
- Wind Power Exploitation to Generate Electricity
- Nuclear Power Exploitation to Generate Electricity
- How Does Magnetic Train Works
- Fossil Fuel, Nuclear Energy, and Alternative Power Sources
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2019, July 9). Nanotechnology: Applications and Implications. https://ivypanda.com/essays/nanotechnology/
"Nanotechnology: Applications and Implications." IvyPanda , 9 July 2019, ivypanda.com/essays/nanotechnology/.
IvyPanda . (2019) 'Nanotechnology: Applications and Implications'. 9 July.
IvyPanda . 2019. "Nanotechnology: Applications and Implications." July 9, 2019. https://ivypanda.com/essays/nanotechnology/.
1. IvyPanda . "Nanotechnology: Applications and Implications." July 9, 2019. https://ivypanda.com/essays/nanotechnology/.
Bibliography
IvyPanda . "Nanotechnology: Applications and Implications." July 9, 2019. https://ivypanda.com/essays/nanotechnology/.
- IEEE Xplore Digital Library
- IEEE Standards Association
- IEEE Spectrum Online
- More IEEE Sites

- The Nanotechnology Council
- NTC Travel Policy
- Frequently Asked Questions
- NTC Fellows
- 2024 Awardees
- 2023 Awardees
Call for Award Nominations
- Conferences 2024
- NTC Conference History
- Newsletter Issues
- Publications
- Distinguished Lecturers 2024
- Distinguished Lecturers 2023
- Past Distinguished Lecturers
- TryNano.org
- Technical Committees
- Technical Committee Award
- Industrial Advisory
- Standards Committee
- Women in Nanotechnology
- Young Professionals
Best PhD Thesis Award
- NTC Publication Awards
- NTC Technical Awards
Join the NTC
Sign up for Mailing List
Visit us on FaceBook
—Latest Publications—
IEEE Transactions on Nanotechnology
IEEE Open Journal of Nanotechnology
IEEE Transactions on NanoBioscience
IEEE Journal of Photovoltaics
IEEE Journal on Exploratory Solid-State Computational Devices and Circuits
IEEE Nanotechnology Magazine
IEEE Nanotechnology e-Newsletter
—-Resources—-
TryNano.org the Nanotech Learning Resource
Latest News
- NTC Young Professionals Update
- Announcing TNANO 2023 Best Paper Award
- Request for Proposals for 2024 IEEE Summer School on Nanotechnology
- Call for Applications – NTC Chapter’s Activity Grant
- Call for 2024 Nominations – IEEE Nanotechnology Council
- CFP: IEEE Journal on Exploratory Solid-State Computational Devices and Circuits (JxCDC)
- Seeking Regional Rep for NTC Young Professionals
News Categories
Recent comments.
- NTC Chapter’s Innovation Grant - Call for Applications - IEEE Nanotechnology Council on DL Program
- Call for Award Nominations 2023 - IEEE Nanotechnology Council on Awards
- Call for Proposals for Future Sites for IEEE-NANO 2026 and IEEE NMDC 2025 - IEEE Nanotechnology Council on NMDC
- Call for Award Nominations 2023 - IEEE Nanotechnology Council on Call for Award Nominations
- Call for Nominations for 2024 Distinguished Lecturers - IEEE Nanotechnology Council on DL Program
CALL FOR NOMINATIONS for:
Ieee nanotechnology council best phd thesis award in nanotechnology.
(established in 2022)
All nomination materials must reach the Education Awards Committee by March 1 st each year.
Download nomination form.
Description: This annual award recognizes a PhD thesis in nanotechnology with remarkable technology innovation or excellence which should have led to publications in NTC venues including journals and conferences. Any member with no conflict of interest (i.e. advisor-advisee relationship) with any member of the NTC ExCom, NTC Education Committee , or NTC Technical Committees can submit a nomination to the Award Committee for this award. Self-nominations are not allowed. The awardee will be selected by a committee composed of the Chair and 3 members. The Chair is appointed by the NTC President with recommendation from the VP of Educational Activities. The Chair appoints the committee members with approval by the NTC President.
Prize: Total honorarium of $500 and a certificate. Only one allowable recipient selected annually
Eligibility: The author of a nominated thesis must be an IEEE member and NTC participant for at least one year. Only materials from the thesis published in NTC sponsored publications (journals/magazines and NTC financially sponsored conferences) will be considered.
The author of the best PhD thesis in nanotechnology must have graduated within the past 3 years , and not be advised by a voting member of the NTC Executive Committee (ExCom) or a member of the Best PhD Awards Evaluation Committee.
A thesis can be nominated for this award only once. Unsuccessful nominations cannot be carried over or re-submitted over the following years.
There are no other requirements.
Basis for Judging : Each member of the Committee, evaluates each nomination on several aspects, including but not limited to: impact, quality of writing and presentation, relevance of the topic, its influence in nanotechnology based communities.
The range of the score by each member of the committee is from 0-5. The Chair will generate for each nomination a scoring table (with averages) to be distributed to voting members of the Committee. The Committee will rank a final pool of up to 3 nominated papers and recommend the winner. The nominations and recommendations will be presented in a report to the NTC ExCom for voting in its May meeting. The Committee Chair will not vote except to break ties.
Presentation: The award will be presented at the annual IEEE Conference on Nanotechnology (NANO) banquet. Attendance is mandatory to receive the award, but no travel allowance is provided.
- Submission of nominations: Email by March 1 st to the Education Awards Chair, Yu Sun Professor of Mechanical Engineering Canada Research Chair Micro & Nano Engineering Systems Robotics Institute Director University of Toronto, M5S 3G8, Canada Email: sun@mie.utoronto.ca
- Committee’s award recommendation(s): April 1 st
- Notification to awardee(s): May 15 th
For further information, please contact the Awards Committee Chair:
Your Name (required)
Your Email (required)
Subject (required)
Your Message (required)
- Entries feed
- Comments feed
- WordPress.org
- Privacy & Security
- Terms & Conditions
- Nondiscrimination Policy

COMMENTS
Nanotechnology is the study of the controlling the matter on an atom and molecular scale. Generally nanotechnology deals with structures sized between 1-100 nanometers in at least one. dimension ...
Nanotechnology is a relatively new field of science and technology that studies tiny objects (0.1-100 nm). Due to various positive attributes displayed by the biogenic synthesis of nanoparticles (NPs) such as cost-effectiveness, none to negligible environmental hazards, and biological reduction served as an attractive alternative to its counterpart chemical methods.
Nanotechnology, contrary to its name, has massively revolutionized industries around the world. This paper predominantly deals with data regarding the applications of nanotechnology in the modernization of several industries. A comprehensive research strategy is adopted to incorporate the latest data driven from major science platforms. Resultantly, a broad-spectrum overview is presented which ...
This review discusses a brief history of nanomaterials and their use throughout history to trigger advances in nanotechnology development. In particular, we describe and define various terms relating to nanomaterials. Various nanomaterial synthesis methods, including top-down and bottom-up approaches, are discussed. The unique features of ...
nanoscience is the key factor. Nanotechnology is multidisciplinary science which deals with physics, chemistry, materials science and other engineering sciences. The applications of Nanotechnology ...
Nanotechnology is starting to play a role in a number of commercial products, though in an evolutionary, rather than revolutionary way, says Peter Dobson. Peter Dobson Thesis 08 Nov 2016
Nano-Selenium: Novel Formulations for Biological and Environmental Applications. Description: This dissertation demonstrates, in multiple ways, that nanotechnology can be used to engineer effective solutions. This dissertation employs the colloidal route for synthesis of elemental ….
Nanotechnology is a multidisciplinary field that covers a vast and diverse array of devices derived from engineering, physics, chemistry, and biology. Nanotechnology has opened up by rapid ...
Nanotechnology is one of the most emerging fields of research within recent decades and is based upon the exploitation of nano-sized materials (e.g., nanoparticles, nanotubes, nanomembranes, nanowires, nanofibers and so on) in various operational fields. Nanomaterials have multiple advantages, including high stability, target selectivity, and plasticity. Diverse biotic (e.g., Capsid of viruses ...
This thesis focuses on the consideration of a question that the advent of what is called nanotechnology makes it increasingly urgent to philosophy in general and the philosophy of science in particular because of the inexistence of the "nanotechnology" stricto sensu, the lack of good definition and the default of something like a ...
This paper focuses on the epistemological status of nanotechnology. It argues a twofold thesis: on the one hand, nanotechnoscience is not just one more chapter in the history of science or technology, but a radical shift that opens new horizons. On the other hand, nanoscience can and must be seen as a new science, namely a cross-disciplinary ...
1. 'Nanotechnology' is a palette of disparate technologies at the nanoscale and does not necessarily constitute a useful category to discuss regulation or technology governance. 2. There is major uncertainty and ignorance regarding the potential impacts of many manufactured nanomaterials on health and the environment.
Graphene, a 2D nanomaterial, has garnered significant attention in recent years due to its exceptional properties, offering immense potential for revolutionizing various technological applications. In the context of the Internet of Things (IoT), which demands seamless connectivity and efficient data processing, graphene's unique attributes have positioned it as a promising candidate to prevail ...
NGs are defined as nano-sized ionic and non-ionic hydrogels made of synthetic or natural polymeric chains, chemically or physically cross-linked ( Molina et al., 2015; Neamtu et al., 2017 ). NGs possess a high water content (70-90% of the entire structure), a high degree of porosity and high load capacity.
The main goal of this thesis has been to devise a nanotechnology-based strategy and tools to spatially and temporally control biologically-relevant phenomena in-vitro which are important in some fields of medical research. 5 Gastrow, M. "Thinking small : the state of nanotechnology research and development in South Africa." ...
Emerging Leaders in Nanotechnology. Marcos H. D. Guimaraes. Giancarlo Franzese. Simona Badilescu. Satyabrata Mohapatra. 537 views. An interdisciplinary journal across nanoscience and nanotechnology, at the interface of chemistry, physics, materials science and engineering. It focuses on new nanofabrication methods and their ap...
The work of historians (Kim 2008, McCray 2005) shows how there may be many different histories of nanotechnology, but McCray documents one increasingly canonical "creation story" which credits Richard Feynman with articulating the guiding vision in his 1959 speech, "There's plenty of room at the bottom."While Feynman laid out the prospects of manipulating matter at the molecular and ...
Thesis. May-2016. POLY (AMIDOAMINE) DENDRIMER BASED NANOMATERIALS FOR DELIVERY OF ANTICANCER AGENTS. Matai, Ishita. Gopinath, P. Theses. Mar-2019. USE OF CARBON BASED NANOFILLERS FOR ENERGY HARVESTING APPLICATIONS. Kaur, Navjot.
crop production and assuring sustainability. Nanotechnology helps to improve agricultural production. by increasing the e ffi ciency of inputs and minimizing relevant losses. Nanomaterials o ff ...
2011. MODELING AND SIMULATION OF NANOWIRE SOLAR CELLS. Kumar, Jitendra. Manhas, Sanjeev; Singh, Dharmendra. M.Tech Dessertation. 2011. INVESTIGATION OF EFFECT OF NANO CRYSTALLIZATION ON MECHANICAL AND SHAPE MEMORY EFFECT OF CuZnAI BASED SHAPE MEMORY ALLOY, SYNTHESIZED BY HIGH ENERGY BALL MILLING (HEBM) Verma, Sujit Kumar. Daniel, B. S. S.
The applications of nanotechnology are as a result of investigating and utilizing these properties (Wickson et al., 2010). There are a host of substances utilised in nanotechnology, the most researched ones are carbon, silicon dioxide and titanium dioxide (Robinson, 2010). Others are aluminum, zinc, silver, copper and gold (Robinson, 2010).
thesis processes have developed their scientific base decades ago, long before other nanotechnology areas have emerged. One finds in this category the pyrol-ysis process for carbon black and the flame reaction for pigments, particle polymerization techniques, self-assembling of micelles in colloidal suspensions, and chemistry selfassembling.
Through nanoscience and nanotechnology it has become possible to study and create very useful functional devices, materials, and systems on the 1-100 nm (one billionth of a meter) length scale.
The author of the best PhD thesis in nanotechnology must have graduated within the past 3 years, and not be advised by a voting member of the NTC Executive Committee (ExCom) or a member of the Best PhD Awards Evaluation Committee. A thesis can be nominated for this award only once. Unsuccessful nominations cannot be carried over or re-submitted ...