

4.2 Names and Structures for Hydrocarbons
As we considered organic structures in the earlier portions of this book, alkanes were presented as examples because they are in many ways the simplest of organic molecules. And yet hydrocarbons can be very small or very large, can include straight chains of carbons or elaborate branching, and can have ring structures and even bridging carbons over rings. The alkenes and alkynes have double and triple bonds that produce new complexities in the structures, as well as reactivity. Then there is the issue of aromaticity, a phenomenon related to structure that is also common among hydrocarbon compounds.
Hydrocarbons are not simple!
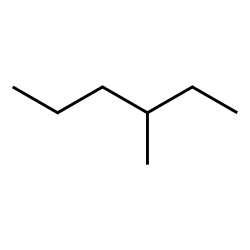
You have already learned the basic naming conventions for small (1-10 carbon) straight chain and somewhat branched alkanes. These include a suffix ‘ane’ to indicate membership in the alkane family, the base of the name related to the number of carbons (e.g. ‘hex’ for a six-carbon parent chain) and indication of branching with a location for the branch and a name for it:
Check your understanding by completing the questions in the short quiz here.
Exercise 4.2.1-3
When faced with a structure containing a functional group such as an alkene, the name of the related alkane can be a good starting point. Most elements of the name will be the same, with the exception that the identity and location of the functional group itself needs to be conveyed somehow. For the alkenes, the suffix used is no longer ‘ane,’ but is now ‘ene.’ The location of the double bond is identified with a number. Count a parent chain that includes the alkene, counting from the end of the chain with the lowest possible number assignment given to the double bond. Then use the number of the carbon where the double bond is first encountered as the location indicator. Current IUPAC rules put the number immediately before the ‘ene’ suffix, but name changes are sometimes accepted rather slowly; it remains very common to see this number earlier in the name:
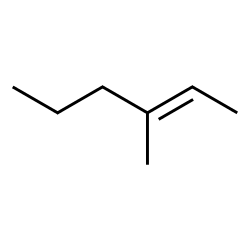
Acceptable names for this molecule include 3-methylhex-2-ene and 3-methyl-2-hexene. IUPAC rules encourage placing the location identifier close to the feature at that location. The first name follows IUPAC rules to the letter. However these names can seem awkward even to chemists, and the second form is used frequently.
Other aspects of naming alkenes are identical to the process used for alkanes: the parent chain is indicated by the base name and the branches are numbered and named just as they are for alkanes.
Geometric Isomers in Alkenes
One other structural variation occurs with alkenes. The geometry of the carbon-carbon double bond is fixed, with no rotation along the axis of the double bond. Thus, two different isomers of a substance are often possible. Just like the structural isomers we have already considered, these are related but different compounds that have the same molecular formula. However, the geometric isomers are also the same in terms of their atom connectivity: each atom in the molecule has the same types of bonds (same connections) to its neighbors. The difference lies only in the 3-dimensional layout of the molecule.
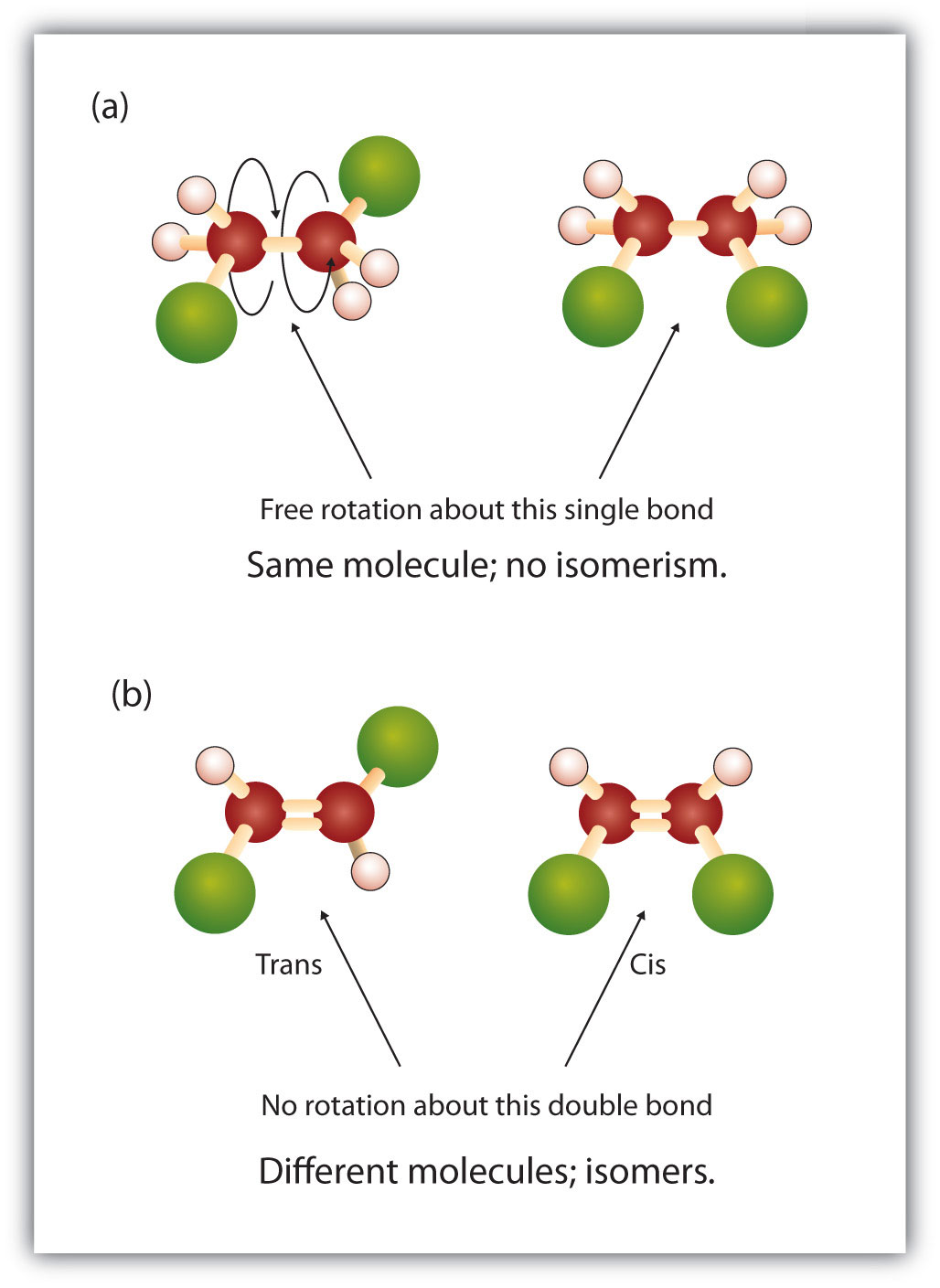
The word prefixes ‘cis’ and ‘trans’ are commonly used to indicate which of the two geometric isomers is being identified.

The ‘cis’ and ‘trans’ nomenclature is based on the parent chain: if the chain comes into the double bond on one side (long axis) of the double bond and leaves on the same side, it is a ‘cis’ isomer. If the parent chain leaves the double bond opposite where it came in, the isomer is termed ‘trans.’
IUPAC has a system for handling the distinction between these geometric isomers that uses the letters E and Z. For IUPAC, E and Z names are derived by applying a set of rules that rank the groups connected to each carbon of the double bond, and assign one with a higher priority than the other. By then following the trail of these groups, from higher priority atom through the two carbons of the double bond and out the other side, the arrangement can be identified and named. If these higher priority groups enter and exit the alkene on the same side (along the axis of the double bond) the molecule is described as ‘Z,’ short for zusammen, German for ‘together.’ If the groups enter and exit the double bond on opposite sides the structure is identified as ‘E’ for entgegen, meaning ‘opposite.’
Z and E isomers often correspond with cis and trans isomers, but not always, since the priority groups are usually but not always in the parent chain.
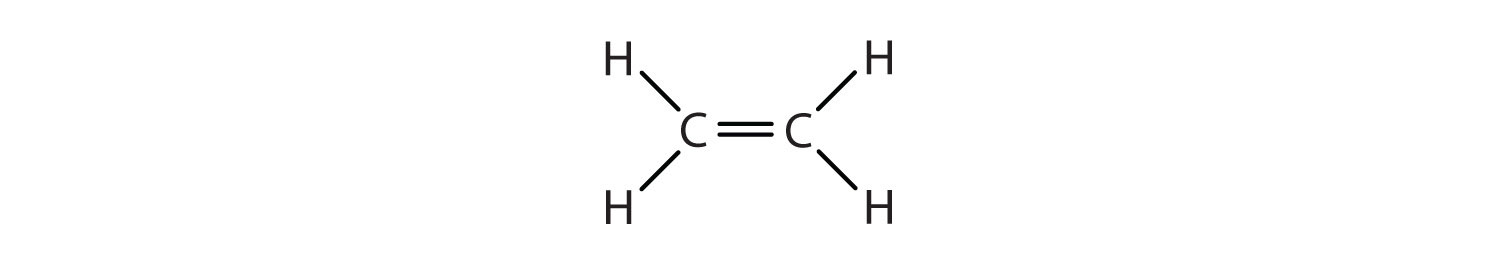
Note that alkenes at the end of a carbon chain will not exhibit this kind of isomerism, because the carbon at the end of the chain has two hydrogens on it:
Why does this geometric distinction matter? The reason is because these isomers are different substances, and will have different characteristics. Specific isomers must be incorporated into pharmaceuticals containing alkenes, for instance, if they are to have the desired characteristics and not deleterious effects.
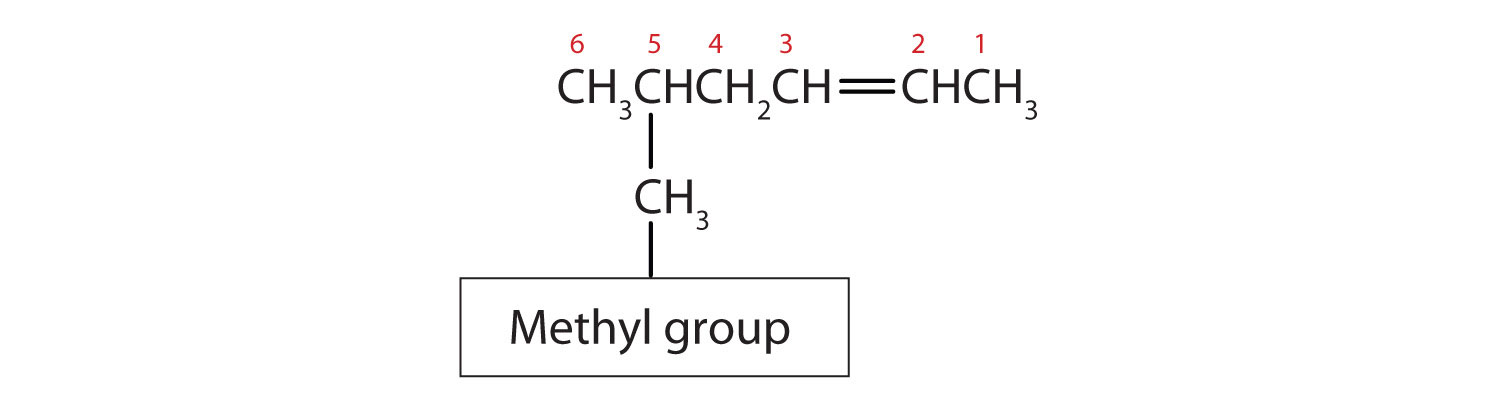
If you reconsider the structure above you should now recognize that it is one of these isomers. Since the parent chain comes into carbon 2 from below and exits from carbon three above the plane of the double bond, this is a trans isomer and could be better named as E-3-Methylhex-2-ene.
Remember these systematic names are coded information, and like any code it takes time and practice (and frequent errors along the way) to learn. With repeated use the code becomes familiar and you can become fluent in reading and understanding the names and structures.
You can practice naming some alkenes by completing the quiz here.
Exercise 4.2.4-6
Alkynes are named similarly to alkenes but without the concern for designating cis or trans isomers.
Exercise 4.2.7-8
An alkene or alkyne having one or more multiple (double or triple) bonds between carbon atoms is called unsaturated. This is because they have fewer hydrogen atoms than does an alkane with the same number of carbon atoms, as is indicated in the following general formulas:
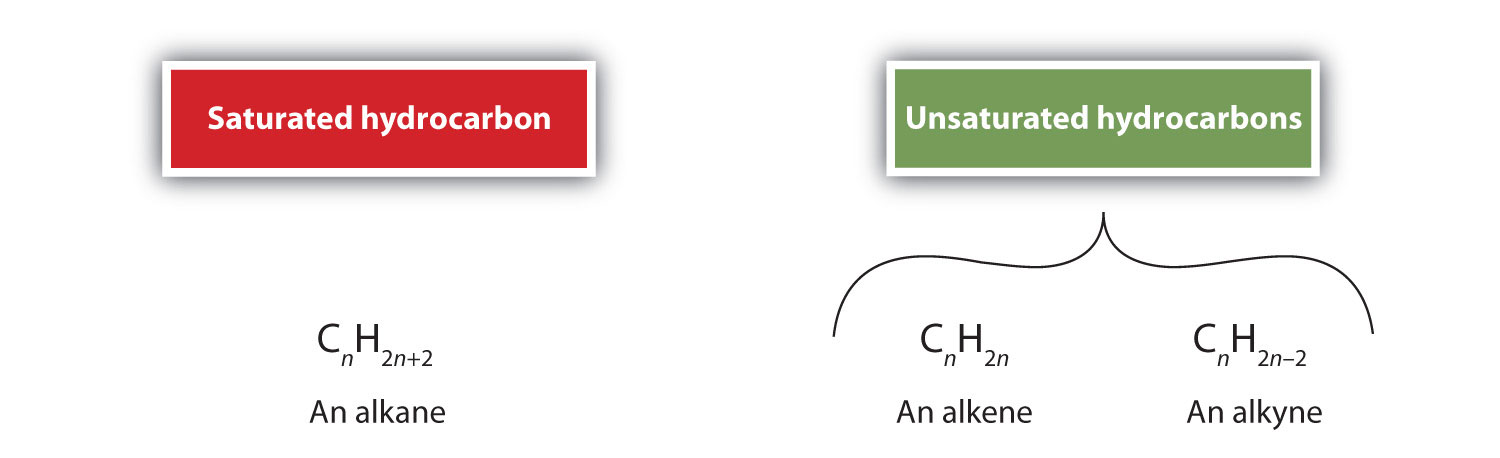
Summary of Naming Rules for Alkenes and Alkynes
The Rules for Naming Alkenes According to the International Union of Pure and Applied Chemistry (IUPAC) are summarized here:
- The longest chain of carbon atoms containing the double or triple bond is considered the parent chain. It is named using the same stem as the alkane having the same number of carbon atoms but ends in – ene to identify it as an alkene. Thus the compound CH 2 =CHCH 3 is propene . Alkynes are similarly indicated, using the suffix -yne.
- If there are four or more carbon atoms in a chain, we must indicate the position of the double or triple bond. The carbons atoms are numbered so that the first of the two that are doubly or triply bonded is given the lower of the two possible numbers. The compound CH 3 CH=CHCH 2 CH 3 , for example, has the double bond between the second and third carbon atoms. Its name is 2-pentene (not 3-pentene).
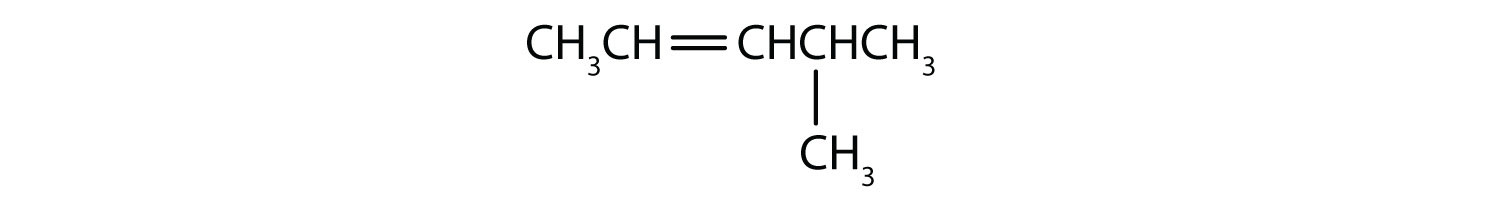
Substituent groups are named as with alkanes, and their position is indicated by a number. Thus,
is 5-methyl-2-hexene. Note that the numbering of the parent chain is always done in such a way as to give the double bond the lowest number, even if that causes a substituent to have a higher number. The double bond always has priority in numbering.
- For alkenes, identify the specific geometric isomer as necessary by using the E or Z tag.
Worked Examples
Name each compound, without concerning yourself with E/Z designation.
- The longest chain containing the double bond has five carbon atoms, so the compound is a pentene (rule 1). To give the first carbon atom of the double bond the lowest number (rule 2), we number from the left, so the compound is a 2-pentene. There is a methyl group on the fourth carbon atom (rule 3), so the compound’s name is 4-methyl-2-pentene.
- The longest chain containing the double bond has four carbon atoms, so the parent compound is a butene (rule 1). (The longest chain overall has five carbon atoms, but it does not contain the double bond, so the parent name is not pentene .) To give the first carbon atom of the double bond the lowest number (rule 2), we number from the left, so the compound is a 1-butene. There is an ethyl group on the second carbon atom (rule 3), so the compound’s name is 2-ethyl-1-butene.
Exercise 4.2.9
Name this compound, without specifying which geometric isomer:
CH 3 CH 2 CH 2 CH 2 CH 2 CH=CHCH 3
Exercise 4.2.10
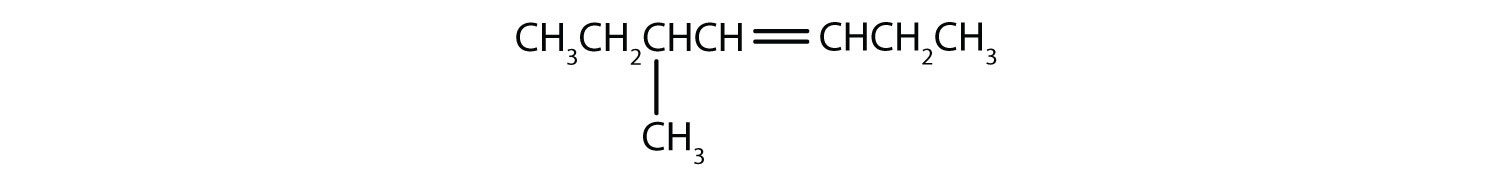
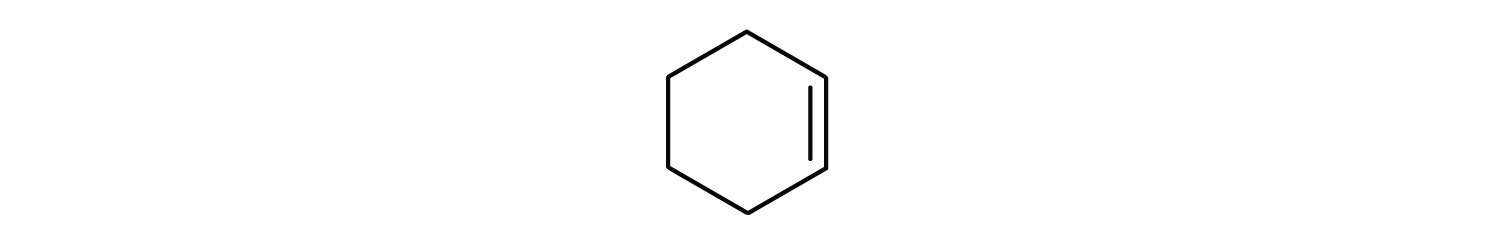
Just as there are cycloalkanes, there are cycloalkenes . These compounds are named like alkenes, but with the prefix cyclo – attached to the beginning of the parent alkene name.
Draw the structure for each compound.
- 3-methyl-2-pentene
cyclohexene
First write the parent chain of five carbon atoms: C–C–C–C–C. Then add the double bond between the second and third carbon atoms:

Now place the methyl group on the third carbon atom and add enough hydrogen atoms to give each carbon atom a total of four bonds.
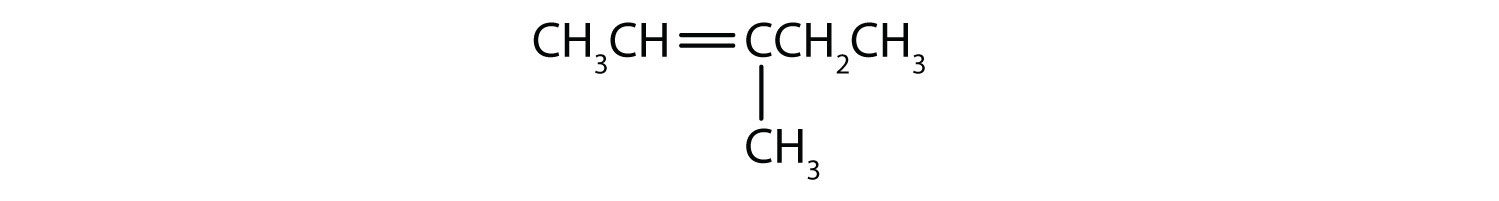
First, consider what each of the three parts of the name means. Cyclo means a ring compound, hex means 6 carbon atoms, and – ene means a double bond.
For each structure listed here, draw a line-bond structure. If you are unable to draw them, describe them in detail with words. Then check your drawing against a reliable source such as Wikipedia, ChemSpider or PubChem. Evaluate the length of the parent chain, the presence and location of branches, and for alkenes, also consider the issue of geometric (cis-trans) isomers.
2-ethyl-1-hexene
cyclopentene
2-methyl-2-pentene
2,3-dimethyl-1-butene
5-methyl-1-hexene
3-ethyl-2-pentene
Exercise 4.2.12
Draw (Z)-4-methyl-2-hexene. If you are unable to draw it, describe it in detail with words. Why is it that the name for this compound requires a (Z) while 3-ethyl-2-pentene (from the prior exercise) does not?
Exercises 4.2.13-15
Name each compound according to the IUPAC system.
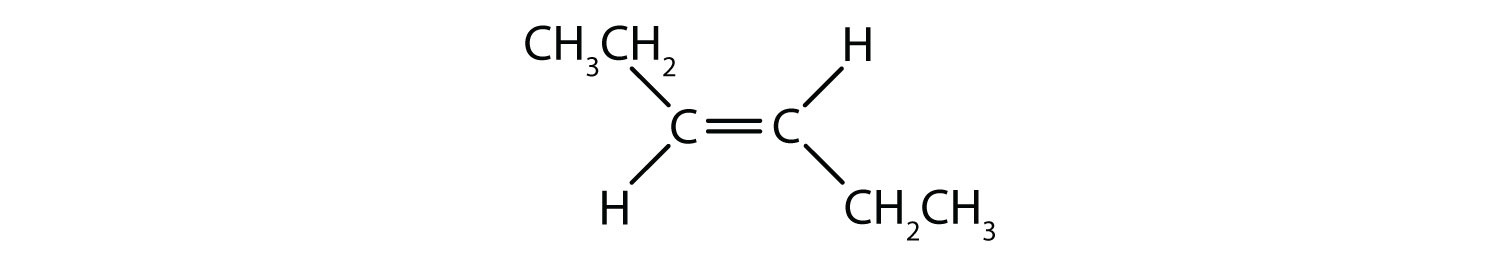
Exercises 4.2.16-19
Classify each compound as a cis isomer, a trans isomer, or neither.
Introductory Organic Chemistry Copyright © 2021 by Carol Higginbotham is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
Chapter 10. Organic Chemistry
10.3 Nomenclature of Hydrocarbons and Alkyl Halides
Learning objectives.
By the end of this section, you will be able to:
- Name saturated and unsaturated hydrocarbons and alkyl halides following the IUPAC rules
- From the name a saturated or unsaturated hydrocarbon or alkyl halides, draw its structure
The largest database [1] of organic compounds lists about 10 million substances, which include compounds originating from living organisms and those synthesized by chemists. The number of potential organic compounds has been estimated [2] at 10 60 —an astronomically high number. The existence of so many organic molecules is a consequence of the ability of carbon atoms to form up to four strong bonds to other carbon atoms, resulting in chains and rings of many different sizes, shapes, and complexities.
The simplest organic compounds contain only the elements carbon and hydrogen, and are called hydrocarbons. Even though they are composed of only two types of atoms, there is a wide variety of hydrocarbons because they may consist of varying lengths of chains, branched chains, and rings of carbon atoms, or combinations of these structures. In addition, hydrocarbons may differ in the types of carbon-carbon bonds present in their molecules. Many hydrocarbons are found in plants, animals, and their fossils; other hydrocarbons have been prepared in the laboratory. We use hydrocarbons every day, mainly as fuels, such as natural gas, acetylene, propane, butane, and the principal components of gasoline, diesel fuel, and heating oil. The familiar plastics polyethylene, polypropylene, and polystyrene are also hydrocarbons. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms.
The Basics of Organic Nomenclature:
Organic chemistry nomenclature is very specific following the general format shown in Figure 1. The International Union of Pure and Applied Chemistry ( IUPAC ) has devised a system of nomenclature that begins with the names of the alkanes and can be adjusted from there to account for more complicated structures.

Naming Alkanes and Alkyl Halides
The IUPAC nomenclature for alkanes and alkyl halides is based on two rules:
Rule 1. Identify the longest chain of carbon atoms (PREFIX+ANE). The longest chain of carbons in the structure is referred to as the parent chain. A two-carbon parent chain is called ethane; a three-carbon parent chain, propane; and a four-carbon parent chain, butane. Longer parent chains are named as follows: pentane (five-carbon chain), hexane (6), heptane (7), octane (8), nonane (9), and decane (10). These prefixes can be seen in the names of the alkanes described in Table 1 .
Rule 2. Names and position of the substituents: Substituents are branches or functional groups that replace hydrogen atoms on a chain. If there are substituents on the parent chain, their names and position on the chain must be included at the front of the name. The position of a substituent or branch is identified by the number of the carbon atom it is bonded to in the chain. We number the carbon atoms in the chain by counting from the end of the chain nearest the substituents. Multiple substituents are named individually and placed in alphabetical order at the front of the name.

When more than one substituent is present, either on the same carbon atom or on different carbon atoms, the substituents are listed alphabetically. Because the carbon atom numbering begins at the end closest to a substituent, the longest chain of carbon atoms is numbered in such a way as to produce the lowest number for the substituents. The ending -o replaces -ine at the end of the name of a halide substituent. For example, an iodine substituent would be called iodo. The number of substituents of the same type is indicated by the prefixes di- (two), tri- (three), tetra- (four), penta- (five) and so on (for example, difluoro- indicates two fluoride substituents).

The four-carbon chain is numbered from the end with the chlorine atom. This puts the substituents on positions 1 and 2 (numbering from the other end would put the substituents on positions 3 and 4). Four carbon atoms means that the base name of this compound will be butane. The bromine at position 2 will be described by adding 2-bromo-; this will come at the beginning of the name, since bromo- comes before chloro- alphabetically. The chlorine at position 1 will be described by adding 1-chloro-, resulting in the name of the molecule being 2-bromo-1-chlorobutane.

3,3-dibromo-2-iodopentane

The open bonds in the methyl and ethyl groups indicate that these alkyl groups are bonded to another atom.
Branched hydrocarbons may have more than one substituent. If the substituents are different, give each substituent a number (using the smallest possible numbers) and list the substituents in alphabetical order, with the numbers separated by hyphens and no spaces in the name. So the molecule shown here is 3-ethyl-2-methylpentane.

If the substituents are the same, use the name of the substituent only once, but use more than one number, separated by a comma and put a numerical prefix before the substituent name that indicates the number of substituents of that type. Consider this molecule:

The longest chain has four C atoms, so it is a butane. There are two substituents, each of which consists of a single C atom; they are methyl groups. The methyl groups are on the second and third C atoms in the chain (no matter which end the numbering starts from), so we would name this molecule 2,3-dimethylbutane. Note the comma between the numbers, the hyphen between the numbers and the substituent name, and the presence of the prefix di – before the methyl . Other molecules—even with larger numbers of substituents—can be named similarly.

Solution The longest carbon chain runs horizontally across the page and contains six carbon atoms (this makes the base of the name hexane, but we will also need to incorporate the name of the branch). In this case, we want to number from right to left (as shown by the blue numbers) so the branch is connected to carbon 3 (imagine the numbers from left to right—this would put the branch on carbon 4, violating our rules). The branch attached to position 3 of our chain contains two carbon atoms (numbered in red)—so we take our name for two carbons eth- and attach -yl at the end to signify we are describing a branch. Putting all the pieces together, this molecule is 3-ethylhexane.

4-propyloctane
Name this molecule.

The longest continuous carbon chain has seven C atoms, so this molecule is named as a heptane. There is a two-carbon substituent on the main chain, which is an ethyl group. To give the substituent the lowest numbering, we number the chain from the right side and see that the substituent is on the third C atom. So this hydrocarbon is 3-ethylheptane.
Test Yourself

2-methylpentane

The longest chain has seven C atoms, so we name this molecule as a heptane. We find two one-carbon substituents on the second C atom and a two-carbon substituent on the third C atom. So this molecule is named 3-ethyl-2,2-dimethylheptane.

4,4,5-tripropyloctane

Want more practice naming alkanes? Watch this brief video tutorial to review the nomenclature process.
Naming Alkenes

Therefore when naming alkenes following IUPAC, you follow the same two rules for alkanes with modification to “rule 1” mentioned above.
Rule 1. Identify the longest chain of carbons which contains the double bond and its position (PREFIX-#-ENE). And when numbering the main chain, the double gets the lowest possible number.
Rule 2. Names and position of the substituents.
For example, this molecule is 2,4-dimethylhept-3-ene. Note the number and the hyphens that indicate the position of the double bond.

Recycling Plastics
Ethylene (the common industrial name for ethene) is a basic raw material in the production of polyethylene and other important compounds. Over 135 million tons of ethylene were produced worldwide in 2010 for use in the polymer, petrochemical, and plastic industries.
Polymers (from Greek words poly meaning “many” and mer meaning “parts”) are large molecules made up of repeating units, referred to as monomers. Polymers can be natural (starch is a polymer of sugar residues and proteins are polymers of amino acids) or synthetic [like polyethylene, polyvinyl chloride (PVC), and polystyrene]. The variety of structures of polymers translates into a broad range of properties and uses that make them integral parts of our everyday lives. Adding functional groups to the structure of a polymer can result in significantly different properties (see the discussion about Kevlar later in this chapter).
An example of a polymerization reaction is shown in Figure 2 . The monomer ethylene (C 2 H 4 ) is a gas at room temperature, but when polymerized, using a transition metal catalyst, it is transformed into a solid material made up of long chains of –CH 2 – units called polyethylene. Polyethylene is a commodity plastic used primarily for packaging (bags and films).

Polyethylene is a member of one subset of synthetic polymers classified as plastics. Plastics are synthetic organic solids that can be molded; they are typically organic polymers with high molecular masses. Most of the monomers that go into common plastics (ethylene, propylene, vinyl chloride, styrene, and ethylene terephthalate) are derived from petrochemicals and are not very biodegradable, making them candidate materials for recycling. Recycling plastics helps minimize the need for using more of the petrochemical supplies and also minimizes the environmental damage caused by throwing away these nonbiodegradable materials.
Plastic recycling is the process of recovering waste, scrap, or used plastics, and reprocessing the material into useful products. For example, polyethylene terephthalate (soft drink bottles) can be melted down and used for plastic furniture, in carpets, or for other applications. Other plastics, like polyethylene (bags) and polypropylene (cups, plastic food containers), can be recycled or reprocessed to be used again. Many areas of the country have recycling programs that focus on one or more of the commodity plastics that have been assigned a recycling code (see Figure 3 ). These operations have been in effect since the 1970s and have made the production of some plastics among the most efficient industrial operations today.

Once you master naming hydrocarbons from their given structures, it is rather easy to draw a structure from a given name. Just draw the parent chain with the correct number of C atoms (putting the double or triple bond in the right position, as necessary) and add the substituents in the proper positions. If you start by drawing the C atom backbone, you can go back and complete the structure by adding H atoms to give each C atom four covalent bonds.
From the name 2,3-dimethyl-4-propylhept-2-ene, we start by drawing the seven-carbon parent chain with a double bond starting at the third carbon:

We add to this structure two one-carbon substituents on the second and third C atoms:

We finish the carbon backbone by adding a three-carbon propyl group to the fourth C atom in the parent chain:

If we so choose, we can add H atoms to each C atom to give each carbon four covalent bonds, being careful to note that the C atoms in the double bond already have an additional covalent bond. Question: How many H atoms do you think are required? There will need to be 24 H atoms to complete the molecule.
Draw the carbon backbone for 2,3,4-trimethylpentane.
First, we draw the five-carbon backbone that represents the pentane chain:
According to the name, there are three one-carbon methyl groups attached to the second, third, and fourth C atoms in the chain. We finish the carbon backbone by putting the three methyl groups on the pentane main chain:

Draw the carbon backbone for 3-ethyl-6,7-dimethyloct-2-ene.

Naming Alkynes
The simplest member of the alkyne series is ethyne, C 2 H 2 , commonly called acetylene.
The IUPAC nomenclature for alkynes is similar to that for alkenes except that the suffix -yne is used to indicate a triple bond in the chain. For example, [latex]\text{CH}_3\text{CH}_2\text{C}\;{\equiv}\;\text{CH}[/latex] is called but-1-yne.
Therefore when naming alkynes following IUPAC, you follow the same two rules for alkanes with modification to “rule 1” mentioned above.
Rule 1. Identify the longest chain of carbons which contains the triple bond and its position (PREFIX-#-YNE). And when numbering the main chain, the triple bond gets the lowest possible number.
Rule 2. Names and position of the substituents

Solution but-2-yne

pent-3-en-1-yne

The longest chain that contains the C–C triple bond has six C atoms, so this is a hexyne molecule. The triple bond starts at the third C atom, so this is a hex-3-yne. Finally, there are two methyl groups on the chain; to give them the lowest possible number, we number the chain from the left side, giving the methyl groups the second position. So the name of this molecule is 2,2-dimethylhex-3-yne.

2,3,4-trimethylpent-2-ene
Naming Arenes
The most commonly known arene is benzene.

Toluene and xylene are important solvents and raw materials in the chemical industry. Styrene is used to produce the polymer polystyrene. Toluene, xylene and styrene are common names for these compounds. The systematic way of naming these benzene derivatives is by following the the two rules:
Rule 1. Identify the arene ring (BENZENE).
Rule 2. Names and position (if more than one) of the substituents: If there are two or more substituents on a benzene molecule, the relative positions must be numbered. The substituent that is first alphabetically is assigned position 1, and the ring is numbered in a circle to give the other substituents the lowest possible number(s).

Therefore the systematic name for toluene is methylbenzene and for xylene is 1,2-dimethylbenzene.
Key Concepts and Summary
Hydrocarbons are organic compounds composed of only carbon and hydrogen. The alkanes are saturated hydrocarbons—that is, hydrocarbons that contain only single bonds. Alkenes and alkynes are unsaturated hydrocarbons. Alkenes contain one or more carbon-carbon double bonds. Alkynes contain one or more carbon-carbon triple bonds. Arenes, also known as aromatic hydrocarbons, contain ring structures with alternating single and double bonds.
The systematic methods of naming the various hydrocarbons follow a similar procedure and the names have three main parts:
1) specifying the information about the substituents,
2) specifying the information about the parent chain (or ring), and
3) the ending which specifies what functional group is present in the structure being named.
Alkanes: #-substituents – PREFIX + ANE
Alkenes: #-substituents – PREFIX -#- ENE
Alkynes: #-substituents – PREFIX -#- YNE
Arenes (specifically benzene derivatives): #-substituents – BENZ ENE
1. Write the chemical formula and Lewis structure of the following, each of which contains five carbon atoms:
a) an alkane b) an alkene c) an alkyne

a) hexane b) 3-methylpentane c) hex-3-ene
d) 4-methylpent-1-ene e) hex-3-yne f) 4-methylpent-2-yne
4. Give the complete IUPAC name for each of the following compounds:
a) [latex]\text{CH}_3\text{CH}_2\text{CBr}_2\text{CH}_3[/latex]
b) [latex](\text{CH}_3)_3\text{CCl}[/latex]

d) [latex]\text{CH}_3\text{CH}_2\text{C}\;{\equiv}\;\text{CH\;CH}_3\text{CH}_2\text{C}\;{\equiv}\;\text{CH}[/latex]

g) [latex](\text{CH}_3)_2\text{CHCH}_2\text{CH} = \text{CH}_2[/latex]
5. Butane is used as a fuel in disposable lighters. Write the Lewis structure for each isomer of butane.
6. Define hydrocarbon . What are the two general types of hydrocarbons?
7. Indicate whether each molecule is an aliphatic (open chain) or an arene. If it is aliphatic, identify the molecule as an alkane, an alkene, or an alkyne.

8. Indicate whether each molecule is an aliphatic or an arene. If it is aliphatic, identify the molecule as an alkane, an alkene, or an alkyne.

9. Name and draw the structural formulas for the four smallest alkanes.
10. Explain why you may see prop-1-ene written just as propene.
11. Name and draw the structural formula of each isomer of pentene.
12. Draw the structure of the product of the reaction of bromine with propene.
13. Draw the structure of the product of the reaction of hydrogen with but-1-ene.
14. How does a branched hydrocarbon differ from a normal hydrocarbon?
15. Name this molecule.

a) 3,4-diethyloctane
b) 2,2-dimethyl-4-propylnonane
26. Draw the carbon backbone for each molecule.
a) 4-ethyl-4-propyloct-2-yne
b) 5-butyl-2,2-dimethyldecane
27. The name 2-ethylhexane is incorrect. Draw the carbon backbone and write the correct name for this molecule.
1. There are several sets of answers; one is:

2. 2-hexene and 2-methylpentane

4. (a) 2,2-dibromobutane; (b) 2-chloro-2-methylpropane; (c) 2-methylbutane; (d) but-1-yne; (e) 4-fluoro-4-methyloct-1-yne; (f) 1-chloropropene; (g) 5-methylpent-1-ene

6. an organic compound composed of only carbon and hydrogen; aliphatic hydrocarbons and aromatic hydrocarbons
7. a) aliphatic; alkane b) arene c) aliphatic; alkene
8. a) aliphatic; alkane b) aliphatic; alkene c) arene d) aliphatic; alkyne e) arene
f) aliphatic; alkene g) aliphatic; alkene h) arene i) aliphatic; alkyne

10. The 1 is not necessary since the double bond is on the first carbon.

15. 3-methyl-hex-2-ene
16. 2,2,3-trimethylpentane
17. 4,4-dimethylpent-1-ene
18. 4,4-dimethylheptane
19. 2,4-dimethylpent-2-ene
20. hex-3-yne
21. 3,4-diethyloctane
22. 4,5-dimethylhept-3-ene
23. 1-bromo-4-chlorobenzene
24. 1-ethyl-2,3-dimethylbenzene

alkane: molecule consisting of only carbon and hydrogen atoms connected by single (σ) bonds
alkene: molecule consisting of carbon and hydrogen containing at least one carbon-carbon double bond
alkyl group: substituent, consisting of an alkane missing one hydrogen atom, attached to a larger structure
alkyne: molecule consisting of carbon and hydrogen containing at least one carbon-carbon triple bond
aromatic hydrocarbon: cyclic molecule consisting of carbon and hydrogen with delocalized alternating carbon-carbon single and double bonds, resulting in enhanced stability
functional group: part of an organic molecule that imparts a specific chemical reactivity to the molecule
organic compound: natural or synthetic compound that contains carbon
saturated hydrocarbon: molecule containing carbon and hydrogen that has only single bonds between carbon atoms
skeletal structure or line structure: shorthand method of drawing organic molecules in which carbon atoms are represented by the ends of lines and bends in between lines, and hydrogen atoms attached to the carbon atoms are not shown (but are understood to be present by the context of the structure)
substituent: branch or functional group that replaces hydrogen atoms in a larger hydrocarbon chain
- This is the Beilstein database, now available through the Reaxys site ( www.elsevier.com/online-tools/reaxys ). ↵
- Peplow, Mark. “Organic Synthesis: The Robo-Chemist,” Nature 512 (2014): 20–2. ↵
CHEM 1114 - Introduction to Chemistry Copyright © 2018 by Shirley Wacowich-Sgarbi is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Post-lockdown teaching support
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More from navigation items
Rules for naming hydrocarbons: alkanes to arenes | 16-18 years
- Four out of five
- No comments
Review the rules for naming hydrocarbon structures, including alkanes, alkenes, alkynes and arenes, using this lesson plan with activities for 16–18 year olds
In this activity, students assemble the names of hydrocarbon structures using component parts written on cards. By discussing the rules for naming hydrocarbons including alkanes, alkenes, alkynes and arenes, students check and clarify their understanding.
This activity is best used after students have spent some time on this topic.
Learning objectives
Students will:
- Understand and be able to use the rules for naming hydrocarbons including alkanes, alkenes, alkynes and arenes.
Sequence of activities
Introductory activity.
- Divide students into groups of three.
- Provide each group with a molecular model kit made up of four carbon atoms, 10 hydrogen atoms and 10 bonds of which four need to be ‘flexible’ bonds.
- Ask them to construct as many different molecules as possible using all or some of the component atoms and bonds.
- Invite groups to show and to name some of their molecules.
- Now share the learning objectives with the students.
Naming hydrocarbons: stage 1
Give each student a ’Student sheet’, and distribute to each group:
- A set of ‘Hydrocarbon naming cards’
- ‘Hydrocarbon structure cards’ from sheet 1, or ’Hydrocarbon structure cards’ from sheets 1 and 2.
Circulate and support groups as they:
- Shuffle the ‘Hydrocarbon structure cards’.
- Take turns in choosing a ‘Hydrocarbon structure card’ and use the ’Hydrocarbon naming cards’ to construct the appropriate name.
- Discuss and agree a name, where a student is unsure.
- Write the name of the hydrocarbon on their ’Student sheet’.
- Ask for a ’Names of structures answer sheet’, when they have named all the hydrocarbons.
- Compare the answers with their group answers and modify their own where necessary.
Naming hydrocarbons: stage 2
Ask students, in their groups, to:
- Identify and make a note of any types of structures that they all found difficult to name correctly.
- Assesses what type of structures each other student was able to name with confidence and what structures caused difficulty.
- Write these assessments on the ‘Student sheet’.
Introducing the learning objectives follows naturally from the initial task of constructing molecular models.
The group work, using cards, involves the students in peer assessment, which is formalised at the end of the session. Comparing their ideas with an answer grid helps students to recognise the standard they are aiming for.
The activity also includes an element of self assessment, both during the naming stage and formalised at the end of the sequence.
- Carbon atoms, x4
- Hydrogen atoms, x10
- Bonds, x10 (including four flexible bonds)
Naming hydrocarbons student sheet
Hydrocarbon naming cards, hydrocarbon structure cards 1, names of hydrocarbon structures answer sheet, hydrocarbon structure cards 2 (optional), additional information.
This lesson plan was originally part of the Assessment for Learning website, published in 2008.
Assessment for Learning is an effective way of actively involving students in their learning. Each session plan comes with suggestions about how to organise activities and worksheets that may be used with students.
- 16-18 years
- Formative assessment
- Lesson planning
- Organic chemistry
- Equations, formulas and nomenclature

Specification
- IUPAC rules for nomenclature.
- Apply IUPAC rules for nomenclature to name organic compounds limited to chains and rings with up to six carbon atoms each.
- Apply IUPAC rules for nomenclature to draw the structure of an organic compound from the IUPAC name limited to chains and rings with up to six carbon atoms each.
- 4. be able to name compounds relevant to this specification using the rules of International Union of Pure and Applied Chemistry (IUPAC) nomenclature Students will be expected to know prefixes for compounds up to C₁₀
- a) application of IUPAC rules of nomenclature for systematically naming organic compounds
- Straight-chain and branched alkanes can be systematically named from structural formulae containing no more than 8 carbons in the longest chain.
- Cycloalkanes (C₃–C₈) can be systematically named from structural formulae. Branched cycloalkanes are not required.
- Straight-chain and branched alkenes can be systematically named indicating the position of the double bond, from structural formulae containing no more than 8 carbon atoms in the longest chain.
- 2.2.2 apply International Union of Pure and Applied Chemistry (IUPAC) rules for nomenclature to name organic compounds with up to six carbon atoms and one or more functional groups;
- 2.2.3 draw and name structural isomers of aliphatic compounds containing up to six carbon atoms, excluding cyclic structures;
- 2.2.6 draw and identify the structural formulae of E and Z isomers.
- Alkenes: non-polar double bond. Structure and nomenclature up to C-4.
- Alkanes, alkenes and alkynes as homologous series. For alkynes only ethyne to be considered.
- Systematic names, stuctural formulas and structural isomers of alkanes to C-5.
- Systematic names, sturctural formulas and structural isomers of alkenes to C-4.
- (a) how to represent simple organic compounds using shortened, displayed and skeletal formulae
- (b) nomenclature rules relating to alkanes, alkenes, halogenoalkanes, alcohols and carboxylic acids
Related articles

Fractional distillation and hydrocarbons | Review my learning worksheets | 14–16 years
By Lyn Nicholls
Identify learning gaps and misconceptions with this set of worksheets offering three levels of support

5 ways to use structure strips effectively
2024-05-08T05:08:00Z By Kristy Turner
Bolster your students’ ability to write independently with these effective strategies

Escape the classroom: and revise chemistry knowledge
2024-05-03T09:21:00Z By Hayley Russell
Challenge your students to break out of the lab and prepare for exams
No comments yet
Only registered users can comment on this article., more from lesson plans.

Determining the structure of compounds | 16–18 years
Examine data relating to the structure and complexity of compounds, including mass, infrared and 1 H NMR spectra

How do scientists grow protein crystals? | 14-16 years
Discover the methods and conditions used by chemical scientists to grow protein crystals in this lesson plan with activities for 14–16 year olds.

How does sodium react with chlorine? | 14-16 years
Investigate the reaction of sodium with chlorine, using students’ understanding of atoms, ions and lattice structure, in this lesson plan for 14–16 year olds.
- Contributors
- Email alerts
Site powered by Webvision Cloud
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Biology library
Course: biology library > unit 4.
- Hydrocarbon overview
- Hydrocarbon structures and isomers
- Functional groups
Hydrocarbons and functional groups
- (Choice A) Hydrocarbons that contain only single covalent bonds between carbon atoms are known as alkynes. A Hydrocarbons that contain only single covalent bonds between carbon atoms are known as alkynes.
- (Choice B) Hydrocarbons can have the same molecular formula but different molecular geometries. B Hydrocarbons can have the same molecular formula but different molecular geometries.
- (Choice C) Hydrocarbons are composed entirely of hydrogen, carbon, and oxygen atoms. C Hydrocarbons are composed entirely of hydrogen, carbon, and oxygen atoms.
- (Choice D) Hydrocarbons can only form linear structures. D Hydrocarbons can only form linear structures.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

8: Hydrocarbons Structure & Nomenclature: Questions
- Last updated
- Save as PDF
- Page ID 44593
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Stan's Academy
Science Rules
Introduction to IUPAC naming of hydrocarbons
Introduction to IUPAC naming of hydrocarbons, in the video that follows you will have an introduction into the naming of hydrocarbons using IUPAC. The introduction includes naming of hydrocarbons based on a simple classification with general formulas, identification of alkyl groups and rules for naming hydrocarbons. Introduction to IUPAC naming of hydrocarbons.
- Organic Conversions
- Balancing of Equations
- Organic chemistry pages
Share this:
- Electrochemistry
- Thermodynamics
- Rates of Rxn
- Chemical Equilibrium
- Acids & Bases
- Organic Chemistry
- ORGANIC PREP
- Science Quizzes
- Video Lessons And Quizzes
- BoylesLawLab
- Order of a reaction
- KMnO4 Titration
- Equilibrium Pblm 01
- Kc using ICE table
- Generating ICE tables
- Hetro & Homogeneous
- Law of Mass Action
- Kc Quadratic Pblm
- Activation Energy – Ea
- Effect of Conc
- Effect of Tempt
- Initial Rate Pblm
- Mechanism in RXN
- Faradays Laws
- IUPAC Naming
- Assignments to submit
- Hydrocarbons
- Nomenclature Saturated Hydrocarbons
Nomenclature of Saturated Hydrocarbons
Nomenclature is a system of terms or rules that are used for forming these terms or names in a distinct field of science and arts. In simple terms it is an assignment of names to organic compounds. Saturated hydrocarbons are organic compounds that consist of carbon and hydrogen single bonds. In these compounds, there is the maximum number of hydrogen atom present for every carbon atoms. For example alkanes.
The saturated hydrocarbons are named according to the following rules:
Longest Chain Rule: The parent chain of the compound is considered the longest chain of carbon atoms.
Lowest Set of Locants: The numbering of the carbon atoms starts from the end which gives the lowest number to the carbon atom carrying the substituent.
Presence of Same Substituent More Than Once: Prefixes such as di, tri, etc are given to the substituents which are present twice, thrice respectively on the parent chain.
Naming Different Substituents: If more than one substituent is present then the substituents are arranged in their alphabetical order.
Naming Different Substituents At Equivalent Positions: If two different substituents are present in the same position from the two ends then the substituents are named so that the substituent which comes first in the alphabetical order gets the lowest number.
Naming The Complex Substituents: Naming of the complex substituent is done when the substituent on the parent chain has a branched structure (i.e complex structure). These substituents are named as a substituted alkyl group and the carbon atom of this substituent attached to the parent chain is numbered 1. The name of this type of substituent is written in brackets.
Let us understand it with the help of an example:

In this case, we have 9 carbon atoms in the straight chain. 5th Carbon atom from both the ends of the straight-chain, consists of substituents having 3 carbon chains. On the first two carbon atoms of the substituent group, there is one additional carbon atom attached.
Now if we consider this as a new parent chain, it has a substituent which has one additional carbon each. For naming them we will firstly number the parent chain. In this case, we have 9 carbon atoms in a straight chain which is also the parent chain. Then we find that the substituent is in the fifth position.

Now taking the substituent we will observe that we have 3 substituent carbons and out of these three, two substituents have additional carbons attached. We find that the longest chain in this can be the first four carbon atom chains but this is wrong as the last carbon is not attached to the parent chain.
So we will consider only three carbon atom chains as the main chain. Thus it can be named propane and in the first and second position, we have methyl group. We can write the name as 1-2 Dimethyl propane, but it will be written as 1-2 Dimethyl propyl as it is a substituent group.

Now taking the substituent with the parent chain we will get 5-(1-2-Dimethyl Propyl) and as the parent chain has 9 carbon atoms so, it will be named nonane. Thus, the final name of the compound will be 5-(1-2-Dimethyl Propyl)nonane.

Watch the video to understand the nomenclature of saturated hydrocarbons and get a deeper knowledge about the concept of nomenclature.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
So good for 🙏children’s and makes students mind so💪💪💪💪
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

The Federal Register
The daily journal of the united states government, request access.
Due to aggressive automated scraping of FederalRegister.gov and eCFR.gov, programmatic access to these sites is limited to access to our extensive developer APIs.
If you are human user receiving this message, we can add your IP address to a set of IPs that can access FederalRegister.gov & eCFR.gov; complete the CAPTCHA (bot test) below and click "Request Access". This process will be necessary for each IP address you wish to access the site from, requests are valid for approximately one quarter (three months) after which the process may need to be repeated.
An official website of the United States government.
If you want to request a wider IP range, first request access for your current IP, and then use the "Site Feedback" button found in the lower left-hand side to make the request.

IMAGES
VIDEO
COMMENTS
This is 3-methyl-hex-2-ene. Acceptable names for this molecule include 3-methylhex-2-ene and 3-methyl-2-hexene. IUPAC rules encourage placing the location identifier close to the feature at that location. The first name follows IUPAC rules to the letter. However these names can seem awkward even to chemists, and the second form is used frequently.
Cyclopropane. ethyne. 2,4-dimethylhexane. 2-methylbutane. 3-Ethyl-2-methylhexane. Cyclohexyne. Cyclohexene. Study with Quizlet and memorize flashcards containing terms like butane, but-1-ene, but-2-ene and more.
C6.1k recall that crude oil is a main source of hydrocarbons and is a feedstock for the petrochemical industry; OCR Chemistry A: Gateway. C6 Global challenges. C6.2 Organic chemistry. C6.2b name and draw the structural formulae, using fully displayed formulae, of the first four members of the straight chain alkanes, alkenes, alcohols and ...
Study with Quizlet and memorize flashcards containing terms like CH4, C3H8, C3H6 and more.
The longest chain of carbons in the structure is referred to as the parent chain. A two-carbon parent chain is called ethane; a three-carbon parent chain, propane; and a four-carbon parent chain, butane. Longer parent chains are named as follows: pentane (five-carbon chain), hexane (6), heptane (7), octane (8), nonane (9), and decane (10).
Basic Naming of Hydrocarbons Hydrocarbon names are based on: 1) type, 2) # of carbons, 3) side chain type and position 1) name will end in -ane, -ene, or -yne 2) the number of carbons is given by a "prefix" 1 meth- 2 eth- 3 prop- 4 but- 5 pent-6 hex- 7 hept- 8 oct- 9 non- 10 dec-Actually, all end in a, but a is dropped when next to a vowel.
Let's see learn how to name hydrocarbons by adding an appropriate suffix to the root word. The root word denotes the number of carbon atoms in the chain and the suffix denotes the presence of single, double, or triple covalent bonds. Few examples: Meth+ane, Eth+ene, Prop+yne. Khan Academy is a nonprofit organization with the mission of ...
Naming hydrocarbons student sheet Author: Derek Denby Subject: Use this handout with the accompanying lesson plan to check and reinforce your students' understanding of the rules for naming hydrocarbon structures. Created Date: 9/14/2020 12:19:40 PM
Prefix: Cyclo-. Ring Structure (Of carbon chain) Nickname for the Ring structure... bensyne ring [Most common ring structure] Suffix: -ane. only single bonds between carbon atoms in a parent chain. Study with Quizlet and memorize flashcards containing terms like Hydrocarbon, Prefixes, Prefix: Meth- and more.
This fact sheet introduces IUPAC naming of alkanes, alkenes and alkynes to students, with examples.
Understand and be able to use the rules for naming hydrocarbons including alkanes, alkenes, alkynes and arenes. Sequence of activities Introductory activity. Divide students into groups of three. Provide each group with a molecular model kit made up of four carbon atoms, 10 hydrogen atoms and 10 bonds of which four need to be 'flexible' bonds.
National Oceanic and Atmospheric Administration
Organic chemistry involves the study of many different compounds that share the same element: carbon. To name and classify these compounds, a systematic nomenclature is needed that can account for their structural and functional diversity. This module covers the basic rules and principles of organic nomenclature, as well as some common naming systems for different classes of organic molecules.
Hydrocarbons can only form linear structures. Report a problem. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere.
Download Page (PDF) Download Full Book (PDF) Resources expand_more. Periodic Table. Physics Constants. Scientific Calculator. Reference expand_more. Reference & Cite. Tools expand_more.
1-Ethyl-2-methylcyclohexane. . 3-Methylpentane. Hexane. 2-Methylbutane. 2,2-Dimethylbutane. Study with Quizlet and memorize flashcards containing terms like 1-Isopropyl-3,5-dimethylcyclohexane, Butane, Cyclopropane and more.
Introduction to IUPAC naming of hydrocarbons, in the video that follows you will have an introduction into the naming of hydrocarbons using IUPAC. The introduction includes naming of hydrocarbons based on a simple classification with general formulas, identification of alkyl groups and rules for naming hydrocarbons.
Nomenclature of Saturated Hydrocarbons. Nomenclature is a system of terms or rules that are used for forming these terms or names in a distinct field of science and arts. In simple terms it is an assignment of names to organic compounds. Saturated hydrocarbons are organic compounds that consist of carbon and hydrogen single bonds.
Student Name: Assignment: Naming Hydrocarbons . Name the following compounds. Structure . Name
ASSIGNMENT 1. The first step in naming hydrocarbons is to identify the longest continuous carbon chain. How many carbons are in the longest chain in each of the following organic? When doing this assignment you should use the naming and structure concepts taught in Chemtutor and the textbook (Davidson Ch 14). DO NOT use the internet to try to find the answers, as you will not have google on ...
How to name hydrocarbons. 1- count the longest chain of carbons (and name it) 2- number so that the branches get the smallest number possible 3- write the "address" and type of branch (methyl, ethyl) {use a prefix when necessary - like di for 2} 4- write the name of the long chain
Student Name: Assignment: Naming Hydrocarbons Name the following compounds. Structure Name 1. 2. Want to read all 2 pages? Previewing 2 of 2 pages Upload your study docs or become a member. View full document. End of preview. Want to read all 2 pages? Upload your study docs or become a member.
2-Methylbutane. hexane. 4-methylpent-2-ene. 3-ethyl-5-methylheptane. What is the IUPAC name? 4-Ethyl-3,5-dimethylheptane. What is the IUPAC name? Study with Quizlet and memorize flashcards containing terms like Cyclopropane, 2,2-Dimethylpentane, Pentane and more.
The Department of the Interior (the Department or DOI), acting through the Bureau of Ocean Energy Management (BOEM) and the Bureau of Safety and Environmental Enforcement (BSEE) ("the agencies"), is finalizing regulatory amendments to its renewable energy regulations under the authority of the...