
- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books

What Is a Chemical Reaction? Definition and Examples

Chemical reactions are the backbone of chemistry and, arguably, life itself. Understanding what a chemical reaction is, how to represent it, how to categorize it, and how to distinguish it from a physical change is vital.
What Is a Chemical Reaction?
A chemical reaction is a process in which the chemical structure of a substance changes, leading to the formation of a new substance with different properties. In other words, the reactants convert into products through the breaking and formation of chemical bonds .
Describing Chemical Reactions Using Chemical Equations
A chemical equation is a symbolic representation of a chemical reaction. Reactants are written on the left-hand side, and products on the right, separated by an arrow indicating the direction of the reaction. Combinations of coefficients, element symbols, subscripts, and superscripts indicate the chemical formulas of the reactants and products and their quantities. For each chemical formula, the cation (positive-charged part) of a compound gets listed before the anion (negative-charged part). For example, you write NaCl for sodium chloride rather than ClNa.
A balanced chemical equation follows conservation of mass and charge. There are exactly the same number of atoms of each element on both the reactant and product sides of the equation. The net electrical charge is also the same for both sides of the equation.
Examples of Chemical Reactions
For example, here are some chemical reactions represented as chemical equations:
- The formation of water from hydrogen and oxygen: 2H 2 + O 2 → 2H 2 O
- The combustion of methane: CH 4 + 2O 2 → CO 2 + 2H 2 O
- The decomposition of calcium carbonate: CaCO 3 → CaO + CO 2
How to Recognize a Chemical Reaction
Not all changes involving matter are chemical reactions. A chemical reaction is a chemical change , which means the starting materials are chemically different from the ending materials. In contrast, matter also changes form via physical changes. But, in a physical change , the chemical identity of matter does not change.
For example, when you melt an ice cube into liquid water, the chemical identity of the ice and the water is the same (H 2 O). Melting (and any other phase transition) is an example of a physical change. No chemical reaction occurs. However, when you combine baking soda (NaHCO 3 ) and vinegar (CH 3 COOH), the two chemical undergo a chemical reaction that produces sodium acetate (NaC 2 H 3 O 2 ), water (H 2 O), and carbon dioxide (CO 2 ).
You can’t see the atoms and molecules in action and in the examples of melting ice and reacting baking soda and vinegar, you start with a transparent substance and end with one. So, how do you know which is a physical change and which is a chemical reaction? There are several indicators of a chemical change:
- Color change
- Forming a gas or bubbles
- Forming a precipitate
- Temperature change
- Releasing or absorbing light or sound
- Irreversibility (Most chemical changes are irreversible, while most physical changes are reversible.)
- Changing chemical properties
Melting ice is reversible and does not really meet the other criteria for a chemical change, so it is a physical change. Mixing baking soda and vinegar results in bubbles, a temperature change, and new chemical properties.
Types of Chemical Reactions
There are many different types of chemical reactions , but there are four main classes:
Synthesis (Combination) Reactions
- Description : Two or more substances combine to form a single product.
- General Reaction: A + B → AB
- Example : N 2 + 3H 2 → 2NH 3
Decomposition Reactions
- Description : A single compound breaks down into two or more simpler substances.
- General Reaction: AB → A + B
- Example : 2H 2 O → 2H 2 + O 2
Single-Replacement Reactions
- Description : One element replaces another element in a compound.
- General Reaction: A + BC → AC + B
- Example : Zn + 2HCl → ZnCl 2 + H 2
Double-Replacement Reactions
- Description : The cations and anions of two different molecules switch places.
- General Reaction: AB + CD → AD + CB
- Example : AgNO 3 + NaCl → AgCl + NaNO 3
Other Types of Reactions
There are many other types of reactions, such as:
- Redox Reactions : Involves electron transfer.
- Acid-Base Reactions : Involves the transfer of a proton.
- Complexation Reactions : Formation of complex ions.
- Polymerization : Formation of polymers from monomers.
Importance of Chemical Reactions
Chemical reactions are at the heart of chemistry. Understanding their mechanisms, types, and representations helps us grasp more complex concepts and applications. From the combustion that powers our cars to the metabolic reactions that keep us alive, chemical reactions are indispensable to our daily lives. Applications include:
- Medication formulation
- Making cleaners
- Making disinfectants
- Waste treatment
- Food processing
- Energy production
- Material design
- Atkins, Peter W.; Julio de Paula (2006). Physical Chemistry (4th ed.). Weinheim: Wiley-VCH. ISBN 978-3-527-31546-8.
- IUPAC (1997). Compendium of Chemical Terminology (the “Gold Book”) (2nd ed.). Oxford: Blackwell Scientific Publications. ISBN 0-9678550-9-8. doi: 10.1351/goldbook
- Wintterlin, J. (1997). “Atomic and Macroscopic Reaction Rates of a Surface-Catalyzed Reaction”. Science . 278 (5345): 1931–4. doi: 10.1126/science.278.5345.1931
- Zumdahl, Steven S.; Zumdahl, Susan A. (2000). Chemistry (5th ed.). Houghton Mifflin. ISBN 0-395-98583-8.
Related Posts

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
1.8 Introduction to Chemical Reactions and Equations
Joey Wu and OpenStax
Learning Objectives
- Define terms used to represent chemical reactions including reactants, products, states, coefficients, and stoichiometry, reaction conditions.
- Differentiate between the liquid phase and the aqueous phase
Chemical Reactions
The space shuttle—and any other rocket-based system—uses chemical reactions to propel itself into space and maneuver itself when it gets into orbit. The rockets that lift the orbiter are of two different types. The three main engines are powered by reacting liquid hydrogen with liquid oxygen to generate water. Then there are the two solid rocket boosters, which use a solid fuel mixture that contains mainly ammonium perchlorate and powdered aluminum. The chemical reaction between these substances produces aluminum oxide, water, nitrogen gas, and hydrogen chloride. Although the solid rocket boosters each have a significantly lower mass than the liquid oxygen and liquid hydrogen tanks, they provide over 80% of the lift needed to put the shuttle into orbit—all because of chemical reactions.

Figure 1 . When we think about chemistry, we usually think about chemical reactions, such as combustion.
Chemistry is largely about chemical changes. Indeed, if there were no chemical changes, chemistry as such would not exist! Chemical changes are a fundamental part of chemistry. Because chemical changes are so central, it may be no surprise that chemistry has developed some special ways of presenting them.
What are Chemical Equations?
Chemical equations are how chemists describe chemical reactions, the process by which one form of matter ( reactants ) turn into another form of matter ( products ). Below is the balanced chemical reaction for the combustion of methane (CH 4 ) with oxygen (the reactants) to produce carbon dioxide and water (the products), see Figure 2.
Figure 2 . Reactants and Products of a chemical equation
Law of Conservation of Mass: Balancing Reactions
CO+O 2 → CO 2
(unbalanced equation)
As matter is made of compounds and elements, and the compounds may change, this means the total number of atoms of each element must be conserved. In the equation below, only atoms of carbon are conserved, and so the equation is not balanced. In the equation below, that atoms of each element are conserved, and so it is balanced.
2CO+O 2 = 2CO 2 (balanced equation)
Stoichiometric Coefficients:
Once the reactants and products are written, you are not able to change the subscripts of the compound to balance the chemical reaction without changing the identity of the reactants and products. However, you may use stoichiometric coefficients in front of each chemical entity (molecule or element) to balance the equations.
2H 2 + O 2 -> 2H 2 O
Extension: Catalyst
Catalysts can be understood by looking at the mechanism of a reaction, that is, how it actually proceeds, and can be considered to be chemicals that function as both a reactant and a product. That is, the catalyst reacts with something and form a new chemical, an intermediate, and that intermediate also reacts, and reproduces the catalyst. Catalysts can speed up reactions , or make reactions happen which without the catalyst are so slow that they don’t really happen.
Key Takeaways
- A chemical reaction is a process in which some substances, called reactants, change into different substances, called products.
- All chemical reactions involve both reactants and products. Reactants are substances that start a chemical reaction, and products are substances that are produced in the reaction.
- Chemical equations should be balanced because the total number of atoms from each elements are conserved.
STEM for Educators Copyright © 2022 by Joey Wu and OpenStax is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book
Introduction to Chemical Reactions
Article objectives.
- The objective of this article is to explain the concept of chemical reactions and the types of chemical reactions.
Introduction
One of the most fundamental concepts of chemistry is something known as the Law of Conservation of Matter , that states that atoms are never introduced or destroyed. This is what allows chemical reactions to work, where one or more chemicals react in such ways that one or more new chemicals are formed, via the moving of electrons between substances. The chemicals present before the reaction are called reactants (for the obvious reason), and the chemicals present after the reaction are called products . No chemical reaction is a chemical reaction without both of these components.
Introduction to Chemical Equations
On paper, a chemical reaction can be written out in the form of a chemical equation , which shows the reaction taking place. Of course there is always a way to describe the reaction in words too.
Example 1: Write a chemical equation for Magnesium Hydroxide and Sulfuric Acid being mixed together in solution to get Magnesium Sulfate and Water.
Solution: The reactants are written on the left, and the products are written on the right, with an arrow in between.
$$Mg(OH)_2 + H_2SO_4 \rightarrow H_2O + MgSO_4$$
States of Matter
Often, it is a good idea to include states of matter in a chemical equation. A state of matter is the phase of the chemical, so either solid, liquid, or gas. In addition, in chemical equations the phrase aqueous is treated as a state of matter. This refers to a chemical being dissolved in a solution with water.
When placing states of matter in chemical equations, use the following symbols:
\((s)\) for a solid
\((l)\) for a liquid (usually water)
\((g)\) for a gas
\((aq)\) for an aqueous substance
Example 2: Write an equation for a reaction between aqueous Gold Perchlorate and solid Titanium which form solid Gold and aqueous Titanium Perchlorate. Include states of matter.
Solution: Use the information given about the chemicals to write the equation:
$$Au(ClO_4)_3 + Ti \rightarrow Ti(ClO_4)_2 + Au$$
Now add the states of matter.
$$Au(ClO_4)_3(aq) + Ti(s) \rightarrow Ti(ClO_4)_2(aq) + Au(s)$$
As a general rule of thumb, always include the states of matter if they are given.
Single Replacement Reactions
There are many different types of chemical reactions, and they are classified based on a combination of the identity of the reactants and products and how many substances/ions are directly involved in the reaction. A single replacement reaction is a reaction where one substance or ion is moved, and nothing else.
Example 3: Based on the following chemical equation, is the reaction a single replacement reaction? How can you tell? The equation is
$$BaCO_3 + Na \rightarrow Na_2CO_3 + Ba$$
Solution: This is a single replacement reaction. The way we can tell is that only the Carbonate ion (\(CO_3^{2-}\)) is moving, and thus influencing the charges of the Sodium and the Barium.
Example 4: Magnesium Chloride and solid Barium react in a single-replacement reaction. What are the products?
Solution: In a single-replacement reaction, one chemical will be moved between substances. Since Magnesium Chloride is an ionic compound, it will likely be an ion. Seeing Magnesium and Barium in a compound together is uncommon because both of these elements are metals, but Barium Chloride is a perfectly plausible ionic compound, so we have the reaction
$$MgCl_2+Ba \rightarrow BaCl_2+Mg$$
So the products are solid Magnesium and Barium Chloride.
Double Replacement Reactions
The counterpart of single replacement reactions, a double replacement reaction is where two substances or ions are moved/inverted to create new substances. Generally double replacement reactions, at least in first-year courses, will have at least two products.
Example 5: Why is the reaction \(HCl + K \rightarrow KCl + H_2\) not a double replacement reaction?
Solution: Only the Chlorine atoms are being moved, so by definition this cannot be a double replacement reaction. It is actually a single replacement reaction.
Example 6: What is one possible double replacement reaction that could theoretically occur between Aluminum Chromate (\(Al(CrO_4)_3\)) and Magnesium Oxide?
Solution: Two components must be inverted to create a double-replacement reaction. It is perfectly valid to allow these substances to be the anions that are present. Therefore we get a chemical equation of
$$Al(CrO_4)_3 + MgO \rightarrow Mg(CrO_4)_2 + Al_2O_3$$
Synthesis Reactions
A synthesis reaction is where two or more chemicals are combined to create one chemical. Generally, if a reaction is known to be a synthesis reaction, predicting the product(s) are easier compared to single or double replacement reactions.
Example 7: The products of a chemical reaction are Sodium Hydroxide and Acetone. Without knowing the products, how do you know that this is not a synthesis reaction?
Solution: There would only be one product, so this must be a different type of reaction.
In the previous example, even though we could tell that it was not a synthesis reaction, we would need to know the reactants in order to determine what type of reaction it actually was.
Example 8: If \(H_2O_2\) and Chromium metal undergo a synthesis reaction, what is one possible product of the reaction?
Solution: Of course there may be multiple answers, but they all must include Chromium, Hydrogen, and Oxygen. This solution creates an ionic compound; the product \(Cr(OH)_3\) appears perfectly valid, as this is indeed a synthesis reaction by definition. For reference, the equation is shown below:
$$H_2O_2 + Cr \rightarrow Cr(OH)_3$$
Decomposition Reaction
A decomposition reaction is a reaction that is in the reverse process of a synthesis reaction; a chemical decomposes into two or more different chemicals. Sometimes the decomposition will be automatic, and other times a trigger such as heat will be necessary.
Example 9: Carbonic acid decomposes naturally. The decomposition reaction is as follows:
$$H_2CO_3 \rightarrow CO_2 + H_2O$$
Oxidation-Reduction Reactions
An oxidation-reduction reaction is a reaction that meets one of two conditions: 1. Elemental oxygen is a reactant. 2. One substance is oxidized , or has electrons taken away from it, and one substance is reduced , where it gets additional electrons.
An oxidation-reduction reaction, or redox reaction, can at the same time be a single replacement reaction, double replacement reaction, or synthesis reaction.
Example 10a: Classify the following chemical reaction in two ways:
$$Sn + O_2 \rightarrow SnO$$
Solution: One of the reactants is elemental oxygen, so it is a redox reaction. In addition, two substances are combined to create one new one, so it is also a synthesis reaction.
The second half of the example generalizes this reaction.
Example 10b: In Example 10a, the Tin metal is being oxidized into Tin cations. All stable metals can undergo basically the same reaction. Here are some specific ones:
$$Li + O_2 \rightarrow Li_2O$$
$$Ca + O_2 \rightarrow CaO$$
$$Al + 3O_2 \rightarrow Al_2O_3$$
In each of these redox reactions, the metal is oxidized and the oxygen is reduced. This property also applies to transition metals (the stable ones):
$$Zr + O_2 \rightarrow ZrO_2$$
Another type of problem is to determine whether a reaction is a redox reaction. As a rule of thumb, check the charges of the ions--redox reactions generally involve ionic compounds.
Example 11: Is this a redox reaction? Explain why.
$$HNO_3 + Cu(OH)_2 \rightarrow Cu(NO_3)_2 + H_2O$$
Solution: There is no elemental oxygen (although substances with oxygen are abundant; don't let this make you think the reaction is redox). Also, there is nothing being oxidized or reduced. Therefore this is not a redox reaction.
Example 12: In the following chemical equation, the element labeled with a question mark is unknown:
$$Mg? + Na \rightarrow Na? + Mg$$
What might this unknown element be if this reaction is a redox reaction?
Solution: There is no room for elemental oxygen in this reaction. However, the "?" can still be oxygen:
$$MgO + Na \rightarrow Na_2O + Mg$$
Here's why: in this reaction, the Magnesium is reduced and the Sodium is Oxidized. Therefore this is a redox reaction.
Basically any anion would work here. For example, if the question mark actually represents Fluorine:
$$MgF_2 + Na \rightarrow NaF + Mg$$
For the same reason, this is still a redox reaction. Even a polyatomic ion would allow this to be a redox reaction (although polyatomic ions are not pure elements). For example, if the ion is Phosphite:
$$Mg_3(PO_3)_2 + Na \rightarrow Na_3PO_3 + Mg$$
Example 13: What type of reaction is this?
$$Mn_2 O_7+Na_2 SO_3 \rightarrow Mn_2 (SO_3)_7+Na_2O$$
Solution: Two different ions, oxide and sulfite, are interchanged. Therefore this is a double-replacement reaction.
Despite being under "Oxidation-Reduction Reactions," the above reaction is not a redox reaction. None of the charges change (nothing is oxidized or reduced). The presence of oxygen may have made you identify the reaction as redox, but that is incorrect because the oxygen is not elemental; it is the anion in the ionic compound Manganese (VII) Oxide.
Combustion Reactions
A combustion reaction is a subtype of redox reactions where a substance is burned with an oxygen flame. Therefore by definition, all combustion reactions are redox reactions, since they always contain elemental oxygen.
Many combustion reactions include elemental oxygen and a hydrocarbon, a chemical comprising only of Carbon and Hydrogen atoms. By default, these reactions always have two products: carbon dioxide and water. The amounts produced are based on the type of hydrocarbon and how much of each reactant are available.
Example 14: Octane is combusted as follows:
$$C_8H_{18} + O_2 \rightarrow CO_2 + H_2O$$
a. Is this reaction a redox reaction?
b. Is this reaction a synthesis reaction?
c. Would the previous answers change if a different hydrocarbon is used?
a. Elemental oxygen is a reactant, so this reaction is a redox reaction.
b. This is not a synthesis reaction, because there are the same number of products as reactants, so two reactants could not combine to form one product.
c. No; regardless, elemental oxygen is still a reactant, and this does nothing to change the products (only the amounts), so this is still not a synthesis reaction.
A carbohydrate is a chemical with only Carbon, Hydrogen, and Oxygen. Combustion of carbohydrates works the same way as the combustion of hydrocarbons.
Example 15: What are the products when \(C_9H_{16}O_2\) is combusted?
Solution: By inspection of the chemical formula \(C_9H_{16}O_2\), it is obvious this is a carbohydrate. As usual, the products will be \(CO_2\) and \(H_2O\). Here is the chemical equation:
$$C_9H_{16}O_2 + O_2 \rightarrow CO_2 + H_2O$$
<div class="article-example" markdown="1">
**Example 16:** Find and balance an equation for the combustion of \(C_7H_{16}O_4\).
**Solution:** "Combustion" implies the addition of elemental oxygen:
$$C_7H_{16}O_4 + O_2 \rightarrow$$
As with any other combustion reaction, the products are carbon dioxide and water:
$$C_7H_{16}O_4 + O_2 \rightarrow CO_2 + H_2O$$
Balance the hydrogen first:
$$C_7H_{16}O_4 + O_2 \rightarrow CO_2 + 8H_2O$$
Now balance carbon:
$$C_7H_{16}O_4 + O_2 \rightarrow 7CO_2 + 8H_2O$$
There are \(22\) carbon atoms on the right side, so we need the same number on the left side. With four oxygen atoms included in the carbohydrate, the remaining \(18\) must come from the elemental oxygen, so
$$C_7H_{16}O_4 + 9O_2 \rightarrow 7CO_2 + 8H_2O$$
</div>
The Law of Conservation of Matter stated that new matter could not be introduced or removed from existence, but many of the equations in this article appeared to violate this concept because the amount of each chemical on both sides of the equation was not the same. However, there is a way to balance equations to make this rule followed yet again; this will be discussed in a different article, as it is too big a concept to include here.
Chemical Reactions and Equations

Chemical Reactions
What is a chemical reaction.
A chemical reaction is in which the bonds are broken within reactant molecules, and new bonds are formed within product molecules in order to form a new substance.
Chemical reactions are all around us, from the metabolism of food in our body to how the light we get from the sun is the result of chemical reactions. Before beginning with chemical reactions, it is important to know about physical and chemical changes.

A burning candle is the best example of physical and chemical change. Take a candle and light it. As time passes, we can observe that the candle changes to wax. If you cover the candle with a jar, it will extinguish.
In the demonstration, burning of the candle is a chemical change while conversion of the candle to wax is a physical change. In a physical change, there is basically a change of state of the substance but in the case of a chemical change mostly a new substance is formed in which either energy is given off or absorbed. Thus, we can conclude that chemical changes are accompanied by certain physical changes.
Table of Content
Basic concepts of chemical reactions.
- Recommended Videos on Chemical Reactions
- Chemical equations
- Types of Chemical equations
Important Points to Remember
- Frequently Asked Questions – FAQs
- A Chemical Reaction is a process that occurs when two or more molecules interact to form a new product(s).
- Compounds that interact to produce new compounds are called reactants whereas the newly formed compounds are called products.
- Chemical reactions play an integral role in different industries, customs and even in our daily life. They are continuously happening in our general surroundings; for example, rusting of iron, pottery, fermentation of wine and so on.
- In a chemical reaction, a chemical change must occur which is generally observed with physical changes like precipitation, heat production, colour change etc.
- A reaction can take place between two atoms or ions or molecules, and they form a new bond and no atom is destroyed or created but a new product is formed from reactants.
- The rate of reaction depends on and is affected by factors like pressure, temperature, the concentration of reactants.
Recommended Videos
Chemical reactions and equations – all activities in one go.
Chemical Equations
Due to the vast amounts of chemical reactions happening around us, a nomenclature was developed to simplify how we express a chemical reaction in the form of a chemical equation. A chemical equation is nothing but a mathematical statement which symbolizes the product formation from reactants while stating certain condition for which how the reaction has been conducted.
The reactants are on the left-hand side whereas the products formed are on the right-hand side. The reactants and products are connected by a one-headed or two-headed arrows. For example, a reaction
A + B → C + D
Here, A and B are the reactants, which react to form the products C and D. In an actual chemical equation, reactants are denoted by their chemical formula . In order to assure the law of conservation of mass, a chemical equation must be balanced i.e. the number of atoms on both sides must be equal. This is the balancing of the equation.
Let us consider an actual chemical reaction between Methane(CH₄) and Oxygen (O 2 ),
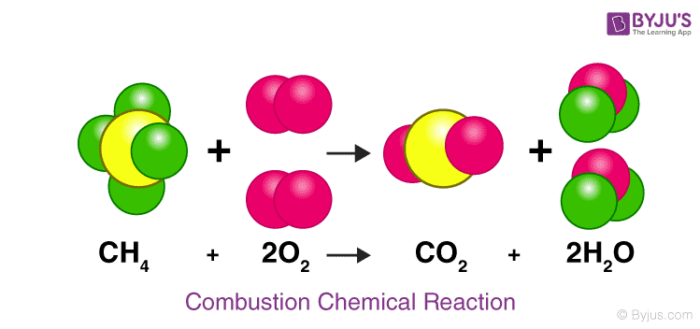
Here we can see how the number of each atom on the left side is balanced on the right side, as stated by the law of conservation of mass.
Types of Chemical Reactions
The basis for different types of reactions is the product formed, the changes that occur, the reactants involved and so on. Different types of reactions are
- Combustion reaction
- Decomposition reaction
- Neutralization reaction
- Redox Reaction
- Precipitation or Double-Displacement Reaction
- Synthesis reaction
1. Combustion Reaction
A combustion reaction is a reaction with a combustible material with an oxidizer to give an oxidized product. An oxidizer is a chemical a fuel requires to burn, generally oxygen. Consider the example of combustion of magnesium metal.
\(\begin{array}{l}2 Mg + O_2 \rightarrow 2 MgO + Heat\end{array} \)
Here, 2 magnesium atoms react with a molecule of oxygen producing 2 molecules of the compound magnesium oxide releasing some heat in the process.
2. Decomposition Reaction
A Decomposition reaction is a reaction in which a single component breaks down into multiple products. Certain changes in energy in the environment have to be made like heat, light or electricity breaking bonds of the compound. Consider the example of the decomposition of calcium carbonate giving out CaO (Quick Lime) which is a major component of cement.
\(\begin{array}{l}Ca C O_3 ( s ) \overset{Heat}{\rightarrow} Ca O ( s ) + CO_2 ( g ) \end{array} \)
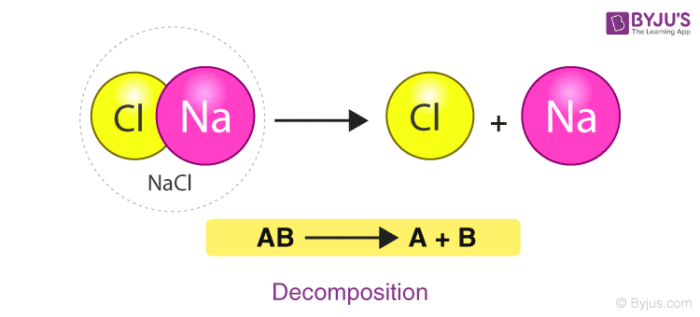
Here, the compound Calcium carbonate when heated breaks down into Calcium Oxide and Carbon Dioxide.
3. Neutralization Reaction
A Neutralization reaction is basically the reaction between an acid and a base giving salt and water as the products. The water molecule formed is by the combination of OH – ions and H + ions. The overall pH of the products when a strong acid and a strong base undergo a neutralization reaction will be 7. Consider the example of the neutralization reaction between Hydrochloric acid and Sodium Hydroxide giving out sodium chloride(Common Salt) and water.
\(\begin{array}{l}H Cl + NaOH \rightarrow NaCl +H_2O\end{array} \)
Here, an acid and a base, Hydrochloric acid and Sodium Hydroxide react in a neutralization reaction to produce Sodium Chloride(Common Salt) and water as the products.
4. Redox Reaction
A RED uction- OX idation reaction is a reaction in which there is a transfer of electrons between chemical species. Let us consider the example of an electrochemical cell-like redox reaction between Zinc and Hydrogen.
\(\begin{array}{l}Zn+2H^{+}\rightarrow Zn^{2+}+H_2\end{array} \)
Here, A Zinc atom reacts with 2 ions of positively charged hydrogen to which electrons get transferred from the zinc atom and hydrogen becomes a stable molecule and Zinc ion is the product.
5. Precipitation or Double-Displacement Reaction
It is a type of displacement reaction in which two compounds react and consequently, their anions and cations switch places forming two new products. Consider the example of the reaction between silver nitrate and sodium chloride. The products will be silver chloride and sodium nitrate after the double-displacement reaction.
\(\begin{array}{l}Ag N O_3 + Na Cl \rightarrow Ag Cl + Na N O_3\end{array} \)
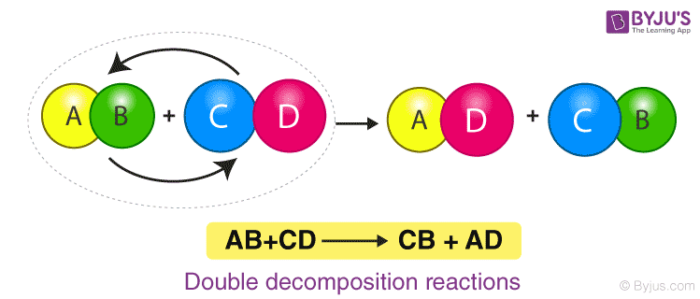
Here, Silver Nitrate and Sodium Chloride undergo a double displacement reaction. Wherein Silver replaces Sodium in Sodium Chloride and Sodium joins with Nitrate becoming Sodium Nitrate along with the Silver Chloride as the product.
6. Synthesis Reaction
A Synthesis reaction is one of the most basic types of reaction wherein multiple simple compounds combine under certain physical conditions giving out a complex product. The product will always be a compound. Let us consider the Synthesis reaction of sodium chloride with reactants solid sodium and chloride gas.
\(\begin{array}{l}2 Na ( s ) + Cl_{2} (g) \rightarrow 2 Na Cl ( s )\end{array} \)
Here, we have 2 Atoms of solid Sodium reacting with Chlorine gas giving out Sodium Chloride viz. Common Salt as the product.
- In a chemical change, a new compound is formed but in a physical change, the substance changes its state of existence.
- Atoms or ions or molecules which react to form a new substance are called reactants; the new atoms or molecules formed are products.
- A chemical reaction follows the law of conservation of mass. That is no atom is destroyed or created but only a new product is formed from reactants.
BYJU’S helps students by delivering chapter wise and detailed solutions to the questions of NCERT books. They can compare their answers with the sample answers given here – NCERT Solutions for class 10 Science Chapter 1 Chemical reactions and equations .
Related Videos

How Will Chemistry Help You Survive Alone on an Island?

Frequently Asked Questions – FAQs
What is meant by a chemical reaction.
A Chemical Reaction is a process that occurs when two or more molecules collide with the right orientation and sufficient force to form a new product. In this process breaking and forming bonds between atoms takes place. Compounds that interact to produce new compounds are called reactants whereas the newly formed compounds are called products.
What is chemical reaction and equation?
A chemical reaction is in which the bonds are broken within reactant molecules, and new bonds are formed within product molecules in order to form a new substance. A chemical equation is nothing but a mathematical statement which symbolizes the product formation from reactants.
What are the chemical reaction types?
On the basis of the product formed, different types of reactions are Combustion reaction, Decomposition reaction, Neutralization reaction, Redox Reaction, Precipitation or Double-Displacement Reaction, Synthesis reaction.
What is a combustion chemical reaction?
A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat.
What is a chemical equation?
What is a decomposition reaction, what is a neutralization reaction, what is a redox reaction, what is precipitation or a double displacement reaction, what is a synthesis reaction.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
so easy to understand thank you for giving these resources for free
It nice😊😊😊😊😊 I was satisfied with this information 😊😊😊😊
So interesting easy way of learning
literally it really helped me a lot it is awsm
It is a very good learning platform
It is a very good learning platform for us
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Biology library
Course: biology library > unit 2.
- Ionic bonds
- Covalent bonds
- Electronegativity
- Electronegativity and bonding
- Intermolecular forces
- Chemical bonds
Chemical reactions introduction
- Chemical reactions
Want to join the conversation?
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

Video transcript

Chapter 5 Introduction to Chemical Reactions
Opening essay.
Although yeast has been used for thousands of years, its true nature has been known only for the last two centuries. Yeasts are single-celled fungi. About 1,000 species are recognized, but the most common species is Saccharomyces cerevisiae , which is used in bread making. Other species are used for the fermentation of alcoholic beverages. Some species can cause infections in humans.
Yeasts live primarily on sugars, such as glucose (C 6 H 12 O 6 ). They convert glucose into carbon dioxide (CO 2 ) and ethanol (C 2 H 5 OH) in a chemical transformation that is represented as follows:
Bread making depends on the production of carbon dioxide. The gas, which is produced in tiny pockets in bread dough, acts as a leavening agent: it expands during baking and makes the bread rise. Leavened bread is softer, lighter, and easier to eat and chew than unleavened bread. The other major use of yeast, fermentation, depends on the production of ethanol, which results from the same chemical transformation. Some alcoholic beverages, such as champagne, can also be carbonated using the carbon dioxide produced by the yeast.
Yeast is among the simplest life forms on Earth, yet it is absolutely necessary for at least two major food industries. Without yeast to turn dough into bread and juice into wine, these foods and food industries would not exist today.
Chemical change is a central concept in chemistry. The goal of chemists is to know how and why a substance changes in the presence of another substance or even by itself. Because there are tens of millions of known substances, there are a huge number of possible chemical reactions. In this chapter, we will find that many of these reactions can be classified into a small number of categories according to certain shared characteristics.
How to Write a Reaction Paper: Guide Full of Tips

Imagine being a writer or an artist and receiving feedback on your work. What words would you cherish most? 'Amazing'? 'Wonderful'? Or perhaps 'Captivating'? While these compliments are nice, they tend to blend into the background noise of everyday praise.
But there's one accolade that truly stands out: 'Thought-provoking.' It's the kind of response every creator dreams of evoking. Thought-provoking pieces don't just passively entertain; they stir something inside us, lingering in our minds long after we've encountered them. In academic circles, a work isn't truly impactful unless it prompts a reaction.
In this article, our research paper writing services will delve into the concept of reaction papers: what they are, how to craft a stellar one, and everything in between. So, let's explore the art of provoking thought together.
What is Reaction Paper
Ever found yourself deeply engrossed in a book, movie, or perhaps an article, only to emerge with a flurry of thoughts and emotions swirling within? That's where a reaction paper comes into play. It helps you articulate those musings to dissect the themes, characters, and nuances of the work that stirred something within you.
A reaction paper is a written response to a book, article, movie, or other media form. It give you an opportunity to critically evaluate what you've experienced and to share your insights with others. Whether you're captivated by a novel's narrative, moved by a film's message, or intrigued by an academic article's argument, it allows you to explore the depths of your reaction.
Don't Enjoy Writing College Essays?
Don't break a sweat. Let our expert writers do the heavy lifting.

How to Write a Reaction Paper with 8 Easy Tips
When learning how to write a reaction paper, it's important to keep an open mind. That means being willing to consider different ideas and perspectives. It's also a good idea to really get into whatever you're reacting to—take notes, highlight important parts, and think about how it makes you feel.
Unlike some other school assignments, like essays or reports, a reaction paper is all about what you think and feel. So, it's kind of easy in that way! You just have to really understand what it's about and how to put it together.
Now, we're going to share some tips to help you write a great paper. And if you're running out of time, don't worry! You can always get some extra help from our essay writing service online .
.webp)
Understand the Point
When you're sharing your thoughts, whether in school or outside of it, it's important to have a good grasp of what you're talking about. So, before you start writing your paper, make sure you understand its goals and purpose. This way, you can give readers what they're looking for—a thoughtful, balanced analysis.
Knowing the purpose of your paper helps you stay on track. It keeps you from wandering off into unrelated subjects and lets you focus on the most important parts of the text. So, when you share your thoughts, they come across as clear and logical.
Read the Text Right After It Has Been Assigned
When you're asked to write a reaction paper, remember that your first reaction might not be your final one. Our initial thoughts can be a bit all over the place—biased, maybe even wrong! So, give yourself some time to really think things through.
Start diving into the material as soon as you get the assignment. Take your time to understand it inside and out. Read it over and over, and do some research if you need to until you've got a handle on everything—from what the author was trying to do to how they did it. Take notes along the way and try to see things from different angles.
When it comes to writing your paper, aim for a thoughtful response, not just a knee-jerk reaction. Back up your points with solid evidence and organize them well. Think of it more like writing a review than leaving a quick comment on a movie website.
Speaking of movies, we've got an example of a movie reaction paper below. Plus, if you're interested, we've got an article on discursive essay format you might find helpful.
Make a Note of Your Early Reactions
When you're diving into a topic, jotting down your initial thoughts is key. These first reactions are like capturing lightning in a bottle—they're raw, honest, and give you a real glimpse into how you're feeling.
Your paper should be like a mirror, reflecting your own experiences and insights. Your instructor wants to see the real you on the page.
Understanding why something makes you feel a certain way is crucial. By keeping track of your reactions, you can spot any biases or assumptions you might have. It's like shining a light in a dark room—you can see things more clearly. And by acknowledging these biases, you can write a paper that's fair and balanced. Plus, it can point you in the direction of further research, like following breadcrumbs through the forest.
Select a Perspective
Your perspective shapes how you see things, and it's like a roadmap for your reaction paper. It keeps you focused and organized and helps you share thoughtful insights.
Before you start writing, think about different angles to approach the topic. Figure out which perspective resonates with you the most. Consider what it does well and where it might fall short.
Putting yourself in the author's shoes can be really helpful. Try to understand why they wrote what they did and how they put it all together. It's like stepping into their world and seeing things from their point of view. This helps you analyze things more clearly and craft a solid paper.
Before we jump into the nitty-gritty of reaction paper templates, there are a few more tips to share. So, keep reading. Or if you're feeling overwhelmed, you can always ask our professional writers - ' do my homework for me ' - to lend a hand with your coursework.
Define Your Thesis
Defining your thesis might feel like trying to untangle a knot at first. Start by gathering all your ideas and main points. Think about which one resonates with you the most. Consider its strengths and weaknesses—does it really capture the essence of what you want to say?
Then, try to distill all those thoughts into a single sentence. It's like taking a handful of puzzle pieces and fitting them together to reveal the big picture. This sentence becomes the heart of your response essay, guiding your reader along with your analysis.
Organize Your Sections
When you're writing a response paper, it's important to organize your thoughts neatly. Papers that are all over the place can confuse readers and make them lose interest.
To avoid this, make sure you plan out your paper first. Create an outline with all the main sections and sub-sections you want to cover. Arrange them in a logical order that makes sense. Then, for each section, start with a clear topic sentence. Back it up with evidence like quotes or examples. After that, share your own opinion and analyze it thoroughly. Keep doing this for each section until your paper is complete. This way, your readers will be able to follow along easily and understand your argument better.
Write the Final Version
Writing a reaction paper isn't a one-shot deal. It takes several tries to get it just right. Your final version should be polished, with a strong thesis and a well-structured layout.
Before calling it done, give your paper a thorough once-over. Make sure it ticks all the boxes for your assignment and meets your readers' expectations. Check that your perspective is crystal clear, your arguments make sense and are backed up with evidence, and your paper flows smoothly from start to finish.
Keep an eye out for any slip-ups. If you catch yourself just summarizing the text instead of offering your own take, go back and rework that section. Your essay should be original but also fair and balanced. So, give it that final polish until it shines.
Check Your Paper for Spelling and Grammar
No matter what type of essay you're writing—whether it's argumentative or a reaction piece—grammar matters. Even if you've got a strong reaction statement and unique opinions, they won't shine if your sentences are hard to read.
Before you hit that submit button, take a moment to check for grammar and spelling mistakes. These little errors might seem minor, but they can really drag down the quality of your work. Plus, they signal a lack of attention to detail, which could hurt how seriously your paper is taken.
Remember, good grammar isn't just about following rules—it's about clarity. If your paper is riddled with mistakes, it'll be harder for readers to grasp your ideas. On the flip side, clean, error-free writing boosts your credibility and ensures that your thoughts come across loud and clear. So, give your paper that final polish—it's worth it.
Reaction Paper Reaction Paper Outline
Now that you've got all those handy tips and tricks under your belt let's talk about the big picture: the outline. It typically consists of three main parts: the introduction, body paragraphs, and conclusion. Each section has its own job to do and is equally crucial to the overall piece. Each part needs to meet the basic requirements of a written assignment, make clear points, and properly credit any direct quotes using the appropriate citation style, like APA format.
.webp)
Introduction
Getting started with writing can feel like trying to climb a mountain. But fear not! It doesn't have to be daunting if you know how to start a reaction paper.
The introduction is your chance to make a strong first impression. It sets the stage for what's to come and gives readers a glimpse of what they can expect. But keep it snappy—nobody likes a long-winded intro!
To craft an effective introduction:
- Provide some context to get readers up to speed.
- Give a brief summary of relevant background information.
- Clearly state the purpose of your paper.
- Explain what you're hoping to achieve and why it matters.
- Wrap it up with a thesis statement that sums up your personal take and outlines the main points you'll be covering.
After your attention-grabbing introduction, it's time to keep the momentum going in the body paragraphs. This is where you really dive into your thoughts and opinions on the key points of the text.
Remember our top tip: divide your ideas into different sections. Each paragraph should kick off with a topic sentence that sums up the main idea you're tackling. Then, give a quick rundown of the specific aspect of the book or article you're discussing. After that, it's your turn to share your honest feelings about it and explain why you feel that way. Back up your ideas with quotes from trustworthy sources, and make sure to cite them correctly. And don't forget to tie your reactions back to the bigger picture.
Wrap up each paragraph by summarizing your thoughts and feelings and linking them back to the main theme of your paper. With this approach, your body paragraphs will flow smoothly and keep your readers engaged every step of the way.
As you wrap up your reaction paper format, don't overlook the importance of a strong conclusion. This is your chance to bring all your thoughts and feelings together in a neat package and leave a lasting impression on your reader.
Kick things off by revisiting your reaction statement. Remind your reader of the main points you've covered in the body paragraphs, and share any fresh insights you've gained along the way. Just remember—keep it focused on what you've already discussed. Your conclusion shouldn't introduce any new information.
Finish off your paper with a memorable closing statement that ties everything together. This is your chance to leave your reader with a final thought that resonates long after they've finished reading. With a well-crafted conclusion, you'll send your paper off on a high note and leave your reader feeling satisfied.
Reaction Paper Example
Sometimes, seeing is believing. That's why we've prepared a reaction paper example to show you exactly what a stellar paper looks like and how paying attention to small details can elevate your essay. While you're at it, you can also check out our pestle analysis example .
Final Words
Our tips and tricks on how to write a compelling reaction paper will get you an A+. Reflect on your thoughts and feelings, be clear, support your ideas with evidence, and remain objective. Review our reaction paper sample and learn how to write a high-quality academic paper.
Get professional research paper writing services from our experienced writers to ensure high grades. We offer a wide range of aid, including nursing essay writing services . Contact us today for reliable and high-quality essay writing services.
Do You Find Writing College Essays Difficult?
Join the ranks of A+ students with our essay writing services!
What Is a Reaction Paper?
How to make an outline for a reaction paper, how do you write a reaction paper.

Daniel Parker
is a seasoned educational writer focusing on scholarship guidance, research papers, and various forms of academic essays including reflective and narrative essays. His expertise also extends to detailed case studies. A scholar with a background in English Literature and Education, Daniel’s work on EssayPro blog aims to support students in achieving academic excellence and securing scholarships. His hobbies include reading classic literature and participating in academic forums.

is an expert in nursing and healthcare, with a strong background in history, law, and literature. Holding advanced degrees in nursing and public health, his analytical approach and comprehensive knowledge help students navigate complex topics. On EssayPro blog, Adam provides insightful articles on everything from historical analysis to the intricacies of healthcare policies. In his downtime, he enjoys historical documentaries and volunteering at local clinics.
Related Articles
.webp)
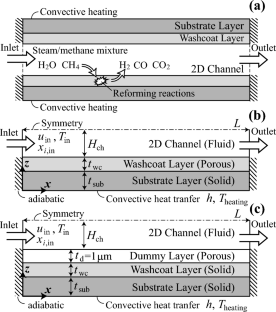
Validation of Effectiveness Factor Correlations for Steam Methane Reforming in Ni-Based Washcoat Catalyst Layers Using Commercial Computational Fluid Dynamics Software
- Original Article
- Published: 21 May 2024
Cite this article

- Yun Seok Oh 1 ,
- Hyun-Joo Oh 1 , 2 &
- Jin Hyun Nam ORCID: orcid.org/0000-0001-8181-0408 3
32 Accesses
Explore all metrics
In this study, the effectiveness factor correlations proposed for steam methane reforming (SMR) in Ni-based washcoat catalyst layers were numerically validated using the commercial computational fluid dynamics (CFD) software, ANSYS Fluent. The SMR process in an exemplary microchannel reformer was simulated, once by fully considering the reaction and diffusion process within the washcoat catalyst layer and again by simplifying the process calculation using the effectiveness factor correlations. It was shown that the proposed effectiveness factor correlations could successfully capture the SMR characteristics in the washcoat catalyst layer, with a discrepancy of approximately 0.1% point in the overall methane conversion ratio in the validation test, while reducing the calculation time by a factor of 1/5 for the same number of iterations. All these results clearly demonstrated that accurate and cost-effective CFD simulation of the steam reformer operation is possible using the proposed effectiveness factor correlations. Finally, this paper also addressed a possible numerical anomaly in the Fluent calculation identified during the present simulation.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Simulation of commercial fixed-bed reactor for maleic anhydride synthesis: application of different kinetic models and industrial process data
Aspen Plus Simulation of Biomass Gasification: a Comprehensive Model Incorporating Reaction Kinetics, Hydrodynamics and Tar Production
Flue gas analysis for biomass and coal co-firing in fluidized bed: process simulation and validation
Data availability.
Upon request to the corresponding author, the user-defined functions (UDFs) used in the present CFD simulation will be made available to the readers.
Abbreviations
Effective diffusivity of species \(i\) in the catalyst layer (m 2 s − 1 )
Binary diffusivity of \(i-j\) species pair (m 2 s − 1 )
Knudsen diffusivity of species \(i\) (m 2 s − 1 )
Effective diffusivity of species \(i\) in the flow channel (m 2 s − 1 )
Mean pore diameter (m)
Equilibrium constant for reaction \(k\) (see Supplement 2 for units)
Adsorption coefficient of species \(i\) (see Supplement 2 for units)
Viscous flow permeability (m 2 )
Effective thermal conductivity (W m − 1 K − 1 )
Reaction rate constant for reaction \(k\) (see Supplement 2 for units)
Half-height of flow channel (m)
Heat transfer coefficient (W m − 2 K − 1 )
Molecular mass of species \(i\) (kg kmol − 1 )
Nominal methane diffusion rate (kmol m − 2 s − 1 )
Molar flow rate of species \(i\) in the flow channel (mol s − 1 )
Partial pressure of species \(i\) (Pa)
Total pressure (Pa)
Area-specific reaction rate of reaction \(k\) (kmol m − 2 s − 1 )
Universal gas constant (8.314 J mol − 1 K − 1 )
Nominal reaction rate of reaction \(k\) (kmol m − 2 s − 1 )
Reaction rate for reaction \(k\) (kmol kg cat − 1 h − 1 )
Steam-to-carbon (S/C) ratio
Volumetric source for species \(i\) (kmol m − 3 s − 1 )
Temperature (K)
Thickness of dummy layer (m)
Thickness of substrate layer (m)
Thickness of the washcoat catalyst layer (m)
Diffusion volumes of species \(i\)
Mole fraction of species \(i\)
Coordinates (m)
Methane conversion ratio
Effective Thiele modulus for reaction \(k\)
Modified Thiele modulus for reaction \(k\)
Effectiveness factor for reaction \(k\)
Apparent catalyst density (kg m − 3 )
At the washcoat layer surface
Species index
Reaction index (I, II, III)
Inlet/outlet
H.S. Fogler, Elements of Chemical Reaction Engineering , 4th edn. (Prentice Hall, Englewood Cliffs, 2006)
Google Scholar
H. Zhang, Z. Sun, Y.H. Hu, Steam reforming of methane: current states of catalyst design and process upgrading. Renew. Sustain. Energy Rev. 149 , 111330 (2021). https://doi.org/10.1016/j.rser.2021.111330
Article CAS Google Scholar
I. Dincer, C. Acar, Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrog. Energy 40 , 11094–11111 (2015). https://doi.org/10.1016/j.ijhydene.2014.12.035
I. Staffell, D. Scamman, A.V. Abad, P. Balcombe, P.E. Dodds, P. Ekins, N. Shah, K.R. Ward, The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 12 , 463–491 (2019). https://doi.org/10.1039/C8EE01157E
D.D. Nguyen, S.I. Ngo, Y.I. Lim, W. Kim, U.D. Lee, D. Seo, W.L. Yoon, Optimal design of a sleeve-type steam methane reforming reactor for hydrogen production from natural gas. Int. J. Hydrog. Energy 44 , 1973–1987 (2019). https://doi.org/10.1016/j.ijhydene.2018.11.188
H. Ishaq, I. Dincer, C. Crawford, A review on hydrogen production and utilization: challenges and opportunities. Int. J. Hydrog. Energy 47 , 26238–26264 (2022). https://doi.org/10.1016/j.ijhydene.2021.11.149
E. Calò, A. Giannini, G. Monteleone, Small stationary reformers for H 2 production from hydrocarbons. Int. J. Hydrog. Energy 35 , 9828–9835 (2010). https://doi.org/10.1016/j.ijhydene.2010.03.067
U.H. Jung, W. Kim, K.Y. Koo, W.L. Yoon, Genuine design of compact natural gas fuel processor for 1-kWe class residential proton exchange membrane fuel cell systems. Fuel Process. Technol. 121 , 32–37 (2014). https://doi.org/10.1016/j.fuproc.2013.12.018
J.I. Yang, T.W. Kim, J.C. Park, T.H. Lim, H. Jung, D.H. Chun, Development of a stand-alone steam methane reformer for on-site hydrogen production. Int. J. Hydrog. Energy 41 , 8176–8183 (2016). https://doi.org/10.1016/j.ijhydene.2015.10.154
J.R. Han, S. Lee, J.M. Lee, Development of 3D CFD model of compact steam methane reforming process for standalone applications. Kor. J. Chem. Eng. 39 , 1182–1193 (2022). https://doi.org/10.1007/s11814-021-1029-4
E. Meloni, M. Martino, V. Palma, A short review on Ni based catalysts and related engineering issues for methane steam reforming. Catalysts 10 , 352 (2020). https://doi.org/10.3390/catal10030352
S.Z. Abbas, V. Dupont, T. Mahmud, Kinetics study and modelling of steam methane reforming process over a NiO/Al 2 O 3 catalyst in an adiabatic packed bed reactor. Int. J. Hydrog. Energy 42 , 2889–2903 (2017). https://doi.org/10.1016/j.ijhydene.2016.11.093
A.G. Dixon, Local transport and reaction rates in a fixed bed reactor tube: endothermic steam methane reforming. Chem. Eng. Sci. 168 , 156–177 (2017). https://doi.org/10.1016/j.ces.2017.04.039
D. Pashchenko, A combined experimental and numerical investigation of flow dynamic in a methane reformer filled with α-Al 2 O 3 -supported catalyst. Int. J. Heat Mass Transf. 133 , 1110–1120 (2019). https://doi.org/10.1016/j.ijheatmasstransfer.2018.12.150
M. Zanfir, A. Gavriilidis, Catalytic combustion assisted methane steam reforming in a catalytic plate reactor. Chem. Eng. Sci. 58 , 3947–3960 (2003). https://doi.org/10.1016/S0009-2509(03)00279-3
D.M. Murphy, A. Manerbino, M. Parker, J. Blasi, R.J. Kee, N.P. Sullivan, Methane steam reforming in a novel ceramic microchannel reactor. Int. J. Hydrog. Energy 38 , 8741–8750 (2013). https://doi.org/10.1016/j.ijhydene.2013.05.014
C. Cao, N. Zhang, Y. Cheng, Numerical analysis on steam methane reforming in a plate microchannel reactor: effect of washcoat properties. Int. J. Hydrog. Energy 41 , 18921–18941 (2016). https://doi.org/10.1016/j.ijhydene.2016.09.034
M. Mundhwa, C.P. Thurgood, Numerical study of methane steam reforming and methane combustion over the segmented and continuously coated layers of catalysts in a plate reactor. Fuel Process. Technol. 158 , 57–72 (2017). https://doi.org/10.1016/j.fuproc.2016.12.002
M.J. Stutz, D. Poulikakos, Optimum washcoat thickness of a monolith reactor for syngas production by partial oxidation of methane. Chem. Eng. Sci. 63 , 1761–1770 (2008). https://doi.org/10.1016/j.ces.2007.11.032
H.S. Roh, D.K. Lee, K.Y. Koo, U.H. Jung, W.L. Yoon, Natural gas steam reforming for hydrogen production over metal monolith catalyst with efficient heat-transfer. Int. J. Hydrog. Energy 35 , 1613–1619 (2010). https://doi.org/10.1016/j.ijhydene.2009.12.051
V. Palma, A. Ricca, E. Meloni, M. Martino, M. Miccio, P. Ciambelli, Experimental and numerical investigations on structured catalysts for methane steam reforming intensification. J. Clean. Prod. 111 , 217–230 (2016). https://doi.org/10.1016/j.jclepro.2015.09.004
P. Inbamrung, T. Sornchamni, C. Prapainainar, S. Tungkamani, P. Narataruksa, G.N. Jovanovic, Modeling of a square channel monolith reactor for methane steam reforming. Energy 152 , 383–400 (2018). https://doi.org/10.1016/j.energy.2018.03.139
D. Pashchenko, R. Mustafin, A. Mustafina, Steam methane reforming in a microchannel reformer: experiment, CFD-modelling and numerical study. Energy 237 , 121624 (2021). https://doi.org/10.1016/j.energy.2021.121624
L. Baharudin, M.J. Watson, Monolithic substrate support catalyst design considerations for steam methane reforming operation. Rev. Chem. Eng. 34 , 481–501 (2018). https://doi.org/10.1515/revce-2016-0048
J. Xu, G.F. Froment, Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics. AIChE J. 35 , 88–96 (1989). https://doi.org/10.1002/aic.690350109
J. Xu, G.F. Froment, Methane steam reforming: II. Diffusional limitations and reactor simulation. AIChE J. 35 , 97–103 (1989). https://doi.org/10.1002/aic.690350110
A. Jeong, D. Shin, S.M. Baek, J.H. Nam, Effectiveness factor correlations from simulations of washcoat nickel catalyst layers for small-scale steam methane reforming applications. Int. J. Hydrog. Energy 43 , 15398–15411 (2018). https://doi.org/10.1016/j.ijhydene.2018.06.059
Y.S. Oh, J.H. Nam, A numerical study on the active reaction thickness of nickel catalyst layers used in a low-pressure steam methane reforming process. Int. J. Hydrog. Energy 46 , 7712–7721 (2021). https://doi.org/10.1016/j.ijhydene.2020.11.280
S.M. Baek, J.H. Kang, K.J. Lee, J.H. Nam, A numerical study of the effectiveness factors of nickel catalyst pellets used in steam methane reforming for residential fuel cell applications. Int. J. Hydrog. Energy 39 , 9180–9192 (2014). https://doi.org/10.1016/j.ijhydene.2014.04.067
J.H. Nam, Effectiveness factor correlations for spherical nickel catalyst pellets used in small-scale steam methane reformers. Int. J. Hydrog. Energy 40 (16), 5644–5652 (2015). https://doi.org/10.1016/j.ijhydene.2015.02.119
ANSYS Fluent User’s Guide (Release 2021 R1). ANSYS Inc.
R.B. Bird, W.E. Stewart, E.N. Lightfoot, Transport Phenomena , 2nd edn. (Wiley, New York, 2002)
E.N. Fuller, P.D. Schettler, J.C. Giddings, New method for prediction of binary gas-phase diffusion coefficients. Ind. Eng. Chem. 58 , 18–27 (1966). https://doi.org/10.1021/ie50677a007
M. Kaviany, Principles of Heat Transfer in Porous Media , 2nd edn. (Springer, New York, 1999)
E.A. Mason, A.P. Malinauskas, Gas Transport in Porous Media: The Dusty Gas Model (Elsevier, New York, 1983)
R. Krishna, J.A. Wesselingh, The Maxwell–Stefan approach to mass transfer. Chem. Eng. Sci. 52 , 861–911 (1997). https://doi.org/10.1016/S0009-2509(96)00458-7
Download references
This research was supported by Daegu University Research Fund (Grant no. 2020-0327).
Author information
Authors and affiliations.
School of Mechanical Engineering, Seoul National University, Seoul, 08826, Republic of Korea
Yun Seok Oh & Hyun-Joo Oh
Home Appliance and Air Solution R&D Center, LG Electronics Inc., Seoul, 08592, Republic of Korea
Hyun-Joo Oh
School of Mechanical and Automotive Engineering, Daegu University, 201 Daegudae-ro, Jinryang-eup, Gyungsan, 38453, Republic of Korea
Jin Hyun Nam
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Jin Hyun Nam .
Ethics declarations
Conflict of interest.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 1321 KB)
Rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Oh, Y.S., Oh, HJ. & Nam, J.H. Validation of Effectiveness Factor Correlations for Steam Methane Reforming in Ni-Based Washcoat Catalyst Layers Using Commercial Computational Fluid Dynamics Software. Korean J. Chem. Eng. (2024). https://doi.org/10.1007/s11814-024-00186-2
Download citation
Received : 12 June 2023
Revised : 27 March 2024
Accepted : 10 May 2024
Published : 21 May 2024
DOI : https://doi.org/10.1007/s11814-024-00186-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Steam methane reforming
- Effectiveness factor correlation
- Washcoat catalyst layer
- Computation fluid dynamics
- Numerical validation
- ANSYS Fluent
- Find a journal
- Publish with us
- Track your research
Help | Advanced Search
Physics > Chemical Physics
Title: design and implementation of a new apparatus for astrochemistry: kinetic measurements of the ch + ocs reaction and frequency comb spectroscopy in a cold uniform supersonic flow.
Abstract: We present the development of a new astrochemical research tool HILTRAC, the Highly Instrumented Low Temperature ReAction Chamber. The instrument is based on a pulsed form of the CRESU (Cinétique de Réaction en Écoulement Supersonique Uniforme, meaning reaction kinetics in a uniform supersonic flow) apparatus, with the aim of collecting kinetics and spectroscopic information on gas phase chemical reactions important in interstellar space or planetary atmospheres. We discuss the apparatus design and its flexibility, the implementation of pulsed laser photolysis followed by laser induced fluorescence (PLP-LIF), and the first implementation of direct infrared frequency comb spectroscopy (DFCS) coupled to the uniform supersonic flow. Achievable flow temperatures range from 32(3) - 111(9) K, characterising a total of five Laval nozzles for use with N2 and Ar buffer gases by pressure impact measurements. These results were further validated using LIF and DFCS measurements of the CH radical and OCS, respectively. Spectroscopic constants and linelists for OCS are reported for the 1001 band near $2890 - 2940 cm^{-1}$ for both $OC^{32}S$ and $OC^{34}S$, measured using DFCS. Additional peaks in the spectrum are tentatively assigned to the OCS-Ar complex. The first reaction rate coefficients for the CH + OCS reaction measured between 32(3) K and 58(5) K are reported. The reaction rate coefficient at 32(3) K was measured to be $3.9(4) \times 10^{10} cm^3 molecule^{-1} s^{-1}$ and the reaction was found to exhibit no observable temperature dependence over this low temperature range.
Submission history
Access paper:.
- Other Formats
References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

8.1: Introduction to Chemical Reaction Rates
- Last updated
- Save as PDF
- Page ID 431435

\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
By the end of this section, you will be able to:
- Define chemical reaction rate
- Derive rate expressions from the balanced equation for a given chemical reaction
- Calculate reaction rates from experimental data
- Describe the effects of chemical nature, physical state, temperature, concentration, and catalysis on reaction rates
Introduction
The lizard in the Figure \(\PageIndex{1}\) photograph is not simply enjoying the sunshine or working on its tan. The heat from the sun’s rays is critical to the lizard’s survival. A warm lizard can move faster than a cold one because the chemical reactions that allow its muscles to move occur more rapidly at higher temperatures. A cold lizard is a slower lizard and an easier meal for predators.
From baking a cake to determining the useful lifespan of a bridge, rates of chemical reactions play important roles in our understanding of processes that involve chemical changes. Two questions are typically posed when planning to carry out a chemical reaction. The first is: “Will the reaction produce the desired products in useful quantities?” The second question is: “How rapidly will the reaction occur?” A third question is often asked when investigating reactions in greater detail: “What specific molecular-level processes take place as the reaction occurs?” Knowing the answer to this question is of practical importance when the yield or rate of a reaction needs to be controlled.
The study of chemical kinetics concerns the second and third questions—that is, the rate at which a reaction yields products and the molecular-scale means by which a reaction occurs. This chapter examines the factors that influence the rates of chemical reactions, the mechanisms by which reactions proceed, and the quantitative techniques used to describe the rates at which reactions occur.
Reaction Rates
A rate is a measure of how some property varies with time. Speed is a familiar rate that expresses the distance traveled by an object in a given amount of time. Wage is a rate that represents the amount of money earned by a person working for a given amount of time. Likewise, the rate of a chemical reaction is a measure of how much reactant is consumed, or how much product is produced, by the reaction in a given amount of time.
The rate of reaction is the change in the amount of a reactant or product per unit time. Reaction rates are therefore determined by measuring the time dependence of some property that can be related to reactant or product amounts. Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in volume or pressure. For reactions involving one or more colored substances, rates may be monitored via measurements of light absorption. For reactions involving aqueous electrolytes, rates may be measured via changes in a solution’s conductivity.
For reactants and products in solution, their relative amounts (concentrations) are conveniently used for purposes of expressing reaction rates. For example, the concentration of hydrogen peroxide, H 2 O 2 , in an aqueous solution changes slowly over time as it decomposes according to the equation:
Figure \(\PageIndex{2}\) provides an example of data collected during the decomposition of H 2 O 2 . To obtain the tabulated results for this decomposition, the concentration of hydrogen peroxide was measured every 6 hours over the course of a day at a constant temperature of 40 °C. The reaction rate between any two measurements is determined by finding the change in concentration, Δ[H 2 O 2 ], and the change in time, Δ t . Reaction rates are, by convention, positive quantities, and so this negative change in concentration is multiplied by −1. Reaction rates were computed for each time interval by dividing the change in concentration by the corresponding time increment, as shown here for the first 6-hour period:
Notice that the reaction rates vary with time, decreasing as the reaction proceeds. Results for the last 6-hour period yield a reaction rate of:
This behavior indicates the reaction continually slows with time. Using the concentrations at the beginning and end of a time period over which the reaction rate is changing results in the calculation of an average rate for the reaction over this time interval.
Instantaneous Reaction Rate
The change in concentration with time shown in Figure \(\PageIndex{2}\) can also be made into a graph, as shown in Figure \(\PageIndex{3}\). The slope of the curve in this graph is another way to find the rate of a reaction. At any specific time, the slope of this line is the rate of the reaction.
Chemistry in Everyday Life
Reaction rates in analysis: test strips for urinalysis.
Physicians often use disposable test strips to measure the amounts of various substances in a patient’s urine ( Figure \(\PageIndex{4}\) ). These test strips contain various chemical reagents, embedded in small pads at various locations along the strip, which undergo changes in color upon exposure to sufficient concentrations of specific substances. The usage instructions for test strips often stress that proper read time is critical for optimal results. This emphasis on read time suggests that kinetic aspects of the chemical reactions occurring on the test strip are important considerations.
The test for urinary glucose relies on a two-step process represented by the chemical equations shown here:
\( \mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6+\mathrm{O}_2 \stackrel{\text {catalyst}}{\longrightarrow} \mathrm{C}_6 \mathrm{H}_{10} \mathrm{O}_6+\mathrm{H}_2 \mathrm{O}_2 \)
\( \mathrm{2 H}_2 \mathrm{O}_{2} +\mathrm{2I}^- \stackrel{\text {catalyst}}{\longrightarrow} \mathrm{I}_2 + \mathrm{2H}_{2} \mathrm{O}+\mathrm{O}_2 \)
+ 2 I − → catalyst I 2 + 2H 2 O + O 2
The first equation depicts the oxidation of glucose in the urine to yield glucolactone and hydrogen peroxide. The hydrogen peroxide produced subsequently oxidizes colorless iodide ion to yield brown iodine, which may be visually detected. Some strips include an additional substance that reacts with iodine to produce a more distinct color change.
The two test reactions shown above are inherently very slow, but their rates are increased by special enzymes embedded in the test strip pad. This is an example of catalysis , a topic discussed later in this chapter. A typical glucose test strip for use with urine requires approximately 30 seconds for completion of the color-forming reactions. Reading the result too soon might lead one to conclude that the glucose concentration of the urine sample is lower than it actually is (a false-negative result). Waiting too long to assess the color change can lead to a false positive due to the slower (not catalyzed) oxidation of iodide ion by other substances found in urine.
Factors Affecting Reaction Rates
The rates at which reactants are consumed and products are formed during chemical reactions vary greatly. Five factors typically affecting the rates of chemical reactions will be explored in this section: the chemical nature of the reacting substances, the state of subdivision (one large lump versus many small particles) of the reactants, the temperature of the reactants, the concentration of the reactants, and the presence of a catalyst.
The Chemical Nature of the Reacting Substances
The rate of a reaction depends on the nature of the participating substances. Reactions that appear similar may have different rates under the same conditions, depending on the identity of the reactants. For example, when small pieces of the metals iron and sodium are exposed to air, the sodium reacts completely with air overnight, whereas the iron is barely affected. The active metals calcium and sodium both react with water to form hydrogen gas and a base. Yet calcium reacts at a moderate rate, whereas sodium reacts so rapidly that the reaction is almost explosive.
The Physical States of the Reactants
A chemical reaction between two or more substances requires intimate contact between the reactants. When reactants are in different physical states, or phases (solid, liquid, gaseous, dissolved), the reaction takes place only at the interface between the phases. Consider the heterogeneous reaction between a solid phase and either a liquid or gaseous phase. Compared with the reaction rate for large solid particles, the rate for smaller particles will be greater because the surface area in contact with the other reactant phase is greater. For example, large pieces of iron react more slowly with acids than they do with finely divided iron powder (Figure \(\PageIndex{5}\)). Large pieces of wood smolder, smaller pieces burn rapidly, and saw dust burns explosively.
Link to Learning
Watch this video to see the reaction of cesium with water in slow motion and a discussion of how the state of reactants and particle size affect reaction rates.
Temperature of the Reactants
Chemical reactions typically occur faster at higher temperatures. Food can spoil quickly when left on the kitchen counter. However, the lower temperature inside of a refrigerator slows that process so that the same food remains fresh for days. Gas burners, hot plates, and ovens are often used in the laboratory to increase the speed of reactions that proceed slowly at ordinary temperatures. For many chemical processes, reaction rates are approximately doubled when the temperature is raised by 10 °C.
Concentrations of the Reactants
The rates of many reactions depend on the concentrations of the reactants. Rates usually increase when the concentration of one or more of the reactants increases. For example, calcium carbonate (CaCO 3 ) deteriorates as a result of its reaction with the pollutant sulfur dioxide. The rate of this reaction depends on the amount of sulfur dioxide in the air (Figure \(\PageIndex{6}\)). An acidic oxide, sulfur dioxide combines with water vapor in the air to produce sulfurous acid in the following reaction:
Calcium carbonate reacts with sulfurous acid as follows:
In a polluted atmosphere where the concentration of sulfur dioxide is high, calcium carbonate deteriorates more rapidly than in less polluted air. Similarly, phosphorus burns much more rapidly in an atmosphere of pure oxygen than in air, which is only about 20% oxygen.
Phosphorus burns rapidly in air, but it will burn even more rapidly if the concentration of oxygen is higher. Watch this video to see an example.
The Presence of a Catalyst
Relatively dilute aqueous solutions of hydrogen peroxide, H 2 O 2 , are commonly used as topical antiseptics. Hydrogen peroxide decomposes to yield water and oxygen gas according to the equation:
Under typical conditions, this decomposition occurs very slowly. When dilute H 2 O 2 (aq) is poured onto an open wound, however, the reaction occurs rapidly and the solution foams because of the vigorous production of oxygen gas. This dramatic difference is caused by the presence of substances within the wound’s exposed tissues that accelerate the decomposition process. Substances that function to increase the rate of a reaction are called catalysts , a topic treated in greater detail later in this chapter.
Chemical reactions occur when molecules collide with each other and undergo a chemical transformation. Before physically performing a reaction in a laboratory, scientists can use molecular modeling simulations to predict how the parameters discussed earlier will influence the rate of a reaction. Use the PhET Reactions & Rates interactive to explore how temperature, concentration, and the nature of the reactants affect reaction rates.
1. Introduction
2. experimental procedure, 4. discussion, 5. conclusion, supporting information.

research papers \(\def\hfill{\hskip 5em}\def\hfil{\hskip 3em}\def\eqno#1{\hfil {#1}}\)

In situ XAFS–XRD study of the Zr–Y 2 O 3 interaction at extra-high temperatures
a Laboratory for Zero-Carbon Energy, Tokyo Institute of Technology, 2-12-1 N1-3 Ookayama, Meguro-ku, Tokyo 152-8550, Japan, b Institute of Materials Research, Tohoku University, 2145-2 Narita-cho, Oarai-cho, Ibaraki-gun, Ibaraki 311-1313, Japan, c Research Institute of Nuclear Engineering, University of Fukui, 1-3-33 Tetsuwa-cho, Tsuruga-shi, Fukui 914-0055, Japan, and d Materials Science Research Center, Japan Atomic Energy Agency, 1-1-1 Kouto, Sayo-cho, Sayo-gun, Hyogo 679-5148, Japan * Correspondence e-mail: [email protected]
The in situ measurement technique for a metal/metal-oxide mixture at extra-high temperature above 2000 K has been desired in the field of nuclear safety engineering. In the present study, we succeeded in simultaneous XAFS–XRD measurements of the Zr oxidation [Zr + O → Zr(O) + ZrO 2 ] up to 1952 K and ZrO 2 –Y 2 O 3 reaction from 1952 to 2519 K. The chemical shift during Zr oxidation was observed in the absorption spectra around the Zr K -edge, and the interatomic cation–cation and cation–oxygen distances obtained by the fitting analysis of EXAFS during the Y 2 O 3 –ZrO 2 reaction are explained. Also, the temperature dependency of the anharmonic effect was investigated by comparing the fitted second- and third-order cumulants with the theoretical ones in which the Morse potential was applied as an interatomic potential, giving a good explanation about the local structure dynamics. Finally, the applicability of the developed system to investigation of nuclear fuel materials, such as UO 2 –Zr, is discussed.
Keywords: in situ XAFS–XRD ; Zr–Y 2 O 3 ; extra-high temperature. ; Zr–Y2O3 .
2.1. Measurement system
2.2. sample preparation.
Specimens were prepared by mixing Y 2 O 3 powder (99.99% purity, Kojundo Chemical Laboratory Co. Ltd) and isopropyl alcohol (extra-pure reagent, Kanto CHEMICAL Co. Inc.) in a mortar to produce a slurry. To test the Zr–Y 2 O 3 mixture (Zr:Y 2 O 3 = 50:50 wt%), Zr powder (99.9% purity, FUJIFILM Wako Pure Chemical Co. Ltd) was mixed with Y 2 O 3 powder. The slurry sample was pasted onto the slit (60 µm wide) of the W holder, dried, and stabilized by heating at 673 K for 20 h. All preparations were conducted in an inert glove box. After the XAFS–XRD measurements, the specimens were removed from the heating chamber. Then metallographic analyses were performed via scanning electron microscopy with electron dispersion spectroscopy (SEM/EDS; JSM-6010 LV, Jeol) and transmission electron microscopy with a spherical aberration corrector (AC-TEM; ARM 200F, Jeol) to investigate the final state of the sample materials.
2.3. Data analysis
3.1. temperature calibration with y 2 o 3 measurements, 3.2. xafs–xrd measurement for zr–y 2 o 3, 3.2.1. xrd profiles and sem/tem of the cooled sample, 3.2.2. chemical shift of the zr- k absorption spectra, 3.2.3. exafs spectra during heating and after cooling.
As the temperature increases, the peak intensity of RSF for Zr—Zr decreases and almost disappears at 1952 K, whereas the appearance of the peak at ∼1.5 Å suggests that the first coordination was changed from Zr—Zr to Zr—O. Regarding the RSF for the Y- K edge, the peak intensity of Y—Y decreases above 1508 K and almost disappears at 2224 K.
4.1. Phase transitions and local structure change during the heating process
4.2. influence of anharmonic phonon oscillations on the local structure.
where σ 2 is the second-order cumulant, C 3 is the third-order cumulant, D and α are parameters in the Morse potential, a is the interatomic distance between the absorbing and scattering atoms, k B is the Boltzmann constant and T is temperature. As described in the equations, σ 2 arising mainly from harmonic phonon oscillation varies linearly on T reflecting attenuation of the EXAFS amplitude, while C 3 from anharmonic effects depends on T 2 reflecting the asymmetry of the interatomic distance distribution (deviation from the symmetric Gaussian distribution).
4.3. State of the cooled sample after the heating process
4.4. applicability of the developed system to the uo 2 –zr reaction.
In the present study, we succeeded in simultaneous XAFS–XRD measurements of reaction processes in metals and metal-oxides at ultra-high temperatures above 2000 K. The structural changes in which Zr was oxidized by the impurity oxygen in Ar gas and the ZrO 2 –Y 2 O 3 reaction proceeded were successfully captured. Since the specimen was very small, it was difficult to measure the temperature using a pyrometer. To solve this problem, temperature calibration was performed using the diffraction pattern of Y 2 O 3 , for which there is some knowledge on the temperature dependence of the lattice parameter at high temperatures. Although this method has the limitation that the same equation cannot be used if the heat transfer characteristics of the sample holder change, we think that this method could be applied to different combinations of materials if temperature calibration is performed against the oxide material in advance. Also, in the present study, Zr was oxidized by residual oxygen in the gas phase. To analyze the melting process of UO 2 –Zr, the oxygen partial pressure in the furnace must be controlled at a sufficiently low level.
The quality of the obtained spectra was acceptable for EXAFS analysis, although the signal-to-noise ratio in the high-energy region tends to deteriorate above ∼2000 K. For diffraction images, it is desirable to be able to scan transmitted X-rays at higher angles to improve the distinguishability of cubic or tetragonal oxide. This can be overcome by improving the equipment, such as widening the glass window at the exit of the chamber's X-ray transmission window. Thus, by implementing some device modifications, it is possible to apply this measurement technique to UO 2 –Zr mixtures and investigate the structural changes leading to the melting process.
In situ simultaneous XAFS–XRD measurements of Y 2 O 3 and Zr–Y 2 O 3 at high temperatures above 2000 K were performed. The XAFS signal was taken in transmission mode for the Zr K - and Y K -edges, and the transmitted X-rays were captured by CCD camera as diffraction images. By combining analysis of the XRD patterns and EXAFS spectra, we succeeded in capturing the structure changes during the Zr oxidation up to 1952 K and the interaction between ZrO 2 and Y 2 O 3 for temperatures from 1952 and 2519 K. The interatomic distances obtained by the EXAFS fitting analysis were confirmed to agree with the literature data for the ZrO 2 –Y 2 O 3 system. Also, we investigated the temperature dependence of the anharmonic effect by comparing the fitted second- and third-order cumulants, leading to the conclusion that thermal disorder was mainly caused by anharmonic effects during the Y 2 O 3 –ZrO 2 reaction. Consequently, the developed system in the present study was confirmed to be applicable for investigation of the metal/metal-oxide mixture at extra-high temperature. For application to the melting of nuclear fuel material, although some modification on the equipment will be required, it is possible to adapt this technique for investigation of the UO 2 –Zr reaction leading to the melting process as future work.
Figures S1 to S3. DOI: https://doi.org/10.1107/S1600577524003321/yn5107sup1.pdf
Acknowledgements
The synchrotron radiation experiments were performed at BL22XU of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal Nos. 2023A3710 and 2023B3740).
Funding information
This work was supported by the Innovative Nuclear Research and Development Program by Ministry of Education, Culture, Sports, Science, and Technology (MEXT) in Japan (JPMXD0223813709).
This is an open-access article distributed under the terms of the Creative Commons Attribution (CC-BY) Licence , which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are cited.

IMAGES
VIDEO
COMMENTS
5: Introduction to Chemical Reactions. 241554. Chemical change is a central concept in chemistry. The goal of chemists is to know how and why a substance changes in the presence of another substance or even by itself. Because there are tens of millions of known substances, there are a huge number of possible chemical reactions.
The state of matter of reactants and products is designated with the symbols (s) for solids, (l) for liquids, and (g) for gases. Chemical reaction, a process in which one or more substances, the reactants, are converted to one or more different substances, the products. Substances are either chemical elements or compounds.
A chemical reaction is a chemical change, which means the starting materials are chemically different from the ending materials. In contrast, matter also changes form via physical changes. But, in a physical change, the chemical identity of matter does not change. For example, when you melt an ice cube into liquid water, the chemical identity ...
Respiration, the process by which we inhale and metabolize oxygen, is a series of redox reactions. In the absence of oxygen, redox reactions still occur in a process called anaerobic metabolism. Antioxidants such as ascorbic acid also play a part in the human diet, acting as reducing agents in various biochemical reactions.
Chemical reactions. Chemical reactions occur when chemical bonds between atoms are formed or broken. The substances that go into a chemical reaction are called the reactants, and the substances produced at the end of the reaction are known as the products. An arrow is drawn between the reactants and products to indicate the direction of the ...
Correctly define a law as it pertains to science. State the law of conservation of matter. Define chemical reaction. Use a balanced chemical equation to represent a chemical reaction.Calculate the amount of one substance that will react with or be produced from a given amount of another substance.Classify a given chemical reaction into a variety of types.Identify a chemical reaction as an ...
Chemical equations are how chemists describe chemical reactions, the process by which one form of matter ( reactants) turn into another form of matter ( products ). Below is the balanced chemical reaction for the combustion of methane (CH 4) with oxygen (the reactants) to produce carbon dioxide and water (the products), see Figure 2. Figure 2.
Opening Essay. Although yeast has been used for thousands of years, its true nature has been known only for the last two centuries. Yeasts are single-celled fungi. ... This is "Introduction to Chemical Reactions", chapter 5 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0).
What a chemical reaction is • A chemical reaction involves the making and breaking of chemical bonds to form new compounds. Since chemical bonds are made of shared or transferred electrons, when a chemical reaction occurs, it is the electrons that change. The nucleus is not changed, so the same atoms are present at the beginning and the end ...
Introduction to the atom: Atoms, compounds, and ions Ions and compounds: Atoms, compounds, ... Molecular composition: Chemical reactions and stoichiometry Types of chemical reactions: Chemical reactions and stoichiometry. Unit 4: More about chemical reactions. Net ionic equations: ...
No chemical reaction is a chemical reaction without both of these components. Introduction to Chemical Equations. On paper, a chemical reaction can be written out in the form of a chemical equation, which shows the reaction taking place. Of course there is always a way to describe the reaction in words too.
Chemical Reactions -A chemical reaction is in which the bonds are broken within reactant molecules, and new bonds are formed within product molecules in order to form a new substance. Chemical reactions are all around us. Chemical reactions are continually taking place on our planet. To learn Definition, Equations, Types, Examples with FAQs of Chemical Reactions.
Carbonic acid, H2CO3, can be formed by at least two different ways. One way is the one you've stated by reacting dissolved carbon dioxide with water: H2O (l) + CO (aq) → H2CO3 (aq). But another is Sal's method in the video where we add bicarbonate to an acidic solution: H^ (+) (aq) + HCO3^ (-) (aq) → H2CO3 (aq).
Chemical change is a central concept in chemistry. The goal of chemists is to know how and why a substance changes in the presence of another substance or even by itself. Because there are tens of millions of known substances, there are a huge number of possible chemical reactions. In this chapter, we will find that many of these reactions can ...
Chemical reactions and physical changes. Physical changes, such as melting, boiling and dissolving, do not make new chemicals. They are usually easy to reverse. In a chemical reaction, chemical ...
a chemical reaction. Reggie and Charlotte are baking oatmeal cookies. They dip the baked cookies in melted chocolate. The chocolate cools to form a hardened coating. Reggie argues the entire chocolate-covered cookie has undergone a chemical change. Charlotte disagrees, saying only the original oatmeal cookie has undergone a chemical change, not ...
Take notes along the way and try to see things from different angles. When it comes to writing your paper, aim for a thoughtful response, not just a knee-jerk reaction. Back up your points with solid evidence and organize them well. Think of it more like writing a review than leaving a quick comment on a movie website.
From the start of a synthetic chemist's training, experiments are conducted based on recipes from textbooks and manuscripts that achieve clean reaction outcomes, allowing the scientist to develop practical skills and some chemical intuition. This procedure is often kept long into a researcher's career, as new recipes are developed based on similar reaction protocols, and intuition-guided ...
reaction is the rearrangement of atoms by breaking and reforming chemical bonds. Reggie and Charlotte are baking oatmeal cookies. They dip the baked cookies in melted chocolate. The chocolate cools to form a hardened coating. Reggie argues the entire chocolate-covered cookie has undergone a chemical change. Charlotte disagrees, saying only the ...
In this study, the effectiveness factor correlations proposed for steam methane reforming (SMR) in Ni-based washcoat catalyst layers were numerically validated using the commercial computational fluid dynamics (CFD) software, ANSYS Fluent. The SMR process in an exemplary microchannel reformer was simulated, once by fully considering the reaction and diffusion process within the washcoat ...
3.2: Types of Chemical Reactions. 3.3: Balancing Chemical Equations. 3.4: Aqueous Reactions. Aqueous solution is any solution where water is present as a solvent. Rain, vinegar, orange juice are all examples of aqueous solutions that you come across in your everyday life. In chemistry aqueous solution indicated by adding " (aq)" to the reactant ...
Understanding the modification mechanism of C9 petroleum resin (C9PR) on styrene-butadiene-styrene (SBS) polymer modified asphalt properties is of significant importance. In this paper, dynamic shear rheometer (DSR), storage stability, fluorescence morphology (FM), scanning electron microscope (SEM), Fourier transform-infrared (FTIR) spectrometer, and molecular dynamic (MD) simulation were ...
We present the development of a new astrochemical research tool HILTRAC, the Highly Instrumented Low Temperature ReAction Chamber. The instrument is based on a pulsed form of the CRESU (Cinétique de Réaction en Écoulement Supersonique Uniforme, meaning reaction kinetics in a uniform supersonic flow) apparatus, with the aim of collecting kinetics and spectroscopic information on gas phase ...
The reaction rate between any two measurements is determined by finding the change in concentration, Δ [H 2 O 2 ], and the change in time, Δ t. Reaction rates are, by convention, positive quantities, and so this negative change in concentration is multiplied by −1. Reaction rates were computed for each time interval by dividing the change ...
1. Introduction. For the safety evaluation of nuclear fuel materials, it is important to obtain knowledge on the high-temperature reactions that cause fuel-cladding melting. In previous fuel-melting experiments, UO 2 -Zr diffusion couples were heated to a target temperature using an induction furnace, held for a certain period of time, cooled to room temperature, and then the samples were ...