

Journal of Clinical Medicine Research Impact, Factor and Metrics, Impact Score, Ranking, h-index, SJR, Rating, Publisher, ISSN, and More
Impact score.
Note: The impact score or impact index shown here is equivalent to the average number of times documents published in a journal/conference in the past two years have been cited in the current year (i.e., Cites / Doc. (2 years)). It is based on Scopus data and can be a little higher or different compared to the impact factor (IF) produced by Journal Citation Report. Please refer to the Web of Science data source to check the exact journal impact factor ™ (Thomson Reuters) metric.
Important Metrics and Factor
| Journal of Clinical Medicine Research | |
| J. Clin. Med. Res. | |
| Journal | |
| Medicine (miscellaneous) (Q2) | |
| 21 | |
| 10200 | |
| 0.53 | |
| 1.58 | |
| Elmer Press | |
| Canada | |
| 19183011, 19183003 | |
| Q2 | |
| 2019-2023 |
About Journal of Clinical Medicine Research
Journal of Clinical Medicine Research is a journal covering the technologies/fields/categories related to Medicine (miscellaneous) (Q2) . It is published by Elmer Press . The overall rank of Journal of Clinical Medicine Research is 10200 . According to SCImago Journal Rank (SJR) , this journal is ranked 0.53 . SCImago Journal Rank is an indicator, which measures the scientific influence of journals. It considers the number of citations received by a journal and the importance of the journals from where these citations come. SJR acts as an alternative to the Journal Impact Factor (or an average number of citations received in last 2 years). This journal has an h-index of 21 . The best quartile for this journal is Q2 .
The ISSN of Journal of Clinical Medicine Research journal is 19183011, 19183003 . An International Standard Serial Number (ISSN) is a unique code of 8 digits. It is used for the recognition of journals, newspapers, periodicals, and magazines in all kind of forms, be it print-media or electronic. Journal of Clinical Medicine Research is cited by a total of 514 articles during the last 3 years (Preceding 2023).
Journal of Clinical Medicine Research Impact IF 2023-2024
The Impact IF 2023 of Journal of Clinical Medicine Research is 1.58 , which is computed in 2024 as per its definition. Journal of Clinical Medicine Research IF is decreased by a factor of 1.09 and approximate percentage change is -40.82% when compared to preceding year 2022, which shows a falling trend. The impact IF , also denoted as Journal impact score (JIS), of an academic journal is a measure of the yearly average number of citations to recent articles published in that journal. It is based on Scopus data.
Journal of Clinical Medicine Research Impact IF 2024 Prediction
Impact IF 2023 of Journal of Clinical Medicine Research is 1.58 . If the same downward trend persists, Impact IF may fall in 2024 as well.
Impact IF Trend
Year wise impact if of journal of clinical medicine research. based on scopus data..
| Year | Impact IF |
|---|---|
| 2023/2024 | Coming Soon |
| 2023 | 1.58 |
| 2022 | 2.67 |
| 2021 | 2.56 |
| 2020 | 1.62 |
| 2019 | 0.00 |
Journal of Clinical Medicine Research h-index
Journal of Clinical Medicine Research has an h-index of 21 . It means 21 articles of this journal have more than 21 number of citations. The h-index is a way of measuring the productivity and citation impact of the publications. The h-index is defined as the maximum value of h such that the given journal/author has published h papers that have each been cited at least h number of times.
Journal of Clinical Medicine Research ISSN
The ISSN of Journal of Clinical Medicine Research is 19183011, 19183003 . ISSN stands for International Standard Serial Number.
An ISSN is a unique code of 8 digits. It is used for the recognition of journals, newspapers, periodicals, and magazines in all kind of forms, be it print-media or electronic.
Journal of Clinical Medicine Research Rank and SCImago Journal Rank (SJR)
The overall rank of Journal of Clinical Medicine Research is 10200 . According to SCImago Journal Rank (SJR), this journal is ranked 0.53 . SCImago Journal Rank is an indicator, which measures the scientific influence of journals. It considers the number of citations received by a journal and the importance of the journals from where these citations come.
SJR of Journal of Clinical Medicine Research by Year
| Year | SJR |
|---|---|
| 2023/2024 | Coming Soon |
| 2023 | 0.53 |
| 2022 | 0.586 |
| 2021 | |
| 2020 | |
| 2019 |
Ranking of Journal of Clinical Medicine Research by Year
| Year | Ranking |
|---|---|
| 2023/2024 | Coming Soon |
| 2023 | 10200 |
| 2022 | 8803 |
| 2021 | 27586 |
| 2020 | 33798 |
| 2019 | 31452 |
Journal of Clinical Medicine Research Publisher
Journal of Clinical Medicine Research is published by Elmer Press . It's publishing house is located in Canada . Coverage history of this journal is as following: 2019-2023 . The organization or individual who handles the printing and distribution of printed or digital publications is known as Publisher.
Call For Papers
Visit the official website of the journal/conference to check the further details about the call for papers.
Abbreviation
The IS0 4 standard abbreviation of Journal of Clinical Medicine Research is J. Clin. Med. Res. . This abbreviation ('J. Clin. Med. Res.') is well recommended and approved for the purpose of indexing, abstraction, referencing and citing goals. It meets all the essential criteria of ISO 4 standard.
ISO 4 (International Organization for Standardization 4) is an international standard that defines a uniform and consistent system for abbreviating serial publication titles and journals.
How to publish in Journal of Clinical Medicine Research
If your research field is/are related to Medicine (miscellaneous) (Q2) , then please visit the official website of this journal .
Acceptance Rate
- The demand or interest of researchers/scientists in publishing in a specific Journal/Conference.
- Peer review complexity and timeline.
- The mix of unsolicited and invited submissions.
- The time it takes from manuscript submission to final publication.
- And Many More.
It is essential to understand that the acceptance rate/rejection rate of papers varies among journals. Some Journals considers all the manuscripts submissions as a basis of acceptance rate computation. On the other hand, few consider the only manuscripts sent for peer review or few even not bother about the accurate maintenance of total submissions. Hence, it can provide a rough estimation only.
The best way to find out the acceptance rate is to reach out to the associated editor or to check the official website of the Journal/Conference.
Frequently Asked Questions (FAQs)
What's the latest impact if of the journal of clinical medicine research.
Journal of Clinical Medicine Research latest impact IF is 1.58 . It's evaluated in the year 2023. The highest and the lowest impact IF or impact score of this journal are 2.67 (2022) and 0.00 (2019) , respectively, in the last 5 years. Moreover, its average IS is 1.69 in the previous 5 years.
What's the SCImago Journal Rank (SJR) of the Journal of Clinical Medicine Research?
The Journal of Clinical Medicine Research has an SJR (SCImago Journal Rank) of 0.53 , according to the latest data. It is computed in the year 2024. In the past 5 years, this journal has recorded a range of SJR, with the highest being 0.586 in 2022 and the lowest being in 2021. Furthermore, the average SJR of the Journal of Clinical Medicine Research over the previous 5-year period stands at 1.69.
What's the latest h-index of the Journal of Clinical Medicine Research?
The latest h-index of the Journal of Clinical Medicine Research is 21 .
Who's the publisher of the Journal of Clinical Medicine Research?
The Journal of Clinical Medicine Research is published by the Elmer Press , with its country of publication being the Canada.
What's the current ranking of the Journal of Clinical Medicine Research?
The Journal of Clinical Medicine Research is currently ranked 10200 out of 27955 Journals, Conferences, and Book Series in the latest ranking. Over the course of the last 5 years, this journal has experienced varying rankings, reaching its highest position of 8803 in 2022 and its lowest position of 33798 in 2020.
What's the abbreviation or short name for the Journal of Clinical Medicine Research?
The standard ISO4 abbreviation for the Journal of Clinical Medicine Research is J. Clin. Med. Res. .
Is the "Journal of Clinical Medicine Research" classified as a Journal, Conference and Proceedings, Trade Journal or Book Series?
Journal of Clinical Medicine Research is classified as a journal that the Elmer Press publishes.
What's the scope or major areas of the Journal of Clinical Medicine Research?
- Medicine (miscellaneous)
For a more comprehensive understanding of its scope, check the official website of this journal.
What's the ISSN of the Journal of Clinical Medicine Research?
The Journal of Clinical Medicine Research is assigned the following International Standard Serial Numbers (ISSN): 19183011, 19183003 .
What's the best quartile of the Journal of Clinical Medicine Research?
The best quartile for the Journal of Clinical Medicine Research is Q2 (2023).
What's the coverage history of the Journal of Clinical Medicine Research?
The Journal of Clinical Medicine Research coverage history can be summarized as follows: 2019-2023 .
Credits and Sources
- Scimago Journal & Country Rank (SJR), https://www.scimagojr.com/
- Journal Impact Factor, https://clarivate.com/
Impact Score, h-Index, and Other Important Details of These Journals, Conferences, and Books
| Journal/Conference/Workshop/Book Title | Type | Ranking | Publisher | h-index | Impact Score |
|---|---|---|---|---|---|
Check complete list
Year wise Impact Score (IS) of Journal of Clinical Medicine Research
| Year | Impact Score (IS) |
|---|---|
| 2024/2025 | Coming Soon |
| 2023 | 1.58 |
| 2022 | 2.67 |
| 2021 | 2.56 |
| 2020 | 1.62 |
| 2019 | 0.00 |
Top Journals/Conferences in Medicine (miscellaneous)
Download Templates
IEEE Article Springer ACM Elsevier Wiley MITPress
Check out Quote of the Day
Journals | Policy | Permission Journal of Clinical Medicine Research
INFORMATION
- For Authors
- For Reviewers
- For Editors
- For Readers
- Admin-login
| MOST READ |
(For the last 6-month published articles)

Original Article Review Case Report Short Communication Letter to the Editor Editorial
| INDEXED IN |
|
|
|
|
| |
| QUICK LINKS | |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
| BROWSE JOURNALS | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

| ARTICLE STATISTICS |
| Submission to First Decision |
| 23 Days |
|
|
| 26% |
|
|
| 19 Days |
| Average article statistics from the last 12 months data |
- Online-First
Journal of Clinical Medicine Research
|
|
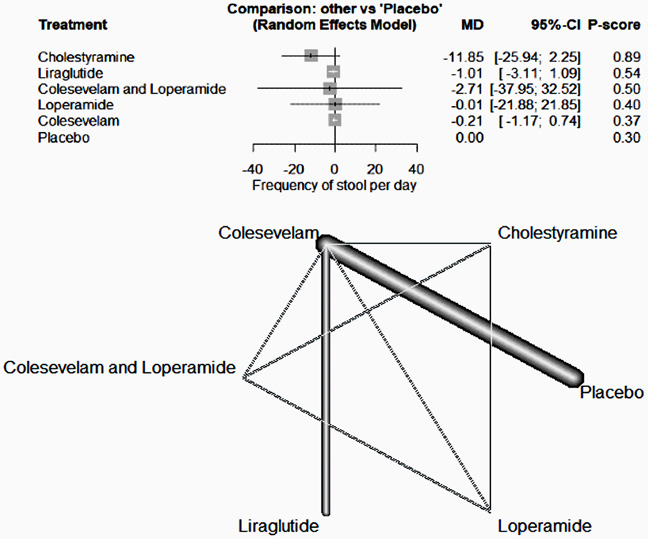
Bile acid malabsorption (BAM) is characterized by chronic watery diarrhea resulting from excessive bile acids in the feces. BAM is often an overlooked cause of chronic diarrhea, with its prevalence not being sufficiently researched.
The field of kidney transplantation is being revolutionized by the integration of artificial intelligence (AI) and machine learning (ML) techniques. AI equips machines with human-like cognitive abilities, while ML enables computers to learn from data.
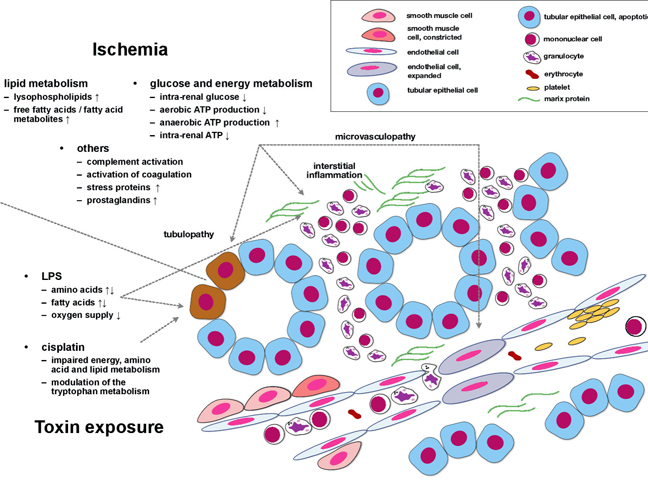
Acute kidney injury (AKI) affects increasing numbers of in-hospital patients in Central Europe and the USA, the prognosis remains poor. Although substantial progress has been achieved in the identification of molecular/cellular processes that induce and perpetuate AKI, more integrated pathophysiological perspectives are missing.

Subjects with mild cognitive impairment (MCI) can progress to dementia. Studies have shown that neuropsychological tests, biological or radiological markers individually or in combination have helped to determine the risk of conversion from MCI to dementia.
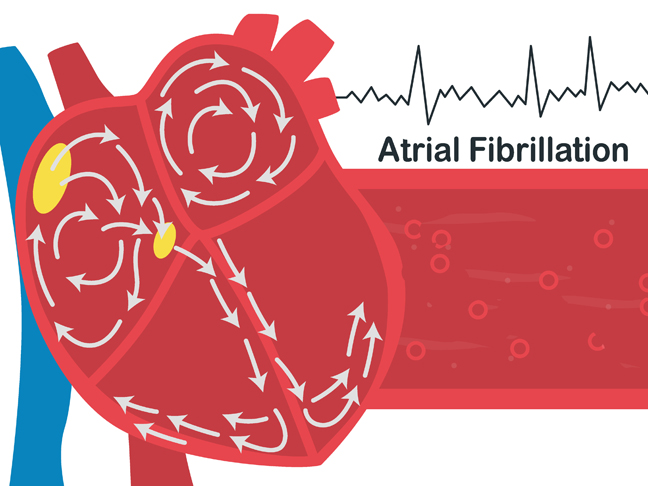
Atrial fibrillation (AF) is the most common arrhythmia with a growing prevalence worldwide, especially in the elderly population. Patients with AF are at higher risk of serious life-threatening events and complications that may lead to long-term sequelae and reduce quality of life.
Viewpoints | � | � | Featured | � |
| Acute kidney injury (AKI) affects up to 30% of all hospitalized patients in Central Europe and the USA. New biomarker molecules have been identified in recent years; most studies performed so far however aimed to identify markers for diagnostic purposes. | | |||
Key Clinical Image |
| |
Current Issue | � |
Vol. 16, No. 6, Jun 2024
Table of contents.
| Marta Maci , Carlotta Fanelli, Mauro Lorusso, Donatella Ferrara, Marino Caroprese, Michele Laurenziello, Michele Tepedino, Domenico Ciavarella | 273-283 |
| doi: https://doi.org/10.14740/jocmr5202 |
Original Article
| Sengottaian Sivakumar, Roni Mendonca, Michael Girshin | 284-292 |
| doi: https://doi.org/10.14740/jocmr5159 |
| Chiara Rosato, Marilena Greco, Giovanni Marciante, Roberta Assunta Lazzari, Floriano Indino, Giambattista Lobreglio | 293-301 |
| doi: https://doi.org/10.14740/jocmr5070 |
| Charlotte Mund, Katharina Asmus, Wajima Safi, Oliver Ritter, Dominique Petrus, Susann Patschan, Daniel Patschan | 302-309 |
| doi: https://doi.org/10.14740/jocmr5190 |
| Anas Elgenidy, Mohammed Al-Mahdi Al-Kurdi, Hoda Atef Abdelsattar Ibrahim , Eman F. Gad, Ahmed K. Awad, Rebecca Caruana, Sheriseane Diacono, Aya Sherif, Tasneem Elattar, Islam E. Al-Ghanam, Asmaa M. Eldmaty, Tareq M. Abubasheer, Ahmed M. Afifi, Amira Elhoufey, Hamad Ghaleb Dailah, Amira M. Osman, Mohamed Ezzat, Doaa Ali Gamal, Rady Elmonier, Ahmed El-Sayed Hammour, Maged T. Abougabal, Khaled Saad | 310-318 |
| doi: https://doi.org/10.14740/jocmr5205 |
Case Report
| Ashley Smith, Sidhant Kalsotra, Joseph D. Tobias | 319-323 |
| doi: https://doi.org/10.14740/jocmr5175 |
 About the Journal Make a SubmissionJournalcover.  InformationFor Authors For Reviewers For Editors For Readers Admin-login Journal IndexingPubMed PubMed Central Scopus (Citescore 5.1) Web of Science (ESCI), Impact Factor 1.6 (2023)
Article StatisticsSubmission to First Decision: 23 Days Acceptance Rate: 26% Acceptance to Publication: 19 Days *Average article statistics from the last 12 months data Tweets by JOCMR_journal COVID-19 ResearchThe COVID-19 outbreak presents the unprecedented challenge for world public and medical practitioners and health care providers, the post COVID-19 condition (or long COVID) includes long term symptoms which may persist for months or years after SARS-CoV-2 infection. We will consider submissions related to all aspects of COVID-19 and Long COVID, and process the manuscripts in priority. Collection of COVID-19 Articles Invite Submissions Website: jocmr.elmerjournals.com Editorial Contact: [email protected] Address: 9225 Leslie Street, Suite 201, Richmond Hill, Ontario, L4B 3H6, Canada View All Journals Journal of Clinical Medicine Research World Journal of Oncology Journal of Medical Cases Cardiology Research Journal of Neurology Research Journal of Endocrinology and Metabolism Gastroenterology Research Journal of Current Surgery World Journal of Nephrology and Urology International Journal of Clinical Pediatrics Journal of Clinical Gynecology and Obstetrics Journal of Hematology Clinical Infection and Immunity Cellular and Molecular Medicine Research 
Data Sharing Statement See More AboutSign up for emails based on your interests, select your interests. Customize your JAMA Network experience by selecting one or more topics from the list below.
Get the latest research based on your areas of interest.Others also liked.
Zissette S , Gautam A , Krumholz HM , Ross JS , Wallach JD. Altmetric Attention Scores and Citations of Published Research With or Without Preprints. JAMA Netw Open. 2024;7(7):e2424732. doi:10.1001/jamanetworkopen.2024.24732 Manage citations:© 2024
Altmetric Attention Scores and Citations of Published Research With or Without Preprints
Use of preprints, defined as preliminary research reports that have not undergone peer review, in clinical and health science research has increased in recent years, partly because of the COVID-19 pandemic. 1 , 2 Although high-impact clinical journals have publication policies supportive of preprints, 3 concerns remain that posting a preprint before submission to a peer-reviewed journal may jeopardize publication, especially if the preprint generates media attention and citations. 4 While biology articles with corresponding preprints have received greater attention than those without, 5 little is known about the highest-impact clinical research. Therefore, we aimed to assess how frequently research articles published in the highest-impact clinical journals are preprinted and whether media attention and citations differed between articles with and without corresponding preprints. In accordance with the Common Rule, this cross-sectional study was exempt from ethics review and informed consent because it used public nonidentifiable data. We followed the STROBE reporting guideline. We identified 25 high-impact journals according to InCites Journal Citation Reports, including the 6 general and internal medicine journals and the 2 clinical medicine journals across 9 subspecialities with the highest impact factors ( Table 1 ). We also included JAMA Network Open as it publishes general and subspecialty clinical articles. We searched PubMed for all records of these journals indexed in 2022 (after the COVID-19 pandemic had largely subsided) and manually identified original research articles. First, we identified preprints automatically linked to published articles in the sample using the bioRxiv/medRxiv application programming interface (API). To locate preprints missed by the API or posted on other platforms, we used WebScrapingAPI to conduct Google searches of published article titles and screened the first 5 records mentioning medRxiv, bioRxiv, or Social Science Research Network. Second, we identified the Altmetric Attention Score (Altmetric API) and citations (Dimensions API) for each article as of March 2024. Within each journal with at least 1 article with a preprint, we calculated the differences in median Altmetric scores and citations between articles with and without preprints. We used Wilcoxon signed-rank test to evaluate whether the median of the distribution of the differences between medians across the 25 journals differed from 0. Two-tailed P < .05 was considered statistically significant. Data analysis was performed with R, version 4.2.1 (R Project for Statistical Computing). Among the 5739 research articles published in 25 journals with high impact factors in 2022, 425 (7.4%) articles in 23 journals had corresponding preprints, ranging from 0 to 41 (26.1%) articles ( Table 1 ). COVID-19–related articles were more likely than non-COVID-19–related articles to have corresponding preprints (257 of 1270 [20.2%] vs 168 of 4469 [3.8%]; P < .001). The median (IQR) difference in medians between articles with and without preprints across journals was not significantly different from 0 for Altmetric Attention Scores (34.1 [6.0-191.0]; P = .33) or citations (8.0 [3.0-30.8]; P = .31) ( Table 2 ). These findings were consistent even after accounting for time from publication to analysis and when stratified by COVID-19–related and non-COVID-19–related articles. The finding that 7.4% of published articles in high-impact journals in 2022 had corresponding preprints is similar to estimates in other fields. 5 However, unlike previous evaluations of publications and corresponding bioRxiv preprints, 5 , 6 this study found no differences in Altmetric Attention Scores or citations between articles with and without preprints in the first years after publication. Study limitations include examining only publications in journals with high impact factors and postings in prominent preprint servers. Furthermore, it is possible that authors are more likely to preprint their most important articles and that the published version of these articles would have received more media attention and citations if they had not been preprinted. However, although not all preprints will subsequently be published in high-impact journals, the results suggest that, among published articles with corresponding preprints, preprinting is not associated with less media attention and lower citation counts. Accepted for Publication: May 31, 2024. Published: July 26, 2024. doi:10.1001/jamanetworkopen.2024.24732 Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Zissette S et al. JAMA Network Open . Corresponding Author: Joshua D. Wallach, PhD, MS, Department of Epidemiology, Rollins School of Public Health, Emory University, 1518 Clifton Rd NE, Claudia Nance Rollins Building, Room 3033 Atlanta, GA 30322 ( [email protected] ). Author Contributions: Mr. Zissette and Dr. Wallach had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Zissette, Gautam, Krumholz, Wallach. Acquisition, analysis, or interpretation of data: Zissette, Gautam, Ross, Wallach. Drafting of the manuscript: Zissette, Wallach. Critical review of the manuscript for important intellectual content: All authors. Statistical analysis: Zissette. Administrative, technical, or material support: Wallach. Supervision: Wallach. Conflict of Interest Disclosures: Dr Krumholz reported being a cofounder of medRxiv; receiving options for Element Science and Identifeye; receiving personal fees from F-Prime; being a cofounder of and holding equity in Hugo Health, Refactor Health, and ENSIGHT-AI; and being associated with research contracts through Yale University from Janssen, Kenvue, and Pfizer outside the submitted work. Dr Ross reported being a cofounder of medRxiv; receiving research support through Yale University from the US Food and Drug Administration (FDA), Johnson & Johnson, Arnold Ventures, Agency for Healthcare Research and Quality, and National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH); being a Deputy Editor at JAMA ; and being an expert witness at the request of Relator’s attorneys (the Greene Law Firm) in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen Inc that was settled in September 2022 outside the submitted work. Dr Wallach reported receiving research support from Arnold Ventures, FDA, Johnson & Johnson through the Yale Open Data Access project, and the National Institute on Alcohol Abuse and Alcoholism of the NIH and serving as a consultant to Hagens Berman Sobol Shapiro LLP and Dugan Law Firm APLC outside the submitted work. No other disclosures were reported. Disclaimer: The contents herein are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. Data Sharing Statement: See the Supplement .
Journal Metrics Reports 2023Clinical medicine, announcement of the latest impact factors from the journal citation reports. Researchers consider a number of factors in deciding where to publish their research, such as journal reputation, readership and community, speed of publication, and citations. See how we share a whole range of information to help the research community decide which journal is the best home for their research as well as what the metrics can tell you about the performance of a journal and its articles. Subdisciplines
Recommended for youLearn more about journal metrics. Read how to measure a journal’s impact. Sign up for new issue alertsNever miss an issue! Have free table of contents alerts for your journals of choice delivered directly to your inbox. Journals metrics by subject
Discover more than 1000 journals


 Follow us on @ScimagoJR Scimago Lab , Copyright 2007-2024. Data Source: Scopus®  Cookie settings Cookie Policy Legal Notice Privacy Policy 
Journal of Biomedical Materials Research Part B: Applied BiomaterialsPrint ISSN: 1552-4973 Online ISSN: 1552-4981 Impact Factor: 3.2 Society for Biomaterials Edited By:Jeremy Gilbert Advertisement Clinical characteristics of KRAS mutation subtypes in non-small cell lung cancer population in Xinjiang, China, and their impact on the prognosis of immunotherapy
Cite this articleYou have full access to this open access article 
Non-small cell lung cancer (NSCLC) is a highly fatal malignancy. The Kirsten rat sarcoma viral oncogene ( KRAS ) gene profoundly impacts patient prognosis. This study aims to explore the correlation between KRAS mutation subtypes, clinical data, and the impact of these subtypes on immunotherapy. Materials and methodsTumor samples from 269 NSCLC patients at the Affiliated Cancer Hospital of Xinjiang Medical University were analyzed. Patients received first- or second-line therapy without targeted therapy. Molecular and clinical data were used to analysis KRAS mutation subtypes and treatment outcomes. KRAS mutations predominantly included G12C, G12D, and G12V subtypes. TP53 had the highest mutation frequency among KRAS mutations, followed by MST1 , STK11 , and KMT2C . Gender differences were noted among KRAS mutation subtypes, with G12C and G12V mutations prevalent in males, while G12D mutations were less common among males. Smokers exhibited varied KRAS mutation subtypes, with G12C and G12V prevalent in smokers and G12D in nonsmokers. KRAS mutations were mainly in lung adenocarcinoma. TTF-1 and PD-L1 expression differed significantly among KRAS mutations. Patients with G12C and G12V mutations showed higher TMB levels and better immunotherapy outcomes compared to those without KRAS mutations. Conversely, patients with G12D mutations had poorer immunotherapy responses. ConclusionsKRAS mutation subtypes exhibit distinct clinical and molecular characteristics and varying responses to immunotherapy. G12C and G12V mutations correlate with better immunotherapy outcomes, while G12D mutations are associated with poorer responses. Avoid common mistakes on your manuscript. IntroductionNon-small cell lung cancer (NSCLC) accounts for the majority of lung cancer cases, making up approximately 85% of diagnoses (Osta et al. 2019 ). In recent years, there have been significant advancements in the treatment of NSCLC, leading to a substantial decrease in mortality rates. This is primarily attributed to the utilization of targeted therapies based on various driver gene mutations such as EGFR , ALK , ROS1 , MET , PIK3CA , RET , BRAF , as well as the approval of immune checkpoint inhibitors (either as monotherapy or in combination with chemotherapy) for patients lacking targetable driver mutations. While advancements in tumor genetic testing have revolutionized cancer diagnosis and treatment protocols, the prognosis of advanced NSCLC patients receiving second-line or subsequent treatment remains unsatisfactory. Kirsten rat sarcoma viral oncogene homolog ( KRAS ) mutations have emerged as significant drivers in human cancers (Jordan et al. 2017 ). KRAS was one of the first human oncogenes discovered, shedding light on the molecular mechanisms underlying cancer development (Pylayeva-Gupta et al. 2011 ; Malumbres and Barbacid 2003 ). Analysis across regions reveals varying frequencies of KRAS mutations, with around 26.1% of Western lung adenocarcinoma (LUAD) patients and approximately 11.2% of Asian patients exhibiting such mutations (Dearden et al. 2013 ). Located on chromosome 12p12.1, the human KRAS gene is prone to mutations, notably at the 12th position in NSCLC, including G12C, G12D, and G12V mutations. These alterations often correlate with poorer prognosis due to the molecular diversity observed in KRAS -mutated tumors, leading to differences in clinical outcomes among patients (Jancík et al. 2010 ; Yu et al. 2015 ; Scheffler et al. 2019 ). Despite over three decades of research, effective drugs targeting KRAS mutations have remained elusive. Consequently, standard treatment for advanced NSCLC patients with KRAS mutations typically involves chemotherapy (Ferrer et al. 2018 ). However, recent developments in immunotherapy offer promising alternatives. While not all KRAS-mutant NSCLC tumors exhibit immune-resistant phenotypes, studies suggest that immune checkpoint inhibitors (ICIs) may enhance survival rates in subsets of KRAS-mutant patients compared to traditional treatments like docetaxel (Qin et al. 2024 ; Sun et al. 2024 ). Furthermore, correlations between KRAS mutation status and tumor mutational burden (TMB), PD-L1 expression, and T-cell infiltration in NSCLC highlight the potential of immunotherapy. High T-cell infiltration and TMB, often observed in smokers with KRAS mutations, suggest a favorable response to immunotherapy in KRAS -mutant NSCLC cases associated with smoking-related lung cancer (Liu et al. 2020a , b ). These findings underscore the importance of exploring immunotherapeutic approaches tailored to KRAS -mutant NSCLC patients for improved clinical outcomes. In numerous studies, patients with KRAS mutations have demonstrated positive responses to immunotherapy (Chen et al. 2024 ; O’Sullivan et al. 2023 ). For instance, in a study evaluating nivolumab in patients with KRAS -mutated NSCLC, response rates remained consistent regardless of KRAS status, indicating that KRAS might not independently influence efficacy, although KRAS -mutated cases exhibited higher PD-L1 expression levels (Passiglia et al. 2019 ). Similarly, a subgroup analysis of the CheckMate057 trial revealed that nivolumab monotherapy conferred greater overall survival (OS) benefits compared to docetaxel monotherapy in second-line treatment for patients with KRAS mutations (Borghaei et al. 2024 ). Additionally, findings from an OS analysis within the OAK study, a randomized phase III trial, suggested potential OS advantages with atezolizumab in patients with KRAS-mutated NSCLC (Christopoulos and Thomas 2017 ). Although overall KRAS mutations may not reliably predict patient prognosis, a nuanced examination of specific subtypes and their interactions with other genetic mutations could offer deeper insights into prognosis and guide subsequent immunotherapy strategies (Sun et al. 2024 ; Cao et al. 1990 ). Given the heterogeneous nature of NSCLC and the diverse molecular subtypes of KRAS mutations, comprehensive evaluation and classification of these subtypes are imperative in clinical management (Ye et al. 2024 ; Zhao et al. 2024 ). Presently, KRAS mutation subtypes are not regarded as independent predictors of ICI response. Considering the variability in KRAS mutation subtypes, the predictive efficacy of immunotherapy may vary considerably. Therefore, this study meticulously categorized primary KRAS mutation subtypes and thoroughly analyzed their correlation with patients’ clinical data. The goal was to elucidate differences among mutation subtypes and uncover the underlying factors contributing to variations in immunotherapy outcomes across these subtypes. Patients and samplesIn this study, from January 2017 to May 2023, we included a total of 269 patients who were tested for KRAS mutations. Among them, 140 patients had KRAS mutations, and the other 129 patients without KRAS mutations served as study controls. A retrospective review was conducted on these patients’ medical records, pathology data, molecular test results, survival status and evaluations of treatment effects. The follow-up period concluded on December 31, 2023. All participants provided informed consent, agreeing to provide their medical records and relevant data for research purposes in accordance with ethical standards. The study received approval from the committee of Affiliated Cancer Hospital of Xinjiang Medical University and adhered to the principles of the Helsinki Declaration, the approval number was K-2,022,040. The tumor stage was determined according to the 8th edition of the Tumor, Node, and Metastasis (TNM) criteria, the histological classification followed the latest standards set by the World Health Organization (WHO). Targeted sequencing and bioinformatics analysisIn this study, we conducted high-throughput sequencing (NGS) on all samples to comprehensively understand the genomic variations in lung cancer patients. Different methods were employed to extract DNA depending on the sample type. For tissue and pleural fluid samples, we used the QIAamp DNA FFPE Tissue Kit from Qiagen, Germany, while peripheral blood samples utilized the QIAamp DNA Blood Mini Kit, also from Qiagen, Germany. cfDNA from plasma was extracted using the MagMAX™ Cell-Free DNA Isolation Kit from Life, USA. We estimated DNA concentration using the Qubit fluorometer and Qubit dsDNA High Sensitivity Assay Kit to ensure sufficient DNA yield for subsequent experiments. Subsequently, DNA library construction was performed using the MGIEasy Universal DNA Library Kit from MGI, China, followed by hybrid capture using the xGen Hybridization and Wash Kit from IDT, USA. Finally, paired-end sequencing with 2 × 100 bp reads was conducted on the MGISEQ-2000 platform, and the sequencing results were aligned to the human reference genome GRCh37/hg19 using BWA-MEM. SNVs and InDels were called using VarScan, and tumor mutation burden (TMB) was evaluated following the method described by Chalmers and colleagues to comprehensively understand the genetic variations in the samples. Statistical analysisFisher’s test and Chi-squared test were employed to assess the significance of differences for categorical data, while the Kruskal-Wallis test was utilized for continuous data. P values were adjusted using the Benjamini and Hochberg (BH) procedure to control the false discovery rate (FDR). Survival curves were generated using the Kaplan-Meier method, and differences in survival curves were compared using the log-rank test. Co-mutations and mutually exclusive mutations were calculated using the Maftools R package. Statistical analysis and data visualization were performed using R software (version 4.0.1). A p-value < 0.05 was considered statistically significant. Clinical characteristics of patients with KRAS mutationsIn this study, a total of 140 patients were identified with KRAS mutations. Among them, 53 patients had KRAS G12C mutations, 38 had KRAS G12D mutations, 19 had KRAS G12V mutations, and 30 had other KRAS mutation subtypes. The average age of our cohort was 65 years, with patients having a median age of 62 years at surgery, ranging from 32 to 81 years. Analysis of clinical data revealed that among all patients with available information, males exhibited a higher prevalence of KRAS mutations (63/140) compared to females (28/140). Specifically, among patients with KRAS mutation subtypes, KRAS G12C mutations were predominantly observed in males (male vs. female: 33 vs. 5), while KRAS G12D mutations were more common in females (male vs. female: 12 vs. 15). Additionally, patients with a history of smoking (51/140) were more likely to have KRAS mutations compared to those who had never smoked (39/140). Interestingly, the smoking status of patients with G12C mutations and G12D mutations showed opposing trends. Among patients with G12C mutations, there were more smokers (Ever vs. Never: 28:10), whereas among those with G12D mutations, more patients had never smoked (Ever vs. Never: 6:20). Furthermore, the majority of samples collected in this study were from patients with stage IV disease (61/140) and those receiving first-line treatment (52/140) (Table 1 ). Additional detailed clinical information can be found in Table 1 and Supplementary Table 1. Molecular characteristics of patients with KRAS mutationsIn this study, all 140 samples with KRAS mutations underwent NGS testing using a cancer-related gene panel. Among them, 90 samples were analyzed using a 616-gene panel sequencing approach, while the remaining 50 patients underwent testing with a 14-gene panel targeting lung cancer-related drug genes. The predominant KRAS mutation subtype observed was G12C, constituting 38.6% (54/140) of all KRAS mutations. The distribution of other major KRAS mutation subtypes were as follows: G12D, 27.1% (38/140); G12V, 14.0% (19/140); and G12A, 6.4% (9/140) (Fig. 1 A). Among the 90 samples analyzed using the 616-gene panel, TP53 exhibited the highest mutation frequency in the KRAS mutation group, followed by MST1 , STK11 , and KMT2C , with mutation frequencies of 56%, 13%, 12%, and 11%, respectively. Conversely, the mutation frequency of EGFR was relatively low at only 9% (Fig. 1 B).  The summary of the KRAS mutation. A . Landscape of somatic mutations identified in the 90 NSCLC patients with KRAS mutation, the top 30 genes with mutation frequency are shown on the graph. B . Pie charts of NSCLC patients with KRAS mutations. Pie charts showing the proportions of different KRAS mutation subtypes Covariation analysis revealed that the most co-mutated genes with KRAS G12C were HGF , PTEN , INHBA , and PIK3CA (Fig. 2 A). For KRAS G12D, the co-mutated gene was primarily STK11 (Fig. 2 B), while for KRAS G12V, c11orf30 and ETV6 were the co-mutated genes (Fig. 2 C). Notably, there were no mutually exclusive genes for KRAS G12C, G12D, or G12V. However, when considering the overall co-mutation status of KRAS mutations, it was observed that KRAS co-mutated with STK11 but was mutually exclusive with SETD2 and EGFR mutations (Fig. 2 D). 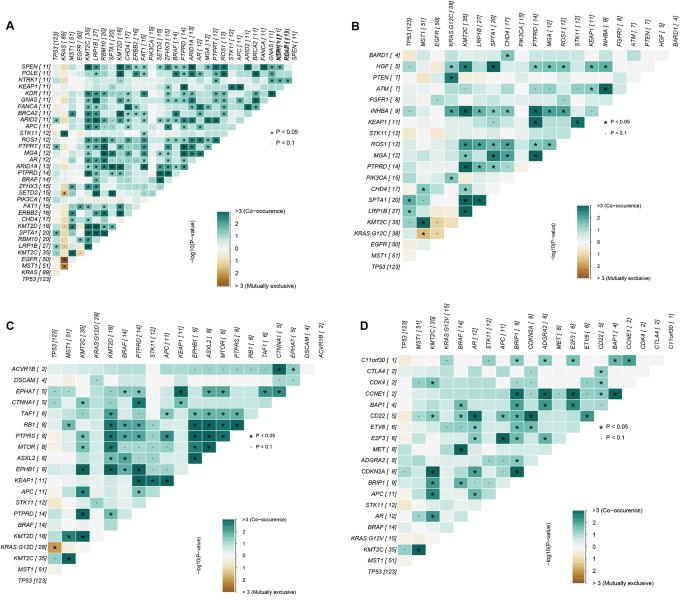 Heatmap of exclusivity and co-occurrence analysis. A . Analysis of genes with co-mutations or mutually exclusive mutations with KRAS mutation. B . Analysis of genes with co-mutations or mutually exclusive mutations with KRAS G12C mutation. C . Analysis of genes with co-mutations or mutually exclusive mutations with KRAS G12D mutations. C . Analysis of genes with co-mutations or mutually exclusive mutations with KRAS G12V mutations Correlation analysis between KRAS mutations and clinical informationBecause KRAS mutations significantly impact patient prognosis, we conducted an in-depth analysis to explore the association between KRAS mutations and patients’ clinical characteristics, aiming to identify factors predisposing certain individuals to develop KRAS mutations. Our analysis revealed that patients harboring KRAS mutations tended to be older, with a higher proportion aged ≥ 65 years compared to those without KRAS mutations, across various KRAS mutation subtypes (Fig. 3 A). Furthermore, significant gender disparities were observed within each KRAS mutation subtypes. Specifically, G12C and G12V mutations predominantly occurred in male patients, whereas G12D mutations were more prevalent among female patients (Fig. 3 B). Notably, the distribution of KRAS mutation subtypes varied among smokers, with G12C and G12V mutations predominantly observed in smokers, while G12D mutations were more common in non-smokers (Fig. 3 C). Additionally, we investigated the correlation between KRAS mutations and pathological indicators, including tumor antigen markers. Our analysis revealed that KRAS mutations were predominantly associated with lung adenocarcinoma, with lung adenocarcinoma representing the majority of KRAS mutation subtypes (Fig. 3 D). Moreover, we observed differential behavior of TTF-1, a tumor antigen marker, across different KRAS mutation subtypes (Fig. 3 E). 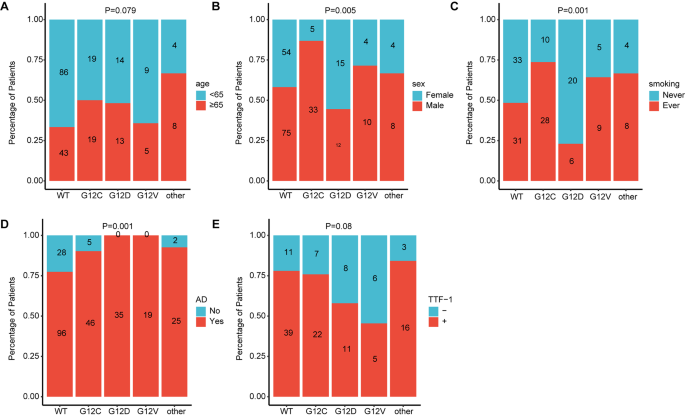 Correlation analysis between KRAS mutations and clinical information. Analysis of correlation between KRAS mutation and age (≥ 65 years and < 65 years) ( A ), gender ( B ), smoking ( C ), Pathological features ( D ) and TTF-1 ( E ) Considering the potential influence of KRAS mutations on immunotherapy efficacy, we examined the relationship between two immune-related molecular markers, PD-L1 expression and TMB, and KRAS mutation subtypes. Our findings indicated significant variations in PD-L1 expression among different KRAS mutation subtypes, G12C and G12V mutations showed a higher proportion of positive PD-L1 expression compared to G12D mutations (Fig. 4 A). Similarly, TMB levels differed significantly across various KRAS mutation subtypes, with higher TMB observed in G12C and G12V patients, while patients with G12D mutations exhibited lower TMB levels (Fig. 4 B).  PD-L1 expression and TMB analysis in different KRAS mutation subtypes. A . Number of patients with different PD-L1 expressions between KRAS mutation and non- KRAS mutation. B . Analysis of TMB in patients with KRAS mutated subtypes and non- KRAS mutated patients The impact of KRAS mutation subtypes on the prognosis of immunotherapyFollowing our investigation, we delved into the influence of distinct KRAS mutation subtypes on immunotherapy outcomes. Our survival analysis unveiled intriguing findings, particularly regarding the response to immunotherapy among patients with different KRAS mutation subtypes. Notably, individuals harboring KRAS G12C mutations demonstrated heightened sensitivity to immunotherapy, as evidenced by significantly improved progression-free survival (PFS) and overall survival (OS) compared to those without KRAS mutations. Kaplan-Meier analysis illustrated a pronounced discrepancy in prognosis between the KRAS G12C mutation group and the KRAS wild-type (WT) group, with KRAS G12C mutant patients exhibiting markedly prolonged PFS (Fig. 5 A). Specifically, the mean PFS duration for KRAS G12C mutant patients was 10.2 months, substantially longer than the 3.6 months observed for non- KRAS -mutant patients ( p = 0.009) (Fig. 5 A and Supplementary Table 2). There is also a difference in OS between patients with G12C mutations and patients without G12C mutations, the mean OS duration was 18.5 months for immunotherapy-treated KRAS G12C mutated patients, surpassing the 14.1 months observed for non- KRAS -mutated patients ( p = 0.367) (Fig. 5 B and Supplementary Table 2). Moreover, patients with KRAS G12V mutations also exhibited favorable survival outcomes following immunotherapy, with extended PFS and OS durations compared to those without KRAS mutations (Fig. 5 C and D). Although the mean PFS duration for KRAS G12V mutant patients (9.22 months) did not significantly differ from non- KRAS -mutant patients ( p = 0.066), the median OS duration was notably longer at 19.3 months compared to 14.1 months for non- KRAS -mutated patients ( p = 0.139) (Supplementary Table 2). In contrast, patients with KRAS G12D mutations displayed poorer responses to immunotherapy, the PFS and OS were shorter than that in the non- KRAS -mutant patients (Fig. 5 E and F). The mean PFS duration for KRAS G12D mutant patients was 1.7 months, significantly inferior to the 3.6 months observed for non-KRAS-mutant patients ( p = 0.03) (Fig. 5 E and Supplementary Table 2). Similarly, the mean OS was 10.8 months which was notably shorter than that 14.1 months of non- KRAS -mutated patients ( p = 0.367) (Fig. 5 F and Supplementary Table 2). However, patients with other KRAS mutation subtypes did not exhibit significant differences in survival outcomes compared to those without KRAS mutations following immunotherapy (Fig. 5 G and H and Supplementary Table 2). These findings underscore the importance of considering specific KRAS mutation subtypes in predicting immunotherapy efficacy and tailoring treatment strategies accordingly.  The Kaplan-Meier analysis for the prognostic value of KRAS mutations subtypes. A . Kaplan-Meier analyses of the PFS between the KRAS G12C mutation and WT patients who received immunotherapy. B . Kaplan-Meier analyses of the OS between the KRAS G12C mutation and WT patients who received immunotherapy. C . Kaplan-Meier analyses of the PFS between the KRAS G12CV mutation and WT patients who received immunotherapy. D . Kaplan-Meier analyses of the OS between the KRAS G12V mutation and WT patients who received immunotherapy. E . Kaplan-Meier analyses of the PFS between the KRAS G12CD mutation and WT patients who received immunotherapy. F . Kaplan-Meier analyses of the OS between the KRAS G12D mutation and WT patients who received immunotherapy. G . Kaplan-Meier analyses of the PFS between the KRAS other mutations and WT patients who received immunotherapy. H . Kaplan-Meier analyses of the OS between the KRAS other mutations and WT patients who received immunotherapy DisscussionImmunotherapy has made significant progress in treating cancers that are difficult to target and has become one of the important treatments for advanced non-small cell lung cancer (Landre et al. 2022 ). Subgroup analyses from clinical trials have highlighted that patients harboring KRAS mutations exhibit heightened sensitivity to PD-1/PD-L1 pathway inhibition therapy, leading to more favorable treatment outcomes. Compared to conventional chemotherapy alone, immunotherapy holds the potential to enhance patients’ PFS and OS (Liu et al. 2020a , b ; Rekowska et al. 2024 ). KRAS mutation-positive patients have a relatively short OS, and further evaluation of the clinical efficacy of these treatment modalities is needed (Stratmann et al. 1990 ; Moldvay and Tímár 2023 ; Kargbo 2024 ). Nevertheless, current research suggests that patients with KRAS mutations face relatively poorer prognoses compared to those without such mutations (Wang et al. 2023 ). Our study revealed that compared with patients lacking KRAS mutations, patients with KRAS G12C and G12V mutations often show longer survival when receiving immunotherapy, which can extend patients’ PFS and OS. Of particular note is the significant effect observed in patients with KRAS G12C mutations, indicating a significant improvement in survival. This phenomenon might be linked to the elevated levels of PD-L1 expression and TMB seen in KRAS G12C and G12V mutations. Conversely, patients carrying KRAS G12D mutations tend to experience inferior outcomes with immunotherapy compared to those without KRAS mutations. Notably, in our study, KRAS G12D patients exhibited lower rates of PD-L1 positive expression and relatively lower TMB levels, potentially influencing the efficacy of immunotherapy. While only a small subset of patients in our samples with KRAS mutations were diagnosed with stage I or II, the presence of KRAS mutations seems to initiate early stages of lung cancer development (Scheffler et al. 2019 ; Izar et al. 2014 ; Nadal et al. 2015 ). Moreover, the KRAS G12C mutation emerges as a promising biomarker for lung cancer immunotherapy. Recent studies have suggested that patients harboring KRAS mutations exhibit favorable responses to ICI therapy (Kaufman and Stinchcombe 2017 ; Skoulidis et al. 2015 ; Dong et al. 2017 ). Another critical aspect under discussion is the categorization of KRAS subtypes based on variations in mutation sites. Variability in mutation sites can influence downstream signaling pathways, thereby affecting overall survival outcomes (Cai et al. 2020 ). Distinct molecular subtypes of KRAS mutations yield diverse consequences, potentially impacting immune evasion mechanisms or the efficacy of immunotherapy (Ihle et al. 2012 ). Ongoing research into the treatment of KRAS mutations remains active. While KRAS mutations alone may not suffice as standalone predictors of immunotherapy outcomes in most studies, the identification of KRAS subtypes could still serve as valuable biomarkers aiding in prognosis prediction. Additionally, we observed a significant association between KRAS mutation subtypes and smoking status. The majority of smokers exhibited KRAS G12C mutations (33/38), while patients with KRAS G12V mutations also had a higher proportion of smokers (9/14). Conversely, the proportion of smokers among patients with KRAS G12D mutations was relatively small (6/26). Several studies have elucidated the link between smoking exposure and KRAS mutations, suggesting that smoking contributes to a higher antigen load, potentially influencing the effect of immunotherapy on KRAS mutations (Cao et al. 1990 ; Amanam et al. 2020 ; Chapman et al. 2016 ). In Caucasian populations, KRAS mutations are more prevalent among females and smokers (Aredo et al. 2019 ). Interestingly, some studies reported that G12C mutations were more frequent in women with a smoking history (Guan et al. 2013 ; Liu et al. 2020a , b ). However, our findings align with Guan et al., indicating that male smokers more commonly harbor KRAS mutations, including G12C mutations. This study primarily investigates the clinical correlation between KRAS mutation subtypes in patients with NSCLC and explores their impact on immunotherapy. These results can facilitate more accurate patient classification, drug development, and offer guidance for future drug usage. Nonetheless, our study has certain limitations. Firstly, the sample is derived from a single source, limiting its generalizability. Secondly, the diverse array of KRAS mutation subtypes may exhibit distinct clinical and molecular characteristics, necessitating a larger sample size for comprehensive research. Lastly, the dataset lacks clinical data for some samples, underscoring the need for future additions to enable a more thorough investigation. Our results indicate that KRAS mutation subtypes differ in their clinical and molecular characteristics. Different KRAS mutation subtypes have different immunotherapy effects. Patients with KRAS G12C and G12V mutations have better immunotherapy effects, while patients with KRAS G12D mutations have relatively poor immunotherapy effects. Data availabilityNo datasets were generated or analysed during the current study. Amanam I, Mambetsariev I, Gupta R, Achuthan S, Wang Y, Pharaon R, Massarelli E, Koczywas M, Reckamp K, Salgia R (2020) Role of immunotherapy and co-mutations on KRAS-mutant non-small cell lung cancer survival. J Thorac Disease 12(9):5086–5095 Article Google Scholar Aredo J, Padda S, Kunder C, Han S, Neal J, Shrager J, Wakelee H (2019) Impact of KRAS mutation subtype and concurrent pathogenic mutations on non-small cell lung cancer outcomes. Lung cancer (Amsterdam Netherlands) 133:144–150 Article PubMed Google Scholar Borghaei H, de Marinis F, Dumoulin D, Reynolds C, Theelen W, Percent I, Gutierrez Calderon V, Johnson M, Madroszyk-Flandin A, Garon E et al (2024) SAPPHIRE: phase III study of sitravatinib plus nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. Annals Oncology: Official J Eur Soc Med Oncol 35(1):66–76 Article CAS Google Scholar Cai D, Hu C, Li L, Deng S, Yang J, Han-Zhang H, Li M (2020) The prevalence and prognostic value of KRAS co-mutation subtypes in Chinese advanced non-small cell lung cancer patients. Cancer Med 9(1):84–93 Article PubMed CAS Google Scholar Cao H, Ma Z, Huang Q, Han H, Li Y, Zhang Y, Chen H (1990) Clinicopathologic features, concurrent genomic alterations, and clinical outcomes of patients with KRAS G12D mutations in resected lung adenocarcinoma. European journal of cancer (Oxford, England : 2024, 202:113985 Chapman A, Sun K, Ruestow P, Cowan D, Madl A (2016) Lung cancer mutation profile of EGFR, ALK, and KRAS: Meta-analysis and comparison of never and ever smokers. Lung cancer (Amsterdam Netherlands) 102:122–134 Chen J, Lu W, Chen M, Cai Z, Zhan P, Liu X, Zhu S, Ye M, Lv T, Lv J et al (2024) Efficacy of immunotherapy in patients with oncogene-driven non-small-cell lung cancer: a systematic review and meta-analysis. Therapeutic Adv Med Oncol 16:17588359231225036 Christopoulos P, Thomas M (2017) A mighty oak in the rapidly expanding field of checkpoint inhibition for NSCLC. J Thorac Disease 9(3):E292–E294 Dearden S, Stevens J, Wu Y, Blowers D (2013) Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Annals Oncology: Official J Eur Soc Med Oncol 24(9):2371–2376 Dong Z, Zhong W, Zhang X, Su J, Xie Z, Liu S, Tu H, Chen H, Sun Y, Zhou Q et al (2017) TP53Potential predictive value of and mutation status for response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin cancer Research: Official J Am Association Cancer Res 23(12):3012–3024 El Osta B, Behera M, Kim S, Berry L, Sica G, Pillai R, Owonikoko T, Kris M, Johnson B, Kwiatkowski D et al (2019) Characteristics and outcomes of patients with metastatic KRAS-Mutant lung adenocarcinomas: the Lung Cancer Mutation Consortium Experience. J Thorac Oncology: Official Publication Int Association Study Lung Cancer 14(5):876–889 Ferrer I, Zugazagoitia J, Herbertz S, John W, Paz-Ares L, Schmid-Bindert G (2018) KRAS-Mutant non-small cell lung cancer: from biology to therapy. Lung cancer (Amsterdam Netherlands) 124:53–64 Guan J, Zhong W, An S, Yang J, Su J, Chen Z, Yan H, Chen Z, Huang Z, Zhang X et al (2013) KRAS mutation in patients with lung cancer: a predictor for poor prognosis but not for EGFR-TKIs or chemotherapy. Ann Surg Oncol 20(4):1381–1388 Ihle N, Byers L, Kim E, Saintigny P, Lee J, Blumenschein G, Tsao A, Liu S, Larsen J, Wang J et al (2012) Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome. J Natl Cancer Inst 104(3):228–239 Article PubMed PubMed Central CAS Google Scholar Izar B, Zhou H, Heist R, Azzoli C, Muzikansky A, Scribner E, Bernardo L, Dias-Santagata D, Iafrate A, Lanuti M (2014) The prognostic impact of KRAS, its codon and amino acid specific mutations, on survival in resected stage I lung adenocarcinoma. J Thorac Oncology: Official Publication Int Association Study Lung Cancer 9(9):1363–1369 Jancík S, Drábek J, Radzioch D, Hajdúch M (2010) Clinical relevance of KRAS in human cancers. Journal of biomedicine & biotechnology 2010:150960 Jordan E, Kim H, Arcila M, Barron D, Chakravarty D, Gao J, Chang M, Ni A, Kundra R, Jonsson P et al (2017) Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Discov 7(6):596–609 Kargbo R (2024) Targeted combination therapies: a New Frontier in the treatment of TP53 and KRAS Mutation-Associated Cancers. ACS Med Chem Lett 15(1):15–16 Kaufman J, Stinchcombe T (2017) Treatment of KRAS-Mutant Non-small Cell Lung Cancer: the end of the beginning for targeted therapies. JAMA 317(18):1835–1837 Landre T, Justeau G, Assié J, Chouahnia K, Davoine C, Taleb C, Chouaïd C, Duchemann B (2022) Anti-PD-(L)1 for KRAS-mutant advanced non-small-cell lung cancers: a meta-analysis of randomized-controlled trials. Cancer Immunol Immunotherapy: CII 71(3):719–726 Liu C, Zheng S, Jin R, Wang X, Wang F, Zang R, Xu H, Lu Z, Huang J, Lei Y et al (2020a) The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett 470:95–105 Liu S, Sun H, Zhou J, Jie G, Xie Z, Shao Y, Zhang X, Ye J, Chen C, Zhang X et al (2020b) KRAS G12CClinical characteristics and prognostic value of the mutation in Chinese non-small cell lung cancer patients. Biomark Res 8:22 Article PubMed PubMed Central Google Scholar Malumbres M, Barbacid M (2003) RAS oncogenes: the first 30 years. Nat Rev Cancer 3(6):459–465 Moldvay J, Tímár J (2023) KRASG12C mutant lung adenocarcinoma: unique biology, novel therapies and new challenges. Pathol Oncol Research: POR 29:1611580 Nadal E, Beer D, Ramnath N (2015) KRAS-G12C mutation is associated with poor outcome in surgically resected lung adenocarcinoma. J Thorac Oncology: Official Publication Int Association Study Lung Cancer 10(2):e9–10 O’Sullivan É, Keogh A, Henderson B, Finn S, Gray S, Gately K (2023) Treatment strategies for KRAS-Mutated non-small-cell Lung Cancer. Cancers 15(6) Passiglia F, Cappuzzo F, Alabiso O, Bettini A, Bidoli P, Chiari R, Defferrari C, Delmonte A, Finocchiaro G, Francini G et al (2019) Efficacy of nivolumab in pre-treated non-small-cell lung cancer patients harbouring KRAS mutations. Br J Cancer 120(1):57–62 Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D (2011) RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer 11(11):761–774 Qin K, Wang K, Li S, Hong L, Padmakumar P, Waree R, Hubert S, Le X, Vokes N, Rai K et al (2024) Clinical Benefit from Docetaxel +/- Ramucirumab Is Not Associated with Mutation Status in Metastatic Non-Small-Cell Lung Cancer Patients Who Progressed on Platinum Doublets and Immunotherapy. Cancers 16(5) Rekowska A, Rola P, Kwiatkowska A, Wójcik-Superczyńska M, Gil M, Krawczyk P, Milanowski J (2024) Abnormalities in the KRAS Gene and Treatment options for NSCLC patients with the G12C mutation in this Gene-A Literature Review and single-center experience. Biomedicines 12(2) Scheffler M, Ihle M, Hein R, Merkelbach-Bruse S, Scheel A, Siemanowski J, Brägelmann J, Kron A, Abedpour N, Ueckeroth F et al (2019) K-ras Mutation subtypes in NSCLC and Associated co-occuring mutations in other oncogenic pathways. J Thorac Oncology: Official Publication Int Association Study Lung Cancer 14(4):606–616 Skoulidis F, Byers L, Diao L, Papadimitrakopoulou V, Tong P, Izzo J, Behrens C, Kadara H, Parra E, Canales J et al (2015) Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov 5(8):860–877 Stratmann J, Althoff F, Doebel P, Rauh J, Trummer A, Hünerlitürkoglu A, Frost N, Yildirim H, Christopoulos P, Burkhard O et al (1990) Sotorasib in KRAS G12C-mutated non-small cell lung cancer: A multicenter real-world experience from the compassionate use program in Germany. European journal of cancer (Oxford, England : 2024, 201:113911 Sun L, Handorf E, Zhou Y, Borghaei H, Aggarwal C, Bauman J (2024) Outcomes in patients treated with frontline immune checkpoint inhibition (ICI) for advanced NSCLC with KRAS mutations and STK11/KEAP1 comutations across PD-L1 levels. Lung cancer (Amsterdam Netherlands) 190:107510 Wang M, Zhang Y, Wu S, Zhang S, Shan H, Yang X, Xu X, Song L, Qu S (2023) KRASClinical outcomes of -mutant non-small cell lung cancer under untargeted therapeutic regimes in the real world: a retrospective observational study. Translational lung cancer Res 12(10):2030–2039 Ye Y, Zhang Y, Luo Y, Xu A, Ji L (2024) Identification of tumor heterogeneity associated with KRAS/TP53 co-mutation status in lung adenocarcinoma based on single-cell RNA sequencing. Am J cancer Res 14(2):655–678 Yu H, Sima C, Shen R, Kass S, Gainor J, Shaw A, Hames M, Iams W, Aston J, Lovly C et al (2015) Prognostic impact of KRAS mutation subtypes in 677 patients with metastatic lung adenocarcinomas. J Thorac Oncology: Official Publication Int Association Study Lung Cancer 10(3):431–437 Zhao L, Wang J, Zhang Y, Wang P, Lv C, Zhao S, Guo T, Li F, Gu C, Zhu Y (2024) Genomic heterogeneity of multiple synchronous lung cancers in Chinese population. Cancer Med 13(2):e6928 Download references AcknowledgementsKey Laboratory of Oncology of Xinjiang Uyghur Autonomous Region, XJKLO-2023U009, Molecular mechanism of the effect of KRAS gene mutation differentiation on immunotherapy of non-small cell lung cancer. Department of Science and Technology of Xinjiang Uygur Autonomous Region, 2022D14010, Innovative team of Xinjiang Lung Cancer Immune Response Mechanism and Immunotherapy Advantageous Population Screening and Prognosis Related Research. Author informationAuthors and affiliations. Department of Pulmonary Medicine, Affiliated Cancer Hospital of Xinjiang Medical University, No. 789 Suzhou East Street, Xincheng District, Urumqi, Xinjiang, 830011, China Guomin Gu, Chunling Liu, Xiaodan Zhu, Yan Yang & Yan Zhao Education and Research Management Office, Affiliated Cancer Hospital of Xinjiang Medical University, No. 789 Suzhou East Street, Xincheng District, Urumqi, Xinjiang, 830011, China Shuming Song Department of Breast and Thyroid Surgery, Affiliated Cancer Hospital of Xinjiang Medical University, No. 789 Suzhou East Street, Xincheng District, Urumqi, Xinjiang, 830011, China Xinjiang Cancer Center/Key Laboratory of Oncology of Xinjiang Uyghur Autonomous Region, Urumqi, Xinjiang, 830011, China You can also search for this author in PubMed Google Scholar ContributionsGG, CL and GS designed the research and supervised the study. GG, XH and YY collected clinical data and sample. GG and SS performed experiments. GG, CL and YZ analyzed the data and designed the figures. GG and CL wrote the paper. CL and GS revised the paper. All authors read and approved the final manuscript. Corresponding authorCorrespondence to Gang Sun . Ethics declarationsEthical approval. This study have been approved by the Affiliated Cancer Hospital of Xinjiang Medical University, and this study was conducted in accordance with the Declaration of Helsinki. A signed written informed consent was obtained from each patient. Consent for publicationWritten informed consent for publication was obtained from each participant. Competing interestsThe authors declare no competing interests.  Additional informationPublisher’s note. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Electronic supplementary materialBelow is the link to the electronic supplementary material. Supplementary Material 1Supplementary material 2, rights and permissions. Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ . Reprints and permissions About this articleGu, G., Liu, C., Zhu, X. et al. Clinical characteristics of KRAS mutation subtypes in non-small cell lung cancer population in Xinjiang, China, and their impact on the prognosis of immunotherapy. J Cancer Res Clin Oncol 150 , 413 (2024). https://doi.org/10.1007/s00432-024-05932-x Download citation Received : 01 August 2024 Accepted : 25 August 2024 Published : 07 September 2024 DOI : https://doi.org/10.1007/s00432-024-05932-x Share this articleAnyone you share the following link with will be able to read this content: Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative
Sign in through your institution
 What is the validity of the Federal Adverse Event Reporting System in contemporary clinical research?
Albert Ha, Ashkan P Langroudi, Michael L Eisenberg, What is the validity of the Federal Adverse Event Reporting System in contemporary clinical research?, The Journal of Sexual Medicine , Volume 21, Issue 9, September 2024, Pages 744–745, https://doi.org/10.1093/jsxmed/qdae072
The Federal Adverse Event Reporting System (FAERS) is a publicly available database sponsored by the US Food and Drug Administration (FDA) that is designed for postmarketing drug safety surveillance through reports of adverse events (AEs), medication errors, and other quality issues for drugs and therapeutic agents released to the market. Even with its limitations, the FAERS repository has played a critical role in the identification of AEs among various medications. In this essay, we examine the development of FAERS, evaluate its strengths and weaknesses within contemporary clinical research, and identify areas for future improvement. Evolution of pharmacovigilanceThe evolution of pharmacovigilance has been driven by key events and reflects the importance of drug safety and monitoring of drug-related AEs. The origins of FAERS date back to 1938, following a tragic event in which the contamination of a sulfanilamide elixir with diethyl glycol led to over 100 deaths. 1 This incident catalyzed the passage of the Federal Food, Drug, and Cosmetic Act of 1938, which empowered the FDA to begin regulating the sale of food, drugs, medical devices, and cosmetics to reduce risks of misbranding and product adulteration. 1 International Society for Sexual Medicine membersPersonal account.
Institutional accessSign in with a library card.
Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways: IP based accessTypically, access is provided across an institutional network to a range of IP addresses. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account. Choose this option to get remote access when outside your institution. Shibboleth/Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic.
If your institution is not listed or you cannot sign in to your institution’s website, please contact your librarian or administrator. Enter your library card number to sign in. If you cannot sign in, please contact your librarian. Society MembersSociety member access to a journal is achieved in one of the following ways: Sign in through society siteMany societies offer single sign-on between the society website and Oxford Academic. If you see ‘Sign in through society site’ in the sign in pane within a journal:
If you do not have a society account or have forgotten your username or password, please contact your society. Sign in using a personal accountSome societies use Oxford Academic personal accounts to provide access to their members. See below. A personal account can be used to get email alerts, save searches, purchase content, and activate subscriptions. Some societies use Oxford Academic personal accounts to provide access to their members. Viewing your signed in accountsClick the account icon in the top right to:
Signed in but can't access contentOxford Academic is home to a wide variety of products. The institutional subscription may not cover the content that you are trying to access. If you believe you should have access to that content, please contact your librarian. For librarians and administrators, your personal account also provides access to institutional account management. Here you will find options to view and activate subscriptions, manage institutional settings and access options, access usage statistics, and more. Short-term AccessTo purchase short-term access, please sign in to your personal account above. Don't already have a personal account? Register
Email alertsRelated articles in.
Citing articles via
Affiliations
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
This Feature Is Available To Subscribers OnlySign In or Create an Account This PDF is available to Subscribers Only For full access to this pdf, sign in to an existing account, or purchase an annual subscription. An official website of the United States government The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site. The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
 Clinical Trials and Clinical Research: A Comprehensive ReviewVenkataramana kandi. 1 Clinical Microbiology, Prathima Institute of Medical Sciences, Karimnagar, IND Sabitha Vadakedath2 Biochemistry, Prathima Institute of Medical Sciences, Karimnagar, IND Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management. Moreover, any research that evaluates the aspects of a disease like the symptoms, risk factors, and pathophysiology, among others may be termed clinical research. However, clinical trials are those studies that assess the potential of a therapeutic drug/device in the management, control, and prevention of disease. In view of the increasing incidences of both communicable and non-communicable diseases, and especially after the effects that Coronavirus Disease-19 (COVID-19) had on public health worldwide, the emphasis on clinical research assumes extremely essential. The knowledge of clinical research will facilitate the discovery of drugs, devices, and vaccines, thereby improving preparedness during public health emergencies. Therefore, in this review, we comprehensively describe the critical elements of clinical research that include clinical trial phases, types, and designs of clinical trials, operations of trial, audit, and management, and ethical concerns. Introduction and backgroundA clinical trial is a systematic process that is intended to find out the safety and efficacy of a drug/device in treating/preventing/diagnosing a disease or a medical condition [ 1 , 2 ]. Clinical trial includes various phases that include phase 0 (micro-dosing studies), phase 1, phase 2, phase 3, and phase 4 [ 3 ]. Phase 0 and phase 2 are called exploratory trial phases, phase 1 is termed the non-therapeutic phase, phase 3 is known as the therapeutic confirmatory phase, and phase 4 is called the post-approval or the post-marketing surveillance phase. Phase 0, also called the micro-dosing phase, was previously done in animals but now it is carried out in human volunteers to understand the dose tolerability (pharmacokinetics) before being administered as a part of the phase 1 trial among healthy individuals. The details of the clinical trial phases are shown in Table Table1 1 . This table has been created by the authors. MTD: maximum tolerated dose; SAD: single ascending dose; MAD: multiple ascending doses; NDA: new drug application; FDA: food and drug administration
Clinical research design has two major types that include non-interventional/observational and interventional/experimental studies. The non-interventional studies may have a comparator group (analytical studies like case-control and cohort studies), or without it (descriptive study). The experimental studies may be either randomized or non-randomized. Clinical trial designs are of several types that include parallel design, crossover design, factorial design, randomized withdrawal approach, adaptive design, superiority design, and non-inferiority design. The advantages and disadvantages of clinical trial designs are depicted in Table Table2 2 .
There are different types of clinical trials that include those which are conducted for treatment, prevention, early detection/screening, and diagnosis. These studies address the activities of an investigational drug on a disease and its outcomes [ 4 ]. They assess whether the drug is able to prevent the disease/condition, the ability of a device to detect/screen the disease, and the efficacy of a medical test to diagnose the disease/condition. The pictorial representation of a disease diagnosis, treatment, and prevention is depicted in Figure Figure1 1 .  This figure has been created by the authors. The clinical trial designs could be improvised to make sure that the study's validity is maintained/retained. The adaptive designs facilitate researchers to improvise during the clinical trial without interfering with the integrity and validity of the results. Moreover, it allows flexibility during the conduction of trials and the collection of data. Despite these advantages, adaptive designs have not been universally accepted among clinical researchers. This could be attributed to the low familiarity of such designs in the research community. The adaptive designs have been applied during various phases of clinical trials and for different clinical conditions [ 5 , 6 ]. The adaptive designs applied during different phases are depicted in Figure Figure2 2 .  The Bayesian adaptive trial design has gained popularity, especially during the Coronavirus Disease-19 (COVID-19) pandemic. Such designs could operate under a single master protocol. It operates as a platform trial wherein multiple treatments can be tested on different patient groups suffering from disease [ 7 ]. In this review, we comprehensively discuss the essential elements of clinical research that include the principles of clinical research, planning clinical trials, practical aspects of clinical trial operations, essentials of clinical trial applications, monitoring, and audit, clinical trial data analysis, regulatory audits, and project management, clinical trial operations at the investigation site, the essentials of clinical trial experiments involving epidemiological, and genetic studies, and ethical considerations in clinical research/trials. A clinical trial involves the study of the effect of an investigational drug/any other intervention in a defined population/participant. The clinical research includes a treatment group and a placebo wherein each group is evaluated for the efficacy of the intervention (improved/not improved) [ 8 ]. Clinical trials are broadly classified into controlled and uncontrolled trials. The uncontrolled trials are potentially biased, and the results of such research are not considered as equally as the controlled studies. Randomized controlled trials (RCTs) are considered the most effective clinical trials wherein the bias is minimized, and the results are considered reliable. There are different types of randomizations and each one has clearly defined functions as elaborated in Table Table3 3 .
Principles of clinical trial/research Clinical trials or clinical research are conducted to improve the understanding of the unknown, test a hypothesis, and perform public health-related research [ 2 , 3 ]. This is majorly carried out by collecting the data and analyzing it to derive conclusions. There are various types of clinical trials that are majorly grouped as analytical, observational, and experimental research. Clinical research can also be classified into non-directed data capture, directed data capture, and drug trials. Clinical research could be prospective or retrospective. It may also be a case-control study or a cohort study. Clinical trials may be initiated to find treatment, prevent, observe, and diagnose a disease or a medical condition. Among the various types of clinical research, observational research using a cross-sectional study design is the most frequently performed clinical research. This type of research is undertaken to analyze the presence or absence of a disease/condition, potential risk factors, and prevalence and incidence rates in a defined population. Clinical trials may be therapeutic or non-therapeutic type depending on the type of intervention. The therapeutic type of clinical trial uses a drug that may be beneficial to the patient. Whereas in a non-therapeutic clinical trial, the participant does not benefit from the drug. The non-therapeutic trials provide additional knowledge of the drug for future improvements. Different terminologies of clinical trials are delineated in Table Table4 4 .
In view of the increased cost of the drug discovery process, developing, and low-income countries depend on the production of generic drugs. The generic drugs are similar in composition to the patented/branded drug. Once the patent period is expired generic drugs can be manufactured which have a similar quality, strength, and safety as the patented drug [ 9 ]. The regulatory requirements and the drug production process are almost the same for the branded and the generic drug according to the Food and Drug Administration (FDA), United States of America (USA). The bioequivalence (BE) studies review the absorption, distribution, metabolism, and excretion (ADME) of the generic drug. These studies compare the concentration of the drug at the desired location in the human body, called the peak concentration of the drug (Cmax). The extent of absorption of the drug is measured using the area under the receiver operating characteristic curve (AUC), wherein the generic drug is supposed to demonstrate similar ADME activities as the branded drug. The BE studies may be undertaken in vitro (fasting, non-fasting, sprinkled fasting) or in vivo studies (clinical, bioanalytical, and statistical) [ 9 ]. Planning clinical trial/research The clinical trial process involves protocol development, designing a case record/report form (CRF), and functioning of institutional review boards (IRBs). It also includes data management and the monitoring of clinical trial site activities. The CRF is the most significant document in a clinical study. It contains the information collected by the investigator about each subject participating in a clinical study/trial. According to the International Council for Harmonisation (ICH), the CRF can be printed, optical, or an electronic document that is used to record the safety and efficacy of the pharmaceutical drug/product in the test subjects. This information is intended for the sponsor who initiates the clinical study [ 10 ]. The CRF is designed as per the protocol and later it is thoroughly reviewed for its correctness (appropriate and structured questions) and finalized. The CRF then proceeds toward the print taking the language of the participating subjects into consideration. Once the CRF is printed, it is distributed to the investigation sites where it is filled with the details of the participating subjects by the investigator/nurse/subject/guardian of the subject/technician/consultant/monitors/pharmacist/pharmacokinetics/contract house staff. The filled CRFs are checked for their completeness and transported to the sponsor [ 11 ]. Effective planning and implementation of a clinical study/trial will influence its success. The clinical study majorly includes the collection and distribution of the trial data, which is done by the clinical data management section. The project manager is crucial to effectively plan, organize, and use the best processes to control and monitor the clinical study [ 10 , 11 ]. The clinical study is conducted by a sponsor or a clinical research organization (CRO). A perfect protocol, time limits, and regulatory requirements assume significance while planning a clinical trial. What, when, how, and who are clearly planned before the initiation of a study trial. Regular review of the project using the bar and Gantt charts, and maintaining the timelines assume increased significance for success with the product (study report, statistical report, database) [ 10 , 11 ]. The steps critical to planning a clinical trial include the idea, review of the available literature, identifying a problem, formulating the hypothesis, writing a synopsis, identifying the investigators, writing a protocol, finding a source of funding, designing a patient consent form, forming ethics boards, identifying an organization, preparing manuals for procedures, quality assurance, investigator training and initiation of the trial by recruiting the participants [ 10 ]. The two most important points to consider before the initiation of the clinical trial include whether there is a need for a clinical trial, if there is a need, then one must make sure that the study design and methodology are strong for the results to be reliable to the people [ 11 ]. For clinical research to envisage high-quality results, the study design, implementation of the study, quality assurance in data collection, and alleviation of bias and confounding factors must be robust [ 12 ]. Another important aspect of conducting a clinical trial is improved management of various elements of clinical research that include human and financial resources. The role of a trial manager to make a successful clinical trial was previously reported. The trial manager could play a key role in planning, coordinating, and successfully executing the trial. Some qualities of a trial manager include better communication and motivation, leadership, and strategic, tactical, and operational skills [ 13 ]. Practical aspects of a clinical trial operations There are different types of clinical research. Research in the development of a novel drug could be initiated by nationally funded research, industry-sponsored research, and clinical research initiated by individuals/investigators. According to the documents 21 code of federal regulations (CFR) 312.3 and ICH E-6 Good Clinical Practice (GCP) 1.54, an investigator is an individual who initiates and conducts clinical research [ 14 ]. The investigator plan, design, conduct, monitor, manage data, compile reports, and supervise research-related regulatory and ethical issues. To manage a successful clinical trial project, it is essential for an investigator to give the letter of intent, write a proposal, set a timeline, develop a protocol and related documents like the case record forms, define the budget, and identify the funding sources. Other major steps of clinical research include the approval of IRBs, conduction and supervision of the research, data review, and analysis. Successful clinical research includes various essential elements like a letter of intent which is the evidence that supports the interest of the researcher to conduct drug research, timeline, funding source, supplier, and participant characters. Quality assurance, according to the ICH and GCP guidelines, is necessary to be implemented during clinical research to generate quality and accurate data. Each element of the clinical research must have been carried out according to the standard operating procedure (SOP), which is written/determined before the initiation of the study and during the preparation of the protocol [ 15 ]. The audit team (quality assurance group) is instrumental in determining the authenticity of the clinical research. The audit, according to the ICH and GCP, is an independent and external team that examines the process (recording the CRF, analysis of data, and interpretation of data) of clinical research. The quality assurance personnel are adequately trained, become trainers if needed, should be good communicators, and must handle any kind of situation. The audits can be at the investigator sites evaluating the CRF data, the protocol, and the personnel involved in clinical research (source data verification, monitors) [ 16 ]. Clinical trial operations are governed by legal and regulatory requirements, based on GCPs, and the application of science, technology, and interpersonal skills [ 17 ]. Clinical trial operations are complex, time and resource-specific that requires extensive planning and coordination, especially for the research which is conducted at multiple trial centers [ 18 ]. Recruiting the clinical trial participants/subjects is the most significant aspect of clinical trial operations. Previous research had noted that most clinical trials do not meet the participant numbers as decided in the protocol. Therefore, it is important to identify the potential barriers to patient recruitment [ 19 ]. Most clinical trials demand huge costs, increased timelines, and resources. Randomized clinical trial studies from Switzerland were analyzed for their costs which revealed approximately 72000 USD for a clinical trial to be completed. This study emphasized the need for increased transparency with respect to the costs associated with the clinical trial and improved collaboration between collaborators and stakeholders [ 20 ]. Clinical trial applications, monitoring, and audit Among the most significant aspects of a clinical trial is the audit. An audit is a systematic process of evaluating the clinical trial operations at the site. The audit ensures that the clinical trial process is conducted according to the protocol, and predefined quality system procedures, following GCP guidelines, and according to the requirements of regulatory authorities [ 21 ]. The auditors are supposed to be independent and work without the involvement of the sponsors, CROs, or personnel at the trial site. The auditors ensure that the trial is conducted by designated professionally qualified, adequately trained personnel, with predefined responsibilities. The auditors also ensure the validity of the investigational drug, and the composition, and functioning of institutional review/ethics committees. The availability and correctness of the documents like the investigational broacher, informed consent forms, CRFs, approval letters of the regulatory authorities, and accreditation of the trial labs/sites [ 21 ]. The data management systems, the data collection software, data backup, recovery, and contingency plans, alternative data recording methods, security of the data, personnel training in data entry, and the statistical methods used to analyze the results of the trial are other important responsibilities of the auditor [ 21 , 22 ]. According to the ICH-GCP Sec 1.29 guidelines the inspection may be described as an act by the regulatory authorities to conduct an official review of the clinical trial-related documents, personnel (sponsor, investigator), and the trial site [ 21 , 22 ]. The summary report of the observations of the inspectors is performed using various forms as listed in Table Table5 5 . FDA: Food and Drug Administration; IND: investigational new drug; NDA: new drug application; IRB: institutional review board; CFR: code of federal regulations
Because protecting data integrity, the rights, safety, and well-being of the study participants are more significant while conducting a clinical trial, regular monitoring and audit of the process appear crucial. Also, the quality of the clinical trial greatly depends on the approach of the trial personnel which includes the sponsors and investigators [ 21 ]. The responsibility of monitoring lies in different hands, and it depends on the clinical trial site. When the trial is initiated by a pharmaceutical industry, the responsibility of trial monitoring depends on the company or the sponsor, and when the trial is conducted by an academic organization, the responsibility lies with the principal investigator [ 21 ]. An audit is a process conducted by an independent body to ensure the quality of the study. Basically, an audit is a quality assurance process that determines if a study is carried out by following the SPOs, in compliance with the GCPs recommended by regulatory bodies like the ICH, FDA, and other local bodies [ 21 ]. An audit is performed to review all the available documents related to the IRB approval, investigational drug, and the documents related to the patient care/case record forms. Other documents that are audited include the protocol (date, sign, treatment, compliance), informed consent form, treatment response/outcome, toxic response/adverse event recording, and the accuracy of data entry [ 22 ]. Clinical trial data analysis, regulatory audits, and project management The essential elements of clinical trial management systems (CDMS) include the management of the study, the site, staff, subject, contracts, data, and document management, patient diary integration, medical coding, monitoring, adverse event reporting, supplier management, lab data, external interfaces, and randomization. The CDMS involves setting a defined start and finishing time, defining study objectives, setting enrolment and termination criteria, commenting, and managing the study design [ 23 ]. Among the various key application areas of clinical trial systems, the data analysis assumes increased significance. The clinical trial data collected at the site in the form of case record form is stored in the CDMS ensuring the errors with respect to the double data entry are minimized. Clinical trial data management uses medical coding, which uses terminologies with respect to the medications and adverse events/serious adverse events that need to be entered into the CDMS. The project undertaken to conduct the clinical trial must be predetermined with timelines and milestones. Timelines are usually set for the preparation of protocol, designing the CRF, planning the project, identifying the first subject, and timelines for recording the patient’s data for the first visit. The timelines also are set for the last subject to be recruited in the study, the CRF of the last subject, and the locked period after the last subject entry. The planning of the project also includes the modes of collection of the data, the methods of the transport of the CRFs, patient diaries, and records of severe adverse events, to the central data management sites (fax, scan, courier, etc.) [ 24 ]. The preparation of SOPs and the type and timing of the quality control (QC) procedures are also included in the project planning before the start of a clinical study. Review (budget, resources, quality of process, assessment), measure (turnaround times, training issues), and control (CRF collection and delivery, incentives, revising the process) are the three important aspects of the implementation of a clinical research project. In view of the increasing complexity related to the conduct of clinical trials, it is important to perform a clinical quality assurance (CQA) audit. The CQA audit process consists of a detailed plan for conducting audits, points of improvement, generating meaningful audit results, verifying SOP, and regulatory compliance, and promoting improvement in clinical trial research [ 25 ]. All the components of a CQA audit are delineated in Table Table6 6 . CRF: case report form; CSR: clinical study report; IC: informed consent; PV: pharmacovigilance; SAE: serious adverse event
Clinical trial operations at the investigator's site The selection of an investigation site is important before starting a clinical trial. It is essential that the individuals recruited for the study meet the inclusion criteria of the trial, and the investigator's and patient's willingness to accept the protocol design and the timelines set by the regulatory authorities including the IRBs. Before conducting clinical research, it is important for an investigator to agree to the terms and conditions of the agreement and maintain the confidentiality of the protocol. Evaluation of the protocol for the feasibility of its practices with respect to the resources, infrastructure, qualified and trained personnel available, availability of the study subjects, and benefit to the institution and the investigator is done by the sponsor during the site selection visit. The standards of a clinical research trial are ensured by the Council for International Organizations of Medical Sciences (CIOMS), National Bioethics Advisory Commission (NBAC), United Nations Programme on Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome (HIV/AIDS) (UNAIDS), and World Medical Association (WMA) [ 26 ]. Recommendations for conducting clinical research based on the WMA support the slogan that says, “The health of my patient will be my first consideration.” According to the International Code of Medical Ethics (ICME), no human should be physically or mentally harmed during the clinical trial, and the study should be conducted in the best interest of the person [ 26 ]. Basic principles recommended by the Helsinki declaration include the conduction of clinical research only after the prior proof of the safety of the drug in animal and lab experiments. The clinical trials must be performed by scientifically, and medically qualified and well-trained personnel. Also, it is important to analyze the benefit of research over harm to the participants before initiating the drug trials. The doctors may prescribe a drug to alleviate the suffering of the patient, save the patient from death, and gain additional knowledge of the drug only after obtaining informed consent. Under the equipoise principle, the investigators must be able to justify the treatment provided as a part of the clinical trial, wherein the patient in the placebo arm may be harmed due to the unavailability of the therapeutic/trial drug. Clinical trial operations greatly depend on the environmental conditions and geographical attributes of the trial site. It may influence the costs and targets defined by the project before the initiation. It was noted that one-fourth of the clinical trial project proposals/applications submit critical data on the investigational drug from outside the country. Also, it was noted that almost 35% of delays in clinical trials owing to patient recruitment with one-third of studies enrolling only 5% of the participants [ 27 ]. It was suggested that clinical trial feasibility assessment in a defined geographical region may be undertaken for improved chances of success. Points to be considered under the feasibility assessment program include if the disease under the study is related to the population of the geographical region, appropriateness of the study design, patient, and comparator group, visit intervals, potential regulatory and ethical challenges, and commitments of the study partners, CROs in respective countries (multi-centric studies) [ 27 ]. Feasibility assessments may be undertaken at the program level (ethics, regulatory, and medical preparedness), study level (clinical, regulatory, technical, and operational aspects), and at the investigation site (investigational drug, competency of personnel, participant recruitment, and retention, quality systems, and infrastructural aspects) [ 27 ]. Clinical trials: true experiments In accordance with the revised schedule "Y" of the Drugs and Cosmetics Act (DCA) (2005), a drug trial may be defined as a systematic study of a novel drug component. The clinical trials aim to evaluate the pharmacodynamic, and pharmacokinetic properties including ADME, efficacy, and safety of new drugs. According to the drug and cosmetic rules (DCR), 1945, a new chemical entity (NCE) may be defined as a novel drug approved for a disease/condition, in a specified route, and at a particular dosage. It also may be a new drug combination, of previously approved drugs. A clinical trial may be performed in three types; one that is done to find the efficacy of an NCE, a comparison study of two drugs against a medical condition, and the clinical research of approved drugs on a disease/condition. Also, studies of the bioavailability and BE studies of the generic drugs, and the drugs already approved in other countries are done to establish the efficacy of new drugs [ 28 ]. Apart from the discovery of a novel drug, clinical trials are also conducted to approve novel medical devices for public use. A medical device is defined as any instrument, apparatus, appliance, software, and any other material used for diagnostic/therapeutic purposes. The medical devices may be divided into three classes wherein class I uses general controls; class II uses general and special controls, and class III uses general, special controls, and premarket approvals [ 28 ]. The premarket approval applications ensure the safety and effectiveness, and confirmation of the activities from bench to animal to human clinical studies. The FDA approval for investigational device exemption (IDE) for a device not approved for a new indication/disease/condition. There are two types of IDE studies that include the feasibility study (basic safety and potential effectiveness) and the pivotal study (trial endpoints, randomization, monitoring, and statistical analysis plan) [ 28 ]. As evidenced by the available literature, there are two types of research that include observational and experimental research. Experimental research is alternatively known as the true type of research wherein the research is conducted by the intervention of a new drug/device/method (educational research). Most true experiments use randomized control trials that remove bias and neutralize the confounding variables that may interfere with the results of research [ 28 ]. The variables that may interfere with the study results are independent variables also called prediction variables (the intervention), dependent variables (the outcome), and extraneous variables (other confounding factors that could influence the outside). True experiments have three basic elements that include manipulation (that influence independent variables), control (over extraneous influencers), and randomization (unbiased grouping) [ 29 ]. Experiments can also be grouped as true, quasi-experimental, and non-experimental studies depending on the presence of specific characteristic features. True experiments have all three elements of study design (manipulation, control, randomization), and prospective, and have great scientific validity. Quasi-experiments generally have two elements of design (manipulation and control), are prospective, and have moderate scientific validity. The non-experimental studies lack manipulation, control, and randomization, are generally retrospective, and have low scientific validity [ 29 ]. Clinical trials: epidemiological and human genetics study Epidemiological studies are intended to control health issues by understanding the distribution, determinants, incidence, prevalence, and impact on health among a defined population. Such studies are attempted to perceive the status of infectious diseases as well as non-communicable diseases [ 30 ]. Experimental studies are of two types that include observational (cross-sectional studies (surveys), case-control studies, and cohort studies) and experimental studies (randomized control studies) [ 3 , 31 ]. Such research may pose challenges related to ethics in relation to the social and cultural milieu. Biomedical research related to human genetics and transplantation research poses an increased threat to ethical concerns, especially after the success of the human genome project (HGP) in the year 2000. The benefits of human genetic studies are innumerable that include the identification of genetic diseases, in vitro fertilization, and regeneration therapy. Research related to human genetics poses ethical, legal, and social issues (ELSI) that need to be appropriately addressed. Most importantly, these genetic research studies use advanced technologies which should be equally available to both economically well-placed and financially deprived people [ 32 ]. Gene therapy and genetic manipulations may potentially precipitate conflict of interest among the family members. The research on genetics may be of various types that include pedigree studies (identifying abnormal gene carriers), genetic screening (for diseases that may be heritable by the children), gene therapeutics (gene replacement therapy, gene construct administration), HGP (sequencing the whole human genome/deoxyribonucleic acid (DNA) fingerprinting), and DNA, cell-line banking/repository [ 33 ]. The biobanks are established to collect and store human tissue samples like umbilical tissue, cord blood, and others [ 34 ]. Epidemiological studies on genetics are attempts to understand the prevalence of diseases that may be transmitted among families. The classical epidemiological studies may include single case observations (one individual), case series (< 10 individuals), ecological studies (population/large group of people), cross-sectional studies (defined number of individuals), case-control studies (defined number of individuals), cohort (defined number of individuals), and interventional studies (defined number of individuals) [ 35 ]. Genetic studies are of different types that include familial aggregation (case-parent, case-parent-grandparent), heritability (study of twins), segregation (pedigree study), linkage study (case-control), association, linkage, disequilibrium, cohort case-only studies (related case-control, unrelated case-control, exposure, non-exposure group, case group), cross-sectional studies, association cohort (related case-control, familial cohort), and experimental retrospective cohort (clinical trial, exposure, and non-exposure group) [ 35 ]. Ethics and concerns in clinical trial/research Because clinical research involves animals and human participants, adhering to ethics and ethical practices assumes increased significance [ 36 ]. In view of the unethical research conducted on war soldiers after the Second World War, the Nuremberg code was introduced in 1947, which promulgated rules for permissible medical experiments on humans. The Nuremberg code suggests that informed consent is mandatory for all the participants in a clinical trial, and the study subjects must be made aware of the nature, duration, and purpose of the study, and potential health hazards (foreseen and unforeseen). The study subjects should have the liberty to withdraw at any time during the trial and to choose a physician upon medical emergency. The other essential principles of clinical research involving human subjects as suggested by the Nuremberg code included benefit to the society, justification of study as noted by the results of the drug experiments on animals, avoiding even minimal suffering to the study participants, and making sure that the participants don’t have life risk, humanity first, improved medical facilities for participants, and suitably qualified investigators [ 37 ]. During the 18th world medical assembly meeting in the year 1964, in Helsinki, Finland, ethical principles for doctors practicing research were proposed. Declaration of Helsinki, as it is known made sure that the interests and concerns of the human participants will always prevail over the interests of the society. Later in 1974, the National Research Act was proposed which made sure that the research proposals are thoroughly screened by the Institutional ethics/Review Board. In 1979, the April 18th Belmont report was proposed by the national commission for the protection of human rights during biomedical and behavioral research. The Belmont report proposed three core principles during research involving human participants that include respect for persons, beneficence, and justice. The ICH laid down GCP guidelines [ 38 ]. These guidelines are universally followed throughout the world during the conduction of clinical research involving human participants. ICH was first founded in 1991, in Brussels, under the umbrella of the USA, Japan, and European countries. The ICH conference is conducted once every two years with the participation from the member countries, observers from the regulatory agencies, like the World Health Organization (WHO), European Free Trade Association (EFTA), and the Canadian Health Protection Branch, and other interested stakeholders from the academia and the industry. The expert working groups of the ICH ensure the quality, efficacy, and safety of the medicinal product (drug/device). Despite the availability of the Nuremberg code, the Belmont Report, and the ICH-GCP guidelines, in the year 1982, International Ethical Guidelines for Biomedical Research Involving Human Subjects was proposed by the CIOMS in association with WHO [ 39 ]. The CIOMS protects the rights of the vulnerable population, and ensures ethical practices during clinical research, especially in underdeveloped countries [ 40 ]. In India, the ethical principles for biomedical research involving human subjects were introduced by the Indian Council of Medical Research (ICMR) in the year 2000 and were later amended in the year 2006 [ 41 ]. Clinical trial approvals can only be done by the IRB approved by the Drug Controller General of India (DGCI) as proposed in the year 2013 [ 42 ]. Current perspectives and future implications A recent study attempted to evaluate the efficacy of adaptive clinical trials in predicting the success of a clinical trial drug that entered phase 3 and minimizing the time and cost of drug development. This study highlighted the drawbacks of such clinical trial designs that include the possibility of type 1 (false positive) and type 2 (false negative) errors [ 43 ]. The usefulness of animal studies during the preclinical phases of a clinical trial was evaluated in a previous study which concluded that animal studies may not completely guarantee the safety of the investigational drug. This is noted by the fact that many drugs which passed toxicity tests in animals produced adverse reactions in humans [ 44 ]. The significance of BE studies to compare branded and generic drugs was reported previously. The pharmacokinetic BE studies of Amoxycillin comparing branded and generic drugs were carried out among a group of healthy participants. The study results have demonstrated that the generic drug had lower Cmax as compared to the branded drug [ 45 ]. To establish the BE of the generic drugs, randomized crossover trials are carried out to assess the Cmax and the AUC. The ratio of each pharmacokinetic characteristic must match the ratio of AUC and/or Cmax, 1:1=1 for a generic drug to be considered as a bioequivalent to a branded drug [ 46 ]. Although the generic drug development is comparatively more beneficial than the branded drugs, synthesis of extended-release formulations of the generic drug appears to be complex. Since the extended-release formulations remain for longer periods in the stomach, they may be influenced by gastric acidity and interact with the food. A recent study suggested the use of bio-relevant dissolution tests to increase the successful production of generic extended-release drug formulations [ 47 ]. Although RCTs are considered the best designs, which rule out bias and the data/results obtained from such clinical research are the most reliable, RCTs may be plagued by miscalculation of the treatment outcomes/bias, problems of cointerventions, and contaminations [ 48 ]. The perception of healthcare providers regarding branded drugs and their view about the generic equivalents was recently analyzed and reported. It was noted that such a perception may be attributed to the flexible regulatory requirements for the approval of a generic drug as compared to a branded drug. Also, could be because a switch from a branded drug to a generic drug in patients may precipitate adverse events as evidenced by previous reports [ 49 ]. Because the vulnerable population like drug/alcohol addicts, mentally challenged people, children, geriatric age people, military persons, ethnic minorities, people suffering from incurable diseases, students, employees, and pregnant women cannot make decisions with respect to participating in a clinical trial, ethical concerns, and legal issues may prop up, that may be appropriately addressed before drug trials which include such groups [ 50 ]. ConclusionsClinical research and clinical trials are important from the public health perspective. Clinical research facilitates scientists, public health administrations, and people to increase their understanding and improve preparedness with reference to the diseases prevalent in different geographical regions of the world. Moreover, clinical research helps in mitigating health-related problems as evidenced by the current Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) pandemic and other emerging and re-emerging microbial infections. Clinical trials are crucial to the development of drugs, devices, and vaccines. Therefore, scientists are required to be up to date with the process and procedures of clinical research and trials as discussed comprehensively in this review. The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus. The authors have declared that no competing interests exist. Journal of Medical Case ReportsIn the era of evidence-based practice, we need practice-based evidence. The basis of this evidence is the detailed information from the case reports of individual people which informs both our clinical research and our daily clinical care. Each case report published in this journal adds valuable new information to our medical knowledge. Prof Michael Kidd AO, Editor-in- Chief  Join the Editorial BoardWe are recruiting Associate Editors to join our Editorial Board. Learn more about the role and how to apply here ! 
Get to know the Editors behind Journal of Medical Case Reports !  megaflopp / Getty Images / iStock Requirements for case reports submitted to JMCR• Patient ethnicity must be included in the Abstract under the Case Presentation section. • Consent for publication is a mandatory journal requirement for all case reports . Written informed consent for publication must be obtained from the patient (or their parent or legal guardian in the case of children under 18, or from the next of kin if the patient has died). For more information, please see our editorial policies . Report of the MonthSuperior mesenteric vein thrombosis due to covid-19 vaccination. Vaccines have made a significant contribute to sowing the spread of the COVID-19 infection. However, side effects of the vaccination are beginning to appear, and one of which, thrombosis, is a particular problem as it can cuase serious complications. While cases of splanchnic venous thrombosis (SVT) after ChAdOx1 nCoV-19 vaccinations have been reported, cases of SVT mRNA-1273 vaccines are rare. In this case report, clinicians describe a patient presenting with superior mesentric vein thrombosis following a COVID-19 vaccination, and examine the relationship between the mRNA-1273 vaccines and intestinal ischemia.
Traumatic encephalocele in the nasal cavity after 6 years of trauma: a case reportAuthors: Kenana Tawashi, Ayham Qatza, Ahmed Sheikh Sobeh and Nizar Sheekh Ahmad Familial adenomatous polyposis: a case reportAuthors: Endeshaw Asaye Kindie, Tigist Desta Beyera, Ephrem Tafesse Teferi, Daniel Zemenfes Ashebir and Henok Bahru Wodajeneh Penetrating cardiac injury after percutaneous breast core-needle biopsy, unusual life-threatening complication: a case reportAuthors: Amonpon Kanlerd, Sasithorn Sujarittanakarn and Wanrudee Lohitvisate Progressive familial intrahepatic cholestasis type 4: a case reportAuthors: Mohamed Abdelmalak Abokandil, Saber Waheeb, Wessam Zaghloul, Manal Abdelgawad, Mona Abdelhady, Mohamed Mansy and Mostafa Kotb Preserving insulin function in diabetes: a case reportAuthors: Masaru Oota Most recent articles RSS View all articles An itchy erythematous papular skin rash as a possible early sign of COVID-19: a case reportAuthors: Alice Serafini, Peter Konstantin Kurotschka, Mariabeatrice Bertolani and Silvia Riccomi Red ear syndrome precipitated by a dietary trigger: a case reportAuthors: Chung Chi Chan and Susmita Ghosh How to choose the best journal for your case reportAuthors: Richard A. Rison, Jennifer Kelly Shepphird and Michael R. Kidd The Erratum to this article has been published in Journal of Medical Case Reports 2017 11 :287 COVID-19 with repeated positive test results for SARS-CoV-2 by PCR and then negative test results twice during intensive care: a case reportAuthors: Masafumi Kanamoto, Masaru Tobe, Tomonori Takazawa and Shigeru Saito Recurrent knee arthritis diagnosed as juvenile idiopathic arthritis with a 10-year asymptomatic period after arthroscopic synovectomy: a case reportAuthors: Atsushi Teramoto, Kota Watanabe, Yuichiro Kii, Miki Kudo, Hidenori Otsubo, Takuro Wada and Toshihiko Yamashita Most accessed articles RSS A Guide to Writing and Using Case ReportsThis thematic series, published in 2016, provides a valuable resource for clinicians who are considered producing a case report. It comprises of a special editorial series of guides on writing, reviewing and using case reports.  Aims and scopeJournal of Medical Case Reports will consider any original case report that expands the field of general medical knowledge, and original research relating to case reports. Case reports should show one of the following:
Suitable research articles include but are not limited to: N of 1 trials, meta-analyses of published case reports, research addressing the use of case reports and the prevalence or importance of case reporting in the medical literature and retrospective studies that include case-specific information (age, sex and ethnicity) for all patients. Article accessesThroughout 2022, articles were accessed from the journal website more than 4.17 million times; an average of over 11 ,400 accesses per day. Latest TweetsYour browser needs to have JavaScript enabled to view this timeline Peer Review Mentoring SchemeThe Editors at Journal of Medical Case Reports endorse peer review mentoring of early career researchers. If you are a senior researcher or professor and supervise an early career researcher with the appropriate expertise, we invite you to co-write and mentor them through the peer review process. Find out how to express your interest in the scheme here . Call for PapersThe Journal of Medical Case Reports is calling for submissions to our Collection on COVID-19 – a look at the past, present and future of the pandemic . Guest Edited by Dr. Jean Karl Soler, The Family Practice Malta, Malta  About the Editor-in-ChiefProfessor Michael Kidd AO FAHMS is foundation Director of the Centre for Future Health Systems at the University of New South Wales in Sydney, Australia, and Professor of Global Primary Care and Future Health Systems with the Nuffield Department of Primary Care Health Sciences at the University of Oxford. During the COVID-19 pandemic, Prof Kidd was the Deputy Chief Medical Officer and Principal Medical Advisor with the Australian Government Department of Health and Aged Care, and Professor of Primary Care Reform at the Australian National University. He holds honorary appointments with the University of Toronto, the University of Melbourne, Flinders University, and the Murdoch Children's Research Institute, and is the Emeritus Director of the World Health Organization Collaborating Centre on Family Medicine and Primary Care. He is an elected Fellow of the Australian Academy of Health and Medical Sciences (FAHMS). In the 2023 King's Birthday Honours List he was made an Officer of the Order of Australia. Prof Kidd served as president of the World Organization of Family Doctors (WONCA) from 2013-2016, and as president of the Royal Australian College of General Practitioners from 2002-2006. He is the founder and Editor-in-Chief of the Journal of Medical Case Reports, the world's first PubMed-listed journal devoted to publishing case reports from all medical disciplines.
Annual Journal MetricsCitation Impact 2023 Journal Impact Factor: 0.9 5-year Journal Impact Factor: N/A Source Normalized Impact per Paper (SNIP): 0.591 SCImago Journal Rank (SJR): 0.304 Speed 2023 Submission to first editorial decision (median days): 33 Submission to acceptance (median days): 148 Usage 2023 Downloads: 4,048,208 Altmetric mentions: 2,745

ISSN: 1752-1947
 Perspectives in Clinical Research
Secondary LogoJournal logo, current issue.  Jul-Sep 2024 - Volume 15 - Issue 3
Colleague's E-mail is Invalid Your message has been successfully sent to your colleague. Save my selection Current Issue HighlightsClinical trial footprint in brics: improvements seen but needs further affirmative action. Perspectives in Clinical Research. 15(3):105-107, Jul-Sep 2024.
 Artificial intelligence at the pen’s edge: Exploring the ethical quagmires in using artificial intelligence models like ChatGPT for assisted writing in biomedical researchPerspectives in Clinical Research. 15(3):108-115, Jul-Sep 2024.
Go to Full Text of this Article  Bio-entrepreneurs’ bugbear: Regulatory rigmarolePerspectives in Clinical Research. 15(3):122-127, Jul-Sep 2024.
 Clinical trial trends over the last 5 years among the BRICS (Brazil, Russia, India, China, and South Africa) nationsPerspectives in Clinical Research. 15(3):128-133, Jul-Sep 2024.
Ethical considerations for real-world evidence studiesPerspectives in Clinical Research. 15(3):152-154, Jul-Sep 2024. Sample size calculation in clinical researchPerspectives in Clinical Research. 15(3):155-159, Jul-Sep 2024.
Experience of participating in community-based clinical trials from rural Maharashtra: Analysis of over 4000 participant feedback formsPerspectives in Clinical Research. 15(3):160-161, Jul-Sep 2024. Information
InitiativesYou are accessing a machine-readable page. In order to be human-readable, please install an RSS reader. All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess . Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers. Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal. Original Submission Date Received: .
 Article Menu
Find support for a specific problem in the support section of our website. Please let us know what you think of our products and services. Visit our dedicated information section to learn more about MDPI. JSmol ViewerCorrelating histopathological microscopic images of creutzfeldt–jakob disease with clinical typology using graph theory and artificial intelligence.  1. Introduction2. background and materials, 2.1. dataset, 2.2. pipeline components for feature extraction, 2.2.1. gaussian filter, 2.2.2. roundness criteria.
2.3. Graph Theory for Feature Extraction
3. A New Method for the Automatic Feature Extraction and Classification of Creutzfeldt–Jakob Disease3.1. image processing pipeline, 3.1.1. preprocessing, 3.1.2. identification of a set of vacuoles, 3.2. feature extraction.
3.3. Coding Details4.1. spongiosis segmentation, 4.2. feature extraction, 4.3. correlation of features with clinical variables, 4.3.1. age and days of evolution, 4.3.2. sex and panencephalic form, 5. discussion, 6. conclusions, supplementary materials, author contributions, institutional review board statement, informed consent statement, data availability statement, acknowledgments, conflicts of interest, abbreviations.
Click here to enlarge figure
Share and CiteMartínez, C.; Teijeira, S.; Domínguez, P.; Campanioni, S.; Busto, L.; González-Nóvoa, J.A.; Alonso, J.; Poveda, E.; San Millán, B.; Veiga, C. Correlating Histopathological Microscopic Images of Creutzfeldt–Jakob Disease with Clinical Typology Using Graph Theory and Artificial Intelligence. Mach. Learn. Knowl. Extr. 2024 , 6 , 2018-2032. https://doi.org/10.3390/make6030099 Martínez C, Teijeira S, Domínguez P, Campanioni S, Busto L, González-Nóvoa JA, Alonso J, Poveda E, San Millán B, Veiga C. Correlating Histopathological Microscopic Images of Creutzfeldt–Jakob Disease with Clinical Typology Using Graph Theory and Artificial Intelligence. Machine Learning and Knowledge Extraction . 2024; 6(3):2018-2032. https://doi.org/10.3390/make6030099 Martínez, Carlos, Susana Teijeira, Patricia Domínguez, Silvia Campanioni, Laura Busto, José A. González-Nóvoa, Jacobo Alonso, Eva Poveda, Beatriz San Millán, and César Veiga. 2024. "Correlating Histopathological Microscopic Images of Creutzfeldt–Jakob Disease with Clinical Typology Using Graph Theory and Artificial Intelligence" Machine Learning and Knowledge Extraction 6, no. 3: 2018-2032. https://doi.org/10.3390/make6030099 Article MetricsArticle access statistics, supplementary material. ZIP-Document (ZIP, 16 KiB) Further InformationMdpi initiatives, follow mdpi.  Subscribe to receive issue release notifications and newsletters from MDPI journals
Africa's Public Service Delivery & Performance Review
 Open Journal SystemsReview article, evidence-based healthcare practice adoption: the impact of electronic health records, about the author(s). Background: For healthcare institutions, proper documentation of the upkeep of patient medical records is imperative. In addition, without a record of the patient’s medical history, the doctors are unable to demonstrate that the treatment was delivered correctly. Aim: The primary objective of this research was to determine the influence of electronic health records (EHR) towards the adoption of evidence-based healthcare practice (EBHP) in South African public healthcare. Methods: The study used a quantitative methodology, and a self-administered questionnaire was used to collect data from 300 healthcare professionals. In all, 450 questionnaires were distributed, and of those, 150 were unfit for data analysis because of insufficient data, leaving a total of 300 responses. Data were analysed using exploratory factor analysis (EFA) to identify latent constructs. Confirmatory factor analysis (CFA) was used to assess the validity and reliability of these constructs. The appropriateness of the measurement model was then assessed using fit indices for a structural equation model. Results: The findings show EHR had a direct influence on information quality, medical error reduction, diagnosis and treatment of diseases as well as better coordination of patient’s care. In addition, the results show that EHR-based clinical decision support is crucial for practising evidence-based healthcare and plays a significant role in the quality of healthcare, particularly in the management of diseases and preventative care. As all requirements for validity and reliability (root mean square error of approximation [RMSEA] = 0.085, comparative fit index [CFI] = 0.956 and χ 2 / df = 2.513) have been satisfied, the model is considered valid and reliable. Conclusion: When healthcare professions such as doctors and nurses accurately record patients’ medical histories, they are able to make successful medical decisions and prescribe medications based on the patients’ past and present medical histories. Electronic health records systems facilitate the easier and more efficient exchange of patient data between medical schools, research labs, specialists, pharmacies and other healthcare institutions. Furthermore, they provide medical professionals with resources and up-to-date information to help them deliver EBHP that can benefit patients by reducing or even eliminating medical errors. Contribution: The study contributes theoretically to the field of information systems by outlining a model that includes the variables that affect the adoption of EBHPs in public hospitals. Sustainable Development GoalCrossref citations.  Subscribe to our newsletterGet specific, domain-collection newsletters detailing the latest CPD courses, scholarly research and call-for-papers in your field. Africa’s Public Service Delivery & Performance Review | ISSN: 2310-2195 (PRINT) | ISSN: 2310-2152 (ONLINE) ISSN: 2310-2152  | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
COMMENTS
SJR acts as an alternative to the Journal Impact Factor (or an average number of citations received in last 2 years). This journal has an h-index of 21. The best quartile for this journal is Q2. The ISSN of Journal of Clinical Medicine Research journal is 19183011, 19183003. An International Standard Serial Number (ISSN) is a unique code of 8 ...
Journal of Clinical Medicine Research, monthly, ISSN 1918-3003 (print), 1918-3011 (online), published by Elmer Press Inc. The content of this site is intended for health care professionals. This is an open-access journal distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits ...
Journal of Clinical Medicine Research
Journal of Clinical Medicine
Journal Rankings on Medicine
Journal Citation Reports - Clarivate
About the Journal About the Journal Journal of Clinical Medicine Research is an international, open access and peer-reviewed journal that concentrates on reports of general medical research and clinical practice. This journal aims to publish scientifically written, evidence-based medicine articles from disciplines of clinical medical sciences ...
Use of preprints, defined as preliminary research reports that have not undergone peer review, in clinical and health science research has increased in recent years, partly because of the COVID-19 pandemic. 1,2 Although high-impact clinical journals have publication policies supportive of preprints, 3 concerns remain that posting a preprint before submission to a peer-reviewed journal may ...
Clinical Medicine. Announcement of the latest impact factors from the Journal Citation Reports. Researchers consider a number of factors in deciding where to publish their research, such as journal reputation, readership and community, speed of publication, and citations. See how we share a whole range of information to help the research ...
2 July 2024. We are pleased to share that Journal of Clinical Medicine (JCM, ISSN: 2077-0383,) was awarded an updated Impact Factor of 3.0 in the 2023 Journal Citation Reports™ released by Clarivate in June 2024. JCM ranks Q1 (58 among 325 titles) in the "Medicine, General & Internal" category.
Journal Rankings on Medicine (miscellaneous) Agricultural and Biological Sciences (miscellaneous) Biochemistry, Genetics and Molecular Biology (miscellaneous) Business, Management and Accounting (miscellaneous) Economics, Econometrics and Finance (miscellaneous) Organizational Behavior and Human Resource Management.
About NEJM
Clinical research is a key factor in healthcare progress, as it contributes toward improving our knowledge on the prevention, etiology, and treatment of different conditions. Healthcare professionals and researchers should be familiar with this specific terminology and procedures of clinical research to understand and be able to evaluate ...
Journal of Biomedical Materials Research Part B: Applied Biomaterials is a highly interdisciplinary journal that focuses on the applied aspects of biomaterials, including their design, development, interactions, and behavior in medical devices and other clinical applications. We are serving the needs of biomaterials professionals who are looking for information on biomaterials applied to the ...
The Journal of Clinical Psychology is a clinical psychology & psychotherapy journal devoted to research and practice in clinical psychological science. Skip to Main Content; Search within. Search term ... Journal of Clinical Psychology: Volume 80, Issue 10. Pages: 2093-2202. October 2024. Previous Issue. GO TO SECTION. Export Citation(s) Export ...
Purpose Non-small cell lung cancer (NSCLC) is a highly fatal malignancy. The Kirsten rat sarcoma viral oncogene (KRAS) gene profoundly impacts patient prognosis. This study aims to explore the correlation between KRAS mutation subtypes, clinical data, and the impact of these subtypes on immunotherapy. Materials and methods Tumor samples from 269 NSCLC patients at the Affiliated Cancer Hospital ...
Journal of International Medical Research
During the study period, 16 faculty mentored 35 medical student and resident research projects and 26 case reports. Research and case reports were primarily performed by residents (88% and 96% respectively). Compared to case reports, research projects were more likely to be presented at national meetings (77% vs 32%, p=0.0009).
The 2020 citation metrics have been officially released in the Journal Citation Reports (JCR)! We are pleased to announce that 85 MDPI journals are included, of which: 10 journals received their first impact factor. 96% of journals increased their impact factor from 2019. 32 journals (38%) ranked among the top 25% of journals, in at least one ...
Clinical Trials: Sage Journals ... Clinical Trials
The Federal Adverse Event Reporting System (FAERS) is a publicly available database sponsored by the US Food and Drug Administration (FDA) that is designed for postmarketing drug safety surveillance through reports of adverse events (AEs), medication errors, and other quality issues for drugs and therapeutic agents released to the market.
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management.
Journal of Clinical and Scientific Research
Canadian Journal of Clinical and Medical Research is an academic journal which aims to publish most complete and reliable source of information on the discoveries and current developments in the mode of Research Articles, Review Articles, Case Reports, Short Communications etc. in all areas of the field making them freely available online ...
Journal of Clinical Medicine is an international, peer-reviewed, open access journal of clinical medicine, published semimonthly online by MDPI.The International Bone Research Association (IBRA), Italian Resuscitation Council (IRC), Spanish Society of Hematology and Hemotherapy (SEHH), Japan Association for Clinical Engineers (JACE), European Independent Foundation in Angiology/ Vascular ...
Journal of Medical Case Reports will consider any original case report that expands the field of general medical knowledge, and original research relating to case reports. Case reports should show one of the following: Unreported or unusual side effects or adverse interactions involving medications. Unexpected or unusual presentations of a disease.
Journal of Clinical Medical Research (ISSN: 2582-6751) is an international scientific open access journal, related to the field of medical and clinical studies. ... Impact Factor as 0.624 for 2021-22 by ISI. Journal Name. Journal of Clinical Medical Research. Journal Short Name. Jour Clin Med Res. DOI. 10.46889/JCMR.2022. ISSN. 2582-6751.
Editor-in-Chief: Dr. Arun Bhatt. ISSN: 2229-3485. Online ISSN: 2229-5488. Frequency: Quarterly. eTOC Alert. Thought you might appreciate this item (s) I saw in Perspectives in Clinical Research. Your message has been successfully sent to your colleague.
Creutzfeldt-Jakob disease (CJD) is a rare, degenerative, and fatal brain disorder caused by abnormal proteins called prions. This research introduces a novel approach combining AI and graph theory to analyze histopathological microscopic images of brain tissues affected by CJD. The detection and quantification of spongiosis, characterized by the presence of vacuoles in the brain tissue ...
Africa's Public Service Delivery & Performance Review is a peer reviewed journal, aimed at the promotion and sharing of knowledge, skills and innovations in government and the wider Public-Sector environment in South Africa and abroad. With a multi-disciplinary outlook, the journal will stimulate service delivery and performance challenges being faced in government.