Advances in Leukemia Research
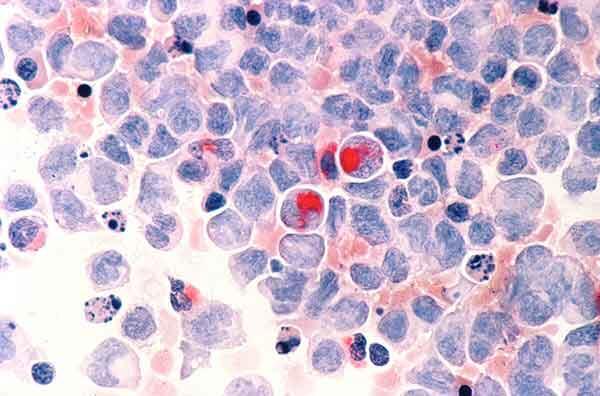
Human cells with acute myelocytic leukemia.
NCI-funded researchers are working to advance our understanding of how to treat leukemia. With progress in both targeted therapies and immunotherapies, leukemia treatment has the potential to become more effective and less toxic.
This page highlights some of the latest research in leukemia, including clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and research findings from recent studies.

Leukemia Treatment for Adults
The mainstays of leukemia treatment for adults have been chemotherapy , radiation therapy , and stem cell transplantation . Over the last two decades, targeted therapies have also become part of the standard of care for some types of leukemia. These treatments target proteins that control how cancer cells grow, divide, and spread. Different types of leukemia require different combinations of therapies. For a complete list of all currently approved drugs, see Drugs Approved for Leukemia.
Although much progress has been made against some types of leukemia, others still have relatively poor rates of survival. And, as the population ages, there is a greater need for treatment regimens that are less toxic .
Acute Lymphoblastic Leukemia (ALL) Treatment
Adult acute lymphoblastic leukemia (ALL) is a type of cancer in which the bone marrow makes too many lymphocytes (a type of white blood cell). It usually gets worse quickly and needs rapid treatment. Some recent research includes:
Combining less-toxic therapies
The intensive chemotherapy treatments used for ALL have serious side effects that many older patients cannot tolerate. Targeted therapies may have fewer side effects than chemotherapy. Clinical trials, including one at NCI , are now testing whether combinations of these types of therapies can be used instead of chemotherapy for older patients with a form of ALL called B-cell ALL.
Immunotherapy
Immunotherapies are treatments that help the body’s immune system fight cancer more effectively. Immunotherapy strategies being used or tested in ALL include:
CAR T-cell therapy
CAR T-cell therapy is a type of treatment in which a patient’s own immune cells are genetically modified to treat their cancer.
- Currently, one type of CAR T cell therapy is approved for the treatment of some children and young adults with ALL. They are now being explored for use in older adults with B-cell ALL.
- A second CAR T-cell therapy has been approved for adults with a type of ALL called B-cell precursor ALL that has not responded to treatment or has returned after previous treatment.
CAR T cell therapies are now being explored for other uses in ALL. For example, scientists hope that it will be possible to use CAR T-cell therapy to delay—or even replace—stem-cell transplantation in older, frailer patients.
Bispecific T-cell engagers
Another immunotherapy being tested in ALL is bispecific T-cell engagers (BiTEs). These drugs attach to immune cells and cancer cells, enabling the immune cells to easily find and destroy the cancer cell by bringing them closer together.
Once such BiTE, called blinatumomab (Blincyto) , was recently shown to improve survival for people with ALL who are in remission after chemotherapy, even when there is no trace of their disease.
Improving treatment for adolescents and young adults (AYAs)
An intensive treatment regimen developed for children with ALL has been found to also improve outcomes for newly diagnosed AYA patients . The pediatric regimen more than doubled the median length of time people lived without their cancer returning compared with an adult treatment regimen. Further studies are now testing the addition of targeted therapies to the combination .
Acute Myeloid Leukemia (AML) Treatment
Acute myeloid leukemia (AML) is the most common type of acute leukemia in adults. It can cause a buildup of abnormal red blood cells, white blood cells, or platelets.
AML tends to be aggressive and is harder to treat than ALL. However, AML cells sometimes have gene changes that cause the tumors to grow but can be targeted with new drugs. Researchers are starting to look at whether genomic sequencing of tumor cells can help doctors choose the best treatment (such as chemotherapy, targeted therapy, stem-cell transplant, or a combination of therapies) for each patient. Scientists are also testing other ways to treat AML.
New Treatment Option for Some People with AML
Combining ivosidenib with chemo is effective for AML with an IDH1 gene mutation.
Targeted therapies
Targeted therapies recently approved to treat AML with certain gene changes include Enasidenib (Idhifa) , Olutasidenib (Rezlidhia) , Ivosidenib (Tibsovo) , Venetoclax (Venclexta) , Gemtuzumab ozogamicin (Mylotarg) , Midostaurin (Rydapt) , Gilteritinib (Xospata) , Glasdegib (Daurismo) , and Quizartinib (Vanflyta) .
Other ways to treat AML
- Testing newer targeted therapies. Researchers continue to develop new drugs to shut down proteins that some leukemias need to grow. For example, new drugs called menin inhibitors stop cancer-promoting genes from being expressed.
- Studying ways to target AML cells indirectly. These include testing ways to make cancer cells more vulnerable to new and existing treatments.
- Targeting AML and related conditions. A type of less-aggressive cancer called myelodysplastic syndrome (MDS) can eventually progress to AML. Researchers are testing HDAC inhibitors and other drugs that alter how genes are switched on and off in both MDS and AML.
- Reducing side effects. Some older adults cannot tolerate the intensive treatments most commonly used for AML. Studies have recently found that several drug combinations can help older people with AML live longer while avoiding many serious side effects. New treatments to relieve symptoms of MDS have also been developed.
- Immunotherapy. CAR T-cells and BiTEs are being tested in people with AML.
Chronic Myelogenous Leukemia (CML) Treatment
Chronic myelogenous leukemia (CML) is a type of cancer in which the bone marrow makes too many granulocytes (a type of white blood cell). These granulocytes are abnormal and can build up in the blood and bone marrow so there is less room for healthy white blood cells, red blood cells, and platelets. CML usually gets worse slowly over time.
Blocking an abnormal protein
Most people with CML have a specific chromosome alteration called the Philadelphia chromosome , which produces an abnormal protein that drives the growth of leukemia cells. Targeted therapies that block this abnormal protein— imatinib (Gleevec) , nilotinib (Tasigna) , dasatinib (Sprycel) , and ponatinib (Iclusig) —have radically changed the outlook for people with CML, who now have close to a normal life expectancy.
Testing new combination therapies
Some people with CML continue to have detectable cancer cells in their body even after long-term treatment with drugs that target the protein produced by the Philadelphia chromosome. NCI-sponsored trials are testing whether the addition of immunotherapy or other targeted therapies to these drugs can reduce the number of CML cells in such patients.
Looking at whether patients can stop taking therapy
Researchers have found that some drugs that target the protein produced by the Philadelphia chromosome can be safely stopped in some CML patients rather than taken for life. These patients must undergo regular testing to ensure the disease has not come back.
Chronic Lymphocytic Leukemia (CLL) Treatment
Like ALL, chronic lymphocytic leukemia (CLL) is a type of cancer in which the bone marrow makes too many lymphocytes (a type of white blood cell). But unlike ALL, CLL is slow growing and worsens over time.
Targeted therapy
Ibrutinib (Imbruvica) . The targeted therapy ibrutinib (Imbruvica) was the first non-chemotherapy drug approved to treat CLL. It shuts down a signaling pathway called the B-cell receptor signaling pathway, which is commonly overactive in CLL cells. Depending on people’s age , ibrutinib may be given in combination with another targeted drug, rituximab (Rituxan) .
Clinical trials have shown that ibrutinib benefits both younger and older patients with CLL.
Venetoclax (Venclexta) and obinutuzumab (Gazyva) . In 2019, the Food and Drug Administration (FDA) approved the second chemotherapy-free initial treatment regimen for CLL , containing the targeted therapies venetoclax (Venclexta) and obinutuzumab (Gazyva) .
Other combinations of these drugs plus ibrutinib are now being used or tested for CLL, including • ibrutinib and venetoclax in people with newly diagnosed CLL • ibrutinib, obinutuzumab, and venetoclax in older adults with newly diagnosed CLL • ibrutinib and obinutuzumab with or without venetoclax in younger adults with newly diagnosed CLL
An ongoing trial at NCI is also testing whether giving the combination of venetoclax and obinutuzumab to some people with CLL before symptoms develop can help them live longer overall.
Zanubrutinib (Brukinsa) . In early 2023, the FDA approved a drug that works in a similar manner to ibrutinib, called zanubrutinib (Brukinsa) , for people with CLL. A large study showed that zanubrutinib alone has fewer side effects and is more effective than ibrutinib for people whose leukemia has returned after initial treatment. More research is now needed to understand how to best combine zanubrutinib with other newer therapies, such as venetoclax.
CAR T-cell therapy is also being tested in adults with CLL. Researchers would like to know if using this type of immunotherapy early in the course of treatment would be more effective than waiting until the cancer recurs.
Hairy Cell Leukemia (HCL) Treatment
Hairy cell leukemia (HCL) is a type of cancer in which the bone marrow makes too many lymphocytes (a type of white blood cell). The disease is called hairy cell leukemia because the abnormal lymphocytes look "hairy" when viewed under a microscope. This rare type of leukemia gets worse slowly, or sometimes does not get worse at all.
Combinations of drugs
Researchers are studying combinations of drugs to treat HCL. For example, in a recent small study, a combination of two targeted therapies— vemurafenib (Zelboraf) and rituximab (Rituxan) — led to long-lasting remissions for most participants with HCL that had come back after previous treatments. More drug combinations are currently being tested in clinical trials.
Leukemia Treatment for Children
For the two most common types of leukemia, AML and ALL, standard leukemia treatments for children have been chemotherapy, radiation therapy, and stem-cell transplant. Despite great improvements in survival for children with many types of leukemia, some treatments don't always work. Also, some children later experience a relapse of their disease. Others live with the side effects of chemotherapy and radiation therapy for the rest of their lives, highlighting the need for less toxic treatments.
Now researchers are focusing on targeted drugs and immunotherapies for the treatment of leukemia in children. Newer chemotherapy drugs are also being tested.
Targeted Therapies
Targeted therapies that have been approved or are being studied for children with leukemia include:
- imatinib (Gleevec) and dasatinib (Sprycel), which are approved for the treatment of children with CML as well as those with a specific type of ALL. The approvals are for children whose cancer cells have the Philadelphia chromosome.
- sorafenib (Nexavar) , which has been studied in combination with standard chemotherapy for children with AML whose leukemia has changes in a gene called FLT3. The addition of sorafenib to standard treatment was safe, and its addition may improve survival time free from leukemia. Other ongoing clinical trials are testing drugs that target FLT3 more specifically than sorafenib (such as gilteritinib).
- larotrectinib (Vitrakvi) , which is being tested in children with leukemia that has a specific change in a gene called NTRK .
More possible targets for the treatment of childhood cancers are discovered every year, and many new drugs that could potentially be used to treat cancers that have these targets are being tested through the Pediatric Preclinical In Vivo Testing Consortium (PIVOT) .
CAR T-cell therapy has recently generated great excitement for the treatment of children with relapsed ALL. One CAR T-cell therapy, tisagenlecleucel (Kymriah) , was approved in 2017 for some children with relapsed ALL.
Researchers continue to address remaining challenges about the use of CAR T-cell therapy in children with leukemia:
- Sometimes, leukemia can become resistant to tisagenlecleucel. Researchers in NCI’s Pediatric Oncology Branch have developed CAR T cells that target leukemia cells in a different way. An ongoing clinical trial is testing whether the combination of these two types of CAR T cells can provide longer-lasting remissions.
- CAR T cells are currently only approved for use in leukemia that has relapsed or proved resistant to standard treatment. A clinical trial from COG is now testing tisagenlecleucel as part of first-line therapy in children with ALL at high risk of relapse.
- More research is needed to understand which children who receive CAR T cells are at high risk of developing resistance to treatment. Researchers also plan to test whether strategies such as combining CAR T-cell therapy with other immunotherapies may help prevent resistance from developing.
- Other research, both in NCI’s Pediatric Oncology Branch and at other institutions, is focused on creating CAR T-cell therapies that work for children with other types of childhood leukemia, such as AML. Several clinical trials of these treatments, including one led by NCI researchers , are now under way.
Two other drugs that use the body’s immune system to fight cancer have shown promise for children with leukemia:
- In clinical trials, the drug was shown to be more effective than chemotherapy in treating ALL that has relapsed in children and young adults.
- An NCI-sponsored trial is now testing the drug as part of treatment for newly diagnosed ALL in children, adolescents, and young adults .
- A drug called inotuzumab ozogamicin (Besponsa) is being tested in children with relapsed B-cell ALL. This drug consists of an antibody that can bind to cancer cells linked to a drug that can kill those cells. An NCI-sponsored trial is also testing the drug as part of treatment for newly diagnosed ALL in children and adolescents at higher risk of relapse.
Chemotherapy
In addition to targeted therapies and immunotherapies, researchers are also working to develop new chemotherapy drugs for leukemia and find better ways to use existing drugs. In 2018, a large clinical trial showed that adding the drug nelarabine (Arranon) to standard chemotherapy improves survival for children and young adults newly diagnosed with T-cell ALL.
Other drugs are being tested that may make standard chemotherapy drugs more effective. These drugs include venetoclax , which has been approved for older adults with some types of leukemia and is now being tested in children .
Survivorship
Children’s developing brains and bodies can be particularly sensitive to the harmful effects of cancer treatment. Because many children treated for cancer go on to live long lives, they may be dealing with these late effects for decades to come.
The NCI-funded Childhood Cancer Survivor Study , ongoing since 1994, tracks the long-term harmful effects of treatments for childhood cancer and studies ways to minimize these effects. NCI also funds research into addressing ways to help cancer survivors cope with and manage health issues stemming from cancer treatment, as well into altering existing treatment regimens to make them less toxic in the long term.
For example, one study found that, in children with ALL, radiation therapy to prevent the cancer from returning in the brain is likely unnecessary . The study found that radiation can even be omitted for children at the highest risk of the cancer coming back, reducing the risk of future problems with thinking and memory, hormone dysfunction, and other side effects of radiation to the brain.
Preventing and Treating Graft Versus Host Disease
Many people with leukemia—both adults and children—have a stem-cell transplant as part of their treatment. If the new stem cells come from a donor, the immune cells they produce may be able to attack any cancer cells that remain in the body.
But sometimes, immune cells produced by donor stem cells attack healthy tissues of the body instead. This condition, called graft versus host disease ( GVHD ), can affect nearly every organ and can cause many painful and debilitating symptoms.
In recent years, several drugs have been approved by the FDA for the treatment of GVHD, including:
• ibrutinib, which is also used as a treatment for some types of leukemia • ruxolitinib (Jakafi) • belumosudil (Rezurock)
Researchers are also testing ways to prevent GVHD from developing in the first place. For example, a recent study found that removing certain immune cells from donated stem cells before they are transplanted may reduce the risk of chronic GVHD without any apparent increase in the likelihood of relapse.
NCI-Supported Research Programs
Many NCI-funded researchers working at the NIH campus and across the United States and the world are seeking ways to address leukemia more effectively. Some research is basic, exploring questions as diverse as the biological underpinnings of cancer. And some is more clinical, seeking to translate this basic information into improving patient outcomes. The programs listed below are a small sampling of NCI’s research efforts in leukemia.
NCI’s Leukemia Specialized Programs of Research Excellence (SPORE) promotes collaborative, interdisciplinary research. SPORE grants involve both basic and clinical/applied scientists working together. They support the efficient movement of basic scientific findings into clinical settings, as well as studies to determine the biological basis for observations made in individuals with cancer or in populations at risk for cancer.
The Pediatric Immunotherapy Discovery and Development Network (PI-DDN) is working to discover and characterize new targets for immunotherapies, design experimental models to test the effectiveness of pediatric immunotherapies, develop new immunotherapy treatments, and improve the understanding of tumor immunity in pediatric cancer patients. The PI-DDN was established as part of the Cancer Moonshot initiative.
The Fusion Oncoproteins in Childhood Cancers (FusOnC2) Consortium is also part of the Cancer Moonshot initiative. The consortium of collaborating research teams will work to advance the understanding of how five important fusion oncoproteins help drive pediatric cancers, including leukemia, and apply this knowledge towards developing drugs that target these proteins.
NCI has also formed partnerships with the pharmaceutical industry, academic institutions, and individual investigators for the early clinical evaluation of innovative cancer therapies. The Experimental Therapeutics Clinical Trials Network (ETCTN) was created to evaluate these therapies using a coordinated, collaborative approach to early-phase clinical trials.
The Pediatric Early-Phase Clinical Trials Network was established to help identify and develop effective new drugs for children and adolescents with cancer. The network’s focus is on phase I and early phase II trials, as well as pilot studies of novel drugs and treatment regimens to determine their tolerability.
NCI’s Pediatric Preclinical In Vivo Testing Consortium (PIVOT) develops mouse models to allow early, rapid testing of new drugs for pediatric cancers, including leukemia. The models are all derived from tissue samples taken from patients’ tumors. The consortium partners both with commercial drug companies and with drug development efforts at universities and cancer centers.
The Therapeutically Applicable Research to Generate Effective Treatments (TARGET) program uses a comprehensive approach to determine the genetic changes that drive childhood cancers. The goal of the program is to use data to guide the development of effective, less toxic therapies. TARGET is organized into disease-specific teams, including those for ALL and AML.
Researchers in NCI’s Division of Cancer Epidemiology and Genetics (DCEG) investigate novel, molecular biomarkers for leukemia, as well as clarify relationships of established risk factors. Studies include those looking at environmental and workplace exposure, families with multiple leukemia cases, and inherited bone marrow failure syndromes to name a few.
Clinical Trials
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Search NCI-Supported Clinical Trials to find leukemia-related trials now accepting patients.
Leukemia Research Results
The following are some of our latest news articles on leukemia research:
- Quizartinib Approval Adds New Treatment Option for AML, Including in Older Patients
- Blinatumomab Increases Survival for Infants with an Aggressive Type of ALL
- Revumenib Shows Promise in Treating Advanced Acute Myeloid Leukemia
- Help Desk for Oncologists Treating People with a Rare Leukemia Pays Big Dividends
- Zanubrutinib’s Approval Improves Targeted Treatment for CLL
- Trial Suggests Expanded Role for Blinatumomab in Treating ALL
View the full list of Leukemia Research Results and Study Updates .
What is new in acute myeloid leukemia classification?
- Open access
- Published: 15 April 2024
- Volume 59 , article number 15 , ( 2024 )
Cite this article
You have full access to this open access article
- Hee Sue Park 1 , 2
70 Accesses
Explore all metrics
Recently, the International Consensus Classification (ICC) and the 5 th edition of the World Health Organization classification (WHO2022) introduced diagnostically similar yet distinct approaches, which has resulted in practical confusion. This review compares these classification systems for acute myeloid leukemia (AML), building up on the revised 4th edition of WHO (WHO2016). Both classifications retain recurrent genetic abnormalities as a primary consideration. However, they differ in terms of blast threshold. The ICC mandates a minimum of 10% blasts in the bone marrow or peripheral blood, whereas the WHO2022 does not specify a blast cut-off. AML with BCR::ABL1 requires > 20% blast count in both classifications. In WHO2022, AML with CEBPA mutation requires > 20% blasts. TP53 mutation, a new entity is exclusive to ICC, diagnosed with > 20% blasts and variant allele frequency > 10%. AML with myelodysplasia-related changes is defined by cytogenetic or gene mutation-based criteria, not morphological dysplasia. Eight genes were common to both groups: ASXL1 , BCOR , EZH2 , SF3B1 , SRSF2 , STAG2 , U2AF1 , and ZRSR2 . An additional gene, RUNX1 , was included in the ICC classification. AML cases defined by differentiation (WHO2022) and AML not otherwise specified (ICC) are categorized as lacking specific defining genetic abnormalities, WHO2022 labels this as a myeloid neoplasm post cytotoxic therapy (MN-pCT), described as an appendix after specific diagnosis. Similarly, in ICC, it can be described as “therapy-related”, without a separate AML category.
Avoid common mistakes on your manuscript.
Introduction
The World Health Organization (WHO) classification of hematolymphoid tumors has long served as an international diagnostic criterion. However, in 2022, the International Consensus Classification (ICC) and the 5 th edition of the WHO classification (WHO2022) offered similar but distinct diagnostic approaches, leading to confusion [ 1 , 2 , 3 , 4 , 5 ]. Since the French-American-British classification in 1976, subsequent updates like WHO2001, WHO2008, and WHO2016, have incorporated new diagnostic criteria that integrate molecular, pathological, and clinical variables into a morphological classification [ 6 , 7 , 8 , 9 ]. The myeloblast threshold in diagnostic criteria has gradually decreased, with genetic abnormalities emerging as a crucial criterion. The evolution has made personalized management more feasible over time. This review explores the changes from the revised 4th edition of WHO2016 to WHO 2022 and the ICC classification, focusing on acute myeloid leukemia (AML).
WHO2016: acute myeloid leukemia with recurrent genetic abnormalities
Genetic abnormalities continue to be key diagnostic criteria. The WHO2016 classification, which defined “AML with recurrent genetic abnormalities”, was renamed “AML with defining genetic abnormalities” in WHO2022 [ 10 ]. While maintaining the same ICC, additional “other rare recurring translocations” subgroups were created [ 5 ]. Both WHO2022 and ICC were broader in scope compared to WHO2016 (Table 1 ).
The key change in WHO2022 is the exclusion of the myeloblast percentage threshold for diagnosis when specific genetic abnormalities are present. Unlike WHO2016, where the myeloblast count was not a significant factor in diagnosing certain AML subtypes, such as AML with t(8;21)(q22;q22.1), AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22), and acute promyelocytic leukemia (APL) with PML-RARA , WHO2022 now applies myeloblast count criteria to additional genetic abnormalities such as t(9;11)(p21.3;q23.2), t(6;9)(p23;q34.1), inv(3)(q21.3q26.2), t(3;3)(q21.3;q26.2) or t(1;22)(p13.3;q13.1), while excluding AML with BCR::ABL1 fusion and CEBPA mutation. A novel structure for “AML with other defined genetic alterations” was introduced, including new and/or uncommon AML subtypes that may be included in future editions. The ICC further categorized subgroups into “AML with recurrent genetic abnormalities” and “other rare recurring translocations”. Notable differences include 1) the incorporation of additional RARA , KMT2A , and MECOME rearrangements and 2) a requirement for blast count exceeding 10% for diagnosis, except in cases of t(9;22)(22)(q34.1;q11.2)/ BCR::ABL1 and TP53 mutations, which require a blast count exceeding 20%.
In WHO2016 classification, AML with mutated NPM1 , AML with biallelic mutations in CEBPA , and AML with mutated RUNX1 were classified as AML with genetic mutations. AML with mutated NPM1 and CEBPA remained classified in both the WHO2022 and ICC classifications. However, AML with mutated RUNX1 was excluded from the provisional diagnosis due to its limited clinical significance.
AML with mutated NPM1
NPM1 -mutated AML has been recognized as a distinct entity since 2008. Morphologically, blasts exhibit monocytic differentiation, and this subtype is frequently observed in young patients with a high prevalence of the normal karyotype [ 11 , 12 , 13 ]. There is a discrepancy in the blast threshold for diagnosis between the ICC and WHO2022. ICC requires a blast count ≥ 10%, whereas WHO2022 does not specify a blast number cutoff. Although an increase in blasts exists in most AML cases with mutated NPM1 , if the blast count is < 10%, the diagnosis is changed to “AML with NPM1” in WHO2022 and “ NPM1 -mutated myelodysplastic syndrome (MDS)” in ICC. NPM1 mutations are also detected in MDS and MDS/MPN [ 14 ], occurring in approximately 2% of MDS, cases with excess blasts [ 15 ], leading to potential confusion in clinical management and treatment decisions.
Especially concerning AML with biallelic mutation of CEBPA
In WHO2022, this category is termed “AML with CEBPA mutation”, encompassing biallelic (bi CEBPA ) and single mutations in the basic leucine zipper region (smbZIP- CEBPA ) [ 10 ]. Conversely, ICC designates the diagnosis as “AML with mutated bZIP CEBPA ”, emphasizing the bZIP domain mutation irrespective of its mono or biallelic nature. This conclusion is supported by recent studies demonstrating that bZIP domain mutations are linked to favorable clinical outcomes [ 4 , 16 ]. The blast count diagnostic criteria in ICC, consistent with other entities, is ≥ 10%. In contrast, WHO2022 suggests a blast count of ≥ 20%. Common morphological features often indicate AML with maturation (FAB M2) or AML without maturation (FAB M1) [ 17 ]. However, distinctive morphological features are lacking and occur at a frequency of 7–16% in adults and 4.5–15% in pediatric patients [ 4 ].
AML with TP53 mutation
Notably , TP53 was not included in the WHO2022 AML with defined genetic abnormalities. Instead, a biallelic TP53 alteration subtype is recognized in MDS, which is considered equivalent to AML. The diagnostic criteria for TP53 alterations in ICC require a blast count ≥ 20%, a higher threshold than in other entities, in conjunction with a variant allele frequency ≥ 10%. Therefore, when the blast count is < 20% in peripheral blood and bone marrow, MDS is characterized by both classifications. In WHO2022, “MDS with biallelic TP53 inactivation (MDS-bi TP53 )” is defined for cases with < 20% blasts, whereas ICC delineates “MDS mutated TP53 ” according to blast count differences. In addition, in cases of monoallelic loss, there was no significant clinical difference compared to the wild type [ 18 ]. Therefore, both classifications focus on biallelic loss.
AML with NUP98 rearrangement
This category is a newly introduced as “AML with NUP98 rearrangement” in WHO2022 and as “AML with t(5;11)(q35.2;p15.4)/ NUP98 :: NSD1 and with t(11;12)(p15.4;p13.3)/ NUP98 : KMD5A and NUP98 and other partners” in ICC. NUP98 exhibits multiple fusion partners and, although infrequent, is associated with a poor prognosis [ 19 ]. The blast count requirement was maintained as a minimum for both classifications.
WHO2016: AML with myelodysplasia-related changes
In WHO2022, this category was named “AML with myelodysplasia-related (AML-MR)”, and ICC classified it as “AML with myelodysplasia-related gene mutation” and “AML with myelodysplasia- related cytogenetic abnormalities”. It was incorporated from an independent category into “AML with defining genetic abnormalities” in WHO2022. Both classification systems exclude morphology-based diagnostic criteria and emphasize molecular abnormalities. Some existing cytogenetic criteria have been updated, and gene mutations have been added. The myeloblast threshold requires ≥ 20% in both peripheral blood or bone marrow for this category.
AML with myelodysplasia-related cytogenetic abnormalities
Although there were no significant differences from the previous WHO2016, some distinctions were observed between the two classification systems (Table 2 ). Complex karyotype (≥ 3 abnormalities) and chromosomal aberrations on chromosomes 5, 7, 12, 17, and X were common in both systems. In the ICC, del(11q) was excluded, and +8 and del(20q) were added. Additionally, balanced abnormalities in WHO2016 were moved to “AML with other rare recurring translocations”.
AML with myelodysplasia-related gene mutations
Eight genes were common to both groups: ASXL1 , BCOR , EZH2 , SF3B1 , SRSF2 , STAG2 , U2AF1 , and ZRSR2 . An additional gene, RUNX1 , was included in ICC. Minimum variant allele frequencies are not required for these genes. They are associated with an adverse prognosis [ 20 , 21 ].
WHO2016: therapy-related myeloid neoplasm
In WHO2022, a category named “Myeloid neoplasms post cytotoxic therapy (MN-pCT)” was introduced, encompassing AML, MDS, and MDS/MPN that develop after cytotoxic therapy inducing DNA damage [ 10 ]. Cytotoxic therapies, such as PARP1 inhibitors and methotrexate, were excluded. It is recommended to append “post cytotoxic therapy” after the specific diagnosis. Similarly, the ICC no longer recognized it as a distinct entity of AML.
WHO2016: acute myeloid leukemia, not otherwise specified
This group lacked genetic abnormalities and was classified based on morphology. Although this subtype has limited prognostic significance, it offers a practical paradigm [ 5 , 10 ]. Both WHO and ICC maintain diagnostic criteria of ≥ 20% myeloblasts. Additionally, this category includes cases with overlapping phenotypic markers of the two lineages, such as mixed phenotype acute leukemia (MPAL) and early T-precursor lymphoblastic leukemia/lymphoma (ETT-ALL). Until recently, the genomics of MPAL has been predominantly associated with KMT2A rearrangement. However, recent findings have highlighted the involvement of the RAS pathway in B/M MPAL, the JAK/STAT pathway in T/M MPAL, ZEB2-BCL11B , NUP214-ABL1 , and ETV6 in T/myeloid cells. These discoveries suggest the potential for future addition of new entities [ 10 , 22 ].
European LeukemiaNet (ELN) risk stratification 2022
Aligned with the updated AML classification system that emphasizes genetic mutations, the ELN has released 2022 risk stratification guidelines based on the ICC classification (Table 3 ) [ 23 , 24 ]. Key changes included: 1) retention of recurrent genetic abnormalities and the addition of new genetic mutations. Eight genes were included in the adverse risk category and designated as AML with myelodysplasia-related gene mutations. 2) The prognostic division based on the allelic ratio of FLT3-ITD in cases of AML coexisting with mutated NPM1 and FLT3-ITD was eliminated in ELN2017. This is due to the lack of standardization in the method for measuring the FLT - ITD allelic ratio. 3) Additionally, NPM1 mutated AML with additional adverse-risk cytogenetic abnormalities was classified as an adverse risk. 4) Mutations in the basic leucine zipper region of CEBPA that affect in-frame confer a favorable prognosis, regardless of whether monoallelic or biallelic mutations. 5) Additional cytogenetic abnormalities such as t(3q26.2;v)/ MECOME -rearranged and t(8;16)(p11.2;p13.3)/ KAT6A :: CREBBP fusion, are now included in the adverse risk group [ 25 , 26 ]. 6) Hyperdiploidy with multiple trisomies is not considered a complex karyotype.
In recent years, studies on the genetic spectrum of AML have increased, expanding the treatment possibilities [ 27 ]. Various gene-targeted therapies, such as FLT3 inhibitors, are being introduced in chemotherapy regimens and are undergoing continuous clinical trials [ 28 , 29 ]. While both the WHO2022 and ICC classification systems are based on these findings, these new classifications have added complexity for researchers and physicians. Differences in terminology and the introduction of updated/new diagnostic entities can cause confusion in the field, affecting diagnosis, management, clinical outcome assessment, and clinical trials [ 2 , 3 ]. Additionally, the diagnosis and risk stratification of AML require various molecular tests, which depend on the adequate economic and diagnostic capacity for their execution. Molecular tests, taking more than two weeks for a formal report, may result in a delayed diagnosis compared to traditional morphological diagnoses, potentially delaying treatment. The new classification system integrates morphological, immunophenotypic, molecular, and cytogenetic information, facilitating the adoption of precision medicine. Consequently, treatment decisions should be based on comprehensive laboratory tests, medical histories, and clinical information.
Availability of data and materials
No datasets were generated or analysed during the current study.
Appelbaum FR. WHO, what, when, where, and why: new classification systems for acute myeloid leukemia and their impact on clinical practice. Best Pract Res Clin Haematol. 2023;36:101518.
Article PubMed Google Scholar
Falini B, Martelli MP. Comparison of the International Consensus and 5th WHO edition classifications of adult myelodysplastic syndromes and acute myeloid leukemia. Am J Hematol. 2023;98:481–92.
Shallis RM, Daver N, Altman JK, et al. Standardising acute myeloid leukaemia classification systems: a perspective from a panel of international experts. Lancet Haematol. 2023;10:e767–76.
Article CAS PubMed Google Scholar
Weinberg OK, Porwit A, Orazi A, et al. The International Consensus Classification of acute myeloid leukemia. Virchows Arch. 2023;482:27–37.
Arber DA, Orazi A, Hasserjian RP, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–28.
Article CAS PubMed PubMed Central Google Scholar
Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the acute leukaemias French-American-British (FAB) co-operative group. Br J Haematol. 1976;33:451–8.
Jaffe ES, Harris NL, Stein H, Vardiman JW. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. 3rd ed. Lyon, France: IARC Press; 2001.
Swerdlow SH, Campo E, Harris NL, et al editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press; 2008.
Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–19.
Article PubMed PubMed Central Google Scholar
Falini B, Brunetti L, Sportoletti P, Martelli MP. NPM1-mutated acute myeloid leukemia: from bench to bedside. Blood. 2020;136:1707–21.
Hwang SM. Classification of acute myeloid leukemia. Blood Res. 2020;55:S1–4.
Falini B, Martelli MP, Bolli N, et al. Acute myeloid leukemia with mutated nucleophosmin (NPM1): is it a distinct entity? Blood. 2011;117:1109–20.
Montalban-Bravo G, Kanagal-Shamanna R, Sasaki K, et al. NPM1 mutations define a specific subgroup of MDS and MDS/MPN patients with favorable outcomes with intensive chemotherapy. Blood Adv. 2019;3:922–33.
Forghieri F, Nasillo V, Paolini A, et al. NPM1-mutated myeloid neoplasms with< 20% blasts: a really distinct clinico-pathologic entity? International J Mol Sci. 2020;21:8975.
Article CAS Google Scholar
Su L, Shi Y-Y, Liu Z-Y, Gao S-J. Acute myeloid leukemia with CEBPA mutations: current progress and future directions. Front Oncol. 2022;12:806137.
Snaddon J, Smith ML, Neat M, et al. Mutations of CEBPA in acute myeloid leukemia FAB types M1 and M2. Genes Chromosomes Cancer. 2003;37:72–8.
Bernard E, Nannya Y, Hasserjian RP, et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat Med. 2020;26:1549–56.
Bertrums EJ, Smith JL, Harmon L, et al. Comprehensive molecular and clinical characterization of NUP98 fusions in pediatric acute myeloid leukemia. Haematologica. 2023;108:2044.
Gao Y, Jia M, Mao Y, et al. Distinct mutation landscapes between acute myeloid leukemia with myelodysplasia-related changes and de novo acute myeloid leukemia. Am J Clin Pathol. 2022;157:691–700.
Tazi Y, Arango-Ossa JE, Zhou Y, et al. Unified classification and risk-stratification in acute myeloid leukemia. Nat Commun. 2022;13:4622.
Alexander TB, Orgel E. Mixed phenotype acute leukemia: current approaches to diagnosis and treatment. Curr Oncol Rep. 2021;23:1–10.
Article Google Scholar
Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77.
Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Kayser S, Hills RK, Langova R, et al. Characteristics and outcome of patients with acute myeloid leukaemia and t (8; 16)(p11; p13): results from an International Collaborative Study. Br J Haematol. 2021;192:832–42.
Ottema S, Mulet-Lazaro R, Beverloo HB, et al. Atypical 3q26/MECOM rearrangements genocopy inv (3)/t (3; 3) in acute myeloid leukemia. Blood. 2020;136:224–34.
Short NJ, Tallman MS, Pollyea DA, Ravandi F, Kantarjian H. Optimizing risk stratification in acute myeloid leukemia: dynamic models for a dynamic therapeutic landscape. J Clin Oncol. 2021;39:2535.
Ahn J-S, Kim H-J. FLT3 mutations in acute myeloid leukemia: a review focusing on clinically applicable drugs. Blood Res. 2022;57(S1):32–3.
Kim D-S, Byun J-M, Shin D-Y, et al. Concomitant ruxolitinib with cytarabine-bassed induction chemotherapy in secondary acute meyloid leukemia evolving from myeloproliferative neoplasm. Blood Res. 2023;58(3):155–7.
Download references
No funding.
Author information
Authors and affiliations.
Department of Laboratory Medicine, Chungbuk National University Hospital, 776, 1 Sunhwan-ro, Seowon-gu, Cheongju, Chungcheongbuk-do, 28644, Republic of Korea
Hee Sue Park
Department of Laboratory Medicine, Chungbuk National University College of Medicine, 776, 1 Sunhwan-ro, Seowon-gu, Cheongju, Chungcheongbuk-do, 28644, Republic of Korea
You can also search for this author in PubMed Google Scholar
Contributions
H.S.Park wrote the main manuscript text.
Corresponding author
Correspondence to Hee Sue Park .
Ethics declarations
Ethics approval and consent to participate.
This review did not require approval from the relevant institutional review board or ethics committee.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Park, H.S. What is new in acute myeloid leukemia classification?. Blood Res. 59 , 15 (2024). https://doi.org/10.1007/s44313-024-00016-8
Download citation
Received : 29 December 2023
Accepted : 05 April 2024
Published : 15 April 2024
DOI : https://doi.org/10.1007/s44313-024-00016-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Acute myeloid leukemia
- International Consensus Classification
- WHO classification
- Find a journal
- Publish with us
- Track your research
Advertisement
Mutation Patterns Predict Drug Sensitivity in Acute Myeloid Leukemia
Clin Cancer Res 2024;XX:XX–XX

- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
- Proof April 15 2024
Guangrong Qin , Jin Dai , Sylvia Chien , Timothy J. Martins , Brenda Loera , Quy H. Nguyen , Melanie L. Oakes , Bahar Tercan , Boris Aguilar , Lauren Hagen , Jeannine McCune , Richard Gelinas , Raymond J. Monnat , Ilya Shmulevich , Pamela S. Becker; Mutation Patterns Predict Drug Sensitivity in Acute Myeloid Leukemia. Clin Cancer Res 2024; https://doi.org/10.1158/1078-0432.CCR-23-1674
Download citation file:
- Ris (Zotero)
- Reference Manager
The inherent genetic heterogeneity of acute myeloid leukemia (AML) has challenged the development of precise and effective therapies. The objective of this study was to elucidate the genomic basis of drug resistance or sensitivity, identify signatures for drug response prediction, and provide resources to the research community.
We performed targeted sequencing, high-throughput drug screening, and single-cell genomic profiling on leukemia cell samples derived from patients with AML. Statistical approaches and machine learning models were applied to identify signatures for drug response prediction. We also integrated large public datasets to understand the co-occurring mutation patterns and further investigated the mutation profiles in the single cells. The features revealed in the co-occurring or mutual exclusivity pattern were further subjected to machine learning models.
We detected genetic signatures associated with sensitivity or resistance to specific agents, and identified five co-occurring mutation groups. The application of single-cell genomic sequencing unveiled the co-occurrence of variants at the individual cell level, highlighting the presence of distinct subclones within patients with AML. Using the mutation pattern for drug response prediction demonstrates high accuracy in predicting sensitivity to some drug classes, such as MEK inhibitors for RAS-mutated leukemia.
Our study highlights the importance of considering the gene mutation patterns for the prediction of drug response in AML. It provides a framework for categorizing patients with AML by mutations that enable drug sensitivity prediction.
Article PDF first page preview
Supplementary data.
Supplementary Table S1-S14
Supplementary Figures S1-S8
Citing articles via
Email alerts.
- Online First
- Collections
- Online ISSN 1557-3265
- Print ISSN 1078-0432
AACR Journals
- Blood Cancer Discovery
- Cancer Discovery
- Cancer Epidemiology, Biomarkers & Prevention
- Cancer Immunology Research
- Cancer Prevention Research
- Cancer Research
- Cancer Research Communications
- Clinical Cancer Research
- Molecular Cancer Research
- Molecular Cancer Therapeutics
- Info for Advertisers
- Information for Institutions/Librarians
- Privacy Policy
- Copyright © 2023 by the American Association for Cancer Research.
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Research article
- Open access
- Published: 15 April 2024
Shared genetic architecture between autoimmune disorders and B-cell acute lymphoblastic leukemia: insights from large-scale genome-wide cross-trait analysis
- Xinghao Yu 1 , 2 na1 ,
- Yiyin Chen 1 , 2 na1 ,
- Jia Chen 1 ,
- Huimin Lu 3 ,
- Depei Wu 1 , 2 &
- Yang Xu 1 , 2
BMC Medicine volume 22 , Article number: 161 ( 2024 ) Cite this article
163 Accesses
Metrics details
To study the shared genetic structure between autoimmune diseases and B-cell acute lymphoblastic leukemia (B-ALL) and identify the shared risk loci and genes and genetic mechanisms involved.
Based on large-scale genome-wide association study (GWAS) summary-level data sets, we observed genetic overlaps between autoimmune diseases and B-ALL, and cross-trait pleiotropic analysis was performed to detect shared pleiotropic loci and genes. A series of functional annotation and tissue-specific analysis were performed to determine the influence of pleiotropic genes. The heritability enrichment analysis was used to detect crucial immune cells and tissues. Finally, bidirectional Mendelian randomization (MR) methods were utilized to investigate the casual associations.
Our research highlighted shared genetic mechanisms between seven autoimmune disorders and B-ALL. A total of 73 pleiotropic loci were identified at the genome-wide significance level ( P < 5 × 10 –8 ), 16 of which had strong evidence of colocalization. We demonstrated that several loci have been previously reported (e.g., 17q21) and discovered some novel loci (e.g., 10p12, 5p13). Further gene-level identified 194 unique pleiotropic genes, for example IKZF1 , GATA3 , IKZF3 , GSDMB , and ORMDL3 . Pathway analysis determined the key role of cellular response to cytokine stimulus, B cell activation, and JAK-STAT signaling pathways. SNP-level and gene-level tissue enrichment suggested that crucial role pleiotropic mechanisms involved in the spleen, whole blood, and EBV-transformed lymphocytes. Also, hyprcoloc and stratified LD score regression analyses revealed that B cells at different developmental stages may be involved in mechanisms shared between two different diseases. Finally, two-sample MR analysis determined causal effects of asthma and rheumatoid arthritis on B-ALL.
Conclusions
Our research proved shared genetic architecture between autoimmune disorders and B-ALL and shed light on the potential mechanism that might involve in.
Peer Review reports
B-cell acute lymphoblastic leukemia (B-ALL) is a prevalent subtype of leukemia characterized by its highly malignant nature, primarily originating from the clonal expansion and abnormal proliferation of B lymphocytes within the hematopoietic system [ 1 ]. Autoimmune disorders are characterized by a disruption in self-tolerance, resulting in pathological alterations and clinical symptoms arising from immune responses targeting self-components [ 2 ]. Concurrently, the pathogenesis of several autoimmune disorders is intricately interwoven with the malfunctioning of B cells within the humoral immune system. The excessive activation of self-reactive B cells precipitates an overproduction of autoantibodies and immune complexes, which, in turn inflict damage upon a multitude of tissues and organs, culminating in the emergence of various autoimmune disorders [ 3 ]. To summarize, B cells assume a pivotal role in the orchestration of humoral immune responses, and their deregulation markedly contributes to the onset of autoimmune diseases and B-cell malignancies [ 4 ].
Epidemiological investigations have discovered associations between autoimmune disorders and B-cell malignancies. For example, rheumatoid arthritis (RA) patients exhibit a twofold increased risk of concomitant B-cell lymphomas when compared to their healthy counterparts [ 5 ]. In the case of systemic lupus erythematosus (SLE) and Sjögren’s syndrome patients, the risk amplifies significantly to 2.7–7.5 times [ 6 ] and 9–18 times [ 6 ], respectively. Previous studies observed that the standardized incidence ratio of ALL was estimated to be 2.77 after RA onset [ 7 ]. Studies also showed that at the time of diagnosis of malignancy, 15–30% of patients present with many of the typical features of rheumatic diseases [ 8 ]. However, current research focused primarily on the onset of autoimmune diseases on hematological malignancies risk, particularly diffuse large B-cell lymphoma and follicular lymphoma. This leaves a clear gap in understanding the pleiotropic mechanisms and bidirectional causations between B-ALL (a disease also derived from B lymphocytes) and autoimmune diseases. Only Li et al. have reported the shared mechanism between autoimmunity and B-ALL, specifically demonstrating the essential role of DYRK1a in mediating the noncanonical NF-κB activation induced by BAFF [ 9 ]. This underscores the existence of substantial knowledge gaps in this field, highlighting the urgent need to ascertain shared risk loci between these two disorders. It is worth noting that traditional clinical or epidemiological research may encounter challenges in ensuring the statistical effectiveness of such investigations.
Recently, the linkage disequilibrium (LD) score regression (LDSC) approach has been developed to indicate whether there exists a genetic correlation between the two types of disease [ 10 ]. It is unclear whether the overall genetic correlation is attributable to a few loci or the entire genome. Few studies to date have systematically evaluated genetic overlap, shared susceptibility genes, and causality between autoimmune diseases and B-ALL. Cross-trait analyses that utilize the correlation of GWAS signals to study polyvalent genetic variants or loci between multiple traits have been shown to accurately identify shared loci between diseases or traits [ 11 , 12 , 13 ]. These pleiotropic loci can be targeted for intervention to potentially prevent or treat these diseases simultaneously. Recently, a novel method called “PLACO” was developed to identify pleiotropy at the SNP-level based on a level-α intersection–union test (IUT) [ 14 ]. Therefore, it is important to determine specific genetic variants or loci that lead to genome-wide genetic correlations or to delve into the shared genetic etiology of these two types of diseases. Our research flowchart is shown in Fig. 1 .
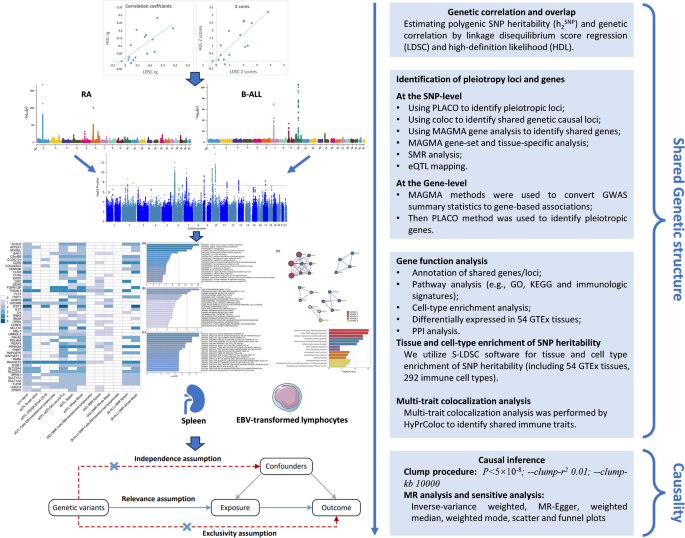
Study workflow
GWAS summary data source
GWAS summary statistics for 16 autoimmune diseases were all publicly available from large-scale GWAS or GWAS meta-analyses: adult-onset asthma (AOA) [ 15 , 16 ], childhood-onset asthma (COA) [ 15 , 16 ], Graves’ disease (GD) [ 17 , 18 ], Hashimoto’s disease (HD) [ 17 , 18 ], hypothyroidism (HT) [ 17 , 18 ], primary biliary cirrhosis (PBC) [ 19 , 20 ], primary sclerosing cholangitis (PSC) [ 21 , 22 ], inflammatory bowel disease (IBD) [ 23 , 24 ], Crohn’s disease (CD) [ 23 , 24 ], ulcerative colitis (UC) [ 23 , 24 ], RA [ 25 , 26 ], SLE [ 27 , 28 ], multiple sclerosis (MS) [ 29 , 30 ], systemic sclerosis (SS) [ 31 , 32 ], type 1 diabetes (T1D) [ 17 , 18 ], and vitiligo [ 33 , 34 ]. GWAS summary statistics for B-ALL were generated in a meta-analysis of four GWAS including a total of 5321 cases and 16,666 controls of European ancestry [ 35 , 36 ]. The same quality control procedure was followed for each study, the association between ALL status and SNP genotypes in each study was assessed using logistic regression, and genetic principal components were used as covariates in the association analysis. Risk estimates were finally combined by fixed-effects inverse variance weighted (IVW) meta-analysis. The sources and details of these datasets are summarized in Additional file 2 : Table S1.
Genetic overlap at the genome-wide level
We used LDSC to evaluate the genetic structure shared between autoimmune disorders and B-ALL [ 10 ]. The LD scores used in LDSC were calculated based on genotypes of common SNPs from European ancestry samples in the 1000 genomes project [ 37 ]. Standard errors (SE) were estimated by the jackknife method in LDSC which was further used to correct for attenuation bias. Intercept of LDSC was used to evaluate potential population overlaps between studies from different consortiums [ 10 ]. It is worth noting that no actual population overlap between autoimmune disorders and B-ALL studies existed in our analysis. A likelihood-based method, called high-definition likelihood (HDL), can utilize GWAS summary statistics to estimate genetic associations, which could reduce the variance of genetic association estimates by about 60% compared with the LDSC method [ 38 ].
We further investigated whether SNP heritability of autoimmune diseases and B-ALL was enriched in specific cells and tissues using hierarchical LDSC regression. Stratified-LDSC (S-LDSC) was applied to different immune cell data to assess whether specific cell types had significant genetic enrichment in these tissues. We downloaded 54 human tissues datasets from GTEx [ 39 ] and 292 immune cell types from the ImmGen consortium [ 40 ] (including B cells, γ δ T cells, α β T cells, innate lymphocytes, myeloid cells, stromal cells, and stem cells). After adjusting for the baseline model and all gene sets, we assessed the significance of the SNP heritability enrichment estimated in each tissue and cell by using the regression coefficient Z-scores and corresponding P values.
Identification of pleiotropic loci and genes by using PLACO
A pleiotropic analysis under composite null hypothesis (PLACO) was used to identify pleiotropy among multiple autoimmune diseases and B-ALL at the SNP-level. SNPs reach genome-wide significant level ( P < 5 × 10 –8 ) and were viewed as pleiotropic variants. The functional mapping and annotation (FUMA) of GWAS was used to determine the genomic regions of these risk variants (i.e., pleiotropic loci) [ 41 ]. Also, a Bayesian colocalization analysis was conducted to determine the pleiotropic loci shared by autoimmune diseases and B-ALL [ 42 ]. To explore the shared mechanisms of the identified loci, nearby genes were mapped based on lead SNPs within each locus. Also, a generalized gene-set analysis of GWAS data (multi-marker analysis of genomic annotation, MAGMA) approach was used to determine the biological function of these pleiotropic loci. Specifically, we performed MAGMA gene analysis to identify pleiotropic genes by properly incorporating LD between markers and to detect multi-marker effects ( P < 0.05/18,345 = 2.73 × 10 –6 ) [ 43 ]. MAGMA gene-set analysis was performed to investigate the biofunction of lead SNPs [ 43 ], and a total of 10,678 gene sets including curated gene sets (c2.all) and go terms (c5.bp, c5.cc, and c5.mf) from Molecular Signatures Database (MSigDB) were finally tested [ 44 ]. Bonferroni correction was performed for all tested gene sets to avoid false positives ( P < 0.05/10,678 = 4.68 × 10 –6 ). Metascape webtools (metascape.org) performed a pathway enrichment analysis to determine the function of mapped genes based on MSigDB [ 44 ]. Genome-wide tissue-specific enrichment analysis was conducted based on 54 GTEx tissues [ 45 ] for the genome-wide pleiotropic results generated by PLACO. We also calculated the average expression (log 2 transformed) of all identified pleiotropic genes in all 54 GTEx tissues [ 45 ] and tested tissue specificity by differentially expressed genes (DEGs) in each tissue (up- and down-regulated DEGs were precomputed by the signs of the t-statistics).
Summary-based Mendelian randomization
Summary-based Mendelian randomization (SMR) [ 46 ] method combined summary-level data from GWAS with data from expression quantitative trait loci (eQTL) studies to identify genes whose expression levels are associated with complex traits due to pleiotropy. It employs SMR and HEIDI methods to test pleiotropic associations between gene expression levels and complex traits of interest using summary-level data from GWAS and eQTL studies. This approach could be interpreted as an analysis to test whether the magnitude of SNP effects on phenotype is mediated by gene expression.
Multi-trait colocalization analysis
We utilized hypothesis prioritization for multi-trait colocalization (HyPrColoc) [ 47 ] method to perform multi-trait colocalization analysis to pinpoint the crucial roles that immune traits played in the onset of autoimmune disorders and B-ALL. Immune-wide GWAS data contains a total of 731 immune cells [ 48 ], which could be publicly available from the GWAS catalog (GCST0001391 ~ GCST0002121). Detailed information on the GWAS summary datasets for immune cells was added to Additional file 1 : Supplementary Methods.
Causal association analysis
We performed a one-directional two-sample Mendelian randomization (MR) analysis to assess possible causal effects of autoimmune disorders on B-ALL risk. The “clumping” procedure in PLINK 1.9 software was used to extract independent significance SNPs for all autoimmune diseases ( P < 5 × 10 –8 ), where r 2 was set to 0.001 and window size was set to a physical distance of 10,000 KB [ 49 ]. Notably, r 2 was calculated based on the 1000 genomes project phase 3 as a reference panel. Proportion of variance explained (PVE) and F statistic ( F > 10) was used to measure the strength of instrumental variables (IVs) (see Additional File 1 : Supplementary Methods) [ 50 ]. To verify causality among these trait pairs, six MR approaches were performed with each set of IVs, i.e., IVW, Debiased-IVW (DIVW) [ 51 ], weighted median approach [ 52 ], MR pleiotropy residual sum and outlier (MR-PRESSO) [ 53 ], MR-Egger [ 54 ], MR robust adjusted profile score (MR-RAPS) [ 55 ], and mode-based estimate [ 56 ] method. Cochran’s Q statistics was used to examine the effect size heterogeneity across the IVs (see Additional File 1 : Supplementary Methods) [ 57 , 58 ]. Additionally, the intercept of MR-Egger regression and global test of MR-PRSSO were utilized to detect horizontal pleiotropy [ 53 , 54 ]. Detailed information on used MR methods was described in Additional file 1 : Supplementary Method.
Software and packages
The main statistical analysis was performed in R (version 3.5.3). LDSC and S-LDSC analysis were implemented with “LDSC” software (v1.0.1) [ 10 ]. PLACO was performed with “PLACO” package [ 14 ]. Bayesian colocalization analysis was performed with the “coloc” package (version 5.2.1) [ 42 ] and HyPrColoc was performed with the “hyprcoloc” package (version 1.0) [ 47 ]. Function analysis was performed by FUMA web tool [ 41 ]. MAGMA gene and gene-set analysis were performed by MAGMA software [ 43 ]. Two-sample MR analysis was conducted with “MendelianRandomization” (version 0.9.0) [ 59 ], mr.raps (version 0.4.1) [ 55 ], and MRPRESSO (version 1.0) [ 53 ] packages. A copy of the main code used in this research is available at: https://github.com/biostatYu/MRcode-/tree/main/AD_BALL .
Shared genetic architecture between autoimmune disorders and B-ALL
We first evaluated the genetic correlation between autoimmune diseases and B-ALL and results from the LDSC and HDL methods were highly consistent (Table 1 and Additional file 2 : Table S2). Specifically, by using the LDSC method, six traits were identified to be genetically correlated with B-ALL, including AOA, HT, IBD, CD, RA, and MS. While implementing the HDL method, significant genetic correlations were observed among AOA, HT, PBC, RA, MS, and B-ALL, leading to a final union set of seven pairwise traits for further analysis. However, we did not find significant genetic correlation between IBD and CD and HDL results. It was noting that only RA remained significantly genetical correlated with B-ALL risk after applying the Bonferroni correction ( P = 0.003 < 0.05/16).
Pleiotropic loci and genes identified for multiple autoimmune disorders and B-ALL
Given the shared genetic mechanisms between autoimmune diseases and B-ALL identified by LDSC and HDL, we used novel pleiotropy analyses (PLACO) to identify potential pleiotropic loci for both diseases (Additional file 1 : Fig. S1). The QQ plots demonstrated no premature divergence between observed and expected values, ruling out the possibility of group stratification (Additional file 1 : Fig. S2). Based on PLACO results, we identified a total of 73 pleiotropic genomic risk loci associated with both autoimmune disorders and B-ALL using FUMA ( P < 5 × 10 –8 ) (Fig. 2 , Additional file 1 : Fig. S1, and Additional file 2 : Table S3). Colocalization analysis finally identified 16 of 73 (21.9%) potential pleiotropic loci with PP.H4 greater than 0.7 (e.g., 5p13) (Table 2 ). The regional plots for each trait pair are presented in Additional file 1 : Fig. S3 ~ S8. Notably, some pleiotropic regions were shared between different pairs, for example, genome regions 7p12.2, 10p14, 6q27, and 10p12.31 were identified in four pairs (Additional file 2 : Table S4). The MAGMA analysis of gene sets suggested that the identified pleiotropic loci may participate in the control of the immune system, hematopoiesis, and various other processes (Fig. 3 A and Additional file 2 : Table S5). Notably, significant monocyte differentiation pathway was found for all trait pairs, and significant leukocyte differentiation was found for all five trait pairs. Further tissue-specific analysis found these risk loci were enriched in several immune-related tissues (e.g., spleen, whole blood, Epstein–Barr virus (EBV)-transformed lymphocytes) (Fig. 3 B and Additional file 2 : Table S6). ANNOVAR category annotation showed that 28 of 73 lead SNPs (38.4%) were intronic variants and 30 of 73 (41.1%) were intergenic variants. Only 2 of 73 (3%) lead SNPs were exonic variants (Additional file 2 : Table S3).
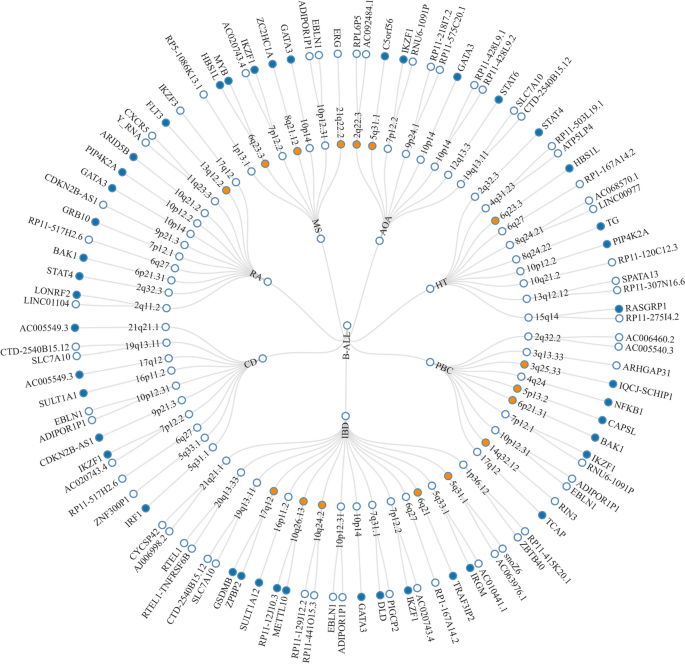
The circular diagram presents pleiotropic loci and genes identified by PLACO among seven trait pairs. Note: Shared loci identified by colocalization analysis are highlighted in orange; shared genes identified by MAGMA analysis are highlighted in blue. B-ALL B-cell acute lymphoblastic leukemia, AOA adult-onset asthma, HT hypothyroidism, PBC primary biliary cirrhosis, IBD inflammatory bowel disease, CD Crohn’s disease, RA rheumatoid arthritis, MS multiple sclerosis
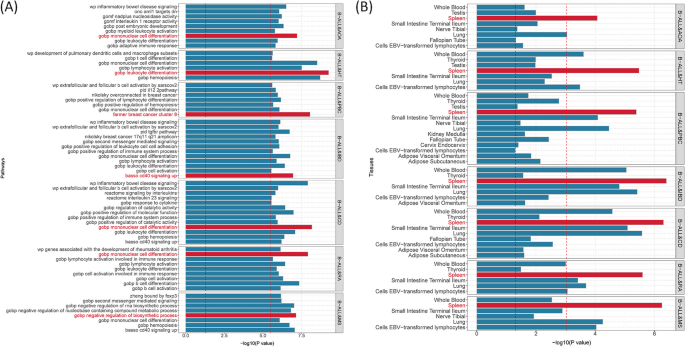
Bar plot of MAGMA gene-set ( A ) and tissue-specific ( B ) analysis for genome-wide pleiotropic results. Note: The red dotted line represents the significance of 0.05 after multiple corrections, and the blue represents the significance of 0.05. B-ALL B-cell acute lymphoblastic leukemia, AOA adult-onset asthma, HT hypothyroidism, PBC primary biliary cirrhosis, IBD inflammatory bowel disease, CD Crohn’s disease, RA rheumatoid arthritis, MS multiple sclerosis
Pleiotropic genes identified and enrichment analysis
We used different methods to map the identified SNP-level signals into the gene-level signals. By using MAGMA gene analysis, a total of 341 significant pleiotropic genes were determined as pleiotropic genes between multiple autoimmune diseases and B-ALL (194 unique) (Additional file 2 : Table S7 and Additional file 1 : Fig. S9). Additional file 2 : Table S8 lists the details of these genes. MAGMA gene analysis detected 92 repeated pleiotropic genes across different trait pairs, with IKZF1 identified as a pleiotropic gene for six pairs, followed by MLLT10 , FIGNL1 , RNASET2 , CCR6 , GATA3 , CLN3 , PIP4K2A , DDC , RP11-514O12.4 , FGFR1OP , and GRB10 in four trait pairs. eQTL analysis identified multiple hits of IKZF1 in blood- and immune-related tissues (e.g., naïve B cell, CD19 B-cell, EBV-transformed lymphocytes cells, cis-eQTLs, trans-eQTLs, spleen, whole blood). Five genes (i.e., TUFM , ZC2HC1A , RNASET2 , GSDMB, and ORMDL3 ) were observed to be significant in five different tissues. We summarized the landscape of pleiotropic genes identified in different methods and tissues in Fig. 4 . We observed several genes ( RNASET2 and FIGNL1 ) were significantly mapped in different tissues with different methods. The IKZF1 gene was also highlighted in whole blood tissues (Fig. 4 ).
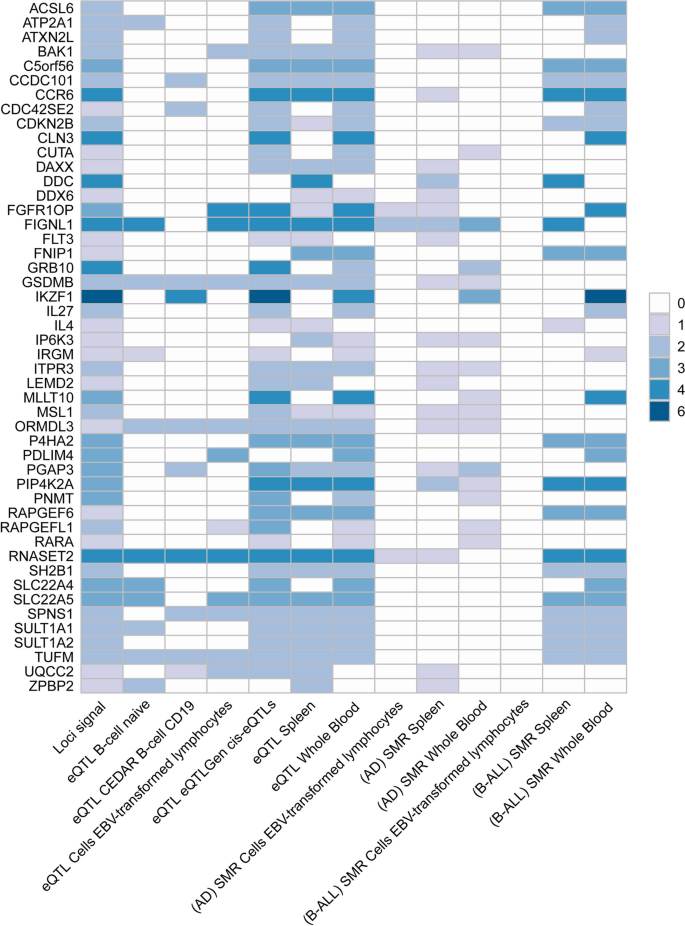
Overview of pleiotropic genes (highlighted in all three signals) for the autoimmune disorders and B-ALL. Note: The signals represent hits of genes across different trait pairs. eQTL expression quantitative trait loci, SMR summary-based Mendelian randomization, AD autoimmune disorders, B-ALL B-cell acute lymphoblastic leukemia, AOA adult-onset asthma, HT hypothyroidism, PBC primary biliary cirrhosis, IBD inflammatory bowel disease, CD Crohn’s disease, RA rheumatoid arthritis, MS multiple sclerosis
The shared mechanism between autoimmune diseases and B-ALL may involve specific organs or tissues involvement. Numerous genes (e.g., TOP2A , IKZF3 , MYB, and CD80 ) showed significant differential expression in EBV-transformed lymphocytes, and APOBR , IKZF1, and IL7R showed significant differential expression in spleen and whole blood tissues (Additional file 1 : Fig. S10 and Additional file 2 : Table S9). Tissue enrichment analysis showed that these genes were also enriched into the spleen and EBV-transformed lymphocytes (Additional file 1 : Fig. S11 and Additional file 2 : Table S10). Additional S-LDSC based on multiple tissues identified significant SNP heritability enrichment for all autoimmune diseases (except AOA) in each of the monocytes, blood cells, and spleen, after adjusting for the baseline model (Additional file 1 : Fig. S12 and Additional file 2 : Table S11). Further enrichment analysis of the GO biological processes associated with these genes indicated higher enrichment in the cellular response to cytokine stimulation, B cell activation, response to tumor necrosis factor, inflammatory response, and receptor signaling pathway via JAK-STAT (Fig. 5 A). These pathways play important roles in immune regulation and leukemogenesis. Cell type enrichment analysis showed the highest significance for bone marrow naïve T cells (Fig. 5 B). Furthermore, we found that these genes were numerically enriched in several immunologic signatures (e.g., MEMORY VS CD21HIGH TRANSITIONAL BCELL DN) (Fig. 5 C). The PPI analysis showed that five PPI networks were constructed, including the JAK-STAT signaling pathway and multiple pathways related to DNA damage were involved. And 22 proteins (e.g., STAT, NFKB1, and GATA3) could participate in these pathways (Fig. 5 D). Also, the results suggest that heritability is enriched in the blood, EBV-transformed lymphocytes, whole blood, and palatine tonsil tissues among five or more autoimmune diseases and B-ALL.
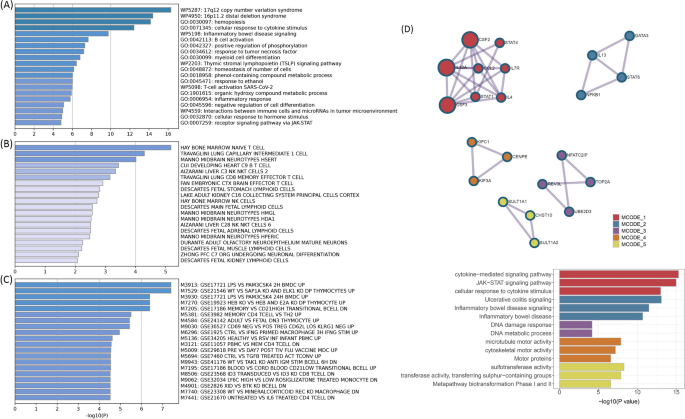
A Pathway enrichments for identified pleiotropic genes (KEGG, GO, Wiki pathways). B Cell-type enrichments for identified pleiotropic genes. C Immune signatures enrichments for identified pleiotropic genes. D Protein–protein interaction analysis based on identified pleiotropic genes
Immune-related mechanisms shared between autoimmune disorders and B-ALL
The shared mechanism involving affected tissues such as the spleen, lymphocytes, and whole blood, suggested an important involvement of immune mechanisms in the inter-disease. We used the S-LDSC method to determine the heritability enrichment of pleiotropy in immune cells and the HyPrColoc method to identify immune cells with co-localization signals with pleiotropic motifs. S-LDSC observed heritability enrichment of B cells in both autoimmune diseases and B-ALL. When analyzing the enrichment of immune traits from ImmGen, we also observed that two cell traits in the B cell panel were enriched: B.FrE.BM (CD19 + IgM + AA4.1 + HSA + ) and preB.FrD.BM (CD19 + IgM − CD45R + CD43 − ). Additionally, numerous cell traits in the T cell panel were also identified, implying the potential immune mechanisms shared (Additional file 1 : Fig. S12 and Additional file 2 : Table S11). Then multi-trait colocalization analysis by using HyPrColoc was performed to pinpoint key immune cells (Additional file 2 : Table S12). Results highlight 59 pleiotropic loci, of which 19 were unique, and these loci support the important role of 42 unique immune cells in autoimmune diseases and B-ALL by sharing causal variants. Our results support the critical influence of BAFF-R, CD4, CD45, and CD28 on different cells. Notably, a total of six BAFF-R-related immune traits were observed, including BAFF-R on B cell, BAFF-R on CD20 − , BAFF-R on CD24 + CD27 + , BAFF-R on IgD + CD24 − , BAFF-R on IgD + CD24 + , and BAFF-R on IgD + CD38 − . Interestingly, BAFF-R on B cell and BAFF-R on CD24 + CD27 + were both shared among three trait pairs (i.e., B-ALL&IBD, B-ALL&PBC, B-ALL&RA).
The causal relationship between autoimmune diseases and B-ALL estimated by MR
MR analyses using the IVW method showed significant positive associations between two autoimmune diseases (AOA and RA) and B-ALL risk (Fig. 6 A and Additional file 2 : Table S13). The risk of B-ALL was found to be able to be increased as the risk of AOA increases, the effect size was estimated by using the IVW method (OR = 1.223, 95%CI = 1.048 ~ 1.426, P = 0.010). Another four methods (DIVW, MR-RAPS, MR-PRESSO, and slope of MR-Egger) are consistent with the results of the IVW method. Although a significant intercept of MR-Egger might indicate the existence of potential horizontal pleiotropy, the global test of MR-PRESSO ruled out this possibility ( P = 0.632). We also observed significant causal effects of RA onset on B-ALL risk by using the IVW method (OR = 1.117, 95%CI = 1.033 ~ 1.208, P = 0.005). DIVW, MR-RAPS, and MR-PRESSO support this association (Fig. 6 B), where the intercept of MR-Egger and the global test of MR-PRESSO ruled out the possibility of horizontal pleiotropy (Additional file 2 : Table S14). Additional scatter and funnel plots eliminate the possibility of potential outliers (Fig. 6 C–D). However, after the Bonferroni adjustment, no causal associations between autoimmune disorders and B-ALL remained statistically significant ( P = 0.003 < 0.05/16). Finally, reverse MR analysis ruled out the possibility of reverse-directional causality.
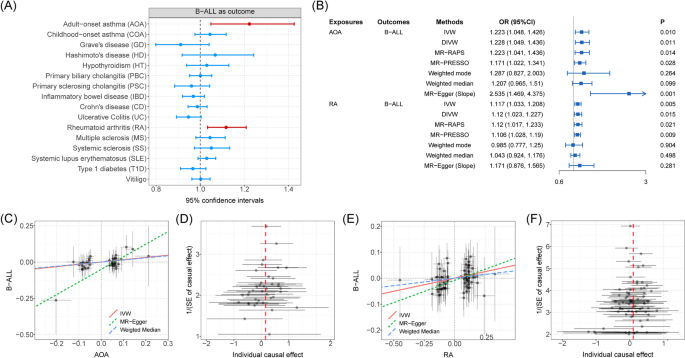
A The forest plot shows causal associations between autoimmune disorders and B-ALL by using one-directional MR analysis. Note: Causal effects were estimated by using IVW method. B Forest plot shows causal effects of AOA and RA on B-ALL risk estimated by using different methods. C Scatter plot shows significant causal association between AOA and B-ALL risk. D Funnel plot shows significant causal association between AOA and B-ALL risk. E Scatter plot shows significant causal association between RA and B-ALL risk. F Funnel plot shows significant causal association between RA and B-ALL risk. Associations highlighted with red represent that associations were significant in more than three main MR methods
Given the critical contribution of B cells to autoimmune disorders and B-ALL, there may be a complex relationship between them [ 60 ]. The study employed comprehensive genetic methodologies to investigate the genetic correlation between autoimmune disorders and B-ALL. The study determined pleiotropic loci using cross-trait PLACO analysis and identified pleiotropic genes through the MAGMA method. Then the key pathways and immunological mechanisms involved were identified. Finally, comprehensive MR analysis and sensitive analysis established the causal relationships between autoimmune diseases and B-ALL.
Through genetic correlation analysis, we observed significant genetic overlap between B-ALL and seven autoimmune disorders, including AOA, HT, IBD, CD, PBC, RA, and MS. We provide strong evidence for a shared genetic mechanism between RA and B-ALL, as well as MR evidence suggesting that patients with RA symptoms should be alerted to the risk of progression to ALL, which is consistent with previous studies [ 7 , 8 ]. Additionally, study have shown that 34 of 699 ALL patients diagnosed and followed had previously received varying doses of steroids for aplastic events or arthritis-based rheumatic diseases [ 61 ]. By using genetic variables, MR methods could well avoid the influence of possible confounding factors. Therefore, we believe that in addition to the effect of immunosuppressants, RA itself will also play an important role in the risk of B-ALL. We also observed significantly causal effects of AOA on B-ALL risk, which was ambiguous in previous studies: a systematic review supported the protective effect of asthma on ALL [ 62 ], two types of research included showed significant high risks of ALL in patients with a history of asthma [ 63 , 64 ].
We identified a series of genetically risk loci associated with both autoimmune diseases and B-ALL, and some of which were observed in multiple phenotype pairs (e.g., 7p12.2, 10p14, 6q27, 10p12.31). Previous studies gave the evidence of key role these loci played in the development of autoimmune disorders and B-ALL. For example, loci on 7p12.2 ( IKZF1 ) had been proven to be associated with risk of childhood B-ALL [ 65 ], which was also identified as susceptibility genes for SLE [ 66 ]. After searching for the GWAS catalog, 7p12.2 had been reported to be associated with multiple autoimmune disorders, including CD [ 23 ], IBD [ 23 ], RA [ 25 ], and MS [ 67 ]. GATA3 (10p14) is a key regulator in the immune system, especially in the differentiation and function of type 2 helper (Th2) cells [ 68 ]. Th2 cells have been demonstrated to play a role in various autoimmune diseases, including SLE and IBD [ 69 , 70 ]. Recent research also highlighted the role of noncoding genetic variation (rs3824462) in GATA3 , linking it to an increased risk of Ph-like ALL, a common subtype of B-ALL. The study revealed that rs3824462 induced local and global changes in chromatin conformation, activating JAK-STAT pathway and promoting disease development [ 71 ].
We searched for the identified risk loci in the GWAS catalog (last update in 2023 December 20) [ 72 ] and found that some of the risk loci have been reported to be associated with both B-ALL and autoimmune disorders (Additional file 1 : Fig. S13 and Additional file 2 : Table S15). For instance, the 17q21 locus is implicated in various autoimmune diseases, including asthma [ 73 , 74 ], IBD [ 75 , 76 ], T1D [ 77 ], and SLE [ 78 ]. This locus, housing IKZF3 , GSDMB , and ORMDL3 , involved in lymphocyte development [ 79 ], pyroptosis [ 80 ], and inflammatory response [ 81 ], has been challenging to dissect. GSDMB and ORMDL3 represent the target genes of rs2290400, and its minor allele is associated with a protective effect against ALL [ 82 ]. IKZF3 polymorphism contributes to B-ALL with a 1.5-fold to twofold increase in relative risk [ 83 ]. Genes previously reported to be associated with leukemia have also been observed in our results to be correlated with autoimmune diseases: MLLT10 (10p12) participates in various chromosomal rearrangements associated with ALL and acute myeloid leukemia (AML) [ 84 ]. It is implicated in chromatin structure regulation and DNA damage response, deemed crucial for early development, maintenance, and differentiation of hematopoietic stem cells. While direct evidence for the impact of MLLT10 on autoimmune diseases has not been established, studies indicated a close association with C-reactive protein levels [ 85 ], widely recognized as a valuable indicator of disease activity in various autoimmune rheumatic diseases [ 86 ]. Simultaneously, certain genes previously reported to be associated with autoimmune disorders have also been found in our results to be associated with B-ALL. IRGM (5q33) encodes a member of the immunity-related GTPase family, crucial in innate immunity and inflammatory responses [ 87 ]. Previous studies have linked IRGM to CD [ 88 , 89 , 90 ], UC [ 91 ], and IBD [ 23 ]. CAPSL (5p13) has been reported to be associated with PBC [ 92 ], T1D [ 93 ], asthma [ 94 ], and SLE [ 95 ]. Although direct evidence of its association with ALL is lacking, increased mRNA levels have been observed in AML patients [ 96 ]. Additionally, the long non-coding RNA C5orf56 (5q31) has been identified for its protective role in IBD [ 97 ]. SCHIP1 (3q25) has been associated with SLE [ 98 ], while RNASET2 (6q27) has been identified as a risk gene for both vitiligo [ 99 ] and GD [ 100 ].
Shared genetic structures observed in our research revealed common mechanisms between autoimmune disease and B-ALL. Identified genes were observed to participate in several pathways, like B cell activation, cellular response to cytokine stimulus, and inflammatory response. For each disease pair, we observed a significant enrichment of pleiotropy to the spleen, a critical site for B cell development. Notably, a substantial presence of BAFF-R-associated immune signature, a key regulator of B cell function and survival, was discerned in a multi-trait colocalization analysis. These findings collectively underscored the pivotal role played by B cells in both autoimmune disorders and B-ALL. In autoimmune conditions, B cells are exposed to antigens, undergo activation, and subsequently proliferate and expand clonally, thereby increasing the risk of accumulating genetic mutations, and finally leads to the emergence and progression of B-ALL [ 60 ]. We can think that ORMDL3 and IKZF3 , mentioned earlier, play crucial roles in this context, as evidenced by prior literature reporting ORMDL3’s vital role in B cell survival [ 101 ], and IKZF3 ’s predominant regulation of B cell differentiation, activation response, and proliferation [ 102 ]. Furthermore, malignancies arising from B cells consistently exhibit concurrent autoimmune disorders at any stage, whereas those derived from T cells are less commonly linked to autoimmune phenomena [ 103 ]. Nevertheless, our findings also identified numerous cell traits in the T cell panel, and we speculate that this may be attributed to interactions between B and T cells. The JAK-STAT pathway may represent a crucial mechanism in this context, as it has been targeted in autoimmune diseases [ 104 ] and its role in B-ALL involves the disruption of preleukemic cells differentiation [ 105 ]. Our results highlighted the critical role of EBV infection as a trigger for both autoimmune disorders and B-ALL: tissue-specific analysis revealed enriched risk loci in EBV-transformed lymphocytes, and the central role of IKZF1 in this cell was also identified by gene-level analyses. EBV remains latent in memory B cells after infection, and reactivation can induce B cell clonal immortalization, promoting lymphomagenesis [ 106 ]. Additionally, EBV-induced autoimmunity has been reported to increase the risk of autoimmune diseases [ 107 ].
Limitations
Our study is not without limitations. Firstly, as with other similar studies, the data used in this study was summary-level, and individual-level datasets were not available. Further stratification of the population (e.g., gender, age, etc.) was therefore not possible. Secondly, the sample size of immune cell GWAS used in this study was limited. Therefore, caution should be exercised in interpreting the role of immune cells and drawing conclusions in our studies. Thirdly, it should be noted that our study was limited to European ancestry and may not be generalizable to other ancestries. It is important to be equally cautious in concluding our findings since the relatively small sample size of B-ALL may result in limited statistical power.
Our research has uncovered the intricate connections between autoimmune disorders, especially AOA, HT, IBD, CD, RA, and MS and B-ALL. Identification of pleiotropic risk loci (7p12, 10p14, 6q27, and 10p12) and genes ( IKZF1 , GATA3 , IKZF3 , GSDMB , and ORMDL3 ) shared between diseases suggested shared mechanisms, such as B cell activation and JAK-STAT pathway, common triggers like EBV infection. Additionally, our findings have shed light on and the causal links between autoimmune disorders (AOA and RA) and B-ALL.
Availability of data and materials
Data are available in public, open access repositories corresponding to the original studies (e.g., GWAS catalog). Main codes used in our research are available at https://github.com/biostatYu/MRcode/tree/main/AD_BALL .
Abbreviations
Acute myeloid leukemia
Adult-onset asthma
- B-cell acute lymphoblastic leukemia
Crohn’s disease
Childhood-onset asthma
Differentially expressed genes
Debiased-inverse variance weighted
Epstein–Barr virus
Expression quantitative trait loci
Functional mapping and annotation
Graves’ disease
Genome-wide association study
Hashimoto's disease
High-definition likelihood
Hypothyroidism
Hypothesis prioritization for multi-trait colocalization
Inflammatory bowel disease
Intersection–union test
Instrumental variables
Inverse variance weighted
Linkage disequilibrium
Linkage disequilibrium score regression
Multi-marker analysis of genomic annotation
- Mendelian randomization
Mendelian randomization pleiotropy residual sum and outlier
Mendelian randomization robust adjusted profile score
Multiple sclerosis
Molecular signatures database
Primary biliary cirrhosis
Pleiotropic analysis under composite null hypothesis
Primary sclerosing cholangitis
Proportion of variance explained
Rheumatoid arthritis
Standard errors
Stratified-linkage disequilibrium score regression
Systemic lupus erythematosus
Systemic sclerosis
Type 1 diabetes
Ulcerative colitis
Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541–52.
Article CAS PubMed Google Scholar
Chi X, Huang M, Tu H, Zhang B, Lin X, Xu H, et al. Innate and adaptive immune abnormalities underlying autoimmune diseases: the genetic connections. Sci China Life Sci. 2023;66(7):1482–517.
Article PubMed Google Scholar
Lin X, Lu L. B Cell-mediated autoimmune diseases. Adv Exp Med Biol. 2020;1254:145–60.
Shaffer AL, Rosenwald A, Staudt LM. Lymphoid malignancies: the dark side of B-cell differentiation. Nat Rev Immunol. 2002;2(12):920–32.
Smedby KE, Hjalgrim H, Askling J, Chang ET, Gregersen H, Porwit-MacDonald A, et al. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst. 2006;98(1):51–60.
Ekström Smedby K, Vajdic CM, Falster M, Engels EA, Martínez-Maza O, Turner J, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111(8):4029–38.
Article PubMed PubMed Central Google Scholar
Hemminki K, Huang W, Sundquist J, Sundquist K, Ji J. Autoimmune diseases and hematological malignancies: exploring the underlying mechanisms from epidemiological evidence. Semin Cancer Biol. 2020;64:114–21.
Cabral DA, Tucker LB. Malignancies in children who initially present with rheumatic complaints. J Pediatr. 1999;134(1):53–7.
Li Y, Xie X, Jie Z, Zhu L, Yang JY, Ko CJ, et al. DYRK1a mediates BAFF-induced noncanonical NF-κB activation to promote autoimmunity and B-cell leukemogenesis. Blood. 2021;138(23):2360–71.
Article CAS PubMed PubMed Central Google Scholar
Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh P-R, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47(11):1236.
Gong W, Guo P, Li Y, Liu L, Yan R, Liu S, et al. Role of the gut-brain axis in the shared genetic etiology between gastrointestinal tract diseases and psychiatric disorders: a genome-wide pleiotropic analysis. JAMA Psychiat. 2023;80(4):360–70.
Article Google Scholar
Yu XH, Yang YQ, Cao RR, Cai MK, Zhang L, Deng FY, et al. Rheumatoid arthritis and osteoporosis: shared genetic effect, pleiotropy and causality. Hum Mol Genet. 2021;30(21):1932–40.
Lu H, Qiao J, Shao Z, Wang T, Huang S, Zeng P. A comprehensive gene-centric pleiotropic association analysis for 14 psychiatric disorders with GWAS summary statistics. BMC Med. 2021;19(1):314.
Ray D, Chatterjee N. A powerful method for pleiotropic analysis under composite null hypothesis identifies novel shared loci between type 2 diabetes and prostate cancer. Plos Genet. 2020;16(12):e1009218.
Ferreira MAR, Mathur R, Vonk JM, Szwajda A, Brumpton B, Granell R, et al. Genetic architectures of childhood- and adult-onset asthma are partly distinct. Am J Hum Genet. 2019;104(4):665–84.
Ferreira MAR, Mathur R, Vonk JM, Szwajda A, Brumpton B, Granell R, et al. Genetic architectures of childhood- and adult-onset asthma are partly distinct. https://www.ebi.ac.uk/gwas/studies/GCST007800 . (2019).
Sakaue S, Kanai M, Tanigawa Y, Karjalainen J, Kurki M, Koshiba S, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. 2021;53(10):1415–24.
Sakaue S, Kanai M, Tanigawa Y, Karjalainen J, Kurki M, Koshiba S, et al. A cross-population atlas of genetic associations for 220 human phenotypes. https://pheweb.jp/downloads . (2021).
Cordell HJ, Fryett JJ, Ueno K, Darlay R, Aiba Y, Hitomi Y, et al. An international genome-wide meta-analysis of primary biliary cholangitis: novel risk loci and candidate drugs. J Hepatol. 2021;75(3):572–81.
Cordell HJ, Fryett JJ, Ueno K, Darlay R, Aiba Y, Hitomi Y, et al. An international genome-wide meta-analysis of primary biliary cholangitis: novel risk loci and candidate drugs. https://www.ebi.ac.uk/gwas/studies/GCST90061440 . (2021).
Ji SG, Juran BD, Mucha S, Folseraas T, Jostins L, Melum E, et al. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat Genet. 2017;49(2):269–73.
Ji SG, Juran BD, Mucha S, Folseraas T, Jostins L, Melum E, et al. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. https://www.ebi.ac.uk/gwas/studies/GCST004030 . (2017).
de Lange KM, Moutsianas L, Lee JC, Lamb CA, Luo Y, Kennedy NA, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet. 2017;49(2):256–61.
de Lange KM, Moutsianas L, Lee JC, Lamb CA, Luo Y, Kennedy NA, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. https://www.ebi.ac.uk/gwas/studies/GCST004131 . (2017).
Ishigaki K, Sakaue S, Terao C, Luo Y, Sonehara K, Yamaguchi K, et al. Multi-ancestry genome-wide association analyses identify novel genetic mechanisms in rheumatoid arthritis. Nat Genet. 2022;54(11):1640–51.
Ishigaki K, Sakaue S, Terao C, Luo Y, Sonehara K, Yamaguchi K, et al. Multi-ancestry genome-wide association analyses identify novel genetic mechanisms in rheumatoid arthritis. https://www.ebi.ac.uk/gwas/studies/GCST90132223 . (2022).
Bentham J, Morris DL, Graham DSC, Pinder CL, Tombleson P, Behrens TW, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet. 2015;47(12):1457–64.
Bentham J, Morris DL, Graham DSC, Pinder CL, Tombleson P, Behrens TW, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. https://www.ebi.ac.uk/gwas/studies/GCST003156 . (2015).
Consortium. IMSG. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science (New York, NY). 2019;365(6460):7188.
Consortium. IMSG. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. https://www.ebi.ac.uk/gwas/studies/GCST009597 . (2019).
López-Isac E, Acosta-Herrera M, Kerick M, Assassi S, Satpathy AT, Granja J, et al. GWAS for systemic sclerosis identifies multiple risk loci and highlights fibrotic and vasculopathy pathways. Nat Commun. 2019;10(1):4955.
López-Isac E, Acosta-Herrera M, Kerick M, Assassi S, Satpathy AT, Granja J, et al. GWAS for systemic sclerosis identifies multiple risk loci and highlights fibrotic and vasculopathy pathways. https://www.ebi.ac.uk/gwas/studies/GCST009131 . (2019).
Jin Y, Andersen G, Yorgov D, Ferrara TM, Ben S, Brownson KM, et al. Genome-wide association studies of autoimmune vitiligo identify 23 new risk loci and highlight key pathways and regulatory variants. Nat Genet. 2016;48(11):1418–24.
Jin Y, Andersen G, Yorgov D, Ferrara TM, Ben S, Brownson KM, et al. Genome-wide association studies of autoimmune vitiligo identify 23 new risk loci and highlight key pathways and regulatory variants. https://www.ebi.ac.uk/gwas/studies/GCST004785 . (2016).
Vijayakrishnan J, Qian M, Studd JB, Yang W, Kinnersley B, Law PJ, et al. Identification of four novel associations for B-cell acute lymphoblastic leukaemia risk. Nat Commun. 2019;10(1):5348.
Vijayakrishnan J, Qian M, Studd JB, Yang W, Kinnersley B, Law PJ, et al. Identification of four novel associations for B-cell acute lymphoblastic leukaemia risk. https://www.ebi.ac.uk/gwas/studies/GCST009638 . (2019).
Consortium GP. A global reference for human genetic variation. Nature. 2015;526(7571):68.
Ning Z, Pawitan Y, Shen X. High-definition likelihood inference of genetic correlations across human complex traits. Nat Genet. 2020;52(8):859–64.
GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45(6):580–5.
ImmGen O-S. mononuclear phagocytes. Nat Immunol. 2016;17(7):741.
Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8(1):1826.
Giambartolomei C, Vukcevic D, Schadt EE, Franke L, Hingorani AD, Wallace C, et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. Plos Genet. 2014;10(5):e1004383.
de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. Plos Comput Biol. 2015;11(4):e1004219.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci. 2005;102(43):15545–50.
Carithers LJ, Ardlie K, Barcus M, Branton PA, Britton A, Buia SA, et al. A novel approach to high-quality postmortem tissue procurement: the GTEx project. Biopreserv Biobanking. 2015;13(5):311–9.
Zhu Z, Zhang F, Hu H, Bakshi A, Robinson MR, Powell JE, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet. 2016;48(5):481–7.
Foley CN, Staley JR, Breen PG, Sun BB, Kirk PDW, Burgess S, et al. A fast and efficient colocalization algorithm for identifying shared genetic risk factors across multiple traits. Nat Commun. 2021;12(1):764.
Orrù V, Steri M, Sidore C, Marongiu M, Serra V, Olla S, et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet. 2020;52(10):1036–45.
Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526(7571):68–74.
Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40(3):755–64.
Ye T, Shao J, Kang H. Debiased inverse-variance weighted estimator in two-sample summary-data Mendelian randomization. Ann Stat. 2021;49(4):2079–100.
Bowden J, Smith GD, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14.
Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693.
Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89.
Zhao Q, Wang J, Hemani G, Bowden J, Small DS. Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. Ann Statist. 2020;48(3):1742–69. https://doi.org/10.1214/19-AOS1866 .
Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(6):1985–98.
Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18(20):2693–708.
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45(6):1961–74.
PubMed PubMed Central Google Scholar
Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734–9.
Baecklund E, Smedby KE, Sutton LA, Askling J, Rosenquist R. Lymphoma development in patients with autoimmune and inflammatory disorders–what are the driving forces? Semin Cancer Biol. 2014;24:61–70.
Révész T, Kardos G, Kajtár P, Schuler D. The adverse effect of prolonged prednisolone pretreatment in children with acute lymphoblastic leukemia. Cancer. 1985;55(8):1637–40.
Zhou MH, Yang QM. Association of asthma with the risk of acute leukemia and non-Hodgkin lymphoma. Mol Clin Oncol. 2015;3(4):859–64.
Chang JS, Tsai YW, Tsai CR, Wiemels JL. Allergy and risk of childhood acute lymphoblastic leukemia: a population-based and record-based study. Am J Epidemiol. 2012;176(11):970–8.
Spector L, Groves F, DeStefano F, Liff J, Klein M, Mullooly J, et al. Medically recorded allergies and the risk of childhood acute lymphoblastic leukaemia. Eur J Cancer (Oxford, England : 1990). 2004;40(4):579–84.
Article CAS Google Scholar
Papaemmanuil E, Hosking FJ, Vijayakrishnan J, Price A, Olver B, Sheridan E, et al. Loci on 7p12.2, 10q21.2 and 14q11.2 are associated with risk of childhood acute lymphoblastic leukemia. Nat Genet. 2009;41(9):1006–10.
Han JW, Zheng HF, Cui Y, Sun LD, Ye DQ, Hu Z, et al. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet. 2009;41(11):1234–7.
Beecham AH, Patsopoulos NA, Xifara DK, Davis MF, Kemppinen A, Cotsapas C, et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013;45(11):1353–60.
Zhu J. GATA3 regulates the development and functions of innate lymphoid cell subsets at multiple stages. Front Immunol. 2017;8:1571.
Ramirez GA, Tassi E, Noviello M, Mazzi BA, Moroni L, Citterio L, et al. Histone‐specific CD4+ T cell plasticity in active and quiescent systemic lupus erythematosus. Arthritis Rheumatol. 2024.
Liu A, Liang X, Wang W, Wang C, Song J, Guo J, et al. Human umbilical cord mesenchymal stem cells ameliorate colon inflammation via modulation of gut microbiota-SCFAs-immune axis. Stem Cell Res Ther. 2023;14(1):271.
Yang H, Zhang H, Luan Y, Liu T, Yang W, Roberts KG, et al. Noncoding genetic variation in GATA3 increases acute lymphoblastic leukemia risk through local and global changes in chromatin conformation. Nat Genet. 2022;54(2):170–9.
Sollis E, Mosaku A, Abid A, Buniello A, Cerezo M, Gil L, et al. The NHGRI-EBI GWAS catalog: knowledgebase and deposition resource. Nucleic Acids Res. 2023;51(D1):D977–85.
Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448(7152):470–3.
Li X, Christenson SA, Modena B, Li H, Busse WW, Castro M, et al. Genetic analyses identify GSDMB associated with asthma severity, exacerbations, and antiviral pathways. J Allergy Clin Immunol. 2021;147(3):894–909.
Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24.
Chao KL, Kulakova L, Herzberg O. Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein. Proc Natl Acad Sci USA. 2017;114(7):E1128–37.
Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41(6):703–7.
Perez RK, Gordon MG, Subramaniam M, Kim MC, Hartoularos GC, Targ S, et al. Single-cell RNA-seq reveals cell type-specific molecular and genetic associations to lupus. Science (New York, NY). 2022;376(6589):eabf1970.
Morgan B, Sun L, Avitahl N, Andrikopoulos K, Ikeda T, Gonzales E, et al. Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation. EMBO J. 1997;16(8):2004–13.
Li X, Zhang T, Kang L, Xin R, Sun M, Chen Q, et al. Apoptotic caspase-7 activation inhibits non-canonical pyroptosis by GSDMB cleavage. Cell Death Differ. 2023;30(9):2120–34.
Zhang Y, Willis-Owen SAG, Spiegel S, Lloyd CM, Moffatt MF, Cookson W. The ORMDL3 asthma gene regulates ICAM1 and has multiple effects on cellular inflammation. Am J Respir Crit Care Med. 2019;199(4):478–88.
Wiemels JL, Walsh KM, de Smith AJ, Metayer C, Gonseth S, Hansen HM, et al. GWAS in childhood acute lymphoblastic leukemia reveals novel genetic associations at chromosomes 17q12 and 8q24.21. Nat Commun. 2018;9(1):286.
Cobaleda C, Vicente-Dueñas C, Sanchez-Garcia I. Infectious triggers and novel therapeutic opportunities in childhood B cell leukaemia. Nat Rev Immunol. 2021;21(9):570–81.
Forgione MO, McClure BJ, Yeung DT, Eadie LN, White DL. MLLT10 rearranged acute leukemia: incidence, prognosis, and possible therapeutic strategies. Genes Chromosomes Cancer. 2020;59(12):709–21.
Said S, Pazoki R, Karhunen V, Võsa U, Ligthart S, Bodinier B, et al. Genetic analysis of over half a million people characterises C-reactive protein loci. Nat Commun. 2022;13(1):2198.
Zhang SL, Lin H, Huang F. Special diagnostic value of C-reactive protein in systemic autoimmune rheumatic diseases complicated with infections. Zhonghua Nei Ke Za Zhi. 2020;59(7):489–92.
CAS PubMed Google Scholar
Singh SB, Davis AS, Taylor GA, Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science (New York, NY). 2006;313(5792):1438–41.
Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42(12):1118–25.
Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–78.
Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohnʼs disease. Nat Genet. 2008;40(8):955–62.
Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47(9):979–86.
Juran BD, Hirschfield GM, Invernizzi P, Atkinson EJ, Li Y, Xie G, et al. Immunochip analyses identify a novel risk locus for primary biliary cirrhosis at 13q14, multiple independent associations at four established risk loci and epistasis between 1p31 and 7q32 risk variants. Hum Mol Genet. 2012;21(23):5209–21.
Baxter AG, Jordan MA. From markers to molecular mechanisms: type 1 diabetes in the post-GWAS era. The review of diabetic studies : RDS. 2012;9(4):201–23.
Johansson Å, Rask-Andersen M, Karlsson T, Ek WE. Genome-wide association analysis of 350 000 Caucasians from the UK Biobank identifies novel loci for asthma, hay fever and eczema. Hum Mol Genet. 2019;28(23):4022–41.
Wang YF, Zhang Y, Lin Z, Zhang H, Wang TY, Cao Y, et al. Identification of 38 novel loci for systemic lupus erythematosus and genetic heterogeneity between ancestral groups. Nat Commun. 2021;12(1):772.
Uhlen M, Zhang C, Lee S, Sjöstedt E, Fagerberg L, Bidkhori G, et al. A pathology atlas of the human cancer transcriptome. Science (New York, NY). 2017;357(6352):eaan2507.
Ma H, Hu T, Tao W, Tong J, Han Z, Herndler-Brandstetter D, et al. A lncRNA from an inflammatory bowel disease risk locus maintains intestinal host-commensal homeostasis. Cell Res. 2023;33(5):372–88.
Khunsriraksakul C, Li Q, Markus H, Patrick MT, Sauteraud R, McGuire D, et al. Multi-ancestry and multi-trait genome-wide association meta-analyses inform clinical risk prediction for systemic lupus erythematosus. Nat Commun. 2023;14(1):668.
Quan C, Ren YQ, Xiang LH, Sun LD, Xu AE, Gao XH, et al. Genome-wide association study for vitiligo identifies susceptibility loci at 6q27 and the MHC. Nat Genet. 2010;42(7):614–8.
Chu X, Pan CM, Zhao SX, Liang J, Gao GQ, Zhang XM, et al. A genome-wide association study identifies two new risk loci for Graves’ disease. Nat Genet. 2011;43(9):897–901.
Dang J, Bian X, Ma X, Li J, Long F, Shan S, et al. ORMDL3 facilitates the survival of splenic B cells via an ATF6α-endoplasmic reticulum stress-Beclin1 autophagy regulatory pathway. J Immunol (Baltimore, Md : 1950). 2017;199(5):1647–59.
John LB, Ward AC. The Ikaros gene family: transcriptional regulators of hematopoiesis and immunity. Mol Immunol. 2011;48(9–10):1272–8.
Porpaczy E, Jäger U. How I manage autoimmune cytopenias in patients with lymphoid cancer. Blood. 2022;139(10):1479–88.
Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77(5):521–46.
Fregona V, Bayet M, Bouttier M, Largeaud L, Hamelle C, Jamrog LA, et al. Stem cell-like reprogramming is required for leukemia-initiating activity in B-ALL. J Exp Med. 2024;221(1):20230279.
Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350(13):1328–37.
Vietzen H, Berger SM, Kühner LM, Furlano PL, Bsteh G, Berger T, et al. Ineffective control of Epstein-Barr-virus-induced autoimmunity increases the risk for multiple sclerosis. Cell. 2023;186(26):5705–18.e13.
Download references
Acknowledgements
We thank all the studies for making the summary association statistics data publicly available. We are also very grateful to the editor and two referees for their insightful and constructive comments, which substantially improved our original manuscript.
This work was supported by National Key Research and Development Program (2022YFC2502700) to Y.X. and National Natural Science Foundation of China (82020108003 to D.W., 82070187 to Y.X.). D.W. was supported by Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Jiangsu Provincial Medical Innovation Center (CXZX202201). Y.C. is also supported by Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX23_3270). D.W. was supported by Suzhou Science and Technology Program Project (SLT201911). X.Y. was supported by Boxi Cultivation Program of the First Affiliated Hospital of Suzhou University (BXQN2023032).
Author information
Xinghao Yu and Yiyin Chen contributed equally to this work.
Authors and Affiliations
National Clinical Research Center for Hematologic Diseases, Jiangsu Institute of Hematology, The First Affiliated Hospital of Soochow University, Suzhou, China
Xinghao Yu, Yiyin Chen, Jia Chen, Yi Fan, Depei Wu & Yang Xu
Collaborative Innovation Center of Hematology, Institute of Blood and Marrow Transplantation, Soochow University, Suzhou, China
Xinghao Yu, Yiyin Chen, Depei Wu & Yang Xu
Department of Outpatient and Emergency, The First Affiliated Hospital of Soochow University, Suzhou, China
You can also search for this author in PubMed Google Scholar
Contributions
DW and YX designed the study. XY obtained the data. XY and YC cleared up the datasets. XY, HL, and YC mainly performed the data analyses. YX, XY, YC, JC, YF, and HL drafted the manuscript. YX, XY, YC, and DW revised the manuscript, and all authors read and approved the final manuscript.
Corresponding authors
Correspondence to Depei Wu or Yang Xu .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
All authors declared no potential conflicts of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:.
Supplementary Methods and Fig. S1-S3. Supplementary Methods - A supplementary document on GWAS quality control, PLACO method, colocalization analysis, MAGMA analysis, HyPrColoc method, immune cell data description, and Mendelian randomization analysis. Fig. S1. Manhattan plot of the PLACO results. Fig. S2. -QQ plots for pleiotropic results performed by PLACO. Fig. S3. Regional plots of each colocalized locus (PP.H4 > 0.7) identified for corresponding trait pair (B-ALL&AOA) by using the PLACO. Fig. S4. Regional plots of each colocalized locus (PP.H4 > 0.7) identified for corresponding trait pair (B- B-ALL&HT) by using the PLACO. Fig. S5. Regional plots of each colocalized locus (PP.H4 > 0.7) identified for corresponding trait pair (B-ALL&PBC) by using the PLACO. Fig. S6. Regional plots of each colocalized locus (PP.H4 > 0.7) identified for corresponding trait pair (B-ALL&IBD) by using the PLACO. Fig. S7. Regional plots of each colocalized locus (PP.H4 > 0.7) identified for corresponding trait pair (B-ALL&MS) by using the PLACO. Fig. S8. Regional plot of each colocalized locus (PP.H4 > 0.7) identified for corresponding trait pair (B- B-ALL&RA) by using the PLACO. Fig. S9. Manhattan plot of MAGMA gene analysis. Fig. S10. Heatmap for expression values of pleiotropic genes in different tissues identified by MAGMA analysis. Fig. S11. Gene-set enrichment for identified pleiotropic genes. Red panels represent significant tissues after Bonferroni adjustment. Fig. S12. Heatmap of tissues and immune traits shared between autoimmune disorders and B-ALL identified by S-LDSC. Fig. S13. Heatmap shows whether the identified risk loci have been reported to be associated with B-ALL and AD in the previous studies after searching the GWAS catalog.

Additional file 2: Table S1.
Data sources. Table S2. Genetic correlation analysis conducted by LDSC and HDL. Table S3. Shared pleiotropic loci identified by PLACO. Table S4. Shared pleiotropic loci among different trait pairs. Table S5. MAGMA Gene-set analysis. Table S6. MAGMA tissue-specific analysis. Table S7. MAGMA gene analysis. Table S8. Information of pleiotropy genes identified by MAGMA. Table S9. Expression value of pleiotropy genes identified by MAGMA in different tissues from GTEx. Table S10. Tissue-specific enrichment of pleiotropy genes identified by MAGMA in different tissues from GTEx. Table S11. S-LDSC cell-type heritability enrichment analysis. Table S12. Multi-trait colocalization analysis highlighted key role of immune cells (PP>0.7). Table S13. Bi-direction MR analysis and sensitive analysis. Table S14. Bi-direction MR analysis and sensitive analysis. Table S15. Identified loci reported in previous GWAS analysis for ALL and AD.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Yu, X., Chen, Y., Chen, J. et al. Shared genetic architecture between autoimmune disorders and B-cell acute lymphoblastic leukemia: insights from large-scale genome-wide cross-trait analysis. BMC Med 22 , 161 (2024). https://doi.org/10.1186/s12916-024-03385-0
Download citation
Received : 08 January 2024
Accepted : 08 April 2024
Published : 15 April 2024
DOI : https://doi.org/10.1186/s12916-024-03385-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Genetic overlap
- Autoimmune disease
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 15, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
New way found to treat early relapse in leukemia
by Peter MacCallum Cancer Centre
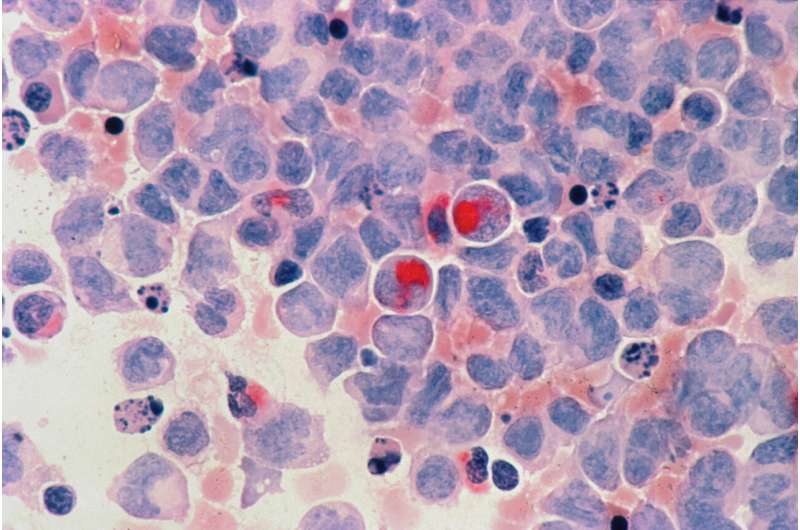
Researchers at Peter Mac have found a new way to treat a form of leukemia that stops the disease in its tracks to prolong remission.
The research, published in the Journal of Clinical Oncology , has shown how a new combination of a molecular technology called measurable residual disease (MRD), medication and low-dose chemotherapy is helping patients live longer with acute myeloid leukemia (AML).
Despite the best treatment , many patients with AML have recurrent disease within the first two years. This MRD test provides advanced warning that the disease is returning several months before the leukemia is visible under the microscope or the patient develops abnormal blood counts.
As soon as MRD was detected to rise, patients were treated with a pill called venetoclax combined with low-dose, under-the-skin injection of chemotherapy that stopped the leukemia in its tracks in the majority of patients.
Dr. Ing-Soo Tiong, hematologist and researcher at Peter Mac, said with the previous approach to treatment, the median survival after first relapse is only 6–8 months. Results of this clinical trial shows 50–70% of AML patients are still alive after two years.
"Prior to this discovery, patients and clinicians face the uncertainty of disease relapse, and the only treatment option then was an even stronger dose of salvage chemotherapy requiring at least a month of stay in hospital associated with a very high risk of infection," he said.
"In this new study we measured a patient's MRD as soon as they finished chemotherapy with the aim of the data telling us which patients were most likely to relapse."
Professor Andrew Wei, co-lead of the AML program at Peter Mac and Royal Melbourne Hospital, explained this option meant patients could be treated as an outpatient or by hospital in the home with results comparable to intensive chemotherapy .
"This is a paradigm-changing clinical trial that utilizes molecular technologies to enable patients to receive their interventional therapy much earlier than normal and with less toxicity," he said.
"The response to treatment was fast and durable, enabling patients to receive a subsequent stem cell transplant with much lower levels of disease burden and enhanced fitness.
"This is the first ever prospective trial using a pre-emptive MRD targeted approach. It has led to the development of a new national trial called INTERCEPT, coordinated by the Australasian Leukemia and Lymphoma Group."
The INTERCEPT trial is currently recruiting patients at Peter Mac and approximately 15 sites nationwide. AML is a type of blood cancer that affects the blood and bone marrow. It is a rare cancer with 1,218 people diagnosed in Australia in 2019.
Explore further
Feedback to editors

Health improvements occurred worldwide since 2010 despite COVID-19 pandemic, but progress was uneven: Study
27 minutes ago

Study shows heart failure, not stroke is the most common complication of atrial fibrillation

Antipsychotics for dementia linked to more harms than previously acknowledged

Protecting brain cells with cannabinol: Research suggests CBN shows promise for treating neurological disorders

Calorie restriction study reveals complexities in how diet impacts aging

Ketamine produces wide variety of responses in the brain, researchers find
2 hours ago

Emotional radio ads may ease listeners' qualms, boosting support for organ donation

To understand cognition and its dysfunction, neuroscientists must learn its rhythms

New insights into the mechanisms of bacterial brain invasion during meningitis

Study finds lower relapse risk in triple-negative breast cancer with high immune cell levels
Related stories.

Novel treatment regimen for FLT3-mutated acute myeloid leukemia shows promise in new study
Feb 8, 2024
New personalized therapy improves survival for patients with CLL leukemia, phase 3 trial finds
Dec 11, 2023

Improving drug therapy for relapsed leukemia
Sep 14, 2022

Improving treatment for hairy cell leukemia
Jan 13, 2023
New CAR T case study shows promise in acute myeloid leukemia
May 9, 2018

Acute myeloid leukemia: Germany-wide clinical trial challenges international standard of care
Dec 12, 2022
Recommended for you

Shape-shifting cancer cell discovery reveals potential skin cancer drug targets
6 hours ago

Targeted therapies outperform hundreds of other drugs in 'priming' lung cancer cells for destruction
5 hours ago

Antibiotics reveal a new way to fight cancer
Apr 16, 2024

A urine-based test that detects tumor DNA fragments could offer early reliable screening for head and neck cancer

An effective drug delivery system for next-generation treatments to hitch a ride in cancer cells
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
EDITORIAL article
This article is part of the research topic.
Allogeneic Transplantation in Pediatric Patients with Hematologic Malignancies
Editorial: Transplant Outcomes and Caregivers Perceptions in Decision-Making Process for Allogeneic HSCT in Pediatric Patients Provisionally Accepted

- 1 Niño Jesús University Children's Hospital, Spain
The final, formatted version of the article will be published soon.
Last but not least, Moreno C et al, addressed the role of haploidentical transplant as alternative to HLA matched donor transplant. The use of alternative donors such as haploidentical donors have expanded the use of allogeneic transplants to pediatric patients lacking a matched sibling donor. Biological parents share at least one haplotype with their children and all of them could be considered as potential donors. Even siblings and other relatives might be potential haploidentical donors, which contributes to almost all pediatric patients can have at least one available donor in a timely manner for allogeneic HSCT. Initially the use of haploidentical transplantation was associated with many clinical problems but the advent of T-cell depletion techniques and the use of post-transplant cyclophosphamide as GvHD prophylaxis have changed the haploidentical transplant landscape leading a rapid increase of its use worldwide over the last few years. As such, it is the allogeneic transplant modality that has the most growth in recent years not only in adults but also in children. However, there are few studies that compare transplant outcomes in between both groups. Authors compared outcomes of children with acute lymphoblastic leukemia undergoing HSCT in second complete remission (CR2) from haploidentical versus HLA-matched donors. A prospective data registry was generated, which allowed them to analyze treatment results. In this study, they focused on the analysis and comparison of treatment outcomes of 76 children undergoing HSCT in CR2 from haploidentical vs. HLAmatched donors. They found no significant differences in the estimate of principal transplant endpoints between both groups. Despite the short follow-up period of study, these results support the role of haploidentical donors as an alternative to HLAcompatible donors in this population.In conclusion, while the promise of HSCT offers a glimmer of hope for pediatric patients with malignant and non-malignant diseases, its realization hinges upon our collective commitment to addressing the social determinants that shape healthcare access and decision-making. Only through concerted efforts to dismantle barriers can we ensure that every child, regardless of background or circumstance, has the opportunity to embrace a future unburdened by disease.
Keywords: Allogeneic HSC transplantation, Pediatric Malignant Diseases, Caregivers perceptions, Transplant outcomes, haploidentical donors
Received: 03 Apr 2024; Accepted: 17 Apr 2024.
Copyright: © 2024 Diaz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Dr. Miguel A. Diaz, Niño Jesús University Children's Hospital, Madrid, Spain
People also looked at
MD Anderson Research Highlights for April 12, 2024
Featuring strategies to overcome treatment resistance, targets for ovarian and braf-mutated cancers, and a novel risk prediction tool for hereditary cancers.

Newswise — HOUSTON ― The University of Texas MD Anderson Cancer Center ’s Research Highlights showcases the latest breakthroughs in cancer care, research and prevention. These advances are made possible through seamless collaboration between MD Anderson’s world-leading clinicians and scientists, bringing discoveries from the lab to the clinic and back.
Recent developments at MD Anderson offer insights into a combination strategy to improve immunotherapy responses, promising trial results for patients with tumors harboring BRAF mutations, a maintenance strategy for patients with acute myeloid leukemia following chemotherapy, a strategy to overcome PARP inhibitor resistance in patients with BRCA1 -deficient cancers, insights into Phase III trial subgroup analyses, a promising therapeutic target for homologous recombination-proficient epithelial ovarian cancers, and a new model to predict cancer risk for patients with Li-Fraumeni syndrome and other hereditary cancers.
Combination strategy remodels tumor microenvironment to improve anti-tumor response Inducible T cell costimulator (ICOS) is a T cell-specific protein, and ICOS agonists enhance the efficacy of CTLA-4 immune checkpoint blockade . Previous studies have shown that, while ICOS pathway activation in combination with CTLA-4 blockade relies on a T cell-mediated response, CD8+ T cells only partially contributes to tumor rejection. In a new study from the James P. Allison Institute , led by Naveen Sharma, Ph.D., and James Allison, Ph.D. , researchers hypothesized that tumor-associated macrophages (TAMs) may play a role in combination therapy activity. Using high-dimensional profiling, they analyzed changes in immune cells that infiltrate tumors and demonstrated that TAMs play a crucial role in remodeling the tumor microenvironment. ICOS co-stimulation combined with anti-CTLA-4 immunotherapy remodeled immunosuppressive TAMs into anti-tumor TAMs in a positive feedback loop with intratumoral effector T cells, suggesting this strategy could improve treatment outcomes. The study recommends further examination of the role of TAMs in immunotherapy treatment efficacy. Learn more in the Journal of Experimental Medicine .
Rechallenge therapy with RAF inhibitors shows promise in BRAF -aberrant cancers Treating patients with a different RAF inhibitor after disease progression, a process known as rechallenging, has demonstrated anti-tumor efficacy in melanoma , but little is known about rechallenging the RAF pathway in other solid tumors. In the RE-RAFFLE study, led by Blessie Nelson, M.B.B.S ., 44 patients with multiple tumors harboring RAF alterations were rechallenged with a second RAF inhibitor. Patients had an overall response rate of 18.1% and a clinical benefit rate of 54.5%, with more than 30% of patients having durable responses lasting longer than six months. However, acquired resistance remained a significant challenge. The researchers noted that future prospective studies are needed to further validate and expand rechallenge targeted therapy options. Learn more in Molecular Cancer .
Low-dose azacitidine plus venetoclax suitable as maintenance therapy for AML patients following chemotherapy A majority of patients with acute myeloid leukemia (AML) experience relapse when they are unable to complete standard consolidation options or receive an allogeneic stem cell transplant . In a Phase II clinical trial led by Tapan Kadia, M.D. , and Alexandre Bazinet, M.D., researchers investigated a maintenance therapy regimen consisting of the BCL-2 inhibitor venetoclax and a low dose of azacitidine. The trial enrolled 35 patients who were in remission following intensive (cohort 1) or low-intensity chemotherapy (cohort 2). Median relapse-free survival – the study’s primary outcome – was not yet reached in cohort 1 and was 30.3 months for cohort 2, indicating encouraging responses. The two-year relapse-free survival rate for all patients was 65%, and all side effects were manageable. The results suggest that the combination therapy is a viable maintenance strategy for this patient population. Learn more in The Lancet Haematology .
GPX4 and PARP co-inhibition may boost PARP inhibitor effectiveness in BRCA1 -deficient cancers Many BRCA1 -deficient cancers develop resistance to Poly (ADP-ribose) polymerase (PARP) inhibitor monotherapy, highlighting a need to understand the underlying mechanisms to improve treatment outcomes. In a new study led by Boyi Gan, Ph.D. , researchers showed that BRCA1 -deficient cancers have a vulnerability that can be exploited using a combination treatment that makes PARP inhibition more effective. They found that BRCA1 has a role in regulating ferroptosis, a type of cell death triggered by lipid peroxidation. In BRCA1 -proficient cancers, the tumor’s adaptive response to PARP inhibition negates its ferroptosis-inducing effects, but BRCA1 deficiency makes tumors vulnerable to ferroptosis induced by GPX4 and PARP co-inhibition. In preclinical models, xenograft tumors from BRCA1 -mutated breast cancer patients with PARP inhibitor resistance were highly sensitive to this combination, supporting this co-inhibition as a therapeutic strategy. Learn more in Cancer Discovery .
Study suggests subgroup analyses of Phase III oncology trials need improvement Phase III randomized controlled trials (RCTs) play a crucial role in evaluating the effectiveness of new cancer treatments. Subgroup analyses often are performed to investigate treatment differences among patient groups in the trial. To assess the reliability of claims about different treatment effects in oncology trials, researchers led by Alexander Sherry, M.D., Pavlos Msaouel, M.D., Ph.D. , and Ethan Ludmir, M.D. , examined 379 published Phase III RCTs with subgroup analyses. The researchers found that 43% of trials had incomplete visual elements on the forest plots needed for interpretation, and the credibility of claims regarding differential treatment effects was assessed as low or very low in 93% of instances. This underscores the importance of improving the quality of subgroup analyses in Phase III cancer trials and carefully interpreting current subgroup findings in ongoing trials. Learn more in JAMA Network Open .
Targeting branched N-glycans and fucosylation sensitizes ovarian tumors to immune checkpoint blockade High-grade serious ovarian cancer accounts for more than 70% of epithelial ovarian cancers, which are the most lethal gynecologic cancer in the U.S. About half of these cancers harbor defects in the homologous recombination DNA repair pathway, making them susceptible to treatment with PARP inhibitors. However, treatment options remain limited for those without these defects. To address this, Rugang Zhang, Ph.D. , and colleagues demonstrated a potential vulnerability in these cancers that sensitizes them to immune checkpoint inhibitors . Aberrant glycosylation – changes in the way sugar molecules are attached to proteins – can help epithelial ovarian cancers evade detection. One type of protein, a branched N-glycan, allows specific cancers to evade CD8+ T cell response. Inhibiting these branched N-glycans in preclinical models sensitized the tumors to immune checkpoint inhibitors. These findings demonstrate that branched N-glycans could be a promising therapeutic target in homologous recombination-proficient epithelial ovarian cancers. Learn more in Nature Communications .
Novel model uses clinical data to predict cancer risk for Li-Fraumeni syndrome Li-Fraumeni syndrome (LFS) is a hereditary disorder characterized by germline mutations in the TP53 tumor suppressor gene, increasing a patient’s risk for various cancer types. There are limited risk prediction models for LFS because research datasets take decades to be completed for model development purposes. These datasets, however, do not reflect model performance in clinical settings since genetic counselors often must base their risk assessments on single counseling visits and incomplete family history. To address this, researchers led by Wenyi Wang, Ph.D. , and Banu Arun, M.D. , trained and validated new risk prediction models by using clinical data from 3,297 patients across 124 families. Their models performed well with patients with either primary or multiple primary cancers who underwent genetic counseling sessions. The researchers developed primary cancer risk and recurring event models for LFS, which performed well in predicting TP53 mutations in untested patients. The models also compared favorably to other validation studies using research cohorts. These models stress the importance of using clinical data and improving data collection within clinic visits to potentially improve care for patients with hereditary cancers. Learn more in the Journal of Clinical Oncology .
Awards and honors
- Padmanee Sharma, M.D., Ph.D. , professor of Genitourinary Medical Oncology , and Raghu Kalluri, M.D., Ph.D. , professor of Cancer Biology , were elected into the Association of American Physicians
- Han Liang, Ph.D. , professor of Bioinformatics and Computational Biology , was inducted into the 2024 Class of the American Institute for Medical and Biological Engineering College of Fellows
- Joshua Kuban, M.D. , associate professor of Interventional Radiology , Rahul Sheth, M.D. , associate professor of Interventional Radiology , Steven Yevich, M.D. , associate professor of Interventional Radiology , and Aaron Kyle Jones, Ph.D. , professor of Imaging Physics , were inducted as Society of Interventional Radiology Fellows
- Padmanee Sharma, M.D., Ph.D. , professor of Genitourinary Medical Oncology , earned the Outstanding Scientist Award from the American Association of Indian Scientists in Cancer Research
- Nicole Vaughan-Adams, B.S.N., associate director of Nursing, and Rebecca Lu, advanced practice provider supervisor in Lymphoma and Myeloma, were awarded the DAISY Nurse Leader Award
MD Anderson at AACR 2024 Read below for highlights from MD Anderson at the American Association for Cancer Research (AACR) Annual Meeting 2024. More information can be found at MDAnderson.org/AACR .
- AACR: Trio of studies highlights promising early results with new cancer therapies and targets
- AACR: PARP1-selective inhibitor demonstrates early efficacy in breast cancers with DNA repair defects
- AACR: Combination treatment is well-tolerated, shows antitumor effects in KRAS G12C-mutated metastatic colorectal cancer
- AACR: Novel immunotherapies show promise for patients with kidney cancer and for solid organ transplant recipients with skin cancer
- MD Anderson Research Highlights AACR 2024 Special Edition
- AACR: MD Anderson’s Scott Kopetz and Elizabeth Travis honored with 2024 Scientific Achievement Awards
- AACR: MD Anderson's Padmanee Sharma elected Fellow of the AACR Academy
In case you missed it Read below to catch up on recent MD Anderson press releases.
- Radiation before mastectomy cuts time delays for reconstructive surgery in breast cancer patients
- MD Anderson’s Institute for Data Science in Oncology announces appointment of inaugural IDSO Affiliates
Read this press release in the MD Anderson Newsroom .
Journal Link: Journal of Experimental
MEDIA CONTACT
Journal of Experimental
TYPE OF ARTICLE
- Experts keyboard_arrow_right Expert Pitch Expert Query Expert Directory
- Journalists
- About keyboard_arrow_right Member Services Accessibility Statement Newswise Live Invoice Lookup Services for Journalists Archived Wires Participating Institutions Media Subscribers Sample Effectiveness Reports Terms of Service Privacy Policy Our Staff Contact Newswise
- Blog FAQ Help
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Blood Cancer J
- v.7(6); 2017 Jun
Acute lymphoblastic leukemia: a comprehensive review and 2017 update
T terwilliger.
1 New York University School of Medicine, New York, USA
M Abdul-Hay
2 Department of Hematology, New York University Perlmutter Cancer Center, New York, USA
Acute lymphoblastic leukemia (ALL) is the second most common acute leukemia in adults, with an incidence of over 6500 cases per year in the United States alone. The hallmark of ALL is chromosomal abnormalities and genetic alterations involved in differentiation and proliferation of lymphoid precursor cells. In adults, 75% of cases develop from precursors of the B-cell lineage, with the remainder of cases consisting of malignant T-cell precursors. Traditionally, risk stratification has been based on clinical factors such age, white blood cell count and response to chemotherapy; however, the identification of recurrent genetic alterations has helped refine individual prognosis and guide management. Despite advances in management, the backbone of therapy remains multi-agent chemotherapy with vincristine, corticosteroids and an anthracycline with allogeneic stem cell transplantation for eligible candidates. Elderly patients are often unable to tolerate such regimens and carry a particularly poor prognosis. Here, we review the major recent advances in the treatment of ALL.
Introduction
Acute lymphoblastic leukemia (ALL) is a malignant transformation and proliferation of lymphoid progenitor cells in the bone marrow, blood and extramedullary sites. While 80% of ALL occurs in children, it represents a devastating disease when it occurs in adults. Within the United States, the incidence of ALL is estimated at 1.6 per 100 000 population. 1 In 2016 alone, an estimated 6590 new cases were diagnosed, with over 1400 deaths due to ALL (American Cancer Society). The incidence of ALL follows a bimodal distribution, with the first peak occurring in childhood and a second peak occurring around the age of 50. 2 While dose-intensification strategies have led to a significant improvement in outcomes for pediatric patients, prognosis for the elderly remains very poor. Despite a high rate of response to induction chemotherapy, only 30–40% of adult patients with ALL will achieve long-term remission. 3
Pathophysiology
The pathogenesis of ALL involves the abnormal proliferation and differentiation of a clonal population of lymphoid cells. Studies in the pediatric population have identified genetic syndromes that predispose to a minority of cases of ALL, such as Down syndrome, Fanconi anemia, Bloom syndrome, ataxia telangiectasia and Nijmegen breakdown syndrome. 4 , 5 , 6 , 7 Other predisposing factors include exposure to ionizing radiation, pesticides, certain solvents or viruses such as Epstein-Barr Virus and Human Immunodeficiency Virus. 8 , 9 , 10 However, in the majority of cases, it appears as a de novo malignancy in previously healthy individuals. Chromosomal aberrations are the hallmark of ALL, but are not sufficient to generate leukemia. Characteristic translocations include t(12;21) [ ETV6-RUNX1 ], t(1;19) [ TCF3-PBX1 ], t(9;22) [ BCR-ABL1 ] and rearrangement of MLL . 11 More recently, a variant with a similar gene expression profile to (Philadelphia) Ph-positive ALL but without the BCR-ABL1 rearrangement has been identified. In more than 80% of cases of this so-called Ph-like ALL, the variant possesses deletions in key transcription factors involved in B-cell development including IKAROS family zinc finger 1 (IKZF1), transcription factor 3 (E2A), early B-cell factor 1 (EBF1) and paired box 5 (PAX5). 12 Similarly, kinase-activating mutations are seen in 90% of the Ph-like ALL. The most common of these include rearrangements involving ABL1, JAK2, PDGFRB, CRLF2 and EPOR, activating mutations of IL7R and FLT3 and deletion of SH2B3, which encodes the JAK2-negative regulator LNK. 13 This has significant therapeutic implications as it suggests that Ph-like ALL, which tends to carry a worse prognosis, may respond to kinase inhibitors. In fact, Roberts et al. 14 showed that cell lines and human leukemic cells expressing ABL1, ABL2, CSF1R and PDGFRB were sensitive in vitro and in vivo human xenograft models to second-generation TKIs (for example, dasatinib.); those with EPOR and JAK2 rearrangements were sensitive to JAK kinase inhibitors (for example, ruxolitinib); and those with ETV6-NTRK3 fusion were sensitive to ALK inhibitors crizotinib. Furthermore, Holmfeldt et al. 15 recently described the genetic basis of another subset with poor outcomes, hypodiploid ALL. In near-haploid (24–31 chromosomes) ALL, alterations in tyrosine kinase or Ras signaling was seen in 71% of cases and in IKAROS family zinc finger 3 (IKZF3) in 13% of cases. In contrast, low-hypodiploid (32–39 chromosomes) ALL, alterations in p53 (91%), IKZF2 (53%) and RB1 (41%) were more common. Both near-haploid and low-hypodiploid exhibited activation of Ras- and PI3K-signaling pathways, suggesting that these pathways may be a target for therapy in aggressive hypodiploid ALL. 15
Most of the clinical manifestations of ALL reflect the accumulation of malignant, poorly differentiated lymphoid cells within the bone marrow, peripheral blood, and, extramedullary sites. Presentation can be nonspecific, with a combination of constitutional symptoms and signs of bone marrow failure (anemia, thrombocytopenia, leukopenia). Common symptoms include ‘B symptoms’ (fever, weight loss, night sweats), easy bleeding or bruising, fatigue, dyspnea and infection. Involvement of extramedullary sites commonly occurs and can cause lymphadenopathy, splenomegaly or hepatomegaly in 20% of patients. 16 , 17 CNS involvement at time of diagnosis occurs in 5–8% of patients and present most commonly as cranial nerve deficits or meningismus. 3 T-cell ALL also may present with a mediastinal mass.
Diagnosis is established by the presence of 20% or more lymphoblasts in the bone marrow or peripheral blood. 16 Evaluation for morphology, flow cytometry, Immunophenotyping and cytogenetic testing is valuable both for confirming the diagnosis and risk stratification. Lumbar puncture with CSF analysis is standard of care at the time of diagnosis to evaluate for CNS involvement. If the CNS is involved, brain MRI should be performed. Other evaluation includes complete blood count with differential and smear to evaluate the other hematopoietic cell lines, coagulation profiles and serum chemistries. Baseline uric acid, calcium, phosphate and lactate dehydrogenase should be recorded to monitor for tumor lysis syndrome.
Classification
The first attempt at classifying ALL was the French American British (FAB) morphological criteria that divided ALL into 3 subtypes (L1, L2 and L3) based on cell size, cytoplasm, nucleoli, vacuolation and basophilia. 18 In 1997, the World Health Organization proposed a composite classification in attempt to account for morphology and cytogenetic profile of the leukemic blasts and identified three types of ALL: B lymphoblastic, T lymphoblastic and Burkitt-cell Leukemia. 19 Later revised in 2008, Burkitt-cell Leukemia was eliminated as it is no longer seen as a separate entity from Burkitt Lymphoma, and B-lymphoblastic leukemia was divided into two subtypes: B-ALL with recurrent genetic abnormalities and B-ALL not otherwise specified. B-ALL with recurrent genetic abnormalities is further delineated based on the specific chromosomal rearrangement present ( Table 1 ). 20 In 2016, two new provisional entities were added to the list of recurrent genetic abnormalities and the hypodiploid was redefined as either low hypodiploid or hypodiploid with TP53 mutations. 21 In adults, B-cell ALL accounts for ~75% of cases while T-cell ALL comprises the remaining cases.
Abbreviations: ALL, acute lymphoblastic leukemia; WHO, World Health Organization.
Prognostic factors
Accurate assessment of prognosis is central to the management of ALL. Risk stratification allows the physician to determine the most appropriate initial treatment regimen as well as when to consider allogeneic stem cell transplantation (Allo-SCT). Historically, age and white blood cell count at the time of diagnosis have been used to risk stratify patients. Increasing age portends a worsening prognosis. Patients over the age of 60 have particularly poor outcomes, with only 10–15% long-term survival. 22 Age is at least in part a surrogate for other prognosticators as the elderly tend to have disease with intrinsic unfavorable biology (for example, Philadelphia chromosome positive, hypodiploidy and complex karyotype), more medical comorbidities and inability to tolerate standard chemotherapy regimens but helps guide therapy nonetheless. In the largest prospective trial to determine optimal treatment, MRC UKALL XII/ECOG E2993 found a significant difference of disease-free (DFS) and overall survival (OS) based on age using a cutoff of 35 in Ph-negative disease. 23 Similarly, they found an elevated white blood cell count at diagnosis, defined as >30 × 10 9 for B-ALL or >100 10 9 for T-ALL, was an independent prognostic factor for DFS and OS. On the basis of these results, Ph-negative disease could be categorized as low risk (no risk factors based on age or WBC count), intermediate risk (age >35 or elevated WBC count), or high risk (age >35 and elevated WBC count). The 5-year OS rates based on these risk categories were 55, 34 and 5%, respectively. 23
Although clinical factors play an important role in guiding therapy, cytogenetic changes have a significant role in risk determination. The cytogenetic aberration with the greatest impact on prognosis and treatment is the presence of the Philadelphia chromosome, t(9;22). The prevalence of t(9;22) in adult ALL can range from 15–50% and increases with age. 24 Ph-positivity has implications both in terms of prognosis and for treatment. Historically, Ph-positive ALL has a 1-year survival of around 10%. However, with the development of TKIs, survival has improved and thus the Ph-status of all patients must be obtained prior to starting therapy. Subsequent analysis of MRC UKALL XII/ECOG E2993, identified cytogenetic subgroups of Ph-negative disease with inferior outcomes. These included t(4;11), KMT2A translocation, t(8;14), complex karyotype (⩾ 5 chromosomal abnormalities) and low hypodiploidy (30–39 chromosomes)/near triploidy (60–78 chromosomes). In contrast, patients with hyperdiploidy and del(9p) had a significantly better outcome. 25 In a later study, the Southwest Oncology Group (SWOG) showed that among the 200 study patients, cytogenetic profile was a more important prognostic factor than age or WBC count. 26 More recently, a subset of high-risk ALL without t(9;22) has been identified with a genetic profile similar to that of Ph-positive ALL. This so called, Ph-like ALL has been associated with poor response to induction chemotherapy, elevated minimal residual disease and poor survival. 13 , 14 , 27
In addition to disease characteristics at the outset, it has long been recognized that response to initial therapy predicts outcome. Historically, treatment response was evaluated morphologically. Recently, it has become standard practice to evaluate patients for minimal residual disease (MRD) using molecular techniques such as flow cytometry and PCR. 28 Several studies have shown the importance of MRD in assigning risk. 29 , 30 , 31 , 32 , 33 , 34 Bruggemann et al. 29 re-stratified standard-risk patients to low risk, intermediate risk and high risk with relapse rates of 0%, 47% and 94%, respectively, based on the persistence of elevated MRD, defined as >10 −4 . In a multivariate analysis of 326 adolescent and adult patients with high-risk Ph-negative ALL treated in The Programa Espanol de Tratamientos en Hematologia (PETHEMA ALL-AR-03), Ribera et al. 35 showed that poor MRD clearance, defined as levels >1 × 10 −3 after induction and levels >5 × 10 −4 after early consolidation by flow cytometry, was the only significant prognostic factor for disease-free and overall survival.
On the basis of what is known about prognostic factors in adult ALL, the National Comprehensive Cancer Network (NCCN) has developed recommendations to approach risk stratification. 16 The National Cancer Institutes defines adolescent and young adults (AYA) to be those aged 15–39 years. The NCCN recognizes that AYA may benefit from treatment with pediatric-inspired regimens and thus are considered separately from adults >40 years. 36 , 37 Both age groups are then stratified into high-risk Ph-positive and standard-risk Ph-negative subgroups. The Ph-negative subgroup can further be categorized as high-risk based on the presence of MRD, elevated WBC (defined above) or unfavorable cytogenetics (defined above).
Established treatments
The structure of treatment of adult ALL has been adapted from pediatric protocols. Unfortunately, while long-term survival approaches 90% for standard-risk pediatric ALL, the success rate is much more modest in adults. Chemotherapy consists of induction, consolidation and long-term maintenance, with CNS prophylaxis given at intervals throughout therapy. The goal of induction therapy is to achieve complete remission and to restore normal hematopoiesis. The backbone of induction therapy typically includes vincristine, corticosteroids and an anthracycline. 38 , 39 In the Cancer and Leukemia Group B 8811 trial, Larsen et al. 40 achieved a complete response rate of 85% and a median survival of 36 months. The 4-week long induction schedule consists of cyclophosphamide on day 1, 3 consecutive days of daunorubicin, weekly vincristine, biweekly l -asparaginase and 3 weeks of prednisone. 40 Due to high induction-related mortality, one-third dose reductions of cyclophosphamide and daunorubicin were implemented for patients older than 60 and the duration of prednisone was shortened to 7 days in this age group. The role of L-asparaginase, while standard in pediatric protocols, is a challenge in adults at times due to the increased rate of adverse events. 41 In fact, in the UKALL 14 Trial, Patel et al. 42 , 43 demonstrated that asparaginase toxicity was the leading cause of induction-related mortality and the protocol was amended to omit asparaginase for patients over the age of 40. The MRC UKALL XII/ECOG 2993 23 regimen utilizes a similar structure to CALGB 8811. Induction is divided into two phases of four weeks. In contrast to CALGB 8811, cyclophosphamide is omitted in phase I of induction, but a single dose of intrathecal methotrexate is added for CNS prophylaxis. In phase II of induction, cyclophosphamide is introduced along with cytarabine, oral 6-mercaptopurine (6-MP), four additional intrathecal doses of methotrexate, and cranial radiation if CNS is positive. After induction therapy, patients received three cycles of intensification therapy of methotrexate with leucovorin rescue and l -asparaginase. Eligible patients with high-risk disease and a matched donor, then underwent Allo-SCT. All others were randomized to standard consolidation/maintenance or autologous stem cell transplant. This study yielded a complete response rate of 91% and an overall 5-year survival of 38%. 23
The Hyper-CVAD (HCVAD)/ Methotrexate-cytarabine regimen is utilizes an alternative structure to the approaches described above. It consists of four cycles of hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone alternated with four cycles of high dose cytarabine and methotrexate. 44 CNS prophylaxis with 4-16 doses of intrathecal chemotherapy depending on predetermined risk of CNS disease. HCVAD has demonstrated similar efficacy to the ECOG trial with a 92% complete response rate and 32% 5-year disease-free survival. 44 Several studies have suggested a benefit to using dexamethasone as opposed to prednisone due to the ability of dexamethasone to achieve higher concentrations in the CNS. Despite a reduction in CNS relapse and improved event-free survival, dexamethasone has increased risk of adverse events compared to prednisone. Since there have been no studies comparing overall survival, the benefit of one corticosteroid over the other has not been established. 45 , 46
After induction, eligible patients may go on to Allo-SCT while all others go on to intensification/consolidation and maintenance. 47 Consolidation varies in the different protocols, but generally utilize similar agents to induction and includes intrathecal chemotherapy and cranial radiation for CNS prophylaxis at times. Maintenance therapy consists of daily 6-MP, weekly methotrexate, and vincristine and a 5-day prednisone pulse every 3 months. Maintenance is administered for 2–3 years after induction, beyond which it has not been shown to have benefit. 17 , 47
Special consideration must be made in the treatment of Ph-positive ALL. Historically, Ph-positive ALL was a very bad player with 5-year survival ~5–20% and Allo-SCT being the only chance for cure. 48 , 49 Various studies have found that matched-sibling Allo-SCT may improve long-term survival to 35–55%, however, availability of matched donors represents a significant limitation. 49 , 50 , 51 The advent of TKIs marked a turning point in the treatment of Ph-positive ALL. Thomas et al. 52 , 53 showed that when added to traditional HCVAD, imatinib resulted in improvement in 3-year OS (54 vs 15%). Despite these promising results, some patients fails treatment due to resistance or relapse, particularly in the CNS where imatinib has limited penetration. 54 Second-generation ABL kinase inhibitor, dasatinib, was developed as a dual src/abl kinase inhibitor for chronic myeloid leukemia with a superior resistance profile to imatinib. Dasatinib was also shown to penetrate the blood-brain barrier and was effective at treating CNS disease in a mouse model and pediatric Ph-positive ALL. 55 In the first study of dasatinib in Ph-positive ALL, Ravandi et al. 56 found a CR rate of 96% when dasatinib was combined with HCVAD, and a 5-year OS of 46%. In a subsequent, multi-center trial HCVAD plus dasatinib achieve a 3-year OS of 71% in adult patients younger than 60. 57 In addition, prior resistance to imatinib did not preclude a response to dasatinib. 58 In addition, dasatinib was shown to be effective in inducing complete remission when used in combination with prednisone and intrathecal methotrexate. 59 In the GIMEMA LAL1205 study, 59 it was noted that the most common cause of relapse was a T315I mutation in the ABL kinase doman. Ponatinib, a third-generation TKI with the ability to inhibit most BCR-ABL1 kinase domain mutations, has recently gained approval for resistant Ph-positive ALL. The PACE trial 60 demonstrated the ability of ponatinib to generate a cytogenetic response in 47% of Ph-positve ALL patients after dasatinib failure. When compared head-to-head with dasatinib, ponatinib achieved significantly better 3-year EFS and OS when used as frontline therapy. 42 , 61 , 62 These data suggest that ponatinib may soon have a role in the frontline therapy of Ph-positive ALL.
Recent studies have suggested that the AYA population, defined as aged 15–39, may benefit from treatment on pediatric-inspired protocols. In an analysis of 262 AYA patients aged 16–21 on pediatric protocol CCG 1961, Nachman et al. 63 reported a 5-year EFS of 68%. Furthermore, patients in the study that were treated on augmented intensity therapy performed better. In a prospective study, Stock et al. 64 treated 317 patients aged 17–39 on Children’s Oncology Group AALL0232 protocol. Median EFS approached 60 months, which was statistically higher than the null hypothesis of 32 months. OS at 2-years was 78%. 64 Similarly, The Group for Research on Adult Acute Lymphoblastic Leukemia (GRAAL), compared 225 patients up to the age of 60 who were treated on pediatric-inspired regimen and historical data from 712 adults treated on standard adult regimen LALA-93. 36 They observed a significant improvement in CR, EFS and OS, which was most marked in patients younger than age of 45 years. In fact, in patients older than 45 years, there was a significantly higher rate of chemotherapy-related events compared to younger patients, suggesting that an age cutoff for pediatric-inspired regimens is appropriate. However still one of the adult regimens is still considered for AYA patients is HCVAD±rituximab. An MD Anderson Cancer Center study revealed no significant difference in CR rate or OS in AYA patients treated with HCVAD±rituximab vs an augmented-Berlin-Frankfurt-Munster regimen. 65
Refractory/relapsed disease
While 85–90% of patients go into remission after induction therapy, there are subsets that are refractory to induction therapy. In addition, a majority of patients that do achieve CR go on to relapse. Options of salvage therapy for relapsed/refractory (r/r) Ph-negative disease include augmented cytotoxic chemotherapy, reformulated single-agent chemotherapy and novel monoclonal antibodies. Augmented-HCVAD for salvage therapy was inspired by pediatric regimens that employ intensified doses of vincristine, corticosteroids and asparaginase in frontline therapy. Faderl et al. 66 treated 90 patients (median age 34) with relapsed or refractory disease with HCVAD in which the dosing of vincristine, dexamethasone and asparaginase where intensified as follows: vincristine 2 mg i.v. weekly on days 1, 8 and 15; dexamethasone 80 mg i.v. or orally (p.o.) on days 1–4 and 15–18, and pegaspargase 2500 units/m 2 i.v. on day 1 of the hyper-CVAD courses (1, 3, 5 and 7) and day 5 of the methotrexate/cytarabine courses (2, 4, 6 and 8). The majority of patients were in first salvage and ten patients were primary refractory, and patients with prior exposure to HCVAD were not excluded. Complete response was observed in 47% of the patients, with a median duration of 5 months. Median DFS and OS were 6.2 and 6 months respectively. 66 It was also noted that the addition of rituximab to HCVAD for B-ALL with high CD20 expression to improve the activity of this salvage regimen.
In patients with relapsed/refractory ALL, particularly those with multiple relapses, toxicity of multi-agent cytotoxic therapy may be limiting. Therefore, attempts have been made at salvage therapy with a single agent. In subgroup analysis of 70 patients receiving second salvage therapy with a single agent (most commonly vinorelbine (6), clofarabine (5), nelarabine (4) and topotecan (4)), only 3 achieved a complete response. 67 , 68 Vincristine sulfate liposomes injection (VSLI) was developed to overcome the dosing and pharmacokinetic limitations of nonliposomal vincristine (VCR). In a phase II study in adults with Ph-negative ALL in their second or greater relapse, VSLI was administered weekly at a dose of 2.25 mg/m 2 . 69 Of the 65 adults enrolled, 20% achieved complete response with a median duration of 23 weeks (range 5–66). Twelve patients were bridged to Allo-SCT, with five long-term survivors. 69 This study led to the accelerated approval of VSLI for salvage therapy in 2012. VSLI was well tolerated with a side effect profile similar to standard-formulation VCR, despite the massive cumulative doses of VCR achieved.
Despite the modest ability of cytotoxic chemotherapy to prolong survival, the only hope for long-term survival in these regimens remains Allo-SCT. However, recently novel monoclonal antibodies have transformed the landscape of salvage therapy by offering a chance at cure may be without Allo-SCT. The first of these is the bispecific anti-T-cell receptor/anti-CD19 antibody, blinatumomab. The proposed mechanism of action of blinatumomab is that it engages T cells to activate a B-cell specific inflammatory and cytolytic response. 70 Blinatumomab was first studied in patients with MRD positive ALL. In one trial, 80% of patients became MRD negative after the first cycle of blinatumomab, with 60% of patients remaining in CR at a median follow-up of 33 months. 71 Importantly, in a multi-center trial (BLAST), Gokbuget et al. 72 confirmed the ability of blinatumomab to eliminate MRD and showed no difference in OS or relapse-free survival (RFS) between patients who received Allo-SCT during the first CR (CR1) and those who did not. Based on these results, blinatumomab was studied for relapsed/refractory Ph-negative ALL. The landmark study was a multi-center, single-arm, open-label phase 2 trial in which 189 patients with primary refractory and relapsed ALL received single-agent therapy with blinatumomab. CR was achieved after 2 cycles in 43 with 82% achieving MRD negativity. The median response duration and the overall survival were 9 and 6 months, respectively. 73 Based on these results, blinatumomab was approved by the FDA for relapsed and refractory ALL in 2016. Subsequently, blinatumomab was compared to investigator’s choice of chemotherapy for r/r Ph-negative ALL in the phase 3 randomized trial (TOWER study). The blinatumomab study group ( n =271) had a median survival of 7.7 months (95% confidence interval (CI): 5.6, 9.6) versus 4.0 months (95% CI: 2.9, 5.3) for standard of care ( n =134) ( P =0.012, hazards ratio (HR), 0.71). 74 The study was terminated early for efficacy based on these results. Blinatumomab has also been investigated for r/r Ph-positive disease. In the ALCANTARA trial, standard dose blinatumomab was given for up to 5 cycles in 45 patients. CR was observed in 36 and 88% of whom were MRD negative, and with a median follow-up of 9 months, the median OS was 7.1 months. 75 Future investigation is planned for the frontline use of blinatumomab for Ph-positive ALL in conjunction with TKIs. 76 The toxicity profile of blinatumomab is acceptable. The most frequent adverse events include fever, chills, neutropenia, anemia and hypogammaglobulinemia. 3 More significant adverse events are rare, but include cytokine release syndrome, altered mental status and seizures. 73 Death from sepsis that is thought to be treatment-related has been reported.
Frontline therapy is the same for B-cell ALL and T-cell ALL. However, owing to different biology of the two subtypes, T-cell ALL is not amenable to salvage treatment with blinatumomab. Fortunately, alternative options for salvage therapy exist. Nelarabine is a T-cell specific purine nucleoside analog that is FDA approved for r/r T-cell ALL. Nelarabine accumulates in T cells at a high rate and incorporates into DNA causing an inhibition of DNA synthesis and subsequent apoptosis. 77 In a phase 2, open-label, multi-center trial, nelarabine was administered on alternate day schedule (days 1, 3 and 5) at 1.5 g/m 2 /day for r/r T-cell ALL. Cycles were repeated every 22 days. The rate of complete remission was 31% (95% CI, 17, 48%), the median DFS and OS were 20 weeks with a 1-year OS of 28%. 77 However, there is still more that needs to be done to achieve a better response and overall survival in patients with relapsed/refractory B- and T-cell ALL.
Future therapies
1-monoclonal antibodies, a-cd22-directed therapy.
CD22 is a B-lineage differentiation antigen expressed in B-cell ALL in 50–100% of adults and 90% of children. 78 , 79 , 80 Upon binding of an antibody, CD22 is rapidly internalized, thus making it an attractive target for delivering immunotoxin to leukemic cells. 81
Epratuzumab
Epratuzumab is an unconjugated monoclonal antibody targeting CD22 that has been studied in pediatric and adult relapsed/refractory ALL. Epratuzumab was evaluated in 15 pediatric patients as part of a salvage therapy regimen. The antibody was administered as a single-agent followed by the antibody in combination with standard re-induction chemotherapy. The treatment resulted in a CR in 9 of the patients, with 7 achieving complete MRD clearance at the end of re-induction. 82 A phase 2 study in adults with relapsed/refractory disease evaluated the addition of epratuzumab to clofaribine/cytarabine. The study demonstrated a superior response rate when compared to historical data of clofaribine/cytarabine alone. 83 More recently, epratuzumab conjugated to the topoisomerase I inhibitor, SN-38, has been shown to have activity against B-cell lymphoma and leukemia cell lines in in vitro and in vivo preclinical studies. 84
Inotuzumab ozogamicin
Inotuzumab ozogamicin (InO) is a monoclonal antibody against CD22 that is conjugated to calicheamicin, a potent cytotoxic compound that induces double-strand DNA breaks. 85 Upon internalization of the immunoconjugate, calicheamicin binds DNA and causes double-stranded DNA breaks, which induces apoptosis. Preclinical studies showed that calicheamicin conjugated to an anti-CD22 antibody resulted in potent cytotoxicity leading to regression of B-cell lymphoma and prevention of xenograft establishment at picomolar concentrations. 86 Phase 1 studies in non-hodgkin lymphoma (NHL) established a maximum tolerated dose of 1.8 mg/m 2 InO given intravenously every 3 to 4 weeks. 87 Subsequently, InO was studied in adults with relapsed/refractory ALL. 88 In this phase 2 trial, 90 patients were treated with either a single infusion every 3 to 4 weeks or weekly InO infusions. Cumulative doses were equivalent among the two treatment strategies. Overall response rate was 58%, with similar response between the two dosing schedules. Median survival was 6.2 months, with a non-significant benefit seen in weekly dosing. However, toxicity was greatly improved by weekly dosing, with a significant reduction in fever, hepatotoxicity and veno-occlusive disease. 89 A second phase 2 study of 35 patients with CD22+ ALL in second salvage or later showed similar complete response rate (66%) and median overall survival (7.4 months). 90 Based on these results, Kantarjian et al. 91 compared weekly dosing of InO to standard chemotherapy for relapsed/refractory ALL. The rate of complete remission was significantly higher in the InO group versus standard chemotherapy 80.7% (95% CI, 72.1–87.7) vs 29.4% (95% CI, 21.0–38.8), P <.001). 91 Progression-free survival (5 months vs 1.8 months) and overall survival (7.7 months vs 6.7 months) were also significantly prolonged with InO compared to standard chemotherapy. The most common adverse events of InO treatment included thrombocytopenia and neutropenia. Veno-occlusive liver disease occurred in 11% of patients treated with InO compared to 1% of those receiving standard chemotherapy. 91 Based on these results, InO was granted Breakthrough Therapy status by the FDA in 2015 and is a strong candidate for expedited approval for relapsed/refractory ALL.
InO has also been studied in frontline therapy in combination with low-intensity HCVAD for elderly patients >60 years. 92 These patients are prone to adverse events from chemotherapy and have poorer outcomes than their younger counterparts. In attempt to reduce toxicity, doxorubicin was eliminated from induction therapy, and cyclophosphamide, prednisone, methotrexate and cytarabine were given at reduced doses. InO was given during each of the first four courses. The regimen was well tolerated and produced superior 1-year OS as compared to historical data among similar patient population (78 vs 60%). 92
Moxetumomab pasudodotox
A third anti-CD22 monoclonal antibody, moxetumomab, is currently in development for treatment of pediatric and adult ALL. Moxetumomab is a reformulation of an older study drug, BL22, which was composed the variable region (F v ) of an anti-CD22 monoclonal antibody fused to Pseudomonas aeruginosa exotoxin A. 93 BL22 was shown to be highly active against Hairy Cell Leukemia in a phase 2 trial. 94 In a phase 1 trial of children with relapsed/refractory ALL, BL22 was well tolerated and exhibited anti-leukemic activity at all doses, but clinical benefits were transient and modest. 95 Therefore, BL22 was reformulated as moxetumomab to contain a F v fragment with greater affinity for CD22. In phase 1 trials, moxetumomab showed an overall activity rate of 70% in children with relapsed/refractory ALL. 96 Enrollment is ongoing for a phase 1/2 trial of moxetumomab pasudodotox for treatment of relapsed/refractory ALL in adults. 97
Combotox is a combination immunotoxin that contains a 1:1 mixture of anti-CD19 and anti-CD22 antibodies, both conjugated to the cytotoxin deglycosylated ricin-A chain. In pediatric patients with relapsed/refractory ALL, combotox led to a CR in 3 of 17 patients. In addition, six additional patients experienced a >95% reduction in peripheral blasts. 98 In adults with relapsed/refractory disease, combotox led to reduction of peripheral blasts in all patients; however, a durable response was not seen as blast count rebounded quickly after the final dose of combotox. 99 A phase I trial is recruiting patients to evaluate combotox in combination with cytarabine for adults with relapsed/refractory ALL ( {"type":"clinical-trial","attrs":{"text":"NCT01408160","term_id":"NCT01408160"}} NCT01408160 ).
CD20 is a B-lineage specific antigen expressed at nearly all stages of differentiation on the surface of both normal and malignant B-cells. Signaling through CD20 plays a role in cell cycle progression, differentiation pathways and regulation of apoptosis. CD20 is expressed in 40–50% of precursor lymphoblasts, and confers a worse prognosis. 100 Moreover, CD20-positive leukemia responds poorly to dose intensification, highlighting the need for targeted therapy. The addition of rituximab, a first-generation anti-CD20 monoclonal antibody, has improved outcomes in these patients, but resistance to rituximab represents a limitation to its use.
Ofatumumab is a second-generation anti-CD20 antibody with a distinct binding site from that of rituximab. Ofatumumab was first showed to have benefit in fludarabine-refractory chronic lymphocytic leukemia, irrespective of prior rituximab exposure. 101 Ofatumumab induces higher levels of complement-dependent cytotoxicity (CDC) and has a slower dissociation rate than rituximab, and thus holds promise for CD20+ lymphoid malignancies both as frontline therapy and as salvage for rituximab-refractory disease. 102 , 103 In a phase 2 study, ofatumumab was used in combination with HCVAD in patients with either newly diagnosed pre-B CD20+ ALL or those who had completed a single course of chemotherapy. In all study patients, CD20+ expression was >1%. 104 Ofatumumab was administered at a dose of 2 grams on days 1 and 11 of the first 4 cycles of induction therapy. All but one patient (98%) achieved CR after cycle 1 and 93% of patients were negative for MRD at end induction. The 3-year CR and OS rates were 78% and 68%, respectively. 105 This is similar to benefits seen when rituximab was used as frontline therapy in CD20+ ALL. 106 Ofatumumab represents a potential alternative frontline therapy for CD20+ pre-B-ALL and an option for patients who failed a rituximab-based regimen.
Obinutuzumab
Another novel anti-CD20 monoclonal antibody, obinutuzumab, has shown promise in preclinical trials for CD20-positive B-ALL. Obinutuzumab was engineered to have enhanced affinity for the FcγRIIIa receptor on effector cells and thus enhanced antibody-dependent cell-mediated cytotoxicity (ADCC). 107 This compromises the ability of obinutuzumab to activate complement and predictably, CDC was inferior to that of rituximab and ofatumumab in vitro . However, obinutuzumab induced direct cell death and ADCC more rapidly and effectively. When all three mechanisms of cell death were evaluated together in B-cell depletion assays, obinutuzumab was more effective than either rituximab or ofatumumab achieving higher maximal depletion and lower EC 50 . Furthermore, obinutuzumab was superior in inhibiting growth in NHL xenograft models. 107 Awasthi et al. 108 compared obinutuzumab to ritixumab in pre-B-ALL cell lines and found obinutuzumab to be superior in inducing cell death and ADCC. In a pre-B-ALL xenograft model, overall survival was improved with obinutuzumab compared to ritixumab. 108 In clinical trials, obinutuzumab has been added to chlorambucil for treatment of adults with CLL and shown to prolong progression-free survival and improve complete response rate when compared to rituximab and chlorambucil. 109 Taken together, these results suggest a role for obinutuzumab in CD20+ pre-B-ALL.
REGN1979 is a biallelic monoclonal antibody targeting CD20 and CD3. The theory of REGN1979 is similar to that of blinatumumab, to engage T cells and B-cells thus resulting in activation of T-cell immune response against B-cells. REGN1979 prevented the establishment of lymphoma xenografts and led to complete tumor regression in murine models. 110 In addition, in a primate model, REGN1979 led to a complete and durable depletion of B-cells. When compared to treatment with rituximab, treatment with REGN1979 led to significantly more profound depletion of B-cells. 110 The safety of REGN1979 was established in a phase 1 trial of 25 patients with NHL and CLL. Dose-dependent antitumor activity was observed. The most significant adverse events include cytokine release syndrome (CRS) and hypotension. 111 A phase 2 trial of REGN1979 in relapsed/refractory ALL is currently open for recruitment ( {"type":"clinical-trial","attrs":{"text":"NCT02651662","term_id":"NCT02651662"}} NCT02651662 ).
CD19 is the most widely expressed B-lineage specific antigen, expressed during all stages of differentiation, but lost on maturation to plasma cells. CD19 serves as a co-receptor for the B-cell surface immunoglobulin and its activation triggers a phosphorylation cascade involving src-family kinases and PI3K as well as the activation of c-myc, leading to proliferation and differentiation. 112 , 113 , 114 CD19 is expressed in nearly all B-cell leukemias, and is rapidly internalized upon binding of an antibody, making it an ideal candidate for immunoconjugate therapy. 115
Coltuximab ravtansine (SAR3419)
Coltuximab ravtansine is an anti-CD19 humanized monoclonal antibody conjugated to a semisynthetic maytansinoid compound, an anti-tubulin molecule similar to vincristine. Maytansinoids are more potent than vinca alkaloids, and thus have been of limited use in systemic therapy due to unacceptable toxicity. 116 However, this potency makes them attractive candidates for targeted delivery. In preclinical studies, SAR3419 monotherapy delayed progression in pre-B-ALL xenografts and provided objective response. When used in combination with a chemotherapy regimen that mimicked pediatric induction protocols, SAR3419 was effective at prolonging the duration of remission. 117 SAR3419 was then evaluated in a Phase 1 clinical trial with CD19+ B-cell lymphoma. Dose-limiting toxicities were reversible blurred vision and neuropathy. A maximum tolerated dose (MTD) of 160 mg/m 2 administered once every three weeks was established. Reduction of tumor size was seen in 74% of patients, including 47% of patients with rituximab-resistant disease. 118 An initial phase 2 clinical trial was terminated early due to low response rate of 25%. 119
Denintuzumab mafodotin (SGN-CD19A)
A second anti-CD19 conjugated monoclonal antibody, denintuzumab mafodotin, is currently in development. In this case, the antibody is linked to the microtubule-disrupting agent monomethyl auristatin F (MMAF). In a phase 1 study of patients with relapsed/refractory B-ALL or aggressive B-cell lymphomas, a complete response rate of 35% was observed. 120 Dosing interval of 3 weeks was shown to be superior to weekly dosing. An MTD was identified at 5 mg/kg q3wk. Interestingly, among Ph-positive B-ALL, the response rate was 63%, leading to recruitment of Ph-positive patients for an expansion cohort. These results warrant further evaluation of denintuzumab mafodotin for relapsed/refractory ALL.
ADCT-402 is the newest anti-CD19 monoclonal antibody to enter development. It is a humanized monoclonal antibody conjugated to a pyrrolobenzodiazepine (PBD). PBDs are a class of natural antibiotics derived from actinomycetes bacteria that inhibit cell division by binding in the minor groove of DNA and cross-linking strands of DNA. In vivo studies show superior antitumor activity of ADCT-402 against CD19-positive lymphoma than maytansinoid or auristatin based therapy. 121 A phase 1 trial of ADCT-402 for relapsed/refractory ALL is underway ( {"type":"clinical-trial","attrs":{"text":"NCT02669264","term_id":"NCT02669264"}} NCT02669264 ).
CD25 is a cell surface antigen and component of the Interleukin-2 receptor (IL-2 R) heterotrimer. 122 Binding of IL-2 R by its ligand activates JAK/STAT, MAP kinase and phosphoinositide 3-kinase (PI3K) signaling pathways, leading to cell proliferation. IL-2 R is rapidly recycled upon binding of its ligand. 123 The IL-2 R signaling pathway is particularly activated in T-cell immune response, and has thus been an attractive target for post-transplant immunosuppression. In some studies, CD25 expression has been as high as 30% of pre-B-ALL lymphoblasts, including 100% expression among the Ph-positive subset. 124
ADCT-301 is a monoclonal antibody against CD25 conjugated to a PBD. In preclinical studies, ADCT-301 has been shown to be potently cytotoxic to CD25-positive anaplastic large cell lymphoma and Hodgkin lymphoma cell lines. In vivo , ADCT-301 exhibited antitumor activity in xenograft and disseminated mouse models. 122 A phase 1 trial is recruiting participants for ADCT-301 in relapsed/refractory AML and ALL ( {"type":"clinical-trial","attrs":{"text":"NCT02588092","term_id":"NCT02588092"}} NCT02588092 ).
2-Proteasome inhibitor (Bortezomib)
Bortezomib, a proteasome inhibitor, was first approved for the treatment of multiple myeloma. Preclinical studies have suggested a synergistic role of bortezomib with dexamethasone and additive effects to standard chemotherapy agents in acute leukemias. 125 As a single agent, bortezomib did not produce durable responses in patients with relapsed/refractory ALL, despite demonstrable proteasomal inhibition. 126 However, in a phase 2 study, bortezomib in combination with vincristine, dexamethasone, pegylated asparaginase and doxorubicin produced a response rate of 80% in children with relapsed/refractory pre-B-ALL. 127 In a recent phase 2 COG trial, re-induction chemotherapy plus bortezomib resulted in a complete response in 68% of children with relapsed pre-B-ALL. 128 Due to it’s ability to inhibit the NF-κB and NOTCH1 signaling pathways, bortezomib is being studied as frontline therapy in T-cell ALL. Recruitment is ongoing for a phase 3 trial of standard chemotherapy with or without bortezomib in children and young adults (age 2–30) with newly diagnosed T-cell ALL or T-cell lymphoblastic lymphoma ( {"type":"clinical-trial","attrs":{"text":"NCT02112916","term_id":"NCT02112916"}} NCT02112916 ). In adults, recruitment has begun for a phase 2 trial of bortezomib with combination chemotherapy in relapsed/refractory ALL ( {"type":"clinical-trial","attrs":{"text":"NCT01769209","term_id":"NCT01769209"}} NCT01769209 ).
3-JAK inhibitor (Ruxolitinib)
The JAK/STAT signaling pathway has been identified as a significant mechanism by which leukemic cells bypass normal growth and proliferation restrictions. 13 In particular, Ph-like ALL appears to be dependent on JAK signaling. The most common rearrangements in Ph-like ALL involve the transmembrane receptor CRLF2, which signals through downstream JAK kinases. Many cytokine receptors, including IL-7 R, act through JAK kinases as well. In addition, JAK1 and JAK2 mutations are found in approximately half of CRLF2-rearranged Ph-like ALL. 12 , 13 , 14 Preclinical studies have suggested benefit of ruxolitinib for the treatment of Ph-like ALL and CRLF2-rearranged ALL. 129 , 130 In addition, ruxolitinib inhibited tumor growth in in vitro and in vivo models of T-ALL with a gain of function in IL-7 R-alpha subunit. 131 A phase 2 trial of ruxolitinib with standard multi-agent chemotherapy is currently open for recruitment of children, adolescents and adults with newly diagnosed high-risk B-ALL with CRLF2 rearrangements ( {"type":"clinical-trial","attrs":{"text":"NCT02723994","term_id":"NCT02723994"}} NCT02723994 ).
4-Hypomethylating agent (Decitabine)
DNA methylation is an important epigenetic modification that regulates gene expression. It has long been reported that DNA methylation may play a role in the development of ALL and that methylation status may be used as part of risk stratification. 132 , 133 , 134 , 135 Decitabine is a cytosine analog that inhibits DNA methyltransferase by targeting it for degredation, thus causing hypomethylation of key regulatory domains on DNA. This leads to differentiation and suppression of tumor growth. 136 Decitabine is currently approved for the treatment of myelodysplastic syndrome (MDS). In a case report, a young girl with her third-relapse of ALL was treated with a decitabine and dexamethasone regimen based on MDS dosing. She was able to undergo Allo-SCT after CR was achieved with re-induction therapy and remained in CR 8 months after transplant. 137 In a MD Anderson phase I trial of decitabine for relapsed/refractory ALL, decitabine was shown to have efficacy when used in combination with Hyper-CVAD for re-induction therapy. 138 In addition, decitabine monotherapy is well tolerated and thus offers a potential treatment option for relapsed disease in patients that cannot tolerate multi-agent chemotherapy. In a phase 2 study, decitabine and vorinostat (a histone deacetylase inhibitor) were given prior to vincristine, prednisone, PEG-L-asparaginase and doxorubicin for relapsed/refractory ALL. 139 Results were promising with a CR rate of 50% (95% CI, 15.7–84.3%) and the OR rate 75% (95% CI, 34.9–96.8%). Decitabine has also been studied in preclinical trials of early T-cell precursor ALL (ETP-ALL), where it has been shown to be synergistic to conventional chemotherapy. 140 Decitabine is currently being studied in the post-Allo-SCT setting ( {"type":"clinical-trial","attrs":{"text":"NCT02264873","term_id":"NCT02264873"}} NCT02264873 ) and in combination with clofarabine, idarubicin and cytarabine for relapsed/refractory AML and ALL ( {"type":"clinical-trial","attrs":{"text":"NCT01794702","term_id":"NCT01794702"}} NCT01794702 ).
5-PI3K/mTOR Inhibitors
The phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) and mammalian target of rapamycin (mTOR) pathways are shown to be constitutively activated in 50–75% of T-ALL. 141 Preclinical studies suggest that inhibition of the PI3K/AKT/mTOR pathways may be an effective treatment for T-ALL. 142 , 143 , 144 , 145 A dual PI3K/mTOR inhibitor, NVP-BEZ235, potently inhibited the proliferation ALL cells in vitro , causing G 0 /G 1 arrest. Moreover, inhibition of proliferation was synergistic when NVP-BEZ235 was combined with cytotoxic agents. 144 On the basis of this promising preclinical data, several clinical trials are underway to evaluate the use of mTOR and PI3K inhibitors in combination with multi-agent chemotherapy in the frontline and relapsed/refractory setting ( {"type":"clinical-trial","attrs":{"text":"NCT01756118","term_id":"NCT01756118"}} NCT01756118 , {"type":"clinical-trial","attrs":{"text":"NCT02484430","term_id":"NCT02484430"}} NCT02484430 , {"type":"clinical-trial","attrs":{"text":"NCT01523977","term_id":"NCT01523977"}} NCT01523977 , {"type":"clinical-trial","attrs":{"text":"NCT01403415","term_id":"NCT01403415"}} NCT01403415 , {"type":"clinical-trial","attrs":{"text":"NCT01614197","term_id":"NCT01614197"}} NCT01614197 and {"type":"clinical-trial","attrs":{"text":"NCT01184885","term_id":"NCT01184885"}} NCT01184885 ).
6-Chimeric antigen receptor (CAR) T cells
Chimeric antigen receptor-modified (CAR) T cells are genetically engineered T cells that express the antigen-binding domain of an immunoglobulin linked via transmembrane domains to the intracellular T-cell receptor signaling moieties. 146 This allows the T cells to recognize unprocessed antigens and to be activated in a major histocompatibility complex (MHC)-independent manner. First generation CAR-Ts contain intracellular signaling moieties derived only from the T-cell receptor/CD3 complex. In contrast, second- and third-generation CAR-Ts include co-stimulatory signals in the CAR gene constructs. More recently, fourth-generation CAR-Ts have been engineered to include a cytokine-expressing cassette.
The process of CAR T-cell therapy involves collecting T cells, introducing the CAR construct, and then an autologous transplant of the modified T cells back into the patient. Options for gene delivery methods include viral vectors and RNA-based methods. 147 Using a viral vector has the benefit of inducing permanent gene expression and thus offering antitumor activity for as long as the transduced T cells persist. Theoretic risks of this method include malignant transformation of the engineered T cells if the CAR construct is inserted in such a way that it deregulates the expression of an oncogene. 148 Another method of gene delivery involves direct transfer of an mRNA construct through electroporation. 149 As no DNA is inserted into the genome of the T-cell, this eliminates the risk of malignant transformation. Given the high replicative potential of these T cells, this methods also offers the advantage of a profound antitumor response. 150 However, the effects of direct mRNA insertion are transient and antitumor activity rarely persists beyond 7 days. Preclinical studies have suggested a role for RNA-based methods with multiple infusions; however, all current clinical trials utilize a viral vector to deliver the CAR construct. 150
As mentioned above, CD19 is an ideal target for immunotherapy against B-cell ALL due to its near universal expression on B-lymphoblasts. In a pilot study at the Children’s Hospital of Philadelphia, Grupp et al. 151 treated 53 children with relapsed/refractory ALL with lymphocyte depleting chemotherapy followed by CD19-directed CAR-Ts. A CR was observed in 50 patients (94%), with a 12-month EFS rate of 45% (95% CI, 31–66%) and OS rate of 78% (95% CI, 67–91%). The CAR-Ts were persistent at 6 months in 68% of the patients. Nearly all of the patients developed cytokine release syndrome (CRS). The 15 patients in which CRS was severe were effectively treated with the anti-IL-6-receptor antibody, tocilizumab. 152 Important causes of treatment failure included the loss of circulating CAR-Ts and the expansion of a CD19-negative clone. CAR-Ts have also shown activity in adults with relapsed/refractory B-ALL. Davila et al. 153 treated 16 adults at Memorial Sloan Kettering Cancer Center (MSKCC) with conditioning chemotherapy followed by CD19-directed CAR T-cell infusion. CR was observed in 88% of patients, with a 1–3 month persistence of CAR-Ts. Lee et al. 154 reported a 66.7%% CR rate in a National Cancer Institute (NCI) intent-to-treat analysis of 20 children and young adults with ALL, with a median CAR-T persistence of 68 days. These data suggest a role for CAR-Ts in the treatment of relapsed/refractory ALL as a bridge to Allo-SCT or to produce durable remission. Limitations include the expansion of CD19-negative clones, the lack of long-term persistence of CAR-Ts after a single infusion, and the risk of CRS. Studies are ongoing to identify factors associated with the development of severe CRS and predict patients that would benefit from pretreatment. 155 , 156
Recently, the application of CAR-T cells has been expanded to CD22-positive B-ALL. Early preclinical studies have showed antitumor activity of CD22-directed CAR-Ts in in vitro and in vivo models that approximates that of CD19-directed CAR-Ts. 157 Based on these findings, phase 1 trials using CD22-directed CAR-Ts are in the recruiting stages ( {"type":"clinical-trial","attrs":{"text":"NCT02650414","term_id":"NCT02650414"}} NCT02650414 ). Preliminary results of nine patients have demonstrated that therapy is well tolerated and produced a sustained remission at 3 months in all three patients treated with a dose level of 1 × 10 6 transduced T cells/kg. 158
Hematopoietic stem cell transplantation
After achieving complete response, treatment options include consolidation and maintenance chemotherapy or Allo-SCT for eligible patients. For high-risk patients and patients with relapsed/refractory disease, Allo-SCT has long been considered the standard of care and best chance for a durable response. While criteria differ between studies, in general high-risk disease is defined as Ph-positive ALL, elevated WBC count, CNS disease, high-risk gene rearrangements, or hypodiploidy. The LALA-94 and City of Hope and Stanford University series have shown a benefit of Allo-SCT over standard chemotherapy in these high-risk patients. 49 , 159 , 160 It is therefore recommended that all high-risk young adults with an available donor undergo Allo-SCT during their first CR (CR1). Recent studies have suggested that patients with ETP-ALL and Ph-like ALL be treated as high-risk and be offered Allo-SCT during CR1 as well. 161 , 162 The role of Allo-SCT in standard-risk adults is less clearly defined. In general, MRD has emerged as a prognostic marker that can restratify patients to high-risk, making them candidates for Allo-SCT. Studies 32 found that MRD-positivity is an independent risk factor for decreased relapse-free and overall survival. Subsequently, other studies 163 evaluated the risk factors in patients treated with Allo-SCT versus standard chemotherapy after CR1. In patients with positive MRD, Allo-SCT was associated with improved relapse-free survival. However, in patients with a complete MRD response, there was no survival benefit to Allo-SCT over standard chemotherapy. 163
Allo-SCT also should be considered in all patients that relapse, optimally after achieving a second CR (CR2). The LALA-94 trial showed a 5-year OS of 33% in patients who were able to undergo Allo-SCT during CR2 compared to 8% in patients who underwent Allo-SCT during active relapse. 164 Patients who are unable to achieve CR2 by conventional methods should be considered for clinical trials with novel agents as a bridge to Allo-SCT. In the MRC/ECOG 2993 study, 5-year survival was highest in the group receiving a sibling donor Allo-SCT compared to unmatched donor or chemotherapy alone (23%, 16% and 4%, respectively). 165
Acute lymphoblastic leukemia has been touted as a major success story in pediatric oncology through the implementation of dose-intensification chemotherapy and Allo-SCT. However, due to high-risk disease characteristics and significant toxicity associated with chemotherapy in adults, outcomes are far less encouraging. There remains much uncertainty about how best to treat adults with ALL, as some studies have shown benefit of pediatric-inspired regimens. However, not all adults are able to tolerate such dose intensification and the exact subset of patients who are likely to benefit has not clearly been defined. Furthermore, elderly patients are particularly susceptible to the dose-limiting toxicities of these agents and are often excluded from Allo-SCT on the basis of performance status and medical comorbidities. Novel targeted therapies offer the promise of effective anti-leukemic activity with reduced toxicity from off-target effects. Given the diverse molecular and genetic alterations occurring in ALL, it is unlikely that a single agent will be effective for all patients with ALL. However, with the ability to characterize the immunophenotype and genotype of each patient’s leukemia, targeted therapy can be expected to lead to improvements in remission and survival as part of individualized treatment strategies. The successes from tyrosine kinase inhibition in CML have been translated to Ph-positive ALL, and second and third generation TKIs are being studied for use in high-risk Ph-like disease. Other signaling pathways, such as PI3K/AKT/mTOR pathway, are also promising targets for small molecule inhibition. In addition to targeting intracellular pathways, monoclonal antibodies recognize cell surface antigens. Immunoconjugates, such as inotuzumab ozogamicin, bind to leukemic cells, are internalized and release a cytotoxin that kills the leukemic cell; whereas dual-specific antibodies, such as blinatumumab, cause the direct activation of T cells against blasts. CAR-Ts involve a similar mechanism, in which a patient’s own T cells are genetically programmed to recognize leukemic cells, inducing an anti-leukemic immune response. Finally, existing agents, such as bortezomib, decitabine and ruxolitinib that are well tolerated in the treatment of various malignancies are now being studied for application in ALL. As the role of these novel agents is further defined and integrated into new treatment strategies, adult ALL may follow pediatric ALL as a major success story in the near future.
The authors declare no conflict of interest.
- National Cancer Institute. SEER cancer statistics review, 1975-2013:Leukemia, annual incidence rates (acute lymphocytic leukemia).
- Paul S, Kantarjian H, Jabbour EJ. Adult Acute Lymphoblastic Leukemia . Mayo Clin Proc 2016; 91 : 1645–1666. [ PubMed ] [ Google Scholar ]
- Jabbour E, O'Brien S, Konopleva M, Kantarjian H. New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia . Cancer 2015; 121 : 2517–2528. [ PubMed ] [ Google Scholar ]
- Shah A, John BM, Sondhi V. Acute lymphoblastic leukemia with treatment—naive Fanconi anemia . Indian Pediatr 2013; 50 : 508–510. [ PubMed ] [ Google Scholar ]
- German J. Bloom's syndrome. XX. The first 100 cancers . Cancer Genet Cytogenet 1997; 93 : 100–106. [ PubMed ] [ Google Scholar ]
- Bielorai B, Fisher T, Waldman D, Lerenthal Y, Nissenkorn A, Tohami T et al. Acute lymphoblastic leukemia in early childhood as the presenting sign of ataxia-telangiectasia variant . Pediatr Hematol Oncol 2013; 30 : 574–582. [ PubMed ] [ Google Scholar ]
- Chessells J, Harrison G, Richards S, Bailey C, Hill F, Gibson B et al. Down's syndrome and acute lymphoblastic leukaemia: clinical features and response to treatment . Arch Dis Child 2001; 85 : 321–325. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Spector LG, R J, Robison LL, Bhatia S. Epidemiology and Etiology Childhood Leukemias, 2nd edition. Cambridge University Press, pp 48–66.
- Sehgal S, Mujtaba S, Gupta D, Aggarwal R, Marwaha RK. High incidence of Epstein Barr virus infection in childhood acute lymphocytic leukemia: a preliminary study . Indian J Pathol Microbiol 2010; 53 : 63–67. [ PubMed ] [ Google Scholar ]
- Geriniere L, Bastion Y, Dumontet C, Salles G, Espinouse D, Coiffier B. Heterogeneity of acute lymphoblastic leukemia in HIV-seropositive patients . Ann Oncol 1994; 5 : 437–440. [ PubMed ] [ Google Scholar ]
- Mullighan CG, Collins-Underwood JR, Phillips LA, Loudin ML, Liu W, Zhang J et al. Rearrangement of CRLF2 in B-progenitor and down syndrome associated acute lymphoblastic leukemia . Nat Genet 2009; 41 : 1243–1246. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia . Nature 2007; 446 : 758–764. [ PubMed ] [ Google Scholar ]
- Roberts KG, Morin RD, Zhang J, Hirst M, Zhao Y, Su X et al. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia . Cancer Cell 2012; 22 : 153–166. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Roberts KG, Li Y, Payne-Turner D, Harvey RC, Yang YL, Pei D et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia . N Engl J Med 2014; 371 : 1005–1015. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Holmfeldt L, Wei L, Diaz-Flores E, Walsh M, Zhang J, Ding L et al. The genomic landscape of hypodiploid acute lymphoblastic leukemia . Nat Genet 2013; 45 : 242–252. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Alvarnas JC, Brown PA, Aoun P, Ballen KK, Barta SK, Borate U et al. Acute lymphoid leukemia (version 2.2015) . Natl Comprehens Cancer Netw 2015; 13 : 1240–1279. [ PubMed ] [ Google Scholar ]
- Jabbour EJ, Faderl S, Kantarjian HM. Adult acute lymphoblastic leukemia . Mayo Clin Proc 2005; 80 : 1517–1527. [ PubMed ] [ Google Scholar ]
- Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group . Br J Haematol 1976; 33 : 451–458. [ PubMed ] [ Google Scholar ]
- Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997 . J Clin Oncol 1999; 17 : 3835–3849. [ PubMed ] [ Google Scholar ]
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes . Blood 2009; 114 : 937–951. [ PubMed ] [ Google Scholar ]
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia . Blood 2016; 127 : 2391–2405. [ PubMed ] [ Google Scholar ]
- Rowe JM. Prognostic factors in adult acute lymphoblastic leukaemia . Br J Haematol 2010; 150 : 389–405. [ PubMed ] [ Google Scholar ]
- Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993 . Blood 2005; 106 : 3760–3767. [ PubMed ] [ Google Scholar ]
- Faderl, S HM Kantarjian, et al, T.U.o.T.M.D.A.C.C. Department of Leukemia, Houston, Texas, T.U.o.T.M.D.A.C.C. Department of Leukemia, P.O. Box 428, 1515 Holcombe Blvd., Houston, TX 77030, S. Jeha, T.U.o.T.M.D.A.C.C. Department of Leukemia, Houston, Texas, The biology and therapy of adult acute lymphoblastic leukemia. Cancer, 2017; 98: 1337–1354. [ PubMed ]
- Moorman AV, Harrison CJ, Buck GA, Richards SM, Secker-Walker LM, Martineau M et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial . Blood 2007; 109 : 3189–3197. [ PubMed ] [ Google Scholar ]
- Pullarkat V, Slovak ML, Kopecky KJ, Forman SJ, Appelbaum FR. Impact of cytogenetics on the outcome of adult acute lymphoblastic leukemia: results of Southwest Oncology Group 9400 study . Blood 2008; 111 : 2563–2572. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hunger SP, Mullighan CG. Redefining ALL classification: toward detecting high-risk ALL and implementing precision medicine . Blood 2015; 125 : 3977–3987. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dongen JJMv, v.d. Velden VHJ, Brüggemann M, Orfao A. Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies . Blood 2015; 125 : 3996–4009. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bruggemann M, Raff T, Flohr T, Gokbuget N, Nakao M, Droese J et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia . Blood 2006; 107 : 1116–1123. [ PubMed ] [ Google Scholar ]
- Jacquy C, Delepaut B, Van Daele S, Vaerman JL, Zenebergh A, Brichard B et al. A prospective study of minimal residual disease in childhood B-lineage acute lymphoblastic leukaemia: MRD level at the end of induction is a strong predictive factor of relapse . Br J Haematol 1997; 98 : 140–146. [ PubMed ] [ Google Scholar ]
- Sutton R, Venn NC, Tolisano J, Bahar AY, Giles JE, Ashton LJ et al. Clinical significance of minimal residual disease at day 15 and at the end of therapy in childhood acute lymphoblastic leukaemia . Br J Haematol 2009; 146 : 292–299. [ PubMed ] [ Google Scholar ]
- Bassan R, Spinelli O, Oldani E, Intermesoli T, Tosi M, Peruta B et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL) . Blood 2009; 113 : 4153–4162. [ PubMed ] [ Google Scholar ]
- Campana D. Minimal residual disease in acute lymphoblastic leukemia . Semin Hematol 2009; 46 : 100–106. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Raff T, Gökbuget N, Lüschen S, Reutzel R, Ritgen M, Irmer S et al. Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: data from the GMALL 06/99 and 07/03 trials . Blood 2007; 109 : 910–915. [ PubMed ] [ Google Scholar ]
- Ribera JM, Oriol A, Morgades M, Montesinos P, Sarra J, Gonzalez-Campos J et al. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial . J Clin Oncol 2014; 32 : 1595–1604. [ PubMed ] [ Google Scholar ]
- Huguet F, Leguay T, Raffoux E, Thomas X, Beldjord K, Delabesse E et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study . J Clin Oncol 2009; 27 : 911–918. [ PubMed ] [ Google Scholar ]
- Stock W, La M, Sanford B, Bloomfield CD, Vardiman JW, Gaynon P et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies . Blood 2008; 112 : 1646–1654. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Scavino HF, George JN, Sears DA. Remission induction in adult acute lymphocytic leukemia. Use of vincristine and prednisone alone . Cancer 1976; 38 : 672–677. [ PubMed ] [ Google Scholar ]
- Gottlieb A, Weinberg V, Ellison R, Henderson E, Terebelo H, Rafla S et al. Efficacy of daunorubicin in the therapy of adult acute lymphocytic leukemia: a prospective randomized trial by cancer and leukemia group B . Blood 1984; 64 : 267–274. [ PubMed ] [ Google Scholar ]
- Larson RA, Dodge RK, Burns CP, Lee EJ, Stone RM, Schulman P et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: cancer and leukemia group B study 8811 . Blood 1995; 85 : 2025–2037. [ PubMed ] [ Google Scholar ]
- Nagura E, Kimura K, Yamada K, Ota K, Maekawa T, Takaku F et al. Nation-wide randomized comparative study of doxorubicin, vincristine and prednisolone combination therapy with and without L-asparaginase for adult acute lymphoblastic leukemia . Cancer Chemother Pharmacol 1994; 33 : 359–365. [ PubMed ] [ Google Scholar ]
- Short NJ, Jabbour E, Sasaki K, Patel K, O'Brien SM, Cortes JE et al. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia . Blood 2016; 128 : 504–507. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Patel B, Kirkwood A, Dey A, Rowntree C, McMillan A, Marks D et al. Feasibility Of pegylated-asparaginase (PEG-ASP) during induction in adults with acute lymphoblastic leukaemia (ALL): results from the UK Phase 3 Multicentre Trial UKALL 14. 2013.
- Kantarjian HM, O'Brien S, Smith TL, Cortes J, Giles FJ, Beran M et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia . J Clin Oncol 2000; 18 : 547–561. [ PubMed ] [ Google Scholar ]
- Hurwitz CA, Silverman LB, Schorin MA, Clavell LA, Dalton VK, Glick KM et al. Substituting dexamethasone for prednisone complicates remission induction in children with acute lymphoblastic leukemia . Cancer 2000; 88 : 1964–1969. [ PubMed ] [ Google Scholar ]
- Jones B, Freeman AI, Shuster JJ, Jacquillat C, Weil M, Pochedly C et al. Lower incidence of meningeal leukemia when prednisone is replaced by dexamethasone in the treatment of acute lymphocytic leukemia . Med Pediatr Oncol 1991; 19 : 269–275. [ PubMed ] [ Google Scholar ]
- Narayanan S, Shami PJ. Treatment of acute lymphoblastic leukemia in adults . Crit Rev Oncol Hematol 2012; 81 : 94–102. [ PubMed ] [ Google Scholar ]
- Faderl S, Kantarjian HM, Thomas DA, Cortes J, Giles F, Pierce S et al. Outcome of Philadelphia chromosome-positive adult acute lymphoblastic leukemia . Leuk Lymphoma 2000; 36 : 263–273. [ PubMed ] [ Google Scholar ]
- Dombret H, Gabert J, Boiron JM, Rigal-Huguet F, Blaise D, Thomas X et al. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia—results of the prospective multicenter LALA-94 trial . Blood 2002; 100 : 2357–2366. [ PubMed ] [ Google Scholar ]
- Laport GG, Alvarnas JC, Palmer JM, Snyder DS, Slovak ML, Cherry AM et al. Long-term remission of Philadelphia chromosome-positive acute lymphoblastic leukemia after allogeneic hematopoietic cell transplantation from matched sibling donors: a 20-year experience with the fractionated total body irradiation-etoposide regimen . Blood 2008; 112 : 903–909. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fielding AK, Rowe JM, Richards SM, Buck G, Moorman AV, Durrant IJ et al. Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993 . Blood 2009; 113 : 4489–4496. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Thomas DA, Faderl S, Ravandi Kashani F, Wierda WG, Andreeff M, Garris RS et al. Long-term outcome after hyper-CVAD and imatinib (IM) for de novo or minimally treated Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph-ALL)[abstract]. 2010; 28(15 s):abstr 8506:[Available from http://meetinglibrary.asco.org/content/52502-74.
- Thomas DA, Faderl S, Cortes J, O'Brien S, Giles FJ, Kornblau SM et al. Treatment of Philadelphia chromosome–positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate . Blood 2004; 103 : 4396–4407. [ PubMed ] [ Google Scholar ]
- Jones D, Thomas D, Yin CC, O'Brien S, Cortes JE, Jabbour E et al. Kinase domain point mutations in Philadelphia chromosome-positive acute lymphoblastic leukemia emerge after therapy with BCR-ABL kinase inhibitors . Cancer 2008; 113 : 985–994. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Porkka K, Koskenvesa P, Lundan T, Rimpilainen J, Mustjoki S, Smykla R et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia . Blood 2008; 112 : 1005–1012. [ PubMed ] [ Google Scholar ]
- Ravandi F, O'Brien SM, Cortes JE, Thomas DM, Garris R, Faderl S et al. Long-term follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia . Cancer 2015; 121 : 4158–4164. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ravandi F, Othus M, O'Brien S, Forman SJ, Ha CS, Wong JYC et al. Multi-Center US Intergroup Study of Intensive Chemotherapy Plus Dasatinib Followed By Allogeneic Stem Cell Transplant in Patients with Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia Younger Than 60 . 2015.
- Ottmann O, Dombret H, Martinelli G, Simonsson B, Guilhot F, Larson RA et al. Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study . Blood 2007; 110 : 2309–2315. [ PubMed ] [ Google Scholar ]
- Foà R, Vitale A, Vignetti M, Meloni G, Guarini A, Propris MSD et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia . Blood 2011; 118 : 6521–6528. [ PubMed ] [ Google Scholar ]
- Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias . N Engl J Med 2013; 369 : 1783–1796. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sasaki K, Jabbour EJ, Ravandi F, Short NJ, Thomas DA, Garcia-Manero G et al. Hyper-CVAD plus ponatinib versus hyper-CVAD plus dasatinib as frontline therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: A propensity score analysis . Cancer 2016; 122 : 3650–3656. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sasaki Koji, Ravandi Farhad, Thomas Deborah A, Cortes Jorge E, Pemmaraju Naveen, Kadia Tapan M et al. Updated results from phase II study of combination of hyper-CVAD (HCVAD) with ponatinib in frontline therapy of patients (pts) with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL)[abstract]. 2016; 34 s; abstr 7036: Available from http://meetinglibrary.asco.org/content/167324-176.
- Nachman J, Siebel N, Sather H, Steinherz P, DeLaat C, Fryer D et al. Outcome for Adolescent and Young Adults 16–21 years of age (AYA) with Acute Lymphoblastic Leukemia (ALL) Treated on the Children’ s Cancer Group (CCG) 1961 Study . 2004.
- Stock W, Luger SM, Advani AS, Geyer S, Harvey RC, Mullighan CG et al. Favorable Outcomes for Older Adolescents and Young Adults (AYA) with Acute Lymphoblastic Leukemia (ALL): Early Results of US Intergroup Trial C10403 , 2014.
- Rytting ME, Jabbour EJ, Jorgensen JL, Ravandi F, Franklin AR, Kadia TM et al. Final results of a single institution experience with a pediatric-based regimen, the augmented Berlin-Frankfurt-Munster, in adolescents and young adults with acute lymphoblastic leukemia, and comparison to the hyper-CVAD regimen . Am J Hematol 2016; 91 : 819–823. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Faderl S, Thomas DA, O'Brien S, Ravandi F, Garcia-Manero G, Borthakur G et al. Augmented hyper-CVAD based on dose-intensified vincristine, dexamethasone, and asparaginase in adult acute lymphoblastic leukemia salvage therapy . Clin Lymphoma Myeloma Leuk 2011; 11 : 54–59. [ PubMed ] [ Google Scholar ]
- O’Brien S, Thomas D, Ravandi F, Faderl S, Cortes J, Borthakur G et al. Outcome of Adults With Acute Lymphocytic Leukemia After Second Salvage Therapy . Cancer 2008; 113 : 3186–3191. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Deitcher OR, O'Brien S, Deitcher SR, Thomas DA, Kantarjian HM. Single-Agent Vincristine Sulfate Liposomes Injection (Marqibo) Compared to Historical Single-Agent Therapy for Adults with Advanced, Relapsed and/or Refractory Philadelphia Chromosome Negative Acute Lymphoblastic Leukemia , 2011.
- O'Brien S, Schiller G, Lister J, Damon L, Goldberg S, Aulitzky W et al. High-Dose Vincristine Sulfate Liposome Injection for Advanced, Relapsed, and Refractory Adult Philadelphia Chromosome–Negative Acute Lymphoblastic Leukemia . in J Clin Oncol 2013; 31 : 676–683. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nagorsen D, Kufer P, Baeuerle PA, Bargou R. Blinatumomab: a historical perspective . Pharmacol Ther 2012; 136 : 334–342. [ PubMed ] [ Google Scholar ]
- Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival . J Clin Oncol 2011; 29 : 2493–2498. [ PubMed ] [ Google Scholar ]
- Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C et al. Long-Term Outcomes after Blinatumomab Treatment: Follow-up of a Phase 2 Study in Patients (Pts) with Minimal Residual Disease (MRD) Positive B-Cell Precursor Acute Lymphoblastic Leukemia (ALL) . Blood 2015; 126 : 680–680. [ Google Scholar ]
- Topp MS, Gokbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study . Lancet Oncol 2015; 16 : 57–66. [ PubMed ] [ Google Scholar ]
- Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera J-M et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia . NEJM 2017; 376 : 836–847. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Martinelli G, Dombret H, Chevallier P, Ottmann OG, Goekbuget N, Topp MS et al. Complete Molecular and Hematologic Response in Adult Patients with Relapsed/Refractory (R/R) Philadelphia Chromosome-Positive B-Precursor Acute Lymphoblastic Leukemia (ALL) Following Treatment with Blinatumomab: Results from a Phase 2 Single-Arm, Multicenter Study (ALCANTARA)[Abstract] . Blood 2015; 126 : 679. [ Google Scholar ]
- D-ALBA Frontline Sequential Dasatinib and Blinatumomab in Adult Philadelphia Positive Acute Lymphoblastic Leukemia - Full Text View - ClinicalTrials.gov. 2017; Available at: https://clinicaltrials.gov/ct2/show/NCT02744768.
- DeAngelo DJ, Yu D, Johnson JL, Coutre SE, Stone RM, Stopeck AT et al. Nelarabine induces complete remissions in adults with relapsed or refractory T-lineage acute lymphoblastic leukemia or lymphoblastic lymphoma: Cancer and Leukemia Group B study 19801 . Blood 2007; 109 : 5136–5142. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shah NN, Stevenson MS, Yuan CM, Richards K, Delbrook C, Kreitman RJ et al. Characterization of CD22 expression in acute lymphoblastic leukemia . Pediatr Blood Cancer 2015; 62 : 964–969. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Piccaluga PP, Arpinati M, Candoni A, Laterza C, Paolini S, Gazzola A et al. Surface antigens analysis reveals significant expression of candidate targets for immunotherapy in adult acute lymphoid leukemia . Leuk Lymphoma 2011; 52 : 325–327. [ PubMed ] [ Google Scholar ]
- Chevallier P, Robillard N, Houille G, Ayari S, Guillaume T, Delaunay J et al. Simultaneous study of five candidate target antigens (CD20, CD22, CD33, CD52, HER2) for antibody-based immunotherapy in B-ALL: a monocentric study of 44 cases in Leukemia 2009; England pp 806–807. [ PubMed ]
- Carnahan J, Wang P, Kendall R, Chen C, Hu S, Boone T et al. Epratuzumab, a humanized monoclonal antibody targeting CD22: characterization of in vitro properties . Clin Cancer Res 2003; 9 (10 Pt 2): 3982s–3990ss. [ PubMed ] [ Google Scholar ]
- Raetz EA, Cairo MS, Borowitz MJ, Blaney SM, Krailo MD, Leil TA et al. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children's Oncology Group Pilot Study . J Clin Oncol 2008; 26 : 3756–3762. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Advani AS, McDonough S, Coutre S, Wood B, Radich J, Mims M et al. SWOG S0910: a phase 2 trial of clofarabine/cytarabine/epratuzumab for relapsed/refractory acute lymphocytic leukaemia . Br J Haematol 2014; 165 : 504–509. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sharkey RM, Govindan SV, Cardillo TM, Goldenberg DM. Epratuzumab–SN-38: A New Antibody–Drug Conjugate for the Therapy of Hematologic Malignancies . Mol Cancer Ther 2012; 11 : 224–234. [ PubMed ] [ Google Scholar ]
- Hinman LM, Hamann PR, Wallace R, Menendez AT, Durr FE, Upeslacis J. Preparation and characterization of monoclonal antibody conjugates of the calicheamicins: a novel and potent family of antitumor antibiotics . Cancer Res 1993; 53 : 3336–3342. [ PubMed ] [ Google Scholar ]
- DiJoseph JF, Armellino DC, Boghaert ER, Khandke K, Dougher MM, Sridharan L et al. Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies . Blood 2004; 103 : 1807–1814. [ PubMed ] [ Google Scholar ]
- Advani A, Coiffier B, Czuczman MS, Dreyling M, Foran J, Gine E et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin's lymphoma: results of a phase I study . J Clin Oncol 2010; 28 : 2085–2093. [ PubMed ] [ Google Scholar ]
- Kantarjian H, Thomas D, Jorgensen J, Jabbour E, Kebriaei P, Rytting M et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study . Lancet Oncol 2012; 13 : 403–411. [ PubMed ] [ Google Scholar ]
- Kantarjian H, Thomas D, Jorgensen J, Kebriaei P, Jabbour E, Rytting M et al. Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia . Cancer 2013; 119 : 2728–2736. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Advani AS, Stein AS, Kantarjian HM, Shustov AR, DeAngelo DJ, Ananthakrishnan R et al. A Phase II Study of Weekly Inotuzumab Ozogamicin (InO) in Adult Patients with CD22-Positive Acute Lymphoblastic Leukemia (ALL) in Second or Later Salvage . Blood 2014; 124 : 2255. [ Google Scholar ]
- Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia . N Engl J Med 2016; 375 : 740–753. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jabbour E, O’Brien S, Thomas D, Sasaki K, Garcia-Manero G, Ravandi F et al. Inotuzumab Ozogamicin (IO) in Combination with Low-Intensity Chemotherapy (mini-hyper-CVD) as Frontline Therapy for Older Patients (pts) and as Salvage Therapy for Adult with Relapsed/Refractory (R/R) Acute Lymphoblastic Leukemia (ALL) . Clin Lymphoma Myeloma Leuk 2015, 15.
- Kreitman RJ, Pastan I. Antibody Fusion Proteins: Anti-CD22 Recombinant Immunotoxin Moxetumomab Pasudotox . Clin Cancer Res 2011; 17 : 6398–6405. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kreitman RJ, Stetler-Stevenson M, Margulies I, Noel P, FitzGerald DJP, Wilson WH et al. Phase II Trial of Recombinant Immunotoxin RFB4(dsFv)-PE38 (BL22) in Patients With Hairy Cell Leukemia . J Clin Oncol 2009; 27 : 2983–2990. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wayne AS, Kreitman RJ, Findley HW, Lew G, Delbrook C, Steinberg SM et al. Anti-CD22 Immunotoxin RFB4(dsFv)-PE38 (BL22) for CD22-Positive Hematologic Malignancies of Childhood: Preclinical Studies and Phase I Clinical Trial . Clin Cancer Res 2010; 16 : 1894–1903. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wayne AS, Bhojwani D, Silverman LB, Richards K, Stetler-Stevenson M, Shah NN et al. A Novel Anti-CD22 Immunotoxin, Moxetumomab Pasudotox: Phase I Study in Pediatric Acute Lymphoblastic Leukemia (ALL) . Blood 2011; 118 : 248. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ravandi F, Kantarjian HM, Goswami T, Wang F, Ibrahim R. Design Of a Phase 1/2 Study Of Moxetumomab Pasudotox In Adult Patients With Relapsed and/Or Refractory Acute Lymphoblastic Leukemia (ALL) . Blood 2013; 122 : 5021. [ Google Scholar ]
- Herrera L, Bostrom B, Gore L, Sandler E, Lew G, Schlegel PG et al. A phase 1 study of Combotox in pediatric patients with refractory B-lineage acute lymphoblastic leukemia . J Pediatr Hematol Oncol 2009; 31 : 936–941. [ PubMed ] [ Google Scholar ]
- Schindler J, Gajavelli S, Ravandi F, Shen Y, Parekh S, Braunchweig I et al. A Phase I Study of a Combination of anti-CD19 and anti-CD22 Immunotoxins (Combotox) in Adult Patients with Refractory B-Lineage Acute Lymphoblastic Leukaemia . Br J Haematol 2011; 154 : 471–476. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Thomas DA, O'Brien S, Jorgensen JL, Cortes J, Faderl S, Garcia-Manero G et al. Prognostic significance of CD20 expression in adults with de novo precursor B-lineage acute lymphoblastic leukemia . Blood 13 : 6330–6337. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wierda WG, Padmanabhan S, Chan GW, Gupta IV, Lisby S, Österborg A et al. Ofatumumab is active in patients with fludarabine-refractory CLL irrespective of prior rituximab: results from the phase 2 international study . Blood 2011; 118 : 5126–5129. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Teeling JL, French RR, Cragg MS, v.d. Brakel J, Pluyter M, Huang H et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas . Blood 2004; 104 : 1793–1800. [ PubMed ] [ Google Scholar ]
- Pawluczkowycz AW, Beurskens FJ, Beum PV, Lindorfer MA, v.d. Winkel JGJ, Parren PWHI et al. Binding of Submaximal C1q Promotes Complement-Dependent Cytotoxicity (CDC) of B Cells Opsonized with Anti-CD20 mAbs Ofatumumab (OFA) or Rituximab (RTX): Considerably Higher Levels of CDC Are Induced by OFA than by RTX . J Immunol 2009; 183 : 749–758. [ PubMed ] [ Google Scholar ]
- Jabbour E, Hagop K, Thomas D, Garcia-Manero G, Hoehn D, Garris R et al. Phase II Study Of The Hyper-CVAD Regimen In Combination With Ofatumumab As Frontline Therapy For Adults With CD-20 Positive Acute Lymphoblastic Leukemia (ALL) . Blood 2013; 122 : 2664. [ Google Scholar ]
- Sasaki K, Kantarjian HM, Ravandi F, Daver N, Kadia TM, Khouri RB et al. Frontline Ofatumumab in Combination with Hyper-CVAD for Adult Patients with CD-20 Positive Acute Lymphoblastic Leukemia (ALL): Interim Result of a Phase II Clinical Trial . Poster presented at the American Society of Hematology 58th Annual Meeting and Exposition . 2 December 2016, San Diego, CA, USA, 2016.
- Thomas DA, O'Brien S, Faderl S, Garcia-Manero G, Ferrajoli A, Wierda W et al. Chemoimmunotherapy With a Modified Hyper-CVAD and Rituximab Regimen Improves Outcome in De Novo Philadelphia Chromosome–Negative Precursor B-Lineage Acute Lymphoblastic Leukemia . J Clin Oncol 2010; 28 : 3880–3889. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Herter S, Herting F, Mundigl O, Waldhauer I, Weinzierl T, Fauti T et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models . Mol Cancer Ther 2013; 12 : 2031–2042. [ PubMed ] [ Google Scholar ]
- Awasthi A, Ayello J, Van de Ven C, Elmacken M, Sabulski A, Barth MJ et al. Obinutuzumab (GA101) compared to rituximab significantly enhances cell death and antibody-dependent cytotoxicity and improves overall survival against CD20(+) rituximab-sensitive/-resistant Burkitt lymphoma (BL) and precursor B-acute lymphoblastic leukaemia (pre-B-ALL): potential targeted therapy in patients with poor risk CD20(+) BL and pre-B-ALL . Br J Haematol 2015; 171 : 763–775. [ PubMed ] [ Google Scholar ]
- Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM et al. Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions . N Engl J Med 2014; 370 : 1101–1110. [ PubMed ] [ Google Scholar ]
- Smith EJ, Olson K, Haber LJ, Varghese B, Duramad P, Tustian AD et al. A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys . Scientific Reports, Published online 2015; 5 : 17943, 2015. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bannerji R, Brown JR, Advani RH, Arnason J, O'Brien SM, Allan JN et al. Phase 1 Study of REGN1979, an Anti-CD20 x Anti-CD3 Bispecific Monoclonal Antibody, in Patients with CD20+ B-Cell Malignancies Previously Treated with CD20-Directed Antibody Therapy . Blood 2016; 128 : 621. [ Google Scholar ]
- Fujimoto M, Poe JC, Jansen PJ, Sato S, Tedder TF. CD19 amplifies B lymphocyte signal transduction by regulating Src-family protein tyrosine kinase activation . J Immunol 1999; 162 : 7088–7094. [ PubMed ] [ Google Scholar ]
- Otero DC, Omori SA, Rickert RC. CD19-dependent Activation of Akt Kinase in B-lymphocytes . J Biol Chem 2001; 276 : 1474–1478. [ PubMed ] [ Google Scholar ]
- Chung EY, Psathas JN, Yu D, Li Y, Weiss MJ, Thomas-Tikhonenko A. CD19 is a major B cell receptor-independent activator of MYC-driven B-lymphomagenesis . J Clin Invest 2012; 122 : 2257–2266. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ning BT, Tang YM, Chen YH, Shen HQ, Qian BQ. Comparison between CD19 and CD20 expression patterns on acute leukemic cells . Zhongguo Shi Yan Xue Ye Xue Za Zhi 2005; 13 : 943–947. [ PubMed ] [ Google Scholar ]
- Widdison Wayne C, Wilhelm Sharon D, Cavanagh Emily E, Whiteman Kathleen R, Leece Barbara A, Kovtun Yelena et al. Semisynthetic Maytansine Analogues for the Targeted Treatment of Cancer . J Med Chem 2006; 49 : 4392–4408. [ PubMed ] [ Google Scholar ]
- Carol H, Szymanska B, Evans K, Boehm I, Houghton PJ, Smith MA et al. The Anti-CD19 Antibody–Drug Conjugate SAR3419 Prevents Hematolymphoid Relapse Postinduction Therapy in Preclinical Models of Pediatric Acute Lymphoblastic Leukemia . Clin Cancer Res 2013; 19 : 1795–1805. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Younes A, Kim S, Romaguera J, Copeland A, Farial Sdc, Kwak LW et al. Phase I Multidose-Escalation Study of the Anti-CD19 Maytansinoid Immunoconjugate SAR3419 Administered by Intravenous Infusion Every 3 Weeks to Patients With Relapsed/Refractory B-Cell Lymphoma . Blood 2009; 114 : 585. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kantarjian HM, Lioure B, Kim SK, Atallah E, Leguay T, Kelly K et al. A Phase II Study of Coltuximab Ravtansine (SAR3419) Monotherapy in Patients With Relapsed or Refractory Acute Lymphoblastic Leukemia . Clin Lymphoma Myeloma Leuk 2016; 16 : 139–145. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fathi AT, Borate U, DeAngelo DJ, O'Brien MM, Trippett T, Shah BD et al. A Phase 1 Study of Denintuzumab Mafodotin (SGN-CD19A) in Adults with Relapsed or Refractory B-Lineage Acute Leukemia (B-ALL) and Highly Aggressive Lymphoma . Blood 2015; 126 : 1328. [ Google Scholar ]
- Zammarchi F, Williams DG, Adams L, Havenith K, Chivers S, D'Hooge F et al. Pre-Clinical Development of Adct-402, a Novel Pyrrolobenzodiazepine (PBD)-Based Antibody Drug Conjugate (ADC) Targeting CD19-Expressing B-Cell Malignancies . Blood 2015; 126 : 1564. [ Google Scholar ]
- Flynn MJ, Pv Berkel, Zammarchi F, Levy J-N, Tiberghien A, Masterson LA et al. Pre-Clinical Activity of Adct-301, a Novel Pyrrolobenzodiazepine (PBD) Dimer-Containing Antibody Drug Conjugate (ADC) Targeting CD25-Expressing Hematological Malignancies . Blood 2014; 124 : 4491. [ PubMed ] [ Google Scholar ]
- Flynn MJ, Berkel PHv, Zammarchi F, Tyrer PC, Akarca AU, Janghra N et al. Pre-Clinical Activity of Adct-301, a Novel Pyrrolobenzodiazepine (PBD) Dimer-Containing Antibody Drug Conjugate (ADC) Targeting CD25-Expressing Hematological Malignancies . Blood 2014; 124 : 4491. [ PubMed ] [ Google Scholar ]
- Owaidah TM, Rawas FI, Al Khayatt MF, Elkum NB. Expression of CD66c and CD25 in acute lymphoblastic leukemia as a predictor of the presence of BCR/ABL rearrangement . Hematol Oncol Stem Cell Ther 2008; 1 : 34–37. [ PubMed ] [ Google Scholar ]
- Horton TM, Gannavarapu A, Blaney SM, D'Argenio DZ, Plon SE, Berg SL. Bortezomib interactions with chemotherapy agents in acute leukemia in vitro . Cancer Chemother Pharmacol 2006; 58 : 13–23. [ PubMed ] [ Google Scholar ]
- Cortes J, Thomas D, Koller C, Giles F, Estey E, Faderl S et al. Phase I Study of Bortezomib in Refractory or Relapsed Acute Leukemias . Clin Cancer Res 2004; 10 : 3371–3376. [ PubMed ] [ Google Scholar ]
- Messinger YH, Gaynon PS, Sposto R, v.d. Giessen J, Eckroth E, Malvar J et al. Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Study . Blood 2012; 120 : 285–290. [ PubMed ] [ Google Scholar ]
- Horton Terzah M, O'Brien Maureen Megan, Borowitz Michael J, Devidas Meenakshi, Raetz Elizabeth A, Brown Patrick Andrew et al. Bortezomib reinduction therapy to improve response rates in pediatric ALL in first relapse: A Childrenâ?'s Oncology Group (COG) study (AALL07P1) [Abstract]. 2013; Available at: http://meetinglibrary.asco.org/content/111816-132.
- Maude SL, Tasian SK, Vincent T, Hall JW, Sheen C, Roberts KG et al. Targeting JAK1/2 and mTOR in murine xenograft models of Ph-like acute lymphoblastic leukemia . Blood 2012; 120 : 3510–3518. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tasian SK, Doral MY, Borowitz MJ, Wood BL, Chen IM, Harvey RC et al. Aberrant STAT5 and PI3K/mTOR pathway signaling occurs in human CRLF2-rearranged B-precursor acute lymphoblastic leukemia . Blood 2012; 120 : 833–842. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Senkevitch E, Hixon J, Andrews C, Barata JT, Li W, Durum S. The JAK Inhibitor Ruxolitinib Is Effective in Treating T Cell Acute Lymphoblastic Leukemia with Gain of Function Mutations in IL-7R Alpha . Blood 2015; 126 : 1330. [ Google Scholar ]
- Nordlund J, Milani L, Lundmark A, Lonnerholm G, Syvanen AC. DNA methylation analysis of bone marrow cells at diagnosis of acute lymphoblastic leukemia and at remission . PLoS One 2012; 7 : e34513. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nordlund J, Backlin CL, Zachariadis V, Cavelier L, Dahlberg J, Ofverholm I et al. DNA methylation-based subtype prediction for pediatric acute lymphoblastic leukemia . Clin Epigenetics 2015; 7 : 11. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chatterton Z, Morenos L, Mechinaud F, Ashley DM, Craig JM, Sexton-Oates A et al. Epigenetic deregulation in pediatric acute lymphoblastic leukemia . Epigenetics 2014; 9 : 459–467. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Milani L, Lundmark A, Kiialainen A, Nordlund J, Flaegstad T, Forestier E et al. DNA methylation for subtype classification and prediction of treatment outcome in patients with childhood acute lymphoblastic leukemia . Blood 2010; 115 : 1214–1225. [ PubMed ] [ Google Scholar ]
- Issa J-PJ. DNA Methylation as a Therapeutic Target in Cancer . Clin Cancer Res 2007; 13 : 1634–1637. [ PubMed ] [ Google Scholar ]
- Yánez L, Bermúdez A, Richard C, Bureo E, Iriondo A. Successful induction therapy with decitabine in refractory childhood acute lymphoblastic leukemia . Leukemia 2009; 23 : 1342–1343. [ PubMed ] [ Google Scholar ]
- Benton CB, Thomas DA, Yang H, Ravandi F, Rytting M, O’Brien S et al. Safety and clinical activity of 5-aza-2’-deoxycytidine (decitabine) with or without Hyper-CVAD in relapsed/refractory acute lymphocytic leukaemia . Br J Haematol 2014; 167 : 356–365. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Burke MJ, Lamba JK, Pounds S, Cao X, Ghodke-Puranik Y, Lindgren BR et al. A therapeutic trial of decitabine and vorinostat in combination with chemotherapy for relapsed/refractory acute lymphoblastic leukemia . Am J Hematol 2014; 89 : 889–895. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lu BY, Thanawala SU, Zochowski KC, Burke MJ, Carroll WL, Bhatla T. Decitabine enhances chemosensitivity of early T-cell precursor-acute lymphoblastic leukemia cell lines and patient-derived samples . Leuk Lymphoma 2016; 57 : 1938–1941. [ PubMed ] [ Google Scholar ]
- Zhao WL. Targeted therapy in T-cell malignancies: dysregulation of the cellular signaling pathways . Leukemia 2010; 24 : 13–21. [ PubMed ] [ Google Scholar ]
- Chiarini F, Fala F, Tazzari PL, Ricci F, Astolfi A, Pession A et al. Dual inhibition of class IA phosphatidylinositol 3-kinase and mammalian target of rapamycin as a new therapeutic option for T-cell acute lymphoblastic leukemia . Cancer Res 2009; 69 : 3520–3528. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bressanin D, Evangelisti C, Ricci F, Tabellini G, Chiarini F, Tazzari PL et al. Harnessing the PI3K/Akt/mTOR pathway in T-cell acute lymphoblastic leukemia: Eliminating activity by targeting at different levels . Oncotarget 2012; 3 : 811–823. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schult C, Dahlhaus M, Glass A, Fischer K, Lange S, Freund M et al. The dual kinase inhibitor NVP-BEZ235 in combination with cytotoxic drugs exerts anti-proliferative activity towards acute lymphoblastic leukemia cells . Anticancer Res 2012; 32 : 463–474. [ PubMed ] [ Google Scholar ]
- Saunders P, Cisterne A, Weiss J, Bradstock KF, Bendall LJ. The mammalian target of rapamycin inhibitor RAD001 (everolimus) synergizes with chemotherapeutic agents, ionizing radiation and proteasome inhibitors in pre-B acute lymphocytic leukemia . Haematologica 2011; 96 : 69–77. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dai H, Wang Y, Lu X, Han W. Chimeric Antigen Receptors Modified T-Cells for Cancer Therapy . Natl Cancer Inst 2016; 108 : pii djv439. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia . Blood 2015; 125 : 4017–4023. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Suerth JD, Schambach A, Baum C. Genetic modification of lymphocytes by retrovirus-based vectors . Curr Opin Immunol 2012; 24 : 598–608. [ PubMed ] [ Google Scholar ]
- Riet T, Holzinger A, Dorrie J, Schaft N, Schuler G, Abken H. Nonviral RNA transfection to transiently modify T cells with chimeric antigen receptors for adoptive therapy . Methods Mol Biol 2013; 969 : 187–201. [ PubMed ] [ Google Scholar ]
- Barrett DM, Liu X, Jiang S, June CH, Grupp SA, Zhao Y. Regimen-specific effects of RNA-modified chimeric antigen receptor T cells in mice with advanced leukemia . Hum Gene Ther 2013; 24 : 717–727. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Grupp SA, Maude SL, Shaw PA, Aplenc R, Barrett DM, Callahan C et al. Durable Remissions in Children with Relapsed/Refractory ALL Treated with T Cells Engineered with a CD19-Targeted Chimeric Antigen Receptor (CTL019) . Blood 2015; 126 : 681–681. [ Google Scholar ]
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ et al. Chimeric antigen receptor T cells for sustained remissions in leukemia . N Engl J Med 2014; 371 : 1507–1517. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K et al. Efficacy and Toxicity Management of 19-28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia . Sci Transl Med 2014; 6 : 224ra25. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial . Lancet 2015; 385 : 517–528. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Maude SL, Barrett D, Teachey DT, Grupp. SA. Managing Cytokine Release Syndrome Associated With Novel T Cell-Engaging Therapies . Cancer J 2014; 20 : 119–122. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M et al. Current concepts in the diagnosis and management of cytokine release syndrome . Blood 2014; 124 : 188–195. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Haso W, Lee DW, Shah NN, Stetler-Stevenson M, Yuan CM, Pastan IH et al. Anti-CD22–chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia . Blood 2013, 1165–1174. [ PMC free article ] [ PubMed ]
- Shah NN, Stetler-Stevenson M, Yuan CM, Shalabi H, Yates B, Delbrook C et al. Minimal Residual Disease Negative Complete Remissions Following Anti-CD22 Chimeric Antigen Receptor (CAR) in Children and Young Adults with Relapsed/Refractory Acute Lymphoblastic Leukemia (ALL) . Blood 2016; 128 : 650. [ Google Scholar ]
- Jamieson CH, Amylon MD, Wong RM, Blume KG. Allogeneic hematopoietic cell transplantation for patients with high-risk acute lymphoblastic leukemia in first or second complete remission using fractionated total-body irradiation and high-dose etoposide: a 15-year experience . Exp Hematol 2003; 31 : 981–986. [ PubMed ] [ Google Scholar ]
- Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial . J Clin Oncol 2004; 22 : 4075–4086. [ PubMed ] [ Google Scholar ]
- Jain N, Lamb AV, O'Brien S, Ravandi F, Konopleva M, Jabbour E et al. Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype . Blood 2016; 127 : 1863–1869. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gokbuget N, Kneba M, Raff T, Trautmann H, Bartram CR, Arnold R et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies . Blood 2012; 120 : 1868–1876. [ PubMed ] [ Google Scholar ]
- Dhedin N, Huynh A, Maury S, Tabrizi R, Beldjord K, Asnafi V et al. Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia . Blood 2015; 125 : 2486–2496, quiz 2586. [ PubMed ] [ Google Scholar ]
- Tavernier E, Boiron JM, Huguet F, Bradstock K, Vey N, Kovacsovics T et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial . Leukemia 2007; 21 : 1907–1914. [ PubMed ] [ Google Scholar ]
- Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study . Blood 2007; 109 : 944–950. [ PubMed ] [ Google Scholar ]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Browse Articles
Autophagy degrades immunogenic endogenous retroelements induced by 5-azacytidine in acute myeloid leukemia
- Nandita Noronha
- Chantal Durette
- Gregory Ehx

Correction to: Histone deacetylase (HDAC) inhibitor ACY241 enhances anti-tumor activities of antigen-specific central memory cytotoxic T lymphocytes against multiple myeloma and solid tumors
- Teru Hideshima
- Kenneth C. Anderson
Combination therapies with ponatinib and asciminib in a preclinical model of chronic myeloid leukemia blast crisis with compound mutations
- Nikola Curik
- Adam Laznicka
- Katerina Machova Polakova
Metabolic reprogramming regulated by TRAF6 contributes to the leukemia progression
- Shinichiro Matsui
- Tomoya Muto
Fluorescence in situ hybridization reveals the evolutionary biology of minor clone of gain/amp(1q) in multiple myeloma
- Yuntong Liu
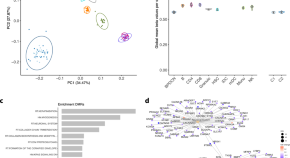
Genome-wide DNA methylation-analysis of blastic plasmacytoid dendritic cell neoplasm identifies distinct molecular features
- Axel Künstner
- Julian Schwarting
- Niklas Gebauer
Management of isocitrate dehydrogenase 1/2 mutated acute myeloid leukemia
- Harry Fruchtman
- Zachary M. Avigan
- John O. Mascarenhas
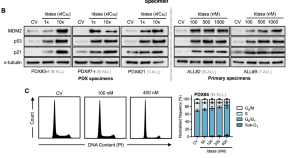
Combination p53 activation and BCL-x L /BCL-2 inhibition as a therapeutic strategy in high-risk and relapsed acute lymphoblastic leukemia
- Hayden L. Bell
- Helen J. Blair
- Julie A. E. Irving
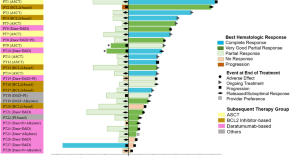
Treatment patterns for AL amyloidosis after frontline daratumumab, bortezomib, cyclophosphamide, and dexamethasone treatment failures
- Saurabh Zanwar
- Morie A. Gertz
- Angela Dispenzieri
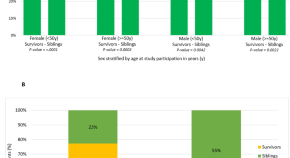
Pre-frailty after blood or marrow transplantation and the risk of subsequent mortality
- Joshua S. Richman
- Smita Bhatia
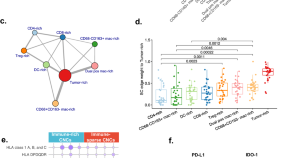
High-plex imaging and cellular neighborhood spatial analysis reveals multiple immune escape and suppression patterns in diffuse large B-cell lymphoma
- David J. Reiss
- Yumi Nakayama
- Anita K. Gandhi
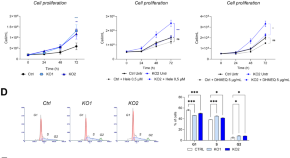
IκBε deficiency accelerates disease development in chronic lymphocytic leukemia
- Jessica Bordini
- Chiara Lenzi
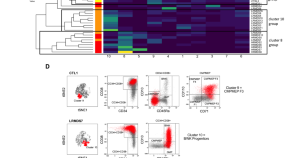
Loss of hematopoietic progenitors heterogeneity is an adverse prognostic factor in lower-risk myelodysplastic neoplasms
- Charles Dussiau
- Thibault Comont
- François Vergez
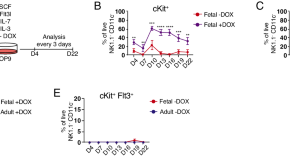
A complex interplay of intra- and extracellular factors regulates the outcome of fetal- and adult-derived MLL-rearranged leukemia
- Maria Jassinskaja
- Sudip Ghosh
- Jenny Hansson
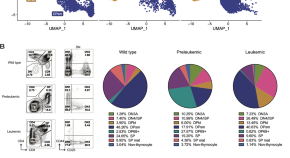
The role of quiescent thymic progenitors in TAL/LMO2-induced T-ALL chemotolerance
- Kevin W. O’Connor
- Kensei Kishimoto
- Michelle A. Kelliher
Mitigating immortal-time bias: exploring osteonecrosis and survival in pediatric ALL - AALL0232 trial insights
- Shyam Srinivasan
- Swaminathan Keerthivasagam
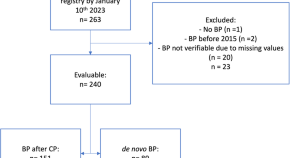
Management and outcome of patients with chronic myeloid leukemia in blast phase in the tyrosine kinase inhibitor era – analysis of the European LeukemiaNet Blast Phase Registry
- Annamaria Brioli
- Elza Lomaia
- Michael Lauseker
ATG or post-transplant cyclophosphamide to prevent GVHD in matched unrelated stem cell transplantation?
- Olaf Penack
- Mouad Abouqateb
- Zinaida Peric
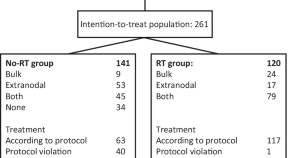
Radiotherapy in younger patients with advanced aggressive B-cell lymphoma—long-term results from the phase 3 R-MegaCHOEP trial
- Michael Oertel
- Marita Ziepert
- Hans Theodor Eich

Molecular ontogeny underlies the benefit of adding venetoclax to hypomethylating agents in newly diagnosed AML patients
- Shai Shimony
- Jacqueline S. Garcia
- R. Coleman Lindsley
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
Posted: January 27, 2023. FDA has approved zanubrutinib (Brukinsa) for chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) based on results from two clinical trials. In both trials, the drug, which blocks a protein called BTK, was more effective and caused fewer side effects than other treatments.
NCI-funded researchers are working to advance our understanding of how to treat leukemia. With progress in both targeted therapies and immunotherapies, leukemia treatment has the potential to become more effective and less toxic. This page highlights some of the latest research in leukemia, including clinical advances that may soon translate ...
A complex interplay of intra- and extracellular factors regulates the outcome of fetal- and adult-derived MLL-rearranged leukemia. Maria Jassinskaja. Sudip Ghosh. Jenny Hansson. Article Open ...
Progress in understanding the pathophysiology and improving the therapy of acute myeloid leukemia (AML) is now occurring at a rapid pace. The discovery of the activity of cytarabine (ara-C) and of ...
2. Age and Race. Age and race are important factors in the incidence of leukemias. For example, in the United Kingdom, 42.8% of all leukemias occur in individuals over 65 years of age [].A review of the subject in the United States reports that the overall age-adjusted leukemia incidence is highest in the White population at 15 per 100,000, followed by Blacks at 11 per 100,000, and Hispanics ...
Read the latest Research articles from Leukemia. Combination p53 activation and BCL-x L /BCL-2 inhibition as a therapeutic strategy in high-risk and relapsed acute lymphoblastic leukemia. Hayden L ...
Despite long periods of stagnation in the 1970s-early 2000s, research efforts over the last 10-20 years have resulted in incredible advances in the types and efficacy of treatment options for many leukemia types. For decades, the mainstay of treatment for AML and ALL remained intensive chemotherapy and stem cell transplant, both highly morbid ...
The production of abnormal leukocytes defines leukemia as either a primary or secondary process. They can be classified as acute or chronic based on the rapidity of proliferation and myeloid or lymphoid based on the cell of origin. Predominant subtypes are acute myeloid leukemia (AML) and chronic myeloid leukemia (CML), involving the myeloid lineage; acute lymphoblastic leukemia (ALL); and ...
Leukemia Research is an international journal which brings comprehensive and current information to all health care professionals involved in basic and applied clinical research in hematological malignancies. The editors encourage the submission of articles relevant to hematological malignancies. The Journal scope includes reporting studies of ...
Research article Full text access Proposal and clinical application of molecular genetic risk scoring system, "MRplus", for BCR-ABL1 negative pediatric B-cell acute lymphoblastic leukemia- report from a single centre
The guidelines of the International Workshop on Chronic Lymphocytic Leukemia (iwCLL) provide unified disease staging and guidance for the clinical management of CLL. 15 Patients with early-stage ...
Leukemia is one of the leading journals in hematology and oncology. It covers all aspects of the research and treatment of leukemia and allied diseases. Studies of normal hemopoiesis are covered ...
select article Corrigendum to "Patient-driven research: Initial results from a prospective health-related quality of life study performed at the request of patients living with hairy cell leukemia" [Leuk. Res. 120 (2022) 106919]
Recently, the International Consensus Classification (ICC) and the 5th edition of the World Health Organization classification (WHO2022) introduced diagnostically similar yet distinct approaches, which has resulted in practical confusion. This review compares these classification systems for acute myeloid leukemia (AML), building up on the revised 4th edition of WHO (WHO2016). Both ...
Introduction. Acute myeloid leukemia (AML) is the most common type of acute leukemia in adults. AML is characterized by the clonal proliferation of abnormal hematopoietic progenitors leading to blood and bone marrow infiltration and consequently hematopoietic failure ().Over the past decades, intensive research has significantly improved our understanding of AML biology, highlighting the role ...
AbstractPurpose:. The inherent genetic heterogeneity of acute myeloid leukemia (AML) has challenged the development of precise and effective therapies. The objective of this study was to elucidate the genomic basis of drug resistance or sensitivity, identify signatures for drug response prediction, and provide resources to the research community.Experimental Design:. We performed targeted ...
To study the shared genetic structure between autoimmune diseases and B-cell acute lymphoblastic leukemia (B-ALL) and identify the shared risk loci and genes and genetic mechanisms involved. Based on large-scale genome-wide association study (GWAS) summary-level data sets, we observed genetic overlaps between autoimmune diseases and B-ALL, and cross-trait pleiotropic analysis was performed to ...
Read the latest Research articles from Leukemia. Inhibitors of Bcl-2 and Bruton's tyrosine kinase synergize to abrogate diffuse large B-cell lymphoma growth in vitro and in orthotopic ...
Phase I trial of the combination of ibrutinib and lenalidomide of the treatment of patients with MDS who have failed standard therapy or who are unfit for or refuse standard therapy. Samantha C. Fisch, Joseph M. Tuscano, Lihong Qi, Brian A. Jonas. Article 106947. View PDF.
Researchers at Peter Mac have found a new way to treat a form of leukemia that stops the disease in its tracks to prolong remission. The research, published in the Journal of Clinical Oncology ...
Despite having an overall survival rate of 94%, B-cell acute lymphoblastic leukemia (B-ALL), the most common childhood cancer, can prove challenging to treat, with survival among relapsed or resistant cases falling between 30-50%. Recent work by St. Jude Children's Research Hospital scientists discovered which tumor cells resist treatment and ...
This article is part of the Research Topic. ... Authors compared outcomes of children with acute lymphoblastic leukemia undergoing HSCT in second complete remission (CR2) from haploidentical versus HLA-matched donors. ... This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use ...
From bone marrow failure syndromes to VEXAS: Disentangling clonal hematopoiesis, immune system, and molecular drivers. Carmelo Gurnari, Valeria Visconte. Article 107038. View PDF. Article preview. Read the latest articles of Leukemia Research at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature.
Introduction. Chronic myeloid leukemia (CML), a malignancy originating in hematopoietic stem cells (HSCs) in the chronic phase of the disease (Table 1) and characterized by myeloid cells of various maturation stages in peripheral blood and bone marrow, is caused by the oncoprotein BCR-ABL1, a dysregulated tyrosine kinase 1.In early 2001, the first targeted therapy against an oncoprotein, the ...
RSS Feed. Leukaemia is a type of blood cancer, which starts in blood-forming tissue, such as the bone marrow, and causes large numbers of immature blood cells to be produced and enter the ...
Younger patients with acute myeloid leukemia (and/or older patients fit for intensive chemotherapy) The median age in AML is 68 years 85. Most of the research with 3 + 7 and other intensive chemotherapy regimens was conducted in younger patients (usual upper age limit 60-65 years).
About the journal. Official journal of the Myelodysplastic Syndromes Foundation . Leukemia Research Reports (LRR), a companion title to Leukemia Research, is a peer-reviewed publication devoted to the rapid publication of short, high-quality papers related to a broad scope of therapeutic areas of hematology, …. View full aims & scope.
MD Anderson Research Highlights for April 12, 2024 Featuring strategies to overcome treatment resistance, targets for ovarian and BRAF-mutated cancers, and a novel risk prediction tool for ...
Abstract. Acute lymphoblastic leukemia (ALL) is the second most common acute leukemia in adults, with an incidence of over 6500 cases per year in the United States alone. The hallmark of ALL is chromosomal abnormalities and genetic alterations involved in differentiation and proliferation of lymphoid precursor cells.
A complex interplay of intra- and extracellular factors regulates the outcome of fetal- and adult-derived MLL-rearranged leukemia. Maria Jassinskaja. Sudip Ghosh. Jenny Hansson. Article Open ...